Filed by Thimble Point Acquisition Corp.
Pursuant to Rule 425 under the Securities Act of 1933
and deemed filed pursuant to Rule 14a-12
under the Securities Exchange Act of 1934, as amended
Subject Company: Thimble Point Acquisition Corp.
Commission File No. 001-39969
Date: July 22, 2021

Pear therapeutics 1

Disclaimer This presentation is provided for informational purposes only and has been prepared to assist interested parties in making their own evaluation with respect to a potential business combination (the “Business Combination”) between Thimble Point Acquisition Corporation (“TPAC”) and Pear Therapeutics, Inc. (the “Company”) and related transactions and for no other purpose. No representations or warranties, express or implied are given in, or in respect of, this presentation. To the fullest extent permitted by law in no circumstances will TPAC, the Company or any of their respective subsidiaries, stockholders, affiliates, representatives, partners, directors, officers, employees, investment banks, advisers or agents be responsible or liable for any direct, indirect or consequential loss or loss of profit arising from the use of this presentation, its contents, its omissions, reliance on the information contained within it, or on opinions communicated in relation thereto or otherwise arising in connection therewith. Industry and market data used in this presentation have been obtained from third-party industry publications and sources as well as from research reports prepared for other purposes. Neither TPAC nor the Company has independently verified the data obtained from these sources and cannot assure you of the data’s accuracy or completeness. This data is subject to change. In addition, this presentation does not purport to be all-inclusive or to contain all of the information that may be required to make a full analysis of TPAC or the Business Combination. Viewers of this presentation should each make their own evaluation of TPAC and of the relevance and adequacy of the information and should make such other investigations as they deem necessary. This Presentation does not constitute (i) a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the proposed Business Combination or (ii) an offer to sell, a solicitation of an offer to sell or buy, or a recommendation to purchase any security of TPAC, the Company, or any of their respective affiliates. No such offering of securities shall be made except by means of a prospectus meeting the requirements of section 10 of the Securities Act of 1933, as amended, and otherwise in accordance with applicable law. Certain statements, estimates, targets and projections in this Presentation may be considered forward-looking statements within the meaning of the “safe harbor” provisions of the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements generally relate to future events or involving, or future performance of, TPAC or the Company. For example, projections of future revenue, prescriptions, and covered lives, statements regarding anticipated growth in the industry in which the Company operates and anticipated growth in demand for the Company’s products, projections of the Company’s future financial results and other metrics, the satisfaction of closing conditions to the Business Combination and the timing of the completion of the Business Combination are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “pro forma”, “may”, “should”, “could”, “might”, “plan”, “possible”, “project”, “strive”, “budget”, “forecast”, “expect”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward-looking statements are subject to risks, uncertainties, and other factors which could cause actual results to differ materially from those expressed or implied by such forward looking statements. These forward-looking statements are based upon estimates and assumptions that, while considered reasonable by TPAC and its management, and the Company and its management, as the case may be, are inherently uncertain. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: the occurrence of any event, change or other circumstances that could give rise to the termination of negotiations and any subsequent definitive agreements with respect to the Business Combination; the outcome of any legal proceedings that may be instituted against TPAC, the Company, the combined company or others following the announcement of the Business Combination and any definitive agreements with respect thereto; the inability to complete the Business Combination due to the failure to obtain approval of the stockholders of TPAC, or the Company to obtain financing to complete the Business Combination or to satisfy other conditions to closing; changes to the proposed structure of the Business Combination that may be required or appropriate as a result of applicable laws or regulations or as a condition to obtaining regulatory approval of the Business Combination; the ability to meet stock exchange listing standards following the consummation of the Business Combination; the risk that the Business Combination disrupts current plans and operations of the Company as a result of the announcement and consummation of the Business Combination; the ability to recognize the anticipated benefits of the Business Combination, which may be affected by, among other things, competition, the ability of the combined company to grow and manage growth profitably, maintain relationships with customers and suppliers and retain its management and key employees; costs related to the Business Combination; changes in applicable laws or regulations; the possibility that the Company or the combined company may be adversely affected by other economic, business, regulatory, and/or competitive factors; the Company’s estimates of expenses and profitability; the evolution of the markets in which the Company competes; the ability of the Company to implement its strategic initiatives and continue to innovate its existing products; the ability of the Company to defend its intellectual property and satisfy regulatory requirements; the impact of the COVID-19 pandemic on the Company’s business; and other risks and uncertainties set forth in the section entitled “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” in TPAC’s final prospectus dated September 14, 2020 relating to its initial public offering. You should also carefully review the other risks and uncertainties set forth under the heading “Risk Factors” in the registration statement on Form S-4 filed by TPAC with the SEC on July 16, 2021. The risks described therein are not exhaustive and are subject to update prior the date when a definitive proxy statement is delivered to TPAC’s stockholders. Additional risks that the Company and TPAC currently do not know about or that they currently believes to be immaterial may also impair the business, financial condition or results of operations of the Company and investors are encouraged to perform their own investigation with respect to the business, prospects, financial condition and operating results of THMA and Pear and the business, prospects, financial condition and operating results of the Post-Combination Company following the completion of the Business Combination. Nothing in this Presentation should be regarded as a representation by any person that the forward-looking statements set forth herein will be achieved or that any of the contemplated results of such forward-looking statements will be achieved. You should not place undue reliance on forward-looking statements, which speak only as of the date they are made. Neither TPAC nor the Company undertakes any duty to update these forward-looking statements.

Disclaimer This Presentation contains financial forecasts for the Company with respect to certain financial results for the Company’s fiscal years 2020 through 2023. Neither TPAC’s nor Company’s independent auditors have audited, studied, reviewed, compiled or performed any procedures with respect to the projections for the purpose of their inclusion in this Presentation, and accordingly, they did not express an opinion or provide any other form of assurance with respect thereto for the purpose of this Presentation. These projections are forward-looking statements and should not be relied upon as being necessarily indicative of future results. In this Presentation, certain of the above-mentioned projected information has been provided for purposes of providing comparisons with historical data. The assumptions and estimates underlying the prospective financial information are inherently uncertain and are subject to a wide variety of significant business, economic and competitive risks and uncertainties that could cause actual results to differ materially from those contained in the prospective financial information. Accordingly, there can be no assurance that the prospective results are indicative of the future performance of the Company or that actual results will not differ materially from those presented in the prospective financial information. Inclusion of the prospective financial information in this Presentation should not be regarded as a representation by any person that the results contained in the prospective financial information will be achieved. This presentation contains trademarks, service marks, trade names and copyrights of TPAC, the Company and other companies, which are the property of their respective owner, including reSET®, reSET-O®, Somryst®, PearConnect™. The financial information and data contained in this presentation is unaudited and does not conform to Regulation S-X. Accordingly, such information and data may not be included in, may be adjusted in or may be presented differently in, any proxy statement, registration statement, or prospectus to be filed by the Company with the SEC. Some of the financial information and data contained in this presentation have not been prepared in accordance with United States generally accepted accounting principles (“GAAP”). The Company and TPAC believe these non-GAAP measures of financial results provide useful information to management and investors regarding certain financial and business trends relating to the Company’s financial condition and results of operations. The Company and TPAC believe that the use of these non-GAAP financial measures provides an additional tool for investors to use in evaluating projected operating results and trends in and in comparing the Company’s financial measures with other similar companies, many of which present similar non-GAAP financial measures to investors. Management does not consider these non-GAAP measures in isolation or as an alternative to financial measures determined in accordance with GAAP. The principal limitation of these non-GAAP financial measures is that they exclude significant expenses and income that are required by GAAP to be recorded in the Company’s financial statements. In addition, they are subject to inherent limitations as they reflect the exercise of judgments by management about which expense and income are excluded or included in determining these non-GAAP financial measures. The Business Combination will be submitted to stockholders of TPAC for their consideration. TPAC filed a Registration Statement on Form S-4 with the SEC on July 16, 2021, which included a preliminary proxy statement, and intends to file a definitive proxy statement, to be distributed to TPAC’s stockholders in connection with TPAC’s solicitation for proxies for the vote by TPAC’s shareholders in connection with the Business Combination and other matters as described in the definitive proxy statement. After the Registration Statement on Form S-4 has been filed and declared effective, TPAC will mail a definitive proxy statement and other relevant documents to its stockholders as of the record date established for voting on the Business Combination. TPAC’s stockholders and other interested persons are advised to read, once available, the preliminary proxy statement and any amendments thereto and, once available, the definitive proxy statement, in connection with TPAC’s solicitation of proxies for its special meeting of stockholders to be held to approve, among other things, the Business Combination, because these documents will contain important information about the Company, TPAC and the Business Combination. Stockholders may also obtain a copy of the preliminary or definitive proxy statement, once available, as well as other documents filed with the SEC regarding the Business Combination and other documents filed with the SEC by TPAC, without charge, at the SEC’s website located at www.sec.gov or by directing a request to meara.murphy@peartherapeutics.com or phone: 650.567.6952. INVESTMENT IN ANY SECURITIES DESCRIBED HEREIN HAS NOT BEEN APPROVED OR DISAPPROVED BY THE SEC OR ANY OTHER REGULATORY AUTHORITY NOR HAS ANY AUTHORITY PASSED UPON OR ENDORSED THE MERITS OF THE OFFERING OR THE ACCURACY OR ADEQUACY OF THE INFORMATION CONTAINED HEREIN. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE. The Company, TPAC and certain of their respective directors, executive officers and other members of management and employees may, under SEC rules, be deemed to be participants in the solicitations of proxies from TPAC’s stockholders in connection with the Business Combination. Information regarding the persons who may, under SEC rules, be deemed participants in the solicitation of TPAC’s stockholders in connection with the Business Combination will be set forth in TPAC’s proxy statement when it is filed with the SEC. You can find more information about TPAC’s directors and executive officers in TPAC’s final prospectus filed with the SEC on September 14, 2020. Additional information regarding the participants in the proxy solicitation and a description of their direct and indirect interests will be included in TPAC’s proxy statement when it becomes available. Stockholders, potential investors and other interested persons should read the proxy statement carefully when it becomes available before making any voting or investment decisions. You may obtain free copies of these documents from the sources indicated above.
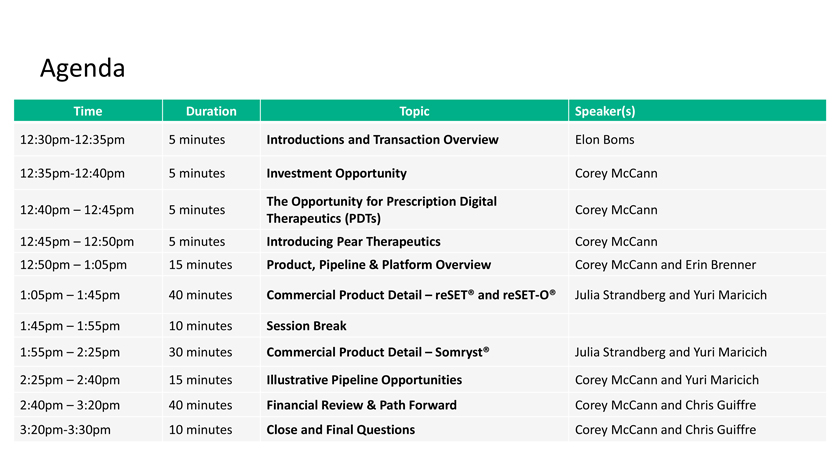
Agenda Time Duration Topic Speaker(s) 12:30pm-12:35pm 5 minutes Introductions and Transaction Overview Elon Boms 12:35pm-12:40pm 5 minutes Investment Opportunity Corey McCann The Opportunity for Prescription Digital 12:40pm – 12:45pm 5 minutes Corey McCann Therapeutics (PDTs) 12:45pm – 12:50pm 5 minutes Introducing Pear Therapeutics Corey McCann 12:50pm – 1:05pm 15 minutes Product, Pipeline & Platform Overview Corey McCann and Erin Brenner 1:05pm – 1:45pm 40 minutes Commercial Product Detail – reSET® and reSET-O® Julia Strandberg and Yuri Maricich 1:45pm – 1:55pm 10 minutes Session Break 1:55pm – 2:25pm 30 minutes Commercial Product Detail – Somryst® Julia Strandberg and Yuri Maricich 2:25pm – 2:40pm 15 minutes Illustrative Pipeline Opportunities Corey McCann and Yuri Maricich 2:40pm – 3:20pm 40 minutes Financial Review & Path Forward Corey McCann and Chris Guiffre 3:20pm-3:30pm 10 minutes Close and Final Questions Corey McCann and Chris Guiffre

The MD, PHD JD, MBA MD, MBA MBA MBA CEO CFO & COO CPDO CMO CCO CEO 5

Pear Therapeutics Transaction Overview Pear is preparing to go public through a SPAC merger with Thimble Point Acquisition Corp. and has raised an oversubscribed $125.8 million PIPE to further support long-term growth Pear Therapeutics Pear is the leader in developing prescription digital therapeutics (POTs) to treat serious disease Founded in 2013 by Dr. Corey McCann. Pear has brought to market the first three Prescription Digital Therapeutics (POTs) ever authorized by the FDA Raised $250M of capital from Softbank, Temasek, Kaiser Permanente. Novartis, Sam Ventures. Jazz Ventures. Arboretum and more Thimble Point Acquisition Corporation Publicly listed SPAC with $276M cash in trust Sponsored by an affiliate of the Pritzker Vlock Family Office (PVFO), Thimble Point invests in high-growth technology-enabled businesses disrupting large and established markets Transaction Pro forma enterprise value of approximately $1.28 Expects $456.8M of cash on Pear’s balance sheet post-transaction, further boosting Pear’s position as category creator and leader $125.8M PIPE anchored by Neuberger Berman, the Pritzker Vlock Family Office. and a leading IDN, along with significant support from existing and new investors Management and insiders rolling over 100% of their equity 6 .··- .PEAR -THERAPEUTICS

Pear Therapeutics Investment Highlights Transformational opportunity to disrupt the $3T global healthcare industry with software-based therapeutics that can address unmet medical needs alone and in combination with pharmaceuticals1 Emerging sector of prescription digital therapeutics (PDTs) = software-based therapeutic interventions with opportunity to treat a wide range of medical conditions for a total addressable market of $2508 in the U.S. Pear is the category creator in POTs with first 3 FDA-authorized POTs ($28+ serviceable available market in the U.S.), deep and broad pipeline, and first end-to-end platform ($158+ serviceable available market in the U.S.) Differentiated platform allows for streamlined discovery, development and commercialization of new POTs, fostering sustainable competitive advantage Data, platform, IP, and regulatory competitive advantages plus capital-efficient business model to pursue software-like margins with therapeutic-like pricing e Management team built to scale led by mix of seasoned life science and tech employees and backed by blue-chip syndicate of cross-disciplinary investors Capital infusion creates opportunity for sustained leadership in a new category with applicability across healthcare 7 .··- .PEAR -THERAPEUTICS

Agenda Introducing Pear Therapeutics Product, Pipeline & Platform Overview Commercial Product Detail Illustrative Pipeline Opportunities Financial Review & Path Forward Appendix 8

Major trends 90% of U.S. $3.8 trillion in annual health care expenditures is for people with chronic converge and mental health conditions1 Number of people who have used telehealth doubled, from 39.4% pre-to highlight to 79.5% post-quarantine2 a need for Across many key disease areas (i.e., substance abuse and insomnia), there are tens of Americans spend an average of millions of patients with only a 5.4 hours on their mobile phones daily as few thousand (or less) trained big data drives deeper insights from specialists3-5 engagement6

20M Americans 30M Americans 50M Americans ~17M Americans struggle with struggle with chronic struggle with acute and struggle with cancer5 addiction1 insomnia2,3 chronic pain4 Drug treatments are Only 10-20% of Sedatives for short Patients left to choose often discontinued patients receive term use only due to between pain and due to side effects treatment1 side effects opiate addiction Smartphone application Smartphone application Virtual reality Smartphone application clinically proven to treat clinically proven to treat application designed to designed to minimize addiction and extend the insomnia as an reduce acute and cancer medication side reach of clinicians alternative to drug chronic pain – and effects by tailoring treatment reduce the use of optimal dosing opiates 10

Prescription Digital Therapeutics (PDTs) are 1900+ 1980+ 2000+ 2020+ Small Biologics Cell/Gene Prescription Digital 11 Molecules Therapies Therapeutics

PDTs are poised to Improve reach allowing disrupt care delivery for broader patient impact to Reimbursable events for dashboard interactions in the healthcare system 24/7 remote ac without fear of Favorable side effect profile vs medications Reduce overall healthcare costs Fill gaps in care across large populations 12

Agenda The Opportunity For Prescription Digital Therapeutics Product, Pipeline & Platform Overview Commercial Product Detail Illustrative Pipeline Opportunities Financial Review & Path Forward Appendix 13

Pear Pear is the first mover and 14 product candidates with Therapeutics leader in the space, FDA-authorized products the potential to improve defining the PDT industry reSET, reSET-O, and care across a range of is via the first 3 FDA- Somryst for the treatment therapeutic areas authorized products of addiction and chronic insomnia address 50M+ US patients and 850M+ patients worldwide1-4* Scalable Strategy to be the primary Demonstrated adoption by infrastructure to discover, platform for PDTs with an patients, clinicians, and develop, and deliver PDTs opportunity to scale from 3 payors and we intend to to patients to 17 to 100+ PDTs apply that playbook across additional geographies and assets 14 *As of July 2021, Pear’s only Ex-US authorization is Singapore for reSET with plans to expand to other Ex-US markets.

Pear’s business is enabled by Payor and provider Foundational estate including integration 20+ owned and licensed patents … Products improve over time Pathway requires clinical with more data evidence 15

…and we believe Pear has similarities to 16

Pear’s team, $250m+ of capital raised culture & investors to date President & CEO represent CFO & COO * Chief Product Development Officer Chief People Officer CMO & Head of Development General Counsel & Secretary person tech + healthcare team in Boston, San Francisco & Raleigh Chief Commercial Officer 17 Note: Perceptive Advisors is Pear’s lender.

Agenda The Opportunity For Prescription Digital Therapeutics Introducing Pear Therapeutics Commercial Product Detail Illustrative Pipeline Opportunities Financial Review & Path Forward Appendix 18

Pear has… P R O D U C T S I N P I P E L I N E L E V E R A G I N G P L A T F O R M 19

Pear’s first designed to redefine care for major medical conditions ( U S ) 1 Only product FDA authorized to treat addiction to alcohol, cannabis, cocaine and stimulants Only FDA-authorized software product that’s proven to help patients with opioid use disorder stay in outpatient treatment longer Only FDA-authorized drug-free and guideline-recommended treatment for chronic insomnia 20

Discovery POC Pivotal Commercial * Substance Use Disorder Pear has Opioid Use Disorder * Chronic Insomnia a Alcohol Use Disorder Schizophrenia Anxiety (GAD) Depression (MDD) of product Bipolar candidates PTSD Acute and Chronic Pain Migraine Multiple Sclerosis Epilepsy IBS ** Specialty GI *** Oncology Cardiovascular Adherence Physiologic Voice Keystroke Sensors Monitoring 21 *Dartmouth transaction is with a researcher employed by Dartmouth. **Karolinska transaction is with individual researchers who are employed by the Karolinska Institute. ***Services agreement with Ironwood to evaluate a PDT in GI diseases.

Our enables the discovery, development, and commercialization of PDTs at scale Component library of therapeutic Clinician dashboard and digital biomarker modalities Patient services center and specialty In-licensing engine to access new pharmacy technologies Data infrastructure and access Remote clinical trials infrastructure to claims data Participant in FDA’s Precertification Pilot Telemedicine integration and Program field salesforce Quality systems compatible with Modular payor contracts and 21CFR 820 and ISO13485 adjudication of value-based agreements 22

Agenda The Opportunity For Prescription Digital Therapeutics Introducing Pear Therapeutics Product, Pipeline & Platform Overview Illustrative Pipeline Opportunities Financial Review & Path Forward Appendix 23

and are designed to redefine treatment of Substance Use Disorder (SUD) and Opioid Use Disorder (OUD) + Cognitive Behavioral Therapy (CBT) Real-World Engagement Fluency Training Concept proficiency Contingency Management Cravings & Triggers Craving & Trigger Assessment Urine Drug Screens & Appointments 24/7, anytime, anywhere addiction Rapid insights into patient engagement and practice treatment with FDA-authorized outcomes performance via single-secure platform 24

and product demo Cognitive behavioral Fluency Craving and trigger therapy lessons training Contingency management assessment 25

and show strong and stronger roved abstinence atients abstinent at 12 reSET1 ks5 of patients retained in of patients retained in therapy at 12 therapy for reSET-O2 weeks5 substitution reduction in inpatient hospital for clinician time3 utilization at 6 months6* of continuous reduction in emergency department Use4 visits at 6 months6* 26 *Results up to 9 months published in June 2021

from the reSET Pivotal Trial * 27 *reSET is indicated for retention in treatment and abstinence.

from the reSET-O Pivotal Trial * 28 *reSET-O is indicated only for retention in treatment.

of the product in the real world + + 29

has published data on real world outcomes Responder Analysis1 Abstinence in Weeks 9-121* (≧80% negative UDS or self-report) 91% 91% 78% 77% % % tinence, Responders, Abs Pivotal Study RWE Pivotal Study RWE (N = 91) (N = 3,144) (N=91) (n=2,269) Analyses of “Abstinence” for each group: reSET-O® is not authorized or promoted to improve abstinence. * Missing Data Removed: No positive UDS and/or self-reported use over the final 4 weeks of the 12-week reSET-O® prescription (weeks 9-12); Patients without any data (UDS or self-reports) over the final four weeks removed from analysis population; Weeks without any data (UDS or self-reports) excluded from analysis

and have demonstrated commercial traction â‰^ â‰^ *As of 6/1/21, upon the effectiveness of Pear’s contract with Prime Therapeutics LLC, Pear has ~23M covered lives for reSET and reSET-O and has the potential to access up to approximately 20M additional covered lives under the Federal Supply Schedule signed in March 2021. In addition, Pear has ~2M covered lives for Somryst. **Providing access means either listing on formulary, as a covered benefit, purchasing product in bulk, or funding a study
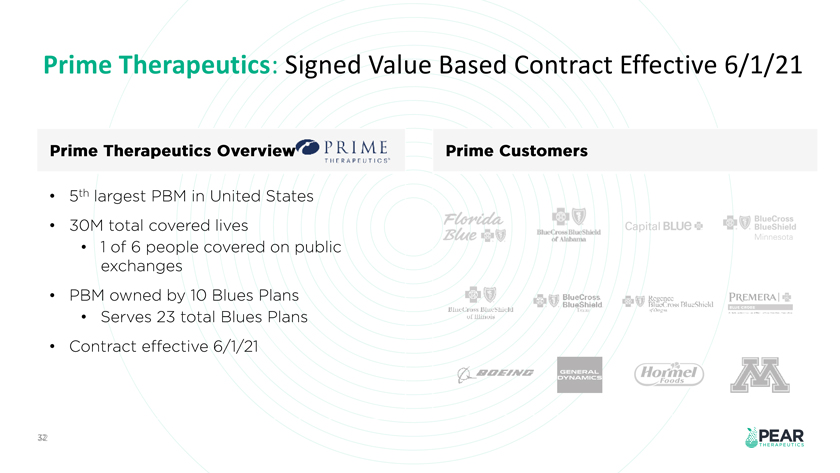
Prime Therapeutics: Signed Value Based Contract Effective 6/1/21 Prime Therapeutics Overview P R I ME Prime Customers T II r RAP r IJT I Sth largest PBM in United States r:t w + {ij BlueCross 30M total covered lives Cap1tal BLUe BlueSh1eld Ewer& BlucCmssBluc hlcld c.t Alnha nn 1 of 6 people covered on public exchanges PBM owned by 10 Blues Plans +tJ ..;. BlueCross +V .Kc-gcnc.:; PR.. EMERAI-i BlueShield Tllu .. Cn, · ... ‘l nlu: .:”.hidd lllt.t l’m Dlw.:hidll Serves 23 total Blues Plans ur llli wb .I Contract effective 6/1/21 fi-- oEING s A 32 .··- .PEAR -THERAPEUTICS

and testimonials Heidi Dr. Carolyn Greer, M.D. Samir Mistry, PharmD reSET-O patient Medical Director Vice President of Pharmacy The Bowen Recovery Center PreferredOne Dr. Greer is a paid consultant of Pear Therapeutics, Inc. 33
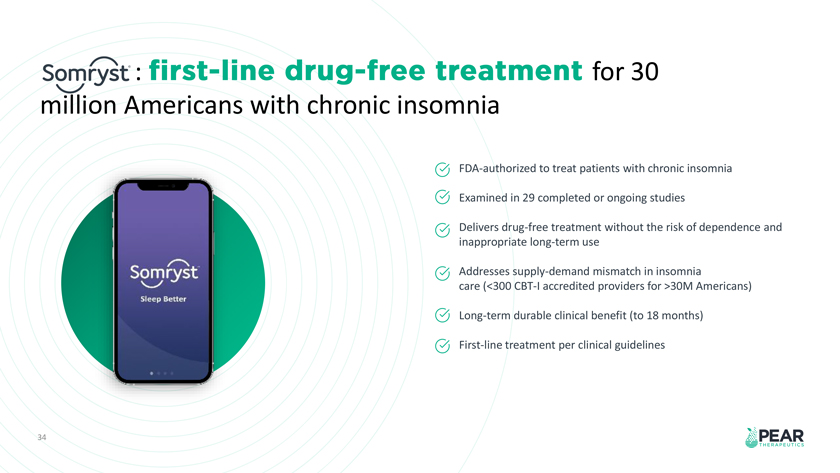
: for 30 million Americans with chronic insomnia FDA-authorized to treat patients with chronic insomnia Examined in 29 completed or ongoing studies Delivers drug-free treatment without the risk of dependence and inappropriate long-term use Addresses supply-demand mismatch in insomnia care ( 300 CBT-I accredited providers for 30M Americans) Long-term durable clinical benefit (to 18 months) First-line treatment per clinical guidelines 34

Cognitive Sleep Restriction & Consolidation Stimulus Control Restructuring 35

data show rease in the severity of insomnia symptoms1 rease in depression ymptoms2 rease in anxiety in insomnia and ymptoms2 Durable effect on insomnia, depression endpoints epression, and anxiety3-4 Decrease in the severity of insomnia symptoms5 Decrease in the time to sleep onset6 Decrease in undesired waking from sleep6 36

from the pivotal trial 37

in the real world Sleep Onset Latency o o o 38

launch Pre-Launch Goals Generate Real World Data Gain Early Advocates Test, Learn and Improve: Commercialization & Product Achieve Strategic Payer Coverage Key Initiatives Launch Somryst Leaders Early Experience Program (SL)EEP Direct To Consumer (DTC) awareness & education All-Digital end to end virtual pilot & optimization Payer Engagement RWE Data generation & collection Outcomes As of July l 2021, over 100 prescribers across 30+ states Real world data generated from 75 leading academic institutions and sleep centers As of July 1, 2021, coverage across â‰^2M covered lives 39

Wendy Dr. Michelle Primeau, M.D. Renee Wallace, PharmD Somryst patient Medical Director Clinical Account Executive Palo Alto Medical Foundation Serve You Rx Sleep Medicine Center 40 Dr. Primeau is a paid consultant of Pear Therapeutics, Inc.

Agenda The Opportunity For Prescription Digital Therapeutics Introducing Pear Therapeutics Product, Pipeline & Platform Overview Commercial Product Detail Financial Review & Path Forward Appendix 41

Illustrative Pipeline Opportunities Alcohol Use Disorder Current Stage: Proof of concept Patient Population: Alcohol Use Disorder WW / US Population Size: 100M / 14.5M Studies and Patients: We believe this product candidate is at a stage ready for potential pivotal randomized clinical trial to support potential FDA submission Depression Current Stage: Discovery Patient Population: Major Depressive Disorder and additional Depression populations WW / US Population Size: 322M / 17.3M Studies and Patients: We believe this product candidate is at the discovery stage of therapeutic development 42
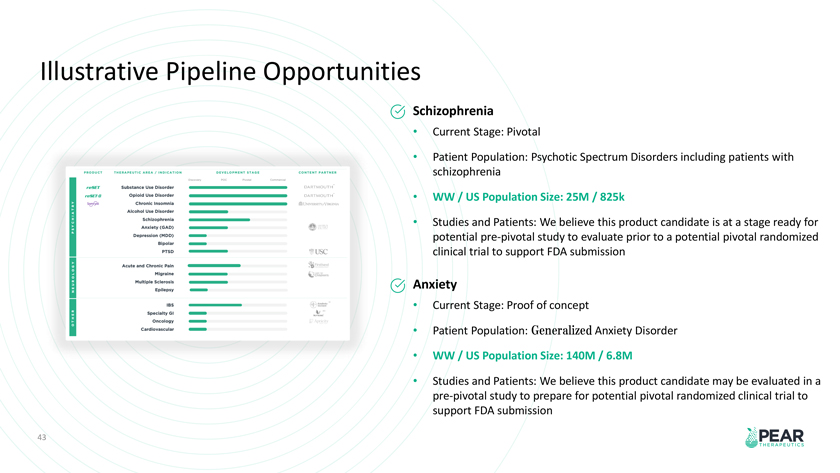
Illustrative Pipeline Opportunities Schizophrenia Current Stage: Pivotal Patient Population: Psychotic Spectrum Disorders including patients with schizophrenia WW / US Population Size: 25M / 825k Studies and Patients: We believe this product candidate is at a stage ready for potential pre-pivotal study to evaluate prior to a potential pivotal randomized clinical trial to support FDA submission Anxiety Current Stage: Proof of concept Patient Population: Generalized Anxiety Disorder WW / US Population Size: 140M / 6.8M Studies and Patients: We believe this product candidate may be evaluated in a pre-pivotal study to prepare for potential pivotal randomized clinical trial to support FDA submission 43

Agenda The Opportunity For Prescription Digital Therapeutics Introducing Pear Therapeutics Product, Pipeline & Platform Overview Commercial Product Detail Illustrative Pipeline Opportunities Appendix 44

S-4 Registration Statement: Operational assumptions underlying projected revenue Revenue Ramp Pear’s Ability To … The aggregate number of covered lives across Pear’s three FDA-authorized products will increase from approximately 3 million at the … continue to attract new customers and retain existing beginning of 2021 to approximately 30-40 million at the beginning of 2022 and approximately 100-120 million at the beginning of 2023. customers. Management believes that these assumptions with respect to the growth in covered lives between the beginning of 2021 and the beginning .. continue to release product . enhancements of 2023 are reasonable because Pear believes that: (i) it will continue to secure new relationships with major pharmacy benefit managers (for example, in June 2021, Pear secured a relationship with Prime Therapeutics, a major pharmacy benefit manager), which can each … partner with other pharmaceutical companies in the account for 30-50 million covered lives, (ii) its products can qualify for coverage by state Medicaid agencies, which currently account for development of new PDTs. approximately 74 million covered lives in the aggregate, and (iii) its products can qualify for Medicare coverage, which currently accounts for approximately 60 million covered lives. … continue to execute real-world studies to collect real world data supporting the clinical utility of its products Pear’s product script volume will increase from approximately 12,500 at the end of 2021 to approximately 50,000-60,000 at the end of and health economic outcomes data supporting the value 2022 and approximately 150,000-190,000 at the end of 2023. Management believes that these assumptions with respect to script volume of its products. are reasonable because: (i) Pear has already generated over 20,000 reSET and reSET-O prescriptions since launch despite limited investment in sales and marketing, (ii) Pear intends to substantially increase its investment in sales and marketing for its products as … continue to drive deeper integration of its products additional covered lives are achieved and (iii) management anticipates that an increase in sales and marketing will lead to additional into health systems infrastructure, including via virtual awareness of Pear’s products, which will increase the number of scripts for its products. care channels and strategic partnerships. During 2021 through 2023, except for minimal revenues from partnerships, no revenue will be generated (i) other than from Pear’s three FDA-authorized products or (ii) outside of the United States. While Pear’s management believes Pear will be able to expand its range of … continue to overcome the challenges associated with COVID-19. products and also offer its products outside of the United States, due to the uncertainty of the timing of Pear’s ability to bring additional products to market and obtain authorizations for the distribution of Pear’s products outside the United States during such period, Pear’s … broaden feature and service offerings centered around management has assumed that there will not be any revenue from either of those potential revenue sources. Pear’s current commercial products. Solely for the purposes of Pear’s projected revenues for 2021 through 2023, Pear’s three FDA-authorized PDTs are forecast to have a 50%- … make effective platform enhancements. 75% script fulfillment rate and are forecast to have a 5%-25% gross-to-net adjustment, which accounts for discounts, rebates and other expenses. For 2021 through 2023, a compound annual growth rate in the digital therapeutics industry of 26.7%, based on projections from a third-party market research report. Management believes that the growth of revenue from Pear’s three FDA-authorized products during this period will outpace the current growth rate of the industry given Pear’s positioning and competitive advantage in the market as the category leader. 45
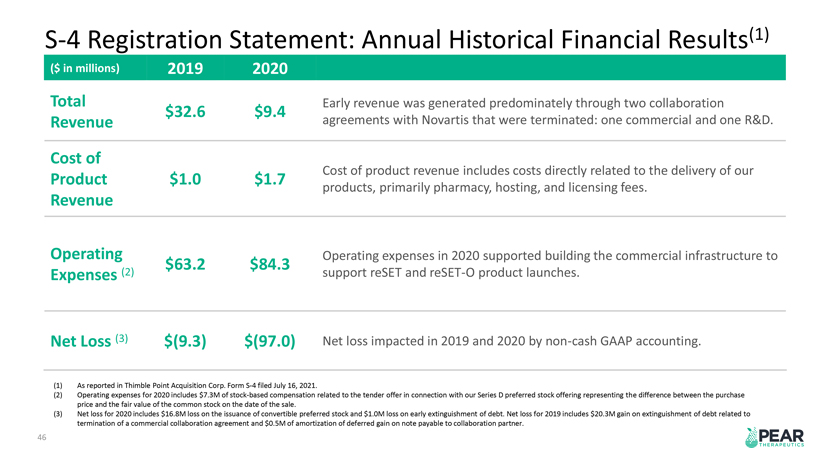
S-4 Registration Statement: Annual Historical Financial Results(1) ($ in millions) 2019 2020 Total Early revenue was generated predominately through two collaboration Attractive Market Trends $32.6 $9.4 Revenue agreements with Novartis that were terminated: one commercial and one R&D. Cost of Embedded Growth Opportunities Cost of product revenue includes costs directly related to the delivery of our Product $1.0 $1.7 products, primarily pharmacy, hosting, and licensing fees. Revenue Operating Operating expenses in 2020 supported building the commercial infrastructure to $63.2 $84.3 Expenses (2) support reSET and reSET-O product launches. Net Loss (3) $(9.3) $(97.0) Net loss impacted in 2019 and 2020 by non-cash GAAP accounting. (1) As reported in Thimble Point Acquisition Corp. Form S-4 filed July 16, 2021. (2) Operating expenses for 2020 includes $7.3M of stock-based compensation related to the tender offer in connection with our Series D preferred stock offering representing the difference between the purchase price and the fair value of the common stock on the date of the sale. (3) Net loss for 2020 includes $16.8M loss on the issuance of convertible preferred stock and $1.0M loss on early extinguishment of debt. Net loss for 2019 includes $20.3M gain on extinguishment of debt related to termination of a commercial collaboration agreement and $0.5M of amortization of deferred gain on note payable to collaboration partner. 46

Company macro-opportunity Growing burden of preventable chronic diseases driving adoption of PDTs Long term effects of pandemic helped accelerate the transition to remote care Pronounced shortage of clinicians Pervasive use of technology by patients and clinicians Abbreviated development timelines with robust probability of clinical success vs drugs Software-like margins with ability to create-drug like medical value Large accessible markets with high unmet medical need Continuous data-collection from commercial product used for demonstration of value First mover advantage in new industry IP portfolio of patents, copyrights and trade secrets Clinical data and software quality requirements Data and platform moats Demonstrated engine for repeated PDT creation Multi-PDT platform for repeated asset commercialization Potential to be horizontal scale player in PDTs We believe we have an opportunity to become the go to developer AND commercializer of PDTs
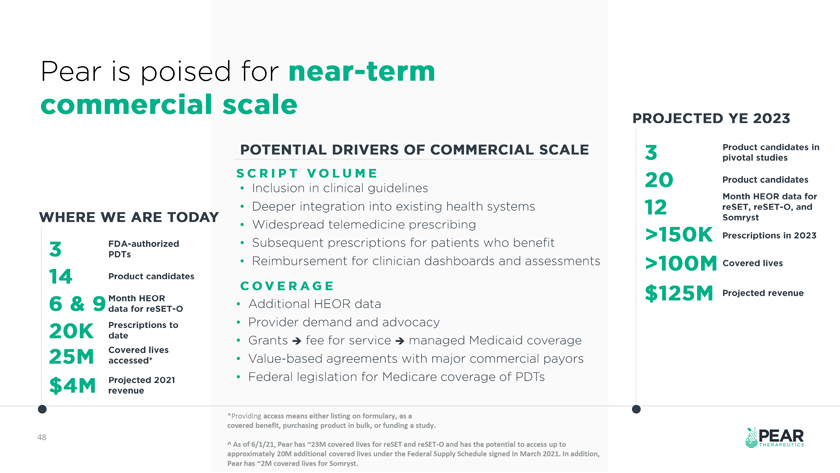
*Providing access means either listing on formulary, as a covered benefit, purchasing product in bulk, or funding a study. 48 ^ As of 6/1/21, Pear has 23M covered lives for reSET and reSET-O and has the potential to access up to approximately 20M additional covered lives under the Federal Supply Schedule signed in March 2021. In addition, Pear has 2M covered lives for Somryst.

Drivers of commercial product ramp Current prescriptions utilized to >20K prescriptions to date in large scalable customers driven by 20 field reps and demonstrate product performance, small marketing budget health economic data, and clinician 2 HEOR pubs and several advocacy letters – more to come demand and advocacy Prescribing institutions represent opportunity for organic script growth Accelerating coverage decisions 2 HEOR pubs demonstrating per patient cost savings in excess of product price following publication of HEOR data Ramp from 3M to 25M covered lives in 1H 2021* for reSET-O Ongoing discussions with payors representing in excess of 100M covered lives Unit Price driven by real-world reSET and reSET-O WAC: $1665 per script medical value in excess of product Somryst WAC: $900 per script price Expect price increases to be tied to product enhancements 49 * Pear also has been added to the Federal Supply Schedule, which could provide access to up to 20M lives

Commercial product ramp through 2023 2021 2022 2023 150K—190K Total Rx 12,500 50K—60K Investment in sales, marketing, medical scales relative to covered Nearing steady lives Predominantly Somryst begins to (product mix) state across Prescribing scales as dashboard CPT codes come online reSET and reSET-O ramp up products Technical process improvements enhance fulfillment Fulfillment 50% 50-65% 75% Fulfillment is higher for chronic insomnia vs addiction Covered Lives Leverage experience with Prime to convert additional PBMs 3M 30—40M 100—120M Leverage wins in IN, OH, KY to accelerate in other states (% Rx Covered) ( 40-50%) ( 50-65%) ( 70-90%) Potential for CMS action via policy and/or legislation Large PBMs, Medicaid, and Medicare may involve higher discounts Gross to Net 8% 20% 22% and rebates than seen to date Revenue $4M $22M $125M 50
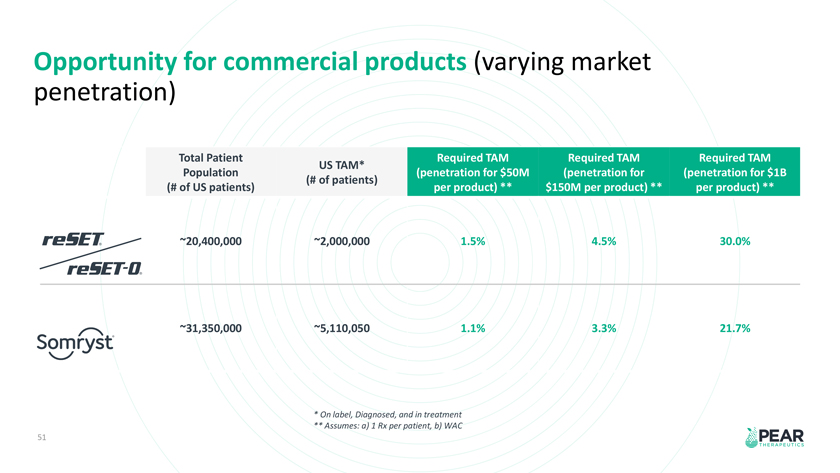
Opportunity for commercial products (varying market penetration) Total Patient Required TAM Required TAM Required TAM US TAM* Population (penetration for $50M (penetration for (penetration for $1B (# of patients) (# of US patients) per product) ** $150M per product) ** per product) ** 20,400,000 2,000,000 1.5% 4.5% 30.0% 31,350,000 5,110,050 1.1% 3.3% 21.7% * On label, Diagnosed, and in treatment ** Assumes: a) 1 Rx per patient, b) WAC 51

Opportunity for commercial products (using varying assumptions for script(s) per patient) Billions $12.0 Billions $30.0 $10.0 $25.0 $8.0 $20.0 $6.0 $15.0 $4.0 $10.0 $2.0 $5.0 $0.0 $0.0 0 20% 40% 60% 80% 100% 0 20% 40% 60% 80% 100% TAM Penetration TAM Penetration 52 1 Rx per patient 2 Rx per patient 3 Rx per patient

Scripts create additional Our commercial patients treated, RWE, and HEOR data points flywheel drives a … Coverage decisions Performance data drive additional drives product prescription growth enhancements Product enhancements support coverage decisions and pricing power 53

…and our engine is Component library of therapeutics Multi-product clinician dashboards and digital biomarkers Patient services center and specialty pharmacy APIs for academics and partner development Integration into EMRs and practice infrastructures Remote clinical trials infrastructure Claims data pipe for continuous FDA Precertification Pilot Program HEOR assessment Quality system compatible with Telemedicine capability 21CFR 820 and ISO13485 and field salesforce 54 *735 assets evaluated since 2018. Pear evaluates approximately 100-200 assets a year.

Pipeline Product Opportunity Alcohol Use Disorder 1,000,000 $3B Schizophrenia 250,000 $750M Anxiety (GAD) 3,000,000 $8B Depression (MDD) 11,000,000 $13B Bipolar 2,300,000 $7B PTSD 6,000,000 $14B 4 product candidates 50,000,000 $70B 4 product candidates 7,750,000 $16B 55

This Developed by Pear and 3rd parties Mono therapies and drug/software combos as the long-term category Multi-product clinician dashboard leader for Integration into payor and provider networks PDTs Data systems for insight generation 56

Platform effects drive increasing efficiencies for our business : : : 57

Pear is the for PDTs Pear is the category creator Pear’s pipeline and platform are poised to PDTs as treatment for most medical conditions and market leader with a drive the space creating a represent a serviceable available market from current serviceable available market from current total addressable market opportunity in the U.S. products in the U.S. pipeline in the U.S. 58

“’ 1. PEAR 59 ·— } .··- .PEAR -THERAPEUTICS

Agenda The Opportunity For Prescription Digital Therapeutics Introducing Pear Therapeutics Product, Pipeline & Platform Overview Commercial Product Detail Illustrative Pipeline Opportunities Financial Review & Path Forward 60
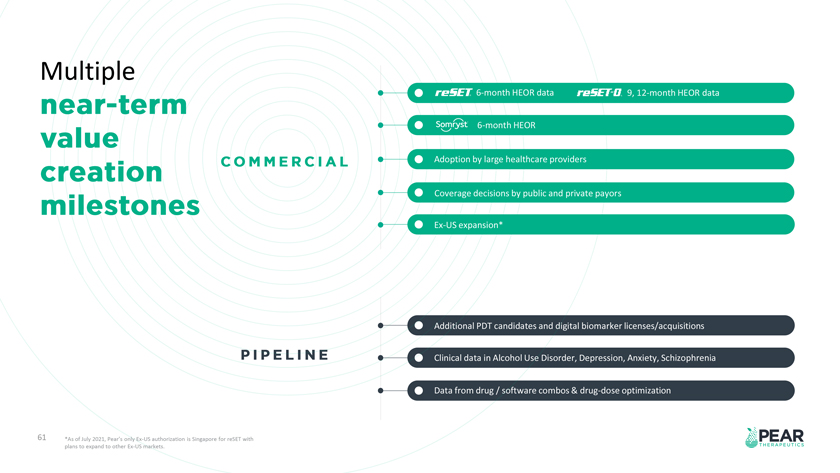
Multiple 6-month HEOR data 9, 12-month HEOR data 6-month HEOR Adoption by large healthcare providers Coverage decisions by public and private payors Ex-US expansion* Additional PDT candidates and digital biomarker licenses/acquisitions Clinical data in Alcohol Use Disorder, Depression, Anxiety, Schizophrenia Data from drug / software combos & drug-dose optimization 61 *As of July 2021, Pear’s only Ex-US authorization is Singapore for reSET with plans to expand to other Ex-US markets.

Summary Estimated Sources and Uses ($ in millions) SPAC Cash in Trust $276.0 Illustrative Price per Share $10.0 Founder Shares(1) 56.3 Pro Forma Shares Outstanding (mm) 165.81 PIPE(2) 125.8 Equity Value 1,658.1 Pear Equity Rollover(3) 1,200.0 (-) Net Cash(4) 456.8 Total Sources $1,658.1 Enterprise Value $1,201.3 Cash to Balance Sheet $361.8 EV / 2023E Revenue(7) 9.61X Founder Shares(1) 56.3 (3) Pear Therapaeutics Shareholders (3) Pear Equity Rollover 1,200.0 (5) SPAC Shares Transaction Costs 40.0 (1) (6) Total Uses $1,658.1 Founder Shares PIPE Shareholders (2) Note: Assumes no redemptions. Ownership at $10.00 per share implies that all warrants are out of the money and therefore are not included. (1) Excludes 1.3mm Founder Shares subject to earnout, vesting ratably at $12.50, $15.00 and $17.50. (2) Includes $23mm FPA from Pritzker Family Office. (3) Excludes 12.4mm Seller Earnout Shares, vesting ratably at $12.50, $15.00 and $17.50. 62 (4) Includes $95mm of existing balance sheet cash. (5) Excludes the impact of 9.2mm Public Warrants with an $11.50 strike price. (6) Excludes the impact of Founder Warrants. Sponsor currently holds 5.0mm warrants, 4.1mm of which will vest at close with an $11.50 strike price. The remaining 1.0mm will be subject to earnout, vesting ratably at $12.50, $15.00 and $17.50. (7) Based on 2023E revenue of $125mm.

Category creators offer Novel Electric Field-based Leading Chronic Disease Leading Liquid Reimagined Connected Oncology Treatment Management Software Biopsy Diagnostics Dialysis Machines Technology 2015 2016 2017 2018 2019 2020 2017 2018 2019 2020 2016 2017 2018 2019 2020 2018 2019 2020 63 Note: Market Cap data as of market close on June 18, 2021. *2020 revenue represents 9M actual and Q4 Estimate from Merger Proxy dated 9/15/20. **Reflects Teladoc acquisition value.

This is a compelling moment to join Pear and the $ in millions Pear’s Implied Value at YE ’22 Discounted to Today vs Current Transaction Value 64 Source: Company projections, Company filings, and FactSet as of June 18, 2021. *Disruptive Healthcare Peers include Novocure, Guardant Health, Inspire, Shockwave Medical, Adaptive Biotechnologies, Schrodinger, Dexom, Outset Medical, and Teladoc Health.

relative to disruptive healthcare peers Category leaders pioneering approaches to address unmet needs using novel and unique ideology First-mover advantage contributing to acceleration of revenue and profitability momentum 33.07x 17.57x 16.88x 16.69x Discount 15.27x to median * 13.21x 12.47x 9.61x 9.82x 8.28x 65 et as of June 18, 2021. *Pear Therapeutics EV is based on valuation assumptions provided on prior pages.

Pear is to life science comparables Therapeutics targeting psychiatric and neurological diseases 66 pany websites and FactSet as of 06/18/21. * Based on Thimble Point Acquisition Corp.’s valuation of Pear Therapeutics of $1.2bn. ** Pro forma for $144mm follow-on offering in April 2021.

analogous to biopharma platforms Pear’s PDT leadership is no less innovative than other biopharma platform companies that brought unorthodox technologies to caregivers, patients and health systems Pioneering developer of Pole position in the development of A sharp departure from prior neuro- First platform to bring cannabinoid CAR-T, the next-generation modality drugs based on mRNA for a vast array degeneration plays, driven by receptor agonists into mainstream in immuno-oncology of therapeutic areas biomarkers and new delivery biopharma for neurology technology JCAR015: Ph 1 (ALL, NHL) VEGF program: Ph 2 (myocardial LRRK2 program: Ph 1 (Parkinson’s) Sativex: Marketed (MS spasticity) ischemia) JCAR017: Ph 1 (ALL, NHL) RIPK1 program: Preclin. (Alzheimer’s, GWP42004: Ph 2 (T2DM) OX40 program: Ph 1 (cancer) ALS) JCAR014: Ph 1 (B-cell GWP42006: Preclin. (Epilepsy) malignancies) Virology programs: Preclin. Enterprise Value: Enterprise Value: Enterprise Value: Enterprise Value: @ IPO: $1.4bn @ IPO: $7.6bn @ IPO: $1.1bn @ IPO: $56mm @ Takeout: $9.0bn @ Current: $78.6bn @ Current: $8.4bn @ Takeout: $6.7bn Shareholder Return: 3.6x Shareholder Return: 8.7x Shareholder Return: 4.1x Shareholder Return: 24.6x 67

and First-ever PDT to achieve are the first PDTs FDA medical claims to treat disease FDA-authorized to treat addiction for 21.2M Americans suffering from addiction to alcohol, cannabis, cocaine, and stimulants1 Two successful RCTs in >1,000 SUD patients (alcohol, cannabis, cocaine, stimulants)2-3 First-ever PDT to receive Breakthrough Designation FDA-authorized for use in combination with buprenorphine to treat 2.1M Americans suffering from addiction to opiates4 3 successful RCTs in >450 OUD patients: 2 with reSET-O + buprenorphine and 1 with reSET-O + methadone5-8 68

ckfacts/fact/table/US/PST045219 Accessed
April 22, 2021.; 4. Atlas on substance use (2010) resources for the prevention and treatment of substance use drsorders. World Health Organization.; https//www.ncbi.nlm.nih.gov/books/NBK553166/
Slide 20 1. TAM = Price x Potential PDT Rx Per Year Sl1de 26 1. Campbell et al., American Journal of Psychiatry. 2014. 171(6);683-690. 2.
Christensen DR Landes RD. Jackson L. et al. Adding an Internet-delivered treatment to an efficacious treatment
package for oprord dependence. J Consult Clin
Psychol. 2014;82(6):964-972. doll0.1037/a0037496. 3. Chaple et al. 2016. The Prrson Journal. 96(3)485-508. 4. Marsch LA Guarino H. Acosta M, Aponte-Melendez Y, Cleland
C. Grabrnski M, Brady
R. Edwards J. Web-based behavioral treatment for substance use disorders as a partial replacement of
standard methadone maintenance treatment. J Subst Abuse Treat. 2014 Jan;46(1)43-51. doi 10 1016/i.Jsat.2013.08.012. Epub 2013 Sep 21. PMID:
24060350; PMCID: PMC3839618. 5. Yuri A Maricich. MD. X1aorui Xiong, PhD, Robert Gerw1en. PhD, Allee Kuo, BA Fulton Velez. MD MBA, Bruce lmbert. MD PhD, Keely Boyer. MBA, H1lary F.
Luderer. PhD, Stephen Braun. BA, Karren Williams.
PhD (2020): Real-World evrdence for a prescription digital therapeutic to treat Oprord Use Disorder, Journal of
Current Medical Research and Opinion, 001:10 1080/03007995.2020.1846023. 6. Fulton F. Velez , Sam Colman . Laura Kauffman , Charles Ruetsch
& Kathryn
Anastassopoulos (2020); Real-world reduction in healthcare resource utilization following treatment of opioid use disorder w1th reSET-0, a novel prescrrption digrtal therapeutic, Expert Revrew of
Pharmacoeconomics & Outcomes Research, DOl: 10.1080/14737167.2021.1840357. 69 -THERAPEUTICS

References 1. Campbell et al., American Journal of Psychiatry. 2014. 171(6):683-690. 2. Yuri A. Maricich, Warren K. Bickel, Lisa A. Marsch, Kirstin Gatchalian, Jeffrey Botbyl & Hilary F. Luderer (2020): Safety and efficacy of a prescription digital therapeutic as an adjunct to buprenorphine for treatment of opioid use disorder, Current Medical Research and Opinion, DOI: 10.1080/03007995.2020.1846022 Slide 28 1. Christensen DR, Landes RD, Jackson L, et al. Adding an Internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol. 2014;82(6):964-972. doi:10.1037/a0037496. Slide 29 1. Fulton F. Velez, Sam Colman, Laura Kauffman, Charles Ruetsch & Kathryn Anastassopoulos (2020) Real-world reduction in healthcare resource utilization following treatment of opioid use disorder with reSET-O, a novel prescription digital therapeutic, Expert Review of Pharmacoeconomics & Outcomes Research, DOI: 10.1080/14737167.2021.1840357 2. Ronquest NA, Willson TM, Montejano LB, Nadipelli VR, Wollschlaeger BA. Relationship between buprenorphine adherence and relapse, health care utilization and costs in privately and publicly insured patients with opioid use disorder. Subst Abuse Rehabil. 2018;9:59- 78. https://doi.org/10.2147/SAR.S150253 Slide 30 1. Maricich YA, Xiong X, Gerwien R, Kuo A, et al. (2020): Real-world evidence for a prescription digital therapeutic to treat opioid use disorder, Current Medical Research and Opinion. DOI: 10.1080/03007995.2020.1846023 Slide 31 1. Yuri A. Maricich, MD, Xiaorui Xiong, PhD, Robert Gerwien, PhD, Alice Kuo, BA Fulton Velez, MD MBA, Bruce Imbert, MD PhD, Keely Boyer, MBA, Hilary F. Luderer, PhD, Stephen Braun, BA, Karren Williams, PhD (2020): Real-World evidence for a prescription digital therapeutic to treat Opioid Use Disorder, Journal of Current Medical Research and Opinion, DOI:10.1080/03007995.2020.1846023. 2. Data on file. reSET-O Patient and Provider Research. December 2020. 3. Fulton F. Velez , Sam Colman , Laura Kauffman , Charles Ruetsch & Kathryn Anastassopoulos (2020): Real-world reduction in healthcare resource utilization following treatment of opioid use disorder with reSET-O, a novel prescription digital therapeutic, Expert Review of Pharmacoeconomics & Outcomes Research, DOI: 10.1080/14737167.2021.1840357. Slide 34 1. Doghrami K. The Epidemiology and Diagnosis of Insomnia. AJMC. 2006. 2. US Census Bureau. United State; QuickFacts. https://www.census.gov/quickfacts/fact/table/US/PST045219 Accessed April 22, 2021. 3.. Batterham P, Christensen H, Mackinnon A, et al. Trajectories of change and long-term outcomes in a randomized controlled trial of internet-based insomnia treatment to prevent depression. BJPsych Open. 2017; 3(5), 228-235. doi:10.1192/bjpo.bp.117.005231. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5611538/ 4 Batterham, P.J., Christensen, H., Thorndike, F. P., Ritterband, L.M., Gerwien, R., Enman, N., Botbyl, J., Maricich, Y. Web-delivered CBT for Insomnia Intervention Improves Sleep Among Adults with Insomnia and Depressive Symptoms. Virtual SLEEP 2020.: https://academic.oup.com/sleep/article-abstract/43/Supplement_1/A200/5847151 Slide 36 1. Ritterband LM, Thorndike FP, Ingersoll KS, et al. Effect of a Web-Based Cognitive Behavior Therapy for Insomnia Intervention With 1-Year Follow-up: A Randomized Clinical Trial. JAMA Psychiatry. 2017;74(1):68–75. doi:10.1001/jamapsychiatry.2016.3249. 2. Christensen H, Batterham PJ, Gosling JA, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;3(4):333-341. 3. Batterham P, Christensen H, Mackinnon A, et al. Trajectories of change and long-term outcomes in a randomized controlled trial of internet-based insomnia treatment to prevent depression. BJPsych Open. 2017; 3(5), 228-235. doi:10.1192/bjpo.bp.117.005231. 4. Batterham, P.J., Christensen, H., Thorndike, F. P., Ritterband, L.M., Gerwien, R., Enman, N., Botbyl, J., Maricich, Y. Web-delivered CBT for Insomnia Intervention Improves Sleep Among Adults with Insomnia and Depressive Symptoms. Virtual SLEEP 2020. 70 5. Real World Impact of a CBT I Digital Therapeutic: Treatment Outcomes and Prescription Sleep Medication Use Among 5,877 Adults. Thorndike FP, Ritterband LM , Gerwien R , Xiong X , Enman NM , Luderer H , Edington, S , Wendorf AR , Maricich YA 6. Ritterband LM, Thorndike, FP, Ingersoll, KS, et al. Effect of a Web-Based Cognitive Behavior Therapy for Insomnia Intervention With 1-Year Follow-up: A Randomized Clinical Trial. JAMA Psychiatry. 2016;74(1),68-75.

References Slide 37 1. Ritterband et al. JAMA Psychiatry. 2017;74(1):68-75. 2. Christensen et al. Lancet Psychiatry. 2016;3(4):333-341. 3. Somryst® Clinician Directions for Use. Boston, MA: Pear Therapeutics, Inc. 2020. Slide 38 1. Thorndike FP et al. Real World Data: Impact of a Digital Therapeutic for Insomnia in Adults. In S. Weiss (Chair), Using eHealth to bridge the gap between research and practice for insomnia: Examples from across the lifespan. Paper presented at: World Sleep Congress; September 2019; Vancouver, CA. 2. Thorndike FP, Gerwien R, Maricich YA, Luderer HF, Enman NM, Xiong R, Edington S, Ritterband L. Evidence From Real-World Users of a Digital Therapeutic for Insomnia. 173rd Annual Meeting of the American Psychiatric Association; April 25-29, 2020; Philadelphia, 3. Maricich YA, Thorndike FP, Gerwien R, Luderer HF, Enman NM, Xiong R, Edington S, Ritterband L. Evidence From Real-World Users of a Digital Therapeutic for Insomnia. Poster presented at: Technology in Psychiatry Summit; October 28-29, 2019; Boston, MA. Slide 67 1. SAMHSA. Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. 2020. 2. Campbell et al., American Journal of Psychiatry. 2014. 171(6):683-690. 3. Chaple et al. 2016. The Prison Journal. 96(3):485-508. 4. https://www.samhsa.gov/sites/default/files/aatod_2018_final.pdf SAMHSA/HHS source provides that 2.1 million Americans suffer from addiction to opiates. 5. Christensen DR, Landes RD, Jackson L, et al. Adding an Internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol. 2014;82(6):964-972. doi:10.1037/a0037496. 6. Internal data, FDA Regulatory filing, K173681, and Maricich Y et al. Safety and Efficacy of reSET in Patients w/ OUD. AAAP Annual Conference, 2018 7. Bickel et al. Exp Clin Psychopharmacol. 2008;16(2):132-143. 8. Marsch et al. Subst Abuse Treat. 2014;46(1):43-51.

Pear therapeutics