UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
OR
For the fiscal year ended
OR
OR
Date of event requiring this shell company report
For the transition period from to
Commission file number:
(Exact name of Registrant as specified in its charter)
N/A
(Translation of Registrant’s name into English)
(Jurisdiction of incorporation or organization)
+86-0796-8403309
(Address of principal executive offices)
Telephone:
People’s Republic of
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act.
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| The |
Securities registered or to be registered pursuant to Section 12(g) of the Act.
None
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act.
None
(Title of Class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
An aggregate of
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes ☐
Note – Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |
| ☒ | Emerging growth company |
If an emerging growth company that prepares its
financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition
period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange
Act.
Indicate by check mark whether the registrant
has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial
reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared
or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error
corrections are restatements that required a recovery analysis of incentive- based compensation received by any of the registrant’s
executive officers during the relevant recovery period pursuant to §240.10D-1(b).
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| International Financial Reporting Standards as issued by the International Accounting Standards Board ☐ | Other ☐ |
| * | If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow. Item 17 ☐ Item 18 ☐ |
If this is an annual report, indicate by check
mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐
No
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court. Yes ☐ No ☐
TABLE OF CONTENTS
i
INTRODUCTION
In this annual report on Form 20-F, unless the context otherwise requires, references to:
| ● | “China” or the “PRC” are to the People’s Republic of China, including the special administrative regions of Hong Kong and Macau and excluding Taiwan for the purposes of this annual report only; | |
| ● | “Exchange Act” are to the Securities Exchange Act of 1934, as amended; | |
| ● | “fiscal year” are to the period from October 1 to September 30 of the next calendar year; | |
| ● | “Jiangxi Universe” are to Jiangxi Universe Pharmaceuticals Co., Ltd., a limited liability company organized under the laws of the PRC, which is wholly owned by Universe Technology (as defined below) and an indirect wholly owned subsidiary of the Company; | |
| ● | “PRC operating entities” are to Jiangxi Universe and its subsidiaries; | |
| ● | “RMB” and Renminbi” are to the legal currency of China; | |
| ● | “shares” or “ordinary shares” are to the ordinary shares of the Company, par value $0.01875 per share; | |
| ● | “SEC” are to the U.S. Securities Exchange Commission; | |
| ● | “Securities Act” are to the Securities Act of 1933, as amended; | |
| ● | “TCM” are to traditional Chinese medicine; | |
| ● | “TCMD” are to traditional Chinese medicine derivatives; | |
| ● | “Universe Hanhe” are to Guangzhou Universe Hanhe Medical Research Co., Ltd., a PRC formed on May 12, 2021, a wholly-owned subsidiary of Jiangxi Universe; | |
| ● | “Universe HK” are to the Company’s wholly owned subsidiary, Universe Pharmaceuticals Group (International) Limited, a company incorporated in Hong Kong; | |
| ● | “Universe Technology” are to Jiangxi Universe Pharmaceuticals Technology Co., Ltd., a limited liability company organized under the laws of the PRC, and is wholly owned by Universe HK; | |
| ● | “Universe Trade” are to Jiangxi Universe Pharmaceuticals Trade Co., Ltd., a PRC company formed in 2010, a wholly-owned subsidiary of Jiangxi Universe; | |
| ● | “US$,” “U.S. dollars,” “$” and “dollars” are to the legal currency of the United States; and | |
| ● | “we,” “us,” “our Company,” or the “Company”, are to one or more of Universe Pharmaceuticals INC, an exempted company incorporated in the Cayman Islands. |
This annual report on Form 20-F includes our audited consolidated financial statements for the fiscal years ended September 30, 2023, 2022, and 2021. In this annual report, we refer to assets, obligations, commitments, and liabilities in our consolidated financial statements in United States dollars. These dollar references are based on the exchange rate of RMB to United States dollars, determined as of a specific date or for a specific period. Changes in the exchange rate will affect the amount of our obligations and the value of our assets in terms of United States dollars which may result in an increase or decrease in the amount of our obligations and the value of our assets.
This annual report contains translations of certain RMB amounts into U.S. dollars at specified rates. Unless otherwise stated, the following exchange rates are used in this annual report:
| September 30, | ||||||
| US$ Exchange Rate | 2023 | 2022 | 2021 | |||
| At the end of the year - RMB | RMB 7.2960 to $1.00 | RMB 7.1135 to $1.00 | RMB 6.4580 to $1.00 | |||
| Average rate for the year - RMB | RMB 7.0533 to $1.00 | RMB 6.5532 to $1.00 | RMB 6.5095 to $1.00 | |||
ii
Part I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not Applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not Applicable.
ITEM 3. KEY INFORMATION
This annual report refers to (i) Universe Pharmaceuticals INC, the Cayman Islands holding company, as “we”, “our”, “us”, or the “Company”, (ii) the Company’s subsidiaries, as “our subsidiaries,” (iii) Jiangxi Universe Pharmaceuticals Co., Ltd., the Company’s indirect wholly owned subsidiary in China (“Jiangxi Universe”) and its subsidiaries, which are domiciled in China and conducting business operations in China, as the “PRC operating entities.” The Company does not conduct any operations.
We are a Cayman Islands holding company with no operations of our own and not a PRC operating company. Our operations are conducted in China by our PRC subsidiaries. Investors in our securities are not purchasing equity interests in our subsidiaries but instead are purchasing equity interests in the ultimate Cayman Islands holding company. Therefore, you will not directly hold any equity interests in our operating companies. The Chinese regulatory authorities could disallow this structure, which would likely result in a material change in our operations and/or a material change in the value of the securities we are registering for sale, including that it could cause the value of such securities to significantly decline or become worthless. For risks facing our Company as a result of our organizational structure and doing business in China, see “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China.” We directly hold 100% equity interests in our subsidiaries, and we do not currently use a variable interest entity (“VIE”) structure.
We face legal and operational risks associated with having the majority of our operations in China, which could significantly limit or completely hinder our ability to offer securities to investors and cause the value of our securities to significantly decline or be worthless. The Chinese government has significant authority to exert influence on the ability of a China-based company, such as us, to conduct its business. Therefore, investors of our company and our business face potential uncertainty from the PRC government. Changes in China’s economic, political or social conditions or government policies could materially adversely affect our business and results of operations. These risks could result in a material change in our operations and/or the value of our ordinary shares or could significantly limit or completely hinder our ability to offer or continue to offer securities to investors and cause the value of such securities to significantly decline or be worthless. In particular, recent statements and regulatory actions by China’s government, such as those related to the use of variable interest entities and data security or anti-monopoly concerns, as well as the PCAOB’s ability to inspect our auditors, may impact our Company’s ability to conduct our business, accept foreign investments, or be listed on a U.S. or other foreign stock exchange. See “Item 3. Key Information — D. Risk Factors — Risks Related to Doing Business in China — The PRC government has significant authority to intervene or influence the China operations of an offshore holding company, such as ours, at any time. The PRC government may exert more control over offerings conducted overseas and/or foreign investment in China-based issuers. If the PRC government exerts more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers and we were to be subject to such oversight and control, it may result in a material adverse change to our business operations, significantly limit or completely hinder our ability to offer or continue to offer securities to investors, and cause the ordinary shares to significantly decline in value or become worthless” and “Item 3. Key Information — D. Risk Factors — Risks Related to Doing Business in China — Uncertainties arising from the legal system in China, including uncertainties regarding the interpretation and enforcement of PRC laws and the possibility that regulations and rules can change quickly with little advance notice, could hinder our ability to offer or continue to offer the ordinary shares, result in a material adverse change to our business operations, and damage our reputation, which would materially and adversely affect our financial condition and results of operations and cause the ordinary shares to significantly decline in value or become worthless.”
1
As of the date of this annual report, we and our subsidiaries have not been involved in any investigations on cybersecurity review initiated by any PRC regulatory authority, nor have any of them received any inquiry, notice, or sanction. As confirmed by our PRC counsel, AllBright Law Offices (Fuzhou), we are not subject to cybersecurity review by the Cyberspace Administration of China, or the CAC, since we currently do not possess any personal information of users in our business operations. It is unlikely for us to have over one million users’ personal information and we do not anticipate that we will be collecting over one million users’ personal information in the foreseeable future, which we understand might otherwise subject us to the Cybersecurity Review Measures. We are not subject to network data security review by the CAC if Draft Regulations on the Network Data Security Administration (Draft for Comments) (the “Security Administration Draft”) are enacted as proposed, because we currently do not have over one million users’ personal information, we do not collect data that affect or may affect national security and we do not anticipate that we will be collecting over one million users’ personal information or data that affect or may affect national security in the foreseeable future, which we understand might otherwise subject us to the Security Administration Draft.
On February 17, 2023, the CSRC issued the Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Enterprises (the “Trial Measures”) and five supporting guidelines (collectively, the “Overseas Listings Rules”), which became effective on March 31, 2023. These rules propose to establish a new filing-based regime to regulate overseas offerings and listings by Chinese domestic companies. Under the Overseas Listings Rules, Chinese domestic companies conducting overseas securities offering and listing activities, either in direct or indirect form, shall complete filing procedures with the CSRC pursuant to the requirements of the Trial Measures within three working days following its submission of initial public offering or listing application. Since the date of effectiveness of the Trial Measures, the domestic enterprises otherwise subject to filing that have been listed overseas or met the following circumstances are considered existing enterprises: the application of such enterprises for indirect overseas securities issuance and listing has been approved by the applicable overseas regulators or overseas stock exchanges (e.g., an applicable registration statement has been declared effective by the SEC) before the effectiveness of the Trial Measures, and are not required to re-perform issuance and listing supervision procedures with the overseas regulators or overseas stock exchanges, and the overseas issuance and listing of such enterprises will be completed by September 30, 2023. Existing enterprises are not required to file immediately, and filing should be made as required if they conduct refinancing activities or other matters requiring filings in the future. In the opinion of our PRC legal counsel, AllBright Law Offices (Fuzhou), as a domestic company listed on Nasdaq since March 2021, and not currently conducting refinancing or other activities that require filings, we are not required to file with the CSRC in accordance with the Trial Measures at this time. However, in the event that we conduct subsequent offerings, we could be subject to filing requirements with the CSRC. In the event that filings with the CSRC are required, we cannot assure you that we can complete the filing procedures, obtain the approvals or complete other compliance procedures in a timely manner, or at all, or that any completion of filing or approval or other compliance procedures would not be rescinded. Any such failure would subject us to sanctions by the CSRC or other PRC regulatory authorities. These regulatory authorities may impose restrictions and penalties on the operations in China, significantly limit or completely hinder our ability to launch any new offering of our securities, limit our ability to pay dividends outside of China, delay or restrict the repatriation of the proceeds from future capital raising activities into China, or take other actions that could materially and adversely affect our business, results of operations, financial condition and prospects, as well as the trading price of our ordinary shares. Furthermore, the PRC government authorities may further strengthen oversight and control over listings and offerings that are conducted overseas. Any such action may adversely affect our operations and significantly limit or completely hinder our ability to offer or continue to offer securities to you and cause the value of such securities to significantly decline or be worthless. See “Item 3. Key Information—Risk Factors—Risks Relating to Doing Business in China— The approval and/or other requirements of the China Securities Regulatory Commission, or the CSRC, or other PRC governmental authorities may be required in connection with an offering under PRC rules, regulations or policies, and, if required, we cannot predict whether or how soon we will be able to obtain such approval.”
2
If we do not receive or maintain any required approvals, or we inadvertently conclude that such approvals are not required, or applicable laws, regulations, or interpretations change such that we are required to obtain approval in the future, we may be subject to an investigation by competent regulators, fines or penalties, ordered to suspend our relevant business and rectify, prohibited from engaging in relevant business, or subject to an order prohibiting us from conducting an offering, and these risks could result in a material adverse change in our operations, significantly limit or completely hinder our ability to continue to offer securities to investors, or cause such securities to significantly decline in value or become worthless. See “Item 3. Key Information—Risk Factors—Risks Relating to Doing Business in China—Failure to comply with cybersecurity, data privacy, data protection, or any other laws and regulations related to data may materially and adversely affect our business, financial condition, and results of operations.”
In addition, trading in our securities may be prohibited under the HFCA Act if the PCAOB determines that it cannot inspect the workpapers prepared by our auditor, and that as a result an exchange may determine to delist our securities. On June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, which, if passed by the U.S. House of Representatives and signed into law, would reduce the period of time for foreign companies to comply with PCAOB audits to two consecutive years instead of three, thus reducing the time period for triggering the prohibition on trading. On December 16, 2021, the PCAOB issued a report on its determination that it is unable to inspect or investigate completely PCAOB-registered public accounting firms headquartered in China and in Hong Kong because of positions taken by PRC and Hong Kong authorities in those jurisdictions. Our auditor, the independent registered public accounting firm that issues the audit report included elsewhere in this annual report, as an auditor of companies that are traded publicly in the U.S. and a firm registered with the PCAOB, is subject to laws in the U.S., pursuant to which the PCAOB conducts regular inspections to assess its compliance with the applicable professional standards. Our auditor, YCM CPA INC., is headquartered in California and has been inspected by the PCAOB. Our auditor is not subject to the determination issued by the PCAOB on December 16, 2021. On August 26, 2022, the CSRC, the Ministry of Finance of the PRC, and the PCAOB signed a Statement of Protocol (the “Protocol”), governing inspections and investigations of audit firms based in China and Hong Kong. The Protocol remains unpublished and is subject to further explanation and implementation. Pursuant to the fact sheet with respect to the Protocol disclosed by the SEC, the PCAOB shall have independent discretion to select any issuer audits for inspection or investigation and has the unfettered ability to transfer information to the SEC. On December 15, 2022, the PCAOB Board determined that the PCAOB was able to secure complete access to inspect and investigate registered public accounting firms headquartered in mainland China and Hong Kong and voted to vacate its previous determinations to the contrary. However, should PRC authorities obstruct or otherwise fail to facilitate the PCAOB’s access in the future, the PCAOB Board will consider the need to issue a new determination. See “Item 3. Key Information — D. Risk Factors — Risks Related to Doing Business in China — Our ordinary shares may be delisted or prohibited from being traded over-the-counter under the Holding Foreign Companies Accountable Act, if the U.S. Public Company Accounting Oversight Board, or the PCAOB, is unable to inspect our auditors. The delisting or the cessation of trading of our ordinary shares, or the threat of their being delisted or prohibited from being traded, may materially and adversely affect the value of your investment. Additionally, the inability of the PCAOB to conduct inspections would deprive our investors with the benefits of such inspections. Our auditor has not been inspected by the PCAOB, but according to our auditor, it will be inspected by the PCAOB on a regular basis.”
Our Cayman Islands holding company has not declared or paid dividends or made any distributions to our shareholders in the past, nor were any dividends or distributions made by a subsidiary to the Cayman Islands holding company. Our board of directors has complete discretion on whether to distribute dividends, subject to applicable laws. We intend to keep any future earnings to finance the expansion of our business, and we do not have any current plan to declare or pay any cash dividends on our ordinary shares in the foreseeable future. See “Item 3. Key Information — D. Risk Factors — Risks Related to Our Ordinary Shares—We currently do not expect to pay dividends on our ordinary shares in the foreseeable future.” If we determine to pay dividends on any of our ordinary shares in the future, as a holding company, we will rely on payments from subsidiaries of Jiangxi Universe to Jiangxi Universe, and from Jiangxi Universe to Universe Technology, and the distribution of such payments to Universe HK, and then to our Company.
3
Subject to certain contractual, legal and regulatory restrictions, and our internal cash management policy, cash and capital contributions may be transferred among our Cayman Islands holding company and our subsidiaries. If needed, our Cayman Islands holding company can transfer cash to our PRC subsidiaries through loans and/or capital contributions, and our PRC subsidiaries can transfer cash to our Cayman Islands holding company through issuing dividends or other distributions. Our finance department supervises cash management, following the instructions of our management. Our finance department is responsible for establishing our cash operation plan and coordinating cash management matters among our subsidiaries and departments. Each subsidiary and department initiate a cash request by putting forward a cash demand plan, which explains the specific amount and timing of cash requested, and submitting it to our finance department. The finance department reviews the cash demand plan and prepares a summary for the management of our Company. Management examines and approves the allocation of cash based on the sources of cash and the priorities of the needs. Other than the above, we currently do not have other cash management policies or procedures that dictate how funds are transferred. Cash flows have occurred between our Cayman Islands holding company and our subsidiaries. From October 1, 2023 to the date of this annual report, the Cayman Islands holding company does not receive cash transfer from its subsidiaries. For the year ended September 30, 2023 and 2022, the Cayman Islands holding company received cash in the amount of $127,827 and $303,746 from its subsidiary in Hong Kong for the payment of directors’ compensation and professional service fees, respectively. The Cayman Islands holding company has not received cash transfer from its subsidiaries for the year ended September 30, 2021. There was no distribution of earnings by our PRC subsidiaries to the Cayman Islands holding company during the years ended September 30, 2021, 2022 and 2023, and from October 1, 2023 to the date of this annual report. In the fiscal year ended September 30, 2021, our Company transferred the net proceeds from its initial public offering, through Universe HK and Universe Technology, to Jiangxi Universe and its subsidiaries, in the amount of $6,807,507, to be used for general corporate purposes. In the years ended September 30, 2022 and 2023, there was no cash transferred from the Cayman Islands holding company to its PRC subsidiaries. From October 1, 2023 to the date of this annual report, there was no cash transferred from the Cayman Islands holding company to its PRC subsidiaries. See also “Item 8. Financial Information—A. Consolidated Statements and Other Financial Information—Dividend Policy” and our audited consolidated financial statements for the fiscal years ended September 30, 2023, 2022, and 2021.
Cash transfers from our Cayman Islands holding company are subject to applicable PRC laws and regulations on loans and direct investment. We may rely on dividends from our subsidiaries in China for our cash requirements, including any payment of dividends to our shareholders. PRC regulations may restrict the ability of our PRC subsidiaries to pay dividends to us, and as a holding company, we will be dependent on receipt of funds from our Hong Kong subsidiary, Universe HK.
Current PRC regulations permit our indirect PRC subsidiaries to pay dividends to Universe HK only out of their respective accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. In addition, each of our subsidiaries in China is required to set aside at least 10% of its after-tax profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of its registered capital. Each of such entity in China is also required to further set aside a portion of its after-tax profits to fund the employee welfare fund, although the amount to be set aside, if any, is determined at the discretion of its board of directors. Although the statutory reserves can be used, among other ways, to increase the registered capital and eliminate future losses in excess of retained earnings of the respective companies, the reserve funds are not distributable as cash dividends except in the event of liquidation.
4
The PRC government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies out of the PRC. Therefore, we may experience difficulties in complying with the administrative requirements necessary to obtain and remit foreign currency for the payment of dividends from our profits, if any. Furthermore, if our subsidiaries and affiliates in the PRC incur debt on their own in the future, the instruments governing the debt may restrict their ability to pay dividends or make other payments. If we or our subsidiaries are unable to receive all of the revenue from our operations, we may be unable to pay dividends on our ordinary shares.
Cash dividends, if any, on our ordinary shares will be paid in U.S. dollars. Universe HK may be considered a non-resident enterprise for tax purposes, so that any dividends our PRC subsidiaries pay to Universe HK may be regarded as China-sourced income and as a result may be subject to PRC withholding tax at a rate of up to 10%. See “Item 10. Additional Information—E. Taxation—People’s Republic of China Taxation.”
In order for us to pay dividends to our shareholders, we will rely on payments made from Universe Technology’s subsidiary, Jiangxi Universe, to Universe Technology and from Universe Technology to Universe HK and then to our Company. According to the EIT Law, such payments from subsidiaries to parent companies in China are subject to the PRC enterprise income tax at a rate of 25%. In addition, if Jiangxi Universe or its subsidiary or branches incur debt on their own behalf in the future, the instruments governing the debt may restrict its ability to pay dividends or make other distributions to us.
Pursuant to the Double Tax Avoidance Arrangement, the 10% withholding tax rate may be lowered to 5% if a Hong Kong resident enterprise owns no less than 25% of a PRC project. The 5% withholding tax rate, however, does not automatically apply and certain requirements must be satisfied, including without limitation that (a) the Hong Kong project must be the beneficial owner of the relevant dividends; and (b) the Hong Kong project must directly hold no less than 25% share ownership in the PRC project during the 12 consecutive months preceding its receipt of the dividends. In current practice, a Hong Kong project must obtain a tax resident certificate from the Hong Kong tax authority to apply for the 5% lower PRC withholding tax rate. As the Hong Kong tax authority will issue such a tax resident certificate on a case-by-case basis, we cannot assure you that we will be able to obtain the tax resident certificate from the relevant Hong Kong tax authority and enjoy the preferential withholding tax rate of 5% under the Double Taxation Arrangement with respect to any dividends paid by our PRC subsidiaries to its immediate holding company, Universe HK. As of the date of this annual report, we have not applied for the tax resident certificate from the relevant Hong Kong tax authority. Universe HK intends to apply for the tax resident certificate if and when Universe Technology plans to declare and pay dividends to Universe HK. See “Item 3. Key Information—D. Risk Factors— There are significant uncertainties under the Enterprise Income Tax Law, or the EIT Law, relating to the withholding tax liabilities of our PRC subsidiaries, and dividends payable by our PRC subsidiaries to our offshore subsidiaries may not qualify to enjoy certain treaty benefits.”
To the extent cash is located in the PRC or within a PRC domiciled entity and may need to be used to fund operations outside of the PRC, the funds may not be available due to limitations placed on us and our subsidiaries by the PRC government. To the extent cash in and assets of the business is in the PRC or a PRC entity, the funds and assets may not be available to fund operations or for other use outside of the PRC due to interventions in or the imposition of restrictions and limitations on the ability of us or our subsidiaries by the PRC government to transfer cash and assets. See “Item 3. Key Information—D. Risk Factors — Risks Related to Doing Business in China — To the extent cash and assets of in the business is in the PRC or a PRC entity, the funds may not be available to fund operations or for other use outside of the PRC due to interventions in or the imposition of restrictions and limitations on the ability of our Company or our subsidiaries by the PRC government to transfer cash and assets.”
5
A. [Reserved]
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
Summary of Risk Factors
Investing in our securities involves significant risks. You should carefully consider all of the information in this annual report before investing in our securities. Below is a summary of the principal risks we face. These risks are discussed more fully under “Item 3. Key Information—D. Risk Factors.”
Risks Related to Our Business and Industry (for a more detailed discussion, see “Item 3. Key Information—D. Risk Factors—Risks Related to Our Business and Industry”)
Risks and uncertainties related to our business include, but are not limited to, the following:
| ● | price increases in raw materials and sourced products could harm our financial results; |
| ● | high quality materials for our products may be difficult to obtain or substantially increase our production costs; |
| ● | we are exposed to a number of risks related to our supply chain for the materials required to manufacture our products which could adversely affect our business operations and future development; |
| ● | we operate in a highly competitive industry. Our failure to compete effectively could adversely affect our market share, revenues and growth prospects; |
| ● | high quality materials for our products may be difficult to obtain or substantially increase our production costs; |
| ● | our future success depends in part on our ability to increase our production capacity, and we may not able to do so in a cost-effective manner. We have engaged a third-party sub-contractor to build manufacturing facilities and an office building for us, and we may encounter challenges relating to the construction, management and operation of such facilities; |
| ● | we are subject to evolving regulatory requirements, non-compliance with which, or changes in which, may adversely affect our business and prospects; and |
| ● | if we fail to maintain or renew requisite licenses, permits, registrations and filings applicable to our business operations, or fail to obtain additional licenses, permits, registrations or filings that become necessary as a result of new enactment or promulgation of government policies, laws or regulations or the expansion of our business, our business and results of operations may be materially and adversely affected. |
6
Risks Related to Doing Business in China (for a more detailed discussion, see “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China”)
We face risks and uncertainties relating to doing business in the PRC in general, including, but not limited to, the following:
| ● | the PRC government has significant authority to intervene or influence the China operations of an offshore holding company, such as ours, at any time. The PRC government may exert more control over offerings conducted overseas and/or foreign investment in China-based issuers. If the PRC government exerts more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers and we were to be subject to such oversight and control, it may result in a material adverse change to our business operations, significantly limit or completely hinder our ability to offer or continue to offer securities to investors, and cause the ordinary shares to significantly decline in value or become worthless; |
| ● | uncertainties arising from the legal system in China, including uncertainties regarding the interpretation and enforcement of PRC laws and the possibility that regulations and rules can change quickly with little advance notice, could hinder our ability to offer or continue to offer the ordinary shares, result in a material adverse change to our business operations, and damage our reputation, which would materially and adversely affect our financial condition and results of operations and cause the ordinary shares to significantly decline in value or become worthless; |
| ● | our ordinary shares may be delisted or prohibited from being traded over-the-counter under the Holding Foreign Companies Accountable Act, if the U.S. Public Company Accounting Oversight Board, or the PCAOB, is unable to inspect our auditors. The delisting or the cessation of trading of our ordinary shares, or the threat of their being delisted or prohibited from being traded, may materially and adversely affect the value of your investment. Additionally, the inability of the PCAOB to conduct inspections would deprive our investors with the benefits of such inspections. Our auditor has not been inspected by the PCAOB, but according to our auditor, it will be inspected by the PCAOB on a regular basis; |
| ● | failure to comply with cybersecurity, data privacy, data protection, or any other laws and regulations related to data may materially and adversely affect our business, financial condition, and results of operations; |
| ● | the approval and/or other requirements of the China Securities Regulatory Commission, or the CSRC, or other PRC governmental authorities may be required in connection with an offering under PRC rules, regulations or policies, and, if required, we cannot predict whether or how soon we will be able to obtain such approval; |
| ● | PRC regulation of loans to, and direct investments in, PRC entities by offshore holding companies may delay or prevent us from using proceeds from our offerings and/or other financing activities to make loans or additional capital contributions to our PRC operating subsidiaries; |
| ● | adverse changes in political, economic and social conditions, as well as government policies in China could have a material adverse effect on our business results of operations, financial conditions and prospects; and |
| ● | changes to the PRC legal system could have an adverse effect on us. |
| ● | recent greater oversight by the Cyberspace Administration of China over data security, particularly for companies seeking to list on a foreign exchange, could adversely impact our business and our offering. See “Risk Factors—Risks Related to Doing Business in China—Recent greater oversight by the Cyberspace Administration of China over data security, particularly for companies seeking to list on a foreign exchange, could adversely impact our business and our offering”; |
| ● | to the extent cash and assets of in the business is in the PRC or a PRC entity, the funds may not be available to fund operations or for other use outside of the PRC due to interventions in or the imposition of restrictions and limitations on the ability of our Company or our subsidiaries by the PRC government to transfer cash and assets. See “Risk Factors—Risks Related to Doing Business in China—To the extent cash and assets of in the business is in the PRC or a PRC entity, the funds may not be available to fund operations or for other use outside of the PRC due to interventions in or the imposition of restrictions and limitations on the ability of our Company or our subsidiaries by the PRC government to transfer cash and assets.” |
7
Risks Relating to Our Ordinary Shares and the Trading Market (for a more detailed discussion, see “Item 3. Key Information—D. Risk Factors—Risks Relating to Our Ordinary Shares”)
In addition to the risks described above, we are subject to general risks and uncertainties relating to our ordinary shares and the trading market, including, but not limited to, the following:
| ● | Our share price has recently declined substantially, and our ordinary shares could be delisted from the Nasdaq or trading could be suspended; | |
| ● | we may issue additional ordinary shares or other equity securities without your approval, which would dilute your ownership interests and may depress the market price of our ordinary shares; and |
| ● | we are a “controlled company” within the meaning of the Nasdaq Stock Market Rules and, as a result, may rely on exemptions from certain corporate governance requirements that provide protection to shareholders of other companies; |
| ● | as a foreign private issuer, we are not subject to certain U.S. securities law disclosure requirements that apply to a domestic U.S. issuer, which may limit the information publicly available to our shareholders; and |
| ● | as a foreign private issuer, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from the Nasdaq listing standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards. |
Risks Related to Our Business and Industry
Price increases in raw materials and sourced products could harm our financial results.
Our principal raw materials include angelica, codonopsis, poria mushroom isatis root, ginseng, and other herbs and plant extracts. These raw materials are subject to price volatility and inflationary pressures. Our success is dependent, in part, on our ability to reduce our exposure to increases in such costs through a variety of ways, while maintaining and improving margins and market share. The manufacturers of such raw materials are also subject to price volatility and labor cost and other inflationary pressures, which may in turn result in an increase in the amount we pay for sourced products. Raw materials and sourced product price increases may offset our productivity gains and price increases and may adversely impact our financial results.
High quality materials for our products may be difficult to obtain or substantially increase our production costs.
Raw materials account for a portion of our manufacturing costs and we rely on third-party suppliers to provide almost all raw materials. Suppliers may be unable or unwilling to provide the raw materials we need in the quantities requested, at prices we are willing to pay, or that meet our quality standards. We are also subject to potential delays in the delivery of raw materials caused by events beyond our control, including transportation interruptions, delivery delays, labor disputes, other supply chain issues, and changes in government regulations. See “—We are exposed to a number of risks related to our supply chain for the materials required to manufacture our products” for details. Our business could be adversely affected if we are unable to obtain reliable sources of the raw materials used in the manufacturing of our products that meet our quality standards. Any significant delay in or disruption of the supply of raw materials could, among other things, substantially increase the cost of such materials, require reformulation or repackaging of products, require the qualification of new suppliers, or result in our inability to meet customer demands, which could in turn adversely affect our financial results.
We are exposed to a number of risks related to our supply chain for the materials required to manufacture our products which could adversely affect our business operations and future development.
We rely on third-party suppliers to provide almost all raw materials, and manufacturing our products is highly complex and requires sourcing specialty materials or materials with quality standards. Many of the risks associated with the complexity of manufacturing our products are applicable to the manufacture and supply of the raw materials. Minor deviations in the manufacturing process for these raw materials could result in supply disruption and reduced production yields for our products, which is beyond our control. In addition, we rely on third parties for the supply of these materials exposing us to similar risks of reliance on third parties for final products. See “—We face risks related to our sales of products obtained from third-party suppliers” for further details.
Our manufacturing processes requires many equipment and raw materials such as medicinal plants. We established supplier qualification procedures to verify the operation conditions, production capabilities, credit-worthiness and quality standard of potential suppliers, in order to procure quality raw materials in a timely manner. Although we are not relying on a single supplier for any of our raw materials, we may in the future rely on sole source vendors or a limited number of vendors for some of our equipment and materials. We currently depend on a limited number of suppliers for certain materials and equipment used in the manufacture of our products. Some of these suppliers are small companies with limited resources and experience to support commercial production and may be ill-equipped to support our needs from time to time. Accordingly, we may experience delays in receiving key materials and equipment to support manufacturing. An inability to continue to source product from any of these suppliers, which could be due to regulatory actions or requirements affecting the supplier, adverse financial or other strategic developments experienced by a supplier, labor disputes or shortages, unexpected demands, or quality issues, could adversely affect our ability to satisfy demand for our products, which could adversely and materially affect our product sales and operating results or our ability to conduct clinical trials, either of which could significantly harm our business.
8
As we continue to develop and scale our manufacturing process, we expect that we will need to obtain supplies of certain raw materials and equipment to be used as part of that process. We may not be able to obtain rights to such materials on commercially reasonable terms, or at all, and if we are unable to alter our process in a commercially viable manner to avoid the use of such materials or find a suitable substitute, it would have a material adverse effect on our business. Even if we are able to alter our process so as to use other materials or equipment, such a change may lead to a delay in our clinical development and/or commercialization plans.
We operate in a highly competitive industry. Our failure to compete effectively could adversely affect our market share, revenues and growth prospects.
The Chinese patent medicine industry in the PRC is subject to significant competition and pricing pressures. We will experience significant competitive pricing pressures as well as competitive products. Several significant competitors may offer products at the same or lower prices than our products. The market is highly sensitive to the introduction of new products, which may rapidly capture a significant share of the market. It is possible that one or more of our competitors could develop a significant research advantage over us that allows them to provide superior products that are more attractive to consumers, which could put us in a competitive disadvantage. Continued pricing pressure or improvements in research and shifts in customer preference could adversely impact our customer base or pricing structure and have a material and adverse effect on our business, financial conditions, results of operations and cash flows.
Failure to maintain or enhance our brands or image could have a material adverse effect on our business and results of operations.
We believe several of our brands, such as “Bai Nian Dan (百年丹)”, “Hu Zhuo Ren (胡卓仁)” and “Long Zhong (龙种)”, are well-recognized among our clients and other Chinese patent medicine industry players. Our brand is integral to our sales and marketing efforts. Our continued success in maintaining and enhancing our brand and image depends to a large extent on our ability to satisfy customer needs by further developing effective and better-quality products, as well as our ability to respond to competitive pressures. If we are unable to satisfy customer needs or if our public image or reputation were otherwise diminished, our business transactions with our customers may decline, which could in turn adversely affect our results of operations.
Our failure to appropriately respond to changing consumer preferences and demand for new products could significantly harm our customer relationships and product sales.
Our business is particularly subject to changing consumer trends and preferences. Our continued success depends in part on our ability to anticipate and respond to these changes, and we may not be able to respond in a timely or commercially appropriate manner to these changes. If we are unable to do so, our customer relationships and product sales could be harmed significantly.
Furthermore, the Chinese patent medicine industry is characterized by rapid and frequent changes in demand and new product introductions. Our failure to accurately depict these trends could negatively impact consumer opinion of our stores as a source for latest products. This could harm our customer relationships and cause losses to our market share. The success of our new product offerings depends upon a number of factors, including our ability to: accurately anticipate customer needs; innovate and develop new products; successfully commercialize new products in a timely manner; price our products competitively; manufacture and deliver our products in sufficient volumes and in a timely manner; and differentiate our product offerings from those our competitors.
If we do not introduce new products or make enhancements to meet the changing needs of our customers in a timely manner, some of our products could become obsolete, which could have a material adverse effect on our revenues and operating results.
If our products do not have the effects intended or cause undesirable side effects, our business may suffer.
Many of the ingredients in our current products have a long history of human consumption, and although we believe that all of these products and the combinations of ingredients in them are safe when taken as directed, the products could have certain undesirable side effects if not taken as directed or if taken by a consumer who has certain medical conditions. In addition, these products may not have the effect intended if they are not taken in accordance with instructions, which may include dietary restrictions. Furthermore, there can be no assurance that any of these products, even when used as directed, will have the effects intended or will not have harmful side effects in an unforeseen way or on an unforeseen cohort. If any of our products or products we develop or commercialize in the future are shown to be harmful or generate negative publicity from perceived harmful effects, our business, financial condition, results of operations, and prospects could be harmed significantly.
9
We have made substantial investment in advertising our products in order to improve our brand awareness and our market position, which efforts may not be successful, and in such event, our financial position and results of operations may be materially and negatively affected.
We have made substantial investment in advertising our products in order to improve our brand awareness and our market position. In particular, in the fiscal year ended September 30, 2021, we started to advertise our products through television advertisement. For example, on September 6, 2021, we entered into a service agreement with an advertising agency, engaging such agency to develop and produce a television advertisement for our signature TCMD products, Bai Nian Dan and Guben Yanling Pill, and to coordinate with a television channel to air the advertisement to audience in certain our target markets, with a term of one year from October 1, 2021 to September 30, 2022. In the fiscal year ended September 30, 2022, we entered into an advertising service agreement with a third-party, Health Headline Technology Co., Ltd. (“Health Headline”), pursuant to which, Health Headline provided media advertising services to promote our brand on the Health Headline website and mobile app, with a service period of ten months from March 1, 2022 to December 31, 2022. For the fiscal year ended September 30, 2023, we renewed the advertising services arrangement with Health Headline by entering a new service agreement with Health Headline with a service period of ten months from March 1, 2023 to December 31, 2023. As of the date of this annual report, there has been no renewal of the advertising service agreement with Health Headline. In addition, we incurred substantial advertising expenditures to maintain and enhance our brand and our products, which may not prove successful. Television advertising and other brand promotion activities may not generate customer awareness or increase revenue, and even if they do, any increase in revenue may not offset the expenses we incur in building our brand. Additionally, there could be a negative reaction to certain advertising campaigns. If we fail to promote our brand, or if we incur excessive expenses in this effort, we may fail to attract or retain customers necessary to realize a sufficient return on our brand-building efforts or to achieve the desired brand awareness that is critical for our success.
Our future success depends in part on our ability to increase our production capacity, and we may not able to do so in a cost-effective manner. We have engaged a third-party sub-contractor to build manufacturing facilities and an office building for us, and we may encounter challenges relating to the construction, management and operation of such facilities.
To the extent we are successful in growing our business, we may need to increase our production capacity. We entered into a construction agreement with a sub-contractor, who will construct four manufacturing factories and an office building for us, with a total maximum budget of approximately RMB165 million (US$23.2 million). The construction started on August 8, 2021, with an originally estimated completion date on August 7, 2023. However, due to resurgence in the number of COVID cases, which resulted in logistic disruptions, material and labor shortage, and domestic travel restriction, the construction work is estimated to be completed in December 2025, and the sub-contractor will bear the increased material and labor costs. Our ability to construct such additional facilities is subject to risks and uncertainties. The construction of any new facilities will be subject to risks inherent in the development and construction, including risks of delays and cost overruns as a result of factors outside of our control, which may include delays in government approvals, burdensome permitting conditions, and delays in the delivery of manufacturing equipment or raw materials required for the construction. Additionally, we also depend on the third-party sub-contractor for the development of new facilities, and as such, we are subject to the risk that such third parties do not fulfill their obligations to us under the contraction agreement.
If the sub-contractor is unable to deliver the new facilities to us on time, or if we are unable to expand our manufacturing facilities in general, we may be unable to further scale our business, which would negatively affect our results of operations and financial condition. If we are unable to transition manufacturing operations to such new facilities in a cost-efficient and timely manner, then we may experience disruptions in operations, which could negatively impact our business and financial results. Further, if the demand for our products decreases or if we do not produce the expected output after any such new facilities are operational, we may not be able to spread a significant amount of our fixed costs over the production volume, thereby increasing our per product fixed cost, which would have a negative impact on our business, financial condition and results of operations.
We have internal control deficiency in our internal control system and deficiencies in our corporate governance. If we fail to improve our internal control function and corporate governance, we may be subject to increased risk of fraud or misuse of corporate assets, which may materially and negatively impact our financial condition and results of operations.
We have internal control deficiency in our internal control system and deficiency in our corporate governance. In the fiscal year ended September 30, 2021, we entered into several material business transactions, including a construction agreement with a third-party sub-contractor to construct manufacturing facilities and an office building for us, a real estate property purchase agreement with a related party for the purchase of certain real properties, and an advertising service agreement with a third-party advertising agency to air our television advertisements. These transactions were not submitted for approval by our board of directors or any of its committees. In the fiscal year ended September 30, 2022, we have implemented measures to improve our internal control procedures and corporate governance as a public company, and obtained board approval prior to entering into all material business transactions, including the entry into a series of definitive agreements with Kitanihon Pharmaceutical Co., Ltd., and a letter of intent for the acquisition of Yunnan Faxi Pharmaceuticals Co., Ltd. (“Yunnan Faxi”). We intend to continue improving our internal control procedures and corporate governance as a public company. Nevertheless, if we fail to communicate with our board of directors on a regular basis or otherwise implement remedial measures to improve our internal control and corporate governance function in this regard, we may be subject to increased risk of fraud or misuse of corporate assets, which may materially and negatively impact our financial condition and results of operations.
10
We are subject to evolving regulatory requirements, non-compliance with which, or changes in which, may adversely affect our business and prospects.
As a manufacturer of products designed for human consumption, we are subject to legal and regulatory requirements applicable to the Chinese patent medicine industry in the PRC. We have been subject to penalties by PRC regulatory authorities in the past due to our failure to comply with their requirements, including noncompliance with the Good Manufacturing Practice for Drugs and the National Drug Standard.
The regulations to which we are subject in this area are evolving. As a result, the interpretation of these laws and their enforcement is often uncertain. Predicting the application of these laws can be difficult, and unexpected outcomes in the interpretation and enforcement of the applicable regulations may have an adverse impact on our business and operations. Additionally, any future changes in regulations may render our business non-compliant or require changes to our business practices or licensing arrangements to ensure compliance. These changes may involve significant costs, which in turn may adversely affect our business and financial prospects.
Various regulatory authorities of the PRC government regulate the manufacturing and trading of Chinese patent medicine. Violations of regulations may lead to the imposition of significant penalties which may affect our business, operations, reputation and financial prospects. See “Item 4. Information on the Company—B. Business Overview—Regulations” for details.
As we introduce new products to our customers, we may be required to comply with additional laws and regulations that are yet to be determined. To comply with such additional laws and regulations, we may be required to obtain necessary certificates, licenses or permits, as well as expend additional resources to monitor regulatory and policy developments. Our failure to adequately comply with such additional laws and regulations may delay, or possibly prevent, some of our products from being offered to customers, which may have a material adverse effect on our business, financial condition and results of operations.
If we fail to maintain or renew requisite licenses, permits, registrations and filings applicable to our business operations, or fail to obtain additional licenses, permits, registrations or filings that become necessary as a result of new enactment or promulgation of government policies, laws or regulations or the expansion of our business, our business and results of operations may be materially and adversely affected.
The Chinese patent medicine industry in China is highly regulated, and multiple licenses, permits, filings and approvals are required to operate our business. Currently, through our PRC subsidiaries, we have obtained a valid pharmaceutical manufacturing license, a medical device selling license, and a pharmaceutical trade license. We have made efforts to obtain all applicable approvals, licenses and permits, but due to the complexities, uncertainties and frequent changes in laws, rules, regulations and their interpretation and implementation, we may not always be able to do so, and we may be penalized by governmental authorities for conducting pharmaceutical manufacturing or sales activities without proper approvals, licenses or permits. Moreover, as we continue to increase our product variety, we may also become subject to new or existing laws and regulations that did not affect us in the past. Failure to obtain, renew or retain requisite licenses, permits or approvals may adversely affect our ability to conduct or expand our business, and may have a material adverse impact on our business prospects, results of operations and financial condition.
Our business is subject to inherent risks relating to product liability and personal injury claims.
As a manufacturer of products designed for human consumption, we are subject to product liability claims if the use of our products is alleged to have resulted in injury. For instance, adverse reactions resulting from human consumption of the ingredients contained in our products could occur. We may also be obligated to recall affected products. If we are found liable for product liability claims, we could be required to pay substantial monetary damages. Furthermore, even if we successfully defend ourselves against this type of claim, we could be required to spend significant management, financial and other resources, which could disrupt our business, and our reputation as well as our brand name may also suffer. We, like many other similar companies in China, do not carry product liability insurance. As a result, any imposition of product liability could materially harm our business, financial condition and results of operations. In addition, we do not have any business interruption insurance, due to the limited coverage of any available business interruption insurance in China, and as a result, any business disruption or natural disaster could severely disrupt our business and operations and significantly decrease our revenue and profitability.
We may not be successful in expanding a distribution network.
Although we intend to expand our distribution network to include additional cities and rural areas in the PRC in an effort to increase our geographic presence, our distribution, logistics and products may encounter competition from various similar or substitutive businesses. Therefore, the success of expansion will depend upon many factors, including our ability to form relationships with, and manage an increasing number of, customers base and optimize our distribution network. If we fail to expand our distribution network as planned, our business, financial condition and results of operations may be materially and adversely affected.
11
The COVID-19 pandemic have been and could continue to materially and adversely affect our business and results of operations.
Our business operations have been affected and may continue to be affected by the ongoing COVID-19 pandemic. Although on December 9, 2022, China announced that it was officially moving towards reopening and gradually lifting travel restrictions, after nearly 3 years of adherering to its dynamic zero-COVID policy, the COVID-19 pandemic continues to threaten global economies and cause significant market volatility and declines in general economic activities of China. In the fiscal year ended September 30, 2023, slowdown in economy caused by the COVID-19 pandemic has led to a decline in customers’ spending power, and we changed our pricing strategy to decrease the per unit price of our TCMD products in an effort to increase sales volume and market share during this challenging period. The average selling price of our TCMD products decreased by 50.0% in the fiscal year ended September 30, 2023 as compared to the fiscal year ended September 30, 2022. In the fiscal year ended September 30, 2022, due to resurgence of COVID-19 pandemic in China and related restrictive measures, including travel restrictions, the PRC operating entities experienced delays in the receipt of purchased raw materials from suppliers and in delivering products to customers. The prices of the raw materials increased by about 5% as compared to the fiscal year ended September 30, 2021. In addition, we granted some customers extended payment terms of 30 days to 120 days. However, based on our present relationship with these customers and our evaluation of their financial conditions, we do not anticipate any material collectability problems. The continued uncertainties associated with the COVID-19 pandemic worldwide may cause our revenue and cash flows to underperform in the next 12 months from the date of this annual report. The extent of the future impact of the COVID-19 pandemic on our business and the results of operations is still uncertain.
We are dependent on certain key personnel and loss of these key personnel could have a material effect on our business, financial condition and results of operations.
Our success is, to a certain extent, attributable to the management, sales and marketing, and research and development expertise of key personnel. We are dependent upon the services of Mr. Gang Lai, the chairman of our board of directors and our chief executive officer, for the continued growth and operation of our Company, due to his industry experiences and management experiences. Although we have no reason to believe that Mr. Gang Lai will discontinue his services with us, the interruption or loss of his services would adversely affect our ability to effectively run our business and pursue our business strategy as well as our results of operation. We currently do not have “key person” insurance on any of our executives or employees. There can be no assurance that we will be able to retain our key personnel after the terms of their employment expire. The loss of the services of one or more of our key personnel could have a material adverse effect upon our business, financial condition, and results of operations.
We may not effectively manage our growth, which could materially harm our business.
We expect that our business will grow in the long run, which may place a significant strain on our management, personnel, systems and resources. We must continue to improve our operational and financial systems and managerial controls and procedures, and we will need to continue to expand, train and manage our technology and workforce. We must also maintain close coordination among our compliance, accounting, finance, marketing and sales organizations. We cannot assure you that we will manage our growth effectively. If we fail to do so, our business could be materially harmed.
Our continued growth will require an increased investment by us in technology, facilities, personnel and financial and management systems and controls. It also will require expansion of our procedures for monitoring and assuring our compliance with applicable regulations, and we will need to integrate, train and manage a growing employee base. The expansion of our existing businesses, any expansion into new businesses and the resulting growth of our employee base will increase our need for internal audit and monitoring processes that are more extensive and broader in scope than those we have historically acquired. We may not be successful in identifying or implementing all of the processes that are necessary. Further, unless our growth results in an increase in our revenues that is proportionate to the increase in our costs associated with this growth, our operating margins and profitability will be adversely affected.
We may not be able to hire and retain qualified personnel to support our growth and if we are unable to retain and hire these personnel in the future, our ability to improve our products and implement our business objects could be adversely affected.
We must attract, recruit and retain a sizeable workforce of technically competent employees. Competition for senior management and personnel in the PRC is intense and the pool of qualified candidates in the PRC is very limited. We may not be able to retain the services of our senior executives or personnel, or attract and retain high-quality senior executives or personnel in the future. This failure could materially and adversely affect our future growth and financial condition.
Our success depends on our ability to protect our intellectual property.
We currently own 73 patents and 99 trademarks in China. We believe that our success depends on our ability to obtain and maintain patent protection for products developed utilizing our technologies, in the PRC and in other countries, and to enforce these patents. There is no assurance that any of our existing and future patents will be deemed to be valid and enforceable against third-party infringement or that our products will not infringe any third-party patent or intellectual property by a court or administrative body having jurisdiction over such matters. Although we have filed additional patent applications with Patent Administration Department of PRC, there is no assurance that they will be granted.
12
Any patents relating to our technologies may not be sufficiently broad to protect our products. In addition, our patents may be challenged, potentially invalidated or potentially circumvented. Our patents may not afford us protection against competitors with similar technology or permit the commercialization of our products without infringing third-party patents or other intellectual property rights.
We also rely, or intend to rely, on our trademarks, trade names and brand names to distinguish our products from the products of our competitors, and have registered or will apply to register a number of these trademarks. However, third parties may oppose our trademark applications or otherwise challenge our use of the trademarks. In the event that our trademarks are successfully challenged, we could be forced to rebrand our products, which could result in loss of brand recognition and could require us to devote resources to advertising and marketing these new brands. Further, our competitors may infringe our trademarks, or we may not have adequate resources to enforce our trademarks.
In addition, we also have trade secrets, non-patented proprietary expertise and continuing technological innovations that we expect to seek to protect, in part, by entering into confidentiality agreements with licensees, suppliers, employees and consultants. These agreements may be breached and there may not be adequate remedies in the event of a breach. Disputes may arise concerning the ownership of intellectual property or the applicability of confidentiality agreements. Moreover, our trade secrets and proprietary technology may otherwise become known or be independently developed by our competitors. If patents are not issued with respect to products arising from research, we may not be able to maintain the confidentiality of information relating to these products.
Further, the application and interpretation of China’s intellectual property laws are still evolving and are uncertain. If we are found to have violated the intellectual property rights of others, we may be subject to liability and penalty for our infringement activities or may be prohibited from using such intellectual property, and we may incur licensing fees or be forced to develop alternatives of our own. In addition, we may incur significant expenses, and may be forced to divert management’s time and other resources from our business and operations to defend against these infringement claims, regardless of their merits. Successful infringement or licensing claims made against us may result in significant monetary liabilities and may materially disrupt our reputation, business and operations by restricting or prohibiting our use of the intellectual property at issue.
Because we rely on our manufacturing operations to produce a significant amount of the products we sell, disruptions in our manufacturing system or losses of manufacturing certifications could adversely affect our sales and customer relationships.
Our manufacturing operations produced approximately 57.5%, 59.8% and 61.6% of the total value of the products we sold for the fiscal year ended September 30, 2023, 2022 and 2021, respectively. Our products are produced in our manufacturing facility located in Jinggangshan, Jiangxi Province, China. For the year ended September 30, 2023, one supplier accounted for 29.6% of our total purchases. For the fiscal year ended September 30, 2022, one supplier accounted for approximately 10.3% of our total purchases. For the fiscal year ended September 30, 2021, no supplier accounted for more than 10% of our total purchases. In the event any of our third-party suppliers or vendors becomes unable or unwilling to continue to provide raw materials in the required volumes or quality levels or in a timely manner, we would be required to identify and obtain acceptable replacement supply sources. If we are unable to identity and obtain alternative supply sources, our business could be adversely affected. Any significant disruption in our operations at our manufacturing facility for any reason, including government-imposed regulatory requirements, the loss of certifications, power interruptions, fires, war, or other force of nature, could disrupt our supply of products, adversely affecting our sales and customer relationships.
We face risks related to our sales of products obtained from third-party suppliers.
We sell a significant number of products that are manufactured by third-party suppliers over which we have no direct control. While we have implemented processes and procedures to try to ensure that the suppliers we use are complying with all applicable regulations, there can be no assurance that such suppliers in all instances will comply with such processes and procedures or otherwise with applicable regulations. Noncompliance could result in our marketing and distribution of contaminated or dangerous products which would subject us to liabilities and could result in the imposition by government authorities of penalties that could restrict or eliminate our ability to purchase products. Any or all of these effects could adversely affect our business, financial condition and results of operation.
The growth of our business depends on our ability to finance new product innovations and these increased costs may reduce our cash flows and, if the products in which we have invested fail, it would reduce our profitability.
We operate in the Chinese patent medicine industry, which is characterized by significant competition and rapid change. New products appear with increasing frequency to supplant existing products. If we fail to adapt to those conditions in a timely and efficient manner, our revenues and profits would likely decline. To remain competitive, we must continue to incur significant costs in product research and development, marketing, equipment and facilities and to make capital investment. These costs may increase, resulting in greater fixed costs and operating expenses.
In the fiscal year ended September 30, 2023, we incurred $4.9 million research and development expenses, a 36.4% decrease compared to the expenses in the fiscal year ended September 30, 2022. In the fiscal year ended September 30, 2022, we incurred $7.6 million of research and development expenses, a 39.9% increase compared to the expenses in the fiscal year ended September 30, 2021. In order to diversify our profit portfolio, a large portion of the research and development expenses in the fiscal year ended September 30, 2022 was spent on developing and testing eight new products. In the fiscal year ended September 30, 2021, we incurred $5.47 million of research and development expenses. In order to diversify our profit portfolio, a large portion of the research and development expenses in the fiscal year ended September 30, 2021 was spent on developing and testing eight new products.
13
Our future operating results will depend to a significant extent on our ability to continue to provide new products that compare favorably based on time to market, cost and performance with the design and manufacturing capabilities and competing third-party suppliers and technologies. Furthermore, our research and development efforts may not produce successful results, and our new products may not achieve market acceptance, create additional revenue for us, or bring us profits. Our failure to increase our net sales sufficient to offset these increased costs would reduce our profitability and may materially and adversely affect our business operations and results of operations.
Future acquisitions may have an adverse effect on our ability to manage our business.
We may acquire businesses, technologies, services or products which are complementary to our core business of manufacturing and selling TCMD products. Future acquisitions may expose us to potential risks, including risks associated with: (i) the integration of new products, services and personnel; unforeseen or hidden liabilities; (ii) the diversion of resources from our existing business; (iii) our potential inability to generate sufficient revenue to offset new costs; the expenses of acquisitions; or (iv) the potential loss of or harm to relationships with both employees and advertising clients resulting from our integration of new businesses.
Any of the potential risks listed above could have a material and adverse effect on our ability to manage our business, our revenues and net income. We may need to raise additional debt funding or sell additional equity securities to make such acquisitions. The raising of additional debt funding by us, if required, would result in increased debt service obligations and could result in additional operating and financing covenants, or liens on our assets, that could restrict our operations. The sale of additional equity securities could result in additional dilution to our shareholders.
Increase in labor costs in the PRC may adversely affect our business and our profitability.
China’s economy has experienced increases in labor costs in recent years, and the average wage in China are expected to continue to grow. The average wage level for our employees has also increased in recent years. We expect that our labor costs, including wages and employee benefits, will continue to increase as we continue to grow our business. Unless we are able to pass on these increased labor costs to our customers by increasing prices for our products or services, our profitability and results of operations may be materially adversely affected.
In addition, we have been subject to stricter regulatory requirements in terms of entering into labor contracts with our employees and paying various statutory employee benefits, including pensions, housing fund, medical insurance, work-related injury insurance, unemployment insurance and childbearing insurance to designated government agencies for the benefit of our employees. Pursuant to the PRC Labor Contract Law (《中华人民共和国劳动法》) (the “Labor Contract Law”) that became effective in January 2008 and its implementing rules that became effective in September 2008 and its amendments that became effective in July 2013, employers are subject to stricter requirements in terms of signing labor contracts, minimum wages, paying remuneration, determining the term of employees’ probation and unilaterally terminating labor contracts. In the event that we decide to terminate some of our employees or otherwise change our employment or labor practices, the Labor Contract Law and its implementation rules may limit our ability to effect any such terminations or those changes in a desirable or cost-effective manner, which could adversely affect our business and results of operations.
As of the date of this annual report, we believe that we are in substantial compliance with labor-related laws and regulations in China, and we have not been notified of any instance of noncompliance. As the interpretation and implementation of labor-related laws and regulations are still evolving, we cannot assure you that our employment practice will not violate labor-related laws and regulations in China, which may subject us to labor disputes or government investigations. If we are deemed to have violated relevant labor laws and regulations, we could be required to provide additional compensation to our employees and our business, financial condition and results of operations could be materially and adversely affected.
Natural disasters (whether or not caused by climate change), unusually adverse weather conditions, pandemic outbreaks, terrorist acts and global political events could cause permanent or temporary distribution center or store closures, impair our ability to purchase, receive or replenish inventory, or cause customer traffic to decline, all of which could result in lost sales and otherwise adversely affect our financial performance.
The occurrence of one or more natural disasters, such as hurricanes, fires, floods and earthquakes (whether or not caused by climate change), unusually adverse weather conditions, pandemic outbreaks, terrorist acts or disruptive global political events, such as civil unrest in countries in which our suppliers are located, or similar disruptions could adversely affect our operations and financial performance. To the extent these events result in the closure of one or more of our distribution centers, a significant number of stores, a manufacturing facility or our corporate headquarters, or impact one or more of our key suppliers, our operations and financial performance could be materially adversely affected through an inability to make deliveries to our stores and through lost sales. In addition, these events could result in increases in fuel (or other energy) prices or a fuel shortage, decrease in available raw materials of sufficient quality and in sufficient amounts, delays in opening new stores, the temporary lack of an adequate work force in a market, the temporary or long-term disruption in the supply of products from some local and overseas suppliers, the temporary disruption in the transport of goods from overseas, delay in the delivery of goods to our distribution centers or stores, the temporary reduction in the availability of products in our stores and disruption to our information systems. These events also could have indirect consequences, such as increases in the cost of insurance, if any of such events was to result in significant loss of property or other insurable damage.
14
Risks Related to Doing Business in China
The PRC government has significant authority to intervene or influence the China operations of an offshore holding company, such as ours, at any time. The PRC government may exert more control over offerings conducted overseas and/or foreign investment in China-based issuers. If the PRC government exerts more oversight and control over offerings that are conducted overseas and/or foreign investment in China-based issuers and we were to be subject to such oversight and control, it may result in a material adverse change to our business operations, significantly limit or completely hinder our ability to offer or continue to offer securities to investors, and cause the ordinary shares to significantly decline in value or become worthless.
Our business, prospects, financial condition, and results of operations may be influenced to a significant degree by political, economic, and social conditions in China generally. The PRC government has significant authority to intervene or influence the China operations of an offshore holding company at any time, which could result in a material adverse change to our operations and the value of the ordinary shares. The PRC government has recently indicated an intent to exert more oversight and control over listings conducted overseas and/or foreign investment in China-based issuers. Any such action may hinder our ability to offer or continue to offer our securities to investors, result in a material adverse change to our business operations, and damage our reputation, which could cause the ordinary shares to significantly decline in value or become worthless. See also “—Failure to comply with cybersecurity, data privacy, data protection, or any other laws and regulations related to data may materially and adversely affect our business, financial condition, and results of operations.”
Uncertainties arising from the legal system in China, including uncertainties regarding the interpretation and enforcement of PRC laws and the possibility that regulations and rules can change quickly with little advance notice, could hinder our ability to offer or continue to offer the ordinary shares, result in a material adverse change to our business operations, and damage our reputation, which would materially and adversely affect our financial condition and results of operations and cause the ordinary shares to significantly decline in value or become worthless.
The legal system in China is a civil law system based on written statutes. Unlike common law systems, it is a system in which decided legal cases may be cited for reference but have less precedential value. The laws, regulations, and legal requirements in China are quickly evolving and their interpretation and enforcement involve uncertainties. These uncertainties could limit the legal protections available to you and us. In addition, we cannot predict the effect of future developments in the PRC legal system, particularly with regard to new economies, including the promulgation of new laws, changes to existing laws or the interpretation or enforcement thereof, or the preemption of local regulations by national laws. Furthermore, the PRC legal system is based in part on government policies and internal rules, some of which are not published on a timely basis or at all. As a result, we may not be aware of our potential violation of these policies and rules. In addition, any administrative and court proceedings in China may be protracted and result in substantial costs and diversion of resources and management attention.
New laws and regulations may be enacted from time to time and substantial uncertainties exist regarding the interpretation and implementation of current and any future PRC laws and regulations applicable to our businesses. In particular, the PRC government authorities may continue to promulgate new laws, regulations, rules and guidelines governing companies in the patent medicine industry with respect to a wide range of issues, such as intellectual property, unfair competition and antitrust, privacy and data protection, and other matters. Compliance with these laws, regulations, rules, guidelines, and implementations may be costly, and any incompliance or associated inquiries, investigations, and other governmental actions may divert significant management time and attention and our financial resources, bring negative publicity, subject us to liabilities or administrative penalties, or materially and adversely affect our business, financial condition, results of operations, and the value of the ordinary shares.
Our ordinary shares may be delisted or prohibited from being traded over-the-counter under the Holding Foreign Companies Accountable Act, if the U.S. Public Company Accounting Oversight Board, or the PCAOB, is unable to inspect our auditors. The delisting or the cessation of trading of our ordinary shares, or the threat of their being delisted or prohibited from being traded, may materially and adversely affect the value of your investment. Additionally, the inability of the PCAOB to conduct inspections would deprive our investors with the benefits of such inspections. Our auditor has not been inspected by the PCAOB, but according to our auditor, it will be inspected by the PCAOB on a regular basis.
The Holding Foreign Companies Accountable Act, or the HFCA Act, was enacted on December 18, 2020. The HFCA Act states if the SEC determines that we have filed audit reports issued by a registered public accounting firm that has not been subject to inspection by the PCAOB for three consecutive years, the SEC shall prohibit our shares from being traded on a national securities exchange or in the over-the-counter trading market in the U.S.
Our auditor, the independent registered public accounting firm that issues the audit report included elsewhere in this annual report, as an auditor of companies that are traded publicly in the United States and a firm registered with the PCAOB, is subject to laws in the United States pursuant to which the PCAOB conducts regular inspections to assess its compliance with the applicable professional standards. Our auditor is headquartered in California and has not been inspected by the PCAOB, but according to our auditor, it will be inspected by the PCAOB on a regular basis.
15
On March 24, 2021, the SEC adopted interim final rules relating to the implementation of certain disclosure and documentation requirements of the Holding Foreign Companies Accountable Act. We will be required to comply with these rules if the SEC identifies us as having a “non-inspection” year under a process to be subsequently established by the SEC. The SEC is assessing how to implement other requirements of the Holding Foreign Companies Accountable Act, including the listing and trading prohibition requirements described above. In May 2021, the PCAOB issued for public comment a proposed rule related to the PCAOB’s responsibilities under the Holding Foreign Companies Accountable Act, which, according to the PCAOB, would establish a framework for the PCAOB to use when determining, as contemplated under the Holding Foreign Companies Accountable Act, whether the PCAOB is unable to inspect or investigate completely registered public accounting firms located in a foreign jurisdiction because of a position taken by one or more authorities in that jurisdiction. The proposed rule was adopted by the PCAOB in September 2021, pending the final approval of the SEC to become effective.
On June 22, 2021, the U.S. Senate passed the Accelerating Holding Foreign Companies Accountable Act, which, if passed by the U.S. House of Representatives and signed into law, would reduce the number of consecutive non-inspection years required for triggering the prohibitions under the Holding Foreign Companies Accountable Act from three years to two.
On December 2, 2021, the SEC issued amendments to finalize rules implementing the submission and disclosure requirements in the Holding Foreign Companies Accountable Act. The rules apply to registrants that the SEC identifies as having filed an annual report with an audit report issued by a registered public accounting firm that is located in a foreign jurisdiction and that PCAOB is unable to inspect or investigate completely because of a position taken by an authority in foreign jurisdictions.
On December 16, 2021, the PCAOB issued a report on its determination that it is unable to inspect or investigate completely PCAOB-registered public accounting firms headquartered in China and in Hong Kong because of positions taken by PRC and Hong Kong authorities in those jurisdictions. The PCAOB has made such determination as mandated under the Holding Foreign Companies Accountable Act. Pursuant to each annual determination by the PCAOB, the SEC will, on an annual basis, identify issuers that have used non-inspected audit firms and thus are at risk of such suspensions in the future. Our auditor is headquartered in California and is not subject to this determination announced by the PCAOB.
The SEC may propose additional rules or guidance that could impact us if our auditor is not subject to PCAOB inspection. For example, on August 6, 2020, the President’s Working Group on Financial Markets, or the PWG, issued the Report on Protecting United States Investors from Significant Risks from Chinese Companies to the then President of the United States. This report recommended that the SEC implement five measures to address companies from jurisdictions that do not provide the PCAOB with sufficient access to fulfil its statutory mandate. Some of the concepts of these recommendations were implemented with the enactment of the Holding Foreign Companies Accountable Act. However, some of the recommendations were more stringent than the Holding Foreign Companies Accountable Act.
The SEC has announced that the SEC staff is preparing a consolidated proposal for the rules regarding the implementation of the Holding Foreign Companies Accountable Act and to address the recommendations in the PWG report. It is unclear when the SEC will complete its rulemaking and when such rules will become effective and what, if any, of the PWG recommendations will be adopted. The SEC has also announced amendments to various annual report forms to accommodate the certification and disclosure requirements of the Holding Foreign Companies Accountable Act. There could be additional regulatory or legislative requirements or guidance that could impact us if our auditor is not subject to PCAOB inspection. The implications of this possible regulation or guidance, in addition to the requirements of the Holding Foreign Companies Accountable Act, are uncertain.
On December 15, 2022, the PCAOB announced that it has completed a test inspection of two selected auditing firms in mainland China and Hong Kong and has voted to vacate its previous Determination Report, which concluded in December 2021 that it could not inspect or investigate completely registered public accounting firms based in mainland China or Hong Kong. Moving forward, the PCAOB will continue to demand complete access in mainland China and Hong Kong. Despite the PCAOB’s announcement, Chinese authorities will need to ensure that the PCAOB continues to have full access for inspections and investigations in 2024 and beyond, or the threat for Chinese companies listed on U.S. stock exchanges has not been relieved.
Our auditor, YCM CPA INC., is headquartered in California. YCM CPA Inc. has been subject to PCAOB inspections and is not subject to the determinations announced by the PCAOB on December 16, 2021. If, for whatever reason, the PCAOB is unable to conduct full inspections of our auditor, uncertainty under the HFCA Act could cause the market price of our ordinary shares to be materially and adversely affected, and our securities could be delisted or prohibited from being traded “over-the-counter”. The risk and uncertainty associated with a potential delisting would have a negative impact on the price of our ordinary shares.
The foregoing recent developments would add uncertainties to our future offerings and may result in prohibitions on the trading of our ordinary shares on the Nasdaq Stock Market, if our auditors fail to meet the PCAOB inspection requirement in time.
Failure to comply with cybersecurity, data privacy, data protection, or any other laws and regulations related to data may materially and adversely affect our business, financial condition, and results of operations.
We may be subject to a variety of cybersecurity, data privacy, data protection, and other laws and regulations related to data, including those relating to the collection, use, sharing, retention, security, disclosure, and transfer of confidential and private information, such as personal information and other data. These laws and regulations apply not only to third-party transactions, but also to transfers of information within our organization. These laws and regulations may restrict our business activities and require us to incur increased costs and efforts to comply, and any breach or noncompliance may subject us to proceedings against us, damage our reputation, or result in penalties and other significant legal liabilities, and thus may materially and adversely affect our business, financial condition, and results of operations.
16
In China, the cybersecurity, data privacy, data protection, or other data-related laws and regulations are relatively new and evolving, and their interpretation and application may be uncertain. For example, on November 14, 2021, the Administration Regulations on Cyber Data Security (Draft for Comments) (the “Draft Regulation”) was proposed by the Cyberspace Administration of China, or the CAC, for public comments until December 13, 2021. The Draft Regulation reiterates that data processors which process the personal information of at least one million users must apply for a cybersecurity review if they plan on listing its securities overseas, and the Draft Regulation further requires the data processors to apply for cybersecurity review in accordance with relevant laws and regulations under the following circumstances: (i) such data processor engages in merger, reorganization or division of internet platform operators that have gathered a large number of data resources related to national security, economic development and public interests affects or may affect national security; (ii) the listing of such data processor overseas affects or may affect national security; and (iii) such data processor engages in other data processing activities that affect or may affect national security. Any failure to comply with such requirements may subject us to, among others, suspension of services, fines, revocation of relevant business permits or business licenses, and/or penalties.
As of the date of this annual report, we have not engaged in the relevant businesses provided in the Draft Regulation. As such, we currently do not expect the draft measures by the CAC or other recent regulations will have an impact on our business or results of operations, and we believe that we are compliant with the regulations and policies that have been issued by the CAC to date. As of the date of this annual report, we have not received any investigation, notice, warning, or sanction from applicable government authorities (including the CAC) with regard to our business operations concerning any issues related to cybersecurity and data security. In addition, we have not been involved in any review, investigation, enquiry, penalty, or other legal proceedings initiated by applicable governmental or regulatory authorities or third parties in relation to in relation to cyber security or data protection. However, we still face uncertainties regarding the interpretation and implementation of these laws and regulations in the future. Cybersecurity review could result in disruption in our operations, negative publicity with respect to our Company, and diversion of our managerial and financial resources. Furthermore, if we were found to be in violation of applicable laws and regulations in China during such review, we could be subject to fines or other government sanctions and reputational damage. Therefore, potential cybersecurity review, if applicable to us, could materially and adversely affect our business, financial condition, and results of operations.
In addition, the PRC Data Security Law, which was promulgated by the Standing Committee of the National People’s Congress (the “SCNPC”) on June 10, 2021 and took effect on September 1, 2021, requires data collection to be conducted in a legitimate and proper manner, and stipulates that, for the purpose of data protection, data processing activities must be conducted based on data classification and hierarchical protection system for data security. Furthermore, the recently issued Opinions on Strictly Cracking Down Illegal Securities Activities require (i) speeding up the revision of the provisions on strengthening the confidentiality and archives management relating to overseas issuance and listing of securities and (ii) improving the laws and regulations relating to data security, cross-border data flow, and management of confidential information. The PRC Personal Information Protection Law, which was promulgated by the SCNPC on August 20, 2021 and took effect on November 1, 2021, integrates the scattered rules with respect to personal information rights and privacy protection and applies to the processing of personal information within China as well as certain personal information processing activities outside China, including those for the provision of products and services to natural persons within China or for the analysis and assessment of acts of natural persons within China. There remain uncertainties regarding the further interpretation and implementation of those laws and regulations, if they are deemed to be applicable to us, we cannot assure you that we will be compliant with such new regulations in all respects, and we may be ordered to rectify and terminate any actions that are deemed illegal by the government authorities and become subject to fines and other government sanctions, which may materially and adversely affect our business, financial condition, and results of operations.
17
Recent greater oversight by the Cyberspace Administration of China over data security, particularly for companies seeking to list on a foreign exchange, could adversely impact our business and operations.
On December 28, 2021, the CAC and other relevant PRC governmental authorities jointly promulgated the Cybersecurity Review Measures, which took effect on February 15, 2022. The Cybersecurity Review Measures provide that, in addition to critical information infrastructure operators (the “CIIOs”) that intend to purchase Internet products and services, net platform operators engaging in data processing activities that affect or may affect national security must be subject to cybersecurity review by the Cybersecurity Review Office of the PRC. According to the Cybersecurity Review Measures, a cybersecurity review assesses potential national security risks that may be brought about by any procurement, data processing, or overseas listing. The Cybersecurity Review Measures require that an online platform operator which possesses the personal information of at least one million users must apply for a cybersecurity review by the CAC if it intends to be listed in foreign countries.
On November 14, 2021, the CAC published the Security Administration Draft, which provides that data processing operators engaging in data processing activities that affect or may affect national security must be subject to network data security review by the relevant Cyberspace Administration of the PRC. According to the Security Administration Draft, data processing operators who possess personal data of at least one million users or collect data that affects or may affect national security must be subject to network data security review by the relevant Cyberspace Administration of the PRC.
As of the date of this annual report, we have not received any notice from any authorities identifying our PRC subsidiaries or the PRC operating entities as CIIOs or requiring us to go through cybersecurity review or network data security review by the CAC. As confirmed by our PRC counsel, AllBright Law Offices (Fuzhou), neither the operations of our PRC subsidiaries, nor of the PRC operating entities are expected to be affected, and that we will not be subject to cybersecurity review by the CAC under the Cybersecurity Review Measures, nor will any such entity be subject to the Security Administration Draft, if it is enacted as proposed, given that our PRC subsidiaries and the PRC operating entities possess personal data of fewer than one million individual clients and do not collect data that affects or may affect national security in their business operations as of the date of this annual report and do not anticipate that they will be collecting over one million users’ personal information or data that affects or may affect national security in the near future. In general, we believe we are compliant with the regulations or policies that have been issued by the CAC to date. There remains uncertainty, however, as to how the Cybersecurity Review Measures and the Security Administration Draft will be interpreted or implemented and whether the PRC regulatory agencies , including the CAC, may adopt new laws, regulations, rules, or detailed implementation and interpretation related to the Cybersecurity Review Measures and the Security Administration Draft. If any such new laws, regulations, rules, or implementation and interpretation come into effect, we will take all reasonable measures and actions to comply and to minimize the adverse effect of such laws on us. We cannot assure you that PRC regulatory agencies, including the CAC, would take the same view as we do. In the event that we are subject to any mandatory cybersecurity review and other specific actions required by the CAC, we face uncertainty as to whether any clearance or other required actions can be timely completed, or at all. If we inadvertently conclude that such approval is not required, fail to obtain and maintain such approvals, licenses, or permits required for our business or respond to changes in the regulatory environment, we could be subject to liabilities, penalties and operational disruption, which may materially and adversely affect our business, operating results, financial condition, and the value of our securities, significantly limit or completely hinder our ability to offer or continue to offer securities to investors, or cause such securities to significantly decline in value or become worthless.
The approval and/or other requirements of the China Securities Regulatory Commission, or the CSRC, or other PRC governmental authorities may be required in connection with an offering under PRC rules, regulations or policies, and, if required, we cannot predict whether or how soon we will be able to obtain such approval.
The Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the M&A Rules, purport to require offshore special purpose vehicles that are controlled by PRC companies or individuals and that have been formed for the purpose of seeking a public listing on an overseas stock exchange through acquisitions of PRC domestic companies or assets to obtain CSRC approval prior to publicly listing their securities on an overseas stock exchange. The interpretation and application of the regulations remain unclear. If a governmental approval is required, it is uncertain how long it will take for us to obtain such approval, and, even if we obtain such approval, the approval could be rescinded. Any failure or delay in obtaining the requisite governmental approval for an offering, or a rescission of such CSRC approval, if obtained by us, may subject us to sanctions imposed by the relevant PRC regulatory authority, which could include fines and penalties on our operations in China, restrictions or limitations on our ability to pay dividends outside of China, and other forms of sanctions that may materially and adversely affect our business, financial condition, and results of operations.
18
On February 17, 2023, the CSRC issued the Trial Administrative Measures of Overseas Securities Offering and Listing by Domestic Companies, or the Overseas Listing Regulations, and five supporting guidelines, which became effective on March 31, 2023. Pursuant to the Overseas Listing Regulations, companies in mainland China that directly or indirectly offer or list their securities in an overseas market, including a company in mainland China limited by shares and an offshore company whose main business operations are in mainland China and intends to offer shares or be listed in an overseas market based on its equities, assets or similar interests in mainland China are required to file with the CSRC within three business days after submitting their listing application documents to the regulator in the place of intended listing. If the company fails to complete the filing procedure or conceals any material fact or falsifies any major content in its filing documents, it may be subject to administrative penalties, such as order to rectify, warnings, fines, and its controlling shareholders, actual controllers, the person directly in charge and other directly liable persons may also be subject to administrative penalties, such as warnings and fines. The Overseas Listing Regulations also provide that a company in mainland China must file with the CSRC within three business days for its follow on offering of securities after it is listed in an overseas market. On February 17, 2023, the CSRC also issued the Notice on Administration of the Filing of Overseas Offering and Listing by Domestic Companies and held a press conference for the release of the Overseas Listing Regulations, which, among others, clarified that the companies in mainland China that have been listed overseas before March 31, 2023 are not required to file with the CSRC immediately, but these companies should complete filing with the CSRC for their refinancing activities in accordance with the Overseas Listing Regulations. A fine between RMB1 million (approximately $157,255) and RMB10 million (approximately $1,572,550) may be imposed if an applicant fails to fulfill the filing requirements with the CSRC or conducts an overseas offering or listing in violation of the Overseas Listings Regulations, and in cases of severe violations, a parallel order to suspend relevant businesses or halt operations for rectification may be issued, and relevant business permits or operational license revoked.
On February 24, 2023, the CSRC, jointly with other relevant governmental authorities, published the Provisions on Strengthening Confidentiality and Archives Management of Overseas Securities Issuance and Listing by Domestic Enterprises, or the Confidentiality and Archives Management Provisions, which became effective on March 31, 2023. Pursuant to the Confidentiality and Archives Management Provisions, China-based companies that offer and list securities in overseas markets shall establish confidentiality and archives system. The “China-based companies” refer to companies in mainland China limited by shares which are directly listed on a foreign stock exchange and the domestic operating entities of an offshore company being indirectly listed on a foreign stock exchange. These China-based companies shall obtain the approvals from relevant authorities and file with the competent confidential administration authorities when providing or publicly filing documents and materials related to state secrets or secrets of the government authorities to the relevant securities companies, securities service agencies or the offshore regulatory authorities, or providing or publicly filing such documents and materials through its offshore listing entity. In addition, China-based companies shall complete corresponding procedures when (i) providing or publicly filing documents and materials which may adversely affect national security and public interests to the relevant securities companies, securities service agencies or the offshore regulatory authorities, (ii) providing or publicly filing such documents and materials through its offshore listing entity, or (iii) providing accounting files or copies to relevant securities companies, securities service institutions, overseas regulators and individuals. These China-based companies are also required to provide written statements as to whether they have completed the approval or filing procedures as above when providing documents and materials to securities companies and securities service providers, and the securities companies and securities service providers should properly retain such written statements for inspection. If a China-based company finds that the documents and materials related to state secrets or secrets of the government authorities or other materials, which may adversely affect national security and public interests, have been leaked or have leakage risks, it should take remedial measures immediately and report to the relevant authorities.
In the opinion of our PRC legal counsel, AllBright Law Offices (Fuzhou), as a domestic company listed on Nasdaq since March 2021, and not currently conducting refinancing or other activities that require filings, we are not required to file with the CSRC in accordance with the Overseas Listing Regulations at this time. However, in the event that we conduct subsequent offerings, we could be subject to filing requirements with the CSRC. In the event that filings with the CSRC are required, we cannot assure you that we can complete the filing procedures, obtain the approvals or complete other compliance procedures in a timely manner, or at all, or that any completion of filing or approval or other compliance procedures would not be rescinded. Any such failure would subject us to sanctions by the CSRC or other PRC regulatory authorities. These regulatory authorities may impose restrictions and penalties on the operations in China, significantly limit or completely hinder our ability to launch any new offering of our securities, limit our ability to pay dividends outside of China, delay or restrict the repatriation of the proceeds from future capital raising activities into China, or take other actions that could materially and adversely affect our business, results of operations, financial condition and prospects, as well as the trading price of our ordinary shares. Furthermore, the PRC government authorities may further strengthen oversight and control over listings and offerings that are conducted overseas. Any such action may adversely affect our operations and significantly limit or completely hinder our ability to offer or continue to offer securities to you and cause the value of such securities to significantly decline or be worthless.
Furthermore, the governmental authorities may impose restrictions and penalties on our operations in China, such as the suspension of our apps and services, revocation of our licenses, shutting down part or all of our operations, limiting our ability to pay dividends outside of China, delaying or restricting the repatriation of the proceeds from an offering into China, or may take other actions that could have a material adverse effect on our business, financial condition, results of operations and prospects, as well as the trading price of the ordinary shares. The PRC governmental authorities may also take actions requiring us, or making it advisable for us, to halt an offering before settlement and delivery of the ordinary shares offered hereby. Consequently, if you engage in market trading or other activities in anticipation of and prior to settlement and delivery, you do so at the risk that settlement and delivery may not occur. In addition, if the PRC governmental authorities later promulgate new rules or requirements that we obtain their approvals for filings, registrations or other kinds of authorizations for an offering, we cannot assure you that we can obtain the approval, authorizations, or complete required procedures or other requirements in a timely manner, or at all, or obtain a waiver of the requisite requirements if and when procedures are established to obtain such a waiver.
19
PRC regulation of loans to, and direct investments in, PRC entities by offshore holding companies may delay or prevent us from using proceeds from our offerings and/or other financing activities to make loans or additional capital contributions to our PRC operating subsidiaries.
As an offshore holding company with PRC subsidiaries, we may transfer funds to our PRC subsidiaries or finance our operating entity by means of loans or capital contributions. Any capital contributions or loans that we, as an offshore entity, make to our Company’s PRC subsidiaries are subject to PRC regulations. Any loans to our PRC subsidiaries, which are foreign-invested enterprises, cannot exceed statutory limits based on the difference between the amount of our investments and registered capital in such subsidiaries, and shall be registered with China’s State Administration of Foreign Exchange (“SAFE”), or its local counterparts. Furthermore, any capital increase contributions we make to our PRC subsidiaries, which are foreign-invested enterprises, are subject to the requirement of making necessary filings in the Foreign Investment Comprehensive Management Information System, or FICMIS, and registration with other government authorities in China. We may not be able to obtain these government registrations or approvals on a timely basis, if at all. If we fail to obtain such approvals or make such registration, our ability to make equity contributions or provide loans to our Company’s PRC subsidiaries or to fund their operations may be negatively affected, which may adversely affect their liquidity and ability to fund their working capital and expansion projects and meet their obligations and commitments. As a result, our liquidity and our ability to fund and expand our business may be negatively affected.
We must remit proceeds of any future offerings to China before they may be used to benefit our business in China, and this process may take several months to complete.
The proceeds of our future offerings must be sent back to China, and the process for sending such proceeds back to China may take as long as six months after the closing of an offering. As an offshore holding company of our PRC operating subsidiaries, we may make loans to our PRC subsidiaries, or we may make additional capital contributions to our PRC subsidiaries. Any loans to our PRC subsidiaries are subject to PRC regulations. For example, loans by us to our subsidiaries in China, which are foreign-invested enterprises, to finance their activities cannot exceed statutory limits and must be registered with SAFE.
To remit the proceeds of our offerings, we must take the following steps:
| ● | First, we will open a special foreign exchange account for capital account transactions. To open this account, we must submit to SAFE certain application forms, identity documents, transaction documents, form of foreign exchange registration of overseas investments of the domestic residents, and foreign exchange registration certificate of the invested company. | |
| ● | Second, we will remit the offering proceeds into this special foreign exchange account. | |
| ● | Third, we will apply for settlement of the foreign exchange. In order to do so, we must submit to SAFE certain application forms, identity documents, payment order to a designated person, and a tax certificate. |
The timing of the process is difficult to estimate because the efficiencies of different SAFE branches can vary significantly. Ordinarily the process takes several months but is required by law to be accomplished within 180 days of application.
We may also decide to finance our subsidiaries by means of capital contributions. These capital contributions must be subject to the requirement of making necessary filings in the FICMIS, and registration with other government authorities in China. We cannot assure you that we will be able to obtain these government approvals on a timely basis, if at all, with respect to future capital contributions by us to our subsidiaries. If we fail to receive such approvals, our ability to use the proceeds of any future offerings and to capitalize our Chinese operations may be negatively affected, which could adversely affect our liquidity and our ability to fund and expand our business. If we fail to receive such approvals, our ability to use the proceeds of our future offerings and to capitalize our Chinese operations may be negatively affected, which could adversely affect our liquidity and our ability to fund and expand our business.
20
We may rely on dividends paid by our subsidiaries for our cash needs, and any limitation on the ability of our subsidiaries to make payments to us could have a material adverse effect on our ability to conduct business.
As a holding company, we conduct all of our business through the PRC operating entities incorporated in China. We may rely on dividends paid by these PRC subsidiaries for our cash needs, including the funds necessary to pay any dividends and other cash distributions to our shareholders, to service any debt we may incur and to pay our operating expenses. The payment of dividends by entities established in China is subject to limitations. Regulations in China currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in China. In accordance with the Article 166, 168 of the Company Law of the PRC (Amended in 2018) (the “PRC Company Law”), each of our PRC subsidiaries is required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year to its general reserves or statutory capital reserve fund until the aggregate amount of such reserves reaches 50% of its respective registered capital. A company may discontinue the contribution when the aggregate sum of the statutory surplus reserve is more than 50% of its registered capital. The statutory common reserve fund of a company shall be used to cover the losses of the company, expand the business and production of the company or be converted into additional capital. As a result, our PRC subsidiaries are restricted in their ability to transfer a portion of their net assets to us in the form of dividends. In addition, if any of our PRC subsidiaries incurs debt on its own behalf in the future, the instruments governing the debt may restrict its ability to pay dividends or make other distributions to us. Any limitations on the ability of our PRC subsidiaries to transfer funds to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends and otherwise fund and conduct our business.
Adverse changes in political, economic and social conditions, as well as government policies in China could have a material adverse effect on our business results of operations, financial conditions and prospects.
All of our business operations are conducted in China. Accordingly, our financial condition, results of operations and prospects are, to a material extent, subject to economic, political and legal developments in China. The economy in China differs from the economies of developed countries in many respects, including, among other things, the degree of government involvement, control of investment, level of economic development, growth rate, foreign exchange controls and resource allocation. Although the economy in China has been transitioning from a planned economy to a more market-oriented economy for about four decades, a substantial portion of productive assets in China is still owned by the PRC government. The PRC government also exercises significant control over the economic growth of China through allocating resources, controlling payments of foreign currency denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. In recent years, the PRC government has implemented measures emphasizing the utilization of market forces in economic reform, the reduction of state ownership of productive assets and the establishment of sound corporate governance practices in business enterprises. Some of these measures benefit the overall economy in China, but may adversely affect us. For example, our financial condition and results of operations may be adversely affected by government policies on the Chinese patent medicine industry in China or changes in tax regulations applicable to us. If the business environment in China deteriorates, our business in China may also be materially and adversely affected.
Changes to the PRC legal system could have an adverse effect on us.
The PRC legal system is a civil law system based on written statutes. Unlike the common law system, prior court decisions under the civil law system may be cited for reference but have limited precedential value. Since these laws and regulations are relatively new and the PRC legal system continues to rapidly evolve, the interpretations of many laws, regulations and rules are not always uniform and the enforcement of these laws, regulations and rules involves uncertainties.
In 1979, the PRC government began to promulgate a comprehensive system of laws and regulations governing economic matters in general. The overall effect of legislation over the past decades has significantly enhanced the protections afforded to various forms of foreign investments in China. However, recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in China. In particular, the interpretation and enforcement of these laws and regulations involve uncertainties. Since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory provisions and contractual terms, it may be difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy. These uncertainties may affect our judgment on the relevance of legal requirements and our ability to enforce our contractual rights or tort claims.
21
Furthermore, the PRC legal system is based in part on government policies and internal rules, some of which are not published on a timely basis or at all and may have retroactive effect. As a result, we may not be aware of our violation of any of these policies and rules until sometime after the violation. In addition, any administrative and court proceedings in China may be protracted, resulting in substantial costs and diversion of resources and management attention.
Labor Contract Law and other labor-related laws in the PRC may adversely affect our business and our results of operations.
On December 28, 2012, the PRC government released the revision of the Labor Contract Law, which became effective on July 1, 2013. Pursuant to the Labor Contract Law, employers are subject to stricter requirements in terms of signing labor contracts, minimum wages, paying remuneration, determining the term of employees’ probation and unilaterally terminating labor contracts. In the event that we decide to terminate some of our employees or otherwise change our employment or labor practices, the Labor Contract Law and its implementation rules may limit our ability to effect those changes in a desirable or cost-effective manner, which could adversely affect our business and results of operations. According to the PRC Social Insurance Law (《中华人民共和国社会保险法》), employees must participate in pension insurance, work-related injury insurance, medical insurance, unemployment insurance and maternity insurance and the employers must, together with their employees or separately, pay the social insurance premiums for such employees. In accordance with the Regulations on the Management of Housing Fund which was promulgated by the State Council in 1999 and amended in 2002 and 2019, employers must register at the designated administrative centers and open bank accounts for depositing employees’ housing funds. As the interpretation and implementation of labor-related laws and regulations are still evolving, we cannot assure you that our employment practices do not and will not violate labor-related laws and regulations in China, which may subject us to labor disputes or government investigations. As of the date of this annual report, we believe that we are in substantial compliance with labor-related laws and regulations in China, and we have not been notified of any instance of noncompliance. We cannot assure you that we will be able to comply with all labor-related law and regulations regarding including those relating to obligations to make social insurance payments and contribute to the housing provident fund. If we are deemed to have violated relevant labor laws and regulations, we could be required to provide additional compensation to our employees and our business, financial condition and results of operations will be adversely affected.
There are significant uncertainties under the Enterprise Income Tax Law, or the EIT Law, relating to the withholding tax liabilities of our PRC subsidiaries, and dividends payable by our PRC subsidiaries to our offshore subsidiaries may not qualify to enjoy certain treaty benefits.
China passed the EIT Law, and it is implementing rules, both of which became effective on January 1, 2008. Under the PRC EIT Law and its implementation rules, the profits of an FIE generated through operations, which are distributed to its immediate holding company outside the PRC, will be subject to a withholding tax rate of 10%. Pursuant to a special arrangement between Hong Kong and the PRC, such rate may be reduced to 5% if a Hong Kong resident enterprise owns more than 25% of the equity interest in the PRC company. Our PRC subsidiaries, Universe Technology, Jiangxi Universe and Universe Trade, are wholly-owned by our Hong Kong subsidiary. Moreover, under the Notice of the State Administration of Taxation on Issues regarding the Administration of the Dividend Provision in Tax Treaties promulgated on February 20, 2009, the taxpayer needs to satisfy certain conditions to enjoy the benefits under a tax treaty. The beneficial owner of the relevant dividends and the corporate shareholder to receive dividends from the PRC subsidiary must have continuously met the direct ownership thresholds during the 12 consecutive months preceding the receipt of the dividends. Further, the State Administration of Taxation (the “SAT”) promulgated the Notice on How to Comprehend and Determine the “Beneficial Owner” in Tax Treaties (《国家税务总局关于税收协定中”受益所有人”有关问题的公告》) on February 3, 2018, which limits the “beneficial owner” to individuals, projects or other organizations normally engaged in substantive operations, and sets forth certain detailed factors in determining the “beneficial owner” status. In current practice, a Hong Kong enterprise must obtain a tax resident certificate from the relevant Hong Kong tax authority to apply for the 5% lower PRC withholding tax rate. As the Hong Kong tax authority will issue such a tax resident certificate on a case-by-case basis, we cannot assure you that we will be able to obtain the tax resident certificate from the relevant Hong Kong tax authority. As of the date of this annual report, we have not commenced the application process for a Hong Kong tax resident certificate from the relevant Hong Kong tax authority, and there is no assurance that we will be granted such a Hong Kong tax resident certificate.
22
Even after we obtain the Hong Kong tax resident certificate, we are required by applicable tax laws and regulations to file required forms and materials with relevant PRC tax authorities to prove that we can enjoy 5% lower PRC withholding tax rate. Universe HK intends to obtain the required materials and file with the relevant tax authorities when it plans to declare and pay dividends, but there is no assurance that the PRC tax authorities will approve the 5% withholding tax rate on dividends received from Universe HK.
Failure to qualify for or obtain any preferential tax treatments that are available in China could adversely affect our results of operations and financial condition.
The EIT Law and its implementation rules generally impose a uniform income tax rate of 25% on all enterprises, but grant preferential treatment to “high and new technology enterprises strongly supported by the state,” or HNTEs, with a preferential enterprise tax rate of 15%. Our subsidiaries, Jiangxi Universe and Universe Trade, were accredited as HNTE and enjoy the reduced income tax rate of 15% for three years through November 2025 and December 2023, respectively. According to the relevant administrative measures, to qualify as an “HNTE,” a company must meet certain financial and non-financial criteria and complete verification procedures with the administrative authorities. Continued qualification as an HNTE is subject to review by the relevant government authorities in China every three years, and in practice, certain local tax authorities may require annual evaluation of the qualification. Universe Trade did not successfully renew its HNTE status at the end of December 2022 and, therefore, will be subject to the standard PRC enterprise income tax rate of 25% starting from January 2024. In the event that Jiangxi Universe fails to renew its status as HNTE with the local tax authority upon expiration of its HNTE status in November 2025, it will be subject to the standard PRC enterprise income tax rate of 25%.
Under the EIT Law, we may be classified as a “Resident Enterprise” of China. Such classification will likely result in unfavorable tax consequences to us and our non-PRC shareholders.
The EIT Law and its implementing rules became effective on December 9, 2019. Under the EIT Law, an enterprise established outside of China with “de facto management bodies” within China is considered a “resident enterprise,” meaning that it can be treated in a manner similar to a Chinese enterprise for enterprise income tax purposes. The implementing rules of the EIT Law define de facto management as “substantial and overall management and control over the production and operations, personnel, accounting, and properties” of the enterprise.
If the PRC tax authorities determine that we are a “resident enterprise” for PRC enterprise income tax purposes, a number of unfavorable PRC tax consequences could follow. First, we may be subject to the enterprise income tax at a rate of 25% on our worldwide taxable income as well as PRC enterprise income tax reporting obligations. In our case, this would mean that income such as non-China source income would be subject to PRC enterprise income tax at a rate of 25%. Currently, we do not have any non-China source income, as we conduct our sales in China. Second, under the EIT Law and its implementing rules, dividends paid to us from our PRC subsidiaries would be deemed as “qualified investment income between resident enterprises” and therefore qualify as “tax-exempt income” pursuant to the clause 26 of the EIT Law. Finally, it is possible that future guidance issued with respect to the new “resident enterprise” classification could result in a situation in which the dividends we pay with respect to our ordinary shares, or the gain our non-PRC shareholders may realize from the transfer of our ordinary shares, may be treated as PRC-sourced income and may therefore be subject to a 10% PRC withholding tax. The EIT Law and its implementing regulations are, however, relatively new and ambiguities exist with respect to the interpretation and identification of PRC-sourced income, and the application and assessment of withholding taxes. If we are required under the EIT Law and its implementing regulations to withhold PRC income tax on dividends payable to our non-PRC shareholders, or if non-PRC shareholders are required to pay PRC income tax on gains on the transfer of their ordinary shares, our business could be negatively impacted and the value of your investment may be materially reduced. Further, if we were to be treated as a “resident enterprise” by PRC tax authorities, we would be subject to taxation in both China and such countries in which we have taxable income, and our PRC tax may not be creditable against such other taxes.
To the extent cash in and assets of the business is in the PRC or a PRC entity, the funds and assets may not be available to fund operations or for other use outside of the PRC due to interventions in or the imposition of restrictions and limitations on the ability of our Company or our subsidiaries by the PRC government to transfer cash and assets.
Relevant PRC laws and regulations permit the companies in mainland China to pay dividends only out of their retained earnings, if any, as determined in accordance with PRC accounting standards and regulations. Additionally, each of the companies in mainland China are required to set aside at least 10% of its after-tax profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of its registered capital. The companies in mainland China are also required to further set aside a portion of their after-tax profits to fund the employee welfare fund, although the amount to be set aside, if any, is determined at their discretion. These reserves are not distributable as cash dividends. Furthermore, if we determine to pay dividends on any of our ordinary shares in the future, as a holding company, we will rely on payments from subsidiaries of Jiangxi Universe to Jiangxi Universe, and from Jiangxi Universe to Universe Technology, and the distribution of such payments to Universe HK, and then to our Company. If our PRC subsidiaries and the PRC operating entities incur debt on their own behalf in the future, the instruments governing the debt may restrict their ability to pay dividends or make other payments to us.
Our cash dividends, if any, will be paid in U.S. dollars. If we are considered a tax resident enterprise of mainland China for tax purposes, any dividends we pay to our overseas shareholders may be regarded as China-sourced income and as a result may be subject to PRC withholding tax. See “—Under the EIT Law, we may be classified as a ‘Resident Enterprise’ of China. Such classification will likely result in unfavorable tax consequences to us and our non-PRC shareholders.”
23
The PRC government also imposes controls on the convertibility of Renminbi into foreign currencies and, in certain cases, the remittance of currency out of mainland China. The majority of our and the PRC operating entities’ income is received in Renminbi and shortages in foreign currencies may restrict our ability to pay dividends or other payments, or otherwise satisfy our foreign currency denominated obligations, if any. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and expenditures from trade-related transactions, can be made in foreign currencies without prior approval from the State Administration of Foreign Exchange as long as certain procedural requirements are met. Approval from appropriate government authorities is required if Renminbi is converted into foreign currency and remitted out of mainland China to pay capital expenses such as the repayment of loans denominated in foreign currencies. The PRC government may, at its discretion, impose restrictions on access to foreign currencies for current account transactions and if this occurs in the future, we may not be able to pay dividends in foreign currencies to our shareholders.
Any limitation on the ability of our PRC subsidiaries and the PRC operating entities to distribute dividends or other payments to their respective shareholders could materially and adversely limit our ability to conduct operations, make investments, engage in acquisitions, or undertake other activities requiring working capital. However, our operations and business, including investment and/or acquisitions by our PRC subsidiaries and the PRC operating entities within mainland China, will not be affected as long as the capital is not transferred in or out of mainland China.
We may be exposed to liabilities under the Foreign Corrupt Practices Act and Chinese anti-corruption law.
We are subject to the U.S. Foreign Corrupt Practices Act (the “FCPA”), and other laws that prohibit improper payments or offers of payments to foreign governments and their officials and political parties by U.S. persons and issuers, as defined by the statute, for the purpose of obtaining or retaining business. We are also subject to Chinese anti-corruption laws, which strictly prohibit the payment of bribes to government officials. We have operations, agreements with third parties, and make sales in China, which may expose us to claims of corruption. Our activities in China create the risk of unauthorized payments or offers of payments by one of the employees of our Company, because these parties are not always subject to our control.
Although we believe that, as of the date of this annual report, we have complied in all material respects with the provisions of the FCPA and Chinese anti-corruption law, our existing safeguards and any future improvements may prove to be less than effective, and the employees of our Company may engage in conduct for which we might be held responsible. Violations of the FCPA or Chinese anti-corruption law may result in severe criminal or civil sanctions, and we may be subject to other liabilities, which could negatively affect our business, operating results and financial condition. In addition, the government may seek to hold our Company liable for successor liability FCPA violations committed by companies in which we invest or that we acquire.
The enforcement of stricter advertisement laws and regulations in the PRC may adversely affect our business and our profitability.
In October 2018, the SCNPC promulgated the PRC Advertising Law, effective on October 26, 2018. According to the Advertising Law, advertisements shall not have any false or misleading content, or defraud or mislead consumers. Furthermore, an advertisement will be deemed as a “false advertisement” if any of the following situations exist: (i) the advertised product or service does not exist; (ii) there is any inconsistency that has a material impact on the decision to purchase in what is included in the advertisement with the actual circumstances with respect to the product’s performance, functions, place of production, uses, quality, specification, ingredient, price, producer, term of validity, sales condition, and honors received, among others, or the service’s contents, provider, form, quality, price, sales condition, and honors received, among others, or any commitments, among others, made on the product or service; (iii) fabricated, forged or unverifiable scientific research results, statistical data, investigation results, excerpts, quotations, or other information have been used as supporting material; (iv) effect or results of using the good or receiving the service are fabricated; or (v) other circumstances where consumers are defrauded or misled by any false or misleading content.
Our current marketing relies on advertisements on media platforms. The laws and regulations of advertising are relatively new and evolving and there is substantial uncertainty as to the interpretation of “false advertisement” by the State Administration for Industry and Commerce of the PRC (the “SAIC”). If any of the advertisements published by our customers is deemed to be a “false advertisement” by the SAIC or its local branch, we could be subject to various penalties, such as discontinuation of publishing the target advertisement, imposition of fines and obligations to eliminate any adverse effects incurred by such false advertisement. Any such penalties may disrupt our business and our competition with competitors, and could affect our results of operations and financial conditions.
We were not in compliance with the PRC’s regulations relating to employee’s social insurance and housing funds prior to April 2020, and as a result, we may be subject to penalties for such non-compliance.
Pursuant to the Social Insurance Law of the PRC (the “Social Insurance Law”), which was promulgated by the SCNPC on October 28, 2010 and amended on December 29, 2018, and the Administrative Regulations on the Housing Provident Funds, which was promulgated by the State Council on April 3, 1999 and last amended on March 24, 2019, employers are required to make contributions, on behalf of their employees, to a number of social security funds, including funds for basic pension insurance, unemployment insurance, basic medical insurance, occupational injury insurance, maternity insurance and to housing provident funds. Prior to April 2020, we only contributed to the social insurance and housing provident funds for some, but not all, of our employees. Since April 2020, we have started contributing to the social insurance and housing funds for our eligible full-time employees in accordance with the aforementioned PRC laws and regulations. Even though we are currently making contributions in accordance with applicable PRC laws, there is a risk that the labor security administration authority may take enforcement action to collect from us all the outstanding contributions of the social insurance required to be made for the employees in the past, and we may be subject to a late charge at the rate of 0.05% per day on the total outstanding contribution.
24
U.S. regulatory bodies may be limited in their ability to conduct investigations or inspections of our operations in China.
The U.S. Securities and Exchange Commission (the “SEC”), the U.S. Department of Justice and other U.S. authorities may also have difficulties in bringing and enforcing actions against us or our directors or executive officers in the PRC. The SEC has stated that there are significant legal and other obstacles to obtaining information needed for investigations or litigation in China. Although the authorities in China may establish a regulatory cooperation mechanism with the securities regulatory authorities of another country or region to implement cross-border supervision and administration, such cooperation with the securities regulatory authorities in Hong Kong or other jurisdictions may not be efficient in the absence of mutual and practical cooperation mechanism. Furthermore, China has recently adopted a revised securities law that became effective on March 1, 2020, Article 177 of which provides, among other things, that no overseas securities regulator is allowed to directly conduct investigation or evidence collection activities within the territory of the PRC. Accordingly, without governmental approval in China, no entity or individual in China may provide documents and information relating to securities business activities to overseas regulators when it is under direct investigation or evidence discovery conducted by overseas regulators. While detailed interpretation of or implementation rules under Article 177 have yet to be promulgated, it could present significant legal and other obstacles to obtaining information needed for investigations and litigation conducted outside of China, which may further increase difficulties faced by you in protecting your interests. See also “—You may experience difficulty in effecting service of process, enforcing foreign judgments or bringing actions against our directors and officers.”
You may experience difficulty in effecting service of process, enforcing foreign judgments or bringing actions against our directors and officers.
We are a Cayman Islands exempted company with limited liability and most of our assets are located outside of the United States. In addition, almost all of our directors and officers are nationals or residents of the PRC, including our chief executive officer and chairman of the board of directors, Mr. Gang Lai, our chief financial officer, Ms. Lin Yang, and our directors, Mr. Jiawen Pang, Mr. Yongping Yu and Mr. Ding Zheng, and all or a substantial portion of their assets are located outside the United States. As a result, it may be difficult or impossible for you to effect service of process within the United States upon our directors and executive officers. It may also be difficult for you to enforce in the United States courts judgments obtained in the United States courts based on the civil liability provisions of the United States federal securities laws against us and our officers and directors who reside and whose assets are located outside the United States.
We have been advised by our Cayman Islands legal counsel, Ogier (Cayman) LLP, that the courts of the Cayman Islands are unlikely (i) to recognize or enforce against us, judgments of courts of the United States obtained against us or our directors or officers predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States; and (ii) in original actions brought in the Cayman Islands, to impose liabilities against us or our directors or officers predicated upon the civil liability provisions of the securities laws of the United States or any state in the United States, so far as the liabilities imposed by those provisions are penal in nature. In those circumstances, although there is currently no statutory enforcement or treaty between the United States and the Cayman Islands providing for enforcement of judgments obtained in the United States. The courts of the Cayman Islands will recognize and enforce a foreign money judgment of a foreign court of competent jurisdiction without retrial on the merits based on the principle that a judgment of a competent foreign court imposes upon the judgment debtor an obligation to pay the sum for which judgment has been given provided certain conditions are met. For a foreign judgment to be enforced in the Cayman Islands, such judgment must be final and conclusive, given by a court of competent jurisdiction (the courts of the Cayman Islands will apply the rules of Cayman Islands private international law to determine whether the foreign court is a court of competent jurisdiction), and must not be in respect of taxes or a fine or penalty, inconsistent with a Cayman Islands judgment in respect of the same matter, impeachable on the grounds of fraud or obtained in a manner, and or be of a kind the enforcement of which is, contrary to natural justice or the public policy of the Cayman Islands. Furthermore, it is uncertain that Cayman Islands courts would enforce: (1) judgments of U.S. courts obtained in actions against us or other persons that are predicated upon the civil liability provisions of the U.S. federal securities laws; or (2) original actions brought against us or other persons predicated upon the Securities Act. Ogier has informed us that there is uncertainty with regard to Cayman Islands law relating to whether a judgment obtained from the U.S. courts under civil liability provisions of the securities laws will be determined by the courts of the Cayman Islands as penal, punitive in nature. A Cayman Islands Court may stay enforcement proceedings if concurrent proceedings are being brought elsewhere.
In addition, there is uncertainty as to whether the courts of the PRC would recognize or enforce judgments of the United States courts against us or such persons predicated upon the civil liability provisions of the securities laws of the United States or any state. The recognition and enforcement of foreign judgments are provided for under the PRC Civil Procedures Law. PRC courts may recognize and enforce foreign judgments in accordance with the requirements of the PRC Civil Procedures Law based either on treaties between China and the country where the judgment is made or on principles of reciprocity between jurisdictions. China does not have any treaties or other forms of reciprocity with the United States that provide for the reciprocal recognition and enforcement of foreign judgments. In addition, according to the PRC Civil Procedures Law, the PRC courts will not enforce a foreign judgment against us or our directors and officers if they decide that the judgment violates the basic principles of PRC laws or national sovereignty, security or public interest. As a result, it is uncertain whether and on what basis a PRC court would enforce a judgment rendered by a court in the United States.
Further, pursuant to the PRC Civil Procedures Law, any matter, including matters arising under U.S. federal securities laws, in relation to assets or personal relationships may be brought as an original action in China, only if the institution of such action satisfies the conditions specified in the PRC Civil Procedures Law. As a result of the conditions set forth in the PRC Civil Procedures Law and the discretion of the PRC courts to determine whether the conditions are satisfied and whether to accept the action for adjudication, there remains uncertainty as to whether an investor will be able to bring an original action in a PRC court based on U.S. federal securities laws.
25
Because our business is conducted in the RMB and the price of our ordinary shares is quoted in United States dollars, changes in currency conversion rates may affect the value of your investments.
Our business is conducted in the PRC, our books and records are maintained in the RMB, the legal currency of the PRC, and the financial statements that we file with the SEC and provide to our shareholders are presented in United States dollars. Changes in the exchange rate between the RMB and U.S. dollars affect the value of our assets and the results of our operations in U.S. dollars. The value of the RMB against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in the PRC’s political and economic conditions and perceived changes in the economy of the PRC and the United States. Any significant revaluation of the RMB may materially and adversely affect our cash flows, revenue and financial condition. Further, our securities will be offered in United States dollars, and we will need to convert any net proceeds we receive into RMB in order to use the funds for our business. Changes in the conversion rate between the United States dollar and the RMB will affect that amount of proceeds we will have available for our business.
Government control in currency conversion may adversely affect our financial condition, our ability to remit dividends, and the value of your investment.
The PRC government imposes controls on the convertibility of the Renminbi into foreign currencies and, in certain cases, the remittance of currency out of China. We receive substantially all of our revenues in Renminbi. Under our current corporate structure, our Cayman Islands holding company may rely on dividend payments from our PRC subsidiaries to fund any cash and financing requirements we may have.
Under existing PRC foreign exchange regulations, the Renminbi cannot be freely converted into any foreign currency, and conversion and remittance of foreign currencies are subject to PRC foreign exchange regulations. It cannot be guaranteed that under a certain exchange rate, we will have sufficient foreign exchange to meet our foreign exchange requirements. Under the current PRC foreign exchange control system, foreign exchange transactions under the current account conducted by us, including the payment of dividends, do not require advance approval from SAFE, but we are required to present documentary evidence of such transactions and conduct such transactions at designated foreign exchange banks within China that have the licenses to carry out foreign exchange business. Foreign exchange transactions under the capital account conducted by us, however, must be approved in advance by SAFE.
Under existing foreign exchange regulations, we are able to pay dividends in foreign currencies without prior approval from SAFE by complying with certain procedural requirements. However, we cannot assure you that these foreign exchange policies regarding payment of dividends in foreign currencies will continue in the future.
In fact, in light of the flood of capital outflows of China in 2016 due to the weakening Renminbi, the PRC government has imposed more restrictive foreign exchange policies and stepped up scrutiny of major outbound capital movement including overseas direct investment. More restrictions and substantial vetting process may be put in place by SAFE to regulate cross-border transactions falling under the capital account. If any of our shareholders regulated by such policies fails to satisfy the applicable overseas direct investment filing or approval requirement timely or at all, it may be subject to penalties from the relevant PRC authorities. The PRC government may, at its discretion, further restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currencies to satisfy our foreign currency demands, we may not be able to pay dividends in foreign currencies to our shareholders, including holders of the ordinary shares. Our capital expenditure plans and our business, operating results and financial condition may be materially and adversely affected.
Our business may be materially and adversely affected if any of our PRC subsidiaries declare bankruptcy or become subject a dissolution or liquidation proceeding.
The Enterprise Bankruptcy Law of the PRC, or the Bankruptcy Law, came into effect on June 1, 2007. The Bankruptcy Law provides that an enterprise will be liquidated if the enterprise fails to settle its debts as and when they fall due and if the enterprise’s assets are, or are demonstrably, insufficient to clear such debts.
Our PRC subsidiaries hold certain assets that are important to our business operations. If any of our PRC subsidiaries undergoes a voluntary or involuntary liquidation proceeding, unrelated third-party creditors may claim rights to some or all of these assets, thereby hindering our ability to operate our business, which could materially and adversely affect our business, financial condition and results of operations.
If any of our PRC subsidiaries undergoes a voluntary or involuntary liquidation proceeding, prior approval from SAFE for remittance of foreign exchange to our shareholders abroad is no longer required, but we still need to conduct a registration process with the SAFE local branch. It is not clear whether “registration” is a mere formality or involves the kind of substantive review process undertaken by SAFE and its relevant branches in the past.
26
Our current corporate structure and business operations may be affected by the newly enacted PRC Foreign Investment Law.
On March 15, 2019, the National People’s Congress approved the Foreign Investment Law, which became effective on January 1, 2020. The PRC Foreign Investment Law defines the “foreign investment” as the investment activities in China conducted directly or indirectly by foreign investors in the following manners: (i) the foreign investor, by itself or together with other investors establishes a foreign invested enterprises in China; (ii) the foreign investor acquires shares, equities, asset tranches, or similar rights and interests of enterprises in China; (iii) the foreign investor, by itself or together with other investors, invests and establishes new projects in China; (iv) the foreign investor invests through other approaches as stipulated by laws, administrative regulations or otherwise regulated by the State Council. If our PRC subsidiaries are recognized as “foreign investment enterprises,” PRC governmental authorities will regulate foreign investment by applying the principle of re-entry national treatment together with a “negative list,” which will be promulgated by or promulgated with approval by the State Council. Foreign investors are prohibited from making any investments in the industries which are listed as “prohibited” in such negative list; and, after satisfying certain additional requirements and conditions as set forth in the “negative list,” are allowed to make investments in industries which are listed as “restricted” in such negative list. For any foreign investor that fails to comply with the negative list, the competent authorities are entitled to ban its investment activities, require such investor to take measures to correct its non-compliance and impose other penalties.
Pharmaceutical production and distribution activities that we conduct through our PRC subsidiaries are not subject to foreign investment restrictions or prohibitions set forth in the Special Administrative Measures for the Access of Foreign Investment (Negative List) (Edition 2022) (the “2022 Negative List”). We do not intend to conduct any types of business activities restricted or prohibited under the 2021 Negative List in the future. However, it is unclear whether any updated “negative list” to be published by the State Council in the future will be different from the 2022 Negative List. If future laws, administrative regulations or provisions of the State Council set forth restrictions or prohibitions on foreign investment in our current business activities, and that our PRC subsidiaries are recognized as “foreign investment enterprises,” we may be required to take appropriate and timely measures to comply with such regulatory requirements. If we fail to do so, our business operations could be materially and adversely affected.
Failure to comply with PRC regulations relating to the establishment of offshore special purpose companies by PRC residents may subject our PRC resident shareholders to personal liability, may limit our ability to acquire PRC companies or to inject capital into our PRC subsidiaries, may limit the ability of our PRC subsidiaries to distribute profits to us or may otherwise materially and adversely affect us.
Pursuant to the Circular on relevant issues concerning Foreign Exchange Administration of Overseas Investment and Financing and Return Investments Conducted by Domestic Residents through Overseas Special Purpose Vehicle (the “Circular 37”), which was promulgated by SAFE, and became effective on July 4, 2014, (1) a PRC resident must register with the local SAFE branch before he or she contributes assets or equity interests in an overseas special purpose vehicle, or an Overseas SPV, that is directly established or indirectly controlled by the PRC resident for the purpose of conducting investment or financing; and (2) following the initial registration, the PRC resident is also required to register with the local SAFE branch for any major change, in respect of the Overseas SPV, including, among other things, a change in the Overseas SPV’s PRC resident shareholder, name of the Overseas SPV, term of operation, or any increase or reduction of the contributions by the PRC resident, share transfer or swap, and merger or division. Additionally, pursuant to the Circular of SAFE on Further Simplifying and Improving the Direct Investment-related Foreign Exchange Administration Policies (the “Circular 13”), which was promulgated on February 13, 2015 and became effective on June 1, 2015, the aforesaid registration shall be directly reviewed and handled by qualified banks in accordance with the Circular 13, and SAFE and its branches shall perform indirect regulation over the foreign exchange registration via qualified banks.
Mr. Gang Lai completed the initial foreign exchange registration on June 3, 2019. As it remains unclear how Circular 37 and Circular 13 will be interpreted and implemented, and how or whether SAFE will apply them to us. Therefore, we cannot predict how they will affect our business operations or future strategies. For example, the ability of our present and prospective PRC subsidiaries to conduct foreign exchange activities, such as the remittance of dividends and foreign currency-denominated borrowings, may be subject to compliance with Circular 37 and Circular 13 by our PRC resident beneficial holders. In addition, as we have little control over either our present or prospective, direct or indirect shareholders or the outcome of such registration procedures, we cannot assure you that these shareholders who are PRC residents will amend or update their registration as required under Circular 37 and Circular 13 in a timely manner or at all. Failure of our present or future shareholders who are PRC residents to comply with Circular 37 and Circular 13 could subject these shareholders to fines or legal sanctions, restrict our overseas or cross-border investment activities, limit the ability of our PRC subsidiaries to make distributions or pay dividends or affect our ownership structure, which could adversely affect our business and prospects.
We may be unable to complete a business combination transaction efficiently or on favorable terms due to complicated merger and acquisition regulations and certain other PRC regulations.
On August 8, 2006, six PRC regulatory authorities, including the Ministry of Commerce of the People’s Republic of China, or MOFCOM, the State Assets Supervision and Administration Commission, the SAT, the SAIC, the CSRC and the SAFE, jointly issued the M&A Rules, which became effective on September 8, 2006 and was amended in June 2009. The M&A Rules, governing the approval process by which a PRC company may participate in an acquisition of assets or equity interests by foreign investors, requires the PRC parties to make a series of applications and supplemental applications to the government agencies, depending on the structure of the transaction. In some instances, the application process may require presentation of economic data concerning a transaction, including appraisals of the target business and evaluations of the acquirer, which are designed to allow the government to assess the transaction. Accordingly, due to the M&A Rules, our ability to engage in business combination transactions has become significantly more complicated, time-consuming and expensive, and we may not be able to negotiate a transaction that is acceptable to our Shareholders or sufficiently protect their interests in a transaction.
27
The M&A Rules allow PRC government agencies to assess the economic terms of a business combination transaction. Parties to a business combination transaction may have to submit to the MOFCOM and other relevant government agencies an appraisal report, an evaluation report and the acquisition agreement, all of which form part of the application for approval, depending on the structure of the transaction. The M&A Rules also prohibit a transaction at an acquisition price obviously lower than the appraised value of the business or assets in China and in certain transaction structures, require that consideration must be paid within defined periods, generally not in excess of a year. In addition, the M&A Rules also limit our ability to negotiate various terms of the acquisition, including aspects of the initial consideration, contingent consideration, holdback provisions, indemnification provisions and provisions relating to the assumption and allocation of assets and liabilities. Transaction structures involving trusts, nominees and similar entities are prohibited. Therefore, such regulation may impede our ability to negotiate and complete a business combination transaction on legal and/or financial terms that satisfy our investors and protect our shareholders’ economic interests.
We face uncertainties with respect to indirect transfers of equity interests in PRC resident enterprises by their non-PRC holding companies.
The SAT released a circular on December 15, 2009 that addresses the transfer of shares by nonresident companies, generally referred to as Circular 698. Circular 698, which became effective retroactively to January 1, 2008, may have a significant impact on many companies that use offshore holding companies to invest in China. Circular 698 has the effect of taxing foreign companies on gains derived from the indirect sale of a PRC company. Where a foreign investor indirectly transfers equity interests in a PRC resident enterprise by selling the shares in an offshore holding company, and the latter is located in a country or jurisdiction that has an effective tax rate less than 12.5% or does not tax foreign income of its residents, the foreign investor must report this indirect transfer to the tax authority in charge of that PRC resident enterprise. Using a “substance over form” principle, the PRC tax authority may disregard the existence of the overseas holding company if it lacks a reasonable commercial purpose and was established for the purpose of avoiding PRC tax. As a result, gains derived from such indirect transfer may be subject to PRC withholding tax at a rate of up to 10.0%.
SAT subsequently released public notices to clarify issues relating to Circular 698, including the Announcement on Several Issues concerning the EIT on the Indirect Transfers of Properties by Nonresident Enterprises (the “SAT Notice 7”), which became effective on February 3, 2015. SAT Notice 7 abolished the compulsive reporting obligations originally set out in Circular 698. Under SAT Notice 7, if a non-resident enterprise transfers its shares in an overseas holding company, which directly or indirectly owns PRC taxable properties, including shares in a PRC company, via an arrangement without reasonable commercial purpose, such transfer shall be deemed as indirect transfer of the underlying PRC taxable properties. Accordingly, the transferee shall be deemed as a withholding agent with the obligation to withhold and remit the EIT to the competent PRC tax authorities. Factors that may be taken into consideration when determining whether there is a “reasonable commercial purpose” include, among other factors, the economic essence of the transferred shares, the economic essence of the assets held by the overseas holding company, the taxability of the transaction in offshore jurisdictions, and economic essence and duration of the offshore structure. SAT Notice 7 also sets out safe harbors for the “reasonable commercial purpose” test.
On October 17, 2017, the SAT released the Notice on Several Issues concerning the Withholding and Collection of Income Tax of Non-resident Enterprises from the Source (the “SAT Notice 37”). SAT Notice 37 clarifies: (1) matters concerning the withholding and collection of corporate income tax, and property transfer of non-resident enterprises based on the EIT Law; (2) the currencies required to be used by the withholding agents (when the payments is made in a currency rather than RMB), as well as the time, venue and business for the performance of the withholding and collection obligations; and (3) the abolishment of Circular 698.
There is little guidance and practical experience regarding the application of SAT Notice 7 and SAT Notice 37 and the related SAT notices. Moreover, the relevant authority has not yet promulgated any formal provisions or formally declared or stated how to calculate the effective tax rates in foreign tax jurisdictions. As a result, due to our complex offshore restructuring, we may become at risk of being taxed under SAT Notice 7 and SAT Notice 37 and we may be required to expend valuable resources to comply with SAT Notice 7 and SAT Notice 37 or to establish that we should not be taxed under SAT Notice 7 and SAT Notice 37, which could have a material adverse effect on our financial condition and results of operations.
28
Risks Related to our Ordinary Shares and the Trading Market
Our share price may be volatile and could decline substantially, which could result in substantial losses to our investors.
The trading price of our ordinary shares is likely to continue to be volatile and could fluctuate widely due to factors beyond our control. This may happen because of broad market and industry factors, including the performance and fluctuation of the market prices of other companies with business operations located mainly in China that have listed their securities in the United States. The securities of some of these companies have experienced significant volatility since their initial public offerings, including, in some cases, substantial price declines in their trading prices. The trading performances of other Chinese companies’ securities after their offerings may affect the attitudes of investors toward Chinese companies listed in the United States in general and consequently may impact the trading performance of our shares, regardless of our actual operating performance.
The market price of our ordinary shares may be volatile, both because of actual and perceived changes in the company’s financial results and prospects, and because of general volatility in the stock market. The factors that could cause fluctuations in our share price may include, among other factors discussed in this section, the following:
| ● | actual or anticipated variations in the financial results and prospects of the company or other companies in the pharmaceutical business; |
| ● | changes in financial estimates by research analysts; |
| ● | changes in the market valuations of other education technology companies; |
| ● | announcements by us or our competitors of new education services, expansions, investments, acquisitions, strategic partnerships or joint ventures; |
| ● | mergers or other business combinations involving us; |
| ● | additions and departures of key personnel and senior management; |
| ● | changes in accounting principles; |
| ● | the passage of legislation or other developments affecting us or our industry; |
| ● | the trading volume of our ordinary shares in the public market; | |
| ● | the release of lockup, escrow or other transfer restrictions on our outstanding equity securities or sales of additional equity securities; |
| ● | potential litigation or regulatory investigations; |
| ● | changes in economic conditions, including fluctuations in global and Chinese economies; |
| ● | financial market conditions; |
| ● | natural disasters, terrorist acts, acts of war or periods of civil unrest; and |
| ● | the realization of some or all of the risks described in this section. |
29
The listing of our ordinary shares on the Nasdaq Global Market is contingent on our compliance with the Nasdaq Global Market’s conditions for continued listing. A decline in the closing price of our ordinary shares could result in a breach of the requirements for listing on the Nasdaq Global Market. If we do not maintain compliance, Nasdaq could commence suspension or delisting procedures in respect of our ordinary shares. The commencement of suspension or delisting procedures by an exchange remains at the discretion of such exchange and would be publicly announced by the exchange. If a suspension or delisting were to occur, there would be significantly less liquidity in the suspended or delisted securities. In addition, our ability to raise additional necessary capital through equity or debt financing would be greatly impaired. Furthermore, with respect to any suspended or delisted ordinary shares, we would expect decreases in institutional and other investor demand, analyst coverage, market making activity and information available concerning trading prices and volume, and fewer broker-dealers would be willing to execute trades with respect to such ordinary shares. A suspension or delisting would likely decrease the attractiveness of our ordinary shares to investors and cause the trading volume of our ordinary shares to decline, which could result in a further decline in the market price of our ordinary shares.
In addition, the stock markets have experienced significant price and trading volume fluctuations from time to time, and the market prices of the equity securities of pharmaceutical companies are sometimes subject to sharp price and trading volume changes. These broad market fluctuations may materially and adversely affect the market price of our ordinary shares.
We may issue additional ordinary shares or other equity securities without your approval, which would dilute your ownership interests and may depress the market price of our ordinary shares.
We may issue additional ordinary shares or our other securities to investors. We may also issue additional ordinary shares or other equity securities of equal or senior rank in the future for any reason or in connection with, among other things, future acquisitions or repayment of outstanding indebtedness, without shareholder approval, in a number of circumstances.
Our issuance of additional ordinary shares or other equity securities of equal or senior rank would have the following effects:
| ● | our existing shareholders’ proportionate ownership interest in us will decrease; |
| ● | the amount of cash available per share, including for payment of dividends in the future, may decrease; |
| ● | the relative voting strength of each previously outstanding share may be diminished; and |
| ● | the market price of our ordinary shares may decline. |
We currently do not expect to pay dividends on our ordinary shares in the foreseeable future.
We currently do not expect to pay dividends on our ordinary shares in the foreseeable future. Instead, for the foreseeable future, it is expected that we will continue to retain any earnings to finance the development and expansion of its business, and not to pay any cash dividends on its ordinary shares. Consequently, you should not rely on an investment in the Company as a source for any future dividend income.
Our board of directors has complete discretion as to whether to distribute dividends, subject to applicable laws. Even if our board of directors decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on, among other things, our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors deemed relevant by our board of directors. Accordingly, the return on your investment in our ordinary shares will likely depend entirely upon any future price appreciation of our ordinary shares. We cannot guarantee that our ordinary shares will appreciate in value or even maintain the price at which you purchased the ordinary shares. You may not realize a return on your investment in our ordinary shares and you may even lose your entire investment in our ordinary shares.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about us or our business, our ordinary share price and trading volume could decline.
The trading market for our ordinary shares will depend in part on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on us. If no securities or industry analysts commence coverage of our Company, the trading price for its ordinary shares would likely be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade its securities or publish inaccurate or unfavorable research about its business, its stock price would likely decline. If one or more of these analysts cease coverage of our Company or fail to publish reports on our Company, demand for its ordinary shares could decrease, which might cause its ordinary share price and trading volume to decline.
30
We are a “controlled company” within the meaning of the Nasdaq Stock Market Rules and, as a result, may rely on exemptions from certain corporate governance requirements that provide protection to shareholders of other companies.
We are a “controlled company” as defined under Rule 5615(c)(1) of the Nasdaq Marketplace Rules because Mr. Gang Lai holds more than 50% of our voting power, and we expect we will continue to be a controlled company. For so long as we remain a controlled company under that definition, we are permitted to elect to rely, and may rely, on certain exemptions from the obligation to comply with certain corporate governance requirements, including:
| ● | the requirement that our director nominees must be selected or recommended solely by independent directors; and | |
| ● | the requirement that we have a corporate governance and nominating committee that is composed entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities. |
As a result, you will not have the same protections afforded to shareholders of companies that are subject to all of the corporate governance requirements of the Nasdaq Stock Market Rules, if we utilize such exemptions. We currently do not intend to utilize the controlled company exemptions.
A sale or perceived sale of a substantial number of our ordinary shares may cause the price of our ordinary shares to decline.
If our shareholders sell substantial amounts of our ordinary shares in the public market, the market price of our ordinary shares could fall. Moreover, the perceived risk of this potential dilution could cause shareholders to attempt to sell their shares and investors to short our ordinary shares. These sales also make it more difficult for us to sell equity-related securities in the future at a time and price that we deem reasonable or appropriate.
We incur substantial increased costs being a public company.
We incur significant legal, accounting and other expenses as a public company that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, as well as rules subsequently implemented by the SEC and Nasdaq, impose various requirements on the corporate governance practices of public companies.
Compliance with these rules and regulations increases our legal and financial compliance costs and makes some corporate activities more time-consuming and costlier. We have incurred additional costs in obtaining director and officer liability insurance. In addition, we incur additional costs associated with our public company reporting requirements. It may also be more difficult for us to find qualified persons to serve on our board of directors or as executive officers.
We are an “emerging growth company,” as defined in the JOBS Act and will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of our initial public offering, (b) in which we have total annual gross revenue of at least $1.235 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our ordinary shares that is held by non-affiliates exceeds $700 million as of the prior March 31, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 in the assessment of the emerging growth company’s internal control over financial reporting and permission to delay adopting new or revised accounting standards until such time as those standards apply to private companies.
After we are no longer an “emerging growth company,” or until five years following the completion of our initial public offering, whichever is earlier, we expect to incur significant additional expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404 and the other rules and regulations of the SEC. For example, as a public company, we have been required to increase the number of independent directors and adopt policies regarding internal controls and disclosure controls and procedures.
31
We are currently evaluating and monitoring developments with respect to these rules and regulations, and we cannot predict or estimate with any degree of certainty the amount of additional costs we may incur or the timing of such costs.
There can be no assurance that we will not be a passive foreign investment company (“PFIC”) for United States federal income tax purposes for any taxable year, which could subject United States holders of our ordinary shares could be subject to adverse United States federal income tax consequences.
A non-United States corporation will be a PFIC for United States federal income tax purposes for any taxable year if either (i) at least 75% of its gross income for such taxable year is passive income or (ii) at least 50% of the value of its assets (based on average of the quarterly values of the assets) during such year is attributable to assets that that produce or are held for the production of passive income. Based on the current and anticipated value of our assets and the composition of our income assets, we are not currently a PFIC under the current PFIC rules for United States federal income tax purposes. However, the determination of whether or not we are a PFIC according to the PFIC rules is made on an annual basis and depends on the composition of our income and assets and the value of our assets from time to time. Therefore, changes in the composition of our income or assets or value of our assets may cause us to become a PFIC. The determination of the value of our assets (including goodwill not reflected on our balance sheet) may be based, in part, on the quarterly market value of ordinary shares, which is subject to change and may be volatile.
The classification of certain of our income as active or passive, and certain of our assets as producing active or passive income, and hence whether we are or will become a PFIC, depends on the interpretation of certain United States Treasury Regulations as well as certain IRS guidance relating to the classification of assets as producing active or passive income. Such regulations guidance is potentially subject to different interpretations. If due to different interpretations of such regulations and guidance the percentage of our passive income or the percentage of our assets treated as producing passive income increases, we may be a PFIC in one or more taxable years.
If we are a PFIC for any taxable year during which a United States person holds ordinary shares, certain adverse United States federal income tax consequences could apply to such United States person. See “Item 10. Additional Information—E. Taxation—United States Federal Income Taxation—PFIC.”
For as long as we are an emerging growth company, we will not be required to comply with certain reporting requirements, including those relating to accounting standards and disclosure about our executive compensation, that apply to other public companies.
We are classified as an “emerging growth company” under the JOBS Act. For as long as we are an emerging growth company, unlike other public companies, we will not be required to, among other things, (i) provide an auditor’s attestation report on management’s assessment of the effectiveness of our system of internal control over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act, (ii) comply with any new requirements adopted by the Public Company Accounting Oversight Board, or the PCAOB, requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the issuer, (iii) provide certain disclosure regarding executive compensation required of larger public companies, or (iv) hold nonbinding advisory votes on executive compensation. We will remain an emerging growth company for up to five years, although we will lose that status sooner if we have more than $1.235 billion of revenues in a fiscal year, have more than $700 million in market value of our ordinary shares held by non-affiliates, or issue more than $1.0 billion of non-convertible debt over a three-year period.
To the extent that we rely on any of the exemptions available to emerging growth companies, you will receive less information about our executive compensation and internal control over financial reporting than issuers that are not emerging growth companies. If some investors find our ordinary shares to be less attractive as a result, there may be a less active trading market for our ordinary shares and our share price may be more volatile.
32
Our ability to produce accurate financial statements have been materially adversely affected by our failure to establish proper internal financial reporting controls. If we fail to establish and maintain proper internal financial reporting controls in a reasonably timely manner, our ability to produce accurate financial statements or comply with applicable regulations may continue to be impaired.
Our independent registered public accounting firm has not conducted an audit of our internal control over financial reporting. In the course of auditing our consolidated financial statements for the year ended September 30, 2023, we identified material weaknesses in our internal control over financial reporting and other control deficiencies as of September 30, 2023. A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis.
The material weakness identified to date relates to a lack of accounting staff and resources with appropriate knowledge of generally accepted accounting principles in the United States (“U.S. GAAP”) and SEC reporting and compliance requirements.
Following the identification of the material weaknesses and control deficiencies, we have undertaken certain remedial steps and plan to continue taking remedial measures, including:
| (i) | recruiting qualified accounting personnel with relevant U.S. GAAP and SEC reporting experience and qualifications to strengthen the financial reporting function and to set up a financial and system control framework. Since very few companies in Ji’an, Jiangxi Province, the area in which our main PRC operating subsidiaries are located, have sought public listing on a U.S. exchange, we have difficulty identifying qualified accounting candidates with U.S. GAAP experience and expertise. We plan to search for qualified personnel in other regions of China; and |
| (ii) | implementing regular and continuous U.S. GAAP accounting and financial reporting training programs for our accounting and financial reporting personnel. |
The implementation of these measures may not fully address the material weaknesses in our internal control over financial reporting, and we cannot conclude that they have been fully remedied. Our failure to correct theses material weaknesses or our failure to discover and address any other material weaknesses could result in inaccuracies in our financial statements and could also impair our ability to comply with applicable financial reporting requirements and related regulatory filings on a timely basis. As a result, our business, financial condition, results of operations and prospects, as well as the trading price of our ordinary shares, may be materially and adversely affected. Moreover, ineffective internal control over financial reporting significantly hinders our ability to prevent fraud.
As a public company, we will be subject to Sarbanes-Oxley Act of 2002, or Sarbanes-Oxley Act. Since we qualify as an “emerging growth company” pursuant to the JOBS Act with less than US$1.235 billion in revenue for our last fiscal year. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404, in the assessment of the emerging growth company’s internal control over financial reporting. Moreover, even if management concludes that our internal control over financial reporting is effective, our independent registered public accounting firm, after conducting its own independent testing, may issue a report that is qualified if it is not satisfied with our internal controls or the level at which our controls are documented, designed, operated or reviewed, or if it interprets the relevant requirements differently from us.
During the course of documenting and testing our internal control procedures, we may identify other weaknesses and deficiencies in its internal control over financial reporting. In addition, if we fail to maintain the adequacy of our internal control over financial reporting, as these standards are modified, supplemented or amended from time to time, we may not be able to conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with Section 404. Generally speaking, if we fail to achieve and maintain an effective internal control environment, we could suffer material misstatements in our financial statements and fail to meet our reporting obligations, which would likely cause investors to lose confidence in our reported financial information. This could in turn limit our access to capital markets, harm our results of operations, and lead to a decline in the trading price of our securities. Additionally, ineffective internal control over financial reporting could expose us to increased risk of fraud or misuse of corporate assets and subject us to potential delisting from the stock exchange on which we list, regulatory investigations and civil or criminal sanctions.
33
As a foreign private issuer, we are not subject to certain U.S. securities law disclosure requirements that apply to a domestic U.S. issuer, which may limit the information publicly available to our shareholders.
As a foreign private issuer, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act and therefore there may be less publicly available information about us than if we were a U.S. domestic issuer. We are exempt from certain provisions of the securities rules and regulations in the United States that are applicable to U.S. domestic issuers, including:
| ● | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q or current reports on Form 8-K; |
| ● | the sections of the Exchange Act regulating the solicitation of proxies, consents, or authorizations in respect of a security registered under the Exchange Act; |
| ● | the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and the selective disclosure rules by issuers of material non-public information under Regulation FD. |
We are required to file an annual report on Form 20-F within four months of the end of each fiscal year. However, the information we are required to file with or furnish to the SEC will be less extensive and less timely compared to that required to be filed with the SEC by U.S. domestic issuers. As a result, you may not be afforded the same protections or information that would be made available to you were you investing in a U.S. domestic issuer.
As a foreign private issuer, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from the Nasdaq listing standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards.
As a foreign private issuer, we are permitted to take advantage of certain provisions in the Nasdaq listing standards that allow us to follow Cayman Islands law for certain governance matters. Certain corporate governance practices in the Cayman Islands may differ significantly from corporate governance listing standards as, except for general fiduciary duties and duties of care, Cayman Islands law has no corporate governance regime which prescribes specific corporate governance standards. Currently, we do not intend to rely on home country practice with respect to our corporate governance. However, if we choose to follow home country practice in the future, our shareholders may be afforded less protection than they otherwise would have under corporate governance listing standards applicable to U.S. domestic issuers.
We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses.
As discussed above, we are a foreign private issuer, and therefore, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter. We would lose our foreign private issuer status if, for example, more than 50% of our ordinary shares are directly or indirectly held by residents of the U.S. and we fail to meet additional requirements necessary to maintain our foreign private issuer status. If we lose our foreign private issuer status on this date, we will be required to file with the SEC periodic reports and registration statements on U.S. domestic issuer forms, which are more detailed and extensive than the forms available to a foreign private issuer. We will also have to mandatorily comply with U.S. federal proxy requirements, and our officers, directors and principal shareholders will become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, we will lose our ability to rely upon exemptions from certain corporate governance requirements under the Nasdaq listing standards. As a U.S. listed public company that is not a foreign private issuer, we will incur significant additional legal, accounting and other expenses that we will not incur as a foreign private issuer, and accounting, reporting and other expenses in order to maintain a listing on a U.S. securities exchange.
34
The laws of the Cayman Islands may not provide our shareholders with benefits comparable to those provided to shareholders of corporations incorporated in the United States.
Our corporate affairs are governed by our amended and restated memorandum and articles of association, by the Companies Act (Revised) of the Cayman Islands and by the common law of the Cayman Islands. The rights of shareholders to take action against our directors, actions by minority shareholders and the fiduciary responsibilities of our directors to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law in the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands and from English common law. Appeals from the Cayman Islands Courts to the Privy Council (which is the final Court of Appeal for British overseas territories such as the Cayman Islands) are binding on courts in the Cayman Islands. Decisions of the English courts, and particularly the Supreme Court and the Court of Appeal are generally of persuasive authority but are not binding in the courts of the Cayman Islands. Decisions of courts in other Commonwealth jurisdictions are similarly of persuasive but not binding authority. The rights of our shareholders and the fiduciary responsibilities of our directors under Cayman Islands law are not as clearly established as they would be under statutes or judicial precedents in the United States. In particular, the Cayman Islands has a less developed body of securities laws relative to the United States. Therefore, our public shareholders may have more difficulty protecting their interests in the face of actions by our management, directors or controlling shareholders than would shareholders of a corporation incorporated in a jurisdiction in the United States.
You may be unable to present proposals before annual general meetings or extraordinary general meetings not called by shareholders.
Cayman Islands law provides shareholders with only limited rights to requisition a general meeting, and does not provide shareholders with any right to put any proposal before a general meeting. These rights, however, may be provided in a company’s amended and restated articles of association. Our amended and restated articles of association allow our shareholders holding shares representing in aggregate not less than 10% of our voting share capital in issue, to requisition a general meeting of our shareholders, in which case our directors are obliged to call such meeting. Advance notice of at least 21 clear days is required for the convening of our annual general shareholders’ meeting and at least 14 days’ notice any other general meeting of our shareholders. A quorum required for a meeting of shareholders consists of at least one shareholder present or by proxy, representing not less than one-third of the total outstanding shares carrying the right to vote at such general meeting of the Company.
The obligation to disclose information publicly may put us at a disadvantage to competitors that are private companies.
We are a public company in the United States. As a public company, we will be required to file periodic reports with the SEC upon the occurrence of matters that are material to our Company and shareholders. Although we may be able to attain confidential treatment of some of our developments, in some cases, we will need to disclose material agreements or results of financial operations that we would not be required to disclose if we were a private company. Our competitors may have access to this information, which would otherwise be confidential. This may give them advantages in competing with our Company. Similarly, as a U.S. public company, we will be governed by U.S. laws that our competitors, which are mostly private Chinese companies, are not required to follow. To the extent compliance with U.S. laws increases our expenses or decreases our competitiveness against such companies, our public company status could affect our results of operations.
Item 4. INFORMATION ON THE COMPANY
A. History and Development of the Company
Our Corporate History and Structure
We are an offshore holding company incorporated in the Cayman Islands. As a holding company with no operations of our own, our operations are conducted in China through our wholly owned indirect PRC subsidiary, Jiangxi Universe, and its subsidiaries. Investors in our securities are not purchasing equity interests in our subsidiaries but instead are purchasing equity interests in the ultimate Cayman Islands holding company. Therefore, you will not directly hold any equity interests in the operating entities. The Chinese regulatory authorities could disallow this structure, which would likely result in a material change in our operations and/or a material change in the value of the securities we are registering for sale, including that it could cause the value of such securities to significantly decline or become worthless. For risks facing our Company as a result of our organizational structure and doing business in China, see “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China.”
We initially conducted our business through Jiangxi Universe, a PRC company formed in 1998 and Universe Trade, a PRC company formed in 2010, a wholly-owned subsidiary of Jiangxi Universe.
With the growth of our business and in order to facilitate international capital investment in our Company, we underwent an offshore reorganization in 2019 and 2020. On December 11, 2019, our holding company, Universe Pharmaceuticals INC, was incorporated under the laws of the Cayman Islands as an exempted company with limited liability. Our wholly owned subsidiary Universe HK was formed in Hong Kong on May 21, 2014 as an intermediate holding company. Universe HK in turn holds all the capital stocks of Universe Technology, a wholly foreign owned enterprise incorporated in China on Aril 8, 2019. Universe Technology holds all the capital stocks and controls Jiangxi Universe. Jiangxi Universe holds 100% of the equity interests in Universe Trade.
35
Our holding company has no business operation other than holding the shares in Universe HK. Universe HK is a pass-through entity with no business operation. Universe Technology is exclusively engaged in the business of managing the operation of Jiangxi Universe. Jiangxi Universe specializes in manufacturing our own TCMD products. Universe Trade specializes in the distribution and sales of our own TCMD products and third-party pharmaceutical products.
Foshan Shangyu Investment Holding Co., Ltd. (“Foshan Shangyu”) is our affiliated entity, 90% owned by and controlled by Mr. Gang Lai, our controlling shareholder. Foshan Shangyu was formed in 2004 in China as a holding company of Mr. Gang Lai. Foshan Shangyu has no business operations.
The following diagram illustrates our corporate structure as of the date of this annual report:
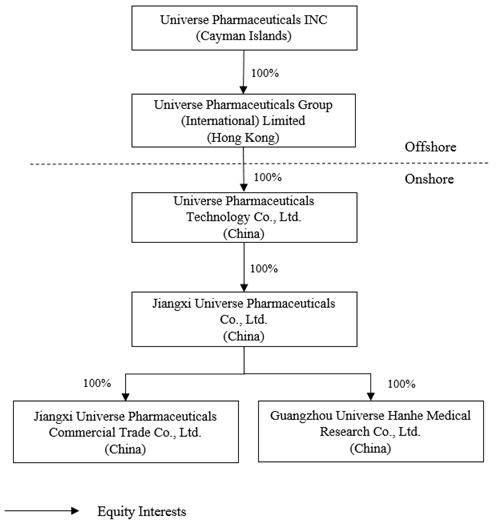
Corporate Information
Our principal executive offices are located at 265 Jingjiu Avenue, Jinggangshan Economic and Technological Development Zone, Ji’an, Jiangxi Province, People’s Republic of China, and our phone number is +86-0796-8403309. Our registered office in the Cayman Islands is located at Vistra (Cayman) Limited, P.O. Box 31119 Grand Pavilion, Hibiscus Way, 802 West Bay Road, Grand Cayman, KYI – 1205 Cayman Islands, and the phone number of our registered office is +1-(345)769-9372. We maintain a corporate website at http://www.universe-pharmacy.com. The information contained in, or accessible from, our website or any other website does not constitute a part of this annual report.
The SEC maintains a website at www.sec.gov that contains reports, proxies, and information statements, and other information regarding issuers that file electronically with the SEC using its EDGAR system.
For information regarding our principal capital expenditures, see “Item 5. Operating and Financial Review and Prospects—B. Liquidity and Capital Resources—Capital Expenditures.”
36
B. Business Overview
Overview
TCM is a comprehensive form of healthcare that has been widely adopted in China for more than 23 centuries. TCM rests upon the assumption that the human body is an ecosystem, embodying the fusion of Shen (psyche), Essence (soma), Qi, Moisture (body fluids), and Blood (tissue). Health in the context of TCM is more than just the absence of diseases, but to identify imbalance in human body and restore harmony. TCM is not only intended to cure diseases but to enhance the capacity for fulfillment, happiness and general well-being of people.
Through the PRC operating entities, we are a pharmaceutical company based in Jiangxi, China, specializing in the manufacturing, marketing, sales and distribution of TCMD products targeting the elderly with the goal of addressing their physical conditions in the aging process and to promote their general well-being. The PRC operating entities have registered and obtained approval for 26 varieties of TCMD products from the National Medical Products Administration (the “NMPA”), and we currently produce 13 varieties of TCMD products, which are sold in approximately 261 cities of 30 provinces in China. In addition, through our subsidiary Universe Trade, we sell not only our own TCMD products, but also biomedical drugs medical instruments, Traditional Chinese Medicine Pieces (“TCMPs”), and dietary supplements manufactured by third-party pharmaceutical companies.
Products manufactured by us. The 13 TCMD products currently manufactured by us fall into two categories: (1) treatment and relief for common chronic health conditions in the elderly designed to achieve physical wellness and longevity (“chronic condition treatments”), and (2) cold and flu medications.
| ● | Chronic condition treatments: Guben Yanling Pill, Shenrong Weisheng Pill, Quanlu Pill, Yangxue Danggui Syrup, Wuzi Yanzong Oral Liquid, Fengtong Medicinal Liquor, Shenrong Medicinal Liquor, Qishe Medicinal Liquor, Fengshitong Medicinal Liquor, and Shiquan Dabu Medicinal Liquor. |
| ● | Cold and flu medicines: Paracetamol Granule for Children, Isatis Root Granule and Qiangli Pipa Syrup. |
As people age, they have an increasing risk of developing chronic health conditions. According to a report published by the Chinese Center for Disease Control and Prevention in March 2019, 75.8% of seniors have at least one chronic health condition, and 35.1% of them have two or more. According to the “Blue Book of Elderly Health (2020-2021)” released in December 2021 by the Chinese Academy of Medical Sciences, the School of Public Health of Peking Union Medical College and the Social Sciences Literature Publishing House, the prevalence of hypertension, diabetes and hypercholesterolemia in Chinese residents aged 60 and above is 58.3%, 19.4% and 10.5%, respectively, and more than 3/4 of the residents have multiple disease coexistence, and with the increase of age, the prevalence of chronic diseases increases. Some of the most common chronic diseases in the elderly include arthritis, chronic kidney disease, fatigue, and low back pain. The PRC operating entities’ products under the category of chronic condition treatments are designed to address some of the aforementioned diseases. The PRC operating entities’ cold and flu medicines, on the other hand, include products designed to treat and relieve symptoms of respiratory illnesses caused by bacteria and viruses.
The PRC operating entities’ third-party products. Through our subsidiary, Universe Trade, we also distribute and sell products manufactured by third-party producers, including biomedical drugs, medical instruments, TCMPs and dietary supplements. For the years ended September 30, 2023, 2022 and 2021, we distributed around 2,239, 2,785 and 2,766 types of third-party products, respectively.
The PRC operating entities’ Customers. The PRC operating entities’ major customers are pharmaceutical distributors, hospitals, clinics and drugstore chains, primarily located in Jiangxi Province, Jiangsu Province, Guangdong Province, Hubei Province, Fujian Province, Guangxi Province and Shandong Province, and 23 other provinces in China.
The PRC operating entities’ customer base decreased slightly from a total of 2,708 as of September 30, 2021 to 2,651 as of September 30, 2022, and further decreased to 2,541 as of September 30, 2023. The revenues from selling the PRC operating entities’ own products decreased from $29,559,286 for the fiscal year ended September 30, 2021 to $23,988,177 for the fiscal year ended September 30, 2022 due to our inability to timely fulfill the orders of our customer in 2022 due to the resurgence of COVID-19 cases in China, and further decreased to $18,572,658 for the fiscal year ended September 30, 2023, as we changed our pricing strategy and decreased selling price of our TCMD products to increase sales volume and market share. The revenues from distributing and selling third-party products decreased from $18,422,745 for the fiscal year ended September 30, 2021 to $16,154,974 in the fiscal year ended September 30, 2022 due to a decrease in the weighted average selling price caused by a change in product mix, and further decreased to $13,736,077 for the fiscal year ended September 30, 2023 due to decreased sales volume and negative impact from foreign currency fluctuation. Our net income was $11,319,952 for the fiscal year ended September 30, 2021, net loss was $8,736,566 for the fiscal year ended September 30, 2022 due to a decrease in our revenue and increase in our selling expense, and our net loss was $6,163,061 for the fiscal year ended September 30, 2023 due to the decrease in our revenue and increase in cost of revenue.
37
Our Competitive Strengths
We believe we have the following competitive strengths:
A recognized manufacturer of TCMD products in China’s rapidly growing health and wellness market
We are a recognized manufacturer of TCMD products in China’s rapidly growing health and wellness market. We own a number of famous brands in the industry, which are also our registered trademarks in China. For instance, our brand “Hu Zhuo Ren (胡卓仁)” is especially well-recognized in Jiangxi Province. Further, our brand “Bai Nian Dan (百年丹)” is famous for specializing in products targeted at the physical wellness of older population. Our other recognized brands include “Long Zhong (龙种)”, “Yi Ke Ting (益克停)”, “Xue Li (血力)”, “Duo Lai Mei (朵来美)”, “Shu Er Kang (舒儿康)”, “Hu Zhuo Ren (胡卓仁)”, “Ai Bi Xin (爱必欣)”, and “Yong He Shuang Feng (永和双凤)”.
The Chinese patent medicine industry is growing rapidly and steadily in China. The primary growth drivers of China’s Chinese patent medicine industry include the increasing disposable income and healthcare awareness in China, growing aging population and the prevalence of chronic diseases, and favorable governmental policies and regulations.
We attribute our success to our recognized brand names, strong relationships with our suppliers, loyal and stable customer base, and proven capability to develop and manufacture TCMD products aligned with the preferences of end consumers.
Rigorous quality control standards and manufacturing protocols
We believe that the quality of our products is crucial to our success as a pharmaceutical company, and we have implemented an overall quality control system, as well as strict manufacturing protocols specifically designed for each product. Our quality control system starts from procurement. The raw materials we source from our suppliers must first be examined and certified for quality. We review the performance of our suppliers based on the quality of their products and adjust future orders from them accordingly. Further, an average of three inspections are made by our personnel throughout the manufacturing process to ensure that the manufacturing protocols are strictly followed, and that the quality of semi-products are at or above standard. After completion of manufacturing, our personnel will perform an overall quality examination. Through the implementation of a quality control system, we are able to identify the weakness in our production process and improve our operations over time. We believe our quality control standards and manufacturing protocols have contributed to the high quality and consistency of our products.
Visionary management team with substantial industry experience
Our visionary management team is the bedrock of our success. Many members of our leadership possess extensive experience in the pharmaceutical, biomedical, chemical and related industries. For instance, our chief executive officer, Mr. Gang Lai, has about 31 years of corporate management experiences. Mr. Xiaojun Deng, the deputy manufacturing manager of Jiangxi Universe, holds a degree in Traditional Chinese Medicine Manufacturing from Jiangxi Medical School with over 26 years of working experience in the Chinese patent medicine industry. Ms. Lin Yang, our chief financial officer, has over 15 years of finance and management experience working in pharmaceutical companies. Mr. Yajun Hu, the general manager of Jiangxi Universe, has over five years of experiences in managing a pharmaceutical company. Mr. Baochang Liu, our chief operating officer, has over 18 years of experience in pharmaceutical marketing and had previously held marketing and management positions at a number of listed pharmaceutical companies in China. Moreover, many members of the team have worked together for an extended period of time and helped build the Company from the ground up. The rapport that the team has built extends beyond the talent and skills of individual team members and contributes to a collective sense of mission.
38
Our Growth Strategy
Build a Strong Brand Image to Achieve National Recognition
We believe that broader recognition and favorable perception of our brand by consumers in our target markets are crucial to our future success. Our brand has gained a solid reputation in Southern China, especially Jiangxi Province, and we plan to increase the awareness of our brand among consumers in other parts of China. Specifically, we plan to build a strong brand image that we are a TCMD producer specializing in the development and manufacture of products designed to address the physical conditions of the elderly during the aging process and to promote their general well-being. To achieve our goal, we plan to spend most of our efforts on the development and marketing of our brand “Bai Nian Dan (百年丹)” as the brand to be associated with our ideal brand image because “Bai Nian” (百年) signifies longevity in the Chinese language, and “Dan” (丹) alludes to our signature product, Guben Yanling Pill. In the fiscal year ended September 30, 2021, we started to advertise our products through television advertisement. We also intend to advertise targeting older customers, including transmitting our advertisements through additional traditional media platforms such as live radio stations, newspapers, as well as in-person marketing at drug stores and clinics. In the fiscal year ended September 30, 2022, our marketing department explored direct selling strategy and increased its efforts to market products directly to customers in Liaoning Province. In the fiscal year ended September 30, 2023, our marketing strategies are primarily focused on digital marketing and brand promotion, and we launched in-app advertising campaigns and created brand films to enhance our recognition among potential customers.
Enhance Our Distribution Network to Increase Market Penetration and Customer Stickiness
Currently, our products are sold in 30 provinces in China. We plan to enter the markets in other parts of China. To achieve this goal, we have made efforts to further strengthen and expand our distribution network through connecting with more local distributors, chain drugstores, malls and supermarkets in other parts of China. Currently, our strategic focus is to attract more marketing talents and build a stronger sales and marketing team to keep us on top of the latest information of local markets, customer preferences and industry trends. We also plan to create an online store to reach a wider consumer demographic. In the future, we plan to start our own retail chain stores, and provide training programs to sales personnel to improve their skills and acquire knowledge of our products, in order to further diversify our distribution channels to increase our market penetration and customer base.
Integrate Our Internal Manufacturing Capability to Ensure Productivity, Supply, and Selection of Products
We plan to further optimize our production facilities to increase the productivity, supply and selection of our products, so that we may gain competitive edges over our competitors. Specifically, we intend to increase productivity and supply by expanding the existing production lines and converting them into automated production lines. To increase the selection of our products, we plan to build additional production facilities for our licensed TCMD products to be launched in the future.
Further Grow Our Research and Development Capacities
The size of the Chinese patent medicine market has been growing steadily. To respond to increasing market demand, we will continue to provide financial and operational resources to focus on the research and development of TCMD products and dietary supplements designed to address the physical conditions of the elderly during the aging process and promote their general well-being.
39
Our Manufactured Products
We manufacture, market and sell 13 different TCMD products to customers in 30 different provinces in China. Our TCMD products fall under two categories: chronic condition treatments and cold and flu medications. The following list outlines our current products:
| Percentage of Gross Sales | ||||||||||||||||||
| Product Category | Product Name | Posology | 2023 | 2022 | 2021 | Intended Uses | ||||||||||||
| Chronic Condition Treatments | Guben Yanling Pill | Pills | 36.4 | % | 42.5 | % | 40.3 | % | To relieve fatigue, palpitation, low back pain, and generalized weakness and soreness. | |||||||||
| Shenrong Weisheng Pill | Pills | 3.5 | % | 2.5 | % | 4.7 | % | To relieve fatigue, dizziness, excessive sweating, and pain in the waist and the knees. | ||||||||||
| Quanlu Pill | Pills | 1.9 | % | 1.7 | % | 1.5 | % | To improve kidney functions and spleen functions, and relieve fatigue, low back pain, and knee pain. | ||||||||||
| Wuzi Yanzong Oral Liquid | Oral liquid | 0.1 | % | 0.5 | % | 0.4 | % | To improve kidney functions. | ||||||||||
| Yangxue Danggui Syrup | Syrup | 0.3 | % | 0.4 | % | 0.9 | % | To improve blood circulation and treating dizziness, headaches and menstrual pains. | ||||||||||
| Fengshitong Medicinal Liquor | Medicinal liquor | 0.8 | % | 0.6 | % | 1.4 | % | To treat low back pain and numbness in the feet and hands, and relieve rheumatoid arthritis pain. | ||||||||||
| Shiquan Dabu Medicinal Liquor | Medicinal liquor | 1.1 | % | 1.0 | % | 1.3 | % | To treat dizziness, palpitation, fatigue, and weakness, and ease menstrual flow. | ||||||||||
| Fengtong Medicinal Liquor | Medicinal liquor | - | 0.2 | % | 0.3 | % | To treat low back pain and numbness in the feet and hands, and relieve symptoms of arthritis. | |||||||||||
| Shenrong Medicinal Liquor | Medicinal liquor | 0.6 | % | 0.5 | % | 0.7 | % | To improve blood circulation and relieve symptoms of fatigue, low back pain and leg pain. | ||||||||||
| Qishe Medicinal Liquor | Medicinal liquor | 0.3 | % | 0.5 | % | 0.4 | % | To treat blood stasis, arthritis, and numbness in the feet and hands. | ||||||||||
| Cold and Flu Medicines Medicinal Liquor | Qiangli Pipa Syrup | Syrup | 4.9 | % | 5.9 | % | 4.6 | % | Relieve cough and reduce mucus and phlegm. | |||||||||
| Paracetamol Granule for Children | Granules | 3.4 | % | 1.9 | % | 2.7 | % | To relieve children’s headaches, muscle aches, toothaches, colds and fevers. | ||||||||||
| Isatis Root Granule | Granules | 4.3 | % | 1.6 | % | 2.4 | % | To treat common colds and other infections of the upper respiratory tract. | ||||||||||
Among the 13 TCMD products we manufacture, Guben Yanling Pill is our signature product. For the fiscal years ended September 30, 2023, 2022, and 2021, the revenue derived from the sale of Guben Yanling Pill represented 36.4%, 42.5% and 40.3% of our total revenue.
40
Our Manufacturing Process
The following chart illustrates our main manufacturing process from raw material purchase to marketing:

Our Raw Materials and Suppliers
We select our raw materials for the manufacturing of our products strictly in accordance with the guidance in Pharmacopoeia of the People’s Republic of China (《中华药典》) (the “PPRC”), an official compendium of drugs covering both TCM and western medications complied by the Pharmacopoeia Commission of the Ministry of Health of People’s Republic of China. The PPRC specifies the standards of description, dosage, purity, storage, and other material information for each drug. In the manufacturing of our TCMD products, more than 110 types of raw materials are regularly used, among which angelica, codonopsis, poria mushroom, isatis root, ginseng loquat leaves, safflower, and Baijiu liquor represent our main raw materials.
Currently, we have stable access to all the raw materials necessary for our production. There are many suppliers in the industry for the regularly used raw materials, and therefore we are not relying on a single supplier for any of our raw materials. If we are unable to purchase any of the raw materials from one supplier, we do not expect to face material difficulties in locating another supplier at substantially the same price. While the prices of such raw materials may vary greatly from time to time due to market forces beyond our control, we believe we can hedge such risk by adjusting our price, or absorbing higher costs if and when necessary.
To source the raw materials required for our products, we regularly contract with our suppliers by placing bulk orders with them at below market prices. Our raw material suppliers include mostly traditional Chinese medicine manufacturers and pharmaceutical trading companies. After years of business cooperation, we believe that our relationships with our current suppliers are strong and stable.
We consider our raw materials suppliers whose sales to us accounted for more than 10% of our overall purchases in any given period to be our major suppliers for such period. For the year ended September 30, 2023, one supplier accounted for 29.6% of the total purchases. For the fiscal year ended September 30, 2022, one supplier, Jiangxi Hongjing Pharmaceutical Co., Ltd., accounted for approximately 10.3% of our total purchases. For the fiscal year ended September 30, 2021, no supplier accounted for more than 10% of our total purchases.
41
Manufacturing Process
The following is a brief description of the manufacturing process of the TCMD products we currently produce by dosage forms.
Pill Products
To make our pill products, the raw materials first go through a preparation process, during which such materials are dried, roughly ground and sterilized. Processed raw materials are then finely ground, mixed with honey, and made into pills before they are finally packaged.
Granule Products
The raw materials of our granule products typically go through a purifying process, during which such materials are stewed, filtered, condensed, and let stand. Processed raw materials are then mixed with supplemental ingredients before they are made into granules, dried, and finally packaged.
Syrup Products
The raw materials of our syrup products are first stewed together and condensed. Condensed liquid is then filtered and mixed with supplemental ingredients before it is bottled and packaged.
Oral Liquid Products
The raw materials of our oral liquid products are first filtered, condensed, and fixed with other supplemental ingredients. The processed materials are then further filtered and sterilized before being bottled and packaged.
Medicinal Liquor Products
The raw materials of our medicinal liquor products first go through a purifying process, during which such materials are selected, cut, rinsed, stewed, and refrigerated. Processed raw materials then go through an extraction process that involves mixing with solvents and filtering. Then, the liquor products are bottled and packaged.
Quality Control and Assurance
We seek to ensure the high quality of our products through our quality control system and by conducting product testing and review. Our entire manufacturing process is strictly supervised pursuant to internal quality control standards that have been set up in strict adherence to the guidelines provided in PPRC. We conduct our quality testing by examining the quality of each and every type of raw materials. If the raw materials meet our quality standards, we start the manufacturing process, during which we continue our quality testing for every substantial procedure, including filtering, grinding, mixing, and pill making. After our products are packaged, we will examine various features of our final products thoroughly, including appearance, weight, taste, water content, and microorganism content.
Third-party Product Distribution
In addition to manufacturing our own products, we also distribute and sell, through our subsidiary Universe Trade, biomedical drugs, medical instruments, TCMPs and dietary supplements manufactured by third-party pharmaceutical companies. For the fiscal year ended September 30, 2023, we had distributed roughly 2,239 third-party products, of which approximately 91.69% are biochemical drugs, such as liquid glucose, prednisolone, and citicoline, approximately 8.29% are medical instruments, such as drug-eluting stents, surgical tubes and syringes, approximately 0.02% are TCMPs, such as red sage tables, Longdan Xiegan pills, and Chinese skullcap capsules. For the fiscal year ended September 30, 2022, we had distributed roughly 2,785 third-party products, of which approximately 85.0% are biochemical drugs, such as liquid glucose, prednisolone, and citicoline, approximately 14.8% are medical instruments, such as drug-eluting stents, surgical tubes and syringes, approximately 0.2% are TCMPs, such as red sage tables, Longdan Xiegan pills, and Chinese skullcap capsules. For the fiscal year ended September 30, 2021, we had distributed roughly 2,766 third-party products, of which approximately 87.30% are biochemical drugs, such as liquid glucose, prednisolone, and citicoline, approximately 12.58% are medical instruments, such as drug-eluting stents, surgical tubes and syringes, approximately 0.11% are TCMPs, such as red sage tables, Longdan Xiegan pills, and Chinese skullcap capsules and approximately 0.01% are dietary supplements, such as vitamins, probiotic powder, and calcium tablets.
42
Our Suppliers of Third-party Products
We source third-party pharmaceutical products from their manufacturers in China. Our third-party product suppliers include mostly medical instrument manufacturers, pharmaceutical product manufacturers and dietary supplement manufacturers. For all of the products that we source and sell, we can generally find similar replacements in the market from the competitors of our current suppliers. Accordingly, we do not have any continuous or long-term supply agreements with any of these suppliers. We purchase third-party medical products from our suppliers on a per purchase order basis.
For the fiscal year ended September 30, 2023, 2022 and 2021, we purchased products from over 661, 658 and 665 suppliers, respectively. For the fiscal years ended September 30, 2023, 2022 and 2021, we did not have any supplier of third-party products whose sales to us accounted for more than 10% of our overall purchases of that fiscal year.
Our Customers
Our customers are mostly pharmaceutical distributors, hospitals, clinics and drugstore chains with pharmaceutical business qualification certificates, awarded and authorized by the NMPA and are authorized to sell and deliver our products to end consumers. As of the date of this annual report, our customers are scattered over 261 cities of 30 provinces in China. We determine whether to establish long-term business relationships with our customers primarily based on two factors, their ability to promote our products and their ability to make payments on time.
As of September 30, 2023, we had total of 2,541 customers, of which 1,267 were pharmaceutical distributors, 279 were clinics, 356 were drug stores, and 639 were hospitals. As of September 30, 2022, we had total of 2,651 customers, of which 1,351 were pharmaceutical distributors, 430 were clinics, 389 were drug stores, and 481 were hospitals.
For the fiscal year ended September 30, 2023, our revenues generated from sales to pharmaceutical distributors, hospitals, clinics and drugstore chains represented 59.26%, 22.02%, 13.50% and 5.22% of our total revenues, respectively. For the fiscal year ended September 30, 2022, our revenues generated from sales to pharmaceutical distributors, hospitals, clinics and drugstore chains represented 49.3%, 10.9%, 8.6%, and 31.2% of our total revenues, respectively. For the fiscal year ended September 30, 2021, our revenues generated from sales to pharmaceutical distributors, hospitals, clinics and drugstore chains represented 59.78%, 29.63%, 10.24% and 0.35% of our total revenues, respectively.
None of our customers generated more than 10% of our revenue for the fiscal years ended September 30, 2023, 2022 and 2021. However, our top 10 customers aggregately accounted for 27.2%, 32.2% and 30.4% of our total revenue for the fiscal years ended September 30, 2023, 2022 and 2021, respectively.
Marketing and Sales
We believe that marketing activities are crucial to our success in the competitive Chinese patent medicine industry. As of September 30, 2023, we had a total of 68 employees in our marketing department. Employees in our marketing department are mainly responsible for performing various marketing activities, including researching the most updated industry and market information, analyzing market trends and consumer preferences, setting up marketing strategies, executing sales contracts, communicating with existing customers and networking with potential customers.
Our marketing and sales initiatives for the next several years will focus on three objectives: developing a strong brand image, building a successful marketing team, and expanding retail channels. To develop our brand image as a producer of TCMD products aiming at addressing the physical conditions of the elderly during the aging process and promoting their general well-being, we seek to promote our brand “Bai Nian Dan (百年丹)” utilizing both online marketing channels such as WeChat official account and other social media and traditional platforms such as television, newspapers, and live radio stations. As part of the efforts to build a successful marketing team, we intend to hire additional sales talents and provide monetary and equity incentives to sales employees. For the purpose of expanding our retail channels, we plan to open an online retail store, and according to the preferences of online shoppers, we may adjust the sizes, packaging, or prices of our products.
43
Research and Development
We established a research and development department in 1998. Our research and development team has been focusing on the upgrade of current products and the development of production techniques to increase productivity. After years of continued development, our research and development department has become the core of our technological innovation efforts. As of September 30, 2023, we had 23 employees dedicated to research and development.
Research and Development (“R&D”) Achievements
Our research and development team has invented patented technologies to enhance the quality of our products and our manufacturing efficiency. For instance, our patented TCM mixer is able to mix powders more evenly and thoroughly compared to a traditional mixing machine, thereby increasing the quality of the mixed medicine powder. The special design of our patented TCM concentration device is able to increase the contact area of the liquid medicine as compared to a regular concentration device, thereby increasing the manufacturing efficiency of our products in liquid dosage form.
As a result of our efforts, our subsidiary, Jiangxi Universe, became certified as a National High-Tech Enterprise by the Science and Technology Department of Jiangxi Province in December 2013, with a term of three years. We successfully renewed the certificate in December 2019 and November 2022. This certification entitles Jiangxi Universe to a favorable corporate income tax of 15%, rather than the unified tax rate of 25% it would pay if it were not certified.
R&D Development Plan
To further our strong brand image, we plan to develop products designed to address the physical conditions of the elderly during the aging process and promoting their general well-being, including TCMD products and dietary supplements. In the upcoming years, we intend to focus on the development of immunity boost products and sleep aids.
In addition to our own efforts, our research and development team also intends to collaborate with other industry professionals and TCM experts with respect to the development of products we plan to launch in the future.
Competition
We compete with pharmaceutical companies in China that manufacture and sell products similar to ours. Furthermore, many of these companies are more established than we are, and have significantly greater financial, technical, and other resources than we presently possess. Some of our competitors may be able to respond more quickly to new opportunities, market changes or changes of customer preferences, and may be able to undertake more extensive promotional activities, offer more attractive terms to distributors, and adopt more aggressive pricing strategies than we are. Despite that, we believe we are well-positioned to compete in this market with our diversified product portfolio, recognized brand name, established sales and marketing network and experienced management team with a proven track record.
44
Competitors of our products
The following table sets forth the competitors of our products.
| Products | Competitors | |
| Guben Yanling Pill | Taiyuan Daningtang Pharmaceuticals Co., Ltd.; Shenyang Dongxin Pharmaceutical Industry Co., Ltd. | |
| Shenrong Weisheng Pill | China Beijing Tong Ren Tang Group Co., Ltd.; Jiangxi Zhongyuan Pharmaceutical Co., Ltd. | |
| Quanlu Pill | Renhe Pharmaceuticals Co.; Guangzhou Pharmaceutical Co., Ltd. | |
| Fuzi Lizhong Pill | China Beijing Tong Ren Tang Group Co., Ltd. | |
| Yangxue Danggui Syrup | Sichuan Tiancheng Pharmaceuticals Co., Ltd. | |
| Qiangli Pipa Syrup | Jiangzhong Pharmaceuticals Co., Ltd.; China Resources Sanjiu Medical & Pharmaceuticals Co., Ltd.; Jiangxi Tengwangge Pharmaceuticals Co., Ltd. | |
| Paracetamol and Chlorpheniramine Maleate Granules for Children |
China Resources Sanjiu Medical & Pharmaceuticals Co., Ltd.; Sunflower Pharmaceutical Group Co., Ltd. | |
| Isatis Root Granules | Guangzhou Pharmaceuticals Co., Ltd.; China Resources Sanjiu Medicine & Pharmaceuticals Co., Ltd.; China Beijing Tong Ren Tang Group Co., Ltd. | |
| Wuzi Yanzong Oral Liquid | China Beijing Tong Ren Tang Group Co., Ltd. | |
| Shuquan Dabu Medicinal Liquor | Jiangxi Puzheng Pharmaceuticals Co., Ltd. | |
| Shenrong Medicinal Liquor | Jiangxi Puzheng Pharmaceuticals Co., Ltd. | |
| Qishe Medicinal Liquor | Jiangxi Zhongyuan Pharmaceuticals Co., Ltd. | |
| Fengtong Medicinal Liquor | Jiangxi Zhongyuan Pharmaceuticals Co., Ltd. |
Competitors of Third-party Products
Our competitors of pharmaceutical products, including biochemical drugs and TCMPs, are many internationally and nationally well-known manufacturers and distributors, including China Beijing Tong Ren Tang Group Co., Ltd., Yunnan Baiyao Group, China Resources Sanjiu Medicine & Pharmaceuticals Co., Ltd., and Guangzhou Baiyunshan Pharmaceutical Holdings Co., Ltd.
Our competitors in the medical instrument market include many well-known manufacturers and distributors of medical instruments, including Shinva Medical Instrument Co., Ltd., Jiangsu Yuyue Medical Equipment & Supply Co., Ltd., Lepu Medical Technology (Beijing) Co., Ltd., and Shanghai Runda Medical Technology Co., Ltd.
Our competitors in the dietary supplement market include internationally and nationally well-known manufacturers and distributors of dietary supplements, such as By-health Co., Ltd., Amway (China) Co., Ltd., and Perfect (China) Co., Ltd.
We intend to compete with these larger companies by appealing to the specific needs and preferences of our customers and offering competitive prices.
45
Employees
As of September 30, 2023, 2022, and 2021, we had a total of 225, 224 and 263 employees, all of whom are located in China. The following table sets forth the number of our employees by function as of September 30, 2023.
| Function | Number of Employees | % of Total | ||||||
| Purchasing Department | 3 | 1 | % | |||||
| Warehouse Department | 10 | 4 | % | |||||
| Manufacturing Department | 89 | 40 | % | |||||
| Quality Control Department | 18 | 8 | % | |||||
| Research and Development Department | 23 | 10 | % | |||||
| Marketing Department | 69 | 30 | % | |||||
| Finance Department | 7 | 3 | % | |||||
| Administration Department | 7 | 3 | % | |||||
| Total | 225 | 100 | % | |||||
Our success depends on our ability to attract, retain and motivate qualified personnel. As part of our human resources strategy, we offer employees competitive salaries and other bonuses and incentives.
We primarily recruit our employees in China through direct hiring. We provide training to new employees that we hire. We also conduct regular and specialized internal training to meet the needs of our employees in different departments. We believe that such training is effective in equipping our employees with the skill set and work ethics we require.
As required under PRC regulations, we participate in various employee social security plans that are organized by applicable local municipal and provincial governments, including housing, pension, medical, work-related injury, maternity and unemployment benefit plans.
We enter into standard contracts and agreements regarding confidentiality, intellectual property, employment, ethic policies and non-competition with most of our executive officers, managers and employees. These contracts typically include a non-competition provision effective during and up to one year after termination of their employment with us and a confidentiality provision effective during and up to one year after their employment with us.
Our employees have not formed any employee union or association. We believe that we maintain a good working relationship with our employees and we have not experienced any difficulty in recruiting staff for our operations as of the date of this annual report.
46
Properties and Facilities
Our corporate headquarters are located in Jinggangshan, Jiangxi Province, China. We own properties in Jingggangshan as office spaces, storage facilities and manufacturing facilities with an aggregate gross floor area of approximately 825,563 square feet. We believe that our existing facilities are generally adequate to meet our current needs, but we expect to seek additional space as needed to accommodate future growth. Following is a list of our properties all of which we own the land use rights to:
| No. | Land Use Right Holder |
Property Address | Use of Property |
Area in Square Feet |
Terms of Use | |||||
| 1 | Jiangxi Universe | 265 Jingjiu Avenue, Economy and Technology Development District, Jinggangshan, Ji’an, Jiangxi, China | Manufacturing | 173,467 | October 2053 | |||||
| 2 | Jiangxi Universe | 265 Jingjiu Avenue, Economy and Technology Development District, Jinggangshan, Ji’an, Jiangxi, China | Manufacturing | 470,921 | October 2053 | |||||
| 3 | Jiangxi Universe | 265 Jingjiu Avenue, Economy and Technology Development District, Jinggangshan, Ji’an, Jiangxi, China | Manufacturing | 57,010 | October 2053 | |||||
| 4 | Jiangxi Universe | 265 Jingjiu Avenue, Economy and Technology Development District, Jinggangshan, Ji’an, Jiangxi, China | Storage | 27,426 | October 2053 | |||||
| 5 | Jiangxi Universe | 265 Jingjiu Avenue, Economy and Technology Development District, Jinggangshan, Ji’an, Jiangxi, China | Manufacturing | 29,276 | October 2053 | |||||
| 6 | Jiangxi Universe | 265 Jingjiu Avenue, Economy and Technology Development District, Jinggangshan, Ji’an, Jiangxi, China | Storage | 57,083 | October 2053 | |||||
| 7 | Jiangxi Universe | 265 Jingjiu Avenue, Economy and Technology Development District, Jinggangshan, Ji’an, Jiangxi, China | Manufacturing | 10,380 | October 2053 |
We manufacture all of our products at the properties listed above. Currently, we are capable of producing a maximum of approximately 12 million bottles of liquid products, 13 million boxes of pill products, and 10 million boxes of solid products annually.
Intellectual Property
We regard our patents, trademarks, domain names and other intellectual property critical to our business operations. We rely on laws and regulations on patents, trademarks and domain names to protect our intellectual property. As of the date of this annual report, we have registered 73 patents in China, including 45 utility model patents, 17 design patents, and 11 invention patents, and 99 trademarks in China, including our well-recognized brands “Bai Nian Dan (百年丹)”, “Hu Zhuo Ren (胡卓仁)” and “Long Zhong (龙种).”
We implement a set of comprehensive measures to protect our intellectual property, in addition to making trademark and patent registration application. Key measures include: (i) timely registration, filing and application for ownership of our intellectual property, (ii) actively tracking the registration and authorization status of our intellectual property and take action in a timely manner if any potential conflicts with our intellectual property are identified, and (iii) clearly stating all rights and obligations regarding the ownership and protection of our intellectual property in all employment contracts and commercial contracts we enter into.
As of the date of this annual report, we have not been subject to any material dispute or claims for infringement upon third parties’ trademarks, licenses and other intellectual property rights in China.
Seasonality
We currently do not experience seasonality in our business.
Environmental Matters
We comply with the Environmental Protection Law of China as well as applicable local regulations. In addition to statutory and regulatory compliance, we actively ensure the environmental sustainability of our operations. Parties may be levied upon us if we fail to adhere to and maintain certain standards. Such failure has not occurred in the past, and we generally do not anticipate that it will occur in the future, but no assurance can be given in this regard.
Insurance
We maintain certain insurance policies to safeguard us against risks and unexpected events. For example, we provide social security insurance including pension insurance, unemployment insurance, work-related injury insurance and medical insurance for our employees in compliance with applicable PRC laws. We also maintain directors and officers’ liability insurance. We do not maintain business interruption insurance or product liability insurance, which are not mandatory under PRC laws. We do not maintain key man insurance, insurance policies covering damages to our network infrastructures or information technology systems nor any insurance policies for our properties. During the fiscal years ended September 30, 2023, 2022 and 2021, we did not make any material insurance claims in relation to our business.
47
Legal Proceedings
We may from time to time become a party to various legal administrative proceedings arising in our ordinary course of our business. As of the date of this annual report, neither we nor any of our subsidiaries is a party of any material legal proceeding.
Regulations
This section sets forth a summary of the principal PRC laws, regulations, and rules relevant to our PRC operating entities’ business and operations in China.
We are a pharmaceutical manufacturer in China. This section sets forth a summary of applicable laws, rules, regulations, government and industry policies and requirement that have a significant impact on our operations and business in China. This summary does not purport to be a complete description of all laws and regulations, which apply to our business and operations. Investors should note that the following summary is based on relevant laws and regulations in force as of the date of this annual report, which may be subject to change.
Major Regulatory Authorities
The pharmaceutical industry in the PRC is mainly administered by four governmental agencies: (i) the NMPA, a department under the State Administration for Market Regulation (the “SAMR”) (国家市场监督管理总局), (ii) the National Health Commission (the “NHC”) (国家卫生健康委员会), (iii) the National Administration of Traditional Chinese Medicine (the “NATCM”) (国家中医药管理局), and (iv) the National Healthcare Bureau (the “NHB”) (国家医疗保障局).
The NMPA, whose predecessor is the China Food and Drug Administration, or the CFDA, is the primary regulator of almost all key stages of the life-cycle of pharmaceutical products, including non-clinical researches, clinical trials, marketing approvals, manufacturing, advertising and promotion, distribution and pharmacovigilance.
The NHC, formerly known as the National Health and Family Planning Commission, is the principal regulator of healthcare in China. It is primarily responsible for drafting national healthcare policies and regulating public health, medical services and health contingency system, coordinating the healthcare reform and overseeing the operation of medical institutions and professional practice of medical personnel. The NHC is responsible for (1) the research, production, circulation and use of Chinese medicines, including traditional Chinese medicine; (2) the preparation and publication of the Chinese Pharmacopoeia; and (3) the supervision of the selection, approval, distribution and revision of the National OTC Drug Catalogue. In addition, the CFDA and its local administrative authorities may take a number of enforcement actions to enforce their regulations.
The NATCM is an agency under the NHC that oversees China’s traditional Chinese medicine industry.
The NHB, a new authority established in May 2018, is responsible for (1) drafting and implementing policies, plans and standards on medical insurance, maternity insurance and medical assistance; (2) administering healthcare fund; (3) formulating a uniform medical insurance catalogue and payment standards on drugs, medical disposables and healthcare services; and (4) formulating and administering the bidding and tendering policies for drugs and medical disposables.
Regulations Related to Pharmaceutical Manufacture
Manufacturing License
Pursuant to the Pharmaceutical Administration Law of the People’s Republic of China (《中华人民共和国药品管理法》), which was promulgated in 1984 by the SCNPC and last amended in August 2019, a pharmaceutical manufacturer is required to obtain its manufacturing license from the NMPA before it starts to manufacture pharmaceutical products. Prior to granting such permit, the relevant government authority will inspect the applicant’s production facilities, and assess whether the sanitary conditions, quality assurance system, management structure and equipment at the production facilities have met the required standards. A manufacturing license is valid for a period of five years and the manufacturer is required to apply for renewal within six months prior to its expiration date. The manufacturer will be subject to reassessment by the authority in accordance with then prevailing legal and regulatory requirements for the purposes of such renewal. Currently, our subsidiary Jiangxi Universe holds a valid manufacturing license from the NMPA issued on December 21, 2020 and valid until December 20, 2025.
48
Contract Manufacturing of Drugs
Pursuant to the Administrative Regulations for the Contract Manufacturing of Drugs (《药品委托生产监督管理规定》) (the “Contract Manufacturing Regulations”) issued by the NMPA in August 2014, in the event that a drug manufacturer in China with drug marketing authorization temporarily lacks manufacturing capability as a result of technology upgrade or is unable to meet market demand due to insufficient manufacturing capacity, it may use contract manufacturer for its drug manufacturing. Contract manufacturing arrangements need to be approved by the provincial branch of the NMPA. The Contract Manufacturing Regulations prohibit contract manufacturing arrangements for certain special drugs, including narcotic drugs, psychoactive drugs, biochemical drugs and active pharmaceutical ingredients.
Other Regulations in relation to the Pharmaceutical Industry
“Two-vote system” for drug sales
The NHC and other six ministries and commissions issued the Notice on the Opinions on the Implementation of the “Two-Invoice System” in Drug Procurement by Public Medical Institutions (for Trial Implementation) (《关于在公立医疗机构药品采购中推行“两票制”的实施意见 (试行) 的通知》) (the “Notice on Two-Invoice System”) on January 11, 2017. “Two-invoice system” means that one invoice shall be issued by a pharmaceutical manufacturer to a distributor, and another invoice shall be issued by the distributor to a hospital. An internal transfer of drugs from a group pharmaceutical distributor to its wholly owned or controlled subsidiary or a transfer of drugs between such wholly owned subsidiaries may not be deemed as “one invoice” however, the invoicing for the whole group can be done only once. Pharmaceutical manufacturers and distributors shall reasonably determine the markup level in the spirit of fairness, legality, legitimacy and integrity. Public medical institutions is encouraged to settle the payment for drugs directly with pharmaceutical manufacturers, and pharmaceutical manufacturers and are encouraged to settle the delivery cost with distributors.
In the sale of drugs, drug manufacturers and distributors shall issue value-added tax (“VAT”) special invoices or normal VAT invoices in accordance with the regulations regarding invoice control. The sold drug shall also be delivered in a way that confirms to the requirements of the Good Supply Practice for Pharmaceutical Products (2016 version) (《药品经营质量管理规范(2016修订)》), and the names of the purchaser and seller on the invoices shall be consistent with the delivery form, payment flow and amount.
Drug Advertisements
Pursuant to the Interim Administrative Measures for the Review of Advertisements for Drugs, Medical Devices, Health Food and Formula Food for Special Medical Purposes (《药品、医疗器械、保健食品、特殊医学用途配方食品广告审查管理暂行办法》) promulgated on December 24, 2019 and effective on March 1, 2020, an enterprise seeking to advertise its drugs must apply for an advertising approval code. An advertising approval code shall expire on the earlier of the expiration dates of the product’s registration certificate, filing certificate or production license. If an expiration date is not prescribed in the product’s registration certificate, filing certificate or production license, the advertising approval code shall be valid for two years. The contents of an approved advertisement shall not be altered without prior approval. Where an advertisement needs to be edited, the enterprise shall submit an application for a new advertisement approval code.
49
Insert Sheet, Labels and Packaging of Pharmaceutical Products
According to the Measures for the Administration of the Insert Sheets and Labels of Drugs (《药品说明书和标签管理规定》) effective on June 1, 2006, the insert sheets and labels of a pharmaceutical product shall be reviewed and approved by the NMPA. A drug insert sheet should include the scientific data, conclusions and information concerning drug safety and efficacy in order to direct the safe and reasonable use of pharmaceutical products. The inner label of a pharmaceutical product shall indicate the product name, indication or function, strength, dose and usage, production date, batch number, expiration date and drug manufacturer, and the outer label of a pharmaceutical product shall indicate the product name, ingredients, description, indication or function, strength, dose and usage and adverse reactions.
According to the Measures for the Administration of Pharmaceutical Packaging (《药品包装管理办法》) effective on September 1, 1988, pharmaceutical packaging must comply with national and professional standards. If no national or professional standards are available, a manufacturer can formulate and implement its own standards after it receives approval from the provincial food and drug administration or bureau of standards. The company shall reapply for approval if it were to change its own packaging standards. Pharmaceutical products with no approved packing standards shall not be sold or traded in the PRC, except for drugs for military use.
Drug Technology Transfer
On August 19, 2009, the NMPA promulgated the Administrative Regulations for Technology Transfer Registration of Drugs (《药品技术转让注册管理规定》) (the “Technology Transfer Regulations”) to regulate the drug technology transfer process, including the application, evaluation, examination, approval and monitoring of, drug technology transfer. Drug technology transfer means that the owner transfers its pharmaceutical manufacturing technology to a pharmaceutical manufacturer and that the transferee applies for drug registration pursuant to the Technology Transfer Regulations. Drug technology transfer includes the transfer of new drug technology and drug manufacturing technology.
Applications for drug technology transfer shall be submitted to the provincial drug regulatory authority where the transferee is located. The drug regulatory authority examines application materials and conducts on-site inspections of the transferee’s manufacturing facilities. If the transferor and the transferee are located in different provinces, the provincial drug regulatory authority where the transferor is located shall issue examination opinions as well. The Center for Drug Evaluation (the “CDE”), a branch of the NMPA, shall further review the application materials, provide technical evaluation opinions and form a comprehensive evaluation opinion based on the on-site inspection reports and the testing results of the samples. The NMPA shall determine whether to approve the application according to the comprehensive evaluation opinion of the CDE. An approval letter of supplementary application and a drug approval number will be issued for qualified applications.
Price of drugs
Pursuant to the Drug Administration Law (《药品管理法》), for those drugs whose prices are determined by market, manufacturers and distributors of pharmaceutical products and medical institutions shall set the prices in accordance with the principles of fairness, rationality, and good faith, and provide consumers with drugs at reasonable prices. Pharmaceutical product manufacturers, distributors and medical institutions shall determine and indicate their products’ retail prices in accordance with the regulations over drug prices promulgated by the pricing department of the State Council of the People’s Republic of China (the “State Council”).
On May 4, 2015, the National Development and Reform Commission, the National Health and Family Planning Commission, the Ministry of Human Resources and Social Security, the Ministry of Industry and Information Technology, the Ministry of Finance, the MOFCOM and the NMPA jointly issued the Notice Regarding Reforms to the Price of Medical Products (《关于印发推进药品价格改革意见的通知》), pursuant to which, since June 1, 2015, other than anesthetics and Class 1 psychotropic drugs, the actual price of pharmaceutical products shall be decided by market instead of by the government. As of the date of this annual report, the actual price of all products we sell, including TCMD products and third-party products, are determined by market.
50
Regulation in relation to medical device registration
Pursuant to the Regulations on Supervision and Administration of Medical Devices (《医疗器械监督管理条例》) promulgated by the State Council of China with the last amendment effective on June 1, 2021, medical devices are classified based on the invasiveness of, and risks associated with, each medical device. Class I medical devices have relatively low risks, whose safety and effectiveness can be guaranteed through routine administration. Class II medical devices have moderate risks and require strict control and administration to ensure their safety and effectiveness. Class III medical devices have relatively high risks and require especially strict control and administration measures to ensure their safety and effectiveness. Pursuant to the Administrative Measures for the Medical Devices Registration and Record (《医疗器械注册与备案管理办法》), which became effective on October 1, 2021, manufacturers of Class I medical devices are required to file with the local food and drug administrative authority at the city level. Manufacturers of Class II medical devices shall obtain product registration certificates from and be subject to the inspection and approval of the drug administrative authority at the provincial level. Manufacturers of Class III medical devices shall obtain product registration certificates from the NMPA and be subject to its inspection and approval.
As of the date of this annual report, we are engaged in the business of selling medical devices. We sell medical devices categorized as Class I, Class II and Class III under the Regulation on Supervision and Administration of Medical Devices in the PRC, and we obtained our medical device selling license in April 2020 in compliance with the applicable PRC laws and regulations.
Regulation relating to Company Establishment and Foreign Investment
The PRC Company Law applies to the establishment, operation and management of both PRC domestic companies and foreign-invested enterprises. Foreign investment in the PRC corporate entities are also regulated by the foreign-Owned Enterprise Law of the PRC (《中华人民共和国外资企业法》) (the “Foreign-Owned Enterprise Law”) promulgated on April 12, 1986 and amended on October 31, 2000 and September 3, 2016, the Implementing Rules for the Foreign-Owned Enterprise Law of the PRC (《中华人民共和国外资企业法实施细则》) promulgated on December 12, 1990 and amended on April 12, 2001 and February 19, 2014, and the Interim Administrative Measures for the Record-filing of the Incorporation and Change of Foreign-invested Enterprises (《外商投资企业设立及变更备案管理暂行办法》) (the “Record-filing Measures”) promulgated on October 8, 2016 and amended on July 30, 2017 and June 29, 2018. Under these laws and regulations, the establishment of a wholly foreign-owned enterprise is subject to the approval of, or the filing with the MOFCOM or its local counterpart, and such wholly foreign-owned enterprises must register and file with the appropriate administrative bureau of industry and commerce.
The Foreign Investment Law of the People’s Republic of China (《中华人民共和国外商投资法》) (the “Foreign Investment Law”), which was promulgated by the National People’s Congress On March 15, 2019, and came into effect on January 1, 2020, provides that foreign investment refers to the investment activities in China carried out directly or indirectly by foreign natural persons, enterprises or other organizations (the “Foreign Investors”), including the following: (1) Foreign Investors establishing foreign-invested enterprises in China alone or collectively with other investors; (2) Foreign Investors acquiring shares, equities, properties or other similar rights of Chinese domestic enterprises; (3) Foreign Investors investing in new projects in China alone or collectively with other investors; and (4) Foreign Investors investing through other ways prescribed by laws and regulations or the State Council. The State adopts the management system of pre-establishment national treatment and negative list for foreign investment. The pre-entry national treatment means the treatment given to Foreign Investors and their investments at the stage of investment access is not lower than that of domestic investors and their investments. The negative list management system means that the state implements special administrative measures for access of foreign investment in specific fields. Foreign investors shall not invest in any forbidden fields stipulated in the negative list and shall mean the conditions stipulated in the negative list before investing in any restricted fields. The negative list is released upon approval of the State Council. After the Foreign Investment Law came into effect, it replaced the Foreign-Owned Enterprise Law.
The Implementation Regulations for the Foreign Investment Law of the PRC (《中华人民共和国外商投资法实施条例》) (the “Implementation Regulations for the FIL”) was adopted at the 74th executive meeting of the State Council on December 12, 2019 and came into effect on January 1, 2020. The purpose of the Implementation Regulations for the FIL is to encourage and promote foreign investment, protect the legitimate rights and interests of investors, regulate the administration of foreign investment, and continuously optimize the foreign investment environment. For those foreign-invested enterprises established prior to the implementation of the Foreign Investment Law and established in accordance with the Law of the People’s Republic of China on Sino-foreign Joint Ventures (《中华人民共和国中外合资经营企业法》), the Law of the People’s Republic of China on Foreign-invested Enterprises, and the Law of the People’s Republic of China on Sino-Foreign Cooperative Enterprises, they can modify or retain their organizational forms and organizational structures in accordance with the PRC Company Law, Partnership Law of the People’s Republic of China and other applicable laws within 5 years since the implementation of the Foreign Investment Law.
51
Foreign investment in China shall comply with the Catalogue for Encouraged Foreign Investment (2022 Revision) (《鼓励外商投资产业目录(2022年版)》), or the 2022 Catalogue, and the Special Administrative Measures for the Access of Foreign Investment (Negative List) (Edition 2022) (《外商投资准入特别管理措施(负面清单) (2022年版)》), or the 2022 Negative List, which were issued on March 12, 2022 and came into effect on the same date. The Catalogue classifies foreign-invested industries into two categories, (1) encouraged foreign-invested industries; and (2) foreign-invested industries that are subject to the 2018 Negative List. The 2018 Negative List set out restrictions such as shareholding requirements and qualifications of the senior management. According to the Record-filing Measures, foreign investments that are not subject to special access administrative measures are only required to complete an online filing with the MOFCOM or its local counterpart. The scope of our business as approved by the licensing authority and the actual scope of our business are not subject to the restrictions set forth in the 2021 Negative List.
The M&A Rules were jointly promulgated by the MOFCOM, the State-Owned Assets Supervision and Administration Commission of the State Council, the SAT, the SAIC, the CSRC, and the SAFE on August 8, 2006 and was amended by MOFCOM on June 22, 2009. The M&A Rules provides that a foreign investor is required to obtain necessary approvals when it: (1) acquires equity interests in a domestic enterprise or subscribes to additional shares in a domestic enterprise; (2) purchases the assets of a domestic enterprise through establishment of a foreign-invested enterprise; or (3) establishes a foreign-invested enterprise through which it purchases the assets of a domestic enterprise and operates these assets. In particular, any PRC company, enterprise or individual is required to obtain approval from the MOFCOM and comply with applicable laws and regulations if it establishes an offshore company and attempts to acquire a domestic enterprise related to such offshore company.
Regulation in relation to Intellectual Property Rights
In terms of international conventions, China has entered into (including but not limited to) the Agreement on Trade-Related Aspects of Intellectual Property Rights (《与贸易有关的知识产权协定》), the Paris Convention for the Protection of Industrial Property (《保护工业产权巴黎公约》), the Madrid Agreement Concerning the International Registration of Marks (《商标国际注册马德里协定》) and the Patent Cooperation Treaty (《专利合作协定》).
Patents
Pursuant to the Patent Law of the PRC (《中华人民共和国专利法》), or the Patent Law, promulgated by the SCNPC on March 12, 1984 and last amended on October 17, 2020 and effective from June 1, 2021 and the Implementation Rules of the Patent Law of the PRC (《中华人民共和国专利法实施细则》), promulgated by the State Council on June 15, 2001 and amended on December 28, 2002 and January 9, 2010, respectively, patents in China fall into three categories: invention patents, utility model patents and design patents. The term of patent protection starts from the date of application and lasts 20 years for invention patents and 10 years for utility model patents and design patents. Any individual or entity that utilizes a patent or conducts any other activity that infringes a patent without the patent holder’s authorization shall pay compensation to the patent holder and be subject to a fine imposed by regulatory authorities and, if such behavior constitutes a crime, shall be held criminally liable in accordance with applicable laws. According to the Patent Law, for public health purposes, the State Intellectual Property Office of the PRC, or SIPO, may grant a compulsory license for manufacturing patented drugs and exporting them to countries or regions covered under relevant international treaties to which PRC has acceded. In addition, under the Patent Law, any organization or individual that applies for a patent in a foreign country for an invention or utility model patent established in China is required to report to the SIPO for confidentiality examination. Patent holders may apply for extending the terms of patented drugs to compensate for commercialization delays due to regulatory review, and may seek punitive damages for willful patent infringement under severe circumstances.
52
Trade Secrets
Pursuant to the PRC Anti-Unfair Competition Law (《中华人民共和国反不正当竞争法》) promulgated by the SCNPC in September 1993, as amended on November 4, 2017 and April 23, 2019 respectively, the term “trade secrets” refers to technical and business information that is unknown to the public, has utility, may create business interests or profits for its legal owners or holders, and is maintained as a secret by its legal owners or holders. Under the PRC Anti-Unfair Competition Law, business persons are prohibited from infringing others’ trade secrets by: (1) obtaining trade secrets from the legal owners or holders by any unfair methods, such as theft, bribery, fraud, coercion, electronic intrusion, or any other illicit means; (2) disclosing, using or permitting others to use the trade secrets obtained illegally under item (1) above; or (3) disclosing, using or permitting others to use the trade secrets, in violation of any contractual agreements or any requirements of the legal owners or holders to keep such trade secrets in confidence. If a third party knows or should have known of the above- mentioned illegal conduct but nevertheless obtains, uses or discloses trade secrets of others, the third party may be deemed to have committed a misappropriation of the others’ trade secrets. The parties whose trade secrets are being misappropriated may petition for administrative corrections, and regulatory authorities may stop any illegal activities and impose fines on the infringing parties.
Trademarks
In accordance with the Trademark Law of the PRC (《中华人民共和国商标法》) (the “Trademark Law”), which was promulgated by the SCNPC on August 23, 1982, and was last amended on April 23, 2019 and came into effect on November 1, 2019, any trademark which is registered with the approval of the Trademark Office is a registered trademark, including commodity trademark, service trademark, collective trademark, certification trademark, and the trademark registrant has the exclusive right to use a registered trademark and such right is protected by law. A registered trademark is valid for a period of 10 years commencing from the date on which the registration is approved. Upon expiration of the trademark, the registrant shall apply for renewal within twelve months prior to the expiration date if it intends to maintain exclusive use of the trademark. If the registrant fails to apply for renewal, a grace period of six months may be granted. In the absence of a renewal upon the expiration of a trademark registration, the registered trademark shall be canceled. Use of a trademark that is identical with or similar to a registered trademark, for the same kind of or similar commodities, without authorization of the trademark registrant, constitutes infringement of the exclusive right to use a registered trademark. Industrial and commercial administrative authorities have the authority to investigate any behavior that may constitute an infringement of the exclusive right under a registered trademark.
Domain names
Domain names are protected under the Administrative Measures on the Internet Domain Names (《互联网域名管理办法》), which was promulgated by the Ministry of Industry and Information Technology, or the MIIT, on August 24, 2017 and came into effect on November 1, 2017, and the Implementing Rules of China Internet Network Information Center on the Registration of Domain Names (《中国互联网络信息中心域名注册实施细则》) issued by China Internet Network Information Center (the “CNNIC”) on May 28, 2012 and came into effect on May 29, 2012. Domain name registrations are handled through domain name service agencies established under the relevant regulations, and the applicant becomes domain name holder upon successful registration. Domain name disputes shall be submitted to an organization authorized by CNNIC for resolution.
Other laws
Product Liability
The Product Quality Law of the PRC (《中华人民共和国产品质量法》), promulgated by the SCNPC on February 22, 1993 and last amended on December 29, 2018, governs the supervision and administration of product quality and specifies the liabilities of manufacturers and sellers. A manufacture shall be liable for compensating for any bodily injuries or property damages, other than the defective product itself, resulting from the defects in the product, unless the manufacturer is able to prove that: (1) the product has never been distributed; (2) the defects causing injuries or damage did not exist at the time when the product was distributed; or (3) the science and technology at the time when the product was distributed was at a level incapable of detecting the defects. A seller shall pay compensation if it fails to indicate either the manufacturer or the supplier of the defective product. A person who is injured or whose property is damaged by the defects in the product may claim for compensation from the manufacturer or the seller.
53
Pursuant to the Civil Code of the People’s Republic of China (《中华人民共和国民法典》), promulgated by the SCNPC on May 28, 2020 and became effective on January 1, 2021, manufacturers shall assume tort liabilities where the defects in products cause damage to others. Sellers shall assume tort liability where the defects in products that have caused damage to others are attributable to the sellers. The aggrieved party may claim for compensation from the manufacturer or the seller of the defected product that has caused damage.
Environmental Protection
Pursuant to the Environmental Protection Law of the PRC (《中华人民共和国环境保护法》) promulgated by the SCNPC on December 26, 1989 and amended on April 24, 2014, the Environmental Impact Assessment Law of the PRC (《中华人民共和国环境影响评价法》), promulgated by the SCNPC on October 28, 2002 and amended on July 2, 2016 and December 29, 2018 respectively, the Administrative Regulations on the Environmental Protection of Construction Project (《建设项目环境保护管理条例》) promulgated by the State Council on November 29, 1998 and amended on July 16, 2017, and other applicable environmental laws and regulations, an enterprise with a project construction plan shall submit an environmental impact assessment report, stating the environmental impacts the project is likely to have, to the administrative authority of environmental protection for approval. Enterprises shall engage qualified institutions to issue the environmental impact assessment reports.
According to the Law of the PRC on the Prevention and Control of Water Pollution (《中华人民共和国水污染防治法》) promulgated by the SCNPC on May 11, 1984 and last amended on June 27, 2017, and effective on January 1, 2018, the Law of the PRC on the Prevention and Control of Atmospheric Pollution (《中华人民共和国大气污染防治法》) promulgated by the SCNPC on September 5, 1987 and last amended on October 26, 2018, the Law of the PRC on the Prevention and Control of Pollution from Environmental Noise (《中华人民共和国环境噪声污染防治法》) promulgated by the SCNPC on October 29, 1996 and amended on December 29, 2018, and the Law of the PRC on the Prevention and Control of Environmental Pollution of Solid Waste (《中华人民共和国固体废物污染环境防治法》), promulgated by the SCNPC on October 30, 1995 and last amended on April 29, 2020, all the enterprises that may cause environmental pollution in the course of their business operation shall implement preventive and curative environmental protection measures in their plants and establish a reliable environmental protection system.
We strictly complied with the applicable environmental laws and regulations in constructing our factory. On December 8, 2004, the environmental protection bureau of local government determined that the environmental protection facilities we constructed for our factory satisfied the relevant standards. According to the certificate issued by the ecological environment bureau of local government (the “Bureau”), our factory has not been assessed on its environmental impacts since its establishment in 2004. Although there were certain procedural defects in the original construction process, we constructed our environmental protection facilities for our factory in strict compliance with the requirements of applicable PRC environmental laws and regulations. Our environmental protection facilities were approved by the Bureau in December 2004 and have since been under normal operations. As of the date of the date of this annual report, we have not been found in violation of applicable environmental laws or regulations, or imposed of administrative penalties by environmental protection authorities in the past three years. No environmental pollution incident has occurred on our manufacturing facility.
54
Foreign Exchange Control
Pursuant to the Foreign Exchange Administration Regulations of the PRC (《中华人民共和国外汇管理条例》), as amended on August 5, 2008, and the Regulation on the Administration of the Foreign Exchange Settlement, Sales and Payment (《结汇、售汇及付汇管理规定》), or the Settlement Regulations, promulgated by the People’s Bank of China on June 20, 1996 and came into effect on July 1, 1996, foreign exchanges required for profit distributions and dividend payments may be purchased from designated foreign exchange banks in the PRC upon presentation of a board resolution authorizing distribution of profits or payment of dividends.
According to the Notice of SAFE on Further Improving and Adjusting Foreign Exchange Administration Policies on Direct Investment (《国家外汇管理局关于进一步改进和调整直接投资外汇管理政策的通知》) (the “Circular No. 59”), promulgated on November 19, 2012 and last amended on December 30, 2019 by the State Administration of Exchange Control, or the SAFE, (1) the opening of and payment into foreign exchange accounts under direct investment accounts are no longer subject to approval by the SAFE; (2) reinvestment with legal income of foreign investors in China is no longer subject to approval by SAFE; (3) the procedures for capital verification and confirmation that foreign-funded enterprises need to go through are simplified; (4) purchase and external payment of foreign exchange under direct investment accounts are no longer subject to approval by SAFE; (5) domestic transfer of foreign exchange under direct investment account is no longer subject to approval by SAFE; and (6) the administration over the conversion of foreign exchange capital of foreign-invested enterprises is improved.
Pursuant to the Circular on Further Simplifying and Improving the Direct Investment-related Foreign Exchange Administration Policies (《关于进一步简化和改进直接投资外汇管理政策的通知》) (the “SAFE Circular No. 13”), which was promulgated on February 13, 2015 and became effective on June 1, 2015, the foreign exchange registration under domestic direct investment and the foreign exchange registration under foreign direct investment is directly reviewed and handled by banks in accordance with the Circular No. 13, and the SAFE and its branches shall perform indirect regulation over the foreign exchange registration via banks.
Pursuant to the Circular on the Reform of the Management Method for the Settlement of Foreign Exchange Capital of Foreign-invested Enterprises (《国家外汇管理局关于改革外商投资企业外汇资本金结汇管理方式的通知》) promulgated by the SAFE on March 30, 2015 and became effective on June 1, 2015, and the Circular on the Reform and Standardization of the Management Policy of the Settlement of Capital Projects (《国家外汇管理局关于改革和规范资本项目结汇管理政策的通知》) promulgated by the SAFE on June 9, 2016, the settlement of foreign exchange by foreign invested enterprises shall be governed by the policy of foreign exchange settlement on a discretionary basis. However, the settlement of foreign exchange shall only be used for its own operation purposes within the business scope of the foreign invested enterprises in accordance with the principle of authenticity.
Pursuant to the Circular 37, a PRC resident shall register with the local SAFE branch before he or she contributes assets or equity interests in an overseas special purpose vehicle (“Overseas SPV”), which is directly established or controlled by the PRC Resident for the purpose of conducting investment for financing. Failure to comply with the SAFE registration requirements could result in penalties for evasion of foreign exchange controls. The Circular No. 13 provides that banks can directly handle the initial foreign exchange registration and amendment registration under the Circular 37. Mr. Gang Lai completed the initial foreign registration on June 3, 2019.
Labor and Social Insurance
Pursuant to the Labor Contract Law (《中华人民共和国劳动合同法》), which was promulgated by the SCNPC on June 29, 2007 and became effective on January 1, 2008, and amended on December 28, 2012 and became effective on July 1, 2013, and the Implementing Regulations of the Labor Contracts Law of the PRC (《中华人民共和国劳动合同法实施条例》), which was promulgated by the State Council on September 18, 2008, labor contracts shall be concluded in writing if employment relationships are to be or have been established between employers and employees. In addition, employee wages cannot be lower than local standards on minimum wages.
Pursuant to the Labor Law of the PRC (《中华人民共和国劳动法》), which was promulgated by the SCNPC on July 5, 1994 and last amended and came into effect on December 29, 2018, employers shall establish and improve their system of workplace safety and sanitation, strictly abide by state rules and standards on workplace safety, educate employees in occupational safety and sanitation in the PRC. Occupational safety and sanitation facilities shall comply with state-fixed standards. Enterprises and institutions shall provide employees whose work involves occupational hazards with health examinations on a regular basis.
According to the Social Insurance Law, the Interim Regulations on the Collection and Payment of Social Security Funds (《社会保险费征缴暂行条例》), which was promulgated by the State Council on January 22, 1999 and amended on March 24, 2019, and the Administrative Regulations on the Housing Provident Funds (《住房公积金管理条例》), which was promulgated by the State Council on April 3, 1999 and last amended on March 24, 2019, employers are required to make contributions, on behalf of their employees, to a number of social security funds, including funds for basic pension insurance, unemployment insurance, basic medical insurance, occupational injury insurance, maternity insurance and to housing provident funds. Any employer who fails to make contributions may be fined and ordered to make good the deficit within a stipulated time limit.
55
Prior to April 2020, we only contributed to the social insurance and housing provident funds for some, but not all, of our employees. There is a risk that the labor security administration authority may take enforcement action to collect from us all the outstanding contributions of the social insurance and housing provident funds required to be made for the employees in the past, and we may be subject to a late charge at the rate of 0.2% per day on the total outstanding contribution. We started to contribute to the social insurance and housing provident funds for all of our employees since April 2020.
Since April 2020, we have been contributing to the social insurance and housing provident funds for our employees in accordance with the minimum contribution requirements. Pursuant to the aforementioned applicable laws and regulations, the government or an employee has the right to demand us to contribute to the employee’s social insurance and housing provident funds calculated based on his or her actual salary, and an employee who has not received contributions from us has the right to demand us to contribute to his or her social insurance and housing provident funds. As confirmed in writing by the local government, our contributions of the social insurance and housing provident funds are in compliance with the PRC laws and the local regulations and policies, and therefore the local government is very unlikely to impose any penalty on us for our contributions since April 2020.
Enterprise Income Tax
According to the EIT Law, promulgated by the National People’s Congress on March 16, 2007, effective on January 1, 2008 and last amended on December 29, 2018, and the Implementation Regulations for the Enterprise Income Tax Law of the PRC (《中华人民共和国企业所得税法实施条例》) promulgated by the State Council on December 6, 2007 and amended on April 23, 2019, other than a few exceptions, the income tax rate for both domestic enterprises and foreign-invested enterprises is 25%. Enterprise taxpayers are classified as either resident enterprises or non-resident enterprises. Resident enterprises are defined as enterprises that are established in China in accordance with PRC laws, or that are established in accordance with the laws of foreign countries but whose actual or de facto management bodies are located in China. Non-resident enterprise refers to an entity established under foreign law whose de facto management bodies are not within the PRC but which have an establishment or place of business in the PRC, or which do not have an establishment or place of business in the PRC but have income sourced within the PRC. An income tax rate of 10% will normally be applicable to dividends declared to non-PRC resident enterprise investors that do not have an establishment or place of business in the PRC, or that have such establishment or place of business but the relevant income is not effectively connected with the establishment or place of business, to the extent such dividends are derived from sources within the PRC.
Pursuant to the Arrangement between the Mainland of China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and Prevention of Fiscal Evasion with respect to Taxes on Incomes (《内地和香港特别行政区关于对所得避免双重征税和防止偷漏税的安排》), or the Double Tax Avoidance Arrangement, and other applicable PRC laws, 5% withholding tax rate shall apply to the dividends paid by a PRC company to a Hong Kong resident, provided that such Hong Kong resident directly holds at least 25% of the equity interests in the PRC company, and 10% of withholding tax rate shall apply if the Hong Kong resident holds less than 25% of the equity interests in the PRC company. However, pursuant to the Circular on Certain Issues Relating to the Implementation of Dividend Provisions in Tax Treaties (《关于执行税收协定股息条款有关问题的通知》) issued on February 20, 2009 by the SAT, if a PRC tax authority determines, in its discretion, that a company benefits from such reduced income tax rate as a result of an arrangement that is primarily tax-driven, such PRC tax authority may adjust the preferential tax treatment of the company. Based on the Announcement on Certain Issues with Respect to the Beneficial Owner in Tax Treaties (《国家税务总局关于税收协定中受益所有人有关问题的公告》) issued by the SAT on February 3, 2018 and effective on April 1, 2018, if an applicant’s business activities do not constitute substantive business activities, it could result in the negative determination of the applicant’s status as a beneficial owner, and consequently, the applicant could be precluded from enjoying the above-mentioned reduced income tax rate of 5% under the Double Tax Avoidance Arrangement.
56
C. Organizational Structure
See “—A. History and Development of the Company.”
D. Property, Plants and Equipment
See “—B. Business Overview—Properties and Facilities.”
Item 4A. UNRESOLVED STAFF COMMENTS
Not applicable.
Item 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
The following discussion of our financial condition and results of operations is based upon and should be read in conjunction with our consolidated financial statements and their related notes included in this annual report. This report contains forward-looking statements. In evaluating our business, you should carefully consider the information provided under the caption “Item 3. Key Information—D. Risk Factors” in this annual report. We caution you that our businesses and financial performance are subject to substantial risks and uncertainties.
A. Operating Results
Overview
Through its subsidiaries (the “PRC operating entities”) in the People’s Republic of China (“China” or the “PRC”), Universe Pharmaceuticals INC (the “Company,” “we,” “our” and “us”) is a pharmaceutical company specializing in the development, manufacturing, marketing and sale of traditional Chinese medicine derivatives (“TCMD”) products targeted to the elderly to address their physical conditions in the aging process and to promote their general well-being. We have registered and obtained approval for 26 varieties of TCMD products from the National Medical Products Administration (the “NMPA”), and we currently produce 13 varieties of TCMD products and sell them in 261 cities in 30 provinces in China as of the date of this report. In addition, we also sell biomedical drugs, medical instruments, traditional Chinese medicine pieces (“TCMPs”) and dietary supplements manufactured by third-party pharmaceutical companies (collectively referred to as “third-party products”).
Our major customers are pharmaceutical companies, hospitals, clinics and drugstore chains, primarily located in Jiangxi Province, Jiangsu Province, Guangdong Province, Hubei Province, Fujian Province, Guangxi Province and Shandong Province, and 23 other provinces in China.
Key Factors that Affect Our Results of Operations
We believe the following key factors may affect our financial condition and results of operations:
Our Ability to Attract Additional Customers and Increase the Spending Per Customer
Our major customers are pharmaceutical distributors, hospitals, clinics and drugstore chains. We currently sell our products to these customers in 261 cities in 30 provinces in China, with significant customers located in Jiangxi Province, Jiangsu Province, Guangdong Province, Hubei Province, Fujian Province, Guangxi Province and Shandong Province in China. For the fiscal years ended September 30, 2023, 2022 and 2021, we had a total of 2,541, 2,651, and 2,708 customers, respectively, and no single customer accounted for more than 10% of our total revenue of any of these fiscal years. However, our top 10 customers in the aggregate accounted for 27.2%, 32.2% and 30.4% of our total revenues for the fiscal years ended September 30, 2023, 2022 and 2021, respectively. Our dependence on a small number of larger customers could expose us to the risk of substantial losses if a single large customer stops purchasing our products, purchases fewer of our products or goes out of business and we cannot find substitute customers on equivalent terms. If any of our significant customers reduces the quantity of the products they purchase from us or stops purchasing from us, our net revenues would be materially and adversely affected. Therefore, the success of our business in the future depends on our effective marketing efforts to expand our distribution network including additional cities and rural areas in the PRC in an effort to increase our geographic appearance. The success of expansion will depend upon many factors, including our ability to form relationships with, and manage an increasing number of, customers base and optimize our distribution network. If our marketing efforts fail to convince customers to accept our products, we may find it difficult to maintain the existing level of sales or to increase such sales. Should this happen, our net revenues would decline and our growth prospects would be severely impaired.
57
Our Ability to Increase Awareness of Our Brand and Develop Customer Loyalty
Our brands, such as “Bai Nian Dan (百年丹)” and “Hu Zhuo Ren (胡卓仁)”, are well-recognized among our clients and other Chinese medicine industry players. Our brand is integral to our sales and marketing efforts. We believe that maintaining and enhancing our brand name recognition in a cost-effective manner is critical to achieving widespread acceptance of our current and future products and is an important element in our effort to increase our customer base. Successful promotion of our brand name will depend largely on our marketing efforts and ability to provide reliable and quality products at competitive prices. Brand promotion activities may not necessarily yield increased revenue, and even if they do, any increased revenue may not offset the expenses we will incur in marketing activities. If we fail to successfully promote and maintain our brand, if we incur substantial expenses in an unsuccessful attempt to promote and maintain our brand, or if we fail to generate the desired level of brand recognition and awareness through our recent television advertising efforts, or at all, we may fail to attract new customers or retain our existing customers, in which case our business, operating results and financial condition, would be materially adversely affected.
Our Ability to Control Costs and Expenses and Improve Our Operating Efficiency
Our business growth is dependent on our ability to attract and retain qualified and productive employees, identify business opportunities, secure new contracts with customers and our ability to control costs and expenses to improve our operating efficiency. Our inventory costs (including raw materials, labor, packaging cost, depreciation and amortization, freight costs, overhead and third-party products purchase costs) have a direct impact on our profitability. The raw materials used in manufacturing of our TCMD products are subject to price volatility and inflationary pressures. Our success is dependent, in part, on our ability to reduce our exposure to increase in those costs through a variety of ways, while maintaining and improving margins and market share. We also rely on third-party manufacturers as a source for a portion of components for a portion of components for our products. These manufacturers are also subject to price volatility and labor cost and other inflationary pressures, which may, in turn, result in an increase in the amount we pay for sourced products. Raw materials and sourced product price increases may offset our productivity gains and price increases and may adversely impact our financial results. In addition, our staffing costs (including payroll and employee benefit expense) and administrative expenses also have a direct impact on our profitability. Our ability to drive the productivity of our staff and enhance our operating efficiency affects our profitability. To the extent that the costs we are required to pay to our suppliers and our staffs exceed our estimates, our profit may be impaired. If we fail to implement initiatives to control costs and improve our operating efficiency over time, our profitability will be negatively impacted.
Our Ability to Compete Successfully
The Chinese patent medicine industry we are in is highly competitive and evolving in China. The development and commercialization of new pharmaceutical products is highly competitive, and the industry currently is characterized by rapidly changing technologies, significant competition and a strong emphasis on intellectual property. We will face competition with respect to our current and future pharmaceutical product candidates from major pharmaceutical companies in China. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization of pharmaceutical products. Some of our current or potential competitors may have significantly greater financial resources and expertise in research and development, manufacturing, product testing, obtaining regulatory approvals and marketing approved products than we do, and in the event that any of these happens, our competitors may be able to establish a strong market position before our new products are able to enter the market. Additionally, technologies developed by our competitors may render our product candidates uneconomical or obsolete. If we do not compete effectively, our operating results could be harmed.
A Severe or Prolonged Slowdown in the Global or Chinese Economy Could Materially and Adversely Affect Our Business and Our Financial Condition
The rapid growth of the Chinese economy has slowed down since 2012 and this slowdown may continue in the future. There is considerable uncertainty over trade conflicts between the United States and China and the long-term effects of the expansionary monetary and fiscal policies adopted by the central banks and financial authorities of some of the world’s leading economies, including the United States and China. The withdrawal of these expansionary monetary and fiscal policies could lead to a contraction. There continue to be concerns over unrest and terrorist threats in the Middle East, Europe, and Africa, which have resulted in volatility in oil and other markets. There are also concerns about the relationships between China and other Asian countries, which may result in or intensify potential conflicts in relation to territorial disputes. The eruption of armed conflict could adversely affect global or Chinese discretionary spending, either of which could have a material and adverse effect on our business, results of operation in financial condition. Economic conditions in China are sensitive to global economic conditions, as well as changes in domestic economic and political policies and the expected or perceived overall economic growth rate in China. Any severe or prolonged slowdown in the global or Chinese economy would likely materially and adversely affect our business, results of operations and financial condition. In addition, continued turbulence in the international markets may adversely affect our ability to access capital markets to meet liquidity needs.
Key Financial Performance Indicators
In assessing our financial performance, we consider a variety of financial performance measures, including principal growth in net revenue and gross profit, our ability to control costs and operating expenses to improve our operating efficiency and net income. Our review of these indicators facilitates timely evaluation of the performance of our business and effective communication of results and key decisions, allowing our business to respond promptly to competitive market conditions and different demands and preferences from our customers. The key measures that we use to evaluate the performance of our business are set forth below.
58
Net Revenue
Our revenue is reported net of all value added taxes (“VAT”). Our products are sold with no right of return and we do not provide other credits or sales incentives to customers. Our revenue is driven by changes in the number of customers, sales volume, selling price, and mix of products sold.
| For the Years Ended September 30, | Variance comparing 2023 to 2022 | Variance comparing 2022 to 2021 | ||||||||||||||||||
| 2023 | 2022 | 2021 | % | % | ||||||||||||||||
| Revenue from sales of self-manufactured TCMD products | 57.5 | % | 59.8 | % | 61.6 | % | (2.3 | )% | (1.8 | )% | ||||||||||
| Revenue from sales of third-party products | 42.5 | % | 40.2 | % | 38.4 | % | 2.3 | % | 1.8 | % | ||||||||||
| Total revenue | 100.0 | % | 100.0 | % | 100.0 | % | ||||||||||||||
| Sales volume by unit- TCMD products | 16,753,432 | 10,799,254 | 17,206,150 | 55.1 | % | (37.2 | )% | |||||||||||||
| Sales volume by unit- third party products | 8,777,877 | 9,226,027 | 8,364,391 | (4.9 | )% | 10.3 | % | |||||||||||||
| Total sales volume | 25,531,309 | 20,025,281 | 25,570,541 | 27.5 | % | (21.7 | )% | |||||||||||||
| Average selling price per unit- TCMD products | $ | 1.11 | $ | 2.22 | $ | 1.72 | (50.0 | )% | 29.1 | % | ||||||||||
| Average selling price per unit- Third-party products | $ | 1.56 | $ | 1.75 | $ | 2.20 | (10.9 | )% | (20.5 | )% | ||||||||||
Revenues from sales of TCMD products manufactured by us accounted for 57.5%, 59.8% and 61.6% of our total revenues for the fiscal years ended September 30, 2023, 2022 and 2021, respectively. The 13 TCMD products manufactured by us fall into two categories: (i) treatment and relief for common chronic health conditions in the elderly designed to achieve physical wellness and longevity (the “Chronic Condition Treatments”) and (ii) cold and flu medications. Our Chronic Condition Treatments primarily include Guben Yanling Pill, Shenrong Weisheng Pill, Quanlu Pill, Yangxue Danggui Syrup, Wuzi Yanzong Oral Liquid, Fengtong Medicinal Liquor, Shenrong Medicinal Liquor, Qishe Medicinal Liquor, Fengshitong Medicinal Liquor, and Shiquan Dabu Medicinal Liquor, and our cold and flu medications primarily include Paracetamol Granule for Children, Isatis Root Granule and Qiangli Pipa Syrup.
In order to diversify our product offerings and product mix, in addition to selling our self-manufactured TCMD products, we also sell products manufactured by third-party pharmaceutical companies, including (i) biomedical drugs, such as liquid glucose, prednisolone, and citicoline, (ii) medical instruments, such as drug-eluting stents, surgical tubes and syringes, (iii) TCMPs, such as red sage tables, Longdan Xiegan pills, and Chinese skullcap capsules, and (iv) dietary supplements, such as vitamins, probiotic powder, and calcium tablets. Revenues from sales of third-party products accounted for 42.5%, 40.2% and 38.4% of our total revenues for the fiscal years ended September 30, 2023, 2022 and 2021, respectively.
Gross Profit
Gross profit is equal to net revenue minus cost of goods sold. Cost of goods sold primarily includes inventory costs (raw materials, labor, packaging cost, depreciation and amortization, third-party products purchase price, freight costs and overhead). Cost of goods sold generally changes as our production costs change, as these are affected by factors including the market price of raw materials, labor productivity, or the purchase price of third-party products, and as the customer and product mix changes. Our cost of revenues accounted for 68.1%, 45.5% and 47.2% of our total revenue for the fiscal years ended September 30, 2023, 2022 and 2021, respectively. We expect our cost of revenues to increase as we further expand our operations in the foreseeable future. We expect our cost of revenues to increase as we further expand our operations in the foreseeable future.
Our gross margin was 31.9%, 54.5% and 52.8% for the fiscal years ended September 30, 2023, 2022 and 2021, respectively. Our gross profit and gross margin are affected by the different product mix and decreased selling price of those products sold during each reporting period. Slowdown in the Chinese economy caused by the COVID-19 pandemic has led to a decline in customers’ spending power, and we changed our pricing strategy to decrease the per unit price of our TCMD products in an effort to increase sales volume and market share during this challenging period. See detailed discussion under “––Results of Operation”.
59
Operating Expenses
Our operating expenses consist of selling expenses, general and administrative expenses and research and development expenses.
Our selling expenses primarily include salary and welfare benefit expenses paid to our sales personnel, advertising expenses to increase the awareness of our brand, shipping and delivery expenses, expenses incurred for our business travel, meals and other sales promotion and marketing activities related expenses. Our selling expenses accounted for 21.0%, 47.5% and 6.2% of our total revenue for the fiscal years ended September 30, 2023, 2022 and 2021, respectively. We expect our overall selling expenses, including but not limited to, advertising expenses and brand promotion expenses and salaries, to increase in the foreseeable future and facilitate the growth of our business, especially when we continue to expand our business and promote our products to customers located at extended geographic areas.
Our general and administrative expenses primarily consist of employee salaries, welfare and insurance expenses, depreciation, bad debt reserve expenses, inspection and maintenance expenses, office supply and utility expenses, business travel and meals expenses, land and property taxes and professional service expenses. General and administrative expenses were 6.8%, 6.5% and 6.9% of our revenue for the fiscal years ended September 30, 2023, 2022 and 2021, respectively. We expect our general and administrative expenses, including, but not limited to, salaries and business consulting expenses, to increase in the foreseeable future, as we plan to hire additional personnel and incur additional expenses in connection with the expansion of our business operations.
The Chinese patent medicine industry is characterized by rapid and frequent changes in customer demand and launches of new products. If we do not launch new products or improve our existing products to meet the changing demands of our customers in a timely manner, some of our products could become uncompetitive in the market, thereby adversely affecting on our revenues and operating results. Our research and development expenses primarily consist of salaries, welfare and insurance expenses paid to our employees involved in the research and development activities, materials and supplies used in the development and testing of new TCMD products, depreciation, and other miscellaneous expenses. Research and development expenses accounted for 15.0%, 19.0% and 11.4% of our revenue for the fiscal years ended September 30, 2023, 2022 and 2021, respectively. As we expect to continue to develop new products and diversify our product offerings to satisfy customer demand, our research and development expenses may increase in the future.
Results of Operations
The following table sets forth a summary of our consolidated results of operations for the periods presented, both in absolute amount and as a percentage of our total operating revenue for the fiscal years presented. This information should be read together with our consolidated financial statements and related notes included elsewhere in this annual report. The results of operations in any period are not necessarily indicative of our future trends.
60
Fiscal year ended September 30, 2023 compared to fiscal year ended September 30, 2022
The following table summarizes the results of our operations during the fiscal years ended September 30, 2023 and 2022, respectively, and provides information regarding the dollar and percentage increase or (decrease) during such periods.
| For the Years Ended September 30, | ||||||||||||||||||||||||
| 2023 | 2022 | Variance | ||||||||||||||||||||||
| Amount | % of revenue | Amount | % of revenue | Amount | % | |||||||||||||||||||
| REVENUE | $ | 32,308,735 | 100.0 | % | $ | 40,143,151 | 100.0 | % | $ | (7,834,416 | ) | (19.5 | )% | |||||||||||
| COST OF REVENUE | 21,993,601 | 68.1 | % | 18,251,815 | 45.5 | % | 3,741,786 | 20.5 | % | |||||||||||||||
| GROSS PROFIT | 10,315,134 | 31.9 | % | 21,891,336 | 54.5 | % | (11,576,202 | ) | (52.9 | )% | ||||||||||||||
| OPERATING EXPENSES | ||||||||||||||||||||||||
| Selling expenses | 6,783,703 | 21.0 | % | 19,083,135 | 47.5 | % | (12,299,432 | ) | (64.5 | )% | ||||||||||||||
| General and administrative expenses | 2,199,657 | 6.8 | % | 2,602,624 | 6.5 | % | (402,967 | ) | (15.5 | )% | ||||||||||||||
| Research and development expenses | 4,858,548 | 15.0 | % | 7,644,375 | 19.0 | % | (2,785,827 | ) | (36.4 | )% | ||||||||||||||
| Total operating expenses | 13,841,908 | 42.8 | % | 29,330,134 | 73.0 | % | (15,488,226 | ) | (52.8 | )% | ||||||||||||||
| INCOME FROM OPERATIONS | (3,526,774 | ) | (10.9 | )% | (7,438,798 | ) | (18.5 | )% | 3,912,024 | (52.6 | )% | |||||||||||||
| OTHER INCOME (EXPENSE) | ||||||||||||||||||||||||
| Interest expense, net | (156,788 | ) | (0.5 | )% | (162,400 | ) | (0.4 | )% | 5,612 | (3.5 | )% | |||||||||||||
| Other income (expense), net | (235,614 | ) | (0.7 | )% | 48,940 | ) | 0.1 | % | (284,554 | ) | (581.4 | )% | ||||||||||||
| Income (loss) from short-term investments | 38,530 | 0.1 | % | (470,477 | ) | (1.2 | )% | 509,007 | (108.2 | )% | ||||||||||||||
| Equity investment income | 31,072 | 0.1 | % | 38,588 | 0.1 | % | (7,516 | ) | (19.5 | )% | ||||||||||||||
| Total other income (expense), net | (322,800 | ) | (1.0 | )% | (545,349 | ) | (1.4 | )% | 222,549 | (40.8 | )% | |||||||||||||
| INCOME (LOSS) BEFORE INCOME TAX | (3,849,574 | ) | (11.9 | )% | (7,984,147 | ) | (19.9 | )% | 4,134,573 | (51.8 | )% | |||||||||||||
| PROVISION FOR INCOME TAXES | 2,313,487 | 7.2 | % | 752,419 | 1.9 | % | 1,561,068 | 207.5 | % | |||||||||||||||
| NET INCOME (LOSS) | $ | (6,163,061 | ) | (19.1 | )% | $ | (8,736,566 | ) | (21.8 | )% | $ | 2,573,505 | (29.5 | )% | ||||||||||
61
Revenues. We currently produce and sell 13 varieties of TCMD products and also sell products manufactured by third-party pharmaceutical companies, to our customers.
| For the Years Ended September 30, | ||||||||||||||||
| 2023 | 2022 | Change | ||||||||||||||
| Amount | Amount | Amount | % | |||||||||||||
| Revenue - TCMD products sales | $ | 18,572,658 | $ | 23,988,177 | $ | (5,415,519 | ) | (22.6 | )% | |||||||
| Revenue – third-party products sales | 13,736,077 | 16,154,974 | (2,418,897 | ) | (15.0 | )% | ||||||||||
| Total revenue | $ | 32,308,735 | $ | 40,143,151 | $ | (7,834,416 | ) | (19.5 | )% | |||||||
Our revenues decreased by $7,834,416, or 19.5%, to $32,308,735 for the fiscal year ended September 30, 2023, from $40,143,151 for the fiscal year ended September 30, 2022.
Revenue from sales of our TCMD products
Sales of TCMD products decreased by $5,415,519, or 22.6%, to $18,572,658 for the fiscal year ended September 30, 2023, from $23,988,177 for the fiscal year ended September 30, 2022. The decrease in the sales of our TCMD product was due to the following specific reasons:
| a) | Slowdown in economy caused by the COVID-19 pandemic has led to a decline in customers’ spending power, and we changed our pricing strategy decrease the per unit price of our TCMD products in an effort to increase sales volume and market share during this challenging period. Average selling price of our TCMD products decreased by $1.11 per unit, or 50.0%, to $1.11 per unit for the fiscal year ended September 30, 2023, from $2.22 per unit for the fiscal year ended September 30, 2022. |
| b) | As a result of our new pricing strategy, sales volume of TCMD products increased by 55.1%, to 16,753,432 units sold for the fiscal year ended September 30, 2023, from 10,799,254 units sold for the fiscal year ended September 30, 2022. |
| c) | The average exchange rate between RMB and US$ was US$1.00 to RMB7.0533 for the fiscal year ended September 30, 2023 as compared to US$1.00 to RMB6.5532 for the fiscal year ended September 30, 2022. The depreciation of RMB against US$ had a 7.6% negative impact on our reported revenues. |
Revenue from sales of third-party products
Sales of third-party products decreased by $2,418,897, or 15.0%, to $13,736,077 for the fiscal year ended September 30, 2023, from $16,154,974 for the fiscal year ended September 30, 2022. Sales volume of third-party products decreased by 4.9%, to 8,777,877 units sold for the fiscal year ended September 30, 2023, from 9,226,027 units sold for the fiscal year ended September 30, 2022. Average selling price of third-party products decreased by $0.19 per unit, or 10.9%, to $1.56 per unit for the fiscal year ended September 30, 2023, from $1.75 per unit for the fiscal year ended September 30, 2022, due to a change in our product mix and the 7.6% negative impact from foreign currency fluctuation as discussed above.
Cost of Revenues. Our cost of revenues primarily consists of inventory costs (raw materials, labor, packaging cost, depreciation and amortization, third-party products purchase price, freight costs and overhead) and business tax. Cost of revenues generally changes as our production costs change, which are affected by factors including the market price of raw materials, labor productivity, and the purchase price of third-party products, and as the customer and product mix changes.
| For the Years Ended September 30, | ||||||||||||||||
| 2023 | 2022 | Change | ||||||||||||||
| Amount | Amount | Amount | % | |||||||||||||
| Cost of revenue- TCMD products | $ | 13,404,804 | $ | 8,187,182 | $ | 5,217,622 | 63.7 | % | ||||||||
| Cost of revenue- third-party products | 8,588,797 | 10,064,633 | (1,475,836 | ) | (14.7 | )% | ||||||||||
| Total cost of revenue | $ | 21,993,601 | $ | 18,251,815 | $ | 3,741,786 | 20.5 | % | ||||||||
Cost of revenues increased by $3,741,786, or 20.5%, to $21,993,601 for the fiscal year ended September 30, 2023, from $18,251,815 for the fiscal year ended September 30, 2022, due to an increase in sales volume and rising prices of traditional Chinese medicine raw materials caused by the imbalance between supply and demand starting from the fourth quarter of 2022.
62
Cost of revenues of TCMD products
Cost of revenues of TCMD products accounted for 60.9% and 44.9% of our total costs of revenues for the fiscal years ended September 30, 2023 and 2022, respectively. Cost of revenues of TCMD products increased by $5,217,622, or 63.7%, from $8,187,182 for the fiscal year ended September 30, 2022 to $13,404,804 for the fiscal year ended September 30, 2023. The increase in cost of revenues of our TCMD products was due to the following reasons:
| (1) | As a result of our new pricing strategy to promote sales, sales volume of TCMD products increased by 55.1%, to 16,753,432 units sold for the fiscal year ended September 30, 2023, from 10,799,254 units sold for the fiscal year ended September 30, 2022. |
| (2) | The average unit cost of our TCMD products increased by $0.04, or 5.5%, from $0.76 per unit for the fiscal year ended September 30, 2022 to $0.80 per unit for the fiscal year ended September 30, 2023. A severe drought in the Yangtze River Basin in China in 2022 led to a decrease in the supply of traditional Chinese medicinal materials, and the COVID-19 pandemic resulted in increased demand of traditional Chinese medicinal materials for recuperating and improving immunity. Due to imbalance between supply and demand starting from the fourth quarter of 2022, prices of the traditional Chinese medicine raw materials increased by about 14% for the fiscal year ended September 30, 2023 as compared to the fiscal year ended September 30, 2022. |
| (3) | The average exchange rate between RMB and US$ was US$1.00 to RMB7.0533 for the fiscal year ended September 30, 2023 as compared to US$1.00 to RMB6.5532 for the fiscal year ended September 30, 2022. The depreciation of RMB against US$ had a 7.6% negative impact on our reported cost of revenues. |
Cost of revenues of third-party products
Cost of revenues of third-party products accounted for 39.1% and 55.1% of our total costs of revenues for the fiscal years ended September 30, 2023 and 2022, respectively. Cost of revenues of third-party products decreased by $1,475,836, or 14.7%, from $10,064,633 for the fiscal year ended September 30, 2022 to $8,588,797 for the fiscal year ended September 30, 2023. Sales volume of third-party products decreased by 4.9% from 9,226,027 units sold for the fiscal year ended September 30, 2022 to 8,777,877 units sold for the fiscal year ended September 30, 2023. Average unit cost of third-party products decreased by $0.11 per unit, or 10.3%, from $1.09 per unit for the fiscal year ended September 30, 2022 to $0.98 per unit for the fiscal year ended September 30, 2023, due to change in product mix and the 7.6% negative impact from foreign currency fluctuation as discussed above.
Gross profit
Our gross profit decreased by $11,576,202 to $10,315,134 for the fiscal year ended September 30, 2023, from $21,891,336 for the fiscal year ended September 30, 2022. Our margin decreased by 22.6% to 31.9% for the fiscal year ended September 30, 2023, from 54.5% for the fiscal years ended September 30, 2022.
| For the Years Ended September 30, | ||||||||||||||||
| 2023 | 2022 | Change | ||||||||||||||
| Amount | Amount | Amount | % | |||||||||||||
| Gross profit- TCMD products | $ | 5,167,854 | $ | 15,800,995 | $ | (10,633,141 | ) | (67.3 | )% | |||||||
| Gross profit- third-party products | 5,147,280 | 6,090,341 | (943,061 | ) | (15.5 | )% | ||||||||||
| Total gross profit | $ | 10,315,134 | $ | 21,891,336 | $ | (11,576,202 | ) | (52.9 | )% | |||||||
| Gross margin- TCMD products | 27.8 | % | 65.9 | % | (38.0 | )% | ||||||||||
| Gross margin- third party products | 37.5 | % | 37.7 | % | (0.2 | )% | ||||||||||
| Total gross margin | 31.9 | % | 54.5 | % | (22.6 | )% | ||||||||||
| Average selling price per unit- TCMD products | $ | 1.11 | $ | 2.22 | $ | (1.11 | ) | (50.0 | )% | |||||||
| Average cost per unit- TCMD products | $ | 0.80 | $ | 0.76 | $ | 0.04 | 5.5 | % | ||||||||
| Average selling price per unit- third party products | $ | 1.56 | $ | 1.75 | $ | (0.19 | ) | (10.9 | )% | |||||||
| Average cost per unit - third party products | $ | 0.98 | $ | 1.09 | $ | (0.11 | ) | (10.3 | )% | |||||||
Gross profit from the sales of our TCMD products decreased by $10,633,141, or 67.3%, from $15,800,995 for the fiscal year ended September 30, 2022 to $5,167,854 for the fiscal year ended September 30, 2023, and the gross margin of our TCMD products decreased by 38.0 percentage point, from 65.9% for the fiscal year ended September 30, 2022 to 27.8% for the fiscal year ended September 30, 2023. The decrease in our gross profit from the sales of TCMD products was due to the following reasons: (i) as discussed above, we changed our pricing strategy to decrease the per unit price of our TCMD products in an effort to promote sales, and average selling price of our TCMD products decreased by $1.11 per unit, or 50.0%, to $1.11 per unit for the fiscal year ended September 30, 2023, from $2.22 per unit for the fiscal year ended September 30, 2022; (ii) average unit cost of our TCMD products increased by $0.04, or 5.5%, from $0.76 per unit for the fiscal year ended September 30, 2022 to $0.80 per unit for the fiscal year ended September 30, 2023, due to increased price of traditional Chinese medicine raw materials, and (iii) the depreciation of RMB against US$ had a 7.6% negative impact on our gross profit from sales of TCMD products.
63
Gross profit from third-party product sales decreased by $943,061, or 15.5%, from $6,090,341 for the fiscal year ended September 30, 2022 to $5,147,280 for the fiscal year ended September 30, 2023, while the gross margin of third-party product sales slightly decreased by 0.2%, from 37.7% for the fiscal year ended September 30, 2022 to 37.5% for the fiscal year ended September 30, 2023. The decrease in our gross profit from third-party products was affected by the decrease in sales volume and the 7.6% negative impact from depreciation of RMB against US$.
Operating expenses
The following table sets forth the breakdown of our operating expenses for the fiscal years ended September 30, 2023 and 2022:
| For the Years Ended September 30, | ||||||||||||||||||||||||
| 2023 | 2022 | Variance | ||||||||||||||||||||||
| Amount | % of revenue | Amount | % of revenue | Amount | % | |||||||||||||||||||
| Total revenue | $ | 32,308,735 | 100.0 | % | $ | 40,143,151 | 100.0 | % | $ | (7,834,416 | ) | (19.5 | )% | |||||||||||
| Operating expenses: | ||||||||||||||||||||||||
| Selling expenses | 6,783,703 | 21.0 | % | 19,083,135 | 47.5 | % | (12,299,432 | ) | (64.5 | )% | ||||||||||||||
| General and administrative expenses | 2,199,657 | 6.8 | % | 2,602,624 | 6.5 | % | (402,967 | ) | (15.5 | )% | ||||||||||||||
| Research and development expenses | 4,858,548 | 15.0 | % | 7,644,375 | 19.0 | % | (2,785,827 | ) | (36.4 | )% | ||||||||||||||
| Total operating expenses | $ | 13,841,908 | 42.8 | % | $ | 29,330,134 | 73.1 | % | $ | (15,488,226 | ) | (52.8 | )% | |||||||||||
Selling expenses
Our selling expenses primarily include salaries and welfare benefit expenses paid to our sales personnel, advertising expenses to increase our brand awareness, shipping and delivery expenses, expenses incurred for our business travel, meals and other sales promotion and marketing activities related expenses.
| For the Years Ended September 30, | ||||||||||||||||||||||||
| 2023 | 2022 | Variance | ||||||||||||||||||||||
| Amount | % | Amount | % | Amount | % | |||||||||||||||||||
| Salary and employee benefit expenses | $ | 778,138 | 11.5 | % | $ | 778,656 | 4.1 | % | $ | (518 | ) | (0.1 | )% | |||||||||||
| Advertising expenses | 4,653,633 | 68.6 | % | 17,527,318 | 91.8 | % | (12,873,685 | ) | (73.4 | )% | ||||||||||||||
| Shipping and delivery expenses | 1,033,213 | 15.2 | % | 638,286 | 3.3 | % | 394,927 | 61.9 | % | |||||||||||||||
| Business travel and meals expenses | 208,108 | 3.1 | % | 125,553 | 0.7 | % | 82,555 | 65.8 | % | |||||||||||||||
| Other sales promotion related expenses | 110,611 | 1.6 | % | 13,322 | 0.1 | % | 97,289 | 730.3 | % | |||||||||||||||
| Total selling expenses | $ | 6,783,703 | 100.0 | % | $ | 19,083,135 | 100.0 | % | $ | (12,299,432 | ) | (64.5 | )% | |||||||||||
Our selling expenses decreased by $12,299,432, or 64.5%, to $6,783,703 for the fiscal year ended September 30, 2023, from $19,083,135 for the fiscal year ended September 30, 2022, primarily attributable to (i) a decrease in advertising expenses by $12,873,685, or 73.4%, from $17,527,318 for the fiscal year ended September 30, 2022, to $4,653,633 for the fiscal year ended September 30, 2023. In September 2021, we entered into an advertising service agreement with a third party, Guangdong Fengyang Legend Consulting Co., Ltd. (“Fengyang Legend”) to promote the sales of our major TCMD products, Bai Nian Dan and Guben Yanling Pill with a service period of one year, from October 1, 2021 to September 30, 2022. In March 2022, we entered into an advertising service agreement with a third-party, Health Headline to promote our brand on the Health Headline’s website and mobile app, with a service period of ten months from March 1, 2022 to December 31, 2022. In March 2023, we renewed the advertising services arrangement with Health Headline by entering a new service agreement with Health Headline with a service period of ten months from March 1, 2023 to December 31, 2023. As our agreement with Fengyang Legend expired in September 2022, advertising expenses decreased significantly during the fiscal year ended September 30, 2023; and (ii) partially offset by an increase in shipping and delivery expenses by $394,927, or 61.9%, from $638,286 for the fiscal year ended September 30, 2022 to $1,033,213 for the fiscal year ended September 30, 2023, due to an increase in our sales volume, increased freight cost and rising fuel prices during the fiscal year ended September 30, 2023.
64
General and Administrative Expenses
Our general and administrative expenses primarily consist of employee salaries, welfare and insurance expenses, depreciation, bad debt reserve expenses, inspection and maintenance expenses, office supply and utility expenses, business travel and meals expenses, land and property taxes and professional service expenses.
| For the Years Ended September 30, | ||||||||||||||||||||||||
| 2023 | 2022 | Variance | ||||||||||||||||||||||
| Amount | % | Amount | % | Amount | % | |||||||||||||||||||
| Salary and employee benefit expense | $ | 728,499 | 33.1 | % | $ | 641,622 | 24.7 | % | $ | 86,877 | 13.5 | % | ||||||||||||
| Depreciation and amortization | 195,451 | 8.9 | % | 185,169 | 7.1 | % | 10,282 | 5.6 | % | |||||||||||||||
| Bad debt reserve expenses (recovery) | (439,327 | ) | (20.0 | )% | 419,353 | 16.1 | % | (858,680 | ) | (204.8 | )% | |||||||||||||
| Land and property tax | 96,412 | 4.4 | % | 103,769 | 4.0 | % | (7,357 | ) | (7.1 | )% | ||||||||||||||
| Office supply and utility expense | 440,873 | 20.0 | % | 307,883 | 11.8 | % | 132,990 | 43.2 | % | |||||||||||||||
| Transportation, business travel and meals expense | 121,122 | 5.5 | % | 83,029 | 3.2 | % | 38,093 | 45.9 | % | |||||||||||||||
| Consulting fee | 926,694 | 42.1 | % | 720,430 | 27.7 | % | 206,264 | 28.6 | % | |||||||||||||||
| Inspection and maintenance fee | 47,154 | 2.1 | % | 39,555 | 1.5 | % | 7,599 | 19.2 | % | |||||||||||||||
| Stamp tax and other expenses | 82,779 | 3.8 | % | 101,814 | 3.9 | % | (19,035 | ) | (18.7 | )% | ||||||||||||||
| Total general and administrative expenses | $ | 2,199,657 | 100.0 | % | $ | 2,602,624 | 100.0 | % | $ | (402,967 | ) | (15.5 | )% | |||||||||||
General and administrative expenses decreased by $402,967, or 15.5%, to $2,199,657 for the fiscal year ended September 30, 2023 from $2,602,624 for the fiscal year ended September 30, 2022, primarily attributable to (i) a decrease in bad debt expense by $858,680, because we accrued less bad debt expenses based on our assessment of the collectability of the accounts receivable and advance to suppliers, partially offset by (ii) an increase in consulting fee by $206,264, or 28.6% due to consulting services retained to improve product knowledge and sales tactics of our employees; and (iii) an increase in office supplies and utility expense by $132,990, or 43.2% due to the registration fees paid to U.S. Securities and Exchange Commission, annual listings fees paid to the Nasdaq Stock Market, and increased investor relations expense for the fiscal year ended September 30, 2023.
Research and development expenses
Our research and development expenses primarily consist of salaries, welfare and insurance expenses paid to our employees involved in the research and development activities, materials and supplies used in the development and testing new TCMD products, depreciation and other miscellaneous expenses.
| For the Years Ended September 30, | ||||||||||||||||||||||||
| 2023 | 2022 | Variance | ||||||||||||||||||||||
| Amount | % | Amount | % | Amount | % | |||||||||||||||||||
| Salary and employee benefit expenses to research and development staff | $ | 140,528 | 2.9 | % | $ | 159,924 | 2.1 | % | $ | (19,396 | )) | (12.1 | )% | |||||||||||
| Materials used in research and development activities | 881,753 | 18.1 | % | 489,692 | 6.4 | % | 392,061 | 80.1 | % | |||||||||||||||
| Expenditure on new product development | 3,794,464 | 78.1 | 6,973,683 | 91.2 | (3,179,219 | ) | (45.6 | )% | ||||||||||||||||
| Depreciation and others | 41,803 | 0.9 | % | 21,076 | 0.3 | % | 20,727 | 98.3 | % | |||||||||||||||
| Total research and development expenses | $ | 4,858,548 | 100.0 | % | $ | 7,644,375 | 100.0 | % | $ | (2,785,827 | ) | (36.4 | )% | |||||||||||
Research and development expenses decreased by $2,785,827, or 36.4%, to $4,858,548 for the fiscal year ended September 30, 2023, from $7,644,375 for the fiscal year ended September 30, 2022, primarily attributable to (i) a decrease in research and development expense of $3,179,219 to develop and test new Chinese medicine products in order to diversify our future product portfolio. During the fiscal year ended September 30, 2022, we cooperated with external academic and research institutions to jointly develop and test eight new Chinese medicine products and accordingly incurred significant amount of research and development expense in connection with such efforts. As these development activities reached their final stage, expenditure on new product development decreased significantly in the fiscal year ended September 30, 2023; and (ii) an increase in the materials used in the research and development activities by $392,061. In order to develop new products and improve the formulation of several existing products, we conducted more testing on product stability and safety, and as a result, more materials were used in R&D activities for the fiscal year ended September 30, 2023 than the fiscal year ended September 30, 2022.
Other income (expenses), net
Total other expenses, net, decreased by $222,549, or 40.8%, to $322,800 for the fiscal year ended September 30, 2023 from $545,349 for the fiscal year ended September 30, 2022. Short-term investment income from wealth management financial products was $38,530 during the fiscal year ended September 30, 2023, compared to short-term investment loss of $470,477 during the fiscal year ended September 30, 2022.
65
Provision for Income Taxes
Provision for income taxes was $2,313,487 for the fiscal year ended September 30, 2023, representing an increase of $1,561,068, or 207.5%, from $752,419 for the fiscal year ended September 30, 2022. As our PRC subsidiaries, Jiangxi Universe and Universe Trade incurred net loss during the years ended September 30, 2023 and 2022, we evaluated the likelihood of the realization of deferred tax assets, determined that deferred tax assets arose from net operating loss carry-forwards in previous years might not be fully realized, and recognized $1,627,550 valuation allowance for deferred tax assets during the fiscal year ended September 30, 2023.
Net Income (Loss)
Net loss was $6,163,061 for the fiscal year ended September 30, 2023, representing a $2,573,505 decrease from net loss of $8,736,566 for the fiscal year ended September 30, 2022.
Year ended September 30, 2022 compared to year ended September 30, 2021
The following table summarizes the results of our operations during years ended September 30, 2022 and 2021, respectively, and provides information regarding the dollar and percentage increase or (decrease) during such periods.
| For the Years Ended September 30, | ||||||||||||||||||||||||
| 2022 | 2021 | Variance | ||||||||||||||||||||||
| Amount | % of revenue | Amount | % of revenue | Amount | % | |||||||||||||||||||
| REVENUE | $ | 40,143,151 | 100.0 | % | $ | 47,982,031 | 100.0 | % | $ | (7,838,880 | ) | (16.3 | )% | |||||||||||
| COST OF REVENUE | 18,251,815 | 45.5 | % | 22,655,854 | 47.2 | % | (4,404,039 | ) | (19.4 | )% | ||||||||||||||
| GROSS PROFIT | 21,891,336 | 54.5 | % | 25,326,177 | 52.8 | % | (3,434,841 | ) | (13.6 | )% | ||||||||||||||
| OPERATING EXPENSES | ||||||||||||||||||||||||
| Selling expenses | 19,083,135 | 47.5 | % | 2,973,531 | 6.2 | % | 16,109,604 | 541.8 | % | |||||||||||||||
| General and administrative expenses | 2,602,624 | 6.5 | % | 3,296,844 | 6.9 | % | (694,220 | ) | (21.1 | )% | ||||||||||||||
| Research and development expenses | 7,644,375 | 19.0 | % | 5,465,662 | 11.4 | % | 2,178,713 | 39.9 | % | |||||||||||||||
| Total operating expenses | 29,330,134 | 73.0 | % | 11,736,037 | 24.5 | % | 17,594,097 | 149.9 | % | |||||||||||||||
| INCOME FROM OPERATIONS | (7,438,798 | ) | (18.5 | )% | 13,590,140 | 28.3 | % | (21,028,938 | ) | (154.7 | )% | |||||||||||||
| OTHER INCOME (EXPENSE) | ||||||||||||||||||||||||
| Interest expense, net | (162,400 | ) | (0.4 | )% | (101,604 | ) | (0.2 | )% | (60,796 | ) | 59.8 | % | ||||||||||||
| Other income (expense), net | 48,940 | 0.1 | % | (80,434 | ) | (0.1 | )% | 129,374 | (160.8 | )% | ||||||||||||||
| Income (loss) from short-term investments | (470,477 | ) | (1.2 | )% | 239,549 | 0.5 | % | (710,026 | ) | (296.4 | )% | |||||||||||||
| Equity investment income | 38,588 | 0.1 | % | 30,827 | 0.1 | % | 7,761 | 25.2 | % | |||||||||||||||
| Total other income (expense), net | (545,349 | ) | (1.4 | )% | 88,338 | 0.2 | % | (633,687 | ) | (717.3 | )% | |||||||||||||
| INCOME (LOSS) BEFORE INCOME TAX | (7,984,147 | ) | (19.9 | )% | 13,678,478 | 28.5 | % | (21,662,625 | ) | (158.4 | )% | |||||||||||||
| PROVISION FOR INCOME TAXES | 752,419 | 1.9 | % | 2,358,526 | 4.9 | % | (1,606,107 | ) | (68.1 | )% | ||||||||||||||
| NET INCOME (LOSS) | $ | (8,736,566 | ) | (21.8 | )% | $ | 11,319,952 | 23.6 | % | $ | (20,056,518 | ) | (177.2 | )% | ||||||||||
66
Revenues: Our total revenues decreased by $7,838,880, or 16.3%, to $40,143,151 for the fiscal year ended September 30, 2022 from $47,982,031 for the fiscal year ended September 30, 2021. The decrease in our revenues was attributable to (i) a decreased number of customers by 2.1%, from 2,708 customers in the fiscal year ended September 30, 2021 to 2,651 customers in the fiscal year ended September 30, 2022; (ii) decreased sales volume of our TCMD products by 37.2%, from 17,206,150 units sold in the fiscal year ended September 30, 2021 to 10,799,254 units sold in the fiscal year ended September 30, 2022 due to our inability to timely fulfill the orders of our customer in 2022 due to the resurgence of COVID-19 cases in China; and (iii) decreased average selling price of our third-party products by 20.5% as affected by changes in product mix based on customer orders.
| For the Years Ended September 30, | ||||||||||||||||
| 2022 | 2021 | Change | ||||||||||||||
| Amount | Amount | Amount | % | |||||||||||||
| Revenue from sales of self-manufactured TCMD products | $ | 23,988,177 | $ | 29,559,286 | $ | (5,571,109 | ) | (18.8 | )% | |||||||
| Revenue from sales of third-party products | 16,154,974 | 18,422,745 | (2,267,771 | ) | (12.3 | )% | ||||||||||
| Total revenue | $ | 40,143,151 | $ | 47,982,031 | $ | (7,838,880 | ) | (16.3 | )% | |||||||
Revenue from sales of our TCMD products
Sales of our TCMD products decreased by $5,571,109, or 18.8%, from $29,559,286 in the fiscal year ended September 30, 2021 to $23,988,177 in the fiscal year ended September 30, 2022. The decrease in the sales of our TCMD products was due to the following reasons:
| a) | Due to the resurgence in the number of COVID cases in China recently, which disrupted the norming functioning of logistics in the country, we experienced difficulties shipping and delivering our products to customers located in certain remote geographic areas. This led to lower sales volume of our TCMD products by 37.2% in the fiscal year ended September 30, 2022 as compared to the fiscal year ended September 30, 2021. The sales volume of our TCMD products decreased by 6,406,896 units from 17,206,150 units in the fiscal year ended September 30, 2021 to 10,799,254 units in the fiscal year ended September 30, 2022. |
| b) | The COVID-19 pandemic led to disruptions of supply chain and transportation and an overall increase in the market prices of raw materials used in manufacturing of our TCMD products. Accordingly, we adjusted the selling prices of our TCMD products in response to the increase in raw material purchase costs and general inflation. The weighted average unit selling price of our TCMD products increased by $0.50 per unit, or 29.1%, from $1.72 per unit in the fiscal year ended September 30, 2021 to $2.22 per unit in the fiscal year ended September 30, 2022. |
Revenue from sales of third-party products
Sales of third-party products decreased by $2,267,771, or 12.3%, from $18,422,745 in the fiscal year ended September 30, 2021 to $16,154,974 in the fiscal year ended September 30, 2022. Sales volume of third-party products increased by 10.3%, from 8,364,391 units sold in the fiscal year ended September 30, 2021 to 9,226,027 units sold in the fiscal year ended September 30, 2022. In addition, due to change in product mix, the weighted average selling price of third-party products decreased by $0.45 per unit, or 20.5%, from $2.20 per unit in the fiscal year ended September 30, 2021 to $1.75 per unit in the fiscal year ended September 30, 2022.
Cost of Revenues. Our cost of revenues primarily consists of inventory costs (raw materials, labor, packaging cost, depreciation and amortization, third-party products purchase price, freight costs and overhead) and business tax. Cost of revenues generally changes as our production costs change, which are affected by factors including the market price of raw materials, labor productivity, or the purchase price of third-party products, and as the customer and product mix changes.
| For the Years Ended September 30, | ||||||||||||||||
| 2022 | 2021 | Change | ||||||||||||||
| Amount | Amount | Amount | % | |||||||||||||
| Cost of revenue- TCMD products | $ | 8,187,182 | $ | 11,162,847 | $ | (2,975,665 | ) | (26.7 | )% | |||||||
| Cost of revenue- third-party products | 10,064,633 | 11,493,007 | (1,428,374 | ) | (12.4 | )% | ||||||||||
| Total cost of revenue | $ | 18,251,815 | $ | 22,655,854 | $ | (4,404,039 | ) | (19.4 | )% | |||||||
Our cost of revenues decreased by $4,404,039, or 19.4%, from $22,655,854 in the fiscal year ended September 30, 2021 to $18,251,815 in the fiscal year ended September 30, 2022. The decrease in our cost of revenues was primarily due to decreased sales volume and increased raw material and third-party product purchase costs.
67
Cost of revenues of TCMD products
Cost of revenues of TCMD products accounted for 44.9% and 49.3% of our total costs of revenues for the fiscal years ended September 30, 2022 and 2021, respectively. Cost of revenues of TCMD products decreased by $2,975,665, or 26.7%, from $11,162,847 in the fiscal year ended September 30, 2021 to $8,187,182 in the fiscal year ended September 30, 2022. The decrease in cost of revenues of our TCMD products was due to the following reasons:
| (1) | Sales volume of TCMD products decreased by 37.2%, from 17,206,150 units sold in the fiscal year ended September 30, 2021 to 10,799,254 units sold in the fiscal year ended September 30, 2022. Due to recent resurgence of the COVID-19 pandemic and related transportation and travel restrictions, we experienced difficulties shipping and delivering our products to customers located in certain remote geographic areas. These factors led to lower sales volume of our TCMD products in the fiscal year ended September 30, 2022 as compared to the fiscal year ended September 30, 2021. As sales volume decreased in the fiscal year ended September 30, 2022, raw materials, labor, packaging, freight and overhead costs associated with our TCMD product sales also decreased. |
| (2) | As a result of inflation and increased market prices of raw materials, the average per unit cost of our TCMD products increased by $0.11, or 16.9%, from $0.65 per unit in the fiscal year ended September 30, 2021 to $0.76 per unit in the fiscal year ended September 30, 2022. Among the 13 varieties of TCMD products, cost of revenues of Guben Yanling Pill, one of our key products, accounted for 20.0% and 23.3% of our total cost of revenues in fiscal years ended September 30, 2022 and 2021, respectively. Unit production costs of Guben Yanling Pill increased by $0.30 per unit or 25.7% from $1.15 per unit in the fiscal year ended September 30, 2021 to $1.45 per unit in the fiscal year ended September 30, 2022. |
The decrease in the cost of revenues of our TCMD products in the fiscal year ended September 30, 2022 as compared to the fiscal year ended September 30, 2021 reflected the above-mentioned factors combined.
Cost of revenues of third-party products
Cost of revenues of third-party products accounted for 55.1% and 50.7% of our total costs of revenues for the fiscal years ended September 30, 2022 and 2021, respectively. Cost of revenues of third-party products decreased by $1,428,374, or 12.4%, from $11,493,007 in the fiscal year ended September 30, 2021 to $10,064,633 in the fiscal year ended September 30, 2022, because of a decrease in the average unit cost of third-party products by $0.29 per unit, or 21.0%, from $1.38 per unit in the fiscal year ended September 30, 2021 to $1.09 per unit in the fiscal year ended September 30, 2022. Sales volume of third-party products increased by 10.3%, from 8,364,391 units sold in the fiscal year ended September 30, 2021 to 9,226,027 units sold in the fiscal year ended September 30, 2022.
These factors led to the decrease in cost of revenues associated with third-party product sales in the fiscal year ended September 30, 2022 as compared to the fiscal year ended September 30, 2021.
Gross profit
Our gross profit decreased by $3,434,841, from $25,326,177 in the fiscal year ended September 30, 2021 to $21,891,336 in the fiscal year ended September 30, 2022. Our gross margin increased by 1.7% from 52.8% in the fiscal year ended September 30, 2021 to 54.5% in the fiscal year ended September 30, 2022.
| For the Years Ended September 30, | ||||||||||||||||
| 2022 | 2021 | Change | ||||||||||||||
| Amount | Amount | Amount | % | |||||||||||||
| Gross profit- TCMD products | $ | 15,800,995 | $ | 18,396,439 | $ | (2,595,444 | ) | (14.1 | )% | |||||||
| Gross profit- third-party products | 6,090,341 | 6,929,738 | (839,397 | ) | (12.1 | )% | ||||||||||
| Total gross profit | $ | 21,891,336 | $ | 25,326,177 | $ | (3,434,841 | ) | (13.6 | )% | |||||||
| Gross margin- TCMD products | 65.9 | % | 62.2 | % | 3.7 | % | ||||||||||
| Gross margin- third party products | 37.7 | % | 37.6 | % | 0.1 | % | ||||||||||
| Total gross margin | 54.5 | % | 52.8 | % | 1.7 | % | ||||||||||
| Average selling price per unit- TCMD products | $ | 2.22 | $ | 1.72 | $ | 0.50 | 29.1 | % | ||||||||
| Average cost per unit- TCMD products | $ | 0.76 | $ | 0.65 | $ | 0.11 | 16.9 | % | ||||||||
| Average selling price per unit- third party products | $ | 1.75 | $ | 2.20 | $ | (0.45 | ) | (20.5 | )% | |||||||
| Average cost per unit - third party products | $ | 1.09 | $ | 1.38 | $ | (0.29 | ) | (21.0 | )% | |||||||
68
Gross profit from the sales of our TCMD products decreased by $2,595,444, or 14.1%, from $18,396,439 in the fiscal year ended September 30, 2021 to $15,800,995 in the fiscal year ended September 30, 2022, and the gross margin of our TCMD products increased by 3.7%, from 62.2% in the fiscal year ended September 30, 2021 to 65.9% in the fiscal year ended September 30, 2022. The decrease in our gross profit from the sales of TCMD products was due to the following reasons: (i) as discussed above, sales volume of TCMD products decreased by 37.2%, from 17,206,150 units sold in the fiscal year ended September 30, 2021 to 10,799,254 units sold in the fiscal year ended September 30, 2022, because we experienced difficulties shipping and delivering our products to our customers in a timely manner due to recent resurgence of the COVID-19 pandemic and related transportation and travel restrictions; and partially offset by (ii) an increase in the weighted average unit selling price of our TCMD products by $0.50 per unit, or 29.1%, from $1.72 per unit in the fiscal year ended September 30, 2021 to $2.22 per unit in the fiscal year ended September 30, 2022, while the average per unit cost of our TCMD products increased by $0.11, or 16.9%, from $0.65 per unit in the fiscal year ended September 30, 2021 to $0.76 per unit in the fiscal year ended September 30, 2022. During the fiscal year ended September 30, 2022, as a result of the decrease in sales volume, our gross profit associated with the sales of Guben Yanling Pill and Shengrong Weisheng Pill decreased by $633,436 and $709,133 respectively, as compared to the fiscal year ended September 30, 2021.
Gross profit from third-party product sales decreased by $839,397, or 12.1%, from $6,929,738 in the fiscal year ended September 30, 2021 to $6,090,341 in the fiscal year ended September 30, 2022, while the gross margin of third-party product sales slightly increased by 0.1%, from 37.6% in the fiscal year ended September 30, 2021 to 37.7% in the fiscal year ended September 30, 2022. The average unit selling price of third-party products decreased by 20.5%, or $0.45 per unit, while the average unit cost of third-party products also decreased by 21.0%, or $0.29 per unit. The decrease in the average selling price outpaced the decrease in average unit cost of third-party products by $0.16 per unit. The decrease in our gross profit from third-party products was affected by changes in sales of different product mix in the fiscal year ended September 30, 2022 as compared to the fiscal year ended September 30, 2021. For example, the largest portion of decrease in gross profit of third-party products was associated with sales of biochemical drugs products, which decreased by $779,342 when sales volume decreased by 10.3%. For future third-party product sales, our strategy is to select and distribute certain high-quality products with higher margin. We do not expect that the sales of third party products will outpace the sales of our own TCMD products going forward.
Operating expenses
The following table sets forth the breakdown of our operating expenses for the fiscal years ended September 30, 2022 and 2021:
| For the Years Ended September 30, | ||||||||||||||||||||||||
| 2022 | 2021 | Variance | ||||||||||||||||||||||
| Amount | % of revenue | Amount | % of revenue | Amount | % | |||||||||||||||||||
| Total revenue | $ | 40,143,151 | 100.0 | % | $ | 47,982,031 | 100.0 | % | $ | (7,838,880 | ) | (16.3 | )% | |||||||||||
| Operating expenses: | ||||||||||||||||||||||||
| Selling expenses | 19,083,135 | 47.5 | % | 2,973,531 | 6.2 | % | 16,109,604 | 541.8 | % | |||||||||||||||
| General and administrative expenses | 2,602,624 | 6.5 | % | 3,296,844 | 6.9 | % | (694,220 | ) | (21.1 | )% | ||||||||||||||
| Research and development expenses | 7,644,375 | 19.0 | % | 5,465,662 | 11.4 | % | 2,178,713 | 39.9 | % | |||||||||||||||
| Total operating expenses | $ | 29,330,134 | 73.1 | % | $ | 11,736,037 | 24.5 | % | $ | 17,594,097 | 149.9 | % | ||||||||||||
Selling expenses
Our selling expenses primarily include salaries and welfare benefit expenses paid to our sales personnel, advertising expenses to increase our brand awareness, shipping and delivery expenses, expenses incurred for our business travel and meals, and other sales promotion and marketing activities related expenses.
| For the Years Ended September 30, | ||||||||||||||||||||||||
| 2022 | 2021 | Variance | ||||||||||||||||||||||
| Amount | % | Amount | % | Amount | % | |||||||||||||||||||
| Salary and employee benefit expenses | $ | 778,656 | 4.1 | % | $ | 859,436 | 28.9 | % | $ | (80,780 | ) | (9.4 | )% | |||||||||||
| Advertising expenses | 17,527,318 | 91.8 | % | 1,316,654 | 44.3 | % | 16,210,664 | 1231.2 | % | |||||||||||||||
| Shipping and delivery expenses | 638,286 | 3.3 | % | 701,997 | 23.6 | % | (63,711 | ) | (9.1 | )% | ||||||||||||||
| Business travel and meals expenses | 125,553 | 0.7 | % | 78,565 | 2.6 | % | 46,988 | 59.8 | % | |||||||||||||||
| Other sales promotion related expenses | 13,322 | 0.1 | % | 16,879 | 0.6 | % | (3,557 | ) | (21.1 | )% | ||||||||||||||
| Total selling expenses | $ | 19,083,135 | 100.0 | % | $ | 2,973,531 | 100.0 | % | $ | 16,109,604 | 541.8 | % | ||||||||||||
69
Our selling expenses increased by $16,109,604, or 541.8%, from $2,973,531 in the fiscal year ended September 30, 2021 to $19,083,135 in the fiscal year ended September 30, 2022, primarily attributable to (i) an increase in advertising expenses by $16,210,664, or 1,231.2%, from $1,316,654 in the fiscal year ended September 30, 2021 to $17,527,318 in the fiscal year ended September 30, 2022. In September 2021, we entered into an advertising service agreement with a third party, Guangdong Fengyang Legend Consulting Co., Ltd. (“Fengyang Legend”), pursuant to which, Fengyang Legend agreed to assist us in developing and producing a TV advertisement for promoting the sales of our major TCMD products, Bai Nian Dan and Guben Yanling Pill, and coordinating with a TV channel to broadcast the advertisement to targeted geographic market areas. In March 2022, we entered into an advertising service agreement with a third-party, Health Headline, pursuant to which, Health Headline provided media advertising services to promote our brand on the Health Headline website and mobile app, with a service period of ten months from March 1, 2022 to December 31, 2022. The advertising form, release cycle and monthly payment depended on the Company’s monthly budget. We recognized $17,501,148 in advertising expense related to these advertising service agreements during the fiscal year ended September 30, 2022; (ii) business travel and meals expense increased by $46,988 or 59.8%, from $78,565 in the fiscal year ended September 30, 2021 to $125,553 in the fiscal year ended September 30, 2022, primarily due to our increased sales activities during the fiscal year ended September 30, 2022; partially offset by (iii) salary and employee benefit expenses decreased by $80,780, or 9.4% due to decreased sale commission during the fiscal year ended September 30, 2022; and (iv) shipping and delivery expenses decreased by $63,711, or 9.1% due to our decreased sales volume during the fiscal year ended September 30, 2022. These above-mentioned factors combined led to the increase in our selling expenses in the fiscal year ended September 30, 2022 as compared to the fiscal year ended September 30, 2021. As a percentage of revenues, our selling expenses accounted for 47.5% and 6.2% of our total revenue for the fiscal years ended September 30, 2022 and 2021, respectively.
General and Administrative Expenses
Our general and administrative expenses primarily consist of employee salaries, welfare and insurance expenses, depreciation, bad debt reserve expenses, inspection and maintenance expenses, office supply and utility expenses, business travel and meals expenses, land and property taxes and professional service expenses.
| For the Years Ended September 30, | ||||||||||||||||||||||||
| 2022 | 2021 | Variance | ||||||||||||||||||||||
| Amount | % | Amount | % | Amount | % | |||||||||||||||||||
| Salary and employee benefit expense | $ | 641,622 | 24.7 | % | $ | 657,543 | 19.9 | % | $ | (15,921 | ) | (2.4 | )% | |||||||||||
| Depreciation and amortization | 185,169 | 7.1 | % | 172,453 | 5.2 | % | 12,716 | 7.4 | % | |||||||||||||||
| Bad debt reserve expenses (recovery) | 419,353 | 16.1 | % | (230,175 | ) | (7.0 | )% | 649,528 | (282.2 | )% | ||||||||||||||
| Land and property tax | 103,769 | 4.0 | % | 104,451 | 3.2 | % | (682 | ) | (0.7 | )% | ||||||||||||||
| Office supply and utility expense | 307,883 | 11.8 | % | 376,204 | 11.4 | % | (68,321 | ) | (18.2 | )% | ||||||||||||||
| Transportation, business travel and meals expense | 83,029 | 3.2 | % | 68,047 | 2.1 | % | 14,982 | 22.0 | % | |||||||||||||||
| Consulting fee | 720,430 | 27.7 | % | 1,967,858 | 59.7 | % | (1,247,428 | ) | (63.4 | )% | ||||||||||||||
| Inspection and maintenance fee | 39,555 | 1.5 | % | 60,939 | 1.8 | % | (21,384 | ) | (35.1 | )% | ||||||||||||||
| Stamp tax and other expenses | 101,814 | 3.9 | % | 119,524 | 3.6 | % | (17,710 | ) | (14.8 | )% | ||||||||||||||
| Total general and administrative expenses | $ | 2,602,624 | 100.0 | % | $ | 3,296,844 | 100.0 | % | $ | (694,220 | ) | (21.1 | )% | |||||||||||
Our general and administrative expenses decreased by $694,220, or 21.1%, from $3,296,844 in the fiscal year ended September 30, 2021 to $2,602,624 in the fiscal year ended September 30, 2022, primarily attributable to (i) a decrease in consulting fees by $1,247,428 because we incurred higher amount of consulting services fees such as legal and audit expenses in the fiscal year ended September 30, 2021 when we completed our initial public offering (the “IPO”); partially offset by (ii) an increase in bad debt expense by $649,528 or 282.2% because we accrued more bad debt expenses based on our assessment of the collectability of the accounts receivable and advance to suppliers. The overall decrease in our general and administrative expenses in the fiscal year ended September 30, 2022 as compared to the fiscal year ended September 30, 2021 reflected the above-mentioned factors combined. As a percentage of revenues, general and administrative expenses were 6.5% and 6.9% of our revenue for the fiscal years ended September 30, 2022 and 2021, respectively.
Research and development expenses
Our research and development expenses primarily consist of salaries, welfare and insurance expenses paid to our employees involved in the research and development activities, materials and supplies used in the development and testing new TCMD products, depreciation and other miscellaneous expenses.
| For the Years Ended September 30, | ||||||||||||||||||||||||
| 2022 | 2021 | Variance | ||||||||||||||||||||||
| Amount | % | Amount | % | Amount | % | |||||||||||||||||||
| Salary and employee benefit expenses to R&D staff | $ | 159,924 | 2.1 | % | $ | 155,940 | 2.9 | % | $ | 3,984 | 2.6 | % | ||||||||||||
| Materials used in R&D activities | 489,692 | 6.4 | % | 836,710 | 15.3 | % | (347,018 | ) | (41.5 | )% | ||||||||||||||
| Expenditure on new product development | 6,973,683 | 91.2 | % | 4,454,400 | 81.5 | % | 2,519,283 | 56.6 | % | |||||||||||||||
| Depreciation and others | 21,076 | 0.3 | % | 18,612 | 0.3 | % | 2,464 | 13.2 | % | |||||||||||||||
| Total R&D expenses | $ | 7,644,375 | 100.0 | % | $ | 5,465,662 | 100.0 | % | $ | 2,178,713 | 39.9 | % | ||||||||||||
70
Research and development expenses increased by $2,178,713, or 39.9%, from $5,465,662 for the fiscal year ended September 30, 2021 to $7,644,375 for the fiscal year ended September 30, 2022, primarily attributable to (i) an increase in research and development expense of $2,519,283 in order to develop and test eight new Chinese medicine products in order to diversify our future product portfolio. In the fiscal year ended September 30, 2021, we entered into several cooperative agreements with external academic and research institutions to jointly conduct the new product development and accordingly incurred significant amount of R&D expense in connection with such efforts; and partially offset by (ii) a decrease in the materials used in the R&D activities by $347,018 as we outsourced part of our R&D activities to external academic and research institutions. As a percentage of revenues, research and development expenses were 19.0% and 11.4% of our revenue in the fiscal years ended September 30, 2022 and 2021, respectively.
Other income (expenses), net
Total other expenses, net, was $545,349 in the fiscal year ended September 30, 2022, compared to a net other income of $88,338 in the fiscal year ended September 30, 2021, due to the following reasons:
| (i) | our short-term investment loss was $470,477 in the fiscal year ended September 30, 2022, compared to short-term investment income of $239,549 in the fiscal year ended September 30, 2021. We had short-term investments of $13.1 million in wealth management financial products from financial institutions to generate investment income, which suffered temporary loss due to fluctuations of the bond market. Such short-term investment can be redeemed anytime at our discretion and is highly liquid. |
| (ii) | interest expenses increased by $60,796, or 59.8%, from $101,604 in the fiscal year ended September 30, 2021 to $162,400 in the fiscal year ended September 30, 2022. The increase in our interest expenses was due to higher weighted average amount of outstanding loans we carried during the fiscal year ended September 30, 2022 as compared to the fiscal year ended September 30, 2021. |
Provision for Income Taxes
Our provision for income taxes was $752,419 in the fiscal year ended September 30, 2022, a decrease of $1,606,107, or 68.1%, from $2,358,526 in the fiscal year ended September 30, 2021 due to our decreased taxable income. Under the EIT Law, domestic enterprises and foreign investment enterprises are usually subject to a unified 25% enterprise income tax rate while preferential tax rates, tax holidays and even tax exemption may be granted on a case-by-case basis. The EIT Law grants preferential tax treatment to HNTEs. Under this preferential tax treatment, HNTEs are entitled to an income tax rate of 15%, subject to a requirement that they re-apply for their HNTE status every three years. Jiangxi Universe, one of our main operating subsidiaries in the PRC, was approved as an HNTE and was entitled to a reduced income tax rate of 15% beginning November 2016 with an initial term of three years. Jiangxi Universe’s HNTE status was successfully renewed in December 2019 and November 2022 for a term of three additional years. Universe Trade, the wholly owned subsidiary of Jiangxi Universe, was approved as an HNTE and was entitled to a reduced income tax rate of 15% beginning December 2020 with an initial term of three years. Universe Trade did not successfully renew its HNTE status at the end of December 2022 and, therefore, will be subject to the standard PRC enterprise income tax rate of 25% starting from January 2024. The EIT Law is typically enforced by the local tax authorities in the PRC. Each local tax authority has the discretion to grant tax holidays to local enterprises as a way to encourage entrepreneurship and stimulate local economy. The corporate income taxes for the fiscal years ended September 30, 2022 and 2021 were reported at a blended reduced rate since Jiangxi Universe and Universe Trade enjoy a 15% reduced income tax rate due to their HNTE status, and Universe Hanhe, the wholly owned subsidiary of Jiangxi Universe, is subject to a standard 25% income tax rate. The impact of the tax holidays noted above decreased PRC corporate income taxes by $694,955 and $1,518,979 for the fiscal years ended September 30, 2022, and 2021, respectively. The benefit of the tax holidays on net income per share (basic and diluted) $0.03 and $0.09 for the fiscal years ended September 30, 2022 and 2021, respectively.
Net Income (Loss)
As a result of the foregoing, we reported a net loss of $8,736,566 for the fiscal year ended September 30, 2022, representing a decrease of $20,056,518 from a net income of $11,319,952 for the fiscal year ended September 30, 2021.
B. Liquidity and Capital Resources
As of September 30, 2023, we had $5.3 million in cash on hand. We also had short-term investments of $13.2 million in wealth management financial products from financial institutions to generate investment income, which we purchased with our IPO proceeds. Such short-term investment can be redeemed anytime at our discretion and is highly liquid. As of September 30, 2023, we also had $10.7 million in accounts receivable. Our accounts receivable primarily include balance due from customers for our pharmaceutical products sold and delivered to customers. Approximately 58.6%, or $6.5 million, of our net accounts receivable balance as of September 30, 2023 has been subsequently collected. Collected accounts receivable will be used as working capital in our operations, if necessary.
71
As of September 30, 2023, our inventory balance amounted to $3.3 million, primarily consisting of raw materials, work-in-progress and finished TCMD products, which we believe are able to be sold quickly based on the analysis of the current trends in demand for our products.
On June 25, 2021, we signed a construction sub-contract with sub-contractor Jiangxi Chenyuan Construction Project Co., Ltd. (“Chenyuan”), pursuant to which, Chenyuan will help us construct four manufacturing factory buildings and an office building with a total estimated budget of RMB165 million (approximately $22.6 million). As of September 30, 2023, we had made a prepayment of approximately RMB69.2 million (approximately $9.5 million) to Chenyuan and future additional capital expenditure on this constriction-in-process (“CIP”) project was estimated to be approximately RMB95.8 million (equivalent to $13.1 million), among which approximately $3.4 million is required for the next 12 months.
On May 6, 2021, we entered into a real estate property purchase agreement with a related party, Jiangxi Yueshang Investment Co., Ltd. (“Jiangxi Yueshang”), an entity in which our chief executive officer, Mr. Gang Lai, owned 5% of its equity interests as of the date of that agreement. Pursuant to this purchase agreement, Jiangxi Yueshang will sell and we will purchase certain residential apartments and commercial office space totaling 2,749.30 square meters, with a total purchase price of RMB32 million (approximately $4.4 million). Pursuant to this purchase agreement, we were required to make a prepayment in the amount of 50% of the total purchase price, with 20% of the total purchase price payable when a certificate of occupancy is available to us, and 30% of the total purchase price payable upon delivery of the property. As of September 30, 2023, we had made a prepayment of RMB16 million (approximately $2.2 million) to Jiangxi Yueshang. The remaining balance is expected to be paid by August 2024.
As of September 30, 2023, we also had short-term bank loans of approximately $5.5 million that we obtained from several PRC banks for working capital purposes. We expect that we will be able to renew all of the existing bank loans upon their maturity based on our past experiences and our outstanding credit history.
As of September 30, 2023, our working capital balance was $23.1 million. In assessing our liquidity, management monitors and analyzes our cash on-hand, our ability to generate sufficient revenue in the future, and our operating and capital expenditure commitments. We believe that our current cash and cash flows provided by operating activities, short-term investments in wealth management financial products, borrowings from banks and from our principal shareholders will be sufficient to meet our working capital needs in the next 12 months from the date our consolidated financial statements for the fiscal year ended September 30, 2023 are released.
The following table sets forth summary of our cash flows for the periods indicated:
| For the Years Ended September 30, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Net cash provided by (used in) operating activities | $ | 1,120,082 | $ | (1,312,346 | ) | $ | (2,055,847 | ) | ||||
| Net cash used in investing activities | (44,169 | ) | (3,908,105 | ) | (27,059,958 | ) | ||||||
| Net cash (used in) provided by financing activities | (1,390,646 | ) | 3,317,943 | 26,581,809 | ||||||||
| Effect of exchange rate change on cash | (111,478 | ) | (463,942 | ) | 553,702 | |||||||
| Net decrease in cash | (426,211 | ) | (2,366,450 | ) | (1,980,294 | ) | ||||||
| Cash, beginning of year | 5,711,458 | 8,077,908 | 10,058,202 | |||||||||
| Cash, end of year | $ | 5,285,247 | $ | 5,711,458 | $ | 8,077,908 | ||||||
Operating Activities
Net cash provided by operating activities was $1,120,082 for the fiscal year ended September 30, 2023, primarily consisted of the following:
| ● | Net loss of $6,163,061 for the period. |
| ● | A decrease in accounts receivable of $4,718,118. Our accounts receivable primarily includes balance due from customers for our pharmaceutical products sold and delivered to customers. We enhanced our accounts receivable management and shortened accounts receivable collection period during the fiscal year ended September 30, 2023. |
72
| ● | An increase in accounts payable of $1,641,426 due to pending invoices from suppliers for raw materials purchased in the third quarter of 2023. |
| ● | An increase in inventory balance of $1,183,823 because we increased inventory stockpiles to reduce the negative impact from increased market price of Chinese traditional medicine raw materials. |
| ● | An decrease in prepaid expenses and other current assets balance of $1,091,022 due to decreased prepaid income tax. |
Net cash used in operating activities was $1,312,346 for the fiscal year ended September 30, 2022, primarily consisted of the following:
| ● | Net loss of $8,736,566 for the fiscal year. |
| ● | An increase in accounts receivable of $1,549,312. Our accounts receivable primarily includes balance due from customers for our pharmaceutical products sold and delivered to customers. As of September 30, 2022, most of our outstanding accounts receivable aged below six months. Collected accounts receivable will be used as working capital in our operations, if necessary. |
| ● | A decrease in advance to suppliers of $2,681,214 because we made significant advance payments to suppliers for raw material purchase as of September 30, 2021 and we received purchased raw materials valued at approximately $2.7 million during the fiscal year ended September 30, 2022. |
| ● | A decrease in prepayment for advertising of $7,385,695. In September 2021, we engaged a third-party advertising agency to develop and produce TV advertisement for promoting the sales of our major TCMD product, Bai Nian Dan and Guben Yanling Pill, and coordinate with a TV channel to broadcast the advertisement to targeted geographic market areas. Such prepayment was charged to expense when our TV advertisement was broadcasted in the fiscal year ended September 30, 2022. |
| ● | An increase in prepaid expenses and other current assets of $1,699,929 related with VAT of advertising expense deductible upon reception of invoice, as well as prepaid income tax deductible from future taxable income. |
| ● | A decrease in accounts payable of $1,896,621 because we made payment to raw material suppliers when we received the invoices from them. |
Net cash used in operating activities was $2,055,847 for the fiscal year ended September 30, 2021, primarily consisting of the following:
| ● | Net income of $11,319,952 for the fiscal year. |
| ● | An increase in accounts receivable of $3,867,457. Our accounts receivable primarily include balance due from customers for our pharmaceutical products sold and delivered to customers. As of September 30, 2021, most of our outstanding accounts receivable aged below six months. Collected accounts receivable will be used as working capital in our operations, if necessary. |
| ● | An increase in inventory balance of $451,634 because we increased the stockpile of raw materials in order to meet the future production need. |
| ● | An increase in advance to suppliers of $2,717,085, representing prepayments made to certain suppliers to ensure continuous supply of raw materials at favorable purchase prices. |
| ● | An increase in prepayment to an advertising agency of $7,434,240 in order to engage this advertising agency to develop and produce TV advertisement for promoting the sales of our major TCMD product, Bai Nian Dan and Guben Yanling Pill, and coordinate with a TV channel to broadcast the advertisement to targeted geographic market areas. Such prepayment has been charged to expense when our TV advertisement was first broadcasted in October 2021. |
| ● | An increase in accounts payable of $2,457,337 primarily due to increased purchase of inventories and we have not received related invoices from the suppliers and accordingly such payable had not been settled as of September 30, 2021. Most of the outstanding accounts payable balance has been subsequently settled as of the date of this annual report. |
73
Investing Activities
Net cash used in investing activities amounted to $44,169 for the fiscal year ended September 30, 2023 due to purchase of fixed assets.
Net cash used in investing activities amounted to $3,908,105 for the fiscal year ended September 30, 2022, which primarily included prepayment for acquisition of $3,814,925. On September 26, 2022, we entered into a letter of intent for an equity transfer with an individual, Mr. Xibo Liu, pursuant to which Mr. Xibo Liu shall transfer his 51% ownership in Yunnan Faxi to us in consideration for RMB72 million (approximately $10.1 million). We prepaid RMB25 million (approximately $3.9 million) in the fiscal year ended September 30, 2022. The intended acquisition is expected to be completed and the remaining balance is expected to be paid by the end of 2024.
Net cash used in investing activities amounted to $27,059,958 for the fiscal year ended September 30, 2021 and primarily included (i) purchase of fixed assets of $444,505; (ii) prepayment of $2,457,600 made to a related party to purchase residential and commercial property; (iii) prepayment of $10,629,120 made to a sub-contractor in order to complete the CIP Project, including four manufacturing factory buildings and an office building, with estimated project completion in December 2025; (iv) purchase of short-term investments of $15,330,660 because we used part of the IPO proceeds to purchase certain wealth management financial products from financial institutions in order to earn investment income; and (v) redemption of short-term investments of $1,801,927.
Financing Activities
Net cash used in financing activities amounted to $1,390,646 for the fiscal year ended September 30, 2023, primarily include the following:
| ● | Proceeds from short-term bank loans of $5,671,104 and repayment of bank loans of $3,969,773. |
| ● | Repayment of related party borrowings of $3,091,977. The balance due to related party mainly consisted of advances from our principal shareholders for working capital purposes during our normal course of business. These advances were non-interest bearing and due on demand. |
Net cash provided by financing activities amounted to $3,317,943 for the fiscal year ended September 30, 2022, primarily including the following:
| ● | Proceeds from short-term bank loans in the amount of $4,272,716 and repayment of bank loans in the amount of $4,272,716. |
| ● | Proceeds from related party borrowings of $3,317,943. The balance due to related party mainly consisted of advances from our principal shareholders for working capital purposes during our normal course of business. These advances were non-interest bearing and due on demand. |
Net cash provided by financing activities amounted to $26,581,809 for the fiscal year ended September 30, 2021, primarily include the following:
| ● | Proceeds from short-term bank loans of $4,300,800 and offset by repayment of bank loans of $2,764,800 upon loan maturity. |
| ● | Net proceeds of $25,957,457 received upon completion of our IPO in March 2021. |
| ● | Repayment of related party borrowings of $911,648. The balance due to related party mainly consisted of advances from our principal shareholder for working capital purposes during our normal course of business. These advances were non-interest bearing and due on demand. |
74
Commitments and contingencies
From time to time, we are a party to various legal actions arising in the ordinary course of business. We accrue costs associated with these matters when they become probable and the amount can be reasonably estimated. Legal costs incurred in connection with loss contingencies are expensed as incurred. For the fiscal years ended September 30, 2023, 2022 and 2021, we did not have any material legal claims or litigation that, individually or in aggregate, could have a material adverse impact on our consolidated financial position, results of operations and cash flows.
As of September 30, 2023, we had the following contractual obligations:
| Payments Due by Period | ||||||||||||||||
| Contractual Obligations | Total | Less than 1 year | 1-2 years | 2-3 years | ||||||||||||
| (1) Debt Obligations | $ | 5,482,456 | $ | 5,482,456 | $ | - | $ | - | ||||||||
| (2) Capital expenditure commitment on CIP project | 13,130,483 | 3,392,270 | 8,607,456 | 1,130,757 | ||||||||||||
| (3) Capital expenditure commitment for purchase of property | 2,192,982 | 2,192,982 | - | - | ||||||||||||
| Total | $ | 20,805,921 | $ | 11,067,708 | $ | 8,607,456 | $ | 1,130,757 | ||||||||
| (1) | As of September 30, 2023, we had total $5,482,456 short-term borrowings from several PRC banks (see Footnote 12 of our consolidated financial statements and footnotes included elsewhere in this annual report, Short-term bank loans, for details). |
| (2) | On June 25, 2021, we signed a construction sub-contract with Chenyuan, pursuant to which, Chenyuan will help us construct four manufacturing factory buildings and an office building with a total estimated budget of RMB165 million (approximately $22.6 million). As of September 30, 2023, we had made a prepayment of approximately RMB69.2 million (approximately $9.5 million) to Chenyuan and future additional capital expenditure on this CIP project was estimated to be approximately RMB95.8 million (equivalent to $13.1 million) (see Footnote 10 of our consolidated financial statements and footnotes included elsewhere in this annual report, Prepayment for CIP project, for details). |
| (3) | On May 6, 2021, we entered into a real estate property purchase agreement with a related party, Jiangxi Yueshang, an entity in which our chief executive officer, Mr. Gang Lai, owned 5% of its equity interests as of the date of that agreement. Pursuant to this purchase agreement, Jiangxi Yueshang will sell and we will purchase certain residential apartments and commercial office space totaling 2,749.30 square meters, with a total purchase price of RMB32 million (approximately $4.4 million). Pursuant to this purchase agreement, we were required to make a prepayment in the amount of 50% of the total purchase price, with 20% of the total purchase price payable when a certificate of occupancy is available to us, and 30% of the total purchase price payable upon delivery of the property. As of September 30, 2023, we had made a prepayment of RMB16 million (approximately $2.2 million) to Jiangxi Yueshang. The remaining balance is expected to be paid by August 2024 (see Footnote 11 of our consolidated financial statements and footnotes included elsewhere in this annual report, Prepayment for purchase of a property, for details). |
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements as of September 30, 2023 and 2022.
Inflation
Inflation does not materially affect our business or the results of our operations.
Seasonality
Seasonality does not materially affect our business or the results of our operations.
75
C. Research and Development, Patents and Licenses, etc.
See “Item 4. Information on the Company—B. Business Overview—Intellectual Property” and “Item 4. Information on the Company—B. Business Overview—Research and Development.”
D. Trend Information
Other than as disclosed below and elsewhere in this annual report on Form 20-F, we are not aware of any trends, uncertainties, demands, commitments, or events for the period from October 1, 2022 to September 30, 2023 that are reasonably likely to have a material adverse effect on our net revenue, income, profitability, liquidity, or capital resources, or that caused the disclosed financial information to be not necessarily indicative of future operating results or financial condition.
E. Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements. These financial statements are prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”), which requires us to make estimates and assumptions that affect the reported amounts of our assets and liabilities and revenues and expenses, to disclose contingent assets and liabilities on the date of the consolidated financial statements, and to disclose the reported amounts of revenues and expenses incurred during the financial reporting period. We continue to evaluate these estimates and assumptions that we believe to be reasonable under the circumstances. We rely on these evaluations as the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Since the use of estimates is an integral component of the financial reporting process, actual results could differ from those estimates. Some of our accounting policies require higher degrees of judgment than others in their application. We believe critical accounting policies as disclosed in this annual report reflect the more significant judgments and estimates used in preparation of our consolidated financial statements.
Risks and Uncertainties
Our main operation is located in the PRC. Accordingly, our business, financial condition, and results of operations may be influenced by political, economic, and legal environments in the PRC, as well as by the general state of the PRC economy. Our results may be adversely affected by changes in the political, regulatory and social conditions in the PRC. Although we have not experienced losses from these situations and believes that we are in compliance with existing laws and regulations including our organization and structure, this may not be indicative of future results.
The development and commercialization of new pharmaceutical products is highly competitive, and the industry currently is characterized by rapidly changing technologies, significant competition and a strong emphasis on intellectual property. We may face competition with respect to our current and future pharmaceutical product candidates from major pharmaceutical companies in China.
Our business, financial condition and results of operations may also be negatively impacted by risks related to natural disasters, extreme weather conditions, health epidemics and other catastrophic incidents, which could significantly disrupt our operations.
The following critical accounting policies rely upon assumptions and estimates and were used in the preparation of our consolidated financial statements:
Uses of estimates
In preparing the consolidated financial statements in conformity with U.S. GAAP, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. These estimates are based on information as of the date of the consolidated financial statements. Significant estimates required to be made by management include, but are not limited to, the allowance for estimated uncollectible receivables, the realizability of advance to suppliers, inventory valuation, useful lives of property and equipment, intangible assets, the recoverability of long-lived assets, provision necessary for contingent liabilities, revenue recognition and realization of deferred tax assets. Actual results could differ from those estimates.
76
Accounts receivable, net
Accounts receivables are presented net of allowance for doubtful accounts. We determine the adequacy of reserves for doubtful accounts based on individual account analysis and historical collection trends. We establish a provision for doubtful receivables when there is objective evidence that we may not be able to collect amounts due. The allowance is based on management’s best estimate of specific losses on individual exposures, as well as a provision on historical trends of collections. Actual amounts received may differ from management’s estimate of credit worthiness and the economic environment. Delinquent account balances are written-off against the allowance for doubtful accounts after management has determined that the likelihood of collection is not probable. Allowance for uncollectable balances amounted to $347,306 and $791,827 as of September 30, 2023 and 2022, respectively.
Inventories, net
Inventories are stated at net realizable value using weighted average method. Costs include the cost of raw materials, freight, direct labor and related production overhead. Any excess of the cost over the net realizable value of each item of inventories is recognized as a provision for diminution in the value of inventories. Net realizable value is the estimated selling price in the normal course of business less any costs to complete and sell products. We evaluate inventories on a quarterly basis for its net realizable value adjustments, and reduces the carrying value of those inventories that are obsolete or in excess of the forecasted usage to their estimated net realizable value based on various factors including aging, expiration dates, as applicable, taking into consideration historical and expected future product sales. We recorded inventory reserve of $69,747 and $120,286 as of September 30, 2023 and 2022, respectively.
Revenue recognition
To determine revenue recognition for contracts with customers, we perform the following five steps : (i) identify the contract(s) with the customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, including variable consideration to the extent that it is probable that a significant future reversal will not occur, (iv) allocate the transaction price to the respective performance obligations in the contract, and (v) recognize revenue when (or as) we satisfy the performance obligation.
We recognize revenue when we transfer our goods and services to customers in an amount that reflects the consideration to which we expect to be entitled in such exchange. We account for the revenue generated from sales of our TCMD and third-party products on a gross basis as we are acting as a principal in these transactions, are subject to inventory risk, have latitude in establishing prices, and are responsible for fulfilling the promise to provide customers the specified goods, which we have control of the goods and has the ability to direct the use of goods to obtain substantially all the benefits. All of our contracts have one single performance obligation as the promise is to transfer the individual goods to customers, and there are no separately identifiable other promises in the contracts. Our revenue streams are recognized at a point in time when title and risk of loss passes and the customer accepts the goods, which generally occurs at delivery. Our products are sold with no right of return and we do not provide other credits or sales incentives to customers. Revenue is reported net of all VAT.
Income Tax
We account for current income taxes in accordance with the laws of the relevant tax authorities. Deferred income taxes are recognized when temporary differences exist between the tax bases of assets and liabilities and their reported amounts in the consolidated financial statements. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period including the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
An uncertain tax position is recognized only if it is “more likely than not” that the tax position would be sustained in a tax examination. The amount recognized is the largest amount of tax benefit that is greater than 50% likely of being realized on examination. For tax positions not meeting the “more likely than not” test, no tax benefit is recorded. Penalties and interest incurred related to underpayment of income tax are classified as income tax expense in the period incurred. No significant penalties or interest relating to income taxes were incurred during the fiscal years ended September 30, 2023, 2022 and 2021. We do not believe there was any uncertain tax provision as of September 30, 2023 and 2022.
Our operating subsidiaries in China are subject to the income tax laws of the PRC. No significant income was generated outside the PRC for the fiscal years ended September 30, 2023, 2022 and 2021. As of September 30, 2023 and 2022, all of our tax returns of our PRC subsidiaries remained open for statutory examination by PRC tax authorities.
77
Recently Issued Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments — Credit Losses” (Topic 326), which will require the measurement of all expected credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts. Subsequently, the FASB issued ASU No. 2018-19, Codification Improvements to Topic 326, to clarify that receivables arising from operating leases are within the scope of lease accounting standards. Further, the FASB issued ASU No. 2019-04, ASU 2019-05, ASU 2019-10, ASU 2019-11 and ASU 2020-02 to provide additional guidance on the credit losses standard. For all other entities, the amendments for ASU 2016-13 are effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years, with early adoption permitted. Adoption of the ASUs is on a modified retrospective basis. We adopted ASU 326 effective October 1, 2022, the first day of our fiscal year ended September 30, 2023. The adoption of ASU 326 did not have a material impact on our financial position, results of operations or cash flows.
Other accounting standards that have been issued by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption. We do not discuss recent standards that are not anticipated to have an impact on or are unrelated to our consolidated financial condition, results of operations, cash flows or disclosures.
Item 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Senior Management
The following table sets forth information regarding our directors and executive officers as of the date of this annual report.
| Name | Age | Position(s) | ||
| Gang Lai | 57 | Chairman of the Board of Directors and Chief Executive Officer | ||
| Lin Yang | 49 | Chief Financial Officer and Director | ||
| Baochang Liu | 44 | Chief Operating Officer | ||
| Jiawen Pang | 57 | Independent Director | ||
| Yongping Yu | 56 | Independent Director | ||
| Ding Zheng | 47 | Independent Director |
The following is a brief biography of each of our executive officers and directors:
Mr. Gang Lai is our chief executive officer and chairman of the board. Mr. Lai has served as the chief executive officer of Jiangxi Universe since 2004 and founded Universe Trade in 2010. Before joining us, Mr. Lai was a successful entrepreneur. He founded Jiangxi Lvzhouyuan Timber Joint Stock Co., Ltd. in 2001, a company listed on PRC National Equities Exchange and Quotations (NEEQ: 838893), and has since served as its chairman of the board. Mr. Lai graduated from Jingdezhen Ceramic Institute with a bachelor’s degree in mechanical engineering.
Ms. Lin Yang is our chief financial officer and director. Ms. Lin Yang has served as the financial director of Jiangxi Universe since April 2006 and the financial director of Universe Trade since its formation in 2010. Before joining us, Ms. Yang served as an accountant at Jiangxi Automobile Engineering Plastic Co., Ltd. from 1998 to March 2006. Ms. Yang graduated from Jiangxi University of Finance and Economics with a bachelor’s degree in accounting.
Mr. Baochang Liu has served as our chief operating officer since December 2021. Mr. Liu has over 17 years of experience in pharmaceutical marketing. From January 2020 to December 2021, Mr. Baochang Liu worked as the vice president of marketing and general manager of OTC department at China Shineway Pharmaceutical Group Ltd. (HKEX: 2877). From November 2015 to December 2019, Mr. Baochang Liu served as the general manager of OTC department at Chengdu Kanghong Pharmaceutical Group Co., Ltd. (SHE: 002773). Prior to that, Mr. Liu worked as the general marketing manager at Chengdu Rongyao Industry (Group) Co., Ltd. from December 2012 to November 2015. Mr. Baochang Liu obtained his bachelor’s degree in accounting and in marketing management from Harbin University of Commerce in 2004 and his master of business administration from Fudan University in 2020.
78
Mr. Jiawen Pang has served as our independent director since March 2021. Since January 1, 2021, Mr. Jiawen Pang has served as the vice president at Guangzhou Dahua Food Technology Co., Ltd., a food manufacturing company, where Mr. Pang is responsible for overseeing the company’s general management and marketing function. From January 1, 2018 to December 2020, Mr. Jiawen Pang served the general manager at Pangbei (Shanghai) Medical Technology Center, a medical device company, where Mr. Pang was responsible for overseeing the general management and marketing function of the company. Mr. Jiawen Pang graduated from Tianjin University of Commerce with a bachelor’s degree in refrigeration and food freezing engineering in 1989 and from Sun Yat-sen University with a master of business administration in healthcare and medicine in 2004.
Mr. Yongping Yu has served as our independent director since May 2023. Mr. Yongping Yu has served as the General Manager of Zhuhai Qirong Venture Capital Investment Management Co., Ltd. since August 2019, a venture capital company focused on the healthcare industry. Mr. Yongping Yu served as a director and Executive Deputy General Manager of Xinhua Kangmei Health Think Tank Co., Ltd. from May 2017 to July 2019. From July 2015 to May 2017, Mr. Yongping Yu served as the General Manager of the Medical Data Division of Xinhua News Agency’s Big Data Business Department. From July 2014 to July 2015, Mr. Yongping Yu served as the Editor-in-Chief and General Manager of Xinhua Cyber Health, a WeChat official account, at Xinhua News Agency. From February 2011 to July 2014, Mr. Yongping Yu served as the Director of the Development Planning Department and Chief Physician of the Aviation General Hospital. From April 2004 to February 2011, Mr. Yongping Yu served as the Deputy Secretary-General of JiangXi Pharmaceutical Association. Mr. Yu obtained a Bachelor of Medicine degree from Nanchang University in the PRC in 1992 and obtained Bachelor of Arts degree in in Chinese language and literature from Tsinghua University in the PRC in 1995.
Mr. Ding Zheng has served as our independent director since March 2021. Mr. Ding Zheng has served as the chairman of the board at Guangzhou Roujing Sunshade Energy-saving Technology Co., Ltd. since 2018. Mr. Zheng has also served as the general manager at Hande Manufacturing (China) Co., Ltd., since 2015. Mr. Zheng obtained a bachelor’s degree in technology economics from Shanghai Jiao Tong University in 2000 and a master of business administration degree from Tsinghua University in 2005. Mr. Zheng is a member of the China Institute of Certified Public Accountants (CICPA).
Board Diversity
The table below provides certain information regarding the diversity of our board of directors as of the date of this annual report.
Board Diversity Matrix
| Country of Principal Executive Offices: | China | |
| Foreign Private Issuer | Yes | |
| Disclosure Prohibited under Home Country Law | No | |
| Total Number of Directors | 5 |
| Female | Male |
Non- Binary |
Did Not Disclose Gender | ||||
| Part I: Gender Identity | |||||||
| Directors | 1 | 4 | 0 | 0 | |||
| Part II: Demographic Background | |||||||
| Underrepresented Individual in Home Country Jurisdiction | 0 | ||||||
| LGBTQ+ | 0 | ||||||
| Did Not Disclose Demographic Background | 0 | ||||||
79
Family Relationships
None of our directors or executive officers has a family relationship as defined in Item 401 of Regulation S-K.
Controlled Company
Mr. Gang Lai, our chief executive officer, director, and chairman of the board of directors, currently beneficially own approximately 57.05% of the aggregate voting power of our outstanding ordinary shares. As a result, we are a “controlled company” within the meaning of the Nasdaq listing rules. As a controlled company, we are permitted to elect to rely on certain exemptions from the obligations to comply with certain corporate governance requirements, including:
| ● | the requirement that a majority of the board of directors consist of independent directors; | |
| ● | the requirement that our director nominees be selected or recommended solely by independent directors; and | |
| ● | the requirement that we have a nominating and corporate governance committee and a compensation committee that are composed entirely of independent directors with a written charter addressing the purposes and responsibilities of the committees. |
Although we do not intend to rely on the controlled company exemptions under the Nasdaq listing rules even if we are a controlled company, we could elect to rely on these exemptions in the future, and if so, you would not have the same protection afforded to shareholders of companies that are subject to all of the corporate governance requirements of Nasdaq.
B. Compensation
For the fiscal year ended September 30, 2023, we paid an aggregate of approximately $90,000 as compensation to our executive officers and directors. None of our non-employee directors have any service contracts with us that provide for benefits upon termination of employment. We have not set aside or accrued any amount to provide pension, retirement, or other similar benefits to our directors and executive officers. Our PRC subsidiaries are required by law to make contributions equal to certain percentages of each employee’s salary for his or her pension insurance, medical insurance, unemployment insurance, and other statutory benefits and a housing provident fund.
C. Board Practices
Board of Directors
Our board of directors consists of five directors, including three independent directors. A director is not required to hold any shares in our company to qualify to serve as a director. A director shall not, as a director, vote in respect of any contract, transaction, arrangement or proposal in which they have an interest which (together with any interest of any person connected with them) is a material interest (otherwise then by virtue of their interests, direct or indirect, in shares or debentures or other securities of, or otherwise in or through, the Company) and if they shall do so their vote shall not be counted, nor in relation thereto shall they be counted in the quorum present at the meeting, but (in the absence of some other material interest than is mentioned below) none of these prohibitions shall apply to: (a) the giving of any security, guarantee or indemnity in respect of: (i) money lent or obligations incurred by them or by any other person for the benefit of the Company or any of its subsidiaries; or (ii) a debt or obligation of the Company or any of its subsidiaries for which a director has assumed responsibility in whole or in part and whether alone or jointly with others under a guarantee or indemnity or by the giving of security; (b) where the Company or any of its subsidiaries is offering securities in which offer the director is or may be entitled to participate as a holder of securities or in the underwriting or sub-underwriting of which the director is to or may participate; (c) any contract, transaction, arrangement or proposal affecting any other body corporate in which he is interested, directly or indirectly and whether as an officer, shareholder, creditor or otherwise howsoever, provided that he (together with persons connected with them) does not to their knowledge hold an interest representing one per cent or more of any class of the equity share capital of such body corporate (or of any third body corporate through which his interest is derived) or of the voting rights available to members of the relevant body corporate; (d) any act or thing done or to be done in respect of any arrangement for the benefit of the employees of the Company or any of its subsidiaries under which they are not accorded as a director any privilege or advantage not generally accorded to the employees to whom such arrangement relates; (e) any matter connected with the purchase or maintenance for any director of insurance against any liability or (to the extent permitted by the Companies Act (Revised) of the Cayman Islands) indemnities in favor of directors, the funding of expenditure by one or more directors in defending proceedings against him or them or the doing of anything to enable such director or directors to avoid incurring such expenditure; or (f) any contract, transaction, arrangement or proposal in which the director has an interest which is not a material interest. The directors may exercise all the powers of the company to borrow money, mortgage its undertaking, property and uncalled capital, and issue debentures or other securities whenever money is borrowed or as security for any obligation of the company or of any third party.
80
Committees of the Board of Directors
We have established three committees under the board of directors: an audit committee, a compensation committee, and a nominating and corporate governance committee. We have adopted a charter for each of the three committees. Each committee’s members and functions are described below.
Audit Committee. Our audit committee consists of Jiawen Pang, Yongping Yu, and Ding Zheng. Ding Zheng serves as the chairman of our audit committee. We have determined that Jiawen Pang, Yongping Yu, and Ding Zheng satisfy the “independence” requirements of Rule 5605(a)(2) of the Listing Rules of the Nasdaq Stock Market and Rule 10A-3 under the Securities Exchange Act. Our board also has determined that Ding Zheng qualifies as an audit committee financial expert within the meaning of the SEC rules or possesses financial sophistication within the meaning of the Nasdaq corporate governance rules. The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The audit committee is responsible for, among other things:
| ● | appointing the independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by the independent auditors; | |
| ● | reviewing with the independent auditors any audit problems or difficulties and management’s response; | |
| ● | discussing the annual audited financial statements with management and the independent auditors; |
| ● | reviewing the adequacy and effectiveness of our accounting and internal control policies and procedures and any steps taken to monitor and control major financial risk exposures; | |
| ● | reviewing and approving all proposed related party transactions; | |
| ● | meeting separately and periodically with management and the independent auditors; and | |
| ● | monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance. |
Compensation Committee. Our compensation committee consists of Jiawen Pang, Yongping Yu, and Ding Zheng. Jiawen Pang serves as the chairperson of our compensation committee. We have determined that Jiawen Pang, Yongping Yu, and Ding Zheng satisfy the “independence” requirements of Rule 5605(a)(2) of the Listing Rules of the Nasdaq Stock Market and Rule 10C-1 under the Securities Exchange Act. The compensation committee assists the board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated. The compensation committee is responsible for, among other things:
| ● | reviewing and approving the total compensation package for our most senior executive officers; | |
| ● | approving and overseeing the total compensation package for our executives other than the most senior executive officers; | |
| ● | reviewing and recommending to the board with respect to the compensation of our directors; | |
| ● | reviewing periodically and approving any long-term incentive compensation or equity plans; | |
| ● | selecting compensation consultants, legal counsel or other advisors after taking into consideration all factors relevant to that person’s independence from management; and | |
| ● | reviewing programs or similar arrangements, annual bonuses, employee pension and welfare benefit plans. |
81
Nominating and Corporate Governance Committee. Our nominating and corporate governance committee consists of Jiawen Pang, Yongping Yu and Ding Zheng. Yongping Yu serves as the chairperson of our nominating and corporate governance committee. Jiawen Pang, Yongping Yu, and Ding Zheng satisfy the “independence” requirements of Rule 5605(a)(2) of the Listing Rules of the Nasdaq Stock Market. The nominating and corporate governance committee assists the board of directors in selecting individuals qualified to become our directors and in determining the composition of the board and its committees. The nominating and corporate governance committee is responsible for, among other things:
| ● | identifying and recommending nominees for election or re-election to our board of directors or for appointment to fill any vacancy; | |
| ● | reviewing annually with our board of directors its current composition in light of the characteristics of independence, age, skills, experience and availability of service to us; | |
| ● | identifying and recommending to our board the directors to serve as members of committees; | |
| ● | advising the board periodically with respect to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations, and making recommendations to our board of directors on all matters of corporate governance and on any corrective action to be taken; and | |
| ● | monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance. |
Duties of Directors
Under Cayman Islands law, all of our directors owe three types of duties to us: (i) statutory duties, (ii) fiduciary duties, and (iii) common law duties. The Companies Act (Revised) of the Cayman Islands imposes a number of statutory duties on a director. A Cayman Islands director’s fiduciary duties are not codified, however, the courts of the Cayman Islands have held that a director owes the following fiduciary duties: (a) a duty to act in what the director bona fide considers to be in the best interests of the company, (b) a duty to exercise their powers for the purposes they were conferred, (c) a duty to avoid fettering his or her discretion in the future and (d) a duty to avoid conflicts of interest and of duty. The common law duties owed by a director are those to act with skill, care and diligence that may reasonably be expected of a person carrying out the same functions as are carried out by that director in relation to the company and, also, to act with the skill, care and diligence in keeping with a standard of care commensurate with any particular skill they have which enables them to meet a higher standard than a director without those skills. In fulfilling their duty of care to us, our directors must ensure compliance with our amended and restated articles of association. We have the right to seek damages if a duty owed by any of our directors is breached.
Our board of directors has all powers necessary for managing, and for directing and supervising, our business affairs. The functions and powers of our board of directors include, among others:
| ● | convening shareholders’ annual general meetings and reporting its work to shareholders at such meetings; | |
| ● | declaring dividends and distributions; | |
| ● | appointing officers and determining the terms of office of the officers; | |
| ● | exercising the borrowing powers of our company and mortgaging the property of our Company; and | |
| ● | approving the transfer of shares in our Company, including the registration of such shares in our share register. |
Terms of Directors and Executive Officers
Our directors may be elected by a resolution of our board of directors or by an ordinary resolution of our shareholders. Unless re-appointed or removed from office pursuant to the provisions of our amended and restated articles of association, each director shall be appointed for a term expiring at the next-following annual general meeting of the Company. A director will cease to be a director if, among other things, the director (i) becomes bankrupt or makes any arrangement or composition with his creditors; (ii) in the opinion of a registered medical practitioner by whom the director is being treated, the director becomes physically or mentally incapable of acting as a director; (iii) resigned his or her office by notice in writing to the company; or (iv) without the consent of the other directors, is absent from meetings of directors for a continuous period of six months.
Our officers are elected by and serve at the discretion of the board of directors.
82
Qualification
There is currently no shareholding qualification for directors, although a shareholding qualification for directors may be fixed by our shareholders by ordinary resolution.
Employment Agreements and Indemnification Agreements
We have entered into employment agreements with each of our executive officers. Pursuant to these employment agreements, we agree to employ each of our executive officers for a specified time period, which may be renewed automatically unless either party gives the other party a two-month prior written notice before the end of the current employment term. We may terminate the employment for cause, at any time, without notice or remuneration, for certain acts of the executive officer, including but not limited to, the commitments of any serious or persistent breach or non-observance of the terms and conditions of the employment, conviction of a criminal offense, willful disobedience of a lawful and reasonable order, fraud or dishonesty, receipt of bribery, or severe neglect of his or her duties. An executive officer may terminate his or her employment at any time with two months’ prior written notice. Each executive officer agrees to hold, both during and after the employment agreement expires, in strict confidence and not to use or disclose to any person, corporation or other entity without written consent, any confidential information.
We have also entered into indemnification agreements with each of our directors and executive officers. Under these agreements, we agree to indemnify our directors and executive officers against certain liabilities and expenses incurred by such persons in connection with claims made by reason of their being a director or officer of our Company.
Insider Participation Concerning Executive Compensation
Our director, Mr. Gang Lai, was making all determinations regarding executive officer compensation from the inception of the Company until our Compensation Committee was set up in March 2021.
Code of Business Conduct and Ethics
Our board of directors has adopted a code of business conduct and ethics, which is applicable to all of our directors, officers, and employees. Our code of business conduct and ethics is publicly available on our website.
Compensation Recovery Policy
We have adopted a compensation recovery policy to provide for the recovery of erroneously-awarded incentive compensation, as required by the Dodd-Frank Wall Street Reform and Consumer Protection Act, final SEC rules and applicable listing standards.
D. Employees
See “Item 4. Information on the Company—B. Business Overview—Employees.”
E. Share Ownership
The following table sets forth information with respect to the beneficial ownership, within the meaning of Rule 13d-3 under the Exchange Act, of our ordinary shares as of the date of this annual report for:
| ● | each of our directors and executive officers; and | |
| ● | each person known to us to own beneficially more than 5% of our ordinary shares. |
Beneficial ownership includes voting or investment power with respect to the securities. Except as indicated below, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all ordinary shares shown as beneficially owned by them. Percentage of beneficial ownership of each listed person is based on 3,645,974 ordinary shares outstanding as of the date of this annual report.
83
Information with respect to beneficial ownership has been furnished by each director, officer, or beneficial owner of 5% or more of our ordinary shares. Beneficial ownership is determined in accordance with the rules of the SEC and generally requires that such person have voting or investment power with respect to securities. In computing the number of ordinary shares beneficially owned by a person listed below and the percentage ownership of such person, ordinary shares underlying options, warrants, or convertible securities held by each such person that are exercisable or convertible within 60 days of the date of this annual report are deemed outstanding, but are not deemed outstanding for computing the percentage ownership of any other person.
| Ordinary Shares Beneficially Owned | Voting Power | |||||||||||
| Number | % | % | ||||||||||
| Directors and Executive Officers*: | ||||||||||||
| Gang Lai(1) | 2,080,000 | 57.05 | % | 57.05 | % | |||||||
| Lin Yang | — | — | — | |||||||||
| Baochang Liu | — | — | — | |||||||||
| Jiawen Pang | — | — | — | |||||||||
| Yongping Yu | — | — | — | |||||||||
| Ding Zheng | — | — | — | |||||||||
| All directors and executive officers as a group: | 2,080,000 | 57.05 | % | 57.05 | % | |||||||
| 5% Shareholders: | ||||||||||||
| Sununion Holding Group Limited(1) | 2,080,000 | 57.05 | % | 57.05 | % | |||||||
| * | Unless otherwise indicated, the business address of each of our directors and officers is 265 Jingjiu Avenue, Jinggangshan Economic and Technological Development Zone, Ji’an, Jiangxi, People’s Republic of China. |
| (1) | Represents 2,080,000 ordinary shares held by Sununion Holding Group Limited, a business company incorporated in the British Virgin Islands, which is owned as to 91.4% and controlled by Gang Lai. The registered address of Sununion Holding Group Limited is Vistra Corporate Services Centre, Wickhams Cay II, Road Town, Tortola, VG1110, British Virgin Islands. |
As of the date of this annual report, approximately 42.95% of our issued and outstanding ordinary shares are held in the United States by one record holder (Cede and Company).
We are not aware of any other arrangement that may, at a subsequent date, result in a change of control of our Company.
F. Disclosure of a Registrant’s Action to Recover Erroneously Awarded Compensation
Not applicable.
Item 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
See “Item 6. Directors, Senior Management and Employees—E. Share Ownership.”
B. Related Party Transactions
Employment Agreements
See “Item 6. Directors, Senior Management and Employees—C. Board Practices—Employment Agreements and Indemnification Agreements.”
84
Material Transactions with Related Parties
(a) Nature of relationships with related parties
| Name | Relationship with the Company | |
| Mr. Gang Lai | Chief Executive Officer and chairman of the Company’s Board of Directors | |
| Ms. Lin Yang | Chief Financial Officer and Director | |
| Ms. Xing Wu | Mr. Gang Lai’s spouse | |
| Mr. Yajun Hu | General Manager of Jiangxi Universe |
(b) Due from related parties
As of September 30, 2023, the balance due from related parties consisted of advanced funds to Mr. Yajun Hu in the amount of $61,678. The advances are non-interest bearing and fully repaid on October 12, 2023. As of September 30, 2022, there was no balances due from related parties.
(c) Due to related parties
As of September 30, 2023 and 2022, the balances due to related parties were $540,096 and $3,379,263, respectively, which mainly consisted of advances from Mr. Gang Lai for working capital purposes in our normal course of business. These advances are non-interest bearing and due on demand.
(d) Loan guarantee provided by related parties
In connection with our bank borrowings from PRC Banks, certain related parties, including Mr. Gang Lai, our controlling shareholder, chairman of the board of directors, chief executive officer, Ms. Lin Yang, our chief financial officer, and Ms. Xing Wu, Mr. Gang Lai’s spouse, jointly signed guarantee agreements with these banks to provide credit guarantee for our certain loans.
(e) Prepayment to related party for property purchase
On May 6, 2021, we entered into a real estate property purchase agreement with a related party, Jiangxi Yueshang, to purchase certain residential apartment and commercial office space totaling 2,749.30 square meters with total purchase price of RMB32 million (approximately $4.4 million). As of September 30, 2023, we had made a prepayment of RMB16 million ($2.2 million) to Jiangxi Yueshang. The remaining balance is expected to be paid by August 2024.
On January 13, 2022, Mr. Gang Lai transferred the 5% equity interest he owned in Jiangxi Yueshang to a third party. As such, after this date, Jiangxi Yueshang is no longer our related party.
C. Interests of Experts and Counsel
Not applicable.
Item 8. FINANCIAL INFORMATION
A. Consolidated Statements and Other Financial Information
We have appended consolidated financial statements filed as part of this annual report. See “Item 18. Financial Statements.”
85
Legal Proceedings
From time to time, we may become a party to various legal or administrative proceedings arising in the ordinary course of our business, including actions with respect to intellectual property infringement, violation of third-party licenses or other rights, breach of contract, and labor and employment claims. We are currently not a party to, and we are not aware of any threat of, any legal or administrative proceedings that, in the opinion of our management, are likely to have any material and adverse effect on our business, financial condition, cash flow, or results of operations.
Dividend Policy
Universe Pharmaceuticals INC has transferred the net proceeds from our initial public offering, through Universe HK and Universe Technology, to Jiangxi Universe in the amount of RMB43,976,156 (approximately $6,807,507).
As of the date of this annual report, none of our subsidiaries have made any dividends or distributions to Universe Pharmaceuticals INC and Universe Pharmaceuticals INC has not made any dividends or distributions to U.S. investors. We intend to keep any future earnings to finance the expansion of our business, and we do not anticipate that any cash dividends will be paid in the foreseeable future. Subject to the PFIC rules, the gross amount of distributions we make to investors with respect to our ordinary shares (including the amount of any taxes withheld therefrom) will be taxable as a dividend, to the extent that the distribution is paid out of our current or accumulated earnings and profits, as determined under U.S. federal income tax principles.
Our board of directors has discretion on whether to distribute dividends. In addition, our shareholders may by ordinary resolution declare a dividend, but no dividend may exceed the amount recommended by our board of directors. In either case, all dividends are subject to certain restrictions under Cayman Islands law, namely that the company may only pay dividends out of profits or share premium, and provided always that in no circumstances may a dividend be paid if this would result in the company being unable to pay its debts as they fall due in the ordinary course of business. Even if we decide to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant.
We are an exempted company with limited liability incorporated in the Cayman Islands. We may rely on dividends from our subsidiaries in China for our cash requirements, including any payment of dividends to our shareholders. PRC regulations may restrict the ability of our PRC subsidiaries to pay dividends to us, and as a holding company, we will be dependent on receipt of funds from our Hong Kong subsidiary, Universe HK.
Current PRC regulations permit our indirect PRC subsidiaries to pay dividends to Universe HK only out of their accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. In addition, each of our subsidiaries in China is required to set aside at least 10% of its after-tax profits each year, if any, to fund a statutory reserve until such reserve reaches 50% of its registered capital. Each of such entity in China is also required to further set aside a portion of its after-tax profits to fund the employee welfare fund, although the amount to be set aside, if any, is determined at the discretion of its board of directors. Although the statutory reserves can be used, among other ways, to increase the registered capital and eliminate future losses in excess of retained earnings of the respective companies, the reserve funds are not distributable as cash dividends except in the event of liquidation.
The PRC government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies out of the PRC. Therefore, we may experience difficulties in complying with the administrative requirements necessary to obtain and remit foreign currency for the payment of dividends from our profits, if any. Furthermore, if our subsidiaries and affiliates in the PRC incur debt on their own in the future, the instruments governing the debt may restrict their ability to pay dividends or make other payments. If we or our subsidiaries are unable to receive all of the revenue from our operations, we may be unable to pay dividends on our ordinary shares.
Cash dividends, if any, on our ordinary shares will be paid in U.S. dollars. Universe HK may be considered a non-resident enterprise for tax purposes, so that any dividends our PRC subsidiaries pay to Universe HK may be regarded as China-sourced income and as a result may be subject to PRC withholding tax at a rate of up to 10%. See “Item 10. Additional Information—E. Taxation—People’s Republic of China Taxation.”
86
In order for us to pay dividends to our shareholders, we will rely on payments made from Universe Technology’s subsidiary, Jiangxi Universe, to Universe Technology and from Universe Technology to Universe HK and then to our Company. According to the EIT Law, such payments from subsidiaries to parent companies in China are subject to the PRC enterprise income tax at a rate of 25%. In addition, if Jiangxi Universe or its subsidiary or branches incur debt on their own behalf in the future, the instruments governing the debt may restrict its ability to pay dividends or make other distributions to us.
Pursuant to the Double Tax Avoidance Arrangement, the 10% withholding tax rate may be lowered to 5% if a Hong Kong resident enterprise owns no less than 25% of a PRC project. The 5% withholding tax rate, however, does not automatically apply and certain requirements must be satisfied, including without limitation that (a) the Hong Kong project must be the beneficial owner of the relevant dividends; and (b) the Hong Kong project must directly hold no less than 25% share ownership in the PRC project during the 12 consecutive months preceding its receipt of the dividends. In current practice, a Hong Kong project must obtain a tax resident certificate from the Hong Kong tax authority to apply for the 5% lower PRC withholding tax rate. As the Hong Kong tax authority will issue such a tax resident certificate on a case-by-case basis, we cannot assure you that we will be able to obtain the tax resident certificate from the relevant Hong Kong tax authority and enjoy the preferential withholding tax rate of 5% under the Double Taxation Arrangement with respect to any dividends paid by our PRC subsidiaries to its immediate holding company, Universe HK. As of the date of this annual report, we have not applied for the tax resident certificate from the relevant Hong Kong tax authority. Universe HK intends to apply for the tax resident certificate if and when Universe Technology plan to declare and pay dividends to Universe HK. See “Item 3. Key Information—D. Risk Factors— There are significant uncertainties under the Enterprise Income Tax Law, or the EIT Law, relating to the withholding tax liabilities of our PRC subsidiaries, and dividends payable by our PRC subsidiaries to our offshore subsidiaries may not qualify to enjoy certain treaty benefits.”
B. Significant Changes
Except as disclosed elsewhere in this annual report, we have not experienced any significant changes since the date of our audited consolidated financial statements included in this annual report.
Item 9. THE OFFER AND LISTING
A. Offer and Listing Details.
Our ordinary shares have been listed on the Nasdaq Global Market since March 23, 2021 under the symbol “UPC.”
B. Plan of Distribution
Not applicable.
C. Markets
Our ordinary shares have been listed on the Nasdaq Global Market since March 23, 2021 under the symbol “UPC.”
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
87
F. Expenses of the Issue
Not applicable.
Item 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
We are an exempted company incorporated under the laws of the Cayman Islands and our affairs are governed by our amended and restated memorandum and articles of association, as amended and restated from time to time, and Companies Act (As Revised) of the Cayman Islands, which we refer to as the Companies Act below, and the common law of the Cayman Islands.
We incorporate by reference into this annual report the description of our fourth amended and restated memorandum of association and our second amended and restated articles of association, which is filed as Exhibit 1.1 to this annual report on Form 20-F.
Registered Office
Our registered office in the Cayman Islands is located at Vistra (Cayman) Limited, P.O. Box 31119 Grand Pavilion, Hibiscus Way, 802 West Bay Road, Grand Cayman, KYI – 1205 Cayman Islands, and the phone number of our registered office is +1-(345)769-9372.
Board of Directors
See “Item 6. Directors, Senior Management and Employees.”
Ordinary Shares
General
All of our issued and outstanding ordinary shares are fully paid and non-assessable. Our ordinary shares are issued in registered form, and are issued when registered in our register of members. Unless the board of directors determine otherwise, each holder of our ordinary shares will not receive a certificate in respect of such ordinary shares. Our shareholders who are non-residents of the Cayman Islands may freely hold and vote their ordinary shares. We may not issue shares or warrants to bearer.
Our authorized share capital is $3,125,000 divided into 150,000,000 ordinary shares, par value $0.01875 per share and 16,666,666.6666 preferred shares, par value $0.01875 per share. Subject to the provisions of the Companies Act and our articles regarding redemption and purchase of the shares, the directors have general and unconditional authority to allot (with or without confirming rights of renunciation), grant options over or otherwise deal with any unissued shares to such persons, at such times and on such terms and conditions as they may decide. Such authority could be exercised by the directors to allot shares which carry rights and privileges that are preferential to the rights attaching to ordinary shares. No share may be issued at a discount except in accordance with the provisions of the Companies Act. The directors may refuse to accept any application for shares, and may accept any application in whole or in part, for any reason or for no reason.
88
Dividends
Subject to the provisions of the Companies Act and any rights attaching to any class or classes of shares under and in accordance with the articles:
| (a) | the directors may declare dividends or distributions out of our funds which are lawfully available for that purpose; and |
| (b) | the Company’s shareholders may, by ordinary resolution, declare dividends but no such dividend shall exceed the amount recommended by the directors. |
Subject to the requirements of the Companies Act regarding the application of a company’s share premium account and with the sanction of an ordinary resolution, dividends may also be declared and paid out of any share premium account. The directors when paying dividends to shareholders may make such payment either in cash or in specie.
Unless provided by the rights attached to a share, no dividend shall bear interest.
Voting Rights
Subject to any rights or restrictions as to voting attached to any shares, unless any share carries special voting rights, on a show of hands every shareholder who is present in person and every person representing a shareholder by proxy shall have one vote per ordinary share. On a poll, every shareholder who is present in person and every person representing a shareholder by proxy shall have one vote for each share of which he or the person represented by proxy is the holder. In addition, all shareholders holding shares of a particular class are entitled to vote at a meeting of the holders of that class of shares. Votes may be given either personally or by proxy.
Variation of Rights of Shares
Whenever our capital is divided into different classes of shares, the rights attaching to any class of share (unless otherwise provided by the terms of issue of the shares of that class) may be varied either with the consent in writing of the holders of not less than two-thirds of the issued shares of that class, or with the sanction of a resolution passed by a majority of not less than two-thirds of the holders of shares of the class present in person or by proxy at a separate general meeting of the holders of shares of that class.
Unless the terms on which a class of shares was issued state otherwise, the rights conferred on the shareholder holding shares of any class shall not be deemed to be varied by the creation or issue of further shares ranking pari passu with the existing shares of that class.
Alteration of Share Capital
Subject to the Companies Act, our shareholders may, by ordinary resolution:
| (a) | increase our share capital by new shares of the amount fixed by that ordinary resolution and with the attached rights, priorities and privileges set out in that ordinary resolution; |
| (b) | consolidate and divide all or any of our share capital into shares of larger amount than our existing shares; |
| (c) | convert all or any of our paid up shares into stock, and reconvert that stock into paid up shares of any denomination; |
| (d) | sub-divide our shares or any of them into shares of an amount smaller than that fixed, so, however, that in the sub-division, the proportion between the amount paid and the amount, if any, unpaid on each reduced share shall be the same as it was in case of the share from which the reduced share is derived; and |
| (e) | cancel shares which, at the date of the passing of that ordinary resolution, have not been taken or agreed to be taken by any person and diminish the amount of our share capital by the amount of the shares so cancelled or, in the case of shares without nominal par value, diminish the number of shares into which our capital is divided. |
Subject to the Companies Act and to any rights for the time being conferred on the shareholders holding a particular class of shares, our shareholders may, by special resolution, reduce its share capital in any way.
89
Calls on Shares and Forfeiture
Subject to the terms of allotment, the directors may make calls on the shareholders in respect of any monies unpaid on their shares including any premium and each shareholder shall (subject to receiving at least 14 clear days’ notice specifying when and where payment is to be made), pay to us the amount called on his shares. Shareholders registered as the joint holders of a share shall be jointly and severally liable to pay all calls in respect of the share. If a call remains unpaid after it has become due and payable the person from whom it is due and payable shall pay interest on the amount unpaid from the day it became due and payable until it is paid at the rate fixed by the terms of allotment of the share or in the notice of the call or if no rate is fixed, at the rate of ten percent per annum. The directors may, at their discretion, waive payment of the interest wholly or in part.
We have a first and paramount lien on all shares (whether fully paid up or not) registered in the name of a shareholder (whether solely or jointly with others). The lien is for all monies payable to us by the shareholder or the shareholder’s estate:
| (a) | either alone or jointly with any other person, whether or not that other person is a shareholder; and |
| (b) | whether or not those monies are presently payable. |
At any time the directors may declare any share to be wholly or partly exempt from the lien on shares provisions of the articles.
We may sell, in such manner as the directors may determine, any share on which the sum in respect of which the lien exists is presently payable, if due notice that such sum is payable has been given (as prescribed by the articles) and, within 14 days of the date on which the notice is deemed to be given under the articles, such notice has not been complied with.
Unclaimed Dividend
A dividend that remains unclaimed for a period of six years after it became due for payment shall be forfeited to, and shall cease to remain owing by, the company.
Forfeiture or Surrender of Shares
If a shareholder fails to pay any capital call, the directors may give to such shareholder not less than 14 clear days’ notice requiring payment and specifying the amount unpaid including any interest which may have accrued, any expenses which have been incurred by us due to that person’s default and the place where payment is to be made. The notice shall also state the place where payment is to be made and contain a warning that if the notice is not complied with, the shares in respect of which the call is made will be liable to be forfeited.
If such notice is not complied with, the directors may, before the payment required by the notice has been received, resolve that any share the subject of that notice be forfeited (which forfeiture shall include all dividends or other monies payable in respect of the forfeited share and not paid before such forfeiture).
A forfeited share may be sold, re-allotted or otherwise disposed of on such terms and in such manner as the directors determine and at any time before a sale, re-allotment or disposition the forfeiture may be cancelled on such terms as the directors think fit.
A person whose shares have been forfeited shall cease to be a shareholder in respect of the forfeited shares, but shall, notwithstanding such forfeiture, remain liable to pay to us all monies which at the date of forfeiture were payable by him to us in respect of the shares, together with all expenses and interest from the date of forfeiture or surrender until payment, but his liability shall cease if and when we receive payment in full of the unpaid amount. The directors, however, may waive payment wholly or in part.
A declaration, whether statutory or under oath, made by a director or the secretary shall be conclusive evidence that the person making the declaration is our director or secretary and that the particular shares have been forfeited or surrendered on a particular date.
Subject to the execution of an instrument of transfer, if necessary, the declaration shall constitute good title to the shares.
90
Share Premium Account
The directors shall establish a share premium account and shall carry the credit of such account from time to time to a sum equal to the amount or value of the premium paid on the issue of any share or capital contributed or such other amounts required by the Companies Act.
Redemption and Purchase of Own Shares
Subject to the Companies Act and any rights for the time being conferred on the shareholders holding a particular class of shares, we may by action of our directors:
| (a) | issue shares that are to be redeemed or liable to be redeemed, at our option or the shareholder holding those redeemable shares, on the terms and in the manner our directors determine before the issue of those shares; | |
| (b) | with the consent by special resolution of the shareholders holding shares of a particular class, vary the rights attaching to that class of shares so as to provide that those shares are to be redeemed or are liable to be redeemed at our option on the terms and in the manner which the directors determine at the time of such variation; and | |
| (c) | purchase all or any of our own shares of any class including any redeemable shares on the terms and in the manner which the directors determine at the time of such purchase. |
We may make a payment in respect of the redemption or purchase of its own shares in any manner authorized by the Companies Act, including out of any combination of capital, our profits and the proceeds of a fresh issue of shares.
When making a payment in respect of the redemption or purchase of shares, the directors may make the payment in cash or in specie (or partly in one and partly in the other) if so authorized by the terms of the allotment of those shares or by the terms applying to those shares, or otherwise by agreement with the shareholder holding those shares.
Transfer of Shares
Provided that a transfer of ordinary shares complies with applicable rules of Nasdaq, a shareholder may transfer ordinary shares to another person by completing an instrument of transfer in a common form or in a form prescribed by Nasdaq or in any other form approved by the directors, executed:
| (a) | where the ordinary shares are fully paid, by or on behalf of that shareholder; and |
| (b) | where the ordinary shares are partly paid, by or on behalf of that shareholder and the transferee. |
The transferor shall be deemed to remain the holder of an ordinary share until the name of the transferee is entered into the register of members of the Company.
Our board of directors may, in its absolute discretion, decline to register any transfer of any ordinary share that has not been fully paid up or is subject to a company lien. Our board of directors may also decline to register any transfer of such ordinary share unless:
| (a) | the instrument of transfer is lodged with the Company, accompanied by the certificate for the ordinary shares to which it relates and such other evidence as our board of directors may reasonably require to show the right of the transferor to make the transfer; |
| (b) | the instrument of transfer is in respect of only one class of shares; | |
| (c) | the instrument of transfer is properly stamped, if required; |
91
| (d) | the ordinary shares transferred is fully paid and free of any lien in favor of us; | |
| (e) | any fee related to the transfer has been paid to us; and | |
| (f) | the transfer is not to more than four joint holders. |
If our directors refuse to register a transfer, they are required, within three months after the date on which the instrument of transfer was lodged, to send to each of the transferor and the transferee notice of such refusal.
This, however, is unlikely to affect market transactions of the ordinary shares purchased by investors in the public offering. The legal title to such ordinary shares and the registration details of those ordinary shares in the Company’s register of members will remain with Depository Trust Company (“DTC”). All market transactions with respect to those ordinary shares will then be carried out without the need for any kind of registration by the directors, as the market transactions will all be conducted through the DTC systems.
The registration of transfers may, on 14 calendar days’ notice being given by advertisement in such one or more newspapers or by electronic means, be suspended and our register of members closed at such times and for such periods as our board of directors may from time to time determine. The registration of transfers, however, may not be suspended, and the register may not be closed, for more than 30 days in any year.
Inspection of Books and Records
Holders of our ordinary shares will have no general right under the Companies Act to inspect or obtain copies of our register of members or our corporate records.
General Meetings
As a Cayman Islands exempted company, we are not obligated by the Companies Act to call shareholders’ annual general meetings; accordingly, we may, but shall not be obliged to, in each year hold a general meeting as an annual general meeting. Any annual general meeting held shall be held at such time and place as may be determined by our board of directors. All general meetings other than annual general meetings shall be called extraordinary general meetings.
The directors may convene general meetings whenever they think fit. General meetings shall also be convened on the written requisition of one or more of the shareholders entitled to attend and vote at our general meetings who (together) hold not less than ten percent of the rights to vote at such general meeting in accordance with the notice provisions in the articles, specifying the purpose of the meeting and signed by each of the shareholders making the requisition. If the directors do not convene such meeting for a date not later than 21 clear days’ after the date of receipt of the written requisition, those shareholders who requested the meeting may convene the general meeting themselves within three months after the end of such period of 21 clear days in which case reasonable expenses incurred by them as a result of the directors failing to convene a meeting shall be reimbursed by us.
At least 14 days’ notice of an extraordinary general meeting and 21 days’ notice of an annual general meeting shall be given to shareholders entitled to attend and vote at such meeting. The notice shall specify the place, the day and the hour of the meeting and the general nature of that business. In addition, if a resolution is proposed as a special resolution, the text of that resolution shall be given to all shareholders. Notice of every general meeting shall also be given to the directors and our auditors.
Subject to the Companies Act and with the consent of the shareholders who, individually or collectively, hold at least 90 percent of the voting rights of all those who have a right to vote at a general meeting, a general meeting may be convened on shorter notice.
A quorum shall consist of the presence (whether in person or represented by proxy) of one or more shareholders holding shares that represent not less than one-third of the outstanding shares carrying the right to vote at such general meeting.
92
If, within 15 minutes from the time appointed for the general meeting, or at any time during the meeting, a quorum is not present, the meeting, if convened upon the requisition of shareholders, shall be cancelled. In any other case it shall stand adjourned to the same time and place seven days or to such other time or place as is determined by the directors.
The chairman may, with the consent of a meeting at which a quorum is present, adjourn the meeting. When a meeting is adjourned for seven days or more, notice of the adjourned meeting shall be given in accordance with the articles.
At any general meeting a resolution put to the vote of the meeting shall be decided on a show of hands, unless a poll is (before, or on, the declaration of the result of the show of hands) demanded by the chairman of the meeting or by at least two shareholders having the right to vote on the resolutions or one or more shareholders present who together hold not less than ten percent of the voting rights of all those who are entitled to vote on the resolution. Unless a poll is so demanded, a declaration by the chairman as to the result of a resolution and an entry to that effect in the minutes of the meeting, shall be conclusive evidence of the outcome of a show of hands, without proof of the number or proportion of the votes recorded in favor of, or against, that resolution.
If a poll is duly demanded it shall be taken in such manner as the chairman directs and the result of the poll shall be deemed to be the resolution of the meeting at which the poll was demanded.
In the case of an equality of votes, whether on a show of hands or on a poll, the chairman of the meeting at which the show of hands takes place or at which the poll is demanded, shall not be entitled to a second or casting vote.
Directors
We may by ordinary resolution, from time to time, fix the maximum and minimum number of directors to be appointed. Under the articles, we are required to have a minimum of one director and the maximum number of directors shall be unlimited.
A director may be appointed by ordinary resolution or by the directors. Any appointment may be to fill a vacancy or as an additional director.
Unless the remuneration of the directors is determined by the shareholders by ordinary resolution, the directors shall be entitled to such remuneration as the directors may determine.
The shareholding qualification for directors may be fixed by our shareholders by ordinary resolution and unless and until so fixed no share qualification shall be required.
Unless removed or re-appointed, each director shall be appointed for a term expiring at the next-following annual general meeting, if one is held. At any annual general meeting held, our directors will be elected by an ordinary resolution of our shareholders. At each annual general meeting, each director so elected shall hold office for a one-year term and until the election of their respective successors in office or removed.
A director may be removed by ordinary resolution.
A director may at any time resign or retire from office by giving us notice in writing. Unless the notice specifies a different date, the director shall be deemed to have resigned on the date that the notice is delivered to us.
Subject to the provisions of the articles, the office of a director may be terminated forthwith if:
| (a) | he is prohibited by the law of the Cayman Islands from acting as a director; |
| (b) | he is made bankrupt or makes an arrangement or composition with his creditors generally; |
| (c) | he resigns his office by notice to us; |
| (d) | he only held office as a director for a fixed term and such term expires; |
93
| (e) | in the opinion of a registered medical practitioner by whom he is being treated he becomes physically or mentally incapable of acting as a director; |
| (f) | he is given notice by the majority of the other directors (not being less than two in number) to vacate office (without prejudice to any claim for damages for breach of any agreement relating to the provision of the services of such director); |
| (g) | he is made subject to any law relating to mental health or incompetence, whether by court order or otherwise; or |
| (h) | without the consent of the other directors, he is absent from meetings of directors for continuous period of six months. |
Each of the compensation committee and the nominating and corporate governance committee shall consist of at least three directors and the majority of the committee members shall be independent within the meaning of Rule 5605(a)(2) of the Listing Rules of the Nasdaq Stock Market. The audit committee shall consist of at least three directors, all of whom shall be independent within the meaning of Rule 5605(a)(2) of the Listing Rules of the Nasdaq Stock Market and will meet the criteria for independence set forth in Rule 10A-3 or Rule 10C-1 of the Exchange Act.
Powers and Duties of Directors
Subject to the provisions of the Companies Act and our memorandum and articles of association, our business shall be managed by the directors, who may exercise all our powers. No prior act of the directors shall be invalidated by any subsequent alteration of our memorandum or articles of association. To the extent allowed by the Companies Act, however, shareholders may by special resolution validate any prior or future act of the directors which would otherwise be in breach of their duties.
The directors may delegate any of their powers to any committee consisting of one or more persons who need not be shareholders and may include non-directors so long as the majority of those persons are directors; any committee so formed shall in the exercise of the powers so delegated conform to any regulations that may be imposed on it by the directors. Our board of directors have established an audit committee, compensation committee, and nomination and corporate governance committee.
The board of directors may establish any local or divisional board of directors or agency and delegate to it its powers and authorities (with power to sub-delegate) for managing any of our affairs whether in the Cayman Islands or elsewhere and may appoint any persons to be members of a local or divisional board of directors, or to be managers or agents, and may fix their remuneration.
The directors may from time to time and at any time by power of attorney or in any other manner they determine appoint any person, either generally or in respect of any specific matter, to be our agent with or without authority for that person to delegate all or any of that person’s powers.
The directors may from time to time and at any time by power of attorney or in any other manner they determine appoint any person, whether nominated directly or indirectly by the directors, to be our attorney or our authorized signatory and for such period and subject to such conditions as they may think fit. The powers, authorities and discretions, however, must not exceed those vested in, or exercisable, by the directors under the articles.
The board of directors may remove any person so appointed and may revoke or vary the delegation.
The directors may exercise all of our powers to borrow money and to mortgage or charge its undertaking, property and assets both present and future and uncalled capital or any part thereof, to issue debentures and other securities whether outright or as collateral security for any debt, liability or obligation of ours or our parent undertaking (if any) or any subsidiary undertaking of us or of any third party.
94
A director shall not, as a director, vote in respect of any contract, transaction, arrangement or proposal in which he has an interest which (together with any interest of any person connected with him) is a material interest (otherwise than by virtue of his interests, direct or indirect, in shares or debentures or other securities of, or otherwise in or through, us) and if he shall do so his vote shall not be counted, nor in relation thereto shall he be counted in the quorum present at the meeting, but (in the absence of some other material interest than is mentioned below) none of these prohibitions shall apply to:
| (a) | the giving of any security, guarantee or indemnity in respect of: |
| (i) | money lent or obligations incurred by him or by any other person for our benefit or any of our subsidiaries; or |
| (ii) | a debt or obligation of ours or any of our subsidiaries for which the director himself has assumed responsibility in whole or in part and whether alone or jointly with others under a guarantee or indemnity or by the giving of security; |
| (b) | where we or any of our subsidiaries is offering securities in which offer the director is or may be entitled to participate as a holder of securities or in the underwriting or sub-underwriting of which the director is to or may participate; |
| (c) | any contract, transaction, arrangement or proposal affecting any other body corporate in which he is interested, directly or indirectly and whether as an officer, shareholder, creditor or otherwise howsoever, provided that he (together with persons connected with him) does not to his knowledge hold an interest representing one percent or more of any class of the equity share capital of such body corporate (or of any third body corporate through which his interest is derived) or of the voting rights available to shareholders of the relevant body corporate; |
| (d) | any act or thing done or to be done in respect of any arrangement for the benefit of the employees of us or any of our subsidiaries under which he is not accorded as a director any privilege or advantage not generally accorded to the employees to whom such arrangement relates; or |
| (e) | any matter connected with the purchase or maintenance for any director of insurance against any liability or (to the extent permitted by the Companies Act) indemnities in favor of directors, the funding of expenditure by one or more directors in defending proceedings against him or them or the doing of anything to enable such director or directors to avoid incurring such expenditure. |
A director may, as a director, vote (and be counted in the quorum) in respect of any contract, transaction, arrangement or proposal in which he has an interest which is not a material interest or as described above.
Capitalization of Profits
The directors may resolve to capitalize:
| (a) | any part of our profits not required for paying any preferential dividend (whether or not those profits are available for distribution); or |
| (b) | any sum standing to the credit of our share premium account or capital redemption reserve, if any. |
The amount resolved to be capitalized must be appropriated to the shareholders who would have been entitled to it had it been distributed by way of dividend and in the same proportions.
95
Liquidation Rights
If we are wound up, the shareholders may, subject to the articles and any other sanction required by the Companies Act, pass a special resolution allowing the liquidator to do either or both of the following:
| (a) | to divide in specie among the shareholders the whole or any part of our assets and, for that purpose, to value any assets and to determine how the division shall be carried out as between the shareholders or different classes of shareholders; and |
| (b) | to vest the whole or any part of the assets in trustees for the benefit of shareholders and those liable to contribute to the winding up. |
The directors have the authority to present a petition for our winding up to the Grand Court of the Cayman Islands on our behalf without the sanction of a resolution passed at a general meeting.
Register of Members
Under the Companies Act, we must keep a register of members and there should be entered therein:
| ● | the names and addresses of our shareholders, a statement of the shares held by each shareholder, and of the amount paid or agreed to be considered as paid, on the shares of each shareholder; | |
| ● | the date on which the name of any person was entered on the register as a shareholder; and | |
| ● | the date on which any person ceased to be a shareholder. |
Under the Companies Act, the register of members of our company is prima facie evidence of the matters set out therein (that is, the register of members will raise a presumption of fact on the matters referred to above unless rebutted) and a shareholder registered in the register of members is deemed as a matter of the Companies Act to have legal title to the shares as set against its name in the register of members.
If the name of any person is incorrectly entered in or omitted from our register of members, or if there is any default or unnecessary delay in entering on the register the fact of any person having ceased to be a shareholder of our company, the person or shareholder aggrieved (or any shareholder of our company or our company itself) may apply to the Grand Court of the Cayman Islands for an order that the register be rectified, and the Court may either refuse such application or it may, if satisfied of the justice of the case, make an order for the rectification of the register.
Preferred Shares
The directors are empowered to designate and issue from time to time one or more classes or series of preference shares and to fix and determine the relative rights, preferences, designations, qualifications, privileges, options, conversion rights, limitations and other special or relative rights of each such class or series so authorized. Such action could adversely affect the voting power and other rights of the holders of our ordinary shares or could have the effect of discouraging any attempt by a person or group to obtain control of us.
As of the date of this annual report, no preferred shares are issued and outstanding.
C. Material Contracts
We have not entered into any material contracts other than in the ordinary course of business and other than those described in “Item 4. Information on the Company” or elsewhere in this annual report.
D. Exchange Controls
See “Item 4. Information on the Company—B. Business Overview—Regulations—Other Laws—Foreign Exchange Control.”
E. Taxation
People’s Republic of China Taxation
The following brief description of Chinese enterprise laws is designed to highlight the enterprise-level taxation on our earnings, which will affect the amount of dividends, if any, we are ultimately able to pay to our shareholders.
96
Enterprise Income Tax
According to the Enterprise Income Tax Law of the People’s Republic of China, or the EIT Law, which was promulgated by the SCNPC on March 16, 2007, and became effective on January 1, 2008, and then amended on February 24, 2017, and the Implementation Rules of the EIT Law, or the Implementation Rules, which were promulgated by the State Council on December 6, 2007, effective on January 1, 2008, and amended on April 23, 2019, enterprises are divided into resident enterprises and non-resident enterprises. Resident enterprises pay enterprise income tax on their incomes obtained in and outside the PRC at the rate of 25%. Non-resident enterprises setting up institutions in the PRC pay enterprise income tax on the incomes obtained by such institutions in and outside the PRC at the rate of 25%. Non-resident enterprises with no institutions in the PRC, and non-resident enterprises with income having no substantial connection with their institutions in the PRC, pay enterprise income tax on their income obtained in the PRC at a reduced rate of 10%.
We are an exempted company incorporated with limited liability in the Cayman Islands and we gain substantial income by way of dividends paid to us from our PRC subsidiaries, Universe Technology, Jiangxi Universe, and Universe Trade. The EIT Law and its implementation rules provide that China-sourced income of foreign enterprises, such as dividends paid by a PRC subsidiary to its equity holders that are non-resident enterprises, will normally be subject to PRC withholding tax at a rate of 10%, unless any such foreign investor’s jurisdiction of incorporation has a tax treaty with China that provides for a preferential tax rate or a tax exemption.
Under the EIT Law, an enterprise established outside of China with a “de facto management body” within China is considered a “resident enterprise,” which means that it is treated in a manner similar to a Chinese enterprise for enterprise income tax purposes. Although the implementation rules of the EIT Law define “de facto management body” as a managing body that actually, comprehensively manage and control the production and operation, staff, accounting, property and other aspects of an enterprise, the only official guidance for this definition currently available is set forth in SAT Notice 82, which provides guidance on the determination of the tax residence status of a Chinese-controlled offshore incorporated enterprise, defined as an enterprise that is incorporated under the laws of a foreign country or territory and that has a PRC enterprise or enterprise group as its primary controlling shareholder. Although the Company does not have a PRC enterprise or enterprise group as our primary controlling shareholder and is therefore not a Chinese-controlled offshore incorporated enterprise within the meaning of SAT Notice 82, in the absence of guidance specifically applicable to us, we have applied the guidance set forth in SAT Notice 82 to evaluate the tax residence status of the Company and its subsidiaries organized outside the PRC.
According to SAT Notice 82, a Chinese-controlled offshore incorporated enterprise will be regarded as a PRC tax resident by virtue of having a “de facto management body” in China and will be subject to PRC enterprise income tax on its worldwide income only if all of the following criteria are met: (i) the places where senior management and senior management departments that are responsible for daily production, operation and management of the enterprise perform their duties are mainly located within the territory of China; (ii) financial decisions (such as money borrowing, lending, financing and financial risk management) and personnel decisions (such as appointment, dismissal and salary and wages) are decided or need to be decided by organizations or persons located within the territory of China; (iii) main property, accounting books, corporate seal, the board of directors and files of the minutes of shareholders’ meetings of the enterprise are located or preserved within the territory of China; and (iv) one half (or more) of the directors or senior management staff having the right to vote habitually reside within the territory of China.
We believe that we do not meet some of the conditions outlined in the immediately preceding paragraph. For example, as a holding company, the key assets and records of the Company, including the resolutions and meeting minutes of our board of directors and the resolutions and meeting minutes of our shareholders, are located and maintained outside the PRC. In addition, we are not aware of any offshore holding companies with a corporate structure similar to ours that has been deemed a PRC “resident enterprise” by the PRC tax authorities. Accordingly, we believe that the Company and its offshore subsidiaries should not be treated as a “resident enterprise” for PRC tax purposes if the criteria for “de facto management body” as set forth in SAT Notice 82 were deemed applicable to us. However, as the tax residency status of an enterprise is subject to determination by the PRC tax authorities and uncertainties remain with respect to the interpretation of the term “de facto management body” as applicable to our offshore entities, we will continue to monitor our tax status.
97
The implementation rules of the EIT law provides that, (i) if the enterprise that distributes dividends is domiciled in the PRC or (ii) if gains are realized from transferring equity interests of enterprises domiciled in the PRC, then such dividends or gains are treated as China-sourced income. It is not clear how “domicile” may be interpreted under the EIT Law, and it may be interpreted as the jurisdiction where the enterprise is a tax resident. Therefore, if we are considered as a PRC tax resident enterprise for PRC tax purposes, any dividends we pay to our overseas shareholders which are non-resident enterprises as well as gains realized by such shareholders from the transfer of our shares may be regarded as China-sourced income and as a result become subject to PRC withholding tax at a rate of up to 10%. AllBright Law Offices (Fuzhou), our PRC counsel, is unable to provide a “will” opinion because it believes that it is more likely than not that we and our offshore subsidiaries would be treated as non-resident enterprises for PRC tax purposes because we do not meet some of the conditions outlined in SAT Notice 82. In addition, AllBright Law Offices (Fuzhou) is not aware of any offshore holding companies with a corporate structure similar to ours that has been deemed a PRC “resident enterprise” by the PRC tax authorities as of the date of this annual report. Therefore, AllBright Law Offices (Fuzhou) believes that it is possible but highly unlikely that the income received by our overseas shareholders will be regarded as China-sourced income. See “Item 3. Key Information—Risk Factors—Risks Related to Doing Business in China—Under the EIT Law, we may be classified as a ‘Resident Enterprise’ of China. Such classification will likely result in unfavorable tax consequences to us and our non-PRC shareholders.”
Currently, as resident enterprises in the PRC, Universe Technology as well as Jiangxi Universe and its subsidiaries in PRC are subject to the enterprise income tax at the rate of 25%, except that once an enterprise meets certain requirements and is identified as a small-scale minimal profit enterprise, the part of its taxable income not more than RMB1 million is subject to a reduced rate of 5% and the part between RMB1 million and 3 million is subject to a reduced rate of 10%. The EIT is calculated based on the entity’s global income as determined under PRC tax laws and accounting standards. If the PRC tax authorities determine that Jiangxi Universe is a PRC resident enterprise for enterprise income tax purposes, we may be required to withhold a 10% withholding tax from dividends we pay to our shareholders that are non-resident enterprises. In addition, non-resident enterprise shareholders may be subject to a 10% PRC withholding tax on gains realized on the sale or other disposition of our ordinary shares, if such income is treated as sourced from within the PRC. It is unclear whether our non-PRC individual shareholders would be subject to any PRC tax on dividends or gains obtained by such non-PRC individual shareholders in the event we are determined to be a PRC resident enterprise. If any PRC tax were to apply to dividends or gains realized by non-PRC individuals, it would generally apply at a rate of 20% unless a reduced rate is available under an applicable tax treaty. However, it is also unclear whether our non-PRC shareholders would be able to claim the benefits of any tax treaties between their country of tax residence and the PRC in the event that we are treated as a PRC resident enterprise. There is no guidance from the PRC government to indicate whether or not any tax treaties between the PRC and other countries would apply in circumstances where a non-PRC company was deemed to be a PRC tax resident, and thus there is no basis for expecting how tax treaty between the PRC and other countries may impact non-resident enterprises.
Value-added Tax
Pursuant to the Provisional Regulations on Value-Added Tax of the PRC (《中华人民共和国增值税暂行条例》), or the VAT Regulations, which were promulgated by the State Council on December 13, 1993, and amended on November 10, 2008, February 6, 2016, and November 19, 2017, respectively, and the Implementation Rules of the Provisional Regulations on Value Added Tax of the PRC (《中华人民共和国增值税暂行条例实施细则》) promulgated by the MOF on December 25, 1993 and amended on December 15, 2008 and October 28, 2011, respectively, entities and individuals that sell goods or labor services of processing, repair or replacement, sell services, intangible assets, or immovables, or import goods within the territory of the People’s Republic of China are taxpayers of value-added tax. The VAT rate is 17% for taxpayers selling goods, labor services, or tangible movable property leasing services or importing goods, except otherwise specified; 11% for taxpayers selling goods, labor services, or tangible movable property leasing services or importing goods, except otherwise specified; 6% for taxpayers selling services or intangible assets.
According to Provisions in the Notice on Adjusting the Value added Tax Rates (Cai Shui [2018] No. 32), or the Notice, issued by the SAT and the MOF, where taxpayers make VAT taxable sales or import goods, the applicable tax rates shall be adjusted from 17% to 16% and from 11% to 10%, respectively. The Notice took effect on May 1, 2018, and the adjusted VAT rates took effect at the same time.
98
On March 23, 2016, the MOF and the SAT jointly issued the Circular of Full Implementation of Business Tax to VAT Reform (the “Circular 36”) (《关于全面推开营业税改征增值税试点的通知》), which confirms that business tax will be completely replaced by VAT from May 1, 2016. The Notice of the MOF and the SAT on the Adjustment to VAT Rates (《关于调整增值税税率的通知》), promulgated on April 4, 2018 and effective as of May 1, 2018, adjusted the applicative rate of VAT. The deduction rates of 17% and 11% applicable to the taxpayers who have VAT taxable sales activities or imported goods are adjusted to 16% and 10%, respectively. For the export goods to which a tax rate of 17% was originally applicable and the export rebate rate was 17%, the export rebate rate is adjusted to 16%. For the export goods and cross-border taxable activities to which a tax rate of 11% was originally applicable and the export rebate rate was 11%, the export rebate rate is adjusted to 10%. Pursuant to such circular, the Value Added Tax Pilot Program has been applicable nationwide since May 1, 2016.
Subsequently, the Notice on Policies for Deepening Reform of Value-added Tax was issued by the SAT, the MOF and the General Administration of Customs on March 30, 2019 and took effective on April 1, 2019, which further adjusted the applicable tax rate for taxpayers making VAT taxable sales or importing goods. The applicable tax rates shall be adjusted from 16% to 13% and from 10% to 9%, respectively.
According to the VAT Regulations and the related rules, as of the date of this annual report, as taxpayers selling goods, Jiangxi Universe and its consolidated affiliated entities are generally subject to 13% VAT rate.
Dividend Withholding Tax
Pursuant to the Arrangement between Mainland China and Hong Kong for the Avoidance of Double Taxation and Prevention of Fiscal Evasion with respect to Taxes on Income (《内地和香港特别行政区关于所得税避免双重征税和防止偷税漏税的安排》) effective on August 21, 2006, no more than 5% withholding tax rate applies to dividends paid by a PRC company to a Hong Kong resident, provided that the recipient is a company that holds at least 25% of the capital of the PRC company. The 10% withholding tax rate applies to dividends paid by a PRC company to a Hong Kong resident if the recipient is a company that holds less than 25% of the capital of the PRC company.
Furthermore, pursuant to the Notice of the SAT on Issues Relating to the Implementation of Dividend Clauses in Tax Treaties (Guo Shui Han [2009] No.81) (《国家税务总局关于执行税收协定股息条款有关问题的通知》(国税函[2009] 81号)), which was promulgated and effective on February 20, 2009, all of the following requirements should be satisfied where a fiscal resident of the other party to the tax agreement needs to be entitled to such tax agreement treatment as being taxed at a tax rate specified in the tax agreement for the dividends paid to it by a PRC resident company: (1) such a fiscal resident who obtains dividends should be a company as provided in the tax agreement; (2) owner’s equity interests and voting shares of the PRC resident company directly owned by such a fiscal resident reaches a specified percentage; and (3) the equity interests of the PRC resident company directly owned by such a fiscal resident, at any time during the 12 months prior to the acquisition of the dividends, reaches a percentage specified in the tax agreement.
In addition, according to the Administrative Measures on Non-resident Taxpayers Enjoying Treaty Benefits (《非居民纳税人享受协定待遇管理办法》) promulgated by the SAT on October 14, 2019 and became effective on January 1, 2020, non-resident taxpayers claiming treaty benefits shall adhere to the principle of “self-assessment, claiming benefits, retention of the relevant materials for future inspection.” Where a non-resident taxpayer self-assesses and concludes that it satisfies the criteria for claiming treaty benefits, it may enjoy treaty benefits at the time of tax declaration or withholding. However such non-resident taxpayers shall retain relevant tax-reporting materials pursuant to the provisions of these Measures for potential future inspection, and accept follow-up administration by relevant tax authorities.
As of the date of this annual report, when considered as a non-PRC resident investor, which is much more likely to happen than not, Universe HK shall be subject to the dividend withholding tax at the rate of 10%. Upon identified as the Hong Kong resident enterprise stipulated by the Double Tax Avoidance Arrangement and other applicable laws, the withholding tax may be reduced to 5%.
Hong Kong Taxation
Entities incorporated in Hong Kong are subject to profits tax in Hong Kong at the rate of 16.5% for each of the fiscal years ended September 30, 2023, 2022 and 2021.
99
United States Federal Income Taxation
The following does not address the tax consequences to any particular investor or to persons in special tax situations such as:
| ● | banks; | |
| ● | financial institutions; | |
| ● | insurance companies; | |
| ● | regulated investment companies; | |
| ● | real estate investment trusts; | |
| ● | broker-dealers; | |
| ● | persons that elect to mark their securities to market; | |
| ● | U.S. expatriates or former long-term residents of the U.S.; | |
| ● | governments or agencies or instrumentalities thereof; | |
| ● | tax-exempt entities; | |
| ● | persons liable for alternative minimum tax; | |
| ● | persons holding our ordinary shares as part of a straddle, hedging, conversion or integrated transaction; | |
| ● | persons that actually or constructively own 10% or more of our voting power or value (including by reason of owning our ordinary shares); | |
| ● | persons who acquired our ordinary shares pursuant to the exercise of any employee share option or otherwise as compensation; | |
| ● | persons holding our ordinary shares through partnerships or other pass-through entities; | |
| ● | beneficiaries of a Trust holding our ordinary shares; or | |
| ● | persons holding our ordinary shares through a Trust. |
The discussion set forth below is addressed only to U.S. Holders that purchased our ordinary shares. Purchasers are urged to consult their own tax advisors about the application of the U.S. federal income tax rules to their particular circumstances as well as the state, local, foreign, and other tax consequences to them of the purchase, ownership, and disposition of our ordinary shares.
Material Tax Consequences Applicable to U.S. Holders of Our Ordinary Shares
The following sets forth the material U.S. federal income tax consequences related to the ownership and disposition of our ordinary shares. It is directed to U.S. Holders (as defined below) of our ordinary shares and is based upon laws and relevant interpretations thereof in effect as of the date of this annual report, all of which are subject to change. This description does not deal with all possible tax consequences relating to ownership and disposition of our ordinary shares or U.S. tax laws, other than the U.S. federal income tax laws, such as the tax consequences under non-U.S. tax laws, state, local, and other tax laws.
The following brief description applies only to U.S. Holders (defined below) that hold ordinary shares as capital assets and that have the U.S. dollar as their functional currency. This brief description is based on the federal income tax laws of the United States in effect as of the date of this annual report and on U.S. Treasury regulations in effect or, in some cases, proposed, as of the date of this annual report, as well as judicial and administrative interpretations thereof available on or before such date. All of the foregoing authorities are subject to change, which change could apply retroactively and could affect the tax consequences described below.
100
The brief description below of the U.S. federal income tax consequences to “U.S. Holders” will apply to you if you are a beneficial owner of ordinary shares and you are, for U.S. federal income tax purposes,
| ● | an individual who is a citizen or resident of the United States; | |
| ● | a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) organized under the laws of the United States, any state thereof or the District of Columbia; | |
| ● | an estate whose income is subject to U.S. federal income taxation regardless of its source; or | |
| ● |
a trust that (1) is subject to the primary supervision of a court within the United States and the control of one or more U.S. persons for all substantial decisions or (2) has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
If a partnership (or other entity treated as a partnership for United States federal income tax purposes) is a beneficial owner of our ordinary shares, the tax treatment of a partner in the partnership will depend upon the status of the partner and the activities of the partnership. Partnerships and partners of a partnership holding our ordinary shares are urged to consult their tax advisors regarding an investment in our ordinary shares. |
Taxation of Dividends and Other Distributions on Our Ordinary Shares
Subject to the PFIC rules discussed below, the gross amount of distributions made by us to you with respect to the ordinary shares (including the amount of any taxes withheld therefrom) will generally be includable in your gross income as dividend income on the date of receipt by you, but only to the extent that the distribution is paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). With respect to corporate U.S. Holders, the dividends will not be eligible for the dividends-received deduction allowed to corporations in respect of dividends received from other U.S. corporations. There were no cash dividends paid during the years ended September 30, 2023 and 2022.
With respect to non-corporate U.S. Holders, including individual U.S. Holders, dividends will be taxed at the lower capital gains rate applicable to qualified dividend income, provided that (1) the ordinary shares are readily tradable on an established securities market in the United States, or we are eligible for the benefits of an approved qualifying income tax treaty with the United States that includes an exchange of information program, (2) we are not a PFIC for either our taxable year in which the dividend is paid or the preceding taxable year, and (3) certain holding period requirements are met. Because there is no income tax treaty between the United States and the Cayman Islands, clause (1) above can be satisfied only if the ordinary shares are readily tradable on an established securities market in the United States. Under U.S. Internal Revenue Service authority, ordinary shares are considered for purpose of clause (1) above to be readily tradable on an established securities market in the United States if they are listed on certain exchanges, which presently includes the NYSE and the Nasdaq Stock Market. Our ordinary shares have been listed on the Nasdaq Global Market since March 23, 2021 under the symbol “UPC.” You are urged to consult your tax advisors regarding the availability of the lower rate for dividends paid with respect to our ordinary shares, including the effects of any change in law after the date of this annual report.
Dividends will constitute foreign source income for foreign tax credit limitation purposes. If the dividends are taxed as qualified dividend income (as discussed above), the amount of the dividend taken into account for purposes of calculating the foreign tax credit limitation will be limited to the gross amount of the dividend, multiplied by the reduced rate divided by the highest rate of tax normally applicable to dividends. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends distributed by us with respect to our ordinary shares will constitute “passive category income” but could, in the case of certain U.S. Holders, constitute “general category income.”
To the extent that the amount of the distribution exceeds our current and accumulated earnings and profits (as determined under U.S. federal income tax principles), it will be treated first as a tax-free return of your tax basis in your ordinary shares, and to the extent the amount of the distribution exceeds your tax basis, the excess will be taxed as capital gain. We do not intend to calculate our earnings and profits under U.S. federal income tax principles. Therefore, a U.S. Holder should expect that a distribution will be treated as a dividend even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above.
101
Taxation of Dispositions of Ordinary Shares
Subject to the PFIC rules discussed below, you will recognize taxable gain or loss on any sale, exchange, or other taxable disposition of a share equal to the difference between the amount realized (in U.S. dollars) for the share and your tax basis (in U.S. dollars) in the ordinary shares. The gain or loss will be capital gain or loss. If you are a non-corporate U.S. Holder, including an individual U.S. Holder, who has held the ordinary shares for more than one year, you will generally be eligible for reduced tax rates. The deductibility of capital losses is subject to limitations. Any such gain or loss that you recognize will generally be treated as United States source income or loss for foreign tax credit limitation purposes which will generally limit the availability of foreign tax credits.
Passive Foreign Investment Company (PFIC) Consequences
A non-U.S. corporation is considered a PFIC, as defined in Section 1297(a) of the U.S. Internal Revenue Code, for any taxable year if either:
| ● | at least 75% of its gross income for such taxable year is passive income; or | |
| ● | at least 50% of the value of its assets (based on an average of the quarterly values of the assets during a taxable year) is attributable to assets that produce or are held for the production of passive income (the “asset test”). |
Passive income generally includes dividends, interest, rents and royalties (other than rents or royalties derived from the active conduct of a trade or business), and gains from the disposition of passive assets. We will be treated as owning our proportionate share of the assets and earning our proportionate share of the income of any other corporation in which we own, directly or indirectly, at least 25% (by value) of the stock. In determining the value and composition of our assets for purposes of the PFIC asset test, the value of our assets must be determined based on the market value of our ordinary shares from time to time, which could cause the value of our non-passive assets to be less than 50% of the value of all of our assets on any particular quarterly testing date for purposes of the asset test.
Based on our operations and the composition of our assets, we are not a PFIC this fiscal year ended, under the current PFIC rules. We must make a separate determination each year as to whether we are a PFIC, however, and there can be no assurance with respect to our status as a PFIC for any future taxable years. Depending on the amount of assets held for the production of passive income, it is possible that, for any subsequent taxable year, more than 50% of our assets may be assets held for the production of passive income. We will make this determination following the end of any particular tax year. In addition, because the value of our assets for purposes of the asset test will generally be determined based on the market price of our ordinary shares, our PFIC status will depend in large part on the market price of our ordinary shares. Accordingly, fluctuations in the market price of the ordinary shares may cause us to become a PFIC. In addition, the application of the PFIC rules is subject to uncertainty in several respects and the composition of our income and assets will be affected by how, and how quickly, we spend our liquid assets. We are under no obligation to take steps to reduce the risk of our being classified as a PFIC, and as stated above, the determination of the value of our assets will depend upon material facts (including the market price of our ordinary shares from time to time) that may not be within our control. If we are a PFIC for any year during which you hold ordinary shares, we will continue to be treated as a PFIC for all succeeding years during which you hold ordinary shares. If we cease to be a PFIC and you did not previously make a timely “mark-to-market” election as described below, you may avoid some of the adverse effects of the PFIC regime by making a “purging election” (as described below) with respect to the ordinary shares.
102
If we are a PFIC for your taxable year(s) during which you hold ordinary shares, you will be subject to special tax rules with respect to any “excess distribution” that you receive and any gain you realize from a sale or other disposition (including a pledge) of the ordinary shares, unless you make a “mark-to-market” election as discussed below. Distributions you receive in a taxable year that are greater than 125% of the average annual distributions you received during the shorter of the three preceding taxable years or your holding period for the ordinary shares will be treated as an excess distribution. Under these special tax rules:
| ● | the excess distribution or gain will be allocated ratably over your holding period for the ordinary shares; | |
| ● | the amount allocated to your current taxable year, and any amount allocated to any of your taxable year(s) prior to the first taxable year in which we were a PFIC, will be treated as ordinary income, and | |
| ● | the amount allocated to each of your other taxable year(s) will be subject to the highest tax rate in effect for that year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
The tax liability for amounts allocated to years prior to the year of disposition or “excess distribution” cannot be offset by any net operating losses for such years, and gains (but not losses) realized on the sale of the ordinary shares cannot be treated as capital, even if you hold the ordinary shares as capital assets.
A U.S. Holder of “marketable stock” (as defined below) in a PFIC may make a mark-to-market election under Section 1296 of the US Internal Revenue Code for such stock to elect out of the tax treatment discussed above. If you make a mark-to-market election for first taxable year which you hold (or are deemed to hold) ordinary shares and for which we are determined to be a PFIC, you will include in your income each year an amount equal to the excess, if any, of the fair market value of the ordinary shares as of the close of such taxable year over your adjusted basis in such ordinary shares, which excess will be treated as ordinary income and not capital gain. You are allowed an ordinary loss for the excess, if any, of the adjusted basis of the ordinary shares over their fair market value as of the close of the taxable year. Such ordinary loss, however, is allowable only to the extent of any net mark-to-market gains on the ordinary shares included in your income for prior taxable years. Amounts included in your income under a mark-to-market election, as well as gain on the actual sale or other disposition of the ordinary shares, are treated as ordinary income. Ordinary loss treatment also applies to any loss realized on the actual sale or disposition of the ordinary shares, to the extent that the amount of such loss does not exceed the net mark-to-market gains previously included for such ordinary shares. Your basis in the ordinary shares will be adjusted to reflect any such income or loss amounts. If you make a valid mark-to-market election, the tax rules that apply to distributions by corporations which are not PFICs would apply to distributions by us, except that the lower applicable capital gains rate for qualified dividend income discussed above under “—Taxation of Dividends and Other Distributions on our Ordinary Shares” generally would not apply.
The mark-to-market election is available only for “marketable stock,” which is stock that is traded in other than de minimis quantities on at least 15 days during each calendar quarter (“regularly traded”) on a qualified exchange or other market (as defined in applicable U.S. Treasury regulations), including the Nasdaq Global Market. If the ordinary shares are regularly traded on the Nasdaq Global Market and if you are a holder of ordinary shares, the mark-to-market election would be available to you were we to be or become a PFIC. Our ordinary shares have been listed on the Nasdaq Global Market since March 23, 2021 under the symbol “UPC.”
Alternatively, a U.S. Holder of stock in a PFIC may make a “qualified electing fund” election under Section 1295(b) of the US Internal Revenue Code with respect to such PFIC to elect out of the tax treatment discussed above. A U.S. Holder who makes a valid qualified electing fund election with respect to a PFIC will generally include in gross income for a taxable year such holder’s pro rata share of the corporation’s earnings and profits for the taxable year. The qualified electing fund election, however, is available only if such PFIC provides such U.S. Holder with certain information regarding its earnings and profits as required under applicable U.S. Treasury regulations. We do not currently intend to prepare or provide the information that would enable you to make a qualified electing fund election. If you hold ordinary shares in any taxable year in which we are a PFIC, you will be required to file U.S. Internal Revenue Service Form 8621 in each such year and provide certain annual information regarding such ordinary shares, including regarding distributions received on the ordinary shares and any gain realized on the disposition of the ordinary shares.
103
If you do not make a timely “mark-to-market” election (as described above), and if we were a PFIC at any time during the period you hold our ordinary shares, then such ordinary shares will continue to be treated as stock of a PFIC with respect to you even if we cease to be a PFIC in a future year, unless you make a “purging election” for the year we cease to be a PFIC. A “purging election” creates a deemed sale of such ordinary shares at their fair market value on the last day of the last year in which we are treated as a PFIC. The gain recognized by the purging election will be subject to the special tax and interest charge rules treating the gain as an excess distribution, as described above. As a result of the purging election, you will have a new basis (equal to the fair market value of the ordinary shares on the last day of the last year in which we are treated as a PFIC) and holding period (which new holding period will begin the day after such last day) in your ordinary shares for tax purposes.
IRC Section 1014(a) provides for a step-up in basis to the fair market value for our ordinary shares when inherited from a decedent that was previously a holder of our ordinary shares. However, if we are determined to be a PFIC and a decedent that was a U.S. Holder did not make either a timely qualified electing fund election for our first taxable year as a PFIC in which the U.S. Holder held (or was deemed to hold) our ordinary shares, or a mark-to-market election and ownership of those ordinary shares are inherited, a special provision in IRC Section 1291(e) provides that the new U.S. Holder’s basis should be reduced by an amount equal to the Section 1014 basis minus the decedent’s adjusted basis just before death. As such if we are determined to be a PFIC at any time prior to a decedent’s passing, the PFIC rules will cause any new U.S. Holder that inherits our ordinary shares from a U.S. Holder to not get a step-up in basis under Section 1014 and instead will receive a carryover basis in those ordinary shares.
You are urged to consult your tax advisors regarding the application of the PFIC rules to your investment in our ordinary shares and the elections discussed above.
Information Reporting and Backup Withholding
Dividend payments with respect to our ordinary shares and proceeds from the sale, exchange, or redemption of our ordinary shares may be subject to information reporting to the U.S. Internal Revenue Service and possible U.S. backup withholding under Section 3406 of the U.S. Internal Revenue Code with at a current flat rate of 24%. Backup withholding will not apply, however, to a U.S. Holder who furnishes a correct taxpayer identification number and makes any other required certification on U.S. Internal Revenue Service Form W-9 or who is otherwise exempt from backup withholding. U.S. Holders who are required to establish their exempt status generally must provide such certification on U.S. Internal Revenue Service Form W-9. U.S. Holders are urged to consult their tax advisors regarding the application of the U.S. information reporting and backup withholding rules.
Backup withholding is not an additional tax. Amounts withheld as backup withholding may be credited against your U.S. federal income tax liability, and you may obtain a refund of any excess amounts withheld under the backup withholding rules by filing the appropriate claim for refund with the U.S. Internal Revenue Service and furnishing any required information. We do not intend to withhold taxes for individual shareholders. Transactions effected through certain brokers or other intermediaries, however, may be subject to withholding taxes (including backup withholding), and such brokers or intermediaries may be required by law to withhold such taxes.
Under the Hiring Incentives to Restore Employment Act of 2010, certain U.S. Holders are required to report information relating to our ordinary shares, subject to certain exceptions (including an exception for ordinary shares held in accounts maintained by certain financial institutions), by attaching a complete Internal Revenue Service Form 8938, Statement of Specified Foreign Financial Assets, with their tax return for each year in which they hold ordinary shares.
F. Dividends and Paying Agents
Not applicable.
G. Statement by Experts
Not applicable.
104
H. Documents on Display
We are subject to the periodic reporting and other informational requirements of the Exchange Act. Under the Exchange Act, we are required to file reports and other information with the SEC. Specifically, we are required to file annually a Form 20-F within four months after the end of each fiscal year. The SEC maintains a website at http://www.sec.gov that contains reports, proxy and information statements, and other information regarding registrants that make electronic filings with the SEC using its EDGAR system. As a foreign private issuer, we are exempt from the rules of the Exchange Act prescribing, among other things, the furnishing and content of proxy statements to shareholders, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
I. Subsidiary Information
Not applicable.
J. Annual Report to Security Holders
Not applicable.
Item 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Foreign Exchange Risk
Substantially all of our revenues are denominated in Renminbi. The Renminbi is not freely convertible into foreign currencies for capital account transactions. The value of the Renminbi against the U.S. dollar and other currencies is affected by, among other things, changes in China’s political and economic conditions and China’s foreign exchange policies. On July 21, 2005, the PRC government changed its decade-old policy of pegging the value of the Renminbi to the U.S. dollar, and the Renminbi appreciated more than 20% against the U.S. dollar over the following three years. Between July 2008 and June 2010, this appreciation halted and the exchange rate between the Renminbi and the U.S. dollar remained within a narrow band. Since June 2010, the Renminbi has fluctuated against the U.S. dollar, at times significantly and unpredictably. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between the Renminbi and the U.S. dollar in the future.
To date, we have not entered into any hedging transactions in an effort to reduce our exposure to foreign currency exchange risk. To the extent that we need to convert U.S. dollars we received from our initial public offering in March 2021 into Renminbi for our operations or capital expenditures, appreciation of the Renminbi against the U.S. dollar would have an adverse effect on the Renminbi amount we would receive from the conversion. Conversely, if we decide to convert our Renminbi into U.S. dollars for the purpose of making payments for dividends on our ordinary shares or for other business purposes, appreciation of the U.S. dollar against the Renminbi would have a negative effect on the U.S. dollar amount available to us.
105
As of September 30, 2023 and 2022, we had U.S. dollar-denominated cash and cash equivalents of US$5.3 million and US$5.7 million, respectively. A 10% depreciation of U.S. dollar against the Renminbi based on the foreign exchange rate on September 30, 2023 would result in a decrease of RMB3.86 million in cash and cash equivalents. A 10% appreciation of U.S. dollar against the Renminbi based on the foreign exchange rate on September 30, 2023 would result in an increase of RMB3.86 million in cash and cash equivalents.
Credit Risk
Accounts receivable are typically unsecured and derived from revenue earned from customers, thereby exposed to credit risk. The risk is mitigated by our assessment of its customers’ creditworthiness and its ongoing monitoring of outstanding balances.
Interest Rate Risk
We have not used derivative financial instruments to hedge interest rate risk. Interest-earning instruments carry a degree of interest rate risk. We have not been exposed, nor do we anticipate being exposed to material risks due to changes in market interest rates. However, our future interest income may fall short of expectations due to changes in market interest rates.
Inflation Risk
In recent years, inflation has not had a material impact on our results of operations. According to the National Bureau of Statistics of China, the consumer price index in China increased by 0.9%, 2.0% and 0.2% in 2021, 2022 and 2023, respectively. Although we have not in the past been materially affected by inflation since our inception, we can provide no assurance that we will not be affected in the future by higher rates of inflation in China. If inflation rises, it may materially and adversely affect our business.
Item 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
D. American Depositary Shares
Not applicable.
106
Part II
Item 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
Item 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
See “Item 10. Additional Information” for a description of the rights of securities holders, which remain unchanged.
Use of Proceeds
The following “Use of Proceeds” information relates to the registration statement on Form F-1 (File Number 333-248067), as amended, which was declared effective by the SEC on March 22, 2021, for our initial public offering, which was closed on March 25, 2021. We issued and sold an aggregate of 5,750,000 ordinary shares, at a price of $5.00 per share with gross proceeds of $28.75 million. Univest Securities, LLC was the underwriter of our initial public offering.
We incurred approximately $2,953,114 in expenses in connection with our initial public offering and $178,758 in expenses in connection with the issuance of overallotment shares. None of the transaction expenses included payments to directors or officers of our company or their associates, persons owning more than 10% or more of our equity securities or our affiliates. None of the net proceeds we received from the initial public offering were paid, directly or indirectly, to any of our directors or officers or their associates, persons owning 10% or more of our equity securities or our affiliates.
The net proceeds raised from the initial public offering were $25,618,128 after deducting underwriting discounts and the offering expenses payable by us. For the period from the effectiveness of the registration statement on Form F-1 to September 30, 2023, we used approximately US$$5.90 million for research and development purposes, approximately US$6.10 million for upgrading and expanding our manufacturing facilities, and approximately US$5.20 million for brand marketing. We still intend to use the remaining proceeds from our initial public offering in the manner disclosed in our registration statement on Form F-1, as amended (File Number 333-248067).
Item 15. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and chief financial officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report, as required by Rule 13a-15(b) under the Exchange Act.
Based upon this evaluation, our management has concluded that, as of September 30, 2023, our existing disclosure controls and procedures were ineffective because of a lack of accounting staff and resources with appropriate knowledge of U.S. GAAP and SEC reporting and compliance requirements.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements in accordance with U.S. GAAP and includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of a company’s assets, (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with generally accepted accounting principles, and that a company’s receipts and expenditures are being made only in accordance with authorizations of a company’s management and directors, and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of a company’s assets that could have a material effect on the consolidated financial statements.
107
Our management, with the participation of our chief executive officer and chief financial officer, conducted an evaluation of the effectiveness of our Company’s internal control over financial reporting as of September 30, 2023 based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO 2013 Framework). Based on this evaluation, we identified one deficiency, which related to a lack of accounting staff and resources with appropriate knowledge of U.S. GAAP and SEC reporting and compliance requirements, and which we believe to be a material weakness as of September 30, 2023.
As a result of the above material weakness, management has concluded that our internal control over financial reporting was not effective as of September 30, 2023. To remedy our identified material weakness as of September 30, 2023, we have undertaken the remedial steps and also plan to adopt certain measures to improve our internal control over financial reporting as set forth below.
Remediation plan of the Material Weakness in Internal Control over Financial Reporting Reported as of September 30, 2023
As of the date of this annual report, we have not fully addressed the above-referenced weakness. However, we have made progress in implementing remedial measures, including:
| (i) | recruiting qualified accounting personnel with relevant U.S. GAAP and SEC reporting experience and qualifications to strengthen the financial reporting function and to set up a financial and system control framework. Since very few companies in Ji’an, Jiangxi Province, the area in which our main PRC operating subsidiaries are located, have sought public listing on a U.S. exchange, we have difficulty identifying qualified accounting candidates with U.S. GAAP experience and expertise. We plan to search for qualified personnel in other regions of China; and |
| (ii) | implementing regular and continuous U.S. GAAP accounting and financial reporting training programs for our accounting and financial reporting personnel. |
Attestation Report of the Registered Public Accounting Firm
This annual report on Form 20-F does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to rules of the SEC where domestic and foreign registrants that are non-accelerated filers, which we are, and “emerging growth companies,” which we also are, are not required to provide the auditor attestation report.
Changes in Internal Control over Financial Reporting
As of September 30, 2022, we identified a material weakness in our internal control over financial reporting, which related to a lack of accounting staff and resources with appropriate knowledge of U.S. GAAP and SEC reporting and compliance requirements.
We implemented the following remedial measures during the fiscal year ended September 30, 2023 to address the material weakness identified as of September 30, 2022:
| i. | providing U.S. GAAP learning materials for our accounting and financial reporting personnel; and |
| ii. | attending online U.S. GAAP courses provided by a leading financial education institutions in China. |
Other than as described above, there were no changes in our internal controls over financial reporting that occurred during the period covered by this annual report on Form 20-F that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 16. [RESERVED]
Item 16A. AUDIT COMMITTEE FINANCIAL EXPERT
Mr. Ding Zheng qualifies as an “audit committee financial expert” as defined in Item 16A of Form 20-F. Mr. Ding Zheng satisfies the “independence” requirements of Section 5605(a)(2) of the Nasdaq Listing Rules as well as the independence requirements of Rule 10A-3 under the Exchange Act.
108
Item 16B. CODE OF ETHICS
Our board of directors has adopted a code of business conduct and ethics, which is applicable to all of our directors, officers, and employees. Our code of business conduct and ethics is publicly available on our website.
Item 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table sets forth the aggregate fees by categories specified below in connection with certain professional services rendered and billed by YCM CPA INC., our independent registered public accounting firm for the periods indicated.
YCM CPA INC.
| For the Years Ended September 30, | ||||||||
| 2022 | 2023 | |||||||
| Audit fees (1) | $ | 210,000 | $ | 265,000 | ||||
| Audit-related fees (2) | - | |||||||
| Tax fees (3) | - | |||||||
| All other fees | - | |||||||
| Total | $ | 210,000 | $ | 265,000 | ||||
| (1) | Audit fees include the aggregate fees billed for each of the fiscal years for professional services rendered by our independent registered public accounting firm for the audit of our annual financial statements or for the audits of our financial statements and review of the interim financial statements. |
| (2) | Audit related fees include the aggregate fees billed for related services by our principal accountant that are reasonably related to the performance of the audit or review of our financial statements and are not reported under audit fees. |
| (3) | Tax fees represent the aggregated fees billed for professional services rendered by our independent registered public accounting firm for tax compliance, tax advice, and tax planning. |
The audit committee of our board of directors has established its pre-approval policies and procedures, pursuant to which the audit committee approved the foregoing audit, tax and non-audit services provided by YCM CPA INC. in fiscal years 2022 and 2023. Consistent with our audit committee’s responsibility for engaging our independent auditors, all audit and permitted non-audit services require pre-approval by the audit committee. The full audit committee approves proposed services and fee estimates for these services. One or more independent directors serving on the audit committee may be delegated by the full audit committee to pre-approve any audit and non-audit services. Any such delegation shall be presented to the full audit committee at its next scheduled meeting. Pursuant to these procedures, the audit committee approved the foregoing audit services provided by YCM CPA INC.
Item 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
Item 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
None.
109
Item 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
Not applicable.
Item 16G. CORPORATE GOVERNANCE
As a Cayman Islands company listed on the Nasdaq Global Market, we are subject to the Nasdaq corporate governance listing standards. Nasdaq rules, however, permit a foreign private issuer like us to follow the corporate governance practices of its home country. Certain corporate governance practices in the Cayman Islands, which is our home country, may differ significantly from the Nasdaq corporate governance listing standards.
Nasdaq Listing Rule 5635 generally provides that shareholder approval is required of U.S. domestic companies listed on Nasdaq prior to issuance (or potential issuance) of securities (i) equaling 20% or more of the company’s common stock or voting power for less than the greater of market or book value (ii) resulting in a change of control of the company; and (iii) which is being issued pursuant to a stock option or purchase plan to be established or materially amended or other equity compensation arrangement made or materially amended. Notwithstanding this general requirement, Nasdaq Listing Rule 5615(a)(3)(A) permits foreign private issuers to follow their home country practice rather than these shareholder approval requirements. The Companies Act does not require shareholder approval prior to any of the foregoing types of issuances. We, therefore, are not required to obtain such shareholder approval prior to entering into a transaction with the potential to issue securities as described above. Specifically, our board of directors has elected to follow our home country rules and be exempt from the requirements to obtain shareholder approval for the issuance of 20% or more of our outstanding ordinary shares under Nasdaq Listing Rule 5635(d).
Nasdaq Listing Rule 5605(b)(1) requires listed companies to have, among other things, a majority of its board members be independent. As a foreign private issuer, however, we are permitted to follow home country practice in lieu of the above requirements. The corporate governance practice in our home country, the Cayman Islands, does not require a majority of our board to consist of independent directors. Currently, a majority of our board members are independent. However, if we change our board composition such that independent directors do not constitute a majority of our board of directors, our shareholders may be afforded less protection than they would otherwise enjoy under Nasdaq’s corporate governance requirements applicable to U.S. domestic issuers. See “Item 3. Key Information—D. Risk Factors—Risks Related to Our Ordinary Shares— As a foreign private issuer, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from the Nasdaq listing standards. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards.”
Other than those described above, there are no significant differences between our corporate governance practices and those followed by U.S. domestic companies under Nasdaq corporate governance listing standards.
Item 16H. MINE SAFETY DISCLOSURE
Not applicable.
Item 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS.
Not applicable.
Item 16J. INSIDER TRADING POLICIES.
Pursuant to applicable SEC transition guidance, the disclosure required by Item 16J will be applicable to the Company from the fiscal year ending September 30, 2024.
Item 16K. CYBERSECURITY.
Pursuant to applicable SEC transition guidance, the disclosure required by Item 16K will be applicable to the Company from the fiscal year ending September 30, 2024.
110
Part III
Item 17. FINANCIAL STATEMENTS
We have elected to provide financial statements pursuant to Item 18.
Item 18. FINANCIAL STATEMENTS
The consolidated financial statements of Universe Pharmaceuticals INC and its subsidiaries are included at the end of this annual report.
Item 19. EXHIBITS
EXHIBIT INDEX
111
| * | Filed with this annual report on Form 20-F |
| ** | Furnished with this annual report on Form 20-F |
112
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| Universe Pharmaceuticals INC | ||
| By: | /s/ Gang Lai | |
| Gang Lai | ||
| Chief Executive Officer, Director, and | ||
| Chairman of the Board of Directors | ||
| Date: January 30, 2024 | ||
113
INDEX TO FINANCIAL STATEMENTS
UNIVERSE PHARMACEUTICALS INC. AND SUBSIDIARIES
TABLE OF CONTENTS
F-1

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Shareholders of Universe Pharmaceuticals INC
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Universe Pharmaceuticals INC and subsidiaries (collectively, the “Company”) as of September 30, 2023 and 2022, and the related consolidated statements of operations and comprehensive income (loss), changes in shareholders’ equity, and cash flows for the years ended September 30, 2023, 2022 and 2021, and the related notes (collectively referred to as the “financial statements”).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of September 30, 2023 and 2022, and the results of its operations and its cash flows for the period ended September 30, 2023, 2022 and 2021, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statement. We believe that our audits provide a reasonable basis for our opinion.
/s/
We have served as the Company’s auditor since 2021.
PCAOB ID
January 30, 2024
F-2
UNIVERSE PHARMACEUTICALS INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| As of September 30, | ||||||||
| 2023 | 2022 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | $ | ||||||
| Short-term investments | ||||||||
| Accounts receivable, net | ||||||||
| Due from related parties | ||||||||
| Inventories, net | ||||||||
| Advance to suppliers | ||||||||
| Prepayment for acquisition | ||||||||
| Prepaid expenses and other current assets | ||||||||
| TOTAL CURRENT ASSETS | ||||||||
| Property and equipment, net | ||||||||
| Prepayments made to a related party for purchase of property | ||||||||
| Prepayments for construction in progress | ||||||||
| Intangible assets, net | ||||||||
| Investment in equity securities | ||||||||
| Deferred tax assets | ||||||||
| TOTAL NONCURRENT ASSETS | ||||||||
| TOTAL ASSETS | $ | $ | ||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Short-term bank loans | $ | $ | ||||||
| Accounts payable | ||||||||
| Taxes payable | ||||||||
| Due to related parties | ||||||||
| Accrued expenses and other current liabilities | ||||||||
| TOTAL CURRENT LIABILITIES | ||||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||
| SHAREHOLDERS’ EQUITY | ||||||||
| Ordinary shares, $ | ||||||||
| Additional paid in capital | ||||||||
| Statutory reserve | ||||||||
| Retained earnings | ||||||||
| Accumulated other comprehensive loss | ( | ) | ( | ) | ||||
| TOTAL SHAREHOLDERS’ EQUITY | ||||||||
| TOTAL LIABILITIES AND SHAREHOLDERS’ EQUITY | $ | $ | ||||||
| * |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
UNIVERSE PHARMACEUTICALS INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
| For the Years Ended September 30, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Revenue | $ | $ | $ | |||||||||
| Cost of revenue | ( | ) | ( | ) | ( | ) | ||||||
| Gross profit | ||||||||||||
| Operating expenses | ||||||||||||
| Selling expenses | ( | ) | ( | ) | ( | ) | ||||||
| General and administrative expenses | ( | ) | ( | ) | ( | ) | ||||||
| Research and development expenses | ( | ) | ( | ) | ( | ) | ||||||
| Total operating expenses | ( | ) | ( | ) | ( | ) | ||||||
| Income from operations | ( | ) | ( | ) | ||||||||
| Other income (expenses) | ||||||||||||
| Interest expense, net | ( | ) | ( | ) | ( | ) | ||||||
| Other (expenses) income, net | ( | ) | ( | ) | ||||||||
Short-term investments income (loss) | ( | ) | ||||||||||
| Equity investment income | ||||||||||||
| Total other (expense) income, net | ( | ) | ( | ) | ||||||||
| (Loss) income before income tax provision | ( | ) | ( | ) | ||||||||
| Income tax provision | ( | ) | ( | ) | ( | ) | ||||||
| Net (loss) income | ( | ) | ( | ) | ||||||||
| Other comprehensive income (loss) | ||||||||||||
| Foreign currency translation adjustment | ( | ) | ( | ) | ||||||||
| Comprehensive (loss) income | $ | ( | ) | $ | ( | ) | $ | |||||
| Earnings per share | ||||||||||||
| $ | ( | ) | $ | ( | ) | $ | ||||||
| Weighted average number of shares outstanding | ||||||||||||
| * |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
UNIVERSE PHARMACEUTICALS INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
FOR THE YEARS ENDED SEPTEMBER 30, 2023, 2022 AND 2021
| Ordinary shares | Additional paid-in | Statutory | Retained | Accumulated other comprehensive | Total shareholders’ | |||||||||||||||||||||||
| Shares * | Amount | capital | reserve | earnings | loss | equity | ||||||||||||||||||||||
| Balance at September 30, 2020 | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||
| Issuance of ordinary shares and additional shares under overallotment option in initial public offerings, gross | ||||||||||||||||||||||||||||
| Capitalized initial public offering costs | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Net income | - | |||||||||||||||||||||||||||
| Foreign currency translation adjustment | - | |||||||||||||||||||||||||||
| Balance at September 30, 2021 | $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation adjustment | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Balance at September 30, 2022 | $ | $ | $ | $ | $ | ( | ) | $ | ||||||||||||||||||||
| Net loss | - | - | - | - | ( | ) | - | ( | ) | |||||||||||||||||||
| Foreign currency translation adjustment | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Balance at September 30, 2023 | $ | $ | $ | $ | $ | ( | ) | $ | ||||||||||||||||||||
| * |
The accompanying notes are an integral part of these consolidated financial statements.
F-5
UNIVERSE PHARMACEUTICALS INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended September 30, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Cash flows from operating activities | ||||||||||||
| Net (loss) income | $ | ( | ) | $ | ( | ) | $ | |||||
| Adjustments to reconcile net income to net cash provided by (used in) operating activities: | ||||||||||||
| Depreciation and amortization | ||||||||||||
| Loss from disposal of fixed assets | ||||||||||||
| Allowance for doubtful accounts | ( | ) | ( | ) | ||||||||
| Inventory reserve | ( | ) | ||||||||||
| Deferred income tax benefit | ( | ) | ( | ) | ||||||||
| Short-term investments income | ( | ) | ( | ) | ||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Accounts receivable | ( | ) | ( | ) | ||||||||
| Inventory, net | ( | ) | ( | ) | ||||||||
| Advance to suppliers, net | ( | ) | ( | ) | ||||||||
| Prepayment for advertising | ( | ) | ||||||||||
| Advances to related parties | ( | ) | ||||||||||
| Prepaid expenses and other current assets | ( | ) | ( | ) | ||||||||
| Accounts payable | ( | ) | ||||||||||
| Taxes payable | ( | ) | ( | ) | ||||||||
| Accrued expenses and other current liabilities | ||||||||||||
| Net cash provided by (used in) operating activities | ( | ) | ( | ) | ||||||||
| Cash flows from investing activities | ||||||||||||
| Purchases of property and equipment | ( | ) | ( | ) | ( | ) | ||||||
| Proceeds from disposal of fixed assets | ||||||||||||
| Prepayments made to a related party for purchase of property | ( | ) | ||||||||||
| Prepayments for construction in progress | ( | ) | ||||||||||
| Prepayment for acquisition | ( | ) | ||||||||||
| Payments for short-term investments | ( | ) | ||||||||||
| Redemption of short-term investments | ||||||||||||
| Net cash used in investing activities | ( | ) | ( | ) | ( | ) | ||||||
| Cash flows from financing activities | ||||||||||||
| Proceeds from short-term bank loans | ||||||||||||
| Repayment of bank loans | ( | ) | ( | ) | ( | ) | ||||||
| Gross proceeds from initial public offerings | ||||||||||||
| Payment for deferred initial public offering costs | ( | ) | ||||||||||
| Proceeds from (prepayments for) related parties borrowings | ( | ) | ( | ) | ||||||||
| Net cash (used in) provided by financing activities | ( | ) | ||||||||||
| Effect of exchange rate changes on cash | ( | ) | ( | ) | ||||||||
| Net (decrease) increase in cash | ( | ) | ( | ) | ( | ) | ||||||
| Cash, beginning of year | ||||||||||||
| Cash, end of year | $ | $ | $ | |||||||||
| Supplemental disclosure information | ||||||||||||
| Cash paid for interest expense | $ | $ | $ | |||||||||
| Cash paid for income tax | $ | $ | $ | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
UNIVERSE PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 — ORGANIZATION AND BUSINESS DESCRIPTION
Universe
Pharmaceuticals Inc. (“Universe INC” or the “Company”) was incorporated under the laws of the Cayman Islands on
Universe
INC owns
Jiangxi Universe Pharmaceuticals Technology Co., Ltd. (“Universe Technology”) was formed on April 8, 2019, as a wholly foreign-owned enterprise (“WFOE”) in the People’s Republic of China (“PRC” or “China”).
Universe INC, Universe HK and Universe Technology are currently not engaging in any active business operations and are merely acting as holding companies.
Jiangxi
Universe Pharmaceuticals Co., Ltd. (“Jiangxi Universe”) was incorporated on
Reorganization
A reorganization
of the Company’s legal structure (the “Reorganization”) was completed on December 11, 2019. The Reorganization involved
the incorporation of Universe INC and Universe Technology, and the transfer of
The Reorganization has been accounted for as a recapitalization among entities under common control, since the same controlling shareholders controlled all these entities before and after the Reorganization. The consolidation of the Company and its subsidiaries has been accounted for at historical cost and prepared on the basis as if the aforementioned transactions had become effective as of the beginning of the first period presented in the accompanying consolidated financial statements. Results of operations for the periods presented comprise those of the previously separate entities combined from the beginning of the period to the end of the period, eliminating the effects of intra-entity transactions.
On March
25, 2021, the Company closed its initial public offering (the “IPO”) of
On May 12, 2021, through the Company’s PRC subsidiary, Jiangxi Universe, the Company established a wholly controlled subsidiary, Guangzhou Universe Hanhe Medical Research Co., Ltd. (“Universe Hanhe”) in Guangzhou City, China, for the business purpose of conducting research and development of new pharmaceutical products in order to diversify the Company’s product offerings in the near future. As of September 30, 2023 and as of the date of this report, Universe Hanhe has no active business operations.
F-7
On July 3, 2023, the Company held an annual general meeting of shareholders at which shareholders, among other things, resolved:
| (a) | with immediate effect, to increase the Company’s authorized share capital from US$ |
| (b) | that, conditional upon the approval of the board of directors of the Company in its sole discretion, with
effect as of the date the board of directors of the Company may determine, the authorized, issued and outstanding shares of the Company
be consolidated by consolidating each 10 shares of the Company, or such lesser whole share amount as the board of directors may determine
in its sole discretion, such amount not to be less than 2, into 1 share of the Company, with such consolidated shares having the same
rights and being subject to the same restrictions (save as to nominal value) as the then existing shares of par value US$ |
| (c) | that, upon the effectiveness of the Share Consolidation, the Company adopt amended and restated articles of association, in substantially the form set out in Annex B in the proxy statement dated 24 May 2023, in substitution for and to the exclusion of, the memorandum of association of the Company in effect immediately prior to effectiveness of the Share Consolidation. |
The board of directors
of the Company resolved to effect the Share Consolidation on July 27, 2023 with the authorized, issued and outstanding shares to be consolidated
on a six (6) for one (1) ratio, which had the effect of reducing the number of: (a) authorized ordinary shares from
F-8
| Date of | Place of | % of | ||||||
| Name of Entity | Incorporation | Incorporation | Ownership | Principal Activities | ||||
| Universe INC | Parent, | |||||||
| Universe HK | ||||||||
| Universe Technology | ||||||||
| Jiangxi Universe | ||||||||
| Universe Trade | ||||||||
| Universe Hanhe |
The Company, through its wholly-owned subsidiaries, is primarily engaged in the development, manufacturing and sale of traditional Chinese medicines derivatives (“TCMD”) products targeted to the elderly to address their physical conditions in the aging process and to promote their general well-being. In addition, the Company also sells biochemical drugs, medical instruments, traditional Chinese medicine pieces products and dietary supplements (collectively, “third-party products”). All of these TCMD and third-party products are currently sold to customers including pharmaceutical companies, hospitals, clinics and drugstore chains throughout China.
NOTE 2 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of consolidation
The accompanying consolidated financial statements include the financial statements of Universe INC, Universe HK, Universe Technology, Jiangxi Universe, Universe Trade and Universe Hanhe. All inter-company balances and transactions are eliminated upon consolidation.
Uses of estimates
In preparing the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America (“US GAAP”), management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. These estimates are based on information as of the date of the consolidated financial statements. Significant estimates required to be made by management include, but are not limited to, the allowance for estimated uncollectible receivables, the realizability of advance to suppliers, inventory valuations, useful lives of property and equipment, intangible assets, the recoverability of long-lived assets, provision necessary for contingent liabilities, revenue recognition and realization of deferred tax assets. Actual results could differ from those estimates.
F-9
Risks and Uncertainties
The business operations of the Company are located in the PRC. Accordingly, the Company’s business, financial condition, and results of operations may be influenced by political, economic, and legal environments in the PRC, as well as by the general state of the PRC economy. The Company’s results may be adversely affected by changes in the political, regulatory and social conditions in the PRC. Although the Company has not experienced losses from these situations and believes that it is in compliance with existing laws and regulations including its organization and structure disclosed in Note 1, this may not be indicative of future results.
The development and commercialization of new pharmaceutical products is highly competitive, and the industry currently is characterized by rapidly changing technologies, significant competition and a strong emphasis on intellectual property. The Company may face competition with respect to its current and future pharmaceutical product candidates from major pharmaceutical companies in China.
The Company’s business, financial condition and results of operations may also be negatively impacted by risks related to natural disasters, extreme weather conditions, health epidemics and other catastrophic incidents, which could significantly disrupt the Company’s operations.
Cash
Cash includes currency on hand and deposits held by banks that can be added or withdrawn without limitation. The Company maintains most of its bank accounts in the PRC. Cash balances in bank accounts in the PRC are not insured by the Federal Deposit Insurance Corporation or other programs.
Accounts receivable, net
Accounts
receivables are presented net of allowance for doubtful accounts. The Company determines the adequacy of reserves for doubtful accounts
based on individual account analysis and historical collection trends. The Company establishes a provision for doubtful receivables when
there is objective evidence that the Company may not be able to collect amounts due. The allowance is based on management’s best
estimate of specific losses on individual exposures, as well as a provision on historical trends of collections. Actual amounts received
may differ from management’s estimate of credit worthiness and the economic environment. Delinquent account balances are written-off
against the allowance for doubtful accounts after management has determined that collection is not probable. Allowance for uncollectable
balances amounted to $
Inventories, net
Inventories
are stated at net realizable value using weighted average method. Costs include the cost of raw materials, freight, direct labor and related
production overhead. Any excess of the cost over the net realizable value of each item of inventories is recognized as a provision for
diminution in the value of inventories. Net realizable value is the estimated selling price in the normal course of business less any
costs to complete and sell products. The Company evaluates inventories on a quarterly basis for its net realizable value adjustments,
and reduces the carrying value of those inventories that are obsolete or in excess of the forecasted usage to their estimated net realizable
value based on various factors including aging, expiration dates, as applicable, taking into consideration historical and expected future
product sales. The Company recorded inventory reserve of $
F-10
Advances to suppliers, net
Advances to suppliers represent prepayments made to ensure continuous high-quality supplies and favorable purchase prices of raw materials. These advances are directly related to the purchases of raw materials used to fulfill sales orders. The Company is required from time to time to make cash advances when placing its purchase orders. These advances are settled upon suppliers delivering raw materials to the Company when the transfer of ownership occurs. The Company reviews its advances to suppliers on a periodic basis and makes general and specific allowances when there is doubt as to the ability of a supplier to provide supplies to the Company or refund an advance. As of September 30, 2023 and 2022, no allowance for doubtful accounts was deemed necessary, as the Company believes that all advances to suppliers are fully realizable.
Short-term investments
The Company’s short-term investments consist of wealth management financial products purchased from a financial institution, which can be redeemed anytime. The financial institution invests the Company’s fund in certain financial instruments including debt and money market securities to generate investment income. The short-term investments are deemed to be trading securities and are measured subsequently at fair value in the statement of financial position. Unrealized holding gains and losses for investment are included in the consolidated statements of operations and comprehensive income over the investment period (see Note 7).
Fair value of financial instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. A three-level fair value hierarchy prioritizes the inputs used to measure fair value. The hierarchy requires entities to maximize the use of observable inputs and minimize the use of unobservable inputs. The three levels of inputs used to measure fair value are as follows:
| ● | Level 1 — | inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. |
| ● | Level 2 — | inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, quoted market prices for identical or similar assets in markets that are not active, inputs other than quoted prices that are observable and inputs derived from or corroborated by observable market data. |
| ● | Level 3 — | inputs to the valuation methodology are unobservable. |
Unless otherwise disclosed, the fair value of the Company’s financial instruments, including cash, accounts receivable, inventories, short-term investments, advances to suppliers, prepaid expenses and other current assets, accounts payable, short-term bank loans, accrued expenses and other current liabilities, taxes payable and due to related parties, approximate the fair value of the respective assets and liabilities as of September 30, 2023 and 2022 based upon the short-term nature of the assets and liabilities. The Company’s investment in equity securities is accounted for using the measurement alternative in accordance with Accounting Standards Codification (“ASC”) 321, “Investments—Equity Securities” (“ASC 321”), which also approximates its recorded value.
The following table presents information about the Company’s assets measured at fair value on a recurring basis as of September 30, 2023 and 2022, and indicates the fair value hierarchy of the valuation techniques that the Company utilized to determine such fair value.
| Fair Value Measured as of September 30, 2023 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Assets: | ||||||||||||||||
| Short-term investments | $ | 13,219,005 | $ | - | $ | - | $ | 13,219,005 | ||||||||
| Fair Value Measured as of September 30, 2022 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Assets: | ||||||||||||||||
| Short-term investments | $ | 13,148,594 | $ | - | $ | - | $ | 13,148,594 | ||||||||
F-11
Property and equipment
| Useful life | ||
| Buildings | ||
| Machinery and equipment | ||
| Automobiles | ||
| Office and electric equipment |
Expenditures for maintenance and repairs, which do not materially extend the useful lives of the assets, are charged to expense as incurred. Expenditures for major renewals and betterments which substantially extend the useful life of assets are capitalized. The cost and related accumulated depreciation of assets retired or sold are removed from the respective accounts, and any gain or loss is recognized in the consolidated statements of income and other comprehensive income in other income or expenses.
The Company reviews the carrying value of property and equipment for impairment whenever events and circumstances indicate that the carrying value of an asset may not be recoverable from the estimated future cash flows expected to result from its use and eventual disposition. In cases where undiscounted expected future cash flows are less than the carrying value, an impairment loss is recognized equal to an amount by which the carrying value exceeds the fair value of assets. The factors considered by management in performing this assessment include current operating results, trends and prospects, the manner in which the property is used, and the effects of obsolescence, demand, competition and other economic factors. Based on this assessment, no impairment expenses for property and equipment were recorded in operating expenses for the years ended September 30, 2023, 2022 and 2021.
Intangible Assets
| Useful life | ||
| Land use rights | ||
| Software |
The Company reviews the carrying value of land use rights for impairment whenever events and circumstances indicate that the carrying value of an asset may not be recoverable from the estimated future cash flows expected to result from its use and eventual disposition. In cases where undiscounted expected future cash flows are less than the carrying value, an impairment loss is recognized equal to an amount by which the carrying value exceeds the fair value of assets. The factors considered by management in performing this assessment include current operating results, trends and prospects, the manner in which the property is used, and the effects of obsolescence, demand, competition and other economic factors. Based on this assessment, no impairment of land use rights was deemed necessary for the years ended September 30, 2023, 2022 and 2021.
F-12
Construction-in-Progress (“CIP”)
CIP represents property and buildings under construction and consists of construction expenditures, equipment procurement, and other direct costs attributable to the construction. CIP is not depreciated. Upon completion and ready for intended use, CIP is reclassified to the appropriate category within property and equipment.
Impairment of long-lived Assets
Long-lived assets with finite lives, primarily property and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. If the estimated undiscounted cash flows from the use of the asset and its eventual disposition below are the asset’s carrying value, then the asset is deemed to be impaired and written down to its fair value. There were no impairments of these assets as of September 30, 2023 and 2022.
Investments in Equity Securities
The Company
accounts for its equity investments in accordance with ASC 321. In accordance with ASC 321, equity investment which the Company has no
significant influence (generally less than a
From March
2009 to September 2017, the Company invested approximately $
The Company
initially recorded the investments at historical cost and subsequently records any dividends received from the net accumulated earnings
of the investee as income. As of September 30, 2023 and 2022, the Company’s investment in JX RBC Bank amounted to $
The investments in equity securities are evaluated for impairment when facts or circumstances indicate that the fair value of the investment is less than its carrying value. An impairment is recognized when a decline in fair value is determined to be other-than-temporary. The Company reviews several factors to determine whether a loss is other-than-temporary. These factors include, but are not limited to, the: (i) nature of the investment; (ii) cause and duration of the impairment; (iii) extent to which fair value is less than cost; (iv) financial condition and near-term prospects of the investments; and (v) ability to hold the investment for a period of time sufficient to allow for any anticipated recovery in fair value. There was no impairment for the Company’s investments in equity securities as of September 30, 2023 and 2022.
F-13
Revenue recognition
To determine revenue recognition for contracts with customers, the Company performs the following five steps : (i) identify the contract(s) with the customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, including variable consideration to the extent that it is probable that a significant future reversal will not occur, (iv) allocate the transaction price to the respective performance obligations in the contract, and (v) recognize revenue when (or as) the Company satisfies the performance obligation.
The Company recognizes revenue when it transfers its goods and services to customers in an amount that reflects the consideration to which the Company expects to be entitled in such exchange. The Company accounts for the revenue generated from sales of its TCMD and third-party products on a gross basis, as the Company is acting as a principal in these transactions, is subject to inventory risk, has latitude in establishing prices, and is responsible for fulfilling the promise to provide customers the specified goods, which the Company has control of the goods and has the ability to direct the use of goods to obtain substantially all the benefits. All of the Company’s contracts have one single performance obligation, namely, the promise is to transfer the individual goods to customers, and there is no separately identifiable other promise in the contracts. The Company’s revenue streams are recognized at a point in time when title and risk of loss passes and the customer accepts the goods, which generally occurs at delivery. The Company’s products are sold with no right of return and the Company does not provide other credits or sales incentive to customers. Revenue is reported net of all value added taxes (“VAT”).
F-14
Disaggregation of Revenues
The Company disaggregates its revenue from contracts by product types, as the Company believes it best depicts how the nature, amount, timing and uncertainty of the revenue and cash flows are affected by economic factors.
Cost of Revenue
Cost of revenue consists primarily of the costs of raw materials, freight charges, direct labor, depreciation of buildings and machinery, warehousing and overhead associated with the manufacturing process.
Research and Development Expenses
The Company
expenses all internal research and development costs as incurred, which primarily comprise of employee costs, internal and external costs
related to execution of studies, manufacturing costs, facility costs of the research center, and amortization and depreciation to intangible
assets and property and equipment used in the research and development activities. For the years ended September 30, 2023, 2022 and 2021,
total research and development expenses were approximately $
Shipping and handling costs
Shipping and handling costs are expensed as incurred. Inbound shipping and handling cost associated with bringing the purchased raw materials and third-party products from suppliers to the Company’s warehouse are included in cost of revenue. Outbound shipping and handling costs associated with shipping and delivery the products to customers are included in selling expenses.
Advertising expense
Advertising
expenses primarily relate to promotion of the Company’s brand name and products through outdoor billboards, social media such as
Weibo and WeChat, and TV advertisement. Advertising costs is expensed as incurred or deferred and then expensed the first time the advertising
takes place. Advertising expenses are included in selling expenses in the consolidated statements of income and comprehensive income.
Advertising expenses amounted to $
F-15
Segment Reporting
The Company uses the management approach in determining reportable operating segments. The management approach considers the internal reporting used by the Company’s chief operating decision maker for making operating decisions about the allocation of resources of the segment and the assessment of its performance in determining the Company’s reportable operating segments. Management has determined that the Company has one operating segment.
Income taxes
The Company accounts for current income taxes in accordance with the laws of the relevant tax authorities. Deferred income taxes are recognized when temporary differences exist between the tax bases of assets and liabilities and their reported amounts in the consolidated financial statements. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period including the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
An uncertain
tax position is recognized only if it is “more likely than not” that the tax position would be sustained in a tax examination.
The amount recognized is the largest amount of tax benefit that is greater than
The Company’s operating subsidiaries in China are subject to the income tax laws of the PRC. No significant income was generated outside the PRC for the years ended September 30, 2023, 2022 and 2021. As of September 30, 2023 and 2022, all of the tax returns of the Company’s PRC subsidiaries remained open for statutory examination by PRC tax authorities.
Value added tax (“VAT”)
Sales revenue
represents the invoiced value of goods, net of VAT. The VAT is based on gross sales price and VAT rates range up to
F-16
Earnings per Share
The Company computes earnings per share (“EPS”) in accordance with ASC 260, “Earnings per Share” (“ASC 260”). ASC 260 requires companies with complex capital structures to present basic and diluted EPS. Basic EPS is measured as net income divided by the weighted average ordinary shares outstanding for the period. Diluted presents the dilutive effect on a per share basis of potential ordinary shares (e.g., convertible securities, options and warrants) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential ordinary shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS. For the years ended September 30, 2023, 2022 and 2021, there were no dilutive shares.
Foreign currency translation
The functional currency for Universe INC is the U.S. Dollar (“US$”). Universe HK uses Hong Kong dollar as its functional currency. However, Universe INC and Universe HK currently only serve as holding companies and did not have active operations as of the date of this report. The Company operates only in the PRC and the Company’s functional currency is the Chinese Yuan (“RMB”). The Company’s consolidated financial statements have been translated into the reporting currency US$.
Assets and liabilities of the Company are translated at the exchange rate at each reporting period end date. Equity is translated at historical rates. Income and expense accounts are translated at the average rate of exchange during the reporting period. The resulting translation adjustments are reported under other comprehensive income. Gains and losses resulting from the translations of foreign currency transactions and balances are reflected in the results of operations.
The RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into US$ at the rates used in translation.
| September 30, 2023 |
September 30, 2022 |
September 30, 2021 | ||||
| Year-end spot rate | US$ |
US$ |
US$ | |||
| Average rate | US$ |
US$ |
US$ |
F-17
Comprehensive Income
Comprehensive income consists of two components, net income and other comprehensive income. The foreign currency translation gain resulting from translation of the financial statements expressed in RMB to US$ is reported in other comprehensive income in the consolidated statements of income and comprehensive income.
Statement of Cash Flows
In accordance with ASC 230, “Statement of Cash Flows”, cash flows from the Company’s operations are formulated based upon the local currencies. As a result, amounts related to assets and liabilities reported on the statements of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheets.
Employee Defined Contribution Plan
The Company’s
subsidiaries in the PRC participate in a government-mandated multi-employer defined contribution plan pursuant to which pension, work-related
injury benefits, maternity insurance, medical insurance, unemployment benefit and housing fund are provided to eligible full-time employees.
The relevant labor regulations require the Company’s subsidiaries in the PRC to pay the local labor and social welfare authorities
monthly contributions based on the applicable benchmarks and rates stipulated by the local government. The contributions to the plan are
expensed as incurred. Employee social security and welfare benefits included as expenses in the accompanying statements of income and
comprehensive income amounted to $
Recent Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, “Financial Instruments — Credit Losses” (Topic 326), which will require the measurement of all expected credit losses for financial assets held at the reporting date based on historical experience, current conditions, and reasonable and supportable forecasts. Subsequently, the FASB issued ASU No. 2018-19, Codification Improvements to Topic 326, to clarify that receivables arising from operating leases are within the scope of lease accounting standards. Further, the FASB issued ASU No. 2019-04, ASU 2019-05, ASU 2019-10, ASU 2019-11 and ASU 2020-02 to provide additional guidance on the credit losses standard. For all other entities, the amendments for ASU 2016-13 are effective for fiscal years beginning after December 15, 2022, including interim periods within those fiscal years, with early adoption permitted. Adoption of the ASUs is on a modified retrospective basis. The Company adopted ASU 326 effective October 1, 2022, the first day of the Company’s fiscal year ending September 30, 2023. The adoption of ASU 326 did not have a material impact on the Company’s financial position, results of operations or cash flows.
F-18
Other accounting standards that have been issued by FASB that do not require adoption until a future date are not expected to have a material impact on the consolidated financial statements upon adoption. The Company does not discuss recent standards that are not anticipated to have an impact on or are unrelated to its consolidated financial condition, results of operations, cash flows or disclosures.
NOTE 3 — ACCOUNTS RECEIVABLE, NET
| As of September 30, | ||||||||
| 2023 | 2022 | |||||||
| Accounts receivable | $ | $ | ||||||
| Less: allowance for doubtful accounts | ( | ) | ( | ) | ||||
| Accounts receivable, net | $ | $ | ||||||
The Company’s
accounts receivable primarily includes the balance due from customers when the Company’s pharmaceutical products are sold and delivered
to customers. As of date of this report, approximately
| As of September 30, | ||||||||
| 2023 | 2022 | |||||||
| Beginning balance | $ | $ | ||||||
| Additions | ||||||||
| Bad debt recovery | ( |
) | ||||||
| Foreign currency translation adjustments | ( |
) | ( |
) | ||||
| Ending balance | $ | $ | ||||||
NOTE 4 — INVENTORY, NET
| As of September 30, | ||||||||
| 2023 | 2022 | |||||||
| Raw materials | $ | $ | ||||||
| Finished goods | ||||||||
| Inventory valuation allowance | ( |
) | ( |
) | ||||
| Total inventory, net | $ | $ | ||||||
F-19
NOTE 5 — ADVANCE TO SUPPLIERS
| As of September 30, | ||||||||
| 2023 | 2022 | |||||||
| Advances to suppliers for inventory raw material purchase | $ | $ | ||||||
| Less: allowance for doubtful accounts | ||||||||
| Advances to suppliers | $ | $ | ||||||
Advances to suppliers represent prepayments made to suppliers to ensure continuous high-quality supplies and favorable purchase prices of raw materials.
NOTE 6 — PREPAYMENT FOR ACQUISITION
On September
26, 2022, the Company entered into a letter of intent for an equity transfer with an individual, Mr. Xibo Liu, pursuant to which, Mr.
Xibo Liu transfers his
NOTE 7 — SHORT-TERM INVESTMENTS
The Company’s
short-term investments consist of wealth management financial products purchased from financial institution, which can be redeemed anytime
at the Company’s discretion.
| As of September 30, | ||||||||
| 2023 | 2022 | |||||||
| Beginning balance | $ | $ | ||||||
| Accrued investment income (loss) | ( |
) | ||||||
| Foreign currency translation adjustments | ( |
) | ||||||
| Ending balance of short-term investments | $ | $ | ||||||
Investment
income (loss) generated from such short-term investments amounted to $
F-20
NOTE 8 — PROPERTY AND EQUIPMENT, NET
| As of September 30, | ||||||||||
| Useful life | 2023 | 2022 | ||||||||
| Buildings | $ | $ | ||||||||
| Machinery and equipment | ||||||||||
| Automobiles | ||||||||||
| Office and electric equipment | ||||||||||
| Subtotal | ||||||||||
| Less: accumulated depreciation | ( | ) | ( | ) | ||||||
| Property and equipment, net | $ | $ | ||||||||
Depreciation
expense was $
NOTE 9 — INTANGIBLE ASSETS, NET
| As of September 30, | ||||||||||
| Useful life | 2023 | 2022 | ||||||||
| Land use rights | $ | $ | ||||||||
| Software | ||||||||||
| Total | ||||||||||
| Less: accumulated amortization | ( | ) | ( | ) | ||||||
| Intangible assets, net | $ | $ | ||||||||
Amortization
expense was $
| Years ending September 30, | Amortization | ||||
| 2024 | $ | ||||
| 2025 | |||||
| 2026 | |||||
| 2027 | |||||
| 2028 | |||||
| Thereafter | |||||
| $ | |||||
F-21
NOTE 10 — PREPAYMENT FOR CIP PROJECT
CIP represents
direct costs of construction incurred for the Company’s manufacturing facilities. On June 25, 2021, the Company signed a construction
sub-contract with sub-contractor Jiangxi Chenyuan Construction Project Co., Ltd. (“Chenyuan”), pursuant to which, Chenyuan
agreed to help the Company construct four manufacturing factory buildings and an office building with a total estimated budget of RMB
As of September
30, 2023, $
As of September
30, 2023, future additional capital expenditure on this CIP project is estimated to be approximately RMB
| Years ending September 30, | Capital | ||||
| 2024 | $ | ||||
| 2025 | |||||
| 2026 | |||||
| Total | $ | ||||
NOTE 11 — PREPAYMENT FOR PURCHASE OF A PROPERTY
On
May 6, 2021, the Company entered into a real estate property purchase agreement with related party Jiangxi Yueshang Investment Co., Ltd.
(“Jiangxi Yueshang”), an entity in which the Company’s chief executive officer, Mr. Gang Lai, owned
As
of September 30, 2023, the Company had made a prepayment of RMB
F-22
NOTE 12 — SHORT-TERM BANK LOANS
| As of September 30, | ||||||||||||
| Note | 2023 | 2022 | ||||||||||
| Short-term bank loans: | ||||||||||||
| Jiangxi Luling Rural Commercial Bank (“LRC Bank”) | (1) | $ | $ | |||||||||
| Bank of Communications Co., Ltd | (2) | |||||||||||
| Zhujiang Rural Bank | (3) | |||||||||||
| Beijing Bank | (4) | |||||||||||
| Total short-term loans | $ | $ | ||||||||||
| (1) | On March 14, 2022, the Company’s subsidiary, Universe
Trade, signed a loan agreement with LRC Bank to borrow RMB |
On June 15, 2022, the Company’s subsidiary,
Jiangxi Universe, entered into a loan agreement with LRC Bank to borrow RMB
On March 13, 2023, a subsidiary of the Company,
Universe Trade, signed a loan agreement with LRC Bank to borrow RMB
On June 15, 2023, a subsidiary of the Company,
Universe Trade, signed a loan agreement with LRC Bank to borrow RMB
F-23
| (2) | On June 24, 2022, the Company’s subsidiary, Jiangxi
Universe, signed a loan agreement with Bank of Communications to borrow RMB |
On June 15, 2023, the Company’s subsidiary,
Jiangxi Universe, signed a loan agreement with Bank of Communications to borrow RMB
| (3) | On May 5, 2023, a subsidiary of the Company, Jiangxi Universe, entered into a loan agreement with Zhujiang Rural Bank to borrow RMB |
| (4) | On July 24, 2023, a subsidiary of the Company, Jiangxi Universe, entered into a loan agreement with Beijing Bank to borrow RMB |
For the
above-mentioned loans, the Company recorded a total interest expense of $
NOTE 13 — RELATED PARTY TRANSACTIONS
| Name | Relationship with the Company | |
| Mr. Gang Lai | ||
| Ms. Lin Yang | ||
| Ms. Xing Wu | ||
| Mr. Yajun Hu |
| As of September 30, | ||||||||
| Name | 2023 | 2022 | ||||||
| Mr. Yajun Hu | $ | $ | ||||||
| Total due to related parties | $ | $ | ||||||
As of September 30, 2023, the balance due from related parties consisted of advanced funds to the Company’s director and general manager, Mr. Yajun Hu. These advances are non-interest bearing and fully repaid on October 12, 2023.
F-24
| As of September 30, | ||||||||
| Name | 2023 | 2022 | ||||||
| Mr. Gang Lai | $ | $ | ||||||
| Total due to related parties | $ | $ | ||||||
As of September 30, 2023 and 2022, the balance due to related parties mainly consisted of advances from the Company’s officers for working capital purposes during the Company’s normal course of business. These advances are non-interest bearing and due on demand.
(d) Loan guarantee provided by related parties
In connection with the Company’s bank borrowings from PRC Banks, certain related parties of the Company, including Mr. Gang Lai, the Company’s controlling shareholder, chairman of the board of directors, chief executive officer, Ms. Lin Yang, the Company’s chief financial officer, and Ms. Xing Wu, Mr. Gang Lai’s spouse, jointly signed guarantee agreements with these banks to provide credit guarantee for the Company’s certain loans (see Note 12).
(e) Prepayment to related party for property purchase
As disclosed
in Note 11, on May 6, 2021, the Company entered into a real estate property purchase agreement with a related party, Jiangxi Yueshang,
to purchase certain residential apartment and commercial office space totaling
On January
13, 2022, Mr. Gang Lai transferred the
NOTE 15 — TAXES
(a) Corporate Income Taxes (“CIT”)
Cayman Islands
Under the current tax laws of the Cayman Islands, Universe INC is not subject to tax on its income or capital gains. In addition, no Cayman Islands withholding tax will be imposed upon the payment of dividends by the Company to its shareholders.
F-25
Hong Kong
Universe
HK is incorporated in Hong Kong and is subject to profit taxes in Hong Kong at a rate of
PRC
Under the
Enterprise Income Tax (“EIT”) Law of PRC, domestic enterprises and Foreign Investment Enterprises (“FIEs”) are
usually subject to a unified
| For the Years Ended September 30, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Current tax provision: | ||||||||||||
| Cayman | $ | $ | $ | |||||||||
| Hong Kong | ||||||||||||
| PRC | ||||||||||||
| Sub-total | ||||||||||||
| Deferred tax provision (benefit): | ||||||||||||
| Cayman | ||||||||||||
| Hong Kong | ||||||||||||
| PRC | ( | ) | ( | ) | ||||||||
| Sub-total | ( | ) | ( | ) | ||||||||
| Total income tax provision | $ | $ | $ | |||||||||
F-26
| For the Years Ended September 30, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Statutory PRC income tax rate | % | % | % | |||||||||
| Effect of income tax holiday | ( | )% | ( | )% | ( | )% | ||||||
| Permanent difference | ( | )% | ( | )% | % | |||||||
| Non-PRC entities not subject to PRC income tax | ( | )% | ( | )% | % | |||||||
| Impact on DTA due to change in applicable income tax rate | ( | )% | % | % | ||||||||
| Change in valuation allowance | ( | )% | % | % | ||||||||
| Effective tax rate | ( | )% | ( | )% | % | |||||||
The Company continually evaluates expiring statutes of limitations, audits, proposed settlements, changes in tax law and new authoritative rulings. As of September 30, 2023, all of the Company’s tax returns of its PRC subsidiaries remain open for statutory examination by PRC tax authorities.
| As of September 30, | ||||||||
| 2023 | 2022 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carry-forwards | $ | $ | ||||||
| Allowance for doubtful accounts | ||||||||
| Total | ||||||||
| Valuation allowance | ||||||||
| Total deferred tax assets | $ | $ | ||||||
The Company periodically evaluates the likelihood of the realization of deferred tax assets, and reduces the carrying amount of the deferred tax assets by a valuation allowance to the extent it believes a portion will not be realized. Management considers new evidence, both positive and negative, that could affect the Company’s future realization of deferred tax assets including its recent cumulative earnings experience, expectation of future income, the carry forward periods available for tax reporting purposes and other relevant factors. Although Jiangxi Universe and Universe Trade incurred net loss during the years ended September 30, 2023 and 2022, the Company expected Jiangxi Universe and Universe Trade would generate net income in the fiscal year ending September 30, 2024, and determined that it was more likely than not that part of its deferred tax assets could be realized due to the estimated future earnings, and recognized valuation allowance for the part of deferred tax assets that was not expected to be realized.
(b) Taxes payable
| As of September 30, | ||||||||
| 2023 | 2022 | |||||||
| Value added tax payable | $ | $ | ||||||
| Other taxes payable | ||||||||
| Total taxes payable | $ | $ | ||||||
F-27
NOTE 15 — CONCENTRATIONS
A majority of the Company’s revenue and expense transactions are denominated in RMB and a significant portion of the Company’s and its subsidiaries’ assets and liabilities are denominated in RMB. RMB is not freely convertible into foreign currencies. In the PRC, certain foreign exchange transactions are required by law to be transacted only by authorized financial institutions at exchange rates set by the People’s Bank of China (“PBOC”). Remittances in currencies other than RMB by the Company in China must be processed through the PBOC or other China foreign exchange regulatory bodies which require certain supporting documentation in order to affect the remittance.
As of September
30, 2023 and 2022, $
For the
years ended September 30, 2023, 2022 and 2021, no single customer accounted for more than
Sales of
one of the Company’s major products, Guben Yanling Pill, accounted for
As of September
30, 2023 and 2022, no customer accounted for more than
For the
year ended September 30, 2023, one supplier accounted for
NOTE 16 — SHAREHOLDERS’ EQUITY
Ordinary Shares
Universe
INC was incorporated under the laws of the Cayman Islands on December 11, 2019. The original authorized number of ordinary shares upon
incorporation was
Initial Public Offering
On March
25, 2021, the Company closed its IPO of
F-28
Increased authorized share capital and share consolidation
On July 3, 2023, the Company held an annual general meeting of shareholders at which shareholders, among other things, resolved:
| (a) | with immediate effect, to increase the Company’s authorized share capital from US$ |
| (b) | that, conditional upon the approval of the board of directors of the Company in its sole discretion, with
effect as of the date the board of directors of the Company may determine, the authorized, issued and outstanding shares of the Company
be consolidated by consolidating each 10 shares of the Company, or such lesser whole share amount as the board of directors may determine
in its sole discretion, such amount not to be less than 2, into 1 share of the Company, with such consolidated shares having the same
rights and being subject to the same restrictions (save as to nominal value) as the then existing shares of par value US$ |
| (c) | that, upon the effectiveness of the Share Consolidation, the Company adopt amended and restated articles of association, in substantially the form set out in Annex B in the proxy statement dated 24 May 2023, in substitution for and to the exclusion of, the memorandum of association of the Company in effect immediately prior to effectiveness of the Share Consolidation. |
The board
of directors of the Company resolved to effect the Share Consolidation at 5:00 p.m. (Cayman Islands time) on July 27, 2023 with the authorized,
issued and outstanding shares to be consolidated on a six (6) for one (1) ratio, which had the effect of reducing the number of: (a) authorized
ordinary shares from
As of September
30, 2023 and 2022, the Company had a total of
Underwriter warrants
In connection
with the Company’s IPO, the Company also agreed to issue warrants to the underwriter, for a nominal consideration of $
F-29
Cash dividends
On September
21, 2016, September 13, 2017, February 2, 2018, September 20, 2018 and February 21, 2019, the board of directors of Jiangxi Universe approved
resolutions to pay cash dividends of RMB
Except for the dividends declared mentioned above, the Company has not declared or paid dividends to its shareholders in the past, and may not choose to make additional distributions in the future. Any decision as to the payment of dividends will depend on the available earnings, the capital requirements of the Company, the Company’s general financial condition and other factors deemed pertinent by the board of directors.
Statutory reserve and restricted net assets
The Company’s PRC subsidiaries are restricted in their ability to transfer a portion of their net assets to the Company. The payment of dividends by entities organized in China is subject to limitations, procedures and formalities. Regulations in the PRC currently permit payment of dividends only out of accumulated profits as determined in accordance with accounting standards and regulations in China.
The Company
is required to make appropriations to certain reserve funds, comprising the statutory surplus reserve and the discretionary surplus reserve,
based on after-tax net income determined in accordance with generally accepted accounting principles of the PRC (“PRC GAAP”).
Appropriations to the statutory surplus reserve are required to be at least
Relevant
PRC laws and regulations restrict the Company’s PRC subsidiaries from transferring a portion of their net assets, equivalent to
their statutory reserves and their share capital, to the Company in the form of loans, advances or cash dividends. Only PRC entities’
accumulated profits may be distributed as dividends to the Company without the consent of a third party. As of September 30, 2023 and
2022, the restricted amounts as determined pursuant to PRC statutory laws totaled $
F-30
NOTE 17 — COMMITMENTS AND CONTINGENCIES
From time to time, the Company is a party to various legal actions arising in the ordinary course of business. The Company accrues costs associated with these matters when they become probable and the amount can be reasonably estimated. Legal costs incurred in connection with loss contingencies are expensed as incurred. For the years ended September 30, 2023, 2022 and 2021, the Company did not have any material legal claims or litigation that, individually or in aggregate, could have a material adverse impact on the Company’s consolidated financial position, results of operations and cash flows.
The Company
has an ongoing CIP project associated with the construction of a new manufacturing facility. As of September 30, 2023, future minimum
capital expenditures on the Company’s CIP project amounted to approximately $
On May 6,
2021, the Company entered into a real estate property purchase agreement with Jiangxi Yueshang, an entity in which the Company’s
chief executive officer, Mr. Gang Lai, owned
NOTE 18 — SUBSEQUENT EVENTS
On November 23, 2023,
a subsidiary of the Company, Jiangxi Universe, entered into a loan agreement with Jiangxi Luling Rural Commercial Bank (“LRC Bank”)
to borrow RMB
NOTE 19 — CONDENSED FINANCIAL INFORMATION OF THE PARENT COMPANY
Pursuant
to the requirements of Rule 12-04(a), 5-04(c) and 4-08(e)(3) of Regulation S-X, the condensed financial information of the parent company
shall be filed when the restricted net assets of consolidated subsidiaries exceed 25 percent of consolidated net assets as of the end
of the most recently completed fiscal year. The Company performed a test on the restricted net assets of consolidated subsidiaries in
accordance with such requirement and concluded that it was applicable to the Company as the restricted net assets of the Company’s
subsidiaries exceeded
For purposes of the above test, restricted net assets of consolidated subsidiaries shall mean that amount of the Company’s proportionate share of net assets of consolidated subsidiaries (after intercompany eliminations) which as of the end of the most recent fiscal year may not be transferred to the parent company by subsidiaries in the form of loans, advances or cash dividends without the consent of a third party.
The condensed financial information of the parent company has been prepared using the same accounting policies as set out in the Company’s consolidated financial statements except that the parent company used the equity method to account for investment in its subsidiaries. Such investment is presented on the condensed balance sheets as “Investment in subsidiaries” and the respective profit or loss as “Equity in earnings of subsidiaries” on the condensed statements of income.
The footnote disclosures contain supplemental information relating to the operations of the Company and, as such, these statements should be read in conjunction with the notes to the consolidated financial statements of the Company. Certain information and footnote disclosures normally included in financial statements prepared in accordance with U.S GAAP have been condensed or omitted.
F-31
UNIVERSE PHARMACEUTICALS INC. AND SUBSIDIARIES
PARENT COMPANY BALANCE SHEETS
| As of September 30, | ||||||||
| 2023 | 2022 | |||||||
| ASSETS | ||||||||
| Cash | $ | $ | ||||||
| Short-term investments | ||||||||
| Total current assets | ||||||||
| Non-current assets | ||||||||
| Investment in subsidiaries | $ | $ | ||||||
| Total assets | $ | $ | ||||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| LIABILITIES | $ | $ | ||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||
| SHAREHOLDERS’ EQUITY | ||||||||
| Ordinary shares, $ | ||||||||
| Additional paid-in capital | ||||||||
| Retained earnings | ||||||||
| Accumulated other comprehensive income | ( | ) | ( | ) | ||||
| Total shareholders’ equity | ||||||||
| Total liabilities and shareholders’ equity | $ | $ | ||||||
| * |
F-32
UNIVERSE PHARMACEUTICALS INC. AND SUBSIDIARIES
| For the Years Ended September 30, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| Operating costs and expenses: | ||||||||||||
| General and administrative expenses | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| Other income (expenses): | ||||||||||||
| Income (loss) from short-term investments | ( | ) | ||||||||||
| Other expenses | ( | ) | ( | ) | ( | ) | ||||||
| Equity in earnings of subsidiaries | ( | ) | ( | ) | ||||||||
| Net (loss) income | ( | ) | ( | ) | ||||||||
| Foreign currency translation adjustments | ( | ) | ( | ) | ||||||||
| Comprehensive (loss) income attributable to the Company | $ | ( | ) | $ | ( | ) | $ | |||||
UNIVERSE PHARMACEUTICALS INC. AND SUBSIDIARIES
| For the Years Ended September 30, | ||||||||||||
| 2023 | 2022 | 2021 | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net income | $ | ( | ) | $ | ( | ) | $ | |||||
| Adjustments to reconcile net cash flows from operating activities: | ||||||||||||
| Equity in earnings of subsidiary | ( | ) | ||||||||||
| Short-term investment income | ( | ) | ||||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ( | ) | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||||||
| Payment for short-term investments | ( | ) | ||||||||||
| Redemption of short-term investments | ||||||||||||
| Net cash used in investing activities | ( | ) | ||||||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||
| Net proceeds from issuance of ordinary shares in IPO | ||||||||||||
| Cash lent to subsidiaries | ( | ) | ||||||||||
| Cash repayment from subsidiaries | ||||||||||||
| Net cash provided by financing activities | ||||||||||||
| EFFECT OF CHANGES OF FOREIGN EXCHANGE RATES ON CASH | ( | ) | ||||||||||
| CHANGES IN CASH | ( | ) | ( | ) | ||||||||
| CASH, beginning of year | ||||||||||||
| CASH, end of year | $ | $ | $ | |||||||||
F-33