Filed Pursuant to Rule 424(b)(3)
Registration No 333-261318

GINKGO BIOWORKS HOLDINGS, INC.
Prospectus for 84,346,092 Shares of
Class A Common Stock, 51,824,925 Shares of
Class A Common Stock Underlying Warrants and 17,325,000 Private Placement Warrants
of Ginkgo Bioworks Holdings, Inc.
This prospectus relates to (i) the resale of 84,346,092 shares of our Class A common stock, par value $0.0001 per share (“Class A common stock”), issued in connection with the Merger (as defined below), including 52,849,353 earn-out shares of New Ginkgo Class A common stock (the “Earn-Out Shares”), which are subject to forfeiture to the extent that certain vesting conditions are not satisfied on or before the fifth anniversary of the closing of the Merger, by certain of the selling securityholders named in this prospectus (each a “Selling Securityholder” and, collectively, the “Selling Securityholders”), (ii) the issuance by us and the resale of up to an aggregate of up to 17,325,000 shares of Class A common stock that are issuable upon the exercise of 17,325,000 private placement warrants (the “Private Placement Warrants”) originally issued in a private placement in connection with the IPO (as defined below) of Soaring Eagle Acquisition Corp., a Delaware corporation (“SRNG”), at an exercise price of $11.50 per share of Class A common stock, (iii) the issuance by us of up to 34,499,925 shares of Class A common stock that are issuable upon the exercise of 34,499,925 warrants issued in connection with the IPO (the “Public Warrants” and, together with the Private Placement Warrants, the “Warrants”) and the resale by the Selling Securityholders of an aggregate of up to 17,325,000 Private Placement Warrants.
On September 16, 2021, we consummated the transactions contemplated by that certain agreement and plan of merger, dated as of May 11, 2021, as amended on May 14, 2021 (the “Merger Agreement”), by and among SRNG, SEAC Merger Sub Inc., a Delaware corporation (“Merger Sub”), and Ginkgo Bioworks, Inc., a Delaware corporation (“Ginkgo”). As contemplated by the Merger Agreement, SRNG effected a deregistration under the Cayman Islands Companies Act (As Revised) and a domestication under Section 388 of the Delaware General Corporation Law, as amended (the “DGCL”), pursuant to which SRNG’s jurisdiction of incorporation was changed from the Cayman Islands to the State of Delaware (the “Domestication”), and, on the terms and subject to the conditions set forth in the Merger Agreement and in accordance with the DGCL, Merger Sub merged with and into Ginkgo, with Ginkgo surviving the merger as a wholly owned subsidiary of SRNG (the “Merger” and, together with the Domestication, the “Business Combination”). In addition, in connection with the consummation of the Business Combination, SRNG was renamed “Ginkgo Bioworks Holdings, Inc.” As used herein, “New Ginkgo” refers to SRNG after the consummation of the Business Combination.
This prospectus provides you with a general description of such securities and the general manner in which the Selling Securityholders may offer or sell the securities. More specific terms of any securities that the Selling Securityholders may offer or sell may be provided in a prospectus supplement that describes, among other things, the specific amounts and prices of the securities being offered and the terms of the offering. The prospectus supplement may also add, update or change information contained in this prospectus.
We will not receive any proceeds from the sale of shares of Class A common stock or Private Placement Warrants by the Selling Securityholders pursuant to this prospectus, except with respect to amounts received by us upon exercise of the Warrants. However, we will pay the expenses, other than any underwriting discounts and commissions, associated with the sale of securities pursuant to this prospectus.
We are registering certain of the securities for resale pursuant to the Selling Securityholders’ registration rights under certain agreements between us and the Selling Securityholders. Our registration of the securities covered by this prospectus does not mean that either we or the Selling Securityholders will issue, offer or sell, as applicable, any of the securities. The Selling Securityholders may offer and sell the securities covered by this prospectus in a number of different ways and at varying prices. We provide more information about how the Selling Securityholders may sell the shares or Private Placement Warrants in the section entitled “Plan of Distribution.”
You should read this prospectus and any prospectus supplement or amendment carefully before you invest in our securities.
Our Class A common stock and Public Warrants are listed on the NYSE under the symbols “DNA” and “DNA.WS,” respectively. On November 22, 2021, the closing price of our Class A common stock was $13.49 and the closing price for our Public Warrants was $3.58.
We are an “emerging growth company” under applicable federal securities laws and will be subject to reduced public company reporting requirements.
INVESTING IN OUR SECURITIES INVOLVES RISKS THAT ARE DESCRIBED IN THE “RISK FACTORS” SECTION BEGINNING ON PAGE 10 OF THIS PROSPECTUS.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of the securities to be issued under this prospectus or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is December 10, 2021.
| SELECTED DEFINITIONS | iv | |||
| vii | ||||
| 1 | ||||
| 1 | ||||
| 1 | ||||
| 3 | ||||
| 3 | ||||
| 6 | ||||
| 6 | ||||
| 8 | ||||
| 10 | ||||
| 10 | ||||
| 20 | ||||
| 21 | ||||
| 25 | ||||
| 27 | ||||
| Risks Related to New Ginkgo’s Personnel, IT and Physical Infrastructure |
34 | |||
| 36 | ||||
| 38 | ||||
| Risks Related to our Organizational Structure and Governance |
51 | |||
| 58 | ||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF GINKGO |
59 | |||
| 59 | ||||
| Generating Economic Value Through Revenue and Downstream Value Share |
60 | |||
| 64 | ||||
| 64 | ||||
| Potential Modification of Equity Awards in Connection with the Business Combination Transaction |
65 | |||
| 66 | ||||
| 69 | ||||
| 74 | ||||
| 76 | ||||
| 78 | ||||
| 80 | ||||
| 80 | ||||
| 87 | ||||
| 87 | ||||
| 87 | ||||
| 88 | ||||
| 88 | ||||
| 88 | ||||
| 90 | ||||
| 95 | ||||
| 100 | ||||
| 101 | ||||
| 103 | ||||
| 109 | ||||
| 119 | ||||
| 122 | ||||
| 124 |
i
| 126 | ||||
| 127 | ||||
| 129 | ||||
| 141 | ||||
| 142 | ||||
| 142 | ||||
| 144 | ||||
| 149 | ||||
| 150 | ||||
| 151 | ||||
| 151 | ||||
| 151 | ||||
| 155 | ||||
| 156 | ||||
| 156 | ||||
| 156 | ||||
| Action by the New Ginkgo Board of Directors to Terminate a Founder |
157 | |||
| 157 | ||||
| 159 | ||||
| 160 | ||||
| 164 | ||||
| Limitations on Liability and Indemnification of Officers and Directors |
164 | |||
| 165 | ||||
| 165 | ||||
| 165 | ||||
| 165 | ||||
| Listing of New Ginkgo Class A Common Stock and New Ginkgo Warrants |
165 | |||
| 166 | ||||
| 166 | ||||
| Restrictions on the Use of Rule 144 by Shell Companies or Former Shell Companies |
166 | |||
| 167 | ||||
| 170 | ||||
| 172 | ||||
| 172 | ||||
| 175 | ||||
| 175 | ||||
| 175 | ||||
| 175 | ||||
| 177 | ||||
| 177 | ||||
| 177 | ||||
| 178 | ||||
| 178 | ||||
| 181 | ||||
| 181 | ||||
| 182 | ||||
| Post-Combination Company Executive Officer and Director Compensation |
182 | |||
| 193 | ||||
| 193 | ||||
| 193 | ||||
| 198 | ||||
| 199 | ||||
| 201 |
ii
| 205 | ||||
| 208 | ||||
| 208 | ||||
| 209 | ||||
| F-1 |
You should rely only on the information contained in this prospectus. No one has been authorized to provide you with information that is different from that contained in this prospectus. This prospectus is dated as of the date set forth on the cover hereof. You should not assume that the information contained in this prospectus is accurate as of any date other than that date.
For investors outside the United States: We have not done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
iii
Unless otherwise stated or unless the context otherwise requires, the term “SRNG” refers to Soaring Eagle Acquisition Corp., the term “New SRNG” refers to Soaring Eagle Acquisition Corp. following the Domestication and the terms “New Ginkgo,” “combined company,” “post-combination company,” “Company,” “we,” “us,” and “our” refer to Ginkgo Bioworks Holdings, Inc. and its subsidiaries following the consummation of the Business Combination.
In this document:
“Business Combination” means the Domestication together with the Merger.
“Bylaws” means New Ginkgo’s amended and restated bylaws.
“Charter” means New Ginkgo’s amended and restated certificate of incorporation.
“Closing” means the closing of the Business Combination on the Closing Date.
“Closing Date” means September 16, 2021.
“Code” means the Internal Revenue Code of 1986, as amended.
“DGCL” means the General Corporation Law of the State of Delaware.
“Earn-out Consideration” means the 188.7 million earn-out shares of New Ginkgo common stock issued in connection with the Business Combination, which are subject to forfeiture to the extent that certain vesting conditions are not satisfied on or before the fifth anniversary of the closing of the Business Combination.
“Exchange Act” means the Securities Exchange Act of 1934, as amended.
“FASB” means the Financial Accounting Standards Board.
“Founder” means any of Jason Kelly, Reshma Shetty, Austin Che, Bartholomew Canton and Thomas F. Knight, Jr.
“Founder Holder” means any Founder or any legal entity or trust through which (directly or indirectly, and by ownership, voting power, contract or otherwise) any Founder exercises exclusive voting control with respect to the shares of capital stock of New Ginkgo owned by such legal entity or trust.
“founder shares” means the SRNG Class B ordinary shares sold prior to SRNG’s initial public offering.
“GDPR” mean the European Union’s General Data Protection Regulation.
“Governing Documents” means the Charter and the Bylaws.
“Old Ginkgo capital stock” means, collectively, the Old Ginkgo Class A common stock, the Old Ginkgo Class B common stock and each other class or series of capital stock of Old Ginkgo (including preferred stock).
“Old Ginkgo Class A common stock” means the Class A common stock, par value $0.0001 per share, of Old Ginkgo.
iv
“Old Ginkgo Class B common stock” means the Class B common stock, par value $0.0001 per share, of Old Ginkgo.
“Old Ginkgo option” means each option to purchase shares of Old Ginkgo capital stock.
“Old Ginkgo shareholder” means each holder of Old Ginkgo capital stock.
“Old Ginkgo warrant” means each warrant to purchase shares of Old Ginkgo capital stock.
“Investment Company Act” means the Investment Company Act of 1940, as amended.
“IPO” means SRNG’s initial public offering, consummated on February 26, 2021, through the sale of 22,500,000 units at $10.00 per unit.
“JOBS Act” means the Jumpstart Our Business Startups Act of 2012.
“Merger Agreement” means that certain Agreement and Plan of Merger, dated as of May 11, 2021, as amended on May 14, 2021, by and among SRNG, Merger Sub and Old Ginkgo.
“Merger Sub” means SEAC Merger Sub Inc., a Delaware corporation and wholly owned subsidiary of SRNG.
“New Ginkgo” means Ginkgo Bioworks Holdings, Inc., a Delaware corporation (which, prior to consummation of the Business Combination, was known as Soaring Eagle Acquisition Corp. (“SRNG” herein)).
“New Ginkgo Board” means the board of directors of New Ginkgo.
“New Ginkgo Class A common stock” means the shares of Class A common stock, par value $0.0001 per share, of New Ginkgo, which shares have the same economic terms as the shares of New Ginkgo Class B common stock, however they are only entitled to one (1) vote per share.
“New Ginkgo Class B common stock” means the shares of Class B common stock, par value $0.0001 per share, of New Ginkgo, which shares have the same economic terms as the shares of New Ginkgo Class A common stock, however they are entitled to ten (10) votes per share and the holders of New Ginkgo Class B common stock, as a class, will have the right, for so long as the outstanding shares of New Ginkgo Class B common stock continue to represent at least 2% of all of the outstanding shares of New Ginkgo common stock, to elect 25% of the directors constituting the New Ginkgo Board.
“New Ginkgo Class C common stock” means the shares of Class C common stock, par value $0.0001 per share, of New Ginkgo, which shares have the same economic terms as the shares of New Ginkgo Class A common stock, but which carry no voting rights (except as otherwise expressly provided in the Charter or required by applicable law).
“New Ginkgo common stock” means, collectively, the New Ginkgo Class A common stock, the New Ginkgo Class B common stock and the New Ginkgo Class C common stock.
“New Ginkgo Management” means the management of New Ginkgo.
“New SRNG” means Soaring Eagle Acquisition Corp., a Delaware corporation, following the Domestication.
v
“NYSE” means the New York Stock Exchange.
“PIPE Investment” means the issuance of an aggregate of 76,000,000 shares of New SRNG Class A common stock pursuant to the Subscription Agreements to the PIPE Investors immediately before the Closing, at a purchase price of $10.00 per share.
“PIPE Investors” means certain institutional investors, including affiliates of the Sponsor, who are party to the Subscription Agreements.
“Private Placement Warrants” means the 17,325,000 warrants held by the Sponsor, which were issued concurrently with the IPO, each of which is exercisable for one share of New Ginkgo Class A common stock.
“Public Warrants” means the warrants included in the units issued in the IPO, each of which is exercisable for one share of New Ginkgo Class A common stock, in accordance with its terms.
“Registration Rights Agreement” means the Amended and Restated Registration Rights Agreement, dated as of September 16, 2021, by and among New Ginkgo, the Sponsor and Viking Global Opportunities Illiquid Investments Sub-Master LP.
“SEC” means the U.S. Securities & Exchange Commission.
“Sponsor” means Eagle Equity Partners III, LLC, a Delaware limited liability company.
“SRNG” means Soaring Eagle Acquisition Corp., a Cayman Islands exempted company, prior to the Domestication.
“SRNG Class A ordinary shares” means the Class A ordinary shares, par value $0.0001 per share, of SRNG.
“SRNG Class B ordinary shares” means the Class B ordinary shares, par value $0.0001 per share, of SRNG.
“SRNG ordinary shares” means, collectively, the SRNG Class A ordinary shares and SRNG Class B ordinary shares.
“SRNG warrants” means, collectively, the SRNG public warrants and the SRNG private placement warrants.
“Subscription Agreements” means the subscription agreements, each dated as of May 11, 2021, between SRNG and the PIPE Investors, pursuant to which SRNG agreed to issue an aggregate of 76,000,000 shares of New SRNG Class A common stock to the PIPE Investors immediately before the Closing at a purchase price of $10.00 per share.
“Transfer Agent” means Computershare Trust Company, N.A.
“Units” means the units of SRNG issued in the IPO, each of which consisted of one SRNG Class A ordinary share and one-fifth of one SRNG public warrant.
“U.S. GAAP” means United States generally accepted accounting principles.
vi
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus includes forward-looking statements regarding, among other things, the plans, strategies and prospects, both business and financial, of New Ginkgo. These statements are based on the beliefs and assumptions of the management of New Ginkgo. Although New Ginkgo believes that its plans, intentions and expectations reflected in or suggested by these forward-looking statements are reasonable, New Ginkgo cannot assure you that it will achieve or realize these plans, intentions or expectations. Forward-looking statements are inherently subject to risks, uncertainties and assumptions. Generally, statements that are not historical facts, including statements concerning possible or assumed future actions, business strategies, events or results of operations, are forward-looking statements. These statements may be preceded by, followed by or include the words “believes”, “estimates”, “expects”, “projects”, “forecasts”, “may”, “will”, “should”, “seeks”, “plans”, “scheduled”, “anticipates” or “intends” or similar expressions. The forward-looking statements are based on projections prepared by, and are the responsibility of, the New Ginkgo Management. Ernst & Young, New Ginkgo’s independent auditor, has not examined, compiled or otherwise applied procedures with respect to the accompanying forward-looking financial information presented herein and, accordingly, expresses no opinion or any other form of assurance on it. The Ernst & Young report included in this prospectus relates to historical financial information of New Ginkgo. It does not extend to the forward-looking information and should not be read as if it does. Forward-looking statements contained in this prospectus include, but are not limited to, statements about:
| • | the ability to maintain the listing of New Ginkgo Class A common stock on the NYSE; |
| • | New Ginkgo’s ability to raise financing in the future and to comply with restrictive covenants related to long-term indebtedness; |
| • | New Ginkgo’s ability to retain or recruit, or adapt to changes required in, its founders, senior executives, key personnel or directors; |
| • | factors relating to the business, operations and financial performance of New Ginkgo, including: |
| • | New Ginkgo’s ability to effectively manage its growth; |
| • | New Ginkgo’s exposure to the volatility and liquidity risks inherent in holding equity interests in certain of its customers; |
| • | rapidly changing technology and extensive competition in the synthetic biology industry that could make the products and processes New Ginkgo is developing obsolete or non-competitive unless it continues to collaborate on the development of new and improved products and processes and pursue new market opportunities; |
| • | New Ginkgo’s reliance on its customers to develop, produce and manufacture products using the engineered cells and/or biomanufacturing processes that New Ginkgo develops; |
| • | New Ginkgo’s ability to comply with laws and regulations applicable to its business; and |
| • | market conditions and global and economic factors beyond New Ginkgo’s control; |
| • | intense competition and competitive pressures from other companies worldwide in the industries in which the combined company will operate; |
| • | litigation and the ability to adequately protect New Ginkgo’s intellectual property rights; and |
| • | other factors detailed under the section entitled “Risk Factors.” |
These and other factors that could cause actual results to differ from those implied by the forward-looking statements in this prospectus are more fully described under the heading “Risk Factors” and elsewhere in this
vii
prospectus. The risks described under the heading “Risk Factors” are not exhaustive. Other sections of this prospectus describe additional factors that could adversely affect the business, financial condition or results of New Ginkgo. New risk factors emerge from time to time and it is not possible to predict all such risk factors, nor can New Ginkgo assess the impact of all such risk factors on the business of New Ginkgo, or the extent to which any factor or combination of factors may cause actual results to differ materially from those contained in any forward-looking statements. Forward-looking statements are not guarantees of performance. You should not put undue reliance on these statements, which speak only as of the date hereof. All forward-looking statements attributable to New Ginkgo or persons acting on its behalf are expressly qualified in their entirety by the foregoing cautionary statements. New Ginkgo undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
viii
This summary highlights selected information included in this prospectus and does not contain all of the information that may be important to you in making an investment decision. This summary is qualified in its entirety by the more detailed information included in this prospectus. Before making your investment decision with respect to our securities, you should carefully read this entire prospectus, including the information under “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations of Ginkgo” and the financial statements included elsewhere in this prospectus.
Ginkgo Bioworks Holdings, Inc. is building a platform to enable customers to program cells as easily as we can program computers. New Ginkgo’s platform is market agnostic and enables biotechnology applications across diverse markets, from food and agriculture to industrial chemicals to pharmaceuticals. New Ginkgo is also actively supporting a number of biosecurity efforts to respond to COVID-19, including vaccine manufacturing optimization, therapeutics discovery, and K-12 pooled testing. New Ginkgo has incurred net losses since its inception. New Ginkgo’s net loss attributable to its stockholders was approximately $229.4 million and $79.7 million for the nine months ended September 30, 2021 and 2020, respectively, and as of September 30, 2021, New Ginkgo had an accumulated deficit of approximately $697.3 million. For more information, see “Risk Factors—Risks Related to New Ginkgo’s Business—We have a history of net losses. We expect to continue to incur losses for the foreseeable future, and we may never achieve or maintain profitability.”
Background and Business Combination
The Company was originally known as Soaring Eagle Acquisition Corp. (“SRNG”).
SRNG (and after the Domestication as described below, “New SRNG”), a Cayman Islands exempted company, previously entered into an agreement and plan of merger, dated as of May 11, 2021, as amended on May 14, 2021 (the “Merger Agreement”), by and among SRNG, SEAC Merger Sub Inc., a Delaware corporation (“Merger Sub”), and Ginkgo Bioworks, Inc., a Delaware corporation (“Old Ginkgo”).
On September 15, 2021, SRNG filed a notice of deregistration with the Cayman Islands Registrar of Companies, together with the necessary accompanying documents, and filed a certificate of incorporation and a certificate of corporate domestication with the Secretary of State of Delaware, pursuant to which SRNG was domesticated and continued as a Delaware corporation, under the name of “Soaring Eagle Acquisition Corp.” (the “Domestication”).
As a result of, and upon the effective time of the Domestication, among other things, (1) each of the then issued and outstanding Class B ordinary shares, par value $0.0001 per share, of SRNG automatically converted, on a one-for-one basis, into a Class A ordinary share, par value $0.0001 per share, of SRNG (a “SRNG Class A ordinary share”); (2) immediately following the conversion described in clause (1), each of the then issued and outstanding SRNG Class A ordinary shares automatically converted, on a one-for-one basis, into a share of Class A common stock, par value $0.0001 per share, of New SRNG (the “New SRNG Class A common stock”); (3) each of the then issued and outstanding redeemable warrant of SRNG (the “SRNG warrants”) automatically converted into a redeemable warrant to acquire one share of New SRNG Class A common stock (the “New SRNG warrants”); and (4) each of the then issued and outstanding units of SRNG that had not been previously separated into the underlying SRNG Class A ordinary shares and underlying SRNG warrants upon the request of the holder thereof (the “SRNG units”), were cancelled and entitled the holder thereof to one share of New SRNG Class A common stock and one-fifth of one New SRNG warrant. No fractional shares will be issued upon exercise of the New SRNG warrants.
1
On September 16, 2021 (the “Closing Date”), as contemplated by the Merger Agreement, New SRNG consummated the merger transaction contemplated by the Merger Agreement (the “Closing”), whereby Merger Sub merged with and into Old Ginkgo, with the separate corporate existence of Merger Sub ceasing and Old Ginkgo being the surviving corporation and a wholly owned subsidiary of New SRNG (the “Merger” and, together with the Domestication, the “Business Combination”). In connection with the consummation of the Business Combination, New SRNG changed its name to “Ginkgo Bioworks Holdings, Inc.” (“New Ginkgo”). The shares of New SRNG Class A common stock and New SRNG warrants described above became shares of New Ginkgo Class A common stock or New Ginkgo warrants, as applicable, upon consummation of the Merger.
Pursuant to the Merger Agreement, SRNG acquired all of the outstanding equity interests of Old Ginkgo for approximately $15.8 billion in aggregate consideration in the form of common stock of New Ginkgo (“New Ginkgo common stock”) valued at $10 per share (the “Base Equity Consideration”), plus approximately 188.7 million earn-out shares of New Ginkgo common stock, which are subject to forfeiture to the extent that the vesting conditions described below are not satisfied on or before the fifth anniversary of the Closing (the “Earn-out Consideration”). Old Ginkgo shareholders received consideration in the form of shares of Class A common stock, par value $0.0001 per share, of New Ginkgo (“New Ginkgo Class A common stock”) and/or Class B common stock, par value $0.0001 per share, of New Ginkgo (“New Ginkgo Class B common stock”), as determined in accordance with the Merger Agreement.
The Base Equity Consideration was allocated among Old Ginkgo equity holders as follows: (1) each share of Class A common stock, par value $0.0001 per share, of Old Ginkgo (“Old Ginkgo Class A common stock”) outstanding immediately prior to the effective time of the Business Combination was converted into approximately 49.080452 shares of New Ginkgo Class A common stock; (2) each share of Class B common stock, par value $0.0001 per share, of Old Ginkgo (“Old Ginkgo Class B common stock”) outstanding immediately prior to the effective time of the Business Combination was converted into approximately 49.080452 shares of New Ginkgo Class B common stock; (3) each option exercisable for one share of Old Ginkgo common stock (each, an “Old Ginkgo option”) under Old Ginkgo’s stock incentive plans outstanding immediately prior to the effective time of the Business Combination was assumed and converted into an option having the same terms and conditions as applied to the Old Ginkgo option so converted but exercisable for approximately 49.080452 shares of New Ginkgo common stock, with appropriate adjustments to the exercise price thereof (each, a “New Ginkgo option”); (4) each award of restricted common stock of Old Ginkgo under Old Ginkgo’s stock incentive plans (each, a “Ginkgo restricted stock award”) outstanding immediately prior to the effective time of the Business Combination was converted into approximately 49.080452 shares of restricted common stock of New Ginkgo (each, a “New Ginkgo restricted stock award”); and (5) each award of restricted stock units of Old Ginkgo under Old Ginkgo’s stock incentive plans (each, an “Old Ginkgo restricted stock unit award”) outstanding immediately prior to the effective time of the Business Combination was assumed and converted into a restricted stock unit having the same terms and conditions as applied to the Old Ginkgo restricted stock unit so converted but relating to approximately 49.080452 shares of common stock of New Ginkgo (each, a “New Ginkgo restricted stock unit award”). No warrants to purchase shares of capital stock of Old Ginkgo were outstanding immediately prior to the effective time of the Business Combination.
In addition to the Base Equity Consideration described above, the holders of Old Ginkgo common stock, Old Ginkgo options, Old Ginkgo restricted stock awards and Old Ginkgo restricted stock unit awards outstanding immediately prior to the effective time of the Business Combination received a proportional amount of the Earn-out Consideration, which is divided into four equal tranches subject to vesting during the five years after the Closing Date (the “Earn-out Period”) based on the conditions below (collectively, the “Earn-out Targets”):
| • | if the trading price per share of New Ginkgo Class A common stock at any point during the trading hours of a trading day is greater than or equal to $12.50 for any 20 trading days within any period of 30 consecutive trading days during the Earn-out Period, 25% of the Earn-out Consideration will immediately vest; |
2
| • | if the trading price per share of New Ginkgo Class A common stock at any point during the trading hours of a trading day is greater than or equal to $15.00 for any 20 trading days within any period of 30 consecutive trading days during the Earn-out Period, an additional 25% of the Earn-out Consideration will immediately vest; |
| • | if the trading price per share of New Ginkgo Class A common stock at any point during the trading hours of a trading day is greater than or equal to $17.50 for any 20 trading days within any period of 30 consecutive trading days, an additional 25% of the Earn-out Consideration will immediately vest; and |
| • | if the trading price per share of New Ginkgo Class A common stock at any point during the trading hours of a trading day is greater than or equal to $20.00 for any 20 trading days within any period of 30 consecutive trading days, the remaining 25% of the Earn-out Consideration will immediately vest. |
Additionally, the vesting of the Earn-out Consideration will be subject to acceleration in the event of certain transactions resulting in a change of control of New Ginkgo or the acquisition by a third party of assets of New Ginkgo representing at least 50% of New Ginkgo’s assets (by value) on a consolidated basis or generating at least 50% of New Ginkgo’s revenues on a consolidated basis, to the extent that the per-share value of the consideration received by New Ginkgo’s stockholders in such transaction or acquisition is greater than or equal to the Earn-out Targets described above.
The First Earn-out Target was achieved on November 15, 2021. To the extent that the subsequent Earn-out Targets described above are not achieved during the Earn-out Period, the portion of the Earn-out Consideration that remains subject to vesting and forfeiture at the end of the Earn-out Period will be forfeited to New Ginkgo for no consideration and cancelled.
The New Ginkgo Class A common stock and Public Warrants are listed on the NYSE under the symbols “DNA” and “DNA.WS”, respectively.
Investing in our securities involves risks. You should carefully consider the risks described in “Risk Factors” beginning on page 10 before making a decision to invest in our Class A common stock. If any of these risks actually occur, our business, financial condition and results of operations would likely be materially adversely affected. Some of the risks related to New Ginkgo’s business and industry are summarized below. References in the summary below to “we,” “us,” “our” and “the Company” generally refer to New Ginkgo.
| • | Our business could have to restructure, we may not meet expectations of investors, or we may have materially different financial results than expected, any of which could have a significant negative effect on our financial condition, results of operations and stock price, which could cause you to lose some or all of your investment. |
| • | Our Class A common stock may not comply with the standards of NYSE. |
| • | Our creditors may have priority over our stockholders in the event of bankruptcy, which could limit the recovery of our stockholders in a liquidation. |
| • | If we were to be deemed an “investment company” under the Investment Company Act, applicable restrictions could make it impractical for us to continue our business as contemplated and could have a material adverse effect on our business. |
| • | The multi-class structure of our common stock could affect our business operations and the price of our stock. |
3
| • | Our multi-class stock structure entitles only our employees and directors to acquire and hold New Ginkgo Class B common stock which has a greater number of votes per share than New Ginkgo Class A common stock, which may affect whether stockholders hold or purchase New Ginkgo Class A common stock. |
| • | Issuing a large number of shares of New Ginkgo Class B common stock in the future may increase the concentration of voting power with our employees and directors, which could have an adverse effect on the trading price of New Ginkgo Class A common stock. |
| • | Issuing New Ginkgo Class C common stock may increase concentration of voting power in New Ginkgo Class B common stock, which could discourage potential acquisitions of our business and could have an adverse effect on the trading price of New Ginkgo Class A common stock. |
| • | Our focus on the long-term best interests of our company and our consideration of the interests of all of our stakeholders may conflict with short-term or medium-term financial interests and business performance, which may adversely impact the value of our common stock. |
| • | Under Delaware law, the language in the Governing Documents limits stockholder actions and the available forums for such actions. |
| • | Our history of net losses is expected to continue, and we may never achieve or maintain profitability. |
| • | We will need substantial additional capital in the future in order to fund our business. |
| • | If we fail to effectively manage our rapid growth, then our business, results of operations and financial condition could be adversely affected. |
| • | Our limited operating history makes it difficult to evaluate our current business and future prospects. |
| • | We currently own and may in the future own equity interests in other operating companies, including certain of our customers; consequently, we have exposure to the volatility and liquidity risks inherent in holding their equity and overall operational and financial performance of these businesses. |
| • | Failure to pursue strategic acquisitions and investments, achieve projected milestones or maintain and expand customer partnerships could have an adverse impact on our business. |
| • | Failure to secure laboratory equipment and third-party suppliers could cause delays in our research, development or production capacity and adversely affect our business. |
| • | We are subject to regulatory and legal scrutiny for our use of genetically modified organisms, biological, hazardous, flammable and/or regulated materials and DNA sequencing synthesis. |
| • | Our reputation could be damaged by third parties’ use of our engineered cells and accompanying production processes. |
| • | International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States. |
| • | Our ability to enter into a definitive agreement with the U.S. International Development Finance Corporation and our overall level of indebtedness could adversely affect liquidity and have an adverse effect on our valuation, operations and business. |
| • | If our customers discontinue using or are not successful in developing, producing and manufacturing products using the engineered cells and/or biomanufacturing processes that we develop, our future financial position may be adversely impacted. |
| • | Our revenue, results of operations, cash flows and reputation in the marketplace may suffer upon the loss of a significant customer. |
4
| • | Our business partners may make announcements about the status of our collaborations, and the price of our common stock may decline as a result of announcements of unexpected results or developments. |
| • | Uncertainty about COVID-19 or another global pandemic could materially affect how we and our business partners are operating and may harm our business and results of operations. |
| • | Decline in COVID-19 testing, decline in our capacity to test or disruption of our telehealth relationships may harm our business and results of operations. |
| • | We may be subject to liability if the COVID-19 tests we utilize in our testing programs provide inaccurate results. |
| • | Failure to pursue new opportunities and develop our platform could make our products obsolete or non-competitive in the market. |
| • | The market may be skeptical of our novel and complex technology and use of genetically modified materials, which could limit public acceptance of our products or processes and limit our revenues. |
| • | Failure to protect or enforce our intellectual property rights, trade secrets and inventions could harm our business, results of operations and financial condition and may result in litigation. |
| • | We may be subject to litigation alleging infringement on the patents of third parties. |
| • | Risks related to intellectual property developed under U.S. federally funded research grants and contracts. |
| • | Our use of genetic resources and sequencing may subject us to obligations under the Nagoya Protocol. |
| • | Our use of in-licensing from third parties and “open source” software could have a material and adverse impact on our business, financial condition and results of operation. |
| • | Loss of key personnel or failure to access infrastructure could delay our programs, harm our development efforts and adversely affect our business and results of operations. |
| • | We rely on our customers, joint venturers, equity investees and other third parties to deliver timely and accurate information in order to accurately report our financial results in the time frame and manner required by law. |
| • | We use estimates in determining the fair value of certain assets and liabilities. If our estimates prove to be incorrect, we may be required to write down the value of these assets or write up the value of these liabilities, which could adversely affect our financial position. |
| • | Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited. |
| • | If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock. |
| • | Our cash and cash equivalents could be adversely affected if the financial institutions in which we hold our cash and cash equivalents fail. |
| • | We are subject to numerous federal, state, local and international laws and regulations related to our business and operations, and the failure to comply with any of these laws and regulations, or failure to comply with new or changed laws and regulations, could adversely affect our business and our financial condition. |
| • | We may incur significant costs complying with environmental, health and safety laws and regulations, and failure to comply with these laws and regulations could expose us to significant liabilities. |
5
| • | We may become subject to the comprehensive laws and rules governing billing and payment, noncompliance with which could result in non-payment or recoupment of overpayments for our services or other sanctions. |
| • | Our receipt of public funds subjects us to the False Claims Act, EKRA (a federal anti-kickback law) and state anti-kickback laws. |
| • | We are engaged in certain research activities involving controlled substances, the making, use, sale, importation, exportation, and distribution of which may be subject to significant regulation. |
| • | Disruptions of information technology systems or data security incidents could result in significant financial, legal, regulatory, business and reputational harm to us. |
| • | Changes in U.S. and foreign tax laws could have a material adverse effect on our business, cash flow, results of operations or financial conditions. |
| • | We may become subject to lawsuits or indemnity claims in the ordinary course of business, which could materially and adversely affect our business and results of operations. |
| • | Short sellers may engage in manipulative activity intended to drive down the market price of New Ginkgo Class A common stock, which could also result in related regulatory and governmental scrutiny, among other effects. |
| • | Our business could be adversely affected by legal challenges to our telehealth partner’s business model. |
We were originally incorporated as a Cayman Islands exempted company in October 2020 as a special purpose acquisition company, formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, recapitalization, reorganization or similar business combination with one or more businesses. SRNG completed its IPO in February 2021. In September 2021, our wholly owned subsidiary merged with and into Old Ginkgo, with Old Ginkgo surviving the merger as a wholly owned subsidiary of SRNG. In connection with the Business Combination, we changed our name to “Ginkgo Bioworks Holdings, Inc.” Our principal executive offices are located at 27 Drydock Avenue, 8th Floor, Boston MA 02210.
Our telephone number is (877) 422-5362. Our website address is www.ginkgobioworks.com. Information contained on our website or connected thereto does not constitute part of, and is not incorporated by reference into, this prospectus or the registration statement of which it forms a part.
Section 102(b)(1) of the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a registration statement under the Securities Act declared effective or do not have a class of securities registered under the Exchange Act) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. We have elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, we, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of New Ginkgo’s financial statements with those of another public company that is neither an emerging growth company nor an emerging growth company that has opted out of using the extended transition period difficult or impossible because of the potential differences in accounting standards used.
6
We will remain an emerging growth company until the earlier of: (1) the last day of the fiscal year (a) following the fifth anniversary of the closing of SRNG’s initial public offering, (b) in which we have total annual gross revenue of at least $1.07 billion or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common equity that is held by non-affiliates exceeds $700 million as of the end of the prior fiscal year’s second fiscal quarter; and (2) the date on which we have issued more than $1.00 billion in non-convertible debt securities during the prior three-year period. References herein to “emerging growth company” have the meaning associated with it in the JOBS Act.
7
|
| ||
| Issuer | Ginkgo Bioworks Holdings, Inc. | |
| Issuance of New Ginkgo Class A common stock |
||
| Shares of our Class A common stock to be issued upon exercise of all Private Placement Warrants and Public Warrants |
Up to 51,824,925 shares of New Ginkgo Class A common stock. | |
| Shares of our Class A common stock outstanding prior to exercise of all Warrants (as of November 8, 2021) | 1,310,783,159 | |
| Use of proceeds | We will receive up to an aggregate of approximately $595,986,638 from the exercise of all 51,824,925 Warrants, assuming the exercise in full of such Warrants for cash. Unless we inform you otherwise in a prospectus supplement or free writing prospectus, we intend to use the net proceeds from the exercise of such Warrants for general corporate purposes which may include acquisitions or other strategic investments or repayment of outstanding indebtedness. | |
| Ticker symbols |
“DNA” and “DNA.WS” for the Class A common stock and Public Warrants, respectively. | |
| Risk factors | Any investment in the securities offered hereby is speculative and involves a high degree of risk. You should carefully consider the information set forth under “Risk Factors” and elsewhere in this prospectus. | |
| Resale of New Ginkgo Class A common stock and Private Placement Warrants | ||
| Shares of Class A common stock offered by the Selling Securityholders (including shares of New Ginkgo Class A common stock that may be issued upon exercise of the Private Placement Warrants) | 385,971,092 shares of New Ginkgo Class A common stock | |
| Private Placement Warrants offered by the Selling Securityholders | 17,325,000 Private Placement Warrants | |
| Exercise price | $11.50 per share, subject to adjustment as described herein. | |
| Redemption | The Warrants are redeemable in certain circumstances. See “Description of New Ginkgo Securities—Warrants” for further discussion. | |
8
| Use of proceeds | We will not receive any proceeds from the sale of the New Ginkgo Class A common stock and the Private Placement Warrants to be offered by the Selling Securityholders. With respect to the issuance of shares of New Ginkgo Class A common stock underlying the Warrants, we will not receive any proceeds from such shares except with respect to amounts received by use upon exercise of such Warrants to the extent such Warrants are exercised for cash. | |
9
An investment in our securities involves a high degree of risk. You should carefully consider the following risk factors, together with all of the other information included in this prospectus, before making an investment decision. Our business, prospects, financial condition or operating results could decline due to any of these risks and, as a result, you may lose all or part of your investment.
Unless the context otherwise requires, all references in this section to the “Company,” “we,” “us” or “our” refer to the business of New Ginkgo and its subsidiaries.
Risks Related to New Ginkgo’s Business
We have a history of net losses. We expect to continue to incur losses for the foreseeable future, and we may never achieve or maintain profitability.
We have incurred significant operating losses since our inception. Our net loss attributable to our stockholders was approximately $229.4 million and $79.7 million for the nine months ended September 30, 2021 and 2020, respectively. As of September 30, 2021, we had an accumulated deficit of approximately $697.3 million. We may incur losses and negative cash flow from operating activities for the foreseeable future as we continue to invest significant additional funds toward further developing our platform, the cell programs we perform on behalf of our customers and otherwise growing our business. We also expect that our operating expenses will increase as a result of becoming a public company and will continue to increase as we grow our business. We have derived a significant portion of our revenues from fees and milestone payments from technical development services provided to customers to advance programs. Historically, these fees have not been sufficient to cover the full cost of our operations. Additionally, if our customers terminate their agreements or development plans with us, our near-term revenues could be adversely affected. In addition, certain of our customer agreements provide for milestone payments, future royalties and other forms of contingent consideration, the payment of which are uncertain, as they are dependent on our ability to successfully develop engineered cells, bioprocesses, or other deliverables and our customers’ ability and willingness to successfully develop and commercialize products and processes.
Our expenses may exceed revenues for the foreseeable future and we may not achieve profitability. If we fail to achieve profitability, or if the time required to achieve profitability is longer than we anticipate, we may not be able to expand or continue our business, and the value of New Ginkgo common stock could be negatively impacted. Our ability to achieve or sustain profitability is based on numerous factors, many of which are beyond our control, including the development of our platform, the initiation of new programs with new and existing customers, the commercial terms of our programs, our ability to advance cell engineering programs in a timely and cost-effective manner, our ability to extend new offerings to customers, our customers’ ability to scale up bioprocesses, the ability of our customers to produce and sell products, the impact of market acceptance of our customers’ products, and our and our customers’ market penetration and margins. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
We will need substantial additional capital in the future in order to fund our business.
We have consumed considerable amounts of capital to date, and we expect to incur continued net losses over the next several years as we continue to develop our business, advance our programs, expand and enhance our platform, and make the capital investments necessary to scale up our Foundry operations and Codebase assets. We may also use additional capital for strategic investments and acquisitions. We believe that our cash and cash equivalents, short-term investments, and interest earned on investments will be sufficient to meet our projected operating requirements for several years and until we reach profitability. However, these assumptions may prove to be incorrect and we could exhaust our available capital resources sooner than we currently expect. Because of the numerous risks and uncertainties associated with our programs, including risks and uncertainties that could impact the rate of progress of our programs, we are unable to estimate with certainty the amounts of capital outlays and operating expenditures associated with these activities.
10
We do not currently have any commitments for future funding. We may receive fees, milestones, and royalty payments under our customer agreements, but these are not guaranteed. Additionally we may be able to sell our equity interests in certain subsidiaries or collaborations but most of these equity stakes are illiquid (e.g. in private companies) and we may not be able to find a buyer or may incur significant impairment if forced to sell these positions for liquidity. We may not receive any further funds under those agreements, the funds we receive may be lower than projected, or our program costs may be higher than projected. In addition, we may not be able to sign new customer agreements or enter into new development plans with existing customers with adequate funds to cover program development expenses. As a result of these and other factors, we do not know whether additional financing will be available when needed, or, if available, whether such financing would be on terms favorable to our stockholders or us.
If future financings involve the issuance of equity securities, our existing stockholders would suffer dilution. If we raise debt financing in the future, we may be subject to restrictive covenants that limit our ability to conduct our business. Our ability to raise funds may be adversely impacted by current or future economic conditions. If we fail to raise sufficient funds and continue to incur losses, our ability to fund our operations, take advantage of strategic opportunities, or otherwise respond to competitive pressures could be significantly limited. If adequate funds are not available, we may not be able to successfully execute our business plan or continue our business.
We have experienced rapid growth and expect our growth to continue, and if we fail to effectively manage our growth, then our business, results of operations, and financial condition could be adversely affected.
We have experienced substantial growth in our business since inception, which has placed and may continue to place significant demands on our company culture, operational infrastructure, and management. We believe that our culture has been a critical component of our success. We have invested substantial time and resources in building our team and nurturing a culture of empowerment of, and active engagement by, our employees. As we expand our business and mature as a public company, we may find it difficult to maintain our culture while managing this growth. Any failure to manage our anticipated growth and organizational changes in a manner that preserves the key aspects of our culture could be detrimental to future success, including our ability to recruit and retain personnel, and effectively focus on and pursue our objectives. This, in turn, could adversely affect our business, results of operations, and financial condition.
In addition, in order to successfully manage our rapid growth, our organizational structure has become more complex and is likely to continue to become more complex. In order to manage these increasing complexities, we will need to continue to scale and adapt our operational, financial, and management controls, as well as our reporting systems and procedures. The expansion of our systems and infrastructure will require us to commit substantial financial, operational, and management resources before our revenue increases and without any assurances that our revenue will increase.
Finally, continued growth could strain our ability to maintain reliable service levels and offerings for our customers. If we fail to achieve the necessary level of capacity, quality and efficiency in performing services and other development activities, or the necessary level of efficiency in our organizational structure as we grow, then our business, results of operations and financial condition could be adversely affected.
Our limited operating history makes it difficult to evaluate our current business and future prospects.
We have a portfolio of cell engineering programs which vary in start date, duration, complexity, and revenue potential. Additionally, our downstream economics in the form of equity interests, milestone payments, or royalty streams add an additional level of uncertainty to our possible future performance. Consequently, predictions about our future success or viability are highly uncertain and may not be as accurate as they could be if we had a longer company history of successfully developing, commercializing and generating revenue from our programs and/or downstream economic participation. With respect to our biosecurity offering, prior to 2020,
11
we had no experience developing or commercializing testing products or services. Moreover, as described above, given the limited operating history of our biosecurity offering, our reliance on school funding for testing, potential disruptions from vaccine rollout generally and for adolescents in the foreseeable future, the impact of summer vacation, and the increased availability of over-the-counter testing options, the future performance of our COVID-19 offerings is unpredictable. Moreover, we cannot predict how long the COVID-19 pandemic will continue and, therefore, we cannot predict the duration of the revenue stream from our COVID-19 testing products and services.
Our long-term objective is to generate free cash flow from the commercialization of programs by customers across a variety of industries, as well as, from our biosecurity-focused offerings. Our estimated costs and timelines for the completion of programs are based on our experiences to date and our expectations for each stage of the program in development. Given the variety of types of programs we support and the continued growth of our platform, there is variability in timelines and costs for launching and executing programs, and completion dates can change over the course of a customer engagement. In addition, our costs and timelines may be greater or subject to variability where regulatory requirements lead to longer timelines, such as in agriculture, food, and therapeutics. In addition, we have equity interests in certain companies and there is and will continue to be variability in the financial performance of these other companies or future companies in which we may have equity interests.
As a business with a limited operating history, we may encounter unforeseen expenses, difficulties, complications, delays, and other known and unknown obstacles. We have encountered in the past, and will encounter in the future, risks and uncertainties frequently experienced by growing companies with limited operating histories in emerging and rapidly changing industries. If our assumptions regarding these risks and uncertainties, which we use to plan and operate our business, are incorrect or change, or if we do not address these risks successfully, our results of operations could differ materially from our expectations, and our business, financial condition, and results of operations could be adversely affected.
If we cannot maintain and expand current customer partnerships and enter into new customer partnerships, our business could be adversely affected.
We do not generate substantial revenue from our own products, and instead generate revenue from customer collaborations in which we provide services, and also receive downstream value in the form of royalties, equity, or milestone payments. As a result, our success depends on our ability to expand the number, size and scope of our customer collaborations. Our ability to win new business depends on many factors, including our reputation in the market, the quality of our service offerings relative to alternatives, the pricing and efficiency of our services relative to alternatives, and our technical capabilities. If we fail to maintain a position of strength in any of these factors, our ability to either sign new customer collaborations or launch new programs with existing customers may suffer and this could adversely affect our prospects. Additionally, in the process of developing programs, we generate Foundry know-how and accumulate meaningful biological and data assets, including optimized proteins and organisms, characterized genetic parts, enhanced understanding of metabolic pathways, biological, chemical, and genetic libraries, and other elements of biological data. Data and know-how generated from our programs provide the basis for expanded capabilities that we believe further supports our customer collaborations. As a result, in addition to reducing our revenue or delaying the development of our programs, the loss of one or more of our customer relationships or the failure to add new customers or programs may hinder our accumulation of such information, thus hindering our efforts to advance our technological differentiation and improve our platform.
We engage in conversations with companies regarding potential customer collaborations on an ongoing basis. We may spend considerable time and money engaging in these conversations and feasibility assessments, including, understanding the technical approach to a program, customer concerns and limitations, and legal or regulatory landscape of a potential program or offering, which may not result in a commercial agreement. Even if an agreement is reached, the resulting relationship may not be successful for many reasons, including our inability to
12
complete a program to our customers’ specifications or within our customers’ time frames, or unsuccessful development or commercialization of products or processes by our customers. In such circumstances, our revenues and downstream value potential from such a collaboration might be meaningfully reduced.
We currently own and may in the future own equity interests in other operating companies, including certain of our customers; consequently, we have exposure to the volatility and liquidity risks inherent in holding their equity and overall operational and financial performance of these businesses.
We currently own equity interests in several of our customers. In the future, we may also own equity interests in other companies. The process by which we receive equity interests and the factors we consider in deciding whether to accept, hold or dispose of these equity positions may differ significantly from those that an independent investor would evaluate when considering equity interests in a company. Owning equity increases our exposure to the risks of the other company and, in the case of customers, beyond the products of our collaborations. Our equity ownership positions expose us to market volatility and the potential for negative returns. We may have restrictions on resale or limited markets to sell our equity ownership. In many cases, our equity position is a minority position which exposes us to further risk, as we are not able to exert control over the companies in which we hold securities.
In connection with future collaborations or joint ventures, we may, from time to time, receive warrants, or options, all of which involve special risks. To the extent we receive warrants or options in connection with future collaborations or joint ventures, we would be exposed to risks involving pricing differences between the market value of underlying securities and our exercise price for the warrants or options, a possible lack of liquidity, and the related inability to close a warrant or option position, all of which could ultimately have an adverse effect on our financial position.
We leverage our own resources and partner with strategic and financial investors in order to help early stage companies and innovators secure funding and benefit from our platform, which exposes us to a number of risks.
Since our founding, we have helped to launch new companies (such as Motif FoodWorks, Inc., Allonnia LLC, Arcaea LLC (FKA Kalo Ingredients LLC), Ayana Bio, LLC and Verb Biotics, LLC) by bringing together strategic and financial investors to secure funding for these early stage and small companies. Going forward, we intend to continue to leverage our own balance sheet and partner with investors in order to enable companies at all stages to benefit from our platform.
Partnering with and investing in early stage and small companies may expose us to a number of risks, including that early stage and small companies may have:
| • | shorter operating histories, narrower product lines and smaller market shares than larger businesses, which tend to render small companies more vulnerable to competitors’ actions and market conditions, as well as general economic downturns; |
| • | more limited access to capital and higher funding costs, may be in a weaker financial position and may need more capital than originally anticipated to expand, compete and operate their business; |
| • | the inability to obtain financing from the public capital markets or other traditional sources, such as commercial banks, in part because loans made to these types of companies entail higher risks than loans made to companies that have larger businesses, greater financial resources or are otherwise able to access traditional credit sources on more attractive terms; |
| • | a higher likelihood of depending on the management talents and efforts of a small group of persons; therefore, the death, disability, resignation or termination of one or more of these persons could have a material adverse impact on such company and, in turn, on us; |
13
| • | less predictable operating results, may be engaged in rapidly changing businesses with products subject to a substantial risk of obsolescence, and may require substantial additional capital to support their operations, finance expansion or maintain their competitive position; |
| • | particular vulnerabilities to changes in customer preferences and market conditions, depend on a limited number of customers, and face intense competition, including from companies with greater financial, technical, managerial and marketing resources; and |
| • | fewer administrative resources, which can lead to greater uncertainty in their ability to generate accurate and reliable financial data, including their ability to deliver audited financial statements. |
Any of these factors or changes thereto could impair an early stage or small company’s financial condition, results of operation, cash flow or result in other adverse events, such as bankruptcy. This, in turn, could result in losses in our investments and a change in our income (loss) on investments.
We may pursue strategic acquisitions and investments that could have an adverse impact on our business if they are unsuccessful.
We have made acquisitions in the past and, if appropriate opportunities become available, we may acquire additional businesses, assets, technologies, or products to enhance our business in the future, but our ability to do so successfully cannot be ensured. We have also made investments in companies that we view as synergistic with our business. Although we conduct due diligence on these acquisitions and investments, such processes may fail to reveal significant liabilities and we could incur losses resulting from undiscovered liabilities of the acquired business that are not covered by indemnification we may obtain from the seller. Even if we identify suitable opportunities, we may not be able to complete such acquisitions on favorable terms or at all. Any acquisitions we make may not strengthen our competitive position, and these transactions may be viewed negatively by customers or investors. We may decide to incur debt or spend cash in connection with an acquisition, which may cause us to face liquidity concerns or be subject to restrictive covenants in the future. We may also issue common stock or other equity securities to the stockholders of the acquired company, which would reduce the percentage ownership of our existing stockholders. In addition, we may not be able to successfully integrate the acquired personnel, assets, technologies, products and/or operations into our existing business in an effective, timely, and non-disruptive manner or retain acquired personnel following an acquisition. Acquisitions may also divert management’s attention from day-to-day responsibilities, increase our expenses and reduce our cash available for operations and other uses. In addition, we may not be able to fully recover the costs of such acquisitions or be successful in leveraging any such strategic transactions into increased business, revenue, or profitability. We also cannot predict the number, timing, or size of any future acquisitions or the effect that any such transactions might have on our operating results.
Accordingly, although there can be no assurance that we will undertake or successfully complete any acquisitions, any transactions that we do complete may not yield the anticipated benefits and may be subject to the foregoing or other risks and have a material and adverse effect on our business, financial condition, results of operations, and prospects. Conversely, any failure to pursue or delay in completing any acquisition or other strategic transaction that would be beneficial to us could delay the development of our platform or advancement of our programs and, thus, potential commercialization of our customer’s products.
Our programs may not achieve milestones and other anticipated key events on the expected timelines or at all, which could have an adverse impact on our business and could cause the price of New Ginkgo common stock to decline.
We may adopt various technical, manufacturing, regulatory, commercial, and other objectives for our programs. These milestones may include our or our customer’s expectations regarding the commencement or completion of technical development, the achievement of manufacturing targets, the submission of regulatory filings, or the realization of other development, regulatory, or commercialization objectives by us or our customers. The
14
achievement of many of these milestones may be outside of our control. All of these milestones are based on a variety of assumptions, including assumptions regarding capital resources, constraints, and priorities, progress of and results from research and development activities, and other factors, including impacts resulting from the COVID-19 pandemic, any of which may cause the timing of achievement of the milestones to vary considerably. If we, our collaborators, or our customers fail to achieve milestones in the expected timeframes, the commercialization of our programs may be delayed, our credibility may be undermined, our business and results of operations may be harmed, and the trading price of New Ginkgo common stock may decline.
We must continue to secure and maintain sufficient and stable supplies of laboratory reagents, consumables, equipment, and laboratory services. We depend on a limited number of suppliers, some of which are single-source suppliers, and contract manufacturers for critical supplies, equipment, and services for research, development, and manufacturing of our products and processes. Our reliance on these third parties exposes us to risks relating to costs, contractual terms, supply, and logistics, and the loss of any one or more of these suppliers or contract manufacturers or their failure to supply us with the necessary supplies, equipment, or services on a timely basis, could cause delays in our research, development, or production capacity and adversely affect our business.
The COVID-19 pandemic has caused substantial disruption in global supply chains and the ability of third parties to provide us services on a timely basis or at all. As a result, we have experienced shortages in some of our key equipment and supplies, including those required in our labs, as well as, disruptions in services provided by third parties, and may continue to do so in the future as a result of the pandemic, or otherwise. We may also experience price increases, quality issues and longer lead times due to unexpected material shortages, service disruptions, and other unanticipated events, which may adversely affect our supply of lab equipment, lab supplies, chemicals, reagents, supplies, and lab services. For some suppliers, we do not enter into long-term agreements and instead secure our materials and services on a purchase order basis. Our suppliers may reduce or cease their supply of materials or services to us at any time in the future. If the supply of materials or services is interrupted, our programs may be delayed.
We depend on a limited number of suppliers for critical items, including lab consumables and equipment, for the development of our programs. Some of these suppliers are single-source suppliers. We do not currently have the infrastructure or capability internally to manufacture these items at the necessary scale or at all. Although we have a reserve of supplies and although alternative suppliers exist for some of these critical products, services, and equipment, our existing processes used in our Foundries have been designed based on the functions, limitations, features, and specifications of the products, services, and equipment that we currently utilize. While we work with a variety of domestic and international suppliers, our suppliers may not be obligated to supply products or services or our arrangements may be terminated with relatively short notice periods. Additionally, we do not have any control over the process or timing of the acquisition or manufacture of materials by our manufacturers and cannot ensure that they will deliver to us the items we order on time, or at all.
In particular, we rely on Twist Bioscience Corporation for custom DNA synthesis and Thermo Fisher Scientific Inc. for certain instruments and consumables. The price and availability of DNA, chemicals, reagents, equipment, consumables, and instruments have a material impact on our ability to provide Foundry services. We also rely on third parties, such as Berkeley Lights, Inc., to develop workflows to use the equipment they provide to us. We may rely on contract manufacturers like Fermic, s.a. de.c.v for scale-up fermentation development, fermentation, and manufacturing of products for some customers.
The loss of the products, services, and equipment provided by one or more of our suppliers could require us to change the design of our research, development, and manufacturing processes based on the functions, limitations, features, and specifications of the replacement items or seek out a new supplier to provide these items. Additionally, as we grow, our existing suppliers may not be able to meet our increasing demand, and we may need to find additional suppliers. We may not be able to secure suppliers who provide lab supplies at, or equipment and services to, the specification, quantity, and quality levels that we demand (or at all) or be able to negotiate acceptable fees and terms of services with any such suppliers.
15
As described above, some lab equipment, lab consumables, and other services and materials that we purchase are purchased from single-source or preferred suppliers, which limits our negotiating leverage and our ability to rely on additional or alternative suppliers for these items. Our dependence on these single-source and preferred suppliers exposes us to certain risks, including the following:
| • | our suppliers may cease or reduce production or deliveries, raise prices, or renegotiate terms; |
| • | we may be unable to locate a suitable replacement on acceptable terms or on a timely basis, if at all; |
| • | if there is a disruption to our single-source or preferred suppliers’ operations, and if we are unable to enter into arrangements with alternative suppliers, we will have no other means of continuing the relevant research, development, or manufacturing operations until they restore the affected facilities or we or they procure alternative sources of supply; |
| • | delays caused by supply issues may harm our reputation, frustrate our customers, and cause them to turn to our competitors for future programs; and |
| • | our ability to progress the development of existing programs and the expansion of our capacity to begin future programs could be materially and adversely impacted if the single-source or preferred suppliers upon which we rely were to experience a significant business challenge, disruption, or failure due to issues such as financial difficulties or bankruptcy, issues relating to other customers such as regulatory or quality compliance issues, or other financial, legal, regulatory, or reputational issues. |
Moreover, to meet anticipated market demand, our suppliers may need to increase manufacturing capacity, which could involve significant challenges. This may require us and our suppliers to invest substantial additional funds and hire and retain the technical personnel who have the necessary experience. Neither we nor our suppliers may successfully complete any required increase to existing research, development, or manufacturing capacity in a timely manner, or at all.
For the nine months ended September 30, 2021, our cost of lab equipment, lab supplies, and lab services accounted for a significant portion of our total research and development expenses. In the event of price increases by suppliers, we may attempt to pass the increased costs to our customers. However, we may not be able to raise the prices of our Foundry services sufficiently to cover increased costs resulting from increases in the cost of our materials and services, or the interruption of a sufficient supply of materials or services. As a result, materials and services costs, including any price increase for our materials and services, may negatively impact our business, financial condition, and results of operations.
Some of our suppliers and contract manufacturers are foreign entities. We may face disruptions due to the inability to obtain customs clearances in a timely manner or restrictions on shipping or international travel due to the COVID-19 pandemic. As a result of ongoing global supply chain challenges resulting in very long lead times for certain products and equipment, we may order in larger volumes in order to secure the supplies we require for our future operations, which may negatively impact our financial conditions, especially if we are unable to use the supplies ordered.
We use biological, hazardous, flammable and/or regulated materials that require considerable training, expertise and expense for handling, storage and disposal and may result in claims against us.
We work with biological and chemical materials that could be hazardous to human, animal, or plant health and safety or the environment. Our operations produce hazardous and biological waste products, and we largely contract with third parties for the disposal of these products. Federal, state, and local laws and regulations govern the use, generation, manufacture, storage, handling, and disposal of these materials and wastes. Compliance with applicable laws and regulations is expensive, and current or future laws and regulations may restrict our operations. If we do not comply with applicable laws and regulations, we may be subject to fines and penalties.
16
In addition, we cannot eliminate the risk of (a) accidental or intentional injury or (b) release, or contamination from these materials or wastes, which could expose us to liability. Furthermore, laws and regulations are complex, change frequently, and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance. Accordingly, in the event of release, contamination, or injury, we could be liable for the resulting harm or penalized with fines in an amount exceeding our resources and our operations could be suspended or otherwise adversely affected. These liabilities could also include regulatory actions, litigation, investigations, remediation obligations, damage to our reputation and brand, supplemental disclosure obligations, loss of customer, consumer, and partner confidence in the safety of our laboratory operations, impairment to our business, and corresponding fees, costs, expenses, loss of revenues, and other potential liabilities, as well as, increased costs or loss of revenue or other harm to our business.
The release of genetically modified organisms or materials, whether inadvertent or purposeful, into uncontrolled environments could have unintended consequences, which may result in increased regulatory scrutiny and otherwise harm our business and financial condition.
The genetically engineered organisms and materials that we develop may have significantly altered characteristics compared to those found in the wild, and the full effects of deployment or release of our genetically engineered organisms and materials into uncontrolled environments may be unknown. In particular, such deployment or release, including an unauthorized release, could impact the environment or community generally or the health and safety of our employees, our customers’ employees, and the consumers of our customers’ products.
In addition, if a high profile biosecurity breach or unauthorized release of a biological agent occurs within our industry, our customers and potential customers may lose trust in the security of the laboratory environments in which we produce genetically modified organisms and materials, even if we are not directly affected. Any adverse effect resulting from such a release, by us or others, could have a material adverse effect on the public acceptance of products from engineered cells and our business and financial condition. Such a release could result in increased regulatory scrutiny of our facilities, platform, and programs, and could require us to implement additional costly measures to maintain our regulatory permits, licenses, authorizations and approvals. To the extent such regulatory scrutiny or changes impact our ability to execute on existing or new programs for our customers, or make doing so more costly or difficult, our business, financial condition, or results of operations may be adversely affected. In addition, we could have exposure to liability for any resulting harm, as well as to regulatory actions, litigation, investigations, remediation obligations, damage to our reputation and brand, supplemental disclosure obligations, loss of customer, consumer, and partner confidence in the safety of engineered cells materials, and organisms, impairment to our business, and corresponding fees, costs, expenses, loss of revenues, and other potential liabilities, as well as, increased costs or loss of revenue or other harm to our business.
We could synthesize DNA sequences or engage in other activity that contravenes biosecurity requirements, or regulatory authorities could promulgate more far-reaching biosecurity requirements that our standard business practices cannot accommodate, which could give rise to substantial legal liability, impede our business, and damage our reputation.
The Federal Select Agent Program (“FSAP”) involves rules administered by the Centers for Disease Control and Prevention and the Animal and Plant Health Inspection Service that regulate possession, use, and transfer of biological select agents and toxins that have the potential to pose a severe threat to public, animal, or plant health or to animal or plant products. In accordance with the International Gene Synthesis Consortium’s (“IGSC”) Harmonized Screening Protocol for screening of synthetic DNA sequence orders, we follow biosafety and biosecurity industry practices and avoid DNA synthesis activities that implicate FSAP rules by screening synthetic DNA sequence orders against the IGSC’s Regulated Pathogen Database; however, we could err in our observance of compliance program requirements in a manner that leaves us in noncompliance with FSAP or
17
other biosecurity rules. In addition, authorities could promulgate new biosecurity requirements that restrict our operations. One or more resulting legal penalties, restraints on our business or reputational damage could have material adverse effects on our business, financial condition, or results of operations.
Third parties may use our engineered cells materials, and organisms and accompanying production processes in ways that could damage our reputation.
After our customers have received our engineered cells materials, and organisms and accompanying production processes, we do not have any control over their use and our customers may use them in ways that are harmful to our reputation. In addition, while we have established a biosecurity program designed to comply with biosafety and biosecurity requirements and export control requirements in an effort to ensure that third parties do not obtain our engineered cells or other biomaterials for malevolent purposes, we cannot guarantee that these preventative measures will eliminate or reduce the risk of the domestic and global opportunities for the misuse or negligent use of our engineered cells materials, and organisms and production processes. Accordingly, in the event of such misuse or negligent use, our reputation, future revenue, and operating results may suffer.
International expansion of our business exposes us to business, regulatory, political, operational, financial, and economic risks associated with doing business outside of the United States.
We currently market our services and deliver our programs, materials, and processes outside of the United States and may market future offerings outside of the United States. We, and our suppliers, collaborators, and customers, currently conduct business outside of the United States. From time to time, our services may include the hiring or secondment of our employees outside the United States at third party facilities or require the hiring or secondment of foreign persons within our facilities. Accordingly, we are subject to a variety of risks inherent in doing business internationally, and our exposure to these risks will increase as we continue to expand our operations and customer base. These risks include:
| • | political, social and economic instability; |
| • | fluctuations in currency exchange rates; |
| • | higher levels of credit risk, corruption, and payment fraud; |
| • | enhanced difficulties of integrating any foreign acquisitions; |
| • | increased expenses and diversion of our management’s attention from advancing programs; |
| • | regulations that might add difficulties in repatriating cash earned outside the United States and otherwise prevent us from freely moving cash; |
| • | import and export controls and restrictions and changes in trade regulations; |
| • | compliance with the U.S. Foreign Corrupt Practices Act, the U.K. Bribery Act, and similar laws in other jurisdictions; |
| • | multiple, conflicting and changing laws and regulations such as privacy, security and data use regulations, tax laws, tariffs, trade regulations, economic sanctions and embargoes, employment laws, anti-corruption laws, regulatory requirements, reimbursement or payor regimes and other governmental approvals, permits and licenses; |
| • | failure by us, our collaborators or our customers to obtain regulatory clearance, authorization or approval for the use of our services in various countries; |
| • | additional potentially relevant third-party patent rights; |
| • | complexities and difficulties in obtaining intellectual property protection and enforcing our intellectual property; |
18
| • | difficulties in staffing and managing foreign operations, including difficulties related to the increased operations, travel, infrastructure and legal compliance costs associated with international locations; |
| • | logistics and regulations associated with shipping chemicals, biomaterials and product samples, including infrastructure conditions and transportation delays; |
| • | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises, on demand and payment for our products and exposure to foreign currency exchange rate fluctuations; |
| • | natural disasters, political and economic instability, including wars, terrorism and political unrest, the outbreak of disease, or public health epidemics, such as COVID-19, which could have an adverse impact on our employees, contractors, customers, partners, travel and the global economy; |
| • | breakdowns in infrastructure, utilities and other services; |
| • | boycotts, curtailment of trade and other business restrictions; and |
| • | the other risks and uncertainties described in this prospectus. |
Additionally, as part of our growth strategy, we will continue to evaluate potential opportunities for international expansion. Operating in international markets requires significant resources and management attention and will subject us to regulatory, economic and political risks in addition to those we face in the United States. However, our international expansion efforts may not be successful, which could limit the size of our market or the ability to provide services or programs internationally.
In addition, due to potential costs from any international expansion efforts and potentially higher supplier costs outside of the United States, our international operations may operate with a lower margin profile. As a result, our margins may fluctuate as we expand our operations and customer base internationally.
Any of these factors could significantly harm our future international expansion and operations and, consequently, our revenue and results of operations.
Our ability to enter into a definitive agreement with the U.S. International Development Finance Corporation and our overall level of indebtedness could adversely affect liquidity and have an adverse effect on our valuation, operations and business.
On November 25, 2020, the U.S. International Development Finance Corporation (“DFC”) announced its approval to extend a loan of up to $1.1 billion to us to aid in the expansion of our commercial biosecurity offering at a global scale, contingent on our entering into a definitive loan agreement with DFC. In the event we finalize and enter into an agreement and accept a loan from the DFC, we may be subject to negative covenants limiting our ability to enter into certain transactions or incur indebtedness. We may also be required to maintain a leverage ratio and other financial metrics within certain limits. Incurring indebtedness could increase our vulnerability to sustained, adverse macroeconomic weakness and limit our ability to obtain further financing. Alternatively, if we do not enter into an agreement and accept a loan from the DFC, we may not have access to capital sufficient to scale our facilities and capabilities to the extent necessary to be competitive in the biosecurity market.
General risks relating to litigation.
Any of the risks identified above could result in significant litigation. In addition to the specific litigation-related risks identified above, litigation of any kind carries certain inherent risks. Because of the substantial amount of discovery required in connection with litigation in U.S. courts, there is a risk that some of our confidential information could be compromised in the discovery process. There could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on our share price.
19
Further, our agreements with some of our customers, suppliers or other entities require us to defend or indemnify these parties if they become involved in infringement claims that target our products, services or technologies, or in certain other situations. If we must defend or indemnify third parties, we could incur significant costs and expenses that could adversely affect our business, operating results or financial condition.
Short sellers may engage in manipulative activity intended to drive down the market price of our Class A common stock, which could also result in related regulatory and governmental scrutiny, among other effects.
Short selling is the practice of selling securities that the seller does not own but rather has borrowed or intends to borrow from a third party with the intention of later buying lower priced identical securities to return to the lender. Accordingly, it is in the interest of a short seller of our Class A common stock for the price to decline. At any time, short sellers may publish, or arrange for the publication of, opinions or characterizations that are intended to create negative market momentum. Issuers, like us, whose securities have historically had limited trading history or volumes and/or have been susceptible to relatively high volatility levels can be vulnerable to such short seller attacks. Short selling reports can cause increased volatility in an issuer’s stock price, and result in regulatory and governmental inquiries. On October 6, 2021, such a report was published about us. Shortly after, we received a preliminary and informal inquiry from the U.S. Department of Justice related to such report. Any related inquiry or formal investigation from a governmental organization or other regulatory body, including any inquiry from the U.S. Securities and Exchange Commission, could result in a material diversion of our management’s time and could have a material adverse effect on our business and results of operations.
Risks Related to New Ginkgo’s Customers
We rely on our customers to develop, produce and manufacture products using the engineered cells and/or biomanufacturing processes that we develop. If these initiatives by our customers are not successful or do not achieve commercial success, or if our customers discontinue their development, production and manufacturing efforts using our engineered cells and/or biomanufacturing processes, our future financial position may be adversely impacted.
We operate as a platform company. As such, we largely rely on our customers to commercialize products that may be enabled by our engineered cells and/or biomanufacturing processes. A portion of the value in our customer collaborations is earned through downstream value sharing in the form of equity, royalty streams, or milestone payments. If our customers are not successful in bringing these products to market, the downstream portion of our value will be adversely impacted. Because we do not directly control manufacturing, product or downstream process development or commercialization, we have limited ability to impact the quality of our partners’ production processes and ultimate commercial success.
In addition, our customers may simply choose not to develop or commercialize a product we have enabled in which we are entitled to downstream value sharing. In our current relationships, we would have limited or no recourse to find alternative methods to monetize these products without the original customer. Because this industry is still nascent and the regulatory environment is evolving, we have limited historical information on the probability of commercial success for bioengineered products or biomanufacturing processes in the market and have limited ability to underwrite the likelihood that our customers will be able to create valuable products or processes in their market using the results of their programs with us. If we overestimate the probability of commercial success, the price of New Ginkgo common stock may be adversely impacted as a result of lower expectations for future cash flows from customer collaborations.
Our revenue is concentrated in a limited number of customers, some of which are related parties, and our revenue, results of operations, cash flows and reputation in the marketplace may suffer upon the loss of a significant customer.
We have derived, and may continue to derive, a significant portion of our revenue from a limited number of large customers. During the nine months ended September 30, 2021, two customers, Cronos Group Inc. and New
20
Jersey Department of Corrections, each represented more than 32.9% of our total revenue and cumulatively represented 39.4% of our total revenue. Due to the significant time required to acquire new customers, to plan and develop new programs for customers, and to satisfactorily execute on existing programs, the loss of one or both of these customers, or the loss of any other significant customer or a significant reduction in the amount of demand from a significant customer would adversely affect our revenue, results of operations, cash flows and reputation in the marketplace. There is always a risk that existing customers will not elect to do business with us in the future or will experience financial difficulties. If our customers experience financial difficulties or business reversals which reduce or eliminate the need for our services, they may be unable or unwilling to fulfill their contracts with us. There is also the risk that our customers will attempt to impose new or additional requirements on us that reduce the profitability of the services performed by Ginkgo. Our customer concentration also increases the concentration of our accounts receivable and our exposure to payment defaults by key customers, which could expose us to substantial and potentially unrecoverable costs if we do not receive payment from key customers. Additionally, the loss of any significant customer could pose reputational harm to Ginkgo and make it more challenging to acquire new customers.
In addition, while our customer collaborations are typically multi-year, we generally do not require our customers to generate a minimum amount of annual demand and without such contracts, our customers are not obligated to use our services beyond the amounts they choose to incur. Our customers may choose to use less of our services depending on program progress, their own technological capabilities, market demand for their products and/or their own internal budget cycles. As a result, we cannot accurately predict our customers’ decisions to reduce or cease utilizing our services. Even where we enter into long-term contracts with our customers, there is no guarantee that such agreements will be negotiated on terms that are commercially favorable to us in the long-term. In addition, existing customers may choose to perform some or all of the services they expect from us internally, with another third-party partner or by using capabilities from acquisitions of assets.
In certain cases, our business partners may have discretion in determining when and whether to make announcements about the status of our collaborations, including about developments and timelines for advancing programs, and the price of New Ginkgo common stock may decline as a result of announcements of unexpected results or developments.
Generally, we and our customers must mutually agree on determining when and whether to make announcements about the status of our collaborations, including developments in our programs and timelines for commercialization of or improvements to products using engineered cells developed using our platform. However, in some cases our customers may report or otherwise may be obligated to disclose certain matters without our consent. Our partners may also wish to report such information more or less frequently than we intend to or may not wish to report such information at all. We or our partners may announce a collaboration or partnership even if there is no guarantee that we will recognize program fees. The price of New Ginkgo common stock may decline as a result of a public announcement of unexpected results or developments in our partnerships, or as a result of our partners not consenting to an announcement or withholding information.
Risks Related to the COVID-19 Pandemic
The recent COVID-19 pandemic and the global attempt to contain it may harm our business and results of operations.
The full impact of the continuing COVID-19 pandemic and related public health measures on our business will depend largely on future developments, including the duration and severity of the pandemic, which remains highly uncertain. On March 11, 2020, the World Health Organization declared the outbreak of COVID-19 caused by a novel strain of coronavirus as a pandemic, which continues to spread throughout the United States and around the world. Since then, extraordinary actions have been taken by international, federal, state and local public health and governmental authorities to contain and combat the outbreak and spread of COVID-19 throughout the world. These actions include travel bans, quarantines, capacity limitations at facilities,
21
“stay-at-home” orders and similar mandates for many individuals to substantially restrict daily activities and for many businesses to curtail or cease normal operations.
The COVID-19 pandemic has had, and is expected to continue to have, an adverse impact on our operations, particularly as a result of preventive and precautionary measures that we, other businesses, and governments are taking. For example, as part of these efforts and in accordance with applicable government directives, we initially temporarily suspended some programs at our facilities in Boston, Massachusetts in late March 2020. In addition, we began restricting non-essential travel. As a result of the travel restrictions, we limited in-person sales and marketing activities. We have continued to operate within the rules and guidance applicable to our business during the pandemic, including by requiring physical distancing, quarantining our personnel and reducing capacity limits in our facilities; however, a continuing implementation of these restrictions could further impact our ability to operate effectively and conduct ongoing research and development, laboratory operations, sales and marketing activities or other activities or operations.
We have also incurred expenses associated with our efforts to accommodate personnel during the COVID-19 pandemic, including costs associated with the provision of COVID-19 testing to our personnel, safety accommodations, providing on-site amenities and enhanced on-site cleaning efforts. During the course of the pandemic, we will continue to incur such expenses associated with our operations.
Governmental mandates and guidelines related to COVID-19, other infectious diseases or public health crises, have impacted and we expect them to continue to impact, our personnel and operations and personnel and operations at third-party facilities in the United States and other countries. The pandemic has caused substantial disruption in global supply chains. These interruptions may require us to suspend operations or delay programs. If we continually delay programs with existing customers, we may be in breach of our contracts with existing customers or customers may decide to cease doing business with us. Difficulties and delays such as those we have experienced and may experience in the future may prevent us from meeting our operating and financial goals, both in general and within our targeted timelines, and may cause our revenues and operating results to fluctuate from period to period.
Our operations rely on the availability of laboratory scientists, engineers and facility, safety, quality and compliance personnel to work on-site. If a critical team member falls ill or needs to quarantine, or if a critical mass of our personnel falls ill or needs to quarantine, we may not be able to continue operations. The COVID-19 pandemic has also had an adverse effect on our ability to attract, recruit, interview and hire at the pace we would typically expect to support our rapidly expanding operations, as well as on our ability to build out facilities to accommodate expanding operations. To the extent that any governmental authority imposes additional regulatory requirements or changes existing laws, regulations and policies that apply to our business and operations, such as additional workplace safety measures, our programs may be delayed, and we may incur further costs in bringing our business and operations into compliance with changing or new laws, regulations and policies.
Further, the effect of the COVID-19 pandemic and mitigation efforts on our customers’ and on consumer demand for their products could materially and adversely affect us, particularly to the extent our customers or potential customers experience declines in demand for their goods or services that contain or use our products or services. We may also experience a slow-down in our pipeline of new programs or a termination of existing programs if our customers or potential customers face disruptions during the pandemic.
The COVID-19 pandemic has negatively impacted the global economy, disrupted global supply chains and created significant volatility and disruption of financial markets. The ultimate impact of the COVID-19 pandemic is highly uncertain and subject to sudden change. We are following, and plan to continue to follow, recommendations from federal, state and local governments regarding workplace policies, practices and procedures. We are continuing to monitor the potential impact of the pandemic, including on global supply chains and manufacturing capacity, but we cannot be certain what the overall impact will be on our business, financial condition, results of operations and prospects on a go-forward basis.
22
Uncertainty regarding the ongoing demand and/or capacity (including capacity at third party clinical testing laboratories) of our COVID-19 individual and pooled sample tests could materially adversely affect our business.
Our COVID-19 testing programs are subject to inherent risks of commercial viability, such as demand for tests, price or market share erosion due to competition and the duration of the COVID-19 pandemic. We are in a highly competitive market – many companies have launched or are seeking to launch COVID-19 testing products and many of these companies already have an existing commercial and technical infrastructure to market and commercialize such offerings. We have limited experience marketing or commercializing diagnostic or pooled sample testing programs and may not be able to sufficiently support operations with our current base of personnel or recruit enough personnel to effectively commercialize COVID-19 testing programs, particularly during a pandemic, at which time the pipeline for experienced personnel will be in high demand. Moreover, as vaccines for COVID-19 and at-home or over-the-counter COVID-19 tests become more widely available, and as infection rates decrease, demand for COVID-19 testing may also decrease.
Our COVID-19 testing business relies heavily on the adoption of pooled testing in schools, which may be hesitant to adopt COVID-19 testing without positive support from parents or teachers. Although we make test validation results and protocols available to parents and teachers, they may not trust the accuracy of the tests or may have concerns about how the tests are performed, how samples are used or tracked and whether appropriate privacy measures are being taken with respect to individually identifiable health information, including genetic information. The ability for schools to pay for COVID-19 testing relies heavily on the availability of federal, state or local funding for testing. If such funding is not available or if there are restrictions on the use of such funding for our pooled sample test offerings, our COVID-19 testing business may not be commercially viable. In addition, as a result of the recent approval of a COVID-19 vaccine for children younger than sixteen years of age, the demand for COVID-19 testing in schools could diminish significantly or be eliminated.
Creating the commercial and technical infrastructure to test on a mass scale is expensive. We may also be limited in our ability to scale up based on expense or unavailability of the required materials, equipment, personnel and infrastructure necessary to deliver diagnostic or pooled sample tests on a mass scale. We may not be able to recover our investment expenses with sufficient revenue generated by our diagnostic and pooled sample testing efforts.
Our ability to commercialize our testing programs is also subject to regulatory or governmental controls, decisions or actions. If the U.S. Department of Health and Human Services terminates its Declaration Justifying Emergency Use of Medical Countermeasures because the circumstances justifying emergency use no longer exist, or if the U.S. Food and Drug Administration (“FDA”) requires premarket approval, clearance or other marketing authorization for the third-party COVID-19 tests that are used in our testing services, we may be unable to market or distribute these COVID-19 tests or generate revenues from our test offerings. We may also experience price erosion if federal or state governments implement price controls.
Finally, the sale of each test is dependent on the supply of the appropriate collection devices authorized for use with the COVID-19 tests we utilize in our testing programs. Disruptions in this supply chain will have a material adverse effect on our ability to sell tests.
Uncertainty regarding the sales and delivery of our COVID-19 individual and pooled sample tests could materially adversely affect our business.
Although we have partnerships with third party clinical testing laboratories to support a high volume of pooled sample testing for COVID-19 nationally, pooled testing has not yet been adopted by all states nor have we established partnerships with clinical testing laboratories in all states. We are continuing to develop processes to scale capacity of COVID-19 pooled sample collection and testing. However, we can give no assurance that we will be able to successfully scale the pooled sample collection and test capacity or that we will be able to
23
establish or maintain the collaborative third party relationships that support such testing capacity. In addition, even if we are able to scale to high volume testing nationwide, there can be no assurance that the testing capacity will be used.
We may be subject to tort liability if the COVID-19 tests we utilize in our testing programs provide inaccurate results.
The Public Readiness and Emergency Preparedness Act (the “PREP Act”) provides immunity for manufacturers, distributors, program planners, qualified persons, and their officials, agents, and employees from certain claims under state or federal law for a “loss” arising out of the administration or use of a “covered countermeasure” in the United States. Distributors are certain persons or entities engaged in the distribution of drugs, biologics, or devices. Program planners include persons who supervise or administer a program with respect to the administration, distribution, provision, or use of a Covered Countermeasure (as defined in the PREP Act). Covered Countermeasures include security countermeasures and “qualified pandemic or epidemic products,” including products intended to diagnose or treat pandemic or epidemic disease, such as COVID-19 diagnostic tests, as well as treatments intended to address conditions caused by such products. Covered Countermeasures must also be approved, cleared, or authorized for emergency use, or otherwise authorized for investigational use, by the FDA in order to be considered Covered Countermeasures under the PREP Act.
For these immunities to apply, the Secretary of HHS must issue a declaration in cases of public health emergency or “credible risk” of a future public health emergency. On March 10, 2020, the Secretary of HHS issued a declaration under the PREP Act and has issued subsequent amendments thereto to provide liability immunity for activities related to certain countermeasures against the ongoing COVID-19 pandemic.
We act as the authorized distributor of certain third-party COVID-19 tests and collection kits that have received Emergency Use Authorization (“EUA”) and supervise testing programs for our COVID-19 testing customers. While we believe our test distribution and program planning activities with respect to these programs would be covered under the provisions of the PREP Act, this cannot be assured. Also, there can be no assurance that the U.S. Congress will not act in the future to reduce coverage under the PREP Act or to repeal it altogether.
Furthermore, some of the third-party tests used as part of our pooled testing program are not covered by an EUA and, at this time, we do not believe that such testing services, administration, or program planning related to our pooled testing program will qualify for PREP Act immunity. If product liability lawsuits are brought against us in connection with allegations of harm connected to our COVID-19 testing services, we may incur substantial liabilities and may be required to limit our testing services. The PREP Act is a complex law with limited judicial precedent, and thus even for the third-party COVID-19 tests and collection kits used in our testing services that are subject to EUAs, we may have to expend significant time and legal resources to obtain dismissal of a lawsuit on the basis of PREP Act immunity.
If we cannot successfully defend ourselves against claims that our COVID-19 testing services caused injuries and if we are not entitled to immunity under the PREP Act, or the U.S. Congress limits or eliminates coverage under the PREP Act, or if the liability protections under the PREP Act are not adequate to cover all claims, we may incur substantial liabilities. Regardless of merit or eventual outcome, product liability claims may result in decreased demand for our services, injury to our reputation, costs to defend litigation, loss of revenue, and substantial money awards to customers.
We are dependent on our relationships with our telehealth partner to provide healthcare services, and our business would be adversely affected if those relationships were disrupted.
Our contractual relationships with our telehealth partner who provides physician authorization for COVID-19 diagnostic and screening testing may implicate certain state laws in the United States that generally prohibit non-physician entities from practicing medicine, exercising control over physicians or engaging in certain
24
practices such as fee-splitting with physicians. There can be no assurance that these laws will be interpreted in a manner consistent with our practices or that other laws or regulations will not be enacted in the future that could have a material and adverse effect on our business, financial condition and results of operations. Regulatory authorities, state medical boards of medicine, state attorneys general and other parties, including our telehealth partner, may assert that we are engaged in the prohibited corporate practice of medicine, and/or that its arrangements with its telehealth partner constitutes unlawful fee-splitting. If a state’s prohibition on the corporate practice of medicine or fee-splitting law is interpreted in a manner that is inconsistent with our practices, we would be required to restructure or terminate our relationship with our telehealth partner to bring our activities into compliance with such laws. A determination of non-compliance, or the termination of or failure to successfully restructure these relationships could result in disciplinary action, penalties, damages, fines, and/or a loss of revenue, any of which could have a material and adverse effect on our business, financial condition and results of operations. State corporate practice of medicine doctrines and fee-splitting prohibitions also often impose penalties on healthcare professionals for aiding the corporate practice of medicine, which could discourage our telehealth partner from providing services to us.
Risks Related to the Synthetic Biology Industry
Rapidly changing technology and emerging competition in the synthetic biology industry could make the platform, programs, and products we and our customers are developing obsolete or non-competitive unless we continue to develop our platform and pursue new market opportunities.
The synthetic biology industry is still emerging and is characterized by rapid and significant technological changes, frequent new product introductions and enhancements, and evolving industry demands and standards. Our future success will depend on our ability to sign and initiate new programs that address the evolving needs of our customers on a timely and cost-effective basis, to advance existing programs and to pursue new market opportunities that develop as a result of technological and scientific advances. Additionally, our customers may face significant competition or other risks which may adversely impact our business and results of operations.
There are a number of companies in the broader synthetic biology industry, and our future success will depend on our ability to maintain a competitive position with respect to technological advances. Technological development by others may result in our platform becoming obsolete. Our ability to compete successfully will depend on our ability to develop proprietary technologies that enable our customers to develop products using our platform in a manner that is either less expensive, faster, superior or otherwise differentiated from what a competitor’s technologies and products might enable. If we are unable to continue to successfully advance our platform or the services it provides at scale, or if our customers are unable to commercialize the products or processes made or improved upon by using our platform, our business and results of operations will be adversely impacted.
Due to the significant lead time involved in launching a new program or developing a new product or process using our platform, our customers are required to make a number of assumptions and estimates regarding the commercial feasibility of a new program, including assumptions and estimates regarding the size of an emerging product category and demand for those end-products and processes which will use our technology, the ability to scale-up manufacturing processes to produce a product on a commercial scale, the ability to penetrate that emerging product category, customer adoption of a downstream product, the existence or non-existence of products being simultaneously developed by competitors, potential market penetration and obsolescence, planned or unplanned. As a result, it is possible that we may commence a new program with a customer who wishes to develop a product or process that has been displaced by the time of launch, addresses a market that no longer exists or is smaller than previously thought, that end-consumers do not like or otherwise is not competitive at the time of launch, in each case, after the incurrence of significant opportunity costs on our part to develop such product. The ultimate success of the products developed by our customers using our services may be dependent on the success of other markets in which we or our customers do not operate in or have knowledge or expertise or which, in each case, may not reach the size anticipated by us or our customers or may be replaced by another emerging product category or eliminated entirely.
25
The market, including customers and potential investors, may be skeptical of our ability to deliver on programs because they are based on a relatively novel and complex technology.
The market, including customers and potential investors, may be skeptical of the viability and benefits of bioengineered products as well as New Ginkgo’s enabling abilities, including our platform and programs, because they are based on a relatively novel approach and the adoption of complex technology. There can be no assurance that our platform and programs will be understood, approved, or accepted by customers, regulators and potential investors or that we will be able to sell our services profitably at competitive prices and with features sufficient to establish demand.
In addition, in order for novel products from our programs to be successfully commercialized, support from the entire relevant supply chain is needed. Relationships with all parts of the supply chain are important in order to gain visibility into market trends and feature and specification requirements and in order to ensure customers are able to successfully manufacture their products, obtain regulatory approval and gain access to key distribution channels. If we are unable to convince these potential customers, their suppliers, or the consumers who purchase products containing or made or developed using engineered cells and/or biomanufacturing processes, of the utility and value of such products or that such products are superior to the products they currently use, we will not be successful in entering these markets and our business and results of operations will be adversely affected. If potential investors are skeptical of the success of our platform or cell programs, our ability to raise capital and the value of New Ginkgo common stock may be adversely affected.
Ethical, legal and social concerns about genetically modified organisms and genetically modified materials and their resulting products could limit or prevent the use of products or processes using our technologies, limit public acceptance of such products or processes and limit our revenues.
Our technologies and the technologies of our customers involve the use of genetically modified cells, organisms and biomaterials, including, without limitation, genetically modified organisms (“GMOs”) and genetically modified microorganisms (“GMMs”), genetically modified plant or animal cells and genetically modified proteins and biomaterials (collectively, “Genetically Modified Materials”), and their respective products. The use, production and marketing of Genetically Modified Materials, are subject to laws and regulations in many countries, some of which are new and some of which are still evolving. In the United States, the FDA, the Environmental Protection Agency (“EPA”) and the U.S. Department of Agriculture (“USDA”) are the primary agencies that regulate the use of GMOs, GMMs and potential products derived from GMOs or GMMs. If regulatory approval of the Genetically Modified Materials or resulting products is not secured, our business operations, financial condition and our ability to grow as a business could be adversely affected. We expect to encounter regulations regarding Genetically Modified Materials in most if not all of the countries in which our customers may seek to establish production capabilities or sell their products and the scope and nature of these regulations will likely be different from country to country. Governmental authorities could, for safety, social or other purposes, impose limits on, or implement regulation of, the use, production or marketing of Genetically Modified Materials. If our customers cannot meet the applicable requirements in other countries in which they intend to produce or sell their products, or if it takes longer than anticipated to obtain such approvals, our business could be adversely affected.
In addition, public perception regarding the safety and environmental hazards of, and ethical concerns over, Genetically Modified Materials or the processes used to create them, including gene editing or gene regulating technologies, could influence public acceptance of our and our customers’ technologies, products and processes. For instance, certain advocacy groups engage in efforts that include regulatory legal challenges and labeling campaigns for genetically modified products, as well as application of pressure to consumer retail outlets seeking a commitment not to carry genetically modified foods. These groups in the past have pressured retail food outlets and grocery store chains to publicly state that they will not carry genetically modified foods and have pressed food brands to publicly state that they will not use ingredients produced by genetically modified microbes. In addition, certain labeling-related initiatives have heightened consumer awareness of GMOs, which may make consumers less likely to purchase products containing GMO ingredients, which could have a negative impact on
26
the commercial success of our customers’ products and programs. These concerns could result in increased expenses, regulatory scrutiny, delays or other impediments to our programs. The subject of Genetically Modified Materials has received negative publicity, which has aroused public debate. This adverse publicity has led to, and could continue to lead to, greater regulation and trade restrictions on imports of Genetically Modified Materials or their resulting products. In addition, with the acquisition of Dutch DNA, we are expanding into the European Union market, which has increased government regulation and scrutiny over genetically modified products. There is a risk that products produced using our technologies could cause adverse health effects or other adverse events, which could also lead to negative publicity, regulatory action or private litigation. If we are unable to overcome the ethical, legal and social concerns relating to genetic engineering, our programs could face increased expenses, regulatory scrutiny, delays or other impediments to deliver our programs or the commercialization of resulting products and processes.
Finally, the COVID-19 pandemic may increase biosecurity concerns by public and/or governmental stakeholders regarding genetic engineering technologies and risks around engineered viruses, microbes and organisms. Such concerns, restrictions, or governmental restrictions could limit the use of Genetically Modified Materials in our customers’ products, which could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to New Ginkgo’s Intellectual Property
Our business could be harmed if we are not able to adequately protect our intellectual property.
Our success depends in part on our ability to obtain and maintain intellectual property protection for our proprietary technologies. We protect our proprietary technologies through patents and trade secrets, both of which entail risk. If we are unable to obtain, maintain or protect intellectual property rights related to our technology, or if our intellectual property rights are inadequate, our competitive position, business, financial conditions, results of operations and prospects may be harmed.
Risks related to our patents and patent applications.
Because of the volume and nature of our inventions, patent protection may not be practicable, available, or appropriate for some aspects of our proprietary technologies. While we own patents and pending patent applications in the United States and in foreign jurisdictions, these applications do not ensure the protection of our intellectual property. There may be prior art of which we are not aware. Additionally, obtaining, maintaining, defending and enforcing patents is costly, time consuming and complex, and we may not be able to file and prosecute all necessary or desirable patent applications, or maintain and enforce any patents that may issue from such patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our technologies before it is too late to obtain patent protection. Although we enter into confidentiality agreements with parties who have access to confidential or patentable aspects of our research and development output, such as our employees, collaborators, consultants, advisors and other third parties, any of these parties may breach the agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection.
Further, pending applications may not be issued or may be issued with claims significantly narrower than we currently seek. Patents for which claims have been allowed may be successfully challenged and invalidated. Unless and until our pending applications issue, their protective scope is impossible to determine and, even after issuance, their protective scope may be limited.
Recent changes in patent law have made patents covering life science inventions more difficult to obtain and enforce. Further legislative changes or changes in the interpretation of existing patent law could increase the uncertainty and cost surrounding the prosecution of our owned patent applications and the maintenance, enforcement or defense of our owned patents. The Leahy-Smith America Invents Act (“the Leahy-Smith Act”) included changes that affect the way patent applications are prosecuted; redefine prior art; enable third-party
27
submission of prior art to the United States Patent and Trademark Office (“USPTO”) during patent prosecution; and provide cost-effective avenues for competitors and other third parties to challenge the validity of patents at USPTO-administered post-grant proceedings, including post-grant review, inter partes review and derivation proceedings. Thus, the Leahy-Smith Act and its continued implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Other changes in the law may further detract from the value of life science patents and facilitate challenges to our patents. In some cases, we use genetic sequence information from naturally occurring organisms, which may not be patentable. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection for naturally occurring sequences and for inventions based on the observation and exploitation of natural phenomena. These decisions have weakened the rights of patent owners in certain situations. The U.S. Court of Appeals for the Federal Circuit has also issued a series of rulings that create obstacles to the patenting of groups of genetic sequences that share functional characteristics, making it more difficult to obtain claims to certain genetic constructs, particularly antibodies. These changes in the law have created uncertainty with respect to the validity and enforceability of patents covering natural and engineered sequences. Depending on future actions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a further material adverse effect on our patent rights and our ability to protect, defend and enforce our patent rights in the future.
Further, the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our patents may be challenged in the courts or patent offices in the United States and abroad. An adverse determination in any such challenge could result in loss of exclusivity, or patent claims being narrowed, invalidated or held unenforceable, in whole or in part. Any of these results could limit our ability to stop others from using or commercializing similar or identical technology to compete directly with us. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, regardless of the outcome, it could dissuade companies from collaborating with us. Such proceedings also may result in substantial cost and require significant time from our scientists and management, even if the eventual outcome is favorable to us.
The laws of some foreign countries do not protect intellectual property rights to the same extent as do the laws of the United States or may apply different rules concerning the assignment of intellectual property rights. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. We may encounter similar difficulties, particularly as we expand to work with foreign employees and contractors and expand our collaboration activities into foreign markets. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents by foreign holders and, in some cases, do not favor the enforcement of patents at all, particularly patents in the life sciences. This could make it difficult for us to stop the infringement of our patents. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business and could be unsuccessful.
Reductions in the scope or enforceability of our patent protection may adversely impact our customers’ ability to commercialize their products and may thus reduce our downstream value from royalties, equity, or commercial milestone payments.
Risks relating to trade secrets and other proprietary information and biomaterials.
Because patent protection may not be available or appropriate for significant aspects of the technology we are developing, our success may depend in large part on our proprietary information, including genetic and other chemical and biological data, processes, know-how, and other trade secrets developed over years of research and development, some of which are embodied in proprietary software. We rely heavily on trade secret protections,
28
especially in cases where we believe patents or other forms of registered intellectual property protection may not be appropriate or obtainable. However, trade secrets are difficult to protect. The secrecy of the Company’s trade secrets must be maintained for them to retain their status and protection as trade secrets. While we strive to protect the secrecy of our trade secrets and other proprietary information, including by requiring our employees, customers, consultants, and contractors to enter into confidentiality agreements and instituting multilayered protections covering our digital environment and biomaterials, we may not be able to adequately protect our trade secrets or other proprietary information. We cannot guarantee that we have entered into such agreements with every party that may have or has had access to our trade secrets, biomaterials or proprietary technology and processes. Further, despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches.
We seek to preserve the integrity and confidentiality of our information by maintaining physical security of our premises and physical and electronic security of our information technology systems, but it is possible that these security measures could be breached. We also rely on systems provided by third parties, which may suffer security breaches or incidents. Such security breaches may be inadvertent or may come about due to intentional misconduct or other malfeasance or by human error or technical malfunctions, including those caused by hackers, employees, contractors, or vendors. It may be difficult or impossible to recover trade secrets or other confidential information once it is hacked, and hackers may operate from jurisdictions that will not cooperate with such efforts. Enforcing any claim that a third party unlawfully obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts in some jurisdictions are less willing or unwilling to protect trade secrets even when a hacker or thief can be identified.
Our competitors may lawfully obtain or independently develop knowledge that is equivalent to one or more of our trade secrets. Were they to do so, we would be unable to prevent them from using that independently developed knowledge. Such a competitor could claim that we had learned the trade secret from them and bring an action against us on that basis. If any of our trade secrets were to be disclosed to or independently developed by a competitor or other third party, our competitive position could be materially and adversely harmed. Moreover, a competitor could file for patent protection covering intellectual property that we have chosen to protect as a trade secret. In such a case, we might be restricted or excluded from using that intellectual property even if we had developed it before our competitor did.
Our facilities hold large collections of microbial strains, cell lines and other biomaterials. Failure to implement adequate controls and protections, failure to implement adequate disposal procedures, unauthorized visitors in the labs, or customers’ failure to adequately protect biological materials can put us and our customers at risk of losing valuable assets through negligence or theft and enabling the use of those lost materials by our competitors. While we believe that we take reasonable measures to protect the security of biomaterials owned by us or our customers, it is possible that our security controls and practices may not prevent unauthorized or other improper access to such genetic material. Any unauthorized access, acquisition, use, destruction, or release of the genetically modified organisms we engineer could result in our having exposure to significant liability under our contracts, as well as to regulatory actions, litigation, investigations, remediation obligations, damage to our reputation and brand, supplemental disclosure obligations, loss of customer, consumer, or partner confidence in the security of our platform, impairment to our business, and corresponding fees, costs, expenses, loss of revenues, and other potential liabilities.
Our customers sometimes provide organisms, genetic material and/or data to us in connection with our collaborations. In the event that we fail to protect customer materials or data or inadvertently use such materials or data for unauthorized purposes, we could be liable to our customers under trade secret laws or contractual provisions.
There could be unintended consequences to the environment generally or the health and safety of our employees or the public as a result of an unauthorized release of genetically modified materials into uncontrolled
29
environments. In addition, if a biosecurity breach or unauthorized release of genetic material were to occur within our industry, our customers and potential customers might lose trust in the security of the laboratory environments in which we produce genetically modified organisms, even if we are not directly affected. Any adverse effect resulting from such a release, by us or others, could have a material adverse effect on the public acceptance of our products and business and our financial condition. Such a release could result in enhanced regulatory activity and we could have exposure to liability for any resulting harm.
Risks relating to ownership of inventions and biomaterials.
Certain of our employees, consultants and contractors were previously employed at universities or other software or biotechnology companies, including our competitors or potential competitors. Additionally, some of our consultants or contractors may have ongoing relationships with universities. Although we try to ensure that our employees, consultants and contractors do not use the intellectual property of others in their work for us, we may be subject to claims that these individuals or other contractors have used or disclosed intellectual property, including trade secrets or other proprietary information, of another. Litigation may result from these claims.
While it is our policy to require that our employees, consultants and contractors who may be involved in the development of intellectual property execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our intellectual property assignment agreements with them may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property. Such claims could have a material adverse effect on our business, financial condition, results of operations and prospects.
If we are unsuccessful in litigating any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel, which could have a material adverse effect on our competitive business position and prospects. Such intellectual property rights could be awarded to a third party, and we could be required to obtain a license from such third party to use or commercialize our technology or products, which license might not be available on commercially reasonable terms, or at all. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to our management and employees.
The life science academic and research community has abided by norms of free exchange of biomaterials, but recently, norms have begun to change so that parties may assert ownership and control over biomaterials that they permitted to be freely disseminated in the past. Thus, despite our best efforts to confirm our right to use biomaterials in our possession, we may use organisms that we believe to be free of encumbrance that are, in fact, subject to claims of title by others. In such a situation, litigation may be required to clear title, if it can be cleared at all. Similarly, we may be subject to claims that we have used biomaterials obtained from licensors or repositories for unauthorized purposes, or purposes not consistent with the licensing terms of the providing organization.
Risks related to the vindication or enforcement of our intellectual property rights.
Competitors and other third parties may infringe or otherwise violate our issued patents or other intellectual property. In addition, our patents may become involved in inventorship, ownership, or priority disputes. We may also become subject to claims by collaboration partners that intellectual property or biomaterials that we believe to be owned by us is actually owned by them. Any litigation concerning any of these issues would be expensive, time consuming and uncertain. There can be no assurances that we would prevail in any suit brought by us or against us by third parties, or successfully settle or otherwise resolve those claims. Significant litigation would have substantial costs, even if the eventual outcome were favorable to us, and would divert management’s attention from our business objectives.
30
Risks related to intellectual property developed under U.S. federally funded research grants and contracts.
Some of our inventions, data, or other intellectual property have been or may be developed during the course of research funded by the U.S. government. The U.S. government may have the right to take title to government-funded inventions if we fail to disclose the inventions to the government in a timely manner or fail to file a patent for the intellectual property within specified time limits. Further, in consequence of our receiving government funding, the U.S. government may have certain rights to intellectual property that we use in our platform or programs pursuant to the Bayh-Dole Act of 1980, as amended (the “Bayh-Dole Act”). Under the Bayh-Dole Act, U.S. government rights in certain “subject inventions” developed under a government-funded program may include a non-exclusive, irrevocable worldwide license to use inventions for any governmental purpose. In some circumstances, the U.S. government may acquire unlimited rights in data we generate. In addition, the U.S. government has the right to require us, or an assignee or exclusive licensee to U.S. Government-funded inventions, to grant licenses to any of these inventions to the government or a third party if the government determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; (iii) government action is necessary to meet requirements for public use under federal regulations; or (iv) the right to use or sell such inventions is exclusively licensed to an entity within the United States and substantially manufactured outside the United States without the U.S. government’s prior approval. Additionally, we may be restricted from granting exclusive licenses for the right to use or sell such inventions unless the licensee agrees to comply with relevant Bayh-Dole Act restrictions (e.g., manufacturing substantially all of the invention in the United States) and reporting requirements. In addition, the U.S. government may acquire title in any country in which a patent application is not filed. Certain technology and inventions are also subject to transfer restrictions during the term of these agreements with the U.S. government and for a period thereafter. These restrictions may limit sales of products or components, transfers to foreign subsidiaries for the purpose of the relevant agreements, and transfers to certain foreign third parties. If any of our intellectual property becomes subject to any of the rights or remedies available to the U.S. government or third parties pursuant to the Bayh-Dole Act, this could impair the value of our intellectual property and could adversely affect our business.
Risks relating to the Nagoya Protocol.
The Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization is a supplemental agreement to the Convention on Biological Diversity. The Protocol is designed to provide for equitable sharing of benefits arising from the utilization of genetic resources and traditional knowledge. Under the Protocol, countries possessing genetic resources (“source countries”) are tasked with setting up procedures and institutional infrastructure for researchers to obtain prior (not post-hoc) informed consent, both from the source country and from any relevant indigenous or traditional communities, for biological research. Many have been slow to adopt workable institutions permitting the rational negotiation of benefit-sharing agreements. Many source countries are now asserting that the use of digital genetic sequence information is subject to the constraints of the Nagoya Protocol or national-level benefit-sharing requirements. It is unclear whether this position will ultimately be adopted or what the implications of such adoption might be. It is unclear what a source country might assert if we used genetic sequences (i) extracted by a third party from a natural resource that was removed from its source country before that source country ratified the CBD or signed the Protocol (ii) extracted by a third party and uploaded to public sequence databases after the source country ratified the CBD; (iii) in a heterologous host organism; or (iv) as a base for further engineering, so that the sequence we use no longer conforms to the natural sequence on which it was based.
We make extensive use of public and proprietary sequence databases to support our work. While we undertake efforts to identify and comply with laws and international protocols relating to the use of genetic resources, the uncertainty surrounding the use of digital sequence information and the lack of workable institutions in many source countries for the efficient negotiation of benefit-sharing agreements may limit our use or cause uncertainty in our use of certain sequences that we obtain from public access databases or natural sources. New financial obligations may arise regarding our use of sequence information. Customers that must certify their compliance with Nagoya Protocol obligations may be reluctant to do business with us unless we engage in
31
expensive and time-consuming benefit-sharing negotiations with source countries of publicly available genetic sequences. These changes could increase our research and development costs and adversely affect our business, financial condition, and results.
Risks that we infringe the patents of third parties or must design around such patents.
There may be patents that affect our freedom to operate in certain areas, and we may as a result choose to design around or license such patents from third parties. If we must spend significant time and money designing around or licensing patents held by others, our business and financial prospects may be harmed. We may be restricted from carrying out certain operations in our foundry, or we may be limited in our ability to design new products for our customers. We may become subject to claims by third parties alleging that we are infringing, misappropriating, or otherwise violating their intellectual property rights.
Risks that we may need to engage in intellectual property litigation.
Any litigation arising from any dispute relating to the intellectual property of third parties would be expensive, time-consuming, and uncertain. There can be no assurance that we would prevail in any such dispute. Parties making claims against us might be able to obtain injunctive or other relief, which could block our or our customers’ ability to develop, commercialize and sell products or use our technologies, and could result in the award of substantial damages against us, including treble damages, attorney’s fees, costs and expenses if we were found to have willfully infringed. In the event of a successful claim against us, we or our customers might be required to pay damages and ongoing royalties, and obtain licenses from third parties, or be prohibited from selling certain products or using certain technologies. We may not be able to obtain these licenses on acceptable or commercially reasonable terms, if at all. In addition, we or our customers could encounter delays in product or service introductions while we attempt to develop alternative or redesign existing products or technologies to avoid or resolve these claims. Our loss in any lawsuit or failure to obtain a license could prevent us from using our platform and technologies. Such a loss or failure could materially affect our business. Any litigation pertaining to these issues would have substantial costs, even if the eventual outcome is favorable to us, and would divert management’s attention from our business objectives.
Risks relating to protection of our trademarks and trade names.
Our registered or unregistered trademarks or trade names may be challenged, infringed, diluted, tarnished, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential collaborators or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement, dilution or tarnishment claims brought by owners of other trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade names, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely affect our business, financial condition, results of operations and prospects.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. The following examples are illustrative:
| • | we may choose not to file a patent in order to maintain certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property; |
32
| • | others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our owned or licensed intellectual property rights; |
| • | the patents of others may harm our business; |
| • | we might not have been the first to make the inventions covered by the issued patents or pending patent applications that we own; |
| • | we might not have been the first to file patent applications covering certain of our inventions; and |
| • | issued patents that we hold rights to may fail to provide us with any competitive advantage, or may be held invalid or unenforceable, including as a result of legal challenges by our competitors. |
Should any of these events occur, they could harm our business, financial condition, results of operations and prospects.
Risks relating to in-licenses.
We rely, and expect to continue to rely on, certain services and intellectual property that we license from third parties for use in our operations, platform, products, services and offerings. We cannot be certain that our licensors are not infringing upon the intellectual property rights of others or that our suppliers and licensors have sufficient rights to the third-party technology used in our business in all jurisdictions in which we may operate. Disputes with licensors over uses or terms could result in the payment of additional royalties or penalties by us, cancellation or non-renewal of the underlying license, or litigation. In the event that we cannot renew and/or expand existing licenses, we may be required to discontinue or limit our use of the operations, platform, products, services or offerings that include or incorporate the licensed intellectual property. Any such discontinuation or limitation could have a material and adverse impact on our business, financial condition and results of operation.
Risks relating to our use of “open-source” software.
We have used “open-source” software in connection with the development and deployment of our software platform, and we expect to continue to use open-source software in the future. Open-source software is licensed by its authors or other third parties under open-source licenses, which in some instances may subject us to certain unfavorable conditions, including requirements that we offer our products that incorporate the open-source software for no cost, that we make publicly available all or part of the source code for any modifications or derivative works we create based upon, incorporating or using the open-source software, or that we license such modifications or derivative works under the terms of the particular open-source license.
Companies that incorporate open-source software into their products have, from time to time, faced claims challenging the use of open-source software and compliance with open-source license terms. We could be subject to similar suits by parties claiming ownership of what we believe to be open-source software or claiming noncompliance with open-source licensing terms. While we monitor our use of open-source software and try to ensure that none is used in a manner that would require us to disclose our proprietary source code or that would otherwise breach the terms of an open-source agreement, we cannot guarantee that we will be successful, that all open-source software is reviewed prior to use in our platform, that our developers have not incorporated open-source software into our products that we are unaware of or that they will not do so in the future.
Furthermore, there are an increasing number of open-source software license types, almost none of which have been interpreted by U.S. or foreign courts, resulting in a dearth of guidance regarding the proper legal interpretation of such licenses. As a result, there is a risk that open-source software licenses could be construed in a manner that imposes unanticipated conditions or restrictions on our ability to market or provide our products and services. If we are held to have breached or failed to fully comply with all the terms and conditions of an open-source software license, we could face infringement claims or other liability, or be required to seek costly licenses from third parties to continue providing our offerings on terms that are not economically feasible, if at
33
all, to re-engineer all or a portion of our platform, to discontinue or delay the provision of our offerings if re-engineering could not be accomplished on a timely basis or to make generally available, in source code form, our proprietary code. Further, in addition to risks related to license requirements, use of certain open-source software carries greater technical and legal risks than does the use of third-party commercial software. For example, open-source software is generally provided without any support or warranties or other contractual protections regarding infringement or the quality of the code, including the existence of security vulnerabilities. To the extent that our platform depends upon the successful operation of open-source software, any undetected errors or defects in open-source software that we use could prevent the deployment or impair the functionality of our systems and injure our reputation. In addition, the public availability of such software may make it easier for others to compromise our platform. Any of the foregoing risks could materially and adversely affect our business, financial condition and results of operations.
Risks Related to New Ginkgo’s Personnel, IT and Physical Infrastructure
Loss of key personnel, including our founders and senior executives, and/or failure to attract, train and retain additional key personnel could delay our cell engineering programs and harm our platform development efforts and our ability to meet our business objectives, particularly given the substantial investment required to train certain of our employees.
Our business involves complex, global operations across a variety of markets and requires a management team and employee workforce that is knowledgeable in the many areas in which we operate. Our future success depends upon our ability to attract, train, retain and motivate highly qualified management, scientific, engineering, information technology, operations, business development and marketing personnel, among others. In addition, the market for qualified personnel is very competitive because of (a) the limited number of people available who have the necessary technical skills and understanding of our technology and products and (b) the nature of our industry which requires certain of our technical personnel to be on-site in our facilities. We compete for qualified technical personnel with other life sciences and information technology companies, as well as academic institutions and research institutions in the markets in which we operate, including Boston, Massachusetts, Cambridge, Massachusetts and Emeryville, California. In addition, as we add international operations, we will increasingly need to recruit qualified personnel outside the United States. However, doing so may also require us to comply with laws to which we are not currently subject, which could cause us to allocate or divert capital, personnel and other resources from our organization, which could adversely affect our business, financial condition, results of operations, prospects and reputation. Establishing international operations and recruiting personnel has and may continue to be impacted by COVID-19 travel and operational restrictions. Our senior leadership team is critical to our vision, strategic direction, platform development, operations and commercial efforts. Our employees, including members of our leadership team, could leave our company with little or no prior notice and would be free to work for a competitor. We also do not maintain “key man” life insurance on any of our employees. The departure of one or more of our founders, senior leadership team members or other key employees could be disruptive to our business until we are able to hire qualified successors.
Our continued platform development, growth and commercial success depends, in part, on recruiting and retaining highly-trained personnel across our various target industries and markets with the necessary background and ability to develop and use our platform and to effectively identify and sell to current and new customers. New hires require significant training and, in most cases, take significant time before they achieve full productivity. Our failure to successfully hire and integrate these key personnel into our business could adversely affect our business. To attract top talent, we believe we will need to offer competitive compensation and benefits packages, including equity incentive programs, which may require significant investment. If we are unable to offer competitive compensation this may make it more difficult for us to attract and retain key employees. Moreover, if the perceived value of our equity awards declines, it may adversely affect our ability to attract and retain key employees. If we do not maintain the necessary personnel to accomplish our business objectives, we may experience staffing constraints that adversely affect our ability to support our programs and operations.
34
In addition, some of our personnel are qualified foreign nationals whose ability to live and work in the U.S. is contingent upon the continued availability of appropriate visas and whose ability to work on some of our technologies may require the procurement of appropriate export licenses. Due to the competition for qualified personnel in the key markets in which we operate, we expect to continue to utilize foreign nationals to fill part of our recruiting needs. As a result, changes to United States immigration policies have and could further restrain the flow of technical and professional talent into the United States and adversely affect our ability to hire and retain qualified personnel.
Our business and results of operations are dependent on adequate access to laboratory and office space and suitable physical infrastructure, including electrical, plumbing, HVAC and network infrastructure, to conduct our operations. Our headquarters and laboratories are located in a flood zone in Boston’s Seaport District. If we are unable to access enough space or we experience failures of our physical infrastructure, our business and results of operations could be adversely affected.
Our business depends on providing customers with technical services. In order to properly conduct our business, we need access to sufficient laboratory space and equipment to perform the activities necessary to advance and complete our programs. Additionally, we need to ensure that our laboratories and corporate offices remain operational at all times, which includes maintaining suitable physical infrastructure, including electrical, plumbing and HVAC, logistics and transportation systems and network infrastructure. We lease our laboratories and office spaces and we rely on the landlords for basic maintenance of our leased laboratories and office buildings. If one of our landlords has not maintained a leased property sufficiently, we may be forced into an early exit from the facility, which could be disruptive to our business. Furthermore, we may continue to acquire laboratories not built by us in order to sufficiently scale and expand our output capacity. If we discover that these buildings and their infrastructure assets are not in the condition we expected when they were acquired, we may be required to incur substantial additional costs to repair or upgrade the laboratories.
Problems in and around one or more of our laboratories or corporate offices, whether or not within our control, could result in service interruptions or significant infrastructure or equipment damage. These could result from numerous factors, including:
| • | human error; |
| • | equipment failure; |
| • | physical, electronic and cybersecurity breaches; |
| • | fire, earthquake, hurricane, flood, tornado and other natural disasters; |
| • | extreme temperatures; |
| • | flood and/or water damage; |
| • | fiber cuts; |
| • | power loss; |
| • | terrorist acts, including acts of bioterrorism; |
| • | sabotage and vandalism; and |
| • | local epidemics or global pandemics such as the COVID-19 pandemic. |
We have timeline obligations to certain customers with respect to their programs. As a result, service interruptions or significant equipment damage in our laboratories could result in difficulty maintaining program timelines for these customers and potential claims related to such failures. Because the services we provide in our laboratories are critical to many of our customers’ businesses, service interruptions or significant equipment damage in our laboratories could also result in lost revenue or other indirect or consequential damages to our customers. We cannot guarantee that a court would enforce any contractual limitations on our liability in the
35
event that one of our customers brings a lawsuit against us as a result of a problem at one of our laboratories and we may decide to reach settlements with affected customers irrespective of any such contractual limitations. Any such settlement may result in a reduction of revenue under U.S. generally accepted accounting principles (“GAAP”). In addition, any loss of service, equipment damage or inability to meet our service obligations could reduce the confidence of our customers and could consequently impair our ability to obtain and retain customers, which would adversely affect both our ability to generate revenues and our operating results.
Furthermore, we are dependent upon internet service providers, telecommunications carriers and other website operators, some of which have experienced significant system failures and electrical outages in the past.
Our customers may in the future experience difficulties due to system failures unrelated to our systems and offerings. If, for any reason, these providers fail to provide the required services, our business, financial condition and results of operations could be materially and adversely impacted.
Risks Related to Financial Reporting
We rely on our customers, joint venturers, equity investees and other third parties to deliver timely and accurate information in order to accurately report our financial results in the time frame and manner required by law.
We need to receive timely, accurate and complete information from a number of third parties in order to accurately report our financial results on a timely basis. If the information that we receive is not accurate, our consolidated financial statements may be materially incorrect and may require restatement. Although we have audit rights with these parties, performing such an audit could be expensive and time consuming and may not be adequate to reveal any discrepancies in a time frame consistent with our reporting requirements. As a result, we may have difficulty completing accurate and timely financial disclosures, which could have an adverse effect on our business.
We use estimates in determining the fair value of certain assets and liabilities. If our estimates prove to be incorrect, we may be required to write down the value of these assets or write up the value of these liabilities, which could adversely affect our financial position.
Our ability to measure and report our financial position and operating results is influenced by the need to estimate the fair value of an asset or liability. Fair value is estimated based on a hierarchy that maximizes the use of observable inputs and minimizes the use of unobservable inputs. Observable inputs are inputs that reflect the assumptions that market participants would use in pricing the asset or liability developed based on market data obtained from sources independent of the reporting entity. Unobservable inputs are inputs that reflect the reporting entity’s own assumptions about the assumptions market participants would use in pricing the asset or liability developed based on the best information available in the circumstances. We estimate the impact or outcome of future events on the basis of information available at the time of the financial statements. An accounting estimate is considered critical if it requires that management make assumptions about matters that were highly uncertain at the time the accounting estimate was made. If actual results differ from management’s judgments and assumptions, then they may have an adverse impact on our results of operations and cash flows.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
We have incurred net losses since our inception and we may never achieve or sustain profitability. Generally, for U.S. federal income tax purposes, net operating losses incurred will carry forward. However, net operating loss carryforwards generated prior to January 1, 2018 are subject to expiration for U.S. federal income tax purposes. As of December 31, 2020, we had federal net operating loss carryforwards of approximately $347.8 million of which $139.2 million will begin to expire in 2029 and $208.6 million, which will carryforward indefinitely. As of December 31, 2020, we had a total state net operating loss carryforward of $282.8 million, of which $278.3 million will begin to expire in 2029. We have approximately $4.5 million of state net operating losses as
36
of December 31, 2020 that can be carried forward indefinitely. As of December 31, 2020, we also had federal and state research and development tax credit carryforwards of approximately $13.8 million and $8.2 million, respectively, which may be available to offset future income tax liabilities. The federal research and development tax credit carryforwards would begin to expire in 2029. The state research and development tax credit carryforwards are not subject to expiration.
Under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended (the “Code”), if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change by value in its equity ownership by certain stockholders over a three-year period, the corporation’s ability to use its pre-ownership change net operating loss carryforwards and other pre-ownership change tax attributes, such as research tax credits, to offset its post- ownership change income or taxes may be limited. Similar provisions of state tax law may also apply to limit the use of our state net operating loss carryforwards and other state tax attributes. We have not performed an analysis to determine whether our past issuances of stock and other changes in our stock ownership may have resulted in one or more ownership changes. If it is determined that we have in the past experienced an ownership change, or if we undergo one or more ownership changes as a result of future transactions in our stock, which may be outside our control, then our ability to utilize our net operating loss carryforwards and other tax attributes may be materially limited. As a result, even if we earn taxable income, we may be unable to use a material portion of our net operating loss carryforwards and other tax attributes, which could adversely affect our future cash flows. There is also a risk that regulatory changes, such as suspensions on the use of net operating losses or other unforeseen reasons, may result in our existing net operating loss carryforwards expiring or otherwise becoming unavailable to offset future taxable income. For these reasons, we may not be able to utilize a material portion of our net operating loss carryforwards and other tax attributes even if we attain profitability.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of New Ginkgo common stock.
As a public reporting company, we are subject to the rules and regulations established by the SEC and NYSE. These rules and regulations require, among other things, that we establish and periodically evaluate procedures with respect to our internal control over financial reporting. Reporting obligations as a public company are likely to place a considerable strain on our financial and management systems, processes and controls, as well as on our personnel, including senior management. In addition, as a public company, we are required to document and test our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 so that our management can certify as to the effectiveness of our internal control over financial reporting. Management’s initial certification under Section 404 of the Sarbanes-Oxley Act of 2002 will be required with our annual report on Form 10-K for the year ending December 31, 2022. In support of such certifications, we are required to document and make significant changes and enhancements, including potentially hiring additional personnel, to our internal control over financial reporting. Likewise, our independent registered public accounting firm is not required to attest to the effectiveness of our internal control over financial reporting until our first annual report is required to be filed with the SEC following the date we are no longer an emerging growth company. At such time as we are required to obtain auditor attestation, if we then have a material weakness, we would receive an adverse opinion regarding our internal control over financial reporting from our independent registered accounting firm.
To achieve compliance with Section 404 within the prescribed period, we will need to continue to dedicate internal resources, including hiring additional financial and accounting personnel and potentially engaging outside consultants. During our evaluation of our internal control, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. We have identified gaps in our internal control environment in the past and cannot provide assurances that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely
37
inhibit our ability to accurately report our financial condition, or results of operations. If we are unable to conclude that our internal control over financial reporting is effective or if our independent registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of shares of New Ginkgo common stock could decline, and we could be subject to sanctions or investigations by NYSE, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
We have identified material weaknesses in our internal control over financial reporting in the past. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. If we are unable to remediate these material weaknesses, or if we identify additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls, we may not be able to accurately or timely report our financial condition or results of operations, which may adversely affect investor confidence in us and, as a result, our stock price.
Our cash and cash equivalents could be adversely affected if the financial institutions in which we hold our cash and cash equivalents fail.
We regularly maintain cash balances at third-party financial institutions in excess of the Federal Deposit Insurance Corporation insurance limit. While we monitor the cash balances in our operating accounts on a daily basis and adjust the balances as appropriate, these balances could be impacted, and there could be a material adverse effect on our business, if one or more of the financial institutions with which we deposit cash fails or is subject to other adverse conditions in the financial or credit markets. To date, we have experienced no loss or lack of access to our invested cash or cash equivalents; however, we can provide no assurance that access to our invested cash and cash equivalents will not be impacted by adverse conditions in the financial and credit markets.
Risks Related to Governmental Regulation and Litigation
Failure to comply with federal, state, local and international laws and regulations could adversely affect our business and our financial condition.
A variety of federal, state, local and international laws and regulations govern certain aspects of our business. For example, we maintain a registration from the U.S. Drug Enforcement Administration (“DEA”) for the research of certain controlled substances and permits from the Boston Public Health Commission to conduct work with recombinant DNA. Some of our programs or products made or developed using our engineered cells and/or biomanufacturing processes are subject to regulations, including those promulgated by the FDA, DEA or USDA. Products utilized in our COVID-19 testing services are subject to regulations promulgated by the FDA, the Centers for Medicare and Medicaid Services, and certain state governments. In addition, we are subject to laws relating to, among other things, anti-bribery, insider trading, sourcing of biological materials and data privacy. The legal and regulatory requirements that apply to our business may be interpreted and applied in a manner that is inconsistent from one jurisdiction to another or may conflict with other rules or our practices. As a result, our practices may not comply, or may not comply in the future with all such laws, regulations, requirements and obligations. Any failure, or perceived failure, by us to comply with any federal, state, local or international laws, regulations, industry self-regulatory principles, industry standards or codes of conduct, regulatory guidance, orders to which we may be subject or other legal obligations could adversely affect our reputation, brand and business, and may result in claims, proceedings or actions against us by governmental entities or others or other liabilities or require us to change our operations. We may also be contractually required to indemnify and hold harmless third parties from the costs or consequences of non-compliance with any laws, regulations or other legal obligations.
38
We may also become subject to increasing regulation in the future as we expand our business. We currently operate a laboratory in California which is subject to a different set of state laws than Massachusetts, including specific laboratory registration requirements. We may also be subject to laws and regulations of the FDA and the states regarding the distribution of COVID-19 tests and test kits in connection with our testing services. We have limited experience operating a business located outside of Massachusetts. As we continue to expand our operations and offerings domestically and globally, we will have to expend significant management and financial resources to maintain compliant practices in those locations. Non-compliance could lead to litigation, which would require substantial management and financial resources.
We may incur significant costs complying with environmental, health and safety laws and regulations, and failure to comply with these laws and regulations could expose us to significant liabilities.
We use hazardous chemical and biological materials in our business and are subject to a variety of federal, state, local and international laws and regulations governing, among other matters, the use, generation, manufacture, transportation, storage, handling, disposal of, and human exposure to these materials, including regulation by governmental regulatory agencies, such as the Occupational Safety and Health Administration and the EPA. We have incurred, and will continue to incur, capital and operating expenditures and other costs in the ordinary course of our business in complying with these laws and regulations.
Although we have implemented safety procedures for storing, handling and disposing of these materials and waste products in an effort to comply with these laws and regulations, we cannot be sure that our safety measures will be compliant or capable of eliminating the risk of injury or contamination from the generation, manufacturing, use, storage, transportation, handling, disposal of and human exposure to hazardous materials and/or flammable chemicals. Failure to comply with environmental, health and safety laws could subject us to liability and resulting damages. There can be no assurance that violations of environmental, health and safety laws will not occur as a result of human error, accident, equipment failure, contamination, intentional misconduct or other causes. Compliance with applicable environmental laws and regulations may be expensive, and the failure to comply with past, present or future laws could result in the imposition of fines, regulatory oversight costs, third party property damage, product liability and personal injury claims, investigation and remediation costs, the suspension of production or a cessation of operations, and our liability may exceed our total assets. Liability under environmental laws can be imposed for the full amount of damages without regard to comparative fault for the investigation and cleanup of contamination and impacts to human health and for damages to natural resources. Contamination at properties we may own and operate and at properties to which we send hazardous materials, may result in liability for us under environmental laws and regulations.
Our business and operations may be affected by other new environmental, health and safety laws and regulations, which may require us to change our operations, or result in greater compliance costs and increasing risks and penalties associated with violations, which could impair our research, development or production efforts and harm our business.
If we fail to comply with healthcare and other governmental regulations, we could face substantial penalties and our business, financial condition and results of operations could be adversely affected.
Our business activities may be subject to regulation and enforcement by the FDA, U.S. Department of Justice, U.S. Department of Health and Human Services (“HHS”) Office of Inspector General, and other federal and state governmental authorities. Although our offerings are not currently billed to any third-party payor, including any commercial payor or government healthcare program, we may, in the future, submit claims for our COVID-19 testing services to third-party payors, including government healthcare programs. If we submit claims to third-party payors, such activity will expand the scope of federal and state healthcare laws applicable to us.
Federal and state healthcare laws and regulations that may affect our ability to conduct business include, without limitation:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting, offering, receiving or paying any remuneration, directly or |
39
| indirectly, overtly or covertly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, lease, order, or arranging for or recommending the purchase, lease or order of, any item or service, for which payment may be made, in whole or in part, under federal healthcare programs such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| • | the federal physician self-referral prohibition, commonly known as the Stark Law, which prohibits a physician, in the absence of an applicable exception, from making a referral for certain designated health services covered by the Medicare or Medicaid program, including clinical laboratory services, if the physician or an immediate family member of the physician has a financial relationship with the entity providing the designated health services. The Stark Law also prohibits the entity furnishing the designated health services from billing, presenting or causing to be presented a claim for the designated health services furnished pursuant to the prohibited referral; |
| • | the federal civil false claims laws, including without limitation the federal False Claims Act (which can be enforced through “qui tam,” or whistleblower actions, by private citizens on behalf of the federal government), and civil monetary penalties laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, false or fraudulent claims for payment of government funds, or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly and improperly avoiding, decreasing or concealing an obligation to pay money to the federal government. In addition, the government may assert that a claim including items and services resulting from a violation of the federal Anti-Kickback Statute or Stark Law constitutes a false or fraudulent claim for purposes of the civil False Claims Act; |
| • | the Eliminating Kickbacks in Recovery Act (“EKRA”), which created a new federal crime for knowingly and willfully: (1) soliciting or receiving any remuneration in return for referring a patient to a recovery home, clinical treatment facility, or laboratory; or (2) paying or offering any remuneration to induce such a referral or in exchange for an individual using the services of a recovery home, clinical treatment facility, or laboratory. Unlike the Anti-Kickback Statute, EKRA is not limited to services reimbursable under a government health care program, but instead extends to all services reimbursed by “health care benefit programs”; |
| • | the healthcare fraud statutes under HIPAA, which impose criminal and civil liability for, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement, in connection with the delivery of, or payment for healthcare benefits, items or services by a healthcare benefit program, which includes both government and privately funded benefits programs. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation; |
| • | federal consumer protection and unfair competition laws, which broadly regulate platform activities and activities that potentially harm consumers; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback, self-referral, and fee-splitting, and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers and self-pay patients. |
Because of the breadth of these laws and the narrowness of available statutory and regulatory exemptions, exceptions, and safe harbors, it is possible that some of our activities could be subject to challenge under one or more of such laws. We may face claims and proceedings by private parties, and claims, investigations and other proceedings by governmental authorities, relating to allegations that our business practices do not comply with current or future laws or regulations involving applicable fraud and abuse or other healthcare laws and regulations, and it is possible that courts or governmental authorities may conclude that we or any of our partners have not
40
complied with them, or that we may find it necessary or appropriate to settle any such claims or other proceedings. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any federal or state laws described above or any other current or future fraud and abuse or other healthcare laws and regulations that apply to us, we may be subject to claims and proceedings by private parties, investigations and other proceedings by governmental authorities, as well as penalties, including significant criminal, civil and administrative penalties, damages and fines, disgorgement, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of noncompliance with these laws or regulations, imprisonment for individuals and exclusion from participation in government programs, such as Medicare and Medicaid, as well as contractual damages and reputational harm. We could also be required to curtail or cease our operations. In addition, if any customers, healthcare professionals we engage, laboratory partners or other entities with whom we do business are found not to be in compliance with applicable laws, they may be subject to the same criminal, civil or administrative sanctions, including exclusion from government-funded healthcare programs. Any of the foregoing could seriously harm our business and financial results.
We may become subject to the comprehensive laws and rules governing billing and payment, noncompliance with which could result in non-payment or recoupment of overpayments for our services or other sanctions.
We may, in the future, submit claims for our COVID-19 testing services to third-party payors. Payors typically have differing and complex billing and documentation requirements. If we fail to comply with these payor-specific requirements, we may not be paid for our services or payment may be substantially delayed or reduced. Numerous state and federal laws would also apply to our claims for payment, including but not limited to (i) “coordination of benefits” rules that dictate which payor must be billed first when a patient has coverage from multiple payors, (ii) requirements that overpayments be refunded within a specified period of time, (iii) “reassignment” rules governing the ability to bill and collect professional fees on behalf of other providers, (iv) requirements that electronic claims for payment be submitted using certain standardized transaction codes and formats, and (v) laws requiring all health and financial information of patients to be maintained in a manner that complies with stringent security and privacy standards.
Audits, inquiries and investigations from government agencies and private insurers and health network partners can occur from time to time in the ordinary course of our business, and could result in costs to us and a diversion of management’s time and attention. New regulations and heightened enforcement activity also could negatively affect our cost of doing business and our risk of becoming the subject of an audit or investigation. Our failure to comply with rules related to billing or adverse findings from audits by government and private payors could result in, among other penalties, non-payment for services rendered or recoupments or refunds of amounts previously paid for such services. We cannot predict whether any future audits, inquiries or investigations, or the public disclosure of such matters, likely would negatively impact our business, financial condition, results of operations, cash flows and the trading price of our securities. See also “Risk Factors—Risks Related to Governmental Regulation and Litigation—If we fail to comply with healthcare and other governmental regulations, we could face substantial penalties and our business, financial condition and results of operations could be adversely affected.”
We and our laboratory partners are subject to a variety of laboratory testing standards, compliance with which is an expensive and time-consuming process, and any failure to comply could result in substantial penalties.
The third-party laboratories that we partner with are subject to the Clinical Laboratory Improvement Amendments of 1988 (“CLIA”). CLIA is a federal law that regulates clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease. CLIA requires virtually all laboratories to be certified by the federal government and mandates compliance with various operational, personnel, facilities administration, quality and proficiency
41
testing requirements depending on the level of complexity for which the laboratory is certified. CLIA certification is also a prerequisite to be eligible to bill state and federal healthcare programs, as well as many private third-party payors, for laboratory testing services. Our partner laboratories hold CLIA certifications for high complexity testing, which mandate compliance with various operational, personnel, facilities administration, quality and proficiency testing requirements depending on the level of complexity for which the laboratory is certified. In addition, we hold CLIA Certificates of Waiver and perform certain CLIA-waived tests on behalf of our clients, which subjects us directly to certain CLIA requirements. Sanctions for failure to comply with CLIA requirements may include suspension, revocation, or limitation of a laboratory’s CLIA certificate, as well as the imposition of significant fines or criminal penalties. Any sanction imposed under CLIA, its implementing regulations, or state or foreign laws or regulations governing licensure, or our partner laboratories’ failure to renew a CLIA certificate, a state or foreign license, or accreditation, could have a material adverse effect on our business.
In addition, our partner laboratories and our laboratories holding CLIA Certificates of Waiver are subject to state laws and regulations governing laboratory licensure. Some states have enacted state licensure laws that are more stringent than CLIA. Our ability to successfully deploy COVID-19 testing at large scale may be adversely impacted if our partner laboratories do not maintain the required regulatory licensure and operate in accordance with CLIA standards. In certain markets such as California, New York, and Pennsylvania, we or our partner laboratories may also need to obtain and maintain additional licensure from such states. It is uncertain that our partner laboratories will be granted such licensure and, in such case, we cannot offer testing to patients located in those states, which could limit our ability to offer testing on a wide scale.
It is possible that additional states may enact laboratory licensure requirements in the future, which could further limit our ability to expand our services.
If any of our partners were to lose or fail to obtain or renew their CLIA certifications or state laboratory licenses, whether as a result of a revocation, suspension or limitation, such laboratories would no longer be able to run the COVID-19 tests we offer to our customers, and our ability to successfully deploy a COVID-19 pooled sample testing program nationwide may be adversely impacted.
The testing industry is subject to complex and costly regulation and if government regulations are interpreted or enforced in a manner adverse to us, we may be subject to enforcement actions, penalties, exclusion, and other material limitations on our operations.
We offer COVID-19 testing services by partnering with third-party laboratories, diagnostic test manufacturers and manufacturers of collection kits, which are subject to extensive and frequently changing federal, state and local laws and regulations governing various aspects of our business, including significant governmental certification and licensing regulations. New laws, regulations and judicial decisions, or new interpretations of existing laws, regulations and decisions, may also limit our potential revenues, and we may need to revise our research and development or commercialization programs. The costs of defending claims associated with violations, as well as any sanctions imposed, could significantly adversely affect our financial performance.
We are required to comply with federal and state genetic testing and privacy laws. We have measures in place to collect clinical data and genetic and other biological samples, and disclose test results, from subjects who have provided appropriate informed consents. However, informed consents could be challenged in the future, and those informed consents could prove invalid, unlawful or otherwise inadequate for our purposes. Any legal challenges could consume our management and financial resources.
Current regulations governing the testing services we offer are shifting and in some cases unclear. If regulators apply different regulations to our pooled testing services or interpret the regulations differently than we do, our ability to deploy the services nationwide will be materially adversely impacted. In addition, our laboratory partners
42
may be unsuccessful in validating, or obtaining or maintaining authorizations for, the tests we rely on to provide our COVID-19 testing services. If any third party manufacturers or laboratories offering tests that we use in our testing services are deemed by the FDA or other regulatory authorities to have violated applicable law or if the tests or test components are marketed, processed or distributed in violation of applicable law, we may be subject to enforcement action or litigation, or we may be required to find alternative tests to support our testing services, which could increase our costs and prevent us from successfully commercializing our COVID-19 testing services.
In addition, we are required to comply with applicable FDA regulations with respect to our distribution of certain COVID-19 diagnostic test kits and collection kits, including, for certain kits, compliance with applicable terms and conditions of an EUA. Such conditions may include requirements related to collection of information on the performance of the product, reporting of adverse events, recordkeeping requirements, and labeling and promotional activities. To the extent that we market or promote third-party tests or test kits outside of the uses authorized for these products or in a false or misleading manner, the tests or collection kits could be considered misbranded or adulterated and in violation of applicable law.
Advertising for any of the tests or collection kits we distribute or the testing services we offer is also subject to regulation by the Federal Trade Commission (“FTC”), under the Federal Trade Commission Act (“FTC Act”). The FTC may take enforcement action for advertising claims that are not adequately substantiated or that are false or misleading. Violations of applicable FDA requirements could result in enforcement actions, such as warning or “untitled” letters, revocation of EUAs, seizures, injunctions, civil penalties and criminal prosecutions and fines, and violation of the FTC Act could result in injunctions and other associated remedies, all of which could have a material adverse effect on our business. Most states also have similar regulatory and enforcement authority for laboratory testing and distribution of related collection kits. For example, many state laws require us to hold a specific form of license to distribute COVID-19 diagnostic test kits and collection kits into such states. These requirements vary from one state to another and frequently change. Complying with state laws and regulations may subject us to similar risks and delays as those we could experience under federal regulation.
We are subject to federal and state laws and regulations governing the protection, use, and disclosure of health information and other types of personal information, and our failure to comply with those laws and regulations or to adequately secure the information we hold could result in significant liability or reputational harm.
Numerous state and federal laws, regulations, standards and other legal obligations, including consumer protection laws and regulations, which govern the collection, dissemination, use, access to, confidentiality, security and processing of personal information, including health-related information, could apply to our operations or the operations of our partners. For example, HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and regulations implemented thereunder (collectively referred to as “HIPAA”) imposes privacy, security and breach notification obligations on certain healthcare providers, health plans, and healthcare clearinghouses, known as covered entities, as well as their business associates that perform certain services that involve creating, receiving, maintaining or transmitting individually identifiable health information for or on behalf of such covered entities, and their covered subcontractors. HIPAA requires covered entities and business associates to develop and maintain policies with respect to the protection of, use and disclosure of protected health information (“PHI”), including the adoption of administrative, physical and technical safeguards to protect such information, and certain notification requirements in the event of a breach of unsecured PHI. If in the future we engage in certain types of standard electronic transactions involving payors, including billing the Medicare or Medicaid programs or commercial health plans, we will be subject to HIPAA as a “covered entity.” We are currently subject to HIPAA as a “business associate” because we perform certain services involving the use or disclosure of PHI on behalf of covered entity customers with respect to our COVID-19 testing service offerings.
43
Additionally, under HIPAA, covered entities must report breaches of unsecured PHI to affected individuals without unreasonable delay, not to exceed 60 days following discovery of the breach by a covered entity or its agents. Notification also must be made to the U.S. Department of Health and Human Services Office for Civil Rights and, in certain circumstances involving large breaches, to the media. Business associates must report breaches of unsecured PHI to covered entities within 60 days of discovery of the breach by the business associate or its agents. A non-permitted use or disclosure of PHI is presumed to be a breach under HIPAA unless the Covered Entity or Business Associate establishes that there is a low probability the information has been compromised consistent with requirements enumerated in HIPAA.
Entities that are found to be in violation of HIPAA as the result of a breach of unsecured PHI, a complaint about privacy practices or an audit by the U.S. Department of Health and Human Services (“HHS”), may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. HIPAA also authorizes state Attorneys General to file suit on behalf of their residents. Courts may award damages, costs and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to sue us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI.
Even when HIPAA or a state law does not apply, according to the Federal Trade Commission (“FTC”), violating consumers’ privacy rights or failing to take appropriate steps to keep consumers’ personal information secure may constitute unfair and/or deceptive acts or practices in violation of Section 5(a) of the Federal Trade Commission Act. The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities.
Several states have enacted privacy laws governing the use and disclosure of health information, such as the California Confidentiality of Medical Information Act; these laws are not preempted by HIPAA to the extent they are more stringent than HIPAA. Such laws and regulations will be subject to interpretation by various courts and other governmental authorities, thus creating potentially complex compliance issues for us and our partners.
Further, in recent years, there have been a number of well-publicized data breaches involving the improper dissemination of personal information of individuals both within and outside of the healthcare industry. Laws in all 50 states require businesses to provide notice to individuals whose personally identifiable information has been disclosed as a result of a data breach. The laws are not consistent, and compliance in the event of a widespread data breach is costly. States are also constantly amending existing laws, and creating new data privacy and security laws, requiring attention to frequently changing regulatory requirements. For example, the California Consumer Privacy Act of 2018 (“CCPA”) went into effect on January 1, 2020. The CCPA creates new transparency requirements and grants California residents several new rights with respect to their personal information. Failure to comply with the CCPA may result in, among other things, significant civil penalties and injunctive relief, or potential statutory or actual damages. On November 3, 2020, California voters passed a ballot initiative for the California Privacy Rights Act (“CPRA”), which will significantly expand the CCPA. Most CPRA provisions will take effect on January 1, 2023, though the obligations will apply to any personal information collected after January 1, 2022. The CPRA will impose additional data protection obligations on covered businesses, including additional consumer rights processes, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. It will also create a new California data protection agency authorized to issue substantive regulations and could result in increased privacy and information security enforcement. The majority of the provisions will go into effect on January 1, 2023, and additional compliance investment and potential business process changes may be required. Similar laws have been proposed or passed in other states, including the Virginia Consumer Data Protection Act, which will take effect on January 1, 2023. We will need to invest substantial resources in putting in place policies and procedures to comply with these evolving state laws.
44
As our operations and business grow, we may become subject to or affected by new or additional data protection laws and regulations and face increased scrutiny or attention from regulatory authorities. For example, the European Union General Data Protection Regulation (“GDPR”), which went into effect in May 2018, imposes strict requirements for processing the personal data of individuals within the European Economic Area (“EEA”). Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater. Among other requirements, the GDPR regulates transfers of personal data subject to the GDPR to third countries that have not been found to provide adequate protection to such personal data, including the United States, and the efficacy and longevity of current transfer mechanisms between the EU and the United States remains uncertain. For example, in 2016, the EU and United States agreed to a transfer framework for data transferred from the EU to the United States, called the Privacy Shield, but the Privacy Shield was invalidated in July 2020 by the Court of Justice of the European Union. Further, from January 1, 2021, companies have to comply with the GDPR and also the United Kingdom GDPR (the “UK GDPR”), which, together with the amended UK Data Protection Act 2018, retains the GDPR in UK national law. The UK GDPR mirrors the fines under the GDPR, i.e., fines up to the greater of €20 million (£17.5 million) or 4% of global turnover. The relationship between the United Kingdom and the European Union in relation to certain aspects of data protection law remains unclear, and it is unclear how United Kingdom data protection laws and regulations will develop in the medium to longer term. On June 28, 2021, the European Commission adopted an adequacy decision in favor of the United Kingdom, enabling data transfers from EU member states to the United Kingdom without additional safeguards. However, the United Kingdom adequacy decision will automatically expire in June 2025 unless the European Commission renews or extends that decision and remains under review by the Commission during this period. These changes may lead to additional costs and increase our overall risk exposure.
Although we work to comply with applicable laws, regulations and standards, contractual obligations and other legal obligations, these requirements are evolving and may be modified, interpreted and applied in an inconsistent manner from one jurisdiction to another, and may conflict with one another or other legal obligations with which Ginkgo must comply. Recently, there has been an increase in public awareness of privacy issues in the wake of revelations about the data-collection activities of various government agencies and in the number of private privacy-related lawsuits filed against companies. Any failure or perceived failure by us or our employees, representatives, contractors, consultants, collaborators, or other third parties to comply with such requirements or adequately address privacy and security concerns, even if unfounded, could result in additional cost and liability to us, damage our reputation, and adversely affect our business and results of operations.
We have pursued in the past and may pursue additional U.S. Government contracting and subcontracting opportunities in the future and as a U.S. Government contractor and subcontractor, we are subject to a number of procurement rules and regulations.
We have entered into agreements with governmental entities and contractors in the past to serve as a U.S. government contractor or subcontractor and may do so again in the future. U.S. government procurement contractors and subcontractors must comply with specific procurement regulations and other requirements. These requirements, although customary in U.S. government contracts, could impact our performance and compliance costs, including by limiting or delaying our ability to share information with business partners, customers and investors. The U.S. government has in the past and may in the future demand contract terms that are less favorable than standard arrangements with private sector customers and may have statutory, contractual, or other legal rights to terminate contracts with us for convenience or for other reasons. Generally, U.S. government contracts contain provisions permitting unilateral termination or modification, in whole or in part, at the government’s convenience. Under general principles of government contracting law, if the government terminates a contract for convenience, the government contractor may recover only its incurred or committed costs, settlement expenses and profit on work completed prior to the termination. If the government terminates a contract for default, the government contractor is entitled to recover costs incurred and associated profits on accepted items only and may be liable for excess costs incurred by the government in procuring undelivered
45
items from another source. Any termination for default may also adversely affect our ability to contract with other government customers, as well as our reputation, business, financial condition and results of operations. In addition, changes in U.S. government budgetary priorities could lead to changes in the procurement environment, affecting availability of U.S. government contracting, subcontracting or funding opportunities, which could lead to modification, reduction or termination of our U.S. government contracts or subcontracts. If and to the extent such changes occur, they could impact our results and potential growth opportunities.
Furthermore, our U.S. government contracts grant the government the right to use technologies developed by us under the government contract or the right to share data related to our technologies, for or on behalf of the government. Under our government contracts, we may not be able to limit third parties, including our competitors, from accessing certain of these technology or data rights, including intellectual property, in providing products and services to the government.
In addition, failure by us, our employees, representatives, contractors, partners, agents, intermediaries, other customers or other third parties to comply with these regulations and requirements could result in reductions of the value of contracts, contract modifications or termination, claims for damages, refund obligations, the assessment of civil or criminal penalties and fines, loss of rights in our intellectual property and temporary suspension or permanent debarment from government contracting, all of which could negatively impact our results of operations and financial condition. Any such damages, penalties, disruptions or limitations in our ability to do business with the public sector could result in reduced sales of our products, reputational damage, penalties and other sanctions, any of which could harm our business, reputation and results of operations.
We are engaged in certain research activities involving controlled substances, including cannabinoids and other chemical intermediates, the making, use, sale, importation, exportation, and distribution of which may be subject to significant regulation by the U.S. Drug Enforcement Administration and other regulatory agencies.
We are engaged in certain research activities involving the development of microbes designed to generate cannabinoids, their precursors and other chemical intermediaries, some of which may be regulated as controlled substances in the United States. Controlled substances are subject to state, federal, and foreign laws and regulations regarding their manufacture, use, sale, importation, exportation, and distribution. Among other things, controlled substances are regulated under the federal Controlled Substances Act of 1970 (“CSA”) and implementing regulations of the U.S. Drug Enforcement Administration (“DEA”). The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have no established medicinal use and may generally not be marketed or sold in the United States. Schedule I substances are subject to the most stringent controls and Schedule V the least controls of the five schedules, based on their relative risk of abuse.
Cannabinoids are naturally occurring compounds found in the cannabis plant. The cannabis plant and its derivatives are highly regulated by the DEA and the USDA. Specifically, marihuana, which is defined as all parts of the plant Cannabis sativa L., whether growing or not, the seeds thereof, the resin extracted therefrom, and every compound, manufacture, salt, derivative, mixture, or preparation, is classified as a Schedule I controlled substance. However, the term does not include “hemp,” which means the cannabis plant and any part of that plant, including the seeds and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol (“THC”) concentration of not more than 0.3% on a dry weight basis. Thus, depending on the THC concentration of the product, the product may or may not be regulated as a controlled substance. The DEA has historically regulated synthetic cannabinoids similarly to naturally-derived cannabinoids. Consequently, even though our cannabinoids that could be produced from microbes may not be derived from the cannabis plant, the DEA may consider them to be controlled substances subject to stringent regulatory controls.
Regulations associated with controlled substances govern manufacturing, labeling, packaging, testing, dispensing, production and procurement quotas, recordkeeping, reporting, handling, shipment and disposal.
46
These regulations include required security measures, such as background checks on employees and physical control of inventory and increase the personnel needs and the expense associated with development and commercialization of products or product candidates including controlled substances. Regulators conduct periodic inspections of entities involved in handling, manufacturing, or otherwise distributing controlled substances, and have broad enforcement authorities. If we are found to be non-compliant with applicable controlled substance registrations and related requirements, we may need to modify its business activities and/or stop handling or producing the products regulated as controlled substances, and could be subject to enforcement action, significant fines or penalties, and/or adverse publicity, among other consequences.
Various states also independently regulate controlled substances. Though state-controlled substances laws often mirror federal law, because the states are separate jurisdictions, they may separately schedule substances, as well. The failure to comply with applicable regulatory requirements could lead to enforcement actions and sanctions from the states in addition to those from the DEA or otherwise arising under federal law.
Changes in government regulations may materially and adversely affect our sales and results of operations.
The markets where we provide our services are heavily influenced by foreign, federal, state and local government regulations and policies. The U.S. or foreign governments may take administrative, legislative, or regulatory action that could materially interfere with our customer’s ability to sell products derived from engineered cells in certain countries and/or to certain customers. The uncertainty regarding future standards and policies may also affect our ability to develop our programs or to license engineered cells to customers and to initiate new programs with our customers, which could have a material adverse effect on our business, financial condition and results of operations.
Changes in U.S. trade policy more generally could trigger retaliatory actions by affected countries, which could impose restrictions on our ability to do business in or with affected countries or prohibit, reduce or discourage purchases of our services by foreign customers, leading to increased program costs, increased costs of developing or manufacturing our customers’ products and higher prices for their products in foreign markets. Changes in, and responses to, U.S. trade policy could reduce the competitiveness of our services or our customers’ products, cause our services to be less in demand and our sales to decline and adversely impact our ability to compete, which could materially and adversely impact our business, financial condition and results of operations.
We are subject to certain U.S. and foreign anti-corruption, anti-bribery and anti-money laundering laws and regulations. We can face serious consequences for violations.
We are subject to the U.S. Foreign Corrupt Practices Act of 1977, as amended (the “FCPA”), the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the U.K. Bribery Act and possibly other anti-corruption, anti-bribery and anti-money laundering laws and regulations in the jurisdictions in which we do business, both domestic and abroad. Anti-corruption and anti-bribery laws have been enforced aggressively in recent years. The FCPA and other anti-corruption laws generally prohibit companies, their employees, agents, representatives, business partners and third-party intermediaries from corruptly promising, authorizing, offering, or providing, directly or indirectly, anything of value to government officials, political parties, or candidates for public office for the purpose of obtaining or retaining business or securing an improper business advantage. The UK Bribery Act and other anti-corruption laws also prohibit commercial bribery not involving government officials, and requesting or accepting bribes; and anti-money laundering laws prohibit engaging in certain transactions involving criminally-derived property or the proceeds of criminal activity.
We and our third-party business partners, representatives and agents may have direct or indirect interactions with officials and employees of government agencies or state-owned or -affiliated universities or other entities (for example, to obtain necessary permits, licenses, patent registrations and other regulatory approvals), which increases our risks under the FCPA and other anti-corruption laws. We also engage contractors, consultants and other third
47
parties from time to time to conduct business development activities abroad. We may be held liable for the corrupt or other illegal activities of our employees or third parties even if we do not explicitly authorize such activities. We expect our non-U.S. activities to increase over time, which may also increase our exposure under these laws.
The FCPA also requires that we keep accurate books and records and maintain a system of adequate internal controls. While we have controls to address compliance with such laws, and will continue to review and enhance our compliance program, we cannot assure you that our employees, agents, representatives, business partners or third-party intermediaries will always comply with our policies and applicable law, for which we may be ultimately held responsible.
Any allegations or violation of the FCPA or other applicable anti-bribery, anti-corruption laws and anti-money laundering laws may result in whistleblower complaints, sanctions, settlements, investigations, prosecution, enforcement actions, substantial criminal fines and civil penalties, disgorgement of profits, imprisonment, debarment, tax reassessments, breach of contract and fraud litigation, loss of export privileges, suspension or debarment from U.S. government contracts, adverse media coverage, reputational harm and other consequences, all of which may have an adverse effect on our reputation, business, financial condition, results of operations and prospects. Responding to an investigation or action can also result in a materially significant diversion of management’s attention and resources and significant defense costs and other professional fees.
Significant disruptions to our and our service providers’ information technology systems or data security incidents could result in significant financial, legal, regulatory, business and reputational harm to us.
We are increasingly dependent on information technology systems and infrastructure, including services licensed, leased or purchased from third parties such as cloud computing infrastructure and operating systems, to operate its business. In the ordinary course of business, we collect, store, process and transmit large amounts of sensitive information, including intellectual property, proprietary business information, personal information and other confidential information. It is critical that we do so in a secure manner to maintain the confidentiality, integrity and availability of such sensitive information. We have also outsourced elements of our operations (including elements of its information technology infrastructure) to third parties, and as a result, we manage a number of third-party vendors who may have access to our networks or our confidential information.
While we take measures to safeguard and protect this information, threats to network and data security are increasingly diverse and sophisticated. As a result of the COVID-19 pandemic, we may also face increased cybersecurity risks due to our reliance on internet technology and the number of our employees working remotely, which may create additional opportunities for cybercriminals to exploit vulnerabilities. Despite our efforts, training and processes to prevent security breaches and incidents, our information technology systems, servers, and those of third parties that we use in our operations are vulnerable to cybersecurity risks, including cyberattacks such as viruses and worms, phishing attacks and other forms of social engineering, denial-of-service attacks, ransomware attacks, physical or electronic break-ins, third-party or employee theft or misuse, and other negligent actions, errors or malfeasance by employees or other third parties, and similar disruptions from unauthorized tampering with its servers and computer systems or those of third parties that we use in its operations, which could lead to interruptions, delays, loss or corruption of critical data, unauthorized access to or acquisition of health-related and other personal information. In addition, we may be the target of email scams and other social engineering attacks that attempt to acquire personal information or company assets or access to our systems. Despite our efforts to create security barriers to such threats, we may not be able to entirely mitigate these risks. Our third-party service providers face similar risks. Any cyberattack that attempts to obtain our data or assets, including data that we maintain on behalf of its customers, disrupt its service, or otherwise access its systems, or those of third parties we use, or any other security breach or incident, could adversely affect our business, financial condition and operating results, be expensive to remedy, and damage our reputation. We and our third-party service providers may face difficulties or delays in identifying or otherwise responding to any attacks or actual or potential security breaches or security incidents. We may incur significant costs and operational consequences of investigating, remediating, eliminating and putting in place additional tools and
48
devices designed to prevent actual or perceived security breaches and other security incidents, including in response to any actual or perceived incident we may suffer, and substantial costs to comply with any notification or other legal obligations resulting from any security breaches or other security incidents. In addition, any such breaches or incidents, or the perception that they have occurred, may result in negative publicity, and could have an adverse effect on our business, financial condition, and operating results.
Although we maintain insurance coverage that may cover certain liabilities in connection with security breaches and other security incidents, we cannot be certain our insurance coverage will be adequate for liabilities actually incurred, that insurance will continue to be available to us on commercially reasonable terms (if at all) or that any insurer will not deny coverage as to any future claim.
Governmental trade controls, including export and import controls, sanctions, customs requirements and related regimes, could subject us to liability or loss of contracting privileges or limit our ability to compete in certain markets.
Our programs and technologies are subject to U.S. and non-U.S. export controls. Export authorizations may be required for biotechnology products, technologies, or services to be exported outside of the United States, to a foreign person, or outside of a foreign jurisdiction. Our current or future programs or technologies are, and may in the future, be subject to the Export Administration Regulations (“EAR”). If a program, technology, or service meets certain criteria for control under the EAR, then that engineered cell, production process, resulting product, technology, or service would be exportable outside the United States or to a foreign person or from one foreign jurisdiction to another foreign jurisdiction only if we obtain the applicable export license or other applicable authorization including qualifying for a license exception, if required. Compliance with the U.S. and foreign export laws and regulations and other applicable regulatory requirements regarding the sales, shipment and use of our engineering cells, bioprocesses and other technology may affect our ability to work with foreign partners, affect the speed at which we can introduce new products into non-U.S. markets, or limit our ability to sell programs or services or license technologies into some countries.
Additionally, certain materials that we use in our programs are subject to U.S. import controls. We currently have, and may in the course of business need to procure, certain import authorizations, for example, related to plant pests, chemicals, biological agents and other controlled materials, including from the USDA, EPA and U.S. Centers for Disease Control. Compliance with applicable regulatory requirements regarding the import of such materials may limit our access to materials critical to our development activities or affect the speed at which we can advance new programs.
Our activities are also subject to the economic sanctions laws and regulations of the United States and other jurisdictions. Such controls prohibit certain transactions, potentially including financial transactions and the transfer of products, technologies and services, to sanctioned countries, governments and persons, without a license or other appropriate authorization. U.S. sanctions policy changes could affect our or our customers’ ability to interact, directly and indirectly, with targeted companies or companies in sanctioned countries.
While we take precautions to comply with U.S. and non-U.S. export control, import control and economic sanctions laws and regulations, we cannot guarantee that such precautions will prevent violations of such laws, including transfers to unauthorized persons or destinations, and including inadvertent violations as a result of a misclassification of a product, technology or service under export control laws. Violations could result in our business being subject to government investigations, denial of export or import privileges, significant fines or penalties, denial of government contracts and reputational harm. Any limitation on our ability to export our engineered cells, production processes, resulting products, technology, or services, or import materials critical to our programs would likely adversely affect our business and financial condition.
49
Changes in U.S. and foreign tax laws could have a material adverse effect on our business, cash flow, results of operations or financial conditions.
We are subject to tax laws, regulations and policies of the U.S. federal, state and local governments. Changes in tax laws, as well as other factors, could cause us to experience fluctuations in our tax obligations and otherwise adversely affect our tax positions and/or our tax liabilities. For example, the results of the 2020 presidential and congressional elections in the United States could result in significant changes in tax law that could adversely impact our effective tax rate. In addition, the Organisation for Economic Co-operation and Development (“OECD”) has published proposals covering various international tax-related issues, including country-by-country reporting, permanent establishment rules, transfer pricing and tax treaties. Future tax reform resulting from these developments may result in changes that could adversely affect our effective tax rate or result in higher cash tax liabilities. There can be no assurance that our tax payments, tax credits, or incentives will not be adversely affected by these or other initiatives.
We may become subject to lawsuits or indemnity claims in the ordinary course of business, which could materially and adversely affect our business and results of operations.
From time to time, we may in the ordinary course of business be named as a defendant in lawsuits, indemnity claims and other legal proceedings. These actions may seek, among other things, compensation for alleged product liability, personal injury, employment discrimination, breach of contract, property damage and other losses or injunctive or declaratory relief.
The marketing, sale and use of our services engineered cells, production processes and resulting products could lead to the filing of product liability claims were someone to allege that our services, engineered cells, production processes or resulting products failed to perform as designed or intended or caused injury or other harms. A product liability claim could result in substantial damages and be costly and time-consuming for us to defend.
Regardless of merit or eventual outcome, product liability claims may result in:
| • | decreased demand for programs and resulting products; |
| • | loss of revenue; |
| • | substantial monetary payments; |
| • | significant time and costs to defend related litigation; |
| • | the inability to commercialize any products from our programs; and |
| • | injury to our reputation and significant negative media attention. |
In the event that such actions, claims or proceedings are ultimately resolved unfavorably to us at amounts exceeding our accrued liability, or at material amounts, the outcome could materially and adversely affect our business and results of operations. In addition, payments of significant amounts, even if reserved, could adversely affect our liquidity position. We maintain product liability insurance, but this insurance may not fully protect us from the financial impact of defending against product liability claims. Any product liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could cause current collaborators to terminate existing agreements or potential collaborators to seek other companies, any of which could impact our business and results of operations.
Our business could be adversely affected by legal challenges to our telehealth partner’s business model.
Certain of our COVID-19 biosecurity offerings rely significantly on healthcare provider orders for testing that are placed on the basis of telemedicine encounters. The ability to conduct telehealth services in a particular state
50
is directly dependent upon the applicable laws governing remote healthcare, the practice of medicine and healthcare delivery in general in such location which are subject to changing political, regulatory and other influences. With respect to telehealth services, state medical boards continue to implement new rules or interpret existing rules in a manner that may limit or restrict the ability of the centers to conduct their business as it has been conducted in the past. Additionally, during the COVID-19 public health emergency, many states enacted waivers and adopted other temporary measures that lifted certain restrictions on out-of-state providers and relaxed licensure requirements to allow greater access to telehealth services during the public health emergency period. At this time, we cannot predict whether these waivers or temporary measures will remain in place after the end of the public health emergency period. Accordingly, we must monitor compliance with laws in every jurisdiction in which we operate, and we cannot provide assurance that government authorities may nonetheless challenge our activities and arrangements with our telehealth partner and consider them non-compliant. Additionally, it is possible that the laws and rules governing the practice of medicine, including remote healthcare, in one or more jurisdictions may change in a manner deleterious to our business. If a successful legal challenge or an adverse change in the relevant laws were to occur, and we are unable to adapt our business model accordingly, our operations as well as the operations of our telehealth partner in the affected jurisdictions would be disrupted, which could have a material adverse effect on our business, financial condition and results of operations.
Risks Related to our Organizational Structure and Governance
We are not, and do not intend to become, regulated as an “investment company” under the Investment Company Act of 1940, as amended (“Investment Company Act”), and if we were deemed an “investment company” under the Investment Company Act, applicable restrictions could make it impractical for us to continue our business as contemplated and could have a material adverse effect on our business.
An entity generally will be deemed to be an “investment company” for purposes of the Investment Company Act if:
| • | it is an “orthodox” investment company because it is or holds itself out as being engaged primarily, or proposes to engage primarily, in the business of investing, reinvesting or trading in securities; or |
| • | it is an inadvertent investment company because, absent an applicable exemption, (i) it owns or proposes to acquire investment securities having a value exceeding 40% of the value of its total assets (exclusive of U.S. government securities and cash items) on an unconsolidated basis, or (ii) it owns or proposes to acquire investment securities having a value exceeding 45% of the value of its total assets (exclusive of U.S. government securities and cash items) and/or more than 45% of its income is derived from investment securities on a consolidated basis with its wholly owned subsidiaries. |
We believe that we are engaged primarily in the business of providing cell engineering services to customers from across a variety of industries and not in the business of investing, reinvesting or trading in securities. We hold ourselves out as a synthetic biology company and do not propose to engage primarily in the business of investing, reinvesting or trading in securities. Accordingly, we do not believe that we are an “orthodox” investment company as defined in Section 3(a)(1)(A) of the Investment Company Act and described in the first bullet point above. Furthermore, we believe that less than 40% of our total assets (exclusive of U.S. government securities and cash items) on an unconsolidated basis after this offering will be composed of assets that could be considered investment securities. Accordingly, we do not believe that we are an inadvertent investment company by virtue of the 40% tests in Section 3(a)(1)(C) of the Investment Company Act as described in the second bullet point above. In addition, we believe that we are not an investment company under Section 3(b)(1) of the Investment Company Act because we are primarily engaged in a non-investment company business.
The Investment Company Act and the rules thereunder contain detailed parameters for the organization and operation of investment companies. Among other things, the Investment Company Act and the rules thereunder limit or prohibit transactions with affiliates, impose limitations on the issuance of debt and equity securities,
51
generally prohibit the issuance of options and impose certain governance requirements. We intend to conduct our operations so that we will not be deemed to be an investment company under the Investment Company Act or otherwise conduct our business in a manner that does not subject us to the registration and other requirements of the Investment Company Act. In order to ensure that we are not deemed to be an investment company, we may be limited in the assets that we may continue to own and, further, may need to dispose of or acquire certain assets at such times or on such terms as may be less favorable to us than in the absence of such requirement. If anything were to happen which would cause us to be deemed to be an investment company under the Investment Company Act (such as significant changes in the value of our programs or a change in circumstance that results in a reclassification of our interests in our programs for purposes of the Investment Company Act), the requirements imposed by the Investment Company Act could make it impractical for us to continue our business as currently conducted, which would materially adversely affect our business, financial condition and results of operations. In addition, if we were to become inadvertently subject to the Investment Company Act, any violation of the Investment Company Act could subject us to material adverse consequences, including potentially significant regulatory penalties and the possibility that certain of our contracts could be deemed unenforceable.
Only our employees and directors are entitled to hold shares of New Ginkgo Class B common stock (including shares of New Ginkgo Class B common stock granted or otherwise issued to our employees and directors in the future), which shares have ten votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of certain amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring stockholder approval.
Shares of New Ginkgo Class B common stock have ten votes per share, whereas shares of New Ginkgo Class A common stock have one vote per share and shares of New Ginkgo Class C common stock have no voting rights (except as otherwise expressly provided in the Charter or required by applicable law). As of November 8, 2021, our directors and executive officers hold in the aggregate approximately 50.6% of the total voting power of our outstanding capital stock, and our directors and employees (including our Founders and executive officers) hold in the aggregate approximately 73.5% of the total voting power of our outstanding capital stock. Accordingly, holders of shares of New Ginkgo Class B common stock are able to significantly influence the outcome of matters submitted to our stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets or other major corporate transaction requiring stockholder approval. This concentrated voting power limits or precludes other stockholders’ ability to influence the outcome of these matters. Holders of New Ginkgo Class B common stock may have interests that differ from holders of New Ginkgo Class A common stock and may vote in a way with which holders of New Ginkgo Class A common stock disagree and which may be adverse to the interests of holders of New Ginkgo Class A common stock. This concentrated voting power is likely to have the effect of limiting the likelihood of an unsolicited merger proposal, unsolicited tender offer or proxy contest for the removal of directors. As a result, our governance structure and Charter may have the effect of depriving our stockholders of an opportunity to sell their shares at a premium over prevailing market prices and make it more difficult to replace our directors and management. Furthermore, this concentrated voting power could discourage a potential investor from acquiring New Ginkgo Class A common stock due to the limited voting power of such stock relative to New Ginkgo Class B common stock, which could also adversely affect the trading price of New Ginkgo Class A common stock.
Our multi-class stock structure is intended to preserve our existing founder-led governance structure, to promote employee retention and engagement, to facilitate continued innovation and the risk-taking that it requires, to permit us to continue to prioritize our long-term goals rather than short-term results, to enhance the likelihood of continued stability in the composition of our board of directors and its policies, and to discourage certain types of transactions that may involve an actual or threatened acquisition of the company, all of which we believe are essential to the long-term success of our company and to long-term stockholder value. We expect to maintain this
52
concentrated voting power among our founders and employees for the foreseeable future, including by issuing additional shares of New Ginkgo Class B common stock to our employees pursuant to our equity compensation plans.
Future transfers of shares of New Ginkgo Class B common stock to persons other than an Eligible Holder, or the holder of shares of New Ginkgo Class B common stock ceasing to be an Eligible Holder, will generally result in those shares converting to shares of Class A common stock on a one-to-one basis, subject to certain exceptions and unless a majority of the independent directors of the New Ginkgo Board determine that such transfer or event will not result in such automatic conversion. Each share of New Ginkgo Class B common stock is also convertible at any time at the option of the holder into one share of New Ginkgo Class A common stock. The conversion of New Ginkgo Class B common stock to New Ginkgo Class A common stock over time will have the effect of increasing the relative voting power of those holders of New Ginkgo Class B common stock who retain their shares of New Ginkgo Class B common stock in the long term. As a result, the relative voting power of holders of New Ginkgo Class A common stock is expected to remain limited for a significant period of time, and it is possible that one or more of the persons or entities holding New Ginkgo Class B common stock could gain significant voting control as other holders of New Ginkgo Class B common stock sell or otherwise convert their shares into New Ginkgo Class A common stock. In addition, the conversion of New Ginkgo Class B common stock to New Ginkgo Class A common stock would dilute holders of New Ginkgo Class A common stock in terms of voting power within the New Ginkgo Class A common stock. Because holders of Class C common stock have no voting rights (except as otherwise expressly provided in the Charter or required by applicable law), if we issue New Ginkgo Class C common stock in the future, the holders of New Ginkgo Class B common stock may be able to significantly influence the outcome of matters submitted to our stockholders for approval for a longer period of time than would be the case if we issued New Ginkgo Class A common stock rather than New Ginkgo Class C common stock in such transactions. See “Description of New Ginkgo Securities” for descriptions of New Ginkgo Class A common stock, New Ginkgo Class B common stock and New Ginkgo Class C common stock and the rights associated with each.
Our share price may change significantly over time, and you may not be able to resell our common stock at or above the price you paid or at all, and you could lose all or part of your investment as a result.
The trading price of New Ginkgo Class A common stock has been in the past and is likely to continue to be volatile. Such volatility may be, in part, attributable to:
| • | future sales of our common stock or other securities by us or our existing stockholders, or the perception of such future sales; |
| • | results of operations of the company or our competitors that vary from the expectations of securities analysts and investors; |
| • | changes in expectations as to our future financial performance and growth, including assessments of our business, prospects, financial estimates and investment recommendations by securities analysts, investors and short sellers; |
| • | additions or departures of key management personnel or members of our board of directors; |
| • | announcements by us or our competitors of significant contracts, new products, acquisitions, joint marketing relationships, joint ventures, other strategic relationships or capital commitments; |
| • | announcements relating to actual or potential civil and non-civil litigation, as well as governmental or regulatory investigations or inquiries; |
| • | guidance that we provide to the public, any changes in this guidance or our failure to meet this guidance; |
| • | changes in the perception of our offerings or the synthetic biology industry more general including changes in regulatory conditions; |
53
| • | the development and sustainability of an active trading market for our common stock; |
| • | changes in accounting principles; |
| • | changes in general economic or market conditions or trends in our industry or markets; |
| • | other events or factors, including those resulting from natural disasters, pandemics, epidemics, war, acts of terrorism or responses to these events. |
These factors among others may materially adversely affect the market price of New Ginkgo Class A common stock, regardless of our actual operating performance. In addition, price volatility may be greater if the public float and trading volume of our common stock are low.
In the past, following periods of market volatility, stockholders have instituted securities class action litigation. If we were involved in securities litigation, it could have a substantial cost and divert resources and the attention of executive management from our business regardless of the outcome of such litigation.
Future sales, or the perception of future sales, by us or our stockholders in the public market could cause the market price for our securities to decline.
The sale of our securities in the public market, or the perception that such sales could occur, could harm the prevailing market price of our securities. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
As of the consummation of the Business Combination, we had a total of approximately 1,959 million shares of common stock outstanding on a fully-diluted basis, consisting of approximately 1,333 million shares of New Ginkgo Class A common stock and approximately 626 million shares of New Ginkgo Class B common stock. All shares issued in the Merger are freely tradable without registration under the Securities Act, and without restriction by persons other than our “affiliates” (as defined under Rule 144 of the Securities Act, “Rule 144”), including our directors, executive officers and other affiliates. Of these shares, approximately 946 million shares of common stock outstanding on a fully-diluted basis are subject to a six month lock-up, which is scheduled to expire on March 15, 2022, however, of these shares up to approximately 7 million shares of common stock may be sold into the market prior to March 15, 2022. Further, approximately 631 million shares of common stock outstanding on a fully-diluted basis are subject to a one-year lock-up, which is scheduled to expire on September 16, 2022, however, of these shares up to approximately 51 million shares of common stock may be sold into the market prior to March 15, 2022. In addition to the above, there are up to approximately 206 million shares of common stock that may be earned if the trading price is greater than or equal to the earn-out price threshold in the table below for any point in a trading day during 20 trading days in a 30 consecutive trading day period. Approximately 17 million of the shares that are part of the earn-out are subject to lock-up.
| Earn-out Price Threshold | Number of Shares Earned | |
| $12.50 |
Approximately 51.5 million (the “First Earn-out Target”) | |
| $15.00 |
Approximately 51.5 million | |
| $17.50 |
Approximately 51.5 million | |
| $20.00 |
Approximately 51.5 million |
The First Earn-out Target was achieved on November 15, 2021. In addition, the shares of our common stock reserved for future issuance under our equity incentive plans will become eligible for sale in the public market once those shares are issued, subject to provisions relating to various vesting agreements and, in some cases, limitations on volume and manner of sale applicable to affiliates under Rule 144, as applicable. Our compensation committee of our board of directors may determine the exact number of shares to be reserved for
54
future issuance under our equity incentive plans at its discretion. We have filed a registration statement on Form S-8 under the Securities Act to register shares of New Ginkgo Class A common stock or securities convertible into or exchangeable for shares of New Ginkgo Class A common stock issued pursuant to our equity incentive plans, and we may file more registration statements on Form S-8 in the future. Any such Form S-8 registration statements will automatically become effective upon filing. Accordingly, shares registered under such registration statements will be available for sale in the open market.
In the future, we may also issue our securities in connection with investments or acquisitions. The amount of shares of New Ginkgo Class A common stock issued in connection with an investment or acquisition could constitute a material portion of our then-outstanding shares of New Ginkgo Class A common stock. Any issuance of additional securities in connection with investments or acquisitions may result in additional dilution to our stockholders.
Our Charter authorizes a large number of shares of New Ginkgo Class B common stock for issuance in the future. The future issuance of shares of New Ginkgo Class B common stock may have the effect of further concentrating voting power with our employees and other Class B stockholders, and could have an adverse effect on the trading price of New Ginkgo Class A common stock.
Under our Charter, we are authorized to issue 4,500,000,000 shares of New Ginkgo Class B common stock, which are entitled to ten votes per share. We currently intend to issue additional shares of New Ginkgo Class B common stock in the future to existing and newly hired employees pursuant to our equity compensation plans. Our authorized but unissued shares of New Ginkgo Class B common stock are available for issuance to Eligible Holders with the approval of our board of directors without stockholder approval, except as may be required by the Listing Rules of the NYSE. In addition, our authorized but unissued shares of New Ginkgo Class B common stock are available for issuance to persons other than Eligible Holders only with the approval of majority of our Class B Directors. If we issue additional shares of New Ginkgo Class B common stock in the future, holders of shares of New Ginkgo Class A common stock, which are entitled to one vote per share, will experience disproportionate voting power dilution relative to economic dilution, and the holders of New Ginkgo Class B common stock may be able to significantly influence the outcome of matters submitted to our stockholders for approval for a longer period of time than would be the case if we issued shares of New Ginkgo Class A common stock.
See “Risk Factors—Risks Relating to our Organizational Structure and Governance—Only our employees and directors are entitled to hold shares of New Ginkgo Class B common stock (including shares of New Ginkgo Class B common stock granted or otherwise issued to our employees and directors in the future), which shares have ten votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets or other major corporate transaction requiring stockholder approval.”
Under our Charter, we are authorized to issue 800,000,000 shares of New Ginkgo Class C common stock, which have no voting rights (except as otherwise expressly provided in the Charter or required by applicable law). Any future issuance of New Ginkgo Class C common stock may have the effect of extending voting power in New Ginkgo Class B common stock, and may discourage potential acquisitions of our business and could have an adverse effect on the trading price of New Ginkgo Class A common stock.
Under our Charter, we are authorized to issue 800,000,000 shares of New Ginkgo Class C common stock, which have no voting rights (except as required by law). We may in the future issue shares of New Ginkgo Class C common stock for a variety of corporate purposes, including financings, acquisitions and investments. Our authorized but unissued shares of New Ginkgo Class C common stock are available for issuance with the approval of our board of directors without stockholder approval, except as may be required by the Listing Rules of the NYSE. Because the New Ginkgo Class C common stock carries no voting rights (except as otherwise
55
expressly provided in the Charter or required by applicable law), is not convertible into any other capital stock, and is not listed for trading on an exchange or registered for sale with the SEC, shares of New Ginkgo Class C common stock may be less liquid and less attractive to any future recipients of these shares than shares of New Ginkgo Class A common stock, although we may seek to list the New Ginkgo Class C common stock for trading and register shares of New Ginkgo Class C common stock for sale in the future. In addition, because our New Ginkgo Class C common stock has no voting rights (except as otherwise expressly provided in the Charter or required by applicable law), if we issue New Ginkgo Class C common stock in the future, the holders of New Ginkgo Class B common stock may be able to significantly influence the outcome of matters submitted to our stockholders for approval for a longer period of time than would be the case if we issued New Ginkgo Class A common stock rather than New Ginkgo Class C common stock in such transactions. In addition, if we issue New Ginkgo Class C common stock in the future, such issuances would have a dilutive effect on the economic interests of New Ginkgo Class A common stock and New Ginkgo Class B common stock. Any such issuance of New Ginkgo Class C common stock could also cause the trading price of New Ginkgo Class A common stock to decline.
We cannot predict the effect the multi-class structure of our common stock may have on the trading price of New Ginkgo Class A common stock.
The holding of low-voting stock, such as New Ginkgo Class A common stock, may not be permitted by the investment policies of certain institutional investors or may be less attractive to the portfolio managers of certain institutional investors. In addition, certain index providers have announced restrictions on including companies with multiple-class share structures in certain of their indices. In July 2017, FTSE Russell and S&P Dow Jones announced that they would cease to allow most newly public companies with dual- or multi-class capital structures to be included in their indices. Affected indices include the Russell 2000 and the S&P 500, S&P MidCap 400 and S&P SmallCap 600, which together make up the S&P Composite 1500. Under the announced policies, our multi-class capital structure would make New Ginkgo Class A common stock ineligible for inclusion in certain indices, and as a result, mutual funds, exchange-traded funds and other investment vehicles that attempt to passively track those indices would not invest in our common stock. These policies may depress our valuation compared to those of other similar companies that are included. Because of our multi-class stock structure, New Ginkgo Class A common stock will likely continue to be excluded from certain of these indices, and we cannot assure you that other stock indices will not take similar actions. Given the sustained flow of investment funds into passive strategies that seek to track certain indices, exclusion from stock indices would likely preclude investment by many of these funds in New Ginkgo Class A common stock and could make shares of New Ginkgo Class A common stock less attractive to other investors. As a result, the trading price of shares of New Ginkgo Class A common stock could be adversely affected.
Our focus on the long-term best interests of our company and our consideration of all of our stakeholders, including our stockholders, workforce, customers, suppliers, academic researchers, governments, communities and other stakeholders that we may identify from time to time, may conflict with short-term or medium-term financial interests and business performance, which may adversely impact the value of our common stock.
We believe that focusing on the long-term best interests of our company and our consideration of all of our stakeholders, including our stockholders, workforce, customers, suppliers, academic researchers, governments, communities and other stakeholders we may identify from time to time, is essential to the long-term success of our company and to long-term stockholder value. Therefore, we have made decisions, and may in the future make decisions, that we believe are in the long-term best interests of our company and our stockholders, even if such decisions may negatively impact the short- or medium-term performance of our business, results of operations, and financial condition or the short- or medium-term performance of New Ginkgo Class A common stock. Our commitment to pursuing long-term value for the company and its stockholders, potentially at the expense of short- or medium-term performance, may materially adversely affect the trading price of New Ginkgo Class A common stock, including by making owning New Ginkgo Class A common stock less appealing to
56
investors who are focused on returns over a shorter time horizon. Our decisions and actions in pursuit of long-term success and long-term stockholder value, which may include our multi-class stock structure, making investments in research and development and our employees, and investing in and introducing new products and services, may not result in the long-term benefits that we expect, in which case our business, results of operations and financial condition, as well as the trading price of New Ginkgo Class A common stock, could be materially adversely affected.
57
All of the shares of New Ginkgo Class A common stock and Private Placement Warrants offered by the Selling Securityholders will be sold by them for their respective accounts. We will not receive any of the proceeds from these sales.
The Selling Securityholders will pay any underwriting fees, discounts, selling commissions, stock transfer taxes and certain legal expenses incurred by such Selling Securityholders in disposing of their shares of New Ginkgo Class A common stock and Private Placement Warrants, and we will bear all other costs, fees and expenses incurred in effecting the registration of such securities covered by this prospectus, including, without limitation, all registration and filing fees, NYSE listing fees and fees and expenses of our counsel and our independent registered public accountants.
We will receive up to an aggregate of approximately $595,986,638 from the exercise of the Warrants, assuming the exercise in full of all of the Warrants for cash, but we will not receive any proceeds from the sale of the shares of New Ginkgo Class A common stock issuable upon such exercise. We expect to use the net proceeds from the exercise of the Warrants for general corporate purposes. We will have broad discretion over the use of proceeds from the exercise of the Warrants. There is no assurance that the holders of the Warrants will elect to exercise any or all of such Warrants. To the extent that the Warrants are exercised on a “cashless basis,” the amount of cash we would receive from the exercise of the Warrants will decrease.
DETERMINATION OF OFFERING PRICE
The offering price of the shares of New Ginkgo Class A common stock underlying the Warrants offered hereby is determined by reference to the exercise price of the Warrants of $11.50 per share. The Public Warrants are listed on the NYSE under the symbol “DNA.WS.”
We cannot currently determine the price or prices at which the shares of New Ginkgo Class A common stock or Private Placement Warrants may be sold by the Selling Securityholders under this prospectus.
MARKET PRICE, TICKER SYMBOL AND DIVIDEND INFORMATION
Market Price and Ticker Symbol
Our Class A common stock and Public Warrants are currently listed on the NYSE under the symbols “DNA” and “DNA.WS”, respectively.
The closing price of the New Ginkgo Class A common stock and Public Warrants on November 22, 2021, was $13.49 and $3.58, respectively.
Holders
As of November 8, 2021, there were 237 holders of record of our New Ginkgo Class A common stock, approximately 2 holders of record of the Public Warrants and one holder of record of the Private Placement Warrants.
Such numbers do not include beneficial owners holding our securities through nominee names. There is no public market for our New Ginkgo Class B common stock.
Dividend Policy
We have not paid any cash dividends on our New Ginkgo Class A common stock to date. The payment of cash dividends in the future will be dependent upon our revenues and earnings, if any, capital requirements and general financial condition. The payment of any cash dividends will be within the discretion of the New Ginkgo Board at such time.
58
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS OF GINKGO
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the consolidated financial statements and related notes thereto included elsewhere in this registration statement and prospectus. This discussion contains forward-looking statements that reflect our plans, estimates and beliefs that involve risks and uncertainties. As a result of many factors, such as those set forth under “Risk Factors” and “Cautionary Note Regarding Forward-Looking Statements” sections elsewhere in this registration statement and prospectus, our actual results may differ materially from those anticipated in these forward-looking statements. References in this section to “Ginkgo” refer to Old Ginkgo and its subsidiaries before the Business Combination and the Company and its subsidiaries after the Business Combination.
Our mission is to make biology easier to engineer.
Ginkgo is building the industry-standard horizontal platform for cell programming. We use our platform to program cells on behalf of our customers. These “cell programs” are designed to enable biological production of products as diverse as novel therapeutics, key food ingredients, and chemicals currently derived from petroleum. We have worked on cell programs in end markets as diverse as specialty chemicals, agriculture, food, consumer products, and pharmaceuticals. Biology did not evolve by end market. All of these applications run on cells which have a common code—DNA—and a common programming platform can enable all of them. Because of this shared platform, we are able to drive scale and learning efficiencies while maintaining flexibility and diversity in our program areas. Ultimately, customers come to us because they believe we maximize the probability of successfully developing their products.
The foundation of our platform includes two core assets that execute a wide variety of cell programs for customers according to their specifications: our Foundry and our Codebase.
| • | Our Foundry wraps proprietary software and automation around core cell engineering workflows— designing DNA, writing DNA, inserting that DNA into cells, testing to measure cell performance—and leverages data analytics and data science to inform each iteration of design. The software, automation and data analysis pipelines we leverage in the Foundry drive a strong scale economic: we have scaled the output of the Foundry by roughly 3X annually since we started measuring it around 2015 and over that time, the average cost per unit operation has fallen by approximately 50% every year. We expect to be able to pass these savings along to our customers, allowing them to take more “shots on goal” with their programs. |
| • | Our Codebase includes both our physical (engineered cells and genetic parts) and digital (genetic sequences and performance data) biological assets, and accumulates as we execute more cell programs on the platform. Every program, whether successful or not, generates valuable Codebase and helps inform future experimental designs and provides reusable genetic parts, making our cell program designs more efficient. |
As the platform scales, we have observed a virtuous cycle between our Foundry, our Codebase, and the value we deliver to customers. We believe this virtuous cycle sustains Ginkgo’s growth and differentiated value proposition.
| • | Foundry: As we take on more work in the Foundry, we benefit from scale economics, which over time may lead to lower program costs. We expect that these lower costs, in turn, will drive additional demand for our cell programming capabilities. |
59
| • | Codebase: Cell programs also generate Codebase, which can drive better experimental direction and improve the odds of technical success, further increasing our customer value proposition, which we believe will result in additional demand. |
Put simply: we believe that as the platform improves with scale, it drives more scale, which drives further platform improvements, and so on. We believe this positive feedback loop has the potential to drive compounding value creation in the future as every new program we add contributes to both near-term revenues and has the potential to add significant downstream economics.
Our business model mirrors the structure of our platform and we are compensated in two primary ways. First, we charge usage fees for Foundry services, in much the same way that cloud computing companies charge usage fees for utilization of computing capacity or contract research organizations (CROs) charge for services. Additionally, we negotiate a value share with our customers (typically in the form of royalties, milestones, and/or equity interests) in order to align our economics with the success of the programs enabled by our platform. As we add new programs, our portfolio of programs with this “downstream” value potential grows.
With a mission to make biology easier to engineer, we have always recognized the imperative to invest in biosecurity as a key component of our platform. We care how our platform is used and investments in biosecurity help us ensure that cell programming is conducted and deployed responsibly.
In the second quarter of 2020, in response to the COVID-19 pandemic, we launched our commercial offering of COVID-19 testing products and services for businesses, academic institutions, and other organizations in which we generate product and service revenue. The near-term growth of the biosecurity sector is highly dependent on international government initiatives and investment and Ginkgo has been supporting and engaging with domestic and international organizations and governments to help shape the understanding of a robust biosecurity program. Given our experience to date, we believe there is a meaningful commercial opportunity in biosecurity that will persist beyond the current COVID-19 pandemic, driven by increased awareness of the need for prevention and response systems. We believe we are well placed to take a leadership position as the biosecurity platform of choice, and we believe that our technology leadership requires that we play an important role in helping the world manage these challenges.
SARS-CoV-2 will not be the last pathogen we face with pandemic potential, but if we make the right investments, it may be the last that catches us unprepared. Industry sources estimate that at steady state, $20 to $40 billion should be spent on pandemic preparedness annually. The near-term growth of this sector is highly dependent on international government initiatives and investment and Ginkgo has been supporting and engaging with domestic and international organizations and governments to help shape the understanding of a robust biosecurity program. Given our experience to date, we believe there is a meaningful commercial opportunity in biosecurity that will persist beyond the current COVID-19 pandemic, driven by increased awareness of the need for prevention and response systems. We are well placed to take a leadership position as the biosecurity platform of choice, and we believe that our technology leadership requires that we play an important role in helping the world manage these challenges.
We believe that cell programming has the potential to be as ubiquitous in the physical world as computer programming has become in the digital world. We believe products in the future will be grown rather than made. To enable that vision, we are building a horizontal platform to make biology easier to engineer. Our business model is aligned with this strategy and with the success of our customers, setting us on what we believe is a path towards sustainable innovation for years to come.
Generating Economic Value Through Revenue and Downstream Value Share
Our cell programming platform is a key enabling technology and source of intellectual property for our customers’ products. We earn Foundry revenue for our research and development (“R&D”) services as well as through a share of the value of products created using our platform.
60
We structure Foundry revenue and downstream value share arrangements to include some combination of the following:
| • | Foundry usage fees in the form of: |
| • | upfront payments upon consummation of an agreement that is recognized over our period of performance; |
| • | reimbursement for costs incurred for R&D services; |
| • | milestone payments upon the achievement of specified technical criteria; |
plus,
| • | downstream value share revenue in the form of: |
| • | milestone payments upon the achievement of specified commercial criteria; |
| • | royalties on sales of products from or comprising engineered organisms; |
| • | royalties related to cost of goods sold reductions realized by our customers; |
or,
| • | downstream value share in the form of equity interests in our customer. |
| • | Downstream value share in the form of equity interest appreciation is not recognized as revenue but is expected to contribute to future cash flows upon liquidation, the amount and timing of which is inherently unpredictable. |
Downstream value share arrangements which involve equity interests fall into two categories: Platform Ventures and Structured Partnerships.
Platform Ventures
Platform Ventures allow leading multinationals to partner with Ginkgo and financial investors to form new ventures in identified market segments with potential to benefit from synthetic biology. In exchange for an equity position in the venture, we contribute license rights to our proprietary cell programming technology and intellectual property, while our partners contribute relevant industry expertise, other resources and venture funding. We also provide R&D services for which we receive cash payments for our costs incurred, plus a margin. Platform Ventures include:
Joyn Bio, LLC
Founded in 2017, Joyn Bio, LLC (“Joyn”) was formed to focus on engineered microbes for use in agricultural applications. Along with certain of our investors, we formed Cooksonia, LLC (“Cooksonia”) which holds a 50% equity interest in Joyn. Bayer CropScience LP contributed cash of $80 million plus intellectual property and holds the remaining 50% equity interest in Joyn. We provided license rights to our intellectual property and platform at inception in return for our equity interest in Joyn, which was recorded at an initial fair value of $97.9 million. The carrying value of our equity method investment in Joyn was $16.4 million as of September 30, 2021. Ginkgo also entered into a Foundry Services Agreement (“Joyn FSA”) with Joyn under which we provide R&D services. Joyn paid us a non-refundable $20.0 million prepayment for services to be provided under the Joyn FSA and made an additional $15.0 million prepayment for services during the year ended December 31, 2019.
61
Motif FoodWorks, Inc.
Founded in 2018, Motif FoodWorks, Inc. (“Motif”) was formed to focus on the application of synthetic biology to reduce the reliance on animal products in the food industry. We entered into an intellectual property contribution agreement that granted Motif rights to our intellectual property, subject to mutually agreed upon technical development plans. In return for our contribution of intellectual property and access to our platform, we received shares of common stock in Motif. The initial fair value of our common stock investment in Motif was $65.1 million which has subsequently been reduced to a carrying value of zero as a result of the allocation of losses under our accounting for equity method investments. Motif was capitalized through Series A preferred stock financings that raised approximately $119 million in gross proceeds from an investor group which included certain of our investors, Louis Dreyfus Company and Fonterra Co-operative Group Limited. In June 2021, Motif raised an additional $226 million through a Series B preferred stock financing. Ginkgo also entered into a Technical Development Agreement with Motif under which we provide R&D services in return for cash consideration on a cost-plus fixed margin basis.
Allonnia, LLC
Founded in 2019, Allonnia, LLC (“Allonnia”) was formed to focus on the application of synthetic biology in the waste bioremediation and biorecovery industries. We entered into an intellectual property contribution agreement that granted Allonnia rights to our intellectual property, subject to mutually agreed upon technical development plans. In return for our contribution of intellectual property and access to our platform, we received common units in Allonnia with a right to additional units subject to additional closings of Allonnia’s Series A preferred units. The initial fair value of our common units received in Allonnia was $24.5 million, subsequently increased by $12.7 million in 2021, all of which has been reduced to a carrying value of zero as a result of the allocation of losses under our accounting for equity method investments. Allonnia was capitalized through Series A preferred unit financings that raised approximately $52 million in gross proceeds from an investor group which included certain of our investors and Battelle Memorial Institute. Ginkgo also entered into a Technical Development Agreement with Allonnia under which we provide R&D services in return for cash consideration on a cost-plus fixed margin basis.
Arcaea, LLC (FKA Kalo Ingredients, LLC)
Founded in 2021, Arcaea, LLC (“Arcaea”) was formed to focus on the application of synthetic biology in the beauty and personal care products industry. In March 2021, we entered into an intellectual property contribution agreement that granted Arcaea rights to our intellectual property, subject to mutually agreed upon technical development plans. In return for our contribution of intellectual property and access to our platform, we received common units in Arcaea with a right to additional units subject to additional closings of Arcaea’s Series A preferred units. The initial fair value of our common units received in Arcaea was $11.9 million which has subsequently been reduced to a carrying value of zero as a result of the allocation of losses under our accounting for equity method investments. Arcaea was capitalized through a Series A preferred unit financing that raised approximately $77 million in gross proceeds from an investor group which included certain of our investors, CHANEL and Givaudan. Upon the closing of the Series A preferred unit financing in July 2021, we received an additional 5,229,900 common units in Arcaea for total consideration of $35.5 million. The fair value of our common units received in Arcaea of $35.5 million has subsequently been reduced to a carrying value of zero as a result of the allocation of losses under our accounting for equity method investments. Ginkgo also entered into a Technical Development Agreement with Arcaea under which we provide R&D services in return for cash consideration on a cost-plus fixed margin basis.
62
Ayana Bio, LLC
Founded in September 2021, Ayana Bio, LLC (“Ayana”) was formed to identify and design new bioactive compounds for use as complementary medicine to support human health and wellness. Ayana was capitalized through a Series A funding that raised $30 million in gross proceeds from an investor group comprising certain of our investors. We hold an interest in 9,000,000 common units (representing 100% of common units at inception) of Ayana and have also provided Ayana with certain licenses to our intellectual property for use in the development or production of products that we have agreed to research and develop under technical development plans. We concluded that we hold a variable interest in and are the primary beneficiary of Ayana, and as a result, we have consolidated the financial statements of Ayana into our consolidated financial statements.
Verb Biotics, LLC
Founded in September 2021, Verb Biotics, LLC (“Verb”) was formed to identify and design new strains of probiotic bacteria with advanced properties for human nutrition, health, and wellness. Verb was capitalized through a Series A funding that raised $30 million in gross proceeds from an investor group comprising certain of our investors. We hold an interest in 9,000,000 common units (representing 100% of common units at inception) of Verb and have also provided Verb with certain licenses to our intellectual property for use in the development or production of products that we have agreed to research and develop under technical development plans. We concluded that we hold a variable interest in and are the primary beneficiary of Verb, and as a result, we have consolidated the financial statements of Verb into our consolidated financial statements.
Structured Partnerships
Structured Partnerships allow Ginkgo to partner with existing entities with complementary assets for high potential synthetic biology applications. Structured Partnerships include:
Genomatica, Inc.
Genomatica, Inc. (“Genomatica”) is a biotechnology company specializing in the development and manufacturing of intermediate and specialty chemicals from both sugar and alternative feedstocks. In 2016 and 2018, we entered into separate preferred stock purchase agreements in which we offered cash and R&D services to Genomatica in exchange for its preferred shares. The initial cost of the investment in Genomatica’s preferred stock was $55.0 million, which is the carrying value of the investment at September 30, 2021 as we account for the investment at historical cost.
Synlogic, Inc.
Synlogic, Inc. (“Synlogic”) is a publicly traded clinical-stage biopharmaceutical company focused on advancing drug discovery and development for synthetic biology-derived medicines. In 2019, we entered into several agreements with Synlogic whereby we purchased Synlogic common shares and warrants to purchase Synlogic common stock and agreed to provide R&D services to Synlogic. At inception, the fair value of Synlogic common stock and warrants was recorded at $35.8 million and $14.4 million, respectively. As of September 30, 2021, the fair value of Synlogic common stock and warrants was $19.4 million and $7.8 million, respectively.
See Notes 8 and 16 of our audited consolidated financial statements and Notes 9 and 17 of our unaudited condensed consolidated financial statements included elsewhere in this registration statement and prospectus for further details of our investments in and the material terms of our agreements with our Platform Ventures and Structured Partnerships.
63
A cell program (or “program”) is the work we do for our customers to enable their product(s) of interest. Programs are defined by a technical development plan. We generally exclude proof-of-concept projects and other exploratory work undertaken on a customer’s behalf from the program count. In the near-term, programs deliver predictable multi-year revenue from platform usage fees. Over the long-term, program growth drives a physical infrastructure scale economic through our Foundry, a data and learning scale economic through our Codebase and accumulation of downstream value share. Our key business metrics comprise New Programs, Current Active Programs, and Cumulative Programs.
| Nine Months Ended September 30, |
LTM1 | Year Ended December 31, |
||||||||||||||||||
| 2021 | 2020 | 2021 | 2020 | 2019 | ||||||||||||||||
| New Programs |
21 | 15 | 24 | 18 | 16 | |||||||||||||||
| Current Active Programs |
61 | 48 | 65 | 49 | 36 | |||||||||||||||
| Cumulative Programs |
95 | 71 | 95 | 74 | 56 | |||||||||||||||
| 1 | Last 12 Months ended September 30, 2021 |
New Programs
New Programs represent the number of unique programs commenced within the reporting period. As new programs have multi-year durations, we view this metric as an indication of future Foundry revenue growth.
Current Active Programs
Current Active Programs represent the number of unique programs for which we performed R&D services in the reporting period. We view this metric as an indication of current period and future Foundry revenue.
Cumulative Programs
Cumulative Programs represent the cumulative number of unique programs Ginkgo has commenced. We view this metric as an indication of our competitive advantage and as a leading indicator of the mid- to long-term potential economic value derived from downstream value share arrangements. The cumulative number of programs also contributes to Codebase, which accumulates with each additional program we conduct over time and drives better experimental direction and improves the odds of technical success in current and future programs.
We believe the preceding metrics are important to understand our current business. These metrics may change or be substituted for additional or different metrics as our business develops. For example, as our program mix changes, our data gathering abilities expand or our understanding of key business drivers develops, we anticipate updating these metrics or their definitions to reflect such changes.
We entered into the Merger Agreement with Soaring Eagle Acquisition Corp. (“SRNG”) on May 11, 2021. On September 14, 2021, the SRNG shareholders approved and adopted the Merger Agreement and the other proposals described in SRNG’s definitive proxy statement/prospectus included in SRNG’s registration statement on Form S-4 (File No. 333-256121), which was declared effective by the SEC on August 11, 2021. Upon the consummation of the Business Combination on September 16, 2021, the separate corporate existence of Merger Sub ceased, and Ginkgo survived the merger as a wholly owned subsidiary of SRNG, which was renamed “Ginkgo Bioworks Holdings, Inc.”
64
The Business Combination was accounted for as a reverse recapitalization in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). Under the guidance in ASC 805, Business Combinations (“ASC 805”), SRNG was treated as the “acquired” company for accounting and financial reporting purposes. We were deemed to be the accounting predecessor of the combined business, and as the parent company of the combined business, the successor SEC registrant, meaning that our financial statements for previous periods will be disclosed in future periodic reports filed with the SEC. The most significant change in our reported financial position and results of operations as a result of the Business Combination was a net increase in cash (as compared to our audited consolidated balance sheet as of December 31, 2020) of $1,509.6 million, including $760.0 million in proceeds from the PIPE Investment that was consummated substantially simultaneously with the closing of the Business Combination. The transaction costs for the Business Combination totaled $108.1 million.
As the successor to an SEC-registered and publicly-listed company, we will need to hire additional personnel and implement procedures and processes to address public company regulatory requirements and customary practices. We expect to incur additional expenses as a public company for, among other things, directors’ and officers’ liability insurance, director fees, and additional internal and external accounting, legal and administrative resources.
Modification of Equity Awards in Connection with the Business Combination Transaction
Our restricted stock units have been granted with both a service-based vesting condition and a performance- based vesting condition. We have historically not recognized any stock-based compensation expense associated with these awards as the achievement of the performance condition associated with the restricted stock units include a change in control or an initial public offering (both as defined in the underlying award agreement) that was not deemed probable of occurring. As a result, a significant amount of stock-based compensation expense related to the restricted stock units remained unrecognized as of September 30, 2021.
The Business Combination did not meet the performance condition required for the vesting of our restricted stock units (“RSUs”). However, our board of directors modified the vesting conditions for the RSUs in November 2021 to allow for those RSUs to vest in the fourth quarter of 2021 and first quarter of 2022. The modification will be accounted for in accordance with ASC 718, Compensation-Stock Compensation (“ASC 718”), which will require us to remeasure the affected awards at the date of modification. As a result of the foregoing, we anticipate a substantial increase in stock-based compensation expense in the fourth quarter of 2021 and first quarter of 2022. Stock-based compensation expense will also increase for any RSU earnout shares that vest upon meeting certain common stock price targets. Additionally, shares of our common stock issued from vested RSUs will be subject to certain transfer restrictions in accordance with our Certificate of Incorporation, as amended, subject to certain exceptions described in the Risk Factors section elsewhere in this registration statement and prospectus under the heading “Future sales, or the perception of future sales, by us or our stockholders in the public market could cause the market price for our securities to decline.” Our board of directors approved the settlement of approximately 6 million shares underlying the RSUs in cash in November 2021 based on the fair market value of the underlying shares of common stock and will allow some employees to use shares received from the RSUs to cover withholding tax obligations, which will result in our using cash to satisfy such tax withholding obligations.
Acquisition of Dutch DNA Biotech B.V.
On July 1, 2021, we acquired 100% of the equity of Dutch DNA Biotech B.V. (“Dutch DNA”), a company based in the Netherlands with a proprietary platform technology focused on the development of fungal strains and fermentation processes for the production of proteins and organic acids. Dutch DNA’s significant expertise and fungal strain assets for the large-scale production of proteins is expected to add a valuable set of tools to Ginkgo’s Codebase and broader platform for cell programming. The total acquisition date fair value of the consideration transferred for Dutch DNA was $35.3 million. Dutch DNA’s results of operations have been included in our condensed consolidated statements of operations and comprehensive loss since the date of acquisition.
65
Components of Results of Operations
Revenue
Foundry Revenue
We generate Foundry revenue through the execution of license and collaboration agreements whereby customers obtain license rights to our proprietary technology and intellectual property for use in the development and commercialization of engineered organisms and derived products. Under these agreements, we typically provide R&D services for cell programming with the goal of producing an engineered cell that meets a mutually agreed specification. Our customers obtain license rights to the output of our services, which are primarily the optimized strains or cell lines, in order to manufacture and commercialize products derived from that licensed strain or cell line. Generally, the terms of these agreements provide that we receive some combination of: (1) Foundry usage fees in the form of (i) upfront payments upon consummation of the agreement or other fixed payments, (ii) reimbursement for costs incurred for R&D services and (iii) milestone payments upon the achievement of specified technical criteria, plus (2) downstream value share payments in the form of (iv) milestone payments upon the achievement of specified commercial criteria, (v) royalties on sales of products from or comprising engineered organisms arising from the collaboration or licensing agreement and (vi) royalties related to cost of goods sold reductions realized by our customers. For the three and nine months ended September 30, 2021 and 2020, royalties did not comprise a material amount of our revenue.
Foundry revenue includes transactions with Platform Ventures (Motif, Joyn, Allonnia and Arcaea) as well as other Structured Partnerships (Genomatica and Synlogic) where, as part of these transactions, we received an equity interest in such entities. Specifically related to the Platform Ventures, in these transactions, we received upfront non-cash consideration in the form of common equity interests in these entities, while the Platform Ventures each received cash equity investments from strategic partners and financial investors. We view the upfront non-cash consideration as prepayments for licenses which will be granted in the future as we complete mutually agreed upon technical development plans. In these instances, we also receive cash payments for our costs incurred for the R&D services performed by us, plus a margin. We are not compensated through additional milestone or royalty payments under these arrangements. Our transactions with Genomatica and Synlogic included the purchase of equity securities and the provision of R&D services. As we perform R&D services under the mutually agreed upon development plans, we recognize a reduction in the prefunded obligation based on a cost incurred, plus margin. Because of our equity holdings in these entities, each is considered a related party. These arrangements are further described in Notes 8, 16 and 20 of our audited consolidated financial statements and in Notes 9, 10, 17 and 19 of our unaudited condensed consolidated financial statements included elsewhere in this registration statement and prospectus.
Downstream value share in the form of equity interest appreciation is not recognized as revenue but is expected to contribute to future cash flows upon liquidation, the amount and timing of which is inherently unpredictable. Equity investees are accounted for as equity method investments, cost method investments or carried at fair value.
Biosecurity Revenue
In the second quarter of 2020, in response to the COVID-19 pandemic, we launched our commercial offering of COVID-19 testing products and services for businesses, academic institutions, and other organizations in which we generate product and service revenue. We generate product revenue primarily through the sale of lateral flow assay (“LFA”) diagnostic test kits, polymerase chain reaction (“PCR”) sample collection kits and pooled test kits, all of which we sell to our customers on a standalone basis. We generate service revenue through the sale of our end-to-end COVID-19 testing services which consist of multiple promised goods and services including sample collection kits, physician authorizations, onsite test administration, outsourced laboratory PCR analysis, and access to results reported through a web-based portal.
66
Generally, the terms of these agreements provide that we receive compensation: (i) upon delivery of diagnostic and sample collection test kits when no service is provided and (ii) when services are included, upon the reporting of results to the customer.
Beginning in the first quarter of 2021, we launched our pooled testing initiative which focuses on providing end-to-end COVID-19 testing and reporting services to groups of individuals. We are currently offering pooled testing services for K-12 schools across the United States; however, we believe that pooled testing services may have a strong value proposition in other use cases including large employers, universities, travel hubs and other congregate settings as it provides a convenient and cost-effective testing option to our customers.
Costs and Operating Expenses
Cost of Biosecurity Product Revenue
Cost of Biosecurity product revenue consists of costs associated with the sale of diagnostic and sample collection test kits, which includes costs incurred to purchase test kits from third parties, as well as shipping, handling and insurance costs.
Cost of Biosecurity Service Revenue
Cost of Biosecurity service revenue consists of costs associated with the provision of our end-to-end COVID-19 testing services, which includes costs incurred to provide sample collection kits, physician authorizations, onsite test administration, outsourced laboratory PCR analysis, access to results reported through our proprietary web-based portal and reporting of results to public health authorities.
Research and Development Expenses
The nature of our business, and primary focus of our activities, generates a significant amount of R&D expenses. R&D expenses represent costs incurred by us for the following:
| • | development, operation, expansion and enhancement of our Foundry and Codebase; and |
| • | development of new offerings, such as Biosecurity. |
The activities above incur the following expenses:
| • | laboratory supplies, consumables and related services provided under agreements with third parties and in-licensing arrangements; |
| • | personnel compensation and benefits; and |
| • | rent, facilities, depreciation, software, professional fees and other direct and allocated overhead expenses. |
We expense R&D costs as incurred. As we grow our Active Programs and customer base and invest in our Foundry and Codebase, we anticipate that our R&D expenses will continue to increase. The nature, timing, and estimated costs required to support our growth will be dependent on advances in technology, our ability to attract new customers and the rate of market penetration within our existing customer industries.
General and Administrative Expenses
General and administrative (“G&A”) expenses consist primarily of costs for personnel in executive, business development, finance, human resources, legal and other corporate administrative functions. G&A expenses also include legal fees incurred relating to corporate, intellectual property and patent matters, professional fees incurred for accounting, auditing, tax and administrative consulting services, insurance costs, and facility-related costs not otherwise included in R&D expenses.
67
We expect our G&A expenses will continue to increase as we pursue organic and inorganic growth initiatives. The increases will likely relate to additional personnel, system costs and increased costs related to finance and legal matters, along with increased expenses related to operating as a publicly traded company, such as fees related to audit, legal and tax services, regulatory compliance programs and investor relations.
Interest Income
Interest income consists primarily of interest earned on our cash and cash equivalents.
Interest Expense
Interest expense consists primarily of interest related to our lease financing obligation.
Loss on Equity Method Investments
Loss on equity method investments includes our share of losses from certain of our equity method investments under the Hypothetical Liquidation at Book Value (“HLBV”) method.
(Loss) Gain on Investments
(Loss) gain on investments includes the change in fair value of Synlogic common stock, warrants to purchase Synlogic common stock and change in fair value of Cronos Group Inc. (“Cronos”) common stock.
Change in Fair Value of Warrant Liabilities
Change in fair value of warrant liabilities includes the change in fair value of the Private Placement Warrants and the Public Warrants, which are classified as liabilities, and were assumed as part of the Business Combination.
Other (Expense) Income, Net
Other (expense) income, net primarily consists of net income generated from achieving milestones under our agreement with the National Institutes of Health (“NIH”), gains related to payments made by Amyris, Inc. (“Amyris”) under a settlement agreement and the change in fair value of our convertible notes with Access Bio, Inc. (“Access Bio”) and promissory note with Glycosyn under which we have elected to account for under the fair value option.
Provision for Income Taxes
Income taxes are recorded in accordance with ASC 740, Income Taxes (“ASC 740”), which provides for deferred taxes using an asset and liability approach. We recognize deferred tax assets and liabilities for the expected future tax consequences of events that have been included in our audited consolidated financial statements or tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse. A valuation allowance against deferred tax assets is recorded if, based on the weight of the available evidence, it is more likely than not that some or all the deferred tax assets will not be realized. For all periods presented, we have recorded a valuation allowance against the deferred tax assets that are not expected to be realized.
We account for uncertain tax positions using a more-likely-than-not threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors, including, but not limited to, changes in the law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or circumstances related to a tax position.
68
Income taxes are determined at the applicable tax rates adjusted for non-deductible expenses, R&D tax credits and other permanent differences. Our income tax provision may be significantly affected by changes to our estimates.
Comparison of the Nine Months Ended September 30, 2021 and 2020
The following table summarizes our unaudited condensed consolidated statements of operations for each period presented:
| Nine Months Ended September 30, |
||||||||||||
| (in thousands) | 2021 | 2020 | Change | |||||||||
| Foundry revenue (related party revenue of $36,746 and $29,784) |
$ | 78,833 | $ | 42,802 | $ | 36,031 | ||||||
| Biosecurity revenue: |
||||||||||||
| Product |
14,622 | — | 14,622 | |||||||||
| Service |
71,888 | 1,797 | 70,091 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
165,343 | 44,599 | 120,744 | |||||||||
|
|
|
|
|
|
|
|||||||
| Costs and operating expenses: |
||||||||||||
| Cost of Biosecurity product revenue |
15,185 | — | 15,185 | |||||||||
| Cost of Biosecurity service revenue |
47,927 | 1,769 | 46,158 | |||||||||
| Research and development |
164,637 | 98,576 | 66,061 | |||||||||
| General and administrative |
81,326 | 25,393 | 55,933 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
309,075 | 125,738 | 183,337 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(143,732 | ) | (81,139 | ) | (62,593 | ) | ||||||
| Other (expense) income, net: |
||||||||||||
| Interest income |
341 | 5,565 | (5,224 | ) | ||||||||
| Interest expense |
(1,822 | ) | (1,795 | ) | (27 | ) | ||||||
| Loss on equity method investments |
(72,621 | ) | (2,151 | ) | (70,470 | ) | ||||||
| (Loss) gain on investments |
3,009 | (4,978 | ) | 7,987 | ||||||||
| Change in fair value of warrant liabilities |
(18,482 | ) | — | (18,482 | ) | |||||||
| Other (expense) income, net |
863 | 6,055 | (5,192 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other (expense) income, net |
(88,712 | ) | 2,696 | (91,408 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Loss before income taxes |
(232,444 | ) | (78,443 | ) | (154,001 | ) | ||||||
| Income tax provision (benefit) |
(797 | ) | 1,881 | (2,678 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (231,647 | ) | $ | (80,324 | ) | $ | (151,323 | ) | |||
|
|
|
|
|
|
|
|||||||
Foundry Revenue
Foundry revenue was $78.8 million and $42.8 million for the nine months ended September 30, 2021 and 2020, respectively. The increase of $36.0 million in Foundry revenue was primarily attributable to the progress of programs with existing and new customers, including downstream value share revenue related to the achievement of a commercial milestone. Revenue from related parties (Platform Ventures and Structured Partnerships) increased from $29.8 million for the nine months ended September 30, 2020 to $36.7 million for the nine months ended September 30, 2021. See Note 19 of our unaudited condensed consolidated financial statements included elsewhere in this registration statement and prospectus for additional information related to transactions with related parties.
69
The total number of Current Active Programs increased from 48 in the nine months ended September 30, 2020 to 61 in the nine months ended September 30, 2021 across 30 customers. In the nine months ended September 30, 2021, 21 New Programs commenced. Cumulative Programs were 95 as of September 30, 2021 and 71 as of September 30, 2020.
As we increase Cumulative Programs and to the extent our customers successfully commercialize products built on our platform, downstream value share is expected to comprise a larger proportion of Foundry revenue. Downstream value share in the form of equity interest appreciation is not recognized as revenue but is expected to contribute to future cash flows upon liquidation, the amount and timing of which is inherently unpredictable. Equity investees are accounted for as equity method investments, cost method investments or carried at fair value.
Biosecurity Revenue
Biosecurity revenue was $86.5 million and $1.8 million for the nine months ended September 30, 2021 and 2020, respectively. Biosecurity revenue for the nine months ended September 30, 2021 consisted of $14.6 million of product revenue from sales of our diagnostic and sample collection test kits and $71.9 million of service revenue from our end-to-end COVID-19 testing services. Biosecurity revenue for the nine months ended September 30, 2020 consisted solely of service revenue from our end-to-end COVID-19 testing services. The amount and components of Biosecurity revenue are dependent on the demand for COVID-19 related testing services which is uncertain in 2021 and beyond.
Cost of Biosecurity Product and Service Revenue
Cost of Biosecurity product and service revenue was $63.1 million and $1.8 million for the nine months ended September 30, 2021 and 2020, respectively. During the nine months ended September 30, 2021, we incurred $15.2 million of product costs associated with purchases of diagnostic and sample collection test kits and $47.9 million of service costs related to our end-to-end COVID-19 testing services.
Research and Development Expenses
Research and development expenses were $164.6 million and $98.6 million for the nine months ended September 30, 2021 and 2020, respectively. The increase of $66.0 million was primarily attributable to increases in personnel-related compensation and benefits expense of $21.2 million, depreciation expense of $11.2 million, rent and facilities expenses of $8.6 million, laboratory supplies and related third-party services expense of $7.6 million, professional fees of $7.1 million, and office supplies, technology and software of $2.5 million. The remaining increase was attributed to other direct and allocated overhead expenses. Increases in research and development expenses supported the Foundry operations, enhancements of Foundry and Codebase and development of our Biosecurity offering.
General and Administrative Expenses
General and administrative expenses were $81.3 million and $25.4 million for the nine months ended September 30, 2021 and 2020, respectively. The increase of $55.9 million was primarily attributable to increases in personnel-related compensation and benefits expense of $30.6 million, of which $14.8 million was stock-based compensation expense related to stock option grants, professional fees of $8.8 million, office supplies, technology and software of $5.4 million, and rent and facilities expenses of $2.6 million. The remaining increase was attributed to marketing and other overhead expenses. Increases in general and administrative expenses supported the growth of Foundry and Biosecurity revenue and activities related to public company readiness.
70
Interest Income
Interest income was $0.3 million and $5.6 million for the nine months ended September 30, 2021 and 2020, respectively. The decrease of $5.3 million was primarily attributable to a $3.5 million decrease in interest income received under our settlement agreement with Amyris and a decrease in interest rates and balance of our cash held in money market accounts.
Interest Expense
Interest expense was $1.8 million for the nine months ended September 30, 2021 and 2020, respectively. There was no change in interest expense between the periods as the expense incurred related to our lease financing obligation remained largely unchanged.
Loss on Equity Method Investments
Loss on equity method investments was $72.6 million and $2.2 million for the nine months ended September 30, 2021 and 2020, respectively. The increase of $70.4 million was attributable to our equity method investments in Joyn, Allonnia and Arcaea. The fair value of the initial equity we received in Arcaea of $47.4 million during the nine months ended September 30, 2021 was reduced to zero during the period as a result of the application of the HLBV method. The fair value of the additional equity we received in Allonnia of $12.7 million during the nine months ended September 30, 2021 was also reduced to zero during the period as a result of the application of the HLBV method. Under the HLBV method, we absorb losses as a common unit holder prior to preferred unit holders due to a substantive profit-sharing agreement where the preferred unit holders receive preferential distribution rights. Because we have no commitment to fund the losses of Arcaea or Allonnia, no further losses on these equity method investments were recognized during the nine months ended September 30, 2021. The loss on our equity method investment in Joyn increased from $2.2 million to $12.6 million during the nine months ended September 30, 2020 and 2021, respectively, representing our share of the investee’s losses under the HLBV method.
(Loss) Gain on Investments
(Loss) gain on investments was $3.0 million and $(5.0) million for the nine months ended September 30, 2021 and 2020, respectively. The change of $8.0 million was attributable to fluctuations in the stock price of our shares of Synlogic and Cronos common stock and warrants to purchase Synlogic common stock.
Change in Fair Value of Warrant Liabilities
The change in fair value of warrant liabilities of $18.5 million for the nine months ended September 30, 2021 was due to an increase in the estimated fair value of the Private Placement Warrants and the Public Warrants assumed as part of the Business Combination.
Other (Expense) Income, Net
Other (expense) income, net was $0.9 million and $6.1 million for the nine months ended September 30, 2021 and 2020, respectively. The decrease of $5.2 million was primarily attributable to a $0.5 million loss resulting from the change in fair value of the Access Bio Convertible Notes, a $3.5 million decrease in payments received under our settlement agreement with Amyris and a $1.2 million decrease in payments received from the NIH.
71
Comparison of the Years Ended December 31, 2020 and 2019
The following table summarizes our consolidated statements of operations for each period presented:
| Year Ended December 31, | ||||||||||||
| (in thousands) | 2020 | 2019 | Change | |||||||||
| Foundry revenue (includes related party revenue of $42,535 and $35,268, respectively) |
$ |
59,221 |
|
$ |
54,184 |
|
$ |
5,037 |
| |||
| Biosecurity revenue: |
||||||||||||
| Product |
8,707 | — | 8,707 | |||||||||
| Service |
8,729 | — | 8,729 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
76,657 | 54,184 | 22,473 | |||||||||
|
|
|
|
|
|
|
|||||||
| Costs and operating expenses: |
||||||||||||
| Cost of Biosecurity product revenue |
6,705 | — | 6,705 | |||||||||
| Cost of Biosecurity service revenue |
8,906 | — | 8,906 | |||||||||
| Research and development |
159,767 | 96,299 | 63,468 | |||||||||
| General and administrative |
38,306 | 29,483 | 8,823 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
213,684 | 125,782 | 87,902 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(137,027 | ) | (71,598 | ) | (65,429 | ) | ||||||
| Other income (expense), net: |
||||||||||||
| Interest income |
2,582 | 5,756 | (3,174 | ) | ||||||||
| Interest expense |
(2,385 | ) | (2,421 | ) | 36 | |||||||
| Loss on equity method investments |
(3,059 | ) | (46,936 | ) | 43,877 | |||||||
| Loss on investments |
(1,070 | ) | (7,797 | ) | 6,727 | |||||||
| Other income, net (includes $721 and $1,794, respectively, from related parties) |
16,125 | 3,161 | 12,964 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total other income (expense), net |
12,193 | (48,237 | ) | 60,430 | ||||||||
|
|
|
|
|
|
|
|||||||
| Loss before provision for income taxes |
(124,834 | ) | (119,835 | ) | (4,999 | ) | ||||||
| Provision for income taxes |
1,889 | 22 | 1,867 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
(126,723 | ) | (119,857 | ) | (6,866 | ) | ||||||
|
|
|
|
|
|
|
|||||||
Foundry Revenue
Foundry revenue was $59.2 million for the year ended December 31, 2020 and $54.2 million for the year ended December 31, 2019. The increase of $5.0 million in Foundry revenue was primarily attributable to the progress of programs with existing and new customers, which was offset by lower utilization of services due to the temporary impact the COVID-19 pandemic had on our Foundry and new business development.
Beginning in 2017, Ginkgo’s commercial growth strategy expanded to include Platform Ventures (Joyn, Motif and Allonnia) and Structured Partnerships (Genomatica and Synlogic). Revenue from equity investees increased from $35.3 million in 2019 to $42.5 million in 2020 and has contributed to greater end market diversification. See Note 20 of our audited consolidated financial statements included elsewhere in this registration statement and prospectus for additional information related to transactions with related parties.
The total number of Current Active Programs increased from 36 in 2019 to 49 in 2020 across 22 customers In 2020, 18 New Programs were commenced. Cumulative Programs increased from 56 in 2019 to 74 in 2020. While downstream value share revenue was immaterial for the years ended December 31, 2020 and 2019, as we increase Cumulative Programs and to the extent our customers successfully commercialize products built on our platform, downstream value share is expected to comprise a larger proportion of Foundry revenue. Downstream
72
value share in the form of equity interest appreciation is not recognized as revenue but is expected to contribute to future cash flows upon liquidation, the amount and timing of which is inherently unpredictable. Equity investees are accounted for as equity method investments or cost method investments.
Biosecurity Revenue
Biosecurity revenue was $17.4 million for the year ended December 31, 2020, which consisted of $8.7 million of product revenue and $8.7 million of service revenue that we recognized in connection with sales of our LFA diagnostic test kits and end-to-end COVID-19 testing services. No Biosecurity revenue was recognized during the year ended December 31, 2019. The amount and components of Biosecurity revenue are dependent on the demand for COVID-19 related testing services which is uncertain in 2021 and beyond.
Cost of Biosecurity Product and Service Revenue
Cost of Biosecurity product and service revenue was $15.6 million for the year ended December 31, 2020. No cost of Biosecurity product and service revenue was incurred during the year ended December 31, 2019. During the year ended December 31, 2020, we incurred $6.7 million of product costs associated with purchases of LFA diagnostic test kits and $8.9 million of service costs related to our end-to-end COVID-19 testing services.
Research and Development Expenses
Research and development expenses were $159.8 million for the year ended December 31, 2020 and $96.3 million for the year ended December 31, 2019. The increase of $63.5 million was primarily attributable to increases in laboratory supplies and related third-party services expense of $31.1 million, personnel-related compensation and benefits expense of $13.2 million, and professional fees of $5.3 million. The remaining increase was attributed to rent, facilities, depreciation, software and other direct and allocated overhead expenses. Increases in research and development expenses supported the Foundry operations, enhancements of Foundry and Codebase, and development of our Biosecurity offering.
General and Administrative Expenses
General and administrative expenses were $38.3 million for the year ended December 31, 2020 and $29.5 million for the year ended December 31, 2019. The increase of $8.8 million was primarily attributable to increases in professional fees of $4.8 million and personnel-related compensation and benefits expense of $2.7 million. Increases in general and administrative expenses supported the growth of Foundry and Biosecurity revenue and activities related to public company readiness.
Interest Income
Interest income was $2.6 million for the year ended December 31, 2020 and $5.8 million for the year ended December 31, 2019. The decrease of $3.2 million was primarily attributable to a decrease in interest rates on our cash held in money market accounts.
Interest Expense
Interest expense was $2.4 million for each of the years ended December 31, 2020 and 2019. There was no change in interest expense between the periods as the expense incurred related to our lease financing obligation remained largely unchanged.
Loss on Equity Method Investments
Loss on equity method investments was $3.1 million for the year ended December 31, 2020, which was primarily attributable to our equity method investments in Synlogic, and $46.9 million for the year ended December 31,
73
2019, which was primarily related to the loss on our equity method investments in Synlogic and Allonnia recognized during the year ended December 31, 2019. The fair value of the equity we received in Allonnia of $24.5 million during the year ended December 31, 2019, which represented the initial carrying value of our equity method investment in Allonnia, was reduced to zero during the period as a result of the application of the HLBV method. Under the HLBV method, we absorb losses as a common unit holder prior to preferred unit holders due to a substantive profit-sharing agreement where the preferred unit holders receive preferential distribution rights. Because we have no commitment to fund the losses of Allonnia, no further losses on this equity method investment were recognized during 2020. The decrease in the loss on the Synlogic equity method investment, which we have elected to account for under the fair value option, resulted from a more significant decrease in the stock price of Synlogic during 2019 as compared to 2020.
Loss on Investments
Loss on investments was $1.1 million for the year ended December 31, 2020 and $7.8 million for the year ended December 31, 2019. The decrease of $6.7 million was attributable to a decrease in the change in fair value of our warrant to purchase Synlogic common stock, which we have elected to account for under the fair value option, which resulted from a more significant decrease in the stock price of Synlogic during 2019 as compared to 2020.
Other Income, net
Other income, net was $16.1 million for the year ended December 31, 2020 and $3.2 million for the year ended December 31, 2019. The increase of $12.9 million was primarily attributable to an increase in the payments received under our settlement agreement with Amyris of $6.7 million and the achievement of milestones under our agreement with the NIH during the year ended December 31, 2020 of $6.6 million, partially offset by a decrease of $1.5 million from the gain on the termination of our collaboration arrangement with Glycosyn during the year ended December 31, 2019.
In addition to our results determined in accordance with U.S. GAAP, we believe that EBITDA and Adjusted EBITDA, each non-GAAP financial measures, are useful in evaluating our operational performance. We use this non-GAAP financial information to evaluate our ongoing operations and for internal planning and forecasting purposes. We believe that this non-GAAP financial information, when taken collectively, may be helpful to investors in assessing our operating performance.
We define EBITDA as net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders before the impact of interest income, interest expense, provision for income taxes and depreciation and amortization.
We define Adjusted EBITDA as EBITDA adjusted for stock-based compensation expense, gain or loss on equity method investments, gain or loss on investments, change in fair value of warrant liabilities and other income and expenses. We believe that the use of EBITDA and Adjusted EBITDA provides an additional tool for investors to use in evaluating ongoing operating results and trends because it eliminates the effect of financing activities, investing activities, and certain non-cash charges and other items. Adjusted EBITDA includes non-cash adjustments such as stock-based compensation, gain or loss on equity method investments, and gain or loss on changes in fair value of our investments, warrant liabilities and loans receivable. Adjusted EBITDA also considers cash components which are not part of our ongoing operating results, such as gains related to settlement payments from Amyris and certain funding received from NIH to invest in our Biosecurity development related to COVID-19. We believe Adjusted EBITDA, although not a replacement for financial performance measures reported under U.S. GAAP, provides investors with a means to compare our financial measures with those of comparable companies, which may present similar non-GAAP financial measures to investors. However, you should be aware that when evaluating EBITDA and Adjusted EBITDA we may generate future income or incur future expenses similar to those excluded when calculating these measures. In addition,
74
our presentation of these measures should not be construed as an inference that our future results will be unaffected by future income or future expenses similar to those excluded when calculating these measures. Our computation of these measures, especially Adjusted EBITDA, may not be comparable to other similarly titled measures computed by other companies because not all companies calculate these measures in the same way.
Because of these limitations, EBITDA and Adjusted EBITDA should not be considered in isolation or as a substitute for performance measures calculated in accordance with U.S. GAAP. We compensate for these limitations by primarily relying on our U.S. GAAP results supplemented by EBITDA and Adjusted EBITDA. You should review the reconciliation of net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders to EBITDA and Adjusted EBITDA below and not rely on any single financial measure to evaluate our business.
The following table reconciles net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders to EBITDA and Adjusted EBITDA for the nine months ended September 30, 2021 and 2020 and for the years ended December 31, 2020 and 2019, respectively:
| Nine Months Ended September 30, |
Year Ended December 31, |
|||||||||||||||
| (in thousands) | 2021 | 2020 | 2020 | 2019 | ||||||||||||
| Net loss attributable to Ginkgo Bioworks, Inc. stockholders |
$ | (229,391 | ) | $ | (79,685 | ) | $ | (126,609 | ) | $ | (119,327 | ) | ||||
| Interest income |
(341 | ) | (5,565 | ) | (2,582 | ) | (5,756 | ) | ||||||||
| Interest expense |
1,822 | 1,795 | 2,385 | 2,421 | ||||||||||||
| Income tax provision (benefit) |
(797 | ) | 1,881 | 1,889 | 22 | |||||||||||
| Depreciation and amortization |
21,073 | 9,860 | 13,864 | 10,755 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| EBITDA |
(207,634 | ) | (71,714 | ) | (111,053 | ) | (111,885 | ) | ||||||||
| Stock-based compensation |
14,764 | 358 | 476 | 771 | ||||||||||||
| Loss on equity method investments(1) |
70,365 | 1,512 | 2,945 | 46,406 | ||||||||||||
| Loss on investments(2) |
(3,009 | ) | 4,978 | 1,070 | 7,797 | |||||||||||
| Change in fair value of warrant liabilities |
18,482 | — | — | — | ||||||||||||
| Other(3) |
421 | (5,804 | ) | (14,860 | ) | (3,118 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Adjusted EBITDA |
(106,611 | ) | (70,670 | ) | (121,422 | ) | (60,029 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | For the nine months ended September 30, 2021 and 2020, represents losses on equity method investments under the HLBV method of $72.6 million and $2.2 million, respectively, net of losses attributable to non-controlling interests. For the years ended December 31, 2020 and 2019, includes i) losses on equity method investments under the HLBV method of $0.4 million and $27.5 million, respectively, net of losses attributable to non-controlling interests and ii) loss on equity method investment under the fair value option of $2.7 million and $19.4 million, respectively. |
| (2) | Includes (gain) loss on the change in fair value of our warrant to purchase Synlogic common stock, which we have elected to account for under the fair value option. |
| (3) | For the nine months ended September 30, 2021, includes $1.0 million received pursuant to our settlement agreement with Amyris offset by $1.4 million in mark-to-market adjustments on Access Bio Convertible Notes and Glycosyn Promissory Note. For the nine months ended September 30, 2020, primarily includes $4.5 million received pursuant to our settlement agreement with Amyris and $1.2 million received from the NIH. For the year ended December 31, 2020, includes $6.6 million in income generated through our agreement with the National Institutes of Health (“NIH”) and $8.3 million received pursuant to our settlement agreement with Amyris. For the year ended December 31, 2019, includes $1.6 million received pursuant to our settlement agreement with Amyris and a $1.5 million gain on the termination of our collaboration arrangement with Glycosyn. |
75
Liquidity and Capital Resources
Since Ginkgo’s formation in 2008, we have incurred significant operating losses. Net losses attributable to us were $229.4 million for the nine months ended September 30, 2021 and $126.6 million for the year ended December 31, 2020. As of September 30, 2021 our accumulated deficit was $697.3 million. We expect to continue to incur significant expenses and operating losses for the foreseeable future.
We anticipate that our expenses will increase significantly in connection with our ongoing activities, as we:
| • | continue our R&D, activities under existing and new programs and further invest in our Foundry and Codebase; |
| • | hire additional personnel and secure facilities to support our expanding R&D efforts; |
| • | develop and expand our offerings, including Biosecurity; |
| • | upgrade and expand our operational, financial and management systems and support our operations; |
| • | acquire companies, assets or intellectual property that advance our company objectives; |
| • | maintain, expand, and protect our intellectual property; and |
| • | incur additional costs associated with operating as a public company. |
Sources of Liquidity
Prior to the Business Combination, our sources of liquidity have been predominantly from proceeds from equity offerings, convertible notes offerings, payments received for R&D services under license and collaboration arrangements; including those received on an upfront basis and upon accomplishment of milestones, payments received from Biosecurity product sales and services provided, and government grants. Upon the closing of the Business Combination in September 2021, we received net proceeds totaling approximately $1,509.6 million from the PIPE Investment. As of September 30, 2021, we had cash and cash equivalents of $1,739.1 million which we believe will be sufficient to enable us to fund our projected operations through at least the next 12 months from the date of the filing of this registration statement and prospectus.
Until required for use in our business, we typically invest our cash in money market funds that are highly liquid and readily convertible to cash. We attempt to minimize the risks related to our cash and cash equivalents by maintaining balances in accounts only with accredited financial institutions and, consequently, we do not believe we are subject to unusual credit risk beyond the normal credit risk associated with ordinary commercial banking relationships.
Cash Flows
The following table provides information regarding our cash flows for each period presented:
| Nine Months Ended September 30, |
Year Ended December 31, |
|||||||||||||||
| (in thousands) | 2021 | 2020 | 2020 | 2019 | ||||||||||||
| Net cash provided by (used in): |
||||||||||||||||
| Operating activities |
$ | (88,277 | ) | $ | (76,491 | ) | $ | (135,830 | ) | $ | (44,663 | ) | ||||
| Investing activities |
(77,485 | ) | (38,291 | ) | (67,121 | ) | (74,602 | ) | ||||||||
| Financing activities |
1,545,188 | 90,504 | 90,318 | 410,385 | ||||||||||||
| Effect of exchange rate changes |
(8 | ) | — | — | — | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net increase (decrease) in cash, cash equivalents and restricted cash |
$ | 1,379,418 | $ | (24,278 | ) | $ | (112,633 | ) | $ | 291,120 | ||||||
|
|
|
|
|
|
|
|
|
|||||||||
76
Operating Activities
Net cash used in operating activities for the nine months ended September 30, 2021 consisted of net loss of $231.6 million, adjusted for net change in our operating assets and liabilities of $30.8 million and non-cash charges of $112.6 million. The net change in our operating assets and liabilities was primarily due to an increase in accounts receivable of $27.8 million and a decrease in deferred revenue of $5.5 million, partially offset by a decrease in prepaid expenses and other current assets of $5.6 million, an increase in accrued expenses and other current liabilities of $29.6 million and an increase in other non-current liabilities of $29.1 million. Non-cash adjustments primarily consisted of depreciation and amortization of $21.1 million, stock-based compensation expense of $14.8 million, loss on equity method investments of $72.6 million, loss on change in fair value of warrant liabilities of $18.5 million, partially offset by a gain on investments of $3.0 million and non-cash equity consideration received of $12.6 million.
Net cash used in operating activities for the nine months ended September 30, 2020 consisted of net loss of $80.3 million, adjusted for net change in our operating assets and liabilities of $13.2 million and non-cash charges of $17.1 million. The net change in our operating assets and liabilities was primarily due to an increase in accounts receivable of $6.3 million and a decrease in deferred revenue of $13.1 million, partially offset by a decrease in other non-current assets of $2.4 million and an increase in accounts payable of $2.7 million. Non-cash adjustments primarily consisted of depreciation and amortization of $9.9 million, loss on equity method investments of $2.2 million and loss on investments of $5.0 million.
Net cash used in operating activities for the year ended December 31, 2020 consisted of net loss of $126.7 million and a net change in our operating assets and liabilities of $26.5 million, offset by non-cash adjustments of $17.4 million. The net change in our operating assets and liabilities was primarily due to a decrease in current and non-current deferred revenue of $19.4 million, an increase in accounts receivable and accounts receivable from related parties of $14.2 million and an increase in prepaid expenses and other current assets of $11.4 million, partially offset by an increase in accounts payable of $7.0 million and an increase in accrued expenses and other current liabilities of $8.7 million. Non-cash adjustments primarily consisted of depreciation and amortization of $13.9 million, loss on equity method investments of $3.1 million and loss on investments of $1.1 million, partially offset by changes in the fair value of loans receivable of $1.1 million.
Net cash used in operating activities for the year ended December 31, 2019 consisted of net loss of $119.9 million, offset by a net change in our operating assets and liabilities of $10.6 million and non-cash adjustments of $64.6 million. The net change in our operating assets and liabilities was primarily due to an increase in non-current deferred rent of $9.1 million, an increase in current and non-current deferred revenue of $4.9 million, an increase in accrued expenses and other current liabilities of $4.2 million, partially offset by an increase in prepaid expenses and other current assets of $4.0 million and an increase in other non-current assets of $2.4 million. Non-cash adjustments primarily consisted of loss on equity method investments of $46.9 million, depreciation and amortization of $10.8 million and loss on investments of $7.8 million, partially offset by the gain on the termination of our collaboration arrangement with Glycosyn of $1.5 million.
Investing Activities
Net cash used in investing activities for the nine months ended September 30, 2021 primarily consisted of purchases of property and equipment of $51.4 million associated with Foundry capacity and capability investments, purchase of non-marketable equity securities of $5.0 million and acquisition of Dutch DNA Biotech B.V. of $21.4 million.
Net cash used in investing activities for the nine months ended September 30, 2020 primarily consisted of purchases of property and equipment of $38.4 million associated with Foundry capacity and capability investments.
77
Net cash used in investing activities for the year ended December 31, 2020 primarily consisted of purchases of property and equipment of $57.8 million, including costs associated with Foundry capacity and capability investments and purchase of Access Bio’s convertible notes of $10.0 million.
Net cash used in investing activities for the year ended December 31, 2019 primarily consisted of purchases of property and equipment of $22.2 million and $50.1 million of cash paid for our investment in Synlogic.
Financing Activities
Net cash provided by financing activities for the nine months ended September 30, 2021 primarily consisted of net proceeds received from the Business Combination of $1,510.9 million, non-controlling interest contributions of $60.0 million related to our consolidated VIEs, Ayana and Verb, partially offset by repurchases of common stock from our founders of $25.0 million.
Net cash provided by financing activities for the nine months ended September 30, 2020 primarily consisted of net proceeds from the issuances of our Series E convertible preferred stock of $91.0 million.
Net cash provided by financing activities for the year ended December 31, 2020 primarily consisted of the net proceeds from the issuance of our Series E convertible preferred stock.
Net cash provided by financing activity for the year ended December 31, 2019 primarily consisted of the net proceeds from the issuance of convertible promissory notes and Series E convertible preferred stock.
Contractual Obligations and Commitments
The following table summarizes our contractual obligations as of December 31, 2020 and the effects that such obligations are expected to have on our liquidity and cash flows in future periods:
| Payments Due by Period | ||||||||||||||||||||
| (in thousands) | Total | Less than 1 Year |
1-3 Years |
3-5 Years |
More than 5 Years |
|||||||||||||||
| Drydock leases(1) |
$ | 124,812 | $ | 10,224 | $ | 24,074 | $ | 27,458 | $ | 63,056 | ||||||||||
| Operating leases, excluding Drydock leases(2) |
56,276 | 6,464 | 16,220 | 17,001 | 16,591 | |||||||||||||||
| Capital leases(3) |
840 | 500 | 340 | — | — | |||||||||||||||
| Purchase obligations(4) |
96,500 | 10,000 | 29,625 | 35,000 | 21,875 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual cash obligations |
$ | 278,428 | $ | 27,188 | $ | 70,259 | $ | 79,459 | $ | 101,522 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | We lease building space at 21, 23, 25 and 27 Drydock Avenue in Boston, Massachusetts where our primary operations are located. The non-cancelable operating leases each expire in January 2030 with options to extend each of the leases for one five-year period at then-market rates. The amounts reflected in the table above represent the minimum rental commitments under the non-cancelable operating leases and do not include the optional extensions. |
| (2) | We have various non-cancelable operating lease and sublease agreements for office and lab space in Boston and Cambridge, Massachusetts and Emeryville, California; which expire at various times through September 2030, subject to certain extension options. The amounts reflected in the table above represent the minimum rental commitments under the non-cancelable operating leases and do not include the optional extensions. |
| (3) | We have various capital leases for lab equipment used in our R&D activities which expire at various times through November 2023. |
78
| (4) | The amounts represent non-cancelable fixed payment obligations under our collaboration agreement with Berkeley Lights, Inc. For the purposes of the above table, due to the differences in timing of the contract years relative to the calendar year, we have assumed that these costs will be incurred ratably over the respective contract years. Refer to Note 11 of our audited consolidated financial statements appearing elsewhere in this registration statement and prospectus for additional details. |
Under our license and collaboration agreements, we are committed to providing certain R&D services related to license rights to our proprietary technology and intellectual property granted to our customers. The expenses we expect to incur as part of our commitments under our license and collaboration agreements, a portion of which are subject to reimbursement from our customers, are not included in the above table as they are contingent upon the occurrence of future events and the timing and likelihood of such potential expenses are not known with certainty.
In March 2018, we entered into a non-cancelable supply agreement with Twist Bioscience Corporation (“Twist”). Pursuant to the supply agreement, we are required to purchase certain products at specified volumes on a quarterly basis over a four-year term. To the extent we fail to meet our quarterly minimum purchase obligations, we are required to pay a fee per unit of shortfall. The products we may purchase that contribute toward achieving the quarterly minimum purchase obligation can vary based on our discretion, subject to advance notice provided to Twist. Our quarterly minimum purchase obligation may be adjusted for the following reasons: (i) due to a lack of availability of certain products for purchase in a given quarter; (ii) due to a lack of certain features available; (iii) delays in shipments over two consecutive quarters beyond the agreed upon lead times; and (iv) if the average yield of certain products measured over two consecutive quarters is greater than a specified yield. We receive volume discounts on purchases based on specified volume thresholds over the term of the supply agreement. Additionally, we receive a discount on each order of certain products, dependent upon the volume of certain other products we purchase in a given order. Refer to Note 11 of our consolidated financial statements appearing elsewhere in this registration statement and prospectus for additional details. As of December 31, 2020, we have incurred approximately $27.1 million under our supply agreement with Twist. We have budgeted approximately $15.0 million as of December 31, 2020 for purchases to be made during the year ended December 31, 2021. We have excluded the cash payments from the table above as the expected timing and amount of our future obligation is uncertain.
Lease Obligations
In April 2021, we entered into a lease consisting of approximately 152,000 square feet of office and laboratory space being developed in Boston, Massachusetts. The lease commencement date is estimated to be June 1, 2024, subject to certain extensions, and expires on the fifteenth anniversary of the lease commencement date. In September 2021, we exercised our expansion option to include the entire rentable area of the building of approximately 262,000 square feet. Annual base rent for the first lease year will be approximately $16.8 million, subject to annual rent increases over the lease term. The lease includes one option to extend the lease for ten years at then-market rates, subject to certain adjustments, and will be secured by a letter of credit of an estimated $14.7 million.
On September 6, 2021, we entered into an amendment to our operating lease at 27 Drydock Avenue in Boston, Massachusetts under which we will lease 47,957 square feet of additional space and extend the term of the lease by six years from January 2030 to January 2036. Minimum rental payments for the additional space will be $0.2 million per month starting in 2021 and $0.1 million per month starting in 2023, increasing by 3% annually. Minimum rental payments for the existing premises during the extended term will be $1.1 million per month, increasing by 3% annually. The table above does not reflect the future cash payments due under the lease amendment. Our letter of credit will increase by $1.0 million and we will continue to have an option to extend the term of the lease beyond the extended term for an additional five-year term.
79
During the nine months ended September 30, 2021, we entered into new capital leases that resulted in total incremental non-cancelable capital lease payments under the new capital leases of $2.0 million through the remainder of the lease terms. The table above does not reflect the future cash payments due under the new capital leases.
Contingent Consideration
In connection with our acquisition of Dutch DNA Biotech B.V. (“Dutch DNA”), we agreed to make additional payments based upon the achievement of certain technical and commercial milestones. We recognized the contingent consideration liability associated with this acquisition at its fair value on the acquisition date and revalue this obligation each reporting period. As of September 30, 2021, the aggregate maximum amount of milestone payments we could be required to make under the Dutch DNA purchase agreement is $20.0 million.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have any off-balance sheet arrangements, as defined in the rules and regulations of the SEC. Although we have holdings in variable interest entities, we are not obligated to fund the losses of such entities. Additionally, there is no obligation arising out of our holdings in variable interest entities where the entity provides material financing, liquidity, market risk or credit risk support to, or engages in leasing, hedging or R&D services with us.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with U.S. GAAP. The preparation of these consolidated financial statements requires us to make judgments and estimates that affect the reported amounts of assets, liabilities, revenues and expenses and the disclosure of contingent assets and liabilities in our consolidated financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. On an ongoing basis, we evaluate our judgments and estimates in light of changes in circumstances, facts and experience. The effects of material revisions in estimates, if any, are reflected in our consolidated financial statements prospectively from the date of change in estimates.
While our significant accounting policies are described in more detail in the notes to our audited consolidated financial statements appearing elsewhere in this registration statement and prospectus, we believe the following accounting policies used in the preparation of our consolidated financial statements require the most significant judgments and estimates.
Revenue Recognition
We account for revenue in accordance with ASC 606, Revenue from Contracts with Customers (“ASC 606”). Under ASC 606, we recognize revenue when the customer obtains control of the promised goods or services, at an amount that reflects the consideration we expect to receive in exchange for those goods or services. To determine revenue recognition for arrangements that are within the scope of ASC 606, we perform the following five steps: (i) identify the contract(s) with a customer, (ii) identify the promises and distinct performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations in the contract, and (v) recognize revenue when (or as) we satisfy the performance obligations.
80
Foundry Revenue
We generate license and service revenue through the execution of license and collaboration agreements whereby customers obtain license rights to our proprietary technology and intellectual property for use in the research, development and commercialization of engineered organisms and derived products. Under these agreements, we typically provide R&D services, which includes the provision of a license to our intellectual property. Additionally, the customer obtains license rights to the output of our services in order to commercialize the resulting output of such services. Generally, the terms of these agreements provide that we receive some combination of: (1) Foundry usage fees in the form of (i) upfront payments upon consummation of the agreement or other fixed payments, (ii) reimbursement for costs incurred for R&D services and (iii) milestone payments upon the achievement of specified technical criteria, plus (2) downstream value share payments in the form of (iv) milestone payments upon the achievement of specified commercial criteria, (v) royalties on sales of products from or comprising engineered organisms arising from the collaboration or licensing agreement and (vi) royalties related to cost of goods sold reductions realized by our customers, or (3) downstream value share in the form of equity interests in our customer.
Our collaboration and licensing agreements often contain multiple promises, including (i) licenses and assignments of intellectual property and materials and (ii) R&D services, and we determine whether each of the promises is a distinct performance obligation based on the nature of each agreement. As we are generally performing R&D services that are highly integrated and interrelated to the licenses and assignments of intellectual property and materials, the promises are generally inseparable. As such, we typically combine the R&D services, licenses, and assignments into a single performance obligation. However, for certain agreements, we only grant licenses or effects such transfers and assignments upon the successful completion of the R&D services or delivery of a developed product. For these agreements, we typically consider (i) the R&D services and (ii) the licenses, transfers, and assignments as distinct performance obligations, as each is transferred separately and has a separately identifiable benefit. Options to acquire additional goods and services are evaluated to determine if such options provide a material right to the counterparty that it would not have received without entering into the contract. If so, the option is accounted for as a separate performance obligation. If not, the option is considered a marketing offer which is accounted for as a separate contract upon the counterparty’s election.
At contract inception, we determine the transaction price, including fixed consideration and any estimated amounts of variable consideration. Any upfront cash payment received upon consummation of the agreement is fixed and generally nonrefundable. Variable consideration is subject to a constraint, and amounts are included in the transaction price to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. Variable consideration may include reimbursement for costs incurred for our R&D efforts, milestone payments upon the achievement of certain technical and commercial criteria, and royalties on sales of products from or comprising engineered organisms arising from the agreement. With respect to the R&D reimbursements and milestone payments, we use the most likely amount method to estimate variable consideration. With respect to agreements that include royalties on sales or other contingent payments based on sales, we apply the royalty recognition constraint which requires a constraint until the royalty or value-sharing transaction occurs. Certain agreements contain payment in the form of equity or other non-cash consideration. Any non-cash consideration is measured at the fair value of the non-cash consideration at contract inception.
For agreements with promises that are combined into a single performance obligation, the entire transaction price is allocated to the single performance obligation. For agreements with multiple performance obligations, the transaction price is allocated to the performance obligations using the relative standalone selling price methodology. For agreements featuring variable consideration, we allocate variable consideration to one or more, but not all, performance obligations if certain conditions are met. Specifically, we assess whether the variable consideration relates solely to our efforts to satisfy the performance obligation and whether allocating such variable consideration entirely to the performance obligation is consistent with the overall allocation objective. If
81
these conditions are not met, we allocate the variable consideration based on the relative standalone selling price methodology. The key assumptions utilized in determining the standalone selling price for each performance obligation include development timelines, estimated R&D costs, commercial markets, likelihood of exercise (in the case of options considered to be material rights), and probabilities of success.
For agreements where the licenses or assignments are considered separate performance obligations or represent the only performance obligation, we recognize revenue at the point in time that we effectively grant the license as the licenses or assignments represent functional intellectual property. For agreements where the licenses and the R&D services represent a combined performance obligation, we recognize revenue over the period of performance based on costs incurred to date as compared to total estimated costs.
We evaluate our measure of progress to recognize revenue at each reporting period and, as necessary, adjust the measure of performance and related revenue recognition. Our measure of performance and revenue recognition involves significant judgment and assumptions, including, but not limited to, estimated costs and timelines to complete our performance obligations. We evaluate contract modifications and amendments to determine whether any changes should be accounted for prospectively or on a cumulative catch-up basis. We utilize the right to invoice practical expedient when we have a right to consideration in an amount that corresponds directly with the value of our performance to date.
Royalties received under the agreements are recognized as revenue when sales have occurred as we apply the sales or usage-based royalties recognition constraint. We have determined the application of this exception is appropriate because the license granted in the agreement is the predominant item to which the royalties relate.
As we receive upfront payments for technical services under certain of our arrangements, we evaluate whether any significant financing components exist given the term over which the fees will be earned may exceed one year. Based on the nature of our agreements, there are no significant financing components as the purpose of the upfront payment is not to provide financing, but rather to secure technical services, exclusivity rights, and Foundry capacity, or the timing of transfer of those goods or services is at the discretion of the customer.
Deferred revenue represents consideration received by us in excess of revenue recognized and primarily results from transactions where we receive upfront payments and non-cash equity consideration. In instances where we have received consideration in advance for an undefined number of technical development plans (“TDPs”) under our customer agreements, we record the advance payments as deferred revenue, net of current portion on our consolidated balance sheets. Upon the execution of a specific TDP, we reclassify the estimated consideration to be earned under that TDP within the next twelve months as current deferred revenue. We also classify unexercised material rights as deferred revenue, net of current portion on our consolidated balance sheets. When a TDP is executed, and the material right is exercised, the amount allocated to the material right, which will be earned within the next twelve months, is reclassified to current deferred revenue. All other deferred revenue is classified as current or non-current based on the timing of when we expect to earn the underlying revenue based upon the projected progress of activities under the TDP.
Biosecurity Revenue
In 2020, we launched our commercial offering of COVID-19 testing products and services for businesses, academic institutions, and other organizations. In the first quarter of 2021, we launched our pooled testing initiative which focuses on providing end-to-end COVID-19 testing services to groups of individuals, with a focus on K-12 schools. We sell COVID-19 test kits on a standalone basis or as part of an end-to-end testing service. We record product revenue from sales of LFA, PCR, and pooled test kits. We record service revenue from sales of our end-to-end COVID-19 testing services, which consist of multiple promised goods and services including sample collection kits, physician authorizations, onsite test administration, outsourced laboratory PCR analysis, and access to results reported through the Company’s proprietary web-based portal. We recognize our product and service revenue using the five-step model under ASC 606.
82
Product revenue is recognized when the test kits are shipped and risk of loss is transferred to the carrier. Our test kits are generally not subject to a customer right of return except for product recalls under the rules and regulations of the U.S. Food and Drug Administration (“FDA”). We have elected to include shipping and handling fees billed to customers as a component of Biosecurity revenue.
Service revenue from our end-to-end COVID-19 testing services is recognized upon completion of the tests and release of the test results on the web-based portal. We have identified one performance obligation in our testing services contracts that represents a series of distinct goods or services that are substantially the same and that have the same pattern of transfer to the customer, with each test as a distinct service within the series. As the price for the testing services is fixed under each customer contract, we have elected the practical expedient to recognize revenue at the amount which we have the right to invoice for services performed. Our testing services contracts are generally one year or less in length and contain fixed unit pricing. Under typical payment terms for testing services, amounts are billed monthly in arrears for services performed or in advance based on contractual billing terms.
Variable Interest Entities
We evaluate our variable interests in variable interest entities (“VIEs”) and consolidate VIEs when we are the primary beneficiary. We determine whether we are the primary beneficiary of each VIE based on our assessment of whether we possess both (i) the power to direct the activities that most significantly affect the VIE’s economic performance and (ii) the obligation to absorb losses that could be significant to the VIE or the right to receive benefits that could be significant to the VIE. We reevaluate the accounting for our VIEs upon the occurrence of events that could change the primary beneficiary conclusion. With respect to our investments in Motif, Allonnia, Genomatica and Arcaea, we have concluded these entities represent variable interest entities. However, although we hold board representation and are involved in the ongoing development activities of the entities via participation on joint steering committees, we have concluded that we are not the primary beneficiary of these entities. We have reached this conclusion due to the fact that: (i) we do not control the board of directors of Motif, Allonnia, Genomatica or Arcaea and no voting or consent agreements exist between ourselves and other members of each respective board of directors or other investors, (ii) the holders of preferred security interests in Motif, Allonnia, Genomatica and Arcaea hold certain rights that require their consent prior to the taking of certain actions, which include certain significant operating and financing decisions and (iii) our representation on the joint steering committee of each respective entity does not give us control over the development activities of either Motif, Allonnia, Genomatica or Arcaea as all votes must pass by consensus and there are no agreements in place that would require either entity to vote in alignment with ourselves. As our involvement in Motif, Allonnia, Genomatica and Arcaea does not give us the power to control the decisions with respect to the development or other activities, which are the most significant activities of Motif, Allonnia, Genomatica or Arcaea, we have accordingly concluded that we are not the primary beneficiary. Additionally, with respect to Cooksonia’s investment in Joyn, as Cooksonia does not control Joyn’s board of directors, it does not have the power to control the decisions related to the development activities of Joyn, which are the most significant activities of Joyn. Accordingly, Cooksonia is not the primary beneficiary of Joyn.
With respect to Cooksonia, we have concluded that we hold a variable interest in this entity through our equity interest and we are the primary beneficiary of Cooksonia as we control the most significant activities of Cooksonia. These conclusions were based on the fact that: (i) we control 100% of the board of directors of Cooksonia and (ii) we hold a controlling financial interest in Cooksonia. Due to the fact that we are the primary beneficiary of Cooksonia, we have consolidated the financial statements of Cooksonia in accordance with ASC 810, Consolidation (“ASC 810”), into our consolidated financial statements and have recognized a non-controlling interest associated with the minority equity interest held by other investors of Cooksonia.
With respect to Ayana and Verb, we have concluded that we hold a variable interest in and are the primary beneficiary of Ayana and Verb as we control the most significant activities of these entities. These conclusions are supported by the fact that as of the primary beneficiary assessment date, for both Verb and Ayana: (i) we
83
have substantive control of the board of directors; (ii) all capital contributions were made by related parties of Ginkgo; and (iii) Ginkgo or its related parties comprise the entirety of the joint steering committee, the governing body which holds significant oversight with respect to the entities’ research and development programs. As a result of being the primary beneficiary of Ayana and Verb, we have consolidated the financial statements of these entities into our condensed consolidated financial statements and have recognized a non-controlling interest associated with the minority equity interest held by other investors.
Impairment of Long-Lived Assets
We review our long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. Recoverability is measured by comparing the book values of the assets to the expected future net undiscounted cash flows that the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the book values of the assets exceed their fair value.
Determination of Fair Value of Non-cash Consideration in Platform Ventures
The fair value of non-cash consideration received in relation to our Platform Ventures is in return for the license rights conveyed to the counterparty. We value the non-cash consideration, which is generally common stock or common units, at inception of the agreements using an option pricing method (“OPM”). The OPM used a back-solve methodology to infer the total equity value based on the pricing of the preferred financing round associated with the formation of the respective Platform Ventures, which was contemporaneous with the intellectual property agreements that conveyed our license rights to such Platform Ventures.
Determination of Fair Value of Loans Receivable
We have elected the fair value option under ASC 825, Financial Instruments (“ASC 825”), to account for our loans receivable. We use various valuation techniques to fair value our loans receivable, which are dependent on the terms of the underlying agreements, and record the gains or loss arising from the change in fair value as a component of other income (expense), net in our consolidated statements of operations and comprehensive loss. As of September 30, 2021 and December 31, 2020, our loans receivable balance primarily consisted of our revolving promissory note with Glycosyn and a series of convertible notes with Access Bio. As of December 31, 2019, the loan receivable balance consisted only of our revolving promissory note with Glycosyn. We used a probability-weighted discounted cash flow valuation approach to value our revolving promissory note with Glycosyn. Under this approach, the present value of the expected cash flows was calculated under four settlement scenarios and then weighted based on the probability of each scenario. A discount rate was also applied. Both the probability and timing of each scenario and the discount rate represented significant inputs used in valuing the revolving promissory note. We used a Monte-Carlo simulation model to determine the value of our convertible notes with Access Bio, which modeled the future stock price of Access Bio over the term of the convertible notes to assess the value of the various settlement features. The significant assumptions used in determining the simulated future stock price included the expected timing of the conversion, which was assumed at maturity, and expected volatility. The significant assumptions used in determining the value of the convertible notes under a redemption at maturity scenario was the discount rate and expected volatility. Refer to Note 3 of our consolidated financial statements appearing elsewhere in this registration statement and prospectus for additional details.
Determination of Fair Value of Common Stock
For awards granted prior to the Business Combination, the fair value of shares of common stock underlying our stock-based awards was determined on each grant date by Ginkgo, considering our most recently available third-party valuations of common stock and our assessment of additional objective and subjective factors that we believed were relevant and which may have changed from the date of the most recent valuation through the grant date. Historically, these independent third-party valuations of our equity instruments were performed
84
contemporaneously with identified value inflection points. The third-party valuations were prepared in accordance with the framework of the American Institute of Certified Public Accountants’ Technical Practice Aid, Valuation of Privately Held Company Equity Securities Issued as Compensation (the “Practice Aid”). The Practice Aid identifies various available methods for allocating enterprise value across classes and series of capital stock to determine the estimated fair value of common stock at each valuation date.
In addition to considering the results of these third-party valuations, we considered various objective and subjective factors to determine the fair value of our equity instruments as of each grant date, which may be later than the most recently available third-party valuation date, including:
| • | the lack of liquidity of our equity as a private company; |
| • | the prices of our convertible preferred stock sold to outside investors in arm’s length transactions and the rights, preferences and privileges of our convertible preferred stock as compared to those of our common stock, including the liquidation preferences of our convertible preferred stock; |
| • | the progress of our R&D efforts to develop our proprietary platform; |
| • | our stage of development and business strategy and the material risks related to our business and industry; |
| • | the valuation of publicly traded companies in the life sciences and biotechnology sectors, as well as recently completed mergers and acquisitions of peer companies; |
| • | any external market conditions and trends within the life sciences industry; |
| • | the likelihood of achieving a liquidity event given prevailing market conditions; and |
| • | the analysis of initial public offerings and the market performance of similar companies in the life sciences industry. |
The assumptions underlying these valuations represent management’s best estimates, which involve inherent uncertainties and the application of management judgment. As a result, if factors or expected outcomes change and we use significantly different assumptions or estimates, the fair value of our stock-based awards could be materially different. The fair value of our common stock is determined based on the quoted market price on the NYSE. We estimate the fair value of our common stock using a hybrid method which uses market approaches to estimate our enterprise value. The hybrid method is a probability-weighted expected return method (“PWERM”) where the equity value in at least one scenario is allocated using an OPM.
Under the PWERM, the value of common stock is estimated based on an analysis of future values assuming various possible future liquidity events. The value of common stock is based on the probability-weighted present value of expected future investment returns considering the possible outcomes and the rights and privileges of each class of equity. The future investment returns are discounted back to the valuation date at a risk-adjusted discount rate which is then weighted based on the probability of the respective outcome.
Under the OPM, each class of stock is treated as a call option on our equity value, with exercise prices based on the liquidation preferences of our convertible preferred stock. Under this methodology, the common stock has value only if the funds available for distribution to the holders exceeds the value of the liquidation preferences of the convertible preferred stock at the time of the liquidity event. The Black-Scholes model is used to price the call options which includes assumptions for the time to liquidity and volatility of equity value. A discount for lack of marketability is then applied to the common stock value.
For awards granted from January 2021 through September 30, 2021, we utilized the hybrid method to estimate the value of our common stock underlying our stock-based awards. We considered two scenarios: (i) a scenario in which the conversion of the convertible preferred stock to common stock occurs through an initial public offering (“IPO”) or a merger with a special purpose acquisition company (“SPAC”), and (ii) a remain-private
85
scenario. With respect to the remain-private scenario, we estimated equity value using the guideline public company method. With respect to the IPO/SPAC scenario, we considered the equity values indicated by preliminary letters of intent received from potential investors or the assumed equity value in the proposed business combination of $15 billion plus contingent consideration in the form of earnout shares. In the IPO/SPAC transaction scenario, conversion of the convertible preferred stock to common stock was assumed. In the remain private scenario, equity value was allocated among the convertible preferred stock and common stock using the OPM. In addition to considering these two scenarios, we considered the prices paid for our common stock and Series B convertible preferred stock in secondary transactions and we included these prices in our weighted average conclusion of value.
For awards granted from August 2020 through December 31, 2020, when using the hybrid method, we considered two scenarios: (i) a scenario in which the conversion of the convertible preferred stock to common stock occurred through an IPO or SPAC transaction, and (ii) a remain private scenario. In both scenarios, we estimated an equity value in a potential IPO or SPAC transaction based on the guideline public company method under a market approach. We then converted the estimated future value to present value using a risk-adjusted discount rate. In the IPO or SPAC transaction scenario, conversion of the convertible preferred stock to common stock was assumed. In the remain private scenario, equity value was allocated among the convertible preferred stock and common stock using the OPM. In addition to considering these two scenarios, we considered the prices paid for our common stock and Series B convertible preferred stock in secondary transactions and we included these prices in our weighted average conclusion of value.
For awards granted from January 1, 2019 through July 2020, when using the hybrid method we considered two scenarios: (i) a fully diluted scenario, in which the per-share common stock value was assumed to equal the price of the convertible preferred stock in a recent round of financing, and (ii) a remain private scenario, in which we used the OPM to back-solve to the price of our convertible preferred stock in a recent round of financing. In the fully diluted scenario, conversion of the convertible preferred stock to common stock was assumed. In the remain private scenario, equity value was allocated among the convertible preferred stock and common stock using the OPM. In addition to considering these two scenarios, for certain valuations during the period, we considered the prices paid for our common stock in secondary transactions and we included these prices in our weighted average conclusion of value.
For awards granted after the Business Combination, we determine the fair value of our common stock on each grant date based on the quoted market price on the New York Stock Exchange (“NYSE”).
We estimate the grant date fair value of stock option awards using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model requires the input of subjective assumptions, including fair value of common stock (for awards granted prior to the Business Combination), expected term, expected volatility, risk-free interest rate and expected dividend yield. The expected term was generally determined using the “simplified” method for “plain vanilla” options. We determined expected volatility using the historical volatility of the stock prices of similar publicly traded peer companies. The risk-free interest rate was based on the yield available on U.S. Treasury zero-coupon issues similar in duration to the expected term of the stock options. We have not paid, and do not expect to pay, dividends in the foreseeable future.
There are significant judgments and estimates inherent in determining the fair value of the common stock. These judgments and estimates include factors, both subjective and objective, including: (i) a discount for lack of marketability; (ii) external market data; (iii) historical activity by us in selling equity to outside investors; (iv) our stage of development; (v) rights and preferences of our equity securities that rank senior to common stock; and (vi) the likelihood of the various scenarios, among others. Changes to these assumptions could result in different fair values of common stock.
86
JOBS Act and Emerging Growth Company Status
In April 2012, the JOBS Act was enacted. As an emerging growth company (“EGC”) under the JOBS Act, we may delay the adoption of certain accounting standards until such time as those standards apply to private companies. Other exemptions and reduced reporting requirements under the JOBS Act for EGCs include presentation of only two years of audited financial statements in a registration statement for an initial public offering, an exemption from the requirement to provide an auditor’s report on internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act of 2002, an exemption from any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation and less extensive disclosure about our executive compensation arrangements. Additionally, the JOBS Act provides that an EGC can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an EGC to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to avail ourselves of the extended transition period and, therefore, while we are an EGC we will not be subject to new or revised accounting standards while they become applicable to other public companies that are not EGCs, unless we choose to early adopt a new or revised accounting standard.
We will remain classified as an EGC until the earlier of: (i) the last day of our first fiscal year in which we have total annual gross revenues of $1.07 billion or more, (ii) the last day of the fiscal year following the fifth anniversary of completion of the IPO of SRNG, (iii) the date on which we have issued more than $1.0 billion of non-convertible debt instruments during the previous three fiscal years or (iv) the date on which we are deemed a “large accelerated filer” under the rules of the SEC.
Recently Issued Accounting Pronouncements
We have reviewed all recently issued standards and have determined that, other than as disclosed in Note 2 of our consolidated financial statements appearing elsewhere in this registration statement and prospectus, such standards will not have a material impact on our financial statements or do not otherwise apply to our current operations.
Quantitative and Qualitative Disclosures about Market Risks
Interest Rate Fluctuation Risk
We are exposed to market risk related to changes in interest rates. Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because our cash equivalents are invested in short-term U.S. Treasury and government obligations. However, because of the short-term nature of the instruments in our portfolio, an immediate change in market interest rates of 100 basis points would not have a material impact on the fair market value of our cash and cash equivalents or on our financial position or results of operations.
Foreign Currency Fluctuation Risk
We are subject to foreign currency exchange risk from the translation of the financial statements of our foreign subsidiary, whose financial condition and results of operations are reported in Euros and then translated into U.S. dollars at the applicable currency exchange rate for inclusion in our consolidated financial statements. Foreign currency translation adjustments were $0.9 million for the nine months ended September 30, 2021. Additionally, we have contracted with and may continue to contract with foreign vendors.
Inflation Fluctuation Risk
Inflation generally affects us by increasing our cost of labor. We do not believe that inflation had a material effect on our business, financial condition or results of operations during the nine months ended September 30, 2021 and 2020 or during the years ended December 31, 2020 and 2019.
87
Unless the context otherwise requires, all references in this section to the “Company,” “we,” “us,” or “our” refer to the business of Ginkgo Bioworks, Inc. and its subsidiaries prior to the Closing and to Ginkgo Bioworks Holdings, Inc. and its subsidiaries following the Closing.
Our mission is to make biology easier to engineer. That has never changed. Every choice we’ve made with respect to our business model, our platform, our people and our culture is grounded in whether it will advance our mission. Biology inherently offers incredible capabilities that we can only imagine in human-made technologies—self-assembly, self-repair, self-replication—capabilities that can enable more renewable and innovative approaches for nearly every industry. To realize this potential, we are building a platform for cell programming by bringing together unparalleled scale, software, automation, data science and reusable biological knowledge, enabling responsible solutions for the next generation of foods, pharmaceuticals, materials and more.
Ginkgo is building the industry-standard horizontal platform for cell programming. Our founders are engineers from diverse fields who, more than 20 years ago, were inspired by an astonishing feature of biology: it runs on digital code. It’s just A, T, C, and G rather than 0 and 1. But where computer bits are used to communicate information, genetic code is inherently physical and as it is read, physical structures are made. We program computers to manipulate bits, but we program cells to manipulate atoms. Cells are the building blocks of our food, our environment and even ourselves.
We use our platform to program cells on behalf of our customers. These “cell programs” are designed to enable biological production of products as diverse as novel therapeutics, key food ingredients, and chemicals currently derived from petroleum. We have worked on 95 major programs through the first nine months of 2021 in end markets as diverse as specialty chemicals, agriculture, food, consumer products, and pharmaceuticals. Biology did not evolve by end market. All of these applications run on cells which have a common code—DNA—and a common programming platform can enable all of them. Because of this shared platform, we are able to drive scale and learning efficiencies while maintaining flexibility and diversity in our program areas. Ultimately, customers come to us because they believe we maximize the probability of successfully developing their products.
Customers look to Ginkgo to overhaul their manufacturing processes or develop new products through biology. They might, for example, be looking to produce a particular chemical via fermentation, at a lower cost, with enhanced supply chain reliability or sustainability. Or perhaps the customer needs a microbe that will live and grow on the roots of corn and convert nitrogen in the air into usable fertilizer for a plant, resulting in improved plant growth. Or a customer might need an antibody that binds to and neutralizes a certain target, along with a way to produce those antibodies at scale. All of these programs and more run on a common platform at Ginkgo.
The foundation of our platform includes two core assets that execute a wide variety of cell programs for customers according to their specifications: our Foundry and our Codebase.
| • | Our Foundry wraps proprietary software and automation around core cell engineering workflows—designing DNA, writing DNA, inserting that DNA into cells, testing to measure cell performance—and leverages data analytics and data science to inform each iteration of design. The software, automation and data analysis pipelines we leverage in the Foundry drive a strong scale economic: we have scaled the output of the Foundry by roughly 3X annually since we started measuring it around 2015 and over that time, the average cost per unit operation has fallen by approximately 50% every year. We expect to be able to pass these savings along to our customers, allowing them to take more “shots on goal” with their programs. |
88
| • | Our Codebase includes both our physical (engineered cells and genetic parts) and digital (genetic sequences and performance data) biological assets, and accumulates as we execute more cell programs on the platform. Every program, whether successful or not, generates valuable Codebase and helps inform future experimental designs and provides reusable genetic parts, making our cell program designs more efficient. |
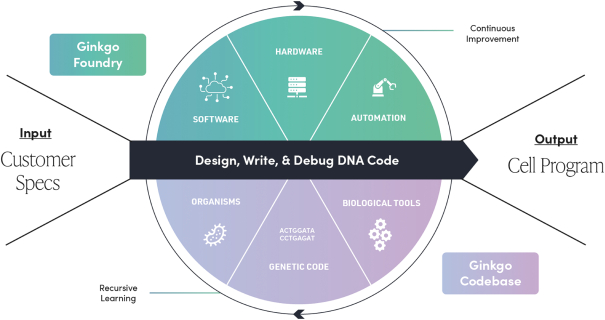
Figure 1: Our platform is used to design, write, and debug DNA code in engineered organisms to execute programs for our customers. Our Foundry leverages proprietary software, automation, and data analytics to reduce the cost of cell programming. Our Codebase consists of reusable biological assets that helps accelerate the engineering process.
As the platform scales, we have observed a virtuous cycle between our Foundry, our Codebase, and the value we deliver to customers. Sketched below, we believe this virtuous cycle sustains Ginkgo’s growth and differentiated value proposition.
| • | Foundry: As we take on more work in the Foundry, we benefit from scale economics, which over time may lead to lower program costs. We expect that these lower costs, in turn, will drive additional demand for our cell programming capabilities. |
| • | Codebase: Cell programs also generate Codebase, which can drive better experimental direction and improve the odds of technical success, further increasing our customer value proposition, which we believe will result in additional demand. |
Put simply: we believe that as the platform improves with scale, it drives more scale, which drives further platform improvements, and so on. We believe this positive feedback loop has the potential to drive compounding value creation in the future, as every new program we add contributes to both near-term revenues and has the potential to add significant downstream economics.
89
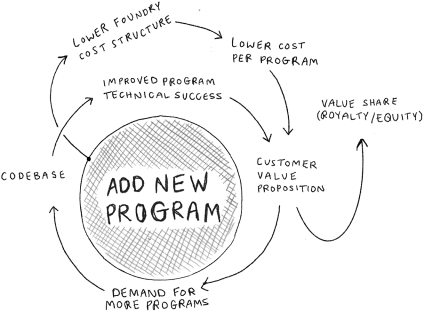
Figure 2: Ginkgo’s virtuous cycle: as we scale, we see greater efficiency and higher odds of technical success, which helps drive further scaling as our value proposition improves.
Our business model mirrors the structure of our platform and we are compensated in two primary ways. First, we charge usage fees for Foundry services, in much the same way that cloud computing companies charge usage fees for utilization of computing capacity or contract research organizations (CROs) charge for services. The total addressable market (TAM) for our Foundry revenue includes the market for biotech labor and tools, which industry sources estimate will be approximately $40 billion in 2021 and which is expected to grow at a CAGR of approximately 20% from 2021 to 2023. This revenue stream represented $59 million in 2020. Additionally, we negotiate a value share with our customers (typically in the form of royalties, milestones, and/or equity interests) in order to align our economics with the success of the programs enabled by our platform. As we add new programs, our portfolio of programs with this “downstream” value potential grows. Through these value shares, we are tapping into what industry sources expect to be a $2 to $4 trillion market for bioengineered products.
We believe that cell programming has the potential to be as ubiquitous in the physical world as computer programming has become in the digital world. We believe products in the future will be grown rather than made. To enable that vision, we are building a horizontal platform to make biology easier to engineer. Our business model is aligned with this strategy and with the success of our customers, setting us on what we believe is a path towards sustainable innovation for years to come.
An Introduction to Synthetic Biology
To fully tell the story of cell programming, we have to start four billion years ago. All living things evolved from a single cell, a tiny bubble containing the code that enabled it to assemble and reproduce itself. But, importantly, that process of reproduction wasn’t perfect; each copy introduced new mutations in the code. These changes are responsible for one of the most powerful and defining features of biology: evolution. Over eons, that first cell and all its progeny copied themselves, and their DNA evolved to create new functions: to eat new kinds of foods and to produce new kinds of chemicals, structures, and behaviors. As reproduction became more, well, interactive, organisms developed tools to borrow DNA from each other, accelerating the pace of evolution. These functions, and thus the genetic code programming the functions, stuck around when they helped the organisms survive and create more descendants. This went on and on for four billion years, leaving us the wild codebase of DNA that enables the diversity of life forms we see on the planet today.
90
Synthetic biology’s story begins mere decades ago, as biologists began to decode the molecular secrets of DNA. The billions-year-old tools of cells—enzymes that cut, copy, and paste sequences of DNA code—are now being leveraged by humans to read, write, and edit DNA in the lab. Polymerases that copy DNA are used to enable PCR tests for COVID-19 and the CRISPR/Cas system from bacteria now enables editing of human genomes to potentially cure genetic diseases.
Today we are using these tools to learn from the full breadth of evolution and biodiversity to write new biological code. Simple soil bacteria produce everything from vital antibiotics to the smell of fresh rain. We can reuse elements of these DNA programs to make new products. Biochemistry is extraordinarily versatile; we’ve reused genetic code libraries across applications as diverse as fine fragrances, baking, and consumer electronics. We may be able to develop programs that can digest human-made “forever chemicals” that biology never encountered before.
As cell programmers, we operate with humility and respect for biology. Our tools are simply borrowed, and the history of biotechnology is a mere blink of an eye compared to the history of living things. Today, we write rudimentary code. We believe that someday our children will write poetry in DNA.
Programming life

Like computers, cells run on digital code. DNA strands are sequences composed of four chemical bases, or nucleotides, represented by the letters A, T, C and G. The letters along the strand encode the proteins that make up the cell and perform biochemical functions. The translation of DNA ☐ RNA ☐ Protein is known as the “central dogma” of molecular biology.
The Central Dogma of Molecular Biology
“Traditional” genetic engineering uses special types of proteins from bacteria that can cut and paste DNA to move sequences from one organism to another. In 1982, Genentech Inc. partnered with Eli Lilly and Company to bring these techniques to market, producing human insulin inside the bacteria, E. coli. Genetic engineers were able to cut the code for the human insulin protein and paste it into the genome of E. coli and “boot up” the sequence: the bacteria could now produce the human protein, which could then be extracted, purified, and used by diabetics. This life-saving development replaced a vastly more expensive and supply-constrained method of extracting insulin from animal pancreases.
Relatively simple proteins like insulin can be produced by transferring one gene sequence into a simple microorganism. Many other biochemicals require much more complex cell programming and are produced by a
91
series of special proteins, called enzymes, working together. These enzymes transform a starting material, or “feedstock,” such as sugar, into a final product, such as an antibiotic, vitamin, or other valuable small molecule. In this way, biology also programs chemistry. Cell programmers can design such multi-enzyme “pathways” and transfer them into a cell to boot up. For example, the cell programs we’re writing for Cronos Group, Inc. to produce cannabinoids require many different enzymes to convert feedstock into cannabinoids such as cannabidiol (CBD).
Once the cell is programmed to produce a new molecule, it can produce the molecule and also replicate itself, creating an exponentially growing number of product-producing cells. Many products of genetic engineering are manufactured in facilities that look like breweries, taking advantage of the centuries old process of industrial fermentation to grow cells at high density, and transforming simple sugars into valuable products that can be extracted and commercialized.
Improved tools for cell programming, including automation, miniaturization, and data science, alongside the decreasing cost of DNA synthesis—writing DNA—are opening up new possibilities for cell programming. For each new program, Ginkgo’s organism engineers design, print, and test hundreds or thousands of different sequences for each step of a pathway, exploring the breadth of biological design space and improving the probability of success. We provide more details about our platform in the sections that follow.
92

93
Figure 3: An overview of a simple cell program.
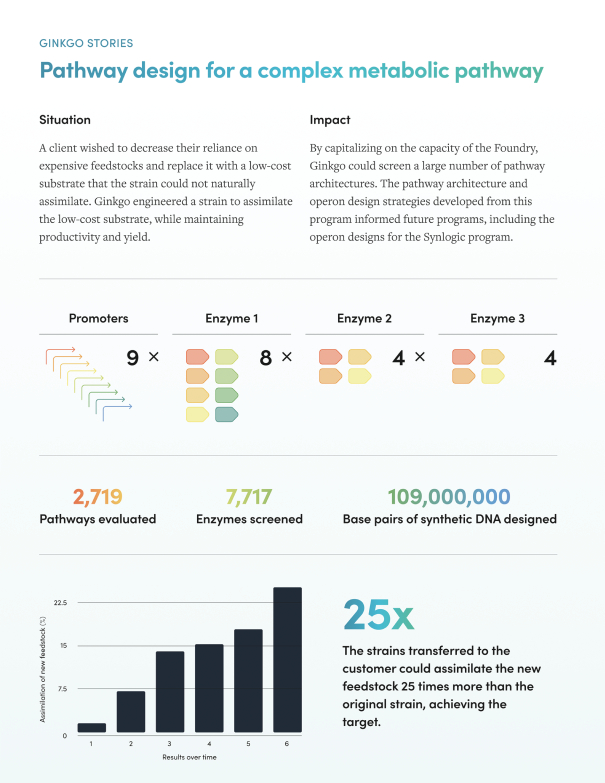
94
The Impact of Cell Programming
The power of biology has never been more apparent. Synthetic biology was featured on the cover of The Economist in April of 2019. Just two years later, hundreds of millions of people are receiving a novel type of vaccine created by companies such as Pfizer and Moderna and made up of a form of biological code, mRNA. Our own cells read that code to produce viral proteins and stimulate our immune response to fight back against the SARS-CoV-2 virus. We no longer question if biotechnology will transform a given industry, we simply question whether we are creative enough to imagine how, and whether we are ready to utilize biology responsibly.
ESG is in our DNA
Biology affects all of us, and we believe cell programming will change the world. Our customers are developing products with far reaching implications in health and the environment. This potential for extraordinary impact, which reaches to the core of who we are and everything about our natural world, requires extraordinary care in how the tools of cell programming are built and used. Technologies reflect the values of the organizations that build them, so our commitment to Environmental, Social, and Governance (ESG) priorities and care must underscore everything we do.
We also must recognize that biotechnologies have not always reflected the values necessary for sustainable and equitable impact and, as a result, remain controversial. Indeed, companies that produce genetically modified organisms (“GMOs”) for human consumption are restricted from certain ESG indices, placing genetic engineering as a major ESG risk alongside the production of weapons, tobacco products, and fossil fuels. We hope to chart a new course built on care so that the world can benefit from the power of biological engineering while avoiding potential risks.
Environmental
We face an urgent environmental crisis that is forcing us to reconsider how we make everything, from our homes, to our food, to our clothing. For centuries, we’ve treated nature as an infinite resource and infinite trash can, extracting raw materials, shaping them through industrial processes that spew out greenhouse gases, and then throwing them away. But these resources are not infinite and there is no “away.” The results have been disastrous—climate change, loss of biodiversity, and pollution have impacted every corner of our world and continue to threaten our way of life.
Cell programming and biological manufacturing are working to address some of the issues that are most contributing to climate change today, from fossil fuel dependency to agricultural emissions, and land use to plastic pollution. Ultimately, biology offers a fundamental shift in how things are made and disposed of: a world where things grow and decay, creating circular, regenerative processes.
There is significant concern that genetic engineering itself creates a form of genetic “pollution” in the environment, with genes from one context introduced into another. This is a concern we take seriously and consider deeply throughout the lifecycle of our programs to ensure that genes introduced will not cause damage—for example, by spreading antibiotic resistance or toxins. We care because the environmental release of certain genetically engineered microbes can also offer tremendous environmental benefit. For example:
| • | Crop-associated microbes programmed with the nitrogen fixing properties of common soil bacteria may be able to reduce the use of chemical fertilizers, which today contribute 5% of global greenhouse gas emissions and account for 4% of natural gas consumption. This is the work of Joyn Bio, LLC our joint venture with Bayer CropScience LP. |
| • | Microbes programmed to clean up wastewater or contaminated land is the work of Allonnia, LLC, a company we formed in partnership with Battelle. |
95
| • | And we are just getting started… we believe biology is our best tool to reverse the damage to our planet and chart us on a path towards sustainability in the future. |
Social
Technology isn’t neutral. Our values and biases are embedded in the technologies we make, in the applications we consider, and in the ways we address problems. Inclusion of those who have historically been left out of the development of new technologies is essential to building equitable and positive outcomes. Just as biological ecosystems thrive with more diversity, the inclusion of many different voices is essential to growing our company and to ensuring that the viewpoints of historically marginalized people are included in the development of our platform. We have many active efforts in recruiting and retaining diverse talent and will continue to invest in this work (see “—Our People & Culture”).
Marginalized people who have been left out of the development of technologies are also the groups most likely to bear the greatest harm, whether from climate change, pollution, or health disparities. The COVID-19 pandemic has made this inequality starkly clear—in the United States, it has been communities of color that have been disproportionately impacted by the pandemic and have had the least access to testing, treatment, and vaccination.
In March of 2020, we committed to $25 million of pro bono work to help accelerate novel diagnostics, therapeutics, and vaccines to help fight COVID-19. Our early work included efforts to improve the manufacturing of vaccines, with a goal to lower costs and increase accessibility of vaccines worldwide. Shortly thereafter, we launched Concentric by Ginkgo, a service to provide public health testing infrastructure for communities that need it most. Our pooled testing service was designed with accessibility and privacy as core design principles, to bring low-cost, easy-to-use testing to K-12 schools in the places that have been most affected by the pandemic. We partnered with school districts such as Baltimore City schools to make sure that our service was designed to serve the community and to build trust with groups who have been excluded, exploited and mistreated by biomedical research in the past.
These values and initiatives are not just a top-down corporate policy, they are an intrinsic part of our culture. Grassroots fundraising challenges to support local and international aid organizations are a regular feature of our internal messaging channels. One of our software engineers even programmed a free tool, @vaccinetime on Twitter, that has helped thousands of Massachusetts residents find vaccine appointments.
Governance
Our culture is built on care, transparency, diversity, employee ownership and engagement, and a deep, humble respect for biology. Transparency is essential to how we operate, to enable sharing of the insights and tools that enable our platform to grow, as well as to build trust and accountability with all of our stakeholders. We have advocated for more transparency in our industry, including supporting GMO labeling, and seek to educate policymakers and the general public about the benefits and risks of synthetic biology through our advocacy efforts.
The individuals who work at Ginkgo and build our platform care deeply about how that platform is used and the impact our company will have in the world. We believe a workforce with strong equity ownership will make the wise decisions needed to build long-term value for our company, and a company whose long-term impacts make them proud. That is why we have implemented a multi-class stock structure that permits all employees (current and future), not just founders, to hold high-vote (10 votes per share) common stock. We believe that our multi-class stock structure will help maintain the long-term mentality we have benefited from as a founder-led company.
For more information, see “Risk Factors—Risks Relating to our Organizational Structure and Governance— Only our employees and directors are entitled to hold shares of New Ginkgo Class B common stock (including
96
shares of our Class B common stock granted or otherwise issued to our employees and directors in the future), which shares will have 10 votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring stockholder approval.”
We have selected independent directors with decades of experience serving as leaders in the life sciences and technology industries. Our board of directors and management team will leverage that experience and consider the interests of stockholders, customers, employees, suppliers, academic researchers, governments, communities, and other stakeholders to pursue long-term value for our company and drive the sustained health of our global community. For more information, see “Risk Factors—Risks Relating to our Organizational Structure and Governance—Our focus on the long-term best interests of our company and our consideration of all of our stakeholders, including our stockholders, workforce, customers, suppliers, academic researchers, governments, communities and other stakeholders that we may identify from time to time, may conflict with short-term or medium-term financial interests and business performance, which may adversely impact the value of our common stock.”
Cell programming is expected to transform all industries
Biology grows. Biology adapts and evolves. Biology heals itself and regenerates. Biology is also, remarkably, programmable, offering us the tools to work with biology to transform how we make stuff. With cell programming, we help our customers across industries grow better products. What does “better” mean? Better products might be more sustainable, have more stable and resilient supply chains, be more accessible, have higher quality and more consistency, and come with lower economic and environmental costs of manufacturing. They can also be truly transformative, fundamentally changing the field of possibilities for what products can do. We have supported many companies that are leveraging our cell programming platform to address some of the world’s most challenging environmental and social issues.
Pharma & Biotech
Biopharma has been a nexus of tremendous innovation in cell programming and synthetic biology. Just in the past year, we have seen the creation and broad adoption of a novel form of biological prophylactic in the form of nucleic acid vaccines. These vaccines contain genetic code that our bodies read to produce viral proteins and stimulate an immune response and antibody production. New nucleic acid vaccines can be programmed quickly, such as the booster vaccines being developed against emerging SARS-CoV-2 variants, offering the potential for rapid response to other future pathogens. They can also be programmed to target a number of other diseases. In the wake of the success of nucleic acid vaccines during the COVID-19 pandemic, new programs for HIV and cancer vaccines, among others, are accelerating.
Biologic medicines like insulin and other protein drugs and antibodies are also produced via cell programming, making a difference in the treatment of countless diseases. Over 30% of the therapies approved by the FDA last year were biologics. New modalities enabled by cell programming, such as cell and gene therapies, microbiome therapies, regenerative medicine, and living medicines are beginning to come online. We believe human health and the ways we treat disease will be transformed by improvements in cell programming technology.
Ginkgo has been active in this field in recent years and we expect to significantly expand our support of therapeutic applications over coming years. From companies developing “living medicines” (Synlogic) to those involved in COVID-19 vaccine production (Moderna and others) to those developing novel antibiotics (Roche), we are using our platform to deliver transformational innovations across a range of disease areas.
97
Industrials & Environment
Since the industrial revolution, manufacturing techniques have been extractive, wasteful, and unsustainable. Not only must we innovate new manufacturing methods in order to keep up with growing demand, we must also
work to remediate issues we have caused historically, by cleaning up our environment and addressing climate change.
Ginkgo is not only working with customers to create cell programs that enable cost-efficient, renewable, and sustainable production of chemicals and materials, such as our work with Genomatica, Inc., but we have also spun out participated in the formation of Allonnia, LLC, a company focused on environmental remediation. Plastic waste and many of the pollutants that plague industrial manufacturing and extraction sites are novel in the course of evolutionary history, so biology has not yet evolved to degrade them efficiently. Cell programming can enable the discovery and development of new enzymes capable of degrading recalcitrant pollutants and recycling waste while entirely reimagining manufacturing for the future.
Food & Agriculture
Food is inherently biological: it comes from life and sustains life. Cell programming can be leveraged to improve the availability of essential food and nutrition to a growing population, decrease the environmental impact and cost of food production, and provide consumers with increased choice.
We are working with some of the largest multinational agriculture companies, including Bayer (through our joint venture, Joyn Bio) and Corteva, to develop cell programs that would make crop production more efficient and sustainable, reducing synthetic nitrogen fertilizer and pesticide usage. In food, we have been active in flavors and sweeteners, and we are the principal cell programming platform for Motif FoodWorks, Inc., a company that is making animal proteins without the need for industrial farming of animals.
Consumer & Technology
Most physical goods have biological origins—from the petrochemicals in our fabrics to fine chemicals extracted from plants—but industry does not necessarily leverage biology, or leverage biology efficiently, to produce these items. Petrochemicals, for example, are used in everything from our fabrics to our cosmetics to our paints. These chemicals and polymers are generally created in complex chemical and physical reactions from crude oil but crude oil is just the result of millions of years of decomposition of previously living matter (they are fossil fuels after all). These biological building blocks can instead be programmed in a living organism to produce these items sustainably, without extracting natural resources. Even in areas where industry does leverage biology, such as extracting raw materials or fine chemicals from plants, the current approaches are woefully inefficient or rife with social consequences.
We have helped some of the world’s largest fragrance companies use fermentation to much more efficiently produce rare molecules typically extracted from plants. In a related field, we are also supporting Cronos in their effort to biosynthesize cannabinoids, with the goal of reducing cost, improving purity and predictability, and enabling production of rare molecules. We have also recently spun out a new company, Arcaea, which is focusing on leveraging biology, from proteins to the microbiome, to build a suite of innovative and efficacious personal care products.
98

Cell programming is addressing our most challenging environmental and social issues Pharma & Biotech Antibody therapeutic development Nucleic acid vaccine production Antibiotic discovery and manufacturing Microbiome therapeutics Gene and cell therapies Industrials & Environment Wastewater remediation Renewable chemicals Pollutant degradation Sustainable building materials Carbon sequestration Food & Agriculture Animal protein replacement Brewing & baking Fertilizer reduction Pest Control Animal feed and aquaculture Consumer & Technology Flavors, fragrances, cannabinoids Skin microbiome Haircare and skincare proteins Textiles and dyes Electronic coatings
Figure 4: Summary of major markets and examples of application areas as well as current and former partners in these fields.
99
For several decades in the computing industry, software ran entirely in local environments: companies built and ran their own servers and customized their applications. The dominance of software-as-a-service (“SaaS”) software and cloud computing over the past decade has demonstrated the value in having common architectures and enabling horizontal platforms. What users may have sacrificed in customizability, they more than gained in
innovation, efficiency, and scalability. We believe Ginkgo is ushering in a similar transition in cell programming, a programming discipline with the power to shape living things and grow applications across the physical world.
The value of these applications will measure in the trillions of dollars
Given the breadth of application areas and the potential of biology (see “—The Impact of Cell Programming”), we believe that the end markets for bioengineered products will be enormous. Industry sources estimate that in the next 10 to 20 years, there will be approximately $2 to $4 trillion of annual direct economic impact from these products, with significant secondary effects. But these applications reflect only what we can already imagine. As we develop a greater ability to program biology and direct it towards novel and more challenging applications, the spectrum of possibilities will undoubtedly grow. Computers were used for little more than counting for decades; we firmly believe the most valuable applications of cell programming are not yet apparent.
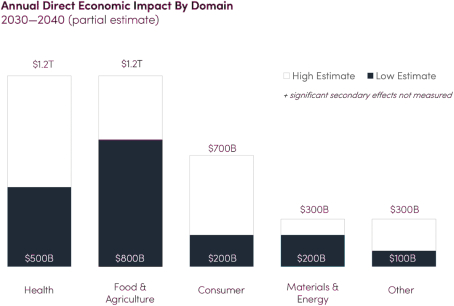
Annual Direct Economic Impact By Domain 2030-2040 (partial estimate) $1.2T $1.2T $700B $300B $300B $500B $800B $200B $200B $100B High estimate low estimate + significant secondary effects not measured Health Food & Agriculture Consumer Materials & Energy Other
Figure 5: Industry sources estimate a $2 to $4 trillion total addressable market for bioengineered products between 2030 and 2040.
Large existing market for “on prem” cell programming research and development
Cell programming today is done in a highly inefficient, distributed manner reminiscent of the early days of computing. Essentially every organization looking to innovate in biology builds its own biology labs in the same way that companies used to set up their own servers. Scientists spend hours moving liquids around rather than designing novel experiments in the same way that computer programmers once spent most of their time physically writing and debugging code (by punching cards, for example) than designing new applications.
100
Intellectual property lies fallow on the shelves of one institution, with no obvious mechanism to explore whether that IP might be useful to researchers in other domains. Ginkgo’s platform breaks down these silos and democratizes access to the most advanced technologies in the field, enabling customers of all sizes to more efficiently drive innovation.
According to industry sources, approximately $40 billion will be spent in 2021 on cell programming research and development. This work is being done in a distributed manner, sacrificing benefits from scale and learning economies. Approximately 60% of the spend today is on labor—scientists designing and executing experiments—while the remaining 40% of this is spent on “tools”—things like DNA synthesis, reagents, and equipment. Ginkgo brings efficiencies to both elements of this existing market.
| 1. | Labor: When scientists are able to leverage advanced automation, they are able to both reduce error rates and free time otherwise spent performing manual work (e.g. pipetting liquids from one plate to another). Freed from the burden of manual programming, scientists have more time to practice the art of cell programming: designing the direction of experimentation, mining data for new insights or exploring new techniques or application areas. This in turn increases the demand for programs as scientists retain a greater capacity for innovation and generate more ideas to test. |
| 2. | Tools: Ginkgo’s scale provides a cost advantage in two primary ways. First, we reduce the amount of capital investment required by our customers—an early stage company building on our platform may never need to build a molecular biology lab. Second, our proprietary technologies and scale economics drive down the marginal cost of each experiment. Combined, this has the impact of transforming what is typically a large fixed cost investment for a cell programmer into a much lower variable cost. This is akin to an IT department not having to build and maintain a costly bank of servers and instead paying a marginal usage-based fee to their cloud computing vendor. Additionally, and perhaps even more impactful, our Codebase provides host cells, genetic parts and associated data for our customers that are unavailable elsewhere and which may reduce the total amount of work required. |
As the cost of compute declined exponentially in computer programming, the demand for compute increased exponentially as developers dreamed up more and more sophisticated applications. We expect the same to be true in cell programming: as our platform scales in capability and capacity, we hope that the range of applications accessible to cell programming will likewise expand in breadth and sophistication.
We believe that Ginkgo is changing the structure of the biotechnology industry. In much the same way that cloud computing centralized hosting services and ushered in a wave of SaaS software companies, Ginkgo is scaling the capabilities needed to program cells. By making these tools more accessible, we hope to usher in a wave of innovation in both “hardware” (life science tools) and “software” (cell programs).
At Ginkgo, we have always admired the symbiotic and regenerative nature of biology, which sits in stark contrast to the often extractive nature of existing technologies. We are often asked who we think the “winners” and “losers” in the industry will be as Ginkgo scales, as if it is a given that our growth must come at the expense of others in the ecosystem. We reject that notion. As our platform scales, we seek to drive benefits for all existing players in this ecosystem:
| • | Innovators—whether in academic labs, startups, or global conglomerates—benefit from faster and more successful R&D efforts |
| • | Scientists are freed to unleash their creativity (we understand the pain of spending years pipetting at the bench too!) |
| • | Life science tools and manufacturing companies benefit from having a clear technical roadmap and known demand to justify investments |
101
| • | Society benefits from responsible innovation, driving more sustainable, cost effective, and high-performance products |

Program Layer Ginkgo provides program management and technical execution to product companies across all industries Ginkgo is building the leading horizontal platform for cell programming across all industries platform layer Ginkgo is the interface between a series of complex technologies and a customer spec Technology Layer Ginkgo integrates standard hardware and wraps it in proprietary software and automation
Figure 6: Schematic of the synthetic biology industry structure. Ginkgo connects and integrates the hardware and tools provided in the technology layer, creating a platform that can be used by cell programming customers who are building products for end-market use.
Program Layer: Ginkgo enables and accelerates product companies, which historically have had to vertically integrate
Ginkgo is not a product company; we are an enabling platform for product companies in a range of end markets. We do not seek to “pick winners” and focus instead on building our platform rather than investing in product-specific risk. Platforms require scale and a relentless focus on innovation while taking a product to market requires many specialized functions that vary depending on the product:
| • | A novel food ingredient requires food scientists to test and enhance taste and functionality |
102
| • | A therapeutic requires clinicians to conduct animal and human studies to test safety and efficacy |
| • | A novel material requires materials scientists to evaluate elasticity, durability, conductivity, or other required features |
| • | An agricultural product requires field trials |
Once the product is developed, major investments are also needed to manufacture, distribute, and market the product. These are the jobs of our customers, the product companies.
Historically, product companies have had to invest in their own R&D capabilities, building their own labs and hiring their own scientists. This investment is inefficient due to lack of scale and drains resources away from application testing and product development. Ginkgo’s platform is not application-specific. The same engineering tools can be used for programs in completely different application areas: cells all run on the same genetic code. As product companies develop their products on Ginkgo’s platform, they gain efficiencies and increase their probability of success. New companies that build on our platform never need to make the fixed capital investments to start a lab from scratch; they are able to leapfrog and compete effectively against established companies.
Technology Layer: Ginkgo collaborates with life science tools companies to drive technology advancements
Because we’re constantly thinking about how to enable the next several years of exponential scaling of our platform, we have good insights into future bottlenecks and welcome the opportunity to collaborate to build technologies that will break through those barriers. We are the largest customer for many of our strategic suppliers and, as such, play an important role in advancing new technologies. As a result, we are often able to secure preferred access, often including custom development and leading economic terms, to next-generation technologies and pass those benefits along to customers.
We expect to continue to invest in and support the development of emerging technologies in this space. In certain areas where Ginkgo has unique needs, we may acquire technologies directly, as we did with Gen9, Inc.’s DNA assembly platform, which was particularly valuable for more complex DNA synthesis needs. In many other areas, we will support new and existing technology companies by placing anchor orders and partnering to develop technology roadmaps that break new ground.
By acting as a horizontal platform, Ginkgo can focus on what we do best (cell programming), our customers can focus on what they do best (bringing products to market in their industry), and our suppliers can focus on what they do best (building great hardware and tools). Biology did not evolve by industry and so cell programming is able to benefit from the scale and efficiency of a horizontal platform. Vertical integration is no longer required, allowing each layer of the ecosystem to flourish as we collectively enable more rapid growth across the industry.
Ginkgo serves diverse customers across a variety of end-markets. Some of these customers may have in-house biological R&D teams and others may have never thought biotechnology applied to their business. In either case, they come to us with a challenge—whether it is supply chain volatility, a race to develop an innovative new product, or an existential threat facing an industry on the wrong side of history—and we partner to enable a biological solution. We begin our relationship by working collaboratively to design the set of specifications for the end product(s) our customer desires. Our cell programmers then take that set of specifications and design an engineering plan to create a cell program that meets or exceeds that set of specifications. When we finish, our customers receive the final engineered organism (which either produces or is their product of interest) and a full “tech transfer” package for manufacturing and downstream processing (which they can implement themselves or pass to a contract manufacturer with our support). Our customers then take these organisms and/or purified products through the final stages of product development (e.g., formulations, clinical trials, field trials, etc.).
103
Our commercial team is organized to both establish new relationships with potential customers (traditional business development) as well as maintain and expand relationships with our existing customers (which we call “alliance management”).
Our business development team has both expertise in relevant industries (Consumer & Technology, Industrial & Environment, Agriculture, Food & Nutrition, Pharma & Biotech and Government & Defense) as well as expertise in our Foundry capabilities and synthetic biology. With this background we are able to identify industry or consumer challenges where biology can serve as a solution. Our categories of customers, independent of industry, include potential customers who have R&D teams with some synthetic biology capabilities where choosing Ginkgo can bring automation, scale and codebase beyond their own; potential customers who are considering but have not yet built lab-scale capabilities where a partnership with Ginkgo allows them to spend their capital on commercialization efforts; and potential customers who are not yet working in synthetic biology whose industries or products stand to be disrupted by biological solutions. Our business development team, with support from our Codebase and Foundry team members, crafts solutions for each of these types of customers through a strategic discussion of customer needs and fit with Ginkgo capabilities.
To grow existing customers, our alliance management team, through close collaboration on our existing programs, seeks technical and business opportunities for our customers that serve as the basis for consideration of future programs. As our programs demonstrate technical success, our existing customers often bring their next strategic R&D needs to our attention.
Over 75 major programs across diverse industries have run on our platform
While most biotechnology companies focus on building products within a fairly narrow scope, Ginkgo has uniquely pursued a partnered strategy across all end-markets. This was not easy. For many years, our platform was less efficient than the status quo of an expert scientist working by-hand at a lab bench. In the early days, the only end markets willing to take a chance on our platform were those without in-house biotechnology capabilities. But as Ginkgo’s platform improved over time and with scale, we were able to win contracts in increasingly sophisticated end markets with more in-house biotechnology expertise. Today, our platform is diversified across all major end-markets with marquee customers and a range of focus areas within each.
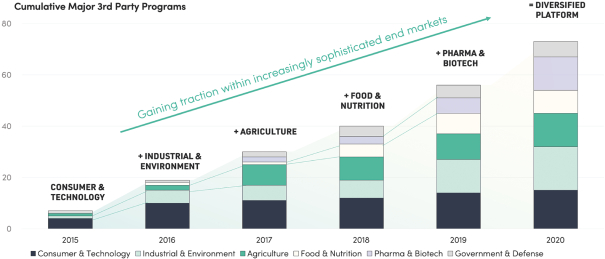
Cumulative Major 3rd Party Programs Gaining traction within increasingly sophisticated end markets Consumer & Technology +Industrial & Environment + Agriculture +Food & Nutrition +Pharma & Biotech Diversified platform 2015 2016 2017 2018 2019 2020 Government & Defense
104
Figure 7: Cumulative “major” programs run by third-party customers on Ginkgo’s platform (excluding proof of concept projects and other exploratory work). Today, Ginkgo has a diverse set of programs across all major end-markets.
Our customers include large multinational organizations with multibillion dollar R&D budgets as well as startups who are depending on us for essentially all of their bioengineering needs. While these customers and their focus areas may look very different, they are all important and valuable to Ginkgo. All of these programs leverage a common infrastructure, and as we demonstrate the value of this platform, we have the ability to grow significantly with our customers.
Ability to grow with our customers and increasingly complement existing R&D budgets
Ginkgo has grown substantially through inside sales with our existing customers. Some of our customers, such as Motif FoodWorks, never needed to build in-house cell engineering capabilities and so as they grow and expand their product pipeline, their demand for our platform should increase and they will benefit from our improving scale efficiencies over time as well. The relative value of our platform compared to the next best option (building a lab, bioengineering team, and intellectual property from scratch) is immense, which yields extremely high retention rates for customers in this category.
Other customers may already have in-house cell programming capabilities. As Ginkgo demonstrates the value-add of our platform by successfully delivering on programs, we have the opportunity to grow our collaborations with them, complementing their core R&D capabilities. We don’t view this as a “replacement” of customer scientists with Ginkgo’s platform. Rather, we hope to expand our customers’ capacity and need for innovation—giving them more “shots on goal” and enabling them to invest more heavily in R&D as the ROI of each dollar spent increases.
We have demonstrated this with several customers. With one customer, an initial proof of concept program has turned into a broader strategic relationship with over nine programs today. With another, we launched a relationship with two programs, quickly expanding it to five by the end of the following year. The growth we have seen with our oldest customers means we continue to have significant customer concentration as it takes time for new customers to ramp up their use of Ginkgo’s platform. During 2020, two of our customers each contributed greater than 10% of revenue and collectively they accounted for 39% of total revenue. We believe customer concentration will decline over time even as we expect to continue to grow our relationships with existing large customers. However, our ability to grow with our customers requires us to maintain satisfied customers, and program or other operational setbacks could impede our ability to meet customer expectations and grow our business.
Powerful proof points across categories
Our platform has now been validated by sophisticated customers across a range of industries. As we launch programs in new areas, those provide a toehold for future sales in that space. As an example, our pro bono project for Moderna, Inc. at the start of the COVID-19 pandemic to enhance production of a key raw ingredient through process engineering provided a proof point and initiated us into this emerging segment, leading to a commercial relationship with another nucleic acid vaccine company, as well as a program to produce a key processing enzyme for mRNA vaccines.
It is still incredibly challenging to break into new industries and our ability to expand into new sectors may be harder than we expect. However, our recent progress in therapeutics has been a significant milestone given that we are ultimately competing against very strong in-house capabilities. We believe that as more proof points emerge across industries, the barriers to adoption will diminish.
While many of the programs we run on the platform are kept highly confidential, below we share some examples of the diverse set of programs running on our platform.
105
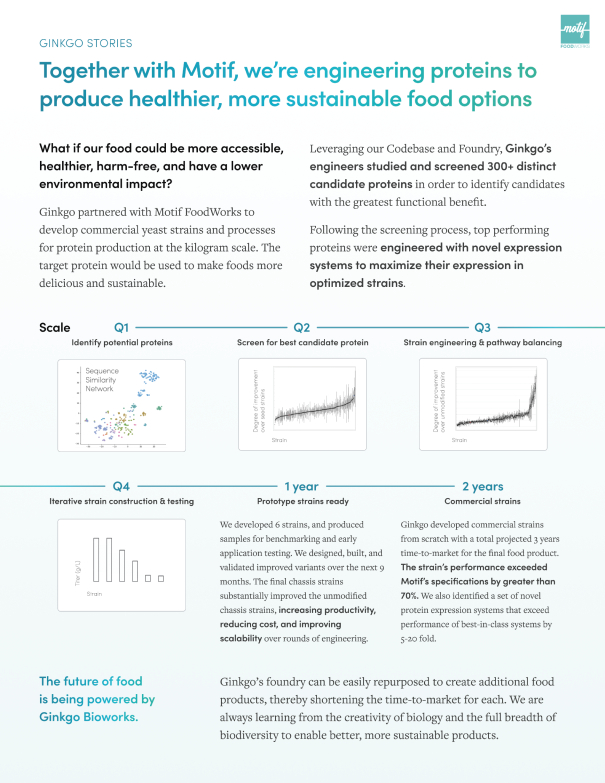
Ginkgo stories together with Motif, were engineering proteins to produce healthier, more sustainable food options what if our food could be more accessible, healthier, harm-free, and have a lower environmental impact? Ginkgo partnered with Motif Foodworks to develop commercial yeast strains and processes for protein production at the kilogram scale. The target protein would be used to make foods more delicious and sustainable. Leveraging our Codebase and Foundry, Ginkgos engineers studied and screened 300+ distinct candidate proteins in order to identify candidates with the greatest functional benefit. Following the screening process, top performing proteins were engineered with novel expression systems to maximize their expression in optimized strains. Scale Q1 Q2 Q3 Identify potential proteins Screen for best candidate protein Strain engineering & pathway balancing Q4 1 year 2 years Iterative strain con
106

GINKGO STORIES Together with Roche, we’re developing novel medicines to combat antibiotic-resistant bacteria What if synthetic biology paired with a genome mining platform could unlock the next-generation of therapeutics? Antibiotic resistance is a growing worldwide problem, endangering thousands of people across the globe and threatening modern medicine. Annually, over 700,000 people die from antibiotic resistant infections. The United Nations projects that this figure could reach 10 million by 20501. Through our collaboration with Roche, a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives, Ginkgo is focused on discovering new classes of antibacterials. Our method is to mine bacterial genomes for novel pathways, and then engineer these pathways to produce molecules that we can test for antibacterial activity. Our partnership with Roche aims to bring the most successful of these molecules to the clinic. Why partner with Ginkgo? The integration of Warp Drive Bio’s genome mining platform with Ginkgo’s state of the art sequencing capabilities, extensive biological codebase, bioinformatics and machine learning tools for gene discovery and strain engineering expertise brings forth
107

108
Ginkgo’s platform combines a strong technical foundation with an ecosystem of supporting resources to maximize our partners’ odds of technical and commercial success. In the nucleus of our platform are our Foundry and Codebase, which our scientists leverage to complete customer programs. The Foundry is, in its simplest form, a very large, highly efficient biology lab, enabled by over a decade of investment in proprietary workflows, custom software, robotic automation, and data science and analytics. It is paired with our Codebase, a collection of biological “parts” and database of biological data, which helps our scientists program cells. But great technology alone is not enough and we are building a community and ecosystem around our technical platform that provides our partners with end-to-end support.
Our Foundry brings a scale economic to cell programming
Cell programming projects involve a conceptually similar engineering cycle regardless of the specific product or market. Based on customer specifications, Ginkgo’s program team develops designs of proteins, pathways and gene networks (see Figure 3) that might meet the specification, leveraging public and proprietary biological knowledge bases (see “—Our Codebase—organizing the world’s biological code”). Those conceptual designs are developed using computer-aided design tools until the exact DNA sequences for those designs have been determined. Those DNA sequences are then “printed,” assembled and inserted into a cell to execute the new DNA code. These prototype cells are then studied and the output or performance of each is measured and compared to the customer’s desired specification. Learnings using data analytics and data science tools inform a new round of prototypes and this cycle is repeated until either the specification has been met or the customer decides to end the program.
The likelihood of technical success increases with each iterative engineering cycle and with the number of prototypes that are explored per cycle. However, with traditional tools for genetic engineering, each of these cycles can be slow, expensive and error prone. Many projects across the industry run out of budget or time. Conventional R&D teams often look to stay within budget by running rapid engineering cycles using largely manual tools and small numbers of prototypes per cycle. However, the inability to broadly explore the potential design space (there are more possible sequences of a 200 amino acid protein encoded in 600 DNA letters than there are stars in the observable universe) and the reliance on manual tools is a difficult handicap to overcome. Since people can only work so hard and since engineering cycles can’t be shortened beyond the duration of the physical steps, this approach has limited potential to improve in the future.
At Ginkgo, we invest in improving the tools and technology for programming cells in order to maximize program success within the constraints of customer timelines and budgets. We do so by scaling the number of prototypes that can be evaluated in each engineering cycle in an effort to reduce the number of cycles required to meet the customer’s specification and ultimately shorten project timelines. A typical screen for one enzyme step in a program might evaluate 1,000 to 2,000 variants to optimize function, of which the top 10 to 100 might be short-listed for further study. A relatively basic program might have 3 to 5 enzymes working in concert, and so in the process of optimizing the entire pathway, thousands or tens of thousands of enzymes and pathway combinations might be designed, built, and tested in the Foundry. The methods we use to increase scale also tend to reduce the average cost per prototype, which means that more prototypes can be evaluated for a given program budget.
Because diverse cell programs share similarities in process and code, many programs can be run simultaneously in a carefully designed centralized facility. This facility, where we use our investments in advanced cell programming technologies to manage diverse programs, is what we call our Foundry.
We make it possible to centralize many cell programming projects in our Foundry by deconstructing programs into a set of common steps and then standardizing those steps. For each step, we have built a specialized functional team that performs that step for all programs. Those teams define a set of standardized services that can be used in concert to execute an end-to-end cell programming process. Each team has access to scientific, software, and robotic engineering resources to replace manual ad hoc operations with standardized, automated,
109
and optimized services. In addition to enabling scale, this approach ensures standard operating procedures, know-how, and human skill become encoded in software that can be more effectively debugged, monitored, controlled, and optimized.
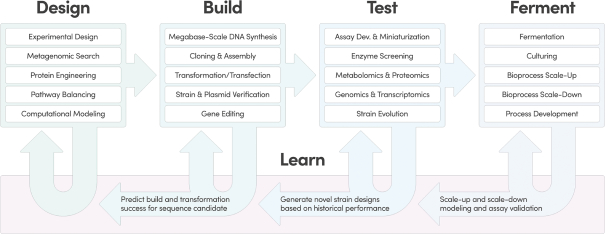
performance Scale-up and Scale-Down modeling and assay validation
Figure 8: A non-exhaustive summary of the functions performed throughout the lifecycle of a program in the Foundry. At each stage, learnings are generated, driving improved design cycles and functional optimizations.
While the engineering strategies described above have historically been relatively uncommon in the life sciences, they are obviously not our invention. Rather, we are inspired by the lessons from other engineering disciplines and seek to apply those to biology. Automotive manufacturing, semiconductor fabrication, and data centers, among many others, demonstrate how automation, data, economies of scale, and continuous improvement can produce compounding gains in scale, costs, and quality. Critically, routine performance of these strategies across dozens of projects gives us the data and experience needed to drive continuous improvement.
As described above, a key strategy in our Foundry is to increase the scale of our operations so that we can run more programs and more prototypes in parallel (i.e., large batch sizes). This approach benefits from operational efficiencies and economies of scale across many dimensions:
| • | Fixed Cost Amortization: Our Foundry is an inherently physical facility and as we scale and improve utilization, we are able to amortize this fixed cost across more work. |
| • | Continuous Learning and Improvement: The cumulative amount of work done as we scale leads to a better understanding about how to program cells. Much of this is then encoded in our Codebase, described below. |
| • | Purchasing Economies: By partnering with Ginkgo, our technology partners and suppliers can generate more value from a single account than they could from multiple smaller accounts, and that extra value is shared with Ginkgo. |
| • | Technology Specialization: Certain technologies that we leverage in the Foundry (such as acoustic liquid handling, automated bioreactors, and advanced mass spectrometry systems) are not easily leveraged or practical for smaller organizations. But for an engineering organization of our size, those investments can drive material improvements in cost efficiency. |
These efficiencies and economies of scale can be observed empirically from a relationship we refer to as “Knight’s Law,” named after Tom Knight, one of our co-founders, and loosely inspired by Moore’s Law for semiconductors. As shown below, we have seen an exponential increase in the output of the Foundry over time alongside an exponential decline in the average cost per unit of output. While this trend was interrupted by
110
temporary lab shutdowns during the COVID-19 pandemic and reduced capacity due to social distancing, we are continuing to drive our business internally towards achieving these metrics. We continue to build our internal metrics around Knight’s Law and believe we can continue to drive this kind of capacity growth in the foreseeable future, though it is dependent on the development of new technologies, which inherently carries risk, and, like Moore’s Law, we will likely hit a limit over time. This feature compares to a conventional facility, where scaling is driven predominantly by the addition of employees, an exponential increase in work would be infeasible and the cost per unit of work would decline little, if at all.
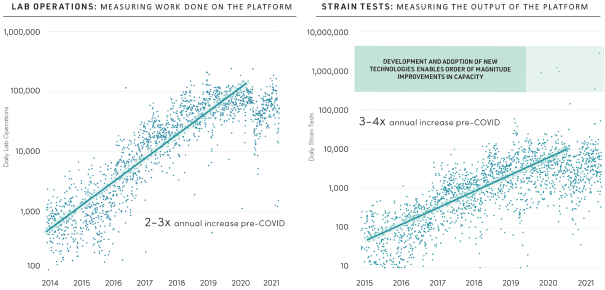
LAB Operations: measuring work done on the platform 1,000,000 100,000 10,000 1,000 100 2014 2015 2016 2017 2018 2019 2020 2021 Doly lab Operation 2-3x annual increase pre-COVID STRAIN TESTS: MEASURING THE OUTPUT OF THE PLATFORM DEVELOPMENT AND ADOPTION OF NEW TECHNOLOGIES ENABLES ORDER OF MAGNITUDE IMPROVEMENTS IN CAPACITY 10,000,000 1,000,000 100,000 10,000 1,000 100 10 2015 2016 2017 2018 2019 2020 2021 Doly Strain Tests 3-x annual increase pre-COVID
Figure 9: The output of the platform increased by over 3X per year for 5 years, and while we expect that kind of scaling to continue, there is no guarantee that we will be able to do so.
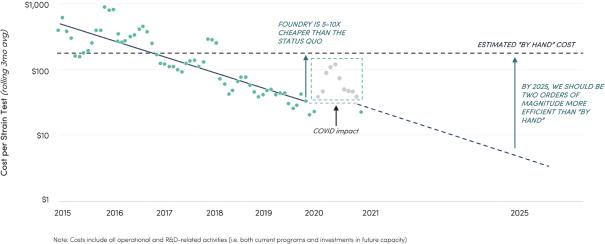
$10,000 $1,000 $100 $10 $1 2015 2016 2017 2018 2019 2020 2021 2025 Cost per Strain Test (rolling 3mo avg) FOUNDRY IS 5-10X CHEAPER THAN THE STATUS QUO ESTIMATED BY HAND COST BY 2025, WE SHOULD BE TWO ORDERS OF MAGNITUDE MORE EFFICIENT THAN BY HAND COVID impact Note; Cov1include all operational and R6D-related activities (i.e both current programs and investments in future capacity)
111
Figure 10: As the output of the platform has increased, our total R&D / operational costs per unit of output has decreased by approximately 50% per year.
We are frequently asked, and spend much time thinking about, whether it will be possible for compounding gains in output and productivity to continue for many years in the future. It is important to note that given significantly advanced tools, most steps in cell programming could be miniaturized to a point where single molecules of DNA and single cells are being manipulated and monitored. At that ultimate degree of miniaturization, the costs and timelines of cell programming could be reduced orders of magnitude from where they are today. Newly available microfluidic technologies, such as those developed by our partner, Berkeley Lights, Inc., point to the reality of this future of cell programming at the single-cell level. Additionally, because many of the enabling tools of cell programming are biological in nature (e.g., polymerases and CRISPR), we are able to point the platform at itself, developing new biological tools to reduce the number of steps or the complexity of a certain operation. For example, we could develop better gene editing enzymes or novel ways to screen cells in a multiplexed format using biological sensors. It is easy to theorize about these types of developments, however they are hard to execute, we will undoubtedly run into roadblocks along the way and we will have to invest significantly in developing new technologies in order to enable the types of improvements we seek to achieve.
Recent advances in machine learning, molecular simulation, and other computational techniques also hold great promise to improve our ability to program cells. We believe our Foundry is well-positioned to build the kind of large, well-structured datasets that such computational approaches need to succeed. In time, we believe computational approaches will reduce the need for certain kinds of experiments (for example, we already use machine learning to make protein and enzyme design projects more efficient). If computational approaches can replace certain sets of experiments, we expect to use the recovered Foundry capacity to work on ever more complex cell programming challenges. The reality is that the cells that we program today accomplish relatively simple functions, such as: “produce as much of molecule X as possible.” Programming cells for complex functions, such as live-cell therapeutics, responsive building materials, multicellular organisms, etc., will require sophisticated sub-systems for environmental sensing, intracellular information processing and feedback, and a multidimensional program that responds to such environmental stimuli. Only when we can deliver such sophisticated programmed cells, will we have truly unlocked the potential of biology and we see the Foundry as being an integral part of the platform for doing so.
Our Codebase—organizing the world’s biological code
Codebase is a familiar term to software developers but is a new concept in biology. Modern software firms develop their own (typically proprietary) codebase of source code and code libraries that can be leveraged by their software developers to more easily create new applications than they could starting from scratch. Additionally, vast repositories of debugged code are shared publicly so that programmers across application areas can leverage prior art in order to innovate faster. This allows software developers to focus their time and effort on developing new features rather than recreating existing logic. Ginkgo’s Codebase is our attempt to characterize functional biological code (reusable genetic parts and strains) that can similarly be repurposed in new cell programs. In addition to the raw performance data we generate through our Foundry experiments (more than 10 million strain tests run to date), we have also incorporated many public databases for genetic sequences and have a proprietary data set of over 440 million additional sequences that we leverage in our designs.
Engineering biology is complex—one of the reasons that Foundry scale is important is that it remains highly difficult to predict the performance of a biological “part” in a given context from a DNA sequence alone. The genomics revolution has outpaced biologists’ ability to test the functionality of each DNA sequence as it was discovered, particularly because most of the community is still performing biological experiments by hand without the benefit of automation. Each program performed at Ginkgo involves testing thousands or millions of DNA sequences; with a small fraction of those ending up in our final engineered cells. For that reason, high-performance biological sequences—the handful of designs from thousands of candidate designs that meet our
112
performance goals for an experiment—are hard-won assets and form a key component of Ginkgo’s Codebase. Not to be discounted, the “losing” designs are still valuable, helping inform more effective campaigns in the future that avoid known failure modes.
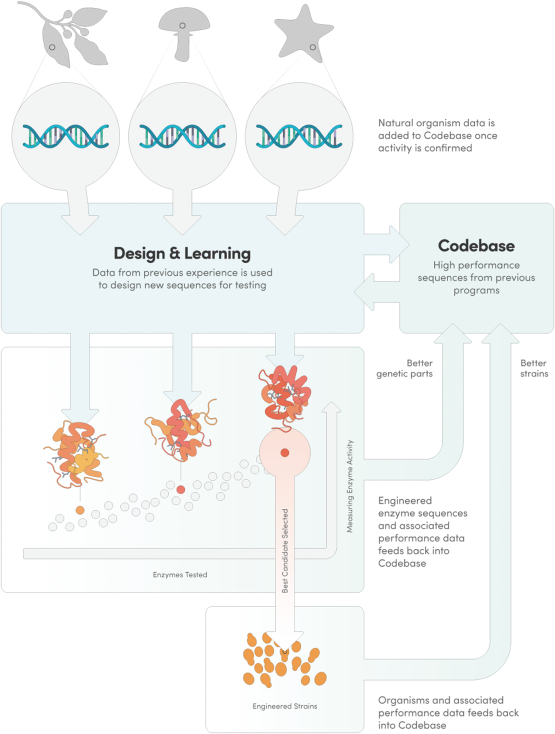
Natural organism data is added to Codebase once activity is confirmed Design & learning Data from previous experience is used to design new sequences for testing Codebase High performance sequences from previous programs Better genetic Parts Better strains Enzymes Tested Best Candidate Selected Measuring Enzyme Activity Engineered enzyme sequences and associated performance data feeds back into Codebase Engineered Strains Organisms and associated performance data feeds back into Codebase
113
Figure 11: Our Codebase incorporates both biological assets from nature as well as engineered assets and data from our Foundry experiments. Because the Foundry enables us to test many thousands of prototype enzymes, pathways, and strains in individual engineering cycles, we are able to quickly expand the range of characterized biological assets in our Codebase.
In some ways Codebase is a “parts catalog” that we can draw from when developing a new organism. As Ginkgo performs more projects, we contribute new parts to our Codebase that can be reused in new contexts. For example, we developed novel synthetic promoters (DNA sequences that can turn on the expression of a gene of interest) that allowed us to increase production of proteins in yeast. Initially, we tested thousands of designs to arrive at a select number of promoters with high performance. Now those high-performing promoters can be reused in any program that involves producing a protein in yeast; they are a modular piece of genetic code. Over the past 20 years, our team has supported efforts to build these kinds of parts libraries—the iGEM Parts Registry and AddGene are two notable examples of initiatives to make reusable parts available to researchers in the community. But despite these efforts, we continue to see intellectual property siloed within organizations across the biotechnology industry, leaving many without the additional intellectual property they need to develop their programs. Ginkgo’s Codebase allows our customers to draw from a broader set of biological assets than any single company would develop for a given application. The scale and diversity of our programs have allowed us to develop a large Codebase that grows with the addition of each new program and can be opened to the broad swath of partners and cell programmers using our platform.
Cell programmers must consider not only the genes in the programs that they design, but also the ways that they interact with the cell that “runs” the program. Therefore, Codebase is more than just the individual modular parts we use to design biological programs. The organisms that have been optimized to run the programs, whether because they have been engineered for robust growth or because they are particularly adept at producing certain classes of products, are known as “chassis” strains. These strains can be reused across multiple programs, significantly reducing the amount of work needed to optimize a program and engineer a commercially viable organism. The breadth of Ginkgo’s customer base allows us to use these chassis strains in many more contexts than traditional industrial biotech players.
For example:
| • | We have developed highly productive organisms for the production of food proteins (incorporating some of the synthetic promoters described above). These same chassis strains can be repurposed for the production of any protein or enzyme, spanning applications as diverse as enzymes for degrading pollutants to structural proteins for personal care products. |
| • | Our collaboration with Cronos Group Inc. involves the production of many different cannabinoids; these cannabinoids share common precursor molecules such that a single chassis strain can be modified to produce each product. |
| • | Ginkgo recently acquired the assets of Novogy, Inc., a company that had been focused on the engineering of oleaginous yeasts to produce fuels and lubricants. At Ginkgo, these assets can be applied not only to fuels and lubricants but also fine flavors and fragrances, food oils, and even materials. A consequence of evolution is that biochemistry has repurposed the same biochemical pathways many times over in different contexts, allowing chassis strains to be redeployed in many similarly diverse contexts at Ginkgo. |
114
Our Foundry and Codebase are inextricably linked. Our Foundry scale allows us to generate unparalleled Codebase assets. These Codebase assets help us improve our designs and provide reusable parts and chassis strains that improve the efficiency and probability of success of our cell programming efforts in the Foundry. As the capabilities of the platform improve, it drives further demand, which increases the rate of learning in our Codebase. The continuous learning and improvements inherent in this relationship is one of the key features of our platform.
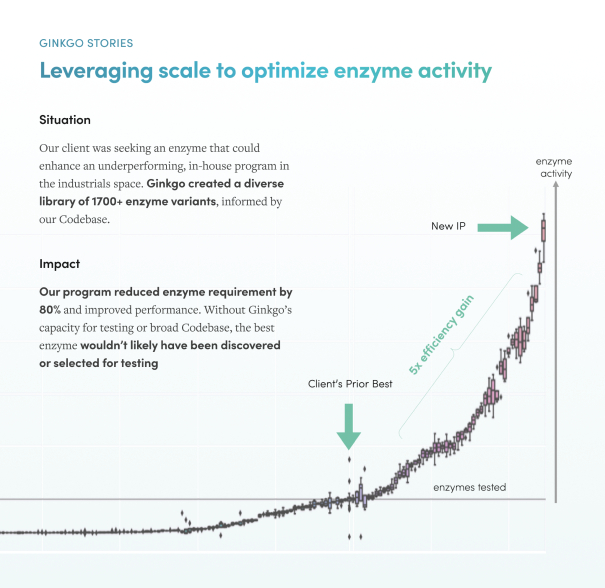
Ginkgo stories Leveraging scale to optimize enzyme activity situation Our Client was seeking an enzyme that could enhance an underperforming, in-house program in the industrials space. Ginkgo created a diverse library of 1700+ enzyme variants, informed by our Codebase. Impact Our program reduced enzyme requirement by 80% and improved performance. Without Ginkgos capacity for testing or broad Codebase, the best enzyme wouldnt likely have been discovered or selected for testing enzyme activity New IP 5x efficiency gain Clients Prior Best enzymes tested
115
An ecosystem to support cell programmers
Ginkgo has long recognized that it is critical to build a true ecosystem around our technical platform. We have been inspired by the leading horizontal platforms in information technology, such as Microsoft Windows and Amazon Web Services, which built real developer communities and provided a range of value-added services on top of their core technology. Like these pathbreakers, who set the stage for a generation of computer developers, we too are trying to ensure that the cell programmers who build applications on our platform have the tools they need to succeed beyond the lab.
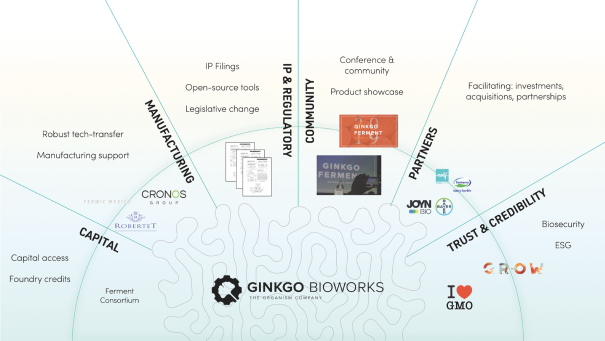
Capital capital access Foundry Credits Ferment Consortium Manufacturing Robust tech-transfer Manufacturing support IP & Regulatory IP Filings Open-Source tools Legislative change Community Conference & community Product showcase Partners Facilitating: Investments, acquisitions, partnerships Trust & credibility Biosecurity ESG
Access to capital
As in the early days of computer programming, it is still extremely expensive to program biology. For that reason, it can be easier for larger companies to make investments in innovation around this space. But Ginkgo’s platform gives small companies and innovators access to the same horsepower as larger players and obviates the need to invest in fixed laboratory assets, providing an even greater strategic benefit. To help address this discrepancy, Ginkgo has assisted in launching new companies (such as Motif FoodWorks, Inc. and Allonnia, LLC) by bringing together strategic and financial investors to secure funding for these early stage companies. Going forward, we intend to leverage our own balance sheet and to partner with investors, enabling companies at all stages to benefit from our platform. We believe that, as Ginkgo’s customers demonstrate increasing success, there will be an explosion of capital for cell programming applications and a recognition of Ginkgo’s platform as the industry standard backbone for these development efforts.
Manufacturing support
Our job is to ensure that our cell programs can be executed at scale and we support our customers to ensure successful commercial scale manufacturing. We have built relationships with a number of leading contract manufacturing organizations and have demonstrated that we can transfer our lab-developed protocols to commercial scale (e.g., 50,000+ L fermentation tanks) with predictable performance. We have an in-house
116
deployment team dedicated to supporting our customers’ scale-up and downstream processing needs. We have even helped certain customers, such as Cronos Group, Inc., acquire and build out their own in-house manufacturing capabilities and certain programs, such as our work with Moderna, Inc., focus on manufacturing process optimization.
Intellectual property protection and regulatory support
Ginkgo takes responsibility for the intellectual property generated through customer collaborations. Our scientific team works continuously with our intellectual property team to file patent applications and monitor for freedom to operate. We are also active in helping shape and influence the evolving regulatory landscape for biological engineering. While our customers are responsible for handling their own regulatory procedures on a product-by-product basis, our broader view and sphere of influence can help build understanding of and support for novel product classes.
Building a community of cell programmers
We launched Ferment, our annual conference, in 2018. The conference highlights developments and thought leadership in the field and brings together scientists, entrepreneurs, investors, and suppliers. Our conference in 2019 brought over 350 participants to our headquarters in Boston. Even prior to launching Ginkgo, our founders focused on building community within the emerging field of cell programming. Tom Knight, one of our founders, was among the professors who launched the International Genetically Engineered Machines (iGEM) Competition in 2004 which has now had over 50,000 students and instructors from over 50 countries go through the competition (including dozens of Ginkgo employees and all five founders!).
Facilitating partnerships within our community
Because Ginkgo serves both large market incumbents and smaller startups, our community also serves to facilitate introductions between innovators and those looking to invest in innovation. We believe that investors and large strategic companies have come to recognize Ginkgo’s platform as a key enabler of innovation and are keen to get to know the companies that are building with us. Those relationships can be the source of funding and go-to-market support for the earlier stage companies building on the platform, increasing the odds that they develop successful products.
We invest in building trust and credibility for the entire industry
The most powerful technologies require the most care. Biology is too powerful for us to not care about how our platform is used. We have and will continue to invest heavily to build and maintain trust in bioengineering as a technology platform across all layers of the industry. At the platform layer, we have focused on building robust biosecurity measures. At the application layer, we are proud to enable a diverse set of programs that drive towards environmental sustainability. We are committed to ESG (i.e., environmental, social, and corporate governance) practices and broad stakeholder engagement at a corporate level. We are also engaged in deep conversations around the implications and ethics of biotechnologies through many public forums, helping shape our platform to promote sustainability in our global community.
117

GINKGO STORIES Producing cultured ingredients at scale A leading company for production of natural ingredients was looking for cost-saving opportunities. Ginkgo identified a plan to fine-tune existing pathways in yeast to produce multiple flavors more efficiently, then optimized the conditions for scale-up. Ginkgos Foundry utilizes the ambr250 disposable reactor system to inform design of experiments and reduce operating costs via automation, allowing us to run hundreds of bioreactors simultaneously. Using the information generated to rationally improve the strain and fermentation process conditions, Ginkgo exceeded the clients desired product titer by 50%. Ginkgos system enables high throughput screening without comprising data quality The conditions optimized at a volume of 250 mL were effectively translated into pilot and commercial scale, and the cultured ingredients reached commercial production with our manufacturing partners.
118
The key input into our unit economics is a cell program. For each of these cell programs, we generate economic value in two primary ways. First, we charge usage-based fees for work done in the Foundry, similar to how one might pay a cloud computing platform based on the amount of compute required to run a SaaS application. Second, we share in the downstream value of the programs that are completed on our platform. This value share can be in the form of royalties on future sales (much like traditional biopharma models), equity, or even lump-sum commercial milestone payments. Because we typically incur no downstream costs (e.g., manufacturing or product development, which our customers manage), these value share payments flow through with approximately 100% contribution margin. This flexible business model allows for more predictable near-term revenue while not sacrificing our ability to create long-term value with asymmetric upside.
Illustrative Program Economics
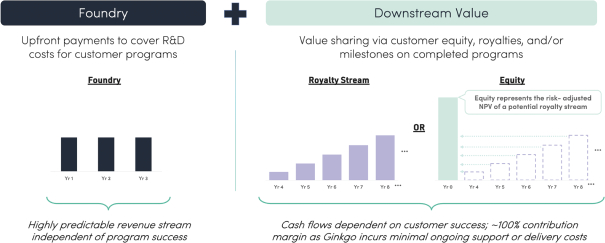
Foundry Upfront payments to cover R&D costs for customer programs Yr 1 Yr 2 Yr 3 Highly predictable revenue stream independent of program success Downstream Value Value sharing via customer equity, royalties, and/or milestones on completed programs Foundry Royalty Stream OR Equity Equity represents the risk-adjusted NPV of a potential royalty stream Yr 4 Yr 5 Yr 6 Yr 7 Yr 8 Yr 0 Yr 4 Yr 5 Yr 6 Yr 7 Yr 8 Cash flows dependent on customer success; ~100% contribution margin as Ginkgo incurs minimal ongoing support or delivery costs
Figure 12: Ginkgo generates economics from programs in two primary ways. First, customers pay upfront fees to cover initial R&D costs for a program. Second, Ginkgo shares in the downstream value (typically in the form of a royalty stream or equity share) generated by programs.
Foundry Revenue
The first stage of a cell program consists of R&D work being performed on Ginkgo’s platform, leveraging our Foundry and Codebase. R&D is inherently risky and our customers recognize that this is a cost they will incur regardless of success and whether they are working on the program in-house or with a partner. Ginkgo provides a much more efficient platform to conduct this R&D work, encouraging companies to build on or adopt our platform.
We estimate that the unit costs of our Foundry cell engineering services are several times less expensive on average than the status quo (a customer doing equivalent R&D in-house, by-hand) and we expect that cost advantage to grow over time. We typically earn revenue tied to the units of work that we perform on behalf of our customers’ programs. Initially, as we were building and validating the platform, these revenue covered less than 20% of the costs incurred to execute a program as the platform was less efficient than the status quo. As our platform has matured and efficiency improved, we have steadily increased the portion of program R&D costs that are covered upfront by customers and we now expect that new programs are structured to fully cover our direct costs, which will eventually enable us to earn a modest margin. Our Foundry revenue provides a strong foundation of predictable revenue that is independent of any commercialization efforts by our partners.
119
As we continue to scale the Foundry and build Codebase, we expect to drive further efficiencies and decrease our average unit costs. This presents us with a strategic choice going forward. We could retain these efficiencies and
increase our margins or we could pass these efficiencies on to our customers, increasing the number of shots on goal and, therefore, the likelihood of program success given a fixed budget. We believe the right choice for long-term value creation is to pass the savings to our customers, reducing the barriers to adoption and driving increased demand for our platform. Our Foundry revenues are thus impacted by a number of drivers:
| • | Number of active programs: We hope to dramatically increase the number of programs working on our platform over time, and if we are successful, we believe this will drive increasing Foundry revenue. |
| • | Units of work per program per year: If our Foundry becomes more efficient and we generate more scale, we expect to be able to do more work per program in a fixed period of time, improving chances of program success. |
| • | Average price per unit of work: If we bring on innovative technologies or step change improvements in existing Foundry services, we plan to pass capability and cost improvements on to our customers. If these new technologies or services are adopted across programs, we believe the average price per unit of work will continue to fall over time. |
| • | Number of years per program: If our platform improves, we expect program duration to decrease over time. Some programs may still be charting new territories and take several years, but programs that are able to leverage substantial pre-existing Codebase (e.g., our Nth program in bulk protein production) should have shorter duration and, in general, greater Foundry capabilities should shorten program durations. |
The expected impact of these drivers is represented below:
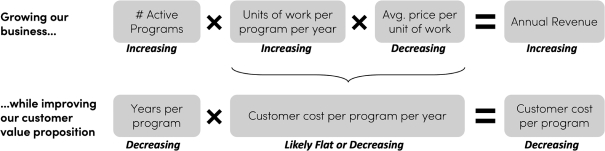
Growing our Business& # Active Programs Increasing Units of work per program per year increasing Avg. price per units of work Decreasing Annual Revenue Increasing &while improving our customer value proposition years per program Decreasing Customer cost per program per year Likely Flat or Decreasing Customer cost per program Decreasing
Figure 13: Illustrative drivers of Ginkgo’s long-term financial model and customer value proposition.
The multi-year nature of an average cell programming project means that our Foundry revenue are predictable and recurring in nature. Additionally, given the lead times inherent in developing technical plans as part of a sales process, we have good visibility into new Foundry revenue bookings. This provides a strong foundation for the business and allows us to be patient while we wait for downstream economics.
120
Downstream Value Share
As the key enabling technology for our customers’ products, we are able to earn a share of the value of the products that are created using our platform. We are quite flexible and have structured a variety of value sharing mechanisms, including royalties, equity, and lump-sum commercial milestone payments. Because the economics to us should be roughly equivalent, we are generally agnostic on which form of downstream value capture we receive and the decision is typically based on customer size and preference, with archetypes described below.

Because Ginkgo typically will have completed the program (and received associated Foundry revenue) prior to realizing downstream value, cash flows from the downstream value capture component generally fall straight to the bottom line as we incur minimal to no ongoing support or delivery costs once the strain is commercialized. This dynamic creates opportunities for outsized returns as our clients successfully commercialize products built on our platform. As we add more programs to the platform over time, we expect downstream value share to contribute income, and therefore we believe our overall margins and cash flow profile will grow significantly.
Biosecurity: A complement to our platform and emerging source of value
With a mission to make biology easier to engineer, we have always recognized the imperative to invest in biosecurity as a key component of our platform. We care how our platform is used and investments in biosecurity help us ensure that cell programming is conducted and deployed responsibly. The COVID-19 pandemic demonstrated the disruptive power of biology and has created a paradigm shift with respect to biosecurity in both public and private institutions that we believe will drive significant growth in demand for these capabilities. Our Biosecurity offering generated $17 million in revenue in 2020 and is expected to continue growing in the near-term, however, demand for COVID-19 testing remains uncertain for the second half of 2021. Our dedication to biosecurity is deeper than our emergency response to the current global pandemic. The rise of the internet and computing capabilities heralded a need for cybersecurity. Learning from this experience, and given the power of biology, we believe innovation in biosecurity must keep pace with innovations in bioengineering.
Consideration for biosecurity is ingrained into our platform tools. For example, we are members of the International Gene Synthesis Consortium (“IGSC”), which has developed harmonized protocols to screen synthetic DNA for concerning sequences. The IGSC protocols are typically used by DNA synthesis providers to help detect and prevent external customers from misusing DNA synthesis services. At Ginkgo, most of the DNA that we synthesize in-house is also designed and used in-house, not shipped to external customers. We still apply the IGSC screening protocols to these Ginkgo developed sequences as an additional biosecurity safeguard. We also have an extensive history working with the Department of Defense, the Defense Advanced Research Projects Agency (“DARPA”), and the Intelligence Advanced Research Projects Agency (“IARPA”) on programs
121
related to building a robust biosecurity infrastructure. Many of these programs, such as the IARPA FELIX program, aim to develop experimental and computational tools that detect or prevent misuse of bioengineering. During the COVID-19 pandemic, we built a robust “endpoint security network” of nationwide K-12 viral testing to help with school reopening plans—and we are now one of the largest providers of K-12 COVID-19 public health testing in the country. This work with educational institutions—organizations that represent the centers of our community—is a meaningful first step in building the pathogen monitoring capabilities critical to the prevention and mitigation of biological risks.
SARS-CoV-2 will not be the last pathogen we face with pandemic potential, but if we make the right investments, it may be the last that catches us unprepared. Industry sources estimate that at steady state, $20 to $40 billion should be spent on pandemic preparedness annually. The near-term growth of this sector is highly dependent on international government initiatives and investment and Ginkgo has been supporting and engaging with domestic and international organizations and governments to help shape the understanding of a robust biosecurity program. Given our experience to date, we believe there is a meaningful commercial opportunity in biosecurity that will persist beyond the current COVID-19 pandemic, driven by increased awareness of the need for prevention and response systems. We are well placed to take a leadership position as the biosecurity platform of choice, and we believe that our technology leadership requires that we play an important role in helping the world manage these challenges.
We have defined a unique business model over the past 12 years. The biotechnology industry has been product-centric for decades, with early horizontal platforms in life sciences frequently vertically integrating upon the development of the first successful product on their platform. As Ginkgo has embarked on this journey, we have studied and learned from innovators and established platform companies in other industries as we built our platform and business. We now benefit from significant historical investments, a virtuous cycle that grows with scale, and a strong business model that is aligned with our customers’ outcomes. These establish a strong sustainable advantage that we believe will help establish Ginkgo as a true industry standard.
Decade-plus head start in creating an industry standard platform
Hardware, software and biological tools need to be tightly integrated to replicate our platform. We have spent over 12 years building the software, automation and data science to best support a high throughput, generalized platform and expect to continue investing in this area. Our software, automation and data infrastructure cannot be easily replicated without bringing together a number of rare, specialized skillsets. In addition, without the scale and demand to stress test a high throughput platform, we expect any newly developed platform would be suboptimal. We estimate that it took us over eight years of investment and iteration to reach cost parity with “by hand” cell programming. We believe competitors will find it difficult to justify the investment in the software, automation and data science needed for high throughput operations before they acquire matching high demand.
Scale economics provide a structural cost advantage
As the only scaled horizontal platform in this space, we have the broadest number of programs that can be run on our platform, providing the highest potential for scale economics. Other companies choose to target specific markets and vertically integrate into products with high expected value. This has a tendency to overfit the capabilities of their R&D team to their targets. As discussed above, our continued scaling and investment in flexible tools that can apply to a broad range of end markets helps us drive efficiencies in the Foundry and Codebase across our diverse programs. Furthermore, as we scale, we are able to leverage advanced technologies that are only practical at scale and also may obtain preferred pricing with a number of suppliers. Competitors may be unable to source equivalent technology or negotiate similar pricing without first achieving scale, a feat that is difficult to do with a narrowly focused R&D platform.
122
Strong network and learning effects
In addition to a raw scale economic, we also accumulate knowledge and reusable Codebase from each program that runs on the platform. Every program benefits from the programs that came before and generates benefits for other current and future programs. These learnings and reusable assets are cumulative, extremely hard to replicate, and increasingly valuable to our customers. Because our learnings are generated by the work we execute in our Foundry, the unmatched scale of our Foundry also means we are learning at a faster rate than any up-and-coming competitor. Thus, there is a recursive element to our platform: as the platform gets better, it also improves faster—we are excited to make this advantage of our platform available to our ecosystem of cell programmers.
Ginkgo’s value creation is aligned closely with customer success
Our platform drives value for customers along two dimensions: reducing the cost of laboratory work via automation and increasing the probability of technical success due to cumulative data and learnings. Our financial model is aligned with those factors. As we gain efficiency, we drive further demand for cell programming, which drives our Foundry revenue up. As both demand and probability of success increase, our risk-adjusted value share also increases. Our model only requires we share in a small fraction of the downstream value created by our programs, providing our customers the opportunity to generate and retain significant value. Ultimately, this encourages broader adoption of our platform across industries.
Furthermore, we seek to maintain close relationships with our customers, supporting their work, and earning their loyalty and satisfaction. The breadth and highly integrated nature of our platform makes it inefficient for a customer to simultaneously work with Ginkgo and any theoretical competitor. As there is not yet a standard interface for cell programming, it requires an upfront investment to learn how to choose and design programs to make the best use of our platform. Thus, customer retention is high and there are substantial switching costs.
We are uniquely positioned to attract the top cell programmers
Just as the top software programmers want to work with the latest technologies, we believe the top cell programmers will be attracted to our industry leading platform and access to its unique capabilities. Our ability to hire and retain the best cell programmers as internal users and developers of our platform pushes us to continually improve and also builds a base of Ginkgo-trained experts. If these Ginkgo trained cell programmers move on to roles and opportunities in product-specific companies, we expect they will become ambassadors for the Ginkgo approach in their next role, expanding our reach into potential customers.
History of investing in credibility and trust
Let’s face it, GMOs have an image problem. This image problem has led to activities by the first generation of genetic engineering companies that backfired: lobbying against transparency in labeling laws, trying to “rebrand” GMOs with different terminology, and other efforts that have failed to build trust and engagement with stakeholders. We have taken a different approach. Rather than avoid the term, we’ve championed transparent labeling, sought to engage and build trust through open dialog, and enthusiastically embraced the potential for genetically modified organisms to do great things. We don’t seek to make GMOs acceptable through branding; we aim to make GMOs that people love.
123

I GMO
Doing so requires care and attention to both the technical and social aspects of our platform and its impacts. This means investing in biosecurity and, as noted above, embedding it into our platform and how we operate. This also means engaging with the social complexities of science and technology with a diverse group of people. We strive for a company culture built on a foundation of diversity, equity and inclusion (see also the sections titled “—The Impact of Cell Programming—ESG is in our DNA” and “—Our People & Culture”), and aim to engage different perspectives through our creative residency and through our magazine, Grow. Through both our internal and external efforts, we seek to engage with the realities of what has made genetic engineering an ESG risk historically, and work towards equitable and positive impact.
We are seeking to usher in a new paradigm for cell programming. It took us over eight years of basic research and investment in software, automation, data science and scale to reach parity with the status quo of individual scientists conducting experiments by hand at a lab bench. It took us several more years to demonstrate business model maturity: delivering a platform with enough value-add to customers that we could cover the cost of cell engineering R&D programs while building Codebase and sharing in the downstream value of our programs. We believe that we are now at an inflection point where we believe we have the opportunity to become the industry standard. We see several drivers of this evolution and growth.
Scale our platform and continue to drive efficiencies and improvements
As discussed above, our platform improves with scale and to date we have observed a positive feedback loop between our Foundry and Codebase. As we scale capacity and demand on the Foundry, we expect our average unit costs to fall, creating a better value proposition for our customers as their program budgets stretch further and drive more demand. Similarly, Foundry output also grows our Codebase, which supports better program execution, creating a better value proposition for our customers as well.
We occupy over 300,000 square feet and maintain state-of-the-art machinery and laboratory equipment. We have built more than 50 custom integrated work cells, consisting of robotic automation systems, mass spectrometry,
fermenters, sequencers, and more. We have the capabilities to engineer dozens of species of organisms from
124
bacteria to fungi to mammalian cells. We have worked on enabling products as varied as polymers, bacterial therapeutics, bulk protein production, novel antibiotics, fine chemicals, and more.
We have been able to work on a diversity of programs while consistently driving efficiencies in the Foundry with scale. We expect to accelerate growth in capacity by integrating new technologies across our existing footprint, building new Foundry space, and investing in software, automation and data to increase utilization.
Leveraging our proof points to grow within all industries
We have now established proof points of success in a diverse set of end markets, in several cases far exceeding our customers’ specifications. When engaging with existing customers or potential new customers in similar or adjacent industry verticals, we can point to these case studies of success to demonstrate the value of our platform. This reduces the barriers to adoption, helps us grow our customer base, and increases the number of new programs under contract. Importantly, the reusable Codebase we generate from these new programs enables us to stay ahead of vertically focused competitors.
Grow with existing customers
Once we establish a relationship with a customer, there is significant room to expand the scope of our program engagements. We are able to grow with our customers and/or expand into other existing pockets of R&D spending. We have seen customers expand from one early program to five or ten programs a few years later and each new logo we add has the potential to become a true platform partner.
When we work with companies from their inception (or at least from the inception of their biotech investments), we enable them to avoid significant fixed cost investments and benefit from our economies of scale. Our relationship with these customers is extremely strong as we are the core technology powering their R&D efforts. As a result, when these customers scale, their usage of our platform typically scales commensurately. For companies with existing, established biological capabilities, as we demonstrate the value of our flexible platform, we are able to grow our relationships to complement their core capabilities and increase the probability of success.
Reduce barriers to adoption by integrating with external R&D teams
It can be easy to fall into the trap of assuming that new disruptive technologies must subsume existing ways of working. When hosted servers and software-as-a-service started rising in prominence, corporate IT teams had to wrestle with changing integrations and demands. Some information technology departments were resistant to moving “off-prem” because they felt they were effectively outsourcing their jobs. In response, the leaders in this field, such as Dell, would sometimes hire their customers’ information technology departments and find them jobs within Dell simply to get past this internal resistance. The reality was that these technologies were ushering in a much more substantial era for information technology, which dramatically increased the demand for this type of talent. This centralization of the model (from every company having large information technology departments building customized code to a broader array of specialized software vendors) didn’t come at the expense of information technology and digital technologies, but enabled its flourishing across all industries. We see something similar happening in biotechnology today. Internal R&D teams are typically both very excited to learn about the power of our platform but are also understandably nervous about what “outsourcing” work to Ginkgo might mean for the future of their teams. We have the opportunity to help them see the benefit in a true partnership with Ginkgo.
The vast majority of programs being run on the platform today are being run and managed by Ginkgo program teams—in-house scientists and engineers who are managing the R&D project to meet a customer’s specifications. But we now have a couple early examples of certain customers, those with more in-house biotech expertise, interacting directly with our platform. Over time, we would like to build in enough standardized interfaces that a distributed network of scientists could access the platform directly through a well-defined
125
integration and self-service layer. This transition will allow our program teams to devote more of their efforts to developing Codebase assets, enabling more rapid scaling, and reducing the barriers to adoption by our customers. There are significant technical hurdles for us to overcome in developing this technology, but it is on our near-term roadmap and we are constantly thinking about how to “productize” individual workflows on the platform.
Build an ecosystem
As described above in “—Our Platform,” we believe we are building the industry standard developer platform for cell programming. In much the same way that early computing platforms and operating systems built real communities around their platforms in the 80s and 90s, we intend to build a community of developers building on the Ginkgo platform. As we invest to expand this ecosystem of services for cell programmers building on the Ginkgo platform, our value proposition to cell programmers increases and we become more ubiquitous.
A company is made of people. We have sought to bring together a diverse and multidisciplinary group of people who share in our mission to make biology easier to engineer. Today, our extensive cross-functional team is collaborating to build our ecosystem, from organism designers to automation engineers, software developers to people operations, business development to facilities management, finance to molecular biology.
A culture built on care
We’ve strived to grow a culture based on care. As engineers, it is easy to fall into the trap of thinking of ourselves simply as tool builders. Tools can be used in many different ways, both good and bad, and engineers often discuss their tools as value neutral. But tools reflect the social beliefs and biases of the people who make them: today this is becoming increasingly apparent, with more and more evidence of algorithmic bias being built into AI systems, facial recognition, and much more.
As designers of the largest horizontal platform for cell programming, we are keenly aware of the need to care about how our platform is used. More significant than the impacts we have seen from digital platforms on our social world, biology is our health, our bodies, our food, and our environment. As we build the tools for programming biology, we must also care how those tools are used, and ensure that the risks and benefits are transparently and equitably shared.
A diverse, world-class team
As of March 31, 2021 we had 495 full time employees. Building a horizontal platform for cell engineering requires collaboration between diverse skills and functions. It also requires deep technical expertise. Our employees are dedicated to the following functions:
| • | Platform functions including organism engineering, design, DNA synthesis and assembly, genome engineering, protein engineering and characterization, transformation and transfection, next generation sequencing, assay development, ultra high throughput screening, analytical chemistry, synthetic chemistry, directed evolution, and fermentation |
| • | Platform infrastructure functions including automation, software, development operations, product management, data engineering, data analysis, and data science |
| • | Deployment functions including upstream and downstream process engineering, project engineering; quality assurance and quality control |
| • | Commercial functions including marketing, business development, alliance management, and corporate development |
126
| • | Shared enabling functions including legal, people operations, finance, information technology, information security, facilities, environmental health and safety, procurement, shipping and receiving, inventory management, laboratory operations and media preparation |
In addition to our full-time employees, our success would not be possible without the collaboration and support of the broad network of partners, contractors, contingent workers and temporary staff who make up the Ginkgo team.
Technologies reflect the values of the people who build them. Diversity, equity, and inclusion are valuable and necessary in their own right, but we believe that it is essential to build a diverse team where people from different backgrounds are included and empowered to speak up and shape the growth of this technology. We are committed to growing a diverse team and continuing to empower an inclusive culture with strong employee ownership and engagement.
The full breadth of Ginkgo’s diversity and inclusion cannot be captured in demographic statistics, just as demographic categories cannot capture the full spectrum of diversity of human experience, however, we collect and report these numbers for transparency and as a lagging indicator of our efforts. As of March 31, 2021, 42% of our full time employees self-identify as an underrepresented gender (not cis male) and 12% self-identify as coming from an underrepresented racial or ethnic group in science and engineering (Blacks or African Americans, Hispanics or Latinos, American Indians or Alaska Natives, and Native Hawaiians and other Pacific Islanders). We are not yet satisfied with these numbers and all teams have objectives around increasing diversity and building a culture of inclusion to ensure that diverse perspectives thrive.
Laying the groundwork for strong employee engagement in the future
As a private, founder-led company, we have been able to infuse the organization with long-term strategic thinking. The long-term engagement and mentality of our employees can be seen in our turnover: voluntary attrition is well below the industry average. As we make the transition to a public company, we are trying to be thoughtful about how to maintain our culture and level of engagement.
The individuals who work at Ginkgo and build our platform care deeply about how that platform is used and the impact our company will have in the world. At the time of the closing of the transaction our team will own over 30% of Ginkgo shares outstanding and we hope to maintain the long-term mentality we have benefited from as a founder-led company even after Ginkgo becomes a public company. We believe a workforce with strong equity ownership will make the wise decisions needed to build long-term value for our company and build a company whose long-term impacts make them proud. That is why we have implemented a multi-class stock structure that permits all employees (current and future), not just founders, to hold high-vote (10 votes per share) common stock. We believe that our multi-class stock structure will help maintain this long-term mentality and encourage long-term equity ownership by our employees, thereby resulting in increasing employee ownership over time. For more information, see “Risk Factors—Risks Relating to our Organizational Structure and Governance—Only our employees and directors are entitled to hold shares of New Ginkgo Class B common stock (including shares of New Ginkgo Class B common stock granted or otherwise issued to our employees and directors in the future), which shares have ten votes per share. This limits or precludes other stockholders’ ability to influence the outcome of matters submitted to stockholders for approval, including the election of directors, the approval of certain employee compensation plans, the adoption of amendments to our organizational documents and the approval of any merger, consolidation, sale of all or substantially all of our assets, or other major corporate transaction requiring stockholder approval.”
To our knowledge, there are currently no other companies that serve all industries covered by our horizontal cell programming platform. The solutions and applications offered by potential competitors vary in size, breadth, and
127
scope, and given our broad set of application areas, we could face competition in many different forms. We also face competition from customers’ internal R&D departments and other research solution providers that largely conduct genetic engineering by-hand. We also compete against companies that seek to utilize synthetic biology technologies to develop specific products or target certain end markets. Additionally, competing platforms may emerge from various sources, including from joint ventures and partnerships between well-capitalized technology and life sciences companies. We identify the following three groups as our principal set of competitors:
The Status Quo: “on prem” cell programming efforts
The main source of competition we encounter is from potential customers choosing to build or maintain in-house cell engineering teams and capabilities. This status quo includes building out laboratory space and then hiring a team of highly trained scientists to conduct research “by-hand” with limited scale efficiencies. Some internal R&D operations maintain a full suite of capabilities and can design, build and test relatively complex pathways while others may have certain internal capabilities and need to outsource other elements to contract research organizations. We believe this is far less efficient for the customer and likely to yield worse outcomes as customers get fewer shots on goal for a given program budget.
That said, it can still be very difficult for companies to choose to trust Ginkgo with their R&D efforts versus building more traditional “on prem” labs. Smaller companies may feel like they’re “betting the farm” on Ginkgo while larger companies may be sensitive to displacing existing R&D teams. As such, a key focus area for us is reducing the barriers to adoption for the platform by de-risking the upfront investment for earlier-stage companies and by helping larger companies integrate their scientists closely into our workflows and empower their scientists to manage requests directly so we feel more like a resource and partner than a fully outsourced provider. Investing in these areas is a key focus area for us going forward.
Examples of traditional “synthetic biology” companies that have been vertically integrated from their founding with a focus on building products using synthetic biology include Amyris, Zymergen, Genomatica, Novozymes, DuPont, and DSM. Additionally, the vast majority of therapeutics companies that are leveraging genetic engineering have in-house capabilities, including Biogen, Novo Nordisk, Vertex, Regeneron, Bayer, and many others. These companies may be viewed as competitors to Ginkgo because they are creating products, using cell programming, that may compete with the products Ginkgo is enabling for our customers. However, as a horizontal platform, we view these companies not as competitors but as potential customers and focus not on “beating” them but rather on demonstrating our incremental value.
Verticalized cell engineering platforms
Within certain end markets, Ginkgo may compete against vertically-focused biotechnology companies providing cell engineering research and development capabilities to customers within a narrow set of end markets. While we believe the siloed nature of these companies limits their long-term potential, in the near-term, we may have a harder time penetrating those end markets given the incumbent vertical specialists in that space. The vast majority of these companies exist within therapeutic end markets given the history of cell engineering in that field. In theory, the expertise and learnings they develop from work in one field could be leveraged into neighboring end markets if these companies decided to adopt (and invest in) a more horizontal strategy. Examples of these vertically-focused platforms include AbCellera (antibody discovery), Codexis (enzymes), Senti Bio (cell therapy for oncology applications) and WuXi biologics (therapeutics).
Other possible entrants
We may also face competition from new entrants in the market, including well-capitalized technology companies with possible strategic interests in synthetic biology and its capabilities. Such companies may emerge as competitors given their access to capital, capacity to create multi-disciplinary teams across biology, chemistry, computer science and engineering, and flexibility to enter strategic ventures with life sciences companies.
128
Overview: Foundry and Codebase
As discussed above, Ginkgo’s two core platform assets include:
| • | Ginkgo’s Foundry, which enables high-throughput cell programming; and |
| • | Ginkgo’s Codebase, which includes reusable biological assets that can be used to accelerate cell programs. |
Ginkgo protects each of these core assets—the Foundry and the Codebase—through a combination of patents and trade secret protections.
Patents
As of July 15, 2021, we had approximately 58 patent “families,” including patents held by the Company as well as by its wholly owned subsidiary Gen9, Inc. Some of these are represented by a Patent Cooperation Treaty (PCT) application with related national applications, as well as 17 pending provisional applications. We have over 45 issued U.S. and over 160 issued foreign patents, which includes European nationalizations, and approximately 40 pending U.S. non-provisional and approximately 115 pending foreign patent applications, including patents and patent applications acquired from third parties.
In addition to our proprietary methods and technologies, we also non-exclusively in-license certain intellectual property assets from third parties.
We intend to pursue additional intellectual property protection to the extent that we believe that it would be beneficial and cost-effective. We cannot provide any assurance that any of our current or future patent applications will result in the issuance of patents. We also cannot assure the scope of any of our future issued patents or warrant that any of our patents will prevent others from commercializing infringing products or technology.
Our patent portfolio is detailed in the chart below:
| Patent Family |
Description |
Application/ |
Filing Date |
Issue Date/ |
Earliest Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| Methods and Devices for High Fidelity Polynucleotide Synthesis | Microfluidic devices and methods for assembling oligonucleotides by merging droplets containing oligonucleotide fragments with regions of complementarity | PCT/US2009/055267; WO/2010/025310 | 08/27/2009 | Nationalized in: US | 01/16/2030 | |||||
| Methods and Apparatuses for Chip-Based DNA Error Reduction | High-fidelity polynucleotide synthesis by generating complementary oligonucleotides to support bound single-stranded oligo (ss-oligo) in a microdroplet using enzymatic processes | PCT/US2010/057405; WO/2011/066186 | 11/19/2010 | Nationalized in: EP, FR, DE, LT, NL, ES, SE, CH, GB, LI, and US | 11/19/2030 | |||||
129
| Patent Family |
Description |
Application/ |
Filing Date |
Issue Date/ |
Earliest Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| Methods and Microfluidic Devices for the Manipulation of Droplets in High Fidelity Polynucleotide Assembly | Methods and devices utilizing droplet-based liquid manipulation on a substrate for assembling nucleic acids including steps of sequence error removal | PCT/US2010/055298; WO/2011/056872 | 11/03/2010 | Nationalized in: US | 11/03/2030 | |||||
| Assembly of High Fidelity Polynucleotides | Methods and apparatuses for preparing and/or assembling high fidelity nucleic acids on a solid support | PCT/US2011/020335; WO/2011/085075 | 01/06/2011 | Nationalized in: US | 01/06/2031 | |||||
| Methods and Devices for Oligonucleotide Synthesis | Devices and methods for the synthesis of polynucleotides and libraries of polynucleotides using manipulation of oligo-containing droplets on a support | US 8,716,467
US 9,388,407
US 9,938,553
US 2018/0195100 |
03/02/2011
03/31/2014
04/08/2016
02/28/2018 |
Issued 5/6/2014
Issued 7/12/2016
Issued 4/10/2018
Published |
05/12/2031
03/02/2031
03/13/2031 | |||||
| Methods for Nucleotide Sequencing and High Fidelity Polynucleotide Synthesis | Methods of obtaining sequence information of target polynucleotides by performing sequencing by ligation and sequencing by polymerase-based reactions | PCT/US2011/036433; WO/2011/143556 | 05/13/2011 | Nationalized in: US | 05/13/2031 | |||||
| Microarray Synthesis and Assembly of Gene-Length Polynucleotides | Processes for in vitro synthesis and on-device assembly of long, gene-length polynucleotides based upon assembly of multiple shorter oligos synthesized in situ on a microarray platform | US 7,563,600; 7,323,320; 8,058,004; 9,023,601; 9,051,666; 10,450,560; 10,640,764; 10,774,325 | 09/12/2002- 02/18/2020 |
Issued 07/21/2009 -09/15/2020 | 09/12/2022 | |||||
| US 2021/0062185 | 09/14/2020 | Published | ||||||||
| PCT/US2003/028946; WO/2004/024886 | 09/12/2003 | Nationalized in: AU, CA, CH, EP, FR, DE, DK, GB, JP, LI, NL | 09/12/2023 | |||||||
| Compositions, Methods, and Apparatus for Oligonucleotides Synthesis | Compositions and methods for high-fidelity polynucleotide assembly on solid support from oligos by adding variable length padding sequences to the ends of the oligos | PCT/US2014/025610; WO/2014/160004 | 03/13/2014 | Nationalized in: EP, US, DE, GB | 03/13/2034 | |||||
130
| Patent Family |
Description |
Application/ |
Filing Date |
Issue Date/ |
Earliest Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| Compositions and Methods for Multiplex Nucleic Acids Synthesis | Methods of producing target nucleic acid using pluralities of oligos with overhangs, in which overhangs of one plurality are designed to be complementary to overhangs of another plurality | PCT/US2014/026261; WO/2014/151696 | 03/13/2014 | Nationalized in: AU, CA, CN, EP, IL, US | 03/13/2034 | |||||
| Methods for the Production of Long Length Clonal Sequence Verified Nucleic Acid Constructs | Methods and compositions for the production and isolation of high fidelity nucleic acids using high throughput sequencing of fragmented oligos which are tagged with unique barcodes at the 5’ and/or 3’ ends | PCT/US2014/048867; WO/2015/017527 | 07/30/2014 | Nationalized in: EP, CH, DE, FR, GB, LI, NL | 07/30/2034 | |||||
| Protein Arrays and Methods of Making and Using the Same | Methods and devices for preparing a protein array to generate and express a plurality of proteins from a plurality of nucleic acids on an array | PCT/US2011/060217; WO/2012/064975 | 11/10/2011 | Nationalized in: EP, US | 11/10/2031 | |||||
| Libraries of Nucleic Acids and Methods for Making the Same (Nucleic Acid Library and its Manufacturing Method) | Methods for designing and producing non-random libraries of nucleic acids using multiplexed polynucleotide synthesis in which complementary overhangs attached to specific sequences are hybridized and ligated to each other | PCT/US2014/067444; WO/2015/081114 | 11/25/2014 | Nationalized in: AU, CA, CN, EP, IL, US | 11/25/2034 | |||||
| Iterative Nucleic Acid Assembly Using Activation of Vector-Encoded Traits | Nucleic acid configurations and cloning strategies for progressively assembling a long nucleic acid product using a plurality of assembly cycles that each include assembling a vector and two or more inserts containing one or more regulatory sequences that activate vector-encoded traits when assembled in a predetermined configuration | PCT/US2007/019209; WO/2008/027558 | 08/31/2007 | Nationalized in: US | 08/31/2027 | |||||
131
| Patent Family |
Description |
Application/ |
Filing Date |
Issue Date/ |
Earliest Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| Methods and Devices for Nucleic Acid Synthesis | Methods and apparatus for the synthesis of polynucleotides on a support using primer extension to generate overlapping construction oligonucleotides and assembly of the polynucleotides of interest by hybridizing construction oligos onto anchor support- bound oligonucleotides | PCT/US2011/060243; WO/2012/078312 | 11/10/2011 | Nationalized in: AU, CA, EP, BE, DE, GB, IE, LT, NL, CH, CN, DE, ES, FR, GB, IL, JP, LI, SE, US | 11/10/2031 | |||||
| Methods for Preparative in Vitro Cloning | Methods and devices for the isolation of nucleic acids from libraries by tagging a population of nucleic acids with unique oligonucleotide tags | US 9,752,176 | 06/15/2012 | Issued 09/05/2017 | 06/15/2032 | |||||
| US 2018/0023120 | 08/01/2017 | Published | ||||||||
| PCT/US2012/042597; WO/2012/174337 | 06/15/2012 | Nationalized in: AU, CA, CN, EP, IL, CH, DE, FR, GB, LI, LT, NL, US | 06/15/2032 | |||||||
| Compositions and Methods for High Fidelity Assembly of Nucleic Acids | Methods, compositions and algorithms for designing and producing a target nucleic acid from blunt-end double stranded nucleic acids generated by digesting the same to create cohesive-end fragments with unique cohesive ends that anneal and are ligated in a predetermined order | US 2013/0059296 | 08/23/2012 | Published | ||||||
| PCT/US2012/052036; WO/2013/032850 | 08/23/2012 | Nationalized in: AU, CA, CH, CN, DE, EP, LI, EP, FR, GB, IL, JP, LT, NL, SE, IE, BE, ES, HK, IS | 08/23/2032 | |||||||
| Device and Method for Nucleic Acid Manipulation | High precision, high selectivity nucleic acid singulation and assembly techniques using mechanical force generated piezoelectrically or acoustically to selectively expel or transfer one or more volumes of nucleic acids from a solid support | PCT/US2018/033823; WO/2018/217702 | 05/22/2018 | Nationalized in: AU, CA, CN, EP, IL, JP, US | 05/22/2038 | |||||
| Compositions and Methods for Site-Directed DNA Nicking and Cleaving | Compositions and methods for site-directed DNA nicking and/or cleaving, and use thereof in, for example, in polynucleotide assembly to create | PCT/US2015/039517; WO/2016/007604 | 07/08/2015 | Nationalized in: EP, DE, GB, US | 07/08/2035 | |||||
132
| Patent Family |
Description |
Application/ |
Filing Date |
Issue Date/ |
Earliest Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| sticky-end breaks in DNA so that the resulting fragments can be used for DNA assembly | ||||||||||
| Methods for Nucleic Acid Assembly and High Throughput Sequencing | Hierarchical assembly of target polybucleotides from construction oligonucleotides | PCT/US2013/047370; WO/2014/004393 | 06/24/2013 | Nationalized in: AU, CA, CN, EP, CH, DE, FR, GB, LT, NL, SE, IL, JP, US | 06/24/2033 | |||||
| Methods for Sorting Nucleic Acids and Preparative in Vitro Cloning | Compositions and methods for sorting and cloning of high fidelity nucleic acids by high throughput sequencing using unique barcode pairs (tag oligos) that may be sequenced to identify a nucleic acid of interest | US 10,081,807 | 04/24/2013 | Issued 09/25/2018 | 04/09/2035 | |||||
| US 10,927,369 | 07/18/2018 | Issued 02/23/2021 | 10/17/2033 | |||||||
| US 2021/0139888 | 01/19/2021 | Published | ||||||||
| PCT/US2013/037921; WO/2013/163263 | 04/24/2013 | Nationalized in: AU, CA, CN, EP, CH, DE, FR, GB, LI, LT, NL, SE, IL | 04/24/2033 | |||||||
| Methods for Screening Proteins Using DNA Encoded Chemical Libraries as Templates for Enzyme Catalysis | Methods, compositions and devices for screening a protein library for proteins having a desired activity | US 9,150,853 | 03/13/2013 | Issued 10/06/2015 | 03/13/2033 | |||||
| US 10,308,931 | 08/31/2015 | Issued 06/04/2019 | 07/27/2033 | |||||||
| US 2019/0249169 | 04/29/2019 | Published | ||||||||
| Owned by Ginkgo Bioworks Holdings, Inc. | ||||||||||
| Methods and Systems for Chemoautotrophic Production of Organic Compounds | Engineered chemoautotrophs (and methods for engineering such chemoautotrophs) including three metabolic modules: energy conversion pathways allowing use of energy from an inorganic energy source, carbon fixation | US 8,349,587 | 10/31/2011 | Issued 01/08/2013 | 10/31/2031 | |||||
133
| Patent Family |
Description |
Application/ |
Filing Date |
Issue Date/ |
Earliest Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| pathways, and product biosynthetic pathways to convert central metabolites into desired products, such as carbon-based products of interest | ||||||||||
| PCT/US2012/62540; WO/2013/066848 | 10/30/2012 | Nationalized in: US | 10/31/2031 | |||||||
| Methods and Systems for Methylotrophic Production of Organic Compounds | Engineered methylotrophs (and methods for selecting such cells) for efficiently converting C1 compounds into various carbon-based products of interest, including systems, mechanisms and methods to confer pathways for energy conversion, methylotrophy, or carbon fixation | PCT/US2013/073582; WO/2014/089436 | 12/06/2013 | Nationalized in: US | 12/06/2033 | |||||
| Methods and Genetic Systems for Cell Engineering | Engineered probiotics comprising a nuclease module designed to specifically target and degrade a nucleic acid, a synthetic mobile genetic element module capable of dispersing the system from one host cell to another, and an antibiotic-free maintenance module | PCT/US2015/022508; WO/2015/148680 | 03/25/15 | Nationalized in: AU, CA, EP, JP, US | 03/25/2035 | |||||
| Methods and Molecules for Yield Improvement Involving Metabolic Engineering | Methods and compositions relating to cells that have been engineered to reduce or eliminate proteins having enzymatic activity that interferes with the expression of a metabolic product | PCT/US2010/036902; WO/2010/141468 | 06/01/2010 | Nationalized in: US | 07/10/2030 | |||||
| Methods and Systems for Cell State Quantification* *(Co-Owned with R. Rettberg) |
Engineered cells, and methods for engineering such cells, for genomic, transcriptomic, or proteomic analysis, using multiple peptide tags | US 9,506,167 | 07/27/2012 | Issued 11/29/2016 | 01/07/2034 | |||||
| US 10,119,975 | 11/29/2016 | Issued 11/06/2018 | 07/27/2032 | |||||||
134
| Patent Family |
Description |
Application/ |
Filing Date |
Issue Date/ |
Earliest Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| Protective Enzymes | Enzymes for protecting polymers from damage caused by fatty acids from secreted biological fluids such as sebum or sweat | PCT/US2018/050718; WO2019/055541 | 09/12/2018 | Nationalized in CN, EP, HK, US | 09/12/2038 | |||||
| Chimeric Terpene Synthases | Cells, enzymes, and methods for production of terpenes (which can be used as fragrances, pheromones, or antimicrobials, among other things) that are partially derived from sequences reconstructed from rare or extinct plants | PCT/US2019/018122; WO2019/161141 | 02/14/2019 | Nationalized in: EP, HK, JP, KR, US | 02/14/2039 | |||||
| Biosynthesis of Mogrosides | Cells, enzymes, and methods for producing mogrosides (high-intensity natural sweeteners) by fermentation | PCT/US2019/060652; WO 2020/097588 |
11/09/2019 | Nationalized in: CA, CN, EP, JP, US | 11/09/2039 | |||||
| Biosynthesis of Mogrosides | Cells, enzymes, and methods for producing mogrosides (high-intensity natural sweeteners) by fermentation | PCT/US2020/057067; WO 2021/081327 |
10/23/2020 | Published | 10/23/2040 | |||||
| Biosynthesis of Mogrosides | Cells, enzymes, and methods for producing mogrosides (high-intensity natural sweeteners) by fermentation | PCT/US2021/032251 | 05/13/2021 | Pending | 05/13/2041 | |||||
| Biosynthesis of Cannabinoids and Cannabinoid Precursors | Cells, enzymes, and methods for producing cannabinoid compounds by fermentation | PCT/US2020/019760; WO2020/176547 | 02/25/2020 | Published | 02/25/2040 | |||||
| Biosynthesis of Cannabinoids and Cannabinoid Precursors | Cells, enzymes, and methods for producing cannabinoid compounds by fermentation | PCT/US2020/046838; WO2021/034848 | 08/18/2020 | Published | 08/18/2040 | |||||
| Biosynthesis of Cannabinoids and Cannabinoid Precursors | Cells, enzymes, and methods for producing cannabinoid compounds by fermentation | PCT/US2021/024398 | 03/26/2021 | Pending | 03/26/2041 | |||||
| Biosynthesis of Cannabinoids and Cannabinoid Precursors | Cells, enzymes, and methods for producing cannabinoid compounds by fermentation | PCT/US2021/37954 | 06/17/2021 | Pending | 06/17/2041 | |||||
135
| Patent Family |
Description |
Application/ |
Filing Date |
Issue Date/ |
Earliest Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| PCT/US2021/37944 | 06/17/2021 | Pending | 06/17/2041 | |||||||
| Biosynthesis of Cannabinoids and Cannabinoid Precursors | Cells, enzymes, and methods for producing cannabinoid compounds by fermentation | PCT/US2021/040941 | 07/08/2021 | Pending | 07/08/2041 | |||||
| Rare Earth Element (REE)-Binding Proteins | Cells, binding proteins, and methods for recovering rare earth elements, including lanthanides | PCT/US2020/038808; WO2020/257702 | 06/19/2020 | Published | 06/19/2040 | |||||
| Biosynthesis of Enzymes for use in Treatment of Maple Syrup Urine Disease (MSUD)* *(Co-Owned with Synlogic, Inc.) |
Methods, enzymes, cells, and compositions for treating maple syrup urine disease (MSUD) and other conditions characterized by excessive branched-chain amino acids | PCT/US2020/038813; WO2020/257707 | 06/19/2020 | Published | 06/19/2040 | |||||
| Optimized Bacteria Engineered to Treat Disorders Involving the Catabolism of Leucine, Isoleucine, and/or Valine* *(Co-Owned with Synlogic, Inc.) |
Methods, enzymes, cells, and compositions engineered to improve leucine catabolism and treat disorders involving the catabolism of leucine, isoleucine, or valine | PCT/US2020/038675; WO 2020/257610 |
06/19/2020 | Published | 06/19/2040 | |||||
| Production of Oligosaccharides | Compositions and methods for producing fructans using sucrose:sucrose 1-fructosyltransferase (1-SST),
fructan:fructan 1-fructosyltransferase (1-FFT), and/or sucrose:fructan-6 -fructosyltransferase (6-SFT) enzymes |
PCT/US2020/052390; WO 2021/061910 |
09/24/2020 | Published | 09/24/2040 | |||||
| Biosynthesis of Histidine/Enhanced Production of Histidine, Purine Pathway Metabolites, and Plasmid DNA | Methods and genetically modified cells for the biosynthetic production of histidine, plasmid DNA, or purine pathway metabolites, including synthetic promoters and genes encoding modified ribose phosphate pyrophosphokinase | PCT/US2020/065286; WO 2021/126961 |
12/16/2020 | Published | 12/16/2040 | |||||
136
| Patent Family |
Description |
Application/ |
Filing Date |
Issue Date/ |
Earliest Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| (RPPK) and/or modified 5,10-methylene-tetrahydrofolate dehydrogenase/5,10-methylene-tetrahydrofolate cyclohydrolase (MTHFDC) enzymes | ||||||||||
| Variant SARS-Cov -2 Proteins and Uses Thereof |
Variant proteins of SARS-CoV-2 nucleocapsid, spike protein, and spike protein receptor binding domain; nucleic acids encoding such variants; and compositions, cells, diagnostic kits containing such variants or its coding nucleic acids; as well as methods of detecting, treating and/or preventing SARS-CoV-2 infection | PCT/US2021/30875 | 05/05/2021 | Pending | 05/05/2041 | |||||
| Compositions and Methods for the Production of Compounds | Host cells, vectors, and nucleic acids encoding recombinant LALs (Large ATP-binding regulators of the LuxR family of transcriptional activators) and LAL binding sites for the production of compounds such as polyketides, and methods for producing such compounds | PCT/US2017/027215; WO 2017/180748 |
04/12/2017 | Nationalized in: US, AU, CA, CN, EP, JP, KR | 04/12/2037 | |||||
| Compositions and Methods for the Production of Compounds | Compositions and methods to facilitate combinatorial biosynthesis of polyketides, with engineered polyketide synthases that include modified domains with altered enzymatic activity | PCT/US2017/058805; WO 2018/081592 |
10/27/2017 | Nationalized in: US, AU, CA, CN, EP, JP, KR | 10/27/2037 | |||||
137
| Patent Family |
Description |
Application/ |
Filing Date |
Issue Date/ |
Earliest Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| Compositions and Methods for the Production of Compounds | Compositions and methods for use in combinatorial biosynthesis of polyketides by module swapping between polyketide synthase genes, with engineered polyketide synthases that include heterologous modules with altered enzymatic activity | PCT/US2017/058800; WO 2018/081590 |
10/27/2017 | Nationalized in: AU, CA, CN, EP, JP, KR, US | 10/27/2037 | |||||
| Enhanced Production of Core Lipids in Oleaginous Yeasts | Engineered cells having genetic modification(s) that increase lipid yield and methods of increasing lipid yield in a cell | PCT/US2015/067805; WO 2016/109494 |
12/29/2015 | Nationalized in: BR, CN, EP, IN, US | 12/29/2035 | |||||
| Heterologous Production of 10-Methylstearic Acid | Engineered gene sequences, cells, and methods for producing branched methyl lipids including 10-methylstearate | PCT/US2017/052491; WO 2018/057607 |
09/20/2017 | Nationalized in: BR, CA, CN, EP, US | 09/20/2037 | |||||
| Heterologous Production of 10-Methylstearic Acid by Cells Expressing Recombinant Methyltransferase | Engineered methyltransferase gene sequences, cells, and methods for producing branched methyl-lipids or exomethylene-substituted lipids | PCT/US2018/051919; WO 2019/060527 |
09/20/2018 | Nationalized in: BR, CA, EP, US | 09/20/2038 | |||||
| Methods and Compositions Involving Promoters Derived From Yarrowia lipolytica | Promoters, recombinant nucleic acids, cells and methods for modulating lipid production in oleaginous microorganisms such as yeasts | 16/942,509; US2021-0032604A1 |
07/29/2020 | Pending | 07/29/2040 | |||||
| Microorganisms Engineered to Use Unconventional Sources of Nitrogen | Microorganisms engineered to grow on an atypical nitrogen source and their use in fermentation to produce a variety of compounds including commodities, fine chemicals, and pharmaceuticals | PCT/US2014/010332; WO 2014/107660 |
01/06/2014 | Nationalized in: AU, CA, BR, IN, US | 01/06/2034 | |||||
138
| Patent Family |
Description |
Application/ |
Filing Date |
Issue Date/ |
Earliest Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| Microorganisms Engineered to Use Unconventional Sources of Phosphorous or Sulfur | Microorganisms engineered to grow on an atypical phosphorus or sulfur source and their use in fermentation to produce a variety of compounds including commodities, fine chemicals, and pharmaceuticals | PCT/US2014/52841; WO 2015/031441 |
08/27/2014 | Nationalized in: CN, AU, CA, BR, IN, EP, FR, DE, GB, US | 08/27/2034 | |||||
| Diacylglycerol Acyltransferase (DGA1) Polynucleotides, and Methods of Increasing Yeast Cell Lipid Production by Overexpression of Heterologous DGA1 | Cells engineered to express heterologous DGA1 enzyme(s) that confer increased lipid production and/or enhanced efficiency of glucose consumption, as well as methods of lipid production using these cells | PCT/US2015/17227; WO 2015/127421 |
02/24/2015 | Nationalized in: CN, AU, IN, FI, EP, BE, DK, FR, DE, LU, SE, CH, GB, US | 02/24/2035 | |||||
| Selective Advantage in Fermentation | Microorganisms engineered to grow on an atypical nitrogen, phosphorus, and/or sulfur source; fermentation compositions composed of such microorganisms and a fermentation medium containing an atypical nitrogen, phosphorus, and/or sulfur source; and fermentation processes thereof | PCT/US2015/024943; WO 2015/157431 |
04/08/2015 | Nationalized in: AU, IN, US | 04/08/2035 | |||||
| Increasing Cellular Lipid Production by Increasing the Activity of Diacylglycerol Acyltransferase and Decreasing the Activity of Triacylglycerol Lipase | Engineered cells having genetic modification(s) that increase lipid yield and methods of increasing lipid yield in a cell | PCT/US15/28760; WO 2015/168531 |
05/01/2015 | Nationalized in: AU, IN, US | 05/01/2035 | |||||
139
| Patent Family |
Description |
Application/ |
Filing Date |
Issue Date/ |
Earliest Date1 | |||||
| Owned by Gen9, Inc. | ||||||||||
| Increasing Lipid Production in Oleaginous Yeast | Engineered cells with genetic modification(s) that increase lipid yields including modifications that increase type 1, type 2, and/or type 3 diacylglycerol acyltransferase activity and modifications that decrease lipase activity, as well as methods of increasing lipid yield | PCT/US2015/033251; WO 2015/184303 |
05/29/2015 | Nationalized in: AU, CN, IN, EP, US | 05/29/2035 | |||||
| Increasing Lipid Production and Optimizing Lipid Composition | Recombinant nucleic acids, engineered cells, and methods for increasing lipid production that involve increasing or decreasing the activity of one or more selected genes | PCT/US2015/033211; WO 2015/184277 |
05/29/2015 | Nationalized in: AU, CN, EP, IN, US | 05/29/2035 | |||||
| Oleic Acid Production in Yeast | Engineered cells having genetic modification(s) that increase oleic acid yield and methods of increasing oleic acid yield in a cell | PCT/US2015/64710; WO 2016/094520 |
12/09/2015 | Nationalized in: CN, BR, IN, EP, US | 12/09/2035 | |||||
| Derivatives of 10-Methylene Lipids, Process for Preparing Such Derivatives and Use Thereof | Tuberculostearic acid (10-methylstearic acid) derivatives, processes for producing such compounds, and their use in processes for preparing polyamides, polyesters, lactams, and lactones | PCT/EP2020/058484; WO 2020/0193681 |
03/26/2020 | Nationalized in: EP | 03/26/2040 | |||||
| 1 | The expiration date of a United States patent may be earlier or later than as listed in this table due to patent term adjustment and/or the existence of a terminal disclaimer. |
Trade secrets
Ginkgo’s technology-related intellectual property that is not patent-protected is maintained as trade secrets. We employ a variety of safeguards to protect our trade secrets, including contractual arrangements that impose obligations of confidentiality and security, digital security measures, and physical security precautions.
With respect to contractual arrangements, we protect our proprietary information by requiring our employees, consultants, contractors, and other advisers to execute nondisclosure and assignment of invention agreements upon commencement of their employment or engagement. Agreements with our employees also bar them from bringing the proprietary rights of third parties to us.
140
We require confidentiality and material transfer agreements from third parties that receive our confidential data or materials, and we also incorporate confidentiality and material transfer precautions into our collaboration agreements. For example, in the course of a cell program, we might transfer samples of intermediate strains to the customer for testing and scale-up work and then transfer a final commercial strain upon completion of our work. To protect both intermediate and final strains, we use strain transfer agreements that document the contractual restrictions and controls we have put into place, typically including, in the case of intermediate strains, covenants requiring the customer to return or destroy all strain samples after testing.
We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. Our privacy and information security program is designed and implemented, both within our internal systems and on our platform, in an effort to address the security and confidentiality of sensitive or confidential data related to our trade secrets, partners, customers, and employees. We maintain a documented information and physical security program with a dedicated team of professionals that focuses on technical measures such as application, network, system, and physical security, as well as policy measures related to privacy compliance, internal training and education, and documented incident response protocols.
Trademarks and domain names
Although our business is directed at sophisticated corporate customers rather than end consumers, we have trademark rights and registrations in our name, logo, and other brand indicia in the United States and other jurisdictions around the world. We also have registered domain names for websites that we use in our business, such as www.ginkgobioworks.com.
Intellectual property transaction structure
We earn revenue from collaboration agreements with customers under which we perform cell programming activities. Through our cell programs, we develop cells that produce or are products for our customers, which they market in their verticals.
With respect to intellectual property, we have relatively standard transaction structures that apply to cell programs for a customer. In this situation, our collaboration agreements typically provide that Ginkgo will own all collaboration-related intellectual property (“Foreground IP”) concerning cell programming. To protect our collaboration partners’ investment in the collaboration and to provide them with a competitive advantage from working with Ginkgo, Ginkgo provides a limited exclusive license to patents within the Foreground IP that cover the product, usually within a specified field. However, our terms may vary in certain special circumstances.
We do not provide exclusive licenses to unpatented Foreground IP (i.e., trade secrets and other knowhow) that results from a collaboration. In our typical deal structure, we also do not provide exclusive licenses to our “background” intellectual property — i.e., the intellectual property, whether patented or unpatented, that we developed before entering into a collaboration or develop independently from our work in the collaboration. We believe that our transaction structures allow us to maximize the reuse of Codebase across programs and ensure that technology we develop does not lie fallow.
Amyris Partnership Agreement
On October 20, 2017, we entered into a partnership agreement (the “Partnership Agreement”) with Amyris, Inc. (“Amyris”), which, as amended from time to time, terminated all prior agreements between Ginkgo and Amyris. In the Partnership Agreement, Amyris, among other things, granted us a non-exclusive license effective as of June 28, 2016 (the date of an earlier agreement between the parties) under all of Amyris’s rights in and to certain
141
specified microbial strains, and under all patents and applications associated with such microbial strains, to make, have made, use, sell, offer to sell and import any products other than farnesene and/or farnesene derivatives that are chemically produced from farnesene. The license is subject to any previous exclusive licenses provided to third parties and is royalty-free, fully paid-up, sublicensable, non-exclusive and perpetual (i.e., it survives termination or expiration of the Partnership Agreement except in the case of our insolvency).
Strateos Collaboration Agreement
On October 2, 2017, we entered into a collaboration agreement (the “Strateos Collaboration Agreement”) with Strateos, Inc. f/k/a Transcriptic, Inc. (“Strateos”), which was amended and restated on April 20, 2021. Under the Strateos Collaboration Agreement, Strateos granted us a non-exclusive, perpetual, irrevocable, fully paid-up, royalty- free license under certain intellectual property rights to use its software platform in a range of activities relating to our business, including, among other things, developing and commercializing cell lines, developing data packages, providing foundry and analytical services and performing diagnostic testing. In connection with the Strateos Collaboration Agreement, we paid Strateos an up-front fee of $3.0 million and agreed to pay an additional $9.0 million in fees during the 5-year term of the agreement in consideration for services provided by Strateos under the agreement, of which more than $7.0 million has already been paid. Either party may terminate the Strateos Collaboration Agreement without cause upon six months’ written notice to the other party. Either party may also terminate the agreement for the other party’s material breach, insolvency or change of control to a direct competitor of the terminating party. After expiration of the agreement, either party’s termination of the agreement for convenience or the other party’s insolvency, or our termination of the agreement for Strateos’ material breach or Strateos’ change of control to one of our direct competitors, we will retain a license to use Strateos’ software. We retain such rights for an 18-month period in the event the agreement is terminated by Strateos for certain material breaches of the agreement, but we do not retain such rights in the event of Strateos’ termination due to our change of control to a Strateos direct competitor, our leak or other unauthorized disclosure of Strateos’ code, or a material breach of our obligations involving payment, intellectual property or confidentiality.
Ginkgo’s headquarters are located in the Seaport district of Boston, Massachusetts and comprise a set of non-cancellable operating leases within a facility totaling approximately 265,000 square feet of office and laboratory space. These lease agreements expire on dates ranging from 2030 to 2036 and each contain one option to extend the lease for a five-year period at then-market rates. We also lease a total of approximately 100,000 square feet of office and lab space in Cambridge, Massachusetts, Emeryville, California, and Utrecht, Netherlands.
In anticipation of expanding facility needs to support future growth, in April 2021, we entered into a lease consisting of approximately 152,000 square feet of new office and laboratory space being developed in Boston, Massachusetts near our headquarters. The lease commencement date is estimated to be June 1, 2024, subject to certain extensions, and expires on the fifteenth anniversary of the lease commencement date. The lease includes one option to extend the lease for ten years at then-market rates as well as certain expansion rights. In September 2021, we exercised our expansion option to include the entire rentable area of the building of approximately 250,000 square feet.
We currently lease all of our facilities and do not own any real property. We believe our facilities are adequate and suitable for our current needs and that the new lease described above provides significant expansion space. To support future organic growth or merger and acquisition activity, we may enter into new leases, assume lease obligations or acquire property both domestically and internationally and believe that, if needed, suitable or alternative space will be available.
Ginkgo’s suppliers for cell programming operations comprise primarily manufacturers and distributors of life science tools, consumables and equipment as well as certain specific providers of contract research, development
142
and manufacturing services. We will sometimes enter into long-term, strategic partnerships with innovative suppliers. Because of the significant scale of our Foundry’s operations, we believe we are often an early adopter and the largest customer at scale of certain new life science tools and technologies. Our supply agreements with Twist Bioscience Corporation (“Twist”) and Berkeley Lights, Inc. (“Berkeley Lights”), as further described below, are examples of such strategic supplier relationships. We will also occasionally acquire technology or Codebase assets for strategic reasons and because we can integrate the technology effectively into our platform — Gen9, Inc. and Warp Drive Bio, Inc. are two examples.
Our suppliers for our biosecurity offering include manufacturers and distributors of lateral flow assay (LFA) test kits, including our collaboration with Access Bio, Inc. and COVID-19 sample collection kits. We have developed a national network of third party labs for provision of COVID-19 molecular testing services. We also utilize third parties for certain other services, including physician authorizations and on-site test administration, in the provision of our end-to-end COVID-19 testing offering.
Our software, automation, data, information technology, development operations (“DevOps”) and information security functions utilize various third party software and information technology service providers, including AWS, for data storage and processing. We also routinely engage a variety of third parties for professional services, contract employment services and consulting services.
Twist Bioscience Corporation
In March 2018, we entered into a non-cancelable supply agreement (the “2018 Agreement”) with Twist, which requires us to purchase synthetic DNA at specified volumes on a quarterly basis over a four-year term. To the extent we fail to meet our quarterly minimum purchase obligations, we are required to pay a fee per unit of shortfall. The products we may purchase that contribute toward achieving our quarterly minimum purchase obligation can vary based on our discretion, subject to advance notice provided to Twist.
Our quarterly minimum purchase obligation may be adjusted for the following reasons: (i) due to a lack of availability of certain products for purchase in a given quarter; (ii) due to a lack of certain features available; (iii) delays in shipments over two consecutive quarters beyond the agreed upon lead times; and (iv) if the average yield of certain products measured over two consecutive quarters is greater than a specified yield. We receive volume discounts on purchases based on specified volume thresholds over the term of the supply agreement.
The 2018 Agreement can only be terminated (i) upon mutual agreement of both parties, (ii) by us upon a specified change of control, (iii) upon a material breach of the contract by either party, or (iv) by Twist in the event that we fail to place orders for more than a certain percentage of our required quarterly minimums under the 2018 Agreement for two consecutive quarters. The purchase minimums in the 2018 Agreement create an enforceable obligation only in conjunction with each purchase order.
Berkeley Lights
In September 2019, we signed a collaboration agreement (the “Berkeley Collaboration”) with Berkeley Lights, a cell biology company focused on enabling and accelerating the rapid development and commercialization of microbial biotherapeutics and other cell-based products for its customers. Under the Berkeley Collaboration, we incorporate Berkeley Lights’ platform into the Foundry to accelerate the engineering of biotherapeutics and cell-based products. Under the Berkeley Collaboration, both parties agree to use diligent efforts to jointly develop certain workflow plans.
We are obligated to pay Berkeley Lights at least $109.0 million, and up to $150.0 million, over the term period of the Berkeley Collaboration from October 2019 through September 2026 for (i) payments for Berkeley Lights’ efforts under the workflow development plans and (ii) payments for purchases of certain equipment, associated consumables, and other goods and services. We have the option to buy down our purchase obligations after the
143
second contract year by making a one-time payment to Berkeley Lights. We are also required to pay to Berkeley Lights certain license fees for the use of Berkeley Lights’ platform and certain milestone payments of up to $11.5 million payable when a therapeutic discovered using certain workflows reaches specified development and regulatory milestones. For more details on the minimum purchase commitments under the Berkeley Collaboration, see Note 10, “Commitments and Contingencies,” of our audited consolidated financial statements included elsewhere in this prospectus.
Under the Berkeley Collaboration, we are granted an exclusivity period for each workflow developed for us by Berkeley Lights, but Berkeley Lights has the option to buy down the exclusivity period, after which the parties will equally share the development costs of the associated workflow.
The Berkeley Collaboration will continue until the seventh anniversary of its effective date, subject to certain automatic extension provisions, including for delays resulting from Berkeley Lights’ failure to supply products or services conforming with the Berkeley Collaboration. The collaboration will automatically terminate if we, at any time after the second contract year, elect to exercise our buy down right. In addition, either party may terminate the Berkeley Collaboration (i) for the material breach by the other party (including, with respect to Ginkgo, a material supply failure), (ii) upon the occurrence of certain insolvency related events of the other party, and (iii) for certain force majeure events.
Our business, or the business of our customers, may be regulated by the U.S. Food and Drug Administration (“FDA”) and other federal authorities in the United States, including the U.S. Federal Trade Commission (“FTC”), U.S. Department of Agriculture (“USDA”), U.S. Drug Enforcement Administration (“DEA”) and Environmental Protection Agency (“EPA”), as well as comparable authorities in foreign jurisdictions and various state and local authorities in the United States. Failure to comply with applicable regulations may result in enforcement actions, civil or criminal sanctions, and adverse publicity.
FDA regulation
We provide cell engineering and product discovery services to customers engaged in the manufacture of foods, cosmetics and pharmaceutical products. The FDA regulates the research, development, testing, quality control, import, export, safety, effectiveness, storage, recordkeeping, premarket review, approval or licensure, processing, formulation, manufacturing, packaging, labeling, advertising, promotion, marketing, distribution, sale, post-market monitoring and reporting of our customers’ pharmaceuticals, cosmetics and food products, and the FTC also regulates the advertising and promotion of these products.
We also act as the authorized distributor of certain COVID-19 diagnostic test and collection kits manufactured by independent third parties, and we work with laboratory partners that provide clinical laboratory testing services as part of the COVID-19 testing services we offer, and these tests and test kits are subject to regulation by the FDA. In particular, the tests and test kits used in our COVID-19 testing services may be subject to regulation by the FDA as medical devices, and may be required to comply with the requirement that such products have obtained clearance, approval, or other marketing authorizations, such as an Emergency Use Authorization (“EUA”), before they can be commercialized, as well as post-market requirements such as adverse event reporting and restrictions on labeling, marketing, and distribution.
Medical products, including COVID-19 tests, that are granted an EUA or other marketing authorization must comply fully with the terms and conditions provided in the EUA or other marketing authorization. For example, EUAs for COVID-19 tests may include conditions of authorization applicable to the EUA holder, authorized distributors and authorized laboratories. Noncompliance with applicable requirements could result in negative consequences, including adverse publicity, judicial or administrative enforcement, warning letters or untitled letters from the FDA, mandated corrective promotional materials, advertising or communications with doctors,
144
and civil or criminal penalties, among others. The FDA can also withdraw marketing authorization for the applicable product, and in the case of a product subject to an EUA, the authorization to market the product under the EUA lasts only as long as the declared public health emergency.
We do not hold any EUAs or other marketing authorizations for COVID-19 tests or perform any COVID-19 testing ourselves as part of our testing services, and we rely on our test suppliers and contract laboratories to comply with the regulatory requirements applicable to their tests and testing activities, including the potential requirement for premarket review and affirmative marketing authorizations, to the extent required. In some cases, however, the third-party laboratories we partner with to conduct pooled testing may not hold EUAs or other marketing authorizations from the FDA for their tests and instead may validate and perform their tests as laboratory developed tests (“LDTs”). An LDT is an in vitro diagnostic test that is intended for clinical use and is designed, manufactured, and used within a single laboratory. Although the FDA takes the position that LDTs are classified as medical devices, the FDA has historically exercised enforcement discretion and has not enforced its requirements, including premarket review, with respect to LDTs. The FDA’s policy toward the regulation of LDTs has been subject to frequent discussion and, in the case of COVID-19 LDTs, has changed throughout the COVID-19 public health emergency.
DEA regulation
We are engaged in the research, development, and export of certain products that may be regulated as controlled substances, including microbes designed to generate precursors to cannabinoids or other chemical intermediates. The Controlled Substances Act of 1970, as amended from time to time, establishes registration, security, recordkeeping, reporting, storage, distribution and other requirements administered by the DEA. The DEA is concerned with the control of handlers of controlled substances, and with the equipment and raw materials used in their manufacture and packaging, in order to prevent loss and diversion into illicit channels of commerce. The DEA regulates controlled substances as Schedule I, II, III, IV or V substances. Schedule I substances by definition have no established medicinal use, and may not be marketed or sold in the United States. Schedule I substances are considered to present the highest risk of abuse, and Schedule V substances the lowest relative risk of abuse among controlled substances. Marijuana is classified as a Schedule I controlled substance. However, the term does not include “hemp,” which means the cannabis plant and any part of that plant, including the seeds and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3% on a dry weight basis.
Annual registration is required for any facility that manufactures, distributes, dispenses, imports or exports any controlled substance. The registration is specific to the particular location, business activity and controlled substance schedule. For example, separate registrations are needed for import and manufacturing, and each registration will specify which controlled substance schedule is authorized for that activity.
The DEA typically inspects a facility to review its security measures prior to issuing a registration. The DEA requires “effective controls and procedures” to guard against theft and diversion of controlled substances. Security requirements vary by controlled substance schedule (with the most stringent requirements applying to Schedule I and Schedule II substances), type of business activity conducted, quantity of substances handled, and a variety of other factors. Required security measures include background checks on employees and physical control of inventory. While the specific means by which effective controls and procedures are achieved may vary, security practices may include use of cages, surveillance cameras and inventory reconciliations. Records must be maintained for the handling of all controlled substances, and, in certain scenarios, periodic reports made to the DEA. Reports must also be made for thefts or losses of any controlled substance, and disposal of controlled substances must adhere to various methods authorized by the regulations. In addition, special authorization and notification requirements apply to imports and exports.
145
Laboratory Licensing and Certification Requirements
The clinical laboratories we partner with for our COVID-19 testing program are subject to federal oversight under Clinical Laboratory Improvement Amendments of 1988 (“CLIA”), which requires all clinical laboratories to meet certain quality assurance, quality control and personnel standards. Laboratories also must undergo proficiency testing and are subject to inspections. Standards for testing under CLIA are based on the complexity of the tests performed by the laboratory, with tests classified as “high complexity,” “moderate complexity,” or “waived.” Laboratories performing high complexity testing are required to meet more stringent requirements than moderate complexity laboratories. Certain of our partner laboratories must undergo on-site surveys at least every two years, which may be conducted by the Centers for Medicare and Medicaid Services (“CMS”) under the CLIA program or by a private CMS approved accrediting agency. In addition, we hold CLIA Certificates of Waiver and perform certain CLIA-waived tests on behalf of our clients, which subjects us directly to certain CLIA requirements. The sanction for failure to comply with CLIA requirements may be suspension, revocation or limitation of a laboratory’s CLIA certificate, which is necessary to conduct business, as well as significant fines and criminal penalties.
The operations of our partner laboratories and our laboratories holding CLIA Certificates of Waiver are also subject to state and local laboratory regulation. CLIA provides that a state may adopt laboratory regulations different from or more stringent than those under federal law, and a number of states have implemented their own laboratory regulatory requirements. State laws may require that laboratory personnel meet certain qualifications, specify certain quality controls, or require maintenance of certain records. No assurances can be given that we or our partner laboratories will pass all future licensure or certification inspections.
Federal Select Agent Regulations
Our research facilities that synthesize DNA sequences or perform other activities could become subject to the Federal Select Agent Program (“FSAP”), which involves rules administered by the Centers for Disease Control and Prevention (“CDC”) and the USDA Animal and Plant Health Inspection Service (“APHIS”). The FSAP regulates the possession, use, and transfer of biological select agents and toxins that have the potential to pose a severe threat to public health, animal or plant health, or animal or plant products. FSAP regulatory requirements include: (i) registration with the CDC and/or APHIS for research facilities that deal with the select agents and toxins; (ii) submission to periodic biosafety and security inspections; and (iii) reporting of theft, loss or release of select agents. Federal agency enforcement actions for violations of FSAP regulations can include the initiation of corrective actions, complete or partial suspension or revocation of select agent registrations or civil or criminal liability.
Genetically Modified Materials Regulations
Our technologies and the technologies of our customers involve the use of genetically modified cells, organisms and biomaterials, including, without limitation, genetically modified organisms (“GMOs”) and genetically modified microorganisms (“GMMs”), genetically modified plant or animal cells and genetically modified proteins and biomaterials (collectively, “Genetically Modified Materials”), and their respective products. In the United States, the FDA, APHIS and the EPA are the primary agencies that regulate the use of GMOs, GMMs and potential products derived from GMOs or GMMs or Genetically Modified Materials, pursuant to the Coordinated Framework for the Regulation of Biotechnology (“Coordinated Framework”).
The FDA reviews the safety of food consumed by humans and of feed consumed by animals under the Federal Food, Drug and Cosmetic Act (“FDCA”). Under the FDCA, food and feed manufacturers are responsible for ensuring that the products they market, including those developed through genetic engineering, are safe and properly labeled. In addition, the FDA must approve the use of any food additives, including GMOs, before marketing.
146
APHIS examines whether a plant itself presents a “plant pest” risk under the Plant Protection Act (“PPA”). Specifically, APHIS is responsible for regulating the introduction (i.e., importation, interstate movement or release into the environment) of certain GMOs and plants under the plant pest provisions in the PPA to ensure that they do not pose a plant pest risk APHIS finalized changes to the PPA’s implementing regulations with respect to certain GMOs in May 2020. A person or organization may request a regulatory status review from APHIS to determine whether a GMO is unlikely to pose a plant pest risk and, therefore, is not regulated under the plant pest provisions of the PPA or the regulations codified at 7 C.F.R. Part 340; requesting a regulatory status review tends to assume the GMO at issue does not otherwise fall within a regulatory exemption. If the GMO does not qualify for an exemption or if the APHIS regulatory status review process finds that the plant poses a plausible plant pest risk, then the GMO may require an APHIS permit regulations were issued in May 2020 to clarify the process. A regulated article may be subject to APHIS for the environmental release, importation, or interstate movement of the GMO or its progeny.
EPA regulates, under the Federal Insecticide, Fungicide and Rodenticide Act (“FIFRA”), the pesticides (including plant incorporated protectants) that are used with crops, including GMO herbicide-tolerant crops.
FIFRA generally requires all pesticides to be registered before distribution or sale, unless they are exempted. Under FIFRA, a pesticide registrant must demonstrate that the pesticide at issue, when used pursuant to its specifications, “will not generally cause unreasonable adverse effects on the environment” to secure a registration. EPA must approve each distinct pesticide product, each distinct use pattern, and each distinct use site. In addition to EPA’s FIFRA authority, EPA also regulates potential human health impacts from pesticides under the FDCA. EPA does so by establishing “tolerance levels” (i.e., “the amount of pesticide that may remain on food products”) under the FDCA.
Telehealth regulation
Our telehealth provider partner is subject to various federal, state and local certification and licensing laws, regulations and approvals, relating to, among other things, the adequacy of health care, the practice of medicine and other health professions (including the provision of remote care and cross-coverage practice), equipment, personnel, operating policies and procedures and the prerequisites for ordering laboratory tests. Some states have enacted regulations specific to providing services to patients via telehealth. Such regulations include, among other things, informed consent requirements that some states require providers to obtain from their patients before providing telehealth services. Health professionals who provide professional services using telehealth modalities must, in most instances, hold a valid license to practice the applicable health profession in the state in which the patient is located. In addition, certain states require a physician providing telehealth to be physically located in the same state as the patient. Any failure to comply with these laws and regulations could result in civil or criminal penalties against telehealth providers.
State corporate practice of medicine and fee splitting laws
Our relationship with our telehealth provider partner, who provides physician oversight and support to individuals seeking COVID-19 diagnostic or screening testing, including evaluating each request for testing, communicating and providing consultation services for certain test results, is subject to various state laws, which are intended to prevent unlicensed persons from interfering with or influencing a physician’s professional judgment, and prohibiting the sharing of professional services income with non-professional or business interests. These laws vary from state to state and are subject to broad interpretation and enforcement by state regulators. A determination of non-compliance could lead to adverse judicial or administrative action against us and/or our telehealth provider partner, civil or criminal penalties, receipt of cease and desist orders from state regulators, loss of provider licenses, or a restructuring of our arrangement with our telehealth provider partner.
Healthcare fraud and abuse laws
Although none of our COVID-testing offerings are currently billed to any third-party payor, including any commercial payor or government healthcare program, by us or any of our laboratory or telehealth provider
147
partners, we may nonetheless be subject to a number of federal and state healthcare regulatory laws that restrict business practices in the healthcare industry. These laws include, but are not limited to, federal and state anti-kickback, false claims, and other healthcare fraud and abuse laws.
The federal Anti-Kickback Statute prohibits, among other things, individuals or entities from knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, overtly or covertly, in cash or in kind to induce or in return for purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any item or service reimbursable under Medicare, Medicaid or other federal healthcare programs. A person or entity does not need to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation.
The federal civil and criminal false claims laws, including the civil False Claims Act, and civil monetary penalties laws prohibit, among other things, any individual or entity from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government, or from knowingly making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute or the federal physician self-referral prohibition, commonly known as the Stark Law, constitutes a false or fraudulent claim for purposes of the civil False Claims Act.
In addition to the Anti-Kickback Statute and the Stark Law, the United States recently enacted a law known as the Eliminating Kickbacks in Recovery Act, or EKRA, which created a new federal crime for knowingly and willfully: (1) soliciting or receiving any remuneration in return for referring a patient to a recovery home, clinical treatment facility, or laboratory; or (2) paying or offering any remuneration to induce such a referral or in exchange for an individual using the services of a recovery home, clinical treatment facility, or laboratory. Unlike the Anti-Kickback Statute, EKRA is not limited to services reimbursable under a government health care program, but instead extends to all services reimbursed by “health care benefit programs.”
The federal Health Insurance Portability and Accountability Act of 1996, as amended by Health Information Technology for Economic and Clinical Health Act (“HIPAA”) created additional federal criminal statutes that prohibit, among other things, knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Similar to the U.S. federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Similar state and local laws and regulations may also restrict business practices in the medical device and clinical laboratory industries, such as state anti-kickback and false claims laws, which may apply to business practices, including but not limited to, research, distribution, sales and marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, or by patients themselves; and state laws that require companies to comply with the industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources.
Violation of any of such laws or any other governmental regulations that apply may result in significant criminal, civil and administrative penalties including damages, fines, imprisonment, disgorgement, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, contractual damages, reputational harm, diminished profits and future earnings, disgorgement, exclusion from participation in government healthcare programs and the curtailment or restructuring of our operations.
148
Federal and state data privacy and security regulations
Numerous state, federal and foreign laws, including consumer protection laws and regulations, govern the collection, dissemination, use, access to, confidentiality and security of personal information, including health-related information. In the United States, numerous federal and state laws and regulations, including data breach notification laws, health information privacy and security laws, including HIPAA, and federal and state consumer protection laws and regulations (e.g., Section 5 of the Federal Trade Commission Act), that govern the collection, use, disclosure, and protection of health-related and other personal information could apply to our operations or the operations of our partners. HIPAA, and its respective implementing regulations, imposes obligations on “covered entities,” including certain health care providers, health plans, and health care clearinghouses, and their respective “business associates” that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, as well as their covered subcontractors with respect to safeguarding the privacy, security and transmission of individually identifiable health information. Violations of the HIPAA privacy and security regulations may result in civil and criminal penalties. The U.S. Department of Health and Human Services (“HHS”) is required to conduct periodic compliance audits of covered entities and their business associates. HIPAA also authorizes State Attorneys General to bring civil actions seeking either an injunction or damages in response to violations of HIPAA privacy and security regulations.
In addition, certain state laws, such as the California Confidentiality of Medical Information Act, the California Consumer Privacy Act of 2018 (“CCPA”) and the California Privacy Rights Act (“CPRA”) govern the privacy and security of personal information, including health-related information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. Privacy and security laws, regulations, and other obligations are constantly evolving, may conflict with each other (thus complicating compliance efforts), and can result in investigations, proceedings, or actions that lead to significant civil or criminal penalties and restrictions on data processing.
We are currently in legal proceedings and may, from time to time, become involved in legal proceedings and regulatory actions arising in the ordinary course of our business. On November 18, 2021, a purported stockholder of the Company, Kevin Stuart, filed a complaint in the United States District Court for the Northern District of California against the Company and certain of its officers and directors, captioned Kevin Stuart v. Ginkgo Bioworks Holdings, Inc. F/K/A Soaring Eagle Acquisition Corp., et al., Case No. 21-cv-08943 (the “Stuart Complaint”). The Stuart Complaint alleges that the Company’s August 13 proxy statement and certain press releases issued between May and September omitted material information related to the Company’s business, operations, and finances and that, as a result, all defendants violated Section 10(b) of the Securities and Exchange Act of 1934 and Rule 10b-5 promulgated thereunder. The Stuart Complaint alleges further that the individual defendants violated Section 20(a) of the Exchange Act. The Stuart Complaint asks the court to determine that the action may be maintained as a class action and to order the defendants to pay damages, prejudgment and post-judgment interest, attorneys’ fees, experts’ fees and other just and proper relief. The Company believes the claims asserted in the Stuart Complaint are without merit. Additional lawsuits may be filed against the Company in connection with its merger with Soaring Eagle Acquisition Corp. which could result in substantial costs to the Company. For additional information on risks relating to litigation, please see the sections titled “Risk Factors—Risks Related to Ginkgo’s Intellectual Property—Risks that we may need to engage in intellectual property litigation,” “—General risks related to litigation” and “Risk Factors—Risks Related to Government Regulation and Litigation.”
149
We were originally incorporated as a Cayman Islands exempted company in October 2020 as a special purpose acquisition company, formed for the purpose of effecting a merger, capital stock exchange, asset acquisition, stock purchase, recapitalization, reorganization or similar business combination with one or more businesses. SRNG completed its IPO in February 2021. In September 2021, our wholly owned subsidiary merged with and into Old Ginkgo, with Old Ginkgo surviving the merger as a wholly owned subsidiary of SRNG. In connection with the Business Combination, we changed our name to “Ginkgo Bioworks Holdings, Inc.” Our principal executive offices are located at 27 Drydock Avenue, 8th Floor, Boston MA 02210.
Our telephone number is (877) 422-5362. Our website address is www.ginkgobioworks.com. Information contained on our website or connected thereto does not constitute part of, and is not incorporated by reference into, this prospectus or the registration statement of which it forms a part. The inclusion of the corporate website address is an inactive textual reference only.
150
The following summary of the material terms of our capital stock is not intended to be a complete summary of the rights and preferences of such securities, and is qualified by reference to our Charter, our Bylaws and the Warrant-related documents described herein, which are exhibits to the registration statement of which this prospectus is a part. We urge you to read each of our Charter, our Bylaws and the Warrant-related documents described herein in their entirety for a complete description of the rights and preferences of our securities.
Authorized and Outstanding Capital Stock
New Ginkgo’s Charter authorizes the issuance of 16,000,000,000 shares of all classes of New Ginkgo’s capital stock, consisting of:
| • | 200,000,000 shares of undesignated preferred stock, par value $0.0001 per share; |
| • | 10,500,000,000 shares of New Ginkgo Class A common stock, par value $0.0001 per share; |
| • | 4,500,000,000 shares of New Ginkgo Class B common stock, par value $0.0001 per share; and |
| • | 800,000,000 shares of New Ginkgo Class C common stock, par value $0.0001 per share. |
As of November 8, 2021, there were 1,310,783,159 shares of New Ginkgo Class A common stock, 361,857,141 shares of New Ginkgo Class B common stock and 51,824,925 warrants to purchase shares of New Ginkgo Class A common stock outstanding.
New Ginkgo has three classes of authorized common stock: New Ginkgo Class A common stock, New Ginkgo Class B common stock, and New Ginkgo Class C common stock. Generally, New Ginkgo Class B common stock can only be issued to, transferred to, and held by New Ginkgo’s directors and employees, or trusts or legal entities through which the right to vote the shares of New Ginkgo Class B common stock held thereby is exercised exclusively by one or more of New Ginkgo’s directors or employees (any such director, employee, trust or legal entity, an “Eligible Holder”), unless otherwise determined by a majority of the Class B Directors then serving.
Voting Rights
New Ginkgo Class A Common Stock
Holders of New Ginkgo Class A common stock are entitled to one (1) vote for each share of New Ginkgo Class A common stock held of record by such holder on all matters voted upon by New Ginkgo stockholders.
New Ginkgo Class B Common Stock
Holders of New Ginkgo Class B common stock are entitled to ten (10) votes for each share of New Ginkgo Class B common stock held of record by such holder on all matters voted upon by New Ginkgo stockholders.
New Ginkgo Class C Common Stock
Except as expressly provided in New Ginkgo’s Charter or required by applicable law, holders of New Ginkgo Class C common stock generally are not entitled to vote on matters voted upon by New Ginkgo stockholders. Solely to the extent that a holder of New Ginkgo Class C common stock is expressly entitled to vote on any matter pursuant to New Ginkgo’s Charter or by applicable law, the holder will be entitled to one (1) vote for each share of New Ginkgo Class C common stock held of record by such holder.
151
Stockholder Votes
Holders of New Ginkgo common stock generally vote together as a single class on all matters submitted to a vote of New Ginkgo stockholders (including the election and removal of directors), unless otherwise provided in New Ginkgo’s certificate of incorporation or required by applicable law. Any action or matter submitted to a vote of the New Ginkgo stockholders will be approved if the number of votes cast in favor of the action or matter exceeds the number of votes cast in opposition to the action or matter, except that New Ginkgo’s directors will be elected by a plurality of the votes cast. Holders of New Ginkgo Class A common stock are not entitled to cumulate their votes in the election of New Ginkgo’s directors.
Delaware law could require holders of a class of New Ginkgo’s capital stock to vote separately as a class on any proposed amendment of New Ginkgo’s certificate of corporation if the amendment would increase or decrease the par value of the shares of that class or would alter or change the powers, preferences or special rights of the shares of that class in a manner that affects them adversely.
Holders of New Ginkgo common stock are not entitled to vote on any amendment to New Ginkgo’s Charter that relates solely to the terms of one or more series of New Ginkgo’s preferred stock and on which the holders of such affected series are entitled to vote, either separately as a class or together with the holders of one or more other series of New Ginkgo’s preferred stock, pursuant to New Ginkgo’s Charter or by applicable law.
Stockholder Action by Written Consent
The Charter provides that New Ginkgo’s stockholders may act by written consent only if (a) the action to be taken or effected has been approved by the affirmative vote of all of the directors of New Ginkgo then serving or (b) the holders of New Ginkgo Class B common stock collectively beneficially own shares representing a majority of the voting power of all of the outstanding shares of capital stock of New Ginkgo. In all other circumstances, any action required or permitted to be taken by New Ginkgo’s stockholders must be effected at a duly called annual or special meeting of stockholders and may not be taken or effected by written consent.
Special Meetings of Stockholders
The Charter provides that, except as otherwise required by applicable law, special meetings of New Ginkgo’s stockholders may be called only by the New Ginkgo Board, the chairman of the New Ginkgo Board, New Ginkgo’s chief executive officer or president, or, at any time that the holders of New Ginkgo Class B common stock collectively beneficially own shares representing a majority of the voting power of all of the outstanding shares of capital stock of New Ginkgo, the holders of shares representing a majority of the voting power of all of the outstanding shares of capital stock of New Ginkgo.
Economic Rights
Except as otherwise expressly provided in New Ginkgo’s Charter or required by applicable law, shares of each class of New Ginkgo common stock have the same rights, powers and preferences and rank equally, share ratably and be identical in all respects as to all matters, including the following:
Dividends and Distributions; Rights upon Liquidation
Subject to the rights of holders of any outstanding series of New Ginkgo preferred stock, the holders of shares of each class of New Ginkgo common stock are entitled to receive ratably, on a per share basis, any dividend or distribution (including upon the liquidation, dissolution or winding up of New Ginkgo) paid by New Ginkgo, unless otherwise approved by the affirmative vote of the holders of a majority of each of the outstanding shares of New Ginkgo Class A common stock, the outstanding shares of New Ginkgo Class B common stock, and the
152
outstanding shares of New Ginkgo Class C common stock, each voting separately as a class, except that, if a dividend or distribution is paid in the form of shares (or options, warrants or other rights to acquire shares) of New Ginkgo common stock, then holders of New Ginkgo Class A common stock will receive shares (or options, warrants or other rights to acquire shares) of New Ginkgo Class A common stock, holders of New Ginkgo Class B common stock will receive shares (or options, warrants or other rights to acquire shares) of New Ginkgo Class B common stock, and holders of shares of Class C common stock will receive shares (or options, warrants or other rights to acquire shares) of New Ginkgo Class C common stock.
Subdivisions, Combinations and Reclassifications
If New Ginkgo subdivides or combines any class of New Ginkgo common stock with any other class of New Ginkgo common stock, then each class of New Ginkgo common stock must be subdivided or combined in the same proportion and manner, unless otherwise approved by the affirmative vote of the holders of a majority of each of the outstanding shares of New Ginkgo Class A common stock, the outstanding shares of New Ginkgo Class B common stock, and the outstanding shares of New Ginkgo Class C common stock, each voting separately as a class.
Mergers and Other Extraordinary Transactions
The Charter provides that, in the event of certain extraordinary transactions affecting New Ginkgo (including certain transactions resulting in a change of control of New Ginkgo, the acquisition by a third party of assets of New Ginkgo generating at least 50% of New Ginkgo’s revenues on a consolidated basis, or any merger or consolidation of New Ginkgo), shares of each class of New Ginkgo common stock will be entitled to receive ratably, on a per share basis, any consideration paid or otherwise distributed to, or rights received by, New Ginkgo stockholders, or into which such shares are converted or for which such shares are exchanged, in connection with such extraordinary transaction (including with respect to the form, amount and timing thereof), unless different treatment of the shares of each such class is approved by the affirmative vote of the holders of a majority of the outstanding shares of New Ginkgo Class A common stock, the holders of a majority of the outstanding shares of New Ginkgo Class B common stock and the holders of a majority of the outstanding shares of New Ginkgo Class C common stock, each voting separately as a class, except that, to the extent that such consideration is paid in the form of securities or other equity interests, holders of New Ginkgo Class B common stock may receive a class, series or other form of such securities or other equity interests each having voting power that is ten (10) times greater than the voting power of any security or other equity interest received by holders of New Ginkgo Class A common stock and holders of New Ginkgo Class C common stock may receive a class, series or other form of such securities or other equity interests having no voting power.
Additionally, the Charter prohibits New Ginkgo from entering into any agreement with respect to a tender or exchange offer by a third party unless such agreement provides for consideration to be paid or distributed to, or rights to be received by, New Ginkgo stockholders in the manner provided in the paragraph immediately above.
Equal Value upon Disposition
The Charter provides that, in the case of any disposition of New Ginkgo Class B common stock for value, the value paid in respect of such share of New Ginkgo Class B common stock must be equal to the prevailing price per share of New Ginkgo Class A common stock at the time of such disposition for value. New Ginkgo may (and expects to) from time to time establish restrictions, policies and procedures relating to transfers and dispositions of shares of New Ginkgo Class B common stock as it deems necessary or advisable.
Transfer Restrictions
Lock-up Applicable to Founders and Employees
The Charter provides that, subject to customary exceptions and the other exceptions described in the following sentences, the Founders and their affiliated trusts and any New Ginkgo stockholder who is an employee of New
153
Ginkgo or any of its wholly owned subsidiaries at the time of the Closing, and any transferee of any of the foregoing, are unable to transfer their shares of New Ginkgo Class A common stock or New Ginkgo Class B common stock received as consideration in the Merger (including upon the settlement of any equity award of New Ginkgo into which any equity award of Old Ginkgo was converted in the Merger), other than the Earn-out Consideration, for a period of one year following the closing of the Business Combination. The transfer restrictions described in the foregoing sentence will not apply to an aggregate of 10% of the total number of shares subject to such transfer restrictions, excluding (from this exception to such transfer restrictions) any shares of New Ginkgo Class A common stock or New Ginkgo Class B common stock received upon the settlement of any equity award of New Ginkgo into which any equity award of Old Ginkgo that was, immediately prior to the effective time of the Merger, subject to any unsatisfied service- or time-based vesting condition was converted in the Merger. Additionally, solely in the case of any holder of any equity award of New Ginkgo (including any such equity award into which any equity award of Old Ginkgo was converted in the merger), the transfer restrictions described in the first sentence of this paragraph will be lifted, beginning on the earlier of March 1, 2022 and the date that is 15 calendar days before the date on which any tax relating to such equity award (other than any equity or portion thereof that is exempted from such transfer restrictions by virtue of the immediately preceding sentence) will become due under applicable law (as reasonably determined by New Ginkgo), solely to the extent necessary to yield aggregate net proceeds to such holder in connection with the transfer of such holder’s shares of New Ginkgo Class A common stock or shares of New Ginkgo Class B common stock (assuming, in each case, that such shares would be sold for value at the prevailing trading price of shares of New Ginkgo Class A common stock at the time of such transfer) sufficient to cover the aggregate amount of ordinary income, employment or similar taxes payable in connection with such equity award (as reasonably determined by New Ginkgo).
Lock-up Applicable to Other Stockholders
The Charter provides that, subject to customary exceptions and the other exceptions described in the following sentence, stockholders of the corporation other than those described in the paragraph immediately above are unable to transfer their shares of New Ginkgo Class A common stock or New Ginkgo Class B common stock received as consideration in the Merger (including upon the settlement of any equity award of New Ginkgo into which an equity award of Ginkgo was converted in the Merger), other than the Earn-out Consideration, for a period of 180 days following the closing of the Business Combination. Solely in the case of any holder of any equity award of New Ginkgo (including any such equity award into which any equity award of Old Ginkgo was converted in the Merger), the transfer restrictions described in the foregoing sentence will be lifted, beginning on the earlier of March 1, 2022 and the date that is 15 calendar days before the date on which any tax relating to such equity award will become due under applicable law (as reasonably determined by New Ginkgo), solely to the extent necessary to yield aggregate net proceeds to such holder in connection with the transfer of such holder’s shares of New Ginkgo Class A common stock or shares of New Ginkgo Class B common stock (assuming, in each case, that such shares would be sold for value at the prevailing trading price of shares of New Ginkgo Class A common stock at the time of such transfer) sufficient to cover the aggregate amount of ordinary income, employment or similar taxes payable in connection with such equity awards (as reasonably determined by New Ginkgo).
Conversion
Optional Conversion
Holders of New Ginkgo Class B common stock have the right to convert shares of their New Ginkgo Class B common stock into fully paid and non assessable shares of New Ginkgo Class A common stock, on a one-to-one basis, at the option of the holder at any time upon written notice to New Ginkgo’s transfer agent.
Automatic Conversion
Generally, shares of New Ginkgo Class B common stock will convert automatically into New Ginkgo Class A common stock upon the holder of such shares ceasing to be an Eligible Holder (whether as a result of the
154
holder’s termination, resignation or removal as a director or employee of New Ginkgo, the transfer of such shares to an individual, trust or entity that is not an Eligible Holder, a person other than a director or employee of New Ginkgo gaining any direct or indirect right to vote such shares, or otherwise), unless otherwise determined by the affirmative vote of a majority of the directors of New Ginkgo then serving who qualify as “independent” in accordance with the requirements of the securities exchange on which equity securities of New Ginkgo are then listed for trading. A determination by the secretary of the New Ginkgo that an event has occurred that triggers the automatic conversion of New Ginkgo Class B common stock into New Ginkgo Class A common stock will be conclusive and binding; however, a holder of New Ginkgo Class B common stock (or New Ginkgo Class A common stock into which New Ginkgo Class B common stock has converted) who believes in good faith that such determination is in error may appeal such determination to the New Ginkgo Board, in which case, the determination of the New Ginkgo Board (including as to whether or not to review such determination) will be conclusive and binding.
Conversion Policies and Procedures
New Ginkgo may (and expects to) establish from time to time certain restrictions, policies and procedures relating to the general administration of its multi-class stock structure and the conversion of New Ginkgo Class B common stock to New Ginkgo Class A common stock. Adoption or amendment of any such policy or procedure must be approved by the affirmative vote of a majority of New Ginkgo’s directors and, if any Class B Director is then serving, at least one Class B Director (defined below).
Registration Rights
Certain New Ginkgo stockholders are party to a registration rights agreement, which grants certain New Ginkgo stockholders the right to require, subject to certain conditions and limitations, that New Ginkgo register for resale securities held by such stockholders and certain “piggback” registration rights with respect to registrations initiated by New Ginkgo. The registration of shares of New Ginkgo Class A common stock pursuant to the exercise of the registration rights provided under the registration rights agreement would enable the applicable New Ginkgo stockholders to resell such shares without restriction under the Securities Act when the applicable registration statement is declared effective. New Ginkgo will bear the expenses incurred in connection with the filing of any registration statements pursuant to the registration rights agreement.
Other Rights
The Charter and the Bylaws do not provide for any preemptive or subscription rights with respect to the New Ginkgo common stock, and there are no redemption or sinking fund provisions applicable to the New Ginkgo common stock. All the outstanding shares of New Ginkgo common stock are validly issued, fully paid and non-assessable.
The Charter authorizes the New Ginkgo Board, to the fullest extent permitted by applicable law, to issue up to an aggregate of 200,000,000 shares of New Ginkgo preferred stock in one or more series from time to time by resolution, without further action by New Ginkgo’s stockholders, and to fix the powers (which may include full, limited or no voting power), designations, preferences and relative, participating, optional or other special rights, if any, of the shares of each such series (which rights may be greater than the rights of any or all of the classes of New Ginkgo common stock) and any qualifications, limitations or restrictions thereof. The issuance of New Ginkgo preferred stock could adversely affect the voting power of holders of New Ginkgo common stock and the likelihood that such holders will receive dividend payments or payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deterring or preventing a change of control or other corporate action. No shares of preferred stock are currently outstanding, and there is no present plan to issue any shares of preferred stock.
155
In acknowledgment of our goal of serving all of our stakeholders over the long term, the Charter provides that, in addition to any other considerations which the New Ginkgo Board, any committee thereof, or any individual director lawfully may take into account in determining whether to take or refrain from taking corporate action on any matter, including making or declining to make any recommendation to our stockholders, our board of directors, any committee thereof, or any individual director may, in his, her, or its discretion, consider the long-term as well as the short-term interests of New Ginkgo, taking into account and considering, as deemed appropriate, the effects of such action on our (a) stockholders and (b) other stakeholders, including our workforce, customers, suppliers, academic researchers, governments and communities, in the case of (b), as may be identified or revised by the New Ginkgo Board from time to time. The Charter also provides that nothing in the Charter or any other governing document, policy, or guideline adopted by us will (i) create any duty owed by any director to any person or entity to consider, or afford any particular weight to, any of the foregoing matters or to limit his or her consideration thereof or (ii) other than as vested in our stockholders to the extent provided under applicable law, be construed as creating any rights against any director or us. These constituency provisions grant discretionary authority only to the extent consistent with and permitted by law, and do not confer third-party beneficiary status on any person or entity.
Election, Appointment and Removal of Directors
Until the time at which the outstanding shares of New Ginkgo Class B common stock cease to represent at least 2% of all of the outstanding shares of New Ginkgo common stock, the holders of New Ginkgo Class B common stock, voting separately as a class, will be entitled to nominate and elect a number of directors equal to 25% (rounded up to the nearest whole number) of the total number of directors constituting the New Ginkgo Board (each such director, a “Class B Director”). All other directors of New Ginkgo will be elected by the holders of all classes of New Ginkgo common stock, voting together as a single class.
The total number of directors constituting the New Ginkgo Board will be fixed from time to time by New Ginkgo’s board of directors, but will be subject to adjustment to ensure that the total number of directors that the holders of New Ginkgo Class B common stock are entitled to nominate and elect is at least 25% of the total number of directors constituting the New Ginkgo Board.
The Charter provides that any Class B Director may be removed from office (a) with cause, only by the affirmative vote of the holders of shares representing a majority of the voting power of all of the outstanding shares of New Ginkgo Class B common stock and (b) without cause, by the affirmative vote of the holders of shares representing a majority of the voting power of all of the outstanding shares of capital stock of New Ginkgo, voting together as a single class. Any director of New Ginkgo other than a Class B Director may be removed from office, with or without cause, by the affirmative vote of the holders of shares representing a majority of the voting power of all of the outstanding shares of capital stock of New Ginkgo, voting together as a single class.
The Charter provides that vacant directorships, including vacancies resulting from any increase in the total number of directors constituting the New Ginkgo Board, may be filled only by the New Ginkgo Board. Vacancies with respect to any Class B Director may be filled only by the remaining Class B Directors.
Committees of the Board of Directors
The New Ginkgo Board has established, and will maintain, an audit committee, a nominating and corporate governance committee and a compensation committee, and may establish such other committees as it determines from time to time. For so long as any Founder serving as a director of New Ginkgo holds shares of New Ginkgo Class B common stock, such director will not be permitted to serve as a member of the compensation committee of the New Ginkgo Board. Subject to applicable requirements of the securities exchange on which equity
156
securities of New Ginkgo are then listed for trading, at any time that any Class B Director is serving as a director of New Ginkgo, each committee (other than the compensation committee) of the New Ginkgo Board must include at least one Class B Director unless a majority of the Class B Directors then serving approve the formation and composition of such committee.
Action by the New Ginkgo Board of Directors to Terminate a Founder
New Ginkgo may not terminate the employment of any Founder for cause, or materially and adversely reduce the responsibilities, title or position of such Founder for cause, without the prior written consent of such Founder, or make any determination that an event has occurred with respect to such Founder that constitutes “cause” (as that term or any similar concept may be defined or used in any agreement relating to the employment of such Founder by New Ginkgo or any of its subsidiaries or any policy of New Ginkgo or any of its subsidiaries applicable to the employment of such Founder), unless such termination, reduction or determination has been approved by at least 75% of the directors of New Ginkgo then in office.
New Ginkgo may not terminate the employment of any Founder other than for cause, or materially and adversely reduce the responsibilities, title or position of such Founder other than for cause, without the prior written consent of such Founder, unless such termination or reduction has been approved by at least 75% of the directors of New Ginkgo then in office and, if any Founder who is not the subject of the action requiring such approval is then serving as a director of New Ginkgo, at least one director of New Ginkgo who is a Founder.
Anti-Takeover Effects of the Charter and the Bylaws
The Charter and Bylaws contain certain provisions that may delay, discourage or impede efforts by another person or entity to acquire control of New Ginkgo. We believe that these provisions, which are summarized below, will discourage coercive takeover practices or inadequate takeover bids. These provisions are also designed to encourage persons or entities seeking to acquire control of us to first negotiate with the New Ginkgo Board, which we believe may result in improvement of the terms of any such acquisition in favor of New Ginkgo’s stockholders. However, these provisions also give the New Ginkgo Board the power to discourage acquisitions that some stockholders may favor.
Authorized but Unissued Capital Stock
The authorized but unissued shares of our common stock and our preferred stock will be available for future issuance without stockholder approval, subject to any limitations imposed by the listing standards of the securities exchange on which New Ginkgo’s equity securities are then listed for trading. These additional shares of capital stock may be used for a variety of corporate purposes, including growth acquisitions, corporate finance transactions, and issuances under our Equity Incentive Plan (“EIP”) and our Employee Stock Purchase Plan (“ESPP”). The existence of authorized but unissued and unreserved capital stock could discourage or impede an attempt to obtain control of New Ginkgo by means of a proxy contest, tender offer, merger, or otherwise.
Amendment of Certificate of Incorporation or Bylaws
The DGCL generally provides that the affirmative vote of a majority of the outstanding shares entitled to vote on amendments to a corporation’s certificate of incorporation or bylaws is required to approve such amendment, unless a corporation’s certificate of incorporation or bylaws, as applicable, imposes a higher voting standard.
The Charter provides that certain provisions thereof may be adopted, amended, altered or repealed only upon the affirmative vote of the holders of at least two-thirds of the voting power of all of the outstanding shares of capital stock of New Ginkgo. Such provisions include those relating to (i) stockholder action by written consent, (ii) special meetings of stockholders, (iii) the New Ginkgo Board (including the election, appointment and removal of directors), (iv) termination of the employment of any Founder, material and adverse reduction of the
157
responsibilities, title or position of any Founder without the prior written consent of such Founder, or determination that an event has occurred with respect to any Founder that constitutes “cause”, (v) limitation of the personal liability of New Ginkgo’s directors, and (vi) New Ginkgo’s waiver of the corporate opportunity doctrine.
The Charter provides that New Ginkgo’s bylaws may be adopted, amended, altered or repealed by the New Ginkgo Board or by the affirmative vote of the holders of at least two-thirds of the voting power of all of the outstanding shares of capital stock of New Ginkgo (or, if the New Ginkgo Board has recommended that stockholders approve such modification to New Ginkgo’s bylaws, the affirmative vote of a majority of the voting power of all of the outstanding shares of capital stock of New Ginkgo).
These provisions may have the effect of deterring hostile takeovers or delaying or preventing changes of control of New Ginkgo or its management such as a merger, reorganization or tender offer. These provisions are intended to enhance the likelihood of continued stability in the composition of the New Ginkgo Board and its policies and to discourage certain types of transactions that may involve an actual or threatened acquisition of New Ginkgo and to reduce New Ginkgo’s vulnerability to an unsolicited acquisition proposal. These provisions are also intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for New Ginkgo’s shares and, as a consequence, may inhibit fluctuations in the market price of the Company’s shares that could result from actual or rumored takeover attempts. Such provisions may also have the effect of preventing changes in management.
Multi-Class Structure
As described above, the Charter provides for a multi-class stock structure, which gives New Ginkgo’s directors and employees (including the Founders) and certain of their affiliated entities and trusts, for so long as they continue to collectively beneficially own shares representing a majority of the voting power of all of the outstanding shares of capital stock of New Ginkgo, significant influence over all matters requiring stockholder approval, including the election of New Ginkgo’s directors and significant corporate transactions, such as a merger or other sale of New Ginkgo or all or substantially all of its assets.
No Cumulative Voting for Directors
The DGCL provides that stockholders are not entitled to cumulate votes in the election of directors unless a corporation’s certificate of incorporation provides otherwise. The Charter does not provide for cumulative voting. As a result, the holders of shares of New Ginkgo common stock representing a majority of the voting power of all of the outstanding shares of capital stock of New Ginkgo will be able to elect all of the directors (other than the Class B Directors) then standing for election.
Vacancies on the New Ginkgo Board
The Charter authorizes only the New Ginkgo Board to fill vacant directorships, including vacancies resulting from any increase in the total number of directors constituting the New Ginkgo Board. In addition, the total number of directors constituting the New Ginkgo Board is permitted to be changed only by the New Ginkgo Board, subject to the requirement that at least 25% of the total number of New Ginkgo’s directors be Class B Directors (for so long as the outstanding shares of New Ginkgo Class B common stock continue to represent at least 2% of all the outstanding shares of New Ginkgo common stock). These provisions could prevent a stockholder from increasing the total number of New Ginkgo’s directors and then gaining control of the New Ginkgo Board.
Requirements to Terminate Employment of Founders
The Charter requires that any termination by New Ginkgo of the employment of any Founder other than for cause, or material and adverse reduction of the responsibilities, title or position of such of such Founder other
158
than for cause without the prior written consent of such Founder, be approved by at least 75% of the directors of New Ginkgo then in office and, if any Founder who is not the subject of the action requiring such approval is then serving as a director of New Ginkgo, at least one director of New Ginkgo who is a Founder. This provision, together with the right of the holders of New Ginkgo Class B common stock to nominate and elect 25% of the New Ginkgo Board, could make it more difficult for a stockholder that gains control of the New Ginkgo Board to effect changes in New Ginkgo’s management.
Special Meetings of Stockholders, Action by Written Consent, and Advance Notice Requirements for Stockholder Proposals
Special Meetings of Stockholders
The Charter permits special meetings of New Ginkgo’s stockholders to be called only by the New Ginkgo Board, the chairman of the New Ginkgo Board, New Ginkgo’s chief executive officer or president, or, at any time that the holders of New Ginkgo Class B common stock collectively beneficially own shares representing a majority of the voting power of all of the outstanding shares of capital stock of New Ginkgo, the holders of shares representing a majority of the voting power of all of the outstanding shares of capital stock of New Ginkgo. These provisions might delay the ability of New Ginkgo’s stockholders to force consideration of a proposal or to take any action, including with respect to the removal of any of New Ginkgo’s directors from office.
Stockholder Action by Written Consent
The Charter provides that New Ginkgo’s stockholders may act by written consent only if (a) the action to be taken or effected has been approved by the affirmative vote of all of the directors of New Ginkgo then serving or (b) the holders of New Ginkgo Class B common stock collectively beneficially own shares representing a majority of the voting power of all of the outstanding shares of capital stock of New Ginkgo. As a result, if the holders of New Ginkgo Class B common stock were to cease to collectively beneficially own shares representing a majority of the voting power of all of the outstanding shares of capital stock of New Ginkgo, New Ginkgo’s stockholders would not be able to take action by written consent on any matter and would only be able to take action at an annual or special meeting of stockholders, unless the New Ginkgo Board had unanimously approved the action to be taken or effected.
Advance Notice Requirement for Stockholder Proposals and Director Nominations
The Bylaws establish an advance notice procedure for stockholder proposals to be brought before an annual meeting of stockholders, including proposed nominations of candidates for election to the New Ginkgo Board. In order for any matter to be “properly brought” before a meeting (and thereby considered or acted upon at such meeting), a stockholder will have to comply with certain advance notice requirements and provide New Ginkgo with certain information. Stockholders at an annual meeting will only be permitted to consider proposals or nominations specified in the notice of meeting or brought before the meeting by or at the direction of the New Ginkgo Board or by a stockholder of record on the record date for the meeting who is entitled to vote at the meeting and has delivered a timely notice, in the form and manner specified in the Bylaws, of such stockholder’s intention to bring such business before the meeting. These provisions might preclude New Ginkgo’s stockholders from bringing matters before our annual meeting of stockholders or from nominating candidates for election to the New Ginkgo Board, or might discourage or impede an attempt by a potential acquirer of New Ginkgo to conduct a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise obtain control of New Ginkgo.
New Ginkgo has elected not to be subject to Section 203 of the DGCL. Under Section 203 of the DGCL, a corporation will not be permitted to engage in a business combination with any interested stockholder for a
159
period of three years following the time that such interested stockholder became an interested stockholder, unless:
(1) prior to such time, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder;
(2) upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (i) by persons who are directors and also officers and (ii) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
(3) at or subsequent to such time the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 662/3% of the outstanding voting stock which is not owned by the interested stockholder.
Generally, a “business combination” includes a merger, asset or stock sale or other transaction resulting in a financial benefit to the interested stockholder. Subject to certain exceptions, an “interested stockholder” is a person who, together with that person’s affiliates and associates, owns, or within the previous three years owned, 15% or more of New Ginkgo’s outstanding voting stock. For purposes of this section only, “voting stock” has the meaning given to it in Section 203 of the DGCL.
Because New Ginkgo has opted out of Section 203 of the DGCL in the Charter, Section 203 of the DGCL will not apply to New Ginkgo.
Ginkgo Warrants
At the effective time of the Merger, each warrant to purchase shares of Old Ginkgo capital stock (each, a “Old Ginkgo Warrant”) that was outstanding and unexercised immediately prior to the effective time of the Merger (after giving effect to the Company Recapitalization, pursuant to which each Old Ginkgo Warrant to purchase shares of Old Ginkgo preferred stock became a Old Ginkgo Warrant to purchase shares of Old Ginkgo Class A common stock), other than such Old Ginkgo Warrants that were automatically exercised in full in accordance with their terms by virtue of the occurrence of the Merger immediately prior to the effective time of the Merger, were assumed by New Ginkgo and converted into a warrant to purchase shares of New Ginkgo Class A common stock on the same terms and subject to the same conditions (including as to vesting and exercisability) as were in effect with respect to such Old Ginkgo Warrant immediately prior to the effective time, with appropriate adjustments to the number of shares of New Ginkgo Class A common stock underlying such warrant and the exercise price applicable thereto to account for the Merger.
SRNG Warrants
At the effective time of the Domestication, each warrant to purchase SRNG ordinary shares (each, a “SRNG Warrant”) that was issued and outstanding immediately prior to the effective time of the Domestication and not terminated pursuant to its terms was converted into a warrant to purchase shares of New Ginkgo common stock on the same terms and conditions (including as to vesting and exercisability) as were in effect with respect to such SRNG Warrant immediately prior to the effective time.
As of November 8, 2021, there are currently outstanding an aggregate of 51,824,925 warrants to acquire New Ginkgo Class A common stock, which comprise 17,325,000 Private Placement Warrants held by the Sponsor and 34,499,925 Public Warrants.
160
Public Warrants
As of November 8, 2021, there were an aggregate of 34,499,925 Public Warrants outstanding, which entitle the holder to acquire New Ginkgo Class A common stock. Each whole Public Warrant entitles the registered holder to purchase one share of New Ginkgo Class A common stock at a price of $11.50 per share, subject to adjustment as discussed below, beginning 30 days after the Closing Date, provided that New Ginkgo has an effective registration statement under the Securities Act covering the New Ginkgo Class A common stock issuable upon exercise of the Public Warrants and a current prospectus relating to such New Ginkgo Class A common stock is available (or New Ginkgo permits holder to exercise their respective warrants on a cashless basis under the circumstances specified in the warrant agreement) and such shares are registered, qualified or exempt from registration under the securities, or blue sky, laws of the state of residence of the holder. Pursuant to the warrant agreement, a holder may exercise its Public Warrants only for a whole number of shares of New Ginkgo Class A common stock. This means only a whole Public Warrant may be exercised at a given time by a warrant holder. No fractional warrants will be issued upon separation of the units and only whole warrants will trade. Accordingly, unless holder has at least five units, such holder will not be able to receive or trade a whole warrant. The Public Warrants will expire five years after the Closing Date, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
Redemption of Warrants for Cash
Once the Warrants become exercisable, New Ginkgo may call the Warrants for redemption for cash:
| • | in whole and not in part; |
| • | at a price of $0.01 per Warrant; |
| • | upon not less than 30 days’ prior written notice of redemption (the “30-day redemption period”) to each warrant holder; and |
| • | if, and only if, the reported closing price of the New Ginkgo Class A common stock equals or exceeds $18.00 per share (as adjusted for stock sub-divisions, stock capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending three business days before New Ginkgo sends the notice of redemption to the warrant holders. |
If and when the Warrants become redeemable by New Ginkgo, New Ginkgo may exercise its redemption right even if New Ginkgo is unable to register or qualify the underlying securities for sale under all applicable state securities laws.
New Ginkgo has established the last of the redemption criterion discussed above to prevent a redemption call unless there is at the time of the call a significant premium to the warrant exercise price. If the foregoing conditions are satisfied and New Ginkgo issues a notice of redemption of the Warrants, each warrant holder will be entitled to exercise his, her or its Warrant prior to the scheduled redemption date. However, the price of the New Ginkgo Class A common stock may fall below the $18.00 redemption trigger price (as adjusted for stock sub-divisions, stock capitalizations, reorganizations, recapitalizations and the like) as well as the $11.50 warrant exercise price after the redemption notice is issued.
Redemption Procedures and Cashless Exercise
If New Ginkgo calls the Warrants for redemption as described above, New Ginkgo’s management will have the option to require any holder that wishes to exercise his, her or its Warrant to do so on a “cashless basis.” In determining whether to require all holders to exercise their Warrants on a “cashless basis,” New Ginkgo’s management will consider, among other factors, New Ginkgo’s cash position, the number of Warrants that are outstanding and the dilutive effect on New Ginkgo’s stockholders of issuing the maximum number of shares of New Ginkgo Class A common stock issuable upon the exercise of its Warrants. If New Ginkgo management takes advantage of this option, all holders of Warrants would pay the exercise price by surrendering their
161
Warrants for that number of shares of New Ginkgo Class A common stock equal to the quotient obtained by dividing (x) the product of the number of New Ginkgo Class A common stock underlying the warrants, multiplied by the excess of the “fair market value” of the New Ginkgo Class A common stock over the exercise price of the warrants by (y) the fair market value. The “fair market value” will mean the average reported closing price of the New Ginkgo Class A common stock for the 10 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of warrants. If the New Ginkgo management takes advantage of this option, the notice of redemption will contain the information necessary to calculate the number of shares of New Ginkgo Class A common stock to be received upon exercise of the warrants, including the “fair market value” in such case. Requiring a cashless exercise in this manner will reduce the number of shares to be issued and thereby lessen the dilutive effect of a warrant redemption.
New Ginkgo believes this feature is an attractive option to New Ginkgo if New Ginkgo does not need the cash from the exercise of the Warrants. If New Ginkgo calls the Warrants for redemption and New Ginkgo’s management does not take advantage of this option, the holders of the Private Placement Warrants and their permitted transferees would still be entitled to exercise their Private Placement Warrants or on a cashless basis using the same formula described above that other warrant holders would have been required to use had all warrant holders been required to exercise their Warrants on a cashless basis, as described in more detail below.
A holder of a Warrant may notify New Ginkgo in writing in the event it elects to be subject to a requirement that such holder will not have the right to exercise such warrant, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates), to the warrant agent’s actual knowledge, would beneficially own in excess of 4.9% or 9.8% (as specified by the holder) of the New Ginkgo Class A common stock outstanding immediately after giving effect to such exercise.
If the number of outstanding shares of New Ginkgo Class A common stock is increased by a share capitalization payable in shares of New Ginkgo Class A common stock, or by a split-up of common stock or other similar event, then, on the effective date of such share capitalization, split-up or similar event, the number of shares of New Ginkgo Class A common stock issuable on exercise of each Warrant will be increased in proportion to such increase in the outstanding shares of New Ginkgo common stock. A rights offering to holders of common stock entitling holders to purchase New Ginkgo Class A common stock at a price less than the fair market value will be deemed a share capitalization of a number of shares of New Ginkgo Class A common stock equal to the product of (i) the number of shares of New Ginkgo Class A common stock actually sold in such rights offering (or issuable under any other equity securities sold in such rights offering that are convertible into or exercisable for New Ginkgo Class A common stock) and (ii) the quotient of (x) the price per share of New Ginkgo Class A common stock paid in such rights offering and (y) the fair market value. For these purposes (i) if the rights offering is for securities convertible into or exercisable for shares of New Ginkgo Class A common stock, in determining the price payable for New Ginkgo Class A common stock, there will be taken into account any consideration received for such rights, as well as any additional amount payable upon exercise or conversion and (ii) fair market value means the volume weighted average price of shares of New Ginkgo Class A common stock as reported during the 10 trading day period ending on the trading day prior to the first date on which the New Ginkgo Class A common stock trades on the applicable exchange or in the applicable market, regular way, without the right to receive such rights.
In addition, if New Ginkgo, at any time while the Warrants are outstanding and unexpired, pays a dividend or make a distribution in cash, securities or other assets to the holders of New Ginkgo Class A common stock on account of such New Ginkgo Class A common stock (or other securities into which the warrants are convertible), other than (a) as described above or (b) certain ordinary cash dividends, then the warrant exercise price will be decreased, effective immediately after the effective date of such event, by the amount of cash and/or the fair market value of any securities or other assets paid on each share of New Ginkgo Class A common stock in respect of such event.
If the number of outstanding shares of New Ginkgo Class A common stock is decreased by a consolidation, combination or reclassification of New Ginkgo Class A common stock or other similar event, then, on the
162
effective date of such consolidation, combination, reverse share split, reclassification or similar event, the number of shares of New Ginkgo Class A common stock issuable on exercise of each Warrant will be decreased in proportion to such decrease in outstanding share of New Ginkgo Class A common stock.
Whenever the number of shares of New Ginkgo Class A common stock purchasable upon the exercise of the warrants is adjusted, as described above, the warrant exercise price will be adjusted by multiplying the Warrant exercise price immediately prior to such adjustment by a fraction (x) the numerator of which will be the number of shares of New Ginkgo Class A common stock purchasable upon the exercise of the Warrants immediately prior to such adjustment, and (y) the denominator of which will be the number of shares of New Ginkgo Class A common stock so purchasable immediately thereafter.
In case of any reclassification or reorganization of the outstanding New Ginkgo Class A common stock (other than those described above or that solely affects the par value of such New Ginkgo Class A common stock), or in the case of any merger or consolidation of New Ginkgo with or into another corporation (other than a consolidation or merger in which New Ginkgo is the continuing corporation and that does not result in any reclassification or reorganization of the issued and outstanding New Ginkgo Class A common stock), or in the case of any sale or conveyance to another corporation or entity of the assets or other property of New Ginkgo as an entirety or substantially as an entirety in connection with which New Ginkgo is dissolved, the holders of the Warrants will thereafter have the right to purchase and receive, upon the basis and upon the terms and conditions specified in the Warrants and in lieu of the New Ginkgo Class A common stock immediately theretofore purchasable and receivable upon the exercise of the rights represented thereby, the kind and amount of shares of New Ginkgo Class A common stock or other securities or property (including cash) receivable upon such reclassification, reorganization, merger or consolidation, or upon a dissolution following any such sale or transfer, that the holder of the Warrants would have received if such holder had exercised their Warrants immediately prior to such event. If less than 70% of the consideration receivable by the holders of New Ginkgo Class A common stock in such a transaction is payable in the form of New Ginkgo Class A common stock in the successor entity that is listed for trading on a national securities exchange or is quoted in an established over-the-counter market, or is to be so listed for trading or quoted immediately following such event, and if the registered holder of the Warrant properly exercises the Warrant within thirty days following public disclosure of such transaction, the Warrant exercise price will be reduced as specified in the warrant agreement based on the Black-Scholes Warrant Value (as defined in the warrant agreement) of the Warrant. The purpose of such exercise price reduction is to provide additional value to holders of the Warrants when an extraordinary transaction occurs during the exercise period of the Warrants pursuant to which the holders of the Warrants otherwise do not receive the full potential value of the Warrants.
The Warrants were issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as warrant agent, and New Ginkgo. The warrant agreement provides that the terms of the Warrants may be amended without the consent of any holder for the purpose of (i) curing any ambiguity or correct any defective provision, or mistake, including to conform the provisions of the warrant agreement to the description of the terms of the Warrants and the warrant agreement set forth herein, (ii) adjusting the provisions relating to cash dividends on New Ginkgo Class A common stock as contemplated by and in accordance with the warrant agreement or (iii) adding or changing any provisions with respect to matters or questions arising under the warrant agreement as the parties to the warrant agreement may deem necessary or desirable and that the parties deem to not adversely affect the rights of the registered holders of the Warrants, provided that the approval by the holders of at least 50% of the then outstanding Public Warrants is required to make any change that adversely affects the interests of the registered holders of Public warrants, and, solely with respect to any amendment to the terms of the Private Placement Warrants, a majority of the then outstanding Private Placement Warrants. You should review a copy of the warrant agreement, which will be filed as an exhibit to the registration statement of which this prospectus is a part, for a complete description of the terms and conditions applicable to the Warrants.
The Warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price (or on a cashless basis, if applicable),
163
by certified or official bank check payable to New Ginkgo, for the number of Warrants being exercised. The Warrant holders do not have the rights or privileges of holders of common stock and any voting rights until they exercise their Warrants and receive New Ginkgo Class A common stock. After the issuance of New Ginkgo Class A common stock upon exercise of the Warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
Private Placement Warrants
The Private Placement Warrants (including the New Ginkgo Class A common stock issuable upon exercise of the Private Placement Warrants) will not be transferable, assignable or salable until thirty (30) days after the Closing Date (except in limited circumstances) and they will not be redeemable by New Ginkgo for cash so long as they are held by the Sponsor, members of the Sponsor or their permitted transferees.
The initial purchasers of the Private Placement Warrants, or their permitted transferees, have the option to exercise the Private Placement Warrants on a cashless basis. Except as described in this section, the Private Placement Warrants have terms and provisions that are identical to those of the Public Warrants sold in the IPO, including that they may be redeemed for shares of New Ginkgo Class A common stock. If the Private Placement Warrants are held by holders other than the Sponsor or their permitted transferees, the Private Placement Warrants will be redeemable by New Ginkgo and exercisable by the holders on the same basis as the Public Warrants included in the units that were sold in the IPO.
The Bylaws provide that, unless New Ginkgo otherwise consents in writing, the Court of Chancery (the “Chancery Court”) of the State of Delaware (or, in the event that the Chancery Court does not have subject matter jurisdiction, another state or federal court located within the State of Delaware) will, to the fullest extent permitted by law, be the sole and exclusive forum for resolution of (a) any derivative action or proceeding brought on behalf of New Ginkgo, (b) any action asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, employee, agent or stockholder of New Ginkgo to New Ginkgo or any of New Ginkgo’s stockholders, or any claim for aiding and abetting such an alleged breach, (c) any action governed by the “internal affairs doctrine” or arising pursuant to any provision of New Ginkgo’s Charter or Bylaws, or to interpret, apply, enforce or determine the validity of New Ginkgo’s Charter or Bylaws, or (d) any action asserting a claim against New Ginkgo or any current or former director, officer, employee, agent or stockholder of New Ginkgo (i) arising pursuant to any provision of the DGCL or (ii) as to which the DGCL confers jurisdiction on the Chancery Court. The foregoing will not apply, however, to any action, claim or proceeding as to which the Chancery Court (or, if applicable, another state or federal court located within the State of Delaware) determines that there is an indispensable party not subject to the jurisdiction of such court (and the indispensable party does not consent to the personal jurisdiction of the such court within ten (10) days following such determination).
Notwithstanding the foregoing, unless New Ginkgo otherwise consents in writing, the federal district courts of the United States will be the exclusive forum for the resolution of any action, claim or proceeding arising under the Securities Act of 1933, as amended.
Limitations on Liability and Indemnification of Officers and Directors
The DGCL authorizes corporations to limit or eliminate the personal liability of directors and stockholders of corporations for monetary damages for breaches of directors’ fiduciary duties, subject to certain exceptions. The Charter includes a provision that eliminates, to the fullest extent permitted by the DGCL (as currently in effect or as it may in the future be amended), the personal liability of New Ginkgo’s directors for damages for any breach of fiduciary duty as a director.
The Bylaws provide that, to the fullest extent permitted by the DGCL (as currently in effect or as it may in the future be amended), New Ginkgo must indemnify and hold harmless and advance expenses to any of its directors
164
and officers who is involved in any action, suit or proceeding by reason of the fact that he or she is or was a director or officer of New Ginkgo or, while serving as a director or officer of New Ginkgo, is or was serving at the request of New Ginkgo as a director, officer, employee or agent of another corporation or of a partnership, joint venture, trust, enterprise or non-profit entity. New Ginkgo also is expressly authorized to carry directors’ and officers’ liability insurance providing indemnification for New Ginkgo’s directors, officers, and certain employees for some liabilities. New Ginkgo believes that these indemnification and advancement provisions and insurance are useful to attract and retain qualified directors and executive officers.
The limitation of liability, advancement and indemnification provisions in the Charter and the Bylaws may discourage stockholders from bringing lawsuits against New Ginkgo’s directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against New Ginkgo’s directors and officers, even though such an action, if successful, might otherwise benefit New Ginkgo and its stockholders. In addition, your investment in New Ginkgo may be adversely affected to the extent that New Ginkgo pays the costs of settlement and damage awards against directors and officer pursuant to these indemnification provisions.
There is currently no pending material litigation or proceeding involving any of New Ginkgo’s directors,
officers, or employees for which indemnification is sought.
The Charter provides for the renouncement by New Ginkgo of any interest or expectancy of New Ginkgo in, or being offered an opportunity to participate, in any matter, transaction, or interest that is presented to, or acquired, created, or developed by, or which otherwise comes into the possession of, any director of New Ginkgo who is not an employee of New Ginkgo or any of its subsidiaries, unless such matter, transaction, or interest is presented to, or acquired, created, or developed by, or otherwise comes into the possession of, that director first in that director’s capacity as a director of New Ginkgo.
Dissenters’ Rights of Appraisal and Payment
Under the DGCL, with certain exceptions, New Ginkgo’s stockholders will have appraisal rights in connection with a merger or consolidation of New Ginkgo. Pursuant to the DGCL, stockholders who properly demand and perfect appraisal rights in connection with such merger or consolidation will have the right to receive payment of the fair value of their shares as determined by the Delaware Court of Chancery.
Stockholders’ Derivative Actions
Under the DGCL, any of New Ginkgo’s stockholders may bring an action in New Ginkgo’s name to procure a judgment in New Ginkgo’s favor, also known as a derivative action, provided that the stockholder bringing the action is a holder of New Ginkgo’s shares at the time of the transaction to which the action relates or such stockholder’s stock thereafter devolved by operation of law.
Transfer Agent and Warrant Agent
Computershare Trust Company, N.A. is the transfer agent for New Ginkgo Class A Common Stock and the warrant agent for the Warrants.
Listing of New Ginkgo Class A Common Stock and New Ginkgo Warrants
The New Ginkgo Class A common stock and the Public Warrants are listed on the NYSE under the symbols “DNA” and “DNA.WS,” respectively.
165
SECURITIES ACT RESTRICTIONS ON RESALE OF SECURITIES
Pursuant to Rule 144 under the Securities Act (“Rule 144”), a person who has beneficially owned restricted Class A common stock or Warrants for at least six months would be entitled to sell their securities provided that (i) such person is not deemed to have been an affiliate of New Ginkgo at the time of, or at any time during the three months preceding, a sale and (ii) New Ginkgo is subject to the Exchange Act periodic reporting requirements for at least three months before the sale and has filed all required reports under Section 13 or 15(d) of the Exchange Act during the 12 months (or such shorter period as it was required to file reports) preceding the sale.
Persons who have beneficially owned restricted Class A common stock or Warrants for at least six months but who are affiliates of New Ginkgo at the time of, or at any time during the three months preceding, a sale would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of:
| • | 1% of the total number of shares of New Ginkgo Class A common stock then outstanding; or |
| • | the average weekly reported trading volume of New Ginkgo’s Class A common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale. |
Sales by affiliates of New Ginkgo under Rule 144 are also limited by manner of sale provisions and notice requirements and by the availability of current public information about New Ginkgo.
Restrictions on the Use of Rule 144 by Shell Companies or Former Shell Companies
Rule 144 is not available for the resale of securities initially issued by shell companies (other than business-combination related shell companies) or issuers that have been at any time previously a shell company. However, Rule 144 also includes an important exception to this prohibition if the following conditions are met:
| • | the issuer of the securities that was formerly a shell company has ceased to be a shell company; |
| • | the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act; |
| • | the issuer of the securities has filed all Exchange Act reports and material required to be filed, as applicable, during the preceding 12 months (or such shorter period that the issuer was required to file such reports and materials) other than Form 8-K reports; and |
| • | at least one year has elapsed from the time that the issuer filed current Form 10-type information with the SEC reflecting its status as an entity that is not a shell company. |
As a result, SRNG’s initial shareholders will be able to sell their founder shares and Private Placement Warrants, as applicable, pursuant to Rule 144 without registration one year after the Closing Date.
New Ginkgo is no longer a shell company, and so, once the conditions listed above are satisfied, Rule 144 will become available for the resale of the above-noted restricted securities.
166
BENEFICIAL OWNERSHIP OF SECURITIES
The following table sets forth information known to the Company regarding the beneficial ownership of New Ginkgo common stock as of November 8, 2021 by:
| • | each person who is a named executive officer or director of New Ginkgo; |
| • | all executive officers and directors of New Ginkgo as a group; and |
| • | each person who is a beneficial owner of more than 5% of New Ginkgo Class A common stock or New Ginkgo Class B common stock. |
Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power over that security, including options and warrants that are currently exercisable or exercisable within 60 days. Unless otherwise indicated, New Ginkgo believes that all persons named in the table below have sole voting and investment power with respect to the voting securities beneficially owned by them.
The beneficial ownership of New Ginkgo common stock is based on 1,310,783,159 shares of New Ginkgo Class A common stock and 361,857,141 shares of New Ginkgo Class B common stock issued and outstanding as of November 8, 2021.
| New Ginkgo Class A common stock |
New Ginkgo Class B common stock |
% of Total Voting Power** |
||||||||||||||||||
| Name and Address of Beneficial Owner |
Shares | % | Shares | % | ||||||||||||||||
| Directors and Executive Officers of New Ginkgo |
| |||||||||||||||||||
| Jason Kelly(1) |
— | — | 82,581,106 | 22.8 | 16.8 | |||||||||||||||
| Reshma Shetty(2) |
— | — | 166,041,730 | 45.9 | 33.7 | |||||||||||||||
| Mark Dmytruk |
— | — | 314,097 | * | — | |||||||||||||||
| Arie Belldegrun |
— | — | — | — | — | |||||||||||||||
| Marijn Dekkers(3) |
6,155,324 | * | — | — | * | |||||||||||||||
| Christian Henry |
234,548 | * | — | — | * | |||||||||||||||
| Reshma Kewalramani |
— | — | — | — | — | |||||||||||||||
| Shyam Sankar |
234,548 | * | — | — | * | |||||||||||||||
| Harry E. Sloan |
— | — | — | — | — | |||||||||||||||
| All Directors and Executive Officers of New Ginkgo as a Group (9 Individuals) |
6,624,420 | * | 248,936,933 | 68.8 | 50.6 | |||||||||||||||
| 5% Beneficial Owners of New Ginkgo |
|
|||||||||||||||||||
| Entities affiliated with Anchorage Capital Group(4) |
74,929,312 | 5.7 | — | — | 1.5 | |||||||||||||||
| Bartholomew Canton(5) |
— | — | 166,041,730 | 45.9 | 33.7 | |||||||||||||||
| Austin Che(6) |
— | — | 83,020,866 | 22.7 | 16.7 | |||||||||||||||
| Entities affiliated with Baillie Gifford & Co.(7) |
89,497,288 | 6.8 | — | — | 1.8 | |||||||||||||||
| Cascade Investment, L.L.C. (8) |
|
151,865,481 |
|
11.6 | — | — | 3.1 | |||||||||||||
| Eagle Equity Partners III, LLC(9) |
48,915,948 | 3.7 | — | — | 1.1 | |||||||||||||||
| General Atlantic (GK), L.P.(10) |
114,886,852 | 8.8 | — | — | 2.3 | |||||||||||||||
| Thomas Knight(11) |
65,963,933 | 5.0 | 9,219,119 | 2.5 | 3.2 | |||||||||||||||
| Senator Global Opportunity Master Fund LP(12) |
80,153,273 | 6.1 | — | — | 1.6 | |||||||||||||||
| Viking Global Opportunities Illiquid Investments Sub-Master LP(13) |
339,055,144 | 25.9 | — | — | 6.9 | |||||||||||||||
| * | Less than one percent. |
| ** | Percentage of total voting power represents voting power with respect to all shares of New Ginkgo Class A common stock and New Ginkgo Class B common stock, as a single class. Each share of New Ginkgo Class B common stock is entitled to 10 votes per share and each share of New Ginkgo Class A common stock is entitled to one vote per share. For more information about the voting rights of New Ginkgo common stock, see “Description of Capital Stock.” |
| (1) | Consists of (a) 70,853,049 shares of New Ginkgo Class B common stock held by Dr. Kelly and (b) 11,728,057 shares of New Ginkgo Class B common stock held by The Kelly 2016 Grantor Retained Annuity Trust, over which Dr. Kelly has sole voting and dispositive power. |
167
| (2) | Consists of (a) 3,093,797 shares of New Ginkgo Class B common stock held by Dr. Shetty, (b) 70,389,783 shares of New Ginkgo Class B common stock held by The Reshma Padmini Shetty Revocable Living Trust – 2014, over which Dr. Shetty has sole voting and dispositive power, (c) 8,245,491 shares of New Ginkgo Class B common stock held by The Reshma Padmini Shetty 2021 Grantor Retained Annuity Trust, over which Dr. Shetty has sole voting and dispositive power, (d) 1,291,794 shares of New Ginkgo Class B common stock held by a family trust, and (e) 79,927,068 shares of New Ginkgo Class B common stock beneficially owned by Dr. Shetty’s spouse, as reported in footnote (5) below. The voting and dispositive power over the shares held by the family trust are held by three or more individuals acting by majority approval and therefore none of the individuals is deemed a beneficial owner of the shares held by such trust. |
| (3) | Consists of (a) 374,960 shares of New Ginkgo Class A common stock held by Mr. Dekkers and (b) 5,780,364 shares of New Ginkgo Class A common stock held by Novalis LifeSciences Investments I, L.P. (“Novalis LifeSciences”). Mr. Dekkers, the Manager of the general partner of Novalis LifeSciences, has sole voting and dispositive power over the shares held by Novalis LifeSciences and, as a result, may be deemed to share beneficial ownership of the shares held by Novalis LifeSciences. The address for this stockholder is 1 Liberty Lane, Suite 100, Hampton, NH 03842. |
| (4) | Consists of (a) 37,464,656 shares of New Ginkgo Class A common stock held by Anchorage Illiquid Opportunities Master VI (A), L.P. and (b) 37,464,656 shares of New Ginkgo Class A common stock held by Anchorage Illiquid Opportunities Offshore Master V, L.P. Anchorage Advisors Management, L.L.C. is the sole managing member of Anchorage Capital Group, L.L.C. (“Anchorage”), which in turn is the investment manager of AIOM VI and AIOM V. Mr. Kevin Ulrich is the Chief Executive Officer of Anchorage and the senior managing member of Anchorage Advisors Management, L.L.C. As such, each of the foregoing persons may be deemed to have voting and dispositive power over the shares held by AIOM VI and AIOM V. Each of the foregoing persons disclaims beneficial ownership of the shares held by AIOM VI and AIOM V, except of any pecuniary interests therein. The address for these stockholders is 610 Broadway, 6th Floor, New York, NY 10012. |
| (5) | Consists of (a) 3,093,797 shares of New Ginkgo Class B common stock held by Dr. Canton, (b) 70,389,783 shares of New Ginkgo Class B common stock held by The Bartholomew Canton Revocable Living Trust – 2014, over which Dr. Canton has sole voting and dispositive power, (c) 8,245,491 shares of New Ginkgo Class B common stock held by The Bartholomew Canton 2021 Grantor Retained Annuity Trust, over which Dr. Canton has sole voting and dispositive power, (d) 1,291,794 shares of New Ginkgo Class B common stock held by a family trust, and (e) 79,927,068 shares of New Ginkgo Class B common stock held by Dr. Canton’s spouse as reported in footnote (2) above. The voting and dispositive power over the shares held by the family trust are held by three or more individuals acting by majority approval and therefore none of the individuals is deemed a beneficial owner of the shares held by such trust. |
| (6) | Consists of (a) 3,093,797 shares of New Ginkgo Class B common stock held by Dr. Che, (b) 79,027,069 shares of New Ginkgo Class B common stock held by Austin Che Revocable Trust, over which Dr. Che has sole voting and dispositive power and (c) 900,000 shares of New Ginkgo Class B common stock held by a family trust. |
| (7) | Consists of (a) 2,581,527 shares of New Ginkgo Class A common stock held by Baillie Gifford US Growth Trust PLC (“USGrowth”) and (b) 86,915,761 shares of New Ginkgo Class A common stock held by Scottish Mortgage Investment Trust PLC. (“SMIT”) As agent for each of USGrowth and SMIT, Baillie Gifford & Co. may be deemed to share the power to direct the disposition and vote of, and therefore to own the shares held by USGrowth and SMIT. Baillie Gifford & Co. disclaims beneficial ownership of all shares held by USGrowth and SMIT. Each of USGrowth and SMIT are publicly traded companies. The address for these stockholders is c/o Baillie Gifford & Co, 1 Greenside Row, Edinburgh EH 1 3 AN, United Kingdom. |
| (8) | Consists of shares of New Ginkgo Class A common stock. All shares of New Ginkgo Class A common stock to be held by Cascade Investment, L.L.C. following the Closing may be deemed to be beneficially owned by William H. Gates III as the sole member of Cascade, L.L.C. The address for this stockholder is 2365 Carillon Point, Kirkland, WA 98033. |
| (9) | Consists of (i) 31,590,948 shares of New Ginkgo Class A common stock and (ii) 17,325,000 New Ginkgo warrants. There are three managing members of Eagle Equity Partners III, LLC. Each managing member has one vote, and the approval of a majority is required to approve an action. Under the so-called “rule of three,” if voting and dispositive decisions regarding an entity’s securities are made by three or more individuals, and voting or dispositive decisions require the approval of a majority of those individuals, then none of the individuals is deemed a beneficial owner of the entity’s securities. Based on the foregoing, no individual managing member of Eagle Equity Partners III, LLC exercises voting or dispositive control over any of the securities held by the entity, even those in which he holds a pecuniary interest. Accordingly, none of them will be deemed to have or share beneficial ownership of such shares. |
| (10) | Consists of shares of New Ginkgo Class A common stock. The limited partners that share beneficial ownership of the shares held by General Atlantic (GK), L.P. (“GA GK”) are the following General Atlantic investment funds (the “GA Funds”): General Atlantic Partners 100, L.P. (“GAP 100”), General Atlantic Partners (Bermuda) EU, L.P. (“GAP Bermuda EU”), GAP Coinvestments III, LLC (“GAPCO III”), GAP Coinvestments IV, LLC (“GAPCO IV”), GAP Coinvestments V, LLC (“GAPCO V”) and GAP Coinvestments CDA, L.P. (“GAPCO CDA”). The general partner of GA GK is General Atlantic (SPV) GP, LLC (“GA SPV”). The general partner of GAP 100 is ultimately controlled by General Atlantic, L.P. (“GA LP”), which is controlled by the Management Committee of GASC MGP, LLC (the “Management Committee”). The general partner of GAP Bermuda EU is ultimately controlled by GAP (Bermuda) L.P. (“GAP Bermuda”), which is also controlled by the Management Committee. GA LP is the managing member of GAPCO III, GAPCO IV and GAPCO V, the general partner of GAPCO CDA and is the sole member of GA SPV. There are nine members of the Management Committee. GA GK, GA LP, GASC MGP, LLC, GAP Bermuda, GA SPV and the GA Funds (collectively, the “GA Group”) are a “group” within the meaning of Rule 13d-5 of the Securities Exchange Act of 1934, as amended. The mailing address of the foregoing General Atlantic entities, other than GAP Bermuda EU and GAP Bermuda, is c/o General Atlantic Service Company, L.P., 55 East 52nd Street, 33rd Floor, New York, NY 10055. The mailing address of GAP Bermuda EU and GAP Bermuda is Clarendon House, 2 Church Street, Hamilton HM 11, Bermuda. Each of the members of the Management Committee disclaims ownership of the shares except to the extent that he has a pecuniary interest therein. |
168
| (11) | Consists of (a) 9,219,119 shares of New Ginkgo Class B common stock held by Mr. Knight; (b) 6,995,255 shares of New Ginkgo Class A common stock held The Knight Family Trust dated August 20, 2019, of which Peter P. Brown and Francis Y. Knight are trustees and have shared voting and dispositive power; (c) 8,992,533 shares of New Ginkgo Class A common stock held The Thomas F. Knight Jr. Grantor Retained Annuity Trust (2) dated December 16, 2020, of which Peter P. Brown and Mr. Knight are trustees and have shared voting and dispositive power; and (d) 49,976,145 shares of New Ginkgo Class A common stock held The Thomas F. Knight Jr. Grantor Retained Annuity Trust, of which Peter P. Brown and Mr. Knight are trustees and have shared voting and dispositive power. |
| (12) | Consists of shares of New Ginkgo Class A common stock. The address for this stockholder is 510 Madison Avenue, 28th Floor, New York, NY 10022. Senator Investment Group LP (“Senator”), is investment manager of the stockholder, Senator Global Opportunity Master Fund LP, and may be deemed to have voting and dispositive power with respect to the shares. The general partner of Senator is Senator Management LLC (the “Senator GP”). Douglas Silverman controls Senator GP, and, accordingly, may be deemed to have voting and dispositive power with respect to the shares held by this stockholder. Mr. Silverman disclaims beneficial ownership of the shares held by the stockholder. |
| (13) | Consists of shares of New Ginkgo Class A common stock. Viking Global Opportunities Illiquid Investments Sub-Master LP (the “Opportunities Fund”) has the authority to dispose of and vote the New Ginkgo Class A common stock that will be directly owned by it, which power may be exercised by its general partner, Viking Global Opportunities Portfolio GP LLC (“Opportunities GP”), and by Viking Global Investors LP (“VGI”), which provides managerial services to Opportunities Fund. O. Andreas Halvorsen, David C. Ott and Rose Shabet, as Executive Committee members of Viking Global Partners LLC (the general partner of VGI) and Viking Global Opportunities GP LLC, the sole member of Opportunities GP, have shared authority to direct the voting and disposition of investments beneficially owned by VGI, Opportunities GP and the Opportunities Fund. The address for each of the entities is c/o Viking Global Investors LP, is 55 Railroad Avenue, Greenwich, CT 06830. |
169
This prospectus relates to the possible resale by the Selling Securityholders of up to 101,671,092 shares of our New Ginkgo Class A common stock, including 17,325,000 shares of our New Ginkgo Class A common stock issuable upon the exercise of the Private Placement Warrants, and up to 17,325,000 Private Placement Warrants. This prospectus also relates to the issuance by us of up to 51,824,925 shares of New Ginkgo Class A common stock upon the exercise of outstanding Warrants. The Selling Securityholders may from time to time offer and sell any or all of the shares of New Ginkgo Class A common stock and Private Placement Warrants set forth below pursuant to this prospectus and any accompanying prospectus supplement. When we refer to the “Selling Securityholders” in this prospectus, we mean the persons listed in the table below, and the pledgees, donees, transferees, assignees, successors, designees and others who later come to hold any of the Selling Securityholders’ interest in the New Ginkgo Class A common stock or Private Placement Warrants other than through a public sale.
The following table sets forth, as of the date of this prospectus, the names of the Selling Securityholders, and the aggregate number of shares of New Ginkgo Class A common stock, including shares of New Ginkgo Class A common stock issuable upon the exercise of the Private Placement Warrants, and Private Placement Warrants that the Selling Securityholders may offer pursuant to this prospectus. The table does not include the issuance by us of up to 51,824,925 shares of Class A common stock upon the exercise of outstanding Warrants, each of which is also covered by this prospectus. For purposes of this table, we have assumed that the Selling Securityholders will have sold all of the securities covered by this prospectus upon the completion of the offering.
| Before the Offering | After the Offering | |||||||||||||||||||||||
| Shares of New Ginkgo Class A Common Stock Beneficially Owned |
Private Placement Warrants Beneficially Owned |
Shares of New Ginkgo Class A Common Stock Beneficially Owned |
Private Placement Warrants Beneficially Owned |
|||||||||||||||||||||
| Shares | % | Shares | % | |||||||||||||||||||||
| Eagle Equity Partners III, LLC(1) |
48,915,948 | 3.7 | 17,325,000 | — | — | — | ||||||||||||||||||
| Viking Global Opportunities Illiquid Investments Sub-Master LP(2) |
339,055,144 | 25.9 | — | 286,300,000 | 21.8 | — | ||||||||||||||||||
| (1) | Includes (1) 31,590,948 shares of New Ginkgo Class A common stock (including 16,737,183 Earn-Out Shares) and (2) 17,325,000 shares of New Ginkgo Class A common stock issuable upon the exercise of the Private Placement Warrants. There are three managing members of Eagle Equity Partners III, LLC. Each managing member has one vote, and the approval of a majority is required to approve an action. Under the so-called “rule of three,” if voting and dispositive decisions regarding an entity’s securities are made by three or more individuals, and voting or dispositive decisions require the approval of a majority of those individuals, then none of the individuals is deemed a beneficial owner of the entity’s securities. Based on the foregoing, no individual managing member of Eagle Equity Partners III, LLC exercises voting or dispositive control over any of the securities held by the entity, even those in which he holds a pecuniary interest. Accordingly, none of them will be deemed to have or share beneficial ownership of such shares. |
| (2) | Includes (1) 337,055,144 shares of New Ginkgo Class A common stock (including 36,112,170 Earn-Out Shares) and (2) 2,000,000 shares of New Ginkgo Class A common stock issued in connection with the PIPE Investment, the resale of which is not covered by this prospectus. Viking Global Opportunities Illiquid Investments Sub-Master LP (the “Opportunities Fund”) has the authority to dispose of and vote the New Ginkgo Class A common stock that will be directly owned by it, which power may be exercised by its general partner, Viking Global Opportunities Portfolio GP LLC (“Opportunities GP”), and by Viking Global Investors LP (“VGI”), which provides managerial services to Opportunities Fund. O. Andreas Halvorsen, David C. Ott and Rose Shabet, as Executive Committee members of Viking Global Partners LLC (the |
170
| general partner of VGI) and Viking Global Opportunities GP LLC, the sole member of Opportunities GP, have shared authority to direct the voting and disposition of investments beneficially owned by VGI, Opportunities GP and the Opportunities Fund. The address for each of the entities is c/o Viking Global Investors LP, is 55 Railroad Avenue, Greenwich, CT 06830. |
Selling Securityholder information for each additional Selling Securityholder, if any, will be set forth by prospectus supplement to the extent required prior to the time of any offer or sale of such Selling Securityholder’s shares of New Ginkgo Class A common stock or Private Placement Warrants pursuant to this prospectus. To the extent permitted by law, a prospectus supplement may add, update, substitute, or change the information contained in this prospectus, including the identity of each Selling Securityholder and the number of shares of New Ginkgo Class A common stock or Private Placement Warrants registered on its behalf. A Selling Securityholder may sell or otherwise transfer all, some or none of such shares of New Ginkgo Class A common stock and Private Placement Warrants in this offering. See “Plan of Distribution.”
For information regarding transactions between us and the Selling Securityholders, see the section entitled “Certain Relationships and Related Person Transactions.”
171
Board of Directors and Management
The following is a list of New Ginkgo’s directors and executive officers and their ages as of November 8, 2021.
| Name | Age |
Position | ||
| Jason Kelly | 40 | Chief Executive Officer and Founder | ||
| Reshma Shetty | 41 | President, Chief Operating Officer and Founder | ||
| Mark Dmytruk | 50 | Chief Financial Officer | ||
| Arie Belldegrun | 71 | Director | ||
| Marijn Dekkers | 64 | Director | ||
| Christian Henry | 53 | Director | ||
| Reshma Kewalramani | 49 | Director | ||
| Shyam Sankar | 39 | Director | ||
| Harry E. Sloan | 71 | Director |
Jason Kelly, one of our Founders, is the Chief Executive Officer and a member of New Ginkgo’s board of directors. Dr. Kelly has served as a director of CM Life Sciences II Inc. (Nasdaq: CMII), a special purpose acquisition company with a focus on the life sciences sector, since its initial public offering in February 2021. Dr. Kelly has a Ph.D. in Biological Engineering and a B.S. in Chemical Engineering and Biology from the Massachusetts Institute of Technology. We believe that Dr. Kelly is qualified to serve on our board of directors as a Founder and due to his knowledge of our company and our business.
Reshma Shetty, one of our Founders, is the President and Chief Operating Officer and a member of New Ginkgo’s board of directors. Dr. Shetty currently serves on the Bio Advisory Group at the non-profit Nuclear Threat Initiative. Dr. Shetty has a Ph.D. in Biological Engineering from the Massachusetts Institute of Technology and a B.S. in Computer Science from the University of Utah. We believe that Dr. Shetty is qualified to serve on our board of directors as a Founder and due to her knowledge of our company and our business.
Mark Dmytruk is the Chief Financial Officer and a member of New Ginkgo’s board of directors. From 2017 to 2020, Mr. Dmytruk served as Executive Vice President, Corporate Strategy and Development, for Syneos Health, a global Contract Research Organization and Contract Commercial Organization serving the biopharmaceutical industry. Syneos Health was formed through the merger of inVentiv Health and INC Research in 2017, and prior to the merger Mr. Dmytruk served at inVentiv Health as Chief of Staff from 2014 to 2017 and President, Patient Outcomes Division, from 2011 to 2014. Prior to inVentiv Health, Mr. Dmytruk served in a variety of roles at Thermo Fisher Scientific (and its predecessor, Fisher Scientific) from 2001 to 2011. As Vice President of Corporate Development, Mr. Dmytruk led the company’s M&A function, contributing to its industry-changing strategy and transformational growth. He also served as Vice President of Finance for the Athena Diagnostics business unit of Thermo Fisher Scientific prior to its sale to Quest Diagnostics. Mr. Dmytruk began his career at Ernst & Young in Canada. Mr. Dmytruk has an M.B.A. from the Sloan School of Management at the Massachusetts Institute of Technology and a Bachelor of Commerce from the University of Alberta.
Arie Belldegrun is a member of New Ginkgo’s board of directors. Dr. Belldegrun is a leader in the field of cell and gene therapy. Dr. Belldegrun is the Executive Chairman and Co-Founder of Allogene Therapeutics, a clinical stage biotechnology company focused on pioneering the development of allogeneic chimeric antigen receptor T cell (AlloCAR T™) therapies for cancer. He also founded Kite Pharma, a biopharmaceutical company engaged in the development of innovative cancer immunotherapies, where he served as Chairman, President and Chief Executive Officer until the acquisition of Kite by Gilead Sciences in October 2017. Dr. Belldegrun is the Chairman of Bellco Capital. He also serves as Chairman of Two River Group, UroGen Pharma and Kronos Bio and as Co-Chairman of Breakthrough Properties. He serves as Co-Founder and Director of IconOVir Bio, Inc and is also co-Founder and Senior Managing Director of Vida Ventures, a life science venture group with offices in
172
Boston and Los Angeles. Dr. Belldegrun is the director of the UCLA Institute of Urologic Oncology at the David Geffen School of Medicine at UCLA, where he also is a Research Professor, holding the Roy and Carol Doumani Chair in Urologic Oncology. Prior to joining UCLA, Dr. Belldegrun was at the National Cancer Institute/National Institute of Health as a research fellow in surgical oncology and immunology. Dr. Belldegrun received his M.D. from the Hebrew University Hadassah Medical School in Israel, after which he completed his post-graduate studies in Immunology at the Weizmann Institute of Science and his residency in urologic surgery at Harvard Medical School. Dr. Belldegrun is certified by the American Board of Urology and is a Fellow of the American College of Surgeons and the American Association of Genitourinary Surgeons. Dr. Belldegrun has authored several books on oncology and more than 500 scientific and medical papers related to urological cancer, immunotherapy, gene therapy and cancer vaccines. We believe that Dr. Belldegrun is qualified to serve on our board of directors due to his extensive knowledge as a leader in the field of cell and gene therapy.
Marijn Dekkers is a member of New Ginkgo’s board of directors. Dr. Dekkers is the Founder and Chairman of Novalis LifeSciences LLC, an investment and advisory firm for the Life Science industry that he founded in 2017. From 2010 to 2016, Dr. Dekkers served as Chief Executive Officer of Bayer AG. Prior to his time at Bayer, from 2002 to 2009, he served as Chief Executive Officer of Thermo Fisher Scientific. Dr. Dekkers serves as a director on several companies in the life sciences industry, which include serving as Chairman at AGBiome and a member of the board of directors at Vizgen, Quanterix, Cerevel Therapeutics, Enko, BioQ Pharma, Georgetown University and the Foundation for the National Institutes of Health. Dr. Dekkers began his career in 1985 as a research scientist at General Electric’s Corporate R&D Center. Dr. Dekkers received his PhD and M.S. in chemical engineering from the University of Eindhoven and his B.S in chemistry from the Radboud University. We believe that Dr. Dekkers is qualified to serve on our board of directors due to his extensive knowledge of the life sciences industry, his familiarity with our company and his prior director service.
Christian Henry is a member of New Ginkgo’s board of directors. Mr. Henry has served as President and Chief Executive Officer of Pacific Biosciences of California, Inc., a leading sequencing company, since September 2020. From 2005 to January 2017, Mr. Henry was a member of the executive team of Illumina, Inc., a global leader in sequencing. During this tenure at Illumina, he served in a number of roles, including Executive Vice President & Chief Commercial Officer, Senior Vice President of Genomic Solutions, Senior Vice President and General Manager of Life Sciences and Senior Vice President and Chief Financial Officer. Prior to joining Illumina in 2005, Mr. Henry served as the Chief Financial Officer of Tickets.com, Inc. from 2003 to 2005. From 1999 to 2003, Mr. Henry served as Vice President, Finance and Corporate Controller of Affymetrix, Inc. (acquired by Thermo Fisher Scientific in 2016). In 1997, Mr. Henry joined Nektar Therapeutics (formerly Inhale Therapeutic Systems, Inc.) as Corporate Controller, and later as its Chief Accounting Officer from 1997 to 1999. In 1996, Mr. Henry served as General Accounting Manager of Sugen, Inc. Mr. Henry began his career in 1992 at Ernst & Young LLP, where he was a Senior Accountant through 1996. Mr. Henry currently serves as a director and Chairman of the board of WAVE Life Sciences Ltd., and as a director of CM Life Sciences Holdings III LLC. Mr. Henry previously served as Chairman of the board of Pacific Biosciences from August 2018 to September 2020. Mr. Henry holds a B.A. in biochemistry and cell biology from the University of California, San Diego and an M.B.A., with a concentration in finance, from the University of California, Irvine. We believe that Mr. Henry is qualified to serve on our board of directors due to his over 20 years of experience in growing companies in the life sciences industry.
Dr. Reshma Kewalramani is a member of New Ginkgo’s board of directors. Dr. Kewalramani has been the Chief Executive Officer and President of Vertex Pharmaceuticals Inc. since April 2020 and a member of Vertex’s Board of Directors since February 2020. Dr. Kewalramani was Vertex’s Executive Vice President and Chief Medical Officer from April 2018 through April 2020. She was Vertex’s Senior Vice President, Late Development from February 2017 until March 2018. From August 2004 to January 2017, she served in roles of increasing responsibility at Amgen Inc., most recently as Vice President, Global Clinical Development, Nephrology & Metabolic Therapeutic Area and as Vice President, U.S. Medical Organization. From 2014 through 2019, Dr. Kewalramani was the industry representative to the FDA’s Endocrine and Metabolic Drug Advisory Committee. She completed her internship and residency in Internal Medicine at the Massachusetts
173
General Hospital and her fellowship in Nephrology at the Massachusetts General Hospital and Brigham and Women’s Hospital combined program. Dr. Kewalramani holds a B.A. from Boston University and an M.D. from Boston University School of Medicine. Dr. Kewalramani also completed the General Management Program at Harvard Business School and is an alumnus of the school. We believe that Dr. Kewalramani is qualified to serve on our board of directors due to her extensive experience serving in senior roles at various pharmaceutical companies.
Shyam Sankar is a member of New Ginkgo’s board of directors. Mr. Sankar is the Chief Operating Officer and Executive Vice President at Palantir Technologies Inc., where he has worked in various positions since 2006. Prior to his time at Palantir, Mr. Sankar served as the Vice President of Network Management and Director of Business Development for Xoom Corporation. Mr. Sankar has a deep operational background overseeing the development of complex technology from near inception to massive scale. Mr. Sankar received his M.S. in management science and engineering from Stanford University and his B.S. in electrical and computer engineering from Cornell University. We believe that Mr. Sankar is qualified to serve on our board of directors due to his business acumen, leadership experience, and operational background, having overseen the development and expansion of a software company from its near inception through its public listing.
Harry E. Sloan is a member of New Ginkgo’s board of directors. Mr. Sloan is the Chairman and Chief Executive Officer of Eagle Equity Partners II, LLC and served as Chief Executive Officer and Chairman of SRNG since October 2020. Most recently Mr. Sloan served as Chief Executive Officer and Chairman of Flying Eagle Acquisition Corp. (NYSE: FEAC) (“Flying Eagle”), which raised $690 million in its initial public offering in March 2020 and in December 2020 completed its initial business combination with Skillz Inc., a technology company that enables game developers to monetize their content through fun and fair multi-player competition. Mr. Sloan remains a director of Skillz Inc. Prior to Flying Eagle, Mr. Sloan was a founding investor of Diamond Eagle Acquisition Corp. (“Diamond Eagle”), which raised $400 million in its initial public offering in May 2019 and in April 2020 completed its initial business combination with DraftKings, Inc., a digital sports entertainment and gaming company known for its industry-leading daily fantasy sports and mobile sports betting platforms, and SBTech (Global) Limited, an international turnkey provider of cutting-edge sports betting and gaming technologies. Mr. Sloan now serves as the Vice Chairman of DraftKings, Inc. Prior to Diamond Eagle, Mr. Sloan was a founding investor of Platinum Eagle Acquisition Corp. (“Platinum Eagle”), which raised $325 million in its initial public offering in January 2018, completed its initial business combination in March 2019 with Target Logistics Management, LLC and RL Signor Holdings, LLC and changed its name to Target Hospitality Corp. Target Hospitality is a vertically integrated specialty rental and hospitality services company. Prior to Platinum Eagle, Mr. Sloan was a founding investor of Double Eagle Acquisition Corp. (“Double Eagle”), which raised $500 million in its initial public offering in September 2015. Double Eagle completed its business combination in November 2017, in which its wholly-owned subsidiary acquired 90% of the shares of Williams Scotsman. In the transaction, Double Eagle changed its name to WillScot Corporation. WillScot Corporation is a specialty rental services market leader providing modular space and portable storage solutions to diverse end markets across North America. Mr. Sloan previously served as chairman and chief executive officer of Silver Eagle Acquisition Corp. from April 2013 until the consummation of its initial business combination in March 2015 with Videocon d2h Limited (“Videocon”). From May 2016 to April 2018, Mr. Sloan served on the board of directors of Videocon, where he was a member of its Nomination, Remuneration and Compensation Committee. Mr. Sloan also served as chairman and chief executive officer of Global Eagle Acquisition Corp. from February 2011 until the consummation of its business combination in January 2013, and he remains a director of the combined company, Global Eagle Entertainment Inc. From October 2005 to August 2009, Mr. Sloan served as chairman and chief executive officer of Metro-Goldwyn-Mayer, Inc. (“MGM”), a motion picture, television, home entertainment, and theatrical production and distribution company, and thereafter continued as non-executive chairman until December 2010. MGM filed for bankruptcy protection in 2010. From 1990 to 2002, Mr. Sloan was chairman and chief executive officer of SBS Broadcasting, S.A., a European broadcasting group, operating commercial television, premium pay channels, radio stations and related print businesses in Western and Central and Eastern Europe, which he founded in 1990 and continued as executive chairman until 2005. In 1999, SBS Broadcasting, S.A. became the largest shareholder of Lions Gate Entertainment Corp. (NYSE: LFG.A) (“Lions
174
Gate”), an independent motion picture and television production company. Mr. Sloan served as chairman of the board of Lions Gate from April 2004 to March 2005. From 1983 to 1989, Mr. Sloan was co-chairman of New World Entertainment Ltd., an independent motion picture and television production company. In January 2011, Mr. Sloan joined the board of Promotora de Informaciones, S.A. (OTCMKTS: PRISY), Spain’s largest media conglomerate which owns El Pais, the leading newspaper in the Spanish-speaking world, as well as pay television, radio and digital properties. He has served on the board of ZeniMax Media Inc., an independent producer of interactive gaming and web content, since 1999. Mr. Sloan is an Associate Professor at the University of California at Los Angeles’s (UCLA) Anderson School of Management and serves on the UCLA Anderson School of Management Board of Visitors and the Executive Board of UCLA Theatre, Film and Television. Mr. Sloan is also a Trustee of The McCain Institute. Mr. Sloan received his B.A. degree from UCLA and J.D. degree from Loyola Law School. We believe that Mr. Sloan is qualified to serve on our board of director due to his public company experience, including with other similarly structured blank check companies, business leadership, operational experience and contacts.
New Ginkgo structures its corporate governance in a manner that it believes closely aligns its interests with those of its stockholders. Notable features of this corporate governance include:
| • | New Ginkgo has independent director representation on its audit, compensation and nominating and corporate governance, and its independent directors will meet regularly in executive sessions without the presence of its corporate officers or non-independent directors; |
| • | at least one of its directors qualifies as an “audit committee financial expert” as defined by the SEC; and |
| • | it implements a range of other corporate governance best practices, including placing limits on the number of directorships held by its directors to prevent “overboarding” and implementing a robust director education program. |
Role of Board in Risk Oversight
The New Ginkgo Board has extensive involvement in the oversight of risk management related to New Ginkgo and its business and will accomplish this oversight through the regular reporting to the board of directors by the audit committee. The audit committee will represent the New Ginkgo Board by periodically reviewing New Ginkgo’s accounting, reporting and financial practices, including the integrity of its financial statements, the surveillance of administrative and financial controls and its compliance with legal and regulatory requirements. Through its regular meetings with management, including the finance, legal, internal audit and information technology functions, the audit committee will review and discuss all significant areas of New Ginkgo’s business and summarize for the New Ginkgo Board all areas of risk and the appropriate mitigating factors. In addition, the New Ginkgo Board will receive periodic detailed operating performance reviews from management.
Composition of the New Ginkgo Board
New Ginkgo’s business and affairs are managed under the direction of its board of directors. Under the Charter, the New Ginkgo Board is declassified and all of New Ginkgo’s directors are elected each year for one-year terms.
The standing committees of the New Ginkgo Board consist of an audit committee, a compensation committee and a nominating and corporate governance committee. The New Ginkgo Board may from time to time establish other committees.
175
New Ginkgo’s president and chief executive officer and other executive officers will regularly report to the non-executive directors and the audit, the compensation and the nominating and corporate governance committees to ensure effective and efficient oversight of our activities and to assist in proper risk management and the ongoing evaluation of management controls.
Audit Committee
New Ginkgo has an audit committee, consisting of Christian Henry, who serves as the chairperson, Marijn Dekkers and Harry Sloan. Each of Messrs. Henry and Sloan qualifies as an independent director under the NYSE corporate governance standards and the independence requirements of Rule 10A-3 under the Exchange Act. The New Ginkgo Board has determined that Mr. Henry qualifies as an “audit committee financial expert” as such term is defined in Item 407(d)(5) of Regulation S-K and possesses financial sophistication, as defined under the rules of NYSE.
The purpose of the audit committee is to prepare the audit committee report required by the SEC to be included in New Ginkgo’s proxy statement and to assist the board of directors in overseeing and monitoring (1) the quality and integrity of the financial statements, (2) compliance with legal and regulatory requirements, (3) New Ginkgo’s independent registered public accounting firm’s qualifications and independence, (4) the performance of New Ginkgo’s internal audit function and (5) the performance of New Ginkgo’s independent registered public accounting firm.
The New Ginkgo Board has adopted a written charter for the audit committee which is available on New Ginkgo’s website.
Compensation Committee
New Ginkgo has a compensation committee, consisting of Shyam Sankar, who serves as the chairperson, Arie Belldegrun and Christian Henry.
The purpose of the compensation committee is to assist the board of directors in discharging its responsibilities relating to (1) setting New Ginkgo’s compensation program and compensation of its executive officers and directors, (2) monitoring New Ginkgo’s incentive and equity-based compensation plans and (3) preparing the compensation committee report required to be included in New Ginkgo’s proxy statement under the rules and regulations of the SEC.
The New Ginkgo Board has adopted a written charter for the compensation committee which is available on New Ginkgo’s website.
Nominating and Corporate Governance Committee
New Ginkgo has a nominating and corporate governance committee, consisting of Marijn Dekkers, who serves as the chairperson, Reshma Kewalramani and Shyam Sankar. The purpose of the nominating and corporate governance committee is to assist the board of directors in discharging its responsibilities relating to (1) identifying individuals qualified to become new board of directors members, consistent with criteria approved by the board of directors, (2) reviewing the qualifications of incumbent directors to determine whether to recommend them for reelection and selecting, or recommending that the board of directors select, the director nominees for the next annual meeting of shareholders, (3) identifying board of directors members qualified to fill vacancies on any board of directors committee and recommending that the board of directors appoint the identified member or members to the applicable committee, (4) reviewing and recommending to the board of directors corporate governance principles applicable to New Ginkgo, (5) overseeing the evaluation of the board of directors and management and (6) handling such other matters that are specifically delegated to the committee by the board of directors from time to time.
176
The New Ginkgo Board has adopted a written charter for the nominating and corporate governance committee which is available on New Ginkgo’s website.
New Ginkgo has adopted a new code of business conduct that applies to all of its directors, officers and employees, including its principal executive officer, principal financial officer and principal accounting officer, which is available on New Ginkgo’s website. New Ginkgo’s code of business conduct is a “code of ethics”, as defined in Item 406(b) of Regulation S-K. Please note that New Ginkgo’s Internet website address is provided as an inactive textual reference only. New Ginkgo will make any legally required disclosures regarding amendments to, or waivers of, provisions of its code of ethics on its Internet website.
Compensation Committee Interlocks and Insider Participation
No member of the compensation committee was at any time during fiscal year 2020, or at any other time, one of our officers or employees. None of our executive officers has served as a director or member of a compensation committee (or other committee serving an equivalent function) of any entity, one of whose executive officers served as a director of our board of directors or member of our compensation committee.
Independence of the Board of Directors
NYSE rules generally require that independent directors must comprise a majority of a listed company’s board of directors. Based upon information requested from and provided by each director concerning his or her background, employment and affiliations, including family relationships, we have determined that each of Arie Belldegrun, Christian Henry, Reshma Kewalramani, Shyam Sankar and Harry Sloan, representing a majority of New Ginkgo’s directors, are “independent” as that term is defined under the applicable rules and regulations of the SEC and the listing requirements and rules of the NYSE.
177
GINKGO’S EXECUTIVE AND DIRECTOR COMPENSATION
This section discusses the material components of the executive compensation program for Ginkgo’s executive officers who are named in the “2020 Summary Compensation Table” below. As an emerging growth company, Ginkgo complies with the executive compensation disclosure rules applicable to “smaller reporting companies,” as such term is defined in the rules promulgated under the Securities Act, which require compensation disclosure for Ginkgo’s principal executive officer and Ginkgo’s two most highly compensated executive officers other than its principal executive officer. These three officers are referred to as Ginkgo’s named executive officers.
In 2020, Ginkgo’s “named executive officers” and their positions were as follows:
| • | Jason Kelly, Chief Executive Officer; |
| • | Reshma Shetty, President and Chief Operating Officer; and |
| • | Mark Dmytruk, Chief Financial Officer. |
On November 9, 2020, Mr. Dmytruk commenced employment with Ginkgo as its Chief Financial Officer.
This discussion may contain forward-looking statements that are based on New Ginkgo’s current plans, considerations, expectations and determinations regarding future compensation programs. The actual compensation programs that New Ginkgo adopts may differ materially from the currently planned programs summarized in this discussion.
2020 Summary Compensation Table
The following table sets forth information concerning the compensation of Ginkgo’s named executive officers for the year ended December 31, 2020.
| Name and Principal Position | Year | Salary ($) |
Bonus ($)(1) |
Stock Awards ($)(2) |
All Other Compensation ($)(3) |
Total ($) | ||||||||||||||||||
| Jason Kelly |
2020 | 250,000 | 414,841 | 9,854,097 | 14,250 | 10,533,188 | ||||||||||||||||||
| Chief Executive Officer |
||||||||||||||||||||||||
| Reshma Shetty |
2020 | 250,000 | 415,386 | 9,854,097 | 14,250 | 10,533,733 | ||||||||||||||||||
| President and Chief Operating Officer |
||||||||||||||||||||||||
| Mark Dmytruk |
2020 | 63,750 | — | — | 2,861 | 66,611 | ||||||||||||||||||
| Chief Financial Officer (4) |
||||||||||||||||||||||||
| (1) | Amounts reflect discretionary bonuses granted to each of Dr. Kelly and Dr. Shetty during 2020. |
| (2) | Amounts reflect the full grant-date fair value of restricted stock units granted during 2020 computed in accordance with ASC Topic 718, rather than the amounts paid to or realized by the named individual. We provide information regarding the assumptions used to calculate the value of all restricted stock units made to named executive officers in Note 15 to the consolidated financial statements included in this prospectus. |
| (3) | For Dr. Kelly, Dr. Shetty and Mr. Dmytruk, amounts represent matching contributions under Ginkgo’s 401(k) plan. |
| (4) | Mr. Dmytruk joined Ginkgo on November 9, 2020 as its Chief Financial Officer, and his salary was pro-rated for his partial year of service during 2020. |
178
NARRATIVE TO SUMMARY COMPENSATION TABLE
2020 Salaries
The named executive officers receive a base salary to provide a fixed component of compensation reflecting the executive’s skill set, experience, role and responsibilities. The 2020 annual base salaries for Ginkgo’s named executives officers were:
| Name |
2020 Annual Base Salary ($) |
|||
| Jason Kelly |
250,000 | |||
| Reshma Shetty |
250,000 | |||
| Mark Dmytruk |
425,000 | |||
In March 2020, Dr. Kelly’s base salary and Dr. Shetty’s base salary were each increased, effective January 1, 2020, from $120,000 to $250,000. Mr. Dmytruk’s base salary was negotiated in connection with his commencement of employment with Ginkgo in November 2020.
2020 Bonuses
Dr. Kelly and Dr. Shetty were each granted one-time bonuses in April 2020, equal to $414,841 and $415,386, respectively. The bonuses were intended to make the executives whole for taxes paid in 2019 on the excess of the price paid by Ginkgo to repurchase certain shares held by the executives over the fair market value of those shares on the date of such repurchase. Mr. Dmytruk did not receive a bonus in 2020.
Equity Compensation
Dr. Kelly and Dr. Shetty each received restricted stock units under Ginkgo’s 2014 Incentive Plan (the “2014 Plan”) during 2020. The restricted stock units vest upon the satisfaction of both an “event condition” and a “service condition” on or before the seventh anniversary of the grant date. The event condition will be satisfied on the first to occur of (i) a change in control or (ii) the earlier of (x) the date six months after the closing of a public offering or (y) March 15 of the calendar year following the effective date of a public offering. The service condition was satisfied on January 1, 2021. See “Post-Combination Company Executive Officer and Director Compensation—Founder Awards” below for a description of proposed changes to the vesting schedule of these restricted stock units in connection with the Business Combination. Mr. Dmytruk did not receive a grant of restricted stock units in 2020.
The following table sets forth the restricted stock units granted to Ginkgo’s named executive officers during 2020 under the 2014 Plan.
| Named Executive Officer |
2020 Restricted Stock Units Granted |
|||
| Jason Kelly |
4,324,037 | |||
| Reshma Shetty |
4,324,037 | |||
| Mark Dmytruk |
0 | |||
Dr. Kelly and Dr. Shetty each entered into a Founder Equity Grant Agreement with Ginkgo on January 1, 2020, which provides for the opportunity to receive future grants of restricted stock units in connection with a financing, a liquidity event, and continued employment following a public offering. A “financing” is defined in
179
the Founder Equity Grant Agreements as Ginkgo’s issuance and sale of its preferred equity interests for bona fide financing purposes. A “liquidity event” is defined in the Founder Equity Grant Agreements as the first to occur of a “change in control” or a “public offering” (each as defined in the restricted stock unit award agreements).
Under their Founder Equity Grant Agreements, Dr. Kelly and Dr. Shetty are eligible to receive grants of restricted stock units in the event that there is a financing or a liquidity event, in each case, prior to the end of the applicable performance period and subject to such executive’s continued service with Ginkgo through such event. The performance period began on January 1, 2020, and will end on the first to occur of a liquidity event and January 1, 2023, unless extended under certain circumstances. The number of restricted stock units granted in connection with a financing or a liquidity event prior to the end of the performance period will be determined according to one or more formulas set forth in the Founder Equity Grant Agreement. Dr. Kelly and Dr. Shetty may waive the grant of certain restricted stock units, in which case, such restricted stock units will be available for restricted stock unit grants to other Ginkgo employees on such terms as determined by the board.
Restricted stock units granted in connection with a financing will vest upon the first to occur of (i) a change in control or (ii) the earlier of (x) the date six months after the closing of a public offering or (y) March 15 of the calendar year following the effective date of a public offering, irrespective of whether the holder is continuously providing services through the vesting date. Restricted stock units granted in connection with a liquidity event will be fully vested on the date of grant.
The maximum number of restricted stock units that may be granted to each of Dr. Kelly and Dr. Shetty under his or her applicable Founder Equity Grant Agreement is 72,142,374, which includes the 4,324,037 restricted stock units granted to each of Dr. Kelly and Dr. Shetty on January 1, 2020 and any restricted stock units Dr. Kelly or Dr. Shetty elects to waive. However, any restricted stock units granted following a public offering (described below) will not count towards the individual award limit.
Following a public offering, Dr. Kelly and Dr. Shetty will be eligible under their Founder Equity Grant Agreements to receive grants of 2,404,746 restricted stock units on each anniversary following such public offering until the tenth anniversary of the public offering, subject to such executive’s continued service with Ginkgo through each applicable grant date and approval by the board or a committee thereof. The restricted stock units granted after a public offering will vest in twelve equal quarterly installments following the applicable grant date, subject to the executive’s continued service with Ginkgo through each applicable vesting date.
As of December 31, 2020, other than the restricted stock units granted on January 1, 2020 described above, no restricted stock units have been granted under the Founder Equity Grant Agreements. See “Post-Combination Company Executive Officer and Director Compensation—Founder Awards” below for a description of the restricted stock units we expect the Ginkgo board to grant to certain of the Founders, including Dr. Kelly and Dr. Shetty, and to Mr. Dmytruk in connection with the Business Combination.
Other Elements of Compensation
Retirement Plan
Ginkgo maintains a 401(k) retirement savings plan for its employees, including Ginkgo’s named executive officers, who satisfy certain eligibility requirements. Ginkgo’s named executive officers are eligible to participate in the 401(k) plan on the same terms as other full-time employees. Under this plan, Ginkgo provides a non-elective safe harbor contribution to all eligible participants equal to up to 5% of eligible compensation, which fully vests once such eligible participant has completed two years of continuous service. Ginkgo believes that providing a vehicle for tax-deferred retirement savings though Ginkgo’s 401(k) plan adds to the overall desirability of Ginkgo’s executive compensation package and further incentivizes Ginkgo’s employees, including Ginkgo’s named executive officers, in accordance with Ginkgo’s compensation policies.
180
Employee Benefits and Perquisites
Health/Welfare Plans. During their employment, Ginkgo’s named executive officers are eligible to participate in Ginkgo’s employee benefit plans and programs, including medical, dental, vision, life, and disability benefits, to the same extent as Ginkgo’s other full-time employees, subject to the terms and eligibility requirements of those plans.
Outstanding Equity Awards at Fiscal Year-End
The following table summarizes the number of shares of Ginkgo common stock underlying outstanding equity incentive plan awards for each named executive officer as of December 31, 2020. Mr. Dmytruk did not hold any outstanding equity awards in Ginkgo as of December 31, 2020.
| Stock Awards(1) | ||||||||||||
| Name |
Grant Date |
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) |
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($)(3) |
|||||||||
| Jason Kelly |
1/1/2020 | 4,324,037 | (2) | 17,088,951 | ||||||||
| Reshma Shetty |
1/1/2020 | 4,324,037 | (2) | |
17,088,951 |
| ||||||
| (1) | The amounts in this table represent grants of restricted stock units to each of the named executive officers. For a description of the restricted stock units, please see the section titled “Narrative to Summary Compensation Table – Equity Compensation” above. |
| (2) | Represents restricted stock units which conditionally vest based on continued service, although the restricted stock units will not become fully vested and payable until the first to occur of (i) a change in control or (ii) the earlier of (x) the date six months after the closing of a public offering or (y) March 15 of the calendar year following the effective date of a public offering. The service condition was satisfied on January 1, 2021. |
| (3) | Amount shown is based on a price per share of $3.95, which is based on a third-party valuation of Ginkgo common stock as of December 31, 2020. |
Executive Compensation Arrangements
Jason Kelly and Reshma Shetty. Neither Dr. Kelly nor Dr. Shetty have entered into employment agreements, offer letters or severance agreements with Ginkgo.
Mark Dmytruk. On November 9, 2020, Mr. Dmytruk commenced employment with Ginkgo under an offer letter dated October 7, 2020. Mr. Dmytruk’s offer letter provides for base salary, eligibility to receive a grant of 2,208,620 restricted stock units, which were granted in March 2021, and participation in our standard benefit plans. Mr. Dmytruk’s offer letter does not contain a fixed employment term.
Pursuant to the terms of Mr. Dmytruk’s offer letter, if Mr. Dmytruk’s employment is terminated by Ginkgo without “cause” (as defined in the offer letter), Mr. Dmytruk will be entitled to receive (i) 12 months’ severance pay based on his base salary rate on the date of such termination, to be paid in installments over the 12-month period following the termination date and (ii) up to 12 months’ company-paid health benefits continuation pursuant to COBRA, in each case subject to Mr. Dmytruk’s execution of a general release of claims in favor of Ginkgo.
181
Mr. Dmytruk also entered into a separate agreement pursuant to which he is subject to employee and customer non-solicitation covenants during the term of his employment or other service with Ginkgo and for one year thereafter. The agreement also includes standard invention assignment and confidential information covenants.
The following table sets forth information concerning the compensation of Ginkgo’s non-employee directors for their service on the board of directors for the year ended December 31, 2020. Ginkgo’s non-employee directors were not entitled to annual fees or other cash compensation during 2020.
| Name |
Stock Awards ($)(1) |
All Other Compensation ($) |
Total ($) | |||||||||
| Marijn Dekkers |
— | — | — | |||||||||
| Shyam Sankar |
671,100 | — | 671,100 | |||||||||
| Christian Henry |
671,100 | — | 671,100 | |||||||||
| Evan Lodes |
— | — | — | |||||||||
| (1) | Amounts reflect the full grant-date fair value of restricted stock units granted during 2020 computed in accordance with ASC Topic 718, rather than the amounts paid to or realized by the named individual. We provide information regarding the assumptions used to calculate the value of all restricted stock units made to our directors in Note 15 to the consolidated financial statements included in this prospectus. |
The restricted stock units granted to Mr. Sankar and Mr. Henry in 2020 vest upon the satisfaction of both an “event condition” and a “service condition” on or before the seventh anniversary of the grant date. The event condition will be satisfied on the first to occur of (i) a change in control or (ii) the earlier of (x) the date six months after the closing of a public offering or (y) March 15 of the calendar year following the effective date of a public offering. The service condition is satisfied in substantially equal monthly installments over three years ending in June 2023. Vesting of the restricted stock units is subject to continued service with Ginkgo through each applicable vesting date; provided that, if Mr. Sankar or Mr. Henry ceases to provide services to Ginkgo prior to the occurrence of the event condition, then all restricted stock units for which the service condition has been satisfied shall vest upon the subsequent occurrence of the event condition if the event condition occurs prior to the seventh anniversary of the grant date.
The table below shows the aggregate numbers of unvested stock awards held as of December 31, 2020 by each non-employee director who was serving as of December 31, 2020.
| Name |
Unvested Restricted Stock Units Outstanding at Fiscal Year End |
|||
| Marjin Dekkers |
1,371,651 | |||
| Shyam Sankar |
196,322 | |||
| Christian Henry |
286,335 | |||
| Evan Lodes |
— | |||
Post-Combination Company Executive Officer and Director Compensation
Founder Awards
As discussed in more detail above under “Equity Compensation”, on January 1, 2020, Ginkgo entered into Founder Equity Grant Agreements with each of the Founders, other than Mr. Knight, pursuant to which such
182
Founders are eligible to receive grants of restricted stock units in connection with a financing or liquidity event on the terms set forth in the Founder Equity Grant Agreements. The Business Combination was not contemplated under the Founder Equity Grant Agreements and does not constitute a financing or liquidity event for purposes of the Founder Equity Grant Agreements. However, we view the SPAC transaction as substantively equivalent to an initial public offering, which would have qualified as a liquidity event under the Founder Equity Grant Agreements.
Notwithstanding the foregoing, in connection with the Business Combination, the Ginkgo board granted 21,458,317 restricted stock units to each of Jason Kelly, Reshma Shetty, Austin Che and Bartholomew Canton under the 2014 Plan. These restricted stock units (the “Founder Awards”) are deemed to satisfy all rights to future grants of restricted stock units that the Founders may have under their respective Founder Equity Grant Agreements.
The Founder Awards are expected to vest upon the satisfaction of both an “event condition” (as described above) and a “service condition” on or before the seventh anniversary of the grant date. The service condition will be satisfied on October 1, 2022, subject to continued service with Ginkgo through such date.
The board and stockholders of Ginkgo approved an increase to the aggregate number of shares available for issuance under the 2014 Plan by 88,344,813 shares to account for such Founder Awards.
In addition, in connection with the grant of the Founder Awards, each of Dr. Kelly, Dr. Shetty, Dr. Che and Dr. Canton agreed to an amendment to such Founder’s restricted stock unit awards granted on January 1, 2020 under the 2014 Plan (the “2020 Awards”), pursuant to which satisfaction of the service condition will be extended to October 1, 2021.
Dmytruk RSU Award
On August 2, the Ginkgo board granted 343,563 restricted stock units to Mr. Dmytruk under the 2014 Plan. These restricted stock units will vest upon the satisfaction of both an “event condition” (as described above) and a “service condition” on or before the seventh anniversary of the grant date. The service condition will generally be satisfied, subject in all cases to Mr. Dmytruk’s continuing to be employed by us through each applicable vesting date, as to 5/48th of the restricted stock units on December 1, 2021 and as to an additional 1/48th of the restricted stock units on the last day of each month thereafter.
Amendment to RSUs under the 2014 Plan
The Ginkgo board amended all awards of restricted stock units outstanding under the 2014 Plan such that the event condition (as described above) will be deemed satisfied in 2021or 2022 as we viewed the SPAC transaction as substantively equivalent to an initial public offering.
Director Compensation
The New Ginkgo Board implemented an annual compensation program for its non-employee directors which became effective on September 16, 2021, pursuant to which the non-employee directors will be entitled to cash and equity compensation in such amounts necessary to attract and retain non-employee directors that have the talent and skills to foster long-term value creation and enhance the sustainable development of the company. The compensation payable under the program is intended to be competitive in relation to both the market in which the company operates and the nature, complexity and size of New Ginkgo’s business.
183
New Ginkgo’s non-employee directors will receive the following amounts for their services on the New Ginkgo Board under the non-employee director compensation program:
Cash Compensation
| • | An annual director fee of $50,000; |
| • | If the director serves as lead independent director or chair or on a committee of the New Ginkgo Board, an additional annual fee as follows: |
| • | Chair of the New Ginkgo Board, $36,000 |
| • | Lead independent director, $25,000; |
| • | Chair of the audit committee, $20,000; |
| • | Audit committee member other than the chair, $10,000; |
| • | Chair of the compensation committee, $15,000; |
| • | Compensation committee member other than the chair, $7,500; |
| • | Chair of the nominating and corporate governance committee, $10,000; |
| • | Nominating and Corporate Governance committee member other than the chair, $5,000. |
Director fees will be payable in arrears in four equal quarterly installments, provided that the amount of each payment will be prorated for any portion of a calendar quarter that a non-employee director is not serving on the New Ginkgo Board and no fee will be payable in respect of any period prior to the closing of the Business Combination. The New Ginkgo Board may permit non-employee directors to elect to receive equity compensation in lieu of cash compensation.
Equity Compensation
| • | Each non-employee director who is initially elected or appointed to the New Ginkgo Board on or after the closing of the Business Combination (other than those non-employee directors who were appointed by SRNG to serve on the New Ginkgo Board or those non-employee directors who served on the board of SRNG or Ginkgo Bioworks, Inc. prior to the closing of the Business Combination) will receive (i) an initial option to purchase shares of New Ginkgo Class A common stock with a grant date fair value of $400,000 (as determined under the program) (the “Initial Option”), (ii) an additional initial option to purchase shares of New Ginkgo Class A common stock with a grant date fair value of $200,000 (as determined under the program) (the “Additional Initial Option”), and (iii) a number of restricted stock units determined by dividing $200,000 by the fair market value of a share of New Ginkgo Class A common stock (the “Additional Initial RSU”). In the event that a non-employee director’s date of initial election or appointment does not occur on the same date as an annual meeting of New Ginkgo’s stockholders, the value of the Additional Initial Option and the Additional Initial RSU will be pro-rated in accordance with the terms of the program. |
| • | If the non-employee director has served on the New Ginkgo Board as of the date of an annual meeting of New Ginkgo’s stockholders that occurs after the closing of the Business Combination and will continue to serve as a non-employee director immediately following such meeting, such non-employee director will receive (i) an option to purchase shares of New Ginkgo Class A common stock with a grant date fair value of $200,000 (as determined under the program) (the “Subsequent Option”) and (ii) a number of restricted stock units determined by dividing $200,000 by the fair market value of a share of New Ginkgo Class A common stock (the “Subsequent RSU”). |
Stock options granted under the program will have an exercise price equal to the fair market value of New Ginkgo’s Class A common stock on the date of grant and will expire not later than ten years after the date of
184
grant. Each Initial Option granted to a non-employee director will vest and become exercisable in substantially equal installments on each of the first three anniversaries of the date of grant. Each Additional Initial Option and the Additional Initial RSUs granted to a non-employee director will vest and become exercisable, as applicable, in a single installment on the day before the next annual meeting of New Ginkgo’s stockholders occurring after the date of the director’s initial election or appointment to the New Ginkgo Board. Each Subsequent Option and the Subsequent RSUs will vest and become exercisable, as applicable, in a single installment on the earlier of the first anniversary of the date of grant or the day before the next annual meeting of New Ginkgo’s stockholders occurring after the date of grant. Vesting of the options and restricted stock units granted under the program is subject to the non-employee director’s continued service through each applicable vesting date. In the event of a change in control of New Ginkgo, the options and restricted stock units granted under the program will vest in full.
Incentive Compensation Plans
In connection with the Business Combination, our stockholders approved the New Ginkgo 2021 Incentive Award Plan, referred to below as the 2021 Plan, and the New Ginkgo 2021 Employee Stock Purchase Plan, referred to below as the ESPP, in order to facilitate the grant of cash and equity incentives to directors, employees (including our named executive officers) and consultants of New Ginkgo. The following summarizes the material terms of the 2021 Plan and the ESPP, as well as the 2014 Plan and the 2008 Stock Incentive Plan, or the 2008 Plan, under which we have previously made periodic grants of equity and equity-based awards to our named executive officers and other key employees prior to the Business Combination.
2021 Incentive Award Plan
Eligibility and Administration
Our employees, consultants and directors, and employees and consultants of our subsidiaries, may be eligible to receive awards under the 2021 Plan. The 2021 Plan will be administered by the New Ginkgo Board, which may delegate its duties and responsibilities to one or more committees of its directors and/or officers (collectively, the “plan administrator”), subject to the limitations imposed under the 2021 Plan, Section 16 of the Exchange Act, stock exchange rules and other applicable laws.
The plan administrator has the authority to take all actions and make all determinations under the 2021 Plan, to interpret the 2021 Plan and award agreements, to impose a mandatory holding period pursuant to which some or all participants may not dispose of or transfer shares issued under the 2021 Plan for a period of time determined by the plan administrator in its discretion, and to adopt, amend and repeal rules for the administration of the 2021 Plan as it deems advisable. The plan administrator also has the authority to determine which eligible service providers receive awards, grant awards and set the terms and conditions of all awards under the 2021 Plan, including any vesting and vesting acceleration provisions, subject to the conditions and limitations in the 2021 Plan.
The plan administrator may, without the approval of the shareholders, grant one or more awards under the 2021 Plan to any employee, director or consultant (including any employee, director or consultant who is a substantial security holder (i.e., those controlling 5% or more of the shares or voting power)) that represent, directly or indirectly, 1% or more of the common stock of New Ginkgo or 1% or more of the voting power of New Ginkgo.
Shares Available for Awards
The aggregate number of shares of New Ginkgo common stock available for issuance under the 2021 Plan, which may be issued as New Ginkgo Class A common stock or New Ginkgo Class B common stock, is initially equal to the sum of (i) 200,000,000 shares of New Ginkgo common stock, (ii) any shares of common stock which remain available for future grants under the 2014 Plan as of immediately prior to approval of the 2021 Plan by the shareholders, (iii) any shares of common stock which are subject to awards under the 2008 Plan and/or 2014 Plan
185
(together, the “Prior Plan”) which become available for issuance under the 2021 Plan pursuant to its terms, (iv) any Remaining Earn-out Shares (as defined in the Merger Agreement), plus (v) an annual increase for ten years on the first day of each calendar year beginning January 1, 2022, equal to the lesser of (A) 4% of the aggregate number of shares of common stock outstanding on the final day of the immediately preceding calendar year and (B) such smaller number of shares as is determined by the New Ginkgo Board. The maximum number of shares of common stock that may be issued pursuant to the exercise of incentive stock options (“ISOs”) granted under the 2021 Plan is 200,000,000 shares. In addition, prior to or in connection with issuing any shares of New Ginkgo common stock under the 2021 Plan, the plan administrator may convert awards previously granted covering shares of Class B common stock to Class A common stock or convert awards previously granted covering shares of Class A common stock to Class B common stock.
If an award under the 2021 Plan or any Prior Plan is forfeited (including repurchases at or below original cost), expires or is settled for cash, any shares subject to such award may, to the extent of such forfeiture, expiration or cash settlement, become, or again be available for, new grants under the 2021 Plan. The payment of dividend equivalents in cash in conjunction with any awards will not reduce the shares available for grant under the 2021 Plan. Furthermore, shares purchased on the open market with the cash proceeds from the exercise of options, and shares tendered or withheld to satisfy the exercise price or tax withholding obligation for any award under the 2021 Plan or any Prior Plan will become, or again be available for, award grants under the 2021 Plan.
Awards granted under the 2021 Plan upon the assumption of, or in substitution for, awards authorized or outstanding under a qualifying equity plan maintained by an entity with which New Ginkgo enters into a merger or similar corporate transaction will not reduce the shares available for grant under the 2021 Plan but will count against the maximum number of shares that may be issued upon the exercise of ISOs.
The 2021 Plan provides that the sum of any cash compensation and the aggregate grant date fair value (determined as of the date of the grant under Financial Accounting Standards Board Accounting Standards Codification Topic 718, or any successor thereto) of all awards granted to a non-employee director as compensation for services as a non-employee director during any fiscal year may not exceed the amount equal to $1,000,000, increased to $1,250,000 in the fiscal year in which the 2021 Plan’s effective date occurs or in the fiscal year of a non-employee director’s initial service as a non-employee director. The value of any cash or equity-based compensation granted prior to the effective date of the 2021 Plan shall not count against these limits. The plan administrator may make exceptions to these limits for individual non-employee directors in extraordinary circumstances, as the plan administrator may determine in its discretion, provided that the non-employee director receiving such additional compensation may not participate in the decision to award such compensation or in other contemporaneous compensation decisions involving non-employee directors.
Awards
The 2021 Plan provides for the grant of stock options, including ISOs and nonqualified stock options (“NSOs”), stock appreciation rights (“SARs”), restricted stock, dividend equivalents, restricted stock units (“RSUs”) and other stock or cash-based awards. Certain awards under the 2021 Plan may constitute or provide for payment of “nonqualified deferred compensation” under Section 409A of the Code, which may impose additional requirements on the terms and conditions of such awards. All awards under the 2021 Plan will be evidenced by award agreements, which will detail the terms and conditions of awards, including any applicable vesting and payment terms and post-termination exercise limitations. Awards other than cash awards generally will be settled in shares of New Ginkgo common stock, but the applicable award agreement may provide for cash settlement of any award. A brief description of each award type follows.
| • | Stock Options and SARs. Stock options provide for the purchase of shares of New Ginkgo common stock in the future at an exercise price set on the grant date. ISOs, in contrast to NSOs, may provide tax deferral beyond exercise and favorable capital gains tax treatment to their holders if certain holding period and other requirements of the Code are satisfied. SARs entitle their holder, upon exercise, to |
186
| receive from us an amount equal to the appreciation of the shares subject to the award between the grant date and the exercise date. Unless otherwise determined by the plan administrator, the exercise price of a stock option or SAR may not be less than 100% of the fair market value of the underlying share on the grant date (or 110% in the case of ISOs granted to certain significant shareholders), except with respect to certain substitute awards granted in connection with a corporate transaction. Unless otherwise determined by the plan administrator, the term of a stock option or SAR may not be longer than ten years (or five years in the case of ISOs granted to certain significant shareholders). |
| • | Restricted Stock. Restricted stock is an award of non-transferable shares of New Ginkgo common stock that remain forfeitable unless and until specified conditions are met and which may be subject to a purchase price. The terms and conditions applicable to restricted stock will be determined by the plan administrator, subject to the conditions and limitations contained in the 2021 Plan. |
| • | Restricted Stock Units, or RSUs. RSUs are contractual promises to deliver shares of New Ginkgo common stock in the future or an equivalent in cash and other consideration determined by the plan administrator, which may also remain forfeitable unless and until specified conditions are met and may be accompanied by the right to receive the equivalent value of dividends paid on shares of New Ginkgo common stock prior to the delivery of the underlying shares (i.e. dividend equivalent rights). The plan administrator may provide that the delivery of the shares (or payment in cash) underlying RSUs will be deferred on a mandatory basis or at the election of the participant. The terms and conditions applicable to RSUs will be determined by the plan administrator, subject to the conditions and limitations contained in the 2021 Plan. |
| • | Other Stock or Cash Based Awards. Other stock or cash-based awards are awards of cash, fully vested shares of New Ginkgo common stock and other awards valued wholly or partially by referring to, or otherwise based on, shares of New Ginkgo common stock. Other stock or cash-based awards may be granted to participants and may also be available as a payment form in the settlement of other awards, as standalone payments and as payment in lieu of compensation to which a participant is otherwise entitled. The plan administrator will determine the terms and conditions of other stock or cash-based awards, which may include any purchase price, performance goal, transfer restrictions and vesting conditions. |
| • | Dividend Equivalents. Dividend equivalents represent the right to receive the equivalent value of dividends paid on shares of New Ginkgo common stock and may be granted in tandem with RSUs. Dividend equivalents are credited as of the dividend record dates during the period between the date an award is granted and the date such award vests, is exercised, is distributed or expires, as determined by the plan administrator. |
Performance Criteria
The plan administrator may select performance criteria for an award to establish performance goals for a performance period. Performance criteria under the 2021 Plan may include, but will not be limited to, the following: net earnings or losses (either before or after one or more of interest, taxes, depreciation, amortization, and non-cash equity-based compensation expense); gross or net sales or revenue or sales or revenue growth; net income (either before or after taxes) or adjusted net income; profits (including but not limited to gross profits, net profits, profit growth, net operation profit or economic profit), profit return ratios or operating margin; budget or operating earnings (either before or after taxes or before or after allocation of corporate overhead and bonus); cash flow (including operating cash flow and free cash flow or cash flow return on capital); return on assets; return on capital or invested capital; cost of capital; return on shareholders’ equity; total stockholder return; return on sales; costs, reductions in costs and cost control measures; expenses; working capital; earnings or loss per share; adjusted earnings or loss per share; price per share or dividends per share (or appreciation in or maintenance of such price or dividends); regulatory achievements or compliance; implementation, completion or attainment of objectives relating to research, development, regulatory, commercial, or strategic milestones or developments; market share; economic value or economic value added models; division, group or corporate
187
financial goals; customer satisfaction/growth; customer service; employee satisfaction; recruitment and maintenance of personnel; human resources management; supervision of litigation and other legal matters; strategic partnerships and transactions; financial ratios (including those measuring liquidity, activity, profitability or leverage); debt levels or reductions; sales-related goals; financing and other capital raising transactions; cash on hand; acquisition activity; investment sourcing activity; and marketing initiatives, any of which may be measured in absolute terms or as compared to any incremental increase or decrease. Such performance goals also may be based solely by reference to our company’s performance or the performance of a subsidiary, division, business segment or business unit of our company or a subsidiary, or based upon performance relative to performance of other companies or upon comparisons of any of the indicators of performance relative to performance of other companies. When determining performance goals, the plan administrator may provide for exclusion of the impact of an event or occurrence which the plan administrator determines should appropriately be excluded, including, without limitation, non-recurring charges or events, acquisitions or divestitures, changes in the corporate or capital structure, events unrelated to the business or outside of the control of management, foreign exchange considerations, and legal, regulatory, tax or accounting changes.
Certain Transactions
In connection with certain corporate transactions and events affecting New Ginkgo common stock, including a change in control, or change in any applicable laws or accounting principles, the plan administrator has broad discretion to take action under the 2021 Plan to prevent the dilution or enlargement of intended benefits, facilitate the transaction or event or give effect to the change in applicable laws or accounting principles. This includes canceling awards for cash (other than with respect to non-employee directors) or property, accelerating the vesting of awards, providing for the assumption or substitution of awards by a successor entity, adjusting the number and type of shares subject to outstanding awards and/or with respect to which awards may be granted under the 2021 Plan and replacing or terminating awards under the 2021 Plan. In the event an award is not assumed or replaced with a comparable award in connection with a change in control, any portion of the award that vests primarily based on providing services for a period of time (as opposed to achieving performance goals) will vest in full in connection with the change in control. In addition, in the event of certain non-reciprocal transactions with shareholders, the plan administrator will make equitable adjustments to the 2021 Plan and outstanding awards as it deems appropriate to reflect the transaction.
Repricing
Stockholder approval will not be required for any amendment that reduces the exercise price or base price of any stock option or SAR, or cancels any stock option or SAR that has an exercise price or base price that is greater than the then-current fair market value of New Ginkgo common stock in exchange for cash, other awards or stock options or SARs with an exercise price or base price per share that is less than the exercise price or base price per share of the original stock options or SARs.
Plan Amendment and Termination
The New Ginkgo Board may amend or terminate the 2021 Plan at any time; however, no amendment, other than an amendment that increases the number of shares available under the 2021 Plan, may materially and adversely affect an award outstanding under the 2021 Plan without the consent of the affected participant, and stockholder approval will be obtained for any amendment to the extent necessary to comply with applicable laws. The 2021 Plan will remain in effect until terminated by the New Ginkgo Board; provided that any portion of the 2021 Plan that constitutes a “formula” under the NYSE listing requirements shall only remain in effect until the tenth anniversary of the date the shareholders last approved the 2021 Plan and ISOs may not be granted after the tenth anniversary of the earlier of the date of the adoption of the 2021 Plan or the date of the approval of the 2021 Plan by the shareholders. No awards may be granted under the 2021 Plan after its termination.
188
Foreign Participants, Claw-back Provisions, Transferability and Participant Payments
The plan administrator may modify award terms, establish subplans and/or adjust other terms and conditions of awards, subject to the share limits described above, in order to facilitate grants of awards subject to the laws and/or stock exchange rules of countries outside of the United States. All awards will be subject to any company claw-back policy. Except as the plan administrator may determine or provide in an award agreement, awards under the 2021 Plan are generally non-transferrable, except by will or the laws of descent and distribution, or, subject to the plan administrator’s consent, pursuant to a domestic relations order, and are generally exercisable only by the participant. With regard to tax withholding, exercise price or repurchase obligations arising in connection with awards, the plan administrator may, in its discretion, accept cash, wire transfer or check, shares of New Ginkgo common stock that meet specified conditions, a “market sell order,” or such other consideration as it deems suitable.
2021 Employee Stock Purchase Plan
The ESPP is comprised of two distinct components in order to provide increased flexibility to grant options to purchase shares under the ESPP. Specifically, the ESPP authorizes (1) the grant of options that are intended to qualify for favorable U.S. federal tax treatment under Section 423 of the Code (the “Section 423 Component”), and (2) the grant of options that are not intended to be tax-qualified under Section 423 of the Code (the “Non-Section 423 Component”). Where permitted under local law and custom, we expect that the Non-Section 423 Component will generally be operated and administered on terms and conditions similar to the Section 423 Component.
Eligibility and Administration
We expect that all of New Ginkgo’s employees will be eligible to participate in the ESPP. However, with respect to the Section 423 Component, an employee may not be granted rights to purchase stock under the ESPP if the employee, immediately after the grant, would own (directly or through attribution) stock possessing 5% or more of the total combined voting power or value of all classes of New Ginkgo’s common stock.
The ESPP is administered by the New Ginkgo Board, which may delegate its duties and responsibilities to one or more committees of its directors (collectively, the “ESPP administrator”). Among other things, the ESPP administrator has authority to interpret the terms of the ESPP, determine eligibility of participants and impose a mandatory holding period pursuant to which employees may not dispose of or transfer shares purchased under the ESPP for a period of time determined by the ESPP administrator in its discretion.
Shares Available for Awards.
A total of 20,000,000 shares of New Ginkgo common stock (which may be issued as New Ginkgo Class A common stock or New Ginkgo Class B common stock) is initially reserved for issuance under the ESPP. In addition, the number of shares available for issuance under the ESPP will increase annually for ten years on the first day of each calendar year beginning January 1, 2022 by an amount equal to the lesser of (A) 1% of the aggregate number of shares of common stock outstanding on the final day of the immediately preceding calendar year and (B) such smaller number of shares as is determined by the New Ginkgo Board, provided that no more than 100,000,000 shares may be issued under the Section 423 Component. Prior to or in connection with issuing any shares of New Ginkgo common stock under the ESPP, the ESPP administrator may convert awards covering shares of New Ginkgo Class B common stock to New Ginkgo Class A common stock or convert awards covering shares of New Ginkgo Class A common stock to New Ginkgo Class B common stock.
Awards
Stock will be offered under the ESPP during offering periods. The length of the offering periods under the ESPP will be determined by the ESPP administrator and may be up to twenty-seven months long. Employee payroll deductions will be used to purchase shares on each purchase date during an offering period. The purchase dates
189
for each offering period will be the final trading day in the offering period. Offering periods under the ESPP will commence when determined by the ESPP administrator. The ESPP administrator may, in its discretion, modify the terms of future offering periods. In non-U.S. jurisdictions where participation in the ESPP through payroll deductions is prohibited, the ESPP administrator may provide that an eligible employee may elect to participate through contributions to the participant’s account under the ESPP in a form acceptable to the ESPP administrator in lieu of or in addition to payroll deductions.
The ESPP permits participants to purchase common stock through payroll deductions of up to a specified percentage of their eligible compensation. The ESPP administrator will establish a maximum number of shares that may be purchased by a participant during any offering period. In addition, no employee will be permitted to accrue the right to purchase stock under the Section 423 Component at a rate in excess of $25,000 worth of shares during any calendar year during which such a purchase right is outstanding (based on the fair market value per share of New Ginkgo’s common stock as of the last trading day prior to the first day of the offering period).
On the first trading day of each offering period, each participant will automatically be granted an option to purchase shares of New Ginkgo’s common stock. The option will expire at the end of the applicable offering period, and will be exercised at that time to the extent of the payroll deductions accumulated during the offering period. The purchase price of the shares, in the absence of a contrary designation, will be 85% of the lower of the fair market value of New Ginkgo’s common stock on the last trading day prior to the first trading day of the offering period or on the last trading day prior to the purchase date. Participants may voluntarily end their participation in the ESPP at any time during a specified period prior to the end of the applicable offering period, and will be paid their accrued payroll deductions that have not yet been used to purchase shares of common stock. Participation ends automatically upon a participant’s termination of employment.
A participant may not transfer rights granted under the ESPP other than by will or the laws of descent and distribution, and are generally exercisable only by the participant.
Certain Transactions
In the event of certain non-reciprocal transactions or events affecting New Ginkgo’s common stock, the ESPP administrator will make equitable adjustments to the ESPP and outstanding rights. In the event of certain unusual or non-recurring events or transactions, including a change in control, the ESPP administrator may provide for (1) either the replacement of outstanding rights with other rights or property or termination of outstanding rights in exchange for cash, (2) the assumption or substitution of outstanding rights by the successor or survivor corporation or parent or subsidiary thereof, if any, (3) the adjustment in the number and type of shares of stock subject to outstanding rights, (4) the use of participants’ accumulated payroll deductions to purchase stock on a new purchase date prior to the next scheduled purchase date and termination of any rights under ongoing offering periods or (5) the termination of all outstanding rights.
ESPP Amendment and Termination
The ESPP administrator may amend, suspend or terminate the ESPP at any time. However, stockholder approval will be obtained for any amendment that increases the aggregate number or changes the type of shares that may be sold pursuant to rights under the ESPP or changes the corporations or classes of corporations whose employees are eligible to participate in the ESPP.
2014 Stock Incentive Plan
Awards
Ginkgo’s board of directors approved the 2014 Plan under which Ginkgo could grant options, stock appreciation rights, restricted stock, restricted stock units and other stock-based awards.
From and after the effective date of the 2021 Plan, no additional awards will be made under the 2014 Plan. However, the 2014 Plan will continue to govern the terms and conditions of the outstanding awards previously
190
granted thereunder. As of the date of this prospectus, awards of restricted stock units and restricted stock are outstanding under the 2014 Plan.
If an award under the 2014 Plan is forfeited (including repurchases at or below original cost), expires or is settled for cash, any shares subject to such award may, to the extent of such forfeiture, expiration or cash settlement, become available for new grants under the 2021 Plan.
Administration
The 2014 Plan is administered by the New Ginkgo Board, which may delegate its duties and responsibilities to one or more committees of its directors. Subject to the terms of the 2014 Plan, the administrator has the power to, among other things, amend and repeal the administrative rules, guidelines and practices relating to the 2014 Plan, to construe and interpret the terms of the 2014 Plan and any award agreements thereunder, to correct any defects, supply any omissions or reconcile any inconsistencies in the 2014 Plan or any award thereunder in the administrator’s discretion and make all other determinations necessary or desirable for the plan administration.
Changes to Capitalization
In the event of any stock split, reverse stock split, stock dividend, recapitalization, combination of shares, reclassification of shares, spinoff or other similar change in capitalization or event, or any dividend or distribution other than an ordinary cash dividend, the number and class of securities available under the 2014 Plan and the number of shares subject to, and, if applicable, the repurchase price per share subject to, each outstanding award will be equitably adjusted (or substituted awards may be made, if applicable) in the manner determined by the administrator.
Reorganization Event
In connection with a reorganization event under the 2014 Plan, the administrator in its discretion may provide for any one or more of the following actions with respect to outstanding awards, other than awards of restricted stock: (i) awards will be assumed, or new rights substituted therefor, by the acquiring or succeeding entity (or an affiliate thereof), (ii) upon written notice to participants, provide that all unexercised awards will terminate immediately prior to such reorganization event unless exercised by the participants, (iii) outstanding awards will become exercisable, realizable, or deliverable, or restrictions applicable to an award will lapse, in whole or in part prior to or upon such reorganization event, (iv) in the event of a reorganization event under the terms of which stockholders will receive a cash payment for each share surrendered in the reorganization event, make or provide for a cash payment to participants with respect to each award held by a participant on the terms set forth in the 2014 Plan or (v) in connection with a liquidation or dissolution, convert awards into the right to receive liquidation proceeds (if applicable, net of the exercise, measurement or purchase price thereof and any applicable tax withholdings).
Upon the occurrence of a reorganization event (other than a liquidation or dissolution), the repurchase and other rights with respect to outstanding restricted stock shall inure to the benefit of the successor and shall, unless the administrator determines otherwise, apply to the cash, securities or other property which the stock was converted into or exchanged for pursuant to such reorganization event in the same manner and to the same extent as they applied to such restricted stock; provided, that the administrator may provide for termination or deemed satisfaction of such repurchase or other rights. Upon the occurrence of a reorganization event involving liquidation or dissolution all restrictions and conditions on all restricted stock will automatically be deemed terminated or satisfied, except as otherwise provided in an award agreement or other agreement with a participant.
Amendment and Termination
The administrator may terminate or amend the 2014 Plan at any time and from time to time, provided that no amendment shall materially or adversely affect any award outstanding at the time of the amendment without the consent of the affected participant.
191
2008 Stock Incentive Plan
Awards
Ginkgo’s board of directors approved the 2008 Plan under which Ginkgo could grant options and restricted stock awards.
From and after the effective date of the 2014 Plan, Ginkgo ceased granting awards under the 2008 Plan. However, the 2008 Plan continues to govern the terms and conditions of the outstanding awards previously granted thereunder. As of the date of this prospectus, options are outstanding under the 2008 Plan.
If an award under the 2008 Plan is forfeited (including repurchases at or below original cost), expires or is settled for cash, any shares subject to such award may, to the extent of such forfeiture, expiration or cash settlement, become available for new grants under the 2021 Plan.
Administration
The 2014 Plan is administered by the New Ginkgo Board, which may delegate its duties and responsibilities to one or more committees of its directors. Subject to the terms of the 2008 Plan, the administrator has the power to, among other things, prescribe, amend and rescind the administrative rules and regulations relating to the 2008 Plan, to determine the terms and conditions of the awards granted under the 2008 Plan, to correct any defects, supply any omissions or reconcile any inconsistencies in the 2008 Plan or any award thereunder in the administrator’s discretion and make all other determinations necessary or desirable for the plan administration.
Changes to Capitalization
If, through or as a result of any merger, consolidation, asset sale, reorganization, recapitalization, reclassification of shares, stock dividend, stock split, reverse stock split or other similar transaction, or other similar change in capitalization or event, the number of outstanding shares are increased, decreased or exchanged for a different number or kind of shares or other securities, an appropriate and proportionate adjustment will be made in the number and class of securities available under the 2008 Plan, the number and class of securities and exercise price per share of each outstanding option, without changing the aggregate purchase price as to which the options remain exercisable.
Reorganization Event
In connection with certain corporate transactions or events, the New Ginkgo Board or the board of directors of the applicable successor entity may take any one or more of the following actions as to some or all outstanding options: (i) provide that such options will be assumed, or new rights substituted therefor, by the acquiring or succeeding entity (or an affiliate thereof), (ii) upon written notice to participants, provide that all unexercised options will terminate immediately following such transaction unless exercised by the participant, provided that any such outstanding options will become fully exercisable prior to or upon such transaction, (iii) in the event of a merger under the terms of which stockholders will receive a cash payment for each share surrendered in the merger, make or provide for a cash payment to participants with respect to each option held by a participant on the terms set forth in the 2008 Plan or (iv) provide that all or any outstanding options shall become exercisable in part or in full immediately prior to such event.
Amendment and Termination
The administrator may terminate or amend the 2008 Plan at any time and from time to time, provided that no amendment shall adversely affect any award outstanding at the time of the amendment without the consent of the affected participant.
192
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
On October 28, 2020, SRNG’s Sponsor purchased an aggregate of 43,125,000 founder shares in exchange for a capital contribution of $25,000, or approximately $0.0006 per share.
SRNG’s Sponsor purchased an aggregate of 19,250,000 Private Placement Warrants in connection with SRNG’s initial public offering, at a price of $1.50 per warrant, or $28,875,000 in the aggregate. See “Description of Capital Stock—Warrants.”
SRNG currently sub-leases its executive offices at 955 Fifth Avenue, New York, NY, 10075 from Global Eagle Acquisition LLC, an affiliate of our Sponsor. From the time of SRNG’s initial public offering until the Closing, SRNG reimbursed Global Eagle Acquisition LLC for office space, secretarial and administrative services provided to members of its management team in an amount that did not exceed $15,000 per month.
Prior to the Closing, SRNG’s officers and directors were entitled to reimbursement for any out-of-pocket expenses incurred in connection with activities on SRNG’s behalf such as identifying potential target businesses and performing due diligence on suitable business combinations. SRNG’s audit committee reviewed on a quarterly basis all payments that were made to SRNG’s Sponsor, SRNG’s officers, directors or its or their affiliates.
On May 10, 2021, an affiliate of SRNG’s Sponsor entered into a Subscription Agreement with the Company, pursuant to which it agreed to purchase an aggregate of 7,500,000 shares of New SRNG Class A common stock at $10.00 per share, for an aggregate purchase price of $75,000,000, from the Company in a private placement to close immediately prior to Closing, but after the Domestication.
The share numbers included in this “Certain Relationships and Related Party Transactions—Ginkgo” section reflect the historical pre-Business Combination shares issued in connection with the relevant transaction, and have not been revised to reflect the accounting of the Business Combination as a reverse recapitalization.
Series E Preferred Stock Financing
From September 2019 through July 2021, Old Ginkgo sold an aggregate of 1,942,610 shares of its Series E preferred stock to the related persons listed below at a purchase price of $150.19 per share, except as described below with respect to the conversion of convertible promissory notes. The following table summarizes purchases of Series E preferred stock from Old Ginkgo by such related persons:
| Name |
Shares of Series E Preferred Stock |
Total Purchase Price (Rounded) |
||||||
| Entities affiliated with Anchorage Capital Group(1) |
105,500 | $ | 15,053,014 | |||||
| Entities affiliated with Baillie Gifford & Co.(2) |
200,479 | $ | 29,104,581 | |||||
| Cascade Investment, L.L.C.(3) |
268,376 | $ | 38,719,465 | |||||
| General Atlantic (GK), L.P.(4) |
513,449 | $ | 76,109,274 | |||||
| Novalis Life Sciences Investments I, LP(5) |
52,755 | $ | 7,527,123 | |||||
| Senator Global Opportunity Master Fund LP(6) |
70,489 | $ | 10,057,534 | |||||
| Viking Global Opportunities Illiquid Investments Sub-Master LP(7) |
731,562 | $ | 106,379,679 | |||||
| (1) | Entities affiliated with Anchorage Capital Group hold more than 5% of Ginkgo’s outstanding capital stock. |
| (2) | Entities affiliated with Baillie Gifford & Co. hold more than 5% of Ginkgo’s outstanding capital stock. |
193
| (3) | Cascade Investment, L.L.C. holds more than 5% of Ginkgo’s outstanding capital stock. |
| (4) | General Atlantic (GK), L.P. holds more than 5% of Ginkgo’s outstanding capital stock. |
| (5) | Marijn Dekkers, a member of Ginkgo’s board of directors, is an affiliate of Novalis Life Sciences Investments I, LP. |
| (6) | Senator Global Opportunity Master Fund LP holds more than 5% of Ginkgo’s outstanding capital stock. Evan Lodes, a member of Ginkgo’s board of directors, is an affiliate of Senator Global Opportunity Master Fund LP. |
| (7) | Viking Global Opportunities Illiquid Investments Sub-Master LP holds more than 5% of Ginkgo’s outstanding capital stock. |
Convertible Note Financing
In certain cases, the payment of the Total Purchase Price above consisted or, or included, the conversion of convertible promissory notes held by the related persons. From June 2019 through July 2019, Old Ginkgo sold an aggregate of $160,500,000 in principal amount of convertible promissory notes to the related persons listed below. Interest on the principal amount of the convertible promissory notes accrued at the rate of 3.0% per year. The outstanding principal and accrued interest of such convertible promissory notes converted into shares of Series E preferred stock at a discounted purchase price of $142.68 per share and are reflected in the above table. The following table summarizes the convertible promissory notes issued by Old Ginkgo to such related persons:
| Name |
Principal Amount |
|||
| Entities affiliated with Anchorage Capital Group(1) |
$ | 15,000,000 | ||
| Entities affiliated with Baillie Gifford & Co.(2) |
$ | 19,000,000 | ||
| Cascade Investment, L.L.C.(3) |
$ | 30,000,000 | ||
| General Atlantic (GK), L.P.(4) |
$ | 19,000,000 | ||
| Novalis Life Sciences Investments I, LP(5) |
$ | 7,500,000 | ||
| Senator Global Opportunity Master Fund LP(6) |
$ | 10,000,000 | ||
| Viking Global Opportunities Illiquid Investments Sub-Master LP(7) |
$ | 60,000,000 | ||
| (1) | Entities affiliated with Anchorage Capital Group hold more than 5% of New Ginkgo’s outstanding capital stock. |
| (2) | Entities affiliated with Baillie Gifford & Co. hold more than 5% of New Ginkgo’s outstanding capital stock. |
| (3) | Cascade Investment, L.L.C. holds more than 5% of New Ginkgo’s outstanding capital stock. |
| (4) | General Atlantic (GK), L.P. holds more than 5% of New Ginkgo’s outstanding capital stock. |
| (5) | Marijn Dekkers, a member of New Ginkgo’s board of directors, is an affiliate of Novalis Life Sciences Investments I, LP. |
| (6) | Senator Global Opportunity Master Fund LP holds more than 5% of New Ginkgo’s outstanding capital stock. Evan Lodes, a member of New Ginkgo’s board of directors, is an affiliate of Senator Global Opportunity Master Fund LP. |
| (7) | Viking Global Opportunities Illiquid Investments Sub-Master LP holds more than 5% of New Ginkgo’s outstanding capital stock. |
Series D Preferred Stock Financing
In June 2018, Old Ginkgo sold 52,400 shares of its Series D preferred stock at a purchase price of $47.71 per share for an aggregate purchase price of $2,500,004 to Novalis Life Sciences Investments I, LP. Marijn Dekkers, a member of New Ginkgo’s board of directors, is an affiliate of Novalis Life Sciences Investments I, LP.
194
Founder Equity Grant Agreements
In January 2020, Old Ginkgo entered into Founder Equity Grant Agreements with each of Jason Kelly, Reshma Shetty, Austin Che and Bartholomew Canton. Dr. Kelly and Dr. Shetty are each a director, officer and holder of more than 5% of New Ginkgo’s outstanding capital stock. Dr. Canton and Dr. Che are each a holder of more than 5% of New Ginkgo’s outstanding capital stock. Dr. Canton is also a family member of Dr. Shetty. Also in January 2020, each of Dr. Kelly, Dr. Shetty, Dr. Canton and Dr. Che received a restricted stock unit award under the 2014 Plan. The terms of the Founder Equity Grants Agreements and the foregoing restricted stock unit awards are described in the section titled “New Ginkgo’s Executive and Director Compensation” in this prospectus.
Founder Equity Repurchases
In July 2018, Old Ginkgo repurchased 90,017 shares of common stock from each of Jason Kelly, Reshma Shetty, Austin Che, Bartholomew Canton and Thomas Knight at a price of $47.71 per share, which was the then most-recent price per share at which Old Ginkgo had sold convertible preferred stock to investors, for a total purchase price for each of $4,294,711. In September 2021, Old Ginkgo repurchased 11,032 shares of common stock from each of Jason Kelly, Reshma Shetty, Austin Che, Bartholomew Canton and Thomas Knight at a price of $453.20 per share, for a total purchase price for each of $4,999,702. Dr. Kelly and Dr. Shetty are each a director, officer and holder of more than 5% of New Ginkgo’s outstanding capital stock. Dr. Che, Dr. Canton and Dr. Knight are each a holder of more than 5% of New Ginkgo’s outstanding capital stock. Dr. Canton is also a family member of Dr. Shetty.
Agreements in Connection with Platform Ventures
In September 2019, Old Ginkgo entered into an agreement with Cascade Investment L.L.C., an affiliated entity of General Atlantic (GK), L.P., and Viking Global Opportunities Illiquid Investments Sub-Master LP, each of which hold more than 5% of New Ginkgo’s outstanding capital stock, pursuant to which such related persons were provided with the first right to invest up to an aggregate of $350.0 million for the financial investment portion of new companies launched by Old Ginkgo as part of its Platform Ventures (such as Allonnia, LLC). The agreement was terminated in May 2021. Initial investments in new companies launched by Old Ginkgo in connection with the agreement were approximately $12.9 million from Cascade Investment L.L.C., $19.5 million from entities affiliated with General Atlantic (GK), L.P., and $57.8 million from entities affiliated with Viking Global Opportunities Illiquid Investments Sub-Master LP.
In September 2021, Old Ginkgo formed 2 new Platform Ventures (Ayana Bio, LLC and Verb Biotics, LLC) and contributed intellectual property rights to these entities. Simultaneously with the arrangements between Old Ginkgo and each of Ayana Bio, LLC and Verb Biotics, LLC, Cascade Investment L.L.C. and entities affiliated with Viking Global Opportunities Illiquid Investments Sub-Master LP, each of which hold more than 5% of New Ginkgo’s outstanding capital stock, invested approximately $7.5 million and $22.5 million, respectively, in each company.
Exchange Agreement
In October 2021, New Ginkgo entered into an amended and restated stockholders agreement with Viking Global Opportunities Illiquid Investments Sub-Master LP, a holder of more than 5% of New Ginkgo’s outstanding capital stock, pursuant to which New Ginkgo agreed, subject to approval of the New Ginkgo Board, to permit such stockholder to exchange a portion of its shares of New Ginkgo Class A common stock for shares of New Ginkgo Class C common stock on a 1-for-1 basis. The New Ginkgo Board approved the exchange in November 2021.
Investors’ Rights Agreement
Old Ginkgo was party to the Third Amended and Restated Investors’ Rights Agreement, dated as of September 9, 2019, which granted registration rights and information rights, among other things, to certain holders of its
195
capital stock, including (i) entities affiliated with Anchorage Capital Group, entities affiliated with Baillie Gifford & Co., Cascade Investment, L.L.C, General Atlantic (GK), L.P., and Viking Global Opportunities Illiquid Investments Sub-Master LP, each of which currently hold more than 5% of New Ginkgo’s capital stock; (ii) Novalis Life Sciences Investments I, L.P., which is affiliated with Marijn Dekkers, a New Ginkgo director; and (iii) Senator Global Opportunity Master Fund LP, which currently holds more than 5% of New Ginkgo’s capital stock and is affiliated with Evan Lodes, a former Old Ginkgo director. This agreement was terminated upon the Closing.
Right of First Refusal and Co-Sale Agreement
Old Ginkgo was party to the Third Amended and Restated Right of First Refusal and Co-Sale Agreement, dated as of September 9, 2019, which granted the right to purchase shares of Old Ginkgo capital stock which certain other stockholders propose to sell to other parties to, among others (i) entities affiliated with Anchorage Capital Group, entities affiliated with Baillie Gifford & Co., Cascade Investment, L.L.C, General Atlantic (GK), L.P., and Viking Global Opportunities Illiquid Investments Sub-Master LP, each of which currently hold more than 5% of New Ginkgo’s capital stock; (ii) Novalis Life Sciences Investments I, L.P., which is affiliated with Marijn Dekkers, a New Ginkgo director; and (iii) Senator Global Opportunity Master Fund LP, which currently holds more than 5% of New Ginkgo’s capital stock and is affiliated with Evan Lodes, a former Old Ginkgo director. This agreement was terminated upon the Closing.
Voting Agreement
Old Ginkgo was party to the Third Amended and Restated Voting Agreement, dated as of September 9, 2019, pursuant to which certain holders of its capital stock, including (i) entities affiliated with Anchorage Capital Group, entities affiliated with Baillie Gifford & Co., Cascade Investment, L.L.C, General Atlantic (GK), L.P., and Viking Global Opportunities Illiquid Investments Sub-Master LP, each of which currently hold more than 5% of New Ginkgo’s capital stock; (ii) Novalis Life Sciences Investments I, L.P., which is affiliated with Marijn Dekkers, a New Ginkgo director; and (iii) Senator Global Opportunity Master Fund LP, which currently holds more than 5% of New Ginkgo’s capital stock and is affiliated with Evan Lodes, a former Old Ginkgo director, agreed to vote their shares in the manner directed by the agreement. This agreement was terminated upon the Closing.
Director and Officer Indemnification
New Ginkgo’s Charter authorizes indemnification and advancement of expenses for its directors and officers to the fullest extent permitted by the DGCL.
PIPE Investment
In May 2021, certain Old Ginkgo related persons entered into Subscription Agreements with SRNG in connection with the Private Placement. The following table summarizes purchases in the Private Placement by such related persons:
| Name |
SRNG Class A ordinary shares |
Total Purchase Price |
||||||
| Entities affiliated with Baillie Gifford & Co.(1) |
10,300,000 | $ | 103,000,000 | |||||
| Cascade Investment, L.L.C.(2) |
3,000,000 | $ | 30,000,000 | |||||
| General Atlantic (GK), L.P.(3) |
250,000 | $ | 2,500,000 | |||||
| Senator Global Opportunity Master Fund LP(4) |
700,000 | $ | 7,000,000 | |||||
| Viking Global Opportunities Illiquid Investments Sub-Master LP(5) |
2,000,000 | $ | 20,000,000 | |||||
196
| (1) | Entities affiliated with Baillie Gifford & Co. hold more than 5% of New Ginkgo’s outstanding capital stock. |
| (2) | Cascade Investment, L.L.C. holds more than 5% of New Ginkgo’s outstanding capital stock. |
| (3) | General Atlantic (GK), L.P. holds more than 5% of New Ginkgo’s outstanding capital stock. |
| (4) | Senator Global Opportunity Master Fund LP holds more than 5% of New Ginkgo’s outstanding capital stock. Evan Lodes, a former member of Old Ginkgo’s board of directors, is an affiliate of Senator Global Opportunity Master Fund LP. |
| (5) | Viking Global Opportunities Illiquid Investments Sub-Master LP holds more than 5% of New Ginkgo’s outstanding capital stock. |
197
UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following is a discussion of certain material U.S. federal income tax consequences relating to the purchase, ownership and disposition of our Class A common stock and warrants, which we collectively refer to as our “securities”. This discussion is limited to certain U.S. federal income tax considerations to investors that will hold securities as capital assets within the meaning of Section 1221 of the U.S. Internal Revenue Code of 1986, as amended (the “Code”) and that purchased such securities from the Selling Securityholders pursuant to this offering.
This discussion is a summary only and does not describe all of the tax consequences that may be relevant to you in light of your particular circumstances, including but not limited to the alternative minimum tax, the Medicare tax on certain net investment income and the different consequences that may apply if you are subject to special rules that apply to certain types of investors, including but not limited to:
| • | banks, financial institutions or financial services entities; |
| • | broker-dealers; |
| • | governments or agencies or instrumentalities thereof; |
| • | regulated investment companies; |
| • | real estate investment trusts; |
| • | expatriates or former long-term residents of the United States; |
| • | persons that actually or constructively own five percent or more (by vote or value) of our shares; |
| • | persons that acquired our securities pursuant to an exercise of employee share options, in connection with employee share incentive plans or otherwise as compensation; |
| • | insurance companies; |
| • | dealers or traders subject to a mark-to-market method of accounting with respect to our securities; |
| • | persons holding our securities as part of a “straddle,” constructive sale, hedge, wash sale, conversion or other integrated or similar transaction; |
| • | U.S. holders (as defined below) whose functional currency is not the U.S. dollar; |
| • | partnerships (or entities or arrangements classified as partnerships or other pass-through entities for U.S. federal income tax purposes) and any beneficial owners of such partnerships; |
| • | tax-exempt entities; |
| • | controlled foreign corporations; and |
| • | passive foreign investment companies. |
If a partnership (including an entity or arrangement treated as a partnership or other pass-thru entity for U.S. federal income tax purposes) holds our securities, the tax treatment of a partner, member or other beneficial owner in such partnership will generally depend upon the status of the partner, member or other beneficial owner, the activities of the partnership and certain determinations made at the partner, member or other beneficial owner level. If you are a partner, member or other beneficial owner of a partnership holding our securities, you are urged to consult your tax advisor regarding the tax consequences of the purchase, ownership and disposition of our securities.
This discussion is based on the Code, and administrative pronouncements, judicial decisions and final, temporary and proposed Treasury Regulations as of the date hereof, which are subject to change, possibly on a retroactive basis, and changes to any of which subsequent to the date of this prospectus may affect the tax consequences described herein. This discussion does not address any aspect of state, local or non-U.S. taxation, or any U.S. federal taxes other than income taxes (such as gift and estate taxes).
198
We have not sought, and do not expect to seek, a ruling from the U.S. Internal Revenue Service (the “IRS”) as to any U.S. federal income tax consequence described herein. The IRS may disagree with the discussion herein, and its determination may be upheld by a court. Moreover, there can be no assurance that future legislation, regulations, administrative rulings or court decisions will not adversely affect the accuracy of the statements in this discussion. You are urged to consult your tax advisor with respect to the application of U.S. federal tax laws to your particular situation, as well as any tax consequences arising under the laws of any state, local or foreign jurisdiction.
THIS DISCUSSION IS ONLY A SUMMARY OF CERTAIN U.S. FEDERAL INCOME TAX CONSIDERATIONS ASSOCIATED WITH THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR SECURITIES ACQUIRED PURSUANT TO THIS OFFERING. EACH PROSPECTIVE INVESTOR IN OUR SECURITIES IS URGED TO CONSULT ITS OWN TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH INVESTOR OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR SECURITIES, INCLUDING THE APPLICABILITY AND EFFECT OF ANY U.S. FEDERAL NON-INCOME, STATE, LOCAL, AND NON-U.S. TAX LAWS OR ANY APPLICABLE INCOME TAX TREATY.
This section applies to you if you are a “U.S. holder.” A U.S. holder is a beneficial owner of our Class A common stock or warrants who or that is, for U.S. federal income tax purposes:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation (or other entity taxable as a corporation) organized in or under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate the income of which is includible in gross income for U.S. federal income tax purposes regardless of its source; or |
| • | a trust, if (i) a court within the United States is able to exercise primary supervision over the administration of the trust and one or more United States persons (as defined in the Code) have authority to control all substantial decisions of the trust or (ii) it has a valid election in effect under Treasury Regulations to be treated as a United States person. |
Taxation of Distributions. If we pay distributions in cash or other property (other than certain distributions of our stock or rights to acquire our stock) to U.S. holders of our Class A common stock, such distributions generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Distributions in excess of current and accumulated earnings and profits will constitute a return of capital that will be applied against and reduce (but not below zero) the U.S. holder’s adjusted tax basis in its Class A common stock. Any remaining excess will be treated as gain realized on the sale or other disposition of its Class A common stock and will be treated as described under “U.S. Holders — Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Class A Common Stock and Warrants” below.
Dividends we pay to a U.S. holder that is treated as a taxable corporation generally will qualify for the dividends received deduction if the requisite holding period is satisfied. With certain exceptions (including, but not limited to, dividends treated as investment income for purposes of investment interest deduction limitations), and provided certain holding period requirements are met, dividends we pay to a non-corporate U.S. holder may constitute “qualified dividend income” that will be subject to tax at the applicable maximum tax rate accorded to long-term capital gains. If the applicable holding period requirements are not satisfied, then a corporation may not be able to qualify for the dividends received deduction and would have taxable income equal to the entire dividend amount, and non-corporate U.S. holders may be subject to tax on such dividend at regular ordinary income tax rates instead of the preferential rate that applies to qualified dividend income.
199
Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Class A Common Stock and Warrants.
Upon a sale or other taxable disposition of our Class A common stock or warrants, a U.S. holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized and the U.S. holder’s adjusted tax basis in its Class A common stock or warrants. Any such capital gain or loss generally will be long-term capital gain or loss if the U.S. holder’s holding period for its Class A common stock or warrants so disposed of exceeds one year. Long-term capital gains recognized by non-corporate U.S. holders may be eligible to be taxed at reduced rates. The deductibility of capital losses is subject to limitations.
Generally, the amount of gain or loss recognized by a U.S. holder is an amount equal to the difference between (i) the sum of the amount of cash and the fair market value of any property received in such disposition and (ii) the U.S. holder’s adjusted tax basis in its Class A common stock or warrants so disposed of. A U.S. holder’s adjusted tax basis in its Class A common stock or warrant generally will equal the U.S. holder’s acquisition cost less, in the case of a share of Class A common stock, any prior distributions treated as a return of capital.
Exercise, Lapse, or Redemption of a Warrant. Except as discussed below with respect to the cashless exercise of a warrant, a U.S. holder generally will not recognize gain or loss upon the exercise of a warrant. A U.S. holder’s tax basis in a share of our Class A common stock received upon exercise of the warrant generally will be an amount equal to the sum of the U.S. holder’s initial investment in the warrant and the exercise price. The U.S. holder’s holding period for the share of Class A common stock received upon exercise of the warrant generally will commence on the date of exercise of the warrant or the date following the date of exercise of the warrant; however, in either case the holding period will not include the period during which the U.S. holder held the warrant. If a warrant is allowed to lapse unexercised, a U.S. holder generally will recognize a capital loss equal to such holder’s tax basis in the warrant.
The tax consequences of a cashless exercise of a warrant are not clear under current tax law. A cashless exercise may be tax-free, either because the exercise is not a gain realization event or because the exercise is treated as a recapitalization for U.S. federal income tax purposes. In either tax-free situation, a U.S. holder’s basis in the share of Class A common stock received would equal the holder’s basis in the warrants used to effect the cashless exercise. If the cashless exercise is not treated as a gain realization event, a U.S. holder’s holding period in the Class A common stock generally would be treated as commencing on the date of exercise of the warrant or the date following the date of exercise of the warrant; however, in either case the holding period will not include the period during which the U.S. holder held the warrant. If the cashless exercise were treated as a recapitalization, the holding period of the Class A common stock would include the holding period of the warrant.
It is also possible that a cashless exercise could be treated in part as a taxable exchange in which gain or loss would be recognized. In such event, a portion of the warrants to be exercised on a cashless basis could, for U.S. federal income tax purposes, be deemed to have been surrendered in consideration for the exercise price of the remaining warrants, which would be deemed to be exercised. For this purpose, a U.S. holder could be deemed to have surrendered warrants having an aggregate fair market value equal to the exercise price for the total number of warrants to be deemed exercised. The U.S. holder would recognize capital gain or loss in an amount equal to the difference between the fair market value of the warrants deemed surrendered and the U.S. holder’s tax basis in such warrants. In this case, a U.S. holder’s tax basis in the Class A common stock received would equal the sum of the U.S. holder’s initial investment in the warrants deemed exercised and the exercise price of such warrants. A U.S. holder’s holding period for the Class A common stock in such case generally would commence on the date following the date of exercise (or possibly the date of exercise) of the warrant.
Due to the absence of authority on the U.S. federal income tax treatment of a cashless exercise, there can be no assurance which, if any, of the alternative tax consequences and holding periods described above would be adopted by the IRS or a court of law. Accordingly, U.S. holders should consult their tax advisors regarding the tax consequences of a cashless exercise.
200
Possible Constructive Distributions. The terms of each warrant provide for an adjustment to the number of shares of Class A common stock for which the warrant may be exercised or to the exercise price of the warrant in certain events as discussed in the section of this registration statement captioned “Description of New Ginkgo Securities—Warrants.” An adjustment which has the effect of preventing dilution generally is not taxable. U.S. holders of the warrants would, however, be treated as receiving a constructive distribution from us if, for example, the adjustment increases the warrant holders’ proportionate interest in our assets or earnings and profits (for instance, through an increase in the number of shares of Class A common stock that would be obtained upon exercise or through a decrease in the exercise price of the warrant) as a result of a distribution of cash or other property such as other securities to the holders of our Class A common stock which is taxable to the U.S. holders of such shares of Class A common stock, or as a result of the issuance of a stock dividend to holders of shares of our common stock as described under “—Taxation of Distributions” above. Such constructive distributions would be subject to tax as described under that section in the same manner as if the U.S. holders of the warrants received a cash distribution from us equal to the fair market value of such increased interest. Generally, a U.S. holder’s adjusted tax basis in its warrants should be increased to the extent of any constructive distribution treated as a dividend. For certain informational reporting purposes, we are required to determine the date and amount of any such constructive distributions and publicly report such information or report such information to the IRS and holders of warrants not exempt from information reporting. Proposed Treasury Regulations, which we may rely on prior to the issuance of final regulations, specify how the date and amount of constructive distributions are determined.
Information Reporting and Backup Withholding. In general, information reporting requirements may apply to distributions paid to a U.S. holder and to the proceeds of the sale or other disposition of our Class A common stock, unless the U.S. holder is an exempt recipient. Backup withholding may apply to such payments if the U.S. holder fails to provide a taxpayer identification number, a certification of exempt status or has been notified by the IRS that it is subject to backup withholding (and such notification has not been withdrawn).
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules will be allowed as a credit against a U.S. holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided the required information is timely furnished to the IRS.
This section applies to you if you are a “Non-U.S. holder.” As used herein, the term “Non-U.S. holder” means a beneficial owner of our Class A common stock or warrants who or that is for U.S. federal income tax purposes:
| • | a non-resident alien individual (other than certain former citizens and residents of the United States subject to U.S. tax as expatriates); |
| • | a foreign corporation; or |
| • | an estate or trust that is not a U.S. holder; |
but generally does not include an individual who is present in the United States for 183 days or more in the taxable year of the disposition of their securities. If you are such an individual, you should consult your tax advisor regarding the U.S. federal income tax consequences of the purchase, ownership or sale or other disposition of our securities.
Taxation of Distributions. In general, any distributions (other than certain distributions of our stock or rights to acquire our stock) we make to a Non-U.S. holder of our Class A common stock, to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles), will constitute dividends for U.S. federal income tax purposes and, provided such dividends are not effectively connected with the Non-U.S. holder’s conduct of a trade or business within the United States, we will be required
to withhold tax from the gross amount of the dividend at a rate of 30%, unless such Non-U.S. holder is eligible
201
for a reduced rate of withholding tax under an applicable income tax treaty and provides proper certification of its eligibility for such reduced rate (usually on an IRS Form W-8BEN or W-8BEN-E). Any distribution not constituting a dividend will be treated first as reducing (but not below zero) the Non-U.S. holder’s adjusted tax basis in its Class A common stock and, to the extent such distribution exceeds the Non-U.S. holder’s adjusted tax basis, as gain realized from the sale or other disposition of its Class A common stock, which will be treated as described under “Non-U.S. Holders — Gain on Sale, Taxable Exchange or Other Taxable Disposition of Class A Common Stock and Warrants” below. In addition, if we determine that we are likely to be classified as a “United States real property holding corporation” (see “Non-U.S. Holders — Gain on Sale, Taxable Exchange or Other Taxable Disposition of Class A Common Stock and Warrants” below), we may withhold 15% of any distribution that exceeds our current and accumulated earnings and profits.
The withholding tax generally does not apply to dividends paid to a Non-U.S. holder who provides an IRS Form W-8ECI, certifying that the dividends are effectively connected with the Non-U.S. holder’s conduct of a trade or business within the United States. Instead, the effectively connected dividends will be subject to regular U.S. federal income tax as if the Non-U.S. holder were a U.S. resident, subject to an applicable income tax treaty providing otherwise. A corporate Non-U.S. holder receiving effectively connected dividends may also be subject to an additional “branch profits tax” imposed at a rate of 30% (or a lower applicable treaty rate).
Gain on Sale, Taxable Exchange or Other Taxable Disposition of Class A Common Stock and Warrants. A Non-U.S. holder generally will not be subject to U.S. federal income or withholding tax in respect of gain recognized on a sale, taxable exchange or other taxable disposition of its Class A common stock or warrants (including an expiration of warrants) unless:
| • | the gain is effectively connected with the conduct by the Non-U.S. holder of a trade or business within the United States (and, under certain income tax treaties, is attributable to a United States permanent establishment or fixed base maintained by the Non-U.S. holder); or |
| • | we are or have been a “United States real property holding corporation” (a “USRPHC”) for U.S. federal income tax purposes at any time during the shorter of the five-year period ending on the date of disposition or the Non-U.S. holder’s holding period for its Class A Common Stock, except, in the case where shares of our Class A common stock and warrants are regularly traded on an established securities market, the Non-U.S. holder has owned, directly or constructively, at all times within the shorter of the five-year period preceding the disposition of its Class A common stock and warrants or such Non-U.S. holder’s holding period for such Class A common stock and warrants, 5% or less of our Class A common stock. It is unclear how the rules for determining the 5% threshold for this purpose would be applied with respect to our Class A common stock and warrants, including how a Non-U.S. holder’s ownership of warrants, if any, impacts the 5% threshold determination with respect to its Class A common stock. There can be no assurance that our Class A common stock and warrants will be treated as regularly traded on an established securities market for this purpose. Non-U.S. holders should consult their own tax advisors regarding the application of the foregoing rules in light of their particular facts and circumstances. |
Unless an applicable treaty provides otherwise, gain described in the first bullet point above will be subject to tax at generally applicable U.S. federal income tax rates as if the Non-U.S. holder were a U.S. resident. Any gains described in the first bullet point above of a Non-U.S. holder that is treated as a foreign corporation for U.S. federal income tax purposes may also be subject to an additional “branch profits tax” imposed at a 30% rate (or lower treaty rate).
If the second bullet point above applies to a Non-U.S. holder, gain recognized by such holder on the sale, exchange or other disposition of its securities will be subject to tax at generally applicable U.S. federal income tax rates. In addition, a buyer of our securities from such holder may be required to withhold U.S. federal income tax at a rate of 15% of the amount realized upon such disposition. We will be classified as a USRPHC if the fair market value of our “United States real property interests” equals or exceeds 50% of the sum of the fair market value of our worldwide real property interests plus our other assets used or held for use in a trade or business, as
202
determined for U.S. federal income tax purposes. We believe we currently are not, and do not anticipate becoming, a USRPHC. However, because our status as a USPRHC depends on the composition our business assets, which may change, no assurance can be provided as to whether we would be treated as a USRPHC in any future year.
Exercise, Lapse, or Redemption of a Warrant. The characterization for U.S. federal income tax purposes of the exercise, redemption or lapse of a warrant held by a Non-U.S. holder will generally correspond to the characterization described under “—U.S. Holders—Exercise, Lapse, or Redemption of a Warrant” above, although to the extent a cashless exercise or redemption results in a taxable exchange, the consequences would follow those described above in “—Gain or Loss on Sale, Taxable Exchange, or Other Taxable Disposition of Class A Common Stock and Warrants.”
Possible Constructive Distributions. The terms of each warrant provide for an adjustment to the number of shares of Class A common stock for which the warrant may be exercised or to the exercise price of the warrant in certain events as discussed in the section of this prospectus captioned “Description of New Ginkgo Securities—Warrants.” An adjustment which has the effect of preventing dilution generally is not taxable. Non-U.S. holders of the warrants would, however, be treated as receiving a constructive distribution from us if, for example, the adjustment increases the warrant holders’ proportionate interest in our assets or earnings and profits (for instance, through an increase in the number of shares of Class A common stock that would be obtained upon exercise or through a decrease in the exercise price of the warrant) as a result of a distribution of cash or other property such as other securities to the holders of shares of our Class A common stock, or as a result of the issuance of a stock dividend to holders of shares of our common stock. Such constructive distribution to a Non-U.S. holder of warrants would be treated as if such Non-U.S. holder had received a cash distribution from us equal to the fair market value of such increased interest (taxed as described above under “— Taxation of Distributions”). For certain informational reporting purposes, we are required to determine the date and amount of any such constructive distributions and publicly report such information or report such information to the IRS and holders of warrants not exempt from information reporting. Proposed Treasury Regulations, which taxpayers may generally rely on prior to the issuance of final regulations, specify how the date and amount of constructive distributions are determined.
Information Reporting and Backup Withholding. Information returns generally will be filed with the IRS in connection with payments to a Non-U.S. holder of distributions on our Class A common stock. Proceeds from a sale or other taxable disposition of our securities within the United States or conducted through certain U.S.-related brokers may be subject to backup withholding or information reporting unless a Non-U.S. holder complies with certification procedures to establish that it is not a United States person. The certification procedures required to claim a reduced rate of withholding under a treaty generally will satisfy such certification requirements. Proceeds of a disposition of our securities conducted through a non-U.S. office of a non-U.S. broker generally will not be subject to backup withholding or information reporting. Backup withholding is not an additional tax. The amount of any backup withholding from a payment to a Non-U.S. holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
FATCA Withholding Taxes. Provisions commonly referred to as “FATCA” impose withholding of 30% on payments of dividends on our Class A common stock (or constructive dividends, if any, with respect to our warrants), or (subject to the proposed Treasury Regulations discussed below) gross proceeds from the sale or other disposition of, our securities paid (or deemed paid) to “foreign financial institutions” (which is broadly defined for this purpose and in general includes investment vehicles) and certain other non-U.S. entities unless various U.S. information reporting and due diligence requirements (generally relating to ownership by United States persons of interests in or accounts with those entities) have been satisfied by, or an exemption applies to, the payee (typically certified as to by the delivery of a properly completed IRS Form W-8BEN-E). Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules. Under certain circumstances, a Non-U.S. holder might be eligible for refunds or credits of such withholding taxes, and a Non-U.S. holder might be required to file
203
a U.S. federal income tax return to claim such refunds or credits. Thirty percent withholding under FATCA was scheduled to apply to payments of gross proceeds from the sale or other disposition of property that produces U.S.-source interest or dividends beginning on January 1, 2019, but on December 13, 2018, the IRS released proposed regulations that, if finalized in their proposed form, would eliminate the obligation to withhold on gross proceeds. Such proposed regulations also delayed withholding on certain other payments received from other foreign financial institutions that are allocable, as provided for under final Treasury Regulations, to payments of U.S.-source dividends, and other fixed or determinable annual or periodic income. Although these proposed Treasury Regulations are not final, taxpayers generally may rely on them until final Treasury Regulations are issued. However, there can be no assurance that final Treasury Regulations will provide the same exceptions from FATCA withholding as the proposed Treasury Regulations. Prospective investors should consult their tax advisors regarding the effects of FATCA on their investment in securities.
204
We are registering the issuance by us of up to 17,325,000 shares of our New Ginkgo Class A common stock issuable upon the exercise of the Private Placement Warrants and 34,499,925 shares of our New Ginkgo Class A common stock issuable upon the exercise of the Public Warrants. We are also registering the resale by the Selling Securityholders of up to 101,671,092 additional shares of our New Ginkgo Class A common stock (including 17,325,000 shares of New Ginkgo Class A common stock issuable upon the exercise of the Private Placement Warrants) and 17,325,000 Private Placement Warrants.
Once issued and upon effectiveness of the registration statement of which this prospectus forms a part, the securities beneficially owned by the Selling Securityholders covered by this prospectus may be offered and sold from time to time by the Selling Securityholders. The term “Selling Securityholders” includes donees, pledgees, transferees or other successors in interest selling securities received after the date of this prospectus from a Selling Securityholder as a gift, pledge, partnership distribution or other transfer. The Selling Securityholders will act independently of us in making decisions with respect to the timing, manner and size of each sale. Such sales may be made on one or more exchanges or in the over-the-counter market or otherwise, at prices and under terms then prevailing or at prices related to the then current market price or in negotiated transactions. Each Selling Securityholder reserves the right to accept and, together with its respective agents, to reject, any proposed purchase of securities to be made directly or through agents. The Selling Securityholders and any of their permitted transferees may sell their securities offered by this prospectus on any stock exchange, market or trading facility on which the securities are traded or in private transactions.
Subject to the limitations set forth in any applicable registration rights agreement, the Selling Securityholders may use any one or more of the following methods when selling the securities offered by this prospectus:
| • | purchases by a broker-dealer as principal and resale by such broker-dealer for its own account pursuant to this prospectus; |
| • | ordinary brokerage transactions and transactions in which the broker solicits purchasers; |
| • | one or more underwritten offerings; |
| • | block trades in which the broker-dealer so engaged will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| • | an exchange distribution in accordance with the rules of the applicable exchange; |
| • | in market transactions, including transactions on a national securities exchange or quotations service or over-the-counter market; |
| • | distributions to their members, partners or stockholders |
| • | settlement of short sales entered into after the date of the registration statement of which this prospectus is a part is declared effective by the SEC; |
| • | agreements with broker-dealers to sell a specified number of the securities at a stipulated price per share; |
| • | in “at the market” offerings, as defined in Rule 415 under the Securities Act, at negotiated prices, at prices prevailing at the time of sale or at prices related to such prevailing market prices, including sales made directly on a national securities exchange or sales made through a market maker other than on an exchange or other similar offerings through sales agents; |
| • | directly to purchasers, including through a specific bidding, auction or other process or in privately negotiated transactions; |
| • | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
205
| • | through a combination of any of the above methods of sale; or |
| • | any other method permitted pursuant to applicable law. |
In addition, a Selling Securityholder that is an entity may elect to make a pro rata in-kind distribution of securities to its members, partners or stockholders pursuant to the registration statement of which this prospectus is a part by delivering a prospectus with a plan of distribution. Such members, partners or stockholders would thereby receive freely tradeable securities pursuant to the distribution through a registration statement. To the extent a distributee is an affiliate of ours (or to the extent otherwise required by law), we may file a prospectus supplement in order to permit the distributees to use the prospectus to resell the securities acquired in the distribution.
The Selling Securityholders also may transfer the securities in other circumstances, in which case the transferees, pledgees or other successors-in-interest will be the selling beneficial owners for purposes of this prospectus. Upon being notified by a Selling Securityholder that a donee, pledgee, transferee, other successor-in-interest intends to sell our securities, we will, to the extent required, promptly file a supplement to this prospectus to name specifically such person as a Selling Securityholder.
To the extent required, the shares of New Ginkgo Class A common stock and Private Placement Warrants to be sold, the names of the Selling Securityholders, the respective purchase prices and public offering prices, the names of any agents, dealer or underwriter, any applicable commissions or discounts with respect to a particular offer will be set forth in an accompanying prospectus supplement or, if appropriate, a post-effective amendment to the registration statement that includes this prospectus.
The Selling Securityholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the shares of New Ginkgo Class A common stock and Private Placement Warrants in the course of hedging the positions they assume. The Selling Securityholders may also sell the shares of New Ginkgo Class A common stock and Private Placement Warrants short and deliver these securities to close out their short positions, or loan or pledge the shares of New Ginkgo Class A common stock and Private Placement Warrants to broker-dealers that in turn may sell these shares. The Selling Securityholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Securityholders also may in the future resell a portion of our New Ginkgo Class A common stock and Private Placement Warrants in open market transactions in reliance upon Rule 144 under the Securities Act, provided that they meet the criteria and conform to the requirements of that rule, or pursuant to other available exemptions from the registration requirements of the Securities Act.
Selling Securityholders may use this prospectus in connection with resales of shares of our New Ginkgo Class A common stock and Private Placement Warrants. This prospectus and any accompanying prospectus supplement will identify the Selling Securityholders, the terms of our New Ginkgo Class A common stock and any material relationships between us and the Selling Securityholders. In offering the securities covered by this prospectus, the Selling Securityholders and any underwriters, broker-dealers or agents who execute sales for the Selling Securityholders may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. Any discounts, commissions, concessions or profit they earn on any resale of those securities may be underwriting discounts and commissions under the Securities Act. Unless otherwise set forth in a prospectus supplement, the Selling Securityholders will receive all the net proceeds from the resale of shares of our New Ginkgo Class A common stock and Private Placement Warrants. If any Selling Securityholder is an “underwriter” within the meaning of Section 2(11) of the Securities Act, then the Selling Securityholder will be subject to the prospectus delivery requirements of the Securities Act. Underwriters and their controlling persons,
206
dealers and agents may be entitled, under agreements entered into with us and the Selling Securityholder, to indemnification against and contribution toward specific civil liabilities, including liabilities under the Securities Act.
In order to comply with the securities laws of certain states, if applicable, the securities must be sold in such jurisdictions only through registered or licensed brokers or dealers. In addition, in certain states the securities may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
We have advised the Selling Securityholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of shares in the market and to the activities of the Selling Securityholders and their affiliates. In addition, to the extent applicable we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the Selling Securityholders for the purpose of satisfying the prospectus delivery requirements of the Securities Act. The Selling Securityholders may indemnify any broker-dealer that participates in transactions involving the sale of the shares of New Ginkgo Class A common stock and Private Placement Warrants against certain liabilities, including liabilities arising under the Securities Act.
207
Latham & Watkins LLP has passed upon the validity of the New Ginkgo Class A common stock and Private Placement Warrants offered by this prospectus and certain other legal matters related to this prospectus.
The consolidated financial statements of Ginkgo Bioworks, Inc. at December 31, 2020 and 2019, and for each of the years then ended, appearing in this Prospectus and Registration Statement have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The financial statements Allonnia, LLC as of December 31, 2020 and for the period from November 27, 2019 (inception) through December 31, 2019 included in this prospectus have been audited by Wolf & Company, P.C., independent public accounting firm, as set forth in their report thereon, appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
CHANGE IN AUDITOR
On the Closing Date, the Audit Committee of the New Ginkgo Board approved the engagement of Ernst & Young LLP (“EY”) as the Company’s independent registered public accounting firm to audit the Company’s consolidated financial statements for the year ending December 31, 2021. WSB served as independent registered public accounting firm of SRNG prior to the consummation of the Business Combination. Accordingly, WSB was informed that it would be replaced by EY as the Company’s independent registered public accounting firm.
The reports of WSB on SRNG’s, the Company’s legal predecessor, balance sheet as of December 31, 2020 and the statements of operations, changes in stockholder’s equity and cash flows for the period from October 22, 2020 (date of inception) through December 31, 2020, did not contain an adverse opinion or a disclaimer of opinion, and were not qualified or modified as to uncertainties, audit scope or accounting principles.
During the period from October 22, 2020 (date of inception) through December 31, 2020 and the subsequent interim period through the Closing Date, there were no disagreements between the Company and WSB on any matter of accounting principles or practices, financial disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of WSB, would have caused it to make reference to the subject matter of the disagreements in its reports on the Company’s financial statements for such period.
During the period from October 22, 2020 (date of inception) through December 31, 2020 and the subsequent interim period through the Closing Date, there were no “reportable events” (as defined in Item 304(a)(1)(v) of Regulation S-K under the Exchange Act).
During the period from October 22, 2020 (inception) to the date the Board approved the engagement of EY as the Company’s independent registered public accounting firm, SRNG did not consult with EY on matters that involved the application of accounting principles to a specified transaction, the type of audit opinion that might be rendered on SRNG’s consolidated financial statements or any other matter that was either the subject of a disagreement or reportable event.
208
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1, including exhibits, under the Securities Act of 1933, as amended, with respect to the New Ginkgo Class A common stock and Private Placement Warrants offered by this prospectus. This prospectus does not contain all of the information included in the registration statement. For further information pertaining to us and our securities, you should refer to the registration statement and our exhibits.
In addition, we file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public on a website maintained by the SEC located at www.sec.gov. We also maintain a website at www.eagleequityptnrs.com. Through our website, we make available, free of charge, annual, quarterly and current reports, proxy statements and other information as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained on, or that may be accessed through, our website is not part of, and is not incorporated into, this prospectus.
209
GINKGO BIOWORKS, INC. AND SUBSIDIARIES
Index to Consolidated Financial Statements as of and for the Years Ended December 31, 2020 and 2019
| Page | ||||
| F-2 | ||||
| F-3 | ||||
| Consolidated Statements of Operations and Comprehensive Loss |
F-5 | |||
| F-6 | ||||
| F-8 | ||||
| F-10 | ||||
Index to Unaudited Interim Condensed Consolidated Financial Statements as of September 30, 2021 and for the Nine Months Ended September 30, 2021 and 2020
| Page | ||||
| F-63 | ||||
| Condensed Consolidated Statements of Operations and Comprehensive Loss |
F-64 | |||
| F-65 | ||||
| F-69 | ||||
| F-71 | ||||
ALLONNIA, LLC(1)
| F-106 | ||||
| Consolidated Financial Statements: |
||||
| F-107 | ||||
| F-108 | ||||
| F-109 | ||||
| F-110 | ||||
| F-111 |
| (1) | As of December 31, 2019 our investment in Allonnia LLC exceeded the 20% threshold in at least one of the tests under SEC’s Regulation S-X, Rule 3-09. Accordingly, we are attaching the audited financial statements of Allonnia LLC. |
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and the Board of Directors of Ginkgo Bioworks, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Ginkgo Bioworks, Inc. and subsidiaries (the Company) as of December 31, 2020 and 2019, the related consolidated statements of operations and comprehensive loss, stockholders’ equity and cash flows for the years then ended, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2020 and 2019, and the results of its operations and its cash flows for the years then ended in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2018.
Boston, Massachusetts
May 14, 2021,
Except for the reverse recapitalization described in Note 1, as to which the date is November 23, 2021
F-2
Ginkgo Bioworks, Inc. and Subsidiaries
(in thousands, except share and per share data)
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 380,801 | $ | 495,287 | ||||
| Accounts receivable, net |
16,694 | 3,461 | ||||||
| Accounts receivable, net from related parties (Note 20) |
5,212 | 4,217 | ||||||
| Inventory, net |
2,736 | — | ||||||
| Prepaid expenses and other current assets |
21,099 | 8,960 | ||||||
|
|
|
|
|
|||||
| Total current assets |
426,542 | 511,925 | ||||||
| Property and equipment, net |
121,435 | 63,132 | ||||||
| Investments |
60,504 | 61,574 | ||||||
| Equity method investments |
42,620 | 45,679 | ||||||
| Intangible assets, net |
3,294 | 3,843 | ||||||
| Goodwill |
1,857 | 1,857 | ||||||
| Loans receivable, net of current portion |
13,298 | 3,724 | ||||||
| Other non-current assets |
5,603 | 5,584 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 675,153 | $ | 697,318 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 13,893 | $ | 2,439 | ||||
| Accrued expenses and other current liabilities |
30,505 | 15,816 | ||||||
| Deferred revenue (includes $22,101 and $16,703, respectively, from related parties) |
28,823 | 21,819 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
73,221 | 40,074 | ||||||
| Non-current liabilities: |
||||||||
| Deferred rent, net of current portion |
12,678 | 11,633 | ||||||
| Deferred revenue, net of current portion (includes $97,977 and $125,628, respectively, from related parties) |
99,652 | 126,079 | ||||||
| Lease financing obligation |
16,518 | 16,767 | ||||||
| Other non-current liabilities |
3,032 | 912 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
205,101 | 195,465 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies (Note 11) |
||||||||
| Stockholders’ equity(1): |
||||||||
| Preferred stock, $0.0001 par value; 200,000,000 shares authorized; none issued and outstanding |
— | — | ||||||
| (1) | Retroactively restated for the reverse recapitalization as described in Note 1. |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
Ginkgo Bioworks, Inc. and Subsidiaries
Consolidated Balance Sheets
(in thousands, except share and per share data)
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Class A, Class B and Class C common stock $0.0001 par value; 15,800,000,000 shares authorized (Class A 10,500,000,000, Class B 4,500,000,000, Class C 800,000,000); 1,289,014,925 (Class A 974,224,443, Class B 314,790,482, Class C 0) and 1,256,237,919 (Class A 956,235,918, Class B 300,002,001, Class C 0) shares issued as of December 31, 2020 and December 31, 2019, respectively; 1,288,595,876 (Class A 974,166,577, Class B 314,429,299, Class C 0) and 1,255,562,032 (Class A 956,120,186, Class B 299,441,846, Class C 0) shares outstanding as of December 31, 2020 and December 31, 2019, respectively |
$ | 129 | $ | 126 | ||||
| Additional paid in capital |
929,125 | 834,206 | ||||||
| Accumulated deficit |
(467,878 | ) | (341,269 | ) | ||||
|
|
|
|
|
|||||
| Total Ginkgo Bioworks, Inc. stockholders’ equity |
461,376 | 493,063 | ||||||
| Non-controlling interest |
8,676 | 8,790 | ||||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
470,052 | 501,853 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 675,153 | $ | 697,318 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
Ginkgo Bioworks, Inc. and Subsidiaries
Consolidated Statements of Operations and Comprehensive Loss
(in thousands, except share and per share data)
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Foundry revenue (includes related party revenue of $42,535 and $35,268, respectively) |
$ | 59,221 | $ | 54,184 | ||||
| Biosecurity revenue: |
||||||||
| Product |
8,707 | — | ||||||
| Service |
8,729 | — | ||||||
|
|
|
|
|
|||||
| Total revenue |
76,657 | 54,184 | ||||||
|
|
|
|
|
|||||
| Costs and operating expenses: |
||||||||
| Cost of Biosecurity product revenue |
6,705 | — | ||||||
| Cost of Biosecurity service revenue |
8,906 | — | ||||||
| Research and development |
159,767 | 96,299 | ||||||
| General and administrative |
38,306 | 29,483 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
213,684 | 125,782 | ||||||
|
|
|
|
|
|||||
| Loss from operations |
(137,027 | ) | (71,598 | ) | ||||
| Other income (expense), net: |
||||||||
| Interest income |
2,582 | 5,756 | ||||||
| Interest expense |
(2,385 | ) | (2,421 | ) | ||||
| Loss on equity method investments |
(3,059 | ) | (46,936 | ) | ||||
| Loss on investments |
(1,070 | ) | (7,797 | ) | ||||
| Other income, net (includes $721 and $1,794, respectively, from related parties) |
16,125 | 3,161 | ||||||
|
|
|
|
|
|||||
| Total other income (expense), net |
12,193 | (48,237 | ) | |||||
|
|
|
|
|
|||||
| Loss before provision for income taxes |
(124,834 | ) | (119,835 | ) | ||||
| Provision for income taxes |
1,889 | 22 | ||||||
|
|
|
|
|
|||||
| Net loss and comprehensive loss |
(126,723 | ) | (119,857 | ) | ||||
| Net loss and comprehensive loss attributable to non-controlling interest |
(114 | ) | (530 | ) | ||||
|
|
|
|
|
|||||
| Net loss and comprehensive loss attributable to Ginkgo Bioworks, Inc. stockholders |
$ | (126,609 | ) | $ | (119,327 | ) | ||
|
|
|
|
|
|||||
| Net loss per share attributable to Ginkgo Bioworks, Inc. common stockholders, basic and diluted (1) |
$ | (0.10 | ) | $ | (0.10 | ) | ||
|
|
|
|
|
|||||
| Weighted average common shares outstanding, basic and diluted (1) |
1,274,766,915 | 1,149,000,417 | ||||||
|
|
|
|
|
|||||
| (1) | Retroactively restated for the reverse recapitalization as described in Note 1. |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
Ginkgo Bioworks, Inc. and Subsidiaries
Consolidated Statements of Stockholders’ Equity
(in thousands, except share data)
| Series B, C, D, E Convertible Preferred Stock |
Old Ginkgo Common Stock |
Common Stock | ||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||
| Balance as of December 31, 2018 (as previously reported) |
14,943,599 | $ | 149 | 8,555,710 | $ | 86 | — | $ | — | |||||||||||||||
| Retroactive application of the reverse recapitalization |
(14,943,599 | ) | (149 | ) | (8,555,710 | ) | (86 | ) | 1,153,356,703 | 116 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2018 |
— | — | — | — | 1,153,356,703 | 116 | ||||||||||||||||||
| Exercise of stock options |
— | — | — | — | 500,621 | — | ||||||||||||||||||
| Issuance of Series E convertible preferred stock, net of issuance costs of $4,830 |
— | — | — | — | 69,812,427 | 7 | ||||||||||||||||||
| Recognition of beneficial conversion feature related to issuance of convertible promissory notes |
— | — | — | — | — | — | ||||||||||||||||||
| Reacquisition of beneficial conversion feature related to convertible promissory notes |
— | — | — | — | — | — | ||||||||||||||||||
| Conversion of convertible promissory notes into Series E convertible preferred stock |
— | — | — | — | 69,151,117 | 7 | ||||||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | 367,858 | — | ||||||||||||||||||
| Repurchase of common stock |
— | — | — | — | — | — | ||||||||||||||||||
| Retirement of treasury stock |
— | — | — | — | (37,626,694 | ) | (4 | ) | ||||||||||||||||
| Issuance of warrants to purchase convertible preferred stock |
— | — | — | — | — | — | ||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | ||||||||||||||||||
| Net loss and comprehensive loss |
— | — | — | — | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2019 |
— | — | — | — | 1,255,562,032 | 126 | ||||||||||||||||||
| Exercise of stock options |
— | — | — | — | 1,921,941 | — | ||||||||||||||||||
| Issuance of Series E convertible preferred stock, net of issuance costs of $0 |
— | — | — | — | 30,855,065 | 3 | ||||||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | 256,838 | — | ||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | ||||||||||||||||||
| Net loss and comprehensive loss |
— | — | — | — | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2020 |
— | $ | — | — | $ | — | 1,288,595,876 | 129 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
Ginkgo Bioworks, Inc. and Subsidiaries
Consolidated Statements of Stockholders’ Equity
(in thousands, except share data)
| Treasury Stock | ||||||||||||||||||||||||
| Shares | Amount | Additional Paid-In Capital |
Accumulated Deficit |
Non-Controlling Interest |
Total Stockholders’ Equity |
|||||||||||||||||||
| Balance as of December 31, 2018 (as previously reported) |
(756,633 | ) | $ | (24,449 | ) | $ | 450,268 | $ | (221,942 | ) | $ | 9,320 | $ | 213,432 | ||||||||||
| Retroactive application of the reverse recapitalization |
(36,379,256 | ) | (4 | ) | 123 | — | — | — | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2018 |
(37,135,889 | ) | (24,453 | ) | 450,391 | (221,942 | ) | 9,320 | 213,432 | |||||||||||||||
| Exercise of stock options |
— | — | 7 | — | — | 7 | ||||||||||||||||||
| Issuance of Series E convertible preferred stock, net of issuance costs of $4,830 |
— | — | 208,794 | — | — | 208,801 | ||||||||||||||||||
| Recognition of beneficial conversion feature related to issuance of convertible promissory notes |
— | — | 198,957 | — | — | 198,957 | ||||||||||||||||||
| Reacquisition of beneficial conversion feature related to convertible promissory notes |
— | — | (211,608 | ) | — | — | (211,608 | ) | ||||||||||||||||
| Conversion of convertible promissory notes into Series E convertible preferred stock |
— | — | 211,601 | — | — | 211,608 | ||||||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | — | — | ||||||||||||||||||
| Repurchase of common stock |
(490,805 | ) | (408 | ) | — | — | — | (408 | ) | |||||||||||||||
| Retirement of treasury stock |
37,626,694 | 24,861 | (24,857 | ) | — | |||||||||||||||||||
| Issuance of warrants to purchase convertible preferred stock |
— | — | 150 | — | — | 150 | ||||||||||||||||||
| Stock-based compensation expense |
— | — | 771 | — | — | 771 | ||||||||||||||||||
| Net loss and comprehensive loss |
— | — | — | (119,327 | ) | (530 | ) | (119,857 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2019 |
— | — | 834,206 | (341,269 | ) | 8,790 | 501,853 | |||||||||||||||||
| Exercise of stock options |
— | — | 26 | — | — | 26 | ||||||||||||||||||
| Issuance of Series E convertible preferred stock, net of issuance costs of $0 |
— | — | 94,417 | — | — | 94,420 | ||||||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | — | — | ||||||||||||||||||
| Stock-based compensation expense |
— | — | 476 | — | — | 476 | ||||||||||||||||||
| Net loss and comprehensive loss |
— | — | — | (126,609 | ) | (114 | ) | (126,723 | ) | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2020 |
— | — | $ | 929,125 | $ | (467,878 | ) | $ | 8,676 | $ | 470,052 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
Ginkgo Bioworks, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(in thousands)
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Cash flow from operating activities: |
||||||||
| Net loss |
$ | (126,723 | ) | $ | (119,857 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation and amortization |
13,864 | 10,755 | ||||||
| Stock-based compensation |
476 | 771 | ||||||
| Loss attributable to equity method investments |
3,059 | 46,936 | ||||||
| Non-cash interest expense related to payments on lease financing obligations |
— | 51 | ||||||
| Non-cash interest expense related to amortization of debt discount on convertible promissory notes |
— | 71 | ||||||
| Gain on extinguishment of convertible promissory notes |
— | (71 | ) | |||||
| Loss attributable to investments |
1,070 | 7,797 | ||||||
| Accrued interest income on loan receivable |
— | (163 | ) | |||||
| Gain on termination of Glycosyn, LLC agreement |
— | (1,530 | ) | |||||
| Change in fair value of loans receivable |
(1,061 | ) | — | |||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable, net |
(13,233 | ) | 378 | |||||
| Accounts receivable, net from related parties (Note 20) |
(995 | ) | (2,221 | ) | ||||
| Prepaid expenses and other current assets |
(11,352 | ) | (4,031 | ) | ||||
| Inventory, net |
(2,736 | ) | — | |||||
| Other non-current assets |
1,834 | (2,361 | ) | |||||
| Accounts payable |
7,019 | 664 | ||||||
| Accrued expenses and other current liabilities |
8,665 | 4,170 | ||||||
| Deferred revenue, current and non-current (includes $(22,253) and $3,112, respectively, from related parties) |
(19,423 | ) | 4,883 | |||||
| Deferred rent, non-current |
1,045 | 9,095 | ||||||
| Other non-current liabilities |
2,661 | — | ||||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(135,830 | ) | (44,663 | ) | ||||
| Cash flow from investing activities: |
||||||||
| Purchases of property and equipment |
(57,821 | ) | (22,219 | ) | ||||
| Purchase of loan receivable from Access Bio, Inc. |
(10,000 | ) | — | |||||
| Issuance of loans receivable |
(100 | ) | (2,250 | ) | ||||
| Cash paid for investment in Synlogic, Inc. |
— | (50,133 | ) | |||||
| Proceeds from loans receivable |
800 | — | ||||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
(67,121 | ) | (74,602 | ) | ||||
| Cash flow from financing activities: |
||||||||
| Proceeds from exercise of stock options |
26 | 7 | ||||||
| Repurchase of common stock |
— | (408 | ) | |||||
| Principal payment on capital lease obligations |
(598 | ) | (736 | ) | ||||
| Proceeds from lease financing obligations |
— | 476 | ||||||
| Principal payment on lease financing obligations |
(150 | ) | (92 | ) | ||||
| Proceeds from issuance of convertible promissory notes, net of issuance costs |
— | 198,957 | ||||||
| Proceeds from issuance of Series E convertible preferred stock, net of issuance costs |
91,040 | 212,181 | ||||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
90,318 | 410,385 | ||||||
| Net increase (decrease) in cash, cash equivalents and restricted cash |
(112,633 | ) | 291,120 | |||||
The accompanying notes are an integral part of these consolidated financial statements.
F-8
Ginkgo Bioworks, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(in thousands)
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Cash, cash equivalents and restricted cash, beginning of period |
$ | 498,510 | $ | 207,390 | ||||
|
|
|
|
|
|||||
| Cash, cash equivalents and restricted cash, end of period |
$ | 385,877 | $ | 498,510 | ||||
|
|
|
|
|
|||||
| Supplemental disclosure of cash flow information: |
||||||||
| Cash paid for interest |
$ | 2,572 | $ | 2,348 | ||||
| Cash paid for income taxes |
$ | — | $ | 31 | ||||
| Supplemental disclosure of non-cash investing and financing activities: |
||||||||
| Purchases of equipment through capital leases |
$ | — | $ | 406 | ||||
| Purchases of property and equipment included in accounts payable and accrued expenses |
$ | 14,458 | $ | 605 | ||||
| Conversion of convertible promissory notes into Series E convertible preferred stock |
$ | — | $ | 211,608 | ||||
| Series E convertible preferred stock issuance costs included in accrued expenses |
$ | — | $ | 3,380 | ||||
| Issuance of loan receivable upon amendment of Glycosyn, LLC agreement |
$ | — | $ | 2,744 | ||||
| Allonnia, LLC equity interest received for intellectual property |
$ | — | $ | 24,480 | ||||
| Loan receivable received as consideration under customer arrangement |
$ | 375 | $ | — | ||||
The following table provides a reconciliation of the cash, cash equivalents and restricted cash balances as of each of the periods shown above:
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Cash and cash equivalents |
$ | 380,801 | $ | 495,287 | ||||
| Restricted cash |
5,076 | 3,223 | ||||||
|
|
|
|
|
|||||
| Total cash, cash equivalents and restricted cash |
$ | 385,877 | $ | 498,510 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these consolidated financial statements.
F-9
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
1. Organization and Basis of Presentation
Business
The mission of Ginkgo Bioworks, Inc. (“Ginkgo Bioworks”, “Ginkgo”, or the “Company”) is to make biology easier to engineer. The Company designs custom cells for customers across multiple markets. Since inception, the Company has devoted its efforts to improving its platform for programming cells to enable customers to leverage biology to create impactful products across a range of industries. The Company’s platform comprises (i) equipment, robotic automation, software, data pipelines and tools, and standard operating procedures for high throughput genetic engineering, fermentation, and analytics (referred to collectively as the “Foundry”), (ii) a library of proprietary genetic assets and associated performance data (referred to collectively as “Codebase”), and (iii) the Company’s team of expert users, developers and operators of the Foundry and Codebase.
Reverse Recapitalization
On September 16, 2021, Soaring Eagle Acquisition Corp. (“SRNG”) consummated the merger transaction contemplated by the agreement and plan of merger, dated as of May 11, 2021, and amended on May 14, 2021 (the “Merger Agreement”), by and among SRNG, SEAC Merger Sub Inc., a Delaware corporation (“Merger Sub”), and Ginkgo Bioworks, Inc., a Delaware corporation (“Old Ginkgo”), whereby Merger Sub merged with and into Ginkgo, the separate corporate existence of Merger Sub ceased and Ginkgo survived the merger as a wholly owned subsidiary of SRNG (the “Business Combination”). In connection with the consummation of the Business Combination, SRNG changed its name to “Ginkgo Bioworks Holdings, Inc.” and, among other transactions contemplated by the Merger Agreement, the existing equity holders of Ginkgo exchanged their equity interests of Ginkgo Bioworks, Inc. for equity interests of Ginkgo Bioworks Holdings, Inc.
The Business Combination was accounted for as a reverse recapitalization, in accordance with U.S. GAAP (the “Reverse Recapitalization”). Under this method of accounting, SRNG was treated as the “acquired” company for financial reporting purposes. Accordingly, for accounting purposes, the Reverse Recapitalization was treated as the equivalent of Ginkgo issuing stock for the net assets of SRNG, accompanied by a recapitalization. Accordingly, all historical financial information presented in these consolidated financial statements represents the accounts of Ginkgo and its subsidiaries “as if” Ginkgo is the predecessor and legal successor to SRNG.
As a result of the Business Combination, the shares and corresponding capital amounts and loss per share related to Ginkgo’s outstanding convertible preferred stock and common stock prior to the Business Combination have been retroactively restated to reflect the exchange ratio of 49.080452 (“Exchange Ratio”) established in the Merger Agreement.
Liquidity and Capital Resources
As of December 31, 2020, the Company had $380.8 million in cash and cash equivalents. The Company’s sources of liquidity have been predominantly from proceeds from equity offerings, convertible note offerings, fees received for research and development services under license and collaboration arrangements, including those received on an upfront basis and upon accomplishment of milestones, fees received from Biosecurity product sales and services provided and government grants. These sources of liquidity have enabled the Company to expand the physical footprint and capacity of the Foundry and grow its personnel to expand capabilities and enter new markets.
The Company has incurred significant operating losses from inception through December 31, 2020, resulting in negative cash flows from operating activities and an accumulated deficit of $467.9 million as of December 31, 2020. The Company expects to continue to incur net losses into the foreseeable future. Successful transition to
F-10
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
profitable operations is dependent upon achieving technical and commercial milestones under existing customer agreements, continuing to increase Foundry output while reducing the unit cost of that output, and expanding the number of engineered organisms under development with customers. The Company plans to continue to fund its losses from operations through future debt and equity financings, liquidation of equity holdings, and new customer and collaborative arrangements. The Company believes that its current cash and cash equivalents will provide adequate liquidity through one year from the date that these consolidated financial statements are issued.
The Company’s future liquidity needs may vary materially from those currently planned and will depend on many factors, including the achievement of technical and commercial milestones under existing customer arrangements, the receipt of cash and equity from new customers and in connection with collaborative arrangements, the investments required to further scale the Foundry and Codebase, and the expenses needed to attract and retain personnel.
Risks and Uncertainties
The Company is subject to a number of risks including rapid technological change, regulatory change, technical feasibility, commercial viability, public perception of genetically modified organisms, uncertain market acceptance of products derived from engineered organisms, alternative means of production, data and cybersecurity breaches, and dependence on key vendors and personnel.
Impact of the COVID-19 Pandemic
In December 2019, an outbreak of a novel strain of coronavirus (“COVID-19”) originated in Wuhan, China, and has since spread globally. On March 11, 2020, the World Health Organization characterized COVID-19 as a pandemic and, on March 13, 2020, the United States declared a national emergency with respect to COVID-19. Since then, extraordinary actions have been taken by authorities to contain and manage the outbreak and spread of COVID-19 around the world.
Consistent with the actions taken by governmental authorities, the Company has taken steps to protect its workforce and support the community efforts. From approximately March 2020 to approximately June 2020, the Company operated at a reduced capacity. The Company also restricted non-essential travel and allowed most of its workforce in general and administration functions to perform their duties remotely. In June 2020, the Company resumed modified on-site operations for its lab workers following the Center for Disease Control and Prevention’s guidance with facial covering requirements, rearranging facilities to follow social distancing protocols, performing active daily health checks, and undertaking regular and thorough disinfection of surfaces and tools.
The COVID-19 pandemic caused some disruption in the Company’s operations and the Company experienced partial suspensions and delays in servicing certain customer contracts. However, the Company believes that the COVID-19 pandemic did not have a material adverse impact to its financial position or results of operations.
The Company continues to monitor and assess the effects of the COVID-19 pandemic on its business, financial condition, results of operations and cash flows.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America (“U.S. GAAP”). Any reference in these notes to
F-11
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
applicable guidance is meant to refer to the authoritative U.S. GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”). All adjustments, consisting of normal recurring adjustments, necessary for a fair presentation have been included.
Emerging Growth Company Status
The Company is an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. The Company has elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that (i) the Company is no longer an emerging growth company or (ii) the Company affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, these consolidated financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates.
Principles of Consolidation
The Company’s wholly owned subsidiaries include Ginkgo Bioworks Security Corporation (“GBSC”), Gen9, Inc. (“Gen9”) and Stegodon Corporation, which, along with Ginkgo Bioworks, Inc., were incorporated under the laws of the State of Delaware. The Company also has a controlling financial interest in Cooksonia, LLC (“Cooksonia”) which is the holding entity for the Company’s investment in Joyn Bio, LLC (“Joyn”). The accompanying consolidated financial statements reflect the Company’s operations and those of subsidiaries in which the Company has a controlling financial interest. All intercompany accounts and transactions have been eliminated.
Variable Interest Entities
The Company evaluates its variable interests in variable interest entities (“VIE”) and consolidates VIEs when the Company is the primary beneficiary. The Company determines whether it is the primary beneficiary of each VIE based on its assessment of whether the Company possesses both (i) the power to direct the activities that most significantly affect the VIE’s economic performance and (ii) the obligation to absorb losses that could be significant to the VIE or the right to receive benefits that could be significant to the VIE. The Company reevaluates the accounting for its VIEs upon the occurrence of events that could change the primary beneficiary conclusion. As of December 31, 2020 and 2019, the maximum risk of loss related to the Company’s VIEs was limited to the carrying value of its investment in such entities.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. Significant estimates and assumptions used in preparation of these consolidated financial statements include, among others, those related to the fair value of equity instruments and equity awards, revenue recognition, the fair value of loans receivable, the fair value of certain investments, including equity method investments, accrued expenses, and income taxes.
F-12
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The Company bases its estimates on historical experience and other market-specific or relevant assumptions that it believes to be reasonable under the circumstances. Reported amounts and disclosures reflect the overall economic conditions that management believes are most likely to occur, and the anticipated measures management intends to take. Actual results could differ materially from those estimates. All revisions to accounting estimates are recognized in the period in which the estimates are revised.
Segment Information
The Company and the Chief Operating Decision Maker (“CODM”), which is comprised of the Chief Executive Officer and the Chief Operating Officer, view the Company’s operations and manage the business as a single operating segment. Strategic decisions are managed centrally, and consistent with this decision-making process, the CODM uses consolidated financial information for purposes of evaluating performance, allocating resources, as well as forecasting future period financial results. The majority of the Company’s long-lived assets are held in the United States.
For the year ended December 31, 2020, two customers, both of which were related parties, accounted for 27.1% and 12.3%, respectively, of the Company’s total revenue. No other customers exceeded more than 10% of the Company’s total revenue during the year ended December 31, 2020.
For the year ended December 31, 2019, three customers that were related parties and one customer that was not a related party accounted for 35.0%, 17.3%, 11.5% and 13.5%, respectively, of the Company’s total revenue. No other customers exceeded more than 10% of the Company’s total revenue during the year ended December 31, 2019.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash and cash equivalents, accounts receivable, and loans receivable. The Company’s cash is maintained in bank deposit accounts and money market funds, which, at times, may exceed federally insured limits. The Company believes that it is not exposed to significant credit risk as its deposits are held in financial institutions in the United States that management believes to be of high credit quality. The Company’s loans receivable are comprised of both collateralized convertible notes, which limits the Company’s credit risk, as well as uncollateralized convertible notes. The Company’s accounts receivable primarily consists of amounts owed under its license and collaboration agreements. The Company has not experienced any material write-offs related to its accounts receivable since inception.
Cash and Cash Equivalents
The Company considers all highly liquid investments with original maturities of three months or less at the date of purchase to be cash equivalents. Cash and cash equivalents include cash held in banks and amounts held in money market accounts. The carrying value of the Company’s cash and cash equivalents approximate fair value due to their short-term maturities.
Restricted Cash
Restricted cash primarily includes cash balances collateralizing letters of credit associated with leases for the Company’s facilities and is included in other non-current assets on the Consolidated Balance Sheets.
F-13
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Accounts Receivable, net
Accounts receivable consists of credit extended to customers in the normal course of business and is reported at the estimated net realizable value. Accounts receivable includes unbilled amounts that have been recognized in revenue but have not yet been invoiced based on timing differences and the terms of the underlying arrangements.
The Company maintains an allowance for doubtful accounts to provide for the estimated amounts of receivables that will not be collected. The allowance is based upon an assessment of customer creditworthiness, historical payment experience, the age of outstanding receivables and collateral to the extent applicable. The Company re-evaluates such allowance on a regular basis and adjusts the allowance as needed. Once a receivable is deemed to be uncollectible, such balance is charged against the allowance.
Inventory, net
Inventory mainly consists of diagnostic testing kits purchased from suppliers. The Company values inventory at the lower of cost or net realizable value using the first-in first-out method. Inventory has been reduced by an allowance for lost and defective inventory based on an analysis of quantities on hand.
Loans Receivable
The Company has elected the fair value option under ASC 825, Financial Instruments (“ASC 825”) to account for its loans receivable. The Company classifies the current portion of the loans receivable balance as a component of prepaid expenses and other current assets on the Consolidated Balance Sheets, with the current portion determined based on the principal balance of the loan that matures within one year from the balance sheet date. The Company records the loans receivable at fair value and recognizes changes in fair value as a component of other income (expense), net in the Consolidated Statements of Operations and Comprehensive Loss.
Property and Equipment, net
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation and amortization are computed using the straight-line method over the estimated useful lives of the assets or the remaining lease term with respect to leasehold improvement assets. Estimated lives of property and equipment are as follows:
| Estimated Useful Life | ||
| Computer equipment and software |
2 to 5 years | |
| Furniture and fixtures |
7 years | |
| Lab equipment |
1 to 5 years | |
| Facilities |
15 to 30 years | |
| Leasehold improvements |
Shorter of useful life or remaining lease term |
Expenditures for maintenance and repairs are expensed as incurred. When assets are retired or otherwise disposed of, the related cost and accumulated depreciation or amortization is removed from the accounts and any resulting gain or loss is reflected in other income, net in the Consolidated Statements of Operations and Comprehensive Loss.
F-14
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Construction in progress relates to assets which have not been placed in service as of period end. Facilities relate to assets acquired under the Company’s build-to-suit arrangement. Refer to Note 11 for discussion of the build-to-suit lease.
Equity Method Investments
The Company utilizes the equity method to account for its investments in common stock, or in-substance common stock, of the Company’s strategic partnerships when it possesses the ability to exercise significant influence, but not control, over the operating and financial policies of the investee. The Company uses judgment when determining the level of influence over the operating and financial policies of the investee considering key factors including, among others, the Company’s ownership interest, representation on the board of directors, participation in policy-making decisions and material contractual arrangements and obligations. Income and losses are allocated based upon relative ownership interest unless there is a substantive profit-sharing agreement in place.
For investments with a substantive profit-sharing agreement, the Company utilizes the Hypothetical Liquidation at Book Value (“HLBV”) method to allocate income and losses from the equity method investment. Under the HLBV method, the Company utilizes the capital account at the end of the period assuming the book value of the entity was liquidated or sold minus the same calculation at the beginning of the period. The difference is the share of earnings or losses attributable to the equity method investment.
Under the equity method, if there is a commitment for the Company to fund the losses of its equity method investees, the Company would continue to record its share of losses resulting in a negative equity method investment, which would be presented as a liability on the Consolidated Balance Sheets. Commitments may be explicit and may include formal guarantees, legal obligations, or arrangements by contract. Implicit commitments may arise from reputational expectations, intercompany relationships, statements by the Company of its intention to provide support, a history of providing financial support or other facts and circumstances. When the Company has no commitment to fund the losses of its equity method investees, the carrying value of its equity method investments will not be reduced below zero. The Company had no commitment to fund additional losses of its equity method investments during the years ended December 31, 2020 and 2019.
The Company evaluates its equity method investments for impairment whenever events or circumstances indicate that the carrying value of the investment may not be recoverable. The Company considers the investee’s financial position, forecasts and economic outlook, and the estimated duration and extent of losses to determine whether a recovery is anticipated. An impairment that is other-than-temporary is recognized in the period identified. The Company has not recognized an impairment loss relative to its equity method investments for the years ended December 31, 2020 and 2019.
The Company may elect the fair value option for its equity method investments on an investment-by-investment basis. For all equity method investments accounted for under the fair value option, the Company carries the equity method investment at fair value. The Company records all subsequent changes in the values of its equity method investments in the Consolidated Statements of Operations and Comprehensive Loss as a component of loss on equity method investments.
Investments
Investments include warrants and non-marketable equity securities where the Company does not possess the ability to exercise significant influence over the investee.
F-15
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The Company has elected to account for the warrants using the fair value option. Subsequent changes in fair values are presented as a component of loss on investments in the Consolidated Statements of Operations and Comprehensive Loss.
Investments in non-marketable equity securities for which the fair value option is not elected and that do not have readily determinable fair values are carried at cost, less any impairments, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer. Each period the Company assesses relevant transactions to identify observable price changes, and the Company regularly monitors these investments to evaluate whether there is an indication an investment is impaired. The Company evaluates whether an investment’s fair value is less than its carrying value using an estimate of fair value, if such an estimate is available. For periods in which there is no estimate of fair value for an investment, the Company evaluates whether an event or change in circumstances has occurred that may have a significant adverse effect on the value of the investment. The Company has not recognized an impairment loss, nor any upward or downward adjustments resulting from observable price changes in identical or similar investments, for the years ended December 31, 2020 and 2019.
Fair Value Measurements
The Company categorizes its assets and liabilities measured at fair value in accordance with the authoritative accounting guidance that establishes a consistent framework for measuring fair value and requires disclosures for each major asset and liability category measured at fair value on either a recurring or nonrecurring basis.
ASC 820, Fair Value Measurement (“ASC 820”), establishes a fair value hierarchy for instruments measured at fair value that distinguishes between assumptions based on market data (observable inputs) and the Company’s own assumptions (unobservable inputs). Observable inputs are inputs that market participants would use in pricing the asset or liability based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the inputs that market participants would use in pricing the asset or liability and are developed based on the best information available in the circumstances.
ASC 820 identifies fair value as the exchange price, or exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As a basis for considering market participant assumptions in fair value measurements, ASC 820 establishes a three-tier fair value hierarchy that distinguishes among the following:
| • | Level 1- Quoted prices (unadjusted) in active markets for identical assets or liabilities; |
| • | Level 2- Inputs other than quoted prices included within Level 1 that are either directly or indirectly observable; and |
| • | Level 3- Unobservable inputs in which little or no market activity exists, therefore requiring an entity to develop its own assumptions about the assumptions that market participants would use in pricing. |
To the extent that the valuation is based on models or inputs that are either less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized in Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
The Company valued its money market fund holdings, loans receivable, and certain equity method investments and investments accounted for pursuant to the fair value option on a recurring basis.
F-16
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The carrying amounts of the Company’s other financial instruments, which include accounts receivable, certain prepaid expenses and other current assets, accounts payable and accrued expenses and other current liabilities, approximate their fair values, primarily due to their short-term nature.
Impairment of Long-Lived Assets
The Company reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying value of an asset may not be recoverable. Recoverability is measured by comparing the book values of the assets to the expected future net undiscounted cash flows that the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the book values of the assets exceed their fair value. The Company has not recognized any impairment losses during the years ended December 31, 2020 and 2019.
Intangible Assets, net
Intangible assets, net consist of certain definite-lived assets including patents, processes and know-how related to technology acquired through a business combination. The Company amortizes such intangible assets on a straight-line basis over their estimated useful life.
The Company reviews intangible assets for impairment whenever events or changes in circumstances have occurred which could indicate that the carrying value of the assets are not recoverable. Recoverability is measured by comparing the carrying value of the intangible assets to the future undiscounted cash flows expected to be generated by the asset. In determining the expected future cash flows, the Company uses assumptions believed to be reasonable, but which are unpredictable and inherently uncertain. Actual future cash flows may differ from the estimates used in impairment testing. The Company recognizes an impairment loss when and to the extent that the estimated fair value of an intangible asset is less than its carrying value. The Company has not recognized an impairment loss for the years ended December 31, 2020 and 2019.
Goodwill
Goodwill represents the excess of acquisition cost over the fair market value of the net assets acquired. The Company assesses the carrying value of goodwill for impairment on at least an annual basis, the assessment of which requires significant judgment. The Company first considers qualitative factors that indicate impairment may have occurred. Such indicators may include deterioration in economic conditions, adverse market conditions, technological obsolescence, other factors that are indicative of negative or declining cash flows, or an increase in costs over multiple periods in excess of those already factored into the fair value assessment. If the qualitative assessment indicates a reduction in the carrying value is more likely than not to have occurred, the Company performs a quantitative assessment, comparing the fair value of the reporting unit to its carrying value, including goodwill. In the Company’s case, the entire organization represents a single reporting unit. If the carrying value of the reporting unit exceeds the fair value, an impairment has occurred, and an impairment loss is recognized. The fair value of the reporting unit is primarily determined based on the income approach. The income approach is a valuation technique in which fair value is based on the forecasted future cash flows, discounted at the appropriate rate of return commensurate with the risk as well as current rates of return for equity and debt capital as of the valuation date. The Company has not recognized an impairment loss for the years ended December 31, 2020 and 2019.
F-17
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Deferred Rent
Deferred rent consists of the difference between cash paid and rent expense recognized on a straight-line basis for the facilities that the Company occupies under operating leases. The Company classifies the current portion of the deferred rent balance as a component of accrued expenses and other current liabilities on the Consolidated Balance Sheets.
Treasury Stock
The Company recorded repurchases of common stock at cost in treasury stock, which is presented as a reduction to stockholders’ equity in the Consolidated Statements of Stockholders’ Equity. When the repurchase price of treasury stock exceeded the fair value of the common stock, the Company recognized the incremental amount as compensation expense in the Consolidated Statements of Operations and Comprehensive Loss. During the year ended December 31, 2019, all shares of treasury stock were returned to authorized and unissued shares of common stock and no shares of common stock remained in treasury as of December 31, 2020 and 2019.
Revenue Recognition
The Company accounts for revenue in accordance with ASC 606, Revenue from Contracts with Customers (“ASC 606”). Under ASC 606, the Company recognizes revenue when the customer obtains control of the promised goods or services at an amount that reflects the consideration the Company expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that are within the scope of ASC 606, the Company performs the following five steps: (i) identify the contract(s) with a customer, (ii) identify the promises and distinct performance obligations in the contract, (iii) determine the transaction price, (iv) allocate the transaction price to the performance obligations in the contract, and (v) recognize revenue when (or as) the Company satisfies the performance obligations.
Foundry Revenue
The Company generates license and service revenue through the execution of license and collaboration agreements whereby customers obtain license rights to the Company’s proprietary technology and intellectual property for use in the research, development and commercialization of engineered organisms, and derived products. Under these agreements, the Company typically provides research and development services, which includes the provision of a license to the Company’s intellectual property. Additionally, the customer obtains license rights to the output of the Company’s services in order to commercialize the resulting output of such services. Generally, the terms of these agreements provide that the Company receives some combination of: (1) Foundry usage fees in the form of (i) upfront payments upon consummation of the agreement or other fixed payments, (ii) reimbursement for costs incurred for research and development services and (iii) milestone payments upon the achievement of specified technical criteria, plus (2) downstream value share payments in the form of (iv) milestone payments upon the achievement of specified commercial criteria, (v) royalties on sales of products from or comprising engineered organisms arising from the collaboration or licensing agreement and (vi) royalties related to cost of goods sold reductions realized by customers.
The Company’s collaboration and licensing agreements often contain multiple promises, including (i) licenses and assignments of intellectual property and materials and (ii) research and development services, and the Company determines whether each of the promises is a distinct performance obligation based on the nature of each agreement. As the Company is generally performing research and development services that are highly integrated and interrelated to the licenses and assignments of intellectual property and materials, the promises are generally inseparable. As such, the Company typically combines the research and development services, licenses,
F-18
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
and assignments into a single performance obligation. However, for certain agreements, the Company only grants licenses or effects such transfers and assignments upon the successful completion of the research and development services or delivery of a developed product. For these agreements, the Company typically considers (i) the research and development services and (ii) the licenses, transfers, and assignments as distinct performance obligations, as each is transferred separately and has a separately identifiable benefit.
Options to acquire additional goods and services are evaluated to determine if such options provide a material right to the counterparty that it would not have received without entering into the contract. If so, the option is accounted for as a separate performance obligation. If not, the option is considered a marketing offer which is accounted for as a separate contract upon the counterparty’s election.
At contract inception, the Company determines the transaction price, including fixed consideration and any estimated amounts of variable consideration. Any upfront cash payment received upon consummation of the agreement is fixed and generally non-refundable. Variable consideration is subject to a constraint, and amounts are included in the transaction price to the extent that it is probable that a significant reversal in the amount of cumulative revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. Variable consideration may include reimbursement for costs incurred for the Company’s research and development efforts, milestone payments upon the achievement of certain technical and commercial criteria, and royalties on sales of products from or comprising engineered organisms arising from the agreement. With respect to the research and development reimbursements and milestone payments, the Company uses the most likely amount method to estimate variable consideration. With respect to agreements that include royalties on sales or other contingent payments based on sales, the Company applies the royalty recognition constraint which requires a constraint until the royalty or value-sharing transaction occurs. Certain agreements contain payment in the form of equity or other non-cash consideration. Any non-cash consideration is measured at the fair value of the non-cash consideration at contract inception.
For agreements with promises that are combined into a single performance obligation, the entire transaction price is allocated to the single performance obligation. For agreements with multiple performance obligations, the transaction price is allocated to the performance obligations using the relative standalone selling price methodology. For agreements featuring variable consideration, the Company allocates variable consideration to one or more, but not all, performance obligations if certain conditions are met. Specifically, the Company assesses whether the variable consideration relates solely to its efforts to satisfy the performance obligation and whether allocating such variable consideration entirely to the performance obligation is consistent with the overall allocation objective. If these conditions are not met, the Company allocates the variable consideration based on the relative standalone selling price methodology. The key assumptions utilized in determining the standalone selling price for each performance obligation include development timelines, estimated research and development costs, commercial markets, likelihood of exercise (in the case of options considered to be material rights), and probabilities of success.
For agreements where the licenses or assignments are considered separate performance obligations or represent the only performance obligation, the Company recognizes revenue at the point in time that the Company effectively grants the license as the licenses or assignments represent functional intellectual property. For agreements where the licenses and the research and development services represent a combined performance obligation, the Company recognizes revenue over the period of performance based on costs incurred to date as compared to total estimated costs.
The Company evaluates its measure of progress to recognize revenue at each reporting period and, as necessary, adjusts the measure of performance and related revenue recognition. The Company’s measure of performance
F-19
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
and revenue recognition involves significant judgment and assumptions, including, but not limited to, estimated costs and timelines to complete its performance obligations. The Company evaluates contract modifications and amendments to determine whether any changes should be accounted for prospectively or on a cumulative catch-up basis. The Company utilizes the right to invoice practical expedient when it has a right to consideration in an amount that corresponds directly with the value of the Company’s performance to date.
Royalties received under the agreements are recognized as revenue when sales have occurred as the Company applies the sales or usage-based royalties recognition constraint. The Company has determined the application of this exception is appropriate because the license granted in the agreement is the predominant item to which the royalties relate.
As the Company receives upfront payments for technical services under certain of its arrangements, the Company evaluates whether any significant financing components exist given the term over which the fees will be earned may exceed one year. Based on the nature of the Company’s agreements, there are no significant financing components as the purpose of the upfront payment is not to provide financing, but rather to secure technical services, exclusivity rights, and Foundry capacity, or the timing of transfer of those goods or services is at the discretion of the customer.
Deferred revenue represents consideration received by the Company in excess of revenue recognized and primarily results from transactions where the Company receives upfront payments and non-cash equity consideration. In instances where the Company has received consideration in advance for an undefined number of technical development plans (“TDPs”) under its customer agreements, the Company records the advance payments as deferred revenue, net of current portion on the Consolidated Balance Sheets. Upon the execution of a specific TDP, the Company reclassifies the estimated consideration to be earned under that TDP within the next twelve months as current deferred revenue. The Company also classifies unexercised material rights as deferred revenue, net of current portion on the Consolidated Balance Sheets. When a TDP is executed, and the material right is exercised, the amount allocated to the material right, which will be earned within the next twelve months, is reclassified to current deferred revenue. All other deferred revenue is classified as current or non-current based on the timing of when the Company expects to earn the underlying revenue based upon the projected progress of activities under the TDP.
Collaboration Arrangements
For arrangements that do not represent contracts with a customer, the Company analyzes its collaboration transactions to assess whether they are within the scope of ASC 808, Collaborative Arrangements (“ASC 808”), to determine whether such arrangements involve joint operating activities performed by parties that are both active participants in the activities and exposed to significant risks and rewards that are dependent on the commercial success of such activities. To the extent the arrangement is within the scope of ASC 808, the Company assesses whether aspects of the arrangement between the Company and its collaboration partner are within the scope of other accounting literature. If the Company concludes that some or all aspects of the arrangement represent a transaction with a customer, the Company accounts for those aspects of the arrangement within the scope of ASC 606.
Biosecurity Revenue
In 2020, the Company launched its commercial offering of COVID-19 testing products and services for businesses, academic institutions, and other organizations. The Company sells COVID-19 test kits on a standalone basis or as part of an end-to-end testing service. The Company records product revenue from sales of
F-20
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
lateral flow assay (“LFA”) diagnostic test kits. The Company records service revenue from sales of its end-to-end COVID-19 testing services, which consist of multiple promised goods and services including sample collection kits, physician authorizations, onsite test administration, outsourced laboratory polymerase chain reaction (“PCR”) analysis, and access to results reported through a web-based portal. The Company recognizes its product and service revenue using the five-step model under ASC 606.
Product revenue from the sale of LFA diagnostic test kits is recognized when the test kits are shipped, and risk of loss is transferred to the carrier. The Company’s diagnostic test kits are generally not subject to a customer right of return except for product recalls under the rules and regulations of the FDA. The Company has elected to include shipping and handling fees billed to customers as a component of Biosecurity revenue.
Service revenue from the Company’s end-to-end COVID-19 testing services is recognized upon completion of the tests and release of the test results on the web-based portal. The Company has identified one performance obligation in its testing services contracts that represents a series of distinct goods or services that are substantially the same and that have the same pattern of transfer to the customer, with each test as a distinct service within the series. As the price for the testing services is fixed under each customer contract, the Company has elected the practical expedient to recognize revenue at the amount which it has the right to invoice for services performed. The Company’s testing services contracts are generally one year or less in length, contain fixed unit pricing and are billed in advance.
Cost of Biosecurity Revenue
Cost of Biosecurity product revenue consists of costs associated with the sale of LFA diagnostic test kits, which includes costs paid to purchase test kits from third parties, as well as shipping, handling, and insurance costs. Cost of Biosecurity service revenue consists of costs associated with the provision of the Company’s end-to-end COVID-19 testing services, which includes costs paid to provide sample collection kits, physician authorizations, onsite test administration, outsourced laboratory PCR analysis, and access to results reported through a web-based portal.
Research and Development Costs
Research and development costs are expensed as incurred. Research and development costs consist of direct and indirect internal costs related to specific projects, acquired intellectual property deemed to be in-process research and development, as well as fees paid to other entities that conduct certain research and development activities on the Company’s behalf.
Patent Costs
The Company expenses all costs as incurred in connection with the filing, prosecution, maintenance, defense, and enforcement of patent applications, including direct application fees and related legal and consulting expenses. Patent costs are included in general and administrative expenses within the Consolidated Statements of Operations and Comprehensive Loss.
Stock-Based Compensation
The Company accounts for equity awards, including grants of restricted stock awards (“RSAs”), restricted stock units (“RSUs”) and stock options in accordance with ASC 718, Compensation – Stock Compensation (“ASC 718”), which requires all equity-based payments to be recognized as stock-based compensation based on their
F-21
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
grant date fair values. The determination of grant date fair value of the RSAs and RSUs is calculated as the fair value of the underlying common stock, less any applicable purchase price.
The Company estimates the fair value of its common stock using a hybrid method which uses market approaches to estimate the Company’s enterprise value. The hybrid method is a probability-weighted expected return method (“PWERM”) where the equity value in at least one scenario is allocated using an option pricing method (“OPM”).
Under the PWERM, the value of the common stock is estimated based on an analysis of future values assuming various possible future liquidity events. The value of the common stock is based on the probability-weighted present value of expected future investment returns considering the possible outcomes and the rights and privileges of each class of equity. The future investment returns are discounted back to the valuation date at a risk-adjusted discount rate which is then weighted based on the probability of the respective outcome.
Under the OPM, each class of stock is treated as a call option on the Company’s equity value, with exercise prices based on the liquidation preferences of the convertible preferred stock. Under this methodology, the common stock has value only if the funds available for distribution to the holders exceeds the value of the liquidation preferences of the convertible preferred stock at the time of the liquidity event. The Black-Scholes model is used to price the call options which includes assumptions for the time to liquidity and volatility of equity value. A discount for lack of marketability is then applied to the common stock value.
For awards granted from August 2020 through December 31, 2020, when using the hybrid method, the Company considered two scenarios: (i) a scenario in which the conversion of the convertible preferred stock to common stock occurred through an initial public offering (“IPO”) or a merger with a special purpose acquisition company (“SPAC”) transaction, and (ii) a remain private scenario. In both scenarios, the Company estimated an equity value in a potential IPO or SPAC transaction based on the guideline public company method under a market approach. The Company then converted the estimated future value to present value using a risk-adjusted discount rate. In the IPO or SPAC transaction scenario, conversion of the convertible preferred stock to common stock was assumed. In the remain private scenario, equity value was allocated among the convertible preferred stock and common stock using the OPM. In addition to considering these two scenarios, the Company considered the prices paid for its common stock and Series B convertible preferred stock in secondary transactions and the Company included these prices in its weighted average conclusion of value.
For awards granted from January 1, 2019 through July 2020, when using the hybrid method the Company considered two scenarios: (i) a fully diluted scenario, in which the per-share common stock value was assumed to equal the price of the convertible preferred stock in a recent round of financing, and (ii) a remain private scenario, in which the Company used the OPM to back-solve to the price of the Company’s convertible preferred stock in a recent round of financing. In the fully diluted scenario, conversion of the convertible preferred stock to common stock was assumed. In the remain private scenario, equity value was allocated among the convertible preferred stock and common stock using the OPM. In addition to considering these two scenarios, for certain valuations during the period, the Company considered the prices paid for its common stock in secondary transactions and included these prices in its weighted average conclusion of value.
There are significant judgments and estimates inherent in determining the fair value of the common stock. These judgments and estimates include factors, both subjective and objective, including: (i) a discount for lack of marketability; (ii) external market data; (iii) historical activity by the Company in selling equity to outside investors; (iv) the Company’s stage of development; (v) rights and preferences of the Company’s equity securities that rank senior to common stock; and (vi) the likelihood of the various scenarios, among others. Changes to these assumptions could result in different fair values of common stock.
F-22
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The Company grants equity awards with both service-based and performance-based vesting conditions. For awards with service-based vesting conditions, the Company recognizes stock-based compensation expense over the requisite service period, which is generally the vesting period, on a straight-line basis. For awards with performance-based vesting conditions, the Company recognizes stock-based compensation only when achievement of the performance condition is deemed probable. The Company classifies stock-based compensation expense in the Consolidated Statements of Operations and Comprehensive Loss in the same manner in which the grantee’s payroll costs are classified or in which the grantee’s service payments are classified. The Company recognizes forfeitures as they occur.
Income Taxes
The Company accounts for income taxes using the asset and liability method, which requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been recognized in the consolidated financial statements or in the Company’s tax returns. Under this method, deferred tax assets and liabilities are determined based on differences between the financial statement carrying amounts and the tax bases of the assets and liabilities using the enacted tax rates in effect in the years in which the differences are expected to reverse. A valuation allowance against deferred tax assets is recorded if, based on the weight of the available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. Potential for recovery of deferred tax assets is evaluated by considering several factors, including estimating the future taxable profits expected, estimating future reversals of existing taxable temporary differences, considering taxable profits in carryback periods, and considering prudent and feasible tax planning strategies.
The Company accounts for uncertain tax positions using a more-likely-than-not threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors including, but not limited to, changes in the law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity, and changes in facts or circumstances related to a tax position. As of December 31, 2020 and 2019, the Company did not have any uncertain tax positions.
Comprehensive Loss
Comprehensive loss is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Comprehensive loss is equal to net loss in all periods presented.
Net Loss per Share
The Company follows the two-class method when computing net loss per share attributable to Ginkgo Bioworks, Inc. common stockholders as the Company has issued shares that meet the definition of participating securities. The two-class method determines net loss per share for each class of common and participating securities according to dividends declared or accumulated and participation rights in undistributed earnings. The two-class method requires earnings for the period to be allocated between common and participating securities based upon their respective rights to share in the earnings as if all earnings for the period had been distributed. The earnings per share amounts are the same for the different classes of common stock because the holders of each class are legally entitled to equal per share distributions whether through dividends or liquidation. During periods of loss, there is no allocation required under the two-class method since the participating securities do not have a contractual obligation to fund the losses of the Company.
Basic net loss per share attributable to Ginkgo Bioworks, Inc. common stockholders is computed by dividing the net loss attributable to Ginkgo Bioworks, Inc. common stockholders by the weighted average number of common shares outstanding for the period. Diluted net loss per share attributable to Ginkgo Bioworks, Inc. common
F-23
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
stockholders is computed by dividing the net loss attributable to Ginkgo Bioworks, Inc. common stockholders by the weighted average number of common shares outstanding for the period, including the effect of potentially dilutive common shares. For purposes of this calculation, outstanding options to purchase shares of common stock, unvested RSAs, unvested RSUs, unvested earnout shares and warrants to purchase Class A common stock are considered potentially dilutive common shares. Treasury stock is excluded from the weighted average number of common shares outstanding used in the calculation of basic and diluted net loss per share attributable to Ginkgo Bioworks, Inc. common stockholders. The Company has generated a net loss in all periods presented, therefore, basic and diluted net loss per share attributable to Ginkgo Bioworks, Inc. common stockholders is the same as the inclusion of the potentially dilutive securities would be anti-dilutive. Net loss and comprehensive loss attributable to Ginkgo Bioworks, Inc. stockholders was equal to net loss attributable to Ginkgo Bioworks, Inc. common stockholders in the periods presented.
Recent Accounting Pronouncements
Recently Adopted Accounting Pronouncements
In November 2019, the FASB issued ASU No. 2019-08, Compensation—Stock Compensation (Topic 718) and Revenue from Contracts with Customers (Topic 606)—Codification Improvements—Share-Based Consideration Payable to a Customer (“ASU 2019-08”), which requires that an entity measure and classify share-based payment awards granted to a customer by applying the guidance in ASC 718. The amount recorded as a reduction of the transaction price is required to be measured on the basis of the grant-date fair value of the share-based payment award in accordance with ASC 718. The Company adopted ASU 2019-08 on January 1, 2020 and the adoption did not have a material impact on the Company’s consolidated financial statements and related disclosures.
In August 2018, the FASB issued ASU 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework—Changes to the Disclosure Requirements for Fair Value Measurement (“ASU 2018-13”), which modifies the disclosure requirements on fair value measurements with respect to Level 3 rollforwards, timing of liquidation of investments in certain entities that calculate net asset value, and measurement uncertainty. The Company adopted ASU 2018-13 on January 1, 2020 and the adoption did not have a material impact on its consolidated financial statements and related disclosures.
In July 2017, the FASB issued ASU 2017-11, Earnings Per Share (Topic 260), Distinguishing Liabilities from Equity (Topic 480), Derivatives and Hedging (Topic 815) I. Accounting for Certain Financial Instruments with Down Round Features II. Replacement of the Indefinite Deferral for Mandatorily Redeemable Financial Instruments of Certain Nonpublic Entities and Certain Mandatorily Redeemable Noncontrolling Interests with a Scope Exception (“ASU 2017-11”). Part I of this standard applies to entities that issue financial instruments such as warrants, convertible debt, or convertible preferred stock that contain down-round features. Part II of this standard replaces the indefinite deferral for certain mandatorily redeemable noncontrolling interests and mandatorily redeemable financial instruments of nonpublic entities contained within ASC 480 with a scope exception and does not impact the accounting for these mandatorily redeemable instruments. The Company adopted ASU 2017-11 on January 1, 2020 and the adoption did not have a material impact on its consolidated financial statements and related disclosures.
In November 2018, the FASB issued ASU 2018-18, Collaborative Arrangements (Topic 808): Clarifying the Interaction between Topic 808 and Topic 606 (“ASU 2018-18”). The provisions of ASU 2018-18 clarify when certain transactions between collaborative arrangement participants should be accounted for under ASC 606 and incorporate unit-of-account guidance consistent with ASC 606 to aid in this determination. The Company early adopted ASU 2018-18 on January 1, 2020 and the adoption did not have a material impact on its consolidated financial statements and related disclosures.
F-24
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Recently Issued Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the FASB and adopted by the Company as of the specified effective date. Unless otherwise discussed, the Company believes that the impact of recently issued standards that are not yet effective will not have a material impact on its financial position or results of operations upon adoption.
In August 2020, the FASB issued ASU 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging- Contracts in Entity’s Own Equity (Subtopic 815-40) (“ASU 2020-06”) which simplifies the accounting for convertible instruments by eliminating the requirement to separate embedded conversion features from the host contract when the conversion features are not required to be accounted for as derivatives under Topic 815, or that do not result in substantial premiums accounted for as paid-in capital. By removing the separation model, a convertible debt instrument will be reported as a single liability instrument with no separate accounting for embedded conversion features. This new standard also removes certain settlement conditions that are required for contracts to qualify for equity classification and simplifies the diluted earnings per share calculations by requiring that an entity use the if-converted method and that the effect of potential share settlement be included in diluted earnings per share calculations. This new standard will be effective for the Company on January 1, 2024, with early adoption permitted no earlier than January 1, 2021. The Company is currently evaluating the impact that the implementation of this standard will have on its consolidated financial statements and related disclosures.
In January 2020, the FASB issued ASU 2020-01, Investments—Equity Securities (Topic 321), Investments—Equity Method and Joint Ventures (Topic 323), and Derivatives and Hedging (Topic 815)—Clarifying the Interactions between Topic 321, Topic 323, and Topic 815 (a consensus of the FASB Emerging Issues Task Force) (“ASU 2020-01”). ASU 2020-01 addresses accounting for the transition into and out of the equity method and provides clarification of the interaction of rules for equity securities, the equity method of accounting, and forward contracts and purchase options on certain types of securities. The guidance is effective for the Company on January 1, 2022. The Company is currently evaluating the impact that the implementation of this standard will have on its consolidated financial statements and related disclosures.
In December 2019, the FASB issued ASU 2019-12, Simplifying the Accounting for Income Taxes (“ASU 2019-12”). The provisions of ASU 2019-12 eliminate certain exceptions related to the approach for intraperiod tax allocation and deferred tax liabilities for outside basis differences and clarify when a step-up in the tax basis of goodwill should be considered part of a business combination or a separate transaction. It also clarifies and simplifies other aspects of the accounting for income taxes. The guidance is effective for the Company on January 1, 2022, with early adoption permitted. The Company is currently evaluating the impact that the implementation of this standard will have on its consolidated financial statements and related disclosures. In October 2018, the FASB issued ASU 2018-17, Consolidation (Topic 810): Targeted Improvements to Related Party Guidance for Variable Interest Entities (“ASU 2018-17”). The provisions of ASU 2018-17 modify the guidance under ASC 810 related to the evaluation of indirect interests held through related parties under common control when determining whether fees paid to decision makers and service providers are variable interests. Indirect interests held through related parties that are under common control are no longer considered to be the equivalent of direct interests in their entirety and instead should be considered on a proportional basis. This guidance more closely aligns with accounting of how indirect interests held through related parties under common control are considered for determining whether a reporting entity must consolidate a VIE. The guidance is effective for the Company on January 1, 2021, with early adoption permitted. The Company is currently evaluating the impact that the implementation of this standard will have on its consolidated financial statements and related disclosures.
F-25
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
In June 2016, the FASB issued ASU 2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments, and subsequently issued multiple amendments to the standard (collectively, “ASU 2016-13”). The provisions of ASU 2016-13 modify the impairment model to utilize an expected loss methodology in place of the currently used incurred loss methodology and require a consideration of a broader range of reasonable and supportable information to inform credit loss estimates. The guidance is effective for the Company on January 1, 2023, with early adoption permitted. The Company is currently evaluating the impact that the implementation of this standard will have on its consolidated financial statements and related disclosures.
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842), Amendments to the FASB Accounting Standards Codification, which supersedes the existing guidance for lease accounting. The FASB has issued several updates to the standard which: (i) clarify how to apply certain aspects of the new standard, (ii) provide an additional transition method for adoption of the new standard, (iii) provide a practical expedient for certain lessor accounting, and (iv) amend certain narrow aspects of the guidance (collectively, “ASC 842”). ASC 842 requires the identification of arrangements that should be accounted for as leases by lessees. In general, for lease arrangements exceeding a twelve-month term, these arrangements must now be recognized as assets and liabilities on the balance sheet of the lessee. Under ASC 842, a right-of-use asset and lease obligation will be recorded for all leases, whether operating or financing, while the income statement will reflect lease expense for operating leases and amortization/interest expense for financing leases. The balance sheet amount recorded for existing leases at the date of adoption of ASC 842 is calculated using the applicable incremental borrowing rate at the date of adoption. The guidance is effective for the Company on January 1, 2022, with early adoption permitted. The Company anticipates the implementation of this standard will have a material impact on its consolidated financial statements and related disclosures.
3. Fair Value Measurements
No transfers between levels have occurred during the periods presented. The following tables present information about the Company’s financial assets measured at fair value on a recurring basis (in thousands):
| As of December 31, 2020 | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets: |
||||||||||||||||
| Money market funds, included in cash and cash equivalents |
$ | 372,537 | $ | 372,537 | $ | — | $ | — | ||||||||
| Synlogic, Inc. common stock, included in equity method investments |
13,696 | 13,696 | — | — | ||||||||||||
| Synlogic, Inc. warrant, included in investments |
5,504 | — | 5,504 | — | ||||||||||||
| Loans receivable, included in prepaid expenses and other current assets |
2,268 | — | — | 2,268 | ||||||||||||
| Loans receivable, net of current portion |
13,298 | — | — | 13,298 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 407,303 | $ | 386,233 | $ | 5,504 | $ | 15,566 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-26
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| As of December 31, 2019 | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets: |
||||||||||||||||
| Money market funds, included in cash and cash equivalents |
$ | 480,178 | $ | 480,178 | $ | — | $ | — | ||||||||
| Synlogic, Inc. common stock, included in equity method investments |
16,359 | 16,359 | — | — | ||||||||||||
| Synlogic, Inc. warrant, included in investments |
6,574 | — | 6,574 | — | ||||||||||||
| Loan receivable, included in prepaid expenses and other current assets |
1,106 | — | — | 1,106 | ||||||||||||
| Loan receivable, net of current portion |
3,724 | — | — | 3,724 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 507,941 | $ | 496,537 | $ | 6,574 | $ | 4,830 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
As of December 31, 2020, loans receivable primarily consisted of a revolving promissory note with Glycosyn, LLC (“Glycosyn”) which is secured by the assets of Glycosyn, including certain intellectual property such as patents and copyrights held by Glycosyn, (“Glycosyn Promissory Note”) and a series of convertible notes with Access Bio, Inc. (“Access Bio Convertible Notes”). As of December 31, 2019, the loan receivable balance consisted of the Glycosyn Promissory Note. The fair value of the Glycosyn Promissory Note and Access Bio Convertible Notes were determined based on significant inputs not observable in the market, which represent a Level 3 measurement within the fair value hierarchy. Significant changes in these unobservable inputs in isolation could have resulted in a significantly lower or higher fair value measurement. Refer to Note 4 for additional details on loans receivable.
The Company used a probability-weighted discounted cash flow valuation approach to determine the fair value of the Glycosyn Promissory Note. Using this approach, the present value of the expected future cash flows were calculated under four settlement scenarios and then were weighted based on the estimated probability of each scenario. The four settlement scenarios considered in the valuation were (i) a qualified financing which resulted in a 20% conversion discount, (ii) repayment upon change in control, (iii) a dissolution scenario and (iv) repayment in accordance with the terms of the note. The significant assumptions used in valuing the Glycosyn Promissory Note during the years ended December 31, 2020 and 2019 included the expected timing and probability of each scenario and the discount rate. For the year ended December 31, 2020, a discount rate of 15% was applied and the probability and timing of each scenario ranged from 10% to 40% and spanned 1 to 2.5 years, respectively. For the year ended December 31, 2019, a discount rate of 15% was applied and the probability and timing of each scenario ranged from 20% to 40% and spanned 1 to 3.5 years, respectively. The weighted average timing of the scenarios weighted based on the probability of each scenario for the years ended December 31, 2020 and 2019 was 1.2 years and 2.3 years, respectively.
The Company used a Monte-Carlo simulation model to determine the fair value of the Access Bio Convertible Notes. The future stock price of Access Bio, Inc. (“Access Bio”) was simulated over the term of the note to assess the value of the settlement features which included (i) conversion into stock at a discount determined under a reset provision tied to the performance of Access Bio’s stock price and (ii) redemption at maturity. The significant assumptions used in determining the simulated future stock price included the expected timing of the conversion, which is assumed at maturity, and expected volatility. The significant assumptions used in determining the fair value of the Access Bio Convertible Notes under a redemption at maturity scenario was the discount rate and expected volatility. For the year ended December 31, 2020 the discount rate that was used to determine fair value of the Access Bio Convertible Notes under the maturity scenario was 32.8%. For the year ended December 31, 2020, the volatility rate used to determine the fair value of the Access Bio Convertible Notes was 83.1% and 88.5% which represented the volatility rate at the inception and as of December 31, 2020, respectively.
F-27
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The following table provides a reconciliation of all assets measured at fair value using Level 3 significant unobservable inputs (in thousands):
| Loans Receivable |
||||
| Balance as of December 31, 2018 |
$ | 750 | ||
| Issuance of loan receivable |
4,994 | |||
| Change in fair value |
(914 | ) | ||
|
|
|
|||
| Balance as of December 31, 2019 |
$ | 4,830 | ||
|
|
|
|||
| Purchase of loan receivable |
10,000 | |||
| Issuance of loans receivable |
475 | |||
| Proceeds from loans receivable |
(800 | ) | ||
| Change in fair value |
1,061 | |||
|
|
|
|||
| Balance as of December 31, 2020 |
$ | 15,566 | ||
|
|
|
|||
4. Loans Receivable
Glycosyn Promissory Note
In October 2018, the Company provided a revolving promissory note to Glycosyn in connection with the Company’s entering into a Foundry Terms of Service Agreement with Glycosyn (Note 16). Under the Glycosyn Promissory Note, the Company provided a revolving promissory note which could have been drawn up to $4.0 million for any purpose through December 31, 2019. The Glycosyn Promissory Note initially matured on the earlier of December 31, 2020, or the termination of the Foundry Terms of Service Agreement. Interest accrued on all outstanding amounts at a rate equal to the prime rate and all payments made on the Glycosyn Promissory Note were applied to accrued interest first. The Glycosyn Promissory Note is convertible at a discount, at the Company’s election, into equity securities of Glycosyn upon Glycosyn’s first issuance of equity securities, other than an underwritten public offering, from which Glycosyn receives gross proceeds of at least $10.0 million. In addition, Glycosyn is obligated to immediately repay the outstanding balance of the loan, plus accrued interest, upon a change in control event. In December 2019, the existing terms of the Glycosyn Promissory Note were amended to add an additional $2.7 million to the principal sum of the note upon Glycosyn exercising its option to terminate the Foundry Terms of Service Agreement (Note 16). In addition, under the amended terms of the Glycosyn Promissory Note, Glycosyn is required to make quarterly payments under the loan commencing in March 2020 with the first two payments as interest-only. The amended Glycosyn Promissory Note accrues interest at a rate of 7.5% per annum and matures in June 2023, unless earlier converted by the Company into equity securities of Glycosyn. The loan conversion and change in control provisions remained unchanged under the amended Glycosyn Promissory Note. As of December 31, 2019, there was $5.7 million outstanding under the Glycosyn Promissory Note, of which the entire portion represented the unpaid principal balance. The fair value of the Glycosyn Promissory Note was $4.8 million as of December 31, 2019, of which $1.1 million was included in prepaid expenses and other current assets with the remaining amount included in loans receivable, net of current portion on the Consolidated Balance Sheet. The fair value adjustment of $0.9 million, which was recognized as part of the gain on the termination of the Glycosyn Foundry Terms of Service Agreement, was recorded as component of other income (expense), net on the Consolidated Statements of Operations and Comprehensive Loss for the year ended December 31, 2019.
During the year ended December 31, 2020, Glycosyn made principal and interest payments totaling $0.8 million against the Glycosyn Promissory Note. As of December 31, 2020, there was $5.4 million outstanding under the
F-28
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Glycosyn Promissory Note, of which $5.3 million represented the unpaid principal balance. The fair value of the Glycosyn Promissory Note was $4.5 million as of December 31, 2020, of which $2.0 million was included in prepaid expenses and other current assets with the remaining amount included in loans receivable, net of current portion on the Consolidated Balance Sheet. The gain on the change in fair value of $0.5 million for the year ended December 31, 2020 was recorded as a component of other income (expense), net on the Consolidated Statements of Operations and Comprehensive Loss.
In January 2021, the Company entered into an amendment to the Glycosyn Promissory Note (Note 21).
Access Bio Convertible Notes
In November 2020, the Company entered into a convertible note subscription agreement with Access Bio, a supplier of the Company’s diagnostic test kits. The Access Bio Convertible Notes are due in November 2022 in the aggregate principal amount of $10.0 million plus a 2% rate of return compounded annually. The Access Bio Convertible Notes are convertible into a number of shares of common stock of Access Bio, a company listed on the Korea Stock Exchange, of up to $10.0 million based on a fixed foreign currency exchange rate and a conversion price subject to certain adjustments, including reset adjustments each quarter based on the trading price of Access Bio’s stock. The adjusted conversion price cannot be reduced to less than 70% of the initial conversion price as a result of the reset adjustments and the reset adjustments cannot increase the effective conversion ratio. The Access Bio Convertible Notes are convertible at the Company’s election any time following the first anniversary of the issuance date of the notes, but prior to the 30th day before the maturity date. Additionally, subject to certain provisions, the Company has the option to cause Access Bio to repurchase, or Access Bio has the option to repurchase, a portion of the outstanding balance under the notes (or up to the entire balance in the case of the Company’s option) at a price to ensure a 2% rate of return compounded annually. As of December 31, 2020, the fair value of the Access Bio Convertible Notes was $10.7 million, the entire balance of which was recorded in loans receivable, net of current portion on the Consolidated Balance Sheet. The gain on the change in fair value of $0.7 million was recorded as a component of other income (expense), net on the Consolidated Statements of Operations and Comprehensive Loss for the year ended December 31, 2020.
5. Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets consisted of the following (in thousands):
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Prepaid expenses |
$ | 10,854 | $ | 2,553 | ||||
| Prepaid inventory |
6,536 | — | ||||||
| Loans receivable |
2,268 | 1,106 | ||||||
| Other current assets |
1,441 | 5,301 | ||||||
|
|
|
|
|
|||||
| Prepaid expenses and other current assets |
$ | 21,099 | $ | 8,960 | ||||
|
|
|
|
|
|||||
F-29
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
6. Inventory, net
Inventory, net consisted of the following (in thousands):
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Finished goods |
$ | 2,756 | $ | — | ||||
| Less: Inventory reserve |
(20 | ) | — | |||||
|
|
|
|
|
|||||
| Inventory, net |
$ | 2,736 | $ | — | ||||
|
|
|
|
|
|||||
7. Property and Equipment, net
Property and equipment, net consisted of the following (in thousands):
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Facilities |
$ | 12,762 | $ | 12,762 | ||||
| Furniture and fixtures |
2,165 | 1,031 | ||||||
| Lab equipment |
51,072 | 38,093 | ||||||
| Computer equipment and software |
6,204 | 2,442 | ||||||
| Leasehold improvements |
40,435 | 29,369 | ||||||
| Construction in progress |
42,575 | 894 | ||||||
|
|
|
|
|
|||||
| Total property and equipment |
155,213 | 84,591 | ||||||
| Less: Accumulated depreciation |
(33,778 | ) | (21,459 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 121,435 | $ | 63,132 | ||||
|
|
|
|
|
|||||
As of December 31, 2020 and 2019, capital leases totaling $3.3 million were included in lab equipment, with related accumulated depreciation of $2.4 million and $1.7 million, respectively. The increase in construction in progress during the year ended December 31, 2020 was primarily due to the build-out of a new high-throughput testing facility.
Depreciation expense related to property and equipment for the years ended December 31, 2020 and 2019 totaled $12.6 million and $9.6 million, respectively, inclusive of $0.7 million and $0.6 million, respectively, related to capital leases.
F-30
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
8. Investments and Equity Method Investments
Investments and equity method investments consisted of the following (in thousands):
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Investments: |
||||||||
| Genomatica, Inc. preferred stock |
$ | 55,000 | $ | 55,000 | ||||
| Synlogic, Inc. warrant |
5,504 | 6,574 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 60,504 | $ | 61,574 | ||||
|
|
|
|
|
|||||
| Equity method investments: |
||||||||
| Joyn Bio, LLC |
$ | 28,924 | $ | 29,320 | ||||
| Synlogic, Inc. |
13,696 | 16,359 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 42,620 | $ | 45,679 | ||||
|
|
|
|
|
|||||
The carrying value of the Company’s equity method investments in Motif Foodworks, Inc. (“Motif”) and Allonnia, LLC (“Allonnia”) as of December 31, 2020 and 2019 was zero and as such, were excluded from the table above.
Loss on investments and equity method investments consisted of the following (in thousands):
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Loss on investments: |
||||||||
| Synlogic, Inc. warrant |
$ | (1,070 | ) | $ | (7,797 | ) | ||
|
|
|
|
|
|||||
| Total |
$ | (1,070 | ) | $ | (7,797 | ) | ||
|
|
|
|
|
|||||
| Loss on equity method investments: |
||||||||
| Joyn Bio, LLC |
$ | (396 | ) | $ | (1,730 | ) | ||
| Glycosyn, LLC |
— | (1,323 | ) | |||||
| Synlogic, Inc. |
(2,663 | ) | (19,403 | ) | ||||
| Allonnia, LLC |
— | (24,480 | ) | |||||
|
|
|
|
|
|||||
| Total |
$ | (3,059 | ) | $ | (46,936 | ) | ||
|
|
|
|
|
|||||
The combined summarized financial information for the Company’s equity method investments, which includes Joyn, Synlogic, Inc. (“Synlogic”), Motif, Allonnia and Glycosyn consisted of the following (in thousands):
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Assets |
$ | 319,311 | $ | 397,280 | ||||
| Liabilities |
$ | 42,441 | $ | 39,832 | ||||
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Revenue |
$ | 545 | $ | 3,579 | ||||
| Total operating expenses |
$ | (125,742 | ) | $ | (134,444 | ) | ||
| Loss from operations |
$ | (125,197 | ) | $ | (130,865 | ) | ||
| Net loss |
$ | (123,480 | ) | $ | (125,290 | ) | ||
F-31
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The summarized financial information for Glycosyn as of and for the year ended December 31, 2020 and as of December 31, 2019 has been excluded from the tables above as the Company no longer held an equity interest in Glycosyn as of December 31, 2019. Refer to Note 16 for additional discussion of the Company’s equity interest in Glycosyn and its other equity method investments.
9. Goodwill and Intangible Assets, net
During the years ended December 31, 2020 and 2019, there was no change in the carrying value of goodwill.
Intangible assets, net consisted of the following (in thousands):
| Gross Carrying Value |
Accumulated Amortization |
Net | ||||||||||
| Balances as of December 31, 2020 |
||||||||||||
| Acquired technology |
$ | 5,490 | $ | (2,196 | ) | $ | 3,294 | |||||
| Balances as of December 31, 2019 |
||||||||||||
| Acquired technology |
$ | 5,490 | $ | (1,647 | ) | $ | 3,843 | |||||
Acquired technology had a weighted average remaining amortization period of 6 and 7 years as of December 31, 2020 and 2019, respectively. Amortization expense was $0.5 million for each of the years ended December 31, 2020 and 2019. Future amortization expense for each of the remaining years in the useful life of the intangible assets will be $0.5 million per year.
| 10. | Accrued Expenses and Other Current Liabilities |
Accrued expenses and other current liabilities consisted of the following (in thousands):
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Accrued compensation and benefits |
$ | 3,037 | $ | 4,864 | ||||
| Accrued professional fees |
6,381 | 4,398 | ||||||
| Accrued financing costs |
— | 3,380 | ||||||
| Capital lease obligation |
485 | 598 | ||||||
| Accrued property and equipment |
10,017 | 597 | ||||||
| Accrued lab supplies |
4,276 | 535 | ||||||
| Accrued external research and development expenses |
3,907 | 423 | ||||||
| Other current liabilities |
2,402 | 1,021 | ||||||
|
|
|
|
|
|||||
| Accrued expenses and other current liabilities |
$ | 30,505 | $ | 15,816 | ||||
|
|
|
|
|
|||||
11. Commitments and Contingencies
Lease Obligations
The Company has entered into various noncancelable operating leases for office and lab space in Boston and Cambridge, Massachusetts and Emeryville, California to support its research and development activities and operations which expire at various dates through September 2030. The Company’s Emeryville, California lease commenced in January 2021. The leases contain periods of free rent, escalating rent, tenant improvement incentives, renewal periods, and expansion options for additional suites. The Company recognizes rent expense on a straight-line basis over the term of each respective lease, inclusive of the free rent periods and reduced by the amortization of the tenant incentives.
F-32
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The Company’s headquarters and primary operations are located in Boston, Massachusetts and are comprised of a number of leases across 21, 23, 25 and 27 Drydock Avenue, which represent the Company’s most significant lease arrangements. The following summarizes the key terms of such leases:
21-23-25 Drydock Avenue
In March 2016, the Company entered into a noncancelable operating lease for approximately 87,000 square feet of office and lab space. The lease is comprised of five separate suites, the first of which was delivered to the Company in April 2016. The Company currently occupies three suites totaling approximately 52,000 square feet and the Company anticipates occupying the remaining suites in 2021 and 2022. The lease contains periods of free rent for each suite and tenant improvement incentives totaling $5.3 million. Base rent is subject to annual increases through the term of the lease. The lease expires in January 2030 and contains one option to extend the lease for a five-year period at then-market rates. The lease is secured by a letter of credit which totaled $1.4 million and $1.7 million as of December 31, 2020 and 2019, respectively. The cash collateralizing the letter of credit is classified in other non-current assets on the Consolidated Balance Sheets. The letter of credit will be increased to $1.5 million upon delivery of the fourth suite.
At the time the Company took possession of the first three suites, the premises were in shell condition and required substantial work prior to occupancy. The Company was deemed the accounting owner during the construction period as the improvements constituted structural elements of the project. Accordingly, the Company capitalized the fair value of the leased space upon delivery from the landlord and recorded a corresponding lease financing obligation. The Company also capitalized the construction costs, leasehold improvements, and interest incurred during the construction period. Construction was complete, and the assets were placed in service, for the first three suites in September 2016, December 2017, and January 2019, respectively. Upon completion of the construction, the Company evaluated the lease and determined it did not meet the criteria for sale-leaseback treatment. Accordingly, the Company depreciates the capitalized assets and recognizes interest expense related to the lease financing obligation using the effective interest rate method over the lease term. For the years ended December 31, 2020 and 2019, the Company recognized $0.4 million of depreciation expense and $2.3 million of interest expense related to the lease.
During the year ended December 31, 2019, the Company recorded leased assets of $3.1 million and tenant improvements of $6.0 million related to assets placed in service during the period. No leased assets were placed in service during the year ended December 31, 2020. As of December 31, 2020 and 2019, the aggregate lease financing obligation for the capitalized suites totaled $16.8 million.
27 Drydock Avenue
Beginning in December 2011, the Company entered into a series of noncancelable operating leases with the same landlord for an aggregate of approximately 130,000 square feet of office and lab space. The Company anticipates occupying approximately 9,000 additional square feet in 2022. The leases contain periods of free rent and provides for aggregate tenant improvement allowances of $13.4 million. As of December 31, 2020 and 2019, the aggregate unamortized balance of tenant improvement allowances under the leases was $8.1 million and $8.9 million, respectively. Base rent for each lease is subject to annual increases through the respective term of the leases. The leases expire in January 2030 and each contain one option to extend the leases for a five-year period at then-market rates. The leases are secured by a letter of credit which totaled $1.6 million and $1.5 million as of December 31, 2020 and 2019, respectively. The cash collateralizing the letter of credit is classified in other non-current assets on the Consolidated Balance Sheets.
The Company subleases a portion of its office and laboratory space to Joyn and Motif. The Company is not relieved of its obligations under the head lease and, therefore, accounts for the arrangements as subleases. The
F-33
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
sublease with Joyn runs coterminous with the Foundry Services Agreement (Note 16) and the sublease with Motif has a five-year term that commenced in November 2020. The sublessees are obligated to pay to the Company base rent plus operating expenses. The Company collects approximately $0.2 million and $0.7 million per year under the subleases with Joyn and Motif, respectively, and presents sublease income as a component of other income (expense), net on the Consolidated Statements of Operations and Comprehensive Loss.
The Company recognized rent expense of $7.0 million and $6.1 million for the years ended December 31, 2020 and 2019, respectively, of which $0.3 million was incurred during the year ended December 31, 2020 under leases in which the Company was a sublessee. The Company incurred no rent expense as a sublessee during the year ended December 31, 2019. Future minimum lease payments under noncancelable operating lease agreements, inclusive of payments for the lease financing obligations, as of December 31, 2020 are as follows (in thousands):
| Years Ending December 31, | Minimum Lease Payments |
|||
| 2021 |
$ | 16,688 | ||
| 2022 |
19,089 | |||
| 2023 |
21,205 | |||
| 2024 |
21,962 | |||
| 2025 |
22,497 | |||
| Thereafter |
79,647 | |||
|
|
|
|||
| Total |
$ | 181,088 | ||
|
|
|
|||
The Company enters into certain capital leases for lab equipment used in research and development activities. Lease terms range from three to five years, may include bargain purchase options, and have fixed monthly rental payments. Future minimum lease payments under capital leases as of December 31, 2020 are as follows (in thousands):
| Years Ending December 31, | Minimum Lease Payments |
|||
| 2021 |
$ | 500 | ||
| 2022 |
238 | |||
| 2023 |
102 | |||
| 2024 |
— | |||
| 2025 |
— | |||
| Thereafter |
— | |||
|
|
|
|||
| Total noncancelable payments |
$ | 840 | ||
| Less: Imputed interest expense |
(43 | ) | ||
|
|
|
|||
| Present value of future minimum lease payments |
$ | 797 | ||
|
|
|
|||
Collaboration Agreement with Berkeley Lights, Inc.
In September 2019, the Company signed a collaboration agreement with Berkeley Lights, Inc. (“Berkeley Lights”), a leading digital cell biology company focused on enabling and accelerating the rapid development and commercialization of microbial biotherapeutics and other cell-based products for its customers. Under the collaboration agreement, the Company has agreed to incorporate Berkeley Lights’ Platform into the Foundry to
F-34
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
accelerate the engineering of biotherapeutics and cell-based products. Under the collaboration agreement, both parties will use diligent efforts to perform their respective responsibilities to develop workflow development plans, including with respect to the Company’s collaborative development of workflows for Berkeley Lights’ Platform. The initial development of workflows will be focused on yeast and mammalian cells. Additionally, the Company is obligated to pay Berkeley Lights at least $109.0 million, and up to $150.0 million, over the term of the collaboration agreement for (i) payments for Berkeley Lights’ efforts under the workflow development plans and (ii) payments for purchases of certain equipment, associated consumables, and other goods and services.
Minimum purchase commitments for contract years one and two, which represents an 18-month period, are binding commitments that must be met each year. For contract years three through seven, the minimum purchase commitments are binding commitments, however the minimum purchase commitment is measured on a cumulative basis. Therefore, any amounts paid by the Company in excess of the given contract year’s purchase commitment may be credited towards subsequent years’ minimum purchase commitment until such excess amount has been fully credited against the minimum cumulative purchase commitment. Minimum purchase commitments under the collaboration agreement are as follows (in thousands):
| Contract Years | Minimum Purchase Commitment |
|||
| October 1, 2019 - September 30, 2020 |
$ | 10,000 | ||
| October 1, 2020 - March 31, 2022 |
15,000 | |||
| April 1, 2022 - March 31, 2023 |
14,000 | |||
| April 1, 2023 - March 31, 2024 |
17,500 | |||
| April 1, 2024 - March 31, 2025 |
17,500 | |||
| Thereafter |
35,000 | |||
|
|
|
|||
| Total |
$ | 109,000 | ||
|
|
|
|||
The collaboration agreement contains provisions requiring the Company to pay to Berkeley Lights certain license fees for the use of Berkeley Lights’ Platform and certain milestone payments of up to $11.5 million payable when a therapeutic discovered using certain workflows reaches specified development and regulatory milestones. License fees owed to Berkeley Lights are variable based on volume usage of Berkeley Lights’ Platform. All such license fees and milestone payments are applied against the satisfaction of the minimum purchase commitment. Further, if Berkeley Lights achieves certain performance targets, the minimum purchase commitment will increase to $150.0 million.
The Company has the option to buy down its purchasing obligations after the second contract year by making a one-time payment to Berkeley Lights. The amount of the buy down payment is dependent upon the cumulative payments made to Berkeley Lights at such time and the number of completed workflows. Additionally, the Company is granted an exclusivity period for each workflow developed for the Company by Berkeley Lights under the collaboration agreement. Berkeley Lights has the option to buy down the exclusivity period by making a one-time payment to the Company equal to a percentage of the development costs incurred by the Company related to the specific workflow. Thereafter, the parties will equally share the development costs of the associated workflow. The Company concluded the payments received from Berkeley Lights related to the buy down of an exclusivity period represent reimbursements for research and development costs and therefore account for the payments as a reduction of research and development expenses.
The collaboration agreement will continue until the seventh anniversary of the effective date, subject to certain automatic extension provisions, including for delays resulting from a Berkeley Lights failure to supply products
F-35
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
or services conforming with the collaboration agreement. The collaboration will automatically terminate if the Company, at any time after the second contract year, elects to exercise its buy down right. In addition, either party may terminate the collaboration agreement (i) for the material breach by the other party (including, with respect to the Company, a material supply failure), (ii) upon the occurrence of certain insolvency related events of the other party, and (iii) for certain force majeure events.
The Company made an upfront payment of $10.0 million, which was fully creditable against certain other payments owed to Berkeley Lights during the term of the collaboration agreement. As of December 31, 2019, $5.2 million of the upfront payment remained in prepaid expenses and other current assets on the Consolidated Balance Sheet and during the year ended December 31, 2020, the Company utilized the remaining portion of the upfront payment. During the years ended December 31, 2020 and 2019, the Company purchased lab equipment from Berkeley Lights totaling $2.0 million and $4.0 million, respectively. Such lab equipment is included in property and equipment, net on the Consolidated Balance Sheets. During the years ended December 31, 2020 and 2019 the Company recorded expense related to services received from Berkeley Lights totaling $7.7 million and $0.8 million, respectively, net of buy downs. Expenses incurred under the collaboration agreement are recorded as research and development expenses, net of Berkeley Lights’ buy down payments, in the Consolidated Statements of Operations and Comprehensive Loss. During the year ended December 31, 2020, Berkeley Lights exercised their option to buy down two workflows for total consideration of $1.7 million. Through December 31, 2020, the Company purchased a total of $14.5 million of equipment, services, and consumables under the collaboration agreement with Berkeley Lights.
Supply Agreement with Twist Bioscience Corporation
In March 2018, the Company signed a supply agreement with Twist Bioscience Corporation (“Twist”) to provide synthetic DNA and certain other services. Under the supply agreement, the Company is obligated to purchase specified volumes of synthetic DNA subject to quarterly minimums over the term of the agreement. The products purchased that contribute to achieving the quarterly minimum purchase commitment can vary based on the Company’s discretion, subject to advanced notice provided to Twist. The term of the supply agreement is four years. The Company’s quarterly minimum purchase commitment may be adjusted for the following reasons: (i) due to a lack of availability of certain products for purchase in a given quarter; (ii) due to lack of certain service features available; (iii) delays in shipments over two consecutive quarters beyond the agreed upon lead times; and (iv) if the average yield of certain products measured over two consecutive quarters is greater than a specified yield. The Company receives volume discounts on purchases based on specified volume thresholds over the term of the supply agreement. Additionally, the Company receives a discount on each order of certain products, dependent upon the volume of certain other products it purchases in a given order. If, at each six-month period over the term of the supply agreement, the Company fails to meet its aggregate quarterly minimum purchase commitment for the prior six months, the Company is obligated to pay Twist a fee per unit of the shortfall.
During the years ended December 31, 2020 and 2019, the Company incurred $10.4 million and $8.3 million, respectively, of research and development expenses under its supply agreement with Twist.
Purchase Orders
The Company has agreements with third parties for certain services for which the Company is not contractually able to terminate for convenience to avoid future obligations to the respective vendors. Such agreements may provide for termination fees, penalties, or costs to wind-down the arrangement. Under such agreements, the Company is contractually obligated to make payments, primarily to reimburse the vendor for their expenditures
F-36
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
that are not recoverable and incurred prior to any cancellation of the respective agreement. The actual amounts the Company could pay in the future to these vendors under the various agreements may differ from the amounts under the purchase orders due to these cancellation provisions.
Indemnification Agreements
The Company enters into standard indemnification agreements and has agreements with indemnification clauses in the ordinary course of business. Under such arrangements, the Company indemnifies, holds harmless and agrees to reimburse the indemnified party for losses suffered or incurred by the indemnified party, who are generally the Company’s business partners. The terms of these indemnification arrangements are generally perpetual and effective any time after contract execution. The maximum potential liability resulting from these indemnification arrangements may be unlimited. The Company has never incurred costs to defend lawsuits or settle claims related to these indemnification arrangements and the Company does not believe that the outcome of any claims under such arrangements will have a material effect on its financial position, results of operations or cash flows, and have not accrued any liabilities related to such obligations as of December 31, 2020 or 2019.
Legal Proceedings
The Company is not currently party to any material legal proceedings. As of each reporting date, the Company evaluates whether or not a potential loss amount or range of loss amounts is reasonably estimable and probable of being incurred and whether such amounts meet the requirements to be accrued or disclosed pursuant to ASC 450, Contingencies (“ASC 450”). The Company expenses costs related to such legal proceedings as incurred.
| 12. | Convertible Promissory Notes |
In June 2019, the Company entered into a Note Purchase Agreement (“NPA”) with certain existing investors. In connection with the NPA, the Company issued convertible promissory notes (“Convertible Promissory Notes”) resulting in aggregate proceeds of $199.0 million, net of issuance costs of $1.0 million. The Convertible Promissory Notes carried interest at the rate of 3% per annum and had a maturity date of June 21, 2021. Pursuant to the NPA, all of the outstanding principal and interest under the Convertible Promissory Notes were to be automatically converted into (i) preferred stock issued in connection with the Company’s next financing that resulted in at least $50.0 million of gross proceeds (“NPA Qualified Financing”) at a 5% discount, (ii) common stock issued in connection with the filing of an effective registration statement pursuant to an initial public offering, or (iii) cash equal to the greater of (x) one and a half times the outstanding principal and interest accrued immediately prior to a sale or change in control event (as defined in the NPA) in which the Company or one of its subsidiaries was a party, or (y) the amount each investor would have received if the outstanding principal and accrued interest had been converted into Series D convertible preferred stock immediately prior to such sale or change in control event. On the maturity date, the Convertible Promissory Notes were to be automatically converted into shares of Series D convertible preferred stock, at a predetermined conversion rate, which was less than the fair value of Series D convertible preferred stock at the date of issuance of the Convertible Promissory Notes. The Company determined that at the Convertible Promissory Notes’ commitment date, this conversion feature was beneficial to the investors and, as such, calculated and recorded a beneficial conversion feature (“BCF”). The intrinsic value of the BCF, which was calculated utilizing the fair value of the underlying Series D convertible preferred stock and effective conversion price on the commitment date, was $199.0 million and was recorded as a debt discount with an offset to additional paid in capital.
The debt discount was amortized to interest expense using the effective interest method through the maturity date of the Convertible Promissory Notes. For the year ended December 31, 2019, the Company recorded interest expense of $0.1 million in the Consolidated Statements of Operations and Comprehensive Loss related to the amortization of the debt discount.
F-37
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The Company’s Series E convertible preferred stock issuance in September 2019 met the criteria of an NPA Qualified Financing. Accordingly, the Convertible Promissory Notes were converted into Series E convertible preferred stock. In connection with the NPA Qualified Financing and the associated conversion, the Company was required to account for the repurchase of the BCF. The total repurchase price associated with the reacquisition of the BCF in connection with the settlement of the Convertible Promissory Notes was the issuance of 69,151,117 shares of Series E convertible preferred stock valued at $211.6 million. The intrinsic value of the BCF upon the NPA Qualified Financing was measured based on the intrinsic value of the conversion option at the settlement date which was in excess of the repurchase price. Therefore, the entire $211.6 million was allocated to the reacquisition of the BCF which was recorded as a reduction to additional paid in capital. As a result of the extinguishment of the Convertible Promissory Notes, the Company recorded a gain of $0.1 million that is reflected in other income (expense), net in the Consolidated Statements of Operations and Comprehensive Loss for the year ended December 31, 2019.
| 13. | Stockholders’ Equity |
The Consolidated Statement of Stockholders’ Equity has been retroactively adjusted for all periods presented to reflect the Business Combination and reverse recapitalization as described in Note 1.
Preferred Stock
Following the closing of the Business Combination, the Company is authorized to issue 200,000,000 shares of preferred stock with a par value $0.0001 per share. The Company’s board of directors are authorized, without stockholder approval, to issue such shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, and to fix the designations, powers, voting, and other rights, preferences and privileges of the shares. There were no issued and outstanding shares of preferred stock as of December 31, 2020 and 2019.
Common Stock
Following the closing of the Business Combination, the Company is authorized to issue 15,800,000,000 shares of common stock, including 10,500,000,000 shares of Class A common stock, par value $0.0001 per share, 4,500,000,000 shares of Class B common stock, par value $0.0001 per share, and 800,000,000 shares of Class C common stock, par value $0.0001 per share.
Voting
Holders of Class A common stock are entitled to one vote per share and holders of Class B common stock are entitled to ten votes per share. Holders of Class C common stock are not entitled to vote except as otherwise expressly provided in the certificate of incorporation or required by applicable law.
Dividends
Common stockholders are entitled to receive dividends, as may be declared by the board of directors. Different classes of common stock are legally entitled to equal per share distributions whether through dividends or liquidation. No dividends have been declared to date.
Conversion
Each share of Class B common stock is convertible at any time at the option of the holder into one share of Class A common stock. Generally, shares of Class B common stock will convert automatically into Class A common stock upon the holder ceasing to be an Eligible Holder (i.e., director, employee, trust or legal entity of Ginkgo), unless otherwise determined by affirmative vote of a majority of independent directors of Ginkgo.
F-38
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Treasury Stock
During the year ended December 31, 2019, the Company repurchased 490,805 shares of common stock from its employees. The Company reclassified the shares as treasury stock, which is shown as a reduction of stockholders’ equity, for the fair value of the common stock repurchased and recorded payroll expense of $0.1 million equal to the difference between the repurchase price and the fair value of the common stock on the repurchase date. Upon the repurchase, the Company returned all shares of treasury stock to authorized and unissued shares of common stock in which the carrying value of the treasury stock was recorded as a reduction to common stock and additional paid-in capital in the Consolidated Balances Sheet. As of December 31, 2020 and 2019, no shares of common stock remained in treasury.
Common Stock Reserved for Future Issuances
| As of December 31, | ||||||||
| 2020 | 2019 | |||||||
| Shares reserved for warrants to purchase common stock |
1,020,187 | 1,020,187 | ||||||
| Shares reserved for exercises of outstanding stock options under the 2008 Stock Incentive Plan |
33,354,871 | 35,276,812 | ||||||
| Shares reserved for vesting of restricted stock units under the 2014 Stock Incentive Plan |
124,932,207 | 70,119,944 | ||||||
| Shares reserved for issuances under the 2014 Stock Incentive Plan |
4,783,479 | 18,122,760 | ||||||
|
|
|
|
|
|||||
| Total common stock reserved for future issuances |
164,090,744 | 124,539,703 | ||||||
|
|
|
|
|
|||||
| 14. | Stock-Based Compensation |
In 2008, the Company adopted the 2008 Stock Incentive Plan (the “2008 Plan”), in 2014 the Company adopted the 2014 Stock Incentive Plan (the “2014 Plan”) and in 2021 the Company adopted the 2021 Incentive Award Plan (the “2021 Plan”, collectively with the 2008 Plan and the 2014 Plan, the “Plans”). Pursuant to the 2021 Plan, the Company may grant incentive and nonqualified stock options, RSUs, RSAs and other stock-based awards to employees, officers, directors, consultants, and advisors. No additional awards may be granted under the 2008 Plan or the 2014 Plan. The Plans are administered by the board of directors or the compensation committee of the board of directors. The shares of common stock underlying any awards that are forfeited, cancelled, repurchased, or otherwise terminated by the Company under the Plans will be added back to the shares available for issuance under the 2021 Plan. As of December 31, 2020, the maximum number of shares of common stock that are reserved for issuance under the 2008 and 2014 Plans is 48,033,713 and 130,759,452, respectively, of which no shares and 4,783,479 shares of common stock are available for future issuance under the 2008 Plan and 2014 Plan, respectively.
Stock Options
As of December 31, 2020, the Company has only issued stock option awards under the 2008 Plan, of which all were granted prior to January 1, 2019 and had a ten-year contractual term. Upon stock option exercise, the Company issues new shares and delivers them to the participant.
F-39
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
A summary of stock option activity under the 2008 Plan is presented below:
| Number of Shares |
Weighted Average Exercise Price per Share |
Weighted Average Remaining Contractual Term |
Aggregate Intrinsic Value(1) |
|||||||||||||
| (in years) | (in thousands) | |||||||||||||||
| Outstanding as of December 31, 2019 |
35,276,812 | $ | 0.02 | 4.20 | $ | 79,915 | ||||||||||
| Exercised |
(1,921,941 | ) | 0.02 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding as of December 31, 2020 |
33,354,871 | $ | 0.02 | 3.20 | $ | 131,370 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Exercisable as of December 31, 2020 |
33,354,871 | $ | 0.02 | 3.20 | $ | 131,370 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) The aggregate intrinsic value of stock options is calculated as the difference between the exercise price of the underlying stock options and the estimated fair value of the common stock for those stock options that had exercise prices lower than the estimated fair value of the common stock as of December 31, 2020 and 2019. |
| |||||||||||||||
The aggregate intrinsic value of stock options exercised during the years ended December 31, 2020 and 2019 was $5.3 million and $1.1 million, respectively. No stock options were granted during the years ended December 31, 2020 and 2019.
Restricted Stock Units
The Company has granted RSUs to employees and non-employees under the 2014 Plan which are subject to two vesting conditions: (i) a service-based vesting condition under which the awards vest based on continued service over a period of time, and (ii) a performance-based vesting condition whereby the awards vest based on a liquidity event in the form of either a change of control or an initial public offering, each as defined in the 2014 Plan. RSUs awarded to new hires generally vest based on service over four years from the date of hire with 25% vesting on the first anniversary of the date of hire, and the remaining on a pro rata basis each month over the next three years. Additional RSU grants generally vest based on service in equal monthly installments over a four-year term. Both new hire and additional RSU grants are also subject to the performance-based vesting condition. Employees are able to retain RSUs vested with respect to the service condition upon departure, and such RSUs remain subject to the performance-based vesting condition. RSUs issued under the 2014 Plan expire seven years from the date of grant.
A summary of the RSU activity under the 2014 Plan is presented below:
| Number of Shares |
Weighted Average Grant Date Fair Value |
|||||||
| Nonvested as of December 31, 2019 |
70,119,944 | $ | 0.99 | |||||
| Granted |
57,184,567 | 2.68 | ||||||
| Forfeited |
(2,372,304 | ) | 1.89 | |||||
|
|
|
|
|
|||||
| Nonvested as of December 31, 2020 |
124,932,207 | $ | 1.74 | |||||
|
|
|
|
|
|||||
The weighted average remaining contractual term for the nonvested RSUs as of December 31, 2020 was 5.13 years. The weighted average grant date fair value of the RSUs granted during the year ended December 31, 2019 was $1.78 per share.
F-40
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Restricted Stock Awards
The Company has granted RSAs to employees and consultants under the 2014 Plan with a service-based condition that generally vest in equal monthly installments over a four-year term.
A summary of the RSA activity under the 2014 Plan is presented below:
| Number of Shares |
Weighted Average Grant Date Fair Value |
|||||||
| Nonvested as of December 31, 2019 |
675,887 | $ | 1.99 | |||||
| Vested |
(256,838 | ) | 1.99 | |||||
|
|
|
|
|
|||||
| Nonvested as of December 31, 2020 |
419,049 | $ | 1.99 | |||||
|
|
|
|
|
|||||
The aggregate fair value of the RSAs that vested during the years ended December 31, 2020 and 2019 was $0.5 million and $0.7 million, respectively.
Stock-Based Compensation
Stock-based compensation expense was allocated as follows (in thousands):
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Research and development |
$ | 79 | $ | 64 | ||||
| General and administrative |
397 | 707 | ||||||
|
|
|
|
|
|||||
| Total |
$ | 476 | $ | 771 | ||||
|
|
|
|
|
|||||
During the years ended December 31, 2020 and 2019, the Company recognized $0.5 million and $0.8 million, respectively, in stock-based compensation expense related to the RSAs. The Company has not recognized any stock-based compensation expense related to the RSUs as of December 31, 2020 as satisfaction of the performance-based vesting condition was not deemed probable. All outstanding stock options were fully vested prior to January 1, 2019 and, accordingly, no stock-based compensation expense was recognized for these awards during the years ended December 31, 2020 and 2019.
As of December 31, 2020, total unrecognized stock-based compensation expense related to the RSUs and RSAs was $218.0 million and $0.8 million, respectively. The total unrecognized stock-based compensation expense related to the RSAs will be recognized over a weighted average period of 1.88 years.
F-41
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
15. Revenue Recognition
Disaggregation of Revenue
The following table sets forth the percentage of Foundry revenues by industry based on total Foundry revenue:
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Food and nutrition |
35 | % | 39 | % | ||||
| Industrial and environmental |
29 | % | 13 | % | ||||
| Agriculture |
13 | % | 18 | % | ||||
| Consumer and technology |
12 | % | 19 | % | ||||
| Other |
11 | % | 11 | % | ||||
|
|
|
|
|
|||||
| Total |
100 | % | 100 | % | ||||
|
|
|
|
|
|||||
The following table sets forth the percentage of revenue by geographic location based on total revenue:
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| North America |
95 | % | 95 | % | ||||
| Rest of world |
5 | % | 5 | % | ||||
|
|
|
|
|
|||||
| Total |
100 | % | 100 | % | ||||
|
|
|
|
|
|||||
Contract Balances
The Company recognizes a contract asset when the Company transfers goods or services to a customer before the customer pays consideration or before payment is due, excluding any amounts presented as accounts receivable. The Company did not have any contract assets as of and for the years ended December 31, 2020 and 2019.
Contract liabilities, or deferred revenue, primarily consist of payments received in advance of performance under the contract or when the Company has an unconditional right to consideration under the terms of the contract before it transfers goods or services to the customer. The Company’s collaborative arrangements with its investees and related parties typically include upfront payments consisting of cash or non-cash consideration for future research and development services and non-cash consideration in the form of equity securities for licenses that will be transferred in the future. The Company records the upfront cash payments and fair value of the equity securities as deferred revenue.
The Company also invoices customers based on contractual billing schedules, which results in the recording of deferred revenue to the extent payment is received prior to the Company’s performance of the related services. Contract liabilities are recognized as revenue as (or when) the Company performs under the contract.
Of the Company’s $147.9 million in deferred revenue at December 31, 2019, $25.5 million was recognized as revenue during 2020. Of the Company’s $127.2 million in deferred revenue at December 31, 2018, $16.8 million was recognized as revenue during 2019.
Performance Obligations
The aggregate amount of the transaction price that was allocated to performance obligations that have not yet been satisfied or are partially satisfied as of December 31, 2020 and 2019 was $20.7 million and $35.3 million,
F-42
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
respectively. The Company has elected the practical expedient not to provide the remaining performance obligation disclosures related to contracts for which the Company recognizes revenue on a cost-plus basis in the amount to which it has the right to invoice. As of December 31, 2020, of the performance obligations not yet satisfied or partially satisfied, 94% is expected to be recognized as revenue during the years ended December 31, 2021 to 2023. The remainder cannot be reasonably estimated due to uncertainty about the timing of development milestones.
16. Significant Collaboration Transactions
Allonnia, LLC
Summary of Arrangement
Allonnia was formed in 2019 and focuses on the application of synthetic biology in the bioremediation space, leveraging Ginkgo’s proprietary platform to develop solutions for waste bioremediation and the biorecovery of rare earth elements or other substances from waste streams or waste deposits. In December 2019, the Company entered into (i) an Intellectual Property Contribution Agreement (“Allonnia IP Agreement”) that granted Allonnia a license to certain of the Company’s intellectual property, (ii) a Technical Development Agreement (“Allonnia TDA”) that establishes the terms under which the Company is providing technical development services, and (iii) a Common Unit Issuance Agreement (“CUIA”) which provides for the issuance of common units of Allonnia to the Company in exchange for the license rights granted under the Allonnia IP Agreement. Contemporaneous with these agreements, Allonnia entered into a Series A Preferred Unit Purchase Agreement under which Allonnia sold 2,970,000 Series A Preferred Units to certain of the Company’s investors, as well as a third-party investor, for aggregate proceeds of approximately $33.0 million. Allonnia also agreed to issue an additional 630,000 Series A Preferred Units to a strategic partner as compensation for the delivery of future services to Allonnia. The Series A Preferred Unit Purchase Agreement also provides for the sale and issuance of up to an additional 5,400,000 Series A Preferred Units subsequent to the initial closing. Subsequently, during the year ended December 31, 2020, Allonnia issued an additional 1,844,911 Series A Preferred Units, 1,664,911 of which were sold for aggregate proceeds of $18.5 million and 180,000 of which were issued in exchange for the rights to certain intellectual property which will vest based on the achievement of milestones associated with the development of the intellectual property received. In 2021, Allonnia issued an additional 22,500 Series A Preferred Units for aggregate proceeds of $0.2 million and closed their Series A Preferred Unit financing. As a result, the Company received an additional 1,867,411 common units in full satisfaction of the additional common unit right described in the following paragraph (Note 21).
Under the Allonnia IP Agreement, the Company licensed intellectual property to Allonnia for use in the development or the production of its products that the parties will subsequently agree to develop under TDPs. The license rights provide Allonnia with the ability to commercialize the specified products from the corresponding strain or enzyme, which can only be developed by the Company under the Allonnia TDA. The Company received 3,600,000 common units as consideration for the license upon execution of the agreement. In addition, the Company is entitled to receive up to an additional 5,400,000 common units upon the issuance of additional Series A Preferred Units by Allonnia.
Under the Allonnia TDA, the parties jointly agree, through equal representation on a joint steering committee, on TDPs for specific strains and enzymes, in which the Company will perform agreed upon development services in return for consideration on a cost-plus basis for all services provided. As of December 31, 2020, the Company has entered into three TDPs with Allonnia.
Accounting Analysis
The Company concluded that Allonnia is a variable interest entity in which it holds a variable interest through its common unit interest. Allonnia was designed to function as a stand-alone entity with its own board of directors,
F-43
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
employees, and operational infrastructure. While the Company was involved with the creation of Allonnia, has board representation, and is involved in the ongoing development activities of Allonnia through its participation on a joint steering committee (as provided for under the Allonnia TDA), the Company concluded this involvement does not give it the power to control the decisions with respect to the development activities of Allonnia, which are the most significant activities of Allonnia. The Company does not control Allonnia’s board of directors and there are no voting or consent agreements between the Company and the other members of Allonnia’s board of directors or the holders of the Series A Preferred Units. Further, the Company’s representation on the joint steering committee does not give it control over Allonnia’s development activities as all votes of the joint steering committee must pass by consensus and there is no agreement in place that would require Allonnia to vote in alignment with the Company. Accordingly, the Company is not the primary beneficiary of Allonnia as it does not control the decisions that most significantly impact Allonnia’s economic performance.
The common unit investment in Allonnia is considered an equity method investment as a result of the Company’s ability to exercise significant influence over the financial and operating policies through its ownership of common units. The initial carrying value of the equity method investment in Allonnia is the fair value of the common units of $24.5 million received in exchange for the Allonnia IP Agreement which, as discussed below, was accounted for as deferred revenue at inception. The fair value of Allonnia’s common units was determined at inception of the agreements using the option pricing method. The option pricing method used a back-solve methodology to infer the total equity value based on the pricing of the Series A Preferred Unit financing, which was contemporaneous with the Allonnia IP Agreement. Further, the Company determined the rights to up to an additional 5,400,000 common units did not meet the definition of a freestanding financial instrument and are not representative of a derivative. The right to the additional common units is considered variable consideration that is fully constrained at inception and until the contingencies related to the issuance of the additional shares are resolved. This contingency was resolved in 2021 when the Company and Allonnia agreed upon the additional 1,867,411 common units to be issued under the agreements (Note 21).
The Series A Preferred Units issued by Allonnia receive a liquidation preference prior to common units. As such, the Company concluded that this represents a substantive profit-sharing arrangement, and the Company is recognizing earnings and losses on the equity method investment using the HLBV method. The Company recorded a loss on equity method investment of $24.5 million from inception through December 31, 2019. The loss allocated to the Company primarily relates to Allonnia’s accounting for the non-cash consideration related to the Allonnia IP Agreement as in-process research and development, which resulted in the full value of the Company’s intellectual property contribution being expensed in the period ended December 31, 2019. As of December 31, 2019, the carrying value of the equity method investment in Allonnia has been reduced to zero. There is no commitment for the Company to provide further financial support to Allonnia and therefore the carrying value of the equity method investment will not be reduced below zero. As a result, no loss was recognized during the year ended December 31, 2020 on the equity method investment.
The relationship with Allonnia is a vendor-customer relationship and is within the scope of ASC 606 as the provision of services and corresponding license rights are considered a part of the Company’s ordinary activities and the common units represent non-cash consideration. While the Allonnia TDA has been executed by the parties and provides the payments terms for future services, the Allonnia TDA does not provide for any transfer of goods or services between the parties. However, the Company will provide licenses and services upon execution of the contemplated TDPs. Accordingly, the Company concluded that the Allonnia TDA met the definition of a contract under ASC 606 and each TDP executed under the Allonnia TDA will be accounted for in accordance with ASC 606. There were no TDPs entered into during the year ended December 31, 2019, therefore, the non-cash consideration of $24.5 million is recorded as deferred revenue, net of current portion on the Consolidated Balance Sheet as of December 31, 2019.
F-44
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The Company’s performance obligations under the contract consist of a combined service and license performance obligation related to the initial TDP executed in February 2020 and nine material rights, related to the estimated additional TDPs the parties expect to execute under the Allonnia TDA. The material rights represent an advance payment for the license rights which will be granted upon the execution of each TDP. As there is no additional payment for these license rights upon execution of a TDP, the Company has determined that there is a material right associated with each of the contemplated future TDPs. The Company has allocated $2.5 million of the upfront non-cash consideration to each of the ten performance obligations under the contract based on the estimated standalone selling price of the performance obligations. Unexercised material rights are recorded as non-current deferred revenue until such time as the parties execute a TDP.
Upon the execution of each TDP, the Company is obligated to provide development services under the TDP and a license to applicable patents and other intellectual property to the ingredient developed under the plan. The license and research and development services under a TDP are highly interdependent and interrelated with one another. Without the Company’s knowledge, expertise, and platform, there would not be a licensable strain or other commercializable product to transfer to Allonnia. Further, Allonnia has rights to all development intellectual property created as part of each TDP, irrespective of the result of the development. Therefore, each executed TDP consists of one combined performance obligation for the license and research and development services to be performed by the Company.
For each TDP, the transaction price consists of variable consideration for the most likely amount of estimated consideration to be received under the cost-plus arrangement and the $2.5 million allocation of the fixed non-cash consideration. As the services performed by the Company create or enhance an asset that Allonnia controls as the asset is created or enhanced, the Company satisfies the performance obligation and recognizes revenue over time. The Company uses an input method that compares total costs incurred relative to total estimated cost to complete to estimate progress under the contract. Any revisions to the estimated total budgeted costs to complete, and the resulting impact to revenue recognition, are reflected in the period of the change through a cumulative catch-up adjustment. In 2021, the constraint was removed from the additional non-cash consideration. The additional consideration of $12.7 million was allocated to all of the performance obligations under its arrangement with Allonnia consistent with the initial relative selling price allocation and a cumulative catch up was recognized for the TDPs in process (Note 21).
As of December 31, 2020 and 2019, the Company had a deferred revenue balance of $26.1 million and $24.5 million, respectively, with Allonnia. During the year ended December 31, 2020, the Company recognized $5.0 million from services provided to Allonnia. No revenue was recognized by the Company during the year ended December 31, 2019.
Glycosyn, LLC
Summary of Arrangement
In October 2018, the Company entered into a series of arrangements with Glycosyn, a biotech company developing components of human milk, to optimize and scale the production of human milk oligosaccharides (“HMOs”) for a suite of products that foster a healthy gut microbial ecology. Glycosyn has developed a portfolio of HMOs that can be produced at lab scale and the focus of the collaboration is to utilize the Company’s platform to more effectively optimize and enhance these existing HMOs-producing strains to scale up production, as well as develop new HMOs products.
The Glycosyn arrangements include (i) a Class C Unit Purchase Agreement (“Glycosyn Purchase Agreement”), (ii) a Foundry Terms of Service Agreement (“Glycosyn FSA”), and (iii) the Glycosyn Promissory Note.
F-45
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Under the Glycosyn Purchase Agreement, the Company purchased 80,142 Class C Units at a purchase price of $124.78 per unit for an aggregate purchase price of $10.0 million. Payment for the Class C Units was made with $1.0 million in cash paid at closing and the right for Glycosyn to utilize up to $9.0 million in Foundry services (“Glycosyn Prepaid Services”). The Class C Units have a liquidation preference over all other outstanding units equal to $10.0 million, plus any accrued or declared and unpaid distributions.
The Glycosyn FSA outlines the general terms and conditions under which the Company will perform services for Glycosyn. These services will, in turn, be performed under an executed TDP agreed to by both parties. Under an executed TDP, the Company will use commercially reasonable efforts to develop strains for the production of Glycosyn products. Further, the Company will grant Glycosyn certain licenses to any resulting product from each TDP to commercialize in the field of biosynthesis of oligosaccharides in microorganisms while the Company retains license rights outside of the field. The Company will charge for services based on its costs plus a fixed margin and apply amounts earned against the Glycosyn Prepaid Services. The first $1.0 million of services will be applied to the Glycosyn Prepaid Services. Thereafter, 25% of every invoice is applied to the Glycosyn Prepaid Services and 75% is payable in cash. Prior to its termination discussed below, the parties had executed one TDP.
The Glycosyn FSA can be terminated by mutual agreement, change in control or insolvency at any time during the term of the agreement. Glycosyn may terminate for convenience following the one-year anniversary, provided notice is received by the Company no later than thirty days following the one-year anniversary. Upon termination by mutual agreement, change in control, or insolvency, the Company is required to repay 50% of any unused Glycosyn Prepaid Services in cash or with Class C Units of Glycosyn at an amount equal to their then-current fair value. Upon termination for convenience by Glycosyn, the Company would be entitled to keep an amount equal to the cumulative amount invoiced against the Glycosyn Prepaid Services and the remainder would be payable to Glycosyn in cash or with Class C Units of Glycosyn at an amount based on their then current fair value.
In 2019, Glycosyn exercised their option to terminate the agreement in accordance with its contractual rights at the one-year anniversary. In connection with the termination notice, the parties negotiated a Unit Repurchase Agreement and Amendment to the FSA which was executed on December 31, 2019 and resulted in (i) the Company returning all of its Class C Units holdings to Glycosyn, (ii) termination of all the Company’s obligations under the Glycosyn FSA, (iii) agreement to perform certain services in the future on a cost-plus fixed margin basis, (iv) an increase to the amount owed on the Glycosyn Promissory Note from $3.1 million to $5.7 million, which was the outstanding balance as of December 31, 2019, and (v) a modification to the terms of the Glycosyn Promissory Note to increase the interest rate, modify the payment terms and extend the maturity. As of December 31, 2020, the outstanding balance on the promissory note was $5.4 million. Refer to Note 4 for discussion of the Glycosyn Promissory Note.
Accounting Analysis
Prior to the termination, the Company accounted for its investment in Glycosyn’s Class C Units as an equity method investment as it held an approximate 18% equity interest in Glycosyn. The Company recorded the initial carrying value of its equity method investment at fair value, which the Company determined was $10.0 million. The fair value was determined by the Company with the assistance of a third-party valuation specialist and utilizes a discounted cash flow analysis of Glycosyn’s projected cash flows and the preferences of the LLC units in a distribution scenario. As the Class C Units receive a preferential distribution, the Company concluded that the shares contain a substantive profit-sharing arrangement. Accordingly, the Company recognized its share of earnings or losses from its equity method investment in Glycosyn using the HLBV method. During the year ended December 31, 2019, prior to the termination, the Company recorded a loss on equity method investment in Glycosyn of $1.3 million. Immediately prior to termination of the Glycosyn FSA, the carrying value of the equity method investment was $8.5 million.
F-46
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
While the Glycosyn FSA has been executed by the parties and provides the payment terms for future services, the Glycosyn FSA does not provide for any transfer of goods or services between the parties. However, there is an obligation that the Company will provide licenses and services upon execution of a TDP. Accordingly, at inception, the Company recorded deferred revenue of $9.0 million equal to the fair value of the equity received less the cash paid. Upon execution of a TDP, the Company will reduce the deferred revenue by the portion of the transaction price funded by the Glycosyn Prepaid Services. During the year ended December 31, 2019, the Company recognized $0.7 million of revenue related to the Glycosyn FSA. At the time of the termination of the Glycosyn FSA, the outstanding balance related to the Glycosyn Prepaid Services was $8.4 million, which was eliminated in conjunction with the termination of the Glycosyn FSA. Upon termination, the Company recognized a gain on termination of $1.5 million primarily attributable to the increase in loan receivable which is carried at fair value. The gain was recorded as a component of other income (expense), net on the Consolidated Statements of Operations and Comprehensive Loss for the year ended December 31, 2019.
Motif FoodWorks, Inc.
Summary of Arrangement
Motif was incorporated in 2018 to focus on the application of synthetic biology in the food industry, leveraging the Ginkgo’s proprietary platform to develop alternative protein ingredients that reduce reliance on animal products. In September 2018, the Company entered into (i) an Intellectual Property Contribution Agreement (“Motif IP Agreement”) with Motif that granted Motif a license to certain of the Company’s intellectual property and (ii) a Technical Development Agreement (“Motif TDA”) that establishes the terms under which the Company is providing technical development services.
Under the Motif IP Agreement, the Company licensed intellectual property to Motif for use in strain development to produce ingredients that the parties will subsequently agree to develop under TDPs. The license rights provide Motif with the ability to commercialize the specified ingredients from the corresponding strain, which can only be developed by the Company under the Motif TDA. In return for the license to the intellectual property, Motif granted the Company 9,000,900 shares of common stock. Concurrent with the Motif IP Agreement, Motif also sold 8,100,720 shares of Series A preferred stock to certain of the Company’s investors, as well as third-party investors, for aggregate proceeds of approximately $90.0 million.
The Motif TDA governs the procurement of the Company’s expertise and technical development services to collaborate in the research, development, and commercialization of specified ingredients. Under the Motif TDA, the parties jointly agree on TDPs for specific ingredients, in which the Company will perform agreed upon development services in return for consideration on a cost-plus fixed margin basis for all services provided. At inception, the Company estimated that it would execute ten TDPs with Motif.
Accounting Analysis
The Company concluded that Motif is a variable interest entity in which it holds a variable interest through its common stock interest. Motif was designed to function as a stand-alone entity with its own board of directors, employees, and operational infrastructure. While the Company was involved with the creation of Motif, has board representation, and is involved in the ongoing development activities of Motif through its participation on a joint steering committee (as provided for under the Motif TDA), the Company concluded this involvement does not give it the power to control the decisions with respect to the development activities of Motif, which are the most significant activities of Motif. The Company does not control Motif’s board of directors and there are no voting or consent agreements between the Company and the other members of Motif’s board of directors or other investors. Further, the Company’s representation on the joint steering committee does not give it control over
F-47
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Motif’s development activities as all votes of the joint steering committee must pass by consensus and there is no agreement in place that would require Motif to vote in alignment with the Company. Accordingly, the Company is not the primary beneficiary of Motif as it does not control the decisions that most significantly impact Motif’s economic performance.
The investment in Motif common stock is considered an equity method investment as a result of the Company’s ability to exercise significant influence over the financial and operating policies through its common stock ownership. The initial carrying value of the equity method investment in Motif is the fair value of the common stock received in exchange for the Motif IP Agreement of $65.1 million which, as discussed below, is being accounted for as non-cash consideration under ASC 606. As Motif’s Series A preferred stockholders receive a liquidation preference prior to common stock, the Company concluded that this represents a substantive profit-sharing arrangement. Accordingly, the Company is recognizing earnings and losses on the equity method investment using the HLBV method. The Company recorded a loss on equity method investment of $65.1 million from inception through December 31, 2018 which reduced the carrying value to zero. The loss allocated to the Company primarily relates to Motif’s accounting for the non-cash consideration related to the Motif IP Agreement as in-process research and development, which resulted in the full value of Company’s intellectual property contribution being expensed in the period ended December 31, 2018, at which time the carrying value of the equity method investment in Motif had been reduced to zero. There is no commitment for the Company to provide further financial support to Motif and therefore the carrying value of the equity method investment will not be reduced below zero. As a result, no loss was recognized during the years ended December 31, 2020 and 2019 on the equity method investment.
The overall arrangement with Motif is a vendor-customer relationship and is within the scope of ASC 606 as the provision of development services and corresponding license rights are considered a part of the Company’s ordinary activities. The licenses contemplated under the Motif IP Agreement are contingent upon a TDP being agreed to by the parties under the Motif TDA and only relate to strains that are developed under a TDP. While the TDPs require approval by the parties, the parties initially estimated that ten TDPs would be negotiated under the arrangement.
The Company’s performance obligations under the Motif IP Agreement consist of ten material rights, related to the initial set of ingredients that the parties desired to develop in the first two years. The material rights represent an advance payment for the license rights which will be granted upon the execution of each TDP. As there is no additional payment for these license rights upon execution of a TDP, the Company has determined that there is a material right associated with each of the contemplated TDPs. The common stock received under the Motif IP Agreement is considered non-cash consideration and has been recognized at fair value. The Company determined the fair value of the common stock was $65.1 million at inception of the agreement with the assistance of a third-party valuation specialist, which was initially recorded as non-current deferred revenue. The option pricing model used a back-solve methodology to determine the total equity value based on the pricing of the Series A financing, which was contemporaneous with the Motif IP Agreement. The Company has allocated $6.5 million to each of the ten material rights. The Company allocated the transaction price based on the estimated standalone selling price of the material rights which is, in turn, based on the intrinsic value of the right and the probability of exercise.
Upon the execution of each TDP, the Company is obligated to provide development services under the TDP and a license to applicable patents and other intellectual property to the ingredient developed under the plan. The license and research and development services under a TDP are highly interdependent and interrelated with one another. Without the Company’s knowledge, expertise and platform, there would not be a licensable strain or other commercializable product to transfer to Motif. Further, Motif has rights to all development intellectual
F-48
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
property created as part of each TDP, irrespective of the result of the development. Therefore, each executed TDP consists of one combined performance obligation for the license and research and development services to be performed by the Company.
For each TDP, the transaction price consists of variable consideration for the most likely amount of estimated consideration to be received under the cost-plus arrangement and the $6.5 million which was allocated to the associated material right under the Motif IP Agreement. As the services performed by the Company create or enhance an asset (i.e., the specified ingredient) that Motif controls as the asset is created or enhanced, the Company satisfies the performance obligation and recognizes revenue over time. The Company uses an input method that compares total costs incurred relative to total estimated cost to complete to estimate progress under the contract. Any revisions to the estimated total budgeted costs to complete, and the resulting impact to revenue recognition, are reflected in the period of the change through a cumulative catch-up adjustment.
As of December 31, 2020 and 2019, the Company had a deferred revenue balance of $54.0 million and $62.5 million, respectively, with Motif. The Company recognized revenue of $20.8 million and $19.0 million from services provided to Motif during the years ended December 31, 2020 and 2019, respectively.
Genomatica, Inc.
2016 Genomatica Agreement
In 2016, the Company purchased Series A preferred stock of Genomatica, Inc. (“Genomatica”), a biotechnology company specializing in the development and manufacturing of intermediate and specialty chemicals from both sugar and alternative feedstocks. The Company also entered into a Collaboration Agreement with Genomatica (“Genomatica Collaboration”) in connection with the financing. The Genomatica Collaboration was entered into to share expertise on biotechnology solutions. Specifically, Genomatica provided the Company with scale-up and process optimization functions, and the Company has provided Genomatica with certain technology development functions generally centered on high throughput strain engineering capabilities. The Genomatica Collaboration’s focus was on obtaining new customers for either party that could benefit from the combined expertise of both parties, and the agreement provides for profit-sharing allocations between Genomatica and the Company depending on the category of the potential product. Each party is responsible for their own costs incurred under an agreed upon TDP.
2018 Genomatica Agreement
In September 2018, the Company entered into a stock purchase agreement with Genomatica under which it received $40.0 million of Series B preferred stock from Genomatica. In lieu of cash consideration, the Company entered into a Foundry Terms of Service Agreement (“Genomatica FSA”) with Genomatica in which the Company would provide up to $40.0 million in services at no charge to Genomatica (“Initial Prepayment”). The Genomatica FSA terminated the Genomatica Collaboration and changed the pricing terms for work performed under TDPs to a cost-plus fixed margin agreement. Genomatica can apply a portion of the $40.0 million in prepaid services to outstanding invoices under the Genomatica FSA, subject to certain limitations that require cash payment for services over certain monthly thresholds. Further, while the Genomatica FSA replaced the Genomatica Collaboration, any fees that would have been paid to or by the Company under contracts previously governed by the Genomatica Collaboration continue to be shared between the parties. These amounts are either (i) added to, if payable to the Company, or (ii) reduced from, if payable to Genomatica, the balance of the prepaid services over the term of the arrangement, with certain restrictions. At the time of the execution of the Genomatica FSA, there was $19.1 million of potential consideration payable to the Company under the Genomatica Collaboration, which upon payment will contribute to the prepaid services balance, and $4.6 million
F-49
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
of potential payments to Genomatica, which upon payment will reduce the prepaid services balance. As of December 31, 2020, and 2019, the Company has received $6.9 million under the Genomatica FSA.
Accounting Analysis
The Company concluded that Genomatica is a variable interest entity in which it holds a variable interest through its preferred stock interest. While the Company holds a seat on Genomatica’s board of directors and participates in board decisions via such participation, it does not have the ability to control the board as there is no voting or consent agreement between the Company and other members of the board or preferred stockholders. Further, while the Company participates on the joint steering committee that governs the Genomatica FSA, all votes must be unanimous and there is no agreement in place that would require Genomatica to vote in alignment with the Company. Accordingly, the Company is not the primary beneficiary of Genomatica as it does not control the decisions that most significantly impact Genomatica’s economic performance.
The Company concluded the preferred stock investment was not in-substance common stock and therefore did not qualify for accounting as an equity method investment. Rather, the Company concluded the preferred stock investment should be accounted for as an equity security as it represents an ownership interest in Genomatica that is not mandatorily redeemable nor does the Company have the unilateral right to redeem the preferred stock. Genomatica’s preferred stock is not exchange-traded and does not have a readily determinable fair value. Therefore, the Company accounts for the Genomatica preferred stock under the measurement alternative for equity investments that do not have a readily determinable fair value, which in this case is at historical cost. As of December 31, 2020, and 2019, the cost of the investment in Genomatica’s preferred stock was $55.0 million and is included in investments on the Consolidated Balance Sheets. As of December 31, 2020 and 2019, no adjustments have been recognized related to the preferred stock investment as a result of the application of the measurement alternative.
Under the Genomatica Collaboration, the Company was entitled to receive a portion of fees earned from third party customers of Genomatica that were within the scope of the agreement. The Company accounted for the collaboration under ASC 808, however the Company applied ASC 606 by analogy for measurement and recognition purposes. Under the Genomatica Collaboration, the Company’s promises consisted of (i) licenses to the Company’s intellectual property, related to the specified development work, and (ii) research and development services. The Company determined that there was a single, combined performance obligation consisting of research services and licenses to certain intellectual property. The Company recognized the revenue for the combined performance obligation using an over-time input method, as the Company’s performance under the contract created or enhanced the target product or strain as such product or strain was developed. The Company measured progress based on the cost incurred relative to total forecasted cost.
The Genomatica FSA represents a modification to the Genomatica Collaboration that resulted in a change in transaction price from milestones to a cost-plus fixed margin structure. The Genomatica FSA did not result in the addition of any distinct promised goods or services, and the Company’s remaining obligation post-modification was to finish the partially satisfied development work that had commenced under the Genomatica Collaboration. This performance obligation was satisfied during the year ended December 31, 2019.
As of December 31, 2020 and 2019, the Company had a deferred revenue balance of $30.1 million and $38.1 million, respectively, with Genomatica. During the years ended December 31, 2020 and 2019, the Company recognized revenue from services provided to Genomatica of $9.4 million and $6.2 million, respectively.
F-50
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Joyn Bio, LLC
Summary of Arrangement
In September 2017, the Company and certain other investors formed Cooksonia for the purposes of holding the Company’s investment in Joyn. Concurrently, Cooksonia entered into a commitment agreement with Bayer CropScience LP (“Bayer”) to form Joyn. Joyn is focused on research, development, discovery, and commercialization of engineered microbes for use in agriculture. The initial program uses advanced techniques in biology to study and engineer naturally occurring soil microbes and their nitrogen-fixing genes to enable crops to produce their own fixed nitrogen and reduce the nitrogen fertilizer required.
The Company contributed $5.0 million in cash and certain intellectual property to Cooksonia in exchange for a 70% equity interest in Cooksonia (“Class A Units”). Cooksonia received $20.0 million in cash from another investor, who is a related party of the Company, for a 20% equity interest in Cooksonia (“Class B Units”). Cooksonia also received certain intellectual property from Genomatica and issued Genomatica a 10% equity interest in Cooksonia (“Cooksonia Class C Units”) and paid Genomatica $5.0 million in cash. Subsequently, Cooksonia contributed $20.0 million and all intellectual property received from the Company and Genomatica in exchange for a 50% equity interest in Joyn. Bayer contributed $20.0 million in cash funding plus specified intellectual property. In addition, Bayer committed to contribute up to an additional $60.0 million to be paid subject to certain funding procedures. In return, Bayer obtained a 50% equity interest in Joyn. The agreements may be terminated by mutual agreement, following a change in control, and for breach.
Joyn is governed by a Board of Managers (“Joyn Board”) comprised of equal representation of the Company and Bayer. The Joyn Board has all the rights, powers, obligations, and authority to manage the business and affairs of Joyn.
The Company also entered into a Foundry Services Agreement (“Joyn FSA”) with Joyn under which the Company will provide Joyn with technical services and preferred access to the Company’s facilities. Joyn paid the Company a non-refundable $20.0 million prepayment for services to be provided under the Joyn FSA (“Joyn Prepaid Services”). The Joyn Prepaid Services can be utilized for technical services performed by the Company, its subcontractors, and third parties involved in the performance of the overall technical services. Amounts due to the Company are applied to the balance of Joyn Prepaid Services as earned. During the year ended December 31, 2019, Joyn made an additional $15.0 million prepayment for services (“Joyn Additional Prepaid Services”). Under certain Joyn termination scenarios, any amount of unused Joyn Additional Prepaid Services shall be repaid by the Company to Joyn. There were no additional prepayments during the year ended December 31, 2020.
Accounting Analysis
From inception, the Company’s investment in Cooksonia has represented a controlling financial interest, resulting in consolidation of Cooksonia within Company’s consolidated financial statements. The Company concluded that Cooksonia is a variable interest entity and that it holds a variable interest in Cooksonia through its Class A Units. The Company is the primary beneficiary of Cooksonia as it controls the decisions that most significantly impact economic performance as the Company controls 100% of the board of directors and holds 70% of the equity in Cooksonia. The initial cash and in-kind contributions the Company made to Cooksonia have been recorded at carrying value as the transaction was with entities under common control. All assets of Cooksonia after the initial investments, net of the amounts paid to Genomatica, were contributed to Joyn for a 50% equity interest in Joyn. The Company presents the non-controlling interest attributable to the other investors’ equity interest in Cooksonia as a component of stockholder’s equity. The initial carrying value of the Company’s equity interest in Cooksonia was $13.1 million, comprised of the initial $5.0 million cash investment
F-51
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
and an $8.1 million adjustment for Cooksonia’s claim on net assets in accordance with ASC 810, Consolidation (“ASC 810”) recognized to reflect a certain investor’s liquidation preference in a termination event that represents a substantive profit-sharing agreement. The initial carrying value of the non-controlling interest was comprised of cash and intellectual property contributions from the other investors of $29.7 million, less the $8.1 million adjustment for the non-controlling interest holders’ claim on the net assets of Cooksonia.
With respect to Cooksonia’s investment in Joyn, as Cooksonia does not control the Joyn Board, it does not have the power to control the decisions related to the development activities of Joyn, which are the most significant activities of Joyn. Accordingly, the Company concluded that Cooksonia is not the primary beneficiary of Joyn as it does not control the decisions that most significantly impact Joyn’s economic performance.
Cooksonia accounts for its 50% equity interest in Joyn as an equity method investment based on the size of its equity interest and its influence on the board of directors. The equity method investment in Joyn was recorded at an initial carrying value of $97.9 million, which is the fair value of Cooksonia’s interest in Joyn. The fair value was determined by management with the assistance of a third-party valuation specialist. The option pricing model used a back-solve methodology to determine the total equity value based on the pricing of the Class B Units which were exchanged for cash. The license of intellectual property to Joyn has been accounted for under ASC 606 as described below. Upon liquidation, the net assets of Joyn are not distributed in accordance with each party’s respective ownership interest. Depending on the circumstances or type of liquidation event, Bayer or Cooksonia may receive certain preference payments or priority in the assets that are distributed. These preferences represent a substantive profit-sharing arrangement and, accordingly, Cooksonia recognizes earnings and losses on its equity method investment using the HLBV method. For the years ended December 31, 2020 and 2019, Cooksonia recognized a loss of $0.4 million and $1.7 million on its equity method investment, comprised of Cooksonia’s changes in claim on the net assets of Joyn as of December 31, 2020 and 2019, respectively.
For the years ended December 31, 2020 and 2019, Cooksonia’s net loss was $0.4 million and $1.7 million (comprised solely of the loss from its equity method investment in Joyn), of which $0.1 million and $0.5 million was attributable to the non-controlling interests, respectively. As of December 31, 2020 and 2019, Cooksonia recognized its equity method investment in Joyn at $28.9 million and $29.3 million, respectively, which was the sole asset held by Cooksonia as of each period end and is included in the Consolidated Balance Sheets for the respective periods. No liabilities were held by Cooksonia as of December 31, 2020 and 2019.
The Company accounts separately under ASC 606 for Cooksonia’s contribution of its intellectual property and the services performed by the Company under technical project plans governed by the Joyn FSA. The Company accounts for the intellectual property sale and the technical services separately as the two agreements were not negotiated with a single commercial objective, the consideration under each agreement is not interdependent, and the intellectual property contribution from Cooksonia is separate and distinct from the research and development services performed under the Joyn FSA.
The Company considers the granting of licenses to the Company’s intellectual property as part of its ordinary business activities and, therefore, Cooksonia’s contribution of intellectual property to Joyn represents a contract with a customer. The intellectual property contains multiple licenses for which control transfers at inception and all revenue associated with the licenses was recognized during the year ended December 31, 2017.
The Joyn FSA functions as a master services agreement that provides the framework for the ongoing research and development services relationship between the Company and Joyn. The Joyn FSA does not create a contract under ASC 606 as it does not identify goods or services to be performed nor does it define consideration under the contract. Upon the execution of a technical project plan under the Joyn FSA, the arrangement qualifies as a contract under ASC 606.
F-52
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The Company accounts for each technical project separately. Each technical project plan provides for distinct services in the context of the contract, has been separately negotiated with Joyn, focuses on different specified strains with separate scopes of work, and has its own budget. The sole performance obligation under each individual technical project plan consists of the research and development services as the requisite licenses were transferred prior to the execution of the technical project plans. The transaction price for each technical project plan is determined at plan inception based on the consideration that the Company negotiated in exchange for the services to be provided. The Company’s performance under each technical project plan creates or enhances assets under Joyn’s control. Joyn receives the benefits of the output of the research and development services which allow Joyn to make strategic business decisions on the direction of each product candidate. Therefore, the Company satisfies the respective performance obligations and recognizes revenue over time.
For the years ended December 31, 2020 and 2019, the Company recognized revenue from services provided to Joyn of $7.3 million and $9.3 million, respectively, for which the balance was applied against deferred revenue. As of December 31, 2020 and 2019, the Company had a deferred revenue balance of $9.9 million and $17.1 million, respectively, with Joyn, which represented the remaining balance of prepaid services as of each respective date. As of December 31, 2020, $9.9 million of the deferred revenue balance remains refundable under certain termination scenarios.
Amyris, Inc.
During 2017, the Company terminated its collaborative relationship with Amyris, Inc. (“Amyris”) as provided in the Amyris Collaboration Agreement and executed a settlement arrangement (“Partnership Agreement”) under which the Company is entitled to receive (i) value share payments owed to the Company under the Amyris Collaboration Agreement, (ii) payments of $0.8 million each quarter commencing on December 31, 2018 through the quarter ended September 30, 2022, and (iii) payments due under an interest bearing $12.0 million promissory note.
The parties amended the agreements during the year ended December 31, 2020 to defer certain payments and provide Amyris waivers for noncompliance with certain covenants. As of December 31, 2020, the Company was owed (i) the $12.0 million principal balance on the promissory note which matures on October 19, 2022 and (ii) payments under the Partnership Agreement, as amended, which includes quarterly payments of $0.2 million to $0.3 million through September 2022 and an end of term payment of $9.8 million on October 19, 2022.
The Company concluded that all amounts due are a settlement for accounting purposes as the payments are being made without any obligation from the Company to Amyris. The balance due on the promissory note and right to payments due under the Partnership Agreement are not recognized in the Company’s financial statements until the gain is realized. The Company recognizes any payments made under the Partnership Agreement and promissory note, including interest, when cash is received as other income (expense), net. During the years ended December 31, 2020 and 2019 the Company received payments of $8.3 million and $1.6 million, respectively, which are recorded as a component of other income (expense), net in the Consolidated Statements of Operations and Comprehensive Loss.
Synlogic, Inc.
Summary of Arrangement
In June 2019, the Company entered into several agreements with Synlogic, a publicly traded clinical-stage biopharmaceutical company focused on advancing drug discovery and development for synthetic biology-derived medicines. The Company entered into a Subscription Agreement with Synlogic whereby it purchased 6,340,771 shares of common stock at $9.00 per share for a total purchase price of $57.1 million, which represented a 19.9%
F-53
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
equity interest in Synlogic. The Company also entered into a Warrant Agreement whereby it received the right to purchase 2,548,117 shares of common stock of Synlogic at an exercise price of $9.00 per share. The Company made a non-refundable prepayment related to the exercise price of the warrant equal to $8.99 per share for a total payment of $22.9 million. The warrant is only exercisable to the extent the Company’s interest in Synlogic does not exceed 19.99%. The Company also entered into a Foundry Services Agreement (“Synlogic FSA”) whereby Synlogic provided $30.0 million in cash as a non-refundable prepayment for Foundry services. The prepaid Foundry services can be utilized for development of collaboration strains. Services performed under the services agreement will be applied to the prepaid amount based on the contractual rates included in the contract, based on costs incurred plus a fixed margin. Work will be performed under the Synlogic FSA pursuant to TDPs. Each TDP will pursue the development of a specific collaboration strain and/or production protocol. The Synlogic FSA will terminate upon the earlier of the exhaustion of the prepayment amount in full or the fifth anniversary of the effective date of the agreement and may be extended in certain circumstances.
Accounting Analysis
The overall arrangement with Synlogic includes the Subscription Agreement whereby the Company purchased shares of Synlogic common stock, the Warrant Agreement whereby the Company prepaid a significant portion of the exercise price of the warrant to purchase Synlogic common stock, which is non-refundable, and the Synlogic FSA whereby the Company will perform services for Synlogic. The Company concluded that these agreements should be considered one arrangement for accounting purposes as they were entered into at the same time and negotiated as a package with a single commercial objective.
The common stock investment in Synlogic is considered an equity method investment as the Company does not have a controlling financial interest in Synlogic but does have the ability to influence the financial and operating policies through its ownership of common stock. The Company has elected to apply the fair value option to account for the equity method investment. At inception, the fair value of the equity method investment in Synlogic was recorded at $35.8 million as a component of equity method investments on the Consolidated Balance Sheet. As of December 31, 2020 and 2019, the fair value of the equity method investment in Synlogic was $13.7 million and $16.4 million, respectively. For the years ended December 31, 2020 and 2019, the Company recorded a loss on its equity method investment of $2.7 million and $19.4 million, respectively, representing the decrease in fair value of Synlogic common stock, which is reflected in loss on equity method investments in the Consolidated Statements of Operations and Comprehensive Loss.
The Company has also elected to apply the fair value option to account for the warrant to purchase Synlogic common stock. At inception, the warrant was recorded at $14.4 million as a component of investments on the Consolidated Balance Sheet. As of December 31, 2020 and 2019, the fair value of the warrant was $5.5 million and $6.6 million, respectively, calculated as the value of the underlying common stock, less the related unpaid exercise price. For the years ended December 31, 2020 and 2019, the Company recorded a loss of $1.1 million and $7.8 million, respectively, representing the decrease in fair value of the warrant, which are reflected in loss on investments in the Consolidated Statements of Operations and Comprehensive Loss.
The Company elected to apply the fair value option to these instruments as the fair value of Synlogic’s common stock is objectively determinable based on quoted market prices in an active market for the identical securities. The Company’s equity method investment in Synlogic is the only equity method investment where the underlying equity instruments are traded in an active market.
For the Synlogic FSA and related TDPs, the Company concluded that the TDPs represent contracts with a customer and will be accounted for under ASC 606. At inception, Synlogic prepaid $30.0 million for services
F-54
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
under the Synlogic FSA. The prepaid services were reduced by $29.8 million, which represents the excess of the aggregate $80.0 million the Company paid to purchase Synlogic’s common stock and warrant over the respective fair values of those instruments. This resulted in deferred revenue at inception of $0.2 million which is being recognized over the period which the Company will provide services to Synlogic. The Company recognized revenue for services provided to Synlogic of $0.1 million for the year ended December 31, 2020 and less than $0.1 million for the year ended 2019. The Company had a deferred revenue balance with Synlogic that totaled $0.1 million each as of December 31, 2020 and 2019.
National Institutes of Health
In July 2020, the Company was awarded a letter contract with the National Institutes of Health (“NIH”) under NIH’s Rapid Acceleration of Diagnostics (“RADx”) initiative. The goal of RADx was to support a range of new lab-based and point-of-care tests that could significantly increase the number, type and availability of COVID-19 tests performed each day in the United States. This contract, which had a total award value of up to $40.5 million, was intended to increase the testing capacity for COVID-19. As of December 31, 2020, the Company had achieved milestone-based payments of $6.6 million under the NIH letter contract, which were recorded in other income, net in the Consolidated Statements of Operations and Comprehensive Loss for the year ended December 31, 2020. In October 2020, the parties agreed not to execute a definitive agreement for the additional milestones and no further amounts are expected to be recognized under this contract.
Octant, Inc.
In November 2020, the Company entered into a development and collaboration agreement with Octant, Inc. (“Octant”) to develop and disseminate a diagnostic test for COVID-19. Under the agreement, the Company made an upfront, non-refundable payment of $5.0 million in exchange for a license to Octant’s proprietary SwabSeq sequencing platform, which can be used to detect the presence of COVID-19. The SwabSeq technology can also be further developed for broader testing efforts for other respiratory illnesses, including the common cold and flu. As part of the arrangement, the Company will pay to Octant profit-sharing fees based on a percentage of the adjusted gross revenues earned at certain of its testing facilities utilizing SwabSeq technology. The $5.0 million upfront payment was determined to be in-process research and development expense and was fully expensed when incurred. There were no profit-sharing payments related to this arrangement for the year ended December 31, 2020.
17. Employee Benefit Plan
The Company has a 401(k) retirement plan covering substantially all employees. Under the retirement plan, employees make voluntary contributions and the Company makes a 5% non-elective contribution for all employees based on compensation, subject to IRS contribution limits. For the years ended December 31, 2020 and 2019, the Company contributed $2.2 million and $1.6 million, respectively, to the retirement plan.
F-55
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
18. Income Taxes
For the years ended December 31, 2020 and 2019, the loss before provision for incomes taxes consisted of the following (in thousands):
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Domestic |
$ | (124,834 | ) | $ | (119,835 | ) | ||
| Foreign |
— | — | ||||||
|
|
|
|
|
|||||
| Total |
$ | (124,834 | ) | $ | (119,835 | ) | ||
|
|
|
|
|
|||||
For the years ended December 31, 2020 and 2019, the Company incurred the following income tax expense (in thousands):
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Income tax expense: |
||||||||
| Current federal income tax |
$ | — | $ | — | ||||
| Current state income tax |
26 | 22 | ||||||
| Deferred federal income tax |
581 | — | ||||||
| Deferred state income tax |
1,282 | — | ||||||
|
|
|
|
|
|||||
| Income tax expense |
$ | 1,889 | $ | 22 | ||||
|
|
|
|
|
|||||
A reconciliation of income tax expense computed at the statutory corporate income tax rate to the effective income tax rate for the years ended December 31, 2020 and 2019 is as follows:
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Tax expense computed at the federal statutory rate |
21.0 | % | 21.0 | % | ||||
| State taxes |
4.5 | % | 4.2 | % | ||||
| Change in valuation allowance |
(31.3 | %) | (25.2 | %) | ||||
| Equity investments |
(0.6 | %) | (5.7 | %) | ||||
| Tax credits |
4.8 | % | 4.4 | % | ||||
| Non-deductible expenses |
(0.2 | %) | (0.1 | %) | ||||
| Other expenses |
0.3 | % | 1.4 | % | ||||
|
|
|
|
|
|||||
| Total income tax expense |
(1.5 | %) | 0.0 | % | ||||
|
|
|
|
|
|||||
F-56
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The Company’s deferred tax assets and liabilities consist of the following (in thousands):
| Years Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
$ | 91,467 | $ | 61,300 | ||||
| Tax credit carryforwards |
20,338 | 14,443 | ||||||
| Accrued expenses |
1,265 | 390 | ||||||
| Deferred revenue |
28,590 | 29,575 | ||||||
| Amortizable intangibles |
4,198 | 3,218 | ||||||
| Tenant allowance |
2,206 | 2,174 | ||||||
|
|
|
|
|
|||||
| Deferred tax assets before valuation allowance |
148,064 | 111,100 | ||||||
| Valuation allowance |
(143,827 | ) | (104,745 | ) | ||||
|
|
|
|
|
|||||
| Deferred tax assets |
4,237 | 6,355 | ||||||
| Deferred tax liabilities: |
||||||||
| Equity-based compensation |
— | (88 | ) | |||||
| Property and equipment |
(830 | ) | (862 | ) | ||||
| Basis differences |
(5,270 | ) | (5,405 | ) | ||||
|
|
|
|
|
|||||
| Deferred tax liabilities |
(6,100 | ) | (6,355 | ) | ||||
|
|
|
|
|
|||||
| Net deferred taxes |
$ | (1,863 | ) | $ | — | |||
|
|
|
|
|
|||||
Activity in the deferred tax assets valuation allowance is summarized as follows (in thousands):
| Beginning of Period |
Additions | Reductions/ Charges |
End of Period |
|||||||||||||
| Deferred tax assets valuation allowance: |
||||||||||||||||
| Year Ended December 31, 2020 |
$ | 104,745 | $ | 39,082 | $ | — | $ | 143,827 | ||||||||
| Year Ended December 31, 2019 |
$ | 74,511 | $ | 30,234 | $ | — | $ | 104,745 | ||||||||
The Company has evaluated the positive and negative evidence bearing upon its ability to realize the deferred tax assets. The Company considered its history of cumulative net losses incurred since inception and has concluded that it is more likely than not that it will not realize the benefits of the deferred tax assets. Accordingly, a valuation allowance has been established against the deferred tax assets as of December 31, 2020 and 2019 that are not expected to be realized. The Company reevaluates the positive and negative evidence at each reporting period. The valuation allowance increased on a net basis by approximately $39.1 million during the year ended December 31, 2020 due primarily to an increase in net operating losses and tax credits.
As of December 31, 2020, the Company had federal net operating loss carryforwards of approximately $347.8 million, of which $139.2 million begin to expire in 2029. The Company has approximately $208.6 million of federal net operating losses as of December 31, 2020 that can be carried forward indefinitely. As of December 31, 2020, the Company had state net operating loss carryforwards of approximately $282.8 million, of which $278.3 million begin to expire in 2029. The Company has approximately $4.5 million of state net operating losses as of December 31, 2020 that can be carried forward indefinitely.
As of December 31, 2020, the Company had federal research and development tax credit carryforwards of approximately $13.8 million which begin to expire in 2029. As of December 31, 2020, the Company also had state research and development tax credit carryforwards of approximately $8.2 million which begin to expire in 2028.
F-57
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Under Sections 382 and 383 of the U.S. Internal Revenue Code, if a corporation undergoes an ownership change, the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes, such as research tax credits, to offset its post-change income and taxes may be limited. In general, an ownership change generally occurs if there is a cumulative change in its ownership by 5% stockholders that exceeds 50 percentage points over a rolling three-year period. Similar rules may apply under U.S. state tax laws. The Company may have experienced an ownership change in the past and may experience ownership changes in the future as a result of future transactions in its share capital, some of which may be outside of the Company’s control. As a result, if the Company earns net taxable income, the Company ability to use its pre-change net operating loss carryforwards, or other pre-change tax attributes, to offset U.S. federal and state taxable income and taxes may be subject to significant limitations.
The Company files tax returns as prescribed by the tax laws of the jurisdictions in which the Company operates. In the normal course of business, the Company is subject to examination by federal and state jurisdictions, where applicable. There are currently no pending tax examinations. As of December 31, 2020, the Company’s tax years are still open under statute from 2017 to the present.
The Company accounts for uncertain tax positions using a more likely than not threshold for recognizing and resolving uncertain tax positions. The evaluation of uncertain tax positions is based on factors that include, but are not limited to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or circumstances related to a tax position. The Company evaluates uncertain tax positions on an annual basis and adjusts the level of the liability to reflect any subsequent changes in the relevant facts surrounding the uncertain positions. The Company accounts for interest and penalties related to uncertain tax positions as part of its provision for income taxes. As of December 31, 2020 and 2019, the Company had no recorded liabilities for uncertain tax positions and had no accrued interest or penalties related to uncertain tax positions. The Company does not expect a material change in unrecognized tax benefits in the next twelve months.
19. Net Loss per Share
The Company computes net loss per share of the Class A common stock and Class B common stock using the two-class method required for participating securities. Basic and diluted loss per share was the same for each period presented as the inclusion of all potential Class A common stock and Class B common stock equivalents would have been antidilutive. The earnings per share amounts are the same for the different classes of common stock because the holders of each class are legally entitled to equal per share distributions whether through dividends or liquidation.
The following potential common shares, presented based on amounts outstanding at each period end, were excluded from the calculation of diluted net loss per share attributable to Ginkgo Bioworks, Inc. common stockholders for the periods indicated because including them would have been anti-dilutive:
| Year Ended December 31, | ||||||||
| 2020 | 2019 | |||||||
| Warrants to purchase common stock |
1,020,187 | 1,020,187 | ||||||
| Outstanding stock options |
33,354,871 | 35,276,812 | ||||||
| Unvested RSUs |
124,932,207 | 70,119,944 | ||||||
| Unvested RSAs |
419,049 | 675,887 | ||||||
F-58
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
| 20. | Related Parties |
Related party transactions included in the Consolidated Balance Sheets, excluding the Company’s investments and equity method investments, are summarized below (in thousands):
| Joyn | Motif | Genomatica | Allonnia | Synlogic | Total | |||||||||||||||||||
| Balances as of December 31, 2020 |
||||||||||||||||||||||||
| Accounts receivable, net |
$ | — | $ | 2,403 | $ | 1,500 | $ | 1,309 | $ | — | $ | 5,212 | ||||||||||||
| Prepaid expenses and other current assets |
$ | 24 | $ | 232 | $ | — | $ | 13 | $ | — | $ | 269 | ||||||||||||
| Deferred revenue, current and non-current |
$ | 9,862 | $ | 53,952 | $ | 30,128 | $ | 26,064 | $ | 72 | $ | 120,078 | ||||||||||||
| Balances as of December 31, 2019 |
||||||||||||||||||||||||
| Accounts receivable, net |
$ | 163 | $ | 4,054 | $ | — | $ | — | $ | — | $ | 4,217 | ||||||||||||
| Deferred revenue, current and non-current |
$ | 17,135 | $ | 62,513 | $ | 38,059 | $ | 24,480 | $ | 144 | $ | 142,331 | ||||||||||||
Related party transactions included in the Consolidated Statements Operations and Comprehensive Loss, excluding the losses on the Company’s investments and equity method investments, are summarized below (in thousands):
| Joyn | Motif | Genomatica | Allonnia | Synlogic | Glycosyn | Total | ||||||||||||||||||||||
| For the Year Ended December 31, 2020 |
||||||||||||||||||||||||||||
| Foundry revenue |
$ | 7,273 | $ | 20,798 | $ | 9,431 | $ | 4,960 | $ | 73 | $ | — | $ | 42,535 | ||||||||||||||
| Other income, net |
$ | 407 | $ | 314 | $ | — | $ | — | $ | — | $ | — | $ | 721 | ||||||||||||||
| For the Year Ended December 31, 2019 |
||||||||||||||||||||||||||||
| Foundry revenue |
$ | 9,349 | $ | 18,986 | $ | 6,248 | $ | — | $ | 17 | $ | 668 | $ | 35,268 | ||||||||||||||
| Interest income |
$ | — | $ | — | $ | — | $ | — | $ | — | $ | 163 | $ | 163 | ||||||||||||||
| Other income, net |
$ | 222 | $ | 42 | $ | — | $ | — | $ | — | $ | 1,530 | $ | 1,794 | ||||||||||||||
Related party transactions included in the changes in operating assets and liabilities in the Consolidated Statements of Cash Flows are summarized below (in thousands):
| Joyn | Motif | Genomatica | Allonnia | Synlogic | Glycosyn | Total | ||||||||||||||||||||||
| For the Year Ended December 31, 2020 |
||||||||||||||||||||||||||||
| Accounts receivable, net |
$ | 163 | $ | 1,651 | $ | (1,500 | ) | $ | (1,309 | ) | $ | — | $ | — | $ | (995 | ) | |||||||||||
| Prepaid expenses and other current assets |
$ | (24 | ) | $ | (232 | ) | $ | — | $ | (13 | ) | $ | — | $ | — | $ | (269 | ) | ||||||||||
| Deferred revenue, current and non-current |
$ | (7,273 | ) | $ | (8,561 | ) | $ | (7,931 | ) | $ | 1,584 | $ | (72 | ) | $ | — | $ | (22,253 | ) | |||||||||
| For the Year Ended December 31, 2019 |
||||||||||||||||||||||||||||
| Accounts receivable, net |
$ | (54 | ) | $ | (2,035 | ) | $ | 8 | $ | — | $ | — | $ | (140 | ) | $ | (2,221 | ) | ||||||||||
| Deferred revenue, current and non-current |
$ | 5,719 | $ | 9 | $ | (2,232 | ) | $ | — | $ | 144 | $ | (528 | ) | $ | 3,112 | ||||||||||||
As the Company no longer held an equity interest in Glycosyn as of December 31, 2019, it was no longer considered a related party of the Company as of that date. Therefore, the related party transactions for Glycosyn
F-59
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
as of and for the year ended December 31, 2020 and as of December 31, 2019 are presented as zero in the tables above. Refer to Note 8 for additional details on the Company’s investments and equity method investments held in its related parties. Refer to Note 16 for additional discussion of the Company’s arrangement with Glycosyn.
21. Subsequent Events
The Company evaluated subsequent events and transactions that occurred after the balance sheet date up to the date that the financial statements were issued. Based on this review, other than as described below, the Company did not identify any subsequent events that would have required adjustment or disclosure in the financial statements.
(a) Amendment to Glycosyn Promissory Note
In January 2021, the existing terms of the Glycosyn Promissory Note were amended to add an additional $0.2 million to the principal balance and to extend the number of interest-only payments to include the quarterly payments due on or before December 31, 2020 and March 31, 2021. The amendment also added a provision to increase the interest rate from 7.5% to 12.5% (or the maximum allowable by law, whichever is less) in the event of default by Glycosyn.
(b) Allonnia Series A Preferred Unit Financing
In January 2021, Allonnia issued an additional 22,500 Series A Preferred Units for aggregate proceeds of $0.2 million and closed their Series A Preferred Unit financing. As a result, the Company received 1,867,411 common units in Allonnia for total consideration of $12.7 million.
(c) Arcaea LLC (“Arcaea”, FKA Kalo Ingredients LLC)
In March 2021, Arcaea, LLC (“Arcaea”) was formed to focus on the application of synthetic biology in the personal care products industry. In March 2021, the Company entered into (i) an IP Property Contribution Agreement (“Arcaea IP Agreement”) that granted Arcaea a license to certain of the Company’s intellectual property, (ii) a Technical Development Agreement (“Arcaea TDA”) that establishes the terms under which the Company will provide technical development services, and (iii) a Common Unit Issuance Agreement (“Arcaea CUIA”) which compensates the Company for its intellectual property contribution and increases Arcaea’s access to the Company’s intellectual property in exchange for more common units.
Under the Arcaea IP Agreement, the Company licensed intellectual property to Arcaea for use in the development or the production of its products that the parties will subsequently agree to develop under TDPs. The license rights provide Arcaea with the ability to commercialize the specified products from the corresponding strain or enzyme, which can only be developed by the Company under the Arcaea TDA. In return for the license to the intellectual property, Arcaea has agreed to issue the Company up to 9,000,000 common units in accordance with certain terms and conditions set forth within the agreements. Upon execution, the Company was issued 1,755,000 common units under the Arcaea CUIA and any additional units will be determined based on the additional closings of the Series A Preferred Units which will be completed within 90 days of execution of the Arcaea CUIA. Under the Arcaea TDA, the parties jointly agree on TDPs for specific strains and enzymes in which the Company will perform agreed upon development services in return for consideration on a cost-plus basis for all services provided.
(d) 2014 Plan Increase
In March 2021, the board of directors approved an increase to the aggregate number of shares reserved for issuance under the 2014 Plan of 39,960,420 shares, raising the total aggregate number of shares reserved for issuance under the 2014 Plan from 130,759,452 to 170,719,873.
F-60
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
(e) Parcel O Lease Agreement
In April 2021, the Company entered into a lease consisting of approximately 152,000 square feet of office and laboratory space being developed in Boston, Massachusetts. The lease commencement date is estimated to be June 1, 2024, subject to certain extensions, and expires on the fifteenth anniversary of the lease commencement date. Annual base rent for the first lease year will be approximately $12.9 million, subject to annual rent increases over the term of the lease. The lease includes one option to extend the lease for ten years at then-market rates, subject to certain adjustments, and will be secured by a letter of credit of $9.1 million.
(f) Acquisition of Dutch DNA Biotech B.V.
In April 2021, the Company entered into a definitive Share Sale and Purchase Agreement (“Purchase Agreement”) with Have Fungi B.V. (“HF”) and a Technical Development Agreement (“TDA”) with Dutch DNA Biotech B.V. (“DDNA”), each a Dutch company located in the Netherlands. Under the Purchase Agreement, the Company will pay HF a purchase price in an amount equal to EUR 10 million, 1,633,937 shares of Ginkgo common stock, plus net debt and working capital adjustments, to acquire 100% ownership in the capital of DDNA. In addition, under the Purchase Agreement, the Company agrees to earn-out payments to HF and certain designees upon achievement of one or more technical and commercialization milestones based on the performance of DDNA, including pursuant to the TDA, in an aggregate amount not to exceed $20.0 million during the earn-out term. The Company expects to finalize the transaction by the beginning of the third quarter of 2021.
(g) Agreement and Plan of Merger
On May 11, 2021, the Company and Soaring Eagle Acquisition Corp. (“SRNG”) entered into an agreement and plan of merger (the “Merger Agreement”) under which Merger Sub, a newly formed subsidiary of SRNG, will be merged with and into Ginkgo with Ginkgo surviving the merger as a wholly owned subsidiary of SRNG (the “Business Combination”). As a result of the proposed merger, SRNG will be renamed “Ginkgo Bioworks Holdings, Inc.” (“New Ginkgo”).
Concurrently with the execution of the Merger Agreement, SRNG entered into subscription agreements with certain accredited investors, pursuant to which, among other things, they agreed to purchase immediately prior to the closing of the Business Combination, an aggregate of 77,500,000 shares of SRNG’s Class A common stock for a purchase price of $10.00 per share, for aggregate gross proceeds of $775 million (the “PIPE Financing”).
Subject to the terms of the Merger Agreement, immediately prior to the effective time of the Business Combination (the “Effective Time”), (i) Ginkgo will effect a recapitalization such that Ginkgo’s authorized capital stock shall consist solely of Ginkgo Class A common stock and Ginkgo Class B common stock and (ii) as of the Effective Time (a) each share of Ginkgo’s Class A common stock or Class B common stock issued and outstanding immediately prior to the Effective Time (including as a result of the automatic exercise of Ginkgo Warrants (defined below) by virtue of the occurrence of the Business Combination pursuant to the terms of such warrants) shall be converted into a share of Class A common stock or Class B common stock, as applicable, of New Ginkgo common stock, calculated, in each case, based on the equity value exchange ratio as set forth in the Merger Agreement, (b) each option exercisable for Class A common stock or Class B common stock of Ginkgo that is outstanding immediately prior to the Effective Time will be assumed and converted into a newly issued option exercisable for shares of Class A common stock or Class B common stock, as applicable, of New Ginkgo (subject to the same terms and conditions as the original Ginkgo option and with appropriate adjustments to the number of shares for which such option is exercisable and the exercise price thereof), (c) each award of restricted common stock of Ginkgo under Ginkgo’s stock incentive plans (a “Ginkgo Restricted Stock Award”) that is outstanding immediately prior to the Effective Time will be converted into the right to receive restricted common
F-61
Ginkgo Bioworks, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
stock of New Ginkgo on the same terms and conditions as applicable to such Ginkgo Restricted Stock Award, (d) each award of restricted stock units of Ginkgo under Ginkgo’s stock incentive plans (a “Ginkgo Restricted Stock Unit Award”) that is outstanding immediately prior to the Effective Time will be converted into the right to receive restricted stock units based on common stock of New Ginkgo on the same terms and conditions as applicable to such Ginkgo Restricted Stock Unit Award and with appropriate adjustments to the number of shares to which each such restricted stock unit relates, and (e) each warrant to purchase shares of Ginkgo capital stock (a “Ginkgo Warrant”) that is outstanding immediately prior to the Effective Time and is not automatically exercised in full in accordance with its terms by virtue of the occurrence of the Business Combination will be assumed and converted into a warrant exercisable for Class A common stock of New Ginkgo (each, a “New Ginkgo assumed warrant”) on the same terms and conditions as applicable to such Ginkgo Warrant immediately prior to the effective time of the Business Combination, with appropriate adjustments to the number of shares for which such New Ginkgo assumed warrant is exercisable and the exercise price thereof.
Completion of the PIPE Financing and Business Combination is subject to approval of SRNG stockholders, Company stockholders and the satisfaction or waiver of certain other customary closing conditions. The approvals from SRNG stockholders and Company stockholders are expected in the third quarter of 2021.
F-62
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Condensed Consolidated Balance Sheets
(unaudited)
(in thousands, except share and per share data)
| As of September 30, |
As of December 31, |
|||||||
| 2021 | 2020 | |||||||
| Assets |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 1,739,056 | $ | 380,801 | ||||
| Accounts receivable, net |
44,675 | 16,694 | ||||||
| Accounts receivable, net from related parties |
6,050 | 5,212 | ||||||
| Inventory, net |
3,474 | 2,736 | ||||||
| Prepaid expenses and other current assets |
17,314 | 21,099 | ||||||
|
|
|
|
|
|||||
| Total current assets |
1,810,569 | 426,542 | ||||||
| Property and equipment, net |
142,704 | 121,435 | ||||||
| Investments |
104,771 | 74,200 | ||||||
| Equity method investments |
16,357 | 28,924 | ||||||
| Intangible assets, net |
22,568 | 3,294 | ||||||
| Goodwill |
16,660 | 1,857 | ||||||
| Loans receivable, net of current portion |
10,161 | 13,298 | ||||||
| Other non-current assets |
26,801 | 5,603 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 2,150,591 | $ | 675,153 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 7,102 | $ | 13,893 | ||||
| Accrued expenses and other current liabilities |
53,554 | 30,505 | ||||||
| Deferred revenue (includes $15,442 and $22,101 from related parties) |
29,649 | 28,823 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
90,305 | 73,221 | ||||||
| Non-current liabilities: |
||||||||
| Deferred rent, net of current portion |
16,960 | 12,678 | ||||||
| Deferred revenue, net of current portion (includes $159,268 and $97,977 from related parties) |
163,042 | 99,652 | ||||||
| Lease financing obligation |
16,268 | 16,518 | ||||||
| Warrant liabilities |
212,935 | — | ||||||
| Other non-current liabilities |
33,110 | 3,032 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
532,620 | 205,101 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies (Note 12) |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.0001 par value; 200,000,000 shares authorized; none issued and outstanding |
— | — | ||||||
| Class A, Class B and Class C common stock $0.0001 par value; 15,800,000,000 shares authorized (Class A 10,500,000,000, Class B 4,500,000,000, Class C 800,000,000); 1,486,954,612 (Class A 1,188,722,056, Class B 298,232,556, Class C 0) and 1,289,014,925 (Class A 974,224,443, Class B 314,790,482, Class C 0) shares issued as of September 30, 2021 and December 31, 2020, respectively; 1,486,712,858 (Class A 1,188,707,589, Class B 298,005,269, Class C 0) and 1,288,595,876 (Class A 974,166,577, Class B 314,429,299, Class C 0) shares outstanding as of September 30, 2021 and December 31, 2020, respectively |
148 | 129 | ||||||
| Additional paid in capital |
2,249,549 | 929,125 | ||||||
| Accumulated deficit |
(697,269 | ) | (467,878 | ) | ||||
| Accumulated other comprehensive income (loss) |
(877 | ) | — | |||||
|
|
|
|
|
|||||
| Total Ginkgo Bioworks Holdings, Inc. stockholders’ equity |
1,551,551 | 461,376 | ||||||
| Non-controlling interest |
66,420 | 8,676 | ||||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
1,617,971 | 470,052 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 2,150,591 | $ | 675,153 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-63
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Condensed Consolidated Statements of Operations and Comprehensive Loss
(unaudited)
(in thousands, except share and per share data)
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
| Foundry revenue (related party revenue of $13,124 and $7,270 for the three months ended September 30, 2021 and 2020, respectively, and $36,746 and $29,784 for the nine months ended September 30 2021 and 2020, respectively) |
$ | 34,737 | $ | 11,505 | $ | 78,833 | $ | 42,802 | ||||||||
| Biosecurity revenue: |
||||||||||||||||
| Product |
8,492 | — | 14,622 | — | ||||||||||||
| Service |
34,381 | 1,797 | 71,888 | 1,797 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
77,610 | 13,302 | 165,343 | 44,599 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Costs and operating expenses: |
||||||||||||||||
| Cost of Biosecurity product revenue |
3,430 | — | 15,185 | — | ||||||||||||
| Cost of Biosecurity service revenue |
18,872 | 1,769 | 47,927 | 1,769 | ||||||||||||
| Research and development |
53,021 | 36,070 | 164,637 | 98,576 | ||||||||||||
| General and administrative |
28,959 | 9,876 | 81,326 | 25,393 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
104,282 | 47,715 | 309,075 | 125,738 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(26,672 | ) | (34,413 | ) | (143,732 | ) | (81,139 | ) | ||||||||
| Other (expense) income, net: |
||||||||||||||||
| Interest income |
121 | 3,318 | 341 | 5,565 | ||||||||||||
| Interest expense |
(649 | ) | (592 | ) | (1,822 | ) | (1,795 | ) | ||||||||
| Loss on equity method investments |
(39,651 | ) | (237 | ) | (72,621 | ) | (2,151 | ) | ||||||||
| (Loss) gain on investments |
(12,368 | ) | (90 | ) | 3,009 | (4,978 | ) | |||||||||
| Change in fair value of warrant liabilities |
(18,482 | ) | — | (18,482 | ) | — | ||||||||||
| Other (expense) income, net: |
(4,911 | ) | 5,894 | 863 | 6,055 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total other (expense) income, net |
(75,940 | ) | 8,293 | (88,712 | ) | 2,696 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before income taxes |
(102,612 | ) | (26,120 | ) | (232,444 | ) | (78,443 | ) | ||||||||
| Income tax provision (benefit) |
(207 | ) | 6 | (797 | ) | 1,881 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(102,405 | ) | (26,126 | ) | (231,647 | ) | (80,324 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to non-controlling interest |
(524 | ) | (71 | ) | (2,256 | ) | (639 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to Ginkgo Bioworks Holdings, Inc. stockholders |
(101,881 | ) | (26,055 | ) | (229,391 | ) | (79,685 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share attributable to Ginkgo Bioworks Holdings, Inc. common stockholders, basic and diluted |
$ | (0.08 | ) | $ | (0.02 | ) | $ | (0.18 | ) | $ | (0.06 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted average common shares outstanding, basic and diluted |
1,323,574,063 | 1,287,053,015 | 1,302,253,729 | 1,270,297,495 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Comprehensive loss: |
||||||||||||||||
| Net loss |
(102,405 | ) | (26,126 | ) | (231,647 | ) | (80,324 | ) | ||||||||
| Other comprehensive income (loss): |
||||||||||||||||
| Foreign currency translation adjustments, net of taxes |
(877 | ) | — | (877 | ) | — | ||||||||||
| Comprehensive loss |
$ | (103,282 | ) | $ | (26,126 | ) | $ | (232,524 | ) | $ | (80,324 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-64
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Condensed Consolidated Statements of Stockholders’ Equity
(unaudited)
(in thousands, except share data)
| Series B, C, D, E Convertible Preferred Stock |
Old Ginkgo Common Stock |
Common Stock | ||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||
| Balance as of December 31, 2019 (as previously reported) |
17,774,941 | $ | 177 | 7,806,772 | $ | 79 | — | $ | — | |||||||||||||||
| Retroactive application of the reverse recapitalization (Note 3) |
(17,774,941 | ) | (177 | ) | (7,806,772 | ) | (79 | ) | 1,255,562,032 | 126 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2019 |
— | — | — | — | 1,255,562,032 | 126 | ||||||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | 79,412 | — | ||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | ||||||||||||||||||
| Net loss |
— | — | — | — | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of March 31, 2020 |
— | — | — | — | 1,255,641,444 | 126 | ||||||||||||||||||
| Exercise of stock options |
— | — | — | — | 814,490 | — | ||||||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | 59,142 | — | ||||||||||||||||||
| Issuance of Series E convertible preferred stock, net of issuance costs of $0 |
— | — | — | — | 23,528,727 | 2 | ||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | ||||||||||||||||||
| Net loss |
— | — | — | — | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of June 30, 2020 |
— | — | — | — | 1,280,043,803 | 128 | ||||||||||||||||||
| Exercise of stock options |
— | — | — | — | 486,878 | — | ||||||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | 59,093 | — | ||||||||||||||||||
| Issuance of Series E convertible preferred stock, net of issuance costs of $0 |
— | — | — | — | 7,326,338 | 1 | ||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | ||||||||||||||||||
| Net loss |
— | — | — | — | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of September 30, 2020 |
— | $ | — | — | $ | — | 1,287,916,112 | $ | 129 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-65
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Condensed Consolidated Statements of Stockholders’ Equity
(unaudited)
(in thousands, except share data)
| Additional Paid-In Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Income (Loss) |
Non- Controlling Interest |
Total Stockholders’ Equity |
||||||||||||||||
| Balance as of December 31, 2019 (as previously reported) |
$ | 834,076 | $ | (341,269 | ) | $ | — | $ | 8,790 | $ | 501,853 | |||||||||
| Retroactive application of the reverse recapitalization (Note 3) |
130 | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as of December 31, 2019 |
834,206 | (341,269 | ) | — | 8,790 | 501,853 | ||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | — | |||||||||||||||
| Stock-based compensation expense |
123 | — | — | — | 123 | |||||||||||||||
| Net loss |
— | (24,972 | ) | — | (496 | ) | (25,468 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as of March 31, 2020 |
834,329 | (366,241 | ) | — | 8,294 | 476,508 | ||||||||||||||
| Exercise of stock options |
12 | — | — | — | 12 | |||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | — | |||||||||||||||
| Issuance of Series E convertible preferred stock, net of issuance costs of $0 |
71,998 | — | — | — | 72,000 | |||||||||||||||
| Stock-based compensation expense |
117 | — | — | — | 117 | |||||||||||||||
| Net loss |
— | (28,658 | ) | — | (72 | ) | (28,730 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as of June 30, 2020 |
906,456 | (394,899 | ) | — | 8,222 | 519,907 | ||||||||||||||
| Exercise of stock options |
7 | — | — | — | 7 | |||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | — | |||||||||||||||
| Issuance of Series E convertible preferred stock, net of issuance costs of $0 |
22,419 | — | — | — | 22,420 | |||||||||||||||
| Stock-based compensation expense |
118 | — | — | — | 118 | |||||||||||||||
| Net loss |
— | (26,055 | ) | — | (71 | ) | (26,126 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as of September 30, 2020 |
$ | 929,000 | $ | (420,954 | ) | $ | — | $ | 8,151 | $ | 516,326 | |||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-66
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Condensed Consolidated Statements of Stockholders’ Equity
(unaudited)
(in thousands, except share data)
| Series B, C, D, E Convertible Preferred Stock |
Old Ginkgo Common Stock |
Common Stock | ||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||
| Balance as of December 31, 2020 (as previously reported) |
18,403,604 | $ | 184 | 7,851,164 | $ | 79 | — | $ | — | |||||||||||||||
| Retroactive application of the reverse recapitalization (Note 3) |
(18,403,604 | ) | (184 | ) | (7,851,164 | ) | (79 | ) | 1,288,595,876 | 129 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2020 |
— | — | — | — | 1,288,595,876 | 129 | ||||||||||||||||||
| Exercise of stock options |
— | — | — | — | 2,758,322 | — | ||||||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | 58,995 | — | ||||||||||||||||||
| Issuance of warrants to purchase Series D convertible preferred stock |
— | — | — | — | — | — | ||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | ||||||||||||||||||
| Net loss |
— | — | — | — | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of March 31, 2021 |
— | — | — | — | 1,291,413,193 | 129 | ||||||||||||||||||
| Exercise of stock options |
— | — | — | — | 1,221,465 | — | ||||||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | 59,142 | — | ||||||||||||||||||
| Issuance of warrants to purchase Series D convertible preferred stock |
— | — | — | — | — | — | ||||||||||||||||||
| Issuance of Series D convertible preferred stock upon exercise of warrants |
— | — | — | — | 771,545 | — | ||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | ||||||||||||||||||
| Net loss |
— | — | — | — | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of June 30, 2021 |
— | — | — | — | 1,293,465,345 | 129 | ||||||||||||||||||
| Exercise of stock options |
— | — | — | — | 245,402 | — | ||||||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | 59,158 | — | ||||||||||||||||||
| Issuance of common stock for a business acquisition |
1,633,937 | — | ||||||||||||||||||||||
| Founder shares repurchase |
— | — | — | — | (2,707,280 | ) | — | |||||||||||||||||
| Issuance of Series B convertible preferred stock upon net exercise of warrants |
— | — | — | — | 242,163 | — | ||||||||||||||||||
| Issuance of Series E convertible preferred stock in exchange for warrants |
— | — | — | — | 408,497 | — | ||||||||||||||||||
| Issuance of common stock upon reverse recapitalization, net of offering costs (Note 3) |
— | — | — | — | 193,365,636 | 19 | ||||||||||||||||||
| Assumption of Public and Private Placement Warrants |
— | — | — | — | — | — | ||||||||||||||||||
| Non-controlling interest contributions |
— | — | ||||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | — | — | ||||||||||||||||||
| Foreign currency translation |
— | — | — | — | — | — | ||||||||||||||||||
| Net loss |
— | — | — | — | — | — | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of September 30, 2021 |
— | $ | — | — | $ | — | 1,486,712,858 | $ | 148 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-67
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Condensed Consolidated Statements of Stockholders’ Equity
(unaudited)
(in thousands, except share data)
| Additional Paid-In Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Income (Loss) |
Non- Controlling Interest |
Total Stockholders’ Equity |
||||||||||||||||
| Balance as of December 31, 2020 (as previously reported) |
$ | 928,991 | $ | (467,878 | ) | $ | — | $ | 8,676 | $ | 470,052 | |||||||||
| Retroactive application of the reverse recapitalization (Note 3) |
134 | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as of December 31, 2020 |
929,125 | (467,878 | ) | — | 8,676 | 470,052 | ||||||||||||||
| Exercise of stock options |
27 | — | — | — | 27 | |||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | — | |||||||||||||||
| Issuance of warrants to purchase Series D convertible preferred stock |
150 | — | — | — | 150 | |||||||||||||||
| Stock-based compensation expense |
118 | — | — | — | 118 | |||||||||||||||
| Net loss |
— | (73,569 | ) | — | (1,209 | ) | (74,778 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as of March 31, 2021 |
929,420 | (541,447 | ) | — | 7,467 | 395,569 | ||||||||||||||
| Exercise of stock options |
12 | — | — | — | 12 | |||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | — | |||||||||||||||
| Issuance of warrants to purchase Series D convertible preferred stock |
150 | — | — | — | 150 | |||||||||||||||
| Issuance of Series D convertible preferred stock upon exercise of warrants |
— | — | — | — | — | |||||||||||||||
| Stock-based compensation expense |
14,519 | — | — | — | 14,519 | |||||||||||||||
| Net loss |
— | (53,941 | ) | — | (523 | ) | (54,464 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as of June 30, 2021 |
944,101 | (595,388 | ) | — | 6,944 | 355,786 | ||||||||||||||
| Exercise of stock options |
2 | — | — | — | 2 | |||||||||||||||
| Vesting of restricted stock awards |
— | — | — | — | — | |||||||||||||||
| Issuance of common stock for a business acquisition |
15,160 | — | — | — | 15,160 | |||||||||||||||
| Founder shares repurchase |
(24,998 | ) | — | — | — | (24,998 | ) | |||||||||||||
| Issuance of Series B convertible preferred stock upon net exercise of warrants |
— | — | — | — | — | |||||||||||||||
| Issuance of Series E convertible preferred stock in exchange for warrants |
— | — | — | — | — | |||||||||||||||
| Issuance of common stock upon reverse recapitalization, net of offering costs (Note 3) |
1,509,610 | — | — | — | 1,509,629 | |||||||||||||||
| Assumption of Public and Private Placement Warrants |
(194,453 | ) | — | — | — | (194,453 | ) | |||||||||||||
| Non-controlling interest contributions |
— | — | — | 60,000 | 60,000 | |||||||||||||||
| Stock-based compensation expense |
127 | — | — | — | 127 | |||||||||||||||
| Foreign currency translation |
— | — | (877 | ) | — | (877 | ) | |||||||||||||
| Net loss |
— | (101,881 | ) | — | (524 | ) | (102,405 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Balance as of September 30, 2021 |
$ | 2,249,549 | $ | (697,269 | ) | $ | (877 | ) | $ | 66,420 | $ | 1,617,971 | ||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-68
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Condensed Consolidated Statements of Cash Flows
(unaudited)
(in thousands)
| Nine Months Ended September 30, |
||||||||
| 2021 | 2020 | |||||||
| Cash flow from operating activities: |
||||||||
| Net loss |
$ | (231,647 | ) | $ | (80,324 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation and amortization |
21,073 | 9,860 | ||||||
| Stock-based compensation |
14,764 | 358 | ||||||
| Non-cash equity consideration |
(12,562 | ) | — | |||||
| Loss on equity method investments |
72,621 | 2,151 | ||||||
| (Gain) loss on investments |
(3,009 | ) | 4,978 | |||||
| Change in fair value of loans receivable |
1,196 | (295 | ) | |||||
| Change in fair value of warrant liabilities |
18,482 | — | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable, net |
(27,832 | ) | (6,338 | ) | ||||
| Accounts receivable, net from related parties |
(838 | ) | (446 | ) | ||||
| Prepaid expenses and other current assets |
5,565 | (1,207 | ) | |||||
| Inventory, net |
(738 | ) | (819 | ) | ||||
| Other non-current assets |
(35 | ) | 2,361 | |||||
| Accounts payable |
(2,771 | ) | 2,700 | |||||
| Accrued expenses and other current liabilities |
29,599 | 1,690 | ||||||
| Deferred revenue, current and non-current (includes $54,632 and $(17,050) from related parties) |
(5,538 | ) | (13,061 | ) | ||||
| Deferred rent, non-current |
4,320 | 39 | ||||||
| Other non-current liabilities |
29,073 | 1,862 | ||||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(88,277 | ) | (76,491 | ) | ||||
|
|
|
|
|
|||||
| Cash flow from investing activities: |
||||||||
| Purchases of property and equipment |
(51,407 | ) | (38,408 | ) | ||||
| Issuance of loan receivable |
— | (100 | ) | |||||
| Proceeds from loan receivable |
304 | 217 | ||||||
| Purchase of non-marketable equity securities |
(5,000 | ) | — | |||||
| Business acquisition, net of cash acquired |
(21,382 | ) | — | |||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
(77,485 | ) | (38,291 | ) | ||||
|
|
|
|
|
|||||
| Cash flow from financing activities: |
||||||||
| Proceeds from reverse recapitalization, net of redemptions of $867,253 and offering costs of $106,838 (Note 3) |
1,510,909 | — | ||||||
| Proceeds from exercise of stock options |
41 | 19 | ||||||
| Repurchase of Founder shares |
(24,998 | ) | — | |||||
| Principal payment on capital lease obligations |
(592 | ) | (457 | ) | ||||
| Non-controlling interest contributions |
60,000 | — | ||||||
| Principal payment on lease financing obligations |
(172 | ) | (98 | ) | ||||
| Proceeds from issuance of Series E convertible preferred stock, net of issuance costs |
— | 91,040 | ||||||
| Net cash provided by financing activities |
1,545,188 | 90,504 | ||||||
|
|
|
|
|
|||||
| Effect of foreign exchange rates on cash and cash equivalents |
(8 | ) | — | |||||
| Net increase (decrease) in cash, cash equivalents and restricted cash |
1,379,418 | (24,278 | ) | |||||
| Cash, cash equivalents and restricted cash, beginning of period |
385,877 | 498,510 | ||||||
|
|
|
|
|
|||||
| Cash, cash equivalents and restricted cash, end of period |
1,765,295 | 474,232 | ||||||
|
|
|
|
|
|||||
| Supplemental disclosure of non-cash investing and financing activities: |
||||||||
| Purchases of equipment through capital leases |
$ | 1,981 | $ | — | ||||
| Purchases of property and equipment included in accounts payable and accrued expenses |
$ | 2,434 | $ | 9,796 | ||||
| Deferred offering costs in accounts payable and accrued expenses |
$ | 1,280 | $ | — | ||||
| Equity received in related parties |
$ | 60,054 | $ | — | ||||
| Purchase of non-marketable equity securities |
$ | 10,000 | $ | — | ||||
| Issuance of common stock for a business acquisition |
$ | 15,087 | $ | — | ||||
| Loan receivable received as consideration under customer arrangement |
$ | — | $ | 300 | ||||
| Initial fair value of warrant liabilities |
$ | 194,453 | $ | — | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-69
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Condensed Consolidated Statements of Cash Flows
(unaudited)
(in thousands)
The following table provides a reconciliation of the cash, cash equivalents and restricted cash balances as of each of the periods shown above:
| As of September 30, | ||||||||
| 2021 | 2020 | |||||||
| Cash and cash equivalents |
$ | 1,739,056 | $ | 471,057 | ||||
| Restricted cash |
26,239 | 3,175 | ||||||
|
|
|
|
|
|||||
| Total cash, cash equivalents and restricted cash |
$ | 1,765,295 | $ | 474,232 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-70
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
1. Organization
Business
The mission of Ginkgo Bioworks Holdings, Inc. (“Ginkgo Bioworks”, “New Ginkgo”, or the “Company”) is to make biology easier to engineer. The Company designs custom cells for customers across multiple markets. Since inception, the Company has devoted its efforts to improving its platform for programming cells to enable customers to leverage biology to create impactful products across a range of industries. The Company’s platform comprises (i) equipment, robotic automation, software, data pipelines and tools, and standard operating procedures for high throughput genetic engineering, fermentation, and analytics (referred to collectively as the “Foundry”), (ii) a library of proprietary genetic assets and associated performance data (referred to collectively as “Codebase”), and (iii) the Company’s team of expert users, developers and operators of the Foundry and Codebase.
On September 16, 2021, Soaring Eagle Acquisition Corp. (“SRNG”) consummated the merger transaction contemplated by the agreement and plan of merger, dated as of May 11, 2021, and amended on May 14, 2021 (the “Merger Agreement”), by and among SRNG, SEAC Merger Sub Inc., a Delaware corporation (“Merger Sub”), and Ginkgo Bioworks, Inc., a Delaware corporation (“Old Ginkgo”), whereby Merger Sub merged with and into Old Ginkgo, the separate corporate existence of Merger Sub ceased and Old Ginkgo survived the merger as a wholly owned subsidiary of SRNG (the “Business Combination”). In connection with the consummation of the Business Combination, SRNG changed its name to “Ginkgo Bioworks Holdings, Inc.” and, among other transactions contemplated by the Merger Agreement, the existing equity holders of Old Ginkgo exchanged their equity interests of Old Ginkgo for equity interests of New Ginkgo.
As a result of the Business Combination, the shares and corresponding capital amounts and loss per share related to Old Ginkgo’s outstanding convertible preferred stock and common stock prior to the Business Combination have been retroactively restated to reflect the Exchange Ratio established in the Merger Agreement. For additional information on the Business Combination, refer to Note 3 to these condensed consolidated financial statements.
Liquidity and Capital Resources
As of September 30, 2021, the Company had $1,739.1 million in cash and cash equivalents, inclusive of approximately $1,509.6 million received in net proceeds from the Business Combination. Prior to the Business Combination, the Company primarily financed its operations through proceeds from equity offerings, convertible note offerings, fees received for research and development services under license and collaboration arrangements, including upfront fees and fees received upon the achievement of milestones, fees received from Biosecurity product sales and services provided and government grants. These sources of liquidity have enabled the Company to expand the physical footprint and capacity of the Foundry and grow its personnel to expand capabilities and enter new markets.
The Company has incurred significant operating losses from inception through September 30, 2021, resulting in negative cash flows from operating activities and an accumulated deficit of $697.3 million as of September 30, 2021. The Company expects to continue to incur net losses into the foreseeable future. The Company’s ability to successfully transition to profitable operations is dependent upon achieving technical and commercial milestones under existing customer agreements, continuing to increase Foundry output while reducing the unit cost of that output, and expanding the number of engineered organisms under development with customers. The Company believes that its current cash and cash equivalents will provide adequate liquidity through at least one year from the date that these condensed consolidated financial statements are issued.
F-71
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
The Company’s future liquidity needs may vary materially from those currently planned and will depend on many factors, including the achievement of technical and commercial milestones under existing customer arrangements, the receipt of cash and equity from new customers and in connection with collaborative arrangements, the investments required to further scale the Foundry and Codebase, and the expenses needed to attract and retain personnel.
Risks and Uncertainties
The Company is subject to a number of risks including rapid technological change, regulatory change, technical feasibility, commercial viability, public perception of genetically modified organisms, uncertain market acceptance of products derived from engineered organisms, alternative means of production, data and cybersecurity breaches, and dependence on key vendors and personnel.
Impact of the COVID-19 Pandemic
In March 2020, the World Health Organization declared the novel strain of coronavirus (“COVID-19”) outbreak as a global pandemic and the United States declared a national emergency as a result of COVID-19. Since then, extraordinary actions have been taken by authorities to contain and manage the outbreak and spread of COVID-19 around the world.
Consistent with the actions taken by governmental authorities, the Company has taken steps to protect its workforce and support the community efforts. From approximately March 2020 to June 2020, the Company operated at a reduced capacity. The Company also restricted non-essential travel and allowed most of its workforce in general and administration functions to perform their duties remotely. In June 2020, the Company resumed modified on-site operations for its lab workers following the Center for Disease Control and Prevention’s guidance with facial covering requirements, rearranging facilities to follow social distancing protocols, performing active daily health checks, and undertaking regular and thorough disinfection of surfaces and tools.
The COVID-19 pandemic caused some disruption in the Company’s operations and the Company experienced partial suspensions and delays in servicing certain customer contracts. However, the Company believes that the COVID-19 pandemic did not have a material adverse impact to its financial position or results of operations.
The Company continues to monitor and assess the effects of the COVID-19 pandemic on its business, financial condition, results of operations and cash flows.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited condensed consolidated financial statements have been prepared in conformity with the rules and regulations of the Securities and Exchange Commission (“SEC”) and generally accepted accounting principles in the United States of America (“U.S. GAAP”) for interim financial reporting. Any reference in these notes to applicable guidance is meant to refer to the authoritative U.S. GAAP as found in the Accounting Standards Codification (“ASC”) and Accounting Standards Updates (“ASU”) of the Financial Accounting Standards Board (“FASB”).
In the opinion of management, the accompanying unaudited condensed consolidated financial statements reflect all normal recurring adjustments necessary to present fairly the financial position, results of operations, comprehensive loss, cash flows, and stockholders’ equity for the interim periods reported, but are not necessarily indicative of the results to be anticipated for the full year 2021 or any future period.
F-72
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
The Business Combination was accounted for as a reverse recapitalization, in accordance with U.S. GAAP (the “Reverse Recapitalization”). Under this method of accounting, SRNG was treated as the “acquired” company for financial reporting purposes. Accordingly, for accounting purposes, the Reverse Recapitalization was treated as the equivalent of Old Ginkgo issuing stock for the net assets of SRNG, accompanied by a recapitalization. The net assets of SRNG are stated at historical cost, with no goodwill or other intangible assets recorded. The determination of Old Ginkgo as the accounting acquirer was primarily based on the fact that Old Ginkgo’s former shareholders currently have the largest voting interest in New Ginkgo, all of the management of New Ginkgo is comprised of Old Ginkgo’s former executive management, Old Ginkgo’s former directors and individuals designated by, or representing, Old Ginkgo shareholders constitute a majority of the initial New Ginkgo Board, and the operations of Old Ginkgo comprise all of the ongoing operations of New Ginkgo.
The consolidated assets, liabilities and results of operations prior to the Reverse Recapitalization are those of Old Ginkgo. The shares and corresponding capital amounts and loss per share, prior to the Reverse Recapitalization, have been retroactively restated to reflect the Exchange Ratio established in the Merger Agreement.
These unaudited condensed consolidated financial statements should be read in conjunction with the audited consolidated financial statements as of and for the years ended December 31, 2020 and 2019, and the notes thereto, included in the Company’s prospectus on Form 424(b)(3) as filed with the SEC on September 17, 2021. The significant accounting policies used in preparation of these unaudited condensed consolidated financial statements are consistent with those described in the Company’s audited consolidated financial statements as of and for the years ended December 31, 2020 and 2019 included in the Company’s prospectus on Form 424(b)(3) as filed with the SEC on September 17, 2021.
Principles of Consolidation
The accompanying condensed consolidated financial statements include the accounts of the Company, its wholly owned subsidiaries and consolidated variable interest entities in which the Company has a controlling financial interest and is the primary beneficiary. Wholly owned subsidiaries include Ginkgo Bioworks, Inc., Ginkgo Bioworks Netherlands B.V., Ginkgo Bioworks Securities Corporation, Gen9, Inc. and Stegodon Corporation. The Company has a controlling financial interest in Cooksonia, LLC (“Cooksonia”), which is the holding entity for the Company’s investment in Joyn Bio, LLC (“Joyn”), and a controlling financial interest in Ayana Bio, LLC and Verb Biotics, LLC. All intercompany accounts and transactions have been eliminated.
Reclassifications
Certain prior year amounts have been reclassified for consistency with the current year presentation. These reclassifications had no effect on the reported results of operations.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the condensed consolidated financial statements, and the reported amounts of revenues and expenses during the reporting period. Significant estimates and assumptions used in the preparation of these condensed consolidated financial statements include, among others, the fair value of equity instruments and equity awards, revenue recognition, the fair value of loans receivable, the fair value of certain investments, including equity method investments, the fair value of warrant liabilities, accrued expenses, and income taxes.
F-73
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
The Company bases its estimates on historical experience and other market-specific or relevant assumptions that it believes to be reasonable under the circumstances. Reported amounts and disclosures reflect the overall economic conditions that management believes are most likely to occur, and the anticipated measures management intends to take. Actual results could differ materially from those estimates. All revisions to accounting estimates are recognized in the period in which the estimates are revised.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash and cash equivalents, accounts receivable, and loans receivable. The Company’s cash and cash equivalents are maintained in bank deposit accounts and money market funds, which, at times, may exceed federally insured limits. The Company believes that it is not exposed to significant credit risk as its deposits are held in financial institutions in the United States that management believes to be of high credit quality. The Company’s loans receivable are comprised of both collateralized convertible notes, which limits the Company’s credit risk, and uncollateralized convertible notes. The Company’s accounts receivable primarily consist of amounts owed under its license and collaboration agreements and its Biosecurity product and service offering. The Company has not experienced any material write-offs related to its accounts receivable since inception.
Restricted Cash
Restricted cash primarily includes cash balances collateralizing letters of credit associated with the Company’s facility leases and a customer prepayment requiring segregation and restrictions in its use in accordance with the customer agreement. Restricted cash is included in other non-current assets on the Consolidated Balance Sheets.
Inventory, net
Inventory mainly consists of diagnostic testing kits purchased from suppliers, testing program supplies and the costs of assembling sample collection kits. Finished goods inventory for lateral flow assay (“LFA”) and polymerase chain reaction (“PCR”) tests are valued at the lower of cost or net realizable value using the first-in first-out method. Raw materials, work in process and finished goods inventory for pooled tests are valued at the lower of cost or net realizable value using the average cost method. Inventory has been reduced by an allowance for excess and obsolete inventory based on the specific identification method.
Equity Method Investments
The Company utilizes the equity method to account for its investments in common stock, or in-substance common stock, when it possesses the ability to exercise significant influence, but not control, over the operating and financial policies of the investee. The Company uses judgment when determining the level of influence over the operating and financial policies of the investee considering key factors including, among others, the Company’s ownership interest, representation on the board of directors, participation in policy-making decisions and material contractual arrangements and obligations. Income and losses are allocated based upon relative ownership interest unless there is a substantive profit-sharing agreement in place.
For investments with a substantive profit-sharing agreement, the Company utilizes the Hypothetical Liquidation at Book Value (“HLBV”) method to allocate income and losses from the equity method investment. Under the HLBV method, the Company utilizes the capital account at the end of the period assuming the book value of the entity was liquidated or sold minus the same calculation at the beginning of the period. The difference is the share of earnings or losses attributable to the equity method investment.
F-74
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
Under the equity method, if there is a commitment for the Company to fund the losses of its equity method investees, the Company would continue to record its share of losses resulting in a negative equity method investment, which would be presented as a liability on the Condensed Consolidated Balance Sheets. Commitments may be explicit and may include formal guarantees, legal obligations, or arrangements by contract. Implicit commitments may arise from reputational expectations, intercompany relationships, statements by the Company of its intention to provide support, a history of providing financial support or other facts and circumstances. When the Company has no commitment to fund the losses of its equity method investees, the carrying value of its equity method investments will not be reduced below zero. The Company had no commitment to fund additional losses of its equity method investments during the three and nine months ended September 30, 2021 and 2020.
The Company evaluates its equity method investments for impairment whenever events or circumstances indicate that the carrying value of the investment may not be recoverable. The Company considers the investee’s financial position, forecasts and economic outlook, and the estimated duration and extent of losses to determine whether a recovery is anticipated. An impairment that is other-than-temporary is recognized in the period identified. The Company has not recognized an impairment loss related to its equity method investments for the three and nine months ended September 30, 2021 and 2020.
The Company may elect the fair value option for its equity method investments on an investment-by-investment basis. For all equity method investments accounted for under the fair value option, the Company carries the equity method investment at fair value. The Company records all subsequent changes in the values of its equity method investments in the Condensed Consolidated Statements of Operations and Comprehensive Loss as a component of loss on equity method investments.
Deferred Offering Costs
The Company capitalizes certain legal, accounting and other third-party fees that are directly associated with in-process equity financings as deferred offering costs until such financings are completed. After completion of the equity financing, such costs are reclassified as a reduction to additional paid-in capital generated as a result of the offering. Should the in-process equity financing be abandoned, the deferred offering costs will be expensed immediately as a charge to operating expenses in the Condensed Consolidated Statements of Operations and Comprehensive Loss. Upon the closing of the Business Combination and as of September 30, 2021, the Company reclassified $6.2 million of deferred offering costs to additional paid-in capital.
Revenue Recognition
Biosecurity Revenue
In 2020, the Company launched its commercial offering of COVID-19 testing products and services for businesses, academic institutions, and other organizations. In the first quarter of 2021, the Company launched its pooled testing initiative which focuses on providing end-to-end COVID-19 testing services to groups of individuals, with a focus on K-12 schools. The Company sells COVID-19 test kits on a standalone basis or as part of an end-to-end testing service. The Company records product revenue from sales of LFA, PCR, and pooled test kits. The Company records service revenue from sales of its end-to-end COVID-19 testing services, which consist of multiple promised goods and services including sample collection kits, physician authorizations, onsite test administration, outsourced laboratory PCR analysis, and access to results reported through the Company’s proprietary web-based portal. The Company recognizes its product and service revenue using the five-step model under ASC 606, Revenue from Contracts with Customers (“ASC 606”).
F-75
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
Product revenue is recognized when the test kits are shipped and risk of loss is transferred to the carrier. The Company’s test kits are generally not subject to a customer right of return except for product recalls under the rules and regulations of the U.S. Food and Drug Administration (“FDA”). The Company has elected to include shipping and handling fees billed to customers as a component of Biosecurity revenue.
Service revenue from the Company’s end-to-end COVID-19 testing services is recognized upon completion of the tests and release of the test results on the web-based portal. The Company has identified one performance obligation in its testing services contracts that represents a series of distinct goods or services that are substantially the same and that have the same pattern of transfer to the customer, with each test as a distinct service within the series. As the price for the testing services is fixed under each customer contract, the Company has elected the practical expedient to recognize revenue at the amount to which it has the right to invoice for services performed. The Company’s testing services contracts are generally one year or less in length and contain fixed unit pricing. Under typical payment terms for testing services, amounts are billed monthly in arrears for services performed or in advance based on contractual billing terms.
Other than as noted herein, there were no other changes to the Company’s revenue recognition policy since the date of the audited consolidated financial statements as of and for the years ended December 31, 2020 and 2019 included in the Company’s prospectus on Form 424(b)(3) as filed with the SEC on September 17, 2021.
Stock-Based Compensation
For awards granted prior to the Business Combination, the Company utilized the hybrid method to estimate the value of its common stock underlying its stock-based awards. The hybrid method is a probability-weighted expected return method (“PWERM”) where the equity value in at least one scenario is allocated using an option pricing method (“OPM”).
For the period from January 2020 through July 2020, when using the hybrid method the Company considered two scenarios: (i) a fully diluted scenario, in which the per-share common stock value was assumed to equal the price of the convertible preferred stock in a recent round of financing, and (ii) a remain private scenario, in which the Company used the OPM to back-solve to the price of the Company’s convertible preferred stock in a recent round of financing. In the fully diluted scenario, conversion of the convertible preferred stock to common stock was assumed. In the remain private scenario, equity value was allocated among the convertible preferred stock and common stock using the OPM. For the period from August 2020 to September 2021, when using the hybrid method the Company considered two scenarios: (i) a scenario in which the conversion of the convertible preferred stock to common stock occurs through an initial public offering (“IPO”) or a merger with a special purpose acquisition company (“SPAC”), and (ii) a remain-private scenario. With respect to the remain-private scenario, the Company estimated equity value using the guideline public company method. With respect to the IPO/SPAC scenario, the Company considered the equity values indicated by preliminary letters of intent received from potential investors or the assumed equity value in the Business Combination. In the IPO/SPAC transaction scenario, conversion of the convertible preferred stock to common stock was assumed. In the remain-private scenario, equity value was allocated among the convertible preferred stock and common stock using the OPM. The Company also considered prices paid for its common stock and Series B convertible preferred stock in secondary transactions and included these prices in its weighted average conclusion of value, if applicable. For awards granted after the Business Combination, the Company determines the fair value of its common stock on each grant date based on the quoted market price on the New York Stock Exchange (“NYSE”).
The Company estimates the grant date fair value of stock option awards using the Black-Scholes option-pricing model. The Black-Scholes option-pricing model requires the input of subjective assumptions, including fair value
F-76
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
of common stock (for awards granted prior to the Business Combination), expected term, expected volatility, risk-free interest rate and expected dividend yield. The expected term was generally determined using the “simplified” method for “plain vanilla” options. The Company determined expected volatility using the historical volatility of the stock prices of similar publicly traded peer companies. The risk-free interest rate was based on the yield available on U.S. Treasury zero-coupon issues similar in duration to the expected term of the stock options. The Company has not paid, and does not expect to pay, dividends in the foreseeable future.
Other than as noted herein, there were no other changes to the Company’s stock-based compensation policy since the date of the audited consolidated financial statements as of and for the years ended December 31, 2020 and 2019 included in the Company’s prospectus on Form 424(b)(3) as filed with the SEC on September 17, 2021.
Warrant Liabilities
The Company classifies Private Placement Warrants and Public Warrants (both defined and discussed in Note 14) as liabilities. At the end of each reporting period, changes in fair value during the period are recognized as change in fair value of warrant liabilities on the Condensed Consolidated Statements of Operations and Comprehensive Loss. The Company will continue to adjust the warrant liability for changes in the fair value until the earlier of (a) the exercise or expiration of the warrants or (b) the redemption of the warrants, at which time the warrants will be reclassified to additional paid-in capital.
Foreign Currency Translation
The Company’s reporting currency is the U.S. dollar while the functional currency of the Company’s non-U.S. subsidiary, Ginkgo Bioworks Netherlands, BV, is the Euro. The financial statements of the non-U.S. subsidiary are translated into U.S. dollars in accordance with ASC 830, Foreign Currency Matters, using period-end exchange rates for assets and liabilities, average exchange rates in the period for revenues and expenses and historical exchange rates for equity. Foreign currency translation adjustments are recorded as a component of other comprehensive income (loss) on the Condensed Consolidated Statements of Operations and Comprehensive Loss and accumulated in other comprehensive income (loss) in stockholders’ equity.
Comprehensive Loss
Comprehensive loss is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources. Comprehensive loss consists of foreign currency translation adjustments.
Recently Adopted Accounting Pronouncements
In October 2018, the FASB issued ASU 2018-17, Consolidation (Topic 810): Targeted Improvements to Related Party Guidance for Variable Interest Entities (“ASU 2018-17”). The provisions of ASU 2018-17 modify the guidance under ASC 810 related to the evaluation of indirect interests held through related parties under common control when determining whether fees paid to decision makers and service providers are variable interests. Indirect interests held through related parties that are under common control are no longer considered to be the equivalent of direct interests in their entirety and instead should be considered on a proportional basis. This guidance more closely aligns with accounting of how indirect interests held through related parties under common control are considered for determining whether a reporting entity must consolidate a variable interest entity. The Company adopted ASU 2018-17 on January 1, 2021 and the adoption did not have a material impact on the Company’s condensed consolidated financial statements and related disclosures.
F-77
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
Recently Issued Accounting Pronouncements
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842): Amendments to the FASB Accounting Standards Codification, which supersedes the existing guidance for lease accounting. The FASB has issued several updates to the standard which: (i) clarify how to apply certain aspects of the new standard, (ii) provide an additional transition method for adoption of the new standard, (iii) provide a practical expedient for certain lessor accounting, and (iv) amend certain narrow aspects of the guidance (collectively, “ASC 842”). ASC 842 requires the identification of arrangements that should be accounted for as leases by lessees. In general, for lease arrangements exceeding a twelve-month term, these arrangements must now be recognized as assets and liabilities on the balance sheet of the lessee. Under ASC 842, a right-of-use asset and lease obligation will be recorded for all leases, whether operating or financing, while the income statement will reflect lease expense for operating leases and amortization/interest expense for financing leases. The balance sheet amount recorded for existing leases at the date of adoption of ASC 842 is calculated using the applicable incremental borrowing rate at the date of adoption. The guidance is effective for the Company on January 1, 2022. The Company is currently evaluating the impact that the adoption of ASU 2016-02 will have on its consolidated financial statements and related disclosures. While the Company continues to evaluate the impact of adopting the new standard, the Company currently anticipates applying the modified retrospective approach effective January 1, 2022. The Company currently expects to elect the package of practical expedients which allows entities to not reassess (i) whether an arrangement is or contains a lease, (ii) the classification of its leases, and (iii) the accounting for initial direct costs. Further, the Company currently anticipates electing, by class of underlying asset, the short-term lease exception for leases with terms of twelve months or less, thereby, not recognizing a lease liability or right-of-use asset on its balance sheets for such short-term leases. The Company expects the impact of adoption to include: (i) the recognition of right-of-use assets and lease liabilities arising from its leases of office, laboratory space and equipment; (ii) the derecognition of deferred rent and unamortized tenant incentives; and (iii) new and expanded disclosure requirements.
3. Business Combination
On September 16, 2021 (the “Closing Date”), the Company and SRNG completed the merger transaction contemplated by the Merger Agreement (the “Closing”), with Old Ginkgo surviving the merger as a wholly owned subsidiary of SRNG.
Pursuant to the Merger Agreement, SRNG acquired all of the outstanding equity interests of Old Ginkgo for approximately $15.8 billion in aggregate consideration in the form of common stock of New Ginkgo valued at $10 per share (the “Base Equity Consideration”). The Base Equity Consideration was allocated among Old Ginkgo equity holders based on an exchange ratio of 49.080452 (“Exchange Ratio”). Accordingly, upon the closing of the Business Combination, all shares of Old Ginkgo Class A common stock and Old Ginkgo Class B common stock issued and outstanding immediately prior to the Business Combination converted into New Ginkgo Class A common stock and New Ginkgo Class B common stock, respectively, each with a par value of $0.0001 per share, based on the Exchange Ratio. All equity awards under Old Ginkgo’s stock incentive plans were assumed by the Company and converted into comparable equity awards that are settled or exercisable for shares of the Company’s common stock. As a result, (i) each outstanding stock option to acquire Old Ginkgo common stock was converted into an option to purchase approximately 49.080452 shares of New Ginkgo common stock, (ii) each outstanding share of restricted common stock was converted into approximately 49.080452 shares of restricted common stock of New Ginkgo and (iii) each outstanding award of restricted stock units was assumed and converted into a restricted stock unit having the same terms and conditions as applied to the Old Ginkgo restricted stock unit so converted but relating to approximately 49.080452 shares of common stock of New Ginkgo.
F-78
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
In addition to the Base Equity Consideration, the equity holders of Old Ginkgo received approximately 188.7 million shares of New Ginkgo common stock (the “Earn-out Consideration”), which are subject to forfeiture to the extent that the vesting conditions described below are not satisfied on or before the fifth anniversary of the Closing (the “Earn-out Period”). If at any point during the trading hours of a trading day, for any 20 trading days within any period of 30 consecutive trading days during the Earn-out Period, the trading price per share of the Company’s Class A common stock is greater than or equal to:
| • | $12.50, then 25% of the Earn-out Consideration will immediately vest; |
| • | $15.00, then an additional 25% of the Earn-out Consideration will immediately vest; |
| • | $17.50, then an additional 25% of the Earn-out Consideration will immediately vest; and |
| • | $20.00, then the remaining 25% of the Earn-out Consideration will immediately vest. |
In connection with the entry into the Merger Agreement, Eagle Equity Partners III, LLC, a Delaware limited liability company (the “Sponsor”), forfeited 11,534,052 of its shares of New Ginkgo Class A common stock and an additional 16,737,183 of its shares of New Ginkgo Class A common stock (the “Sponsor Earn-out Shares”) became subject to vesting and forfeiture conditions identical to those applicable to the Earn-out Consideration issued to Old Ginkgo equity holders. The Earn-out Consideration and the Sponsor Earn-out Shares are accounted for as equity classified equity instruments, were included as merger consideration as part of the Reverse Recapitalization and recorded in additional paid-in capital.
The Business Combination is accounted for as a reverse recapitalization, in accordance with U.S. GAAP. Under this method of accounting, SRNG was treated as the “acquired” company for financial reporting purposes. Accordingly, the Business Combination was treated as the equivalent of Old Ginkgo issuing stock for the net assets of SRNG, accompanied by a recapitalization. The net assets of SRNG are stated at historical cost, with no goodwill or other intangible assets recorded.
PIPE Investment
On May 11, 2021, concurrently with the execution of the Merger Agreement, SRNG entered into subscription agreements with certain accredited investors (the “PIPE Investors”). In connection with the consummation of the Business Combination on September 16, 2021, the PIPE Investors collectively consummated investments for 76,000,000 shares of the Company’s Class A common stock at a price of $10.00 per share (the “PIPE Shares”) for an aggregate amount of $760.0 million (the “PIPE Investment”).
Summary of Net Proceeds
The following table summarizes the elements of the net proceeds from the Business Combination as of September 30, 2021 (in thousands):
| Cash—SRNG Trust and cash (net of redemptions) |
$ | 857,747 | ||
| Cash—PIPE Investment |
760,000 | |||
| Less: Payment of underwriter fees and other offering costs |
(106,838 | ) | ||
|
|
|
|||
| Proceeds from Business Combination, net of offering costs paid at Closing |
1,510,909 | |||
| Less: Other offering costs included in accounts payable and accrued expenses at September 30, 2021 |
(1,280 | ) | ||
|
|
|
|||
| Net proceeds from the Business Combination |
$ | 1,509,629 | ||
|
|
|
F-79
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
Summary of Shares Issued
The following table summarizes the number of shares of common stock outstanding immediately following the consummation of the Business Combination:
| SRNG shares outstanding prior to the Business Combination |
215,625,000 | |||
| Less: redemption of SRNG shares prior to the Business Combination |
(86,725,312 | ) | ||
| Less: SRNG shares forfeited |
(11,534,052 | ) | ||
|
|
|
|||
| Common stock of SRNG(1) |
117,365,636 | |||
| Shares issued pursuant to the PIPE Investment |
76,000,000 | |||
|
|
|
|||
| Business Combination and PIPE Investment shares |
193,365,636 | |||
| Conversion of Old Ginkgo Series B preferred stock to common stock |
203,346,152 | |||
| Conversion of Old Ginkgo Series C preferred stock to common stock |
228,641,430 | |||
| Conversion of Old Ginkgo Series D preferred stock to common stock |
302,464,716 | |||
| Conversion of Old Ginkgo Series E preferred stock to common stock |
170,227,108 | |||
| Conversion of Old Ginkgo common stock(2) |
387,016,194 | |||
|
|
|
|||
| Total shares of New Ginkgo common stock outstanding immediately following the Business Combination |
1,485,061,236 | |||
|
|
|
| (1) | Includes 16,737,183 shares of Class A common stock, the Sponsor Earn-out Shares, that are subject to forfeiture if certain earnout conditions are not met, as the shares are legally outstanding as of the Closing of the Business Combination. |
| (2) | Excludes 283,396,094 shares of Class A and Class B common stock underlying rollover equity instruments (i.e., restricted stock units and stock options) and 259,440 shares of Class A and Class B common stock underlying unvested restricted stock awards. |
4. Acquisition
On July 1, 2021, the Company completed an acquisition of 100% of the equity of Dutch DNA Biotech B.V. (“Dutch DNA”), a company based in the Netherlands with a proprietary platform technology focused on the development of fungal strains and fermentation processes for the production of proteins and organic acids. Dutch DNA’s significant expertise and fungal strain assets for the large-scale production of proteins is expected to add a valuable set of tools to the Company’s Codebase and broader platform for cell programming.
F-80
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
The following table summarizes the preliminary acquisition date fair value of the consideration transferred for Dutch DNA (in thousands):
| Cash |
$ | 11,451 | ||
| Fair value of Class A common stock |
15,087 | |||
| Contingent consideration |
8,760 | |||
|
|
|
|||
| Total Dutch DNA consideration |
$ | 35,298 | ||
|
|
|
The fair value of the Class A common stock issued as part of the consideration paid for Dutch DNA was determined using the then-most recently available third-party valuation of the Company’s common stock. The contingent consideration arrangement requires the Company to pay up to a maximum of $20.0 million to the seller upon the achievement of certain technical and commercial milestones by Dutch DNA pursuant to a Technical Development Agreement executed between the Company and Dutch DNA prior to the close of the acquisition. Refer to Note 5 for further discussion of the fair value of the contingent consideration liability.
The acquisition was accounted for in accordance with ASC 805, Business Combinations. Dutch DNA’s results of operations have been included in the Condensed Consolidated Statements of Operations and Comprehensive Loss since the date of acquisition, which were not material. The Dutch DNA acquisition does not represent a material business combination, and therefore pro forma financial information is not provided. The Company allocated the purchase price to the tangible and identifiable intangible assets acquired and liabilities assumed based on their respective estimated fair values on the acquisition date. The fair value of the intangible assets was determined using the replacement cost method which estimates the cost the Company would incur in rebuilding the technology. The excess purchase price consideration was recorded as goodwill and is made up of the future potential value of the acquired intellectual property and the assembled workforce. The Company incurred $0.6 million of acquisition-related costs which were included in general and administrative expenses in the Condensed Consolidated Statements of Operations and Comprehensive Loss.
The following table summarizes the preliminary acquisition date fair values of assets acquired and liabilities assumed as of the acquisition date (in thousands):
| Cash |
$ | 387 | ||
| Accounts receivable |
149 | |||
| Prepaid expenses and other current assets |
170 | |||
| Property, plant and equipment |
234 | |||
| Intangibles(1) |
20,500 | |||
| Goodwill(2) |
15,177 | |||
| Accounts payable |
(194 | ) | ||
| Accrued expenses and other current liabilities |
(137 | ) | ||
| Other non-current liabilities |
(988 | ) | ||
|
|
|
|||
| Net assets acquired |
$ | 35,298 | ||
|
|
|
| (1) | Estimated useful life of 15 years. |
| (2) | Non-deductible for tax purposes. |
F-81
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
5. Fair Value Measurements
No transfers between levels have occurred during the periods presented. The following tables present information about the Company’s financial assets and liabilities measured at fair value on a recurring basis (in thousands):
| As of September 30, 2021 | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets: |
||||||||||||||||
| Money market funds, included in cash and cash equivalents |
$ | 1,667,271 | $ | 1,667,271 | $ | — | $ | — | ||||||||
| Synlogic, Inc. common stock, included in investments |
19,339 | 19,339 | — | — | ||||||||||||
| Synlogic, Inc. warrants, included in investments |
7,772 | — | 7,772 | — | ||||||||||||
| Cronos Group Inc. common stock, included in investments |
7,660 | — | 7,660 | — | ||||||||||||
| Loans receivable, included in prepaid expenses and other current assets |
3,905 | — | — | 3,905 | ||||||||||||
| Loans receivable, net of current portion |
10,161 | — | — | 10,161 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets |
$ | 1,716,108 | $ | 1,686,610 | $ | 15,432 | $ | 14,066 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Liabilities: |
||||||||||||||||
| Public Warrants, included in warrant liabilities |
$ | 116,955 | $ | 116,955 | $ | — | $ | — | ||||||||
| Private Placement Warrants, included in warrant liabilities |
95,980 | — | — | 95,980 | ||||||||||||
| Contingent consideration, included in other non-current liabilities |
8,737 | — | — | 8,737 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total liabilities |
$ | 221,672 | $ | 116,955 | $ | — | $ | 104,717 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| As of December 31, 2020 | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets: |
||||||||||||||||
| Money market funds, included in cash and cash equivalents |
$ | 372,537 | $ | 372,537 | $ | — | $ | — | ||||||||
| Synlogic, Inc. common stock, included in investments |
13,696 | 13,696 | — | — | ||||||||||||
| Synlogic, Inc. warrants, included in investments |
5,504 | — | 5,504 | — | ||||||||||||
| Loans receivable, included in prepaid expenses and other current assets |
2,268 | — | — | 2,268 | ||||||||||||
| Loans receivable, net of current portion |
13,298 | — | — | 13,298 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total assets |
$ | 407,303 | $ | 386,233 | $ | 5,504 | $ | 15,566 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The fair value of the warrants to purchase Synlogic common stock (Note 9) is calculated as the value of the underlying common stock, less the related unpaid exercise price and represents a Level 2 measurement within the fair value hierarchy.
F-82
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
During the three months ended September 30, 2021, the Company received 1,467,490 shares of Cronos Group Inc. (“Cronos”) common stock as consideration for the achievement of the first target productivity milestone under the collaboration agreement with Cronos to produce eight cultured cannabinoids. The fair value of the Cronos common stock is calculated as the quoted price of the common stock adjusted for short-term marketability restrictions. Accordingly, the shares are classified as Level 2 financial instruments.
Loans Receivable
As of September 30, 2021 and December 31, 2020, loans receivable primarily consisted of a revolving promissory note with Glycosyn, LLC (“Glycosyn”) which is secured by the assets of Glycosyn, including certain intellectual property such as patents and copyrights held by Glycosyn, (“Glycosyn Promissory Note”) and a series of convertible notes with Access Bio, Inc. (“Access Bio Convertible Notes”). The fair value of the Glycosyn Promissory Note and Access Bio Convertible Notes were determined based on significant inputs not observable in the market, which represent a Level 3 measurement within the fair value hierarchy. Significant changes in these unobservable inputs in isolation could have resulted in a significantly lower or higher fair value measurement. Refer to Note 6 for additional details on the Company’s loans receivable.
The Company used a probability-weighted discounted cash flow valuation approach to determine the fair value of the Glycosyn Promissory Note. Using this approach, the present value of the expected future cash flows was calculated under four settlement scenarios and then were weighted based on the estimated probability of each scenario. The four settlement scenarios considered in the valuation were (i) a qualified financing which resulted in a 20% conversion discount, (ii) repayment upon change in control, (iii) a dissolution scenario and (iv) repayment in accordance with the terms of the note. The significant assumptions used in valuing the Glycosyn Promissory Note included the expected timing and probability of each scenario and the discount rate. As of September 30, 2021, a discount rate of 15% was applied, the probability of each scenario ranged from 5% to 85% and the time to event-date was 0.25 years. As of December 31, 2020, a discount rate of 15% was applied and the probability and timing of each scenario ranged from 10% to 40% and from 1 to 2.5 years. The weighted average timing of the scenarios weighted based on the probability of each scenario as of September 30, 2021 and December 31, 2020 was 0.25 years and 1.2 years, respectively.
The Company used a Monte-Carlo simulation model to determine the fair value of the Access Bio Convertible Notes. The future stock price of Access Bio, Inc. (“Access Bio”) was simulated over the term of the note to assess the value of the settlement features which included (i) conversion into stock at a discount determined under a reset provision tied to the performance of Access Bio’s stock price and (ii) redemption at maturity. The significant assumptions used in determining the simulated future stock price included the expected timing of the conversion, which is assumed at maturity, and expected volatility. The significant assumptions used in determining the fair value of the Access Bio Convertible Notes under a redemption at maturity scenario was the discount rate and expected volatility. As of September 30, 2021, the key assumptions used were a discount rate of 30.7% and volatility of 90.0%. As of December 31, 2020, the key assumptions used were a discount rate of 32.8% and volatility of 88.5%.
F-83
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
The following table provides a reconciliation of loans receivable measured at fair value using Level 3 significant unobservable inputs (in thousands):
| 2021 | 2020 | |||||||
| Balance at January 1 |
$ | 15,566 | $ | 4,830 | ||||
| Issuance of loans receivable |
— | 400 | ||||||
| Proceeds from loans receivable |
(304 | ) | (217 | ) | ||||
| Change in fair value |
(1,196 | ) | 295 | |||||
|
|
|
|
|
|||||
| Balance at September 30 |
$ | 14,066 | $ | 5,308 | ||||
|
|
|
|
|
|||||
Warrant Liabilities
The fair value of the Private Placement Warrants have been estimated using a Monte Carlo simulation model as of the date the Company assumed the warrants from SRNG and subsequently as of the balance sheet date. The fair value of the Public Warrants have been measured based on the quoted price of such warrants on the NYSE. The estimated fair value of the Private Placement Warrants is determined using Level 3 inputs. Inherent in a Monte Carlo simulation are assumptions related to expected stock-price volatility, expected life, risk-free interest rate and dividend yield. Material increases (or decreases) in any of those inputs may result in a significantly higher (or lower) fair value measurement. The Company estimates the volatility of its Private Placement Warrants based on implied volatility from the Company’s Public Warrants and from historical volatility of select peer company’s common stock that matches the expected remaining life of the warrants. The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve for a maturity similar to the expected remaining life of the warrants. The expected life of the warrants is assumed to be equivalent to their remaining contractual term. The dividend yield is based on the historical rate, which the Company anticipates remaining at zero. Refer to Note 14 for additional details on the Company’s warrant liabilities.
The following table provides quantitative information regarding Level 3 inputs used in the recurring valuation of the Private Placement Warrants as of their measurement dates:
| September 16, 2021 (Closing) |
September 30, 2021 |
|||||||
| Exercise price |
$ | 11.50 | $ | 11.50 | ||||
| Stock price |
$ | 11.42 | $ | 11.59 | ||||
| Volatility |
53.1 | % | 55.4 | % | ||||
| Term |
5 years | 4.96 years | ||||||
| Risk-free rate |
0.84 | % | 0.98 | % | ||||
The following table provides a reconciliation of the Private Placement Warrants measured at fair value using Level 3 significant unobservable inputs (in thousands):
| 2021 | ||||
| Balance at January 1 |
$ | — | ||
| Additions pursuant to the Business Combination |
90,263 | |||
| Change in fair value |
5,717 | |||
|
|
|
|||
| Balance at September 30 |
$ | 95,980 | ||
|
|
|
|||
F-84
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
Contingent Consideration
In connection with the acquisition of Dutch DNA, the Company recorded contingent consideration liabilities for the estimated fair value of earn-out payments up to a maximum of $20.0 million payable to the seller upon the achievement of certain technical and commercial milestones by Dutch DNA pursuant to a Technical Development Agreement executed between the Company and Dutch DNA prior to the close of the acquisition. The contingent consideration liabilities are measured at fair value and are based on significant inputs not observable in the marketplace, which represent a Level 3 measurement. The fair value of the earn-outs was estimated using a combination of a probability-based approach and a discounted cash flow model. The key valuation inputs used as of the acquisition date were management’s estimate of the probability of achieving each milestone ranging from 10% to 80%, discount rates ranging from 6.93% to 9% and projections related to Dutch DNA’s after-tax revenues for each of the calendar years 2021 through 2046. Material increases (or decreases) in any of those inputs may result in a significantly higher (or lower) fair value measurement.
There were no material changes to the valuation assumptions as of September 30, 2021, therefore, the change in fair value is not significant.
6. Loans Receivable
Glycosyn Promissory Note
In October 2018, the Company provided a revolving promissory note to Glycosyn which has been amended several times since inception. The Glycosyn Promissory Note is convertible at a discount, at the Company’s election, into equity securities of Glycosyn upon Glycosyn’s first issuance of equity securities, other than an underwritten public offering, from which Glycosyn receives gross proceeds of at least $10.0 million. In addition, Glycosyn is obligated to immediately repay the outstanding balance of the loan, plus accrued interest, upon a change in control event. In January 2021, the existing terms of the Glycosyn Promissory Note were amended to add an additional $0.2 million to the principal balance, extend the number of interest-only payments through June 30, 2021 and to increase the interest rate from 7.5% to 12.5% in the event of default by Glycosyn. In July 2021, the parties entered into an additional amendment to extend the number of interest-only payments through the end of 2021 and to accelerate the maturity date to December 31, 2021. In the event that Glycosyn were to execute a term sheet on or prior to December 31, 2021 with an investor or acquirer for a bona fide equity financing or change of control transaction that, upon its consummation, will provide Glycosyn with gross proceeds of at least $10 million, then the maturity date will instead be the earlier of the consummation date of such transaction or March 31, 2022.
As of September 30, 2021 and December 31, 2020, the unpaid principal balance under the Glycosyn Promissory Note was $5.4 million and $5.3 million, respectively. The fair value of the Glycosyn Promissory Note was $3.7 million as of September 30, 2021, of which $3.7 million was included in prepaid expenses and other current assets, and $4.5 million as of December 31, 2020, of which $2.0 million was included in prepaid expenses and other current assets and the remaining amount within loans receivable, net of current portion on the Condensed Consolidated Balance Sheets. The loss on the change in fair value of $0.8 million for the three and nine months ended September 30, 2021 was recorded as a component of other (expense) income, net on the Condensed Consolidated Statements of Operations and Comprehensive Loss. The change in fair value for the three and nine months ended September 30, 2020 was immaterial.
Access Bio Convertible Notes
In November 2020, the Company entered into a convertible note subscription agreement with Access Bio, a supplier of the Company’s diagnostic test kits. The Access Bio Convertible Notes are due in November 2022 in
F-85
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
the aggregate principal amount of $10.0 million plus a 2% rate of return compounded annually. The Access Bio Convertible Notes are convertible into a number of shares of common stock of Access Bio, a company listed on the Korea Stock Exchange, of up to $10.0 million based on a fixed foreign currency exchange rate and a conversion price subject to certain adjustments, including reset adjustments each quarter based on the trading price of Access Bio’s stock. The adjusted conversion price cannot be reduced to less than 70% of the initial conversion price and the reset adjustments cannot increase the effective conversion ratio. The Access Bio Convertible Notes are convertible at the Company’s election any time following the first anniversary of the issuance date of the notes and prior to the 30th day before the maturity date. Additionally, subject to certain provisions, the Company has the option to cause Access Bio to repurchase, or Access Bio has the option to repurchase, a portion of the outstanding balance under the notes (up to the entire balance in the case of the Company’s option) at a price to ensure a 2% rate of return compounded annually.
As of September 30, 2021 and December 31, 2020, the fair value of the Access Bio Convertible Notes was $10.2 million and $10.7 million, respectively, which was recorded in loans receivable, net of current portion on the Condensed Consolidated Balance Sheets. The loss from the change in fair value of the Access Bio Convertible Notes during the three and nine months ended September 30, 2021 was $4.9 million and $0.5 million, respectively, and was recorded as a component of other (expense) income, net on the Condensed Consolidated Statements of Operations and Comprehensive Loss.
7. Inventory, net
Inventory, net consisted of the following (in thousands):
| As of September 30, |
As of December 31, |
|||||||
| 2021 | 2020 | |||||||
| Finished goods |
$ | 2,532 | $ | 2,756 | ||||
| Raw materials |
828 | — | ||||||
| Work in process |
163 | — | ||||||
| Less: Inventory reserve |
(49 | ) | (20 | ) | ||||
|
|
|
|
|
|||||
| Inventory, net |
$ | 3,474 | $ | 2,736 | ||||
|
|
|
|
|
|||||
F-86
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
8. Property and Equipment, net
Property and equipment, net consisted of the following (in thousands):
| As of September 30, |
As of December 31, |
|||||||
| 2021 | 2020 | |||||||
| Facilities |
$ | 12,762 | $ | 12,762 | ||||
| Furniture and fixtures |
4,302 | 2,165 | ||||||
| Lab equipment |
106,213 | 51,072 | ||||||
| Computer equipment and software |
9,822 | 6,204 | ||||||
| Leasehold improvements |
52,941 | 40,435 | ||||||
| Construction in progress |
10,459 | 42,575 | ||||||
| Vehicles |
41 | — | ||||||
|
|
|
|
|
|||||
| Total property and equipment |
196,540 | 155,213 | ||||||
| Less: Accumulated depreciation |
(53,836 | ) | (33,778 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 142,704 | $ | 121,435 | ||||
|
|
|
|
|
|||||
9. Investments and Equity Method Investments
The Company partners with other investors to form new ventures, including Joyn, Motif Foodworks, Inc. (“Motif”), Allonnia, LLC (“Allonnia”) and Arcaea LLC (“Arcaea”, FKA Kalo Ingredients LLC) (collectively “Platform Ventures”). The Company also partners with existing entities, including Genomatica, Inc. (“Genomatica”) and Synlogic, Inc. (“Synlogic”) (collectively, “Structured Partnerships”), with complementary assets for high potential synthetic biology applications. The Company or its subsidiaries hold equity interests in these Platform Ventures and Structured Partnerships. The Company’s investments in Platform Ventures are accounted for under the equity method. The Company’s investments in Synlogic, a publicly traded company, are carried at fair value. As of September 30, 2021 and December 31, 2020, the Company held 6,340,771 shares of Synlogic common stock and warrants to purchase an aggregate of 2,548,117 shares of Synlogic common stock. Prior to the third quarter of 2021, the Company’s investment in Synlogic common stock was classified as an equity method investment and accounted for under the fair value option. Due to a decrease in the level of ownership, the investment no longer qualifies for the equity method and was reclassified from equity method investments to investments on the Condensed Consolidated Balance Sheets, and from loss on equity method investments to (loss) gain on investments on the Condensed Consolidated Statements of Operations and Comprehensive Loss for all periods presented. However, the Company continues to apply the fair value option to account for its investments in Synlogic.
The Company’s non-marketable equity securities are investments in privately held companies without readily determinable fair values. The investment in Genomatica preferred stock and other non-marketable equity securities are initially recorded using the measurement alternative at cost and subsequently adjusted to fair value for any impairment and observable price change in orderly transactions for the identical or a similar investment of the same issuer. As of September 30, 2021 and December 31, 2020, no impairment or adjustment from observable price changes have been recognized related to investments accounted for under the measurement alternative.
F-87
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
Investments and equity method investments consisted of the following (in thousands):
| As of September 30, |
As of December 31, |
|||||||
| 2021 | 2020 | |||||||
| Investments: |
||||||||
| Genomatica, Inc. preferred stock |
$ | 55,000 | $ | 55,000 | ||||
| Synlogic, Inc. common stock |
19,339 | 13,696 | ||||||
| Synlogic, Inc. warrants |
7,772 | 5,504 | ||||||
| Cronos Group Inc. common stock |
7,660 | — | ||||||
| Non-marketable equity securities |
15,000 | — | ||||||
|
|
|
|
|
|||||
| Total |
$ | 104,771 | $ | 74,200 | ||||
|
|
|
|
|
|||||
| Equity method investments(1): |
||||||||
| Joyn Bio, LLC |
$ | 16,357 | $ | 28,924 | ||||
|
|
|
|
|
|||||
| Total |
$ | 16,357 | $ | 28,924 | ||||
|
|
|
|
|
|||||
| (1) | Equity method investments in Platform Ventures with a carrying value of zero as of September 30, 2021 and December 31, 2020 were excluded from the table. |
(Losses) gains on investments and equity method investments consisted of the following (in thousands):
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
| (Loss) gain on investments: |
||||||||||||||||
| Synlogic, Inc. common stock |
$ | (5,327 | ) | $ | (64 | ) | $ | 5,642 | $ | (3,551 | ) | |||||
| Synlogic, Inc. warrants |
(2,140 | ) | (26 | ) | 2,268 | (1,427 | ) | |||||||||
| Cronos Group Inc. |
(4,901 | ) | — | (4,901 | ) | — | ||||||||||
| Total |
$ | (12,368 | ) | $ | (90 | ) | $ | 3,009 | $ | (4,978 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss on equity method investments: |
||||||||||||||||
| Joyn Bio, LLC |
$ | (4,192 | ) | $ | (237 | ) | $ | (12,567 | ) | $ | (2,151 | ) | ||||
| Allonnia, LLC |
— | — | (12,698 | ) | — | |||||||||||
| Arcaea LLC |
(35,459 | ) | — | (47,356 | ) | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | (39,651 | ) | $ | (237 | ) | $ | (72,621 | ) | $ | (2,151 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
In addition, the Company must determine which of its equity method investments, if any, is considered a “significant subsidiary” pursuant to SEC Rule 10-01(b)(1) of Regulation S-X for interim reporting. As of September 30, 2021, the Company’s equity method investment in Arcaea met at least one of the significance tests. As a result, the following table shows summarized unaudited financial information for Arcaea for the nine months ended September 30, 2021(1) (in thousands):
| Revenue |
$ | — | ||
| Total operating expenses |
$ | (3,596 | ) | |
| Loss from operations |
$ | (3,596 | ) | |
| Net loss |
$ | (3,551 | ) |
| (1) | Arcaea was founded in 2021, therefore, prior year comparative periods are not applicable. |
F-88
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
Refer to Notes 10 and 17 for additional details on the Company’s investments and equity method investments.
10. Variable Interest Entities
Consolidated Variable Interest Entities
The Company holds a 70% equity interest in Cooksonia, which was formed by the Company and certain other investors for the purposes of holding the Company’s investment in Joyn. The Company concluded that it holds a variable interest in and is the primary beneficiary of Cooksonia as it controls the most significant activities of Cooksonia. These conclusions were reached considering that: (i) the Company controls 100% of the board of directors of Cooksonia and (ii) the Company holds a controlling financial interest in Cooksonia. Due to the fact that the Company is the primary beneficiary of Cooksonia, the Company has consolidated the financial statements of Cooksonia in accordance with ASC 810, Consolidation (“ASC 810”) into its condensed consolidated financial statements and has recognized a non-controlling interest associated with the minority equity interest held by other investors of Cooksonia, which together hold the remaining 30% equity interest in Cooksonia. The Company presents the non-controlling interest attributable to the other investors’ equity interest in Cooksonia as a component of stockholder’s equity.
The sole asset held by Cooksonia as of September 30, 2021 and December 31, 2020 was its equity method investment in Joyn, which was included in the Company’s Condensed Consolidated Balance Sheets as of each period end. The balance of Cooksonia’s equity method investment in Joyn as of September 30, 2021 and December 31, 2020 was $16.4 million and $28.9 million, respectively. No liabilities were held by Cooksonia as of September 30, 2021 and December 31, 2020. The net loss incurred by Cooksonia was $4.2 million and $0.2 million for the three months ended September 30, 2021 and 2020, respectively, and $12.6 million and $2.2 million for the nine months ended September 30, 2021 and 2020, respectively, which was comprised solely of the loss from its equity method investment in Joyn and was included in the Company’s Condensed Consolidated Statements of Operations and Comprehensive Loss for the respective periods. The net loss incurred by Cooksonia attributable to the non-controlling interest was $0.5 million and $0.1 million for the three months ended September 30, 2021 and 2020, respectively, and $2.3 million and $0.6 million for the nine months ended September 30, 2021 and 2020, respectively.
The Company holds an interest in 9,000,000 common units (representing 100% of common units at inception) of Ayana Bio, LLC (“Ayana”), which was formed in September 2021 to focus on harnessing bioactive compounds for use as complementary medicine to support human health and wellness. The Company has also provided Ayana with certain licenses to intellectual property for use in the development or production of products that the parties agree to research and develop under technical development plans. Additionally, in September 2021, Ayana entered into a Series A Preferred Unit Purchase Agreement under which it sold 9,000,000 Series A preferred units to certain of the Company’s investors for aggregate proceeds of approximately $30.0 million.
The Company holds an interest in 9,000,000 common units (representing 100% of common units at inception) of Verb Biotics, LLC (“Verb”), which was formed in September 2021 to focus on identifying and designing new strains of probiotic bacteria with advanced properties for human nutrition, health and wellness. The Company has also provided Verb with certain licenses to intellectual property for use in the development or production of products that the parties agree to research and develop under technical development plans. Additionally, in September 2021, Verb entered into a Series A Preferred Unit Purchase Agreement under which it sold 9,000,000 Series A preferred units to certain of the Company’s investors for aggregate proceeds of approximately $30.0 million.
F-89
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
The Company concluded that it holds a variable interest in and is the primary beneficiary of Ayana and Verb as it controls the most significant activities of these entities. These conclusions are supported by the fact that as of the primary beneficiary assessment date, for both Verb and Ayana: (i) the Company has substantive control of the board of directors; (ii) all capital contributions were made by related parties of New Ginkgo; and (iii) New Ginkgo or its related parties comprise the entirety of the Joint Steering Committee, the governing body which holds significant oversight with respect to the entities’ research and development programs.
Due to the fact that the Company is the primary beneficiary of Ayana and Verb, the Company has consolidated the financial statements of these entities into its condensed consolidated financial statements and has recognized a non-controlling interest associated with the minority equity interest held by other investors. The Company presents the non-controlling interest attributable to the other investors’ equity interest as a component of Stockholders’ equity, which at September 30, 2021 was $30.0 million for each of Ayana and Verb.
The sole asset held by Ayana and Verb was the cash proceeds raised from the Series A Preferred stock financing of $60.0 million in total for both entities which remained unchanged at September 30, 2021 due to the limited operations of these entities from inception through September 30, 2021. These cash balances can only be used to settle the obligations of the respective variable interest entity (“VIEs”). There were no significant liabilities recognized and no significant income or loss from operations during the period ended September 30, 2021.
Unconsolidated Variable Interest Entities
With respect to the Company’s investments in Motif, Allonnia, Genomatica and Arcaea, the Company has concluded these entities represent VIEs. However, although the Company holds board representation and is involved in the ongoing development activities of the entities via its participation on joint steering committees, the Company has concluded that it is not the primary beneficiary of these entities. This conclusion is supported by the fact that: (i) it does not control the board of directors of any of Motif, Allonnia, Genomatica or Arcaea, and no voting or consent agreements exist between the Company and other members of each respective board of directors or other investors, (ii) the holders of preferred security interests in Motif, Allonnia, Genomatica and Arcaea hold certain rights that require their consent prior to the taking of certain actions, which include certain significant operating and financing decisions, and (iii) the Company’s representation on the joint steering committee of each respective entity does not give it control over the development activities of any of Motif, Allonnia, Genomatica or Arcaea, as all votes must pass by consensus and there are no agreements in place that would require any of the entities to vote in alignment with the Company. As the Company’s involvement in Motif, Allonnia, Genomatica and Arcaea does not give it the power to control the decisions with respect to their development or other activities, which are their most significant activities, the Company has concluded that it is not the primary beneficiary of Motif, Allonnia, Genomatica or Arcaea.
With respect to Cooksonia’s investment in Joyn, as Cooksonia does not control Joyn’s board of directors, it does not have the power to control the decisions related to the development activities of Joyn, which are its most significant activities. Accordingly, the Company has concluded that Cooksonia is not the primary beneficiary of Joyn.
Additionally, the Company holds equity interests in certain privately-held companies that are not consolidated as the Company is not the primary beneficiary. As of September 30, 2021 and December 31, 2020, the maximum risk of loss related to the Company’s unconsolidated VIEs was limited to the carrying value of its investment in such entities.
Refer to Notes 9 and 17 for additional details on the Company’s investments and equity method investments.
F-90
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
11. Goodwill and Intangible Assets, net
Goodwill consisted of the following (in thousands):
| Balances as of December 31, 2020 |
$ | 1,857 | ||
| Goodwill acquired in Dutch DNA acquisition |
15,177 | |||
| Currency translation adjustment |
(374 | ) | ||
|
|
|
|||
| Balances as of September 30, 2021 |
$ | 16,660 | ||
|
|
|
Intangible assets, net consisted of the following (in thousands):
| Weighted Average Amortization Period |
Gross Carrying Value |
Accumulated Amortization |
Net | |||||||||||||
| Balances as of September 30, 2021 |
||||||||||||||||
| Acquired developed technology |
13.5 | $ | 25,509 | $ | (2,941 | ) | $ | 22,568 | ||||||||
| Balances as of December 31, 2020 |
||||||||||||||||
| Acquired developed technology |
6.0 | $ | 5,490 | $ | (2,196 | ) | $ | 3,294 | ||||||||
Amortization expense was $0.4 million and $0.1 million for the three months ended September 30, 2021 and 2020, respectively, and $0.7 million and $0.4 million for the nine months ended September 30, 2021 and 2020, respectively. Future amortization expense will be $0.4 million for the remainder of 2021 and $1.8 million per year thereafter over the remaining estimated useful life of the intangible assets.
12. Commitments and Contingencies
The Company is party to a number of agreements with certain collaborators and suppliers that require the Company to meet minimum purchase obligations over the term of such agreements. During the nine months ended September 30, 2021, there were no material changes to the Company’s obligations under these agreements. For a description of the arrangements and the related accounting conclusions, refer to Note 11 to the audited consolidated financial statements as of and for the years ended December 31, 2020 and 2019 included in the Company’s prospectus on Form 424(b)(3) as filed with the SEC on September 17, 2021.
Lease Obligations
In April 2021, the Company entered into a lease consisting of approximately 152,000 square feet of office and laboratory space being developed in Boston, Massachusetts. The lease commencement date is estimated to be June 1, 2024, subject to certain extensions, and expires on the fifteenth anniversary of the lease commencement date. In September 2021, the Company exercised its expansion option to include the entire rentable area of the building of approximately 262,000 square feet. Annual base rent for the first lease year will be approximately $16.8 million, subject to annual rent increases over the lease term. The lease includes one option to extend the lease for ten years at then-market rates, subject to certain adjustments, and will be secured by a letter of credit of an estimated $14.7 million.
On September 6, 2021, the Company entered into an amendment to its operating lease at 27 Drydock Avenue in Boston, Massachusetts under which the Company will lease an additional 47,957 square feet of space and extend the term of the lease by six years from January 2030 to January 2036. The Company anticipates occupying approximately 29,552 square feet of additional space in 2021 and the remainder in 2023. The minimum monthly
F-91
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
rent for the expansion premises will be $0.2 million starting in 2021 and $0.1 million starting in 2023, increasing by 3% annually. The minimum monthly rent for the existing premises during the extended term will be $1.1 million, increasing by 3% annually. The Company’s letter of credit will increase by $1.0 million. The Company will continue to have an option to extend the term of the lease beyond the extended term for an additional five-year term.
Indemnification Agreements
The Company enters into standard indemnification agreements and has agreements with indemnification clauses in the ordinary course of business. Under such arrangements, the Company indemnifies, holds harmless and agrees to reimburse the indemnified party for losses suffered or incurred by the indemnified party, who are generally the Company’s business partners. The terms of these indemnification arrangements are generally perpetual and effective any time after contract execution. The maximum potential liability resulting from these indemnification arrangements may be unlimited. The Company has never incurred costs to defend lawsuits or settle claims as a result of such indemnifications and the Company is not aware of any indemnification arrangements that could have a material effect on its financial position, results of operations or cash flows, and it has not accrued any liabilities related to such obligations as of September 30, 2021.
Legal Proceedings
The Company currently does not believe it is party to any legal proceedings where the potential loss amount is material. As of each reporting date, the Company evaluates whether or not a potential loss amount or range of loss amounts is reasonably estimable and probable of being incurred and whether such amounts meet the requirements to be accrued or disclosed pursuant to ASC 450, Contingencies (“ASC 450”). The Company expenses costs related to such legal proceedings as incurred.
Registration Rights
In connection with the closing of the Business Combination, the Company entered into an amended and restated registration rights agreement (the “Registration Rights Agreement”) among the Company, SRNG and certain Old Ginkgo stockholders. Pursuant to the Registration Rights Agreement, the Company will be required to register for resale securities held by the stockholders. The Company will have no obligation to facilitate more than two demands per calendar year for each of the SRNG or the Ginkgo Holders (as defined in the Registration Rights Agreement) that the Company register such stockholders’ securities. In addition, the holders have certain “piggyback” registration rights with respect to registrations initiated by the Company. The Company will bear the expenses incurred in connection with the filing of any registration statements pursuant to the Registration Rights Agreement.
13. Stockholders’ Equity
The Condensed Consolidated Statement of Stockholders’ Equity has been retroactively adjusted for all periods presented to reflect the Business Combination and reverse recapitalization as described in Note 3.
Preferred Stock
The Company is authorized to issue 200,000,000 shares of preferred stock with a par value $0.0001 per share. The Company’s board of directors are authorized, without stockholder approval, to issue such shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, and to fix the designations, powers, voting, and other rights, preferences and privileges of the shares. There were no issued and outstanding shares of preferred stock as of September 30, 2021.
F-92
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
Upon closing of the Business Combination, all Series B, C, D, and E convertible preferred stock issued and outstanding prior to September 16, 2021 were converted and exchanged for Class A common stock of the Company pursuant to the Exchange Ratio established in the Merger Agreement. All fractional shares were rounded down.
Common Stock
The Company is authorized to issue 15,800,000,000 shares of common stock, including 10,500,000,000 shares of Class A common stock, par value $0.0001 per share, 4,500,000,000 shares of Class B common stock, par value $0.0001 per share, and 800,000,000 shares of Class C common stock, par value $0.0001 per share.
Voting
Holders of Class A common stock are entitled to one vote per share and holders of Class B common stock are entitled to ten votes per share. Holders of Class C common stock are not entitled to vote except as otherwise expressly provided in the certificate of incorporation or required by applicable law.
Dividends
Common stockholders are entitled to receive dividends, as may be declared by the board of directors. Different classes of common stock are legally entitled to equal per share distributions whether through dividends or liquidation. No dividends have been declared to date.
Conversion
Each share of Class B common stock is convertible at any time at the option of the holder into one share of Class A common stock. Generally, shares of Class B common stock will convert automatically into Class A common stock upon the holder ceasing to be an Eligible Holder (i.e., director, employee, trust or legal entity of New Ginkgo), unless otherwise determined by affirmative vote of a majority of independent directors of New Ginkgo.
Common Stock Reserved for Future Issuances
The Company had the following shares of common stock reserved for future issuances:
| September 30, 2021 |
||||
| Stock options issued and outstanding |
29,199,113 | |||
| Restricted stock units outstanding |
254,254,142 | |||
| Shares available for grant under the 2021 Plan |
200,386,956 | |||
| Shares available for grant under the ESPP |
20,000,000 | |||
| Warrants to purchase Class A common stock |
51,824,925 | |||
| New Ginkgo Earn-out shares |
188,865,133 | |||
|
|
|
|||
| Total common stock reserved for future issuances |
744,530,269 | |||
|
|
|
|||
14. Warrant Liabilities
Upon the closing of the Business Combination, the Company assumed 34,499,925 publicly-traded warrants (“Public Warrants”) and 17,325,000 private placement warrants (the “Private Placement Warrants”) held by the
F-93
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
Sponsor. Both the Public Warrants and the Private Placement Warrants were issued in conjunction with the consummation of SRNG’s initial public offering on February 26, 2021. Each whole warrant entitles the holder to purchase one share of the Company’s Class A common stock at a price of $11.50 per share, subject to adjustments. The warrants will expire five years from the completion of the Business Combination, or earlier upon redemption or liquidation.
No Public Warrants will be exercisable for cash unless the Company has an effective and current registration statement covering the issuance of the shares of common stock issuable upon exercise of the Public Warrants. Once the Public Warrants become exercisable, the Company may redeem the outstanding Public Warrants:
| ● | in whole and not in part |
| ● | at a price of $0.01 per Public Warrant; |
| ● | upon not less than 30 days’ prior written notice of redemption to each warrant holder; and |
| ● | if, and only if, the reported closing price of the ordinary shares equals or exceeds $18.00 per share (as adjusted for share sub-divisions, share capitalizations, reorganizations, recapitalizations and the like) for any 20 trading days within a 30-trading day period ending three business days before the Company send to the notice of redemption to the warrant holders. |
If and when the warrants become redeemable by the Company, the Company may exercise its redemption right even if it is unable to register or qualify the underlying securities for sale under all applicable state securities laws. If the Company calls the Public Warrants for redemption, as described above, its management will have the option to require any holder that wishes to exercise the Public Warrants to do so on a “cashless basis,” as described in the warrant agreement. The exercise price and number of ordinary shares issuable upon exercise of the Public Warrants may be adjusted in certain circumstances including in the event of a share dividend, extraordinary dividend or recapitalization, reorganization, merger or consolidation. However, except as described above, the Public Warrants will not be adjusted for issuances of ordinary shares at a price below its exercise price. Additionally, in no event will the Company be required to net cash settle the Public Warrants.
The Private Placement Warrants are identical to the Public Warrants, except that (i) the Private Placement Warrants will be exercisable on a cashless basis and be non-redeemable so long as they are held by the initial purchasers or their permitted transferees and (ii) the Private Placement Warrants and the Class A ordinary shares issuable upon exercise of the Private Placement Warrants will be entitled to registration rights. If the Private Placement Warrants are held by someone other than the initial purchasers or their permitted transferees, the Private Placement Warrants will be redeemable by the Company and exercisable by such holders on the same basis as the Public Warrants.
As of September 30, 2021, the aggregate values of the Public Warrants and the Private Placement Warrants was $117.0 million and $96.0 million, respectively, representing warrants outstanding to purchase 34.5 million shares and 17.3 million shares, respectively, of the Company’s Class A common stock. The warrants are accounted for as liabilities in accordance with ASC 815-40 and are presented within warrant liabilities on the Condensed Consolidated Balance Sheet. The warrants are recorded as liabilities due to a provision in the warrant agreement related to certain tender or exchange offers precludes the warrants from being accounted for as components of equity. The warrant liabilities are measured at fair value at inception and on a recurring basis, with changes in fair value presented within change in fair value of warrant liabilities on the Condensed Consolidated Statement of Operations and Comprehensive Loss. See Note 5 for additional information.
15. Stock-Based Compensation
2008 Stock Incentive Plan
The 2008 Stock Incentive Plan (the “2008 Plan”) provided for the Company to grant options and restricted stock awards (“RSAs”). From and after the effective date of the 2014 Stock Incentive Plan, the Company ceased
F-94
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
granting awards under the 2008 Plan. However, the 2008 Plan continues to govern the terms and conditions of the outstanding awards previously granted thereunder. Shares of common stock underlying any awards that are forfeited, cancelled, repurchased, or otherwise terminated by the Company under the 2008 Plan will be added back to the shares available for issuance under the 2021 Incentive Award Plan.
2014 Stock Incentive Plan
The 2014 Stock Incentive Plan (the “2014 Plan”) provided for the Company to grant options, stock appreciation rights, restricted stock, restricted stock units (“RSUs”) and other stock-based awards. From and after the effective date of the 2021 Incentive Award Plan, the Company ceased granting awards under the 2014 Plan. However, the 2014 Plan continues to govern the terms and conditions of the outstanding awards previously granted thereunder. Shares of common stock underlying any awards that are forfeited, cancelled, repurchased, or otherwise terminated by the Company under the 2014 Plan will be added back to the shares available for issuance under the 2021 Incentive Award Plan.
2021 Incentive Award Plan
On September 16, 2021, the 2021 Incentive Award Plan (the “2021 Plan”) became effective. The 2021 Plan provides for the grant of stock options, including incentive stock options (“ISOs”) and nonqualified stock options, stock appreciation rights, restricted stock, dividend equivalents, RSUs and other stock or cash-based awards to employees, consultants and directors of New Ginkgo and its subsidiaries.
The aggregate number of shares of common stock available for issuance under the 2021 Plan, which may be issued as Class A common stock and/or Class B common stock, was initially 200,440,957 shares. As of September 30, 2021, 200,386,956 shares remain available for future issuance under the 2021 Plan. The number of shares of common stock reserved for issuance under the 2021 Plan will automatically increase for ten years on January 1 of each year, starting on January 1, 2022, in an amount equal to the lesser of (a) 4.0% of the aggregate number of shares of common stock outstanding on the final day of the immediately preceding calendar year and (b) such smaller number of shares as is determined by the Board. The maximum number of shares of common stock that may be issued pursuant to the exercise of incentive stock options granted under the 2021 Plan will be 200,000,000 shares.
2021 Employee Stock Purchase Plan
On September 16, 2021, the 2021 Employee Stock Purchase Plan (the “ESPP”) became effective. The ESPP authorizes (i) the grant of options that are intended to qualify for favorable U.S. federal tax treatment under Section 423 of the Internal Revenue Code of 1986 (the “Section 423 Component”) and (ii) the grant of options that are not intended to be tax-qualified (the “Non-Section 423 Component”). All of the Company’s employees are expected to be eligible to participate in the ESPP. However, with respect to the Section 423 Component, an employee may not be granted rights to purchase stock under the ESPP if the employee, immediately after the grant, would own (directly or through attribution) stock possessing 5% or more of the total combined voting power or value of all classes of the Company’s common stock.
The ESPP permits the Company to deliver up to 20,000,000 shares of common stock pursuant to awards issued under the ESPP, which may be Class A common stock and/or Class B common stock. The number of shares of common stock reserved for issuance under the ESPP will automatically increase each January 1, beginning on January 1, 2022, by an amount equal to the lesser of (a) 1% of the aggregate number of shares of common stock outstanding on the final day of the immediately preceding calendar year and (b) such smaller number of shares as
F-95
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
is determined by the Board, provided that no more than 100,000,000 shares may be issued under the Section 423 Component. Prior to or in connection with issuing any shares of common stock under the ESPP, the ESPP administrator may convert awards covering shares of Class B common stock to Class A common stock. As of September 30, 2021, no awards have been granted under the ESPP.
Stock Options
During the nine months ended September 30, 2021, the Company granted options with an aggregate fair value of $14.9 million. No stock options were granted during the nine months ended September 30, 2020.
A summary of stock option activity for the nine months ended September 30, 2021 is presented below:
| Number of Shares |
Weighted Average Exercise Price per Share |
Weighted Average Remaining Contractual Term |
Aggregate Intrinsic Value(1) |
|||||||||||||
| (in years) | (in thousands) | |||||||||||||||
| Outstanding as of December 31, 2020 |
33,354,871 | $ | 0.02 | 3.20 | $ | 386,017 | ||||||||||
| Granted |
1,664,251 | 0.50 | ||||||||||||||
| Exercised |
(4,225,189 | ) | 0.01 | |||||||||||||
| Forfeited |
(1,594,820 | ) | 0.02 | |||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Outstanding as of September 30, 2021 |
29,199,113 | 0.04 | 2.43 | 337,104 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Exercisable as of September 30, 2021 |
29,129,682 | $ | 0.02 | 2.41 | $ | 337,092 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | The aggregate intrinsic value of stock options is calculated as the difference between the exercise price of the underlying stock options and the estimated fair value of the common stock for those stock options that had exercise prices lower than the estimated fair value of the common stock as of September 30, 2021 and December 31, 2020. |
The aggregate intrinsic value of stock options exercised during the nine months ended September 30, 2021 and 2020 was $48.9 million and $15.1 million, respectively. The weighted-average fair value of options granted during the nine months ended September 30, 2021 was $8.97 per share and was calculated using the following estimated assumptions:
| Nine Months Ended September 30, 2021 |
||||
| Risk-free interest rate |
0.11 | % | ||
| Expected dividend yield |
0 | % | ||
| Expected volatility |
88.6 | % | ||
| Expected term |
0.96 years | |||
Restricted Stock Units
Under the 2014 Plan, RSUs granted to employees are subject to two vesting conditions: (i) a service-based vesting condition under which the awards vest based on continued service over a period of time, and (ii) a performance-based vesting condition whereby the awards vest based on a liquidity event in the form of either a change of control or an initial public offering, each as defined in the 2014 plan. Under the 2021 Plan, RSUs granted to employees are subject to a service-based vesting condition only.
F-96
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
A summary of the RSU activity during the nine months ended September 30, 2021 is presented below:
| Number of Shares |
Weighted Average Grant Date Fair Value |
|||||||
| Nonvested as of December 31, 2020 |
124,932,207 | $ | 1.74 | |||||
| Granted |
132,512,654 | 8.78 | ||||||
| Forfeited |
(3,190,719 | ) | 3.97 | |||||
|
|
|
|
|
|||||
| Nonvested as of September 30, 2021 |
254,254,142 | $ | 5.38 | |||||
|
|
|
|
|
|||||
The weighted average remaining contractual term for the nonvested RSUs as of September 30, 2021 was 5.62 years. The weighted average grant date fair value of RSUs granted during the nine months ended September 30, 2021 and 2020 was $8.78 and $2.68 per share, respectively.
Restricted Stock Awards
A summary of the RSA activity during the nine months ended September 30, 2021 is presented below:
| Number of Shares |
Weighted Average Grant Date Fair Value |
|||||||
| Nonvested as of December 31, 2020 |
419,049 | $ | 1.99 | |||||
| Vested |
(177,295 | ) | 1.99 | |||||
|
|
|
|
|
|||||
| Nonvested as of September 30, 2021 |
241,754 | $ | 1.99 | |||||
|
|
|
|
|
|||||
The aggregate fair value of the RSAs that vested during the nine months ended September 30, 2021 and 2020 was $0.4 million.
Stock-Based Compensation
Stock-based compensation expense was allocated as follows (in thousands):
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
| Research and development |
$ | 20 | $ | 21 | $ | 60 | $ | 66 | ||||||||
| General and administrative |
107 | 97 | 14,704 | 292 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 127 | $ | 118 | $ | 14,764 | $ | 358 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
During the nine months ended September 30, 2021 and 2020, the Company recognized $14.8 million and $0.4 million, respectively, of stock-based compensation expense related to stock options and RSAs. The Company has not recognized any stock-based compensation expense related to RSUs as of September 30, 2021 as satisfaction of the performance-based vesting condition was not deemed probable. As of September 30, 2021, total unrecognized stock-based compensation expense related to RSUs was $1,369.0 million.
F-97
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
As of September 30, 2021, total unrecognized stock-based compensation expense related to stock options was $0.5 million and is expected to be recognized over a weighted-average period of 2.46 years. As of September 30, 2021, total unrecognized stock-based compensation expense related to RSAs was $0.5 million and is expected to be recognized over a weighted-average period of 1.21 years.
16. Revenue Recognition
Disaggregation of Revenue
The following table sets forth the percentage of Foundry revenues by industry based on total Foundry revenue:
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
| Food and nutrition |
19 | % | 27 | % | 22 | % | 36 | % | ||||||||
| Industrial and environmental |
12 | % | 27 | % | 17 | % | 27 | % | ||||||||
| Agriculture |
8 | % | 20 | % | 9 | % | 14 | % | ||||||||
| Consumer and technology |
52 | % | 15 | % | 34 | % | 15 | % | ||||||||
| Other |
9 | % | 11 | % | 18 | % | 8 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total Foundry revenue |
100 | % | 100 | % | 100 | % | 100 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The following table sets forth the percentage of revenue by geographic location based on total revenue:
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
| North America |
97 | % | 91 | % | 96 | % | 93 | % | ||||||||
| Rest of world |
3 | % | 9 | % | 4 | % | 7 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
100 | % | 100 | % | 100 | % | 100 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Contract Balances
The Company recognizes a contract asset when the Company transfers goods or services to a customer before the customer pays consideration or before payment is due, excluding any amounts presented as accounts receivable. The Company had no contract asset balances as of September 30, 2021 and December 31, 2020.
Contract liabilities, or deferred revenue, primarily consist of payments received in advance of performance under the contract or when the Company has an unconditional right to consideration under the terms of the contract before it transfers goods or services to the customer. The Company’s collaborative arrangements with its investees and related parties typically include upfront payments consisting of cash or non-cash consideration for future research and development services and non-cash consideration in the form of equity securities for licenses that will be transferred in the future. The Company records the upfront cash payments and fair value of the equity securities as deferred revenue.
The Company also invoices customers based on contractual billing schedules, which results in the recording of deferred revenue to the extent payment is received prior to the Company’s performance of the related services. Contract liabilities are recognized as revenue as (or when) the Company performs under the contract.
F-98
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
During the nine months ended September 30, 2021, the Company recognized $23.7 million of revenue that was included in the contract liabilities balance of $128.5 million as of December 31, 2020. During the nine months ended September 30, 2020, the Company recognized $19.0 million of revenue that was included in the contract liabilities balance of $147.9 million as of December 31, 2019.
Performance Obligations
The aggregate amount of the transaction price that was allocated to performance obligations that have not yet been satisfied or are partially satisfied as of September 30, 2021 and December 31, 2020 was $25.3 million and $20.7 million, respectively. The Company has elected the practical expedient not to provide the remaining performance obligation disclosures related to contracts for which the Company recognizes revenue in the amount to which it has the right to invoice. As of September 30, 2021, of the performance obligations not yet satisfied or partially satisfied, nearly all is expected to be recognized as revenue during the years 2021 to 2026.
17. Significant Collaboration Transactions
Arcaea LLC (FKA Kalo Ingredients, LLC)
Summary of Arrangement
Arcaea was formed in March 2021 to focus on the application of synthetic biology in the personal care products industry. In March 2021, the Company entered into (i) an Intellectual Property Contribution Agreement (“Arcaea IP Agreement”) that granted Arcaea a license to certain of the Company’s intellectual property, (ii) a Technical Development Agreement (“Arcaea TDA”) that establishes the terms under which the Company will provide technical research and development services, and (iii) a Common Unit Issuance Agreement (“Arcaea CUIA”) which compensates the Company for its intellectual property contribution. Contemporaneous with these transactions, Arcaea entered into a Series A Preferred Unit Purchase Agreement under which it sold 1,755,000 Series A preferred units to certain of the Company’s investors, for aggregate proceeds of approximately $19.5 million. The Series A Preferred Unit Purchase Agreement provided for the sale and issuance of up to an additional 7,245,000 Series A preferred units subsequent to the initial closing. In subsequent closings through September 30, 2021, Arcaea issued an additional 5,139,900 Series A preferred units to existing and third-party investors for aggregate proceeds of approximately $57.1 million and closed its Series A preferred unit financing. As a result, the Company received an additional 5,229,900 common units in Arcaea for total consideration of $35.5 million.
Under the Arcaea IP Agreement, the Company licensed certain intellectual property to Arcaea for use in the development or the production of Arcaea’s products that the parties will subsequently agree to research and develop under technical development plans (“TDPs”). The license rights provide Arcaea with the ability to commercialize the specified products from the corresponding TDP under the Arcaea TDA. In return for the license to the intellectual property, Arcaea has agreed to issue the Company up to 9,000,000 common units in accordance with certain terms and conditions set forth within the agreements. The Company received 1,755,000 common units upon execution of the Arcaea CUIA and an additional 5,229,900 common units upon closing of the Series A preferred unit financing in July 2021 (as discussed above). No additional common units are expected to be issued to the Company.
Under the Arcaea TDA, the parties jointly agree on TDPs, through equal representation on a joint steering committee, under which the Company will perform agreed-upon research and development services in return for consideration on a cost-plus basis for all services provided.
F-99
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
Accounting Analysis
The common unit investment in Arcaea is considered an equity method investment as a result of the Company’s ability to exercise significant influence over Arcaea’s financial and operating policies through its ownership of common units. The initial carrying value of the equity method investment in Arcaea is the fair value of the common units of $11.9 million received in exchange for the Arcaea IP Agreement which, as discussed below, was accounted for as deferred revenue at inception. The fair value of Arcaea’s common units was determined at inception of the agreements using the option pricing method. The option pricing method used a back-solve methodology to infer the total equity value based on the pricing of the Series A preferred unit financing, which was contemporaneous with the Arcaea IP Agreement. Further, the Company determined the rights to up to an additional 7,245,000 common units did not meet the definition of a freestanding financial instrument and are not representative of a derivative. The right to the additional common units is considered variable consideration that is fully constrained at inception and until the contingencies related to the issuance of the additional shares are resolved.
The Series A preferred units issued by Arcaea receive a liquidation preference prior to common units. As such, the Company concluded that this represents a substantive profit-sharing arrangement, and the Company is recognizing earnings and losses on the equity method investment using the HLBV method. The Company recorded a $11.9 million loss on its equity method investment in Arcaea during the first quarter in 2021. The loss allocated to the Company primarily relates to Arcaea’s accounting for the non-cash consideration related to the Arcaea IP Agreement as in-process research and development, which resulted in the full value of the Company’s intellectual property contribution being expensed in the first quarter of 2021. As of September 30, 2021, the carrying value of the equity method investment in Arcaea has been reduced to zero. There is no commitment for the Company to provide further financial support to Arcaea, and therefore the carrying value of the equity method investment will not be reduced below zero.
The relationship with Arcaea is a vendor-customer relationship and is within the scope of ASC 606, as the provision of services and corresponding license rights are considered a part of the Company’s ordinary activities. The common units issued to the Company represent non-cash consideration. While the Arcaea TDA has been executed by the parties and provides the payments terms for future services, the Arcaea TDA does not provide for any transfer of goods or services between the parties. However, the Company will provide licenses and services upon execution of the contemplated TDPs. Accordingly, the Company concluded that the Arcaea TDA, in combination with the Arcaea CUIA, met the definition of a contract under ASC 606. Each TDP executed under the Arcaea TDA will be accounted for in accordance with ASC 606.
The Company’s performance obligations under the contract consist of ten material rights to future technical research and development services and commercial licenses under individual TDPs that the Company expects to execute under the Arcaea TDA. The material rights represent an advance payment for the license rights, which will be granted upon the execution of future TDPs. As there is no additional payment for these license rights when future TDPs are executed, the Company has determined that there is a material right associated with each of the contemplated additional TDPs under the Arcaea TDA. The Company has allocated approximately $1.2 million of the upfront non-cash consideration to each of the ten material rights based on the estimated standalone selling price of the performance obligations. During the nine months ended September 30, 2021, the additional non-cash consideration received of $35.5 million, which is representative of previously constrained variable consideration, was allocated to each of the ten performance obligations under the arrangement with Arcaea of $3.6 million each consistent with the initial relative selling price allocation. Unexercised material rights are recorded as non-current deferred revenue until such time as the parties execute a TDP conveying a commercial license.
F-100
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
Upon the execution of a TDP underlying a material right, the Company is obligated to provide technical research and development services under the TDP and a license to applicable patents and other intellectual property designed and developed under the TDP. The technical research and development services and license provided under a TDP are highly interdependent and interrelated with one another. Without the Company’s knowledge, expertise, and platform, there would not be a licensable strain or other commercializable product to transfer to Arcaea. Further, Arcaea has rights to development intellectual property created as part of each TDP, irrespective of the result of the development. Therefore, each executed TDP underlying a material right consists of one combined performance obligation for the technical research and development services and license to be provided by the Company.
For each TDP underlying a material right, the transaction price consists of variable consideration for the most likely amount of estimated consideration to be received under the cost-plus arrangement and non-cash consideration allocated to the material rights. As the services performed by the Company under a TDP create or enhance an asset that Arcaea controls as the asset is created or enhanced, the Company satisfies the performance obligation and recognizes revenue over time. The Company uses an input method that compares total costs incurred relative to total estimated cost to complete to estimate progress under the contract. Any revisions to the estimated total budgeted costs to complete, and the resulting impact to revenue recognition, are reflected in the period of the change through a cumulative catch-up adjustment.
As of September 30, 2021, the Company had a deferred revenue balance of $47.4 million with Arcaea, consisting of non-cash equity consideration received. During the three and nine months ended September 30, 2021, the Company recognized $1.3 million and $2.5 million, respectively, from services provided to Arcaea.
Allonnia, LLC
Summary of Arrangement
Allonnia was formed in 2019 and focuses on the application of synthetic biology in the bioremediation space, leveraging the Company’s proprietary platform to develop solutions to treat waste streams through degrading or metabolizing contaminants of concern and recover and upcycle valuable materials from waste. In December 2019, the Company entered into (i) an Intellectual Property Contribution Agreement (“Allonnia IP Agreement”) that granted Allonnia a license to certain of the Company’s intellectual property, (ii) a Technical Development Agreement (“Allonnia TDA”) that establishes the terms under which the Company is providing technical development services, and (iii) a Common Unit Issuance Agreement (“Allonnia CUIA”) which provides for the issuance of common units of Allonnia to the Company in exchange for the license rights granted under the Allonnia IP Agreement. Contemporaneous with these agreements, Allonnia entered into a Series A Preferred Unit Purchase Agreement under which Allonnia sold 2,970,000 Series A preferred units to certain of the Company’s investors, as well as a third-party investor. Allonnia also agreed to issue an additional 630,000 Series A preferred units to a strategic partner as compensation for the delivery of future services to Allonnia. The Series A Preferred Unit Purchase Agreement also provided for the sale and issuance of up to an additional 5,400,000 Series A preferred units subsequent to the initial closing. Through December 31, 2020, Allonnia issued an additional 1,844,911 Series A preferred units, 180,000 of which were issued in exchange for the rights to certain intellectual property which will vest based on the achievement of milestones associated with the development of the intellectual property received. During the nine months ended September 30, 2021, Allonnia closed their Series A preferred unit financing and issued 22,500 Series A preferred units to an additional third-party investor.
Under the Allonnia IP Agreement, the Company licensed intellectual property to Allonnia for use in the development or the production of its products that the parties will subsequently agree to develop under TDPs.
F-101
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
The license rights provide Allonnia with the ability to commercialize the specified products from the corresponding strain or enzyme, which can only be developed by the Company under the Allonnia TDA. The Company received 3,600,000 common units as consideration for the license upon execution of the agreement and an additional 1,867,411 common units during the nine months ended September 30, 2021 in connection with the closing of the Series A preferred unit financing.
Under the Allonnia TDA, the parties jointly agree, through equal representation on a joint steering committee, on TDPs for specific strains and enzymes, in which the Company will perform agreed upon development services in return for consideration on a cost-plus basis for all services provided.
Accounting Analysis
The common unit investment in Allonnia is considered an equity method investment as a result of the Company’s ability to exercise significant influence over Allonnia’s financial and operating policies through its ownership of common units. The initial carrying value of the equity method investment in Allonnia, which was equal to the fair value of the common units received in exchange for the Allonnia IP Agreement of $24.5 million, was subsequently reduced to zero as a result of the application of the HLBV method and was recorded as a loss on equity method investments. In the second quarter of 2021, Allonnia issued an additional 22,500 Series A preferred units and closed their Series A preferred unit financing. As a result, the Company received an additional 1,867,411 common units for total consideration of $12.7 million. The additional consideration received resulted in an increase in the Company’s equity method investment in Allonnia of $12.7 million, which the Company subsequently reduced to zero as a result of the application of the HLBV method. Accordingly, the Company recorded a loss on its equity method investment in Allonnia of $12.7 million during the nine months ended September 30, 2021. As of September 30, 2021, the carrying value of the equity method investment in Allonnia remained at zero. There is no commitment for the Company to provide further financial support to Allonnia and therefore the carrying value of the equity method investment will not be reduced below zero. Therefore, no additional loss was recognized during the nine months ended September 30, 2021.
The relationship with Allonnia is a vendor-customer relationship and is within the scope of ASC 606 whereby the Company will provide licenses and services upon execution of the TDPs as outlined under the terms of the Allonnia TDA. The Company’s performance obligations under the contract consist of a combined service and license performance obligation related to the initial TDP executed in February 2020 and nine material rights, related to the estimated additional TDPs the parties expect to execute under the Allonnia TDA. The material rights represent an advance payment for the license rights which will be granted upon the execution of each TDP. As there is no additional payment for these license rights upon execution of a TDP, the Company has determined that there is a material right associated with each of the contemplated future TDPs. The Company initially allocated $2.5 million of the upfront non-cash consideration to each of the ten performance obligations under the contract based on the estimated standalone selling price of the performance obligations. Unexercised material rights are recorded as non-current deferred revenue until such time as the parties execute a TDP.
Upon the execution of each TDP, the Company is obligated to provide development services under the TDP and a license to applicable patents and other intellectual property to the products developed under the plan. Each executed TDP consists of one combined performance obligation for the license and research and development services to be performed by the Company.
The transaction price for each TDP consists of variable consideration for the most likely amount of estimated consideration to be received under the cost-plus arrangement and the non-cash consideration allocated to the material rights. The Company recognizes revenue over time as it satisfies the respective performance obligations
F-102
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
using an input method that compares total costs incurred relative to total estimated cost to complete to estimate progress under the contract. During the nine months ended September 30, 2021, the additional non-cash consideration received of $12.7 million, which is representative of previously constrained variable consideration, was allocated to each of the ten performance obligations under the arrangement with Allonnia of $1.3 million each consistent with the initial relative selling price allocation. Additionally, a cumulative catch up in revenue was recognized for the TDPs in process.
As of September 30, 2021 and December 31, 2020, the Company had a deferred revenue balance of $38.0 million and $26.1 million, respectively, with Allonnia. During the three months ended September 30, 2021 and 2020, the Company recognized $0.9 million and $1.3 million, respectively, from services provided to Allonnia. During the nine months ended September 30, 2021 and 2020, the Company recognized $4.3 million and $2.8 million, respectively, from services provided to Allonnia.
Other Significant Collaboration Transactions
In addition to the activity discussed above related to Arcaea and Allonnia, the Company provided research and development services under existing collaboration arrangements with Joyn, Motif, Synlogic and Genomatica. During the three and nine months ended September 30, 2021, there were no material changes to the Company’s arrangements with its Platform Ventures and Structured Partnerships except as noted above. For a description of these arrangements and the related accounting conclusions, refer to Note 17 to the audited consolidated financial statements as of and for the years ended December 31, 2020 and 2019 included in the Company’s prospectus on Form 424(b)(3) as filed with the SEC on September 17, 2021. Refer to Notes 9 and 10 for additional details on the Company’s investments and Note 19 for a summary of transactions with related parties.
18. Net Loss per Share
As a result of the Business Combination, the Company has retroactively restated the weighted average shares outstanding prior to September 16, 2021 to give effect to the Exchange Ratio.
The Company computes net loss per share of the Class A common stock and Class B common stock using the two-class method required for participating securities. Basic and diluted loss per share was the same for each period presented as the inclusion of all potential Class A common stock and Class B common stock equivalents would have been antidilutive. The earnings per share amounts are the same for the different classes of common stock because the holders of each class are legally entitled to equal per share distributions whether through dividends or liquidation.
The following potential common shares, presented based on amounts outstanding at each period end, were excluded from the calculation of diluted net loss per share attributable to Ginkgo Bioworks Holdings, Inc. common stockholders for the periods presented because including them would have been anti-dilutive:
| As of September 30, | ||||||||
| 2021 | 2020 | |||||||
| Warrants to purchase Class A common stock |
51,824,925 | 1,013,708 | ||||||
| Outstanding stock options |
29,199,113 | 33,975,444 | ||||||
| Unvested RSUs |
254,254,142 | 125,586,745 | ||||||
| Unvested RSAs |
241,754 | 478,240 | ||||||
| New Ginkgo and Sponsor earn-out shares |
205,602,316 | — | ||||||
|
|
|
|
|
|||||
| 541,122,250 | 161,054,137 | |||||||
|
|
|
|
|
|||||
F-103
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
19. Related Parties
The Company’s significant transactions with its related parties are primarily comprised of revenue generating activities under collaboration and license agreements.
Significant related party transactions included in the Condensed Consolidated Balance Sheets are summarized below (in thousands):
| September 30, | December 31, | |||||||
| 2021 | 2020 | |||||||
| Accounts receivable, net: |
||||||||
| Joyn |
$ | 1 | $ | — | ||||
| Motif |
3,750 | 2,403 | ||||||
| Genomatica |
— | 1,500 | ||||||
| Allonnia |
971 | 1,309 | ||||||
| Synlogic |
— | — | ||||||
| Arcaea |
1,328 | — | ||||||
|
|
|
|
|
|||||
| $ | 6,050 | $ | 5,212 | |||||
|
|
|
|
|
|||||
| Deferred revenue, current and non-current: |
||||||||
| Joyn |
$ | 5,595 | $ | 9,862 | ||||
| Motif |
53,184 | 53,952 | ||||||
| Genomatica |
20,499 | 30,128 | ||||||
| Allonnia |
38,016 | 26,064 | ||||||
| Synlogic |
60 | 72 | ||||||
| Arcaea |
47,356 | — | ||||||
| Other equity investee |
10,000 | — | ||||||
|
|
|
|
|
|||||
| $ | 174,710 | $ | 120,078 | |||||
|
|
|
|
|
|||||
Significant related party transactions included in the Condensed Consolidated Statements of Operations and Comprehensive Loss are summarized below (in thousands):
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
| Foundry revenue: |
||||||||||||||||
| Joyn |
$ | 1,515 | $ | 2,151 | $ | 4,267 | $ | 5,733 | ||||||||
| Motif |
6,099 | 3,034 | 16,203 | 15,036 | ||||||||||||
| Genomatica |
3,279 | 748 | 9,480 | 6,169 | ||||||||||||
| Allonnia |
913 | 1,322 | 4,277 | 2,784 | ||||||||||||
| Synlogic |
2 | 15 | 12 | 62 | ||||||||||||
| Arcaea |
1,316 | — | 2,507 | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| $ | 13,124 | $ | 7,270 | $ | 36,746 | $ | 29,784 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
In September 2021, prior to the closing of the Business Combination, the Company repurchased a total of 2,707,280 common shares from the Company’s founders at a price of $9.23 per share, which was then the most-recent fair value per share based on a third-party valuation, for a total purchase price of $25.0 million. Upon
F-104
Ginkgo Bioworks Holdings, Inc. and Subsidiaries
Notes to Condensed Consolidated Financial Statements
(unaudited)
repurchase, the shares were immediately retired and were recorded as a reduction to common stock and additional paid-in capital in the Condensed Consolidated Balances Sheet.
Refer to Notes 9 and 17 for additional details on the Company’s investments and equity method investments held in its related parties.
20. Subsequent Events
On October 1, 2021, Viking Global Opportunities Illiquid Investments Sub-Master LP (“Viking”), who owns 339,055,114 shares of Class A common stock of the Company, signed an agreement to exchange a portion of its shares of Class A common stock for an equal number of shares of Class C common stock. On November 13, 2021, the Board approved the exchange and Viking will surrender to the Company, at no cost, the number of shares of Class A common stock to be determined within 5 business days after the date on which the stockholder receives written notice that the board has approved the exchange.
On November 17, 2021 the Board of Directors modified the vesting terms of RSUs granted under the Company’s equity incentive plans to allow 10% of the RSUs that were vested as of the closing of the Business Combination with respect to the service condition (the “10% RSUs”) to immediately and automatically accelerate and vest with respect to the performance condition, effective as of November 19, 2021, the date on which the Form S-8 registration statement covering such shares became effective. The Company will cash settle the 10% RSUs. The remaining RSUs will accelerate and vest in full with respect to the performance condition on or before March 15, 2022. Additionally, on November 17, 2021, the Board of Directors approved 100% of the earnout shares, received by the equity holders of Old Ginkgo pursuant to the Merger Agreement, to automatically accelerate and vest with respect to the performance condition, effective as of November 19, 2021, the date on which the Form S-8 registration statement covering such shares became effective. Additionally, on November 15, 2021, 25% of the earnout shares became vested with respect the first market condition of $12.50 per share, as the trading price of the Company’s Class A common stock equaled or exceeded $12.50 per share for at least 20 trading days within the 30 day trading period ending November 15, 2021. As a result of the modification of the 10% RSUs and the earnout shares, as well as achievement of the $12.50 per share market condition, the Company will record a significant amount of equity-based compensation expense under ASC 718, Stock Compensation, in the fourth quarter of 2021 and the first quarter of 2022.
F-105
To the Board of Directors of Allonnia, LLC:
Report on the Financial Statements
We have audited the accompanying consolidated financial statements of Allonnia, LLC (the “Company”), which comprise the consolidated balance sheets as of December 31, 2020 and 2019 and the related statements of operations, changes in members’ equity and cash flows for the year ended December 31, 2020 and the period from November 27, 2019 (inception) through December 31, 2019, and the related notes to the consolidated financial statements.
Management’s Responsibility for the Financial Statements
Management is responsible for the preparation and fair presentation of these consolidated financial statements in accordance with accounting principles generally accepted in the United States of America; this includes the design, implementation, and maintenance of internal control relevant to the preparation and fair presentation of financial statements that are free from material misstatement, whether due to fraud or error.
Auditors’ Responsibility
Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the financial statements. The procedures selected depend on the auditor’s judgment, including the assessment of the risks of material misstatement of the financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity’s preparation and fair presentation of the financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. Accordingly, we express no such opinion. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluating the overall presentation of the financial statements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Allonnia, LLC as of December 31, 2020 and 2019, and the results of its operations and its cash flows for the periods then ended in accordance with accounting principles generally accepted in the United States of America.
/s/ Wolf & Company, P.C.
Boston, Massachusetts
June 23, 2021
F-106
Allonnia, LLC
December 31, 2020 and 2019
| Assets | ||||||||
| 2020 | 2019 | |||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 43,520,543 | $ | 32,828,673 | ||||
| Prepaid expenses and other current assets |
3,015,817 | 3,384 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 46,536,360 | $ | 32,832,057 | ||||
|
|
|
|
|
|||||
| Liabilities and Members’ Equity | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 728,580 | $ | 23,706 | ||||
| Accrued expenses |
901,784 | 117,848 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
1,630,364 | 141,554 | ||||||
| Members’ equity |
44,905,996 | 32,690,503 | ||||||
|
|
|
|
|
|||||
| Total liabilities and members’ equity |
$ | 46,536,360 | $ | 32,832,057 | ||||
|
|
|
|
|
|||||
See accompanying notes to the consolidated financial statements.
F-107
Allonnia, LLC
Consolidated Statements of Operations
For the Year Ended December 31, 2020 and Period from November 27, 2019 (Inception) Through December 31, 2019
| 2020 | 2019 | |||||||
| Operating expenses: |
||||||||
| Research and development |
$ | 3,966,936 | $ | 24,481,519 | ||||
| General and administrative |
2,106,245 | 162,978 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
6,073,181 | 24,644,497 | ||||||
|
|
|
|
|
|||||
| Net operating loss |
(6,073,181 | ) | (24,644,497 | ) | ||||
| Interest income |
61,663 | — | ||||||
|
|
|
|
|
|||||
| Net loss |
$ | (6,011,518 | ) | $ | (24,644,497 | ) | ||
|
|
|
|
|
|||||
See accompanying notes to the consolidated financial statements.
F-108
Allonnia, LLC
Consolidated Statements of Changes in Members’ Equity
For the Year Ended December 31, 2020 and Period from November 27, 2019 (Inception) Through December 31, 2019
| Units | Members’ Equity |
|||||||||||||||||||||||
| Series A-1 Preferred |
Series A-2 Preferred |
Series A-3 Preferred |
Common | Incentive | ||||||||||||||||||||
| Balance at November 27, 2019 (Inception) |
— | — | — | — | — | — | ||||||||||||||||||
| Issuance of Common Units |
— | — | — | 3,600,000 | — | 24,480,000 | ||||||||||||||||||
| Issuance of Series A-1 Preferred Units net of issuance costs of $145,000 |
2,970,000 | — | — | — | — | 32,855,000 | ||||||||||||||||||
| Net loss |
— | — | — | — | — | (24,644,497 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2019 |
2,970,000 | — | — | 3,600,000 | — | 32,690,503 | ||||||||||||||||||
| Issuance of Series A-1 Preferred Units net of issuance costs of $272,000 |
1,664,911 | — | — | — | — | 18,227,011 | ||||||||||||||||||
| Issuance of Series A-2 Preferred Units |
— | 180,000 | — | — | — | — | ||||||||||||||||||
| Issuance of Series A-3 Preferred Units |
— | — | 180,000 | — | — | — | ||||||||||||||||||
| Issuance of Incentive Units |
— | — | — | — | 140,000 | — | ||||||||||||||||||
| Net loss |
— | — | — | — | — | (6,011,518 | ) | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance as of December 31, 2020 |
4,634,911 | 180,000 | 180,000 | 3,600,000 | 140,000 | $ | 44,905,996 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
See accompanying notes to the consolidated financial statements.
F-109
Allonnia, LLC
Consolidated Statements of Cash Flows
For the Year Ended December 31, 2020 and Period from November 27, 2019 (Inception) Through December 31, 2019
| 2020 | 2019 | |||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | (6,011,518 | ) | $ | (24,644,497 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Fair value common stock issued for acquiring intellectual property |
— | 24,480,000 | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Prepaid expenses and other current assets |
(3,012,433 | ) | (3,384 | ) | ||||
| Accounts payable |
704,874 | 23,706 | ||||||
| Accrued expenses |
783,936 | 117,848 | ||||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(7,535,141 | ) | (26,327 | ) | ||||
|
|
|
|
|
|||||
| Cash flows from financing activities: |
||||||||
| Proceeds from issuance of Series A-1 Preferred Units, net of issuance costs |
18,227,011 | 32,855,000 | ||||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
18,227,011 | 32,855,000 | ||||||
|
|
|
|
|
|||||
| Increase in cash and cash equivalents |
10,691,870 | 32,828,673 | ||||||
| Cash and cash equivalents, beginning of period |
32,828,673 | — | ||||||
|
|
|
|
|
|||||
| Cash and cash equivalents, end of period |
$ | 43,520,543 | $ | 32,828,673 | ||||
|
|
|
|
|
|||||
See accompanying notes to the consolidated financial statements.
F-110
Allonnia, LLC
Notes to Consolidated Financial Statements
For the Year Ended December 31, 2020 and Period from November 27, 2019 (Inception) Through December 31, 2019
| 1. | NATURE OF OPERATIONS |
Business
Allonnia, LLC (“Allonnia”) commenced activities in November 2019 and is formed under the laws of the State of Delaware. Allonnia is a bioremediation company that uses advanced technology and biology to engineer breakthrough systems to develop and commercialize novel waste remediation and management solutions. Allonnia works to identify microbes capable of breaking down waste, then uses the tools of synthetic biology to amplify their abilities both to clean up toxic pollution and bind to valuable materials in the waste stream for reuse.
Allonnia designs and deploys at-scale transformative innovations in water and wastewater treatment, soil redemption, and solid waste management and upcycling. By creating the next generation of enzymes, proteins, and microbes that degrade or metabolize contaminants of concern, Allonnia can recover and upcycle valuable materials from waste and augment existing biological treatment processes.
Since its inception, Allonnia has devoted its efforts to business planning, research and development, recruiting management and technical staff, and raising capital. Allonnia is subject to risks similar to other companies in their industry including rapid technological change, uncertainty of market acceptance of the product, competition from larger companies, and dependence of key personnel and strategic partners.
Allonnia Management Pool Co., LLC, a wholly-owned subsidiary of Allonnia, was formed on December 9, 2020 under the laws of the State of Delaware to facilitate Allonnia’s administration of grants of incentive units to officers, employees and consultants of Allonnia.
Principles of Consolidation
The accompanying consolidated financial statements include the operations of Allonnia and its wholly-owned subsidiary Allonnia Management Pool Co., LLC (collectively referred to as the “Company”). All intercompany accounts and transactions have been eliminated in consolidation.
| 2. | SIGNIFICANT ACCOUNTING POLICIES |
A summary of the significant accounting policies followed by the Company in the preparation of the consolidated financial statements is as follows:
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates and changes in estimates may occur.
Cash and Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less at the date of purchase to be cash equivalents. The Company maintains its cash in bank deposit accounts which, at times, may exceed the federal insurance limit.
F-111
Allonnia, LLC
Notes to Consolidated Financial Statements (Continued)
| 2. | SIGNIFICANT ACCOUNTING POLICIES (continued) |
Research and Development Expenses
Costs incurred for research and development are expensed as incurred. Research and development expenses primarily consist of salaries and related expenses for personnel, outside consulting services and sponsored research and the costs of materials and supplies used.
Income Taxes
The Company is taxed as a partnership. As such, the results of operations are included in the income tax returns of its members. Accordingly, no provision for income taxes has been recorded in these financial statements.
The Company follows accounting guidance regarding the recognition, measurement, presentation, and disclosure of uncertain tax positions in the financial statements. Tax positions taken or expected to be taken, including the position that the Company qualifies as a pass-through entity, are required to be evaluated to determine whether the tax positions are “more-likely-than-not” to be upheld under regulatory review. The resulting tax impact of these tax positions are recognized in the financial statements based on the result of this evaluation. There are no such provisions for uncertain tax positions as of December 31, 2020 and 2019. The Company is subject to federal and state tax examination by tax authorities for all years since inception.
The Company records interest and penalties, if any, as part of other income (expense). No interest or penalties were recorded for the periods ended December 31, 2020 and 2019.
Unit-based Compensation
The Company accounts for unit-based awards in accordance with the Financial Accounting Standards Board (FASB) ASC 718, Compensation – Stock Compensation. Unit-based compensation expense for all incentive unit awards made to employees, officers and other key individuals is measured based on the grant-date fair value of the award. Unit-based compensation expense for awards granted to non-employees is determined using the fair value of the consideration received or the fair value of the unit-based award issued, whichever is more reliably measured.
The Company uses the Black-Scholes option pricing model to determine the fair value of awards granted. The Company recognizes the compensation cost of unit-based awards on a straight-line basis over the vesting period of the award.
The determination of the fair value of unit-based payment awards utilizing the Black-Scholes model is affected by the unit price and a number of assumptions, including expected volatility, expected life, risk-free interest rate and expected dividends. The Company does not have a history of market prices of its Common Units as it is not a public company, and as such, volatility is estimated using historical volatilities of similar public entities. The expected life of the awards is estimated based on the requisite service period. The risk-free interest rate assumption is based on observed interest rates appropriate for the terms of the awards. The dividend yield assumption is based on the history and expectation of paying no dividends. The Company recognizes forfeitures related to unit-based payments when they occur. Forfeited options are recorded as a reduction to unit-based compensation expense.
F-112
Allonnia, LLC
Notes to Consolidated Financial Statements (Continued)
| 2. | SIGNIFICANT ACCOUNTING POLICIES (concluded) |
Unit-based Compensation (concluded)
There were no incentive unit awards granted in 2019. For the year ended December 31, 2020, the Company calculated the fair value of incentive unit awards using the following assumptions:
| Risk-free interest rate | 1.67 | % | ||
| Expected dividend yield | 0.00 | % | ||
| Volatility factor | 70.00 | % | ||
| Expected life | 3 years |
| 3. | LICENSE, RESEARCH AND DEVELOPMENT AND CONSULTING AGREEMENTS |
Ginkgo BioWorks, Inc.
On December 18, 2019, the Company and Ginkgo BioWorks, Inc. (“Ginkgo”) entered into a) an intellectual property contribution agreement (“IPCA”), b) a Common Unit Issuance Agreement (“CUIA”), c) a G&A Services Agreement for general and administrative services (“G&A Services Agreement”), and d) a Technical Development Agreement (“TDA”), collectively referred to as the Ginkgo Agreements.
Pursuant to the IPCA, Ginkgo licensed and contributed certain intellectual property to the Company in exchange for up to 9,000,000 Common Units of the Company, as specified in CUIA (see Note 4). The Company issued 3,600,000 Common Units as consideration for the license upon execution of the CUIA, the fair value of which was determined to be $24,480,000 at inception of the agreements using the option pricing method. The Company accounted for the non-cash consideration related to the CUIA as research and development expense in the period ended December 31, 2019.
Pursuant to the G&A Services Agreement, Ginkgo will perform its obligations as an independent contractor and the Company will pay Ginkgo for services at the fully burdened full time equivalent cost, plus pass-through costs, materials and services.
Pursuant to the TDA, Ginkgo will provide the Company with technical services and grant the Company access to the Ginkgo Foundry for the Company’s development of its products. The Company will pay Ginkgo an amount covering (i) all direct and indirect costs, as defined, incurred by Ginkgo in connection with the technical services provided to the Company and (ii) a 5% arm’s length mark-up on total costs consisting of (a) third party costs, (b) direct costs and (c) the G&A Estimate (see Note 5). Pursuant to the TDA, the parties jointly agree, through equal representation on a joint steering committee, on technical development plans (“TDPs”) for specific strains and enzymes, in which Ginkgo will perform agreed upon development services in return for consideration on a cost-plus basis, as noted above, for all services provided. As of December 31, 2020, the Company has entered into three TDPs with Ginkgo.
Battelle Memorial Institute
On October 1, 2020, the Company and Battelle Memorial Institute (“Battelle”) entered into a research and development consulting service agreement (“Battelle Agreement”). In consideration of the 180,000 Series A-2 Preferred Units issued to Battelle pursuant to the Series A-2 Preferred Unit Subscription Agreement (see Note 4), Battelle will perform $2,000,000 worth of technical and consulting services to the Company through agreed-upon task orders within a period of three years from the date of the Battelle Agreement. The value of any conforming services and deliverables in the task orders provided by Battelle will be deducted from the $2,000,000 noted above. The Company will record a unit-based research and
F-113
Allonnia, LLC
Notes to Consolidated Financial Statements (Continued)
LICENSE, RESEARCH AND DEVELOPMENT AND CONSULTING AGREEMENTS (concluded)
Battelle Memorial Institute (concluded)
development expense in the statement of operations for the fair value of the conforming services and deliverables as they are being received and the related amount in members’ equity. No research and development services have been provided as of December 31, 2020.
Each party in the Battelle Agreement retains the entire rights of the intellectual property in existence prior to this agreement or developed independently of this agreement (“Background IP”). Battelle irrevocably assigns to the Company all rights of intellectual property developed in the course of providing consulting services to the Company (“Program Technology”). In addition, if Battelle incorporates its Background IP into a deliverable or Program Technology, Battelle grants to the Company a perpetual, non-exclusive, sublicensable, (through multiple tiers), royalty-free, worldwide license to such incorporated Background IP to allow lawful use of the Program Technology or deliverable that incorporates it.
Metabolik Technologies Inc.
On December 18, 2020, the Company and Metabolik Technologies Inc. (“Metabolik”) entered into an asset purchase agreement (“Metabolik Agreement”). Pursuant to this agreement, Metabolik will transfer certain intellectual property in exchange for potential royalty payments from Allonnia in the amounts of a single digit percentage of Operating Profit, as defined in this agreement. The Company’s royalty obligation begins once the cumulative aggregate Operating Profit is greater than zero and ends fifteen years thereafter, provided that the royalties will terminate in the event that Metabolik or its affiliates ceases to hold any equity interest in the Company, other than as a result of a change of control of the Company. At any time during the term of this agreement, the Company may, upon written notice to Metabolik, provide written notice of the Company’s interest in buying out its royalty obligations under this agreement.
On December 18, 2020, the Company issued in accordance with the Series A-3 Preferred Unit Issuance Agreement 180,000 Series A-3 Preferred Units (see Note 4). In exchange, Metabolik will transfer certain acquired assets, as defined, pursuant to the terms of Metabolik Agreement.
| 4. | MEMBERS’ EQUITY |
Series A Preferred Units
As of December 31, 2020, the Company was authorized to issue 9,000,000 of Series A Preferred Units, of which 8,190,000 units were designated as Series A-1 Preferred Units (“Series A-1”), 630,000 were designated Series A-2 Preferred Units (“Series A-2”), and 180,000 were designated Series A-3 Preferred Units (“Series A-3”) (collectively, referred to as “Series A Preferred Units”). The original issue price of Series A Preferred Units is $11.111111 per unit.
Pursuant to the Series A-1 Unit Purchase Agreement, as amended, the Company issued 2,970,000 and 1,664,911 units of Series A-1 at a purchase price of $11.111111 per unit in 2019 and 2020, respectively, for the total gross proceeds of $51,499,011.
On December 18, 2019, Battelle entered into the Series A-2 Preferred Unit Subscription Agreement with the Company. In accordance with this agreement, Battelle would be issued up to 630,000 Series A-2 units upon achievement of the following milestones:
| a) | 180,000 units of Series A-2 will be issued upon execution by Battelle and the Company of a consulting agreement within 12 months following the date of this agreement, pursuant to |
F-114
Allonnia, LLC
Notes to Consolidated Financial Statements (Continued)
MEMBERS’ EQUITY (continued)
Series A Preferred Units (concluded)
| which Battelle would provide $2,000,000 worth of technical and consulting services (see Note 3). |
| b) | Upon achieving the milestone (a) above, the Company will issue additional 180,000 units of Series A-2 in 30,000 increments on each of the following dates so long as the Company has not determined that Battelle did not provide the Company with services or that the services were not satisfactory pursuant to the Battelle Agreement: June 18, 2021; December 18, 2021; June 18, 2022; December 18, 2022; June 18, 2023; and December 18, 2023. |
| c) | Upon achieving the milestone (a) above, the Company will issue additional 270,000 units of Series A-2 in 45,000 increments on the following dates so long as the Company has not determined that Battelle did not provide the Company with services or that the services were not satisfactory pursuant to the Battelle Agreement: June 18, 2024; December 18, 2024; June 18, 2025; December 18, 2025; June 18, 2026; and December 18, 2026. Notwithstanding the foregoing, on August 18, 2021 Battelle may elect to purchase all 270,000 units of Series A-2 in exchanges for a price per unit of $11.111111 and a total purchase price of $3,000,000 in lieu of the issuances of such units described above so long as Battelle has achieved milestone (a) above and the Battelle Agreement remains in effect. |
On December 18, 2020, Metabolik entered into the Series A-3 Preferred Unit Issuance Agreement with the Company. In accordance with this agreement, Metabolik was issued 180,000 Series A-3 units that become “eligible” capital accounts upon achievement of the following milestones:
| a) | 45,000 units of Series A-3 will be treated as “eligible” if the Company enters into a joint development agreement (“JDA”) with a certain party within one year following the date on which Ginkgo delivers an intermediate strain to the Company pursuant to the Technical Development Plan between Ginkgo and the Company. Notwithstanding the foregoing, if the Company determines, in its reasonable discretion, within thirty days following the execution of the JDA, that the JDA was not substantially related to the contributed assets, as defined, or certain services pursuant to the Metabolik Agreement (see Note 3) then such 45,000 units of Series A-3 will instead be automatically forfeited for no consideration. |
| b) | 135,000 units of Series A-3 will be treated as “eligible” in the event that the Company enters into a certain commercialization agreement, as defined in the Metabolik Agreement, and receives revenue under the commercialization agreement in excess of $10,000,000 in the aggregate within five years following the execution of the commercialization agreement. |
The Company will monitor milestones on an ongoing basis, and will record a unit-based research and development expense and a related amount to members’ equity as these milestones become probable of being achieved. No unit-based research and development expenses have been recognized in 2020 in connection with the issuance of Series A-3 units.
Common Units
As of December 31, 2020, the Company was authorized to issue 9,000,000 Common Units. As specified in the CUIA, the authorized Common Units will be issued to Ginkgo as follows:
| a) | 3,600,000 Common Units in partial consideration for the license and transfer of intellectual property in accordance with IPCA |
F-115
Allonnia, LLC
Notes to Consolidated Financial Statements (Continued)
MEMBERS’ EQUITY (continued)
Common Units (concluded)
| b) | 5,400,000 Common Units in partial consideration for the additional obligations set forth in the IPCA and TDA (as defined in the IPCA); provided that Ginkgo will not receive such additional Common Units unless the Company has sold and issued at least 8,370,000 Series A-1 Preferred Units pursuant to a Series A-1 Preferred Unit Purchase Agreement. |
| c) | In the event the Company sells and issues less than 8,370,000 but more than 2,970,000 Series A-1 Preferred Units, the Company and Ginkgo will collaborate to determine how many additional Common Units will be issued. |
As of December 31, 2020 and 2019, there were 3,600,000 of Common Units issued and outstanding.
The rights and preferences of the Series A Preferred Units and Common units, collectively, Capital Units are as follows:
Voting Rights
Any action to be taken by the members will be taken by the members holding a majority of the capital units then outstanding, voting together as a single class.
Distributions
In the event of a Liquidation Event, as defined, distributions will have the following priority: (i) first, to the holders of Series A-1, in proportion to their respective number of Series A-1 Preferred Units, until the Company has distributed a cumulative amount in respect of each Series A-1 Preferred Unit; (ii) second, to the holders of Series A-2 Preferred Units and eligible Series A-3 Preferred Units, in proportion to their respective number of Series A-2 Preferred Units and eligible Series A-3 Preferred Units, until the Company has distributed a cumulative amount in respect of each Series A-2 Preferred Unit and eligible Series A-3 Preferred Unit; (iii) third, to the holders of Common Units, in proportion to their respective number of Common Units, until the Company has distributed a cumulative amount in respect of each Common Unit equal to the Series A preference amount.
After payment in full of the amounts above, any remaining amount is distributed to the holders of Series A-1, Series A-2, eligible Series A-3, Common Units, and Incentive Units, on a per unit pro rata basis; provided, however, that no distributions will be paid with respect to any Incentive Unit until the aggregate amount of all distributions from and after the date of issuance of such Incentive Unit exceeds the applicable Threshold Amount associated with such Incentive Unit.
Distributions
The holders of the Series A-3 are not entitled to any distributions with respect to any Series A-3 Preferred Units other than eligible Series A-3 Preferred Units.
Incentive Units
As of December 31, 2020, the Company was authorized to issue up to 2,000,000 Incentive Units. Incentive Units and the rights and privileges associated with them, collectively, are intended to be “profits interests”. Incentive Units may be issued within a series, with each series having a separate Threshold Amount. The Threshold Amount will initially mean the amount equal to the amount that would be received on all
F-116
Allonnia, LLC
Notes to Consolidated Financial Statements (Continued)
MEMBERS’ EQUITY (concluded)
Incentive Units (concluded)
outstanding Units if, immediately prior to the issuance of an Incentive Unit, all the assets of the Company were sold for their respective fair value (as determined by the Board in good faith), the liabilities of the Company were paid in full, and the remaining proceeds were distributed. An Incentive Unit’s Threshold Amount will subsequently be increased in an amount equal to the aggregate amount of any Capital Contribution made to the Company after the issuance of such Incentive Unit. Incentive Units are interests solely in profits and will have Capital Accounts associated therewith at the time of their issuance of zero dollars.
The Incentive Units do not have any voting rights and are subject to vesting.
Allocations of profits will be made with respect to any Incentive Units that are Unvested Units in the same manner as if they were Vested Units. Any distributions made with respect to any Incentive Units that are Unvested Units will not be distributed and instead be recorded by the Company (such amount, an “Unvested Distribution Amount”) in the Company’s books and records until such Unvested Units vest and become Vested Units. Any Unvested Distribution Amounts that relate to Incentive Units that are Unvested Units that are forfeited or fail to vest for whatever reason will be allocated and distributed as a new distribution.
In December 2020, there were 140,000 Incentive Units granted to employees of the Company. These units vest over a period of four years. The 2020 unit-based compensation expense in connection with these units was immaterial.
There were no Incentive Units granted in 2019.
| 5. | RELATED PARTY TRANSACTIONS |
The Company receives various services from companies that own either Common Units (Ginkgo) or Series A-1 Preferred Units (Battelle and Metabolik) in the Company.
In 2020 and 2019, the Company paid Ginkgo $4,164,292 and $28,519, respectively, pursuant to the TDA and G&A Service Agreement (see Note 3).
| 6. | SUBSEQUENT EVENTS |
Management has evaluated subsequent events through June 23, 2021, which is the date the consolidated financial statements were available to be issued.
In January 2021, the Company issued 1,867,411 of Common Units to Ginkgo, pursuant to IPCA (see Note 3) and 22,500 Series A-1 Units to an investor for $250,000.
Other than the above, there were no subsequent events that require adjustments to or disclosure in the consolidated financial statements.
F-117