Table of Contents
Filed Pursuant to Rule 424(b)(5)
Registration No. 333-266875
The information in this preliminary prospectus supplement is not complete and may be changed. This preliminary prospectus supplement and the accompanying prospectus are not an offer to sell the securities and are not soliciting an offer to buy the securities in any jurisdiction where the offer or sale is not permitted.
Subject to Completion, dated January 24, 2024
Preliminary Prospectus Supplement
(To Prospectus dated August 25, 2022)

Shares of Common Stock
We are offering shares of our common stock to be sold in the offering. Our common stock is listed on The Nasdaq Global Select Market under the symbol “TRML.” On January 23, 2024, the last reported sale price of our common stock as reported on The Nasdaq Global Select Market was $38.46 per share.
We are an “emerging growth company” under applicable Securities and Exchange Commission rules and will be subject to reduced public company reporting requirements for this prospectus supplement and future filings.
Investing in our common stock involves a high degree of risk. See “Risk Factors” on page S-9 of this prospectus supplement and in the documents incorporated by reference into this prospectus supplement before investing in our common stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||||
| Proceeds, before expenses, to us |
$ | $ | ||||||
| (1) | See the section titled “Underwriting” for additional information regarding underwriter compensation and estimated offering expenses. |
We have granted the underwriters an option for a period of 30 days from the date of this prospectus supplement, to purchase, from time to time, in whole or in part, up to additional shares of our common stock. If the underwriters exercise the option in full, the total underwriting discounts and commissions payable by us will be $ , and the total proceeds to us, before expenses, will be $ .
The underwriters expect to deliver the shares to purchasers on or about , 2024.
Joint Book-Running Managers
| Jefferies | Piper Sandler | Guggenheim Securities | Truist Securities | |||
Prospectus Supplement dated , 2024.
Table of Contents
PROSPECTUS SUPPLEMENT
| Page | ||||
| S-iii | ||||
| S-1 | ||||
| S-7 | ||||
| S-9 | ||||
| S-11 | ||||
| S-12 | ||||
| S-13 | ||||
| S-14 | ||||
| S-16 | ||||
| S-18 | ||||
| CERTAIN MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS FOR NON-U.S. HOLDERS OF COMMON STOCK |
S-23 | |||
| S-27 | ||||
| S-36 | ||||
| S-36 | ||||
| S-36 | ||||
| S-37 | ||||
PROSPECTUS
| i | ||||
| 1 | ||||
| 2 | ||||
| 4 | ||||
| 6 | ||||
| 7 | ||||
| 8 | ||||
| 14 | ||||
| 21 | ||||
| 22 | ||||
| 25 | ||||
| 28 | ||||
| 28 | ||||
| 28 | ||||
| 29 | ||||
We and the underwriters have not authorized anyone to provide you with information different than that contained in or incorporated by reference in this prospectus supplement, the accompanying prospectus and in any free writing prospectus that we have authorized for use in connection with this offering. We and the underwriters take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. We and the underwriters are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume
S-i
Table of Contents
that the information appearing in this prospectus supplement, the accompanying prospectus, the documents incorporated by reference in this prospectus supplement and the accompanying prospectus, and in any free writing prospectus that we have authorized for use in connection with this offering, is accurate only as of the date of those respective documents. Our business, financial condition, results of operations and prospects may have changed since those dates. You should read this prospectus supplement, the accompanying prospectus, the documents incorporated by reference in this prospectus supplement and the accompanying prospectus, and any free writing prospectus that we have authorized for use in connection with this offering, in their entirety before making an investment decision. You should also read and consider the information in the documents to which we have referred you in the sections of this prospectus supplement entitled “Where You Can Find More Information” and “Incorporation of Certain Information by Reference.”
We are not, and the underwriters are not, making an offer to sell our common stock in any jurisdiction where the offer or sale is not permitted. We have not, and the underwriters have not, done anything that would permit this offering or possession or distribution of this prospectus supplement, the accompanying prospectus or any free writing prospectus we have authorized for use in connection with this offering in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of common stock and the distribution of this prospectus outside the United States.
S-ii
Table of Contents
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying prospectus are part of a “shelf” registration statement on Form S-3 (File No. 333-266875) that we filed with the Securities and Exchange Commission (the “SEC”) on August 15, 2022 and which became effective on August 25, 2022. The prospectus supplement describes the specific terms of this offering and also adds to and updates the information contained in the accompanying prospectus and the documents incorporated by reference into this prospectus supplement and the accompanying prospectus. The accompanying prospectus gives more general information, some of which may not apply to this offering. This prospectus supplement may add, update or change information contained in the accompanying prospectus. To the extent that any statement we make in this prospectus supplement is inconsistent with statements made in the accompanying prospectus, or any documents incorporated by reference, the statements made in this prospectus supplement will be deemed to modify or supersede those made in the accompanying prospectus, including the documents incorporated by reference therein. Information in any document we subsequently file that is incorporated by reference herein shall modify or supersede the information in this prospectus supplement, the accompanying prospectus and documents incorporated by reference prior to such subsequent filing. Generally, when we refer to the prospectus, we are referring to this prospectus supplement and the accompanying prospectus combined.
We have not authorized anyone to provide you with information different than or inconsistent with the information contained in or incorporated by reference in this prospectus supplement, the accompanying prospectus and in any free writing prospectus that we have authorized for use in connection with this offering. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. You should assume that the information appearing in this prospectus supplement, the accompanying prospectus, the documents incorporated by reference in this prospectus supplement and the accompanying prospectus, and in any free writing prospectus that we have authorized for use in connection with this offering, is accurate only as of the date of those respective documents, regardless of the time of delivery of those respective documents. Our business, financial condition, results of operations and prospects may have changed since those dates. You should read this prospectus supplement, the accompanying prospectus, the documents incorporated by reference in this prospectus supplement and the accompanying prospectus, and any free writing prospectus that we have authorized for use in connection with this offering, in their entirety before making an investment decision. You should also read and consider the information in the documents to which we have referred you in the sections of this prospectus supplement entitled “Where You Can Find Additional Information” and “Incorporation of Certain Information by Reference.”
We are offering to sell, and seeking offers to buy, our securities only in jurisdictions where offers and sales are permitted. The distribution of this prospectus supplement and the accompanying prospectus and the offering of our securities in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement and the accompanying prospectus must inform themselves about, and observe any restrictions relating to, the offering of our securities and the distribution of this prospectus supplement and the accompanying prospectus outside the United States. This prospectus supplement and the accompanying prospectus do not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus supplement and the accompanying prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
Unless the context requires otherwise, references in this prospectus to the “Company,” “Tourmaline,” “we,” “us” and “our” refer to Tourmaline Bio, Inc. (formerly known as Talaris Therapeutics, Inc.) and its consolidated subsidiaries.
This prospectus supplement, the accompanying prospectus and the information incorporated herein and therein by reference may include trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated by reference into this prospectus supplement or the accompanying prospectus are the property of their respective owners.
S-iii
Table of Contents
This summary highlights selected information contained elsewhere or incorporated by reference in this prospectus supplement and the accompanying prospectus. This summary does not contain all the information you should consider before investing in our common stock. You should read and consider carefully the more detailed information in this prospectus supplement and the accompanying prospectus, including the factors described under the heading “Risk Factors” in this prospectus supplement and the financial and other information incorporated by reference in this prospectus supplement and the accompanying prospectus, as well as the information included in any free writing prospectus that we have authorized for use in connection with this offering, before making an investment decision.
Company Overview
We are a late-stage clinical biotechnology company developing transformative medicines to dramatically improve the lives of patients with life-altering immune and inflammatory diseases. In doing so, we seek to identify and develop medicines that have the potential to establish new standards-of-care in areas of high unmet medical need.
Our initial product candidate is TOUR006, a fully human monoclonal antibody that selectively binds to interleukin-6 (“IL-6”), a key proinflammatory cytokine involved in the pathogenesis of many autoimmune and inflammatory disorders. The anti-IL-6 and anti-IL-6 receptor (“IL-6R”) antibody class (“IL-6 class”) has over two decades of clinical and commercial experience treating over a million patients with a variety of autoimmune and inflammatory diseases. To date, four anti-IL-6 or anti-IL-6R antibodies have been approved in the United States. These four anti-IL-6 or anti-IL-6R antibodies together generated more than $3.5 billion in global sales in 2022.
TOUR006 is a long-acting anti-IL-6 antibody which we believe has best-in-class properties including a high binding affinity to IL-6, long half-life, and low observed immunogenicity. These characteristics may allow TOUR006 to achieve substantial IL-6 pathway suppression with relatively low amounts of drug exposure, potentially enabling delivery in a convenient, low volume, infrequently administered, subcutaneous injection.
We are pursuing two strategic paths for TOUR006, the first of which we refer to as “FcRn+”. Neonatal Fc receptor (“FcRn”) inhibitors have emerged as a novel therapeutic class to treat autoantibody-driven diseases. However, FcRn inhibitors have significant limitations including suboptimal efficacy, lack of durable efficacy, high burden dosing profile, and an unknown long-term safety profile. We believe TOUR006 has the potential to be a superior therapy for a wide range of autoantibody-driven diseases, compared to FcRn inhibitors. We have identified thyroid eye disease (“TED”) as our beachhead indication for our FcRn+ strategy. TED is an autoimmune disease characterized by autoantibody-mediated activation of the tissues surrounding the eye, causing inflammation and disfigurement which can be sight-threatening in severe cases. We have identified a substantial body of published clinical observations characterizing the beneficial off-label use of currently marketed IL-6 pathway inhibitors, namely Actemra® (tocilizumab), an anti-IL-6R monoclonal antibody, in reducing inflammation, eye-bulging, and levels of autoantibodies in patients with TED. However, no formal, industry-sponsored development effort studying the IL-6 class for the treatment of TED has been completed to date.
We are currently evaluating TOUR006 in a pivotal Phase 2b trial in first-line TED, which we refer to as the spiriTED trial. We initiated the spiriTED trial in September 2023 and expect to report topline data in the first half of 2025. Further, we expect to commence a pivotal Phase 3 trial of TOUR006 in first-line TED in 2024, with topline data expected in 2026. This second pivotal trial will replace the previously-planned open-label basket study in additional TED patient cohorts.
S-1
Table of Contents
Our second strategic path is cardiovascular inflammation. We believe TOUR006 has the potential to transform the care of high-risk patients by targeting key inflammatory pathways driving cardiovascular disease. Atherosclerotic cardiovascular disease (“ASCVD”) is a leading cause of death globally. Preventing major adverse cardiovascular events (“MACE”), such as death, nonfatal myocardial infarction or nonfatal stroke, has the potential to significantly reduce global cardiovascular disease burden. IL-6 has been identified as a promising drug target for addressing the risk of MACE in ASCVD and multiple external Phase 3 cardiovascular outcome trials investigating IL-6 blockade are ongoing. We believe that TOUR006 potentially offers a meaningfully enhanced product profile to these competitor programs with a potential for subcutaneous dosing once every three months. As previously announced in January 2024, we have reached alignment with the U.S. Food and Drug Administration (“FDA”) on the ASCVD clinical development program, including a Phase 2 trial evaluating the reduction of C-reactive protein (“CRP”), a validated biomarker for inflammation, with quarterly dosing of TOUR006 in patients with elevated cardiovascular risk. This trial is targeted to commence in the first half of 2024, and we expect to report topline data in the first half of 2025. Pending successful initiation and completion, positive results from the Phase 2 trial are expected to position us to be ready in 2025 to commence a pivotal Phase 3 trial for TOUR006 in cardiovascular disease.
Our Pipeline
The following figure summarizes our current development programs:
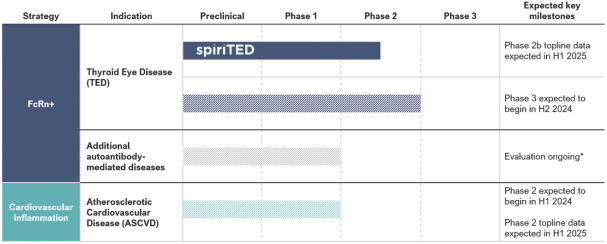
Note: Hatched bars represent trials that have not yet commenced. The timing of regulatory submissions and clinical trial milestones are subject to change and additional discussion with the FDA
*Clinical development plan for additional indications subject to change upon indication selection and discussion with the FDA
As can be seen in the chart above, we plan to identify additional indication opportunities for TOUR006. In addition, we continue to evaluate new in-licensing and acquisition opportunities for assets that we believe have standard-of-care changing potential for patients with immune, inflammatory and other diseases.
Our Strategy
We seek to identify and develop transformative medicines that have the potential to establish new standards-of-care in areas of high unmet medical need. We plan to apply a human data-focused approach to
S-2
Table of Contents
indication selection, identifying diseases where IL-6 pathway inhibitors have been used successfully in practice despite limited formal industry development and where we believe TOUR006 can potentially bring significant improvements over existing standards of care. We also plan to leverage insights from clinical trials of competitor IL-6 pathway inhibitor programs with a goal of rapidly bringing TOUR006 into indications that have already been externally de-risked. We believe this focus on leveraging existing human data could allow us identify indications with high potential for clinical and commercial success and can maximize the value of TOUR006.
The key elements of our strategy include:
| • | In our FcRn+ strategy, advance TOUR006 through clinical development in patients with TED as our beachhead indication in autoantibody-driven diseases. Our initial product candidate, TOUR006, has the potential for a differentiated product profile for the treatment of TED based on the literature supporting IL-6 pathway inhibition in active TED, a favorable long-term safety profile of the IL-6 class observed to date, and the potentially low administrative burden offered by infrequent, subcutaneous dosing. In September 2023, we initiated our pivotal Phase 2b spiriTED trial to assess the safety and efficacy of TOUR006 for the treatment of TED, and we expect to report topline results from this trial in the first half of 2025. Further, we expect to commence a pivotal Phase 3 trial for TOUR006 in TED in 2024. Topline data from this planned Phase 3 trial are expected in 2026. |
| • | In our cardiovascular inflammation strategy, advance TOUR006 through clinical development in patients with ASCVD. We believe that TOUR006 has the potential to provide a differentiated product profile for the treatment of inflammatory risk in ASCVD with the potential for subcutaneous dosing once every three months. We plan to initiate a Phase 2 clinical trial to assess the safety, pharmacokinetics (“PK”), and pharmacodynamics (“PD”) of TOUR006 for the treatment of ASCVD in the first half of 2024, with topline data expected in the first half of 2025. |
| • | Maximize the potential of TOUR006 in additional indications where IL-6 inhibition has shown compelling evidence of clinical benefit. We believe that TOUR006 has broad application beyond TED and ASCVD. We aim to identify and develop in additional indications where IL-6 inhibition has shown evidence of clinical benefit, but has not entered industry-led clinical development, as well as indications where we could bring TOUR006 forward, capitalizing on external de-risking events. |
| • | Explore business development opportunities to selectively expand our product portfolio. We continue to evaluate new in-licensing and acquisition opportunities for assets that we believe have standard-of-care changing potential for patients with immune, inflammatory and other diseases. We also plan to strategically evaluate potential collaborations with external parties to maximize the potential of TOUR006. |
Summary of Selected Risks Associated with this Offering and Our Business
Our business is subject to numerous risks and uncertainties, including those highlighted in the section titled “Risk Factors” below in this prospectus supplement and those described under similar headings in the documents incorporated by reference into this prospectus supplement. These risks include:
| • | If you purchase the common stock sold in this offering, you will experience immediate and substantial dilution in your investment. You will experience further dilution if we issue additional equity securities in the future. |
| • | Our management will have broad discretion as to the use of the net proceeds from the shares of common stock we are selling in this offering, and we may not use these proceeds effectively. |
| • | We have incurred net losses every year since our inception and have no source of product revenue. We expect to continue to incur significant operating losses and may never become profitable. |
S-3
Table of Contents
| • | Our business is highly dependent on the success of TOUR006 as well as any other potential future product candidates. If we are unable to successfully complete clinical development of, obtain regulatory approval for, or commercialize, TOUR006 or any other potential future product candidates, or if we experience delays in doing so, our business will be materially harmed. |
| • | We will need significant additional capital to proceed with development and commercialization of TOUR006 and any potential future product candidates and our other operations. We may not be able to access sufficient capital on acceptable terms, if at all, and, as a result, we may be required to delay, scale back or discontinue development of such product candidates or other operations. |
| • | We have a limited operating history and no history of commercializing products, which may make it difficult for an investor to evaluate the success of our business to date and to assess our future viability. |
| • | We will incur additional costs and increased demands upon management as a result of complying with the laws and regulations appliable to public companies. |
| • | We may not be able to obtain and maintain the relationships with third parties that are necessary to develop, commercialize and manufacture TOUR006 and any potential future product candidates. |
| • | We rely completely on contract development and manufacturing organization (“CDMOs”) for the manufacture and testing of TOUR006 and any potential future product candidates under current good manufacturing practices, and we are subject to many manufacturing risks, any of which could substantially increase our costs and limit supply of any potential product candidates and any future products. |
| • | Our manufacturing and testing of bulk drug substance for TOUR006 currently takes place in China through a global CDMO with facilities in China and around the world. A significant disruption in the operation of the manufacturing facility in China, a trade war or political unrest could materially adversely affect our business, financial condition and results of operations. |
| • | We may seek to establish business development arrangements, and, if we are not able to establish them on commercially reasonable terms, or at all, we may have to alter our development and commercialization plans. |
| • | TOUR006 and any other of our future product candidates must undergo rigorous clinical trials before seeking regulatory approvals, and clinical trials may be delayed, suspended or terminated at any time for many reasons, any of which could delay or prevent regulatory approval and, if approval is granted, commercialization of our product candidates. |
| • | If clinical trials of TOUR006 or any potential future product candidates fail to timely initiate, enroll, complete, or produce positive results or fail to demonstrate safety and efficacy to the satisfaction of the FDA or comparable health authorities or sufficiently to demonstrate differentiation from other approved therapies or therapies in development, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of our product candidates. |
| • | If we experience delays or difficulties in the enrollment of patients in clinical trials, development of TOUR006, or any potential future product candidates, may be delayed or prevented, which would have a material adverse effect on our business. |
| • | Even if we obtain approval to market TOUR006 or other potential future product candidates, these products may become subject to unfavorable pricing regulations, reimbursement practices from third-party payors or healthcare reform initiatives in the U.S. and abroad, which could harm our business. |
| • | We expect to expand our clinical development, manufacturing and regulatory capabilities and potentially implement sales, marketing and distribution capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations. |
S-4
Table of Contents
| • | Healthcare reform may negatively impact our ability to profitably sell TOUR006 and any potential future product candidates, if approved. |
| • | Our international operations may expose us to business, regulatory, political, operational, financial, pricing and reimbursement risks associated with doing business outside of the U.S. |
| • | Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop. |
| • | Our relationships with healthcare providers, customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse, transparency and other healthcare laws and regulations, which, if violated, could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, administrative burdens and diminished profits and future earnings. |
| • | Our business could be materially and adversely affected in the future by the effects of disease outbreaks, epidemics and pandemics. |
| • | Our ability to use our U.S. net operating loss carryforwards and certain other U.S. tax attributes may be limited. |
| • | We have identified material weaknesses in our internal control over financial reporting. If we are unable to remediate these material weaknesses, or if we identify additional material weaknesses in the future or otherwise fail to maintain effective internal control over financial reporting, we may not be able to accurately or timely report our financial condition or results of operations, which may adversely affect our business. |
| • | Our internal control over financial reporting may not meet the standards required by Section 404 of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), and failure to achieve and maintain effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act, could have a material adverse effect on our business and share price. |
Preliminary Financial Information for the Year Ended December 31, 2023
We have not finalized our financial statements as of and for the year ended December 31, 2023. Based on our current estimates, we expect to report that we had approximately $203.0 million in total unrestricted cash and investments as of December 31, 2023. The actual amounts that we report will be subject to our financial closing procedures and any final adjustments that may be made prior to the time our financial results for the year ended December 31, 2023 are finalized and filed with the SEC. The preliminary financial data included herein has been prepared by, and is the responsibility of, our management. Our independent registered public accounting firm has not audited, reviewed, compiled, or applied agreed-upon procedures with respect to the preliminary financial data and, accordingly, does not express an opinion or any other form of assurance with respect thereto. This estimate should not be viewed as a substitute for financial statements prepared in accordance with accounting principles generally accepted in the United States and it is not necessarily indicative of the results to be achieved in any future period. Accordingly, you should not place undue reliance on this preliminary estimate. We assume no duty to update this preliminary estimate except as required by law.
Corporate Information
Prior to October 19, 2023, we were a clinical-stage biopharmaceutical company known as Talaris Therapeutics, Inc. (“Talaris”) that had historically been focused on applying allogenic hematopoietic stem cell transplantation to solid organ transplantation, autoimmune diseases and certain severe blood, immune and metabolic disorders. Talaris was originally incorporated in the State of Delaware in February 2002.
S-5
Table of Contents
On October 19, 2023, we completed our business combination with Tourmaline Bio, Inc. (“Legacy Tourmaline”) in accordance with the terms and conditions of the Agreement and Plan of Merger, dated as of June 22, 2023 (the “Merger Agreement”), by and among Talaris, Legacy Tourmaline and Terrain Merger Sub, Inc., a direct wholly owned subsidiary of Talaris (“Merger Sub”), pursuant to which Merger Sub merged with and into Legacy Tourmaline, with Legacy Tourmaline surviving as a direct wholly owned subsidiary of Talaris and the surviving corporation of the merger (the “Merger”). On October 19, 2023, in connection with and prior to the completion of the Merger, Talaris effected a 1-for-10 reverse stock split of its common stock (the “Reverse Stock Split”). Upon the completion of the Merger on October 19, 2023, Legacy Tourmaline changed its name from “Tourmaline Bio, Inc.” to “Tourmaline Sub, Inc.”, and Talaris changed its name from “Talaris Therapeutics, Inc.” to “Tourmaline Bio, Inc.”
Following the completion of the Merger, we began conducting the business conducted by Legacy Tourmaline, which is a late-stage clinical biotechnology company developing transformative medicines to dramatically improve the lives of patients with life-altering immune and inflammatory diseases. Our common stock began trading on The Nasdaq Global Select Market under the symbol “TRML” on October 20, 2023.
Our principal executive office is located at 27 West 24th Street, Suite 702, New York, New York 10010. Our telephone number is (646) 481-9832. Our website address is www.tourmalinebio.com. The information contained in, or that can be accessed through, our website is not incorporated by reference herein and is not part of this prospectus supplement.
Implications of Being an Emerging Growth Company and Smaller Reporting Company
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). We may take advantage of certain exemptions from various public company reporting requirements, including not being required to have our internal control over financial reporting audited by our independent registered public accounting firm under Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and any golden parachute payments. We may take advantage of these exemptions until December 31, 2026 or until we are no longer an “emerging growth company,” whichever is earlier. We will cease to be an emerging growth company prior to the end of such period if certain earlier events occur, including if we become a “large accelerated filer” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), our annual gross revenues exceed $1.235 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have elected to use this extended transition period under the JOBS Act until the earlier of the date we (i) are no longer an emerging growth company or (ii) affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act.
We are also a “smaller reporting company” as defined in the Exchange Act. We may continue to be a smaller reporting company even after we are no longer an emerging growth company, which would allow us to take advantage of many of the same exemptions available to emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation. We will be able to take advantage of the scaled disclosures available to smaller reporting companies for so long as our voting and non-voting common stock held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter, or our annual revenue is less than $100.0 million during the most recently completed fiscal year and our voting and non-voting common stock held by non-affiliates is less than $700.0 million measured on the last business day of our second fiscal quarter.
S-6
Table of Contents
| Common stock offered by us |
shares of our common stock. |
| Common stock to be outstanding immediately after this offering |
shares (or shares if the underwriters exercise their option to purchase additional shares in full). |
| Option to purchase additional shares |
We have granted the underwriters an option to purchase up to an additional shares of our common stock. This option is exercisable, in whole or in part, for a period of 30 days from the date of this prospectus supplement. |
| Use of proceeds |
We estimate that the net proceeds we will receive from this offering will be approximately $ million (or approximately $ million if the underwriters exercise their option to purchase additional shares of our common stock in full), after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
| We currently intend to use the net proceeds of this offering, together with our existing cash and cash equivalents, to fund continued clinical development of pipeline products, as well as for working capital and general corporate purposes. See section entitled “Use of Proceeds” on page S-12 of this prospectus supplement. |
| Risk factors |
Investing in our common stock involves a high degree of risk. See section entitled “Risk Factors” on page S-9 of this prospectus supplement and in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2023 and in the other documents which are incorporated by reference in this prospectus supplement in their entirety. |
| Nasdaq Global Select Market symbol |
“TRML.” |
The number of shares of our common stock to be outstanding immediately after this offering is based on 20,337,571 shares outstanding as of December 31, 2023, and excludes:
| • | 2,445,698 shares of our common stock issuable upon the exercise of options outstanding as of December 31, 2023, at a weighted-average exercise price of $9.29 per share; |
| • | 19,113 shares of our common stock issuable upon the settlement of restricted stock units outstanding as of December 31, 2023; |
| • | 971,444 shares of our common stock reserved for future issuance pursuant to our 2023 Equity Incentive Plan (the “2023 Plan”), as of December 31, 2023, as well as any shares reserved pursuant to provisions in our 2023 Plan that automatically increase the number of shares of common stock reserved for issuance under the 2023 Plan; and |
| • | 203,367 shares of our common stock reserved for issuance under our 2023 Employee Stock Purchase Plan (the “ESPP”), as of December 31, 2023, as well as any shares reserved pursuant to provisions in the ESPP that automatically increase the number of shares of common stock reserved for issuance under the ESPP. |
S-7
Table of Contents
In addition, the number of shares of our common stock to be outstanding immediately after this offering as shown above does not include the up to $75.0 million of shares issuable as of December 31, 2023 pursuant to our Sales Agreement with Leerink Partners LLC (formerly known as SVB Securities LLC) (the “Sales Agreement”). As of December 31, 2023, no shares of common stock have been sold pursuant to the Sales Agreement.
Except as otherwise indicated, all share and per share amounts included in this prospectus supplement reflect the issuance of shares of common stock in the Merger and the Reverse Stock Split. In addition, except as otherwise indicated, all information in this prospectus supplement assumes no exercise of the outstanding options referred to above, and assumes no exercise by the underwriters of their option to purchase additional shares.
S-8
Table of Contents
Investing in our common stock involves a high degree of risk. Our business, prospects, financial condition or operating results could be materially adversely affected by the risks identified below and in the documents incorporated by reference in this prospectus supplement, as well as other risks not currently known to us or that we currently consider immaterial. The trading price of our common stock could decline due to any of these risks, and you may lose all or part of your investment. In assessing the risks described below, you should also refer to the information, including under the heading “Risk Factors,” contained in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2023, and in the other documents which are incorporated by reference in this prospectus supplement in their entirety.
Risks Related to this Offering
Management will have broad discretion as to the use of the net proceeds from the shares of common stock we are selling in this offering, and we may not use these proceeds effectively.
Our management will have broad discretion in the application of the net proceeds from the shares of common stock we are selling in this offering and could spend these proceeds in ways that do not improve our results of operations or enhance the value of our common stock. Our failure to apply these funds effectively could have a material adverse effect on our business, delay the development of our product candidates, and cause the price of our common stock to decline.
If you purchase the common stock sold in this offering, you will experience immediate and substantial dilution in your investment. You will experience further dilution if we issue additional equity securities in the future.
Since the price per share of our common stock being offered is substantially higher than the net tangible book value per share of our common stock, you will suffer substantial dilution as described below. Based on the public offering price of $ per share, and our pro forma as adjusted net tangible book value as of September 30, 2023, after giving effect to this offering, if you purchase shares of common stock in this offering, you will suffer immediate and substantial dilution of $ per share with respect to the pro forma as adjusted net tangible book value per share of the common stock. See the section of this prospectus supplement entitled “Dilution” for a more detailed discussion of the dilution you will incur if you purchase common stock in this offering.
To the extent that outstanding stock options have been or may be exercised, investors purchasing our common stock in this offering may experience further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations or other factors even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders or result in downward pressure on the price of our common stock.
A sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. If our stockholders sell, or the market perceives that our stockholders intend to sell, substantial amounts of our common stock in the public market, the market price of our common stock could decline significantly.
We cannot predict what effect, if any, sales of our shares in the public market or the availability of shares for sale will have on the market price of our common stock. However, future sales of substantial amounts of our common stock in the public market, including shares issued upon exercise of outstanding options or warrants, or
the perception that such sales may occur, could adversely affect the market price of our common stock.
S-9
Table of Contents
In addition, Jefferies LLC and Piper Sandler & Co. may, in their sole discretion, release all or some portion of the shares subject to the lock-up agreements associated with this offering at any time and for any reason. In addition, in connection with the Merger, certain securityholders of Talaris and Legacy Tourmaline, collectively holding approximately 99,742,166 shares of common stock, entered into lock-up agreements with Talaris. Such shares will become available for sale in the public market on April 16, 2024, 180 days after the closing of the Merger, as a result of the expiration of the lock-up agreements. Sales of a substantial number of such shares upon expiration of the lock-up and market stand-off agreements, the perception that such sales may occur, or early release of these agreements, could cause our market price to fall or make it more difficult for you to sell your common stock at a time and price that you deem appropriate.
S-10
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Statements contained in this prospectus supplement, the accompanying prospectus, the documents incorporated by reference herein and therein, or any free writing prospectus that we authorized that are not strictly historical in nature are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). These forward-looking statements are subject to the “safe harbor” created by Section 27A of the Securities Act and Section 21E of the Exchange Act and may include, but are not limited to, statements related to future events or to our future operating or financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. All statements other than statements of historical facts contained in this prospectus supplement, the accompanying prospectus, and the documents incorporated by reference herein and therein, including statements regarding our strategy, future financial condition, future operations, research and development, planned clinical trials and the expected timing for the data from these clinical trials, the timing and likelihood of regulatory filings and approvals for our product candidate and any potential future product candidates, our ability to commercialize our product candidate and any potential future product candidates, the potential benefits of collaborations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements.
In some cases, you can identify forward-looking statements by terminology such as “aim,” “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “design,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “positioned,” “potential,” “predict,” “seek,” “should,” “target,” “will,” “would” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These statements reflect our views as of the date on which they were made with respect to future events and are based on assumptions and subject to risks and uncertainties. The underlying information and expectations are likely to change over time. In addition, statements that “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date the statement is made, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and you are cautioned not to unduly rely upon these statements.
Given these uncertainties, you should not place undue reliance on these forward-looking statements as actual events or results may differ materially from those projected in the forward-looking statements due to various factors, including, but not limited to, those set forth under the heading “Risk Factors” in this prospectus supplement, the accompanying prospectus, and the documents incorporated by reference herein and therein, or any free writing prospectus that we authorized. These forward-looking statements represent our estimates and assumptions only as of the date of the document containing the applicable statement. Our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements contained in this prospectus supplement, the accompanying prospectus, and the documents incorporated by reference herein and therein by these cautionary statements. Unless required by law, we undertake no obligation to update or revise any forward-looking statements to reflect new information or future events or developments. Thus, you should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. Before deciding to purchase our common stock, you should carefully consider the risk factors discussed herein or incorporated by reference, in addition to the other information set forth in this prospectus supplement, the accompanying prospectus, and the documents incorporated by reference herein and therein. or any free writing prospectus that we authorized.
S-11
Table of Contents
We estimate that the net proceeds we will receive from this offering will be approximately $ million (or approximately $ million if the underwriters exercise their option to purchase additional shares of our common stock in full), after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
We currently intend to use the net proceeds of this offering, together with our existing cash and cash equivalents, to fund continued clinical development of pipeline products, as well as for working capital and general corporate purposes. We may also use a portion of the remaining net proceeds to in-license, acquire or invest in complementary businesses, technologies, products or assets. However, we have no current commitments or obligations to do so.
The amounts and timing of these expenditures will depend on a number of factors, such as the timing and progress of our research and development efforts and the competitive environment for our product candidate. We may temporarily invest the net proceeds in a variety of capital preservation instruments, including short-term, investment-grade, interest-bearing instruments and U.S. government securities, or may hold such proceeds as cash, until they are used for their stated purpose. As of the date of this prospectus supplement, we cannot specify with certainty all of the particular uses of the net proceeds from this offering. Accordingly, we will retain broad discretion over the use of such proceeds.
S-12
Table of Contents
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. We do not intend to pay cash dividends on our common stock for the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors and will depend upon, among other factors, our results of operations, financial condition, capital requirements, contractual restrictions, business prospects and other factors our board of directors may deem relevant.
S-13
Table of Contents
The following table sets forth our cash, cash equivalents, and marketable securities and our capitalization as of September 30, 2023:
| • | on an actual basis, which gives effect to the Reverse Stock Split; |
| • | on a pro forma basis to give effect to the Merger (including the Pre-Merger Financing Transaction (as defined below)) and the Special Dividend (as defined below); and |
| • | on a pro forma as adjusted basis to give further effect to the issuance and sale of shares of our common stock in this offering at a public offering price of $ per share of common stock, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. |
On October 19, 2023, we completed the Merger with Legacy Tourmaline. In connection with the Merger, we declared a special cash dividend to our stockholders on October 6, 2023 (the “Special Dividend”). The Special Dividend was $15.118 per share of our common stock, payable in cash. The ex-dividend date in respect to the Special Dividend was before market open on October 20, 2023, and only pre-Merger stockholders of record as of October 16, 2023, the record date for the Special Dividend, that continued to hold their eligible shares until market open on October 20, 2023 were entitled to the dividend payment. The aggregate amount of the Special Dividend was $64.7 million (representing $67.5 million less $2.8 million paid to certain pre-Merger holders of Talaris stock options, stock appreciation rights and restricted stock units as described below) and was contingent upon closing of the Merger.
Subject to the terms of the Merger Agreement, immediately prior to effective time of the Merger, (a) all unexpired and unvested Talaris stock options, stock appreciation rights, and restricted stock units were accelerated, (b) all outstanding Talaris restricted stock units and all outstanding and in-the-money Talaris stock options and stock appreciation rights were settled for consideration equal to (i) 55% of each award’s value in shares of Talaris common stock and (ii) 45% of each award’s value in cash, and (c) all out-of-the-money outstanding Talaris stock options and stock appreciation rights were cancelled for no consideration. As a result, Talaris issued 176,803 shares of Talaris common stock and paid an aggregate amount of $2.8 million to certain pre-Merger holders of Talaris stock options, stock appreciation rights and restricted stock units.
Subject to the terms of the Merger Agreement, immediately prior to the effective time of the Merger, (a) certain new and current investors of Legacy Tourmaline purchased an aggregate of $75.0 million of common stock of Legacy Tourmaline (the “Pre-Merger Financing Transaction”) and (b) each share of Legacy Tourmaline preferred stock was converted into one share of Legacy Tourmaline common stock. At the effective time of the Merger, we issued an aggregate of approximately 15,877,090 shares of our common stock to Legacy Tourmaline stockholders, based on an exchange ratio of 0.07977 (as effected by the Reverse Stock Split) shares of our common stock for each share of Legacy Tourmaline common stock outstanding immediately prior to the Merger, including those shares of common stock issued upon the conversion of Legacy Tourmaline preferred stock and those shares of Legacy Tourmaline common stock issued in the Pre-Merger Financing Transaction.
S-14
Table of Contents
This table should be read together with our consolidated financial statements and related notes and the other financial information included or incorporated by reference in this prospectus supplement and the accompanying prospectus.
| As of September 30, 2023 | ||||||||||||
| Actual | Pro Forma(1) |
Pro Forma As Adjusted |
||||||||||
| (In thousands, except share and per share data) |
||||||||||||
| Cash, cash equivalents and marketable securities(2) |
$ | 147,024 | $ | 219,433 | $ | |||||||
|
|
|
|
|
|
|
|||||||
| Stockholders’ equity: |
||||||||||||
| Common stock, $0.0001 par value per share, 140,000,000 shares authorized and 10,000,000 non-voting shares authorized, actual, pro forma and pro forma as adjusted; 4,282,848 shares issued and outstanding, actual; 20,336,741 shares issued and outstanding, pro forma; shares issued and outstanding, pro forma as adjusted |
— | 2 | ||||||||||
| Additional paid-in capital |
351,980 | 273,199 | ||||||||||
| Accumulated deficit |
(208,991 | ) | (58,904 | ) | ||||||||
| Accumulated other comprehensive loss |
(115 | ) | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total stockholders’ equity |
142,874 | 214,297 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | 289,898 | $ | 433,730 | $ | |||||||
|
|
|
|
|
|
|
|||||||
| (1) | Pro forma to give effect to the Merger and associated transactions, including the Pre-Merger Financing Transaction and the Reverse Stock Split. See Exhibit 99.3 to our Current Report on Form 8-K that we filed with the SEC on January 24, 2024 for details on the calculations of pro forma amounts. Legacy Tourmaline was determined to be the accounting acquirer. |
| (2) | Includes $79.9 million of marketable securities as of September 30, 2023. |
The number of shares of our common stock to be outstanding after this offering is based on an aggregate of 20,336,741 shares of our common stock outstanding as of September 30, 2023, pro forma, and excludes:
| • | 1,403,408 shares of our common stock issuable upon the exercise of options outstanding as of September 30, 2023, at a weighted-average exercise price of $8.36 per share; |
| • | 2,033,677 shares of our common stock reserved for future issuance pursuant to the 2023 Plan as of September 30, 2023, as well as any shares reserved pursuant to provisions in our 2023 Plan that automatically increase the number of shares of common stock reserved for issuance under the 2023 Plan; and |
| • | 203,367 shares of our common stock reserved for issuance under the ESPP as of September 30, 2023, as well as any shares reserved pursuant to provisions in the ESPP that automatically increase the number of shares of common stock reserved for issuance under the ESPP. |
In addition, the number of shares of our common stock to be outstanding immediately after this offering as shown above does not include the up to $75.0 million of shares issuable as of December 31, 2023 pursuant to the Sales Agreement. As of December 31, 2023, no shares of common stock have been sold pursuant to the Sales Agreement.
Except as otherwise indicated, all information in this prospectus supplement assumes no exercise of the outstanding options referred to above, and assumes no exercise by the underwriters of their option to purchase additional shares.
S-15
Table of Contents
Investors purchasing shares of our common stock in this offering will experience immediate and substantial dilution in the pro forma net tangible book value of their shares of common stock. Dilution in net tangible book value represents the difference between the public offering price per share and the adjusted net tangible book value per share of our common stock immediately after this offering.
The historical net tangible book value as of September 30, 2023 was $142.9 million, or $33.36 per share. Historical net tangible book value per share of our common stock represents our total tangible assets (total assets less intangible assets) less total liabilities, divided by the number of shares of common stock outstanding as of that date.
The pro forma net tangible book value as of September 30, 2023 was $214.3 million, or $10.54 per share. Pro forma net tangible book value per share of our common stock represents our total tangible assets (total assets less intangible assets) less total liabilities, after giving effect to the Merger (including the Pre-Merger Financing Transaction) and the Special Dividend, divided by the number of shares of common stock outstanding as of that date, after giving effect to the Merger, Pre-Merger Financing Transaction and Reverse Stock Split.
After giving effect to the issuance of shares of common stock by us at the public offering price of $ per share, after deducting underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of September 30, 2023 would have been approximately $ million, or $ per share of common stock. This represents an immediate increase in pro forma net tangible book value of $ per share to existing stockholders and an immediate dilution of $ per share to new investors purchasing our common stock in this offering.
The following table illustrates this dilution on a per share basis to new investors, assuming the underwriters do not exercise their option to purchase additional shares of our common stock:
| Public offering price per share |
$ | |||||||
| Historical net tangible book value per share as of September 30, 2023 |
$ | 33.36 | ||||||
|
|
|
|||||||
| Pro forma net tangible book value per share as of September 30, 2023 |
$ | 10.54 | ||||||
| Increase in pro forma net tangible book value per share attributable to new investors purchasing shares in this offering |
$ | |||||||
|
|
|
|||||||
| Pro forma as adjusted net tangible book value per share after giving effect to this offering |
$ | |||||||
|
|
|
|||||||
| Dilution per share to new investors participating in this offering |
$ | |||||||
|
|
|
If the underwriters exercise their option in full to purchase an additional shares of common stock from us in this offering, the pro forma as adjusted net tangible book value per share after the offering would be $ per share, the increase in the pro forma as adjusted net tangible book value per share to existing stockholders would be $ per share and the dilution to new investors purchasing our common stock in this offering would be $ per share.
Except as noted otherwise, the above discussion and table are based on an aggregate of 20,336,741 shares of our common stock outstanding as of September 30, 2023, pro forma, and excludes:
| • | 1,403,408 shares of our common stock issuable upon the exercise of options outstanding as of September 30, 2023, at a weighted-average exercise price of $8.36 per share; |
S-16
Table of Contents
| • | 2,033,677 shares of our common stock reserved for future issuance pursuant to the 2023 Plan as of September 30, 2023, as well as any shares reserved pursuant to provisions in our 2023 Plan that automatically increase the number of shares of common stock reserved for issuance under the 2023 Plan; and |
| • | 203,367 shares of our common stock reserved for issuance under the ESPP as of September 30, 2023, as well as any shares reserved pursuant to provisions in the ESPP that automatically increase the number of shares of common stock reserved for issuance under the ESPP. |
In addition, the number of shares of our common stock to be outstanding immediately after this offering as shown above does not include the up to $75.0 million of shares issuable as of December 31, 2023 pursuant to the Sales Agreement. As of December 31, 2023, no shares of common stock have been sold pursuant to the Sales Agreement.
Except as otherwise indicated, all information in this prospectus supplement assumes no exercise of the outstanding options referred to above, and assumes no exercise by the underwriters of their option to purchase additional shares.
In addition, we may choose to raise additional capital due to market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans. To the extent that we raise additional capital by issuing equity securities or convertible debt, your ownership will be further diluted.
To the extent that existing options are exercised, new options or other equity awards are issued under the 2023 Plan or shares of common stock are issued under the ESPP in the future, there will be further dilution to investors participating in this offering.
S-17
Table of Contents
The following description of our capital stock is intended as a summary only and therefore is not a complete description of our capital stock. This description is based upon, and is qualified by reference to, our certificate of incorporation, our bylaws, and applicable provisions of Delaware corporate law. You should read our certificate of incorporation and our bylaws, in each case, as amended and supplemented, which are filed as exhibits to our Quarterly Report for the quarter ended September 30, 2023, incorporated by reference into this prospectus supplement, for the provisions that are important to you.
General
Our authorized capital stock consists of 140,000,000 shares of common stock, par value $0.0001 per share, 10,000,000 shares of non-voting common stock, par value $0.0001 per share and 10,000,000 shares of undesignated preferred stock, par value $0.0001 per share, all of which shares of preferred stock are undesignated. The following description of our capital stock and provisions of our certificate of incorporation and bylaws are summaries and are qualified by reference to our certificate of incorporation and our bylaws, in each case, as amended and supplemented.
Common Stock
As of December 31, 2023, we had outstanding 20,337,571 shares of common stock.
The holders of our common stock and non-voting common stock have identical rights subject to two exceptions. First, except as otherwise expressly provided in our certificate of incorporation or as required by applicable law, on any matter that is submitted to a vote by our stockholders, holders of our common stock are entitled to one vote per share of common stock, and holders of our non-voting common stock are not entitled to any votes per share of non-voting common stock, including for the election of directors. Second, holders of our common stock have no conversion rights, while holders of our non-voting common stock shall have the right to convert each share of our non-voting common stock held into one share of common stock at such holder’s election, provided that as a result of such conversion, such holder, together with its affiliates and any members of a Schedule 13(d) group with such holder, would not beneficially own in excess of 9.9% of our common stock following such conversion, unless otherwise expressly provided for in our certificate of incorporation. However, this ownership limitation may be increased to any other percentage designated by such holder of non-voting common stock upon 61 days’ notice to us or decreased at any time. Holders of our non-voting common stock are also permitted to make certain transfers to non-affiliates upon which, such transferred shares would immediately convert to shares of our common stock.
Except as otherwise provided by law, our third amended and restated certificate of incorporation or our bylaws, in all matters other than the election of directors, the affirmative vote of the majority of the shares present in person or represented by proxy at a meeting at which a quorum is present and entitled to vote on the subject matter shall be the act of the stockholders. Directors shall be elected by a plurality of the shares present in person or represented by proxy at a meeting at which a quorum is present and entitled to vote on the election of directors.
Holders of our common stock and non-voting common stock are entitled to receive ratably any dividends declared by our board of directors out of funds legally available for that purpose, subject to any preferential dividend rights of any outstanding preferred stock. Our common stock and non-voting common stock have no preemptive rights or other subscription rights or redemption or sinking fund provisions.
In the event of our liquidation, dissolution or winding up, holders of our common stock and non-voting common stock will be entitled to share ratably in all assets remaining after payment of all debts and other liabilities and any liquidation preference of any outstanding preferred stock. Holders of shares of our common stock and non-voting common stock are not required to make additional capital contributions. The shares to be issued by us in this offering will be, when issued and paid for, validly issued, fully paid and non-assessable.
S-18
Table of Contents
Preferred Stock
As of December 31, 2023, no shares of our preferred stock were issued and outstanding.
Our board of directors will have the authority, without further action by our stockholders, to issue up to 10,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting, or the designation of, such series, any or all of which may be greater than the rights of common stock. The issuance of our preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon our liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change in control of our Company or other corporate action.
Anti-takeover Effects of our Certificate of Incorporation and Bylaws and Delaware Law
Our certificate of incorporation and bylaws include a number of provisions that may have the effect of delaying, deferring or preventing another party from acquiring control of us and encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our board of directors rather than pursue non-negotiated takeover attempts. These provisions include the items described below.
Board Composition and Filling Vacancies
Our certificate of incorporation provides for the division of our board of directors into three classes serving staggered three-year terms, with one class being elected each year. Our certificate of incorporation also provides that directors may be removed only for cause and then only by the affirmative vote of the holders of two-thirds or more of the shares then entitled to vote at an election of directors. Furthermore, any vacancy on our board of directors, however occurring, including a vacancy resulting from an increase in the size of our board, may only be filled by the affirmative vote of a majority of our directors then in office even if less than a quorum. The classification of directors, together with the limitations on removal of directors and treatment of vacancies, has the effect of making it more difficult for stockholders to change the composition of our board of directors.
No Written Consent of Stockholders
Our certificate of incorporation provides that all stockholder actions are required to be taken by a vote of the stockholders at an annual or special meeting, and that stockholders may not take any action by written consent in lieu of a meeting. This limit may lengthen the amount of time required to take stockholder actions and would prevent the amendment of our bylaws or removal of directors by our stockholders without holding a meeting of stockholders.
Meetings of Stockholders
Our certificate of incorporation and bylaws provide that only a majority of the members of our board of directors then in office may call special meetings of stockholders and only those matters set forth in the notice of the special meeting may be considered or acted upon at a special meeting of stockholders. Our bylaws limit the business that may be conducted at an annual meeting of stockholders to those matters properly brought before the meeting.
Advance Notice Requirements
Our bylaws establish advance notice procedures with regard to stockholder proposals relating to the nomination of candidates for election as directors or new business to be brought before meetings of our
S-19
Table of Contents
stockholders. These procedures provide that notice of stockholder proposals must be timely given in writing to our corporate secretary prior to the meeting at which the action is to be taken. Generally, to be timely, notice must be received at our principal executive offices not less than 90 days nor more than 120 days prior to the first anniversary date of the annual meeting for the preceding year. Our bylaws specify the requirements as to form and content of all stockholders’ notices. These requirements may preclude stockholders from bringing matters before the stockholders at an annual or special meeting.
Amendment to Certificate of Incorporation and Bylaws
Any amendment of our certificate of incorporation must first be approved by a majority of our board of directors, and if required by law or our certificate of incorporation, must thereafter be approved by a majority of the outstanding shares of capital stock (other than the non-voting common stock) entitled to vote on the amendment and a majority of the outstanding shares of each class entitled to vote thereon as a class, except that the amendment of the provisions relating to stockholder action, board composition, and limitation of liability must be approved by not less than two-thirds of the outstanding shares entitled to vote on the amendment, and not less than two-thirds of the outstanding shares of each class entitled to vote thereon as a class. Our bylaws may be amended by the affirmative vote of a majority of the directors then in office, subject to any limitations set forth in the bylaws; and may also be amended by the affirmative vote of not less than two-thirds of the outstanding shares entitled to vote on the amendment, and not less than two-thirds of the outstanding shares of each class entitled to vote thereon as a class, or, if our board of directors recommends that the stockholders approve the amendment, by the affirmative vote of the majority of the outstanding shares entitled to vote on the amendment, in each case voting together as a single class. Any amendments of the provisions relating to the rights on non-voting common stock requires the unanimous approval of all outstanding shares of non-voting common stock.
Undesignated Preferred Stock
Our certificate of incorporation provides for 10,000,000 authorized shares of preferred stock. The existence of authorized but unissued shares of preferred stock may enable our board of directors to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise. For example, if in the due exercise of its fiduciary obligations, our board of directors were to determine that a takeover proposal is not in the best interests of our stockholders, our board of directors could cause shares of preferred stock to be issued without stockholder approval in one or more private offerings or other transactions that might dilute the voting or other rights of the proposed acquirer or insurgent stockholder or stockholder group. In this regard, our certificate of incorporation grants our board of directors broad power to establish the rights and preferences of authorized and unissued shares of preferred stock. The issuance of shares of preferred stock could decrease the amount of earnings and assets available for distribution to holders of shares of common stock and non-voting common stock. The issuance may also adversely affect the rights and powers, including voting rights, of these holders and may have the effect of delaying, deterring or preventing a change in control of us.
Choice of Forum
Our bylaws provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for any state law claims for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers, and employees to us or our stockholders, (3) any action asserting a claim arising pursuant to the Delaware General Corporation Law, our certificate of incorporation or our bylaws, (4) any action to interpret, apply, enforce or determine the validity of our certificate of incorporation or bylaws, or (5) any action asserting a claim that is governed by the internal affairs doctrine; provided, however, that the this provision shall not apply to any causes of action arising under the Securities Act or Exchange Act. In addition, our bylaws provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the sole and exclusive forum for resolving any complaint
S-20
Table of Contents
asserting a cause or causes of action arising under the Securities Act, including all causes of action asserted against any defendants to such complaint. For the avoidance of doubt, this provision is intended to benefit and may be enforced by us, our officers and directors, the underwriters to any offering giving rise to such complaint, and any other professional entity whose profession gives authority to a statement made by that person or entity and who has prepared or certified any part of the documents underlying the offering. While the Delaware courts have determined that such choice of forum provisions are facially valid, a stockholder may nevertheless seek to bring a claim in a venue other than those designated in the exclusive forum provisions, and there can be no assurance that such provisions will be enforced by a court in those other jurisdictions. Any person or entity purchasing or otherwise acquiring any interest in our securities shall be deemed to have notice of and consented to these forum provisions. These forum provisions may impose additional costs on stockholders, may limit our stockholders’ ability to bring a claim in a forum they find favorable, and the designated courts may reach different judgments or results than other courts.
Section 203 of the Delaware General Corporation Law
We are subject to the provisions of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. Under Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions:
| • | before the stockholder became interested, our board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| • | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee stock plans, in some instances, but not the outstanding voting stock owned by the interested stockholder; or |
| • | at or after the time the stockholder became interested, the business combination was approved by our board of directors and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, lease, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| • | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| • | subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; and |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges, or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
S-21
Table of Contents
Listing on the Nasdaq Global Select Market
Our common stock is listed on the Nasdaq Global Select Market under the symbol “TRML.”
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A. The transfer agent and registrar’s address is 150 Royall Street, Canton, MA 02021, and its telephone number is (800) 962-4284.
S-22
Table of Contents
CERTAIN MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS FOR NON-U.S. HOLDERS OF COMMON STOCK
The following discussion is a summary of the material U.S. federal income tax considerations applicable to non-U.S. holders (as defined below) with respect to their purchase, ownership and disposition of shares of our common stock issued pursuant to this offering, but does not purport to be a complete analysis of all potential tax effects. This discussion is based on current provisions of the U.S. Internal Revenue Code of 1986, as amended, which we refer to as the Code, existing and proposed U.S. Treasury Regulations promulgated thereunder, current administrative rulings and judicial decisions, all as in effect as of the date of this prospectus and, all of which are subject to change or to differing interpretation, possibly with retroactive effect. Any such change or differing interpretation could alter the tax consequences to non-U.S. holders described in this prospectus. There can be no assurance that the Internal Revenue Service, which we refer to as the IRS, will not challenge one or more of the tax consequences described herein.
This discussion is limited to non-U.S. holder that hold our common stock as a capital asset within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all aspects of U.S. federal income taxation that may be relevant to a particular non-U.S. holder in light of that non-U.S. holder’s individual circumstances, nor does it address any aspects of U.S. state, local or non-U.S. taxes, the alternative minimum tax provisions of the Code, the special accounting rules in Section 451(b) of the Code, the Medicare tax on net investment income or any U.S. federal tax other than the income tax (including, for example, the gift or estate tax). This discussion also does not consider any specific facts or circumstances that may apply to a non-U.S. holder and does not address the special tax rules applicable to particular non-U.S. holders, such as:
| • | banks, insurance companies and other financial institutions; |
| • | tax-exempt or governmental organizations; |
| • | brokers, dealers or traders in securities; |
| • | tax-qualified retirement plans; |
| • | corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | “qualified foreign pension funds,” or entities wholly owned by a “qualified foreign pension fund”; |
| • | persons that hold, actually and constructively, more than 5% of our common stock; |
| • | persons deemed to sell our common stock under the constructive sale provisions of the Code; |
| • | persons that hold our common stock as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment; |
| • | partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes; and |
| • | U.S. expatriates and former citizens or long-term residents of the United States. |
If a person is a partner in an entity treated as a partnership for U.S. federal income tax purposes that holds our common stock, the tax treatment of such partner will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding our common stock and the partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences to them.
For purposes of this discussion, a “non-U.S. holder” means a beneficial owner of our common stock that is neither a U.S. person nor an entity treated as a partnership for U.S. federal income tax purposes. A U.S. person is any person that, for U.S. federal income tax purposes, is or is treated as any of the following:
| • | an individual who is a citizen or resident of the United States; |
S-23
Table of Contents
| • | a domestic corporation; |
| • | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| • | a trust that (1) is subject to the primary supervision of a U.S. court and the control of one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code), or (2) has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
This discussion is for general information only and is not intended to be, and does not constitute, tax advice. Accordingly, all prospective investors of our common stock should consult their own tax advisors with respect to the U.S. federal, state, local and non-U.S. tax consequences of the purchase, ownership and disposition of our common stock.
Distributions on Our Common Stock
Distributions, if any, on our common stock generally will constitute dividends for U.S. federal income tax purposes to the extent made out of our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Distributions in excess of our current or accumulated earnings and profits will constitute, first, a return of capital and reduce a non-U.S. holder’s adjusted tax basis in its common stock, but not below zero, and thereafter as capital gain, subject to the tax treatment described below in “Gain on Sale or Other Taxable Disposition of Our Common Stock.”
Subject to the discussion below, dividends paid to a non-U.S. holder generally will be subject to withholding of U.S. federal income tax at a 30% rate (or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence, provided the non-U.S. holder furnishes a valid IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) certifying qualification for the lower treaty rate). A non-U.S. holder that does not timely furnish the required documentation, but that qualifies for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
Dividends that are treated as effectively connected with a trade or business conducted by a non-U.S. holder within the United States and, if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed base maintained by such non-U.S. holder in the United States, are generally exempt from the 30% withholding tax described above if the non-U.S. holder satisfies applicable certification and disclosure requirements. However, such U.S. effectively connected income, net of specified deductions and credits, is taxed at the same U.S. federal income tax rates applicable to U.S. persons. Any U.S. effectively connected income received by a non-U.S. holder that is a corporation may also, under certain circumstances, be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence. Non-U.S. holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
Gain on Sale or Other Taxable Disposition of Our Common Stock
Subject to the discussions below under “Backup Withholding and Information Reporting” and “FATCA,” a non-U.S. holder generally will not be subject to any U.S. federal income tax on any gain realized upon such holder’s sale or other taxable disposition of shares of our common stock unless:
| • | the gain is effectively connected with the non-U.S. holder’s conduct of a U.S. trade or business within the United States and, if an applicable income tax treaty so provides, is attributable to a permanent establishment or a fixed base maintained by such non-U.S. holder in the United States, in which case the non-U.S. holder generally will be taxed on a net income basis at the U.S. federal income tax rates |
S-24
Table of Contents
| applicable to U.S. persons and, if the non-U.S. holder is a foreign corporation, the branch profits tax described above in “Distributions on Our Common Stock” also may apply; |
| • | the non-U.S. holder is a nonresident alien individual who is present in the United States for 183 days or more in the taxable year of the disposition and certain other conditions are met, in which case the non-U.S. holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty between the United States and such holder’s country of residence) on the net gain derived from the disposition, which may be offset by certain U.S. source capital losses of the non-U.S. holder, if any (even though the individual is not considered a resident of the United States), provided that the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses; or |
| • | our common stock constitutes a U.S. real property interest, or USRPI, by reason of our status as a U.S. real property holding corporation, or USRPHC, for U.S. federal income tax purposes and our common stock is not regularly traded on an established securities market, as defined by applicable Treasury Regulations. We believe we currently are not, and we do not anticipate becoming, a USRPHC. Because the determination of whether we are a USRPHC depends, however, on the fair market value of our USRPIs relative to the fair market value of our worldwide real property interests and our assets used or held for use in a trade or business, there can be no assurance we currently are not a USRPHC or will not become one in the future. . |
Non-U.S. holders should consult their tax advisors regarding potentially applicable income tax treaties that may provide for different rules.
Backup Withholding and Information Reporting
Payments of dividends on our common stock will not be subject to backup withholding, provided the applicable withholding agent does not have actual knowledge or reason to know the holder is a U.S. person and the holder either certifies its non-U.S. status, such as by furnishing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI, or otherwise establishes an exemption. However, information returns are required to be filed with the IRS in connection with any dividends on our common stock paid to the non-U.S. holder, regardless of whether any tax was actually withheld. In addition, proceeds of the sale or other taxable disposition of our common stock within the United States or conducted through certain U.S.-related brokers generally will not be subject to backup withholding or information reporting, if the applicable withholding agent receives the certification described above and does not have actual knowledge or reason to know that such holder is a U.S. person, or the holder otherwise establishes an exemption. Proceeds of a disposition of our common stock conducted through a non-U.S. office of a non-U.S. broker generally will not be subject to backup withholding or information reporting.
Copies of information returns that are filed with the IRS may also be made available under the provisions of an applicable treaty or agreement to the tax authorities of the country in which the non-U.S. holder resides or is established.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
FATCA
Withholding taxes may be imposed under Sections 1471 to 1474 of the Code (such Sections commonly referred to as the Foreign Account Tax Compliance Act, or FATCA) on certain types of payments made to non-U.S. financial institutions and certain other non-U.S. entities. Specifically, a 30% withholding tax may be imposed on dividends on, or (subject to the proposed Treasury Regulations discussed below) gross proceeds from the sale or other disposition of, our common stock paid to a “foreign financial institution” or a “non-financial
S-25
Table of Contents
foreign entity” (each as defined in the Code), unless (1) the foreign financial institution undertakes certain diligence and reporting obligations, (2) the non-financial foreign entity either certifies it does not have any “substantial United States owners” (as defined in the Code) or furnishes identifying information regarding each substantial United States owner, or (3) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules. If the payee is a foreign financial institution and is subject to the diligence and reporting requirements in (1) above, it must enter into an agreement with the U.S. Department of the Treasury requiring, among other things, that it undertake to identify accounts held by certain “specified United States persons” or “United States owned foreign entities” (each as defined in the Code), annually report certain information about such accounts, and withhold 30% on certain payments to non-compliant foreign financial institutions and certain other account holders. Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules.
Under the applicable Treasury Regulations and administrative guidance, withholding under FATCA generally applies to payments of dividends on our common stock. While withholding under FATCA would have applied also to payments of gross proceeds from the sale or other disposition of stock, proposed Treasury Regulations eliminate FATCA withholding on payments of gross proceeds entirely. Taxpayers generally may rely on these proposed Treasury Regulations until final Treasury Regulations are issued. Prospective investors should consult their tax advisors regarding the potential application of withholding under FATCA to their investment in our common stock.
S-26
Table of Contents
Subject to the terms and conditions set forth in the underwriting agreement, dated , 2024, among us and Jefferies LLC, Piper Sandler & Co., Guggenheim Securities, LLC and Truist Securities, Inc., as the representatives of the underwriters named below and the joint book-running managers of this offering, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us, the respective number of shares of our common stock shown opposite its name below:
| Underwriter |
Number of Shares | |||
| Jefferies LLC |
||||
| Piper Sandler & Co. |
||||
| Guggenheim Securities, LLC |
||||
| Truist Securities, Inc. |
||||
|
|
|
|||
| Total |
||||
|
|
|
|||
The underwriting agreement provides that the obligations of the several underwriters are subject to certain conditions precedent such as the receipt by the underwriters of officers’ certificates and legal opinions and approval of certain legal matters by their counsel. The underwriting agreement provides that the underwriters will purchase all of the shares of our common stock if any of them are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the nondefaulting underwriters may be increased or the underwriting agreement may be terminated. We have agreed to indemnify the underwriters and certain of their controlling persons against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make in respect of those liabilities.
The underwriters have advised us that, following the completion of this offering, they currently intend to make a market in our common stock as permitted by applicable laws and regulations. However, the underwriters are not obligated to do so, and the underwriters may discontinue any market-making activities at any time without notice in their sole discretion. Accordingly, no assurance can be given as to the liquidity of the trading market for our common stock, that you will be able to sell any of the common stock held by you at a particular time or that the prices that you receive when you sell will be favorable.
The underwriters are offering the shares of our common stock subject to their acceptance of the shares of common stock from us and subject to prior sale. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part. In addition, the underwriters have advised us that they do not intend to confirm sales to any account over which they exercise discretionary authority.
Commissions and Expenses
The underwriters have advised us that they propose to offer the shares of our common stock to the public at the public offering price set forth on the cover page of this prospectus supplement and to certain dealers, which may include the underwriters, at that price less a concession not in excess of $ per share of common stock. The underwriters may allow, and certain dealers may reallow, a discount from the concession not in excess of $ per share of common stock to certain brokers and dealers. After the offering, the public offering price, concession and reallowance to dealers may be reduced by the representatives. No such reduction will change the amount of proceeds to be received by us as set forth on the cover page of this prospectus supplement.
S-27
Table of Contents
The following table shows the public offering price, the underwriting discounts and commissions that we are to pay the underwriters and the proceeds, before expenses, to us in connection with this offering. Such amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares.
| Per Share | Total | |||||||||||||||
| Without Option to Purchase Additional Shares |
With Option to Purchase Additional Shares |
Without Option to Purchase Additional Shares |
With Option to Purchase Additional Shares |
|||||||||||||
| Public offering price |
$ | $ | $ | $ | ||||||||||||
| Underwriting discounts and commissions paid by us |
$ | $ | $ | $ | ||||||||||||
| Proceeds to us, before expenses |
$ | $ | $ | $ | ||||||||||||
We estimate expenses payable by us in connection with this offering, other than the underwriting discounts and commissions referred to above, will be approximately $595,000. We have agreed to reimburse the underwriters up to $20,000 for certain expenses incurred in connection with this offering. In accordance with FINRA Rule 5110 these reimbursed expenses are deemed underwriting compensation for this offering.
Listing
Our common stock is listed on The Nasdaq Global Select Market under the trading symbol “TRML.”
Option to Purchase Additional Shares
We have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus supplement, to purchase, from time to time, in whole or in part, up to an aggregate of shares from us at the public offering price set forth on the cover page of this prospectus supplement, less underwriting discounts and commissions. If the underwriters exercise this option, each underwriter will be obligated, subject to specified conditions, to purchase a number of additional shares of our common stock proportionate to that underwriter’s initial purchase commitment as indicated in the table above.
No Sales of Similar Securities
We have agreed, subject to specified exceptions, not to directly or indirectly:
| • | sell, offer to sell, contract to sell or lend or grant any option to sell (including any short sale), pledge, hypothecate or grant any security interest in, or transfer any shares of our common stock, options or warrants to acquire shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock (collectively with the common stock, the “lock-up securities”); |
| • | establish or increase an open “put equivalent position” within the meaning of Rule 16a-l(h) under the Exchange Act, or liquidate or decrease any “call equivalent position” (as defined in Rule 16a-1(b) under the Exchange Act); |
| • | otherwise dispose of any lock-up securities; |
| • | enter into any swap, hedge or similar arrangement or agreement that transfers, in whole or in part, the economic risk of ownership of any lock-up securities; |
| • | submit or file any registration statement under the Securities Act in respect of any lock-up securities; |
S-28
Table of Contents
| • | effect a reverse stock split, recapitalization, share consolidation, reclassification or similar transaction affecting the outstanding shares of our common stock; or |
| • | publicly announce an intention to do any of the foregoing for a period of 90 days after the date of this prospectus supplement without the prior written consent of Jefferies LLC and Piper Sandler & Co. |
Notwithstanding the foregoing, we may (i) issue shares of our common stock or options to purchase shares of common stock, or issue shares upon exercise of options, pursuant to any stock option, stock bonus, employee stock purchase plan or other stock plan or arrangement described in the registration statement of which this prospectus supplement forms a part, (ii) issue lock-up securities to any non-employee director pursuant to any non-employee director compensation plan or program described in the registration statement of which this prospectus supplement forms a part, (iii) issue shares of our common stock pursuant to the exercise or settlement of any options or warrants or other rights to acquire lock-up securities, or upon the conversion of convertible securities outstanding on the date hereof, (iv) file one or more registration statements on Form S-8 to register lock-up securities issued or issuable pursuant to any plans or programs described in (i) or (ii) above and (v) issue lock-up securities, or enter into an agreement to issue lock-up securities, in connection with any merger, joint venture, strategic alliances, commercial, lending or other collaborative or strategic transaction, or the acquisition or license of the business, property, technology or other assets of another individual or entity or the assumption of an employee benefit plan in connection with a merger or acquisition; provided that the aggregate number of lock-up securities (on an as-converted or as-exercised basis, as the case may be) that we may issue or agree to issue pursuant to this clause (v) shall not exceed 5% of the total number of shares of our common stock immediately following the completion of the transactions contemplated by the underwriting agreement and that each recipient thereof provides to the underwriter a signed lock-up agreement.
Our directors and executive officers, and certain of our significant stockholders (such persons, the “lock-up parties”) have entered into lock up agreements with the underwriters prior to the commencement of this offering pursuant to which each lock-up party, with limited exceptions, for a period of 90 days after the date of this prospectus supplement (such period, the “restricted period”), may not (and may not cause any of their direct or indirect affiliates to), without the prior written consent of Jefferies LLC and Piper Sandler & Co.
| • | sell, offer to sell, contract to sell or lend or grant any option to sell (including any short sale), pledge, hypothecate or grant any security interest in, or transfer any lock-up securities; |
| • | enter into any swap, hedge or similar arrangement or agreement that transfers, in whole or in part, the economic risk of ownership of the lock-up securities, whether any such transaction is to be settled by delivery of lock-up securities, in cash or otherwise, |
| • | establish or increase an open “put equivalent position” within the meaning of Rule 16a-l(h) under the Exchange Act, or liquidate or decrease any “call equivalent position” (as defined in Rule 16a-1(b) under the Exchange Act) |
| • | make any demand for or exercise any right with respect to, the registration of any lock-up securities, or |
| • | publicly disclose the intention to do any of the foregoing. |
The restrictions described in the immediately preceding paragraph and contained in the lock-up agreements between the underwriters and the lock-up parties do not apply, subject in certain cases to various conditions, to certain transactions, including (i) sales or transfers of shares of our common stock solely in connection with the “cashless” exercise of stock options outstanding on the date hereof for the purpose of exercising such stock options that will expire during the restricted period pursuant to certain employee benefit plans, options or other rights, provided that (a) in connection therewith, shares of our common stock are not sold into the public market by a facilitating broker-dealer or otherwise and (b) any shares received upon such exercise will be subject to the terms hereof, (ii) the disposition of shares of our common stock solely to satisfy estimated tax obligations related to the delivery of shares pursuant to the vesting of restricted stock units or restricted common stock granted to the
S-29
Table of Contents
lock-up party, (iii) distributions of lock-up securities to general or limited partners, members, shareholders, affiliates or wholly owned subsidiaries of the lock-up party or any investment fund or other entity controlled or managed by the lock-up party (subject to each transferee signing a lock-up agreement), (iv) transfers or dispositions of lock-up securities to any corporation, partnership, limited liability company or other entity all of the beneficial ownership interests of which are held by the lock-up party or their family member (subject to each transferee signing a lock-up agreement), (v) transfers to us, pursuant to a contractual arrangement, of lock-up securities in connection with termination of employment or other termination of a service provider and pursuant to agreements wherein we have the option to repurchase such lock-up securities, (vi) the establishment of any contract, instruction or plan pursuant to Rule 10b5-1 under the Exchange Act, provided that (x) no sales or transfers of lock-up securities shall be made pursuant to such a plan prior to the expiration of the restricted period, (y) no filing under the Exchange Act or other public announcement shall be made voluntarily in connection therewith during the restricted period, and (z) any required public disclosure of such plan includes the restrictions set forth in the lock-up agreement, (vii) pursuant to a bona fide third-party tender offer, merger, consolidation or other similar transaction that is approved by our Board of Directors and made to all holders of our capital stock involving a change of control, provided that in the event that such tender offer, merger, consolidation or other similar transaction is not completed, the lock-up securities shall remain subject to the provisions of the lock-up agreement, (viii) transfers of lock-up securities by gift, or by will, other testamentary document or intestate succession to a family member or to a trust whose beneficiaries consist exclusively of one or more of the lock-up party and/or a family member of the lock-up party (subject to each transferee signing a lock-up agreement) and (ix) transfers of lock-up securities as a bona fide gift or charitable contributions (subject to each transferee signing a lock-up agreement).
This restrictions described above terminate after the close of trading of the common stock on and including the 90th day after the date of this prospectus supplement. Jefferies LLC and Piper Sandler & Co. may, in their sole discretion and at any time or from time to time before the termination of the 90-day period release all or any portion of the securities subject to lock-up agreements. There are no existing agreements between the underwriters and any of our stockholders who will execute a lock-up agreement, providing consent to the sale of shares prior to the expiration of the lock-up period.
Stabilization
The underwriters have advised us that they, pursuant to Regulation M under the Securities Exchange Act of 1934, as amended, certain persons participating in the offering may engage in short sale transactions, stabilizing transactions, syndicate covering transactions or the imposition of penalty bids in connection with this offering. These activities may have the effect of stabilizing or maintaining the market price of our common stock at a level above that which might otherwise prevail in the open market. Establishing short sales positions may involve either “covered” short sales or “naked” short sales.
“Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares of our common stock in this offering. The underwriters may close out any covered short position by either exercising their option to purchase additional shares of our common stock or purchasing shares of our common stock in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the option to purchase additional shares.
“Naked” short sales are sales in excess of the option to purchase additional shares of our common stock. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares of our common stock in the open market after pricing that could adversely affect investors who purchase in this offering.
S-30
Table of Contents
A stabilizing bid is a bid for the purchase of shares of common stock on behalf of the underwriters for the purpose of fixing or maintaining the price of the common stock. A syndicate covering transaction is the bid for or the purchase of shares of common stock on behalf of the underwriters to reduce a short position incurred by the underwriters in connection with the offering. Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. A penalty bid is an arrangement permitting the underwriters to reclaim the selling concession otherwise accruing to a syndicate member in connection with the offering if the common stock originally sold by such syndicate member are purchased in a syndicate covering transaction and therefore have not been effectively placed by such syndicate member.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. The underwriters are not obligated to engage in these activities and, if commenced, any of the activities may be discontinued at any time.
The underwriters may also engage in passive market making transactions in our common stock on The Nasdaq Global Select Market in accordance with Rule 103 of Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, that bid must then be lowered when specified purchase limits are exceeded.
Electronic Distribution
A prospectus in electronic format may be made available by e-mail or on the websites or through online services maintained by one or more of the underwriters or their affiliates. In those cases, prospective investors may view offering terms online and may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of shares of common stock for sale to online brokerage account holders. Any such allocation for online distributions will be made by the underwriters on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters’ web sites and any information contained in any other web site maintained by any of the underwriters is not part of this prospectus, has not been approved and/or endorsed by us or the underwriters and should not be relied upon by investors.
Other Activities and Relationships
The underwriters and certain of their affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriters and certain of their affiliates have, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for us and our affiliates, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the underwriters and certain of their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments issued by us and our affiliates. If the underwriters or their respective affiliates have a lending relationship with us, they routinely hedge their credit exposure to us consistent with their customary risk management policies. The underwriters and their respective affiliates may hedge such exposure by entering into transactions which consist of either the purchase of credit default swaps or the creation of short positions in our securities or the securities of
S-31
Table of Contents
our affiliates, including potentially the common stock offered hereby. Any such short positions could adversely affect future trading prices of the common stock offered hereby. The underwriters and certain of their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Selling Restrictions
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of our common stock offered by this prospectus supplement in any jurisdiction where action for that purpose is required. Our common stock offered by this prospectus supplement may not be offered or sold, directly or indirectly, nor may this prospectus supplement or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus supplement comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus supplement. This prospectus supplement does not constitute an offer to sell or a solicitation of an offer to buy any shares of common stock offered by this prospectus supplement in any jurisdiction in which such an offer or a solicitation is unlawful.
Notice to Prospective Investors in Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus supplement (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
Notice to Prospective Investors in the European Economic Area
The securities are not intended to be offered, sold or otherwise made available to and should not be offered, sold or otherwise made available to any retail investor in the European Economic Area (“EEA”). For these purposes, a retail investor means a person who is one (or more) of: (i) a retail client as defined in point (11) of Article 4(1) of Directive 2014/65/EU (as amended, “MiFID II”); (ii) a customer within the meaning of Directive (EU) 2016/97 (as amended, the “Insurance Distribution Directive”), where that customer would not qualify as a professional client as defined in point (10) of Article 4(1) of MiFID II; or (iii) not a qualified investor as defined in Regulation (EU) 2017/1129 (as amended, the “Prospectus Regulation”). Consequently no key information document required by Regulation (EU) No 1286/2014 (as amended, the “PRIIPs Regulation”) for offering or selling the securities or otherwise making them available to retail investors in the EEA has been prepared and therefore offering or selling the securities or otherwise making them available to any retail investor in the EEA may be unlawful under the PRIIPs Regulation.
S-32
Table of Contents
The offer of securities shall not require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation, and each person who initially acquires any securities or to whom any offer is made will be deemed to have represented, acknowledged and agreed to and with each of the underwriters and us that it is a “qualified investor” within the meaning of Article 2(e) of the Prospectus Regulation. In the case of any securities being offered to a financial intermediary as that term is used in the Prospectus Regulation, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the securities acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any securities to the public other than their offer or resale in a Relevant State to qualified investors as so defined or in circumstances in which the prior consent of the underwriters have been obtained to each such proposed offer or resale.
For the purposes of this provision, the expression an “offer to the public” in relation to securities in any Relevant State means the communication in any form and by any means of sufficient information on the terms of the offer and any securities to be offered so as to enable an investor to decide to purchase or subscribe for any securities, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129, as amended.
Notice to Prospective Investors in the United Kingdom
No securities have been offered or will be offered pursuant to this offering to the public in the UK prior to the publication of a prospectus in relation to the securities which has been approved by the Financial Conduct Authority, except that the securities may be offered to the public in the UK at any time:
| (a) | to any legal entity which is a qualified investor as defined under Article 2 of the UK Prospectus Regulation; |
| (b) | to fewer than 150 natural or legal persons (other than qualified investors as defined under Article 2 of the UK Prospectus Regulation), subject to obtaining the prior consent of underwriters for any such offer; or |
| (c) | in any other circumstances falling within Section 86 of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the “Order”), |
provided that no such offer of the securities shall require us or any underwriter to publish a prospectus pursuant to Section 85 of the FSMA or supplement a prospectus pursuant to Article 23 of the UK Prospectus Regulation. For the purposes of this provision, the expression an “offer to the public” in relation to the securities in the UK means the communication in any form and by any means of sufficient information on the terms of the offer and any securities to be offered so as to enable an investor to decide to purchase or subscribe for any securities and the expression “UK Prospectus Regulation” means Regulation (EU) 2017/1129 as it forms part of domestic law by virtue of the European Union (Withdrawal) Act 2018.
In addition, in the UK, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Regulation) (i) who have professional experience in matters relating to investments falling within Article 19(5) of the Order and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”) or otherwise in circumstances which have not resulted and will not result in an offer to the public of the securities in the UK within the meaning of the Order.
Any person in the UK that is not a relevant person should not act or rely on the information included in this document or use it as basis for taking any action. In the UK, any investment or investment activity that this document relates to may be made or taken exclusively by relevant persons.
S-33
Table of Contents
Notice to Prospective Investors in Australia
This prospectus supplement:
| • | does not constitute a disclosure document or a prospectus under Chapter 6D.2 of the Corporations Act 2001 (Cth), or the Corporations Act; |
| • | has not been, and will not be, lodged with the Australian Securities and Investments Commission, or ASIC, as a disclosure document for the purposes of the Corporations Act and does not purport to include the information required of a disclosure document for the purposes of the Corporations Act; and |
| • | may only be provided in Australia to select investors who are able to demonstrate that they fall within one or more of the categories of investors available under section 708 of the Corporations Act (“Exempt Investors”). |
The securities may not be directly or indirectly offered for subscription or purchased or sold, and no invitations to subscribe for or buy the securities may be issued, and no draft or definitive offering memorandum, advertisement or other offering material relating to any securities may be distributed in Australia, except where disclosure to investors is not required under Chapter 6D of the Corporations Act or is otherwise in compliance with all applicable Australian laws and regulations. By submitting an application for the securities, you represent and warrant to us that you are an Exempt Investor.
As any offer of securities under this document will be made without disclosure in Australia under Chapter 6D.2 of the Corporations Act, the offer of those securities for resale in Australia within 12 months may, under section 707 of the Corporations Act, require disclosure to investors under Chapter 6D.2 if none of the exemptions in section 708 applies to that resale. By applying for the securities you undertake to us that you will not, for a period of 12 months from the date of issue of the securities, offer, transfer, assign or otherwise alienate those securities to investors in Australia except in circumstances where disclosure to investors is not required under Chapter 6D.2 of the Corporations Act or where a compliant disclosure document is prepared and lodged with ASIC.
Notice to Prospective Investors in Japan
The securities have not been and will not be registered pursuant to Article 4, Paragraph 1 of the Financial Instruments and Exchange Act of Japan. Accordingly, none of the securities nor any interest therein may be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any “resident” of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to or for the benefit of a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Act and any other applicable laws, regulations and ministerial guidelines of Japan in effect at the relevant time.
Notice to Prospective Investors in Hong Kong
The securities have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571 of the Laws of Hong Kong) (the “SFO”) of Hong Kong and any rules made thereunder; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32) of Hong Kong (the “CO”) or which do not constitute an offer to the public within the meaning of the CO. No advertisement, invitation or document relating to the securities has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other
S-34
Table of Contents
than with respect to securities which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the SFO and any rules made thereunder.
Notice to Prospective Investors in Singapore
This prospectus supplement has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus supplement or any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the securities, may not be circulated or distributed, nor may the securities be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to any person in Singapore other than:
| (a) | to an institutional investor (as defined in Section 4A of the Securities and Futures Act (Chapter 289) of Singapore, as modified or amended from time to time (the “SFA”)) pursuant to Section 274 of the SFA; |
| (b) | to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA; or |
| (c) | otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA. |
Where the securities are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| (a) | a corporation (which is not an accredited investor as defined in Section 4A of the SFA) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| (b) | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, |
securities or securities-based derivatives contracts (each term as defined in Section 2(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the securities pursuant to an offer made under Section 275 of the SFA except:
| (i) | to an institutional investor or to a relevant person, or to any person arising from an offer referred to in Section 276(4)(i)(B) of the SFA; |
| (ii) | where no consideration is or will be given for the transfer; |
| (iii) | where the transfer is by operation of law; |
| (iv) | as specified in Section 276(7) of the SFA; or |
| (v) | as specified in Regulation 37A of the Securities and Futures (Offers of Investments) (Securities and Securities-based Derivatives Contracts) Regulations 2018. |
S-35
Table of Contents
The validity of the shares of common stock being offered by this prospectus will be passed upon for us by Cooley LLP, New York, New York. Covington & Burling LLP, New York, New York, is counsel to the underwriters in connection with this offering.
The financial statements of Talaris Therapeutics, Inc. (subsequently renamed Tourmaline Bio, Inc.) as of December 31, 2022 and 2021, and for each of the years then ended, incorporated by reference in this prospectus, have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report. Such financial statements are incorporated by reference in reliance upon the report of such firm given their authority as experts in accounting and auditing.
The financial statements of Tourmaline Bio, Inc. (subsequently renamed Tourmaline Sub, Inc.) as of December 31, 2022 and 2021, and for the year ended December 31, 2022 and the period from September 17, 2021 (inception) through December 31, 2021, incorporated by reference in this prospectus, have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report. Such financial statements are incorporated by reference in reliance upon the report of such firm given their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus supplement and the accompanying prospectus are part of the registration statement on Form S-3 we filed with the SEC under the Securities Act and do not contain all the information set forth in the registration statement. Whenever a reference is made in this prospectus supplement or the accompanying prospectus to any of our contracts, agreements or other documents, the reference may not be complete and you should refer to the exhibits that are a part of the registration statement or the exhibits to the reports or other documents incorporated by reference in this prospectus supplement and the accompanying prospectus for a copy of such contract, agreement or other document. Because we are subject to the information and reporting requirements of the Exchange Act, we file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov. We maintain a website at www.tourmalinebio.com. The information contained in, or that can be accessed through, our website is not incorporated by reference herein and is not part of this prospectus.
S-36
Table of Contents
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” the information we file with it, which means that we can disclose important information to you by referring you to those documents. Information incorporated by reference is part of this prospectus supplement and the accompanying prospectus. Later information filed with the SEC and incorporated by reference in this prospectus supplement and the accompanying prospectus will update and supersede this information.
We incorporate by reference the documents listed below and any future filings made with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus supplement until the termination of the offering of the shares covered by this prospectus supplement (other than any information furnished under Item 2.02 or Item 7.01 of any current report on Form 8-K and exhibits filed on such form that are related to such items):
| • | our Annual Report on Form 10-K for the year ended December 31, 2022, filed with the SEC on March 31, 2023; |
| • | the portions of our Definitive Proxy Statement on Schedule 14A for our 2023 Annual Meeting of Stockholders, filed with the SEC on April 28, 2023, specifically incorporated by reference into our Annual Report on Form 10-K for the year ended December 31, 2022; |
| • | our Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2023, filed with the SEC on May 15, 2023; |
| • | our Quarterly Report on Form 10-Q for the fiscal quarter ended June 30, 2023, filed with the SEC on August 14, 2023; |
| • | our Quarterly Report on Form 10-Q for the fiscal quarter ended September 30, 2023, filed with the SEC on November 14, 2023; |
| • | our Current Reports on Form 8-K (other than information furnished rather than filed) filed with the SEC on February 16, 2023, April 14, 2023, May 30, 2023, June 14, 2023, June 22, 2023, October 6, 2023, October 10, 2023, October 18, 2023, October 20, 2023 (excluding Item 9.01(b) thereof), October 27, 2023, November 14, 2023, November 30, 2023, December 14, 2023, January 8, 2024 and January 24, 2024; |
| • | Exhibits 10.4 and 10.5 to our registration statement on Form S-4/A filed with the SEC on September 13, 2023; |
| • | the audited and unaudited historical financial statements and related notes thereto set under the caption “Tourmaline Bio, Inc. Index to Financial Statements” on pages F-1 through F-40 of the definitive proxy statement/prospectus filed with the SEC on September 15, 2023; and |
| • | the description of our common stock contained in our registration statement on Form 8-A filed with the SEC on May 4, 2021, including any amendments or reports filed for the purposes of updating this description, including Exhibit 4.3 to our Annual Report on Form 10-K for the year ended December 31, 2022. |
We will provide to each person, including any beneficial owner, to whom a prospectus is delivered, without charge upon written or oral request, a copy of any or all of the documents that are incorporated by reference into this prospectus but not delivered with the prospectus, including exhibits which are specifically incorporated by reference into such documents. You should direct any requests for documents to Tourmaline Bio, Inc., Attn: Investor Relations, 27 West 24th Street, Suite 702, New York, New York 10010.
Exhibits to the filings will not be sent, however, unless those exhibits have specifically been incorporated by reference in this prospectus.
S-37
Table of Contents
In accordance with Rule 412 of the Securities Act, any statement contained in a document incorporated by reference herein shall be deemed modified or superseded to the extent that a statement contained herein or in any other subsequently filed document which also is or is deemed to be incorporated by reference herein modifies or supersedes such statement.
S-38
Table of Contents
PROSPECTUS
$250,000,000

Common Stock
Preferred Stock
Debt Securities
Warrants
Units
We may from time to time issue, in one or more series or classes, up to $250,000,000 in aggregate principal amount of our common stock, preferred stock, debt securities, warrants and/or units, in any combination, together or separately, in one or more offerings in amounts, at prices and on the terms that we will determine at the time of the offering and which will be set forth in a prospectus supplement to this prospectus and any related free writing prospectus.
We may offer these securities separately or together in units. Each time we sell securities described herein, we will provide prospective investors with a supplement to this prospectus that will specify the terms of the securities being offered. We may sell these securities to or through underwriters or dealers and also to other purchasers or through agents. We will set forth the names of any underwriters or agents, and any fees, conversions, or discount arrangements, in the applicable prospectus supplement. We may not sell any securities under this prospectus without delivery of the applicable prospectus supplement. You should read this document and any prospectus supplement or amendment carefully before you invest in our securities.
Our common stock is listed on The Nasdaq Global Market under the symbol “TALS.” On August 11, 2022, the closing price for our common stock, as reported on The Nasdaq Global Market, was $4.69 per share. Our principal executive offices are located at 93 Worcester Street, Wellesley, MA 02481.
Investing in these securities involves certain risks. See “Risk Factors” on page 1 of this prospectus as well as those included in any accompanying prospectus supplement and in the documents incorporated by reference in this prospectus for a discussion of the factors you should carefully consider before deciding to purchase these securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is August 25, 2022.
Table of Contents
TABLE OF CONTENTS
| i | ||||
| 1 | ||||
| 2 | ||||
| 4 | ||||
| 6 | ||||
| 7 | ||||
| 8 | ||||
| 14 | ||||
| 21 | ||||
| 22 | ||||
| 25 | ||||
| 28 | ||||
| 28 | ||||
| 28 | ||||
| 29 | ||||
Table of Contents
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission (the “SEC”) utilizing a “shelf” registration process. Under this shelf registration process, we may from time to time sell any combination of the securities described in this prospectus in one or more offerings for an aggregate initial offering price of up to $250,000,000.
This prospectus provides you with a general description of the securities we may offer. Each time we sell securities, we will provide one or more prospectus supplements that will contain specific information about the terms of the offering. The prospectus supplement may also add, update or change information contained in this prospectus. This prospectus, together with any accompanying prospectus supplement, contains important information you should know before investing in our securities, including important information about us and the securities being offered. You should read both this prospectus and the accompanying prospectus supplement together with the additional information described under the headings “Where You Can Find More Information” and “Incorporation by Reference” beginning on page 28 of this prospectus.
You should rely only on the information contained in or incorporated by reference in this prospectus, any accompanying prospectus supplement or in any related free writing prospectus filed by us with the SEC. We have not authorized anyone to provide you with different information. This prospectus and the accompanying prospectus supplement do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the securities described in the accompanying prospectus supplement or an offer to sell or the solicitation of an offer to buy such securities in any circumstances in which such offer or solicitation is unlawful. You should assume that the information appearing in this prospectus, any prospectus supplement, the documents incorporated by reference and any related free writing prospectus is accurate only as of their respective dates. Our business, financial condition, results of operations and prospects may have changed materially since those dates. This prospectus, any applicable prospectus supplement and the information incorporated herein or therein by reference contains market data, industry statistics and other data that have been obtained or compiled from information made available by independent third parties. We have not independently verified the accuracy and completeness of such data.
THIS PROSPECTUS MAY NOT BE USED TO OFFER AND SELL SECURITIES UNLESS IT IS ACCOMPANIED BY AN ADDITIONAL PROSPECTUS OR A PROSPECTUS SUPPLEMENT.
As used in this prospectus, unless the context otherwise requires, references to the “Talaris,” “company,” “we,” “us” and “our” refer to Talaris Therapeutics, Inc. and, where appropriate, our subsidiaries.
We own various U.S. federal trademark applications and unregistered trademarks, including our company name. All other trademarks or trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus are referred to without the symbols ® and ™, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
i
Table of Contents
Investing in our securities involves a high degree of risk. You should carefully consider the risks set forth in our filings with the SEC that are incorporated by reference herein and any prospectus supplement, as well as other information we include or incorporate by reference into this prospectus and any applicable prospectus supplement, before making an investment decision. Our business, financial condition or results of operations could be materially adversely affected by the materialization of any of these risks. The trading price of our securities could decline due to the materialization of any of these risks, and you may lose all or part of your investment. This prospectus and the documents incorporated herein by reference also contain forward-looking statements that involve risks and uncertainties. Actual results could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks described in the documents incorporated herein by reference, including our most recent Annual Report on Form 10-K, our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K and the other documents we file with the SEC that are deemed incorporated by reference into this prospectus.
1
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, including the documents that we incorporate by reference, includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Forward-looking statements relate to, among others, our plans, objectives and expectations for our business, operations and financial performance and condition, and can be identified by terminology such as “may,” “will,” “could,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “project,” “seek,” “endeavor,” “target,” “continue” and similar expressions that do not relate solely to historical matters. Forward-looking statements are based on management’s belief and assumptions and on information currently available to management. Although we believe that the expectations reflected in forward-looking statements are reasonable, such statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by forward-looking statements.
Forward-looking statements include, but are not limited to, statements about:
| • | the success, cost and timing of our product development activities and clinical trials, including statements regarding the timing of initiation and completion of studies or trials and related preparatory work, the period during which the results of the trials will become available, and our research and development programs; |
| • | the potential for COVID-19 or other pandemic, epidemic or outbreak of an infectious disease, to disrupt our business plans, product development activities, ongoing clinical trials, including the timing and enrollment of patients, the health of our employees and the strength of our supply chain; |
| • | our expectations regarding the safety or efficacy profile of our product candidates |
| • | our ability to advance any product candidate into or successfully complete any clinical trial; |
| • | our ability to obtain regulatory approval for any of our candidates; |
| • | our ability to successfully manufacture our product candidates for future clinical trials or for commercial use, if approved; |
| • | the ability to license additional intellectual property relating to any future product candidates and to comply with our existing license agreement; |
| • | our ability to commercialize our products in light of the intellectual property rights of others; |
| • | the success of competing therapies that are or become available; |
| • | our ability to obtain funding for our operations, including funding necessary to complete further development and commercialization of our product candidates; |
| • | the commercialization of our product candidates, if approved; |
| • | our plans to research, develop and commercialize our product candidates; |
| • | our ability to attract collaborators with development, regulatory and commercialization expertise; |
| • | future agreements with third parties in connection with the development or commercialization of our product candidates and any other approved product; |
| • | the size and growth potential of the markets for our product candidates, and our ability to serve those markets; |
| • | the rate and degree of market acceptance of our product candidates; |
| • | regulatory and political developments in the United States and foreign countries, including but not limited to the Russia-Ukraine conflict and associated sanctions; |
2
Table of Contents
| • | our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately; |
| • | our ability to attract and retain key scientific or management personnel; |
| • | the accuracy of our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
| • | the impact of laws and regulations; and |
| • | our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates. |
The forward-looking statements in this prospectus represent our views as of the date of this prospectus. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should therefore not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this prospectus.
We may from time-to-time provide estimates, projections and other information concerning our industry, our business and the markets for our programs and product candidates. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. Unless otherwise expressly stated, we obtained this industry, business, market, and other data from our own internal estimates and research as well as from reports, research surveys, studies, and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data and similar sources. While we are not aware of any misstatements regarding any third-party information presented in this prospectus, their estimates, in particular, as they relate to projections, involve numerous assumptions, are subject to risks and uncertainties and are subject to change based on various factors. You should read this prospectus, any applicable prospectus supplement and any related free writing prospectus and the documents that we reference therein and have filed with the SEC as exhibits thereto completely and with the understanding that our actual future results may be materially different from any future results expressed or implied by these forward-looking statements. These estimates involve numerous assumptions, are subject to risks and uncertainties and are subject to change based on various factors, including those discussed under Part I, Item 1A “Risk Factors” and elsewhere in our most recent Annual Report on Form 10-K, our subsequent Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K, and the section of any accompanying prospectus supplement entitled “Risk Factors.”
3
Table of Contents
Overview
We are a late-clinical stage, cell therapy company developing an innovative method of allogeneic hematopoietic stem cell transplantation (“allo-HSCT”) that we believe has the potential to transform the standard of care in solid organ transplantation, certain severe autoimmune diseases and certain severe blood, immune and metabolic disorders. In the organ transplant setting, which is our initial focus, we believe our proprietary therapeutic approach, which we call Facilitated Allo-HSCT Therapy, could prevent organ rejection without the morbidity and mortality that has been associated with the use of lifelong anti-rejection medicines, also known as chronic immunosuppression. Beyond the organ transplant setting, our Facilitated Allo-HSCT Therapy also has the potential to treat a range of severe autoimmune diseases and severe blood, immune and metabolic disorders, in each case with potential for similar outcomes to what has previously been observed with HSCT, while mitigating the toxicities, morbidities and extended hospital stay associated with the conditioning regimen typically required by HSCT. We believe that our target indications, individually and collectively, represent a significant unmet need and commercial opportunity.
Corporate History
We were incorporated under the laws of the state of Delaware in February 2002. Our executive offices are located at 93 Worcester Street, Wellesley, MA 02481 and our telephone number at that address is (502) 398-9250. We maintain an Internet website at the following address: www.talaristx.com. The information on our website is not incorporated by reference in this prospectus or in any other filings we make with the SEC. We do not incorporate the information on or accessible through our website into this prospectus, and you should not consider any information on, or that can be accessed through, our website as part of this prospectus.
We use various trademarks and trade names in our business, including without limitation our corporate name and logo. All other trademarks or trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, the trademarks and trade names in this prospectus may be referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
Implications of Being an Emerging Growth Company and a Smaller Reporting Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, as amended (the “JOBS Act”). As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
| • | being permitted to only disclose two years of audited financial statements in addition to any required unaudited interim financial statements with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure; |
| • | reduced disclosure about our executive compensation arrangements; |
| • | not being required to hold advisory votes on executive compensation or to obtain stockholder approval of any golden parachute arrangements not previously approved; and |
| • | an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting. |
We may take advantage of these exemptions until the fifth anniversary of our initial public offering or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company on the date that is the earliest of (i) the last day of the fiscal year in which we have total annual gross
4
Table of Contents
revenue of $1.07 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of this offering; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the last day of the fiscal year in which we are deemed to be a large accelerated filer under the rules of the SEC, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th. We may choose to take advantage of some but not all of these exemptions. We have taken advantage of reduced reporting requirements in this prospectus. Accordingly, the information contained herein may be different from the information you receive from other public companies in which you hold stock. We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements under the JOBS Act. Subject to certain conditions, as an emerging growth company, we may rely on certain of these exemptions, including without limitation, providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act of 2002.
We are also a “smaller reporting company” as defined in the Exchange Act. We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies until the fiscal year following the determination that our voting and non-voting common stock held by non-affiliates is more than $250 million measured on the last business day of our second fiscal quarter, or our annual revenues are less than $100 million during the most recently completed fiscal year and our voting and non-voting common stock held by non-affiliates is more than $700 million measured on the last business day of our second fiscal quarter.
5
Table of Contents
We intend to use the net proceeds from the sale of any securities offered under this prospectus for general corporate purposes unless otherwise indicated in the applicable prospectus supplement. General corporate purposes may include research and development and clinical development costs to support the advancement of our product candidates and the expansion of our research and development programs; working capital; capital expenditures; office expansion; and other general corporate purposes. We may temporarily invest the net proceeds in a variety of capital preservation instruments, including short-term, investment-grade, interest-bearing instruments and U.S. government securities, or may hold such proceeds as cash, until they are used for their stated purpose. We have not determined the amount of net proceeds to be used specifically for such purposes. As a result, management will retain broad discretion over the allocation of net proceeds.
6
Table of Contents
SECURITIES THAT MAY BE OFFERED
This prospectus contains summary descriptions of the securities we may offer from time to time. These summary descriptions are not meant to be complete descriptions of each security. The particular terms of any security will be described in the applicable prospectus supplement. This prospectus provides you with a general description of the securities we may offer. Each time we sell securities described herein, we will provide prospective investors with a supplement to this prospectus that will contain specific information about the terms of that offering, including the specific amounts, prices and terms of the securities offered.
We may sell the securities to or through underwriters, dealers or agents, directly to purchasers or through a combination of any of these methods of sale or as otherwise set forth below under “Plan of Distribution.” We, as well as any agents acting on our behalf, reserve the sole right to accept and to reject in whole or in part any proposed purchase of securities. Any prospectus supplement will set forth the names of any underwriters, dealers, agents or other entities involved in the sale of securities described in that prospectus supplement and any applicable fee, commission or discount arrangements with them.
7
Table of Contents
The following description of our capital stock is intended as a summary only and therefore is not a complete description of our capital stock. This description is based upon, and is qualified by reference to, our certificate of incorporation, our bylaws, and applicable provisions of Delaware corporate law. You should read our certificate of incorporation and our bylaws, in each case, as amended and supplemented, which are filed as exhibits to the registration statement of which this prospectus forms a part, for the provisions that are important to you.
General
Our authorized capital stock consists of 140,000,000 shares of common stock, par value $0.0001 per share, 10,000,000 shares of non-voting common stock, par value $0.0001 per share and 10,000,000 shares of undesignated preferred stock, par value $0.0001 per share, all of which shares of preferred stock are undesignated. The following description of our capital stock and provisions of our certificate of incorporation and bylaws are summaries and are qualified by reference to our certificate of incorporation and our bylaws, in each case, as amended and supplemented.
Common Stock
As of June 30, 2022, we had outstanding 40,333,944 shares of common stock and 1,150,000 shares of non-voting common stock, held of record by 28 stockholders.
The holders of our common stock and non-voting common stock have identical rights subject to two exceptions. First, except as otherwise expressly provided in our certificate of incorporation or as required by applicable law, on any matter that is submitted to a vote by our stockholders, holders of our common stock are entitled to one vote per share of common stock, and holders of our non-voting common stock are not entitled to any votes per share of non-voting common stock, including for the election of directors. Second, holders of our common stock have no conversion rights, while holders of our non-voting common stock shall have the right to convert each share of our non-voting common stock held into one share of common stock at such holder’s election, provided that as a result of such conversion, such holder, together with its affiliates and any members of a Schedule 13(d) group with such holder, would not beneficially own in excess of 9.9% of our common stock following such conversion, unless otherwise expressly provided for in our certificate of incorporation. However, this ownership limitation may be increased to any other percentage designated by such holder of non-voting common stock upon 61 days’ notice to us or decreased at any time. Holders of our non-voting common stock are also permitted to make certain transfers to non-affiliates upon which, such transferred shares would immediately convert to shares of our common stock.
Except as otherwise provided by law, our third amended and restated certificate of incorporation or our bylaws, in all matters other than the election of directors, the affirmative vote of the majority of the shares present in person or represented by proxy at a meeting at which a quorum is present and entitled to vote on the subject matter shall be the act of the stockholders. Directors shall be elected by a plurality of the shares present in person or represented by proxy at a meeting at which a quorum is present and entitled to vote on the election of directors.
Holders of our common stock common stock and non-voting common stock are entitled to receive ratably any dividends declared by our board of directors out of funds legally available for that purpose, subject to any preferential dividend rights of any outstanding preferred stock. Our common stock and non-voting common stock have no preemptive rights or other subscription rights or redemption or sinking fund provisions.
In the event of our liquidation, dissolution or winding up, holders of our common stock and non-voting common stock will be entitled to share ratably in all assets remaining after payment of all debts and other liabilities and any liquidation preference of any outstanding preferred stock. Holders of shares of our common stock and
8
Table of Contents
non-voting common stock are not required to make additional capital contributions. The shares to be issued by us in this offering will be, when issued and paid for, validly issued, fully paid and non-assessable.
Preferred Stock
As of June 30, 2022, no shares of our preferred stock were issued and outstanding.
Our board of directors will have the authority, without further action by our stockholders, to issue up to 10,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting, or the designation of, such series, any or all of which may be greater than the rights of common stock. The issuance of our preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon our liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change in control of our Company or other corporate action.
Registration Rights
Certain holders of our common stock are entitled to rights with respect to the registration of these securities under the Securities Act. These rights are provided under the terms of an investor rights agreement between us and certain holders of our common stock (the “Investor Rights Agreement Parties”). The investor rights agreement includes demand registration rights, short-form registration rights, and piggyback registration rights. All fees, costs and expenses of underwritten registrations under this agreement will be borne by us and all selling expenses, including underwriting discounts and selling commissions, will be borne by the holders of the shares being registered.
Demand Registration Rights
Beginning six months after our initial public offering, certain holders of our voting common stock and non-voting common stock are entitled to demand registration rights. Under the terms of the investors’ rights agreement, we will be required, upon the written request of a majority of holders of the registerable securities then outstanding that would result in an aggregate offering price of at least $10 million, to file a registration statement on Form S-1 with respect to at least 30% of the registrable securities then outstanding and to use commercially reasonable efforts to effect the registration of all or a portion of these shares for public resale.
Short-form Registration Rights
Certain holders of our voting common stock and non-voting common stock are also entitled to short-form registration rights. Pursuant to the investors’ rights agreement, if we are eligible to file a registration statement on Form S-3, upon the written request of at least 20% in interest of these holders to sell registrable securities at an aggregate price of at least $5 million, we will be required to use commercially reasonable efforts to effect a registration of such shares. We are required to effect only two registrations in any twelve month period pursuant to this provision of the investor rights agreement.
Piggyback Registration Rights
Certain holders of our voting common stock and non-voting common stock are entitled to piggyback registration rights. If we register any of our securities either for our own account or for the account of other security holders, the holders of these shares are entitled to include their shares in the registration. Subject to certain exceptions contained in the investors’ rights agreement, we and the underwriters may limit the number of shares included in the underwritten offering to the number of shares which we and the underwriters determine in our sole discretion will not jeopardize the success of the offering.
9
Table of Contents
Indemnification
Our investors’ rights agreement contains customary cross-indemnification provisions, under which we are obligated to indemnify holders of registrable securities in the event of material misstatements or omissions in the registration statement attributable to us, and they are obligated to indemnify us for material misstatements or omissions attributable to them.
Expiration of Registration Rights
The demand registration rights and short-form registration rights granted under the investor rights agreement will terminate on the third anniversary of the completion of our initial public offering.
Anti-takeover Effects of our Certificate of Incorporation and Bylaws and Delaware Law
Our certificate of incorporation and bylaws include a number of provisions that may have the effect of delaying, deferring or preventing another party from acquiring control of us and encouraging persons considering unsolicited tender offers or other unilateral takeover proposals to negotiate with our board of directors rather than pursue non-negotiated takeover attempts. These provisions include the items described below.
Board Composition and Filling Vacancies
Our certificate of incorporation provides for the division of our board of directors into three classes serving staggered three-year terms, with one class being elected each year. Our certificate of incorporation also provides that directors may be removed only for cause and then only by the affirmative vote of the holders of two-thirds or more of the shares then entitled to vote at an election of directors. Furthermore, any vacancy on our board of directors, however occurring, including a vacancy resulting from an increase in the size of our board, may only be filled by the affirmative vote of a majority of our directors then in office even if less than a quorum. The classification of directors, together with the limitations on removal of directors and treatment of vacancies, has the effect of making it more difficult for stockholders to change the composition of our board of directors.
No Written Consent of Stockholders
Our certificate of incorporation provides that all stockholder actions are required to be taken by a vote of the stockholders at an annual or special meeting, and that stockholders may not take any action by written consent in lieu of a meeting. This limit may lengthen the amount of time required to take stockholder actions and would prevent the amendment of our bylaws or removal of directors by our stockholders without holding a meeting of stockholders.
Meetings of Stockholders
Our certificate of incorporation and bylaws provide that only a majority of the members of our board of directors then in office may call special meetings of stockholders and only those matters set forth in the notice of the special meeting may be considered or acted upon at a special meeting of stockholders. Our bylaws limit the business that may be conducted at an annual meeting of stockholders to those matters properly brought before the meeting.
Advance Notice Requirements
Our bylaws establish advance notice procedures with regard to stockholder proposals relating to the nomination of candidates for election as directors or new business to be brought before meetings of our stockholders. These procedures provide that notice of stockholder proposals must be timely given in writing to our corporate secretary prior to the meeting at which the action is to be taken. Generally, to be timely, notice must be received
10
Table of Contents
at our principal executive offices not less than 90 days nor more than 120 days prior to the first anniversary date of the annual meeting for the preceding year. Our bylaws specify the requirements as to form and content of all stockholders’ notices. These requirements may preclude stockholders from bringing matters before the stockholders at an annual or special meeting.
Amendment to Certificate of Incorporation and Bylaws
Any amendment of our certificate of incorporation must first be approved by a majority of our board of directors, and if required by law or our certificate of incorporation, must thereafter be approved by a majority of the outstanding shares entitled to vote on the amendment and a majority of the outstanding shares of each class entitled to vote thereon as a class, except that the amendment of the provisions relating to stockholder action, board composition, and limitation of liability must be approved by not less than two-thirds of the outstanding shares entitled to vote on the amendment, and not less than two-thirds of the outstanding shares of each class entitled to vote thereon as a class. Our bylaws may be amended by the affirmative vote of a majority of the directors then in office, subject to any limitations set forth in the bylaws; and may also be amended by the affirmative vote of a majority of the outstanding shares entitled to vote on the amendment, voting together as a single class, except that the amendment of the provisions relating to notice of stockholder business and nominations and special meetings must be approved by not less than two-thirds of the outstanding shares entitled to vote on the amendment, and not less than two-thirds of the outstanding shares of each class entitled to vote thereon as a class, or, if our board of directors recommends that the stockholders approve the amendment, by the affirmative vote of the majority of the outstanding shares entitled to vote on the amendment, in each case voting together as a single class.
Undesignated Preferred Stock
Our certificate of incorporation provides for 10,000,000 authorized shares of preferred stock. The existence of authorized but unissued shares of preferred stock may enable our board of directors to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or otherwise. For example, if in the due exercise of its fiduciary obligations, our board of directors were to determine that a takeover proposal is not in the best interests of our stockholders, our board of directors could cause shares of preferred stock to be issued without stockholder approval in one or more private offerings or other transactions that might dilute the voting or other rights of the proposed acquirer or insurgent stockholder or stockholder group. In this regard, our certificate of incorporation grants our board of directors broad power to establish the rights and preferences of authorized and unissued shares of preferred stock. The issuance of shares of preferred stock could decrease the amount of earnings and assets available for distribution to holders of shares of common stock and non-voting common stock. The issuance may also adversely affect the rights and powers, including voting rights, of these holders and may have the effect of delaying, deterring or preventing a change in control of us.
Choice of Forum
Our bylaws provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for any state law claims for (1) any derivative action or proceeding brought on our behalf, (2) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers, and employees to us or our stockholders, (3) any action asserting a claim arising pursuant to the Delaware General Corporation Law, our certificate of incorporation or our bylaws, (4) any action to interpret, apply, enforce or determine the validity of our certificate of incorporation or bylaws, or (5) any action asserting a claim that is governed by the internal affairs doctrine; provided, however, that the this provision shall not apply to any causes of action arising under the Securities Act or Exchange Act. In addition, our bylaws provide that, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States shall be the sole and exclusive forum for resolving any complaint asserting a cause or causes of action arising under the Securities Act, including all causes of action asserted against any defendants to such complaint. For the avoidance of doubt, this provision is intended to benefit and
11
Table of Contents
may be enforced by us, our officers and directors, the underwriters to any offering giving rise to such complaint, and any other professional entity whose profession gives authority to a statement made by that person or entity and who has prepared or certified any part of the documents underlying the offering. While the Delaware courts have determined that such choice of forum provisions are facially valid, a stockholder may nevertheless seek to bring a claim in a venue other than those designated in the exclusive forum provisions, and there can be no assurance that such provisions will be enforced by a court in those other jurisdictions. Any person or entity purchasing or otherwise acquiring any interest in our securities shall be deemed to have notice of and consented to these forum provisions. These forum provisions may impose additional costs on stockholders, may limit our stockholders’ ability to bring a claim in a forum they find favorable, and the designated courts may reach different judgments or results than other courts.
Section 203 of the Delaware General Corporation Law
We are subject to the provisions of Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless the business combination is approved in a prescribed manner. Under Section 203, a business combination between a corporation and an interested stockholder is prohibited unless it satisfies one of the following conditions:
| • | before the stockholder became interested, our board of directors approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; |
| • | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, shares owned by persons who are directors and also officers, and employee stock plans, in some instances, but not the outstanding voting stock owned by the interested stockholder; or |
| • | at or after the time the stockholder became interested, the business combination was approved by our board of directors and authorized at an annual or special meeting of the stockholders by the affirmative vote of at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder. |
Section 203 defines a business combination to include:
| • | any merger or consolidation involving the corporation and the interested stockholder; |
| • | any sale, transfer, lease, pledge or other disposition involving the interested stockholder of 10% or more of the assets of the corporation; |
| • | subject to exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
| • | subject to exceptions, any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder; and |
| • | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges, or other financial benefits provided by or through the corporation. |
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
Listing on the Nasdaq Global Market
Our common stock is listed on the Nasdaq Global Market under the symbol “TALS.”
12
Table of Contents
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Computershare Trust Company, N.A. The transfer agent and registrar’s address is 250 Royall Street, Canton, MA 02021, and its telephone number is (800) 962-4284.
13
Table of Contents
DESCRIPTION OF DEBT SECURITIES
We may issue debt securities from time to time, in one or more series, as either senior or subordinated debt or as senior or subordinated convertible debt. While the terms we have summarized below will apply generally to any debt securities that we may offer under this prospectus, we will describe the particular terms of any debt securities that we may offer in more detail in the applicable prospectus supplement. The terms of any debt securities offered under a prospectus supplement may differ from the terms described below. Unless the context requires otherwise, whenever we refer to the indenture, we also are referring to any supplemental indentures that specify the terms of a particular series of debt securities.
We will issue the debt securities under the indenture that we will enter into with the trustee named in the indenture. The indenture will be qualified under the Trust Indenture Act of 1939, as amended (the “Trust Indenture Act”). We have filed the form of indenture as an exhibit to the registration statement of which this prospectus is a part, and supplemental indentures and forms of debt securities containing the terms of the debt securities being offered will be filed as exhibits to the registration statement of which this prospectus is a part, or will be incorporated by reference from, reports that we file with the SEC.
The following summary of material provisions of the debt securities and the indenture is subject to, and qualified in its entirety by reference to, all of the provisions of the indenture applicable to a particular series of debt securities. We urge you to read the applicable prospectus supplements and any related free writing prospectuses related to the debt securities that we may offer under this prospectus, as well as the complete indenture that contains the terms of the debt securities.
General
The indenture does not limit the amount of debt securities that we may issue. It provides that we may issue debt securities up to the principal amount that we may authorize and may be in any currency or currency unit that we may designate. Except for the limitations on consolidation, merger and sale of all or substantially all of our assets contained in the indenture, the terms of the indenture do not contain any covenants or other provisions designed to give holders of any debt securities protection against changes in our operations, financial condition or transactions involving us.
We may issue the debt securities issued under the indenture as “discount securities,” which means they may be sold at a discount below their stated principal amount. These debt securities, as well as other debt securities that are not issued at a discount, may be issued with “original issue discount” (“OID”) for U.S. federal income tax purposes because of interest payment and other characteristics or terms of the debt securities. Material U.S. federal income tax considerations applicable to debt securities issued with OID will be described in more detail in any applicable prospectus supplement.
We will describe in the applicable prospectus supplement the terms of the series of debt securities being offered, including:
| • | the title of the series of debt securities; |
| • | any limit upon the aggregate principal amount that may be issued; |
| • | the maturity date or dates; |
| • | the form of the debt securities of the series; |
| • | the applicability of any guarantees; |
| • | whether or not the debt securities will be secured or unsecured, and the terms of any secured debt; |
| • | whether the debt securities rank as senior debt, senior subordinated debt, subordinated debt or any combination thereof, and the terms of any subordination; |
14
Table of Contents
| • | if the price (expressed as a percentage of the aggregate principal amount thereof) at which such debt securities will be issued is a price other than the principal amount thereof, the portion of the principal amount thereof payable upon declaration of acceleration of the maturity thereof, or if applicable, the portion of the principal amount of such debt securities that is convertible into another security or the method by which any such portion shall be determined; |
| • | the interest rate or rates, which may be fixed or variable, or the method for determining the rate and the date interest will begin to accrue, the dates interest will be payable and the regular record dates for interest payment dates or the method for determining such dates; |
| • | our right, if any, to defer payment of interest and the maximum length of any such deferral period; |
| • | if applicable, the date or dates after which, or the period or periods during which, and the price or prices at which, we may, at our option, redeem the series of debt securities pursuant to any optional or provisional redemption provisions and the terms of those redemption provisions; |
| • | the date or dates, if any, on which, and the price or prices at which we are obligated, pursuant to any mandatory sinking fund or analogous fund provisions or otherwise, to redeem, or at the holder’s option to purchase, the series of debt securities and the currency or currency unit in which the debt securities are payable; |
| • | the denominations in which we will issue the series of debt securities, if other than denominations of $1,000 and any integral multiple thereof; |
| • | any and all terms, if applicable, relating to any auction or remarketing of the debt securities of that series and any security for our obligations with respect to such debt securities and any other terms which may be advisable in connection with the marketing of debt securities of that series; |
| • | whether the debt securities of the series shall be issued in whole or in part in the form of a global security or securities; the terms and conditions, if any, upon which such global security or securities may be exchanged in whole or in part for other individual securities; and the depositary for such global security or securities; |
| • | if applicable, the provisions relating to conversion or exchange of any debt securities of the series and the terms and conditions upon which such debt securities will be so convertible or exchangeable, including the conversion or exchange price, as applicable, or how it will be calculated and may be adjusted, any mandatory or optional (at our option or the holders’ option) conversion or exchange features, the applicable conversion or exchange period and the manner of settlement for any conversion or exchange; |
| • | if other than the full principal amount thereof, the portion of the principal amount of debt securities of the series which shall be payable upon declaration of acceleration of the maturity thereof; |
| • | additions to or changes in the covenants applicable to the particular debt securities being issued, including, among others, the consolidation, merger or sale covenant; |
| • | additions to or changes in the events of default with respect to the securities and any change in the right of the trustee or the holders to declare the principal, premium, if any, and interest, if any, with respect to such securities to be due and payable; |
| • | additions to or changes in or deletions of the provisions relating to covenant defeasance and legal defeasance; |
| • | additions to or changes in the provisions relating to satisfaction and discharge of the indenture; |
| • | additions to or changes in the provisions relating to the modification of the indenture both with and without the consent of holders of debt securities issued under the indenture; |
| • | the currency of payment of debt securities if other than U.S. dollars and the manner of determining the equivalent amount in U.S. dollars; |
15
Table of Contents
| • | whether interest will be payable in cash or additional debt securities at our or the holders’ option and the terms and conditions upon which the election may be made; |
| • | the terms and conditions, if any, upon which we will pay amounts in addition to the stated interest, premium, if any and principal amounts of the debt securities of the series to any holder that is not a “United States person” for federal tax purposes; |
| • | any restrictions on transfer, sale or assignment of the debt securities of the series; and |
| • | any other specific terms, preferences, rights or limitations of, or restrictions on, the debt securities, any other additions or changes in the provisions of the indenture, and any terms that may be required by us or advisable under applicable laws or regulations. |
Conversion or Exchange Rights
We will set forth in the applicable prospectus supplement the terms on which a series of debt securities may be convertible into or exchangeable for our common stock or our other securities. We will include provisions as to settlement upon conversion or exchange and whether conversion or exchange is mandatory, at the option of the holder or at our option. We may include provisions pursuant to which the number of shares of our common stock or our other securities that the holders of the series of debt securities receive would be subject to adjustment.
Consolidation, Merger or Sale
Unless we provide otherwise in the prospectus supplement applicable to a particular series of debt securities, the indenture will not contain any covenant that restricts our ability to merge or consolidate, or sell, convey, transfer or otherwise dispose of our assets as an entirety or substantially as an entirety. However, any successor to or acquirer of such assets (other than any subsidiary of ours) must assume all of our obligations under the indenture or the debt securities, as appropriate.
Events of Default under the Indenture
Unless we provide otherwise in the prospectus supplement applicable to a particular series of debt securities, the following are events of default under the indenture with respect to any series of debt securities that we may issue:
| • | if we fail to pay any installment of interest on any series of debt securities, as and when the same shall become due and payable, and such default continues for a period of 90 days; provided, however, that a valid extension of an interest payment period by us in accordance with the terms of any indenture supplemental thereto shall not constitute a default in the payment of interest for this purpose; |
| • | if we fail to pay the principal of, or premium, if any, on any series of debt securities as and when the same shall become due and payable whether at maturity, upon redemption, by declaration or otherwise, or in any payment required by any sinking or analogous fund established with respect to such series; provided, however, that a valid extension of the maturity of such debt securities in accordance with the terms of any indenture supplemental thereto shall not constitute a default in the payment of principal or premium, if any; |
| • | if we fail to observe or perform any other covenant or agreement contained in the debt securities or the indenture, other than a covenant specifically relating to another series of debt securities, and our failure continues for 90 days after we receive written notice of such failure, requiring the same to be remedied and stating that such is a notice of default thereunder, from the trustee or holders of at least 25% in aggregate principal amount of the outstanding debt securities of the applicable series; and |
| • | if specified events of bankruptcy, insolvency or reorganization occur. |
If an event of default with respect to debt securities of any series occurs and is continuing, other than an event of default specified in the last bullet point above, the trustee or the holders of at least 25% in aggregate principal
16
Table of Contents
amount of the outstanding debt securities of that series, by notice to us in writing, and to the trustee if notice is given by such holders, may declare the unpaid principal of, premium, if any, and accrued interest, if any, due and payable immediately. If an event of default specified in the last bullet point above occurs with respect to us, the principal amount of and accrued interest, if any, of each issue of debt securities then outstanding shall be due and payable without any notice or other action on the part of the trustee or any holder.
The holders of a majority in principal amount of the outstanding debt securities of an affected series may waive any default or event of default with respect to the series and its consequences, except defaults or events of default regarding payment of principal, premium, if any, or interest, unless we have cured the default or event of default in accordance with the indenture. Any waiver shall cure the default or event of default.
Subject to the terms of the indenture, if an event of default under an indenture shall occur and be continuing, the trustee will be under no obligation to exercise any of its rights or powers under such indenture at the request or direction of any of the holders of the applicable series of debt securities, unless such holders have offered the trustee reasonable indemnity. The holders of a majority in principal amount of the outstanding debt securities of any series will have the right to direct the time, method and place of conducting any proceeding for any remedy available to the trustee, or exercising any trust or power conferred on the trustee, with respect to the debt securities of that series, provided that:
| • | the direction so given by the holder is not in conflict with any law or the applicable indenture; and |
| • | subject to its duties under the Trust Indenture Act, the trustee need not take any action that might involve it in personal liability or might be unduly prejudicial to the holders not involved in the proceeding. |
A holder of the debt securities of any series will have the right to institute a proceeding under the indenture or to appoint a receiver or trustee, or to seek other remedies only if:
| • | the holder has given written notice to the trustee of a continuing event of default with respect to that series; |
| • | the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series have made written request, |
| • | such holders have offered to the trustee indemnity satisfactory to it against the costs, expenses and liabilities to be incurred by the trustee in compliance with the request; and |
| • | the trustee does not institute the proceeding, and does not receive from the holders of a majority in aggregate principal amount of the outstanding debt securities of that series other conflicting directions within 90 days after the notice, request and offer. |
These limitations do not apply to a suit instituted by a holder of debt securities if we default in the payment of the principal, premium, if any, or interest on, the debt securities.
We will periodically file statements with the trustee regarding our compliance with specified covenants in the indenture.
Modification of Indenture; Waiver
We and the trustee may change an indenture without the consent of any holders with respect to specific matters:
| • | to cure any ambiguity, defect or inconsistency in the indenture or in the debt securities of any series; |
| • | to comply with the provisions described above under “Description of Debt Securities—Consolidation, Merger or Sale”; |
| • | to provide for uncertificated debt securities in addition to or in place of certificated debt securities; |
17
Table of Contents
| • | to add to our covenants, restrictions, conditions or provisions such new covenants, restrictions, conditions or provisions for the benefit of the holders of all or any series of debt securities, to make the occurrence, or the occurrence and the continuance, of a default in any such additional covenants, restrictions, conditions or provisions an event of default or to surrender any right or power conferred upon us in the indenture; |
| • | to add to, delete from or revise the conditions, limitations, and restrictions on the authorized amount, terms, or purposes of issue, authentication and delivery of debt securities, as set forth in the indenture; |
| • | to make any change that does not adversely affect the interests of any holder of debt securities of any series in any material respect; |
| • | to provide for the issuance of and establish the form and terms and conditions of the debt securities of any series as provided above under “Description of Debt Securities—General” to establish the form of any certifications required to be furnished pursuant to the terms of the indenture or any series of debt securities, or to add to the rights of the holders of any series of debt securities; |
| • | to evidence and provide for the acceptance of appointment under any indenture by a successor trustee; or |
| • | to comply with any requirements of the SEC in connection with the qualification of any indenture under the Trust Indenture Act. |
In addition, under the indenture, the rights of holders of a series of debt securities may be changed by us and the trustee with the written consent of the holders of at least a majority in aggregate principal amount of the outstanding debt securities of each series that is affected. However, unless we provide otherwise in the prospectus supplement applicable to a particular series of debt securities, we and the trustee may make the following changes only with the consent of each holder of any outstanding debt securities affected:
| • | extending the fixed maturity of any debt securities of any series; |
| • | reducing the principal amount, reducing the rate of or extending the time of payment of interest, or reducing any premium payable upon the redemption of any series of any debt securities; or |
| • | reducing the percentage of debt securities, the holders of which are required to consent to any amendment, supplement, modification or waiver. |
Discharge
Each indenture provides that we can elect to be discharged from our obligations with respect to one or more series of debt securities, except for specified obligations, including obligations to:
| • | provide for payment; |
| • | register the transfer or exchange of debt securities of the series; |
| • | replace stolen, lost or mutilated debt securities of the series; |
| • | pay principal of and premium and interest on any debt securities of the series; |
| • | maintain paying agencies; |
| • | hold monies for payment in trust; |
| • | recover excess money held by the trustee; |
| • | compensate and indemnify the trustee; and |
| • | appoint any successor trustee. |
18
Table of Contents
In order to exercise our rights to be discharged, we must deposit with the trustee money or government obligations sufficient to pay all the principal of, any premium, if any, and interest on, the debt securities of the series on the dates payments are due.
Form, Exchange and Transfer
We will issue the debt securities of each series only in fully registered form without coupons and, unless we provide otherwise in the applicable prospectus supplement, in denominations of $1,000 and any integral multiple thereof. The indenture provides that we may issue debt securities of a series in temporary or permanent global form and as book-entry securities that will be deposited with, or on behalf of, The Depository Trust Company (“DTC”) or another depositary named by us and identified in the applicable prospectus supplement with respect to that series. To the extent the debt securities of a series are issued in global form and as book-entry, a description of terms relating to any book-entry securities will be set forth in the applicable prospectus supplement.
At the option of the holder, subject to the terms of the indenture and the limitations applicable to global securities described in the applicable prospectus supplement, the holder of the debt securities of any series can exchange the debt securities for other debt securities of the same series, in any authorized denomination and of like tenor and aggregate principal amount.
Subject to the terms of the indenture and the limitations applicable to global securities set forth in the applicable prospectus supplement, holders of the debt securities may present the debt securities for exchange or for registration of transfer, duly endorsed or with the form of transfer endorsed thereon duly executed if so required by us or the security registrar, at the office of the security registrar or at the office of any transfer agent designated by us for this purpose. Unless otherwise provided in the debt securities that the holder presents for transfer or exchange, we will impose no service charge for any registration of transfer or exchange, but we may require payment of any taxes or other governmental charges.
We will name in the applicable prospectus supplement the security registrar, and any transfer agent in addition to the security registrar, that we initially designate for any debt securities. We may at any time designate additional transfer agents or rescind the designation of any transfer agent or approve a change in the office through which any transfer agent acts, except that we will be required to maintain a transfer agent in each place of payment for the debt securities of each series.
If we elect to redeem the debt securities of any series, we will not be required to:
| • | issue, register the transfer of, or exchange any debt securities of that series during a period beginning at the opening of business 15 days before the day of mailing of a notice of redemption of any debt securities that may be selected for redemption and ending at the close of business on the day of the mailing; or |
| • | register the transfer of or exchange any debt securities so selected for redemption, in whole or in part, except the unredeemed portion of any debt securities we are redeeming in part. |
Information Concerning the Trustee
The trustee, other than during the occurrence and continuance of an event of default under an indenture, undertakes to perform only those duties as are specifically set forth in the applicable indenture. Upon an event of default under an indenture, the trustee must use the same degree of care as a prudent person would exercise or use in the conduct of his or her own affairs. Subject to this provision, the trustee is under no obligation to exercise any of the powers given it by the indenture at the request of any holder of debt securities unless it is offered reasonable security and indemnity against the costs, expenses and liabilities that it might incur.
19
Table of Contents
Payment and Paying Agents
Unless we otherwise indicate in the applicable prospectus supplement, we will make payment of the interest on any debt securities on any interest payment date to the person in whose name the debt securities, or one or more predecessor securities, are registered at the close of business on the regular record date for the interest.
We will pay principal of and any premium and interest on the debt securities of a particular series at the office of the paying agents designated by us, except that unless we otherwise indicate in the applicable prospectus supplement, we will make interest payments by check that we will mail to the holder or by wire transfer to certain holders. Unless we otherwise indicate in the applicable prospectus supplement, we will designate the corporate trust office of the trustee as our sole paying agent for payments with respect to debt securities of each series. We will name in the applicable prospectus supplement any other paying agents that we initially designate for the debt securities of a particular series. We will maintain a paying agent in each place of payment for the debt securities of a particular series.
All money we pay to a paying agent or the trustee for the payment of the principal of or any premium or interest on any debt securities that remains unclaimed at the end of two years after such principal, premium or interest has become due and payable will be repaid to us, and the holder of the debt security thereafter may look only to us for payment thereof.
Governing Law
The indenture and the debt securities will be governed by and construed in accordance with the laws of the state of New York, except to the extent that the Trust Indenture Act is applicable.
20
Table of Contents
The following description, together with the additional information we may include in any applicable prospectus supplements, summarizes the material terms and provisions of the warrants that we may offer under this prospectus and the related warrant agreements and warrant certificates. While the terms summarized below will apply generally to any warrants that we may offer, we will describe the particular terms of any series of warrants in more detail in the applicable prospectus supplement. If we indicate in the prospectus supplement, the terms of any warrants offered under that prospectus supplement may differ from the terms described below. Specific warrant agreements will contain additional important terms and provisions and will be incorporated by reference as an exhibit to the registration statement, which includes this prospectus.
General
We may issue warrants for the purchase of common stock, preferred stock in one or more series. We may issue warrants independently or together with common stock, preferred stock, and the warrants may be attached to or separate from these securities.
We will evidence each series of warrants by warrant certificates that we will issue under a separate warrant agreement. We will enter into the warrant agreement with a warrant agent. We will indicate the name and address of the warrant agent in the applicable prospectus supplement relating to a particular series of warrants.
We will describe in the applicable prospectus supplement the terms of the series of warrants, including:
| • | the offering price and aggregate number of warrants offered; |
| • | the currency for which the warrants may be purchased; |
| • | if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security; |
| • | if applicable, the date on and after which the warrants and the related securities will be separately transferable; |
| • | in the case of warrants to purchase common stock or preferred stock, the number of shares of common stock or preferred stock, as the case may be, purchasable upon the exercise of one warrant and the price at which these shares may be purchased upon such exercise; |
| • | in the case of warrants to purchase debt securities, the principal amount of debt securities purchasable upon exercise of one warrant and the price at, and currency in which, this principal amount of debt securities may be purchased upon such exercise; |
| • | the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreement and the warrants; |
| • | the terms of any rights to redeem or call the warrants; |
| • | any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants; |
| • | the periods during which, and places at which, the warrants are exercisable; |
| • | the manner of exercise; |
| • | the dates on which the right to exercise the warrants will commence and expire; |
| • | the manner in which the warrant agreement and warrants may be modified; |
| • | federal income tax consequences of holding or exercising the warrants; |
| • | the terms of the securities issuable upon exercise of the warrants; and |
| • | any other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
21
Table of Contents
We may issue units comprised of shares of common stock, shares of preferred stock and warrants in any combination. We may issue units in such amounts and in as many distinct series as we wish. This section outlines certain provisions of the units that we may issue. If we issue units, they will be issued under one or more unit agreements to be entered into between us and a bank or other financial institution, as unit agent. The information described in this section may not be complete in all respects and is qualified entirely by reference to the unit agreement with respect to the units of any particular series. The specific terms of any series of units offered will be described in the applicable prospectus supplement. If so described in a particular supplement, the specific terms of any series of units may differ from the general description of terms presented below. We urge you to read any prospectus supplement related to any series of units we may offer, as well as the complete unit agreement and unit certificate that contain the terms of the units. If we issue units, forms of unit agreements and unit certificates relating to such units will be incorporated by reference as exhibits to the registration statement, which includes this prospectus.
Each unit that we may issue will be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or at any time before a specified date. The applicable prospectus supplement may describe:
| • | the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; |
| • | any provisions of the governing unit agreement; |
| • | the price or prices at which such units will be issued; |
| • | the applicable United States federal income tax considerations relating to the units; |
| • | any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units; and |
| • | any other terms of the units and of the securities comprising the units. |
The provisions described in this section, as well as those described under “Description of Capital Stock,” and “Description of Warrants” will apply to the securities included in each unit, to the extent relevant and as may be updated in any prospectus supplements.
Issuance in Series
We may issue units in such amounts and in as many distinct series as we wish. This section summarizes terms of the units that apply generally to all series. Most of the financial and other specific terms of a particular series of units will be described in the applicable prospectus supplement.
Unit Agreements
We will issue the units under one or more unit agreements to be entered into between us and a bank or other financial institution, as unit agent. We may add, replace or terminate unit agents from time to time. We will identify the unit agreement under which each series of units will be issued and the unit agent under that agreement in the applicable prospectus supplement.
22
Table of Contents
The following provisions will generally apply to all unit agreements unless otherwise stated in the applicable prospectus supplement:
Modification without Consent
We and the applicable unit agent may amend any unit or unit agreement without the consent of any holder:
| • | to cure any ambiguity; any provisions of the governing unit agreement that differ from those described below; |
| • | to correct or supplement any defective or inconsistent provision; or |
| • | to make any other change that we believe is necessary or desirable and will not adversely affect the interests of the affected holders in any material respect. |
We do not need any approval to make changes that affect only units to be issued after the changes take effect. We may also make changes that do not adversely affect a particular unit in any material respect, even if they adversely affect other units in a material respect. In those cases, we do not need to obtain the approval of the holder of the unaffected unit; we need only obtain any required approvals from the holders of the affected units.
Modification with Consent
We may not amend any particular unit or a unit agreement with respect to any particular unit unless we obtain the consent of the holder of that unit, if the amendment would:
| • | impair any right of the holder to exercise or enforce any right under a security included in the unit if the terms of that security require the consent of the holder to any changes that would impair the exercise or enforcement of that right; or |
| • | reduce the percentage of outstanding units or any series or class the consent of whose holders is required to amend that series or class, or the applicable unit agreement with respect to that series or class, as described below. |
Any other change to a particular unit agreement and the units issued under that agreement would require the following approval:
| • | If the change affects only the units of a particular series issued under that agreement, the change must be approved by the holders of a majority of the outstanding units of that series; or |
| • | If the change affects the units of more than one series issued under that agreement, it must be approved by the holders of a majority of all outstanding units of all series affected by the change, with the units of all the affected series voting together as one class for this purpose. |
These provisions regarding changes with majority approval also apply to changes affecting any securities issued under a unit agreement, as the governing document.
In each case, the required approval must be given by written consent.
Unit Agreements Will Not Be Qualified under Trust Indenture Act
No unit agreement will be qualified as an indenture, and no unit agent will be required to qualify as a trustee, under the Trust Indenture Act. Therefore, holders of units issued under unit agreements will not have the protections of the Trust Indenture Act with respect to their units.
Mergers and Similar Transactions Permitted; No Restrictive Covenants or Events of Default
The unit agreements will not restrict our ability to merge or consolidate with, or sell our assets to, another corporation or other entity or to engage in any other transactions. If at any time we merge or consolidate with, or
23
Table of Contents
sell our assets substantially as an entirety to, another corporation or other entity, the successor entity will succeed to and assume our obligations under the unit agreements. We will then be relieved of any further obligation under these agreements.
The unit agreements will not include any restrictions on our ability to put liens on our assets, nor will they restrict our ability to sell our assets. The unit agreements also will not provide for any events of default or remedies upon the occurrence of any events of default.
Governing Law
The unit agreements and the units will be governed by New York law.
Form, Exchange and Transfer
We will issue each unit in global—i.e., book-entry—form only. Units in book-entry form will be represented by a global security registered in the name of a depositary, which will be the holder of all the units represented by the global security. Those who own beneficial interests in a unit will do so through participants in the depositary’s system, and the rights of these indirect owners will be governed solely by the applicable procedures of the depositary and its participants. We will describe book-entry securities, and other terms regarding the issuance and registration of the units in the applicable prospectus supplement.
Each unit and all securities comprising the unit will be issued in the same form.
If we issue any units in registered, non-global form, the following will apply to them.
The units will be issued in the denominations stated in the applicable prospectus supplement. Holders may exchange their units for units of smaller denominations or combined into fewer units of larger denominations, as long as the total amount is not changed.
| • | Holders may exchange or transfer their units at the office of the unit agent. Holders may also replace lost, stolen, destroyed or mutilated units at that office. We may appoint another entity to perform these functions or perform them ourselves. |
| • | Holders will not be required to pay a service charge to transfer or exchange their units, but they may be required to pay for any tax or other governmental charge associated with the transfer or exchange. The transfer or exchange, and any replacement, will be made only if our transfer agent is satisfied with the holder’s proof of legal ownership. The transfer agent may also require an indemnity before replacing any units. |
| • | If we have the right to redeem, accelerate or settle any units before their maturity, and we exercise our right as to less than all those units or other securities, we may block the exchange or transfer of those units during the period beginning 15 days before the day we mail the notice of exercise and ending on the day of that mailing, in order to freeze the list of holders to prepare the mailing. We may also refuse to register transfers of or exchange any unit selected for early settlement, except that we will continue to permit transfers and exchanges of the unsettled portion of any unit being partially settled. We may also block the transfer or exchange of any unit in this manner if the unit includes securities that are or may be selected for early settlement. |
Only the depositary will be entitled to transfer or exchange a unit in global form, since it will be the sole holder of the unit.
Payments and Notices
In making payments and giving notices with respect to our units, we will follow the procedures as described in the applicable prospectus supplement.
24
Table of Contents
We may sell the securities offered through this prospectus and any accompanying prospectus supplement, if required, in any of the following ways: (i) to or through underwriters or dealers, (ii) directly to purchasers, including our affiliates, (iii) through agents, (iv) in an “at the market offering,” within the meaning of Rule 415(a)(4) of the Securities Act or (v) through a combination of any of these methods or any other method permitted by law. The securities may be distributed at a fixed price or prices, which may be changed, market prices prevailing at the time of sale, prices related to the prevailing market prices, or negotiated prices, either:
| • | on or through the facilities of The Nasdaq Global Market or any other securities exchange or quotation or trading service on which such securities may be listed, quoted or traded at the time of sale; and/or |
| • | to or through a market maker otherwise than on The Nasdaq Global Market or such other securities exchanges or quotation or trading services. |
In addition, we may issue the securities as a dividend or distribution or in a subscription rights offering to our existing security holders.
We may directly solicit offers to purchase securities, or agents may be designated to solicit such offers. In the prospectus supplement relating to such offering, we will name any agent that could be viewed as an underwriter under the Securities Act and describe any commissions that we must pay to any such agent. Any such agent will be acting on a best efforts basis for the period of its appointment or, if indicated in the applicable prospectus supplement, on a firm commitment basis. This prospectus may be used in connection with any offering of our securities through any of these methods or other methods described in the applicable prospectus supplement.
Each prospectus supplement will describe the method of distribution of the securities and any applicable restrictions.
The prospectus supplement with respect to the securities of a particular series will describe the terms of the offering of the securities, including the following:
| • | the name of the agent or any underwriters; |
| • | the public offering or purchase price; |
| • | any discounts and commissions to be allowed or paid to the agent or underwriters; |
| • | all other items constituting underwriting compensation; |
| • | any discounts and commissions to be allowed or paid to dealers; and |
| • | any exchanges on which the securities will be listed. |
If any underwriters or agents are used in the sale of the securities in respect of which this prospectus is delivered, we will enter into an underwriting agreement, sales agreement or other agreement with them at the time of sale to them, and we will set forth in the prospectus supplement relating to such offering the names of the underwriters or agents and the terms of the related agreement with them.
In connection with the offering of securities, we may grant to the underwriters an option to purchase additional securities with an additional underwriting commission, as may be set forth in the accompanying prospectus supplement. If we grant any such option, the terms of such option will be set forth in the prospectus supplement for such securities.
If a dealer is used in the sale of the securities in respect of which the prospectus is delivered, we will sell such securities to the dealer, as principal. The dealer, who may be deemed to be an “underwriter” as that term is defined in the Securities Act, may then resell such securities to the public at varying prices to be determined by such dealer at the time of resale.
25
Table of Contents
If we offer securities in a subscription rights offering to our existing security holders, we may enter into a standby underwriting agreement with dealers, acting as standby underwriters. We may pay the standby underwriters a commitment fee for the securities they commit to purchase on a standby basis. If we do not enter into a standby underwriting arrangement, we may retain a dealer-manager to manage a subscription rights offering for us.
Agents, underwriters, dealers and other persons may be entitled under agreements which they may enter into with us to indemnification by us against certain civil liabilities, including liabilities under the Securities Act, and may be customers of, engage in transactions with or perform services for us in the ordinary course of business.
If so indicated in the applicable prospectus supplement, we will authorize underwriters or other persons acting as our agents to solicit offers by certain institutions to purchase securities from us pursuant to delayed delivery contracts providing for payment and delivery on the date stated in the prospectus supplement. Each contract will be for an amount not less than, and the aggregate amount of securities sold pursuant to such contracts shall not be less nor more than, the respective amounts stated in the prospectus supplement. Institutions with whom the contracts, when authorized, may be made include commercial and savings banks, insurance companies, pension funds, investment companies, educational and charitable institutions and other institutions, but shall in all cases be subject to our approval. Delayed delivery contracts will not be subject to any conditions except that:
| • | the purchase by an institution of the securities covered under that contract shall not at the time of delivery be prohibited under the laws of the jurisdiction to which that institution is subject; and |
| • | if the securities are also being sold to underwriters acting as principals for their own account, the underwriters shall have purchased such securities not sold for delayed delivery. The underwriters and other persons acting as our agents will not have any responsibility in respect of the validity or performance of delayed delivery contracts. |
Offered securities may also be offered and sold, if so indicated in the prospectus supplement, in connection with a remarketing upon their purchase, in accordance with a redemption or repayment pursuant to their terms, or otherwise, by one or more remarketing firms, acting as principals for their own accounts or as agents for us. Any remarketing firm will be identified and the terms of its agreement, if any, with us and its compensation will be described in the applicable prospectus supplement. Remarketing firms may be deemed to be underwriters in connection with their remarketing of offered securities.
Certain agents, underwriters and dealers, and their associates and affiliates, may be customers of, have borrowing relationships with, engage in other transactions with, or perform services, including investment banking services, for us or one or more of our respective affiliates in the ordinary course of business.
In order to facilitate the offering of the securities, any underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of the securities or any other securities the prices of which may be used to determine payments on such securities. Specifically, any underwriters may overallot in connection with the offering, creating a short position for their own accounts. In addition, to cover overallotments or to stabilize the price of the securities or of any such other securities, the underwriters may bid for, and purchase, the securities or any such other securities in the open market. Finally, in any offering of the securities through a syndicate of underwriters, the underwriting syndicate may reclaim selling concessions allowed to an underwriter or a dealer for distributing the securities in the offering if the syndicate repurchases previously distributed securities in transactions to cover syndicate short positions, in stabilization transactions or otherwise. Any of these activities may stabilize or maintain the market price of the securities above independent market levels. Any such underwriters are not required to engage in these activities and may end any of these activities at any time.
We may engage in at the market offerings into an existing trading market in accordance with Rule 415(a)(4) under the Securities Act. In addition, we may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If the applicable
26
Table of Contents
prospectus supplement so indicates, in connection with those derivatives, the third parties may sell securities covered by this prospectus and the applicable prospectus supplement, including in short sale transactions. If so, the third party may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of stock, and may use securities received from us in settlement of those derivatives to close out any related open borrowings of stock. The third party in such sale transactions will be an underwriter and, if not identified in this prospectus, will be named in the applicable prospectus supplement (or a post-effective amendment). In addition, we may otherwise loan or pledge securities to a financial institution or other third party that in turn may sell the securities short using this prospectus and an applicable prospectus supplement. Such financial institution or other third party may transfer its economic short position to investors in our securities or in connection with a concurrent offering of other securities.
Under Rule 15c6-1 of the Exchange Act, trades in the secondary market generally are required to settle in two business days, unless the parties to any such trade expressly agree otherwise. The applicable prospectus supplement may provide that the original issue date for your securities may be more than two scheduled business days after the trade date for your securities. Accordingly, in such a case, if you wish to trade securities on any date prior to the second business day before the original issue date for your securities, you will be required, by virtue of the fact that your securities initially are expected to settle in more than two scheduled business days after the trade date for your securities, to make alternative settlement arrangements to prevent a failed settlement.
The securities may be new issues of securities and may have no established trading market. The securities may or may not be listed on a national securities exchange. We can make no assurance as to the liquidity of or the existence of trading markets for any of the securities.
The specific terms of any lock-up provisions in respect of any given offering will be described in the applicable prospectus supplement.
Any underwriters, dealers and agents may engage in transactions with us, or perform services for us, in the ordinary course of business for which they receive compensation.
The anticipated date of delivery of offered securities will be set forth in the applicable prospectus supplement relating to each offer.
27
Table of Contents
Unless the applicable prospectus supplement indicates otherwise, the validity of the securities in respect of which this prospectus is being delivered will be passed upon by Goodwin Procter LLP, Boston, Massachusetts. Any underwriters will also be advised about the validity of the securities and other legal matters by their own counsel, which will be named in the prospectus supplement.
The financial statements of Talaris Therapeutics, Inc. incorporated by reference in this prospectus, have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report. Such financial statements are incorporated by reference in reliance upon the report of such firm, given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at www.sec.gov and on our investor website at www.talaristx.com. Our website is not a part of this prospectus and is not incorporated by reference in this prospectus.
We have the authority to designate and issue more than one class or series of stock having various preferences, conversion and other rights, voting powers, restrictions, limitations as to dividends, qualifications, and terms and conditions of redemption. See “Description of Capital Stock.” We will furnish a full statement of the relative rights and preferences of each class or series of our stock which has been so designated and any restrictions on the ownership or transfer of our stock to any stockholder upon request and without charge. Written requests for such copies should be directed to Talaris Therapeutics, Inc., 93 Worcester Steet, Wellesley, MA 02481, Attention: Corporate Secretary.
This prospectus is part of a registration statement on Form S-3 we filed with the SEC. This prospectus omits some information contained in the registration statement in accordance with SEC rules and regulations. You should review the information and exhibits in the registration statement for further information about us and our consolidated subsidiaries and the securities we are offering. Statements in this prospectus concerning any document we filed as an exhibit to the registration statement or that we otherwise filed with the SEC are not intended to be comprehensive and are qualified by reference to these filings. You should review the complete document to evaluate these statements.
28
Table of Contents
The SEC allows us to incorporate by reference much of the information we file with the SEC, which means that we can disclose important information to you by referring you to those publicly available documents. The information that we incorporate by reference in this prospectus is considered to be part of this prospectus. Because we are incorporating by reference future filings with the SEC, this prospectus is continually updated and those future filings may modify or supersede some of the information included or incorporated in this prospectus. This means that you must look at all of the SEC filings that we incorporate by reference to determine if any of the statements in this prospectus or in any document previously incorporated by reference have been modified or superseded. This prospectus incorporates by reference the documents listed below and any future filings we make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, including all filings made after the date of the filing of this registration statement and prior to the effectiveness of this registration statement, except as to any portion of any future report or document that is not deemed filed under such provisions until we sell all of the securities:
| • | Annual Report on Form 10-K for the fiscal year ended December 31, 2021, filed with the SEC on March 17, 2022; |
| • | The information specifically incorporated by reference into our Annual Report on Form 10-K for the year ended December 31, 2021 from our definitive proxy statement on Schedule 14A (other than information furnished rather than filed), which was filed with the SEC on April 29, 2022; |
| • | Quarterly Reports on Form 10-Q filed with the SEC on May 12, 2022 and August 15, 2022; |
| • | Current Reports on Form 8-K filed with the SEC on May 17, 2022, June 13, 2022 and June 30, 2022; and |
| • | The description of our common stock contained in our Registration Statement on Form 8-A (File No. 001-40384) as filed with the SEC on May 4, 2021, including any amendments or reports filed for the purpose of updating such description, including Exhibit 4.3 to our Annual Report on Form 10-K for the year ended December 31, 2020, as filed with the SEC on March 25, 2021. |
In addition, all reports and other documents filed by us pursuant to the Exchange Act after the date of the initial registration statement and prior to effectiveness of the registration statement shall be deemed to be incorporated by reference into this prospectus.
You may request a copy of these filings, at no cost, by writing or telephoning us at the following address or telephone number:
Talaris Therapeutics, Inc.
93 Worcester Steet
Wellesley, MA 02481
Attn: Investor Relations
(502) 398-9250
You may also access these documents, free of charge on the SEC’s website at www.sec.gov or on our website at www.talaristx.com. Information contained on our website is not incorporated by reference into this prospectus, and you should not consider any information on, or that can be accessed from, our website as part of this prospectus or any accompanying prospectus supplement.
Notwithstanding the foregoing, unless specifically stated to the contrary, information that we furnish (and that is not deemed “filed” with the SEC) under Items 2.02 and 7.01 of any Current Report on Form 8-K, including the related exhibits under Item 9.01, is not incorporated by reference into this prospectus or the registration statement of which this prospectus is a part.
29
Table of Contents
This prospectus is part of a registration statement we filed with the SEC. We have incorporated exhibits into this registration statement. You should read the exhibits carefully for provisions that may be important to you.
You should rely only on the information incorporated by reference or provided in this prospectus or any prospectus supplement. We have not authorized anyone to provide you with different information. We are not making an offer of these securities in any state where the offer is not permitted. You should not assume that the information in this prospectus or in the documents incorporated by reference is accurate as of any date other than the date on the front of this prospectus or those documents.
30
Table of Contents

Shares of Common Stock
PRELIMINARY PROSPECTUS SUPPLEMENT
Joint Book-Running Managers
Jefferies
Piper Sandler
Guggenheim Securities
Truist Securities
, 2024