
Baudax Bio Corporate Presentation September 2021 Exhibit 99.1

Forward Looking Statements This presentation contains forward-looking statements that involve risks and uncertainties. Such forward-looking statements reflect Baudax Bio’s expectations about its future performance and opportunities that involve substantial risks and uncertainties. When used herein, the words “anticipate,” “believe,” “estimate,” “may,” “upcoming,” “plan,” “target,” “goal,” “intend,” and “expect,” and similar expressions, as they relate to Baudax Bio or its management, are intended to identify such forward-looking statements. These forward-looking statements are based on information available to Baudax Bio as of the date of publication on this internet site and are subject to a number of risks, uncertainties, and other factors that could cause Baudax Bio’s performance to differ materially from those expressed in, or implied by, these forward-looking statements. These risks and uncertainties include, among other things, risks related to the ongoing economic and social consequences of the COVID-19 pandemic, including any adverse impact on the commercial launch of ANJESO® or disruption in supply chain, Baudax Bio’s ability to maintain regulatory approval for ANJESO, Baudax Bio’s ability to successfully commercialize ANJESO; the acceptance of ANJESO by the medical community, including physicians, patients, health care providers and hospital formularies; Baudax Bio’s ability and that of Baudax Bio’s third party manufacturers to successfully scale-up the commercial manufacturing process for ANJESO, Baudax Bio’s ability to produce commercial supply in quantities and quality sufficient to satisfy market demand for ANJESO, Baudax Bio’s ability to raise future financing for continued product development, payment of milestones and ANJESO commercialization, Baudax Bio’s ability to pay its debt and satisfy conditions necessary to access future tranches of debt, Baudax Bio’s ability to comply with the financial and other covenants under its credit facility, Baudax Bio’s ability to manage costs and execute on its operational and budget plans, the accuracy of Baudax Bio’s estimates of the potential market for ANJESO, Baudax Bio’s ability to achieve its financial goals; and Baudax Bio’s ability to obtain, maintain and successfully enforce adequate patent and other intellectual property protection. These forward-looking statements should be considered together with the risks and uncertainties that may affect Baudax Bio’s business and future results included in Baudax Bio’s filings with the Securities and Exchange Commission at www.sec.gov. These forward-looking statements are based on information currently available to Baudax Bio, and Baudax Bio assumes no obligation to update any forward-looking statements except as required by applicable law.

Company Highlights ANJESO® (meloxicam) injection Product launched 2020 Approved for use in adults for the management of moderate-to-severe pain, alone or in combination with non-NSAID analgesics* Significant potential commercial opportunity Additional pipeline candidates in clinical stage development for acute care settings Baudax financial position Cash, cash equivalents and short-term investments as of 6/30/21 : $37.6 Million Experienced management team with significant commercial, development, and regulatory experience Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.ANJESO.com * Limitation of Use: Because of delayed onset of analgesia, ANJESO® alone is not recommended for use when rapid onset of analgesia is required.

Experienced Commercial Management & Launch Leadership Team Gerri Henwood – President and CEO Founded Recro Pharma (REPH), Auxilium Pharmaceuticals (AUXL – NASDAQ then Endo) and IBAH (NASDAQ then Omnicare); GSK Richard Casten – Chief Financial Officer 25 years diversified financial experience – Lupin Pharmaceuticals (LUPIN - NSE), Endo International (ENDP - NASDAQ), Campbell Soup Company (CPB - NYSE), Ernst & Young LLP Jyrki Mattila, MD, PhD –EVP, Business Development Over 30 years of Pharma executive leadership and business development experience -Lipocine, iCeutica, Auxilium, Orion Pharma Greg Gangemi – Chief Commercial Officer and Sr. Vice President Over 25 years of industry, launch and operations experience – Recro, Sepracor/Sunovion, Cubist, Ferring and Ocular Therapeutix Janeese Carter – Vice President, Marketing Over 15 years of marketing, market research, new business strategy, and sales – Recro Pharma, CSL Behring, Pfizer/Wyeth Paul Baddeley – Executive Director, Commercial Operations 20 years of industry and consulting experience in commercial operations & analytics – Recro, Collegium, IMS Health, Endo

Commercial Opportunity

1. Data on file. Baudax Bio, Inc. *The mechanism of action of IV meloxicam, like other NSAIDs, is not completely understood, but involves inhibition of both COX-1 and COX-2 pathways. COX-1 = cyclooxygenase 1; COX-2 = cyclooxygenase 2; IV = intravenous; NSAID = nonsteroidal anti-inflammatory drug; MMA = multimodal analgesia ANJESO® (meloxicam) Injection: The First and Only Once-Daily IV Analgesic Up to 24-hour pain relief Demonstrated Safety & Tolerability COX-2 Preferential IV NSAID* Once-daily IV push Efficacy in orthopedic & soft tissue procedures Evaluated in more than 1500 patients1 That can be incorporated into MMA protocols Ready-to-use, no reconstitution or refrigeration

ANJESO® (meloxicam) injection Overview Proprietary non-opioid, long-acting IV form Incorporates Alkermes’ NanoCrystal® technology Once daily, long-acting, preferential COX-2 inhibitor for moderate to severe acute pain Commercial Launch ongoing; Q2’21 reported metrics: Total number of vials sold to all customers up 30% Vials sold to hospitals and ASCs combined up 46% Vials sold to existing hospitals up 55% Vials sold to new ambulatory surgery centers up 150% Orange Book Listed patents run until 2030 Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.ANJESO.com * Quarter over quarter metrics based on second quarter of 2021 compared to first quarter 2021

*SPID (Sum of Pain Intensity Differences) is calculated by the sum of the difference between current pain and baseline pain at each post-dose time point. SPID48 = summed pain intensity difference from 0-48 hours, SPID24 = summed pain intensity difference from 0-24 hours. aAll studies completed with efficacy, safety and opioid reduction data. 1. Pollak RA et al. Clin J Pain. 2018;34(10):918-926. 2. Singla N et al. Plast Reconstr Surg Glob Open. 2018;6:e1846. 3. Bergese SD et al. Clin Pharmacol Drug Dev. 2019;8(8) 1062-1072. ANJESO® Evaluated in Three Phase 3 Studies Study Populationa ANJESO 30 mg Placebo Primary Endpoint Outcome Bunionectomy1 n=100 n=101 SPID48* 31% greater pain reduction vs placebo (p=0.0034) Abdominoplasty2 n=110 n=109 SPID24* 17% greater pain reduction vs placebo (p=0.0145) Safety study; multiple hard & soft tissue procedures3 n=538 n=183 Safety, including number of patients with adverse events up to 28 days after dosing Adverse Events comparable to placebo

Source: ANJESO Prescribing Information ANJESO® Adverse Events Across All Phase 3 Studies Adverse Reactions in Placebo-Controlled Phase 3 Clinical Trials occurring in ≥2% of Patients Treated with ANJESO® and at a greater frequency than Placebo ANJESO 30 mg (n=748) Placebo (n=393) % (n) % (n) Constipation 57 (7.6%) 24 (6.1%) Gamma-Glutamyl Transferase Increased 21 (2.8%) 6 (1.5%) Anemia 18 (2.4%) 4 (1.0%) ANJESO (n=748)

*Preoperative dosing = ANJESO 30mg was administered prior to surgical incision (TKA) or 30 minutes prior to the start of surgery (bowel resection), then once-daily while in hospital until discharge or IV analgesic was no longer appropriate. 1. Studies completed with efficacy, safety, opioid reduction and healthcare resource utilization measures. 2. Data on file. Baudax Bio, Inc. Abstracts and publications pending. MMA = multimodal analgesia; LOS = length of stay Two Phase 3b Health Economic Studies Completed with Preoperative Administration of ANJESO® Study Population1 ANJESO® 30 mg Placebo Primary Endpoint Selection of Secondary Endpoints Selection of Results2 Total Knee Arthroplasty (TKA) n=93 n=88 Evaluate efficacy of preoperative* administration measured by total opioid consumption Evaluate impact on pain control and healthcare resource utilization Preoperative administration of ANJESO as part of a MMA regimen was associated with lower total mean hospital costs >$2,500 during the hospital stay than patients in the placebo group Bowel Resection Surgery n=27 n=28 Evaluate safety and tolerability of preoperative* administration Evaluate impact on hospital LOS, opioid consumption and healthcare resource utilization Preoperative administration of ANJESO as part of a MMA regimen was well tolerated and decreased mean LOS by 1.1 days (3.6 vs 4.7 days)

Source: ANJESO Prescribing Information. *Vial size approximately 16 X 34.5 mm ANJESO® (meloxicam) Injection: The First and Only Once-Daily IV Analgesic Dosing and Administration Highlights Once-daily, IV bolus injection push over 15 seconds Administered as a 30-mg (1 mL) Available as a small* (2 mL) single dose vial Ready-to-use No reconstitution required Room temperature storage – no need to refrigerate When initiating ANJESO, monitor patient analgesic response. Because the median time to meaningful pain relief was 2 and 3 hours after ANJESO administration in two clinical studies, a non-NSAID analgesic with a rapid onset of effect may be needed, for example, upon anesthetic emergence or resolution of local or regional anesthetic blocks. Some patients may not experience adequate analgesia for the entire 24-hour dosing interval and may require administration of a short-acting, non-NSAID, immediate-release analgesic.

Commercial Launch

Sales continue to show growth through Q2 2021 Source: Baudax Bio Sales Data Launch Progress is being made despite varying levels of COVID-19 disruption of account access, formulary meetings, and elective surgeries Vials sold grew 30% in Q2’21 compared to Q1’21 Sales to hospitals and ASCs combined up 46% Strong end user demand during 2Q’21 based on key metrics for hospital-based drugs

Q2 2021 units sold of ANJESO matched cumulative units for 2020
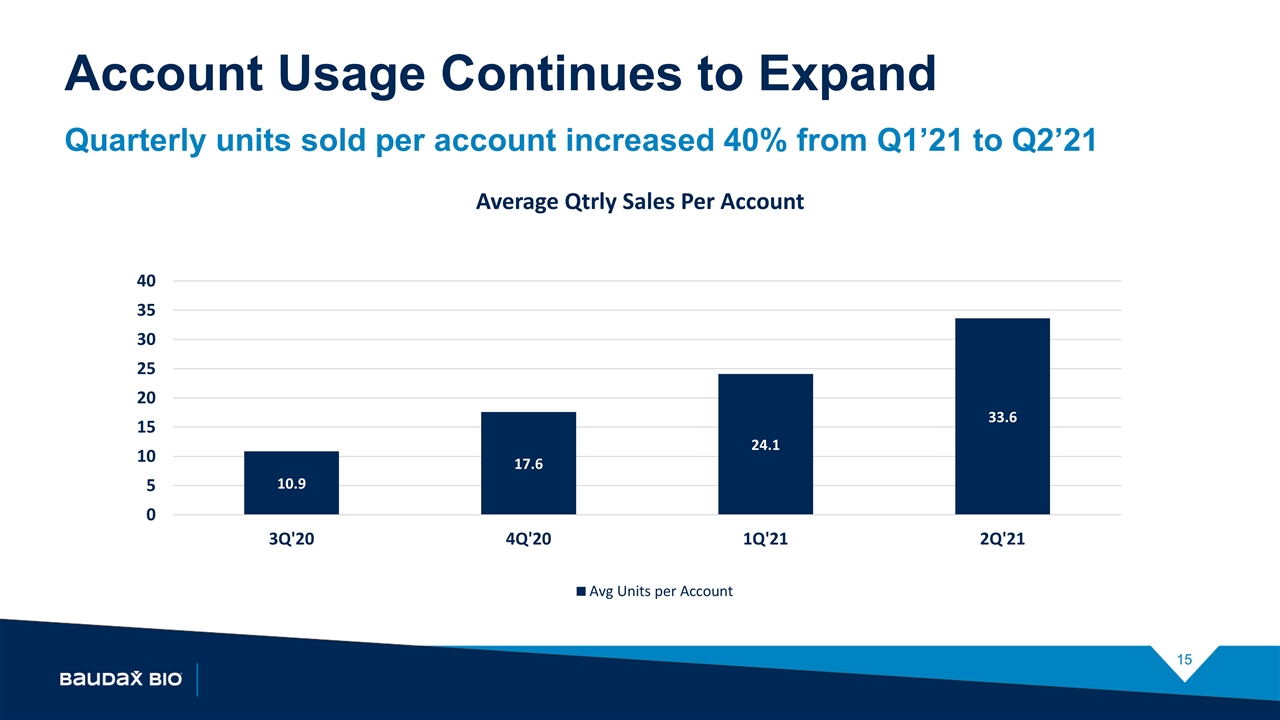
Account Usage Continues to Expand Quarterly units sold per account increased 40% from Q1’21 to Q2’21

ATU (Awareness, Trial & Usage) Market Research Reports: Messaging Rated Highly Compelling & Future Utilization Encouraging ANJESO demonstrated up to 24-hours of pain relief…. ANJESO is the first and only once-daily IV analgesic Safety was demonstrated in more than 1500 surgical patients …. Messages related to duration of effect & safety were seen to be highly compelling % of MDs selecting messages as highly compelling More than 1 in 3 MDs suggest increase utilization of ANJESO in next 3- 6 mos. Top 3 Drivers of ANJESO Increase Inclusion within Formulary Strong efficacy/safety vs. alternatives Adoption of MMA protocols Key ANJESO® Messaging Future ANJESO Utilization Source: Baudax Bio ANJESO Launch ATU Wave 1 Initial Findings; December 2020; n = 150

2021 ANJESO® Strategic Imperatives Maintain Focus On Building Advocates Accelerate Formulary Adoption & Gain New Accounts Facilitate pull through at accounts which have ANJESO on formulary

Cost-Effective & Innovative Approach Continues Current team focused on developing advocacy, P&T submissions and pull through with core customer. Baudax Field Teams Deployed virtual reps in December for outreach to hospitals not currently targeted Tele Sales: Extend Hospital Reach Surgical & Medical Device Consultants engaged to accelerate access and uptake with targeted customers and accounts Territory Advisors Accelerate Access Deployed virtual Ortho reps in late January with previous relationships to expand access and reach in Orthopedics Tele Sales: Extend Ortho Reach

Field Engaging Customers In-Person & Virtually with Comprehensive Resources Publication Resources Speaker Program Market Access/ Reimbursement Detail Aids & Collateral Core Visual Aid Promotional Leave Behind Rep Inservice Deck Tabletop Panels Baudax Bio Brochure Phase 3 Publication Flashcard Phase 3 Abdominoplasty Reprint Phase 3 Bunionectomy Reprint Phase 3 Safety Study Reprint Pharmacoeconomic Materials Virtual and In-Personal Speaker Programs Site specific billing resources Comprehensive Billing Guides NDC Announcements HUB flashcard Coverage Announcements Commercial Claim Forms Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.ANJESO.com

Company Highlights ANJESO® (meloxicam) injection Product launched 2020 Approved for use in adults for the management of moderate-to-severe pain, alone or in combination with non-NSAID analgesics* Significant potential commercial opportunity Additional pipeline candidates in clinical stage development for acute care settings Baudax financial position Cash, cash equivalents and short-term investments as of 6/30/21 : $37.6 Million Experienced management team with significant commercial, development, and regulatory experience Please see Important Safety Information including BOXED WARNING at the end of presentation. Full Prescribing Information at www.ANJESO.com * Limitation of Use: Because of delayed onset of analgesia, ANJESO® alone is not recommended for use when rapid onset of analgesia is required.

Thank you!

Important Safety Information

Indication and Boxed Warning INDICATION ANJESO is indicated for use in adults for the management of moderate-to-severe pain, alone or in combination with non-NSAID analgesics. Limitation of Use: Because of delayed onset of analgesia, ANJESO alone is not recommended for use when rapid onset of analgesia is required. IMPORTANT SAFETY INFORMATION WARNING: RISK OF SERIOUS CARDIOVASCULAR AND GASTROINTESTINAL EVENTS Cardiovascular Risk Non-steroidal anti-inflammatory drugs (NSAIDs) cause an increased risk of serious cardiovascular thrombotic events, including myocardial infarction and stroke, which can be fatal. This risk may occur early in treatment and may increase with duration of use. ANJESO is contraindicated in the setting of coronary artery bypass graft (CABG) surgery. Gastrointestinal Risk NSAIDs cause an increased risk of serious gastrointestinal (GI) adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients and patients with a prior history of peptic ulcer disease and/or GI bleeding are at greater risk for serious GI events.

Important Safety Information (cont) CONTRAINDICATIONS ANJESO is contraindicated in patients with: Known hypersensitivity (eg, anaphylactic reactions and serious skin reactions) to meloxicam or any components of the drug product. History of asthma, urticaria, or other allergic-type reactions after taking aspirin or other NSAIDs. In the setting of coronary artery bypass graft (CABG) surgery. Moderate to severe renal insufficiency patients who are at risk for renal failure due to volume depletion. WARNINGS AND PRECAUTIONS Hepatotoxicity: Elevations of ALT or AST have been reported in patients with NSAIDs. In addition, rare, sometimes fatal, cases of severe hepatic injury including fulminant hepatitis, liver necrosis, and hepatic failure have been reported. Inform patients of warning signs and symptoms of hepatotoxicity. Discontinue ANJESO immediately if abnormal liver tests persist or worsen or if clinical signs and symptoms of liver disease develop. Hypertension: NSAIDs including ANJESO can lead to new onset of hypertension or worsening of preexisting hypertension, which may contribute to the increased incidence of cardiovascular (CV) events. Patients taking some antihypertensive medications may have impaired response to these therapies when taking NSAIDs. Monitor blood pressure. Heart Failure and Edema: NSAID use increased the risk of myocardial infarction (MI), hospitalization for heart failure, and death. Avoid use of ANJESO in patients with severe heart failure unless benefits are expected to outweigh risk of worsening heart failure. If ANJESO is used in patients with severe heart failure, monitor patients for signs of worsening heart failure.

Important Safety Information (cont) Post MI Patients: Avoid the use of ANJESO in patients with recent MI unless the benefits are expected to outweigh the risk of recurrent CV thrombotic events. If ANJESO is used in these patients, monitor for signs of cardiac ischemia. Renal Toxicity: Long-term administration of NSAIDs has resulted in renal papillary necrosis, renal insufficiency, acute renal failure, and other renal injury. ANJESO is not recommended in patients with moderate to severe renal insufficiency and is contraindicated in patients with moderate to severe renal insufficiency who are at risk for renal failure due to volume depletion. Correct volume status in dehydrated or hypovolemic patients prior to initiating ANJESO. Monitor renal function in patients with renal or hepatic impairment, heart failure, dehydration, or hypovolemia. Avoid use of ANJESO in patients with advanced renal disease unless benefits are expected to outweigh risk of worsening renal function. If ANJESO is used in patients with advanced renal disease, monitor patients for signs of worsening renal function. Anaphylactic Reactions: Meloxicam has been associated with anaphylactic reactions in patients with and without known hypersensitivity to meloxicam and in patients with aspirin-sensitive asthma. Seek emergency help if an anaphylactic reaction occurs. Exacerbation of Asthma Related to Aspirin Sensitivity: ANJESO is contraindicated in patients with aspirin-sensitive asthma. Monitor patients with preexisting asthma (without aspirin sensitivity). Serious Skin Reactions: NSAIDs, including ANJESO, can cause serious skin reactions, including exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal and can occur without warning. Discontinue ANJESO at first appearance of skin rash or other signs of hypersensitivity. Hematologic Toxicity: Anemia has occurred in NSAID-treated patients. Monitor hemoglobin or hematocrit in patients with any signs or symptoms of anemia. NSAIDs, including ANJESO, may increase the risk of bleeding events. Monitor patients for signs of bleeding.

Important Safety Information (cont) DRUG INTERACTIONS Drugs That Interfere With Hemostasis (eg, warfarin, aspirin, SSRIs/SNRIs): Monitor patients for bleeding who are concomitantly taking ANJESO with drugs that interfere with hemostasis. Concomitant use of ANJESO and analgesic doses of aspirin is not generally recommended. Angiotensin Converting Enzyme (ACE) Inhibitors, Angiotensin Receptor Blockers (ARB), or Beta-Blockers: Concomitant use with ANJESO may diminish the antihypertensive effect of these drugs. Monitor blood pressure. ACE Inhibitors and ARBs: Concomitant use with ANJESO in elderly, volume depleted, or those with renal impairment may result in deterioration of renal function. In such high-risk patients, monitor for signs of worsening renal function. Diuretics: NSAIDs can reduce natriuretic effect of furosemide and thiazide diuretics. Monitor patients to ensure diuretic efficacy including antihypertensive effects. ADVERSE REACTIONS The most common adverse reactions in controlled clinical trials occurring in ≥2% of patients treated with ANJESO and at a greater frequency than placebo included: constipation, gamma-glutamyl transferase increased and anemia. USE IN SPECIFIC POPULATIONS Pregnancy: Use of NSAIDs during the third trimester of pregnancy increases the risk of premature closure of the fetal ductus arteriosus. Avoid use of NSAIDs in pregnant women starting at 30 weeks gestation. Infertility: NSAIDs are associated with reversible infertility. Consider withdrawal of ANJESO in women who have trouble conceiving. Please see full Prescribing Information, including Boxed Warning, at www.baudaxbio.com.

APPENDIX: Additional information for ANJESO®

Source: Wholesale Acquisition Cost Prices from Red Book accessed August 2020, which may not represent a customer’s cost. Price per day equals dosing schedule times price per dose. Dosing schedule according to product prescribing information for 24-hour coverage. Generic ketorolac has multiple manufacturers, price reflects the lowest manufacturer WAC. Wholesale Acquisition Cost: ANJESO and Other Non-Opioids Note: For illustration only. Not drawn to scale Strong Economic Evidence Available at Launch Economic Analysis of two Phase 3b studies completed with positive data available Budget Impact & Cost Effectiveness Models to address ANJESO cost effectiveness vs. other IV analgesics Retrospective Analyses of claims database that models real-world AE rates and costs $47.37 $173.84 $334.18 Ofirmev (4X dose) Ofirmev (per dose) Exparel (per dose) $9.84 IV Ketorolac (per day) Caldolor (4X dose) $78.60 $94.74 $142.11 Ofirmev (2X dose) Ofirmev (3X dose) $94.00

AWP=average wholesale price; DRG=diagnosis related group. Surgical Setting Coding and Reimbursement Medicare Unique Code, C9059 (Injection, Meloxicam 1mg) Reimbursed at 80% of 95% of AWP Medicare Use J3490 Reimbursement bundled into DRG payment Commercial Permanent J-code J1738 effective 10/1/2020 May be bundled with procedure or separately reimbursed based on the facility contract Commercial Permanent J-code J1738 effective 10/1/2020 May be bundled with procedure or separately reimbursed based on the facility contract Commercial Use J3490 Bundled and part of a case rate Medicare Unique Code, C9059 (Injection, Meloxicam 1mg) Reimbursed at 80% of 95% of AWP Permanent J-code J1738 "Injection, meloxicam, 1 mg“ effective 10/1/2020 and replaced all other codes Ambulatory Surgery Centers Hospital Outpatient Hospital Inpatient