Exhibit 99.2

For Investor Use Only For Investor Use Only Maximizing Immune Integrity Investor Event Presentation July 2023

For Investor Use Only Disclaimer Disclosures This investor presentation (this “Presentation”) has been prepared by NKGen Biotech, Inc . (“ NKGen ,” “ NKGen Biotech” or the “Company”) for informational purposes only and to assist interested parties in making their own evaluation with respect to the business combination (the “Business Combination”) between the Company and Graf Acquisition Corp . IV (“Graf”) and related transactions and not for any other purpose . Nothing contained in this Presentation is or should be construed as a recommendation promise or representation by the presenter, Graf or the Company or any officer director employee agent or advisor of the Company or Graf . This Presentation shall not constitute ( i ) a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the Business Combination or (ii) an offer to sell or the solicitation of an offer to buy securities, nor shall there be any sale of securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such U . S . state or jurisdiction . The Company and Graf disclaim any and all liability for any loss or damage (whether foreseeable or not) suffered or incurred by any person or entity as a result of anything contained or omitted from this Presentation and such liability is expressly disclaimed . The recipient agrees that it shall not seek to sue or otherwise hold the Company, Graf or any of their respective directors, officers, employees, affiliates, agents, advisors or representatives liable in any respect for the provision of this Presentation, the information contained in this Presentation, or the omission of any information from this Presentation . Limitations Information provided in this Presentation speaks only as of the date hereof . Nothing set forth herein should be regarded or relied upon as a representation, warranty or prediction that the Company will achieve or is likely to achieve any particular future result . While neither the Company nor Graf is not aware of any misstatements regarding any information in this Presentation, neither the Company, nor Graf, nor any of their respective affiliates or representatives makes any representation or warranty, express or implied, as to the accuracy or completeness thereof . This Presentation does not purport to contain all the information or factors that may be required to make a full analysis of the Company or the Business Combination . Viewers of this Presentation should each make their own evaluation of the Company and of the relevance and adequacy of the information and should make such other investigations as they deem necessary . Industry and Market Data Certain information contained herein is based on information released by third party sources . Industry publications and other published industry sources generally indicate that the information contained therein was obtained from sources believed to be reliable . Forecasts and other forward - looking information obtained from these sources are subject to the same qualifications and uncertainties as the other forward - looking statements in this presentation . In addition, the Company does not undertake any obligation to update any information or forward - looking statement, or to update the reasons why actual results could differ materially from those anticipated herein, even if new information becomes available in the future . This Presentation also contains estimates and other statistical data made by independent third parties and by the Company relating to market size and growth and other data about the Company's industry and results of peer companies . This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates . In addition, projections, assumptions, and estimates of the Company's future performance and the future performance of the markets in which the Company competes are necessarily subject to a high degree of uncertainty and risk . Any trademarks, servicemarks , trade names and copyrights of the Company and other companies, publications and individuals contained in this Presentation are the property of their respective owners . 2

For Investor Use Only Disclaimer Important Information About the Business Combination and Where to Find It The proposed Business Combination will be submitted to stockholders of Graf for their consideration . Graf has filed a registration statement on Form S - 4 , dated May 15 , 2023 , and amended on June 26 , 2023 (as amended from time to time, the “Registration Statement”), relating to the proposed Business Combination, with the Securities and Exchange Commission (the “SEC”), which includes both a preliminary prospectus with respect to the combined company’s securities to be issued in connection with the proposed Business Combination and a proxy statement to be distributed to Graf's stockholders in connection with Graf's solicitation of proxies for the vote by its stockholders in connection with the proposed Business Combination and other matters as described in the Registration Statement . Graf urges its investors, stockholders and other interested persons to read the preliminary proxy statement/prospectus and, when available, any amendments thereto and the definitive proxy statement/prospectus, as well as other documents filed by Graf with the SEC, because these documents will contain important information about Graf, the Company and the proposed Business Combination . After the Registration Statement is declared effective, Graf will mail the definitive proxy statement/prospectus to its stockholders as of a record date to be established for voting on the proposed Business Combination . Stockholders will also be able to obtain a copy of the Registration Statement, including the preliminary proxy statement/prospectus and, once available, the definitive proxy statement/prospectus, as well as other documents filed with the SEC regarding the proposed Business Combination and other documents filed by Graf with the SEC, without charge, at the SEC's website located at www . sec . gov or by directing a request to : Graf Acquisition Corp . IV, 1790 Hughes Landing Blvd . , Suite 400 , The Woodlands, TX 77380 . Participants in the Solicitation Graf and the Company and their respective directors and executive officers may be considered participants in the solicitation of proxies with respect to the proposed Business Combination under the rules of the SEC . Information about the directors and executive officers of Graf is set forth in the Registration Statement (and will be included in the definitive proxy statement/prospectus) . Information regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of Graf stockholders in connection with the proposed Business Combination is set forth in the Registration Statement (and will be included in the definitive proxy statement/prospectus) . Stockholders, potential investors and other interested persons should read the proxy statement/prospectus carefully before making any voting or investment decisions . These documents can be obtained free of charge from the sources indicated above . 3

For Investor Use Only Disclaimer Forward - Looking Statements This Presentation includes forward - looking statements regarding, among other things, the plans, strategies and prospects, both business and financial, of Graf and the Company . These statements are based on the beliefs and assumptions of the management of Graf and the Company . Although Graf and the Company believe that their respective plans, intentions and expectations reflected in or suggested by these forward - looking statements are reasonable, neither Graf nor the Company can assure you that either will achieve or realize these plans, intentions or expectations . Forward - looking statements are inherently subject to risks, uncertainties and assumptions . Generally, statements that are not historical facts, including statements concerning possible or assumed future actions, business strategies, events or results of operations, are forward - looking statements . These statements may be preceded by, followed by or include the words “believes,” “estimates,” “anticipates,” “expects,” “projects,” “forecasts,” “outlook,” “future,” “may,” “will,” “should,” “seeks,” “seems,” “targets,” “plans,” “scheduled,” “anticipates,” “intends” or similar expressions . The forward - looking statements are based on projections prepared by, and are the responsibility of, Graf’s or the Company’s management . These forward - looking statements are not guarantees of future performance, conditions or results, and involve a number of known and unknown risks, uncertainties, assumptions and other important factors, including changes in domestic and foreign business, market, financial, political and legal conditions, many of which are outside the control of the parties, that could cause actual results or outcomes to differ materially from those discussed in the forward - looking statements . Important factors that may affect actual results or outcomes include, among others, the inability of the parties to successfully or timely consummate the proposed Business Combination ; the failure to obtain stockholder approval of the extension of time Graf has to consummate the proposed Business Combination at any future meeting of Graf stockholders at which such proposal is presented ; the failure to satisfy the conditions to the consummation of the proposed Business Combination, including the approval of the Merger Agreement by Graf’s stockholders, the satisfaction of the minimum cash condition and the receipt of certain governmental and regulatory approvals ; the inability to obtain any PIPE investments ; the effect of the announcement or pendency of the proposed Business Combination on the Company’s business relationships, operating results, and business generally ; the risk that the proposed Business Combination disrupts the current plans and operations of the Company ; the risk that regulatory approvals for the Company’s product development are not obtained, are delayed or are subject to unanticipated conditions that could adversely affect the post - Business Combination entity (“New NKGen”) or the expected benefits of the proposed Business Combination ; the Company’s ability to manage future growth ; the Company’s ability to manage clinical trials or studies, including any compassionate use programs and product pipeline ; the risk associated with the use and reliance on the initial or preliminary results and data from the compassionate use programs and the ongoing Phase I clinical trial ; the dependence on the success of the Company’s SNK natural killer cell technology platform ; New NKGen’s ability to meet the listing standards of the New York Stock Exchange, NYSE American or Nasdaq Stock Market ; the amount of redemption requests made by Graf’s public stockholders ; the complexity of numerous regulatory and legal requirements that the Company needs to comply with to operate its business ; the failure to obtain, adequately protect, maintain or enforce the Company’s intellectual property rights ; the ability of Graf or New NKGen to issue equity or equity - linked securities in connection with the proposed Business Combination or in the future ; the concentrated ownership of New NKGen common stock among the Company’s existing executive officers, directors and principal stockholders ; and those factors discussed under the heading “Risk Factors” in the Registration Statement and other documents of Graf filed, or to be filed, with the SEC . New risk factors emerge from time to time and it is not possible to predict all such risk factors, nor can Graf or the Company assess the impact of all such risk factors on the businesses of Graf and the Company prior to the proposed Business Combination, and the combined company following the proposed Business Combination, or the extent to which any factor or combination of factors may cause actual results to differ materially from those contained in any forward - looking statements . Forward - looking statements are not guarantees of performance . You should not put undue reliance on these statements, which speak only as of the date hereof . All forward - looking statements attributable to Graf or the Company or persons acting on their behalf are expressly qualified in their entirety by the foregoing cautionary statements . Graf and the Company prior to the proposed Business Combination, and the combined company following the proposed Business Combination, undertake no obligations to update or revise publicly any forward - looking statements, whether as a result of new information, future events or otherwise, except as required by law . 4

For Investor Use Only NKGen Biotech Is A Clinical Stage Company Focused On Neurodegenerative and Oncological Diseases With Innovative Natural Killer (NK) Cells • Large expansion capacity, enhanced cytotoxicity, minimal degradation with cryopreservation • Ready - to - scale manufacturing capabilities • Can be made readily available as off - the - shelf therapies • Broad applicability across multiple diseases 5 1. Based on $145M pre - money equity value plus the aggregate amount of principal and accrued interest underlying of convertible s ecurities as of May 22, 2023 ( assuming $15.8 million) that are converted into common stock immediately prior to the closing. Transaction equity value is exclusive of $5 million of pr o forma debt with NKMAX. 2. $25M committed by NKMax Co., Ltd. ("NKMAX"), the majority shareholder of the Company. 3. Based on at least $50 million in net proceeds from the proposed business combination including up to $25 million backstop com mitment from NKMAX, which proceeds are expected to be used toward the funding of the Company's clinical trials, operations and transaction expenses. • Three Phase I autologous SNK01 trials in oncological and neurodegenerative diseases • Recent FDA IND clearance for SNK02 in solid tumors; allogeneic Phase I monotherapy trial to begin as early as second half of 2023 • Clinical/preclinical readouts for multiple indications in mid - 2023, before the expected de - SPAC closing • Compassionate use in neurodegenerative disease patients with clinical improvements* • Encouraging anti - tumor activity in solid tumors • Proposed business combination announced with Graf Acquisition Corp. IV • ~$161M transaction equity value 1 • Minimum $50 million net proceeds at closing anticipated 3Q23 2 • Cash runway expected to fund trials and operations into 2025 3 * The results from compassionate use studies have not been verified or validated. Only randomized and controlled clinical tri als can support benefit for treatments for patients. These studies may not be predictive of clinical trial results and cannot be used to establish safety or efficacy for regulatory approval.

For Investor Use Only Executive Team 6 *Board of Directors of NKGen Biotech Sangwoo Park* Founder & Executive Chairman Founder of NKMAX, Korean Parent Company Pierre Gagnon Chief Operating Officer NKMAX, Korean Parent Company Paul Y. Song, MD* CEO and Vice Chairman Jack Tsai, MD, MBA SVP, Business Development & Portfolio Strategy Ryan Park, CFA VP, Finance and Accounting Yong Man Kim, PhD* CIO & Chief Scientific Officer Paul Chang, MPH VP, Development Operations Denise Chua, MBA, CLS, MT - ASCP VP, Investor Relations and Corporate Communications James Graf Chief Executive Officer Sabrina McKee CFO and EVP, Strategy Tony Kuznik EVP, General Counsel

For Investor Use Only Natural Killer Cells Innate Lymphoid Cells: • 5 - 15% of circulating lymphocytes Surface Phenotype: • CD3 - CD56+ Can Distinguish Non - Self/Dangerous Cells From Healthy Cells: • Ability to kill a broad range of “dangerous” cells • Mediate antibody - dependent cellular cytotoxicity (ADCC) 7 Weak and/or deficient NK cells have been shown to be correlated with various disease conditions. Liu, S., Galat , V., Galat4, Y. et al. NK cell - based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol 14, 7 (2021). https://doi.org/10.1186/s13045 - 020 - 01014 - w
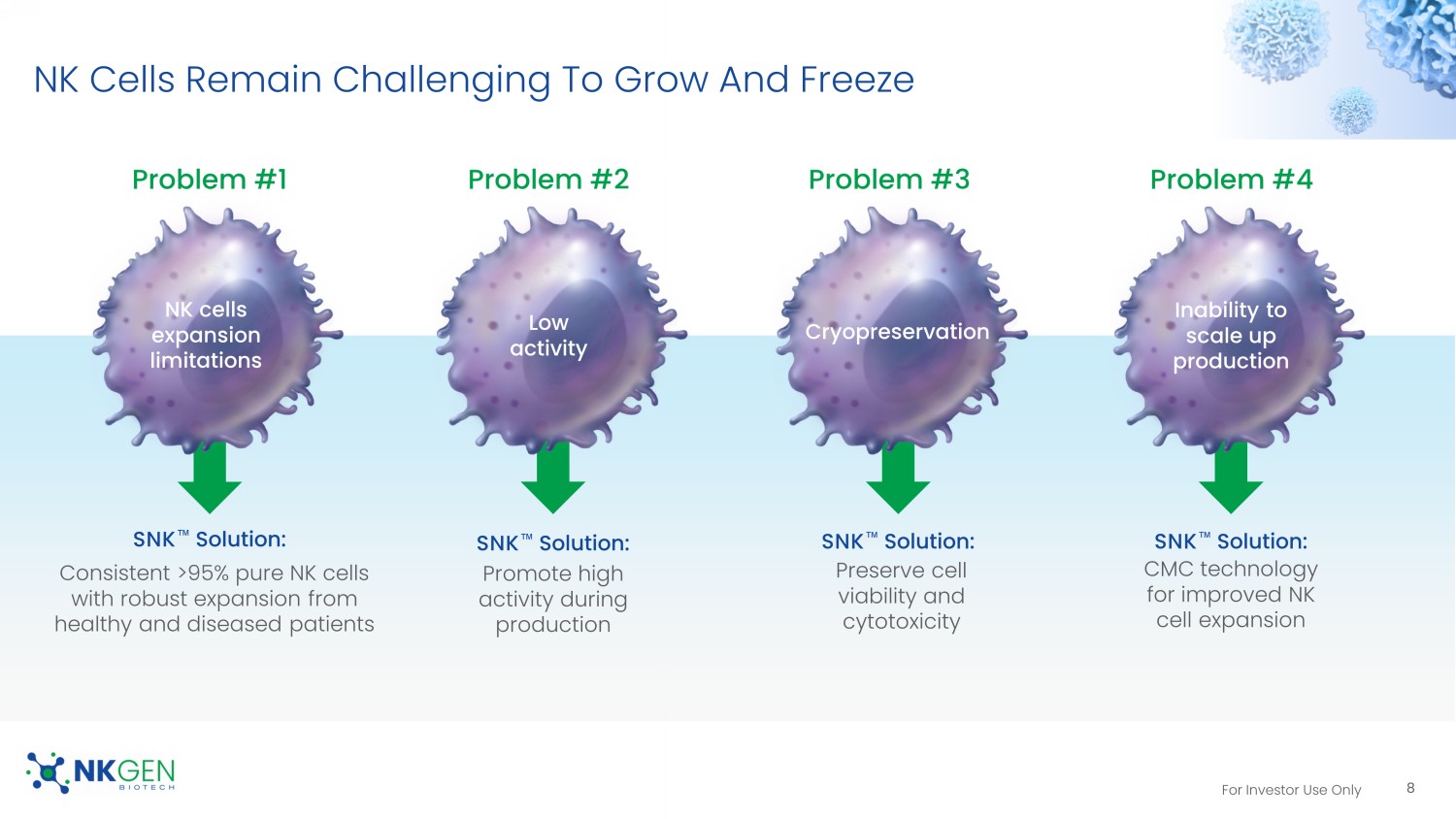
For Investor Use Only NK Cells Remain Challenging To Grow And Freeze NK cells expansion limitations Low activity Inability to scale up production Problem #2 Problem #4 SNK ΠSolution: Consistent >95% pure NK cells with robust expansion from healthy and diseased patients Promote high activity during production CMC technology for improved NK cell expansion Problem #1 Cryopreservation Preserve cell viability and cytotoxicity Problem #3 SNK ΠSolution: SNK ΠSolution: SNK ΠSolution: 8

For Investor Use Only State - Of - The - Art GMP Manufacturing Facility Licensed cell therapy manufacturing facility • 25,000 sq ft facility (12,000 sq ft for GMP) completed in 2019 • CAP/CLIA Laboratory • Facility owned and operated by NKGen Biotech 9

For Investor Use Only SNK01 (Autologous) Superior Expansion, Cytotoxicity, And Activating Receptor Expression Cytotoxicity Expansion NK cell activating receptors and ligands Receptor Expression Levels 10 1100 3915 0 2000 4000 6000 FOLD (X) Culture Day Expansion Fold (11 Cancer Patients) 5022 D0 D6 D10 D14 D17 D0 D6 D10 D14 D17 E:T Ratio E:T Ratio = NK Cell : Cancer Cell Ratio Target Cell = Myelogenous Leukemia 10:1 3:1 1:1 0.5:1 Lysis (%) 120 100 80 60 40 20 0 D0 D17 50 % Receptor Expression NKp30 Culture Day % Receptor Expression NKp46 NKp44 NKG2D CD16 Culture Day 100 50 0 Culture Day Culture Day Culture Day 100 0 60 30 0 % Receptor Expression % Receptor Expression 100 50 0 % Receptor Expression 100 50 0 D - 0 D - 17 D - 0 D - 17 D - 0 D - 17 D - 0 D - 17 D - 0 D - 17 Donor 1 Donor 2 Donor 3 1096 5060 3140 0 2000 4000 6000 FOLD (X) Culture Day Expansion Fold (8 Healthy Donors)

For Investor Use Only NKGen Can Produce Over 100,000 Doses And Maintain High Viability And Cytotoxicity 11 98.0 98.0 96.5 94.6 94.7 89.8 0.0 20.0 40.0 60.0 80.0 100.0 after thawing in infusion buffer after 4 hours (%) Viability Trypan blue 7AAD 89.8 67.3 42.1 94.5 74.4 52.0 90.9 62.9 44.5 0.0 20.0 40.0 60.0 80.0 100.0 10:1 3:1 1:1 Lysis(%) E:T Ratio Cytotoxicity after thawing in infusion buffer after 4 hours Process I ~14 - 15 days Screening Point Process II Storage Period Process III ~31 - 32 days • After thawing of cryopreserved SNK. • After dilution of thawed SNK with infusion buffer. • After 4 hours storing of diluted SNK. Process Whole Blood Collection (~100 mL) Drug Substance (WCB) Cryopreserved Drug Product Donor selection (eligible, KIR, haplotype) Release to hospitals for clinical trials (KIR haplotype, CD16 genotype, etc.) Stage Scale Starting Material Drug Substance Process I & II (1 - 1.5 x 10 7 cells/vial) Drug Product (3 x10 9 cells/dose) Note (Actual Production Scale) Process III / Batch Total Doses (Drug Substance X Process III) Whole Blood ( ~100 mL ) ~400 vials 270 - 340 108,000 – 136,000 Estimated Dose 35 - 70 14,000 – 28,000 Stage I (Preclinical – Phase I ) 135 - 200 54,000 – 80,000 Stage II (Phase II) > 270 108,000 Stage III (Phase III – NDA) Not All Companies Can Produce Large Numbers of Doses And Preserve Viability and Cytotoxicity With Their Cryopreservation
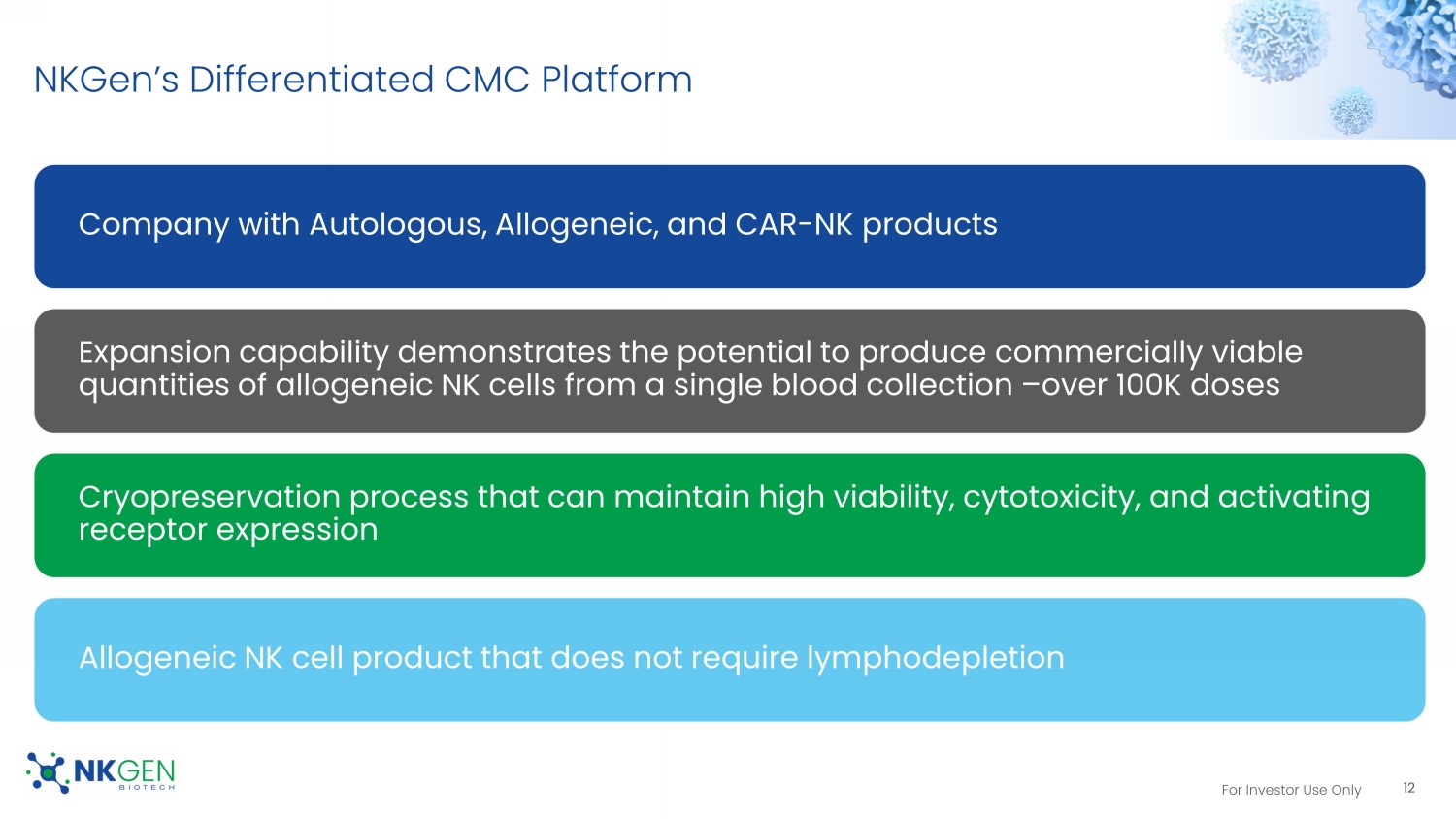
For Investor Use Only NKGen’s Differentiated CMC Platform Company with Autologous, Allogeneic, and CAR - NK products Expansion capability demonstrates the potential to produce commercially viable quantities of allogeneic NK cells from a single blood collection – over 100K doses Cryopreservation process that can maintain high viability, cytotoxicity, and activating receptor expression Allogeneic NK cell product that does not require lymphodepletion 12

For Investor Use Only NKGen Biotech Pipeline Product IND Enabling Pre - Clinical Phase I Clinical Phase II Clinical Phase III Clinical Anticipated Milestones Autologous SNK01 Monotherapy • US IND Submission for AD – 2H2023 • US IND submission for PD – 1H2024 avelumab or pembrolizumab • Final CSR Q3 2023 Allogeneic SNK02 x IND clearance 2022 • FPI 2H2023 HER2 - CAR SNK02 • US IND submission 2025 13 Refractory PD - L1+ and PD - L1 - solid tumors Neurodegenerative Disease Oncology Targets HER2+ solid tumors

For Investor Use Only Scientific/Clinical Advisors And Collaborators Neurology Oncology Craig Blackstone, MD, PhD Advisor Ming Guo, MD, PhD Advisor Anthony T. Reder, MD Advisor Yong Ben, MD, MBA Advisor Evren Alici , MD, PhD Advisor Sant Chawla, MD Collaborator 14

For Investor Use Only Neuro Program Alzheimer’s and Parkinson’s Diseases 15

For Investor Use Only Market Opportunity in Neurodegenerative Disease NKGen’s SNK01, not yet FDA approved, will seek to address a multi - billion/year market 16 1. Dementia Statistics published by Alzheimer’s Disease International, last accessed July 6, 2023 2. "Global Alzheimer’s Disease Market $6.3 Billion by 2029" and "Global Parkinson’s Disease Therapeutics Market $10.5 Billion by 2029", February 22, 2023 by iHealthcareAnalyst , Inc. 3. Reports of patient experiences from the compassionate use case studies have not been verified or validated. Only randomize d a nd controlled clinical trials can support benefit for patients. These compassionate case study results and early trials may not be predictive of cli nic al trial results and cannot be used to establish safety or efficacy for regulatory approval. We are aware of no therapies currently on the market that halt or reverse progression of AD or PD Alzheimer’s and Parkinson’s patients are estimated to account for ~ 46 million of the 55 million people with dementia worldwide 2 Over 55 million people worldwide had dementia in 2020 , with a new case diagnosed every 3 seconds; nearly 10 million new cases every year 1 Evidence of actual cognitive improvement in early trials and compassionate use AD and PD patients treated with SNK01 3 Global Parkinson’s and Alzheimer’s disease therapeutics markets forecast to be ~$16.8 billion by 2029 2 The annual global cost of dementia is >$1.3 trillion 1

For Investor Use Only Chronic protein deposition leads to an autoinflammatory cascade and damage Activation of Autoreactive CD4+ and CD8+ T cells 1 - 5 which migrate to the brain via CXCR3 6 17 1. Lindestam Arlehamn - NATURE Communications (2020) 11:1875 1 - 11. 2. Stojić - Vukanić Z - Front Immunol (2020) 11: 566225. 3. Monsonego - J. Clin. Invest. (2003) 112:415 – 422. 4. Machhi - Journal of Neuroinflammation (2021) 18:272. 5. Heneka - Lancet Neurol. (2015 ) 14(4): 388 – 405. 6. Zhou - Current Neuropharmacology, (2019) 17:142 - 150 Autoreactive T cells Cause Neuroinflammation And Damage

For Investor Use Only NK Cells Regulate Autoreactive T cells 1 - 4 18 1. Rabinovich - J Immunol (2003) 170 (7): 3572 – 3576. 2. Lu - Immunity. 2007 May ; 26(5): 593 – 604. 3. Nielsen - PLoS ONE 7(2): e31959. 4. Ardolino - Blood (2011) 117 (18): 4778 – 4786. DNAM - 1 NKG2D CD56 DNAM - 1

For Investor Use Only Autoreactive T cells And SNK01 Cross BBB Via CXCR3 CXCR3+ T cells migrate to CXCL10 positive astrocytes that frequently are associated with amyloid deposits. 1 CXCR3 was highly expressed on a subpopulation of neurons and neuronal processes in the neocortex, hippocampus, striatum, cerebellum , and spinal cord. 1 1. Xia - Neuroimmunol ., 2000, 108(1 - 2), 227 - 235. 19 CXCR3 Expression

For Investor Use Only Proteins And Damaged Neurons NK cells have been found to prevent and reduce protein accumulation 1,2 NK cells have also been found to identify and degenerate intact sensory axons after nerve injury 3 20 1. Earls - PNAS - January 2020 117 (3) 1762 - 1771. 2. Marsh et al. PNAS February 2016 - E1317. 3. Davies et al., 2019, Cell 176, 716 – 728.

For Investor Use Only Compassionate Case Study # 1 - 38 Y.O. With PSEN1 Mutation And Advanced Alzheimer’s Treated With SNK01 THESE REPORTS OF PATIENT EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPP ORT BENEFIT FOR PATIENTS. THESE MAY NOT BE PREDICTIVE OF CLINICAL TRIAL RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY OR EFFICACY FOR REGULATORY APPRO VAL . 21 Patient was independently seen and evaluated by Dr. Ming Guo (at UCLA Medical Center) before initiating SNK01 treatment. Prior to treatment, patient was unable to get out of a wheelchair by himself or feed himself. After 5 monthly doses, patient can be seen slowly eating using chopsticks and unsteadily getting up out of a car without any assistance. IMPRESSION: Striking decreased parietal activity with moderate diffuse temporal, occipital and posterior fossa activity. RESULTS AND INTERPRETATIONS: This patient is heterozygous in the PSEN1 gene for a variant designated c.338T>A, which is predicted to result in the amino acid substitution p.Leu113Gln. This variant has been reported in a patients with Alzheimer’s Disease ( Finckh et al. 2005. PubMed ID: 15776278). A different amino acid change at the same position, c.338T>C (p.Leu113Pro), has previously been reported to be causative for frontotemporal dementia and early - onset Alzheimer’s Disease ( Raux et al. 2000. PubMed ID: 11094121). Together we classify the c.338T>A (p.Leu113Gln) variant as likely pathogenic.

For Investor Use Only Compassionate Use Case Study # 2 – 70 Y.O. With Advanced Alzheimer’s Treated With SNK01 22 Patient was originally diagnosed in 2016 at Columbia University Medical Center with Advanced Alzheimer’s Disease. Patient received 6 monthly SNK01 treatments in 2020 under compassionate use and the family and treating physician have reported improvements. Patient had marked improvement in cognitive function which lasted for about six months after the end of the patient’s initial course of treatments which were stopped during COVID. Over the past 2.5 years, patient continued to decline back to a single digit MMSE score suggesting lack of durability and need for continued treatment. THESE REPORTS OF PATIENT EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPP ORT BENEFIT FOR PATIENTS. THESE MAY NOT BE PREDICTIVE OF CLINICAL TRIAL RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY OR EFFICACY FOR REGULATORY APPROVAL. Technical Results: A β 42 369.1 pg /ml T - Tau 692.6 pg /ml P - Tau 83.5 pg /ml ATI 0.35 Consistent with AD

For Investor Use Only Patient Was Granted Single Compassionate Use IND Approval By US FDA To Resume SNK01 Treatment 23 THESE REPORTS OF PATIENT EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPP ORT BENEFIT FOR PATIENTS. THESE MAY NOT BE PREDICTIVE OF CLINICAL TRIAL RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY OR EFFICACY FOR REGULATORY APPROVAL. Patient resumed treatment on 1/27/2023 and has had 3 monthly doses to date at the Neurologic Associates of Long Island Clinic under the care of Dr. Vincent DeOrchis . Currently plan to treat indefinitely until maximum benefit. Patient’s MMSE score could not be determined prior to treatment as patient was not very verbal. After restart of second course of treatment, patient was able to speak much more. Family and treating neurologist see improved energy, eye contact, and verbalization. Patient is now able to stand up/walk without assistance and to repeat words, both of which she was unable to do prior to start of treatment, according to the patient’s family.

For Investor Use Only Compassionate Case Study # 3 - Age: 79 Y.O. With Advanced Alzheimer’s Treated With SNK01 Legend MMSE Score Stage ≤12 Severe Dementia 13 - 20 Moderate Dementia 20 - 25 Mild Dementia ≥25 No Cognitive Impairment THESE REPORTS OF PATIENT EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPP ORT BENEFIT FOR PATIENTS. THESE MAY NOT BE PREDICTIVE OF CLINICAL TRIAL RESULTS AND CANNOT BE USED TO ESTABLISH SAFETY OR EFFICACY FOR REGULATORY APPROVAL. 24

For Investor Use Only Phase I Study Abstract Accepted for the 2023 Alzheimer’s Association International Conference in July 2023 Phase I Study Abstract Accepted For The 2023 Alzheimer’s Association International Conference (AAIC) In July 2023 25 No observed SAEs or dose limiting toxicity in this ongoing trial, to date

For Investor Use Only Decreases in Alzheimer’s - Related Protein Biomarkers Following SNK01 Treatment Biomarker Interim Data 1 From MX04 Phase I Trial 2 26 • Following SNK01 treatment, decreases were observed across several protein biomarkers related to Alzheimer’s pathology • 12 weeks after the last SNK01 infusion, some biomarkers return to pre - treatment levels, suggesting the need for chronic dosing • Optimal dosing regimen is currently being evaluated Worsening A β 42 Change from the baseline A β 42/40 Change from the baseline Worsening pTau 181 Change from the baseline Worsening At Week 11 50% had improved levels 3 At Week 11 90% had improved or stable levels 70% had improved levels At Week 11 60% had improved or stable levels 4 30% had improved 1. Biomarker data collection cut off as of May 24, 2023. 2. Data analysis are based on a small sample size which is not indicative of overall results. 3. Patients with increased level of Aβ 42 in cerebrospinal fluid after treatment are considered "improved" 4. Patients with unchanged or increased ratio of Aβ 42 and Aβ 40 (Aβ 42/40) are considered as "improved or stable levels." • Ten subjects with mild or moderate to severe AD were enrolled in the MX04 study as of May 23, 2023. • No treatment related adverse events have been observed to date. • Preliminary results indicated that SNK01 was well tolerated.

For Investor Use Only Dose Response From Baseline Following SNK01 Treatment Biomarker Interim Data From MX04 Phase I Trial 27 Worsening Worsening 1 WEEK AFTER THE LAST DOSE 1 WEEK AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE

For Investor Use Only Tau > Amyloid? 1. Rapoport - PNAS (2002) 99:9 6365 – 6372. 2. Brier – Sci. Trans. Med. (2016) 8:338 p. 338ra66. 3. LaJoie - Sci. Transl. Med. eaau5732 (2020) 12:1. 4. Kametani - Frontiers in Neuroscience (2018) 12:25 1 - 11. 28 Clinical pathological studies have linked tau pathology closely to the onset and progression of cognitive symptoms in patients with Alzheimer's disease 1 - 3 Tau proteins , not amyloid, may be a key driver of Alzheimer's symptoms as accumulation of tau in the brain can predict cognitive decline 3,4

For Investor Use Only Decreases In Alzheimer’s - Related Inflammatory Markers Following SNK01 Treatment Biomarker Interim Data From MX04 Phase I Trial 29 • Following SNK01 treatment, reductions were observed across several neuroinflammatory markers related to Alzheimer’s pathology • 12 weeks after the last SNK01 infusion, some neuroinflammation markers return to pre - treatment levels, suggesting the need for chronic dosing • NF - L marker also indicates a decrease in damaged neurons Neuroinflammation GFAP Change from the baseline NF - L Change from the baseline YKL - 40 Change from the baseline Neuroinflammation Worsening At Week 11 60% had improved levels At Week 11 50% had improved or stable levels 30% had improved At Week 11 60% had improved or stable levels 50% improved

For Investor Use Only Dose Response From Baseline Following SNK01 Treatment Biomarker Interim Data From MX04 Phase I Trial 30 Neuroinflammation Decrease in AD Patient 1 WEEK AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE 1 WEEK AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE

For Investor Use Only Changes In Cognitive Function Following SNK01 Treatment Interim Data From MX04 Phase I Trial 31 were stable or improved one week after final dose remained stable or improved 12 weeks after last dose were stable or improved one week after final dose remained stable or improved 12 weeks after last dose were stable or improved one week after final dose remained stable or improved 12 weeks after last dose Worsening Worsening Worsening 60% 83% 70% 67% 50% 83%

For Investor Use Only 32 Dose Response From Baseline Following SNK01 Treatment Cognitive Function Interim Data From MX04 Phase I Trial Worsening Worsening Worsening 1 WEEK AFTER THE LAST DOSE 1 WEEK AFTER THE LAST DOSE 1 WEEK AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE 12 WEEKS AFTER THE LAST DOSE

For Investor Use Only Parkinson’s Compassionate Use Case Study # 1 : Qualitative Evaluation Of 47 Y.O. After Treatment with SNK01 Patient was independently seen and evaluated by Dr. Ming Guo (at UCLA Medical Center) 33 SNK dose dates THESE REPORTS OF PATIENT EXPERIENCES AND IMAGES HAVE NOT BEEN VERIFIED OR VALIDATED. ONLY CONTROLLED CLINICAL TRIALS CAN SUPPORT BENEFIT FOR PATIENTS. THESE MAY NOT BE PREDICTIVE OF CLINICAL TRIAL RESULTS AND CANNOT BE USED TO ESTABLISH SAFET Y O R EFFICACY FOR REGULATORY APPROVAL. 0% 10% 20% 30% 40% 50% 60% Baseline 8/4/2020 8/25/2020 9/15/2020 9/29/2020 11/10/2020 11/24/2020 Percent Improvement from Baseline Mental sharpness/reaction Fatigue/energy Speech Left Leg Movement Left two fingers stickiness Face stiffness/smiling Slight depression Neck stiffness Left side weakness Left Hand Movement Slight balance

For Investor Use Only Oncology Program 34
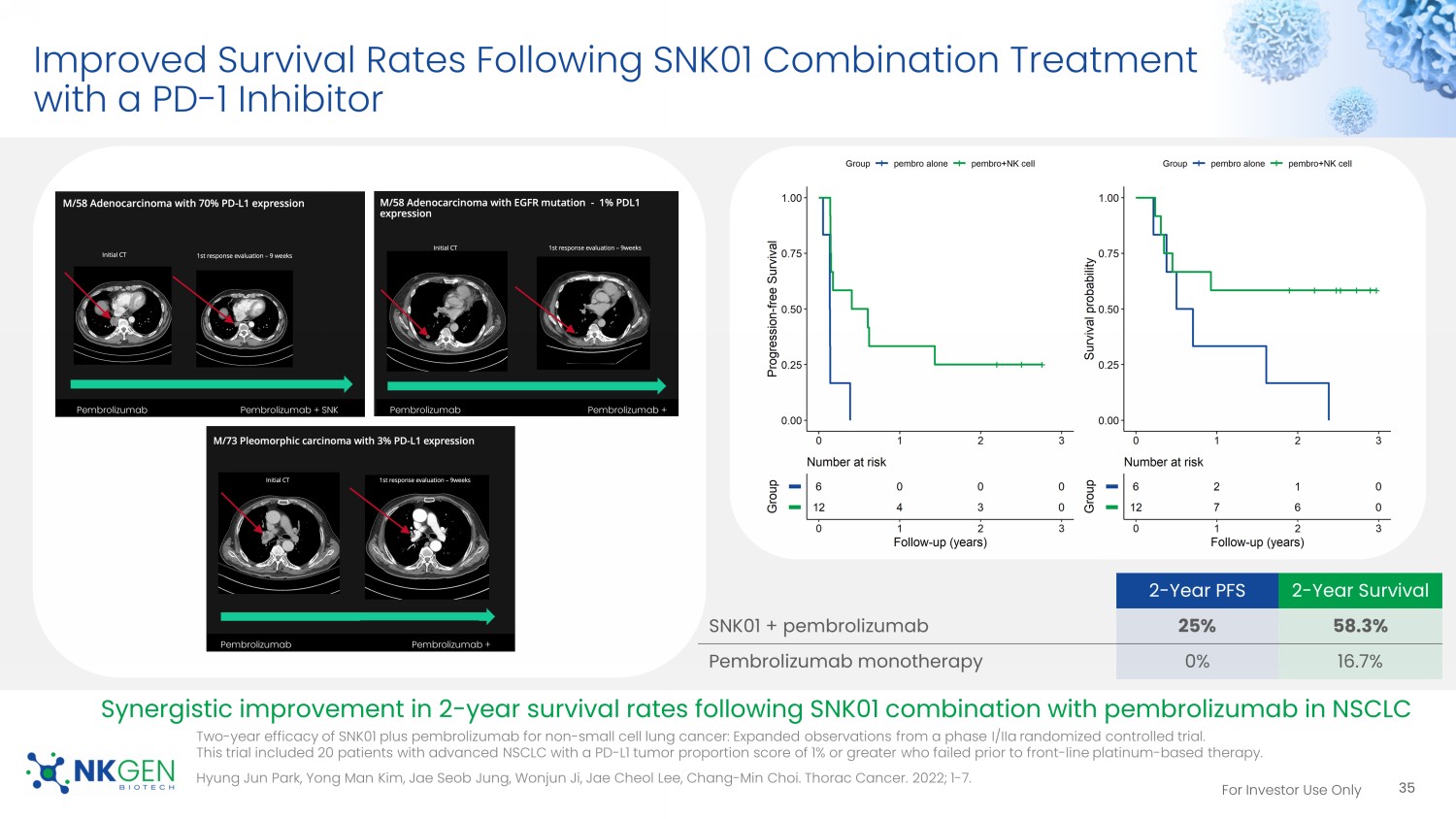
For Investor Use Only Improved Survival Rates Following SNK01 Combination Treatment with a PD - 1 Inhibitor 2 - Year PFS 2 - Year Survival SNK01 + pembrolizumab 25% 58.3% Pembrolizumab monotherapy 0% 16.7% Synergistic improvement in 2 - year survival rates following SNK01 combination with pembrolizumab in NSCLC Pembrolizumab Pembrolizumab + SNK #5 Pembrolizumab Pembrolizumab + SNK #5 Pembrolizumab Pembrolizumab + SNK #5 35 Two - year efficacy of SNK01 plus pembrolizumab for non - small cell lung cancer: Expanded observations from a phase I/ IIa randomized controlled trial. This trial included 20 patients with advanced NSCLC with a PD - L1 tumor proportion score of 1% or greater who failed prior to fro nt - line platinum - based therapy. Hyung Jun Park, Yong Man Kim, Jae Seob Jung, Wonjun Ji, Jae Cheol Lee, Chang - Min Choi. Thorac Cancer. 2022; 1 - 7.

For Investor Use Only Can SNK Change The TME For PDL1 - Tumors? NK cells can positively affect the TME to help make PD - L1 negative tumors respond to ICIs* . 1 . Erlinda M. Gordon, et al. (2022). Durable Responses Using SNK01 Autologous Enhanced Natural Killer Cells and Pembrolizumab for Chemotherapy - Resistant Advanced Sarcoma: Case Reports, Literature Review and Future Perspectives. J. Cancer Research and Cellular Therapeutics. 6(5). 2 . Victoria S. Chua, Poster Presentation: USFDA Authorized Compassionate Use of SNK01 (Autologous Non - Genetically Modified Natura l Killer Cells With Enhanced Cytotoxicity) and Immune Checkpoint Inhibitors in Advanced Heavily Pre - treated Sarcoma. A Promising Regimen. ESMO Annual Meeting 2022. Baseline September 2020 After 2 nd SNK October 2020 After 11 th SNK July 2021 36 Patient Age: 58 2 • Stage IV Chondrosarcoma • Diagnosed 2019 • Disease included lung, abdomen and pelvis with extensive liver disease. Non - healing large pelvic wound due to tumor progression. • <10% PD - L1+, Microsatellite stable Failed Therapies 1. Opdivo ® 2. Ibrance ® 3. panzopanib Patient Age: 32 1 • Stage IVB Desmoplastic Small Round Cell Sarcoma • Diagnosed 2017 • Disease included lung, abdomen and pelvis with extensive disease in abdominal/ pelvic lymph nodes and liver • PD - L1 Negative Tumor Failed Therapies 1. doxorubicin, cytoxan, and vincristine 2. etoposide and ifosfamide 3. aldoxorubicin and ifosfamide 4. irinotecan, vincristine, and Temodar ® 5. Yondelis ® and Keytruda ®

For Investor Use Only Effect Of NK Cells On Cetuximab - mediated ADCC Preliminary Phase I data Presented at ASCO 2023 Cetuximab: anti - EGFR antibody. A Study on the Anti - Tumor Activity of SNK01 in EGFR - TKI - Resistant Non - small Cell Lung Cancer, NKMax Co., Ltd., Internal In Vitro Test Report , NKMAX - R20 - 04 , October 30, 2020 [page 30]. 37 IgG Media EGFR Lysis (%) Lysis (%) parental PC - 9 PC - 9/GR PC - 9/OR parental HCC827 HCC827/GR HCC827/OR Con GC Con GC Con GC Con GC Con GC Con GC Cetuximab - mediated ADCC activity of NK cells in EGFR - TKI - resistant NSCLC cells. Parental PC - 9, PC - 9/GR, and PC - 9/OR cells (A) as well as parental HCC827, HCC827/GR and HCC827/OR NSCLC cells (B) were treated w ith (GC) or without (Con) gemcitabine plus carboplatin for 24 hours and then co - cultured with NK cells for 2 hours at the E:T ratio of 3:1 in the pr esence of media alone (Media), isotype control IgG antibody (IgG) or cetuximab (EGFR). The cytotoxic activity of NK cells against NSCLC cells was shown as mean ̃ SD. One - way ANOVA with Tukey’s post hoc analysis was applied for statistical analysis. *p<0.05, **p<0.01, and ***p<0.005, relati ve to untreated cells (Media). A B ** ** * * *** *** *** *** *** ** * 100 80 60 40 20 0 80 60 40 20 0

For Investor Use Only ASCO 2023 Poster Presentation SNK01 Phase I/ IIa Clinical Trial Results ClinicalTrials.gov Identifier: NCT04872634 12 patients with TKI resistant metastatic NSCLC were evaluated Dose - limiting toxicity was not observed, therefore the maximum tolerated dose of SNK01 was determined to be 6 ̩ 10 9 cells/dose No SNK01 - related adverse events of Grade 3 or higher were observed The objective response rate was 25% (3/12), disease control rate (DCR) was 100%, with 3/12 patients experiencing a partial response (25%) and 9/12 with stable disease (75%) for the total of the 12 patients Median progression free survival (PFS) was 143 days. Some patients are still being followed and an updated PFS will be provid ed at a later date 38 Choi, M.G. et al. Poster #45, ASCO 2023.

For Investor Use Only NK Cells: The Missing Piece In Cancer Therapeutics? We believe that the future of cancer therapies will be in combination therapies that enable precision targeting, immune activation and immune effector response simultaneously. SNK cells may provide the “missing” piece for modular treatment approach – regimens that can be easily adapted to include new agents as data become available. 39 Tumor targeting Immune activation Immune effectors Tri/ BiKEs , BiTEs mAbs , ADCs PD - 1/L1, CTLA - 4, TIGIT Missing piece: Adoptive, enhanced NK effector cells
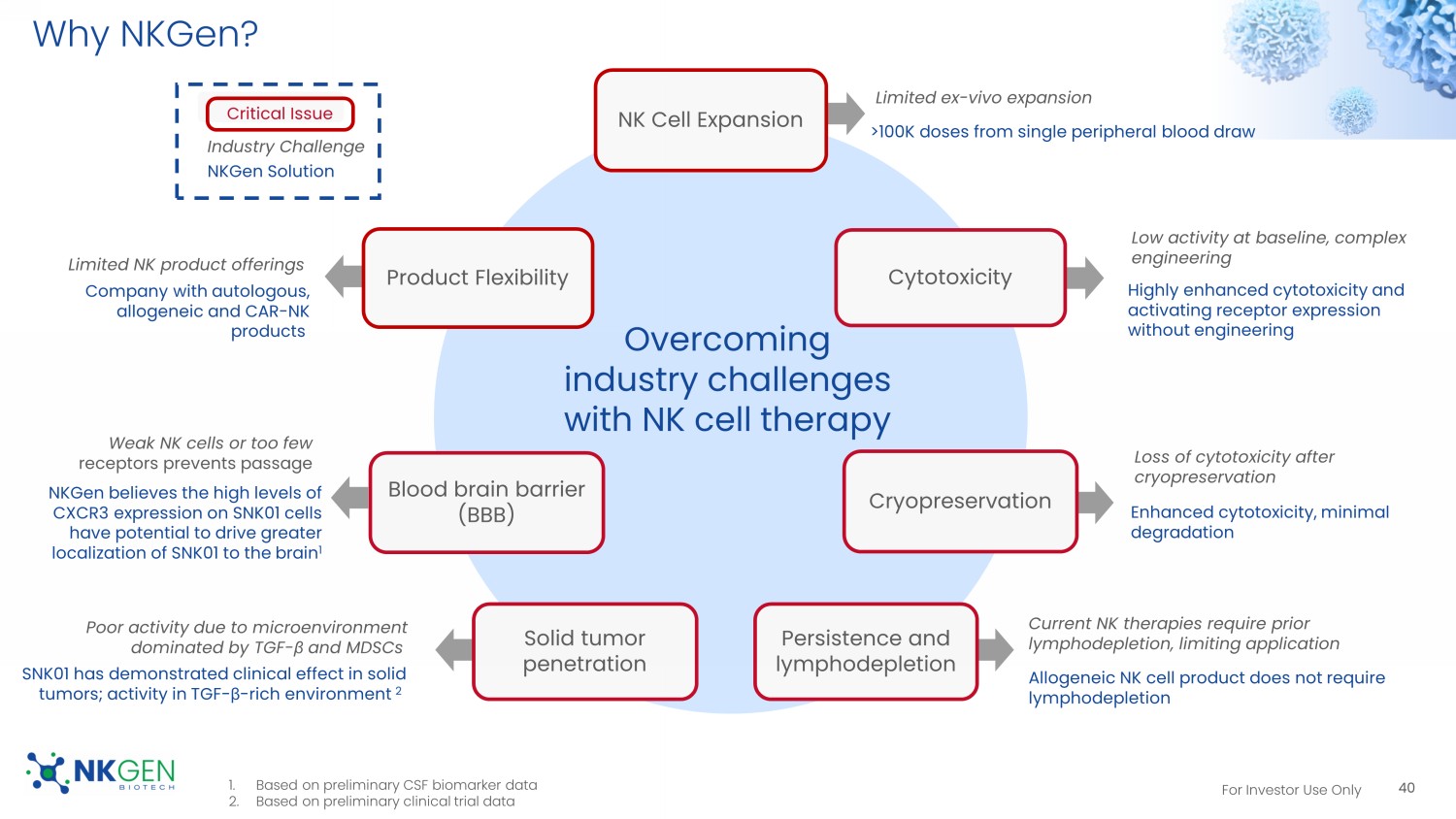
For Investor Use Only Why NKGen? 40 NK Cell Expansion Cytotoxicity Cryopreservation Persistence and lymphodepletion Solid tumor penetration Blood brain barrier (BBB) Product Flexibility Overcoming industry challenges with NK cell therapy Limited ex - vivo expansion >100K doses from single peripheral blood draw Low activity at baseline, complex engineering Highly enhanced cytotoxicity and activating receptor expression without engineering Loss of cytotoxicity after cryopreservation Enhanced cytotoxicity, minimal degradation Current NK therapies require prior lymphodepletion, limiting application Allogeneic NK cell product does not require lymphodepletion Poor activity due to microenvironment dominated by TGF - β and MDSCs SNK01 has demonstrated clinical effect in solid tumors; activity in TGF - β - rich environment 2 Critical Issue Industry Challenge NKGen Solution Weak NK cells or too few receptors prevents passage NKGen believes the high levels of CXCR3 expression on SNK01 cells have potential to drive greater localization of SNK01 to the brain 1 Limited NK product offerings Company with autologous, allogeneic and CAR - NK products 1. Based on preliminary CSF biomarker data 2. Based on preliminary clinical trial data

For Investor Use Only Transaction Overview 1 Key transaction Terms • ~$161M transaction equity value 2 • 100% equity rollover • $50M minimum net cash closing condition 3 • Proceeds expected to fund trials and operations into 2025 4 • $25M backstop commitment from majority shareholder 5 • Expected closing in Q3 2023 • Potential Sponsor forfeiture of up to ~70% of Founder Shares 6 41 NKGen Shareholders 68% Graf Shareholders 15% Graf Sponsor & Directors 6% Backstop 11% NKGen Shareholders 68% Graf Shareholders 26% Graf Sponsor & Directors 6% 1. All calculations are based on a $10/share price, except for the redemption scenarios (including SPAC Cash in Trust), which is ba sed on actuals following the May 22, 2023 extension vote. Both scenarios exclude shares of Common Stock issuable upon the exercise of Warrants held by the Graf Insiders and Deferred Founder shares. 2 . Based on $145M pre - money equity value plus the aggregate amount of principal and accrued interest underlying of convertible securities as of May 22, 2023 ( assuming $15.8 million) that are converted into common stock immediately prior to the closing. Transaction equity value is exclusive of $5 million of pro forma debt with NKMAX. 3. Calculated after paying any outstanding transaction expenses incurred by Graf. 4 . Based on current base trial plan expected requirements. 5 . Concurrently with the execution of the Merger Agreement, Graf entered into a backstop agreement with NKMAX, pursuant to which , N KMAX agreed to purchase, contingent upon the Closing and subject to the terms contained herein, at a price of $10.00 per share, subject to most favored nations adjustments, an amount of common shar es of the combined company not to exceed $25 million without the consent of NKMAX or Graf. 6 . 2,947,262 Deferred Founder Shares are subject to vesting and forfeiture following Closing, as follows: (A) 1,473,631 shares of the Deferred Founder Shares will vest if, at any time during the Vesting Period, VWAP equals or exceeds $14.00 per share (as adjusted for share splits, share dividends, reorganizations, and rec apitalizations) at any 20 trading days in a 30 consecutive trading - day period beginning from the Closing Date until the fifth anniversary of the Closing Date, and (B) 1,473,631 shares of the Deferred Fou nde r Shares will vest if, at any time during the Vesting Period, the VWAP of the Common Stock equals or exceeds $16.00 per share (as adjusted for share splits, share dividends, reorganizations, and recapitalizatio ns) at any 20 trading days in a 30 consecutive trading - day period during the Vesting Period. If the First Triggering Event and/or Second Triggering Event does not occur, the respective Deferred Founder Shares w ill be forfeited. The Deferred Founder Shares do not have voting rights during the Vesting Period. 7. After paying estimated outstanding Graf expenses. 8 . NKMAX represents 83% of total NKGen Shareholders. 9. This does not account for shares underlying assumed options. No Redemption Max Redemption 8 8 9 9

For Investor Use Only For Investor Use Only Thank you

For Investor Use Only Appendix 43

For Investor Use Only Allogeneic NK cell product that does not require lymphodepletion. US Phase I Allogeneic Trial Expected To Begin In 2H2023 44 Confidential

For Investor Use Only Autologous Next Gen Manufacturing NKGen “Off - the - Shelf Autologous” SNK Cell Therapy • Cryopreserved autologous manufacturing process takes ~18 days from NK cell isolation to cryopreserved product release • One - time production for all the doses; SNK cells are frozen and stored on site, ready for release • Ability to produce multiple doses (6 x 10 9 cells each) from single leukapheresis to fulfill 4 – 6 months of weekly treatments • Multiple doses are produced at once; approx. 20 doses=1 batch release and cryopreserved • Cryopreserved autologous process fully developed 45 Superior Cell Expansion and Activation Our Cryoprocess preserves cell features Day 0 Up to 18 Days Auto - SNK No Lymphodepletion Collect Leukapheresis Isolate Isolate NK cells from PBMC 2 Activate & Expand Stimulate NK cells to activate & expand to enhance cytotoxicity 3 4 Deliver to Clinic Doses in cryopreserved state released to clinic 1 Thaw and Administer Doses thawed, as needed, and administered to patient 5 5 Cryopreserve Multiple doses cryopreserved

For Investor Use Only Intellectual Property NK Cell Expansion Platform Patents 46 • Highly cytotoxic NK Cells • Consistently large yields (including unhealthy donors) • Granted in key territories -- North America, • Still alive (continuing application pending) • Expiry 2033 • Larger yields -- ideal for allogeneic production • Even stronger cytotoxicity • Method patent granted in the U.S. • Composition of matter granted in the U.S. • Ongoing national phase in several key territories - North America, EU • Still alive (continuing application pending) • Expiry 2039 Patent Family 1 Patent Family 2 Additional Patent Families • Cryopreservation and recovery of highly cytotoxic NK cells • Method for reduction of immune checkpoint therapy - related adverse events • Treatment of neurodegenerative and/or neurological disease • Ongoing national phase, or potential to enter national phase in several key territories - North America, EU • Estimated Expiry 2040 - 2043

For Investor Use Only Cash Used in Operating Activities 47 NKGen expenses 2020 2021 2022 Total cash burn 1 $17.3M $19.9M $22.5M RND - only cash expenses 2 $10.6M $14.0M $15.7M 1. Total cash burn amount equals net cash used in operating activities, which is a GAAP financial measure. 2. R&D - only cash expenses represent R&D expenses of $11.0M, $14.7M , $16.7M and $3.6M for the years ended December 31, 2020, 2021 , 2022 and for the three months ended March 31 2023, less R&D related depreciation expenses of $0.3M, $0.6M ,$1.0M and $0.2M and R&D related stock - based compensation expenses of $0.1M, $0.1M , $0 and $0.3M for the years ended December 31, 2020, 2021 and 2022 and for the three months ended March 31 2023.

For Investor Use Only Significant Opportunity With Funding Potential From Partnering Our Platform And In Oncology 48 1. Total market sales US, EU5, JPN, according to Global Data. Parkinson’s (2029) and Alzheimer’s (2028). 2. Total NK market sales WW by 2026, according to Allied Market Research. 3. BiosciDB aggregate cell therapy CDMO deals since 2019. Autologous SNK for Parkinson's Autologous SNK for Alzheimer’s NK cells for Oncology CDMO for NK Cellular Processes Development objectives Demonstrate safety and superior efficacy of NK autologous NNK: Build evidence SNK as treatment to treat neurodegeneration in Parkinson patients. Demonstrate safety and efficacy for use of autologous NK cells in Alzheimer's Disease. SNK01 proof of concept in diseases with few treatment options. Partnerships in combination of other agents to improve efficacy. Pivot to allogeneic for oncology programs. Superior manufacturing process which can be leveraged for use with all NK Cells. Addresses Cytotoxicity, Expansion, Freeze/Thaw, CAR - NK, yields> 100k doses. Partners / Development lead Total addressable market ~$5B 2 ~1B+ 3 ~$12B 1 ~$13B 1

For Investor Use Only Allogeneic Anti - HER2 - CAR SNK02 49 (US IND submission anticipated in 2025) CONFIDENTIAL

For Investor Use Only NKGen – Engineering, Expansion, And Cryopreservation 50 Analysis / Final product and cell banking Collection NK cell stimulation Engineering Expansion & Enrichment Collection D0 D3 - 6 D10 - 11 D14 D45 IL - 2 IL - 21 Feeder

For Investor Use Only Expression Level Of Anti - HER2 - CAR on NK Cell 51 51 Anti - HER2 scFv Co - stimulatory CD3 ζ Cytokine leader Hinge TM Promoter P2A 1.11 1.05 2.3 2.47 2.39 1.89 2.42 1.38 1.64 85.67 89.03 87.93 86.31 87.16 86.03 83.03 81.62 82.74 0 10 20 30 40 50 60 70 80 90 100 D10 D14 S1 D6 S1 D10 S1 D14 S2 D6 S2 D10 S2 D14 S2 D17 % expression CAR Expression SNK anti-HER2-CAR NK

For Investor Use Only NK Cell Expansion Rate 0 50 100 150 200 250 300 350 400 450 D0 D4 D10 D14 D17 Expansion Rate (P1) SNK Anti-HER2-CAR-SNK 0 200 400 600 800 1000 1200 S1 D0 S1 D6 S1 D10 S1 D14 S1 D17 Expansion Rate (S1) SNK Anti-HER2-CAR SNK 0 500 1000 1500 2000 2500 3000 S2 D0 S2 D6 S2 D10 S2 D14 S2 D17 Expansion Rate (S2) SNK anti-HER2-CAR SNK Expansion fold Population doubling level (2 n ) SNK: non - transduced NK cells 0.00 5.00 10.00 15.00 20.00 25.00 30.00 D0 D4 D10 D14 S1 D6 S1 D10 S1 D14 S2 D6 S2 D10 S2 D14 S2 D17 PDL SNK anti-HER2-CAR SNK SNK: 427.8 - fold CAR SNK: 399.1 - fold SNK: 1012.4 - fold CAR SNK: 993.3 - fold SNK: 2489.5 - fold CAR SNK: 2383.9 - fold SNK: 2 27.8 (2.31 x 10 8 - fold) CAR SNK: 2 27.6 (2.01 x 10 8 - fold) 52

For Investor Use Only 1.89 2.42 1.38 1.65 1.29 86.03 83.2 81.6 82.84 84.02 0 10 20 30 40 50 60 70 80 90 100 S2 D6 S2 D10 S2 D14 S2 D17 THAWING Persistence 0 20 40 60 80 10:1 5:1 2.5:1 1.25:1 0.63:1 0.3:1 Thawing (SK - BR3) 0 10 20 30 40 50 10:1 5:1 2.5:1 1.25:1 0.63:1 0.3:1 Thawing (SK - OV3) 0 20 40 60 80 10:1 5:1 2.5:1 1.25:1 0.63:1 0.3:1 Fresh (SK - OV3) 0 20 40 60 80 100 10:1 5:1 2.5:1 1.25:1 0.63:1 0.3:1 Fresh (SK - BR3) Cytotoxicity (2 hours) SK - OV3 SK - BR3 SNK02 Anti - HER2 - CAR SNK02 SNK02 Anti - HER2 - CAR SNK02 SNK02 Anti - HER2 - CAR SNK02 CAR Expression The Effect Of Freezing/Thawing On CAR Expression & Cytotoxicity 53 SNK02 Anti - HER2 - CAR SNK02 SNK02 Anti - HER2 - CAR SNK02

For Investor Use Only Partnerships With Merck And Pfizer In September 2020, NKGen (formerly NKMax America) entered into a clinical trial collaboration and supply agreement with Germany - based Merck KGaA to evaluate the safety and tolerability of SNK01, NKGen's autologous NK cell therapy in combination with avelumab (BAVENCIO), a human anti - PD - L1 therapy co - developed and co - commercialized by Merck KGaA and Pfizer, Inc. in solid tumor. 54

For Investor Use Only SNK01 – MX04 Phase I Trial Dosing and Administration: To identify the Maximum Tolerated Dose (MTD), the subjects were given SNK01 in an open - label setting per the following treatment plan, using a 3 + 3 design: • Cohort 1 - 1.0 x 10 9 cells; infusions Q3W, total of four doses. • Cohort 2 - 2.0 x 10 9 cells; infusions Q3W, total of four doses. • Cohort 3 - 4.0 x 10 9 cells; infusions Q3W, total of four doses. • Once the MTD is identified, a final cohort, Cohort 4 , including 12 subjects, will receive the MTD to study safety, tolerability, and preliminary efficacy 55

For Investor Use Only References For NK Cells And PD - 1/PD - L1 • Hasim, M., et al. (2022). When killers become thieves: trogocytosed PD - 1 inhibits NK cells in cancer. ScienceAdvances , 8(15). • Hsu, J., et al. (2018). Contribution of NK cells to immunotherapy mediated by PD - 1/PD - L1 blockade. J Clin Invest, 128(10):4654 - 4 668. • Ben - Shmuel, A., et al. (2020). Unleashing natural killer cells in the tumor microenvironment – the next generation of immunother apy? Front Immunol, 11:275. • Dunai, C., et al. (2018). NK cells for PD - 1/PD - L1 blockade immunotherapy: pinning down the NK cell. J Clin Invest, 128(10):4251 - 4253. • Pesce , S., et al. (2019). PD/1 - PD - Ls checkpoint: Insight on the potential role of NK cells. Front Immunol, 10:1242. • Bi, J., et al. (2019). NK cell dysfunction and checkpoint immunotherapy. Front Immunol, 10:1999. • Benson, D.M., et al. (2010) The PD - 1/PD - L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT - 011, a novel monoclonal anti PD - 1 antibody. Blood, 116(13):2286 - 94. 56

For Investor Use Only References For CI Tolerability • Trinh, S., et al. (2019). Management of Immune - Related Adverse Events Associated with Immune Checkpoint Inhibitor Therapy: a Min ireview of Current Clinical Guidelines. Asia Pac J Oncol Nurs , 6(2):154 - 160. • Berti , A., et al. (2021). Meta - analysis of immune - related adverse events in phase 3 clinical trials assessing immune checkpoint inhib itors for lung cancer. Crit Rev Oncol Hematol , 162:10335.1 • Rabinovich , B.A., et al. (2003). Activated but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol, 170(7) :3572 - 6. • Nielsen, N., et al. (2012). Cytotoxicity of CD56 bright NK cells towards autologous activate CD4+ T cells is mediated through NK G2D, LFA - 1 and TRAIL and dampened via CD94/NKG2A. PLoS One, 7(2):e:31959. • Lu, L., et al. (2007). Regulation of activated CD4+ T cells by NK cells via the Qa - 1 - NKG2A inhibitory pathway. Immunity, (5):593 - 604. Cerboni , C., et al. Antigen - activated human T lymphocytes express cell - surface NKG2D ligands via ATM/ATR - dependent mechanism and become susceptible to autologous NK - cell lysis. Blood, 110(2):6060 - 15. • Kelly, K., et al. (2020). Efficacy and Immune - related adverse event associations in avelumab - treated patients. Journal for ImmunoTherapy of Cancer, (8)2:e001427. 57

For Investor Use Only References For Neurodegenerative Disease • Zhou, Y - Q, et al. (2019). The role of CXCR3 in Neurological Diseases. Curr Neuropharmacol , 17(2): 142 - 150. • Marsh, S.E., et al. (2016). The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial fu nct ion. Proc Natl Acad Sci U S A,113(9):E1316 - 25. • Alves, S., et al. (2017). Interleukin - 2 improves amyloid pathology, synaptic failure and memory in Alzheimer’s disease mice. Bra in, 140(3):826 - 842. • Mate, I., et al. (2015). Function and Redox State of Peritoneal leukocytes as preclinical and prodromic markers in a longitud ina l study of triple - transgenic mice for Alzheimer’s disease. J Alzheimers Dis, 43(1):213 - 26. • Davies, A.J., et al. (2019). Natural Killer cells degenerate intact sensory afferents following nerve injury. Cell, 176(4), 7 16 - 728. • Hoxha, E., et al. (2018). The emerging role of altered cerebellar synaptic processing in Alzheimer’s disease. Front Aging Neurosci , 10:396. • Desikan , R.S., et al. (2010). Selective disruption of the cerebral neocortex in Alzheimer’s disease. PLoS One, 5(9):e12853. • Rao, Y.L., et al. (2022). Hippocampus and its involvement in Alzheimer’s disease: a review. 3 Biotech, 12(2):55. • Qin, Q., et al. (2021). Prominent striatum amyloid retention in early - onset familial Alzheimer’s disease with PSEN1 mutations: a pilot PET/MR study. Front Aging Neurosci , 13:732159. 58

For Investor Use Only References Neurodegenerative Disease (Cont.) • Earls, R.H., et al., (2020). NK cells clear alpha - synuclein and the depletion of NK cells exacerbates synuclein pathology in a m ouse model of alpha - synucleinopathy . Proc Natl Acad Sci U S A, 117(3):1762 - 1771. • Rabinovich , B.A., et al. (2003). Activated but not resting, T cells can be recognized and killed by syngeneic NK cells. J Immunol, 170 (7) :3572 - 6. • Betourney , Charlene. “‘Natural Killer’ cells could halt Parkinson’s progression.” UGA Today, March 12, 2020 • La Jolla Institute for Immunology. “New Therapies could stop T cells from attacking brain cells in Parkinson’s disease.” SciT ech Daily, March 21, 2022. • Lindestam Arlehamn , C.S., et al. (2020). Alpha - Synuclein - specific T cell reactivity is associated with preclinical and early Parkinson’s disease. Nat Commun , (11)1:1875. • Earls, R.H. and Lee, J - K., (2020). The role of natural killer cells in Parkinson’s disease. Experimental & Molecular Medicine, 52:1517 - 1525. 59