UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM
(Mark One)
QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number:
(Exact Name of Registrant as Specified in its Charter)
( State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer |
(Address of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area code: (
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
|
The |
||
|
|
The |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
|||
|
☒ |
|
Smaller reporting company |
|
||
|
|
|
|
|
|
|
Emerging growth company |
|
|
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes
As of May 9, 2022, there were
Table of Contents
|
|
Page |
PART I. |
|
|
Item 1. |
1 |
|
|
Unaudited Condensed Consolidated Balance Sheets as of March 31, 2022 and December 31, 2021 |
1 |
|
Unaudited Condensed Consolidated Statements of Operations and Comprehensive Loss for the three months ended March 31, 2022 and 2021 |
2 |
|
Unaudited Condensed Consolidated Statements of Redeemable Convertible Preferred Stock and Stockholders’ Equity for the three months ended March 31, 2022 and 2021 |
3 |
|
Unaudited Condensed Consolidated Statements of Cash Flows for the three months ended March 31, 2022 and 2021 |
4 |
|
Notes to Unaudited Condensed Consolidated Financial Statements |
5 |
Item 2. |
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
16 |
Item 3. |
22 |
|
Item 4. |
22 |
|
|
|
|
PART II. |
|
|
Item 1. |
24 |
|
Item 1A. |
24 |
|
Item 2. |
24 |
|
Item 3. |
24 |
|
Item 4. |
24 |
|
Item 5. |
24 |
|
Item 6. |
25 |
|
26 |
||
i
PART I—FINANCIAL INFORMATION
Item 1. Financial Statements
SURROZEN, INC.
Condensed Consolidated Balance Sheets
(In thousands, except per share amounts)
|
|
March 31, |
|
|
December 31, 2021 |
|
||
|
|
(Unaudited) |
|
|
|
|
||
Assets |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
|
|
$ |
|
||
Short-term marketable securities |
|
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
|
|
|
|
||
Total current assets |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Property and equipment, net |
|
|
|
|
|
|
||
Operating lease right-of-use assets |
|
|
|
|
|
|
||
Long-term marketable securities |
|
|
|
|
|
|
||
Restricted cash |
|
|
|
|
|
|
||
Other assets |
|
|
|
|
|
|
||
Total assets |
|
$ |
|
|
$ |
|
||
|
|
|
|
|
|
|
||
Liabilities and stockholders’ equity |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
|
|
$ |
|
||
Accrued and other liabilities |
|
|
|
|
|
|
||
Lease liabilities, current portion |
|
|
|
|
|
|
||
Total current liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
Lease liabilities, noncurrent portion |
|
|
|
|
|
|
||
Warrant liabilities |
|
|
|
|
|
|
||
Total liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
||
Stockholders’ equity: |
|
|
|
|
|
|
||
Preferred stock, $ |
|
|
|
|
|
|
||
Common stock, $ |
|
|
|
|
|
|
||
Additional paid-in capital |
|
|
|
|
|
|
||
Accumulated other comprehensive loss |
|
|
( |
) |
|
|
( |
) |
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
Total stockholders’ equity |
|
|
|
|
|
|
||
Total liabilities and stockholders’ equity |
|
$ |
|
|
$ |
|
||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
1
SURROZEN, INC.
Condensed Consolidated Statements of Operations and Comprehensive Loss
(Unaudited)
(In thousands, except per share amounts)
|
|
Three Months Ended March 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Operating expenses: |
|
|
|
|
|
|
||
Research and development |
|
$ |
|
|
$ |
|
||
General and administrative |
|
|
|
|
|
|
||
Total operating expenses |
|
|
|
|
|
|
||
Loss from operations |
|
|
( |
) |
|
|
( |
) |
Interest income |
|
|
|
|
|
|
||
Other income |
|
|
|
|
|
|
||
Net loss |
|
|
( |
) |
|
|
( |
) |
Unrealized loss on marketable securities, net of tax |
|
|
( |
) |
|
|
|
|
Comprehensive loss |
|
$ |
( |
) |
|
$ |
( |
) |
|
|
|
|
|
|
|
||
Net loss per share attributable to common |
|
$ |
( |
) |
|
$ |
( |
) |
|
|
|
|
|
|
|
||
Weighted-average shares used in computing net |
|
|
|
|
|
|
||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
2
SURROZEN, INC.
Condensed Consolidated Statements of Redeemable Convertible Preferred Stock and Stockholders’ Equity
(Unaudited)
(In thousands)
|
|
|
|
|
|
|
|
Additional |
|
|
Accumulated |
|
|
|
|
|
Total |
|
||||||
|
|
Common stock |
|
|
paid-in |
|
|
comprehensive |
|
|
Accumulated |
|
|
stockholders’ |
|
|||||||||
|
|
Shares |
|
|
Amount |
|
|
capital |
|
|
loss |
|
|
deficit |
|
|
equity |
|
||||||
Balance at December 31, 2021 |
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
||||
Issuance of common stock under Equity Purchase Agreement |
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Repurchase of early exercised stock options |
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Vesting of early exercised stock options |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Other comprehensive loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
( |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Balance at March 31, 2022 |
|
|
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
|
$ |
|
||||
|
|
Redeemable convertible |
|
|
|
|
|
|
|
|
Additional |
|
|
Accumulated |
|
|
|
|
|
Total |
|
|||||||||||
|
|
preferred stock |
|
|
Common stock |
|
|
paid-in |
|
|
comprehensive |
|
|
Accumulated |
|
|
stockholders’ |
|
||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
capital |
|
|
loss |
|
|
deficit |
|
|
equity |
|
||||||||
Balance at December 31, 2020, as previously reported |
|
|
|
|
$ |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
( |
) |
||||||
Retroactive application of recapitalization |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||||
Balance at December 31, 2020, after effect of Business |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
( |
) |
|
|
|
||||
Exercises of stock options |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|||
Restricted stock granted |
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Reclassification to liability for early exercised stock |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
— |
|
|
|
— |
|
|
|
( |
) |
Vesting of early exercised stock options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
||
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
( |
) |
|
|
( |
) |
Balance at March 31, 2021, after effect of Business |
|
|
— |
|
|
$ |
— |
|
|
|
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
3
SURROZEN, INC.
Condensed Consolidated Statements of Cash Flows
(Unaudited)
(In thousands)
|
Three Months Ended March 31, |
|
|||||
|
2022 |
|
|
2021 |
|
||
Operating activities: |
|
|
|
|
|
||
Net loss |
$ |
( |
) |
|
$ |
( |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
||
Depreciation |
|
|
|
|
|
||
Stock-based compensation |
|
|
|
|
|
||
Non-cash operating lease expense |
|
|
|
|
|
||
Amortization of premium on marketable securities, net |
|
|
|
|
|
||
Change in fair value of warrant liabilities |
|
( |
) |
|
|
|
|
Changes in operating assets and liabilities: |
|
|
|
|
|
||
Prepaid expenses and other current assets |
|
|
|
|
( |
) |
|
Other assets |
|
( |
) |
|
|
( |
) |
Accounts payable |
|
( |
) |
|
|
|
|
Accrued and other liabilities |
|
( |
) |
|
|
|
|
Operating lease liabilities |
|
( |
) |
|
|
( |
) |
Net cash used in operating activities |
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
||
Investing activities: |
|
|
|
|
|
||
Purchases of property and equipment |
|
( |
) |
|
|
( |
) |
Purchases of marketable securities |
|
|
|
|
( |
) |
|
Net cash used in investing activities |
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
||
Financing activities: |
|
|
|
|
|
||
Proceeds from exercise of stock options |
|
|
|
|
|
||
Net cash provided by financing activities |
|
|
|
|
|
||
|
|
|
|
|
|
||
Net decrease in cash, cash equivalents and restricted cash |
|
( |
) |
|
|
( |
) |
Cash, cash equivalents and restricted cash at beginning of period |
|
|
|
|
|
||
Cash, cash equivalents and restricted cash at end of period |
$ |
|
|
$ |
|
||
|
|
|
|
|
|
||
Supplemental disclosure of noncash investing and financing activities: |
|
|
|
|
|
||
Deferred costs related to Equity Purchase Agreement included in accounts |
$ |
|
|
$ |
|
||
Transaction costs in Business Combination included in accrued liabilities |
$ |
|
|
$ |
|
||
Purchases of property and equipment included in accounts payable |
$ |
|
|
$ |
|
||
Vesting of early exercises of stock options |
$ |
|
|
$ |
|
||
Reclassification of early exercised stock options to liability |
$ |
|
|
$ |
|
||
The following table presents a reconciliation of the Company’s cash, cash equivalents and restricted cash in the Company’s unaudited condensed consolidated balance sheets:
|
March 31, |
|
|||||
|
2022 |
|
|
2021 |
|
||
Cash and cash equivalents |
$ |
|
|
$ |
|
||
Restricted cash |
|
|
|
|
|
||
Cash, cash equivalents and restricted cash |
$ |
|
|
$ |
|
||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
4
SURROZEN, INC.
Notes to the Unaudited Condensed Consolidated Financial Statements
Note 1. Organization and Business
Organization
Surrozen, Inc., or the Company, formerly known as Consonance-HFW Acquisition Corp., or Consonance, is a preclinical stage biotechnology company committed to discovering and developing drug candidates to selectively modulate the Wnt pathway, a critical mediator of tissue repair, in a broad range of organs and tissues, for human diseases. The Company, a Delaware corporation, is located in South San Francisco, California.
Business Combination and Private Investment in Public Entity Financing
Consonance was a blank check company incorporated as a Cayman Islands exempted company on August 21, 2020. It was formed for the purpose of effecting a merger, share exchange, asset acquisition, share purchase, reorganization or similar business combination with one or more businesses.
On August 11, 2021, Consonance consummated a business combination, or the Business Combination, among Consonance, Perseverance Merger Sub Inc., a subsidiary of Consonance, and Surrozen, Inc., or Legacy Surrozen, a Delaware company incorporated on August 12, 2015. Upon closing of the Business Combination, Consonance became a Delaware corporation and was renamed to Surrozen, Inc., Legacy Surrozen, was renamed to Surrozen Operating, Inc., and Legacy Surrozen continued as a wholly-owned subsidiary of the Company. See Note 3, "Recapitalization" for additional details.
Liquidity
The Company has incurred net operating losses each period since inception. During the three months ended March 31, 2022 and 2021, the Company incurred a net loss of $
In February 2022, the Company entered into a purchase agreement, or the Equity Purchase Agreement, and a registration rights agreement with Lincoln Park Capital Fund, LLC, or Lincoln Park, pursuant to which Lincoln Park is obligated to purchase up to $
Management believes that the existing cash, cash equivalents, and marketable securities are sufficient for the Company to continue operating activities for at least the next 12 months from the date of issuance of its unaudited condensed consolidated financial statements. However, if the Company’s anticipated cash burn is greater than anticipated, the Company could use its capital resources sooner than expected which may result in the need to reduce future planned expenditures and/or raise additional capital to continue to fund the operations.
Note 2. Summary of Significant Accounting Policies
Basis of Presentation
The Company’s unaudited condensed consolidated financial statements and accompanying notes have been prepared in accordance with generally accepted accounting principles in the United States of America, or GAAP, and pursuant to the regulations of the U.S. Securities and Exchange Commission, or SEC. As permitted under those rules, certain footnotes or other financial information that are normally required by GAAP have been condensed or omitted, and accordingly the condensed consolidated balance sheet as of December 31, 2021 has been derived from the Company’s audited consolidated financial statements at that date but does not include all of the information required by GAAP for complete consolidated financial statements. These unaudited condensed consolidated financial statements have been prepared on the same basis as the Company’s annual consolidated financial statements and, in the opinion of management, reflect all adjustments (consisting of normal recurring adjustments) that are necessary for a fair presentation of the Company’s consolidated financial statements. The results of operations for the three months ended March 31, 2022 are not necessarily indicative of the results to be expected for the year ended December 31, 2022 or for any other interim period or for any other future year.
5
The unaudited condensed consolidated financial statements include the accounts of the Company and its subsidiary. All intercompany transactions and balances have been eliminated.
The Business Combination discussed in Note 1 was accounted for as a reverse recapitalization with Legacy Surrozen as the accounting acquirer and Consonance as the acquired company for accounting purposes. Accordingly, all historical financial information presented in the unaudited condensed consolidated financial statements represents the accounts of Legacy Surrozen at their historical cost as if Legacy Surrozen is the predecessor to the Company. The unaudited condensed consolidated financial statements following the closing of the Business Combination reflect the results of the combined entity’s operations. All issued and outstanding common stock, redeemable convertible preferred stock and stock awards of Legacy Surrozen and per share amounts contained in the unaudited condensed consolidated financial statements for the periods presented prior to the closing of the Business Combination have been retroactively restated to reflect the exchange ratio established in the Business Combination. See Note 3, “Recapitalization” for additional details.
The accompanying condensed consolidated financial statements and related financial information should be read in conjunction with the audited consolidated financial statements and the related notes thereto for the year ended December 31, 2021 included in the Company’s Annual Report on Form 10-K, filed with the SEC on March 28, 2022.
Use of Estimates
The preparation of unaudited condensed consolidated financial statements in conformity with GAAP requires management to make judgments, estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the unaudited condensed consolidated financial statements and the reported amounts of expenses during the reporting period. Significant estimates and assumptions made in the accompanying unaudited condensed consolidated financial statements include, but are not limited to, certain accruals for research and development activities, the fair value of common stock prior to the Business Combination, stock-based compensation expense and income taxes. Management bases its estimates on historical experience and on various other market-specific and relevant assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results could materially differ from those estimates.
Concentration of Credit Risk
Financial instruments, which potentially subject the Company to significant concentration of credit risk, consist of cash, cash equivalents and marketable securities. The Company's cash is held by one financial institution that management believes is creditworthy. Such deposits held with the financial institution may at times exceed federally insured limits, however, its exposure to credit risk in the event of default by the financial institution is limited to the extent of amounts recorded on the unaudited condensed consolidated balance sheets. The Company performs evaluations of the relative credit standing of these financial institutions to limit the amount of credit exposure. The Company's policy is to invest cash in institutional money market funds and marketable securities with high credit quality to limit the amount of credit exposure. The Company currently maintains a portfolio of cash equivalents and marketable securities in a variety of securities, including money market funds, U.S. government bonds, foreign bonds, commercial paper and corporate debt securities. The Company has not experienced any losses on its cash equivalents and marketable securities.
Marketable Securities
The Company invests its excess cash in marketable U.S. government bonds, foreign bonds, commercial paper and corporate debt securities. All marketable securities have been classified as available-for-sale and are carried at estimated fair value as determined based upon quoted market prices or pricing models for similar securities. The Company does not buy or hold securities principally for the purpose of selling them in the near future. The Company’s policy is focused on the preservation of capital, liquidity, and return. From time to time, the Company may sell certain securities, but the objectives are generally not to generate profits on short-term differences in price.
Short-term marketable securities have maturities less than or equal to one year as of the balance sheet date. Long-term marketable securities have maturities greater than one year as of the balance sheet date. These marketable securities are carried at estimated fair value with unrealized holding gains and losses included in accumulated other comprehensive loss in stockholders’ equity until realized. Gains and losses on marketable security transactions are reported on the specific-identification method. Interest income is recognized in the unaudited condensed consolidated statements of operations and comprehensive loss when earned.
The Company periodically evaluates its available-for-sale marketable securities for impairment. When the fair value of a marketable security is below its amortized cost, the amortized cost is reduced to its fair value if it is more likely than not that the Company is
6
Warrant Liabilities
The Company's Public Warrants, Private Placement Warrants and PIPE Warrants were classified as liabilities (see Note 8). At the end of each reporting period, any changes in fair value during the period are recognized in other income within the unaudited condensed consolidated statements of operations and comprehensive loss. The Company will continue to adjust the warrant liabilities for changes in the fair value until the earlier of a) the exercise or expiration of the warrants or b) the redemption of the warrants, at which time such warrants will be reclassified to additional paid-in capital.
Net Loss Per Share
Basic net loss per share is calculated by dividing the net loss attributable to common stock by the weighted-average number of shares of common stock outstanding for the period, without consideration for potential dilutive securities. Since the Company was in a loss position for the periods presented, basic net loss per share is the same as diluted net loss per share as the effects of potentially dilutive securities are antidilutive.
|
|
March 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
|
|
|
|
|
|
|
||
Options outstanding |
|
|
|
|
|
|
||
Unvested restricted stock |
|
|
|
|
|
|
||
Unvested common stock subject to repurchase |
|
|
|
|
|
|
||
Warrants to purchase common stock |
|
|
|
|
|
|
||
Total |
|
|
|
|
|
|
||
Note 3. Recapitalization
On August 11, 2021, Consonance consummated the Business Combination (see Note 1). Immediately after the consummation of the Business Combination, certain investors subscribed for and purchased an aggregate of
Accordingly, for accounting purposes, the reverse recapitalization was treated as the equivalent of Legacy Surrozen issuing stock for the net assets of Consonance, accompanied by a recapitalization. The net assets of Consonance are stated at historical cost, with
7
Pursuant to the Business Combination Agreement, upon the closing of the Business Combination, (i) each share of redeemable convertible preferred stock of Legacy Surrozen (on an as converted to common stock basis) and each share of common stock of Legacy Surrozen, whether vested or unvested, was converted into
Note 4. Fair Value Measurement
The following tables summarize the Company’s financial assets and liabilities that are measured at fair value on a recurring basis (in thousands):
|
|
March 31, 2022 |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds(1) |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Commercial paper |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Corporate bonds |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Government bonds |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Foreign bonds |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total financial assets measured at fair value |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Liabilities(2): |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Public Warrants |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Private Placement Warrants |
|
|
|
|
|
|
|
|
|
|
|
|
||||
PIPE Warrants |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total financial liabilities measured at fair value |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
December 31, 2021 |
|
|||||||||||||
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
|
Total |
|
||||
Assets: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds(1) |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Commercial paper |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Corporate bonds |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Government bonds |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Foreign bonds |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total financial assets measured at fair value |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Liabilities(2): |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Public Warrants |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Private Placement Warrants |
|
|
|
|
|
|
|
|
|
|
|
|
||||
PIPE Warrants |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Total financial liabilities measured at fair value |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
There were
Corporate bonds, commercial paper, foreign bonds and government bonds are classified as Level 2 as they were valued based upon quoted market prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active, and model-based valuation techniques for which all significant inputs are observable in the market or can be corroborated by observable market data for substantially the full term of the assets.
8
The Public Warrants are classified as Level 1 due to the use of an observable market quote in an active market. The Private Placement Warrants and PIPE Warrants are classified as Level 2 due to the use of observable market data for identical or similar liabilities. The fair value of each Private Placement Warrant and PIPE Warrant was determined to be consistent with that of a Public Warrant because the Private Placement Warrants and PIPE Warrants are also subject to the make-whole redemption feature, which allows the Company to redeem both types of warrants on similar terms when the stock price is in the range of $
The following tables provide the Company’s marketable securities by security type (in thousands):
|
|
March 31, 2022 |
|
|||||||||||||
|
|
Amortized Cost |
|
|
Gross Unrealized Gains |
|
|
Gross Unrealized Losses |
|
|
Fair Value |
|
||||
Commercial paper |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Corporate bonds |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|||
Government bonds |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|||
Foreign bonds |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|||
Total short-term marketable securities |
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Government bonds |
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||
Total long-term marketable securities |
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||
|
|
December 31, 2021 |
|
|||||||||||||
|
|
Amortized Cost |
|
|
Gross Unrealized Gains |
|
|
Gross Unrealized Losses |
|
|
Fair Value |
|
||||
Commercial paper |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Corporate bonds |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|||
Foreign bonds |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|||
Total short-term marketable securities |
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
Government bonds |
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||
Corporate bonds |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|||
Total long-term marketable securities |
|
$ |
|
|
$ |
|
|
$ |
( |
) |
|
$ |
|
|||
The following table indicates the length of the time that individual securities have been in a continuous unrealized loss position as of March 31, 2022 (dollars in thousands):
|
|
|
|
|
|
|
Less Than 12 Months |
|
||||||
|
|
|
|
Number of Investments |
|
|
Fair Value |
|
|
Unrealized Losses |
|
|||
Corporate bonds |
|
|
|
|
|
|
$ |
|
|
$ |
|
|||
Government bonds |
|
|
|
|
|
|
|
|
|
|
|
|||
Foreign bonds |
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
$ |
|
|
$ |
|
|||
As of March 31, 2022 and December 31, 2021, all short-term marketable securities had maturities of one year or less. All long-term marketable securities as of March 31, 2022 and December 31, 2021 had maturities of greater than one year but less than two years. There have been no significant realized gains or losses on the short-term and long-term marketable securities during the three months ended March 31, 2022 and 2021. The Company periodically reviews the available-for-sale investments for other-than-temporary impairment loss. All investments with unrealized losses have been in a loss position for less than 12 months. The Company determined that the unrealized loss was primarily attributed to changes in current market interest rates and not to credit quality. The Company does not intend to sell the marketable securities that are in an unrealized loss position, nor is it more likely than not that the Company will be required to sell the marketable securities before the recovery of the amortized cost basis, which may be at maturity. As a result, the Company did recognize any other-than-temporary impairment losses as of March 31, 2022.
9
Note 5. Balance Sheet Components
Accrued and Other Liabilities
Accrued and other liabilities consist of the following (in thousands):
|
|
March 31, |
|
|
December 31, 2021 |
|
||
Accrued research and development expenses |
|
$ |
|
|
$ |
|
||
Accrued payroll and related expenses |
|
|
|
|
|
|
||
Accrued professional service fees |
|
|
|
|
|
|
||
Liability for early exercised stock options |
|
|
|
|
|
|
||
Other |
|
|
|
|
|
|
||
Accrued and other liabilities |
|
$ |
|
|
$ |
|
||
Note 6. License Agreements
Stanford License Agreements
In March 2016, the Company entered into a license agreement with Stanford University, or the 2016 Stanford Agreement, which was amended in July 2016, October 2016 and January 2021, pursuant to which the Company obtained from Stanford a worldwide, exclusive, sublicensable license under certain patents, rights, or licensed patents and technology related to its engineered Wnt surrogate molecules to make, use, import, offer to sell and sell products that are claimed by the licensed patents or that use or incorporate such technology, or licensed products, for the treatment, diagnosis and prevention of human and veterinary diseases. The Company agreed to pay Stanford an aggregate of up to $
In June 2018, the Company entered into another license agreement with Stanford, or the 2018 Stanford Agreement, pursuant to which the Company obtained from Stanford a worldwide, exclusive, sublicensable license under certain patent rights related to its surrogate R-spondin proteins, or the licensed patents, to make, use, import, offer to sell and sell products that are claimed by the licensed patents, or licensed products, for the treatment, diagnosis and prevention of human and veterinary diseases, or the exclusive field. Additionally, Stanford granted the Company a worldwide, non-exclusive, sublicensable license under the licensed patents to make and use licensed products for research and development purposes in furtherance of the exclusive field and a worldwide, non-exclusive license to make, use and import, but not to offer to sell or sell, licensed products in any other field of use. The Company agreed to pay Stanford an aggregate of up to $
For the three months ended March 31, 2022 and 2021, the Company incurred de minimis research and development expenses under the Stanford agreements.
UCSF License and Option Agreements
In September and October 2016, the Company entered into two separate license and option agreements with The Regents of the University of California, or the UCSF Agreements, pursuant to which the Company obtained exclusive licenses from UCSF for internal research and antibody discovery purposes and an option to negotiate with UCSF to obtain an exclusive license under UCSF’s rights in the applicable library to make, use, sell, offer for sale and import products incorporating antibodies identified or resulting from the Company’s use of such library, or licensed products.
In January 2020, the Company amended and restated the UCSF Agreements to provide non-exclusive licenses to make and use a certain human Fab naïve phage display library and to make and use a certain phage display llama VHH single domain antibody library for internal research and antibody discovery purposes and an option to negotiate with UCSF to obtain a non-exclusive commercial license under UCSF’s rights in the applicable library to make, use, sell, offer for sale and import products incorporating antibodies identified or resulting from the Company’s use of such library, or licensed products.
10
In March 2022, the Company exercised the option under the UCSF Agreements and entered into a non-exclusive commercial license agreement to make and use licensed products derived from the phage display llama VHH single domain antibody library. Under the commercial license agreement, the Company paid UCSF a nominal license issue fee and agreed to pay a nominal annual license maintenance fee, five- to six-digit payments per licensed product upon achievement of a regulatory milestone, nominal minimum annual royalties, and earned royalties equal to a sub-single digit percentage of the Company’s and the Company’s sublicensees’ net sales of licensed products.
For the three months ended March 31, 2022 and 2021, the Company incurred de minimis research and development expenses under the UCSF Agreements and the commercial license agreement.
Distributed Bio Subscription Agreement
In September 2016, the Company entered into, and in January 2019, the Company amended, an antibody library subscription agreement with Charles River Laboratories International, Inc., formerly known as Distributed Bio, or the Distributed Bio Agreement, in which the Company obtained from Distributed Bio a non-exclusive license to use Distributed Bio’s antibody library to identify antibodies directed to an unlimited number of the Company’s proprietary targets and to make, use, sell, offer for sale, import and exploit products incorporating the antibodies that the Company identifies, or licensed products. The Company agreed to pay Distributed Bio an annual fee in the low six figures after the first three years. Additionally, the Company agreed to pay Distributed Bio an aggregate of $
For the three months ended March 31, 2022 and 2021, the Company incurred de minimis research and development expenses under the Distributed Bio Agreement.
Note 7. Commitments and Contingencies
Lease Agreements
In August 2016, the Company entered into a lease agreement for office and lab space, which consists of approximately
In January 2020, the Company entered into a lease agreement for a term of
The operating lease expense for the three months ended March 31, 2022 and 2021 was $
Aggregate future minimum rental payments under the operating leases as of March 31, 2022, were as follows (in thousands):
Remaining nine months ending December 31, 2022 |
|
$ |
|
|
Year ending December 31, 2023 |
|
|
|
|
Year ending December 31, 2024 |
|
|
|
|
Year ending December 31, 2025 |
|
|
|
|
Total lease payments |
|
|
|
|
Less: Imputed interest |
|
|
( |
) |
Operating lease liabilities |
|
$ |
|
11
Note 8. Stockholders’ Equity
Equity Purchase Agreement
In February 2022, the Company entered into the Equity Purchase Agreement with Lincoln Park, pursuant to which Lincoln Park is obligated to purchase up to $
Upon execution of the Equity Purchase Agreement, the Company issued
As contemplated by the Equity Purchase Agreement, and so long as the closing price of the Company’s common stock exceeds $
The Company may also direct Lincoln Park to purchase additional shares no less than the Regular Purchase Share Limit and no greater than
As of March 31, 2022, the Company has
Note 9. Common Stock Warrants
In connection with the Business Combination, Legacy Surrozen, as the accounting acquirer, was deemed to assume warrants held by Consonance’s stockholders, or the Public Warrants, and warrants held by Consonance's sponsor, or the Private Placement Warrants. In addition, in the PIPE Financing, certain investors subscribed for and purchased an aggregate of
Type |
|
Classification |
|
Expiration Date |
|
Exercise Price per Warrant |
|
|
Number of Warrants |
|
|
Fair Value |
|
|||
Public Warrants |
|
|
|
$ |
|
|
|
|
|
$ |
|
|||||
Private Placement Warrants |
|
|
|
|
|
|
|
|
|
|
|
|||||
PIPE Warrants |
|
|
|
|
|
|
|
|
|
|
|
|||||
Total |
|
|
|
|
|
|
|
|
|
|
|
$ |
|
|||
Public Warrants
Each whole Public Warrant entitles the holder to purchase one share of the Company’s common stock at a price of $
12
effective. The registration statement on Form S-1 to register for resale under the Securities Act of 1933, as amended, was effective in November 2021. The Company shall use its efforts to maintain the effectiveness of the registration statement until the expiration or redemption of the Public Warrants. If the Company fails to have maintained an effective registration statement, the Public Warrant holders have the right to exercise the Public Warrants on a cashless basis until such time as there is an effective registration statement.
The Company may redeem the outstanding Public Warrants at a price of $
In no event will the Company be required to net cash settle the Public Warrants. The Public Warrant holders do not have the rights or privileges of common stockholders and any voting rights until they exercise their Public Warrants and receive common stock.
Private Placement Warrants
The Private Placement Warrants have terms and provisions that are identical to those of the Public Warrants, except that so long as they are held by Consonance's sponsor or any of its permitted transferees, the Private Placement Warrants: (i) may be exercised for cash or on a cashless basis, (ii) may not be transferred, assigned or sold until
PIPE Warrants
Each whole PIPE Warrant entitles the holder to purchase one share of the Company’s common stock at a price of $
Classification
The Public Warrants, Private Placement Warrants and PIPE Warrants are not considered indexed to the Company’s common stock as certain provisions of the warrant agreements could change the settlement amount of these warrants. As a result, they were classified as liabilities and recorded at fair value with subsequent change in their respective fair value recognized in the other income within the unaudited condensed consolidated statements of operations and comprehensive loss at each reporting date. See Note 4 for the discussion of warrant valuations.
Note 10. Stock-Based Compensation Plan
The Company maintains the 2021 Equity Incentive Plan, or the 2021 Plan, which provides for the granting of stock awards to employees, directors and consultants. Options granted under the 2021 Plan may be either incentive stock options, or ISOs, or nonqualified stock options, or NSOs. Options granted under the 2021 Plan expire no later than
13
Stock Options
A summary of stock option activity is set forth below:
|
|
Options outstanding |
|
|||||||||||||
|
|
|
|
|
|
|
|
Weighted |
|
|
|
|
||||
|
|
|
|
|
Weighted |
|
|
Average |
|
|
Aggregate |
|
||||
|
|
Number of |
|
|
Average |
|
|
Remaining |
|
|
Intrinsic |
|
||||
|
|
Options |
|
|
Exercise |
|
|
Contractual Life |
|
|
Value |
|
||||
|
|
(In thousands) |
|
|
Price |
|
|
(In years) |
|
|
(In thousands) |
|
||||
Outstanding – December 31, 2021 |
|
|
|
|
$ |
|
|
|
|
|
|
|
||||
Granted |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Exercised |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Cancelled |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|||
Outstanding – March 31, 2022 |
|
|
|
|
|
|
|
|
|
|
$ |
|
||||
Exercisable – March 31, 2022 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
The aggregate intrinsic values of options outstanding, exercisable, vested and expected to vest is the difference between the exercise price of the options and the fair value of the Company’s common stock at March 31, 2022.
During the three months ended March 31, 2022, the Company granted options with a weighted-average grant-date fair value of $
The fair value of options is estimated at the grant date using the Black-Scholes option-pricing model with the following weighted-average assumptions:
|
|
Three Months Ended March 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Expected term (in years) |
|
|
|
|
|
|
||
Expected volatility |
|
|
% |
|
|
% |
||
Risk-free rate |
|
|
% |
|
|
% |
||
Dividend yield |
|
|
|
|
|
|
||
Restricted Stock Awards
The following table summarizes the Company’s restricted stock award activity:
|
|
|
|
|
Weighted |
|
||
|
|
Number of |
|
|
Average |
|
||
|
|
Shares |
|
|
Grant Date |
|
||
|
|
(In thousands) |
|
|
Fair Value |
|
||
RSAs, unvested at December 31, 2021 |
|
|
|
|
$ |
|
||
Granted |
|
|
|
|
|
|
||
Vested |
|
|
( |
) |
|
|
|
|
Forfeited |
|
|
|
|
|
|
||
RSAs, unvested at March 31, 2022 |
|
|
|
|
|
|
||
The fair value of restricted stock awards vested during the three months ended March 31, 2022 was $
14
Stock-Based Compensation
Total stock-based compensation recorded in the unaudited condensed consolidated statements of operations and comprehensive loss related to stock options and restricted stock awards was as follows (in thousands):
|
|
Three Months Ended March 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Research and development |
|
$ |
|
|
$ |
|
||
General and administrative |
|
|
|
|
|
|
||
Total stock-based compensation expense |
|
$ |
|
|
$ |
|
||
As of March 31, 2022, there was approximately $
15
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis should be read in conjunction with our unaudited condensed consolidated financial statements and related notes included elsewhere in this Quarterly Report on Form 10-Q, or this Report, and our consolidated financial statements and related notes thereto for the year ended December 31, 2021 included in the Annual Report on Form 10-K filed on March 28, 2022. Unless otherwise indicated, the terms “Surrozen,” “we,” “us,” or “our” refer to Surrozen Operating, Inc., or Legacy Surrozen prior to the Business Combination with Consonance-HFW Acquisition Corp. and Surrozen, Inc., formerly known as Consonance-HFW Acquisition Corp., together with its consolidated subsidiaries after giving effect to the Business Combination.
Forward-Looking Statements
The following discussion of our financial condition and results of operations contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. All statements other than statements of historical facts are “forward-looking statements” for purposes of these provisions, including those relating to future events or our future financial performance and financial guidance. In some cases, you can identify forward-looking statements by terminology such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “project,” “believe,” “estimate,” “predict,” “potential,” “intend” or “continue,” the negative of terms like these or other comparable terminology, and other words or terms of similar meaning in connection with any discussion of future operating or financial performance. These statements are only predictions.
All forward-looking statements included in this document are based on information available to us on the date hereof, and we assume no obligation to update any such forward-looking statements. Any or all of our forward-looking statements in this document may turn out to be wrong. Actual events or results may differ materially. Our forward-looking statements can be affected by inaccurate assumptions we might make or by known or unknown risks, uncertainties and other factors. In evaluating these statements, you should specifically consider various factors, including the risks outlined under the caption “Risk Factors” set forth in Item 1A of Part II of this Report, as well as those contained from time to time in our other filings with the SEC. We caution investors that our business and financial performance are subject to substantial risks and uncertainties.
Overview
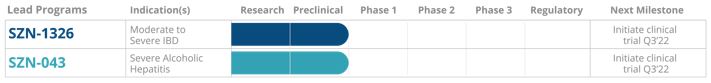
We are discovering and developing biologic drug candidates to selectively modulate the Wnt pathway, a critical mediator of tissue repair, in a broad range of organs and tissues, for human diseases. Building upon the seminal work of our founders and scientific advisors who discovered the Wnt gene and key regulators of the Wnt pathway, we have made breakthrough discoveries that we believe will overcome previous limitations in harnessing the potential of Wnt biology. These breakthroughs enable us to rapidly and flexibly design tissue-targeted therapeutics that modulate Wnt signaling. As a result of our discoveries, we are pioneering the selective activation of Wnt signaling, designing and engineering Wnt pathway mimetics, and advancing tissue-specific Wnt candidates. Our lead product candidates are multi-specific, antibody-based therapeutics that mimic the roles of naturally occurring Wnt or R-spondin proteins, which are involved in activation and enhancement of the Wnt pathway, respectively. Given Wnt signaling is essential in tissue maintenance and regeneration throughout the body, we have the potential to target a wide variety of severe diseases, including certain diseases that afflict the intestine, liver, retina, cornea, lung, kidney, cochlea, skin, pancreas and central nervous system. In each of these areas, we believe our approach has the potential to change the treatment paradigm for the disease and substantially impact patient outcomes. Our strategy is to exploit the full potential of Wnt signaling by identifying disease states responsive to Wnt modulation, design tissue-specific therapeutics, and advance candidates into clinical development in targeted indications with high unmet need. Our unique approach and platform technologies have led to the discovery and advancement of two lead product candidates. We plan to initiate a Phase 1 clinical trial in the third quarter of 2022 for SZN-1326, our candidate in development for moderate to severe inflammatory bowel disease, or IBD, with ulcerative colitis, or UC, as our first proposed indication. Furthermore, we plan to initiate a Phase 1 clinical trial in the third quarter of 2022 for SZN-043, our candidate in development for severe alcoholic hepatitis, or AH. We expect to nominate additional lead candidates and advance them into the clinic in 2023 and beyond. In January 2022, we nominated SZN-413, as a development candidate for the treatment of retinal vascular -associated diseases.
The chart below represents a summary of our wholly owned product candidates:

By leveraging our scientific capabilities and approach, we have identified more than 20 potential tissue types to explore. In our most advanced research programs, we are developing potential therapeutics for ocular diseases such as retinal vascular diseases. Genetic
16
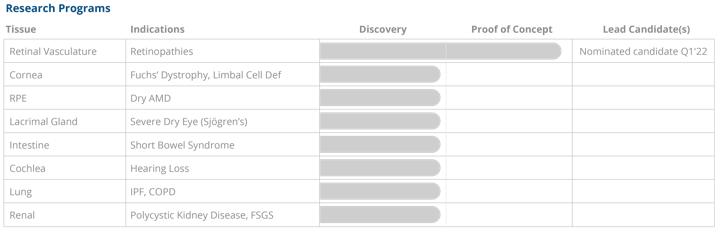
studies in the literature have identified that the Wnt signaling pathway is critical for maintenance of healthy retinal blood vessels. We have shown that activation of Wnt-pathway signaling can potentially reverse vascular damage through a mechanism that is distinct from the mechanisms of currently approved therapeutics that target angiogenesis. We also have identified the potential for regeneration of retinal pigment epithelium, or RPE, an important cell type in the retina. RPE cells are required for maintenance and viability of photoreceptors and as such are a potential target for the treatment of dry AMD. We are also assessing the potential to drive tissue repair in conditions such as hearing loss and diseases resulting in tissue injury to organs including the cornea, lacrimal gland, lung and kidney.
The chart below represents a summary of our wholly-owned research programs:
Since our inception in 2015, we have devoted substantially all of our efforts and financial resources to organizing and staffing our company, business planning, raising capital, developing and optimizing our Wnt therapeutics platform, identifying potential product candidates, undertaking research and development activities, engaging in strategic transactions, establishing and enhancing our intellectual property portfolio, and providing general and administrative support for these operations. We have incurred net losses since inception. During the three months ended March 31, 2022 and 2021, we incurred net losses of $7.9 million and $13.0 million. As of March 31, 2022, we had an accumulated deficit of $150.6 million and cash, cash equivalents and marketable securities of $104.3 million.
We expect to continue to incur losses for the foreseeable future and expect to incur increased expenses as we expand our pipeline and advance our product candidates through clinical development and regulatory submissions. Specifically, in the near term we expect to incur substantial expenses relating to our planned Phase 1 clinical trials, the development and validation of our manufacturing processes, and other research and development activities.
Impacts of the Conflict between Russia and Ukraine and the COVID-19 Pandemic
Russia invaded Ukraine in February 2022 and is still engaged in active armed conflict against the country. The global COVID-19 pandemic continues to evolve rapidly, and we will continue to monitor developments closely. To date, our financial condition and operations have not been significantly impacted by the conflict between Russia and Ukraine and the COVID-19 pandemic. The extent of the impact on our business, operations and clinical development timelines and plans remains uncertain and will depend on certain developments, including the actions of U.S. and foreign governments to impose sanctions on Russia and to slow the spread of the COVID-19 and their impact on our preclinical development activities, regulatory agencies, clinical research organizations, or CROs, third-party manufacturers, other third parties with whom we do business, and, if we obtain regulatory approval to commence dosing in humans, trial enrollment and trial sites. We will continue to actively monitor the rapidly evolving situation and may take actions that alter our operations, including those that may be required by federal, state or local authorities or that we determine are in the best interests of our employees and other third parties with whom we do business.
UCSF Commercial License Agreement
In March 2022, we exercised the option under the amended and restated license and option agreements with The Regents of the University of California executed in January 2020 and entered into a non-exclusive commercial license agreement to make and use licensed products derived from the phage display llama VHH single domain antibody library. Under the commercial license agreement, we paid UCSF a nominal license issue fee and agreed to pay a nominal annual license maintenance fee, five- to six-digit payments per licensed product upon achievement of a regulatory milestone, nominal minimum annual royalties, and earned royalties equal to a sub-single digit percentage of our and our sublicensees’ net sales of licensed products.
17
Components of Results of Operations
Revenue
We have not generated any revenue from the sale of our products, and we do not expect to generate any revenue unless and until we obtain regulatory clearance or approval of, and commercialize, our product candidates.
Operating Expenses
We classify operating expenses into two main categories: (i) research and development expenses and (ii) general and administrative expenses.
Research and Development Expenses
Since our inception, we have focused significant resources on our research and development activities. Our research and development expenses consist of external and internal expenses incurred in connection with our research activities and development programs.
External expenses include:
Internal expenses include:
We expect our research and development expenses will increase significantly for the foreseeable future as we identify and develop product candidates, in particular as we seek to initiate clinical trials and pursue regulatory approval and commercialization for SZN-1326 and SZN-043.
The successful development of our product candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing or costs required to complete the remaining development of SZN-1326 and SZN-043 or any future product candidates. This is due to the numerous risks and uncertainties associated with the development of product candidates, many of which are outside of our control, including those associated with:
18
Any changes in the outcome of any of these variables could mean a significant change in the costs and timing associated with the development of our drug candidates.
General and Administrative Expenses
General and administrative expenses consist primarily of personnel-related costs, including salaries, bonuses, benefits and stock-based compensation expense for personnel in executive, finance, human resources, business and corporate development, legal, and other administrative functions. General and administrative expenses also include legal fees, professional fees paid for accounting, auditing, consulting, tax, investor relations services, insurance costs, and facility costs not otherwise included in research and development expenses, and costs associated with compliance with the rules and regulations of the SEC and those of the Nasdaq. We expect that our general and administrative expenses will increase significantly for the foreseeable future to support our expanding headcount and operations.
Interest Income
Interest income consists primarily of interest earned on our cash equivalents and marketable securities.
Other Income
Other income consists of the gain on the change in fair value of warrant liabilities.
Results of Operations
Comparison of the Three Months Ended March 31, 2022 and 2021
The following table summarizes results of operations for the periods presented (dollars in thousands):
|
|
Three Months Ended March 31, |
|
|
$ |
|
|
% |
|
|||||||
|
|
2022 |
|
|
2021 |
|
|
Change |
|
|
Change |
|
||||
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Research and development |
|
$ |
9,371 |
|
|
$ |
8,601 |
|
|
$ |
770 |
|
|
|
9 |
% |
General and administrative |
|
|
5,122 |
|
|
|
4,430 |
|
|
|
692 |
|
|
|
16 |
% |
Total operating expenses |
|
|
14,493 |
|
|
|
13,031 |
|
|
|
1,462 |
|
|
|
11 |
% |
Loss from operations |
|
|
(14,493 |
) |
|
|
(13,031 |
) |
|
|
(1,462 |
) |
|
|
11 |
% |
Interest income |
|
|
49 |
|
|
|
9 |
|
|
|
40 |
|
|
|
444 |
% |
Other income |
|
|
6,497 |
|
|
|
— |
|
|
|
6,497 |
|
|
* |
|
|
Net loss |
|
$ |
(7,947 |
) |
|
$ |
(13,022 |
) |
|
$ |
5,075 |
|
|
|
-39 |
% |
*Percentage is not meaningful
Research and Development Expenses
The following table summarizes research and development expenses for the periods presented (dollars in thousands):
|
|
Three Months Ended March 31, |
|
|
$ |
|
|
% |
|
|||||||
|
|
2022 |
|
|
2021 |
|
|
Change |
|
|
Change |
|
||||
External expenses(1) |
|
$ |
3,366 |
|
|
$ |
4,314 |
|
|
$ |
(948 |
) |
|
|
-22 |
% |
Internal costs: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Personnel expenses (including |
|
|
4,194 |
|
|
|
2,971 |
|
|
|
1,223 |
|
|
|
41 |
% |
Facilities and other expenses |
|
|
1,811 |
|
|
|
1,316 |
|
|
|
495 |
|
|
|
38 |
% |
Total research and |
|
$ |
9,371 |
|
|
$ |
8,601 |
|
|
$ |
770 |
|
|
|
9 |
% |
19
(1) In future periods when clinical trial expenses are incurred, external expenses will be broken out between our clinical programs and preclinical programs.
The increase of $0.8 million, or 9%, in research and development expenses for the three months ended March 31, 2022, compared to the three months ended March 31, 2021, is due in part to the $1.2 million increase in personnel-related expenses as a result of a higher headcount and options granted to our employees and the increase of $0.5 million in facilities and other expenses is attributable to the increase in headcount and corporate insurance, offset by the $0.3 million decrease in external expenses primarily due to the completion of manufacturing drug substance for our SZN-1326 and SZN-043 programs.
General and Administrative Expenses
The increase of $0.7 million, or 16%, in general and administrative expenses for the three months ended March 31, 2022, compared to the three months ended March 31, 2021, is primarily attributable to the $1.3 million increase in personnel-related expenses due to an increase in headcount and options granted to our employees and the $0.5 million increase in corporate insurance, offset by the $1.1 million decrease in professional and consulting service fees related to the potential initial public offering prior to our decision to commence the business combination with Consonance-HFW Acquisition Corp.
Interest Income
The increase of $40,000, or 444%, in interest income for the three months ended March 31, 2022, compared to the three months ended March 31, 2021, is primarily due to the increase in investments in money market funds and marketable securities.
Other Income
The increase of $6.5 million in other income for the three months ended March 31, 2022, compared to the three months ended March 31, 2021, is related to the gain on the change in fair value of warrant liabilities.
Liquidity and Capital Resources
Since inception, we have incurred significant net operating losses and negative cash flows from operations. Historically, we financed our operations primarily from the sale of our redeemable convertible preferred stock. As of March 31, 2022, we had cash, cash equivalents and marketable securities of $104.3 million and an accumulated deficit of $150.6 million.
In February 2022, we entered into a purchase agreement and a registration rights agreement with Lincoln Park, pursuant to which Lincoln Park is obligated to purchase up to $50.0 million of our common stock from time to time at our sole discretion over a 36-month period commencing on April 27, 2022.
We believe, based on our current operating plan, that our existing cash, cash equivalents, and marketable securities will be sufficient to fund our operations for at least the next 12 months from the date of this Report. However, if the anticipated operating results are not achieved in future periods, we could use our capital resources sooner than expected which may result in the need to reduce future planned expenditures and/or raise additional capital to continue to fund the operations.
Future Funding Requirements
To date, we have not generated any revenue. We do not expect to generate any meaningful revenue unless and until we obtain regulatory approval and commercialize SZN-1326 and SZN-043 or any future product candidates, and we do not know when, or if, that will occur. We will continue to require substantial additional capital to develop SZN-1326 and SZN-043 and fund operations for the foreseeable future. Since our inception in 2015, we have devoted substantially all of our efforts and financial resources to organizing and staffing our company, business planning, raising capital, developing and optimizing our Wnt therapeutics platform, identifying potential product candidates, undertaking research and development activities, engaging in strategic transactions, establishing and enhancing our intellectual property portfolio, and providing general and administrative support for these operations. We expect our expenses to continue to increase in connection with our ongoing activities as we continue to advance SZN-1326 and SZN-043 into clinical development and regulatory approval. In addition, we will continue to incur additional costs associated with operating as a public company.
We expect that our cash, cash equivalents and marketable securities, will provide the capital needed to fund our operations in the short-term. We expect that in the long-term we will need to raise additional capital through public or private equity offerings, debt financings or other capital sources, including government grants, potential collaborations with other companies or other strategic transactions as we do not expect sales of common stock to Lincoln Park to be sufficient to provide all necessary financing until we are able to generate revenue on our own. There can be no assurance that sufficient funds will be available to us at all or on attractive terms when needed from these sources. If we are unable to obtain additional funding from these or other sources when needed, it may be necessary to significantly reduce expenses through reductions in staff and delaying, scaling back operations, or stopping certain research and development programs.
20
We have based our projections of operating capital requirements on assumptions that may prove to be incorrect and we may use all our available capital resources sooner than we expect. Because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
In addition, any future financing through sales of equity securities, including sales to Lincoln Park under the Equity Purchase Agreement, will cause our stockholders to experience dilution. If we raise additional capital through debt financing, we may be subject to covenants that restrict our operations including limitations on our ability to incur liens or additional debt, pay dividends, repurchase our common stock, make certain investments, and engage in certain merger, consolidation, or asset sale transactions. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our stockholders. If we are unable to raise additional funds when needed, we may be required to delay, reduce, or terminate some or all of our development programs and clinical trials. We may also be required to sell or license to others our rights to SZN-1326 and SZN-043 and any future product candidates or discovery programs in certain territories or indications that we would prefer to develop and commercialize ourselves.
Summary of Cash Flows
The following table sets forth the primary sources and uses of cash, cash equivalents and restricted cash for the periods presented below (in thousands):
|
|
Three Months Ended March 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
|
|
|
|
|
|
|
||
Net cash used in operating activities |
|
$ |
(18,374 |
) |
|
$ |
(10,332 |
) |
Net cash used in investing activities |
|
|
(412 |
) |
|
|
(1,442 |
) |
Net cash provided by financing activities |
|
|
— |
|
|
|
196 |
|
Net decrease in cash, cash equivalents |
|
$ |
(18,786 |
) |
|
$ |
(11,578 |
) |
Cash Used in Operating Activities
Cash used in operating activities of $18.4 million for the three months ended March 31, 2022 was primarily due to the use of funds in our operations and the resulting net loss of $7.9 million, a net change of $5.9 million in our net operating assets and liabilities and $4.6 million in non-cash charges. The net change in our operating assets and liabilities was primarily due to a net increase in prepaid expenses, accounts payable and accrued liabilities. Cash used in operating activities of $10.3 million for the three months ended March 31, 2021 was primarily due to the use of funds in our operations and the resulting net loss of $13.0 million, partially offset by $1.3 million in
21
non-cash charges and a net change of $1.4 million in our net operating assets and liabilities. The net change in our operating assets and liabilities was primarily due to a net increase in prepaid expenses, accounts payable and accrued liabilities.
Cash Used in Investing Activities
Cash used in investing activities of $0.4 million for the three months ended March 31, 2022 was for the purchase of laboratory and computer equipment. Cash used in investing activities of $1.4 million for the three months ended March 31, 2021 consisted primarily of $1.1 million of cash used for the purchase of marketable securities and $0.3 million of cash used for the purchase of lab equipment.
Cash Provided by Financing Activities
Cash provided by financing activities of $0.2 million for the three months ended March 31, 2021 consisted primarily of the proceeds from the exercise of options.
Contractual Obligations and Commitments
Our contractual obligations as of March 31, 2022 have not materially changed from what we presented in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in our Annual Report on Form 10-K for the year ended December 31, 2021.
Critical Accounting Policies, Significant Judgments and Use of Estimates
Our unaudited condensed consolidated financial statements have been prepared in accordance with U.S. GAAP. The preparation of these unaudited condensed consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the unaudited condensed consolidated financial statements, as well as the reported expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ materially from these estimates.
During the three months ended March 31, 2022, there were no material changes to our critical accounting policies or in the methodology used for estimates from those described under “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Annual Report on Form 10-K for the year ended December 31, 2021.
Emerging Growth Company Status
We are an emerging growth company, or EGC, as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. The JOBS Act permits companies with EGC status to take advantage of an extended transition period to comply with new or revised accounting standards, delaying the adoption of these accounting standards until they would apply to private companies. We have elected to use this extended transition period to enable us to comply with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date the Company (i) is no longer an EGC or (ii) affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. As a result, our unaudited condensed consolidated financial statements may not be comparable to companies that comply with the new or revised accounting standards as of public company effective dates.
In addition, we intend to rely on the other exemptions and reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, if, as an EGC, we intend to rely on such exemptions, we are not required to, among other things: (i) provide an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act; (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act; (iii) comply with any requirement that may be adopted by the Public Company Accounting Oversight Board; and (iv) disclose certain executive compensation-related items such as the correlation between executive compensation and performance and comparisons of the Chief Executive Officer’s compensation to median employee compensation.
We will remain an EGC under the JOBS Act until the earliest of (i) the last day of the fiscal year (a) of 2025, (b) the year in which we have total annual gross revenue of at least $1.07 billion, or (c) the year in which we are deemed to be a large accelerated filer; or (ii) the date on which we have issued more than $1.00 billion in non-convertible debt securities during the prior three-year period.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and are not required to provide the information otherwise required under this item.
Item 4. Controls and Procedures.
Management’s Evaluation of Disclosure Controls and Procedures
22
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rule 13a-15(f). Our management, under the supervision and with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this Report. Based on the evaluation of our disclosure controls and procedures as required by Rule 13a-15 under the Exchange Act, our Chief Executive Officer and Chief Financial Officer have concluded that, as of the end of the period covered by this Report, our disclosure controls and procedures were not effective at the reasonable assurance level as a result of the material weakness described below.
Material Weakness
As previously reported, in connection with the audit of our financial statements for the years ended December 31, 2020, we and our independent registered public accounting firm identified one material weakness in our internal control over financial reporting. This material weakness continued to exist as of March 31, 2022. A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. The material weakness that we identified relates to a lack of sufficient accounting and financial reporting personnel with requisite knowledge and experience in application of U.S. GAAP and SEC rules.
To respond to the material weakness, we have devoted, and plan to continue to devote, significant effort and resources to the remediation and improvement of our internal control over financial reporting. We are in the process of implementing measures designed to improve our internal control over financial reporting and remediate the control deficiencies that led to the material weakness, including hiring additional accounting personnel, obtaining advisory services from professional consultants with U.S. GAAP and SEC reporting experience in their industry, research materials and documents and increased communication among our personnel and third-party professionals with whom we consult regarding complex accounting applications and expanding the capabilities of the existing accounting and financial personnel through continuous training and education in the accounting and reporting requirements under U.S. GAAP and the SEC rules and regulations. The process of designing and implementing effective internal controls is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a system of internal controls that is adequate to satisfy our reporting obligations as a public company.
Changes in Internal Control Over Financial Reporting
There has been no change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Effectiveness of Controls and Procedures
We do not expect that our disclosure controls and procedures will prevent all errors and all instances of fraud. Disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the disclosure controls and procedures are met. Further, the design of disclosure controls and procedures must reflect the fact that there are resource constraints, and the benefits must be considered relative to their costs. Because of the inherent limitations in all disclosure controls and procedures, no evaluation of disclosure controls and procedures can provide absolute assurance that we have detected all our control deficiencies and instances of fraud, if any. The design of disclosure controls and procedures also is based partly on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
23
PART II—OTHER INFORMATION
Item 1. Legal Proceedings
From time to time, we may be subject to legal proceedings. We are not currently a party to or aware of any proceedings that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or results of operations. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources, and other factors.
Item 1A. Risk Factors.
As a smaller reporting company (as defined in Rule 12b-2 of the Exchange Act), we are not required to provide the information called for by this Item 1A. Risk factors describing the major risks to our business can be found under Item 1A, “Risk Factors”, in our Annual Report on Form 10-K for the year ended December 31, 2021.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
All unregistered sales of our securities during the three months ended March 31, 2022, were previously disclosed in a Current Report on Form 8-K.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Other Information.
None.
24
Item 6. Exhibits.
Exhibit Number |
|
Description |
|
|
|
2.1† |
|
|
3.1 |
|
|
3.2 |
|
|
4.1 |
|
|
4.2 |
|
|
4.3 |
|
|
4.4 |
|
|
4.5 |
|
|
10.1† |
|
|
10.2 |
|
|
10.3* |
|
|
31.1* |
|
|
31.2* |
|
|
32.1* |
|
|
32.2* |
|
|
101.INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. |
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
|
|
|
* Filed herewith.
+ Indicates management contract or compensatory plan or arrangement.
† Schedules and exhibits to this agreement have been omitted pursuant to Item 601(b)(2) of Regulation S-K. A copy of any omitted schedule and/or exhibit will be furnished to the SEC upon request.
†† The Company has redacted provisions or terms of this Exhibit pursuant to Regulation S-K Item 601(b)(10). The Company agrees to furnish an unredacted copy of the Exhibit to the SEC upon its request.
25
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
|
|
SURROZEN, INC. |
|
|
|
|
|
Date: May 11, 2022 |
|
By: |
/s/ Craig Parker |
|
|
|
Craig Parker |
|
|
|
President and Chief Executive Officer and Director (Principal Executive Officer)
|
|
|
|
|
Date: May 11, 2022 |
|
By: |
/s/ Charles Williams |
|
|
|
Charles Williams |
|
|
|
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer)
|
26