FALSE000181577612/3100018157762024-03-212024-03-210001815776dei:FormerAddressMember2024-03-212024-03-21
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of The
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): March 21, 2024
LENZ THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| | | | | | | | | | | | | | |
| Delaware | | 001-40532 | | 84-4867570 |
(State or other jurisdiction of incorporation) | | (Commission File Number) | | (I.R.S. Employer Identification No.) |
| | | | | | | | |
| 445 Marine View Ave., Ste. #320 | | |
Del Mar, California | | 92014 |
(Address of principal executive offices) | | (Zip code) |
(858) 925-7000
(Registrant’s telephone number, including area code)
Graphite Bio, Inc.
611 Gateway Blvd., Suite 120
South San Francisco, CA 94080
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| | | | | |
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered |
| Common Stock, par value $0.00001 per share | | LENZ | | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Introductory Note
On March 21, 2024 (the “Closing Date”), Graphite Bio, Inc., a Delaware corporation and our predecessor company (“Graphite”), consummated the previously announced merger pursuant to the terms of the Agreement and Plan of Merger, dated as of November 14, 2023 (the “Merger Agreement”), by and among Graphite, Generate Merger Sub, Inc., a Delaware corporation and wholly-owned subsidiary of Graphite (“Generate Merger Sub”) and LENZ Therapeutics Operations, Inc. (previously named Lenz Therapeutics, Inc.), a Delaware corporation (“LENZ OpCo”).
Pursuant to the Merger Agreement, on the Closing Date, (i) Graphite effected a reverse stock split of Graphite’s issued common stock at a ratio of 1:7, (ii) Graphite changed its name to “LENZ Therapeutics, Inc.”, and (iii) Generate Merger Sub merged with and into LENZ OpCo (the “Merger”), with LENZ OpCo as the surviving company in the Merger and, after giving effect to such Merger, LENZ OpCo becoming a wholly-owned subsidiary of LENZ Therapeutics, Inc. (together with its consolidated subsidiary, “New LENZ” or “LENZ”).
Unless the context otherwise requires, “LENZ,” “we,” “us,” “our,” and the “Company” refer to New LENZ. All references herein to the “Board” refer to the board of directors of New LENZ. All references herein to the “Closing” refer to the closing of the transactions contemplated by the Merger Agreement (the “Transactions” or the “Merger”), including the Merger and the transactions contemplated by the subscription agreement entered into by Graphite and certain investors (the “PIPE Investors”) pursuant to which the PIPE Investors collectively subscribed for and purchased shares of Common Stock for an aggregate purchase price of approximately $53.5 million (the “PIPE Financing”).
Item 2.01. Completion of Acquisition or Disposition of Assets.
As previously reported, on March 14, 2024, Graphite held a special meeting (the “Special Meeting”) at which the Graphite stockholders considered and approved, among other matters, (i) the issuance of Common Stock, which represented more than 20% of the shares of Common Stock outstanding immediately prior to the Merger, to stockholders of LENZ OpCo, pursuant to the terms of the Merger Agreement, (ii) the change of control of Graphite resulting from the Merger pursuant to Nasdaq Listing Rule 5635(b), and (iii) the issuance of shares of Common Stock to the PIPE Investors pursuant to Nasdaq Listing Rule 5635(d), which shares of Common Stock represented more than 20% of the shares of Common Stock outstanding as of the date of the execution of the Subscription Agreement.
On March 21, 2024, the parties to the Merger Agreement completed the Merger and the other transactions contemplated thereby in accordance with the terms of the Merger Agreement. Effective at 4:01 p.m. eastern time on March 21, 2024, the Company effected a reverse stock split at a ratio of 1:7 and changed its name to “LENZ Therapeutics, Inc.”, and effective at 4:02 p.m. eastern time on March 21, 2024 the parties to the Merger Agreement consummated the Merger.
In accordance with the terms and subject to the conditions of the Merger Agreement, at the effective time of the Merger (the “Effective Time”), (i) each share of LENZ OpCo outstanding as of immediately prior to the Effective Time was exchanged for shares of common stock of New LENZ, par value $0.00001 per share (“Common Stock”), (ii) all vested and unvested options to purchase shares of LENZ OpCo were exchanged for comparable options to purchase shares of Common Stock, and (iii) each warrant to purchase shares of LENZ OpCo outstanding as of immediately prior to the Effective Time was converted into a warrant to purchase shares of Common Stock, in each case, based on an exchange ratio of 0.2022. Each share of Common Stock and each option to purchase shares of Common Stock that was issued and outstanding as of immediately prior to the Effective Time remain issued and outstanding in accordance with its terms and such shares and options, subject to the special cash dividend and reverse stock split (each described below), were unaffected by the Merger; provided that, each outstanding and unexercised option to purchase shares of Common Stock with a per share exercise price equal to or greater than $3.00 (prior to giving effect to the special cash dividend and reverse stock split) (the “Out-of-the-Money Graphite Options”) were accelerated in full immediately prior to the Effective Time and each such Out-of-the-Money Graphite Option not exercised as of immediately prior to the Effective Time was cancelled at the Effective Time for no consideration. All options to purchase shares of Common Stock immediately prior to the Effective Time with a per share exercise price of less than $3.00 (prior to giving effect to the special cash dividend and reverse stock split)
continue to be subject to the same terms and conditions after the Effective Time as were applicable to such options as of immediately prior to the Effective Time.
Upon the closing of the Transactions, (i) an aggregate of 13,654,408 shares of Common Stock were issued in exchange for the shares of LENZ OpCo outstanding as of immediately prior to the Effective Time, (ii) warrants to purchase 164,676 shares of Common Stock were issued pursuant to the conversion of warrants to purchase shares of LENZ OpCo outstanding as of immediately prior to the Effective Time, and (iii) an aggregate of 3,559,565 shares of Common Stock were issued to the PIPE Investors in the PIPE Financing. Moreover, at the Closing, all options to purchase shares of LENZ OpCo were exchanged for comparable options to purchase shares of Common Stock based on an exchange ratio of 0.2022. Immediately after giving effect to the Transactions, there were approximately 25,533,533 shares of Common Stock outstanding, 164,676 shares of Common Stock subject to outstanding warrants, and 1,590,018 shares of Common Stock subject to outstanding options under LENZ OpCo’s 2020 Equity Incentive Plan. The Common Stock, which was previously listed on The Nasdaq Stock Market LLC (“Nasdaq”) and traded under the ticker symbol “GRPH” through the close of business on March 21, 2024, will commence trading on Nasdaq under the ticker symbol “LENZ” on March 22, 2024.
The material terms and conditions of the Merger Agreement are described in the definitive proxy statement/prospectus (the “Proxy Statement/Prospectus”) included in Graphite’s Registration Statement on Form S-4 (File No. 333-275919), filed with the Securities and Exchange Commission (the “SEC”) on February 9, 2024, in the section entitled “Merger Agreement” beginning on page 203 of the Proxy Statement/Prospectus, which is incorporated herein by reference.
FORM 10 INFORMATION
Forward-Looking Statements
Certain statements in this Current Report on Form 8-K and the information incorporated herein by reference may constitute “forward-looking statements” for purposes of the federal securities laws. Our forward-looking statements include, but are not limited to, statements regarding our or our management team’s expectations, hopes, beliefs, intentions or strategies regarding the future, including those relating to the Transactions and their expected benefits; New LENZ’s performance following the Transactions; our plans relating to the clinical development of our product candidates, including the size, number and areas to be evaluated; our plans relating to commercializing our product candidates, if approved, including the geographic areas of focus and strategy; and New LENZ’s ability to obtain funding for its operations. Forward-looking statements include statements relating to our management team’s expectations, hopes, beliefs, intentions or strategies regarding the future, including those relating to the Transactions. In addition, any statements that refer to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumptions, are forward-looking statements. The words “anticipate,” “believe,” “contemplate,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions may identify forward-looking statements, but the absence of these words does not mean that a statement is not forward-looking.
These forward-looking statements are based on current expectations and beliefs concerning future developments and their potential effects. There can be no assurance that future developments affecting us will be those that we have anticipated. Forward-looking statements include, but are not limited to, statements concerning the following:
•the likelihood of our clinical trials demonstrating safety and efficacy of our product candidates, and other positive results;
•the timing, progress and results of our clinical trials for our current product candidates, including statements regarding the timing of completion of trials, and the reporting of data from our current trials;
•our plans relating to the clinical development of our product candidates, including the size, number and areas to be evaluated;
•the size of the market opportunity for our product candidates, including our estimates of the size of the affected population and potential adoption rate;
•our plans relating to commercializing our product candidates, if approved, including the geographic areas of focus and sales strategy;
•our competitive position and the success of competing therapies that are or may become available;
•the beneficial characteristics, and the potential safety, efficacy and therapeutic effects of our product candidates;
•the need to hire additional personnel and our ability to attract and retain such personnel;
•the timing, scope and likelihood of regulatory filings and approvals for our current product candidates;
•our ability to obtain and maintain regulatory approval of our product candidates;
•our plans relating to the further development and manufacturing of our product candidates;
•the expected potential benefits of strategic collaborations with third parties and our ability to attract collaborators with development, regulatory and commercialization expertise;
•the rate and degree of market acceptance and clinical utility of our current product candidates and other product candidates we may develop;
•the impact of existing laws and regulations and regulatory developments in the United States and other jurisdictions;
•our intellectual property position, including the scope of protection we are able to establish and maintain for intellectual property rights covering our current product candidates, including the extensions of existing patent terms where available, the validity of intellectual property rights held by third parties, and our ability not to infringe, misappropriate or otherwise violate any third-party intellectual property rights;
•our continued reliance on third parties to conduct additional clinical trials of our product candidates, and for the manufacture of our product candidates for clinical trials;
•the accuracy of our estimates regarding expenses, future revenue, capital requirements and needs for additional financing;
•our financial performance;
•costs related to the Merger;
•our ability to recognize the anticipated benefits of the Merger;
•the period over which we estimate our existing cash and cash equivalents will be sufficient to fund our future operating expenses and capital expenditure requirements;
•our expectations regarding the period during which we will remain an emerging growth company under the JOBS Act; and
•our anticipated use of our existing resources and the proceeds from the Transactions.
These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. As a result of a number of known and unknown risks and uncertainties, including but not limited to those described under the heading "Risk Factors" beginning on page 26 of the Proxy Statement/Prospectus, which is incorporated herein by reference, our actual results or performance may be materially different from those expressed or implied by these forward-looking statements.
Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. There may be additional risks that we consider immaterial or which are unknown. It is not possible to predict or identify all such risks. We do not undertake any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
Business
We are a late-stage biopharmaceutical company focused on developing and commercializing innovative therapies to improve vision. Our initial focus is the treatment of presbyopia, the inevitable loss of near vision that impacts the daily lives of nearly all people over 45. In the United States, the estimated addressable population who suffer from this condition, known as presbyopes, is 128 million, almost four times the number of individuals suffering from dry eye disease and three times the number of individuals suffering from childhood myopia, macular degeneration, diabetic retinopathy and glaucoma combined. We believe that a once-daily pharmacological eye drop that can effectively and safely improve near vision throughout the full workday, without the need for reading glasses, will be a highly attractive commercial product with an estimated U.S. market opportunity in excess of $3 billion. It is our goal to develop and commercialize such a product, and we have assembled an executive team with extensive clinical and commercial experience to execute this goal and become the category leader.
Our product candidates LNZ100 and LNZ101 are preservative-free, single-use, once-daily eye drops containing aceclidine and aceclidine plus brimonidine, respectively. We believe our product candidates are differentiated based on rapid onset, degree and duration of near vision improvement, as well as their ability to be used across the full age range of presbyopes, from their mid-40s to well into their mid-70s, as well as the broadest refractive range. Aceclidine’s pupil-selective mechanism of action was demonstrated in our clinical trials where near vision improved while avoiding blurry distance vision. Our product candidates were well-tolerated in clinical trials, and their active ingredients have favorable tolerability profiles that have been well-established empirically. Our product candidates have patent protection until 2039, at a minimum, due to a robust intellectual property portfolio underpinned by issued patents. If one of our product candidates is approved, we believe that it could be the first aceclidine-based product approved by the U.S. Food and Drug Administration (“FDA”) and would then be eligible for five years of new chemical entity (“NCE”) exclusivity in the United States.
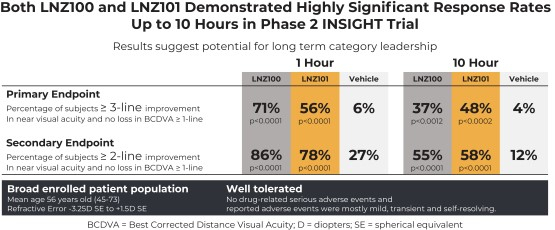
In our Phase 2 trial (NCT05294328, the “INSIGHT”, or “Phase 2” trial), both LNZ100 and LNZ101 achieved the primary endpoint of three-lines or greater improvement in near visual acuity without losing one or more lines in Best Corrected Distance Visual Acuity (“BCDVA”) at one-hour post-treatment, with a response rate of 71% (p<0.0001) and 56% (p<0.0001), respectively, compared to 6% for vehicle, with three-lines or greater improvement being observed as early as 30 minutes post-treatment, the earliest timepoint measured, and lasting up to 10 hours, the last timepoint measured. BCDVA in this context refers to the best possible distance vision that an individual’s eye can see using corrective lenses. For more details, see the section entitled “INSIGHT: Phase 2 Clinical Trial — Trial Design” in the Proxy Statement/Prospectus in the section titled “LENZ’s Business” beginning on page 311.
Based on the positive results in our Phase 2 trial, we conducted three Phase 3 clinical trials (the “CLARITY” or “Phase 3” trials) with top-line results expected to be announced in April 2024. Subject to successful completion of these trials, we plan to submit a New Drug Application (“NDA”) to the FDA for one or both of our product candidates in mid-2024. If approval is granted, we will rigorously evaluate the results of our Phase 3 data, especially patient reported outcomes, and FDA feedback to select and commercialize the product we believe will have the greatest commercial potential, with a launch target date in second half of 2025.
It is estimated that there are 1.8 billion presbyopes globally and 128 million presbyopes in the United States. As people age, the crystalline lens in their eyes gradually hardens, resulting in a loss of lens elasticity that reduces the ability of the lens to increase its curvature and refractive power to focus incoming light for near vision onto the retina, known as accommodation. Although the progression of presbyopia is gradual, presbyopes often experience an abrupt change in their daily life as the symptoms become more pronounced starting in their mid-40s, when reading glasses or other corrective aids are suddenly necessary to read text or conduct close-up work. Presbyopia is typically self-diagnosed and self-managed with over-the-counter reading glasses, or managed, after evaluation by an eye care professional (“ECP”), with prescription reading or bifocal glasses or multifocal contact lenses. Currently, the only approved and marketed pharmaceutical treatment for presbyopia is marketed by AbbVie under the brand Vuity.
Based on data collected in a third-party study commissioned by us in early 2023 that is further described in the section “Market Opportunity” in the Proxy Statement/Prospectus in the section titled “LENZ’s Business” beginning on page 311, we found that presbyopes have high willingness to use a daily prescription eye drop that improves their near vision throughout the full workday. We expect that there will be a wide range of presbyopes that will be interested in using the eye drops at least four times a week. The large initial demand seen for Vuity during its launch in late 2021 and early 2022 corroborates the market demand for a pharmaceutical option for the treatment of presbyopia. However, despite a promising initial launch, Vuity’s user uptake has been limited by reportedly lower-than-expected efficacy and duration of effect across users. Additionally, Vuity use is associated with some side effects, including retinal tears and detachments, induced by the stimulation of the ciliary muscle. These limitations on efficacy and safety subsequently resulted in lower than anticipated prescription refill rates and a label amendment reflecting the risk of retinal tears and detachment specifically associated with Vuity. We believe that our once-daily eye drop, if approved, could become the leading brand for presbyopes, by improving near vision throughout the full workday.
Our product candidates LNZ100 and LNZ101 are formulated with aceclidine, a miotic, and designed to achieve optimal pupil diameter without impacting distance vision, a key limitation of other miotics. Miotics are compounds that cause pupil constriction, or miosis, creating a pinhole effect that enables better focus of incoming light from near objects onto the retina. Research has shown that a pupil diameter below two millimeters (2 mm) is optimal for presbyopia treatment and results in clinically meaningful improvement in near vision. Unlike other miotics such as pilocarpine and carbachol, aceclidine’s mechanism of action is pupil-selective, meaning it can activate the iris sphincter muscle and cause miosis of the pupil to a diameter below 2 mm without overstimulating the ciliary muscles that can cause a myopic shift and impair distance vision. As a result, aceclidine does not require any remaining accommodation to improve near vision, broadening its benefits to older presbyopes whose lens has lost this capacity. Therefore, we expect that users may be able to benefit from treatment even as they age from mid-40s to well into their mid-70s and across a broader range of refractive errors, as demonstrated in clinical testing to date.
LNZ101 contains the active ingredient brimonidine in addition to aceclidine. Brimonidine is an alpha 2 (“α2”) adrenergic receptor agonist that has also been used for lowering intraocular pressure as a treatment for glaucoma since the 1990s. Brimonidine causes vasoconstriction, prolonging the presence of aceclidine on the ocular surface and increasing aceclidine’s penetration into the anterior chamber. As a result, brimonidine extends the duration of the miotic effect of aceclidine.
While aceclidine is new to the United States, it has a long-established history outside the United States having been approved in Europe since the 1970s for the treatment of glaucoma, and marketed by Merck under the brand Glaucostat, at higher concentrations than in LENZ’s product candidates and up to four times a day. Similarly, brimonidine also has a long-established history of use. It is the active ingredient in Alphagan and Alphagan P, products initially marketed by Allergan (now AbbVie) for the treatment of glaucoma, in each case at higher concentrations than in our product candidates, and is also used in Lumify (“OTC”), invented by the founders of LENZ OpCo and marketed by Bausch & Lomb. Given the known favorable tolerability profile of both active ingredients used for decades, and the unique mechanism of action of aceclidine, we believe LNZ100 and LNZ101 have the potential to treat the broadest population of presbyopes and become the category leader.
In the INSIGHT trial, both product candidates demonstrated rapid onset with 73% and 62% three-lines or greater improvement in near visual acuity within 30 min for LNZ100 and LNZ101, respectively, compared to 8% for vehicle, and sustained the statistically significant three-lines or greater improvement in near visual acuity over an extended duration of 10 hours post-treatment, the last measured timepoint. Both LNZ100 and LNZ101 were well-tolerated with no serious drug-related adverse events.
In December 2022, we initiated our three Phase 3 multi-center, double-masked, randomized, active and vehicle-controlled, U.S.-based efficacy and safety trials for LNZ100 and LNZ101, for which we expect to announce top-line results in April 2024. The Phase 3 study consists of two six-week safety and efficacy trials, CLARITY-1 and CLARITY-2, and a six-month safety trial, CLARITY-3. The primary efficacy endpoints and the study population for the CLARITY trials are similar to that of the INSIGHT trial, enrolling participants in the same age range from 45 to 75 years and with a refractive range of -4.0 diopters (“D”) spherical equivalent (“SE”) to
+1.0D SE. As with the INSIGHT trial, the CLARITY trials will also permit enrollment of users who had previously undergone prior vision correction, such as LASIK, or cataract extraction with lens implant. Subject to successful completion of the CLARITY trials, we plan to submit an NDA for at least one of our product candidates to the FDA in mid-2024. If approval is granted, we will rigorously evaluate the results of its Phase 3 data, especially patient reported outcomes, and FDA feedback to select and commercialize the product we believe will have the greatest commercial potential, with a launch target date in second half of 2025.
Given our goal to develop and commercialize the leading, once-daily eye drop for presbyopia that can effectively and safely improve near vision throughout the full workday, we continue to build a robust commercial strategy in the United States to be launch-ready upon expected timing of FDA approval. We retain the flexibility to not only seek commercialization of our product candidates, but to also remain opportunistic in developing, in-licensing or partnering other products or product candidates to further leverage our commercial infrastructure to drive growth and operating leverage. Our product candidates have patent protection until 2039, at a minimum, due to a robust intellectual property portfolio underpinned by issued patents. If one of our product candidates is approved, we believe that it could be the first FDA-approved aceclidine-based product and would then be eligible for five years of NCE exclusivity in the United States. As of March 21, 2024, we had at least 18 issued U.S. patents, at least 25 issued patents outside the United States and at least 74 pending applications globally.
To execute our vision, we have assembled a team with extensive experience building successful life science and consumer product companies. Our team has helped launch and commercialize over a dozen ophthalmic products and therapies, including Acuvue, Alphagan P, Combigan, Dailies AquaComfort Plus, Durysta, Latisse, Lumigan, Pred Forte, Refresh, Restasis, Truetear, and Vuity, as well as major consumer-focused brands such as Botox, Herbalife and Ray-Ban. Members of our management team have held senior positions at Alcon, Allergan, Alvotech, Avanir, Bausch + Lomb, Herbalife, Hospira, Johnson & Johnson, Pfenex, Pfizer, VISX and others. We have also engaged a strong team of medical advisors across the ophthalmology and optometry fields. Our team is further supported by a strong group of investors that share our commitment to helping the millions of people experiencing symptoms of presbyopia in the United States and globally.
Our business is further described in the Proxy Statement/Prospectus in the section titled “LENZ’s Business” beginning on page 311 of the Proxy Statement/Prospectus and that information is incorporated herein by reference.
Risk Factors
The risk factors related to LENZ’s business and operations and the Transactions are set forth in the Proxy Statement/Prospectus in the section titled “Risk Factors” beginning on page 26 of the Proxy Statement/Prospectus and that information is incorporated herein by reference.
Audited Financial Statements
The audited financial statements as of and for the years ended December 31, 2023 and 2022 of LENZ OpCo set forth in Exhibit 99.1 hereto have been prepared in accordance with U.S. generally accepted accounting principles and pursuant to the regulations of the SEC.
These audited financial statements should be read in conjunction with the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included herein.
Unaudited Pro Forma Condensed Combined Financial Information
The unaudited pro forma condensed combined financial information of the Company for the year ended December 31, 2023 is set forth in Exhibit 99.2 hereto and is incorporated herein by reference.
Management’s Discussion and Analysis of Financial Condition and Results of Operations
Reference is made to the disclosure contained in Graphite's Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on February 27, 2024, in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” beginning on page 62 of the Annual Report on Form 10-K, which is incorporated herein by reference.
Management’s discussion and analysis of the financial condition and results of operation of LENZ OpCo as of and for the year ended December 31, 2023 is set forth below.
The following discussion and analysis provides information that the Company’s management believes is relevant to an assessment and understanding of the Company’s results of operations and financial condition. The discussion should be read together with the audited financial statements and related notes and unaudited pro forma condensed financial information that are included elsewhere in this Current Report on Form 8-K. This discussion may contain forward-looking statements based upon current expectations that involve risks and uncertainties. The Company’s actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth in the Proxy Statement/Prospectus in the section entitled “Risk Factors” beginning on page 26 of the Proxy Statement/Prospectus or in other parts of this Current Report on Form 8-K. Unless the context otherwise requires, references in this “Management’s Discussion and Analysis of Financial Condition and Results of Operations” to “LENZ OpCo,” “the Company,” “we,” “us” and “our” refer to the business and operations of LENZ OpCo prior to the Merger and to New LENZ and its consolidated subsidiary following the Closing.
Overview
We are a late-stage biopharmaceutical company focused on developing and commercializing innovative therapies to improve vision. Our initial focus is the treatment of presbyopia, the inevitable loss of near vision that impacts the daily lives of nearly all people over 45. In the United States, the estimated addressable population which suffers from this condition, known as presbyopes, is 128 million, almost four times the number of individuals suffering from dry eye disease and three times the number of individuals suffering from childhood myopia, macular degeneration, diabetic retinopathy and glaucoma combined. We believe that a once-daily pharmacological eye drop that can effectively and safely improve near vision throughout the full workday, without the need for reading glasses, will be a highly attractive commercial product with an estimated U.S. market opportunity in excess of $3 billion. It is our goal to develop and commercialize such a product, and we have assembled an executive team with extensive clinical and commercial experience to execute this goal and become the category leader.
Our product candidates LNZ100 and LNZ101 are preservative-free, single-use, once-daily eye drops containing aceclidine and aceclidine plus brimonidine, respectively. We believe our product candidates are differentiated based on rapid onset, degree and duration of near vision improvement, as well as their ability to be used across the full age range of presbyopes, from their mid-40s to well into their mid-70s, as well as the broadest refractive range. Aceclidine’s pupil-selective mechanism of action was demonstrated in our clinical trials where near vision improved while avoiding blurry distance vision. Our product candidates were well-tolerated in clinical trials, and their active ingredients have favorable tolerability profiles that have been well-established empirically.
In our INSIGHT Phase 2 trial, both LNZ100 and LNZ101 achieved the primary endpoint of three-lines or greater improvement in near visual acuity without losing one or more lines in Best Corrected Distance Visual Acuity at one hour post-treatment, with a response rate of 71% and 56%, respectively, compared to 6% for vehicle. Based on the positive results in our Phase 2 trial, we conducted three Phase 3 clinical trials (the CLARITY or Phase 3 trials) with results expected to be announced in April 2024. Subject to successful completion of these trials, we plan to submit an NDA to the FDA for one or both of our product candidates in mid-2024. If approval is granted, we will rigorously evaluate the results of our Phase 3 data, especially patient reported outcomes, and FDA feedback to select and commercialize the product that we believe will have the greatest commercial potential, with a launch target date in second half of 2025.
For more information regarding our business, see the section titled “Business” of this Current Report on Form 8-K.
As of December 31, 2023, we had $65.8 million of cash, cash equivalents and short-term investments. Based on our current plans, we believe our existing cash, cash equivalents and short-term investments, together with the proceeds from the merger and the Graphite private placement will allow the company to continue to build infrastructure and commercialize our lead product candidate, subject to successful completion of the ongoing Phase 3 trials, the NDA submission and FDA approval. We do not expect to generate any revenues from product sales unless and until we successfully complete development and obtain regulatory approval for one or more of our product candidates. We have incurred net losses in each year since inception, and as of December 31, 2023, we had an accumulated deficit
of $95.2 million. These losses have resulted principally from costs incurred in connection with research and development activities and selling, general and administrative costs associated with our operations. We expect to continue to incur significant expenses and operating losses for the foreseeable future due to the cost of research and development, including conducting clinical trials, the regulatory approval process and preparation for and the commercial launch of LNZ100 or LNZ101, subject to FDA approval, including expenses related to product sales, marketing and distribution, and additional costs associated with being a public company, including audit, legal, regulatory and tax-related services associated with maintaining compliance with an exchange listing and SEC requirements. As a result of these and other factors, it is possible that we may require additional financing to fund our operations and planned growth.
Through the completion of the Merger, Lenz OpCo financed its operations primarily through private placements of its common stock and convertible preferred stock, including the following financings during the periods presented:
•In March 2023, LENZ OpCo issued and sold an aggregate of 28,019,181 shares of Series B preferred stock at a purchase price of $2.9801 per share for an aggregate purchase price of approximately $83.5 million.
•In October 2022, LENZ OpCo issued and sold an aggregate of 9,899,340 shares of Series A Preferred Stock at a purchase price of $2.15 per share for an aggregate purchase price of approximately $21.3 million as part of a milestone closing of the 2021 Series A Preferred Stock financing.
•In April 2022, LENZ OpCo issued and sold an aggregate of 2,950,548 shares of Series A-1 Preferred Stock at a purchase price of $3.3892 per share for an aggregate purchase price of approximately $10.0 million.
Until such time as we can generate significant revenue from sales of our product candidates, if ever, we expect to finance our cash needs through equity offerings, debt financings or other capital sources, including potential collaborations, licenses and other similar arrangements. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. Our failure to raise capital or enter into such other arrangements when needed would have a negative impact on our financial condition and could force us to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourself.
Ji Xing License and Collaboration Agreement
In April 2022, we entered into a License and Collaboration Agreement with Ji Xing Pharmaceuticals Hong Kong Limited (“Ji Xing”) granting Ji Xing an exclusive license (the “Ji Xing License”) to certain of our intellectual property rights to develop, use, import, and sell products containing LNZ100 or LNZ101 (“Products”) for the treatment of presbyopia in humans in mainland China, Hong Kong Special Administrative Region, Macau Special Administrative Region, and Taiwan (collectively, “Greater China). We also granted Ji Xing (i) the right to negotiate in good faith and enter into agreements to purchase Products from us for clinical and commercial uses at cost plus a negotiated percentage and (ii) the right of first negotiation to obtain a regional license from us on other products we might develop outside of the field of presbyopia for commercialization in Greater China.
We received nonrefundable, non-creditable upfront payments totaling $15.0 million as initial consideration under the Ji Xing License. In addition, we are also eligible to receive (i) up to $95.0 million in regulatory and sales milestone payments, (ii) tiered, escalating royalties in the range of 5% to 15% on net sales of Products in Greater China by Ji Xing, its affiliates and sublicensees, and (iii) tiered, deescalating royalties in the range of 15% to 5% of sublicensing income received by Ji Xing prior to the regulatory approval of the first Product in Greater China.
The $15.0 million upfront payments allocated to that single performance obligation was recognized on execution of the Ji Xing License in the year ended December 31, 2022. No additional amounts under the Ji Xing License were received during the year ended December 31, 2022.
In connection with the Ji Xing License, RTW Investments, LP (“RTW”), a significant investor in Ji Xing, through three funds managed or advised by RTW, invested approximately $10.0 million in exchange for 2,950,548 shares of LENZ OpCo's Series A-1 Preferred Stock at a purchase price of $3.3892 per share.
Key Trends and Factors Affecting Comparability Between Periods
•The Ji Xing License was signed in April 2022, and we recorded $15.0 million of revenue for the year ended December 31, 2022. We did not generate any revenue related to the Ji Xing License or from other sources for the year ending December 31, 2023.
•We continue to build out our research and development team and our research and development costs increased in 2023, relative to 2022, as a result of significant expenses related to the CLARITY trials. See the section of this Current Report on Form 8-K titled “Business” for more information about the CLARITY trials.
•We have built a cross-functional commercial team consisting of marketing, market access and commercial operations and will continue to strategically build our sales and our commercial infrastructure with capabilities designed to scale when necessary to support a commercial launch if approval is received. These expenses increased in 2023 as compared to 2022.
•Following the Merger, the combined company’s expenses will increase from those that we incurred in prior years as a privately held company, including (i) costs to comply with the rules and regulations of the SEC and those of Nasdaq, (ii) legal, accounting and other professional services, (iii) insurance, (iv) investor relations activities, and (v) other administrative and professional services.
Recent Developments
The Merger and PIPE Financing
On November 14, 2023, we entered into the Merger Agreement with Graphite and Generate Merger Sub, pursuant to which LENZ OpCo merged with and into Generate Merger Sub at the Effective Time on March 21, 2024, with LENZ OpCo continuing after the Merger as the surviving company and a wholly-owned subsidiary of Graphite. At the Effective Time, each outstanding share of LENZ OpCo capital stock was converted into the right to receive shares of Graphite common stock, par value $0.00001, as set forth in the Merger Agreement. Upon closing of the Merger, the combined company was named “LENZ Therapeutics, Inc.” and will continue to be listed on the Nasdaq.
Under the exchange ratio formula in the Merger Agreement, immediately following the Effective Time, the LENZ OpCo securityholders owned approximately 65% of the outstanding shares of the combined company’s common stock on a fully-diluted basis and securityholders of Graphite as of immediately prior to the Effective Time owned approximately 35% of the outstanding shares of the combined company’s common stock on a fully-diluted basis (prior to giving effect to the PIPE Financing and excluding shares reserved for future grants under the 2024 Plan and the 2024 ESPP, each described elsewhere in this Current Report on Form 8-K).
Concurrently with the execution of the Merger Agreement, Graphite entered into the subscription agreement with the PIPE Investors, pursuant to which, immediately following the Effective Time, the PIPE Investors subscribed for and purchased an aggregate of 3,559,565 shares of Common Stock at a price of $15.0299 per share for aggregate gross proceeds of approximately $53.5 million.
Basis of Presentation
The following discussion highlights our results of operations and the principal factors that have affected our financial condition as well as our liquidity and capital resources for the periods described and provides information that management believes is relevant for an assessment and understanding of the balance sheets and statements of operations and comprehensive loss presented herein. The following discussion and analysis are based on our audited financial statements and related notes thereto, which we have prepared in accordance with GAAP. You should read the discussion and analysis together with such audited financial statements and the related notes thereto.
Components of Statements of Operations and Comprehensive Loss
Revenue
We currently have no products approved for sale, and we have not generated any revenue from product sales to date. We have generated revenue related to the Ji Xing License, and in the future may generate revenue from payments received under licenses or collaboration agreements we may enter into with respect to our product candidates.
We recorded $15.0 million of license revenue related to the Ji Xing License for the year ended December 31, 2022.
We did not generate any revenue related to the Ji Xing License or from other sources for the year ended December 31, 2023.
Operating Expenses
Research and Development
Research and development expenses, which consist primarily of costs associated with our product research and development efforts, are expensed as incurred. Research and development expenses consist primarily of: (i) employee related costs, including salaries, benefits and share-based compensation expense for employees engaged in research and development activities; (ii) third-party contract costs relating to research, formulation, manufacturing, nonclinical studies and clinical trial activities; (iii) external costs of outside consultants who assist with technology development, regulatory affairs, clinical development and quality assurance; and (iv) allocated facility-related costs. We track research and development costs collectively for LNZ100 or LNZ101 because expenses incurred are interrelated and disaggregation would not be meaningful.
Costs for certain activities, such as manufacturing, nonclinical studies and clinical trials are generally recognized based on the evaluation of the progress of completion of specific tasks using information and data provided by our vendors and collaborators. Research and development activities are central to our business.
We continue to build out our research and development team and we expect our research and development costs will decrease in 2024, relative to 2023, given the completion of the CLARITY trials expected to occur in April 2024.
Selling, General and Administrative
Selling, general and administrative expenses consist primarily of salaries and related benefits, including share-based compensation, related to our executive, finance, business development, sales and marketing, and other corporate functions. Other general and administrative expenses include professional fees for legal, auditing, tax and business consulting services, insurance costs, intellectual property and patent costs, facility costs and travel costs. We expect that selling, general and administrative expenses will increase in the future as we expand our operating activities. Additionally, we expect that the combined company will incur significant additional expenses associated with being a public company that LENZ OpCo did not incur as a privately-held company, including (i) costs to comply with the rules and regulations of the SEC and those of Nasdaq, (ii) legal, accounting and other professional services, (iii) insurance, (iv) investor relations activities, and (v) other administrative and professional services.
Other Income (Expense), Net
Other income (expense), net consists of the change in fair value of preferred stock warrants liability and interest income earned on cash, cash equivalents and short-term investments.
Provision for Income Taxes
Income tax expense (benefit) consists of U.S. federal and state income taxes.
Results of Operations
Comparison of the Year Ended December 31, 2022 and 2023
The following table presents the results of operations for the periods indicated (amounts in thousands, except percentages):
| | | | | | | | | | | | | | | | | | | | | | | |
| Year Ended
December 31, | | | | |
| 2022 | | 2023 | | $ Change | | % Change |
Revenue: | | | | | | | |
License revenue | $ | 15,000 | | | $ | — | | | $ | (15,000) | | | (100) | % |
Total revenue | 15,000 | | | — | | | (15,000) | | | (100) | % |
Operating expenses: | | | | | | | |
Research and development | 21,125 | | | 59,504 | | | 38,379 | | | 182 | % |
Selling, general and administrative | 4,358 | | | 12,925 | | | 8,567 | | | 197 | % |
Total operating expenses | 25,483 | | | 72,429 | | | 46,946 | | | 184 | % |
Loss from operations | (10,483) | | | (72,429) | | | (61,946) | | | 591 | % |
Other income: | | | | | | | |
Other | 15 | | | 93 | | | 78 | | | 520 | % |
Interest income | 4 | | | 2,189 | | | 2,185 | | | 54,625 | % |
Total other income (expense), net | 19 | | | 2,282 | | | 2,263 | | | 11,911 | % |
Net loss before income taxes | (10,464) | | | (70,147) | | | (59,683) | | | 570 | % |
Income tax expense (benefit) | 347 | | | (179) | | | (526) | | | (152) | % |
Net loss | $ | (10,811) | | | $ | (69,968) | | | $ | (59,157) | | | 547 | % |
License Revenue
During the year ended December 31, 2022, we recognized $15.0 million of license revenue related to the Ji Xing License. This revenue was recognized upon completion of the related performance obligation. We did not recognize any revenue for the year ended December 31, 2023.
Research and Development
Substantially all of our research and development expenses incurred for the years ended December 31, 2022 and 2023 were related to the development of LNZ100 and LNZ101, which were both included together in our INSIGHT and CLARITY trials.
The following table presents a detailed breakdown of our research and development expenses for the periods indicated (in thousands):
| | | | | | | | | | | |
| Year Ended
December 31, |
| 2022 | | 2023 |
| Contract clinical research expense | $ | 11,598 | | | $ | 37,949 | |
| Contract manufacturing expense | 6,006 | | | 8,339 | |
| Contract nonclinical research expense | 1,216 | | | 6,835 | |
| Contract regulatory consulting expense | 237 | | | 1,107 | |
| Employee salaries and related expense | 1,810 | | | 4,131 | |
| Other expense | 258 | | | 1,143 | |
| Total research and development | $ | 21,125 | | | $ | 59,504 | |
Research and development expenses increased $38.4 million, or 182%, from $21.1 million for the year ended December 31, 2022 to $59.5 million for the year ended December 31, 2023. The increase was primarily driven by a $26.4 million increase in contract research expense for our clinical trials, a $2.3 million increase in contract manufacturing expenses for clinical drug product manufacturing, a $5.6 million increase in contract nonclinical research expense, and a $2.3 million increase in employee salaries and related expenses. These increases were primarily related to our CLARITY trials and preparation for our potential NDA filing.
Selling, General and Administrative
Selling, general and administrative expenses increased $8.6 million, or 197%, from $4.4 million for the year ended December 31, 2022 to $12.9 million for the year ended December 31, 2023. The increase was primarily driven by a $4.6 million increase in legal and other professional services (principally related to legal fees incurred in connection with our abandoned initial public offering), a $2.2 million increase in employee salaries and related expenses due to increased headcount, and a $1.4 million increase in sales infrastructure and marketing expenses.
Other Income (Expense), Net
Other income, net for the year ended December 31, 2022, was $19,000, compared to $2.3 million for the year ended December 31, 2023. The change was primarily driven by a $2.2 million increase in interest income and a reduction in fair value of preferred stock warrants liability of $0.1 million.
Provision for Income Taxes
During the year ended December 31, 2022, we recognized income tax expense $0.3 million due to the requirement to capitalize research and development expenses under the Tax Cuts and Jobs Act (the “TCJA”), compared to an income tax benefit of $0.2 million for the year ended December 31, 2023 primarily due to our income derived in foreign markets, which is subject to a lower tax rate as a result of the foreign-derived intangible income deduction that was introduced as part of the TCJA. The TCJA requires taxpayers to capitalize and amortize research and development expenditures under section 174 for tax years beginning after December 31, 2021. This rule became effective for us during the year ended December 31, 2022 resulting in a gross deferred tax asset for capitalized research and development expenditures of approximately $66.0 million as of December 31, 2023. We will continue to amortize these costs for tax purposes over 5 years for R&D performed in the U.S. and over 15 years for R&D performed outside the U.S.
Liquidity and Capital Resources
Sources of Liquidity
As of December 31, 2023, we had $65.8 million of cash, cash equivalents and marketable securities. Based on our current operating plans, we believe our existing cash, cash equivalents and short-term investments will be sufficient to fund our planned operations into 2025. Further, based on our current operating plans, we believe our cash, cash equivalents, and short-term investments as of December 31, 2023, together with the proceeds from the merger and the Graphite private placement, will be sufficient to fund our planned operations through several anticipated value-creating milestones and through at least 2026, and will allow us to continue to build infrastructure and commercialize our lead product candidate, subject to successful completion of the ongoing Phase 3 trials, NDA submission and FDA approval.
We have incurred net losses in each year since inception and as of December 31, 2023, we had an accumulated deficit of $95.2 million. Our net losses were $10.8 million and $70.0 million for the years ended December 31, 2022 and 2023, respectively. These losses have resulted principally from costs incurred in connection with research and development activities and selling, general and administrative costs associated with our operations. We expect to continue to incur significant expenses and operating losses for the foreseeable future due to the cost of research and development, the regulatory approval process for either LNZ100 or LNZ101, and the commercial launch of either product, if approved.
From inception through December 31, 2023, we received funding of $13.0 million from our initial seed financing, $47.0 million from the sale of Series A Convertible Preferred Stock, $10.0 million from the sale of Series A-1 Convertible Preferred Stock, and gross proceeds of $83.5 million from the sale of Series B Convertible Preferred Stock.
Funding Requirements
We believe that our existing cash, cash equivalents and marketable securities will be sufficient to fund our planned operations into 2025. Further, based on our current operating plans, we believe our existing cash, cash equivalents, and short-term investments, together with the proceeds from the Merger and the PIPE Financing, will be sufficient to fund our planned operations through several anticipated value-creating milestones and through at least 2026, and will allow us to continue to build infrastructure and commercialize our lead product candidate, subject to successful completion of the ongoing Phase 3 trials, NDA submission and FDA approval. This belief is based on assumptions that may prove to be wrong, and we could use our available capital resources sooner than expected. Changing circumstances, some of which may be beyond our control, could cause us to consume capital significantly faster than currently anticipated, and we may need to seek additional funds sooner than planned.
Our future capital requirements will depend on many factors, including but not limited to:
•the results, costs, and timing of our ongoing clinical trials for LNZ100 and LNZ101;
•the costs and timing of manufacturing for our product candidates and commercial manufacturing if any product candidate is approved;
•costs associated with establishing a sales, marketing, and distribution infrastructure to commercialize any products for which we may obtain marketing approval;
•the costs, timing, and outcome of regulatory review of our product candidates;
•the legal costs of obtaining, maintaining, and enforcing our patents and other intellectual property rights;
•our efforts to enhance operational systems and hire additional personnel to satisfy our obligations as a public company;
•the terms and timing of establishing and maintaining licenses and other similar arrangements;
•our ability to achieve sufficient market acceptance and adequate market share and revenue for any approved products; and
•costs associated with any products or technologies that we may in-license or otherwise acquire or develop.
Prior to the Merger, LENZ OpCo funded its operations primarily through the sale and issuance of convertible preferred stock and it is possible that, following the Merger, we may require additional financing. We intend to evaluate financing opportunities from time to time, and our ability to obtain financing will depend, among other things, on our development efforts, business plans, operating performance and the condition of the capital markets at the time we seek financing. We cannot assure you that additional financing will be available to us on favorable terms when required, or at all. If we raise additional funds through the issuance of equity or equity-linked securities, those securities may have rights, preferences or privileges senior to the rights of our Common Stock, and our stockholders may experience dilution. If we raise additional funds through the incurrence of indebtedness, then we may be subject to increased fixed payment obligations and could be subject to restrictive covenants, such as limitations on our ability to incur additional debt, and other operating restrictions that could adversely impact our ability to conduct our business.
Cash Flows
The following table summarizes our cash flows for the years presented (amounts in thousands):
| | | | | | | | | | | |
| Years Ended December 31, |
| 2022 | | 2023 |
| Net cash (used in) provided by: | | | |
| Operating activities | $ | (4,091) | | | $ | (60,380) | |
| Investing activities | (37) | | | (29,621) | |
| Financing activities | 30,262 | | | 80,700 | |
| Net increase in cash and cash equivalents | $ | 26,134 | | | $ | (9,301) | |
Net Cash Used in Operating Activities
Our net cash used in operating activities primarily results from our net loss adjusted for non-cash expenses, changes in working capital components, amounts due to contract research organizations to conduct our clinical programs, manufacturing of drug product and employee-related expenditures for research and development and selling, general and administrative activities. Our cash flows from operating activities will continue to be affected by spending to develop and pursue regulatory approval for either LNZ100 or LNZ101 and commercialization activities, if approval is obtained. Our cash flows will also be affected by other operating and general administrative activities, including operating as a public company.
For the year ended December 31, 2022, cash used in operating activities was $4.1 million and resulted from (i) our net loss of $10.8 million plus a $2.1 million increase in operating assets, offset by (ii) a $8.2 million increase in accounts payable and accrued liabilities and $0.7 million in non-cash adjustments primarily related to share-based compensation expense.
For the year ended December 31, 2023, cash used in operating activities was $60.4 million and resulted from (i) our net loss of $70.0 million, plus $1.1 million in amortization of premiums and discounts on marketable securities, offset by (ii) an $8.6 million increase in accounts payable and accrued liabilities, $1.3 million of share-based compensation expense, and a $0.8 million decrease in operating assets.
Net Cash Used in Investing Activities
Cash used in investing activities for the year ended December 31, 2022 was $37,000 and related to the purchase of property and equipment.
For the year ended December 31, 2023, cash used in investing activities was $29.6 million and related primarily to the purchase of marketable securities of $52.1 million offset by proceeds from maturities of marketable securities of $22.5 million.
Net Cash Provided by Financing Activities
Cash provided by financing activities for the year ended December 31, 2022 includes $20.2 million in net cash proceeds from the sale by LENZ OpCo of Series A Convertible Preferred Stock, $9.9 million in net cash proceeds from the sale by LENZ OpCo of Series A-1 Convertible Preferred Stock, and $0.1 million in net cash proceeds from the exercise of LENZ OpCo stock options.
For the year ended December 31, 2023, cash provided by financing activities was $80.7 million and consisted of $83.0 million in net cash proceeds from the sale by LENZ OpCo of Series B Convertible Preferred Stock, and $0.2 million in net cash proceeds from the exercise of LENZ OpCo stock options, offset by an increase in deferred offering costs of $2.5 million.
Material Cash Requirements from Contractual Obligations
In February 2022, we entered into a lease for 2,930 square feet of office space in Del Mar, California. In March 2023, we entered into a lease amendment for a 647 square feet expansion of our office space at the same facility. The term of the lease, as amended, is forty-eight months from the original commencement date, terminating March 31, 2026, unless terminated sooner.
Rent expense is recorded on a straight-line basis. Rent expense related to the Del Mar lease was $0.1 million for the years ended December 31, 2022 and 2023, respectively. See Note 6 to our audited financial statements for details related to future lease payments.
We also have contracts with various organizations to conduct research and development activities, including clinical trial organizations to manage clinical trial activities and manufacturing companies to manufacture the drug product used in the clinical trials. The scope of the services under these research and development contracts can be modified and the contracts cancelled by us upon written notice. In the event of a cancellation, the company would be liable for the cost and expenses incurred to date as well as any close out costs of the service arrangement.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements as defined in the rules and regulations of the SEC.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of the financial condition and results of operations is based on our financial statements, which have been prepared in accordance with GAAP. The preparation of our financial statements requires us to make certain estimates, judgements and assumptions that affect the reported amounts of assets and liabilities as of the date of the financial statements, as well as the reported amounts of revenues and expenses during the periods presented. We believe that the estimates, judgments and assumptions are reasonable based upon information available to us at the time that these estimates, judgments and assumptions are made. To the extent there are material differences between these estimates, judgments or assumptions and actual results, our financial statements will be affected. Historically, revisions to our estimates have not resulted in a material change to our financial statements.
While our significant accounting policies are described in more detail in the notes to our financial statements, we believe the following accounting policies to be most critical to the judgments and estimates used in the preparation of our financial statements.
Prepaid and Accrued Research and Development Expenses
As part of the process of preparing our financial statements, we are required to estimate our prepaid and accrued research and development expenses. This process involves reviewing open contracts and purchase orders, communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. We make estimates of our prepaid and accrued research and development expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us at the time.
Although we do not expect our estimates to be materially different from amounts actually incurred, if our estimates of the status and timing of services performed differ from the actual status and timing of services performed, we may report amounts that are too high or too low in any particular period. To date, there have been no material differences between our estimates and amounts actually incurred.
Preferred Stock Warrants Liability
LENZ OpCo had freestanding warrants to purchase shares of Series A convertible preferred stock, referred to herein as the Series A Warrants. Upon certain change in control events that were outside of LENZ OpCo's control, including liquidation, sale or transfer of control, holders of the preferred stock could cause redemption of such
warrants. The Series A Warrants are revalued at each subsequent balance sheet date, with fair value changes recognized as increases or reductions to other income (expense), net in the accompanying statements of operations. See Note 3 to our audited financial statements for information concerning certain of the specific assumptions we used in determining the value of the Series A Warrants at each reporting period. Upon completion of the Merger, the Series A Warrants became exercisable into shares of the Common Stock and will no longer continue to be remeasured at each reporting date.
Stock-Based Compensation Expense
Stock-based compensation expense represents the cost of the grant date fair value of equity awards recognized over the requisite service period of the awards (usually the vesting period) on a straight-line basis. We estimate the fair value of equity awards using the Black-Scholes option pricing model and recognize forfeitures as they occur. Estimating the fair value of equity awards as of the grant date using valuation models, such as the Black-Scholes option pricing model, is affected by assumptions regarding a number of variables, including the risk-free interest rate, the expected stock price volatility, the expected term of stock options, the expected dividend yield and the fair value of the underlying common stock on the date of grant. Changes in the assumptions can materially affect the fair value and ultimately how much stock-based compensation expense is recognized. These inputs are subjective and generally require significant analysis and judgment to develop. See Note 10 to our audited financial statements for information concerning certain of the specific assumptions we used in applying the Black-Scholes option pricing model to determine the estimated fair value of our stock options granted, if any, during the years ended December 31, 2022 and 2023.
Common Stock Valuations
LENZ OpCo was required to estimate the fair value of the common stock underlying its equity awards when performing fair value calculations. The fair value of the common stock underlying such equity awards was determined on the grant date by the LENZ OpCo board of directors considering the most recently available third-party valuations of LENZ OpCo common stock and the LENZ OpCo board of directors’ assessment of additional objective and subjective factors that it believed were relevant, and factors that may have changed from the date of the most recent valuation through the date of the grant. All options were intended to be granted with an exercise price per share no less than the fair value per share of common stock underlying those options on the date of grant, based on the information known to the LENZ OpCo board of directors on the date of grant. In the absence of a public trading market for the LENZ OpCo common stock prior to the Merger, on each grant date LENZ OpCo developed an estimate of the fair value of our common stock in order to determine an exercise price for the option grants.
The various objective and subjective factors the LENZ OpCo board of directors considered, along with input from management, to determine the fair value of the LENZ OpCo common stock, included:
•valuations of the LENZ OpCo common stock performed by independent third-party valuation specialists;
•LENZ OpCo's stage of development and business strategy, including the status of research and development efforts of its platforms, programs and product candidates, and the material risks related to its business and industry;
•LENZ OpCo's results of operations and financial position, including levels of available capital resources;
•the valuation of publicly traded companies in the life sciences and biotechnology sectors, as well as recently completed mergers and acquisitions of peer companies;
•the lack of marketability of the LENZ OpCo common stock as a private company;
•the prices of LENZ OpCo convertible preferred stock sold to investors in arm’s length transactions and the rights, preferences, and privileges of LENZ OpCo convertible preferred stock relative to those of LENZ OpCo common stock;
•the likelihood of achieving a liquidity event for the holders of LENZ OpCo common stock, such as an initial public offering or a sale of the company, given prevailing market conditions;
•trends and developments in LENZ OpCo's industry; and
•external market conditions affecting the life sciences and biotechnology industry sectors.
Prior to the Merger, determinations of the fair value of LENZ OpCo common stock have included the consideration by the LENZ OpCo board of directors of valuations prepared by an independent third-party valuation specialist using methodologies, approaches and assumptions consistent with the American Institute of Certified Public Accountants Accounting and Valuation Guide: Valuation of Privately Held Company Equity Securities Issued as Compensation (the “Practice Aid”).
The Practice Aid prescribes several valuation approaches for setting the value of an enterprise, such as the cost, income and market approaches, and various methodologies for allocating the value of an enterprise to the LENZ OpCo common stock. The cost approach establishes the value of an enterprise based on the cost of reproducing or replacing the property less depreciation and functional or economic obsolescence, if present. The income approach establishes the value of an enterprise based on the present value of future cash flows that are reasonably reflective of LENZ OpCo's future operations, discounting to the present value with an appropriate risk adjusted discount rate or capitalization rate. The market approach is based on the assumption that the value of an asset is equal to the value of a substitute asset with the same characteristics. Each valuation methodology was considered in LENZ OpCo's valuations.
The Practice Aid also identifies various available methods for allocating enterprise value across classes and series of capital stock to determine the estimated fair value of common stock at each valuation date. In accordance with the Practice Aid, the LENZ OpCo board of directors considered the following methods:
•Probability-weighted expected return method (“PWERM”). The PWERM is a scenario-based analysis that estimates the fair value of common stock based upon an analysis of future values for the business, assuming various outcomes. The common stock value is based on the probability-weighted present value of expected future investment returns considering each of the possible forecasted outcomes as well as the rights of each class of stock. The future value of the common stock under each outcome is discounted back to the valuation date at an appropriate risk-adjusted discount rate and probability weighted to arrive at a non-marketable indication of value for the common stock.
•Option Pricing Method (“OPM”). Under the OPM, shares are valued by creating a series of call options, representing the present value of the expected future returns to the stockholders, with exercise prices based on the liquidation preferences and conversion terms of each equity class. The estimated fair values of the preferred and common stock are inferred by analyzing these options.
•Hybrid Return Method. The hybrid return method is a blended approach using aspects of both the PWERM and OPM, in which the equity value in one of the scenarios is calculated using an OPM.
LENZ OpCo generally estimated the enterprise value of its business using a market approach. For each of the valuations conducted as of October 30, 2020, May 1, 2022 and October 31, 2022, LENZ OpCo used the precedent transaction method (one of the three general methodologies of the market approach) to determine enterprise value. The precedent transaction method considers the sale price of shares in a recent financing and then back- solves using an option pricing model that gives consideration to the company's capitalization structure and rights of the preferred and common stockholders as well as an assumption for a discount for lack of marketability (“DLOM”). For the March 6, 2023, June 30, 2023, and September 6, 2023 valuations, LENZ OpCo examined two scenarios to estimate enterprise value: (1) the “IPO Scenario,” which represents the future value of LENZ OpCo common stock upon an initial public offering, based on certain assumptions, and then risk-adjusts to estimate the present value of LENZ OpCo's common stock excluding any DLOM, and (2) the “Stay Private Scenario,” in which LENZ OpCo would remain an independent and private company and for which it used the precedent transaction method including a DLOM. We then probability weighted the valuations for the IPO Scenario and the Stay Private Scenario to estimate enterprise value.
Given the uncertainty associated with both the timing and type of any future exit scenario, and based on LENZ OpCo's stage of development and other relevant factors, for the valuations conducted as of October 30, 2020, May 1, 2022, and October 31, 2022, LENZ OpCo concluded that the OPM was most appropriate for allocating enterprise value. LENZ OpCo believed the OPM was the most appropriate given the expectation of various potential liquidity outcomes and the difficulty of selecting and supporting appropriate enterprise values given LENZ OpCo's early stage of development. Under the OPM, shares are valued by creating a series of call options with exercise prices based on the liquidation preferences and conversion terms of each equity class. The values of LENZ OpCo's preferred stock and common stock are inferred by analyzing these options. For the valuations conducted as of March 6, 2023, June 30, 2023, and September 6, 2023, LENZ OpCo determined to allocate enterprise value using the hybrid return method, which is a hybrid between PWERM and OPM and estimates the probability weighted value across multiple scenarios but uses the OPM to estimate the allocation of value within one or more of those scenarios. The hybrid method is useful when certain discrete future outcomes can be predicted, but also accounts for uncertainty regarding the timing or likelihood of specific alternative exit events.
The assumptions underlying these valuations represented LENZ OpCo management’s best estimates, which involved inherent uncertainties and the application of management’s judgment. As a result, if LENZ OpCo had used significantly different assumptions or estimates, the fair value of LENZ OpCo's common stock and LENZ OpCo's stock-based compensation expense could have been materially different. Following the closing of the Merger, our board of directors will determine the fair value of our common stock based on our closing price as reported on the date of grant on the primary stock exchange on which our common stock is traded.
Other Company Information
Jumpstart Our Business Startups Act (“JOBS Act”)
We will be an emerging growth company, as defined in the JOBS Act, and we may remain an emerging growth company until the last day of the fiscal year following the fifth anniversary of the initial public offering of Graphite’s common stock. For so long as we remain an emerging growth company, we are permitted and intend to rely on certain exemptions from various public company disclosure and reporting requirements, including not being required to have our internal control over financial reporting audited by our independent registered public accounting firm pursuant to Section 404(b) of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and any golden parachute payments not previously approved. In particular, we have provided only two years of audited financial statements and have not included all of the executive compensation-related information that would be required if we were not an emerging growth company. Accordingly, the information contained herein may not be comparable with the information stockholders receive from other public companies in which they may hold stock.
Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. Prior to the Merger, Graphite has elected to use, and we intend to continue to use, this extended transition period for complying with certain or new or revised accounting standards until the earlier of (i) the last day of the fiscal year (a) following the fifth anniversary of the closing of Graphite's initial public offering, (b) in which we have total annual gross revenue of at least $1.235 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700.0 million as of the prior June 30th, (ii) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period, or (iii) if we affirmatively and irrevocably opt out of the extended transition period provided by the JOBS Act.
We will also be a “smaller reporting company” because the market value of Graphite’s stock held by non-affiliates was less than $700 million as of June 30, 2023 and its annual revenue was less than $100 million during the fiscal year ended December 31, 2022. We may continue to be a smaller reporting company after the Merger in any given year if either (i) the market value of our stock held by non-affiliates is less than $250 million as of June 30 in the most recently completed fiscal year or (ii) our annual revenue is less than $100 million during the most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700 million as of June 30
in the most recently completed fiscal year. If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
Recent Accounting Pronouncements
See Note 2 to our audited financial statements included elsewhere in this Current Report on Form 8-K for a discussion of recent accounting pronouncements.
Properties
Our corporate headquarters is located in Del Mar, California, and consists of 3,577 square feet of office space pursuant to a lease that expires in March 2026. We neither own nor lease any other material real property.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth information as of the Closing Date regarding the beneficial ownership of Common Stock immediately following consummation of the Transactions by:
•each person known by New LENZ to be the beneficial owner of more than 5% of New LENZ’s outstanding Common Stock immediately following the consummation of the Transactions;
•each of New LENZ’s executive officers and directors; and
•all of New LENZ’s executive officers and directors as a group after the consummation of the Transactions.
Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if such person possesses sole or shared voting or investment power over that security. Under those rules, beneficial ownership includes securities that such person has the right to acquire, such as through the exercise of stock options, within 60 days of the Closing Date. Shares subject to warrants and options that are currently exercisable or exercisable within 60 days of the Closing Date are considered outstanding and beneficially owned by the person holding such warrant and/or options for the purpose of computing the percentage ownership of that person but are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Except as noted by footnote, and subject to community property laws where applicable, based on the information provided to New LENZ, New LENZ believes that the individuals and entities named in the table below have sole voting and investment power with respect to all shares shown as beneficially owned by them. Unless otherwise noted, the business address of each of the directors and executive officers of New LENZ is 445 Marine View Ave., Ste. #320, Del Mar, CA 92014. The percentage of beneficial ownership of New LENZ is
calculated based on an estimated 25,533,533 shares of Common Stock outstanding immediately after giving effect to the Transactions.
| | | | | | | | | | | | | | |
| Name of Beneficial Owners | | Number of Shares | | % |
| Greater than 5% Stockholders | | | | |
Alpha Wave Ventures II, LP(1) | | 3,612,211 | | | 14.1 | % |
Entities affiliated with Point72 Asset Management(2) | | 1,882,693 | | | 7.4 | % |
Entities affiliated with RA Capital Management(3) | | 4,249,356 | | | 16.6 | % |
Entities affiliated with Versant Management(4) | | 4,612,684 | | | 18.0 | % |
| Executive Officers and Directors | | | | |
Evert Schimmelpennink(5) | | 785,693 | | | 3.0 | % |
Shawn Olsson(6) | | 149,355 | | | * |
Marc Odrich(7) | | 166,071 | | | * |
| Daniel Chevallard | | — | | | — | % |
Frederic Guerard(8) | | 37,772 | | | * |
James McCollum(9) | | 595,842 | | | 2.3 | % |
Zach Scheiner(10) | | 4,249,356 | | | 16.6 | % |
| Shelley Thunen | | — | | | — | % |
| Jeff George | | — | | | — | % |
Kimberlee C. Drapkin(11) | | 5,714 | | | * |
All directors and executive officers as a group (10 persons)(12) | | 5,989,803 | | | 22.5 | % |
____________
* Less than 1%
(1)Consists of (i) 2,714,002 shares of Common Stock received by Alpha Wave Ventures II, LP in the Merger as an equityholder of record of LENZ OpCo, and (ii) 898,209 shares of Common Stock purchased by Alpha Wave Ventures II, LP in the PIPE Financing. Alpha Wave Ventures GP, Ltd is the general partner of Alpha Wave Ventures II, LP and therefore may be deemed to have beneficial ownership over these shares. The address of Alpha Wave Ventures GP, Ltd is 667 Madison Ave, 19th Floor, New York, New York 10065.
(2)Consists of (i) 1,017,751 shares of Common Stock received by Point72 Biotech Private Investments, LLC – Series LT (“Point72 Biotech”) in the Merger as an equityholder of LENZ OpCo, and (ii) 864,942 shares of Common Stock purchased by Point72 Associates, LLC (“Point72 Associates”) in the PIPE Financing. Differentiated Ventures Investments, LLC (“Differentiated Ventures”), a Delaware limited liability company, is the managing member of Point72 Biotech and may be deemed to share beneficial ownership of the shares held by Point72 Biotech. 72 Investment Holdings, LLC (“72 Investment Holdings”), a Delaware limited liability company, is the sole member of Differentiated Ventures and may be deemed to share beneficial ownership of the shares of which Differentiated Ventures may be deemed to share beneficial ownership. Steven A. Cohen (“Mr. Cohen”) is the sole member of 72 Investment Holdings and may be deemed to share beneficial ownership of the shares of which 72 Investment Holdings may be deemed to share beneficial ownership. Each of Differentiated Ventures, 72 Investment Holdings, and Mr. Cohen disclaims beneficial ownership of the shares held by Point72 Biotech. Pursuant to an investment management agreement, Point72 Asset Management, L.P. (“Point72 Asset Management”), a Delaware limited partnership, maintains investment and voting power with respect to the shares held by Point72 Associates and therefore may be deemed to share beneficial ownership of such shares. Point72 Capital Advisors, Inc. (“Point72 Capital Advisors”) a Delaware corporation, is the general partner of Point72 Asset Management and may be deemed to share beneficial ownership of the shares of which Point72 Asset Management may be deemed to share beneficial ownership. Mr. Cohen is the sole member of Point72 Capital Advisors and may be deemed to share beneficial ownership of the shares of which Point72 Capital Advisors may be deemed to share beneficial ownership. Each of Point72 Asset Management, Point72 Capital Advisors and Mr. Cohen disclaims beneficial ownership of the shares held by Point72 Associates. The address for these entities and individuals is c/o Point72, L.P., 72 Cummings Point Road, Stamford, CT 06902.
(3)Consists of (i) 2,386,301 shares of Common Stock received by RA Capital Healthcare Fund, L.P. (“RACHF”) in the Merger as an equityholder of LENZ OpCo, (ii) 629,784 shares of Common Stock received by RA Capital Nexus Fund II, L.P. (“Nexus II”) in the Merger as an equityholder of LENZ OpCo, (iii) 164,729 shares of Common Stock received by a separately managed account (the “Account,” and together with RACHF and Nexus II, the “RA Funds”) in the Merger as an equityholder of LENZ OpCo, (iv) 54,582 shares of Common Stock subject to warrants to purchase shares of Common Stock held by RACHF as an equityholder of LENZ OpCo, (v) 10,580 shares of Common Stock subject to warrants to purchase shares of Common Stock held by Nexus II as an equityholder of LENZ OpCo, (vi) 5,371 shares of Common Stock subject to warrants to purchase shares of Common Stock held by the Account as an equityholder of LENZ OpCo, (vii) 933,038 shares of Common Stock purchased by RACHF in the PIPE Financing, and (viii) 64,971 shares of Common Stock purchased by Nexus II in the PIPE Financing. RA Capital Management, L.P. is the investment manager for the RA Funds. The general partner of RA Capital Management, L.P. is RA Capital Management GP, LLC, of which Peter Kolchinsky, Ph.D. and Rajeev Shah are the managing members. Each of RA Capital Management, L.P., RA Capital Management GP, LLC, Mr. Kolchinsky and Mr. Shah may be deemed to have voting and investment power over the securities held by the RA Funds. RA Capital Management, L.P., RA Capital Management GP, LLC,
Mr. Kolchinsky and Mr. Shah disclaim beneficial ownership of such securities, except to the extent of any pecuniary interest therein. The principal business address of the persons and entities listed above is 200 Berkeley Street, 18th Floor, Boston, MA 02116.
(4)Consists of (i) 598,203 shares of Common Stock received by Versant Vantage II, L.P. (“Versant Vantage II”) in the Merger as an equityholder of LENZ OpCo, (ii) 1,598,789 shares of Common Stock received by Versant Venture Capital VII, L.P. (“Versant VII”) in the Merger as an equityholder of LENZ OpCo, (iii) 70,534 shares of Common Stock subject to warrants to purchase shares of Common Stock held by Versant VII as an equityholder of LENZ OpCo, (iv) 2,101,199 shares of Common Stock held by Versant Venture Capital VI, LP (“Versant Capital VI”), and (v) 243,959 shares of Common Stock held by Versant Vantage II. Versant Vantage II GP-GP is the general partner of Versant Vantage II GP, which is the general partner of Versant Vantage II. Each of Versant Vantage II GP and Versant Vantage II GP-GP share voting and dispositive power with respect to the shares held by Versant Vantage II. Versant Ventures VI GP-GP, LLC (“Versant Ventures VI GP-GP”) is the general partner of Versant Ventures VI GP, L.P. (“Versant Ventures VI GP”), which is the general partner of Versant VI. Each of Versant Ventures VI GP-GP and Versant Ventures VI GP share voting and dispositive power with respect to the shares held by Versant VI. Versant Ventures VII GP-GP is the general partner of Versant Ventures VII GP, which is the general partner of Versant VII. Each of Versant Ventures VII GP and Versant Ventures VII GP-GP share voting and dispositive power with respect to the securities held by Versant VII. The address for each of the entities mentioned in this footnote is One Sansome Street, Suite 1650, San Francisco, CA 94104.
(5)Consists of shares of Common Stock subject to options held by Mr. Schimmelpennink exercisable within 60 days of the Closing Date.
(6)Consists of shares of Common Stock subject to options held by Mr. Olsson exercisable within 60 days of the Closing Date.
(7)Consists of (i) 99,599 shares of Common Stock received by Dr. Odrich in the Merger as an equityholder of LENZ OpCo, and (ii) 66,472 shares of Common Stock subject to options held by Dr. Odrich exercisable within 60 days of the Closing Date.
(8)Consists of shares of Common Stock subject to options held by Dr. Guerard exercisable within 60 days of the Closing Date.
(9)Consists of (i) 95,034 shares of Common Stock received by James McCollum in the Merger as an equityholder of LENZ OpCo, (ii) 477,600 shares of Common Stock received by the McCollum Living Trust in the Merger as an equityholder of LENZ OpCo, (iii) 6,575 shares of Common Stock subject to warrants to purchase shares of Common Stock held by the McCollum Living Trust as an equityholder of LENZ OpCo, and (iv) 16,633 shares of common stock purchased by the McCollum Living Trust in the PIPE Financing. Mr. McCollum is a trustee of the McCollum Living Trust and as such has voting and investment control over the shares held by the McCollum Living Trust.
(10)Consists of the shares of Common Stock set forth in footnote 3 above. Dr. Scheiner is employed as a principal at RA Capital Management, L.P. Dr. Scheiner disclaims beneficial ownership of such shares, except to the extent of his pecuniary interest therein, if any.
(11)Consists of shares of Common Stock subject to options held by Ms. Drapkin exercisable within 60 days of the Closing Date.
(12)Consists of (i) 4,867,689 shares of Common Stock beneficially owned by our executive officers and directors, (ii) 1,045,006 shares of Common Stock subject to options held our executive officers and directors and exercisable within 60 days of the Closing Date, and (iii) 77,108 shares of Common Stock subject to warrants beneficially owned by our executive officers and directors.
Directors and Executive Officers
New LENZ’s directors and executive officers after the consummation of the Transactions are described in the Proxy Statement/Prospectus in the section titled “Management Following the Merger” beginning on page 380 of the Proxy Statement/Prospectus and that information is incorporated herein by reference.
In addition to the directors and executive officers described in the Proxy Statement/Prospectus, on March 21, 2024, the Board appointed Daniel Chevallard as the Company’s Chief Financial Officer. Mr. Chevallard will serve as the principal financial officer and principal accounting officer of the Company.
From July 2019 to March 2024, Mr. Chevallard served as Chief Financial Officer, Treasurer and Secretary of Viracta Therapeutics, Inc. (NASDAQ: VIRX), a biotechnology company, and served as its Chief Operating Officer from March 2021 to March 2024. Previously, Mr. Chevallard served as the Chief Financial Officer and principal financial officer at Regulus Therapeutics (NASDAQ: RGLS) from May 2017 to July 2019. Mr. Chevallard joined Regulus Therapeutics in December 2012 as Vice President, Accounting and Financial Reporting and served as Vice President, Finance from May 2013 to April 2017. Prior to joining Regulus Therapeutics, Mr. Chevallard held various senior roles in corporate finance, accounting and financial reporting including Controller and Senior Director, Finance of Prometheus Laboratories Inc. (acquired by Nestlé Health Science in July 2011). Prior to joining Prometheus, Mr. Chevallard spent approximately five years in public accounting at Ernst & Young, LLP in their assurance services practice. Mr. Chevallard received his Bachelor of Accountancy from the University of San Diego and is a Certified Public Accountant (inactive) in the state of California.
Independence of our Board of Directors
Under the Nasdaq listing standards, a majority of the members of the New LENZ Board must qualify as “independent,” as affirmatively determined by the New LENZ Board. Under the rules of Nasdaq, a director will only qualify as an “independent director” if, in the opinion of that company’s board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. The New LENZ Board has determined that each individual serving on the New LENZ
Board upon consummation of the Merger other than Evert Schimmelpennink qualifies as an independent director under Nasdaq listing standards.
Committees of the Board of Directors
Information with respect to the composition of the committees of the Board immediately after the Closing is set forth in the Proxy Statement/Prospectus in the subsection titled “Committees of the Combined Company’s Board of Directors” in the section titled “Management Following the Merger” beginning on page 383 of the Proxy Statement/Prospectus and that information is incorporated herein by reference.
Executive Compensation
A description of the compensation of the named executive officers of LENZ OpCo and the compensation of the executive officers of Graphite before the consummation of the Transactions is set forth in the Proxy Statement/Prospectus in the sections titled “LENZ Executive Compensation” beginning on page 246 of the Proxy Statement/Prospectus and “Graphite Executive Compensation” beginning on page 236 of the Proxy Statement/Prospectus, respectively, and that information is incorporated herein by reference.
At the Special Meeting, the Graphite stockholders approved the 2024 Plan and the 2024 ESPP (as defined below). The summary of the 2024 Plan set forth in the Proxy Statement/Prospectus in the section titled “Proposal No. 2 – The 2024 Plan Proposal” beginning on page 265 of the Proxy Statement/Prospectus and the summary of the 2024 ESPP set forth in the Proxy Statement/Prospectus in the section titled “Proposal No. 4 – The 2024 ESPP Proposal” beginning on page 275 of the Proxy Statement/Prospectus are each incorporated herein by reference. A copy of the full text of the 2024 Plan is attached hereto as Exhibit 10.9 and a copy of the full text of the 2024 ESPP is attached hereto as Exhibit 10.10, each of which is incorporated herein by reference.
Director Compensation
A description of the compensation of the directors of LENZ OpCo and of Graphite before the consummation of the Transactions is set forth in the Proxy Statement/Prospectus in the sections titled “LENZ Director Compensation” beginning on page 254 of the Proxy Statement/Prospectus and “Graphite Director Compensation,” beginning on page 242 of the Proxy Statement/Prospectus, respectively, and that information is incorporated herein by reference.
Certain Relationships and Related Person Transactions
Certain relationships and related person transactions are described in the Proxy Statement/Prospectus in the section titled “Certain Relationships and Related Party Transactions of the Combined Company,” beginning on page 401 of the Proxy Statement/Prospectus and that information is incorporated herein by reference.
Legal Proceedings
In connection with the Merger, one complaint has been filed in the United States District Court for the Northern District of California captioned Glen Chew v. Graphite Bio, Inc. et al., Case No. 3:24-cv-00613 (filed February 1, 2024) and one complaint has been filed in the United States District Court for the District of Delaware captioned Kevin Turner v. Graphite Bio, Inc. et al., Case No. 1:24-cv-00241-UNA (filed February 22, 2024) (collectively, the “Complaints”). The Complaints generally allege that the Proxy Statement/Prospectus filed by Graphite with the SEC misrepresents and/or omits certain purportedly material information relating to LENZ’s financial projections, the analyses performed by the financial advisor to Graphite’s Board of Directors in connection with the Merger, potential conflicts of interest of the financial advisor to Graphite’s Board of Directors, potential conflicts of interest of Graphite’s officers, and Graphite’s liquidation analysis. The Complaints assert violations of Section 14(a) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and Rule 14a-9 promulgated thereunder against all defendants (Graphite, its Board of Directors and certain officers) and violations of Section 20(a) of the Exchange Act against Graphite’s directors and officers. The Complaints seek orders enjoining the proposed Merger, or in the event that the proposed Merger is consummated, an order rescinding the Merger or awarding rescissory damages, as well as costs, including attorneys’ and experts’ fees.
Graphite has also received twelve additional demand letters by purported Graphite stockholders from December 14, 2023 to March 20, 2024 seeking additional disclosures in the Proxy Statement/Prospectus (the “Demands”).
We cannot predict the outcome of any litigation or the Demands. The Company and the individual defendants intend to vigorously defend against the Complaints, the Demands, and any subsequently filed similar actions. It is possible additional lawsuits may be filed or additional demand letters may be received arising out of the Merger.
Graphite and LENZ believe that the disclosures set forth in the Proxy Statement/Prospectus comply fully with all applicable laws, and deny the allegations in the Complaints and Demands described above. Nevertheless, in order to moot plaintiffs’ disclosure claims, avoid nuisance and possible expense and business delays, and provide additional information to its stockholders, Graphite voluntarily supplemented certain disclosures in the Proxy Statement/Prospectus on March 5, 2024 (the “Supplemental Disclosures”). Nothing in the Supplemental Disclosures shall be deemed an admission of the legal merit of the Complaints or the Demands described above, or of the necessity or materiality under applicable laws of any of the disclosures set forth herein. To the contrary, we specifically deny all allegations in the Complaints and the Demands that any additional disclosure was or is required or is material.
Market Price of and Dividends on Common Equity and Related Stockholder Matters
The Common Stock will begin trading on Nasdaq under the symbol “LENZ” on March 22, 2024. As of immediately after the Closing Date, there were approximately 103 registered holders of Common Stock.
LENZ OpCo has not paid any cash dividends on shares of its Common Stock. In connection with the consummation of the Merger, the Graphite board of directors declared a special cash dividend to its stockholders (the “Special Dividend”), which was paid on March 21, 2024. The Special Dividend was in the amount of $1.03 per share of Graphite’s common stock and was payable in cash to the stockholders of record of Graphite as of March 18, 2024. Other than the Special Dividend, Graphite had not declared or paid any dividends prior to the Merger. Any decision to declare and pay dividends in the future will be made at the sole discretion of the Board and will depend on, among other things, LENZ’s results of operations, cash requirements, financial condition, contractual restrictions and other factors that the Board may deem relevant.
Recent Sales of Unregistered Securities
Reference is made to the disclosure set forth below under Item 3.02 of this Current Report on Form 8-K concerning the issuance and sale of certain unregistered securities, which is incorporated herein by reference.
Description of Company’s Securities
The description of New LENZ’s securities is contained in the Proxy Statement/Prospectus in the section titled “Description of Graphite Capital Stock” beginning on page 408 of the Proxy Statement/Prospectus and that information is incorporated herein by reference.
Indemnification of Officers and Directors
New LENZ has entered into indemnification agreements with each of its directors and executive officers, in each case effective as of the Closing Date. Each indemnification agreement provides for indemnification and advancements by New LENZ of certain expenses and costs relating to claims, suits or proceedings arising from such person's service to New LENZ or, at our request, service to other entities, as officers or directors to the maximum extent permitted by applicable law.
The foregoing description of the indemnification agreements does not purport to be complete and is qualified in its entirety by the terms and conditions of the indemnification agreements, a form of which is filed as Exhibit 10.18 to this Current Report on Form 8-K and is incorporated herein by reference.
Change in the Registrant’s Certifying Accountant
The information set forth under Item 4.01 of this Current Report on Form 8-K is incorporated herein by reference.
Financial Statements and Exhibits
The information set forth under Item 9.01 of this Current Report on Form 8-K is incorporated herein by reference.
Item 3.02. Unregistered Sales of Equity Securities
Concurrently with the execution of the Merger Agreement, Graphite entered into a subscription agreement (the “Subscription Agreement”) with the PIPE Investors, pursuant to which, at the Closing, the PIPE Investors subscribed for and purchased an aggregate of 3,559,565 shares of Common Stock at a price of $15.0299 per share for aggregate gross proceeds of approximately $53.5 million. The shares of Common Stock issued pursuant to the Subscription Agreement (the “PIPE Financing Shares”) have not been registered under the Securities Act in reliance upon the exemption provided in Section 4(a)(2) of the Securities Act. In connection with the consummation of the Merger, Graphite entered into a registration rights agreement with the PIPE Investors, pursuant to which Graphite agreed that, within ten (10) calendar days after the Closing Date, we will file with the SEC (at our sole cost and expense) a registration statement (the “Resale Registration Statement”) registering the resale of the PIPE Financing Shares. The foregoing description of the Subscription Agreement does not purport to be complete and is qualified in its entirety by the terms and conditions thereof, the form of which is attached hereto as Exhibit 10.5 and is incorporated herein by reference.
Item 3.03. Material Modifications to Rights of Security Holders.
At the Special Meeting, the Graphite stockholders approved an amendment to the amended and restated certificate of incorporation of the Company to, among other things, effect a reverse stock split of the Common Stock at a ratio of 1:7 (the “Reverse Stock Split”). On March 21, 2024, in connection with the Merger and effective immediately prior to the Effective Time, the Company filed a certificate of amendment to the amended and restated certificate of incorporation to effect the Reverse Stock Split and to change the name of the Company from Graphite Bio, Inc. to LENZ Therapeutics, Inc. As of the opening of trading on Nasdaq on March 22, 2024, the Common Stock will begin to trade on a Reverse Stock Split-adjusted basis (as well as under the new ticker symbol “LENZ”).
As a result of the Reverse Stock Split, the number of issued and outstanding shares of Common Stock immediately prior to the Reverse Stock Split was reduced into a smaller number of shares, such that every seven shares of Common Stock held by a stockholder immediately prior to the Reverse Stock Split were combined and reclassified into one share of Common Stock after the Reverse Stock Split. Immediately following the Reverse Stock Split, the Merger and the PIPE Financing, there were approximately 25,533,533 million shares of Common Stock outstanding.
No fractional shares were issued in connection with the Reverse Stock Split. Stockholders of record who otherwise would be entitled to receive fractional shares because they held a number of pre-split shares not evenly divisible by the number of pre-split shares for which each post-split share is to be reclassified, is entitled, upon surrender to the exchange agent of certificates representing such shares, to a cash payment in lieu thereof in an amount equal to such fractional shares of Common Stock multiplied by the then fair value of the Common Stock as determined by the Board. For the foregoing purposes, all shares of Common Stock held by a holder are aggregated (thus resulting in no more than one fractional share per holder). The ownership of a fractional interest will not give the holder thereof any voting, dividend or other rights except to receive payment therefor as described herein.
In accordance with the amended and restated certificate of incorporation of the Company, as amended, no corresponding adjustment was made with respect to the Company’s authorized Common Stock or preferred stock. The Reverse Stock Split has no effect on the par value of the Common Stock or preferred stock of the Company. Immediately after the Reverse Stock Split, prior to giving effect to the Merger, each stockholder’s percentage ownership interest in the Company and proportional voting power remained unchanged, other than as a result of the rounding to eliminate fractional shares, as described in the preceding paragraph. The rights and privileges of the holders of shares of Common Stock will be unaffected by the Reverse Stock Split.
The foregoing description of the certificate of amendment to the amended and restated certificate of incorporation of the Company to effect the Reverse Stock Split is not complete and is subject to and qualified in its entirety by reference to the amended and restated certificate of incorporation, as amended, a copy of which is attached as Exhibit 3.1 hereto and is incorporated herein by reference.
In accordance with Rule 12g-3(a) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), New LENZ is the successor issuer to Graphite and has assumed the attributes of Graphite as the registrant. In addition, the shares of Common Stock of New LENZ, as the successor to Graphite, are deemed to be registered under Section 12(b) of the Exchange Act. Holders of uncertificated shares of Graphite’s common stock prior to the Closing have continued as holders of shares of uncertificated shares of New LENZ’s Common Stock. After consummation of the Transactions, the Common Stock was listed on the Nasdaq under the symbols “LENZ” and the CUSIP number relating to the Common Stock was changed to 52635N103. Holders of Graphite’s common stock who have filed reports under the Exchange Act with respect to those shares should indicate in their next filing, or any amendment to a prior filing, filed on or after the Closing Date that New LENZ is the successor to Graphite.
Item 4.01. Change in the Registrant’s Certifying Accountant.
(a) Dismissal of Independent Registered Accounting Firm
On March 21, 2024, the audit committee of the Board approved a resolution dismissing Deloitte & Touche LLP (“Deloitte”), Graphite’s independent registered public accounting firm prior to the Merger, as New LENZ’s independent registered public accounting firm.
The reports of Deloitte on Graphite’s financial statements as of and for the most fiscal years ending December 31, 2023 and 2022 did not contain an adverse opinion or a disclaimer of opinion, and were not qualified or modified as to uncertainties, audit scope or accounting principles.
During Graphite’s fiscal years ending December 31, 2023 and 2022 and the subsequent interim period through March 21, 2024, there were no disagreements between Graphite and Deloitte on any matter of accounting principles or practices, financial disclosure or auditing scope or procedure, which disagreements, if not resolved to the satisfaction of Deloitte, would have caused it to make reference to the subject matter of the disagreements in its reports on Graphite’s financial statements for such year.
During Graphite’s fiscal years ending December 31, 2023 and 2022 and the subsequent interim period through March 21, 2024, there were no “reportable events” (as defined in Item 304(a)(1)(v) of Regulation S-K under the Exchange Act).
New LENZ provided Deloitte with a copy of the foregoing disclosures and has requested that Deloitte furnish New LENZ with a letter addressed to the SEC stating whether it agrees with the statements made by New LENZ set forth above. A copy of Deloitte’s letter, dated March 21, 2024, is filed as Exhibit 16.1 to this Current Report on Form 8-K.
(b) Engagement of New Independent Registered Accounting Firm
On March 21, 2024, the audit committee of the Board approved a resolution appointing Ernst & Young LLP ("EY") as New LENZ's independent registered public accounting firm to audit New LENZ's consolidated financial statements for the fiscal year ending December 31, 2024. EY served as the independent registered public accounting firm of LENZ OpCo prior to the Merger. During the fiscal years ending December 31, 2023 and 2022 and the subsequent interim period through March 21, 2024, neither New LENZ, nor any party on behalf of New LENZ, consulted with EY with respect to either (i) the application of accounting principles to a specified transaction, either completed or proposed, or the type of the audit opinion that might be rendered with respect to New LENZ’s consolidated financial statements, and no written report or oral advice was provided to New LENZ by EY that was an important factor considered by New LENZ in reaching a decision as to any accounting, auditing or financial reporting issue, or (ii) any matter that was subject to any disagreement (as that term is defined in Item 304(a)(1)(iv) of Regulation S-K and the related instructions) or a reportable event (as that term is defined in Item 304(a)(1)(v) of Regulation S-K).
Item 5.01. Changes in Control of Registrant.
Reference is made to the disclosure in the Proxy Statement/Prospectus in the section titled “Proposal No. 1 – The Nasdaq Stock Issuance Proposal” beginning on page 255 of the Proxy Statement/Prospectus, which is incorporated
herein by reference. Further reference is made to the information contained in Item 2.01 to this Current Report on Form 8-K, which is incorporated herein by reference.
Item 5.02. Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers.
Board of Directors
Upon the consummation of the Transactions, and in accordance with the terms of the Merger Agreement, each director of Graphite, other than Kimberlee C. Drapkin, and each executive officer of Graphite ceased serving in such capacities and six new directors were appointed to the Board. In accordance with the amended and restated certificate of incorporation of the Company, the Board is divided into three staggered classes of directors and each director was assigned to one of the three classes. At each annual meeting of the stockholders, a class of directors will be elected for a three-year term to succeed the directors of the same class whose terms are then expiring. The terms of the directors will expire upon the election and qualification of successor directors at the annual meeting of stockholders to be held during the year 2024 for Class III directors (or 2025 if no annual meeting of stockholders is held in 2024), 2025 for Class I directors, and 2026 for Class II directors. Effective as of the Effective Time, Frederic Guerard and James McCollum were appointed as Class I directors, Jeff George, Shelley Thunen, and Evert Schimmelpennink were appointed as Class II directors and Kimberlee C. Drapkin and Zach Scheiner were appointed as Class III directors.
Furthermore, also on March 21, 2024, the Board confirmed that there would continue to be three standing committees of the Board: an audit committee, a compensation committee and a nominating and corporate governance committee. The Board appointed the following members to the audit committee: Ms. Thunen, Dr. Guerard, and Dr. Scheiner, with Ms. Thunen appointed as the chairperson of the audit committee. The Board appointed the following members to the compensation committee: Dr. Guerard, Ms. Drapkin and Ms. Thunen, with Dr. Guerard appointed as the chairperson of the compensation committee. The Board appointed the following members to the nominating and corporate governance committee: Mr. George, Ms. Drapkin and Dr. Scheiner, with Mr. George appointed as the chairperson of the nominating and corporate governance committee.
Also on March 21, 2024, Mr. George was appointed as the Chair of the Board.
A description of the compensation of the directors of LENZ OpCo and of Graphite before the consummation of the Transactions is set forth in the Proxy Statement/Prospectus in the sections titled “LENZ Director Compensation” beginning on page 254 of the Proxy Statement/Prospectus and “Graphite Director Compensation,” beginning on page 242 of the Proxy Statement/Prospectus, respectively, and that information is incorporated herein by reference.
Following the Transactions, pursuant to New LENZ’s outside director compensation policy (the “Outside Director Compensation Policy”), each non-employee director will receive an annual retainer of $40,000, an annual retainer of $30,000 for serving as chair of the Board, a $15,000 annual retainer for serving as the chair of the audit committee, a $7,500 annual retainer for serving as a member of the audit committee, a $12,000 annual retainer for serving as the chair of the compensation committee, a $6,000 annual retainer for serving as a member of the compensation committee, a $10,000 annual retainer for serving as the chair of the nominating and corporate governance committee, and a $5,000 annual retainer for serving on the nominating and corporate governance committee, in each case to be paid quarterly in arrears and prorated based on the number of actual days served on the Board or applicable committee. Each non-employee director who serves as a committee chair of the Board will receive the cash retainer fee as the chair of the committee but not the cash retainer fee as a member of that committee, provided that the non-employee director who serves as the non-employee chair of the Board will receive the annual retainer fees for such role as well as the annual retainer fee for service as a non-employee director. The above-listed fees for service as non-employee chair of the Board or a chair or member of any committee are payable in addition to the non-employee director retainer.
In addition, the Outside Director Compensation Policy provides that, each individual who is a non-employee director as of the effective date of the policy will be granted an award of stock options to purchase 27,000 shares of New LENZ common stock, with such number of shares subject to equitable adjustment by the Board in the event of a capitalization adjustment following the Reverse Stock Split and the closing of the Merger (the “Merger Award”).
The Merger Award will be granted automatically on the first trading day on or after the effective date of the policy. Each Merger Award will be scheduled to vest in equal monthly installments over the next thirty-six (36) months on the same day of each relevant month as the applicable vesting date, in each case subject to the non-employee director continuing to be a non-employee director through the applicable vesting date.
The Outside Director Compensation Policy also provides that, each individual who first becomes a non-employee director following the effective date of the policy and who does not receive a Merger Award will be granted an award of stock options to purchase 27,000 shares of New LENZ common stock, with such number of shares subject to equitable adjustment by the Board in the event of a capitalization adjustment following the Reverse Stock Split and the closing of the Merger (the “Initial Award”). The Initial Award will be granted automatically on the first trading day on or after the date on which such individual first becomes a non-employee director (the first date as a non-employee director, the “Initial Start Date”), whether through election by LENZ stockholders or appointment by the Board to fill a vacancy. Each Initial Award will be scheduled to vest in equal monthly installments over the next thirty-six (36) months following the Initial Start Date on the same day of each relevant month as the applicable Initial Start Date, in each case subject to the non-employee director continuing to be a non-employee director through the applicable vesting date.
On the first trading day immediately following each annual meeting of New LENZ stockholders following the Merger (each, an “Annual Meeting”), each non-employee director automatically will be granted an award of stock options (an “Annual Award”) to purchase 13,500 shares of New LENZ common stock, with such number of shares subject to equitable adjustment by the Board in the event of a capitalization adjustment following the Reverse Stock Split and the closing of the Merger; provided that the first Annual Award granted to an individual who first becomes a non-employee director following the effective date of the Outside Director Compensation Policy will cover a number of shares equal to the product of (A) 13,500 multiplied by (B) a fraction, (i) the numerator of which is the number of fully completed months between the applicable Initial Start Date and the date of the first Annual Meeting to occur after such individual first becomes a non-employee director, and (ii) the denominator of which is twelve (12), subject to equitable adjustment by the Board in the event of a capitalization adjustment. Each Annual Award will be scheduled to vest in full on the first anniversary of the date on which the Annual Award is granted, in each case subject to the non-employee director continuing to be a non-employee director through the applicable vesting date.
The foregoing description of the Outside Director Compensation Policy is not complete and is subject to and qualified in its entirety by reference to the Outside Director Compensation Policy, a copy of which is attached as Exhibit 10.16 hereto and is incorporated herein by reference.
Executive Officers
On March 21, 2024, the following individuals were appointed to serve as executive officers of New LENZ:
| | | | | | | | |
| Name | | Position |
| Evert Schimmelpennink | | Chief Executive Officer, President, and Secretary |
| Shawn Olsson | | Chief Commercial Officer |
| Marc Odrich, M.D. | | Chief Medical Officer |
| Daniel Chevallard | | Chief Financial Officer |
Reference is made to the disclosure described in the Proxy Statement/Prospectus in the section titled “Management Following the Merger,” beginning on page 380 of the Proxy Statement/Prospectus, which is incorporated herein by reference.
In addition to the disclosure described in the Proxy Statement/Prospectus, on March 21, 2024, the Board appointed Daniel Chevallard as the Company’s Chief Financial Officer. Mr. Chevallard will serve as the principal financial officer and principal accounting officer of the Company.
There are no family relationships among any of the Company’s directors and executive officers.
LENZ Therapeutics, Inc. 2024 Equity Incentive Plan
At the Special Meeting held on March 14, 2024, Graphite stockholders considered and approved the LENZ Therapeutics, Inc. 2024 Equity Incentive Plan (the “2024 Plan”). The 2024 Plan allows New LENZ to make equity and equity-based incentive awards to officers, employees, non-employee directors and consultants. The Board anticipates that providing such persons with a direct stake in New LENZ will assure a closer alignment of the interests of such individuals with those of New LENZ and its stockholders, thereby stimulating their efforts on New LENZ’s behalf and strengthening their desire to remain with New LENZ.
Subject to the adjustment provisions contained in the 2024 Plan and the evergreen provision described below, a total of 3,011,948 shares of New LENZ Common Stock are initially reserved for issuance pursuant to the 2024 Plan. In addition, the shares reserved for issuance under the 2024 Plan include any shares subject to awards granted under LENZ OpCo’s 2020 Equity Incentive Plan (including, but not limited to, awards granted under LENZ OpCo’s 2020 Equity Incentive Plan that were assumed in the Merger), Graphite’s 2021 Stock Option and Incentive Plan and Graphite’s 2020 Stock Option and Grant Plan, each as amended from time to time, that, on or after the effective date of the Merger, expire or terminate without having been exercised in full, are tendered to or withheld for payment of an exercise price or for tax withholding obligations, are forfeited or repurchased due to failure to vest, with a maximum number of shares that may be added to the 2024 Plan equal to 1,607,930 shares. The number of shares available for issuance under the 2024 Plan also will include an annual increase, or the evergreen feature, on the first day of each of New LENZ’s fiscal years, beginning with New LENZ’s fiscal year 2025, equal to the least of:
•4,517,922 shares of New LENZ Common Stock;
•a number of shares equal to 5% of the number of shares of all classes of New LENZ Common Stock outstanding as of the last day of the immediately preceding fiscal year; or
•such number of shares as the Board or its designated committee may determine no later than the last day of New LENZ’s immediately preceding fiscal year.
If an award of options or stock appreciation rights expire or become unexercisable without having been exercised in full, is surrendered pursuant to an exchange program, or, with respect to restricted stock, restricted stock units, or stock-settled performance awards, are reacquired by New LENZ due to failure to vest or forfeited to New LENZ, the unpurchased or unissued shares will become available for future issuance under the 2024 Plan (unless the 2024 Plan has terminated). With respect to stock appreciation rights, only the net shares actually issued will cease to be available under the 2024 Plan and all remaining shares of New LENZ Common Stock subject to the stock appreciation right will remain available for future issuance under the 2024 Plan (unless the 2024 Plan has terminated). Shares that have actually been issued under the 2024 Plan will not be returned to the 2024 Plan. Shares used to pay the exercise price of an award or satisfy the tax withholding obligations related to an award will become available for future issuance under the 2024 Plan. To the extent an award is paid out in cash rather than shares, such cash payment will not result in a reduction in the number of shares available for issuance under the 2024 Plan.
If any extraordinary dividend or other extraordinary distribution (whether in cash, shares, other securities, or other property), recapitalization, stock split, reverse stock split, reorganization, merger, consolidation, split-up, spin-off, combination, reclassification, repurchase, or exchange of shares or other securities of New LENZ, other change in the corporate structure of New LENZ affecting the shares of New LENZ Common Stock, or any similar equity restructuring transaction, as that term is used in Statement of FASB ASC 718 (or any of its successors) affecting the shares of Common Stock occurs, to prevent diminution or enlargement of the benefits or potential benefits available under the 2024 Plan, the administrator will adjust the number and class of shares that may be delivered under the 2024 Plan and/or the number, class and price of shares covered by each outstanding award and the numerical share limits set forth in the 2024 Plan.
A more complete summary of the terms of the 2024 Plan is set forth in the Proxy Statement/Prospectus in the section titled “Proposal No. 3 – The 2024 Plan Proposal” beginning on page 265 of the Proxy Statement/Prospectus. That summary and the foregoing description of the 2024 Plan does not purport to be complete and is qualified in its entirety by reference to the text of the 2024 Plan, which is attached as Exhibit 10.9 hereto and incorporated herein by reference.
LENZ Therapeutics, Inc. 2024 Employee Stock Purchase Plan
At the Special Meeting of Graphite stockholders held on March 14, 2024, Graphite stockholders considered and approved the LENZ Therapeutics, Inc. 2024 Employee Stock Purchase Plan (the “2024 ESPP”). An aggregate of 250,995 shares of Common Stock will be reserved and available for issuance under the 2024 ESPP. The number of shares of New LENZ Common Stock available for issuance under the 2024 ESPP will be increased on the first day of each fiscal year beginning with the fiscal year following the fiscal year in which the first enrollment date under the 2024 ESPP (if any) occurs, in an amount equal to the least of (a) 376,493 shares of New LENZ Common Stock, (b) a number of shares of New LENZ Common Stock equal to 1% of the outstanding shares of common stock of New LENZ on the last day of the immediately preceding fiscal year, or (c) an amount determined by the administrator. Shares issuable under the 2024 ESPP will be authorized, but unissued, or reacquired shares of New LENZ Common Stock. If our capital structure changes because of a stock dividend, stock split or similar event, the number of shares that can be issued under the 2024 ESPP will be appropriately adjusted.
A more complete summary of the terms of the 2024 ESPP is set forth in the Proxy Statement/Prospectus in the section titled “Proposal No. 4 – The 2024 ESPP Proposal” beginning on page 275 of the Proxy Statement/Prospectus. That summary and the foregoing description of the 2024 ESPP does not purport to be complete and is qualified in its entirety by reference to the text of the 2024 ESPP, which is attached as Exhibit 10.10 hereto and incorporated herein by reference.
Employment Arrangements with Executive Officers
The Company is party to confirmatory employment letters with each of Evert Schimmelpennink, its Chief Executive Officer, Marc Odrich, its Chief Medical Officer and Shawn Olsson, its Chief Commercial Officer, and an employment agreement with Daniel Chevallard, its Chief Financial Officer. The material terms of these agreements with Mr. Schimmelpennink, Dr. Odrich, Mr. Olsson and Mr. Chevallard are described below.
Evert Schimmelpennink
In connection with the closing of the Transactions, LENZ entered into a confirmatory employment letter with Mr. Schimmelpennink, its Chief Executive Officer. The confirmatory employment letter has no specific term and provides that Mr. Schimmelpennink is an at-will employee. The confirmatory employment letter supersedes all pre-existing agreements and understandings that Mr. Schimmelpennink may have entered into concerning his employment relationship with LENZ. Prior to the Closing, Mr. Schimmelpennink’s annual base salary was $538,500 and he was eligible for an annual target cash bonus opportunity equal to 50% of his base salary. As of the Closing Date, pursuant to the confirmatory employment letter, Mr. Schimmelpennink’s annual base salary was increased to $630,000 and he is eligible for a target annual cash bonus opportunity equal to 55% of his annual base salary.
Marc Odrich
In connection with the closing of the Transactions, LENZ entered into a confirmatory employment letter with Dr. Odrich, its Chief Medical Officer. The confirmatory employment letter has no specific term and provides that Dr. Odrich is an at-will employee. The confirmatory employment letter supersedes all pre-existing agreements and understandings that Dr. Odrich may have entered into concerning his employment relationship with LENZ. Prior to the Closing, Dr. Odrich’s annual base salary was $410,000 and he was eligible for an annual target cash bonus opportunity equal to 30% of his base salary. As of the Closing Date, pursuant to the confirmatory employment letter, Dr. Odrich’s annual base salary was increased to $485,000 and he is eligible for a target annual cash bonus opportunity equal to 40% of his annual base salary.
Shawn Olsson
In connection with the closing of the Transactions, LENZ entered into a confirmatory employment letter with Mr. Olsson, its Chief Commercial Officer. The confirmatory employment letter has no specific term and provides that Mr. Olsson is an at-will employee. The confirmatory employment letter supersedes all pre-existing agreements and understandings that Mr. Olsson may have entered into concerning his employment relationship with LENZ. Prior to the Closing, Mr. Olsson’s annual base salary was $361,725 and he was eligible for an annual target cash bonus
opportunity equal to 35% of his base salary. As of the Closing Date, pursuant to the confirmatory employment letter, Mr. Olsson’s annual base salary was increased to $433,000 and he is eligible for a target annual cash bonus opportunity equal to 40% of his annual base salary.
Daniel Chevallard
Effective as of March 21, 2024, LENZ entered into an employment agreement with Mr. Chevallard, who was appointed Chief Financial Officer of LENZ effective as of March 21, 2024. The employment agreement has no specific term and provides that Mr. Chevallard is an at-will employee. The employment agreement provides that as of March 21, 2024, Mr. Chevallard’s annual base salary is $485,000 and he is eligible for a target annual cash bonus opportunity equal to 40% of his annual base salary. In addition, pursuant to the employment agreement with Mr. Chevallard, on March 21, 2024 the Board granted Mr. Chevallard an option to purchase 200,000 shares of Common Stock.
The foregoing descriptions of the confirmatory employment letters with each of Mr. Schimmelpennink, Dr. Odrich and Mr. Olsson, and the employment agreement with Mr. Chevallard, do not purport to be complete and are qualified in their entirety by the terms and conditions of the employment letters, which are filed herewith as Exhibits 10.11, 10.12, 10.13, and 10.14 respectively, and incorporated herein by reference.
Executive Incentive Compensation Plan
Our Board approved an Employee Incentive Compensation Plan (the “Incentive Compensation Plan”) to provide periodic incentive bonus opportunities to our employees, effective upon Closing.
The Incentive Compensation Plan allows the plan’s administrator to grant incentive awards, generally payable in cash, to employees selected by the compensation committee, including the executive officers of the Company, based upon performance goals established by the compensation committee.
Under the Incentive Compensation Plan, the plan’s administrator will determine the performance goals, if any, applicable to any award, which goals may include, without limitation, goals related to: attainment of research and development milestones; sales bookings; business divestitures and acquisitions; capital raising; cash flow; cash position; contract awards or backlog; corporate transactions; customer renewals; customer retention rates from an acquired company, subsidiary, business unit or division; earnings (which may include any calculation of earnings, including but not limited to earnings before interest and taxes, earnings before taxes, earnings before interest, taxes, depreciation and amortization and net taxes); earnings per share; expenses; financial milestones; gross margin; growth in stockholder value relative to the moving average of the S&P 500 Index or another index; internal rate of return; leadership development or succession planning; license or research collaboration arrangements; market share; net income; net profit; net sales; new product or business development; new product invention or innovation; number of customers; operating cash flow; operating expenses; operating income; operating margin; overhead or other expense reduction; patents; procurement; product defect measures; product release timelines; productivity; profit; regulatory milestones or regulatory-related goals; retained earnings; return on assets; return on capital; return on equity; return on investment; return on sales; revenue; revenue growth; sales results; sales growth; savings; stock price; time to market; total stockholder return; working capital; unadjusted or adjusted actual contract value; unadjusted or adjusted total contract value; and individual objectives such as peer reviews or other subjective or objective criteria. As determined by the administrator, the performance goals may be based on U.S. generally accepted accounting principles (“GAAP”) or non GAAP results and any actual results may be adjusted by the administrator for one-time items or unbudgeted or unexpected items and/or payments of actual awards under the Incentive Compensation Plan when determining whether the performance goals have been met. The performance goals may be based on any factors the administrator determines relevant, including without limitation on an individual, divisional, portfolio, project, business unit, segment or company-wide basis. Any criteria used may be measured on such basis as the administrator determines, including without limitation: (a) in absolute terms, (b) in combination with another performance goal or goals (for example, but not by way of limitation, as a ratio or matrix), (c) in relative terms (including, but not limited to, results for other periods, passage of time and/or against another company or companies or an index or indices), (d) on a per-share basis, (e) against the performance of the Company as a whole or a segment of the Company and/or (f) on a pre-tax or after-tax basis. The performance goals may differ
from participant to participant and from award to award. The administrator also may determine that a target award (or portion thereof) will not have a performance goal associated with it but instead will be granted (if at all) as determined by the administrator.
The compensation committee of the board of directors will administer the Incentive Compensation Plan and may, in its sole discretion and at any time prior to payment of an actual award, increase, reduce or eliminate a participant’s actual award, and/or increase, reduce or eliminate the amount allocated to the bonus pool for a particular performance period. The actual award may be below, at or above a participant’s target award, as determined by the administrator. The administrator may determine the amount of any increase, reduction or elimination on the basis of such factors as it deems relevant, and it will not be required to establish any allocation or weighting with respect to the factors it considers.
Actual awards generally will be paid in cash (or its equivalent) in a single lump sum only after they are earned, and, unless otherwise determined by the administrator, to earn an actual award a participant must be employed by us through the date the actual award is paid. The administrator of the Incentive Compensation Plan may reserve the right to settle an actual award with a grant of an equity award, which equity award may have such terms and conditions, including vesting, as the administrator determines. Payment of awards will occur as soon as practicable after the end of the performance period to which the award relates and after the actual award is approved by the administrator, but no later than the dates set forth in the Incentive Compensation Plan.
Awards under the Incentive Compensation Plan will be subject to the clawback policy of the Company. The administrator also may impose such other clawback, recovery or recoupment provisions with respect an award under the Incentive Compensation Plan as the administrator determines necessary or appropriate, including, without limitation, a reacquisition right in respect of previously acquired cash, stock or other property provided with respect to an award. Certain participants may be required to reimburse us for certain amounts paid under an award under the Incentive Compensation Plan in connection with certain accounting restatements we may be required to prepare due to our material noncompliance with any financial reporting requirements under applicable securities laws, as a result of misconduct.
The administrator will have the authority to amend, suspend or terminate the Incentive Compensation Plan, provided such action does not alter or impair the existing rights of any participant with respect to any earned awards.
The foregoing description of the Incentive Compensation Plan does not purport to be complete and is qualified in its entirety by the text of the Incentive Compensation Plan, which is filed herewith as Exhibit 10.17 and incorporated herein by reference.
Potential Payments Upon Termination or Change of Control
Regardless of the manner in which an officer’s service terminates, that officer is entitled to receive amounts earned during his or her term of service, including unpaid salary and unused vacation, as applicable.
Each of Mr. Schimmelpennikn, Mr. Odrich and Mr. Olsson holds stock options granted subject to the general terms of LENZ OpCo’s 2020 Equity Incentive Plan (the “2020 Plan”). A description of the termination and change in control provisions in the 2020 Plan and applicable to the stock options granted to such officers is set forth in the Proxy Statement/Prospectus in the section titled “Employee Benefit and Stock Plans – 2020 Equity Incentive Plan” beginning on page 249 of the Proxy Statement/Prospectus. That summary does not purport to be complete and is qualified in its entirety by reference to the text of the 2020 Plan, which is attached as Exhibit 10.4 hereto and incorporated herein by reference.
In connection with the Closing, New LENZ adopted an executive change in control and severance policy (the “Severance Policy”) for eligible employees of New LENZ, including the executive officers and other key employees, effective as of the Closing. The Severance Policy is designed to be an “employee welfare benefit plan” (as defined in Section 3(1) of the Employee Retirement Income Security Act of 1974, as amended). The compensation committee of the board of directors of New LENZ will administer the Severance Policy, and designate individuals as eligible to participate in the Severance Policy, whether individually or by position or
category of position. Each participant in the Severance Policy must execute a participation agreement (each an “Eligible Employee”).
Pursuant to the Severance Policy, upon a termination of an Eligible Employee’s employment (x) by New LENZ without Cause (as such term is defined in the Severance Policy) (excluding by reason of the Eligible Employee’s death or disability) or (y) by the Eligible Employee for Good Reason (as such term is defined in the Severance Policy) (such termination, a “Qualified Termination”), in either case, outside of the period beginning 3 months prior to a Change in Control (as such term is defined in the Severance Policy) and ending 12 months following a Change in Control (the “Change in Control Period”), (the “Non-CIC Qualified Termination”), Eligible Employees will be eligible to receive (i) a lump sum payment equal to (A) 12 months of annualized base salary with respect to New LENZ’s Chief Executive Officer, (B) 9 months of annualized base salary with respect to New LENZ’s Senior Vice Presidents and Executive Officers other than New LENZ’s Chief Executive Officer, and (C) 3 months plus 2 weeks per year of continuous service of annualized base salary with respect to New LENZ’s Vice Presidents, and (ii) subject to a valid election under the Consolidated Omnibus Budget Reconciliation Act of 1985, as amended (“COBRA”), the cost of such continuation coverage for the Eligible Employee and any of the Eligible Employee’s eligible dependents that were covered under New LENZ’s health care plans immediately prior to the date of his or her Non-CIC Qualified Termination until the earliest of the date which the Eligible Employee or their eligible dependents become covered under similar plans, the date which the Eligible Employee ceases to be eligible for coverage under COBRA, or (A) 12 months following the Non-CIC Qualified Termination with respect to New LENZ’s Chief Executive Officer, (B) 9 months following the Non-CIC Qualified Termination with respect to New LENZ’s Senior Vice Presidents and Executive Officers other than New LENZ’s Chief Executive Officer, and (C) 3 months plus 2 weeks per year of continuous service following the Non-CIC Qualified Termination with respect to New LENZ’s Vice Presidents. Any unvested portion of the Eligible Employee’s then-outstanding equity awards will remain outstanding until the earlier of (A) 3 months following the Non-CIC Qualified Termination or (B) the occurrence of a Change in Control, provided that, if no Change in Control occurs within the 3 months following a Non-CIC Qualified Termination, any unvested portion of the Eligible Employee’s equity awards automatically and permanently will be forfeited on the 3-month anniversary following such termination date without having vested.
If a Qualified Termination occurs during a Change in Control Period (the “CIC Qualified Termination”), the Eligible Employee will be entitled to receive (i) a lump sum payment equal to (A) 18 months of annualized base salary with respect to New LENZ’s Chief Executive Officer, (B) 12 months of annualized base salary with respect to New LENZ’s Senior Vice Presidents and Executive Officers other than New LENZ’s Chief Executive Officer, and (C) 6 months of annualized base salary with respect to New LENZ’s Vice Presidents, (ii) subject to a valid election under COBRA, the cost of such continuation coverage for the Eligible Employee and any of the Eligible Employee’s eligible dependents that were covered under New LENZ’s health care plans immediately prior to the date of his or her CIC Qualified Termination until the earliest of the date which the Eligible Employee or their eligible dependents become covered under similar plans, the date which the Eligible Employee ceases to be eligible for coverage under COBRA, or (A) 18 months following the CIC Qualified Termination with respect to New LENZ’s Chief Executive Officer, (B) 12 months following the CIC Qualified Termination with respect to New LENZ’s Senior Vice Presidents and Executive Officers other than New LENZ’s Chief Executive Officer, and (C) 6 months following the CIC Qualified Termination with respect to New LENZ’s Vice Presidents, (iii) a lump-sum payment equal to a percentage of the Eligible Employee’s target bonus in effect for the fiscal year which the CIC Qualified Termination occurs in, which such percentage is (A) 150% with respect to New LENZ’s Chief Executive Officer, (B) 100% with respect to New LENZ’s Senior Vice Presidents and Executive Officers other than New LENZ’s Chief Executive Officer, and (C) 50% with respect to New LENZ’s Vice Presidents, and (iv) acceleration of vesting as to 100% of the then-unvested shares or rights subject to all of the Eligible Employee’s equity awards. In the case of an equity award subject to performance-based vesting conditions, unless otherwise specified in the applicable equity award agreement governing the award, all performance goals and other vesting criteria will be deemed achieved at target.
The Severance Policy further provides that if any payment or benefit that an Eligible Employee would receive from New LENZ or any other party (the “Payment”) would (i) constitute a “parachute payment” within the meaning of Section 280G of the Code, and (ii) be subject to the excise tax imposed by Section 4999 of the Code (the “Excise Tax”), then such Payment will be equal to the Best Results Amount. The “Best Results Amount” will be either (x) the full amount of such Payment or (y) such lesser amount as would result in no portion of the Payment being
subject to the Excise Tax, whichever of the foregoing amounts, taking into account the applicable federal, state and local employment taxes, income taxes and the Excise Tax, results in the Eligible Employee’s receipt, on an after-tax basis, of the greater amount notwithstanding that all or some portion of the Payment may be subject to the Excise Tax.
The receipt of payments and benefits under the Severance Policy is subject to the Eligible Employee signing and not revoking a separation agreement and release of claims no later than the sixtieth (60th) day following the Eligible Employee’s termination.
For purposes of the Severance Policy, for the avoidance of doubt, Mr. Schimmelpennink will participate at the level of benefits provided to New LENZ’s Chief Executive officer, and Mr. Olsson, Dr. Odrich and Mr. Chevallard will participate at the level of benefits provided to New LENZ’s Senior Vice Presidents and Executive Officers other than New LENZ’s Chief Executive Officer.
The foregoing description of the Severance Policy does not purport to be complete and is qualified in its entirety by reference to the terms and conditions of the Severance Policy, which is filed herewith as Exhibit 10.15 and incorporated herein by reference.
Indemnification Agreements
Effective as of the Closing Date, New LENZ entered into indemnification agreements with each of its directors and executive officers. Each indemnification agreement provides for indemnification and advancements by New LENZ of certain expenses and costs relating to claims, suits or proceedings arising from his or her service to New LENZ or, at our request, service to other entities, as officers or directors to the maximum extent permitted by applicable law.
The foregoing description of the indemnification agreements does not purport to be complete and is qualified in its entirety by the terms and conditions thereof, a form of which is filed herewith as Exhibit 10.18 and is incorporated herein by reference.
A description of the compensation of the named executive officers of LENZ OpCo and the compensation of the executive officers of Graphite before the consummation of the Transactions is set forth in the Proxy Statement/Prospectus in the sections titled “LENZ Executive Compensation” beginning on page 246 of the Proxy Statement/Prospectus and the section titled “Graphite Executive Compensation,” beginning on page 236 of the Proxy Statement/Prospectus, respectively, and that information is incorporated herein by reference. Our executive officers are also eligible to receive bonuses under the Employee Incentive Compensation Plan, which is filed herewith as Exhibit 10.17 and is incorporated herein by reference.
Certain Relationships and Related Person Transactions
Certain relationships and related person transactions are described in the Proxy Statement/Prospectus in the section titled “Certain Relationships and Related Party Transactions of the Combined Company,” beginning on page 401 of the Proxy Statement/Prospectus and that information is incorporated herein by reference.
Item 5.03. Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year
In connection with the Merger, the Board adopted, and the stockholders approved, an amendment to the Company’s amended and restated certificate of incorporation, to effect the Reverse Stock Split and to change the Company’s name from “Graphite Bio, Inc.” to “LENZ Therapeutics, Inc.” The certificate of amendment to the amended and restated certificate of incorporation was effective March 21, 2024.
The foregoing description of the amended and restated certificate of incorporation is not complete and is subject to and qualified in its entirety by reference to the amended and restated certificate of incorporation, a copy of which is attached hereto as Exhibit 3.1 and incorporated herein by reference.
Item 5.05. Amendments to the Registrant’s Code of Ethics, or Waiver of a Provision of the Code of Ethics.
In connection with the Transactions, on March 21, 2024, the Board approved and adopted a new Code of Business Conduct and Ethics applicable to all employees, officers and directors of the Company. A copy of the Code of Business Conduct and Ethics can be found on the Company’s website at https://www.lenz-tx.com/. New LENZ intends to disclose future amendments to such code, or any waivers of its requirements, applicable to any principal executive officer, principal financial officer, principal accounting officer or controller or persons performing similar functions or its directors on its website identified above or in a current report on Form 8-K. Information contained on the website is not incorporated by reference herein and should not be considered to be part of this Current Report on Form 8-K. The inclusion of New LENZ’s website address in this Current Report on Form 8-K is an inactive textual reference only.
Item 5.06. Change in Shell Company Status.
As a result of the Transactions, New LENZ ceased to be a shell company upon the Closing. The material terms of the Transactions are described in the section entitled “Proposal No. 1 – The Nasdaq Stock Issuance Proposal” beginning on page 255 of the Proxy Statement/Prospectus and are incorporated herein by reference.
Item 7.01. Regulation FD Disclosure.
On March 21, 2024, LENZ issued a press release announcing the consummation of its previously announced Merger. A copy of such press release is furnished as Exhibit 99.3 hereto.
The information set forth under this Item 7.01, including Exhibit 99.3, shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or incorporated by reference in any filing under the Securities Act of 1933, as amended, unless expressly incorporated by specific reference in such filing.
Item 9.01. Financial Statements and Exhibits.
(a) Financial statements of businesses acquired.
The audited financial statements of Graphite as of and for the fiscal years ended December 31, 2023 and 2022 and the related notes are included in Graphite’s annual report on Form 10-K for the fiscal year ended December 31, 2023 (the “Form 10-K”) that was filed with the SEC on February 27, 2024 and are incorporated herein by reference.
The audited financial statements of LENZ OpCo as of and for the fiscal years ended December 31, 2023 and 2022 are set forth in Exhibit 99.1 hereto and are incorporated herein by reference.
(b) Pro forma financial information.
The unaudited pro forma condensed combined financial information of the Company for the year ended December 31, 2023 is set forth in Exhibit 99.2 hereto and is incorporated herein by reference.
(d)Exhibits
| | | | | | | | |
Exhibit Number | | Description |
| | |
| 2.1† | | |
| 3.1 | | |
| 3.2 | | |
| 4.1 | | |
| 4.2 | | |
| | | | | | | | |
| 10.1+ | | |
| 10.2+ | | |
| 10.3+ | | |
| 10.4+ | | |
| 10.5 | | |
| 10.6 | | |
| 10.7 | | |
| 10.8 | | |
| 10.9+ | | |
| 10.10+ | | |
| 10.11+ | | |
| 10.12+ | | |
| 10.13+ | | |
| 10.14+ | | |
| 10.15+ | | |
| 10.16+ | | |
| 10.17+ | | |
| 10.18 | | |
10.19# | | |
| 10.20 | | |
10.21 | | |
| 16.1 | | |
| 23.1 | | |
| 99.1 | | |
| 99.2 | | |
| 99.3 | | |
| 104 | | Cover Page Interactive Data File (the cover page XBRL tags are embedded within the inline XBRL document). |
____________
+ Indicates management contract or compensatory plan.
† Schedules and exhibits to this Exhibit omitted pursuant to Regulation S-K Item 601(b)(2). The Registrant agrees to furnish supplementally a copy of any omitted schedule or exhibit to the SEC upon request.
# Portions of the exhibit were omitted pursuant to Regulation S-K Item 601(b)(10). The Registrant agrees to furnish to the SEC a copy of any omitted portions of the exhibit upon request.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Dated: March 21, 2024
| | | | | |
| LENZ THERAPEUTICS, INC. |
| |
| By: | /s/ Evert Schimmelpennink |
| Name: | Evert Schimmelpennink |
| Title: | Chief Executive Officer |

