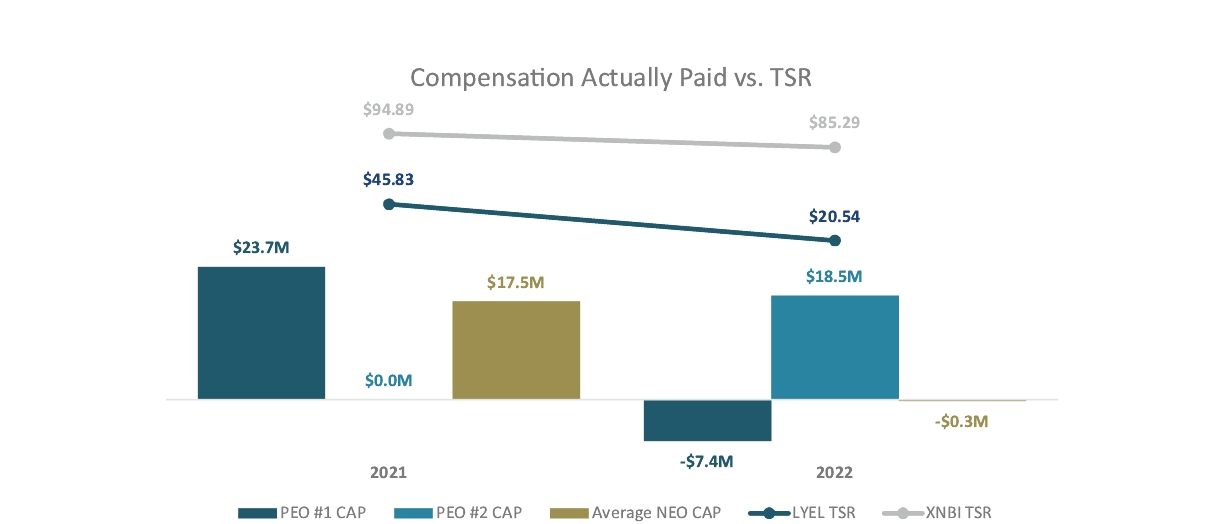
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Related Person Transactions Policy and
Procedures
We have adopted a written policy that our executive officers, directors, nominees for
election as a director, beneficial owners of more than 5% of any class of our common stock and any members of the immediate family of any of the foregoing persons are not permitted to enter into a related person transaction with us without the
approval or ratification of our Audit Committee (or, where Audit Committee approval would be inappropriate, to another independent body of the Board of Directors). Any request for us to enter into a transaction with an executive officer,
director, nominee for election as a director, beneficial owner of more than 5% of any class of our common stock, or any member of the immediate family of any of the foregoing persons, in which the amount involved exceeds $120,000 (or, if less, 1%
of the average of our total assets in a fiscal year) and such person would have a direct or indirect interest, must be presented to our Audit Committee for review, consideration and approval or ratification.
Under the policy, where a transaction has been identified as a related person
transaction, management must present information regarding the proposed related person transaction to our Audit Committee (or, where Audit Committee approval would be inappropriate, to another independent body of the Board of Directors) for
consideration and approval or ratification. The presentation must include a description of, among other things, the material facts (including the proposed aggregate value), the interests, direct and indirect, of the related persons, the benefits
to us of the transaction, the availability of other sources of comparable products or services, an assessment of whether the proposed related person transaction is on terms that are comparable to the terms available to or from, as the case maybe,
an unrelated third party, and management’s recommendation.
To identify related person transactions in advance, we rely on information supplied by
our executive officers, directors and certain significant stockholders. In approving or rejecting any proposed related person transaction, our Audit Committee considers all relevant available facts and circumstances, including, but not limited to
(a) whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances, (b) the risks, costs and benefits to us, (c) the extent of the related person’s
interest in the transaction, including, without limitation, the impact on a director’s independence in the event the related person is a director, immediate family member of a director or an entity with which a director is affiliated, and (d) the
availability of other sources for comparable services or products. In the event a director has an interest in the proposed transaction, the director must recuse himself or herself from the deliberations and approval or ratification. The policy
requires that, in determining whether to approve, ratify or reject a related person transaction, our Audit Committee approves only those related person transactions that, in light of known circumstances, are in, or are not inconsistent with, our
best interests and the best interests of our stockholders, as the Committee determines in the good faith exercise of its discretion.
Certain Related Person Transactions
The following includes a summary of transactions since January 1, 2022 and any
currently proposed transactions to which we have been or are to be a party in which the amount involved exceeded or will exceed $120,000, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than
5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change in control and other
arrangements, which are described under the sections titled “Executive Compensation” and “Non-Employee Director Compensation.” We also describe below certain other transactions with our directors, executive officers and stockholders.
Collaboration and
License Agreement with GSK
In May 2019, we entered into a Collaboration and License Agreement with GSK, amended
in June 2020 and December 2021 (the “GSK Agreement”), for potential T-cell therapies that apply our platform technologies and cell therapy innovations to T-cell receptors or chimeric antigen receptors under distinct collaboration programs. GSK
terminated the GSK Agreement effective December 24, 2022, and we have discontinued any further work on our collaboration programs. GSK is a holder of more than 10% of our common stock. Under the GSK Agreement, we were entitled to certain payments
upon the achievement of specified development and commercial milestones (for each selected target that is already within GSK’s pipeline and meet certain criteria, we were eligible to receive up to an aggregate of approximately $400.0 million, and
for each selected target that is not already within GSK’s