Table of Contents
Delaware |
2836 |
87-2652913 | ||
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
| Marko Zatylny Ropes & Gray LLP Prudential Tower 800 Boylston Street Boston, MA 02199-3600 (617) 951-7000 |
Karen Tepichin General Counsel Ginkgo Bioworks Holdings, Inc. 27 Drydock Avenue 8th Floor Boston, MA 02210 (877) 422-5362 |
Large accelerated filer |
☐ |
Accelerated filer |
☐ | |||
☒ |
Smaller reporting company |
|||||
Emerging growth company |
||||||
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. These securities may not be issued until the registration statement filed with the U.S. Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and does not constitute the solicitation of an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED SEPTEMBER 1, 2022
PRELIMINARY PROSPECTUS

GINKGO BIOWORKS HOLDINGS, INC.
Prospectus for 292,051,107 Shares of
Class A Common Stock of Ginkgo Bioworks Holdings, Inc.
This prospectus relates to the resale from time to time by the selling securityholders named in this prospectus (which term as used in this prospectus includes their respective transferees, pledgees, distributees, donees, and successors-in-interest, each a “Selling Securityholder” and, collectively, the “Selling Securityholders”) of 292,051,107 shares of Class A common stock, par value of $0.0001 per share (“Class A common stock”), of Ginkgo Bioworks Holdings, Inc.
This prospectus provides a general description of such securities and the general manner in which the Selling Securityholders may offer or sell the securities. More specific terms of any securities that the Selling Securityholders may offer or sell may be provided in a prospectus supplement that describes, among other things, the specific amounts and prices of the securities being offered and the terms of the offering. The prospectus supplement may also add, update or change information contained in this prospectus.
We are registering certain of the securities for resale pursuant to the Selling Securityholders’ registration rights under certain agreements between us and the Selling Securityholders. The Selling Securityholders may offer all or part of the shares for resale from time to time through public or private transactions, at either prevailing market prices or at privately negotiated prices. We will pay certain offering fees and expenses and fees in connection with the registration of our Class A common stock. We will not receive any of the proceeds from the resale of the shares of Common Stock by the Selling Securityholders. Class A common stock is currently listed on the NYSE and trades under the symbol “DNA.” The last reported sale price of Class A common stock on the NYSE on August 8, 2022 was $4.20 per share.
We are an “emerging growth company” under applicable federal securities laws and will be subject to reduced public company reporting requirements.
INVESTING IN OUR SECURITIES INVOLVES RISKS THAT ARE DESCRIBED IN THE “RISK FACTORS” SECTION BEGINNING ON PAGE 6 OF THIS PROSPECTUS.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of the securities to be issued under this prospectus or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2022.
Table of Contents
TABLE OF CONTENTS
| iv | ||||
| vi | ||||
| 1 | ||||
| 1 | ||||
| 1 | ||||
| 1 | ||||
| 3 | ||||
| 4 | ||||
| 5 | ||||
| 6 | ||||
| 6 | ||||
| 16 | ||||
| 17 | ||||
| 20 | ||||
| 22 | ||||
| 30 | ||||
| 32 | ||||
| 34 | ||||
| Risks Related to our Common Stock, Organizational Structure and Governance |
46 | |||
| 53 | ||||
| 53 | ||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
54 | |||
| 54 | ||||
| 56 | ||||
| 59 | ||||
| 60 | ||||
| 60 | ||||
| 60 | ||||
| Modification of Equity Awards in Connection with Business Combination |
61 | |||
| 61 | ||||
| 65 | ||||
| 74 | ||||
| 77 | ||||
| 84 | ||||
| 84 | ||||
| 85 | ||||
| 85 | ||||
| 85 | ||||
| 87 | ||||
| 89 | ||||
| 90 | ||||
| 94 | ||||
| 96 | ||||
| 97 | ||||
| 99 | ||||
| 110 | ||||
| 111 | ||||
| 113 | ||||
| 113 |
i
Table of Contents
| 115 | ||||
| 117 | ||||
| 118 | ||||
| 120 | ||||
| 134 | ||||
| 134 | ||||
| 135 | ||||
| 135 | ||||
| 136 | ||||
| 141 | ||||
| 141 | ||||
| 142 | ||||
| 142 | ||||
| 142 | ||||
| 146 | ||||
| 146 | ||||
| 146 | ||||
| 147 | ||||
| Action by the Ginkgo Board of Directors to Terminate a Founder |
147 | |||
| 147 | ||||
| 150 | ||||
| 150 | ||||
| 154 | ||||
| Limitations on Liability and Indemnification of Officers and Directors |
155 | |||
| 155 | ||||
| 155 | ||||
| 155 | ||||
| 156 | ||||
| 156 | ||||
| 157 | ||||
| 157 | ||||
| Restrictions on the Use of Rule 144 by Shell Companies or Former Shell Companies |
157 | |||
| 158 | ||||
| 162 | ||||
| 163 | ||||
| 165 | ||||
| 165 | ||||
| 168 | ||||
| 168 | ||||
| 168 | ||||
| 168 | ||||
| 169 | ||||
| 170 | ||||
| 170 | ||||
| 170 | ||||
| 170 | ||||
| 170 | ||||
| 173 | ||||
| 174 | ||||
| 174 | ||||
| 177 | ||||
| 177 |
ii
Table of Contents
| 177 | ||||
| 181 | ||||
| 182 | ||||
| 183 | ||||
| 184 | ||||
| 187 | ||||
| 190 | ||||
| 190 | ||||
| 190 | ||||
| 191 | ||||
| F-1 |
You should rely only on the information contained in this prospectus. We have not and the Selling Securityholders have not authorized anyone to provide you with information that is different from that contained in this prospectus. This prospectus is dated as of the date set forth on the cover hereof. You should not assume that the information contained in this prospectus is accurate as of any date other than that date.
For investors outside the United States, neither we, nor the Selling Securityholders, have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
iii
Table of Contents
SELECTED DEFINITIONS
Unless otherwise stated or unless the context otherwise requires, the terms “combined company,” “Company,” “Ginkgo,” “New Ginkgo,” “we,” “us,” “our” and similar terms refer to Ginkgo Bioworks Holdings, Inc. and its subsidiaries.
In this document:
“Business Combination” means the Domestication together with the Merger.
“Business Combination Registration Rights Agreement” means the Amended and Restated Registration Rights Agreement, dated as of September 16, 2021, by and among Ginkgo, the Sponsor and Viking Global Opportunities Illiquid Investments Sub-Master LP.
“Bylaws” means our amended and restated bylaws.
“Charter” means our amended and restated certificate of incorporation. “Code” means the Internal Revenue Code of 1986, as amended. “DGCL” means the General Corporation Law of the State of Delaware.
“Class A common stock” means the shares of Class A common stock, par value $0.0001 per share, of Ginkgo, which shares have the same economic terms as the shares of Class B common stock, however they are only entitled to one (1) vote per share.
“Class B common stock” means the shares of Class B common stock, par value $0.0001 per share, of Ginkgo, which shares have the same economic terms as the shares of Class A common stock, however they are entitled to ten (10) votes per share and the holders of Class B common stock, as a class, will have the right, for so long as the outstanding shares of Class B common stock continue to represent at least 2% of all of the outstanding shares of Common Stock, to elect 25% of the directors constituting the Ginkgo Board.
“Class C common stock” means the shares of Class C common stock, par value $0.0001 per share, of Ginkgo, which shares have the same economic terms as the shares of Class A common stock, but which carry no voting rights (except as otherwise expressly provided in the Charter or required by applicable law).
“Closing” means the closing of the Business Combination on the Closing Date.
“Closing Date” means September 16, 2021.
“Common Stock” means, collectively, the Class A common stock, the Class B common stock and the Class C common stock.
“Earn-out Consideration” means the 188.7 million Earn-Out Shares of Common Stock issued in connection with the Business Combination, which are subject to forfeiture to the extent that certain vesting conditions are not satisfied on or before the fifth anniversary of Closing Date.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“Founder” means any of Jason Kelly, Reshma Shetty, Austin Che, Bartholomew Canton and Thomas F. Knight, Jr. “founder shares” means the SRNG Class B ordinary shares sold prior to SRNG’s initial public offering.
“Ginkgo Board” means the board of directors of Ginkgo.
iv
Table of Contents
“Ginkgo Management” means the management of Ginkgo. “Governing Documents” means the Charter and the Bylaws.
“IPO” means SRNG’s initial public offering, consummated on February 26, 2021, through the sale of 22,500,000 units at $10.00 per unit.
“JOBS Act” means the Jumpstart Our Business Startups Act of 2012.
“Merger Agreement” means that certain Agreement and Plan of Merger, dated as of May 11, 2021, as amended on May 14, 2021, by and among SRNG, Merger Sub and Old Ginkgo.
“Merger Sub” means SEAC Merger Sub Inc., a Delaware corporation and wholly owned subsidiary of SRNG. “NYSE” means the New York Stock Exchange.
“Old Ginkgo” means Ginkgo Bioworks, Inc., a Delaware corporation.
“Old Ginkgo capital stock” means, collectively, the Old Class A common stock, the Old Class B common stock and each other class or series of capital stock of Old Ginkgo (including preferred stock).
“Old Class A common stock” means the Class A common stock, par value $0.0001 per share, of Old Ginkgo.
“Old Class B common stock” means the Class B common stock, par value $0.0001 per share, of Old Ginkgo.
“Old Ginkgo warrant” means each warrant to purchase shares of Old Ginkgo capital stock.
“Private Placement Warrants” means the 17,325,000 warrants held by the Sponsor, which were issued concurrently with the IPO, each of which is exercisable for one share of Class A common stock.
“Public Warrants” means the warrants included in the units issued in the IPO, each of which is exercisable for one share of Class A common stock, in accordance with its terms.
“SEC” means the U.S. Securities & Exchange Commission.
“Sponsor” means Eagle Equity Partners III, LLC, a Delaware limited liability company.
“SRNG” means Soaring Eagle Acquisition Corp., a Cayman Islands exempted company, prior to the Domestication.
“SRNG Class A ordinary shares” means the Class A ordinary shares, par value $0.0001 per share, of SRNG.
“SRNG Class B ordinary shares” means the Class B ordinary shares, par value $0.0001 per share, of SRNG.
“SRNG ordinary shares” means, collectively, the SRNG Class A ordinary shares and SRNG Class B ordinary shares.
“SRNG warrants” means, collectively, the SRNG Public Warrants and the SRNG Private Placement Warrants.
“Transfer Agent” means Computershare Trust Company, N.A.
“Warrants” means the Private Placement Warrants and the Public Warrants.
v
Table of Contents
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This report includes forward-looking statements regarding, among other things, the plans, strategies and prospects, both business and financial, of Ginkgo Bioworks Holdings, Inc. (“Ginkgo”). These statements are based on the beliefs and assumptions of the management of Ginkgo. Although Ginkgo believes that its plans, intentions and expectations reflected in or suggested by these forward-looking statements are reasonable, Ginkgo cannot assure you that it will achieve or realize these plans, intentions or expectations. Forward-looking statements are inherently subject to risks, uncertainties and assumptions. Generally, statements that are not historical facts, including statements concerning possible or assumed future actions, business strategies, events or results of operations, are forward-looking statements. These statements may be preceded by, followed by or include the words “believes”, “estimates”, “expects”, “projects”, “forecasts”, “may”, “will”, “should”, “seeks”, “plans”, “scheduled”, “anticipates” or “intends” or similar expressions. Forward-looking statements contained in this prospectus include, but are not limited to, statements about:
| • | Ginkgo’s ability to raise financing in the future and to comply with restrictive covenants related to long-term indebtedness; |
| • | Ginkgo’s ability to retain or recruit, or adapt to changes required in, its founders, senior executives, key personnel or directors; |
| • | factors relating to the business, operations and financial performance of Ginkgo, including: |
| • | Ginkgo’s ability to effectively manage its growth; |
| • | Ginkgo’s exposure to the volatility and liquidity risks inherent in holding equity interests in certain of its customers; |
| • | rapidly changing technology and extensive competition in the synthetic biology industry that could make the products and processes Ginkgo is developing obsolete or non-competitive unless it continues to collaborate on the development of new and improved products and processes and pursue new market opportunities; |
| • | Ginkgo’s reliance on its customers to develop, produce and manufacture products using the engineered cells and/or biomanufacturing processes that Ginkgo develops; |
| • | Ginkgo’s ability to comply with laws and regulations applicable to its business; and |
| • | market conditions and global and economic factors beyond Ginkgo’s control; |
| • | intense competition and competitive pressures from other companies worldwide in the industries in which Ginkgo operates; |
| • | litigation and the ability to adequately protect Ginkgo’s intellectual property rights; |
| • | the success of Ginkgo’s programs and their potential to contribute revenue; |
| • | Ginkgo’s ability to close and realize the benefits of pending merger and acquisition transactions; and |
| • | other factors detailed under the section entitled “Risk Factors.” |
These and other factors that could cause actual results to differ from those implied by the forward-looking statements in this prospectus are more fully described under the heading “Risk Factors” and elsewhere in this report. The risks described under the heading “Risk Factors” are not exhaustive. Other sections of this prospectus describe additional factors that could adversely affect the business, financial condition or results of Ginkgo. New risk factors emerge from time to time and it is not possible to predict all such risk factors, nor can Ginkgo assess the impact of all such risk factors on the business of Ginkgo, or the extent to which any factor or combination of factors may cause actual results to differ materially from those contained in any forward-looking statements. Forward-looking statements are not guarantees of performance. You should not put undue reliance on these statements, which speak only as of the date hereof. All forward-looking statements attributable to Ginkgo or persons acting on its behalf are expressly qualified in their entirety by the foregoing cautionary statements. Ginkgo undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
vi
Table of Contents
SUMMARY OF THE PROSPECTUS
This summary highlights selected information included in this prospectus and does not contain all of the information that may be important to you in making an investment decision. This summary is qualified in its entirety by the more detailed information included in this prospectus. Before making your investment decision with respect to our securities, you should carefully read this entire prospectus, including the information under “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements included elsewhere in this prospectus.
The Company
Ginkgo Bioworks Holdings, Inc. is building a platform to enable customers to program cells as easily as we can program computers. Ginkgo’s platform is market agnostic and enables biotechnology applications across diverse markets, from food and agriculture to industrial chemicals to pharmaceuticals. Ginkgo is also actively supporting a number of biosecurity efforts to respond to COVID-19, including vaccine manufacturing optimization, therapeutics discovery, and K-12 pooled testing. Ginkgo has incurred net losses since its inception. Ginkgo’s net loss attributable to its stockholders was approximately $668.8 million and $1,259.3 million for the three and six months ended June 30, 2022, respectively, and $1,830.0 million, $126.6 million and $119.3 million for the fiscal years ended December 31, 2021, 2020 and 2019, respectively. As of June 30, 2022, Ginkgo had an accumulated deficit of approximately $3,557.3 million, and as of December 31, 2021, Ginkgo had an accumulated deficit of approximately $2,297.9 million. For more information, see “Risk Factors—Risks Related to Ginkgo’s Business—We have a history of net losses. We expect to continue to incur losses for the foreseeable future, and we may never achieve or maintain profitability.”
Stock Exchange Listing
Ginkgo’s Class A common stock and public warrants are listed on the NYSE under the symbols “DNA” and
“DNA.WS”, respectively.
Summary of Risk Factors
Investing in our securities involves risks. You should carefully consider the risks described in “Risk Factors” beginning on page 8 before making a decision to invest in our Class A common stock. If any of these risks actually occur, our business, financial condition and results of operations would likely be materially adversely affected. Some of the risks related to Ginkgo’s business and industry are summarized below. References in the summary below to “we,” “us,” “our” and “the Company” generally refer to Ginkgo.
| • | We have a history of net losses. We expect to continue to incur losses for the foreseeable future, and we may never achieve or maintain profitability. |
| • | We are not, and do not intend to become, regulated as an “investment company” under the Investment Company Act of 1940, as amended (“Investment Company Act”), and if we were deemed an “investment company” under the Investment Company Act, applicable restrictions could make it impractical for us to continue our business as contemplated and could have a material adverse effect on our business. |
| • | Only our employees and directors are entitled to hold shares of Class B common stock (including shares of Class B common stock granted or otherwise issued to our employees and directors in the future), which have ten votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of certain amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring stockholder approval. |
1
Table of Contents
| • | Outstanding Class C common stock may have the effect of extending voting power in Class B common stock, and may discourage potential acquisitions of our business and could have an adverse effect on the trading price of Class A common stock. |
| • | We may need substantial additional capital in the future in order to fund our business. |
| • | We have experienced rapid growth and expect our growth to continue, and if we fail to effectively manage our growth, then our business, results of operations, and financial condition could be adversely affected. |
| • | Our limited operating history makes it difficult to evaluate our current business and future prospects. |
| • | We currently own and may in the future own equity interests in other operating companies, including certain of our customers; consequently, we have exposure to the volatility and liquidity risks inherent in holding their equity and overall operational and financial performance of these businesses. |
| • | We may pursue strategic acquisitions and investments that are dilutive to our stockholders and that could have an adverse impact on our business if they are unsuccessful. |
| • | We must continue to secure and maintain sufficient and stable supplies of laboratory reagents, consumables, equipment, and laboratory services. We depend on a limited number of suppliers, some of which are single-source suppliers, and contract manufacturers for critical supplies, equipment, and services for research, development, and manufacturing of our products and processes. Our reliance on these third parties exposes us to risks relating to costs, contractual terms, supply, and logistics, and the loss of any one or more of these suppliers or contract manufacturers or their failure to supply us with the necessary supplies, equipment, or services on a timely basis, could cause delays in our research, development, or production capacity and adversely affect our business. |
| • | We use biological, hazardous, flammable and/or regulated materials that require considerable training, expertise and expense for handling, storage and disposal and may result in claims against us. |
| • | Third parties may use our engineered cells, materials, and organisms and accompanying production processes in ways that could damage our reputation. |
| • | If our customers discontinue their development, production and manufacturing efforts using our engineered cells and/or biomanufacturing processes, our future financial position may be adversely impacted. |
| • | Our revenue is concentrated in a limited number of customers, some of which are related parties, and our revenue, results of operations, cash flows and reputation in the marketplace may suffer upon the loss of a significant customer. |
| • | In certain cases, our business partners may have discretion in determining when and whether to make announcements about the status of our collaborations, including about developments and timelines for advancing programs, and the price of our common stock may decline as a result of announcements of unexpected results or developments. |
| • | Uncertainty regarding the ongoing demand and/or capacity (including capacity at third party clinical testing laboratories) of our COVID-19 individual and pooled sample tests could materially adversely affect our business. |
| • | Uncertainty regarding the sales and delivery of our COVID-19 individual and pooled sample tests could materially adversely affect our business. |
| • | We may be subject to tort liability if the COVID-19 tests we utilize in our testing programs provide inaccurate results. |
2
Table of Contents
| • | Rapidly changing technology and emerging competition in the synthetic biology industry could make the platform, programs, and products we and our customers are developing obsolete or non-competitive unless we continue to develop our platform and pursue new market opportunities. |
| • | Ethical, legal and social concerns about GMOs and Genetically Modified Materials and their resulting products could limit or prevent the use of products or processes using our technologies, limit public acceptance of such products or processes and limit our revenues. |
| • | If we are unable to obtain, maintain and defend patents protecting our intellectual property, our competitive position will be harmed. |
| • | Under certain circumstances, we may share or lose rights to intellectual property developed under U.S. federally funded research grants and contracts. |
| • | The use of digital genetic sequence information may be subject to the Nagoya Protocol, which could increase our costs and adversely affect our business. |
| • | We rely on our customers, joint venturers, equity investees and other third parties to deliver timely and accurate information in order to accurately report our financial results in the time frame and manner required by law. |
| • | Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited. |
| • | Failure to comply with federal, state, local and international laws and regulations could adversely affect our business and our financial condition. |
| • | We may incur significant costs complying with environmental, health and safety laws and regulations, and failure to comply with these laws and regulations could expose us to significant liabilities. |
| • | We may become subject to the comprehensive laws and rules governing billing and payment, noncompliance with which could result in non-payment or recoupment of overpayments for our services or other sanctions. |
| • | We and our laboratory partners are subject to a variety of laboratory testing standards, compliance with which is an expensive and time-consuming process, and any failure to comply could result in substantial penalties and disruptions to our business. |
| • | If we fail to comply with healthcare and other governmental regulations, we could face substantial penalties and our business, financial condition and results of operations could be adversely affected. |
| • | We are engaged in certain research activities involving controlled substances, including cannabinoids and other chemical intermediates, the making, use, sale, importation, exportation, and distribution of which may be subject to significant regulation by the DEA and other regulatory agencies. |
| • | Significant disruptions to our and our service providers’ information technology systems or data security incidents could result in significant financial, legal, regulatory, business and reputational harm to us. |
| • | Our business could be adversely affected by legal challenges to our telehealth partner’s business model. |
Corporate Information
We were originally incorporated as a Cayman Islands exempted company in October 2020 as a special purpose acquisition company, formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, recapitalization, reorganization or similar business combination with one or more businesses. SRNG completed its IPO in February 2021. In September 2021, our wholly owned subsidiary merged with and into Old Ginkgo, with Old Ginkgo surviving the merger as a wholly owned subsidiary of SRNG. In connection with the Business Combination, we changed our name to “Ginkgo Bioworks Holdings, Inc.” Our principal executive offices are located at 27 Drydock Avenue, 8th Floor, Boston MA 02210.
3
Table of Contents
Our telephone number is (877) 422-5362. Our website address is www.ginkgobioworks.com. Information contained on our website or connected thereto does not constitute part of, and is not incorporated by reference into, this prospectus or the registration statement of which it forms a part.
Emerging Growth Company
Section 102(b)(1) of the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a registration statement under the Securities Act declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. We have elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company (“EGC”), can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of Ginkgo’s financial statements with those of another public company that is neither an EGC nor an EGC that has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
We will remain an EGC until the earlier of: (1) the last day of the fiscal year (a) following the fifth anniversary of the Closing of SRNG’s initial public offering, (b) in which we have total annual gross revenue of at least $1.07 billion or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common equity that is held by non-affiliates exceeds $700 million as of the end of the prior fiscal year’s second fiscal quarter; and (2) the date on which we have issued more than $1.00 billion in non- convertible debt securities during the prior three-year period. References herein to “EGC” have the meaning associated with it in the JOBS Act.
4
Table of Contents
THE OFFERING
| Issuer |
Ginkgo Bioworks Holdings, Inc. |
| Class A common stock offered by the Selling Securityholders |
Up to 292,051,107 shares of Class A common stock |
| Shares of our Class A common stock outstanding prior to exercise of all Warrants (as of August 8, 2022) |
1,099,693,341 |
| Use of proceeds |
The Selling Securityholders will receive all of the proceeds from this offering. We will not receive any of the proceeds from the sale of the shares of common stock by the Selling Securityholders. |
| Ticker symbols |
“DNA” and “DNA.WS” for the Class A common stock and Public Warrants, respectively. |
| Risk factors |
Any investment in the securities offered hereby is speculative and involves a high degree of risk. You should carefully consider the information set forth under “Risk Factors” and elsewhere in this prospectus. |
5
Table of Contents
RISK FACTORS
An investment in our securities involves a high degree of risk. You should carefully consider the following risk factors, together with all of the other information included in this prospectus, before making an investment decision. Our business, prospects, financial condition or operating results could decline due to any of these risks and, as a result, you may lose all or part of your investment.
Unless the context otherwise requires, all references in this section to the “Company,” “we,” “us” or “our” refer to the business of Ginkgo and its subsidiaries.
Risks Related to Ginkgo’s Business
We have a history of net losses. We expect to continue to incur losses for the foreseeable future, and we may never achieve or maintain profitability.
We have incurred significant operating losses since our inception. Our net loss attributable to our stockholders was approximately $668.8 million and $1,259.3 million for the three and six months ended June 30, 2022, respectively. As of June 30, 2022, we had an accumulated deficit of approximately $3,557.3 million. We may incur losses and negative cash flow from operating activities for the foreseeable future as we continue to invest significant additional funds toward further developing our platform, the cell programs we perform on behalf of our customers and otherwise growing our business, including our biosecurity offering. Our operating expenses have increased as a result of becoming a public company, and we expect that our operating expenses will continue to increase as we grow our business. We have derived a significant portion of our revenues from fees and milestone payments from technical development services provided to customers to advance programs, as well as a significant portion of our revenues from our biosecurity offering. Historically, these fees have not been sufficient to cover the full cost of our operations. Additionally, if our customers terminate their agreements or development plans with us, our near-term revenues could be adversely affected. In addition, certain of our customer agreements provide for milestone payments, future royalties and other forms of contingent consideration, the payment of which are uncertain, as they are dependent on our ability to successfully develop engineered cells, bioprocesses, or other deliverables and our customers’ ability and willingness to successfully develop and commercialize products and processes.
Our expenses may exceed revenues for the foreseeable future and we may not achieve profitability. If we fail to achieve profitability, or if the time required to achieve profitability is longer than we anticipate, we may not be able to expand or continue our business, and the value of our common stock could be negatively impacted. Our ability to achieve or sustain profitability is based on numerous factors, many of which are beyond our control, including the development of our platform, the initiation of new programs with new and existing customers, the commercial terms of our programs, our ability to advance cell engineering programs in a timely and cost-effective manner, our ability to extend new offerings to customers, our customers’ ability to scale up bioprocesses, the ability of our customers to produce and sell products, the impact of market acceptance of our customers’ products, and our customers’ market penetration and margins. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
We may need substantial additional capital in the future in order to fund our business.
We have consumed considerable amounts of capital to date, and we expect to incur continued net losses over the next several years as we continue to develop our business, advance our programs, expand and enhance our platform, and make the capital investments necessary to scale up our Foundry operations and Codebase assets. We may also use additional capital for our biosecurity offering, strategic investments and acquisitions. We believe that our cash and cash equivalents, short-term investments, and interest earned on investments will be sufficient to meet our projected operating requirements for several years and until we reach profitability. However, these assumptions may prove to be incorrect and we could exhaust our available capital resources
6
Table of Contents
sooner than we currently expect. Because of the numerous risks and uncertainties associated with our programs, including risks and uncertainties that could impact the rate of progress of our programs, we are unable to estimate with certainty the amounts of capital outlays and operating expenditures associated with these activities.
We do not currently have any commitments for future funding. We may receive fees, milestones, and royalty payments under our customer agreements, but these are not guaranteed. Additionally, we may be able to sell our equity interests in certain subsidiaries or collaborations but most of these equity stakes are illiquid (e.g., in private companies) and we may not be able to find a buyer or may incur significant impairment if forced to sell these positions for liquidity. We may not receive any further funds under those agreements, the funds we receive may be lower than projected, or our program costs may be higher than projected. In addition, we may not be able to sign new customer agreements or enter into new development plans with existing customers with adequate funds to cover program development expenses. As a result of these and other factors, we do not know whether additional financing will be available when needed, or, if available, whether such financing would be on terms favorable to our stockholders or us.
If future financings involve the issuance of equity securities, our existing stockholders would suffer dilution. If we raise debt financing in the future, we may be subject to restrictive covenants that limit our ability to conduct our business. Our ability to raise funds may be adversely impacted by current or future economic conditions. If we fail to raise sufficient funds and continue to incur losses, our ability to fund our operations, take advantage of strategic opportunities, or otherwise respond to competitive pressures could be significantly limited. If adequate funds are not available, we may not be able to successfully execute our business plan or continue our business.
We have experienced rapid growth and expect our growth to continue, and if we fail to effectively manage our growth, then our business, results of operations, and financial condition could be adversely affected.
We have experienced substantial growth in our business since inception, which has placed and may continue to place significant demands on our company culture, operational infrastructure, and management. We believe that our culture has been a critical component of our success. We have invested substantial time and resources in building our team and nurturing a culture of empowerment of, and active engagement by, our employees. As we expand our business and mature as a public company, we may find it difficult to maintain our culture while managing this growth. Any failure to manage our anticipated growth and organizational changes in a manner that preserves the key aspects of our culture could be detrimental to future success, including our ability to recruit and retain personnel, and effectively focus on and pursue our objectives. This, in turn, could adversely affect our business, results of operations, and financial condition.
In addition, in order to successfully manage our rapid growth, our organizational structure has become more complex and is likely to continue to become more complex. In order to manage these increasing complexities, we will need to continue to scale and adapt our operational, financial, and management controls, as well as our reporting systems and procedures. The expansion of our systems and infrastructure will require us to commit substantial financial, operational, and management resources before our revenue increases and without any assurances that our revenue will increase.
Finally, continued growth could strain our ability to maintain reliable service levels and offerings for our customers. If we fail to achieve the necessary level of capacity, quality and efficiency in performing services and other development activities, or the necessary level of efficiency in our organizational structure as we grow, then our business, results of operations, and financial condition could be adversely affected.
Our limited operating history makes it difficult to evaluate our current business and future prospects.
We have a portfolio of cell engineering programs which vary in start date, duration, complexity, and revenue potential. Additionally, our downstream economics in the form of equity interests, milestone payments, or royalty streams add an additional level of uncertainty to our possible future performance. Consequently,
7
Table of Contents
predictions about our future success or viability are highly uncertain and may not be as accurate as they could be if we had a longer company history of successfully developing, commercializing and generating revenue from our programs and/or downstream economic participation. With respect to our biosecurity offering, prior to 2020, we had no experience developing or commercializing testing services. Moreover, as described above, given the limited operating history of our biosecurity offering, our reliance on government funding for testing, potential disruptions from vaccine rollout generally, the availability of COVID-19 therapeutics, the impact of summer vacation and other school breaks, and the increased availability of over-the-counter testing options, the future performance of our COVID-19 testing program is unpredictable. Moreover, we cannot predict how long the COVID-19 pandemic will continue and, therefore, we cannot predict the duration of the revenue stream, which could diminish significantly, from our COVID-19 testing services.
Our long-term objective is to generate free cash flow from the commercialization of programs by customers across a variety of industries, as well as from our biosecurity-focused offerings. Our estimated costs and timelines for the completion of programs are based on our experiences to date and our expectations for each stage of the program in development. Given the variety of types of programs we support and the continued growth of our platform, there is variability in timelines and costs for launching and executing programs, and completion dates can change over the course of a customer engagement. In addition, our costs and timelines may be greater or subject to variability where regulatory requirements lead to longer timelines, such as in agriculture, food, and therapeutics. In addition, we have equity interests in certain companies and there is and will continue to be variability in the financial performance of these other companies or future companies in which we may have equity interests.
As a business with a limited operating history, we may encounter unforeseen expenses, difficulties, complications, delays, and other known and unknown obstacles. We have encountered in the past, and will encounter in the future, risks and uncertainties frequently experienced by growing companies with limited operating histories in emerging and rapidly changing industries. If our assumptions regarding these risks and uncertainties, which we use to plan and operate our business, are incorrect or change, or if we do not address these risks successfully, our results of operations could differ materially from our expectations, and our business, financial condition, and results of operations could be adversely affected.
If we cannot maintain and expand current customer partnerships and enter into new customer partnerships, our business could be adversely affected.
We do not generate substantial revenue from our own products, and instead generate revenue from customer collaborations in which we provide services, and also receive downstream value in the form of royalties, equity, or milestone payments. As a result, our success depends on our ability to expand the number, size and scope of our customer collaborations. Our ability to win new business depends on many factors, including our reputation in the market, the quality of our service offerings relative to alternatives, the pricing and efficiency of our services relative to alternatives, and our technical capabilities. If we fail to maintain a position of strength in any of these factors, our ability to either sign new customer collaborations or launch new programs with existing customers may suffer and this could adversely affect our prospects. Additionally, in the process of developing programs, we generate Foundry know-how and accumulate meaningful biological and data assets, including optimized proteins and organisms, characterized genetic parts, enhanced understanding of metabolic pathways, biological, chemical, and genetic libraries, and other elements of biological data. Data and know-how generated from our programs provide the basis for expanded capabilities that we believe further supports our customer collaborations. As a result, in addition to reducing our revenue or delaying the development of our programs, the loss of one or more of our customer relationships or the failure to add new customers or programs may hinder our accumulation of such information, thus hindering our efforts to advance our technological differentiation and improve our platform.
We engage in conversations with companies regarding potential customer collaborations on an ongoing basis. We may spend considerable time and money engaging in these conversations and feasibility assessments,
8
Table of Contents
including understanding the technical approach to a program, customer concerns and limitations, and legal or regulatory landscape of a potential program or offering, which may not result in a commercial agreement. Even if an agreement is reached, the resulting relationship may not be successful for many reasons, including our inability to complete a program to our customers’ specifications or within our customers’ time frames, or unsuccessful development or commercialization of products or processes by our customers. In such circumstances, our revenues and downstream value potential from such a collaboration might be meaningfully reduced.
We currently own and may in the future own equity interests in other operating companies, including certain of our customers; consequently, we have exposure to the volatility and liquidity risks inherent in holding their equity and overall operational and financial performance of these businesses.
We currently own equity interests in several of our customers. In the future, we may also own equity interests in other companies. The process by which we receive equity interests and the factors we consider in deciding whether to accept, hold or dispose of these equity positions may differ significantly from those that an independent investor would evaluate when considering equity interests in a company. Owning equity increases our exposure to the risks of the other company and, in the case of customers, beyond the products of our collaborations. Our equity ownership positions expose us to market volatility and the potential for negative returns. We may have restrictions on resale or limited markets to sell our equity ownership. In many cases, our equity position is a minority position which exposes us to further risk, as we are not able to exert control over the companies in which we hold securities.
In connection with future collaborations or joint ventures, we may, from time to time, receive warrants or options, all of which involve special risks. To the extent we receive warrants or options in connection with future collaborations or joint ventures, we would be exposed to risks involving pricing differences between the market value of underlying securities and our exercise price for the warrants or options, a possible lack of liquidity, and the related inability to close a warrant or option position, all of which could ultimately have an adverse effect on our financial position.
We leverage our own resources and partner with strategic and financial investors in order to help early stage companies and innovators secure funding and benefit from our platform, which exposes us to a number of risks.
Since our founding, we have helped to launch new companies (such as BiomEdit, LLC, Motif FoodWorks, Inc., Allonnia, LLC, Arcaea, LLC (formerly known as Kalo Ingredients, LLC), Ayana Bio, LLC, Joyn Bio LLC and Verb Biotics, LLC) by bringing together strategic and financial investors to secure funding for these early stage and small companies. Going forward, we intend to continue to leverage our own balance sheet and partner with investors to enable companies at all stages to benefit from our platform.
Partnering with and investing in early stage and small companies may expose us to a number of risks, including that early stage and small companies may have:
| • | shorter operating histories, narrower product lines and smaller market shares than larger businesses, which tend to render small companies more vulnerable to competitors’ actions and market conditions, as well as general economic downturns; |
| • | more limited access to capital and higher funding costs, may be in a weaker financial position and may need more capital than originally anticipated to expand, compete and operate their business; |
| • | the inability to obtain financing from the public capital markets or other traditional sources, such as commercial banks, in part because loans made to these types of companies entail higher risks than loans made to companies that have larger businesses, greater financial resources or are otherwise able to access traditional credit sources on more attractive terms; |
9
Table of Contents
| • | a higher likelihood of depending on the management talents and efforts of a small group of persons; therefore, the death, disability, resignation or termination of one or more of these persons could have a material adverse impact on such company and, in turn, on us; |
| • | less predictable operating results, may be engaged in rapidly changing businesses with products subject to a substantial risk of obsolescence, and may require substantial additional capital to support their operations, finance expansion or maintain their competitive position; |
| • | particular vulnerabilities to changes in customer preferences and market conditions, depend on a limited number of customers, and face intense competition, including from companies with greater financial, technical, managerial and marketing resources; and |
| • | fewer administrative resources, which can lead to greater uncertainty in their ability to generate accurate and reliable financial data, including their ability to deliver audited financial statements. |
Any of these factors or changes thereto could impair an early stage or small company’s financial condition, results of operation, cash flow or result in other adverse events, such as bankruptcy. This, in turn, could result in losses in our investments and a change in our income (loss) on investments.
We may be unable to complete pending strategic acquisitions or successfully integrate strategic acquisitions which could adversely affect our business and financial condition.
Our inability to complete any pending strategic acquisitions or to successfully integrate any new or previous strategic acquisitions could have a material adverse effect on our business. Our business strategy includes the acquisition of technologies and businesses that complement or augment our existing products and services. We may continue to seek attractive opportunities to acquire businesses, enter into joint ventures and make other investments that are complementary to our existing strengths. There are no assurances, however, that any strategic acquisition opportunities will arise or, if they do, that they will be consummated. Certain acquisitions may be difficult to complete for a number of reasons, including the need to satisfy customary closing conditions, the need for antitrust and/or other regulatory approvals, as well as disputes or litigation. For example, our announced and pending Zymergen merger and Bayer acquisition are subject to a number of closing conditions, as described in Note 15 to our consolidated financial statements. Any strategic acquisition we may complete may be made at a substantial premium over the fair value of the net identifiable assets of the acquired company and thus our realization of this value relies on successful integration and continued operations. We may not be able to integrate acquired businesses successfully into our existing businesses, make such businesses profitable, retain key employees or realize anticipated cost savings or synergies, if any, from these acquisitions, which could adversely affect our business and financial condition. Further, our ongoing business may be disrupted, and our management’s attention may be diverted by acquisitions, investments, transition and/or integration activities.
We may pursue strategic acquisitions and investments that are dilutive to our stockholders and that could have an adverse impact on our business if they are unsuccessful.
We have made acquisitions in the past and, as appropriate opportunities become available, we may acquire additional businesses, assets, technologies, or products to enhance our business in the future, but our ability to do so successfully cannot be ensured. We have also made investments in companies that we view as synergistic with our business. Although we conduct due diligence on these acquisitions and investments, such processes may underestimate or fail to reveal significant liabilities and we could incur losses resulting from liabilities of the acquired business that are not covered by indemnification we may obtain from the seller. Even if we identify suitable opportunities, including pending transactions, we may not be able to complete such acquisitions on favorable terms or at all, which could damage our business. Additionally, pursuing acquisitions, whether successful or unsuccessful, could result in civil litigation and regulatory penalties. Any acquisitions we make may not strengthen our competitive position, and these transactions may be viewed negatively by customers or investors. We may decide to incur debt or spend cash in connection with a strategic acquisition, which may cause
10
Table of Contents
us to face liquidity concerns or be subject to restrictive covenants in the future. We have, and in the future may, also issue common stock or other equity securities to the stockholders of the acquired company, which could constitute a material portion of our then-outstanding shares of common stock and may reduce the percentage ownership of our existing stockholders.
In addition, we may not be able to successfully integrate the acquired personnel, assets, technologies, products and/or operations into our existing business in an effective, timely, and non-disruptive manner or retain acquired personnel following an acquisition. Acquisitions may also divert management’s attention from day-to-day responsibilities, increase our expenses and reduce our cash available for operations and other uses. In addition, we may not be able to fully recover the costs of such acquisitions or be successful in leveraging any such strategic transactions into increased business, revenue, or profitability. We also cannot predict the number, timing, or size of any future acquisitions or the effect that any such transactions might have on our operating results.
Accordingly, although there can be no assurance that we will undertake or successfully complete any future acquisitions, any transactions that we do complete may not yield the anticipated benefits and may be subject to the foregoing or other risks and have a material and adverse effect on our business, financial condition, results of operations, and prospects. Conversely, any failure to pursue or delay in completing any acquisition or other strategic transaction that would be beneficial to us, including those caused by competing parties, could delay the development of our platform or advancement of our programs and, thus, potential commercialization of our customer’s products.
Our programs may not achieve milestones and other anticipated key events on the expected timelines or at all, which could have an adverse impact on our business and could cause the price of our common stock to decline.
We may adopt various technical, manufacturing, regulatory, commercial, and other objectives for our programs. These milestones may include our or our customers’ expectations regarding the commencement or completion of technical development, the achievement of manufacturing targets, the submission of regulatory filings, or the realization of other development, regulatory, or commercialization objectives by us or our customers. The achievement of many of these milestones may be outside of our control. All of these milestones are based on a variety of assumptions, including assumptions regarding capital resources, constraints, and priorities, progress of and results from research and development (“R&D”) activities, and other factors, including impacts resulting from the COVID-19 pandemic, any of which may cause the timing of achievement of the milestones to vary considerably. If we, our collaborators, or our customers fail to achieve milestones in the expected timeframes, the commercialization of our programs may be delayed, our credibility may be undermined, our business and results of operations may be harmed, and the trading price of our common stock may decline.
We must continue to secure and maintain sufficient and stable supplies of laboratory reagents, consumables, equipment, and laboratory services. We depend on a limited number of suppliers, some of which are single-source suppliers, and contract manufacturers for critical supplies, equipment, and services for research, development, and manufacturing of our products and processes. Our reliance on these third parties exposes us to risks relating to costs, contractual terms, supply, and logistics, and the loss of any one or more of these suppliers or contract manufacturers or their failure to supply us with the necessary supplies, equipment, or services on a timely basis, could cause delays in our research, development, or production capacity and adversely affect our business.
The COVID-19 pandemic has caused substantial disruption in global supply chains and the ability of third parties to provide us services on a timely basis or at all. As a result, we have experienced shortages in some of our key equipment and supplies, including those required in our labs, as well as disruptions in services provided by third parties, and may continue to do so in the future as a result of the pandemic, or otherwise. We may also experience price increases, quality issues and longer lead times due to unexpected material shortages, service
11
Table of Contents
disruptions, and other unanticipated events, which may adversely affect our supply of lab equipment, lab supplies, chemicals, reagents, supplies, and lab services. For some suppliers, we do not enter into long-term agreements and instead secure our materials and services on a purchase order basis. Our suppliers may reduce or cease their supply of materials or services to us at any time in the future. If the supply of materials or services is interrupted, our programs may be delayed.
We depend on a limited number of suppliers for critical items, including lab consumables and equipment, for the development of our programs. Some of these suppliers are single-source suppliers. We do not currently have the infrastructure or capability internally to manufacture these items at the necessary scale or at all. Although we have a reserve of supplies and although alternative suppliers exist for some of these critical products, services, and equipment, our existing processes used in our Foundries have been designed based on the functions, limitations, features, and specifications of the products, services, and equipment that we currently utilize. While we work with a variety of domestic and international suppliers, our suppliers may not be obligated to supply products or services or our arrangements may be terminated with relatively short notice periods. Additionally, we do not have any control over the process or timing of the acquisition or manufacture of materials by our manufacturers and cannot ensure that they will deliver to us the items we order on time, or at all.
In particular, we rely on Twist Bioscience Corporation for custom DNA synthesis and Thermo Fisher Scientific Inc. and others for certain instruments and consumables. The price and availability of DNA, chemicals, reagents, equipment, consumables, and instruments have a material impact on our ability to provide Foundry services. We may rely on contract manufacturers like Fermic, s.a. de.c.v for scale-up fermentation development, fermentation, and manufacturing of products for some customers.
The loss of the products, services, and equipment provided by one or more of our suppliers could require us to change the design of our research, development, and manufacturing processes based on the functions, limitations, features, and specifications of the replacement items or seek out a new supplier to provide these items. Additionally, as we grow, our existing suppliers may not be able to meet our increasing demand, and we may need to find additional suppliers. We may not be able to secure suppliers who provide lab supplies at, or equipment and services to, the specification, quantity, and quality levels that we demand (or at all) or be able to negotiate acceptable fees and terms of services with any such suppliers.
As described above, some lab equipment, lab consumables, and other services and materials that we purchase are purchased from single-source or preferred suppliers, which limits our negotiating leverage and our ability to rely on additional or alternative suppliers for these items. Our dependence on these single-source and preferred suppliers exposes us to certain risks, including the following:
| • | our suppliers may cease or reduce production or deliveries, raise prices, or renegotiate terms; |
| • | we may be unable to locate a suitable replacement on acceptable terms or on a timely basis, if at all; |
| • | if there is a disruption to our single-source or preferred suppliers’ operations, and if we are unable to enter into arrangements with alternative suppliers, we will have no other means of continuing the relevant research, development, or manufacturing operations until they restore the affected facilities or we or they procure alternative sources of supply; |
| • | delays caused by supply issues may harm our reputation, frustrate our customers, and cause them to turn to our competitors for future programs; and |
| • | our ability to progress the development of existing programs and the expansion of our capacity to begin future programs could be materially and adversely impacted if the single-source or preferred suppliers upon which we rely were to experience a significant business challenge, disruption, or failure due to issues such as financial difficulties or bankruptcy, issues relating to other customers such as regulatory or quality compliance issues, or other financial, legal, regulatory, or reputational issues. |
Moreover, to meet anticipated market demand, our suppliers may need to increase manufacturing capacity, which could involve significant challenges. This may require us and our suppliers to invest substantial additional funds
12
Table of Contents
and hire and retain the technical personnel who have the necessary experience. Neither we nor our suppliers may successfully complete any required increase to existing research, development, or manufacturing capacity in a timely manner, or at all.
For the quarter ended June 30, 2022, our cost of lab equipment, lab supplies, and lab services accounted for a significant portion of our total R&D expenses. In the event of price increases by suppliers, whether as a result of inflationary pressures or otherwise, we may attempt to pass the increased costs to our customers. However, we may not be able to raise the prices of our Foundry services sufficiently to cover increased costs resulting from increases in the cost of our materials and services, or the interruption of a sufficient supply of materials or services. As a result, materials and services costs, including any price increase for our materials and services, may negatively impact our business, financial condition, and results of operations.
Some of our suppliers and contract manufacturers are foreign entities. We may face disruptions due to the inability to obtain customs clearances in a timely manner or restrictions on shipping or international travel due to the COVID-19 pandemic. As a result of ongoing global supply chain challenges resulting in very long lead times for certain products and equipment, we may order in larger volumes in order to secure the supplies we require for our future operations, which may negatively impact our financial conditions, especially if we are unable to use the supplies ordered.
We use biological, hazardous, flammable and/or regulated materials that require considerable training, expertise and expense for handling, storage and disposal and may result in claims against us.
We work with biological and chemical materials that could be hazardous to human, animal, or plant health and safety or the environment. Our operations produce hazardous and biological waste products, and we largely contract with third parties for the disposal of these products. Federal, state, and local laws and regulations govern the use, generation, manufacture, storage, handling, and disposal of these materials and wastes. Compliance with applicable laws and regulations is expensive, and current or future laws and regulations may restrict our operations. If we do not comply with applicable laws and regulations, we may be subject to fines and penalties.
In addition, we cannot eliminate the risk of (a) accidental or intentional injury or (b) release, or contamination from these materials or wastes, which could expose us to liability. Furthermore, laws and regulations are complex, change frequently, and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance. Accordingly, in the event of release, contamination, or injury, we could be liable for the resulting harm or penalized with fines in an amount exceeding our resources and our operations could be suspended or otherwise adversely affected. These liabilities could also include regulatory actions, litigation, investigations, remediation obligations, damage to our reputation and brand, supplemental disclosure obligations, loss of customer, consumer, and partner confidence in the safety of our laboratory operations, impairment to our business, and corresponding fees, costs, expenses, loss of revenues, and other potential liabilities, as well as increased costs or loss of revenue or other harm to our business.
The release of GMOs or Genetically Modified Materials, whether inadvertent or purposeful, into uncontrolled environments could have unintended consequences, which may result in increased regulatory scrutiny and otherwise harm our business and financial condition.
The genetically engineered organisms and materials that we develop may have significantly altered characteristics compared to those found in the wild, and the full effects of deployment or release of our genetically engineered organisms and materials into uncontrolled environments may be unknown. In particular, such deployment or release, including an unauthorized release, could impact the environment or community generally or the health and safety of our employees, our customers’ employees, and the consumers of our customers’ products.
In addition, if a high profile biosecurity breach or unauthorized release of a biological agent occurs within our industry, our customers and potential customers may lose trust in the security of the laboratory environments in
13
Table of Contents
which we produce genetically modified organisms (“GMOs”) and genetically modified plant or animal cells and genetically modified proteins and biomaterials (collectively, “Genetically Modified Materials”), even if we are not directly affected. Any adverse effect resulting from such a release, by us or others, could have a material adverse effect on the public acceptance of products from engineered cells and our business and financial condition. Such a release could result in increased regulatory scrutiny of our facilities, platform, and programs, and could require us to implement additional costly measures to maintain our regulatory permits, licenses, authorizations and approvals. To the extent such regulatory scrutiny or changes impact our ability to execute on existing or new programs for our customers, or make doing so more costly or difficult, our business, financial condition, or results of operations may be adversely affected. In addition, we could have exposure to liability for any resulting harm, as well as to regulatory actions, litigation, investigations, remediation obligations, damage to our reputation and brand, supplemental disclosure obligations, loss of customer, consumer, and partner confidence in the safety of engineered cells materials and organisms, impairment to our business, and corresponding fees, costs, expenses, loss of revenues, and other potential liabilities, as well as increased costs or loss of revenue or other harm to our business.
We could synthesize DNA sequences or engage in other activity that inadvertently contravenes biosecurity requirements, or regulatory authorities could promulgate more far-reaching biosecurity requirements that our standard business practices cannot accommodate, which could give rise to substantial legal liability, impede our business, and damage our reputation.
The Federal Select Agent Program (“FSAP”) involves rules administered by the Centers for Disease Control and Prevention and the Animal and Plant Health Inspection Service that regulate possession, use, and transfer of biological select agents and toxins that have the potential to pose a severe threat to public, animal, or plant health or to animal or plant products. In accordance with the International Gene Synthesis Consortium’s (“IGSC”) Harmonized Screening Protocol for screening of synthetic DNA sequence orders, we follow biosafety and biosecurity industry practices and avoid DNA synthesis activities that implicate FSAP rules by screening synthetic DNA sequence orders against the IGSC’s Regulated Pathogen Database; however, we could err in our observance of compliance program requirements in a manner that leaves us in noncompliance with FSAP or other biosecurity rules. In addition, authorities could promulgate new biosecurity requirements that restrict our operations. One or more resulting legal penalties, restraints on our business or reputational damage could have material adverse effects on our business, financial condition, or results of operations.
Third parties may use our engineered cells, materials, and organisms and accompanying production processes in ways that could damage our reputation.
After our customers have received our engineered cells, materials, and organisms and accompanying production processes, we do not have any control over their use and our customers may use them in ways that are harmful to our reputation. In addition, while we have established a biosecurity program designed to comply with biosafety and biosecurity requirements and export control requirements in an effort to ensure that third parties do not obtain our engineered cells or other biomaterials for malevolent purposes, we cannot guarantee that these preventative measures will eliminate or reduce the risk of the domestic and global opportunities for the misuse or negligent use of our engineered cells materials, and organisms and production processes. Accordingly, in the event of such misuse or negligent use, our reputation, future revenue, and operating results may suffer.
International expansion of our business exposes us to business, regulatory, political, operational, financial, and economic risks associated with doing business outside of the United States.
We currently market our services and deliver our programs, materials, and processes outside of the United States and may market future offerings outside of the United States. We, and our suppliers, collaborators, and customers, currently conduct business outside of the United States. From time to time, our services may include the hiring or secondment of our employees outside the United States at third party facilities or require the hiring or secondment of foreign persons within our facilities, including as a result of foreign acquisitions. Accordingly,
14
Table of Contents
we are subject to a variety of risks inherent in doing business internationally, and our exposure to these risks will increase as we continue to expand our operations and customer base. These risks include:
| • | political, social and economic instability; |
| • | fluctuations in currency exchange rates; |
| • | higher levels of credit risk, corruption, and payment fraud; |
| • | enhanced difficulties of integrating any foreign acquisitions; |
| • | increased expenses and diversion of our management’s attention from advancing programs; |
| • | regulations that might add difficulties in repatriating cash earned outside the United States and otherwise prevent us from freely moving cash; |
| • | import and export controls and restrictions and changes in trade regulations; |
| • | compliance with the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, and similar laws in other jurisdictions; |
| • | multiple, conflicting and changing laws and regulations such as privacy, security and data use regulations, tax laws, tariffs, trade regulations, economic sanctions and embargoes, employment laws, anti-corruption laws, regulatory requirements, reimbursement or payor regimes and other governmental approvals, permits and licenses; |
| • | failure by us, our collaborators or our customers to obtain regulatory clearance, authorization or approval for the use of our services in various countries; |
| • | additional potentially relevant third-party patent rights; |
| • | complexities and difficulties in obtaining intellectual property protection and enforcing our intellectual property; |
| • | difficulties in staffing and managing foreign operations, including difficulties related to the increased operations, travel, infrastructure and legal compliance costs associated with international locations; |
| • | logistics and regulations associated with shipping chemicals, biomaterials and product samples, including infrastructure conditions and transportation delays; |
| • | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises, on demand and payment for our products and exposure to foreign currency exchange rate fluctuations; |
| • | natural disasters, political and economic instability, including wars (including the Russian invasion of Ukraine), terrorism and political unrest, the outbreak of disease, or public health epidemics, such as COVID-19, which could have an adverse impact on our employees, contractors, customers, partners, travel and the global economy; |
| • | breakdowns in infrastructure, utilities and other services; |
| • | boycotts, curtailment of trade and other business restrictions; and |
| • | the other risks and uncertainties described in this prospectus. |
Additionally, as part of our growth strategy, we will continue to evaluate potential opportunities for international expansion. Operating in international markets requires significant resources and management attention and will subject us to regulatory, economic and political risks in addition to those we face in the United States. However, our international expansion efforts may not be successful, which could limit the size of our market or the ability to provide services or programs internationally.
15
Table of Contents
In addition, due to potential costs from any international expansion efforts and potentially higher supplier costs outside of the United States, our international operations may operate with a lower margin profile. As a result, our margins may fluctuate as we expand our operations and customer base internationally.
Any of these factors could significantly harm our future international expansion and operations and, consequently, our revenue and results of operations.
Risks Related to Our Customers
We rely on our customers to develop, produce and manufacture products using the engineered cells and/or biomanufacturing processes that we develop. If these initiatives by our customers are not successful or do not achieve commercial success, or if our customers discontinue their development, production and manufacturing efforts using our engineered cells and/or biomanufacturing processes, our future financial position may be adversely impacted.
We operate as a platform company. As such, we rely on our customers to commercialize products that may be enabled by our engineered cells and/or biomanufacturing processes. A portion of the value in our customer collaborations is earned through downstream value sharing in the form of equity, royalty streams, or milestone payments. If our customers are not successful in bringing these products to market, the downstream portion of our value will be adversely impacted. Because we do not directly control manufacturing, product or downstream process development or commercialization, we have limited ability to impact the quality of our partners’ production processes and ultimate commercial success.
In addition, our customers may simply choose not to develop or commercialize a product we have enabled in which we are entitled to downstream value sharing. In our current relationships, we would have limited or no recourse to find alternative methods to monetize these products without the original customer. Because this industry is still nascent and the regulatory environment is evolving, we have limited historical information on the probability of commercial success for bioengineered products or biomanufacturing processes in the market and have limited ability to underwrite the likelihood that our customers will be able to create valuable products or processes in their market using the results of their programs with us. If we overestimate the probability of commercial success, the price of our common stock may be adversely impacted as a result of lower expectations for future cash flows from customer collaborations.
Our revenue is concentrated in a limited number of customers, some of which are related parties, and our revenue, results of operations, cash flows and reputation in the marketplace may suffer upon the loss of a significant customer.
We have derived, and may continue to derive, a significant portion of our revenue from a limited number of large customers. During the quarter ended June 30, 2022, three customers each represented more than 10% of our total revenue and cumulatively represented 41% of our total revenue. Due to the significant time required to acquire new customers, to plan and develop new programs for customers, and to satisfactorily execute on existing programs, the loss of any of these customers, or the loss of any other significant customer or a significant reduction in the amount of demand from a significant customer would adversely affect our revenue, results of operations, cash flows and reputation in the marketplace. There is always a risk that existing customers will not elect to do business with us in the future or will experience financial difficulties. If our customers experience financial difficulties or business reversals which reduce or eliminate the need for our services, they may be unable or unwilling to fulfill their contracts with us. There is also the risk that our customers will attempt to impose new or additional requirements on us that reduce the profitability of the services performed by us. Our customer concentration also increases the concentration of our accounts receivable and our exposure to payment defaults by key customers, which could expose us to substantial and potentially unrecoverable costs if we do not receive payment from key customers. Additionally, the loss of any significant customer could pose reputational harm to us and make it more challenging to acquire new customers.
16
Table of Contents
In addition, while our customer collaborations are typically multi-year, we generally do not require our customers to generate a minimum amount of annual demand and without such contracts, our customers are not obligated to use our services beyond the amounts they choose to incur. Our customers may choose to use fewer of our services depending on program progress, their own technological capabilities, market demand for their products and/or their own internal budget cycles. As a result, we cannot accurately predict our customers’ decisions to reduce or cease utilizing our services. Even where we enter into long-term contracts with our customers, there is no guarantee that such agreements will be negotiated on terms that are commercially favorable to us in the long-term. In addition, existing customers may choose to perform some or all of the services they expect from us internally, with another third-party partner or by using capabilities from acquisitions of assets.
In certain cases, our business partners may have discretion in determining when and whether to make announcements about the status of our collaborations, including about developments and timelines for advancing programs, and the price of our common stock may decline as a result of announcements of unexpected results or developments.
Generally, we and our customers must mutually agree on determining when and whether to make announcements about the status of our collaborations, including developments in our programs and timelines for commercialization of or improvements to products using engineered cells developed using our platform. However, in some cases our customers may report or otherwise may be obligated to disclose certain matters without our consent. Our partners may also wish to report such information more or less frequently than we intend to or may not wish to report such information at all. We or our partners may announce a collaboration or partnership even if there is no guarantee that we will recognize program fees. The price of our common stock may decline as a result of a public announcement of unexpected results or developments in our partnerships, or as a result of our partners not consenting to an announcement or withholding information.
Risks Related to the COVID-19 Pandemic
The recent COVID-19 pandemic and the global attempt to contain it may harm our business and results of operations.
The full impact of the continuing COVID-19 pandemic and related public health measures on our business will depend largely on future developments, including the duration and severity of the pandemic, which remains highly uncertain. Extraordinary actions have been taken by international, federal, state and local public health and governmental authorities to contain and combat the outbreak and spread of COVID-19 throughout the world, including travel bans, quarantines, capacity limitations at facilities, “stay-at-home” orders and similar mandates for many individuals to substantially restrict daily activities and for many businesses to curtail or cease normal operations. Additionally, our operations rely on the availability of laboratory scientists, engineers and facility, safety, quality and compliance personnel to work on-site. If a critical team member falls ill or needs to quarantine, or if a critical mass of our personnel falls ill or needs to quarantine, we may not be able to continue operations. The COVID-19 pandemic has also had an adverse effect on our ability to attract, recruit, interview and hire at the pace we would typically expect to support our rapidly expanding operations, as well as on our ability to build out facilities to accommodate expanding operations.
The COVID-19 pandemic has had, and is expected to continue to have, an adverse impact on our operations, particularly as a result of preventive and precautionary measures that we, other businesses, and governments are taking. For example, as part of these efforts and in accordance with applicable government directives, we initially temporarily suspended some programs at our facilities in Boston, Massachusetts in late March 2020. We have continued to operate within the rules and guidance applicable to our business during the pandemic, including by requiring physical distancing, quarantining our personnel and reducing capacity limits in our facilities, and operations at third-party facilities have been similarly impacted by governmental mandates and guidelines; however, a continuing implementation of these restrictions, or the implementation of additional restrictions,
17
Table of Contents
could further impact our ability to operate effectively and conduct ongoing R&D, laboratory operations, sales and marketing activities or other activities or operations, or lead to further compliance costs.
We have also incurred expenses associated with our efforts to accommodate personnel during the COVID-19 pandemic, including costs associated with the provision of COVID-19 testing to our personnel, safety accommodations, providing on-site amenities and enhanced on-site cleaning efforts, and we will continue to incur such expenses associated with our operations.
The pandemic has also caused substantial disruption in global supply chains. These interruptions may require us to suspend operations or delay programs. If we continually delay programs with existing customers, we may be in breach of our contracts with existing customers or customers may decide to cease doing business with us or have decreased demand for our products. We may also experience a slow-down in our pipeline of new programs or a termination of existing programs if our customers or potential customers face disruptions during the pandemic. Difficulties and delays such as those we have experienced and may experience in the future may prevent us from meeting our operating and financial goals, both in general and within our targeted timelines, and may cause our revenues and operating results to fluctuate from period to period.
Uncertainty regarding the ongoing demand and/or capacity (including capacity at third party clinical testing laboratories) of our COVID-19 individual and pooled sample tests could materially adversely affect our business.
Our biosecurity offering consists of COVID-19 testing programs, which are subject to inherent risks of commercial viability, such as demand for tests, price or market share erosion due to competition and the duration of the COVID-19 pandemic. We are in a highly competitive market – many companies have launched or are seeking to launch COVID-19 testing products and many of these companies already have an existing commercial and technical infrastructure to market and commercialize such offerings. We have limited experience marketing or commercializing diagnostic or pooled sample testing programs and may not be able to sufficiently support operations with our current base of personnel or recruit enough personnel to effectively commercialize COVID-19 testing programs, particularly during a pandemic, at which time the pipeline for experienced personnel will be in high demand. Moreover, as vaccines for COVID-19 and at-home or over-the-counter COVID-19 tests become more widely available, and as infection rates decrease, demand for COVID-19 testing may also decrease.
Our COVID-19 testing business relies heavily on the adoption of pooled testing in schools, which may be hesitant to adopt COVID-19 testing without positive support from parents or teachers. Although we make test validation results and protocols available to parents and teachers, they may not trust the accuracy of the tests or may have concerns about how the tests are performed, how samples are used or tracked and whether appropriate privacy measures are being taken with respect to individually identifiable health information, including genetic information. The ability for schools to pay for COVID-19 testing relies heavily on the availability of federal, state or local funding for testing. If such funding is depleted, discontinued or otherwise becomes unavailable, or if there are restrictions on the use of such funding for our pooled sample test offerings, our COVID-19 testing business may not be commercially viable. Our COVID-19 testing business is subject to seasonality concerns as the demand for COVID-19 testing in schools is diminished during summer vacations, as well as other school breaks. In addition, as a result of the recent FDA emergency use authorization of a COVID-19 vaccine for children five through eleven years of age, the demand for COVID-19 testing in schools could diminish significantly or be eliminated.
Creating the commercial and technical infrastructure to test on a mass scale is expensive. We may also be limited in our ability to scale up based on expense or unavailability of the required materials, equipment, personnel and infrastructure necessary to deliver diagnostic or pooled sample tests on a mass scale. We may not be able to recover our investment expenses with sufficient revenue generated by our diagnostic and pooled sample testing efforts.
18
Table of Contents
Our ability to commercialize our testing programs is also subject to regulatory or governmental controls, decisions or actions. If the U.S. Department of Health and Human Services (“HHS”) terminates its Declaration Justifying Emergency Use of Medical Countermeasures because the circumstances justifying emergency use no longer exist and, if the third-party COVID-19 tests that are used in our testing services are not able to obtain premarket approval, clearance or other marketing authorization from the U.S. Food and Drug Administration (“FDA”), we may be unable to market or distribute these COVID-19 tests, fulfill our contractual testing requirements or generate revenues from our test offerings. We may also experience price erosion if federal or state governments implement price controls or if the price of supply inputs increase.
Finally, the sale of each test is dependent on the supply of the appropriate collection devices authorized for use with the COVID-19 tests we utilize in our testing programs. Disruptions in this supply chain will have a material adverse effect on our ability to sell tests.
Uncertainty regarding the sales and delivery of our COVID-19 individual and pooled sample tests could materially adversely affect our business.
Although we have partnerships with third party clinical testing laboratories to support a high volume of pooled sample testing for COVID-19 nationally, pooled testing has not yet been adopted by all states nor have we established partnerships with clinical testing laboratories in all states. We are continuing to develop processes to scale capacity of COVID-19 pooled sample collection and testing. However, we can give no assurance that we will be able to successfully scale the pooled sample collection and test capacity or that we will be able to establish or maintain the collaborative third party relationships that support such testing capacity. In addition, even if we are able to scale to high volume testing nationwide, there can be no assurance that the testing capacity will be used.
We may be subject to tort liability if the COVID-19 tests we utilize in our testing programs provide inaccurate results.
The Public Readiness and Emergency Preparedness Act (the “PREP Act”) provides immunity for manufacturers, distributors, program planners, qualified persons, and their officials, agents, and employees from certain claims under state or federal law for a “loss” arising out of the administration or use of a “covered countermeasure” in the United States. Distributors are certain persons or entities engaged in the distribution of drugs, biologics, or devices. Program planners include persons who supervise or administer a program with respect to the administration, distribution, provision, or use of a Covered Countermeasure (as defined in the PREP Act). Covered Countermeasures include security countermeasures and “qualified pandemic or epidemic products,” including products intended to diagnose or treat pandemic or epidemic disease, such as COVID-19 diagnostic tests, as well as treatments intended to address conditions caused by such products. Covered Countermeasures must also be approved, cleared, or authorized for emergency use, or otherwise authorized for investigational use, by the FDA in order to be considered Covered Countermeasures under the PREP Act.
For these immunities to apply, the Secretary of HHS must issue a declaration in cases of public health emergency or “credible risk” of a future public health emergency. On March 10, 2020, the Secretary of HHS issued a declaration under the PREP Act and has issued subsequent amendments thereto to provide liability immunity for activities related to certain countermeasures against the ongoing COVID-19 pandemic.
We act as the authorized distributor of certain third-party COVID-19 tests and collection kits that have received Emergency Use Authorization (“EUA”) and supervise testing programs for our COVID-19 testing customers. There can be no assurance that our test distribution and program planning activities regarding these programs would be covered under the provisions of the PREP Act. Also, there can be no assurance that the U.S. Congress will not act in the future to reduce coverage under the PREP Act or to repeal it altogether.
Furthermore, some of the third-party tests used as part of our pooled testing program are not covered by an EUA and, at this time, we do not believe that such testing services, administration, or program planning related to our
19
Table of Contents
pooled testing program will qualify for PREP Act immunity. If product liability lawsuits are brought against us in connection with allegations of harm connected to our COVID-19 testing services, we may incur substantial liabilities and may be required to limit our testing services. The PREP Act is a complex law with limited judicial precedent, and thus even for the third-party COVID-19 tests and collection kits used in our testing services that are subject to EUAs, we may have to expend significant time and legal resources to obtain dismissal of a lawsuit on the basis of PREP Act immunity.
If we cannot successfully defend ourselves against claims that our COVID-19 testing services caused injuries and if we are not entitled to immunity under the PREP Act, or the U.S. Congress limits or eliminates coverage under the PREP Act, or if the liability protections under the PREP Act are not adequate to cover all claims, we may incur substantial liabilities. Regardless of merit or eventual outcome, product liability claims may result in decreased demand for our services, injury to our reputation, costs to defend litigation, loss of revenue, and substantial money awards to customers.
We are dependent on our relationships with our telehealth partner to provide healthcare services, and our business would be adversely affected if those relationships were disrupted.
Our contractual relationships with our telehealth partner who provides physician authorization for COVID-19 diagnostic and screening testing may implicate certain state laws in the United States that generally prohibit non-physician entities from practicing medicine, exercising control over physicians or engaging in certain practices such as fee-splitting with physicians. There can be no assurance that these laws will be interpreted in a manner consistent with our practices or that other laws or regulations will not be enacted in the future that could have a material and adverse effect on our business, financial condition and results of operations. Regulatory authorities, state medical boards of medicine, state attorneys general and other parties, including our telehealth partner, may assert that we are engaged in the prohibited corporate practice of medicine, and/or that its arrangements with its telehealth partner constitutes unlawful fee-splitting. If a state’s prohibition on the corporate practice of medicine or fee-splitting law is interpreted in a manner that is inconsistent with our practices, we would be required to restructure or terminate our relationship with our telehealth partner to bring our activities into compliance with such laws. A determination of non-compliance, or the termination of or failure to successfully restructure these relationships could result in disciplinary action, penalties, damages, fines, and/or a loss of revenue, any of which could have a material and adverse effect on our business, financial condition and results of operations. State corporate practice of medicine doctrines and fee-splitting prohibitions also often impose penalties on healthcare professionals for aiding the corporate practice of medicine, which could discourage our telehealth partner from providing services to us.
Risks Related to the Synthetic Biology Industry
Rapidly changing technology and emerging competition in the synthetic biology industry could make the platform, programs, and products we and our customers are developing obsolete or non-competitive unless we continue to develop our platform and pursue new market opportunities.
The synthetic biology industry is still emerging and is characterized by rapid and significant technological changes, frequent new product introductions and enhancements, and evolving industry demands and standards. Our future success will depend on our ability to sign and initiate new programs that address the evolving needs of our customers on a timely and cost-effective basis, to advance existing programs and to pursue new market opportunities that develop as a result of technological and scientific advances. Additionally, our customers may face significant competition or other risks which may adversely impact our business and results of operations.
There are a number of companies in the broader synthetic biology industry, and our future success will depend on our ability to maintain a competitive position with respect to technological advances. Technological development by others may result in our platform becoming obsolete. Our ability to compete successfully will depend on our ability to develop proprietary technologies that enable our customers to develop products using our platform in a
20
Table of Contents
manner that is either less expensive, faster, superior or otherwise differentiated from what a competitor’s technologies and products might enable. If we are unable to continue to successfully advance our platform or the services it provides at scale, or if our customers are unable to commercialize the products or processes made or improved upon by using our platform, our business and results of operations will be adversely impacted.
Due to the significant lead time involved in launching a new program or developing a new product or process using our platform, our customers are required to make a number of assumptions and estimates regarding the commercial feasibility of a new program, including assumptions and estimates regarding the size of an emerging product category and demand for those end-products and processes which will use our technology, the ability to scale-up manufacturing processes to produce a product on a commercial scale, the ability to penetrate that emerging product category, customer adoption of a downstream product, the existence or non-existence of products being simultaneously developed by competitors, potential market penetration and obsolescence, planned or unplanned. As a result, it is possible that we may commence a new program with a customer who wishes to develop a product or process that has been displaced by the time of launch, addresses a market that no longer exists or is smaller than previously thought, that end-consumers do not like or otherwise is not competitive at the time of launch, in each case, after the incurrence of significant opportunity costs on our part to develop such product. The ultimate success of the products developed by our customers using our services may be dependent on the success of other markets in which we or our customers do not operate in or have knowledge or expertise or which, in each case, may not reach the size anticipated by us or our customers or may be replaced by another emerging product category or eliminated entirely.
The market, including customers and potential investors, may be skeptical of our ability to deliver on programs because they are based on a relatively novel and complex technology.
The market, including customers and potential investors, may be skeptical of the viability and benefits of bioengineered products as well as our enabling abilities, including our platform and programs, because they are based on a relatively novel approach and the adoption of complex technology. There can be no assurance that our platform and programs will be understood, approved, or accepted by customers, regulators and potential investors or that we will be able to sell our services profitably at competitive prices and with features sufficient to establish demand.
In addition, in order for novel products from our programs to be successfully commercialized, support from the entire relevant supply chain is needed. Relationships with all parts of the supply chain are important in order to gain visibility into market trends and feature and specification requirements and in order to ensure customers are able to successfully manufacture their products, obtain regulatory approval and gain access to key distribution channels. If we are unable to convince these potential customers, their suppliers, or the consumers who purchase products containing or made or developed using engineered cells and/or biomanufacturing processes, of the utility and value of such products or that such products are superior to the products they currently use, we will not be successful in entering these markets and our business and results of operations will be adversely affected. If potential investors are skeptical of the success of our platform or cell programs, our ability to raise capital and the value of our common stock may be adversely affected.
Ethical, legal and social concerns about GMOs and Genetically Modified Materials and their resulting products could limit or prevent the use of products or processes using our technologies, limit public acceptance of such products or processes and limit our revenues.
Our technologies and the technologies of our customers involve the use of genetically modified cells, organisms and biomaterials, including, without limitation, GMOs, genetically modified microorganisms (“GMMs”), Genetically Modified Materials and their respective products. The use, production and marketing of Genetically Modified Materials, are subject to laws and regulations in many countries, some of which are new and some of which are still evolving. In the United States, the FDA, the Environmental Protection Agency (“EPA”) and the U.S. Department of Agriculture (“USDA”) are the primary agencies that regulate the use of GMOs, GMMs and
21
Table of Contents
potential products derived from GMOs or GMMs. If regulatory approval of the Genetically Modified Materials or resulting products is not secured, our business operations, financial condition and our ability to grow as a business could be adversely affected. We expect to encounter regulations regarding Genetically Modified Materials in most, if not all, of the countries in which our customers may seek to establish production capabilities or sell their products and the scope and nature of these regulations will likely be different from country to country. Governmental authorities could, for safety, social or other purposes, impose limits on, or implement regulation of, the use, production or marketing of Genetically Modified Materials. If our customers cannot meet the applicable requirements in other countries in which they intend to produce or sell their products, or if it takes longer than anticipated to obtain such approvals, our business could be adversely affected.
In addition, public perception regarding the safety and environmental hazards of, and ethical concerns over, Genetically Modified Materials or the processes used to create them, including gene editing or gene regulating technologies, could influence public acceptance of our and our customers’ technologies, products, and processes. For instance, certain advocacy groups engage in efforts that include regulatory legal challenges and labeling campaigns for genetically modified products, as well as application of pressure to consumer retail outlets seeking a commitment not to carry genetically modified foods. These groups in the past have pressured retail food outlets and grocery store chains to publicly state that they will not carry genetically modified foods and have pressured food brands to publicly state that they will not use ingredients produced by genetically modified microbes. In addition, certain labeling-related initiatives have heightened consumer awareness of GMOs, which may make consumers less likely to purchase products containing GMO ingredients, which could have a negative impact on the commercial success of our customers’ products and programs. These concerns could result in increased expenses, regulatory scrutiny, delays or other impediments to our programs. The subject of Genetically Modified Materials has received negative publicity, which has aroused public debate. This adverse publicity has led to, and could continue to lead to, greater regulation and trade restrictions on imports of Genetically Modified Materials or their resulting products. In addition, with the acquisitions of Dutch DNA Biotech B.V. and FGen AG (“FGen”), we are expanding into the European Union market, which has increased government regulation and scrutiny over genetically modified products. There is a risk that products produced using our technologies could cause adverse health effects or other adverse events, which could also lead to negative publicity, regulatory action or private litigation. If we are unable to overcome the ethical, legal and social concerns relating to genetic engineering, our programs could face increased expenses, regulatory scrutiny, delays or other impediments to deliver our programs or the commercialization of resulting products and processes.
Finally, the COVID-19 pandemic may increase biosecurity concerns by public and/or governmental stakeholders regarding genetic engineering technologies and risks around engineered viruses, microbes and organisms. Such concerns, restrictions, or governmental restrictions could limit the use of Genetically Modified Materials in our customers’ products, which could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Intellectual Property
If we are unable to obtain, maintain and defend patents protecting our intellectual property, our competitive position will be harmed.
Our success depends in part on our ability to obtain and maintain intellectual property protection for our proprietary technologies. We protect our proprietary technologies through patents and trade secrets, both of which entail risk. If we are unable to obtain, maintain or protect intellectual property rights related to our technology, or if our intellectual property rights are inadequate, our competitive position, business, financial conditions, results of operations and prospects may be harmed.
Because of the volume and nature of our inventions, patent protection may not be practicable, available, or appropriate for some aspects of our proprietary technologies. While we own patents and pending patent applications in the United States and in foreign jurisdictions, these applications do not ensure the protection of
22
Table of Contents
our intellectual property. There may be prior art of which we are not aware. Additionally, obtaining, maintaining, defending and enforcing patents is costly, time consuming and complex, and we may not be able to file and prosecute all necessary or desirable patent applications, or maintain and enforce any patents that may issue from such patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our technologies before it is too late to obtain patent protection. Although we enter into confidentiality agreements with parties who have access to confidential or patentable aspects of our R&D output, such as our employees, collaborators, consultants, advisors and other third parties, any of these parties may breach the agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection.
Further, pending applications may not be issued or may be issued with claims significantly narrower than we currently seek. Patents for which claims have been allowed may be successfully challenged and invalidated. Unless and until our pending applications issue, their protective scope is impossible to determine and, even after issuance, their protective scope may be limited.
Recent changes in patent law have made patents covering life science inventions more difficult to obtain and enforce. Further legislative changes or changes in the interpretation of existing patent law could increase the uncertainty and cost surrounding the prosecution of our owned patent applications and the maintenance, enforcement or defense of our owned patents. The Leahy-Smith America Invents Act (“the Leahy-Smith Act”) included changes that affect the way patent applications are prosecuted; redefine prior art; enable third-party submission of prior art to the United States Patent and Trademark Office (“USPTO”) during patent prosecution; and provide cost-effective avenues for competitors and other third parties to challenge the validity of patents at USPTO-administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Thus, the Leahy-Smith Act and its continued implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Other changes in the law may further detract from the value of life science patents and facilitate challenges to our patents. In some cases, we use genetic sequence information from naturally occurring organisms, which may not be patentable. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection for naturally occurring sequences and for inventions based on the observation and exploitation of natural phenomena. These decisions have weakened the rights of patent owners in certain situations. The U.S. Court of Appeals for the Federal Circuit has also issued a series of rulings that create obstacles to the patenting of groups of genetic sequences that share functional characteristics, making it more difficult to obtain claims to certain genetic constructs, particularly antibodies. These changes in the law have created uncertainty with respect to the validity and enforceability of patents covering natural and engineered sequences. Depending on future actions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a further material adverse effect on our patent rights and our ability to protect, defend and enforce our patent rights in the future.
Further, the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in the United States and abroad. An adverse determination in any such challenge could result in loss of exclusivity, or patent claims being narrowed, invalidated or held unenforceable, in whole or in part. Any of these results could limit our ability to stop others from using or commercializing similar or identical technology to compete directly with us. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us. Such proceedings also may result in substantial cost and require significant time from our scientists and management, even if the eventual outcome is favorable to us.
The laws of some foreign countries do not protect intellectual property rights to the same extent as do the laws of the United States or may apply different rules concerning the assignment of intellectual property rights. Many
23
Table of Contents
companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. We may encounter similar difficulties, particularly as we expand to work with foreign employees and contractors and expand our collaboration activities into foreign markets. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents by foreign holders and, in some cases, do not favor the enforcement of patents at all, particularly patents in the life sciences. This could make it difficult for us to stop the infringement of our patents. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business and could be unsuccessful.
Reductions in the scope or enforceability of our patent protection may adversely affect our customers’ ability to commercialize their products and may thus reduce our downstream value from royalties, equity, or commercial milestone payments.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position will be harmed.
Because patent protection may not be available or appropriate for significant aspects of the technology we are developing, our success may depend in large part on our proprietary information, including genetic and other chemical and biological data, processes, know-how, and other trade secrets developed over years of R&D, some of which are embodied in proprietary software. We rely heavily on trade secret protections, especially in cases where we believe patents or other forms of registered intellectual property protection may not be appropriate or obtainable. However, trade secrets are difficult to protect. The secrecy of the Company’s trade secrets must be maintained for them to retain their status and protection as trade secrets. While we strive to protect the secrecy of our trade secrets and other proprietary information, including by requiring our employees, customers, consultants, and contractors to enter into confidentiality agreements and instituting multilayered protections covering our digital environment and biomaterials, we may not be able to adequately protect our trade secrets or other proprietary information. We cannot guarantee that we have entered into such agreements with every party that may have or has had access to our trade secrets, biomaterials or proprietary technology and processes. Further, despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches.
We seek to preserve the integrity and confidentiality of our information by maintaining physical security of our premises and physical and electronic security of our information technology systems, but it is possible that these security measures could be breached. We also rely on systems provided by third parties, which may suffer security breaches or incidents. Such security breaches may be inadvertent or may come about due to intentional misconduct or other malfeasance or by human error or technical malfunctions, including those caused by hackers, employees, contractors, or vendors. It may be difficult or impossible to recover trade secrets or other confidential information once it is hacked, and hackers may operate from jurisdictions that will not cooperate with such efforts. Enforcing any claim that a third party unlawfully obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts in some jurisdictions are less willing or unwilling to protect trade secrets even when a hacker or thief can be identified.
Our competitors may lawfully obtain or independently develop knowledge that is equivalent to one or more of our trade secrets. Were they to do so, we would be unable to prevent them from using that independently developed knowledge. Such a competitor could claim that we had learned the trade secret from them and bring an action against us on that basis. If any of our trade secrets were to be disclosed to or independently developed by a competitor or other third party, our competitive position could be materially and adversely harmed. Moreover, a competitor could file for patent protection covering intellectual property that we have chosen to protect as a trade secret. In such a case, we might be restricted or excluded from using that intellectual property even if we had developed it before our competitor did.
Our facilities hold large collections of microbial strains, cell lines and other biomaterials. Failure to implement adequate controls and protections, failure to implement adequate disposal procedures, unauthorized visitors in the
24
Table of Contents
labs, or customers’ failure to adequately protect biological materials can put us and our customers at risk of losing valuable assets through negligence or theft and enabling the use of those lost materials by our competitors. While we believe that we take reasonable measures to protect the security of biomaterials owned by us or our customers, it is possible that our security controls and practices may not prevent unauthorized or other improper access to such genetic material. Any unauthorized access, acquisition, use, destruction, or release of the GMOs we engineer could result in our having exposure to significant liability under our contracts, as well as to regulatory actions, litigation, investigations, remediation obligations, damage to our reputation and brand, supplemental disclosure obligations, loss of customer, consumer, or partner confidence in the security of our platform, impairment to our business, and corresponding fees, costs, expenses, loss of revenues, and other potential liabilities.
Our customers sometimes provide organisms, genetic material and/or data to us in connection with our collaborations. In the event that we fail to protect customer materials or data or inadvertently use such materials or data for unauthorized purposes, we could be liable to our customers under trade secret laws or contractual provisions.
There could be unintended consequences to the environment generally or the health and safety of our employees or the public as a result of an unauthorized release of Genetically Modified Materials into uncontrolled environments. In addition, if a biosecurity breach or unauthorized release of genetic material were to occur within our industry, our customers and potential customers might lose trust in the security of the laboratory environments in which we produce GMOs, even if we are not directly affected. Any adverse effect resulting from such a release, by us or others, could have a material adverse effect on the public acceptance of our products and business and our financial condition. Such a release could result in enhanced regulatory activity, and we could have exposure to liability for any resulting harm.
We may be subject to claims challenging the inventorship or ownership of our patents, biomaterials and other intellectual property.
Certain of our employees, consultants and contractors were previously employed at universities or other software or biotechnology companies, including our competitors or potential competitors. Additionally, some of our consultants or contractors may have ongoing relationships with universities. Although we try to ensure that our employees, consultants and contractors do not use the intellectual property of others in their work for us, we may be subject to claims that these individuals or other contractors have used or disclosed intellectual property, including trade secrets or other proprietary information, of another. Litigation may result from these claims.
While it is our policy to require that our employees, consultants and contractors who may be involved in the development of intellectual property execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our intellectual property assignment agreements with them may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property. Such claims could have a material adverse effect on our business, financial condition, results of operations and prospects.
If we are unsuccessful in litigating any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could have a material adverse effect on our competitive business position and prospects. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from such third party to use or commercialize our technology or products, which license might not be available on commercially reasonable terms, or at all. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our management and employees.
The life science academic and research community has abided by norms of free exchange of biomaterials, but recently, norms have begun to change so that parties may assert ownership and control over biomaterials that
25
Table of Contents
they permitted to be freely disseminated in the past. Thus, despite our best efforts to confirm our right to use biomaterials in our possession, we may use organisms that we believe to be free of encumbrance that are, in fact, subject to claims of title by others. In such a situation, litigation may be required to clear title, if it can be cleared at all. Similarly, we may be subject to claims that we have used biomaterials obtained from licensors or repositories for unauthorized purposes, or purposes not consistent with the licensing terms of the providing organization.
We may become involved in lawsuits or other enforcement proceedings to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and potentially unsuccessful.
Competitors and other third parties may infringe or otherwise violate our issued patents or other intellectual property. In addition, our patents may become involved in inventorship, ownership, or priority disputes. We may also become subject to claims by collaboration partners that intellectual property or biomaterials that we believe to be owned by us are actually owned by them. Any litigation concerning any of these issues would be expensive, time consuming and uncertain. There can be no assurances that we would prevail in any suit brought by us or against us by third parties, or successfully settle or otherwise resolve those claims. Significant litigation would have substantial costs, even if the eventual outcome were favorable to us, and would divert management’s attention from our business objectives.
Under certain circumstances, we may share or lose rights to intellectual property developed under U.S. federally funded research grants and contracts.
Some of our inventions, data, or other intellectual property have been or may be developed during the course of research funded by the U.S. government. The U.S. government may have the right to take title to government-funded inventions if we fail to disclose the inventions to the government in a timely manner or fail to file a patent for the intellectual property within specified time limits. Further, in consequence of our receiving government funding, the U.S. government may have certain rights to intellectual property that we use in our platform or programs pursuant to the Bayh-Dole Act of 1980, as amended (the “Bayh-Dole Act”). Under the Bayh-Dole Act, U.S. government rights in certain “subject inventions” developed under a government-funded program may include a non-exclusive, irrevocable worldwide license to use inventions for any governmental purpose. In some circumstances, the U.S. government may acquire unlimited rights in data we generate. In addition, the U.S. government has the right to require us, or an assignee or exclusive licensee to U.S. Government-funded inventions, to grant licenses to any of these inventions to the government or a third party if the government determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; (iii) government action is necessary to meet requirements for public use under federal regulations; or (iv) the right to use or sell such inventions is exclusively licensed to an entity within the United States and substantially manufactured outside the United States without the U.S. government’s prior approval. Additionally, we may be restricted from granting exclusive licenses for the right to use or sell such inventions unless the licensee agrees to comply with relevant Bayh-Dole Act restrictions (e.g., manufacturing substantially all of the invention in the United States) and reporting requirements. In addition, the U.S. government may acquire title in any country in which a patent application is not filed. Certain technology and inventions are also subject to transfer restrictions during the term of these agreements with the U.S. government and for a period thereafter. These restrictions may limit sales of products or components, transfers to foreign subsidiaries for the purpose of the relevant agreements, and transfers to certain foreign third parties. If any of our intellectual property becomes subject to any of the rights or remedies available to the U.S. government or third parties pursuant to the Bayh-Dole Act, this could impair the value of our intellectual property and could adversely affect our business.
The use of digital genetic sequence information may be subject to the Nagoya Protocol, which could increase our costs and adversely affect our business.
The Nagoya Protocol is a supplemental agreement to the Convention on Biological Diversity (“CBD”). The Nagoya Protocol is designed to provide for equitable sharing of benefits arising from the utilization of genetic
26
Table of Contents
resources and traditional knowledge. Under the Nagoya Protocol, countries possessing genetic resources (“source countries”) are tasked with setting up procedures and institutional infrastructure for researchers to obtain prior informed consent, both from the source country and from any relevant indigenous or traditional communities, for biological research. Many have been slow to adopt workable institutions permitting the rational negotiation of benefit-sharing agreements. Many source countries are now asserting that the use of digital genetic sequence information is subject to the constraints of the Nagoya Protocol or similar national- or local-level benefit-sharing requirements. It is unclear whether this position will ultimately be adopted or what the implications of such adoption might be. It is unclear what a source country might assert if we used genetic sequences (i) extracted by a third party from a natural resource that was removed from its source country before that source country ratified the CBD or signed the Nagoya Protocol (ii) extracted by a third party and uploaded to public sequence databases after the source country ratified the CBD; (iii) in a heterologous host organism; or (iv) as a base for further engineering, so that the sequence we use no longer conforms to the natural sequence on which it was based.
We make extensive use of public and proprietary sequence databases to support our work. While we undertake efforts to identify and comply with laws and international protocols relating to the use of genetic resources, the uncertainty surrounding the use of digital sequence information and the lack of workable institutions in many source countries for the efficient negotiation of benefit-sharing agreements may limit our use or cause uncertainty in our use of certain sequences that we obtain from public access databases or natural sources. New financial obligations may arise regarding our use of sequence information. Customers that must certify their compliance with Nagoya Protocol obligations may be reluctant to do business with us unless we engage in expensive and time-consuming benefit-sharing negotiations with source countries of publicly available genetic sequences. These changes could increase our R&D costs and adversely affect our business, financial condition, and results.
Third party patents may limit our freedom to operate in certain areas, which may adversely affect our business.
There may be patents that affect our freedom to operate in certain areas, and we may as a result choose to design around or license such patents from third parties. If we must spend significant time and money designing around or licensing patents held by others, our business and financial prospects may be harmed. We may be restricted from carrying out certain operations in our Foundry, or we may be limited in our ability to design new products for our customers. We may become subject to claims by third parties alleging that we are infringing, misappropriating, or otherwise violating their intellectual property rights.
If we are sued for infringing intellectual property rights of third parties, such litigation could be costly and time consuming and could prevent or delay us from using our platform and technologies.
Any litigation arising from any dispute relating to the intellectual property of third parties would be expensive, time-consuming, and uncertain. There can be no assurance that we would prevail in any such dispute. Parties making claims against us might be able to obtain injunctive or other relief, which could block our or our customers’ ability to develop, commercialize and sell products or use our technologies, and could result in the award of substantial damages against us, including treble damages, attorney’s fees, costs and expenses if we were found to have willfully infringed. In the event of a successful claim against us, we or our customers might be required to pay damages and ongoing royalties, and obtain licenses from third parties, or be prohibited from selling certain products or using certain technologies. We may not be able to obtain these licenses on acceptable or commercially reasonable terms, if at all. In addition, we or our customers could encounter delays in product or service introductions while we attempt to develop alternative designs or redesign existing products or technologies to avoid or resolve these claims. Our loss in any lawsuit or failure to obtain a license could prevent us from using our platform and technologies. Such a loss or failure could materially affect our business and reputation. Any litigation pertaining to these issues would have substantial costs, even if the eventual outcome were favorable to us, and would divert management’s attention from our business objectives.
27
Table of Contents
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, diluted, tarnished, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential collaborators or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement, dilution or tarnishment claims brought by owners of other trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade names, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely affect our business, financial condition, results of operations and prospects.
Any claims or lawsuits relating to infringement of intellectual property rights brought by or against us will be costly and time consuming and may adversely affect our business, financial condition and results of operations.
Any of the risks identified above could result in significant litigation. In addition to the specific litigation-related risks identified above, litigation of any kind carries certain inherent risks. Because of the substantial amount of discovery required in connection with litigation in U.S. courts, there is a risk that some of our confidential information could be compromised in the discovery process. There could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on our share price.
Further, our agreements with some of our customers, suppliers or other entities require us to defend or indemnify these parties if they become involved in infringement claims that target our products, services or technologies, or in certain other situations. If we must defend or indemnify third parties, we could incur significant costs and expenses that could adversely affect our business, operating results or financial condition.
Intellectual property rights do not necessarily address all potential threats to our business.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. The following examples are illustrative:
| • | we may choose not to file a patent in order to maintain certain intellectual property as trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property; |
| • | others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our owned or licensed intellectual property rights; |
| • | the patents of others may harm our business; |
| • | we might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own; |
| • | we might not have been the first to file patent applications covering certain of our inventions; and |
| • | issued patents that we hold rights to may fail to provide us with any competitive advantage, or may be held invalid or unenforceable, including as a result of legal challenges by our competitors. |
Should any of these events occur, they could harm our business, financial condition, results of operations and prospects.
28
Table of Contents
Intellectual property disputes of third parties and customers could have a material adverse effect on our business, financial condition, and results.
We rely, and expect to continue to rely on, certain capital equipment, machinery, consumables, reagents, software, services and intellectual property that we purchase or license from third parties for use in our operations, platform, products, services and offerings. We cannot be certain that our vendors, suppliers, and licensors are not infringing upon the intellectual property rights of others or that they have sufficient rights to the third-party technology used in our business in all jurisdictions in which we may operate. Disputes with any of these third parties over uses or terms could result in the payment of additional royalties or penalties by us, cancellation or non-renewal of the underlying license, termination of supplies or rights to use, or litigation. In the event that we cannot resolve issues of this kind, we may be required to discontinue or limit our use of the operations, platform, products, services or offerings that include or incorporate the licensed intellectual property. Any such discontinuation or limitation could have a material and adverse impact on our business, financial condition and results of operation.
Our customers may become involved in intellectual property disputes with third parties that are related or unrelated to any products or services we have supplied or rendered to them. Such disputes could result in a customer being unable to market its products, thus depriving us of license, milestone, or other revenues. Such deprivation could have a material adverse impact on our financial condition and results.
Our use of “open-source” software could negatively affect our ability to market or provide our services and could subject us to possible litigation.
We have used “open-source” software in connection with the development and deployment of our software platform, and we expect to continue to use open-source software in the future. Open-source software is licensed by its authors or other third parties under open-source licenses, which in some instances may subject us to certain unfavorable conditions, including requirements that we offer our products that incorporate the open-source software for no cost, that we make publicly available all or part of the source code for any modifications or derivative works we create based upon, incorporating or using the open-source software, or that we license such modifications or derivative works under the terms of the particular open-source license.
Companies that incorporate open-source software into their products have, from time to time, faced claims challenging the use of open-source software and compliance with open-source license terms. We could be subject to similar suits by parties claiming ownership of what we believe to be open-source software or claiming noncompliance with open-source licensing terms. While we monitor our use of open-source software and try to ensure that none is used in a manner that would require us to disclose our proprietary source code or that would otherwise breach the terms of an open-source agreement, we cannot guarantee that we will be successful, that all open-source software is reviewed prior to use in our platform, that our developers have not incorporated open- source software into our products that we are unaware of or that they will not do so in the future.
Furthermore, there are an increasing number of open-source software license types, almost none of which have been interpreted by U.S. or foreign courts, resulting in a dearth of guidance regarding the proper legal interpretation of such licenses. As a result, there is a risk that open-source software licenses could be construed in a manner that imposes unanticipated conditions or restrictions on our ability to market or provide our products and services. If we are held to have breached or failed to fully comply with all the terms and conditions of an open-source software license, we could face infringement claims or other liability, or be required to seek costly licenses from third parties to continue providing our offerings on terms that are not economically feasible, if at all, to re-engineer all or a portion of our platform, to discontinue or delay the provision of our offerings if re-engineering could not be accomplished on a timely basis or to make generally available, in source code form, our proprietary code. Further, in addition to risks related to license requirements, use of certain open-source software carries greater technical and legal risks than does the use of third-party commercial software. For example, open-source software is generally provided without any support or warranties or other contractual protections
29
Table of Contents
regarding infringement or the quality of the code, including the existence of security vulnerabilities. To the extent that our platform depends upon the successful operation of open-source software, any undetected errors or defects in open-source software that we use could prevent the deployment or impair the functionality of our systems and injure our reputation. In addition, the public availability of such software may make it easier for others to compromise our platform. Any of the foregoing risks could materially and adversely affect our business, financial condition and results of operations.
Risks Related to Personnel, IT and Physical Infrastructure
Loss of key personnel, including our founders and senior executives, and/or failure to attract, train and retain additional key personnel could delay our cell engineering programs, harm our platform development efforts, limit our biosecurity offerings, and harm our ability to meet our business objectives, particularly given the substantial investment required to train certain of our employees.
Our business involves complex, global operations across a variety of markets and requires a management team and employee workforce that is knowledgeable in the many areas in which we operate. Our future success depends upon our ability to attract, train, retain and motivate highly qualified management, scientific, engineering, information technology, operations, business development and marketing personnel, among others. In addition, the market for qualified personnel is very competitive because of (a) the limited number of people available who have the necessary technical skills and understanding of our technology and products and (b) the nature of our industry which requires certain of our technical personnel to be on-site in our facilities. We compete for qualified technical personnel with other life sciences and information technology companies, as well as academic institutions and research institutions in the markets in which we operate, including Boston, Massachusetts, Cambridge, Massachusetts, Emeryville, California, Utrecht, Netherlands, Basel, Switzerland and Melbourne, Australia. In addition, as we add international operations, we will increasingly need to recruit qualified personnel outside the United States. However, doing so may also require us to comply with laws to which we are not currently subject, which could cause us to allocate or divert capital, personnel and other resources from our organization, which could adversely affect our business, financial condition, results of operations, prospects and reputation. Establishing international operations and recruiting personnel has and may continue to be impacted by COVID-19 travel and operational restrictions. Our senior leadership team is critical to our vision, strategic direction, platform development, operations and commercial efforts. Our employees, including members of our leadership team, could leave our company with little or no prior notice and would be free to work for a competitor. We also do not maintain “key person” life insurance on any of our employees. The departure of one or more of our founders, senior leadership team members or other key employees could be disruptive to our business until we are able to hire qualified successors.
Our continued platform development, growth and commercial success depends, in part, on recruiting and retaining highly-trained personnel across our various target industries and markets with the necessary background and ability to develop and use our platform and to effectively identify and sell to current and new customers. New hires require significant training and, in most cases, take significant time before they achieve full productivity. Our failure to successfully hire and integrate these key personnel into our business could adversely affect our business. To attract top talent, we believe we will need to offer competitive compensation and benefits packages, including equity incentive programs, which may require significant investment. If we are unable to offer competitive compensation this may make it more difficult for us to attract and retain key employees. Moreover, if the perceived value of our equity awards declines, it may adversely affect our ability to attract and retain key employees. If we do not maintain the necessary personnel to accomplish our business objectives, we may experience staffing constraints that adversely affect our ability to support our programs and operations.
In addition, some of our personnel are qualified foreign nationals whose ability to live and work in the U.S. is contingent upon the continued availability of appropriate visas and whose ability to work on some of our technologies may require the procurement of appropriate export licenses. Due to the competition for qualified personnel in the key markets in which we operate, we expect to continue to utilize foreign nationals to fill part of
30
Table of Contents
our recruiting needs. As a result, changes to United States immigration policies have and could further restrain the flow of technical and professional talent into the United States and adversely affect our ability to hire and retain qualified personnel.
Our business and results of operations are dependent on adequate access to laboratory and office space and suitable physical infrastructure, including electrical, plumbing, HVAC and network infrastructure, to conduct our operations. Our headquarters and laboratories are located in a flood zone in Boston’s Seaport District. If we are unable to access enough space or we experience failures of our physical infrastructure, our business and results of operations could be adversely affected.
Our business depends on providing customers with technical services. In order to properly conduct our business, we need access to sufficient laboratory space and equipment to perform the activities necessary to advance and complete our programs. Additionally, we need to ensure that our laboratories and corporate offices remain operational at all times, which includes maintaining suitable physical infrastructure, including electrical, plumbing and HVAC, logistics and transportation systems and network infrastructure. We lease our laboratories and office spaces and we rely on the landlords for basic maintenance of our leased laboratories and office buildings. If one of our landlords has not maintained a leased property sufficiently, we may be forced into an early exit from the facility, which could be disruptive to our business. Furthermore, we may continue to acquire laboratories not built by us in order to sufficiently scale and expand our output capacity. If we discover that these buildings and their infrastructure assets are not in the condition we expected when they were acquired, we may be required to incur substantial additional costs to repair or upgrade the laboratories.
Problems in and around one or more of our laboratories or corporate offices, whether or not within our control, could result in service interruptions or significant infrastructure or equipment damage. These could result from numerous factors, including:
| • | human error; |
| • | equipment failure; |
| • | physical, electronic and cybersecurity breaches; |
| • | fire, earthquake, hurricane, flood, tornado and other natural disasters; |
| • | extreme temperatures; |
| • | flood and/or water damage; |
| • | fiber cuts; |
| • | power loss; |
| • | terrorist acts, including acts of bioterrorism; |
| • | sabotage, vandalism and cyberattacks; and |
| • | local epidemics or global pandemics such as the COVID-19 pandemic. |
We have timeline obligations to certain customers with respect to their programs. As a result, service interruptions or significant equipment damage in our laboratories could result in difficulty maintaining program timelines for these customers and potential claims related to such failures. Because the services we provide in our laboratories are critical to many of our customers’ businesses, service interruptions or significant equipment damage in our laboratories could also result in lost revenue or other indirect or consequential damages to our customers. We cannot guarantee that a court would enforce any contractual limitations on our liability in the event that one of our customers brings a lawsuit against us as a result of a problem at one of our laboratories and we may decide to reach settlements with affected customers irrespective of any such contractual limitations. In addition, any loss of service, equipment damage or inability to meet our service obligations could reduce the confidence of our customers and could consequently impair our ability to obtain and retain customers, which would adversely affect both our ability to generate revenues and our operating results.
31
Table of Contents
Furthermore, we are dependent upon internet service providers, telecommunications carriers and other website operators, some of which have experienced significant system failures and electrical outages in the past.
Our customers may, in the future, experience difficulties due to system failures unrelated to our systems and offerings. If, for any reason, these providers fail to provide the required services, our business, financial condition and results of operations could be materially and adversely impacted.
Risks Related to Financial Reporting
We rely on our customers, joint venturers, equity investees and other third parties to deliver timely and accurate information in order to accurately report our financial results in the time frame and manner required by law.
We need to receive timely, accurate, and complete information from a number of third parties in order to accurately report our financial results on a timely basis. If the information that we receive is not accurate, our consolidated financial statements may be materially incorrect and may require restatement. Although we have audit rights with these parties, performing such an audit could be expensive and time consuming and may not be adequate to reveal any discrepancies in a time frame consistent with our reporting requirements. As a result, we may have difficulty completing accurate and timely financial disclosures, which could have an adverse effect on our business.
We use estimates in determining the fair value of certain assets and liabilities. If our estimates prove to be incorrect, we may be required to write down the value of these assets or write up the value of these liabilities, which could adversely affect our financial position.
Our ability to measure and report our financial position and operating results is influenced by the need to estimate the fair value of an asset or liability. Fair value is estimated based on a hierarchy that maximizes the use of observable inputs and minimizes the use of unobservable inputs. Observable inputs are inputs that reflect the assumptions that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the reporting entity. Unobservable inputs are inputs that reflect the reporting entity’s own assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. We estimate the impact or outcome of future events on the basis of information available at the time of the financial statements. An accounting estimate is considered critical if it requires that management make assumptions about matters that were highly uncertain at the time the accounting estimate was made. If actual results differ from management’s judgments and assumptions, then they may have an adverse impact on our results of operations and cash flows.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
We have incurred net losses since our inception and we may never achieve or sustain profitability. Generally, for U.S. federal income tax purposes, net operating losses incurred will carry forward. However, net operating loss carryforwards generated prior to January 1, 2018 are subject to expiration for U.S. federal income tax purposes. As of December 31, 2021, we had federal net operating loss carryforwards of approximately $665.2 million, of which $139.2 million begin to expire in 2029. We have approximately $526.0 million of federal net operating losses as of December 31, 2021 that can be carried forward indefinitely. As of December 31, 2021, we had state net operating loss carryforwards of approximately $529.3 million, of which $485.9 million begin to expire in 2029. We have approximately $43.4 million of state net operating losses as of December 31, 2021 that can be carried forward indefinitely. As of December 31, 2021, we had federal research and development tax credit carryforwards of approximately $23.3 million which begin to expire in 2029. As of December 31, 2021, we also had state research and development and investment tax credit carryforwards of approximately $18.0 million which begin to expire in 2030.
Under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change by value in its equity ownership by certain
32
Table of Contents
stockholders over a three-year period, the corporation’s ability to use its pre-ownership change net operating loss carryforwards and other pre-ownership change tax attributes, such as research tax credits, to offset its post- ownership change income or taxes may be limited. Similar provisions of state tax law may also apply to limit the use of our state net operating loss carryforwards and other state tax attributes. We have not performed an analysis to determine whether our past issuances of stock and other changes in our stock ownership may have resulted in one or more ownership changes. If it is determined that we have in the past experienced an ownership change, or if we undergo one or more ownership changes as a result of future transactions in our stock, which may be outside our control, then our ability to utilize our net operating loss carryforwards and other tax attributes may be materially limited. As a result, even if we earn taxable income, we may be unable to use a material portion of our net operating loss carryforwards and other tax attributes, which could adversely affect our future cash flows. There is also a risk that regulatory changes, such as suspensions on the use of net operating losses or other unforeseen reasons, may result in our existing net operating loss carryforwards expiring or otherwise becoming unavailable to offset future taxable income. For these reasons, we may not be able to utilize a material portion of our net operating loss carryforwards and other tax attributes even if we attain profitability.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
As a public reporting company, we are subject to the rules and regulations established by the SEC and the New York Stock Exchange (“NYSE”). These rules and regulations require, among other things, that we establish and periodically evaluate procedures with respect to our internal control over financial reporting. Reporting obligations as a public company are likely to place a considerable strain on our financial and management systems, processes and controls, as well as on our personnel, including senior management. In addition, as a public company, we are required to document and test our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 so that our management can certify as to the effectiveness of our internal control over financial reporting. Management’s initial certification under Section 404 of the Sarbanes-Oxley Act of 2002 will be required with our Annual Report on Form 10-K for the year ending December 31, 2022. In support of such certifications, we are required to document and make significant changes and enhancements, including potentially hiring additional personnel, to our internal control over financial reporting. Likewise, our independent registered public accounting firm is not required to attest to the effectiveness of our internal control over financial reporting until our first annual report is required to be filed with the SEC following the date we are no longer an EGC. At such time as we are required to obtain auditor attestation, if we then have a material weakness, we would receive an adverse opinion regarding our internal control over financial reporting from our independent registered accounting firm.
To achieve compliance with Section 404 within the prescribed period, we will need to continue to dedicate internal resources, including hiring additional financial and accounting personnel and potentially engaging outside consultants. During our evaluation of our internal control, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. We have identified material weaknesses in our internal control environment in the past and cannot provide assurances that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, or results of operations. If we are unable to conclude that our internal control over financial reporting is effective or if our independent registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of shares of our common stock could decline, and we could be subject to sanctions or investigations by NYSE, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
33
Table of Contents
We have identified material weaknesses in our internal control over financial reporting in the past. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. If we are unable to remediate these material weaknesses, or if we identify additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls, we may not be able to accurately or timely report our financial condition or results of operations, which may adversely affect investor confidence in us and, as a result, our stock price.
Our cash and cash equivalents could be adversely affected if the financial institutions in which we hold our cash and cash equivalents fail.
We regularly maintain cash balances at third-party financial institutions in excess of the Federal Deposit Insurance Corporation insurance limit. While we monitor the cash balances in our operating accounts on a daily basis and adjust the balances as appropriate, these balances could be impacted, and there could be a material adverse effect on our business, if one or more of the financial institutions with which we deposit cash fails or is subject to other adverse conditions in the financial or credit markets. To date, we have experienced no loss or lack of access to our invested cash or cash equivalents; however, we can provide no assurance that access to our invested cash and cash equivalents will not be impacted by adverse conditions in the financial and credit markets.
Risks Related to Governmental Regulation and Litigation
Failure to comply with federal, state, local and international laws and regulations could adversely affect our business and our financial condition.
A variety of federal, state, local and international laws and regulations govern certain aspects of our business. For example, we maintain a registration from the U.S. Drug Enforcement Administration (“DEA”) for the research of certain controlled substances and permits from the Boston Public Health Commission to conduct work with recombinant DNA. Some of our programs or products made or developed using our engineered cells and/or biomanufacturing processes are subject to regulations, including those promulgated by the FDA, DEA, EPA or USDA. Products utilized in our COVID-19 testing services are subject to regulations promulgated by the FDA, the Centers for Medicare and Medicaid Services, and certain state governments. In addition, we are subject to laws relating to, among other things, anti-bribery, insider trading, sourcing of biological materials and data privacy. The legal and regulatory requirements that apply to our business may be interpreted and applied in a manner that is inconsistent from one jurisdiction to another or may conflict with other rules or our practices. As a result, our practices may not comply, or may not comply in the future with all such laws, regulations, requirements and obligations. Any failure, or perceived failure, by us to comply with any federal, state, local or international laws, regulations, industry self-regulatory principles, industry standards or codes of conduct, regulatory guidance, orders to which we may be subject or other legal obligations could adversely affect our reputation, brand and business, and may result in claims, proceedings or actions against us by governmental entities or others or other liabilities or require us to change our operations. We may also be contractually required to indemnify and hold harmless third parties from the costs or consequences of non-compliance with any laws, regulations or other legal obligations.
We may also become subject to increasing regulation in the future as we expand our business. We have limited experience operating a business located outside of Massachusetts. As we continue to expand our operations and offerings domestically and globally, we will have to expend significant management and financial resources to maintain compliant practices in those locations. Non-compliance could lead to litigation, which would require substantial management and financial resources.
We may incur significant costs complying with environmental, health and safety laws and regulations, and failure to comply with these laws and regulations could expose us to significant liabilities.
We use hazardous chemical and biological materials in our business and are subject to a variety of federal, state, local and international laws and regulations governing, among other matters, the use, generation, manufacture,
34
Table of Contents
transportation, storage, handling, disposal of, and human exposure to these materials, including regulation by governmental regulatory agencies, such as the Occupational Safety and Health Administration and the EPA. We have incurred, and will continue to incur, capital and operating expenditures and other costs in the ordinary course of our business in complying with these laws and regulations.
Although we have implemented safety procedures for storing, handling and disposing of these materials and waste products in an effort to comply with these laws and regulations, we cannot be sure that our safety measures will be compliant or capable of eliminating the risk of injury or contamination from the generation, manufacturing, use, storage, transportation, handling, disposal of and human exposure to hazardous materials and/or flammable chemicals. Failure to comply with environmental, health and safety laws could subject us to liability and resulting damages. There can be no assurance that violations of environmental, health and safety laws will not occur as a result of human error, accident, equipment failure, contamination, intentional misconduct or other causes. Compliance with applicable environmental laws and regulations may be expensive, and the failure to comply with past, present or future laws could result in the imposition of fines, regulatory oversight costs, third party property damage, product liability and personal injury claims, investigation and remediation costs, the suspension of production or a cessation of operations, and our liability may exceed our total assets. Liability under environmental laws can be imposed for the full amount of damages without regard to comparative fault for the investigation and cleanup of contamination and impacts to human health and for damages to natural resources. Contamination at properties we may own and operate and at properties to which we send hazardous materials, may result in liability for us under environmental laws and regulations.
Our business and operations may be affected by other new environmental, health and safety laws and regulations, which may require us to change our operations, or result in greater compliance costs and increasing risks and penalties associated with violations, which could impair our research, development or production efforts and harm our business.
If we fail to comply with healthcare and other governmental regulations, we could face substantial penalties and our business, financial condition and results of operations could be adversely affected.
Our business activities may be subject to regulation and enforcement by the FDA, U.S. Department of Justice, HHS, Office of Inspector General, and other federal and state governmental authorities. Although our offerings are not currently billed to any third-party payor, including any commercial payor or government healthcare program, we may, in the future, submit claims for our COVID-19 testing services to third-party payors, including government healthcare programs. If we submit claims to third-party payors, such activity will expand the scope of federal and state healthcare laws applicable to us.
Federal and state healthcare laws and regulations that may affect our ability to conduct business include, without limitation:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving or paying any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, lease, order, or arranging for or recommending the purchase, lease or order of, any item or service, for which payment may be made, in whole or in part, under federal healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| • | the federal physician self-referral prohibition, commonly known as the Stark Law, which prohibits a physician, in the absence of an applicable exception, from making a referral for certain designated health services covered by the Medicare or Medicaid program, including clinical laboratory services, if the physician or an immediate family member of the physician has a financial relationship with the entity providing the designated health services. The Stark Law also prohibits the entity furnishing the designated health services from billing, presenting or causing to be presented a claim for the designated health services furnished pursuant to the prohibited referral; |
35
Table of Contents
| • | the federal civil false claims laws, including without limitation the federal False Claims Act (which can be enforced through “qui tam,” or whistleblower actions, by private citizens on behalf of the federal government), and civil monetary penalties laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, false or fraudulent claims for payment of government funds, or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government. In addition, the government may assert that a claim including items and services resulting from a violation of the federal Anti-Kickback Statute or Stark Law constitutes a false or fraudulent claim for purposes of the civil False Claims Act; |
| • | the Eliminating Kickbacks in Recovery Act (“EKRA”), which created a new federal crime for knowingly and willfully: (1) soliciting or receiving any remuneration in return for referring a patient to a recovery home, clinical treatment facility, or laboratory; or (2) paying or offering any remuneration to induce such a referral or in exchange for an individual using the services of a recovery home, clinical treatment facility, or laboratory. Unlike the Anti-Kickback Statute, EKRA is not limited to services reimbursable under a government health care program, but instead extends to all services reimbursed by “health care benefit programs”; |
| • | the healthcare fraud statutes under the Health Information Technology for Economic and Clinical Health Act (“HIPAA”), which impose criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement, in connection with the delivery of, or payment for healthcare benefits, items or services by a healthcare benefit program, which includes both government and privately funded benefits programs. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| • | federal consumer protection and unfair competition laws, which broadly regulate platform activities and activities that potentially harm consumers; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback, self-referral, and fee-splitting, and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers and self-pay patients. |
Because of the breadth of these laws and the narrowness of available statutory and regulatory exemptions, exceptions, and safe harbors, it is possible that some of our activities could be subject to challenge under one or more of such laws. We may face claims and proceedings by private parties, and claims, investigations and other proceedings by governmental authorities, relating to allegations that our business practices do not comply with current or future laws or regulations involving applicable fraud and abuse or other healthcare laws and regulations, and it is possible that courts or governmental authorities may conclude that we or any of our partners have not complied with them, or that we may find it necessary or appropriate to settle any such claims or other proceedings. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any federal or state laws described above or any other current or future fraud and abuse or other healthcare laws and regulations that apply to us, we may be subject to claims and proceedings by private parties, investigations and other proceedings by governmental authorities, as well as penalties, including significant criminal, civil and administrative penalties, damages and fines, disgorgement, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of noncompliance with these laws or regulations, imprisonment for individuals and exclusion from participation in government programs, such as Medicare and Medicaid, as well as contractual damages and reputational harm. We could also be required to curtail or cease our operations. In addition, if any customers, healthcare professionals we engage,
36
Table of Contents
laboratory partners or other entities with whom we do business are found not to be in compliance with applicable laws, they may be subject to the same criminal, civil or administrative sanctions, including exclusion from government-funded healthcare programs. Any of the foregoing could seriously harm our business and financial results.
We may become subject to the comprehensive laws and rules governing billing and payment, noncompliance with which could result in non-payment or recoupment of overpayments for our services or other sanctions.
We may, in the future, submit claims for our COVID-19 testing services to third-party payors. Payors typically have differing and complex billing and documentation requirements. If we fail to comply with these payor-specific requirements, we may not be paid for our services or payment may be substantially delayed or reduced. Numerous state and federal laws would also apply to our claims for payment, including but not limited to (i) “coordination of benefits” rules that dictate which payor must be billed first when a patient has coverage from multiple payors, (ii) requirements that overpayments be refunded within a specified period of time, (iii) “reassignment” rules governing the ability to bill and collect professional fees on behalf of other providers, (iv) requirements that electronic claims for payment be submitted using certain standardized transaction codes and formats, and (v) laws requiring all health and financial information of patients to be maintained in a manner that complies with stringent security and privacy standards.
Audits, inquiries and investigations from government agencies and health network partners can occur from time to time in the ordinary course of our business, and could result in costs to us and a diversion of management’s time and attention. New regulations and heightened enforcement activity also could negatively affect our cost of doing business and our risk of becoming the subject of an audit or investigation. If we bill for our service in the future, our failure to comply with rules related to billing or adverse findings from audits by government and private payors could result in, among other penalties, non-payment for services rendered or recoupments or refunds of amounts previously paid for such services. We cannot predict whether any future audits, inquiries or investigations, or the public disclosure of such matters, likely would negatively impact our business, financial condition, results of operations, cash flows and the trading price of our securities. See also “Risk Factors—Risks Related to Governmental Regulation and Litigation—If we fail to comply with healthcare and other governmental regulations, we could face substantial penalties and our business, financial condition and results of operations could be adversely affected.”
We and our laboratory partners are subject to a variety of laboratory testing standards, compliance with which is an expensive and time-consuming process, and any failure to comply could result in substantial penalties and disruptions to our business.
We and the third-party laboratories that we partner with are subject to the Clinical Laboratory Improvement Amendments of 1988 (“CLIA”). CLIA is a federal law that regulates clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease. CLIA requires virtually all laboratories to be certified by the federal government and mandates compliance with various operational, personnel, facilities administration, quality and proficiency testing requirements depending on the level of complexity for which the laboratory is certified. CLIA certification is also a prerequisite to be eligible to bill state and federal healthcare programs, as well as many private third-party payors, for laboratory testing services. Our partner laboratories hold CLIA certifications for high complexity testing, which mandate compliance with various operational, personnel, facilities administration, quality and proficiency testing requirements depending on the level of complexity for which the laboratory is certified. In addition, we hold a CLIA high complexity testing certification and perform certain CLIA-waived tests on behalf of our clients, which subjects us directly to certain CLIA requirements. Sanctions for failure to comply with CLIA requirements may include suspension, revocation, or limitation of a laboratory’s CLIA certificate, as well as the imposition of significant fines or criminal penalties. Any sanction imposed under CLIA, its implementing regulations, or state or foreign laws or regulations governing licensure, or our partner laboratories’ failure to renew a CLIA certificate, a state or foreign license, or accreditation, could have a material adverse effect on our business.
37
Table of Contents
In addition, our partner laboratories and our laboratories holding CLIA Certificates of Waiver are subject to state laws and regulations governing laboratory licensure. Some states have enacted state licensure laws that are more stringent than CLIA. Our ability to successfully deploy COVID-19 testing at large scale may be adversely impacted if our partner laboratories do not maintain the required regulatory licensure and operate in accordance with CLIA standards. In certain markets such as California, New York, and Pennsylvania, we or our partner laboratories may also need to obtain and maintain additional licensure from such states. It is uncertain that our partner laboratories will be granted such licensure and, in such case, we cannot offer testing to patients located in those states, which could limit our ability to offer testing on a wide scale.
It is possible that additional states may enact laboratory licensure requirements in the future, which could further limit our ability to expand our services.
We rely on third-party laboratories in the conduct of our biosecurity business offering. If any of our partners cease working with us, or face supply chain disruptions or other difficulties, our business could be harmed. Specifically, if any of our partners were to lose or fail to obtain or renew their CLIA certifications or state laboratory licenses, whether as a result of a revocation, suspension or limitation, such laboratories would no longer be able to run the COVID-19 tests we offer to our customers, and our ability to successfully deploy a COVID-19 pooled sample testing program nationwide may be adversely impacted.
The testing industry is subject to complex and costly regulation and if government regulations are interpreted or enforced in a manner adverse to us, we may be subject to enforcement actions, penalties, exclusion, and other material limitations on our operations.
We offer COVID-19 testing services by partnering with third-party laboratories, diagnostic test manufacturers and manufacturers of collection kits, which are subject to extensive and frequently changing federal, state and local laws and regulations governing various aspects of our business, including significant governmental certification and licensing regulations. New laws, regulations and judicial decisions, or new interpretations of existing laws, regulations and decisions, may also limit our potential revenues, and we may need to revise our R&D or commercialization programs. The costs of defending claims associated with violations, as well as any sanctions imposed, could significantly adversely affect our financial performance.
We are required to comply with federal and state genetic testing and privacy laws. We have measures in place to collect clinical data and genetic and other biological samples, and disclose test results, from subjects who have provided appropriate informed consents. However, informed consents could be challenged in the future, and those informed consents could prove invalid, unlawful or otherwise inadequate for our purposes. Any legal challenges could consume our management and financial resources.
Current regulations governing the testing services we offer are shifting and in some cases unclear. In addition, our laboratory partners may be unsuccessful in validating, or obtaining or maintaining authorizations for, the tests we rely on to provide our COVID-19 testing services. If any third-party manufacturers or laboratories offering tests that we use in our testing services are deemed by the FDA or other regulatory authorities to have violated applicable law or if the tests or test components are marketed, processed or distributed in violation of applicable law, we may be subject to enforcement action or litigation, or we may be required to find alternative tests to support our testing services, which could increase our costs and prevent us from successfully commercializing our COVID-19 testing services.
In addition, we are required to comply with applicable FDA regulations with respect to our distribution of certain COVID-19 diagnostic test kits and collection kits, including, for certain kits, compliance with applicable terms and conditions of an EUA. Such conditions may include requirements related to collection of information on the performance of the product, reporting of adverse events, recordkeeping requirements, and labeling and promotional activities. To the extent that we market or promote third-party tests or test kits outside of the uses authorized for these products or in a false or misleading manner, the tests or collection kits could be considered misbranded or adulterated and in violation of applicable law.
38
Table of Contents
Advertising for any of the tests or collection kits we distribute or the testing services we offer is also subject to regulation by the Federal Trade Commission (“FTC”), under the Federal Trade Commission Act (“FTC Act”). The FTC may take enforcement action for advertising claims that are not adequately substantiated or that are false or misleading. Violations of applicable FDA requirements could result in enforcement actions, such as warning or “untitled” letters, revocation of EUAs, seizures, injunctions, civil penalties and criminal prosecutions and fines, and violation of the FTC Act could result in injunctions and other associated remedies, all of which could have a material adverse effect on our business. Most states also have similar regulatory and enforcement authority for laboratory testing and distribution of related collection kits. For example, many state laws require us to hold a specific form of license to distribute COVID-19 diagnostic test kits and collection kits into such states. These requirements vary from one state to another and frequently change. Complying with state laws and regulations may subject us to similar risks and delays as those we could experience under federal regulation.
We are subject to federal and state laws and regulations governing the protection, use, and disclosure of health information and other types of personal information, and our failure to comply with those laws and regulations or to adequately secure the information we hold could result in significant liability or reputational harm.
Numerous state and federal laws, regulations, standards and other legal obligations, including consumer protection laws and regulations, which govern the collection, dissemination, use, access to, confidentiality, security and processing of personal information, including health-related information, could apply to our operations or the operations of our partners. For example, HIPAA imposes privacy, security and breach notification obligations on certain healthcare providers, health plans, and healthcare clearinghouses, known as covered entities, as well as their business associates that perform certain services that involve creating, receiving, maintaining or transmitting individually identifiable health information for or on behalf of such covered entities, and their covered subcontractors. HIPAA requires covered entities and business associates to develop and maintain policies with respect to the protection of, use and disclosure of protected health information (“PHI”), including the adoption of administrative, physical and technical safeguards to protect such information, and certain notification requirements in the event of a breach of unsecured PHI. If in the future we engage in certain types of standard electronic transactions involving payors, including billing the Medicare or Medicaid programs or commercial health plans, we will be subject to HIPAA as a “covered entity.” We are currently subject to HIPAA as a “business associate” because we perform certain services involving the use or disclosure of PHI on behalf of covered entity customers with respect to our COVID-19 testing service offerings. Implementation of the infrastructure necessary to meet HIPAA standards requires substantial investment. Being subject to HIPAA as a covered entity or business associate exposes us to significant fines and penalties, including criminal fines and penalties.
Additionally, under HIPAA, covered entities must report breaches of unsecured PHI to affected individuals without unreasonable delay, not to exceed 60 days following discovery of the breach by a covered entity or its agents. Notification also must be made to the HHS Office for Civil Rights and, in certain circumstances involving large breaches, to the media. Business associates must report breaches of unsecured PHI to covered entities within 60 days of discovery of the breach by the business associate or its agents. A non-permitted use or disclosure of PHI is presumed to be a breach under HIPAA unless the Covered Entity or Business Associate establishes that there is a low probability the information has been compromised consistent with requirements enumerated in HIPAA.
Entities that are found to be in violation of HIPAA as the result of a breach of unsecured PHI, a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. HIPAA also authorizes state attorneys general to file suit on behalf of their residents. Courts may award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to sue us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI.
39
Table of Contents
Even when HIPAA or a state law does not apply, according to the FTC, violating consumers’ privacy rights or failing to take appropriate steps to keep consumers’ personal information secure may constitute unfair and/or deceptive acts or practices in violation of Section 5(a) of the FTC Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities.
Several states have enacted privacy laws governing the use and disclosure of health information, such as the California Confidentiality of Medical Information Act; these laws are not preempted by HIPAA to the extent they are more stringent than HIPAA. Such laws and regulations will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our partners. Further, in recent years, there have been a number of well-publicized data breaches involving the improper dissemination of personal information of individuals both within and outside of the healthcare industry. Laws in all 50 states require businesses to provide notice to individuals whose personally identifiable information has been disclosed as a result of a data breach. The laws are not consistent, and compliance in the event of a widespread data breach is costly. States are also constantly amending existing laws, and creating new data privacy and security laws, requiring attention to frequently changing regulatory requirements. For example, the California Consumer Privacy Act of 2018 (“CCPA”) went into effect on January 1, 2020. The CCPA creates new transparency requirements and grants California residents several new rights with respect to their personal information. Failure to comply with the CCPA may result in, among other things, significant civil penalties and injunctive relief, or potential statutory or actual damages. On November 3, 2020, California voters passed a ballot initiative for the California Privacy Rights Act (“CPRA”), which will significantly expand the CCPA. Most CPRA provisions will take effect on January 1, 2023, though the obligations will apply to any personal information collected after January 1, 2022. The CPRA will impose additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. It will also create a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. The majority of the provisions will go into effect on January 1, 2023, and additional compliance investment and potential business process changes may be required. Similar laws have been proposed or passed in other states, including the Virginia Consumer Data Protection Act, which will take effect on January 1, 2023. We will need to invest substantial resources in putting in place policies and procedures to comply with these evolving state laws.
As our operations and business grow, we may become subject to or affected by new or additional data protection laws and regulations and face increased scrutiny or attention from regulatory authorities. For example, the European Union General Data Protection Regulation (“GDPR”), which went into effect in May 2018, imposes strict requirements for processing the personal data of individuals within the European Economic Area. Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater. Among other requirements, the GDPR regulates transfers of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data, including the United States, and the efficacy and longevity of current transfer mechanisms between the EU and the United States remains uncertain. For example, in 2016, the EU and United States agreed to a transfer framework for data transferred from the EU to the United States, called the Privacy Shield, but the Privacy Shield was invalidated in July 2020 by the Court of Justice of the European Union. Further, from January 1, 2021, companies have to comply with the GDPR and also the United Kingdom GDPR (the “UK GDPR”), which, together with the amended UK Data Protection Act 2018, retains the GDPR in UK national law. The UK GDPR mirrors the fines under the GDPR, i.e., fines up to the greater of €20 million (£17.5 million) or 4% of global turnover. The relationship between the United Kingdom and the European Union in relation to certain aspects of data protection law remains unclear, and it is unclear how United Kingdom data protection laws and regulations will develop in the medium to longer term. On June 28, 2021, the European Commission adopted an adequacy decision in favor of the United Kingdom,
40
Table of Contents
enabling data transfers from EU member states to the United Kingdom without additional safeguards. However, the United Kingdom adequacy decision will automatically expire in June 2025 unless the European Commission renews or extends that decision and remains under review by the Commission during this period. These changes may lead to additional costs and increase our overall risk exposure.
Although we work to comply with applicable laws, regulations and standards, contractual obligations and other legal obligations, these requirements are evolving and may be modified, interpreted and applied in an inconsistent manner from one jurisdiction to another, and may conflict with one another or other legal obligations with which Ginkgo must comply. Recently, there has been an increase in public awareness of privacy issues in the wake of revelations about the data-collection activities of various government agencies and in the number of private privacy-related lawsuits filed against companies. Any failure or perceived failure by us or our employees, representatives, contractors, consultants, collaborators, or other third parties to comply with such requirements or adequately address privacy and security concerns, even if unfounded, could result in additional cost and liability to us, damage our reputation, and adversely affect our business and results of operations.
We have pursued in the past and may pursue additional U.S. Government contracting and subcontracting opportunities in the future and as a U.S. Government contractor and subcontractor, we are subject to a number of procurement rules and regulations.
We have entered into agreements with governmental entities and contractors in the past to serve as a U.S. government contractor or subcontractor and may do so again in the future. U.S. government procurement contractors and subcontractors must comply with specific procurement regulations and other requirements. These requirements, although customary in U.S. government contracts, could impact our performance and compliance costs, including by limiting or delaying our ability to share information with business partners, customers and investors. The U.S. government has in the past and may in the future demand contract terms that are less favorable than standard arrangements with private sector customers and may have statutory, contractual, or other legal rights to terminate contracts with us for convenience or for other reasons. Generally, U.S. government contracts contain provisions permitting unilateral termination or modification, in whole or in part, at the government’s convenience. Under general principles of government contracting law, if the government terminates a contract for convenience, the government contractor may recover only its incurred or committed costs, settlement expenses and profit on work completed prior to the termination. If the government terminates a contract for default, the government contractor is entitled to recover costs incurred and associated profits on accepted items only and may be liable for excess costs incurred by the government in procuring undelivered items from another source. Any termination for default may also adversely affect our ability to contract with other government customers, as well as our reputation, business, financial condition and results of operations. In addition, changes in U.S. government budgetary priorities could lead to changes in the procurement environment, affecting availability of U.S. government contracting, subcontracting or funding opportunities, which could lead to modification, reduction or termination of our U.S. government contracts or subcontracts. If and to the extent such changes occur, they could impact our results and potential growth opportunities.
Furthermore, our U.S. government contracts grant the government the right to use technologies developed by us under the government contract or the right to share data related to our technologies, for or on behalf of the government. Under our government contracts, we may not be able to limit third parties, including our competitors, from accessing certain of these technology or data rights, including intellectual property, in providing products and services to the government.
In addition, failure by us, our employees, representatives, contractors, partners, agents, intermediaries, other customers or other third parties to comply with these regulations and requirements could result in reductions of the value of contracts, contract modifications or termination, claims for damages, refund obligations, the assessment of civil or criminal penalties and fines, loss of rights in our intellectual property and temporary suspension or permanent debarment from government contracting, all of which could negatively impact our results of operations and financial condition. Any such damages, penalties, disruptions or limitations in our
41
Table of Contents
ability to do business with the public sector could result in reduced sales of our products, reputational damage, penalties and other sanctions, any of which could harm our business, reputation and results of operations.
We are engaged in certain research activities involving controlled substances, including cannabinoids and other chemical intermediates, the making, use, sale, importation, exportation, and distribution of which may be subject to significant regulation by the DEA and other regulatory agencies.
We are engaged in certain research activities involving the development of microbes designed to generate cannabinoids, their precursors and other chemical intermediaries, some of which may be regulated as controlled substances in the United States. Controlled substances are subject to state, federal, and foreign laws and regulations regarding their manufacture, use, sale, importation, exportation, and distribution. Among other things, controlled substances are regulated under the federal Controlled Substances Act of 1970 and implementing regulations of the DEA. The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have no established medicinal use and may generally not be marketed or sold in the United States. Schedule I substances are subject to the most stringent controls and Schedule V the least controls of the five schedules, based on their relative risk of abuse.
Cannabinoids are naturally occurring compounds found in the cannabis plant. The cannabis plant and its derivatives are highly regulated by the DEA and the USDA. Specifically, marihuana, which is defined as all parts of the plant Cannabis sativa L., whether growing or not, the seeds thereof, the resin extracted therefrom, and every compound, manufacture, salt, derivative, mixture, or preparation, is classified as a Schedule I controlled substance. However, the term does not include “hemp,” which means the cannabis plant and any part of that plant, including the seeds and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol (“THC”) concentration of not more than 0.3% on a dry weight basis. Thus, depending on the THC concentration of the product, the product may or may not be regulated as a controlled substance. The DEA has historically regulated synthetic cannabinoids similarly to naturally-derived cannabinoids. Consequently, even though our cannabinoids that could be produced from microbes may not be derived from the cannabis plant, the DEA may consider them to be controlled substances subject to stringent regulatory controls.
Regulations associated with controlled substances govern manufacturing, labeling, packaging, testing, dispensing, production and procurement quotas, recordkeeping, reporting, handling, shipment and disposal. These regulations include required security measures, such as background checks on employees and physical control of inventory and increase the personnel needs and the expense associated with development and commercialization of products or product candidates including controlled substances. Regulators conduct periodic inspections of entities involved in handling, manufacturing, or otherwise distributing controlled substances, and have broad enforcement authorities. If we are found to be non-compliant with applicable controlled substance registrations and related requirements, we may need to modify its business activities and/or stop handling or producing the products regulated as controlled substances, and could be subject to enforcement action, significant fines or penalties, and/or adverse publicity, among other consequences.
Various states also independently regulate controlled substances. Though state-controlled substances laws often mirror federal law, because the states are separate jurisdictions, they may separately schedule substances, as well. The failure to comply with applicable regulatory requirements could lead to enforcement actions and sanctions from the states in addition to those from the DEA or otherwise arising under federal law.
Changes in government regulations may materially and adversely affect our sales and results of operations.
The markets where we provide our services are heavily influenced by foreign, federal, state and local government regulations and policies. The U.S. or foreign governments may take administrative, legislative, or regulatory action that could materially interfere with our customer’s ability to sell products derived from engineered cells in certain countries and/or to certain customers. The uncertainty regarding future standards and policies may also
42
Table of Contents
affect our ability to develop our programs or to license engineered cells to customers and to initiate new programs with our customers, which could have a material adverse effect on our business, financial condition and results of operations.
Changes in U.S. trade policy more generally could trigger retaliatory actions by affected countries, which could impose restrictions on our ability to do business in or with affected countries or prohibit, reduce or discourage purchases of our services by foreign customers, leading to increased program costs, increased costs of developing or manufacturing our customers’ products and higher prices for their products in foreign markets. Changes in, and responses to, U.S. trade policy could reduce the competitiveness of our services or our customers’ products, cause our services to be less in demand and our sales to decline and adversely impact our ability to compete, which could materially and adversely impact our business, financial condition and results of operations.
We are subject to certain U.S. and foreign anti-corruption, anti-bribery and anti-money laundering laws and regulations. We can face serious consequences for violations.
We are subject to the U.S. Foreign Corrupt Practices Act of 1977, as amended (the “FCPA”), the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the U.K. Bribery Act and possibly other anti-corruption, anti-bribery and anti-money laundering laws and regulations in the jurisdictions in which we do business, both domestic and abroad. Anti-corruption and anti-bribery laws have been enforced aggressively in recent years. The FCPA and other anti-corruption laws generally prohibit companies, their employees, agents, representatives, business partners and third-party intermediaries from corruptly promising, authorizing, offering, or providing, directly or indirectly, anything of value to government officials, political parties, or candidates for public office for the purpose of obtaining or retaining business or securing an improper business advantage. The UK Bribery Act and other anti-corruption laws also prohibit commercial bribery not involving government officials, and requesting or accepting bribes; and anti-money laundering laws prohibit engaging in certain transactions involving criminally-derived property or the proceeds of criminal activity.
We and our third-party business partners, representatives and agents may have direct or indirect interactions with officials and employees of government agencies or state-owned or -affiliated universities or other entities (for example, to obtain necessary permits, licenses, patent registrations and other regulatory approvals), which increases our risks under the FCPA and other anti-corruption laws. We also engage contractors, consultants and other third parties from time to time to conduct business development activities abroad. We may be held liable for the corrupt or other illegal activities of our employees or third parties even if we do not explicitly authorize such activities. We have increased and, in the future, expect our non-U.S. activities to increase over time, which may also increase our exposure under these laws.
The FCPA also requires that we keep accurate books and records and maintain a system of adequate internal controls. While we have controls to address compliance with such laws, and will continue to review and enhance our compliance program, we cannot assure you that our employees, agents, representatives, business partners or third-party intermediaries will always comply with our policies and applicable law, for which we may be ultimately held responsible.
Any allegations or violation of the FCPA or other applicable anti-bribery, anti-corruption laws and anti-money laundering laws may result in whistleblower complaints, sanctions, settlements, investigations, prosecution, enforcement actions, substantial criminal fines and civil penalties, disgorgement of profits, imprisonment, debarment, tax reassessments, breach of contract and fraud litigation, loss of export privileges, suspension or debarment from U.S. government contracts, adverse media coverage, reputational harm and other consequences, all of which may have an adverse effect on our reputation, business, financial condition, results of operations and prospects. Responding to an investigation or action can also result in a materially significant diversion of management’s attention and resources and significant defense costs and other professional fees.
43
Table of Contents
Significant disruptions to our and our service providers’ information technology systems or data security incidents could result in significant financial, legal, regulatory, business and reputational harm to us.
We are increasingly dependent on information technology systems and infrastructure, including services licensed, leased or purchased from third parties such as cloud computing infrastructure and operating systems, to operate its business. In the ordinary course of business, we collect, store, process and transmit large amounts of sensitive information, including intellectual property, proprietary business information, personal information and other confidential information. It is critical that we do so in a secure manner to maintain the confidentiality, integrity and availability of such sensitive information. We have also outsourced elements of our operations (including elements of its information technology infrastructure) to third parties, and as a result, we manage a number of third-party vendors who may have access to our networks or our confidential information. While we take measures to safeguard and protect this information, threats to network and data security are increasingly diverse and sophisticated. We may also face increased cybersecurity risks due to our reliance on internet technology and the number of our employees working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities. Despite our efforts, training and processes to prevent security breaches and incidents, our information technology systems, servers, and those of third parties that we use in our operations are vulnerable to cybersecurity risks, including cyberattacks such as viruses and worms, phishing attacks and other forms of social engineering, denial-of-service attacks, ransomware attacks, physical or electronic break-ins, third-party or employee theft or misuse, and other negligent actions, errors or malfeasance by employees or other third parties, and similar disruptions from unauthorized tampering with its servers and computer systems or those of third parties that we use in its operations, which could lead to interruptions, delays, loss or corruption of critical data, unauthorized access to or acquisition of health-related and other personal information. In addition, we may be the target of email scams and other social engineering attacks that attempt to acquire personal information or company assets or access to our systems. Despite our efforts to create security barriers to such threats, we may not be able to entirely mitigate these risks. Our third-party service providers face similar risks. Any cyberattack that attempts to obtain our data or assets, including data that we maintain on behalf of its customers, disrupt its service, or otherwise access its systems, or those of third parties we use, or any other security breach or incident, could adversely affect our business, financial condition and operating results, be expensive to remedy, and damage our reputation. We and our third-party service providers may face difficulties or delays in identifying or otherwise responding to any attacks or actual or potential security breaches or security incidents. We may incur significant costs and operational consequences of investigating, remediating, eliminating and putting in place additional tools and devices designed to prevent actual or perceived security breaches and other security incidents, including in response to any actual or perceived incident we may suffer, and substantial costs to comply with any notification or other legal obligations resulting from any security breaches or other security incidents. In addition, any such breaches or incidents, or the perception that they have occurred, may result in negative publicity, and could have an adverse effect on our business, financial condition, and operating results.
Although we maintain insurance coverage that may cover certain liabilities in connection with security breaches and other security incidents, we cannot be certain our insurance coverage will be adequate for liabilities actually incurred, that insurance will continue to be available to us on commercially reasonable terms (if at all) or that any insurer will not deny coverage as to any future claim.
Governmental trade controls, including export and import controls, sanctions, customs requirements and related regimes, could subject us to liability or loss of contracting privileges or limit our ability to compete in certain markets.
Our programs and technologies are subject to U.S. and non-U.S. export controls. Export authorizations may be required for biotechnology products, technologies, or services to be exported outside of the United States, to a foreign person, or outside of a foreign jurisdiction. Our current or future programs or technologies are, and may in the future, be subject to the Export Administration Regulations (“EAR”). If a program, technology, or service meets certain criteria for control under the EAR, then that engineered cell, production process, resulting product, technology, or service would be exportable outside the United States or to a foreign person or from one foreign
44
Table of Contents
jurisdiction to another foreign jurisdiction only if we obtain the applicable export license or other applicable authorization including qualifying for a license exception, if required. Compliance with the U.S. and foreign export laws and regulations and other applicable regulatory requirements regarding the sales, shipment and use of our engineering cells, bioprocesses and other technology may affect our ability to work with foreign partners, affect the speed at which we can introduce new products into non-U.S. markets, or limit our ability to sell programs or services or license technologies into some countries.
Additionally, certain materials that we use in our programs are subject to U.S. import controls. We currently have, and may in the course of business need to procure, certain import authorizations, for example, related to plant pests, chemicals, biological agents and other controlled materials, including from the USDA, EPA and CDC. Compliance with applicable regulatory requirements regarding the import of such materials may limit our access to materials critical to our development activities or affect the speed at which we can advance new programs.
Our activities are also subject to the economic sanctions laws and regulations of the United States and other jurisdictions. Such controls prohibit certain transactions, potentially including financial transactions and the transfer of products, technologies and services, to sanctioned countries, governments and persons, without a license or other appropriate authorization. U.S. sanctions policy changes could affect our or our customers’ ability to interact, directly and indirectly, with targeted companies or companies in sanctioned countries.
While we take precautions to comply with U.S. and non-U.S. export control, import control and economic sanctions laws and regulations, we cannot guarantee that such precautions will prevent violations of such laws, including transfers to unauthorized persons or destinations, and including inadvertent violations as a result of a misclassification of a product, technology or service under export control laws. Violations could result in our business being subject to government investigations, denial of export or import privileges, significant fines or penalties, denial of government contracts and reputational harm. Any limitation on our ability to export our engineered cells, production processes, resulting products, technology, or services, or import materials critical to our programs would likely adversely affect our business and financial condition.
Changes in U.S. and foreign tax laws could have a material adverse effect on our business, cash flow, results of operations or financial condition.
We are subject to income and non-income based taxes in the U.S. and foreign jurisdictions. Changes in tax laws, regulations and policies, or their interpretation and application, in the jurisdictions where we are subject to tax, could have a material adverse effect on our business, cash flow, results of operations or financial condition. The U.S. Congress continually debates changes to U.S. corporate income tax laws, which could result in significant changes to these laws. In addition, the Group of Twenty (G20), the Organization for Economic Co-operation and Development (OECD), the European Commission (EC) and individual taxing jurisdictions have published proposals covering various international tax-related issues, including country-by-country reporting, permanent establishment rules, transfer pricing and tax treaties. It is possible that any future tax legislation that may be enacted could materially impact our effective tax rate and cash tax liability as well as tax credits and incentives.
We may become subject to lawsuits or indemnity claims in the ordinary course of business, which could materially and adversely affect our business and results of operations.
From time to time, we may in the ordinary course of business be named as a defendant in lawsuits, indemnity claims and other legal proceedings. These actions may seek, among other things, compensation for alleged product liability, personal injury, employment discrimination, breach of contract, property damage and other losses or injunctive or declaratory relief.
The marketing, sale and use of our services engineered cells, production processes and resulting products could lead to the filing of product liability claims were someone to allege that our services, engineered cells, production
45
Table of Contents
processes or resulting products failed to perform as designed or intended or caused injury or other harms. A product liability claim could result in substantial damages and be costly and time-consuming for us to defend.
Regardless of merit or eventual outcome, product liability claims may result in:
| • | decreased demand for programs and resulting products; |
| • | loss of revenue; |
| • | substantial monetary payments; |
| • | significant time and costs to defend related litigation; |
| • | the inability to commercialize any products from our programs; and |
| • | injury to our reputation and significant negative media attention. |
In the event that such actions, claims or proceedings are ultimately resolved unfavorably to us at amounts exceeding our accrued liability, or at material amounts, the outcome could materially and adversely affect our business and results of operations. In addition, payments of significant amounts, even if reserved, could adversely affect our liquidity position. We maintain product liability insurance, but this insurance may not fully protect us from the financial impact of defending against product liability claims. Any product liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could cause current collaborators to terminate existing agreements or potential collaborators to seek other companies, any of which could impact our business and results of operations.
Our business could be adversely affected by legal challenges to our telehealth partner’s business model.
Certain of our COVID-19 biosecurity offerings rely significantly on healthcare provider orders for testing that are placed on the basis of telemedicine encounters. The ability to conduct telehealth services in a particular state is directly dependent upon the applicable laws governing remote healthcare, the practice of medicine and healthcare delivery in general in such location which are subject to changing political, regulatory and other influences. With respect to telehealth services, state medical boards continue to implement new rules or interpret existing rules in a manner that may limit or restrict the ability of the centers to conduct their business as it has been conducted in the past. Additionally, during the COVID-19 public health emergency, many states enacted waivers and adopted other temporary measures that lifted certain restrictions on out-of-state providers and relaxed licensure requirements to allow greater access to telehealth services during the public health emergency period. At this time, we cannot predict whether these waivers or temporary measures will remain in place after the end of the public health emergency period. Accordingly, we must monitor compliance with laws in every jurisdiction in which we operate, and we cannot provide assurance that government authorities may nonetheless challenge our activities and arrangements with our telehealth partner and consider them non-compliant. Additionally, it is possible that the laws and rules governing the practice of medicine, including remote healthcare, in one or more jurisdictions may change in a manner deleterious to our business. If a successful legal challenge or an adverse change in the relevant laws were to occur, and we are unable to adapt our business model accordingly, our operations as well as the operations of our telehealth partner in the affected jurisdictions would be disrupted, which could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to our Common Stock, Organizational Structure and Governance
We are not, and do not intend to become, regulated as an “investment company” under the Investment Company Act, and if we were deemed an “investment company” under the Investment Company Act, applicable restrictions could make it impractical for us to continue our business as contemplated and could have a material adverse effect on our business.
An entity generally will be deemed to be an “investment company” for purposes of the Investment Company Act if:
| • | it is an “orthodox” investment company because it is or holds itself out as being engaged primarily, or proposes to engage primarily, in the business of investing, reinvesting or trading in securities; or |
46
Table of Contents
| • | it is an inadvertent investment company because, absent an applicable exemption, (i) it owns or proposes to acquire investment securities having a value exceeding 40% of the value of its total assets (exclusive of U.S. government securities and cash items) on an unconsolidated basis, or (ii) it owns or proposes to acquire investment securities having a value exceeding 45% of the value of its total assets (exclusive of U.S. government securities and cash items) and/or more than 45% of its income is derived from investment securities on a consolidated basis with its wholly owned subsidiaries. |
We believe that we are engaged primarily in the business of providing cell engineering services to customers from across a variety of industries and not in the business of investing, reinvesting or trading in securities. We hold ourselves out as a synthetic biology company and do not propose to engage primarily in the business of investing, reinvesting or trading in securities. Accordingly, we do not believe that we are an “orthodox” investment company as defined in Section 3(a)(1)(A) of the Investment Company Act of 1940, as amended (the “Investment Company Act”) and described in the first bullet point above. Furthermore, we believe that less than 40% of our total assets (exclusive of U.S. government securities and cash items) on an unconsolidated basis will be composed of assets that could be considered investment securities. Accordingly, we do not believe that we are an inadvertent investment company by virtue of the 40% tests in Section 3(a)(1)(C) of the Investment Company Act as described in the second bullet point above. In addition, we believe that we are not an investment company under Section 3(b)(1) of the Investment Company Act because we are primarily engaged in a non-investment company business.
The Investment Company Act and the rules thereunder contain detailed parameters for the organization and operation of investment companies. Among other things, the Investment Company Act and the rules thereunder limit or prohibit transactions with affiliates, impose limitations on the issuance of debt and equity securities, generally prohibit the issuance of options and impose certain governance requirements. We intend to conduct our operations so that we will not be deemed to be an investment company under the Investment Company Act or otherwise conduct our business in a manner that does not subject us to the registration and other requirements of the Investment Company Act. In order to ensure that we are not deemed to be an investment company, we may be limited in the assets that we may continue to own and, further, may need to dispose of or acquire certain assets at such times or on such terms as may be less favorable to us than in the absence of such requirement. If anything were to happen which would cause us to be deemed to be an investment company under the Investment Company Act (such as significant changes in the value of our programs or a change in circumstance that results in a reclassification of our interests in our programs for purposes of the Investment Company Act), the requirements imposed by the Investment Company Act could make it impractical for us to continue our business as currently conducted, which would materially adversely affect our business, financial condition and results of operations. In addition, if we were to become inadvertently subject to the Investment Company Act, any violation of the Investment Company Act could subject us to material adverse consequences, including potentially significant regulatory penalties and the possibility that certain of our contracts could be deemed unenforceable.
Only our employees and directors are entitled to hold shares of Class B common stock (including shares of Class B common stock granted or otherwise issued to our employees and directors in the future), which shares have ten votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of certain amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring stockholder approval.
Shares of our Class B common stock have ten votes per share, whereas shares of our Class A common stock have one vote per share and shares of our Class C common stock have no voting rights (except as otherwise expressly provided in our amended and restated certificate of incorporation (the “Charter”) or required by applicable law). As of June 30, 2022, our directors and executive officers hold in the aggregate approximately 49.2% of the total voting power of our outstanding capital stock, and our directors, founders and executive officers hold in the aggregate approximately 68.7% of the total voting power of our outstanding capital stock. Accordingly, holders
47
Table of Contents
of shares of Class B common stock are able to significantly influence the outcome of matters submitted to our stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets or other major corporate transaction requiring stockholder approval. This concentrated voting power limits or precludes other stockholders’ ability to influence the outcome of these matters. Holders of Class B common stock may have interests that differ from holders of Class A common stock and may vote in a way with which holders of Class A common stock disagree and which may be adverse to the interests of holders of Class A common stock. This concentrated voting power is likely to have the effect of limiting the likelihood of an unsolicited merger proposal, unsolicited tender offer or proxy contest for the removal of directors. As a result, our governance structure and Charter may have the effect of depriving our stockholders of an opportunity to sell their shares at a premium over prevailing market prices and make it more difficult to replace our directors and management. Furthermore, this concentrated voting power could discourage a potential investor from acquiring Class A common stock due to the limited voting power of such stock relative to Class B common stock, which could also adversely affect the trading price of Class A common stock.
Our multi-class stock structure is intended to preserve our existing founder-led governance structure, to promote employee retention and engagement, to facilitate continued innovation and the risk-taking that it requires, to permit us to continue to prioritize our long-term goals rather than short-term results, to enhance the likelihood of continued stability in the composition of our board of directors and its policies, and to discourage certain types of transactions that may involve an actual or threatened acquisition of the company, all of which we believe are essential to the long-term success of our company and to long-term stockholder value. We expect to maintain this concentrated voting power among our founders and employees for the foreseeable future, including by issuing additional shares of Class B common stock to our employees pursuant to our equity compensation plans.
Future transfers of shares of Class B common stock to persons other than Ginkgo directors and employees, or trusts or legal entities through which the right to vote the shares of Class B common stock held thereby is exercised exclusively by one or more of Ginkgo’s directors or employees (any such director, employee, trust or legal entity, an “Eligible Holder”), or the holder of shares of Class B common stock ceasing to be an Eligible Holder, will generally result in those shares converting to shares of Class A common stock on a one-to-one basis, subject to certain exceptions and unless a majority of the independent directors of our board of directors determine that such transfer or event will not result in such automatic conversion. Each share of Class B common stock is also convertible at any time at the option of the holder into one share of Class A common stock. The conversion of Class B common stock to Class A common stock over time will have the effect of increasing the relative voting power of those holders of Class B common stock who retain their shares of Class B common stock in the long term. As a result, the relative voting power of holders of Class A common stock is expected to remain limited for a significant period of time, and it is possible that one or more of the persons or entities holding Class B common stock could gain significant voting control as other holders of Class B common stock sell or otherwise convert their shares into Class A common stock. In addition, the conversion of Class B common stock to Class A common stock would dilute holders of Class A common stock in terms of voting power within the Class A common stock. Because holders of Class C common stock have no voting rights (except as otherwise expressly provided in the Charter or required by applicable law), the holders of Class B common stock may be able to significantly influence the outcome of matters submitted to our stockholders for approval for a longer period of time than would be the case if we issued Class A common stock rather than Class C common stock in such transactions.
Our share price may change significantly over time, and you may not be able to resell our common stock at or above the price you paid or at all, and you could lose all or part of your investment as a result.
The trading price of our Class A common stock has been in the past and is likely to continue to be volatile. Such volatility may be, in part, attributable to:
| • | future sales of our common stock or other securities by us or our existing stockholders, or the perception of such future sales; |
48
Table of Contents
| • | results of operations of the company or our competitors that vary from the expectations of securities analysts and investors; |
| • | changes in expectations as to our future financial performance and growth, including assessments of our business, prospects, financial estimates and investment recommendations by securities analysts, investors and short sellers; |
| • | additions or departures of key management personnel or members of our board of directors; |
| • | announcements by us or our competitors of significant contracts, new products, acquisitions, joint marketing relationships, joint ventures, other strategic relationships or capital commitments; |
| • | announcements relating to actual or potential civil and non-civil litigation, as well as governmental or regulatory investigations or inquiries; |
| • | guidance that we provide to the public, any changes in this guidance or our failure to meet this guidance; |
| • | changes in the perception of our offerings or the synthetic biology industry more general including changes in regulatory conditions; |
| • | the development and sustainability of an active trading market for our common stock; |
| • | changes in accounting principles; |
| • | changes in general economic or market conditions or trends in our industry or markets; |
| • | other events or factors, including those resulting from natural disasters, pandemics, epidemics, war (including Russia’s invasion of Ukraine), acts of terrorism or responses to these events. |
These factors among others may materially adversely affect the market price of our Class A common stock, regardless of our actual operating performance. In addition, price volatility may be greater if the public float and trading volume of our common stock are low.
In the past, following periods of market volatility, stockholders have instituted securities class action litigation. If we were involved in securities litigation, it could have a substantial cost and divert resources and the attention of executive management from our business regardless of the outcome of such litigation.
Future sales, or the perception of future sales, by us or our stockholders in the public market could cause the market price for our securities to decline.
The sale of our securities in the public market, including by entities to which we have issued shares in connection with transactions, or the perception that such sales could occur, could harm the prevailing market price of our securities. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
As of the consummation of the Business Combination, we had a total of approximately 1,959 million shares of common stock outstanding on a fully-diluted basis, consisting of approximately 1,333 million shares of Class A common stock and approximately 626 million shares of Class B common stock. All shares issued in the merger are freely tradable without registration under the Securities Act, and without restriction by persons other than our “affiliates” (as defined under Rule 144 of the Securities Act, “Rule 144”), including our directors, executive officers and other affiliates. Of these shares, approximately 631 million shares of common stock outstanding on a fully-diluted basis are subject to a one-year lock-up, which is scheduled to expire on September 16, 2022. In addition to the above, there are up to approximately 206 million shares of common stock that may be earned if the trading price is greater than or equal to the earnout price threshold in the table below for any point in a trading day during 20 trading days in a 30 consecutive trading day period, of which approximately 51.5 million
49
Table of Contents
shares were earned as of June 30, 2022. The vast majority of the shares that are part of the earnout will not be subject to lock-up once the earnout conditions are met.
| Earnout Price Threshold |
Number of Shares Earned | |||
| $12.50 (earnout condition has been met) |
Approximately 51.5 million | |||
| $15.00 |
Approximately 51.5 million | |||
| $17.50 |
Approximately 51.5 million | |||
| $20.00 |
Approximately 51.5 million | |||
In connection with the Business Combination, in September 2021, Jason Kelly, Reshma Shetty, Austin Che and Bartholomew Canton were each granted 21,458,317 restricted stock units, pursuant to Founder Equity Grant Agreements dated January 1, 2020. Each named founder agreed to extend the vesting on these restricted stock units and their 4,324,037 restricted stock units granted in 2020 such that these restricted stock units and their associated earnout shares will not fully vest until October 1, 2022, and such vesting is subject to the founder’s continued service at Ginkgo through such date. When those restricted stock units and earnouts vest, if any of such shares are sold into the market (including to cover the income tax obligations associated with this vesting event or otherwise), such sales could harm the prevailing market price of our securities.
In addition, the shares of our common stock reserved for future issuance under our equity incentive plans will become eligible for sale in the public market once those shares are issued, subject to provisions relating to various vesting agreements and, in some cases, limitations on volume and manner of sale applicable to affiliates under Rule 144, as applicable. Our compensation committee of our board of directors may determine the exact number of shares to be reserved for future issuance under our equity incentive plans at its discretion. We are expected to file one or more registration statements on Form S-8 under the Securities Act to register shares of Class A common stock or securities convertible into or exchangeable for shares of Class A common stock issued pursuant to our equity incentive plans. Any such Form S-8 registration statements will automatically become effective upon filing. Accordingly, shares registered under such registration statements will be available for sale in the open market.
Short sellers may engage in manipulative activity intended to drive down the market price of our Class A common stock, which could also result in related regulatory and governmental scrutiny, among other effects.
Short selling is the practice of selling securities that the seller does not own but rather has borrowed or intends to borrow from a third party with the intention of later buying lower priced identical securities to return to the lender. Accordingly, it is in the interest of a short seller of our Class A common stock for the price to decline. At any time, short sellers may publish, or arrange for the publication of, opinions or characterizations that are intended to create negative market momentum. Issuers, like us, whose securities have historically had limited trading history or volumes and/or have been susceptible to relatively high volatility levels can be vulnerable to such short seller attacks. Short selling reports can cause increased volatility in an issuer’s stock price, and result in regulatory and governmental inquiries. On October 6, 2021, such a report was published about us. Shortly after, we received a preliminary and informal inquiry from the U.S. Department of Justice related to such report. Any related inquiry or formal investigation from a governmental organization or other regulatory body, including any inquiry from the SEC, could result in a material diversion of our management’s time and could have a material adverse effect on our business and results of operations.
Our Charter authorizes a large number of shares of Class B common stock for issuance in the future. The future issuance of shares of Class B common stock may have the effect of further concentrating voting power with our employees and other Class B stockholders, and could have an adverse effect on the trading price of Class A common stock.
Under our Charter, we are authorized to issue 4,500,000,000 shares of Class B common stock, which are entitled to ten votes per share. We currently intend to issue additional shares of Class B common stock in the future to
50
Table of Contents
existing and newly hired employees pursuant to our equity compensation plans. Our authorized but unissued shares of Class B common stock are available for issuance to Eligible Holders with the approval of our board of directors without stockholder approval, except as may be required by the Listing Rules of the NYSE. In addition, our authorized but unissued shares of Class B common stock are available for issuance to persons other than Eligible Holders only with the approval of a majority of our directors elected by the holders of Class B common stock, voting separately as a class. If we issue additional shares of Class B common stock in the future, holders of shares of Class A common stock, which are entitled to one vote per share, will experience disproportionate voting power dilution relative to economic dilution, and the holders of Class B common stock may be able to significantly influence the outcome of matters submitted to our stockholders for approval for a longer period of time than would be the case if we issued shares of Class A common stock.
See “Risk Factors—Risks Relating to our Organizational Structure and Governance—Only our employees and directors are entitled to hold shares of Class B common stock (including shares of Class B common stock granted or otherwise issued to our employees and directors in the future), which shares have ten votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of certain amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets or other major corporate transaction requiring stockholder approval.”
Under our Charter, we are authorized to issue 800,000,000 shares of Class C common stock, which have no voting rights (except as otherwise expressly provided in the Charter or required by applicable law). Outstanding Class C common stock may have the effect of extending voting power in Class B common stock, and may discourage potential acquisitions of our business and could have an adverse effect on the trading price of Class A common stock.
Under our Charter, we are authorized to issue 800,000,000 shares of Class C common stock, which have no voting rights (except as required by law). Class C common stock may be used for a variety of corporate purposes, including financings, acquisitions and investments. Our authorized but unissued shares of Class C common stock are available for issuance with the approval of our board of directors without stockholder approval, except as may be required by the Listing Rules of the NYSE. Because the Class C common stock carries no voting rights (except as otherwise expressly provided in the Charter or required by applicable law), is not convertible into any other capital stock, and is not listed for trading on an exchange or registered for sale with the SEC, shares of Class C common stock may be less liquid and less attractive to any future recipients of these shares than shares of Class A common stock, although we may seek to list the Class C common stock for trading and register shares of Class C common stock for sale in the future. In addition, because our Class C common stock has no voting rights (except as otherwise expressly provided in the Charter or required by applicable law), the holders of Class B common stock may be able to significantly influence the outcome of matters submitted to our stockholders for approval for a longer period of time than would be the case if we issued Class A common stock rather than Class C common stock in such transactions. In addition, further issuances of Class C common stock would have a dilutive effect on the economic interests of Class A common stock and Class B common stock. Any such issuance could also cause the trading price of Class A common stock to decline.
We cannot predict the effect the multi-class structure of our common stock may have on the trading price of our Class A common stock.
The holding of low-voting stock, such as Class A common stock, may not be permitted by the investment policies of certain institutional investors or may be less attractive to the portfolio managers of certain institutional investors. In addition, certain index providers have announced restrictions on including companies with multiple-class share structures in certain of their indices. In July 2017, S&P Dow Jones announced that they would cease to allow most newly public companies with dual- or multi-class capital structures to be included in their indices. Affected indices include the S&P 500, S&P MidCap 400 and S&P SmallCap 600, which together make up the
51
Table of Contents
S&P Composite 1500. Under the announced policies, our multi-class capital structure would make our Class A common stock ineligible for inclusion in certain indices, and as a result, mutual funds, exchange-traded funds and other investment vehicles that attempt to passively track those indices would not invest in our common stock. These policies may depress our valuation compared to those of other similar companies that are included. Because of our multi-class stock structure, our Class A common stock will likely continue to be excluded from certain of these indices, and we cannot assure you that other stock indices will not take similar actions. Given the sustained flow of investment funds into passive strategies that seek to track certain indices, exclusion from stock indices would likely preclude investment by many of these funds in our Class A common stock and could make shares of our Class A common stock less attractive to other investors. As a result, the trading price of shares of our Class A common stock could be adversely affected.
Our focus on the long-term best interests of our company and our consideration of all of our stakeholders, including our stockholders, workforce, customers, suppliers, academic researchers, governments, communities and other stakeholders that we may identify from time to time, may conflict with short-term or medium-term financial interests and business performance, which may adversely impact the value of our common stock.
We believe that focusing on the long-term best interests of our company and our consideration of all of our stakeholders, including our stockholders, workforce, customers, suppliers, academic researchers, governments, communities and other stakeholders we may identify from time to time, is essential to the long-term success of our company and to long-term stockholder value. Therefore, we have made decisions, and may in the future make decisions, that we believe are in the long-term best interests of our company and our stockholders, even if such decisions may negatively impact the short- or medium-term performance of our business, results of operations, and financial condition or the short- or medium-term performance of our Class A common stock. Our commitment to pursuing long-term value for the company and its stockholders, potentially at the expense of short- or medium-term performance, may materially adversely affect the trading price of our Class A common stock, including by making owning our Class A common stock less appealing to investors who are focused on returns over a shorter time horizon. Our decisions and actions in pursuit of long-term success and long-term stockholder value, which may include our multi-class stock structure, making investments in R&D and our employees, and investing in and introducing new products and services, may not result in the long-term benefits that we expect, in which case our business, results of operations and financial condition, as well as the trading price of our Class A common stock, could be materially adversely affected.
52
Table of Contents
USE OF PROCEEDS
All of the shares of Class A common stock offered by the Selling Securityholders pursuant to this prospectus will be sold by the Selling Securityholders for their respective accounts. We will not receive any of the proceeds from these sales.
The Selling Securityholders will pay any underwriting fees, discounts, selling commissions, stock transfer taxes and certain legal expenses incurred by such Selling Securityholders in disposing of their shares of Ginkgo Class A common stock, and we will bear all other costs, fees and expenses incurred in effecting the registration of such securities covered by this prospectus, including, without limitation, all registration and filing fees, NYSE listing fees and fees and expenses of our counsel and our independent registered public accountants.
MARKET PRICE, TICKER SYMBOL AND DIVIDEND INFORMATION
Market Price and Ticker Symbol
Our Class A common stock and Public Warrants are currently listed on the NYSE under the symbols “DNA” and
“DNA.WS”, respectively.
The closing price of the Class A common stock and Public Warrants on August 8, 2022, was $3.24 and $.68, respectively.
Holders
As of June 30, 2022, there were approximately 241 holders of record of our Class A Common Stock, 1 holder of record of the Public Warrants and 24 holders of record of the Private Placement Warrants.
Such numbers do not include beneficial owners holding our securities through nominee names. There is no public market for our Class B common stock.
Dividend Policy
We have not paid any cash dividends on our Class A common stock to date. The payment of cash dividends in the future will be dependent upon our revenues and earnings, if any, capital requirements and general financial condition. The payment of any cash dividends will be within the discretion of the Ginkgo Board at such time.
53
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the audited consolidated financial statements and unaudited condensed consolidated financial statements and respective related notes thereto included elsewhere in this prospectus. This discussion contains forward-looking statements that reflect our plans, estimates and beliefs that involve risks and uncertainties. Actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including those discussed in “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” elsewhere in this prospectus.
References to “we,” “us” and “our” in this section captioned “Management’s Discussion & Analysis of Financial Condition and Results of Operations of Ginkgo” refer solely to Ginkgo and its consolidated subsidiaries.
Overview
Our mission is to make biology easier to engineer.
Ginkgo is building the industry-standard horizontal platform for cell programming. We use our platform to program cells on behalf of our customers. These “cell programs” are designed to enable biological production of products as diverse as novel therapeutics, key food ingredients, and chemicals currently derived from petroleum. We have worked on cell programs in end markets as diverse as specialty chemicals, agriculture, food, consumer products, and pharmaceuticals. Biology did not evolve by end market. All of these applications run on cells which have a common code—DNA—and a common programming platform can enable all of them. Because of this shared platform, we are able to drive scale and learning efficiencies while maintaining flexibility and diversity in our program areas. Ultimately, customers come to us because they believe we maximize the probability of successfully developing their products.
The foundation of our platform includes two core assets that execute a wide variety of cell programs for customers according to their specifications: our Foundry and our Codebase.
| • | Our Foundry wraps proprietary software and automation around core cell engineering workflows— designing DNA, writing DNA, inserting that DNA into cells, testing to measure cell performance—and leverages data analytics and data science to inform each iteration of design. The software, automation and data analysis pipelines we leverage in the Foundry drive a strong scale economic: we have scaled the output of the Foundry by roughly 3X annually since we started measuring it around 2015 (with the exception of 2020 during the COVID-19 pandemic) and over that time, the average cost per unit operation has fallen by approximately 50% every year. We expect to be able to pass some of these savings along to our customers, allowing them to take more “shots on goal” with their programs. |
| • | Our Codebase includes both our physical (engineered cells and genetic parts) and digital (genetic sequences and performance data) biological assets, and accumulates as we execute more cell programs on the platform. Every program, whether successful or not, generates valuable Codebase and helps inform future experimental designs and provides reusable genetic parts, making our cell program designs more efficient. |
As the platform scales, we have observed a virtuous cycle between our Foundry, our Codebase, and the value we deliver to customers. We believe this virtuous cycle sustains Ginkgo’s growth and differentiated value proposition.
| • | Foundry: As we take on more work in the Foundry, we benefit from scale economics, which over time may lead to lower program costs. We expect that these lower costs, in turn, will drive additional demand for our cell programming capabilities. |
54
Table of Contents
| • | Codebase: Cell programs also generate Codebase, which can drive better experimental direction and improve the odds of technical success, further increasing our customer value proposition, which we believe will result in additional demand. |
Put simply: we believe that as the platform improves with scale, it drives more scale, which drives further platform improvements, and so on. We believe this positive feedback loop has the potential to drive compounding value creation in the future as every new program we add contributes to both near-term revenues and has the potential to add significant downstream economics.
Our business model mirrors the structure of our platform and we are compensated in two primary ways. First, we charge usage fees for Foundry services, in much the same way that cloud computing companies charge usage fees for utilization of computing capacity or contract research organizations charge for services. Additionally, we negotiate a value share with our customers (typically in the form of royalties, milestones, and/or equity interests) in order to align our economics with the success of the programs enabled by our platform. As we add new programs, our portfolio of programs with this “downstream” value potential grows.
With a mission to make biology easier to engineer, we have always recognized the imperative to invest in biosecurity as a key component of our platform. We care how our platform is used, and biosecurity is a necessary complement to synthetic biology that helps us ensure our cell programming work is conducted and deployed responsibly. The near-term growth of the biosecurity sector is highly dependent on domestic and international government initiatives and investment and Ginkgo has been supporting and engaging with domestic and international organizations and governments to help shape the understanding of a robust biosecurity program.
In the second quarter of 2020, in response to the COVID-19 pandemic, we launched our commercial offering of COVID-19 testing products and services for businesses, academic institutions, and other organizations in which we generate product and service revenue. Beginning in the first quarter of 2021, we launched our pooled testing initiative which focuses on providing end-to-end COVID-19 testing and reporting services to public health authorities. We are currently offering pooled testing and reporting services for K-12 schools across the United States, at airports through our partnership with XpresCheck and the CDC, as well as through other congregate settings such as our partnership with Eurofins. In the future, we believe that testing services may have a value proposition internationally and in other use cases including wastewater monitoring and air monitoring.
Prior to 2022, we operated as a single reportable segment. In the first quarter of 2022, we reorganized our operations into two operating and reportable segments: Foundry and Biosecurity. The reorganization reflects changes made to our internal management structure and how our chief operating decision makers evaluate operating results and make decisions on how to allocate resources. Our two operating and reportable segments are described below:
| • | Foundry consists of research and development services performed under collaboration and license agreements relating to our cell programming platform. Our cell programming platform includes two core assets: the Foundry, highly efficient biology lab facilities, enabled by investment in proprietary workflows, custom software, robotic automation, and data science and analytics, which is paired with our Codebase, a collection of biological “parts” and a database of biological data used to program cells. The Foundry segment includes costs incurred for the development, operation, expansion and enhancement of the Foundry and Codebase. Foundry revenue is derived from Foundry usage fees and downstream value share in the form of milestone payments, royalties or equity interests. |
| • | Biosecurity consists of COVID-19 testing products and services primarily provided to public health authorities. Biosecurity revenue is derived from sales of test kits and testing and reporting services fees. |
55
Table of Contents
Generating Economic Value Through Cell Programs
Our cell programming platform is a key enabling technology and source of intellectual property for our customers’ products. We earn Foundry revenue for our research and development (“R&D”) services as well as through a share of the value of products created using our platform.
We structure Foundry revenue to include some combination of the following:
| • | Foundry usage fees in the form of: |
| • | upfront payments upon consummation of an agreement or other fixed payments that are generally recognized over our period of performance; |
| • | reimbursement for costs incurred for R&D services; |
| • | milestone payments upon the achievement of specified technical criteria; |
plus,
| • | downstream value share payments in the form of: |
| • | milestone payments upon the achievement of specified commercial criteria; |
| • | royalties on sales of products from or comprising engineered organisms; |
| • | royalties related to cost of goods sold reductions realized by our customers; |
or,
| • | downstream value share in the form of equity interests in our customer. |
| • | downstream value share in the form of equity interest appreciation is not recognized as revenue but is expected to contribute to future cash flows upon liquidation, the amount and timing of which is inherently unpredictable. |
Downstream value share arrangements which involve equity interests generally fall into two categories: Platform Ventures and Structured Partnerships.
Platform Ventures
Platform Ventures allow Ginkgo to partner with leading multinationals and financial investors to form new ventures in identified market segments with potential to benefit from synthetic biology. In exchange for an equity position in the venture, we contribute license rights to our proprietary cell programming technology and intellectual property, while our partners contribute relevant industry expertise, other resources and venture funding. We also provide R&D services for which we receive cash payments for our costs incurred, plus a margin. Platform Ventures include:
Joyn Bio, LLC
Founded in 2017, Joyn Bio, LLC (“Joyn”) was formed to focus on engineered microbes for use in agricultural applications. Along with certain of our investors, we formed Cooksonia, LLC (“Cooksonia”) which holds a 50% equity interest in Joyn. Bayer CropScience LP (“Bayer”) contributed cash of $80 million plus intellectual property and holds the remaining 50% equity interest in Joyn. We provided license rights to our intellectual property and platform at inception in return for our equity interest in Joyn, which was recorded at an initial fair value of $97.9 million. The carrying value of our equity method investment in Joyn was $1.3 million as of June 30, 2022. Ginkgo also entered into a Foundry Services Agreement (“Joyn FSA”) with Joyn under which we provide R&D services. Joyn paid us a total of $35.0 million in non-refundable prepayments for services to be provided under the Joyn FSA. In July 2022, we entered into an APA with Bayer to integrate certain R&D platform assets of Joyn. Refer to the section entitled “Pending Acquisition” below for additional discussion.
56
Table of Contents
Motif FoodWorks, Inc.
Founded in 2018, Motif FoodWorks, Inc. (“Motif”) was formed to focus on the application of synthetic biology to reduce the reliance on animal products in the food industry. We entered into an intellectual property contribution agreement that granted Motif rights to our intellectual property, subject to mutually agreed upon technical development plans. In return for our contribution of intellectual property and access to our platform, we received shares of common stock in Motif. The initial fair value of our common stock investment in Motif was $65.1 million which has subsequently been reduced to a carrying value of zero as a result of the allocation of losses under our accounting for equity method investments. Motif was capitalized through Series A preferred stock financings that raised approximately $119 million in gross proceeds from an investor group which included certain of our investors, Louis Dreyfus Company and Fonterra Co-operative Group Limited. In June 2021, Motif raised an additional $226 million through a Series B preferred stock financing. Ginkgo also entered into a Technical Development Agreement with Motif under which we provide R&D services in return for cash consideration on a cost-plus fixed margin basis. Motif launched its first product, HEMAMI, in 2021.
Allonnia, LLC
Founded in 2019, Allonnia was formed to focus on the application of synthetic biology in the waste bioremediation and biorecovery industries. We entered into an intellectual property contribution agreement that granted Allonnia rights to our intellectual property, subject to mutually agreed upon technical development plans. In return for our contribution of intellectual property and access to our platform, we received common units in Allonnia with a right to additional units subject to additional closings of Allonnia’s Series A preferred units. The initial fair value of our common units received in Allonnia was $24.5 million, subsequently increased by $12.7 million in 2021, all of which has been reduced to a carrying value of zero as a result of the allocation of losses under our accounting for equity method investments. Allonnia was capitalized through Series A preferred unit financings that raised approximately $52 million in gross proceeds from an investor group which included certain of our investors and Battelle Memorial Institute. Ginkgo also entered into a Technical Development Agreement with Allonnia under which we provide R&D services in return for cash consideration on a cost-plus fixed margin basis.
Arcaea, LLC (FKA Kalo Ingredients, LLC)
Founded in 2021, Arcaea, LLC (“Arcaea”) was formed to focus on the application of synthetic biology in the beauty and personal care products industry. In March 2021, we entered into an intellectual property contribution agreement that granted Arcaea rights to our intellectual property, subject to mutually agreed upon technical development plans. In return for our contribution of intellectual property and access to our platform, we received common units in Arcaea with a right to additional units subject to additional closings of Arcaea’s Series A preferred units. The initial fair value of our common units received in Arcaea was $11.9 million which has subsequently been reduced to a carrying value of zero as a result of the allocation of losses under our accounting for equity method investments. Arcaea was capitalized through a Series A preferred unit financing that raised approximately $77 million in gross proceeds from an investor group which included certain of our investors, CHANEL and Givaudan. Upon the closing of the Series A preferred unit financing in July 2021, we received an additional 5,229,900 common units in Arcaea. The fair value of our Arcaea common units received in July 2021 of $35.5 million has subsequently been reduced to a carrying value of zero as a result of the allocation of losses under our accounting for equity method investments. Ginkgo also entered into a Technical Development Agreement with Arcaea under which we provide R&D services in return for cash consideration on a cost-plus fixed margin basis.
Ayana Bio, LLC
Founded in September 2021, Ayana Bio, LLC (“Ayana”) was formed to identify and design new bioactive compounds for use as complementary medicine to support human health and wellness. Ayana was capitalized
57
Table of Contents
through a Series A funding that raised $30 million in gross proceeds from an investor group comprising certain of our investors. We hold an interest in 9,000,000 common units (representing 100% of common units at inception) of Ayana and have also provided Ayana with certain licenses to our intellectual property for use in the development or production of products that we have agreed to research and develop under technical development plans. We concluded that we hold a variable interest in and are the primary beneficiary of Ayana, and as a result, we have consolidated the financial statements of Ayana into our consolidated financial statements.
Verb Biotics, LLC
Founded in September 2021, Verb Biotics, LLC (“Verb”) was formed to identify and design new strains of probiotic bacteria with advanced properties for human nutrition, health, and wellness. Verb was capitalized through a Series A funding that raised $30 million in gross proceeds from an investor group comprising certain of our investors. We hold an interest in 9,000,000 common units (representing 100% of common units at inception) of Verb and have also provided Verb with certain licenses to our intellectual property for use in the development or production of products that we have agreed to research and develop under technical development plans. Prior to the first quarter of 2022, we consolidated Verb as a variable interest entity. In the first quarter of 2022, we deconsolidated Verb and began accounting for our retained investment in Verb as an equity method investment. The initial carrying value of the equity method investment in Verb was equal to the fair value of our retained interest of $15.9 million as of the deconsolidation date which has been subsequently reduced to a carrying value of zero due to a basis difference associated with in-process research and development identified as part of the initial accounting for the equity method investment.
BiomEdit, LLC
Founded in April 2022, BiomEdit, LLC (BiomEdit”) was formed to discover, design and develop novel probiotics, microbiome derived bioactives and engineered microbial medicines in the animal health industry. BiomEdit was capitalized through a Series A preferred unit financing that raised approximately $32.5 million in gross proceeds from an investor group which included one of our investors. In April 2022, we entered into an intellectual property contribution agreement that granted BiomEdit rights to our intellectual property, subject to mutually agreed upon technical development plans and, in return, we received 3.9 million voting common units in BiomEdit. In addition, Elanco also contributed intellectual property in exchange for 3.9 million non-voting common units in BiomEdit. The initial fair value of our common units received in BiomEdit was $8.9 million which has subsequently been reduced to a carrying value of $4.3 million as a result of the allocation of losses under our accounting for equity method investments. Ginkgo also entered into a Technical Development Agreement with BiomEdit under which we will provide R&D services in return for cash consideration on a fixed fee or cost-plus basis.
Structured Partnerships
Structured Partnerships allow Ginkgo to: (i) partner with early stage synthetic biology product companies to adopt our Foundry as their cell programming R&D platform, in which we offer flexible commercial terms on the Foundry usage fees including the ability to pay a portion or all of such upfront fees in the form of equity, in addition to downstream value share consideration (“Startup Structured Partnership”); and (ii) partner with existing entities with complementary assets for high potential synthetic biology applications in a large-scale, multi-program collaboration (“Legacy Structured Partnership”). In the three and six months ended June 30, 2022, we entered into five and seven, respectively, Startup Structured Partnerships, which provided for payments of Foundry usage fees with convertible financial instruments and equity securities in the aggregate amount of $18.1 million, which will be recognized as revenue over our period of performance. Prior to 2022, we entered into five Startup Structured Partnerships in which we received $16.5 million in upfront consideration in the form of equity interests that is recognized as revenue over our period of performance. Our Legacy Structured Partnerships are described below:
58
Table of Contents
Genomatica, Inc.
Genomatica, Inc. (“Genomatica”) is a biotechnology company specializing in the development and manufacturing of intermediate and specialty chemicals from both sugar and alternative feedstocks. In 2016 and 2018, we entered into separate preferred stock purchase agreements in which we offered cash and R&D services to Genomatica in exchange for its preferred shares. The initial cost of the investment in Genomatica’s preferred stock was $55.0 million. As of June 30, 2022, the carrying value of the investment is $44.9 million and reflects the historical cost less an impairment loss recognized in the second quarter of 2022.
Synlogic, Inc.
Synlogic, Inc. (“Synlogic”) is a publicly traded clinical-stage biopharmaceutical company focused on advancing drug discovery and development for synthetic biology-derived medicines. In 2019, we entered into several agreements with Synlogic whereby we purchased Synlogic common stock and warrants to purchase Synlogic common stock and agreed to provide R&D services to Synlogic. At inception, the fair value of Synlogic common stock and warrants was recorded at $35.8 million and $14.4 million, respectively. As of June 30, 2022, the fair value of Synlogic common stock and warrants was $7.3 million and $2.9 million, respectively.
See Notes 10, 11 and 20 to our audited consolidated financial statements and Notes 4, 5, 12 and 14 to our unaudited condensed consolidated financial statements included elsewhere in this prospectus for further details of our investments in and the material terms of our agreements with our Platform Ventures and Structured Partnerships.
Key Business Metrics
A cell program (or “program”) is the work we do for our customers to enable their product(s) of interest. Programs are defined by a technical development plan. We generally exclude proof-of-concept projects and other exploratory work undertaken on a customer’s behalf from the program count. In the near-term, programs deliver multi-year revenue from platform usage fees. Over the long-term, program growth drives a physical infrastructure scale economic through our Foundry, a data and learning scale economic through our Codebase and accumulation of downstream value share. Our key business metrics comprise New Programs, Current Active Programs, and Cumulative Programs.
| Three Months Ended June 30, |
Six Months Ended June 30, |
LTM1 June 30, |
Years Ended December 31, |
|||||||||||||||||||||||||
| 2022 | 2021 | 2022 | 2021 | 2022 | 2021 | 2020 | ||||||||||||||||||||||
| New Programs |
13 | 7 | 24 | 11 | 44 | 31 | 18 | |||||||||||||||||||||
| Current Active Programs |
73 | 46 | 77 | 51 | 88 | 71 | 49 | |||||||||||||||||||||
| Cumulative Programs |
129 | 85 | 129 | 85 | 129 | 105 | 74 | |||||||||||||||||||||
| (1) | Last 12 months |
New Programs
New Programs represent the number of unique programs commenced within the reporting period. As new programs have multi-year durations, we view this metric as an indication of future Foundry revenue growth.
Current Active Programs
Current Active Programs represent the number of unique programs for which we performed R&D services in the reporting period. We view this metric as an indication of current period and future Foundry revenue.
59
Table of Contents
Cumulative Programs
Cumulative Programs represent the cumulative number of unique programs Ginkgo has commenced. We view this metric as an indication of our competitive advantage and as a leading indicator of the mid- to long-term potential economic value derived from downstream value share arrangements. The cumulative number of programs also contributes to Codebase, which accumulates with each additional program we conduct over time and drives better experimental direction and improves the odds of technical success in current and future programs.
We believe the preceding metrics are important to understand our current business. These metrics may change or be substituted for additional or different metrics as our business develops. For example, as our program mix changes, our data gathering abilities expand or our understanding of key business drivers develops, we anticipate updating these metrics or their definitions to reflect such changes.
Pending Merger
On July 24, 2022, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Zymergen Inc. (“Zymergen”), and Pepper Merger Subsidiary Inc., an indirect wholly owned subsidiary of Ginkgo (“Merger Sub”), providing for the merger of Merger Sub with and into Zymergen (the “Merger”), with Zymergen surviving the Merger as a wholly owned subsidiary of Ginkgo. Under the Merger Agreement, at the effective time of the Merger, each outstanding share of common stock of Zymergen (other than excluded shares specified in the Merger Agreement) will be canceled and converted into the right to receive 0.9179 of a share of Class A common stock of Ginkgo. The transaction is expected to be completed by the first quarter of 2023 subject to approval by the stockholders of Zymergen, receipt of regulatory approvals and satisfaction or waiver of other closing conditions.
For a summary of the transaction, see Note 15, Subsequent Events, to our condensed consolidated financial statements included elsewhere in this prospectus or reference our Form 8-K filed with the SEC on July 25, 2022.
Pending Acquisition
On July 24, 2022, we entered into the APA, pursuant to which we will acquire certain assets and liabilities of Bayer, which are expected to expand our platform capabilities in agricultural biologicals. Under the APA, we will acquire Bayer’s 175,000-square-foot West Sacramento Biologics Research & Development site, team, and internal discovery and lead optimization platform as well as integrate certain R&D platform assets of Joyn, the joint-venture between our holding company Cooksonia and Bayer formed in 2017. As consideration for the assets acquired in the transaction and subject to the terms and conditions of the APA, we have agreed to pay approximately $83.0 million, which may be satisfied, at our discretion, in shares of Ginkgo Class A common stock and/or cash. The transaction is expected to close in the fourth quarter of 2022 subject to regulatory approvals and the satisfaction of customary closing conditions.
Business Combination
We entered into the Merger Agreement with Soaring Eagle Acquisition Corp. (“SRNG”) on May 11, 2021. On September 14, 2021, the SRNG shareholders approved and adopted the Merger Agreement and the other proposals described in SRNG’s definitive proxy statement/prospectus included in SRNG’s registration statement on Form S-4 (File No. 333-256121), which was declared effective by the SEC on August 11, 2021. Upon the consummation of the Business Combination on September 16, 2021, SEAC Merger Sub Inc., a wholly owned subsidiary of SRNG (“Merger Sub”), merged with and into Ginkgo, the separate corporate existence of Merger Sub ceased, and Ginkgo survived the merger as a wholly owned subsidiary of SRNG, which was renamed “Ginkgo Bioworks Holdings, Inc.”
The Business Combination was accounted for as a reverse recapitalization in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). Under the guidance in ASC 805,
60
Table of Contents
Business Combinations (“ASC 805”), SRNG was treated as the “acquired” company for accounting and financial reporting purposes. We were deemed the accounting predecessor of the combined business, and as the parent company of the combined business, are the successor SEC registrant, meaning that our financial statements for previous periods will be disclosed in future periodic reports filed with the SEC. The most significant change in our reported financial position and results of operations as a result of the Business Combination was a net increase in cash (as compared to our audited consolidated balance sheet as of December 31, 2020) of $1,509.6 million, including $760.0 million in proceeds from investments by certain accredited investors for 76,000,000 shares of Ginkgo’s Class A common stock (the “PIPE Investment”) that was consummated substantially simultaneously with the closing of the Business Combination. The transaction costs for the Business Combination totaled $108.1 million.
As the successor to an SEC-registered and publicly-traded company, we will need to hire additional personnel and implement procedures and processes to address public company regulatory requirements and customary practices. We expect to incur additional expenses as a public company for, among other things, directors’ and officers’ liability insurance, director fees, and additional internal and external accounting, legal and administrative resources.
Modification of Equity Awards in Connection with Business Combination
Prior to the Business Combination, our restricted stock units (“RSUs”) were granted with both a service-based vesting condition and a performance-based vesting condition. We have historically not recognized any stock-based compensation expense associated with these awards as the achievement of the performance condition required a change in control or an initial public offering (both as defined in the underlying award agreement) that was not deemed probable of occurring. The Business Combination did not meet the performance condition required for vesting of our RSUs.
On November 17, 2021 our board of directors modified the vesting terms of RSUs to allow 10% of the RSUs that met the service condition as of the closing of the Business Combination (the “10% RSU”) to vest with respect to the performance condition, effective as of November 19, 2021, the date on which the Form S-8 registration statement covering such shares became effective. The remaining RSUs vested in full with respect to the performance condition on or before March 15, 2022. The change to the vesting terms was accounted for as a modification and resulted in approximately $533.2 million and $1,115.0 million of stock-based compensation expense recognized in the three and six months ended June 30, 2022, respectively, and $1,492.2 million of incremental stock-based compensation expense in the fourth quarter of 2021. The 10% RSUs representing appoximately 5.7 million shares were settled in cash for a total cash payment of $76.5 million equal to fair value of the stock on the Form S-8 effective date. Stock-based compensation expense also included $60.8 million and $129.1 million in the three and six months ended June 30, 2022, respectively, and $173.5 million in the fourth quarter of 2021, related to RSU earnout shares which were subject to the same performance condition as the underlying RSUs, in addition to achieving certain target stock price thresholds. The first target stock price of $12.50 per share was achieved on November 15, 2021.
Components of Results of Operations
Revenue
Foundry Revenue
We generate Foundry revenue through the execution of license and collaboration agreements whereby customers obtain license rights to our proprietary technology and intellectual property for use in the development and commercialization of engineered organisms and derived products. Under these agreements, we typically provide R&D services for cell programming with the goal of producing an engineered cell that meets a mutually agreed specification. Our customers obtain license rights to the output of our services, which are primarily the optimized strains or cell lines, in order to manufacture and commercialize products derived from that licensed strain or cell line. Generally, the terms of these agreements provide that we receive some combination of: (1) Foundry usage
61
Table of Contents
fees in the form of (i) upfront payments upon consummation of the agreement or other fixed payments, (ii) reimbursement for costs incurred for R&D services and (iii) milestone payments upon the achievement of specified technical criteria, plus (2) downstream value share payments in the form of (i) milestone payments upon the achievement of specified commercial criteria, (ii) royalties on sales of products from or comprising engineered organisms arising from the collaboration or licensing agreement and (iii) royalties related to cost of goods sold reductions realized by our customers. For the three and six months ended June 30, 2022 and 2021 and the years ended December 31, 2021 and 2020, royalties did not comprise a material amount of our revenue.
Foundry revenue includes transactions with Platform Ventures as well as Structured Partnerships where, as part of these transactions, we received an equity interest in such entities. Specifically related to the Platform Ventures, in these transactions, we received upfront non-cash consideration in the form of common equity interests in these entities, while the Platform Ventures each received cash equity investments from strategic partners and financial investors. We view the upfront non-cash consideration as prepayments for licenses which will be granted in the future as we complete mutually agreed upon technical development plans. In these instances, we also receive cash payments for our costs incurred for the R&D services performed by us, plus a margin. We are not compensated through additional milestone or royalty payments under these arrangements. Our transactions with Genomatica and Synlogic included the purchase of equity securities and the provision of R&D services. Our transactions with Startup Structured Partnerships included the provision of R&D services in exchange for equity interests or financial instruments that are convertible into equity and other downstream value share consideration. As we perform R&D services under the mutually agreed upon development plans, we recognize a reduction in the prefunded obligation based on costs incurred, plus margin basis. These arrangements are further described in Notes 10, 11, 20 and 24 to our audited consolidated financial statements and Notes 4, 5, 12 and 14 to our unaudited condensed consolidated financial statements included elsewhere in this prospectus.
Downstream value share in the form of equity interest appreciation is not recognized as revenue but is expected to contribute to future cash flows upon liquidation, the amount and timing of which is inherently unpredictable. Equity investees are accounted for as equity method investments, cost method investments or carried at fair value.
Biosecurity Revenue
In the second quarter of 2020, in response to the COVID-19 pandemic, we launched our commercial offering of COVID-19 testing products and services for businesses, academic institutions, and other organizations in which we generate product and service revenue. We generate product revenue through the sale of lateral flow assay (“LFA”) diagnostic test kits, polymerase chain reaction (“PCR”) sample collection kits and pooled test kits, all of which we sell to our customers on a standalone basis. We generate service revenue primarily through the sale of our end-to-end COVID-19 testing services which consist of multiple promised goods and services including sample collection kits, physician authorizations, onsite test administration, outsourced laboratory PCR analysis, and access to results reported through a web-based portal.
Generally, the terms of these agreements provide that we are entitled to compensation: (i) upon delivery of diagnostic test kits when no service is provided and (ii) when services are included, upon the reporting of results to the customer.
Beginning in the first quarter of 2021, we launched our pooled testing initiative which focuses on providing end-to-end COVID-19 testing and reporting services to public health authorities. We are currently offering pooled testing and reporting services for K-12 schools across the United States, at airports through our partnership with XpresCheck and the CDC, as well as through other congregate settings such as our partnership with Eurofins and Quest Laboratories in the state of Texas. In the future, we believe that testing services may have a value proposition internationally and in other use cases including wastewater monitoring and air monitoring. The amount and components of Biosecurity revenue are dependent on the demand for COVID-19 testing products and services which is uncertain for the remainder of 2022 and beyond and is subject to seasonality as the demand for COVID-19 testing in schools is diminished during summer vacations and other school breaks.
62
Table of Contents
Costs and Operating Expenses
Cost of Biosecurity Product Revenue
Cost of Biosecurity product revenue consists of costs associated with the sale of diagnostic and sample collection test kits which includes costs incurred to purchase test kits from third parties.
Cost of Biosecurity Service Revenue
Cost of Biosecurity service revenue consists of costs associated with the provision of our end-to-end COVID-19 testing services, which includes costs incurred to provide sample collection kits, physician authorizations, onsite test administration, outsourced laboratory PCR analysis, access to results reported through our proprietary web-based portal and reporting of results to public health authorities.
Research and Development Expenses
The nature of our business, and primary focus of our activities, generates a significant amount of R&D expenses. R&D expenses represent costs incurred by us for the following:
| • | development, operation, expansion and enhancement of our Foundry and Codebase; and |
| • | development of new offerings, such as Biosecurity. |
The activities above incur the following expenses:
| • | laboratory supplies, consumables and related services provided under agreements with third parties and in-licensing arrangements; |
| • | personnel compensation and benefits; and |
| • | rent, facilities, depreciation, software, professional fees and other direct and allocated overhead expenses. |
We expense R&D costs as incurred. As we grow our active programs and customer base and invest in our Foundry and Codebase, we anticipate that our R&D expenses will continue to increase. The nature, timing, and estimated costs required to support our growth will be dependent on advances in technology, our ability to attract new customers and the rate of market penetration within our existing customer industries.
In the first half of 2022, R&D expenses included a significant charge for stock-based compensation expense as a result of the modification of vesting terms of RSUs and the vesting of certain earnout shares (as further described above in “Modification of Equity Awards in Connection with Business Combination”).
General and Administrative Expenses
General and administrative (“G&A”) expenses consist primarily of costs for personnel in executive, business development, finance, human resources, legal and other corporate administrative functions. G&A expenses also include legal fees incurred relating to corporate, intellectual property and patent matters, professional fees incurred for accounting, auditing, tax and administrative consulting services, insurance costs, and facility-related costs not otherwise included in R&D expenses.
We expect our G&A expenses will continue to increase as we pursue organic and inorganic growth initiatives. The increases will likely relate to additional personnel, system costs and increased costs related to business development, finance and legal matters, along with increased expenses related to operating as a publicly traded company, such as fees related to audit, legal and tax services, regulatory compliance programs and investor relations.
In the first half of 2022, G&A expenses included a significant charge for stock-based compensation expense as a result of the modification of vesting terms of RSUs and the vesting of certain earnout shares (as further described above in “Modification of Equity Awards in Connection with Business Combination”).
63
Table of Contents
Interest Income (Expense), Net
Interest income (expense), net primarily consists of interest earned on our cash and cash equivalents offset by interest expense related to our lease financing obligation.
Loss on Equity Method Investments
Loss on equity method investments includes our share of losses from certain of our equity method investments under the hypothetical liquidation at book value (“HLBV”) method.
(Loss) Gain on Investments
(Loss) gain on investments includes the change in fair value of our marketable equity securities in publicly-traded companies and an impairment loss recognized on non-marketable equity securities in privately-held companies.
Change in Fair Value of Warrant Liabilities
Change in fair value of warrant liabilities includes the change in fair value of private placement warrants (“Private Placement Warrants”) and publicly-traded warrants (“Public Warrants”), which are classified as liabilities and were assumed as part of the Business Combination.
Gain on Deconsolidation of Subsidiary
Gain on deconsolidation of subsidiary relates to our deconsolidation of Verb, a variable interest entity, in the first quarter of 2022. The deconsolidation resulted in the removal of Verb’s assets, liabilities and non-controlling interest balances from our balance sheet and the recognition of our retained interest in Verb measured at fair value as of the deconsolidation date.
Other (Expense) Income, Net
Other (expense) income, net primarily consists of change in fair value of our convertible notes with Access Bio, Inc. (“Access Bio”) and promisorry note with Glycosyn which we have elected to account for under the fair value option, income generated from achieving milestones under our agreement with the National Institutes of Health (“NIH”) and sublease rent income.
Provision for Income Taxes
Income taxes are recorded in accordance with ASC 740, Income Taxes (“ASC 740”), which provides for deferred taxes using an asset and liability approach. We recognize deferred tax assets and liabilities for the expected future tax consequences of events that have been included in our financial statements or tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. A valuation allowance against deferred tax assets is recorded if, based on the weight of the available evidence, it is more likely than not that some or all the deferred tax assets will not be realized. For all periods presented, we have recorded a valuation allowance against the deferred tax assets that are not expected to be realized.
We account for uncertain tax positions using a more-likely-than-not threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors, including, but not limited to, changes in the law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or circumstances related to a tax position.
Income taxes are determined at the applicable tax rates adjusted for non-deductible expenses, R&D tax credits and other permanent differences. Our income tax provision may be significantly affected by changes to our estimates.
64
Table of Contents
Results of Operations
Comparison of the Three and Six Months Ended June 30, 2022 and 2021
The following table summarizes our consolidated statements of operations for each period presented:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||||||||||
| (in thousands) | 2022 | 2021 | Change | 2022 | 2021 | Change | ||||||||||||||||||
| Foundry revenue |
$ | 44,242 | $ | 21,592 | $ | 22,650 | $ | 65,730 | $ | 44,096 | $ | 21,634 | ||||||||||||
| Biosecurity revenue: |
||||||||||||||||||||||||
| Product |
3,887 | 355 | 3,532 | 17,834 | 6,130 | 11,704 | ||||||||||||||||||
| Service |
96,489 | 21,689 | 74,800 | 229,459 | 37,507 | 191,952 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total revenue |
144,618 | 43,636 | 100,982 | 313,023 | 87,733 | 225,290 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Costs and operating expenses: |
||||||||||||||||||||||||
| Cost of Biosecurity product revenue |
2,444 | 1,820 | 624 | 10,539 | 11,755 | (1,216 | ) | |||||||||||||||||
| Cost of Biosecurity service revenue |
61,467 | 15,290 | 46,177 | 138,804 | 29,055 | 109,749 | ||||||||||||||||||
| Research and development(1) |
289,188 | 52,031 | 237,157 | 611,908 | 111,616 | 500,292 | ||||||||||||||||||
| General and administrative(1) |
438,427 | 34,440 | 403,987 | 873,195 | 52,367 | 820,828 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
791,526 | 103,581 | 687,945 | 1,634,446 | 204,793 | 1,429,653 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss from operations |
(646,908 | ) | (59,945 | ) | (586,963 | ) | (1,321,423 | ) | (117,060 | ) | (1,204,363 | ) | ||||||||||||
| Other (expense) income: |
||||||||||||||||||||||||
| Interest income (expense), net |
1,674 | (478 | ) | 2,152 | 1,277 | (953 | ) | 2,230 | ||||||||||||||||
| Loss on equity method investments |
(10,166 | ) | (4,346 | ) | (5,820 | ) | (31,053 | ) | (32,970 | ) | 1,917 | |||||||||||||
| (Loss) gain on investments |
(38,673 | ) | 2,755 | (41,428 | ) | (38,223 | ) | 15,377 | (53,600 | ) | ||||||||||||||
| Change in fair value of warrant liabilities |
23,509 | — | 23,509 | 108,544 | — | 108,544 | ||||||||||||||||||
| Gain on deconsolidation of subsidiary |
— | — | — | 15,900 | — | 15,900 | ||||||||||||||||||
| Other (expense) income, net |
(51 | ) | 7,119 | (7,170 | ) | 1,586 | 5,774 | (4,188 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total other (expense) income, net |
(23,707 | ) | 5,050 | (28,757 | ) | 58,031 | (12,772 | ) | 70,803 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss before income taxes |
(670,615 | ) | (54,895 | ) | (615,720 | ) | (1,263,392 | ) | (129,832 | ) | (1,133,560 | ) | ||||||||||||
| Income tax benefit |
(45 | ) | (431 | ) | 386 | (229 | ) | (590 | ) | 361 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss |
(670,570 | ) | (54,464 | ) | (616,106 | ) | (1,263,163 | ) | (129,242 | ) | (1,133,921 | ) | ||||||||||||
| Net loss attributable to non-controlling interest |
(1,745 | ) | (523 | ) | (1,222 | ) | (3,833 | ) | (1,732 | ) | (2,101 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders |
$ | (668,825 | ) | $ | (53,941 | ) | $ | (614,884 | ) | $ | (1,259,330 | ) | $ | (127,510 | ) | $ | (1,131,820 | ) | ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| (1) | In the first half of 2022, R&D and G&A expenses included a significant charge to stock-based compensation expense as a result of the modification of the vesting terms of RSUs and all related earnout shares (as further described above in “Modification of Equity Awards in Connection with Business |
65
Table of Contents
| Combination”). Total stock-based compensation expense, inclusive of $0.8 million and $7.0 million in employer payroll taxes for the three and six months ended June 30, 2022, respectively, was as follows: |
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) | 2022 | 2021 | 2022 | 2021 | ||||||||||||
| Research and development |
$ | 217,291 | $ | 22 | $ | 483,631 | $ | 40 | ||||||||
| General and administrative |
389,979 | 14,497 | 782,674 | 14,597 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 607,270 | $ | 14,519 | $ | 1,266,305 | $ | 14,637 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Foundry Revenue
Foundry revenue increased $22.7 million and $21.6 million in the three and six months ended June 30, 2022 compared to the same periods in 2021. The increase was primarily attributable to progress of Current Active Programs with existing and new customers, including a downstream value share payment related to the achievement of a commercial milestone in the second quarter of 2022. Additionally, revenue increased due to the launch of New Programs and was partially offset by the completion of certain programs. Programs typically require a ramp-up period and/or the achievement of technical and/or commercial milestones before contributing in a meaningful way to revenue.
The total number of Current Active Programs increased from 46 to 73 in the three months ended June 30, 2021 and 2022, respectively. In the second quarter of 2022, 13 New Programs commenced compared to 7 New Programs in the comparable prior year period. Cumulative Programs increased from 85 to 129 in the three months ended June 30, 2021 and 2022, respectively. The number of active customers increased from 22 to 36 in the three months ended June 30, 2021 and 2022, respectively.
The total number of Current Active Programs increased from 51 to 77 in the six months ended June 30, 2021 and 2022, respectively. In the first half of 2022, 24 New Programs commenced compared to 11 New Programs in the comparable prior year period. Cumulative Programs increased from 85 to 129 in the six months ended June 30, 2021 and 2022, respectively. The number of active customers increased from 24 to 37 in the six months ended June 30, 2021 and 2022, respectively.
While the majority of Foundry revenue today is made up of Foundry usage fees, as we increase Cumulative Programs and to the extent our customers successfully commercialize products built on our platform, downstream value share is expected to comprise a larger proportion of Foundry revenue. Downstream value share in the form of equity interest appreciation is not recognized as revenue but is expected to contribute to future cash flows upon liquidation, the amount and timing of which is inherently unpredictable.
Biosecurity Revenue
Biosecurity revenue increased $78.3 million in the three months ended June 30, 2022 compared to the same period in 2021 and was comprised of an increase in product revenue of $3.5 million and an increase in service revenue of $74.8 million.
Biosecurity revenue increased $203.7 million in the six months ended June 30, 2022 compared to the same period in 2021 and was comprised of an increase in product revenue of $11.7 million and an increase in service revenue of $192.0 million.
The amount and components of Biosecurity revenue are dependent on the demand for COVID-19 testing products and services which is uncertain in 2022 and beyond.
66
Table of Contents
Cost of Biosecurity Product and Service Revenue
Cost of Biosecurity product and service revenue increased $46.8 million and $108.5 million in the three and six months ended June 30, 2022, respectively, compared to the same periods in 2021.
The increase was driven by increased demand for our COVID-19 testing products and services.
Research and Development Expenses
Research and development expenses increased $237.2 million in the three months ended June 30, 2022 compared to the same period in 2021. The increase was attributable to increases in personnel-related compensation and benefits expense of $5.4 million, professional fees of $3.6 million, rent and facilities expense of $2.8 million, software and technology expense of $2.4 million, depreciation and amortization expense of $1.9 million, outside services expense of $1.7 million and lab supplies and other direct and allocated overhead expenses of $2.1 million. The remaining change was attributable to an increase in stock-based compensation expense of $217.3 million (inclusive of employer payroll taxes) primarily as a result of the modification of the vesting terms of RSUs and certain earnout shares in the fourth quarter of 2021 (as further described above in “Modification of Equity Awards in Connection with Business Combination”).
Research and development expenses increased $500.3 million in the six months ended June 30, 2022 compared to the same period in 2021. The increase was attributable to increases in depreciation and amortization expense of $5.2 million, personnel-related compensation and benefits expense of $4.6 million, rent and facilities expense of $3.5 million, professional fees of $3.5 million, software and technology expense of $3.2 million, outside services expense of $2.3 million and non-capitalized equipment expense of $2.1 million, partially offset by a decrease in lab supplies of $7.5 million as a result of lower R&D expenses related to our Biosecurity offering and a decrease in other direct and allocated overhead expenses of $0.2 million. The remaining change was attributable to an increase in stock-based compensation expense of $483.6 million (inclusive of employer payroll taxes) primarily as a result of the modification of the vesting terms of RSUs and certain earnout shares in the fourth quarter of 2021 (as further described above in “Modification of Equity Awards in Connection with Business Combination”).
General and Administrative Expenses
General and administrative expenses increased $404.0 million in the three months ended June 30, 2022 compared to the same period in 2021. The increase was attributable to increases in professional fees of $12.6 million primarily as a result of becoming a public company and supporting business scaling, personnel-related compensation and benefits expense of $8.3 million, insurance expense of $3.2 million, marketing expense of $2.1 million, travel and entertainment expense of $1.3 million and increases in software and information technology, depreciation, rent and facilities and other direct and allocated overhead expenses of $1.0 million. The remaining increase was attributable to stock-based compensation expense of $375.5 million (inclusive of employer payroll taxes) primarily as a result of the modification of the vesting terms of RSUs and certain earnout shares in the fourth quarter of 2021 (as further described above in “Modification of Equity Awards in Connection with Business Combination”).
General and administrative expenses increased $820.8 million in the six months ended June 30, 2022 compared to the same period in 2021. The increase was attributable to increases in professional fees of $21.7 million primarily as a result of becoming a public company and supporting business scaling, personnel-related compensation and benefits expense of $15.7 million, insurance expense of $6.5 million, marketing expense of $2.6 million, software and technology expense of $2.1 million, travel and entertainment expense of $1.8 million, depreciation and amortization expense of $1.0 million, rent and facilities expense of $0.8 million and increases in business taxes and other direct and allocated overhead expenses of $0.5 million. The remaining increase was attributable to stock-based compensation expense of $768.1 million (inclusive of employer payroll taxes) primarily as a result of the modification of the vesting terms of RSUs and certain earnout shares in the fourth quarter of 2021 (as further described above in “Modification of Equity Awards in Connection with Business Combination”).
67
Table of Contents
Increases in general and administrative expenses not attributable to stock-based compensation expense supported the growth of Foundry and Biosecurity revenue, merger and acquisition activity and activities related to becoming a public company.
Interest Income (Expense), Net
Interest income (expense), net increased $2.2 million in the three and six months ended June 30, 2022 compared to the same periods in 2021. The increase was due to higher cash balances and increases in interest rates on cash held in money market accounts.
Loss on Equity Method Investments
Loss on equity method investments increased $5.8 million in the three months ended June 30, 2022 compared to the same period in 2021. The increase was primarily attributable to our equity method investments in BiomEdit and Joyn. The initial fair value of our common units received in BiomEdit of $8.9 million during the three months ended June 30, 2022 has been subsequently reduced to $4.3 million as a result of the application of the HLBV method. The loss on our equity method investment in Joyn increased from $4.3 million in the three months ended June 30, 2021 to $5.4 million in the three months ended June 30, 2022, representing our share of the investee’s losses under the HLBV method.
Loss on equity method investments decreased $1.9 million in the six months ended June 30, 2022 compared to the same period in 2021. The decrease was primarily attributable to our equity method investments in BiomEdit, Verb, Joyn, Arcaea and Allonnia. During the six months ended June 30, 2022, we recorded a $4.6 million loss on our equity method investment in BiomEdit as a result of the application of the HLBV method. Upon the deconsolidation of Verb in the first quarter of 2022, we recorded a $15.9 million loss on our retained investment in Verb due to a basis difference associated with in-process research and development identified as part of the initial accounting for the equity method investment. The loss on our equity method investment in Joyn increased from $8.4 million in the six months ended June 30, 2021 to $10.4 million in the six months ended June 30, 2022, representing our share of the investee’s losses under the HLBV method. The increase in losses related to BiomEdit, Verb and Joyn were offset by a decrease in losses related to Arcaea and Allonnia. The fair value of the additional equity we received in Arcaea of $11.9 million during the six months ended June 30, 2021 was reduced to zero during the period as a result of the application of the HLBV method. The fair value of the additional equity we received in Allonnia of $12.7 million during the six months ended June 30, 2021 was also reduced to zero during the period as a result of the application of the HLBV method.
Under the HLBV method, we absorb losses as a common unit holder prior to preferred unit holders due to a substantive profit-sharing agreement where the preferred unit holders receive preferential distribution rights. Because we have no commitment to fund the losses of Verb, Arcaea or Allonnia, no further losses on these equity method investments were recognized during the periods presented.
(Loss) Gain on Investments
(Loss) gain on investments decreased $41.4 million and $53.6 million in the three and six months ended June 30, 2022, respectively, compared to the same periods in 2021. The decrease was driven by decreases in the stock prices of our marketable equity securities and a $10.1 million impairment loss recognized on our investment in Genomatica preferred stock during the second quarter of 2022.
Change in Fair Value of Warrant Liabilities
The change in fair value of warrant liabilities of $23.5 million and $108.5 million in the three and six months ended June 30, 2022, respectively, was due to a decrease in the estimated fair value of the Private Placement Warrants and a decrease in the quoted price of the Public Warrants primarily driven by a decrease in our stock price.
68
Table of Contents
Gain on Deconsolidation of Subsidiary
Gain on deconsolidation of subsidiary relates to our deconsolidation of Verb and consisted of our $15.9 million retained interest in Verb measured at fair value as of the deconsolidation date.
Other (Expense) Income, Net
Other (expense) income, net decreased $7.2 million in the three months ended June 30, 2022 compared to the same period in 2021. The decrease was primarily attributable to a $7.3 million decrease in the change in fair value of the Access Bio Convertible Notes.
Other (expense) income, net decreased $4.2 million in the six months ended June 30, 2022 compared to the same period in 2021. The decrease was primarily attributable to a $4.7 million decrease in the change in fair value of the Access Bio Convertible Notes.
Comparison of the Years Ended December 31, 2021 and 2020
The following table summarizes our consolidated statements of operations for each period presented:
| Year Ended December 31, | ||||||||||||
| (in thousands) | 2021 | 2020 | Change | |||||||||
| Foundry revenue (related party revenue of $47,161 and $42,535 for the years ended 2021 and 2020, respectively) |
$ | 112,989 | $ | 59,221 | $ | 53,768 | ||||||
| Biosecurity revenue: |
||||||||||||
| Product |
23,040 | 8,707 | 14,333 | |||||||||
| Service |
177,808 | 8,729 | 169,079 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
313,837 | 76,657 | 237,180 | |||||||||
|
|
|
|
|
|
|
|||||||
| Costs and operating expenses: |
||||||||||||
| Cost of Biosecurity product revenue |
20,017 | 6,705 | 13,312 | |||||||||
| Cost of Biosecurity service revenue |
109,673 | 8,906 | 100,767 | |||||||||
| Research and development(1) |
1,149,662 | 159,767 | 989,895 | |||||||||
| General and administrative(1) |
862,952 | 38,306 | 824,646 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
2,142,304 | 213,684 | 1,928,620 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(1,828,467 | ) | (137,027 | ) | (1,691,440 | ) | ||||||
| Other (expense) income: |
||||||||||||
| Interest income |
837 | 2,582 | (1,745 | ) | ||||||||
| Interest expense |
(2,373 | ) | (2,385 | ) | 12 | |||||||
| Loss on equity method investments |
(77,284 | ) | (396 | ) | (76,888 | ) | ||||||
| Loss on investments |
(11,543 | ) | (3,733 | ) | (7,810 | ) | ||||||
| Change in fair value of warrant liabilities |
58,615 | — | 58,615 | |||||||||
| Gain on settlement of partnership agreement |
23,826 | 8,286 | 15,540 | |||||||||
| Other (expense) income, net |
(1,733 | ) | 7,839 | (9,572 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Total other (expense) income, net |
(9,655 | ) | 12,193 | (21,848 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
(1,838,122 | ) | (124,834 | ) | (1,713,288 | ) | ||||||
| Income tax (benefit) provision |
(1,480 | ) | 1,889 | (3,369 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(1,836,642 | ) | (126,723 | ) | (1,709,919 | ) | ||||||
| Net loss attributable to non-controlling interest |
(6,595 | ) | (114 | ) | (6,481 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders |
$ | (1,830,047 | ) | $ | (126,609 | ) | $ | (1,703,438 | ) | |||
|
|
|
|
|
|
|
|||||||
69
Table of Contents
| (1) | In the fourth quarter of 2021, R&D and G&A expenses included a significant adjustment for stock-based compensation expense as a result of the modification of the vesting terms of RSUs and all related earnout shares (as further described above in “Modification of Equity Awards in Connection with Business Combination”). Total stock-based compensation expense inclusive of employer payroll taxes was allocated as follows (in thousands): |
| Year Ended December 31, | ||||||||||||
| 2021 | 2020 | 2019 | ||||||||||
| Research and development |
$ | 930,360 | $ | 79 | $ | 64 | ||||||
| General and administrative |
757,247 | 397 | 707 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
$ | 1,687,607 | $ | 476 | $ | 771 | ||||||
Foundry Revenue
Foundry revenue was $113.0 million for the year ended December 31, 2021 and $59.2 million for the year ended December 31, 2020. The increase of $53.8 million in Foundry revenue was primarily attributable to the progress of programs with existing and new customers, including downstream value share revenue related to the achievement of commercial milestones.
The total number of Current Active Programs increased from 49 in 2020 to 71 in 2021. In 2021, 31 New Programs were commenced. Cumulative Programs increased from 74 in 2020 to 105 in 2021. The number of customers increased from 22 in 2020 to 33 in 2021.
While the majority of Foundry revenue today is made up of Foundry usage fees, as we increase Cumulative Programs and to the extent our customers successfully commercialize products built on our platform, downstream value share is expected to comprise a larger proportion of Foundry revenue. Downstream value share in the form of equity interest appreciation is not recognized as revenue but is expected to contribute to future cash flows upon liquidation, the amount and timing of which is inherently unpredictable.
Biosecurity Revenue
Biosecurity revenue was $200.8 million for the year ended December 31, 2021 and $17.4 million for the year ended December 31, 2020. The increase of $183.4 million in Biosecurity revenue was comprised of an increase in product revenue of $14.3 million and an increase in service revenue of $169.1 million.
The amount and components of Biosecurity revenue are dependent on the demand for COVID-19 testing products and services which is uncertain in 2022 and beyond.
Cost of Biosecurity Product and Service Revenue
Cost of Biosecurity product and service revenue was $129.7 million for the year ended December 31, 2021 and $15.6 million for the year ended December 31, 2020. The increase of $114.1 million was driven by the increase in the demand for our COVID-19 testing products and services.
Research and Development Expenses
Research and development expenses were $1,149.7 million for the year ended December 31, 2021 and $159.8 million for the year ended December 31, 2020. The increase of $989.9 million was primarily attributable to increases in stock-based compensation expense of $930.3 million (inclusive of employer payroll taxes) as a result of the modification of the vesting terms of RSUs and certain earnout shares in the fourth quarter of 2021 (as further described above in “Modification of Equity Awards in Connection with Business Combination”), personnel-related compensation and benefits expense of $22.6 million, rent and facilities expense of $11.8 million, and professional fees of $6.0 million. The remaining increase was attributable to depreciation and amortization expense of
70
Table of Contents
$14.9 million and other direct and allocated overhead expenses. Increases in research and development expenses not attributable to stock-based compensation expense supported the Foundry operations, enhancements of Foundry and Codebase, and development of our Biosecurity offering.
General and Administrative Expenses
General and administrative expenses were $863.0 million for the year ended December 31, 2021 and $38.3 million for the year ended December 31, 2020. The increase of $824.6 million was primarily attributable to increases in stock-based compensation expense of $756.9 million (inclusive of employer payroll taxes) as a result of the modification of the vesting terms of RSUs and certain earnout shares in the fourth quarter of 2021 (as further described above in “Modification of Equity Awards in Connection with Business Combination”), personnel-related compensation and benefits expense of $23.5 million, professional fees of $19.9 million, software and technology expense of $7.2 million, rent and facilities expense of $4.2 million, insurance expense of $3.5 million and marketing expense of $2.7 million. The remaining increase was attributable to business taxes and other direct and allocated overhead expenses. Increases in general and administrative expenses not attributable to stock-based compensation expense supported the growth of Foundry and Biosecurity revenue and activities related to public company readiness.
Interest Income (Expense), Net
Interest income was $0.8 million and $2.6 million for the years ended December 31, 2021 and 2020, respectively.
The decrease of $1.8 million was primarily due to a decrease in interest rates on our cash held in money market accounts.
Interest expense was $2.4 million for each of the years ended December 31, 2021 and 2020 and was related to our lease financing obligation.
Loss on Equity Method Investments
Loss on equity method investments was $77.3 million and $0.4 million for the years ended December 31, 2021 and 2020, respectively. The increase of $76.9 million was attributable to our equity method investments in Joyn, Allonnia and Arcaea. The fair value of the initial equity we received in Arcaea of $47.4 million during the year ended December 31, 2021 was reduced to zero during the period as a result of the application of the HLBV method. The fair value of the additional equity we received in Allonnia of $12.7 million during the year ended December 31, 2021 was also reduced to zero during the period as a result of the application of the HLBV method. Under the HLBV method, we absorb losses as a common unit holder prior to preferred unit holders due to a substantive profit-sharing agreement where the preferred unit holders receive preferential distribution rights. Because we have no commitment to fund the losses of Arcaea or Allonnia, no further losses on these equity method investments were recognized during the year ended December 31, 2021. The loss on our equity method investment in Joyn increased from $0.4 million to $17.2 million during the years ended December 31, 2020 and 2021, respectively, representing our share of the investee’s losses under the HLBV method.
Loss on Investments
Loss on investments was $11.5 million and $3.7 million for the years ended December 31, 2021 and 2020, respectively. The change of $7.8 million was attributable to fluctuations in the stock price of our shares of Synlogic and Cronos common stock and warrants to purchase Synlogic common stock.
Change in Fair Value of Warrant Liabilities
The change in fair value of warrant liabilities of $58.6 million for the year ended December 31, 2021 was due to a decrease in the estimated fair value of the Private Placement Warrants and a decrease in the quoted price of the Public Warrants.
71
Table of Contents
Gain on Settlement of Partnership Agreement
Gain on settlement of partnership agreement was $23.8 million and $8.3 million for the years ended December 31, 2021 and 2020, respectively. The increase of $15.5 million was due to Amyris making a prepayment in 2021 in full settlement of all amounts due under the partnership agreement.
Other (Expense) Income, Net
Other (expense) income, net was $(1.7) million and $7.8 million for the years ended December 31, 2021 and 2020, respectively. The decrease of $9.5 million was primarily attributable to a $6.6 million decrease in payments received from the NIH, a $4.5 million change in fair value of the Access Bio convertible notes and Glycosyn promissory note, partially offset by net increase in other miscellaneous income.
Non-GAAP Information
In addition to our results determined in accordance with U.S. GAAP, we believe that EBITDA and Adjusted EBITDA, each non-GAAP financial measures, are useful in evaluating our operational performance. We use this non-GAAP financial information to evaluate our ongoing operations and for internal planning and forecasting purposes. We believe that this non-GAAP financial information, when taken collectively, may be helpful to investors in assessing our operating performance.
We define EBITDA as net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders before the impact of interest income, interest expense, provision for income taxes and depreciation and amortization.
We define Adjusted EBITDA as EBITDA adjusted for stock-based compensation expense, gain or loss on equity method investments, gain or loss on investments, change in fair value of warrant liabilities, gain on deconsolidation of subsidiary, acquired in-process research and development in connection with asset acquisitions, and other income and expenses. In the second quarter of 2022, we redefined Adjusted EBITDA to exclude transaction and integration costs associated with planned, completed or terminated mergers and acquisitions and have recast our previous first quarter 2022 Adjusted EBITDA calculation to exclude these costs and conform to the new presentation. We believe that the use of EBITDA and Adjusted EBITDA provides an additional tool for investors to use in evaluating ongoing operating results and trends because it eliminates the effect of financing activities, investing activities, and certain non-cash charges and other items. Adjusted EBITDA includes non-cash adjustments such as stock-based compensation, gain or loss on equity method investments, and gain or loss on changes in fair value of our investments, warrant liabilities, loans receivable and contingent consideration liability. Adjusted EBITDA also considers cash components which are not part of our ongoing operating results such as gains related to settlement payments from Amyris, certain funding received from the NIH to invest in our biosecurity development related to COVID-19 and transaction and integration costs incurred in connection with mergers and acquisitions.
We believe Adjusted EBITDA, although not a replacement for financial performance measures reported under U.S. GAAP, provides investors with a means to compare our financial measures with those of comparable companies, which may present similar non-GAAP financial measures to investors. However, you should be aware that when evaluating EBITDA and Adjusted EBITDA we may generate future income or incur future expenses similar to those excluded when calculating these measures. In addition, our presentation of these measures should not be construed as an inference that our future results will be unaffected by future income or future expenses similar to those excluded when calculating these measures. Our computation of these measures, especially Adjusted EBITDA, may not be comparable to other similarly titled measures computed by other companies because not all companies calculate these measures in the same way. Because of these limitations, EBITDA and Adjusted EBITDA should not be considered in isolation or as a substitute for performance measures calculated in accordance with U.S. GAAP. We compensate for these limitations by primarily relying on our U.S. GAAP results supplemented by EBITDA and Adjusted EBITDA. You should review the reconciliation of net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders to EBITDA and Adjusted EBITDA below and not rely on any single financial measure to evaluate our business.
72
Table of Contents
The following table reconciles net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders to EBITDA and Adjusted EBITDA for the three and six months ended June 30, 2022 and 2021, respectively:
| Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
| (in thousands) | 2022 | 2021 | 2022 | 2021 | ||||||||||||
| Net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders |
$ | (668,825 | ) | $ | (53,941 | ) | $ | (1,259,330 | ) | $ | (127,510 | ) | ||||
| Interest (income) expense, net |
(1,674 | ) | 478 | (1,277 | ) | 953 | ||||||||||
| Income tax benefit |
(45 | ) | (431 | ) | (229 | ) | (590 | ) | ||||||||
| Depreciation and amortization |
9,608 | 7,165 | 19,096 | 12,794 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| EBITDA |
(660,936 | ) | (46,729 | ) | (1,241,740 | ) | (114,353 | ) | ||||||||
| Stock-based compensation(1) |
607,270 | 14,519 | 1,266,305 | 14,637 | ||||||||||||
| Loss on equity method investments(2) |
9,952 | 3,823 | 30,216 | 31,238 | ||||||||||||
| Loss (gain) on investments |
38,673 | (2,755 | ) | 38,223 | (15,377 | ) | ||||||||||
| Change in fair value of warrant liabilities |
(23,509 | ) | — | (108,544 | ) | — | ||||||||||
| Gain on deconsolidation of subsidiary |
— | — | (15,900 | ) | — | |||||||||||
| Merger and acquisition related expenses(3) |
2,716 | — | 6,562 | — | ||||||||||||
| In-process research and development(4) |
1,605 | — | 1,605 | — | ||||||||||||
| Other(5) |
906 | (6,406 | ) | 332 | (4,831 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Adjusted EBITDA |
$ | (23,323 | ) | $ | (37,548 | ) | $ | (22,941 | ) | $ | (88,686 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | For the three and six months ended June 30, 2022, includes employer payroll taxes of $0.8 million and $7.0 million, respectively. |
| (2) | Represents losses on equity method investments under the HLBV method, net of losses attributable to non-controlling interests. |
| (3) | Represents transaction and integration costs directly related to mergers and acquisitions including (i) due diligence, legal and other professional fees associated with acquisitions and (ii) the fair value adjustments to contingent consideration liabilities resulting from acquisitions. In the second quarter of 2022, we redefined Adjusted EBITDA to exclude the impact of merger and acquisition related expenses. We elected to recast our previous first quarter 2022 Adjusted EBITDA calculation to exclude these costs and conform to the new presentation. |
| (4) | Represents acquired intangible assets expensed to research and development associated with an asset acquisition. |
| (5) | For the three and six months ended June 30, 2022, includes change in fair value of Access Bio Convertible Notes. For the three and six months ended June 30, 2021, includes change in fair value of Access Bio Convertible Notes and gain related to a settlement payment from Amyris. |
73
Table of Contents
The following table reconciles net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders to EBITDA and Adjusted EBITDA for the years ended December 31, 2021 and 2020, respectively:
| Year Ended December 31, | ||||||||
| (in thousands) | 2021 | 2020 | ||||||
| Net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders |
$ | (1,830,047 | ) | $ | (126,609 | ) | ||
| Interest income |
(837 | ) | (2,582 | ) | ||||
| Interest expense |
2,373 | 2,385 | ||||||
| Income tax (benefit) provision |
(1,480 | ) | 1,889 | |||||
| Depreciation and amortization |
29,076 | 13,864 | ||||||
|
|
|
|
|
|||||
| EBITDA |
(1,800,915 | ) | (111,053 | ) | ||||
| Stock-based compensation(1) |
1,687,607 | 476 | ||||||
| Loss on equity method investments(2) |
74,445 | 282 | ||||||
| Loss on investments(3) |
11,543 | 3,733 | ||||||
| Change in fair value of warrant liabilities |
(58,615 | ) | — | |||||
| Gain on settlement of partnership agreement |
(23,826 | ) | (8,286 | ) | ||||
| Other(4) |
3,712 | (6,574 | ) | |||||
|
|
|
|
|
|||||
| Adjusted EBITDA |
$ | (106,049 | ) | $ | (121,422 | ) | ||
|
|
|
|
|
|||||
| (1) | For the year ended December 31, 2021, includes $5.0 million in employer payroll taxes related to stock-based compensation. |
| (2) | For the years ended December 31, 2021 and 2020, represents losses on equity method investments under the HLBV method of $77.3 million and $0.4 million, respectively, net of losses attributable to non-controlling interests. |
| (3) | Includes loss on the change in fair value of our common stock investments in Synlogic and Cronos and warrants to purchase Synlogic common stock, which are all carried at fair value. |
| (4) | For the year ended December 31, 2021, includes $3.7 million in mark-to-market adjustments on Access Bio convertible notes and the Glycosyn Promissory Note. For the year ended December 31, 2020, includes $6.6 million received from the NIH. |
Liquidity and Capital Resources
Sources of Liquidity
Prior to the Business Combination, our sources of liquidity have been predominantly cash flows from equity offerings, convertible notes offerings, payments received for R&D services under license and collaboration arrangements including those received on an upfront basis and upon accomplishment of milestones, payments received from Biosecurity product sales and services, and government grants. Upon the closing of the Business Combination in September 2021, we received net proceeds totaling approximately $1,509.6 million, inclusive of $760.0 million from the PIPE Investment. As of June 30, 2022, we had cash and cash equivalents of $1,377.2 million which we believe will be sufficient to enable us to fund our projected operations through at least the next 12 months from the date of filing of this prospectus.
Material Cash Requirements
We anticipate that our expenditures will increase significantly in connection with our ongoing activities, as we:
| • | continue our R&D activities under existing and new programs and further invest in our Foundry and Codebase; |
| • | hire additional personnel and secure facilities to support our expanding R&D efforts; |
| • | develop and expand our offerings, including Biosecurity; |
74
Table of Contents
| • | upgrade and expand our operational, financial and management systems and support our operations; |
| • | acquire and integrate companies, assets or intellectual property that advance our company objectives; |
| • | maintain, expand, and protect our intellectual property; and |
| • | incur additional costs associated with operating as a public company. |
Other than as described below, during the first six months of 2022, there were no significant changes to our material cash requirements, including contractual and other obligations.
Purchase Obligations
In March 2022, we entered into a four-year noncancelable supply agreement for the purchase of diverse products including synthetic DNA. Under the agreement, we are obligated to spend a minimum of $58.0 million over the four-year term, with approximately $10.8 million payable in the next 12 months.
We also have a noncancelable purchase obligation with a supplier under which we are obligated to pay at least $109.0 million, and up to $150.0 million, over a seven-year term ending in 2026 for minimum purchases of certain equipment, associated consumables and other goods and services. The purchase obligation includes variable license fees and milestone payments of up to $11.5 million upon achievement of development and regulatory milestones. As of December 31, 2021, we had approximately $86.5 million remaining under the purchase obligation, with approximately $13 million payable within 12 months. Additionally, we have minimum purchase volume commitments with certain suppliers.
Operating Leases
We have various noncancelable operating leases for office and laboratory space that begin to expire on dates ranging from 2030 through 2036. As of December 31, 2021, we have minimum rental commitments under noncancelable operating leases of $20.6 million in 2022 and $664.8 million thereafter. In June 2022, we entered into an amendment to our headquarters lease in Boston, Massachusetts, which is expected to increase our total base rent payments by approximately $23.1 million over the life of the lease beginning in April 2023 through January 2036, with approximately $0.4 million due in the next twelve months.
Capital Expenditures
As of December 31, 2021, we anticipate our cumulative spending on capital expenditures to be in the range of $115 million over the next twelve months, subject to management’s ongoing reassessment, to support our commercial plan as we strategically invest in capacity and technology to deliver new cell programs.
Cash Flows
The following table provides information regarding our cash flows for each period presented:
| Six Months Ended June 30, | Year Ended December 31, | |||||||||||||||
| (in thousands) | 2022 | 2021 | 2021 | 2020 | ||||||||||||
| Net cash (used in) provided by: |
||||||||||||||||
| Operating activities |
$ | (119,195 | ) | $ | (83,042 | ) | $ | (253,818 | ) | $ | (135,830 | ) | ||||
| Investing activities |
(50,648 | ) | (46,977 | ) | (73,257 | ) | (67,121 | ) | ||||||||
| Financing activities |
(2,146 | ) | (2,556 | ) | 1,534,145 | 90,318 | ||||||||||
| Effect of exchange rate changes |
(104 | ) | — | (19 | ) | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net increase (decrease) in cash, cash equivalents and restricted cash |
$ | (172,093 | ) | $ | (132,575 | ) | $ | 1,207,051 | $ | (112,633 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
75
Table of Contents
Operating Activities
Net cash used in operating activities for the six months ended June 30, 2022 consisted of net loss of $1,263.2 million, adjusted for net change in operating assets and liabilities of $63.1 million and non-cash charges of $1,207.1 million. The net change in operating assets and liabilities was primarily due to a $38.6 million increase in accounts receivable driven by an increase in Biosecurity revenue, a $4.7 million increase in inventory from increased purchases of LFA and pooled test kits in response to higher demand, a $12.8 million decrease in accrued expenses and other current liabilities, a $19.7 million decrease in deferred revenue, a $4.0 million decrease in other non-current liabilities, partially offset by a $10.7 million increase in accounts payable and a $5.0 million decrease in prepaid expenses and other current assets. Non-cash charges primarily consisted of $19.1 million in depreciation and amortization expense, $1,259.3 million in stock-based compensation expense, $31.1 million loss on equity method investments, $38.2 million loss on investments, partially offset by $18.1 million of non-cash equity consideration received from a customer upon achievement of a milestone, $108.5 million gain on the change in fair value of warrant liabilities and $15.9 million gain on the deconsolidation of Verb.
Net cash used in operating activities for the six months ended June 30, 2021 consisted of net loss of $129.2 million, adjusted for net change in operating assets and liabilities of $5.6 million and non-cash charges of $40.6 million. The net change in operating assets and liabilities was primarily due to a $4.9 million decrease in prepaid expenses and other current assets and a $19.1 million increase in accrued expenses and other current liabilities, partially offset by a $6.5 million increase in accounts receivable, a $7.3 million decrease in accounts payable and a $6.1 million decrease in deferred revenue. Non-cash charges primarily consisted of $12.8 million in depreciation and amortization expense, $14.6 million in stock-based compensation expense, $33.0 million loss on equity method investments, partially offset by a $15.4 million gain on investments and a $4.4 million gain on the change in fair value of loans receivable.
Net cash used in operating activities for the year ended December 31, 2021 consisted of net loss of $1,836.6 million, adjusted for net change in operating assets and liabilities of $61.5 million and non-cash charges of $1,644.4 million. The net change in operating assets and liabilities was primarily due to an increase in accounts receivable of $114.1 million driven by an increase in biosecurity revenue and a decrease in deferred revenue of $10.5 million, partially offset by an increase in accrued expenses and other current liabilities of $44.8 million primarily due to biosecurity revenue accruals, an increase in deferred rent of $6.0 million as a result of entering into new leases and expanding the terms of existing leases and an increase in other non-current liabilities of $18.6 million primarily due to a $20.0 million customer deposit liability. Non-cash adjustments primarily consisted of depreciation and amortization of $29.1 million, stock-based compensation expense of $1,606.0 million, loss on equity method investments of $77.3 million and loss on investments of $11.5 million, partially offset by gain on change in fair value of warrant liabilities of $58.6 million and non-cash equity consideration of $24.2 million from milestones associated with a customer collaboration arrangement.
Net cash used in operating activities for the year ended December 31, 2020 consisted of net loss of $126.7 million and a net change in operating assets and liabilities of $26.5 million, offset by non-cash adjustments of $17.4 million. The net change in operating assets and liabilities was primarily due to a decrease in current and non-current deferred revenue of $19.4 million, an increase in accounts receivable and accounts receivable from related parties of $14.2 million and an increase in prepaid expenses and other current assets of $11.4 million, partially offset by an increase in accounts payable of $7.0 million and an increase in accrued expenses and other current liabilities of $8.7 million. Non-cash adjustments primarily consisted of depreciation and amortization of $13.9 million, loss on equity method investments of $0.4 million and loss on investments of $3.7 million, partially offset by changes in the fair value of loans receivable of $1.1 million.
Investing Activities
Net cash used in investing activities for the six months ended June 30, 2022 primarily consisted of purchases of property and equipment of $13.2 million associated with Foundry capacity and capability investments, purchase
76
Table of Contents
of marketable equity securities of $3.7 million, relinquishment of $28.8 million in cash upon the deconsolidation of Verb, $6.5 million convertible note financing provided to Joyn and $1.4 million of cash acquired in acquisition.
Net cash used in investing activities for the six months ended June 30, 2021 primarily consisted of purchases of property and equipment of $46.0 million associated with Foundry capacity and capability investments.
Net cash used in investing activities for the year ended December 31, 2021 primarily consisted of purchases of property and equipment of $56.5 million associated with Foundry capacity and capability investments, purchase of non-marketable equity securities of $5.0 million and acquisition of Dutch DNA for $12.0 million.
Net cash used in investing activities for the year ended December 31, 2020 primarily consisted of purchases of property and equipment of $57.8 million, including costs associated with Foundry capacity and capability investments and purchase of Access Bio’s convertible notes of $10.0 million.
Financing Activities
Net cash used in financing activities for the six months ended June 30, 2022 primarily consisted of principal payments on capital leases and lease financing obligation, tax withholding payments related to net share settlement of equity awards and payment of contingent consideration related to a business acquisition.
Net cash used in financing activities for the six months ended June 30, 2021 primarily consisted of principal payments on capital leases and lease financing obligation and payments of deferred offering costs.
Net cash provided by financing activities for the year ended December 31, 2021 primarily consisted of net proceeds received from the Business Combination of $1,509.6 million, non-controlling interest contributions of $59.9 million related to our consolidated variable interest entities (“VIEs”), Ayana and Verb, partially offset by repurchases of common stock from our founders of $25.0 million and tax withholding payments related to net share settlement of equity awards of $9.5 million.
Net cash provided by financing activities for the year ended December 31, 2020 primarily consisted of the net proceeds from the issuance of our Series E convertible preferred stock.
Critical Accounting Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these consolidated financial statements requires us to make judgments and estimates that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our consolidated financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. On an ongoing basis, we evaluate our judgments and estimates in light of changes in circumstances, facts and experience. The effects of material revisions in estimates, if any, are reflected in our consolidated financial statements prospectively from the date of change in estimates.
While our significant accounting policies are described in more detail in Note 2 to our audited annual consolidated financial statements appearing elsewhere in this prospectus, we believe the following accounting policies used in the preparation of our consolidated financial statements require the most significant judgments and estimates.
77
Table of Contents
Revenue Recognition
We account for revenue in accordance with ASC 606, Revenue from Contracts with Customers (“ASC 606”). Under ASC 606, we recognize revenue when the customer obtains control of the promised goods or services, at an amount that reflects the consideration we expect to receive in exchange for those goods or services. To determine revenue recognition for arrangements that are within the scope of ASC 606, we perform the following five steps: (i) identify the contract(s) with a customer, (ii) identify the promises and distinct performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations in the contract, and (v) recognize revenue when (or as) we satisfy the performance obligations.
Foundry Revenue
We generate license and service revenue through the execution of license and collaboration agreements whereby customers obtain license rights to our proprietary technology and intellectual property for use in the research, development and commercialization of engineered organisms and derived products. Under these agreements, we typically provide R&D services, which includes the provision of a license to our intellectual property. Additionally, the customer obtains license rights to the output of our services in order to commercialize the resulting output of such services. Generally, the terms of these agreements provide that we receive some combination of: (1) Foundry usage fees in the form of (i) upfront payments upon consummation of the agreement or other fixed payments, (ii) reimbursement for costs incurred for R&D services and (iii) milestone payments upon the achievement of specified technical criteria, plus (2) downstream value share payments in the form of (iv) milestone payments upon the achievement of specified commercial criteria, (v) royalties on sales of products from or comprising engineered organisms arising from the collaboration or licensing agreement and (vi) royalties related to cost of goods sold reductions realized by our customers, or (3) downstream value share in the form of equity interests in our customer.
Our collaboration and licensing agreements often contain multiple promises, including (i) licenses and assignments of intellectual property and materials and (ii) R&D services, and we determine whether each of the promises is a distinct performance obligation based on the nature of each agreement. As we are generally performing R&D services that are highly integrated and interrelated to the licenses and assignments of intellectual property and materials, the promises are generally inseparable. As such, we typically combine the R&D services, licenses, and assignments into a single performance obligation. However, for certain agreements, we only grant licenses or effects such transfers and assignments upon the successful completion of the R&D services or delivery of a developed product. For these agreements, we typically consider (i) the R&D services and (ii) the licenses, transfers, and assignments as distinct performance obligations, as each is transferred separately and has a separately identifiable benefit. Options to acquire additional goods and services are evaluated to determine if such options provide a material right to the counterparty that it would not have received without entering into the contract. If so, the option is accounted for as a separate performance obligation. If not, the option is considered a marketing offer which is accounted for as a separate contract upon the counterparty’s election.
At contract inception, we determine the transaction price, including fixed consideration and any estimated amounts of variable consideration. Any upfront cash payment received upon consummation of the agreement is fixed and generally nonrefundable. Variable consideration is subject to a constraint, and amounts are included in the transaction price to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. Variable consideration may include reimbursement for costs incurred for our R&D efforts, milestone payments upon the achievement of certain technical and commercial criteria, and royalties on sales of products from or comprising engineered organisms arising from the agreement. With respect to the R&D reimbursements and milestone payments, we use the most likely amount method to estimate variable consideration. With respect to agreements that include royalties on sales or other contingent payments based on
78
Table of Contents
sales, we apply the royalty recognition constraint which requires a constraint until the royalty or value-sharing transaction occurs. Certain agreements contain payment in the form of equity or other non-cash consideration. Any non-cash consideration is measured at the fair value of the non-cash consideration at contract inception.
For agreements with promises that are combined into a single performance obligation, the entire transaction price is allocated to the single performance obligation. For agreements with multiple performance obligations, the transaction price is allocated to the performance obligations using the relative standalone selling price methodology. For agreements featuring variable consideration, we allocate variable consideration to one or more, but not all, performance obligations if certain conditions are met. Specifically, we assess whether the variable consideration relates solely to our efforts to satisfy the performance obligation and whether allocating such variable consideration entirely to the performance obligation is consistent with the overall allocation objective. If these conditions are not met, we allocate the variable consideration based on the relative standalone selling price methodology. The key assumptions utilized in determining the standalone selling price for each performance obligation include development timelines, estimated R&D costs, commercial markets, likelihood of exercise (in the case of options considered to be material rights), and probabilities of success.
For agreements where the licenses or assignments are considered separate performance obligations or represent the only performance obligation, we recognize revenue at the point in time that we effectively grant the license as the licenses or assignments represent functional intellectual property. For agreements where the licenses and the R&D services represent a combined performance obligation, we recognize revenue over the period of performance based on costs incurred to date as compared to total estimated costs.
We evaluate our measure of progress to recognize revenue at each reporting period and, as necessary, adjust the measure of performance and related revenue recognition. Our measure of performance and revenue recognition involves significant judgment and assumptions, including, but not limited to, estimated costs and timelines to complete our performance obligations. We evaluate contract modifications and amendments to determine whether any changes should be accounted for prospectively or on a cumulative catch-up basis. We utilize the right to invoice practical expedient when we have a right to consideration in an amount that corresponds directly with the value of our performance to date.
Royalties received under the agreements are recognized as revenue when sales have occurred as we apply the sales or usage-based royalties recognition constraint. We have determined the application of this exception is appropriate because the license granted in the agreement is the predominant item to which the royalties relate.
As we receive upfront payments for technical services under certain of our arrangements, we evaluate whether any significant financing components exist given the term over which the fees will be earned may exceed one year. Based on the nature of our agreements, there are no significant financing components as the purpose of the upfront payment is not to provide financing, but rather to secure technical services, exclusivity rights, and Foundry capacity, or the timing of transfer of those goods or services is at the discretion of the customer.
Deferred revenue represents consideration received by us in excess of revenue recognized and primarily results from transactions where we receive upfront payments and non-cash equity consideration. In instances where we have received consideration in advance for an undefined number of technical development plans (“TDPs”) under our customer agreements, we record the advance payments as deferred revenue, net of current portion on our consolidated balance sheets. Upon the execution of a specific TDP, we reclassify the estimated consideration to be earned under that TDP within the next twelve months as current deferred revenue. We also classify unexercised material rights as deferred revenue, net of current portion on our consolidated balance sheets. When a TDP is executed, and the material right is exercised, the amount allocated to the material right, which will be earned within the next twelve months, is reclassified to current deferred revenue. All other deferred revenue is classified as current or non-current based on the timing of when we expect to earn the underlying revenue based upon the projected progress of activities under the TDP.
79
Table of Contents
Biosecurity Revenue
In 2020, we launched our commercial offering of COVID-19 testing products and services for businesses, academic institutions, and other organizations. In the first quarter of 2021, we launched our pooled testing initiative which focuses on providing end-to-end COVID-19 testing services to groups of individuals, with a focus on K-12 schools. We sell COVID-19 test kits on a standalone basis or as part of an end-to-end testing service. We record product revenue from sales of LFA, PCR, and pooled test kits. We record service revenue from sales of our end-to-end COVID-19 testing services, which consist of multiple promised goods and services including sample collection kits, physician authorizations, onsite test administration, outsourced laboratory PCR analysis, and access to results reported through the Company’s proprietary web-based portal. We recognize our product and service revenue using the five-step model under ASC 606.
Product revenue is recognized when the test kits are shipped and risk of loss is transferred to the carrier. Our test kits are generally not subject to a customer right of return except for product recalls under the rules and regulations of the U.S. Food and Drug Administration (“FDA”). We have elected to include shipping and handling fees billed to customers as a component of Biosecurity revenue.
Service revenue from our end-to-end COVID-19 testing services is recognized upon completion of the tests and release of the test results on the web-based portal. We have identified one performance obligation in our testing services contracts that represents a series of distinct goods or services that are substantially the same and that have the same pattern of transfer to the customer, with each test as a distinct service within the series. As the price for the testing services is fixed under each customer contract, we have elected the practical expedient to recognize revenue at the amount which we have the right to invoice for services performed. Our testing services contracts are generally one year or less in length and contain fixed unit pricing. Under typical payment terms for testing services, amounts are billed monthly in arrears for services performed or in advance based on contractual billing terms.
Variable Interest Entities
We evaluate our variable interests in variable interest entities (“VIEs”) and consolidate VIEs when we are the primary beneficiary. We determine whether we are the primary beneficiary of each VIE based on our assessment of whether we possess both (i) the power to direct the activities that most significantly affect the VIE’s economic performance and (ii) the obligation to absorb losses that could be significant to the VIE or the right to receive benefits that could be significant to the VIE. We reevaluate the accounting for our VIEs upon the occurrence of events that could change the primary beneficiary conclusion. With respect to our investments in Motif, Allonnia, Genomatica and Arcaea, we have concluded these entities represent variable interest entities. However, although we hold board representation and are involved in the ongoing development activities of the entities via participation on joint steering committees, we have concluded that we are not the primary beneficiary of these entities. We have reached this conclusion due to the fact that: (i) we do not control the board of directors of Motif, Allonnia, Genomatica or Arcaea and no voting or consent agreements exist between ourselves and other members of each respective board of directors or other investors, (ii) the holders of preferred security interests in Motif, Allonnia, Genomatica and Arcaea hold certain rights that require their consent prior to the taking of certain actions, which include certain significant operating and financing decisions and (iii) our representation on the joint steering committee of each respective entity does not give us control over the development activities of either Motif, Allonnia, Genomatica or Arcaea as all votes must pass by consensus and there are no agreements in place that would require either entity to vote in alignment with ourselves. As our involvement in Motif, Allonnia, Genomatica and Arcaea does not give us the power to control the decisions with respect to the development or other activities, which are the most significant activities of Motif, Allonnia, Genomatica or Arcaea, we have accordingly concluded that we are not the primary beneficiary. Additionally, with respect to Cooksonia’s investment in Joyn, as Cooksonia does not control Joyn’s board of directors, it does not have the power to control the decisions related to the development activities of Joyn, which are the most significant activities of Joyn. Accordingly, Cooksonia is not the primary beneficiary of Joyn.
80
Table of Contents
With respect to Cooksonia, we have concluded that we hold a variable interest in this entity through our equity interest and we are the primary beneficiary of Cooksonia as we control the most significant activities of Cooksonia. These conclusions were based on the fact that: (i) we control 100% of the board of directors of Cooksonia and (ii) we hold a controlling financial interest in Cooksonia. Due to the fact that we are the primary beneficiary of Cooksonia, we have consolidated the financial statements of Cooksonia in accordance with ASC 810, Consolidation (“ASC 810”), into our consolidated financial statements and have recognized a non-controlling interest associated with the minority equity interest held by other investors of Cooksonia.
With respect to Ayana and Verb, as of December 31, 2021, we concluded that we held a variable interest in and were the primary beneficiary of Ayana and Verb as we controlled the most significant activities of these entities. These conclusions were reached because, as of the primary beneficiary assessment dates in 2021, for both Verb and Ayana: (i) we had substantive control of the board of directors; (ii) all capital contributions were made by related parties of Ginkgo; and (iii) Ginkgo or its related parties comprised the entirety of the Joint Steering Committee, the governing body which holds significant oversight with respect to the entities’ research and development programs.
As of June 30, 2022, there has been no change in the consolidation status of Cooksonia and Ayana. During the first quarter of 2022, Verb hired a new chief executive officer who was not an affiliate, related party or agent of Ginkgo. The chief executive officer was also appointed to Verb’s Joint Steering Committee and board of directors. As a result, we concluded we no longer had substantive control of the board of directors or the Joint Steering Committee. Accordingly, we concluded that we were no longer the primary beneficiary of Verb as we no longer controlled the most significant activities of the entity. As a result of this change in the primary beneficiary determination, we deconsolidated the entity in the first quarter of 2022.
Impairment of Long-Lived Assets
We review our long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. Recoverability is measured by comparing the book values of the assets to the expected future net undiscounted cash flows that the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the book values of the assets exceed their fair value.
Determination of Fair Value of Non-cash Consideration in Platform Ventures
The fair value of non-cash consideration received in relation to our Platform Ventures is in return for the license rights conveyed to the counterparty. We value the non-cash consideration, which is generally common stock or common units, at inception of the agreements using an option pricing method (“OPM”). The OPM used a back-solve methodology to infer the total equity value based on the pricing of the preferred financing round associated with the formation of the respective Platform Ventures, which was contemporaneous with the intellectual property agreements that conveyed our license rights to such Platform Ventures.
Determination of Fair Value of Loans Receivable
We have elected the fair value option under ASC 825, Financial Instruments (“ASC 825”), to account for our loans receivable. We use various valuation techniques to fair value our loans receivable, which are dependent on the terms of the underlying agreements, and record the gains or loss arising from the change in fair value as a component of other income (expense), net in our consolidated statements of operations and comprehensive loss. As of June 30, 2022 and December 31, 2021, our loans receivable balance primarily consisted of our revolving promissory note with Glycosyn and a series of convertible notes with Access Bio. We used a probability-weighted discounted cash flow valuation approach to value our revolving promissory note with Glycosyn. Under this approach, the present value of the expected cash flows was calculated under four settlement scenarios and then weighted based on the probability of each scenario. A discount rate was also applied. Both the probability
81
Table of Contents
and timing of each scenario and the discount rate represented significant inputs used in valuing the revolving promissory note. We used a Monte-Carlo simulation model to determine the value of our convertible notes with Access Bio, which modeled the future stock price of Access Bio over the term of the convertible notes to assess the value of the various settlement features. The significant assumptions used in determining the simulated future stock price included the expected timing of the conversion, which was assumed at maturity, and expected volatility. The significant assumptions used in determining the value of the convertible notes under a redemption at maturity scenario was the discount rate and expected volatility. Refer to Notes 2, 5 and 6 to our consolidated financial statements and Note 3 to our unaudited condensed consolidated financial statements appearing elsewhere in this registration statement and prospectus for additional details.
Determination of Fair Value of Common Stock
For awards granted prior to the Business Combination, the fair value of shares of common stock underlying our stock-based awards was determined on each grant date by Ginkgo, considering our most recently available third-party valuations of common stock and our assessment of additional objective and subjective factors that we believed were relevant and which may have changed from the date of the most recent valuation through the grant date. Historically, these independent third-party valuations of our equity instruments were performed contemporaneously with identified value inflection points. The third-party valuations were prepared in accordance with the framework of the American Institute of Certified Public Accountants’ Technical Practice Aid, Valuation of Privately Held Company Equity Securities Issued as Compensation (the “Practice Aid”). The Practice Aid identifies various available methods for allocating enterprise value across classes and series of capital stock to determine the estimated fair value of common stock at each valuation date.
In addition to considering the results of these third-party valuations, we considered various objective and subjective factors to determine the fair value of our equity instruments as of each grant date, which may be later than the most recently available third-party valuation date, including:
| • | the lack of liquidity of our equity as a private company; |
| • | the prices of our convertible preferred stock sold to outside investors in arm’s length transactions and the rights, preferences and privileges of our convertible preferred stock as compared to those of our common stock, including the liquidation preferences of our convertible preferred stock; |
| • | the progress of our R&D efforts to develop our proprietary platform; |
| • | our stage of development and business strategy and the material risks related to our business and industry; |
| • | the valuation of publicly traded companies in the life sciences and biotechnology sectors, as well as recently completed mergers and acquisitions of peer companies; |
| • | any external market conditions and trends within the life sciences industry; |
| • | the likelihood of achieving a liquidity event given prevailing market conditions; and |
| • | the analysis of initial public offerings and the market performance of similar companies in the life sciences industry. |
The assumptions underlying these valuations represent management’s best estimates, which involve inherent uncertainties and the application of management judgment. As a result, if factors or expected outcomes change and we use significantly different assumptions or estimates, the fair value of our stock-based awards could be materially different. The fair value of our common stock is determined based on the quoted market price on the NYSE. We estimate the fair value of our common stock using a hybrid method which uses market approaches to estimate our enterprise value. The hybrid method is a probability-weighted expected return method (“PWERM”) where the equity value in at least one scenario is allocated using an OPM.
Under the PWERM, the value of common stock is estimated based on an analysis of future values assuming various possible future liquidity events. The value of common stock is based on the probability-weighted present
82
Table of Contents
value of expected future investment returns considering the possible outcomes and the rights and privileges of each class of equity. The future investment returns are discounted back to the valuation date at a risk-adjusted discount rate which is then weighted based on the probability of the respective outcome.
Under the OPM, each class of stock is treated as a call option on our equity value, with exercise prices based on the liquidation preferences of our convertible preferred stock. Under this methodology, the common stock has value only if the funds available for distribution to the holders exceeds the value of the liquidation preferences of the convertible preferred stock at the time of the liquidity event. The Black-Scholes model is used to price the call options which includes assumptions for the time to liquidity and volatility of equity value. A discount for lack of marketability is then applied to the common stock value.
For awards granted from January 2021 through September 30, 2021, we utilized the hybrid method to estimate the value of our common stock underlying our stock-based awards. We considered two scenarios: (i) a scenario in which the conversion of the convertible preferred stock to common stock occurs through an initial public offering (“IPO”) or a merger with a special purpose acquisition company (“SPAC”), and (ii) a remain-private scenario. With respect to the remain-private scenario, we estimated equity value using the guideline public company method. With respect to the IPO/SPAC scenario, we considered the equity values indicated by preliminary letters of intent received from potential investors or the assumed equity value in the proposed business combination of $15 billion plus contingent consideration in the form of earnout shares. In the IPO/SPAC transaction scenario, conversion of the convertible preferred stock to common stock was assumed. In the remain private scenario, equity value was allocated among the convertible preferred stock and common stock using the OPM. In addition to considering these two scenarios, we considered the prices paid for our common stock and Series B convertible preferred stock in secondary transactions and we included these prices in our weighted average conclusion of value.
For awards granted from August 2020 through December 31, 2020, when using the hybrid method, we considered two scenarios: (i) a scenario in which the conversion of the convertible preferred stock to common stock occurred through an IPO or SPAC transaction, and (ii) a remain private scenario. In both scenarios, we estimated an equity value in a potential IPO or SPAC transaction based on the guideline public company method under a market approach. We then converted the estimated future value to present value using a risk-adjusted discount rate. In the IPO or SPAC transaction scenario, conversion of the convertible preferred stock to common stock was assumed. In the remain private scenario, equity value was allocated among the convertible preferred stock and common stock using the OPM. In addition to considering these two scenarios, we considered the prices paid for our common stock and Series B convertible preferred stock in secondary transactions and we included these prices in our weighted average conclusion of value.
For awards granted from January 1, 2019 through July 2020, when using the hybrid method we considered two scenarios: (i) a fully diluted scenario, in which the per-share common stock value was assumed to equal the price of the convertible preferred stock in a recent round of financing, and (ii) a remain private scenario, in which we used the OPM to back-solve to the price of our convertible preferred stock in a recent round of financing. In the fully diluted scenario, conversion of the convertible preferred stock to common stock was assumed. In the remain private scenario, equity value was allocated among the convertible preferred stock and common stock using the OPM. In addition to considering these two scenarios, for certain valuations during the period, we considered the prices paid for our common stock in secondary transactions and we included these prices in our weighted average conclusion of value.
For awards granted after the Business Combination, we determine the fair value of our common stock on each grant date based on the quoted market price on the NYSE.
We estimate the grant date fair value of stock option awards using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model requires the input of subjective assumptions, including fair value of common stock (for awards granted prior to the Business Combination), expected term, expected volatility, risk-free
83
Table of Contents
interest rate and expected dividend yield. The expected term was generally determined using the “simplified” method for “plain vanilla” options. We determined expected volatility using the historical volatility of the stock prices of similar publicly traded peer companies. The risk-free interest rate was based on the yield available on U.S. Treasury zero-coupon issues similar in duration to the expected term of the stock options. We have not paid, and do not expect to pay, dividends in the foreseeable future.
There are significant judgments and estimates inherent in determining the fair value of the common stock. These judgments and estimates include factors, both subjective and objective, including: (i) a discount for lack of marketability; (ii) external market data; (iii) historical activity by us in selling equity to outside investors; (iv) our stage of development; (v) rights and preferences of our equity securities that rank senior to common stock; and (vi) the likelihood of the various scenarios, among others. Changes to these assumptions could result in different fair values of common stock.
JOBS Act and Emerging Growth Company Status
In April 2012, the JOBS Act was enacted. As an EGC under the JOBS Act, we may delay the adoption of certain accounting standards until such time as those standards apply to private companies. Other exemptions and reduced reporting requirements under the JOBS Act for EGCs include presentation of only two years of audited financial statements in a registration statement for an initial public offering, an exemption from the requirement to provide an auditor’s report on internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act of 2002, an exemption from any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation and less extensive disclosure about our executive compensation arrangements. Additionally, the JOBS Act provides that an EGC can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an EGC to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to avail ourselves of the extended transition period and, therefore, while we are an EGC we will not be subject to new or revised accounting standards while they become applicable to other public companies that are not EGCs, unless we choose to early adopt a new or revised accounting standard.
We will remain classified as an EGC until the earlier of: (i) the last day of our first fiscal year in which we have total annual gross revenues of $1.07 billion or more, (ii) the last day of the fiscal year following the fifth anniversary of completion of the IPO of SRNG, (iii) the date on which we have issued more than $1.0 billion of non-convertible debt instruments during the previous three fiscal years or (iv) the date on which we are deemed a “large accelerated filer” under the rules of the SEC.
Recently Issued Accounting Pronouncements
See Note 2, “Summary of Significant Accounting Policies,” to our audited consolidated financial statements and Note 1, “Basis of Presentation and Summary of Significant Accounting Policies,” to our unaudited condensed consolidated financial statements contained elsewhere in this prospectus for a discussion of recently issued accounting pronouncements.
84
Table of Contents
BUSINESS OF GINKGO
Unless the context otherwise requires, all references in this section to the “Company,” “Ginkgo,” “we,” “us,” or “our” refer to the business of Ginkgo Bioworks Holdings, Inc. and its subsidiaries.
Mission
Our mission is to make biology easier to engineer. That has never changed. Every choice we’ve made with respect to our business model, our platform, our people and our culture is grounded in whether it will advance our mission. Biology inherently offers incredible capabilities that we can only imagine in human-made technologies—self-assembly, self-repair, self-replication—capabilities that can enable more renewable and innovative approaches for nearly every industry. To realize this potential, we are building a platform for cell programming by bringing together unparalleled scale, software, automation, data science and reusable biological knowledge, enabling responsible solutions for the next generation of foods, pharmaceuticals, materials and more.
Overview
Ginkgo is building the industry-standard horizontal platform for cell programming. Our founders are engineers from diverse fields who, more than 20 years ago, were inspired by an astonishing feature of biology: it runs on digital code. It’s just A, T, C, and G rather than 0 and 1. But where computer bits are used to communicate information, genetic code is inherently physical and as it is read, physical structures are made. We program computers to manipulate bits, but we program cells to manipulate atoms. Cells are the building blocks of our food, our environment and even ourselves.
We use our platform to program cells on behalf of our customers. These “cell programs” (or “programs”) are designed to enable biological production of products as diverse as novel therapeutics, key food ingredients, and chemicals currently derived from petroleum. Through June 30, 2022, we have worked on 129 cumulative programs, which represent the cumulative number of unique programs Ginkgo has commenced in end markets as diverse as specialty chemicals, agriculture, food, consumer products, and pharmaceuticals (the “Cumulative Programs”). Biology did not evolve by end market. All of these applications run on cells which have a common code—DNA—and a common programming platform can enable all of them. Because of this shared platform, we are able to drive scale and learning efficiencies while maintaining flexibility and diversity in our program areas. Ultimately, customers come to us because they believe we maximize the probability of successfully developing their products.
Customers look to Ginkgo to overhaul their manufacturing processes or develop new products through biology. They might, for example, be looking to produce a particular chemical via fermentation, at a lower cost, with enhanced supply chain reliability or sustainability. Or perhaps the customer needs a microbe that will live and grow on the roots of corn and convert nitrogen in the air into usable fertilizer for a plant, resulting in improved plant growth. Or a customer might need an antibody that binds to and neutralizes a certain target, along with a way to produce those antibodies at scale. All of these programs and more run on a common platform at Ginkgo.
We care deeply how our platform is used and recognize biosecurity as a necessary complement to our cell programming work. Biosecurity is ingrained in our platform and in our ethos—we work collaboratively to help ensure responsible research conduct and deployment within our own work and within the broader synthetic biology community. We have additionally invested in developing targeted biosecurity and public health capabilities, including an initiative for pathogen biomonitoring at scale. Through this initiative, known as Concentric by Ginkgo, we serve as a system integrator to implement customizable and accessible biosecurity programs for monitoring and mitigating the spread of COVID-19 in thousands of communities across the country. We are dedicated to setting the industry standard for biosecurity as we continue to scale our platform.
The foundation of our platform includes two core assets that execute a wide variety of cell programs for customers according to their specifications: our Foundry and our Codebase.
| • | Our Foundry wraps proprietary software and automation around core cell engineering workflows— designing DNA, writing DNA, inserting that DNA into cells, testing to measure cell performance—and |
85
Table of Contents
| leverages data analytics and data science to inform each iteration of design. The software, automation and data analysis pipelines we leverage in the Foundry drive a strong scale economic: we have scaled the output of the Foundry by roughly 3X annually since we started measuring it around 2015 (with the exception of 2020 during the COVID-19 pandemic) and over that time, the average cost per unit operation has fallen by approximately 50% every year. We expect to be able to pass these savings along to our customers, allowing them to take more “shots on goal” with their programs. |
| • | Our Codebase includes both our physical (engineered cells and genetic parts) and digital (genetic sequences and performance data) biological assets, and accumulates as we execute more cell programs on the platform. Every program, whether successful or not, generates valuable Codebase and helps inform future experimental designs and provides reusable genetic parts, making our cell program designs more efficient. |
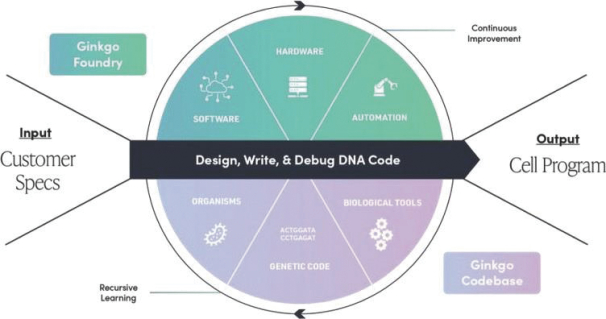
Figure 1: Our platform is used to design, write, and debug DNA code in engineered organisms to execute programs for our customers. Our Foundry leverages proprietary software, automation, and data analytics to reduce the cost of cell programming. Our Codebase consists of reusable biological assets that helps accelerate the engineering process.
As the platform scales, we have observed a virtuous cycle between our Foundry, our Codebase, and the value we deliver to customers. Sketched below, we believe this virtuous cycle sustains Ginkgo’s growth and differentiated value proposition.
| • | Foundry: As we take on more work in the Foundry, we benefit from scale economics, which over time may lead to lower program costs. We expect that these lower costs, in turn, will drive additional demand for our cell programming capabilities. |
| • | Codebase: Cell programs also generate Codebase, which can drive better experimental direction and improve the odds of technical success, further increasing our customer value proposition, which we believe will result in additional demand. |
86
Table of Contents
Put simply: we believe that as the platform improves with scale, it drives more scale, which drives further platform improvements, and so on. We believe this positive feedback loop has the potential to drive compounding value creation in the future, as every new program we add contributes to both near-term revenues and has the potential to add significant downstream economics.
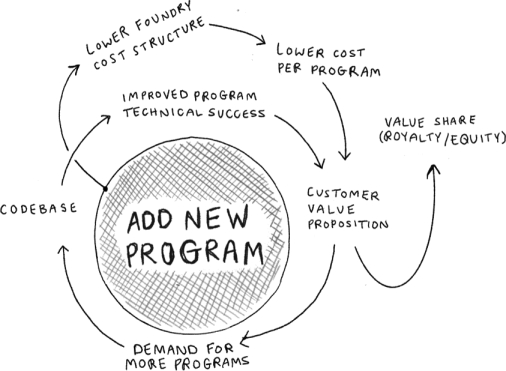
Figure 2: Ginkgo’s virtuous cycle: as we scale, we see greater efficiency and higher odds of technical success, which helps drive further scaling as our value proposition improves.
Our business model mirrors the structure of our platform and we are compensated in two primary ways. First, we charge usage fees for Foundry services, in much the same way that cloud computing companies charge usage fees for utilization of computing capacity or contract research organizations (“CROs”) charge for services. The total addressable market for biological research and development (“R&D”) services, including labor and tools, was estimated to be over $40 billion in 2021 and is growing strongly. Additionally, we negotiate a value share with our customers (typically in the form of royalties, milestones, and/or equity interests) in order to align our economics with the success of the programs enabled by our platform. As we add new programs, our portfolio of programs with this “downstream” value potential grows. Through these value shares, we are tapping into what industry sources expect to be a $2 to $4 trillion market for bioengineered products in the next 10 to 20 years.
We believe that cell programming has the potential to be as ubiquitous in the physical world as computer programming has become in the digital world. We believe products in the future will be grown rather than made. To enable that vision, we are building a horizontal platform to make biology easier to engineer. Our business model is aligned with this strategy and with the success of our customers, setting us on what we believe is a path towards sustainable innovation for years to come.
An Introduction to Synthetic Biology
To fully tell the story of cell programming, we have to start four billion years ago. All living things evolved from a single cell, a tiny bubble containing the code that enabled it to assemble and reproduce itself. But, importantly,
87
Table of Contents
that process of reproduction wasn’t perfect; each copy introduced new mutations in the code. These changes are responsible for one of the most powerful and defining features of biology: evolution. Over eons, that first cell and all its progeny copied themselves, and their DNA evolved to create new functions: to eat new kinds of foods and to produce new kinds of chemicals, structures, and behaviors. As reproduction became more, well, interactive, organisms developed tools to borrow DNA from each other, accelerating the pace of evolution. These functions, and thus the genetic code programming the functions, stuck around when they helped the organisms survive and create more descendants. This went on and on for four billion years, leaving us the wild codebase of DNA that enables the diversity of life forms we see on the planet today.
Synthetic biology’s story begins mere decades ago, as biologists began to decode the molecular secrets of DNA. The billions-year-old tools of cells—enzymes that cut, copy, and paste sequences of DNA code—are now being leveraged by humans to read, write, and edit DNA in the lab. Polymerases that copy DNA are used to enable polymerase chain reaction (“PCR”) tests for COVID-19 and the CRISPR/Cas system from bacteria now enables editing of human genomes to potentially cure genetic diseases.
Today we are using these tools to learn from the full breadth of evolution and biodiversity to write new biological code. Simple soil bacteria produce everything from vital antibiotics to the smell of fresh rain. We can reuse elements of these DNA programs to make new products. Biochemistry is extraordinarily versatile; we’ve reused the same genetic code libraries across applications as diverse as fine fragrances, baking, and consumer electronics. We may be able to develop programs that can digest human-made “forever chemicals” that biology never encountered before.
As cell programmers, we operate with humility and respect for biology. Our tools are simply borrowed, and the history of biotechnology is a mere blink of an eye compared to the history of living things. Today, we write rudimentary code. We believe that someday our children will write poetry in DNA.
Programming life

Figure 3: DNA strands are sequences composed of four chemical bases, or nucleotides, represented by the letters A, T, C and G.
Like computers, cells run on digital code. DNA strands are sequences composed of four chemical bases, or nucleotides, represented by the letters A, T, C and G. The letters along the strand encode the proteins that make up the cell and perform biochemical functions. The translation of DNA to RNA to Protein is known as the “central dogma” of molecular biology.
88
Table of Contents
The Central Dogma of Molecular Biology
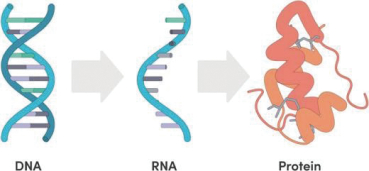
Figure 4: The translation of DNA to RNA to Protein is known as the “central dogma” of molecular biology.
“Traditional” genetic engineering uses special types of proteins from bacteria that can cut and paste DNA to move sequences from one organism to another. In 1982, Genentech Inc. partnered with Eli Lilly and Company to bring these techniques to market, producing human insulin inside the bacteria E. coli. Genetic engineers were able to cut the code for the human insulin protein and paste it into the genome of E. coli and “boot up” the sequence: the bacteria could now produce the human protein, which could then be extracted, purified, and used by diabetics. This life-saving development replaced a vastly more expensive and supply-constrained method of extracting insulin from animal pancreases.
Relatively simple proteins like insulin can be produced by transferring one gene sequence into a simple microorganism. Many other biochemicals require much more complex cell programming and are produced by a series of special proteins, called enzymes, working together. These enzymes transform a starting material, or “feedstock,” such as sugar, into a final product, such as an antibiotic, vitamin, or other valuable small molecule. In this way, biology also programs chemistry. Cell programmers can design such multi-enzyme “pathways” and transfer them into a cell to boot up. For example, the cell programs we’re writing for Cronos Group, Inc. to produce cannabinoids require many different enzymes to convert feedstock into cannabinoids such as cannabidiol.
Once the cell is programmed to produce a new molecule, it can produce the molecule and also replicate itself, creating an exponentially growing number of product-producing cells. Many products of genetic engineering are manufactured in facilities that look like breweries, taking advantage of the centuries old process of industrial fermentation to grow cells at high density, and transforming simple sugars into valuable products that can be extracted and commercialized.
Improved tools for cell programming, including automation, miniaturization, and data science, alongside the decreasing cost of DNA synthesis—writing DNA—are opening up new possibilities for cell programming. For each new program, Ginkgo’s organism engineers design, print, and test hundreds or thousands of different
89
Table of Contents
sequences for each step of a pathway, exploring the breadth of biological design space and improving the probability of success. We provide more details about our platform in the sections that follow.
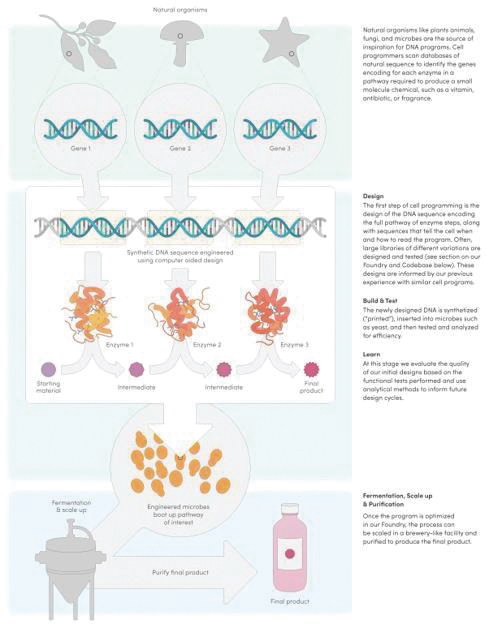
Figure 5: An overview of a simple cell program.
The Impact of Cell Programming
The power of biology has never been more apparent. Synthetic biology was featured on the cover of The Economist in April of 2019. A few years later, billions of doses of a novel type of vaccine have been distributed, created by companies such as Pfizer and Moderna and made up of a form of biological code, mRNA. Our own cells read that code to produce viral proteins and stimulate our immune response to fight back against the SARS-CoV-2 virus. We no longer question if biotechnology will transform a given industry, we simply question whether we are creative enough to imagine how, and whether we are ready to utilize biology responsibly.
90
Table of Contents
ESG is in our DNA
Biology affects all of us, and we believe cell programming will change the world. Our customers are developing products with far reaching implications in health and the environment. This potential for extraordinary impact, which reaches to the core of who we are and everything about our natural world, requires extraordinary care in how the tools of cell programming are built and used. Technologies reflect the values of the organizations that build them, so our commitment to Environmental, Social, and Governance (“ESG”) priorities and care must underscore everything we do.
We also must recognize that biotechnologies have not always reflected the values necessary for sustainable and equitable impact and, as a result, remain controversial. Indeed, companies that produce genetically modified organisms (“GMOs”) for human consumption are restricted from certain ESG indices, placing genetic engineering as a major ESG risk alongside the production of weapons, tobacco products, and fossil fuels. We hope to chart a new course built on care so that the world can benefit from the power of biological engineering while avoiding potential risks.
Environmental
We face an urgent environmental crisis that is forcing us to reconsider how we make everything, from our homes, to our food, to our clothing. For centuries, we’ve treated nature as an infinite resource and infinite trash can, extracting raw materials, shaping them through industrial processes that spew out greenhouse gases, and then throwing them away. But these resources are not infinite and there is no “away.” The results have been disastrous—climate change, loss of biodiversity, and pollution have impacted every corner of our world and continue to threaten our way of life.
Cell programming and biological manufacturing are working to address some of the issues that are most contributing to climate change today, from fossil fuel dependency to agricultural emissions, and land use to plastic pollution. Ultimately, biology offers a fundamental shift in how things are made and disposed of: a world where things grow and decay, creating circular, regenerative processes.
There is significant concern that genetic engineering itself creates a form of genetic “pollution” in the environment, with genes from one context introduced into another. This is a concern we take seriously and consider deeply throughout the lifecycle of our programs to ensure that genes introduced will not cause damage—for example, by spreading antibiotic resistance or toxins. We care because the environmental release of certain genetically engineered microbes can also offer tremendous environmental benefit. For example:
| • | Crop-associated microbes programmed with the nitrogen fixing properties of common soil bacteria may be able to reduce the use of chemical fertilizers, which have been estimated to contribute 5% of global greenhouse gas emissions and account for 4% of natural gas consumption. This is the work of Joyn Bio, LLC (“Joyn”) our joint venture with Bayer CropScience LP. |
| • | Microbes programmed to clean up wastewater or contaminated land is the work of Allonnia, LLC (“Allonnia”), a company we formed in partnership with Battelle. |
And we are just getting started… we believe biology is our best tool to reverse the damage to our planet and chart us on a path towards sustainability in the future.
Social
Technology isn’t neutral. Our values and biases are embedded in the technologies we make, in the applications we consider, and in the ways we address problems. Inclusion of those who have historically been left out of the development of new technologies is essential to building equitable and positive outcomes. Just as biological ecosystems thrive with more diversity, the inclusion of many different voices is essential to growing our company and to ensuring that the viewpoints of historically marginalized people are included in the development
91
Table of Contents
of our platform. We have many active efforts in recruiting and retaining diverse talent and will continue to invest in this work (see “—Our People & Culture”).
Marginalized people who have been left out of the development of technologies are also the groups most likely to bear the greatest harm, whether from climate change, pollution, or health disparities. The COVID-19 pandemic has made this inequality starkly clear—in the United States, it has been communities of color that have been disproportionately impacted by the pandemic and have had the least access to testing, treatment, and vaccination.
In March of 2020, we committed to $25 million of pro bono work to help accelerate novel diagnostics, therapeutics, and vaccines to help fight COVID-19. Our early work included efforts to improve the manufacturing of vaccines, with a goal to lower costs and increase accessibility of vaccines worldwide. Shortly thereafter, we launched Concentric by Ginkgo, a service to provide public health testing infrastructure for communities. Our pooled testing service was designed with accessibility and privacy as core design principles, to bring low-cost, easy-to-use testing to K-12 schools in the places that have been most affected by the pandemic. We partnered with school districts such as Baltimore City Public Schools to make sure that our program was designed to serve the community and to build trust with groups who have been excluded, exploited and mistreated by biomedical research in the past.
These values and initiatives are not just a top-down corporate policy, they are an intrinsic part of our culture. Grassroots fundraising challenges to support local and international aid organizations are a regular feature of our internal messaging channels. One of our software engineers even programmed a free tool, @vaccinetime on Twitter, that helped thousands of Massachusetts residents find vaccine appointments.
Governance
Our culture is built on care, transparency, diversity, employee ownership and engagement, and a deep, humble respect for biology. Transparency is essential to how we operate, to enable sharing of the insights and tools that enable our platform to grow, as well as to build trust and accountability with all of our stakeholders. We have advocated for more transparency in our industry, including supporting GMO labeling, and seek to educate policymakers and the general public about the benefits and risks of synthetic biology through our advocacy efforts.
The individuals who work at Ginkgo and build our platform care deeply about how that platform is used and the impact our company will have in the world. We believe a workforce with strong equity ownership will make the wise decisions needed to build long-term value for our company, and a company whose long-term impacts make them proud. That is why we have implemented a multi-class stock structure that permits all employees (current and future), not just founders, to hold high-vote (10 votes per share) common stock. We believe that our multi- class stock structure will help maintain the long-term mentality we have benefited from as a founder-led company.
For more information, see “Risk Factors—Risks Relating to our Organizational Structure and Governance— Only our employees and directors are entitled to hold shares of Ginkgo Class B common stock (including shares of our Class B common stock granted or otherwise issued to our employees and directors in the future), which shares have 10 votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of certain amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring stockholder approval.”
We have selected directors with decades of experience serving as leaders in the life sciences and technology industries. Our board of directors and management team will leverage that experience and consider the interests of stockholders, customers, employees, suppliers, academic researchers, governments, communities, and other
92
Table of Contents
stakeholders to pursue long-term value for our company and drive the sustained health of our global community. For more information, see “Risk Factors—Risks Relating to our Organizational Structure and Governance—Our focus on the long-term best interests of our company and our consideration of all of our stakeholders, including our stockholders, workforce, customers, suppliers, academic researchers, governments, communities and other stakeholders that we may identify from time to time, may conflict with short-term or medium-term financial interests and business performance, which may adversely impact the value of our common stock.”
Cell programming is expected to transform all industries
Biology grows. Biology adapts and evolves. Biology heals itself and regenerates. Biology is also, remarkably, programmable, offering us the tools to work with biology to transform how we make stuff. With cell programming, we help our customers across industries grow better products. What does “better” mean? Better products might be more sustainable, have more stable and resilient supply chains, be more accessible, have higher quality and more consistency, and come with lower economic and environmental costs of manufacturing. They can also be truly transformative, fundamentally changing the field of possibilities for what products can do. We have supported many companies that are leveraging our cell programming platform to address some of the world’s most challenging environmental and social issues.
Pharma & Biotech
Biopharma has been a nexus of tremendous innovation in cell programming and synthetic biology. Just in the past year, we have seen the creation and broad adoption of a novel form of biological prophylactic in the form of nucleic acid vaccines. These vaccines contain genetic code that our bodies read to produce viral proteins and stimulate an immune response and antibody production. New nucleic acid vaccines can be programmed quickly, such as the booster vaccines being developed against emerging SARS-CoV-2 variants, offering the potential for rapid response to other future pathogens. They can also be programmed to target a number of other diseases. In the wake of the success of nucleic acid vaccines during the COVID-19 pandemic, new programs for HIV and cancer vaccines, among others, are accelerating.
Biologic medicines like insulin and other protein drugs and antibodies are also produced via cell programming, making a difference in the treatment of countless diseases. Over 20% of the therapies approved by the U.S. Food and Drug Administration (“FDA”) in 2021 were biologics. In addition, new modalities enabled by cell programming, such as cell and gene therapies, microbiome therapies, and regenerative medicines, are beginning to come online. We believe human health and the ways we treat disease will be transformed by improvements in cell programming technology.
Ginkgo has been active in this field in recent years and we expect to significantly expand our support of therapeutic applications over coming years. From companies developing “living medicines” (Synlogic, Inc. (“Synlogic”)) to those involved in COVID-19 vaccine production (Moderna and others) to those developing novel antibiotics (Roche), we are using our platform to deliver transformational innovations across a range of disease areas.
Industrials & Environment
Since the industrial revolution, manufacturing techniques have been extractive, wasteful, and unsustainable. Not only must we innovate new manufacturing methods in order to keep up with growing demand, we must also work to remediate issues we have caused historically, by cleaning up our environment and addressing climate change.
Ginkgo is not only working with customers to create cell programs that enable cost-efficient, renewable, and sustainable production of chemicals and materials, such as our work with Genomatica, Inc. (“Genomatica”), but we have also participated in the formation of Allonnia, a company focused on environmental remediation. Plastic
93
Table of Contents
waste and many of the pollutants that plague industrial manufacturing and extraction sites are novel in the course of evolutionary history, so biology has not yet evolved to degrade them efficiently. Cell programming can enable the discovery and development of new enzymes capable of degrading recalcitrant pollutants and recycling waste while entirely reimagining manufacturing for the future.
Food & Agriculture
Food is inherently biological: it comes from life and sustains life. Cell programming can be leveraged to improve the availability of essential food and nutrition to a growing population, decrease the environmental impact and cost of food production, and provide consumers with increased choice.
We are working with some of the largest multinational agriculture companies, including Bayer (through our joint venture, Joyn Bio) and Corteva, to develop cell programs that would make crop production more efficient and sustainable, reducing synthetic nitrogen fertilizer and pesticide usage. In food, we have been active in flavors and sweeteners, and we are the principal cell programming platform for Motif FoodWorks, Inc. (“Motif”), a company that is making animal proteins without the need for industrial farming of animals, which launched its first product, HEMAMI™, in 2021.
Consumer & Technology
Most physical goods have biological origins—from the petrochemicals in our fabrics to fine chemicals extracted from plants—but industry does not necessarily leverage biology, or leverage biology efficiently, to produce these items. Petrochemicals, for example, are used in everything from our fabrics to our cosmetics to our paints. These chemicals and polymers are generally created in complex chemical and physical reactions from crude oil, but crude oil is just the result of millions of years of decomposition of previously living matter (they are fossil fuels after all). These biological building blocks can instead be programmed in a living organism to produce these items sustainably, without extracting natural resources. Even in areas where industry does leverage biology, such as extracting raw materials or fine chemicals from plants, we believe the current approaches are woefully inefficient or rife with social consequences.
We have helped some of the world’s largest fragrance companies such as Givaudan use fermentation to much more efficiently produce rare molecules typically extracted from plants. In a related field, we are also supporting Cronos in their effort to biosynthesize cannabinoids, with the goal of reducing cost, improving purity and predictability, and enabling production of rare molecules. We also helped launch a new company in 2021, Arcaea (formerly Kalo Ingredients, LLC), which is focusing on leveraging biology, from proteins to the microbiome, to build a suite of innovative and efficacious personal care products.
Market Opportunity
For several decades in the computing industry, software ran entirely in local environments: companies built and ran their own servers and customized their applications. The dominance of software-as-a-service (“SaaS”) software and cloud computing over the past decade has demonstrated the value in having common architectures and enabling horizontal platforms. What users may have sacrificed in customizability, they more than gained in innovation, efficiency, and scalability. We believe Ginkgo is ushering in a similar transition in cell programming, a programming discipline with the power to shape living things and grow applications across the physical world.
The value of these applications will measure in the trillions of dollars
Given the breadth of application areas and the potential of biology (see “—The Impact of Cell Programming”), we believe that the end markets for bioengineered products will be enormous. Industry sources estimate that in the next 10 to 20 years, there will be approximately $2 to $4 trillion of annual direct economic impact from these products, with significant secondary effects. But these applications reflect only what we can already imagine. As
94
Table of Contents
we develop a greater ability to program biology and direct it towards novel and more challenging applications, the spectrum of possibilities will undoubtedly grow. Computers were used for little more than counting for decades; we firmly believe the most valuable applications of cell programming are not yet apparent.
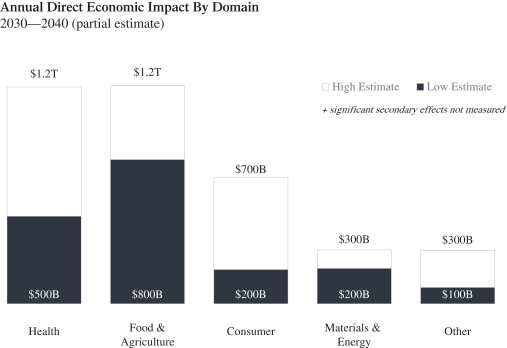
Figure 6: Industry sources estimate a $2 to $4 trillion total addressable market for bioengineered products between 2030 and 2040.
Large existing market for “on prem” cell programming research and development
Cell programming today is done in a highly inefficient, distributed manner reminiscent of the early days of computing. Essentially every organization looking to innovate in biology builds its own biology labs in the same way that individual tech companies used to set up their own servers instead of using cloud computing. Scientists spend hours moving liquids around rather than designing novel experiments just as computer programmers once spent most of their time physically writing and debugging code (by punching cards, for example) rather than designing new applications.
Because of the way cell programming is done today, intellectual property that could be useful for multiple applications is tied up by exclusivities that delay the progress of the field overall. Ginkgo’s platform breaks down these silos and democratizes access to the most advanced technologies in the field, enabling customers of all sizes to more efficiently drive innovation.
According to industry sources, the market for cell programming R&D is approximately $40 billion and growing rapidly. This work is being done in a distributed manner, sacrificing benefits from scale and learning economies. Approximately 60% of the spend today is on labor—scientists designing and executing experiments—while the remaining 40% of this is spent on “tools”—things like DNA synthesis, reagents, and equipment. Ginkgo brings efficiencies to both elements of this existing market.
| • | Labor: When scientists leverage advanced automation, they can both reduce error rates and free time otherwise spent performing manual work (e.g. pipetting liquids from one plate to another). Freed from the burden of manual programming, scientists have more time to practice the art of cell programming: designing the direction of experimentation, mining data for new insights or exploring new techniques or |
95
Table of Contents
| application areas. This in turn increases the demand for programs as scientists retain a greater capacity for innovation and generate more ideas to test. |
| • | Tools: Ginkgo’s scale provides a cost advantage in two primary ways. First, we reduce the amount of capital investment required by our customers—an early stage company building on our platform may never need to build a molecular biology lab. Second, our proprietary technologies and scale economics drive down the marginal cost of each experiment. Combined, these factors have the impact of transforming what is typically a large fixed cost investment for a cell programmer into a much lower variable cost. This is akin to an IT department not having to build and maintain a costly bank of servers and instead paying a marginal usage- based fee to their cloud computing vendor. Additionally, and perhaps even more impactful, our Codebase provides host cells, genetic parts and associated data for our customers that are unavailable elsewhere and which may reduce the total amount of work required. |
As the cost of computing power declined exponentially in computer programming, the demand for computing power increased exponentially as developers dreamed up more and more sophisticated applications. We expect the same to be true in cell programming: as our platform scales in capability and capacity, we hope that the range of applications accessible to cell programming will likewise expand in breadth and sophistication.
Industry Overview
We believe that Ginkgo is changing the structure of the biotechnology industry. In much the same way that cloud computing centralized hosting services and ushered in a wave of SaaS software companies, Ginkgo is scaling the capabilities needed to program cells. By making these tools more accessible, we hope to usher in a wave of innovation in both “hardware” (life science tools) and “software” (cell programs).
At Ginkgo, we have always admired the symbiotic and regenerative nature of biology, which sits in stark contrast to the often extractive nature of existing technologies. We are often asked who we think the “winners” and “losers” in the industry will be as Ginkgo scales, as if it is a given that our growth must come at the expense of others in the ecosystem. We reject that notion. As our platform scales, we seek to drive benefits for all existing players in this ecosystem:
| • | Innovators—whether in academic labs, startups, or global conglomerates—benefit from faster and more successful R&D efforts |
| • | Scientists are freed to unleash their creativity (we understand the pain of spending years pipetting at the bench too!) |
| • | Life science tools and manufacturing companies benefit from having a clear technical roadmap and known demand to justify investments |
| • | Society benefits from responsible innovation, driving more sustainable, cost effective, and high-performance products |
Program Layer: Ginkgo enables and accelerates product companies, which historically have had to vertically integrate
Ginkgo is not a product company; we are an enabling platform for product companies in a range of end markets. We do not seek to “pick winners” and focus instead on building our platform rather than investing in product- specific risk. Platforms require scale and a relentless focus on innovation while taking a product to market requires many specialized functions that vary depending on the product:
| • | A novel food ingredient requires food scientists to test and enhance taste and functionality |
| • | A therapeutic requires clinicians to conduct animal and human studies to test safety and efficacy |
96
Table of Contents
| • | A novel material requires materials scientists to evaluate elasticity, durability, conductivity, or other required features |
| • | An agricultural product requires field trials |
Once the product is developed, major investments are also needed to manufacture, distribute, and market the product. These are the jobs of our customers, the product companies.
Historically, product companies have had to invest in their own R&D capabilities, building their own labs and hiring their own scientists. This investment is inefficient due to lack of scale and drains resources away from application testing and product development. Ginkgo’s platform is not application-specific. The same engineering tools can be used for programs in completely different application areas: cells all run on the same genetic code. As product companies develop their products on Ginkgo’s platform, they gain efficiencies and increase their probability of success. New companies that build on our platform never need to make the fixed capital investments to start a lab from scratch; they are able to leapfrog and compete effectively against established companies.
Technology Layer: Ginkgo collaborates with life science tools companies to drive technology advancements
Because we’re constantly thinking about how to enable the next several years of exponential scaling of our platform, we have good insights into future bottlenecks and welcome the opportunity to collaborate to build technologies that will break through those barriers. We are the largest customer for many of our strategic suppliers and, as such, play an important role in advancing new technologies. As a result, we are often able to secure preferred access, often including custom development and leading economic terms, to next-generation technologies and pass those benefits along to customers.
We expect to continue to invest in and support the development of emerging technologies in this space. In certain areas where Ginkgo has unique needs, we may acquire technologies directly, as we did with Gen9, Inc.’s DNA assembly platform, which was particularly valuable for more complex DNA synthesis needs. In many other
areas, we will support new and existing technology companies by placing anchor orders and partnering to develop technology roadmaps that break new ground.
By acting as a horizontal platform, Ginkgo can focus on what we do best (cell programming), our customers can focus on what they do best (bringing products to market in their industry), and our suppliers can focus on what they do best (building great hardware and tools). Biology did not evolve by industry and so cell programming is able to benefit from the scale and efficiency of a horizontal platform. Vertical integration is no longer required, allowing each layer of the ecosystem to flourish as we collectively enable more rapid growth across the industry.
Enabling Customer Success
Ginkgo serves diverse customers across a variety of end markets. Some of these customers may have in-house biological R&D teams and others may have never thought biotechnology applied to their business. In either case, they come to us with a challenge—whether it is supply chain volatility, a race to develop an innovative new product, or an existential threat facing an industry on the wrong side of history—and we partner to enable a biological solution. We begin our relationship by working collaboratively to design the set of specifications for the end product(s) our customer desires. Our cell programmers then take that set of specifications and design an engineering plan to create a cell program that meets or exceeds that set of specifications. When we finish, our customers receive the final engineered organism (which either produces or is their product of interest) and a full “tech transfer” package for manufacturing and downstream processing (which they can implement themselves or pass to a contract manufacturer with our support). Our customers then take these organisms and/or purified products through the final stages of product development (e.g., formulations, clinical trials, field trials, etc.).
97
Table of Contents
Our commercial team is organized to both establish new relationships with potential customers (traditional business development) as well as maintain and expand relationships with our existing customers (which we call “alliance management”).
Our business development team has both expertise in relevant industries (Consumer & Technology, Industrial & Environment, Agriculture, Food & Nutrition, Pharma & Biotech and Government & Defense) as well as expertise in our Foundry capabilities and synthetic biology. With this background we are able to identify industry or consumer challenges where biology can serve as a solution. Our categories of customers, independent of industry, include potential customers who have R&D teams with some synthetic biology capabilities where choosing Ginkgo can bring automation, scale, and codebase beyond their own; potential customers who are considering but have not yet built lab-scale capabilities where a partnership with Ginkgo allows them to spend their capital on commercialization efforts; and potential customers who are not yet working in synthetic biology whose industries or products stand to be disrupted by biological solutions. Our business development team, with support from our Codebase and Foundry team members, crafts solutions for each of these types of customers through a strategic discussion of customer needs and fit with Ginkgo capabilities.
To grow existing customers, our alliance management team, through close collaboration on our existing programs, seeks technical and business opportunities for our customers that serve as the basis for consideration
of future programs. As our programs demonstrate technical success, our existing customers often bring their next strategic R&D needs to our attention.
Over 100 Cumulative Programs across diverse industries have run on our platform
While most biotechnology companies focus on building products within a fairly narrow scope, Ginkgo has uniquely pursued a partnered strategy across all end markets. This was not easy. For many years, our platform was less efficient than the status quo of an expert scientist working by-hand at a lab bench. In the early days, the only end markets willing to take a chance on our platform were those without in-house biotechnology capabilities. But as Ginkgo’s platform improved over time and with scale, we were able to win contracts in increasingly sophisticated end markets with more in-house biotechnology expertise. Today, our platform is diversified across all major end markets with marquee customers and a range of focus areas within each.
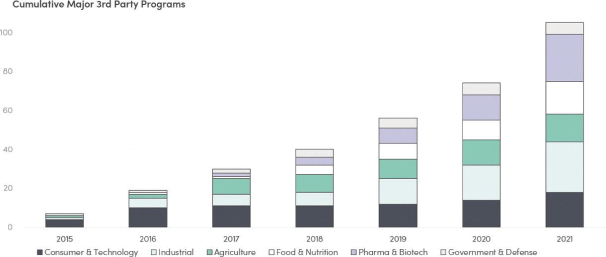
Figure 7: Cumulative Programs run by third-party customers on Ginkgo’s platform (excluding proof of concept projects and other exploratory work). Today, Ginkgo has a diverse set of programs across all major end markets.
98
Table of Contents
Our customers include large multinational organizations with multibillion dollar R&D budgets as well as startups who are depending on us for essentially all of their bioengineering needs. While these customers and their focus areas may look very different, they are all important and valuable to Ginkgo. All of these programs leverage a common infrastructure, and as we demonstrate the value of this platform, we have the ability to grow
significantly with our customers.
Ability to grow with our customers and increasingly complement existing R&D budgets
Ginkgo has grown substantially through inside sales with our existing customers. Some of our customers, such as Motif FoodWorks, never needed to build in-house cell engineering capabilities and so as they grow and expand their product pipeline, we expect their demand for our platform will increase and they will benefit from our improving scale efficiencies over time as well. The relative value of our platform compared to the next best option (building a lab, bioengineering team, and intellectual property from scratch) is immense, which yields extremely high retention rates for customers in this category.
Other customers may already have in-house cell programming capabilities. As Ginkgo demonstrates the value-add of our platform by successfully delivering on programs, we have the opportunity to grow our collaborations with them, complementing their core R&D capabilities. We don’t view this as a “replacement” of customer scientists with Ginkgo’s platform. Rather, we hope to expand our customers’ capacity and need for innovation—giving them more “shots on goal” and enabling them to invest more heavily in R&D as the return on investment of each dollar spent increases.
We have demonstrated this with several customers. With one customer, an initial proof of concept program has turned into a broader strategic relationship with over nine programs today. With another, we launched a relationship with two programs, quickly expanding it to five by the end of the following year. The growth we have seen with our oldest customers means we continue to have significant customer concentration as it takes time for new customers to ramp up their use of Ginkgo’s platform. During 2021, two of our customers each contributed greater than 10% of revenue and collectively they accounted for 28% of total revenue, down from
39% in 2020. We believe customer concentration will decline over time even as we expect to continue to grow our relationships with existing large customers. However, our ability to grow with our customers requires us to maintain satisfied customers, and program or other operational setbacks could impede our ability to meet customer expectations and grow our business.
Powerful proof points across categories
Our platform has now been validated by sophisticated customers across a range of industries. As we launch programs in new areas, those provide a toehold for future sales in that space. As an example, our pro bono project for Moderna, Inc. at the start of the COVID-19 pandemic to enhance production of a key raw ingredient through process engineering provided a proof point and initiated us into this emerging segment, leading to a commercial relationship with another nucleic acid vaccine company, as well as a program to produce a key processing enzyme for mRNA vaccines. Similarly, the technical success of programs with Motif FoodWorks, Aldevron, and Cronos Group in 2021 attracted inbound interest from other potential customers.
It is still incredibly challenging to break into new industries and our ability to expand into new sectors may be harder than we expect. However, our recent progress in therapeutics has been a significant milestone given that we are ultimately competing against very strong in-house capabilities. We believe that as more proof points emerge across industries, the barriers to adoption will diminish.
Our Platform
Ginkgo’s platform combines a strong technical foundation with an ecosystem of supporting resources to maximize our partners’ odds of technical and commercial success. In the nucleus of our platform are our Foundry and Codebase, which our scientists leverage to complete customer programs. The Foundry is, in its simplest
99
Table of Contents
form, a very large, highly efficient biology lab, enabled by over a decade of investment in proprietary workflows, custom software, robotic automation, and data science and analytics. It is paired with our Codebase, a collection of biological “parts” and a database of biological data, which helps our scientists program cells. But great technology alone is not enough, and we are building a community and ecosystem around our technical platform that provides our partners with end-to-end support.
Our Foundry brings economies of scale to cell programming
Cell programming projects involve a conceptually similar engineering cycle regardless of the specific product or market. Based on customer specifications, Ginkgo’s program team develops designs of proteins, pathways and gene networks (see Figure 5) that might meet the specification, leveraging public and proprietary biological knowledge bases (see “—Our Codebase—organizing the world’s biological code”). Those conceptual designs are developed using computer-aided design tools until the exact DNA sequences for those designs have been determined. Those DNA sequences are then “printed,” assembled and inserted into a cell to execute the new
DNA code. These prototype cells are then studied and the output or performance of each is measured and compared to the customer’s desired specification. Learnings using data analytics and data science tools inform a new round of prototypes and this cycle is repeated until either the specification has been met or the customer decides to end the program.
The likelihood of technical success increases with each iterative engineering cycle and with the number of prototypes that are explored per cycle. However, with traditional tools for genetic engineering, each of these cycles can be slow, expensive and error prone. Many projects across the industry run out of budget or time. Conventional R&D teams often look to stay within budget by running rapid engineering cycles using largely manual tools and small numbers of prototypes per cycle. However, the inability to broadly explore the potential design space (there are more possible sequences of a 200 amino acid protein encoded in 600 DNA letters than there are stars in the observable universe) and the reliance on manual tools is a difficult handicap to overcome. Since people can only work so hard and since engineering cycles can’t be shortened beyond the duration of the physical steps, this approach has limited potential to improve in the future.
At Ginkgo, we invest in improving the tools and technology for programming cells in order to maximize program success within the constraints of customer timelines and budgets. We do so by scaling the number of prototypes that can be evaluated in each engineering cycle in an effort to reduce the number of cycles required to meet the customer’s specification and ultimately shorten project timelines. A typical screen for one enzyme step in a program might evaluate 1,000 to 2,000 variants to optimize function, of which the top 10 to 100 might be short- listed for further study. A relatively basic program might have 3 to 5 enzymes working in concert, and so in the process of optimizing the entire pathway, thousands or tens of thousands of enzymes and pathway combinations might be designed, built, and tested in the Foundry. The methods we use to increase scale also tend to reduce the average cost per prototype, which means that more prototypes can be evaluated for a given program budget.
Because diverse cell programs share similarities in process and code, many programs can be run simultaneously in a carefully designed centralized facility. This facility, where we use our investments in advanced cell programming technologies to manage diverse programs, is what we call our Foundry.
We make it possible to centralize many cell programming projects in our Foundry by deconstructing programs into a set of common steps and then standardizing those steps. For each step, we have built a specialized functional team that performs that step for all programs. Those teams define a set of standardized services that can be used in concert to execute an end-to-end cell programming process. Each team has access to scientific, software, and robotic engineering resources to replace manual ad hoc operations with standardized, automated, and optimized services. In addition to enabling scale, this approach ensures standard operating procedures, know-
100
Table of Contents
how, and human skill become encoded in software that can be more effectively debugged, monitored, controlled, and optimized.
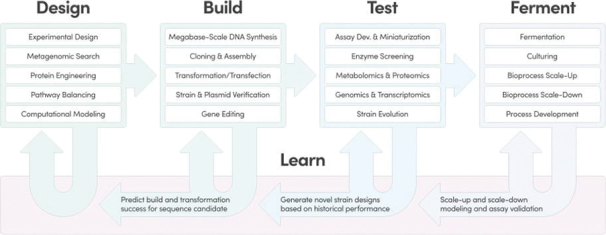
Figure 8: A non-exhaustive summary of the functions performed throughout the lifecycle of a program in the Foundry. At each stage, learnings are generated, driving improved design cycles and functional optimizations.
While the engineering strategies described above have historically been relatively uncommon in the life sciences, they are obviously not our invention. Rather, we are inspired by the lessons from other engineering disciplines and seek to apply those to biology. Automotive manufacturing, semiconductor fabrication, and data centers, among many other industries, demonstrate how automation, data, economies of scale, and continuous improvement can produce compounding gains in scale, costs, and quality. Critically, routine performance of these strategies across dozens of projects gives us the data and experience needed to drive continuous improvement.
As described above, a key strategy in our Foundry is to increase the scale of our operations so that we can run more programs and more prototypes in parallel (i.e., large batch sizes). This approach benefits from operational efficiencies and economies of scale across many dimensions:
| • | Fixed Cost Amortization: Our Foundry is an inherently physical facility and as we scale and improve utilization, we are able to amortize this fixed cost across more work. |
| • | Continuous Learning and Improvement: The cumulative amount of work done as we scale leads to a better understanding about how to program cells. Much of this is then encoded in our Codebase, described below. |
| • | Purchasing Economies: By partnering with Ginkgo, our technology partners and suppliers can generate more value from a single account than they could from multiple smaller accounts, and that extra value is shared with Ginkgo. |
| • | Technology Specialization: Certain technologies that we leverage in the Foundry (such as acoustic liquid handling, automated bioreactors, and advanced mass spectrometry systems) are not easily leveraged or practical for smaller organizations. But for an engineering organization of our size, those investments can drive material improvements in cost efficiency. |
These efficiencies and economies of scale can be observed empirically from a relationship we refer to as “Knight’s Law,” named after Tom Knight, one of our co-founders, and loosely inspired by Moore’s Law for semiconductors. As shown below, we have seen an exponential increase in the output of the Foundry over time alongside an exponential decline in the average cost per unit of output. While this trend was interrupted by temporary lab shutdowns during the COVID-19 pandemic and reduced capacity due to social distancing, we have since continued to drive our business towards achieving these metrics. While Knight’s Law does not provide the full story on our development, it is a useful tool that allows us to continue to build efficiencies of scale. We continue to build our internal metrics around Knight’s Law and believe we can continue to drive this kind of
101
Table of Contents
capacity growth in the foreseeable future, though it is dependent on the development of new technologies, which inherently carries risk, and, like Moore’s Law, we will likely hit a limit over time. This feature compares to a conventional facility, where scaling is driven predominantly by the addition of employees, an exponential
increase in work would be infeasible and the cost per unit of work would decline little, if at all.
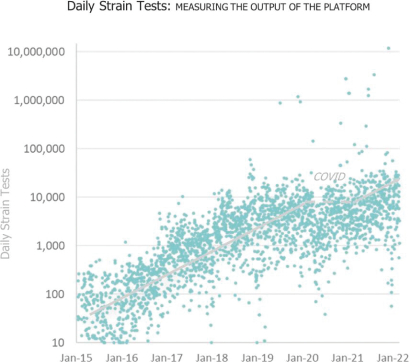
Figure 9: The output of the platform increased by over 3X per year for 5 years (with the exception of 2020), and while we expect that kind of scaling to continue, there is no guarantee that we will be able to do so.
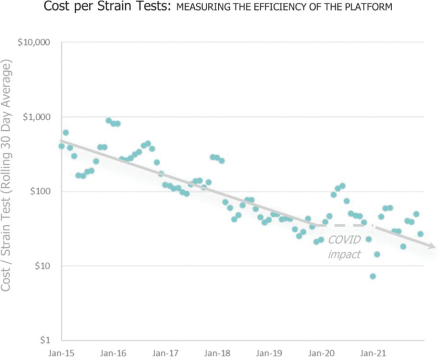
Figure 10: As the output of the platform has increased, our total R&D / operational costs per unit of output has decreased by approximately 50% per year.
102
Table of Contents
We are frequently asked, and spend much time thinking about, whether it will be possible for compounding gains in output and productivity to continue for many years in the future. It is important to note that given significantly advanced tools, most steps in cell programming could be miniaturized to a point where single molecules of DNA and single cells are being manipulated and monitored. At that ultimate degree of miniaturization, the costs and timelines of cell programming could be reduced orders of magnitude from where they are today. Newly available microfluidic technologies point to the reality of this future of cell programming at the single-cell level. Additionally, because many of the enabling tools of cell programming are biological in nature (e.g., polymerases and CRISPR), we are able to point the platform at itself, developing new biological tools to reduce the number of steps or the complexity of a certain operation. For example, we could develop better gene editing enzymes or novel ways to screen cells in a multiplexed format using biological sensors. It is easy to theorize about these types of developments, however they are hard to execute, we will undoubtedly run into roadblocks along the way and we will have to invest significantly in developing new technologies in order to enable the types of improvements we seek to achieve.
Recent advances in machine learning, molecular simulation, and other computational techniques also hold great promise to improve our ability to program cells. We believe our Foundry is well-positioned to build the kind of large, well-structured datasets that such computational approaches need to succeed. In time, we believe computational approaches will reduce the need for certain kinds of experiments (for example, we already use machine learning to make protein and enzyme design projects more efficient). If computational approaches can replace certain sets of experiments, we expect to use the recovered Foundry capacity to work on ever-more complex cell programming challenges. The reality is that the cells that we program today accomplish relatively simple functions, such as: “produce as much of molecule X as possible.” Programming cells for complex functions, such as live-cell therapeutics, responsive building materials, multicellular organisms, etc., will require sophisticated sub-systems for environmental sensing, intracellular information processing and feedback, and a multidimensional program that responds to such environmental stimuli. Only when we can deliver such
sophisticated programmed cells will we have truly unlocked the potential of biology, and we see the Foundry as being an integral part of the platform for doing so.
Our Codebase—organizing the world’s biological code
Codebase is a familiar term to software developers but is a new concept in biology. Modern software firms develop their own (typically proprietary) codebase of source code and code libraries that can be leveraged by their software developers to more easily create new applications than they could starting from scratch. Additionally, vast repositories of debugged code are shared publicly so that programmers across application areas can leverage prior art in order to innovate faster. This allows software developers to focus their time and effort on developing new features rather than recreating existing logic. Ginkgo’s Codebase is comprised of reusable genetic parts and strains that can be repurposed in new cell programs. We are continually investing in better ways to characterize functional biological code to drive increased reusability. In addition to the raw performance data we generate through our Foundry experiments (more than 24.8 million strain tests run as of December 31, 2021), we have also incorporated many public databases for genetic sequences and have a proprietary data set of over 3.8 billion additional sequences that we leverage in our designs.
Engineering biology is complex—one of the reasons that Foundry scale is important is that it remains highly difficult to predict the performance of a biological “part” in a given context from a DNA sequence alone. The genomics revolution has outpaced biologists’ ability to test the functionality of each DNA sequence as it was discovered, particularly because most of the community is still performing biological experiments by hand without the benefit of automation. Each program performed at Ginkgo involves testing thousands or millions of DNA sequences; with a small fraction of those ending up in our final engineered cells. For that reason, high- performance biological sequences—the handful of designs from thousands of candidate designs that meet our performance goals for an experiment—are hard-won assets and form a key component of Ginkgo’s Codebase.
103
Table of Contents
Not to be discounted, the “losing” designs are still valuable, helping inform more effective campaigns in the future that avoid known failure modes.
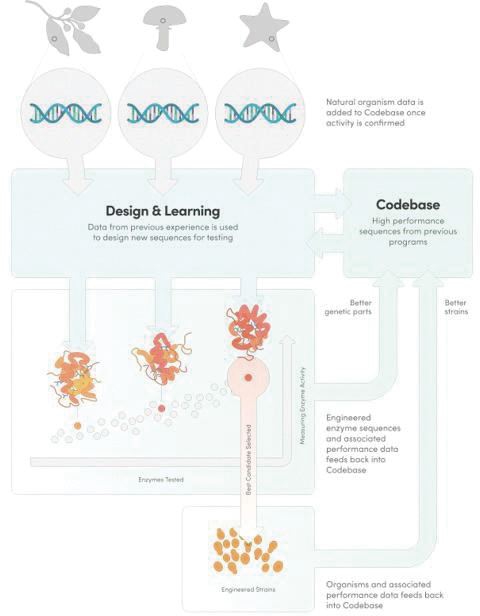
Figure 11: Our Codebase incorporates both biological assets from nature as well as engineered assets and data from our Foundry experiments. Because the Foundry enables us to test many thousands of prototype enzymes, pathways, and strains in individual engineering cycles, we are able to quickly expand the range of characterized biological assets in our Codebase.
In some ways Codebase is a “parts catalog” that we can draw from when developing a new organism. As Ginkgo performs more projects, we contribute new parts to our Codebase that can be reused in new contexts. For example, we developed novel synthetic promoters (DNA sequences that can turn on the expression of a gene of interest) that allowed us to increase production of proteins in yeast. Initially, we tested thousands of designs to arrive at a select number of promoters with high performance. Now those high-performing promoters can be
104
Table of Contents
reused in any program that involves producing a protein in yeast; they are a modular piece of genetic code. Over the past 20 years, our team has supported efforts to build these kinds of parts libraries—the International
Genetically Engineered Machine (“iGEM”) Parts Registry and AddGene are two notable examples of initiatives to make reusable parts available to researchers in the community. But despite these efforts, we continue to see intellectual property siloed within organizations across the biotechnology industry, leaving many without the additional intellectual property they need to develop their programs. Ginkgo’s Codebase allows our customers to draw from a broader set of biological assets than any single company would develop for a given application. The scale and diversity of our programs have allowed us to develop a large Codebase that grows with the addition of each new program and can be opened to the broad swath of partners and cell programmers using our platform.
Cell programmers must consider not only the genes in the programs that they design, but also the ways that they interact with the cell that “runs” the program. Therefore, Codebase is more than just the individual modular parts we use to design biological programs. The organisms that have been optimized to run the programs, whether because they have been engineered for robust growth or because they are particularly adept at producing certain classes of products, are known as “chassis” strains. These strains can be reused across multiple programs, significantly reducing the amount of work needed to optimize a program and engineer a commercially viable organism. The breadth of Ginkgo’s customer base allows us to use these chassis strains in many more contexts than traditional industrial biotech players.
For example:
| • | Our collaboration with Cronos Group Inc. involves the production of many different cannabinoids; these cannabinoids share common precursor molecules such that a single chassis strain can be modified to produce each product. |
| • | In 2020, Ginkgo acquired the assets of Novogy, Inc., a company that had been focused on the engineering of oleaginous yeasts to produce fuels and lubricants. At Ginkgo, these assets can be applied not only to fuels and lubricants but also fine flavors and fragrances, food oils, and even materials. A consequence of evolution is that biochemistry has repurposed the same biochemical pathways many times over in different contexts, allowing chassis strains to be redeployed in diverse contexts at Ginkgo. |
| • | In 2021, Ginkgo acquired Dutch DNA Biotech B.V. (“Dutch DNA”). This team specializes in the development of filamentous fungi for protein production. Traditionally, filamentous fungi have been used for the production of industrial enzymes—typically enzymes that are native to fungi or close relatives. At Ginkgo, these fungal strains are being engineered to produce a broad range of proteins, including bacterial and mammalian proteins. These proteins are applicable to a wide range of end products, such as food and materials. |
| • | In 2022, Ginkgo announced a new cell programming project with Sumitomo Chemical Co., Ltd, to produce a target molecule for the personal care and cosmetic industries. The target molecule is envisioned to augment or replace one that is otherwise currently gathered from animal sources. This program was selected in part due to the availability of a precursor molecule in Ginkgo’s Codebase. |
| • | We aim to re-deploy Codebase assets in new contexts. In 2021, we launched our “Cell Development Kit” (“CDK”) product offering. Inspired by “Software Development Kits” used in the software industry, CDKs offer a standardized entry point for Ginkgo to input new projects on the Ginkgo platform. The first CDKs to launch are focused on protein expression programs. CDKs provide cell programmers access to the toolkit needed to get started developing commercial proteins, including access to pre-engineered host cells optimized for such protein production, specialized equipment, automation capabilities, genetic engineering expertise, insights garnered from Ginkgo’s Codebase and the applicable infrastructure to design, build and test a custom protein production microbe. |
| • | Ginkgo’s CDKs are designed to cut the cost of launching a cell program and speed up development timelines to build engineered microbes, for example, to determine whether a protein may be successfully and commercially produced. By simplifying the pathway for companies to get started on the Ginkgo |
105
Table of Contents
| platform with standard terms, a phased approach, low costs and clear deliverables, the CDK can help de-risk projects prior to full scale technical development. In effect, CDKs allow us to more efficiently deploy useful Codebase and the cell engineering know-how we have accumulated to add new projects to the platform. |
Our Foundry and Codebase are inextricably linked. Our Foundry scale allows us to generate unparalleled Codebase assets. These Codebase assets help us improve our designs and provide reusable parts and chassis strains that improve the efficiency and probability of success of our cell programming efforts in the Foundry. As the capabilities of the platform improve, it drives further demand, which increases the rate of learning in our Codebase. The continuous learning and improvements inherent in this relationship is one of the key features of our platform.
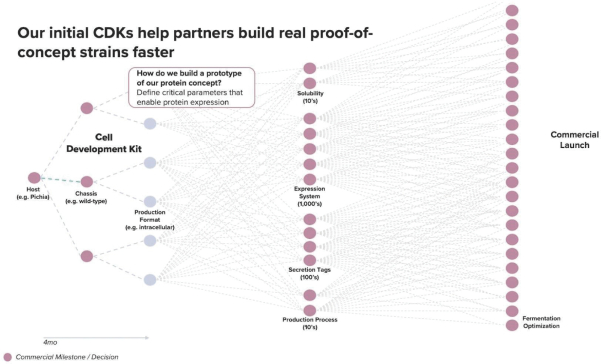
Figure 12: We believe our initial CDKs can help cell programmers build proof-of-concept strains faster.
An ecosystem to support cell programmers
Ginkgo has long recognized that it is critical to build a true ecosystem around our technical platform. We have been inspired by the leading horizontal platforms in information technology, such as Microsoft Windows and Amazon Web Services, which built real developer communities and provided a range of value-added services on top of their core technology. Like these pathbreakers, who set the stage for a generation of computer developers,
106
Table of Contents
we too are trying to ensure that the cell programmers who build applications on our platform have the tools they need to succeed beyond the lab.
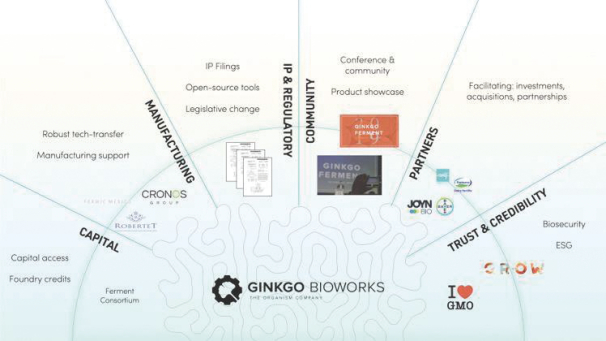
Figure 13: Ginkgo strives to create an ecosystem to ensure that cell programmers have the tools they need to succeed.
Access to capital
As in the early days of computer programming, it is still extremely expensive to program biology. For that reason, it can be easier for larger companies to make investments in innovation around this space. But Ginkgo’s platform gives small companies and innovators access to the same horsepower as larger players and obviates the need to invest in fixed laboratory assets, providing an even greater strategic benefit. To help address this discrepancy, Ginkgo has assisted in launching new companies (such as Motif and Allonnia) by bringing together strategic and financial investors to secure funding for these early stage companies. Going forward, we intend to leverage our own balance sheet and to partner with investors, enabling companies at all stages to benefit from our platform. We believe that, as Ginkgo’s customers demonstrate increasing success, there will be an explosion of capital for cell programming applications and a recognition of Ginkgo’s platform as setting the industry standard and providing the backbone for these development efforts.
Manufacturing support
Our job is to ensure that our cell programs can be executed at scale and we support our customers to ensure successful commercial scale manufacturing. We have built relationships with a number of leading contract manufacturing organizations and have demonstrated that we can transfer our lab-developed protocols to commercial scale (e.g., 50,000+ L fermentation tanks) with predictable performance. We have an in-house deployment team dedicated to supporting our customers’ scale-up and downstream processing needs. We have
107
Table of Contents
even helped certain customers, such as Cronos Group, Inc., acquire and build out their own in-house manufacturing capabilities and certain programs, such as our work with Moderna, Inc., focus on manufacturing process optimization.
Intellectual property protection and regulatory support
Ginkgo takes responsibility for the cell engineering intellectual property generated through customer collaborations. Our scientific team works continuously with our intellectual property team to file patent applications and monitor for freedom to operate. We are also active in helping shape and influence the evolving regulatory landscape for biological engineering. While our customers are responsible for handling their own regulatory procedures on a product-by-product basis, our broader view and sphere of influence can help build understanding of and support for novel product classes.
Building a community of cell programmers
We launched Ferment, our annual conference, in 2018. The conference highlights developments and thought leadership in the field and brings together scientists, entrepreneurs, investors, and suppliers. Our conference in
2021 brought over 700 participants to our headquarters in Boston. Even prior to launching Ginkgo, our founders focused on building community within the emerging field of cell programming. Tom Knight, one of our founders, was among the professors who launched the iGEM Competition in 2004, which has now had over
70,000 participants from over 40 countries take part in the competition (including dozens of Ginkgo employees and all five founders!).
Facilitating partnerships within our community
Because Ginkgo serves both large market incumbents and smaller startups, our community also serves to facilitate introductions between innovators and those looking to invest in innovation. We believe that investors and large strategic companies have come to recognize Ginkgo’s platform as a key enabler of innovation and are keen to get to know the companies that are building with us. Those relationships can be the source of funding and go-to-market support for the earlier stage companies building on the platform, increasing the odds that they develop successful products.
We invest in building trust and credibility for the entire industry
The most powerful technologies require the most care. Biology is too powerful for us to not care about how our platform is used. We have and will continue to invest heavily to build and maintain trust in bioengineering as a technology platform across all layers of the industry. At the platform layer, we have focused on building robust biosecurity measures. At the application layer, we are proud to enable a diverse set of programs that drive
towards environmental sustainability. We are committed to ESG practices and broad stakeholder engagement at a corporate level. We are also engaged in deep conversations around the implications and ethics of biotechnologies through many public forums, helping shape our platform to promote sustainability in our global community.
Biosecurity: A complement to our platform and demonstrated source of value
With a mission to make biology easier to engineer, we have always recognized the imperative to invest in biosecurity as a key component of our platform. We care how our platform is used, and biosecurity is a necessary complement to synthetic biology that helps us ensure our cell programming work is conducted and deployed responsibly. Consideration for biosecurity is ingrained into our cell engineering platform tools, as evidenced through our membership in the International Gene Synthesis Consortium (“IGSC”), and our application of their harmonized protocols to screen synthetic DNA for concerning sequences, as well as our ongoing work with national defense agencies aiming to develop experimental and computational tools that detect or prevent misuse of bioengineering.
108
Table of Contents
During the COVID-19 pandemic, we collectively witnessed the disruptive effects of a biological threat. The pandemic created a renewed sense of urgency for the need to counter biological risk, and triggered a paradigm shift for biosecurity in public and private institutions. Here at Ginkgo, this pandemic has deepened our resolve to advance global biosecurity and accelerated our capabilities to do so. At the start of the pandemic, we made a commitment of $25 million of free access to our platform for partner COVID-19 projects and launched a partnership with Moderna on process optimization for mRNA vaccine manufacturing. Since then, we’ve worked with Aldevron to deliver a breakthrough in the manufacturing of vaccinia capping enzyme for mRNA vaccines, and we’ve advanced discovery of therapeutics. We’ve also participated in the largest wastewater sequencing effort in the United States, supporting Biobot Analytics and the U.S. Department for Health and Human Services (“HHS”) with genomic sequencing capacity.
Most strikingly within the biosecurity space, we’ve grown Concentric by Ginkgo into a trusted brand and successful business as a large-scale systems integrator to facilitate COVID-19 testing in communities across the country. We tackled a moment of global crisis head-on through the growth of an innovative, scalable, community-centered apparatus for robust health security.
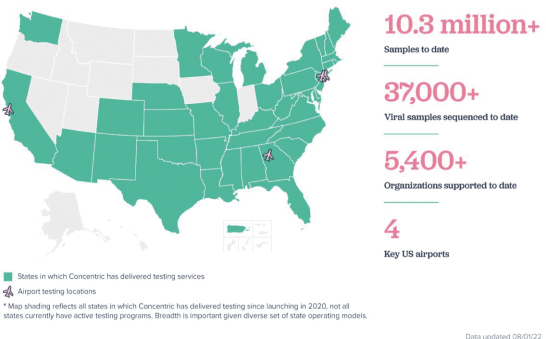
Figure 14: Concentric’s pathogen monitoring network has conducted testing across most of the U.S. Map shading reflects all states in which Concentric has delivered testing since launching in 2020. Not all states have active testing programs as of June 2022.
Through this pathogen monitoring network, we’ve empowered communities to make informed public health decisions through the many waves of the pandemic. Our tools provide early warning of infection and provide communities with tools that can mitigate outbreaks. We’ve worked closely with national, state, and local public health authorities and educational leaders to make sure that our programs are aligned with broader COVID-19 guidance and mitigation strategies, and are capable of delivering timely data to inform public health decision- making. As a systems integrator, we developed customizable, low-burden programs based on the particular needs of educators, students, parents, guardians, and community members, and grew a world-class customer service system to support each local program in context. This work with educational institutions—organizations that represent the centers of our community—is a meaningful first step in building the pathogen monitoring capabilities critical to the prevention and mitigation of biological risks.
109
Table of Contents
As we sought to evolve our COVID-19 monitoring platform to serve communities in other contexts, we developed strategic partnerships to complement our operational and technical expertise.
| • | Our partnership with Eurofins’ Clinical Enterprise, serving the Northeast and South regions of the United States through the federally-funded Operation Expanded Testing program, enabled us to offer free testing to senior living centers, correctional facilities, early education centers, and other congregate settings, many in underserved communities. |
| • | Another partnership, with XpresCheck and the U.S. Centers for Disease Control and Prevention (“CDC”), to monitor incoming international passengers at four of the nation’s busiest international airports, allowed us to leverage our viral sequencing capabilities and led us to identify the first U.S. cases of two Omicron sub-variants, BA.2 and BA.3. |
| • | Earlier this year, we acquired Project Beacon COVID-19 LLC, a Boston-based social benefit organization focused on helping to increase the capacity, availability, accessibility and affordability of COVID-19 testing, and assumed responsibility for providing COVID-19 monitoring tools to communities across Massachusetts. |
We are continually exploring where public and private partnerships can help us scale our platform and further innovate in pathogen biomonitoring.
Our dedication to biosecurity is deeper than our emergency response to the current global pandemic.
SARS-CoV-2 will not be the last pathogen we face with pandemic potential, but if we take the necessary steps, it may be the last that catches us unprepared. Ginkgo has been supporting and engaging with domestic and international organizations and governments to help shape the understanding of a robust biosecurity program beyond the COVID-19 pandemic, and we believe there is a meaningful commercial opportunity in such a
program driven by increased awareness of the need for prevention and response systems. Given our experience to date, we believe we are well placed to take a leadership position as the biosecurity platform of choice. If we are
to harness biology to engineer products for our health, food, energy, and environment as we drive the growth of a sustainable bioeconomy, we must also move with the same speed and vigor to secure biology.
Our Business Model
The key input into our unit economics is a cell program. For each of these cell programs, we generate economic value in two primary ways. First, we charge usage fees for Foundry services, in much the same way that cloud computing companies charge usage fees for utilization of computing capacity or contract research organizations (CROs) charge for services. Additionally, we negotiate a value share with our customers (typically in the form of royalties, milestones, and/or equity interests) in order to align our economics with the success of the programs enabled by our platform. As we add new programs, our portfolio of programs with this “downstream” value potential grows. Because we typically incur no downstream costs (e.g., manufacturing or product development, which our customers manage), these value share payments flow through with approximately 100% contribution margin. This flexible business model allows for more predictable near-term revenue while not sacrificing our ability to create long-term value with asymmetric upside.
110
Table of Contents
Foundry Revenue
Illustrative Program Economics
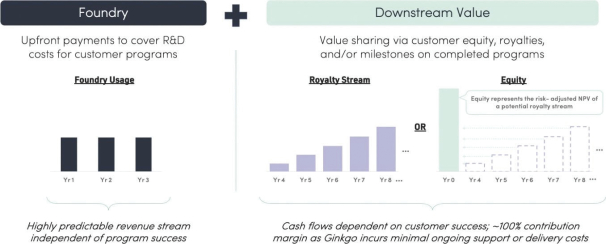
Figure 15: Ginkgo generates economics from programs in two primary ways. First, customers pay upfront fees to cover initial R&D costs for a program. Second, Ginkgo shares in the downstream value (typically in the form of a royalty stream, milestone, and/or equity share) generated by programs.
Foundry Usage Fees
The first stage of a cell program consists of R&D work being performed on Ginkgo’s platform, leveraging our Foundry and Codebase. R&D is inherently risky and our customers recognize that this is a cost they will incur regardless of success and whether they are working on the program in-house or with a partner. Ginkgo provides a much more efficient platform to conduct this R&D work, encouraging companies to build on or adopt our platform.
We estimate that the unit costs of our Foundry cell engineering services are several times less expensive on average than the status quo (a customer doing equivalent R&D in-house, by-hand) and we expect that cost advantage to grow over time. We typically earn usage fees tied to the units of work that we perform on behalf of our customers’ programs. Initially, as we were building and validating the platform, these fees covered less than
20% of the costs incurred to execute a program as the platform was less efficient than the status quo. As our platform has matured and efficiency improved, we have steadily increased the portion of program R&D costs that are covered upfront by customers and we now expect that new programs are structured to fully cover our direct costs, which will eventually enable us to earn a modest margin. Foundry usage fees provide a strong foundation of predictable revenue that is independent of any commercialization efforts by our partners.
As we continue to scale the Foundry and build Codebase, we expect to drive further efficiencies and decrease our average unit costs. This presents us with a strategic choice going forward. We could retain these efficiencies and increase our margins or we could pass these efficiencies on to our customers, increasing the number of shots on goal and, therefore, the likelihood of program success given a fixed budget. We believe the right choice for long- term value creation is to pass the savings to our customers, reducing the barriers to adoption and driving increased demand for our platform. Our Foundry usage fees are thus impacted by a number of drivers:
| • | Number of active programs: We hope to dramatically increase the number of programs working on our platform over time, and if we are successful, we believe this will drive increasing usage fees. |
111
Table of Contents
| • | Units of work per program per year: If our Foundry becomes more efficient and we generate more scale, we expect to be able to do more work per program in a fixed period of time, improving chances of program success. |
| • | Average price per unit of work: If we bring on innovative technologies or step change improvements in existing Foundry services, we plan to pass capability and cost improvements on to our customers. If these new technologies or services are adopted across programs, we believe the average price per unit of work will continue to fall over time. |
| • | Number of years per program: If our platform improves, we expect program duration to decrease over time. Some programs may still be charting new territories and take several years, but programs that are able to leverage substantial pre-existing Codebase (e.g., our Nth program in bulk protein production) should have shorter duration and, in general, greater Foundry capabilities should shorten program durations. |
The expected impact of these drivers is represented below:
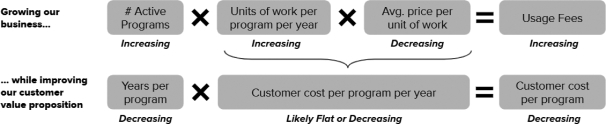
Figure 16: Illustrative drivers of Ginkgo’s long-term financial model and customer value proposition.
The multi-year nature of an average cell programming project means that our usage fees are predictable and recurring in nature. Additionally, given the lead times inherent in developing technical plans as part of a sales process, we have good visibility into new Foundry usage fee bookings. This provides a strong foundation for the business and allows us to be patient while we wait for downstream economics.
Downstream Value Share
As the key enabling technology for our customers’ products, we are able to earn a share of the value of the products that are created using our platform. We are quite flexible and have structured a variety of value sharing mechanisms, including royalties, equity, and lump-sum commercial milestone payments. Because the economics to us should be roughly equivalent, we are generally agnostic on which form of downstream value capture we receive and the decision is typically based on customer size and preference, with archetypes described below.
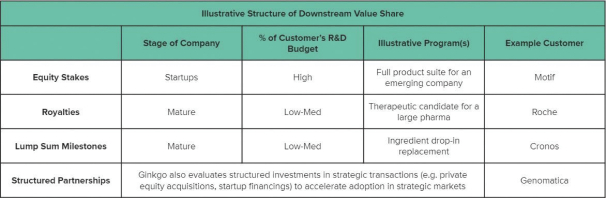
Figure 17: Illustrative of the forms of downstream value capture received by Ginkgo, based on customer size and preference.
112
Table of Contents
Because Ginkgo typically will have completed the program (and received associated usage fees) prior to realizing downstream value, cash flows from the downstream value capture component generally fall straight to the bottom line as we incur minimal to no ongoing support or delivery costs once the strain is commercialized. This dynamic creates opportunities for outsized returns as our clients successfully commercialize products built on our platform. As we add more programs to the platform over time, we expect downstream value share to contribute income, and therefore we believe our overall margins and cash flow profile will grow significantly.
Biosecurity Revenue
In the second quarter of 2020, in response to the COVID-19 pandemic, we launched our commercial offering of COVID-19 testing products and services for businesses, academic institutions, and other organizations in which we generate product and service revenue. We generate product revenue through the sale of lateral flow assay (“LFA”) diagnostic test kits, polymerase chain reaction (“PCR”) sample collection kits and pooled test kits, all of which we sell to our customers on a standalone basis. We generate service revenue primarily through the sale of our end-to-end COVID-19 testing services which consist of multiple promised goods and services including sample collection kits, physician authorizations, onsite test administration, outsourced laboratory PCR analysis, and access to results reported through a web-based portal.
Beginning in the first quarter of 2021, we launched our pooled testing initiative which focuses on providing end-to-end COVID-19 testing and reporting services to public health authorities. We are currently offering pooled testing and reporting services for K-12 schools across the United States, at airports through our partnership with XpresCheck and the CDC, as well as through other congregate settings such as our partnership with Eurofins. In the future, we believe that testing services may have a value proposition internationally and in other use cases including wastewater monitoring and air monitoring.
Our Sustainable Advantage
We have defined a unique business model over the past 14 years. The biotechnology industry has been product- centric for decades, with early horizontal platforms in life sciences frequently vertically integrating upon the development of the first successful product on their platform. As Ginkgo has embarked on this journey, we have studied and learned from innovators and established platform companies in other industries as we built our platform and business. We now benefit from significant historical investments, a virtuous cycle that grows with scale, and a strong business model that is aligned with our customers’ outcomes. These establish a strong sustainable advantage that we believe will help establish Ginkgo as a true industry standard.
Decade-plus head start in creating an industry standard platform
Hardware, software and biological tools need to be tightly integrated to replicate our platform. We have spent over 14 years building the software, automation and data science to best support a high throughput, generalized platform and expect to continue investing in this area. Our software, automation and data infrastructure cannot be easily replicated without bringing together a number of rare, specialized skillsets. In addition, without the scale and demand to stress test a high throughput platform, we expect any newly developed platform would be suboptimal. We estimate that it took us over eight years of investment and iteration to reach cost parity with “by hand” cell programming. We believe competitors will find it difficult to justify the investment in the software, automation and data science needed for high throughput operations before they acquire matching high demand.
Scale economics provide a structural cost advantage
As the only scaled horizontal platform in this space, we have the broadest number of programs that can be run on our platform, providing the highest potential for scale economics. Other companies choose to target specific markets and vertically integrate into products with high expected value. This has a tendency to overfit the capabilities of their R&D team to their targets. As discussed above, our continued scaling and investment in
113
Table of Contents
flexible tools that can apply to a broad range of end markets helps us drive efficiencies in the Foundry and Codebase across our diverse programs. Furthermore, as we scale, we are able to leverage advanced technologies that are only practical at scale and also may obtain preferred pricing with a number of suppliers. Competitors may be unable to source equivalent technology or negotiate similar pricing without first achieving scale, a feat that is difficult to do with a narrowly focused R&D platform.
Strong network and learning effects
In addition to a raw scale economic, we also accumulate knowledge and reusable Codebase from each program that runs on the platform. Every program benefits from the programs that came before and generates benefits for other current and future programs. These learnings and reusable assets are cumulative, extremely hard to replicate, and increasingly valuable to our customers. Because our learnings are generated by the work we execute in our Foundry, the scaling in our Foundry drives a scaling in our rate of learning. Thus, there is a recursive element to our platform: as the platform gets better, it also improves faster—we are excited to make this advantage of our platform available to our ecosystem of cell programmers.
Ginkgo’s value creation is aligned closely with customer success
Our platform drives value for customers along two dimensions: reducing the cost of laboratory work via automation and increasing the probability of technical success due to cumulative data and learnings. Our financial model is aligned with those factors. As we gain efficiency, we drive further demand for cell programming, which drives our Foundry revenue up. As both demand and probability of success increase, our risk-adjusted value share also increases. Our model only requires we share in a small fraction of the downstream value created by our programs, providing our customers the opportunity to generate and retain significant value. Ultimately, this encourages broader adoption of our platform across industries.
Furthermore, we seek to maintain close relationships with our customers, supporting their work, and earning their loyalty and satisfaction. The breadth and highly integrated nature of our platform makes it inefficient for a customer to simultaneously work with Ginkgo and any theoretical competitor. As there is not yet a standard interface for cell programming, it requires an upfront investment to learn how to choose and design programs to make the best use of our platform. Thus, customer retention is high and there are substantial switching costs.
We are uniquely positioned to attract the top cell programmers
Just as the top software programmers want to work with the latest technologies, we believe the top cell programmers will be attracted to our industry leading platform and access to its unique capabilities. Our ability to hire and retain the best cell programmers as internal users and developers of our platform pushes us to
continually improve and also builds a base of Ginkgo-trained experts. If these Ginkgo trained cell programmers move on to roles and opportunities in product-specific companies, we expect they will become ambassadors for the Ginkgo approach in their next role, expanding our reach into potential customers.
History of investing in credibility and trust
Let’s face it, GMOs have an image problem. This image problem has led to activities by the first generation of genetic engineering companies that backfired: lobbying against transparency in labeling laws, trying to “rebrand” GMOs with different terminology, and other efforts that have failed to build trust and engagement with stakeholders. We have taken a different approach. Rather than avoid the term, we’ve championed transparent labeling, sought to engage and build trust through open dialog, and enthusiastically embraced the potential for
114
Table of Contents
GMOs to do great things. We don’t seek to make GMOs acceptable through branding; we aim to make GMOs that people love.

Figure 18: Ginkgo seeks to make GMOs that people love.
Doing so requires care and attention to both the technical and social aspects of our platform and its impacts. This means investing in biosecurity and, as noted above, embedding it into our platform and how we operate. This
also means engaging with the social complexities of science and technology with a diverse group of people. We strive for a company culture based on a foundation of Diversity, Equity and Inclusion (see also the sections titled “—The Impact of Cell Programming—ESG is in our DNA” and “—Our People & Culture”), and aim to engage different perspectives through our creative residency and through our magazine, Grow. Through both our internal and external efforts, we seek to engage with the realities of what has made genetic engineering an ESG risk historically, and work towards equitable and positive impact.
Our Growth Strategy
We are seeking to usher in a new paradigm for cell programming. It took us over eight years of basic research and investment in software, automation, data science and scale to reach parity with the status quo of individual scientists conducting experiments by hand at a lab bench. It took us several more years to demonstrate business model maturity: delivering a platform with enough value-add to customers that we could cover the cost of cell engineering R&D programs while building Codebase and sharing in the downstream value of our programs. We believe that we are now at an inflection point where we believe we have the opportunity to become the industry standard. We see several drivers of this evolution and growth.
Scale our platform and continue to drive efficiencies and improvements
As discussed above, our platform improves with scale and to date we have observed a positive feedback loop between our Foundry and Codebase. As we scale capacity and demand on the Foundry, we expect our average unit costs to fall, creating a better value proposition for our customers as their program budgets stretch further and drive more demand. Similarly, Foundry output also grows our Codebase, which supports better program execution, creating a better value proposition for our customers as well.
We occupy over 300,000 square feet and maintain state-of-the-art machinery and laboratory equipment. We have built more than 50 custom integrated work cells, consisting of robotic automation systems, mass spectrometry, fermenters, sequencers, and more. We have the capabilities to engineer dozens of species of organisms from
115
Table of Contents
bacteria to fungi to mammalian cells. We have worked on enabling products as varied as polymers, bacterial therapeutics, bulk protein production, novel antibiotics, fine chemicals, and more.
We have been able to work on a diversity of programs while consistently driving efficiencies in the Foundry with scale. We expect to accelerate growth in capacity by integrating new technologies across our existing footprint, building new Foundry space, and investing in software, automation and data to increase utilization.
Leveraging our proof points to grow within all industries
We have now established proof points of success in a diverse set of end markets, in several cases far exceeding our customers’ specifications. When engaging with existing customers or potential new customers in similar or adjacent industry verticals, we can point to these case studies of success to demonstrate the value of our platform. This reduces the barriers to adoption, helps us grow our customer base, and increases the number of new programs under contract. Importantly, the reusable Codebase we generate from these new programs enables us to stay ahead of vertically focused competitors.
Grow with existing customers
Once we establish a relationship with a customer, there is significant room to expand the scope of our program engagements. We are able to grow with our customers and/or expand into other existing pockets of R&D spending. We have seen customers expand from one early program to five or ten programs a few years later and each new logo we add has the potential to become a true platform partner.
When we work with companies from their inception (or at least from the inception of their biotech investments), we enable them to avoid significant fixed cost investments and benefit from our economies of scale. Our relationship with these customers is extremely strong, as we are the core technology powering their R&D efforts. As a result, when these customers scale, their usage of our platform typically scales commensurately. For companies with existing, established biological capabilities, as we demonstrate the value of our flexible platform, we are able to grow our relationships to complement their core capabilities and increase the probability of
success.
Reduce barriers to adoption by integrating with external R&D teams
It can be easy to fall into the trap of assuming that new disruptive technologies must subsume existing ways of working. When hosted servers and SaaS started rising in prominence, corporate IT teams had to wrestle with changing integrations and demands. Some information technology departments were resistant to moving
“off-prem” because they felt they were effectively outsourcing their jobs. In response, the leaders in this field, such as Dell, would sometimes hire their customers’ information technology departments and find them jobs within Dell simply to get past this internal resistance. The reality was that these technologies were ushering in a much more substantial era for information technology, which dramatically increased the demand for this type of talent. This centralization of the model (from every company having large information technology departments building customized code to a broader array of specialized software vendors) didn’t come at the expense of information technology and digital technologies, but enabled its flourishing across all industries. We see something similar happening in biotechnology today. Internal R&D teams are typically both very excited to learn about the power of our platform but are also understandably nervous about what “outsourcing” work to Ginkgo might mean for the future of their teams. We have the opportunity to help them see the benefit in a true partnership with Ginkgo.
The vast majority of programs being run on the platform today are being run and managed by Ginkgo program teams—in-house scientists and engineers who are managing the R&D project to meet a customer’s specifications. But we now have a couple early examples of certain customers, those with more in-house biotech expertise, interacting directly with our platform. Over time, we would like to build in enough standardized interfaces that a distributed network of scientists could access the platform directly through a well-defined integration and self-service layer. This transition will allow our program teams to devote more of their efforts to developing Codebase assets, enabling more rapid scaling, and reducing the barriers to adoption by our customers.
116
Table of Contents
There are significant technical hurdles for us to overcome in developing this technology, but it is on our near- term roadmap and we are constantly thinking about how to “productize” individual workflows on the platform.
Build an ecosystem
As described above in “—Our Platform,” we believe we are building the industry standard developer platform for cell programming. In much the same way that early computing platforms and operating systems built real communities around their platforms in the 80s and 90s, we intend to build a community of developers building on the Ginkgo platform. As we invest to expand this ecosystem of services for cell programmers building on the
Ginkgo platform, our value proposition to cell programmers increases and we become more ubiquitous.
Our People & Culture
A company is made of people. We have sought to bring together a diverse and multidisciplinary group of people who share in our mission to make biology easier to engineer. Today, our extensive cross-functional team is collaborating to build our ecosystem, from organism designers to automation engineers, software developers to people operations, business development to facilities management, finance to molecular biology.
A culture built on care
We’ve strived to grow a culture based on care. As engineers, it is easy to fall into the trap of thinking of ourselves simply as tool builders. Tools can be used in many different ways, both good and bad, and engineers often discuss their tools as value neutral. But tools reflect the social beliefs and biases of the people who make them: today this is becoming increasingly apparent, with more and more evidence of algorithmic bias being built into AI systems, facial recognition, and much more.
As designers of the largest horizontal platform for cell programming, we are keenly aware of the need to care about how our platform is used. More significant than the impacts we have seen from digital platforms on our social world, biology is our health, our bodies, our food, and our environment. As we build the tools for programming biology, we must also care how those tools are used, and ensure that the risks and benefits are transparently and equitably shared.
A diverse, world-class team
As of June 30, 2022 we had 830 employees. Building a horizontal platform for cell engineering and a biosecurity offering requires collaboration between diverse skills and functions. It also requires deep technical expertise. Our employees are dedicated to the following functions:
| • | Platform functions including organism engineering, design, DNA synthesis and assembly, genome engineering, protein engineering and characterization, transformation and transfection, next generation sequencing, assay development, ultra high throughput screening, analytical chemistry, synthetic chemistry, directed evolution, and fermentation |
| • | Platform infrastructure functions including automation, software, development operations (“DevOps”), product management, data engineering, data analysis, and data science |
| • | Deployment functions including upstream and downstream process engineering, project engineering, quality assurance and quality control |
| • | Commercial functions including marketing, business development, alliance management, and corporate development |
| • | Operational functions including bioinformatics, lab network management, delivery logistics and customer support |
117
Table of Contents
| • | Shared enabling functions including legal, people, operations, finance, information technology, information security, facilities, environmental health and safety, procurement, shipping and receiving, inventory management, laboratory operations and media preparation |
In addition to our employees, our success would not be possible without the collaboration and support of the broad network of partners, contractors, contingent workers and temporary staff who make up the Ginkgo team.
Technologies reflect the values of the people who build them. Diversity, Equity, and Inclusion are valuable and necessary in their own right, but we believe that it is essential to build a diverse team where people from different backgrounds are included and empowered to speak up and shape the growth of this technology. We are
committed to growing a diverse team and continuing to empower an inclusive culture with strong employee ownership and engagement.
The full breadth of Ginkgo’s diversity and inclusion cannot be captured in demographic statistics, just as demographic categories cannot capture the full spectrum of diversity of human experience; however, we collect and report these numbers for transparency and as a lagging indicator of our efforts. As of June 30, 2022,
43% of our U.S. employees self-identify as an underrepresented gender (not cis male) and 13% self-identify as coming from an underrepresented racial or ethnic group in science and engineering (Black or African American, Hispanic or Latino, American Indian or Alaska Native, and Native Hawaiian and other Pacific Islander). We are not yet satisfied with these numbers and all teams have objectives around increasing diversity and building a culture of inclusion to ensure that diverse perspectives thrive.
Laying the groundwork for strong employee engagement in the future
As a private, founder-led company, we were able to infuse the organization with long-term strategic thinking. The long-term engagement and mentality of our employees can be seen in our turnover: voluntary attrition is well below the industry average.
The individuals who work at Ginkgo and build our platform care deeply about how that platform is used and the impact our company will have in the world. We hope to maintain the long-term mentality we have benefited from as a founder-led company now that Ginkgo is a public company. We believe a workforce with strong equity ownership will make the wise decisions needed to build long-term value for our company and build a company whose long-term impacts make them proud. That is why we have implemented a multi-class stock structure that permits all employees (current and future), not just founders, to hold high-vote (10 votes per share) common stock. We believe that our multi-class stock structure will help maintain this long-term mentality and encourage long-term equity ownership by our employees, thereby resulting in increasing employee ownership over time. For more information, see “Risk Factors—Risks Relating to our Organizational Structure and Governance—Only our employees and directors are entitled to hold shares of Ginkgo Class B common stock (including shares of Ginkgo Class B common stock granted or otherwise issued to our employees and directors in the future), which shares have ten votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of certain amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring stockholder approval.”
Competition
To our knowledge, there are currently no other companies that serve all industries covered by our horizontal cell programming platform. The solutions and applications offered by potential competitors vary in size, breadth, and scope, and given our broad set of application areas, we could face competition in many different forms. We also face competition from customers’ internal R&D departments and other research solution providers that largely conduct genetic engineering by-hand. We also compete against companies that seek to utilize synthetic biology
118
Table of Contents
technologies to develop specific products or target certain end markets. Additionally, competing platforms may emerge from various sources, including from joint ventures and partnerships between well-capitalized technology and life sciences companies. We identify the following three groups as our principal set of competitors:
The Status Quo: “on prem” cell programming efforts
The main source of competition we encounter is from potential customers choosing to build or maintain in-house cell engineering teams and capabilities. This status quo includes building out laboratory space and then hiring a team of highly trained scientists to conduct research “by-hand” with limited scale efficiencies. Some internal R&D operations maintain a full suite of capabilities and can design, build and test relatively complex pathways while others may have certain internal capabilities and need to outsource other elements to CROs. We believe
this is far less efficient for the customer and likely to yield worse outcomes as customers get fewer shots on goal for a given program budget.
That said, it can still be very difficult for companies to choose to trust Ginkgo with their R&D efforts versus building more traditional “on prem” labs. Smaller companies may feel like they’re “betting the farm” on Ginkgo, while larger companies may be sensitive to displacing existing R&D teams. As such, a key focus area for us is reducing the barriers to adoption for the platform by de-risking the upfront investment for earlier-stage
companies and by helping larger companies integrate their scientists closely into our workflows and empower their scientists to manage requests directly so we feel more like a resource and partner than a fully outsourced provider. Investing in these areas is a key focus area for us going forward.
Examples of traditional “synthetic biology” companies that have been vertically integrated from their founding with a focus on building products using synthetic biology include Amyris, Inc. (“Amyris”), Zymergen, Genomatica, Novozymes, DuPont, and DSM. Additionally, the vast majority of therapeutics companies that are leveraging genetic engineering have in-house capabilities, including Biogen, Novo Nordisk, Vertex, Regeneron, Bayer, and many others. These companies may be viewed as competitors to Ginkgo because they are creating products, using cell programming, that may compete with the products Ginkgo is enabling for our customers. However, as a horizontal platform, we view these companies not as competitors but as potential customers and focus not on “beating” them but rather on demonstrating our value proposition.
Verticalized cell engineering platforms
Within certain end markets, Ginkgo may compete against vertically-focused biotechnology companies providing cell engineering R&D capabilities to customers within a narrow set of end markets. While we believe the siloed nature of these companies limits their long-term potential, in the near-term, we may have a harder time penetrating those end markets given the incumbent vertical specialists in that space. The vast majority of these companies exist within therapeutic end markets given the history of cell engineering in that field. In theory, the expertise and learnings they develop from work in one field could be leveraged into neighboring end markets if these companies decided to adopt (and invest in) a more horizontal strategy. Examples of these vertically-focused platforms include AbCellera (antibody discovery), Codexis (enzymes), Senti Bio (cell therapy for oncology applications) and WuXi biologics (therapeutics).
Other possible entrants
We may also face competition from new entrants in the market, including well-capitalized technology companies with possible strategic interests in synthetic biology and its capabilities. Such companies may emerge as competitors given their access to capital, capacity to create multi-disciplinary teams across biology, chemistry, computer science and engineering, and flexibility to enter strategic ventures with life sciences companies.
119
Table of Contents
Intellectual Property
Overview: Foundry and Codebase
As discussed above, Ginkgo’s two core platform assets include:
| • | Ginkgo’s Foundry, which enables high-throughput cell programming; and |
| • | Ginkgo’s Codebase, which includes reusable biological assets that can be used to accelerate cell programs. |
Ginkgo protects each of these core assets—the Foundry and the Codebase—through a combination of patents and trade secret protections.
Patents
As of December 31, 2021, we had approximately 63 patent “families,” including patents held by the Company as well as by its wholly owned subsidiary Gen9, Inc. Some of these are represented by a Patent Cooperation Treaty (“PCT”) application with related national applications, as well as 16 pending provisional applications. We have over 58 issued U.S. and over 202 issued foreign patents, which includes European nationalizations, and approximately 69 pending U.S. non-provisional and approximately 150 pending foreign patent applications, including patents and patent applications acquired from third parties.
In addition to our proprietary methods and technologies, we also non-exclusively in-license certain intellectual property assets from third parties.
We intend to pursue additional intellectual property protection to the extent that we believe that it would be beneficial and cost-effective. We may abandon applications that are no longer relevant to our business. We cannot provide any assurance that any of our current or future patent applications will result in the issuance of patents. We also cannot assure the scope of any of our future issued patents or warrant that any of our patents will prevent others from commercializing infringing products or technology.
Our patent portfolio is detailed in the chart below:
| Patent Family |
Description |
Application/ Publication/ Patent Number |
Filing Date | Issue Date/ Status |
Earliest Expected Expiration Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| Methods and Devices for High Fidelity Polynucleotide Synthesis | Microfluidic devices and methods for assembling oligonucleotides by merging droplets containing oligonucleotide fragments with regions of complementarity | PCT/US2009/055267; WO/2010/025310 |
08/27/2009 | Nationalized in: US |
01/16/2030 | |||||
| Methods and Apparatuses for Chip-Based DNA Error Reduction | High-fidelity polynucleotide synthesis by generating complementary oligonucleotides to support bound single-stranded oligo (ss-oligo) in a microdroplet using enzymatic processes | PCT/US2010/057405; WO/2011/066186 |
11/19/2010 | Nationalized in: EP, FR, DE, LT, NL, ES, SE, CH, GB, LI, and US |
11/19/2030 | |||||
120
Table of Contents
| Methods and Microfluidic Devices for the Manipulation of Droplets in High Fidelity Polynucleotide Assembly | Methods and devices utilizing droplet-based liquid manipulation on a substrate for assembling nucleic acids including steps of sequence error removal | PCT/US2010/055298; WO/2011/056872 |
11/03/2010 | Nationalized in: US |
11/03/2030 | |||||
| Assembly of High Fidelity Polynucleotides | Methods and apparatuses for preparing and/or assembling high fidelity nucleic acids on a solid support | PCT/US2011/020335; WO/2011/085075 |
01/06/2011 | Nationalized in: US |
01/06/2031 | |||||
| Methods and Devices for Oligonucleotide Synthesis | Devices and methods for the synthesis of polynucleotides and libraries of polynucleotides using manipulation of oligo-containing droplets on a support | US 8,716,467 | 03/02/2011 | Issued 5/6/2014 |
05/12/2031 | |||||
| US 9,388,407 | 03/31/2014 | Issued 7/12/2016 |
03/02/2031 | |||||||
| US 9,938,553 | 04/08/2016 | Issued 4/10/2018 |
03/13/2031 | |||||||
| US 15/908,726; US 2018/0195100 |
02/28/2018 | Published | ||||||||
| Methods for Nucleotide Sequencing and High Fidelity Polynucleotide Synthesis | Methods of obtaining sequence information of target polynucleotides by performing sequencing by ligation and sequencing by polymerase-based reactions | PCT/US2011/036433; WO/2011/143556 |
05/13/2011 | Nationalized in: US |
05/13/2031 | |||||
| Microarray Synthesis and Assembly of Gene-Length Polynucleotides | Processes for in vitro synthesis and on-device assembly of long, gene-length polynucleotides based upon assembly of multiple shorter oligos synthesized in situ on a microarray platform | US 7,563,600; 7,323,320; 8,058,004; 9,023,601; 9,051,666; 10,450,560; 10,640,764; 10,774,325 |
09/12/2002- 02/18/2020 |
Issued 07/21/2009 - 09/15/2020 |
09/12/2022 | |||||
| US 17/019,448; US 2021/0062185 |
09/14/2020 | Published | ||||||||
| PCT/US2003/028946; WO/2004/024886 |
09/12/2003 | Nationalized in: AU, CA, CH, EP, FR, DE, DK, GB, JP, LI, NL |
09/12/2023 | |||||||
121
Table of Contents
| Compositions, Methods, and Apparatus for Oligonucleotides Synthesis | Compositions and methods for high-fidelity polynucleotide assembly on solid support from oligos by adding variable length padding sequences to the ends of the oligos | PCT/US2014/025610; WO/2014/160004 |
03/13/2014 | Nationalized in: EP, US, DE, GB |
03/13/2034 | |||||
| Compositions and Methods for Multiplex Nucleic Acids Synthesis | Methods of producing target nucleic acid using pluralities of oligos with overhangs, in which overhangs of one plurality are designed to be complementary to overhangs of another plurality | PCT/US2014/026261; WO/2014/151696 |
03/13/2014 | Nationalized in: AU, CA, CN, EP, IL, US |
03/13/2034 | |||||
| Methods for the Production of Long Length Clonal Sequence Verified Nucleic Acid Constructs | Methods and compositions for the production and isolation of high fidelity nucleic acids using high throughput sequencing of fragmented oligos which are tagged with unique barcodes at the 5’ and/or 3’ ends | PCT/US2014/048867; WO/2015/017527 |
07/30/2014 | Nationalized in: EP, CH, DE, FR, GB, LI, NL |
07/30/2034 | |||||
| Protein Arrays and Methods of Making and Using the Same | Methods and devices for preparing a protein array to generate and express a plurality of proteins from a plurality of nucleic acids on an array | PCT/US2011/060217; WO/2012/064975 |
11/10/2011 | Nationalized in: EP, US |
11/10/2031 | |||||
| Libraries of Nucleic Acids and Methods for Making the Same (Nucleic Acid Library and its Manufacturing Method) | Methods for designing and producing non-random libraries of nucleic acids using multiplexed polynucleotide synthesis in which complementary overhangs attached to specific sequences are hybridized and ligated to each other | PCT/US2014/067444; WO/2015/081114 |
11/25/2014 | Nationalized in: AU, CA, CN, EP, IL, US |
11/25/2034 | |||||
122
Table of Contents
| Iterative Nucleic Acid Assembly Using Activation of Vector-Encoded Traits | Nucleic acid configurations and cloning strategies for progressively assembling a long nucleic acid product using a plurality of assembly cycles that each include assembling a vector and two or more inserts containing one or more regulatory sequences that activate vector-encoded traits when assembled in a predetermined configuration | PCT/US2007/019209; WO/2008/027558 |
08/31/2007 | Nationalized in: US |
08/31/2027 | |||||
| Methods and Devices for Nucleic Acid Synthesis | Methods and apparatus for the synthesis of polynucleotides on a support using primer extension to generate overlapping construction oligonucleotides and assembly of the polynucleotides of interest by hybridizing construction oligos onto anchor support-bound oligonucleotides | PCT/US2011/060243; WO/2012/078312 |
11/10/2011 | Nationalized in: AU, CA, EP, BE, DE, GB, IE, LT, NL, CH, CN, DE, ES, FR, GB, IL, JP, LI, SE, US |
11/10/2031 | |||||
| Methods for Preparative in Vitro Cloning | Methods and devices for the isolation of nucleic acids from libraries by tagging a population of nucleic acids with unique oligonucleotide tags | US 9,752,176 | 06/15/2012 | Issued 09/05/2017 |
06/15/2032 | |||||
| US 15/666,345; US 2018/0023120 |
08/01/2017 | Published | ||||||||
| US 17/536,828 | 11/29/2021 | Pending | ||||||||
| PCT/US2012/042597; WO/2012/174337 |
06/15/2012 | Nationalized in: AU, CA, CN, EP, IL, CH, DE, FR, GB, LI, LT, NL, US |
06/15/2032 | |||||||
123
Table of Contents
| Compositions and Methods for High Fidelity Assembly of Nucleic Acids | Methods, compositions and algorithms for designing and producing a target nucleic acid from blunt-end double stranded nucleic acids generated by digesting the same to create cohesive-end fragments with unique cohesive ends that anneal and are ligated in a predetermined order | US 13/592,827; US 2013/0059296 US
17/373,324; US
PCT/US2012/052036; |
08/23/2012 07/12/2021
08/23/2012
|
Published
Published
Nationalized |
08/23/2032 | |||||
| Device and Method for Nucleic Acid Manipulation | High precision, high selectivity nucleic acid singulation and assembly techniques using mechanical force generated piezoelectrically or acoustically to selectively expel or transfer one or more volumes of nucleic acids from a solid support | PCT/US2018/033823; WO/2018/217702 |
05/22/2018 | Nationalized in: AU, CA, CN, EP, IL, JP, US |
05/22/2038 | |||||
| Compositions and Methods for Site-Directed DNA Nicking and Cleaving | Compositions and methods for site-directed DNA nicking and/or cleaving, and use thereof in, for example, polynucleotide assembly to create sticky-end breaks in DNA so that the resulting fragments can be used for DNA assembly | PCT/US2015/039517; WO/2016/007604 |
07/08/2015 | Nationalized in: EP, DE, GB, US |
07/08/2035 | |||||
| Methods for Nucleic Acid Assembly and High Throughput Sequencing | Hierarchical assembly of target polynucleotides from construction oligonucleotides | PCT/US2013/047370; WO/2014/004393 |
06/24/2013 | Nationalized in: AU, CA, CN, EP, CH, DE, FR, GB, LT, NL, SE, IL, JP, US |
06/24/2033 | |||||
124
Table of Contents
| Methods for Sorting Nucleic Acids and Preparative in Vitro Cloning | Compositions and methods for sorting and cloning of high fidelity nucleic acids by high throughput sequencing using unique barcode pairs (tag oligos) that may be sequenced to identify a nucleic acid of interest | US 10,081,807 | 04/24/2013 | Issued 09/25/2018 |
04/09/2035 | |||||
| US 10,927,369 | 07/18/2018 | Issued 02/23/2021 |
10/17/2033 | |||||||
| US 17/152,202; US 2021/0139888 |
01/19/2021 | Published | ||||||||
| PCT/ US2013/037921; WO/2013/163263 |
04/24/2013 | Nationalized in: AU, CA, CN, EP, CH, DE, FR, GB, LI, LT, NL, SE, IL |
04/24/2033 | |||||||
| Methods for Screening Proteins Using DNA Encoded Chemical Libraries as Templates for Enzyme Catalysis |
Methods, compositions and devices for screening a protein library for proteins having a desired activity | US 9,150,853 | 03/13/2013 | Issued 10/06/2015 |
03/13/2033 | |||||
| US 10,308,931 | 08/31/2015 | Issued 06/04/2019 |
07/27/2033 | |||||||
| US 16/397,314; US 2019/0249169 |
04/29/2019 | Published | ||||||||
| Owned by Ginkgo Bioworks, Inc. | ||||||||||
| Methods and Systems for Chemoautotrophic Production of Organic Compounds | Engineered chemoautotrophs (and methods for engineering such chemoautotrophs) including three metabolic modules: energy conversion pathways allowing use of energy from an inorganic energy source, carbon fixation | US 8,349,587 | 10/31/2011 | Issued 01/08/2013 |
10/31/2031 | |||||
| pathways, and product biosynthetic pathways to convert central metabolites into desired products, such as carbon-based products of interest | ||||||||||
| PCT/ US2012/62540; WO/2013/066848 |
10/30/2012 | Nationalized in: US |
10/31/2031 | |||||||
| Methods and Systems for Methylotrophic Production of Organic Compounds | Engineered methylotrophs (and methods for selecting such cells) for efficiently converting C1 compounds into various carbon-based products of interest, including systems, mechanisms and methods to confer pathways for energy conversion, methylotrophy, or carbon fixation | PCT/ US2013/073582; WO/2014/089436 |
12/06/2013 | Nationalized in: US |
12/06/2033 | |||||
125
Table of Contents
| Methods and Genetic Systems for Cell Engineering | Engineered probiotics comprising a nuclease module designed to specifically target and degrade a nucleic acid, a synthetic mobile genetic element module capable of dispersing the system from one host cell to another, and an antibiotic-free maintenance module | PCT/US2015/022508; WO/2015/148680 |
03/25/15 | Nationalized in: AU, CA, EP, JP, US |
03/25/2035 | |||||
| Methods and Molecules for Yield Improvement Involving Metabolic Engineering | Methods and compositions relating to cells that have been engineered to reduce or eliminate proteins having enzymatic activity that interferes with the expression of a metabolic product | PCT/US2010/036902; WO/2010/141468 |
06/01/2010 | Nationalized in: US |
07/10/2030 | |||||
| Methods and Systems for Cell State Quantification* *(Co-Owned with R. Rettberg) |
Engineered cells, and methods for engineering such cells, for genomic, transcriptomic, or proteomic analysis, using multiple peptide tags | US 9,506,167 | 07/27/2012 | Issued 11/29/2016 |
01/07/2034 | |||||
| US 10,119,975 | 11/29/2016 | Issued 11/06/2018 |
07/27/2032 | |||||||
| Protective Enzymes | Enzymes for protecting polymers from damage caused by fatty acids from secreted biological fluids such as sebum or sweat | PCT/US2018/050718; WO2019/055541 |
09/12/2018 | Nationalized in CN, EP, HK, US |
09/12/2038 | |||||
| Chimeric Terpene Synthases | Cells, enzymes, and methods for production of terpenes (which can be used as fragrances, pheromones, or antimicrobials, among other things) that are partially derived from sequences reconstructed from rare or extinct plants | PCT/US2019/018122; WO2019/161141 |
02/14/2019 | Nationalized in: EP, HK, JP, KR, US |
02/14/2039 | |||||
126
Table of Contents
| Biosynthesis of Mogrosides | Cells, enzymes, and methods for producing mogrosides (high-intensity natural sweeteners) by fermentation | PCT/US2019/060652; WO 2020/097588 |
11/09/2019 | Nationalized in: CA, CN, EP, JP, US |
11/09/2039 | |||||
| Biosynthesis of Mogrosides | Cells, enzymes, and methods for producing mogrosides (high-intensity natural sweeteners) by fermentation | PCT/US2020/057067; WO 2021/081327 |
10/23/2020 | Published | 10/23/2040 | |||||
| Biosynthesis of Mogrosides | Cells, enzymes, and methods for producing mogrosides (high-intensity natural sweeteners) by fermentation | PCT/US2021/032251; WO2021/231728 |
05/13/2021 | Published | 05/13/2041 | |||||
| Biosynthesis of Cannabinoids and Cannabinoid Precursors | Cells, enzymes, and methods for producing cannabinoid compounds by fermentation | PCT/US2020/019760; WO2020/176547 |
02/25/2020 | Nationalized in CA, EP, IL, US |
02/25/2040 | |||||
| Biosynthesis of Cannabinoids and Cannabinoid Precursors | Cells, enzymes, and methods for producing cannabinoid compounds by fermentation | PCT/US2020/046838; WO2021/034848 |
08/18/2020 | Published | 08/18/2040 | |||||
| Biosynthesis of Cannabinoids and Cannabinoid Precursors | Cells, enzymes, and methods for producing cannabinoid compounds by fermentation | PCT/US2021/024398; WO2021/195520 |
03/26/2021 | Published | 03/26/2041 | |||||
| Biosynthesis of Cannabinoids and Cannabinoid Precursors | Cells, enzymes, and methods for producing cannabinoid compounds by fermentation | PCT/US2021/037954; WO2021/257915 |
06/17/2021 | Published | 06/17/2041 | |||||
| Biosynthesis of Cannabinoids and Cannabinoid Precursors | Cells, enzymes, and methods for producing cannabinoid compounds by fermentation | PCT/US2021/040941 | 07/08/2021 | Pending | 07/08/2041 | |||||
| Biosynthesis of Cannabinoids and Cannabinoid Precursors | Cells, enzymes, and methods for producing cannabinoid compounds by fermentation | PCT/US2021/054641 | 10/12/2021 | Pending | 10/12/2041 | |||||
| Rare Earth Element (REE)-Binding Proteins | Cells, binding proteins, and methods for recovering rare earth elements, including lanthanides | PCT/US2020/038808; WO2020/257702 |
06/19/2020 | Nationalized in AU, CA, CN, EP, JP, KR, US |
06/19/2040 | |||||
127
Table of Contents
| Biosynthesis of Enzymes for use in Treatment of Maple Syrup Urine Disease (MSUD)* *(Co-Owned with Synlogic, Inc.) |
Methods, enzymes, cells, and compositions for treating maple syrup urine disease (MSUD) and other conditions characterized by excessive branched-chain amino acids | PCT/US2020/038813; WO2020/257707 |
06/19/2020 | Nationalized in IL, JP, US |
06/19/2040 | |||||
| Optimized Bacteria Engineered to Treat Disorders Involving the Catabolism of Leucine, Isoleucine, and/or Valine* *(Co-Owned with Synlogic, Inc.) |
Methods, enzymes, cells, and compositions engineered to improve leucine catabolism and treat disorders involving the catabolism of leucine, isoleucine, or valine | PCT/US2020/038675; WO 2020/257610 |
06/19/2020 | Nationalized in CA, US |
06/19/2040 | |||||
| Production of Oligosaccharides | Compositions and methods for producing fructans using sucrose:sucrose 1-fructosyltransferase (1-SST), fructan:fructan 1-fructosyltransferase (1-FFT), and/or sucrose:fructan-6 -fructosyltransferase (6-SFT) enzymes | PCT/US2020/052390; WO 2021/061910 |
09/24/2020 | Published | 09/24/2040 | |||||
| Biosynthesis of Histidine/Enhanced Production of Histidine, Purine Pathway Metabolites, and Plasmid DNA | Methods and genetically modified cells for the biosynthetic production of histidine, plasmid DNA, or purine pathway metabolites, including synthetic promoters and genes encoding modified ribose phosphate pyrophosphokinase | PCT/US2020/065286; WO 2021/126961 |
12/16/2020 | Published | 12/16/2040 | |||||
| (RPPK) and/or modified 5,10-methylene-tetrahydrofolate dehydrogenase/5,10-methylene-tetrahydrofolate cyclohydrolase (MTHFDC) enzymes | ||||||||||
128
Table of Contents
| Variant SARS-Cov -2 Proteins and Uses Thereof | Variant proteins of SARS-CoV-2 nucleocapsid, spike protein, and spike protein receptor binding domain; nucleic acids encoding such variants; and compositions, cells, diagnostic kits containing such variants or its coding nucleic acids; as well as methods of detecting, treating and/or preventing SARS-CoV-2 infection | PCT/US2021/030875 | 05/05/2021 | Pending | 05/05/2041 | |||||
| Methanol Utilization | Methods and genetically modified cells for the biosynthetic production of amino acids such as lysine using methanol as a feedstock. | PCT/US2020/028746; WO 2020/214940 |
04/17/2020 | Nationalized in: CA, CN, EP, JP, KR, US |
04/17/2040 | |||||
| Use of Bone Morphogenetic Proteins and Their Receptors for Aesthetics and Cosmetics | Methods for preventing or reducing skin wrinkles and/or enhancing or preserving facial contours using one or more Bone Morphogenetic Proteins (BMPs), or an associated BMP protein | PCT/US2021/049768 | 9/10/2021 | Pending | 9/10/2041 | |||||
| Compositions and Methods for the Production of Compounds | Host cells, vectors, and nucleic acids encoding recombinant LALs (Large ATP-binding regulators of the LuxR family of transcriptional activators) and LAL binding sites for the production of compounds such as polyketides, and methods for producing such compounds | PCT/US2017/027215; WO 2017/180748 |
04/12/2017 | Nationalized in: US, AU, CA, CN, EP, JP, KR |
04/12/2037 | |||||
129
Table of Contents
| Compositions and Methods for the Production of Compounds | Compositions and methods to facilitate combinatorial biosynthesis of polyketides, with engineered polyketide synthases that include modified domains with altered enzymatic activity | PCT/US2017/058805; WO 2018/081592 |
10/27/2017 | Nationalized in: US, AU, CA, CN, EP, JP, KR |
10/27/2037 | |||||
| Compositions and Methods for the Production of Compounds | Compositions and methods for use in combinatorial biosynthesis of polyketides by module swapping between polyketide synthase genes, with engineered polyketide synthases that include heterologous modules with altered enzymatic activity | PCT/US2017/058800; WO 2018/081590 |
10/27/2017 | Nationalized in: AU, CA, CN, EP, JP, KR, US |
10/27/2037 | |||||
| Enhanced Production of Core Lipids in Oleaginous Yeasts | Engineered cells having genetic modification(s) that increase lipid yield and methods of increasing lipid yield in a cell | PCT/US2015/067805; WO 2016/109494 |
12/29/2015 | Nationalized in: BR, CN, EP, IN, US |
12/29/2035 | |||||
| Heterologous Production of 10-Methylstearic Acid | Engineered gene sequences, cells, and methods for producing branched methyl lipids including 10-methylstearate | PCT/US2017/052491; WO 2018/057607 |
09/20/2017 | Nationalized in: BR, CA, CN, EP, US |
09/20/2037 | |||||
| Heterologous Production of 10-Methylstearic Acid by Cells Expressing Recombinant Methyltransferase | Engineered methyltransferase gene sequences, cells, and methods for producing branched methyl-lipids or exomethylene-substituted lipids | PCT/US2018/051919; WO 2019/060527 |
09/20/2018 | Nationalized in: BR, CA, EP, US |
09/20/2038 | |||||
| Methods and Compositions Involving Promoters Derived From Yarrowia lipolytica | Promoters, recombinant nucleic acids, cells and methods for modulating lipid production in oleaginous microorganisms such as yeasts | 16/942,509; US2021-0032604A1 |
07/29/2020 | Pending | 07/29/2040 | |||||
130
Table of Contents
| Microorganisms Engineered to Use Unconventional Sources of Nitrogen | Microorganisms engineered to grow on an atypical nitrogen source and their use in fermentation to produce a variety of compounds including commodities, fine chemicals, and pharmaceuticals | PCT/US2014/010332; WO 2014/107660 |
01/06/2014 | Nationalized in: AU, CA, BR, IN, US |
01/06/2034 | |||||
| Microorganisms Engineered to Use Unconventional Sources of Phosphorous or Sulfur | Microorganisms engineered to grow on an atypical phosphorus or sulfur source and their use in fermentation to produce a variety of compounds including commodities, fine chemicals, and pharmaceuticals | PCT/US2014/52841; WO 2015/031441 |
08/27/2014 | Nationalized in: CN, AU, CA, BR, IN, EP, FR, DE, GB, US |
08/27/2034 | |||||
| Diacylglycerol Acyltransferase (DGA1) Polynucleotides, and Methods of Increasing Yeast Cell Lipid Production by Overexpression of Heterologous DGA1 | Cells engineered to express heterologous DGA1 enzyme(s) that confer increased lipid production and/or enhanced efficiency of glucose consumption, as well as methods of lipid production using these cells | PCT/US2015/17227; WO 2015/127421 |
02/24/2015 | Nationalized in: CN, AU, IN, FI, EP, BE, DK, FR, DE, LU, SE, CH, GB, US |
02/24/2035 | |||||
| Selective Advantage in Fermentation | Microorganisms engineered to grow on an atypical nitrogen, phosphorus, and/or sulfur source; fermentation compositions composed of such microorganisms and a fermentation medium containing an atypical nitrogen, phosphorus, and/or sulfur source; and fermentation processes thereof | PCT/US2015/024943; WO 2015/157431 |
04/08/2015 | Nationalized in: AU, IN, US |
04/08/2035 | |||||
| Increasing Cellular Lipid Production by Increasing the Activity of Diacylglycerol Acyltransferase and Decreasing the Activity of Triacylglycerol Lipase | Engineered cells having genetic modification(s) that increase lipid yield and methods of increasing lipid yield in a cell | PCT/US15/28760; WO 2015/168531 |
05/01/2015 | Nationalized in: AU, IN, US |
05/01/2035 | |||||
131
Table of Contents
| Increasing Lipid Production in Oleaginous Yeast | Engineered cells with genetic modification(s) that increase lipid yields including modifications that increase type 1, type 2, and/or type 3 diacylglycerol acyltransferase activity and modifications that decrease lipase activity, as well as methods of increasing lipid yield | PCT/US2015/033251; WO 2015/184303 |
05/29/2015 | Nationalized in: AU, CN, IN, EP, US |
05/29/2035 | |||||
| Increasing Lipid Production and Optimizing Lipid Composition | Recombinant nucleic acids, engineered cells, and methods for increasing lipid production that involve increasing or decreasing the activity of one or more selected genes | PCT/US2015/033211; WO 2015/184277 |
05/29/2015 | Nationalized in: AU, CN, EP, IN, US |
05/29/2035 | |||||
| Oleic Acid Production in Yeast | Engineered cells having genetic modification(s) that increase oleic acid yield and methods of increasing oleic acid yield in a cell | PCT/US2015/64710; WO 2016/094520 |
12/09/2015 | Nationalized in: CN, BR, IN, EP, US |
12/09/2035 | |||||
| Derivatives of 10-Methylene Lipids, Process for Preparing Such Derivatives and Use Thereof | Tuberculostearic acid (10-methylstearic acid) derivatives, processes for producing such compounds, and their use in processes for preparing polyamides, polyesters, lactams, and lactones | PCT/EP2020/058484; WO 2020/0193681 |
03/26/2020 | Nationalized in: EP |
03/26/2040 | |||||
| Synthetic Expression Systems | Transcriptional units, synthetic expression systems, and host cells capable of expressing a gene of interest; methods for the production of bioproducts in methanol-free culture conditions. | PCT/US2021/049180 | 09/05/2021 | Pending | 09/05/2041 | |||||
132
Table of Contents
| Synthetic Methanol Inducible Promoters and Uses Thereof | Synthetic promoters capable of facilitating the high-yield synthesis of proteins and molecules; transcriptional units and host cells comprising such synthetic promoters; and methods of use thereof | PCT/US2021/059135 | 11/12/2021 | Pending | 11/12/2041 |
| 1. | The expiration date of a United States patent may be earlier or later than as listed in this table due to patent term adjustment and/or the existence of a terminal disclaimer. |
Trade secrets
Ginkgo’s technology-related intellectual property that is not patent-protected is maintained as trade secrets. We employ a variety of safeguards to protect our trade secrets, including contractual arrangements with our employees, consultants, contractors and other advisors that impose obligations of confidentiality, assignment of inventions, and security; digital security measures; and physical security precautions.
We require confidentiality and material transfer agreements from third parties that receive our confidential data or materials, and we also incorporate confidentiality and material transfer precautions into our collaboration agreements. For example, in the course of a cell program, we might transfer samples of intermediate strains to the customer for testing and scale-up work and then transfer a final commercial strain upon completion of our work. To protect both intermediate and final strains, we use strain transfer agreements that document the contractual restrictions and controls we have put into place, typically including, in the case of intermediate strains, covenants requiring the customer to return or destroy all strain samples after testing.
Trademarks and domain names
Although our business is directed at sophisticated corporate customers rather than end consumers, we have trademark rights and registrations in our name, logo, and other brand indicia in the United States and other jurisdictions around the world. We also have registered domain names for websites that we use in our business, such as www.ginkgobioworks.com.
Intellectual property transaction structure
We earn revenue from collaboration agreements with customers under which we perform cell programming activities. Through our cell programs, we develop cells that produce or are products for our customers, which they market in their verticals.
With respect to intellectual property, we have relatively standard transaction structures that apply to cell programs for a customer. In this situation, our collaboration agreements typically provide that Ginkgo will own all collaboration-related intellectual property (“Foreground IP”) concerning cell programming. To protect our collaboration partners’ investment in the collaboration and to provide them with a competitive advantage from working with Ginkgo, Ginkgo provides a limited exclusive license to patents within the Foreground IP that cover the product, usually within a specified field. However, our terms may vary.
We typically do not provide exclusive licenses to unpatented Foreground IP (i.e., trade secrets and other know-how) that results from a collaboration. In our typical deal structure, we also do not provide exclusive licenses to our “background” intellectual property—i.e., the intellectual property, whether patented or unpatented, that we developed before entering into a collaboration or develop independently from our work in the collaboration. We believe that our transaction structures allow us to maximize the reuse of Codebase across programs and ensure that technology we develop does not lie fallow.
133
Table of Contents
In-License Agreements
Amyris Partnership Agreement
On October 20, 2017, we entered into a partnership agreement (the “Partnership Agreement”) with Amyris, which, as amended from time to time, terminated all prior agreements between Ginkgo and Amyris. In the Partnership Agreement, Amyris, among other things, granted us a non-exclusive license effective as of June 28, 2016 (the date of an earlier agreement between the parties) under all of Amyris’s rights in and to certain specified microbial strains, and under all patents and applications associated with such microbial strains, to make, have made, use, sell, offer to sell and import any products other than farnesene and/or farnesene derivatives that are chemically produced from farnesene. The license is subject to any previous exclusive licenses provided to third parties and is royalty-free, fully paid-up, sublicensable, non-exclusive and perpetual (i.e., it survives termination or expiration of the Partnership Agreement except in the case of our insolvency).
Strateos Collaboration Agreement
On October 2, 2017, we entered into a collaboration agreement with Strateos, Inc. f/k/a Transcriptic, Inc. (“Strateos”), which was amended and restated on April 20, 2021 (the “Strateos Collaboration Agreement”). Under the Strateos Collaboration Agreement, Strateos granted us a non-exclusive, perpetual, irrevocable, fully paid-up, royalty-free license under certain intellectual property rights to use its software platform in a range of activities relating to our business, including, among other things, developing and commercializing cell lines, developing data packages, providing foundry and analytical services and performing diagnostic testing. In connection with the Strateos Collaboration Agreement, we paid Strateos an up-front fee of $3.0 million and agreed to pay an additional $9.0 million in fees during the five-year term of the agreement in consideration for services provided by Strateos under the agreement, of which $8.5 million had already been paid as of June 30, 2022. Either party may terminate the Strateos Collaboration Agreement without cause upon six months’ written notice to the other party. Either party may also terminate the agreement for the other party’s material breach, insolvency or change of control to a direct competitor of the terminating party. After expiration of the agreement, either party’s termination of the agreement for convenience or the other party’s insolvency, or our termination of the agreement for Strateos’ material breach or Strateos’ change of control to one of our direct competitors, we will retain a license to use Strateos’ software. We retain such rights for an 18-month period in the event the agreement is terminated by Strateos for certain material breaches of the agreement, but we do not retain such rights in the event of Strateos’ termination due to our change of control to a Strateos direct competitor, our leak or other unauthorized disclosure of Strateos’ code, or a material breach of our obligations involving payment, intellectual property or confidentiality.
Properties
Ginkgo’s headquarters are located in the Seaport district of Boston, Massachusetts and comprise a set of non-cancellable operating leases within a facility totaling over 300,000 square feet of office and laboratory space. These lease agreements expire on dates ranging from 2030 to 2036 and each contain one option to extend the lease for a five-year period at then-market rates. We also lease a total of approximately 100,000 square feet of office and lab space in Cambridge, Massachusetts, Emeryville, California, and Utrecht, Netherlands.
In anticipation of expanding facility needs to support future growth, in April 2021, we entered into a lease, as amended, consisting of approximately 260,000 rentable square feet of new office and laboratory space being developed in Boston, Massachusetts near our headquarters. The lease commencement date is estimated to be June 1, 2024, subject to certain extensions, and expires on the fifteenth anniversary of the lease commencement date. The lease includes one option to extend the lease for ten years at then-market rates as well as an expansion option if the owner constructs an additional building on the property.
We currently lease all of our facilities and do not own any real property. We believe our facilities are adequate and suitable for our current needs and that the new lease described above provides significant expansion space.
134
Table of Contents
To support future organic growth or merger and acquisition activity, we may enter into new leases, assume lease obligations or acquire property both domestically and internationally and believe that, if needed, suitable or alternative space will be available
Suppliers
Ginkgo’s suppliers for cell programming operations comprise primarily manufacturers and distributors of life science tools, consumables and equipment as well as certain specific providers of contract research, development and manufacturing services. We will sometimes enter into long-term, strategic partnerships with innovative suppliers. Because of the significant scale of our Foundry’s operations, we believe we are often an early adopter and the largest customer at scale of certain new life science tools and technologies. Our supply agreements with Twist Bioscience Corporation (“Twist”), as further described below, are examples of such strategic supplier relationships. We will also occasionally acquire technology or Codebase assets for strategic reasons and because we can integrate the technology effectively into our platform—FGen is a recent example.
Our suppliers for our biosecurity offering include multiple manufacturers and distributors of lateral flow assay (“LFA”) test kits and COVID-19 sample collection kits. We have developed a national network of third party labs for provision of COVID-19 molecular testing services. We also utilize third parties for certain other services, including physician authorizations and on-site test administration, in the provision of our end-to-end COVID-19 testing offering.
Our software, automation, data, information technology, DevOps and information security functions utilize various third party software and information technology service providers, including AWS, for data storage and processing. We also routinely engage a variety of third parties for professional services, contract employment services and consulting services.
Twist
In April 2022, we entered into a non-cancelable supply agreement (the “2022 Agreement”), with Twist, which requires us to purchase synthetic DNA at specified volumes on an annual basis over a four-year term. To the extent we fail to meet our annual minimum purchase obligations, we are required to pay a fee per unit of shortfall. The products we may purchase that contribute toward achieving our annual minimum purchase obligation can vary based on our discretion, subject to advance notice provided to Twist.
Our annual minimum purchase obligation may be adjusted for the following reasons: (i) due to a lack of availability of certain products for purchase in a given quarter; (ii) due to a lack of certain features available; (iii) delays in shipments; and (iv) lack of performance. We receive volume discounts on purchases based on specified volume thresholds over the term of the 2022 Agreement.
The 2022 Agreement can only be terminated (i) upon mutual agreement of both parties, (ii) by us upon a specified change of control, (iii) upon a material breach of the contract by either party, or (iv) by Twist in the event that we fail to place orders for more than a certain percentage of our required annual minimums under the 2022 Agreement. The purchase minimums in the 2022 Agreement create an enforceable obligation only in conjunction with each purchase order.
Government Contracts
We have entered into agreements with governmental entities and contractors in the past to serve as a U.S. government contractor or subcontractor and may do so again in the future. See “Risk Factors—We have pursued in the past and may pursue additional U.S. government contracting and subcontracting opportunities in the future and as a U.S. government contractor and subcontractor, we are subject to a number of procurement rules and regulations”.
135
Table of Contents
Government Regulations
Our business, or the business of our customers, may be regulated by the FDA and other federal authorities in the United States, including the U.S. Federal Trade Commission (“FTC”), U.S. Department of Agriculture (“USDA”), U.S. Drug Enforcement Administration (“DEA”) and Environmental Protection Agency (“EPA”), as well as comparable authorities in foreign jurisdictions and various state and local authorities in the United States. Failure to comply with applicable regulations may result in enforcement actions, civil or criminal sanctions, and adverse publicity.
FDA regulation
We provide cell engineering and product discovery services to customers engaged in the manufacture of foods, cosmetics and pharmaceutical products. The FDA regulates the research, development, testing, quality control, import, export, safety, effectiveness, storage, recordkeeping, premarket review, approval or licensure, processing, formulation, manufacturing, packaging, labeling, advertising, promotion, marketing, distribution, sale, post- market monitoring and reporting of our customers’ pharmaceuticals, cosmetics and food products, and the FTC also regulates the advertising and promotion of these products.
We also act as a systems integrator and authorized distributor of certain COVID-19 diagnostic test and collection kits manufactured by independent third parties, and we work with laboratory partners that provide clinical laboratory testing services as part of the COVID-19 testing services we offer, and these tests and test kits may be subject to regulation by the FDA. In particular, the tests and test kits used in our COVID-19 testing services may be subject to regulation by the FDA as medical devices, and may be required to comply with the requirement that such products have obtained clearance, approval, or other marketing authorizations, such as an Emergency Use Authorization (“EUA”), before they can be commercialized, as well as post-market requirements such as adverse event reporting and restrictions on labeling, marketing, and distribution.
The HHS and FDA issued several policy statements in November 2021 governing the regulation of COVID-19 Laboratory Developed Tests (“LDTs”) that resume FDA premarket review of COVID-19 LDTs that HHS halted in August 2020. Pursuant to these new policy statements, FDA expects laboratories to seek FDA marketing authorization and otherwise comply with FDA device regulations when marketing COVID-19 LDTs. An LDT is an in vitro diagnostic test that is intended for clinical use and is designed, manufactured, and used within a single laboratory. LDTs are classified as medical devices, but the FDA has historically exercised enforcement discretion and has generally not enforced FDA requirements, including premarket review, with respect to laboratories that offer LDTs. While HHS and FDA have announced their intention to require premarket review of COVID-19 LDTs, either agency may change its position in the future.
Medical products, including COVID-19 tests, that are granted an EUA or other marketing authorization must comply fully with the terms and conditions provided in the EUA or other marketing authorization. For example, EUAs for COVID-19 tests may include conditions of authorization applicable to the EUA holder, authorized distributors and authorized laboratories. Noncompliance with applicable requirements could result in negative consequences, including adverse publicity, judicial or administrative enforcement, warning letters or untitled letters from the FDA, mandated corrective promotional materials, advertising or communications with doctors, and civil or criminal penalties, among others. The FDA can also withdraw marketing authorization for the applicable product, and in the case of a product subject to an EUA, the authorization to market the product under the EUA lasts only as long as the declared public health emergency.
DEA regulation
We are engaged in the research, development, and export of certain products that may be regulated as controlled substances, including microbes designed to generate precursors to cannabinoids or other chemical intermediates. The Controlled Substances Act of 1970 (“CSA”), as amended from time to time, establishes registration,
136
Table of Contents
security, recordkeeping, reporting, storage, distribution and other requirements administered by the DEA. The DEA is concerned with the control of handlers of controlled substances, and with the equipment and raw materials used in their manufacture and packaging, in order to prevent loss and diversion into illicit channels of commerce. The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have no established medicinal use, and may not be marketed or sold in the United States. Schedule I substances are considered to present the highest risk of abuse, and Schedule V substances the lowest relative risk of abuse among controlled substances. Marijuana is classified as a Schedule I controlled substance. However, the term does not include “hemp,” which means the cannabis plant and any part of that plant, including the seeds and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol (“THC”) concentration of not more than 0.3% on a dry weight basis.
Annual registration is required for any facility that manufactures, distributes, dispenses, imports or exports any controlled substance. The registration is specific to the particular location, business activity and controlled substance schedule. For example, separate registrations are needed for import and manufacturing, and each registration will specify which controlled substance schedule is authorized for that activity.
The DEA typically inspects a facility to review its security measures prior to issuing a registration. The DEA requires “effective controls and procedures” to guard against theft and diversion of controlled substances. Security requirements vary by controlled substance schedule (with the most stringent requirements applying to Schedule I and Schedule II substances), type of business activity conducted, quantity of substances handled, and a variety of other factors. Required security measures include background checks on employees and physical control of inventory. While the specific means by which effective controls and procedures are achieved may vary, security practices may include use of cages, surveillance cameras and inventory reconciliations. Records must be maintained for the handling of all controlled substances, and, in certain scenarios, periodic reports made to the DEA. Reports must also be made for thefts or losses of any controlled substance, and disposal of controlled substances must adhere to various methods authorized by the regulations. In addition, special authorization and notification requirements apply to imports and exports.
Failure by registered establishments to maintain compliance with applicable requirements, particularly as manifested in loss or diversion, can result in enforcement action. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could eventuate in criminal proceedings. Individual states also regulate controlled substances.
Laboratory Licensing and Certification Requirements
The clinical laboratories we partner with for our COVID-19 testing program are subject to federal oversight under Clinical Laboratory Improvement Amendments of 1988 (“CLIA”), which requires all clinical laboratories to meet certain quality assurance, quality control and personnel standards. Laboratories also must undergo proficiency testing and are subject to inspections. Standards for testing under CLIA are based on the complexity of the tests performed by the laboratory, with tests classified as “high complexity,” “moderate complexity,” or “waived.” Laboratories performing high complexity testing are required to meet more stringent requirements than moderate complexity laboratories. Certain of our partner laboratories must undergo on-site surveys at least every two years, which may be conducted by the Centers for Medicare and Medicaid Services (“CMS”) under the CLIA program or by a private CMS approved accrediting agency. In addition, we hold CLIA Certificates of Waiver and may perform certain CLIA-waived tests on behalf of our clients, which subjects us to certain CLIA requirements. The sanction for failure to comply with CLIA requirements may be suspension, revocation or limitation of a laboratory’s CLIA certificate, which is necessary to conduct business, as well as significant fines and criminal penalties.
The operations of our partner laboratories and our laboratories holding CLIA Certificates of Waiver are also subject to state and local laboratory regulation. CLIA provides that a state may adopt laboratory regulations different from or more stringent than those under federal law, and a number of states have implemented their
137
Table of Contents
own laboratory regulatory requirements. State laws may require that laboratory personnel meet certain qualifications, specify certain quality controls, or require maintenance of certain records. No assurances can be given that we or our partner laboratories will pass all future licensure or certification inspections.
Federal Select Agent Regulations
Our research facilities that synthesize DNA sequences or perform other activities could become subject to the Federal Select Agent Program (“FSAP”), which involves rules administered by the Centers for Disease Control and Prevention (“CDC”) and the USDA Animal and Plant Health Inspection Service (“APHIS”). The FSAP regulates the possession, use, and transfer of biological select agents and toxins that have the potential to pose a severe threat to public health, animal or plant health, or animal or plant products. FSAP regulatory requirements include: (i) registration with the CDC and/or APHIS for research facilities that deal with the select agents and toxins; (ii) submission to periodic biosafety and security inspections; and (iii) reporting of theft, loss or release of select agents. Federal agency enforcement actions for violations of FSAP regulations can include the initiation of corrective actions, complete or partial suspension or revocation of select agent registrations or civil or criminal liability.
Genetically Modified Materials Regulations
Our technologies and the technologies of our customers involve the use of genetically modified cells, organisms and biomaterials, including, without limitation, GMOs and genetically modified microorganisms (“GMMs”), Genetically Modified Materials”), and their respective products. In the United States, the FDA, APHIS and the EPA are the primary agencies that regulate the use of GMOs, GMMs and potential products derived from GMOs or GMMs or Genetically Modified Materials, pursuant to the Coordinated Framework for the Regulation of Biotechnology.
The FDA reviews the safety of food consumed by humans and of feed consumed by animals under the Federal Food, Drug and Cosmetic Act (“FDCA”). Under the FDCA, food and feed manufacturers are responsible for ensuring that the products they market, including those developed through genetic engineering, are safe and properly labeled. In addition, the FDA must approve the use of any food additives, including GMOs, before marketing.
APHIS examines whether a plant itself presents a “plant pest” risk under the Plant Protection Act (“PPA”). Specifically, APHIS is responsible for regulating the introduction (i.e., importation, interstate movement or release into the environment) of certain GMOs and plants under the plant pest provisions in the PPA to ensure that they do not pose a plant pest risk. APHIS finalized changes to the PPA’s implementing regulations with respect to certain GMOs in May 2020. A person or organization may request a regulatory status review from APHIS to determine whether a GMO is unlikely to pose a plant pest risk and, therefore, is not regulated under the plant pest provisions of the PPA or the regulations codified at 7 C.F.R. Part 340; requesting a regulatory status review tends to assume the GMO at issue does not otherwise fall within a regulatory exemption. If the GMO does not qualify for an exemption or if the APHIS regulatory status review process finds that the plant poses a plausible plant pest risk, then the GMO may require an APHIS permit, i.e., be a regulated article under Part 340. A regulated article may be subject to APHIS for the environmental release, importation, or interstate movement of the GMO or its progeny.
EPA regulates, under the Federal Insecticide, Fungicide and Rodenticide Act (“FIFRA”), the pesticides (including plant incorporated protectants) that are used with crops, including GMO herbicide-tolerant crops. FIFRA generally requires all pesticides to be registered before distribution or sale, unless they are exempted. Under FIFRA, a pesticide registrant must demonstrate that the pesticide at issue, when used pursuant to its specifications, “will not generally cause unreasonable adverse effects on the environment” to secure a registration. EPA must approve each distinct pesticide product, each distinct use pattern, and each distinct use site. In addition to EPA’s FIFRA authority, EPA also regulates potential human health impacts from pesticides
138
Table of Contents
under the FDCA. EPA does so by establishing “tolerance levels” (i.e., “the amount of pesticide that may remain on food products”) under the FDCA.
Telehealth regulation
Our telehealth provider partner is subject to various federal, state and local certification and licensing laws, regulations and approvals, relating to, among other things, the adequacy of health care, the practice of medicine and other health professions (including the provision of remote care and cross-coverage practice), equipment, personnel, operating policies and procedures and the prerequisites for ordering laboratory tests. Some states have enacted regulations specific to providing services to patients via telehealth. Such regulations include, among other things, informed consent requirements that some states require providers to obtain from their patients before providing telehealth services. Health professionals who provide professional services using telehealth modalities must, in most instances, hold a valid license to practice the applicable health profession in the state in which the patient is located. In addition, certain states require a physician providing telehealth to be physically located in the same state as the patient. Any failure to comply with these laws and regulations could result in civil or criminal penalties against telehealth providers.
State corporate practice of medicine and fee splitting laws
Our relationship with our telehealth provider partner, who provides physician oversight and support to individuals seeking COVID-19 diagnostic or screening testing, including evaluating each request for testing, communicating and providing consultation services for certain test results, is subject to various state laws, which are intended to prevent unlicensed persons from interfering with or influencing a physician’s professional judgment, and prohibiting the sharing of professional services income with non-professional or business interests. These laws vary from state to state and are subject to broad interpretation and enforcement by state regulators. A determination of non-compliance could lead to adverse judicial or administrative action against us and/or our telehealth provider partner, civil or criminal penalties, receipt of cease and desist orders from state regulators, loss of provider licenses, or a restructuring of our arrangement with our telehealth provider partner.
Healthcare fraud and abuse laws
Although none of our COVID-testing offerings are currently billed to any third-party payor, including any commercial payor or government healthcare program, by us or any of our laboratory or telehealth provider partners, we may nonetheless be subject to a number of federal and state healthcare regulatory laws that restrict business practices in the healthcare industry. These laws include, but are not limited to, federal and state anti- kickback, false claims, and other healthcare fraud and abuse laws.
The federal Anti-Kickback Statute prohibits, among other things, individuals or entities from knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, overtly or covertly, in cash or in kind to induce or in return for purchasing, leasing, ordering or arranging for or recommending the purchase,
lease or order of any item or service reimbursable under Medicare, Medicaid or other federal healthcare programs. A person or entity does not need to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation.
The federal physician self-referral prohibition, commonly known as the Stark Law, prohibits a physician, in the absence of an applicable exception, from making a referral for certain designated health services covered by the Medicare or Medicaid program, including clinical laboratory services, if the physician or an immediate family member of the physician has a financial relationship with the entity providing the designated health services. The Stark Law also prohibits the entity furnishing the designated health services from billing, presenting or causing to be presented a claim for the designated health services furnished pursuant to the prohibited referral.
The federal civil and criminal false claims laws, including the civil False Claims Act, and civil monetary penalties laws prohibit, among other things, any individual or entity from knowingly presenting, or causing to be
139
Table of Contents
presented, a false claim for payment to the federal government, knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government, or from knowingly making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute or the Stark Law, constitutes a false or fraudulent claim for purposes of the civil False Claims Act.
In addition to the Anti-Kickback Statute and the Stark Law, the United States recently enacted a law known as the Eliminating Kickbacks in Recovery Act (“EKRA”), which created a new federal crime for knowingly and willfully: (1) soliciting or receiving any remuneration in return for referring a patient to a recovery home, clinical treatment facility, or laboratory; or (2) paying or offering any remuneration to induce such a referral or in exchange for an individual using the services of a recovery home, clinical treatment facility, or laboratory. Unlike the Anti-Kickback Statute, EKRA is not limited to services reimbursable under a government health care program, but instead extends to all services reimbursed by “health care benefit programs.”
The federal Health Insurance Portability and Accountability Act of 1996, as amended by Health Information Technology for Economic and Clinical Health Act (“HIPAA”) created additional federal criminal statutes that prohibit, among other things, knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the U.S. federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Similar state and local laws and regulations may also restrict business practices in the medical device and clinical laboratory industries, such as state anti-kickback and false claims laws, which may apply to business practices, including but not limited to, research, distribution, sales and marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, or by patients themselves; and state laws that require companies to comply with the industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources.
Violation of any of such laws or any other governmental regulations that apply may result in significant criminal, civil and administrative penalties including damages, fines, imprisonment, disgorgement, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, contractual damages, reputational harm, diminished profits and future earnings, disgorgement, exclusion from participation in government healthcare programs and the curtailment or restructuring of our operations.
Federal and state data privacy and security regulations
Numerous state, federal and foreign laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality and security of personal information, including health- related information. In the United States, numerous federal and state laws and regulations, including data breach notification laws, health information privacy and security laws, including HIPAA, and federal and state consumer protection laws and regulations (e.g., Section 5 of the Federal Trade Commission Act (“FTC Act”)), that govern the collection, use, disclosure, and protection of health-related and other personal information could apply to our operations or the operations of our partners. HIPAA, and its respective implementing regulations, imposes obligations on “covered entities,” including certain health care providers, health plans, and health care clearinghouses, and their respective “business associates” that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, as well as their covered subcontractors with respect to safeguarding the privacy, security and transmission of individually identifiable health information.
140
Table of Contents
Violations of the HIPAA privacy and security regulations may result in civil and criminal penalties. The U.S. Department of Health and Human Services (“HHS”) is required to conduct periodic compliance audits of covered entities and their business associates. HIPAA also authorizes State Attorneys General to bring civil actions seeking either an injunction or damages in response to violations of HIPAA privacy and security regulations.
In addition, certain state laws, such as the California Confidentiality of Medical Information Act, the California Consumer Privacy Act of 2018 (“CCPA”) and the California Privacy Rights Act (“CPRA”) govern the privacy and security of personal information, including health-related information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. Privacy and security laws, regulations, and other obligations are constantly evolving, may conflict with each other (thus complicating compliance efforts), and can result in investigations, proceedings, or actions that lead to significant civil or criminal penalties and restrictions on data processing.
Legal Proceedings
From time to time, we may in the ordinary course of business be named as a defendant in lawsuits, indemnity claims and other legal proceedings. We do not believe any pending litigation to be material, the outcome of which would, in management’s judgment based on information currently available, have a material adverse effect on our results of operations or financial condition. See Note 14, “Commitments and Contingencies,” to the audited consolidated financial statements and Note 8, “Commitments and Contingencies,” to the unaudited condensed consolidated financial statements included elsewhere in this registration statement and proxy statement/prospectus.
Ginkgo Corporate Information
Ginkgo’s principal executive office is located at 27 Drydock Avenue, Boston, Massachusetts 02210, and Ginkgo’s telephone number is (877) 422-5362. Ginkgo’s corporate website address is www.ginkgobioworks.com. We make available on the Investor Relations section of our website, free of charge, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, proxy statements, and Forms 3, 4 and 5, and amendments to those reports as soon as reasonably practicable after filing such documents with, or furnishing such documents to, the U.S. Securities and Exchange Commission (the “SEC”). The SEC maintains a website (www.sec.gov) that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC.
The information contained on, or accessible through, our corporate website or connected thereto does not constitute part of, and is not incorporated by reference into this prospectus and should not be considered part of this prospectus. The inclusion of the corporate website address is an inactive textual reference only.
141
Table of Contents
DESCRIPTION OF CAPITAL STOCK
The following summary of the material terms of our capital stock is not intended to be a complete summary of the rights and preferences of such securities, and is qualified by reference to our Charter, our Bylaws and the Warrant- related documents described herein, which are exhibits to the registration statement of which this prospectus is a part. We urge you to read each of our Charter, our Bylaws and the Warrant-related documents described herein in their entirety for a complete description of the rights and preferences of our securities.
Authorized Capital Stock
Ginkgo’s Charter authorizes the issuance of 16,000,000,000 shares of all classes of Ginkgo’s capital stock, consisting of:
| • | 200,000,000 shares of undesignated preferred stock, par value $0.0001 per share; |
| • | 10,500,000,000 shares of Class A common stock, par value $0.0001 per share; |
| • | 4,500,000,000 shares of Class B common stock, par value $0.0001 per share; and |
| • | 800,000,000 shares of Class C common stock, par value $0.0001 per share. |
Common Stock
Ginkgo has three classes of authorized common stock: Class A common stock, Class B common stock, and Class C common stock. Generally, Class B common stock can only be issued to, transferred to, and held by Ginkgo’s directors and employees, or trusts or legal entities through which the right to vote the shares of Class B common stock held thereby is exercised exclusively by one or more of Ginkgo’s directors or employees (any such director, employee, trust or legal entity, an “Eligible Holder”), unless otherwise determined by a majority of the Class B Directors then serving.
Voting Rights
Class A Common Stock
Holders of Class A common stock are entitled to one (1) vote for each share of Class A common stock held of record by such holder on all matters voted upon by Ginkgo stockholders.
Class B Common Stock
Holders of Class B common stock are entitled to ten (10) votes for each share of Class B common stock held of record by such holder on all matters voted upon by Ginkgo stockholders.
Class C Common Stock
Except as expressly provided in Ginkgo’s Charter or required by applicable law, holders of Class C common stock generally are not entitled to vote on matters voted upon by Ginkgo stockholders. Solely to the extent that a holder of Class C common stock is expressly entitled to vote on any matter pursuant to Ginkgo’s Charter or by applicable law, the holder will be entitled to one (1) vote for each share of Class C common stock held of record by such holder.
Stockholder Votes
Holders of Common Stock generally vote together as a single class on all matters submitted to a vote of Ginkgo stockholders (including the election and removal of directors), unless otherwise provided in Ginkgo’s certificate
142
Table of Contents
of incorporation or required by applicable law. Any action or matter submitted to a vote of the Ginkgo stockholders will be approved if the number of votes cast in favor of the action or matter exceeds the number of votes cast in opposition to the action or matter, except that Ginkgo’s directors will be elected by a plurality of the votes cast. Holders of Class A common stock are not entitled to cumulate their votes in the election of Ginkgo’s directors.
Delaware law could require holders of a class of Ginkgo’s capital stock to vote separately as a class on any proposed amendment of Ginkgo’s certificate of corporation if the amendment would increase or decrease the par value of the shares of that class or would alter or change the powers, preferences or special rights of the shares of that class in a manner that affects them adversely.
Holders of Common Stock are not entitled to vote on any amendment to Ginkgo’s Charter that relates solely to the terms of one or more series of Ginkgo’s preferred stock and on which the holders of such affected series are entitled to vote, either separately as a class or together with the holders of one or more other series of Ginkgo’s preferred stock, pursuant to Ginkgo’s Charter or by applicable law.
Stockholder Action by Written Consent
The Charter provides that Ginkgo’s stockholders may act by written consent only if (a) the action to be taken or effected has been approved by the affirmative vote of all of the directors of Ginkgo then serving or (b) the holders of Class B common stock collectively beneficially own shares representing a majority of the voting power of all of the outstanding shares of capital stock of Ginkgo. In all other circumstances, any action required or permitted to be taken by Ginkgo’s stockholders must be effected at a duly called annual or special meeting of stockholders and may not be taken or effected by written consent.
Special Meetings of Stockholders
The Charter provides that, except as otherwise required by applicable law, special meetings of Ginkgo’s stockholders may be called only by the Ginkgo Board, the chairman of the Ginkgo Board, Ginkgo’s chief executive officer or president, or, at any time that the holders of Class B common stock collectively beneficially own shares representing a majority of the voting power of all of the outstanding shares of capital stock of Ginkgo, the holders of shares representing a majority of the voting power of all of the outstanding shares of capital stock of Ginkgo.
Economic Rights
Except as otherwise expressly provided in Ginkgo’s Charter or required by applicable law, shares of each class of Common Stock have the same rights, powers and preferences and rank equally, share ratably and be identical in all respects as to all matters, including the following:
Dividends and Distributions; Rights upon Liquidation
Subject to the rights of holders of any outstanding series of Ginkgo preferred stock, the holders of shares of each class of Common Stock are entitled to receive ratably, on a per share basis, any dividend or distribution (including upon the liquidation, dissolution or winding up of Ginkgo) paid by Ginkgo, unless otherwise approved by the affirmative vote of the holders of a majority of each of the outstanding shares of Class A common stock, the outstanding shares of Class B common stock, and the outstanding shares of Class C common stock, each voting separately as a class, except that, if a dividend or distribution is paid in the form of shares (or options, warrants or other rights to acquire shares) of Common Stock, then holders of Class A common stock will receive shares (or options, warrants or other rights to acquire shares) of Class A common stock, holders of Class B common stock will receive shares (or options, warrants or other rights to acquire shares) of Class B common stock, and holders of shares of Class C common stock will receive shares (or options, warrants or other rights to acquire shares) of Class C common stock.
143
Table of Contents
Subdivisions, Combinations and Reclassifications
If Ginkgo subdivides or combines any class of Common Stock with any other class of Common Stock, then each class of Common Stock must be subdivided or combined in the same proportion and manner, unless otherwise approved by the affirmative vote of the holders of a majority of each of the outstanding shares of Class A common stock, the outstanding shares of Class B common stock, and the outstanding shares of Class C common stock, each voting separately as a class.
Mergers and Other Extraordinary Transactions
The Charter provides that, in the event of certain extraordinary transactions affecting Ginkgo (including certain transactions resulting in a change of control of Ginkgo, the acquisition by a third party of assets of Ginkgo generating at least 50% of Ginkgo’s revenues on a consolidated basis, or any merger or consolidation of Ginkgo), shares of each class of Common Stock will be entitled to receive ratably, on a per share basis, any consideration paid or otherwise distributed to, or rights received by, Ginkgo stockholders, or into which such shares are converted or for which such shares are exchanged, in connection with such extraordinary transaction (including with respect to the form, amount and timing thereof), unless different treatment of the shares of each such class is approved by the affirmative vote of the holders of a majority of the outstanding shares of Class A common stock, the holders of a majority of the outstanding shares of Class B common stock and the holders of a majority of the outstanding shares of Class C common stock, each voting separately as a class, except that, to the extent that such consideration is paid in the form of securities or other equity interests, holders of Class B common stock may receive a class, series or other form of such securities or other equity interests each having voting power that is ten (10) times greater than the voting power of any security or other equity interest received by holders of Class A common stock and holders of Class C common stock may receive a class, series or other form of such securities or other equity interests having no voting power.
Additionally, the Charter prohibits Ginkgo from entering into any agreement with respect to a tender or exchange offer by a third party unless such agreement provides for consideration to be paid or distributed to, or rights to be received by, Ginkgo stockholders in the manner provided in the paragraph immediately above.
Equal Value upon Disposition
The Charter provides that, in the case of any disposition of Class B common stock for value, the value paid in respect of such share of Class B common stock must be equal to the prevailing price per share of Class A common stock at the time of such disposition for value. Ginkgo may (and expects to) from time to time establish restrictions, policies and procedures relating to transfers and dispositions of shares of Class B common stock as it deems necessary or advisable.
Transfer Restrictions
Lock-up Applicable to Founders and Employees
The Charter provides that, subject to customary exceptions and the other exceptions described in the following sentences, the Founders and their affiliated trusts and any Ginkgo stockholder who is an employee of Ginkgo or any of its wholly owned subsidiaries at the time of the Closing, and any transferee of any of the foregoing, are unable to transfer their shares of Class A common stock or Class B common stock received as consideration in the Merger (including upon the settlement of any equity award of Ginkgo into which any equity award of Old Ginkgo was converted in the Merger), other than the Earn-out Consideration, for a period of one year following the closing of the Business Combination. The transfer restrictions described in the foregoing sentence will not apply to an aggregate of 10% of the total number of shares subject to such transfer restrictions, excluding (from this exception to such transfer restrictions) any shares of Class A common stock or Class B common stock received upon the settlement of any equity award of Ginkgo into which any equity award of Old Ginkgo that was, immediately prior to the effective time of the Merger, subject to any unsatisfied service- or time-based
144
Table of Contents
vesting condition was converted in the Merger. Additionally, solely in the case of any holder of any equity award of Ginkgo (including any such equity award into which any equity award of Old Ginkgo was converted in the merger), the transfer restrictions described in the first sentence of this paragraph no longer apply.
Conversion
Optional Conversion
Holders of Class B common stock have the right to convert shares of their Class B common stock into fully paid and non assessable shares of Class A common stock, on a one-to-one basis, at the option of the holder at any time upon written notice to Ginkgo’s transfer agent.
Automatic Conversion
Generally, shares of Class B common stock will convert automatically into Ginkgo Class A common stock upon the holder of such shares ceasing to be an Eligible Holder (whether as a result of the holder’s termination, resignation or removal as a director or employee of Ginkgo, the transfer of such shares to an individual, trust or entity that is not an Eligible Holder, a person other than a director or employee of Ginkgo gaining any direct or indirect right to vote such shares, or otherwise), unless otherwise determined by the affirmative vote of a majority of the directors of Ginkgo then serving who qualify as “independent” in accordance with the requirements of the securities exchange on which equity securities of Ginkgo are then listed for trading. A determination by the secretary of the Ginkgo that an event has occurred that triggers the automatic conversion of Class B common stock into Class A common stock will be conclusive and binding; however, a holder of Class B common stock (or Class A common stock into which Class B common stock has converted) who believes in
good faith that such determination is in error may appeal such determination to the Ginkgo Board, in which case, the determination of the Ginkgo Board (including as to whether or not to review such determination) will be conclusive and binding.
Conversion Policies and Procedures
Ginkgo may (and expects to) establish from time to time certain restrictions, policies and procedures relating to the general administration of its multi-class stock structure and the conversion of Class B common stock to Class A common stock. Adoption or amendment of any such policy or procedure must be approved by the affirmative vote of a majority of Ginkgo’s directors and, if any Class B Director is then serving, at least one Class B Director (defined below).
Registration Rights
Each of the Amended and Restated Registration Rights Agreement, dated as of September 16, 2021, by and among Ginkgo, the SRNG Sponsor and Viking Global Opportunities Illiquid Investments Sub-Master LP (the “SRNG Business Combination Registration Rights Agreement”) and the Share Purchase Agreement, dated March 11, 2022, by and among Ginkgo International Holdings, Inc. (our indirect, wholly owned subsidiary), FGen, and the sellers named therein (the “FGen Purchase Agreement”) granted registration rights to certain Ginkgo stockholders. Each agreement grants certain Ginkgo stockholders the right to require, subject to certain conditions and limitations, that Ginkgo register for resale securities held by such stockholders, and the SRNG Business Combination Registration Rights Agreement includes “piggyback” registration rights with respect to registrations initiated by Ginkgo. The registration of shares of Class A common stock pursuant to the exercise of any of these registration rights would enable the applicable Ginkgo stockholders to resell such shares without restriction under the Securities Act when the applicable registration statement is declared effective. Ginkgo will bear the expenses incurred in connection with the filing of any registration statements pursuant to the SRNG Business Combination Registration Rights Agreement and FGen Purchase Agreement
145
Table of Contents
Other Rights
The Charter and the Bylaws do not provide for any preemptive or subscription rights with respect to the Common Stock, and there are no redemption or sinking fund provisions applicable to the Common Stock. All the outstanding shares of Common Stock are validly issued, fully paid and nonassessable.
Preferred Stock
The Charter authorizes the Ginkgo Board, to the fullest extent permitted by applicable law, to issue up to an aggregate of 200,000,000 shares of Ginkgo preferred stock in one or more series from time to time by resolution, without further action by Ginkgo’s stockholders, and to fix the powers (which may include full, limited or no voting power), designations, preferences and relative, participating, optional or other special rights, if any, of the shares of each such series (which rights may be greater than the rights of any or all of the classes of Common Stock) and any qualifications, limitations or restrictions thereof. The issuance of Ginkgo preferred stock could adversely affect the voting power of holders of Common Stock and the likelihood that such holders will receive dividend payments or payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deterring or preventing a change of control or other corporate action. No shares of preferred stock are currently outstanding, and there is no present plan to issue any shares of preferred stock.
Other Constituencies
In acknowledgment of our goal of serving all of our stakeholders over the long term, the Charter provides that, in addition to any other considerations which the Ginkgo Board, any committee thereof, or any individual director lawfully may take into account in determining whether to take or refrain from taking corporate action on any matter, including making or declining to make any recommendation to our stockholders, our board of directors, any committee thereof, or any individual director may, in his, her, or its discretion, consider the long-term as well as the short-term interests of Ginkgo, taking into account and considering, as deemed appropriate, the effects of such action on our (a) stockholders and (b) other stakeholders, including our workforce, customers, suppliers, academic researchers, governments and communities, in the case of (b), as may be identified or revised by the Ginkgo Board from time to time. The Charter also provides that nothing in the Charter or any other governing document, policy, or guideline adopted by us will (i) create any duty owed by any director to any person or entity to consider, or afford any particular weight to, any of the foregoing matters or to limit his or her consideration thereof or (ii) other than as vested in our stockholders to the extent provided under applicable law, be construed
as creating any rights against any director or us. These constituency provisions grant discretionary authority only to the extent consistent with and permitted by law, and do not confer third-party beneficiary status on any person or entity.
Election, Appointment and Removal of Directors
Until the time at which the outstanding shares of Class B common stock cease to represent at least 2% of all of the outstanding shares of Common Stock, the holders of Class B common stock, voting separately as a class, will be entitled to nominate and elect a number of directors equal to 25% (rounded up to the nearest whole number) of the total number of directors constituting the Ginkgo Board (each such director, a “Class B Director”). All other directors of Ginkgo will be elected by the holders of all classes of Common Stock, voting together as a single class.
The total number of directors constituting the Ginkgo Board will be fixed from time to time by Ginkgo’s Board, but will be subject to adjustment to ensure that the total number of directors that the holders of Class B common stock are entitled to nominate and elect is at least 25% of the total number of directors constituting the Ginkgo Board.
The Charter provides that any Class B Director may be removed from office (a) with cause, only by the affirmative vote of the holders of shares representing a majority of the voting power of all of the outstanding
146
Table of Contents
shares of Class B common stock and (b) without cause, by the affirmative vote of the holders of shares representing a majority of the voting power of all of the outstanding shares of capital stock of Ginkgo, voting together as a single class. Any director of Ginkgo other than a Class B Director may be removed from office, with or without cause, by the affirmative vote of the holders of shares representing a majority of the voting power of all of the outstanding shares of capital stock of Ginkgo, voting together as a single class. The Charter provides that vacant directorships, including vacancies resulting from any increase in the total number of directors constituting the Ginkgo Board, may be filled only by the Ginkgo Board. Vacancies with respect to any Class B Director may be filled only by the remaining Class B Directors.
Committees of the Board of Directors
The Ginkgo Board has established, and will maintain, an audit committee, a nominating and corporate governance committee and a compensation committee, and may establish such other committees as it determines from time to time. For so long as any Founder serving as a director of Ginkgo holds shares of Class B common stock, such director will not be permitted to serve as a member of the compensation committee of the Ginkgo Board. Subject to applicable requirements of the securities exchange on which equity securities of Ginkgo are then listed for trading, at any time that any Class B Director is serving as a director of Ginkgo, each committee (other than the compensation committee) of the Ginkgo Board must include at least one Class B Director unless a majority of the Class B Directors then serving approve the formation and composition of such committee.
Action by the Ginkgo Board of Directors to Terminate a Founder
Ginkgo may not terminate the employment of any Founder for cause, or materially and adversely reduce the responsibilities, title or position of such Founder for cause, without the prior written consent of such Founder, or make any determination that an event has occurred with respect to such Founder that constitutes “cause” (as that term or any similar concept may be defined or used in any agreement relating to the employment of such Founder by Ginkgo or any of its subsidiaries or any policy of Ginkgo or any of its subsidiaries applicable to the employment of such Founder), unless such termination, reduction or determination has been approved by at least
75% of the directors of Ginkgo then in office.
Ginkgo may not terminate the employment of any Founder other than for cause, or materially and adversely reduce the responsibilities, title or position of such Founder other than for cause, without the prior written consent of such Founder, unless such termination or reduction has been approved by at least 75% of the directors of Ginkgo then in office and, if any Founder who is not the subject of the action requiring such approval is then serving as a director of Ginkgo, at least one director of Ginkgo who is a Founder.
Anti-Takeover Effects of the Charter and the Bylaws
The Charter and Bylaws contain certain provisions that may delay, discourage or impede efforts by another person or entity to acquire control of Ginkgo. We believe that these provisions, which are summarized below, will discourage coercive takeover practices or inadequate takeover bids. These provisions are also designed to encourage persons or entities seeking to acquire control of us to first negotiate with the Ginkgo Board, which we believe may result in improvement of the terms of any such acquisition in favor of Ginkgo’s stockholders. However, these provisions also give the Ginkgo Board the power to discourage acquisitions that some stockholders may favor.
Authorized but Unissued Capital Stock
The authorized but unissued shares of our common stock and our preferred stock will be available for future issuance without stockholder approval, subject to any limitations imposed by the listing standards of the securities exchange on which Ginkgo’s equity securities are then listed for trading. These additional shares of capital stock may be used for a variety of corporate purposes, including growth acquisitions, corporate finance
147
Table of Contents
transactions, and issuances under our Equity Incentive Plan (“EIP”) and our Employee Stock Purchase Plan (“ESPP”). The existence of authorized but unissued and unreserved capital stock could discourage or impede an attempt to obtain control of Ginkgo by means of a proxy contest, tender offer, merger, or otherwise.
Amendment of Certificate of Incorporation or Bylaws
The DGCL generally provides that the affirmative vote of a majority of the outstanding shares entitled to vote on amendments to a corporation’s certificate of incorporation or bylaws is required to approve such amendment, unless a corporation’s certificate of incorporation or bylaws, as applicable, imposes a higher voting standard.
The Charter provides that certain provisions thereof may be adopted, amended, altered or repealed only upon the affirmative vote of the holders of at least two-thirds of the voting power of all of the outstanding shares of capital stock of Ginkgo. Such provisions include those relating to (i) stockholder action by written consent, (ii) special meetings of stockholders, (iii) the Ginkgo Board (including the election, appointment and removal of directors), (iv) termination of the employment of any Founder, material and adverse reduction of the responsibilities, title or position of any Founder without the prior written consent of such Founder, or determination that an event has occurred with respect to any Founder that constitutes “cause”, (v) limitation of the personal liability of Ginkgo’s directors, and (vi) Ginkgo’s waiver of the corporate opportunity doctrine.
The Charter provides that Ginkgo’s Bylaws may be adopted, amended, altered or repealed by the Ginkgo Board or by the affirmative vote of the holders of at least two-thirds of the voting power of all of the outstanding shares of capital stock of Ginkgo (or, if the Ginkgo Board has recommended that stockholders approve such modification to Ginkgo’s Bylaws, the affirmative vote of a majority of the voting power of all of the outstanding shares of capital stock of Ginkgo).
These provisions may have the effect of deterring hostile takeovers or delaying or preventing changes of control of Ginkgo or its management such as a merger, reorganization or tender offer. These provisions are intended to enhance the likelihood of continued stability in the composition of the Ginkgo Board and its policies and to discourage certain types of transactions that may involve an actual or threatened acquisition of Ginkgo and to reduce Ginkgo’s vulnerability to an unsolicited acquisition proposal. These provisions are also intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for Ginkgo’s shares and, as a consequence, may inhibit fluctuations in the market price of the Company’s shares that could result from actual or rumored takeover attempts. Such provisions may also have the effect of preventing changes in management.
Multi-Class Structure
As described above, the Charter provides for a multi-class stock structure, which gives Ginkgo’s directors and employees (including the Founders) and certain of their affiliated entities and trusts, for so long as they continue to collectively beneficially own shares representing a majority of the voting power of all of the outstanding shares of capital stock of Ginkgo, significant influence over all matters requiring stockholder approval, including the election of Ginkgo’s directors and significant corporate transactions, such as a merger or other sale of Ginkgo or all or substantially all of its assets.
No Cumulative Voting for Directors
The DGCL provides that stockholders are not entitled to cumulate votes in the election of directors unless a corporation’s certificate of incorporation provides otherwise. The Charter does not provide for cumulative voting. As a result, the holders of shares of Common Stock representing a majority of the voting power of all of the outstanding shares of capital stock of Ginkgo will be able to elect all of the directors (other than the Class B Directors) then standing for election.
148
Table of Contents
Vacancies on the Ginkgo Board
The Charter authorizes only the Ginkgo Board to fill vacant directorships, including vacancies resulting from any increase in the total number of directors constituting the Ginkgo Board. In addition, the total number of directors
constituting the Ginkgo Board is permitted to be changed only by the Ginkgo Board, subject to the requirement that at least 25% of the total number of Ginkgo’s directors be Class B Directors (for so long as the outstanding shares of Class B common stock continue to represent at least 2% of all the outstanding shares of Common Stock). These provisions could prevent a stockholder from increasing the total number of Ginkgo’s directors and then gaining control of the Ginkgo Board.
Requirements to Terminate Employment of Founders
The Charter requires that any termination by Ginkgo of the employment of any Founder other than for cause, or material and adverse reduction of the responsibilities, title or position of such of such Founder other than for cause without the prior written consent of such Founder, be approved by at least 75% of the directors of Ginkgo then in office and, if any Founder who is not the subject of the action requiring such approval is then serving as a director of Ginkgo, at least one director of Ginkgo who is a Founder. This provision, together with the right of the holders of Class B common stock to nominate and elect 25% of the Ginkgo Board, could make it more difficult for a stockholder that gains control of the Ginkgo Board to effect changes in Ginkgo’s management.
Special Meetings of Stockholders, Action by Written Consent, and Advance Notice Requirements for Stockholder Proposals
Special Meetings of Stockholders
The Charter permits special meetings of Ginkgo’s stockholders to be called only by the Ginkgo Board, the chairman of the Ginkgo Board, Ginkgo’s chief executive officer or president, or, at any time that the holders of Class B common stock collectively beneficially own shares representing a majority of the voting power of all of the outstanding shares of capital stock of Ginkgo, the holders of shares representing a majority of the voting power of all of the outstanding shares of capital stock of Ginkgo. These provisions might delay the ability of Ginkgo’s stockholders to force consideration of a proposal or to take any action, including with respect to the removal of any of Ginkgo’s directors from office.
Stockholder Action by Written Consent
The Charter provides that Ginkgo’s stockholders may act by written consent only if (a) the action to be taken or effected has been approved by the affirmative vote of all of the directors of Ginkgo then serving or (b) the holders of Class B common stock collectively beneficially own shares representing a majority of the voting power of all of the outstanding shares of capital stock of Ginkgo. As a result, if the holders of Class B common stock were to cease to collectively beneficially own shares representing a majority of the voting power of all of the outstanding shares of capital stock of Ginkgo, Ginkgo’s stockholders would not be able to take action by written consent on any matter and would only be able to take action at an annual or special meeting of stockholders, unless the Ginkgo Board had unanimously approved the action to be taken or effected.
Advance Notice Requirement for Stockholder Proposals and Director Nominations
The Bylaws establish an advance notice procedure for stockholder proposals to be brought before an annual meeting of stockholders, including proposed nominations of candidates for election to the Ginkgo Board. In order for any matter to be “properly brought” before a meeting (and thereby considered or acted upon at such meeting), a stockholder will have to comply with certain advance notice requirements and provide Ginkgo with certain information. Stockholders at an annual meeting will only be permitted to consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of the Ginkgo Board or by a stockholder of record on the record date for the meeting who is entitled to vote at the meeting and has delivered
149
Table of Contents
a timely notice, in the form and manner specified in the Bylaws, of such stockholder’s intention to bring such business before the meeting. These provisions might preclude Ginkgo’s stockholders from bringing matters before our annual meeting of stockholders or from nominating candidates for election to the Ginkgo Board, or might discourage or impede an attempt by a potential acquirer of Ginkgo to conduct a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise obtain control of Ginkgo.
Business Combinations
Ginkgo has elected not to be subject to Section 203 of the DGCL. Under Section 203 of the DGCL, a corporation will not be permitted to engage in a business combination with any interested stockholder for a period of three years following the time that such interested stockholder became an interested stockholder, unless:
(1) prior to such time, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder;
(2) upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (i) by persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
(3) at or subsequent to such time the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2⁄3% of the outstanding voting stock which is not owned by the interested stockholder.
Generally, a “business combination” includes a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. Subject to certain exceptions, an “interested stockholder” is a person who, together with that person’s affiliates and associates, owns, or within the previous three years owned, 15% or more of Ginkgo’s outstanding voting stock. For purposes of this section only, “voting stock” has the meaning given to it in Section 203 of the DGCL.
Because Ginkgo has opted out of Section 203 of the DGCL in the Charter, Section 203 of the DGCL will not apply to Ginkgo.
Warrants
Ginkgo Warrants
At the effective time of the Merger, each warrant to purchase shares of Old Ginkgo capital stock (each, an “Old Ginkgo Warrant”) that was outstanding and unexercised immediately prior to the effective time of the Merger (after giving effect to the Company Recapitalization, pursuant to which each Old Ginkgo Warrant to purchase shares of Old Ginkgo preferred stock became an Old Ginkgo Warrant to purchase shares of Old Class A common stock), other than such Old Ginkgo Warrants that were automatically exercised in full in accordance with their terms by virtue of the occurrence of the Merger immediately prior to the effective time of the Merger, were assumed by Ginkgo and converted into a warrant to purchase shares of Class A common stock on the same terms and subject to the same conditions (including as to vesting and exercisability) as were in effect with respect to such Old Ginkgo Warrant immediately prior to the effective time, with appropriate adjustments to the number of shares of Class A common stock underlying such warrant and the exercise price applicable thereto to account for the Merger.
150
Table of Contents
SRNG Warrants
At the effective time of the Domestication, each warrant to purchase SRNG ordinary shares (each, a “SRNG Warrant”) that was issued and outstanding immediately prior to the effective time of the Domestication and not terminated pursuant to its terms was converted into a warrant to purchase shares of Common Stock on the same terms and conditions (including as to vesting and exercisability) as were in effect with respect to such SRNG Warrant immediately prior to the effective time.
As of June 30, 2022, there are currently outstanding an aggregate of 51,824,895 warrants to acquire Class A common stock, which comprise 17,325,000 Private Placement Warrants held by the Sponsor and 34,499,895
Public Warrants.
Public Warrants
As of June 30, 2022, there were an aggregate of 34,499,895 Public Warrants outstanding, which entitle the holder to acquire Class A common stock. Each whole Public Warrant entitles the registered holder to purchase one share of Class A common stock at a price of $11.50 per share, subject to adjustment as discussed below, beginning 30 days after the Closing Date, provided that Ginkgo has an effective registration statement under the Securities Act covering the Class A common stock issuable upon exercise of the Public Warrants and a current prospectus relating to such Class A common stock is available (or Ginkgo permits holder to exercise their respective warrants on a cashless basis under the circumstances specified in the warrant agreement) and such shares are registered, qualified or exempt from registration under the securities, or blue sky, laws of the state of residence of the holder. Pursuant to the warrant agreement, a holder may exercise its Public Warrants only for a whole number of shares of Class A common stock. This means only a whole Public Warrant may be exercised at a given time by a warrant holder. No fractional warrants will be issued upon separation of the units and only whole warrants will trade. Accordingly, unless holder has at least five units, such holder will not be able to receive or trade a whole warrant. The Public Warrants will expire five years after the Closing Date, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
Redemption of Warrants for Cash
Once the Warrants become exercisable, Ginkgo may call the Warrants for redemption for cash:
| • | in whole and not in part; |
| • | at a price of $0.01 per Warrant; |
| • | upon not less than 30 days’ prior written notice of redemption (the “30-day redemption period”) to each warrant holder; and |
| • | if, and only if, the reported closing price of the Class A common stock equals or exceeds $18.00 per share (as adjusted for stock sub-divisions, stock capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending three business days before Ginkgo sends the notice of redemption to the warrant holders. |
Ginkgo may exercise its redemption right even if Ginkgo is unable to register or qualify the underlying securities for sale under all applicable state securities laws.
Ginkgo has established the last of the redemption criterion discussed above to prevent a redemption call unless there is at the time of the call a significant premium to the warrant exercise price. If the foregoing conditions are satisfied and Ginkgo issues a notice of redemption of the Warrants, each warrant holder will be entitled to exercise his, her or its Warrant prior to the scheduled redemption date. However, the price of the Class A common stock may fall below the $18.00 redemption trigger price (as adjusted for stock sub-divisions, stock capitalizations, reorganizations, recapitalizations and the like) as well as the $11.50 warrant exercise price after the redemption notice is issued.
151
Table of Contents
Redemption Procedures and Cashless Exercise
If Ginkgo calls the Warrants for redemption as described above, Ginkgo’s management will have the option to require any holder that wishes to exercise his, her or its Warrant to do so on a “cashless basis.” In determining whether to require all holders to exercise their Warrants on a “cashless basis,” Ginkgo’s management will consider, among other factors, Ginkgo’s cash position, the number of Warrants that are outstanding and the dilutive effect on Ginkgo’s stockholders of issuing the maximum number of shares of Class A common stock issuable upon the exercise of its Warrants. If Ginkgo Management takes advantage of this option, all holders of Warrants would pay the exercise price by surrendering their Warrants for that number of shares of Class A common stock equal to the quotient obtained by dividing (x) the product of the number of Class A common stock underlying the warrants, multiplied by the excess of the “fair market value” of the Class A common stock over the exercise price of the warrants by (y) the fair market value. The “fair market value” will mean the average reported closing price of the Class A common stock for the 10 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants. If the Ginkgo Management takes advantage of this option, the notice of redemption will contain the information necessary to calculate the number of shares of Class A common stock to be received upon exercise of the warrants, including the “fair market value” in such case. Requiring a cashless exercise in this manner will reduce the number of shares to be issued and thereby lessen the dilutive effect of a warrant redemption.
Ginkgo believes this feature is an attractive option to Ginkgo if Ginkgo does not need the cash from the exercise of the Warrants. If Ginkgo calls the Warrants for redemption and Ginkgo’s management does not take advantage of this option, the holders of the Private Placement Warrants and their permitted transferees would still be entitled to exercise their Private Placement Warrants or on a cashless basis using the same formula described above that other warrant holders would have been required to use had all warrant holders been required to exercise their Warrants on a cashless basis, as described in more detail below.
A holder of a Warrant may notify Ginkgo in writing in the event it elects to be subject to a requirement that such holder will not have the right to exercise such warrant, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates), to the warrant agent’s actual knowledge, would beneficially own in excess of 4.9% or 9.8% (as specified by the holder) of the Class A common stock outstanding immediately after giving effect to such exercise.
If the number of outstanding shares of Class A common stock is increased by a share capitalization payable in shares of Class A common stock, or by a split-up of common stock or other similar event, then, on the effective date of such share capitalization, split-up or similar event, the number of shares of Class A common stock issuable on exercise of each Warrant will be increased in proportion to such increase in the outstanding shares of Common Stock. A rights offering to holders of common stock entitling holders to purchase Class A common stock at a price less than the fair market value will be deemed a share capitalization of a number of shares of Class A common stock equal to the product of (i) the number of shares of Class A common stock actually sold in such rights offering (or issuable under any other equity securities sold in such rights offering that are convertible into or exercisable for Class A common stock) and (ii) the quotient of (x) the price per share of Class A common stock paid in such rights offering and (y) the fair market value. For these purposes (i) if the rights offering is for securities convertible into or exercisable for shares of Class A common stock, in determining the price payable for Class A common stock, there will be taken into account any consideration received for such rights, as well as any additional amount payable upon exercise or conversion and (ii) fair market value means the volume weighted average price of shares of Class A common stock as reported during the 10 trading day period ending on the trading day prior to the first date on which the Class A common stock trades on the applicable exchange or in the applicable market, regular way, without the right to receive such rights.
In addition, if Ginkgo, at any time while the Warrants are outstanding and unexpired, pays a dividend or make a distribution in cash, securities or other assets to the holders of Class A common stock on account of such Class A common stock (or other securities into which the warrants are convertible), other than (a) as described above or
152
Table of Contents
(b) certain ordinary cash dividends, then the warrant exercise price will be decreased, effective immediately after the effective date of such event, by the amount of cash and/or the fair market value of any securities or other assets paid on each share of Class A common stock in respect of such event.
If the number of outstanding shares of Class A common stock is decreased by a consolidation, combination or reclassification of Class A common stock or other similar event, then, on the effective date of such consolidation, combination, reverse share split, reclassification or similar event, the number of shares of Class A common stock issuable on exercise of each Warrant will be decreased in proportion to such decrease in outstanding share of Class A common stock.
Whenever the number of shares of Class A common stock purchasable upon the exercise of the warrants is adjusted, as described above, the warrant exercise price will be adjusted by multiplying the Warrant exercise price immediately prior to such adjustment by a fraction (x) the numerator of which will be the number of shares of Class A common stock purchasable upon the exercise of the Warrants immediately prior to such adjustment, and (y) the denominator of which will be the number of shares of Class A common stock so purchasable immediately thereafter.
In case of any reclassification or reorganization of the outstanding Class A common stock (other than those described above or that solely affects the par value of such Class A common stock), or in the case of any merger or consolidation of Ginkgo with or into another corporation (other than a consolidation or merger in which Ginkgo is the continuing corporation and that does not result in any reclassification or reorganization of the issued and outstanding Class A common stock), or in the case of any sale or conveyance to another corporation or entity of the assets or other property of Ginkgo as an entirety or substantially as an entirety in connection with which Ginkgo is dissolved, the holders of the Warrants will thereafter have the right to purchase and receive, upon the basis and upon the terms and conditions specified in the Warrants and in lieu of the Class A common stock immediately theretofore purchasable and receivable upon the exercise of the rights represented thereby, the kind and amount of shares of Class A common stock or other securities or property (including cash) receivable upon such reclassification, reorganization, merger or consolidation, or upon a dissolution following any such sale or transfer, that the holder of the Warrants would have received if such holder had exercised their Warrants immediately prior to such event. If less than 70% of the consideration receivable by the holders of Class A common stock in such a transaction is payable in the form of Class A common stock in the successor entity that is listed for trading on a national securities exchange or is quoted in an established over-the-counter market, or is to be so listed for trading or quoted immediately following such event, and if the registered holder of the Warrant properly exercises the Warrant within thirty days following public disclosure of such transaction, the Warrant exercise price will be reduced as specified in the warrant agreement based on the Black-Scholes Warrant Value (as defined in the warrant agreement) of the Warrant. The purpose of such exercise price reduction is to provide additional value to holders of the Warrants when an extraordinary transaction occurs during the exercise period of the Warrants pursuant to which the holders of the Warrants otherwise do not receive the full potential value of the Warrants.
The Warrants were issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as warrant agent, and Ginkgo. In connection with the Merger, Continental Stock Transfer & Trust Company assigned the warrant agreement to Computershare Trust Company, N.A. The warrant agreement provides that the terms of the Warrants may be amended without the consent of any holder for the purpose of (i) curing any ambiguity or correct any defective provision, or mistake, including to conform the provisions of the warrant agreement to the description of the terms of the Warrants and the warrant agreement set forth herein, (ii) adjusting the provisions relating to cash dividends on Class A common stock as contemplated by and in accordance with the warrant agreement or (iii) adding or changing any provisions with respect to matters or questions arising under the warrant agreement as the parties to the warrant agreement may deem necessary or desirable and that the parties deem to not adversely affect the rights of the registered holders of the Warrants, provided that the approval by the holders of at least 50% of the then outstanding Public Warrants is required to make any change that adversely affects the interests of the registered holders of Public Warrants, and, solely with
153
Table of Contents
respect to any amendment to the terms of the Private Placement Warrants, a majority of the then outstanding Private Placement Warrants. You should review a copy of the warrant agreement for a complete description of the terms and conditions applicable to the Warrants.
The Warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price (or on a cashless basis, if applicable), by certified or official bank check payable to Ginkgo, for the number of Warrants being exercised. The Warrant holders do not have the rights or privileges of holders of common stock and any voting rights until they exercise
their Warrants and receive Class A common stock. After the issuance of Ginkgo Class A common stock upon exercise of the Warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
Private Placement Warrants
The Private Placement Warrants (including the Class A common stock issuable upon exercise of the Private Placement Warrants) will not be transferable, assignable or salable until thirty (30) days after the Closing Date (except in limited circumstances) and they will not be redeemable by Ginkgo for cash so long as they are held by the Sponsor, members of the Sponsor or their permitted transferees.
The initial purchasers of the Private Placement Warrants, or their permitted transferees, have the option to exercise the Private Placement Warrants on a cashless basis. Except as described in this section, the Private Placement Warrants have terms and provisions that are identical to those of the Public Warrants sold in the IPO, including that they may be redeemed for shares of Class A common stock. If the Private Placement Warrants are held by holders other than the Sponsor or their permitted transferees, the Private Placement Warrants will be redeemable by Ginkgo and exercisable by the holders on the same basis as the Public Warrants included in the units that were sold in the IPO.
Exclusive Forum
The Bylaws provide that, unless Ginkgo otherwise consents in writing, the Court of Chancery (the “Chancery Court”) of the State of Delaware (or, in the event that the Chancery Court does not have subject matter jurisdiction, another state or federal court located within the State of Delaware) will, to the fullest extent permitted by law, be the sole and exclusive forum for resolution of (a) any derivative action or proceeding brought on behalf of Ginkgo, (b) any action asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, employee, agent or stockholder of Ginkgo to Ginkgo or any of Ginkgo’s stockholders, or any claim for aiding and abetting such an alleged breach, (c) any action governed by the “internal affairs doctrine” or arising pursuant to any provision of Ginkgo’s Charter or Bylaws, or to interpret, apply, enforce or determine the validity of Ginkgo’s Charter or Bylaws, or (d) any action asserting a claim against Ginkgo or any current or former director, officer, employee, agent or stockholder of Ginkgo (i) arising pursuant to any provision of the DGCL or (ii) as to which the DGCL confers jurisdiction on the Chancery Court. The foregoing will not apply, however, to any action, claim or proceeding as to which the Chancery Court (or, if applicable, another state or federal court located within the State of Delaware) determines that there is an indispensable party not subject to the jurisdiction of such court (and the indispensable party does not consent to the personal jurisdiction of the such court within ten (10) days following such determination).
Notwithstanding the foregoing, unless Ginkgo otherwise consents in writing, the federal district courts of the United States will be the exclusive forum for the resolution of any action, claim or proceeding arising under the Securities Act of 1933, as amended.
154
Table of Contents
Limitations on Liability and Indemnification of Officers and Directors
The DGCL authorizes corporations to limit or eliminate the personal liability of directors and stockholders of corporations for monetary damages for breaches of directors’ fiduciary duties, subject to certain exceptions. The Charter includes a provision that eliminates, to the fullest extent permitted by the DGCL (as currently in effect or as it may in the future be amended), the personal liability of Ginkgo’s directors for damages for any breach of fiduciary duty as a director.
The Bylaws provide that, to the fullest extent permitted by the DGCL (as currently in effect or as it may in the future be amended), Ginkgo must indemnify and hold harmless and advance expenses to any of its directors and officers who is involved in any action, suit or proceeding by reason of the fact that he or she is or was a director
or officer of Ginkgo or, while serving as a director or officer of Ginkgo, is or was serving at the request of Ginkgo as a director, officer, employee or agent of another corporation or of a partnership, joint venture, trust, enterprise or non-profit entity. Ginkgo also is expressly authorized to carry directors’ and officers’ liability insurance providing indemnification for Ginkgo’s directors, officers, and certain employees for some liabilities. Ginkgo believes that these indemnification and advancement provisions and insurance are useful to attract and retain qualified directors and executive officers.
The limitation of liability, advancement and indemnification provisions in the Charter and the Bylaws may discourage stockholders from bringing lawsuits against Ginkgo’s directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against Ginkgo’s directors and officers, even though such an action, if successful, might otherwise benefit Ginkgo and its stockholders. In addition, your investment in Ginkgo may be adversely affected to the extent that Ginkgo pays the costs of settlement and damage awards against directors and officer pursuant to these indemnification provisions.
There is currently no pending material litigation or proceeding involving any of Ginkgo’s directors, officers, or employees for which indemnification is sought.
Corporate Opportunities
The Charter provides for the renouncement by Ginkgo of any interest or expectancy of Ginkgo in, or being offered an opportunity to participate, in any matter, transaction, or interest that is presented to, or acquired, created, or developed by, or which otherwise comes into the possession of, any director of Ginkgo who is not an employee of Ginkgo or any of its subsidiaries, unless such matter, transaction, or interest is presented to, or acquired, created, or developed by, or otherwise comes into the possession of, that director first in that director’s capacity as a director of Ginkgo.
Dissenters’ Rights of Appraisal and Payment
Under the DGCL, with certain exceptions, Ginkgo’s stockholders will have appraisal rights in connection with a merger or consolidation of Ginkgo. Pursuant to the DGCL, stockholders who properly demand and perfect appraisal rights in connection with such merger or consolidation will have the right to receive payment of the fair value of their shares as determined by the Delaware Court of Chancery.
Stockholders’ Derivative Actions
Under the DGCL, any of Ginkgo’s stockholders may bring an action in Ginkgo’s name to procure a judgment in Ginkgo’s favor, also known as a derivative action, provided that the stockholder bringing the action is a holder of Ginkgo’s shares at the time of the transaction to which the action relates or such stockholder’s stock thereafter devolved by operation of law.
155
Table of Contents
Transfer Agent and Warrant Agent
Computershare Trust Company, N.A. is the transfer agent for Class A common stock and the warrant agent for the Warrants.
Listing of Class A common stock and Ginkgo Warrants
The Class A common stock and the Public Warrants are listed on the NYSE under the symbols “DNA” and
“DNA.WS,” respectively.
156
Table of Contents
SECURITIES ACT RESTRICTIONS ON RESALE OF SECURITIES
Rule 144
Pursuant to Rule 144 of the Securities Act (“Rule 144”), a person who has beneficially owned restricted Class A common stock or Warrants for at least six months would be entitled to sell their securities provided that (i) such person is not deemed to have been an affiliate of Ginkgo at the time of, or at any time during the three months preceding, a sale and (ii) Ginkgo is subject to the Exchange Act periodic reporting requirements for at least three months before the sale and has filed all required reports under Section 13 or 15(d) of the Exchange Act during the 12 months (or such shorter period as it was required to file reports) preceding the sale.
Persons who have beneficially owned restricted Class A common stock or Warrants for at least six months but who are affiliates of Ginkgo at the time of, or at any time during the three months preceding, a sale would be subject to additional restrictions, by which such person would be entitled to sell within any three- month period only a number of securities that does not exceed the greater of:
| • | 1% of the total number of shares of Class A common stock then outstanding; or |
| • | the average weekly reported trading volume of Ginkgo’s Class A common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Sales by affiliates of Ginkgo under Rule 144 are also limited by manner of sale provisions and notice requirements and by the availability of current public information about Ginkgo.
Restrictions on the Use of Rule 144 by Shell Companies or Former Shell Companies
Rule 144 is not available for the resale of securities initially issued by shell companies (other than business-combination related shell companies) or issuers that have been at any time previously a shell company. However, Rule 144 also includes an important exception to this prohibition if the following conditions are met:
| • | the issuer of the securities that was formerly a shell company has ceased to be a shell company; |
| • | the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act; |
| • | the issuer of the securities has filed all Exchange Act reports and material required to be filed, as applicable, during the preceding 12 months (or such shorter period that the issuer was required to file such reports and materials) other than Form 8-K reports; and |
| • | at least one year has elapsed from the time that the issuer filed current Form 10-type information with the SEC reflecting its status as an entity that is not a shell company. |
As a result, SRNG’s initial shareholders will be able to sell their founder shares and Private Placement Warrants, as applicable, pursuant to Rule 144 without registration one year after the Closing Date.
Ginkgo is no longer a shell company, and so, once the conditions listed above are satisfied, Rule 144 will become available for the resale of the above-noted restricted securities.
157
Table of Contents
BENEFICIAL OWNERSHIP OF SECURITIES
The following table sets forth information known to the Company regarding the beneficial ownership of Ginkgo common stock as of June 30, 2022 (unless otherwise specified) by:
| • | each person who is a named executive officer or director of Ginkgo; |
| • | all executive officers and directors of Ginkgo as a group; and |
| • | each person who is a beneficial owner of more than 5% of Ginkgo Class A common stock or Ginkgo Class B common stock. |
Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power over that security, including options and warrants that are currently exercisable or exercisable within 60 days. Ginkgo common stock issuable upon exercise of options and warrants currently exercisable within 60 days are deemed outstanding solely for purposes of calculating the percentage of total voting power of the beneficial owner thereof. Unless otherwise indicated, Ginkgo believes that all persons named in the table below have sole voting and investment power with respect to the voting securities beneficially owned by them.
The beneficial ownership of Ginkgo common stock is based on 1,095,736,657 shares of Ginkgo Class A common stock and 396,077,560 shares of Ginkgo Class B common stock issued and outstanding as of June 30, 2022.
| Class A common stock | Class B common stock | % of Total Voting Power** | ||||||||||||||||||
| Name of Beneficial Owner | Shares | % | Shares | % | % | |||||||||||||||
| Directors and Executive Officers of Ginkgo |
||||||||||||||||||||
| Jason Kelly(1) |
— | — | 82,431,106 | 20.8 | % | 16.3 | % | |||||||||||||
| Reshma Shetty(2) |
— | — | 165,841,730 | 41.9 | % | 32.8 | % | |||||||||||||
| Mark Dmytruk(3) |
— | — | 674,494 | * | * | |||||||||||||||
| Arie Belldegrun(4) |
589,662 | * | — | — | * | |||||||||||||||
| Marijn Dekkers(5) |
7,972,951 | * | — | — | * | |||||||||||||||
| Christian Henry(6) |
1,376,864 | * | — | — | * | |||||||||||||||
| Reshma Kewalramani(7) |
70,921 | — | — | — | — | |||||||||||||||
| Shyam Sankar(8) |
1,331,874 | * | — | — | * | |||||||||||||||
| Harry E. Sloan(9) |
70,921 | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| All Directors and Executive Officers of Ginkgo as a Group (9 individuals) |
11,413,193 | 1.0 | % | 248,947,330 | 62.9 | % | 49.5 | % | ||||||||||||
| 5% Beneficial Owners of Ginkgo |
||||||||||||||||||||
| Entities affiliated with Anchorage Capital Group(10) |
69,208,354 | 6.3 | % | — | — | 1.4 | % | |||||||||||||
| Bartholomew Canton(11) |
— | — | 165,841,730 | 41.9 | % | 32.8 | % | |||||||||||||
| Austin Che(12) |
— | — | 82,920,866 | 20.9 | % | 16.4 | % | |||||||||||||
| Entities affiliated with Baillie Gifford & Co.(13) |
167,752,680 | 15.3 | % | — | — | 3.3 | % | |||||||||||||
| Cascade Investment, L.L.C.(14) |
151,865,481 | 13.9 | % | — | — | 3.0 | % | |||||||||||||
| General Atlantic (GK), L.P.(15) |
111,566,297 | 10.2 | % | — | — | 2.2 | % | |||||||||||||
| Thomas Knight(16) |
66,110,869 | 6.0 | % | 8,972,183 | 2.3 | % | 3.1 | % | ||||||||||||
| Senator Global Opportunity Master Fund LP(17) |
76,595,199 | 7.0 | % | — | — | 1.5 | % | |||||||||||||
| Viking Global Investors LP(18) |
115,084,128 | 10.5 | % | — | — | 2.3 | % | |||||||||||||
| * | Less than one percent. |
| ** | Percentage of total voting power represents voting power with respect to all shares of Ginkgo Class A common stock and Ginkgo Class B common stock, as a single class. Each share of Ginkgo Class B common |
158
Table of Contents
| stock is entitled to 10 votes per share and each share of Ginkgo Class A common stock is entitled to one vote per share. For more information about the voting rights of Ginkgo common stock, see “Description of Capital Stock”. |
| (1) | Consists of (a) 70,703,049 shares of Ginkgo Class B common stock held by Dr. Kelly and (b) 11,728,057 shares of Ginkgo Class B common stock held by The Kelly 2016 Grantor Retained Annuity Trust, over which Dr. Kelly has sole voting and dispositive power. |
| (2) | Consists of (a) 3,093,797 shares of Ginkgo Class B common stock held by Dr. Shetty, (b) 70,389,783 shares of Ginkgo Class B common stock held by The Reshma Padmini Shetty Revocable Living Trust – 2014, over which Dr. Shetty has sole voting and dispositive power, (c) 8,245,491 shares of Ginkgo Class B common stock held by The Reshma Padmini Shetty 2021 Grantor Retained Annuity Trust, over which Dr. Shetty has sole voting and dispositive power, (d) 2,583,588 shares of Ginkgo Class B common stock held by a family trust, and (e) 81,529,071 shares of Ginkgo Class B common stock beneficially owned by Dr. Shetty’s spouse, as reported in footnote (5) below. The voting and dispositive power over the shares held by the family trust are held by three or more individuals acting by majority approval and therefore none of the individuals is deemed a beneficial owner of the shares held by such trust. |
| (3) | Consists of (a) 674,494 shares of Ginkgo Class B common stock held by Mr. Dmytruk. |
| (4) | Consists of (a) 89,662 shares of Ginkgo Class A common stock held directly by Dr. Belldegrun, and (c) 500,000 shares of Ginkgo Class A common stock held by Bellco Legacy LLC. Bellco Legacy LLC is owned and managed by trusts controlled by Dr. Belldegrun. |
| (5) | Consists of (a) 2,192,587shares of Ginkgo Class A common stock held by Dr. Dekkers and (b) 5,780,364 shares of Ginkgo Class A common stock held by Novalis LifeSciences Investments I, L.P. (“Novalis LifeSciences”). Dr. Dekkers, the Manager of the general partner of Novalis LifeSciences, has sole voting and dispositive power over the shares held by Novalis LifeSciences and, as a result, may be deemed to share beneficial ownership of the shares held by Novalis LifeSciences. The address for this stockholder is 1 Liberty Lane, Suite 100, Hampton, NH 03842. |
| (6) | Consists of (a) 1,376,864 shares of Ginkgo Class A common stock held by Mr. Henry. |
| (7) | Consists of (a) 70,921 shares of Ginkgo Class A common stock held by Dr. Kewalramani |
| (8) | Consists of (a) 1,331,874 shares of Ginkgo Class A common stock held by Mr. Sankar. |
| (9) | Consists of (a) 70,921 shares of Ginkgo Class A common stock held by Mr. Sloan |
| (10) | Consists of (a) 34,454,177 shares of Ginkgo Class A common stock held by Anchorage Illiquid Opportunities Master VI (A), L.P. and (b) 34,454,177 shares of Ginkgo Class A common stock held by Anchorage Illiquid Opportunities Offshore Master V, L.P. (c) 250,000 shares of Ginkgo Class A common stock held for the account of ACMO (d) 50,000 Shares the Reporting Persons have the right to acquire upon exercise of warrants held for the account of ACMO Anchorage Advisors Management, L.L.C. is the sole managing member of Anchorage Capital Group, L.L.C. (“Anchorage”), which in turn is the investment manager of AIOM VI and AIOM V. Mr. Kevin Ulrich is the Chief Executive Officer of Anchorage and the senior managing member of Anchorage Advisors Management, L.L.C. As such, each of the foregoing persons may be deemed to have voting and dispositive power over the shares held by AIOM VI and AIOM V. Each of the foregoing persons disclaims beneficial ownership of the shares held by AIOM VI and AIOM V, except of any pecuniary interests therein. The address for these stockholders is 610 Broadway, 6th Floor, New York, NY 10012. Data was obtained from 13G/A that was filed with the Securities and Exchange Commission on February 14, 2022. |
| (11) | Consists of (a) 3,093,797 shares of Ginkgo Class B common stock held by Dr. Canton, (b) 70,389,783 shares of Ginkgo Class B common stock held by The Bartholomew Canton Revocable Living Trust – 2014, over which Dr. Canton has sole voting and dispositive power, (c) 8,245,491 shares of Ginkgo Class B common stock held by The Bartholomew Canton 2021 Grantor Retained Annuity Trust, over which Dr. Canton has sole voting and dispositive power, (d) 2,583,588 shares of Ginkgo Class B common stock held by a family trust, and (e) 81,529,071 shares of Ginkgo Class B common stock held by Dr. Canton’s spouse as reported in footnote (2) above. The voting and dispositive power over the shares held by the family trust are held by three or more individuals acting by majority approval and therefore none of the individuals is deemed a beneficial owner of the shares held by such trust. |
159
Table of Contents
| (12) | Consists of (a) 3,093,797 shares of Ginkgo Class B common stock held by Dr. Che, (b) 78,927,069 shares of Ginkgo Class B common stock held by Austin Che Revocable Trust, over which Dr. Che has sole voting and dispositive power and (c) 900,000 shares of Ginkgo Class B common stock held by a family trust. |
| (13) | Consists of shares of Ginkgo Class A common stock. All shares of Ginkgo Class A common stock are held by Baillie Gifford & Co. and/or one or more of its investment adviser subsidiaries, which may include Baillie Gifford Overseas Limited, on behalf of investment advisory clients, which may include investment companies registered under the Investment Company Act, employee benefit plans, pension funds or other institutional clients. Securities representing more than 5% of the class are held on behalf of Scottish Mortgage Investment Trust PLC, a closed-ended investment trust which is managed by Baillie Gifford & Co Limited, a wholly owned subsidiary of Baillie Gifford & Co. The address for these stockholders is c/o Baillie Gifford & Co, Calton Square 1 Greenside Row. Edinburgh Scotland, UK EH1 3AN. Data was obtained from 13G/A that was filed with the Securities and Exchange Commission on April 6, 2022. |
| (14) | Consists of shares of Ginkgo Class A common stock. All shares of Ginkgo Class A common stock to be held by Cascade Investment, L.L.C. following the Business Combination Closing may be deemed to be beneficially owned by William H. Gates III as the sole member of Cascade, L.L.C. The address for this stockholder is 2365 Carillon Point, Kirkland, WA 98033. Data was obtained from 13G/A that was filed with the Securities and Exchange Commission on September 24, 2021. |
| (15) | Consists of shares of Ginkgo Class A common stock. The limited partners that share beneficial ownership of the shares held by General Atlantic (GK), L.P., General Atlantic Partners 100, L.P. (“GAP 100”), General Atlantic Partners (Bermuda) EU, L.P. (“GAP Bermuda EU”), GAP Coinvestments III, LLC (“GAPCO III”), GAP Coinvestments IV, LLC (“GAPCO IV”), GAP Coinvestments V, LLC (“GAPCO V”) and GAP Coinvestments CDA, L.P. (“GAPCO CDA”) are collectively referred to as the “GA Funds”. The address of GA LP, GAP 100, GAPCO III, GAPCO IV, GAPCO V, GAPCO CDA, GA GenPar, GA SPV and GA GK is c/o General Atlantic Service Company, L.P., 55 East 52nd Street, 33rd Floor, New York, NY 10055. The address of GAP Bermuda EU, GenPar Bermuda and GAP Bermuda is Clarendon House, 2 Church Street, Hamilton HM 11, Bermuda. The general partner of GA GK is General Atlantic (SPV) GP, LLC (“GA SPV”). The general partner of GAP 100 is ultimately controlled by General Atlantic, L.P. (“GA LP”), which is controlled by the Management Committee of GASC MGP, LLC (the “Management Committee”). The general partner of GAP Bermuda EU is ultimately controlled by GAP (Bermuda) L.P. (“GAP Bermuda”), which is also controlled by the Management Committee. GA LP is the managing member of GAPCO III, GAPCO IV and GAPCO V, the general partner of GAPCO CDA and is the sole member of GA SPV. There are nine members of the Management Committee. GA GK, GA LP, GASC MGP, LLC, GAP Bermuda, GA SPV and the GA Funds. The GA Funds share beneficial ownership of the shares of common stock held by GA GK. The general partner of GA GK is GA SPV. The general partner of GAP 100 is GA GenPar. The general partner of GAP Bermuda EU is GenPar Bermuda. GA LP, which is controlled by the Management Committee of GASC MGP, LLC (the “GA Management Committee”), is the managing member of GAPCO III, GAPCO IV and GAPCO V, the general partner of GAPCO CDA and GA GenPar, and the sole member of GA SPV. The general partner of GenPar Bermuda is GAP Bermuda, which is also controlled by the GA Management Committee. There are nine members of the GA Management Committee. By virtue of the foregoing, the Reporting Persons may be deemed to share voting power and the power to direct the disposition of the shares that each owns of record. Each of the members of the GA Management Committee disclaims ownership of the shares of common stock reported herein except to the extent he or she has a pecuniary interest therein. Data was obtained from 13G/A that was filed with the Securities and Exchange Commission on February 11, 2022. |
| (16) | Consists of (a) 9,182,067 shares of Ginkgo Class A common stock held of record by the Reporting Person; (b) 8,972,183 shares of Ginkgo Class B common stock, $0.0001 per share, of the Issuer (the “Class B common stock”) held of record by the Reporting Person; (c) 6,995,255 shares of Ginkgo Class A common stock held of record by the Knight Family Trust dated August 20, 2019; (d) 47,423,785 shares of Ginkgo Class A common stock held of record by the Thomas F. Knight, Jr., Trustee of The Thomas F. Knight Jr. Grantor Retained Annuity Trust or his/her successor in trust; and (e) 2,509,762 shares of Ginkgo Class A common stock held of record by the Thomas F. Knight Jr. Grantor Retained Annuity Trust (2) dated December 16, 2020. The Reporting Person serves as co-trustee for the Thomas F. Knight, Jr., Trustee of The |
160
Table of Contents
| Thomas F. Knight Jr. Grantor Retained Annuity Trust or his/her successor in trust and the Thomas F. Knight Jr. Grantor Retained Annuity Trust (2) dated December 16, 2020, and the Reporting Person’s spouse serves as co-trustee for the Knight Family Trust dated August 20, 2019. As such, the Reporting Person may be deemed to share beneficial ownership over the shares held of record by each of the trusts. The shares of Ginkgo Class B common stock may be redeemed by the holder at any time for shares of Ginkgo Class A common stock on a one-to-one basis. |
| (17) | Consists of shares of Ginkgo Class A common stock. The address for this stockholder is 510 Madison Avenue, 28th Floor, New York, NY 10022. Senator Investment Group LP (“Senator”), is investment manager of the stockholder, Senator Global Opportunity Master Fund LP, and may be deemed to have voting and dispositive power with respect to the shares. The general partner of Senator is Senator Management LLC (the “Senator GP”). Douglas Silverman controls Senator GP, and, accordingly, may be deemed to have voting and dispositive power with respect to the shares held by this stockholder. Mr. Silverman disclaims beneficial ownership of the shares held by the stockholder. Data was obtained from 13G/A that was filed with the Securities and Exchange Commission on February 10, 2022. |
| (18) | Consists of (a) 115,084,128 shares of Ginkgo Class A common stock held by Viking Global Investors LP (“VGI”), Viking Global Opportunities Parent GP LLC (“Opportunities Parent”), Viking Global Opportunities GP LLC (“Opportunities GP”), Viking Global Opportunities Portfolio GP LLC (“Opportunities Portfolio GP”), Viking Global Opportunities Illiquid Investments Sub-Master LP (“VGOP”), O. Andreas Halvorsen, David C. Ott and Rose S. Shabet (collectively, the “Reporting Persons”). (b) 27,084,128 shares of Ginkgo Class A Common Stock directly and beneficially owned by VGOP that remain subject to forfeiture to the extent certain price targets are not satisfied and (c) 88,000,000 shares of Ginkgo Class A Common Stock that will be issued to VGOP in exchange for an equivalent quantity of the Issuer’s Class C common stock. The address for these stockholders is 55 Railroad Avenue, Greenwich, Connecticut 06830. Data was obtained from 13G that was filed with the Securities and Exchange Commission on July 1, 2022. |
Securities Authorized for Issuance Under Equity Compensation Plans
The following table sets forth information as of December 31, 2021 regarding shares of common stock that may be issued under our equity compensation plans.
| Number of securities to be issued upon exercise of outstanding options and vesting of outstanding restricted stock units (#) |
Weighted- average exercise price of outstanding options ($) |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in the first column) (#) |
||||||||||
| Equity compensation plans approved by securityholders(1) |
193,550,805 | (2) | $ | 0.05 | 200,569,979 | (3)(4) | ||||||
| Equity compensation plans not approved by securityholders |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
193,550,805 | $ | 0.05 | 200,569,979 | ||||||||
|
|
|
|
|
|
|
|||||||
| (1) | Includes the Ginkgo Bioworks Holdings, Inc. 2021 Equity Incentive Plan (the “2021 Plan”). |
| (2) | Includes 25,228,853 shares of common stock issuable upon the exercise of outstanding stock options and 168,321,952 shares of common stock issuable upon settlement of outstanding restricted stock units. |
| (3) | As of December 31, 2021, there were 200,569,979 shares available for grant under the 2021 Plan. |
| (4) | The 2021 Plan provides that the number of shares of common stock reserved and available for issuance under the 2021 Plan shall be cumulatively increased on January 1 of each year. The number of shares of common stock increased each year will be equal to the lesser of: (i) 4% of the number of shares of common stock issued and outstanding on the immediately preceding December 31 or (ii) such lesser amount as determined by our board of directors. |
161
Table of Contents
DESCRIPTION OF PRIVATE PLACEMENTS OF COMMON STOCK
The Company was originally incorporated as a Cayman Islands exempted company in October 2020 as a special purpose acquisition company, formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, recapitalization, reorganization or similar business combination with one or more businesses. We were originally known as Soaring Eagle Acquisition Corp. SRNG completed its IPO in February
2021.
SRNG (and after the Domestication as described below, “New SRNG”), previously entered into the Merger Agreement, by and among SRNG, Merger Sub, and Old Ginkgo.
On September 15, 2021, SRNG filed a notice of deregistration with the Cayman Islands Registrar of Companies, together with the necessary accompanying documents, and filed a certificate of incorporation and a certificate of corporate domestication with the Secretary of State of Delaware, pursuant to which SRNG was domesticated and continued as a Delaware corporation, under the name of “Soaring Eagle Acquisition Corp.” (the “Domestication”).
As a result of, and upon the effective time of the Domestication, among other things, (1) each of the then issued and outstanding Class B ordinary shares, par value $0.0001 per share, of SRNG automatically converted, on a one-for- one basis, into a Class A ordinary share, par value $0.0001 per share, of SRNG (a “SRNG Class A ordinary share”); (2) immediately following the conversion described in clause (1), each of the then issued and outstanding SRNG Class A ordinary shares automatically converted, on a one-for-one basis, into a share of Class A common stock, par value $0.0001 per share, of New SRNG (the “New SRNG Class A common stock”); (3) each of the then issued and outstanding redeemable warrant of SRNG (the “SRNG warrants”) automatically converted into a redeemable warrant to acquire one share of New SRNG Class A common stock (the “New SRNG warrants”); and (4) each of the then issued and outstanding units of SRNG that had not been previously separated into the underlying SRNG Class A ordinary shares and underlying SRNG warrants upon the request of the holder thereof (the “SRNG units”), were cancelled and entitled the holder thereof to one share of New SRNG Class A common stock and on-fifth of one New SRNG warrant. No fractional shares will be issued upon exercised of the New SRNG warrants.
On September 16, 2021, as contemplated by the Merger Agreement, New SRNG consummated the merger transaction contemplated by the Merger Agreement, whereby Merger Sub merged with and into Old Ginkgo, with the separate corporate existence of Merger Sub ceasing and Old Ginkgo being the surviving corporation and a wholly owned subsidiary of New SRNG (the “Merger” and, together with the Domestication, the “Business Combination”). In connection with the consummation of the Business Combination, New SRNG changed its name to “Ginkgo Bioworks Holdings, Inc.”. The shares of New SRNG Class A common stock and New SRNG warrants described above became shares of Class A common stock or Ginkgo warrants, as applicable, upon consummation of the Merger.
162
Table of Contents
SELLING SECURITYHOLDERS
The Selling Securityholders may from time to time offer and sell any or all of the shares of Class A common stock set forth below pursuant to this prospectus and any accompanying prospectus supplement, including the shares of Class A common stock that may be issued in exchange for shares of Class C common stock pursuant to the Exchange Agreement between Ginkgo and Viking Global Opportunities Illiquid Investments Sub-Master LP. When we refer to the “Selling Securityholders” in this prospectus, we mean the persons listed in the table below, and the pledgees, donees, transferees, assignees, successors, designees and others who later come to hold any of the Selling Securityholders’ interest in Class A common stock other than through a public sale.
The following table and the accompanying footnote are based primarily on information provided to us by the Selling Securityholders indicating the shares of Class A common stock they wished to be covered by this registration statement and that are eligible for sale under this prospectus. A Selling Securityholder may have sold or transferred some or all of the shares of Class A common stock indicated below with respect to such Selling Securityholder and may in the future sell or transfer some or all of the Class A common stock indicated below, in each case in transactions exempt from the registration requirements of the Securities Act, rather than under this prospectus. We cannot advise you as to whether the Selling Securityholder will in fact sell any or all of such shares of Class A common stock. For purposes of this table, we have assumed that the Selling Securityholder will have sold all of the shares of Class A common stock covered by this prospectus upon the completion of the offering. We have based percentage ownership on 1,099,693,341 shares of Class A common stock outstanding as of August 8, 2022.
We have determined beneficial ownership in accordance with the rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Unless otherwise indicated below, to our knowledge, the persons and entities named in the tables have sole voting and sole investment power with respect to all securities that they beneficially own, subject to community property laws where applicable.
| Before the Offering | Shares of Ginkgo Class A Common Stock being Offered |
After the Offering | ||||||||||||||||||||||||||
| Shares of Class A Shares of Class C | Shares of Class A | |||||||||||||||||||||||||||
| Common Stock beneficially owned |
Common Stock beneficially owned |
Common Stock beneficially owned |
||||||||||||||||||||||||||
| Shares | % | Shares | % | Shares | % | |||||||||||||||||||||||
| Viking Global Opportunities Illiquid Investments Sub-Master LP(1) |
51,055,144 | 4.7 | % | 288,000,000 | 100 | % | 288,000,000 | 51,055,144 | 4.6 | % | ||||||||||||||||||
| Other Selling Securityholders |
4,051,107 | <1 | % | — | — | 4,051,107 | — | — | ||||||||||||||||||||
| (1) | Includes 51,055,144 shares of Class A common stock and 288,000,000 shares of Class C common stock that may be exchanged pursuant to the Exchange Agreement into Gingko Class A common stock upon at least 61 days’ notice. The resale of the 51,055,144 shares of Class A common stock beneficially owned by Viking Global Opportunities Illiquid Investments Sub-Master LP (the “Opportunities Fund”) is not covered by this prospectus. The Opportunities Fund has the authority to dispose of and vote the Class A common stock that is or will be directly owned by it, which power may be exercised by its general partner, Viking Global Opportunities Portfolio GP LLC (“Opportunities GP”), and by Viking Global Investors LP (“VGI”), which provides managerial services to Opportunities Fund. O. Andreas Halvorsen, David C. Ott and Rose Shabet, as Executive Committee members of Viking Global Partners LLC (the general partner of VGI) and Viking Global Opportunities GP LLC, the sole member of Opportunities GP, have shared authority to direct the voting and disposition of investments beneficially owned by VGI, Opportunities GP and the Opportunities Fund. The address for each of the entities is c/o Viking Global Investors LP, is 55 Railroad Avenue, Greenwich, CT 06830. |
Selling Securityholder information for each additional Selling Securityholder, if any, will be set forth by prospectus supplement to the extent required prior to the time of any offer or sale of such Selling
163
Table of Contents
Securityholder’s shares of Class A common stock pursuant to this prospectus. To the extent permitted by law, a prospectus supplement may add, update, substitute, or change the information contained in this prospectus, including the identity of each Selling Securityholder and the number of shares of Class A common stock registered on its behalf. A Selling Securityholder may sell or otherwise transfer all, some or none of such shares of Class A common stock in this offering. See “Plan of Distribution.”
For information regarding transactions between us and the Selling Securityholders, see the section entitled “Certain Relationships and Related Party Transactions.”
164
Table of Contents
GINKGO MANAGEMENT
Board of Directors and Management
The following is a list of Ginkgo’s directors and executive officers and their ages as of August 8, 2022.
| Name |
Age | Position | ||||
| Jason Kelly |
41 | Chief Executive Officer and Founder; Director | ||||
| Reshma Shetty |
42 | President, Chief Operating Officer and Founder; Director | ||||
| Mark Dmytruk |
50 | Chief Financial Officer | ||||
| Arie Belldegrun |
72 | Director | ||||
| Marijn Dekkers |
64 | Director | ||||
| Kathy Hopinkah Hannan |
61 | Director | ||||
| Christian Henry |
54 | Director | ||||
| Reshma Kewalramani |
49 | Director | ||||
| Shyam Sankar |
40 | Director | ||||
| Harry E. Sloan |
72 | Director | ||||
Jason Kelly, one of our Founders, is the Chief Executive Officer and a member of Ginkgo’s board of directors. Dr. Kelly has served as a director of CM Life Sciences II Inc. (Nasdaq: CMII), a SPAC with a focus on the life sciences sector, since its initial public offering in February 2021. Dr. Kelly has a Ph.D. in Biological Engineering and a B.S. in Chemical Engineering and Biology from the Massachusetts Institute of Technology. We believe that Dr. Kelly is qualified to serve on our board of directors as a Founder and due to his knowledge of our company and our business.
Reshma Shetty, one of our Founders, is the President and Chief Operating Officer and a member of Ginkgo’s board of directors. Dr. Shetty currently serves on the Bio Advisory Group at the non-profit Nuclear Threat Initiative. Dr. Shetty has a Ph.D. in Biological Engineering from the Massachusetts Institute of Technology and a B.S. in Computer Science from the University of Utah. We believe that Dr. Shetty is qualified to serve on our board of directors as a Founder and due to her knowledge of our company and our business.
Mark Dmytruk has served as our Chief Financial Officer since joining Ginkgo in 2020. From 2017 to 2020, Mr. Dmytruk served as Executive Vice President, Corporate Strategy and Development, for Syneos Health, a global CRO and contract commercial organization serving the biopharmaceutical industry. Syneos Health was formed through the merger of inVentiv Health and INC Research in 2017, and prior to the merger Mr. Dmytruk served at inVentiv Health as Chief of Staff from 2014 to 2017 and President, Patient Outcomes Division, from 2011 to 2014. Prior to inVentiv Health, Mr. Dmytruk served in a variety of roles at Thermo Fisher Scientific (and its predecessor, Fisher Scientific) from 2001 to 2011. As Vice President of Corporate Development, Mr. Dmytruk led the company’s M&A function, contributing to its industry-changing strategy and transformational growth. He also served as Vice President of Finance for the Athena Diagnostics business unit of Thermo Fisher Scientific prior to its sale to Quest Diagnostics. Mr. Dmytruk began his career at Ernst & Young in Canada. Mr. Dmytruk has an M.B.A. from the Sloan School of Management at the Massachusetts Institute of Technology and a Bachelor of Commerce from the University of Alberta.
Arie Belldegrun, M.D., is a member of Ginkgo’s board of directors. Dr. Belldegrun is a leader in the field of cell and gene therapy. Dr. Belldegrun is a co-founder of Allogene Therapeutics, a public biopharmaceutical company, and has served as Executive Chairman of its board of directors since November 2017. From March 2014 until October 2017, Dr. Belldegrun served as the President and Chief Executive Officer of Kite Pharma, Inc. and as a member of its board of directors from June 2009 until October 2017. Dr. Belldegrun currently serves as Chairman of Bellco Capital LLC, a position he has held since 2004, Chairman of Kronos Bio, a position he has held since November 2017, Chairman of UroGen Pharma, Ltd., a position he has held since December 2012, as Chairman and Partner of Two River Group, a position he has held since June 2009, as co-Chairman of
165
Table of Contents
Breakthrough Properties LLC and Breakthrough Services, L.L.C., a position he has held since April 2019, as a director of ByHeart, Inc., a position he has held since October 2019, and as a director of IconOVir Bio, Inc., a position he has held since June 2020. Dr. Belldegrun has also served as Senior Managing Director of Vida Ventures, LLC since November 2017. He is certified by the American Board of Urology and the American Association of Genitourinary Surgeons. Dr. Belldegrun is Research Professor, holds the Roy and Carol Doumani Chair in Urologic Oncology, and Director of the Institute of Urologic Oncology at the David Geffen School of Medicine at the University of California, Los Angeles. Prior to joining UCLA in October of 1988, he was a research fellow at NCI/NIH in surgical oncology and immunotherapy from July 1985 to August 1988 under Dr. Steven Rosenberg. Dr. Belldegrun received his M.D. from the Hebrew University Hadassah Medical School in Jerusalem before completing his post graduate studies in Immunology at the Weizmann Institute of Science and his residency in Urologic Surgery at Harvard Medical School. We believe that Dr. Belldegrun is qualified to serve on our board of directors due to his extensive knowledge as a leader in the field of cell and gene therapy.
Marijn Dekkers is chairman of Ginkgo’s board of directors. Dr. Dekkers is the Founder and Chairman of Novalis LifeSciences LLC, an investment and advisory firm for the Life Science industry that he founded in 2017. Before that, from 2010 to 2016, Dr. Dekkers served as Chief Executive Officer of Bayer AG. Prior to his time at Bayer, from 2002 to 2009, he served as Chief Executive Officer of Thermo Fisher Scientific. Dr. Dekkers currently serves as a director on the boards of Quantum-SI, Inc. and Cerevel Therapeutics Holdings, Inc., as well as Georgetown University and the Foundation for the National Institutes of Health. He is a former director of Unilever, General Electric and Biogen. Dr. Dekkers began his career in 1985 as a research scientist at General Electric’s Corporate R&D Center. Dr. Dekkers received his PhD and M.S. in chemical engineering from the University of Eindhoven and his B.S in chemistry from the Radboud University. We believe that Dr. Dekkers is qualified to serve on our board of directors due to his extensive knowledge of the life sciences industry, his familiarity with our company and his prior director service.
Kathy Hopinkah Hannan is a member of the Ginkgo Board. Dr. Hannan is a former Global Lead Partner, National Managing Partner and Vice Chair of KPMG, LLP, the U.S. member firm of the global audit, tax and advisory services firm KPMG International. Dr. Hannan has over 30 years of industry experience and held numerous leadership roles during her distinguished career with KPMG. From 2015 until her 2018 retirement, Dr. Hannan served as Global Lead Partner, Senior Advisor for KPMG’s Board Leadership Center and National Leader Total Impact Strategy. Dr. Hannan also served as the Midwest Area Managing Partner for KPMG’s Tax Services from 2004 to 2009. Subsequent to that role, from 2009 to 2015, Dr. Hannan served as the National Managing Partner of Diversity and Corporate Responsibility. While at KPMG, Dr. Hannan also founded the KPMG Women’s Advisory Board. In addition to her roles at KPMG, as a Native American Indian and member of the Ho-Chunk Nation Tribe, Dr. Hannan served on President George W. Bush’s National Advisory Council on Indian Education. She also served as a member of the Committee to establish the Board of Directors for the Ho-Chunk Tribe’s corporation under Section 17 of the Indian Reorganization Act. Currently, Dr. Hannan serves on the boards of directors of Annaly Capital Management, Inc. (NYSE: NLY), Otis Worldwide Corporation (NYSE: OTIS), and Carpenter Technology Corporation (NYSE: CRS), is Chair of the Board of Trustees and a member of the Executive Committee of the Smithsonian National Museum of the American Indian, is a Trustee of the Conference Board and is an active member of Women Corporate Directors. From 2014 to 2020, Dr. Hannan served as Chair of the Board & National President for Girl Scouts of the USA. Dr. Hannan received a Ph.D. in Leadership Studies from Benedictine University and a B.A. from Loras College. She is also a graduate of the Chicago Management Institute at the University of Chicago, Booth School of Business and the Institute of Comparative Political & Economic Systems at Georgetown University. In addition, Dr. Hannan has completed the Carnegie Mellon/NACD Cyber-Risk Oversight Program, the CERT Certificate in Cybersecurity Oversight, and the NACD Directorship Certification. We believe that Dr. Hannan is qualified to serve on the Ginkgo Board due to her business acumen and prior director experience.
Christian Henry is a member of Ginkgo’s board of directors. Mr. Henry has served as President and Chief Executive Officer of Pacific Biosciences of California, Inc., a leading sequencing company, since September 2020. From 2005 to January 2017, Mr. Henry was a member of the executive team of Illumina, Inc., a global
166
Table of Contents
leader in sequencing. During this tenure at Illumina, he served in a number of roles, including Executive Vice President & Chief Commercial Officer, Senior Vice President of Genomic Solutions, Senior Vice President and General Manager of Life Sciences and Senior Vice President and Chief Financial Officer. Prior to joining Illumina in 2005, Mr. Henry served as the Chief Financial Officer of Tickets.com, Inc. from 2003 to 2005. From 1999 to 2003, Mr. Henry served as Vice President, Finance and Corporate Controller of Affymetrix, Inc. (acquired by Thermo Fisher Scientific in 2016). In 1997, Mr. Henry joined Nektar Therapeutics (formerly Inhale Therapeutic Systems, Inc.) as Corporate Controller, and later as its Chief Accounting Officer from 1997 to 1999. In 1996, Mr. Henry served as General Accounting Manager of Sugen, Inc. Mr. Henry began his career in 1992 at Ernst & Young LLP, where he was a Senior Accountant through 1996. Mr. Henry currently serves as a director and Chairman of the board of WAVE Life Sciences Ltd., and as a director of CM Life Sciences Holdings III LLC. Mr. Henry previously served as Chairman of the board of Pacific Biosciences from August 2018 to September 2020. Mr. Henry holds a B.A. in biochemistry and cell biology from the University of California, San Diego and an M.B.A., with a concentration in finance, from the University of California, Irvine. We believe that Mr. Henry is qualified to serve on our board of directors due to his over 20 years of experience in growing companies in the life sciences industry.
Reshma Kewalramani is a member of Ginkgo’s board of directors. Dr. Kewalramani has been the Chief Executive Officer and President of Vertex Pharmaceuticals Inc. since April 2020 and a member of Vertex’s Board of Directors since February 2020. Dr. Kewalramani was Vertex’s Executive Vice President and Chief Medical Officer from April 2018 through April 2020. She was Vertex’s Senior Vice President, Late Development from February 2017 until March 2018. From August 2004 to January 2017, she served in roles of increasing responsibility at Amgen Inc., most recently as Vice President, Global Clinical Development, Nephrology & Metabolic Therapeutic Area and as Vice President, U.S. Medical Organization. From 2014 through 2019, Dr. Kewalramani was the industry representative to the FDA’s Endocrine and Metabolic Drug Advisory Committee. She completed her internship and residency in Internal Medicine at the Massachusetts General Hospital and her fellowship in Nephrology at the Massachusetts General Hospital and Brigham and Women’s Hospital combined program. Dr. Kewalramani holds a B.A. from Boston University and an M.D. from Boston University School of Medicine. Dr. Kewalramani also completed the General Management Program at Harvard Business School and is an alumnus of the school. We believe that Dr. Kewalramani is qualified to serve on our board of directors due to her extensive experience serving in senior roles at various pharmaceutical companies.
Shyam Sankar is a member of Ginkgo’s board of directors. Mr. Sankar is the Chief Operating Officer and Executive Vice President at Palantir Technologies Inc., where he has worked in various positions since 2006. Prior to his time at Palantir, Mr. Sankar served as the Vice President of Network Management and Director of Business Development for Xoom Corporation. Mr. Sankar has a deep operational background overseeing the development of complex technology from near inception to massive scale. Mr. Sankar received his M.S. in management science and engineering from Stanford University and his B.S. in electrical and computer engineering from Cornell University. We believe that Mr. Sankar is qualified to serve on our board of directors due to his business acumen, leadership experience, and operational background, having overseen the development and expansion of a software company from its near inception through its public listing.
Harry E. Sloan is a founder, public company CEO and a leading investor in the media, entertainment and technology industries. Mr. Sloan is the Chairman and CEO of Eagle Equity Partners II, LLC. Under Mr. Sloan’s leadership, the company has acquired and taken public, through SPACs, several digital media companies including, during 2020, DraftKings and mobile gaming company Skillz. Mr. Sloan has been at the forefront and evolution of the video gaming industry as one of the founding investors and a Board Member of Zenimax/ Bethesda Game Studios, the award-winning studio acquired by Microsoft in March 2021. Mr. Sloan co-founded Soaring Eagle Acquisition Corp. (Nasdaq: SRNGU), which raised $1.725 billion in its initial public offering in February 2021 and three months later announced a merger with Boston-based Ginkgo Bioworks Inc. in a deal valued at $17.5 billion. In January 2022, Mr. Sloan and his partners launched Screaming Eagle Acquisition Corp. With a closing of its initial public offering of 75,000,000 units, at a price of $10 per unit, Screaming Eagle is the
167
Table of Contents
largest initial public offering of a public acquisition vehicle since March 2021. Mr. Sloan has served as a director of Skillz, Inc. since December 2020 and DraftKings Inc. since April 2020. He was a director of Soaring Eagle Acquisition Corp. from October 2020 until September 2021, Flying Eagle Acquisition Corp. from March 2020 until December 2020, Diamond Eagle Acquisition Corp. from May 2019 until April 2020, and Videocon d2h Limited from May 2016 until April 2018. We believe that Mr. Sloan is qualified to serve on our board of directors due to his public company experience, business leadership, and operational experience.
Corporate Governance
Ginkgo structures its corporate governance in a manner that it believes closely aligns its interests with those of its stockholders. Notable features of this corporate governance include:
| • | Ginkgo has independent director representation on its audit, compensation and nominating and corporate governance committees, and its independent directors will meet regularly in executive sessions without the presence of its corporate officers or non-independent directors; |
| • | at least one of its directors qualifies as an “audit committee financial expert” as defined by the SEC; and |
| • | it implements a range of other corporate governance practices, including notifications around proposed membership on outside boards to prevent conflicts of interest and implementing a director orientation and continuing education program. |
Role of Board in Risk Oversight
The Ginkgo Board has extensive involvement in the oversight of risk management related to Ginkgo and its business and will accomplish this oversight through the regular reporting to the board of directors by the audit committee. The audit committee will represent the Ginkgo Board by periodically reviewing Ginkgo’s accounting, reporting and financial practices, including the integrity of its financial statements, the surveillance of administrative and financial controls and its compliance with legal and regulatory requirements. Through its regular meetings with management, including the finance, legal, internal audit and information technology functions, the audit committee will review and discuss all significant areas of Ginkgo’s business and summarize for the Ginkgo Board all areas of risk and the appropriate mitigating factors. In addition, the Ginkgo Board will receive periodic detailed operating performance reviews from management.
Composition of the Ginkgo Board
Ginkgo’s business and affairs are managed under the direction of its board of directors. Under the Charter, the Ginkgo Board is declassified and all of Ginkgo’s directors are elected each year for one-year terms.
Board Committees
The standing committees of the Ginkgo Board consist of an audit committee, a compensation committee and a nominating and corporate governance committee. The Ginkgo Board may from time to time establish other committees. Ginkgo’s chief executive officer, its president and chief operating officer, and other executive officers will regularly report to the non-executive directors and the audit, the compensation and the nominating and corporate governance committees to ensure effective and efficient oversight of our activities and to assist in proper risk management and the ongoing evaluation of management controls.
Audit Committee
Ginkgo has an audit committee, consisting of Christian Henry, who serves as the chairperson, Harry Sloan, and Shyam Sankar. Each of Messrs. Henry, Sankar and Sloan qualify as an independent director under the NYSE corporate governance standards and the independence requirements of Rule 10A-3 under the Securities Exchange
168
Table of Contents
Act of 1934, as amended (the “Exchange Act”). The Ginkgo Board has determined that Mr. Henry qualifies as an “audit committee financial expert” as such term is defined in Item 407(d)(5) of Regulation S-K and possesses financial sophistication, as defined under the rules of NYSE.
The purpose of the audit committee is to prepare the audit committee report required by the SEC to be included in Ginkgo’s proxy statement and to assist the board of directors in overseeing and monitoring (1) the quality and integrity of the financial statements, (2) compliance with legal and regulatory requirements, (3) Ginkgo’s independent registered public accounting firm’s qualifications and independence, (4) the performance of Ginkgo’s internal audit function and (5) the performance of Ginkgo’s independent registered public accounting firm.
The Ginkgo Board has adopted a written charter for the audit committee, which is available on Ginkgo’s website.
Compensation Committee
Ginkgo has a compensation committee, consisting of Shyam Sankar, who serves as the chairperson, Arie
Belldegrun and Christian Henry.
The purpose of the compensation committee is to assist the board of directors in discharging its responsibilities relating to (1) setting Ginkgo’s compensation program and compensation of its executive officers and directors, (2) monitoring Ginkgo’s incentive and equity-based compensation plans and (3) preparing the compensation committee report required to be included in Ginkgo’s proxy statement under the rules and regulations of the SEC.
The Ginkgo Board has adopted a written charter for the compensation committee, which is available on Ginkgo’s website.
Nominating and Corporate Governance Committee
Ginkgo has a nominating and corporate governance committee, consisting of Reshma Kewalramani and Shyam Sankar. Dr. Kewalramani serves as the chairperson. The purpose of the nominating and corporate governance committee is to assist the board of directors in discharging its responsibilities relating to (1) identifying individuals qualified to become new board of directors members, consistent with criteria approved by the board of directors, (2) reviewing the qualifications of incumbent directors to determine whether to recommend them for reelection and selecting, or recommending that the board of directors select, the director nominees for the next annual meeting of shareholders, (3) identifying board of directors members qualified to fill vacancies on any board of directors committee and recommending that the board of directors appoint the identified member or members to the applicable committee, (4) reviewing and recommending to the board of directors corporate governance principles applicable to Ginkgo, (5) overseeing the evaluation of the board of directors and management and (6) handling such other matters that are specifically delegated to the committee by the board of directors from time to time.
The Ginkgo Board has adopted a written charter for the nominating and corporate governance committee, which is available on Ginkgo’s website.
Code of Business Conduct
Ginkgo has adopted a code of business conduct and ethics that applies to all of its directors, officers and employees, including its principal executive officer, principal financial officer and principal accounting officer, which is available on Ginkgo’s website. Ginkgo’s code of business conduct and ethics is a “code of ethics”, as defined in Item 406(b) of Regulation S-K. Please note that Ginkgo’s website address is provided as an inactive textual reference only. Ginkgo will make any legally required disclosures regarding amendments to, or waivers of, provisions of its code of ethics on its website.
169
Table of Contents
EXECUTIVE AND DIRECTOR COMPENSATION
Compensation Committee Interlocks and Insider Participation
No member of the compensation committee was at any time during fiscal year 2021, or at any other time, one of our officers or employees. None of our executive officers has served as a director or member of a compensation committee (or other committee serving an equivalent function) of any entity, one of whose executive officers served as a director of our board of directors or member of our compensation committee.
Independence of the Board of Directors
NYSE rules generally require that independent directors must comprise a majority of a listed company’s board of directors. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, we have determined that each of Arie Belldegrun, Kathy Hopinkah Hannan, Christian Henry, Reshma Kewalramani, Shyam Sankar and Harry Sloan, representing a majority of Ginkgo’s directors, are “independent” as that term is defined under the applicable rules and regulations of the SEC and the listing requirements and rules of the NYSE.
Ginkgo’s Executive and Director Compensation
This section discusses the material components of the executive compensation program for Ginkgo’s executive officers who are named in the “2021 Summary Compensation Table” below. As an EGC, Ginkgo complies with the executive compensation disclosure rules applicable to “smaller reporting companies,” as such term is defined in the rules promulgated under the Securities Act, which require compensation disclosure for Ginkgo’s principal executive officer and Ginkgo’s two most highly compensated executive officers other than its principal executive officer. These three officers are referred to as Ginkgo’s named executive officers.
In 2021, Ginkgo’s “named executive officers” and their positions were as follows:
| • | Jason Kelly, Chief Executive Officer; |
| • | Reshma Shetty, President and Chief Operating Officer; and |
| • | Mark Dmytruk, Chief Financial Officer. |
This discussion may contain forward-looking statements that are based on Ginkgo’s current plans, considerations, expectations and determinations regarding future compensation programs. The actual compensation programs that Ginkgo adopts may differ materially from the currently planned programs summarized in this discussion.
2021 Summary Compensation Table
The following table sets forth information concerning the compensation of Ginkgo’s named executive officers for the years ended December 31, 2021 and December 31, 2020.
| Name and Principal Position |
Year | Salary ($)(1) |
Bonus ($) | Stock Awards ($)(2) |
All Other Compensation ($)(3) |
Total ($) | ||||||||||||||||||
| Jason Kelly |
2021 | $ | 250,000 | $ | — | $ | 380,479,776 | (4) | $ | 12,500 | $ | 380,742,276 | ||||||||||||
| Chief Executive Officer |
2020 | $ | 250,000 | $ | 414,841 | $ | 9,854,097 | $ | 14,250 | $ | 10,533,188 | |||||||||||||
| Reshma Shetty |
2021 | $ | 250,000 | $ | — | $ | 380,479,776 | (4) | $ | 12,500 | $ | 380,742,276 | ||||||||||||
| President and Chief Operating Officer |
2020 | $ | 250,000 | $ | 415,386 | $ | 9,854,097 | $ | 14,250 | $ | 10,533,733 | |||||||||||||
| Mark Dmytruk |
2021 | $ | 425,000 | $ | — | $ | 39,629,178 | (4) | $ | 14,500 | $ | 40,068,678 | ||||||||||||
| Chief Financial Officer |
2020 | $ | 63,750 | $ | — | $ | — | $ | 2,861 | $ | 66,611 | |||||||||||||
170
Table of Contents
| (1) | Mr. Dmytruk participated in a salary exchange program during 2021 and elected to be paid $50,000 of his 2021 base salary in the form of restricted stock units and was granted 65,375 restricted stock units in April 2021 with a grant date fair value of $888,446. The modification date fair value is computed in accordance with ASC Topic 718 and reflects the fair value on November 17, 2022 (i.e. the modification date), rather than the historical grant date fair value, the current fair value, or the amounts paid to or realized by Mr. Dmytruk.See “Narrative to Summary Compensation Table—2021 Salaries” below for a description of this salary exchange program. |
| (2) | Amounts reflect the modification date fair value of restricted stock units and associated earnouts granted during 2021 and 2020. For Mr. Dmytruk, this includes $838,446, which represents the modification date fair value of the restricted stock units that were granted as part of the salary exchange program in excess of the $50,000 portion of his base salary that he elected to exchange. |
| (3) | Amounts represent matching contributions under Ginkgo’s 401(k) plan. |
| (4) | Amounts for Dr. Kelly ($158,652,934), Dr. Shetty ($158,652,934) and Mr. Dmytruk ($23,125,369) reflect the incremental fair value over the historical grant date fair value attributable to the modification of restricted stock units and associated earnouts on November 17, 2021, rather than the historical grant date fair value, the current fair value, or the amounts paid to or realized by the named individual. For Dr. Kelly and Dr. Shetty, the amounts differ from the amounts disclosed in the Company’s Form 10-K filed on March 29, 2022 ($142,097,631 for each) as a result of an administrative error, which had no impact on the Company’s financial statements. We provide information regarding the assumptions used to calculate the value of all restricted stock units made to named executive officers in Note 18 to the consolidated financial statements and Note 9 to the unaudited condensed financial statements included in this prospectus. |
Narrative To Summary Compensation Table
2021 Salaries
The named executive officers receive a base salary to provide a fixed component of compensation reflecting the executive’s skill set, experience, role and responsibilities. No changes were made to the annual base salaries of the named executive officers during 2021.
The 2021 annual base salaries for Ginkgo’s named executive officers were:
| Name |
2021 Annual Base Salary ($) |
|||
| Jason Kelly |
$ | 250,000 | ||
| Reshma Shetty |
$ | 250,000 | ||
| Mark Dmytruk |
$ | 425,000 | ||
During 2021, our employees were given the opportunity to participate in a salary exchange program under which they could exchange a portion of their annual base salaries for restricted stock units. The salary to restricted stock units exchange took place on a one-to-four basis such that for each $1 of salary contributed to the program, the employee would receive a number of restricted stock units valued at $4. Mr. Dmytruk was our only named executive officer to participate in this program during 2021.
Equity Compensation
During 2021, Mr. Dmytruk was granted restricted stock units under our 2014 Incentive Plan (the “2014 Plan”). These restricted stock units vest upon the satisfaction of both an “event condition” and a “service condition” on or before the seventh anniversary of the applicable grant date. At the time of grant, the event condition would be satisfied on the first to occur of (i) a change in control or (ii) the earlier of (x) the date six months after the closing of a public offering or (y) March 15 of the calendar year following the effective date of a public offering. See the “Outstanding Equity Awards at Fiscal Year-End” table below for details regarding the service-based vesting schedule applicable to these awards.
171
Table of Contents
In connection with the Business Combination in September 2021, Dr. Kelly and Dr. Shetty were each granted 21,458,317 restricted stock units and agreed to extend the vesting on their restricted stock units granted in 2020. These restricted stock units will vest upon the satisfaction of both an “event condition” (as described above) and a “service condition” on or before the seventh anniversary of the grant date. The service condition will be satisfied on October 1, 2022, subject to continued service with Ginkgo through such date. The 2021 grant of restricted stock units was deemed to satisfy all rights that Dr. Kelly and Dr. Shetty may have had under their Founder Equity Grant Agreements with Ginkgo that were entered into in January 2020.
In connection with the Business Combination, the restricted stock units held by the named executive officers were converted into restricted stock units in Ginkgo and, with respect to the earn-out portion of the consideration paid in the Business Combination, restricted stock. This restricted stock is subject to the service-based vesting schedule of the related restricted stock units and, in addition, only vest based on the attainment of certain stock price thresholds. See the “Outstanding Equity Awards at Fiscal Year-End” table below for additional information. In November 2021, the Ginkgo Board authorized the amendment of all outstanding restricted stock units and shares of restricted stock, including those held by the named executive officers, such that the event condition was deemed satisfied no later than March 15, 2022.
In connection with the Business Combination, we adopted the 2021 Incentive Award Plan (the “2021 Plan”) in order to facilitate the grant of cash and equity incentives to directors, employees (including our named executive officers) and consultants of Ginkgo. We ceased granting awards under the 2014 Plan when the 2021 Plan became effective.
Other Elements of Compensation
Retirement Plan
Ginkgo maintains a 401(k) retirement savings plan for its employees, including Ginkgo’s named executive officers, who satisfy certain eligibility requirements. Ginkgo’s named executive officers are eligible to participate in the 401(k) plan on the same terms as other full-time employees. Under this plan, Ginkgo provides a non-elective safe harbor contribution to all eligible participants equal to up to 5% of eligible compensation, which fully vests once such eligible participant has completed two years of continuous service. Ginkgo believes that providing a vehicle for tax-deferred retirement savings though Ginkgo’s 401(k) plan adds to the overall desirability of Ginkgo’s executive compensation package and further incentivizes Ginkgo’s employees, including Ginkgo’s named executive officers, in accordance with Ginkgo’s compensation policies.
Employee Benefits and Perquisites
Health/Welfare Plans. During their employment, Ginkgo’s named executive officers are eligible to participate in Ginkgo’s employee benefit plans and programs, including medical, dental, vision, life, and disability benefits, to the same extent as Ginkgo’s other full-time employees, subject to the terms and eligibility requirements of those plans.
172
Table of Contents
Outstanding Equity Awards at Fiscal Year-End
The following table summarizes the number of shares of Common Stock underlying outstanding equity incentive plan awards for each named executive officer as of December 31, 2021.
| Stock Awards | ||||||||||||||||||||
| Name |
Grant Date | Number of Shares or Units of Stock That Have Not Vested (#) |
Market Value of Shares or Units of Stock That Have Not Vested ($)(7) |
Equity Incentive Plan Awards: Unearned Shares, Units or Other Rights That Have Not Vested (#) |
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(7) |
|||||||||||||||
| Jason Kelly |
1/1/2020 | 4,324,037 | (1) | $ | 35,932,747 | 389,151 | (6) | $ | 3,233,845 | |||||||||||
| 1/1/2020 | 129,719 | (2) | $ | 1,077,965 | — | $ | — | |||||||||||||
| 8/18/2021 | 21,458,317 | (1) | $ | 178,318,614 | 1,931,193 | (6) | $ | 16,048,214 | ||||||||||||
| 8/18/2021 | 643,734 | (2) | $ | 5,349,430 | — | $ | — | |||||||||||||
| Reshma Shetty |
1/1/2020 | 4,324,037 | (1) | $ | 35,932,747 | 389,151 | (6) | $ | 3,233,845 | |||||||||||
| 1/1/2020 | 129,719 | (2) | $ | 1,077,965 | — | $ | — | |||||||||||||
| 8/18/2021 | 21,458,317 | (1) | $ | 178,318,614 | 1,931,193 | (6) | $ | 16,048,214 | ||||||||||||
| 8/18/2021 | 643,734 | (2) | $ | 5,349,430 | — | $ | — | |||||||||||||
| Mark Dmytruk |
3/2/2021 | 1,564,440 | (3) | $ | 13,000,496 | 198,768 | (6) | $ | 1,651,762 | |||||||||||
| 3/2/2021 | 48,315 | (2) | $ | 401,498 | — | $ | — | |||||||||||||
| 4/4/2021 | 47,670 | (4) | $ | 396,138 | 5,883 | (6) | $ | 48,888 | ||||||||||||
| 4/4/2021 | 1,472 | (2) | $ | 12,232 | — | $ | — | |||||||||||||
| 8/2/2021 | 300,619 | (5) | $ | 2,498,144 | 30,918 | (6) | $ | 256,929 | ||||||||||||
| 8/2/2021 | 9,236 | (2) | $ | 76,751 | — | $ | — | |||||||||||||
| (1) | The restricted stock units granted to Dr. Kelly and Dr. Shetty will fully vest on October 1, 2022, subject to the named executive officer’s continued service to Ginkgo through such date. |
| (2) | Represents restricted stock for which the event condition had been satisfied as of December 31, 2021. The restricted stock granted to Dr. Kelly and Dr. Shetty will fully vest on October 1, 2022, subject to the named executive officer’s continued service to Ginkgo through such date. The restricted stock granted to Mr. Dmytruk will vest pursuant to the vesting schedule applicable to the restricted stock units that have the same grant date as such restricted stock, provided that any restricted stock scheduled to vest on or after January 1, 2022 and prior to October 1, 2022 will not vest until October 1, 2022, subject to Mr. Dmytruk’s continued service to Ginkgo through each applicable vesting date and accelerated vesting of some shares in the event his employment is terminated without cause or due to his death. |
| (3) | The restricted stock units granted to Mr. Dmytruk began vesting November 9, 2021 and will continue to vest in substantially equal monthly installments until November 1, 2024, subject to his continued service to Ginkgo through such dates. In addition, 644,180 restricted stock units were conditionally vested as of December 31, 2021, subject to the satisfaction of the “event condition”, which was subsequently met on March 15, 2022. |
| (4) | The restricted stock units granted to Mr. Dmytruk began vesting November 30, 2021 and will continue to vest in substantially equal monthly installments until December 1, 2024, subject to his continued service to Ginkgo through such dates. In addition, 17,705 restricted stock units were conditionally vested as of December 31, 2021, subject to the satisfaction of the “event condition”, which was subsequently met on March 15, 2022. |
| (5) | The restricted stock units granted to Mr. Dmytruk began vesting December 1, 2021 and will continue to vest in substantially equal monthly installments until July 1, 2025, subject to his continued service to Ginkgo through such dates. In addition, 42,944 restricted stock units were conditionally vested as of December 31, 2021, subject to the satisfaction of the “event condition”, which was subsequently met on March 15, 2022. |
| (6) | Represents restricted stock which vest in three substantially equal installments if the trading price per share of Class A common stock at any point during the trading hours of a trading day is greater than or equal to |
173
Table of Contents
| the following thresholds for any 20 trading days within any period of 30 consecutive trading days during the five year period after the closing of the Business Combination: $15.00, $17.50 and $20.00. Once the condition has been satisfied, the shares will be subject to the same service-based vesting applicable to the named executive officer’s restricted stock units, as described in the footnotes to this table. |
| (7) | Amount shown is based on the closing price of our Class A common stock of $8.31 on December 31, 2021. |
Executive Compensation Arrangements
Jason Kelly and Reshma Shetty. Neither Dr. Kelly nor Dr. Shetty have entered into employment agreements, offer letters or severance agreements with Ginkgo.
Mark Dmytruk. We have entered into an offer letter with Mr. Dmytruk pursuant to which he will be entitled to receive the following payments and benefits in the event his employment is terminated by Ginkgo without “cause” (as defined in the offer letter), (i) 12 months’ severance pay based on his base salary rate on the date of such termination, to be paid in installments over the 12-month period following the termination date and (ii) up to 12 months’ company-paid health benefits continuation pursuant to COBRA, in each case subject to Mr. Dmytruk’s execution of a general release of claims in favor of Ginkgo.
Mr. Dmytruk also entered into a separate agreement pursuant to which he is subject to employee and customer non-solicitation covenants during the term of his employment or other service with Ginkgo and for one year thereafter. The agreement also includes standard invention assignment and confidential information covenants.
Director Compensation
In connection with the Business Combination, the Ginkgo Board implemented an annual compensation program for its non-employee directors which became effective on September 16, 2021, pursuant to which the non-employee directors are entitled to cash and equity compensation in such amounts necessary to attract and retain non-employee directors that have the talent and skills to foster long-term value creation and enhance the sustainable development of the company. The compensation payable under the program is intended to be competitive in relation to both the market in which the company operates and the nature, complexity and size of Ginkgo’s business.
Ginkgo’s non-employee directors receive the following amounts for their services on the Ginkgo Board under the non-employee director compensation program:
Cash Compensation
| • | An annual director fee of $50,000; |
| • | If the director serves as lead independent director or chair or on a committee of the Ginkgo Board, an additional annual fee as follows: |
| • | Chair of the Ginkgo Board, $36,000 |
| • | Lead independent director, $25,000; |
| • | Chair of the audit committee, $20,000; |
| • | Audit committee member other than the chair, $10,000; |
| • | Chair of the compensation committee, $15,000; |
| • | Compensation committee member other than the chair, $7,500; |
| • | Chair of the nominating and corporate governance committee, $10,000; |
| • | Nominating and Corporate Governance committee member other than the chair, $5,000. |
174
Table of Contents
Director fees are payable in arrears in four equal quarterly installments, provided that the amount of each payment will be prorated for any portion of a calendar quarter that a non-employee director is not serving on the Ginkgo Board. The Ginkgo Board may permit non-employee directors to elect to receive equity compensation in lieu of cash compensation.
Equity Compensation
| • | Generally each non-employee director who is initially elected or appointed to the Ginkgo Board (other than those non-employee directors who were appointed by SRNG to serve on the Ginkgo Board or those non-employee directors who served on the board of SRNG or Ginkgo Bioworks, Inc. prior to the closing of the Business Combination) will receive (i) an initial option to purchase shares of Class A common stock with a grant date fair value of $400,000 (as determined under the program) (the “Initial Option”), (ii) an additional initial option to purchase shares of Class A common stock with a grant date fair value of $200,000 (as determined under the program) (the “Additional Initial Option”), and (iii) a number of restricted stock units determined by dividing $200,000 by the fair market value of a share of Class A common stock (the “Additional Initial RSU”). In the event that a non-employee director’s date of initial election or appointment does not occur on the same date as an annual meeting of stockholders, the value of the Additional Initial Option and the Additional Initial RSU will be prorated in accordance with the terms of the program. |
| • | If the non-employee director has served on the Ginkgo Board as of the date of an annual meeting of stockholders and will continue to serve as a non-employee director immediately following such meeting, such non-employee director will receive (i) an option to purchase shares of Class A common stock with a grant date fair value of $200,000 (as determined under the program) (the “Subsequent Option”) and (ii) a number of restricted stock units determined by dividing $200,000 by the fair market value of a share of Ginkgo Class A common stock (the “Subsequent RSU”). |
Stock options granted under the program have an exercise price equal to the fair market value of Class A common stock on the date of grant and expire not later than ten years after the date of grant. Each Initial Option granted to a non-employee director will vest and become exercisable in substantially equal installments on each of the first three anniversaries of the date of grant. Each Additional Initial Option and the Additional Initial RSUs granted to a non-employee director will vest and become exercisable, as applicable, in a single installment on the day before the next annual meeting of stockholders occurring after the date of the director’s initial election or appointment to the Ginkgo Board. Each Subsequent Option and the Subsequent RSUs will vest and become exercisable, as applicable, in a single installment on the earlier of the first anniversary of the date of grant or the day before the next annual meeting of stockholders occurring after the date of grant. Vesting of the options and restricted stock units granted under the program is subject to the non-employee director’s continued service through each applicable vesting date. In the event of a change in control of Ginkgo, the options and restricted stock units granted under the program will vest in full.
In connection with the Business Combination, Dr. Kewalramani was granted an Initial Option (51,782 shares), an Additional Initial Option (17,649 shares) and an Additional Initial RSU (9,818 restricted stock units) under the program.
175
Table of Contents
The following table sets forth information concerning the compensation of Ginkgo’s non-employee directors for their service on the board of directors for the year ended December 31, 2021.
| Name |
Fees Earned or Paid in Cash ($)(1) |
Stock Awards ($)(2) |
Option Awards ($)(2) |
Total ($) | ||||||||||||
| Arie Belldegrun |
16,563 | — | — | 16,563 | ||||||||||||
| Marijn Dekkers |
30,533 | 30,430,090 | — | 30,460,623 | ||||||||||||
| Christian Henry |
22,323 | 20,000,194 | — | 20,022,517 | ||||||||||||
| Reshma Kewalramani |
15,842 | 132,052 | 532,053 | 679,947 | ||||||||||||
| Evan Lodes |
— | — | — | — | ||||||||||||
| Shyam Sankar |
20,163 | 20,625,832 | — | 20,645,995 | ||||||||||||
| Harry Sloan |
17,283 | — | — | 17,283 | ||||||||||||
| (1) | Amounts shown include annual fees earned for service on the board of directors and committees of the board, pro-rated for the portion of the year following the Business Combination. No compensation was earned by any non-employee director during 2021 prior to the Business Combination. |
| (2) | Amounts reflect the full grant-date fair value of restricted stock units and options granted during 2021 computed in accordance with ASC Topic 718, rather than the amounts paid to or realized by the named individual. Amounts shown for Dr. Dekkers ($30,430,090), Mr. Henry ($20,000,194) and Mr. Sankar ($20,625,832) reflect the incremental fair value attributable to the modification of equity awards during 2021. We provide information regarding the assumptions used to calculate the value of all restricted stock units and options made to our directors in Note 18 to the consolidated financial statements included in this prospectus. |
The table below shows the aggregate numbers of options (exercisable and unexercisable), unvested restricted stock and restricted stock units held as of December 31, 2021 by each non-employee director who was serving as of December 31, 2021.
| Name |
Options Outstanding at Fiscal Year End (#) |
Restricted Stock Outstanding at Fiscal Year End (#) |
Restricted Stock Units Outstanding at Fiscal Year End (#) |
|||||||||
| Arie Belldegrun |
— | — | — | |||||||||
| Marijn Dekkers |
— | 281,217 | 3,124,756 | |||||||||
| Christian Henry |
— | 175,911 | 1,954,629 | |||||||||
| Reshma Kewalramani |
69,431 | — | 9,818 | |||||||||
| Shyam Sankar |
— | 175,911 | 1,954,629 | |||||||||
| Harry Sloan |
— | — | — | |||||||||
176
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
SRNG
On October 28, 2020, SRNG’s Sponsor purchased an aggregate of 43,125,000 founder shares in exchange for a capital contribution of $25,000, or approximately $0.0006 per share. SRNG’s Sponsor purchased an aggregate of 19,250,000 Private Placement Warrants in connection with SRNG’s IPO, at a price of $1.50 per warrant, or $28,875,000 in the aggregate. See the descripton of Ginkgo’s securities in the section titled “Description of Ginkgo’s Capital Stock” in this prospectus.
Prior to the Closing, SRNG sub-leased its executive offices at 955 Fifth Avenue, New York, NY, 10075 from Global Eagle Acquisition LLC, an affiliate of SRNG’s Sponsor. Also prior to the Closing, SRNG reimbursed Global Eagle Acquisition LLC for office space, secretarial and administrative services provided to members of its management team in an amount that did not exceed $15,000 per month.
Prior to the Closing, SRNG’s officers and directors were entitled to reimbursement for any out-of-pocket expenses incurred in connection with activities on SRNG’s behalf such as identifying potential target businesses and performing due diligence on suitable business combinations. SRNG’s audit committee reviewed on a quarterly basis all payments that were made to SRNG’s Sponsor, SRNG’s officers, directors or its or their affiliates.
On May 10, 2021, an affiliate of SRNG’s Sponsor entered into a subscription agreement with SRNG, pursuant to which it agreed to purchase an aggregate of 7,500,000 shares of New SRNG Class A common stock at $10.00 per share, for an aggregate purchase price of $75,000,000, from the Company in a private placement which closed immediately prior to Closing, but after the Domestication.
Ginkgo
The share numbers included in this “Certain Relationships and Related Party Transactions—Ginkgo” section reflect the historical pre-Business Combination shares issued in connection with the relevant transaction, and have not been revised to reflect the accounting of the Business Combination as a reverse recapitalization.
Series E Preferred Stock Financing
From September 2019 through July 2021, Old Ginkgo sold an aggregate of 1,942,610 shares of its Series E preferred stock to the related persons listed below at a purchase price of $150.19 per share, except as described below with respect to the conversion of convertible promissory notes. The following table summarizes purchases of Series E preferred stock from Old Ginkgo by such related persons:
| Name |
Shares of Series E Preferred Stock |
Total Purchase Price (Rounded) |
||||||
| Entities affiliated with Anchorage Capital Group(1) |
105,500 | $ | 15,053,014 | |||||
| Entities affiliated with Baillie Gifford & Co.(2) |
200,479 | $ | 29,104,581 | |||||
| Cascade Investment, L.L.C.(3) |
268,376 | $ | 38,719,465 | |||||
| General Atlantic (GK), L.P.(4) |
513,449 | $ | 76,109,274 | |||||
| Novalis Life Sciences Investments I, LP(5) |
52,755 | $ | 7,527,123 | |||||
| Senator Global Opportunity Master Fund LP(6) |
70,489 | $ | 10,057,534 | |||||
| Viking Global Opportunities Illiquid Investments Sub-Master LP(7) |
731,562 | $ | 106,379,679 | |||||
177
Table of Contents
| (1) | Entities affiliated with Anchorage hold more than 5% of Ginkgo’s outstanding capital stock. |
| (2) | Entities affiliated with Baillie Gifford & Co. hold more than 5% of Ginkgo’s outstanding capital stock. |
| (3) | Cascade Investment, L.L.C. holds more than 5% of Ginkgo’s outstanding capital stock. |
| (4) | GA GK holds more than 5% of Ginkgo’s outstanding capital stock. |
| (5) | Marijn Dekkers, a member of Ginkgo’s board of directors, is an affiliate of Novalis LifeSciences. |
| (6) | Senator Global Opportunity Master Fund LP holds more than 5% of Ginkgo’s outstanding capital stock. Evan Lodes, a former Old Ginkgo director, is an affiliate of Senator Global Opportunity Master Fund LP. |
| (7) | Viking Global Opportunities Illiquid Investments Sub-Master LP holds more than 5% of Ginkgo’s outstanding capital stock. |
Convertible Note Financing
In certain cases, the payment of the Total Purchase Price above consisted or, or included, the conversion of convertible promissory notes held by the related persons. From June 2019 through July 2019, Old Ginkgo sold an aggregate of $160,500,000 in principal amount of convertible promissory notes to the related persons listed below. Interest on the principal amount of the convertible promissory notes accrued at the rate of 3.0% per year. The outstanding principal and accrued interest of such convertible promissory notes converted into shares of Series E preferred stock at a discounted purchase price of $142.68 per share and are reflected in the above table. The following table summarizes the convertible promissory notes issued by Old Ginkgo to such related persons:
| Name |
Principal Amount |
|||
| Entities affiliated with Anchorage Capital Group(1) |
$ | 15,000,000 | ||
| Entities affiliated with Baillie Gifford & Co.(2) |
$ | 19,000,000 | ||
| Cascade Investment, L.L.C.(3) |
$ | 30,000,000 | ||
| General Atlantic (GK), L.P.(4) |
$ | 19,000,000 | ||
| Novalis Life Sciences Investments I, LP(5) |
$ | 7,500,000 | ||
| Senator Global Opportunity Master Fund LP(6) |
$ | 10,000,000 | ||
| Viking Global Opportunities Illiquid Investments Sub-Master LP(7) |
$ | 60,000,000 | ||
| (1) | Entities affiliated with Anchorage hold more than 5% of Ginkgo’s outstanding capital stock. |
| (2) | Entities affiliated with Baillie Gifford & Co. hold more than 5% of Ginkgo’s outstanding capital stock. |
| (3) | Cascade Investment, L.L.C. holds more than 5% of Ginkgo’s outstanding capital stock. |
| (4) | GA GK holds more than 5% of Ginkgo’s outstanding capital stock. |
| (5) | Marijn Dekkers, a member of Ginkgo’s board of directors, is an affiliate of Novalis LifeSciences. |
| (6) | Senator Global Opportunity Master Fund LP holds more than 5% of Ginkgo’s outstanding capital stock. Evan Lodes, a former Old Ginkgo director, is an affiliate of Senator Global Opportunity Master Fund LP. |
| (7) | Viking Global Opportunities Illiquid Investments Sub-Master LP holds more than 5% of Ginkgo’s outstanding capital stock. |
Series D Preferred Stock Financing
In June 2018, Old Ginkgo sold 52,400 shares of its Series D preferred stock at a purchase price of $47.71 per share for an aggregate purchase price of $2,500,004 to Novalis LifeSciences. Marijn Dekkers, a member of Ginkgo’s board of directors, is an affiliate of Novalis LifeSciences.
Founder Equity Grant Agreements
In January 2020, Old Ginkgo entered into Founder Equity Grant Agreements with each of Jason Kelly, Reshma Shetty, Austin Che and Bartholomew Canton. Dr. Kelly and Dr. Shetty are each a director, officer and holder of more than 5% of Ginkgo’s outstanding capital stock. Dr. Canton and Dr. Che are each a holder of more than 5%
178
Table of Contents
of Ginkgo’s outstanding capital stock. Dr. Canton is also a family member of Dr. Shetty. Also in January 2020, each of Dr. Kelly, Dr. Shetty, Dr. Canton and Dr. Che received a restricted stock unit award under the 2014 Plan. The terms of the Founder Equity Grants Agreements and the foregoing restricted stock unit awards are described in the section titled “Executive and Director Compensation” in this prospectus.
Founder Equity Repurchases
In July 2018, Old Ginkgo repurchased 90,017 shares of common stock from each of Dr. Kelly, Reshma Shetty, Austin Che, Dr. Canton and Dr. Knight at a price of $47.71 per share, which was the then most- recent price per share at which Old Ginkgo had sold convertible preferred stock to investors, for a total purchase price for each of $4,294,711. In September 2021, Old Ginkgo repurchased 11,032 shares of common stock from each of Dr. Kelly, Reshma Shetty, Austin Che, Dr. Canton and Dr. Knight at a price of $453.20 per share, for a total purchase price for each of $4,999,702. Dr. Kelly and Dr. Shetty are each a director, officer and holder of more than 5% of Ginkgo’s outstanding capital stock. Dr. Che, Dr. Canton and Dr. Knight are each a holder of more than 5% of Ginkgo’s outstanding capital stock. Dr. Canton is also a family member of Dr. Shetty.
Agreements in Connection with Platform Ventures
In September 2019, Old Ginkgo entered into an agreement with Cascade Investment L.L.C., an affiliated entity of GA GK, and Viking Global Opportunities Illiquid Investments Sub-Master LP, each of which hold more than 5% of Ginkgo’s outstanding capital stock, pursuant to which such related persons were provided with the first right to invest up to an aggregate of $350.0 million for the financial investment portion of new companies launched by Old Ginkgo as part of its Platform Ventures (such as Allonnia). The agreement was terminated in May 2021. Initial investments in new companies launched by Old Ginkgo in connection with the agreement were approximately $12.9 million from Cascade Investment L.L.C., $19.5 million from entities affiliated with GA GK., and $57.8 million from entities affiliated with Viking Global Opportunities Illiquid Investments Sub-Master LP.
In September 2021, Old Ginkgo formed 2 new Platform Ventures (Ayana Bio, LLC and Verb Biotics, LLC) and contributed intellectual property rights to these entities. Simultaneously with the arrangements between Old Ginkgo and each of Ayana Bio, LLC and Verb Biotics, LLC, Cascade Investment L.L.C. and entities affiliated with Viking Global Opportunities Illiquid Investments Sub-Master LP, each of which hold more than 5% of Ginkgo’s outstanding capital stock, invested approximately $7.5 million and $22.5 million, respectively, in each company.
Exchange Agreement
In October 2021 and March 2022, Ginkgo entered into a first and second amended and restated stockholders agreement, respectively, with Viking Global Opportunities Illiquid Investments Sub-Master LP, a holder of more than 5% of Ginkgo’s outstanding capital stock at the time of each agreement, pursuant to which Ginkgo agreed, subject to approval of the Ginkgo Board, to permit such stockholder to exchange a portion of its shares of Ginkgo Class A common stock for shares of Ginkgo Class C common stock on a 1-for-1 basis. The Ginkgo Board approved the exchange and Viking Global Opportunities Illiquid Investments Sub-Master LP effected the exchange in March 2022.
Investors’ Rights Agreement
Old Ginkgo was party to the Third Amended and Restated Investors’ Rights Agreement, dated as of September 9, 2019, which granted registration rights and information rights, among other things, to certain holders of its capital stock, including (i) entities affiliated with Anchorage, entities affiliated with Baillie Gifford & Co., Cascade Investment, L.L.C, GA GK, L.P., and Viking Global Opportunities Illiquid Investments Sub-Master LP, each of which currently holds more than 5% of Ginkgo’s capital stock; (ii) Novalis LifeSciences, which is
179
Table of Contents
affiliated with Marijn Dekkers, a Ginkgo director; and (iii) Senator Global Opportunity Master Fund LP, which currently holds more than 5% of Ginkgo’s capital stock and is affiliated with Evan Lodes, a former Old Ginkgo director. This agreement was terminated upon the Closing.
Right of First Refusal and Co-Sale Agreement
Old Ginkgo was party to the Third Amended and Restated Right of First Refusal and Co-Sale Agreement, dated as of September 9, 2019, which granted the right to purchase shares of Old Ginkgo capital stock which certain other stockholders propose to sell to other parties to, among others (i) entities affiliated with Anchorage, entities affiliated with Baillie Gifford & Co., Cascade Investment, L.L.C, GA GK, and Viking Global Opportunities Illiquid Investments Sub-Master LP, each of which currently hold more than 5% of Ginkgo’s capital stock; (ii) Novalis LifeSciences, which is affiliated with Marijn Dekkers, a Ginkgo director; and (iii) Senator Global Opportunity Master Fund LP, which currently holds more than 5% of Ginkgo’s capital stock and is affiliated with Evan Lodes, a former Old Ginkgo director. This agreement was terminated upon the Closing.
Voting Agreement
Old Ginkgo was party to the Third Amended and Restated Voting Agreement, dated as of September 9, 2019, pursuant to which certain holders of its capital stock, including (i) entities affiliated with Anchorage, entities affiliated with Baillie Gifford & Co., Cascade Investment, L.L.C, GA GK, and Viking Global Opportunities Illiquid Investments Sub-Master LP, each of which currently hold more than 5% of Ginkgo’s capital stock; (ii) Novalis LifeSciences, which is affiliated with Marijn Dekkers, a Ginkgo director; and (iii) Senator Global Opportunity Master Fund LP, which currently holds more than 5% of Ginkgo’s capital stock and is affiliated with Evan Lodes, a former Old Ginkgo director, agreed to vote their shares in the manner directed by the agreement. This agreement was terminated upon the Closing.
Director and Officer Indemnification
Ginkgo’s Charter authorizes indemnification and advancement of expenses for its directors and officers to the fullest extent permitted by the DGCL.
PIPE Investment
In May 2021, certain Old Ginkgo related persons entered into Subscription Agreements with SRNG in connection with the Private Placement. The following table summarizes purchases in the Private Placement by such related persons:
| Name |
SRNG Class A ordinary shares |
Total Purchase Price |
||||||
| Entities affiliated with Baillie Gifford & Co.(1) |
10,300,000 | $ | 103,000,000 | |||||
| Cascade Investment, L.L.C.(2) |
3,000,000 | $ | 30,000,000 | |||||
| General Atlantic (GK), L.P.(3) |
250,000 | $ | 2,500,000 | |||||
| Senator Global Opportunity Master Fund LP(4) |
700,000 | $ | 7,000,000 | |||||
| Viking Global Opportunities Illiquid Investments Sub-Master LP(5) |
2,000,000 | $ | 20,000,000 | |||||
| (1) | Entities affiliated with Baillie Gifford & Co. hold more than 5% of Ginkgo’s outstanding capital stock. (2) Cascade Investment, L.L.C. holds more than 5% of Ginkgo’s outstanding capital stock. |
| (3) | GA GK holds more than 5% of Ginkgo’s outstanding capital stock. |
| (4) | Senator Global Opportunity Master Fund LP holds more than 5% of Ginkgo’s outstanding capital stock. Evan Lodes, a former Old Ginkgo director, is an affiliate of Senator Global Opportunity Master Fund LP. |
| (5) | Viking Global Opportunities Illiquid Investments Sub-Master LP holds more than 5% of Ginkgo’s outstanding capital stock. |
180
Table of Contents
Policies and Procedures for Related Person Transactions
Our written related person transaction policy sets forth the following policies and procedures for the review and approval or ratification of related person transactions.
A “Related Person Transaction” is a material transaction, arrangement or relationship (or any series of similar transactions, arrangements or relationships) in which the Company (including any of its subsidiaries) was, is or will be a participant and in which any related person had, has or will have a direct or indirect material interest. A transaction involving an amount exceeding $120,000 is presumed to be a “material transaction.” A “Related Person” means:
| • | any person who is, or at any time since the beginning of the Company’s last fiscal year was, a director or executive officer of the Company or a nominee to become a director of the Company; |
| • | any person who is known to be the beneficial owner of more than 5% of any class of the Company’s voting securities; |
| • | any immediate family member of any of the foregoing persons, which means any child, stepchild, parent, stepparent, spouse, sibling, mother-in-law, father-in-law, son-in-law, daughter-in-law, brother-in-law or sister-in-law of a director, executive officer, nominee or beneficial owner of more than 5% of any class of the Company’s voting securities, and any other person (other than a tenant or employee) sharing the same household of such director, executive officer, nominee or beneficial owner of more than 5% of any class of the Company’s voting securities; and |
| • | any firm, corporation or other entity in which any of the foregoing persons is employed or is a general partner or principal or in a similar position or in which such person has a 5% or greater beneficial ownership interest in any class of the Company’s voting securities. |
We have policies and procedures designed to minimize potential conflicts of interest arising from any dealings we may have with our affiliates and to provide appropriate procedures for the disclosure of any real or potential conflicts of interest that may exist from time to time. Specifically, pursuant to its charter, the audit committee has the responsibility to review related person transactions.
181
Table of Contents
UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a discussion of certain material U.S. federal income tax consequences relating to the purchase, ownership and disposition of Class A common stock. This discussion is limited to certain U.S. federal income tax considerations to investors that will hold Class A common stock as capital assets within the meaning of Section 1221 of the U.S. Internal Revenue Code of 1986, as amended, and that purchased such Class A common stock from the Selling Securityholders pursuant to this offering.
This discussion is a summary only and does not describe all of the tax consequences that may be relevant to you in light of your particular circumstances, including but not limited to the alternative minimum tax, the Medicare tax on certain net investment income and the different consequences that may apply if you are subject to special rules that apply to certain types of investors, including but not limited to:
| • | banks, financial institutions or financial services entities; |
| • | broker-dealers; |
| • | governments or agencies or instrumentalities thereof; |
| • | regulated investment companies; |
| • | real estate investment trusts; |
| • | expatriates or former long-term residents of the United States; |
| • | persons that actually or constructively own five percent or more (by vote or value) of our shares; |
| • | persons that acquired Class A common stock pursuant to an exercise of employee share options, in connection with employee share incentive plans or otherwise as compensation; |
| • | insurance companies; |
| • | dealers or traders subject to a mark-to-market method of accounting with respect to Class A common stock; |
| • | persons holding Class A common stock as part of a “straddle,” constructive sale, hedge, wash sale, conversion or other integrated or similar transaction; |
| • | U.S. holders (as defined below) whose functional currency is not the U.S. dollar; |
| • | partnerships (or entities or arrangements classified as partnerships or other pass-through entities for U.S. federal income tax purposes) and any beneficial owners of such partnerships; |
| • | tax-exempt entities; |
| • | controlled foreign corporations; and |
| • | passive foreign investment companies. |
If a partnership (including an entity or arrangement treated as a partnership or other pass-thru entity for U.S. federal income tax purposes) holds Class A common stock, the tax treatment of a partner, member or other beneficial owner in such partnership will generally depend upon the status of the partner, member or other beneficial owner, the activities of the partnership and certain determinations made at the partner, member or other beneficial owner level. If you are a partner, member or other beneficial owner of a partnership holding Class A common stock, you are urged to consult your tax advisor regarding the tax consequences of the purchase, ownership and disposition of Class A common stock.
This discussion is based on the Code, and administrative pronouncements, judicial decisions and final, temporary and proposed Treasury Regulations as of the date hereof, which are subject to change, possibly on a retroactive basis, and changes to any of which subsequent to the date of this prospectus may affect the tax consequences
182
Table of Contents
described herein. This discussion does not address any aspect of state, local or non-U.S. taxation, or any U.S. federal taxes other than income taxes (such as gift and estate taxes).
We have not sought, and do not expect to seek, a ruling from the U.S. Internal Revenue Service (the “IRS”) as to any U.S. federal income tax consequence described herein. The IRS may disagree with the discussion herein, and its determination may be upheld by a court. Moreover, there can be no assurance that future legislation, regulations, administrative rulings or court decisions will not adversely affect the accuracy of the statements in this discussion. You are urged to consult your tax advisor with respect to the application of U.S. federal tax laws to your particular situation, as well as any tax consequences arising under the laws of any state, local or foreign jurisdiction.
THIS DISCUSSION IS ONLY A SUMMARY OF CERTAIN U.S. FEDERAL INCOME TAX CONSIDERATIONS ASSOCIATED WITH THE PURCHASE, OWNERSHIP AND DISPOSITION OF CLASS A COMMON STOCK ACQUIRED PURSUANT TO THIS OFFERING. EACH PROSPECTIVE INVESTOR IN CLASS A COMMON STOCK IS URGED TO CONSULT ITS OWN TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH INVESTOR OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF CLASS A COMMON STOCK, INCLUDING THE APPLICABILITY AND EFFECT OF ANY U.S. FEDERAL NON- INCOME, STATE, LOCAL, AND NON-U.S. TAX LAWS OR ANY APPLICABLE INCOME TAX TREATY.
U.S. Holders
This section applies to you if you are a “U.S. holder.” A U.S. holder is a beneficial owner of Class A common stock who or that is, for U.S. federal income tax purposes:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation (or other entity taxable as a corporation) organized in or under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate the income of which is includible in gross income for U.S. federal income tax purposes regardless of its source; or |
| • | a trust, if (i) a court within the United States is able to exercise primary supervision over the administration of the trust and one or more United States persons (as defined in the Code) have authority to control all substantial decisions of the trust or (ii) it has a valid election in effect under Treasury Regulations to be treated as a United States person. |
Taxation of Distributions. If we pay distributions in cash or other property (other than certain distributions of our stock or rights to acquire our stock) to U.S. holders of Class A common stock, such distributions generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Distributions in excess of current and accumulated earnings and profits will constitute a return of capital that will be applied against and reduce (but not below zero) the U.S. holder’s adjusted tax basis in its Class A common stock. Any remaining excess will be treated as gain realized on the sale or other disposition of its Class A common stock and will be treated as described under “U.S. Holders—Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Class A Common Stock” below.
Dividends we pay to a U.S. holder that is treated as a taxable corporation generally will qualify for the dividends received deduction if the requisite holding period is satisfied. With certain exceptions (including, but not limited to, dividends treated as investment income for purposes of investment interest deduction limitations), and provided certain holding period requirements are met, dividends we pay to a non-corporate U.S. holder may constitute “qualified dividend income” that will be subject to tax at the applicable maximum tax rate accorded to long-term capital gains. If the applicable holding period requirements are not satisfied, then a corporation may not be able to qualify for the dividends received deduction and would have taxable income equal to the entire
183
Table of Contents
dividend amount, and non-corporate U.S. holders may be subject to tax on such dividend at regular ordinary income tax rates instead of the preferential rate that applies to qualified dividend income.
Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Class A Common Stock. Upon a sale or other taxable disposition of Class A common stock, a U.S. holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized and the U.S. holder’s adjusted tax basis in its Class A common stock. Any such capital gain or loss generally will be long-term capital gain or loss if the U.S. holder’s holding period for its Class A common stock so disposed of exceeds one year. Long-term capital gains recognized by non-corporate U.S. holders may be eligible to be taxed at reduced rates. The deductibility of capital losses is subject to limitations.
Generally, the amount of gain or loss recognized by a U.S. holder is an amount equal to the difference between (i) the sum of the amount of cash and the fair market value of any property received in such disposition and (ii) the U.S. holder’s adjusted tax basis in its Class A common stock so disposed of. A U.S. holder’s adjusted tax basis in its Class A common stock generally will equal the U.S. holder’s acquisition cost less, in the case of a share of Class A common stock, any prior distributions treated as a return of capital.
Information Reporting and Backup Withholding. In general, information reporting requirements may apply to distributions paid to a U.S. holder and to the proceeds of the sale or other disposition of Class A common stock, unless the U.S. holder is an exempt recipient. Backup withholding may apply to such payments if the U.S. holder fails to provide a taxpayer identification number, a certification of exempt status or has been notified by the IRS that it is subject to backup withholding (and such notification has not been withdrawn).
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules will be allowed as a credit against a U.S. holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided the required information is timely furnished to the IRS.
Non-U.S. Holders
This section applies to you if you are a “Non-U.S. holder.” As used herein, the term “Non-U.S. holder” means a beneficial owner of Class A common stock who or that is for U.S. federal income tax purposes:
| • | a non-resident alien individual (other than certain former citizens and residents of the United States subject to U.S. tax as expatriates); |
| • | a foreign corporation; or |
| • | an estate or trust that is not a U.S. holder; |
but generally does not include an individual who is present in the United States for 183 days or more in the taxable year of the disposition of their Class A common stock. If you are such an individual, you should consult your tax advisor regarding the U.S. federal income tax consequences of the purchase, ownership or sale or other disposition of Class A common stock.
Taxation of Distributions. In general, any distributions (other than certain distributions of our stock or rights to acquire our stock) we make to a Non-U.S. holder of Class A common stock, to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles), will constitute dividends for U.S. federal income tax purposes and, provided such dividends are not effectively connected with the Non-U.S. holder’s conduct of a trade or business within the United States, we will be required to withhold tax from the gross amount of the dividend at a rate of 30%, unless such Non-U.S. holder is eligible for a reduced rate of withholding tax under an applicable income tax treaty and provides proper certification of its eligibility for such reduced rate (usually on an IRS Form W-8BEN or W-8BEN-E). Any distribution not constituting a dividend will be treated first as reducing (but not below zero) the Non-U.S. holder’s adjusted tax basis in its Class A common stock and, to the extent such distribution exceeds the Non-U.S. holder’s adjusted tax basis, as
184
Table of Contents
gain realized from the sale or other disposition of its Class A common stock, which will be treated as described under “Non-U.S. Holders—Gain on Sale, Taxable Exchange or Other Taxable Disposition of Class A Common Stock” below. In addition, if we determine that we are likely to be classified as a “United States real property holding corporation” (see “Non-U.S. Holders—Gain on Sale, Taxable Exchange or Other Taxable Disposition of Class A Common Stock” below), we may withhold 15% of any distribution that exceeds our current and accumulated earnings and profits.
The withholding tax generally does not apply to dividends paid to a Non-U.S. holder who provides an IRS Form W- 8ECI, certifying that the dividends are effectively connected with the Non-U.S. holder’s conduct of a trade or business within the United States. Instead, the effectively connected dividends will be subject to regular U.S. federal income tax as if the Non-U.S. holder were a U.S. resident, subject to an applicable income tax treaty providing otherwise. A corporate Non-U.S. holder receiving effectively connected dividends may also be subject to an additional “branch profits tax” imposed at a rate of 30% (or a lower applicable treaty rate).
Gain on Sale, Taxable Exchange or Other Taxable Disposition of Class A Common Stock. A Non-U.S. holder generally will not be subject to U.S. federal income or withholding tax in respect of gain recognized on a sale, taxable exchange or other taxable disposition of its Class A common stock unless:
| • | the gain is effectively connected with the conduct by the Non-U.S. holder of a trade or business within the United States (and, under certain income tax treaties, is attributable to a United States permanent establishment or fixed base maintained by the Non-U.S. holder); or |
| • | we are or have been a “United States real property holding corporation” (a “USRPHC”) for U.S. federal income tax purposes at any time during the shorter of the five-year period ending on the date of disposition or the Non-U.S. holder’s holding period for its Class A common stock, except, in the case where shares of Class A common stock are regularly traded on an established securities market, the Non-U.S. holder has owned, directly or constructively, at all times within the shorter of the five-year period preceding the disposition of its Class A common stock or such Non-U.S. holder’s holding period for such Class A common stock, 5% or less of Ginkgo Class A common stock. It is unclear how the rules for determining the 5% threshold for this purpose would be applied with respect to Class A common stock. There can be no assurance that Class A common stock will be treated as regularly traded on an established securities market for this purpose. Non-U.S. holders should consult their own tax advisors regarding the application of the foregoing rules in light of their particular facts and circumstances. |
Unless an applicable treaty provides otherwise, gain described in the first bullet point above will be subject to tax at generally applicable U.S. federal income tax rates as if the Non-U.S. holder were a U.S. resident. Any gains described in the first bullet point above of a Non-U.S. holder that is treated as a foreign corporation for U.S. federal income tax purposes may also be subject to an additional “branch profits tax” imposed at a 30% rate (or lower treaty rate).
If the second bullet point above applies to a Non-U.S. holder, gain recognized by such holder on the sale, exchange or other disposition of its Class A common stock will be subject to tax at generally applicable U.S. federal income tax rates. In addition, a buyer of Class A common stock from such holder may be required to withhold U.S. federal income tax at a rate of 15% of the amount realized upon such disposition. We will be classified as a USRPHC if the fair market value of our “United States real property interests” equals or exceeds 50% of the sum of the fair market value of our worldwide real property interests plus our other assets used or held for use in a trade or business, as determined for U.S. federal income tax purposes. We believe we currently are not, and do not anticipate becoming, a USRPHC. However, because our status as a USPRHC depends on the composition our business assets, which may change, no assurance can be provided as to whether we would be treated as a USRPHC in any future year.
185
Table of Contents
Information Reporting and Backup Withholding. Information returns generally will be filed with the IRS in connection with payments to a Non-U.S. holder of distributions on Class A common stock. Proceeds from a sale or other taxable disposition of Class A common stock within the United States or conducted through certain U.S.-related brokers may be subject to backup withholding or information reporting unless a Non-U.S. holder complies with certification procedures to establish that it is not a United States person. The certification procedures required to claim a reduced rate of withholding under a treaty generally will satisfy such certification requirements. Proceeds of a disposition of Class A common stock conducted through a non-U.S. office of a non-U.S. broker generally will not be subject to backup withholding or information reporting. Backup withholding is not an additional tax. The amount of any backup withholding from a payment to a Non-U.S. holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
FATCA Withholding Taxes. Provisions commonly referred to as “FATCA” impose withholding of 30% on payments of dividends on Class A common stock, or (subject to the proposed Treasury Regulations discussed below) gross proceeds from the sale or other disposition of, Class A common stock paid (or deemed paid) to “foreign financial institutions” (which is broadly defined for this purpose and in general includes investment vehicles) and certain other non-U.S. entities unless various U.S. information reporting and due diligence requirements (generally relating to ownership by United States persons of interests in or accounts with those entities) have been satisfied by, or an exemption applies to, the payee (typically certified as to by the delivery of a properly completed IRS Form W-8BEN-E). Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules. Under certain circumstances, a Non-U.S. holder might be eligible for refunds or credits of such withholding taxes, and a Non-U.S. holder might be required to file a U.S. federal income tax return to claim such refunds or credits. Thirty percent withholding under FATCA was scheduled to apply to payments of gross proceeds from the sale or other disposition of property that produces U.S.- source interest or dividends beginning on January 1, 2019, but on December 13, 2018, the IRS released proposed regulations that, if finalized in their proposed form, would eliminate the obligation to withhold on gross proceeds. Such proposed regulations also delayed withholding on certain other payments received from other foreign financial institutions that are allocable, as provided for under final Treasury Regulations, to payments of U.S.-source dividends, and other fixed or determinable annual or periodic income. Although these proposed Treasury Regulations are not final, taxpayers generally may rely on them until final Treasury Regulations are issued. However, there can be no assurance that final Treasury Regulations will provide the same exceptions from FATCA withholding as the proposed Treasury Regulations. Prospective investors should consult their tax advisors regarding the effects of FATCA on their investment in Class A common stock.
186
Table of Contents
PLAN OF DISTRIBUTION
We are registering the offer and sale, from time to time, by the Selling Securityholders of up to 292,051,107 shares of Class A common stock, par value $0.0001 per share. We will not receive any of the proceeds from the sale of Class A common stock by the Selling Securityholders.
Once issued and upon effectiveness of the registration statement of which this prospectus forms a part, the Class A common stock beneficially owned by the Selling Securityholders covered by this prospectus may be offered and sold from time to time by the Selling Securityholders. The term “Selling Securityholders” includes donees, pledgees, transferees or other successors in interest selling securities received after the date of this prospectus from a Selling Securityholder as a gift, pledge, partnership distribution or other transfer. The Selling Securityholders will act independently of us in making decisions with respect to the timing, manner and size of each sale. Such sales may be made on one or more exchanges or in the over-the-counter market or otherwise, at prices and under terms then prevailing or at prices related to the then current market price or in negotiated transactions. Each Selling Securityholder reserves the right to accept and, together with its respective agents, to reject, any proposed purchase of securities to be made directly or through agents. The Selling Securityholders and any of their permitted transferees may sell their securities offered by this prospectus on any stock exchange, market or trading facility on which the securities are traded or in private transactions.
Subject to the limitations set forth in the Purchase Agreement, the Selling Securityholders may use any one or more of the following methods when selling the securities offered by this prospectus:
| • | purchases by a broker-dealer as principal and resale by such broker-dealer for its own account pursuant to this prospectus; |
| • | ordinary brokerage transactions and transactions in which the broker solicits purchasers; |
| • | one or more underwritten offerings; |
| • | block trades in which the broker-dealer so engaged will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| • | an exchange distribution in accordance with the rules of the applicable exchange; |
| • | in market transactions, including transactions on a national securities exchange or quotations service or over-the-counter market; |
| • | distributions to their members, partners or stockholders |
| • | settlement of short sales entered into after the date of the registration statement of which this prospectus is a part is declared effective by the SEC; |
| • | agreements with broker-dealers to sell a specified number of the securities at a stipulated price per share; |
| • | in “at the market” offerings, as defined in Rule 415 under the Securities Act, at negotiated prices, at prices prevailing at the time of sale or at prices related to such prevailing market prices, including sales made directly on a national securities exchange or sales made through a market maker other than on an exchange or other similar offerings through sales agents; |
| • | directly to purchasers, including through a specific bidding, auction or other process or in privately negotiated transactions; |
| • | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| • | through a combination of any of the above methods of sale; or |
| • | any other method permitted pursuant to applicable law. |
187
Table of Contents
In addition, a Selling Securityholder that is an entity may elect to make a pro rata in-kind distribution of securities to its members, partners or stockholders pursuant to the registration statement of which this prospectus is a part by delivering a prospectus with a plan of distribution. Such members, partners or stockholders would thereby receive freely tradeable securities pursuant to the distribution through a registration statement. To the extent a distributee is an affiliate of ours (or to the extent otherwise required by law), we may file a prospectus supplement in order to permit the distributees to use the prospectus to resell the securities acquired in the distribution.
The Selling Securityholders also may transfer the securities in other circumstances, in which case the transferees, pledgees or other successors-in-interest will be the selling beneficial owners for purposes of this prospectus. Upon being notified by a Selling Securityholder that a donee, pledgee, transferee, other successor-in-interest intends to sell our securities, we will, to the extent required, promptly file a supplement to this prospectus to name specifically such person as a Selling Securityholder.
To the extent required, the shares of Class A common stock to be sold, the names of the Selling Securityholders, the respective purchase prices and public offering prices, the names of any agents, dealer or underwriter, any applicable commissions or discounts with respect to a particular offer will be set forth in an accompanying prospectus supplement or, if appropriate, a post-effective amendment to the registration statement that includes this prospectus.
The Selling Securityholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the shares of Class A common stock in the course of hedging the positions they assume. The Selling Securityholders may also sell the shares of Class A common stock short and deliver these securities to close out their short positions, or loan or pledge the shares of Class A common stock to broker-dealers that in turn may sell these shares. The Selling Securityholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Securityholders also may in the future resell a portion of Class A common stock in open market transactions in reliance upon Rule 144 under the Securities Act, provided that they meet the criteria and conform to the requirements of that rule, or pursuant to other available exemptions from the registration requirements of the Securities Act.
Selling Securityholders may use this prospectus in connection with resales of shares of Class A common stock. This prospectus and any accompanying prospectus supplement will identify the Selling Securityholders, the terms of Class A common stock and any material relationships between us and the Selling Securityholders. In offering the securities covered by this prospectus, the Selling Securityholders and any underwriters, broker-dealers or agents who execute sales for the Selling Securityholders may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. Any discounts, commissions, concessions or profit they earn on any resale of those securities may be underwriting discounts and commissions under the Securities Act. Unless otherwise set forth in a prospectus supplement, the Selling Securityholders will receive all the net proceeds from the resale of shares of Class A common stock. If any Selling Securityholder is an “underwriter” within the meaning of Section 2(11) of the Securities Act, then the Selling Securityholder will be subject to the prospectus delivery requirements of the Securities Act. Underwriters and their controlling persons, dealers and agents may be entitled, under agreements entered into with us and the Selling Securityholder, to indemnification against and contribution toward specific civil liabilities, including liabilities under the Securities Act.
In order to comply with the securities laws of certain states, if applicable, the Class A common stock must be sold in such jurisdictions only through registered or licensed brokers or dealers. In addition, in certain states the Class A common stock may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
188
Table of Contents
We have advised the Selling Securityholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of shares in the market and to the activities of the Selling Securityholders and their affiliates. In addition, to the extent applicable we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the Selling Securityholders for the purpose of satisfying the prospectus delivery requirements of the Securities Act. The Selling Securityholders may indemnify any broker-dealer that participates in transactions involving the sale of the shares of Class A common stock against certain liabilities, including liabilities arising under the Securities Act.
189
Table of Contents
LEGAL MATTERS
Ropes & Gray LLP has passed upon the validity of the Class A common stock offered by this prospectus and certain other legal matters related to this prospectus.
EXPERTS
The consolidated financial statements of Ginkgo Bioworks Holdings, Inc. at December 31, 2021 and 2020, and for each of the three years in the period ended December 31, 2021, appearing in this Prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The financial statements of Allonnia, LLC at December 31, 2021 and 2020, and for each of the two years in the period ended December 31, 2021, and for the period from November 27, 2019 (inception) through December 31, 2019 included in this prospectus have been audited by Wolf & Company, P.C., independent public accounting firm, as set forth in their reports thereon, appearing elsewhere herein, and are included in reliance upon such reports given on the authority of such firm as experts in accounting and auditing.
CHANGE IN AUDITOR
On the Closing Date, the Audit Committee of the Ginkgo Board approved the engagement of Ernst & Young LLP (“EY”) as the Company’s independent registered public accounting firm to audit the Company’s consolidated financial statements for the year ending December 31, 2021. WithumSmith+Brown, PC (“WSB”) served as independent registered public accounting firm of SRNG prior to the consummation of the Business Combination. Accordingly, WSB was informed that it would be replaced by EY as the Company’s independent registered public accounting firm.
The reports of WSB on SRNG’s, the Company’s legal predecessor, balance sheet as of December 31, 2020 and the statements of operations, changes in stockholder’s equity and cash flows for the period from October 22, 2020 (date of inception) through December 31, 2020, did not contain an adverse opinion or a disclaimer of opinion, and were not qualified or modified as to uncertainties, audit scope or accounting principles.
During the period from October 22, 2020 (date of inception) through December 31, 2020 and the subsequent interim period through the Closing Date, there were no disagreements between the Company and WSB on any matter of accounting principles or practices, financial disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of WSB, would have caused it to make reference to the subject matter of the disagreements in its reports on the Company’s financial statements for such period.
During the period from October 22, 2020 (date of inception) through December 31, 2020 and the subsequent interim period through the Closing Date, there were no “reportable events” (as defined in Item 304(a)(1)(v) of Regulation S-K under the Exchange Act).
During the period from October 22, 2020 (inception) to the date the Board approved the engagement of EY as the Company’s independent registered public accounting firm, SRNG did not consult with EY on matters that involved the application of accounting principles to a specified transaction, the type of audit opinion that might be rendered on SRNG’s consolidated financial statements or any other matter that was either the subject of a disagreement or reportable event.
190
Table of Contents
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1, including exhibits, under the Securities Act of 1933, as amended, with respect to the common stock offered by this prospectus. This prospectus does not contain all of the information included in the registration statement. For further information pertaining to us and our securities, you should refer to the registration statement and our exhibits.
In addition, we file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public on a website maintained by the SEC located at www.sec.gov. We also maintain a website at www.ginkgobioworks.com. Through our website, we make available, free of charge, annual, quarterly and current reports, proxy statements and other information as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained on, or that may be accessed through, our website is not part of, and is not incorporated into, this prospectus.
191
Table of Contents
Page |
||||
F-2 |
||||
F-3 |
||||
F-4 |
||||
F-5 |
||||
F-7 |
||||
F-9 |
||||
Page |
||||
| F-68 | ||||
| F-69 | ||||
| F-70 | ||||
| F-72 | ||||
| F-73 | ||||
Page |
||||
| F-101 | ||||
| Consolidated Financial Statements: |
||||
| F-103 | ||||
| F-104 | ||||
| F-105 | ||||
| F-106 | ||||
| F-107 | ||||
| F-115 | ||||
| Consolidated Financial Statements: |
||||
| F-116 | ||||
| F-117 | ||||
| F-118 | ||||
| F-119 | ||||
| F-120 |
As of December 31, |
||||||||
2021 |
2020 |
|||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | $ | ||||||
| Accounts receivable, net |
||||||||
| Accounts receivable - related parties |
||||||||
| Inventory, net |
||||||||
| Prepaid expenses and other current assets |
||||||||
| |
|
|
|
|||||
| Total current assets |
||||||||
| Property and equipment, net |
||||||||
| Investments |
||||||||
| Equity method investments |
||||||||
| Intangible assets, net |
||||||||
| Goodwill |
||||||||
| Loans receivable, net of current portion |
||||||||
| Other non-current assets |
||||||||
| |
|
|
|
|||||
| Total assets |
$ | $ | ||||||
| |
|
|
|
|||||
| Liabilities and Stockholders’ Equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | $ | ||||||
| Deferred revenue (includes $ |
||||||||
| Accrued expenses and other current liabilities |
||||||||
| |
|
|
|
|||||
| Total current liabilities |
||||||||
| Non-current liabilities: |
||||||||
| Deferred rent, net of current portion |
||||||||
| Deferred revenue, net of current portion (includes $ |
||||||||
| Lease financing obligation |
||||||||
| Warrant liabilities |
||||||||
| Other non-current liabilities |
||||||||
| |
|
|
|
|||||
| Total liabilities |
||||||||
| |
|
|
|
|||||
| Commitments and contingencies (Note 14) |
||||||||
| Stockholders’ equity (1) : |
||||||||
| Preferred stock, $ |
||||||||
| Class A, Class B and Class C common stock $ |
||||||||
| Additional paid-in capital |
||||||||
| Accumulated deficit |
( |
) | ( |
) | ||||
| Accumulated other comprehensive loss |
( |
) | ||||||
| |
|
|
|
|||||
| Total Ginkgo Bioworks Holdings, Inc. stockholders’ equity |
||||||||
| Non-controlling interest |
||||||||
| |
|
|
|
|||||
| Total stockholders’ equity |
||||||||
| |
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | $ | ||||||
| |
|
|
|
|||||
| (1) | Balances as of December 31, 2020 have been retroactively restated for the reverse recapitalization as described in Note 2. |
Year Ended December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
| Foundry revenue (related party revenue of $ 2021, 2020 and 2019, respectively) |
$ |
$ |
$ |
|||||||||
| Biosecurity revenue: |
||||||||||||
| Product |
||||||||||||
| Service |
||||||||||||
| |
|
|
|
|
|
|||||||
| Total revenue |
||||||||||||
| |
|
|
|
|
|
|||||||
| Costs and operating expenses: |
||||||||||||
| Cost of Biosecurity product revenue |
||||||||||||
| Cost of Biosecurity service revenue |
||||||||||||
| Research and development |
||||||||||||
| General and administrative |
||||||||||||
| |
|
|
|
|
|
|||||||
| Total operating expenses |
||||||||||||
| |
|
|
|
|
|
|||||||
| Loss from operations |
( |
) |
( |
) |
( |
) | ||||||
| Other (expense) income: |
||||||||||||
| Interest income |
||||||||||||
| Interest expense |
( |
) |
( |
) |
( |
) | ||||||
| Loss on equity method investments |
( |
) |
( |
) |
( |
) | ||||||
| Loss on investments |
( |
) |
( |
) |
( |
) | ||||||
| Change in fair value of warrant liabilities |
||||||||||||
| Gain on settlement of partnership agreement |
||||||||||||
| Other (expense) income, net |
( |
) |
||||||||||
| |
|
|
|
|
|
|||||||
| Total other (expense) income, net |
( |
) |
( |
) | ||||||||
| |
|
|
|
|
|
|||||||
| Loss before income taxes |
( |
) |
( |
) |
( |
) | ||||||
| Income tax (benefit) provision |
( |
) |
||||||||||
| |
|
|
|
|
|
|||||||
| Net loss |
( |
) |
( |
) |
( |
) | ||||||
| Net loss attributable to non-controlling interest |
( |
) |
( |
) |
( |
) | ||||||
| |
|
|
|
|
|
|||||||
| Net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders |
$ |
( |
) |
$ |
( |
) |
$ |
( |
) | |||
| |
|
|
|
|
|
|||||||
| Net loss per share attributable to Ginkgo Bioworks Holdings, Inc. (1) common stockholders: |
||||||||||||
| Basic |
$ |
( |
) |
$ |
( |
) |
$ |
( |
) | |||
| Diluted |
$ | ( |
) |
$ |
( |
) |
$ |
( |
) | |||
| Weighted average common shares outstanding (1) |
||||||||||||
| Basic |
||||||||||||
| Diluted |
||||||||||||
| Comprehensive loss: |
||||||||||||
| Net loss |
$ |
( |
) | $ |
( |
) | $ |
( |
) | |||
| Other comprehensive loss: |
||||||||||||
| Foreign currency translation adjustment |
( |
) |
||||||||||
| |
|
|
|
|
|
|||||||
| Total other comprehensive loss |
( |
) |
||||||||||
| |
|
|
|
|
|
|||||||
| Comprehensive loss |
$ |
( |
) |
$ |
( |
) |
$ |
( |
) | |||
| |
|
|
|
|
|
|||||||
| (1) | Amounts for the year ended December 31, 2020 and 2019 have been retroactively restated for the reverse recapitalization as described in Note 2. |
Series B, C, D, E Convertible Preferred Stock |
Old Ginkgo Common Stock |
Common Stock |
||||||||||||||||||||||
Shares |
Amount |
Shares |
Amount |
Shares |
Amount |
|||||||||||||||||||
| Balance as of December 31, 2018 (as previously reported) |
$ |
$ |
$ |
|||||||||||||||||||||
| Retroactive application of the reverse recapitalization |
( |
) |
( |
) |
( |
) |
( |
) |
||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2018 |
— |
— |
— |
— |
||||||||||||||||||||
| Exercise of stock options |
— |
— |
— |
— |
— |
|||||||||||||||||||
| Issuance of Series E convertible preferred stock, net of issuance costs of $ |
— |
— |
— |
— |
||||||||||||||||||||
| Beneficial conversion feature of convertible promissory notes |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| Conversion of convertible promissory notes into Series E convertible preferred stock |
— |
— |
— |
— |
||||||||||||||||||||
| Vesting of restricted stock awards |
— |
— |
— |
— |
— |
|||||||||||||||||||
| Repurchase of common stock |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| Retirement of treasury stock |
— |
— |
— |
— |
( |
) |
( |
) | ||||||||||||||||
| Issuance of warrants to purchase convertible preferred stock |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| Stock-based compensation expense |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| Net loss and comprehensive loss |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2019 |
— |
— |
— |
— |
||||||||||||||||||||
| Exercise of stock options |
— |
— |
— |
— |
— |
|||||||||||||||||||
| Issuance of Series E convertible preferred stock, net of issuance costs of $ |
— |
— |
— |
— |
||||||||||||||||||||
| Vesting of restricted stock awards |
— |
— |
— |
— |
— |
|||||||||||||||||||
| Stock-based compensation expense |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| Net loss and comprehensive loss |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| Balance as of December 31, 2020 |
— |
— |
— |
— |
||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Issuance of common stock upon exercise or vesting of equity awards |
— |
— |
— |
— |
||||||||||||||||||||
| Vesting of restricted stock - earnouts |
— |
— |
— |
— |
||||||||||||||||||||
| Tax withholdings related to net share settlement of equity awards |
— |
— |
— |
— |
( |
) |
— |
|||||||||||||||||
| Founder shares repurchase |
— |
— |
— |
— |
( |
) |
— |
|||||||||||||||||
| Issuance of warrants to purchase Series D convertible preferred stock |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| Issuance of Series D and B convertible preferred stock upon exercise of warrants |
— |
— |
— |
— |
— |
|||||||||||||||||||
| Issuance of Series E convertible preferred stock in exchange for warrants |
— |
— |
— |
— |
— |
|||||||||||||||||||
| Issuance of common stock for a business acquisition |
— |
— |
— |
— |
— |
|||||||||||||||||||
| Issuance of common stock upon reverse recapitalization, net of offering costs (Note 3) |
— |
— |
— |
— |
||||||||||||||||||||
| Assumption of Public and Private Placement Warrants |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| Contributions from non-controlling interests |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| Stock-based compensation expense |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| Foreign currency translation |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| Net loss |
— |
— |
— |
— |
— |
— |
||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2021 |
— |
$ |
— |
— |
$ |
— |
$ |
|||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Treasury Stock |
Additional Paid-In Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Loss |
Non- Controlling Interest |
Total Stockholders’ Equity |
|||||||||||||||||||||||
Shares |
Amount |
|||||||||||||||||||||||||||
| Balance as of December 31, 2018 (as previously reported) |
( |
) | $ | ( |
) | $ | $ | ( |
) | $ | — | $ | $ | |||||||||||||||
| Retroactive application of the reverse recapitalization |
( |
) | ( |
) | — | — | — | — | ||||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance as of December 31, 2018 |
( |
) |
( |
) |
( |
) | — | |||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | — | |||||||||||||||||||||||
| Issuance of Series E convertible preferred stock, net of issuance costs of $ |
— | — | — | — | — | |||||||||||||||||||||||
| Beneficial conversion feature of convertible promissory notes |
— | — | ( |
) |
— | — | — | ( |
) | |||||||||||||||||||
| Conversion of convertible promissory notes into Series E convertible preferred stock |
— | — | — | — | — | |||||||||||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Repurchase of common stock |
( |
) | ( |
) | — | — | — | — | ( |
) | ||||||||||||||||||
| Retirement of treasury stock |
( |
) | — | |||||||||||||||||||||||||
| Issuance of warrants to purchase convertible preferred stock |
— | — | — | — | — | |||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | |||||||||||||||||||||||
| Net loss and comprehensive loss |
— | — | — | ( |
) | — | ( |
) | ( |
) | ||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance as of December 31, 2019 |
( |
) | — | |||||||||||||||||||||||||
| Exercise of stock options |
— | — | — | — | — | |||||||||||||||||||||||
| Issuance of Series E convertible preferred stock, net of issuance costs of $ |
— | — | — | — | — | |||||||||||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | |||||||||||||||||||||||
| Net loss and comprehensive loss |
— | — | — | ( |
) | — | ( |
) | ( |
) | ||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance as of December 31, 2020 |
( |
) | ||||||||||||||||||||||||||
| Issuance of common stock upon exercise or vesting of equity awards |
— | — | — | — | — | |||||||||||||||||||||||
| Vesting of restricted stock - earnouts |
— | — | ( |
) | — | — | — | — | ||||||||||||||||||||
| Tax withholdings related to net share settlement of equity awards |
— | — | ( |
) | — | — | — | ( |
) | |||||||||||||||||||
| Founder shares repurchase |
— | — | ( |
) | — | — | — | ( |
) | |||||||||||||||||||
| Issuance of warrants to purchase Series D convertible preferred stock |
— | — | — | — | — | |||||||||||||||||||||||
| Issuance of Series D and B convertible preferred stock upon exercise of warrants |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Issuance of Series E convertible preferred stock in exchange for warrants |
— | — | — | — | — | — | — | |||||||||||||||||||||
| Issuance of common stock for a business acquisition |
— | — | — | — | — | |||||||||||||||||||||||
| Issuance of common stock upon reverse recapitalization, net of offering costs (Note 3) |
— | — | — | — | — | |||||||||||||||||||||||
| Assumption of Public and Private Placement Warrants |
— | — | ( |
) | — | — | — | ( |
) | |||||||||||||||||||
| Contributions from non-controlling interests |
— | — | — | — | — | |||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | |||||||||||||||||||||||
| Foreign currency translation |
— | — | — | — | ( |
) | — | ( |
) | |||||||||||||||||||
| Net loss |
— | — | — | ( |
) | — | ( |
) | ( |
) | ||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance as of December 31, 2021 |
$ | $ | $ | ( |
) | $ | ( |
) | $ | $ | ||||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
Year Ended December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net loss |
$ |
( |
) |
$ |
( |
) |
$ |
( |
) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
||||||||||||
| Stock-based compensation |
||||||||||||
| Non-cash equity consideration |
( |
) |
||||||||||
| Loss on equity method investments |
||||||||||||
| Loss on investments |
||||||||||||
| Change in fair value of loans receivable |
( |
) |
( |
) | ||||||||
| Change in fair value of warrant liabilities |
( |
) |
||||||||||
| Other non-cash activity |
( |
) |
( |
) | ||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable ($ |
( |
) |
( |
) |
( |
) | ||||||
| Prepaid expenses and other current assets |
( |
) |
( |
) |
( |
) | ||||||
| Inventory |
( |
) |
( |
) |
||||||||
| Other non-current assets |
( |
) |
( |
) | ||||||||
| Accounts payable |
( |
) |
||||||||||
| Accrued expenses and other current liabilities |
||||||||||||
| Deferred revenue, current and non-current ($ |
( |
) |
( |
) |
||||||||
| Deferred rent, non-current |
||||||||||||
| Other non-current liabilities |
||||||||||||
| |
|
|
|
|
|
|||||||
| Net cash used in operating activities |
( |
) | ( |
) | ( |
) | ||||||
| |
|
|
|
|
|
|||||||
| Cash flows from investing activities: |
||||||||||||
| Purchases of property and equipment |
( |
) |
( |
) |
( |
) | ||||||
| Purchases and issuances of loan receivable |
( |
) |
( |
) | ||||||||
| Proceeds from loans receivable |
||||||||||||
| Purchase of investments |
( |
) |
( |
) | ||||||||
| Business acquisition, net of cash acquired |
( |
) | ||||||||||
| |
|
|
|
|
|
|||||||
| Net cash used in investing activities |
( |
) | ( |
) | ( |
) | ||||||
| |
|
|
|
|
|
|||||||
| Cash flows from financing activities: |
||||||||||||
| Proceeds from reverse recapitalization, net of redemptions of $ |
||||||||||||
| Proceeds from exercise of stock options |
||||||||||||
| Repurchases of common stock |
( |
) |
( |
) | ||||||||
| Taxes paid related to net share settlement of equity awards |
( |
) |
||||||||||
| Principal payments on capital leases and lease financing obligation |
( |
) |
( |
) |
( |
) | ||||||
| Proceeds from lease financing obligation |
||||||||||||
| Contributions from non-controlling interests |
||||||||||||
| Proceeds from issuance of convertible promissory notes, net of issuance costs |
||||||||||||
| Proceeds from issuance of Series E convertible preferred stock, net of issuance costs |
||||||||||||
| |
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
||||||||||||
| |
|
|
|
|
|
|||||||
| Effect of foreign exchange rates on cash and cash equivalents |
( |
) | ||||||||||
| |
|
|
|
|
|
|||||||
| Net increase (decrease) in cash, cash equivalents and restricted cash |
( |
) |
||||||||||
| Cash, cash equivalents and restricted cash, beginning of period |
||||||||||||
| |
|
|
|
|
|
|||||||
| Cash, cash equivalents and restricted cash, end of period |
$ | $ | $ | |||||||||
| |
|
|
|
|
|
|||||||
Year Ended December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
| Supplemental disclosure of cash flow information: |
||||||||||||
| Cash paid for interest |
$ | $ | $ | |||||||||
| Cash paid for income taxes |
$ | $ | $ | |||||||||
| Supplemental disclosure of non-cash investing and financing activities: |
||||||||||||
| Purchases of equipment through capital leases |
$ | $ | $ | |||||||||
| Purchases of property and equipment included in accounts payable and accrued expenses |
$ | $ | $ | |||||||||
| Equity received in related parties |
$ | $ | $ | |||||||||
| Purchase of non-marketable equity securities |
$ | $ | $ | |||||||||
| Issuance of common stock for a business acquisition |
$ | $ | $ | |||||||||
| Acquisition date fair value of contingent consideration |
$ | $ | $ | |||||||||
| Purchases and issuances of loans receivable |
$ | $ | $ | |||||||||
| Initial fair value of warrant liabilities |
$ | $ | $ | |||||||||
| Conversion of convertible promissory notes to preferred stock |
$ | $ | $ | |||||||||
| Series E convertible preferred stock issuance costs included in accrued expenses |
$ | $ | $ | |||||||||
| Lease financing obligation for build-to-suit |
$ | $ | $ | |||||||||
As of December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
| Cash and cash equivalents |
$ | $ | $ | |||||||||
| Restricted cash |
||||||||||||
| |
|
|
|
|
|
|||||||
| Total cash, cash equivalents and restricted cash |
$ | $ | $ | |||||||||
| |
|
|
|
|
|
|||||||
Estimated Useful Life | ||
| Computer equipment and software |
||
| Furniture and fixtures |
||
| Lab equipment |
||
| Facilities |
||
| Vehicles |
||
| Leasehold improvements |
| • | Level 1- Quoted prices (unadjusted) in active markets for identical assets or liabilities; |
| • | Level 2- Inputs other than quoted prices included within Level 1 that are either directly or indirectly observable; and |
| • | Level 3- Unobservable inputs in which little or no market activity exists, therefore requiring an entity to develop its own assumptions about the assumptions that market participants would use in pricing. |
| • | $ |
| • | $ |
| • | $ |
| • | $ |
| Cash - |
$ | |||
| Cash - |
||||
| Less: Payment of underwriter fees and other offering costs |
( |
) | ||
| |
|
|||
| Net proceeds from the Business Combination |
$ | |||
| |
|
| SRNG shares outstanding prior to the Business Combination |
||||
| Less: redemption of SRNG shares prior to the Business Combination |
( |
) | ||
| Less: SRNG shares forfeited |
( |
) | ||
| |
|
|||
| Common stock of SRNG (1) |
||||
| Shares issued pursuant to the PIPE Investment |
||||
| |
|
|||
| Business Combination and PIPE Investment shares |
||||
| Conversion of Old Ginkgo Series B preferred stock to common stock |
||||
| Conversion of Old Ginkgo Series C preferred stock to common stock |
||||
| Conversion of Old Ginkgo Series D preferred stock to common stock |
||||
| Conversion of Old Ginkgo Series E preferred stock to common stock |
||||
| Conversion of Old Ginkgo common stock (2) |
||||
| |
|
|||
| Total shares of New Ginkgo common stock outstanding immediately following the Business Combination |
||||
| |
|
| (1) | Includes |
| (2) | Excludes |
| Cash |
$ | |||
| Fair value of Class A common stock |
||||
| Contingent consideration |
||||
| |
|
|||
| Total Dutch DNA consideration |
$ | |||
| |
|
| Cash |
$ | |||
| Accounts receivable |
||||
| Prepaid expenses and other current assets |
||||
| Property, plant and equipment |
||||
| Intangibles (1) |
||||
| Goodwill (2) |
||||
| Accounts payable |
( |
) | ||
| Accrued expenses and other current liabilities |
( |
) | ||
| Other non-current liabilities |
( |
) | ||
| |
|
|||
| Net assets acquired |
$ | |||
| |
|
| (1) | Estimated useful life of |
| (2) | Non-deductible for tax purposes. |
As of December 31, 2021 |
||||||||||||||||
Total |
Level 1 |
Level 2 |
Level 3 |
|||||||||||||
Assets: |
||||||||||||||||
Money market funds, included in cash and cash equivalents |
$ | $ | $ | $ | ||||||||||||
Synlogic, Inc. common stock, included in investments |
||||||||||||||||
Synlogic, Inc. warrants, included in investments |
||||||||||||||||
Cronos Group Inc. common stock, included in investments |
||||||||||||||||
Loans receivable, included in prepaid expenses and other current assets |
||||||||||||||||
Total assets |
$ | $ | $ | $ | ||||||||||||
Liabilities: |
||||||||||||||||
Public Warrants, included in warrant liabilities |
$ | $ | $ | $ | ||||||||||||
Private Placement Warrants, included in warrant liabilities |
||||||||||||||||
Contingent consideration, included in other non-current liabilities |
||||||||||||||||
Total liabilities |
$ | $ | $ | $ | ||||||||||||
As of December 31, 2020 |
||||||||||||||||
Total |
Level 1 |
Level 2 |
Level 3 |
|||||||||||||
Assets: |
||||||||||||||||
Money market funds, included in cash and cash equivalents |
$ | $ | $ | $ | ||||||||||||
Synlogic, Inc. common stock, included in investments |
||||||||||||||||
Synlogic, Inc. warrants, included in investments |
||||||||||||||||
Loans receivable, included in prepaid expenses and other current assets |
||||||||||||||||
Loans receivable, net of current portion |
||||||||||||||||
Total assets |
$ | $ | $ | $ | ||||||||||||
2021 |
2020 |
|||||||
Balance at January 1 |
$ | $ | ||||||
Purchases and issuances |
||||||||
Proceeds from loans receivable |
( |
) | ( |
) | ||||
Conversion of promissory notes |
( |
) | ||||||
Change in fair value |
( |
) | ||||||
Balance at December 31 |
$ | $ | ||||||
September 16, 2021 (Closing) |
December 31, 2021 |
|||||||
Exercise price |
$ | $ | ||||||
Stock price |
$ | $ | ||||||
Volatility |
% | % | ||||||
Term (in years) |
||||||||
Risk-free interest rate |
% | % | ||||||
2021 |
||||
Balance at January 1 |
$ |
|||
Additions pursuant to the Business Combination |
||||
Change in fair value |
( |
) | ||
Balance at December 31 |
$ |
|||
2021 |
||||
Balance at January 1 |
$ |
|||
Acquisition |
||||
Change in fair value |
( |
) | ||
Balance at December 31 |
$ |
|||
As of December 31, |
||||||||
2021 |
2020 |
|||||||
| Prepaid expenses |
$ |
$ |
||||||
| Prepaid insurance |
||||||||
| Prepaid inventory |
||||||||
| Loans receivable |
||||||||
| Other receivables |
||||||||
| Other current assets |
||||||||
| |
|
|
|
|||||
| Prepaid expenses and other current assets |
$ |
$ |
||||||
| |
|
|
|
|||||
As of December 31, |
||||||||
2021 |
2020 |
|||||||
| Finished goods |
$ |
$ |
||||||
| Raw materials |
||||||||
| Work in process |
||||||||
| Less: Inventory reserve |
( |
) |
( |
) | ||||
| |
|
|
|
|||||
| Inventory, net |
$ |
$ |
||||||
| |
|
|
|
|||||
As of December 31, |
||||||||
2021 |
2020 |
|||||||
| Facilities |
$ | $ | ||||||
| Furniture and fixtures |
||||||||
| Lab equipment |
||||||||
| Computer equipment and software |
||||||||
| Leasehold improvements |
||||||||
| Construction in progress |
||||||||
| Vehicles |
||||||||
| |
|
|
|
|||||
| Total property and equipment |
||||||||
| Less: Accumulated depreciation |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Property and equipment, net |
$ | $ | ||||||
| |
|
|
|
|||||
As of December 31, |
||||||||
2021 |
2020 |
|||||||
| Investments: |
||||||||
| Genomatica, Inc. preferred stock |
$ | $ | ||||||
| Synlogic, Inc. common stock |
||||||||
| Synlogic, Inc. warrants |
||||||||
| Cronos Group Inc. common stock |
||||||||
| Non-marketable equity securities |
||||||||
| |
|
|
|
|||||
| Total |
$ | $ | ||||||
| |
|
|
|
|||||
| Equity method investments (1) : |
||||||||
| Joyn Bio, LLC |
$ | $ | ||||||
| Other |
||||||||
| |
|
|
|
|||||
| Total |
$ |
$ |
||||||
| |
|
|
|
|||||
| (1) | Equity method investments in Platform Ventures with a carrying value of |
Year Ended December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
| (Loss) gain on investments: |
||||||||||||
| Synlogic, Inc. common stock |
$ | $ | ( |
) | $ | ( |
) | |||||
| Synlogic, Inc. warrants |
( |
) | ( |
) | ||||||||
| Cronos Group Inc. |
( |
) | ||||||||||
| |
|
|
|
|
|
|||||||
| Total |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| |
|
|
|
|
|
|||||||
| Loss on equity method investments: |
||||||||||||
| Joyn Bio, LLC |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| Allonnia, LLC |
( |
) | ( |
) | ||||||||
| Arcaea, LLC |
( |
) | ||||||||||
| Glycosyn, LLC |
( |
) | ||||||||||
| |
|
|
|
|
|
|||||||
| Total |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
| |
|
|
|
|
|
|||||||
As of December 31, |
||||||||
2021 |
2020 |
|||||||
| Cash and cash equivalents |
$ | $ | — | |||||
| Prepaid expenses and other current assets |
— | |||||||
| Equity method investments |
||||||||
| |
|
|
|
|||||
| Total assets |
$ | $ | ||||||
| |
|
|
|
|||||
| Accounts payable |
$ |
$ |
— |
|||||
| Accrued expenses and other current liabilities |
— |
|||||||
| |
|
|
|
|||||
| Total liabilities |
$ |
$ |
— |
|||||
| |
|
|
|
|||||
As of December 31, |
||||||||
2021 |
2020 |
|||||||
Beginning balance |
$ | $ | ||||||
Goodwill acquired in Dutch DNA acquisition |
||||||||
Measurement period adjustment (see Note 4) |
||||||||
Currency translation |
( |
) | ||||||
Ending balance |
$ | $ | ||||||
Weighted Average Amortization Period |
Gross Carrying Value |
Accumulated Amortization |
Net (1) |
|||||||||||||
Balances as of December 31, 2021 |
||||||||||||||||
Acquired technology |
$ |
$ |
( |
) |
$ |
|||||||||||
Balances as of December 31, 2020 |
||||||||||||||||
Acquired technology |
$ |
$ |
( |
) |
$ |
|||||||||||
| (1) | Includes a decrease of $ |
As of December 31, |
||||||||
2021 |
2020 |
|||||||
Employee compensation and benefits |
$ | $ | ||||||
Professional fees |
||||||||
Property and equipment |
||||||||
Product revenue accruals |
||||||||
Biosecurity service revenue accruals |
||||||||
Inventory related accruals |
||||||||
Lab supplies |
||||||||
External research and development expenses |
||||||||
Liability classified stock-based compensation (Note 18) |
||||||||
Capital lease obligation |
||||||||
Other current liabilities |
||||||||
Accrued expenses and other current liabilities |
$ | $ | ||||||
Years Ending December 31, |
Minimum Lease Payments (1) |
|||
| 2022 |
$ | |||
| 2023 |
||||
| 2024 |
||||
| 2025 |
||||
| 2026 |
||||
| Thereafter |
||||
| |
|
|||
| Total |
$ | |||
| |
|
|||
| (1) | Excluded from the table above is $ build-to-suit |
Years Ending December 31, |
Minimum Lease Payments |
|||
| 2022 |
$ | |||
| 2023 |
||||
| 2024 |
||||
| 2025 |
||||
| 2026 |
||||
| Thereafter |
||||
| |
|
|||
| Total noncancelable payments |
$ | |||
| Less: Imputed interest expense |
( |
) | ||
| |
|
|||
| Present value of future minimum lease payments |
$ | |||
| |
|
|||
Contract Years |
Minimum Purchase Commitment |
|||
| October 1, 2020 - March 31, 2022 |
||||
| April 1, 2022 - March 31, 2023 |
||||
| April 1, 2023 - March 31, 2024 |
||||
| April 1, 2024 - March 31, 2025 |
||||
| Thereafter |
||||
| |
|
|||
| Total |
$ |
|||
| |
|
|||
| • | in whole and not in part |
| • | at a price of $ |
| • | upon not less than |
| • | if, and only if, the reported closing price of the ordinary shares equals or exceeds $ sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) for any 30 -trading day period ending three business days before the Company sends the notice of redemption to the warrant holders. |
December 31, 2021 |
||||
| Stock options issued and outstanding |
||||
| Restricted stock units outstanding |
||||
| Shares available for grant under the 2021 Plan |
||||
| Shares available for grant under the ESPP |
||||
| Warrants to purchase Class A common stock |
||||
| |
|
|||
| Total common stock reserved for future issuances (1) |
||||
| |
|
|||
(1) |
Excludes unvested earnout shares which are restricted shares issued to equity holders of Old Ginkgo as part of the Business Combination (Note 3) and are recorded in equity as shares outstanding upon satisfying the vesting conditions. |
Number of Shares |
Weighted Average Exercise Price per Share |
Weighted Average Remaining Contractual Term |
Aggregate Intrinsic Value (1) |
|||||||||||||
(in years) |
(in thousands) |
|||||||||||||||
Outstanding as of December 31, 2020 |
$ | |||||||||||||||
Granted |
$ | |||||||||||||||
Exercised |
( |
) | $ | |||||||||||||
Forfeited |
( |
) | $ | |||||||||||||
Outstanding as of December 31, 2021 |
$ | $ | ||||||||||||||
Exercisable as of December 31, 2021 |
$ | $ | ||||||||||||||
| (1) | The aggregate intrinsic value is calculated as the difference between the Company’s closing stock price on the last trading day of the year and the exercise prices, multiplied by the number of in-the-money |
Year Ended December 31, 2021 |
||||
Risk-free interest rate |
% | |||
Dividend yield |
% | |||
Expected volatility |
% | |||
Expected term (in years) |
||||
Restricted Stock Units |
Restricted Stock Awards |
|||||||||||||||
Number of Shares |
Weighted Average Grant Date Fair Value (1) |
Number of Shares |
Weighted Average Grant Date Fair Value |
|||||||||||||
Nonvested as of December 31, 2020 |
$ | $ | ||||||||||||||
Granted |
$ | — | ||||||||||||||
Vested |
( |
) | $ | ( |
) | $ | ||||||||||
Forfeited |
( |
) | $ | |||||||||||||
Nonvested as of December 31, 2021 |
$ | $ | ||||||||||||||
| (1) | The weighted average grant date fair value of awards nonvested as of December 31, 2020 and awards forfeited prior to the modification date reflect the original grant date fair value and not the modification-date fair value. |
Year Ended December 31, 2021 |
||||
Risk-free interest rate |
||||
Expected volatility |
||||
Expected term (in years) |
||||
Dividend yield |
||||
Number of Shares |
Weighted Average Grant Date Fair Value |
|||||||
Nonvested as of December 31, 2020 |
||||||||
Granted |
$ | |||||||
Vested |
( |
) | $ | |||||
Forfeited |
( |
) | $ | |||||
Nonvested as of December 31, 2021 |
$ | |||||||
Year Ended December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
Research and development |
$ | $ | $ | |||||||||
General and administrative |
||||||||||||
Total |
$ | $ | $ | |||||||||
Year Ended December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
Consumer and technology |
% | % | % | |||||||||
Food and nutrition |
% | % | % | |||||||||
Industrial and environment |
% | % | % | |||||||||
Agriculture |
% | % | % | |||||||||
Pharma and Biotech |
% | % | % | |||||||||
Government and Defense |
% | % | % | |||||||||
Total Foundry revenue |
% | % | % | |||||||||
Year Ended December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
Domestic |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
Foreign |
( |
) | ||||||||||
Total |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
Year Ended December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
Current state income tax |
$ | $ | $ | |||||||||
Deferred federal income tax |
( |
) | ||||||||||
Deferred state income tax |
( |
) | ||||||||||
Deferred foreign income tax |
( |
) | ||||||||||
Income tax (benefit) expense |
$ | ( |
) | $ | $ | |||||||
Year Ended December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
Federal income tax at statutory rate |
% | % | % | |||||||||
State income tax |
% | % | % | |||||||||
Change in valuation allowance |
( |
)% | ( |
)% | ( |
)% | ||||||
Executive compensation |
( |
)% | ||||||||||
Equity investments |
( |
)% | ( |
)% | ( |
)% | ||||||
Tax credits |
% | % | % | |||||||||
Non-deductible expenses and change in fair value of warrant liability |
% | ( |
)% | ( |
)% | |||||||
Other expenses |
( |
)% | % | % | ||||||||
Effective tax rate |
% | ( |
)% | |||||||||
Year Ended December 31, |
||||||||
2021 |
2020 |
|||||||
Deferred tax assets: |
||||||||
Net operating loss carryforwards |
$ | $ | ||||||
Tax credit carryforwards |
||||||||
Accrued expenses |
||||||||
Deferred revenue |
||||||||
Stock-based compensation |
||||||||
Amortizable intangibles |
||||||||
Tenant allowance |
||||||||
Deferred tax assets before valuation allowance |
||||||||
Valuation allowance |
( |
) | ( |
) | ||||
Deferred tax assets, net of valuation allowance |
||||||||
Deferred tax liabilities: |
||||||||
Amortizable intangibles |
( |
) | ||||||
Property and equipment |
( |
) | ( |
) | ||||
Basis differences |
( |
) | ( |
) | ||||
Deferred tax liabilities |
( |
) | ( |
) | ||||
Net deferred taxes |
$ | ( |
) | $ | ( |
) | ||
Beginning of Period |
Additions |
End of Period |
||||||||||
Deferred tax assets valuation allowance: |
||||||||||||
Year ended December 31, 2021 |
$ |
$ |
$ |
|||||||||
Year ended December 31, 2020 |
$ |
$ |
$ |
|||||||||
Year ended December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
Numerator: |
||||||||||||
Net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders, basic |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
Change in fair value of warrant liabilities |
$ | |||||||||||
Net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders, diluted |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
Denominator |
||||||||||||
Weighted average common shares outstanding, basic |
||||||||||||
Weighted average effect of dilutive outstanding warrants |
||||||||||||
Weighted average common shares outstanding, diluted |
||||||||||||
Basic net loss per share |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
Diluted net loss per share |
$ | ( |
) | $ | ( |
) | $ | ( |
) | |||
As of December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
Warrants to purchase Class A common stock |
||||||||||||
Outstanding stock options |
||||||||||||
Unvested RSUs |
||||||||||||
Unvested RSAs |
||||||||||||
New Ginkgo and Sponsor earnout shares (1) |
||||||||||||
(1) |
Represents earnout shares for which the vesting conditions have not been satisfied. |
As of December 31, |
||||||||
2021 |
2020 |
|||||||
| Accounts receivable: |
||||||||
| Joyn |
$ | $ | ||||||
| Motif |
||||||||
| Genomatica |
||||||||
| Allonnia |
||||||||
| Arcaea |
||||||||
| |
|
|
|
|||||
$ |
$ |
|||||||
| |
|
|
|
|||||
| Deferred revenue, current and non-current: |
||||||||
| Joyn |
$ | $ | ||||||
| Motif |
||||||||
| Genomatica |
||||||||
| Allonnia |
||||||||
| Arcaea |
||||||||
| Other equity investees |
||||||||
| |
|
|
|
|||||
| $ | $ | |||||||
| |
|
|
|
|||||
Year Ended December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
| Foundry revenue: |
||||||||||||
| Joyn |
$ | $ | $ | |||||||||
| Motif |
||||||||||||
| Genomatica |
||||||||||||
| Allonnia |
— | |||||||||||
| Arcaea |
— | — | ||||||||||
| Other equity investees |
||||||||||||
| |
|
|
|
|
|
|||||||
| $ | $ | $ | ||||||||||
| |
|
|
|
|
|
|||||||
• |
Foundry consists of research and development services performed under collaboration and license agreements relating to the Company’s cell programming platform. The Company’s cell programming platform includes two core assets: the Foundry, highly efficient biology lab facilities, enabled by investment in proprietary workflows, custom software, robotic automation, and data science and analytics, which is paired with the Company’s Codebase, a collection of biological “parts” and a database of biological data used to program cells. In addition to costs incurred under collaboration and license agreements with customers, the Foundry segment also includes costs incurred for the development, operation, expansion and enhancement of the Foundry and Codebase. Foundry revenue is derived from Foundry usage fees and downstream value share in the form of milestone payments, royalties or equity interests. |
• |
Launched in 2020, Biosecurity consists of COVID-19 testing products and services primarily provided to public health authorities. Biosecurity revenue is derived from sales of test kits and testing and reporting services fees. |
Year ended December 31, |
||||||||||||
2021 |
2020 |
2019 |
||||||||||
| Revenue: |
||||||||||||
| Foundry |
$ |
$ |
$ |
|||||||||
| Biosecurity |
— |
|||||||||||
| |
|
|
|
|
|
|||||||
| Total revenue |
||||||||||||
| Segment cost of revenue: |
||||||||||||
| Biosecurity |
— |
|||||||||||
| Segment research and development expense: |
||||||||||||
| Foundry |
||||||||||||
| Biosecurity |
— |
|||||||||||
| |
|
|
|
|
|
|||||||
| Total segment research and development expense |
||||||||||||
| Segment general and administrative expense: |
||||||||||||
| Foundry |
||||||||||||
| Biosecurity |
— |
|||||||||||
| |
|
|
|
|
|
|||||||
| Total segment general and administrative expense |
||||||||||||
| Segment operating income (loss): |
||||||||||||
| Foundry |
( |
) |
( |
) |
( |
) | ||||||
| Biosecurity |
( |
) |
— |
|||||||||
| |
|
|
|
|
|
|||||||
| Total segment operating loss |
( |
) |
( |
) |
( |
) | ||||||
| |
|
|
|
|
|
|||||||
| Operating expenses not allocated to segments: |
||||||||||||
| Stock-based compensation (1) |
||||||||||||
| Depreciation and amortization |
||||||||||||
| Change in fair value of contingent consideration liability |
( |
) |
— |
— |
||||||||
| |
|
|
|
|
|
|||||||
| Loss from operations |
$ |
( |
) |
$ |
( |
) |
$ |
( |
) | |||
| |
|
|
|
|
|
|||||||
(1) |
Includes $ |
As of June 30, 2022 |
As of December 31, 2021 |
|||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | $ | ||||||
| Accounts receivable, net |
||||||||
| Accounts receivable - related parties |
||||||||
| Inventory, net |
||||||||
| Prepaid expenses and other current assets ($ |
||||||||
| |
|
|
|
|||||
| Total current assets |
||||||||
| Property and equipment, net |
||||||||
| Investments |
||||||||
| Equity method investments |
||||||||
| Intangible assets, net |
||||||||
| Goodwill |
||||||||
| Other non-current assets |
||||||||
| |
|
|
|
|||||
| Total assets |
$ | $ | ||||||
| |
|
|
|
|||||
| Liabilities and Stockholders’ Equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | $ | ||||||
| Deferred revenue ($ |
||||||||
| Accrued expenses and other current liabilities |
||||||||
| |
|
|
|
|||||
| Total current liabilities |
||||||||
| Non-current liabilities: |
||||||||
| Deferred rent, net of current portion |
||||||||
| Deferred revenue, net of current portion ($ |
||||||||
| Lease financing obligation |
||||||||
| Warrant liabilities |
||||||||
| Other non-current liabilities |
||||||||
| |
|
|
|
|||||
| Total liabilities |
||||||||
| |
|
|
|
|||||
| Commitments and contingencies (Note 8) |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $ |
||||||||
| Common stock, $ |
||||||||
| Additional paid-in capital |
||||||||
| Accumulated deficit |
( |
) | ( |
) | ||||
| Accumulated other comprehensive loss |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Total Ginkgo Bioworks Holdings, Inc. stockholders’ equity |
||||||||
| Non-controlling interest |
||||||||
| |
|
|
|
|||||
| Total stockholders’ equity |
||||||||
| |
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | $ | ||||||
| |
|
|
|
|||||
Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
2022 |
2021 |
2022 |
2021 |
|||||||||||||
| Foundry revenue (1) |
$ | $ | $ | $ | ||||||||||||
| Biosecurity revenue: |
||||||||||||||||
| Product |
||||||||||||||||
| Service |
||||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total revenue |
||||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Costs and operating expenses: |
||||||||||||||||
| Cost of Biosecurity product revenue |
||||||||||||||||
| Cost of Biosecurity service revenue |
||||||||||||||||
| Research and development |
||||||||||||||||
| General and administrative |
||||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
||||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
| Other (expense) income: |
||||||||||||||||
| Interest income (expense), net |
( |
) | ( |
) | ||||||||||||
| Loss on equity method investments |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
| (Loss) gain on investments |
( |
) | ( |
) | ||||||||||||
| Change in fair value of warrant liabilities |
||||||||||||||||
| Gain on deconsolidation of subsidiary |
||||||||||||||||
| Other (expense) income, net |
( |
) | ||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total other (expense) income, net |
( |
) | ( |
) | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Loss before income taxes |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
| Income tax benefit |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Net loss |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
| Net loss attributable to non-controlling interest |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
| |
|
|
|
|
|
|
|
|||||||||
| Net loss per share attributable to Ginkgo Bioworks Holdings, Inc. common stockholders, basic and diluted (2) |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
| Weighted average common shares outstanding, basic and diluted (2) |
||||||||||||||||
| Comprehensive loss: |
||||||||||||||||
| Net loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
| Other comprehensive loss: |
||||||||||||||||
| Foreign currency translation adjustment |
( |
) | ( |
) | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Total other comprehensive loss |
( |
) | ( |
) | ||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| Comprehensive loss |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
| |
|
|
|
|
|
|
|
|||||||||
| (1) | Includes related party revenue of $ |
| (2) | Amounts for the three and six months ended June 30, 2021 have been retroactively restated for the reverse recapitalization as described in Note 1. |
Three Months Ended June 30, 2022 |
||||||||||||||||||||||||||||
Common Stock |
||||||||||||||||||||||||||||
Shares |
Amount |
Additional Paid-In Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Loss |
Non- Controlling Interest |
Total Stockholders’ Equity |
||||||||||||||||||||||
Balance as of March 31, 2022 |
$ | $ | $ | ( |
) | $ | ( |
) | $ | $ | ||||||||||||||||||
Issuance of common stock upon exercise or vesting of equity awards |
— | — | — | |||||||||||||||||||||||||
Issuance of common stock for business acquisitions |
— | — | — | |||||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | ||||||||||||||||||||||||
Foreign currency translation |
— | — | — | — | ( |
) | — | ( |
) | |||||||||||||||||||
Net loss |
— | — | — | ( |
) | — | ( |
) | ( |
) | ||||||||||||||||||
Balance as of June 30, 2022 |
$ | $ | $ | ( |
) | $ | ( |
) | $ | $ | ||||||||||||||||||
Six Months Ended June 30, 2022 |
||||||||||||||||||||||||||||
Common Stock |
||||||||||||||||||||||||||||
Shares |
Amount |
Additional Paid-In Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Loss |
Non- Controlling Interest |
Total Stockholders’ Equity |
||||||||||||||||||||||
Balance as of December 31, 2021 |
$ | $ | $ | ( |
) | $ | ( |
) | $ | $ | ||||||||||||||||||
Issuance of common stock upon exercise or vesting of equity awards |
— | — | — | |||||||||||||||||||||||||
Tax withholdings related to net share settlement of equity awards |
( |
) | — | ( |
) | — | — | — | ( |
) | ||||||||||||||||||
Issuance of common stock upon exercise of Public Warrants |
— | — | — | — | — | — | ||||||||||||||||||||||
Issuance of common stock for business acquisitions |
— | — | — | |||||||||||||||||||||||||
Deconsolidation of subsidiary |
— | — | — | — | — | ( |
) | ( |
) | |||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | ||||||||||||||||||||||||
Foreign currency translation |
— | — | — | — | ( |
) | — | ( |
) | |||||||||||||||||||
Net loss |
— | — | — | ( |
) | — | ( |
) | ( |
) | ||||||||||||||||||
Balance as of June 30, 2022 |
$ | $ | $ | ( |
) | $ | ( |
) | $ | $ | ||||||||||||||||||
Three Months Ended June 30, 2021 |
||||||||||||||||||||||||||||
Common Stock (1) |
||||||||||||||||||||||||||||
Shares |
Amount |
Additional Paid-In Capital (1) |
Accumulated Deficit |
Accumulated Other Comprehensive Loss |
Non- Controlling Interest |
Total Stockholders’ Equity |
||||||||||||||||||||||
Balance as of March 31, 2021 |
$ | $ | $ | ( |
) | $ | |
$ | $ | |||||||||||||||||||
Issuance of common stock upon exercise or vesting of equity awards |
— | — | — | — | ||||||||||||||||||||||||
Issuance of warrants to purchase Series D convertible preferred stock |
— | — | — | — | — | |||||||||||||||||||||||
Issuance of Series D convertible preferred stock upon exercise of warrants |
— | — | — | — | — | — | ||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | |||||||||||||||||||||||
Net loss |
— | — | — | ( |
) | — | ( |
) | ( |
) | ||||||||||||||||||
Balance as of June 30, 2021 |
$ | $ | $ | ( |
) | $ | $ | $ | ||||||||||||||||||||
Six Months Ended June 30, 2021 |
||||||||||||||||||||||||||||
Common Stock (1) |
||||||||||||||||||||||||||||
Shares |
Amount |
Additional Paid-In Capital (1) |
Accumulated Deficit |
Accumulated Other Comprehensive Loss |
Non- Controlling Interest |
Total Stockholders’ Equity |
||||||||||||||||||||||
Balance as of December 31, 2020 |
$ | $ | $ | ( |
) | $ | $ | $ | ||||||||||||||||||||
Issuance of common stock upon exercise or vesting of equity awards |
— | — | — | — | ||||||||||||||||||||||||
Issuance of warrants to purchase Series D convertible preferred stock |
— | — | — | — | — | |||||||||||||||||||||||
Issuance of Series D convertible preferred stock upon exercise of warrants |
— | — | — | — | — | — | ||||||||||||||||||||||
Stock-based compensation expense |
— | — | — | — | — | |||||||||||||||||||||||
Net loss |
— | — | — | ( |
) | — | ( |
) | ( |
) | ||||||||||||||||||
Balance as of June 30, 2021 |
$ | $ | $ | ( |
) | $ | $ | $ | ||||||||||||||||||||
| (1) | Balances presented were retroactively restated for the reverse recapitalization as described in Note 1. |
Six Months Ended June 30, |
||||||||
2022 |
2021 |
|||||||
Cash flows from operating activities: |
||||||||
Net loss |
$ | ( |
) | $ | ( |
) | ||
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
Depreciation and amortization |
||||||||
Stock-based compensation |
||||||||
Loss on equity method investments |
||||||||
Loss (gain) on investments |
( |
) | ||||||
Non-cash customer consideration |
( |
) | ||||||
Change in fair value of loans receivable |
( |
) | ||||||
Change in fair value of warrant liabilities |
( |
) | ||||||
Gain on deconsolidation of subsidiary (Note 5) |
( |
) | ||||||
In-process research and development |
||||||||
Other non-cash activity |
||||||||
Changes in operating assets and liabilities: |
||||||||
Accounts receivable ($ ( ) from related parties) |
( |
) | ( |
) | ||||
Prepaid expenses and other current assets |
||||||||
Inventory |
( |
) | ||||||
Other non-current assets |
( |
) | ( |
) | ||||
Accounts payable |
( |
) | ||||||
Accrued expenses and other current liabilities |
( |
) | ||||||
Deferred revenue, current and non-current ($( |
( |
) | ( |
) | ||||
Deferred rent, non-current |
||||||||
Other non-current liabilities |
( |
) | ||||||
Net cash used in operating activities |
( |
) | ( |
) | ||||
Cash flows from investing activities: |
||||||||
Cash acquired in acquisition |
||||||||
Purchase of convertible note (related party) |
( |
) | ||||||
Purchases of property and equipment |
( |
) | ( |
) | ||||
Purchase of marketable equity securities |
( |
) | ||||||
Deconsolidation of subsidiary - cash |
( |
) | ||||||
Prepayment for business acquisition |
( |
) | ||||||
Other |
||||||||
Net cash used in investing activities |
( |
) | ( |
) | ||||
Cash flows from financing activities: |
||||||||
Proceeds from exercise of stock options |
||||||||
Taxes paid related to net share settlement of equity awards |
( |
) | ||||||
Principal payments on capital leases and lease financing obligation |
( |
) | ( |
) | ||||
Contingent consideration payment |
( |
) | ||||||
Payment of deferred offering costs |
( |
) | ||||||
Net cash used in financing activities |
( |
) | ( |
) | ||||
Effect of foreign exchange rates on cash and cash equivalents |
( |
) | ||||||
Net decrease in cash, cash equivalents and restricted cash |
( |
) | ( |
) | ||||
Cash and cash equivalents, beginning of period |
||||||||
Restricted cash, beginning of period |
||||||||
Cash, cash equivalents and restricted cash, beginning of period |
||||||||
Cash and cash equivalents, end of period |
||||||||
Restricted cash, end of period |
||||||||
Cash, cash equivalents and restricted cash, end of period |
$ | $ | ||||||
Supplemental disclosure of non-cash investing and financing activities: |
||||||||
Purchases of equipment through capital leases |
$ | $ | ||||||
Purchases of property and equipment included in accounts payable and accrued expenses |
$ | $ | ||||||
Equity received in related parties |
$ | $ | ||||||
Convertible financial instruments received for Foundry services |
$ | $ | ||||||
Equity securities and warrants received for Foundry services |
$ | $ | ||||||
Lease financing obligation for build-to-suit |
$ | $ | ||||||
Common stock issued for business acquisition |
$ | $ | ||||||
Contingent consideration for business acquisition |
$ | $ | ||||||
Deferred offering costs in accounts payable and accrued expenses |
$ | $ | ||||||
Fair value of Class A common stock |
$ | |||
Contingent consideration - restricted stock |
||||
Contingent consideration - milestones |
||||
Total FGen consideration |
$ | |||
Cash and cash equivalents |
$ | |||
Accounts receivable |
||||
Other non-current assets |
||||
Property and equipment |
||||
Intangible assets (1) |
||||
Goodwill (2) |
||||
Accounts payable and accrued expenses |
( |
) | ||
Deferred revenue |
( |
) | ||
Deferred tax liability |
( |
) | ||
Net assets acquired |
$ | |||
| (1) | Estimated useful life of |
| (2) | Non-deductible for tax purposes. |
As of June 30, 2022 |
||||||||||||||||||
Classification |
Total |
Level 1 |
Level 2 |
Level 3 |
||||||||||||||
Assets: |
||||||||||||||||||
Money market funds |
Cash and cash equivalents |
$ | $ | $ | — | $ | — | |||||||||||
Synlogic, Inc. warrants (1) |
Investments |
— | — | |||||||||||||||
| Marketable equity securities (2) |
Investments | — | ||||||||||||||||
Loans receivable |
Prepaid expenses and other current assets | — | — | |||||||||||||||
Total assets |
$ | $ | $ | $ | ||||||||||||||
Liabilities: |
||||||||||||||||||
Public Warrants |
Warrant liabilities |
$ | $ | $ | — | $ | — | |||||||||||
Private Placement Warrants |
Warrant liabilities |
— | — | |||||||||||||||
Contingent consideration |
Accrued Expenses and Other Current Liabilities | — | — | |||||||||||||||
Contingent consideration |
Other non-current liabilities |
— | — | |||||||||||||||
Total liabilities |
$ | $ | $ | — | $ | |||||||||||||
As of December 31, 2021 |
||||||||||||||||||
Classification |
Total |
Level 1 |
Level 2 |
Level 3 |
||||||||||||||
Assets: |
||||||||||||||||||
Money market funds |
Cash and cash equivalents |
$ | $ | $ | — | $ | — | |||||||||||
Synlogic, Inc. warrants (1) |
Investments |
— | — | |||||||||||||||
| Marketable equity securities (2) |
Investments |
— | ||||||||||||||||
Loans receivable |
Prepaid expenses and other current assets | — | — | |||||||||||||||
Total assets |
$ | $ | $ | $ | ||||||||||||||
Liabilities: |
||||||||||||||||||
Public Warrants |
Warrant liabilities |
$ | $ | $ | — | $ | — | |||||||||||
Private Placement Warrants |
Warrant liabilities |
— | — | |||||||||||||||
Contingent consideration |
Other non-current liabilities |
— | — | |||||||||||||||
Total liabilities |
$ | $ | $ | — | $ | |||||||||||||
| (1) | The fair value of Synlogic, Inc. warrants is calculated as the quoted price of the underlying common stock, less the unpaid exercise price of the warrants. |
| (2) | Marketable equity securities classified as Level 2 reflect a discount for lack of marketability due to regulatory sales restrictions, which lapsed on a portion of the shares held during the six months ended June 30, 2022 and were reclassified as Level 1. |
2022 |
2021 |
|||||||
Balance at January 1 |
$ | $ | ||||||
Proceeds from loans receivable |
— | ( |
) | |||||
Change in fair value |
( |
) | ||||||
Balance at June 30 |
$ | $ | ||||||
June 30, 2022 |
December 31, 2021 |
|||||||
Exercise price |
$ | $ | ||||||
Stock price |
$ | $ | ||||||
Volatility |
% | % | ||||||
Term (in years) |
||||||||
Risk-free interest rate |
% | % | ||||||
2022 |
||||
Balance at January 1 |
$ | |||
Change in fair value |
( |
) | ||
Balance at June 30 |
$ | |||
June 30, 2022 |
December 31, 2021 |
|||||||||||
Contingent Consideration Liability |
Valuation Technique |
Unobservable Input |
Range |
Range |
||||||||
| Earnout payments (FGen and Dutch DNA acquisitions) | Probability-weighted present value | Probability of payment | ||||||||||
| Discount rate | ||||||||||||
| Earnout payments (Dutch DNA acquisition) | Discounted cash flow | Projected years of payments | ||||||||||
| Discount rate | ||||||||||||
2022 |
||||
Balance at January 1 |
$ | |||
Additions |
||||
Change in fair value |
||||
Settlements and payments |
( |
) | ||
Balance at June 30 |
$ | |||
As of June 30, |
As of December 31, |
|||||||
2022 |
2021 |
|||||||
Investments: |
||||||||
Genomatica, Inc. preferred stock |
$ | $ | ||||||
Synlogic, Inc. common stock |
||||||||
Synlogic, Inc. warrants |
||||||||
Marketable equity securities |
||||||||
Non-marketable equity securities |
||||||||
Total |
$ | $ | ||||||
Equity method investments (1) : |
||||||||
Joyn Bio, LLC |
$ | $ | ||||||
BiomEdit, LLC |
||||||||
Other |
||||||||
Total |
$ | $ | ||||||
| (1) | Equity method investments in Platform Ventures with a carrying value of |
Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
2022 |
2021 |
2022 |
2021 |
|||||||||||||
(Loss) gain on investments: |
||||||||||||||||
Synlogic, Inc. common stock |
$ | ( |
) | $ | $ | ( |
) | $ | ||||||||
Synlogic, Inc. warrants |
( |
) | ( |
) | ||||||||||||
Genomatica |
( |
) | ( |
) | ||||||||||||
Marketable equity securities |
( |
) | ( |
) | ||||||||||||
Total |
$ | ( |
) | $ | $ | ( |
) | $ | ||||||||
Loss on equity method investments: |
||||||||||||||||
Joyn Bio, LLC |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
Allonnia, LLC |
( |
) | ||||||||||||||
Arcaea, LLC |
( |
) | ||||||||||||||
Verb Biotics, LLC |
( |
) | ||||||||||||||
BiomEdit, LLC |
( |
) | ( |
) | ||||||||||||
Other |
( |
) | ( |
) | ||||||||||||
Total |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
As of June 30, |
As of December 31, |
|||||||
2022 |
2021 |
|||||||
Cash and cash equivalents |
$ | $ | ||||||
Prepaid expenses and other current assets |
||||||||
Equity method investments |
||||||||
Property and equipment, net |
— | |||||||
Other non-current assets |
— | |||||||
Total assets |
$ | $ | ||||||
Accounts payable |
$ | $ | ||||||
Accrued expenses and other current liabilities |
||||||||
Total liabilities |
$ | $ | ||||||
As of June 30, |
||||||||
2022 |
2021 |
|||||||
Cash and cash equivalents |
$ | $ | ||||||
Restricted cash included in prepaid expenses and other current assets (1) |
||||||||
Restricted cash included in other non-current assets (1) |
||||||||
Total cash, cash equivalents and restricted cash |
$ | $ | ||||||
| (1) | Includes cash balances collateralizing letters of credit associated with the Company’s facility leases and a customer prepayment requiring segregation and restrictions in its use in accordance with the customer agreement. |
As of June 30, |
As of December 31, |
|||||||
2022 |
2021 |
|||||||
Finished goods |
$ | $ | ||||||
Raw materials |
||||||||
Work in process |
||||||||
Less: inventory reserve |
( |
) | ( |
) | ||||
Inventory, net |
$ | $ | ||||||
As of June 30, |
As of December 31, |
|||||||
2022 |
2021 |
|||||||
Facilities |
$ | $ | ||||||
Furniture and fixtures |
||||||||
Lab equipment |
||||||||
Computer equipment and software |
||||||||
Leasehold improvements |
||||||||
Construction in progress |
||||||||
Vehicles |
||||||||
Total property and equipment |
||||||||
Less: Accumulated depreciation |
( |
) | ( |
) | ||||
Property and equipment, net |
$ | $ | ||||||
Authorized |
Issued |
Outstanding |
||||||||||
Common stock as of June 30, 2022: |
||||||||||||
Class A |
||||||||||||
Class B |
||||||||||||
Class C |
||||||||||||
Common stock as of December 31, 2021: |
||||||||||||
Class A |
||||||||||||
Class B |
||||||||||||
Class C |
||||||||||||
Balance as of December 31, 2021 |
$ | |||
Goodwill acquired in FGen acquisition |
||||
Impact of foreign currency translation |
( |
) | ||
Measurement period adjustment (1) |
||||
Balance as of June 30, 2022 |
$ | |||
| (1) | Reflects the final determination of net-working capital adjustments as of the acquisition date related to the Dutch DNA acquisition. |
Weighted Average Amortization Period |
Gross Carrying Amount (1) |
Accumulated Amortization (1) |
Net |
|||||||||||||
Balances as of June 30, 2022 |
||||||||||||||||
Acquired technology |
$ | $ | ( |
) | $ | |||||||||||
Balances as of December 31, 2021 |
||||||||||||||||
Acquired technology |
$ | $ | ( |
) | $ | |||||||||||
| (1) | Gross carrying amount and accumulated amortization include the impact of cumulative foreign currency translation adjustments. |
Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
2022 |
2021 |
2022 |
2021 |
|||||||||||||
Research and development |
$ | $ | $ | $ | ||||||||||||
General and administrative |
||||||||||||||||
Total |
$ | $ | $ | $ | ||||||||||||
Number of Shares |
Weighted Average Exercise Price per Share |
Weighted Average Remaining Contractual Term |
Aggregate Intrinsic Value (1) |
|||||||||||||
| (in years) | (in thousands) | |||||||||||||||
Outstanding as of December 31, 2021 |
$ | |||||||||||||||
Granted |
$ | |||||||||||||||
Exercised |
( |
) | $ | |||||||||||||
Outstanding as of June 30, 2022 |
$ | $ | ||||||||||||||
Exercisable as of June 30, 2022 |
$ | $ | ||||||||||||||
| (1) | The aggregate intrinsic value is calculated as the difference between the Company’s closing stock price on the last trading day of the quarter and the exercise prices, multiplied by the number of in-the-money |
Risk -free interest rate |
% | |||
Expected dividend yield |
% | |||
Expected volatility |
% | |||
Expected term |
Restricted Stock Units |
Restricted Stock Awards |
|||||||||||||||
Number of Shares |
Weighted Average Grant Date Fair Value (1) |
Number of Shares |
Weighted Average Grant Date Fair Value |
|||||||||||||
Nonvested as of December 31, 2021 |
$ | $ | ||||||||||||||
Granted |
$ | |||||||||||||||
Vested |
( |
) | $ | ( |
) | $ | ||||||||||
Forfeited |
( |
) | $ | |||||||||||||
Nonvested as of June 30, 2022 |
$ | $ | ||||||||||||||
| (1) | The weighted average grant date fair value of awards granted prior to the modification date reflect the modification-date fair value and not the original grant date fair value. |
Number of Shares |
Weighted Average Grant Date Fair Value |
|||||||
Nonvested as of December 31, 2021 |
$ | |||||||
Vested |
( |
) | $ | |||||
Forfeited |
( |
) | $ | |||||
Nonvested as of June 30, 2022 |
$ | |||||||
Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
2022 |
2021 |
2022 |
2021 |
|||||||||||||
Consumer and technology |
% | % | % | % | ||||||||||||
Pharma and biotech |
% | % | % | % | ||||||||||||
Food and nutrition |
% | % | % | % | ||||||||||||
Industrial and environment |
% | % | % | % | ||||||||||||
Agriculture |
% | % | % | % | ||||||||||||
Government and defense |
% | % | % | % | ||||||||||||
Total Foundry revenue |
% | % | % | % | ||||||||||||
| • | Foundry consists of research and development services performed under collaboration and license agreements relating to the Company’s cell programming platform. The Company’s cell programming platform includes two core assets: the Foundry, highly efficient biology lab facilities, enabled by investment in proprietary workflows, custom software, robotic automation, and data science and analytics, which is paired with the Company’s Codebase, a collection of biological “parts” and a |
database of biological data used to program cells. The Foundry segment includes costs incurred for the development, operation, expansion and enhancement of the Foundry and Codebase. Foundry revenue is derived from Foundry usage fees and downstream value share in the form of milestone payments, royalties or equity interests. |
| • | Biosecurity consists of COVID-19 testing products and services primarily provided to public health authorities. Biosecurity revenue is derived from sales of test kits and testing and reporting services fees. |
Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
2022 |
2021 |
2022 |
2021 |
|||||||||||||
Revenue: |
||||||||||||||||
Foundry |
$ | $ | $ | $ | ||||||||||||
Biosecurity |
||||||||||||||||
Total revenue |
||||||||||||||||
Segment cost of revenue: |
||||||||||||||||
Biosecurity |
||||||||||||||||
Segment research and development expense: |
||||||||||||||||
Foundry |
||||||||||||||||
Biosecurity |
||||||||||||||||
Total segment research and development expense |
||||||||||||||||
Segment general and administrative expense: |
||||||||||||||||
Foundry |
||||||||||||||||
Biosecurity |
||||||||||||||||
Total segment general and administrative expense |
||||||||||||||||
Segment operating income (loss): |
||||||||||||||||
Foundry |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
Biosecurity |
( |
) | ( |
) | ||||||||||||
Total segment operating income (loss) |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||
Operating expenses not allocated to segments: |
||||||||||||||||
Stock-based compensation (1) |
||||||||||||||||
Depreciation and amortization |
||||||||||||||||
Change in fair value of contingent consideration liability |
( |
) | ||||||||||||||
Loss from operations |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | ||||
| (1) | Includes $ |
As of June 30, |
||||||||
2022 |
2021 |
|||||||
| Warrants to purchase Class A common stock |
||||||||
| Outstanding stock options |
||||||||
| Unvested RSUs |
||||||||
| Unvested RSAs |
||||||||
| Earnout shares (1) |
||||||||
| |
|
|
|
|||||
| |
|
|
|
|||||
| (1) | Represents earnout shares for which the service-based vesting conditions and/or market conditions have not been met. |
As of June 30, 2022 |
As of December 31, 2021 |
|||||||
| Accounts receivable: |
||||||||
| Joyn |
$ | $ | ||||||
| Motif |
||||||||
| Allonnia |
||||||||
| Arcaea |
||||||||
| Verb |
||||||||
| |
|
|
|
|||||
| $ | $ | |||||||
| |
|
|
|
|||||
| Deferred revenue, current and non-current: |
||||||||
| Joyn |
$ | $ | ||||||
| Motif |
||||||||
| Genomatica |
||||||||
| Allonnia |
||||||||
| Arcaea |
||||||||
| Other equity investees |
||||||||
| |
|
|
|
|||||
| $ | $ | |||||||
| |
|
|
|
|||||
Three Months Ended June 30, |
Six Months Ended June 30, |
|||||||||||||||
2022 |
2021 |
2022 |
2021 |
|||||||||||||
| Foundry revenue: |
||||||||||||||||
| Joyn |
$ | $ | $ | $ | ||||||||||||
| Motif |
||||||||||||||||
| Genomatica |
||||||||||||||||
| Allonnia |
||||||||||||||||
| Arcaea |
||||||||||||||||
| Verb |
||||||||||||||||
| Other equity investees |
||||||||||||||||
| |
|
|
|
|
|
|
|
|||||||||
| $ | $ | $ | $ | |||||||||||||
| |
|
|
|
|
|
|
|
|||||||||

| • | Exercise professional judgment and maintain professional skepticism throughout the audit. |
| • | Identify and assess the risks of material misstatement of the financial statements, whether due to fraud or error, and design and perform audit procedures responsive to those risks. Such procedures include examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. |
| • | Obtain an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control. Accordingly, no such opinion is expressed. |
| • | Evaluate the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluate the overall presentation of the financial statements. |
| • | Conclude whether, in our judgment, there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s ability to continue as a going concern for a reasonable period of time. |
 |
Boston, Massachusetts |
February 28, 2022 |
| 2021 | 2020 | |||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | 34,801,297 | $ | 43,520,543 | ||||
Accounts receivable |
7,200 | — | ||||||
Prepaid expenses and other current assets |
3,015,017 | 3,015,817 | ||||||
Total assets |
$ | 37,823,514 | $ | 46,536,360 | ||||
Liabilities and Members’ Equity |
||||||||
Current liabilities: |
||||||||
Accounts payable |
$ | 631,291 | $ | 728,580 | ||||
Accrued expenses |
1,134,128 | 901,784 | ||||||
Total liabilities |
1,765,419 | 1,630,364 | ||||||
Members’ equity |
36,058,095 | 44,905,996 | ||||||
Total liabilities and members’ equity |
$ | 37,823,514 | $ | 46,536,360 | ||||
| 2021 | 2020 | |||||||
| Revenue: |
||||||||
| Revenue from consulting services |
$ | 507,200 | $ | — | ||||
| |
|
|
|
|||||
| Total revenue |
507,200 | — | ||||||
| |
|
|
|
|||||
| Operating expenses: |
||||||||
| Research and development: |
5,989,879 | 3,966,936 | ||||||
| General and administrative |
5,042,650 | 2,106,245 | ||||||
| |
|
|
|
|||||
| Total operating expenses |
11,032,529 | 6,073,181 | ||||||
| |
|
|
|
|||||
| Net operating loss |
(10,525,329 | ) | (6,073,181 | ) | ||||
| Interest income |
76,164 | 61,663 | ||||||
| |
|
|
|
|||||
| Net Loss |
$ | (10,449,165 | ) | $ | (6,011,518 | ) | ||
| |
|
|
|
|||||
| Units | Members’ Equity |
|||||||||||||||||||||||
| Series A-l Preferred |
Series A-2 Preferred |
Series A-3 Preferred |
Common | Incentive | ||||||||||||||||||||
| Balance as of December 31, 2019 |
2,970,000 | — | — | 3,600,000 | — | $ | 32,690,503 | |||||||||||||||||
| Issuance of Series A-1 Preferred Units net of issuance costs of $272,000 |
1,664,911 | — | — | — | — | 18,227,011 | ||||||||||||||||||
| Issuance of Series A-2 Preferred Units |
— | 180,000 | — | — | — | — | ||||||||||||||||||
| Issuance of Series A-3 Preferred Units |
— | — | 180,000 | — | — | — | ||||||||||||||||||
| Issuance of Incentive Units |
— | — | — | 140,000 | — | |||||||||||||||||||
| Net loss |
— | — | — | — | — | (6,011,518 | ) | |||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2020 |
4,634,911 | 180,000 | 180,000 | 3,600,000 | 140,000 | 44,905,996 | ||||||||||||||||||
| Issuance of Common Units |
— | — | — | 1,867,411 | — | — | ||||||||||||||||||
| Issuance of Series A-1 Preferred Units |
22,500 | — | — | — | — | 250,000 | ||||||||||||||||||
| Issuance of Series A-2 Preferred Units |
— | 60,000 | — | — | — | 666,667 | ||||||||||||||||||
| Issuance of Incentive Units |
— | — | — | — | 267,294 | — | ||||||||||||||||||
| Unit based compensation expense |
— | — | — | — | — | 684,597 | ||||||||||||||||||
| Net loss |
— | — | — | — | — | (10,449,165 | ) | |||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2021 |
4,657,411 | 240,000 | 180,000 | 5,467,411 | 407,294 | $ | 36,058,095 | |||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| 2021 | 2020 | |||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | (10,449,165 | ) | $ | (6,011,518 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Fair value of Series A-2 issued for services |
666,667 | — | ||||||
| Unit-based compensation expense |
684,597 | — | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable |
(7,200 | ) | — | |||||
| Prepaid expenses and other current assets |
800 | (3,012,433 | ) | |||||
| Accounts payable |
(97,289 | ) | 704,874 | |||||
| Accrued expenses |
232,344 | 783,936 | ||||||
| |
|
|
|
|||||
| Net cash used in operating activities |
(8,969,246 | ) | (7,535,141 | ) | ||||
| |
|
|
|
|||||
| Cash flows from financing activities: |
||||||||
| Proceeds from issuance of Series A-l Preferred Units, net of issuance costs |
250,000 | 18,227,011 | ||||||
| |
|
|
|
|||||
| Net cash provided by financing activities |
250,000 | 18,227,011 | ||||||
| Increase in cash and cash equivalents |
(8,719,246 | ) | 10,691,870 | |||||
| Cash and cash equivalents, beginning of year |
43,520,543 | 32,828,673 | ||||||
| |
|
|
|
|||||
| Cash and cash equivalents, end of year |
$ | 34,801,297 | $ | 43,520,543 | ||||
| |
|
|
|
|||||
1. |
NATURE OF OPERATIONS |
2. |
SIGNIFICANT ACCOUNTING POLICIES |
2. |
SIGNIFICANT ACCOUNTING POLICIES (continued) |
2. |
SIGNIFICANT ACCOUNTING POLICIES (concluded) |
| 2021 | 2020 | |||||||
| Risk-free interest rate |
1.26 | % | 1.67 | % | ||||
| Expected dividend yield |
0.00 | % | 0.00 | % | ||||
| Volatility factor |
70.00 | % | 70.00 | % | ||||
| Expected life |
3 years | 3 years | ||||||
3. |
LICENSE, RESEARCH AND DEVELOPMENT AND CONSULTING AGREEMENTS |
3. |
LICENSE, RESEARCH AND DEVELOPMENT AND CONSULTING AGREEMENTS (continued) |
3. |
LICENSE, RESEARCH AND DEVELOPMENT AND CONSULTING AGREEMENTS (concluded) |
4. |
MEMBERS’ EQUITY |
| a) | 180,000 units of Series A-2 will be issued upon execution by Battelle and the Company of a consulting agreement within 12 months following the date of this agreement, pursuant to which Battelle would provide $2,000,000 worth of technical and consulting services (see Note 3). |
| b) | Upon achieving the milestone (a) above, the Company will issue additional 180,000 units of Series A-2 in 30,000 increments on each of the following dates so long as the Company has not determined that Battelle did not provide the Company with services or that the services were not satisfactory pursuant to the Battelle Agreement: June 18, 2021; December 18, 2021; June 18, 2022; December 18, 2022; June 18, 2023; and December 18, 2023 (see Note 3). |
| c) | Upon achieving the milestone (a) above, the Company will issue additional 270,000 units of Series A-2 in 45,000 increments on the following dates so long as the Company has not determined that Battelle did not provide the Company with services or that the services were not satisfactory pursuant to the Battelle Agreement: June 18, 2024; December 18, 2024; June 18, 2025; December 18, 2025; June 18, 2026; and December 18, 2026. |
| a) | 45,000 units of Series A-3 will be treated as “eligible” if the Company enters into a joint development agreement (“JDA”) with a certain party within one year following the date on which |
4. |
MEMBERS’ EQUITY (continued) |
| Ginkgo delivers an intermediate strain to the Company pursuant to the Technical Development Plan between Ginkgo and the Company. Notwithstanding the foregoing, if the Company determines, in its reasonable discretion, within thirty days following the execution of the JDA, that the JDA was not substantially related to the contributed assets, as defined, or certain services pursuant to the Metabolik Agreement (see Note 3) then such 45,000 units of Series A-3 will instead be automatically forfeited for no consideration. |
| b) | 135,000 units of Series A-3 will be treated as “eligible” in the event that the Company enters into a certain commercialization agreement, as defined in the Metabolik Agreement, and receives revenue under the commercialization agreement in excess of $10,000,000 in the aggregate within five years following the execution of the commercialization agreement. |
| a) | 3,600,000 Common Units in partial consideration for the license and transfer of intellectual property in accordance with IPCA |
| b) | 5,400,000 Common Units in partial consideration for the additional obligations set forth in the IPCA and TDA (as defined in the IPCA); provided that Ginkgo will not receive such additional Common Units unless the Company has sold and issued at least 8,370,000 Series A-1 Preferred Units pursuant to a Series A-1 Preferred Unit Purchase Agreement. |
| c) | In the event the Company sells and issues less than 8,370,000 but more than 2,970,000 Series A-1 Preferred Units, the Company and Ginkgo will collaborate to determine how many additional Common Units will be issued. |
| Voting Rights |
| Distributions |
4. |
MEMBERS’ EQUITY (continued) |
4. |
MEMBERS’ EQUITY (concluded) |
| Incentive Units |
Weighted Average Grant Date Fair Value |
|||||||
Balance at December 31, 2020 |
140,000 | $ | 2.00 | |||||
Granted |
267,294 | 2.36 | ||||||
Exercised |
— | — | ||||||
Balance at December 31, 2021 |
407,294 | $ | 2.25 | |||||
5. |
RELATED PARTY TRANSACTIONS |
6. |
SUBSEQUENT EVENTS |
| /s/ Wolf & Company, P.C. |
| Boston, Massachusetts |
| June 23, 2021 |
Assets |
||||||||
| 2020 | 2019 | |||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | 43,520,543 | $ | 32,828,673 | ||||
Prepaid expenses and other current assets |
3,015,817 | 3,384 | ||||||
Total assets |
$ | 46,536,360 | $ | 32,832,057 | ||||
Liabilities and Members’ Equity |
||||||||
Current liabilities: |
||||||||
Accounts payable |
$ | 728,580 | $ | 23,706 | ||||
Accrued expenses |
901,784 | 117,848 | ||||||
Total liabilities |
1,630,364 | 141,554 | ||||||
Members’ equity |
44,905,996 | 32,690,503 | ||||||
Total liabilities and members’ equity |
$ | 46,536,360 | $ | 32,832,057 | ||||
| 2020 | 2019 | |||||||
Operating expenses: |
||||||||
Research and development |
$ | 3,966,936 | $ | 24,481,519 | ||||
General and administrative |
2,106,245 | 162,978 | ||||||
Total operating expenses |
6,073,181 | 24,644,497 | ||||||
Net operating loss |
(6,073,181 | ) | (24,644,497 | ) | ||||
Interest income |
61,663 | — | ||||||
Net loss |
$ | (6,011,518 | ) | $ | (24,644,497 | ) | ||
| Units | ||||||||||||||||||||||||
| Series A-1 Preferred |
Series A-2 Preferred |
Series A-3 Preferred |
Common | Incentive | Members’ Equity |
|||||||||||||||||||
| Balance at November 27, 2019 (Inception) |
— | — | — | — | — | $ |
— | |||||||||||||||||
| Issuance of Common Units |
— | — | — | 3,600,000 | — | 24,480,000 | ||||||||||||||||||
| Issuance of Series A-1 Preferred Units net of issuance costs of $145,000 |
2,970,000 | — | — | — | — | 32,855,000 | ||||||||||||||||||
| Net loss |
— | — | — | — | — | (24,644,497 | ) | |||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2019 |
2,970,000 | — | — | 3,600,000 | — | 32,690,503 | ||||||||||||||||||
| Issuance of Series A-1 Preferred Units net of issuance costs of $272,000 |
1,664,911 | — | — | — | — | 18,227,011 | ||||||||||||||||||
| Issuance of Series A-2 Preferred Units |
— | 180,000 | — | — | — | — | ||||||||||||||||||
| Issuance of Series A-3 Preferred Units |
— | — | 180,000 | — | — | — | ||||||||||||||||||
| Issuance of Incentive Units |
— | — | — | — | 140,000 | — | ||||||||||||||||||
| Net loss |
— | — | — | — | — | (6,011,518 | ) | |||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2020 |
4,634,911 | 180,000 | 180,000 | 3,600,000 | 140,000 | $ | 44,905,996 | |||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| 2020 | 2019 | |||||||
Cash flows from operating activities: |
||||||||
Net loss |
$ | (6,011,518 | ) | $ | (24,644,497 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
Fair value common stock issued for acquiring intellectual property Changes in operating assets and liabilities: |
— | 24,480,000 | ||||||
Prepaid expenses and other current assets |
(3,012,433 | ) | (3,384 | ) | ||||
Accounts payable |
704,874 | 23,706 | ||||||
Accrued expenses |
783,936 | 117,848 | ||||||
Net cash used in operating activities |
(7,535,141 | ) | (26,327 | ) | ||||
Cash flows from financing activities: |
||||||||
Proceeds from issuance of Series A-1 Preferred Units, net of issuance costs |
18,227,011 | 32,855,000 | ||||||
Net cash provided by financing activities |
18,227,011 | 32,855,000 | ||||||
Increase in cash and cash equivalents |
10,691,870 | 32,828,673 | ||||||
Cash and cash equivalents, beginning of period |
32,828,673 | — | ||||||
Cash and cash equivalents, end of period |
$ | 43,520,543 | $ | 32,828,673 | ||||
1. |
NATURE OF OPERATIONS |
2. |
SIGNIFICANT ACCOUNTING POLICIES |
2. |
SIGNIFICANT ACCOUNTING POLICIES (continued) |
2. |
SIGNIFICANT ACCOUNTING POLICIES (concluded) |
| Risk-free interest rate |
1.67 | % | ||
| Expected dividend yield |
0.00 | % | ||
| Volatility factor |
70.00 | % | ||
| Expected life |
3 years |
3. |
LICENSE, RESEARCH AND DEVELOPMENT AND CONSULTING AGREEMENTS |
3. |
LICENSE, RESEARCH AND DEVELOPMENT AND CONSULTING AGREEMENTS (concluded) |
4. |
MEMBERS’ EQUITY |
| a) | 180,000 units of Series A-2 will be issued upon execution by Battelle and the Company of a consulting agreement within 12 months following the date of this agreement, pursuant to which Battelle would provide $2,000,000 worth of technical and consulting services (see Note 3). |
4. |
MEMBERS’ EQUITY (continued) |
| b) | Upon achieving the milestone (a) above, the Company will issue additional 180,000 units of Series A-2 in 30,000 increments on each of the following dates so long as the Company has not determined that Battelle did not provide the Company with services or that the services were not satisfactory pursuant to the Battelle Agreement: June 18, 2021; December 18, 2021; June 18, 2022; December 18, 2022; June 18, 2023; and December 18, 2023. |
| c) | Upon achieving the milestone (a) above, the Company will issue additional 270,000 units of Series A-2 in 45,000 increments on the following dates so long as the Company has not determined that Battelle did not provide the Company with services or that the services were not satisfactory pursuant to the Battelle Agreement: June 18, 2024; December 18, 2024; June 18, 2025; December 18, 2025; June 18, 2026; and December 18, 2026. Notwithstanding the foregoing, on August 18, 2021 Battelle may elect to purchase all 270,000 units of Series A-2 in exchanges for a price per unit of $11.111111 and a total purchase price of $3,000,000 in lieu of the issuances of such units described above so long as Battelle has achieved milestone (a) above and the Battelle Agreement remains in effect. |
| a) | 45,000 units of Series A-3 A-3 |
| b) | 135,000 units of Series A-3 |
| a) | 3,600,000 Common Units in partial consideration for the license and transfer of intellectual property in accordance with IPCA |
4. |
MEMBERS’ EQUITY (continued) |
| b) | 5,400,000 Common Units in partial consideration for the additional obligations set forth in the IPCA and TDA (as defined in the IPCA); provided that Ginkgo will not receive such additional Common Units unless the Company has sold and issued at least 8,370,000 Series A-1 Preferred Units pursuant to a Series A-1 Preferred Unit Purchase Agreement. |
| c) | In the event the Company sells and issues less than 8,370,000 but more than 2,970,000 Series A-1 Preferred Units, the Company and Ginkgo will collaborate to determine how many additional Common Units will be issued. |
4. |
MEMBERS’ EQUITY (concluded) |
5. |
RELATED PARTY TRANSACTIONS |
6. |
SUBSEQUENT EVENTS |
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 13. | Other Expenses of Issuance and Distribution. |
The following table sets forth the estimated expenses to be borne by the registrant in connection with the issuance and distribution of the shares of common stock being registered hereby.
| Expense |
Estimated Amount |
|||
| Securities and Exchange Commission registration fee |
$ | 83,876.08 | ||
| Accounting fees and expenses |
||||
| Legal fees and expenses |
||||
| Financial printing and miscellaneous expenses |
||||
|
|
|
|||
| Total |
$ | |||
|
|
|
|||
| Item 14. | Indemnification of Directors and Officers. |
Section 145 of the Delaware General Corporation Law, or the DGCL, permits a corporation to indemnify its directors and officers against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlements actually and reasonably incurred by them in connection with any action, suit or proceeding brought by third parties. The directors or officers must have acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reason to believe their conduct was unlawful. In a derivative action, an action only by or in the right of the corporation, indemnification may be made only for expenses actually and reasonably incurred by directors and officers in connection with the defense or settlement of an action or suit, and only with respect to a matter as to which they must have acted in good faith and in a manner they reasonably believed to be in or not opposed to the best interests of the corporation. No indemnification may be made if such person must have been adjudged liable to the corporation, unless and only to the extent that the court in which the action or suit was brought must determine upon application that the defendant officers or directors are fairly and reasonably entitled to indemnity for such expenses despite such adjudication of liability. The current certificate of incorporation and the bylaw of the registrant provide for indemnification by the registrant of its directors, senior officers and employees to the fullest extent permitted by applicable law.
Section 102(b)(7) of the DGCL permits a corporation to provide in its charter that a director of the corporation must not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for liability (1) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (2) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (3) for payments of unlawful dividends or unlawful stock purchases or redemptions or (4) for any transaction from which the director derived an improper personal benefit. The current certificate of incorporation of the registrant provide for such limitation of liability.
We have entered into indemnification agreements with each of our directors and officers in which we have agreed to indemnify, defend and hold harmless, and also advance expenses as incurred, to the fullest extent permitted under applicable law, from damage arising from the fact that such person is or was an officer or director of our company or our subsidiaries.
The indemnification rights set forth above shall not be exclusive of any other right which an indemnified person may have or hereafter acquire under any statute, our amended and restated certificate of incorporation, our amended and restated bylaws, any agreement, any vote of stockholders or disinterested directors or otherwise. We maintain standard policies of insurance that provide coverage (1) to our directors and officers against loss rising from claims made by reason of breach of duty or other wrongful act and (2) to us with respect to indemnification payments that we may make to such directors and officers.
II-1
Table of Contents
We have purchased and intend to maintain insurance on behalf of the registrant and any person who is or was a director or officer against any loss arising from any claim asserted against him or her and incurred by him or her in that capacity, subject to certain exclusions and limits of the amount of coverage.
| Item 15. | Recent Sales of Unregistered Securities. |
In connection with SRNG’s initial formation in January 2020, Eagle Equity Partners III, LLC (the “Sponsor”) was issued 43,125,000 founder shares.
Simultaneously with the closing of SRNG’s IPO, the Sponsor purchased an aggregate of 19,250,000 Private Placement Warrants at $1.50 per Private Placement Warrant ($28,875,000 in the aggregate). In connection with the Business Combination, the Sponsor agreed to forfeit 1,925,000 Private Placement Warrants.
Prior to the consummation of the Business Combination, SRNG entered into Subscription Agreements, each dated as of May 11, 2021, in connection with the Private Placement.
The Private Placement closed immediately prior to the Business Combination. The shares of New SRNG Class A common stock issued to the PIPE Investors became shares of Ginkgo Class A common stock upon consummation of the Business Combination.
In April 2022, in connection with the FGen Purchase Agreement, we issued a total of 5,749,957 shares of our Class A common stock to FGen, valued at approximately $20.9 million, as consideration in connection with the acquisition of the outstanding equity interests of FGen, in a private transaction exempt from the registration requirements of the Securities Act pursuant to Section 4(a)(2) of the Securities Act.
In June 2022, in connection with the Bitome Purchase Agreement, Bitome’s outstanding convertible debt was repaid in full pursuant to, among other things, the issuance of 388,649 shares of Ginkgo Class A common stock (valued at approximately $1.2 million as of the acquisition date).
| Item 16. | Exhibits and Financial Statement Schedules. |
The following exhibits are filed as part of this registration statement:
II-2
Table of Contents
II-3
Table of Contents
II-4
Table of Contents
II-5
Table of Contents
| 101.DEF* | Inline XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB* | Inline XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE* | Inline XBRL Taxonomy Extension Presentation Linkbase Document | |
| 104* | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | |
| † | The annexes, schedules, and certain exhibits to this Exhibit have been omitted pursuant to Item 601(b)(2) of Regulation S-K. The Registrant hereby agrees to furnish supplementally a copy of any omitted annex, schedule or exhibit to the SEC upon request. |
| ‡ | Certain confidential information contained in this Exhibit has been omitted because it is (i) not material and (ii) of the type that the registrant treats as private or confidential. |
| + | Indicates a management contract of compensatory plan. |
| * | Filed herewith. |
| Item 17. | Undertakings. |
The undersigned registrant, hereby undertakes:
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933, as amended; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| (2) | That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (4) | That, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
II-6
Table of Contents
| (5) | That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
Insofar as indemnification for liabilities arising under the Securities may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-7
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Post-Effective Amendment No. 1 to this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Boston, Commonwealth of Massachusetts, on the 1st day of September, 2022.
| GINKGO BIOWORKS HOLDINGS, INC. | ||
| By: | /s/ Jason Kelly | |
| Name: | Jason Kelly | |
| Title: | Chief Executive Officer | |
Each person whose signature appears below constitutes and appoints each of Jason Kelly and Mark Dmytruk, acting alone or together with another attorney-in-fact, as his or her true and lawful attorney-in-fact and agent, with full power of substitution and resubstitution, for such person and in his or her name, place and stead, in any and all capacities, to sign any or all further amendments (including post-effective amendments) to this registration statement (and any additional registration statement related hereto permitted by Rule 426(b) promulgated under the Securities Act (and all further amendments, including post-effective amendments thereto)), and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Post-Effective Amendment No. 1 to this Registration Statement has been signed below by the following persons in the capacities and on the dates indicated.
| Name | Title | Date | ||
| /s/ Jason Kelly Jason Kelly |
Chief Executive Officer and Director (Principal Executive Officer) | September 1, 2022 | ||
| * Mark Dmytruk |
Chief Financial Officer (Principal Financial Officer) |
September 1, 2022 | ||
| * Marie Fallon |
Chief Accounting Officer (Principal Accounting Officer) | September 1, 2022 | ||
| * Reshma Shetty |
President, Chief Operating Officer and Director | September 1, 2022 | ||
| * Marijn Dekkers |
Director, Chair of the Board | September 1, 2022 | ||
| * Arie Belldegrun |
Director | September 1, 2022 | ||
| /s/ Kathy Hopinkah Hannan Kathy Hopinkah Hannan |
Director | September 1, 2022 | ||
Table of Contents
| * Christian Henry |
Director | September 1, 2022 | ||
| * Reshma Kewalramani |
Director | September 1, 2022 | ||
| * Shyam Sankar |
Director | September 1, 2022 | ||
| * Harry E. Sloan |
Director | September 1, 2022 | ||
| *By: | /s/ Jason Kelly | |
| Jason Kelly, attorney-in-fact |