
December 8, 2020
Securities and Exchange Commission
Division of Corporation Finance
Office of Life Sciences
100 F Street, N.E.
Washington, D.C. 20549
Attention: Deanna Virginio
Suzanne Hayes
Nudrat Salik
Sasha Parikh
Re: BioAtla, Inc.
Registration Statement on Form S-1
Filed on November 13, 2020
File No. 333-250093
Ladies and Gentlemen,
On behalf of our client, BioAtla, Inc. (the “Company”), we are responding to the comments from the Staff (the “Staff”) of the Securities and Exchange Commission (the “Commission”) relating to the Company’s Registration Statement on Form S-1 (the “Registration Statement”) contained in the Staff’s letter dated December 3, 2020 (the “Comment Letter”). In response to the comments set forth in the Comment Letter, the Company has revised the Registration Statement and is filing an Amendment No. 1 to the Registration Statement on Form S-1 (the “Amended Registration Statement”) together with this response letter. The Amended Registration Statement also contains certain additional updates and revisions.
Set forth below are the Company’s responses to the Staff’s comments in the Comment Letter. The responses and information below are based on information provided to us by the Company. For convenience, the Staff’s comments are repeated below in bold, followed by the Company’s response to the comments as well as a summary of the responsive actions taken. We have included page numbers to refer to the location in the Amended Registration Statement submitted herewith where the revised language addressing a particular comment appears. Capitalized terms used but not defined herein are used herein as defined in the Amended Registration Statement.

|
Page 2 |
Registration Statement on Form S-1 filed November 13, 2020
LLC Conversion, page 82
1. We note your response to comment 82 and clarify that a diagram depicting the transactions resulting in the single legal entity will assist the reader in understanding the series of transactions.
Response: The Company respectfully advises the Staff that it has revised disclosure on page 84 of the Amended Registration Statement.
BA3011 Phase 1 clinical trial
Antitumor Activity, page 135
2. We note your revised disclosure in response to prior comment 7. Please expand your disclosure to discuss your results in terms of the clinical endpoints described on page 135. Please also disclose whether any of the results related to antitumor activity were considered statistically significant. Please include similar disclosure for BA3021.
Response: The Company respectfully advises the Staff that it has revised disclosure on pages 138-139, 142 and 147-149 of the Amended Registration Statement.
Safety, page 140
3. We note your revised disclosure in response to prior comment 20, including SAEs observed in patients receiving the Phase 2 dose level and the 2.4 mg/kg Q3W does level. We also note your disclosure on page 134 that patients were enrolled into 9 dose cohorts. Please revise your disclosure to clarify whether any SAEs were observed in any of the other dose cohorts.
Response: The Company respectfully advises the Staff that it has revised disclosure on pages 142-143 of the Amended Registration Statement.
Bispecific candidates, page 154
4. We note your response to prior comment 5 and your revised disclosure regarding the EpCAM x CD3 bispecific candidates. Please revise your disclosure to include a

|
Page 3 |
description of preclinical studies or other development activities conducted for BA3142, EGFR x CD3 or Nectin-4 x CD3 bispecific antibody product candidates. Alternatively, please remove any programs that are not material from your pipeline table.
Response: The Company respectfully advises the Staff that it has revised disclosure on page 157 and the pipeline table on pages 3, 119-120 and 131 of the Amended Registration Statement.
Global Co-Development and Collaboration Agreement with BeiGene, Ltd., page 156
5. Please disclose the royalty term under the Global Co-Development and Collaboration Agreement with BeiGene.
Response: The Company respectfully advises the Staff that it has revised disclosure on page 160 of the Amended Registration Statement.
Amended and Restated Exclusive Rights Agreement with Himalaya Therapeutics SEZC, page 157
6. Please revise your disclosure to clarify the nature of the rights granted to Himalaya Therapeutics SEZC. We also note your disclosure that payments to the Company may include upfront payments, milestone payments and double-digit royalties. Please disclose the amounts paid to date, aggregate future potential milestone payments to be received, and the royalty term.
Response: The Company respectfully advises the Staff that it has revised disclosure on page 161 of the Amended Registration Statement.
Exclusive License Agreement with BioAtla Holdings, LLC, page 157
7. Please revise your disclosure to describe the nature of the rights granted to BioAtla LLC. Please also describe any obligations of BioAtla under the agreement. For example, will BioAtla owe any potential future milestone or royalty payments in connection with the rights granted. With respect to your option to acquire the ACT preparations and ACT treatments, please expand your disclosure to describe the nature of the rights you may acquire. Please also disclose the term of the royalty under your option.

|
Page 4 |
Response: The Company respectfully advises the Staff that it has revised disclosure on pages 161-162 of the Amended Registration Statement.
Exclusive License Agreement with Inversagen, page 157
8. Please revise your disclosure to clarify the nature of the rights acquired by Inversagen. For example, did the Company grant a worldwide exclusive license to develop and commercialize the antibodies. We also note your disclosure that Inversagen will pay you milestone payments and royalties. Please disclose the aggregate future potential milestone payments to be received. Please also disclose the royalty rates or range and the royalty term. With respect to your option to acquire rights to the immuno-oncology antibody please specify the nature of those rights and disclose the royalty term referenced.
Response: The Company respectfully advises the Staff that it has revised disclosure on pages 160-161 of the Amended Registration Statement.
Notes to the Financial Statements
1. Organization and summary of significant accounting policies, page F-8
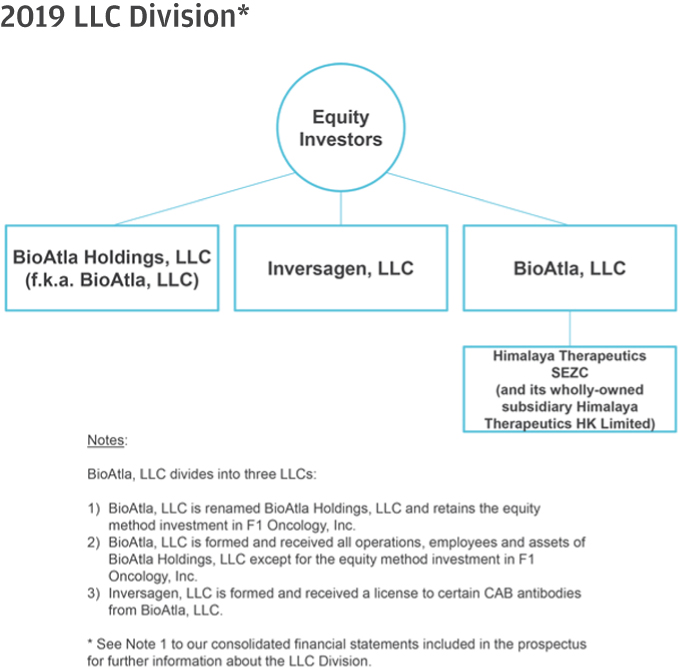
9. We note your response to comments 14 and 27 and revised disclosure. Regarding the March 2019 Division, please address the following:
| • | When describing the “exceptions” related to the operating agreement and capital structure between the companies, please identify, in revised disclosure, which of the three “Post-Division LLC’s” you are referring to as the “Post-Division Successor”. In this regard, it would appear from your disclosure here that the “Post-Division Successor” is the new legal entity named BioAtla, LLC rather than BioAtla Holdings, LLC. |
and
| • | Your response to comment 12 indicates that prior to July 2020, you had two consolidated subsidiaries, Himalaya Therapeutics SEZC and its wholly owned subsidiary Himalaya Therapeutics HK Limited. Please clarify whether these entities existed at the time of the March 2019 Division. If so, please include a discussion of these subsidiaries under the ‘Organization’ and “LLC Division’ headers in this footnote as applicable. |

|
Page 5 |
Response: The Company respectfully advises the Staff that it has revised disclosure on page F-8 of the Amended Registration Statement. Both Himalaya Therapeutics SEZC and its wholly owned subsidiary Himalaya Therapeutics HK Limited existed at both the time of the Division and the Corporate Reorganization and the disclosure on page F-8 has been updated to clarify this.
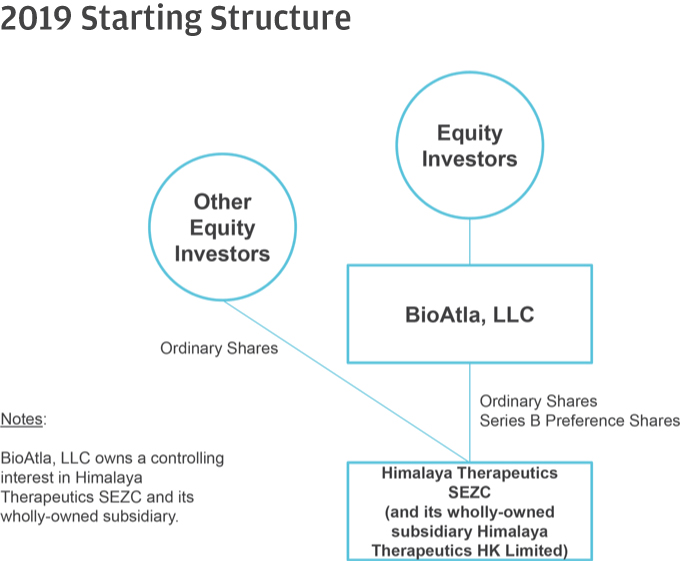
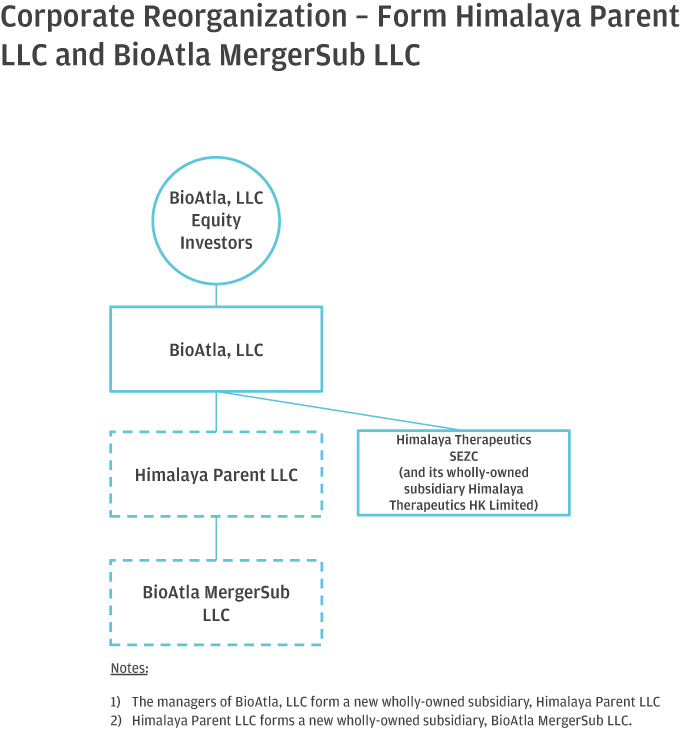
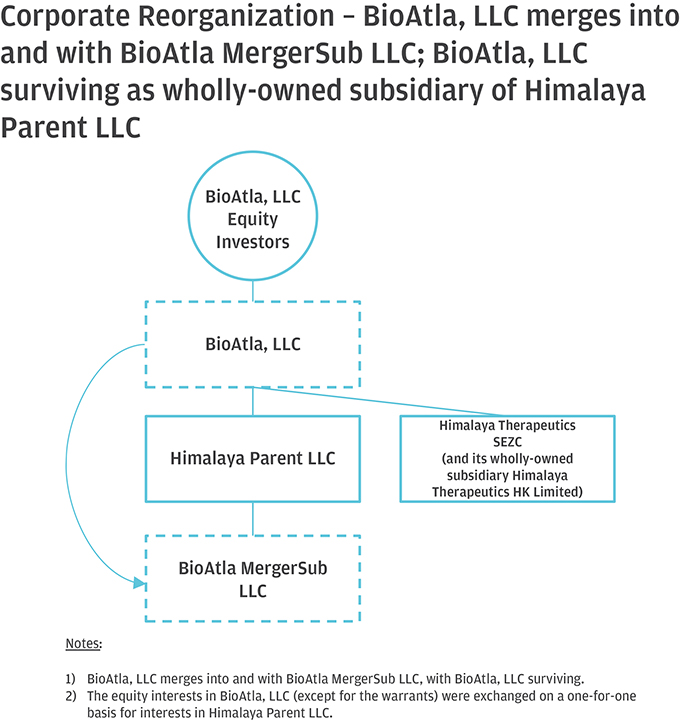
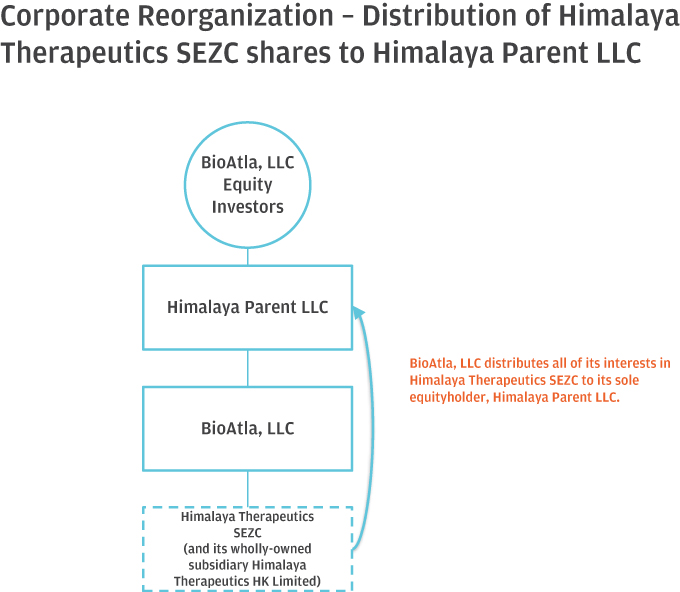
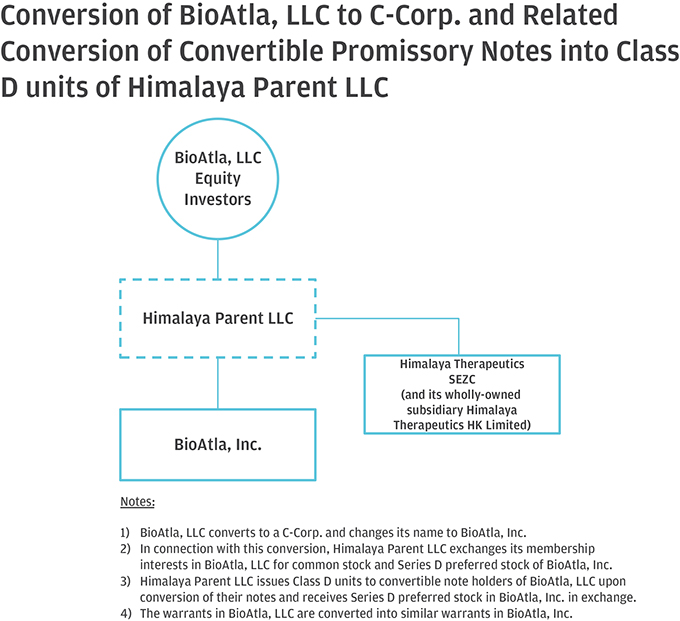
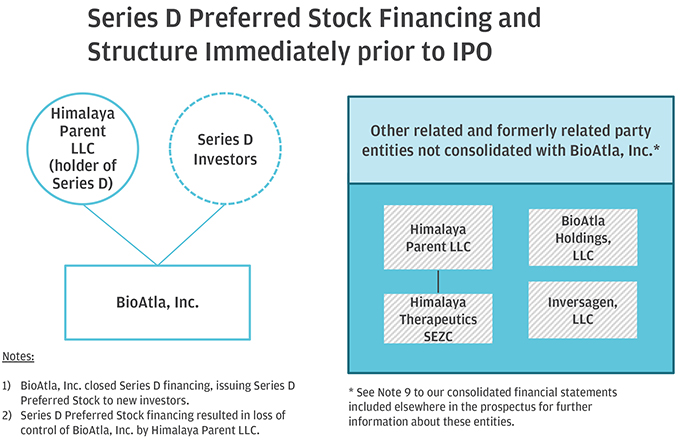
10. Please help us better understand and further clarify your disclosures related to the series of transactions that took place as part of your Corporate Reorganization. Please clarify in your disclosure that Himalaya Parent, LLC became the parent to BioAtla, LLC after it was merged into BioAtla MergerSub LLC, with BioAtla, LLC surviving, if true. If this is not a true statement, please clarify. Also, clarify how you subsequently separated from Himalaya Parent, LLC. Providing us a diagram of your organization from the Pre-Division Predecessor to the “Division” and lastly, the corporate reorganization.
Response: The Company respectfully advises the Staff that it has revised disclosure on pages F-8-F-9 of the Amended Registration Statement. Himalaya Parent briefly became the parent to BioAtla, LLC after BioAtla, LLC was merged with BioAtla Merger Sub LLC, with BioAtla, LLC surviving. Following the issuance of BioAtla, Inc.’s Series D Preferred Shares to the crossover investors, however, Himalaya Parent no longer controlled BioAtla, Inc. The disclosure on pages F-8–F-9 have been updated to clarify this. The following is a graphical depiction of the LLC Division, Corporate Reorganization, LLC Conversion and related transactions.
11. Please tell us how your equity accounts reflect compliance with SAB Topic 4B relating to undistributed earnings or losses of the limited liability company for periods prior to becoming a taxable corporation.
Response: The Company respectfully acknowledges the Staff’s comment and has reclassified its pre-LLC Conversion accumulated deficit to additional paid-in capital (up to the aggregate amount of paid-in capital at that date) in accordance with SAB Topic 4B. Accordingly, the Company has revised its presentation on pages F-3 and F-5.

|
Page 6 |
* * *
Please contact the undersigned at (202) 261-3440 if you have any questions regarding the foregoing
Sincerely,
| /s/ David E. Schulman |
| David E. Schulman |
| cc: | Jay M. Short, PhD, BioAtla, Inc. |
David S. Rosenthal, Dechert LLP