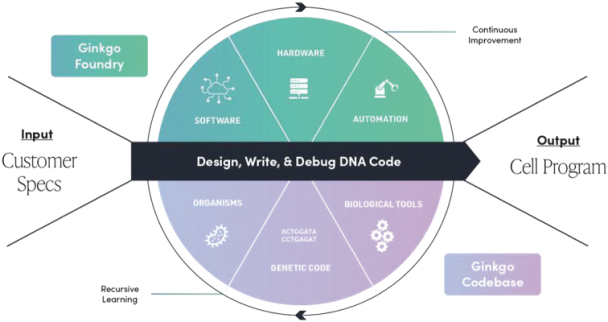
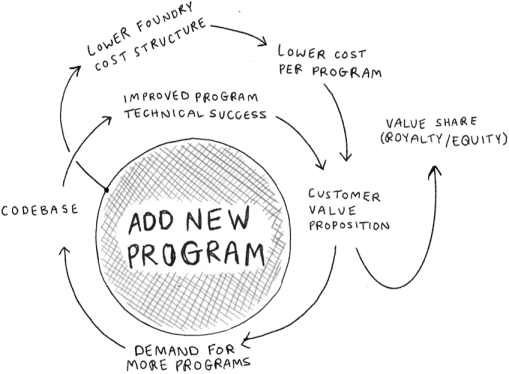
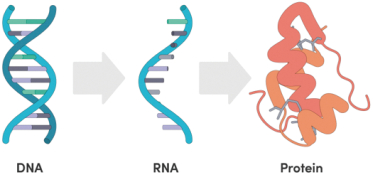
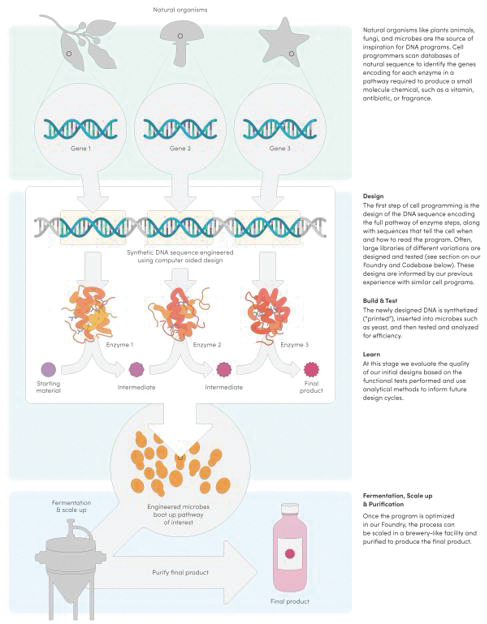
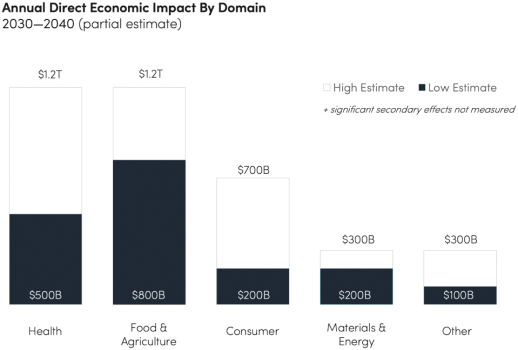
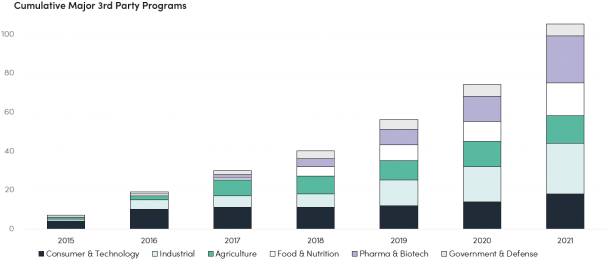
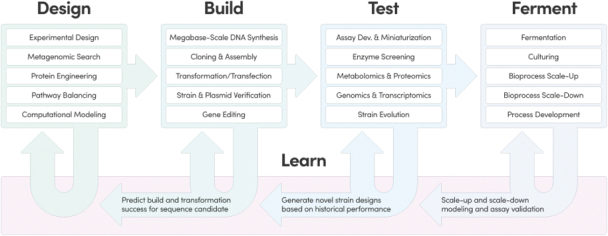
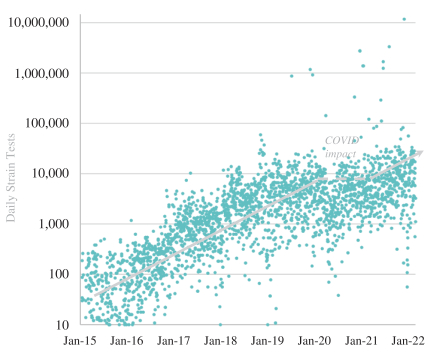
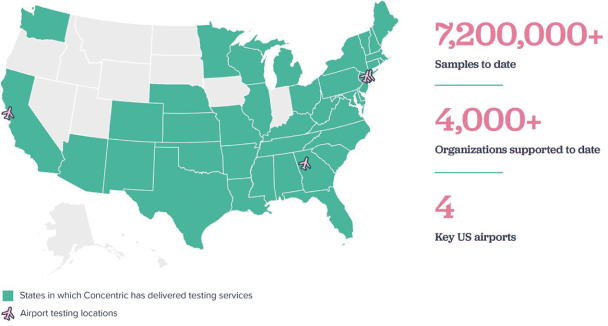
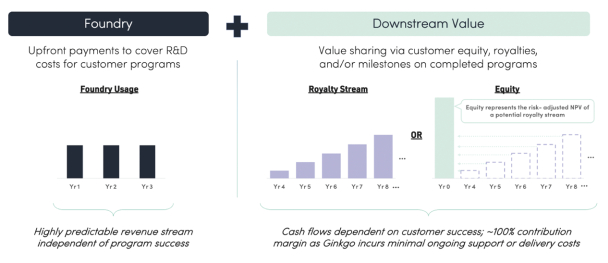
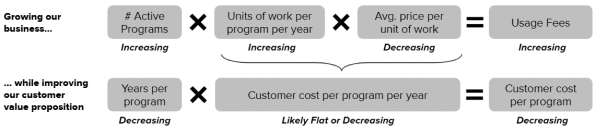
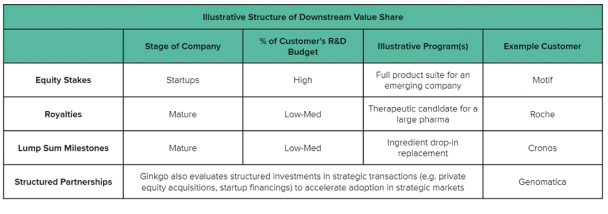
The information in this preliminary prospectus is not complete and may be changed. These securities may not be issued until the registration statement filed with the U.S. Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and does not constitute the solicitation of an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED APRIL 4, 2022
PRELIMINARY PROSPECTUS

GINKGO BIOWORKS HOLDINGS, INC.
Prospectus for 292,051,107 Shares of
Class A Common Stock of Ginkgo Bioworks Holdings, Inc.
This prospectus relates to the resale from time to time by the selling securityholders named in this prospectus (which term as used in this prospectus includes their respective transferees, pledgees, distributees, donees, and each a “Selling Securityholder” and, collectively, the “Selling Securityholders”) of 292,051,107 shares of Class A common stock, par value of $0.0001 per share (“Ginkgo Class A common stock”), of Ginkgo Bioworks Holdings, Inc.
successors-in-interest,
This prospectus provides a general description of such securities and the general manner in which the Selling Securityholders may offer or sell the securities. More specific terms of any securities that the Selling Securityholders may offer or sell may be provided in a prospectus supplement that describes, among other things, the specific amounts and prices of the securities being offered and the terms of the offering. The prospectus supplement may also add, update or change information contained in this prospectus.
We are registering certain of the securities for resale pursuant to the Selling Securityholders’ registration rights under certain agreements between us and the Selling Securityholders. The Selling Securityholders may offer all or part of the shares for resale from time to time through public or private transactions, at either prevailing market prices or at privately negotiated prices. We will pay certain offering fees and expenses and fees in connection with the registration of our Ginkgo Class A common stock. We will not receive any of the proceeds from the resale of the shares of Ginkgo common stock by the Selling Securityholders. Ginkgo Class A common stock is currently listed on the NYSE and trades under the symbol “DNA.” The last reported sale price of Ginkgo Class A common stock on the NYSE on April 1, 2022 was $4.20 per share.
We are an “emerging growth company” under applicable federal securities laws and will be subject to reduced public company reporting requirements.
INVESTING IN OUR SECURITIES INVOLVES RISKS THAT ARE DESCRIBED IN THE “
” SECTION BEGINNING ON PAGE 6 OF THIS PROSPECTUS.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of the securities to be issued under this prospectus or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2022.