An Offering Statement pursuant to Regulation A relating to these securities has been filed with Securities and Exchange Commission. Information contained in this Preliminary Offering Circular is subject to completion or amendment. These securities may not be sold nor may offers to buy be accepted before the Offering Statement filed with the Commission is qualified. This Preliminary Offering Circular shall not constitute an offer to sell or the solicitation of an offer to buy nor may there be any sales of these securities in any state in which such offer, solicitation or sale would be unlawful before registration or qualification under the laws of such state. The company may elect to satisfy its obligation to deliver a Final Offering Circular by sending you a notice within two business days after the completion of the company’s sale to you that contains the URL where the Final Offering Circular or the Offering Statement in which such Final Offering Circular was filed may be obtained.
PRELIMINARY OFFERING CIRCULAR DATED MAY 27, 2020
Quara Devices Inc.

1712 Pearl Street
Boulder, CO 80302
+1 (888) 887-6658
quaralife.com
UP TO 3,082,779 SHARES OF COMMON STOCK OFFERED BY THE ISSUER
UP TO 365,497 SHARES OF COMMON STOCK OFFERED BY THE SELLING SHAREHOLDERS
We are seeking to raise up to $17,880,118 and our selling shareholders are seeking to raise $2,119,883 from the sale of Common Stock to the public. As a result, the maximum offering amount is $20,000,001. There is no minimum offering dollar amount.
SEE “SECURITIES BEING OFFERED” AT PAGE 33
| Price | Underwriting discount and commissions (1) | Proceeds to Issuer (2) | Proceeds to Selling Shareholders (2) | |||||||||||||
| Per share | $ | 5.80 | $ | 0.058 | $ | 5.742 | $ | 5.742 | ||||||||
| Total Maximum | $ | 20,000,001 | $ | 200,000 | $ | 17,701,317 | $ | 2,098,684 | ||||||||
| (1) | We have not engaged any placement agent or underwriter in connection with this offering. To the extent that we do so, we will file a supplement to the Offering Statement of which this Offering Circular is a part. The company has engaged Dalmore Group, LLC, member FINRA/SIPC (“Dalmore”), to perform administrative and technology related functions in connection with this offering, but not for underwriting or placement agent services. This includes the 1% commission, but it does not include the one-time set-up fees payable by the company to Dalmore. See “Plan of Distribution and Selling Security Holders” for details. |
| (2) | Does not include other expenses of the offering. See “Plan of Distribution and Selling Security Holders” for a description of these expenses. |
The minimum investment amount for shares of our Common Stock is $580.00, or 100 shares.
We expect that, not including state filing fees, the amount of expenses of the offering that we will pay will be approximately $900,000.
The offering will terminate at the earlier of: (1) the date at which the maximum offering amount has been sold, (2) the date which is three years from this offering being qualified by the United States Securities and Exchange Commission (the “SEC”), or (3) the date at which the offering is earlier terminated by the company in its sole discretion. The company may undertake one or more closings on a rolling basis. After each closing, funds tendered by investors will be available to the company and the selling shareholders. The offering is being conducted on a best-efforts basis.
The company has engaged Prime Trust, LLC as an escrow agent to hold funds tendered by investors. We may hold a series of closings at which we and the selling shareholders receive the funds from the Escrow Agent and issue or sell the shares to investors.
Each holder of Common Stock is entitled to one vote for each share on all matters submitted to a vote of the shareholders. See “Securities Being Offered.”
THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION DOES NOT PASS UPON THE MERITS OR GIVE ITS APPROVAL OF ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SOLICITATION MATERIALS. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE COMMISSION; HOWEVER, THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED ARE EXEMPT FROM REGISTRATION.
GENERALLY, NO SALE MAY BE MADE TO YOU IN THIS OFFERING IF THE AGGREGATE PURCHASE PRICE YOU PAY IS MORE THAN 10% OF THE GREATER OF YOUR ANNUAL INCOME OR NET WORTH. DIFFERENT RULES APPLY TO ACCREDITED INVESTORS AND NON-NATURAL PERSONS. BEFORE MAKING ANY REPRESENTATION THAT YOUR INVESTMENT DOES NOT EXCEED APPLICABLE THRESHOLDS, WE ENCOURAGE YOU TO REVIEW RULE 251(d)(2)(i)(C) OF REGULATION A. FOR GENERAL INFORMATION ON INVESTING, WE ENCOURAGE YOU TO REFER TO www.investor.gov.
This offering is inherently risky. See “Risk Factors” on page 5.
Sales of these securities will commence on approximately _______, 2020.
The company is following the “Offering Circular” format of disclosure under Regulation A.
In the event that we become a reporting company under the Securities Exchange Act of 1934, we intend to take advantage of the provisions that relate to “Emerging Growth Companies” under the JOBS Act of 2012. See “Implications of Being an Emerging Growth Company.
TABLE OF CONTENTS
In this Offering Circular, the term “Quara,” “we,” “us”, “our” or “the company” refers to Quara Devices Inc.
THIS OFFERING CIRCULAR MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY’S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS “ESTIMATE,” “PROJECT,” “BELIEVE,” “ANTICIPATE,” “INTEND,” “EXPECT” AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS, WHICH CONSTITUTE FORWARD LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT’S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY’S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.
| 1 |
Implications of Being an Emerging Growth Company
We are not subject to the ongoing reporting requirements of the Exchange Act of 1934, as amended (the “Exchange Act”) because we are not registering our securities under the Exchange Act. Rather, we will be subject to the more limited reporting requirements under Regulation A, including the obligation to electronically file:
| ● | annual reports (including disclosure relating to our business operations for the preceding three fiscal years, or, if in existence for less than three years, since inception, related party transactions, beneficial ownership of the issuer’s securities, executive officers and directors and certain executive compensation information, management’s discussion and analysis (“MD&A”) of the issuer’s liquidity, capital resources, and results of operations, and two years of audited financial statements), | |
| ● | semiannual reports (including disclosure primarily relating to the issuer’s interim financial statements and MD&A) and | |
| ● | current reports for certain material events. |
In addition, at any time after completing reporting for the fiscal year in which our offering statement was qualified, if the securities of each class to which this offering statement relates are held of record by fewer than 300 persons and offers or sales are not ongoing, we may immediately suspend our ongoing reporting obligations under Regulation A.
If and when we become subject to the ongoing reporting requirements of the Exchange Act, as an issuer with less than $1.07 billion in total annual gross revenues during our last fiscal year, we will qualify as an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”) and this status will be significant. An emerging growth company may take advantage of certain reduced reporting requirements and is relieved of certain other significant requirements that are otherwise generally applicable to public companies. In particular, as an emerging growth company we:
| ● | will not be required to obtain an auditor attestation on our internal controls over financial reporting pursuant to the Sarbanes-Oxley Act of 2002; | |
| ● | will not be required to provide a detailed narrative disclosure discussing our compensation principles, objectives and elements and analyzing how those elements fit with our principles and objectives (commonly referred to as “compensation discussion and analysis”); | |
| ● | will not be required to obtain a non-binding advisory vote from our shareholders on executive compensation or golden parachute arrangements (commonly referred to as the “say-on-pay,” “say-on-frequency” and “say-on-golden-parachute” votes); | |
| ● | will be exempt from certain executive compensation disclosure provisions requiring a pay-for-performance graph and CEO pay ratio disclosure; | |
| ● | may present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations, or MD&A; and | |
| ● | will be eligible to claim longer phase-in periods for the adoption of new or revised financial accounting standards. |
We intend to take advantage of all of these reduced reporting requirements and exemptions, including the longer phase-in periods for the adoption of new or revised financial accounting standards under Section 107 of the JOBS Act. Our election to use the phase-in periods may make it difficult to compare our financial statements to those of non-emerging growth companies and other emerging growth companies that have opted out of the phase-in periods under Section 107 of the JOBS Act.
Under the JOBS Act, we may take advantage of the above-described reduced reporting requirements and exemptions for up to five years after our initial sale of common equity pursuant to a registration statement declared effective under the Securities Act of 1933, as amended (the “Securities Act”), or such earlier time that we no longer meet the definition of an emerging growth company. Note that this offering, while a public offering, is not a sale of common equity pursuant to a registration statement, since the offering is conducted pursuant to an exemption from the registration requirements. In this regard, the JOBS Act provides that we would cease to be an “emerging growth company” if we have more than $1.07 billion in annual revenues, have more than $700 million in market value of our common stock held by non-affiliates, or issue more than $1 billion in principal amount of non-convertible debt over a three-year period.
Certain of these reduced reporting requirements and exemptions are also available to us due to the fact that we may also qualify, once listed, as a “smaller reporting company” under the SEC’s rules. For instance, smaller reporting companies are not required to obtain an auditor attestation on their assessment of internal control over financial reporting; are not required to provide a compensation discussion and analysis; are not required to provide a pay-for-performance graph or CEO pay ratio disclosure; and may present only two years of audited financial statements and related MD&A disclosure.
| 2 |
The Company
Quara is an emerging med-tech & biotech company focusing on the development and commercialization of a handheld bacterial quorum sensing device to provide rapid early warning to the presence of harmful pathogens. This PathogenometerTM, which we call QuaraSenseTM is a handheld, battery operated device that uses proprietary fluorescent detection proteins to detect the molecules bacteria release when they become virulent. Once testing of QuaraSense is complete, we intend to commercialize it, focusing initially on shrimp diseases in the aquaculture market and urinary tract infections (“UTIs” or “UTI” in the singular) in the human health market, subject to regulatory approval. Thereafter, we intend to further develop QuaraSense to be used more broadly in the aquaculture market as well as in the veterinary, health care, food processing and home monitoring markets, among others.
Control of virulent bacteria is currently heavily reliant on the use of antibiotics. Unfortunately, this overuse of antibiotics has led to the evolution of bacteria known as superbugs that are resistant to several antibiotics. Superbugs are expected to cost the global economy $100 trillion in health care and lost productivity by 2050 (as per the International Federation of Pharmaceutical Manufacturers & Associations, November, 2018). The current over-use of antibiotics is due, in part, to a failure to detect infections early enough and the lack of timely feedback on the effectiveness of drugs that are administered.
Quara seeks to mitigate costly and sometimes deadly challenges with proprietary technology combined with the following key attributes:
| ● | handheld and portable, | |
| ● | simple to use, | |
| ● | cost effective, | |
| ● | broad detection of over 160 bacterial species, and | |
| ● | rapid, early warning of a bacterial infection, providing results in minutes. |
We have a functioning prototype that is ready for commercialization for detection of shrimp disease in the aquaculture market. We anticipate it will take us 9 to 12 months from funding to begin shipping QuaraSense to shrimp producers that are capable of detecting the signaling molecule that is a general indicator of many of the most common types of bacteria. We expect that further development of our technology to be able to detect additional types of virulent bacteria specific to our other target markets, and to expand our technology portfolio to the detection of pathogenic viruses.
Our mission and strategy is strongly supported by our high quality, knowledgeable and successful team of officers, directors and advisors, including scientists who are experts in the fields of biology, biophysics and bioengineering and senior management and advisors with extensive experience in bringing new products to market and expertise in capital markets, marketing and executive leadership.
The Offering
| Securities offered by us: | Maximum of 3,082,779 shares of Common Stock. | |
| Securities offered by the selling shareholders (1): | Maximum of 365,497 shares of Common Stock | |
| Common Stock outstanding before the offering (2): | 38,457,361 shares | |
| Common Stock outstanding after the offering (2): | 41,540,140 shares, assuming we raise the maximum offering amount | |
| Use of proceeds: | Purchase of intellectual property, product commercialization, marketing and brand development, payment of deferred salaries and working capital reserves. |
| (1) | See “Plan of Distribution and Selling Security Holders” |
| (2) | We have granted 3,500,000 options under our 2019 Stock Option Plan (“Stock Option Plan”). Our Stock Option Plan reserves for issuance a number of shares equal to 15% of the number of shares of Common Stock that are issued, or 5,768,604 shares of Common Stock currently. The number of shares of Common Stock outstanding before and after the offering shown above does not include shares of Common Stock issuable upon exercise of options issue under our Stock Option Plan or any shares of Common Stock that will remain reserved for issuance pursuant to our Stock Option Plan. It also does not reflect shares of common stock that we have agreed to issue to Colorado State University Research Foundation as discussed under “The Company’s Business—Intellectual Property.” |
| 3 |
Selected Risks Associated with Our Business
Our business is subject to a number of risks and uncertainties, including those highlighted in the section titled “Risk Factors” immediately following this summary. These risks include, but are not limited to, the following:
| ● | We have a limited history upon which an investor can evaluate our performance and future prospects. | |
| ● | We may not be able to develop commercially viable sensor products on the timetable we anticipate, or at all, or successfully execute on our business plan. | |
| ● | We may not be able to raise enough capital to acquire the intellectual property from Pebble Labs, Inc. (“Pebble Labs”) by the contractual due date or to commercialize our product and begin generating revenue. | |
| ● | Our ability to raise capital and to commercialize our sensor products may be materially impacted by the COVID-19 pandemic. | |
| ● | We may not be able to effectively manage our growth, and any failure to do so may have an adverse effect on our business viability. | |
| ● | We will compete with other companies that are developing or have developed analyzers designed to exploit similar markets to those in which we intend to penetrate. Many of these other companies have substantially greater resources than we do. | |
| ● | We expect to be highly dependent on third party suppliers and contractors that will need to have a high level of expertise and meet strict quality standards. | |
| ● | Failure to obtain approval to market our sensor products for human health applications may limit our prospects for growth. | |
| ● | Adverse regulatory or policy changes could have a material impact on our business. | |
| ● | If we fail to effectively protect our intellectual property, our business may suffer. | |
| ● | Our business and its prospects for success are dependent on key personnel who are not easy to recruit and retain, especially in the life sciences industry which requires a high level of expertise. | |
| ● | We may be subject to product liability claims which could have a material adverse effect on our business, our prospects and our reputation. | |
| ● | We have identified a significant deficiency in our internal controls over financial reporting. | |
| ● | Our valuation has been established by us, is difficult to assess and you may risk overpaying for your investment. | |
| ● | Because this is a “best efforts” offering with no minimum, any investment made could be the only investment in this offering, leaving the company without adequate capital to pursue its business plan or even to cover the expenses of this offering. | |
| ● | This offering involves “rolling closings,” which may mean that earlier investors may not have the benefit of information that later investors have. | |
| ● | The value of your investment may be diluted if we issue additional options or shares of Common Stock. |
| 4 |
The SEC requires the company to identify risks that are specific to its business and its financial condition. The company is still subject to all the same risks that all companies in its business, and all companies in the economy, are exposed to. These include risks relating to economic downturns, political and economic events and technological developments (such as hacking and the ability to prevent hacking). Additionally, relatively early-stage companies are inherently riskier than more developed companies. You should consider general risks as well as specific risks when deciding whether to invest.
Risks Related to our Business
We may not be able to develop commercially viable sensor products on the timetable we anticipate, or at all. Our sensor technology may be difficult to scale to a commercially viable level since it must meet expectations that it is equivalent or superior to traditional diagnostic technology in terms of reliability and cost efficiency. We still need to develop and refine the technology necessary to ensure that our sensors meet performance goals and cost targets. We need to perform additional laboratory and field testing, and we may encounter problems and delays. If the tests reveal technical defects or reveal that our products do not meet performance goals and cost targets, our commercialization schedule could be delayed as we attempt to devise solutions to the defects or problems. If we are unable to find solutions, our business may not be viable.
We recently entered into an agreement with the Colorado State University Research Foundation to license certain intellectual property that we believe will allow us to develop a device to detect virulent viral pathogens such as HIV, hepatitis C, dengue, Zika and severe acute respiratory syndrome coronavirus 2 (“SARS-Cov-2”), responsible for COVID-19. We strongly caution you that our ability to integrate this technology into our device portfolio, conduct sufficient testing to establish the safety and efficacy of using our device for viral testing, obtain required approval to market our device from the FDA and to successfully commercialize it for this use is highly uncertain at this early stage of development, and is likely to be a lengthy process. Even if we were able to successfully test and obtain FDA approval, by that time many other competing testing methods may be so prevalent that we would not be able to capture enough market share to make commercialization financially feasible.
We may not be able to successfully execute our business plan. In addition to the requirement to successfully develop the technology for commercially viable sensors, we must also raise significant amounts of capital, foster relationships with key suppliers and attract customers. There is no guarantee that we will be able to achieve or sustain any of the foregoing within our anticipated timeframe or at all. We may exceed our budget, encounter obstacles in research and development activities, or be hindered or delayed in implementing our commercialization plans, any of which could imperil our ability to secure customer contracts and begin generating revenues. In addition, any such delays or problems would require us to secure additional funding over and above what we currently anticipate we require to sustain our business, which we may not be able to raise.
We may not be able to raise enough capital to acquire intellectual property from Pebble Labs by the contractual due date or to commercialize our product and begin generating revenue. We are required to pay Pebble Labs $500,000 by September 30, 2020 in order to perfect the purchase of the intellectual property related to their propriety fluorescent detection proteins. If we fail to raise a sufficient amount of net proceeds prior to September 30, 2020, we will need to ask Pebble Labs for an extension. They have no obligation to grant an extension and if they fail to do so, we will not have access to the protein developed by Pebble Labs that may be needed to expand the capabilities of our current prototype device for use in certain markets, and we may not have access to some of their employees who currently act as advisors to us. With the purchase of the intellectual property from OptiEnz Sensors, LLC we do have a detection protein, hardware and software providing us with a viable prototype to commercialize for the aquaculture and other markets. In addition, if we fail to raise at least $900,000 we may not have sufficient funds to commercialize our product at all. There is no assurance that we will be able to raise additional capital in the future. As the Company may make potential acquisitions or expand its product line, the need to raise additional capital may be even more important for our future growth, and if we are unable to raise the necessary money on acceptable terms, we may be unable to pursue or realize our objectives and be hindered in our growth.
| 5 |
Our ability to raise capital and to commercialize our sensor products may be materially impacted by the COVID-19 pandemic. The full impact on the economy and the capital markets in the U.S. and the rest of the world from the COVID-19 pandemic are uncertain, in terms of both scale and duration. The high level of volatility in the capital markets may make it difficult to raise funds, especially for early stage companies that involve higher risk. If we are able to raise sufficient funds to begin the work of commercializing our sensor products, we may have difficulty securing supplies needed or manufacturing and distribution partners. The impact of social distancing measures and related workforce reductions may negatively impact the ability of suppliers to deliver us the components we need for manufacture or the ability of any of our potential partners to operate effectively to meet our requirements. In addition, many of the third parties that we would rely on for production and distribution are likely to be highly engaged in manufacturing products aimed at combatting the pandemic by manufacturing testing supplies and equipment, medical equipment and/or potential treatments. We cannot assure you that, should we raise sufficient funds, we will be able to contract with suppliers, manufacturing partners or distribution partners at a level that would allow us to achieve profitability, or at all.
We are an early stage company with a limited operating history. The company was formed on February 5, 2019. Accordingly, we have a limited history upon which an investor can evaluate our performance and future prospects. Our activities to date have focused on research and development activity to create a prototype of our sensor and as a result, we have incurred only net losses to date. Our financial statements do not reflect any operating revenues. We cannot assure you that we will be in a position to generate revenues or profits in the foreseeable future.
We may not be able to effectively manage our growth, and any failure to do so may have an adverse effect on our business viability. We intend to use the proceeds of this offering to help us achieve commercialization of our sensing device for the aquaculture market and further develop it to address other markets. We have no experience in producing sensors for market and may face significant challenges in developing, staffing and managing the production of sensing devices reliably and efficiently on a high-volume, low-cost basis. Manufacturing a sophisticated high-tech product with exacting specifications requires expertise and experience which we currently do not have and may have difficulty in securing. In addition, our future operating results will depend on our ability to effectively build and manage supplier and customer relationships across a broad geographic footprint. Managing growth is made more difficult by the fact that we currently have no corporate offices or permanent physical locations and, therefore, our senior management team generally coordinates through electronic communications and by phone. Our failure to effectively manage our growth could negatively impact our business results and prospects as well as our reputation.
The diagnostics market is highly complex and competitive. We will compete with other companies that are developing or have developed analyzers designed to exploit similar markets to those in which we intend to penetrate. Many of these other companies have substantially greater resources than we do. We cannot assure you that developments by other companies will not adversely affect the competitiveness of our products. The diagnostic industry is also characterized by extensive research efforts and rapid technological change. Competition can be expected to increase as technological advances are made and commercial applications for diagnostic technologies increase. Our competitors may use different technologies or approaches to develop products similar to the products which we are seeking to develop or may develop new or enhanced products or processes that may be more effective and less expensive. We may not be able to market our products to compete successfully in the existing competitive environment. Moreover, national laboratories and universities around the world are also researching as well as developing similar sensors. New developments may render the Company’s products obsolete or uneconomical. Competition in all these forms may impede the Company’s ability to produce and sell a commercially viable product or be disadvantaged in some other manner which could materially impact the Company’s business prospects.
We expect to be highly dependent on third party suppliers and contractors. We expect to rely heavily on manufacturing partners to co-develop and produce the necessary technology and components for our sensing devices. Due to the complexity of the technology in our devices, our suppliers and manufacturing partners require a high level of expertise and will need to meet strict quality standards. We have established good working relationships with prospective partners; however, if we are unable to secure contracts with them, or if any contract is terminated for reasons outside of our control, it may be difficult for us to find new suppliers or contractors that are able to meet these standards. Furthermore, to the extent that any of our suppliers provides us with products that prove to be defective or fail to meet our specifications, or there are failures in manufacturing our devices by a third party, our business and reputation will likely suffer.
| 6 |
Failure to obtain approval to market our sensor products for human health applications may limit our prospects for growth. We will require approval from the U.S. Food and Drug Administration (“FDA”) and similar agencies in other countries prior to marketing our sensor products for human health applications. We will need to establish, to the satisfaction of those organizations, that our products are safe and effective for use. Because our technology tests for the presence of virulent bacteria in a different manner than currently exists, we cannot refer to existing technology to support our application and, as a result, cannot assure you that we will receive approval. In addition, we believe that the resources of the FDA are heavily engaged in monitoring, reviewing and approving testing solutions for COVID-19 and the underlying virus, SARS-Cov-2, and they may not have sufficient staffing or other resources to review our application in a timely manner. As a result, we may not be in a position to pursue human health applications for a significant period of time, if at all.
Adverse regulatory or policy changes could have a material impact on our business. Our business is premised on our bacterial detection systems being able to meet regulations and policies in our target markets. If the regulatory framework in these markets becomes more restrictive to the point where our products are unable to meet these standards, the Company will have difficulty in selling its products and potential customers may seek alternative technologies altogether.
If we fail to effectively protect our intellectual property, our business may suffer. We will rely on patents pending to protect our intellectual property. There is no assurance that any patents will be issued with the desired breadth of claim coverage or at all. The failure to obtain patents for our current technology or any future technology could materially impair our business prospects or, in the case of future development, impair our ability to expand our business into other markets. If any patents are granted, they will, as is generally the case with patents, be subject to uncertainty with respect to their validity, scope and enforceability and thus we cannot guarantee you that our patents, or patents that we may license from third parties, will not be invalidated, circumvented, challenged, or become unenforceable. In cases where the Company must license intellectual property from third parties, there is no guarantee that the Company will be able to do so on acceptable terms.
Some of our proprietary processes, technologies and know-how are not under patent protection. Although we intend to seek patent protection where possible and in the best interests of the company, in some cases we must rely on the law of trade secrets to protect our intellectual property. Accordingly, there is a risk that such trade secrets may not stay secret. This risk also applies to confidentiality agreements and inventors’ rights agreements with our strategic partners and employees. There is no assurance that these agreements will not be breached, that we will have adequate remedies for any breach, or that such persons or institutions will not assert rights to intellectual property arising out of these relationships. Finally, effective patent, trade secret, trademark and copyright protection may be unavailable, limited or not applied for in certain countries.
We may also be subject to allegations of infringement of other parties’ intellectual property, or conversely, be forced to sue those who infringe our intellectual property. Such litigation is usually costly, time-consuming, and would divert resources away from the Company. If we lose such lawsuits, we may be compelled to pay damages or to cease development, manufacture, use or sale of the infringing product.
Our business and its prospects for success are dependent on key personnel. We rely on key personnel in management, research and development, operations, manufacturing and marketing who are not easy to recruit and retain, especially in the life sciences industry which requires a high level of expertise. We believe that we have and will continue to offer key personnel competitive compensation packages, but we cannot assure you that our key personnel will remain with the company or that we will be able to hire additional personnel with the correct skill sets and qualifications in the future. In particular, our Chief Executive Officer does not receive any compensation in connection with his position. We do not maintain any key person insurance and the loss of any of our key personnel could significantly impair our ability to establish a viable business.
In addition, our key personnel are serial entrepreneurs. It is possible that some, if not all, of our key personnel may exit the business within the next three years. In the event one or more of our key personnel exit the business the company may experience financial loss, disruption to our operations and technology development, damage to our brand and reputation and, if any departing person joins a competitor, a weakening of our competitive position.
| 7 |
We may be subject to product liability claims as product malfunction is always a possibility. Depending on the magnitude of the damage, any of these occurrences could lead to civil lawsuits for which our insurance policies may not be adequate or available, and in certain cases, may even lead to criminal sanctions. We may be forced to pay significant damages, curtail operations or shut down, which could have a material adverse effect on our business, our prospects and our reputation.
We have identified a significant deficiency in our internal controls over financial reporting. Ensuring that we have adequate internal financial and accounting controls and procedures in place to produce accurate financial statements on a timely basis is a costly and time-consuming effort that needs to be re-evaluated frequently. Our management has identified a significant deficiency in our internal controls. While management is working to remediate the deficiencies, there is no assurance that such changes, when economically feasible and sustainable, will remediate the identified deficiencies or that the controls will prevent or detect future significant deficiencies. If we are not able to maintain effective internal control over financial reporting, our financial statements, including related disclosures, may be inaccurate, which could have a material adverse effect on our business. We may discover additional deficiencies in our internal financial and accounting controls and procedures that need improvement from time to time.
Management is responsible for establishing and maintaining adequate internal control over financial reporting to provide reasonable assurance regarding the reliability of our financial reporting and the preparation of financial statements for external purposes in accordance with United States generally accepted accounting principles. Management does not expect that our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, within the company will have been detected.
Risks Related to the Securities and the Offering
Any valuation at this stage is difficult to assess. The valuation for the offering was established by the company. Unlike listed companies that are valued publicly through market-driven stock prices, the valuation of private companies, especially startups, is difficult to assess and you may risk overpaying for your investment.
There is no minimum amount set as a condition to closing this offering. Because this is a “best efforts” offering with no minimum, we will have access to any funds tendered. This might mean that any investment made could be the only investment in this offering, leaving the company without adequate capital to pursue its business plan or even to cover the expenses of this offering.
This offering involves “rolling closings,” which may mean that earlier investors may not have the benefit of information that later investors have. We may conduct closings on funds tendered in the offering at any time. At that point, investors whose subscription agreements have been accepted will become our shareholders. We may file supplements to our Offering Circular reflecting material changes and investors whose subscriptions have not yet been accepted will have the benefit of that additional information. These investors may withdraw their subscriptions and get their money back. Investors whose subscriptions have already been accepted, however, will already be our shareholders and will have no such right.
This investment is illiquid. There is no currently established market for reselling these securities. If you decide that you want to resell these securities in the future, you may not be able to find a buyer.
The value of your investment may be diluted if the company issues additional options or shares of Common Stock. Our Articles of Incorporation provides that we can issue an unlimited number of shares of our Common Stock, whether in a subsequent offering, in connection with an acquisition or otherwise. We have granted 3,500,000 options under our Stock Option Plan. Our Stock Option Plan reserves for issuance a number of shares equal to 15% of the number of shares of Common Stock that are issued, or 5,768,604 shares of Common Stock currently and we may in the future increase the number or percentage of shares reserved for issuance under this plan or adopt another plan. We have also agreed to issue shares of common stock to Colorado State University Research Foundation as discussed under “The Company’s Business—Intellectual Property.” The issuance of additional shares of Common Stock, or additional option grants under our Stock Option Plan or other stock based incentive program may dilute the value of your holdings. The company views stock based incentive compensation as an important competitive tool, particularly in attracting both managerial and technological talent.
| 8 |
Dilution means a reduction in value, control or earnings of the shares the investor owns.
Immediate dilution
An early-stage company typically sells its shares (or grants options over its shares) to its founders and early employees at a very low cash cost, because they are, in effect, putting their “sweat equity” into the company. When the company seeks cash investments from outside investors, like you, the new investors typically pay a much larger sum for their shares than the founders or earlier investors, which means that the cash value of your stake is diluted because all the shares are worth the same amount, and you paid more than earlier investors for your shares.
The following table demonstrates the price that new investors are paying for their shares with the effective cash price paid by the existing shareholder. The table gives effect to the sale of shares by us at $5,000,000, $10,000,000 and $20,000,001 (the maximum amount offered), in each case excluding shares being offered by the selling shareholders.
| $5,000,000 Raise | $10,000,000 Raise | $20,000,001 Raise | ||||||||||
| Price per Share for new investors | $ | 5.80 | $ | 5.80 | $ | 5.80 | ||||||
| Shares issued to new investors | 603,448 | 1,358,640 | 3,082,779 | |||||||||
| Gross proceeds raised | $ | 3,499,998 | $ | 7,880,112 | $ | 17,880,118 | ||||||
| Less: Offering costs | $ | (718,000 | ) | $ | (775,000 | ) | $ | (900,000 | ) | |||
| Net offering proceeds | $ | 2,781,998 | $ | 7,105,112 | $ | 16,980,118 | ||||||
| Adjusted net tangible book value pre-financing (as of 12/31/2019) | $ | (9,891 | ) | $ | (9,891 | ) | $ | (9,891 | ) | |||
| Adjusted net tangible book value post-financing | $ | 2,772,107 | $ | 7,095,221 | $ | 16,970,227 | ||||||
| Shares issued and outstanding pre-financing | 38,457,361 | 38,457,361 | 38,457,361 | |||||||||
| Post-financing shares issued and outstanding | 39,060,809 | 39,816,001 | 41,540,140 | |||||||||
| Net tangible book value per share prior to offering | $ | (0.000 | ) | $ | (0.000 | ) | $ | (0.000 | ) | |||
| Increase/(Decrease) per share attributable to new investors | $ | 0.071 | $ | 0.178 | $ | 0.409 | ||||||
| Net tangible book value per share after offering | $ | 0.071 | $ | 0.178 | $ | 0.409 | ||||||
| Dilution per share to new investors | $ | 5.73 | $ | 5.62 | $ | 5.39 | ||||||
| Dilution per share to new investors | 98.8 | % | 96.9 | % | 93.0 | % | ||||||
As of December 31, 2019, the Company had not issued any options pursuant to the Company’s Stock Option Plan. In 2020 to date, the Company has issued 3,500,000 options to its directors, consultants and advisors. The above table excludes the future issuance of up to 3,500,000 shares of Common Stock that will be underlying those options. If all options to be issued were exercised, the adjusted net tangible book value post-financing would increase by $4,812,500, the post-financing shares issued and outstanding would be 42,560,809, 43,316,001 and 45,040,140 at the 25%, 50% and 100% levels, the net tangible book value per share after offering would be $0.178, $0.275 and $0.484 at those levels and the dilution per share to new investors would be $5.62 (96.9%), $5.53 (95.3%), and $5.32 (91.7%), respectively.
Future dilution
Another important way of looking at dilution is the dilution that happens due to future actions by the company. The investor’s stake in a company could be diluted due to the company issuing additional shares. In other words, when the company issues more shares, the percentage of the company that you own will go down, even though the value of the company may go up. You will own a smaller piece of a larger company. This increase in number of shares outstanding could result from a stock offering (such as an initial public offering, another Regulation A round, a venture capital round, angel investment), employees exercising stock options, or by conversion of certain instruments (e.g. convertible bonds, preferred shares or warrants) into stock.
| 9 |
If the company decides to issue more shares, an investor could experience value dilution, with each share being worth less than before, and control dilution, with the total percentage an investor owns being less than before. There may also be earnings dilution, with a reduction in the amount earned per share (though this typically occurs only if the company offers dividends, and most early stage companies are unlikely to offer dividends, preferring to invest any earnings into the company).
The type of dilution that hurts early-stage investors most occurs when the company sells more shares in a “down round,” meaning at a lower valuation than in earlier offerings. An example of how this might occur is as follows (numbers are for illustrative purposes only):
| ● | In June 2019 Jane invests $20,000 for shares that represent 2% of a company valued at $1 million. | |
| ● | In December 2019 the company is doing very well and sells $5 million in shares to venture capitalists on a valuation (before the new investment) of $10 million. Jane now owns only 1.3% of the company but her stake is worth $200,000. | |
| ● | In June 2020 the company has run into serious problems and in order to stay afloat it raises $1 million at a valuation of only $2 million (the “down round”). Jane now owns only 0.89% of the company and her stake is worth only $26,660. |
This type of dilution might also happen upon conversion of convertible notes into shares. Typically, the terms of convertible notes issued by early-stage companies provide that in the event of another round of financing, the holders of the convertible notes get to convert their notes into equity at a “discount” to the price paid by the new investors, i.e., they get more shares than the new investors would for the same price. Additionally, convertible notes may have a “price cap” on the conversion price, which effectively acts as a share price ceiling. Either way, the holders of the convertible notes get more shares for their money than new investors. In the event that the financing is a “down round” the holders of the convertible notes will dilute existing equity holders, and even more than the new investors do, because they get more shares for their money. Investors should pay careful attention to the number of shares of Common Stock underlying convertible notes that the company may issue in the future, and the terms of those notes.
If you are making an investment expecting to own a certain percentage of the company or expecting each share to hold a certain amount of value, it’s important to realize how the value of those shares can decrease by actions taken by the company. Dilution can make drastic changes to the value of each share, ownership percentage, voting control, and earnings per share.
| 10 |
We estimate that the net proceeds from this offering will be approximately $16,980,118 assuming we raise the maximum offering amount and after deducting the estimated offering expenses of approximately $900,000 (excluding state filing fees).
The following table below sets forth the uses of proceeds assuming an offering amount of $5,000,000, $10,000,000, and $20,000,001 (the maximum offering amount), excluding in each case the shares to be sold by the selling shareholders. For further discussion, see the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Plan of Operations.”
$5,000,000 Offering | $10,000,000 Offering | $20,000,001 Offering | ||||||||||
| Offering Proceeds | ||||||||||||
| Shares Sold | 603,448 | 1,358,640 | 3,082,779 | |||||||||
| Gross Proceeds to the Company from this Offering | $ | 3,499,998 | $ | 7,880,112 | $ | 17,880,118 | ||||||
| Offering Expenses (1) | $ | 718,000 | $ | 775,000 | $ | 900,000 | ||||||
| Total Offering Proceeds Available for Use | $ | 2,781,998 | $ | 7,105,112 | $ | 16,980,118 | ||||||
| Estimated Expenditures | ||||||||||||
| Purchase of Intellectual Property from Pebble Labs | $ | 500,000 | $ | 500,000 | $ | 500,000 | ||||||
| Commercialization of QuaraSense Device | $ | 700,000 | $ | 1,500,000 | $ | 1,500,000 | ||||||
| Commercialization of Viral Detection Technology | 700,000 | 1,500,000 | 1,500,000 | |||||||||
| Brand Development | $ | 400,000 | $ | 2,000,000 | $ | 2,000,000 | ||||||
| Payment of Deferred Compensation | $ | 444,000 | $ | 444,000 | $ | 444,000 | ||||||
| Total Expenditures | $ | 2,744,000 | $ | 5,944,000 | $ | 5,944,000 | ||||||
| Working Capital Reserves | $ | 37,998 | $ | 1,161,112 | $ | 11,036,118 | ||||||
| (1) | Excludes state filing fees of approximately $12,000. |
For a discussion of our agreement to acquire intellectual property from Pebble Labs Inc. please see “The Company’s Business.” We anticipate that expenditures for commercialization of the QuaraSense device will include development of production version of prototype device, improvement of the performance of the fluorescent resonance energy transfer (“FRET”) detection protein, development of sample pretreatment protocol development and other customer feedback dependent tasks. In addition, we intend to invest in marketing and branding activities to develop our brand and market our product. At this time, we estimate that the expenditures for commercialization of the viral detection technology will be similar to the QuaraSense device.
The above figures represent only estimated costs. This expected use of net proceeds from this offering represents our intentions based upon our current plans and business conditions. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors. As a result, our management will retain broad discretion over the allocation of the net proceeds from this offering. We may find it necessary or advisable to use the net proceeds from this offering for other purposes, and we will have broad discretion in the application of net proceeds from this offering. Furthermore, if we fail to raise at least $900,000 we anticipate that we will need to secure additional funding to fully commercialize our QuaraSense device. Please see “Risk Factors.”
We reserve the right to change the above use of proceeds if management believes it is in the best interests of the Company.
| 11 |
Overview
Quara is an emerging med-tech & biotech company focusing on the development and commercialization of a handheld bacterial quorum sensing device to provide rapid early warning to the presence of harmful pathogens. This PathogenometerTM, which we call QuaraSenseTM is a handheld, battery operated device that uses proprietary fluorescent detection proteins to detect the molecules bacteria release when they become virulent. Once testing of QuaraSense is complete, we intend to commercialize it, focusing initially on shrimp diseases in the aquaculture market and UTIs in the human health market, subject to regulatory approval. Thereafter, we intend to further develop QuaraSense to be used more broadly in the aquaculture market as well as in the veterinary, health care, food processing and home monitoring markets, among others.
Control of virulent bacteria is currently heavily reliant on the use of antibiotics. Unfortunately, this overuse of antibiotics has led to the evolution of bacteria known as superbugs that are resistant to several antibiotics. Superbugs are expected to cost the global economy $100 trillion in health care and lost productivity by 2050 (as per the International Federation of Pharmaceutical Manufacturers & Associations, November 2018). The current over-use of antibiotics is due, in part, to a failure to detect infections early enough and the lack of timely feedback on the effectiveness of drugs that are administered.
Quara seeks to mitigate costly and sometimes deadly challenges with proprietary technology combined with the following key attributes:
| ● | handheld and portable, | |
| ● | simple to use, | |
| ● | cost effective, | |
| ● | broad detection of over 160 bacterial species including most common pathogens, and | |
| ● | rapid, early warning of a bacterial infection, providing results in minutes. |
We have a functioning prototype that is ready for commercialization for detection of shrimp disease in the aquaculture market. We believe QuaraSense has substantial potential for the broader aquaculture market as well as the veterinary, health care, food processing and home health monitoring markets. We believe QuaraSense will help to improve people’s lives and reduce food production costs as we seek to provide an early warning of harmful pathogens in our bodies, our pets, and food production systems and processing equipment. We believe that potential market is massive, and we believe we have the management and scientific teams to launch our QuaraSense successfully.
Our History
Quara was formed in 2019 to acquire and commercialize intellectual property from OptiEnz Sensors, LLC (www.optienz.com), which initially developed our device and was founded by our Chief Science Officer, and from Pebble Labs, Inc. (www.pebblelabs.com) of Los Alamos, New Mexico. OptiEnz and Pebble Labs have highly educated and experienced research teams. Pebble Labs has emerged as one of North America’s top privately-owned bio-tech R&D organizations. In addition, both the OptiEnz team and the Pebble Labs team have committed to supporting our company, providing ongoing research into related sensors, regulatory expertise, industry support and expanding the technology of QuaraSense.
The work of Pebble Labs in conjunction with OptiEnz Sensors, LLC, resulted in development of a prototype device, QuaraSense, that we are using for ongoing testing purposes. These achievements were led by the OptiEnz founder and Chief Technology Officer, Ken Reardon Ph.D. and Dr. Reardon now serves as our Chief Science Officer.
Market Opportunity
Bacterial infections have enormous impact upon the entire human population as well as those of other species: pets, livestock, and the shrimp and fish. produced by the aquaculture industry that are essential to the world’s future food security. Currently combatting harmful bacteria relies on extensive use of antibiotics across all these segments. For a variety of reasons, primarily overuse and preventative use, more and more bacteria are developing resistance to antibiotics. When a bacterium is resistant to several commonly used antibiotics, it is referred to as a “superbug”.
| 12 |
The traditional and still primary method of detecting a bacterial infection is to take a sample (for example, blood, urine, or saliva) from the patient — whether human, pet, or other animal — and put it in a special environment in a laboratory to grow any bacteria that are present. Since the test relies on bacterial growth, several days may be required to obtain results. For many diseases, the time required to test for an infection represents a critical period when the infection could grow to dangerous levels. For example, it takes between 48 and 72 hours to diagnose a sepsis causing infection. Current testing based upon growth may fail to detect evasive strains of bacteria. Furthermore, not all bacteria can be cultured in the laboratory.
Other laboratory-based methods of bacterial detection and identification typically require long processing times, can lack sensitivity and specificity, and/or require highly specialized equipment and trained technicians and are therefore costly and not available in all countries. These other methods include biochemical assays, immunological tests, and genetic analyses.
Aquaculture
It has been reported by the Marine Science Agency of the United Kingdom that more than half of all seafood consumed globally (160 million metric tons per year) is from aquaculture as opposed to wild-capture fisheries. Aquaculture production is trending to increasingly dominate the market over wild capture.
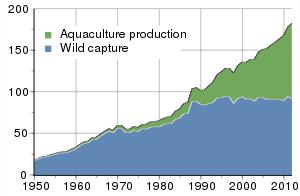
Global harvest of aquatic organisms
in million tons 1950-2010
FishStat Database 2014
In specific sectors, such as shrimp, disease losses may exceed 40% of global yield capacity with emergent diseases, such as Early Mortality Syndrome, threatening to collapse production in all nations. The global shrimp consumption market is in excess of $40 billion and is expected to reach $68 billion in the next ten years. It is estimated that there are over 50,000 shrimp producers with over 500,000 ponds and the numbers are expanding each year to keep pace with demand. QuaraSense would allow in situ testing in ponds with results available in minutes rather than days, increasing the ability for early detection and remediation.
Concerns surrounding the ability to rapidly confirm disease is the major constricting factor for expansion of the aquaculture industry to 2050 and could potentially cost the sector $6 billion in yield loss each year. Currently, shrimp diseases are detected by taking shrimp to a laboratory, dissecting them, and examining their organs under a microscope. This is time consuming and subject to sampling challenges as well as substantial environmental and biological perturbations.
QuaraSense has been developed to help reduce the more than $6 billion in aquaculture products (shrimp, salmon and others) lost annually to disease. Early notice of the outbreak of virulent bacteria can reduce overuse of antibiotics, thereby diminishing antibiotic resistance. Quara will also provide data services to allow users to track bacterial challenge over time to determine efficacy of treatment and key relationships between weather, feeding and other variables important for aquaculture production processes. With the cost effective and rapid response time that QuaraSense will be able to offer, we expect the demand for QuaraSense technology to be significant and to increase as aquaculture grows to meet world food demand.
| 13 |
Human Health
Superbugs are expected to cost the global economy $100 trillion in health care and lost productivity by 2050. Globally, more than 700,000 people die from superbug bacterial infections annually, a number that is increasing every year. For example, sepsis, a condition resulting from the body’s overwhelming and life-threatening response to infection, can lead to tissue damage, organ failure, and death. According to the Sepsis Alliance, on average approximately 30% of patients diagnosed with severe sepsis do not survive. Up to 50% of survivors suffer from post-sepsis syndrome. According to a 2006 study published in Critical Care Medicine, the risk of death from sepsis increases by an average of up to 7.6% with every hour that passes before treatment begins, often using a broad-spectrum antibiotic. Sepsis has been named as the most expensive in-patient cost in American hospitals by the Healthcare Cost and Utilization Project. One report stated the costs were $24 billion in 2014. Though not all sepsis-related infections are bacterial, it is the most common cause. Early detection and treatment are essential for survival and limiting disability for survivors.
Urinary tract infections, or UTIs are a severe public health problem exacerbated by the rise in multidrug resistant strains of bacteria and high recurrence rates. They are some of the most common bacterial infections, affecting 150 million people each year worldwide.1 In 2007, in the United States alone, there were an estimated 10.5 million office visits for UTI symptoms. Currently, the societal costs of these infections, including health care costs and time missed from work, are approximately USD $3.5 billion per year in the United States alone. UTIs are a significant cause of morbidity in infant boys, older men and females of all ages. UTI is a common catheter-induced illness as the result of patients staying in a hospital or clinic and these catheter-inducted UTIs are a major cause of extended hospital stays. In the US, Medicare is penalizing hospitals that fail to address UTI on the length of the hospital stay by reducing overall reimbursement.
UTIs are caused by a wide range of pathogens, including Gram-negative and Gram-positive bacteria, as well as fungi. Uncomplicated UTIs typically affect women, children and elderly patients who are otherwise healthy. Complicated UTIs are usually associated with indwelling catheters, urinary tract abnormalities, immunosuppression or exposure to antibiotics.
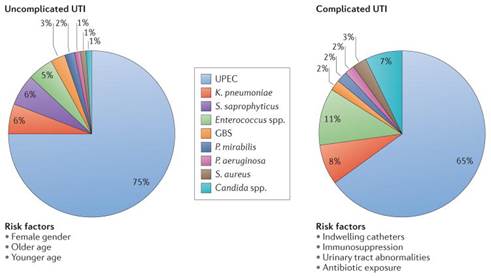
1 Flores-Mireles, A., Walker, J., Caparon, M. et al. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13, 269–284 (2015). https://doi.org/10.1038/nrmicro3432.
| 14 |
UTIs are responsible for a surprisingly diverse array of symptoms which are frequently misinterpreted. If an infection can be detected or ruled out quickly in the hospital setting, a patient may receive treatment before the infection becomes life threatening or, if ruled out, medical professionals can more quickly turn to analyzing other causes. If left untreated, a UTI can lead to death. UTIs may lead to a form of sepsis called urosepsis. Infections are a major cause of hospitalization and death in nursing homes. Nearly 380,000 people die of infections in US nursing homes every year.
QuaraSense is designed to quickly detect an infection and the need for treatment which is critically important for a number of infections. A follow up test with QuaraSense several hours after the start of treatment may also show whether the approach is effective. In cases where a different antibiotic is required, this confirmation of efficacy may help reduce the use of unnecessary antibiotics as well as help to limit the time of suffering or discomfort for patients. QuaraSense is also expected to be much more cost effective than current testing regimes and systems.
We estimate the total addressable market at $6.5 billion for medical device sales, $3.3 billion in annual data services revenue and $35 billion per year. These internal estimates are based on various external studies for home based medical device sales.
Animal Health
Global livestock populations are significantly endangered by disease. In order to avoid epidemics and spread of infection from animal to animal (and animal to humans), these infections should be monitored effectively. There are several examples of animal diseases such as brucellosis, respiratory and reproductive disorders and tuberculosis being commonly found in animals. Currently, these various disorders in livestock are detected using veterinary diagnostics carried out in laboratories using various techniques relying on samples of blood, feces and tissue. New techniques and techniques developed for human diagnosis are also being widely used in veterinary diagnostics. Immunoassay, hematology, and DNA test are various techniques currently used for detection of infection in animals. All of these are limited in throughput, response time, and expense.
We expect that veterinarians and animal owners will be able to use QuaraSense to quickly determine if their livestock or pet has a harmful bacterial infection. Seventy percent of antibiotics in the U.S. are given to livestock for prophylactic reasons. With QuaraSense, antibiotics may be given only when a bacterial threat is present, saving millions of dollars and limiting the rise of superbugs caused by the overuse of antibiotics. And early detection of infection will help mitigate suffering and will reduce veterinary costs for pets and their owners.
The global veterinary diagnostics market size is estimated to grow at a compound annual growth rate of over 7% from 2019 to 2026 and reach a global market value around $5.4 billion by 2026. Of that, the pet diagnostic market size was valued at over USD 2.1 billion in 2018 and the global market is expected to be $4 billion by 2026, according to a 2019 study by Grand View Research. Increasing demand for point of care diagnostics is expected further propel the growth of the pet diagnostics market.
Food Processing
Biofilms form when bacteria adhere to surfaces in aqueous environments and begin to excrete a slimy, glue-like substance that can anchor them to a variety of materials including metals, plastics, soil particles, medical implant materials and, most significantly, human or animal tissue. For example, biofilms can develop on the interiors of pipes, which can lead to clogging and corrosion. Biofilms on floors and counters can make sanitation difficult particularly in food preparation areas.
Bacterial infections that go undetected can have devastating impacts on businesses and the economy. The Center for Disease Control in a 2011 study estimated that each year roughly 1 in 6 Americans (48 million people) get sick, 128,000 are hospitalized, and 3,000 die as a result of foodborne diseases. In 2015, some customers of Blue Bell Ice Cream became ill and some died. The CDC detected Listeria bacteria in the manufacturer’s plants, resulting in the company having to issue a national recall of over 8 million gallons of ice cream, lay off 1,450 of its 3,900 employees and furlough another 1,400, and borrow $125 million to undertake a decontamination of its plants and the replacement of some equipment that could not be cleaned as a result of the said biofilm having been created by bacteria. The temporary shutdown of this one company led to hundreds of layoffs across other direct and indirect local supporting industries as the 200,000 tourists to the plant also disappeared.
| 15 |
Lately, nearly every month the Centers for Disease Control and Prevention report similar listeria infections linked to food products, such as milk, cheese, ice cream, and pork products. Inadequate detection and control of bacteria costs billions in food production losses each year. The annual global cost of food-borne illnesses is estimated at over $110 billion and over 48 million Americans are stricken ill each year with over 23,000 deaths from antibiotic-resistant bacteria.
These human and financial tolls were due to not detecting the virulent bacterial infection early enough. QuaraSense is expected to provide cost effective, near real-time testing for bacterial outbreaks in equipment, from food processing to water and oil pipelines. Early detection of a bacterial threat will help to minimize the creation of harmful biofilms that in some cases necessitates significant rebuilds of food infrastructure systems, often the only way to get rid of the infection within the processing equipment.
Our Technology
Our planned offering is centered around three product offerings:
QuaraSenseTM: handheld bacterial detection through proprietary quorum-sensing technology
QuaraViewTM: QuaraSense data services and consultation
QuaraTestTM: QuaraSense consumable cartridges
Quara Technology is Quorum Sensing
Bacteria communicate with each other in a population-dependent manner using a variety of chemical signal molecules called autoinducers, some of these molecules are species-specific and others are more general. The process is known as quorum sensing (“QS”). QS molecules are synthesized inside bacterial cells and are exported into the bacterial surroundings, where they may accumulate in increasing concentrations. Bacteria have receptors on their outer surface that bind QS molecules. At a certain level of receptor binding a cascade of events is triggered that change bacterial gene expression patterns, followed by changes in bacterial metabolism and operational mode. QS signal molecules may regulate a diverse array of functions, including antibiotic production, virulence, biofilm formation, stress and defense responses, motility, metabolism, and activities involved in interactions with hosts. The proprietary Quara technology focuses upon the same quorum sensing cues that the bacteria themselves rely upon to shift behavior from a minor to a major threat to the host organism (people and animals).
For example, one of the most serious pathogens of marine fish and invertebrates, particularly shrimp, is the bacterium Vibrio harveyi. This bacterium uses (2S,4S)-2-methyl-2,3,3,4- tetrahydroxy-tetrahydrofuran borate (BAI-2) as its QS signal. Hence, detection of BAI-2 may be a means to detect the presence of V. harveyi in industrial shrimp aquaculture operations. Other bacteria that affect shrimp may also use BAI-2 or the related AI-2, and Quara has evidence that AI-2 can be used as an indicator to detect gram-negative bacterial pathogens generally. Quara also has evidence that this approach can be used to detect other bacterial pathogens and pathogenic yeast.
Fluorescence Resonance Energy Transfer or “FRET” Technology
FRET is a physical phenomenon that is increasingly being used in biomedical research and drug discovery. It is the distance-dependent transfer of energy from one fluorescent molecule (the donor) to another fluorescent molecule (the acceptor). The transfer of energy leads to a reduction in the donor’s fluorescence intensity and an increase in the acceptor’s emission intensity. Due to its sensitivity to distance, FRET has been used to investigate molecular interactions. Quara technology uses FRET to rapidly measure the presence and magnitude of autoinducers or messaging molecules.
Quara Innovation Technology
A former Los Alamos National Laboratory Level 6 scientist, and the rest of his Pebble Labs Inc. research team developed and validated a protein that allows the bacterial QS molecule, BAI-2, to be detected using FRET. Further work by Pebble Labs in conjunction with OptiEnz Sensors, LLC, led by our Chief Science Officer Ken Reardon Ph.D., resulted in development of a prototype hardware unit that is being used for ongoing testing purposes. This operational prototype device has demonstrated the ability to obtain quantitative measurements of BAI-2 over a wide concentration range in laboratory solutions. We have also established a method of extending the functional lifetime of the detection protein, setting the stage for a measurement device with replaceable FRET protein cartridges, or QuaraTest, our primary consumable product.
| 16 |
Strategy
Once testing of our QuaraSense product is complete, we intend to commercialize our product, initially focusing on shrimp diseases in the aquaculture market and UTIs in the human health market. This will involve:
| ● | Contracting with manufacturers to produce the device and the QuaraTest consumable products to scale. We have identified several manufacturers capable of meeting our quality standards and believe we will be able to move forward quickly into production. | |
| ● | Signing customers who have indicated an interest. | |
| ● | Hiring sales and marketing personnel. | |
| ● | Entering into licensing agreement with distributors to expand our customer outreach. |
We have given priority to preparing QuaraSense for these markets for the following reasons:
Aquaculture
| ● | The aquaculture market needs better disease diagnostics. | |
| ● | Given the large economic losses due to bacterial infection in aquaculture, we may be able to demonstrate a substantial economic benefit. | |
| ● | QuaraSense portability is a good fit for potential aquaculture customers. | |
| ● | Quara and its partners have strong relationships existing players in the aquaculture market. | |
| ● | There are few regulatory hurdles compared with human health applications. | |
| ● | The market is currently underserved. |
UTIs in the Human Health Market
| ● | They are some of the most common bacterial infections, affecting 150 million people each year worldwide. | |
| ● | There are large economic losses each year due to infections. | |
| ● | Quara has been invited to participate in a clinical study along with the University of Utah School of Medicine. |
We anticipate it will take us 9 to 12 months from funding to begin shipping QuaraSense to shrimp producers that are capable of detecting the signaling molecule that is a general indicator of many of the most common types of bacteria. We expect that further development of our technology to be able to detect additional types of bacteria specific to the broader aquaculture market as well as the veterinary, health care, food processing and home health monitoring markets.
We have established advisory relationships with key opinion leaders and researchers in the field of urology at the University of Utah Medical School for the purpose of conducting clinical research structured around QuaraSense’s potential to detect UTIs and catheter induced UTI infections in human patients. This large market has substantial unmet needs. Quara is examining time frames required to commercialize its technology for this market in either remote point-of-care or hospital applications. All applications will require approval of the U.S. FDA (or other authority such as the CE marking in the EU) following regulatory classification, regulatory risk assessment and favorable clinical outcomes. We believe that this application is an appropriate application of Quara technology that, if successful, could yield significant benefits to patients and revenues for our company.
We recently entered into an agreement with Colorado State University Research Foundation to license certain intellectual property rights that we believe will enable us to develop a viral detection device to detect specific nucleic acids in patient samples. This is a platform technology that can be used for the diagnosis of infectious virus-based diseases such as HIV/AIDS, hepatitis C, dengue, Zika and COVID-19. Commercialization of a device with this functionality will depend on a significant amount of field testing, and approval to market the product from the FDA, which is a time consuming process and is far from certain at this stage.
| 17 |
In parallel with the above efforts, we will begin work to further develop our sensing technology to address the veterinary services and animal diagnostics market as there are few significant regulatory hurdles and it is also an underserved, large and growing market.
Intellectual Property
We have purchased, for a payment of $50,000, all of the intellectual property rights of OptiEnz Sensors, LLC in a portable instrument and associated software for measuring FRET between pairs of fluorophores in the field of the detection of microbial pathogens. The instrument, software, and methods developed can be used to measure FRET between any fluorophore pair and can make simultaneous measurements of multiple fluorophore pairs. We have agreed to grant OptiEnz Sensors, LLC an exclusive perpetual license to use that intellectual property for research purposes and to pay OptiEnz Sensors, LLC a royalty payment of 5% of our net sales up to a total payment of $450,000 and thereafter a royalty payment of 1.5% of net sales. Our Chief Science Officer, Dr. Ken Reardon is a founder and principal of OptiEnz.
We have an agreement to acquire, for a payment of $500,000, which is due by September 30, 2020, all of the intellectual property rights of Pebble Labs related to the “Improved Fluorescent Resonance Energy Transfer Based Biosensor Proteins and Their Methods of Use Thereof”, U.S. Provisional Patent No. 6273,0424 filed on September 12, 2018. On September 12, 2019, the provisional patent was converted to Patent Application US 19/50813. Following payment of the purchase price for that intellectual property, as discussed in “Use of Proceeds Below,” we have agreed to grant Pebble Labs an exclusive perpetual license to use that intellectual property for research purposes and to pay Pebble Labs a royalty payment of 1.5% of our net sales derived from products resulting from the Patent Application. If we fail to raise sufficient funds to make the payment to Pebble Labs by September 30, 2020, we will need to request an extension of the payment due date, which Pebble Labs is under no obligation to grant.
On May 14, 2020, the Company licensed from the Colorado State University Research Foundation an exclusive right in all territories and for all fields to the patent rights and know-how relating to technology known as PadLock-RCA-Nuclease Protection Lateral Flow Assay for the detection of pathogen sequences at the point of care. The Company will pay an upfront fee of $5,000 and pay royalties ranging from 3% to 4% based on volume of annual net sales. The Company will be subject to minimum royalty payments beginning in 2023 of $5,000 and $10,000 beginning in 2025. The Company has also agreed to milestone payments based on net sales ranging from $10,000 to $1 million. In addition, the Company will issue common shares to Colorado State University Research Foundation upon the Company completing proof of concept work demonstrating utility in detecting SARS-CoV-2 in an amount equal to 1% of all issued and outstanding shares on a fully diluted basis calculated on a post-closing basis.
Competition
Nearly all medical bacterial assays use the approach of trying to determine whether (and at what concentration) a specific bacterial species/strain is present. There are many types of pathogenic bacteria and, since bacteria can be beneficial, harmful, or neutral, it is difficult to find a feature that differentiates pathogenic from non-pathogenic bacteria.
Traditional testing relied on culturing cells from samples of blood, urine, or saliva. Culturing cells for this purpose is slow and insensitive. New technologies based on the Polymerase Chain Reaction (“PCR”) are being developed for medical microbial tests, and some are on the market. PCR is a way to make copies of a specific part of the bacterial DNA. This requires identification of a gene or other part of the DNA that is unique to the targeted bacterium (meaning that the user needs to know what species and strain to look for). This is potentially a challenge for diseases such as UTIs that are caused by a wide range of pathogens. Furthermore, the DNA must be extracted from the cells for processing. Relatively large, complex, and expensive equipment is required to process the samples, make copies via PCR, and quantify the outcome, and the procedure typically takes several hours. False negative measurements are frequently an issue. Current PCR-based devices must be used in a laboratory setting with highly trained technicians.
| 18 |
QuaraSense is designed to determine whether, and to what level, pathogenic bacteria are virulent. In certain states, pathogenic bacteria release QS molecules, such as AI-2 into their environment. These signaling molecules regulate many functions, including virulence. AI-2 is common to a wide array of pathogenic bacteria, so QuaraSense would have utility sensing the virulent state of many species of bacteria, and the technology has the potential to be expanded to other QS molecules used by other groups of pathogenic microorganisms. Because the signaling molecules are released into the surroundings, there is no need to collect samples that directly contain the bacteria. This allows less invasive and simpler testing; for example, aquaculture pond water could be tested rather than the shrimp or salmon. QuaraSense is small and robust, so it can be used where the samples are obtained, such as a shrimp pond, a livestock pen, or an urgent care clinic.
Comparison: Quara technology vs. PCR-based assays
QuaraSense FRET Technology |
Species-Specific (PCR Based) Assay | |||
| What is detected? | Detects living cells that are in a virulent state | Detects living and dead cells of the specific strain that is targeted | ||
| Sample requirements | Non-invasive; Fluid near the site of infection | Material containing bacteria | ||
| Sample processing | Automated, rapid removal of impurities | Cell disruption, sample cleanup, and amplification of DNA | ||
| Response time | Rapid (5 minutes) | Slow (hours) | ||
| Specificity | General detection of virulent bacteria. Can be multiplexed. | Highly specific; user must know what strain to look for. Can be multiplexed. | ||
| Equipment: ease of use | Simple | Requires training | ||
| Equipment: complexity | Simple | Complex; service contract likely needed | ||
| Equipment: size | Small, Portable and handheld | Large; must be used in a Laboratory | ||
| Cost | Cost effective | Significant cost | ||
| Consumables | Cost effective | Significant cost |
Competitors
Various competitors are developing and commercializing bacterial detection devices. Nearly all rely on PCR amplification of DNA, with the shortcomings mentioned previously.
| 19 |
LexaGene is developing a PCR-based analyzer for pathogen detection that is designed to be placed at the site of sample collection. The company states that the device will detect up to 15 pathogens at once with sensitivity and specificity and return results in about 1 hour. The LX2™ Genetic Analyzer is being designed for testing in veterinary diagnostics and human clinical diagnostics, as well as food safety testing, water quality monitoring, and other markets. As of May 2019, they were targeting a selling price of around $50,000 per unit. As reported in LexaGene’s third quarter Management’s Discussion and Analysis report the Company is still pre-revenue.
ElectroNucleics is a startup company working on feasibility testing of a new device based on electromechanical signal transduction for the low cost, amplification-free detection of DNA and RNA at low concentrations. They hope that an integrated microfluidic device eventually can be produced for pathogen detection.
Regulation
To market our QuaraSense and related technology for use in human health and to a lesser extent veterinary health, we will become subject to regulation and oversight by the Food and Drug Administration (“FDA”) and similar organizations in other countries. When a product is a medical device, a Section 510(k) marketing application must be submitted to the FDA. A 510(k) is a premarketing submission made to the FDA to demonstrate that the device to be marketed is as safe and effective. We will not be able to submit an application to the FDA related to human health applications until we have completed our final design and additional testing and the FDA may not grant its approval. If our application is approved, we will be subject to oversight by the FDA. The FDA also exercises oversight of veterinary health medical devices.
Employees
The company has a total of 6 persons who work for the company under consulting arrangements on a part time basis, apart from our full time Chief Executive Officer, as the company is still in the development phase. In addition, we expect to hire up to 10 people, primarily in product development, testing, marketing, and oversight of manufacturing partnerships to assist us in reaching commercialization of our product and in expanding our business thereafter.
| 20 |
Quara is a fully remote company in that each person employed or contracted by us works remotely. As a result, we do not have any offices or properties.
| 21 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements and the related notes included in this report. The following discussion contains forward-looking statements that reflect our plans, estimates, and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Unless otherwise indicated, the latest results discussed below are as of December 31, 2019.
Overview
Our company was incorporated under the laws of the State of Wyoming February 5, 2019. Quara is an emerging med-tech & biotech company focusing on the development and commercialization of a handheld bacterial quorum sensing device to provide rapid early warning to the presence of harmful pathogens. This PathogenometerTM, which we call QuaraSenseTM is a handheld, battery operated device that uses proprietary fluorescent detection proteins to detect the molecules bacteria release when they become virulent. Once testing of QuaraSense is complete, we intend to commercialize it, focusing initially on shrimp diseases in the aquaculture market and UTIs in the human health market, subject to regulatory approval. Thereafter, we intend to further develop QuaraSense to be used more broadly in the aquaculture market as well as in the veterinary, health care, food processing and home monitoring markets, among others.
We are a pre-revenue company with a limited operating history upon which to base an evaluation of our business and prospects. Our short operating history may hinder our ability to successfully meet our objectives and makes it difficult for potential investors to evaluate our business or prospective operations. We have not generated any revenues since inception, and we are not currently profitable and may never become profitable.
Our financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. Our ability to continue as a going concern is contingent upon its ability to raise additional capital as required. During the period from February 5, 2019 (inception) through December 31, 2019, the Company incurred net losses of $911,927. The Company does not currently generate any cash on its own. We have funded operations exclusively in the form of capital raised from the issuance of our equity securities.
Results of operations
| Period from February 5, 2019 (Inception) to December 31, 2019 | ||||
| Operating Expenses | ||||
| General and administrative | $ | 805,284 | ||
| Sales and marketing | 106,643 | |||
| Total operating expenses | 911,927 | |||
| Net loss | $ | (911,927 | ) | |
To date, we have not generated any revenues from our planned operations. We incurred a net loss of $911,927 during the period from February 5, 2019 (inception) to December 31, 2019, primarily consisting of consulting services of $613,215, legal and professional fees of $84,500, sales and marketing fees of $106,643 and other general and administrative fees of $107,569. We anticipate that operating expenses will continue to rise in connection with the continued development of our business operations.
| 22 |
Liquidity and Capital Resources
To date, we have generated no cash from operations and negative cash flows from operating activities. The Company has financed its activities to date by raising capital from private placements. These factors raise substantial doubt about our ability to continue as a going concern. Our future expenditures and capital requirements will depend on numerous factors, including the success of this offering and the ability to execute our business plan. We may encounter difficulty sourcing future financing.
We had cash in the amount of $360,359 as of December 31, 2019, and a working capital deficiency of $29,891 as of December 31, 2019.
We have contractual obligations for capital expenditures in the amount of $500,000, representing amounts payable to Pebble Labs under our agreement with them to acquire certain intellection property as described under “The Company’s Business – Intellectual Property.” We expect to use the proceeds from this offering to fulfill such commitments.
Plan of Operation
As noted above, the continuation of our current plan of operations requires us to raise significant additional capital. If we are successful in raising the maximum offering amount through our issuance of common shares in this offering, we believe that we will have sufficient cash resources to fund our plan of operations for the next 24 months. If we are unable to do so, we may have to curtail and possibly cease some operations. Furthermore, if we fail to raise at least $900,000 we anticipate that we will need to secure additional funding to fully commercialize our QuaraSense device.
We are a pre-revenue company in the development stage. We began operations in February 2019 and have a very limited operating history. Our plan of operations for the next few years includes completing the development work and additional testing of our QuaraSense device, development and optimized production of our planned products, developing, executing and monitoring sales and marketing campaigns.
We continually evaluate our plan of operations to determine the manner in which we can most effectively utilize our limited cash resources. The timing of completion of any aspect of our plan of operations is highly dependent upon the availability of cash to implement that aspect of the plan and other factors beyond our control. There is no assurance that we will successfully obtain the required capital or revenues, or, if obtained, that the amounts will be sufficient to fund our ongoing operations.
These circumstances raise substantial doubt about our ability to continue as a going concern. Our financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or amounts and classification of liabilities that might result from this uncertainty.
Trend Information
Because we are still in the startup phase and have only recently commenced operations, we are unable to identify any recent trends in revenue or expenses. Thus, we are unable to identify any known trends, uncertainties, demands, commitments or events involving our business that are reasonably likely to have a material effect on our revenues, income from operations, profitability, liquidity or capital resources, or that would cause the reported financial information in this Offering to not be indicative of future operating results or financial condition.
See the section entitled “Implications of Being an Emerging Growth Company” at the beginning of this Offering Circular for a discussion of the modified reporting requirements for “emerging growth” companies that we may take advantage of should be become a public reporting company.
| 23 |
DIRECTORS, EXECUTIVE OFFICERS AND SIGNIFICANT EMPLOYEES
The company’s executive officers and directors are listed below:
| Name | Position | Age | Date Appointed to Current Position |
Approximate Hours Per Week (if part- time) / full-time | ||||
| Executive Officers | ||||||||
| Rodney W. Reum | Executive Chairman | 64 | June 2019 | 30 | ||||
| Steven C. Eror | Chief Executive Officer | 66 | November 2019 | full-time | ||||
| Nicolette A. Keith | Chief Financial Officer | 49 | June 2019 | 30 | ||||
| David W. Smalley | General Counsel | 59 | May 2020 | 20 | ||||
| Kenneth F. Reardon | Chief Science Officer | 61 | June 2019 | 10 | ||||
| Yu-Cheng (Mike) Kao | Vice President, Finance | 55 | November 2019 | 10 | ||||
| Directors | ||||||||
| Rodney W. Reum | Executive Chairman | 64 | June 2019 | |||||
| Steven C. Eror | Chief Executive Officer and Director | 66 | May 2020 | |||||
| Michael B. Harrison | Director | 63 | May 2020 | |||||
| David W. Smalley | Director | 59 | May 2020 | |||||
| Larry K. Doan | Director | 66 | May 2020 |
Rodney W. Reum, Director and Executive Chairman
Mr. Reum has 35 years of senior executive leadership of both public and private companies. For over 10 years he has been the chief executive officer of Caballarius Global Holdings Inc., a company specializing in consulting services specializing in corporate financing, structuring and governance. He has played a key role in management of the financing of many enterprises up to CAD $1.3 billion dollars for one project. He has been an officer and director of several public companies assisting a number of them through the “going public” phase of their growth. He has also been instrumental in bringing several new technologies from the development stage to market in the alternative energy, military and law enforcement sectors. He was a founder, CEO and Chairman of Mission Ready Solutions Inc. from 2011 to 2017 and is currently the CFO of Fabled Copper Corp, a position he has held since 2018, and is a board member of the following public companies: Ponderous Capital Corp. (since 2018), and Efficacious Elk Capital Corp (since 2018). Mr. Reum is also a director of the following private corporations: Britec Computing Systems Ltd. (since 2005), Veridyne Power Corp. (since 2018) and Roxcel Cloud Inc (since 2018).
| 24 |
Steven C. Eror, Director and Chief Executive Officer
Mr. Eror is an experienced chief executive officer, director and investor in public and private companies with successes in the healthcare industry in cancer, inflammation and infection control. He is a strong business builder and business development professional with specific experience in predictive analytics, drug delivery, medical devices, business development, entrepreneurship, and strategic planning. Steven has a lifelong interest in technology funding and investment. His experience includes serving as CEO of ProLung, Inc. (2005 - 2018), MacroMed, Inc. (2002 - 2004), Consonus, Inc. (2001 - 2002) and as an Adjunct Professor of Finance at the University of Utah (2004-2006) where he also received a BA in Economics and French and an MBA. He is also Fellow at the National Association of Corporate Directors. In 2018, Mr. Eror was terminated as CEO of ProLung for cause as claimed by the company and he resigned from its board of directors along with 4 other directors of the company. Mr. Eror and seven other persons then initiated an unsuccessful proxy solicitation requesting that the shareholders of ProLung increase the size of the board and elect each of them as directors to the ProLung board. Mr. Eror and ProLung subsequently resolved their differences and agreed that Mr. Eror would provide consulting services to ProLung. Our Board of Directors believes that Mr. Eror’s business education, expertise, and extensive executive experience in the biotechnology industry qualify him for service as a member of our Board of Directors.
Nicolette A. Keith, Chief Financial Officer
Ms. Keith brings over 25 years of accounting and managerial experience in both the public and private sectors Ms. Keith has acted as Chief Financial Officer of public companies listed on the TSX Venture Exchange and the Frankfurt Exchange as well as held a senior accounting role for a company listed on the New York Stock Exchange. Areas of focus for Ms. Keith will include regulatory reporting, capital management, business process improvements, system optimization, internal controls and management reporting. Ms. Keith earned an Arts and Science Bachelor’s degree from the University of Victoria and subsequently obtained the Certified General Accountants (CPA, CGA) designation. She is currently the CFO for the Village of Keremeos, BC (2015-present); acting CFO for the following Exchange listed companies: Ximen Mining Corp (2018-present), GGX Gold Corp. (2018-present), Fort St James Nickel Corp. (2018-present), Golden Dawn Minerals Inc. (2019-present) and formerly Mission Ready Solutions Inc. (2012-2017). She is also currently a contributing board member for Ponderous Panda Capital Corp. and the CEO of privately held 2K Services Ltd. (2017-present).
David W. Smalley, Director and General Counsel
Mr. Smalley has nearly 30 years’ experience practicing corporate and securities law, providing legal services for financing private and public companies. Mr. Smalley has been and continues to be a director of a number of capital pool companies listed for trading on the TSX-Venture Exchange. He has served as director and officer of numerous public and private companies including: Fabled Copper Corp. (Director, 2017 to present), Flying Monkey Capital Corp. (Director, 2014 to 2017), Empower Environmental Solutions Inc. (Director, 2012 to 2017), Ponderous Panda Capital Corp. (Director, President and CEO, 2017 to present), Efficacious Elk Capital Corp. (Director and Corporate Secretary, 2018), Avidian Gold Inc. (formerly Marching Moose Capital Corp.) (Director, President and CEO, 2014 to 2015), Scorpio Gold Corporation (Director, 2009 to 2018), Trait Biosciences Inc. (Director and Chief Legal Counsel, 2017 to present), Pebble Labs (Director and Chief Counsel, 2016 to 2020). Mr. Smalley is also a principal in David Smalley Law Corp. (2013 to present).
Kenneth F. Reardon, Chief Science Officer
Dr. Reardon is a Professor (since 1988) and Jud and Pat Harper Chair of Chemical and Biological Engineering (since 2013) and holds joint appointments in several other programs at Colorado State University, including Cell and Molecular Biology and Biomedical Engineering. In 2010, Dr. Reardon founded OptiEnz Sensors, LLC (“OptiEnz”) and remains its Chief Technology Officer. OptiEnz produces biosensors that continuously monitor organic chemicals in aqueous solutions. His research combines sensor development, bioreactor analysis, systems biology, and applied microbiology and microbial ecology. Dr. Reardon received his B.S. degree from the University of Pennsylvania and his Ph.D. from California School of Technology and Applied Science, both in chemical engineering.
| 25 |
Yu-Cheng (Mike) Kao, Vice President, Finance
Mr. Kao is the principal partner of WDM Chartered Professional Accountants, a Vancouver-based firm, and has been a partner with the firm since 1998 and has been with the firm since 1991. He is proficient with both personal and corporate accounting, providing client services such as business advisory, consultancy, accounting, and tax services. WDM is registered with the Canadian Public Accountability Board, (CPAB), and the U.S. Public Company Accounting Oversight Board, (PCAOB), requiring a commitment to maintain the highest standards of professional objectivity, audit quality, and technical excellence. Mr. Yao is also the CFO and board member of Ponderous Panda Capital Corporation.
Michael B. Harrison, Director
Mr. Harrison has 35 years of experience in investment banking with interests in resource, energy, and biotechnology sectors. He has served as CEO of two companies that he led to successful exits. He has been on the board of directors for numerous international publicly listed companies and has raised millions of dollars in funding for private and publicly traded companies. Since 2016, Mr. Harrison has been the Chairman and CEO of Pebble Labs and, since 2017, the Chairman of Trait Bio Sciences Inc. Mr. Harrison is also the Chairman of Pique Capital, Hong Kong, ROC (since 2017), a Director and CEO of Fabled Copper Corp. (since 2018) and a Director of Efficacious Elk Capital Corp. (since 2018).
Larry K. Doan, Director
Mr. Doan is a retired executive who was a director/founder and Vice President of Extreme CCTV (1999-2008), a company that he helped take public on the Toronto Stock Exchange and was part of the Directors committee that saw the takeover of the company in 2007. His focus had been on developing sales channels in North America and Europe. Mr. Doan has served as a Director of Mission Ready Services Inc. (2013-2014), a TSX-Venture Exchange listed company that develops and manufactures products for use by militaries and first responders. Has been a director of a number of capital pool companies including: Flying Monkey Capital Corp., (2015 to 2018) and was a director of Avidian Gold Inc. (formerly Marching Moose Capital Corp.) (2014 to 2015). He is currently also a contributing director of Ponderous Panda Capital Corp. (2018-present).
Our Advisors
Our business benefits from the advice and support of a strong team of advisors.
Our Scientific Advisors
Dr. Mikhail Sinev - Senior Scientist Advisor
Dr. Sinev has a Ph.D. in Biophysics from Moscow Institute of Physics and Technology, Russia. Dr. Sinev has an expertise in protein purification techniques, and application of fluorescence techniques for ligand biding studies. He is the author of 17 publications in refereed journals and is currently actively involved with Pebbles Labs.
Dr. Pedro Miguel Melo Da Costa Nunes - Senior Scientist Advisor
Dr. Costa-Nunes has a Ph.D. in Biology from the Instituto Superior de Agronomia, Technical University of Lisbon, Portugal. His expertise is in plant Argonaute protein diversity and function, and he is the author of 22 publications. He is currently actively involved with Pebbles Labs.
Dr. Aristobulo Loaiza - Advisor – Aquaculture
Dr. Loaiza is the former Senior Manager of BASF New Business. He is known as a natural leader who leverages systems thinking and networking to drive business results. He has extensive chemicals, biotechnology and commercial training with deep knowledge and a solid network in various value chains including Biotech, Agriculture, and Nutrition and Food Safety. Dr. Loaiza received his M.S. in Bioinorganic Chemistry from UCLA and his Ph.D. in chemistry/ biochemistry from Purdue University.
Dr. Rick DeRose - Product Development
Dr. DeRose has more than 25 years of experience in Ag Biotech at Syngenta and Bayer developing innovation strategies for R&D projects. He was co-inventor of the first Roundup Ready Corn varieties. Dr. DeRose received the ASPB 2017 Innovation Prize for Agricultural Technology for translating discovery research into real-world outcomes in Global Agriculture and is an Industry Fellow for the Center for Innovation Management Studies at North Carolina State University. He received a Ph.D. in Medical Microbiology and Immunology from Wake Forest School of Medicine.
| 26 |
Dr. Brian Heinze – Advisor – R&D
Dr. Heinze is the R&D Director at OptiEnz, responsible for research, product development, and project management. He has been actively involved in researching and developing optical biosensors for more than eight years, receiving numerous awards including the National SMART Grant, NASA Space Grant, and a National Science Foundation Small Business Innovation Research Award. Dr. Heinze earned a B.S. degree in biology and a Ph.D. in biosystems engineering, both from the University of Arizona with honors.
Dr. Anne Lo - Advisor – Animal Diagnostics
Dr. Anne Lo trained as a veterinary surgeon and worked in a number of clinical positions. She subsequently joined the management consulting firm Bain & Co. in London, before moving to a strategy role with WorldPay. Dr. Lo is now with Horizons Ventures based in Hong Kong, where she primarily covered science and healthcare investments. Dr. Lo received her B.Sc. and BVM&S degrees from the University of Edinburgh and her Ph.D. from the University of Cambridge.
Dr. James M. Hotaling, MD, MS, FECSM – Urinary Tract Infection Clinical Trial Advisor
Dr. Hotaling is a fellowship-trained urologist specializing in Male Infertility and Men’s Health. He completed his undergraduate work at Dartmouth, graduating magna cum laude with a double major in history and biophysical chemistry. He then went to Duke for medical school and completed a 6-year residency at the University of Washington, where he trained with one of the top penile reconstructive surgeons in the world, Dr. Hunter Wessells. Dr. Hotaling elected to pursue an additional year of training under Dr. Craig Niederberger at the University of Illinois at Chicago, focusing on Male Infertility and Men’s Health. He is also one of the only Men’s Health and Infertility experts in the United States to have undergone additional training to become a Fellow of European College of Sexual Medicine (FECSM). He has over 85 publications, is funded by the NIH to study Erectile Dysfunction and Male Infertility and is regularly invited to speak at conferences all over the United States on Male Infertility, Men’s Health and Erectile Dysfunction. He has been on the faculty at the University of Utah since 2013 and is currently the medical director of the fertility integrated practice unit, the director of the Men’s Health program and a co-director of the fellowship in reconstructive urology and men’s health. In addition, Dr. Hotaling is an editor of Fertility and Sterility, the premier journal in the field.
Dr. David Dandy– Senior Science Advisor
Prior to joining Colorado State University in 1992, Dr. Dandy spent four years as a Senior Staff Member in the Advanced Materials Department at Sandia National Laboratories. In the mid-2000s, Dr. Dandy switched his primary focus to the development of novel miniaturized biosensing devices. That work has involved detection and identification of biomarkers associated with bacterial and viral infection in humans, and it has recently expanded to plant pathogens. Target biomarkers have included nucleic acids, antigens, host antibodies, and intact virus particles. Dr. Dandy’s research in diagnostics focuses on developing and implementing microfluidic solutions for passive and active mixing strategies, passive pumping, automated flow control in microfluidic networks, and microparticle concentration and separation. He holds a total of five US patents on two label-free biosensing technologies, the first employing an integrated optical waveguide and the second an optical method based on enzymatic conversion of target analyte. Dr. Dandy also has three pending patent applications. Dr. Dandy earned a BS in chemical engineering at the University of California, Davis, and MS and PhD degrees in chemical engineering from the California Institute of Technology. He is currently Professor and Department Head of Chemical and Biological Engineering and has a joint appointment as Professor of Biomedical Engineering
Dr. Charles Henry – Senior Science Advisor
Dr. Henry joined Colorado State University in 2002 and is now Professor of Chemistry with a joint appointment as Professor of Chemical and Biological Engineering. He served as Chair of the Department of Chemistry from 2014-2018. Dr. Henry’s research interests lie broadly in the development of lab-on-a-chip technologies to study environmental and biological phenomena. Major techniques used include microfabrication, chromatography, electrochemistry, electrophoresis, microfluidics, microscopy, and 3D printing. Dr. Henry has published over 180 peer-reviewed publications and generated eight issued patents. In addition, Dr. Henry has been involved in five spin-out companies from Colorado State University with products ranging from industrial water quality sensors to low-cost environmental diagnostics. Dr. Henry’s current research includes projects to develop low-cost capillary flow driven diagnostic assays and biosensors for infectious diseases (bacterial and viral) and disease biomarkers, and the creation of new tissue-on-a-chip systems that integrate living ex vivo tissue into microfluidic devices.
| 27 |
Dr. Brian Geiss– Senior Science Advisor
Dr. Geiss is Associate Professor in Microbiology, Immunology, and Pathology at Colorado State University. He has a wide range of experience, from protein biochemistry and structural biology to molecular virology and in vivo pathogenesis analyses. Since 2005, he has studied RNA viruses including flaviviruses, alphaviruses, and coronaviruses. Dr. Geiss has been supported by the NIH since 2006 to develop novel antiviral targeting flavivirus RNA capping and define the mechanisms of viral RNA capping. Among other accomplishments, Dr. Geiss identified the first patented guanylyltransferase-targeted antiviral molecule that can suppress the replication of multiple different flaviviruses. He has also developed a number of tools for virology, including several alphavirus replicon and infectious virus launch systems. Recently, Dr. Geiss has focused on development of novel biosensors for the detection of infectious diseases, including pathogen nucleic acids, virus particles, intact bacterial cells, and pathogen-specific antibody responses in a variety of sample matrices.
Our Business Advisors
Keara Sauber - Advisor– Human Health Devices
Ms. Sauber began her career at GlaxoSmithKline in a top sales role and has won multiple awards for running a $20M+ per quarter territory while performing an analyst role while on a rotation. After working in the healthcare space, Ms. Sauber joined a fintech specialty lender backed by Goldman Sachs as Vice President. As an executive at a fast growth fintech company, Ms. Sauber built strategic partnerships with companies in various verticals to build proprietary lending solutions, helping businesses achieve their financial goals. In 2017, she and her team originated more than $50 million in capital needs.
Cynthia Ekberg Tsai - Advisor – Business Development
Ms. Tsai is known for her unique skills in working with executives from companies that desire strategic advice and hands on experience in partnering, investment and brand building in the US, Europe, South America and Asia. In 2020, she was designated one of the top 100 people in finance. She is currently Chief Executive Officer of Tana Systems, a global software and IT company based in the U.S. and India. Ms. Tsai leads a team of 50 engineers in the U.S. and 500 engineers in India. She is also the Chief Executive Officer of Healthquest, a global biotechnology and medical technologies advisory firm.
Dr. Tichafa Munyikwa - Regulatory Affairs
Dr. Munyikwa has over 20 years’ experience at major Ag Biotech companies as a Senior Research Scientist and Global Regulatory Expert. He has worked in the fields of agribusiness, biotechnology, plant molecular and cell biology, biologicals, RNA interference, genome editing, and synthetic biology. Dr. Munyikwa received his Ph.D. in Cell and Molecular Biology from Wageningen University (The Netherlands).
Rod Turner - Advisor – Financing
Mr. Turner is the Chief Executive Officer of Manhattan Street Capital specializing in Reg A+, Reg D and US STO advisory services. He was a senior executive for two IPOs to NASDAQ (Ashton-Tate and Symantec). Mr. Turner built a VC firm and was an angel investor in Ask Jeeves, INFN, AMRS, eASIC, and Bloom Energy. His background is as an engineer and he has skills in all areas of business. Mr. Turner is a sought-after speaker in the areas of Reg A+ and Reg D financings.
Miro Cernetig - Advisor – Brand Strategy & Communications
Mr. Cernetig is the founder of Catalytico ~ ideas in motion. He has built brands for companies and projects with values measured in the billions of dollars. An award-winning journalist and filmmaker, Mr. Cernetig brings deep research and story-making to brands and strategy, thanks to decades of travel throughout Canada, Asia, Europe and the United States. He has written for The Globe & Mail as bureau chief in Beijing, New York, Vancouver, Alberta and The Arctic. His writing has appeared in The International Herald Tribune, The Economist and The Toronto Star, where he was Montreal bureau chief. His films have been broadcast internationally on the BBC, CBC, National Geographic and other networks.
Arnold Peinado – Advisor – Business Development
Mr. Peinado is a retired partner of the international law firm of Milbank, Tweed, Hadley & McCloy. He was a senior partner in the Firm’s Global Securities group and Transportation and Space Finance team. He joined the firm in 1982 as an associate, having received a JD from Harvard Law School and an MBA Harvard Business School. Mr. Peinado has been a member of the board of the Urban Justice Center since 1995 and is also: a trustee of the New Jersey Chapter of The Nature Conservancy, a leading conservation and environmentally focused NGO, a director of iCivics, the largest provider of on-line civic education in the US and a director of the Alleluia Fund, the grants-making arm of the Episcopal Diocese of Newark, N.J.
| 28 |
COMPENSATION OF DIRECTORS AND EXECUTIVE OFFICERS
For the period from February 5, 2019 (Inception) to December 31, 2019, we compensated our executive officers as follows. We did not pay cash compensation to our directors in connection with their board service in 2019.
| Name | Capacities in which compensation was received |
Cash compensation ($) (1) |
Other compensation ($) (2) |
Total compensation ($) | ||||
| Rodney W. Reum | Executive Chairman | 144,000 | Nil | 144,000 | ||||
| Steven C. Eror | Chief Executive Officer (3) | Nil | Nil | Nil | ||||
| Nicolette A. Keith | Chief Financial Officer | 144,000 | Nil | 144,000 | ||||
| Kenneth F. Reardon | Chief Science Officer | 45,000 | Nil | 45,000 | ||||
| David W. Smalley | General Counsel (4) | Nil | Nil | Nil | ||||
| Yu-Cheng (Mike) Kao | Vice President Finance | Nil | Nil | Nil |
| (1) | Payment of these amounts has been deferred as discussed below. | |
| (2) | Equity-based compensation that we have agreed to grant to the named individuals is discussed below. | |
| (3) | Appointed as CEO on December 1, 2019. | |
| (4) | Appointed as General Counsel on May 1, 2020. |
Mr. Reum serves as Executive Chairman pursuant to a Consulting Agreement we have entered into with Caballarius Global Holdings Inc. Under that Agreement, Caballarius, of which Mr. Reum is a principal, is entitled to fees of $16,000 per month and a one-time signing bonus of $32,000. These amounts are deferred pending our completing a financing of not less than $2.5 million. If we become a reporting company on any recognized public exchange or our shares are qualified under Regulation A of the Securities Act, this monthly fee increases to $20,000. Mr. Reum was issued 1,340,000 shares for services provided to the Company prior to April 2019. In addition, under this agreement we have agreed to grant to Mr. Reum incentive stock options exercisable for 150,000 shares of our Common Stock at $0.25 per share for a period of 10 years and an additional incentive stock options exercisable into 150,000 shares of our Common Stock at $2.50 per share for a period of 10 years. Mr. Reum is also eligible to participate in any future benefit or bonus programs that we may establish for senior executives.
Mr. Eror was appointed to be our Chief Executive Officer by our board of directors on December 1, 2019. Mr. Eror receives no salary, bonus or other form of earned income in connection with his service as our Chief Executive Officer. We have agreed to reimburse Mr. Eror for his expenses incurred in connection with his services to our company, including an expense allowance of $3,000 per month.
Ms. Keith serves as Chief Financial Officer pursuant to a Consulting Agreement we have entered into with 2K Services Ltd. Under that Agreement, 2K, of which Ms. Keith is a principal, is entitled to fees of $16,000 per month and a one-time signing bonus of $32,000. These amounts are deferred pending our completing a financing of not less than $2.5 million. If we become a reporting company on any recognized public exchange or our shares are qualified under Regulation A of the Securities Act, this monthly fee increases to $20,000. Ms. Keith was issued 740,000 shares for services provided to the Company prior to April 2019. In addition, under this agreement we have agreed to grant to Ms. Keith incentive stock options exercisable for 150,000 shares of our Common Stock at $0.25 per share for a period of 10 years and an additional incentive stock options exercisable into 150,000 shares of our Common Stock at $2.50 per share for a period of 10 years. Ms. Keith is also eligible to participate in any future benefit or bonus programs that we may establish for senior executives.
| 29 |
Mr. Smalley serves as General Counsel pursuant to a Consulting Agreement we have entered into with David Smalley Law Corp. Under that Agreement, David Smalley Law Corp., of which Mr. Smalley is a principal, is entitled to fees of $10,000 per month. These amounts are deferred pending our completing a financing of not less than $2.5 million. If Mr. Smalley provides more than 40 hours of service a month, he is entitled to receive $600 per hour for each hour over 40 hours. Mr. Smalley is also eligible to participate in any future benefit or bonus programs that we may establish for senior executives
Dr. Reardon serves as Chief Science Officer pursuant to a Consulting Agreement we have entered into with KFR Tech, LLC, Under that Agreement, KFR Tech, of which Dr. Reardon is a principal, is entitled to fees of $5,000 per month for 40 hours of service each month and a one-time signing bonus of $10,000. These amounts are deferred pending our completing a financing of not less than $2.5 million. If Dr. Reardon provides more than 40 hours of service a month, he is entitled to receive $125 per hour for each hour over 40. Dr. Reardon was issued 300,000 shares for services provided to the Company prior to April 2019. In addition, under this agreement we have agreed to grant to Dr. Reardon incentive stock options exercisable for 150,000 shares of our Common Stock at $0.25 per share for a period of 10 years and an additional incentive stock options exercisable into 150,000 shares of our Common Stock at $2.50 per share for a period of 10 years. Dr. Reardon is also eligible to participate in any future benefit or bonus programs that we may establish for senior executives.
Mr. Kao serves as Vice President Finance pursuant to a Consulting Agreement we have entered into and he is entitled to fees of $5,000 per month for 40 hours of service each month beginning on the date that our Offering Statement for this offering is qualified by the SEC. The payment of these amounts is deferred pending our completing a financing of not less than $2.5 million. If Mr. Kao provides more than 40 hours of service a month, he is entitled to receive $125 per hour for each hour over 40. In addition, under this agreement we have agreed to grant to Mr. Kao incentive stock options exercisable for 100,000 shares of our Common Stock at $0.25 per share for a period of 10 years and an additional incentive stock options exercisable into 100,000 shares of our Common Stock at $2.50 per share for a period of 10 years. Mr. Kao is also eligible to participate in any future benefit or bonus programs that we may establish for senior executives.
We intend to award our non-management board members each 200,000 shares of options to purchase our company’s stock for their services in 2020.
| 30 |
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITY HOLDERS
The following table sets out, as of May 25, 2020, the voting securities of the company that are owned by executive officers and directors or that they have a right to acquire. No other person holds more than 10% of any class of the company’s voting securities or has the right to acquire those securities. The address of each officer and director is the Company’s address as set forth on the cover page of this Offering Circular.
Name and address of beneficial owner | Title of class | Amount and nature of beneficial ownership | Amount and nature of beneficial ownership acquirable (1) | Percent of class | ||||||||||
| Executive Officers and Directors | ||||||||||||||
| Rodney W. Reum Director and Executive Chairman | Common Stock | 3,600,000 | 300,000 | 10.1 | % | |||||||||
| Steven C. Eror Director and Chief Executive Officer | Common Stock | nil | nil | nil | ||||||||||
| Nicolette A. Keith Chief Financial Officer | Common Stock | 740,000 | 300,000 | 2.7 | % | |||||||||
| Kenneth F. Reardon Chief Science Officer | Common Stock | 600,000 | 300,000 | 2.3 | % | |||||||||
| Yu-Cheng (Mike) Kao Vice President Finance | Common Stock | 1,000,000 | 200,000 | 3.1 | % | |||||||||
| Michael B. Harrison Director | Common Stock | 2,561,618 | 200,000 | 7.1 | % | |||||||||
| David W. Smalley Director and General Counsel | Common Stock | 1,657,296 | 200,000 | 4.8 | % | |||||||||
| Larry K. Doan Director | Common Stock | 500,000 | 200,000 | 1.8 | % | |||||||||
| All current executive officers and directors as a group (8 people) | Common Stock | 10,658,914 | 1,700,000 | 30.8 | % | |||||||||
| (1) | Represents options to purchase the identified number of shares of Common Stock and assumes that all such options are vested. |
| 31 |
INTEREST OF MANAGEMENT AND OTHERS IN CERTAIN TRANSACTIONS
On March 26, 2019, we entered into an assignment of Intellectual Property Rights and a Research License and Royalty Calculation Agreement with Pebble Labs. The Chief Executive Officer and Chief Counsel and both directors of Pebble Labs are now directors of the company. Pebble Labs has assigned to us any and all intellectual property rights relating to the Improved Fluorescent Resonance Energy Transfer Based Biosensor Proteins and Their Methods of Use Thereof, U.S. Provisional Patent No. 6273,0424 filed on September 12, 2018, for a one-time payment of $500,000 and an ongoing royalty fee equal to 1.5% of net sales derived from products resulting from the Provisional Patent. The one-time fee is payable on or before September 30, 2020. On September 12, 2019, the Provisional Patent was converted to Patent Application US 19/50813.
On April 13, 2020 we entered into an assignment of intellectual property rights from OptiEnz Sensors, LLC for consideration of $50,000, previously advanced to OptiEnz during 2019. Our Chief Science Officer, Dr. Ken Reardon is a founder and principal of OptiEnz. Under the assignment, OptiEnz has assigned any and all intellectual property rights relating to a portable instrument and associated software for measuring fluorescence resonance energy transfer (“FRET”) between pairs of fluorophores. The instrument, software, and methods developed can be used to measure FRET between any fluorophore pair and can make simultaneous measurements of multiple fluorophore pairs. In addition, we will pay a royalty to OptiEnz of 5% of the net sales of the product up to a total royalty payment of $450,000, after which the royalty payment will be 1.5% of the net sales of the product. During 2019, we advanced $50,000 to OptiEnz for development work on QuaraSense product, which was applied towards the purchase of intellectual property under the assignment.
As noted under “Compensation of Directors and Executive Officers,” the cash compensation payable to our executive officers has been deferred pending completion of an offering of not less than $2.5 million,
| 32 |
The company is offering up to 3,082,779 shares of Common Stock and the selling shareholders are offering up to 365,497 shares of Common Stock. See “Plan of Distribution and Selling Security Holders.”
CAPITAL STOCK
General
Our company was incorporated in the State of Wyoming on February 5, 2019. The following description summarizes the most important terms of the company’s capital stock. This summary does not purport to be complete and is qualified in its entirety by the provisions of our articles of incorporation (“Articles”), a copy of which has been filed as an exhibit to the Offering Statement of which this Offering Circular is a part. For a complete description of our capital stock, you should refer to the Articles and to the applicable provisions of Wyoming law.
We are authorized to issue and unlimited number of shares of Common Stock, without par value. As of May 25, 2020, our outstanding shares of capital stock consisted of 38,457,361 shares. We have also agreed to issue shares of common stock to Colorado State University Research Foundation as discussed under “The Company’s Business—Intellectual Property” or In addition, we have granted 3,500,000 options under our Stock Option Plan. Our Stock Option Plan reserves for issuance a number of shares equal to 15% of the number of shares of Common Stock that are issued, or 5,768,604 shares of Common Stock as of May 25, 2020.
Voting Rights
Each holder of the company’s Common Stock is entitled to one vote for each share on all matters submitted to a vote of the shareholders, including the election of directors. Directors are elected by a plurality of the votes cast by the shares entitled to vote; shareholders do not have a right to cumulate their votes for directors.
Dividend Rights
Holders of Common Stock are entitled to receive dividends, as may be declared from time to time by the Board of Directors out of legally available funds. The company has never declared or paid cash dividends on any of its capital stock and currently does not anticipate paying any cash dividends after this offering or in the foreseeable future.
Liquidation Rights
In the event of a voluntary or involuntary liquidation, dissolution, or winding up of the company, the holders of Common Stock are entitled to share ratably in the net assets legally available for distribution to shareholders after the payment of all debts and other liabilities of the company.
Preferred Stock
The company is not authorized to issue any preferred stock.
| 33 |
PLAN OF DISTRIBUTION AND SELLING SECURITY HOLDERS
We are offering a maximum of 3,082,779 shares of Common Stock to the public and certain of our shareholders are offering a maximum of 365,497 shares of Common Stock, in each case at a price of $5.80 per share on a “best efforts” basis. There is no minimum offering amount; however, the minimum investment for each investor is $580.00, or 100 shares. Potential investors should be aware that there can be no assurance that any other funds will be invested in this offering other than their own funds.
We plan to market the securities in this offering both through online and offline means. Online marketing may take the form of contacting potential investors through electronic media and posting our Offering Circular or “testing the waters” materials on an online investment platform.
The offering will terminate at the earliest of: (1) the date at which the maximum offering amount has been sold, (2) the date which is three years from this offering being qualified by the SEC, and (3) the date at which the offering is earlier terminated by us at our sole discretion.
The company may undertake one or more closings on a rolling basis. At each closing 70% of the shares sold to new investors will be newly issued shares sold by us and 30% will be shares sold by the selling shareholders on a pro rata basis (rounding to eliminate fractional shares) until all of the shares offered by the selling shareholders have been sold. After each closing, funds tendered by investors will be available to the company and the selling shareholders.
We and the selling shareholders are offering securities in all states.
The company has engaged Dalmore Group, LLC (“Dalmore”) a broker-dealer registered with the SEC and a member of FINRA, to perform the following administrative and technology related functions in connection with this offering, but not for underwriting or placement agent services:
| ● | Review investor information, including KYC (“Know Your Customer”) data, AML (“Anti Money Laundering”) and other compliance background checks, and provide a recommendation to the company whether or not to accept investor as a customer. | |
| ● | Review each investors subscription agreement to confirm such investors participation in the offering and provide a determination to the company whether or not to accept the use of the subscription agreement for the investor’s participation. | |
| ● | Contact and/or notify the company, if needed, to gather additional information or clarification on an investor; | |
| ● | Not provide any investment advice nor any investment recommendations to any investor. | |
| ● | Keep investor details and data confidential and not disclose to any third-party except as required by regulators or pursuant to the terms of the agreement (e.g. as needed for AML and background checks). | |
| ● | Coordinate with third party providers to ensure adequate review and compliance. |
As compensation for the services listed above, the company has agreed to pay Dalmore a $25,000 advance fee plus a $3,500 FINRA corporate filing fee. The advance fee will cover reasonable out-of-pocket accountable expenses actually anticipated to be incurred by Dalmore, such as preparing the FINRA filing, working with our SEC counsel in providing information to the extent necessary, coordination with any third party vendors involved in this offering and any other services necessary and required. Dalmore will refund to us any portion of the advance fee related that is not used. In addition, the company and the selling shareholders will pay their proportional share of commissions to Dalmore equal to 1% of the amount raised in the offering to support the offering once the SEC has qualified the Offering Statement and the offering commences. The company estimates that total fees due to pay Dalmore would be $225,000 for a fully subscribed offering, in addition to the payment of the FINRA corporate filing fee. These assumptions were used in estimating the expenses of this offering.
The Company has engaged the Creative Direct Marketing Group, In. (“CDMG”) to design and carry out an integrated marketing strategy for this offering including branding, direct mail, digital market integration, social media, video, TV and radio. We have agreed to pay CDMG approximately $210,000 for these services.
| 34 |
Incentives
The company intends to offer marketing promotions to encourage potential investors to invest, which may include offers such as branded promotional merchandise and discounts on the purchase of the Company’s products. Details on the company’s current incentives, if any, can be found on the company’s offering page found at www.manhattanstreetcapital.com/Quara.
TAX CONSEQUENCES FOR RECIPIENT (INCLUDING FEDERAL, STATE, LOCAL AND FOREIGN INCOME TAX CONSEQUENCES) WITH RESPECT TO THE INVESTMENT BENEFIT PACKAGES ARE THE SOLE RESPONSIBILITY OF THE INVESTOR. INVESTORS MUST CONSULT WITH THEIR OWN PERSONAL ACCOUNTANT(S) AND/OR TAX ADVISOR(S) REGARDING THESE MATTERS.
The Online Platform
The company entered into an engagement agreement (the “Engagement Agreement”) with Manhattan Street Capital. In connection with this offering, the company will pay Manhattan Street Capital fees of $2,000 per month for its services in hosting the Offering of the shares on its online platform, including during any “testing the waters” phase. Further, the company will pay Manhattan Street Capital a technology and administration fee of $25 per investor, in cash, paid by the company when each investor deposits funds into the escrow account. The above fees do not include fees for back-end services including, but not limited to: payment processing, digital currency conversion, escrow and technology fees, AML check, and accredited investor verification. These fees may include:
| ● | AML check fees between $2 and $6 per investor, depending on the location of the investor and | |
| ● | A technology license fee of $300 per month. |
For general advisory services, the company will pay Manhattan Street Capital $90,000 in cash. The company has also issued Manhattan Street Capital 100,000 shares of Common Stock in connection with the services provided under this agreement.
All fees are due to Manhattan Street Capital regardless of the success of the offerings.
Manhattan Street Capital does not directly solicit or communicate with investors with respect to offerings posted on its site, although it does advertise the existence of its platform, which may include identifying issuers listed on the platform. Our Offering Circular will be furnished to prospective investors in this offering via download 24 hours a day, 7 days a week on the www.manhattanstreetcapital.com website.
Investors’ Tender of Funds
We and the selling shareholders will conduct multiple closings on investments (so not all investors will receive their shares on the same date). The funds tendered by potential investors will be held by our escrow agent, Prime Trust, LLC (the “Escrow Agent”) and will be transferred to us and the selling shareholders at each Closing. The escrow agreement can be found in Exhibit 8 to the Offering Statement of which this Offering Circular is a part.
Process of Subscribing
You will be required to complete a subscription agreement in order to invest. The subscription agreement includes a representation by the investor to the effect that, if you are not an “accredited investor” as defined under securities law, you are investing an amount that does not exceed the greater of 10% of your annual income or 10% of your net worth (excluding your principal residence).
If you decide to subscribe for the Common Stock in this offering, you should complete the following steps:
| 1. | Go to www.manhattanstreetcapital.com/Quara, click on the “Invest Now” button | |
| 2. | Complete the online investment form. | |
| 3. | Deliver funds directly by check, wire, debit card, or electronic funds transfer via ACH to the specified account or deliver evidence of cancellation of debt. | |
| 4. | Once funds or documentation are received an automated AML check will be performed to verify the identity and status of the investor. | |
| 5. | Once AML is verified, investor will electronically receive, review, execute and deliver to us a subscription agreement. |
| 35 |
Any potential investor will have ample time to review the subscription agreement, along with their counsel, prior to making any final investment decision. Dalmore will review all subscription agreements completed by the investor. After Dalmore has completed its review of a subscription agreement for an investment in the company, the funds may be released by the Escrow Agent.
If the subscription agreement is not complete or there is other missing or incomplete information, the funds will not be released until the investor provides all required information. In the case of a debit card payment, provided the payment is approved, Dalmore will have up to three days to ensure all the documentation is complete. Dalmore will generally review all subscription agreements on the same day, but not later than the day after the submission of the subscription agreement.
All funds tendered (by check, wire, debit card, or electronic funds transfer via ACH to the specified account or deliver evidence of cancellation of debt) by investors will be deposited into an escrow account at the Escrow Agent for the benefit of the company and the selling shareholders. All funds received by wire transfer will be made available immediately while funds transferred by ACH will be restricted for a minimum of three days to clear the banking system prior to deposit into an account at the Escrow Agent.
The company and the selling shareholders maintain the right to accept or reject subscriptions in whole or in part, for any reason or for no reason, including, but not limited to, in the event that an investor fails to provide all necessary information, even after further requests, in the event an investor fails to provide requested follow up information to complete background checks or fails background checks, and in the event the offering is oversubscribed in excess of the maximum offering amount.
In the interest of allowing interested investors as much time as possible to complete the paperwork associated with a subscription, there is no maximum period of time to decide whether to accept or reject a subscription. If a subscription is rejected, funds will not be accepted by wire transfer or ACH, and payments made by debit card or check will be returned to subscribers within 30 days of such rejection without deduction or interest. Upon acceptance of a subscription, the company will send a confirmation of such acceptance to the subscriber.
Dalmore has not investigated the desirability or advisability of investment in the shares nor approved, endorsed or passed upon the merits of purchasing the shares. Dalmore is not participating as an underwriter and under no circumstance will it solicit any investment in the company, recommend the company’s securities or provide investment advice to any prospective investor, or make any securities recommendations to investors. Dalmore is not distributing any offering circulars or making any oral representations concerning this Offering Circular or this offering. Based upon Dalmore’s anticipated limited role in this offering, it has not and will not conduct extensive due diligence of this offering and no investor should rely on the involvement of Dalmore in this offering as any basis for a belief that it has done extensive due diligence. Dalmore does not expressly or impliedly affirm the completeness or accuracy of the Offering Statement and/or Offering Circular. All inquiries regarding this offering should be made directly to the company.
Upon confirmation that an investor’s funds have cleared, the company and the selling shareholders will instruct the Transfer Agent to issue shares to the investor, or transfer such shares, in the case of shares sold by the selling shareholders. The Transfer Agent will notify an investor when shares are ready to be issued or transferred and the Transfer Agent has set up an account for the investor.
Escrow Agent
The company has agreed to pay the Escrow Agent:
| ● | $400 escrow account set-up fee, | |
| ● | $30 per month escrow account fee for so long as the offering is being conducted, | |
| ● | a cash management fee of 0.5% of funds processed (up to a maximum of $8,000), | |
| ● | a technology platform license fee of $400.00 per month, | |
| ● | a transaction fee of $10.00 per investor, | |
| ● | an ACH processing fee of $2.00 per transaction, | |
| ● | a wire processing fee of $15.00 per transaction (domestic), | |
| ● | a check processing of $5.00 per transaction and | |
| ● | debit/credit card fees of $0.35 + 3% as charged by credit/debit card processor. |
| 36 |
The Escrow Agent has not investigated the desirability or advisability of investment in the shares nor approved, endorsed or passed upon the merits of purchasing the securities.
Transfer Agent
The company has also engaged Colonial Stock Transfer Company, Inc. (“Colonial”), a registered transfer agent with the SEC, who will serve as transfer agent to maintain shareholder information on a book-entry basis; there are no set up costs for this service, fees for this service will be limited to secondary market activity. The company estimates the aggregate fee due to Colonial for the above services to be $6,000 annually.
Selling Security Holders
The selling shareholders that invested in Quara in 2019 will sell up to a maximum of 365,497 shares of Common Stock, representing 1% of our outstanding shares of Common Stock.
The following table sets forth the name of the selling shareholders, the number of shares of Common Stock beneficially owned by them prior to this offering, the number of shares being offered by them in this offering and the number of shares and percentage of outstanding shares of Common Stock to be beneficially owned by them after this offering, assuming that all of the selling shareholder shares are sold in the offering.
We will pay all of the expenses of the offering (other than the 1% fee charged by Dalmore and any other selling agents’ discounts and commissions, payable with respect to the selling shareholder shares sold in the offering) but will not receive any of the proceeds from the sale of selling shareholder shares in the offering.
| Selling Shareholder | Amount Owned Prior to the Offering | Amount Offered by Selling Shareholder | Amount Owned after the Offering | |||||||||
| Rodney W. Reum* | 3,600,000 | 62,069 | 3,537,931 | |||||||||
| David W. Smalley* | 1,657,296 | 19,569 | 1,637,727 | |||||||||
| Michael B. Harrison* | 2,561,618 | 4,828 | 2,555,790 | |||||||||
| Larry K. Doan* | 500,000 | 8,621 | 491,379 | |||||||||
| Nicolette A. Keith* | 740,000 | 12,759 | 727,241 | |||||||||
| Yu-Cheng (Mike) Kao* | 1,000,000 | 17,241 | 982,759 | |||||||||
| Kenneth F. Reardon* | 600,000 | 10,345 | 589,655 | |||||||||
| Tich Munyikwa** | 500,000 | 8,621 | 491,379 | |||||||||
| Richard DeRose** | 200,000 | 3,448 | 196,552 | |||||||||
| Andrew Hunter* | 246,234 | 3,534 | 242,701 | |||||||||
| Keara Sauber* | 800,000 | 13,793 | 786,207 | |||||||||
| Steve Witt*** | 100,000 | 1,724 | 98,276 | |||||||||
| Brian Heinze*** | 100,000 | 1,724 | 98,276 | |||||||||
| Miro Cernetig* | 1,000,000 | 17,241 | 982,759 | |||||||||
| Richard Sayre | 3,261,278 | 1,724 | 3,259,554 | |||||||||
| Aristobulo Loaiza* | 100,000 | 1,724 | 98,276 | |||||||||
| Arnold Peinado* | 400,000 | 6,897 | 393,103 | |||||||||
| Gina Lupino* | 300,000 | 5,172 | 294,828 | |||||||||
| White Tree Ventures LLC (1) | 1,217,107 | 3,448 | 1,213,659 | |||||||||
| Kimberly Landry** | 2,486,597 | 2,586 | 2,484,011 | |||||||||
| David Chu | 500,000 | 8,621 | 491,379 | |||||||||
| Peter McDonough | 254,979 | 3,448 | 251,531 | |||||||||
| James Berliner | 400,000 | 6,897 | 393,103 | |||||||||
| Eric Briones | 126,223 | 991 | 125,232 | |||||||||
| New Mexico Consortium (2) | 798,838 | 10,345 | 788,493 | |||||||||
| Steve Buelow | 400,000 | 6,897 | 393,103 | |||||||||
| ATP Management Company, LLC (3) | 1,000,000 | 17,241 | 982,759 | |||||||||
| Bernard Ofstehage | 200,000 | 3,448 | 196,552 | |||||||||
| Deirdre Kenney | 600,000 | 25,862 | 574,138 | |||||||||
| Tom Herdman | 500,000 | 21,552 | 478,448 | |||||||||
| David Blaeser | 200,000 | 8,621 | 191,379 | |||||||||
| Monica Blaeser | 200,000 | 8,621 | 191,379 | |||||||||
| Debra Lewis | 200,000 | 8,621 | 191,379 | |||||||||
| Kristy Towson | 200,000 | 8,621 | 191,379 | |||||||||
| Tara Hutzal | 200,000 | 8,621 | 191,379 | |||||||||
| Rachel Stubbert | 200,000 | 8,621 | 191,379 | |||||||||
| Svilen Stoyanov | 32,500 | 1,401 | 31,099 | |||||||||
| * | These persons are directors, members of the company’s management or advisors to the company and to which we have granted options in connection with those services. These options are not included in the amount owned prior to or after the offering. |
| ** | These persons are affiliated with Pebble Labs and to which we have granted options in connection with their efforts in developing the intellectual property that supports the QuaraSense device. Shares underlying those options are not including the amount owned prior to or after the offering. |
| *** | These persons are affiliated with OptiEnz Sensors, LLC and to which we have granted options in connection with their efforts in developing the intellectual property that serves as the foundation for the QuaraSense device. Shares underlying those options are not including the amount owned prior to or after the offering. |
| (1) | The beneficial owner of White Tree Ventures LLC is Jon Bloodworth, the Managing Partner. |
| (2) | The beneficial owner of New Mexico Consortium is Steve Buelow, the CEO. |
| (3) | The beneficial owner of ATP Fund is Kyle Cox, the Managing Partner. |
| 37 |
ONGOING REPORTING AND SUPPLEMENTS TO THIS OFFERING CIRCULAR
We will be required to make annual and semi-annual filings with the SEC. We will make annual filings on Form 1-K, which will be due by the end of April each year and will include audited financial statements for the previous fiscal year. We will make semi-annual filings on Form 1-SA, which will be due by September 28 each year, which will include unaudited financial statements for the six months to June 30. We will also file a Form 1-U to announce important events such as the loss of a senior officer, a change in auditors or certain types of capital-raising. We will be required to keep making these reports unless we file a Form 1-Z to exit the reporting system, which we will only be able to do if we have less than 300 shareholders of record and have filed at least one Form 1-K.
We may supplement the information in this Offering Circular by filing a Supplement with the SEC. All these filings will be available on the SEC’s EDGAR filing system. You should read all the available information before investing.
| 38 |
FINANCIAL STATEMENTS
For the period ended December 31, 2019
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and the Board of Directors of
Quara Devices, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheet of Quara Devices, Inc. (the “Company”) as of December 31, 2019, and the related statements of operations, stockholders’ deficit, and cash flows, for the period from February 5, 2020 (Inception) to December 31, 2019, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019, and the results of its operations and its cash flows for the period then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has not achieved positive earnings and operating cash flows to enable the Company to finance its operations internally, which raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ dbbmckennon
We have served as the Company’s auditor since 2020
Newport Beach, CA
May 27, 2020
| F-1 |
QUARA DEVICES INC.
BALANCE SHEET
| December
31, 2019 | ||||
| Assets | ||||
| Current assets | ||||
| Cash | $ | 360,359 | ||
| Loan receivable – related party | 50,000 | |||
| Total current assets | 410,359 | |||
| Deferred offering costs | 20,000 | |||
| Total assets | 430,359 | |||
| Liabilities and stockholders’ deficit | ||||
Current liabilities | ||||
| Accounts payable | 202 | |||
| Related party payables | 440,048 | |||
| Total liabilities | 440,250 | |||
| Commitments and contingencies (Note 4) | - | |||
| Stockholders’ deficit | ||||
| Common stock, no par value, unlimited authorized, 38,357,361 shares issued and outstanding | 902,036 | |||
| Accumulated Deficit | (911,927 | ) | ||
| Total stockholders’ deficit | (9,891 | ) | ||
| Total liabilities and stockholders’ deficit | $ | 430,359 | ||
The accompanying notes are an integral part of these financial statements.
| F-2 |
QUARA DEVICES INC.
STATEMENTS OF OPERATIONS
| Period
from February 5, 2019 (Inception) to December 31, 2019 | ||||
| Operating Expenses | ||||
| General and administrative | $ | 805,284 | ||
| Sales and marketing | 106,643 | |||
| Total operating expenses | 911,927 | |||
| Net loss | $ | (911,927 | ) | |
| Basic and diluted loss per common share | $ | (0.035 | ) | |
| Weighted average number of common shares outstanding – basic and diluted | 25,986,288 | |||
The accompanying notes are an integral part of these financial statements.
| F-3 |
QUARA DEVICES INC.
STATEMENT OF STOCKHOLDERS’ DEFICIT
| Common Stock | Total | |||||||||||||||
Number of Shares | Amount | Accumulated Deficit | Stockholders’ Deficit | |||||||||||||
| Balance, February 5, 2019 (Inception) | - | - | - | - | ||||||||||||
| Founders’ shares | 20,357,361 | $ | 2,036 | $ | - | $ | 2,036 | |||||||||
| Shares issued for cash | 10,740,000 | 537,000 | - | 537,000 | ||||||||||||
| Shares issued for services | 5,200,000 | 260,000 | - | 260,000 | ||||||||||||
| Shares issued to settle related party payables | 2,060,000 | 103,000 | - | 103,000 | ||||||||||||
| Net loss | - | - | (911,927 | ) | (911,927 | ) | ||||||||||
| Balance, December 31, 2019 | 38,357,361 | $ | 902,036 | $ | (911,927 | ) | $ | (9,891 | ) | |||||||
The accompanying notes are an integral part of these financial statements.
| F-4 |
QUARA DEVICES INC.
STATEMENTS OF CASH FLOWS
| Period
from February 5, 2019 (Inception) to December 31, 2019 | ||||
| OPERATING ACTIVITIES | ||||
| Net loss | $ | (911,927 | ) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||
| Stock-based compensation | 260,000 | |||
| Changes in operating assets and liabilities: | ||||
| Accounts payable | 202 | |||
| Related party payables | 543,048 | |||
| Net cash used in operating activities | (108,677 | ) | ||
| INVESTING ACTIVITIES | ||||
| Loan receivable – related party | (50,000 | ) | ||
| Net cash used in investing activities | (50,000 | ) | ||
| FINANCING ACTIVITIES | ||||
| Proceeds from issuance of common shares | 539,036 | |||
| Deferred offering costs | (20,000 | ) | ||
| Net cash provided by financing activities | 519,036 | |||
| Change in cash during the period | 360,359 | |||
| Cash, beginning of period | - | |||
| Cash, end of period | $ | 360,359 | ||
| Supplemental disclosure of cash flow information: | ||||
| Cash paid for interest | $ | - | ||
| Cash paid for income taxes | $ | - | ||
| Non-cash investing and financing activities: | ||||
Related party payables settled in common stock | $ | 103,000 | ||
The accompanying notes are an integral part of these financial statements.
| F-5 |
QUARA DEVICES INC.
NOTES TO THE FINANCIAL STATEMENTS
| 1. | NATURE OF OPERATIONS |
Quara Devices Inc. (the “Company”) was incorporated by Articles of Incorporation issued pursuant to the provisions of the Wyoming Business Corporations Act on February 5, 2019 (“Inception”). The Company is an emerging med-tech & biotech company focusing on the development of revolutionary sensors including a handheld bacterial quorum sensing device to provide rapid early warning to the presence of harmful pathogens. The Company’s head office 1712 Pearl Street, Boulder, CO 80302 and its registered and records office address is 1623 Central Avenue, Suite 204, Cheyenne, WY 82001.
Risks and Uncertainties
The Company has a limited operating history and has not generated revenue from intended operations. The Company’s business and operations are sensitive to general business and economic conditions in the U.S. and worldwide along with local, state, and federal governmental policy decisions. A host of factors beyond the Company’s control could cause fluctuations in these conditions. Adverse conditions may include: changes in biotechnology regulatory environment, technological advances that render our technologies obsolete, availability of resources for testing, acceptance of technologies into the intended communities, and competition from larger, more well-funded companies. These adverse conditions could affect the Company’s financial condition and the results of its operations.
| 2. | GOING CONCERN |
We will rely on debt and equity financing for working capital until positive cash flows from operations can be achieved and have incurred operating losses since Inception. These matters raise substantial doubt about the Company’s ability to continue as a going concern. These financial statements are prepared on the basis that the Company will continue as a going concern, which assumes that the Company will be able to continue in operation for the foreseeable future and will be able to realize its assets and discharge its liabilities in the normal course of operations. The Company’s ability to continue as a going concern is dependent upon the financial support from its shareholders and other related parties, its ability to obtain financing for the continuing exploration and development of its sensors.
| 3. | SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation
The accounting and reporting policies of the Company conform to accounting principles generally accepted in the United States of America (“GAAP”). These financial statements of the Company are presented in United States dollars, which is the Company’s functional currency
Use of estimates and judgements
The preparation of these financial statements in conformity with GAAP requires management to make certain estimates, judgments and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported expenses during the period. Actual results could differ from these estimates. The preparation of these financial statements requires management to make judgments regarding the going concern of the Company, as discussed in Note 2.
Significant assumptions about the future and other sources of estimation uncertainty that management has made at the end of the reporting period, that could result in a material adjustment to the carrying amounts of assets and liabilities in the event that actual results differ from assumptions made, relate to, but are not limited to, deferred tax assets and liabilities and valuation of stock-based compensation.
| F-6 |
QUARA DEVICES INC.
NOTES TO THE FINANCIAL STATEMENTS
Fair Value of Financial Instruments
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants as of the measurement date. Applicable accounting guidance provides an established hierarchy for inputs used in measuring fair value that maximizes the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available. Observable inputs are inputs that market participants would use in valuing the asset or liability and are developed based on market data obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions about the factors that market participants would use in valuing the asset or liability. There are three levels of inputs that may be used to measure fair value:
| Level 1 | - | Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets. | |
| Level 2 | - | Include other inputs that are directly or indirectly observable in the marketplace. | |
| Level 3 | - | Unobservable inputs which are supported by little or no market activity. |
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
Fair-value estimates discussed herein are based upon certain market assumptions and pertinent information available to management as of December 31, 2019. The respective carrying value of certain on-balance-sheet financial instruments approximated their fair values. These financial instruments include cash and cash equivalents, loan receivable – related party, accounts payable, and related party payables. Fair values for these items were assumed to approximate carrying values because of their short-term nature or they are payable on demand.
Cash and Cash Equivalents
For purpose of the statement of cash flows, the Company considers institutional money market funds and all highly liquid debt instruments purchased with an original maturity of three months or less to be cash equivalents.
Offering Costs
The Company accounts for offering costs in accordance with Accounting Standards Codification (“ASC”) 340, Other Assets and Deferred Costs. Prior to the completion of an offering, offering costs were capitalized as deferred offering costs on the balance sheet. The deferred offering costs are netted against the proceeds of the offering in stockholders’ equity (deficit) or the related debt, as applicable. As of December 31, 2019, $20,000 in deferred offering costs were included in the accompanying balance sheet.
Stock-Based Compensation
The Company accounts for stock options issued to employees under ASC 718, Compensation – Stock Compensation. Under ASC 718, stock-based compensation cost to employees is measured at the grant date, based on the estimated fair value of the award. Stock-based compensation is recognized as expense over the employee’s requisite vesting period and over the nonemployee’s period of providing goods or services. The fair value of each stock option or warrant award is estimated on the date of grant using the Black-Scholes option valuation model. Restricted shares are measured based on the fair market value of the underlying stock on the grant date.
Income taxes
The Company applies ASC 740, Income Taxes (“ASC 740”). Deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial statement reported amounts at each period end, based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized. The provision for income taxes represents the tax expense for the period, if any, and the change during the period in deferred tax assets and liabilities.
| F-7 |
QUARA DEVICES INC.
NOTES TO THE FINANCIAL STATEMENTS
ASC 740 also provides criteria for the recognition, measurement, presentation and disclosure of uncertain tax positions. A tax benefit from an uncertain position is recognized only if it is “more likely than not” that the position is sustainable upon examination by the relevant taxing authority based on its technical merit.
Loss Per Share
The Company presents basic and diluted loss per share data for its common shares, calculated by dividing the loss attributable to common shareholders of the Company by the weighted average number of common shares outstanding during the year. Diluted loss per share does not adjust the loss attributable to common shareholders or the weighted average number of common shares outstanding when the effect is anti-dilutive.
Concentration of Credit Risk
The Company maintains its cash with a major financial institution located in the United States of America which it believes to be credit worthy. Balances are insured by the Federal Deposit Insurance Corporation up to $250,000. At times, the Company maintains balances in excess of the federally insured limits.
New Accounting Standards
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-02, Leases (Topic 842), specifying the accounting for leases, which supersedes the leases requirements in Topic 840, Leases. The objective of Topic 842 is to establish the principles that lessees and lessors shall apply to report useful information to users of consolidated financial statements about the amount, timing, and uncertainty of cash flows arising from a lease. Lessees are permitted to make an accounting policy election to not recognize the asset and liability for leases with a term of twelve months or less. Lessors’ accounting is largely unchanged from the previous accounting standard. In addition, Topic 842 expands the disclosure requirements of lease arrangements. Lessees and lessors will use a modified retrospective transition approach, which includes several practical expedients. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020 for emerging growth companies, with early adoption permitted. The Company has reviewed the provisions of the new standard, but it is not expected to have a significant impact on the Company.
In June 2016, the FASB issued guidance that sets forth a current expected credit loss impairment model for financial assets, which replaces the current incurred loss model, and in 2018 and 2019 issued amendments and updates to the new standard. This model requires a financial asset (or group of financial assets), including trade receivables, measured at amortized cost to be presented at the net amount expected to be collected with an allowance for credit losses deducted from the amortized cost basis. The allowance for credit losses should reflect management’s current estimate of credit losses that are expected to occur over the remaining life of a financial asset. This guidance is effective for annual periods beginning after December 15, 2019 and interim periods within those annual periods using a modified retrospective transition method. The Company has reviewed the provisions of the new standard, but it is not expected to have a significant impact on the Company.
| F-8 |
QUARA DEVICES INC.
NOTES TO THE FINANCIAL STATEMENTS
In December 2019, the FASB issued guidance that simplifies the accounting for income taxes by removing certain exceptions in existing guidance and improves consistency in application by clarifying and amending existing guidance. This guidance is effective for annual periods beginning after December 15, 2020, and interim periods within those annual periods, where the transition method varies depending upon the specific amendment. Early adoption is permitted, including adoption in any interim period. An entity that elects to early adopt the amendments in an interim period should reflect any adjustments as of the beginning of the annual period that includes that interim period, and all amendments must be adopted in the same period. The Company has reviewed the provisions of the new standard, but it is not expected to have a significant impact on the Company.
The FASB issues ASUs to amend the authoritative literature in ASC. There have been several ASUs to date, including those above, that amend the original text of ASC. Management believes that those issued to date either (i) provide supplemental guidance, (ii) are technical corrections, (iii) are not applicable to us or (iv) are not expected to have a significant impact our financial statements.
| 4. | COMMITMENTS AND CONTIGENCIES |
We are not a party to any legal proceedings, and we are not aware of any claims or actions pending or threatened against us. In the future, we might from time to time become involved in litigation relating to claims arising from our ordinary course of business, the resolution of which we do not anticipate would have a material adverse impact on our financial position, results of operations or cash flows.
Intellectual Property Purchase Agreement
On March 26, 2019, the Company entered into an assignment of Intellectual Property Rights and a Research License and Royalty Calculation Agreement with Pebble Labs Inc. (Pebble). Pebble will assign any and all intellectual property rights, including all inventions and patent rights therein, copyrights, design rights, trade secrets, confidential information, and any other analogous intangible proprietary rights, whether registered or unregistered, which may subsist anywhere in the world, and all applications for registration or issuance of any of same, including all divisions, continuations, reissues, and extensions thereof, and all rights to file any such applications, and all registrations for any of same; relating to the Improved Fluorescent Resonance Energy Transfer Based Biosensor Proteins and Their Methods of Use Thereof, U.S. Provisional Patent No. 6273,0424 filed on September 12, 2018 for a one-time payment of $500,000 and an ongoing royalty fee equal to 1.5% of net sales derived from products resulting from the Provisional Patent. The one-time fee was originally payable by November 30, 2019 and was subsequently extended to September 30, 2020. In the absence of the required one-time payment or agreed upon extension, the assignment of rights becomes null and void and Pebble will retain all rights. There is no penalty for non-payment other than the loss of these rights.
On September 12, 2019, the Provisional Patent was converted to Patent Application US 19/50813.
| 5. | SHAREHOLDERS’ DEFICIT |
Common Stock
The Company is authorized to issue unlimited common shares with no par value.
On or near Inception, the Company issued 20,357,361 common shares for $0.0001 per share to founders.
During the period ended December 31, 2019, the Company issued 10,740,000 common shares at $0.05 per common share, for total cash proceeds of $537,000.
During the period, the Company issued 5,200,000 common shares at $0.05, each, for services valued at $260,000 including marketing services of $15,000, legal services of $82,000 and consulting services of $163,000. The shares were valued based on the sale price to third parties described above. Of the total, $15,000 is included in sales and marketing and $245,000 is included in general and administrative expenses in the accompanying statement of operations, respectively.
On September 30, 2019, the Company issued 2,060,000 common shares at $0.05 per common share, for settlement of related-party advances of $103,000.
| F-9 |
QUARA DEVICES INC.
NOTES TO THE FINANCIAL STATEMENTS
Stock options
The Company has established the Quara Devices, Inc. 2019 Stock Option Plan (the “Plan”) under which it is authorized to grant stock options to executive Officers, Directors, employees, and consultants. Under the Plan, the number of options that may be issued is limited to no more than 15% of the Company’s issued and outstanding shares immediately prior to the grant. The options can be granted for a maximum term of ten (10) years and vest at the discretion of the Board of Directors. No options have been granted as of December 31, 2019. See Note 8 for subsequent events.
| 6. | INCOME TAXES |
At December 31, 2019, the Company had approximately $540,000, of net operating losses (“NOL”) carry forwards for federal and state income tax purposes. These losses are available for future years and have no expiration under current federal regulations. Utilization of these losses may be severely or completely limited if the Company undergoes an ownership change pursuant to Internal Revenue Code Section 382.
The provision for income taxes for continuing operations consists of the following components for the period ended December 31, 2019:
| Current | $ | - | ||
| Deferred | - | |||
| Total tax provision for (benefit from) income taxes | $ | - |
A comparison of the provision for income tax expense at the federal statutory rate of 21% for the period ended December 31, 2019, the Company’s effective rate is as follows:
| Federal statutory rate | 21.0 | % | ||
| State tax, net of federal benefit | (0.0 | ) | ||
| Permanent differences | (9.0 | ) | ||
| Valuation allowance | (12.0 | ) | ||
| Effective tax rate | 0.0 | % |
At December 31, 2019, the Company had deferred tax assets of approximately $113,000 and has established a full allowance against all deferred tax assets.
| 7. | RELATED-PARTY TRANSACTIONS |
Key management personnel include those persons having the authority and responsibility of planning, directing and executing the activities of the Company. The Company has determined that its key management personnel consist of its Executive Officers and Directors. Other related parties to the Company include companies in which key management has control or significant influence. Key management personnel have received no paid salaries and have deferred compensation due them for services until specified levels of funding have been obtained. Key management and certain directors were issued 4,395,000 common shares for services provided to the Company.
The Company has common ownership with Pebble Labs Inc. The Company has assessed the common ownership as well as the voting rights of common shareholders and determined that the common shareholders do not represent a control group.
Related-party payables:
During the period ended December 31, 2019, the Company entered into agreements with certain executive officers of the Company. The agreements require that all consulting fees be accrued and deferred until the Company has completed a financing of at least $2.5 million.
| F-10 |
QUARA DEVICES INC.
NOTES TO THE FINANCIAL STATEMENTS
| 2019 | ||||
| Compensation deferred | $ | 423,000 | ||
| Due to related parties for reimbursable expenses | 17,048 | |||
| $ | 440,048 | |||
Loan receivable – related party
During the period the Company advanced $50,000 to a company controlled by an executive officer of the Company for development work on the Company’s QuaraSense product. Subsequent to the period end, the advance was applied towards the purchase of intellectual property, see Note 8.
| 8. | SUBSEQUENT EVENTS |
On April 13, 2020 the Company entered into an assignment of intellectual property rights from OptiEnz Sensors, LLC (OptiEnz) for consideration of $50,000 previously advanced to OptiEnz during 2019 and reflected as a Loan receivable – related party on the accompanying balance sheet of the Company as at December 31, 2019. OptiEnz has assigned any and all intellectual property rights, including all inventions and patent rights therein, copyrights, design rights, trade secrets, confidential information, and any other analogous intangible proprietary rights, whether registered or unregistered, which may subsist anywhere in the world, and all applications for registration or issuance of any of same, including all divisions, continuations, reissues, and extensions thereof, and all rights to file any such applications, and all registrations for any of same; relating to a portable instrument and associated software for measuring fluorescence resonance energy transfer (FRET) between pairs of fluorophores. The instrument, software, and methods developed can be used to measure FRET between any fluorophore pair and can make simultaneous measurements of multiple fluorophore pairs. In addition, the Company will pay a royalty to OptiEnz of 5% of the net sales of the product as reserved in the Assignment up to a total royalty payment of $450,000 after which the Royalty shall be calculated at 1.5% of the net sales of the product.
On May 14, 2020, the Company licensed from the Colorado State University Research Foundation an exclusive right in all territories and for all fields to the patent rights and know-how relating to technology known as PadLock-RCA-Nuclease Protection Lateral Flow Assay for the detection of pathogen sequences at the point of care. The Company will pay an upfront fee of $5,000 and pay royalties ranging from 3% to 4% based on volume of annual net sales. The Company will be subject to minimum royalty payments beginning in 2023 of $5,000 and $10,000 beginning in 2025. The Company has also agreed to milestone payments based on net sales ranging from $10,000 to $1,000,000. In addition, the Company will issue common shares upon the Company completing proof of concept work demonstrating utility in diagnosing SARS-CoV-2 in an amount equal to 1% of all issued and outstanding shares on a fully diluted basis calculated on a post-closing basis.
Under the Company’s stock option plan, on January 15, 2020 the Company granted 1,650,000 stock options to its directors, officers and advisors with an exercise price of $0.25 per share and exercisable for 10 years. The options vest quarterly in equal amounts over 24 months. In addition, the Company issued 1,650,000 stock options to its directors, officers and advisors with an exercise price of $2.50 per share and exercisable for 10 years. The options vest quarterly in equal amounts over 24 months.
Under the Company’s stock option plan, on April 15, 2020 the Company issued 100,000 stock options to advisors of the Company with an exercise price of $0.25 per share and exercisable for 10 years. The options vest quarterly in equal amounts over 24 months. In addition, the Company issued 100,000 stock options to advisors of the Company with an exercise price of $2.50 per share and exercisable for 10 years. The options vest quarterly in equal amounts over 24 months.
On May 23, 2020 the Company issued 100,000 common shares at $0.05 each to FundAthena, Inc (DBA as Manhattan Street Capital) for services valued at $5,000.
The Company has evaluated subsequent events that occurred after December 31, 2019 through May 27, 2020, the issuance date of these financial statements. There have been no other events or transactions during this time which would have a material effect on these financial statements, other than those disclosed.
| F-11 |
PART III
INDEX TO EXHIBITS
* To be filed by amendment.
| 40 |
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has duly caused this Offering Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Boulder, Colorado, on May 27, 2020.
| Quara Devices Inc. | ||
| By: | /s/ Steven C. Eror | |
| Steven C. Eror, Chief Executive Officer | ||
The following persons in the capacities and on the dates indicated have signed this Offering Statement.
| /s/ Steven C. Eror | |
| Steven C. Eror, Chief Executive Officer and Director | |
| Date: May 27, 2020 | |
| /s/ Nicolette A. Keith | |
| Nicolette A. Keith, Chief Financial Officer | |
| Principal Financial Officer, Principal Accounting Officer | |
| Date: May 27, 2020 | |
| /s/ Rodney W. Reum | |
| Rodney W. Reum, Executive Chairman and Director | |
| Date: May 27, 2020 | |
| /s/ David W. Smalley | |
| David W. Smalley, Director | |
| Date: May 27, 2020 | |
| /s/ Michael B. Harrison | |
| Michael B. Harrison, Director | |
| Date: May 27, 2020 | |
| /s/ Larry K. Doan | |
| Larry K. Doan, Director | |
| Date: May 27, 2020 |
| 41 |








