UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM
(Mark One)
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from __________________ to __________________
Commission File Number:
(Exact Name of Registrant as Specified in its Charter)
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer |
NA |
|
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code:
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
The |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
☒ |
|
Smaller reporting company |
|
||
|
|
|
|
Emerging growth company |
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes
As of August 10, 2023, the registrant had
Table of Contents
|
|
Page |
PART I. |
1 |
|
Item 1. |
1 |
|
|
1 |
|
|
Condensed Consolidated Statements of Operations and Comprehensive Loss |
2 |
|
3 |
|
|
4 |
|
|
5 |
|
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
14 |
Item 3. |
28 |
|
Item 4. |
28 |
|
PART II. |
29 |
|
Item 1. |
29 |
|
Item 1A. |
29 |
|
Item 2. |
30 |
|
Item 3. |
30 |
|
Item 4. |
30 |
|
Item 5. |
30 |
|
Item 6. |
31 |
|
32 |
||
i
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q contains forward-looking statements within the meaning of the federal securities laws. All statements other than statements of historical fact contained in this Quarterly Report on Form 10-Q, including statements regarding our future results of operations and financial position, business strategy and plans and objectives of management for future operations, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “may,” “should,” “would,” “expects,” “plans,” “anticipates,” “could,” “intends,” “target,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this Quarterly Report on Form 10-Q are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak only as of the date of this Quarterly Report on Form 10-Q and are subject to a number of risks, uncertainties and assumptions described in the section titled “Risk Factors” and elsewhere in this Quarterly Report on Form 10-Q, in our Annual Report on Form 10-K for the year ended December 31, 2022 and in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2023. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Some of the key factors that could cause actual results to differ from our expectations include:
ii
These statements relate to future events or to our future financial performance and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by these forward-looking statements. Factors that may cause actual results to differ materially from current expectations include, among other things, those set forth in Part I, Item 1A of our Annual Report on Form 10-K for the year ended December 31, 2022, Part II, Item 1A - “Risk Factors” in this Quarterly Report on Form 10-Q, and those described elsewhere in this Quarterly Report on Form 10-Q and in our Quarterly Report on Form 10-Q for the quarter ended March 31, 2023. Any forward-looking statement in this Quarterly Report on Form 10-Q reflects our current view with respect to future events and is subject to these and other risks, uncertainties, and assumptions relating to our operations, results of operations, industry, and future growth. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Except as required by law, we assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
This Quarterly Report on Form 10-Q also contains estimates, projections, and other information concerning our industry, our business, and the markets for certain drugs, including data regarding the estimated size of those markets, their projected growth rates, and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections, or similar methodologies is inherently subject to uncertainties, and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained these industry, business, market, and other data from reports, research surveys, studies, and similar data prepared by third parties, industry, medical and general publications, government data, and similar sources. In some cases, we do not expressly refer to the sources from which these data are derived.
Except where the context otherwise requires, in this Quarterly Report on Form 10-Q, “we,” “us,” “our,” “IO Biotech,” and the “Company” refer to IO Biotech, Inc. and, where appropriate, its consolidated subsidiaries.
Trademarks
The Company has applied for various trademarks that we use in connection with the operation of its business. This Quarterly Report on Form 10-Q includes trademarks, service marks, and trade names owned by us or other companies. All trademarks, service marks, and trade names included in this Quarterly Report on Form 10-Q are the property of their respective owners. Solely for convenience, the trademarks and trade names in this report may be referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
iii
PART I—FINANCIAL INFORMATION
Item 1. Interim Financial Statements (Unaudited).
IO BIOTECH, INC.
Condensed Consolidated Balance Sheets
(In thousands, except share and per share amounts)
(unaudited)
|
|
June 30, |
|
|
December 31, |
|
||
Assets |
|
|
|
|
|
|
||
Current assets |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
Prepaid expenses and other current assets |
|
|
|
|
|
|
||
Total current assets |
|
|
|
|
|
|
||
Restricted cash |
|
|
|
|
|
|
||
Property and equipment, net |
|
|
|
|
|
|
||
Right of use lease asset |
|
|
|
|
|
|
||
Other non-current assets |
|
|
|
|
|
|
||
Total non-current assets |
|
|
|
|
|
|
||
Total assets |
|
$ |
|
|
$ |
|
||
Liabilities and stockholders’ equity |
|
|
|
|
|
|
||
Current liabilities |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
|
|
$ |
|
||
Lease liability - current |
|
|
|
|
|
|
||
Accrued expenses and other current liabilities |
|
|
|
|
|
|
||
Total current liabilities |
|
|
|
|
|
|
||
Lease liability - non-current |
|
|
|
|
|
|
||
Total non-current liabilities |
|
|
|
|
|
|
||
Total liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|||
Stockholders’ equity |
|
|
|
|
|
|
||
Preferred stock, par value of $ |
|
|
|
|
|
|
||
Common stock, par value of $ |
|
|
|
|
|
|
||
Additional paid-in capital |
|
|
|
|
|
|
||
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
Accumulated other comprehensive loss |
|
|
( |
) |
|
|
( |
) |
Total stockholders’ equity |
|
|
|
|
|
|
||
Total liabilities and stockholders’ equity |
|
$ |
|
|
$ |
|
||
See accompanying notes to the unaudited interim condensed consolidated financial statements.
1
IO BIOTECH, INC.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
(unaudited)
|
|
Three Months Ended |
|
|
Six Months Ended |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
||||
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
General and administrative |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Loss from operations |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Other income (expense) |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Currency exchange gain (loss), net |
|
|
|
|
|
( |
) |
|
|
|
|
|
( |
) |
||
Interest income |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Interest expense |
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Total other income (expense), net |
|
|
|
|
|
( |
) |
|
|
|
|
|
( |
) |
||
Loss before income tax expense |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Income tax expense |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Net loss |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Net loss attributable to common shareholders |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Net loss per common share, basic and diluted |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
Weighted-average number of shares used in computing net loss per common share, basic and diluted |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Other comprehensive (loss) income |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
Foreign currency translation |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
( |
) |
|
Total comprehensive loss |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
See accompanying notes to the unaudited interim condensed consolidated financial statements.
2
IO BIOTECH, INC.
(In thousands, except share amounts)
(unaudited)
|
|
Common Stock |
|
|
Additional |
|
|
Accumulated Other |
|
|
Accumulated |
|
|
Total |
|
|||||||||
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
Loss |
|
|
Deficit |
|
|
Equity |
|
||||||
Balance, January 1, 2022 |
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
||||
Equity-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Foreign currency translation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Balance, June 30, 2022 |
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Balance, January 1, 2023 |
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
||||
Equity-based compensation |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Foreign currency translation |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
||
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Balance, June 30, 2023 |
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
||||
See accompanying notes to the unaudited interim condensed consolidated financial statements.
3
IO BIOTECH, INC.
Condensed Consolidated Statements of Cash Flows
(In thousands)
(unaudited)
|
|
Six Months Ended |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
Cash flows from operating activities |
|
|
|
|
|
|
||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
Adjustment to reconcile net loss to net cash used in operating activities |
|
|
|
|
|
|
||
Depreciation |
|
|
|
|
|
|
||
Equity-based compensation |
|
|
|
|
|
|
||
Amortization of right of use lease asset |
|
|
|
|
|
|
||
Foreign currency (gain) loss |
|
|
( |
) |
|
|
|
|
Changes in operating assets and liabilities |
|
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
|
|
|
|
||
Other non-current assets |
|
|
( |
) |
|
|
( |
) |
Accounts payable |
|
|
|
|
|
|
||
Lease liability |
|
|
( |
) |
|
|
( |
) |
Accrued expenses and other current liabilities |
|
|
( |
) |
|
|
( |
) |
Net cash used in operating activities |
|
|
( |
) |
|
|
( |
) |
Cash flows from investing activities |
|
|
|
|
|
|
||
Purchase of property and equipment |
|
|
( |
) |
|
|
( |
) |
Net cash used in investing activities |
|
|
( |
) |
|
|
( |
) |
Cash flows from financing activities |
|
|
|
|
|
|
||
Net cash provided by financing activities |
|
|
— |
|
|
|
— |
|
Net decrease in cash, cash equivalents and restricted cash |
|
|
( |
) |
|
|
( |
) |
Effect of exchange rate changes on cash, cash equivalents and restricted cash |
|
|
|
|
|
( |
) |
|
Cash, cash equivalents and restricted cash, beginning of period |
|
|
|
|
|
|
||
Cash, cash equivalents and restricted cash, end of period |
|
$ |
|
|
$ |
|
||
Components of cash, cash equivalents, and restricted cash |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
Restricted cash |
|
|
|
|
|
|
||
Total cash, cash equivalents and restricted cash |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
See accompanying notes to the unaudited interim condensed consolidated financial statements.
4
Notes to Condensed Consolidated Financial Statements
(unaudited)
1. Description of Business, Organization and Liquidity
Business
IO Biotech, Inc. is a clinical-stage biotechnology company dedicated to the identification and development of disruptive immune therapies for the treatment of cancer. We are developing novel, immune-modulating cancer vaccines based on our T-win technology platform. As used in these financial statements, unless the context otherwise requires, references to the “Company,” “we,” “us,” and “our” refer to IO Biotech, Inc. and its subsidiaries.
Corporate Reorganization
IO Biotech ApS was incorporated in Denmark in December 2014. In November 2021, we completed a corporate reorganization whereby IO Biotech ApS became a wholly-owned subsidiary of the Company. In connection with the corporate reorganization, each issued and outstanding Class A ordinary share ($
Initial Public Offering (IPO)
In November 2021, we completed our IPO, selling an aggregate of
Immediately prior to the consummation of the IPO, all outstanding shares of our Class A ordinary shares and Class B and Class C convertible preference shares were converted into
On November 9, 2021, we amended and restated the certificate of incorporation of IO Biotech, Inc. to authorize the issuance of
At-The-Market Equity Program
On February 15, 2023, we filed a new prospectus supplement with the U.S. Securities and Exchange Commission (the “SEC”) with respect to the offer and sale of shares of our common stock, par value $
Risks and Uncertainties
We are subject to risks common to companies in the biotechnology industry including, but not limited to, new technological innovations, protection of proprietary technology, dependence on key personnel, compliance with government regulations and the need to obtain additional financing. Product candidates currently under development will require significant additional research and development efforts, including extensive pre-clinical and clinical testing and regulatory approval, prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel infrastructure and extensive compliance reporting capabilities.
5
Our product candidates are in development. There can be no assurance that our research and development will be successfully completed, that adequate protection for our intellectual property will be obtained, that any products developed will obtain necessary government regulatory approval or that any approved products will be commercially viable. Even if our product development efforts are successful, it is uncertain when, if ever, we will generate significant revenue from product sales. We operate in an environment of rapid change in technology and substantial competition from pharmaceutical and biotechnology companies. In addition, we are dependent upon the services of our employees and consultants.
Liquidity Considerations and Going Concern Basis of Accounting
Since inception, we have devoted substantially all of our efforts to business planning, conducting research and development, recruiting management and technical staff, and raising capital. We have financed our operations primarily through the issuance of convertible preference shares, convertible notes and our IPO. In addition, on August 7, 2023, we entered into a Securities Purchase Agreement (the “Purchase Agreement”) with certain institutional investors (the “Purchasers”), pursuant to which we raised an additional $75.1 million in gross proceeds through a private placement transaction (the “Private Placement”), before deducting offering expenses. The offering closed on August 9, 2023.
Our continued discovery and development of product candidates will require significant additional research and development efforts, including extensive preclinical and clinical testing and regulatory approval prior to commercialization. These efforts require significant amounts of additional capital, adequate personnel and infrastructure and extensive compliance-reporting capabilities. Even if product development efforts are successful, it is uncertain when, if ever, we will realize significant revenue from product sales.
As of June 30, 2023, we had an accumulated deficit of $
Coronavirus Pandemic
2. Summary of Significant Accounting Policies
There have been no changes to the significant accounting policies disclosed in Note 2 to the Company’s annual financial statements for the years ended December 31, 2022 and 2021 included in its Annual Report on Form 10-K filed with the SEC, other than those described below.
Unaudited Financial Information
The accompanying unaudited interim condensed consolidated financial statements included herein have been prepared in conformity with generally accepted accounting principles in the United States of America ("U.S. GAAP"), and pursuant to the rules and regulations of the SEC. In the Company’s opinion, the information furnished herein reflects all adjustments, all of which are of a normal and recurring nature and necessary for a fair presentation of the financial position and results of operations for the reported interim periods. The Company considers events or transactions that occur after the balance sheet date but before the financial statements are issued to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure. The results of operations for interim periods are not necessarily indicative of results to be expected for the full year or any other interim period. Unaudited interim financial statements and footnotes should be read in conjunction with the audited financial statements and footnotes included in the Company’s Annual Report filed on Form 10-K for the fiscal year ended December 31, 2022.
6
Recently Adopted Accounting Standards
In June 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-13, Financial Instruments—Credit Losses: Measurement of Credit Losses on Financial Instruments. ASU 2016-13 requires measurement and recognition of expected credit losses for financial assets. In April 2019, the FASB issued clarification to ASU 2016-13 within , Codification Improvements to Topic 326, Financial Instruments—Credit Losses, Topic 815, Derivatives and Hedging, and Topic 825, Financial Instruments, or ASU 2016-13. The guidance is effective for fiscal years beginning after December 15, 2022. The Company has
Recently Issued Accounting Standards
In August 2020, the FASB issued ASU No. 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging Contracts in Entity's Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. ASU 2020-06 will simplify the accounting for convertible instruments by reducing the number of accounting models for convertible debt instruments and convertible preferred stock. Limiting the accounting models results in fewer embedded conversion features being separately recognized from the host contract as compared with current U.S. GAAP. Convertible instruments that continue to be subject to separation models are (i) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting and (ii) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. ASU 2020-06 also amends the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. ASU 2020-06 will be effective for us beginning after December 15, 2023. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020, including interim periods within those fiscal years. We are currently assessing the impact adoption of ASU 2020-06 will have on our financial statements and disclosures.
Other than the items noted above, there have been no new accounting pronouncements not yet effective or adopted in the current year that we believe have a significant impact, or potential significant impact, to our unaudited interim condensed consolidated financial statements.
3. Fair Value Measurements
The following table presents information about our financial assets and liabilities measured at fair value on a recurring basis and indicate the level of the fair value hierarchy utilized to determine such fair values (in thousands):
|
|
June 30, 2023 |
|
|||||||||||||
|
|
Total |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds(1) |
|
$ |
|
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
||
Total assets measured at fair value |
|
$ |
|
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
December 31, 2022 |
|
|||||||||||||
|
|
Total |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||
Assets |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds(1) |
|
$ |
|
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
||
Total assets measured at fair value |
|
$ |
|
|
$ |
|
|
$ |
— |
|
|
$ |
— |
|
||
(1) Money market funds with maturities of 90 days or less at the date of purchase are included within cash and cash equivalents in the accompanying condensed consolidated balance sheets and are recognized at fair value.
As of June 30, 2023 and December 31, 2022, the Company only held Level 1 financial instruments, respectively.
There were
7
4. License and Collaboration Agreements
In February 2018, we entered into a clinical collaboration with MSD International GmbH (MSDIG) to evaluate IO102 in combination with KEYTRUDA® (pembrolizumab) in first-line treatment of patients with metastatic non-small cell lung cancer. Under the terms of the collaboration with MSDIG, we will conduct an international Phase 1/2 study to evaluate a combination therapy of IO102 and KEYTRUDA®. We will sponsor the clinical trials and MSDIG will provide KEYTRUDA® to be used in the clinical trials free of charge. We and MSDIG will be responsible for our own internal costs and expenses to support the study and we shall bear all other costs associated with conducting the study, including costs of providing IO102 for use in the study. The rights to the data from the clinical trials will be shared by us and MSDIG and we will maintain global commercial rights to IO102.
In September 2021, we entered into a clinical collaboration with MSDIG and MSD International Business GmbH (MSDIB), another affiliate of Merck (collectively, “MSD”) to evaluate IO102-IO103 in combination with KEYTRUDA® versus KEYTRUDA® alone in treatment of patients with metastatic (advanced) melanoma. Under the terms of the collaboration with MSD, we will conduct an international Phase 3 study to evaluate a combination therapy of IO102-IO103 and KEYTRUDA®. We will sponsor the clinical trials and MSD will provide KEYTRUDA® to be used in the clinical trials free of charge. We and MSD will be responsible for our own internal costs and expenses to support the study and we shall bear all other costs associated with conducting the study, including costs of providing IO102-IO103 for use in the study. The rights to the data from the clinical trials will be shared by us and MSD and we will maintain global commercial rights to IO102-IO103.
In December 2021, we entered into a clinical collaboration with MSD to evaluate IO102-IO103 in combination with KEYTRUDA® in previously untreated patients with three different tumor types— metastatic non-small cell lung cancer (NSCLC), squamous cell carcinoma of the head and neck (SCCHN), and urothelial bladder cancer (UBC). Under the terms of the collaboration with MSD, we will conduct an international Phase 2 study to evaluate a combination therapy of IO102-IO103 and KEYTRUDA®. We will sponsor the clinical trials and MSD will provide KEYTRUDA® to be used in the clinical trials free of charge. We and MSD will be responsible for our own internal costs and expenses to support the study and we shall bear all other costs associated with conducting the study, including costs of providing IO102-IO103 for use in the study. The rights to the data from the clinical trials will be shared by us and MSD and we will maintain global commercial rights to IO102-IO103.
In November 2022, we entered into a clinical collaboration with MSD to evaluate IO102-IO103 in combination with KEYTRUDA® as a neo-adjuvant/adjuvant therapy for patients with metastatic melanoma and SCCHN. Under the terms of the collaboration with MSD, we will conduct an international Phase 2 study to evaluate a combination therapy of IO102-IO103 and KEYTRUDA®. We will sponsor the clinical trials and MSD will provide KEYTRUDA® to be used in the clinical trials free of charge. We and MSD will be responsible for our own internal costs and expenses to support the study and we shall bear all other costs associated with conducting the study, including costs of providing IO102-IO103 for use in the study. The rights to the data from the clinical trials will be shared by us and MSD and we will maintain global commercial rights to IO102-IO103.
5. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consist of the following (in thousands):
|
|
June 30, |
|
|
December 31, |
|
||
Prepaid contract research and development costs |
|
$ |
|
|
$ |
|
||
Insurance |
|
|
|
|
|
|
||
Research and development tax credit receivable |
|
|
|
|
|
|
||
Value-added tax refund receivable |
|
|
|
|
|
|
||
Other |
|
|
|
|
|
|
||
Total prepaid expenses and other current assets |
|
$ |
|
|
$ |
|
||
8
6. Property and Equipment, Net
Property and equipment, net consist of the following (in thousands):
|
|
June 30, |
|
|
December 31, |
|
||
Laboratory equipment |
|
$ |
|
|
$ |
|
||
Computer hardware |
|
|
|
|
|
|
||
Office furniture |
|
|
|
|
|
|
||
Less: accumulated depreciation |
|
|
( |
) |
|
|
( |
) |
Total property and equipment, net |
|
$ |
|
|
$ |
|
||
For the three months ended June 30, 2023 and 2022, the Company recognized $
7. Accrued Expenses and Other Current Liabilities
Accrued expenses and other current liabilities consist of the following (in thousands):
|
|
June 30, |
|
|
December 31, |
|
||
Accrued contract research and development costs |
|
$ |
|
|
$ |
|
||
Professional fees |
|
|
|
|
|
|
||
Employee compensation costs |
|
|
|
|
|
|
||
Other liabilities |
|
|
|
|
|
|
||
Total accrued expenses and other current liabilities |
|
$ |
|
|
$ |
|
||
8. Leases
On January 1, 2022, the Company adopted Accounting Standards Codification (“ASC”) 842 using the modified retrospective transition approach allowed under ASU 2018-11 which releases companies from presenting comparative periods and related disclosures under ASC 842 and requires a cumulative-effect adjustment to the opening balance of accumulated deficit in the period of adoption. The Company had an immaterial cumulative-effect adjustment to the opening balance of accumulated deficit as of January 1, 2022. As of June 30, 2023, the Company is party to
The Company is party to an operating lease in Copenhagen, Denmark for office space that commenced in March 2021 with the initial term set to expire in January 2025. Base rent for this initial lease was approximately $
9
Quantitative information regarding the Company’s leases for the six months ended June 30, 2023 and 2022 is as follows (in thousands):
|
|
Six Months Ended |
|
|
Six Months Ended |
|
||
Lease Cost |
|
June 30, 2023 |
|
|
June 30, 2022 |
|
||
Operating lease cost |
|
$ |
|
|
$ |
|
||
Operating cash flows paid for amounts included in the measurement of lease liabilities |
|
$ |
|
|
$ |
|
||
Operating lease liabilities arising from obtaining right‑of‑use assets |
|
$ |
|
|
$ |
|
||
Remaining lease term (years) |
|
|
|
|
|
|||
Weighted average discount rate |
|
|
% |
|
|
% |
||
Future lease payments (undiscounted) under noncancelable leases are as follows at June 30, 2023 (in thousands):
Future Lease Payments |
|
Amount |
|
|
Remainder of 2023 |
|
$ |
|
|
2024 |
|
|
|
|
2025 |
|
|
|
|
2026 |
|
|
|
|
2027 |
|
|
|
|
Thereafter |
|
|
|
|
Total |
|
$ |
|
|
The Company’s leases do not provide an implicit rate. Therefore, the Company used its incremental borrowing rate based on the information available at commencement date in determining the present value of lease payments. The Company used the incremental borrowing rate on January 1, 2022 for operating leases that commenced prior to that date, which is the rate incurred to borrow on a collateralized basis over a similar term an amount equal to the lease payments in a similar economic environment.
9. Commitments and Contingencies
Legal Proceedings
From time to time, we may be party to litigation arising in the ordinary course of business. We were not subject to any material legal proceedings during the six months ended June 30, 2023 and the year ended December 31, 2022, and, to our knowledge, no material legal proceedings are currently pending or threatened.
Contractual Obligations and Commitments
We enter into contracts in the ordinary course of business with third-party service providers for clinical trials, preclinical research studies and testing, manufacturing and other services and products for operating purposes. These contracts generally provide for termination upon notice of
Indemnification Agreements
We enter into certain types of contracts that contingently requires us to indemnify various parties against claims from third parties. These contracts primarily relate to procurement, service, consultancy or license agreements under which we may be required to indemnify vendors, service providers or licensees for certain claims, including claims that may be brought against them arising from our acts or omissions with respect to our products, technology, intellectual property or services. The Company, as permitted under Delaware law and in accordance with its amended and restated certificate of incorporation and amended and restated bylaws and pursuant to indemnification agreements with certain of its officers and directors, indemnifies its officers and directors for certain events or occurrences, subject to certain limits, which the officer or director is or was serving at the Company’s request in such capacity. At the 2023 Annual Meeting of Stockholders, the Company’s stockholders approved an amendment to, and the Company subsequently amended, its amended and restated certificate of incorporation to extend the indemnification of officers pursuant to recent amendments to the General Corporation Law of the State of Delaware.
10
From time to time, we may receive indemnification claims under existing contracts in the normal course of business. In the event that one or more of these matters were to result in a claim against us, an adverse outcome, including a judgment or settlement, may cause a material adverse effect on our future business, operating results or financial condition. It is not possible to estimate the maximum amount potentially payable under these contracts since we have no history of prior indemnification claims and the unique facts and circumstances involved in each particular claim will be determinative.
10. Stockholders' Equity
Common and Preferred Stock
Upon the closing of our IPO in November 2021, we filed an amended and restated certificate of incorporation, which authorized us to issue
As of June 30, 2023 and December 31, 2022, the Company had
11. Equity-Based Compensation
2021 Equity Incentive Plan
In November 2021, our Board adopted, and our stockholders approved, the 2021 Equity Incentive Plan (“2021 Equity Plan”), which became effective on November 4, 2021. The 2021 Equity Plan provides for the grant of incentive stock options, non-statutory stock options, stock appreciation rights, awards of restricted stock, restricted stock units and other stock-based awards. The number of shares of our common stock reserved for issuance under the 2021 Equity Plan is equal to
The following table summarizes our stock options activity for the six months ended June 30, 2023:
|
|
Number of |
|
|
Weighted- |
|
|
Weighted- |
|
|
Aggregate |
|
||||
Outstanding, January 1, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
— |
|
|||
Granted |
|
|
|
|
$ |
|
|
|
— |
|
|
$ |
— |
|
||
Cancelled or forfeited |
|
|
( |
) |
|
$ |
|
|
|
— |
|
|
$ |
— |
|
|
Outstanding, June 30, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
— |
|
|||
Exercisable, June 30, 2023 |
|
|
|
|
$ |
|
|
|
|
|
$ |
— |
|
|||
2021 Employee Stock Purchase Plan
In November 2021, our Board adopted and our stockholders approved the 2021 Employee Stock Purchase Plan (2021 ESPP), which became effective on November 4, 2021. The number of shares of our common stock reserved for issuance under the 2021 ESPP is equal to
Equity-Based Compensation
All share-based awards granted are measured based on the fair value on the date of the grant and compensation expense is recognized with respect to those awards over the requisite service period, which is generally the vesting period of the respective award. Forfeitures related to equity-based compensation awards are recognized as they occur, and we reverse any previously recognized compensation cost associated with forfeited awards in the period the forfeiture occurs.
For the three months ended June 30, 2023 and 2022, we recorded equity-based compensation expense of $
11
equity-based compensation expense of $
The fair values of the options granted during the period ended June 30, 2023 were estimated based on the Black-Scholes model, using the following assumptions:
|
|
June 30, |
|
Expected volatility |
|
|
|
Risk-free interest rate |
|
|
|
Expected term (in years) |
|
|
|
Expected dividend yield |
|
|
Equity-based compensation expense recorded as research and development and general and administrative expenses is as follows (in thousands):
|
|
Three Months Ended June 30, |
|
|
Six Months Ended June 30, |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
||||
Research and development |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
General and administrative |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total equity-based compensation |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
We did
12. Income Taxes
We are subject to taxes for earnings generated in multiple jurisdictions, both inside and outside of the United States and our tax expense is primarily affected by unrecognized tax benefits in Denmark. We recorded a provision for income taxes of $
We have evaluated the positive and negative evidence involving our ability to realize our deferred tax assets. We have considered our history of cumulative net losses incurred since inception and our lack of any commercial products. We have concluded that it is more likely than not that we will not realize the benefits of our deferred tax assets in IO Biotech ApS, IO Bio US, Inc. and IO Biotech, Inc. We reevaluate the positive and negative evidence at each reporting period.
12
13. Net Loss Per Share
Basic and diluted net loss per common share is calculated as follows (in thousands except share and per share amounts):
|
|
Three Months Ended |
|
|
Six Months Ended |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
||||
Net loss |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
Net loss attributable to common shareholders |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
Net loss per common share, basic and diluted |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
( |
) |
Weighted-average number of shares used in computing net loss per common share, basic and diluted |
|
|
|
|
|
|
|
|
|
|
|
|
||||
The following outstanding potentially dilutive securities have been excluded from the calculation of diluted net loss per common share, as their effect is anti-dilutive:
|
|
Three Months Ended |
|
|
Six Months Ended |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
2023 |
|
|
2022 |
|
||||
Stock options to purchase common stock |
|
|
|
|
|
|
|
|
|
|
|
|
||||
14. Subsequent Events
On August 7, 2023, the Company entered into the Purchase Agreement, pursuant to which the Company agreed to sell and issue (i) 37,065,647 shares of the Company’s common stock (“Common Stock”), and (ii) 37,065,647 warrants to purchase up to 37,065,647 shares of Common Stock (the “Warrants”) in the Private Placement. Each Purchaser’s Warrant is exercisable for a number of shares of Common Stock equal to one hundred percent of the aggregate number of shares of Common Stock purchased by such Purchaser. The purchase price per share of Common Stock and Warrant is $2.025 per share (the “Purchase Price”).
The Warrants will be immediately exercisable upon issuance at an exercise price of $2.47 per share, subject to adjustment as set forth therein. The Warrants will be exercisable until the earlier of (i) February 9, 2027, and (ii) one day prior to the closing of an acquisition, as defined within the Form of Common Stock Purchase Warrant agreement. The Warrants may be exercised on a cashless basis if there is no effective registration statement registering the shares underlying the Warrants.
In connection with the execution of the Purchase Agreement, the Company also entered into a registration rights agreement (the “Registration Rights Agreement”) with the Purchasers. Under the terms of the Registration Rights Agreement, the Company has agreed to prepare and file, by September 8, 2023 (the “Filing Deadline”), one or more registration statements with the SEC to register for resale the Common Stock issued under the Purchase Agreement and the shares of Common Stock issuable upon conversion of the Warrants issued pursuant to the Purchase Agreement (the “Registrable Securities”), and to cause the applicable registration statements to become effective within a specified period after the Filing Deadline. Certain cash penalties will apply to the Company in the event of registration failures, as described in the Registration Rights Agreement.
The Private Placement closed on August 9, 2023. The Company received approximately $75.1 million in gross proceeds from the Private Placement, before deducting offering expenses that are expected to amount to approximately $3.0 million. The Company intends to use the net proceeds from the Private Placement for general corporate purposes.
13
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes included in this Quarterly Report on Form 10-Q. Some of the information contained in this discussion and analysis or set forth elsewhere in this Quarterly Report, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in Part I, Item 1A. “Risk Factors” of our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on March 14, 2023, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis. You should carefully read the section entitled “Risk Factors” in our Annual Report on Form 10-K to gain an understanding of the important factors that could cause actual results to differ materially from our forward-looking statements. Please also see the section entitled “Cautionary Note Regarding Forward-Looking Statements.”
Overview
We are a clinical-stage biopharmaceutical company developing novel, immune-modulating cancer vaccines based on our T-win technology platform. Our product candidates are designed to induce the immune system to simultaneously target and disrupt multiple pathways that regulate tumor-induced immunosuppression. We believe this represents a paradigm shift in the management of cancer and that our product candidates have the potential to become cornerstones of the treatment regimens of multiple solid tumors. Our lead product candidate, the IO102-IO103 cancer vaccine, is designed to target the immunosuppressive mechanisms mediated by key immunosuppressive proteins such as indoleamine 2,3-dioxygenase (IDO) and programmed death ligand (PD-L1). In a single-arm Phase 1/2 clinical trial of 30 patients with metastatic melanoma with the primary objective of investigating safety and tolerability, the secondary objective of investigating immune response, and the tertiary objective of investigating clinical efficacy, IO102-IO103, in combination with nivolumab, demonstrated an ability to induce meaningful tumor regression and establish durable anti-tumor response while achieving a manageable tolerability profile for patients. The clinical efficacy endpoints in this trial included objective response (OR), progression free survival (PFS) and overall survival (OS). In this trial, we observed a confirmed overall response rate (ORR) of 73% as per RECIST 1.1 and a complete response rate (CRR) of 50%. Based on the results from this trial, IO102-IO103, in combination with pembrolizumab, was granted BTD by the FDA for treatment of unresectable/metastatic melanoma.
We enrolled the first patient in a potentially registrational Phase 3 trial for IO102-IO103 in combination with pembrolizumab as a potential first-line treatment in advanced melanoma, the IOB-013/KN-D18 trial, in May of 2022. We randomized 225 patients as of June 2023 and expect to fully enroll the trial by the end of 2023. On June 14, 2023, we announced that we will increase the number of patients to be enrolled in the Phase 3 trial to 380 patients, which could potentially accelerate the time to reach the primary endpoint of PFS, which is an event-based driven analysis, and will be assessed when 226 events (progression or death) are registered in the trial. The primary endpoint is powered at 89% to detect a hazard ratio of 0.65. The Phase 3 trial protocol calls for an interim analysis of overall response rate one year after 225 patients have been randomized; if we achieve an 18% point or greater benefit in ORR, this interim analysis could allow for submission of a Biologics License Application (BLA) for accelerated approval in the US. The trial design and discussions with FDA are aimed at potentially pursuing accelerated approval, if the trial data are favorable, based on the interim analysis of ORR, supported by other data. If the data are supportive, we also plan to file a Marketing Authorization Application (MAA) with the European Medicines Agency (EMA) based on the primary endpoint of PFS.
Our T-win platform is a novel approach to cancer vaccines designed to activate pre-existing T cells to target immunosuppressive mechanisms. Our T-win product candidates are designed to employ a dual mechanism of action: (1) direct killing of immunosuppressive cells, including both tumor cells and genetically stable cells in the tumor microenvironment (TME), that express IDO and PD-L1 and (2) modulation of the TME into a more pro-inflammatory, anti-tumor environment. Our T-win technology is built upon our team’s deep understanding of both TME and a tumor’s ability to evade surveillance and destruction by the immune system. Our approach is in contrast to previous methods that have sought to either block singular immunosuppressive pathways or to direct the immune system against specific identified antigens expressed by tumor cells.
14
We are developing a pipeline of product candidates that leverage our T-win technology platform to address targets within the TME. In addition to melanoma, we plan to evaluate IO102-IO103 in multiple solid tumor indications to potentially expand the market opportunity for IO102-IO103. We are also focusing on additional targets that play key roles in immunosuppression and that are expressed in a broad range of solid tumors. Our current pipeline of product candidates is summarized in the table below.
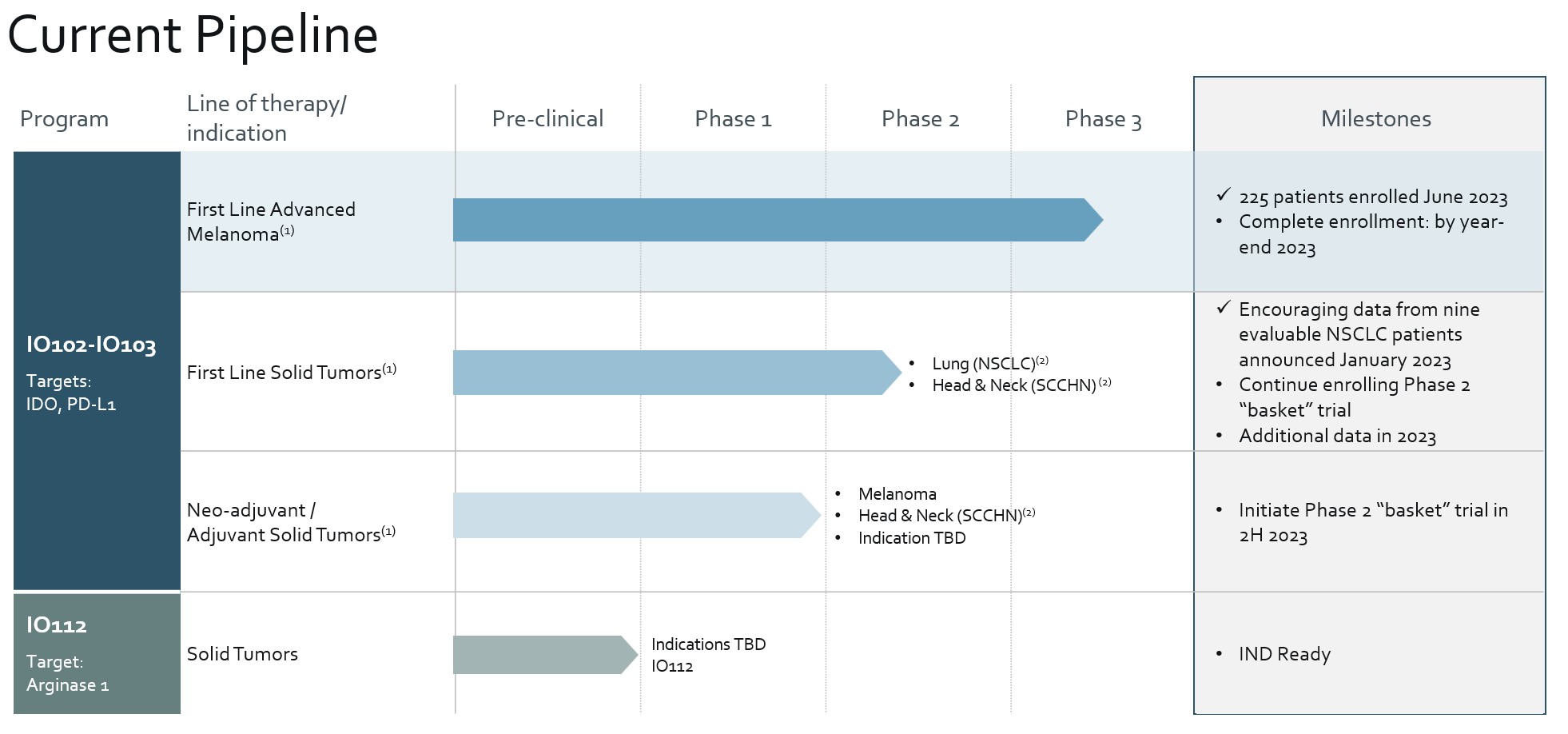
Our lead product candidate, IO102-IO103, combines our two fully-owned, novel immune-modulating vaccines, IO102 and IO103, which are designed to target IDO+ and PD-L1+ target cells, respectively. IDO and PD-L1 are often dysregulated and over-expressed in a wide range of solid tumors, and result in the inhibition of the body’s natural pro-inflammatory anti-tumor response within the TME. IO102-IO103 is designed to employ our novel dual mechanism of action approach. This is in contrast to previous approaches which have sought to block singular immunosuppressive pathways or to direct the immune system against specific identified antigens expressed by tumor cells. By combining IO102 and IO103 in a single treatment regimen, we also aim to provide a synergistic therapeutic effect on tumors.
On December 14, 2020, the FDA granted us BTD for the IO102-IO103 cancer vaccine in combination with pembrolizumab for the treatment of patients with unresectable or metastatic melanoma based on data from the Phase 1/2 clinical trial, MM1636. A BTD enables us to solicit more frequent and intensive guidance from the FDA as to how to conduct an efficient development program for IO102-IO103. The MM1636 trial was an investigator-initiated, single-arm Phase 1/2 trial of 30 anti PD-1/PD-L1 naïve patients with metastatic melanoma receiving IO102-IO103 and nivolumab, an anti-PD-1 monoclonal antibody. In this trial, investigators initially observed an ORR of 80% (24 out of 30 patients); however, 2 of 24 patients in which a response was observed progressed before subsequent radiological confirmation, which resulted in a confirmed ORR of 73% based on RECIST 1.1. Of these 80% responders, 50% achieved a complete response (CR), or complete elimination of their tumors. While a total of five patients (17%) experienced a treatment-related high-grade adverse event (grade 3-5), and 17% discontinued treatment with both nivolumab and IO102-IO103, data from this trial suggests a manageable tolerability profile for patients. In addition, we have observed treatment-induced infiltration of CD3+/CD8+ T cells into the tumor site in responding patients and detected IO102 and/or IO103-specific T cells in tumors after treatment in correlative biomarker data where this was analyzed. Consistent with the earlier reported data, with an additional 15 months of patient follow-up, results from the January 5, 2023 data cut as published in the May 2023 Journal for ImmunoTherapy of Cancer for the MM1636 Phase 1/2 study of IO102-IO103 in combination with nivolumab for metastatic melanoma continue to be encouraging. As of that data cut-off, 30 PD-1 naïve patients were enrolled with a minimum follow-up time of 49.8 months. Median OS was not reached, median PFS was 25.5 months, and 50% of patients (15/30) achieved a CR, or complete disappearance of their tumors and the ORR was 80% (73% confirmed ORR per RECIST 1.1). Patients who were anti-PD-1 antibody therapy resistant and enrolled in cohort B in this study had no response to therapy, which we believe shows that our vaccine works best in front-line metastatic melanoma patients, as we expected in this setting.
15
We are currently recruiting for a Phase 3 potentially registrational trial for IO102-IO103, the IOB-013/KN-D18 trial, in combination with pembrolizumab in anti PD-1/PD-L1 treatment naïve patients with unresectable or metastatic melanoma that we expect to fully enroll by the end of 2023. While the MM1636 trial investigated IO102-IO103 in combination with nivolumab, we have made the commercial decision to investigate IO102-IO103 in combination with pembrolizumab in the Phase 3 trial. Nivolumab and pembrolizumab are both IgG4 subclass antibodies that target the PD-1 receptor. In a comparative data analysis by Moser (Annals of Oncology 2020), researchers found no difference between the effectiveness of frontline pembrolizumab and nivolumab in patients with advanced melanoma. The Phase 3 trial also includes a concurrent evaluation of the initial participants to allow for an assessment of safety (a safety run-in) for the combination of IO102-IO103 and pembrolizumab. The pembrolizumab for this trial is being supplied by Merck pursuant to a Clinical Trial Collaboration and Supply Agreement that we entered into in September 2021. The independent data monitoring committee for the IOB-013/KN-D18 trial convened its second meeting in May 2023 to review safety data from the first 86 randomized patients and recommended that the trial continue without modifications.
We are also investigating the IO102-IO103 cancer vaccine in several other solid tumor indications. We are conducting a Phase 2 basket trial, the IOB-022 trial, which will enable us to investigate multiple first-line solid tumor indications treatment naïve patients for metastatic disease. This basket trial is designed to investigate the safety and efficacy of IO102-IO103 in combination with pembrolizumab in metastatic non-small cell lung cancer (NSCLC), adenocarcinoma histology, with PD-L1 TPS ≥ 50% (cohort A), recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) with CPS ≥ 20 (cohort B), and metastatic urothelial bladder carcinoma (UBC) with CPS ≥ 10 (cohort C). We initiated the IOB-022 trial in April 2022 and as of April 12th, 2023, 30 patients are enrolled (24 in cohort A and 6 in cohort B). Consistent with the earlier reported data, results from the June 12, 2023 for the IOB-022/KN-D38 Phase 1/2 study of IO102-IO103 in combination with pembrolizumab in metastatic NSCLC and recurrent and/or metastatic SCCHN continue to be encouraging. Of the 24 patients enrolled in cohort A, 17 were efficacy evaluable per protocol having received at least two full cycles of treatment. Among the 17 evaluable patients, 9 patients had a partial response while 5 had stable disease; 3 patients had progressive disease. Of the 6 patients enrolled in cohort B, 5 were efficacy evaluable per protocol having received at least two full cycles of treatment. Among the 5 evaluable patients, 3 patients had a partial response while 2 patients had progressive disease. The safety profile observed to date in this study is consistent with prior clinical experience with IO102-IO103. We expect to report additional data from this study by the end of 2023.
In addition to first-line cancer indications, we also plan to investigate IO102-IO103 when used before or after curatively intended surgery as a neo-adjuvant/adjuvant therapy. In April 2023, the FDA cleared the Company’s Investigational New Drug Application (IND) for the evaluation of IO102-IO103 in the neo-adjuvant / adjuvant treatment of solid tumors. As with our targeted first-line cancer indications, we plan to conduct a Phase 2 basket trial, the IOB-032 trial, which will enable us to investigate IO102-IO103 in combination with pembrolizumab in multiple solid tumor indications in anti PD-1/PD-L1 naïve settings focused initially on melanoma and SCCHN. We expect to initiate this trial in the second half of 2023.
Our development of IO102-IO103 is based on our prior separate development of IO102 and IO103. IO102 is our fully-owned novel product candidate containing a single IDO-derived peptide sequence designed to engage and activate IDO-specific human T cells. IDO small molecule inhibitors have shown clinical potential in combination with PD-1 antibodies in early clinical trials, but have not been able to demonstrate the same level of efficacy in later-stage clinical trials. Our Phase 1 non-randomized trial of IO101, our first-generation IDO therapy, in NSCLC resulted in proof of concept for our approach, with 47% of patients displaying clinical benefit and an OS of 26 months in the treatment arm compared to 8 months in the group receiving standard of care. There were no grade 3 or higher adverse events (AEs). We have completed testing of IO102 in a randomized Phase 1/2 trial in combination with pembrolizumab standard-of-care in first-line treatment of patients with metastatic NSCLC. IO103 is our fully-owned, novel product candidate containing a single PD-L1-derived peptide designed to engage and activate PD-L1 specific human T cells. Continued clinical development of IO102 and IO103 will be focused on their use in our dual- and multi-antigen approaches.
IO112 is our fully-owned, novel product candidate containing a single Arginase 1-derived peptide designed to engage and activate Arginase 1-specific human T cells. IO112 is designed to target T cells that recognize epitopes derived from Arginase 1, which is an immunoregulatory enzyme highly expressed in difficult-to-treat tumors associated with high levels of myeloid-derived suppressor cells (MDSCs) including colorectal, breast, prostate and pancreatic and ovarian cancers. Arginase overexpression is a well-documented tumor escape mechanism. IO112 is currently being tested in a single arm first-in-human Phase 1 trial in patients with Arginase-positive solid tumors conducted in an investigator-initiated trial at the University of Copenhagen. We plan to be ready to file an IND for IO112 by the end of 2023.
16
In addition to IO102, IO103 and IO112, we are evaluating additional product candidates that we believe have potential for use in solid tumors. All our compounds in preclinical development are designed to target well-documented immunosuppressive molecules that are known to be overexpressed in the TME across a wide range of tumors. These targets provide additional opportunities across multiple cancer indications.
Targeting TGFβ1 expressing cells in the TME via a vaccine approach presents a novel and attractive way to modulate the pathway and drive therapeutic benefit in cancer setting. We are developing and characterizing TGFβ1-selective peptide vaccines capable of inducing strong immune responses. Preliminary evidence in mouse models showed that treatment with a TGFβ1 vaccine drives CD4+ T cell infiltration in the TME and might promote in vivo targeted cell killing. Further experimental work to elucidate the cellular and molecular mechanisms of a TGFβ1 vaccine is ongoing to support further development of a TGFβ1 vaccine to modulate the TME for therapeutic benefit in a wide range of cancer indications.
We were established in December 2014 as a spin-off of the National Center for Cancer Immune Therapy at Herlev University Hospital in Denmark. We have assembled a seasoned management team and Board with extensive experience in developing novel oncology therapies, including advancing product candidates from preclinical research through to clinical development and ultimately to regulatory approval. Our team is led by our founder, President and Chief Executive Officer, Mai-Britt Zocca, Ph.D., who has close to 20 years of experience as a biotech executive. Amy Sullivan, our Chief Financial Officer, joined us in October 2022 and brings 30 years of public company industry experience to IO Biotech. Qasim Ahmad, MD, our Chief Medical Officer, is a trained internist and clinical oncologist and has more than 20 years of experience in strategic clinical development, medical affairs, and obtaining marketing authorizations. Mads Hald Andersen, Ph.D., our scientific founder and advisor, is a Professor and director at the National Center for Cancer Immune Therapy, Herlev University Hospital and an internationally recognized immunology researcher. Our Board has deep expertise in the fields of immuno-oncology, business and finance.
On November 9, 2021, we completed an IPO of our common stock and issued and sold 8,222,500 shares of common stock at a public offering price of $14.00 per share, including 1,072,500 shares of common stock sold pursuant to the underwriters’ exercise of their option to purchase additional shares of common stock, resulting in net proceeds of $103.3 million after deducting underwriting discounts and commissions and estimated offering expenses.
On August 7, 2023, the Company entered into the Purchase Agreement, pursuant to which the Company agreed to sell and issue (i) 37,065,647 shares of the Company’s Common Stock, and (ii) 37,065,647 warrants to purchase up to 37,065,647 shares of Warrants in the Private Placement. Each Purchaser’s Warrant is exercisable for a number of shares of Common Stock equal to one hundred percent of the aggregate number of shares of Common Stock purchased by such Purchaser. The purchase price per share of Common Stock and Warrant is $2.025 per share.
The Warrants will be immediately exercisable upon issuance at an exercise price of $2.47 per share, subject to adjustment as set forth therein. The Warrants will be exercisable until the earlier of (i) February 9, 2027, and (ii) one day prior to the closing of an acquisition, as defined within the Form of Common Stock Purchase Warrant agreement. The Warrants may be exercised on a cashless basis if there is no effective registration statement registering the shares underlying the Warrants.
In connection with the execution of the Purchase Agreement, the Company also entered into the Registration Rights Agreement with the Purchasers. Under the terms of the Registration Rights Agreement, the Company has agreed to prepare and file, by the Filing Deadline, one or more registration statements with the SEC to register for resale the Common Stock issued under the Purchase Agreement and the shares of Common Stock issuable upon conversion of the Warrants issued pursuant to the Purchase Agreement, and to cause the applicable registration statements to become effective within a specified period after the Filing Deadline. Certain cash penalties will apply to the Company in the event of registration failures, as described in the Registration Rights Agreement.
The Private Placement closed on August 9, 2023. The Company received approximately $75.1 million in gross proceeds from the Private Placement, before deducting offering expenses that are expected to amount to approximately $3.0 million. The Company intends to use the net proceeds from the Private Placement for general corporate purposes.
Since inception, we have had significant operating losses. Our net loss was $38.2 million for the six months ended June 30, 2023 and $71.5 million and $67.9 million for the years ended December 31, 2022 and December 31, 2021, respectively. As of June 30, 2023, we had an accumulated deficit of $216.0 million and $110.1 million in cash and cash equivalents.
Our ability to generate revenue from product sales sufficient to achieve profitability will depend heavily on the successful development and eventual commercialization of one or more of our product candidates. Our operations to date have been financed primarily by aggregate net proceeds of $288.7 million from the issuance of convertible preference shares, convertible notes, ordinary shares, and our IPO in addition to the estimated $75.1 million gross proceeds raised through the Private Placement, before deducting estimated offering expenses.
17
Cash used to fund operating expenses is impacted by the timing of when we pay these expenses, as reflected in the change in our accounts payable and accrued expenses. We expect to continue to incur net losses for the foreseeable future, and we expect our research and development expenses, general and administrative expenses, and capital expenditures will continue to increase. In particular, we expect our expenses to increase as we continue our development of, and seek regulatory approvals for, our product candidates, as well as hire additional personnel, pay fees to outside consultants, lawyers and accountants, and incur other increased costs associated with being a public company. In addition, if and when we seek and obtain regulatory approval to commercialize any product candidate, we will also incur increased expenses in connection with commercialization and marketing of any such product. Our net losses may fluctuate significantly from quarter-to-quarter and year-to-year, depending on the timing of our clinical trials and our expenditures on other research and development activities.
Based upon our current operating plan, we believe that our existing cash and cash equivalents of $110.1 million as of June 30, 2023, taken together with the $75.1 million in gross proceeds raised through the Private Placement, which closed on August 9, 2023, will be sufficient to continue funding our development activities into the fourth quarter of 2025. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect. To finance our operations beyond that point we will need to raise additional capital, which, though we were successful in raising additional capital through the Private Placement cannot be assured, and which we may not be able to pursue successfully again in the future.
To date, we have not had any products approved for sale and, therefore, have not generated any product revenue. We do not expect to generate any revenues from product sales unless and until we successfully complete development and obtain regulatory approval for one or more of our product candidates. If we obtain regulatory approval for any of our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution, and associated regulatory and compliance costs. As a result, until such time, if ever, that we can generate substantial product revenue, we expect to finance our cash needs through equity offerings, debt financings or other capital sources, including collaborations, licenses or similar arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed or on favorable terms, if at all. Any failure to raise capital as and when needed could have a negative impact on our financial condition and on our ability to pursue our business plans and strategies, including our research and development activities. If we are unable to raise capital, we will need to delay, reduce or terminate planned activities to reduce costs.
Coronavirus Pandemic
Although the World Health Organization has declared that COVID-19 no longer represents a global health emergency, the actual and perceived impact of COVID-19 and any effect on our business cannot be predicted. As a result, there can be no assurance that we will not experience additional negative impacts associated with COVID-19, which could be significant. The COVID-19 pandemic may negatively impact our business, financial condition and results of operations causing interruptions or delays in the Company’s programs and services.
Components of Operating Results
Operating Expenses
Our operating expenses since inception have consisted primarily of research and development expenses and general and administrative costs.
Research and Development
Our research and development expenses consist primarily of costs incurred for the development of our product candidates and our drug discovery efforts, which include:
18
We expense all research and development costs in the periods in which they are incurred. Costs for certain research and development activities are recognized based on an evaluation of the progress to completion of specific tasks using information and data provided to us by our vendors and third-party service providers.
From time to time, we obtain grants from public and private funds for our research and development projects. The grant income for a given period is recognized as a cost reimbursement and is typically based on the time and the costs that we have spent on the specific project during that period.
We have historically met the requirements to receive a tax credit in Denmark of up to 5.5 million Danish Kroner per year for losses resulting from research and development costs of up to 25 million Danish Kroner per year. The tax credit is presented as a reduction to research and development expense in the statements of operations.
We use our personnel and infrastructure resources across multiple research and development programs directed toward identifying and developing product candidates. We generally have not tracked our research and development expenses on a program-by-program basis. Substantially all of our direct research and development expenses in the years ended December 31, 2022 and 2021 and in the six months ended June 30, 2023 were on IO102-IO103 and consisted primarily of external costs, such as consultants, third-party contract organizations that conduct research and development activities on our behalf, costs related to production of preclinical and clinical materials, including fees paid to contract manufacturers, and laboratory and vendor expenses related to the execution of our ongoing and planned preclinical studies and clinical trials.
We expect our research and development expenses to increase substantially for the foreseeable future as we continue to invest in research and development activities related to developing our product candidates, including investments in conducting clinical trials, manufacturing and otherwise advancing our programs. The process of conducting the necessary clinical research to obtain regulatory approval is costly and time-consuming, and the successful development of our product candidates is highly uncertain.
Because of the numerous risks and uncertainties associated with product development and the current stage of development of our product candidates and programs, we cannot reasonably estimate or know the nature, timing and estimated costs necessary to complete the remainder of the development of our product candidates or programs. We are also unable to predict if, when, or to what extent we will obtain approval and generate revenues from the commercialization and sale of our product candidates. The duration, costs and timing of preclinical studies and clinical trials and development of our product candidates will depend on a variety of factors, including:
19
We may never succeed in achieving regulatory approval for any of our product candidates. We may obtain unexpected results from our preclinical studies and clinical trials. We may elect to discontinue, delay or modify clinical trials of some product candidates or focus on others. A change in the outcome of any of these factors could mean a significant change in the costs and timing associated with the development of our current and future preclinical and clinical product candidates. For example, if the FDA or another regulatory authority were to require us to conduct clinical trials beyond those that we currently anticipate will be required for the completion of clinical development, or if we experience significant delays in execution of or enrollment in any of our preclinical studies or clinical trials, we could be required to expend significant additional financial resources and time on the completion of preclinical and clinical development.
Research and development activities account for a significant portion of our operating expenses. We expect our research and development expenses to increase for the foreseeable future as we continue to implement our business strategy, which includes advancing IO102-IO103 through clinical development and other product candidates further into clinical development, expanding our research and development efforts, including hiring additional personnel to support our research and development efforts, and seeking regulatory approvals for our product candidates that successfully complete clinical trials. In addition, product candidates in later stages of clinical development generally incur higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. As a result, we expect our research and development expenses to increase as our product candidates advance into later stages of clinical development. However, we do not believe that it is possible at this time to accurately project total program-specific expenses through commercialization. There are numerous factors associated with the successful commercialization of any of our product candidates, including future trial design and various regulatory requirements, many of which cannot be determined with accuracy at this time based on our stage of development.
General and Administrative Expenses
Our general and administrative expenses consist primarily of personnel costs, depreciation expense and other expenses for outside professional services, including legal fees relating to patent and corporate matters, human resources, audit and accounting services and facility-related fees not otherwise included in research and development expenses. Personnel costs consist of salaries, benefits and equity-based compensation expense, for our personnel in executive, finance and accounting, business operations and other administrative functions. We expect our general and administrative expenses to increase over the next several years to support our continued research and development activities, manufacturing activities, increased costs of expanding our operations and operating as a public company. These increases will likely include increases related to the hiring of additional personnel, fees to outside consultants, lawyers and accountants, and increased costs associated with being a public company such as expenses related to services associated with maintaining compliance with Nasdaq listing rules and SEC requirements, director and officer insurance premiums and investor relations costs.
Other Income (Expense), Net
Our other income (expense), net is comprised of:
20
Results of Operations
Comparison of the three months ended June 30, 2023 and 2022
The following sets forth our results of operations:
|
|
For the Three Months |
|
|
Change |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
Amount |
|
|
Percent |
|
||||
|
|
(in thousands) |
|
|||||||||||||
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
$ |
16,504 |
|
|
$ |
12,226 |
|
|
$ |
4,278 |
|
|
|
35.0 |
% |
General and administrative |
|
|
5,348 |
|
|
|
5,935 |
|
|
|
(587 |
) |
|
|
(9.9 |
)% |
Total operating expenses |
|
|
21,852 |
|
|
|
18,161 |
|
|
|
3,691 |
|
|
|
20.3 |
% |
Loss from operations |
|
|
(21,852 |
) |
|
|
(18,161 |
) |
|
|
(3,691 |
) |
|
|
20.3 |
% |
Other income (expense) |
|
|
1,206 |
|
|
|
(230 |
) |
|
|
1,436 |
|
|
|
(624.3 |
)% |
Loss before income tax expense |
|
|
(20,646 |
) |
|
|
(18,391 |
) |
|
|
(2,255 |
) |
|
|
12.3 |
% |
Income tax expense |
|
|
532 |
|
|
|
104 |
|
|
|
428 |
|
|
|
411.5 |
% |
Net loss |
|
$ |
(21,178 |
) |
|
$ |
(18,495 |
) |
|
$ |
(2,683 |
) |
|
|
14.5 |
% |
Research and Development Expenses
Research and development expenses were comprised of:
|
|
For the Three Months |
|
|
Change |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
Amount |
|
|
Percent |
|
||||
|
|
(in thousands) |
|
|||||||||||||
Preclinical studies and clinical trial-related activities |
|
$ |
7,283 |
|
|
$ |
5,875 |
|
|
$ |
1,408 |
|
|
|
24.0 |
% |
Chemistry, manufacturing and control |
|
|
4,933 |
|
|
|
1,596 |
|
|
|
3,337 |
|
|
|
209.1 |
% |
Personnel |
|
|
3,804 |
|
|
|
3,755 |
|
|
|
49 |
|
|
|
1.3 |
% |
Consultants and other costs |
|
|
484 |
|
|
|
1,000 |
|
|
|
(516 |
) |
|
|
(51.6 |
)% |
Total research and development expenses |
|
$ |
16,504 |
|
|
$ |
12,226 |
|
|
$ |
4,278 |
|
|
|
35.0 |
% |
Research and development expenses were $16.5 million for the three months ended June 30, 2023, compared to $12.2 million for the three months ended June 30, 2022. The increase of $4.3 million was primarily related to an increase in preclinical studies and clinical trial-related activities for our IO102-IO103 product candidate, including the continued execution of our Phase 3 clinical trial, of $1.4 million and an increase in costs for chemistry, manufacturing and control activities of $3.3 million related to manufacturing activities of our IO102-IO103 product candidate, which were offset by a decrease in consultants and other costs of $0.5 million.
General and Administrative Expenses
General and administrative expenses were comprised of:
|
|
For the Three Months |
|
|
Change |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
Amount |
|
|
Percent |
|
||||
|
|
(in thousands) |
|
|||||||||||||
Personnel |
|
$ |
2,196 |
|
|
$ |
1,756 |
|
|
$ |
440 |
|
|
|
25.1 |
% |
Professional services |
|
|
948 |
|
|
|
878 |
|
|
|
70 |
|
|
|
8.0 |
% |
Consultants and other costs |
|
|
2,204 |
|
|
|
3,301 |
|
|
|
(1,097 |
) |
|
|
(33.2 |
)% |
Total general and administrative expenses |
|
$ |
5,348 |
|
|
$ |
5,935 |
|
|
$ |
(587 |
) |
|
|
(9.9 |
)% |
General and administrative expenses were $5.3 million for the three months ended June 30, 2023, compared to $5.9 million for the three months ended June 30, 2022. The decrease of $0.6 million was primarily related to a decrease in consultants and other costs of $1.1 million due to a decrease in consultant spend and insurance premiums, which was offset by an increase in personnel costs of $0.4 million primarily related to an increase in headcount.
21
Other Income (Expense), Net
Other income (expense), net was comprised of:
|
|
For the Three Months |
|
|
Change |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
Amount |
|
|
Percent |
|
||||
|
|
(in thousands) |
|
|||||||||||||
Currency exchange gain (loss), net |
|
$ |
10 |
|
|
$ |
(286 |
) |
|
$ |
296 |
|
|
|
(103.5 |
)% |
Interest income |
|
|
1,196 |
|
|
|
158 |
|
|
|
1,038 |
|
|
|
657.0 |
% |
Interest expense |
|
|
— |
|
|
|
(102 |
) |
|
|
102 |
|
|
|
(100.0 |
)% |
Other income (expense), net |
|
$ |
1,206 |
|
|
$ |
(230 |
) |
|
$ |
1,436 |
|
|
|
(624.3 |
)% |
Other income (expense), net was $1.2 million for the three months ended June 30, 2023, compared to ($0.2) million for the three months ended June 30, 2022. The increase of $1.4 million was primarily due to the increase in interest income recognized on our money market fund.
Comparison of the six months ended June 30, 2023 and 2022
The following sets forth our results of operations:
|
|
For the Six Months |
|
|
Change |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
Amount |
|
|
Percent |
|
||||
|
|
(in thousands) |
|
|||||||||||||
Operating expenses |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
$ |
28,404 |
|
|
$ |
22,531 |
|
|
$ |
5,873 |
|
|
|
26.1 |
% |
General and administrative |
|
|
11,372 |
|
|
|
12,639 |
|
|
|
(1,267 |
) |
|
|
(10.0 |
)% |
Total operating expenses |
|
|
39,776 |
|
|
|
35,170 |
|
|
|
4,606 |
|
|
|
13.1 |
% |
Loss from operations |
|
|
(39,776 |
) |
|
|
(35,170 |
) |
|
|
(4,606 |
) |
|
|
13.1 |
% |
Other income (expense) |
|
|
2,492 |
|
|
|
(358 |
) |
|
|
2,850 |
|
|
|
(796.1 |
)% |
Loss before income tax expense |
|
|
(37,284 |
) |
|
|
(35,528 |
) |
|
|
(1,756 |
) |
|
|
4.9 |
% |
Income tax expense |
|
|
938 |
|
|
|
171 |
|
|
|
767 |
|
|
|
448.5 |
% |
Net loss |
|
$ |
(38,222 |
) |
|
$ |
(35,699 |
) |
|
$ |
(2,523 |
) |
|
|
7.1 |
% |
Research and Development Expenses
Research and development expenses were comprised of:
|
|
For the Six Months |
|
|
Change |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
Amount |
|
|
Percent |
|
||||
|
|
(in thousands) |
|
|||||||||||||
Preclinical studies and clinical trial-related activities |
|
$ |
12,629 |
|
|
$ |
9,811 |
|
|
$ |
2,818 |
|
|
|
28.7 |
% |
Chemistry, manufacturing and control |
|
|
7,751 |
|
|
|
4,624 |
|
|
|
3,127 |
|
|
|
67.6 |
% |
Personnel |
|
|
7,595 |
|
|
|
7,177 |
|
|
|
418 |
|
|
|
5.8 |
% |
Consultants and other costs |
|
|
429 |
|
|
|
919 |
|
|
|
(490 |
) |
|
|
(53.3 |
)% |
Total research and development expenses |
|
$ |
28,404 |
|
|
$ |
22,531 |
|
|
$ |
5,873 |
|
|
|
26.1 |
% |
Research and development expenses were $28.4 million for the six months ended June 30, 2023, compared to $22.5 million for the six months ended June 30, 2022. The increase of $5.9 million was primarily related to an increase in preclinical studies and clinical trial-related activities for our IO102-IO103 product candidate, including the continued execution of our Phase 3 clinical trial of $2.8 million and an increase in costs for chemistry, manufacturing and control activities of $3.1 million related to manufacturing activities of our IO102-IO103 product candidate.
22
General and Administrative Expenses
General and administrative expenses were comprised of:
|
|
For the Six Months |
|
|
Change |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
Amount |
|
|
Percent |
|
||||
|
|
(in thousands) |
|
|||||||||||||
Personnel |
|
$ |
4,851 |
|
|
$ |
3,587 |
|
|
$ |
1,264 |
|
|
|
35.2 |
% |
Professional services |
|
|
2,457 |
|
|
|
2,664 |
|
|
|
(207 |
) |
|
|
(7.8 |
)% |
Consultants and other costs |
|
|
4,064 |
|
|
|
6,388 |
|
|
|
(2,324 |
) |
|
|
(36.4 |
)% |
Total general and administrative expenses |
|
$ |
11,372 |
|
|
$ |
12,639 |
|
|
$ |
(1,267 |
) |
|
|
(10.0 |
)% |
General and administrative expenses were $11.4 million for the six months ended June 30, 2023, compared to $12.6 million for the six months ended June 30, 2022. The decrease of $1.3 million was primarily related to a decrease in consultants and other costs of $2.3 million primarily related to a decrease in consultant spend and insurance premiums, which was offset by an increase in personnel costs of $1.3 million primarily related to an increase in headcount.
Other Income (Expense), Net
Other income (expense), net was comprised of:
|
|
For the Six Months |
|
|
Change |
|
||||||||||
|
|
2023 |
|
|
2022 |
|
|
Amount |
|
|
Percent |
|
||||
|
|
(in thousands) |
|
|||||||||||||
Currency exchange gain (loss), net |
|
$ |
268 |
|
|
$ |
(305 |
) |
|
$ |
573 |
|
|
|
(187.9 |
)% |
Interest income |
|
|
2,224 |
|
|
|
173 |
|
|
|
2,051 |
|
|
|
1185.5 |
% |
Interest expense |
|
|
— |
|
|
|
(226 |
) |
|
|
226 |
|
|
|
(100.0 |
)% |
Other income (expense), net |
|
$ |
2,492 |
|
|
$ |
(358 |
) |
|
$ |
2,850 |
|
|
|
(796.1 |
)% |
Other income (expense), net was $2.5 million for the six months ended June 30, 2023, compared to $(0.4) million for the six months ended June 30, 2022. The increase of $2.9 million was primarily due to the increase in interest income recognized on our money market fund.
Liquidity and Capital Resources
Sources of Liquidity
Our operations to date have been financed primarily by aggregate net proceeds of $288.7 million from the issuance of convertible preference shares, convertible notes, class A ordinary shares, and our IPO. In addition, on August 7, 2023, we entered into the Purchase Agreement with the Purchasers, pursuant to which we raised an additional $75.1 million in gross proceeds through the Private Placement, before deducting estimated offering expenses.
On November 9, 2021, we completed an IPO of our common stock and issued and sold 8,222,500 shares of common stock at a public offering price of $14.00 per share, including 1,072,500 shares of common stock sold pursuant to the underwriters’ exercise of their option to purchase additional shares of common stock, resulting in net proceeds of $103.3 million after deducting underwriting discounts and commissions and estimated offering expenses. In addition, on August 7, 2023, we entered into the Purchase Agreement with the Purchasers, pursuant to which we raised an additional $75.1 million in gross proceeds through the Private Placement, before deducting estimated offering expenses that are expected to amount to approximately $3.0 million. Since inception, we have had significant operating losses. Our net loss was $38.2 million for the six months ended June 30, 2023 and $71.5 million and $67.9 million for the years ended December 31, 2022 and 2021, respectively. As of June 30, 2023, we had an accumulated deficit of $216.0 million and $110.1 million in cash and cash equivalents. Our primary use of cash is to fund operating expenses, which consist primarily of research and development expenditures, and to a lesser extent, general and administrative expenditures. Cash used to fund operating expenses is impacted by the timing of when we pay these expenses, as reflected in the change in our outstanding accounts payable and accrued expenses.
23
In addition, on February 15, 2023, we filed a new prospectus supplement with the SEC with respect to the offer and sale of shares of our common stock, par value $0.001 per share, with an aggregate offering price of up to $19,500,000, establishing an at-the-market equity program. On February 15, 2023, we also entered into a Sales Agreement by and between the Company and Cowen and Company, LLC for shares with an aggregate offering price of up to $75,000,000 through which we may, from time to time, sell shares to Cowen and Company, LLC. Any shares offered and sold through the at-the-market equity program will be issued pursuant to the Company’s Registration Statement on Form S-3 (File No. 333-269569), which was declared effective on February 10, 2023, the prospectus supplement related to the offering that forms a part of the registration statement, and any applicable prospectus supplements that may form a part of the registration statement in the future. The aggregate market value of shares eligible for sale under the prospectus supplement and under the Sales Agreement will be subject to the limitations of General Instruction I.B.6 of Form S-3, to the extent required under such instruction.
We currently expect that our cash and cash equivalents of $110.1 million as of June 30, 2023, taken together with the $75.1 million in gross proceeds raised through the Private Placement, which closed on August 9, 2023, will be sufficient to fund our operating expenses and capital requirements into the fourth quarter of 2025. However, additional funding will be necessary to fund our future clinical and pre-clinical activities. If we are unable to obtain funding, we could be forced to delay, reduce or eliminate our research and development programs, product portfolio expansion or commercialization efforts, which could adversely affect our business prospects and our ability to continue operations.
Cash Flows
The following table summarizes our cash flows for the periods indicated:
|
|
For the Six Months |
|
|||||
|
|
2023 |
|
|
2022 |
|
||
|
|
(in thousands) |
|
|||||
Net cash used in operating activities |
|
$ |
(32,952 |
) |
|
$ |
(33,855 |
) |
Net cash used in investing activities |
|
|
(187 |
) |
|
|
(223 |
) |
Net cash provided by financing activities |
|
|
— |
|
|
|
— |
|
Net decrease in cash and cash equivalents |
|
$ |
(33,139 |
) |
|
$ |
(34,078 |
) |
Net Cash Used in Operating Activities
Cash used in operating activities of $33.0 million for the six months ended June 30, 2023 was primarily attributable to our net loss of $38.2 million, partially offset by a decrease of $1.7 million in our working capital accounts and an increase in non-cash items of $3.6 million primarily due to share-based compensation.
Cash used in operating activities of $33.9 million for the six months ended June 30, 2022 was primarily attributable to our net loss of $35.7 million and a net increase of $1.7 million in our working capital accounts, partially offset by non-cash items of $3.5 million primarily due to share-based compensation.
Net Cash Used in Investing Activities
Cash used in investing activities of $0.2 million for both the six months ended June 30, 2023 and 2022, respectively, was related to the purchase of property and equipment.
Net Cash Provided by Financing Activities
We had no cash provided by financing activities for the six months ended June 30, 2023 and 2022.
Funding Requirements
Any product candidates we may develop may never achieve commercialization and we anticipate that we will continue to incur losses for the foreseeable future. We expect that our research and development expenses, general and administrative expenses, and capital expenditures will continue to increase. As a result, until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity offerings, debt financings or other capital sources, including potentially collaborations, licenses and other similar arrangements. Our primary uses of capital are, and we expect will continue to be, compensation and related expenses; costs related to third-party clinical research, manufacturing and development services; costs relating to the build-out of our headquarters and our other offices, laboratories and manufacturing facility; license payments or milestone obligations that may arise; laboratory expenses and costs for related supplies; clinical costs; manufacturing costs; legal and other regulatory expenses; and general overhead costs.
24
Based upon our current operating plan, we believe that our existing cash and cash equivalents of $110.1 million as of June 30, 2023, taken together with the $75.1 million in gross proceeds raised through the Private Placement, which closed on August 9, 2023, will be sufficient to continue funding our development activities into the fourth quarter of 2025. To finance our operations beyond that point we will need to raise additional capital, which, though we were successful in raising additional capital through the Private Placement, cannot be assured and which we may not be able to pursue successfully again in the future. We have based this estimate on assumptions that may prove to be wrong, and we could utilize our available capital resources sooner than we currently expect. We will continue to require additional financing to advance our current product candidates through clinical development, to develop, acquire or in-license other potential product candidates and to fund operations for the foreseeable future. We will continue to seek funds through equity offerings, debt financings or other capital sources, potentially including collaborations, licenses and other similar arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. If we do raise additional capital through public or private equity offerings, the ownership interest of our existing stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect our stockholders’ rights. If we raise additional capital through debt financing, we may be subject to covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Any failure to raise capital as and when needed could have a negative impact on our financial condition and on our ability to pursue our business plans and strategies. If we are unable to raise capital, we may need to delay, reduce or terminate planned activities to reduce costs.
Because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
Further, our operating plans may change, and we may need additional funds to meet operational needs and capital requirements for clinical trials and other research and development activities. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated product development programs.
25
Contractual Obligations and Commitments
In March 2021, we entered into a new lease for our office space in Copenhagen, Denmark that was amended in September 2022 and set to expire in December 2027. The lease for our office space in Copenhagen, Denmark is terminable upon six months' notice. In January 2023, we entered into a new lease for laboratory space in Copenhagen, Denmark that expires in December 2027. In August 2021, we entered into a new lease for laboratory facilities and office space in Rockville, Maryland that expires in April 2027. In October 2021, we entered into a new lease for office space in New York, New York that expires in January 2027. In June 2023, we entered into a new lease for office space in Newport, United Kingdom that expires in May 2025.
We enter into contracts in the ordinary course of business with third-party service providers for clinical trials, preclinical research studies and testing, manufacturing and other services and products for operating purposes. These contracts generally provide for termination upon notice of 30 to 90 days, and therefore, we believe that our non-cancelable obligations under these agreements are not material and we cannot reasonably estimate whether they will occur. However, in the event of a termination of any contracts with CROs or other institutions and with respect to active patients enrolled in our clinical trials, we may be financially obligated for a period beyond the contractual termination notice periods.
We may also enter into additional research, manufacturing, supplier, lease and other agreements in the future, which may require up-front payments and even long-term commitments of cash.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our condensed consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these condensed consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management’s judgments and estimates.
Going Concern
Our evaluation of our ability to continue as a going concern requires us to evaluate our future sources and uses of cash sufficient to fund our currently expected operations in conducting research and development activities. We evaluate the probability associated with each source and use of cash resources in making our going concern determination. The research and development of pharmaceutical products is inherently subject to uncertainty. We currently expect that our cash and cash equivalents of $110.1 million as of June 30, 2023 will be sufficient to fund our operating expenses and capital requirements for at least 12 months from the date the condensed consolidated financial statements are issued.
Research and Development Costs
We incur substantial expenses associated with clinical trials. Accounting for clinical trials relating to activities performed by CMOs, CROs and other external vendors requires management to exercise significant estimates in regard to the timing and accounting for these expenses. We estimate costs of research and development activities conducted by service providers, which include costs associated with the conduct of sponsored research, preclinical studies, contract manufacturing activities and pass-through costs. The diverse nature of services being provided under CRO and other arrangements, the different compensation arrangements that exist for each type of service and the lack of timely information related to certain clinical activities complicates the estimation of accruals for services rendered by CROs and other vendors in connection with clinical trials. We record the estimated costs of research and development activities based upon the estimated amount of services provided but not yet invoiced and include these costs in the accrued and other current liabilities or prepaid expenses on the balance sheets and within research and development expense on the statements of operations. In estimating the duration of a clinical study, we evaluate the start-up treatment and wrap-up periods, compensation arrangements and services rendered attributable to each clinical trial. Fluctuations are regularly tested against payment plans and trial completion assumptions.
We estimate these costs based on factors such as estimates of the work completed and budget provided and in accordance with agreements established with our collaboration partners and third-party service providers. We make significant judgments and estimates in determining the accrued liabilities and prepaid expense balances in each reporting period. As actual costs become known, we adjust our accrued liabilities or prepaid expenses. We have not experienced any material differences between accrued costs and actual costs incurred since our inception.
26
Our expenses related to clinical trials are based on estimates of patient enrollment and related expenses at clinical investigator sites as well as estimates for the services received and efforts expended pursuant to contracts with multiple research institutions, CMOs and CROs that may be used to conduct and manage clinical trials, chemistry and testing and manufacturing services on our behalf. We generally accrue expenses related to clinical trials based on contracted amounts applied to the level of patient enrollment and activity. If timelines or contracts are modified based upon changes in the clinical trial protocol or scope of work to be performed, we modify our estimates of accrued expenses accordingly on a prospective basis.
Equity-based Compensation
We issued stock-based compensation awards through the granting of warrants and stock options, which generally vest over a four-year period. We issued 1,211,155 options with a weighted average exercise price of $5.34 to certain employees, board members and advisors during the year ended December 31, 2022. We also issued 1,996,365 options with a weighted average exercise price of $2.07 under our 2021 Equity Plan during the six months ended June 30, 2023.
We account for equity-based compensation in accordance with ASC 718, Compensation-Stock Compensation (718). In accordance with ASC 718, compensation cost is measured at estimated fair value and is included as compensation expense over the vesting period during which service is provided in exchange for the award. The Company reverses any previously recognized compensation cost associated with forfeited awards in the period of the forfeiture occurs.
We use a Black-Scholes option pricing model to determine fair value of our warrants and options. The Black-Scholes option pricing model includes various assumptions, including the fair value of common shares, expected life of warrants and options, the expected volatility and the expected risk-free interest rate. These assumptions reflect our best estimates, but they involve inherent uncertainties based on market conditions generally outside our control. As a result, if other assumptions had been used, equity-based compensation cost could have been materially impacted. Furthermore, if we use different assumptions for future grants, share-based compensation cost could be materially impacted in future periods.
We will continue to use judgment in evaluating the assumptions utilized for our equity-based compensation expense calculations on a prospective basis. In addition to the assumptions used in the Black-Scholes model, the amount of equity-based compensation expense we recognize in our financial statements includes warrant forfeitures as they occurred.
Income Taxes
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and operating losses and tax credit carry forwards. Deferred tax assets and liabilities are measured using enacted statutory tax rates expected to apply to taxable income in the jurisdictions and years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
Based on the level of historical operating results and projections for the taxable income for the future, we have determined that it is more likely than not that our net deferred tax assets will not be realized. Accordingly, we have recorded a full valuation allowance to reduce our net deferred tax assets in IO Biotech ApS, IO Bio US, Inc. and IO Biotech, Inc.
We recognize tax benefits from uncertain tax positions only if, based on the technical merits of the position, it is more likely than not that the tax positions will be sustained on examination by the tax authority. The tax benefits recognized in the financial statements from such positions are measured based on the largest amount that is more than 50% likely to be realized upon ultimate settlement. We recognize interest and penalties related to unrecognized tax benefits within the provision for taxes in our statements of operations and comprehensive loss.
We operate in Denmark and the United States and may be subject to audits from various tax authorities. Management’s judgment is required in determining our provision for income taxes, our deferred tax assets and liabilities, liabilities for uncertain tax positions, and any valuation allowance recorded against our net deferred tax assets. We will monitor the extent to which our deferred tax assets may be realized and adjust the valuation allowance accordingly.
Recently Adopted Significant Accounting Policies
Refer to Note 2, “Summary of Significant Accounting Policies,” in the accompanying notes to our condensed consolidated financial statements for the six months ended June 30, 2023 and 2022 appearing elsewhere in this Quarterly Report on Form 10-Q for a discussion of recently issued accounting standards.
Off-Balance Sheet Arrangements
During the periods presented, we did not have, nor do we currently have, any off-balance sheet arrangements as defined under SEC rules.
27
Emerging Growth Company Status
As an EGC under the JOBS Act, we may delay the adoption of certain accounting standards until such time as those standards apply to private companies. We have elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date we (1) are no longer an emerging growth company or (2) affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. As a result, our condensed consolidated financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
Other exemptions and reduced reporting requirements under the JOBS Act for EGCs include presentation of only two years of audited financial statements in a registration statement for an IPO, an exemption from the requirement to provide an auditor’s report on internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act, an exemption from any requirement that may be adopted by the Public Company Accounting Oversight Board (PCAOB) regarding mandatory audit firm rotation, and less extensive disclosure about our executive compensation arrangements.
We may remain classified as an EGC until December 31, 2026, although if the market value of our common stock that is held by non-affiliates exceeds $700 million as of June 30 of any year before that time, or if we have annual gross revenues of $1.235 billion or more in any fiscal year, we would cease to be an EGC as of December 31 of the applicable year. We also would cease to be an EGC if we issue more than $1.0 billion of non-convertible debt over a three-year period.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
We are a smaller reporting company as defined by Item 10 of Regulation S-K and are not required to provide the information otherwise required under this item.
Item 4. Controls and Procedures.
Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer, Chief Financial Officer and Chief Accounting Officer, evaluated the effectiveness of our disclosure controls and procedures as of June 30, 2023. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended (the Exchange Act), means controls and other procedures of a company that are designed to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company on the reports that it files or submits under the Exchange Act is accumulated and communicated to management, including, our principal executive and principal financial officers, as appropriate, to allow timely decisions regarding required disclosure.
Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures. Based on the evaluation of our disclosure controls and procedures as of June 30, 2023, our Chief Executive Officer, Chief Financial Officer and Chief Accounting Officer concluded that our disclosure controls and procedures were effective as of June 30, 2023.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting as defined under the Exchange Act and by the PCAOB, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
The Company previously disclosed a material weakness in internal control over financial reporting as of December 31, 2021 in Item 9A. of our Annual Report on Form 10-K for the year ended December 31, 2021 related to our financial statement close process, primarily related to the lack of required finance capacity, knowledge or expertise to perform the financial statement close in a timely and accurate manner or to account for certain complex areas of U.S. GAAP. The material weakness was remediated effective December 31, 2022 through processes described in Item 9A of our Annual Report on Form 10-K for the year ended December 31, 2022.
Changes in Internal Control
There has been no change in our internal control over financial reporting as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act during our most recently completed fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
28
PART II—OTHER INFORMATION
Item 1. Legal Proceedings.
From time to time, we may be a party to litigation or subject to claims incident to the ordinary course of business. Although the results of litigation and claims cannot be predicted with certainty, we currently believe that the final outcome of these ordinary course matters will not have a material adverse effect on our business. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors. We are not currently a party to any material legal proceedings.
Item 1A. Risk Factors.
Our business is subject to risks and events that, if they occur, could adversely affect our financial condition and results of operations and the trading price of our securities. In addition to the other information set forth in this Quarterly Report on Form 10-Q, you should carefully consider the factors described in Part I, Item 1A. “Risk Factors” of our Annual Report on Form 10-K for the fiscal year ended December 31, 2022, filed with the SEC on March 14, 2023, and Part II, Item 1A. “Risk Factors” of our Quarterly Report on Form Q for the fiscal quarter ended March 31, 2023, filed with the SEC on May 11, 2023. There have been no material changes to the risk factors described in those reports.
29
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
Use of Proceeds From Registered Securities
On November 9, 2021, we completed our IPO in which we issued and sold 8,222,500 shares of common stock, $0.001 par value per share, including 1,072,500 shares of common stock sold pursuant to the underwriters’ exercise of their option to purchase additional shares of common stock. The offer and sale of the shares in the IPO was registered under the Securities Act pursuant to a registration statement on Form S-1 (File No. 333-260301), which was filed with the SEC on October 15, 2021 and subsequently amended and declared effective on November 4, 2021, and the prospectus included therein (the “Prospectus”). The underwriters of the IPO were Morgan Stanley & Co. LLC, Jefferies LLC, Cowen and Company, LLC and Kempen & Co U.S.A, Inc.
We raised approximately $103.3 million in net proceeds after deducting underwriting discounts and commissions of $8.0 million and other offering expenses of approximately $3.8 million payable by us. No underwriting discounts and commissions or offering expenses were paid directly or indirectly to any of our directors of officers (or their associates) or persons owning ten percent or more of any class of our equity securities or to any other affiliates.
There has been no material change in the planned use of proceeds from our IPO, as described in the Prospectus.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
None.
Item 5. Other Information.
None.
30
Item 6. Exhibits.
Exhibit Number |
|
Description |
3.1* |
|
Amended and Restated Certificate of Incorporation of IO Biotech, Inc. |
31.1* |
|
|
31.2* |
|
|
32.1* |
|
|
32.2* |
|
|
101.INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
* Filed herewith.
31
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
IO Biotech, Inc. |
|
|
|
|
|
Date: August 11, 2023 |
|
By: |
/s/ Mai-Britt Zocca |
|
|
|
Mai-Britt Zocca, Ph.D. |
|
|
|
Chief Executive Officer and Director |
|
|
|
(Principal Executive) |
|
|
|
|
Date: August 11, 2023 |
|
By: |
/s/ Amy Sullivan |
|
|
|
Amy Sullivan |
|
|
|
Chief Financial Officer |
|
|
|
(Principal Financial Officer) |
|
|
|
|
32