
OmniAb, Inc. Nasdaq: OABI January 2023 Exhibit 99.1
We caution you that this presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, expected cash runway, business strategy, our expectations regarding the application of, and the rate and degree of market acceptance of, our technology platform and other technologies, our expectations regarding the addressable markets for our technologies, including the growth rate of the markets in which we operate, the timing of the initiation or completion of preclinical studies and clinical trials by our partners, expectations regarding product approvals and potential for future revenue growth, launches by our partners and the timing thereof, and the potential for and timing of receipt of milestones and royalties under our license agreements with partners, are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions. The inclusion of forward-looking statements should not be regarded as a representation by us that any of our plans will be achieved. Actual results may differ from those set forth in this presentation due to the risks and uncertainties inherent in our business, including, without limitation: the anticipated benefits of our separation from Ligand may not be achieved; our future success is dependent on acceptance of our technology platform by new and existing partners, as well as on the eventual development, approval and commercialization of products developed by our partners for which we have no control over the development plan, regulatory strategy or commercialization efforts; biopharmaceutical development is inherently uncertain, risks arising from changes in technology; the competitive environment in the life sciences and biotechnology platform market; our failure to maintain, protect and defend our intellectual property rights; difficulties with performance of third parties we will rely on for our business; regulatory developments in the United States and foreign countries; unstable market and economic conditions may have serious adverse consequences on our business, financial condition and stock price; we may use our capital resources sooner than we expect; and other risks described in our press releases and filings with the SEC. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date made, and except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise. All forward-looking statements are qualified in their entirety by this cautionary statement, which is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Information regarding partnered products and programs comes from information publicly released by our partners. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about the antibody industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. Disclaimer
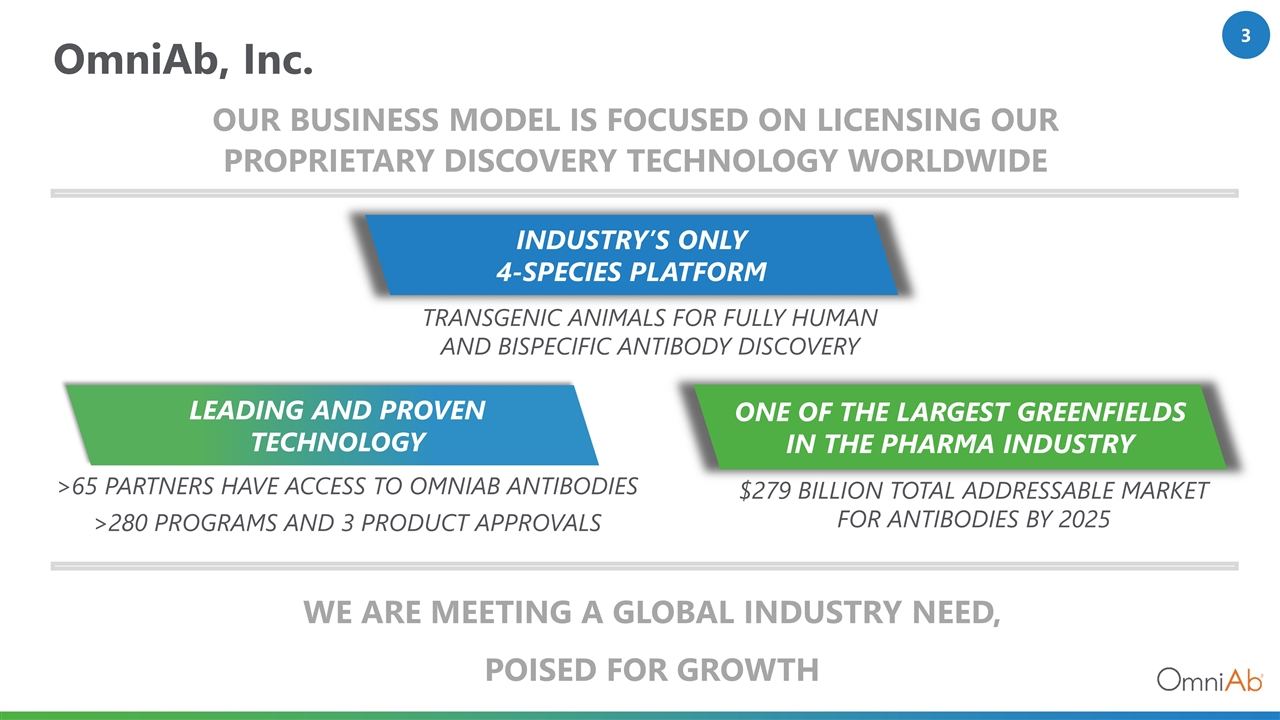
OmniAb, Inc. ONE OF THE LARGEST GREENFIELDS IN THE PHARMA INDUSTRY >65 partners HAVE ACCESS TO OMNIAB ANTIBODIES >280 Programs and 3 PRODUCT approvals Transgenic animals for fully human and Bispecific antibody Discovery LEADING AND PROVEN TECHNOLOGY INDUSTRY’S ONLY 4-SPECIES PLATFORM $279 Billion Total Addressable Market for Antibodies by 2025 OUR BUSINESS MODEL IS FOCUSED ON Licensing our proprietary DISCOVERY TECHNOLOGY Worldwide WE ARE MEETING A GLOBAL INDUSTRY NEED, POISED FOR GROWTH

Our mission is to enable the rapid development of innovative therapeutics by pushing the frontiers of drug discovery technologies. Mission
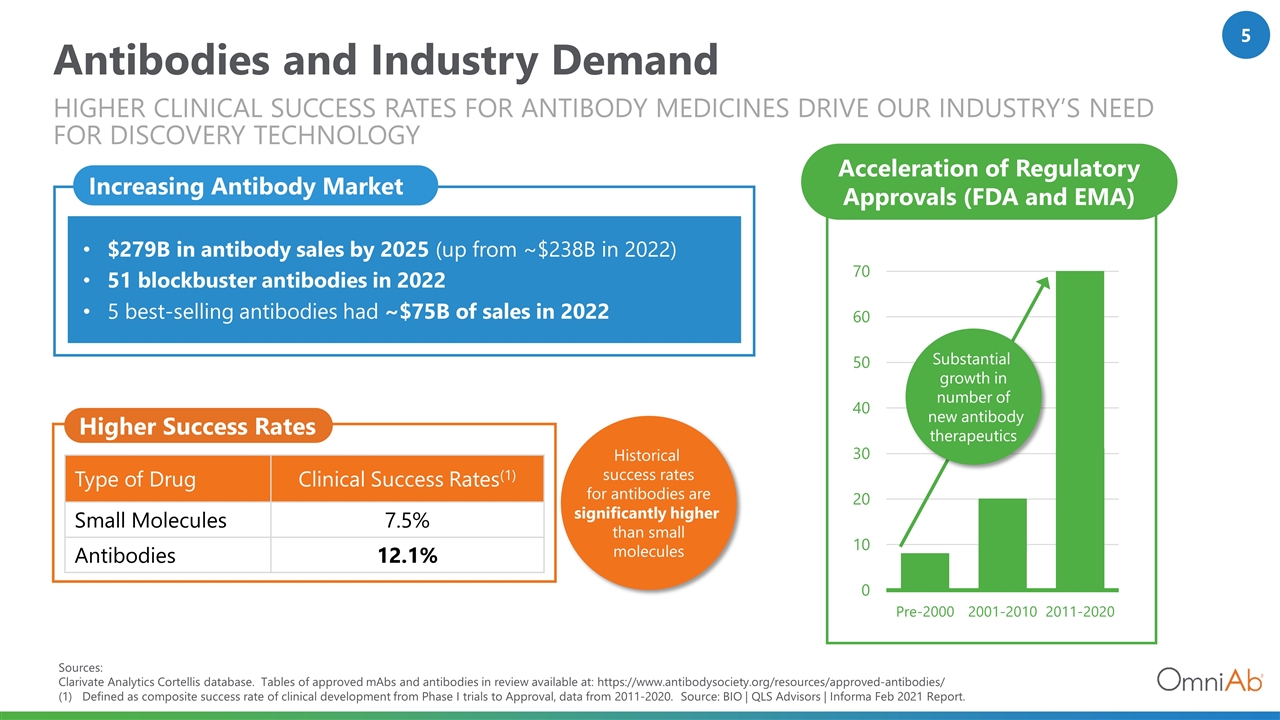
Type of Drug Clinical Success Rates(1) Small Molecules 7.5% Antibodies 12.1% Sources: Clarivate Analytics Cortellis database. Tables of approved mAbs and antibodies in review available at: https://www.antibodysociety.org/resources/approved-antibodies/ (1) Defined as composite success rate of clinical development from Phase I trials to Approval, data from 2011-2020. Source: BIO | QLS Advisors | Informa Feb 2021 Report. Higher Success Rates Historical success rates for antibodies are significantly higher than small molecules Antibodies and Industry Demand Higher clinical Success Rates for Antibody Medicines Drive our Industry’s Need for Discovery Technology Increasing Antibody Market $279B in antibody sales by 2025 (up from ~$238B in 2022) 51 blockbuster antibodies in 2022 5 best-selling antibodies had ~$75B of sales in 2022 Acceleration of Regulatory Approvals (FDA and EMA) Substantial growth in number of new antibody therapeutics
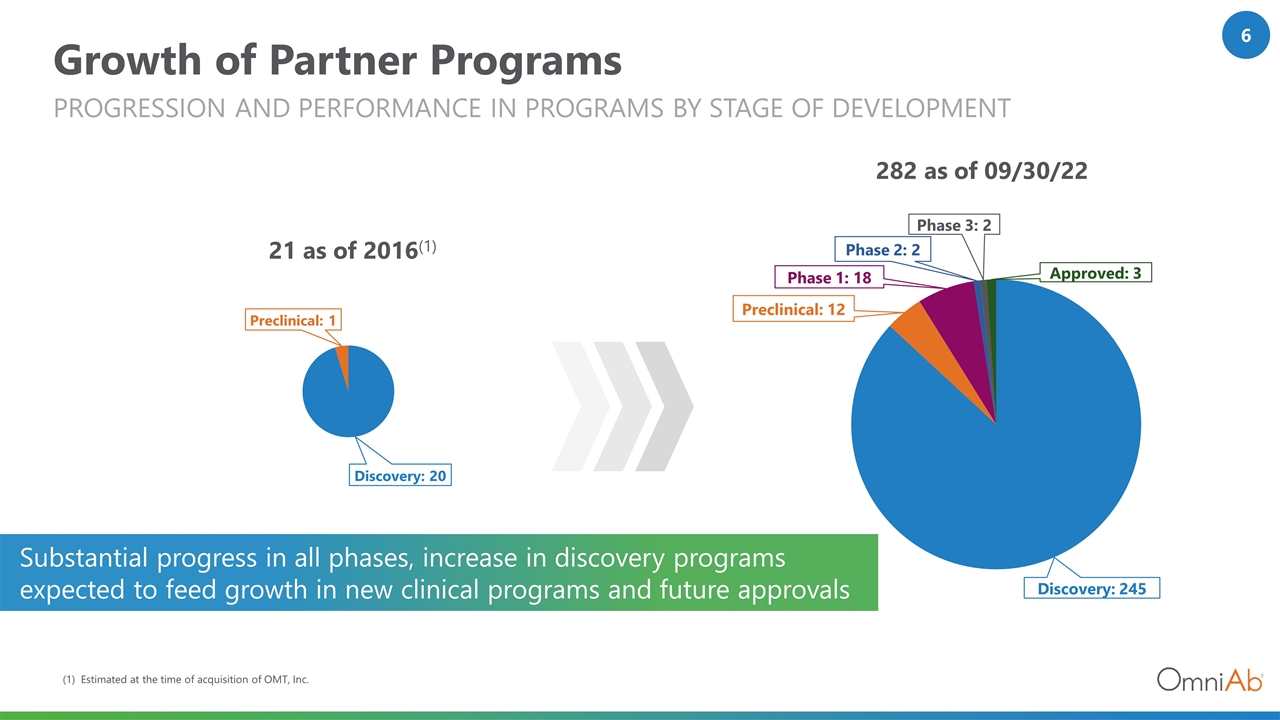
Growth of Partner Programs Progression and Performance in Programs by Stage of Development 21 as of 2016(1) 282 as of 09/30/22 (1) Estimated at the time of acquisition of OMT, Inc. Substantial progress in all phases, increase in discovery programs expected to feed growth in new clinical programs and future approvals
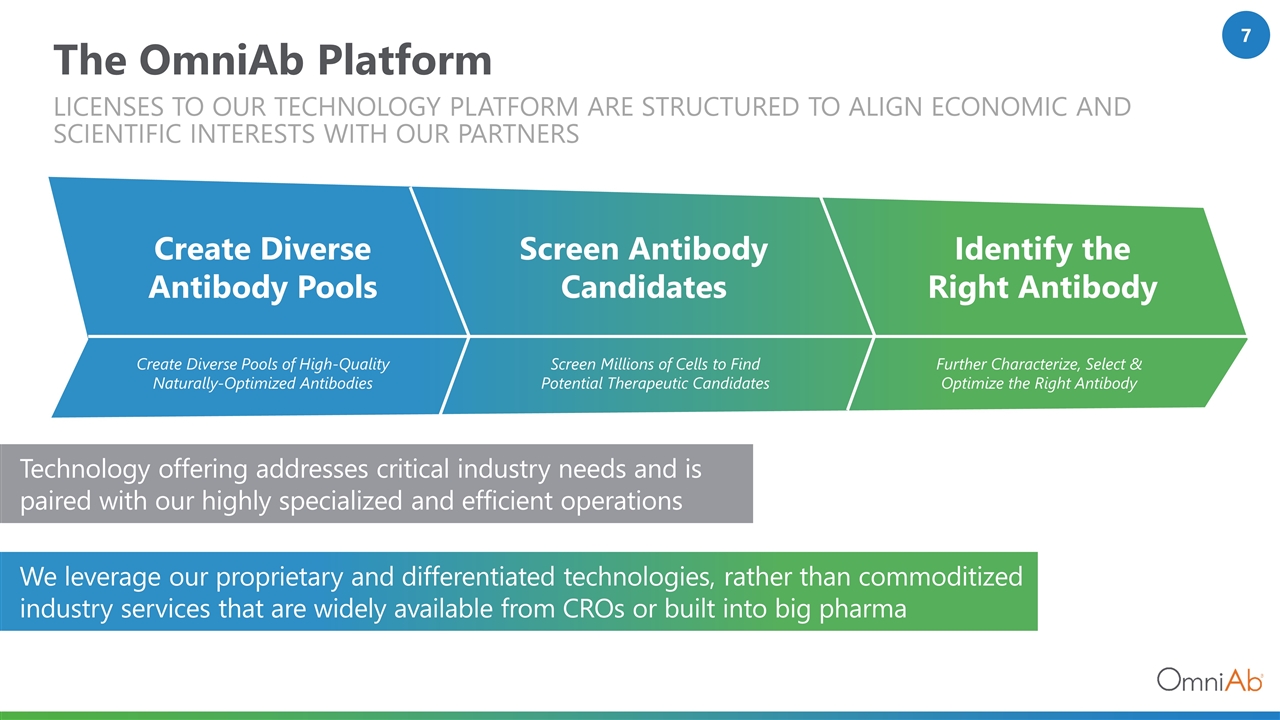
The OmniAb Platform We leverage our proprietary and differentiated technologies, rather than commoditized industry services that are widely available from CROs or built into big pharma Create Diverse Pools of High-Quality Naturally-Optimized Antibodies Screen Millions of Cells to Find Potential Therapeutic Candidates Further Characterize, Select & Optimize the Right Antibody Create Diverse Antibody Pools Screen Antibody Candidates Identify the Right Antibody Technology offering addresses critical industry needs and is paired with our highly specialized and efficient operations Licenses to our technology platform are structured to align economic and scientific interests with our partners
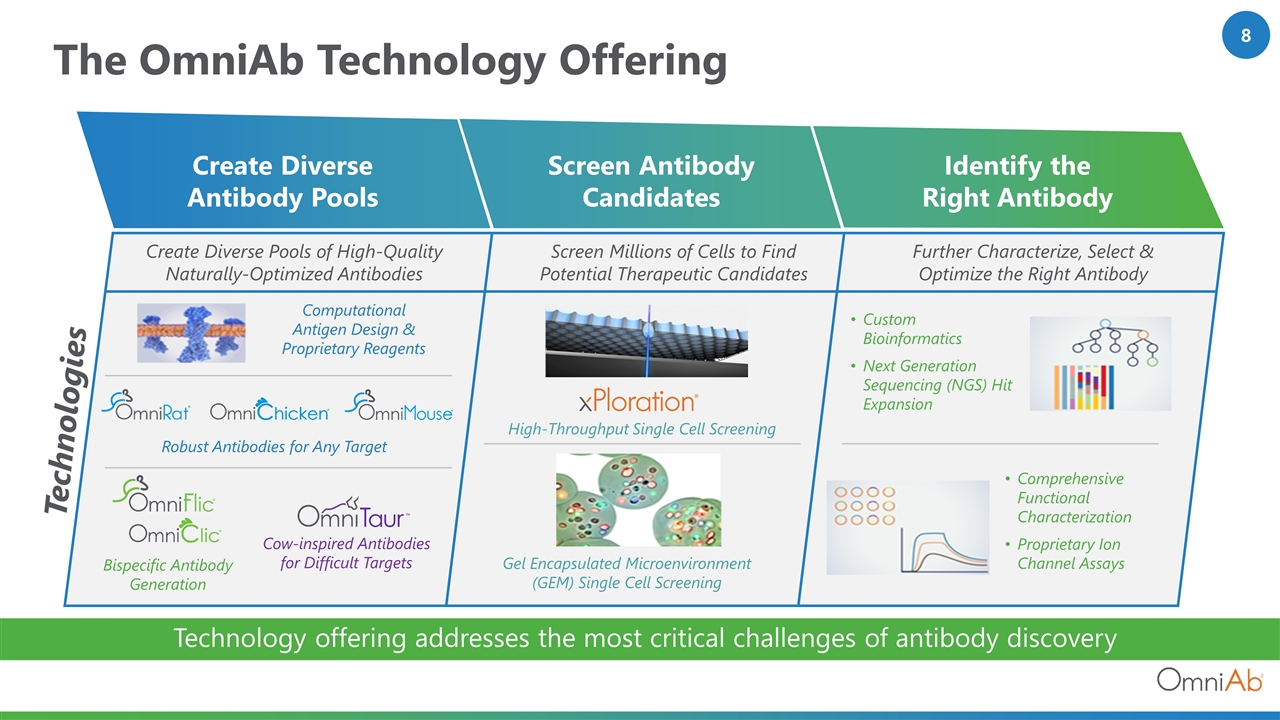
The OmniAb Technology Offering Technology offering addresses the most critical challenges of antibody discovery Technologies Create Diverse Antibody Pools Screen Antibody Candidates Identify the Right Antibody Create Diverse Pools of High-Quality Naturally-Optimized Antibodies Screen Millions of Cells to Find Potential Therapeutic Candidates Further Characterize, Select & Optimize the Right Antibody Computational Antigen Design & Proprietary Reagents Robust Antibodies for Any Target Bispecific Antibody Generation Cow-inspired Antibodies for Difficult Targets High-Throughput Single Cell Screening Gel Encapsulated Microenvironment (GEM) Single Cell Screening Custom Bioinformatics Next Generation Sequencing (NGS) Hit Expansion Comprehensive Functional Characterization Proprietary Ion Channel Assays
What is Biological Intelligence™? We believe that antibodies generated in vivo are superior to ones from other sources because they are naturally optimized through an iterative process that preferentially selects for antibodies with excellent specificity and developability profiles The ability of the immune system in our engineered transgenic animals to create optimized antibodies for human therapeutics is what we call Biological Intelligence We believe this approach increases the efficiency and probability of success of therapeutic antibody discovery and may help limit the attrition of antibody product candidates in the clinic
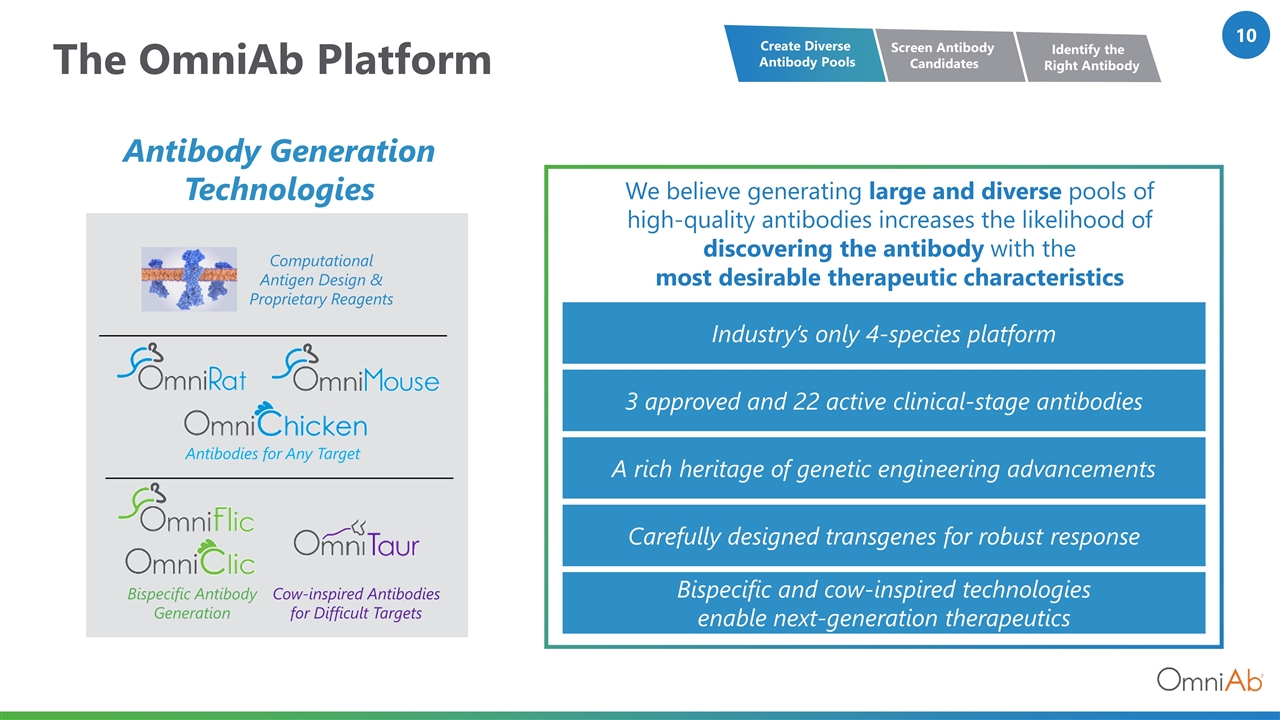
The OmniAb Platform We believe generating large and diverse pools of high-quality antibodies increases the likelihood of discovering the antibody with the most desirable therapeutic characteristics Antibodies for Any Target Bispecific Antibody Generation Cow-inspired Antibodies for Difficult Targets Computational Antigen Design & Proprietary Reagents Antibody Generation Technologies Industry’s only 4-species platform A rich heritage of genetic engineering advancements Bispecific and cow-inspired technologies enable next-generation therapeutics 3 approved and 22 active clinical-stage antibodies Carefully designed transgenes for robust response Screen Antibody Candidates Create Diverse Antibody Pools Identify the Right Antibody
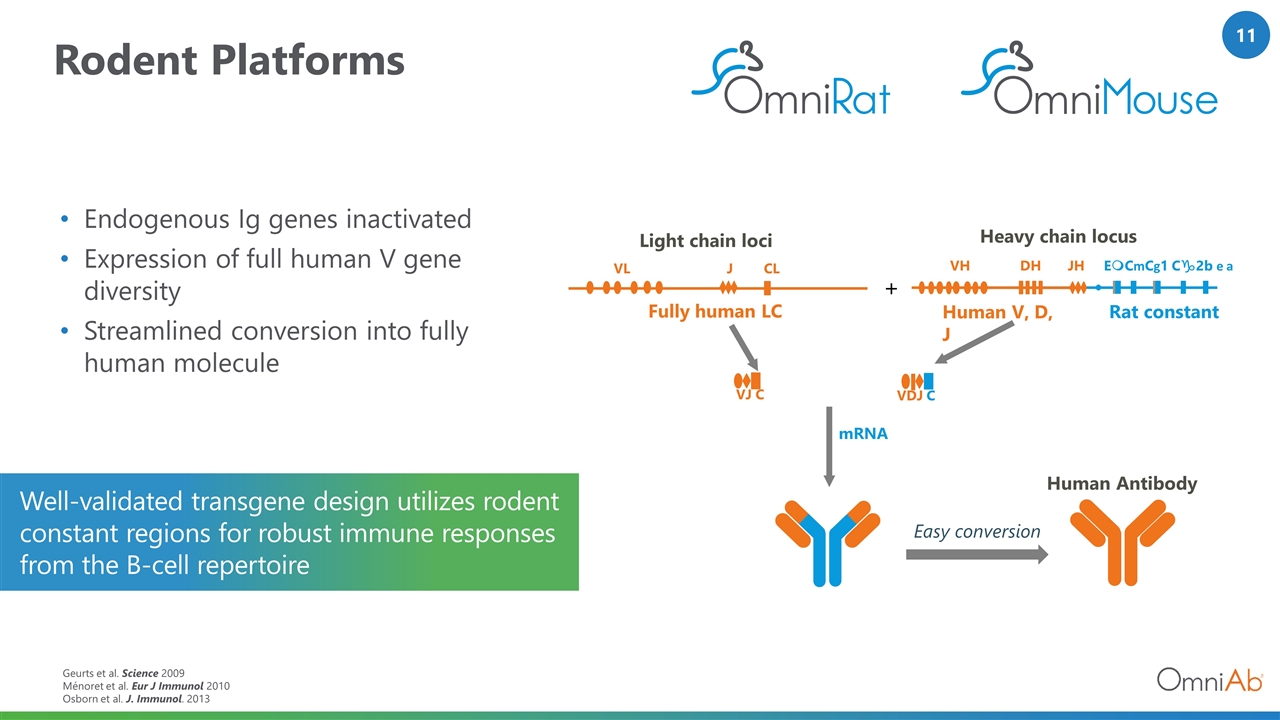
Rodent Platforms Endogenous Ig genes inactivated Expression of full human V gene diversity Streamlined conversion into fully human molecule Geurts et al. Science 2009 Ménoret et al. Eur J Immunol 2010 Osborn et al. J. Immunol. 2013 Well-validated transgene design utilizes rodent constant regions for robust immune responses from the B-cell repertoire mRNA Human Antibody Light chain loci VL J CL + VJ C VH DH JH EmCmCg1 Cg2b e a Easy conversion Heavy chain locus VDJ C Rat constant Human V, D, J Fully human LC
OmniChicken Powered By Evolution
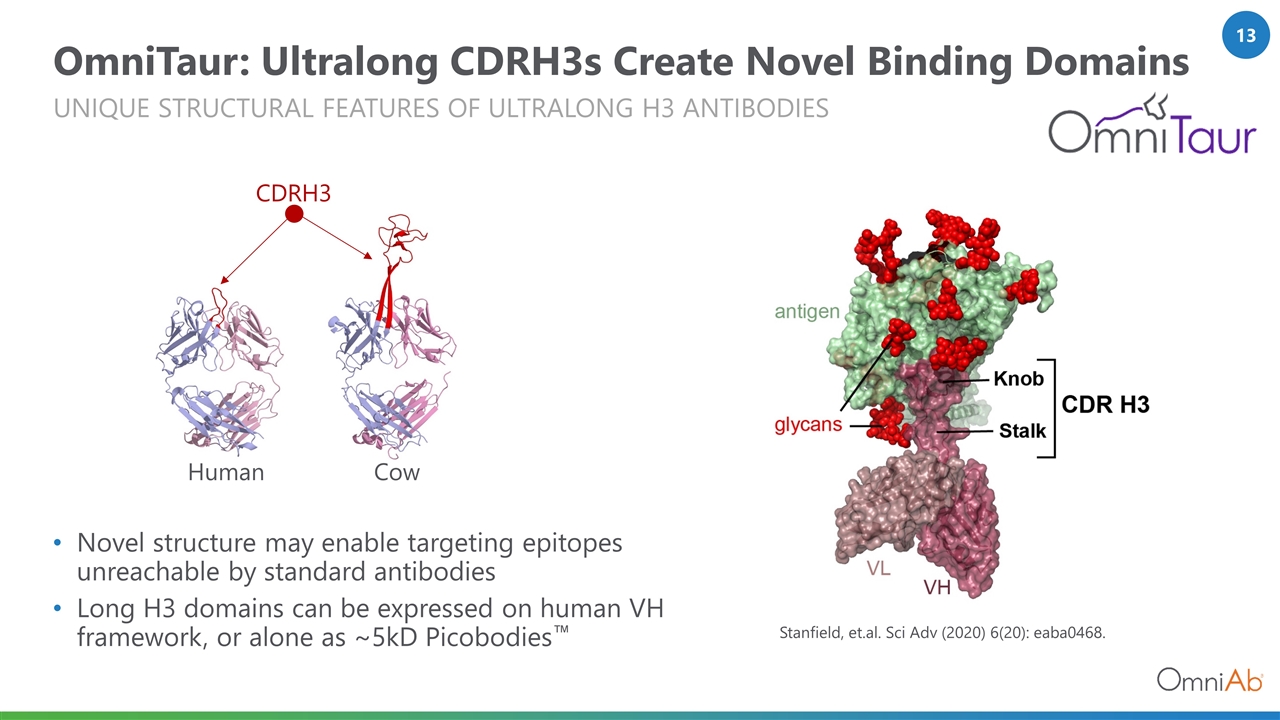
OmniTaur: Ultralong CDRH3s Create Novel Binding Domains Unique structural features of ultralong H3 antibodies Novel structure may enable targeting epitopes unreachable by standard antibodies Long H3 domains can be expressed on human VH framework, or alone as ~5kD Picobodies™ CDRH3 Human Cow Stanfield, et.al. Sci Adv (2020) 6(20): eaba0468.
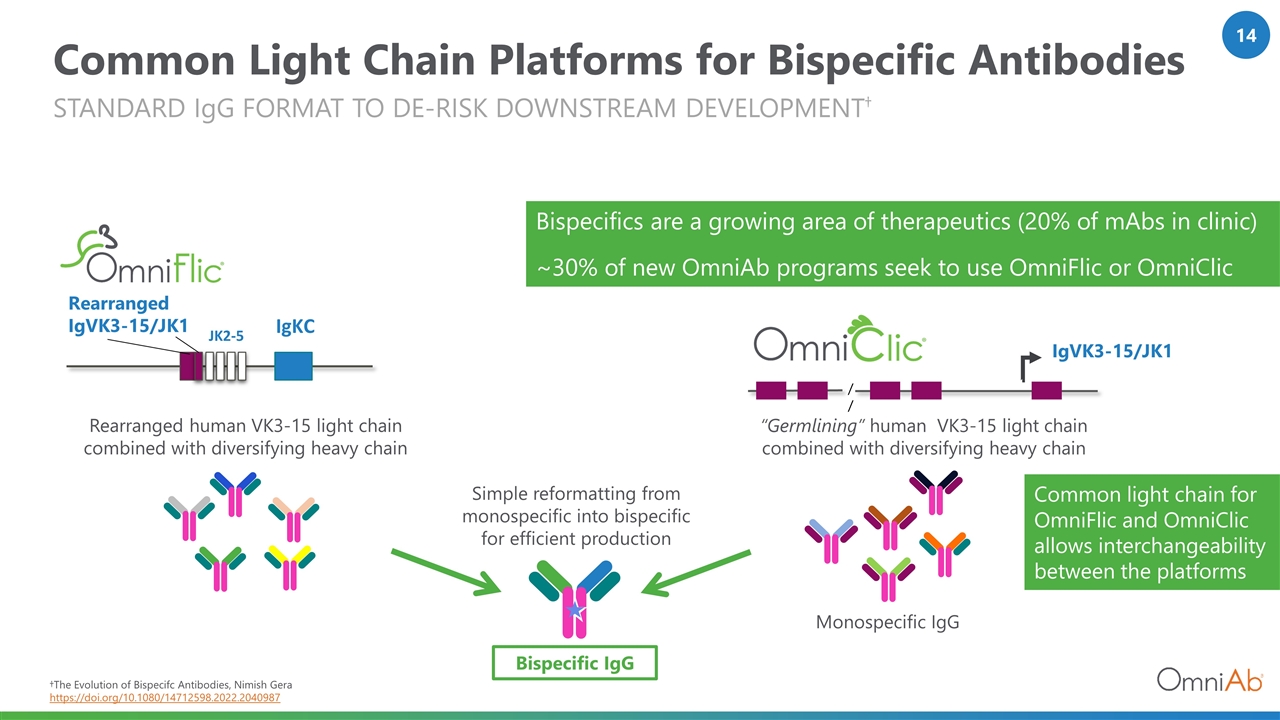
Common Light Chain Platforms for Bispecific Antibodies standard IgG format to de-risk downstream development† †The Evolution of Bispecifc Antibodies, Nimish Gera https://doi.org/10.1080/14712598.2022.2040987 Monospecific IgG Bispecific IgG Rearranged human VK3-15 light chain combined with diversifying heavy chain JK2-5 Rearranged IgVK3-15/JK1 IgKC IgVK3-15/JK1 // Simple reformatting from monospecific into bispecific for efficient production “Germlining” human VK3-15 light chain combined with diversifying heavy chain Common light chain for OmniFlic and OmniClic allows interchangeability between the platforms Bispecifics are a growing area of therapeutics (20% of mAbs in clinic) ~30% of new OmniAb programs seek to use OmniFlic or OmniClic
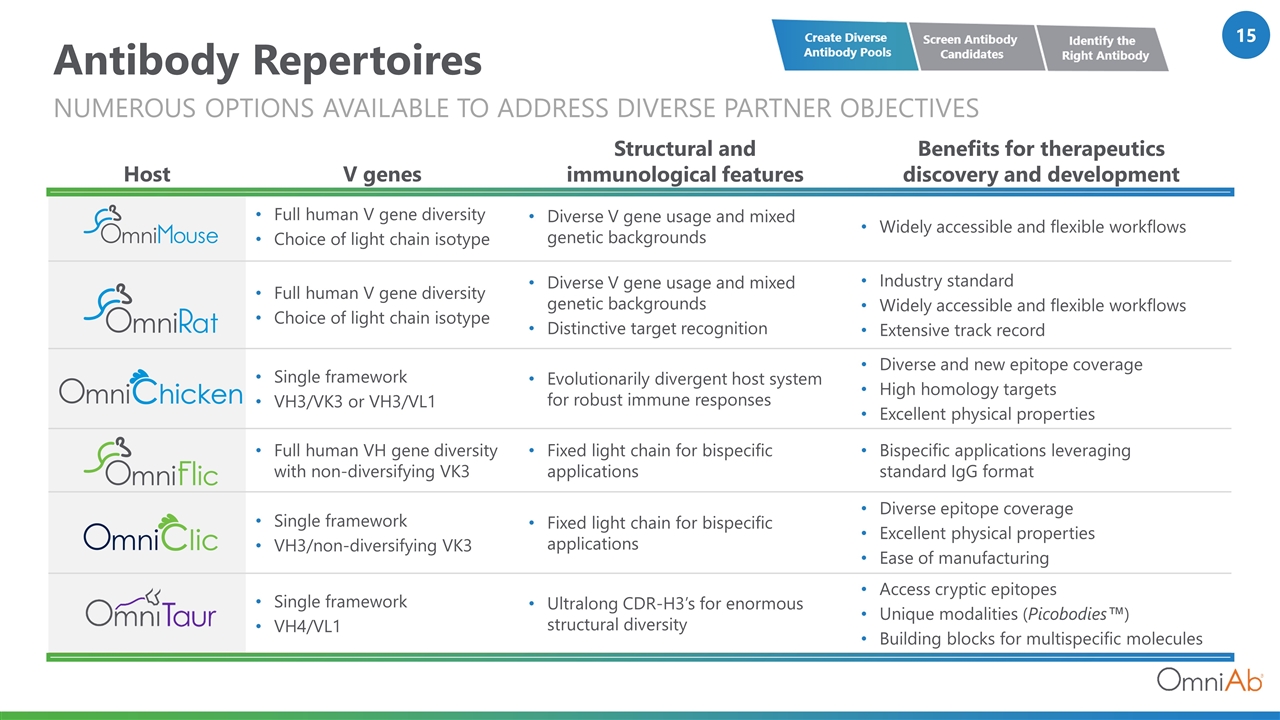
Antibody Repertoires numerous options available to address diverse partner objectives Host V genes Structural and immunological features Benefits for therapeutics discovery and development Full human V gene diversity Choice of light chain isotype Diverse V gene usage and mixed genetic backgrounds Widely accessible and flexible workflows Full human V gene diversity Choice of light chain isotype Diverse V gene usage and mixed genetic backgrounds Distinctive target recognition Industry standard Widely accessible and flexible workflows Extensive track record Single framework VH3/VK3 or VH3/VL1 Evolutionarily divergent host system for robust immune responses Diverse and new epitope coverage High homology targets Excellent physical properties Full human VH gene diversity with non-diversifying VK3 Fixed light chain for bispecific applications Bispecific applications leveraging standard IgG format Single framework VH3/non-diversifying VK3 Fixed light chain for bispecific applications Diverse epitope coverage Excellent physical properties Ease of manufacturing Single framework VH4/VL1 Ultralong CDR-H3’s for enormous structural diversity Access cryptic epitopes Unique modalities (Picobodies™) Building blocks for multispecific molecules
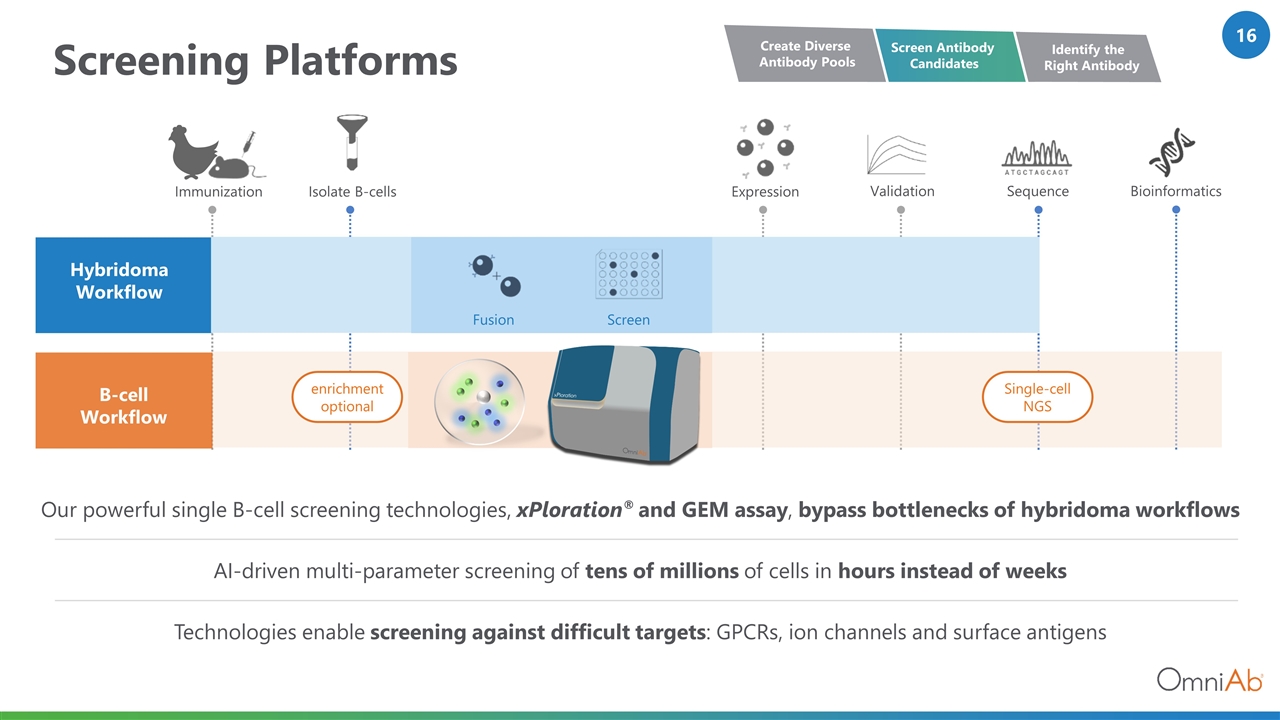
Screening Platforms Hybridoma Workflow B-cell Workflow Immunization Isolate B-cells Sequence Bioinformatics enrichment optional Single-cell NGS Expression Validation Screen Fusion Technologies enable screening against difficult targets: GPCRs, ion channels and surface antigens Our powerful single B-cell screening technologies, xPloration® and GEM assay, bypass bottlenecks of hybridoma workflows AI-driven multi-parameter screening of tens of millions of cells in hours instead of weeks Screen Antibody Candidates Create Diverse Antibody Pools Identify the Right Antibody
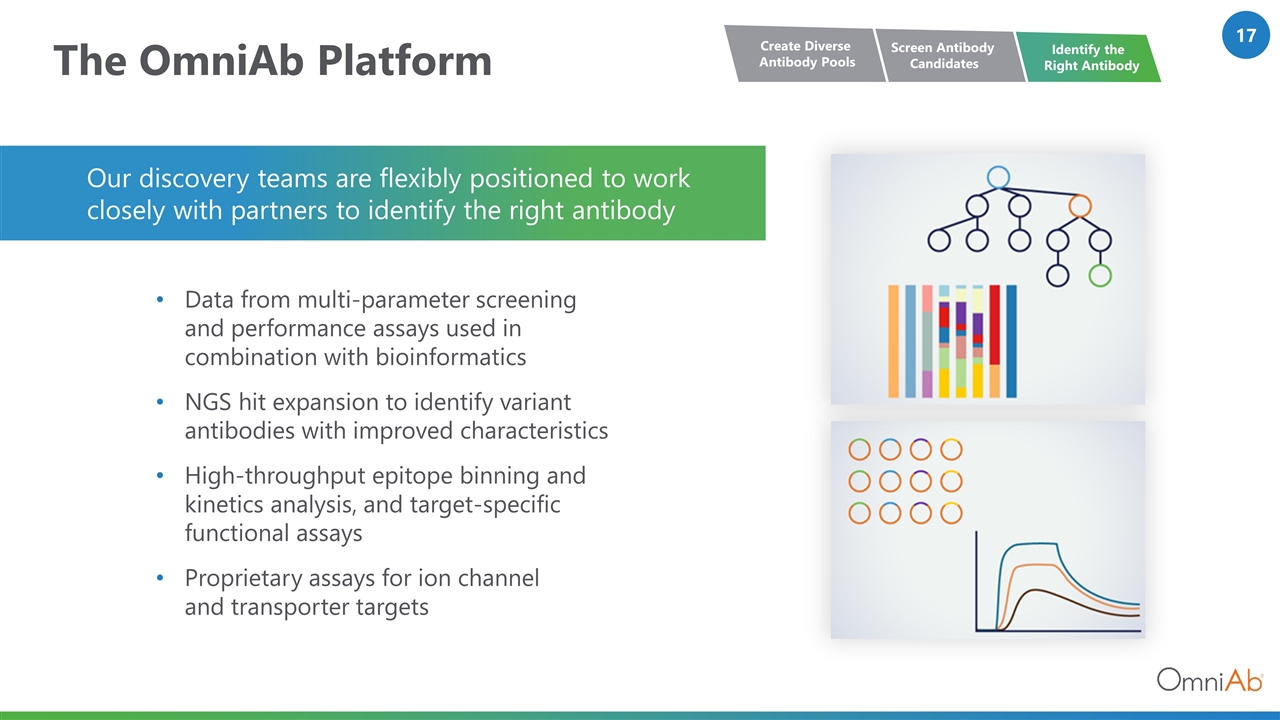
The OmniAb Platform Data from multi-parameter screening and performance assays used in combination with bioinformatics NGS hit expansion to identify variant antibodies with improved characteristics High-throughput epitope binning and kinetics analysis, and target-specific functional assays Proprietary assays for ion channel and transporter targets Our discovery teams are flexibly positioned to work closely with partners to identify the right antibody Screen Antibody Candidates Create Diverse Antibody Pools Identify the Right Antibody
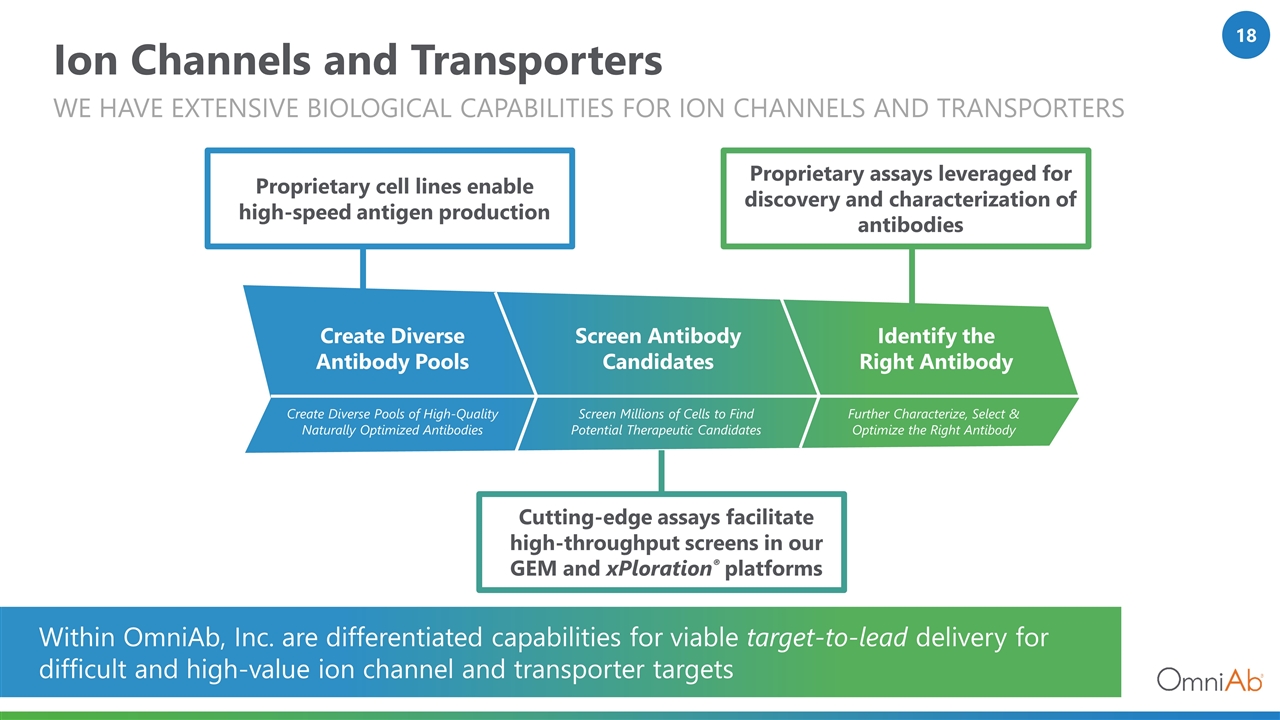
Create Diverse Pools of High-Quality Naturally Optimized Antibodies Screen Millions of Cells to Find Potential Therapeutic Candidates Further Characterize, Select & Optimize the Right Antibody Create Diverse Antibody Pools Screen Antibody Candidates Identify the Right Antibody Ion Channels and Transporters We Have Extensive biological capabilities FOR ion channels and transporters Proprietary cell lines enable high-speed antigen production Cutting-edge assays facilitate high-throughput screens in our GEM and xPloration® platforms Proprietary assays leveraged for discovery and characterization of antibodies Within OmniAb, Inc. are differentiated capabilities for viable target-to-lead delivery for difficult and high-value ion channel and transporter targets
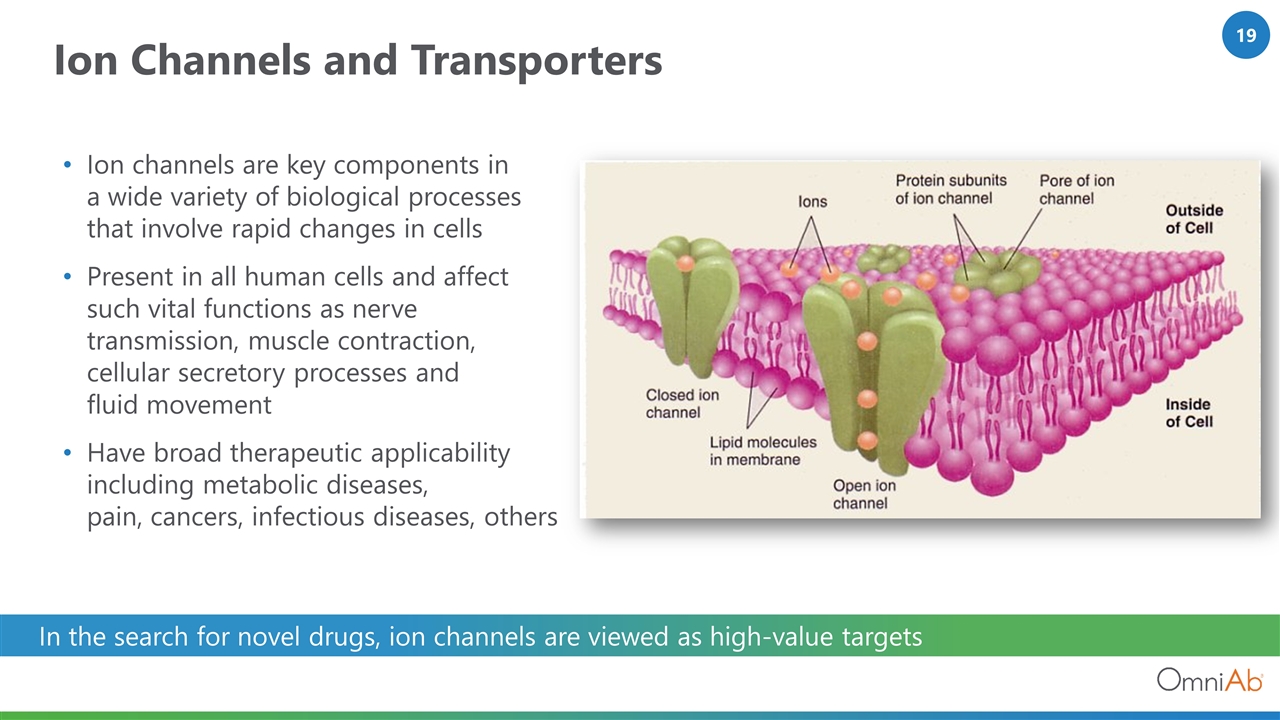
Ion Channels and Transporters Ion channels are key components in a wide variety of biological processes that involve rapid changes in cells Present in all human cells and affect such vital functions as nerve transmission, muscle contraction, cellular secretory processes and fluid movement Have broad therapeutic applicability including metabolic diseases, pain, cancers, infectious diseases, others In the search for novel drugs, ion channels are viewed as high-value targets
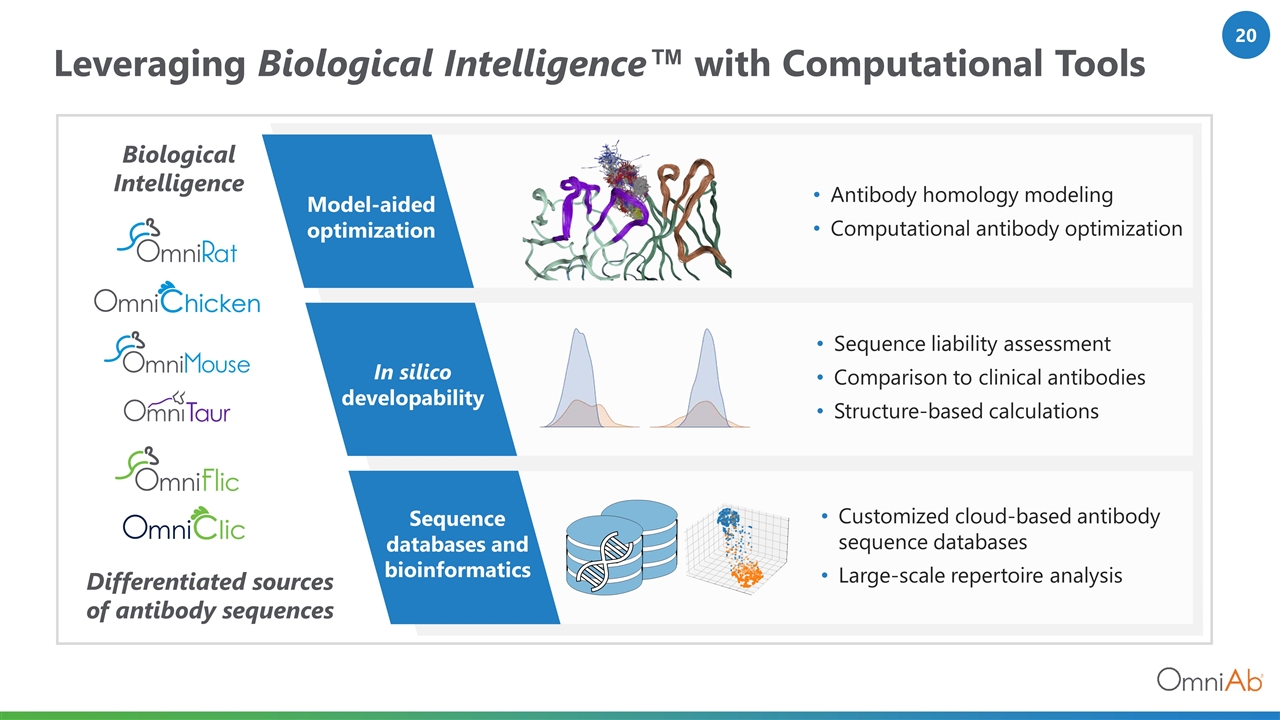
Leveraging Biological Intelligence™ with Computational Tools Biological Intelligence Differentiated sources of antibody sequences In silico developability Sequence liability assessment Comparison to clinical antibodies Structure-based calculations Sequence databases and bioinformatics Customized cloud-based antibody sequence databases Large-scale repertoire analysis Model-aided optimization Antibody homology modeling Computational antibody optimization
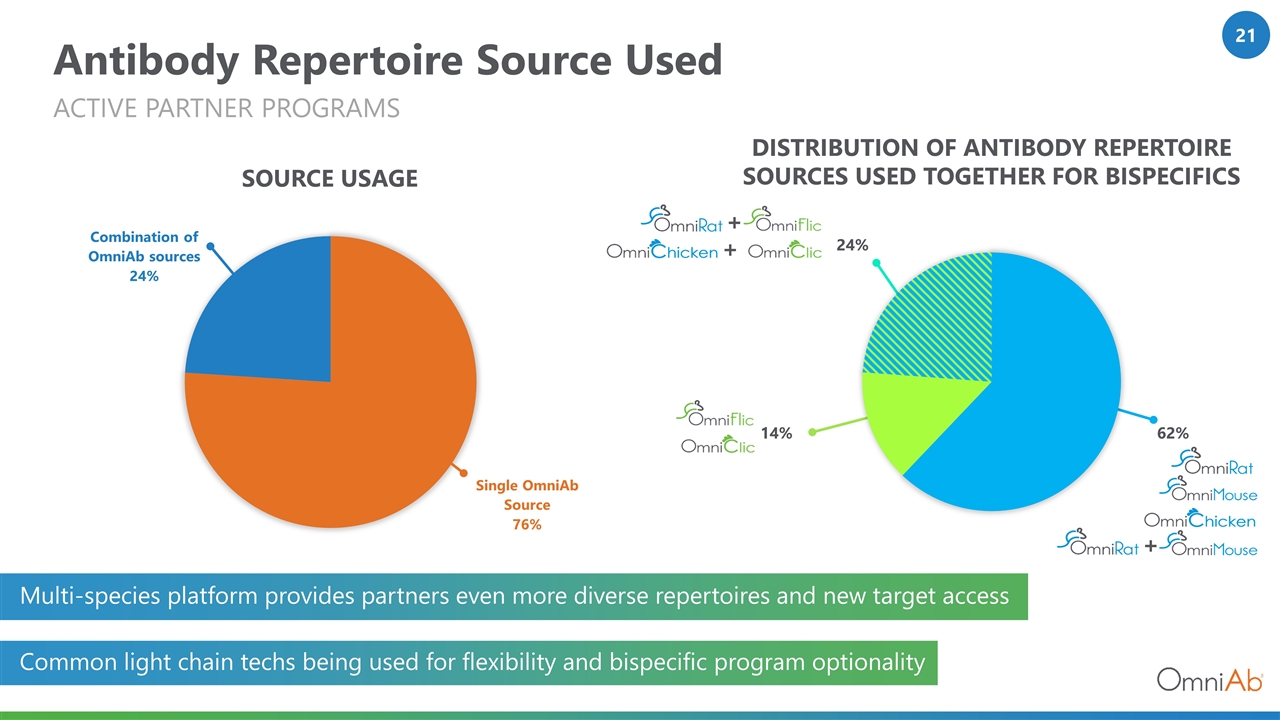
Antibody Repertoire Source Used ACTIVE Partner PROGRAMS DISTRIBUTION OF ANTIBODY REPERTOIRE SOURCES USED TOGETHER FOR BISPECIFICS SOURCE USAGE 24% 62% 14% + + + Multi-species platform provides partners even more diverse repertoires and new target access Common light chain techs being used for flexibility and bispecific program optionality
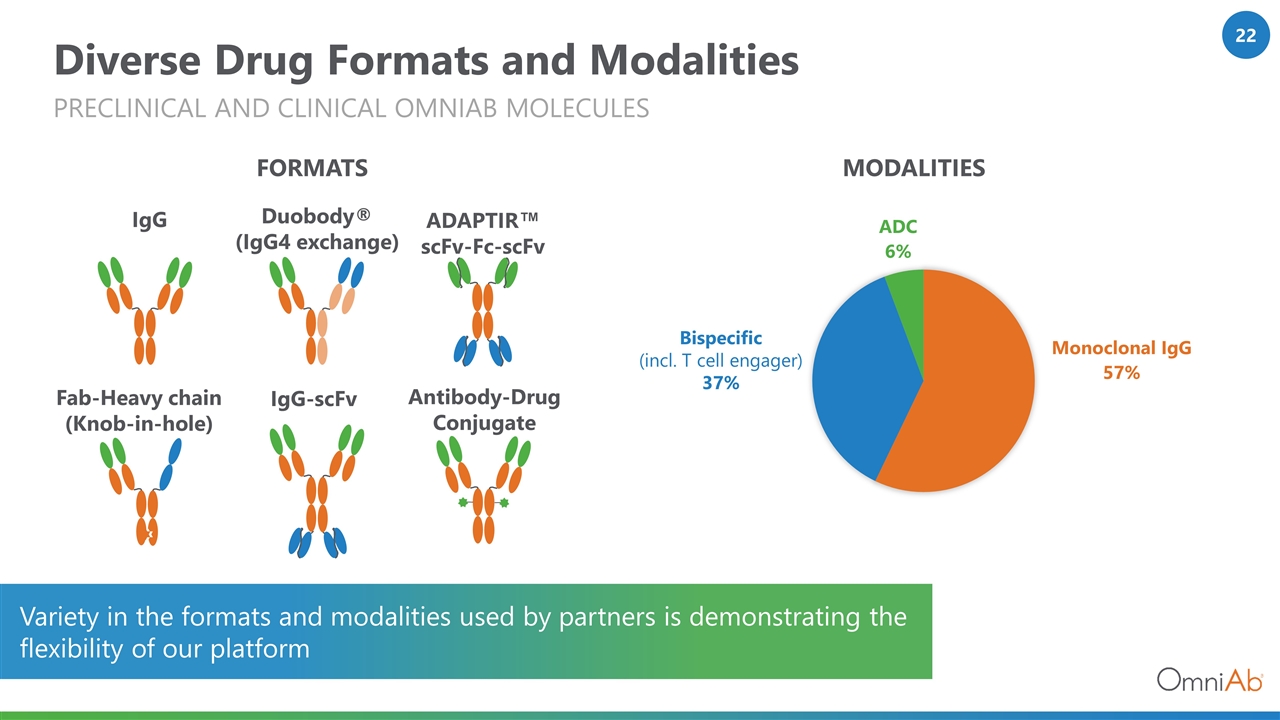
Diverse Drug Formats and Modalities PRECLINICAL AND CLINICAL OMNIAB MOLECULES Duobody® (IgG4 exchange) ADAPTIR™ scFv-Fc-scFv IgG IgG-scFv Antibody-Drug Conjugate Fab-Heavy chain (Knob-in-hole) Variety in the formats and modalities used by partners is demonstrating the flexibility of our platform MODALITIES FORMATS Bispecific (incl. T cell engager) 37%

Select OmniAb Partners >65 companies currently have access to omniab ANTIBODIES
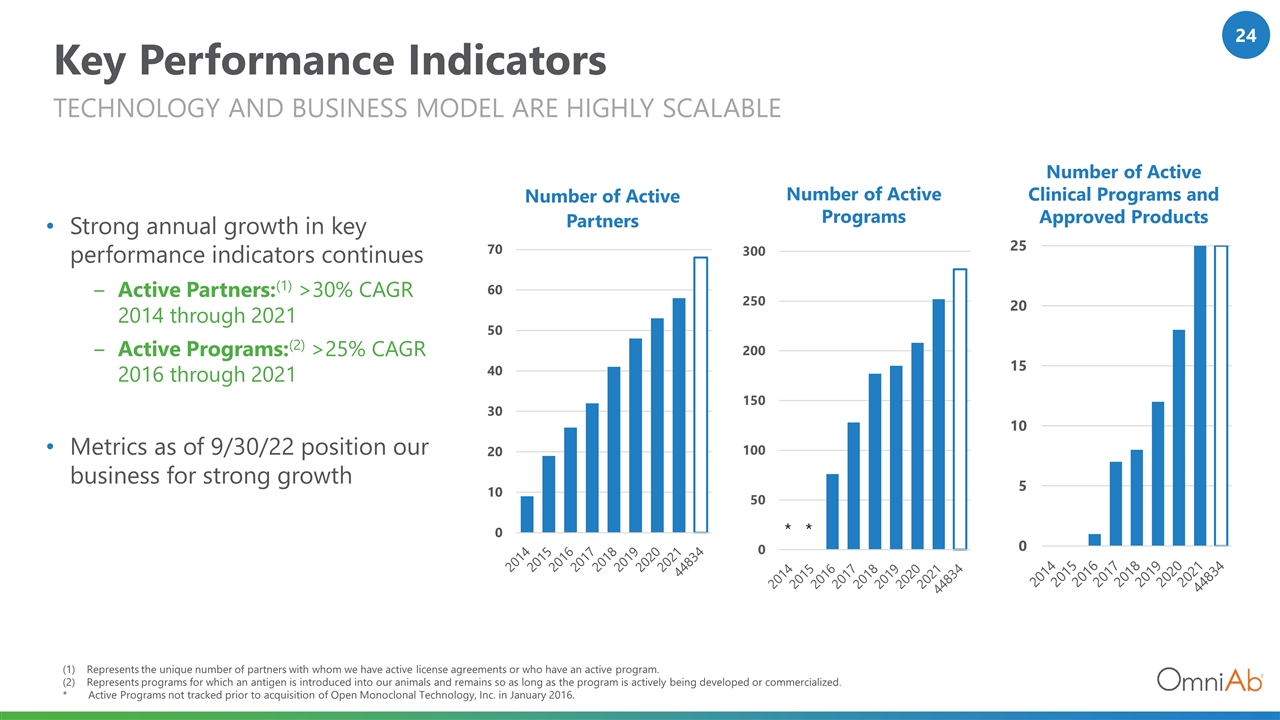
Key Performance Indicators Strong annual growth in key performance indicators continues Active Partners:(1) >30% CAGR 2014 through 2021 Active Programs:(2) >25% CAGR 2016 through 2021 Metrics as of 9/30/22 position our business for strong growth Technology and business model are highly scalable Represents the unique number of partners with whom we have active license agreements or who have an active program. Represents programs for which an antigen is introduced into our animals and remains so as long as the program is actively being developed or commercialized. * Active Programs not tracked prior to acquisition of Open Monoclonal Technology, Inc. in January 2016. Number of Active Programs Number of Active Clinical Programs and Approved Products * *
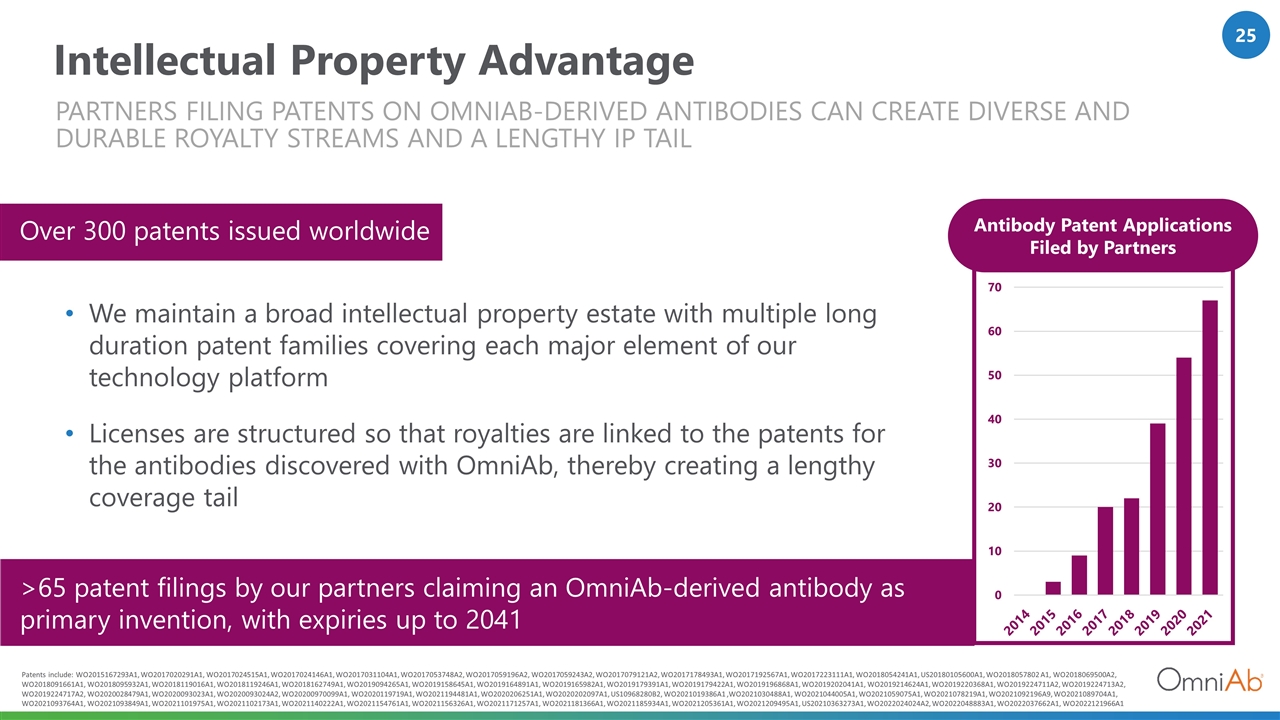
Intellectual Property Advantage Antibody Patent Applications Filed by Partners PARTNERS FILING PATENTS ON OMNIAB-DERIVED ANTIBODIES can CREATE DIVERSE AND DURABLE ROYALTY STREAMS AND A LENGTHY IP TAIL >65 patent filings by our partners claiming an OmniAb-derived antibody as primary invention, with expiries up to 2041 We maintain a broad intellectual property estate with multiple long duration patent families covering each major element of our technology platform Licenses are structured so that royalties are linked to the patents for the antibodies discovered with OmniAb, thereby creating a lengthy coverage tail Over 300 patents issued worldwide Patents include: WO2015167293A1, WO2017020291A1, WO2017024515A1, WO2017024146A1, WO2017031104A1, WO2017053748A2, WO2017059196A2, WO2017059243A2, WO2017079121A2, WO2017178493A1, WO2017192567A1, WO2017223111A1, WO2018054241A1, US20180105600A1, WO2018057802 A1, WO2018069500A2, WO2018091661A1, WO2018095932A1, WO2018119016A1, WO2018119246A1, WO2018162749A1, WO2019094265A1, WO2019158645A1, WO2019164891A1, WO2019165982A1, WO2019179391A1, WO2019179422A1, WO2019196868A1, WO2019202041A1, WO2019214624A1, WO2019220368A1, WO2019224711A2, WO2019224713A2, WO2019224717A2, WO2020028479A1, WO2020093023A1, WO2020093024A2, WO20200970099A1, WO2020119719A1, WO2021194481A1, WO2020206251A1, WO2020202097A1, US10968280B2, WO2021019386A1 ,WO2021030488A1, WO2021044005A1, WO2021059075A1, WO2021078219A1, WO2021092196A9, WO2021089704A1, WO2021093764A1, WO2021093849A1, WO2021101975A1, WO2021102173A1, WO2021140222A1, WO2021154761A1, WO2021156326A1, WO2021171257A1, WO2021181366A1, WO2021185934A1, WO2021205361A1, WO2021209495A1, US20210363273A1, WO2022024024A2, WO2022048883A1, WO2022037662A1, WO2022121966A1

Business Model Our agreements are structured to align economic and scientific interests with our partners License partnerships designed to include: Upfront and collaboration/access fees Milestones Royalties on commercial sales
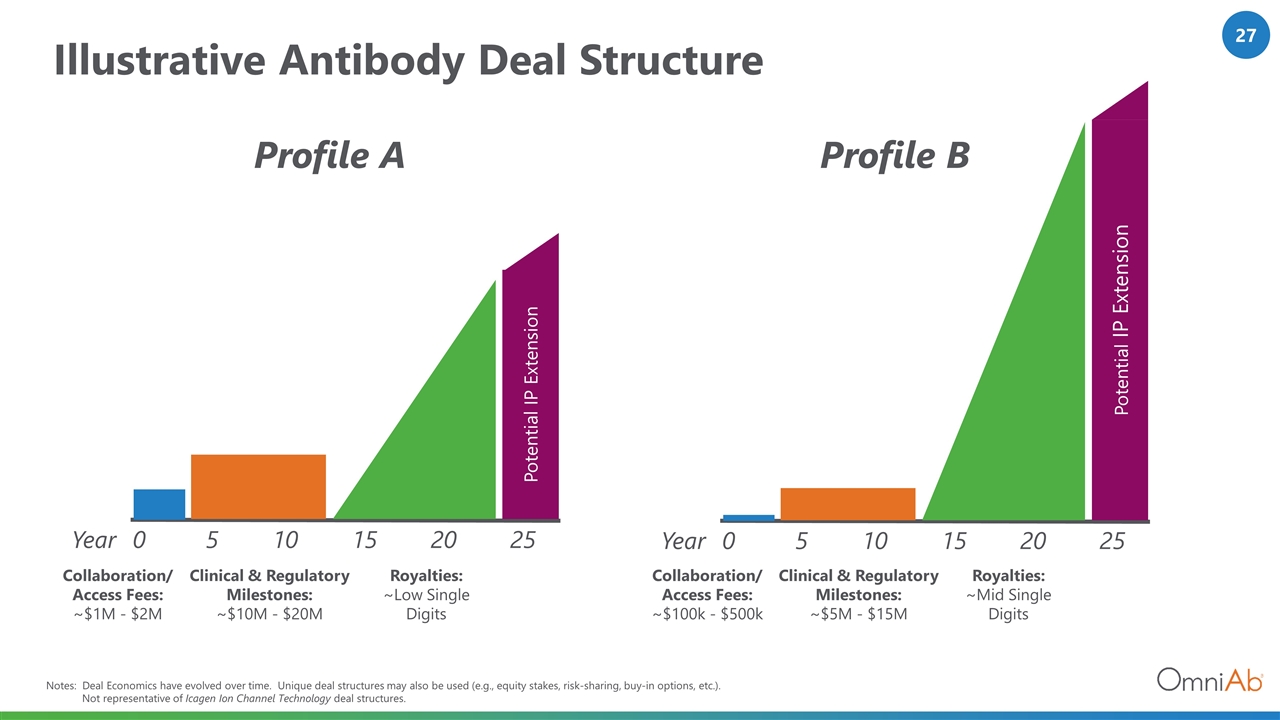
Illustrative Antibody Deal Structure Year 0 5 10 15 20 25 Collaboration/ Access Fees: ~$1M - $2M Potential IP Extension Notes: Deal Economics have evolved over time. Unique deal structures may also be used (e.g., equity stakes, risk-sharing, buy-in options, etc.). Not representative of Icagen Ion Channel Technology deal structures. Clinical & Regulatory Milestones: ~$10M - $20M Royalties: ~Low Single Digits Profile A Profile B Year 0 5 10 15 20 25 Collaboration/ Access Fees: ~$100k - $500k Potential IP Extension Clinical & Regulatory Milestones: ~$5M - $15M Royalties: ~Mid Single Digits
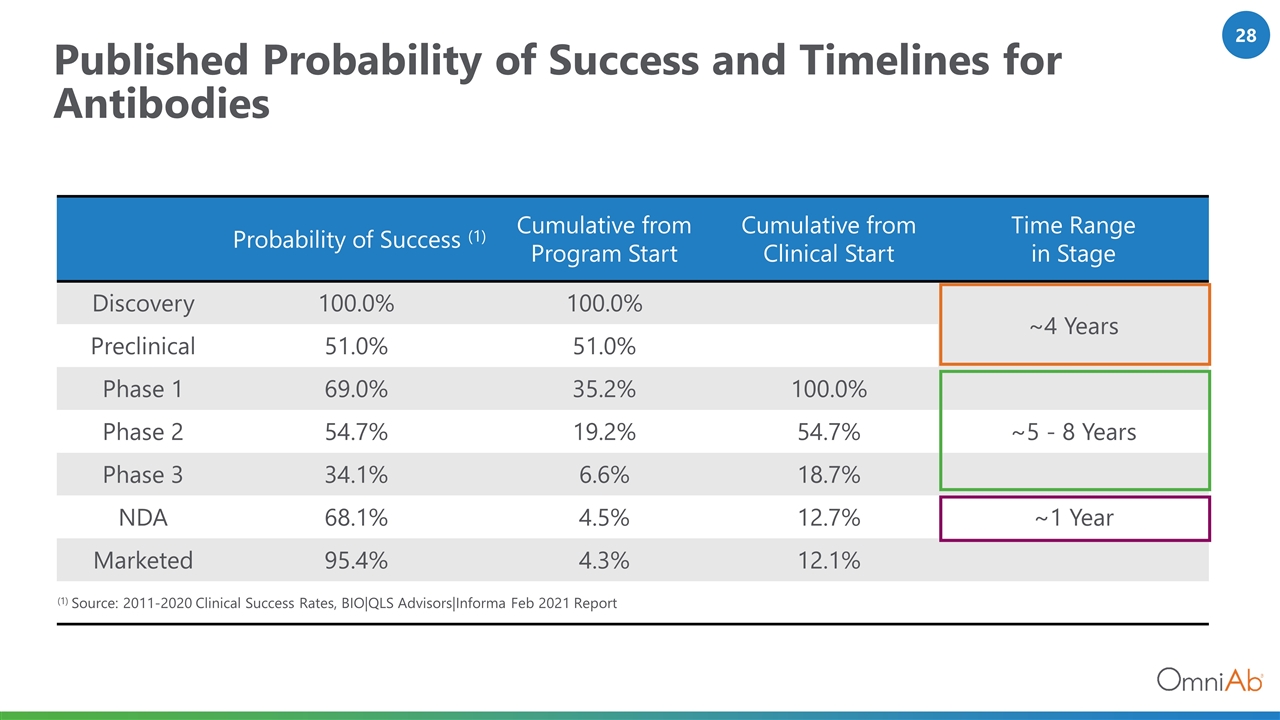
Published Probability of Success and Timelines for Antibodies Probability of Success (1) Cumulative from Program Start Cumulative from Clinical Start Time Range in Stage Discovery 100.0% 100.0% ~4 Years Preclinical 51.0% 51.0% Phase 1 69.0% 35.2% 100.0% Phase 2 54.7% 19.2% 54.7% ~5 - 8 Years Phase 3 34.1% 6.6% 18.7% NDA 68.1% 4.5% 12.7% ~1 Year Marketed 95.4% 4.3% 12.1% (1) Source: 2011-2020 Clinical Success Rates, BIO|QLS Advisors|Informa Feb 2021 Report
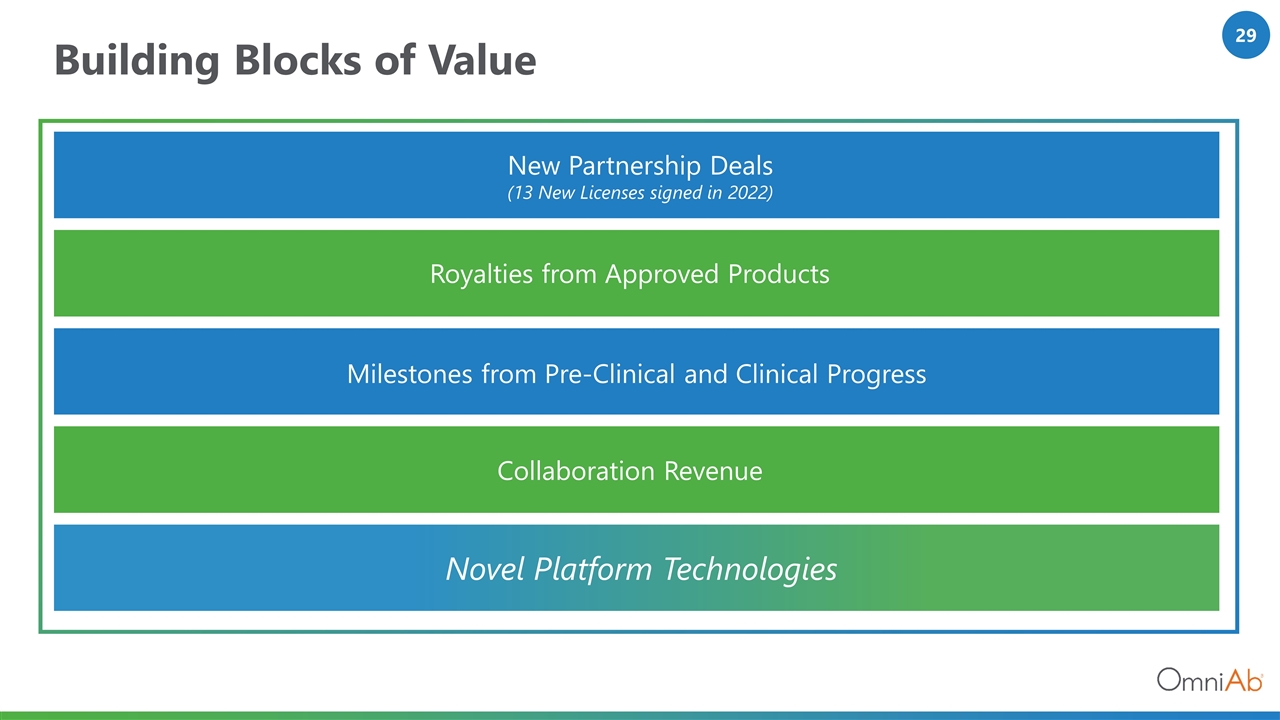
Building Blocks of Value Novel Platform Technologies Collaboration Revenue Royalties from Approved Products Milestones from Pre-Clinical and Clinical Progress New Partnership Deals (13 New Licenses signed in 2022)
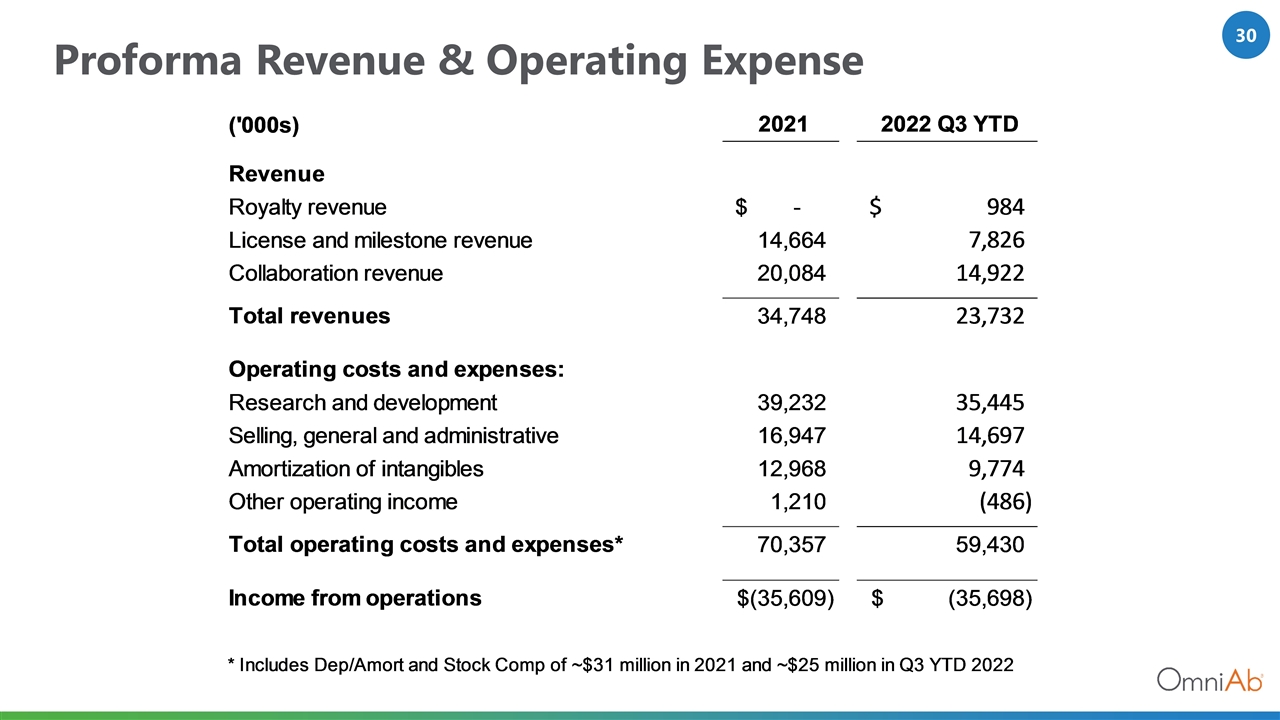
Proforma Revenue & Operating Expense

Cash Position Provides Long Operational Runway ($ millions) 12/31/22 Cash Balance* ~ $88 Teclistamab/TECVAYLI First Commercial Sale Milestones Received in January 2023 $35 Proforma Cash Position ~ $123 * Includes cash, cash equivalents and marketable securities Business is close to break-even cash flow Recent transaction and milestone Payments provide sufficient capital to fund operations for foreseeable future
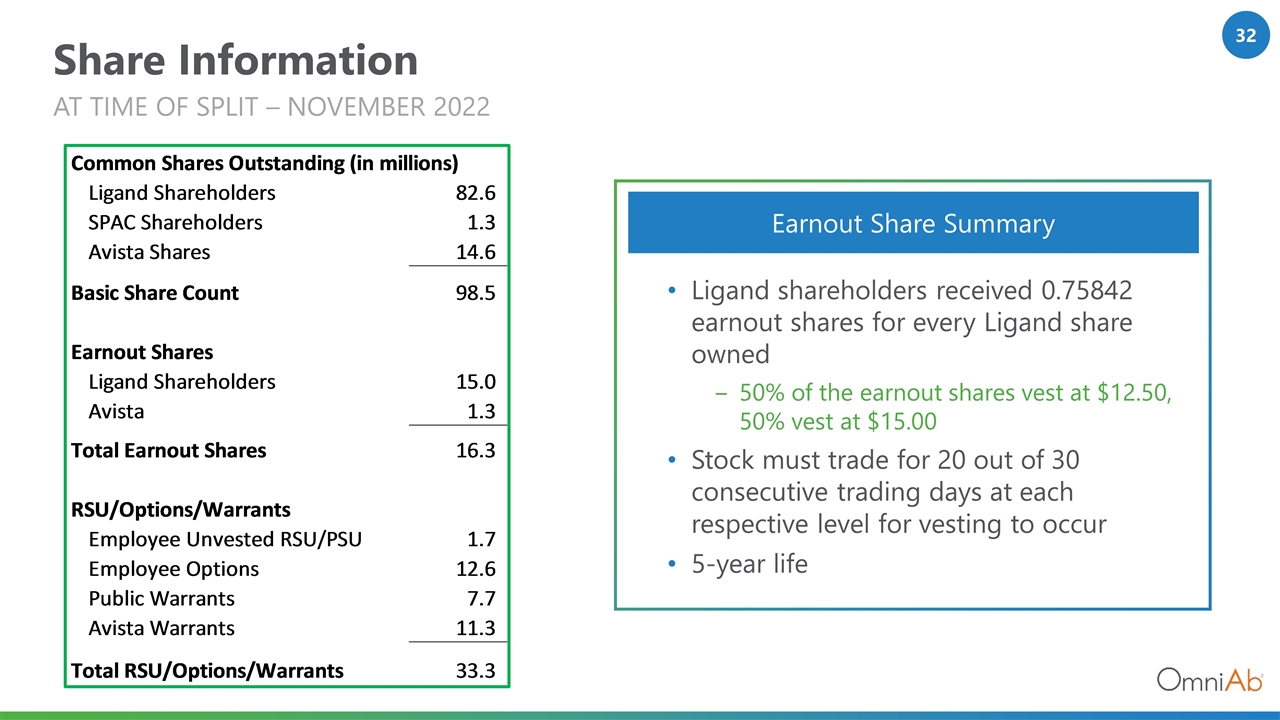
Share Information Ligand shareholders received 0.75842 earnout shares for every Ligand share owned 50% of the earnout shares vest at $12.50, 50% vest at $15.00 Stock must trade for 20 out of 30 consecutive trading days at each respective level for vesting to occur 5-year life Earnout Share Summary AT TIME OF SPLIT – NOVEMBER 2022

Christel Iffland, PhD SVP, Antibody Technologies Former Associate Director at Merck KGaA / EMD Serono Co-inventor of Avelumab Dana Farber, Albert Einstein College Marie-Cecile Van De Lavoir, PhD, DVM SVP, Technical Operations & Genetics Co-Founder/COO, Crystal Bioscience Origen Therapeutics, Inventor Germ Cell Technology Fulbright Scholar, UCSF, Utrecht, Guelph, Cornell Bill Harriman, PhD SVP, Antibody Discovery Co-Founder/CSO, Crystal Bioscience Trellis, Roche, Abgenix UCSF-Immunology, Haas MBA Doug Krafte, PhD SVP, Ion Channels Former Exec at Icagen Inc., Pfizer Pain & Sensory Disorders, Boehringer Ingelheim, Aurora Biosciences Univ. Rochester, Vanderbilt Matt Foehr Chief Executive Officer Board Member Viking Therapeutics Former Exec at Ligand Pharmaceuticals, GlaxoSmithKline, Stiefel Labs, Connetics Corp. Kurt Gustafson Chief Financial Officer Board Member Xencor, Inc Former CFO/Exec at Spectrum Pharmaceuticals, Halozyme Therapeutics, Amgen Charles Berkman Chief Legal Officer Former General Counsel at Ligand Pharmaceuticals, Attorney at Baker & McKenzie, Lyon & Lyon Carolyn Bertozzi, PhD Professor of Humanities & Sciences Stanford University Investigator HHMRI 2022 Nobel Laureate Board Member Alnylam Former Board Member Eli Lilly, Founder of multiple companies Nom/Gov Sarah Boyce CEO, Avidity Former Akcea Therapeutics, Ionis Pharmaceuticals, Forest Labs HC&C Chair Sunil Patel Public & Private Biotech Executive Former Abgenix, Gilead, BiPar and OncoMed AC Chair, HC&C Jennifer Cochran, PhD Professor of Bioengineering Stanford University Founder of multiple companies, including xCella Biosciences Nom/Gov Chair, AC John Higgins OmniAb, Inc. Board Chair CEO and Board Member, Ligand Pharmaceuticals Former CFO, Connetics Corp. Board Member Bio-Techne HC&C Josh Tamaroff Partner, Avista Capital Partners Barclays Capital Lehman Brothers AC, Nom/Gov Management Board of Directors Matt Foehr Chief Executive Officer Board Member Viking Therapeutics Former Exec at Ligand Pharmaceuticals, GlaxoSmithKline, Stiefel Labs, Connetics Corp.
Intelligent expansion of our technology platform is informed by deep relationships with our broad base of partners
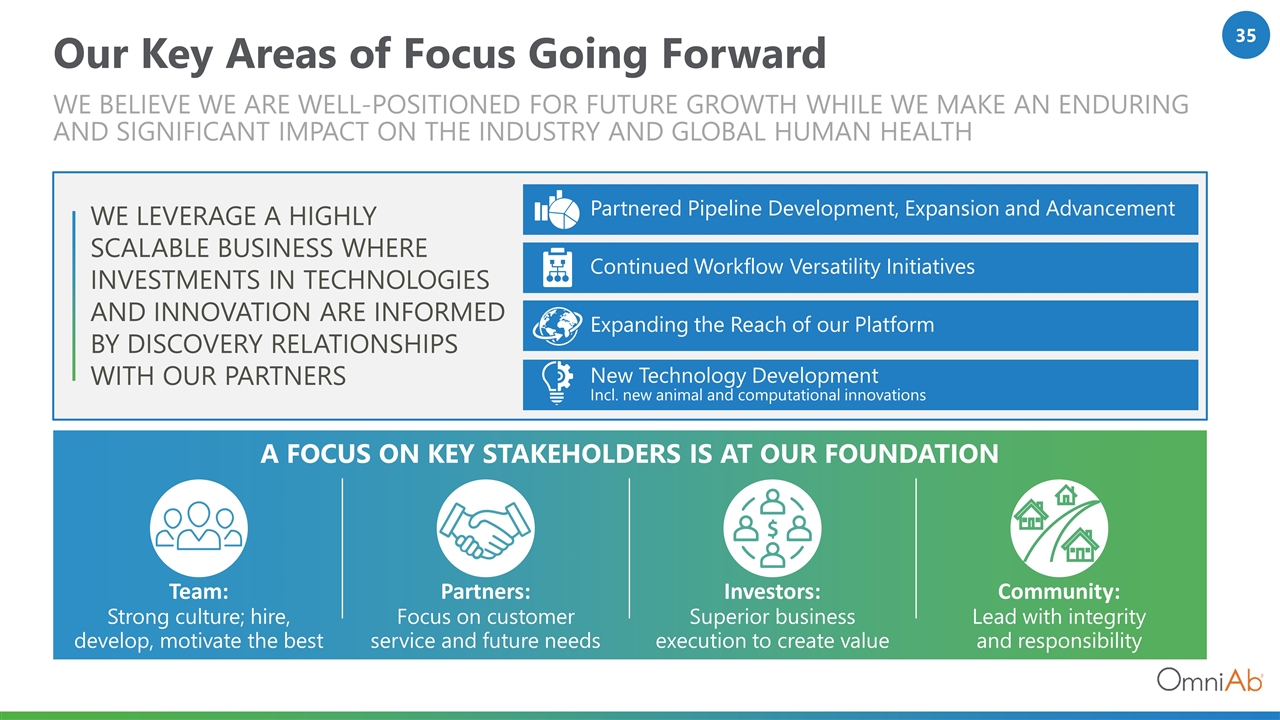
Our Key Areas of Focus Going Forward We believe we are Well-positioned for future growth while WE MAKE an enduring and significant impact on the industry and global human health Community: Lead with integrity and responsibility Investors: Superior business execution to create value We LEVERAGE A Highly scalable business where investments in TECHNOLOGIES and innovation are informed by DISCOVERY relationships with our partners Partnered Pipeline Development, Expansion and Advancement Expanding the Reach of our Platform Continued Workflow Versatility Initiatives New Technology Development Incl. new animal and computational innovations A focus on key stakeholders IS at our foundation Team: Strong culture; hire, develop, motivate the best Partners: Focus on customer service and future needs

For more information, please visit www.omniab.com
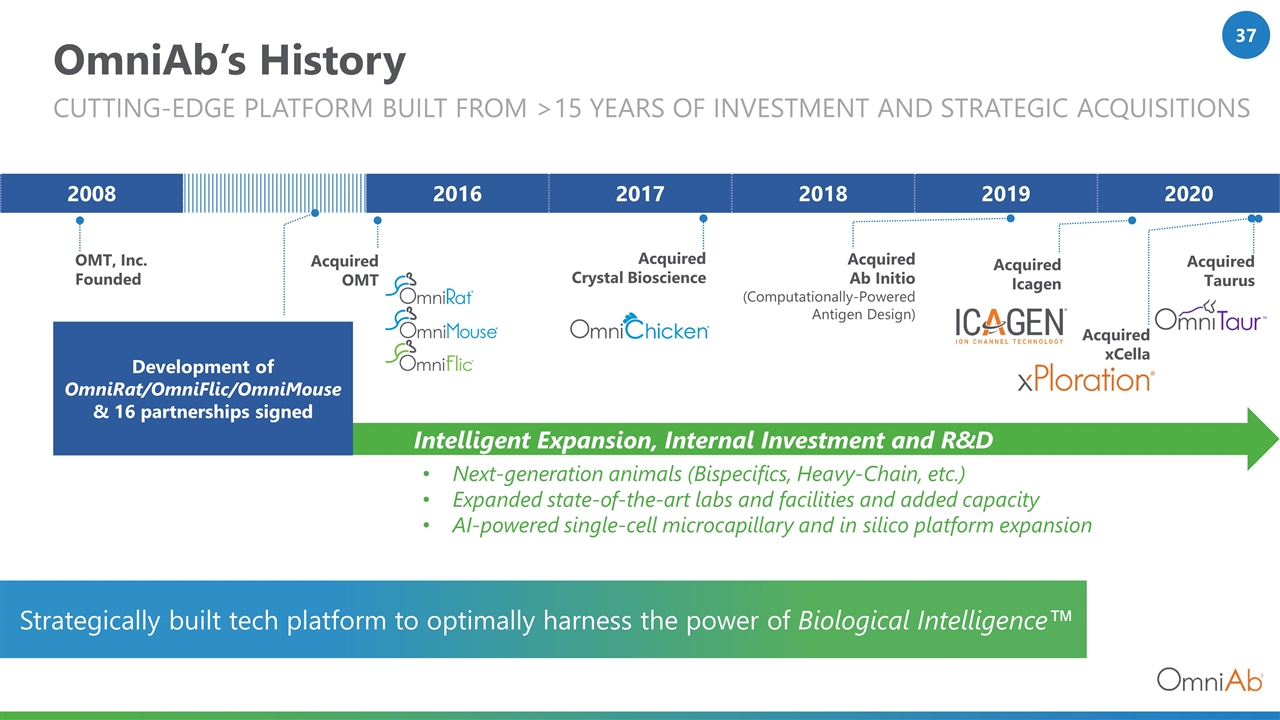
OmniAb’s History Cutting-Edge platform BUILT FROM >15 YEARS of investment and strategic acquisitions Acquired OMT Acquired Crystal Bioscience 2008 2016 2017 2018 2019 2020 OMT, Inc. Founded Acquired Ab Initio (Computationally-Powered Antigen Design) Acquired xCella Acquired Taurus Next-generation animals (Bispecifics, Heavy-Chain, etc.) Expanded state-of-the-art labs and facilities and added capacity AI-powered single-cell microcapillary and in silico platform expansion Development of OmniRat/OmniFlic/OmniMouse & 16 partnerships signed Acquired Icagen Intelligent Expansion, Internal Investment and R&D Strategically built tech platform to optimally harness the power of Biological Intelligence™
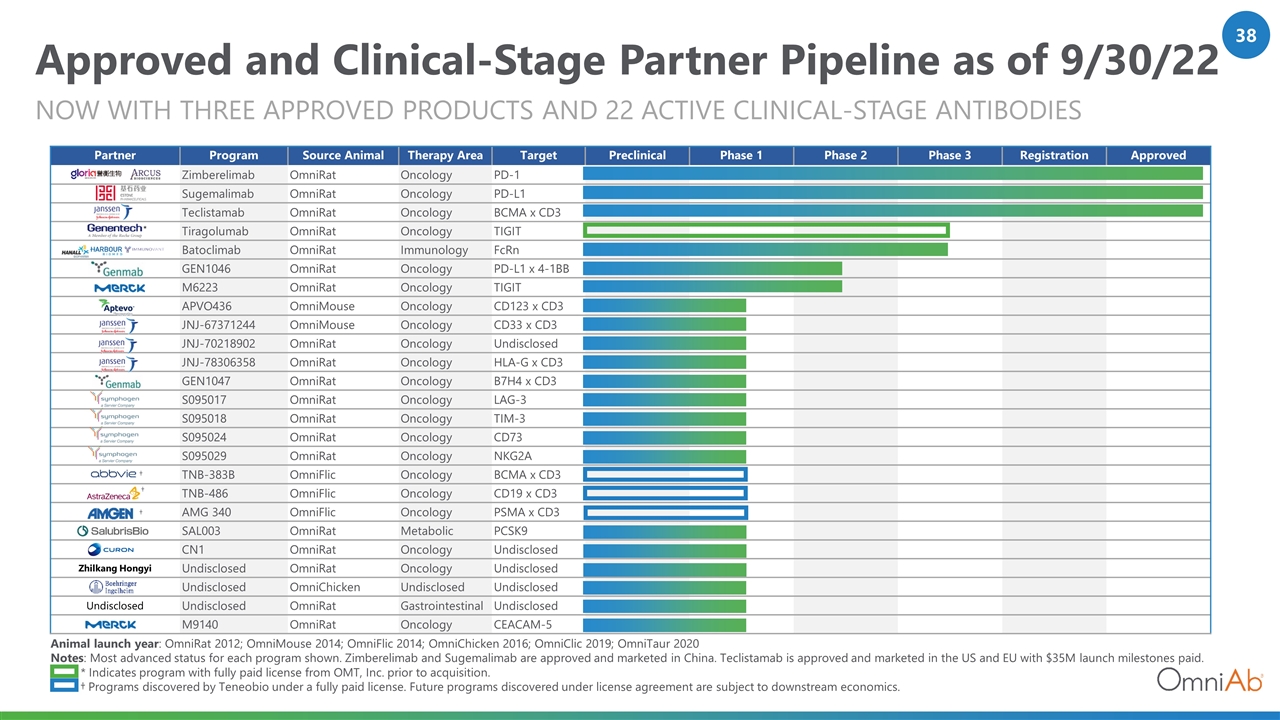
Approved and Clinical-Stage Partner Pipeline as of 9/30/22 Now with Three APPROVED PRODUCTS AND 22 Active CLINICAL-Stage ANTIBODIES Partner Program Source Animal Therapy Area Target Preclinical Phase 1 Phase 2 Phase 3 Registration Approved Zimberelimab OmniRat Oncology PD-1 Sugemalimab OmniRat Oncology PD-L1 Teclistamab OmniRat Oncology BCMA x CD3 Tiragolumab OmniRat Oncology TIGIT Batoclimab OmniRat Immunology FcRn GEN1046 OmniRat Oncology PD-L1 x 4-1BB M6223 OmniRat Oncology TIGIT APVO436 OmniMouse Oncology CD123 x CD3 JNJ-67371244 OmniMouse Oncology CD33 x CD3 JNJ-70218902 OmniRat Oncology Undisclosed JNJ-78306358 OmniRat Oncology HLA-G x CD3 GEN1047 OmniRat Oncology B7H4 x CD3 S095017 OmniRat Oncology LAG-3 S095018 OmniRat Oncology TIM-3 S095024 OmniRat Oncology CD73 S095029 OmniRat Oncology NKG2A TNB-383B OmniFlic Oncology BCMA x CD3 TNB-486 OmniFlic Oncology CD19 x CD3 AMG 340 OmniFlic Oncology PSMA x CD3 SAL003 OmniRat Metabolic PCSK9 CN1 OmniRat Oncology Undisclosed Zhilkang Hongyi Undisclosed OmniRat Oncology Undisclosed Undisclosed OmniChicken Undisclosed Undisclosed Undisclosed Undisclosed OmniRat Gastrointestinal Undisclosed M9140 OmniRat Oncology CEACAM-5 * † † † Animal launch year: OmniRat 2012; OmniMouse 2014; OmniFlic 2014; OmniChicken 2016; OmniClic 2019; OmniTaur 2020 Notes: Most advanced status for each program shown. Zimberelimab and Sugemalimab are approved and marketed in China. Teclistamab is approved and marketed in the US and EU with $35M launch milestones paid. * Indicates program with fully paid license from OMT, Inc. prior to acquisition. † Programs discovered by Teneobio under a fully paid license. Future programs discovered under license agreement are subject to downstream economics. †