INFORMATION ABOUT AEON
Unless the context otherwise requires, all references in this subsection to “we,” “us,” or “our” refer to AEON prior to the consummation of the Business Combination.
Overview
We are a clinical stage biopharmaceutical company focused on developing our proprietary botulinum toxin complex, ABP-450 (prabotulinumtoxinA) injection, or ABP-450, for debilitating medical conditions, with an initial focus on the neurosciences market. We recently completed a Phase 2 study of ABP-450 for the treatment of cervical dystonia and have an ongoing Phase 2 study of ABP-450 for the treatment of both chronic and episodic migraine. ABP-450 is the same botulinum toxin complex that is currently approved and marketed for cosmetic indications by Evolus under the name Jeuveau. ABP-450 is manufactured by Daewoong in compliance with current Good Manufacturing Practice, or cGMP, in a facility that has been approved by the FDA, Health Canada and EMA. We have exclusive development and distribution rights for therapeutic indications of ABP-450 in the United States, Canada, the European Union, the United Kingdom, and certain other international territories. We have built a highly experienced management team with specific experience in biopharmaceutical and botulinum toxin development and commercialization.
Botulinum toxins have proven to be a highly versatile therapeutic biologic, with over 230 therapeutic uses documented in published scientific literature and nine approved therapeutic indications in the United States. Our initial development programs for ABP-450 are directed at migraine, cervical dystonia and gastroparesis. We selected these initial indications based on a comprehensive product assessment screen designed to identify indications where we believe ABP-450 can deliver significant value to patients, physicians and payors and where its clinical, regulatory and commercial characteristics suggest viability. We believe that ABP-450 has application in a broad range of indications and we plan to continue to explore additional indications that satisfy our product assessment screens. The following table depicts the development status of ABP-450 across our current indications:
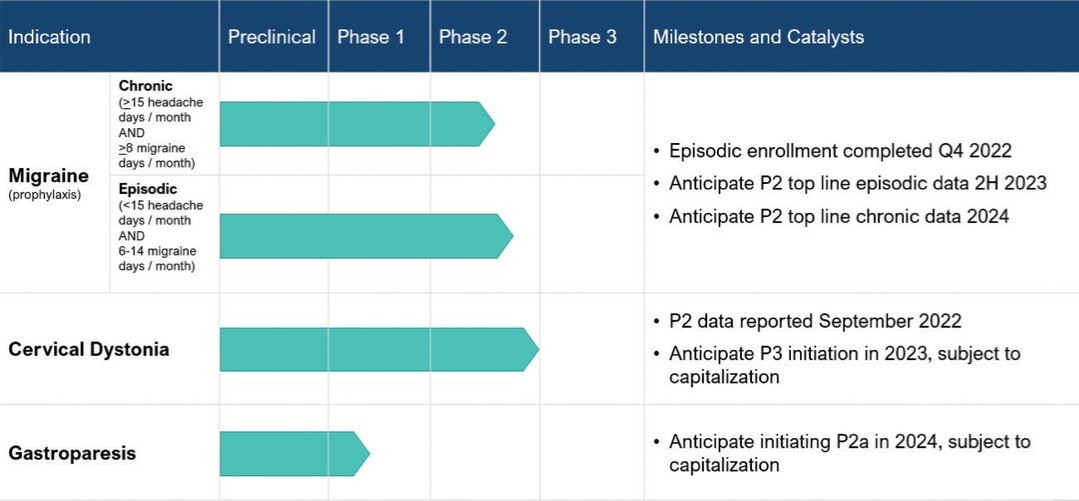
The FDA accepted our IND application for ABP-450 as a preventative treatment for migraine in October 2020, and we began treating patients in our Phase 2 clinical study beginning in March 2021. Prior to commencing this Phase 2 study, no Phase 1 clinical studies of ABP-450 had been performed in regards to migraine by us or any other party. Nevertheless, given the extensive pre-clinical toxicology and other data developed by our licensing partner, Daewoong, and the aesthetic licensor of ABP-450, Evolus, the FDA permitted us to proceed directly to this Phase 2 clinical trial. We plan to enroll approximately 765 patients in this randomized, double-blind, placebo-controlled study across approximately 60 study sites in the United States, Canada and Australia. This study includes migraine patients that experience six or more migraines per month, which is inclusive of chronic migraine patients that experience 15 or more headache days and eight or more migraines per month, as well as certain episodic migraine patients that experience less than 15 headache days and six to 14 migraines per month. Patients enrolled in the study receive two injection cycles using our patent-
191