UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
(Mark One)
OR
For the fiscal year ended
OR
For the transition period from to
OR
Date of event requiring this shell company report
Commission file number
(Exact name of Registrant as specified in its charter and translation of Registrant’s name into English)
(Jurisdiction of incorporation or organization)
(Address of principal executive offices)
Phone:
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| The |
Securities registered or to be registered pursuant to Section 12(g) of the Act. None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act. None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
The number of common shares outstanding as of
December 31, 2023 was
Indicate by check mark if the registrant is a well-known
seasoned issuer, as defined in Rule 405 of the Securities Act. ☐ Yes ☒
If this report is an annual or transition report,
indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange
Act of 1934. ☐ Yes ☒
Indicate by check mark whether the registrant (1) has
filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months
(or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements
for the past 90 days. ☒
Indicate by check mark whether the registrant has
submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405
of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| ☐ | Emerging growth company |
If an emerging growth company that prepares its
financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition
period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange
Act.
Indicate by check mark whether the registrant has
filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting
under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or
issued its audit report.
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP ☐ | by the International Accounting Standards Board ☒ | Other ☐ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. ☐ Item 17 ☐ Item 18
If securities are registered pursuant
to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect
the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
If this is an annual report,
indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). ☐ Yes
TABLE OF CONTENTS
i
ii
INTRODUCTION
Akanda Corp. was incorporated on July 16, 2021 in the Province of Ontario, Canada under the Business Corporations Act (Ontario). The principal listing of our ordinary shares is The Nasdaq Capital Market, or Nasdaq. We filed a registration statement on Form F-1 (File No.: 333-262436) with respect to our common shares with the U.S. Securities and Exchange Commission, or SEC, which was declared effective on March 14, 2022. Our common shares are listed on The Nasdaq Capital Market, under the symbol “AKAN”. As used in this Annual Report on Form 20-F, the terms “we,” “us,” “our”, “Akanda” and the “Company” mean Akanda Corp. and its subsidiaries, unless otherwise indicated.
FINANCIAL AND OTHER INFORMATION
Our financial statements appearing in this Annual Report on Form 20-F are prepared in accordance with the International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB. Our fiscal year ends on December 31 of each year as does our reporting year.
We have made rounding adjustments to some of the figures included in this Annual Report. Accordingly, numerical figures shown as totals in some tables may not be an arithmetic aggregation of the figures that precede them.
Statements made in this Annual Report concerning the contents of any contract, agreement or other document are summaries of such contracts, agreements or documents and are not complete descriptions of all of their terms. If we filed any of these documents as an exhibit to this Annual Report or to any registration statement that we previously filed, you may read the document itself for a complete description of its terms.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Various statements contained in this Annual Report on Form 20-F, including those that express a belief, expectation or intention, as well as those that are not statements of historical fact, are forward-looking statements. These forward-looking statements may include projections and estimates concerning our possible or assumed future results of operations, financial condition, business strategies and plans, market opportunity, competitive position, industry environment, and potential growth opportunities. In some cases, you can identify forward-looking statements by terms such as “may”, “might”, “will”, “should”, “believe”, “expect”, “could”, “would”, “intend”, “plan”, “anticipate”, “estimate”, “continue”, “predict”, “project”, “potential”, “target,” “goal” or other words that convey the uncertainty of future events or outcomes. You can also identify forward-looking statements by discussions of strategy, plans or intentions. We have based these forward-looking statements on our current expectations and assumptions about future events. While our management considers these expectations and assumptions to be reasonable, because forward-looking statements relate to matters that have not yet occurred, they are inherently subject to significant business, competitive, economic, regulatory and other risks, contingencies and uncertainties, most of which are difficult to predict and many of which are beyond our control. These and other important factors may cause our actual results, performance or achievements to differ materially from any future results, performance or achievements expressed or implied by the forward-looking statements in this Annual Report, including among other things:
| ● | our limited operating history; |
| ● | unpredictable events, such as the COVID-19 outbreak, and associated business disruptions; |
| ● | changes in cannabis laws, regulations and guidelines; |
| ● | decrease in demand for cannabis and cannabis-derived products; |
| ● | exposure to product liability claims and actions; |
iii
| ● | damage to our reputation due to negative publicity; |
| ● | risks associated with product recalls; |
| ● | the viability of our product offerings; |
| ● | our ability to attract and retain skilled personnel; |
| ● | maintenance of effective quality control systems; |
| ● | regulatory compliance risks; |
| ● | risks inherent in an agricultural business; |
| ● | increased competition in the markets in which we operate and intend to operate; |
| ● | the success of our continuing research and development efforts; |
| ● | risks associated with expansion into new jurisdictions; |
| ● | risks related to our international operations in Europe, including the implications of the United Kingdom’s recent withdrawal from the European Union; |
| ● | our ability to obtain and maintain adequate insurance coverage; |
| ● | our ability to identify and integrate strategic acquisitions, investments and partnerships and to manage our growth; |
| ● | our ability to raise capital and the availability of future financing; |
| ● | global economy risks; and |
| ● | our ability to maintain the listing of our securities on Nasdaq. |
Given the foregoing risks and uncertainties, you are cautioned not to place undue reliance on the forward-looking statements in this Annual Report. The forward-looking statements contained in this Annual Report are not guarantees of future performance, and our actual results of operations and financial condition may differ materially from such forward-looking statements. In addition, even if our results of operations and financial condition are consistent with the forward-looking statements in this Annual Report, they may not be predictive of results or developments in future periods. Except as required by applicable law, including the securities laws of the United States, we undertake no obligation to publicly release any update or revision to any forward-looking statements to reflect new information, future events or circumstances, or otherwise after the date hereof. Please see the Risk Factors section that appears in “Item 3. Key Information – D. Risk Factors.”
iv
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable.
ITEM 3. KEY INFORMATION
| A. | [Reserved] |
| B. | Capitalization and Indebtedness |
Not applicable.
| C. | Reasons for the Offer and Use of Proceeds |
Not applicable.
| D. | Risk Factors |
The following risks relate specifically to our business and should be considered carefully. Our business, financial condition and results of operations could be harmed by any of the following risks. As a result, the trading price of our common shares could decline and shareholders could lose part or all of their investment.
Risks Related to Our Business and Industry
We are an early-stage company with limited operating history and may never become profitable.
Akanda was only recently incorporated to be a holding company. Each of our operating subsidiaries, has a very limited operating history and has generated minimal revenue. Bophelo was formed and commenced operations in 2018 and was primarily engaged in construction and preparation activities since its inception. Bophelo made only one sale of cannabis flower to a local buyer in April 2022 and generated sales revenue of $31,123 in the twelve-month period ended December 31, 2022. During the year ended December 31, 2022, the Company determined that it no longer controlled Bophelo as a result of an insolvent liquidation. As a result of the loss of control, the Company derecognized the net assets of Bophelo and accounted for the operating results as discontinued operation. Canmart was formed in 2018 and commenced operations in 2020. Canmart generated sales revenue of approximately $423,683 in the twelve-month period ended December 31, 2023 (2022 — $101,778). During the year ended December 31, 2022, the Company acquired Holigen Limited (“Holigen”) and its wholly-owned subsidiary, RPK Biopharma Unipessoal, LDA (“RPK”). RPK generated sales revenue of approximately $1,736,369 during the year ended December 31, 2023 (2022 — $1,933,203). We remain an early-stage company and have limited financial resources and minimal operating cash flow. If we cannot successfully develop, manufacture and distribute our products, or if we experience difficulties in the development process, such as capacity constraints, quality control problems or other disruptions, we may not be able to develop or offer market-ready commercial products at acceptable costs, which would adversely affect our ability to effectively enter the market or expand our market share. A failure by us to achieve a low-cost structure through economies of scale or improvements in cultivation, manufacturing or distribution processes would have a material adverse effect on our commercialization plans and our business, prospects, results of operations and financial condition.
1
We expect to require additional funding to maintain and expand our operations and develop our sales and distribution channels. However, there can be no assurance that additional funding will be available to us for the development of our business, which will require the commitment of substantial resources. Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in the early stages of development. Potential investors should carefully consider the risks and uncertainties that an early stage company with a very limited operating history will face. In particular, potential investors should consider that we may be unable to:
| ● | successfully implement or execute our business plan, or that our business plan is sound; |
| ● | effectively pursue business opportunities, including potential acquisitions; |
| ● | adjust to changing conditions or keep pace with increased demand; |
| ● | attract and retain an experienced management team; or |
| ● | raise sufficient funds in the capital markets to effectuate our business plan, including expanding production capacity. licensing and approvals. |
Our financial situation creates doubt as to whether we will continue as a going concern.
Each of Akanda and Canmart has generated no revenue or, only minimal revenue, while RPK generated revenue of $1,736,369, and incurred a consolidated net loss for the fiscal year ending December 31, 2023, primarily as a result of increased operating expenses to execute our business plan and growth strategy. There can be no assurances that we will be able to achieve a level of revenues adequate to generate sufficient cash flow from operations or obtain funding from additional financing through private placements, public offerings and/or bank financing necessary to support our working capital requirements.
To the extent that funds generated from any private placements, public offerings and/or bank financing are insufficient, we will have to raise additional working capital. No assurance can be given that additional financing will be available, or if available, will be on acceptable terms. These conditions raise substantial doubt about our ability to continue as a going concern. If adequate working capital is not available, we may be forced to discontinue operations, which would cause investors to lose their entire investment.
Our subsidiary, Bophelo, is currently in insolvency proceedings.
Our indirect wholly-owned subsidiary, Bophelo, was placed into liquidation by the High Court of Lesotho (the “Lesotho Court”) in July 2022 pursuant to an unauthorized application and request (the “Liquidation Application”) that was filed by Louisa Mojela, our former Executive Chairman, who was terminated as Executive Chairman of Akanda, and the Mophuti Matsoso Development Trust (“MMD Trust”), which we believe was established by Ms. Mojela. Mr. Chavonnes Cooper of Cape Town, South Africa, was appointed by the Lesotho Court as liquidator of Bophelo for purposes of maintaining the value of the assets owned or managed by Bophelo. While we intend to contest and seek to reverse the determination by the Lesotho Court to place Bophelo in liquidation, and will seek to recover significant loans made to Bophelo to fund the execution of Bophelo’s business plan, including payment of rents and staffing costs in the event that the Lesotho Court does not reverse its determination to place Bophelo in liquidation, there can be no assurance that we will be successful in reversing the Lesotho Court’s determination to place Bophelo in liquidation.
As a result of Bophelo’s liquidation, during the year ended December 31, 2022, we determined that we no longer controlled Bophelo. As a result of the loss of control, we derecognized the net assets of Bophelo and accounted for the operating results as discontinued operation.
2
We may become involved in litigation matters that are expensive and time consuming, and, if resolved adversely, could harm our reputation, business, financial condition or results of operations.
We may become involved in litigation matters that are expensive and time consuming, and, if resolved adversely, could harm our reputation, business, financial condition or results of operations.
In February 2023, Tejinder Virk, our former Chief Executive Officer, notified us of his resignation. Mr. Virk’s resignation was a result of disagreement with us regarding contractual obligations owed pursuant to the Service Agreement dated June 2, 2021 (the “Service Agreement”) between Mr. Virk, Halo Labs Inc., as guarantor, and Canmart Limited. According to Mr. Virk, the Company and Canmart committed a breach of the Service Agreement by failing to pay him monies and benefits owed following his placement on a paid leave of absence in November 2022 due to an internal investigation into Mr. Virk’s conduct as our Chief Executive Officer and as a director of Canmart. While we have informed Mr. Virk that he has been summarily dismissed and will be paid through February 2023. On May 12, 2023 Tejinder Virk issued a claim for Detriment and dismissal for alleged protected disclosures totaling £1,630,302.22. The claim has been denied in its entirety.
In addition, in July 2022, our former Executive Chairman, Louisa Mojela was summarily terminated as Chairman of Bophelo for Cause, as a “bad leaver”. In the event that the Lesotho Court does not reverse its determination to place Bophelo in liquidation, we plan to seek to recover significant loans that we have made to Bophelo to fund the execution of Bophelo’s business plan, including payment of rents and staffing costs. On October 20, 2022 Ms. Mojela filed a claim against Canmart and Akanda for wrongful termination of her Service Agreement. Ms. Mojela sought £1,832,150.62 plus further administrative and legal fees. The Company denied her claim and lodged a counterclaim lodged for losses caused by Ms. Mojela, including a loan of US $6,849,935.69 Akanda advanced to Bophelo. As at December 31, 2023, Ms. Mojela’s entire application failed. At the Consequentials hearing on January 15, 2024 Canmart and Akanda were awarded £60,000 in legal costs. Ms. Mojela is pursuing an appeal.
On April 29, 2023, Trevor Scott, our former Chief Financial Officer, filed a claim against the Company for amounts owing under his employment agreement totaling £420,659.95. This claim was resolved via a confidential settlement on January 15, 2024 and no amounts beyond this settlement are further owing to Mr. Scott.
On May 15, 2023, Vidya Iyer, our former SVP of Finance, filed a claim against the Company for amounts owing under her employment agreement totaling £151,774. This claim was resolved via a confidential settlement on March 27, 2024 and no amounts beyond this settlement are further owing to Ms. Iyer.
On January 29, 2024, Shailesh Bhushan, our former Chief Financial Officer, filed a complaint with the Employment Standards Branch of British Columbia claiming unpaid salary and invoices in the aggregate amount of CAD $271,990 from the period December 2022 through November 2023. The Company previously offered to Mr. Bhushan an annual salary of CAD $60,000 and as such, believes the claim to be frivolous, strongly disputes the amount claimed, and intends to vigorously defend itself.
Please refer to Note 22 of the Audited Consolidated Financial Statements as of and for the years ended December 31, 2023 and 2022 filed on April 30, 2024 for details of the legal proceedings with Mr. Virk, Ms. Mojela and Mr. Bhushan.
3
Future acquisitions and strategic investments could be difficult to integrate, divert the attention of key management personnel, disrupt our business, dilute shareholder value, and harm our results of operations and financial condition.
We may in the future seek to acquire or invest in, businesses, products, or technologies that we believe could complement our operations or expand our breadth, enhance our capabilities, or otherwise offer growth opportunities. While our growth strategy includes broadening our product offerings, implementing an aggressive marketing plan and employing product diversification, there can be no assurance that our systems, procedures and controls will be adequate to support our operations as they expand. We cannot assure you that our personnel, systems, procedures or controls will be adequate to support our operations in the future or that we will be able to successfully implement appropriate measures consistent with our growth strategy. As part of our planned growth and diversified product offerings, we may have to implement new operational and financial systems, procedures and controls to expand, train and manage our employee base, and maintain close coordination among our staff. We cannot guarantee that we will be able to do so, or that if we are able to do so, we will be able to effectively integrate them into our existing staff and systems. Additionally, the integration of our acquisitions and pursuit of potential future acquisitions may divert the attention of management and cause us to incur various expenses in identifying, investigating, and pursuing suitable acquisitions, whether or not they are consummated. Any acquisition, investment or business relationship may result in unforeseen operating difficulties and expenditures. In addition, we have limited experience in acquiring other businesses. Specifically, we may not successfully evaluate or utilize the acquired products, assets or personnel, or accurately forecast the financial impact of an acquisition transaction, including accounting charges. Moreover, the anticipated benefits of any acquisition, investment, or business relationship may not be realized, or we may be exposed to unknown risks or liabilities associated with our acquisitions.
We may not be able to find and identify desirable acquisition targets or we may not be successful in entering into an agreement with any one target. Acquisitions could also result in dilutive issuances of equity securities or the incurrence of debt, which could harm our results of operations. In addition, if an acquired business fails to meet our expectations, our business, results of operations, and financial condition may suffer. In some cases, minority shareholders may exist in certain of our non-wholly-owned acquisitions (for businesses we do not purchase as an 100% owned subsidiary) and may retain minority shareholder rights which could make a future change of control or necessary corporate approvals for actions more difficult to achieve and/or more costly.
We may also make strategic investments in early-stage companies developing products or technologies that we believe could complement our business or expand our breadth, enhance our technical capabilities, or otherwise offer growth opportunities. These investments may be in early-stage private companies for restricted stock. Such investments are generally illiquid and may never generate value. Further, the companies in which we invest may not succeed, and our investments could lose their value.
Demand for cannabis and its derivative products could be adversely affected and significantly influenced by scientific research or findings, regulatory proceedings, litigation, or media attention.
The legal cannabis industry in the United Kingdom, the European Union and in many other potential international markets for us is at an early stage of its development. Consumer perceptions regarding legality, morality, consumption, safety, efficacy and quality of medicinal cannabis are mixed and evolving and can be significantly influenced by scientific research or findings, regulatory investigations, litigation, media attention and other publicity regarding the consumption of medicinal cannabis products. There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the medicinal cannabis market or any particular product, or consistent with earlier publicity. Future research reports, findings, regulatory proceedings, litigation, media attention or other publicity that are perceived as less favorable than, or that question, earlier research reports, findings or publicity, could have a material adverse effect on the demand for medicinal cannabis and on our business, results of operations, financial condition and cash flows. Public opinion and support for medicinal cannabis use has traditionally been inconsistent and varies from jurisdiction to jurisdiction. Our ability to gain and increase market acceptance of our business may require substantial expenditures on investor relations, strategic relationships and marketing initiatives. There can be no assurance that such initiatives will be successful, and their failure to materialize into significant demand may have an adverse effect on our financial condition.
4
Our success will depend, in part, on our ability to continue to enhance our product offerings to respond to technological and regulatory changes and emerging industry standards and practices.
Rapidly changing markets, technology, emerging industry and regulatory standards and frequent introduction of new products characterize our business. The process of cultivating and processing our cannabis products to meet applicable standards and successfully marketing such products and obtaining necessary licenses requires significant continuing costs, marketing efforts, third-party commitments and regulatory approvals. Canmart has made a limited number of sales. From 2023 and beyond, we plan to expand our product offering to include cannabis oils and extracts, and ultimately, to produce consumer branded cannabis products for discerning patients. We may not be successful in timely expanding our production capacity, or obtaining any required regulatory approvals or licenses, to implement our growth plans, which, together with any capital expenditures made in our operations, may have a material adverse effect on our business, financial condition and operating results.
We are subject to the inherent risk of exposure to product liability claims.
As a cultivator and distributor of products designed to be ingested by humans, we face an inherent risk of exposure to product liability claims, regulatory action and litigation if our products are alleged to have caused bodily harm or injury. In addition, the sale of our products involves the risk of injury to consumers due to tampering by unauthorized third parties or product contamination. Adverse reactions resulting from human consumption of our products alone or in combination with other medications or substances could occur. We may be subject to various product liability claims, including, among others, that our products caused injury or illness, include inadequate instructions for use or include inadequate warnings concerning health risks, possible side effects or interactions with other substances. Product liability claims or regulatory actions against us could result in increased costs, could adversely affect our reputation with our clients and consumers generally, and could have a material adverse effect on our results of operations and financial condition. There can be no assurances that we will be able to obtain or maintain product liability insurance on acceptable terms or with adequate coverage against potential liabilities. Such insurance is expensive and may not be available in the future on acceptable terms, or at all. The inability to obtain sufficient insurance coverage on reasonable terms or to otherwise protect against potential product liability claims could prevent or inhibit the commercialization of our products.
We are subject to the inherent risks involved with product recalls.
Manufacturers and distributors of products are sometimes subject to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packaging safety and inadequate or inaccurate labelling disclosure. If any of our products are recalled due to an alleged product defect or for any other reason, we could be required to incur the unexpected expense of the recall and any legal proceedings that might arise in connection therewith. There can be no assurance that any quality, potency or contamination problems will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuits. Additionally, if our products are subject to recall, our reputation could be harmed. A recall for any of the foregoing reasons could lead to decreased demand for our products and could have a material adverse effect on our results of operations and financial condition. Additionally, product recalls may lead to increased scrutiny of our operations by regulatory agencies, requiring further management attention, potential loss of applicable licenses, and increased legal fees and other expenses.
Research regarding the medical benefits, viability, safety, efficacy, use and social acceptance of cannabis or isolated cannabinoids (such as cannabidiol and tetrahydrocannabinol) remains in early stages.
There have been relatively few clinical trials on the benefits of cannabis or isolated cannabinoids (such as cannabidiol and tetrahydrocannabinol). Although we believe that the articles, reports and studies published support our beliefs regarding the medical benefits, viability, safety, efficacy, dosing and social acceptance of cannabis, future research and clinical trials may prove such statements to be incorrect, or could raise concerns regarding, and perceptions relating to, cannabis. Given these risks, uncertainties and assumptions, investors should not place undue reliance on such articles and reports. Future research studies and clinical trials may draw opposing conclusions to those stated herein or reach negative conclusions related to medical cannabis, which could have a material adverse effect on the demand for our products and could result in a material adverse effect on our business, financial condition and results of operations or prospects.
5
We may not be able to maintain effective quality control systems.
We may not be able to maintain an effective quality control system. The effectiveness of our quality control system and our ability to maintain EU Good Manufacturing Practice (“EU GMP”) and Good Agricultural and Collecting Practices (“GACP”) certifications with respect to our manufacturing, processing and testing facilities depend on a number of factors, including the design of our quality control procedures, training programs, and the ability to ensure that our employees adhere to our policies and procedures. We also may depend on third party service providers to manufacture, process or test our products, that are subject to EU GMP and GACP requirements.
We expect that regulatory agencies will periodically inspect our and our service providers’ facilities to evaluate compliance with applicable EU GMP and GACP requirements. Failure to comply with these requirements may subject us or our service providers to possible regulatory enforcement actions. Any failure or deterioration of our or our service providers’ quality control systems, including loss of EU GMP and GACP certifications, may have a material adverse effect on our business, results of operations and financial condition.
The medical cannabis industry and market may not continue to exist or develop as we anticipate and we may ultimately be unable to succeed in this industry and market.
We are operating our current business in a relatively new industry, and our success depends on the continued growth of this market as well as our ability to attract and retain patients. Demand for pharmaceutical-grade cannabis and cannabis-based products is dependent on a number of social, political and economic factors that are beyond our control. Our projections on the number of people who have the potential to benefit from treatment with pharmaceutical-grade cannabis or cannabis-based products are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, and market research, and may prove to be incorrect. There is no assurance that an increase in existing demand will occur, that we will benefit from any such increased demand, or that our business will remain profitable even in the event of such an increase in demand.
In addition to being subject to the general business risks applicable to a business involving an agricultural product and a regulated medical product, we need to continue to build brand awareness within the medical cannabis industry and make significant investments in our business strategy and production capacity. These investments include introducing new medical cannabis products into the markets in which we operate, adopting quality assurance protocols and procedures, building our international presence and undertaking regulatory compliance efforts. These activities may not promote our products as effectively as intended, or at all, and we expect that our competitors will undertake similar investments to compete with us for market share.
Competitive conditions, physician preferences, patient requirements and spending patterns in the medical cannabis industry and market are relatively unknown and may have been uniquely impacted by circumstances unlike those in other existing industries and markets. Our target patient population may be smaller than expected, may not be otherwise amenable to treatment with our products, or may become increasingly difficult to identify and access. Further, we may not be successful in our efforts to attract and retain patients, develop new pharmaceutical-grade cannabis and cannabis-based products, produce and distribute these products to the markets in which we operate or to which we export in time to be effectively commercialized. In order to be successful in these activities, we may be required to expend significantly more resources than we currently anticipate, which could adversely affect our business, financial condition, results of operations and prospects.
The cannabis and cannabinoid industries face strong opposition.
Many political and social organizations oppose hemp and cannabis and their legalization, and many people, even those who support legalization, oppose the sale of hemp, cannabis and their derivatives in their geographies. Our business will need support from local governments, industry participants, consumers and residents to be successful. Additionally, there are large, well-funded businesses and industry groups that may have a strong opposition to the cannabis industry. For example, the pharmaceutical and alcohol industries have traditionally opposed cannabis legalization. Any efforts by these or other industries opposed to cannabis to halt or impede the cannabis industry could have detrimental effects on our business.
6
We, or the medical cannabis industry more generally, may receive unfavorable publicity or become subject to negative patient, physician or investor perception.
We believe that the medical cannabis industry is highly dependent upon positive patient, physician or investor perception regarding the benefits, safety, efficacy and quality of the cannabis distributed to patients for medical use. Perception of the medical cannabis industry, medicinal cannabis products, currently and in the future, may be significantly influenced by scientific research or findings, regulatory investigations, litigation, political statements, media attention and other publicity (whether or not accurate or with merit) both in Europe and elsewhere relating to the use of cannabis or cannabis-based products for medical purposes, including unexpected safety or efficacy concerns arising with respect to pharmaceutical-grade cannabis or cannabis-based products or the activities of medical-use cannabis industry participants.
There can be no assurance that future scientific research, findings, regulatory proceedings, litigation, media attention or other research findings or publicity will be favorable to the medical-use cannabis market or any particular medicinal cannabis products or will be consistent with prior publicity. Adverse future scientific research reports, findings and regulatory proceedings that are, or litigation, media attention or other publicity that is, perceived as less favorable than, or that questions, earlier research reports, findings or publicity (whether or not accurate or with merit) could result in a significant reduction in the demand for our medicinal cannabis products or cannabis for medical use more generally. Further, adverse publicity reports or other media attention regarding the safety, efficacy and quality of cannabis for medical purposes, or our current or future products specifically, or associating the use of cannabis with illness or other negative effects or events, could adversely affect us. This adverse publicity could arise even if the adverse effects associated with cannabis or cannabis-based products resulted from products that are not derived from medicinal cannabis or a patient’s failure to use such products legally, appropriately or as directed.
We are subject to the risks inherent in an agricultural business.
Our business involves the growing of cannabis, which is an agricultural product. The occurrence of severe adverse weather conditions, especially droughts, fires, storms or floods is unpredictable and may have a potentially devastating impact on agricultural production and may otherwise adversely affect the supply of cannabis. Adverse weather conditions may be exacerbated by the effects of climate change and may result in the introduction and increased frequency of pests and diseases. The effects of severe adverse weather conditions may reduce our yields or require us to increase our level of investment to maintain yields. Additionally, higher than average temperatures and rainfall can contribute to an increased presence of insects and pests, which could negatively affect cannabis crops. Future droughts could reduce the yield and quality of our cannabis production, which could materially and adversely affect our business, financial condition and results of operations.
The occurrence and effects of plant disease, insects and pests can be unpredictable and devastating to agricultural production, potentially rendering all or a substantial portion of the affected harvests unsuitable for sale. Even when only a portion of the production is damaged, our results of operations could be adversely affected because all or a substantial portion of the production costs may have been incurred. Although some plant diseases are treatable, the cost of treatment can be high and such events could adversely affect our operating results and financial condition. Furthermore, if we fail to control a given plant disease and the production is threatened, we may be unable to adequately supply our customers, which could adversely affect our business, financial condition and results of operations. There can be no assurance that natural elements will not have a material adverse effect on production.
Our business will be reliant upon third party suppliers, service providers and distributors.
As our business grows, we will need a supply chain for certain material portions of the production and distribution process of our products. Our suppliers, service providers and distributors may elect, at any time, to breach or otherwise cease to participate in supply, service or distribution agreements, or other relationships, on which our operations rely. Loss of our suppliers, service providers or distributors would have a material adverse effect on our business and operational results.
Part of our strategy is to enter into and maintain arrangements with third parties related to the development, testing, marketing, manufacturing, distribution and commercialization of our products. Our revenues are dependent on the successful efforts of these third parties, including the efforts of our distribution partners. Entering into strategic relationships can be a complex process and the interests of our distribution partners may not be or remain aligned with our interests. Some of our current and future distribution partners may decide to compete with us, refuse or be unable to fulfill or honor their contractual obligations to us, or change their plans to reduce their commitment to, or even abandon, their relationships with us. There can be no assurance that our distribution partners will market our products successfully or that any such third-party collaboration will be on favorable terms.
Our profit margins and the timely delivery of our products are dependent upon the ability of our outside suppliers and manufacturers to supply us with products in a timely and cost-efficient manner. Our ability to develop our business and enter new markets and sustain satisfactory levels of sales in each market depends upon the ability of our outside suppliers and manufacturers to produce the ingredients and products and to comply with all applicable regulations. The failure of our primary suppliers or manufacturers to supply ingredients or produce our products could adversely affect our business operations.
7
There is no assurance that our sales and promotional activities will be successful.
Our future growth and profitability will depend on the effectiveness and efficiency of sales and promotional expenditures, including our ability to (i) create greater awareness of our products, (ii) determine the appropriate creative message and media mix for future marketing expenditures and (iii) effectively manage sales and promotional costs in order to maintain acceptable operating margins. We plan to continue to develop the direct sale model of Canmart, which may require us to establish our own clinics and pharmacies. There can be no assurance that our sales and promotional expenditures will result in revenues in the future or will generate awareness of our products and services. In addition, no assurance can be given that we will be able to manage our sales and promotional expenditures on a cost-effective basis.
We believe that maintaining and promoting our brand is critical to expanding our customer base. Maintaining and promoting our brand will depend largely on our ability to continue to provide quality, reliable and innovative products, which we may not do successfully. We may introduce new products or services that our customers do not like, which may negatively affect our brand and reputation. Maintaining and enhancing our brand may require us to make substantial investments, and these investments may not achieve the desired goals. If we fail to successfully promote and maintain our brand or if we incur excessive expenses in this effort, our business and financial results from operations could be materially adversely affected.
We may be unable to sustain our pricing model.
Significant price fluctuations or shortages in the cost of materials may increase our cost of goods sold and cause our results of operations and financial condition to suffer. If we are unable to secure materials at a reasonable price, we may have to alter or discontinue selling some of our products or attempt to pass along the cost to our customers, any of which could adversely affect our results of operations and financial condition.
Additionally, increasing costs of labor, freight and energy could increase our and our suppliers’ cost of goods. If our suppliers are affected by increases in their costs of labor, freight and energy, they may attempt to pass these cost increases on to us. If we pay such increases, we may not be able to offset them through increases in its pricing, which could adversely affect our results of operations and financial condition.
We may be unable to effectively manage future growth.
We may be subject to growth-related risks, including capacity constraints and pressure on our internal systems and controls. Our ability to manage growth effectively will require us to continue to implement and improve our operational and financial systems and to expand, train and manage our employee base. Rapid growth of our business may significantly strain our management, operations and technical resources. If we are successful in obtaining large orders for our products, we will be required to deliver large volumes of products to our customers on a timely basis and at a reasonable cost. We may not obtain large-scale orders for our products and if we do, we may not be able to satisfy large-scale production requirements on a timely and cost-effective basis. Our inability to deal with this growth may have a material adverse effect on our business, financial condition, results of operations and prospects.
We are subject to significant competition by new and existing competitors in the cannabis industry.
The industry in which we operate is subject to intense and increasing competition. Many of our competitors have greater resources that may enable them to compete more effectively than us in the cannabis industry, or they have a longer operating history and greater capital resources and facilities, which may enable them to compete more effectively in this market. We expect to face additional competition from existing licensees and new market entrants who are granted licenses in the jurisdictions in which we operate, including the United Kingdom and other jurisdictions in which we intend to expand our operations. If a significant number of new licenses are granted in the near term, we may experience increased competition for market share and may experience downward pricing pressure on our products as new entrants increase production. Such competition may cause us to encounter difficulties in generating revenues and market share, and in positioning our products in the market. If we are unable to successfully compete with existing companies and new entrants to the market, our lack of competitive advantage will have a negative effect on our business and financial condition.
8
The legalization of adult-use, recreational cannabis may reduce sales of medical cannabis.
Legalization of the sale to adults of recreational, non-medical cannabis in any country may increase competition in the medical cannabis market. We currently do not plan to sell recreational, non-medical cannabis products. We may not be able to achieve our business plan in a highly competitive market where recreational, adult-use cannabis is legal, or the market may experience a drop in the price of cannabis and cannabis products over time, decreasing our profit margins.
We are dependent upon our management and key employees, and the loss of any member of our management team or any key employee could have a material adverse effect on our operations.
Our success is dependent upon the ability, expertise, judgment, discretion and good faith of our senior management and key employees, including, without limitation, Katie Field, our Interim Chief Executive Officer and Executive Director, and Gucharn Deol, our Chief Financial Officer. The loss of any member of our management team or any of our key employees could have a material adverse effect on our business and results of operations. While employment agreements and incentive programs are customarily used as primary methods of retaining the services of key employees, these agreements and incentive programs cannot assure the continued services of such employees. Any loss of the services of such individuals, or an inability to attract other suitably qualified persons when needed, could have a material adverse effect on our business, operating results or financial condition. We do not currently maintain key-person insurance on the lives of any of our key employees or members of management. Competition for qualified technical, sales and marketing staff, as well as officers and directors can be intense, and no assurance can be provided that we will be able to attract or retain such qualified individuals in the future, which may adversely affect our operations.
Our directors and officers may have conflicts of interest in conducting their duties.
We may be subject to various potential conflicts of interest because of the fact that some of our officers and directors may be engaged in the cannabis industry through their participation in corporations, partnership or joint ventures, which are potential competitors of our company. Situations may arise in connection with potential acquisitions in investments where the other interests of these directors and officers may conflict with the interests of our company. Our directors and officers with conflicts of interest will be subject to the procedures set out in the related Canadian law and regulations.
Our executive officers are engaged in other business activities and, accordingly, may not devote sufficient time to our business affairs, which may affect our ability to conduct operations.
In addition, our executive officers and directors may devote time to their outside business interests, so long as such activities do not materially or adversely interfere with their duties to us. In some cases, our executive officers and directors may have fiduciary obligations associated with these business interests that interfere with their ability to devote time to our business and affairs and that could adversely affect our operations. These business interests could require significant time and attention of our executive officers and directors. For example, our Interim Chief Executive Officer and Executive Director, Ms. Katie Field, is the Chief Executive Officer and Chairman of Halo.
The Coronavirus (“COVID-19”) outbreak and similar disease outbreaks or public health emergencies could adversely affect our future operations.
Our operations could be significantly and adversely affected by the effects of a widespread global outbreak of a contagious disease and other unforeseen events, including the outbreak of a respiratory illness caused by COVID-19 and the related economic repercussions. We cannot accurately predict the effects COVID-19 will have on our operations and the ability of others to meet their obligations with us, including uncertainties relating to the ultimate geographic spread of the virus, the severity of the disease, the duration of the outbreak, and the length of travel and quarantine restrictions imposed by governments of affected countries. In light of the COVID-19 pandemic, there could be a negative impact on sourcing medical cannabis products for our distribution in the United Kingdom. Additionally, COVID-19 has caused significant disruptions to the global financial markets, which could impact our ability to raise additional capital. The ultimate impact on us and our significant suppliers and prospective customers is unknown, but our operations and financial condition could suffer in the event of any of these types of unpredictable events. Further, any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our business, results of operations, financial condition and cash flows.
9
The U.K. and EU populations have reached a significant level of vaccination. There is no guarantee, however, that the continued development of COVID-19 will not affect our operations negatively. During the year ended December 31, 2023, both the UK government and governments inside the EU have substantially reduced COVID-19 restrictions.
We could be subject to a security breach that could result in significant damage or theft of products and equipment.
Breaches of security at our facilities may occur and could result in damage to or theft of products and equipment. A security breach at our facilities could result in a significant loss of inventory or work in process, expose us to liability under applicable regulations and increase expenses relating to the investigation of the breach and implementation of additional preventative security measures, any of which could have an adverse effect on our business, financial condition and results of operations.
We may incur significant costs to defend our intellectual property and other proprietary rights.
The ownership and protection of trademarks, patents, trade secrets and intellectual property rights are significant aspects of our future success. Unauthorized parties may attempt to replicate or otherwise obtain and use our products and technology. Policing the unauthorized use of our current or future trademarks, patents, trade secrets or intellectual property rights could be difficult, expensive, time-consuming and unpredictable, as may be enforcing these rights against unauthorized use by others.
In addition, other parties may claim that our products infringe on their proprietary rights such as trade secrets. Such claims, regardless of their merit, may result in the expenditure of significant financial and managerial resources, legal fees, injunctions, temporary restraining orders and/or require the payment of damages. Additionally, we may need to obtain licenses from third parties who allege that we have infringed on their lawful rights. Such licenses may not be available on terms acceptable to us or at all. In addition, we may not be able to obtain or utilize on terms that are favorable to us, or at all, licenses or other rights with respect to intellectual property that we do not own.
If we sustain cyber-attacks or other privacy or data security incidents that result in security breaches that disrupt our operations or result in the unintended dissemination of protected personal information or proprietary or confidential information, or if we are found by regulators to be non-compliant with statutory requirements for the protection and storage of personal data, we could suffer a loss of revenue, increased costs, exposure to significant liability, reputational harm and other serious negative consequences.
As our operations expand, we may process, store and transmit large amounts of data in our operations, including protected personal information as well as proprietary or confidential information relating to our business and third parties. Experienced computer programmers and hackers may be able to penetrate our layered security controls and misappropriate or compromise our protected personal information or proprietary or confidential information or that of third parties, create system disruptions or cause system shutdowns. They also may be able to develop and deploy viruses, worms and other malicious software programs that attack our systems or otherwise exploit any security vulnerabilities. Hardware, software, or applications we develop or procure from third parties may contain defects in design or manufacture or other problems that could unexpectedly compromise information security. Our facilities may also be vulnerable to security incidents or security attacks, acts of vandalism or theft, coordinated attacks by activist entities, misplaced or lost data, human errors, or other similar events that could negatively affect our systems and our customer’s data.
10
Risks Related to Our International Operations
As a company based outside of the United States, we are subject to economic, political, regulatory and other risks associated with international operations.
Our business is subject to risks associated with conducting business outside of the United States. Our operations are based primarily in the United Kingdom and Canada. Our principal office and Canmart’s operations are located in the United Kingdom and we own certain property in Canada. Accordingly, our future results could be harmed by a variety of factors, including, without limitation, the following:
| ● | economic weakness, including inflation, or political instability in non-U.S. economies and markets; |
| ● | differing and changing regulatory requirements for product licenses and approvals; |
| ● | differing jurisdictions could present different issues for securing, maintaining or obtaining freedom to operate in such jurisdictions; |
| ● | difficulties in compliance with different, complex and changing laws, regulations and court systems of multiple jurisdictions and compliance with a wide variety of foreign laws, treaties and regulations; |
| ● | changes in applicable non-U.S. regulations and customs, tariffs and trade barriers; |
| ● | changes in applicable non-U.S. currency exchange rates and currency controls; |
| ● | changes in a specific country’s or region’s political or economic environment, including the implications of the decision of the United Kingdom to withdraw from the European Union; |
| ● | trade protection measures, import or export licensing requirements or other restrictive actions by governments; |
| ● | differing reimbursement regimes and price controls in certain non-U.S. markets; |
| ● | negative consequences from changes in tax laws; |
| ● | compliance with applicable tax, employment, immigration and labor laws for employees living or traveling abroad, including, for example, the variable tax treatment in different jurisdictions of options granted under our share option schemes or equity incentive plans; |
| ● | workforce uncertainty in countries where labor unrest is more common than in the United States; |
| ● | difficulties associated with staffing and managing international operations, including differing labor relations; |
| ● | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| ● | business interruptions resulting from geo-political actions, including war and terrorism, or natural disasters, including droughts, floods and fires. |
11
Our business could suffer as a result of the United Kingdom’s withdrawal from the European Union.
While we are incorporated in the Province of Ontario in Canada, our principal office, a number of our executive officers and key employees, and Canmart’s operations and assets are primarily located in the United Kingdom. The United Kingdom formally exited the European Union, commonly referred to as Brexit, on January 31, 2020. Under the terms of its departure, the United Kingdom entered into a transition period during which it continued to follow all European Union rules, and the trading relationship remained the same, until December 31, 2020. On December 24, 2020, the European Union and the United Kingdom entered into a new trade agreement to govern their relationship following Brexit. However, substantial uncertainty remains concerning which EU laws and regulations will continue to be implemented in the United Kingdom after Brexit (including financial laws and regulations, tax and free trade agreements, intellectual property rights, data protection laws, supply chain logistics, environmental, health and safety laws and regulations, immigration laws and employment laws).
The uncertainty concerning the United Kingdom’s legal, political and economic relationship with the European Union after Brexit may negatively impact direct foreign investment in the United Kingdom, increase costs, depress economic activity and restrict access to capital. It may also be a source of instability in the international markets, create significant currency fluctuations, and/or otherwise adversely affect trading agreements or similar cross-border cooperation arrangements (whether economic, tax, fiscal, legal, regulatory or otherwise) beyond the date of Brexit. We may also face new regulatory costs and challenges that could have an adverse effect on our operations.
The United Kingdom’s withdrawal from the European Union could lead to increased market volatility, which could make it more difficult for us to do business in Europe or have other adverse effects on our business.
As a result of the United Kingdom’s withdrawal from the European Union, the United Kingdom now has third country status outside of the European Union. Before the end of 2020, the United Kingdom and the European concluded a Trade and Cooperation Agreement (“TCA”) which took effect January 1, 2021. The terms of the TCA allow for tariff-free and quota-free access to the EU market for the United Kingdom so long as the United Kingdom does not diverge from EU laws. To the extent the United Kingdom does diverge from EU laws, access to EU markets may be made more restricted than it currently is. In addition, the TCA does not allow U.K. institutions access to EU markets, so it is possible that there will be a period of considerable uncertainty, particularly in relation to U.K. financial and banking markets, as well as in relation to the regulatory process in Europe. As a result of this uncertainty, financial markets could experience volatility. We may also face new regulatory costs and challenges that could have a material adverse effect on our operations. In this regard, the European Medicines Agency has already issued a notice reminding marketing authorization holders of centrally authorized medicinal products for human and veterinary use of certain legal requirements that need to be considered as part of Brexit, such as the requirement for the marketing authorization holder of a product centrally approved by the European Commission to be established in the European Union, and the requirement for some activities relating to centrally approved products to be performed in the European Union. As a third country, the United Kingdom will lose the benefits of global trade agreements negotiated by the European Union on behalf of its members, which may result in increased trade barriers which could make our doing business worldwide more difficult. In addition, currency exchange rates in the pound sterling and the euro with respect to each other and the U.S. dollar have already been adversely affected by Brexit. Should this foreign exchange volatility continue, it could cause volatility in our financial results.
We expect to increase our international sales in the future, and such sales may be subject to unexpected exchange rate fluctuations, regulatory requirements and other barriers.
We currently expect that our sales will be denominated in U.S. Dollars and Euros and that we may, in the future, have sales denominated in the currencies of additional countries in which we establish operations or distribution. In addition, we expect to incur the majority of our operating expenses in U.S. Dollars and Euros. Our international sales may be subject to unexpected regulatory requirements and other barriers. Any fluctuation in the exchange rates of foreign currencies may negatively affect our business, financial condition and results of operations. We have not previously engaged in foreign currency hedging. If we decide to hedge our foreign currency exposure, we may not be able to hedge effectively due to lack of experience, unreasonable costs or illiquid markets. In addition, those activities may be limited in the protection they provide from foreign currency fluctuations and can themselves result in losses.
12
Tax regulations and challenges by tax authorities could have a material adverse effect on our business.
We expect to operate in a number of countries and will therefore be regularly examined by and remain subject to numerous tax regulations. Changes in our global mix of earnings could affect our effective tax rate. Furthermore, changes in tax laws could result in higher tax-related expenses and payments. Legislative changes in any of the countries in which we operate could materially impact our tax receivables and liabilities as well as deferred tax assets and deferred tax liabilities. Additionally, the uncertain tax environment in some regions in which we operate may limit our ability to successfully challenge an adverse determination by any local tax authorities. We expect to operate in countries with complex tax rules, which may be interpreted in a variety of ways and could affect our effective tax rate. Future interpretations or developments of tax regimes or a higher than anticipated effective tax rate could have a material adverse effect on our tax liability, return on investments and business operations.
In addition, our subsidiaries operate in, are incorporated in and are tax residents of, various jurisdictions. The tax authorities in the various jurisdictions in which we and our subsidiaries operate, or are incorporated, may disagree with and challenge our assessments of our transactions, tax position, deductions, exemptions, where we or our subsidiaries are tax resident, or other matters. If we are unsuccessful in responding to any such challenge from a tax authority, we may be required to pay additional taxes, interest, fines or penalties, we may be subject to taxes for the same business in more than one jurisdiction or may also be subject to higher tax rates, withholding or other taxes. A successful challenge could potentially result in payments to the relevant tax authority of substantial amounts that could have a material adverse effect on our financial condition and results of operations.
Even if we are successful in responding to challenges by taxing authorities, responding to such challenges may be expensive, consume time and other resources, or divert management’s time and focus from our business operations. Therefore, a challenge as to our tax position or status or transactions, even if unsuccessful, may have a material adverse effect on our business, financial condition, results of operations or liquidity or the business, financial condition, and results of operations.
We face the risk of disruption from labor disputes and changes to labor laws, which could result in significant additional operating costs or alter our relationship with our employees.
We are required to comply with extensive labor regulations in each of the countries in which we have employees, including with respect to wages, social security benefits and termination payments. Labor or employee led disruptions could have a material adverse effect on our business, results of operations and financial condition.
Risks Related to our Regulatory Framework
The medicinal cannabis regulatory regime is restrictive and new in the United Kingdom and Europe, and laws and enforcement could rapidly change again.
There are significant legal restrictions and regulations that govern the cannabis industry in the United Kingdom. The legislative changes made to allow for the prescription and possession of medicinal cannabis without Home Office licenses are very narrow. “Cannabis” remains a Class B controlled drug under the Misuse of Drugs Act 1973 and remains a Schedule 1 drug under the Misuse of Drugs Regulation 2001 (“MDR 2001”). Schedule 1 contains drugs which are not used medically. Cultivation, distribution and possession of Schedule 1 controlled drug is illegal without appropriate licenses. It is only CBPMs that have been moved to various other schedules under the MDR 2001, which then allows for the prescription and possession of CBPMs without a license.
However, there are also strict requirements that need to be met for the supply of CBPMs to patients to be compliant with the regulations. Despite the demand for CBPMs, there has been great reluctance from the medical establishment in general to prescribe “medicinal products” for which there are no official prescribing guidelines and a lack of clinical data. In particular, the Royal College of Physicians and NHS England have issued guidelines for medical practitioners stating that there is currently limited evidence of the effectiveness of CBPMs, except in very limited cases. This appears to have made specialist doctors loathe to prescribe CBPMs against this explicit guidance. As the medical establishment and regulators are still firming up their approaches to guidance and enforcement, this can create a level of operating uncertainty.
Our activities are, and will continue to be, subject to evolving regulation by governmental authorities. Due to the current regulatory environment in the United Kingdom, new risks may emerge; management may not be able to predict all such risks.
13
U.K. based companies also need to be aware of the potential difficulties posed by the U.K. Proceeds of Crime Act 2002 (“POCA”). POCA prohibits dealing with any benefit (directly or indirectly) arising from criminal conduct. Conduct is criminal if it:
| ● | constitutes an offence in any part of the United Kingdom, or |
| ● | would constitute an offence in part of the United Kingdom if it occurred there. |
This principle of “dual criminality” means that measures to legalize cannabis overseas can be potentially irrelevant when it comes to investing in the United Kingdom, and medicinal cannabis companies operating in the United Kingdom.
Although the risk of action being taken against a U.K. investor by law enforcement may be considered low when dealing with the indirect proceeds of cannabis, U.K. companies and investors should be sure to understand the precise nature of their investments or transactions and to keep in mind that investing in, or doing business with, companies involved in recreational cannabis, even where their activity is legal under the laws applicable to them, may nonetheless cause the U.K.-based investor or counterparty to violate U.K. money laundering laws.
Our activities are, and will continue to be, subject to evolving regulation by governmental authorities.
Cannabis laws, regulations, and guidelines are dynamic and subject to changes.
Cannabis laws and regulations are dynamic and subject to evolving interpretations which could require us to incur substantial costs associated with compliance or alter certain aspects of our business plan. It is also possible that regulations may be enacted in the future that will be directly applicable to certain aspects of our businesses. We cannot predict the nature of any future laws, regulations, interpretations or applications, nor can we determine what effect additional governmental regulations or administrative policies and procedures, when and if promulgated, could have on our business. Management expects that the legislative and regulatory environment in the cannabis industry in the United Kingdom and internationally will continue to be dynamic and will require innovative solutions to try to comply with this changing legal landscape in this nascent industry for the foreseeable future. Failure to comply with any such legislation may have a material adverse effect on our business, financial condition and results of operations.
Public opinion can also exert a significant influence over the regulation of the cannabis industry. A negative shift in the public’s perception of the cannabis industry could affect future legislation or regulation in different jurisdictions.
There are risks associated with the regulatory regime and permitting requirements of our operations.
Achievement of our business objectives is contingent, in part, upon compliance with regulatory requirements enacted by governmental authorities and obtaining all regulatory approvals, where necessary, for the cultivation, processing and sale of our products. Canmart currently holds the licenses required to conduct its operations. We may not be able to obtain or maintain the necessary licenses, permits, quotas, authorizations, certifications or accreditations to operate our business going forward, or may only be able to do so at great cost. We cannot predict the time required to secure all appropriate regulatory approvals for our products, or the extent of testing and documentation that may be required by local governmental authorities.
Our officers and directors must rely, to a great extent, on our local legal counsel and local consultants retained in the United Kingdom in order to keep abreast of material legal, regulatory and governmental developments as they pertain to and affect our business operations, and to assist us with governmental relations. We must rely, to some extent, on those members of management and the Board who have previous experience working and conducting business in the United Kingdom in order to enhance our understanding of and appreciation for the local business culture and practices in such jurisdictions.
We also rely on the advice of local experts and professionals in connection with any current and new regulations that develop in respect of banking, financing and tax matters in the jurisdictions in which we operate. Any developments or changes in such legal, regulatory or governmental requirements or in local business practices in such jurisdictions are beyond our control and may adversely affect our business.
We will incur ongoing costs and obligations related to regulatory compliance. Failure to comply with applicable laws, regulations and permitting requirements may result in enforcement actions thereunder, including orders issued by regulatory or judicial authorities causing operations to cease or be curtailed, and may include corrective measures requiring capital expenditures, installation of additional equipment, or remedial actions. We may be required to compensate those suffering loss or damage by reason of our operations and may have civil or criminal fines or penalties imposed for violations of applicable laws or regulations. In addition, changes in regulations, more vigorous enforcement thereof or other unanticipated events could require extensive changes to our operations, increased compliance costs or give rise to material liabilities, which could have a material adverse effect on our business, results of operations and financial condition.
14
Any failure on our part to comply with applicable regulations or to obtain and maintain the necessary licenses and certifications could prevent us from being able to carry on our business, and there may be additional costs associated with any such failure.
Our business activities are heavily regulated in all jurisdictions where we do business. Our operations are subject to various laws, regulations and guidelines by governmental authorities relating to the cultivation, processing, manufacture, marketing, management, distribution, transportation, storage, sale, packaging, labelling, pricing and disposal of cannabis and cannabis products. In addition, we are subject to laws and regulations relating to employee health and safety, insurance coverage and the environment. Laws and regulations, applied generally, grant government agencies and self-regulatory bodies broad administrative discretion over our activities, including the power to limit or restrict business activities as well as impose additional disclosure requirements on our products and services.
Any failure by us to comply with applicable regulatory requirements could:
| ● | require extensive changes to our operations; |
| ● | result in regulatory or agency proceedings or investigations; |
| ● | result in the revocation of our licenses and permits, the imposition of additional conditions on licenses to operate our business, and increased compliance costs; |
| ● | result in product recalls or seizures; |
| ● | result in damage awards, civil or criminal fines or penalties; |
| ● | result in the suspension or expulsion from a particular market or jurisdiction of our key personnel; |
| ● | result in restrictions on our operations or the imposition of additional or more stringent inspection, testing and reporting requirements; |
| ● | harm our reputation; or |
| ● | give rise to material liabilities. |
There can be no assurance that any future regulatory or agency proceedings, investigations or audits will not result in substantial costs, a diversion of management’s attention and resources or other adverse consequences to our business.
In addition, changes in regulations, government or judicial interpretation of regulations, or more vigorous enforcement thereof or other unanticipated events could require extensive changes to our operations, increase compliance costs or give rise to material liabilities or a revocation of our licenses and other permits. Furthermore, governmental authorities may change their administration, application or enforcement procedures at any time, which may adversely affect our ongoing regulatory compliance costs. There is no assurance that we will be able to comply or continue to comply with applicable regulations.
The legal cannabis market is a relatively new industry. As a result, the size of our target market is difficult to quantify, and investors will be reliant on their own estimates on the accuracy of market data.
Because the cannabis industry is in a nascent stage, there is a lack of information about comparable companies available for potential investors to review in deciding whether to invest in us and, few, if any, established companies whose business model we can follow or upon whose success we can build. Accordingly, investors should rely on their own estimates regarding the potential size, economics and risks of the cannabis market in deciding whether to invest in our Common Shares. We are an early-stage company that has not generated net income. There can be no assurance that our growth estimates are accurate or that the cannabis market will be large enough for our business to grow as projected.
15
Although we are committed to researching and developing new markets and products and improving existing products, there can be no assurances that such research and market development activities will prove profitable or that the resulting markets or products, if any, will be commercially viable or successfully produced and marketed. We must rely largely on our own market research to forecast sales and design products as detailed forecasts and consumer research are not generally obtainable from reliable third-party sources in the United Kingdom, the European market, Canada and in other international jurisdictions.
In addition, there is no assurance that the industry and market will continue to exist and grow as currently estimated or anticipated or function and evolve in the manner consistent with management’s expectations and assumptions. We could also be subject to other events or circumstances that adversely affect the cannabis industry, such as the imposition of further restrictions on sales and marketing or further restrictions on sales in certain areas and markets.
Marijuana remains illegal under U.S. federal law, and the enforcement of U.S. cannabis laws could change.
There are significant legal restrictions and regulations that govern the cannabis industry in the United States. Marijuana remains a Schedule I drug under the Controlled Substances Act, making it illegal under federal law in the United States to, among other things, cultivate, distribute or possess cannabis in the United States. In those states in which the use of marijuana has been legalized, its use remains a violation of federal law pursuant to the Controlled Substances Act. The Controlled Substances Act classifies marijuana as a Schedule I controlled substance, and as such, medical and adult use cannabis use is illegal under U.S. federal law. Unless and until the U.S. Congress amends the Controlled Substances Act with respect to marijuana (and the President approves such amendment), there is a risk that federal authorities may enforce current federal law. Financial transactions involving proceeds generated by, or intended to promote, cannabis-related business activities in the United States may form the basis for prosecution under applicable U.S. federal money laundering legislation. While the approach to enforcement of such laws by the federal government in the United States has trended toward non-enforcement against individuals and businesses that comply with medical or adult-use cannabis regulatory programs in states where such programs are legal, strict compliance with state laws with respect to cannabis will neither absolve us of liability under U.S. federal law, nor will it provide a defense to any federal proceeding which may be brought against us should we expand our operations into the U.S. Since U.S. federal law criminalizing the use of marijuana pre-empts state laws that legalize its use, enforcement of federal law regarding marijuana may be a significant risk and could greatly harm our business, prospects, revenue, results of operation and financial condition if we were to expand our operations into the United States. We currently have no operations in the United States and no plans to expand our operations into the United States in the foreseeable future.
Our activities are, and will continue to be, subject to evolving regulation by governmental authorities. The legality of the production, cultivation, extraction, distribution, retail sales, transportation and use of cannabis differs among states in the United States. Due to the current regulatory environment in the United States, new risks may emerge; management may not be able to predict all such risks. Due to the conflicting views between state legislatures and the federal government regarding cannabis, cannabis businesses are subject to inconsistent laws and regulations. There can be no assurance that the federal government will not enforce federal laws relating to marijuana and seek to prosecute cases involving marijuana businesses that are otherwise compliant with state laws in the future. To date, federal enforcement agencies have taken little or no action against state-compliant cannabis businesses in the United States. However, the DOJ may change its enforcement policies at any time, with or without advance notice. The uncertainty of U.S. federal enforcement practices going forward and the inconsistency between U.S. federal and state laws and regulations may present risks for us if we expand our operations into the United States in the future.
Risks Related to Financials and Accounting
There are tax risks we may be subject to in carrying out our business in multiple jurisdictions.
We will operate and, accordingly, will be subject to income tax and other forms of taxation in multiple jurisdictions. We may be subject to income taxes and non-income taxes in a variety of jurisdictions and our tax structure may be subject to review by both domestic and foreign taxation authorities. Those tax authorities may disagree with our interpretation and/or application of relevant tax rules. A challenge by a tax authority in these circumstances might require us to incur costs in connection with litigation against the relevant tax authority or reaching a settlement with the tax authority and, if the tax authority’s challenge is successful, could result in additional taxes (perhaps together with interest and penalties) being assessed on us, and as a result an increase in the amount of tax payable by us. In addition, we may be subject to different taxes imposed by the local governments in the jurisdictions where we operate, and changes within such tax, legal and regulatory framework may have an adverse effect on our financial results.
16
Taxation laws and rates which determine taxation expenses may vary significantly in different jurisdictions, and legislation governing taxation laws and rates are also subject to change. Therefore, our earnings may be affected by changes in the proportion of earnings taxed in different jurisdictions, changes in taxation rates, changes in estimates of liabilities and changes in the amount of other forms of taxation. The determination of our provision for income taxes and other tax liabilities will require significant judgment (including based on external advice) as to the interpretation and application of these rules. We may have exposure to greater than anticipated tax liabilities or expenses.
There is a risk that we will be a passive foreign investment company (“PFIC”) for U.S. federal income tax purposes for the current or any future taxable year, which could result in material adverse U.S. federal income tax consequences if you are a U.S. Holder.
If we (or any of our non-U.S. subsidiaries) are a PFIC for any taxable year during which a U.S. Holder owns Common Shares, certain adverse U.S. federal income tax consequences could apply to such U.S. Holder. The determination of whether a corporation is a PFIC for a taxable year depends, in part, on the application of complex U.S. federal income tax rules that are subject to differing interpretations. In addition, the determination of whether a corporation will be a PFIC for any taxable year generally can only be made after the close of such taxable year. Therefore, it is possible that we could be classified as a PFIC for our initial taxable year or in future years due to changes in the nature of our business, composition of our assets or income, as well as changes in our market capitalization. In particular, our PFIC status will depend, in part, on the amount of cash that we raise in this offering and how quickly we utilize the cash in our business. Based upon the foregoing, it is uncertain whether we will be a PFIC for our current taxable year or any future taxable year. We have not determined, if we (or any of our non-U.S. subsidiaries) were to be classified as a PFIC for a taxable year, whether we will provide information necessary for a U.S. Holder to make a “qualified electing fund” election which, if available, would result in tax treatment different from (and generally less adverse than) the general tax treatment for PFICs. Accordingly, U.S. Holders should assume that they will not be able to make a qualified electing fund election with respect to our Common Shares. The PFIC rules are complex, and each U.S. Holder should consult his, her or its own tax advisor regarding the PFIC rules, the elections which may be available, and how the PFIC rules may affect the U.S. federal income tax consequences relating to the ownership and disposition of our Common Shares.
Failure to develop our internal controls over financial reporting as we grow could have an adverse effect on our operations.
As we mature, we will need to continue to develop and improve our current internal control systems and procedures to manage our growth. We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish appropriate controls, or any failure of those controls once established, could adversely affect our public disclosures regarding our business, financial condition or results of operations. In addition, management’s assessment of internal controls over financial reporting may identify weaknesses and conditions that need to be addressed in our internal controls over financial reporting or other matters that may raise concerns for investors.
Risks Related to Our Common Shares
We will need to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product and business development efforts or other operations.
We expect to have sufficient capital to fund our current operations at least through the middle of 2024. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances or a combination of these approaches. Raising funds in the current economic environment may present additional challenges. It is not certain that we have accounted for all costs and expenses of future development and regulatory compliance. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
Any additional fundraising efforts may divert the attention of our management team from their day-to-day activities, which may adversely affect our ability to launch our business and develop and commercialize our products. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any future financing may adversely affect the holdings or the rights of our shareholders, and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our shares to decline. The sale of additional equity or convertible securities may dilute our existing shareholders.
17
The incurrence of indebtedness would result in increased fixed payment obligations, and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely affect our ability to conduct our business. For example, as of March 31, 2024, the Company has incurred aggregated outstanding loans, including accrued interest, of approximately $1,326,682 from Halo (the “Halo Debt”), making Halo a significant creditor of the Company. Even though the Halo Debt is unsecured, Halo may be able to influence our strategic goals, management and affairs, including use of available capital, capital raising opportunities, borrowing, recapitalization, and any acquisition, sale, merger, or consolidation transactions. The interests of Halo may not always coincide with the interests of the Company or the interests of shareholders and Halo may act in a manner that advances its best interests and not necessarily our Company. Additionally, there may be an inherent conflict of interest because our interim Chief Executive Officer and director, Ms. Katie Field, is serving as the Chief Executive Officer and Chairman of Halo.
We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable and we may be required to relinquish rights to some of our technologies or products or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any product, or be unable to expand our operations or otherwise capitalize on our business opportunities, as desired, which could materially affect our business, financial condition and results of operations.
Future sales and issuances of our capital stock or rights to purchase capital stock could result in additional dilution of the percentage ownership of our shareholders and could cause the price of our Common Shares to decline.
We may issue additional securities. Future sales and issuances of our capital stock or rights to purchase our capital stock could result in substantial dilution to our existing shareholders. We may sell Common Shares, convertible securities, and other equity securities in one or more transactions at prices and in a manner as we may determine from time to time. If we sell any such securities in subsequent transactions, investors may be materially diluted. New investors in such subsequent transactions could gain rights, preferences, and privileges senior to those of holders of our Common Shares.
We incur increased costs as a result of operating as a public company and our management is required to devote substantial time to new compliance initiatives.
As a public company, particularly after we are no longer an emerging growth company, we incur and will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, and rules implemented by the SEC and Nasdaq, impose various requirements on public companies, including requirements to file periodic and event-driven reports with respect to our business and financial condition and operations and establish and maintain effective disclosure and financial controls and corporate governance practices. Our management and other personnel have limited experience operating a public company, which may result in operational inefficiencies or errors, or a failure to improve or maintain effective internal controls over financial reporting (“ICFR”) and disclosure controls and procedures necessary to ensure timely and accurate reporting of operational and financial results. Our existing management team will need to devote a substantial amount of time to these compliance initiatives, and we may need to hire additional personnel to assist us with compliance. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time consuming and costly.
Pursuant to Section 404 of the Sarbanes-Oxley Act (“Section 404”), we are required to furnish a report by our management on our ICFR, which, after we are no longer an emerging growth company, must be accompanied by an attestation report on ICFR issued by our independent registered public accounting firm. To achieve compliance with Section 404 within the prescribed period, we will document and evaluate our ICFR, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants, and adopt a detailed work plan to assess and document the adequacy of our ICFR, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented, and implement a continuous reporting and improvement process for ICFR. If our management and/or auditors determine that there are one or more material weaknesses in our ICFR, such a determination could cause an adverse reaction in the financial markets due to a loss of confidence in the reliability of our consolidated financial statements.
18
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some public company required activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and divert management’s time and attention from revenue generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
Being a public company and complying with applicable rules and regulations make it more expensive for us to obtain director and officer liability insurance. These factors could also make it more difficult for us to attract and retain qualified executive officers and members of our Board.
We may not be able to maintain a listing of our Common Shares on Nasdaq. If we fail to meet applicable listing requirements, Nasdaq may delist our Common Shares from trading, in which case the liquidity and market price of our Common Shares could decline.
Although our Common Shares are listed on Nasdaq, we must meet certain financial and liquidity criteria to maintain such listing. If we violate Nasdaq’s listing requirements, or if we fail to meet any of Nasdaq’s listing standards, our Common Shares may be delisted. In addition, our board of directors may determine that the cost of maintaining our listing on a national securities exchange outweighs the benefits of such listing. A delisting of our Common Shares from Nasdaq may materially impair our shareholders’ ability to buy and sell our Common Shares and could have an adverse effect on the market price of, and the efficiency of the trading market for, our Common Shares. The delisting of our Common Shares could significantly impair our ability to raise capital and the value of your investment.
We cannot assure you that we will be able to meet the continued listing standards of Nasdaq in the future. If we fail to comply with the applicable listing standards and Nasdaq delists our Common Shares, we and our shareholders could face significant material adverse consequences, including:
| ● | a limited availability of market quotations for our Common Shares; |
| ● | reduced liquidity for our Common Shares; |
| ● | a determination that our Common Shares are “penny stock”, which would require brokers trading in our Common Shares to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our Common Shares; |
| ● | a limited amount of news about us and analyst coverage of us; and |
| ● | a decreased ability for us to issue additional equity securities or obtain additional equity or debt financing in the future. |
On September 27, 2022, we received a letter from the staff of the Listing Qualifications Department of the Nasdaq, notifying the Company that it is not in compliance with the minimum bid price requirement set forth under Nasdaq Listing Rule 5550(a)(2). It resulted from the fact that the closing bid price of the Company’s Common Shares, was below $1.00 per share for a period of 30 consecutive business days. The letter also states that Nasdaq will provide the Company 180 calendar days, or until March 27, 2023 to regain compliance with Nasdaq Listing Rule 5550(a)(2). We effected a reverse share split at a ratio of 1-for-10 shares on March 9, 2023 in order to regain compliance.
On July 3, 2023, we received a new letter from the staff of the Listing Qualifications Department of the Nasdaq, notifying the Company that it is again not in compliance with the minimum bid price requirement set forth under Nasdaq Listing Rule 5550(a)(2). It resulted from the fact that the closing bid price of the Company’s Common Shares, below $1.00 per share for a period of 30 consecutive business days. The letter also states that Nasdaq will provide the Company 180 calendar days, or until January 1, 2024 to regain compliance with Nasdaq Listing Rule 5550(a)(2). To regain compliance, the Company’s Common Shares must have a closing bid price of at least $1.00 for a minimum of 10 consecutive business days. In the event the Company does not regain compliance by January 1, 2024 the Company may be eligible for additional time to regain compliance or may face delisting.
19
The receipt of the Notification Letter has no immediate effect on the listing of the Company’s Common Shares, which will continue to trade uninterrupted on Nasdaq under the ticker “AKAN”. We intend to monitor the closing bid price of its Common Shares and may, if appropriate, consider implementing available options, including, but not limited to, implementing a reverse stock split of its Common Shares to regain compliance with the minimum bid price requirement under the Nasdaq listing rules.
Receiving financial benefit directly or indirectly as a result of ownership of Common Shares may be subject to anti-money laundering laws in the United Kingdom.
Cannabis-related financial transactions, including investment in the securities of cannabis companies and receipt of any associated benefits, such as dividends, could be subject to anti-money laundering laws in the U.K., specifically the Proceeds of Crime Act, however the application of these laws is still developing. In the U.K., financial benefit directly or indirectly arising from conduct that would be considered unlawful if it were to take place in the U.K. may be viewed to be within the purview of these laws, and persons receiving any such benefit, including investors may be subject to liability under such laws. Each prospective investor should therefore contact his, her or its own legal advisor regarding the ownership of our Common Shares and any related potential liability.
We are a foreign private issuer and take advantage of the less frequent and detailed reporting obligations applicable to foreign private issuers.
We are a “foreign private issuer”, as such term is defined in Rule 405 under the Securities Act, and are not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. Under the Exchange Act, we are subject to reporting obligations that, in certain respects, are less detailed and less frequent than those of U.S. domestic reporting companies. As a result, we do not file the same reports that a U.S. domestic issuer files with the SEC, although we are required to file with or furnish to the SEC the continuous disclosure documents that we are required to file in Canada under Canadian securities laws, if applicable. In addition, our officers, directors, and principal shareholders are exempt from the reporting and “short swing” profit recovery provisions of Section 16 of the Exchange Act. Therefore, our shareholders may not know on as timely a basis when our officers, directors and principal shareholders purchase or sell shares.
As a foreign private issuer, we are exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements. We are also exempt from Regulation FD, which prohibits issuers from making selective disclosures of material non-public information. While we comply with the corresponding requirements relating to proxy statements and disclosure of material non-public information under Canadian securities laws, if and when applicable, these requirements differ from those under the Exchange Act and Regulation FD and shareholders should not expect to receive the same information at the same time as such information is provided by U.S. domestic companies. In addition, we have more time than U.S. domestic companies after the end of each fiscal year to file our annual report with the SEC and are not required under the Exchange Act to file quarterly reports with the SEC.
In addition, as a foreign private issuer, we have the option to follow certain Canadian corporate governance practices, except to the extent that such laws would be contrary to U.S. securities laws, and provided that we disclose the requirements we are not following and describe the Canadian practices we follow instead. We may in the future elect to follow home country practices in Canada with regard to certain corporate governance matters.
As a result, our shareholders may not have the same protections afforded to shareholders of U.S. domestic companies that are subject to all corporate governance requirements.
20
We may lose our status as a foreign private issuer in the United States, which would result in increased costs related to regulatory compliance under United States securities laws.
We will cease to qualify as a “foreign private issuer,” as defined in Rule 405 under the Securities Act and Rule 3b-4 under the Exchange Act, if, as of the last business day of our second fiscal quarter, more than 50% of our outstanding Common Shares are directly or indirectly owned by residents of the United States and any of the following three circumstances applies: (i) the majority of our executive officers or directors are U.S. citizens or residents; (ii) more than 50% of our assets are located in the United States; or (iii) our business is administered principally in the United States. If we determine that we fail to qualify as a foreign private issuer, we will cease to be eligible to avail ourselves of the forms and rules designated for foreign private issuers beginning on the first day of the fiscal year following such determination. Among other things, this will result in loss of the exemption from registration under the Exchange Act provided by Rule 12g3-2(b) thereunder, and, if we are required to register our Common Shares under section 12(g) of the Exchange Act, we will have to do so as a domestic issuer. Further, any securities that we issue in unregistered or unqualified offerings both within and outside the United States will be “restricted securities” (as defined in Rule 144(a)(3) under the Securities Act) and will continue to be subject to United States resale restrictions notwithstanding their resale in “offshore transactions” pursuant to Regulation S under the Securities Act. As a practical matter, this will likely require us to register more offerings of our securities under the Securities Act on either a primary offering or resale basis, even if they take place entirely outside the United States. The resulting legal and administrative costs of complying with the resulting regulatory requirements are anticipated to be substantial, and to subject us to additional exposure to liability for which we may not be able to obtain insurance coverage on favorable terms, or at all.
If our share price fluctuates, you could lose a significant part of your investment.
The market price of our Common Shares could be subject to wide fluctuations in response to, among other things, the risk factors described in this section of the Annual Report on Form 20-F, and other factors beyond our control, such as fluctuations in the valuation of companies perceived by investors to be comparable to us.
Furthermore, the stock markets have experienced price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political, and market conditions, such as recessions, interest rate changes or international currency fluctuations, may negatively affect the market price of our Common Shares. In the past, many companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business.
The public offering price of our Common Shares has been determined by negotiations between us and the underwriter based upon many factors and may not be indicative of prices that will prevail following the closing of this offering. Volatility in the market price of our Common Shares may prevent investors from being able to sell their shares at or above the public offering price. As a result, you may suffer a loss on your investment.
Investors may be unable to enforce judgments against our directors and officers because our directors and officers reside outside of the United States.
We are incorporated under the laws of the Province of Ontario, Canada and most of our assets are located outside of the United States. Furthermore, most of our directors and officers reside outside of the United States in Canada and the United Kingdom. As a result, investors may not be able to effect service of process within the United States upon our directors or officers or enforce against them in U.S. courts, judgments predicated on U.S. securities laws. Likewise, it may also be difficult for an investor to enforce in U.S. courts, judgments obtained against these persons in courts located in jurisdictions outside of the United States.
21
As a result of the above, public shareholders may have more difficulty in protecting their interests in the face of actions taken by management, members of our Board of Directors or controlling shareholders than they would as public shareholders of a U.S. based company.
We do not intend to pay dividends on our Common Shares in the near future, and, consequently, your ability to achieve a return on your investment will depend on appreciation in the price of our Common Shares.
We have never declared or paid any cash dividend on our Common Shares and do not currently intend to do so in the foreseeable future. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends in the foreseeable future. Therefore, the success of an investment in our Common Shares will depend upon any future appreciation in their value. There is no guarantee that our Common Shares will appreciate in value or even maintain the price at which you purchased them.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about us, our share price and trading volume could decline.
The trading market for our Common Shares will depend, in part, on the research and reports that securities or industry analysts publish about us or our operations. We do not have any control over these analysts and their research and reports. Securities and industry analysts do not currently, and may never, publish research on our business. If no security or industry analysts commence coverage on us, the trading price for our Common Shares would likely be negatively affected. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade our shares or publish inaccurate or unfavorable research about our business, our share price would likely decline. In addition, if our operating results fail to meet the forecast of analysts, our share price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, demand for our shares could decrease, which might cause our share price and trading volume to decline.
ITEM 4. INFORMATION ON THE COMPANY
| A. | History and Development of the Company |
Akanda Corp. was incorporated in the Province of Ontario, Canada on July 16, 2021 in connection with the plan of Halo to reorganize its medical cannabis market focused international business assets. In September 2021, we entered into a share purchase agreement with Halo, a publicly-traded, vertically integrated multinational cannabis company (NEO: HALO) (OTCQX: HCANF) (Germany: A9KN). Pursuant to this agreement, we acquired all the issued and outstanding equity interests of Cannahealth Limited, a Republic of Malta company (“Cannahealth”), from Halo (the “Cannahealth Acquisition”).
At the closing of the Cannahealth Acquisition on November 3, 2021, Cannahealth owned all the issued and outstanding equity interests of Canmart and Bophelo Holdings, which owned all the issued and outstanding equity interests of Bophelo. As a result of the Acquisition, both Bophelo and Canmart became our indirect wholly-owned subsidiaries. As consideration for this Acquisition, we issued 1,312,921 Common Shares to Halo at a price of $10.00 per share, resulting in Halo owning approximately 68.3% of all our outstanding Common Shares at the closing of the Cannahealth Acquisition.
On November 12, 2021, Halo transferred 210,000 Common Shares to an unaffiliated party, 1306077B.C. LTD. (the “Halo Transferee”), which resulted in Halo owning 49.6% of our issued and outstanding Common Shares (the “Halo Transfer”) at the time. On 14 March 2022, and pursuant to a convertible debenture agreement between Akanda and Halo, Akanda issued 164,574 Common Shares to Halo to settle the principal amount and accrued interest (at the time of conversion) of $6,582,980 owing to Halo in terms of the convertible debenture agreement.
On April 20, 2022, Akanda, Cannahealth, The Flowr Corporation (“Flowr”) and Holigen Holdings Limited (“Holigen”), a wholly-owned subsidiary of Flowr entered into a share purchase agreement (the “Holigen Agreement”) whereby Cannahealth would acquire 100% of the ordinary shares of Holigen, which is the holding company of RPK Biopharma, Unipessoal, LDA (“RPK”), a cultivator and manufacturer of medical cannabis products based in Portugal (the “Holigen Acquisition”). The Holigen Acquisition closed on April 29, 2022.
22
The Purchase Price for the Holigen Acquisition was comprised of (i) of $3,000,000 in cash and (ii) 190,000 Common Shares, no par value per share, of Akanda (the “Akanda Shares”). The Akanda Shares were issued pursuant to Regulation S of the Securities Act. Concurrent to the closing of the Holigen Acquisition, Akanda purchased 14,285,714 Common Shares of Flowr for an aggregate purchase price of CAD$999,999.98.
RPK’s operations consist of a 20,000 square foot indoor EU GMP certified grow facility located near Sintra, Lisbon, Portugal, dedicated to the cultivation of high-THC premium cannabis as well as a large seven million square foot (180+ acre) outdoor facility located in Aljustrel. In 2020, Holigen grew more than 20 high-THC strains at the Aljustrel facility making it the largest outdoor medical cannabis grow in the EU. Combined, these facilities provide the flexibility of capacity in Portugal to produce approximately seven tonnes annually, and have consistently been harvesting with THC levels of approximately at least 25%.
Flowr entered into a lock-up agreement with Akanda, pursuant to which they agreed not to sell any Akanda Shares for a period of eleven (11) months following the closing date of the Holigen Acquisition, with limited exceptions as discussed in the Holigen Agreement. Flowr also entered into restrictive covenant agreements pursuant to which they agreed that they will not compete with the business conducted by Holigen nor solicit its employees for a period of two years following the closing date. Pursuant to the Holigen Agreement, Flowr also agreed to customary indemnifications for the benefit of Cannahealth and Akanda. Capitalized terms not defined herein shall have the meanings ascribed to them in the Holigen Agreement which is filed as Exhibit 2.1 to Akanda’s Report Form 6-K filed on April 27, 2022 and is incorporated by reference herein.
On July 15, 2022, our indirect wholly-owned subsidiary Bophelo, a Lesotho company, was placed into liquidation by the High Court of Lesotho (the “Lesotho Court”) pursuant to an unauthorized application and request (the “Liquidation Application”) that was filed by Louisa Mojela, our former Executive Chairman, who was terminated as Executive Chairman of Akanda in July 2022, and the Mophuti Matsoso Development Trust (“MMD Trust”), which we believe was established by Ms. Mojela. Mr. Chavonnes Cooper of Cape Town, South Africa, was appointed by the Lesotho Court as liquidator of Bophelo for purposes of maintaining the value of the assets owned or managed by Bophelo. We intend to seek to recover significant loans made to Bophelo to fund the execution of Bophelo’s business plan, including payment of rents and staffing costs in the event that the Lesotho Court does not reverse its determination to place Bophelo in liquidation. As a result of Bophelo’s liquidation, during the year ended December 31, 2022, we determined that we no longer controlled Bophelo. As a result of the loss of control, we derecognized the net assets of Bophelo and accounted for the operating results as discontinued operation.
On August 9, 2022, we entered into a cooperation agreement with Cansativa GmbH (“Cansativa Group”) to allow the Cansativa platform to supply the German market with dried flowers from Akanda’s EU-GMP certified indoor grow facility in Sintra, Portugal. All pharmacies in Germany will be able to purchase these products through the Cansativa platform.
On September 13, 2022, we entered into an exclusive license agreement with international cannabis lifestyle brand Cookies. The multi-year agreement enables us to pursue the current medical and future adult-use opportunities in Europe with what we believe is one of the best-known cannabis brands and highest quality genetics in the world. Under the terms of the license agreement, Akanda gains the ability to cultivate, manufacture and distribute Cookies strains, and the rights to sell Cookies branded products, including non-cannabis merchandise, in Portugal. Akanda intends to initially produce EU GMP certified Cookies branded high THC medical cannabis products at its flagship indoor premium cultivation and manufacturing facility in Sintra, Portugal. Additionally, Akanda is able to exclusively open and operate flagship Cookies branded pharmacy outlets throughout the country. Cookies, founded in 2010 by Billboard-charting rapper and entrepreneur Berner and Bay Area breeder and cultivator Jai, offers a collection of over 70 proprietary cannabis cultivars and more than 2,000 products.
Akanda’s EU GMP certified indoor grow facility in Sintra received its first purchase order in September 2022. Akanda recently entered into an agreement to deliver 1,000 kilograms of high-grade medical cannabis flower to German pharmacies through the Cansativa platform. Cansativa is the only company in Germany permitted to distribute domestically grown cannabis. Cansativa will have a right of first refusal (ROFR) to take on additional quantities that could result in the full capacity utilization of RPK’s 2,000 kilograms per annum indoor production capacity. Akanda is targeting a leading 10% market share position of German medical cannabis imports, with room for additional expansion and growth. Germany has imported medical cannabis from numerous countries, with Portugal becoming an increasingly larger player gaining ground on Canada, the leader in this space. Holigen is one of few publicly traded companies that is a Europe-based cultivator, manufacturer and distributor of EU GMP certified medical cannabis. Holigen hosts a one-of-a-kind 20,000 square foot indoor cultivation site in Sintra dedicated to the cultivation of high THC premium cannabis as well as a 600,000 square foot outdoor facility located two hours south in Aljustrel.
23
On November 30, 2022, our former Chief Executive Officer, Tejinder Virk was placed on a paid leave of absence due to an internal investigation into Mr. Virk’s conduct as our Chief Executive Officer and as a director of Canmart. In February 2023, Mr. Virk notified us of his resignation as our Chief Executive Officer. Mr. Virk’s resignation was a result of disagreement with us regarding contractual obligations owed pursuant to the Service Agreement between Mr. Virk, Halo Labs Inc., as guarantor, and Canmart Limited. According to Mr. Virk, the Company and Canmart committed a breach of the Service Agreement by failing to pay him monies and benefits owed following his placement on a paid leave of absence in November 2022. On February 13, 2023, we notified Mr. Virk that he has was summarily dismissed. On May 12, 2023, Mr. Virk issued a claim for detriment and dismissal for alleged protected disclosures totaling £1,630,302.22. The claim has been denied in it’s entirely. As of December 28, 2023, the list of issues to be agreed upon are outstanding and pending further discovery, bundle to be agreed by February 1, 2024, witness statements due for exchange by March 28, 2024.
On March 9, 2023, we implemented a 1-for-10 reverse stock split of our Common Shares. Every 10 shares of our issued and outstanding Common Shares were automatically converted into one issued and outstanding common share. No fractional shares were issued as a result of the reverse stock split. Instead, any fractional shares that resulted from the split were rounded down to the next whole number. The reverse stock split affected all shareholders uniformly and did not alter any shareholder’s percentage interest in the Company’s outstanding Common Shares, except for adjustments that resulted from the treatment of fractional shares.
In July 2022, our former Executive Chairman, Louisa Mojela was summarily terminated as Chairman of Bophelo for Cause, as a “bad leaver”. In the event that the Lesotho Court does not reverse its determination to place Bophelo in liquidation, we plan to seek to recover significant loans that we have made to Bophelo to fund the execution of Bophelo’s business plan, including payment of rents and staffing costs. On October 20, 2022 Ms. Mojela filed a claim against Canmart and Akanda for wrongful termination of her Service Agreement. Ms. Mojela sought £1,832,150.62 plus further administrative and legal fees. The Company denied her claim and lodged a counterclaim lodged for losses caused by Ms. Mojela including a loan of US $6,849,935.69 Akanda advanced to Bophelo. As at December 31, 2023, Ms. Mojela’s entire application failed. At the Consequentials hearing on January 15, 2024 Canmart and Akanda were awarded £60,000 in legal costs. Ms. Mojela is pursuing an appeal.
On September 20, 2023, the Company announced the signing of an option to develop Canadian THC and CBD farming facility. The Company and 1107385 B.C. LTD, have agreed upon terms to purchase farming land and related operations and licenses, pursuant to which, the key deal terms are as follows:
| ● | Akanda will issue a non-refundable payment equal to One Million Eight Hundred Thousand United States Dollars (USD1,800,000) and if paid in Common Shares of Akanda will be based on formula to calculate the per share price as set forth in the agreement. The initial payment will be broken up into the First Option Payment, the Second Option Payment and the Third Option Payment, upon signing, 15 days after signing, 30 days after signing respectively. |
| ● | This buys Akanda the right to develop the property for two years. The Company plans during this time period to develop Tetrahydrocannabinol (THC) and CBD facilities at this site. Additional payments will be made based upon milestones achieved from the development. Additional milestones include THC cultivation, sales of product, CBD cultivation, and Hemp cultivation. This is similar to a mining agreement where operators buy the right to mine a site. In this case the Company has purchased the right to develop the farming land. |
Recent Developments since January 1, 2024
On January 4, 2024, the Company announced that it received an extension of 180 calendar days from The Nasdaq Stock Market LLC to regain compliance with the Nasdaq’s minimum $1.00 bid price requirement set forth in Nasdaq Listing Rule 5550(a)(2) for continued listing on The Nasdaq Capital Market, following the expiration of the initial 180 calendar days period to regain compliance on January 2, 2024.
Sale of RPK
On February 28, 2024, the Company entered into a share purchase agreement with Somai Pharmaceuticals Ltd (“Somai”), Cannahealth and Holigen to sell all the shares of RPK to Somai for a consideration of $2,000,000, subject to certain closing conditions, customary representations and warranties, covenants and indemnification obligations. On April 1, 2024, the Company completed the transaction with Somai for the sale of RPK. For a detailed description of the transaction, please see below under “Business Overview.”
24
Financing Transactions
On February 1, 2024, the Company entered into a securities purchase agreement with an accredited investor in connection with the issuance and sale by the Company in a registered direct offering (the “Registered Direct Offering”) at market price in accordance with Nasdaq rules of 280,851 Common Shares at a purchase price of $0.406 per share, and pre-funded warrants to purchase 1,462,991 Common Shares at a purchase price of $0.4059 per pre-funded warrant, and the exercise price of each pre-funded warrant is $0.0001 per share, pursuant to the Company’s effective shelf registration statement on Form F-3 (File No. 333-276577) and a related base prospectus, together with the related prospectus supplement dated as of February 2, 2024, filed with the Securities and Exchange Commission. The pre-funded warrants were immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full, subject to certain beneficial ownership limitations as set forth in the pre-funded warrant. The closing of the issuance of the Common Shares and pre-funded warrants occurred on February 2, 2024. The gross proceeds from the offering were approximately $708,000 before deducting the financial advisor’s fees and other estimated expenses relating to such offering. All of the pre-funded warrants have been exercised in accordance with their terms.
On March 1, 2024, the Company entered into a securities purchase agreement with an accredited investor in connection with the issuance and sale by the Company in a Registered Direct Offering of 367,870 Common Shares at a purchase price of $0.20544 per share, and pre-funded warrants to purchase 361,972 Common Shares at a purchase price of $0.20534 per pre-funded warrant, and the exercise price of each pre-funded warrant is $0.0001 per share, pursuant to the Company’s effective shelf registration statement on Form F-3 (File No. 333-276577) and a related base prospectus, together with the related prospectus supplement dated as of March 1, 2024, filed with the Securities and Exchange Commission. The pre-funded warrants were immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full, subject to certain beneficial ownership limitations as set forth in the pre-funded warrant. The closing of the issuance of the Common Shares and pre-funded warrants occurred on March 4, 2024. The gross proceeds from the offering were approximately $150,000 before deducting the financial advisor’s fees and other estimated expenses relating to such offering. All of the pre-funded warrants have been exercised in accordance with their terms.
On March 4, 2024, the Company entered into a securities purchase agreement with an accredited investor in connection with the issuance and sale by the Company in a Registered Direct Offering of 367,870 Common Shares at a purchase price of $0.16872 per share, and pre-funded warrants to purchase 373,002 Common Shares at a purchase price of $0.16862 per pre-funded warrant, and the exercise price of each pre-funded warrant is $0.0001 per share, pursuant to the Company’s effective shelf registration statement on Form F-3 (File No. 333-276577) and a related base prospectus, together with the related prospectus supplement dated as of March 4, 2024, filed with the Securities and Exchange Commission. The pre-funded warrants were immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full, subject to certain beneficial ownership limitations as set forth in the pre-funded warrant. The closing of the issuance of the Common Shares and pre-funded warrants occurred on March 5, 2024. The gross proceeds from the offering were approximately $125,000 before deducting the financial advisor’s fees and other estimated expenses relating to such offering. All of the pre-funded warrants have been exercised in accordance with their terms.
On March 25, 2024, the Company entered into a firm commitment underwriting agreement with Univest Securities, LLC (“Univest”), as the underwriter in connection with the issuance and sale by the Company in an underwritten public offering of 3,087,443 Common Shares at a purchase price of $0.1217 per share, and pre-funded warrants to purchase 37,997,190 Common Shares at a purchase price of $0.1216 per pre-funded warrant, pursuant to the Company’s effective registration statement on Form F-1 (File No.: 333-277182) and a related preliminary prospectus, together with the related final prospectus dated as of March 26, 2024, filed with the Securities and Exchange Commission. The pre-funded warrants were immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full, subject to certain beneficial ownership limitation as set forth in the pre-funded warrant. The gross proceeds from the underwritten public offering were $5,000,000 before deducting underwriting discounts and commissions, and estimated expenses payable by the Company. The closing of the underwritten public offering occurred on March 27, 2024. 20,085,234 of the pre-funded warrants have been exercised in accordance with their terms.
Shareholder Meeting
On March 22, 2024, the Company held its 2023 annual shareholder meeting and a special meeting of shareholders. The shareholders of the Company approved the following proposals, amongst others:
| ● | authorize the board of directors to select one or more share Consolidation ratios of between 10 pre-consolidation Common Shares for one (1) post-consolidation Common Share and 100 pre-consolidation Common Shares for one (1) post- consolidation Common Share, provided that, (A) the cumulative effect of such share consolidation shall not result in a consolidation ratio that exceeds 100 pre-share consolidation Common Shares for one (1) post-share consolidation Common Share, and (B) such share consolidation occurs prior to the earlier of the 12 month anniversary of the shareholders meeting and the next annual meeting of shareholders. |
| ● | a new thirty percent (30%) evergreen 2024 Equity Incentive Plan, which was adopted by the board of directors on February 26, 2024. |
| ● | authorize the proposed RPK sale transaction to Somai. |
25
Resignation of Harvinder Singh
On April 24, 2024, Mr. Harvinder Singh resigned as an independent director of the Board of Directors of the Company. A Resignation and Mutual Release Agreement dated April 24, 2024 was entered between the Company and Mr. Singh, pursuant to which the Company agreed to pay Harvinder Singh a separation and release amount of $50,000.
Our principal executive offices and mailing address are located at 1a, 1b Learoyd Road New Romney TN28 8XU, United Kingdom, and our telephone number is +44 (203) 488-9514.
The SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC on www.sec.gov. You can also find information on our website akandacorp.com. The information contained on our website is not a part of this annual report.
| B. | Business Overview |
Background
We are a cannabis cultivation, manufacturing and distribution company whose mission is to provide premium quality medical cannabis products to patients worldwide. We are an early stage, emerging growth company headquartered in London, the United Kingdom. We have a limited operating history and minimal revenues to date. Canmart is our operating and wholly-owned subsidiary. We expect to expand their local operations and develop sales channels of our medicinal-grade cannabis products and cannabis based medical and wellness products in international markets and in particular, in Europe.
Canmart Ltd
Our indirect wholly-owned subsidiary Canmart, a company incorporated under the laws of England and Wales, is a licensed importer and distributor of CBPMs in the United Kingdom (UK). Canmart holds a Controlled Drug License issued by the Home Office to possess and supply CBPMs in the UK.
This license expired on February 3, 2022 and needs to be renewed annually. We applied for the license renewal in January 2022, coinciding with an application to increase import capabilities to Schedule 1 (bulk product) and we are currently awaiting a response from the UK Home Office. We have decided to not take up the Schedule 1 license and focus on where our expertise lie in the distribution of goods safely and effectively. Until such time that the renewal application is approved by the UK Home Office, Canmart can continue with its day to day business under the conditions of its existing license. Canmart continues to receive new import licenses issued by the UK Home Office for every specific shipment of CBPMs and Canmart has thus far successfully imported over 100kgs of product for distribution in the UK. Canmart holds both a Manufacturer’s Specials License for importation of CBPMs and a Wholesale Distribution Authorization from the Medicines and Healthcare Products Regulatory Agency.
Canmart commenced importing and distributing CBPMs in 2020. Under the current controlled drugs regulatory regime, Canmart is only able to supply to dispensing pharmacists and other wholesale distributors, tied in with prescribing and clinic partners. However, Canmart’s has reduced its plan for Canmart-owned and operated clinics and pharmacies to instead provide third party and specialist import and distribution services for Schedule 2 products including CBPM’s. Canmart continues to work further with premium product suppliers to bring safe, effective and required products to market that patients demand, and working with existing and new clinical cannabis operations in the UK to provide third party products.
Cannahealth Limited
Our direct wholly-owned subsidiary Cannahealth, a Republic of Malta company, is a holding company of all the ownership interests in Canmart and Holigen. Cannahealth does not engage in any operations.
Holigen Holdings Limited
In May 2022, our wholly owned subsidiary, Cannahealth, acquired 100% of the ordinary shares of Holigen and its wholly-owned operating subsidiary, RPK Biopharma Unipessoal, LDA from the Flowr Corporation. Through its operations in RPK, Holigen is a producer of premium EU GMP grade indoor grown cannabis flower. The acquisition of Holigen enabled us to produce EU GMP grade cannabis flower for the European market, in particular Germany and the UK.
26
RPK Biopharma Unipessoal, LDA
RPK Biopharma Unipessoal, LDA’s operations consist of a 20,000 square foot indoor EU GMP certified grow facility located near Sintra, Lisbon, Portugal, dedicated to the cultivation of high-tetrahydrocannabinol (THC) premium cannabis and yielding over 2,000 kg of flower per year. This outdoor and greenhouse expansion site in Portugal affords scaliable cultivation as well as a 600,000 square foot outdoor facility located in Aljustrel, Portugal. RPK currently sells over 25 percent high THC flower into Germany, the largest medical cannabis market in Europe as well as selling into the United Kingdom generating revenues and ready to scale. RPK also features a genetics and retail partnership with Cookies, one of the world’s top cannabis brands and genetics have already been received at our Portuguese facility and ready to cultivate. In addition, we believe the Sintra facility is the only commercial scale premium indoor EU GMP certified and licensed grow in Portugal. RPK generates revenue through a German company’s offtake agreement of its clean testing non-irradiated quality flower. The facility has a purpose-built design, with all unnecessary equipment residing outside of the rooms to reduce contamination risk and self-contained modular grow rooms result in better environmental control. There is no irradiation required and we believe less than 10% of licensed producer’s have this ability. RPK’s other business lines include contract manufacturing services and distribution services. RPK has a senior bank lender with a mortgage secured by the facility and an equipment leasing agreement. The Company is currently in discussions with the bank to obtain more time to restructure and bring current these agreements.
On January 21, 2024, the Company entered into an amended non-binding letter of intent with Somai Pharmaceuticals Ltd. (“Somai”) for the sale of RPK (the “LOI”). The term of the LOI was extended through March 31, 2024, and the purchase price therein was amended to an aggregate of $2,000,000 from $2,700,000, which include up to approximately $4,000,000 Euros of RPK’s liabilities. In addition, a deposit of $500,000 was placed in an escrow account with the remainder of the balance due upon successful completion of said the proposed transaction. The precise terms of the proposed transaction will be negotiated and contained in a definitive agreement. On February 28, 2024, the Company entered in a definitive share purchase agreement with Somai, Cannahealth and Holigen. Under the terms of the share purchase agreement, Somai is acquiring all of the shares of RPK for a consideration of $2,000,000, subject to certain closing conditions, customary representations and warranties, covenants and indemnification obligations.
On April 1, 2024, the Company completed the transaction with Somai for the sale of RPK. Pursuant to the provisions set forth in the definitive share purchase agreement, the cash purchase price is $2,000,000. In addition Somai is assuming up to 1,000,000 Euros of current liabilities and RPK’s debt with the senior secured lender bank, Caixa Agricola. In total Somai is assuming approximately 4,000,000 Euros of debt. In accordance with the terms of the proposed transaction, a deposit amounting to $500,000 was released from a joint escrow account and the remainder of the purchase price was paid directly to the Company.
Bophelo Bio Science and Wellness (Pty) Ltd
Our indirect wholly-owned subsidiary Bophelo, a Lesotho company, is focused on the cultivation of cannabis, the production of medical cannabis products including dried flower, oils, and other concentrates and the supply of such medical cannabis products to wholesalers in international markets. As a result of Bophelo’s liquidation, during the year ended December 31, 2022, we determined that we no longer controlled Bophelo. As a result of the loss of control, we derecognized the net assets of Bophelo and accounted for the operating results as discontinued operation.
1900 Ferne Road, Gabriola Island, British Columbia
On September 20, 2023, Akanda acquired the right to develop a Canadian farming property in British Columbia, including farming land and related operations and licenses, from 1107385 B.C. LTD. We plan to develop THC and CBD facilities at this site. We agreed to issue a non-refundable payment equal to $1,800,000 and if paid in our Common Shares will be based on formula to calculate the per share price as set forth in the agreement. The initial payment will be broken up into the First Option Payment, the Second Option Payment and the Third Option Payment, upon signing, 15 days after signing, 30 days after signing respectively. On September 22, 2023, we paid the First Option Payment by issuing 879,895 Common Shares. We have paid the Second Option Payment of $600,000 and the Third Option Payment of $600,000. Additional payments to the seller will be made based upon milestones achieved from the development, including THC cultivation, sales of product, CBD cultivation, and hemp cultivation.
27
Competition
European Union
Our main competitors in Portugal are the other 34 companies licensed by INFARMED for activities with cannabis, but specifically the others that are EU-GMP certified (as of Jan. 1, 2023) such as Tilray Portugal, Unipessoal, Lda, Portocanna SA, Agrivab Medical Cannabis, and Clever Leaves Portugal Unipessoal Lda. (as of April 2023, Tilray and Clever Leaves have decided to close or reduce operations in Portugal). There is an increasing number of companies with applications to enter the cultivation market in Portugal, many of them at this time were not able secure sales contracts which has led to closure of many competitors. The German market has just recently voted in favor legalizing cannabis but it does not yet have cultivation facilities in the country and is dependent on import from other nations.
United Kingdom
The United Kingdom’s CBPM market is a highly regulated and restricted operational environment. CBPMs is supplied as a “special’s medication” with no marketing authorization for medical claims. Canmart has identified an opportunity to grow its business in the expanding CBPM sector by establishing direct sales channels to patients. Our primary competition is from three established suppliers including The Lyphe Group, the pioneer in this sector which operates The Medical Cannabis Clinics, Grow Pharma and IPS Pharma which are established specials formulators and have moved to supplying medical cannabis through their associated clinics. Canmart is establishing sales channels to communicate and market directly to patients as an innovative and disruptive sales model in this field. Canmart’s strategy is to expand its customer base and market size by identifying patients with specific conditions and needs and providing easy-to-access education and consultations to patients about medical benefits of CBPMs based on observational clinical studies from international studies.
Canada
CBD
The industry in which we operate is subject to intense and increasing competition. The Canadian hemp industry has grown to over 130,000 acres, and Canada has established a global leadership position in producing hemp grain for food. Hemp could become Canada’s next “canola”. Innovation and technology development in this industry has been almost exclusively privately funded and enabled to date. CBD competitors include Charlotte’s Web, Cresco Labs, and the Valens Company.
THC
Some of the larger competitors in this segment include Aleafia Health/ Red White and Bloom, WeedMD, and 48 North. We believe this market is worthwhile to enter as long as we can keep costs down and deliver high yields. There remains 63.2 million square feet of outdoor area licensed for cannabis production, according to Health Canada down from 76.7 million square feet in 2021.
Business Partnerships
In August 2022, we entered into a cooperation agreement with Cansativa Group to allow the Cansativa platform to supply the German market with dried flowers from Akanda’s EU-GMP certified indoor grow facility in Sintra, Portugal. All pharmacies in Germany will be able to purchase these products through the Cansativa platform.
In September 2022, we entered into an exclusive license agreement with international cannabis lifestyle brand Cookies. The multi-year agreement enables us to pursue the current medical and future adult-use opportunities in Europe with what we believe is one of the best-known cannabis brands and highest quality genetics in the world. Under the terms of the license agreement, Akanda gains the ability to cultivate, manufacture and distribute Cookies strains, and the rights to sell Cookies branded products, including non-cannabis merchandise, in Portugal. Akanda intends to initially produce EU GMP certified Cookies branded high THC medical cannabis products at its flagship indoor premium cultivation and manufacturing facility in Sintra, Portugal. Additionally, Akanda is able to exclusively open and operate flagship Cookies branded pharmacy outlets throughout the country.
In April 2024, the Company completed the transaction with Somai for the sale of RPK and as a result will not continue with the cooperation agreements stated above.
Regulation
Regulatory Framework in the United Kingdom
CBD Regulation — Overview
Cannabis, cannabis resin, cannabinol and other cannabinol derivatives (among others) are listed as Class B controlled drugs under Schedule 2, of the Misuse of Drugs Act 1971, or the MDA, based on a harms assessment. They are also listed under Schedule 1 to the Misuse of Drugs Regulations 2001, or the MDR, together with other chemical constituents such as the cannabinoid THC (defined below). As such, it is unlawful to cultivate, possess, supply, produce, import or export these controlled drugs except under license. The Hemp (Third Country Imports) Regulations 2002 also require, except in specified circumstances, that hemp from non-EU countries be imported under a license and, in the case of hemp seeds other than for sowing, under an authorization.
28
CBD is one of the main chemical compounds found in the cannabis plant, together with THC. CBD, as an isolated substance (i.e. containing no THC) is not a controlled drug under the MDA/MDR. Unlike CBD, THC is the main “psychoactive” component of cannabis and is a controlled drug.
A CBD product containing THC (in any amount), or any other controlled cannabinoid under the regulations cannot be practically prescribed, administered or supplied to the public unless it is an ‘exempt product’ or a cannabis based product for medicinal use in humans, or CBPM. CBPMs are subject to further regulation and licensing given the medicinal purpose for which they are marketed and prescribed.
The U.K. Home Office (specifically, the Drugs & Firearms Licensing Unit, or DFLU) prescribes two separate licensing regimes relating to cannabis cultivation, according to whether the varieties are high THC (above 0.2% THC content) or low THC (below 0.2% THC content). A license is required to cover both cultivation and possession.
The sale of CBD products (i.e. the “finished products” following extraction and processing of CBD into products) is subject to additional regulations and licensing regimes — see below CBD Extraction for General Commercial Purposes and CBD Extraction for Medicinal Purposes for a more detailed discussion.
Controlled Drugs License
Companies wishing to possess, supply, produce/manufacture, import or export ‘controlled drugs’ can only lawfully do so under Controlled Drugs Licenses issued by the Home Office. Licenses are issued for specific drugs, entities and locations and cannot be transferred to other drugs, entities or locations.
Applications for a controlled drug license are submitted online and prospective licensees are advised that it can take up to 16 weeks for the Home Office to review and ensure that various security and record- keeping requirements have been met. Where an enhanced DBS check has been obtained within the last three years for all persons named on the application, such checks do not need to be repeated. The DFLU may also conduct site visits, where needed. The term of the license is one year from the date of issuance and further applications are required to be submitted for each license to be renewed.
Companies wishing to import ‘controlled drugs’ will need to apply to the Home Office for a separate import license for each shipment.
Canmart holds a Controlled Drug Licenses issued by the Home Office to possess and supply CBPMs in the United Kingdom. This license expires on February 3, 2022. Canmart is required to apply for import licenses from the Home Office for each individual shipment and has, as of 2021 when shipping of CBPMs to Canmart commenced, been successful in applying for those import licenses as required.
CBD Extraction for General Commercial (Retail) Purposes
CBD products such as CBD oil are becoming increasingly prevalent in the U.K. retail market. Where a CBD product contains a controlled drug (in any quantity) such as THC, the product needs to satisfy the requirements for an ‘exempt product’ under the MDR to be lawfully available to the public.
In general, an exempt product is a product containing a controlled drug that is: (a) not designed to be administered to a human being or animal, (b) not packaged in such a way that it can be recovered by readily applicable means, and (c) does not contain more than 1 mg (per container) of the controlled drug. All three prongs are required to be established, including significant testing by an independent and licensing U.K. company and the provision of comprehensive and independently verifiable research and information. Notably, the 0.2% THC threshold for the cultivation of industrial hemp does not apply to CBD finished products, Rather, only 1 mg of THC (per container) is permissible in any given product that is placed on the U.K. market.
CBD Extraction for Medicinal Purposes (Medical Cannabis)
CBPMs are preparations or products that: (a) contain cannabis or other cannabinol derivatives, (b) are produced for medicinal use in humans, and (c) are a medicinal product, substance or preparation for use as an ingredient in a medicinal product. A CBD preparation or product containing controlled cannabinoids (e.g., THC) which meets the three prongs of this definition may be classed as a CBPM.
29
Companies wishing to possess, supply and or import/export CBPMs will require a controlled drug license in addition to a high-THC cannabis cultivation license if they are involved in production/manufacturing, which are both issued by the Home Office, unless an exemption applies to that licensing requirement.
In addition, the regulation of CBPMs in the United Kingdom is undertaken by the Medicines and Healthcare Products Regulatory Agency, or MHRA. The MHRA is responsible for ensuring all medicines and medical devices in the United Kingdom are safe and appropriate in accordance with the Human Medicines Regulations 2012 (SI 2012/1916), or HMR. Under the HMR, CBPMs can only be manufactured and assembled in accordance with the specifications of a doctor listed on the General Medical Council Specialist Register and must meet a ‘special’ clinical need of the individual patient.
The manufacturer, assembler or importer/exporter of a CBPM must also hold a Manufacturer’s “Specials” License, which is granted by the Licensing Authority (specifically, the U.K. Ministers designated under the HMR) for all medicinal products for human use. The manufacturing, storage and/or assembly site and its operations will be inspected for compliance with the European Union’s “good manufacturing practice” and the conditions of the license. These require that the manufacture, storage and/or assembly is carried out under the supervision of appropriately qualified staff, including a named quality controller and production manager, who are acceptable to the Licensing Authority. License applications are submitted online to the MHRA and take approximately 90 business days to process.
The distributer of a CBPM must also hold a Distribution Authorization issued by the Licensing Authority. All distributers of medicinal products for human use must have a similar license. Canmart holds both a Manufacturer’s Specials License for importation of CBPMs and a Wholesale Distribution Authorization.
CBD Sales and “Novel Food” Status
In the United Kingdom, the sale of CBD products falls under the regulatory purview of the U.K. Food Standards Agency, or the FSA. The FSA, in turn, follows the guidance and regulations set by the European Union, specifically the European Food Standards Agency, the EFSA, and the European Commission, or the EC, respectively.
In November 2015, the European Parliament and the Council of the European Union adopted a new regulation on novel food, Regulation (EU) 2015/2283, or the Novel Food Regulation, with the intent of making the novel food authorization process more efficient while ensuring high standards of food safety for consumers. The Novel Food Regulation came into force on January 1, 2018.
The Novel Food Regulation provides that a food is “novel” if it has not been used for human consumption to a significant degree within the European Union before May 15, 1997. The regulations further provide that a food stuff will be authorized only if it can be demonstrated that the product is safe, properly labeled so as to not mislead consumers and is not nutritionally disadvantageous.
On January 15, 2019, the EC updated the European Union’s Novel Foods Catalogue, specifically, the entries relating to cannabis sativa and cannabinoids, to include other cannabinoids extracts used in food and food supplements and hemp-derived products in food.
While the Novel Foods Catalogue is non-exhaustive and carries no legal effect, it is frequently updated and amended with input from Member States and is used as reference by authorities in EU countries to aid enforcement of the Novel Food Regulation. A novel food can only be sold in the European Union once it has successfully gone through the authorization process (involving a safety risk assessment) and an implementing act is published authorizing the addition of the novel food to the Novel Foods Catalogue. This process can take up to 18 months from receipt of the initial application. The United Kingdom has adopted the EU Novel Foods regime as retained EU law and the FSA has confirmed it will follow the EFSA. Therefore products new to market will require novel food authorization from the FSA prior to being made available and marketing. There is also a currently a regime in place to allow for products that were already on the market prior to early 2020 to apply for retroactive novel food authorization.
30
Proceeds of Crime Act or POCA
POCA addresses money-laundering offences in the United Kingdom. Under POCA, a person or entity commits a principal money laundering offence if they:
| (a) | conceal, disguise, convert or transfer criminal property; |
| (b) | enter into or become concerned in an arrangement which he knows or suspects facilitates the acquisition, retention use of control of criminal property by or on behalf of another person; or |
| (c) | acquire criminal property. |
Property is criminal property if it constitutes a person’s benefit from criminal conduct and the alleged offender knows or suspects that it constitutes such a benefit.
Criminal conduct is conduct that constitutes an offence in any part of the United Kingdom or in the case of overseas conduct would constitute an offence in any part of the United Kingdom if it occurred there. This is relevant for U.K. companies that operate in the medicinal cannabis industry as they may have relationships with medicinal or recreational cannabis companies that operate legally in non-U.K. jurisdictions, however whose activities would be caught by POCA, and for U.K based investors who are investing in cannabis-related investments.
There are defenses available to the money laundering offences, principally making an authorized disclosure prior to the offence being committed and gaining appropriate consent from the National Crime Agency (“NCA”) to receive the criminal property in question, and receiving the property for adequate consideration, as in the case of a contract.
Canmart will take into consideration any potential POCA applications when dealing with non-U.K. companies and will act accordingly to ensure compliance with POCA.
Regulatory Framework in the European Union
Germany
In March 2017, Germany legalized cannabis for medicinal purposes. Since then, Germany has rapidly become Europe’s leading medical cannabis market. Germany’s federal regulator, BfArM, takes a progressive approach to legal cannabis reimbursing patients for treatment via public insurers. Apart from dentists and veterinarians, any physician is authorized to prescribe medical cannabis. There are no limitations on which medical conditions are eligible for cannabis treatment. Together with the affordability granted by reimbursement and clear regulations around distribution and dispensation, patient access is greater than anywhere else in Europe.
Portugal
In 2001, Portugal decriminalized possession and consumption of personal amounts of drugs, including up to 25 grams of cannabis. Further, in 2018, Portuguese authorities passed a bill allowing the consumption of medical cannabis for domestic patients. Medicine is licensed under INFARMED, the country’s primary regulatory body, which has responsibility for licensing the cultivation of high THC plants. Although cultivation of cannabis for medical purposes has been taking place in Portugal for years, legalization in 2018 has caused more companies to take advantage of Portugal’s favorable climate as it relates to cannabis cultivation.
Regulatory Framework in Canada
On October 17, 2018, the Cannabis Act (S.C. 2018, c. 16) (the “Cannabis Act”) and the regulations enacted under the Cannabis Act, which set out the rules and standards that apply to the production, distribution, sale, importation and exportation of cannabis by federal licence holders (the “Cannabis Regulations”), came into force, legalizing the sale of cannabis for adult recreational use. Cannabis in Canada is subject to a complex regulatory framework arising from federal, provincial, and territorial legislation. The Cannabis Act and the Cannabis Regulations provide the framework for legal access to medical and non-medical cannabis, and control and regulate its production, distribution, sale, import and export. The provinces and territories of Canada have enacted legislation to control and regulate how non-medical cannabis is distributed and sold within their respective jurisdictions.
On October 17, 2019, October 17, 2020 and December 2, 2022, subsequent amending regulations titled the came into force that, among other things, expanded the scope of the Cannabis Act and Cannabis Regulations to enable the sale of certain categories of cannabis, including cannabis extracts, topicals and edibles, and set THC content limits for certain categories of cannabis products.
31
Prior to the Cannabis Act and the Cannabis Regulations coming into force, only the sale of medical cannabis was permitted and it was regulated by the Access to Cannabis for Medical Purposes Regulations (the “ACMPR”) made under the Controlled Drugs and Substances Act (Canada) (the “CDSA”). The Cannabis Act and the Cannabis Regulations replaced the CDSA and the ACMPR as the governing laws and regulations in respect of the cultivation, processing, sale and distribution of cannabis (including cannabis oil extract) in Canada.
Canada’s regulatory framework for cannabis is constantly evolving and both the Canadian Ministry of Health for Canada (“Health Canada”), which has regulatory oversight over and administration of the Cannabis Act, and provincial and territorial regulators frequently release and update guidance to assist the industry in interpreting and applying the applicable laws to their operations.
Licensing
The Cannabis Regulations establish six classes and various sub-classes of licenses that authorize specific activities, namely: (1) cultivation (standard cultivation, micro-cultivation, nursery); (2) processing (standard processing, micro-processing); (3) sales (sale for medical purposes); (4) analytical testing; (5) research; and (6) and cannabis drug license. Licensing requirements and authorized activities vary by class and sub-class, and authorized activities can also be narrowed by conditions described in individual licenses when they are issued.
Health Canada is responsible for reviewing and approving all federal licensing applications. While Health Canada does provide service standards for new applications, renewals, and amendments, they are not guaranteed and may not always be met. The volume of applications in queue or under review by Health Canada, the complexity of an application or amendment, and the quality of the submission, among other factors, can impact the duration of the review process, creating uncertainty in timelines. After a license is issued, it is the holder’s responsibility to comply with all applicable requirements in the Cannabis Act and Cannabis Regulations, including periodic inspections by Health Canada to ensure continued compliance.
Security Clearances
Certain people associated with cannabis licensees, including individuals occupying a “key position” such as directors, officers, large shareholders, and individuals identified by the Minister of Health (the “Minister”), must hold a valid security clearance issued by the Minister. The Minister may refuse to grant security clearances to individuals with organized crime associations or past convictions for, or in association with, drug trafficking, corruption, or violent offences. Individuals who have a history of nonviolent, lower-risk criminal activity (for example, simple possession of cannabis, or small-scale cultivation of cannabis plants) are not precluded by legislation from participating in the legal cannabis industry, and the granting of security clearance to such individuals is at the discretion of the Minister.
Cannabis Tracking System
The Cannabis Tracking and Licensing System (“CTLS”) was established by Health Canada to, among other things, track cannabis throughout the supply chain to help prevent diversion of cannabis into, and out of, the illicit market. Under the CTLS, holders of a cultivation, processing and/or sale for medical purposes licenses are required to submit monthly reports to Health Canada setting out inventory levels of finished and unfinished cannabis for each cannabis class.
Cannabis Products
The Cannabis Act differentiates between cannabis depending on its form (referred to as “classes” of cannabis in the Cannabis Act) and only permits the sale of specified classes of cannabis. Upon enactment of the Cannabis Act on October 17, 2018, these classes included dried cannabis, fresh cannabis, cannabis plants, cannabis seeds, and cannabis oil. On October 17, 2019, edible cannabis, cannabis extracts and cannabis topicals were added to the authorized classes of cannabis, also known as “Cannabis 2.0”). Cannabis oil was subsumed into cannabis extracts and ceased to exist as a standalone class as of October 17, 2020.
Health Products and Cosmetics Containing Cannabis
Health Canada has taken a scientific, evidence-based approach to the oversight of health products containing cannabis that are approved with health claims, including prescription and non-prescription drugs, natural health products, veterinary drugs and veterinary health products, and medical devices. Per Health Canada’s Cosmetic Ingredient Hotlist, the use of cannabis species (hemp) derivatives (other than certain hemp seed derivatives containing no more than 10 parts per million THC) in cosmetics, are permitted, subject to the provisions of the Cosmetic Ingredient Hotlist and the Industrial Hemp Regulations.
32
Packaging and Labelling
The Cannabis Regulations set out a comprehensive approach to the packaging and labelling of cannabis products. This approach helps to promote informed consumer choice and encourage the safe handling and storage of cannabis. All cannabis products must be packaged in plain packaging that is child-resistant and tamper-evident and displays a variety of information such as the standardized cannabis symbol, THC and CBD potency, and prescribed health warning messages.
Promotion
The Cannabis Act and Cannabis Regulations outline several prohibitions that can potentially apply to anyone who may be involved in the promotion of cannabis, cannabis accessories and services related to cannabis, or related activities. These prohibitions are intended to protect public health and safety, including by protecting the health of young persons by restricting their access to cannabis, and young persons and others from inducements to use cannabis.
Cannabis for Medical Purposes
With the Cannabis Act and the Cannabis Regulations coming into force on October 17, 2018, the medical cannabis regime migrated from the CDSA and the ACMPR to the Cannabis Act and the Cannabis Regulations. The medical cannabis regulatory framework under the Cannabis Act and the Cannabis Regulations remains substantively the same as under the CDSA and the ACMPR, with adjustments to create consistency with rules for non-medical use, improve patient access, and reduce the risk of abuse within the medical access system.
Under Part 14 of the Cannabis Regulations, patients maintain three options for obtaining cannabis for medical purposes: (i) they can continue to access cannabis by registering with licensees holding a licence to sell for medical purposes; (ii) they can register with Health Canada to produce a limited amount of cannabis for their own medical purposes; or (iii) they can designate someone else to produce cannabis for them. With respect to (ii) and (iii), starting materials, such as plants or seeds, must be obtained from licensees. Starting materials for personal production, such as plants or seeds, must be obtained from a license holder.
Provincial and Territorial Regulatory Regimes
Provinces and territories of Canada are authorized to license and oversee the distribution and sale of non-medical cannabis to adult consumers in their respective jurisdictions. As a result, regulations pertaining to the sale and distribution of non-medical cannabis vary from province to province and territory to territory.
The Cannabis Act prohibits individuals aged 18 years or older from possessing more than 30 grams of dried cannabis (or its equivalent) in public and from the personal cultivation of more than four plants at any one time. Provinces and territories have the flexibility to increase the minimum age of consumption, lower possession limits, and set added requirements on personal cultivation within their respective jurisdictions. Provinces and territories can also restrict where cannabis can be consumed in public.
The following chart outlines basic details regarding the current regulatory regime by province and territory. The possession limit of 30 grams remains unchanged in all provinces.
| Province/Territory | Legal Age | Where it is Legal to Purchase | ||
| Alberta | 18 | Private licensed stores or government-operated online store | ||
| British Columbia | 19 | Government-operated stores or online, or private licensed stores | ||
| Manitoba | 19 | Private licensed stores or online | ||
| New Brunswick | 19 | Government-operated stores or online | ||
| Newfoundland and Labrador | 19 | Private licensed stores or government-operated online store | ||
| Northwest Territories | 19 | Government-operated stores or online | ||
| Nova Scotia | 19 | Government-operated stores or online | ||
| Nunavut | 19 | Government-operated online store | ||
| Ontario | 19 | Private licensed stores or government-operated online store | ||
| Prince Edward Island | 19 | Government-operated stores or online | ||
| Quebec | 21 | Government-operated stores or online | ||
| Saskatchewan | 19 | Private licensed stores or online | ||
| Yukon | 19 | Government-operated online store or private licensed stores |
33
Industrial Hemp
The regulatory framework for industrial hemp is set out in the Industrial Hemp Regulations. Industrial hemp is defined under the Industrial Hemp Regulations as a cannabis plant – or any part of the plant – in which the concentration of THC is 0.3% (weight by weight) or less in the flowering heads and leaves.
Under this framework, a license from Health Canada is required in order to conduct various activities with industrial hemp. These activities include the cultivation, sale, import, export, cleaning, preparing, and processing of certain parts of the industrial hemp plant. Not every activity that involves industrial hemp falls within the scope of the Industrial Hemp Regulations and may instead fall under the Cannabis Regulations. For example, the extraction of phytocannabinoids from the flowering heads, leaves and branches of the plant requires a processing license under the Cannabis Regulations. Additionally, only seeds of approved industrial hemp varieties which have a THC level lower than 0.3% in their leaves and flowering heads, can be planted.
In addition to obtaining a license, industrial hemp license holders must comply with the Cannabis Act and Cannabis Regulations, and with other applicable federal, provincial and territorial legislation and municipal by-laws.
Inflation and Seasonality
The facility in Portugal is an indoor facility, therefore seasonality has no effect on the harvest month to month or season to season. Inflation has increased operating and production cost for the Portugal facility. Increased labor costs, as well electrical and material costs such as fertilizer have had an impact on net revenue. See Item 5 of this Annual Report for a discussion of our operating and financial results.
| C. | Legal Entity Structure |
The following chart reflects our legal entity structure (including the jurisdiction of formation or incorporation of the various entities).
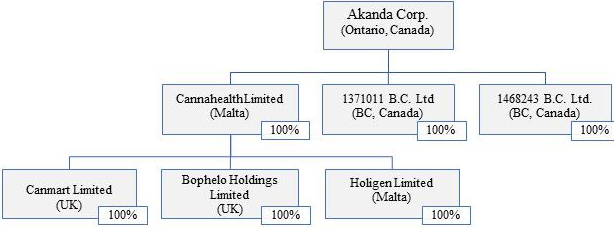
34
| D. | Property, Plants and Equipment |
Canmart currently has access to use 30,000 square foot warehouse in Somerset, United Kingdom, which is owned by D&D Investments Limited which is owned by certain directors of Canmart. Canmart does not pay any rent for the use of the warehouse and the owner may demand the vacation of the warehouse by Canmart at any time. Canmart expects to enter into a lease agreement with the owner with specified lease terms and to pay rent under the lease.
On September 19, 2023, the Company entered into an option to purchase agreement with 1107385 B.C. LTD. (the “Owner”), a British Columbia company, to purchase certain land property located at 1900 Ferne Road, Gabriola Island, British Columbia from the Owner (the “Prior Agreement”) for USD$4,300,000, payable in three installments. Pursuant to the Prior Agreement, the first option payment (US$600,000) shall be paid either in cash or by the issuance of Common Shares of the Company, and if the Company elects to issue any Common Share to the Owner, the total number of the Common Share shall not exceed 4.99% of the total outstanding Common Shares of the Company (the “Share Cap”) on the date of such issuance. The second and third option payment, each US$600,000, will be satisfied by the issuance of Common Shares of the Company in the name of the Owner or payment in cash in accordance with the Prior Agreement. Once Hemp Cultivation Approval (defined therein) has been issued or obtained, the Company will pay the Owner the Hemp Cultivation Approval Payment (US$750,000) within ten(10) business days. Once CBD Cultivation Approval (defined therein) has been issued or obtained, the Company will pay the Owner the CBD Cultivation Approval Payment (US$750,000) within ten(10) business days. Once THC Cultivation Approval (defined therein) has been issued or obtained, the Company will pay the Owner the THC Cultivation Approval Payment (US$500,000) within ten(10) business days. Once THC Sales Approval (defined therein) has been issued or obtained, the Company will pay the Owner the THC Sales Approval Payment (US$500,000) within ten(10) business days. On September 22, 2023, the Company and the Owner amended and restated the Prior Agreement (the “Amended and Restated Agreement”) to remove the cash payment option and Share Cap for the first option payment, and agreed to issue the Owner 879,895 Common Shares in full consideration for the first option payment. Pursuant to the Amended and Restated Agreement, the Company issued such Common Shares to the Owner. The remaining terms and conditions of the Prior Agreement remain in full force and effect.
ITEM 4A. UNRESOLVED STAFF COMMENTS
None.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
Overview
Our fiscal year begins on January 1 and ends on December 31. Unless otherwise noted, references to year pertain to our fiscal year. For example, 2023 refers to fiscal 2023 which is the period from January 1, 2023 and to December 31, 2023.
Our Audited Financial Statements for the years ended December 31, 2023 and 2022, respectively, for Akanda Corp. as a group (the “Akanda Group”), have been prepared in accordance with International Financial Reporting Standards (IFRS) and are presented in millions of US dollars except where otherwise indicated. Our historical results are not necessarily indicative of the results that should be expected in any future period.
We have derived the consolidated statements of operations data for Akanda Group for the years ended December 31, 2023 and 2022, respectively, and the consolidated financial position information as at December 31, 2023 and 2022, respectively, from the Akanda Group’s Audited Financial Statements included under Item 18 of this Annual Report on Form 20-F.
Akanda was incorporated in the Province of Ontario, Canada on July 16, 2021 in connection with the plan of Halo to reorganize its medical cannabis market focused international business assets. On November 3, 2021, Akanda acquired Cannahealth, which owned all the issued and outstanding equity interests of Canmart and Bophelo Holdings, which, in turn, owned all the issued and outstanding equity interests of Bophelo. As a result of the Acquisition, both Bophelo and Canmart became our indirect wholly-owned subsidiaries. On April 29, 2022, the Company, through its wholly owned subsidiary, Cannahealth acquired Holigen, which owned all the issued and outstanding equity interests of RPK. As a result of the acquisition, RPK became our indirect wholly-owned subsidiary. We have consolidated all our subsidiary companies, Cannahealth in Malta, Bophelo in the UK, Canmart in the UK, Holigen in Portugal, RPK in Portugal and 1371011 B.C. Ltd in Canada, in the Akanda Group Audited Financial Statements and financial information presented on December 31, 2023.
As a result of Bophelo’s liquidation, during the year ended December 31, 2022, we determined that we no longer controlled Bophelo. As a result of the loss of control, we derecognized the net assets of Bophelo and accounted for the operating results as discontinued operation.
35
| A. | Operating Results |
Results of Operations
The discussion below summarizes Akanda Group’s consolidated historical operation results.
On April 20, 2022, Akanda, Cannahealth, The Flowr Corporation (“Flowr”) and Holigen Holdings Limited (“Holigen”), a wholly-owned subsidiary of Flowr entered into a share purchase agreement (the “Holigen Agreement”) whereby Cannahealth would acquire 100% of the ordinary shares of Holigen, which is the holding company of RPK Biopharma, Unipessoal, LDA (“RPK”), a cultivator and manufacturer of medical cannabis products based in Portugal (the “Holigen Acquisition”). The Holigen Acquisition closed on April 29, 2022.
Year Ended December 31, 2023 Compared to the Year Ended December 31, 2022.
The following table sets forth key components of Akanda Group’s results of operations for the year ended December 31, 2023 compared to the year ended December 31, 2022.
| Years ended | ||||||||
| December 31, | ||||||||
| 2023 | 2022 | |||||||
| Sales | $ | 2,160,052 | $ | 2,619,682 | ||||
| Cost of sales | 1,908,515 | 566,252 | ||||||
Gross Profit before gain (loss) on change in fair value biological assets and inventory | 251,537 | 2,053,430 | ||||||
| Gain (Loss) on change in fair value of biological assets and inventory | (140,088 | ) | 1,216,129 | |||||
| Gross Profit | 111,449 | 3,269,559 | ||||||
| Operating expenses | ||||||||
| Depreciation and amortization | 4,283,731 | 3,598,323 | ||||||
| Consulting and professional fees | 2,978,321 | 7,759,824 | ||||||
| Personnel expenses | 2,395,583 | 6,593,527 | ||||||
| Share-based payment expenses to social development trust | — | 2,124,615 | ||||||
| General and administrative expenses | 505,187 | 3,369,659 | ||||||
| Total operating expenses | 10,162,822 | 23,445,948 | ||||||
| Operating loss | (10,051,373 | ) | (20,176,389 | ) | ||||
| Other (expense) income: | ||||||||
| Finance income | 39 | 886 | ||||||
| Finance expense | (255,656 | ) | (115,324 | ) | ||||
| Foreign exchange gain (loss), net | 148,823 | (256,431 | ) | |||||
| Gain on bargain purchase | — | 12,760,356 | ||||||
| Gain on debt settlement | 113,037 | 67,075 | ||||||
| Other income | 7,055 | 659 | ||||||
| Change in fair value of financial assets measured at FVTPL | (264,655 | ) | (516,281 | ) | ||||
| Loss on disposal of assets | (4,495 | ) | — | |||||
| Write-off of AP, net | 2,697,719 | — | ||||||
| Impairment loss | (24,665,564 | ) | — | |||||
| (22,223,697 | ) | 11,940,940 | ||||||
| Net loss from continuing operations | (32,275,070 | ) | (8,235,449 | ) | ||||
| Loss from discontinued operations | — | (3,422,225 | ) | |||||
| Net loss | $ | (32,275,070 | ) | $ | (11,657,674 | ) | ||
| Translation adjustment | (39,412 | ) | (1,395,508 | ) | ||||
| Comprehensive loss | $ | (32,314,482 | ) | $ | (13,053,182 | ) | ||
| Loss per share from continuing operations - basic and diluted | (7.16 | ) | (2.75 | ) | ||||
| Loss per share - basic and diluted | $ | (7.16 | ) | $ | (3.86 | ) | ||
| Weighted average common shares outstanding | 4,505,263 | 2,993,009 | ||||||
36
Revenue
The revenue of $2,160,052 for the year ended December 31, 2023 as compared to $2,619,682 for 2022 came from Canmart Ltd. in the UK and RPK in Portugal. The significant revenue decrease in 2023 is due to higher sales discounts, rebates and/or returns recognized in the current year. Akanda remains focus on ramping up of its operations by securing CBPM products from international suppliers in Canada, importing these products and applying additional efforts on expanding sales of these imported products to patients, through dispensing clinics and physicians. Canmart sold, during the current year, a variety of different CBPM product types, including cannabis oil and cannabis flower for medical use. Canmart has access to use a 30,000 square foot logistics warehouse in SE England. Canmart has obtained the necessary licenses to import CBPMs to supply the patients in the U.K. domestic market. Canmart is further focusing on tapping other international markets for the import of various CBPM products.
Cost of Sales
Cost of sales increased from $566,252 in 2022 to $1,908,515 in 2023. The increase is directly related to the increase in sales activities and its continuous effort in selling and marketing its products during the year 2023. This increase in sales represents the associated cost of CBPMs imported by Canmart and supplied to its patients. The increase is essentially commensurate with increased purchases and sales of CBPMs in the current year due to the fact that Canmart commenced more meaningful sales activity in the year ended December 31, 2023 compared to the low level of sales in the previous periods. The increase in cost of sales is also due to the purchase and sales activities by RPK.
Gain or loss on change in fair value of biological assets and inventory
The gain or loss on change in fair value of biological assets and inventory was recognized in RPK Biopharma Unipessoal Lda. The fair value of the biological assets was determined based on the most recent available evidence for the selling price of similar biological assets. The valuation was done by an independent valuator considering the selling price available for cannabis biomass in Portugal and the historical cost paid by the Company for the cannabis seeds. The gain decreased from $1,216,129 in 2022 to $(140,088) in 2023.
Amortization and Depreciation
Amortization and depreciation expenses increased from $3,598,323 for 2022 to $4,283,731 for the year ended December 31, 2023. The increase in the amortization and depreciation expenses recorded during the year ended December 31, 2023 was due to depreciation charges on additional items of property, plant and equipment that were acquired and brought into use during 2023 and additional amortization of intangible assets (the Company’s cannabis operating and distribution licenses), as well as due to full depreciation on property, plant and equipment that took place during the year ended December 31, 2022.
Consulting and Professional Fees
The consulting and professional fees incurred decreased from $7,759,824 in 2022 to $2,2978,321 for the year ended December 31, 2023. This significant decrease in consulting and professional fees resulted from the decreased corporate activity during the current year as compared to higher activities in the previous year due to the engagement of various professional advisors and consultants in relation to Akanda Group’s plans for completion of business acquisition, plans for a public offering and listing. The decreased was also due to lower RSUs granted to consultants in the current year.
Personnel Expenses
The Akanda group incurred personnel expenses of $2,395,583 for the year ended December 31, 2023 compared to $6,593,527 for 2022. For the year ended December 31, 2023, the group experienced a significant decrease in personnel expenses primarily due to the changes in key management personnel in relation to the resignation and appointment of senior management and board of directors during the previous year resulting for lower fees paid or accrued to the current executive team of Akanda in the current year, as well as significant decreased in workers in Lesotho. As Bophelo was considered no longer operational due to liquidation in 2022, no more salaries expenses were paid or accrued to casual workers and laborers.
37
General and Administration Expenses
The Akanda group incurred general and administration expenses of $505,187 and $3,369,659 for the years ended December 31, 2023 and 2022, respectively. These costs consisted mainly of a broad range of site related operational expenses such as utilities, fuel costs, import duties, security expenses, repairs and maintenance and consumables and office related operational expenses for its day to day business activities. These costs decreased compared to the prior period mainly due to decreased corporate activities, as well as it had no longer incurred any site or office expenses in Bophelo .
Interest Expense
The group incurred interest expense of $255,656 for the year ended December 31, 2023 compared to interest expense of $115,324 for 2022. The significant increase in expense during the year ended December 31, 2023 was primarily due to the interest or financing costs incurred in relation the increase in interest-bearing loans borrowed by Akanda, Canmart and RPK during the current year.
Interest income
Interest income for the year ended December 31, 2023 was $39 compared to $886 for 2022. This decrease was related to interest received during the year 2023 as a result of experiencing increased average cash balances throughout the year.
Foreign Currency Translation
The foreign exchange gain (loss) is recognized on the translation of the consolidated financial statements from their functional currencies to United States Dollar. The Euro is the functional currency of Cannahealth, Holigen and RPK, Great British Pounds is the functional currency of Canmart and Canadian dollars is the functional currency of Akanda and 1371011 B.C. Ltd. while the United States Dollar is its reporting currency. The exchange gains and losses have not been incurred on any transactions or balances held by these companies in a different currency.
Net Loss and Total Comprehensive Loss
For the years ended December 31, 2023 and 2022, respectively, the group incurred a net loss of $32,275,070 and $11,657,674, respectively, and a comprehensive loss of $32,314,482 and $13,053,182, respectively, which consisted primarily of depreciation and amortization of $4,283,731 and $3,598,323, respectively, consulting and professional fee expenses of $2,978,321 and $7,759,824, respectively, personnel expenses of $2,395,583 and $6,593,527, respectively, share-based payment expenses to ASDT of $nil and $2,124,615, respectively, general and administrative expenses of $505,187 and $3,369,659, respectively, and loss from discontinued operations of $nil and $3,422,225, respectively. The increase in losses for the year ended December 31, 2023 compared to 2022 was mainly due to the impairment loss of $24,665,564 incurred during the year ended December 31, 2023.
Off-Balance Sheet Arrangements
Akanda did not have, during the reporting period, and we do not currently have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditure or capital resources that is material to investors.
| B. | Liquidity and Capital Resources |
Cash Flows
The Akanda Group’s principal liquidity requirements are for corporate operating expenses, working capital and capital expenditures. Historically, we have funded our liquidity requirements primarily through shareholder loans, loans from third parties and from the issuance of shares. We did not have, during the reporting period, and we do not currently have any contractual obligations for ongoing capital expenditures.
38
The following table summarizes our cash flows from operating, investing and financing activities for the years ended December 31, 2023 and 2022:
| Year Ended | ||||||||||||
| December 31, 2023 | ||||||||||||
| 2023 | Change | 2022 | ||||||||||
| Cash used by operating activities | $ | (1,500,574 | ) | $ | 9,968,822 | $ | (11,469,396 | ) | ||||
| Cash used in investing activities | $ | 24,888 | $ | 4,243,550 | $ | (4,218,662 | ) | |||||
| Cash provided by financing activities | $ | 1,486,567 | $ | (12,652,069 | ) | $ | 14,138,636 | |||||
| Year Ended | ||||||||||||
| December 31, 2022 | ||||||||||||
| 2022 | Change | 2021 | ||||||||||
| Cash used by operating activities | $ | (11,469,396 | ) | $ | (4,912,334 | ) | $ | (6,557,062 | ) | |||
| Cash used in investing activities | $ | (4,218,662 | ) | $ | (3,642,190 | ) | $ | (576,472 | ) | |||
| Cash provided by financing activities | $ | 14,138,636 | $ | 2,902,945 | $ | 11,235,691 | ||||||
Cash Flows from Operating Activities
For the year ended December 31, 2023, Akanda Group’s cash flow from operating activities decreased by $9,968,822 due to decreased operating expenses at Bophelo, as well as due to lower corporate expenses incurred as a result of decreased corporate activity. The significant increase in the previous year was due to gain recognized in the acquisition of Holigen (and its subsidiary, RPK).
Cash Flows from Investing Activities
Cash provided by investing activities was $24,888 for the year ended December 31, 2023, which was attributable to leasehold improvements and purchase of computer and furniture and fixtures and loan receivable, offset by proceeds from disposal of property, plant and equipment. The cash used during the year ended December 31, 2022 were attributable to purchase of marketable securities from Flowr Corporation, purchase of property, plant and equipment, cash surrendered on loss of control of Bophelo and investments in relation to acquisition of Holigen (and its subsidiary, RPK).
Cash Flows from Financing Activities
Share Capital and Financing
During the year ended December 31, 2022, Akanda completed its initial public offering of 400,000 Common Shares at a price of $40.00 per share to the public for a total of $16,000,000 of gross proceeds to Akanda, prior to deducting underwriting discounts, commissions, and other offering expenses on March 17, 2022. The Common Shares began trading on The Nasdaq Capital Market on March 15, 2022, under the symbol “AKAN.” Akanda used and intends to use the proceeds from the initial public offering primarily for property, plant and equipment, operations, working capital and general corporate purposes. Akanda issued 16,200 Common Shares upon gross receipts of $405,000 net of issuance costs of $126,519.
No equity financing was made during the year ended December 31, 2023.
Short Term Loans
During the year ended December 31, 2023, Akanda received total loans of $2,313,167 for its capital as well as working capital needs, of which $1,570,145 was advances from related parties.
Cash provided by financing activities was $1,486,567 for the year ended December 31, 2023, which was mainly attributable to proceeds from above short term loans, partially offset by repayment of loans and lease payments. Cash provided by financing activities during the year ended December 31, 2022 was attributable to proceeds from IPO, proceeds from private placements, proceeds from loans, and partially offset by repayment of loans and lease payments.
39
Disclosure of Contractual Arrangements
On December 31, 2023, Akanda Group was committed to minimum lease payments as follows:
| Less than | 1 – 5 | Over | ||||||||||
| Contractual Obligation | One Year | Years | 5 Years | |||||||||
| Land lease | $ | 140,000 | $ | — | $ | — | ||||||
The amounts above are undiscounted and include the total amounts due, including the interest component.
On December 31, 2022, Akanda Group was committed to minimum lease payments as follows:
| Less than | 1 – 5 | Over | ||||||||||
| Contractual Obligation | One Year | Years | 5 Years | |||||||||
| Land lease | $ | 240,000 | $ | 140,000 | $ | — | ||||||
The amounts above are undiscounted and include the total amounts due, including the interest component.
Subsequent to the year ended December 31, 2023, the Company:
| i. | Completed a Share Purchase Agreement for Sale of RPK |
On April 1, 2024, pursuant to the signed definitive Share Purchase Agreement and Escrow Agreement with Somai on February 29, 2024, the Company completed the transaction with Somai for the sale of RPK, its indirect wholly-owned Portuguese subsidiary.
Under the terms of the Share Purchase Agreement, Somai acquired RPK for a total cash consideration of Two Million United States Dollars ($2,000,000). In addition, Somai assumed up to One Million Euros of current liabilities and RPK’s debt with the senior secured lender Bank, Caixa Agricola. In total, Somai assumed approximately 4,000,000 Euros of debt. In accordance with the agreement, a deposit of Five Hundred Thousand United States Dollars ($500,000) was released from a joint escrow account and the remainder of the purchase price was paid directly to the Company in March 2024.
In connection with the closing, the Company paid a cash finder’s fee of 5% of the gross sales price or an aggregate of $446,250, inclusive of taxes to Cannera Holdings LTD, a British Columbia company.
| ii. | Issued the following shares: |
| a. | On February 2, 2024, the Company entered into a securities purchase agreement with Corbo Capital Inc. pursuant to which the Company issued 280,851 common shares at a purchase price of $0.406 per share and 1,462,991 pre-funded warrants for gross proceeds of $708,000. The purchase price of each pre-funded warrant is equal to the price at which one common share is sold in the offering, minus $0.0001, and the exercise price of each pre-funded warrant is $0.0001 per share. The pre-funded warrants were immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full, subject to certain beneficial ownership limitations as set forth in the pre-funded warrant. On the same day, the Company issued 560,100 common shares pursuant the exercise of 560,100 pre-funded warrants. |
| b. | On February 13, 2024, the Company issued 560,000 commons shares pursuant the exercise of 560,000 pre-funded warrants. |
| c. | On February 20, 2024, the Company issued 280,000 commons shares pursuant the exercise of 280,000 pre-funded warrants. |
| d. | On February 28, 2024, the Company issued 62,891 commons shares pursuant the exercise of the remaining 62,891 pre-funded warrants. |
40
| e. | On March 4, 2024, the Company entered into a securities purchase agreement with certain investors pursuant to which the Company issued 367,870 common shares at a purchase price of $0.2054 per share and 361,972 pre-funded warrants for gross proceeds of $150,000. The purchase price of each pre-funded warrant is equal to the price at which one common share is sold in the offering, minus $0.0001, and the exercise price of each pre-funded warrant is $0.0001 per share. The pre-funded warrants were immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full, subject to certain beneficial ownership limitations as set forth in the pre-funded warrant. On the same day, the Company issued 361,972 commons shares pursuant the exercise of 361,972 pre-funded warrants. |
| f. | On March 5, 2024, the Company entered into a securities purchase agreement with certain investors pursuant to which the Company issued 367,870 common shares at a purchase price of $0.16872 per share and 373,002 pre-funded warrants for gross proceeds of $125,000. The purchase price of each pre-funded warrant is equal to the price at which one common share is sold in the offering, minus $0.0001, and the exercise price of each pre-funded warrant is $0.0001 per share. The pre-funded warrants were immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full, subject to certain beneficial ownership limitations as set forth in the pre-funded warrant. On March 6, 2024, the Company issued 373,002 commons shares pursuant the exercise of 373,002 pre-funded warrants. |
| g. | On March 24, 2024, the Company entered into an underwriting agreement with Univest Securities, LLC (“Univest”) as the underwriter in connection with the issuance and sale by the Company in an underwritten public offering of 3,087,443 common shares at a purchase price of $0.1217 per share and 37,997,190 pre-funded warrants for gross proceeds of $5,000,000. The purchase price of each pre-funded warrant is equal to the price at which one common share is sold in the offering, minus $0.0001, and the exercise price of each pre-funded warrant is $0.0001 per share. The pre-funded warrants were immediately exercisable and may be exercised at any time until all of the pre-funded warrants are exercised in full, subject to certain beneficial ownership limitations as set forth in the pre-funded warrant. As of April 30, 2024, 17,991,956 pre-funded warrants remained unexercised. |
| iii. | Received the following loans: |
| a. | On January 10, 2024, the Company received a loan of CAD $40,000 from 1226053 BC Ltd., an arm’s length party. The loan is unsecured, bears interest of 7% per annum and payable within 12 months. |
| b. | On January 10, 2024, the Company received a loan of CAD $40,000 from Mercantile Holdings Inc., an arm’s length party. The loan is unsecured, bears interest of 7% per annum and payable within 12 months. |
| c. | On February 1, 2024, the Company received a loan of $5,500 from Alson Niu, an arm’s length party. The loan is unsecured, bears interest of 7% per annum and payable within 12 months. |
| iv. | Outstanding claims |
On January 29, 2024, Shailesh Bhushan, the former Chief Financial Officer of the Company, filed a complaint with the Employment Standards Branch of British Columbia claiming unpaid salary and invoices in the aggregate amount of CAD $271,990 from the period December 2022 through November 2023. The Company previously offered to Mr. Bhushan an annual salary of CAD $60,000 and as such, believes the claim to be frivolous, strongly disputes the amount claimed, and intends to vigorously defend itself.
On March 27, 2024, the Company entered into a settlement agreement with Vidya Iyer, the former SVP of Finance to settle the claims for a sum of £30,000 to be paid in installment.
| C. | Research and Development, Patents and Licenses |
Not applicable.
| D. | Trend Information |
Because we are still in the startup phase and have only recently commenced operations, we are unable to identify any recent trends in revenue or expenses. Thus, we are unable to identify any known trends, uncertainties, demands, commitments or events involving our business that are reasonably likely to have a material effect on our revenues, income from operations, profitability, liquidity or capital resources, or that would cause the reported financial information in this prospectus to not be indicative of future operating results or financial condition.
| E. | Material Accounting Policies and Estimates |
Please refer to Note 3 of Akanda Group’s audited consolidated financial statements included in Item 18 of this Annual Report on Form 20-F.
| B. | Research and Development, Patents and Licenses |
Not applicable.
41
| C. | Trend Information |
Because we are still in the startup phase and have only recently commenced operations, we are unable to identify any recent trends in revenue or expenses. Thus, we are unable to identify any known trends, uncertainties, demands, commitments or events involving our business that are reasonably likely to have a material effect on our revenues, income from operations, profitability, liquidity or capital resources, or that would cause the reported financial information in this prospectus to not be indicative of future operating results or financial condition.
| D. | Critical Accounting Policies and Estimates |
Please refer to Note 3 of Akanda Group’s audited consolidated financial statements included in this Form 20-F.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
| A. | Directors and Senior Management |
The following table sets forth our directors and senior management, their age and the positions they held as of April 30, 2024.
Name |
Age | Position | ||
| Katharyn Field(1) | 41 | Interim Chief Executive Officer and Executive Director | ||
| Gurcharn Deol | 56 | Chief Financial Officer | ||
| Jatinder Dhaliwal(2) | 35 | Director | ||
| David Jenkins(2) | 43 | Director | ||
| Christopher Cooper(2) | 53 | Director |
| (1) | Ms. Field is the Chief Executive Officer of Halo, which is a shareholder and creditor of the Company. |
| (2) | Independent Director. |
Biographical Information
The following is a summary of certain biographical information concerning our executive officers and directors.
Katharyn Field has served as our Executive Director since July 2022 and Interim Chief Executive Officer since February 2023. Ms. Field is also currently CEO and Chairman of Halo, a position she has held since June 2022. From February 2020 to June 2022, Ms. Field served as the president of Halo and from April 2019 through February 2020, she served as the Chief Strategy Officer. Ms. Field’s resume includes positions at the Brookings Institution, from October 2005 to May 2009 and Bain & Company from September 2011 to March 2013. In 2014, she entered the cannabis industry and led the procurement, build out, and sale of one of five original vertically integrated companies with state licenses in Florida. Subsequently, Ms. Field operated a strategy consulting practice focused on cannabis and also worked at MariMed from May 2018 to January 2019 as Executive Vice President of Corporate Development. Ms. Field was elected as a member of Sway Energy Corporation’s board of directors from February 2021 to May 2022. Ms. Field also completed an internship in the public liaison’s office of The White House during the George W. Bush administration. Ms. Field holds an MBA from Columbia Business School and a BA with honors from Stanford University.
Gurcharn Deol has served as our Chief Financial Officer since December 4, 2023. Mr. Deol is a multi-industry executive with over 35 years of public company management experience. Mr. Deol is currently the CEO of Bayridge Resources Corp and currently director of Green Battery Metals Corp, Amber Brands Inc and Neotech Metals Corp, which are all public companies trading on the Canadian Stock Exchanges. Mr. Deol’s recent experience includes being a director or in management of numerous Canadian private and public companies including CEO of Bayridge Resources Inc., a director of Amber Brands Inc., Green Battery Minerals Inc., and Neotech Metals Inc. He has been involved in initial IPOs being established which required taking private companies in Canada through the regulatory process of going public. Mr. Deol holds B.A., M.A., PhD in Physiological and Counseling Psychology.
Jatinder Dhaliwal has served as one of our directors and as a member of our Audit Committee, our Compensation Committee and our Nominating and Corporate Governance Committee since July 2022. Mr. Dhaliwal is a registered pharmacist and has significant capital markets experience, having served as CEO and director of multiple publicly traded cannabis companies. Mr. Dhaliwal is currently a director and Chief Executive and Financial Officer at Binovi Technologies Corp., a position that he has held since January 2022; currently a director and Chief Executive and Financial Officer at Kiaro Holdings Corp., a position that he has held since August 2022; was a director at Makara Mining Corp., a position that he held from August 2021 to March 2022; a director and CEO at Global Health Clinics Ltd., a position that he has held since March 2019; a director and CEO at EGF Theramed Health Corp., a position that he held from January 2022 to August 2022; a director at Ravenquest Biomed Inc, a position that he has held since November 2019; and a director at Intact Gold Corp., a position that he has held since November 2019. Mr. Dhaliwal holds a Bachelor of Pharmacy from the University of British Columbia and a Bachelor of Science in Biology from the University of Victoria.
42
David Jenkins has served as one of our directors and as a member of our Audit Committee and our Compensation Committee since February 2023. Mr. Jenkins is currently a director at Binovi Technologies Corp., a position that he has held since December 2021; a director at Kiaro Holdings Corp. (TSXV: KO), a position that he has held since August 2022; a director at Levitee Labs., a position that he has held since January 2022; a director at Pontus Protein Ltd. (TSXV: HULK), a position that he has held since March 2022; a director at Boundary Gold & Copper Mining Ltd., a position that he has held since July 2020; a director at Montego Resources Inc., a position that he has held since January 2020; and a director at Quantum Battery Metals Corp., a position that he has held since January 2020.
Christopher Cooper has served as one of our directors and as a member of our Audit Committee, our Compensation Committee and our Nominating and Corporate Governance Committee since April 2024. Mr. Cooper has over 20 years of extensive business experience in all facets of corporate development, senior management, finance and operations, in both the private and public sectors. His experience includes spearheading growth strategies, financial reporting, quarterly and annual budgets, overseeing corporate administration, while achieving company objectives and maintaining internal cost controls. Mr. Cooper’s current primary occupation is a business consultant, and he serves as the president of Number 2 Capital Corp. Mr. Cooper has been serving as a director and CEO of Reparo Energy Partners Corp. since 2003, a director and CFO of Sweet Earth Holdings Corp since 2020, and was a director of Bullion Gold Resources Corp. from 2018 to 2020, and a director of StartMonday Technology Corp. from 2019 to 2021. Mr. Cooper was also a director of Counterpath Corporation (Nasdaq: CPAH) from 2005 to 2021, a Nasdaq listed company which was acquired by Alianza, Inc. in March 2021 for USD$25.6 million. Mr. Cooper was a director of Alpha Lithium Corporation from 2018 to 2023, which was acquired by Tecpetrol in October 2023 for approximately CAD$313 million. In addition, Mr. Cooper received his Bachelor of Business Administration from Hofstra University and his Master’s in Business Administration from Dowling College in New York.
| B. | Compensation |
Compensation of our Executive Officers and Directors
The following table sets forth information concerning the compensation of our executive officers and members of our Board of Directors for the fiscal years ended December 31, 2023 and 2022.
| Year | Salary ($) | Stock awards ($) | Option awards ($) | Non-equity incentive plan compensation ($) | pension value and nonqualified deferred compensation earnings | All other compensation ($) | Total ($) | |||||||||||||||||||||||||
| Tejinder Virk | 2023 | 73,341 | — | — | — | — | — | 73,341 | ||||||||||||||||||||||||
| (Former Chief Executive Officer) | 2022 | 1,298,967 | 170,144 | — | — | — | — | 1,469,111 | ||||||||||||||||||||||||
| Katharyn Field | 2023 | 111,968 | — | — | — | — | — | 111,968 | ||||||||||||||||||||||||
| (Interim CEO and Executive Director) | 2022 | 34,798 | — | — | — | — | — | 34,798 | ||||||||||||||||||||||||
| Jatinder Dhaliwal | 2023 | 111,968 | — | — | — | — | — | 111,968 | ||||||||||||||||||||||||
| (Director) | 2022 | 34,798 | — | — | — | — | — | 34,798 | ||||||||||||||||||||||||
| Harvinder Singh | 2023 | 111,968 | — | — | — | — | — | 111,968 | ||||||||||||||||||||||||
| (Former Director) | 2022 | 34,798 | — | — | — | — | — | 34,798 | ||||||||||||||||||||||||
| David Jenkins | 2023 | 79,949 | — | — | — | — | — | 79,949 | ||||||||||||||||||||||||
| (Director) | 2022 | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Christopher Cooper | 2023 | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| (Director) | 2022 | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Yuying Liang | 2023 | 27,697 | — | — | — | — | — | 27,697 | ||||||||||||||||||||||||
| (Former Director) | 2022 | 26,585 | — | — | — | — | 26,585 | |||||||||||||||||||||||||
| Trevor Scott | 2023 | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| (Former Chief Financial Officer) | 2022 | 631,352 | 417,755 | — | — | — | 7,761 | 1,056,868 | ||||||||||||||||||||||||
| Shailesh Bushan | 2023 | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| (Former Chief Financial Officer) | 2022 | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Gurcharn Deol | 2023 | 3,407 | — | — | — | — | — | 3,407 | ||||||||||||||||||||||||
| (Chief Financial Officer) | 2022 | — | — | — | — | — | — | — | ||||||||||||||||||||||||
| Total | 2023 | 520,298 | — | — | — | — | — | 520,298 | ||||||||||||||||||||||||
| 2022 | 2,061,298 | 587,899 | — | — | — | 7,761 | 2,656,958 | |||||||||||||||||||||||||
43
Director Compensation
We have four directors We currently do not pay our directors who are executive officers additional compensation. We expect to compensate our non-executive directors for their director services. which will be settled in cash or partly in equity awards at the election of the non-executive director. Total non-executive director compensation is as follows:
| ● | an annual retainer of $75,000 ($112,500 for the lead independent director); |
| ● | an initial equity award equal to the value of $100,000; |
| ● | an additional $5,000 per annum for any non-executive director serving on a board committee ($6,000 if serving as chair of a board committee other than the audit and risk committee and $10,000 if serving as chair of the audit and risk committee). |
Executive Employment Agreements, Arrangements or Plans
There are currently no executive employment agreements entered into and currently in place between the Company and its executive officers.
Stock Option Plan
The Company has a 2021 Equity Incentive Plan (the “2021 Plan”) whereby it may grant options for the purchase of Common Shares, or Restricted Share Units, to any director, consultant, employee or officer of the Company or its subsidiaries. The aggregate number of shares that may be issuable pursuant to options granted under the Plan will not exceed 20% of the issued Common Shares of the Company. The options are non-transferable and non-assignable and may be granted for a term not exceeding 5 years. The exercise price of the options will be determined by our Board of Directors at the time of grant but may not be less than the closing price of such shares on Nasdaq on the trading date immediately precedent the date of grant, subject to all applicable regulatory requirements.
Please refer to Note 15 of Akanda Group’s audited financial statements included in Item 18 of this Annual Report on Form 20-F for a discussion of Restricted Share Units issued in Fiscal 2023.
On March 22, 2024, the shareholders of the Company approved 2024 Equity Incentive Plan (the “2024 Plan”, together with the 2021 Plan, the “Stock Option Plan”) whereby it may grant options for the purchase of Common Shares, or Restricted Share Units, to any director, consultant, employee or officer of the Company or its subsidiaries. The aggregate number of shares that may be issuable pursuant to options granted under the Plan will not exceed 30% of the issued Common Shares of the Company. The options are non-transferable and non-assignable and may be granted for a term not exceeding 10 years. The exercise price of the options will be determined by our Board of Directors at the time of grant but may not be less than the closing price of such shares on Nasdaq on the trading date immediately precedent the date of grant, subject to all applicable regulatory requirements.
44
| C. | Board Practices |
Introduction
Our business and affairs are managed under the direction of our Board of Directors. Our Board of Directors is composed of four directors. When considering whether directors have the experience, qualifications, attributes or skills, taken as a whole, to enable our Board of Directors to effectively satisfy its oversight responsibilities in light of our business and structure, the Board of Directors focuses primarily on each person’s background and experience as reflected in the information discussed in the directors’ respective biographies set forth above. We believe that our directors provide an appropriate mix of experience and skills relevant to the size and nature of our business.
Election of Directors
Each of our officers holds office until his or her successor is appointed. Directors are elected to serve until the close of the next annual meeting of shareholders or until their successors have been elected or appointed.
We do not have any defined policy or procedural requirements for shareholders to submit recommendations or nominations for directors. Our Board of Directors believes that, given the stage of our development, a specific nominating policy would be premature and of little assistance until our business operations develop to a more advanced level. The Board of Directors, with the help of its nominating and ESG committee, will assess all candidates, whether submitted by management or shareholders, and make recommendations for election or appointment.
Corporate Governance
We are a “foreign private issuer” under the federal securities laws of the United States and The Nasdaq Stock Market listing standards. Under the federal securities laws of the United States, foreign private issuers are subject to different disclosure requirements than U.S.-domiciled registrants. We intend to take all actions necessary for us to maintain compliance as a foreign private issuer under the applicable corporate governance requirements of the Sarbanes-Oxley Act, the rules adopted by the SEC and the Nasdaq listing standards.
Under the SEC rules and the Nasdaq listing standards, a foreign private issuer is subject to less stringent corporate governance requirements. Subject to certain exceptions, the SEC and the Nasdaq permit a foreign private issuer to follow its home country practice in lieu of their respective rules and listing standards. Following our home country governance practices, as opposed to the requirements that would otherwise apply to a company listed on Nasdaq, may provide less protection than is accorded to investors under the Nasdaq Rules applicable to U.S. domestic issuers.
In particular, as a foreign private issuer, in accordance with and pursuant to the authority contained in Nasdaq Listing Rule 5615(a)(3), we may follow certain Canadian law and corporate practice in lieu of certain corporate governance provisions set out under the Nasdaq Rule 5600 Series, the requirement in Listing Rule 5250(b)(3) to disclose third party director and nominee compensation, and the requirement in Listing Rule 5250(d) to distribute annual and interim reports. Of particular note, the following rules under the Nasdaq Listing Rule 5600 Series may differ from Canadian law requirements:
| ● | Nasdaq Listing Rule 5605(b)(1) requires that at least a majority of the Company’s Board of Directors shall be independent directors, and Nasdaq Listing Rule 5605(b)(2) requires that independent directors regularly meet in executive session, where only independent directors are present. We have two independent directors. Our independent directors meet regularly with other members of the Board and meet in executive session at least two (2) times per year. |
| ● | Nasdaq Listing Rule 5620(c) sets out a quorum requirement of at least 33-1/3% of the outstanding shares with respect to meetings of shareholders. In accordance with Canadian law and generally accepted business practices, our bylaws (the “Bylaws”) provide that a quorum is met when holders of not less than 10% of the shares entitled to vote at the meeting of shareholders are present in person or represented by proxy. The quorum requirement provided in our Bylaws is consistent with applicable Canadian laws and corporate practices. |
45
| ● | Nasdaq Listing Rule 5605(c)(2)(A) requires that the Company shall have an audit committee composed entirely of not less than three directors, each of whom must be independent. Our audit and risk committee is comprised of three directors, and each member of the audit and risk committee meets the independence requirements of Nasdaq Listing Rule 5605(a)(2) and Rule 10A-3(b)(1) under the Exchange Act. |
| ● | Nasdaq Listing Rule 5605(d)(2)(A) requires, among other things, that the Company’s compensation committee include at least two members, each of whom is an independent director as defined under Nasdaq Listing Rule 5605(a)(2). Our compensation committee is comprised of three directors, and each member of the compensation committee meets the independence requirements of Nasdaq Listing Rule 5605(a)(2). |
| ● | Nasdaq Listing Rule 5605(e) requires that the nominations committee include solely independent directors or is constituted by a majority of independent directors in a vote in which only independent directors participate. Our nominating committee is comprised of two directors, who meets the independence requirements of Nasdaq Listing Rule 5605(a)(2). |
We followed home country rules with regard to the requirement to hold an annual shareholders meeting no later than one year after the Company’s fiscal year end, under Nasdaq Listing Rule 5620. In accordance with the laws in the Province of Ontario, Canada, we did not hold our annual shareholders meeting in the fiscal year 2023 and instead held it in fiscal year 2024 pursuant to an order to delay the calling of the annual meeting granted by the Ontario Superior Court of Justice pursuant to subsection 106(1) of the Business Corporations Act (Ontario). In addition, with regard to the underwritten public offering on March 27, 2024, we followed home country rules with regard to the requirement for shareholder approval for transactions other than “public offerings” under Nasdaq Listing Rule 5635. We may in the future elect to follow additional home country practices in Canada with regard to certain corporate governance matters.
Indemnification of Directors and Officers
In accordance with the Business Corporations Act (Ontario) and pursuant to the Bylaws of the Company, subject to certain conditions, the Company shall indemnify a director or officer, a former director or officer, or another individual who acts or acted at the Company’s request as a director or officer, or an individual acting in a similar capacity, of another entity, against all costs, charges and expenses, including any amount paid to settle an action or satisfy a judgment, reasonably incurred by the individual in respect of any civil, criminal, administrative, investigative or other proceeding in which the individual is involved because of that association with the Company or other entity. The Company shall not indemnify an individual unless the individual:
| ● | acted honestly and in good faith with a view to the best interests of the Company or, as the case may be, to the best interests of the other entity for which the individual acted as a director or officer or in a similar capacity at the Company’s request; and |
| ● | in the case of a criminal or administration action or proceeding enforced by a monetary penalty, had reasonable grounds to believe the conduct was lawful. |
| ● | An individual referred to above is entitled to an indemnity from the Company in respect of all costs, charges and expenses reasonably incurred by the individual in connection with the defense of any civil, criminal, administrative, investigative or other proceeding to which the individual is subject because of the individual’s association with the Company or other entity as described above, if the individual seeking an indemnity: |
| o | acted honestly and in good faith with a view to the best interests of the Company or, as the case may be, to the best interests of the other entity for which the individual acted as a director or officer or in a similar capacity at the Company’s request; |
| o | in the case of a criminal or administration action or proceeding enforced by a monetary penalty, had reasonable grounds to believe the conduct was lawful; and |
| o | was not judged by a court or other competent authority to have committed any fault or omitted to do anything that the individual ought to have done. |
46
Committees of the Board
Audit Committee
Jatinder Dhaliwal, David Jenkins and Christopher Cooper currently serve as the members of our audit committee. Our board of directors has determined that each of Jatinder Dhaliwal, David Jenkins and Christopher Cooper meet the independent director standard under Nasdaq listing standards and under Rule 10-A-3(b)(1) of the Exchange Act. David Jenkins serves as the chairman of the audit committee.
Our audit committee is responsible for, among other things:
| ● | appointing, compensating, retaining, evaluating, terminating and overseeing our independent registered public accounting firm; |
| ● | discussing with our independent registered public accounting firm their independence from management; |
| ● | reviewing, with our independent registered public accounting firm, the scope and results of their audit; |
| ● | approving all audit and permissible non-audit services to be performed by our independent registered public accounting firm; |
| ● | overseeing the financial reporting process and discussing with management and our independent registered public accounting firm any financial statements that we file with the SEC; |
| ● | overseeing our financial and accounting controls and compliance with legal and regulatory requirements; |
| ● | reviewing our policies on risk assessment and risk management; |
| ● | reviewing related person transactions; and |
| ● | establishing procedures for the confidential anonymous submission of concerns regarding questionable accounting, internal controls or auditing matters. |
Compensation Committee
Jatinder Dhaliwal, David Jenkins and Christopher Cooper currently serve as the members of our compensation committee. Our board of directors has determined that each of Jatinder Dhaliwal, David Jenkins and Christopher Cooper meet the independent director standard under Nasdaq listing standards and under Rule 10-A-3(b)(1) of the Exchange Act. Jatinder Dhaliwal serves as the chairman of the compensation committee.
Our compensation committee is responsible for, among other things:
| ● | determining and recommending to the Board of Directors for approval, the corporate goals and objectives, evaluating the performance and reviewing and approving the compensation of our executive officers; |
| ● | reviewing or making recommendations to our Board of Directors regarding our incentive compensation and equity-based plans, policies and programs; |
| ● | reviewing all employment agreement and severance arrangements for our executive officers; |
| ● | reviewing and making recommendations to our Board of Directors regarding the compensation of our directors; and |
| ● | retaining and overseeing any compensation consultants. |
47
Nominating Committee
Jatinder Dhaliwal and Christopher Cooper currently serve as members of our nominating and corporate governance committee. Our board of directors has determined that Jatinder Dhaliwal and Christopher Cooper meet the independent director standard under Nasdaq listing standards and under Rule 10-A-3(b)(1) of the Exchange Act. Christopher Cooper is the chairman of the nominating and corporate governance committee.
Our nominating and corporate governance committee is responsible for, among other things:
| ● | identifying individuals qualified to become members of our Board of Directors consistent with criteria approved by our Board of Directors; |
| ● | overseeing succession planning for our executive officers; |
| ● | periodically reviewing our Board of Directors’ leadership structure and recommending any proposed changes to our Board of Directors; |
| ● | overseeing an annual evaluation of the effectiveness of our Board of Directors and its committees; |
| ● | developing and recommending to our Board of Directors a set of corporate governance guidelines; |
| ● | developing and recommending to our Board of Directors our ESG initiatives and programs. |
Board Leadership Structure and Risk Oversight
Our Board of Directors oversees our business and considers the risks associated with our business strategy and decisions. Our Board of Directors currently implements its risk oversight function as a whole. Each of the Board committees also provides risk oversight in respect of its areas of concentration and report material risks to the Board for further consideration.
Conflicts of Interest
There are potential conflicts of interest to which the directors, officers, and insiders of our company will be subject in connection with the operations of our company. Some of the directors, officers and insiders are engaged in and will continue to be engaged in corporations or businesses which may be in competition with the business of our company. Accordingly, situations may arise where the directors, officers and insiders will be in direct competition with our company. The directors and officers of our company have a fiduciary obligation to act in the best interests of our company, avoid conflicts of interest and to disclose to all other board members any relevant information about potential conflicts. They have the same obligations to the other companies in respect of which they act as directors and officers. Discharge by the directors and officers of their obligations to our company may result in a breach of their obligations to the other companies, and in certain circumstances this could expose our company to liability to those companies. Similarly, discharge by the directors and officers of their obligations to the other companies could result in a breach of their obligation to act in the best interests of our company. Such conflicting legal obligations may expose our company to liability to others and impair our ability to achieve our business objectives. All of the directors or officers of our company have entered into non-competition or non-disclosure agreements with our company. Conflicts, if any, will be subject to the procedures and remedies as provided under the Code of Ethics and Related Party Transaction Policy and applicable securities laws, regulations and policies.
Terms of Office
Each of our officers holds office until his or her successor is appointed. Directors are elected to serve until the close of the next annual meeting of shareholders or until their successors have been elected or appointed.
Director Independence
We use the definition of “independence” under applicable Nasdaq Listing Rules to make determinations regarding director independence. Under such definitions, the Company’s Board of Directors has affirmatively determined that each of Jatinder Dhaliwal, David Jenkins and Christopher Cooper is independent.
Shareholder Communications
We do not have a formal policy regarding shareholder communications with our Board of Directors. A shareholder who wishes to communicate with our Board of Directors may do so by directing a written request addressed to our Chief Financial Officer, at Akanda Corp., 1a, 1b Learoyd Road New Romney TN28 8XU, United Kingdom.
| D. | Employees |
As of April 29, 2024, we had a total of 5 employees.
| E. | Share Ownership |
See Item 6.B. – “Compensation” and Item 7 – “Major Shareholders and Related Party Transactions.”
48
| F. | Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation |
Not applicable.
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
| A. | Major Shareholders |
Share Ownership
The following table sets forth information about the beneficial ownership of our Common Shares as of April __, 2024 by:
| ● | each of our executive officers and directors; |
| ● | all of our executive officers and directors; and |
| ● | each person or entity (or group of affiliated persons or entities) known by us to be the beneficial owner of 5% or more of our Common Shares. |
To our knowledge, each shareholder named in the table has sole voting and investment power with respect to all of our Common Shares shown as “beneficially owned” (as determined by the rules of the SEC) by such shareholder, subject to applicable community property laws and except as otherwise set forth in the footnotes to the table. The SEC has defined “beneficial” ownership of a security to mean the possession, directly or indirectly, of voting power and/or investment power.
Percentages beneficial ownership (as determined in accordance with Rule 13d-3 under the Exchange Act) are based on 32,015,528 Common Shares outstanding as of April 30, 2024.
Common Shares which may be acquired upon conversion of convertible securities or exercise of stock options or warrants which are currently exercisable or convertible or which become exercisable or convertible within 60 days after the date indicated in the table are deemed beneficially owned by the holder thereof.
Except as noted in the footnotes to the table below, the address for all of the shareholders in the table below is c/o Akanda, 1a, 1b Learoyd Road New Romney TN28 8XU, United Kingdom.
| Percentage of Common Shares Beneficially Owned(1) | ||||||||
| Name of Beneficial Owner | Number of Common Shares Beneficially Owned | Percentage | ||||||
| Executive Officers and Directors: | ||||||||
| Katharyn Field(2) | — | — | ||||||
| Gurcharn Deol | — | — | ||||||
| Jatinder Dhaliwal | — | — | ||||||
| David Jenkins | — | — | ||||||
| Christopher Cooper | — | — | ||||||
| All executive officers and directors as a group | — | — | ||||||
| 5% or more Shareholders: | — | — | ||||||
| — | — | — | ||||||
| * | Represents less than 1% of the number of Common Shares outstanding. |
| (1) | Beneficial ownership is determined in accordance with Rule 13d-3 under the Exchange Act. A person is deemed to be the beneficial owner of any Common Shares if that person has or shares voting power or investment power with respect to those shares or has the right to acquire beneficial ownership at any time within 60 days. |
| (2) | Katharyn Field is the CEO and Chairman of Halo but does not have voting or dispositive power over the Common Shares held of record and beneficially owned by Halo. Ms. Field disclaims beneficial ownership of the Common Shares held by Halo. |
For additional information about our principal shareholders, please see Item 7.B. – Related Party Transactions.
49
| B. | Related Party Transactions |
Secured convertible debenture
On November 3, 2021 (the “Issuance Date”), the Company entered into an agreement with Halo in which the Company issued Halo a secured convertible debenture with an initial value of $6,559,294. The notes were convertible into Common Shares of the Company’s capital receiving the number of shares, at its current market price, required to satisfy the principal and interest payable. The obligation to convert the note within six months of the Issuance Date is triggered by (a) an initial public offering by the Company on a stock exchange; (b) an amalgamation, arrangement, merger, reverse takeover, reorganization or similar event; (c) a sale or conveyance of all or substantially all of the property and assets of the Company to any arm’s length third party for consideration consisting of free trading securities and the subsequent distribution of all of such consideration to all of the holders of Common Shares, on a pro rata basis; (d) the sale or exchange of all or substantially all of shares of the Borrower for free trading securities. The debt bears interest at one percent per annum, matures on November 3, 2022 and is secured by all of the assets of the Company other than the interests in the securities of Bophelo Bio Science and Wellness (Pty) Ltd. and ranks ahead of all other debt issued by the Company. On March 15, 2022, upon completion of the Company’s initial public offering the Company issued 164,574 Common Shares to Halo Collective, Inc. (“Halo”) at a price of $40 each to settle the principal amount of $6,559,294 plus accrued interest $23,686 owing to Halo in terms of a convertible debenture agreement, which totaled $6,582,980 at the time of conversion.
Unsecured debenture
On January 26, 2023, the Company issued a promissory note to Halo for a principal amount of $328,000.00 (the “Halo Note”). The Halo Note bears an interest rate of 7% per annum and has matured on June 25, 2023. If the amount payable is due, whether at stated maturity, by acceleration or otherwise, the overdue amount shall bear and accrue an interest rate of 1.25%. On July 25, 2023, the Company and Halo entered into a Note Conversion Agreement to convert $360,960 of the total remaining outstanding balance, including accrued interest, under the Halo Note into 582,193 Common Shares of the Company at $0.62 per share (the “Conversion Shares”). The Company and Halo agreed that after the issuance of the Conversion Shares, the principal balance outstanding under the Halo Note is $0.
During the period from February 1, 2023 to March 31, 2024, the Company received additional loans from Halo in the aggregate principal amount of $1,539,949. These loans are unsecured and bears the same interest rate of 7% per annum and have no specific terms of repayment. The Company made a partial payment amounting to $26,897 in January 2024 and $110,791 in March 2024. As of March 31, 2024, the outstanding balance to Halo, including accrued interest and other loans was $1,326,682.
Transactions with Key Management Personnel
The Company has identified its Board of Directors, Executive Chairman, Chief Executive Officer (“CEO”), Chief Operating Officer (“COO”), Chief Financial Officer (“CFO”) and its President as its key management personnel who have the authority and responsibility for planning, directing and controlling the Company’s main activities.
For the fiscal year ended December 31, | 2023 | |||
| Key Management Remuneration | $ | 522,669 | ||
| Stock-based compensation | — | |||
| Short term accommodation expense | — | |||
| $ | 522,669 | |||
The Key Management remuneration is included in Professional and Consulting fees and Personnel Expenses in the Statement of Operations.
As of December 31, 2023, the Company has balances payable to related parties of $2,255,522 (2022 — $679,617) as below:
| a. | Included within accounts payable and accrued liabilities at December 31, 2023 is remuneration payable to key management totaling $889,367 (2022 — $679,617), which includes amounts owing to the following current and former directors and officers of the Company: |
| ● | current directors and officers: |
| i. | $99,051 owing to J Dhaliwal (2022 — $nil); |
| ii. | $81,384 owing to K Field (2022 — $nil); |
| iii. | $81,384 owing to H Singh (2022 — $nil); and |
| iv. | $81,384 owing to D Jenkins (2022 — $nil). |
50
| ● | former directors and officers: |
| i. | $85,733 owing to T Scott (2022 — $507,326); |
| ii. | $28,196 owing to Y Liang (2022 — $nil); |
| iii. | $nil owing to T Virk (2022 — $15,092); |
| iv. | $nil owing to T Flow (2022 — $49,617); |
| v. | $nil owing to Dr. Akkar-Schenkl (2022 — $17,223); |
| vi. | $nil owing to L Mojela (2022 — $15,092); |
| vii. | $8,417 owing to P van den Berg (2022 — $16,344); |
| viii. | $15,418 owing to C Kié (2022 — $22,335); |
| ix. | $8,333 owing to G Jones (2022 — $10,379); |
| x. | $10,039 owing to P Freyre (2022 — $7,619); |
| xi. | $8,333 owing to G Dingaan (2022 — $10,379); and |
| xii. | $6,250 owing to B Baker (2022 — $8,211). |
| b. | The sole director and officer of RPK, Kiranjit Sidhu is also the owner of Catalyst Capital LLC (“Catalyst”). |
| i. | On November 14, 2022, the Company received a loan of £25,000 ($30,224) from Catalyst. The loan is unsecured and bears interest of £200 per week. The loan has matured on January 31, 2023 and is due on demand. Any unpaid amount is charged with late fees of £200 for each week the payment is late. As of December 31, 2023, the loan balance including accrued interest of $46,811 (2022 — $30,224) remains outstanding. |
| ii. | On January 17, 2023, the Company received an additional loan of €45,000 ($48,666) from Catalyst. The loan is unsecured and bears interest of 0.75% per day, compounding daily. The loan has matured on February 1, 2023 and is due on demand. Any unpaid amount is charged with late fees of 1% compounding interest for each day the payment is late. During the year ended December 31, 2023, the lender has willingly forgone any interest arising from this loan. As of December 31, 2023, the loan balance including accrued interest of $49,664 (2022 — $nil) remains outstanding. |
| iii. | On January 23, 2023, the Company entered into an independent contractor agreement with Mr. Sidhu, pursuant to which, he agreed to provide services regarding the business operations, business development and strategic matters to the Company for $550,000. The payment for the services was settled by the issuance of 289,473 RSU converted to 289,473 Common Shares of the Company to Mr. Sidhu in January 2023. |
| iv. | On February 5, 2023, the Company entered into another independent contractor agreement with Mr. Sidhu, pursuant to which, he agreed to provide services regarding the business operations, business development, legal and strategic matters to the Company for $650,000. The payment for the services was partially settled by the issuance of 161,295 RSUs converted to 161,295 Common Shares in May 2023 and 140,746 RSUs converted to 140,746 Common Shares in July 2023. As of the December 31, 2023, the balance of the payment is $350,395. |
| c. | The Company has the following loans outstanding to 1248787 B.C. Ltd. (“1248787”), a company controlled by Jatinder Dhaliwal, a director of the Akanda: |
| i. | On August 18, 2023, the Company received a loan of C$24,000 ($17,714) from 1248787. The loan is unsecured, bears interest of 18% per annum and payable within 12 months. As of the December 31, 2023, the loan balance including accrued interest of $19,306 (2022 – $nil) remains outstanding. |
| ii. | On September 27, 2023, the Company received a loan of C$3,000 ($2,219) from 1248787. The loan is unsecured, bears interest of 18% per annum and payable within 12 months. As of the December 31, 2023, the loan balance including accrued interest of $2,369 (2022 – $nil) remains outstanding. |
51
| iii. | On October 13, 2023, the Company received a loan of C$40,000 ($29,258) from 1248787. The loan is unsecured, bears interest of 18% per annum and payable within 12 months. As of the December 31, 2023, the loan balance including accrued interest of $31,346 (2022 – $nil) remains outstanding. |
The Company’s related party transactions are measured at the exchange amount which is the amount of consideration established and agreed to by the related parties.
Outstanding Claims
On January 29, 2024, the Company was informed that Mr. Shailesh Bhushan, the former Chief Financial Officer of the Company, filed a complaint with the Employment Standards Branch of British Columbia claiming unpaid salary and invoices in the aggregate amount of CAD $271,990 from the period December 2022 through November 2023. The Company previously offered to Mr. Bhushan an annual salary of CAD $60,000 and as such, believes the claim to be frivolous, strongly disputes the amount claimed, and intends to vigorously defend itself.
See Notes 5 and 22 of our Audited Financial Statements for the year ended December 31, 2023 for a description of additional outstanding claims by and against the Company.
On April 24, 2024, Mr. Harvinder Singh resigned as an independent director of the Board of Directors of the Company. A Resignation and Mutual Release Agreement dated April 24, 2024 was entered between the Company and Mr. Singh, pursuant to which the Company agreed to pay Harvinder Singh a separation and release amount of $50,000.
Additionally, please refer to Note 16 of Akanda Group’s audited financial statements included in Item 18 of this Annual Report on Form 20-F.
| C. | Interests of Experts and Counsel |
Not applicable.
ITEM 8. FINANCIAL INFORMATION
| A. | Consolidated Statements and Other Financial Information |
See Item 18. — “Financial Statements.”
| B. | Significant Changes |
No significant changes occurred since the date of the annual financial statements.
ITEM 9. THE OFFER AND LISTING
| A. | Offer and Listing Details |
Our Common Shares have traded on The Nasdaq Capital Market under the symbol “AKAN” from our initial public offering on March 15, 2022.
| B. | Plan of Distribution |
Not applicable.
| C. | Markets |
Our ordinary shares are listed and traded on The Nasdaq Capital Market.
| D. | Selling Shareholders |
Not applicable.
| E. | Dilution |
Not applicable.
52
| F. | Expenses of the Issue |
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
| A. | Share Capital |
Not applicable.
| B. | Memorandum and Articles of Association |
General
The Company’s Articles of Incorporation, as amended by Articles of Amendment dated as of August 30, 2021, and as further amended by Articles of Amendment dated as of March 8, 2023 (the “Articles”), provide that our authorized capital consists of an unlimited number of Common Shares, and an unlimited number of preferred shares (the “Preferred Shares”), issuable in series.
As of April 30, 2024, the Company has 32,015,528 Common Shares issued and outstanding and no Preferred Shares issued and outstanding.
Rights, Preferences and Restrictions Attaching to Our Common Shares
The Articles provide the following rights, privileges, restrictions and conditions attaching to our Common Shares:
| ● | to vote at meetings of shareholders, except meetings at which only holders of a specified class of shares other than the Common Shares are entitled to vote; |
| ● | subject to the prior rights of the holders of the Preferred Shares, to share equally in the remaining property of our Company on liquidation, dissolution or winding-up of our Company; and |
| ● | subject to the prior rights of the holders of the Preferred Shares, the Common Shares are entitled to receive dividends if, as, and when declared by the Board of Directors. |
The holders of Common Shares are entitled to receive notice of and to attend all annual and special meetings of our shareholders and to one vote in respect of each Common Share held at the record date for each such meeting, except a meeting of holders of a particular class of shares other than Common Shares who are entitled to vote separately as a class at such meeting. Subject to the prior rights of the holders of the Preferred Shares, the holders of Common Shares are entitled, at the discretion of our Board of Directors, to receive out of any or all of our profits or surplus properly available for the payment of dividends, any dividend declared by our Board of Directors and payable by the Company on the Common Shares. The holders of the Common Shares will participate in any distribution of the assets of the Company upon liquidation, dissolution or winding-up or other distribution of the assets of the Company, subject to the prior rights of the holder of the Preferred Shares.
Pre-emptive Rights
Our Common Shares do not contain any pre-emptive purchase rights to any of our securities.
Shareholder Meetings
The Business Corporations Act (Ontario) provides that: (i) a general meeting of shareholders shall be held at such place in or outside Ontario as the directors determine or, in the absence of such a determination, at the place where the registered office of our Company is located; (ii) directors must call an annual meeting of shareholders not later than 18 months after the date of incorporation and no later than 15 months after the last preceding annual meeting; (iii) for the purpose of determining shareholders entitled to receive notice of or vote at meetings of shareholders, the directors may fix in advance a date as the record date for that determination, provided that such date shall not precede by more than 60 days or by less than 30 days, the date on which the meeting is to be held; (iv) the holders of not less than 5% of the issued shares entitled to vote at a meeting may requisition the directors to call a meeting of shareholders for the purposes stated in the requisition; and (v) if for any reasons it impracticable to call a meeting of shareholders in the matter in which it may be called or to conduct the meeting in the matter prescribed by the by-laws, the Articles or the Business Corporations Act (Ontario), or for any other reason the court thinks fit, the court, upon the application of a director or shareholder entitled to vote at the meeting, may order a meeting to be called, held and conducted in a manner that the court directs. The Company’s by-laws provide that a quorum is met when at least two persons present in person and holding or representing by proxy not less than 10% of the votes attached to all shares entitled to be voted at the meeting.
The holders of our Common Shares are entitled to attend and vote at all meetings of the shareholders of the Company, except a meeting of holders of a particular class of shares other than the Common Shares who are entitled to vote separately as a class at such meeting.
53
Fully Paid and Non-assessable
All outstanding Common Shares are duly authorized, validly issued, fully paid and non-assessable.
Resale Restrictions
Our Articles do not impose restrictions on the transfer of Common Shares by a shareholder.
Preferred Shares
The Preferred Shares may at any time and from time to time be issued in one or more series. The Board of Directors will, by resolution, from time to time, before the issue thereof, fix the designation, rights, privileges, restrictions and conditions attaching to the Preferred Shares of each series.
Transfer Agent and Registrar
The transfer agent and registrar for our Common Shares is Vstock Transfer, LLC.
Listing
Our Common Shares are listed on The Nasdaq Capital Market under the symbol “AKAN”.
| C. | Material Contracts |
See Item 6.B – Compensation and Item 7.B. – Related Party Transactions.
| D. | Exchange Controls |
We are not aware of any governmental laws, decrees, regulations or other legislation in Canada that restrict the export or import of capital, including the availability of cash and cash equivalents for use by our affiliated companies, or that affect the remittance of dividends, interest or other payments to non-resident holders of our securities. Any remittances of dividends to residents of the United States and to other non-resident holders are, however, subject to withholding tax. See Item 10.E. – “Taxation”.
| E. | Taxation |
Material Canadian Federal Income Tax Considerations
The following summary describes the principal Canadian federal income tax considerations pursuant to the Income Tax Act (Canada) and the regulations thereunder (the “Tax Act”) generally applicable to the acquisition, holding and disposition of the Common Shares by a holder who acquires, as beneficial owner, our Common Shares and who, for purposes of the Tax Act and at all relevant times, holds the Common Shares as capital property, deals at arm’s length with the Company and is not affiliated with the Company (a “Holder”). Generally, the Common Shares will be considered to be capital property to a Holder provided the Holder does not acquire or hold the Common Shares in the course of carrying on a business of trading or dealing in securities and has not acquired them in one or more transactions considered to be an adventure or concern in the nature of trade.
This summary does not apply to a Holder (i) that is a “financial institution” for the purposes of the mark-to-market rules contained in the Tax Act; (ii) that is a “specified financial institution” as defined in the Tax Act; (iii), an interest in which would be, or for whom a Common Share would be, a “tax shelter investment” as defined in the Tax Act; (iv) that has made a functional currency reporting election under the Tax Act to report in a currency other than the Canadian currency; (v) that has or will enter into a “derivative forward agreement”, a “synthetic disposition arrangement” or a “dividend rental arrangement”, each as defined under the Tax Act, with respect to the Common Shares. (vi) that carries on, or is deemed to carry on, an insurance business in Canada or elsewhere. Such Holders should consult their own tax advisors with respect to an investment in the Common Shares.
Additional considerations, not discussed herein, may be applicable to a Holder that is a corporation resident in Canada, and that is or becomes, or does not deal at arm’s length for purposes of the Tax Act with a corporation resident in Canada that is or becomes, as part of a transaction or event or series of transactions or events that includes the acquisition of the Common Shares, controlled by a non-resident person or group of non-resident persons not dealing with each other at arm’s length for purposes of the “foreign affiliate dumping” rules in section 212.3 of the Tax Act. Such Holders should consult their own tax advisors with respect to the possible application of these rules.
In addition, this summary does not address the deductibility of interest by a Holder who has borrowed money or otherwise incurred debt in connection with the acquisition of the Common Shares.
54
This summary is based upon the provisions of the Tax Act in force as of the date hereof, all specific proposals to amend the Tax Act that have been publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Proposed Amendments”) and counsel’s understanding of the current administrative policies and assessing practices of the Canada Revenue Agency (“CRA”) made publicly available prior to the date hereof. This summary assumes the Proposed Amendments will be enacted in the form proposed, however, no assurance can be given that the Proposed Amendments will be enacted in the form proposed, if at all. This summary is not exhaustive of all possible Canadian federal income tax considerations and, except for the Proposed Amendments, does not take into account or anticipate any changes in law or the administrative policies or assessing practices of the CRA, whether by legislative, governmental or judicial action or decision, nor does it take into account provincial, territorial or foreign tax considerations, which may differ significantly from those discussed herein.
Subject to certain exceptions that are not discussed in this summary, for the purposes of the Tax Act, all amounts relating to the acquisition, holding or disposition of Common Shares, including dividends, must be determined in Canadian dollars using the relevant exchange rate determined in accordance with the Tax Act.
This summary is of a general nature only and is not intended to be, nor should it be construed to be, legal or tax advice to any particular holder or prospective holder of the Common Shares, and no representations with respect to the income tax consequences to any holder or prospective holder are made. Consequently, holders and prospective holders of the Common Shares should consult their own tax advisors for advice with respect to the tax consequences to them of acquiring the Common Shares, having regard to their particular circumstances.
Holders Resident in Canada
This portion of the summary applies to a Holder who, at all relevant times, for purposes of the Tax Act and any applicable income tax treaty or convention, is or is deemed to be resident in Canada (a “Resident Holder”).
Certain Resident Holders who might not otherwise be considered to hold their Common Shares as capital property may, in certain circumstances, be entitled to have the Common Shares, and all other “Canadian securities” (as defined in the Tax Act) owned by such Resident Holders in the taxation year of the election and any subsequent taxation year, treated as capital property by making the irrevocable election permitted by subsection 39(4) of the Tax Act. Resident Holders should consult their own tax advisors regarding the availability or advisability of this election.
Dividends on the Common Shares
Dividends received or deemed to be received on the Common Shares by a Resident Holder who is an individual (other than certain trusts) will generally be included in the individual’s income and will be subject to the gross-up and dividend tax credit rules applicable to taxable dividends received from taxable Canadian corporations, including the enhanced dividend tax credit rules applicable to any dividends designated by the Company as “eligible dividends” in accordance with the Tax Act. There may be limitations on the ability of the Company to designate dividends as “eligible dividends.”
In the case of a Resident Holder that is a corporation, the amount of any such taxable dividend that is included in its income for a taxation year will generally also be deductible in computing its taxable income for that taxation year. In certain circumstances, a dividend received or deemed to be received by a Resident Holder that is a corporation may be deemed to be proceeds of disposition or a capital gain pursuant to subsection 55(2) of the Tax Act. Resident Holders that are corporations should consult their own tax advisors having regard to their own particular circumstances.
A Resident Holder that is a “private corporation” or a “subject corporation”, each as defined in the Tax Act will generally be liable to pay a refundable tax under Part IV of the Tax Act on dividends received or deemed to be received on the Common Shares to the extent such dividends are deductible in computing its taxable income for the taxation year. Such additional tax may be refundable in certain circumstances.
Dispositions of Common Shares
Upon a disposition (or a deemed disposition) of a Common Share, a Resident Holder generally will realize a capital gain (or a capital loss) equal to the amount, if any, by which the proceeds of disposition of such Common Share, net of any reasonable costs of disposition, are greater (or are less) than the adjusted cost base of such Common Share to the Resident Holder.
The adjusted cost base to a Resident Holder of Common Shares acquired hereunder will be determined by averaging the cost of such Common Shares to the Resident Holder with the adjusted cost base of all other Common Shares, if any, held by the Resident Holder as capital property immediately before the acquisition.
55
Taxation of Capital Gains and Capital Losses
Generally, one-half of any capital gain (a “taxable capital gain”) realized by a Resident Holder in a taxation year must be included in the Resident Holder’s income for the year and one-half of any capital loss (an “allowable capital loss”) realized by a Resident Holder in a taxation year must be deducted from taxable capital gains realized by the Resident Holder in that year. Allowable capital losses in excess of taxable capital gains realized in a taxation year generally may be carried back and deducted in any of the three preceding taxation years or carried forward and deducted in any subsequent taxation year against net taxable capital gains realized in such years, to the extent and under the circumstances described in the Tax Act.
The amount of any capital loss realized by a Resident Holder that is a corporation on the disposition of a Common Share may be reduced by the amount of dividends received or deemed to be received by it on such Common Share, to the extent and under the circumstances described in the Tax Act. Similar rules may apply where a Common Share is owned by a partnership or trust of which a corporation, trust or partnership is a member or beneficiary. Resident Holders to whom these rules may be relevant should consult their own tax advisors.
Aggregate Investment Income
A Resident Holder that is, throughout the relevant taxation year, a “Canadian-controlled private corporation”, as defined in the Tax Act, may be liable to pay a refundable tax on its “aggregate investment income”, which is defined in the Tax Act to include an amount in respect of taxable capital gains and dividends or deemed dividends that are not deductible in computing such corporation’s income. Pursuant to Proposed Amendments released on April 7, 2022 in a Notice of Ways and Means Motion (the “Notice”) released with the federal budget, it is proposed that the refundable tax on investment income will also apply to corporations that are “substantive CCPCs” as defined in the Notice.
Alternative Minimum Tax
Capital gains realized and dividends received or deemed to be received by an individual (including certain trusts) may give rise to liability for alternative minimum tax as calculated under the detailed rules set out in the Tax Act. Resident Holders who are individuals should consult their own tax advisors in this regard.
Holders Not Resident in Canada
This portion of the summary applies to a Holder who, at all relevant times, for purposes of the Tax Act and any applicable income tax treaty or convention (i) is neither resident nor deemed to be resident in Canada, and (ii) does not, and is not deemed to, use or hold the Common Shares in a business carried on in Canada (a “Non-Resident Holder”). In addition, this portion of the summary does not apply to an insurer who carries on an insurance business in Canada and elsewhere or an “authorized foreign bank” (as defined in the Tax Act) and such Non-Resident Holders should consult their own tax advisors.
Dividends on the Common Shares
Any dividends paid or credited, or deemed to be paid or credited, on the Common Shares, as the case may be, to a Non-Resident Holder will generally be subject to Canadian withholding tax at the rate of 25% of the gross amount of the dividend, subject to any reduction in the rate of withholding to which that Non-Resident Holder may be entitled under an applicable income tax treaty or convention. For instance, where the Non-Resident Holder is a resident of the United States that is entitled to applicable benefits under the Canada-United States Income Tax Convention (1980), as amended, and is the beneficial owner of the dividends, the rate of Canadian withholding tax applicable to dividends is generally reduced to 15%. The rate of withholding tax is generally further reduced to 5% if the beneficial owner of such dividend is a company that owns, directly or indirectly, at least 10% of the voting stock of the Company. Non-Resident Holders should consult their own tax advisors to determine their entitlement to relief under an applicable income tax treaty or convention.
Disposition of the Common Shares
A Non-Resident Holder will not be subject to tax under the Tax Act in respect of any capital gain realized by such Non-Resident Holder on a disposition of a Common Share unless such share constitutes “taxable Canadian property” (as defined in the Tax Act) of the Non-Resident Holder at the time of disposition and the Non-Resident Holder is not entitled to relief under an applicable income tax treaty or convention.
56
Generally, the Common Shares will not constitute “taxable Canadian property” of a Non-Resident Holder at any particular time provided that the Common Shares are then listed on a “designated stock exchange” for the purposes of the Tax Act (which currently includes the Nasdaq), unless at any time during the 60-month period immediately preceding such time: (i) at least 25% or more of the issued shares of any class or series of the capital stock of the Company were owned by or belonged to any combination of (x) the Non-Resident Holder, (y) persons with whom the Non-Resident Holder did not deal at arm’s length (for the purposes of the Tax Act), and (z) partnerships in which the Non-Resident Holder or a person described in (y) holds a membership interest directly or indirectly through one or more partnerships; and (ii) more than 50% of the fair market value of such shares was derived directly or indirectly from one, or any combination of, real or immovable property situated in Canada, Canadian resource property (as defined in the Tax Act), timber resource property (as defined in the Tax Act) or options in respect of, interests in or for civil law rights in, any such property (whether or not such property exists). Notwithstanding the foregoing, the Common Shares may also be deemed to be “taxable Canadian property” in certain circumstances.
In cases where a Non-Resident Holder disposes (or is deemed to have disposed) of a Common Share that is “taxable Canadian property” to that Non-Resident Holder, and the Non-Resident Holder is not entitled to an exemption under an applicable income tax treaty or convention, the consequences described above under the headings “Holders Resident in Canada — Dispositions of Common Shares” and “Taxation of Capital Gains and Capital Losses” will generally be applicable to such disposition. Non-Resident Holders for whom a Common Share is, or may be, “taxable Canadian property” should consult their own tax advisors.
Material U.S. Federal Income Tax Considerations
The following discussion is a general summary of certain material U.S. federal income tax considerations with respect to the ownership and disposition of shares of our Common Shares. This summary is based on current U.S. federal income tax laws (including provisions of the U.S. Internal Revenue Code of 1986, as amended (the “Code”), U.S. Treasury regulations promulgated thereunder and administrative rulings and court decisions, all in effect as of the date hereof), all of which are subject to change at any time, possibly with retroactive effect.
For purposes of this discussion, the term “U.S. Holder” means a beneficial owner of one or more of our Common Shares that is for U.S. federal income tax purposes one of the following:
| ● | an individual citizen or resident of the United States, including individuals treated as residents of the United States solely for tax purposes; |
| ● | a corporation created or organized in or under the laws of the United States or any political subdivision thereof; |
| ● | an estate the income of which is subject to U.S. federal income taxation regardless of its source, or; |
| ● | a trust if (1) a court within the United States can exercise primary supervision over it, and one or more United States persons have the authority to control all substantial decisions of the trust, or (2) the trust has a valid election in effect under applicable U.S. Treasury regulations to be treated as a United States person. |
This discussion applies only to a U.S. Holder that holds Common Shares as “capital assets” within the meaning of Section 1221 of the Code (generally, property held for investment). Unless otherwise provided, this summary does not discuss reporting requirements. In addition, this discussion does not address any tax consequences other than U.S. federal income tax consequences, such as U.S. state and local tax consequences, U.S. estate and gift tax consequences, and non-U.S. tax consequences, and does not describe all of the U.S. federal income tax consequences that may be relevant in light of a U.S. Holder’s particular circumstances, including alternative minimum tax consequences, the net investment income tax, and tax consequences to holders that are subject to special provisions under the Code, including, but not limited to, holders that:
| ● | are tax exempt organizations, qualified retirement plans, individual retirement accounts, or other tax deferred accounts; |
| ● | are financial institutions, underwriters, insurance companies, real estate investment trusts, or regulated investment companies; |
| ● | are brokers or dealers in securities or currencies or holders that are traders in securities that elect to apply a mark-to-market accounting method; |
57
| ● | have a “functional currency” for U.S. federal income tax purposes that is not the U.S. dollar; |
| ● | own Common Shares as part of a straddle, hedging transaction, conversion transaction, constructive sale, or other arrangement involving more than one position; |
| ● | acquire Common Shares in connection with the exercise of employee stock options or otherwise as compensation for services; |
| ● | are partnerships or other pass-through entities for U.S. federal income tax purposes (or investors in such partnerships and entities); |
| ● | are required to accelerate the recognition of any item of gross income with respect to the Common Shares as a result of such income being recognized on an applicable financial statement; |
| ● | own or will own (directly, indirectly, or constructively) 10% or more of our total combined voting power or value; |
| ● | are controlled foreign corporations; |
| ● | are passive foreign investment companies; |
| ● | hold the Common Shares in connection with trade or business conducted outside of the United States or in connection with a permanent establishment or other fixed place of business outside of the United States; or |
| ● | are former U.S. citizens or former long-term residents of the United States. |
If an entity or arrangement treated as a partnership for U.S. federal income tax purposes holds shares of our securities, the tax treatment of a person treated as a partner for U.S. federal income tax purposes generally will depend on the status of the partner and the activities of the partnership. Persons that for U.S. federal income tax purposes are treated as a partner in a partnership holding shares of our securities should consult their tax advisors.
We have not sought, and do not expect to seek, a ruling from the United States Internal Revenue Service (the “IRS”), as to any United States federal income tax consequence described herein. The IRS may disagree with the discussion herein, and its determination may be upheld by a court. Moreover, there can be no assurance that future legislation, regulations, administrative rulings or court decisions will not adversely affect the accuracy of the statements in this discussion.
Except as otherwise noted, this summary assumes that the Company (nor any of its subsidiaries) is not a passive foreign investment company (a “PFIC”) for U.S. federal income tax purposes. A non-U.S. entity’s possible status as a PFIC must be determined annually and therefore may be subject to change. If the Company (or any of its subsidiaries) were to be a PFIC in any year, materially adverse consequences could result for U.S. Holders.
All prospective investors should consult with their own tax advisors regarding the U.S. federal, state, local, non-U.S. income and other tax considerations of acquiring, holding and disposing of the Common Shares.
WE RECOMMEND THAT PROSPECTIVE HOLDERS OF OUR COMMON SHARES CONSULT WITH THEIR TAX ADVISORS REGARDING THE TAX CONSEQUENCES TO THEM (INCLUDING THE APPLICATION AND EFFECT OF ANY FEDERAL, STATE, LOCAL, NON-U.S. INCOME AND OTHER TAX LAWS) OF THE OWNERSHIP AND DISPOSITION OF OUR COMMON SHARES.
U.S. Holders
Taxation of Distributions to U.S. Holders
Subject to the PFIC rules discussed below, a U.S. Holder generally will be required to include in gross income, in accordance with such U.S. Holder’s method of accounting for United States federal income tax purposes, as dividends the amount of any distribution of cash or other property (other than certain distributions of the Company’s shares or rights to acquire the Company’s shares) paid on the Company’s Common Shares to the extent the distribution is paid out of the Company’s current or accumulated earnings and profits (as determined under United States federal income tax principles). Distributions in excess of such earnings and profits generally will be applied against and reduce the U.S. Holder’s basis in its Common Shares (but not below zero) and, to the extent in excess of such basis, will be treated as gain from the sale or exchange of such Common Shares (the treatment of which is described under “— Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Common Shares to U.S. Holders” below). Because we do not intend to determine our earnings and profits on the basis of U.S. federal income tax principles, we expect that distributions, if issued, will generally be reported to U.S. Holders as dividends.
58
Dividends paid by us will be taxable to a corporate U.S. Holder at regular rates and will not be eligible for the dividends-received deduction generally allowed to domestic corporations in respect of dividends received from other domestic corporations. With respect to individuals and other non-corporate U.S. Holders, dividends generally will be taxed at the lower applicable long-term capital gains rate (see “— Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Common Shares to U.S. Holders” below) applicable to “qualified dividend income,” provided that certain conditions are satisfied, including that (1) our Common Shares on which the dividends are paid are readily tradable on an established securities market in the United States or the Company is eligible for the benefits of the U.S.-Canada income tax treaty (the “Treaty”), (2) we are not a PFIC (nor treated as such with respect to a U.S. Holder) at the time the dividend was paid or in the previous year, and (3) certain other requirements are met. U.S. Holders should consult their tax advisors regarding the availability of such lower rate for any dividends paid with respect to our Common Shares.
For U.S. foreign tax credit purposes, dividends paid on our Common Shares generally will be treated as foreign source income and generally will constitute passive category income. The amount of a dividend will include any amounts withheld by us in respect of Canadian income taxes. Subject to applicable limitations, some of which vary depending upon the U.S. Holder’s particular circumstances, Canadian income taxes withheld from dividends on the Common Shares, at a rate not exceeding any reduced rate pursuant to the Treaty, will be creditable against the U.S. Holder’s U.S. federal income tax liability. In lieu of claiming a foreign tax credit, U.S. Holders may, at their election, deduct foreign taxes, including any Canadian income taxes, in computing their taxable income, subject to generally applicable limitations under U.S. law. An election to deduct foreign taxes instead of claiming foreign tax credits applies to all foreign taxes paid or accrued in the taxable year. The rules governing foreign tax credits are complex and U.S. Holders should consult their tax advisers regarding the creditability or deductibility of foreign taxes in their particular circumstances.
The amount of any dividend paid in Canadian dollars will equal the U.S. dollar value of the Canadian dollars received, calculated by reference to the exchange rate in effect on the date the dividend is received by you, in the case of Common Shares, regardless of whether the Canadian dollars are converted into U.S. dollars. If the Canadian dollars received as a dividend are converted into U.S. dollars on the date of receipt, a U.S. Holder generally will not be required to recognize foreign currency gain or loss in respect of the dividend income. If the Canadian dollars received as a dividend are not converted into U.S. dollars on the date of receipt, a U.S. Holder will have a basis in the Canadian dollars equal to their U.S. dollar value on the date of receipt. Any gain or loss realized on a subsequent conversion or other disposition of the Canadian dollar will be treated as U.S. source ordinary income or loss.
Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Common Shares to U.S. Holders
Subject to the PFIC rules discussed below, a U.S. Holder generally will recognize capital gain or loss on the sale or other taxable disposition of our Common Shares. The amount of gain or loss recognized by a U.S. Holder on a sale or other taxable disposition generally will be equal to the difference between (i) the sum of the amount of cash and the fair market value of any property received in such disposition and (ii) the U.S. Holder’s adjusted tax basis in its Common Shares so disposed of. A U.S. Holder’s adjusted tax basis in its Common Shares generally will equal the U.S. Holder’s acquisition cost reduced by any prior distributions treated as a return of capital.
Any capital gain or loss recognized generally will be long-term capital gain or loss if the U.S. Holder’s holding period for such Common Shares exceeds one year. Long-term capital gain realized by a non-corporate U.S. Holder may be taxed at rates of taxation lower than the rates applicable to ordinary income and short-term capital gains, while short-term capital gains are subject to U.S. federal income tax at the rates applicable to ordinary income. The deductibility of capital losses is subject to various limitations.
Any gain or loss recognized by a U.S. Holder will generally be U.S. source gain or loss for foreign tax credit purposes. Consequently, a U.S. Holder may not be able to use the foreign tax credit arising from any non-U.S. tax imposed on the disposition of the Common Shares unless such credit can be applied (subject to applicable limitations) against tax due on other income treated as derived from non-U.S. sources.
59
Passive Foreign Investment Company (“PFIC”) Rules
A non-U.S. corporation will be classified as a PFIC for United States federal income tax purposes if either (i) at least 75% of its gross income in a taxable year, including its pro rata share of the gross income of any corporation in which it is considered to own at least 25% of the shares by value, is passive income (ii) at least 50% of its assets in a taxable year (ordinarily determined based on fair market value and averaged quarterly over the year), including its pro rata share of the assets of any corporation in which it is considered to own at least 25% of the shares by value, are held for the production of, or produce, passive income. Passive income generally includes, among other things, dividends, interest, rents and royalties (other than rents or royalties derived from the active conduct of a trade or business) and gains from the disposition of assets giving rise to passive income.
Although our PFIC status is determined annually, an initial determination that our Company is a PFIC generally will apply for subsequent years to a U.S. Holder who held Company Shares while we were a PFIC, whether or not we meet the test for PFIC status in those subsequent years. If we are determined to be a PFIC for any taxable year (or portion thereof) that is included in the holding period of a U.S. Holder of our Common Shares and the U.S. Holder did not make either a timely mark-to-market election or a qualified electing fund (“QEF”) election for our first taxable year as a PFIC in which the U.S. Holder held (or was deemed to hold) Common Shares, as described below, such U.S. Holder generally will be subject to special rules with respect to (i) any gain recognized by the U.S. Holder on the sale or other disposition of its Common Shares (which may include gain realized by reason of transfers of Common Shares that would otherwise qualify as nonrecognition transactions for United States federal income tax purposes) and (ii) any “excess distribution” made to the U.S. Holder (generally, any distributions to such U.S. Holder during a taxable year of the U.S. Holder that are greater than 125% of the average annual distributions received by such U.S. Holder in respect of the Common Shares during the three preceding taxable years of such U.S. Holder or, if shorter, such U.S. Holder’s holding period for the Common Shares). Under these special tax rules:
| ● | the U.S. Holder’s gain or excess distribution will be allocated ratably over the U.S. Holder’s holding period for the Common Shares; |
| ● | the amount allocated to the U.S. Holder’s taxable year in which the U.S. Holder recognized the gain or received the excess distribution, or to the period in the U.S. Holder’s holding period before the first day of our first taxable year in which we are a PFIC, will be taxed as ordinary income; |
| ● | the amount allocated to other taxable years (or portions thereof) of the U.S. Holder and included in its holding period will be taxed at the highest tax rate in effect for that year and applicable to the U.S. Holder without regard to the U.S. Holder’s other items of income and loss for such year; and |
| ● | an additional amount equal to the interest charge generally applicable to underpayments of tax will be imposed on the U.S. Holder with respect to the tax attributable to each such other taxable year of the U.S. Holder. |
In general, if we are determined to be a PFIC, a U.S. Holder may be able to avoid application of the PFIC tax consequences described above in respect to our Common Shares by making a timely and valid QEF election (if eligible to do so) to include in income its pro rata share of our net capital gains (as long-term capital gain) and other earnings and profits (as ordinary income), on a current basis, in each case whether or not distributed, in the taxable year of the U.S. Holder in which or with which our taxable year ends. A U.S. Holder generally may make a separate election to defer the payment of taxes on undistributed income inclusions under the QEF rules, but if deferred, any such taxes will be subject to an interest charge. There is no assurance that we will have timely knowledge of our status as a PFIC in the future or of the required information to be provided. We, therefore, have not determined whether, if we were to be classified as a PFIC for a taxable year, we will provide information necessary for a U.S. Holder to make a QEF election which, if available, would result in tax treatment different from (and generally less adverse than) the general tax treatment for PFICs. Accordingly, U.S. Holders should assume that they will not be able to make a QEF election with respect to the Common Shares.
Alternatively, if a U.S. Holder, at the close of its taxable year, owns shares in a PFIC that are treated as marketable stock, the U.S. Holder may make a mark-to-market election with respect to such shares for such taxable year. If the U.S. Holder makes a valid mark-to-market election for the first taxable year of the U.S. Holder in which the U.S. Holder holds (or is deemed to hold) Common Shares in us and for which we are determined to be a PFIC, such U.S. Holder generally will not be subject to the PFIC rules described above in respect to its Common Shares. Instead, in general, the U.S. Holder will include as ordinary income in each taxable year the excess, if any, of the fair market value of Common Shares at the end of its taxable year over its adjusted basis in its Common Shares. These amounts of ordinary income would not be eligible for the favorable tax rates applicable to qualified dividend income or long-term capital gains. The U.S. Holder also generally will recognize an ordinary loss in respect of the excess, if any, of its adjusted basis in its Common Shares over the fair market value of its Common Shares at the end of its taxable year (but only to the extent of the net amount of previously included income as a result of the mark-to-market election). The U.S. Holder’s basis in its Common Shares will be adjusted to reflect any such income or loss amounts, and any further gain recognized on a sale or other taxable disposition of its Common Shares will be treated as ordinary income.
60
The mark-to-market election is available only for stock that is regularly traded on a national securities exchange that is registered with the Securities and Exchange Commission or on a foreign exchange or market that the IRS determines has rules sufficient to ensure that the market price represents a legitimate and sound fair market value. If made, a mark-to-market election would be effective for the taxable year for which the election was made and for all subsequent taxable years unless the Common Shares ceased to qualify as “marketable stock” for purposes of the PFIC rules or the IRS consented to the revocation of the election. U.S. Holders are urged to consult their own tax advisors regarding the availability and tax consequences of a mark-to-market election in respect to our Common Shares under their particular circumstances.
If we are or become a PFIC and, at any time, have a non-U.S. subsidiary that is classified as a PFIC, U.S. Holders generally would be deemed to own a portion of the shares of such lower-tier PFIC, and generally could incur liability for the deferred tax and interest charge described above if we receive a distribution from, or dispose of all or part of our interest in, the lower-tier PFIC or the U.S. Holders otherwise were deemed to have disposed of an interest in the lower-tier PFIC. There can be no assurance that we will have timely knowledge of the status of any such lower-tier PFIC. In addition, we may not hold a controlling interest in any such lower-tier PFIC and thus there can be no assurance we will be able to cause the lower- tier PFIC to provide such required information. A mark-to-market election generally would not be available with respect to such lower-tier PFIC. U.S. Holders are urged to consult their tax advisors regarding the tax issues raised by lower-tier PFICs.
A U.S. Holder that owns (or is deemed to own) shares in a PFIC during any taxable year of the U.S. Holder, may have to file an IRS Form 8621, or any successor form, (whether or not a QEF or mark-to- market election is made) and such other information as may be required by the U.S. Treasury Department. Failure to do so, if required, will extend the statute of limitations until such required information is furnished to the IRS (potentially including with respect to items that do not relate to a U.S. Holder’s investment in Common Shares).
The rules dealing with PFICs and with the QEF and mark-to-market elections are very complex and are affected by various factors in addition to those described above. Accordingly, U.S. Holders of our Common Shares should consult their own tax advisors concerning the application of the PFIC rules to our Common Shares under their particular circumstances.
Information Reporting and Backup Withholding
Payments of dividends or sales proceeds that are made within the United States or through certain U.S.- related financial intermediaries may be subject to information reporting and backup withholding, unless (i) the U.S. Holder is a corporation or other exempt recipient, or (ii) in the case of backup withholding, the U.S. Holder provides a correct U.S. taxpayer identification number and certifies that it is not subject to backup withholding.
Backup withholding is not an additional tax. Any amounts withheld under the U.S. backup withholding rules will be allowed as a credit against a U.S. Holder’s U.S. federal income tax liability, if any, or will be refunded, if such U.S. Holder furnishes required information to the IRS in a timely manner. Each U.S. Holder should consult its own tax advisor regarding the information reporting and backup withholding rules in their particular circumstances and the availability of and procedures for obtaining an exemption from backup withholding.
Reporting Obligations for Certain Owners of Foreign Financial Assets
Certain U.S. Holders may be required to file an IRS Form 926 (Return by a U.S. Transferor of Property to a Foreign Corporation) to report a transfer of property (including cash) to us. Substantial penalties may be imposed on a U.S. Holder that fails to comply with this reporting requirement, and the period of limitations on assessment and collection of United States federal income taxes will be extended in the event of a failure to comply. Furthermore, certain U.S. Holders who are individuals and certain entities will be required to report information with respect to such U.S. Holder’s investment in “specified foreign financial assets” on IRS Form 8938 (Statement of Specified Foreign Financial Assets), subject to certain exceptions. Specified foreign financial assets generally include any financial account maintained with a non-U.S. financial institution and should also include the Common Shares if they are not held in an account maintained with a U.S. financial institution. Persons who are required to report specified foreign financial assets and fail to do so may be subject to substantial penalties, and the period of limitations on assessment and collection of United States federal income taxes may be extended in the event of a failure to comply. Potential investors are urged to consult their tax advisors regarding the foreign financial asset and other reporting obligations and their application to an investment in our Common Shares.
61
The discussion of reporting obligations set forth above is not intended to constitute an exhaustive description of all reporting obligations that may apply to a U.S. Holder. A failure to satisfy certain reporting obligations may result in an extension of the period during which the IRS can assess a tax, and under certain circumstances, such an extension may apply to assessments of amounts unrelated to any unsatisfied reporting obligation. Penalties for failure to comply with these reporting obligations are substantial. U.S. Holders should consult with their own tax advisors regarding their reporting obligations under these rules, including the requirement to file an IRS Form 8938.
Non-U.S. Holders
This section applies to you if you are a “Non-U.S. Holder.” As used herein, the term “Non-U.S. Holder” means a beneficial owner of our Common Shares that is for United States federal income tax purposes that is not a U.S. Holder, as defined above.
Taxation of Distributions to Non-U.S. Holders
A Non-U.S. Holder of our Common Shares will generally not be subject to U.S. federal income or withholding tax on dividends received on our Common Shares unless such income is effectively connected with the conduct by the holder of a U.S. trade or business.
Any distribution not constituting a dividend will be treated as first reducing the adjusted basis in the Non-U.S. Holder’s shares of our Common Shares and, to the extent it exceeds the adjusted basis in the Non-U.S. Holder’s shares of our Common Shares, as gain from the sale or exchange of such shares. Any such gain will be subject to the treatment described below under “— Gain on Sale or Other Disposition of our Common Shares to Non-U.S. Holders”.
Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Common Shares to Non-U.S. Holders
A Non-U.S. Holder of our Common Shares will not be subject to U.S. federal income or withholding tax on gain realized on the sale or other taxable disposition of our Common Shares of Common Shares, unless: such gain is effectively connected with the conduct by the holder of a U.S. trade or business; or in the case of gain realized by an individual holder, the holder is present in the United States for 183 days or more in the taxable year of the sale and certain other conditions are met.
| F. | Dividends and Paying Agents |
Not applicable.
| G. | Statement by Experts |
Not applicable.
| H. | Documents on Display |
We are subject to the reporting requirements of the U.S. Securities Exchange Act of 1934, as amended, or the Exchange Act, as applicable to “foreign private issuers” as defined in Rule 3b-4 under the Exchange Act. As a foreign private issuer, we are exempt from certain provisions of the Exchange Act. Accordingly, our proxy solicitations are not subject to the disclosure and procedural requirements of regulation 14A under the Exchange Act, transactions in our equity securities by our officers and directors are exempt from reporting and the “short-swing” profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. However, we file with the U.S. Securities and Exchange Commission an Annual Report on Form 20-F containing financial statements that have been examined and reported on, with and opinion expressed by an independent registered public accounting firm, and we submit reports to the U.S. Securities and Exchange Commission on Form 6-K containing (among other things) press releases and unaudited financial information for the first six months of each fiscal year. We post our Annual Report on Form 20-F on our website promptly following the filing of our Annual Report with the U.S. Securities and Exchange Commission. The information on our website is not incorporated by reference into this Annual Report.
This document and the exhibits thereto and any other document we file pursuant to the Exchange Act may be inspected without charge and copied at prescribed rates at the U.S. Securities and Exchange Commission public reference room at 100 F Street, N.E., Room 1580, Washington D.C. 20549. You may obtain information on the operation of the Securities and Exchange Commission’s public reference room in Washington, D.C. by calling the U.S. Securities and Exchange Commission at 1-800-SEC-0330.
The U.S. Securities and Exchange Commission maintains a website at www.sec.gov that contains reports, proxy and information statements and other information regarding registrants that make electronic filings with the U.S. Securities and Exchange Commission using its EDGAR (Electronic Data Gathering, Analysis, and Retrieval) system.
The documents concerning our company which are referred to in this document may also be inspected at our office located at 1a, 1b Learoyd Road, New Romney TN28 8XU, United Kingdom.
62
| I. | Subsidiary information |
We currently have the following significant subsidiaries:
| ● | Canmart Limited |
| ● | Cannahealth Limited |
| ● | Holigen Holdings Limited |
| ● | Bophelo Holdings Limited |
| ● | Bophelo Bio Science and Wellness (Pty) Limited* |
| ● | 1371011 BC Limited |
| * | Bophelo Bio Science and Wellness (Pty) Ltd.is in the process of being liquidated. |
| J. | Annual report to security holders |
Not Applicable.
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
The Company’s activities expose it to a variety of financial risks: market risk (including foreign exchange and interest rate risks), credit risk and liquidity risk. Risk management is the responsibility of the Company, which identifies, evaluates and, where appropriate, mitigates financial risks.
Foreign exchange risk
Foreign exchange risk: is the risk that the fair value of future cash flows for financial instruments will fluctuate because of changes in foreign exchange rates. The Company has not entered into any foreign exchange hedging contracts. The Company is exposed to currency risk from the British Pound (“GBP”), the European Union Euro (“EUR”) and Canadian dollar (“CAD”) through the following foreign currency denominated financial assets and liabilities:
| As at (expressed in GBP) | December
31, 2023 | December 31, 2022 | ||||||
| Financial assets | ||||||||
| Cash and cash held in trust | £ | 20,087 | £ | 75,315 | ||||
| Trade and other receivables | 104,753 | 149,223 | ||||||
| Loan receivable | 466,000 | 400,000 | ||||||
| £ | 590,840 | £ | 624,538 | |||||
| Financial liabilities | ||||||||
| Trade and other payables | £ | 998,092 | £ | 923,725 | ||||
| Loans and borrowings | 36,771 | 25,000 | ||||||
| £ | 1,034,863 | £ | 948,725 | |||||
| As at (expressed in EUR) | December
31, 2023 | December 31, 2022 | ||||||
| Financial assets | ||||||||
| Cash | € | 46,202 | € | 42,664 | ||||
| Trade and other receivables | 136,963 | 986,320 | ||||||
| € | 183,165 | € | 1,028,984 | |||||
| Financial liabilities | ||||||||
| Trade and other payables | € | 2,171,878 | € | 3,201,180 | ||||
| Loans and borrowings | 3,225,389 | 3,307,633 | ||||||
| € | 5,397,267 | € | 6,508,813 | |||||
63
| As at (expressed in CAD) | December
31, 2023 | December 31, 2022 | ||||||
| Financial assets | ||||||||
| Cash | $ | 22,949 | $ | 140,423 | ||||
| Marketable securities | — | 357,143 | ||||||
| $ | 22,949 | $ | 497,566 | |||||
| Financial liabilities | ||||||||
| Trade and other payables | $ | 3,356,916 | $ | 3,629,380 | ||||
| Due to related party | 2,744,510 | 810,206 | ||||||
| Holdback payable | 511,238 | 511,238 | ||||||
| Lease liabilities | 179,412 | 448,064 | ||||||
| Loans and borrowings | 340,469 | — | ||||||
| $ | 7,132,545 | $ | 5,398,888 | |||||
Based on the above net exposures as at December 31, 2023, assuming that all other variables remain constant, a 5% appreciation or deterioration of the USD against the GBP would result in a corresponding increase or decrease, respectively on the Company’s net income of approximately $17,000 (2022 — $13,000), EUR — $236,000 (2022 — $256,000) and CAD — $268,000 (2022 — $181,000).
Credit risk
Credit risk is the risk of financial loss to the Company if a partner or counterparty to a financial instrument fails to meet its contractual obligation and arises principally from the Company’s cash and accounts receivable. The carrying amounts of the financial assets represents the maximum credit exposure. The Company limits its exposure to credit risk on cash by placing these financial instruments with high-credit quality financial institutions.
At December 31, 2023, the Company was subject to a concentration of credit risk related to its accounts receivable as 81% (2022 — 85% from one customer) of the balance of amounts owing is from one customer. As at December 31, 2022, the Company recorded a bad debt expense of $332,715, within general and administrative expenses, as the amounts were deemed not collectible from the customer. The Company did not record any bad debt expense during the year ended December 31, 2023. As at December 31, 2023 and 2022, the expected credit lifetime credit losses for accounts receivable aged as current were nominal amounts. The Company considers a financial asset in default when internal or external information indicates that the Company is unlikely to receive the outstanding contractual amounts in full. A financial asset is written off when there is no reasonable expectation of recovering the contractual cash flows.
Liquidity risk
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they come due. The Company manages its liquidity risk by continuously monitoring forecasted and actual cash flows, as well as anticipated investing and financing activities and to ensure that it will have sufficient liquidity to meet its liabilities and commitments when due and to fund future operations. The Company’s trade and other payables are due within the current operating year.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
| A. | Debt Securities |
Not applicable.
| B. | Warrants and Rights |
Not applicable.
| C. | Other Securities |
Not applicable.
| D. | American Depositary Shares |
Not applicable.
64
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
Not applicable.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
Not applicable.
ITEM 15. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Financial Officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of December 31, 2023, as required by Rule 13a-15(b) under the Exchange Act. Based on that evaluation, our management has concluded that, as of December 31, 2023, our disclosure controls and procedures were not effective, because of a material weakness in our internal control over financial reporting, as discussed below.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Under the supervision and with the participation of our management, including our Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2023, based on the criteria set forth in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO 2013). Based on our evaluation under the criteria set forth in Internal Control — Integrated Framework, our management concluded that our internal control over financial reporting was not effective as of December 31, 2023 due to the material weakness described below.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
Management did not maintain effective internal control over financial reporting over the accounting for impairment of long-lived assets. Specifically, the internal controls with respect to the assessment of impairment indicators were not designed to effectively account for the impairment in our property and equipment and intangible assets. Additionally, the internal controls over identifying and disclosing related party transactions were determined to be ineffective. The Company will implement immediately an appropriate remedial measure whereby an additional review related to the identification of impairment triggering events will be put in place. Additionally, management will implement further controls to ensure proper disclosure and accounting for related party transactions. We will continue to monitor and evaluate the effectiveness of our internal controls and procedures over financial reporting on an ongoing basis and are committed to taking further action and implementing additional improvements as necessary.
This Annual Report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the Company’s registered public accounting firm pursuant to rules of the SEC that permit the Company to provide only management’s report in this Annual Report.
Inherent Limitations on Effectiveness of Controls
In designing and evaluating our disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Changes in Internal Control Over Financial Reporting
There has been no change in our internal control over financial reporting (as defined in Rule 13a-15(f) of the Exchange Act) for the fiscal year ended December 31, 2023 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
In January 2023, the Company terminated the employment of Trevor Scott and have stepped down as Chief Financial Officer of the Company immediately. On March 7, 2023, the Company appointed Shailesh Bhushan as Chief Financial Officer of the Company. On December 4, 2023, Mr. Bhushan resigned from his position as Chief Financial Officer of the Company and the Company appointed Gurcharn Deol as Chief Financial Officer of the Company to replace Mr. Bhushan.
65
ITEM 16. RESERVED
Not applicable.
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT
David Jenkins is a member of our board of directors and serves on our audit committee. Our board has determined that David Jenkins is an audit committee financial expert and satisfies the “independence” requirements of the U.S. Securities and Exchange Commission, the NASDAQ Marketplace Rules.
ITEM 16B. CODE OF ETHICS
We have adopted a code of conduct that applies to our directors, chief executive officer and all senior financial officers of our company, including the chief financial officer, chief accounting officer or controller, or persons performing similar functions. The code of conduct is publicly available on our website at www.akandacorp.com/investors.
Written copies are available upon request. If we make any substantive amendment to the code of conduct or grant any waivers, including any implicit waiver, from a provision of the code of conduct, we will disclose the nature of such amendment or waiver on our website.
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
We engaged Green Growth CPAs as our independent registered public accounting firm. Set forth below is a summary of the fees paid to Green Growth CPAs for services provided in fiscal year 2023 and 2022.
Accountant Fees and Services
| Period | 2023 | 2022 | ||||||
| Audit Fees | $ | 107,450.00 | $ | 140,968.00 | ||||
| Audit Related Fees (F1 and S3 review) | 20,000.00 | 15,000.00 | ||||||
| Tax Fees | — | — | ||||||
| All Other Fees | — | — | ||||||
| $ | 127,450.00 | $ | 155,968.00 | |||||
Audit Fees. Audit fees consists of services rendered by an independent registered public accounting firm for the audit of our consolidated financial statements and our internal control over financial reporting, review of the interim financial statements.
Audit-Related Fees. Audit-related fees relate to assurance and associated services that traditionally are performed by the independent auditor including SEC filings, comfort letter, consents and comment letters in connection with regulatory filings.
Tax Fees. Tax fees consist of services rendered by an external auditor for tax compliance, tax consulting and tax planning.
All Other Fees. All other fees are for any other permissible work that is not an Audit, Audit-Related or Tax Fee.
Pre-Approval Policies and Procedures
Our Audit Committee has adopted policies and procedures for the pre-approval of audit and non-audit services rendered by our independent registered public accounting firm. Pre-approval of an audit or non-audit service may be given as a general pre-approval, as part of the audit committee’s approval of the scope of the engagement of our independent registered public accounting firm, or on an individual basis. Any proposed services exceeding general pre-approved levels also requires specific pre-approval by our audit committee. The policy prohibits retention of the independent registered public accounting firm to perform the prohibited non-audit functions defined in Section 201 of the Sarbanes-Oxley Act or the rules of the Securities and Exchange Commission, and also requires the audit committee to consider whether proposed services are compatible with the independence of the registered public accounting firm.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
66
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
Not applicable.
ITEM 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
The
Company dismissed
The Company’s change of auditor is described on its Current Report on Form 6-K filed with the SEC on March 21, 2023, which is incorporated herein by reference.
ITEM 16G. CORPORATE GOVERNANCE
Under NASDAQ Stock Market Rule 5615(a)(3), foreign private issuers, such as our company, are permitted to follow certain home country corporate governance practices instead of certain provisions of the NASDAQ Stock Market Rules. A foreign private issuer that elects to follow a home country practice instead of any such NASDAQ rules must submit to NASDAQ, in advance, a written statement from an independent counsel in such issuer’s home country certifying that the issuer’s practices are not prohibited by the home country’s laws. We submitted such a written statement to NASDAQ. See “Item 6. Directors, Senior Management and Employees—C. Board Practices—Corporate Governance Requirements — Nasdaq Global Market Marketplace Rules” for a summary of such differences.
ITEM 16H. MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
ITEM 16J. INSIDER TRADING POLICIES
We have an Insider Trading Policy pursuant to SEC Rule 10b-5. See Exhibit 11.4 of this annual report on Form 20-F.
ITEM 16K. CYBERSECURITY
We use SaaS based information technology (IT) systems/services which are provided by reputable vendors, our IT environment is audited by third party IT auditors for IT risk management regularly. The Company takes care of access management and related threats. Logs, threats, and alerts are monitored and dealt with by the Company on a regular basis. All system/services being used (Microsoft (email/SharePoint/teams) are all cloud-based provided by reputable cloud vendors and their IT environments are audited by third party auditors for IT risk management. Information is analyzed on potential threats to the organization, identifying what threats each of our IT assets may face and from where those may originate. All server level threats are taken care of by systems providers under SaaS model. There were no cyber-attacks, virus infection or security breach during the prior fiscal year. The Company is not aware of or been informed of instances of noncompliance with any regulatory requirements associated with IT. The information system has not experienced a significant loss of data that could not be restored from backup systems.
67
PART III
ITEM 17. FINANCIAL STATEMENTS
We have elected to furnish financial statements and related information specified in Item 18.
ITEM 18. FINANCIAL STATEMENTS
The following financial statements are filed as part of this annual report on Form 20-F.
68
Index to Financial Statements
Akanda Corp.
F-1
Report of Independent Registered Public Accounting Firm

To the shareholders and the board of directors of Akanda Corp.
Opinion on the Financial Statements
We have audited the accompanying consolidated statement of financial position of Akanda Corp. (the “Company”), as of December 31, 3023 and 2022, the related consolidated statements of comprehensive loss, changes in shareholders’ equity (deficit) and cash flows for the years then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2023 and 2022, and the results of its operations and its cash flows for the years then ended, in conformity with the International Financial Reporting Standards as issued by the International Accounting Standards Board.
Consideration of the Company’s Ability to Continue as a Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company’s significant operating losses raise substantial doubt about its ability to continue as a going concern. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provides a reasonable basis for our opinion.

We have served as the Company’s auditor since 2022
April 30, 2024
PCAOB ID Number
F-2
Akanda Corp.
Consolidated Statements of Financial Position
(Expressed in United States Dollars)
| As at | December 31, | December 31, | ||||||||
| Note | 2023 | 2022 | ||||||||
| ASSETS | ||||||||||
| Current | ||||||||||
| Cash | $ | $ | ||||||||
| Cash held in trust | ||||||||||
| Trade and other receivables | 6 | |||||||||
| Prepayments | ||||||||||
| Biological assets | 7 | |||||||||
| Inventory | 7 | |||||||||
| Marketable securities | 8 | |||||||||
| Total Current Assets | ||||||||||
| Non-Current | ||||||||||
| Property, plant and equipment | 9 | |||||||||
| Intangible assets | 11 | |||||||||
| Loan receivable | 12 | |||||||||
| Right-of-use assets | 10 | |||||||||
| Total Non-Current Assets | ||||||||||
| Total Assets | $ | $ | ||||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY (DEFICIT) | ||||||||||
| Current | ||||||||||
| Trade and other payables | $ | $ | ||||||||
| Lease liability | 13 | |||||||||
| Loans and borrowings | 14 | |||||||||
| Holdback payable | 4 | |||||||||
| Due to related parties | 16 | |||||||||
| Total Current Liabilities | ||||||||||
| Non-Current | ||||||||||
| Lease liability | 13 | |||||||||
| Loans and borrowings | 14 | |||||||||
| Total Non-Current Liabilities | ||||||||||
| Total Liabilities | ||||||||||
| Shareholders’ Equity (Deficit) | ||||||||||
| Share capital | 15 | |||||||||
| Other reserves | 15 | |||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||||
| Accumulated other comprehensive loss | ( | ) | ( | ) | ||||||
| Total Shareholders’ Equity (Deficit) | ( | ) | ||||||||
| Total Liabilities and Shareholders’ Equity (Deficit) | $ | $ | ||||||||
Subsequent Events (Note 23)
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Akanda Corp.
Consolidated Statements of Loss and Comprehensive Loss
(Expressed in United States Dollars)
| Year ended | ||||||||||
| December 31, | ||||||||||
| Note | 2023 | 2022 | ||||||||
| Sales | $ | $ | ||||||||
| Cost of sales | 7 | |||||||||
| Gross Profit before gain (loss) on change in fair value of biological assets and inventory | ||||||||||
| Gain (Loss) on change in fair value of biological assets and inventory | 7 | ( | ) | |||||||
| Gross Profit | ||||||||||
| Operating expenses | ||||||||||
| Depreciation and amortization | 9,10,11 | |||||||||
| Consulting and professional fees | ||||||||||
| Personnel expenses | 16 | |||||||||
| Share-based payment expenses to social development trust | 15 | |||||||||
| General and administrative expenses | ||||||||||
| Total operating expenses | ||||||||||
| Operating loss | ( | ) | ( | ) | ||||||
| Other income (expenses): | ||||||||||
| Finance income | ||||||||||
| Finance expense | ( | ) | ( | ) | ||||||
| Foreign exchange gain (loss), net | ( | ) | ||||||||
| Gain on bargain purchase | 4 | |||||||||
| Gain on debt settlement | ||||||||||
| Other income | ||||||||||
| Change in fair value of financial assets measured at FVTPL | 8 | ( | ) | ( | ) | |||||
| Loss on disposal of assets | 9 | ( | ) | |||||||
| Write-off of AP, net | ||||||||||
| Impairment loss | 9,11 | ( | ) | |||||||
| ( | ) | |||||||||
| Net loss from continuing operations | ( | ) | ( | ) | ||||||
| Loss from discontinued operations | 5 | ( | ) | |||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||||
| Translation adjustment | ( | ) | ( | ) | ||||||
| Comprehensive loss | $ | ( | ) | $ | ( | ) | ||||
| 15 | $ | ( | ) | $ | ( | ) | ||||
| 15 | $ | ( | ) | $ | ( | ) | ||||
| Weighted average common shares outstanding | 15 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Akanda Corp.
Consolidated Statements of Shareholders’ Equity (Deficit)
(Expressed in United States Dollars)
| Note | Share capital | Other reserves | Accumulated deficit | Accumulated other comprehensive loss | Total | |||||||||||||||||
| Balance, December 31, 2021 | $ | $ | $ | ( | ) | $ | $ | ( | ) | |||||||||||||
| Issuance of shares for Holigen Acquisition | 4,15 | |||||||||||||||||||||
| Issuance of shares to ASDT | 15 | |||||||||||||||||||||
| Issuance of shares from private placement | 15 | |||||||||||||||||||||
| Issuance of shares from IPO | 15 | |||||||||||||||||||||
| Issuance of shares upon conversion of note | 15,16 | |||||||||||||||||||||
| Fair value of RSUs issued at $ | 15 | |||||||||||||||||||||
| Fair value of RSUs issued at $ | 15 | |||||||||||||||||||||
| Fair value of RSUs issued at $ | 15 | |||||||||||||||||||||
| Fair value of RSUs issued at $ | 15 | |||||||||||||||||||||
| Fair value of RSUs issued at $ | 15 | |||||||||||||||||||||
| Loss of control of Bophelo Bio | 5 | ( | ) | ( | ) | ( | ) | ( | ) | |||||||||||||
| Net loss | ( | ) | ( | ) | ||||||||||||||||||
| Translation adjustment | ( | ) | ( | ) | ||||||||||||||||||
| Balance, December 31, 2022 | ( | ) | ( | ) | ||||||||||||||||||
| Fair value of RSUs issued at $ | 15 | |||||||||||||||||||||
| Fair value of RSUs issued at $ | 15 | |||||||||||||||||||||
| Fair value of RSUs issued at $ | 15 | |||||||||||||||||||||
| Cancelled shares | 15 | ( | ) | ( | ) | |||||||||||||||||
| Issuance of shares upon conversion of note | 15 | |||||||||||||||||||||
| Issuance of shares pursuant to the first option payment to acquire a certain land property | 15 | |||||||||||||||||||||
| Net loss | ( | ) | ( | ) | ||||||||||||||||||
| Translation adjustment | ( | ) | ( | ) | ||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Akanda Corp.
Consolidated Statements of Cash Flows
(Expressed in United States Dollars)
| Years ended December 31, | ||||||||||
| Note | 2023 | 2022 | ||||||||
| Cash flows from operating activities: | ||||||||||
| Net loss from continuing operations | $ | ( | ) | $ | ( | ) | ||||
| Net loss from discontinued operations | ( | ) | ||||||||
| Net loss for the year | ( | ) | ( | ) | ||||||
| Adjustments for non-cash items: | ||||||||||
| Loss on loss of control of Bophelo Bio, net of cash surrendered | 5 | |||||||||
| Gain on bargain purchase | 4 | ( | ) | |||||||
| Share based payments to social development trust | 15 | |||||||||
| Depreciation and amortization | 9,10,11 | |||||||||
| Change in fair value of biological assets | 7 | ( | ) | |||||||
| Change in fair value of financial asset at fair value through profit or loss | 8 | |||||||||
| Stock-based compensation | 15 | |||||||||
| Interest expenses | ||||||||||
| Fair value of RSUs exercised, net of cancelled shares | 15 | |||||||||
| Gain on settlement on debt | ( | ) | ( | ) | ||||||
| Loss on disposal of asset | 9 | |||||||||
| Write-off of AP, net | ( | ) | ||||||||
| Impairment loss | 9,11 | |||||||||
| Working capital adjustments (net of amounts acquired/disposed): | ||||||||||
| Trade and other receivables | ( | ) | ||||||||
| Prepayments | ( | ) | ||||||||
| Inventory | ||||||||||
| Trade and other payables | ||||||||||
| Due to related parties | ||||||||||
| Cash flows used in operating activities | ( | ) | ( | ) | ||||||
| Cash flows from investing activities: | ||||||||||
| Acquisition of Holigen, net of cash acquired and holdback | 4 | ( | ) | |||||||
| Purchase of financial assets at fair value through profit or loss | 8 | ( | ) | |||||||
| Additions to property, plant and equipment | 9 | ( | ) | ( | ) | |||||
| Cash surrendered on loss of control of Bophelo Bio Sciences | 5 | ( | ) | |||||||
| Loan receivable | 12 | ( | ) | |||||||
| Proceeds from disposal of property, plant and equipment | ||||||||||
| Cash flows provided by (used in) investing activities | ( | ) | ||||||||
| Cash flows from financing activities: | ||||||||||
| Proceeds from IPO, net of costs | ||||||||||
| Proceeds from private placement, net of costs | ||||||||||
| Advances from related parties | ||||||||||
| Loans received | ||||||||||
| Loans repaid | ( | ) | ( | ) | ||||||
| Lease payments | ( | ) | ( | ) | ||||||
| Cash flows provided by financing activities | ||||||||||
| Net increase (decrease) in cash and cash equivalents | ( | ) | ||||||||
| Effects of exchange rate changes on cash and cash equivalents | ( | ) | ( | ) | ||||||
| Cash and cash equivalents at the beginning of the year | ||||||||||
| Cash and cash equivalents at the end of the year | $ | $ | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 1. | Nature of Operations and Going Concern |
Akanda Corp. (the “Company”) is domiciled in Canada and was incorporated on July 16, 2021. The Company’s registered office is 77 King Street West, Suite 400, Toronto-Dominion Centre, Toronto Canada, Ontario, M5K 0A1.
Prior to the liquidation event on July
15, 2022 described below, the Company, through its indirectly held subsidiary, Bophelo Bio Science and Wellness (Pty) Ltd. is in the business
of cultivating and manufacturing cannabis biomass and medical cannabis products in Lesotho (specifically near Ts’akholo, in the
Mafeteng district of the Kingdom of Lesotho, Southern Africa), for export to international markets. At December 31, 2022, the Company
determined that it no longer controlled Bophelo Bio Science and Wellness (Pty) Ltd. as a result of the insolvent liquidation order signed
by the Lesotho Court on July 15, 2022 (note 5). As a result of the loss of control, the Company derecognized all assets and liabilities
at their book values on December 31, 2022 and wrote down all balances receivable from the entity to $. During the year ended December
31, 2022, the Company recorded a loss on loss of control of Bophelo Bio Science and Wellness (Pty) Ltd. of $
The Company was incorporated for the designed purpose of becoming the ultimate parent company of Cannahealth Ltd. (“Cannahealth”), through a reorganization of entities with common control. The share purchase agreement became unconditional on or about November 3, 2021 and the Company acquired the shares in the aforementioned entities from Halo Collective Inc. (“Halo”).
On April 29, 2022, the Company, through
its wholly owned subsidiary, Cannahealth, acquired
The Company’s consolidated financial
statements have been prepared on a going concern basis which assumes that the Company will be able to realize its assets and discharge
its liabilities in the normal course of business for the foreseeable future. The Company incurred a net cash outflow of $
The Company is an early-stage company and is primarily dependent on externally provided financing to continue as a going concern. Additional funds will be required to enable the Company to pursue such an initiative and the Company may be unable to obtain such financing on satisfactory terms. Furthermore, there is no assurance that the Company will be profitable. Management intends to finance operating costs over the next twelve months with its cash on hand, and/or additional cash that will be generated from operations. The Company does not at this stage have any firm plans or commitments regarding further financing.
These uncertainties may cast significant doubt upon the Company’s ability to continue as a going concern. These consolidated financial statements do not include any adjustments relating to the recoverability and classification of assets and liabilities which might be necessary should the Company be unable to continue in existence.
F-7
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 2. | Basis of Preparation |
| (a) | Statement of compliance |
These consolidated financial statements, including comparatives, have been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standards Board (“IASB”) and interpretations issued by the International Financial Reporting Interpretations Committee (“IFRIC”).
| (b) | Basis of preparation |
These consolidated financial statements have been prepared on an accrual basis, except for cash flow information, and are based on the historical cost, modified where applicable and related to the valuation of certain financial assets and financial liabilities to fair value.
Cannahealth and its subsidiaries, Bophelo Holdings Ltd, Bophelo Bio Science and Wellness (Pty) Ltd, and Canmart were under the common control of Halo until the acquisition by the Company had occurred. As of November 2021, shareholdings in each of the three separate entities were made consistent through the issuance of shares or the repurchase of shares for cash to the relevant shareholders (the ‘Reorganization Transactions’). As of November 2021, shareholdings in each of the four entities were identical. When the Company was formed in July 2021 with a view to ultimately acquiring Cannahealth and its subsidiaries, its majority shareholders were also consistent with each of the three existing entities. Therefore, immediately prior to the acquisition, the majority shareholder ownership of the Company and Cannahealth were demonstrated common control, and immediately after the acquisition, the shareholdings held in the Company by each individual shareholder were also identical.
The Company performed an assessment and determined Bophelo Bio Science and Wellness (Pty) Ltd to be the predecessor entity to the Company, and that the corporate restructuring in which Akanda became the parent company did not have economic substance. As such, in preparing the Company’s consolidated financial statements, the Company accounted for the acquisition in as a transaction between entities under common control combining the Company and Cannahealth from the earliest reporting date using the ‘pooling of interests method’ of accounting, where assets for the Companies that came under common control were transferred into the consolidated group at the book value on the date in which common control was achieved.
In the acquisition described above, shares were issued to existing shareholders for no consideration. Therefore, the number of shares outstanding was increased without an increase in resources. The number of shares outstanding before the exchange have been adjusted for the change in shares as if the issuance had occurred at the beginning of the earliest period presented. All share and per share information presented herein has been retrospectively adjusted to give effect to the culmination of the reorganization and the issuance of shares on incorporation of Akanda on January 1, 2019.
| (c) | Functional and presentation currency |
The Company and its subsidiaries are measured using the currency of the primary economic environment in which each subsidiary operates - the functional currency. The Euro is the functional currency of RPK, Holigen and Cannahealth, Great British Pounds is the functional currency of Canmart and Canadian Dollars is the functional currency of 1371011 and Akanda while the United States Dollars is its reporting currency.
These consolidated financial statements are prepared and presented in United States Dollars (“USD” or “$”), which is the Company’s reporting currency. All financial information has been rounded to the nearest dollar except where indicated otherwise.
F-8
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 2. | Basis of Preparation (continued) |
| (d) | Use of estimates and judgments |
The preparation of consolidated financial statements in conformity with IFRS requires management to make estimates, judgements and assumptions that affect the application of accounting policies and the reported amounts of assets, liabilities, revenue and expenses during the year. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any future periods affected. Areas in which management has made critical judgments in the process of applying accounting policies and that have the most significant effect on the amounts recognized in the consolidated financial statements include the determination of the Company’s and its subsidiaries’ functional currencies. Information about key assumptions and estimation uncertainties that have a significant risk of resulting in a material adjustment to the carrying amount of assets and liabilities within the next financial year are included in the following notes to the consolidated financial statements for the years ended December 31, 2023 and 2022:
| ● | Note 3(d): Estimates of variable consideration receivable from revenue from contracts with customers |
| ● | Note 3(f): Estimates of the net realizable value of the Company’s inventories |
| ● | Note 3(g): Estimates of the fair value of the Company’s biological assets |
| ● | Note 3(h): Measurement and useful lives of the Company’s property, plant and equipment |
| ● | Note 3(i): Measurement and useful lives of the Company’s intangible assets |
| ● | Note 3(k): Estimates and assessment of the income tax assets/liabilities |
| ● | Note 3(l): Estimates of the Company’s incremental borrowing rate used in the valuation of its leases |
| 3. | Material Accounting Policies |
| (a) | Basis of consolidation |
These consolidated financial statements include the accounts of the Company and its subsidiaries. Subsidiaries are entities that are controlled by the Company. Control exists when the Company has power over the investee and the Company is exposed or has the rights to variable returns from the investee. Subsidiaries are included in the consolidated financial results of the Company from the effective date of acquisition up to the effective date of disposition or loss of control. The financial statements of the subsidiaries are prepared for the same reporting period as the parent company, using consistent accounting policies. All intercompany transactions and balances and unrealized gains and losses from intercompany transactions have been eliminated.
| Country of Incorporation | Holding | Functional Currency | ||||
| Cannahealth Ltd. (“Cannahealth”) | ||||||
| Bophelo Holdings Ltd. (“Bophelo H”) | ||||||
| Bophelo Bio Science and Wellness (Pty) Ltd. (“Bophelo”) | ||||||
| Canmart Ltd. (“Canmart”) | ||||||
| Holigen Holdings Limited (“Holigen”) | ||||||
| RPK Biopharma Unipessoal Lda. (“RPK”) | ||||||
| 1371011 BC Ltd. (“1371011”) |
F-9
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 3. | Material Accounting Policies (continued) |
| (b) | Foreign currency |
Items included in the financial statements of each of the Company’s consolidated subsidiaries are measured using the currency of the primary economic environment in which each subsidiary operates (the functional currency). The consolidated financial statements are presented in USD. All assets and liabilities in each statement of financial position are translated at the closing rate at the date of that statement of financial position. All income and expenses are translated at exchange rates at the dates of the transactions.
Foreign currency transactions are translated into the respective functional currencies of the Company and its subsidiaries using the exchange rates prevailing at the dates of the transactions. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation at period-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognized in profit and loss. Non-monetary items that are not carried at fair value are translated using the exchange rates as at the date of the initial transaction. Non-monetary items measured at fair value in a foreign currency are translated using the exchange rates at the date when the fair value is determined.
The results and financial position of the Company’s foreign subsidiaries that have a different functional currency from the Company’s functional and presentation currency are translated into USD as follows:
| (i) | Assets and liabilities of the foreign subsidiary are translated at the closing exchange rate on the date of the consolidated statement of financial position; |
| (ii) | Revenue and expenses of the foreign subsidiary are translated at the average closing exchange rate for the period reported in the consolidated statement of profit or loss. When the average exchange rate does not provide a reasonable approximation of the cumulative effect of the rates prevailing on the transaction date, the Company utilizes the closing exchange rate on the date of the transaction; |
| (iii) | The exchange rate differences for foreign subsidiaries are recognized in other comprehensive income in the cumulative translation account |
| (c) | Financial instruments |
| (i) | Financial assets |
The Company initially recognizes a financial asset on the trade date at which the Company becomes a party to the contractual provisions of the instrument.
Upon recognition of a financial asset, classification is made based on the business model for managing the asset and the asset’s contractual cash flow characteristics. The financial asset is initially recognized at its fair value and subsequently classified and measured as (i) amortized cost; (ii) fair value through other comprehensive income (“FVOCI”); or (iii) FVTPL. Financial assets are classified as FVTPL if they have not been classified as measured at amortized cost or FVOCI.
The Company derecognizes a financial asset when the contractual rights to the cash flows from the asset expire, or it transfers the rights to receive the contractual cash flows on the financial asset in a transaction in which substantially all the risks and rewards of ownership of the financial asset are transferred. Financial assets and liabilities are offset and the net amount presented in the consolidated statements of financial position when, and only when, the Company has a legal right to offset the amounts and intends either to settle on a net basis or to realize the asset and settle the liability simultaneously. The Company has classified all of its financial assets as financial assets measured at amortized cost or FVTPL. The Company has not classified any financial assets as FVTPL or FVOCI.
F-10
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 3. | Material Accounting Policies (continued) |
| (c) | Financial instruments (continued) |
Financial assets measured at amortized cost
A non-derivative financial asset is measured at amortized cost when both of the following conditions are met: (i) the asset is held within a business model whose objective is to hold assets in order to collect the contractual cash flows; and (ii) the contractual terms of the financial asset give rise on specified dates to cash flows that are solely payments of principal and interest on the principal amount outstanding. Such assets are recognized initially at fair value plus any directly attributable transaction costs and measured at amortized cost using the effective interest method subsequent to initial recognition, loans and receivables are measured at amortized cost. Financial assets measured at amortized cost are comprised of cash and trade and other receivables.
| (ii) | Financial liabilities |
The Company recognizes a financial liability on the trade date in which it becomes a party to the contractual provisions of the instrument at fair value plus any directly attributable costs. Financial liabilities are subsequently measured at amortized cost or FVTPL and are not subsequently reclassified. The Company’s financial liabilities are trade and other payables and loans and borrowings which are recognized on an amortized cost basis.
Financial liabilities measured at amortized cost
All financial liabilities are recognized initially on the trade date at which the Company becomes a party to the contractual provisions of the instrument. Such financial liabilities are recognized initially at fair value plus any directly attributable transaction costs. All financial liabilities are measured at amortized cost, except for financial liabilities measured at FVTPL. A financial liability may no longer be reclassified subsequent to initial recognition. Subsequent to initial recognition, financial liabilities are measured at amortized cost using the effective interest method.
The Company derecognizes a financial liability when its contractual obligations are discharged or cancelled, or when they expire. The Company has the following non-derivative financial liabilities which are classified as financial liabilities measured at amortized cost: trade and other payables and loans and borrowings.
| (d) | Revenue from contracts with customers |
Revenue is measured based on the consideration specified in a contract with a customer. The Company recognizes revenue when it transfers control over a good or service to a customer. The Company records revenue upon transfer of promised goods or services to customers in amounts that reflect the consideration to which the Company expects to be entitled in exchange for those goods or services based on the following five step approach:
Step 1: Identify the contracts with customers;
Step 2: Identify the performance obligations in the contract;
Step 3: Determine the transaction price;
Step 4: Allocate the transaction price to the performance obligations in the contract; and
Step 5: Recognize revenue as performance obligations are satisfied.
The Company typically satisfies its performance obligations at a point in time, upon completion of sale. The Company primarily acts as principal in contracts with its customers. The Company does not have material obligations for returns, refunds and other similar obligations, nor warranties and related obligations.
F-11
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 3. | Material Accounting Policies (continued) |
| (d) | Revenue from contracts with customers (continued) |
Revenue is recognized at the amount of the transaction price that is allocated to the performance obligation. The transaction price is the amount of consideration to which an entity expects to be entitled in exchange for transferring promised goods or services to a customer.
The Company has a single revenue stream currently that relates to the sale of cannabis-based products for medicinal use. This revenue stream is assessed as one performance obligation. Revenue from cannabis based medicinal product sales is recognized once the performance obligation has been satisfied, which would be upon the customer taking the delivery of the product. The transaction price for each product and service will be determined based on the respective invoice.
The Company exercises judgments in determining the amount of the costs incurred to obtain or fulfil a contract with a customer, which includes, but is not limited to (a) the likelihood of obtaining the contract, (b) the estimate of the profitability of the contract, and (c) the credit risk of the customer. An impairment loss will be recognized in profit or loss to the extent that the carrying amount of the asset exceeds (a) the remaining amount of consideration that the entity expects to receive in exchange for the goods or services to which the asset relates, less (b) the costs that relate directly to providing those goods or services and that have not been recognized as expenses.
| (e) | Cash and cash equivalents |
The Company considers all liquid investments purchased with a maturity of three months or less at acquisition to be cash and cash equivalents, which are carried and classified at amortized cost. The Company did not hold any cash equivalents as of December 31, 2023 and 2022.
| (f) | Inventories |
Inventories consist of raw materials and are measured at the lower of cost and net realizable value. The cost of inventories is based on the first-in first-out principle, and includes expenditures incurred in acquiring the inventories and other costs incurred in bringing them to their existing location and condition. Inventories are written down to net realizable value when the cost of inventories is estimated to be unrecoverable due to obsolescence, damage, or declining selling prices. Net realizable value is the estimated selling price in the ordinary course of business, less the estimated costs of completion and selling expenses. When the circumstances that previously caused inventories to be written down below cost no longer exist, or when there is clear evidence of an increase in selling prices, the amount of the write-down previously recorded is reversed.
| (g) | Biological assets |
Biological assets are measured at their fair value less costs to sell in the consolidated statement of financial position. The Company’s method of accounting for biological assets attributes value accretion on a straight-line basis throughout the life of the biological asset from initial cloning to the point of harvest. All direct and indirect costs of biological assets are capitalized as they are incurred.
Biological assets and produce held by the Company is planned to be used in four possible ways:
| ● | Sale to the export market; | |
| ● | Sale to the local market; | |
| ● | Repurposed for use in research and development; and | |
| ● | Written off for being obsolete. |
F-12
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 3. | Material Accounting Policies (continued) |
| (h) | Property, plant and equipment |
| (i) | Recognition and measurement |
Items of property and equipment are measured at cost less accumulated depreciation and accumulated impairment losses. When parts of an item of property and equipment have different estimated useful lives, they are accounted for as separate items within property and equipment. The costs of the ongoing regular servicing of property and equipment are recognized in tin the period in which they are incurred.
| (ii) | Depreciation |
| Plant and equipment | ||
| Leasehold improvements | ||
| Motor Vehicles | ||
| Computers | ||
| Furniture and fixtures |
| (i) | Intangible assets |
Intangible assets are recorded at cost
less amortization and impairment losses, if any. The Company had a cannabis operator’s license in Lesotho, held by its subsidiary
Bophelo, which was valid for 10 years and was subject to a renewal at the end of the 10 years. The license is automatically
renewed annually on payment of necessary fees as well as submission of operational documents to the Ministry of Health. As a result of
loss of control of Bophelo, the license was derecognized and recorded as a loss on the consolidated statement of loss and comprehensive
loss. The Company also has a cannabis API manufacturing and GMP license in Portugal, held by its newly acquired subsidiary Holigen-RPK,
which is valid for 10 years. Pursuant to the Company’s upcoming sale of RPK (note 23), its Portuguese subsidiary, the Company impaired
a portion of its intangible assets and recognized an impairment loss of $
Additionally, the Company has cannabis distribution licenses in the United Kingdom held by its subsidiary, Canmart which have been assessed as having an indefinite useful life. As such, these licenses are not amortized but their recoverable amounts are tested annually for impairment. The indefinite intangible assets are recorded at cost less impairment losses, if any. The Company capitalizes the initial license application cost as the cost of intangible assets while the annual license renewal fees are expensed in the year during which they occur.
| (j) | Impairment of non-financial assets |
The Company assesses at each reporting period whether there is an indication that a non-financial asset may be impaired. An impairment loss is recognized when the carrying amount of an asset, or its cash generating unit (“CGU”), exceeds its recoverable amount. A CGU is the smallest identifiable group of assets that generates cash inflows that are largely independent of the cash inflows from other assets or groups of assets. The recoverable amount is the greater of the assets or CGU’s fair value less costs to sell and value in use. In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset or CGU. In determining fair value less cost to sell, an appropriate valuation model is used. For an asset that does not generate largely independent cash inflows, the recoverable amount is determined for the CGU to which the asset belongs.
Impairment losses recognized in prior periods are assessed at each reporting date for any indications that the loss has decreased or no longer exists. An impairment loss is reversed if there has been a change in the estimates used to determine the recoverable amount. An impairment loss is reversed only to the extent that the asset’s carrying amount does not exceed the carrying amount that would have been determined, net of amortization, if no impairment loss had been recognized.
F-13
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 3. | Material Accounting Policies (continued) |
| (k) | Income taxes |
Income tax expense comprises current and deferred taxes. Current taxes and deferred taxes are recognized in profit or loss except to the extent that it relates to a business combination, or items recognized directly in equity or in other comprehensive loss.
Current taxes are the expected tax receivable or payable on the taxable income or loss for the year, using tax rates enacted or substantively enacted at the reporting date, and any adjustment to tax receivable or payable in respect of previous years. Deferred taxes are recognized in respect of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for taxation purposes.
Deferred taxes are not recognized for the following temporary differences: the initial recognition of assets or liabilities in a transaction that is not a business combination and that affects neither accounting nor taxable profit or loss, and differences relating to investments in subsidiaries and jointly controlled entities to the extent that it is probable that they will not reverse in the foreseeable future.
In addition, deferred taxes are not recognized for taxable temporary differences arising on the initial recognition of goodwill. Deferred taxes are measured at the tax rates that are expected to be applied to temporary differences when they reverse, based on the tax laws that have been enacted or substantively enacted by the reporting date. Deferred tax assets and liabilities are offset if there is a legally enforceable right to offset current tax assets and liabilities, and they relate to income taxes levied by the same tax authority on the same taxable entity, or on different tax entities, but they intend to settle current tax assets and liabilities on a net basis or their tax assets and liabilities will be realized simultaneously.
A deferred tax asset is recognized for unused tax losses, tax credits and deductible temporary differences, to the extent that it is probable that future taxable profits will be available against which they can be utilized. Deferred tax assets are reviewed at each reporting date and are reduced to the extent that it is no longer probable that the related tax benefit will be realized.
| (l) | Leases |
At inception of a contract, the Company assesses whether a contract is, or contains, a lease. A contract is, or contains, a lease if the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration. The Company assesses whether the contract involves the use of an identified asset, whether the right to obtain substantially all of the economic benefits from use of the asset during the term of the arrangement exists, and if the Company has the right to direct the use of the asset. At inception or on reassessment of a contract that contains a lease component, the Company allocates the consideration in the contract to each lease component on the basis of their relative standalone prices.
As a lessee, the Company recognizes a right-of-use asset and a lease liability at the commencement date of a lease. The right-of-use asset is initially measured at cost, which is comprised of the initial amount of the lease liability adjusted for any lease payments made at or before the commencement date, plus any decommissioning and restoration costs, less any lease incentives received.
The right-of-use asset is subsequently depreciated from the commencement date to the earlier of the end of the lease term, or the end of the useful life of the asset. In addition, the right-of-use asset may be reduced due to impairment losses, if any, and adjusted for certain remeasurements of the lease liability.
F-14
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 3. | Material Accounting Policies (continued) |
| (l) | Leases (continued) |
A lease liability is initially measured at the present value of the lease payments that are not paid at the commencement date, discounted by the interest rate implicit in the lease, or if that rate cannot be readily determined, the incremental borrowing rate. Lease payments included in the measurement of the lease liability are comprised of:
| a) | fixed payments, including in-substance fixed payments, less any lease incentives receivable; |
| b) | variable lease payments that depend on an index or a rate, initially measured using the index or rate as at the commencement date; |
| c) | amounts expected to be payable under a residual value guarantee; |
| d) | exercise prices of purchase options if the Company is reasonably certain to exercise that option; and |
| e) | payments of penalties for terminating the lease, if the lease term reflects the lessee exercising an option to terminate the lease. |
The lease liability is measured at amortized cost using the effective interest method. It is remeasured when there is a change in future lease payments arising from a change in an index or rate, or if there is a change in the estimate or assessment of the expected amount payable under a residual value guarantee, purchase, extension or termination option.
Variable lease payments not included in the initial measurement of the lease liability are charged directly to profit or loss.
| (m) | Loss per share |
The Company presents basic loss per share (“LPS”) data for its ordinary shares. Basic LPS is calculated by dividing the profit or loss attributable to ordinary shareholders of the Company by the weighted average number of ordinary shares outstanding during the year, adjusted for the Company’s own shares held. Diluted LPS is computed similar to basic LPS except that the weighted average shares outstanding are increased to include additional shares for the assumed exercise of any exercisable instruments, if dilutive. The number of additional shares is calculated by assuming that outstanding exercisable instruments were exercised and that the proceeds from such exercise were used to acquire common shares at the average market price during the reporting periods.
| (n) | New standards issued and adopted |
Amendments to IAS 1 and IFRS Practice Statement 2 – Disclosure of Accounting policies
The amendments require that an entity discloses its material accounting policy information, instead of its significant accounting policies. Further amendments explain how an entity can identify a material accounting policy. Examples of when an accounting policy is likely to be material are added. To support the amendment, the IASB has also developed guidance and examples to explain and demonstrate the application of the ‘four-step materiality process’ described in IFRS Practice Statement 2. The amendments are effective for annual reporting periods beginning on or after January 1, 2023. The adoption of this amendment did not have a significant impact to the Company’s consolidated financial statements.
F-15
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 3. | Material Accounting Policies (continued) |
| (o) | New standards issued, not yet adopted |
In January 2020, the IAS issued an amendment to IAS 1 Presentation of Financial Statements that clarifies the criterion for classifying a liability as non-current relating to the right to defer settlement of a liability for at least 12 months after the reporting period.
| 1. | Liabilities are classified as non-current if the entity has a substantive right to defer settlement for at least 12 months at the end of the reporting period. The amendment no longer refers to unconditional rights. The assessment determines whether a right exists, but it does not consider whether the entity will exercise the right. |
| 2. | ‘Settlement’ is defined as the extinguishment of a liability with cash, other economic resources or an entity’s own equity instruments. There is an exception for convertible instruments that might be converted into equity, but only for those instruments where the conversion option is classified as an equity instrument as a separate component of a compound financial instrument. |
In October 2022, the IASB issued amendments to IAS 1 that specified how an entity assesses whether it has the right to defer settlement of a liability when that right is subject to compliance with covenants within twelve months after the reporting period. The amendment applies to annual reporting periods beginning on or after January 1, 2024 and is applied retrospectively upon adoption. The Company does not expect the amendments to have a significant impact on the consolidated financial statements upon adoption.
| 4. | Business Combination |
On April 29, 2022, the Company, through
its wholly owned subsidiary, Cannahealth, acquired
RPK’s operations consist of a
| Cash | $ | |||
| Holdback payable | ||||
| Fair value of | ||||
| Total consideration | $ |
The cash purchase price of the acquisition
is $
The Company incurred acquisition-related
costs of approximately $
F-16
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 4. | Business Combination (continued) |
| Cash | $ | |||
| Accounts receivable | ||||
| Biological assets | ||||
| Inventory | ||||
| Prepayments | ||||
| Intangible assets | ||||
| Property, plant and equipment, net | ||||
| Trade and other payables | ( | ) | ||
| Loans and borrowing | ( | ) | ||
| Net assets acquired | $ |
During the year ended December 31,
2022, the Company recorded a bargain purchase gain of $
From the date of acquisition, the operations
of Holigen contributed a net loss of $
During the year ended December 31,
2023, the acquired business contributed $
| 5. | Loss of Control of Bophelo Bio Science and Wellness (Pty) Ltd. |
On July 26, 2022, the Company announced that the High Court of Lesotho (the “Lesotho Court”) has placed in liquidation the Company’s, wholly-owned subsidiary, Bophelo Bio Science and Wellness (Pty) Ltd. (“Bophelo”). The action to place Bophelo in liquidation was taken by the Lesotho Court pursuant to an application and request (the “Liquidation Application”) that was filed by Louisa Mojela, the former Executive Chairman of the Company, who was recently terminated as Executive Chairman of Akanda, and the Mophuti Matsoso Development Trust (“MMD Trust”). Akanda intends to convene a special committee to investigate Ms. Mojela’s actions and conduct, including actions and conduct taken by her prior to her filing of the Liquidation Application, and will pursue all of its available legal rights and remedies against Ms. Mojela and the MMD Trust for taking this unauthorized action. The Company also intends to contest and seek to reverse the determination by the Lesotho Court to place Bophelo in liquidation. In addition, Akanda will seek to recover significant loans that it has made to Bophelo to fund the execution of Bophelo’s business plan, including payment of rents and staffing costs, in the event that the Lesotho Court does not reverse its determination to place Bophelo in liquidation. Finally, Ms. Mojela has been summarily terminated as Chairman of Bophelo for Cause, as a “bad leaver”, as a result of her action to seek to place Bophelo in liquidation. Ms. Mojela has instituted legal proceedings against the Company as a result of the termination of her employment. In an action taken without the Company’s knowledge, the Lesotho Court has ordered an insolvent liquidation of Bophelo, and has appointed Mr. Chavonnes Cooper of Cape Town, South Africa, as liquidator of Bophelo for purposes of maintaining the value of the assets owned or managed by Bophelo. The order was signed by the Honorable Mr. Justice Mokhesi on July 15, 2022.
At December 31, 2022, the Company determined
that it no longer controlled Bophelo Bio Science and Wellness (Pty) Ltd. as a result of the above insolvent liquidation. As a result of
the loss of control, the Company derecognized all assets and liabilities at their book values on December 31, 2022 and wrote down all
balances receivable from the entity to $. During the year ended December 31, 2022, the Company recorded a loss on loss of control of
Bophelo Bio Science and Wellness (Pty) Ltd. of $
F-17
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 5. | Loss of Control of Bophelo Bio Science and Wellness (Pty) Ltd. (continued) |
| Year ended December 31, | 2022 | |||
| Revenue | $ | |||
| Operating expenses | ( | ) | ||
| Other expenses | ( | ) | ||
| ( | ) | |||
| Loss on loss of control of subsidiary | ( | ) | ||
| Loss on discontinued operations | $ | ( | ) | |
| Exchange differences on translation of discontinued operations | $ | ( | ) | |
| Other comprehensive income from discontinued operations | $ | ( | ) | |
| Cash flows provided by operating activities | $ | |||
| Cash flows used in investing activities | ( | ) | ||
| Cash flows used in financing activities | ( | ) | ||
| Effects of exchange rate changes on cash and cash equivalents | ||||
| Net change in cash used in the subsidiary | $ | ( | ) | |
| Carrying amount of net assets immediately prior to loss of control of subsidiary | $ | |||
| Reclassification of foreign currency translation reserve | ( | ) | ||
| Loss on loss of control of subsidiary | $ | |||
| Cash | $ | |||
| Accounts receivable | ||||
| Prepayments | ||||
| Property, plant and equipment | ||||
| Right-of-use assets | ||||
| Intangible assets | ||||
| Total assets | $ | |||
| Trade and other payables | ||||
| Lease liability | ||||
| Long-term debt | ||||
| Total liabilities | $ | |||
| Net assets | $ |
| 6. |
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Trade accounts receivable | $ | $ | ||||||
| Sales taxes receivable | ||||||||
| Other receivables | ||||||||
| $ | $ | |||||||
As at December 31, 2023, there were four customers
(2022 – one customer) with an amount greater than
F-18
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 7. | Inventory |
The Company’s inventory as of
December 31, 2023 included consumer packaging inventory and dried cannabis flower finished product at RPK in Portugal with a total carrying
amount of $
During the year ended December 31,
2023, the Company assessed the fair value of its biological assets and inventory and recognized a total of $
Biological assets
| For the years ended December 31, | 2023 | 2022 | ||||||
| Balance, beginning of the year | $ | $ | ||||||
| Acquisition (note 4) | ||||||||
| Gain (loss) on change in fair value of biological assets | ( | ) | ||||||
| Movement in exchange rate | ||||||||
| Balance, end of the year | $ | $ | ||||||
At December 31, 2023, there are no cannabis plants in the ground and only the mother plants remained. Hence, there are no biological assets recognized during the year ended December 31, 2023.
Biological assets at December 31, 2022
consisted of approximately
| 8. | Marketable Securities |
During the year ended December 31,
2022, concurrent to the acquisition of Holigen (note 4), the Company subscribed for, and purchased
| Balance, December 31, 2021 | $ | |||
| Purchase of investment | ||||
| Change in fair value | ( | ) | ||
| Movement in exchange rate | ( | ) | ||
| Balance, December 31, 2022 | ||||
| Change in fair value | ( | ) | ||
| Movement in exchange rate | ||||
| Balance, December 31, 2023 (*) | $ |
| (*) |
F-19
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 9. | Property, Plant and Equipment |
| Cost | Land | Plant and equipment | Leasehold Improvements | Motor Vehicles | Computers | Furniture and fixtures | Capital work- in-progress | Total | ||||||||||||||||||||||||
| Balance, December 31, 2021 | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Acquisitions (note 4) | ||||||||||||||||||||||||||||||||
| Additions | ||||||||||||||||||||||||||||||||
| Impact of loss of control of Bophelo Bio Science & Wellness (Pty) Ltd. | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||
| Foreign exchange movements | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||
| Balance, December 31, 2022 | ||||||||||||||||||||||||||||||||
| Additions | ||||||||||||||||||||||||||||||||
| Disposal | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||
| Foreign exchange movements | ( | ) | ||||||||||||||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Accumulated depreciation | Land | Plant and equipment | Leasehold Improvements | Motor Vehicles | Computers | Furniture and fixtures | Capital work- in-progress | Total | ||||||||||||||||||||||||
| Balance, December 31, 2021 | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Depreciation | ||||||||||||||||||||||||||||||||
| Depreciation – Bophelo | ||||||||||||||||||||||||||||||||
| Impact of loss of control of Bophelo Bio Science & Wellness (Pty) Ltd. | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||
| Foreign exchange movements | ( | ) | ( | ) | ||||||||||||||||||||||||||||
| Balance, December 31, 2022 | ||||||||||||||||||||||||||||||||
| Depreciation | ||||||||||||||||||||||||||||||||
| Disposal | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||
| Foreign exchange movements | ( | ) | ||||||||||||||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
F-20
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 9. | Property, Plant and Equipment (continued) |
| Impairment | Land | Plant and equipment | Leasehold Improvements | Motor Vehicles | Computers | Furniture and fixtures | Capital work- in-progress | Total | ||||||||||||||||||||||||
| Balance, December 31, 2022 and 2021 | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Impairment | ||||||||||||||||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Net book value | Land | Plant and equipment | Leasehold Improvements | Motor Vehicles | Computers | Furniture and fixtures | Capital work- in-progress | Total | ||||||||||||||||||||||||
| Balance, December 31, 2022 | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||
During the year ended December 31,
2022, the Company derecognized property, plant and equipment with a net book value of $
During the year ended December 31,
2023, the Company recorded depreciation of its property, plant and equipment of $
During the year ended December 31,
2023, the Company sold some of its equipment held in Portugal and recognized a loss on disposal of $
Pursuant to the Company’s upcoming
sale of RPK (note 23), its Portuguese subsidiary, the Company impaired a portion of its property, plant and equipment and recognized an
impairment loss of $
1900 Ferne Road, Gabriola Island, British Columbia
On September 19, 2023, and as amended on September 22, 2023, the Company entered into an option agreement with 1107385 B.C. Ltd (“1107385”) to purchase farming land property and related operations and licenses from 1107385. To acquire the property, the Company must pay the following:
| A. | The Company will issue a non-refundable payment equal to
$ |
| ● | the
First Option Payment, upon signing (issued |
|
| ● | the
Second Option Payment, 15 days after signing (paid $ |
|
| ● | the
Third Option Payment, 30 days after signing (paid $ |
This buys the Company the right to develop the property for two years. The Company plans during this time period to develop Tetrahydrocannabinol (THC) and CBD facilities at this site.
F-21
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 9. | Property, Plant and Equipment (continued) |
| B. | Additional payments will be made based upon milestones achieved from the development. Further payment milestones include: |
| ● | Upon approval or a license for THC cultivation
on the property from the applicable regulatory authority, $ | |
| ● | Upon sale of THC product cultivated from the
property, $ | |
| ● | Upon Hemp cultivation approval from the application
regulatory authority, $ | |
| ● | Upon CBD cultivation approval from the application
regulatory authority, $ |
| 10. | Right-of-use Assets |
On August 1, 2022, the Company entered
into a lease agreement for an office space with a monthly lease payment of $
| Land lease | Office lease | Total | ||||||||||
| Balance, December 31, 2021 | $ | $ | – | $ | ||||||||
| Additions | ||||||||||||
| Amortization | ( | ) | ( | ) | ||||||||
| Amortization – Bophelo | ( | ) | ( | ) | ||||||||
| Impact of loss of control of Bophelo Bio Science & Wellness (Pty) Ltd. | ( | ) | ( | ) | ||||||||
| Movement in exchange rates | ( | ) | ( | ) | ( | ) | ||||||
| Balance, December 31, 2022 | ||||||||||||
| Amortization | ( | ) | ( | ) | ||||||||
| Movement in exchange rates | ||||||||||||
| Balance, December 31, 2023 | $ | – | $ | $ | ||||||||
During the year ended December 31,
2023, the Company recorded amortization on its right-of-use assets of $
At December 31, 2022, the Company derecognized
right-of-use assets with a net book value of $
| 11. | Intangible Assets |
| Cost: | Software | Licences | Total | |||||||||
| Balance, December 31, 2021 | $ | $ | $ | |||||||||
| Acquisitions (note 4) | ||||||||||||
| Impact of loss of control of Bophelo Bio Science & Wellness (Pty) Ltd. | ( | ) | ( | ) | ||||||||
| Movement in exchange rates | ( | ) | ( | ) | ||||||||
| Balance, December 31, 2022 | ||||||||||||
| Movement in exchange rates | ||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | |||||||||
F-22
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 11. | Intangible Assets (continued) |
| Accumulated amortization: | Software | Licences | Total | |||||||||
| Balance, December 31, 2021 | $ | $ | $ | |||||||||
| Amortization | ||||||||||||
| Impact of loss of control of Bophelo Bio Science & Wellness (Pty) Ltd. | ( | ) | ( | ) | ||||||||
| Movement in exchange rates | ||||||||||||
| Balance, December 31, 2022 | ||||||||||||
| Amortization | ||||||||||||
| Movement in exchange rates | ||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | |||||||||
| Impairment: | Software | Licences | Total | |||||||||
| Balance, December 31, 2022 and 2021 | $ | $ | $ | |||||||||
| Impairment | ||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | |||||||||
| Net book value | Software | Licences | Total | |||||||||
| Balance, December 31, 2022 | $ | $ | $ | |||||||||
| Balance, December 31, 2023 | $ | $ | $ | |||||||||
The
Company’s intangible assets consists of computer software program with a carrying value of $
Pursuant
to the Company’s upcoming sale of RPK (note 23), its Portuguese subsidiary, the Company impaired a portion of its intangible assets
and recognized an impairment loss of $
At December 31, 2023, the remaining useful life of the Company’s finite life intangible assets is approximately years. The Company’s cannabis distribution license has been classified as an indefinite-life intangible asset as the Company expects to maintain this asset and the end point of the useful life of such asset cannot be determined. The Company evaluates the assumption of the indefinite life of the cannabis distribution license at least annually.
| 12. | Loan Receivable |
| December 31, | December 31, | |||||||
| 2023 | 2022 | |||||||
| Loan to Cellen Life Sciences Limited | $ | $ | ||||||
| Loan to an arm’s length party | ||||||||
| $ | $ | |||||||
Included
in the loan receivable at December 31, 2023 is an amount of $
F-23
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 12. | Loan Receivable (continued) |
On
November 10, 2022, the Company entered into an agreement (the “Loan Restructuring Agreement”) with Cellen Life Sciences Limited
and Cellen Biotech Limited (collectively referred to as “Cellen”) which entails the restructuring of the payment terms applicable
to the $
During
the year ended December 31, 2023, the Company loaned an amount of $
| 13. | Lease Liability |
| Maturity | Incremental borrowing rate | December
31, | December
31, | |||||||||||||
| Current | % | $ | $ | |||||||||||||
| Non-current | % | |||||||||||||||
| $ | $ | |||||||||||||||
On
August 1, 2022, the Company entered into a lease agreement for an office space with a monthly lease payment of $
| Cost: | Land Lease | Office Lease | Total | |||||||||
| Balance, December 31, 2021 | $ | $ | $ | |||||||||
| Present value of lease payments | ||||||||||||
| Accrued interest | ||||||||||||
| Cash payments | ( | ) | ( | ) | ||||||||
| Accounts payable | ( | ) | ( | ) | ||||||||
| Impact of loss of control of Bophelo Bio Science & Wellness (Pty) Ltd. (note 5) | ( | ) | ( | ) | ||||||||
| Movement in exchange rates | ( | ) | ( | ) | ( | ) | ||||||
| Balance, December 31, 2022 | ||||||||||||
| Accrued interest | ||||||||||||
| Cash payments | ( | ) | ( | ) | ||||||||
| Accounts payable | ( | ) | ( | ) | ||||||||
| Movement in exchange rates | ||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | |||||||||
During
the year ended December 31, 2022, the Company derecognized the lease liability with a net book value of $
F-24
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 13. | Lease Liability (continued) |
| Year ended December 31: | ||||
| 2023 | $ | |||
| 2024 | ||||
| $ | ||||
| 14. | Loans and Borrowings |
| (a) | Louisa Mojela loans: |
The loans described below have been granted to the Company to fulfill its capital and operational requirements. The terms of the loans are described below:
| (i) | Short-term loan #1 |
This
is a short-term loan facility of approximately $
| (ii) | Short-term loan #2 |
This
is a short-term loan facility of approximately $
| (iii) | Short-term loan #3 |
During
the year ended December 31, 2021, the Company received short term loan facility of approximately $
| (b) | Bank loans: |
The loans below have been granted to Holigen Ltd. and its subsidiaries in order to fund their capital and operational needs on site.
| (i) | Short term loans |
As
at December 31, 2023, the balance of the loans from Caixa was $
F-25
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 14. | Loans and Borrowings (continued) |
| (b) | Bank loans: (continued) |
| (ii) | Long term loans |
As
at December 31, 2023, the balance of the loans from Caixa was $
As
at December 31, 2023, the loans balance including accrued interest, which is recorded within accounts payable, was $
| (c) | Other loans: |
| (i) | During the year ended December 31, 2022, the Company received a loan of £ |
On
January 17, 2023, the Company received an additional loan of €
During
the year ended December 31, 2023, the Company recorded interest expense of $
| (ii) | On April 26, 2023, the Company received loan of € |
| (iii) | During the year ended December 31, 2023, the Company received a total loan of $ |
| (iv) | During the year ended December 31, 2023, the Company received loans of $ |
| (v) | During the year ended December 31, 2023, the Company received loans of $ |
F-26
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 15. | Share Capital |
| (a) | Authorized |
The Company has authorized share capital of an unlimited number of common shares with par value.
On March 9, 2023, the Company implemented a 1-for-10 reverse stock split on its ordinary shares.
| (b) |
| Cost: | Number of shares | Capital | ||||||
| Balance, December 31, 2021 | $ | |||||||
| Issuance of shares to ASDT | ||||||||
| Issuance of shares from private placement | ||||||||
| Issuance of shares upon conversion of note | ||||||||
| Issuance of shares from IPO | ||||||||
| Issuance of shares in Holigen acquisition (note 4) | ||||||||
| Loss of control of Bophelo Bio | ( | ) | ||||||
| Fair value of RSUs issued at $ | ||||||||
| Fair value of RSUs issued at $ | ||||||||
| Fair value of RSUs issued at $ | ||||||||
| Fair value of RSUs issued at $ | ||||||||
| Fair value of RSUs issued at $ | ||||||||
| Balance, December 31, 2022 | ||||||||
| Fair value of RSUs issued at $ | ||||||||
| Fair value of RSUs issued at $ | ||||||||
| Fair value of RSUs issued at $ | ||||||||
| Cancelled shares | ( | ) | ( | ) | ||||
| Issuance of shares upon conversion of note | ||||||||
| Issuance of shares pursuant to the first option payment to acquire a certain land property (note 9) | ||||||||
| Balance, December 31, 2023 | $ | |||||||
During the year ended December 31, 2023, the Company had the following share capital transactions:
| (i) | On January 26, 2023, the Company issued |
| (ii) | On May 2, 2023, the Company issued |
| (iii) | On June 6, 2023, the Company cancelled |
| (iv) | On July 26, 2023, the Company issued |
| (v) | On August 14, 2023, the Company issued |
| (vi) | On October 11, 2023, the Company issued |
F-27
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 15. | Share Capital (continued) |
| (b) | Shares issued and outstanding (continued) |
During the year ended December 31, 2022, the Company had the following share capital transactions:
| (i) | On March 14, 2022, the Company issued |
| (ii) | On March 14, 2022, the Company completed a private placement, issuing |
| (iii) | On March 15, 2022, the Company issued |
| (iv) | On March 15, 2022, the Company issued |
| (v) | On April 29, 2022, the Company issued |
| (vi) | On August 4, 2022, the Company issued |
| (vii) | On August 11, 2022, the Company issued |
| (viii) | On August 25, 2022, the Company issued |
| (ix) | On September 8, 2022, the Company issued |
| (x) | On October 28, 2022, the Company issued |
| (xi) | On November 22, 2022, the Company issued |
| (c) | Loss per share |
The
weighted average number of common shares outstanding for basic and diluted loss per share for the year ended December 31, 2023 was
| (d) | Restricted stock units |
In
order to incentivize senior executive management and key staff, the Company makes use of equity incentives awarded pursuant to the Employee
Share Ownership Plan (“ESOP”). In terms of the ESOP, the Company may award up to
On
April 22, 2022, the Company granted
F-28
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 15. | Share Capital (continued) |
| (d) | Restricted stock units (continued) |
On
July 29, 2022, the Company granted
On
August 11, 2022, the Company granted
On
August 18, 2022, the Company granted
On
September 6, 2022, the Company granted
On
September 21, 2022, the Company granted
On
September 22, 2022, the Company granted
On
October 28, 2022, the Company granted
On
November 21, 2022, the Company granted
On
January 24, 2023, the Company granted
On
May 2, 2023, the Company granted
F-29
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 15. | Share Capital (continued) |
| (d) | Restricted stock units (continued) |
On
July 26, 2023, the Company granted
| Number of RSUs | ||||
| Balance, December 31, 2021 | ||||
| Granted | ||||
| Exercised | ( | ) | ||
| Balance, December 31, 2022 | ||||
| Granted | ||||
| Exercised | ( | ) | ||
| Forfeited/Cancelled | ( | ) | ||
| Balance, December 31, 2023 | ||||
During
the year ended December 31, 2023, the Company recorded $
| 16. | Related Party Transactions |
Secured convertible debenture
On November 3, 2021 (the “Issuance Date”), the Company
entered into an agreement with Halo in which the Company issued Halo a secured convertible debenture with an initial value of $
Unsecured debenture
On
January 26, 2023, the Company issued a promissory note to Halo for a principal amount of $
During
the year ended December 31, 2023, the Company received additional loans from Halo in the aggregate principal amount of $
F-30
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 16. | Related Party Transactions (continued) |
Transactions with Key Management Personnel
| For the years ended December 31, | 2023 | 2022 | ||||||
| Key Management Remuneration | $ | $ | ||||||
| Stock-based compensation | ||||||||
| $ | $ | |||||||
The Key Management remuneration is included in Professional and Consulting fees and Personnel Expenses in the Statement of Operations.
As
of December 31, 2023, the Company has balances payable to related parties of $
| a. | Included within accounts payable and accrued liabilities at December 31, 2023 is remuneration payable to key management totaling $ |
| ● | current directors and officers: |
| i. | $ |
| ii. | $ |
| iii. | $ |
| iv. | $ |
| v. | $ |
| ● | former directors and officers: |
| i. | $ |
| ii. | $ |
| iii. | $ owing to T Virk (2022 – $ |
| iv. | $ owing to T Flow (2022 – $ |
| v. | $ owing to Dr. Akkar-Schenkl (2022 – $ |
| vi. | $ owing to L Mojela (2022 – $ |
| vii. | $ |
| viii. | $ |
| ix. | $ |
| x. | $ |
F-31
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 16. | Related Party Transactions (continued) |
| xi. | $ |
| xii. | $ |
| b. | The sole director and officer of RPK, Kiranjit Sidhu is also the owner of Catalyst Capital LLC (“Catalyst”). |
| i. | On November 14, 2022, the Company received a loan of £ |
| ii. | On January 17, 2023, the Company received an additional loan of € |
| iii. | On January 23, 2023, the Company entered into an independent contractor agreement with Mr. Sidhu, pursuant to which, he agreed to provide services regarding the business operations, business development and strategic matters to the Company for $ |
| iv. | On February 5, 2023, the Company entered into another independent contractor agreement with Mr. Sidhu, pursuant to which, he agreed to provide services regarding the business operations, business development, legal and strategic matters to the Company for $ |
| c. | The Company has the following loans outstanding to 1248787 B.C. Ltd. (“1248787”), a company controlled by Jatinder Dhaliwal, a director of the Akanda: |
| i. | On August 18, 2023, the Company received a loan of C$ |
| ii. | On September 27, 2023, the Company received a loan of C$ |
| iii. | On October 13, 2023, the Company received a loan of C$ |
| d. | The Company has the following loans transactions with Halo, a company controlled by Katharyn Field, the executive director and interim CEO of the Akanda: |
On
January 26, 2023, the Company issued a promissory note to Halo for a principal amount of $
During
the year ended December 31, 2023, the Company received additional loans from Halo in the aggregate principal amount of $
The Company’s related party transactions are measured at the exchange amount which is the amount of consideration established and agreed to by the related parties.
F-32
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 17. | Income Taxes |
| Years ended December 31, | 2023 | 2022 | ||||||
| Current: | $ | – | $ | – | ||||
| Kingdom of Lesotho | ||||||||
| Republic of Malta | ||||||||
| United Kingdom | ||||||||
| $ | $ | |||||||
A reconciliation of the expected income tax recovery to the actual income tax recovery is as follows:
| Years ended December 31, | 2023 | 2022 | ||||||
| Net loss before income taxes: | $ | ( | ) | $ | ( | ) | ||
| Statutory income tax rate | % | % | ||||||
| Income tax benefit | ( | ) | ( | ) | ||||
| Non-deductible items | ||||||||
| Non-taxable items | ( | ) | ( | ) | ||||
| Foreign rate differential | ( | ) | ||||||
| Unrecognized loss carryforwards | ||||||||
| $ | $ | |||||||
Deferred tax assets
| At December 31, | 2023 | 2022 | ||||||
| Net operating loss before carryforwards | $ | $ | ||||||
| Unrecognized loss carryforwards | $ | $ | ||||||
Deferred tax assets have not been recognized in respect of unutilized tax losses carried forward because it is not probable that future taxable profit will be available against which the Company can use the benefits therefrom.
| 18. | Financial Instruments |
Determination of Fair Values
IFRS 13, Fair Value Measurement, establishes a fair value hierarchy that reflects the significance of the inputs used in measuring fair value. The fair value hierarchy has the following levels:
Level 1 – Quoted prices in active markets for identical assets or liabilities;
Level 2 – Inputs other than quoted prices included within Level 1 that are either directly or indirectly observable;
Level 3 – Unobservable inputs in which little or no market activity exists, therefore requiring an entity to develop its own assumptions about the assumptions market participants would use in pricing.
F-33
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 18. | Financial Instruments (continued) |
A number of the Company’s accounting policies and disclosures require the determination of fair value, for both financial and non-financial assets and liabilities. Fair values have been determined for measurement and/or disclosure purposes based on the following models. When applicable, further information about the assumptions made in determining fair values is disclosed in the notes specific to that asset or liability.
| December 31, 2023 | December 31, 2022 | |||||||||||||||||
| Level | Carrying amount | Fair value | Carrying amount | Fair value | ||||||||||||||
| Financial assets | ||||||||||||||||||
| Financial assets measured at amortised cost: | ||||||||||||||||||
| Cash and cash held in trust | 1 | |||||||||||||||||
| Marketable securities | 1 | |||||||||||||||||
| Trade and other receivables | 2 | |||||||||||||||||
| Loan receivable | 2 | |||||||||||||||||
| Financial liabilities | ||||||||||||||||||
| Financial liabilities measure at amortised cost: | ||||||||||||||||||
| Trade and other payables | 2 | |||||||||||||||||
| Loans and borrowings | 2 | |||||||||||||||||
| Holdback payable | 2 | |||||||||||||||||
| Lease liabilities | 2 | |||||||||||||||||
| Due to related parties | 2 | |||||||||||||||||
| 19. | Risks Arising from Financial Instruments and Risk Management |
The Company’s activities expose it to a variety of financial risks: market risk (including foreign exchange and interest rate risks), credit risk and liquidity risk. Risk management is the responsibility of the Company, which identifies, evaluates and, where appropriate, mitigates financial risks.
| (a) | Market risk |
Foreign
exchange risk: is the risk that the fair value of future cash flows for financial instruments will fluctuate because of changes in foreign
exchange rates.
| As at (expressed in GBP) | December
31, 2023 | December
31, 2022 | ||||||
| Financial assets | ||||||||
| Cash and cash held in trust | £ | £ | ||||||
| Trade and other receivables | ||||||||
| Loan receivable | ||||||||
| £ | £ | |||||||
| Financial liabilities | ||||||||
| Trade and other payables | £ | £ | ||||||
| Loans and borrowings | ||||||||
| £ | £ | |||||||
F-34
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 19. | Risks Arising from Financial Instruments and Risk Management |
| (a) | Market risk (continued) |
| As at (expressed in EUR) | December 31, 2023 | December 31, 2022 | ||||||
| Financial assets | ||||||||
| Cash | € | € | ||||||
| Trade and other receivables | ||||||||
| € | € | |||||||
| Financial liabilities | ||||||||
| Trade and other payables | € | € | ||||||
| Loans and borrowings | ||||||||
| € | € | |||||||
| As at (expressed in CAD) | December
31, 2023 | December
31, 2022 | ||||||
| Financial assets | ||||||||
| Cash | $ | $ | ||||||
| Marketable securities | ||||||||
| $ | $ | |||||||
| Financial liabilities | ||||||||
| Trade and other payables | $ | $ | ||||||
| Due to related party | ||||||||
| Holdback payable | ||||||||
| Lease liabilities | ||||||||
| Loans and borrowings | ||||||||
| $ | $ | |||||||
Based
on the above net exposures as at December 31, 2023, assuming that all other variables remain constant, a
| (b) | Credit risk |
Credit risk is the risk of financial loss to the Company if a partner or counterparty to a financial instrument fails to meet its contractual obligation and arises principally from the Company’s cash and accounts receivable. The carrying amounts of the financial assets represents the maximum credit exposure. The Company limits its exposure to credit risk on cash by placing these financial instruments with high-credit quality financial institutions.
At
December 31, 2023, the Company was subject to a concentration of credit risk related to its accounts receivable as
| (c) | Liquidity risk |
Liquidity risk is the risk that the Company will not be able to meet its financial obligations as they come due. The Company manages its liquidity risk by continuously monitoring forecasted and actual cash flows, as well as anticipated investing and financing activities and to ensure that it will have sufficient liquidity to meet its liabilities and commitments when due and to fund future operations. The Company’s trade and other payables are due within the current operating year.
F-35
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 20. | Capital Management |
The Company manages its capital structure and makes adjustments to it, based on the funds available to the Company, in order to continue the business of the Company. The Company, upon approval from its Board of Directors, will balance its overall capital structure through new share and warrant issuances, granting of stock options, the issuance of debt or by undertaking other activities as deemed appropriate under the specific circumstance. The Board of Directors does not establish a quantitative return on capital criteria for management, but rather relies on the expertise of the Company’s management to sustain future development of the business.
The Company’s objectives when managing capital are to safeguard the Company’s ability to continue as a going concern and to provide capital to pursue the development and commercialization of its products. In the management of capital, the Company includes cash, long-term debt and capital. The Company manages the capital structure and makes adjustments to it in light of changes in economic conditions and the risk characteristics of the underlying assets. To maintain or adjust the capital structure, the Company may attempt to issue new shares or new debt.
At the current stage of the Company’s development, in order to maximize its current business activities, the Company does not pay out dividends. Management reviews its capital management approach on an ongoing basis and believes that this approach, given the relative size of the Company, is reasonable.
The Company’s overall strategy with respect to capital risk management remains unchanged for the years ended December 31, 2023 and 2022.
| 21. | Segmented Information |
The
Company has
| As at December 31, 2023 | ||||||||||||||||
| Financial statement line item: | Cultivation | Distribution | Corporate | Total | ||||||||||||
| Reportable segment assets | $ | $ | $ | $ | ||||||||||||
| Reportable segment liabilities | ||||||||||||||||
F-36
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 21. | Segmented Information (continued) |
| As at December 31, 2022 | ||||||||||||||||
| Financial statement line item: | Cultivation | Distribution | Corporate | Total | ||||||||||||
| Reportable segment assets | $ | $ | $ | $ | ||||||||||||
| Reportable segment liabilities | ||||||||||||||||
| For the year ended December 31, 2023 | ||||||||||||||||
| Financial statement line item: | Cultivation | Distribution | Corporate | Total | ||||||||||||
| Revenues from external customers | $ | $ | $ | $ | ||||||||||||
| Intersegment revenues | ||||||||||||||||
| Other income (expense) | ( | ) | ( | ) | ||||||||||||
| Finance income | ||||||||||||||||
| Finance expense | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Depreciation & amortization | ||||||||||||||||
| Discontinued operations | ||||||||||||||||
| Reportable segment income (loss) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| For the year ended December 31, 2022 | ||||||||||||||||
| Financial statement line item: | Cultivation | Distribution | Corporate | Total | ||||||||||||
| Revenues from external customers | $ | $ | $ | $ | ||||||||||||
| Intersegment revenues | ||||||||||||||||
| Other expense | ( | ) | ( | ) | ||||||||||||
| Finance income | ||||||||||||||||
| Finance expense | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Depreciation & amortization | ||||||||||||||||
| Discontinued operations | ( | ) | ( | ) | ||||||||||||
| Reportable segment loss | ( | ) | ( | ) | ( | ) | ||||||||||
| For the year ended December 31, 2023 | ||||||||||||||||
| Revenues | Cultivation | Distribution | Corporate | Total | ||||||||||||
| Total revenues | $ | $ | $ | $ | ||||||||||||
| Elimination of inter segment revenue | ||||||||||||||||
| Total revenue | $ | $ | $ | $ | ||||||||||||
| For the year ended December 31, 2022 | ||||||||||||||||
| Revenues | Cultivation | Distribution | Corporate | Total | ||||||||||||
| Total revenues | $ | $ | $ | $ | ||||||||||||
| Elimination of inter segment revenue | ||||||||||||||||
| Total revenue | $ | $ | $ | $ | ||||||||||||
| For the year ended December 31, 2023 | ||||||||||||||||
| Loss | Cultivation | Distribution | Corporate | Total | ||||||||||||
| Total loss for reportable segments | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| Total loss on discontinued operations | ||||||||||||||||
| Elimination of inter segment profit or loss | ||||||||||||||||
| Loss before income tax expense | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
F-37
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 21. | Segmented Information (continued) |
| For the year ended December 31, 2022 | ||||||||||||||||
| Loss | Cultivation | Distribution | Corporate | Total | ||||||||||||
| Total profit or loss for reportable segments | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
| Total loss on discontinued operations | ( | ) | ( | ) | ||||||||||||
| Elimination of inter segment profit or loss | ||||||||||||||||
| Income (Loss) before income tax expense | $ | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||||
| As at December 31, 2023 | ||||||||||||||||
| Assets | Cultivation | Distribution | Corporate | Total | ||||||||||||
| Total assets for reportable segments | $ | $ | $ | $ | ||||||||||||
| Elimination of inter segment assets | ( | ) | ( | ) | ( | ) | ||||||||||
| Segments’ assets | $ | $ | $ | $ | ||||||||||||
| As at December 31, 2022 | ||||||||||||||||
| Assets | Cultivation | Distribution | Corporate | Total | ||||||||||||
| Total assets for reportable segments | $ | $ | $ | $ | ||||||||||||
| Elimination of inter segment assets | ( | ) | ( | ) | ( | ) | ||||||||||
| Segments’ assets | $ | $ | $ | $ | ||||||||||||
| As at December 31, 2023 | ||||||||||||||||
| Liabilities | Cultivation | Distribution | Corporate | Total | ||||||||||||
| Total liabilities for reportable segments | $ | $ | $ | ( | ) | $ | ||||||||||
| Elimination of inter segment liabilities | ( | ) | ( | ) | ( | ) | ||||||||||
| Entity’s liabilities | $ | $ | $ | $ | ||||||||||||
| As at December 31, 2022 | ||||||||||||||||
| Liabilities | Cultivation | Distribution | Corporate | Total | ||||||||||||
| Total liabilities for reportable segments | $ | $ | $ | ( | ) | $ | ||||||||||
| Elimination of inter segment liabilities | ( | ) | ( | ) | ( | ) | ||||||||||
| Entity’s liabilities | $ | $ | $ | $ | ||||||||||||
| 22. | Contingencies |
On October 20, 2022, Louisa Mojela
filed a claim against Canmart and the Company for wrongful termination of her Service Agreement. The claimant sought £
On
April 29, 2023, Trevor Scott, former CFO of the Company, issued a claim against the Company for amounts owing under his employment agreement
totaling £
F-38
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 22. | Contingencies (continued) |
On May 12, 2023, Tejinder Virk, former
CEO of the Company, issued a claim for Detriment and dismissal for alleged protected disclosures totaling £
On
May 15, 2023, Vidya Iyer, the Company’s former SVP of Finance issued a claim for amounts owing under her employment agreement
totaling £
Subsequent to the year ended December 31, 2023, the Company’s former CFO filed a claim against the Company for unpaid CFO fees and invoices from the period December 2022 through November 2023 (note 23).
| 23. | Subsequent Events |
Subsequent to the year ended December 31, 2023, the Company:
| i. | Completed a Share Purchase Agreement for Sale of RPK |
On April 1, 2024, pursuant to the signed definitive Share Purchase Agreement and Escrow Agreement with Somai Pharmaceuticals Ltd. (“Somai”) on February 29, 2024, the Company completed the transaction with Somai for the sale of RPK, its indirect wholly-owned Portuguese subsidiary.
Under the terms of the Share Purchase Agreement, Somai acquired RPK
for a total cash consideration of Two Million United States Dollars ($
In connection with the closing, the Company paid a cash finder’s
fee of
| ii. | Issued the following shares: |
| a. | On February 2, 2024, the Company entered into a securities purchase agreement with Corbo Capital Inc. pursuant to which the Company issued |
| b. | On February 13, 2024, the Company issued |
| c. | On February 20, 2024, the Company issued |
| d. | On February 28, 2024, the Company issued |
F-39
Akanda Corp.
Notes to the Consolidated Financial Statements
(Expressed in United States Dollars)
| 23. | Subsequent Events (continued) |
| e. | On March 4, 2024, the Company entered into a securities purchase agreement with certain investors pursuant to which the Company issued
On the same day, the Company issued |
| f. | On March 5, 2024, the Company entered into a securities purchase agreement with certain investors pursuant to which the Company issued
On March 6, 2024, the Company issued |
| g. | On March 24, 2024, the Company entered into an underwriting agreement with Univest Securities, LLC (“Univest”) as the underwriter in connection with the issuance and sale by the Company in an underwritten public offering of |
| iii. | Received the following loans: |
| a. | On January 10, 2024, the Company received a loan of CAD $ |
| b. | On January 10, 2024, the Company received a loan of CAD $ |
| c. | On February 1, 2024, the Company received a loan of $ |
| iv. | Outstanding Claims |
On
January 29, 2024, the Company was informed that Mr. Shailesh Bhushan, the former Chief Financial Officer of the Company, filed
a complaint with the Employment Standards Branch of British Columbia claiming unpaid salary and invoices in the aggregate amount of CAD
$
On
March 27, 2024, the Company entered into a settlement agreement with Vidya Iyer, the former SVP of Finance to settle the claims for a
sum of £
On April 24, 2024, Mr. Harvinder Singh resigned as an independent director
of the Board of Directors of the Company. A Resignation and Mutual Release Agreement dated April 24, 2024 was entered between the Company
and Mr. Singh, pursuant to which the Company agreed to pay Harvinder Singh a separation and release amount of $
| v. | Incorporated a new subsidiary |
On February 28, 2024, the Company incorporated
a new subsidiary –
F-40
ITEM 19. EXHIBITS
The following exhibits are filed as part of this Annual Report on Form 20-F:
EXHIBIT INDEX
69
| + | Indicates management contract or compensatory plan. |
| # | Filed herewith. |
| Ω | Certain portions of this exhibit (indicated by “[***]”) have been omitted pursuant to Regulation S-K, Item (601)(b)(10). |
| ^ | Certain of the exhibits and schedules to this exhibit have been omitted in accordance with Regulation S-K Item 601(a)(5). The Company agrees to furnish a copy of all omitted exhibits and schedules to the SEC upon its request. |
In accordance with SEC Release Nos. 33-8238 and 34-47986, Final Rule: Management’s Reports on Internal Control Over Financial Reporting and Certification of Disclosure in Exchange Act Periodic Reports, and the instruction to Form 20-F, the certifications furnished in Exhibit 13.1 and Exhibit 13.2 hereto are deemed to accompany this Annual Report on Form 20-F and will not be deemed “filed” for purpose of Section 18 of the Exchange Act. Such certifications will not be deemed to be incorporated by reference into any filing under the U.S. Securities Act of 1933, as amended, or the U.S. Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate them by reference.
70
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| Akanda Corp. | ||
| /s/ Katie Field | ||
| By: | Katie Field | |
| Title: | Interim Chief Executive Officer and Director | |
Date: May 1, 2024
71