UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended
Commission File No.:
(Exact name of registrant as specified in its charter)
Translation of registrant’s name into English: Not applicable
| Tel: + | ||
| (Jurisdiction of incorporation or organization) | (Address of principal executive offices) |
President and Chief Executive Officer
+972 8 958 4777
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| Title of each class to be registered | Trading Symbol(s) | Name of each exchange on which each class is to be registered | ||
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
Number of outstanding shares
of each of the issuer’s classes of capital or common stock as of December 31, 2022:
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Exchange Act of 1934.
Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T during the preceding 12 months.
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company.
| Large accelerated filer ☐ | Accelerated filer ☐ | ☒ | |
| Emerging Growth Company |
If an emerging growth company
that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the
extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B)
of the Securities Act.
Indicate by check mark whether
the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control
over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that
prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing.
U.S. GAAP ☐
☒
Other ☐
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
☐ Item 17 ☐ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company.
Yes ☐
No
TABLE OF CONTENTS
i
INTRODUCTION
We are a next-generation mobile health, or mHealth, and digital health company that develops and markets clinical and consumer medical-grade health monitoring solutions and offers end-to-end support for remote monitoring and telemedicine projects. With extensive experience in the field of medical devices, digital health and patients monitoring, we are committed to raising the global level of healthcare by empowering caregivers and patients to better monitor, manage and improve clinical and personal health outcomes. We believe that we are at the forefront of the digital health revolution in developing the next generation of mobile technologies and services that are designed to empower consumers, patients, and providers to better monitor, manage and improve clinical and personal health outcomes, especially for those who suffer from cardiovascular disease, or CVD, pulmonary disease and diabetes. Our business and future revenue stream can be broken down into two business areas: monitoring services and home collection kits for lab testing.
Unless the context requires otherwise, the terms “G Medical Innovations Holdings,” “we,” “us,” “our,” the “Company” and “G Medical Innovations Holdings” refer to G Medical Innovations Holdings Ltd., a Cayman Islands exempted company, and its subsidiaries: G Medical Innovations Ltd., an Israeli corporation, G Medical Innovations USA Inc., a Delaware corporation, G Medical Health and Wellness, Inc, a Delaware corporation, G Medical Health and Wellness Lab, Inc., a Delaware corporation, G Medical Innovations MK Ltd., a Macedonian corporation, G Medical Innovations Asia Limited, a Hong Kong corporation, G Medical Diagnostic Services, Inc., a Texas corporation, G Medical Mobile Health Solutions, Inc., an Illinois corporation, Telerhythmics, LLC , a company formed under the laws of the state of Tennessee, G Medical Tests and Services, Inc., a Delaware corporation, G Medical Lab Services, Inc. a Delaware corporation, G Medical Innovations UK Ltd., a UK corporation, all of which are wholly-owned subsidiaries, G-Medical Lab Services Inc., a Delaware corporation and 80%-owned subsidiary, and Guangzhou Yimei Innovative Medical Science and Technology Co., Ltd., or G Medical China, a 70%-owned subsidiary of G Medical Innovations Asia Limited.
References to “Ordinary Shares”, “warrants” and “share capital” refer to our ordinary shares, warrants and share capital, respectively, of G Medical Innovations Holdings.
Our reporting currency and functional currency is the U.S. dollar. Unless otherwise expressly stated or the context otherwise requires, references in this annual report to “dollars” or “$” mean U.S. dollars, and references to “A$” are to Australian dollars. Unless derived from our consolidated financial statements or otherwise indicated, U.S. dollar translations of A$ amounts presented in this annual report are translated using the rate of A$1.469 to $1.00, based on the exchange rates certified for customs purposes by the Federal Reserve Bank of New York on December 31, 2022. References to “Ordinary Shares” are to our ordinary shares, par value $3.15 per share. We report under International Financial Reporting Standards (or IFRS), as issued by the International Accounting Standards Board (or the IASB). None of the financial statements were prepared in accordance with generally accepted accounting principles in the United States.
Our shareholders have approved three reverse stock splits since 2020: on October 29, 2020, our shareholders approved, at an extraordinary general shareholders meeting, a one-for-18 consolidation (the “October 2020 Reverse Stock Split”) of our Ordinary Shares pursuant to which holders of our Ordinary Shares received one Ordinary Share for every 18 Ordinary Shares held; on March 25, 2021, our shareholders approved, at an extraordinary general shareholders meeting, a one-for-five consolidation (the “March 2021 Reverse Stock Split”) of our Ordinary Shares pursuant to which holders of our Ordinary Shares received one Ordinary Share for every five Ordinary Shares held; and on November 15, 2022, our shareholders approved, at an extraordinary general shareholders meeting, a 35-for-one consolidation of our Ordinary Shares pursuant to which holders of our Ordinary Shares received one Ordinary Share for every 35 Ordinary Shares held which took effect on November 16, 2022 (the “November 2022 Reverse Stock Split”). Unless the context expressly dictates otherwise, all reference to share and per share amounts referred to herein reflect the October 2020 Reverse Stock Split, the March 2021 Reverse Stock Split and the November 2022 Reverse Stock Split.
ii
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain information included or incorporated by reference in this annual report on Form 20-F may be deemed to be “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 and other securities laws. Forward-looking statements are often characterized by the use of forward-looking terminology such as “may,” “will,” “expect,” “anticipate,” “estimate,” “continue,” “believe,” “should,” “intend,” “project” or other similar words, but are not the only way these statements are identified.
These forward-looking statements may include, but are not limited to, statements relating to our objectives, plans and strategies, statements that contain projections of results of operations or of financial condition, expected capital needs and expenses, statements relating to the research, development, completion and use of our products, and all statements (other than statements of historical facts) that address activities, events or developments that we intend, expect, project, believe or anticipate will or may occur in the future.
Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties. We have based these forward-looking statements on assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments and other factors they believe to be appropriate.
Important factors that could cause actual results, developments and business decisions to differ materially from those anticipated in these forward-looking statements include, among other things:
| ● | our expectation regarding the sufficiency of our existing cash and cash equivalents to fund our current operations; | |
| ● | our ability and plans to manufacture, market and sell our products and services, including those related to our new direct to customer at-home health testing kit business; | |
| ● | our expectation regarding government and third-party payors providing adequate coverage and reimbursement for the use of our products and services, including our testing services; | |
| ● | the commercial launch and future sales of our existing products or services or any other future potential product candidates or services; | |
| ● | planned pilot programs with healthcare providers for our products; | |
| ● | our plan to further expand by targeting healthcare providers who can benefit from our comprehensive service offerings; | |
| ● | our intention to drive multiple recurring revenue streams, across consumer and professional healthcare verticals and in geographical territories; | |
| ● | our expectations regarding future growth; | |
| ● | our planned level of capital expenditures; | |
| ● | our plans to continue to invest in research and development to develop technology for both existing and new products; | |
| ● | our anticipation that we will penetrate a higher number of distribution channels and markets with a relatively low overhead; |
iii
| ● | our anticipation that the monitoring services will continue to grow thereby increasing monthly recurring revenues payable to us; | |
| ● | anticipated actions of the U.S. Food and Drug Administration, or the U.S. FDA, state regulators, if any, or other similar foreign regulatory agencies, including approval to conduct clinical trials, the timing and scope of those trials and the prospects for regulatory approval or clearance of, or other regulatory action with respect to our products or services; | |
| ● | our ability to launch and penetrate markets in new locations, including taking steps to expand our activities; | |
| ● | our ability to retain key executive members; | |
| ● | our ability to internally develop new inventions and intellectual property; | |
| ● | interpretations of current laws and the passages of future laws; | |
| ● | acceptance of our business model by investors; and | |
| ● | those factors referred to in “Item 3.D. Risk Factors,” “Item 4. Information on the Company,” and “Item 5. Operating and Financial Review and Prospects”, as well as in this annual report on Form 20-F generally. |
Readers are urged to carefully review and consider the various disclosures made throughout this annual report on Form 20-F which are designed to advise interested parties of the risks and factors that may affect our business, financial condition, results of operations and prospects.
You should not put undue reliance on any forward-looking statements. Any forward-looking statements in this annual report on Form 20-F are made as of the date hereof and are expressly qualified in their entirety by the cautionary statements included in this annual report. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
In addition, the section of this annual report on Form 20-F entitled “Item 4. Information on the Company” contains information obtained from independent industry sources and other sources that we have not independently verified.
iv
EXPLANATORY NOTE
Market data and certain industry data and forecasts used throughout this annual report on Form 20-F were obtained from internal company surveys, market research, publicly available information, reports of governmental agencies and industry publications and surveys. Industry surveys, publications and forecasts generally state that the information contained therein has been obtained from sources believed to be reliable. However, this information may prove to be inaccurate because of the method by which some of the data for the estimates is obtained or because this information cannot always be verified with complete certainty due to the limits on the availability and reliability of raw data, the voluntary nature of the data gathering process and other limitations and uncertainties. As a result, the market and industry data and forecasts included or incorporated by reference in this annual report, and estimates and beliefs based on that data, may not be reliable. We have relied on certain data from third-party sources, including internal surveys, industry forecasts and market research, which we believe to be reliable based on our management’s knowledge of the industry. However, we have not ascertained the underlying economic assumptions relied upon therein. Forecasts are particularly likely to be inaccurate, especially over long periods of time. In addition, we do not necessarily know what assumptions regarding general economic growth were used in preparing the forecasts we cite. Statements as to our market position are based to the best of our knowledge on the most currently available data. While we are not aware of any misstatements regarding the industry data presented in this annual report, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” in this annual report.
Statements made in this annual report on Form 20-F concerning the contents of any agreement, contract or other document are summaries of such agreements, contracts or documents and are not a complete description of all of their terms. If we filed any of these agreements, contracts or documents as exhibits to this Report or to any previous filing with the Securities and Exchange Commission (or the SEC), you may read the document itself for a complete understanding of its terms.
v
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
Not applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not applicable.
ITEM 3. KEY INFORMATION
A. Reserved.
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
You should carefully consider the risks described below, together with all of the other information in this annual report on Form 20-F. The risks and uncertainties described below are those significant risk factors, currently known and specific to us, that we believe are relevant to an investment in our securities. Additional risks and uncertainties not currently known to us or that we now deem immaterial may also harm us. If any of these risks materialize our business, results of operations or financial condition could suffer, and the price of our Ordinary Shares could decline substantially.
Summary Risk Factors
Our business is subject to numerous risks, as more fully described in the section entitled “Risk Factors” immediately following this annual report summary. You should read these risks before you invest in the Securities. In particular, our risks include, but are not limited to, the following:
| ● | we have identified material weaknesses in our internal control over financial reporting that could, if not remediated, result in material misstatements in our financial statements. If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, shareholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our Ordinary Shares; |
| ● | we have a limited operating history on which to assess the prospects for our business, have generated little revenue from sales of our products, have incurred losses since inception and we anticipate that we will continue to incur significant losses until we are able to successfully commercialize our products and services globally; |
| ● | we expect that we will need to raise substantial additional funding before we can expect to become profitable from sales of our products and services and this additional financing may not be available on acceptable terms, or at all; |
| ● | raising additional capital would cause dilution to holders of our equity securities, and may affect the rights of existing holders of equity securities; |
| ● | we may not succeed in completing the development and commercialization of our products and services and generating significant revenues; |
| ● | the expansion of our home testing kit business presents important challenges to our ability to manage our business; |
| ● | our success depends upon market acceptance of our products and services, our ability to develop and commercialize new products and services and generate revenues and our ability to identify new markets for our technology; |
| ● | medical device development is costly and involves continual technological change which may render our current or future products obsolete; |
| ● | we will be dependent upon success in our customer acquisition strategy and successfully integrating acquired companies and technology; |
1
| ● | we have recently invested significant capital in our COVID-19 related services, including the purchase of COVID-19 testing kits, however, the future of COVID-19 related services is uncertain and we have stopped operations in our COVID-19 testing related business, which has led to the write off of inventory and fixed assets in our fiscal year ended December 31, 2022; | |
| ● | we are dependent upon third-party manufacturers and suppliers making us vulnerable to supply shortages and problems and price fluctuations, which could harm our business, and we have no timely ability to replace our current manufacturing capabilities; |
| ● | we have limited manufacturing history on which to assess the prospects for our business, and we anticipate that we will incur significant losses once we initiate our in-house manufacturing until we are able to successfully commercialize our products globally; |
| ● | we have applied for various patents, but there is a risk that our patent applications will not be granted or that we will receive enforceable patent rights, which could leave us at a competitive disadvantage; |
| ● | we face intense competition in the market, and as a result we may be unable to effectively compete in our industry; |
| ● | the level of our commercial success will depend in part on our ability to generate and grow sales with our sales and marketing team, strategies and partnerships, and we may be unsuccessful in these efforts; |
| ● | if third-party payors do not provide adequate coverage and reimbursement for the use of our products and services, our revenue will be negatively impacted; |
| ● | we may not be able to obtain the necessary clearance(s) or approval(s) of the U.S. FDA or any applicable state equivalents, or similar foreign regulatory agencies, such as European Economic Area (or EEA) Notified Bodies, or the NMPA or may not be able to obtain such approvals in a timely fashion; |
| ● | the operation of our monitoring centers is subject to rules and regulations governing Independent Diagnostic Testing Facility (or IDTF) and state licensure requirements; failure to comply with these rules could prevent us from receiving reimbursement from Medicare and some commercial payors; |
| ● | changes in the regulatory environment may constrain or require us to restructure our operations, which may delay or prevent us from marketing our products and services and as a result harming our revenue and operating results; |
| ● | we may be party to or target of lawsuits, investigations and proceedings arising out of claims alleging negligence, product liability, breach of warranty or malpractice that may involve large claims and significant defense costs whether or not such liability is imposed. Such potential claims may be costly to defend, could consume management resources and could adversely affect our reputation and business; |
| ● | we are a Cayman Islands exempted company with limited liability. The rights of our shareholders may be different from the rights of shareholders governed by the laws of U.S. jurisdictions. In addition, our shareholders may face difficulties in protecting their interests because we are a Cayman Islands exempted company and United States civil liabilities and certain judgments obtained against us by our shareholders may not be enforceable; |
2
| ● | failure to meet Nasdaq’s continued listing requirements could result in the delisting of our Ordinary Shares, negatively impact the price of our Ordinary Shares and negatively impact our ability to raise additional capital; |
| ● | our principal manufacturing facility is located in China and we plan to operate in the Chinese market. Changes in the Chinese government’s macroeconomic policies or its public policy could have a negative effect on our business and results of operations. The Chinese government exerts substantial influence over the manner in which we must conduct our business activities. In addition, uncertainties with respect to the Chinese legal system could adversely affect us; |
| ● | we maintain material operations in Israel. It may be difficult to enforce a judgment of a U.S. court against us and our officers and directors and the Israeli experts named in this annual report in Israel or the United States, to assert U.S. securities laws claims in Israel or to serve process on our officers and directors and these experts. In addition, potential political, economic and military instability in the State of Israel, where our management team and our research and development facilities are located, may adversely affect our results of operations; and |
| ● | we may be required to pay monetary remuneration to our Israeli employees for their inventions, even if the rights to such inventions have been duly assigned to us. |
Risks Related to Our Financial Condition and Capital Requirements
We have identified material weaknesses in our internal control over financial reporting that could, if not remediated, result in material misstatements in our financial statements. If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, shareholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our Ordinary Shares.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures are designed to prevent fraud. Our management will be required to assess the effectiveness of our internal controls and procedures and disclose changes in these controls on an annual basis. However, for as long as we are an “emerging growth company” under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act (or Section 404).
Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404, or any subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our Ordinary Shares.
We have identified material weaknesses in our internal control over financial reporting as of December 31, 2022. As defined in Regulation 12b-2 under the Exchange Act, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual financial statements will not be prevented or detected on a timely basis. Specifically, we determined that the material weaknesses are related to having an insufficient number of financial reporting personnel with an appropriate level of knowledge, experience and training in the application of IFRS and SEC rules and regulations commensurate with our reporting requirements.
We have taken action toward remediating these material weaknesses by providing enhanced training to existing financial and accounting employees on related IFRS issues. In addition, to remediate these material weaknesses, we are implementing measures and developing, communicating, and implementing an accounting policy manual for our financial reporting personnel for recurring transactions, period-end closing processes and policy relating to segregation of duties.
If we are unable to certify that our internal control over financial reporting is effective and in compliance with Section 404, we may be subject to sanctions or investigations by regulatory authorities, such as the SEC or stock exchanges, and we could lose investor confidence in the accuracy and completeness of our financial reports, which could hurt our business, the price of our Ordinary Shares and our ability to access the capital market.
We have a limited operating history on which to assess the prospects for our business, have generated little revenue from sales of our products, and have incurred losses since inception. We anticipate that we will continue to incur significant losses until we are able to successfully commercialize our products and services globally.
Since inception, we have devoted substantially all of our financial resources to develop our products and their related services. We have financed our operations primarily through the issuance of equity securities and loans, have incurred losses since inception including net losses of approximately $25.1 million in 2022 and $14.9 million in 2021 and $12.7 million in 2020. Our accumulated deficit as of December 31, 2022 was $115,263 thousand. We have financed our operations primarily through the issuance of equity securities. We have generated little revenue from the sale of our products to date and have incurred significant losses. The amount of our future net losses will depend, in part, on on-going development of our products and their related services, the success of our new home testing kit related business, the rate of our future expenditures and our ability to obtain funding through the issuance of our securities, strategic collaborations or grants. We expect to continue to incur significant losses until we are able to successfully commercialize our products and services globally. We anticipate that our expenses will increase substantially if and as we:
| ● | continue the development of our products and services, including with respect to our new home testing kit business; |
3
| ● | establish a sales, marketing and distribution infrastructure to commercialize our products and services; |
| ● | seek to identify, assess, acquire, license and/or develop other products and services and subsequent generations of our current products and services; |
| ● | seek to maintain, protect and expand our intellectual property portfolio; |
| ● | seek to attract and retain skilled personnel; and |
| ● | continue to support our operations as a public company, our product development and planned future commercialization efforts. |
| Our ability to generate future revenue from product and service sales depends heavily on our success in many areas, including but not limited to: |
| ● | Successfully establishing and our ability to manage our home testing kit business; |
| ● | addressing any competing technological and market developments; |
| ● | negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter; |
| ● | establishing and maintaining resale and distribution relationships with third-parties that can provide adequate (in amount and quality) infrastructure to support market demand for our products; |
| ● | launching and commercializing current and future products and services, either directly or with a collaborator or distributor; and |
| ● | maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how. |
We expect that we will need to raise substantial additional funding before we can expect to become profitable from sales of our products and services. This additional financing may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
As of December 31, 2022, we had approximately $0.295 million in cash and cash equivalents and an accumulated deficit of $115,263 thousands. Based upon our currently expected level of operating expenditures, we expect that our current existing cash and cash equivalents, future fund raisings and the Company’s major shareholder commitment to continue and support the Company’s ongoing operation for the foreseeable future (if other sources of funding would not be available to the Company and under certain conditions) will be sufficient to fund our current operations for the foreseeable future. We will require substantial additional capital to fund our current operation and to grow our business and commercialize our products and services. In addition, our operating plans may change as a result of many factors that may currently be unknown to us, and we may need to seek additional funds sooner than planned.
We cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our stockholders and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our Ordinary Shares to decline. The incurrence of indebtedness could result in increased fixed payment obligations, and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable, and we may be required to relinquish rights to some of our technologies or products or otherwise agree to terms unfavorable to us, any of which may have a material adverse effect on our business, operating results and prospects. Even if we believe that we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
Raising additional capital would cause dilution to holders of our equity securities, and may affect the rights of existing holders of equity securities.
We may seek additional capital through a combination of private and public equity offerings, debt financings and collaborations and strategic and licensing arrangements. To the extent that we raise additional capital through the issuance of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a holder of the Ordinary Shares.
4
Risks Related to Our Business
We may not succeed in completing the development and commercialization of our products and services and generating significant revenues.
While we recently expanded our business to provide home test kits related services, including hormones, sexual transmitted diseases, nutrition, colon cancer, food sensitivity and allergies testing, since commencing our operations, we have focused on the research and development and limited clinical trials of our products and services. Some of our products and services are not approved for commercialization and have never generated any revenues. Our ability to generate revenues and achieve profitability depends on our ability to successfully complete the development of these products and services, obtain regulatory approvals and generate significant revenues. The future success of our business cannot be determined at this time, and we do not anticipate generating revenues from some of our products and services for the foreseeable future. In addition, we have limited experience in commercializing our products and services and we may face several challenges with respect to our commercialization efforts, including, among others, that:
| ● | we may not have adequate financial or other resources to complete the development of our products or services associated with a given product; |
| ● | we may not have adequate financial or other resources to complete the development of our lab and the equipment involved for such operations; |
| ● | we may not have adequate financial or other resources to support the ongoing operation of our lab, including materials required for the tests analysis, physician network costs and other unforeseen expenses |
| ● | we may not be able to manufacture our products in commercial quantities, at an adequate quality or at an acceptable cost; |
| ● | we may not be able to establish adequate sales, marketing and distribution channels; |
| ● | healthcare professionals and patients may not accept our products or fully utilize our products’ services; |
| ● | we may not be aware of possible complications from the continued use of our products or services since we have limited clinical experience with respect to the actual use of our products and services; |
| ● | technological breakthroughs in the mobile and e-health solutions and services may reduce the demand for our products; |
| ● | changes in the market for mobile and e-health solutions and services, new alliances between existing market participants and the entrance of new market participants may interfere with our market penetration efforts; |
| ● | third-party payors may not agree to reimburse patients for any or all of the purchase price of our products, which may adversely affect patients’ willingness to purchase our products; |
| ● | uncertainty as to market demand may result in inefficient pricing of our products and services; |
| ● | we may face third-party claims of intellectual property infringement for any of our technologies, services and at home testing kits; and |
| ● | we may fail to obtain or maintain regulatory approvals for our products or services in our target markets or may face adverse regulatory or legal actions relating to our products or services even if regulatory approval is obtained. |
5
If we are unable to meet any one or more of these challenges successfully, our ability to effectively commercialize our products and services could be limited, which in turn could have a material adverse effect on our business, financial condition and results of operations.
The expansion of our home testing kit business presents important challenges to our ability to manage our business.
In July 2022, we launched our home testing kit related business with seven types of at home testing kits. In October 2022, we expanded our business to offer an additional 24 different at-home health tests kits. Since the COVID-19 pandemic, there has been an increase in home testing for medical conditions and there is existing competition in the home testing kit market and we expect new entrants to enter the market, and these competitors may cause price declines or reduced market share for us. We expect this competition to intensify in the future. We face competition from a variety of sources, including, among others, an increasing number of companies seeking to develop and commercialize, or who have developed and commercialized home testing kit services, such as specialty and reference laboratories, and established and emerging healthcare, information technology and service companies that may develop and sell competitive products or services. There can be no assurance that our investments in our home testing kit business and capabilities will result in desirable returns, and if our operating results continue to decline as a result of decreased demand, our stock price could decline.
We may be subject to liability and our insurance may not be sufficient to cover damages.
Our business exposes us to potential liability risks that are inherent in the marketing and sale of testing kits and diagnostics. The use of our products may expose us to professional and product liability claims and possible adverse publicity. We may be subject to claims resulting from incorrect results of analysis with respect to our products and services. Litigation of such claims could be costly. Further, if a court were to require us to pay damages to a plaintiff, the amount of such damages could be significant and severely damage our financial condition. Although we have public and product liability insurance coverage under broad form liability and professional indemnity policies, the level or breadth of our coverage may not be adequate to fully cover any potential liability claims. In addition, we may not be able to obtain additional liability coverage in the future at an acceptable cost. A successful claim or series of claims brought against us in excess of our insurance coverage and the effect of professional and/or product liability litigation upon the reputation and marketability of our technology and products, together with the diversion of the attention of key personnel, could negatively affect our business.
Our success depends upon market acceptance of our products and services, our ability to develop and commercialize new products and services and generate revenues and our ability to identify new markets for our technology.
We have developed, and are engaged in the development of, mobile and e-health solutions and services using our suite of devices and software solutions. Our success will depend on the acceptance of our products and services in the healthcare market. We are faced with the risk that the marketplace will not be receptive to our products and services over competing products and that we will be unable to compete effectively. Factors that could affect our ability to successfully commercialize our current and any potential future products and services include:
| ● | the challenges of developing (or acquiring externally) technology solutions that are adequate and competitive in meeting the requirements of next-generation design challenges; |
| ● | our ability to successfully complete the validation process of each home test kit to be able to launch it to the market. Delays in launching kits might affect our ability to meet the sales forecast; |
| ● | the dependence upon physicians’ acceptance of our products and their willingness to prescribe our product to their patients for the sale of our products and provision of our services; and |
| ● | retailer acceptance of our products and their interest in selling it in their stores. There might be cases that due to private labelling of home testing kits by the retailer, the retailers won’t be interested in promoting our product but its own product, or in case a retailer signed an exclusive agreement with a large competitor, we might receive a negative response to our interest to work with a retailer in a specific area or stores. |
We cannot assure that our current products or any future products, and services, will gain broad market acceptance. If the market for our current products in development fails to develop or develops more slowly than expected, or if any of the services and standards supported by us do not achieve or sustain market acceptance, our business and operating results would be materially and adversely affected.
6
Medical device development is costly and involves continual technological change which may render our current or future products obsolete.
The market for monitoring services and products is characterized by rapid technological change, medical advances, changing consumer requirements, short device lifecycles and evolving industry standards. Any one of these factors could reduce the demand for our services and devices or require substantial resources and expenditures for research, design and development to avoid technological or market obsolescence.
Our success will depend on our ability to enhance our current technology, services and systems and develop or acquire and market new technologies to keep pace with technological developments and evolving industry standards, while responding to changes in customer needs. A failure to adequately develop or acquire device enhancements or new devices that will address changing technologies and customer requirements adequately, or to introduce such devices on a timely basis, may have a material adverse effect on our business, financial condition and results of operations.
We might have insufficient financial resources to improve existing devices, advance technologies and develop new devices at competitive prices. Technological advances by one or more competitors or future entrants into the field may result in our present services or devices becoming non-competitive or obsolete, which may decrease revenues and profits and adversely affect our business and results of operations.
We will encounter significant competition across our product lines and in each market in which we will sell our products and services from various companies, some of which may have greater financial and marketing resources than we do. Our primary competitors include Biotelemetry, Inc., iRhythm Technologies, Preventice Solutions, Inc., Bardy Diagnostics, Inc. and other arrhythmia service providers, as well as a wide range of medical device companies that sell a single or limited number of competitive products and services, such as Teledoc Health, Inc., DarioHealth Corp. and Itamar Medical, Inc., or participate in only a specific market segment.
We will be dependent upon success in our customer acquisition strategy.
Our business will be dependent upon success in our customer acquisition strategy. If we fail to maintain a high quality of service or a high quality of device technology, we may fail to retain existing users or add new users. If users decrease their level of engagement, our revenue, financial results and business may be significantly harmed. Our future success depends upon building a commercial operation in the United States and China, as well as entering additional markets to commercialize our products and services. We believe that our expanded growth will depend on the further development, regulatory approval and commercialization of our products and services, which we anticipate that can be used by nearly all targeted individuals. If we fail to expand the use of our product and services in a timely manner, we may not be able to expand our markets or to grow our revenue, and our business may be adversely impacted. The size of our user base and our users’ level of engagement are critical to our success. Our financial performance will be significantly determined by our success in adding, retaining and engaging active users. If people do not perceive our products or services to be useful, reliable and trustworthy, we may not be able to attract or retain users or otherwise maintain or increase the frequency and duration of their engagement. A decrease in user retention, growth or engagement could render less attractive to developers, which may have a material and adverse impact on our revenue, business, financial condition and results of operations.
Any number of factors could negatively affect user retention, growth and engagement, including:
| ● | users increasingly engaging with competing products; |
| ● | users not actively using the services associated with each of our respective services; |
| ● | failure to introduce new and improved products and services; |
7
| ● | inability to successfully balance efforts to provide a compelling user experience with the decisions made with respect to the added value services provided; |
| ● | inability to continue to develop products for mobile devices that users find engaging, that work with a variety of mobile operating systems and networks and that achieve a high level of market acceptance; |
| ● | changes in user sentiment about the quality or usefulness of our products and services or concerns related to privacy and sharing, safety, security or other factors; |
| ● | inability to manage and priorities information to ensure users are presented with content that is interesting, useful and relevant to them; |
| ● | adverse changes in our products that are mandated by legislation or regulatory agencies, both in the United States and across the globe; or |
| ● | technical or other problems preventing us from delivering products or services in a rapid and reliable manner or otherwise affecting the user experience. |
We have recently invested significant capital in our COVID-19 related services, including the purchase of COVID-19 testing kits, however, the future of COVID-19 related services is uncertain and we have stopped operations in our COVID-19 testing related business, which has led to the write off of inventory and fixed assets in our fiscal year ended December 31, 2022.
In December 2021 we launched our COVID-19 testing business, which entailed a significant investment of capital, including, among others, to establish several of testing facilities and laboratories throughout the state of California and the purchase of COVID-19 testing kits. We also entered into various agreements and arrangements regarding our COVID-19 testing business. The level of demand for COVID-19 testing has varied depending on, among other things, changes in the number of reported cases of COVID-19, discoveries of new variants or subvariants of the virus, different COVID-19 mitigation efforts and policies adopted by various governments or businesses, all of which are subject to change and beyond our control. Moreover, the future of COVID-19 related services may be dependent on changes to laws and regulations governing healthcare service providers, including measures to control costs, or reductions in reimbursement levels from government payors or insurance companies. These changes are difficult to predict and may also impact our revenue recognition or collecting accounts receivables. We currently have no expectations to open any new COVID-19 testing centers as the volume of COVID-19 testing has decreased significantly since April 2022. Given the decrease in COVID-19 cases, in the second half of fiscal year 2022 we decided to stop our COVID-19 testing related business and have closed all the locations performing point-of-care tests in communities in Southern and Northern California. We have incurred a write off charge of approximately $2.1 million related to our COVID-19 test inventory and fixed assets in our audited financial statements for the year ended December 31, 2022.
If we are unable to successfully integrate acquired companies and technology, we may not realize the benefits anticipated and our future growth may be adversely affected.
We have grown through acquisitions of companies and technology, including our acquisitions of CardioStaff, in November 2017 and Telerhythmics in November 2018. Acquisitions bring risks associated with our assumption of the liabilities of an acquired company, which may be liabilities that we were or are unaware of at the time of the acquisition, potential write-offs of acquired assets and potential loss of the acquired company’s key employees or customers. Physician, patient and customer satisfaction or performance problems with an acquired business, technology, service or device could also have a material adverse effect on our reputation. Additionally, potential disputes with the seller of an acquired business or its employees, suppliers or customers could adversely affect our business, operating results and financial condition. If we fail to properly evaluate and execute acquisitions, our business may be disrupted and our operating results and prospects may be harmed.
8
Furthermore, integrating acquired companies or new technologies into our business may prove more difficult than we anticipate. We may encounter difficulties in successfully integrating our operations, technologies, services and personnel with that of the acquired company, and our financial and management resources may be diverted from our existing operations. Offices in multiple states create a strain on our ability to effectively manage our operations and key personnel. If we elect to consolidate our facilities, we may lose key personnel unwilling to relocate to the consolidated facility, may have difficulty hiring appropriate personnel at the consolidated facility and may have difficulty providing continuity of service through the consolidation.
We are dependent upon third-party manufacturers and suppliers making us vulnerable to supply shortages and problems and price fluctuations, which could harm our business.
We do not manufacture our products in-house. Rather, we rely on a limited number of third parties to manufacture and assemble our products. Our suppliers and manufacturers may encounter problems during manufacturing for a variety of reasons, including, for example, failure to follow specific protocols and procedures, failure to comply with applicable legal and regulatory requirements, equipment malfunction and environmental factors, failure to properly conduct their own business affairs, and infringement of third-party intellectual property rights, any of which could delay or impede their ability to meet our requirements. Our reliance on these third-party suppliers also subjects us to other risks that could harm our business, including:
| ● | we are not a major customer of many of our suppliers and manufacturers, and these third parties may therefore give other customers’ needs higher priority than ours; |
| ● | third parties may threaten or enforce their intellectual property rights against our suppliers, which may cause disruptions or delays in shipment, or may force our suppliers to cease conducting business with us; |
| ● | we may not be able to obtain an adequate supply in a timely manner or on commercially reasonable terms; |
| ● | our suppliers and manufacturers, especially new suppliers and manufacturers, may make errors in manufacturing that could negatively affect the efficacy or safety of our products or cause delays in shipment; |
| ● | we may have difficulty locating and qualifying alternative suppliers and manufacturers; |
| ● | switching components, suppliers or manufacturers may require product redesign and possibly submission to the U.S. FDA, the European Medicines Agency, EEA Notified Bodies, and the Chinese National Medical Products Administration (or NMPA) or other similar foreign regulatory agencies, which could significantly impede or delay our commercial activities; |
| ● | the occurrence of a fire, natural disaster or other catastrophe impacting one or more of our suppliers and manufacturers may affect their ability to deliver products to us in a timely manner; and |
| ● | our suppliers and manufacturers may encounter financial or other business hardships unrelated to our demand, which could inhibit their ability to fulfill our orders and meet our requirements. |
We expect that our Prizma device and Extended Holter Patch System will be primarily manufactured by a third party in China. However, due to the current complexities of traveling to China as a result of the COVID-19 pandemic, we use a contract manufacturer in Israel to meet our manufacturing requirements. Manufacturers in Israel are generally more expensive that in China. In the future, we may not be able to quickly establish additional or alternative suppliers and manufacturers if necessary, in part because we may need to undertake additional activities to establish such suppliers as required by the regulatory approval process. Any interruption or delay in obtaining products from our third-party suppliers, or our inability to obtain products from qualified alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our customers and cause them to switch to competing products.
9
Regarding our home testing kits, our sole manufacturer is currently located in China and the current COVID-19 situation in China might pose delays on supply chain interruptions which might have an adverse effect on our revenues. We are exploring other supplier located in North America to reduce such possible delays and interruptions. In addition, government restrictions on goods originating from China and increase of import fees might lead to delays in goods delivered to the United States and adversely affect the Company’s operations and revenues.
We are dependent upon third-party service providers for the provision of certain services that we provide. Interruptions or delays in the services provided by these third-parties could impair the delivery of certain services and utility of our products, which could adversely affect the penetration of our products and services, our business, operating results and reputation.
The success of certain services that we provide are dependent upon third-party service providers. For instance, we are dependent upon third-party service providers to provide analysis of medical results. If we fail to maintain these relationships, we would be forced to seek alternative providers to provide such analyses, which we may not find available on commercially reasonable terms, or at all.
As we expand our commercial activities, an increased burden will be placed upon the quality of medical results analyses. Interruptions or delays, for any length of time, could have a material adverse effect on our business and operating results. Frequent or persistent interruptions in our ability to provide quality and timely analyses could cause permanent harm to our reputation and could cause current or potential users of our products and services, or prescribing physicians, to believe that our systems are unreliable, leading them to switch to our competitors. Such interruptions could result in liability claims and litigation against us for damages or injuries resulting from the disruption in service.
We expect to be exposed to fluctuations in currency exchange rates, which could adversely affect our results of operations.
We incur expenses in U.S. dollars, NIS, Chinese yuan (RMB), and Macedonian denars, but our financial statements are denominated in U.S. dollars. Accordingly, we face exposure to adverse movements in currency exchange rates. Our foreign operations will be exposed to foreign exchange rate fluctuations as the financial results are translated from the local currency into U.S. dollars upon consolidation. Specifically, the U.S. dollar cost of our operations in Israel is influenced by any movements in the currency exchange rate of the NIS. Such movements in the currency exchange rate may have a negative effect on our financial results. If the U.S. dollar weakens against foreign currencies, the translation of these foreign currency denominated transactions will result in increased operating expenses. Similarly, if the U.S. dollar strengthens against foreign currencies, the translation of these foreign currency denominated transactions will result in decreased operating expenses. As exchange rates vary, operating results, when translated, may differ materially from our or the capital market’s expectations.
Non-U.S. governments often impose strict price controls, which may adversely affect our future profitability.
We intend to seek approval to market products and their associated services in both the United States and in non-U.S. jurisdictions. Accordingly, we are subject to rules and regulations in those jurisdictions relating to our products and services. In some countries, particularly countries of the European Union (or the EU) and those of the EEA and China, each of which has developed its own rules and regulations, pricing may be subject to governmental control under certain circumstances. In these countries, pricing negotiations with governmental agencies can take considerable time after the receipt of marketing approval for a medical device candidate. To obtain reimbursement or pricing approval in some countries, we may be required to conduct a clinical trial that compares the cost-effectiveness of our product to other available products. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, we may be unable to achieve or sustain profitability.
10
We are dependent on our employees, including notably our Chief Executive Officer, the loss of whom could have an adverse effect on our company.
As of May 15, 2023, we had 85 employees. Our future performance depends to a large extent on the continued services of members of our current management including, in particular, Dr. Yacov Geva, our Chief Executive Officer. Any of our employees and consultants may leave our company at any time, subject to certain notice periods. The loss of the services of any of our executive officers or any key employees or consultants would adversely affect our ability to execute our business plan and harm our operating results.
International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States or Cayman Islands.
We currently have significant international operations, and our business strategy incorporates additional significant international expansion, particularly in anticipated expansion of regulatory approvals of our products. Doing business internationally involves a number of risks, including but not limited to:
multiple, conflicting and changing laws and regulations such as privacy regulations, tax laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses;
| ● | failure by us to obtain regulatory approvals for the use of our products and services in various countries; |
| ● | additional potentially relevant third-party patent rights; |
| ● | complexities and difficulties in obtaining protection and enforcing our intellectual property; |
| ● | difficulties in staffing and managing foreign operations; |
| ● | complexities associated with managing multiple regulatory, governmental and reimbursement regimes; |
| ● | limits in our ability to penetrate international markets; |
| ● | financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the impact of local and regional financial crises on demand and payment for our products and exposure to foreign currency exchange rate fluctuations; |
| ● | natural disasters, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade and other business restrictions; |
| ● | an outbreak of a contagious disease, such as the novel coronavirus pandemic of 2019 which may cause us, third party vendors and manufacturers and/or customers to temporarily suspend our or their respective operations in the affected city or country; |
| ● | certain expenses including, among others, expenses for travel, translation and insurance; and |
| ● | regulatory and compliance risks that relate to maintaining accurate information and control over sales and activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, its books and records provisions or its anti-bribery provisions. |
Any of these factors could significantly harm our future international expansion and operations and, consequently, our results of operations.
11
We face intense competition in the market, and as a result we may be unable to effectively compete in our industry.
With respect to our products and monitoring services we compete directly and primarily with arrhythmia monitoring providers such as Philips Healthcare, iRhythm Technologies and other smaller companies. These companies hold significant market share in the United States. Their dominant market position and significant control over the market could significantly limit our ability to introduce or effectively market and generate sales of our products and service offerings. We will also compete with numerous second-tier and third-tier competitors, such as Teledoc Health, Inc., DarioHealth Corp. and Itamar Medical, Inc., and may in the future face further competition from smartwatch makers such as Apple and Samsung, and app developers using the smartwatch platforms for products that will compete with our current and future products.
Many of our competitors have long histories and strong reputations within the industry. They have significantly greater brand recognition, financial and human resources than we do. They also have more experience and capabilities in researching and developing testing devices, obtaining and maintaining regulatory clearances and other requirements, manufacturing and marketing those products than we do. There is a significant risk that we may be unable to overcome the advantages held by our competition, and our inability to do so could lead to the failure of our business and the loss of your investment.
Competition in the electronic health devices and more specifically mobile health devices markets is extremely intense, which can lead to, among other things, price reductions, longer selling cycles, lower product margins, loss of market share and additional working capital requirements. To succeed, we must, among other critical matters, gain consumer acceptance for our minimally invasive solutions as compared to other solutions currently available in the market, and potential future devices incorporating our principal technology and offer better strategic concepts, technical solutions, prices and response time, or a combination of these factors, than those of other competitors. If our competitors offer significant discounts on certain products or services, we may need to lower our prices or offer other favorable terms in order to compete successfully. Moreover, any broad-based changes to our prices and pricing policies could make it difficult to generate revenues or cause our revenues to decline. Moreover, if our competitors develop and commercialize products and services that are more effective or desirable than products and services that we may develop, we may not convince our customers to use our products and services. Any such changes would likely reduce our commercial opportunity and revenue potential and could materially adversely impact our operating results.
In addition, the home kit testing market has become increasingly competitive and, following the COVID-19 pandemic, competitive home kit testing solutions have entered and may continue to enter the market. We expect this competition to intensify in the future. We face competition from a variety of sources, including, among others, an increasing number of companies seeking to develop and commercialize, or who have developed and commercialized home testing services, such as specialty and reference laboratories, and established and emerging healthcare, information technology and service companies that may develop and sell competitive products or services.
The level of our commercial success will depend in part on our ability to generate and grow sales with our sales and marketing team, strategies and partnerships, and we may be unsuccessful in these efforts.
We may not be able to market or sell our testing and services in order to drive demand sufficiently to support our desired growth. We are currently marketing our testing and services through a small internal sales team. Although we have made efforts to enhance and improve our internal sales department, it remains significantly smaller than many of our competitors’ sales teams. We have historically relied significantly on organic growth but our ability to rely on this type of interest in future periods is uncertain.
12
We believe our ability to maintain and grow sales volume in the future will depend in large part on our ability to further develop our sales team and create and implement effective sales and marketing strategies. We plan to focus on these objectives and to take steps to pursue them, including hiring new key members and restructuring the organization of our sales and marketing team, re-focusing our sales and marketing initiatives and strategies, and increasing the overall scope of our marketing activities. These efforts will require and involve significant time and expense. Moreover, these efforts may be unsuccessful. For instance, we may not be able to attract and hire the qualified personnel we need to grow or otherwise improve our sales and marketing team for our testing services as quickly or as successfully as we would like for various reasons, including intense competition in our industry for qualified personnel and our relative lack of experience selling and marketing our tests. Even if we are able to further develop our sales and marketing team and strategy, we may not be successful in growing our customer base or increasing testing volumes from our existing customers.
If third-party payors do not provide adequate coverage and reimbursement for the use of our products and services, our revenue will be negatively impacted.
We will be highly dependent on reimbursement by third parties in relation to our revenue streams. Such reimbursement may vary based on the particular service or device used in providing services and is based on the identity of the third-party. Our ability to maintain a leading position in the monitoring market depends on our relationships with private third parties.
We expect to engage with private third parties to allow us to receive reimbursement from insurance companies for monitoring fees. The loss of a significant number of private third-party contracts may have an adverse effect on our revenues that derives from monitoring services, which could have an adverse effect on our business, financial condition and results of operations.
Over the past few years, reimbursement rates from certain third parties have declined, in some cases significantly. There can be no assurance that this trend will not continue or apply on more third parties. In addition, there is no assurance that third parties’ reimbursement will continue to cover our monitoring services at all, or, if covered, will reimburse us at commercially viable rates.
In addition, private third parties may not reimburse any new services offered by us or reimburse those new services at commercially viable rates. The failure to receive reimbursement at adequate levels for our existing or future services may adversely affect demand for those services, our products, our revenues and expected growth. This could have an adverse effect on our business, financial condition and results of operations.
In addition, collecting revenue from third party payors can be time consuming, may be subject to delays and invoicing third parties may not lead to immediate revenue recognition in connection with tests that have been performed. The timing of invoicing and collection of payments from third party payors may have a material adverse effect on our business, financial condition and results of operations. For example, during the six month period ended June 30, 2022, we performed over 100,000 tests, for which we expected to submit approximately $5 million in invoices related to COVID-19 to collection from payors; however, as of December 31, 2022, we recorded only $200,000 in recorded revenue from COVID-19 tests performed during the period.
Risks Related to Product Development and Regulatory Approval
The regulatory clearance process which we must navigate is expensive, time-consuming, and uncertain and may prevent us from obtaining clearance for the commercialization of our current products and services in additional jurisdictions, or any future product.
We are not permitted to market our products and their associated services until we receive regulatory clearance. For example, we have applied for NMPA clearance for our Prizma device in China, but we may not commence marketing or sales activity until such time that we receive NMPA clearance.
The research, design, testing, manufacturing, labelling, selling, marketing and distribution of medical devices, such as our products and product candidates, are subject to extensive regulation by the U.S. FDA and similar foreign regulatory agencies, with regulations that differ from country to country. There can be no assurance that, even after such time and expenditures, we will be able to obtain necessary regulatory clearances or approvals for our products and product candidates. In addition, during the regulatory process, other companies may develop other technologies with the same intended use as our products.
13
We are also subject to numerous post-marketing regulatory requirements, which include labelling regulations and medical device reporting regulations, which may require us to report to different regulatory agencies if our device causes or contributes to a death or serious injury, or malfunctions in a way that would likely cause or contribute to a death or serious injury. In addition, these regulatory requirements may change in the future in a way that adversely affects us. If we fail to comply with present or future regulatory requirements that are applicable to us, we may be subject to enforcement action by regulatory agencies, which may include, among others, any of the following sanctions:
| ● | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| ● | customer notification, or orders for repair, replacement or refunds; |
| ● | voluntary or mandatory recall or seizure of our current or future products; |
| ● | imposing operating restrictions, suspension or shutdown of production; |
| ● | refusing our requests for 510(k) and CE clearances or pre-market approval of new products, new intended uses or modifications to existing products or future products; |
| ● | rescinding 510(k) and CE clearances or suspending or withdrawing pre-market approvals that have already been granted; and |
| ● | criminal prosecution. |
The occurrence of any of these events may have a material adverse effect on our business, financial condition and results of operations.
Changes in the regulatory environment may constrain or require us to restructure our operations, which may delay or prevent us from marketing our products and services and as a result harming our revenue and operating results.
Healthcare laws and regulations and review procedures change frequently and may change significantly in the future. We may not be able to adapt our operations to address every new regulation, and new regulations may adversely affect our business. For instance, although our Chinese subsidiary was granted acceptance to the “Green Channel” expedited Guangdong Provincial NMPA regulatory approval process for the Prizma device, if the process becomes more onerous, costly or time-consuming, we will need to re-evaluate our Chinese commercialization strategy and may need to invest more of our limited resources before even entering the Chinese market with our products. Our products and product candidates are also subject to the European Union Medical Device Regulations. We cannot assure you that a review of our business by courts or regulatory agencies would not result in a determination that adversely affects our revenue and operating results, or that the healthcare regulatory environment review procedures of the U.S. FDA, NMPA and EEA Notified Bodies, among other similar foreign regulatory agencies, will not change in a way delays or prevents us from marketing our products and services and as a result harming our revenue and operating results.
In addition, there is risk that the U.S. Congress may implement changes in laws and regulations governing healthcare service providers, including measures to control costs, or reductions in reimbursement levels, which may adversely affect our business and results of operations.
Government payors, such as CMS as well as insurers, have increased their efforts to control the cost, utilization and delivery of healthcare services. From time to time, the U.S. Congress has considered and implemented changes in the CMS fee schedules in conjunction with budgetary legislation. Further reductions of reimbursement by CMS for services or changes in policy regarding coverage of tests or other requirements for payment, such as prior authorization or a physician or qualified practitioner’s signature on test requisitions, may be implemented from time to time. Reductions in the reimbursement rates and changes in payment policies of other third-party payors may occur as well. Similar changes in the past have resulted in reduced payments as well as added costs and have added more complex regulatory and administrative requirements. Further changes in federal, state, local and third-party payor regulations or policies in the United States or our primary foreign markets may have a material adverse impact on our business. Actions by the U.S. FDA, CMS, and similar foreign regulatory agencies regulating insurance or changes in other laws, regulations, or policies may also have a material adverse effect on our business.
14
Moreover, changes in regulation associated with operation of labs, might also lead to interruptions in the operation of the Company’s lab and/or generate revenues and profit.
If we, our affiliates, manufacturers or suppliers fail to comply with the U.S. FDA’s Quality System Regulation, or QSR, or any applicable state or foreign equivalent, our operations could be interrupted and our operating results could suffer.
We, our affiliates, manufacturers and suppliers must, unless specifically exempt by regulation, follow the QSR and, to the extent required, the equivalent regulation enacted in other foreign jurisdictions, such as the EU (and if necessary, the regulations of its member states) and China, regarding the manufacturing process. If we, our affiliates, our manufacturers or suppliers are found to be in significant non-compliance or fail to take satisfactory corrective action in response to adverse QSR inspectional findings, or to findings of similar foreign regulatory agencies, the U.S. FDA and these other similar foreign regulatory agencies could take enforcement actions against us, our affiliates, manufacturers and suppliers which could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. Accordingly, our operating results could suffer.
Product and services liability suits, whether or not meritorious, could be brought against us. These suits could result in expensive and time-consuming litigation, payment of substantial damages and an increase in our insurance rates.
If any of our current or future products and services that we make or sell (including items that we source from third-parties) are defectively designed or manufactured contain defective components, are misused, have safety or quality issues, have inadequate operating guidelines, or if someone claims any of the foregoing, whether or not meritorious, we may become subject to substantial and costly litigation. Misusing our devices or their services or failing to adhere to the operating guidelines could cause significant harm to patients, including death. The foregoing events could lead to recalls or safety alerts, result in the removal of a product or service from the market and result in product liability or similar claims being brought against us.
Any product liability claims brought against us could divert management’s attention from our core business, be expensive to defend and result in sizable damage awards against us. While we maintain product liability insurance, we may not have sufficient insurance coverage for all future claims. Any product liability claims brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing continuing coverage, could harm our reputation in the industry and could reduce revenue. Product and services liability claims in excess of our insurance coverage would be paid out of cash reserves harming our financial condition and adversely affecting our results of operations.
Broad-based domestic and international government initiatives to reduce spending, particularly those related to healthcare costs may reduce reimbursement rates for medical procedures, which will reduce the cost-effectiveness of our products and services.
Healthcare reforms, changes in healthcare policies and changes to third-party coverage and reimbursements, including legislation enacted reforming the U.S. healthcare system, and any future changes to such legislation, may affect demand for our products and services and may have a material adverse effect on our financial condition and results of operations. There can be no assurance that current levels of reimbursement will not be decreased in the future, or that future legislation, regulation, or reimbursement policies of third-parties will not adversely affect the demand for our products and services or our ability to sell products and provide services on a profitable basis. The adoption of significant changes to the healthcare system in the United States, Europe, the EEA or other jurisdictions in which we may market our products and services, could limit the prices we are able to charge for our products and services or the amounts of reimbursement available for our products and services, could limit the acceptance and availability of our products and services, reduce medical procedure volumes and increase operational and other costs. President Trump has stated that he intends to “repeal and replace” the Affordable Care Act, and Congress has taken initial steps to repeal the law. In December 2017, Congress passed and the President signed into law tax reform legislation that made significant changes to the Affordable Care Act including the repeal of the “individual mandate” that was in place to strongly encourage broad participation in the health insurance markets. Given these changes and other statements of political leaders, we cannot predict the ultimate impact on the Affordable Care Act and the subsequent effect on our business at this time. While we are unable to predict what changes may ultimately be enacted, to the extent that future changes affect how our products and services are paid for and reimbursed by government and private payers our business could be adversely impacted.
15
We cannot predict whether future healthcare initiatives will be implemented at the federal or state level or internationally, or the effect that any future legislation or regulation will have on us. The expansion of government’s role in any country’s healthcare industry may result in decreased profits to us, lower reimbursements by third-parties for procedures in which our products and services are used, and reduced medical procedure volumes, all of which may adversely affect our business, financial condition and results of operations.
We are or may be subject to federal, state and foreign healthcare fraud and abuse laws and regulations.
Many federal, state and foreign healthcare laws and regulations apply to medical devices. We are or may be subject to certain federal and state regulations, including the federal Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully soliciting, offering, receiving, or paying any remuneration, directly or indirectly, in cash or in kind, to induce or reward purchasing, ordering or arranging for or recommending the purchase or order of any item or service for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid; HIPAA which imposes criminal and civil liability for knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of, or payment for, healthcare benefits, items or services; the federal Civil Monetary Penalties Law, which authorizes the imposition of substantial civil monetary penalties against an entity that engages in activities including, among others (1) knowingly presenting, or causing to be presented, a claim for services not provided as claimed or that is otherwise false or fraudulent in any way; (2) arranging for or contracting with an individual or entity that is excluded from participation in federal healthcare programs to provide items or services reimbursable by a federal healthcare program; (3) violations of the federal Anti-Kickback Statute; or (4) failing to report and return a known overpayment; the federal False Statements Statute, which prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry, in connection with the delivery of or payment for healthcare benefits, items, or services; the federal civil False Claims Act (or FCA) which prohibits, among other things, knowingly presenting, or causing to be presented claims for payment of government funds that are false or fraudulent, or knowingly making, using or causing to be made or used a false record or statement material to such a false or fraudulent claim, or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government; and other federal and state false claims laws. The FCA prohibits anyone from knowingly presenting, conspiring to present, making a false statement in order to present, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services, including medical devices, that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. This law also prohibits anyone from knowingly underpaying an obligation owed to a federal program. Increasingly, U.S. federal agencies are requiring nonmonetary remedial measures, such as corporate integrity agreements in FCA settlements. The U.S. Department of Justice announced in 2016 its intent to follow the “Yates Memo,” taking a far more aggressive approach in pursuing individuals as FCA defendants in addition to the corporations.
The majority of states also have statutes similar to the federal anti-kickback law and false claims laws that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, that apply regardless of whether the payer is a government entity or a private commercial entity. The Federal Open Payments, or Physician Payments Sunshine Act, program requires manufacturers of products for which payment is available under Medicare, Medicaid or the State Children’s Health Insurance Program, to track and report annually to the federal government (for disclosure to the public) certain payments and other transfers of value made to physicians and teaching hospitals as well as disclosure of payments and other transfers of value provided to physicians and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations. Our failure to appropriately track and report payments to the government could result in civil fines and penalties, which could adversely affect the results of our operations. In addition, several U.S. states and localities have enacted legislation requiring medical device companies to establish marketing compliance programs, file periodic reports with the state, and/or make periodic public disclosures on sales, marketing, pricing, clinical trials, and other activities. Other state laws prohibit certain marketing-related activities including the provision of gifts, meals or other items to certain healthcare providers. Many of these laws and regulations contain ambiguous requirements that government officials have not yet clarified. Given the lack of clarity in the laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent federal and state laws and regulations.
16
The medical device industry has been under heightened scrutiny as the subject of government investigations and enforcement actions involving manufacturers who allegedly offered unlawful inducements to potential or existing customers in an attempt to procure their business, including arrangements with physician consultants. If our operations or arrangements are found to be in violation of such governmental regulations, we may be subject to civil and criminal penalties, damages, fines, exclusion from the Medicare and Medicaid programs and the curtailment of our operations. All of these penalties could adversely affect our ability to operate our business and our financial results.
The operation of our monitoring centers is subject to rules and regulations governing IDTF and state licensure requirements; failure to comply with these rules could prevent us from receiving reimbursement from Medicare and some commercial payors.
We operate two monitoring centers in the United States that receive and analyze the data obtained from our patient monitors and generate preliminary reports that are delivered to physicians. To receive reimbursement from Medicare and some commercial payors, our monitoring centers must be certified as IDTFs. As a certified IDTF, we must adhere to strict regulations governing how our monitoring centers operate, and how our technicians are trained and certified on analyzing the data received from our monitors. These rules and regulations are subject to change, and vary from location to location. Changes may require modifications at our monitoring centers, which could increase our costs significantly. If we fail to maintain IDTF certification, our services may no longer be reimbursed by Medicare and some commercial payors, which could have a material adverse impact on our business.
Audits or denials of our claims by government agencies and private payors could reduce our revenue and have an adverse effect on our results of operations.
Our business operations submit claims on behalf of patients to, and receive payments from, Medicare, Medicaid and other third-party payors. We must submit reimbursement claims under appropriate codes and maintain certain documentation to support our claims. Medicare contractors and Medicaid agencies periodically conduct reviews and other audits of claims and are under increasing pressure to more closely scrutinize health care claims and supporting documentation. Such reviews and similar audits of our claims could result in material delays in payment, as well as material recoupments or denials, which would reduce our net sales and profitability, or may result in our exclusion from participation in the Medicare or Medicaid programs. We are also subject to similar review and audits from private payors, which may result in material delays in payment and material recoupments and denials. In addition, state agencies may conduct investigations or submit requests for information relating to claims data submitted to private payors.
Losing a payor would impact our sales and adversely affect our business and operating results.
Our three largest payors in the aggregate accounted for approximately 50% of our monitoring service revenue for the year ended December 31, 2022. Our contracts with commercial payors may allow either party to terminate the agreement by providing between 60 and 120 days’ prior written notice to the other party at any time following the end of the initial term of the contract. Commercial payors may choose to terminate or not renew their contracts with us for any reason and, in some instances, can change the reimbursement rates they agree to pay. A commercial payor who terminates or does not renew their contract with us may, or may not, alter their coverage of our services. In the event any of our key commercial payors terminate their agreements with us, elect not to renew or enter into new agreements with us upon expiration of their current agreements, or do not renew or establish new agreements on terms as favorable as are currently contracted, our business, operating results and prospects would be adversely affected.
17
If we are found to have violated laws protecting the confidentiality and privacy of patient health information, we could be subject to civil or criminal penalties, which could increase our liabilities and harm our reputation or our business.
As part of our clinical trials and the use of our products, we may have access to medical data of patients. There are a number of federal and state laws protecting the confidentiality and privacy of certain patient health information, including patient records, and restricting the use and disclosure of that protected information. In particular, the U.S. Department of Health and Human Services promulgated patient privacy and security rules under the HIPAA. These privacy and security rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their own health information and limiting most use and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose. We may face difficulties in holding such information in compliance with applicable law. If we are found to be in violation of the privacy and security rules under HIPAA, we could be subject to civil or criminal penalties, which could increase our liabilities, harm our reputation and have a material adverse effect on our business, financial condition and results of operations.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain effective patent rights for our products and services, we may not be able to compete effectively in our markets. If we are unable to protect the confidentiality of our trade secrets or know-how, such proprietary information may be used by others to compete against us.
We have applied for various patents, in different territories. We presently have seven patents that have been granted or allowed and four patent applications which are still pending. In addition to the protection afforded by any patents that may be granted, historically, we have relied on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes that are not easily known, knowable or easily ascertainable, and for which patent infringement is difficult to monitor and enforce and any other elements of our product candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors, and contractors. We also seek to preserve the integrity and confidentiality of our data, trade secrets and intellectual property by maintaining physical security of our premises and physical and electronic security of our information technology systems. Agreements or security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets and intellectual property may otherwise become known or be independently discovered by competitors.
Also, there is a risk that the patent applications that were submitted by us with regards to our technologies will not be granted. In the event of failure to obtain granted patents, our developments will not be proprietary, subject to publication, which might allow other entities to manufacture our products or develop our methods of use as services and compete with our products, technologies, and/or services, which could leave us at a competitive disadvantage.
Further, there is no assurance that all potentially relevant prior art relating to our patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue, and even if such patents cover our products or services, third parties may challenge their validity, enforceability, or scope, which may result in such patents being narrowed, found unenforceable or invalidated. Furthermore, even if they are unchallenged, our patent applications and any future patents may not adequately protect our products or services and provide patent exclusivity for our new products or services, or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
If we cannot obtain and maintain effective patent rights for our products and services, we may not be able to compete effectively, and our business and results of operations would be harmed.
We cannot provide any assurances that our trade secrets and other confidential proprietary information will not be disclosed in violation of our confidentiality agreements or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Also, misappropriation or unauthorized and unavoidable disclosure of our trade secrets and intellectual property could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets and intellectual property are deemed inadequate, we may have insufficient recourse against third parties for misappropriating any trade secret.
18
Intellectual property rights of third parties could adversely affect our ability to commercialize our products and services, and we might be required to litigate or obtain licenses from third parties in order to develop or market our product candidates. Such litigation or licenses could be costly or not available on commercially reasonable terms.
It is inherently difficult to conclusively assess our freedom to operate without infringing on third-party rights. Our competitive position may be adversely affected if existing patents or patents resulting from patent applications issued to third parties or other third-party intellectual property rights are held to cover our products or services or elements thereof, or our manufacturing or uses relevant to our development plans. In such cases, we may not be in a position to develop or commercialize products or services or our product candidates (and any relevant services) unless we successfully pursue litigation to nullify or invalidate the third-party intellectual property right concerned, or enter into a license agreement with the intellectual property right holder, if available on commercially reasonable terms. There may also be pending patent applications that if they result in issued patents, could be alleged to be infringed by our new products or services. If such an infringement claim should be brought and be successful, we may be required to pay substantial damages, be forced to abandon our new products or services or seek a license from any patent holders. No assurances can be given that a license will be available on commercially reasonable terms, if at all.
It is also possible that we have failed to identify relevant third-party patents or applications. For example, U.S. patent applications filed before November 29, 2000 and certain U.S. patent applications filed after that date that will not be filed outside the United States remain confidential until patents issue. Patent applications in the United States and elsewhere are published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our new products or services could have been filed by others without our knowledge. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our services, our new products or the use of our new products. Third-party intellectual property right holders may also actively bring infringement claims against us. We cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims. If we are unable to successfully settle future claims on terms acceptable to us, we may be required to engage in or continue costly, unpredictable and time-consuming litigation and may be prevented from or experience substantial delays in pursuing the development of and/or marketing our new products or services. If we fail in any such dispute, in addition to being forced to pay damages, we may be temporarily or permanently prohibited from commercializing our new products or services that are held to be infringing. We might, if possible, also be forced to redesign our new products so that we no longer infringe the third-party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing new products and services. As our industries expand and more patents are issued, the risk increases that our products and services may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, designs or methods of manufacture related to the use or manufacture of our products or services. There may be currently pending patent applications or continued patent applications that may later result in issued patents that our products or services may infringe. In addition, third parties may obtain patents or services in the future and claim that use of our technologies infringes upon these patents.
If any third-party patents were held by a court of competent jurisdiction to cover aspects of our processes for designs, or methods of use, the holders of any such patents may be able to block our ability to develop and commercialize the applicable product candidate unless we obtain a license or until such patent expires or is finally determined to be invalid or unenforceable. In either case, such a license may not be available on commercially reasonable terms or at all.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our products or services. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or services, or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
19
Patent policy and rule changes could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any issued patents.
Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of any patents that may issue from our patent applications, or narrow the scope of our patent protection. The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. We therefore cannot be certain that we were the first to file the invention claimed in our owned and licensed patent or pending applications, or that we or our licensor were the first to file for patent protection of such inventions. Assuming all other requirements for patentability are met, in the United States prior to March 15, 2013, the first to make the claimed invention without undue delay in filing, is entitled to the patent, while generally outside the United States, the first to file a patent application is entitled to the patent. After March 15, 2013, under the Leahy-Smith America Invents Act (or the Leahy-Smith Act) enacted on September 16, 2011, the United States has moved to a first to file system. The Leahy-Smith Act also includes a number of significant changes that affect the way patent applications will be prosecuted and may also affect patent litigation. In general, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of any issued patents, all of which could have a material adverse effect on our business and financial condition.
We may be involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming, and unsuccessful.
Competitors may infringe our intellectual property. If we were to initiate legal proceedings against a third-party to enforce a patent covering one of our new products or services, the defendant could counterclaim that the patent covering our product candidate is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness, or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the United States Patents Trademarks Office (or USPTO), or made a misleading statement, during prosecution. Under the Leahy-Smith Act, the validity of U.S. patents may also be challenged in post-grant proceedings before the USPTO. The outcome following legal assertions of invalidity and unenforceability is unpredictable.
Derivation proceedings initiated by third parties or brought by us may be necessary to determine the priority of inventions and/or their scope with respect to our patent or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. In addition, the uncertainties associated with litigation could have a material adverse effect on our ability to raise the funds necessary to continue our clinical trials, continue our research programs, license necessary technology from third parties, or enter into development partnerships that would help us bring our new products or services to market.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of the Ordinary Shares.
20
We may be subject to claims challenging the inventorship of our intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an interest in, or right to compensation, with respect to our current patent and patent applications, future patents or other intellectual property as an inventor or co-inventor. For example, we may have inventorship disputes arise from conflicting obligations of consultants or others who are involved in developing our products or services. Litigation may be necessary to defend against these and other claims challenging inventorship or claiming the right to compensation. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, and defending patents on products and services, as well as monitoring their infringement in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States.
Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products or services and may also export otherwise infringing products or services to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products or services may compete with our products or services. Future patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, which could make it difficult for us to stop the marketing of competing products or services in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions, whether or not successful, could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our future patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to monitor and enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to Cayman Law and Our Incorporation in the Cayman Islands
We are a Cayman Islands exempted company with limited liability. The rights of our shareholders may be different from the rights of shareholders governed by the laws of U.S. jurisdictions.
We are a Cayman Islands exempted company with limited liability. Our corporate affairs are governed by our Amended and Restated Memorandum and Articles of Association adopted by special resolution passed on February 16, 2023 (or the Amended and Restated Memorandum and Articles of Association) and by the laws of the Cayman Islands. The rights of shareholders and the responsibilities of members of our board of directors may be different from the rights of shareholders and responsibilities of directors in companies governed by the laws of U.S. jurisdictions. In the performance of its duties, the board of directors of a solvent Cayman Islands exempted company is required to consider that company’s interests, and the interests of its shareholders as a whole, which may differ from the interests of one or more of its individual shareholders.
21
Our shareholders may face difficulties in protecting their interests because we are a Cayman Islands exempted company.
Our corporate affairs are governed by our Amended and Restated Memorandum and Articles of Association, by the Companies Act (as amended) of the Cayman Islands (or the Cayman Islands Companies Act) and the common law of the Cayman Islands. The rights of our shareholders and the fiduciary responsibilities of our directors under the laws of the Cayman Islands are not as clearly defined as under statutes or judicial precedent in existence in jurisdictions in the United States. Therefore, you may have more difficulty protecting your interests than would shareholders of a corporation incorporated in a jurisdiction in the United States, due to the comparatively less formal nature of Cayman Islands law in this area.
While Cayman Islands law allows a dissenting shareholder to express the shareholder’s view that a court-sanctioned reorganization of a Cayman Islands company would not provide fair value for the shareholder’s shares, Cayman Islands statutory law does not specifically provide for shareholder appraisal rights in connection with a merger or consolidation of a company. This may make it more difficult for you to assess the value of any consideration you may receive in a merger or consolidation or to require that the acquirer gives you additional consideration if you believe the consideration offered is insufficient. However, Cayman Islands statutory law provides a mechanism for a dissenting shareholder in a merger or consolidation to apply to the Grand Court of the Cayman Islands (or the Grand Court) for a determination of the fair value of the dissenter’s shares if it is not possible for the company and the dissenter to agree on a fair price within the time limits prescribed.
Shareholders of Cayman Islands exempted companies (such as ours) have no general rights under Cayman Islands law to inspect corporate records and accounts or to obtain copies of lists of shareholders. This may make it more difficult for you to obtain information needed to establish any facts necessary for a shareholder motion or to solicit proxies from other shareholders in connection with a proxy contest.
Under Cayman Islands’ law, a minority shareholder may bring a derivative action against the board of directors only in very limited circumstances, or seek to wind up the company on the just and equitable ground. Class actions are not recognized in the Cayman Islands, but groups of shareholders with identical interests may bring representative proceedings, which are similar.
Under Cayman Islands statutory law, a transferee to a scheme or contract involving the transfer of shares in a Cayman Islands company, which has been approved by holders of not less than 90% in value of the shares affected, has the power to compulsorily acquire the shares of any dissenting shareholders. An objection to such acquisition can be made to the Grand Court by any dissenting shareholder but this is unlikely to succeed in the case of an offer which has been so approved unless there is evidence of fraud, bad faith or collusion. A Cayman Islands company may also propose a compromise or arrangement with its shareholders or any class of them. If a majority in number, representing at least 75% in value, of shareholders agrees to the compromise or arrangement then, subject to Grand Court approval of the same, it is binding on all of the shareholders. A shareholder may appear at the Grand Court hearing by which the company seeks the Grand Court’s approval of the compromise or arrangement to oppose it.
United States civil liabilities and certain judgments obtained against us by our shareholders may not be enforceable.
We are a Cayman Islands exempted company and substantially all of our assets are located outside the United States. In addition, the majority of our directors and officers are nationals and residents of countries other than the United States. A substantial portion of the assets of these persons is located outside the United States. As a result, it may be difficult to effect service of process within the United States upon these persons. It may also be difficult to enforce in judgments obtained in U.S. courts based on the civil liability provisions of U.S. federal securities laws against us and our officers and directors who are not resident in the United States.
Further, it is unclear if original actions predicated on civil liabilities based solely upon U.S. federal securities laws are enforceable in courts outside the United States, including in the Cayman Islands. Courts of the Cayman Islands may not, in an original action in the Cayman Islands, recognize or enforce judgments of U.S. courts predicated upon the civil liability provisions of the securities laws of the United States or any state of the United States on the grounds that such provisions are penal in nature. Although there is no statutory enforcement in the Cayman Islands of judgments obtained in the United States, courts of the Cayman Islands will recognize and enforce a foreign judgment of a court of competent jurisdiction if such judgment is final, for a liquidated sum, provided it is not in respect of taxes or a fine or penalty, is not inconsistent with a Cayman Islands’ judgment in respect of the same matters, and is not impeachable under Cayman Islands law for fraud, being in breach of public policy of the Cayman Islands or being contrary to natural justice. In addition, a Cayman Islands court may stay proceedings if concurrent proceedings are being brought elsewhere.
22
Risks Related to Conducting Business in China
We expect that our Prizma will be manufactured by a third party in China. Operating in the Chinese market subjects us to the following and similar risks:
Changes in the Chinese government’s macroeconomic policies or its public policy could have a negative effect on our business and results of operations.
The Chinese government has implemented various measures to control the rate of economic growth in China. Some of these measures may have a negative effect on us over the short or long term. Previously, to cope with high inflation and financial imbalances, the Chinese government sharply tightened monetary policy and, in addition, enacted a series of social programs and anti-inflationary measures. These measures have, in conjunction, increased the costs on the financial and manufacturing sectors, destabilized the real estate sector and significantly slowed down the rate of economic growth in China. The Chinese government may be forced to engage in further macroeconomic policy shifts in order to limit instability and/or damage to certain business models or markets, or engage other, new practices in order to re-stimulate the rate of economic growth in China. The Chinese government’s continued attempts at managing the Chinese economy through a variety of macroeconomic policies, even if effected properly, or new practices, which the Chinese government does not have experience with, may further slow China’s economy growth and/or cause great social unrest, all of which would have a negative effect on our business and results of operations.
The Chinese government exerts substantial influence over the manner in which we must conduct our business activities.
China has recently only permitted provincial and local economic autonomy and private economic activities. The Chinese government has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Our ability to operate in China may be harmed by changes in its economic policies and regulations, including those relating to taxation, import and export tariffs, environmental regulations, land use rights, property and other matters. We believe that our operations in China are in material compliance with all applicable legal and regulatory requirements. However, the central or local governments of these jurisdictions may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures and efforts on our part to ensure compliance with such regulations or interpretations.
Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in China or particular regions thereof, and could require us to divest of any interest we hold in Chinese properties or joint ventures.
Uncertainties with respect to the Chinese legal system could adversely affect us.
The Chinese legal system is a civil law system based on written statutes. Unlike the common law system, prior court decisions under the civil law system may be cited for reference but have limited precedential value. Since these laws and regulations are relatively new and the Chinese legal system continues to rapidly evolve, the interpretations of many laws, regulations and rules are not always uniform and the enforcement of these laws, regulations and rules involves uncertainties.
In 1979, the Chinese government began to promulgate a comprehensive system of laws and regulations governing economic matters in general. The overall effect of legislation over the past three decades has significantly enhanced the protections afforded to various forms of foreign investments in China. However, China has not developed a fully integrated legal system, and recently enacted laws and regulations may not sufficiently cover all aspects of economic activities in China. In particular, the interpretation and enforcement of these laws and regulations involve uncertainties. Since Chinese administrative and court authorities have significant discretion in interpreting and implementing statutory provisions and contractual terms, it may be difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy. These uncertainties may affect our judgment on the relevance of legal requirements and our ability to enforce our contractual rights or tort claims. In addition, the regulatory uncertainties may be exploited through unmerited or frivolous legal actions or threats in attempts to extract payments or benefits from us.
23
Risks Related to Our Operations in Israel
We maintain material operations in Israel, which subjects us to the following and similar risks:
It may be difficult to enforce a judgment of a U.S. court against us and our officers and directors and the Israeli experts named in this annual report in Israel or the United States, to assert U.S. securities laws claims in Israel or to serve process on our officers and directors and these experts.
The vast majority of our executive officers and directors and the Israeli experts named in this annual report are located in Israel. All of our assets and most of the assets of these persons are located in Israel. Therefore, a judgment obtained against us, or any of these persons, including a judgment based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not necessarily be enforced by an Israeli court. It also may be difficult to affect service of process on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Additionally, it may be difficult for an investor, or any other person or entity, to initiate an action with respect to U.S. securities laws in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws reasoning that Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact by expert witnesses, which can be a time consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated with enforcing a judgment against us in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign court.
Potential political, economic and military instability in the State of Israel, where our management team and our research and development facilities are located, may adversely affect our results of operations.
Our operating subsidiary, along with our management team and our research and development facilities are located in Israel. In addition, the vast majority of our officers and directors are residents of Israel. Accordingly, political, economic and military conditions in Israel and the surrounding region may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its neighboring Arab countries, the Hamas militant group and the Hezbollah. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its trading partners could adversely affect our operations and results of operations. Ongoing and revived hostilities or other Israeli political or economic factors, such as, an interruption of operations at the Tel Aviv airport, could prevent or delay our regular operation, product development and delivery of products. If continued or resumed, these hostilities may negatively affect business conditions in Israel in general and our business in particular. In the event that hostilities disrupt the ongoing operation of our facilities and our operations may be materially adversely affected.
In addition, since 2010 political uprisings and conflicts in various countries in the Middle East, including Egypt and Syria, are affecting the political stability of those countries. It is not clear how this instability will develop and how it will affect the political and security situation in the Middle East. This instability has raised concerns regarding security in the region and the potential for armed conflict. In Syria, a country bordering Israel, a civil war is taking place. In addition, it is widely believed that Iran, which has previously threatened to attack Israel, has been stepping up its efforts to achieve nuclear capability. Iran is also believed to have a strong influence among extremist groups in the region, such as Hamas in Gaza and Hezbollah in Lebanon. Additionally, the Islamic State of Iraq and Levant, a violent jihadist group, is involved in hostilities in Iraq and Syria. The tension between Israel and Iran and/or these groups may escalate in the future and turn violent, which could affect the Israeli economy in general and us in particular. Any potential future conflict could also include missile strikes against parts of Israel, including our offices and facilities. Such instability may lead to deterioration in the political and trade relationships that exist between the State of Israel and certain other countries. Any armed conflicts, terrorist activities or political instability in the region could adversely affect business conditions, could harm our results of operations and could make it more difficult for us to raise capital. Parties with whom we do business may sometimes decline to travel to Israel during periods of heightened unrest or tension, forcing us to make alternative arrangements when necessary in order to meet our business partners face to face. Several countries, principally in the Middle East, still restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies if hostilities in Israel or political instability in the region continues or increases. Similarly, Israeli companies are limited in conducting business with entities from several countries. For instance, the Israeli legislature passed a law forbidding any investments in entities that transact business with Iran. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements.
24
Our employees and consultants in Israel, including members of our senior management, may be obligated to perform one month, and in some cases longer periods, of military reserve duty until they reach the age of 40 (or older, for citizens who hold certain positions in the Israeli armed forces reserves) and, in the event of a military conflict or emergency circumstances, may be called to immediate and unlimited active duty. In the event of severe unrest or other conflict, individuals could be required to serve in the military for extended periods of time. In response to increases in terrorist activity, there have been periods of significant call-ups of military reservists. It is possible that there will be similar large-scale military reserve duty call-ups in the future. Our operations could be disrupted by the absence of a significant number of our officers, directors, employees and consultants related to military service. Such disruption could materially adversely affect our business and operations. Additionally, the absence of a significant number of the employees of our Israeli suppliers and contractors related to military service or the absence for extended periods of one or more of their key employees for military service may disrupt their operations.
Our insurance does not cover losses that may occur as a result of an event associated with the security situation in the Middle East or for any resulting disruption in our operations. Although the Israeli government has in the past covered the reinstatement value of direct damages that were caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained or, if maintained, will be sufficient to compensate us fully for damages incurred and the government may cease providing such coverage or the coverage might not suffice to cover potential damages. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflicts or political instability in the region would likely negatively affect business conditions generally and could harm our results of operations and product development.
Further, in the past, the State of Israel and Israeli companies have been subjected to economic boycotts. Several countries still restrict business with the State of Israel and with Israeli companies. These restrictive laws and policies may have an adverse impact on our operating results, financial conditions or the expansion of our business. Similarly, Israeli corporations are limited in conducting business with entities from several countries.
Additionally, the newly elected Israeli government has announced plans to significantly reduce the Israeli Supreme Court’s judicial oversight, including reducing its ability to strike down legislation that it deems unreasonable, and plans to increase political influence over the selection of judges. These plans have prompted protests of Israeli citizens and criticism of leading Israeli business leaders as well as some foreign leaders. If such government plans are eventually enacted, they may cause operational challenges for us since we are headquartered in Israel and almost all of our employees are located in Israel. In addition, if foreign policy is negatively impacted with regard to Israel, this could impact our business with suppliers and customers which could in turn adversely impact our reputation, results of operations or financial condition.
We may be required to pay monetary remuneration to our Israeli employees for their inventions, even if the rights to such inventions have been duly assigned to us.
We enter into agreements with our Israeli employees pursuant to which such individuals agree that any inventions created in the scope of their employment are assigned to us or owned exclusively by us, depending on the jurisdiction, without the employee retaining any rights. A significant portion of our intellectual property has been developed by our Israeli employees during the course of their employment for us. Under the Israeli Patent Law, 5727-1967 (or the Patent Law) inventions conceived by an employee during the scope of his or her employment with a company, and as a consequence of such employment, are regarded as “service inventions,” which belong to the employer by default, absent a specific agreement between the employee and employer giving the employee ownership rights. The Patent Law also provides that if there is no agreement between an employer and an employee, regarding the remuneration for the service inventions, even if the ownership rights were assigned to the employer, the Israeli Compensation and Royalties Committee (or the Committee), a body constituted under the Patent Law, shall determine whether the employee is entitled to remuneration for these inventions. The Committee has not yet determined the method for calculating this Committee-enforced remuneration. While it has been held that an employee may waive his or her rights to remuneration, and that a waiver of such rights may be concluded like any other agreement, in writing, orally or by conduct, pending litigation in the Israeli labor court is questioning whether such waiver under an employment agreement is enforceable. Although our Israeli employees have agreed that any rights related to their inventions are owned exclusively by us, we may face claims demanding remuneration in consideration for employees’ service inventions. As a consequence of such claims, we could be required to pay additional remuneration or royalties to our current and/or former employees, or be forced to litigate such claims, which could negatively affect our business.
25
Risks Related to the Ownership of our Ordinary Shares
Failure to meet Nasdaq’s continued listing requirements could result in the delisting of our Ordinary Shares, negatively impact the price of our Ordinary Shares and negatively impact our ability to raise additional capital.
On November 22, 2022, we received deficiency letter from the Listing Qualifications Department of the Nasdaq Stock Market (or the Staff) notifying us that, based on our shareholders’ deficit of $1,240 thousand as of June 30, 2022, as reported in our Report of Foreign Private Issuer on Form 6-K on November 18, 2022, we were no longer in compliance with the minimum shareholders’ equity requirement for continued listing on the Nasdaq Capital Market under Nasdaq Listing Rule 5550(b)(1), which requires listed companies to maintain shareholders’ equity of at least $2.5 million. We had 45 calendar days, or until January 6, 2023, to submit a plan to regain compliance. We submitted our compliance plan on January 6, 2023 and made a subsequent submission on January 19, 2023.
On February 16, 2023, we received a letter from the Staff of the Nasdaq notifying us that Nasdaq had determined to delist our Ordinary Shares and tradeable warrants from Nasdaq based on (i) our non-compliance with the minimum $2,500,000 stockholders’ equity requirement for continued listing set forth in Nasdaq Listing Rule 5550(b) and (ii) providing the Staff a submission of information that contained material misrepresentations, in violation of Nasdaq Listing Rule 5250(a)(1), in relation to our extraordinary general meeting of shareholders, which was adjourned on February 9, 2023 and ultimately held and concluded on February 16, 2023. On February 23, 2023, we requested a hearing before a Nasdaq Hearings Panel (or the Panel), which stays any delisting or suspension action pending the hearing and the expiration of any additional extension period granted by the Panel following the hearing.
On May 11, 2023, the Panel granted the request for continued listing on Nasdaq subject to the requirement that we, by no later than August 4, 2023 (or the Exception Period), provide the Panel with unaudited interim financial statements for the second quarter of 2023 and demonstrate to the Panel that we continue to meet the equity requirement. Upon review of our interim financial statements for the second quarter of 2023, the Panel will decide if we demonstrated an ability to maintain compliance with the continued listing requirements on a long-term basis. In addition, from May 11, 2023 until the end of the Exception Period, the Panel reserves the right to reconsider the terms of this exception based on any event, condition or circumstance that exists or develops that would, in the opinion of the Panel, make the continued listing of our securities on Nasdaq inadvisable or unwarranted. Until the end of the Exception Period, we are required to provide prompt notification to the Panel of any significant events that may affect our compliance with Nasdaq requirements.
In addition, on May 15, 2023 we received a written notice from the Staff indicating that we were no longer in compliance with the minimum bid price requirement for continued listing set forth in Nasdaq Listing Rule 5550(a)(2), which requires listed securities to maintain a minimum bid price of $1.00 per share. Under Nasdaq Listing Rule 5810(c)(3)(A), we have been granted a period of 180 calendar days, which can be extended by Nasdaq, to regain compliance with the minimum bid price requirement.
There can be no assurance that we will ultimately regain compliance with all applicable requirements for continued listing on the Nasdaq Capital Market in the future. If our Ordinary Shares are delisted from Nasdaq, it will have material negative impact on the actual and potential liquidity of our securities, as well as material negative impact on our ability to raise future capital.
If, for any reason, Nasdaq should delist our Ordinary Shares from trading on its exchange and we are unable to obtain listing on another national securities exchange or take action to restore our compliance with the Nasdaq continued listing requirements, a reduction in some or all of the following may occur, each of which could have a material adverse effect on our shareholders:
| ● | the liquidity of our Ordinary Shares; |
| ● | the market price of our Ordinary Shares; |
| ● | our ability to obtain financing for the continuation of our operations; |
| ● | the number of institutional and general investors that will consider investing in our Ordinary Shares; |
| ● | the number of investors in general that will consider investing in our Ordinary Shares; |
| ● | the number of market makers in our Ordinary Shares; |
| ● | the availability of information concerning the trading prices and volume of our Ordinary Shares; and |
| ● | the number of broker-dealers willing to execute trades in shares of our Ordinary Shares. |
Further, we would likely become a “penny stock”, which would make trading of our Ordinary Shares much more difficult.
We do not know whether a market for the Ordinary Shares will be sustained or what the trading price of the Ordinary Shares will be and as a result it may be difficult for you to sell your Ordinary Shares.
Although our Ordinary Shares are listed on the Nasdaq Capital Market, an active trading market for the Ordinary Shares may not be sustained. It may be difficult for you to sell your Ordinary Shares without depressing the market price for the Ordinary Shares. As a result of these and other factors, you may not be able to sell your Ordinary Shares. Further, an inactive market may also impair our ability to raise capital by selling Ordinary Shares and may impair our ability to enter into strategic partnerships or acquire companies, products, or services by using our equity securities as consideration.
26
We have never paid cash dividends on our share capital, and we do not anticipate paying any cash dividends in the foreseeable future.
We have never declared or paid cash dividends, and we do not anticipate paying cash dividends in the foreseeable future. Therefore, you should not rely on an investment in Ordinary Shares as a source for any future dividend income. Our board of directors has complete discretion as to whether to distribute dividends. Even if our board of directors decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors deemed relevant by our board of directors.
Our principal shareholders, officers and directors currently beneficially own approximately 14.7 % of our Ordinary Shares . They will therefore be able to exert significant control over matters submitted to our shareholders for approval.
As of the date of this annual report, our principal shareholders, officers and directors beneficially own approximately 14.7% of our Ordinary Shares. This significant concentration of share ownership may adversely affect the trading price for our Ordinary Shares because investors often perceive disadvantages in owning shares in companies with controlling shareholders. As a result, these shareholders, if they acted together, could significantly influence or even unilaterally approve matters requiring approval by our shareholders, including the election of directors and the approval of mergers or other business combination transactions. The interests of these shareholders may not always coincide with our interests or the interests of other shareholders.
The market price of our Ordinary Shares may be highly volatile and such volatility could cause you to lose some or all of your investment and also subject us to litigation.
The market price of our Ordinary Shares may fluctuate significantly in response to numerous factors, some of which are beyond our control, such as:
| ● | the announcement of new products or product enhancements by us or our competitors; | |
| ● | government and third-party payors providing adequate coverage and reimbursement for the use of our products and services; | |
| ● | developments concerning intellectual property rights; | |
| ● | changes in legal, regulatory, and enforcement frameworks impacting our technology or the application of our technology; | |
| ● | variations in our and our competitors’ results of operations; | |
| ● | fluctuations in earnings estimates or recommendations by securities analysts, if our securities are covered by analysts; | |
| ● | the results of product liability or intellectual property lawsuits; | |
| ● | future issuances of securities; | |
| ● | the addition or departure of key personnel; | |
| ● | announcements by us or our competitors of acquisitions, investments or strategic alliances; and | |
| ● | general market conditions and other factors, including factors unrelated to our operating performance. | |
| ● | other events or factors, including those resulting from war, incidents of terrorism or responses to these events; and | |
| ● | general economic and market conditions. |
Furthermore, in recent years, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies, including medical device related technology companies in particular. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political and market conditions such as recessions, interest rate changes or international currency fluctuations, may negatively impact the market price of our Ordinary Shares.
In the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management’s attention from other business concerns, which could also harm our business.
27
The JOBS Act will allow us to postpone the date by which we must comply with some of the laws and regulations intended to protect investors and to reduce the amount of information we provide in our reports filed with the SEC, which could undermine investor confidence in our company and adversely affect the market price of the Ordinary Shares.
For so long as we remain an “emerging growth company” as defined in the JOBS Act, we intend to take advantage of certain exemptions from various requirements that are applicable to public companies that are not “emerging growth companies” including:
| ● | the provisions of the Sarbanes-Oxley Act requiring that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting; | |
| ● | Section 107 of the JOBS Act, which provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. This means that an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We are electing to delay such adoption of new or revised accounting standards. As a result of this adoption, our financial statements may not be comparable to companies that comply with the public company effective date; | |
| ● | any rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements; and | |
| ● | our ability to furnish two rather than three years of income statements and statements of cash flows in various required filings. |
We intend to take advantage of these exemptions until we are no longer an “emerging growth company.” We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the date of our first sale of common equity securities pursuant to an effective registration statement under the Securities Act, (b) in which we have total annual gross revenue of at least $1.235 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our Ordinary Shares that is held by non-affiliates exceeds $700 million as of the prior June 30, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period. We cannot predict if investors will find our securities less attractive because we may rely on these exemptions. If some investors find our securities less attractive as a result, there may be a less active trading market for the Ordinary Shares, and our market prices may be more volatile and may decline.
As a “foreign private issuer” we are subject to less stringent disclosure requirements than domestic registrants and are permitted, and may in the future elect to follow certain home country corporate governance practices instead of otherwise applicable SEC and Nasdaq requirements, which may result in less protection than is accorded to investors under rules applicable to domestic U.S. registrants.
As a foreign private issuer and emerging growth company, we may be subject to different disclosure and other requirements than domestic U.S. registrants and non-emerging growth companies. For example, as a foreign private issuer, in the United States, we are not subject to the same disclosure requirements as a domestic U.S. registrant under the Exchange Act, including the requirements to prepare and issue quarterly reports on Form 10-Q or to file current reports on Form 8-K upon the occurrence of specified significant events, the proxy rules applicable to domestic U.S. registrants under Section 14 of the Exchange Act or the insider reporting and short-swing profit rules applicable to domestic U.S. registrants under Section 16 of the Exchange Act. In addition, we intend to rely on exemptions from certain U.S. rules which will permit us to follow Cayman Islands legal requirements rather than certain of the requirements that are applicable to U.S. domestic registrants.
We follow Cayman Islands laws and regulations that are applicable to Cayman Islands companies. However, Cayman Islands laws and regulations applicable to Cayman Islands companies do not contain any provisions comparable to the U.S. proxy rules, the U.S. rules relating to the filing of reports on Form 10-Q or 8-K or the U.S. rules relating to liability for insiders who profit from trades made in a short period of time, as referred to above.
28
Furthermore, foreign private issuers are required to file their annual report on Form 20-F within 120 days after the end of each fiscal year, while U.S. domestic registrants that are non-accelerated filers are required to file their annual report on Form 10-K within 90 days after the end of each fiscal year. Foreign private issuers are also exempt from Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information, although we will be subject to Cayman Islands laws and regulations having substantially the same effect as Regulation Fair Disclosure. As a result of the above, even though we are required to file reports on Form 6-K disclosing the limited information which we have made or are required to make public pursuant to Cayman Islands law, or are required to distribute to shareholders generally, and that is material to us, you may not receive information of the same type or amount that is required to be disclosed to shareholders of a U.S. registrant. These exemptions and leniencies will reduce the frequency and scope of information and protections to which you are entitled as an investor.
The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter and, accordingly, the next determination will be made with respect to us on June 30, 2023. In the future, we would lose our foreign private issuer status if a majority of our shareholders, directors or management are U.S. citizens or residents and we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic registrant may be significantly higher.
We may be a “passive foreign investment company,” or PFIC, for U.S. federal income tax purposes in the current taxable year or may become one in any subsequent taxable year. There generally would be negative tax consequences for U.S. taxpayers that are holders of the Ordinary Shares if we are or were to become a PFIC.
Based on the projected composition of our income and valuation of our assets, we do not expect to be a PFIC for 2022, and we do not expect to become a PFIC in the future, although there can be no assurance in this regard. The determination of whether we are a PFIC is made on an annual basis and will depend on the composition of our income and assets from time to time. We will be treated as a PFIC for U.S. federal income tax purposes in any taxable year in which either (1) at least 75% of our gross income is “passive income” or (2) on average at least 50% of our assets by value produce passive income or are held for the production of passive income. Passive income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in a public offering. In determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account. The tests for determining PFIC status are applied annually, and it is difficult to make accurate projections of future income and assets which are relevant to this determination. In addition, our PFIC status may depend in part on the market value of the Ordinary Shares. Accordingly, there can be no assurance that we currently are not or will not become a PFIC in the future. If we are a PFIC in any taxable year during which a U.S. taxpayer holds the Ordinary Shares, such U.S. taxpayer would be subject to certain adverse U.S. federal income tax rules. In particular, if the U.S. taxpayer did not make an election to treat us as a “qualified electing fund”, or QEF, or make a “mark-to-market” election, then “excess distributions” to the U.S. taxpayer, and any gain realized on the sale or other disposition of the Ordinary Shares by the U.S. taxpayer: (1) would be allocated ratably over the U.S. taxpayer’s holding period for the Ordinary Shares; (2) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, if the U.S. Internal Revenue Service, or the IRS, determines that we are a PFIC for a year with respect to which we have determined that we were not a PFIC, it may be too late for a U.S. taxpayer to make a timely QEF or mark-to-market election. U.S. taxpayers that have held the Ordinary Shares during a period when we were a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC in subsequent years, subject to exceptions for U.S. taxpayer who made a timely QEF or mark-to-market election. A U.S. taxpayer can make a QEF election by completing the relevant portions of and filing IRS Form 8621 in accordance with the instructions thereto. We do not intend to notify U.S. taxpayers that hold the Ordinary Shares if we believe we will be treated as a PFIC for any taxable year in order to enable U.S. taxpayers to consider whether to make a QEF election. In addition, we do not intend to furnish such U.S. taxpayers annually with information needed in order to complete IRS Form 8621 and to make and maintain a valid QEF election for any year in which we or any of our subsidiaries are a PFIC. U.S. taxpayers that hold the Ordinary Shares are strongly urged to consult their tax advisors about the PFIC rules, including tax return filing requirements and the eligibility, manner, and consequences to them of making a QEF or mark-to-market election with respect to the Ordinary Shares in the event that we are a PFIC (see “Item 10.E. Taxation—U.S. Federal Income Tax Considerations—Passive Foreign Investment Companies” for additional information).
29
We may be subject to securities litigation, which is expensive and could divert management attention.
In the past, companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Litigation of this type could result in substantial costs and diversion of management’s attention and resources, which could seriously hurt our business. Any adverse determination in litigation could also subject us to significant liabilities.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they adversely change their recommendations or publish negative reports regarding our business or the Ordinary Shares, our share price and trading volume could decline.
The trading market for the Ordinary Shares will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. We do not have any control over these analysts and we cannot provide any assurance that analysts will cover us or provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation regarding the Ordinary Shares, or provide more favorable relative recommendations about our competitors, the price of our Ordinary Shares would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the price of our Ordinary Shares or trading volume to decline.
Future sales of our Ordinary Shares could reduce the market price of our Ordinary Shares.
Substantial sales of our Ordinary Shares on Nasdaq may cause the market price of our Ordinary Shares to decline. Sales by us or our security holders of substantial amounts of our Ordinary Shares, or the perception that these sales may occur in the future, could cause a reduction in the market price of our Ordinary Shares.
The issuance of any additional Ordinary Shares or any securities that are exercisable for or convertible into Ordinary Shares, may have an adverse effect on the market price of our Ordinary Shares and will have a dilutive effect on our existing shareholders and holders of Ordinary Shares.
Other General Risk Factors
If we are not able to attract and retain highly skilled managerial, scientific and technical personnel, we may not be able to implement our business model successfully.
We believe that our management team must be able to act decisively to apply and adapt our business model in the rapidly changing markets in which we will compete. In addition, we will rely upon technical and scientific employees or third-party contractors to effectively establish, manage and grow our business. Consequently, we believe that our future viability will depend largely on our ability to attract and retain highly skilled managerial, sales, scientific and technical personnel. In order to do so, we may need to pay higher compensation or fees to our employees or consultants than we currently expect and such higher compensation payments would have a negative effect on our operating results. Competition for experienced, high-quality personnel is intense and we cannot assure that we will be able to recruit and retain such personnel. We may not be able to hire or retain the necessary personnel to implement our business strategy. Our failure to hire and retain such personnel could impair our ability to develop new products and services and manage our business effectively.
30
We will need to expand our organization and we may experience difficulties in recruiting needed additional employees and consultants, which could disrupt our operations.
As our development and commercialization plans and strategies develop and because we are so leanly staffed, we will need additional managerial, operational, sales, marketing, financial, legal and other resources. The competition for qualified personnel in the medical device industry is intense. Due to this intense competition, we may be unable to attract and retain qualified personnel necessary for the development of our business or to recruit suitable replacement personnel.
Security breaches, loss of data and other disruptions could compromise sensitive information related to our business or patients, or prevent us from accessing critical information and expose us to liability, which could adversely affect our business and our reputation.
In the ordinary course of our business, we and certain of our third-party service providers, collect, process, and store sensitive data, including intellectual property, personal and medical information about our patients and our proprietary business information. The secure maintenance and transmission of this information is critical to our operations and business strategy. We rely on commercially available systems, software, tools and domestically available monitoring to provide security for processing, transmitting and storing this sensitive data.
In the event that patients authorize or enable us to sell their personal data to third-parties and/or access their data on our systems, we cannot ensure the complete integrity or security of such data in our systems as we would not control that access. Third-parties may also attempt to fraudulently induce our employees, patients or physicians who use our technology, into disclosing sensitive information. Third-parties may also otherwise compromise our security measures in order to gain unauthorized access to the information we store. This could result in significant legal and financial exposure, a loss in confidence in the security of our services, interruptions or malfunctions in our services, and, ultimately, harm to our future business prospects and revenue.
A security breach or privacy violation that leads to disclosure or modification of, or prevents access to, patient information, including protected health information, could harm our reputation, compel us to comply with disparate state breach notification laws, require us to verify the correctness of database contents and otherwise subject us to liability under laws that protect personal data, resulting in increased costs or loss of revenue. If we are unable to prevent such security breaches or privacy violations or implement satisfactory remedial measures in a timely manner, the market perception of the effectiveness of our security measures could be harmed, our operations could be disrupted, our brand could be adversely affected, demand for our products and services may decrease, we may be unable to provide the our service, we may lose sales and customers, and we may suffer loss of reputation, financial loss and other regulatory penalties because of lost or misappropriated information, including sensitive patient data. We may be required to expend significant capital and financial resources to invest in security measures, protect against such threats or to alleviate problems caused by breaches in security. In addition, these breaches and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm. Although we have invested in our systems and the protection of our data to reduce the risk of an intrusion or interruption, and we monitor our systems on an ongoing basis for any current or potential threats, we can give no assurances that these measures and efforts will prevent all intrusions, interruptions, or breakdowns.
Because techniques used to obtain unauthorized access or to sabotage systems change frequently and generally are not recognized until launched, we may be unable to anticipate these techniques or to implement adequate preventive measures.
With respect to medical information, we follow HIPAA guidelines and, among others, separate personal information from medical information, and further employ additional encryption tools to protect the privacy of our patients and medical data. However, hackers may attempt to penetrate our computer systems, and, if successful, misappropriate personal or confidential business information. In addition, an associate, contractor or other third-party with whom we do business may attempt to circumvent our security measures in order to obtain such information, and may purposefully or inadvertently cause a breach involving such information. While we continue to implement additional protective measures to reduce the risk of and detect cyber incidents, cyber-attacks are becoming more sophisticated and frequent, and the techniques used in such attacks change rapidly.
Also, our information technology networks and infrastructure may still be vulnerable to damage, disruptions or shutdowns due to attack by hackers or breaches, employee error or malfeasance, power outages, computer viruses, telecommunication or utility failures, systems failures, natural disasters or other catastrophic events. Any such compromise could disrupt our operations, damage our reputation and subject us to additional costs and liabilities, any of which could adversely affect our business.
31
Depending on the nature of the information compromised, in the event of a data breach or other unauthorized access to or acquisition of our user data, we may also have obligations to notify users about the incident and we may need to provide some form of remedy for the individuals affected by the incident. A growing number of legislative and regulatory bodies have adopted consumer notification requirements in the event of unauthorized access to or acquisition of certain types of personal data. Such breach notification laws continue to evolve and may be inconsistent from one jurisdiction to another. Complying with these obligations could cause us to incur substantial costs and could increase negative publicity surrounding any incident that compromises user data. In addition, the interpretation and application of consumer, health-related and data protection laws, rules and regulations in the United States, Europe and elsewhere are often uncertain, contradictory and in flux. It is possible that these laws, rules and regulations may be interpreted and applied in a manner that is inconsistent with our practices or those of our distributors and partners. If we or these third parties are found to have violated such laws, rules or regulations, it could result in government-imposed fines, orders requiring that we or these third parties change our or their practices, or criminal charges, which could adversely affect our business. Complying with these various laws could cause us to incur substantial costs or require us to change our business practices, systems and compliance procedures in a manner adverse to our business.
Our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional medical device products. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate and/or grow revenue could be reduced and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize medical device products and services and compete effectively will depend, in part, on our ability to effectively manage any future growth.
ITEM 4. INFORMATION ON THE COMPANY
A. History and Development of the Company
Our legal and commercial name is G Medical Innovations Holdings Ltd. We were incorporated in the Cayman Islands in 2014 as an exempted company with limited liability. Our principal offices are located at 5 Oppenheimer Street, Rehovot 7670105, Israel, and our telephone number in the United States is +1.800.595.2898. Our internet address is http://gmedinnovations.com/. None of the information on our website is incorporated by reference herein. G Medical Innovations USA Inc. serves as our agent for service of process in the United States for certain limited matters, and its address is 12708 Riata Vista Cir Ste A-103, Austin, TX 78727.
We use our website (http://gmedinnovations.com/) as a channel of distribution of Company information. The information we post on our website may be deemed material. Accordingly, investors should monitor our website, in addition to following our press releases, SEC filings and public conference calls and webcasts. The contents of our website are not, however, a part of this annual report.
We are an emerging growth company, as defined in Section 2(a) of the Securities Act, as implemented under the JOBS Act. While we currently qualify as an “emerging growth company” under the JOBS Act, we will cease to be an emerging growth company on or before June 25, 2026. As such, we are eligible to, and intend to, take advantage of certain exemptions from reporting requirements that generally apply to public companies, including the auditor attestation requirements with respect to internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act, compliance with new standards adopted by the Public Company Accounting Oversight Board which may require mandatory audit firm rotation or auditor discussion and analysis, exemption from say on pay, say on frequency, and say on golden parachute voting requirements, and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We will be an emerging growth company until the earliest of: (i) the last day of the fiscal year during which we had total annual gross revenues of $1.235 billion or more, (ii) the last day of the fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement (i.e. June 25, 2026), (iii) the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt, or (iv) the date on which we are deemed a “large accelerated filer” as defined in Regulation S-K under the Securities Act, which means the market value of our Ordinary Shares that is held by non-affiliates exceeds $700 million as of the prior June 30th.
32
As a foreign private issuer, we are exempt from certain rules and regulations under the Exchange Act that are applicable to other public companies that are not foreign private issuers. For example, although we intend to report our financial results on a quarterly basis, we will not be required to issue quarterly reports, proxy statements that comply with the requirements applicable to U.S. domestic reporting companies, or individual executive compensation information that is as detailed as that required of U.S. domestic reporting companies. We will also have four months after the end of each fiscal year to file our annual report with the SEC and will not be required to file current reports as frequently or promptly as U.S. domestic reporting companies. Our senior management, directors, and principal shareholders will be exempt from the requirements to report transactions in our equity securities and from the short-swing profit liability provisions contained in Section 16 of the Exchange Act. As a foreign private issuer, we will also not be subject to the requirements of Regulation FD (Fair Disclosure) promulgated under the Exchange Act.
Our capital expenditures for December 31, 2022, 2021 and 2020 amounted to $2,121,000, $550,000, and $507,000, respectively. Our purchases of fixed assets primarily include laboratory equipment and establishment of our production site in Rehovot, Israel. We financed these expenditures primarily from cash on hand.
B. Business Overview
Overview
We are a next-generation mobile health (mHealth) and digital health company that develops and markets clinical and consumer medical-grade health monitoring solutions and offers end-to-end support for remote monitoring and telemedicine projects. With extensive experience in the field of medical devices, digital health and patients monitoring, we are committed to raising the global level of healthcare by empowering caregivers and patients to better monitor, manage and improve clinical and personal health outcomes. We believe that we are at the forefront of the digital health revolution in developing the next generation of mobile technologies and services that are designed to empower consumers, patients, and providers to better monitor, manage and improve clinical and personal health outcomes, especially for those who suffer from CVD, pulmonary disease and diabetes. Our business and future revenue stream can be broken down into two business areas: monitoring services and home collection kits for lab testing.
Monitoring Services
We possess innovative MCT monitors purchased from third parties that utilize sophisticated Deep Neural Network based Artificial Intelligence (AI) backend ECG processing software--Cardiologs, which we use as a powerful secondary analysis tool once the monitoring session ends. This dual analysis approach is not offered by most of our competitors. Further, we have two CMS-approved IDTFs, each staffed with experienced clinical staff and billing teams, nationwide insurance contracts, and a full portfolio of diagnostic monitoring tools, including the G-Patch devices for extended holter (AECG), the Spider device for mobile cardiac telemetry (MCT), and the Prizma device for remote patient monitoring (RPM).
As medicine and technology become integrated, we believe we are well positioned to capture market share and grow in this business area. Our product line consists of our Prizma medical device, a clinical grade device that can transform a smartphone into a medical monitoring device, enabling both healthcare providers and individuals to monitor, manage and share a wide range of vital signs and biometric indicators. In addition, we are developing a Wireless Vital Signs Monitoring System, which is expected to provide full, continuous, and real time monitoring of a wide range of vital signs and biometrics. The monitoring services include Independent Diagnostic Testing Facility monitoring services and private monitoring services.
33
Home Collection Kits for Lab Testing
In the second half of 2022, we took a strategic decision to divert to more comprehensive home testing solutions as an expansion of our patient services and as part of our vision to move towards a home-based health care system. On July 13, 2022, we announced that our wholly owned subsidiary, G Medical Health and Wellness, Inc., has developed seven different at-home tests to collect samples of blood, saliva, stool, urine or a vaginal swab which we expect will be available to be purchased privately by the user in a retail store or online. Once purchased, the user will be able to collect the sample in the privacy and comfort of his or her home and send the sample to our CLIA certified lab for analysis. Once it arrives at the lab, the sample will be analyzed, and results typically will be available to the customer within days in our secured and HIPAA compliant portal. In addition, on October 6, 2022, we introduced a Monkeypox consumer home health test kit, along with 23 additional new direct to consumer home collection kits with 24 to 48 hours results, all of which are expected to be available to consumers the third quarter of 2023 through popular big-box retailers, pharmacies and online. The collection kits are developed for testing health issues related to hormones, sexual transmitted disease, nutrition, colon cancer, food sensitivity, and allergies, with results going directly to the user within days. The entire data, from both vital signs and laboratory tests, is collected, and managed under one database, and give the user the ability to create, manage and control their EMR, with the ability to retrieve and share it with third parties anywhere and anytime. The prices for our at-home testing kits will range from $49 to $259.
During the first half of 2022, we operated six locations performing point-of-care tests in communities in Southern and Northern California. The volume of COVID-19 testing has decreased significantly since April 2022. Given the decrease in COVID-19 cases, in the second half of fiscal year 2022 we decided to discontinue our COVID-19 related business activities. We have written off inventory and fixed assets related to our COVID-19 business for the fiscal year ended December 31, 2022.
Our management team is led by individuals with over 25 years of experience in developing mobile embedded medical sensors and software, algorithms and ambulatory medical devices, and with extensive experience filing for, and obtaining approval from, the FDA for medical devices, including devices approved when the members of our management team were employed at other companies. Our management has proven their ability to execute our go-to-market strategy as described below, with a proven track record medical device development and commercialization experience in the United States, China, Europe, Australia, South Africa, Japan, the Asia Pacific region and Brazil.
Our Products and Product Candidates
Our product platforms are positioned to reduce inefficiencies in healthcare delivery, improve access, reduce costs, increase quality of care and make healthcare more personalized and precise. We believe that early detection and diagnosis, as well as accessibility for all patients and providers, will positively impact the direction and cost of healthcare today.
Our current product lines consist of our Prizma device, the G-Patch for extended holter, the Spider for MCT as well as our new Patch which has received an Emergency Use Authorization (or EUA) from the FDA for QT analysis . In addition, we are developing our VSMS.
34
Prizma Medical Device
The innovative Prizma is a “plug-and-play” medical device that measures vital signs with electronic medical records functionality and clinical grade reporting standards. Our Prizma can transform almost any smartphone into a medical monitoring device using wireless Bluetooth connection. Prizma presents a comprehensive health profile of the user, measuring a wide range of vital signs and biometrics including ECG, oxygen saturation, temperature, heart rate, pain levels and stress levels. Blood pressure, body weight and blood glucose measurements may be entered via Bluetooth connection to the Prizma from an external device or manually entered and tracked on the Prizma app. All of the measurements are saved and tracked on the Prizma app and on the cloud portal. Users may generate reports and share with third-parties (i.e., medical providers, family members).
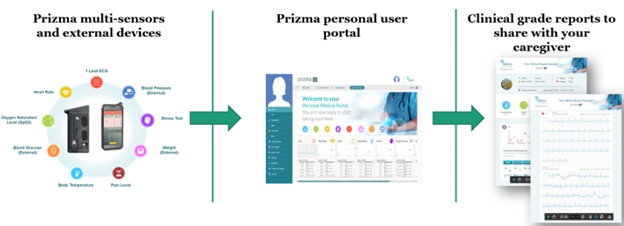
The Prizma device has been designed as an invaluable tool to help manage an individuals’ health and wellness, and allow healthcare providers to analyze the collected data in order to make better treatment decisions for conditions such as diabetes and heart disease, and vital signs functions for users with a range of health management needs. The Prizma app is available for download on the Apple Store and on Google Play. Prizma is powered by an independent battery, and activated by the user when needed, making usage and transport efficient and comfortable. The user simply places his or her index fingers on sensors embedded on the Prizma device, and the software seamlessly analyzes the data using various measurement methods and algorithms. Prizma can be used on almost any mobile phone, allowing us to quickly adapt the application to new smartphones.
Along with our Prizma device, we offer a remote patient monitoring service (or RPM) for chronic patients. The Prizma device can record various vital signs as frequently as needed on the go and such recordings are being analyzed and presented on a monthly report for physicians to keep track of their patients’ vitals. This service can be provided as a monthly recurring service as long as prescribed by the physician. Prizma is reimbursed monthly per patient under CPT code 99453, 99454, 99457 and additional codes, at a onetime rate of approximately $17 and a monthly rate of approximately $50 to the Company.
35
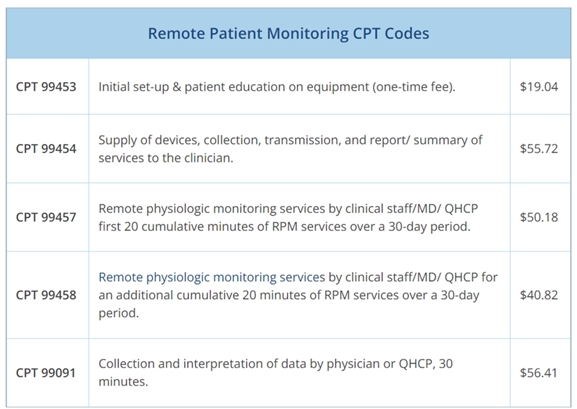
The Prizma is capable of monitoring the following:
 |
 |
 |
 |
 |
 |
 |
 |
| ECG | Body temperature |
Heart rate | Sp02 | Stress | Blood pressure |
Weight | Blood glucose |
36
The Prizma device is currently available as a standalone model, as pictured below.

The Prizma platform provides clients with a one-stop, multi-function and multi-account platform providing secure access to a private cloud healthcare solution powered from a remote cloud infrastructure. The portal syncs all Prizma device measurements to the doctor’s dashboard automatically and notifies of any abnormalities. Patient reports can be generated and downloaded to integrate seamlessly within the physician’s Electronic Health Record. Prizma is compatible with iOS 11.0 or above, Android 6.0 or above and Smartphone Bluetooth BT SIG 4.2.
Monitoring Station:
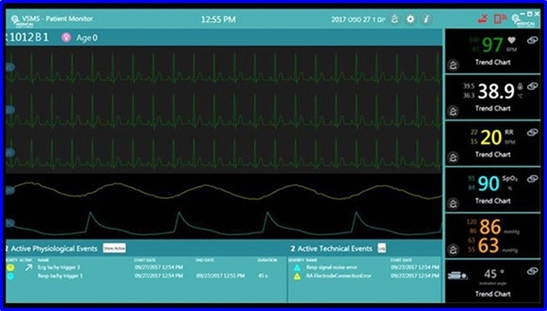
37
Physician’s Portal:
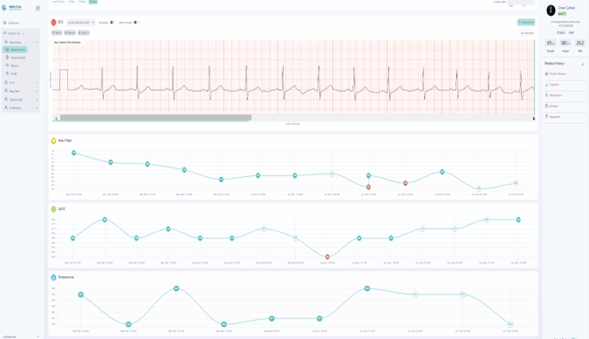
User Portal:
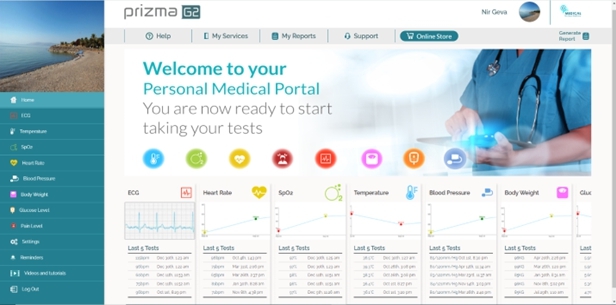
38
Prizma Reports:
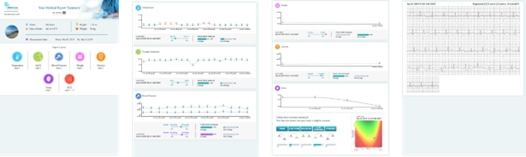
This Prizma platform allows our customers to enter and see all their medical information in a single platform. The information is stored on the platform and is also accessible to physicians. In addition, the system allows users to see and print reports with personal medical information and share such reports with third parties. This makes each interaction and use easy and simple.
Our Prizma device addresses the monitoring needs of many individuals, including those suffering from a wide range of diseases:
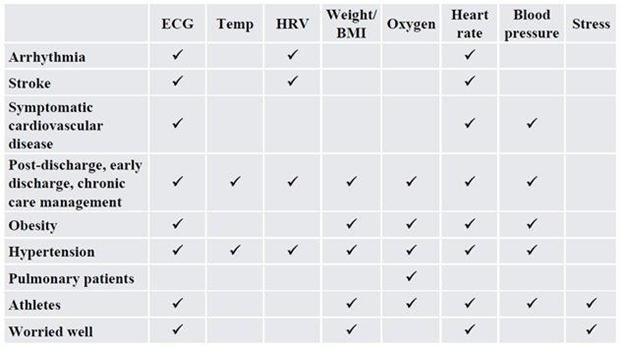
In August 2017, we received 510(k) clearance (under prescription) for our Prizma device from the U.S. FDA, and in September 2017 we received CE mark. These clearances were followed by approval received in November 2017 from the Therapeutic Goods Administration (or the TGA). In March 2020, we received registration with the Italian Health Ministry’s database of medical products. In April 2020, the U.S. FDA granted us OTC authorization based on an EUA policy which remained in force during the public health emergency related to COVID-19 until May 11, 2023, during which time we were allowed to sell the Prizma device directly to consumers without a physician’s prescription. In April 2020, we received Taiwan FDA approval for our Prizma device. We are also preparing our application to the Chinese National Medical Products Administration (or NMPA) for our Prizma device.
Despite the fact that regulatory clearances and approvals were received in 2017, since the Prizma device is a novel and innovative product, and due to first to market penetration, the market acceptance of the product was slow. In addition, the Current Procedural Terminology codes covering Prizma RPM were only approved by the Centers for Medicare & Medicaid Services (or CMS) in November 2019. We intend to continue our market education efforts for our Prizma device and other products, in order to enhance market acceptance.
39
Extended Holter Patch System
Our Extended Holter Patch System is a multi-channel patient-worn biosensor that captures ECG data continuously for up to 14 days. We believe that multi-channel ECGs can deliver higher predictive values with more actionable data, which enables a more accurate diagnosis. In addition, the Extended Holter Patch System allows patients to capture any symptomatic event by tapping a button on the recorder and documenting their activity and symptom in the patient diary. This correlates the ECG activity and provides physicians with more contextual data to make a diagnosis. Following the monitoring session, the device is returned to us and the data is uploaded to our secure cloud for analysis. A concise clinical report of preliminary findings is generated by certified cardiac technicians, validated through a quality assurance (or QA) process, and made available to the physicians on our secure physician portal.
The modular and easy-to-use patch solution utilizes a body worn sensor, a removable recorder, a secure cloud platform and a clinical reporting system. The patch has a very soft fabric format, and its water-resistant technology allows showering without removing the patch. These attributes encourage patients to wear the sensor all day long, resulting in more meaningful data, enabling more accurate diagnosis and timely treatment decisions. Clinical studies have demonstrated that wearable patch technologies can improve a physician’s ability to more accurately detect arrhythmia, allowing them to change the course of treatment. In November 2017, we received the CE mark for our Extended Holter Patch System, and in May 2020 we received TGA approval. In May 2021, we received emergency clearance from the U.S. FDA to use our Extended Holter Patch System for patients with suspected or identified QT prolongation syndrome.
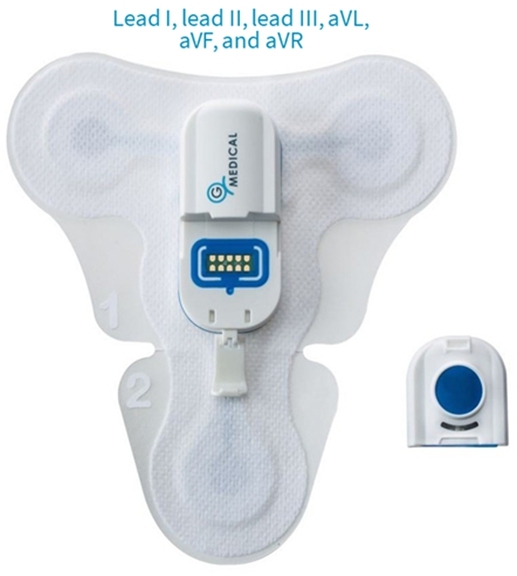
Our Patch recorder provides up to six channels of ECG and can be used for up to 14 days of ECG monitoring. The Patch records and transmits the ECG data to a smartphone which acts as a gateway to our call center. The base line ECG signal, system and electrode status are verified before starting the enrollment process. The frequency of transmissions can be set by the healthcare provider while the default setting of the device is to record and transmit 10 minutes of ECG data every hour. The data is saved and wirelessly transmitted by the user’s smartphone to our monitoring call center for QT prolongation syndrome detection capabilities analysis. The call center in turn sends the report to the prescribing physician at the hospital.
40
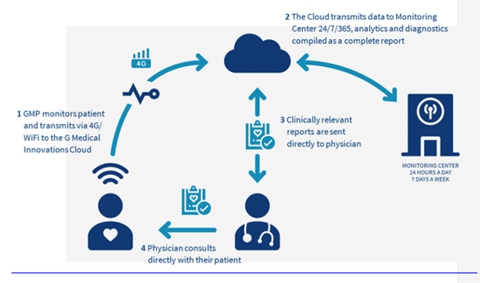
In May 2020, the U.S. FDA approved under EUA the use of our Patch in order to detect QT prolongation syndrome in a hospital setting for remote monitoring of the QT interval prolongation of an ECG. The EUA policy remained in force during the public health emergency related to COVID-19 until May 11, 2023.
Our Market Opportunity
Telemedicine and Mobile Health Market Opportunity
In 2019, health care spending in the United States topped $3.8 trillion—nearly 18% of the gross domestic product, or GDP—as projected by the CMS Office of the Actuary. Prior to the COVID-19 pandemic, Deloitte projected health spending would continue to grow at a rate of 5.3% a year, reaching nearly $6.2 trillion by 2028. If those increases were to continue unabated over the next 20 years, health spending could reach a staggering $11.8 trillion by 2040. Although the pandemic caused people to defer care throughout much of 2020, a rebound and then continuation of the trends beyond 2021 would drive health care spending to historic levels in the coming years.
According to data published by the American Hospital Association, in 2020 there were approximately 33 million admissions in all U.S. hospitals. The total expenses for all U.S. hospitals were approximately $1 trillion.
According to data published by the Centers for Disease Control and Prevention, in 2019 there were approximately 147 million hospital visits and approximately 884 million doctor visits in the United States alone, and an equal number of diagnostic data sets collected. Healthcare providers require diagnostic data to evaluate patients before, during and after medical interactions. A standard set of health diagnostics data is routinely collected at each healthcare interaction, including patients’ temperature, blood pressure, weight, heartrate, SPO2, blood glucose, ECG and stress.
Telemedicine provides potential answers to major healthcare challenges, including improved productivity and efficiency, and better utilization of centralized assets and scarce talent resources. A recent report by the OECD states that several elements must be in place to ensure the widespread delivery of teleconsultations and other telemedicine applications:
| ● | a clearly defined regulatory environment; | |
| ● | treatment of health data; | |
| ● | medical liability; | |
| ● | policies governing the establishment and use of telemedicine services; and | |
| ● | national and regional strategies that address telemedicine. |
41
Recent developments in the COVID-19 pandemic enabled the breaking of some of the barriers associated with the use of telemedicine technologies, and we believe that telemedicine is recognized as an essential instrument in healthcare by patients and healthcare providers and it is now widely believed that there will be a significant increase in the use of telemedicine services. While prior to COVID-19, patients and healthcare providers often preferred in-person meetings and expressed hesitation to the use of telemedicine as a substitute, the situation created following the spread of COVID-19, has demonstrated that telemedicine enables safe, efficient and cost-effective treatment and monitoring. Telemedicine services have the potential to facilitate medical care for both confirmed COVID-19 patients and non-COVID-19 patients, while protecting patients and healthcare providers. According to a consumer survey from McKinsey & Company from April 2020, consumer adoption of telehealth products has skyrocketed in light of COVID-19, from 11% of U.S. consumers using telehealth in 2019 to 46% of consumers using telehealth after April 2020. In addition, it is estimated that approximately $250 billion, which represents approximately 20% of all Medicare, Medicaid, and Commercial outpatient, office, and home health spend, could potentially be virtualized.
Even before COVID-19, mHealth was a fast-growing market, and data suggests this trend is going to continue. The World Health Organization (or WHO) defines mHealth as “medical and public health practice supported by mobile devices such as mobile phones, patient monitoring devices, personal digital assistants, and other wireless devices.” According to a report published by research2guidnace, mHealth is projected to have the highest positive impact on reducing costs associated with:
| ● | readmission in hospitals and duration of stay; | |
| ● | patients’ non-adherence to treatments; | |
| ● | doctor visits and consultation costs; | |
| ● | redundant examination and medical trial costs; | |
| ● | prevention costs; | |
| ● | labor costs; and | |
| ● | investment in technologies. |
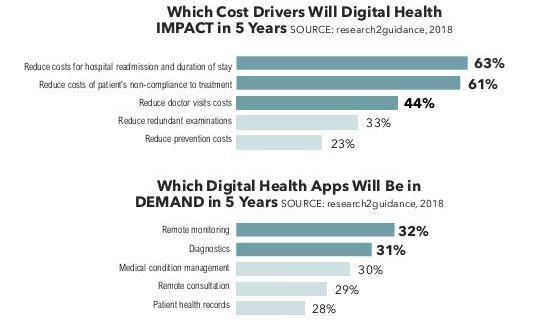
With the growing penetration of smartphones and internet connectivity, the adoption of mHealth technologies by physicians and patients has increased considerably. According to research by Grand View Research, the global mHealth market size was valued at $56.8 billion in 2022 and is expected to expand at a compound annual growth rate of 10.8% from 2023 to 2030. The mHealth apps segment dominated the market for mHealth and accounted for the largest revenue share of 76.5% in 2022, that was a result of increasing penetration of the internet and smartphones and the growing adoption of mobile health apps by healthcare professionals and the patient.
42
According to a report published by Precedence Research in August 2022, the IoT healthcare market (meaning the market for interrelated computing devices and mechanical and digital machines targeting healthcare such as our products) is projected to reach approximately $261.7 billion by 2023 and $960 billion by 2030. According to SEC filings made by Fitbit, Inc., it was able to sell 16 million of its devices in 2019 alone. We believe that a rising adoption of wearable technology and a growing geriatric population coupled with the rising prevalence of chronic conditions, which affects six in ten Americans, are among the key factors driving the market expansion.
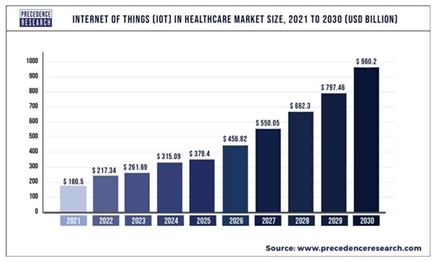
With the growing penetration of smartphones and internet connectivity, the adoption of mHealth technologies by physicians and patients has increased considerably. This specially holds true for mobile healthcare apps, including fitness and medical apps, with fitness and wellness holding a significant share of the total mHealth apps market. Moreover, the healthcare industry exhibits a high growth potential for the IT industry due to supportive government initiatives across all regions.
According to a March 2020 report from MarketsandMarkets, the global telehealth market is projected to reach $55.6 billion by 2025, which is a significant increase from $25.4 billion in 2020. The growth is projected at a CAGR of 16.9% during the forecast period. According to the report, North America dominates the telehealth market by region, due to factors such as the rising prevalence of chronic conditions, the need to reduce healthcare expenditure, increasing overall and geriatric population. However, the Asia Pacific market is expected to grow at the highest rate during the forecast period, owing to the prevalence of chronic diseases and the overcrowding of hospitals.
According to a May 2020 report from McKinsey & Company, in order to realize the full potential of telehealth, the market requires, among others, increased access to remote monitoring devices for specific clinical conditions (glucose monitoring for diabetes; heartbeat and blood pressure monitors for cardiovascular conditions). Also, providers may be required to integrate these types of devices into care plans. Payers may need to offer reimbursement, and solutions may need to enable integrated access between, for example, primary care physicians, care managers, and at-home caregivers. These services could also require the deployment of supportive patient engagement tools (for example, digital coaching, care plan navigation tools), tailored to patients’ needs and integrated with communication channels to providers, care managers, and others involved in their care.
United States
According to the Deloitte 2020 Survey of US Health Care Consumers, which surveyed 4,522 consumers between February 24 and March 14, 2020, and the Health Care Consumer Response to COVID-19 Survey (together, the Deloitte Surveys), which surveyed 1,510 consumers about their health, experiences, and behavior in mid-April to early May 2020, the percentage of consumers using virtual visits increased from 19% at the beginning of 2020 to 28% in April 2020. Moreover, the data shows an increase in consumer willingness to share data in every scenario measured in the graph below.
43
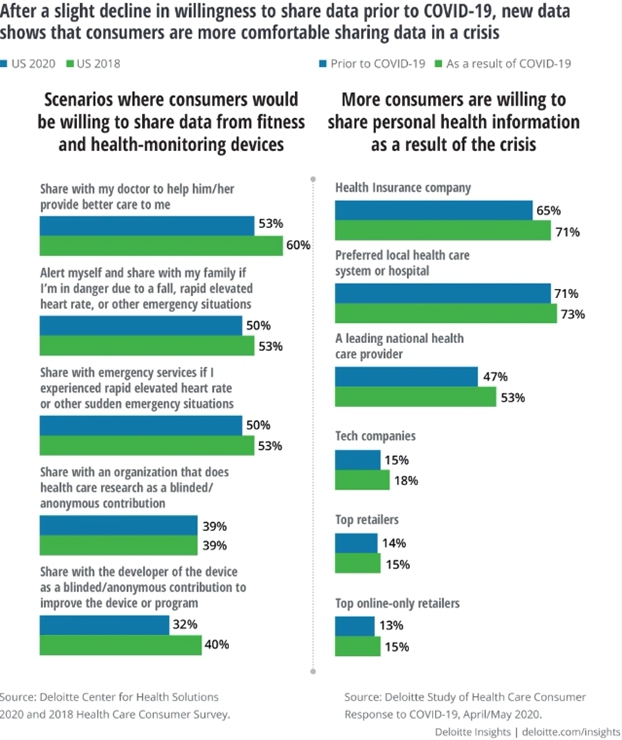
44
In addition, the Deloitte surveys reveal that about a third to half of consumers are comfortable using at-home diagnostics for various reasons, as demonstrated in the graph below. Younger generations are more comfortable across the board. The largest gaps in comfort levels by generation are for genetic tests (24% of seniors as opposed to 47% of Gen Z and millennials) and at-home blood tests to track overall health (28% of seniors as opposed to 47/48% of Gen Z and millennials).
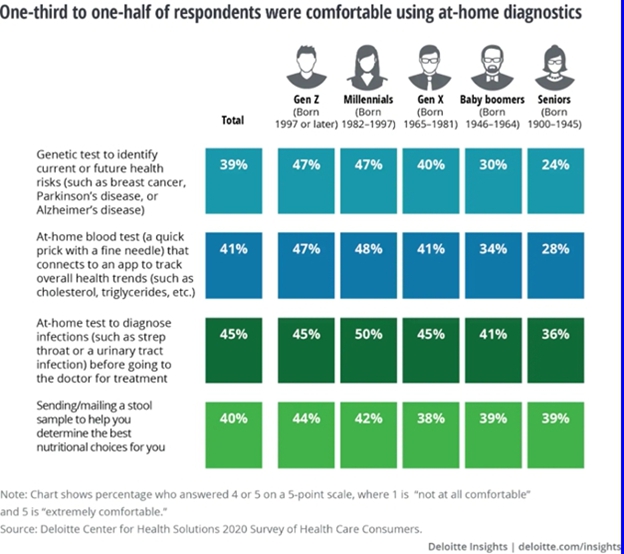
A study conducted by the Society for Cardiovascular Angiography & Intervention, that surveyed 1,068 responses from a nationally representative sample over age 30, found that more than 50% of Americans are avoiding care for medical emergencies such as heart attack, due to the fear of contracting COVID-19. Health systems, independent practices and others telehealth providers are reporting 50 to 175 times more telehealth visits compared to the number of telehealth visits pre-COVID-19. In addition, 57% of health providers view telehealth more favorably than they did before COVID-19 and 64% are more comfortable using it.
CMS have recently expanded access to Medicare telemedicine services on a temporary and emergency basis under the 1135 waiver authority and Coronavirus Preparedness and Response Supplemental Appropriations Act (Phase 1), so that beneficiaries can receive a wider range of services from their physicians without the need to attend a healthcare facility. Under the COVID-19 new guidelines, beginning March 6, 2020 and for the duration of the COVID-19 public health emergency, Medicare is able to pay for office, hospital, and other visits furnished via telemedicine across the country, including in patients’ homes.
45
In addition, in November 2018, CMS finalized changes to the 2019 Physician Fee Schedule and the Quality Payment Program and will now pay providers for communication technology-based services. Through the rule, CMS is also expanding the number of Medicare-covered telemedicine services to include “prolonged preventive service(s)”. CMS will pay physicians for their time when they check in with Medicare beneficiaries via telephone or another telecommunications device. Physicians will also be paid for the time it takes to review a video or image sent by a patient to assess whether a visit is needed.
China
According to a March 2020 report from Statista and data from the Organization for Economic Co-operation and Development, China is facing a significant shortage of physicians with two doctors for every 1,000 people, compared to 2.6 doctors per 1,000 people in the United States and 3.7 per 1,000 people in Australia. Accordingly, the Chinese government has made healthcare a priority.
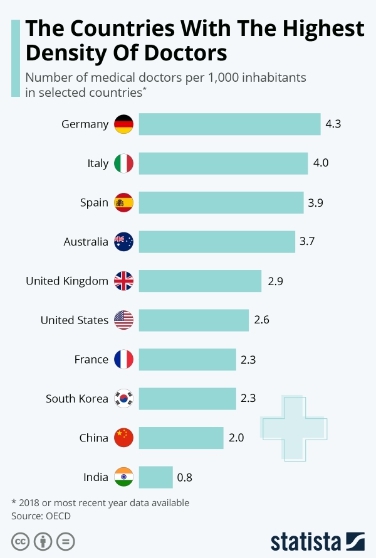
In addition, in China the distribution of superior medical resources is extremely uneven. More than 70% of the third-level grade-A hospitals are located in the eastern region, making the potential demand for telemedicine in the central and western regions disproportionately much larger.
46
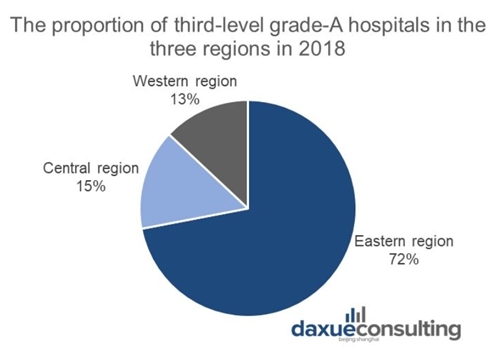
Data source: chyxx.com - The proportion of third-level grade-A hospitals in the three regions in 2018
According to the China Power Project, China’s population is growing old at a faster rate than almost all other countries, due to increased life expectancy and decreased birth rate as a result of China’s 36-year one-child policy. In 2017, in China, the proportion of Chinese citizens above 60 years old obtained 17.3 percent, approximately above 241 million. It is expected that China’s 65-year-old population will reach 487 million, or nearly 35 percent in 2050. China’s looming demographic shift presents considerable social and economic challenges. According to China Briefing, the changing of demographics in China have led to a population where chronic disease is more prevalent than in the past. As a result, there is a higher demand for disease management and ongoing monitoring.
In addition, a draft of China’s Law on the Promotion of Basic Medical and Health Care approved by the National People’s Congress became effective in June 2020. The draft law contemplates the promotion of telemedicine services, which has been expedited by the COVID-19 outbreak. In 2016, the government launched Healthy China 2030. There is currently a government mandate that prioritizes healthcare which AI and digital health will help to fill the gap, with telemedicine making it possible to increase the number of people receiving real time consultation.
Both local and foreign companies are investing in the development of specialized and differentiated private hospitals, along with innovative aged care. According to Research and Markets, China, the world second largest economy, is forecast to reach an estimated mHealth market size of $25.6 billion in 2027, representing a CAGR of 22.1% from 2020 through 2027.
mHealth is being driven by increase in aging population over the age of 60 and a lack of access to quality healthcare-services and limited resources that hinder a physicians’ ability to provide sufficient time beyond treatment and diagnosis.
47
mHealth can also be used in more rural parts of China to help resolve accessibility challenges. The current policy environment in China is playing an important role in promoting industry development and facilitating medical treatment through combining Internet technology with China’s health industry. Internet hospitals have become a service platform that integrates online appointments, consultations, prescribing and delivering medicine, and online and offline diagnosis and treatment with the help of Internet technology. Integrating physical hospitals into the platform provides strong support for Internet hospitals in conducting consultations, follow-up visits, and chronic disease management on the Internet. Remote monitoring and other mHealth solutions break through space limitations of physical hospitals but also work synergistically with physical hospitals, so people in remote areas can also have access to high-quality medical treatment.
According to a July 2020 article in the Journal of Medical Internet Research titled “The Internet Hospital as a Telehealth Model in China: Systematic Search and Content Analysis”, by January 2019, the number of registered internet hospitals expanded to approximately 130 in 25 provinces, accounting for 73.5% of all provinces or province-level municipalities in China. Internet hospitals, as a new telehealth model, are distinct but overlap with online health, telemedicine, and mobile medical.
Source: J Med Internet Res (July 2020)
48
The figure below, included in a September 2021 article in the Journal of Medical Internet Research titled “Running an Internet Hospital in China: Perspective Based on a Case Study”, shows the number of patients that received services from internet hospitals from October 2019 to September 2020. From January to April 2020, due to the COVID-19 epidemic, the number of patients using internet hospitals soared. Since then, the epidemic in China has been stable, and most patients have returned to offline treatment; however, the overall number has increased.
Source: Running an Internet Hospital in China: Perspective Based on a Case Study. J Med Internet Res 2021
Source: Asian Hospital & Healthcare Management. mHealth in China
49
Monitoring Services Market Opportunity
According to CMS, two-thirds of Medicare beneficiaries have two or more chronic conditions. Such conditions often require continuous medical monitoring. In addition, an estimated 5.86 million ambulatory ECG diagnostic tests were prescribed by physicians in the United States in 2020, which represents a $2.21 billion market opportunity. With an estimated 13% CAGR (from 2015 to 2025), this represents a very healthy market for our arrhythmia monitoring technologies and service platform. Although there is little data outside of the United States, we believe additional markets exist due to an ageing global population, unhealthy lifestyles and prevalence of Atrial fibrillation. Clinical research has also shown that traditional ambulatory cardiac monitoring tools, such as Holter and event monitors, do not collect the required amount of data for making a definitive diagnosis, as these older devices may have too short of a monitoring time, may not continuously collect ECG data, or patients will not wear the device (low patient compliance). Hospitals and physicians are also outsourcing more of their ambulatory ECG monitoring needs, in order to minimize costs and workflow burden. According to a report on Mobile Cardiac Telemetry Systems Market Size, Share and Trends by Grand View Research, the global mobile cardiac telemetry systems market size was estimated at $833.8 million in 2021 and is expected to register a compound annual growth rate (CAGR) of 12.1% from 2022 to 2030.
Our innovative technologies and proprietary software and algorithms have the ability to improve patient compliance, provides continuous monitoring and multiple channels of ECG, which ensures higher diagnostic yields that can deliver better clinical outcomes.
Integrated Delivery Networks
Third-party research estimates that the global healthcare big data analytics market was worth $19.6 billion in 2018 and is projected to reach a value of $47.7 billion by 2024, registering a CAGR of around 16% from 2019 to 2024. This data will be augmented by patient-generated health data (or PGHD), which is data primarily captured and recorded by the patient themselves, allowing them greater ownership over their own health. Integrating PGHD into electronic health records will help providers understand the patient experience, increase efficiency and productivity of clinical trials, improve the prediction of addressable treatment toxicities, and ultimately improve quality of care and clinical outcomes. The United States could reach a “critical mass” of physicians using PGHD from devices such as wearables by 2020, according to new research released by the Consumer Technology Association. Additionally, insurers are offering free remote monitors/wearables and cash incentives to subscribers who meet certain health goals.
50
Concierge Medicine
Concierge medicine can be defined as a B2B2C model. Currently, about 12,000 physicians in the United States offer concierge healthcare services. This niche market is growing due in part to organizations that recruit physicians to manage concierge practices. Concierge physicians have fewer patients, offer same-day appointments and longer office visits, and participate in email and phone communication. Studies have found that patients with concierge medicine physicians are more satisfied and have fewer visits to ERs and specialists, thus resulting fewer hospitalizations, fewer emergency department visits, and better control of hypertension and diabetes. A study published in The American Journal of Managed Care, found a 79% reduction in hospital admissions for Medicare patients in concierge medicine affiliated practices, compared with those in traditional practices.
Direct Primary Care
Direct primary care (or DPC) can also be defined as a B2C model. DPC practices have a direct financial relationship with patients and provide comprehensive care and preventive services. This is a mass-market variant of concierge medicine, with the biggest difference being that the DPC model charges a flat rate fee that often includes most or all physician services. The monthly fee typically includes basic checkups, same-day or next-day appointments, and the ability to obtain medications and lab tests at or near wholesale prices. The DPC model does not rely on insurance co-pays, deductibles or co-insurance fees. All DPC providers recommended patients have some form of insurance, or take part in a healthcare sharing plan that functions like insurance, as a patient is not protected financially if they have a health issue outside the scope of primary care.
Our Ecosystem
The following is a depiction of the ecosystem in which our products and services are intended to play a role:
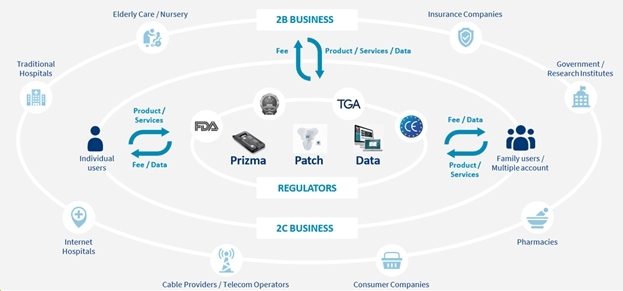
We anticipate that our next generation mobile technologies will empower both users and businesses to better utilize their business processes by merging them into a coherent ecosystem. We plan to provide users (individuals or families) products and services, and collect valuable data and monetary inflows at the same time. We also plan to serve businesses (including hospitals, pharmacies, elderly care institutions, research institutions, etc.) by establishing a direct linkage using mobile technology to maximize the value of user data, which is more than ever needed by businesses.
51
Our Strategy
Our strategic objective is to participate in the large and growing worldwide mHealth marketplace by developing and commercializing innovative next generation telemedicine solutions and monitoring service platforms. Using our proprietary and patented suite of devices and software solutions, we are implementing a go-to-market strategy aimed at establishing and growing multiple recurring revenue streams across consumer and professional healthcare verticals, with a primary geographical focus on the United States and China. Our business activities in the United Kingdom, Europe, Australia and elsewhere in Asia have been paused to focus on the United States and China.
Our current strategic commercial activities are focused on:
| ● | investing in cardiac monitoring service centers in the United States; | |
| ● | commercializing the Prizma device and Patch Extended Holter Patch System monitoring solution in the United States, China and other markets; | |
| ● | completing the development of our VSMS; and | |
| ● | cultivating various channels of distribution. Such channels include hospitals, insurance companies, chronic care management companies, concierge medicine groups, Telcos, specialized mobile virtual network operators (or MVNOs) distribution houses, original design manufacturer (or ODM) handsets and wireless design centers. |
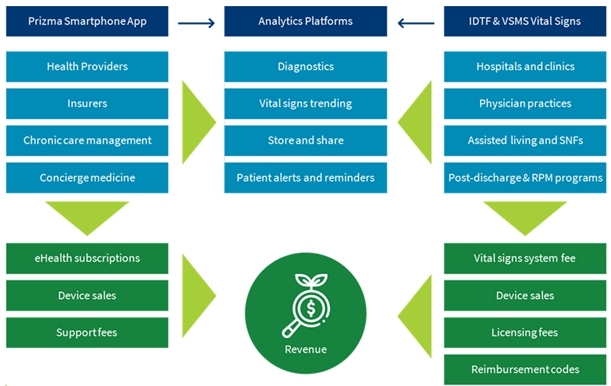
| ● | Hospitals, Clinics and Physician Practices. Our cardiac monitoring services are marketed to healthcare institutions, hospitals, clinics and physician practices. We employ highly educated sales professionals in the United States who regularly call on these stakeholders and educate them on the clinical value of multi-lead ECG monitoring solutions. We believe our comprehensive range of technologies appeals to many healthcare providers, as they can order the right device for each patient. |
52
We will lease the Vital Signs System to hospitals on a per day fee model. The device can then be prescribed by a physician for monitoring patients in the hospital, and in pre-admission and post discharge programs. Once a patient is discharged, we anticipate that patients may continue monitoring services by using the Prizma device.
| ● | Insurance Companies. We propose to sell the Prizma device and lease the monitoring center diagnostic services on a monthly fee per patient. Healthcare insurers typically have high-acuity patients who would benefit from participation in chronic care management programs. | |
| ● | Chronic Care Management. We propose to sell the Prizma device and lease customized back end analytics platforms to these companies who manage high-acuity patients of healthcare insurers, homecare agencies, nursing homes, and skilled nursing facilities (SNFs). | |
| ● | Concierge medicine. We will explore purchase and lease programs of the Prizma solution to concierge medicine practices. This model may resemble those provided to healthcare insurers or chronic care management companies. |
In addition, cloud monitoring services from the Prizma device are anticipated to be offered based on a two tier program: monitoring over the cloud by sending the data and receiving automatic feedback; and monitoring with access to a professional clinical call center that can provide real time feedback to the customer and ability to triage with a healthcare professional.
Implementation of Our Strategy in the United States
Our monitoring center strategy is to be the go-to provider of innovative cardiac monitoring services in the United States. We plan to further expand by targeting all healthcare providers who can benefit from our comprehensive service offerings, which include our Extended Holter Patch System, MCT, event and traditional Holter devices. Our customers demand a wider range of offerings as one device type does not fit all needs.
To penetrate this market and drive growth, we plan to:
| ● | educate stakeholders in the healthcare environment on the benefits of multi-lead technologies that deliver more comprehensive clinical results and high-patient compliance; | |
| ● | provide in-house clinical evidence of diagnostic results generated from our service platform; | |
| ● | contract with more commercial payors in order to increase patient access. We currently have contracts with CMS, some Blue Cross Blue Shield entities, a key veterans affairs medical center, and other commercial payors across the country; | |
| ● | offer our clinical expertise and patient-centric services 24/7; | |
| ● | expand our sales force to drive growth in targeted territories; and | |
| ● | utilize our platform to introduce innovative solutions such as the Prizma device, Extended Holter Patch System and VSMS. |
In addition to the 2017 acquisition of CardioStaff, in November 2018 we executed on our acquisition strategy with the purchase of Tennessee-based Telerhythmics, in the amount of $1.95 million. Founded in 1996, Telerhythmics provides on-site and remote arrhythmia-monitoring services to doctors and physicians. These services include MCT, event and Holter monitoring. The acquisition brings additional payer contracts, clinical and logistical scalability, access to additional monitoring technologies and an existing platform for launching our proprietary technologies into the important digital health space. We are working on a smooth integration and will disclose these plans in the coming months. For the foreseeable future, Telerhythmics, a wholly-owned subsidiary of G Medical Innovations USA Inc., will run operations in a separate facility, but with synergistic opportunities that will help us achieve higher profitability.
53
In April 2020, we entered into a distribution agreement with LiveCare Corp. (or LiveCare), a U.S.-based remote patient solutions company. Pursuant to the agreement, LiveCare will promote the sale of and distribute our Prizma device in the United States. As part of the agreement, we will work with LiveCare to integrate the Prizma device into LiveCare’s Link+ platform, which will be offered directly to consumers. The Link+ platform is a 4G smart home gateway that integrates an array of medical devices into a patient’s home using a simple, touch-free syncing process. The agreement is for an initial period of three years, unless notice of termination is provided by either party, and will automatically renew for successive one year terms thereafter, unless either party provides notice of its intent not to renew the agreement at least 30 days prior to the applicable anniversary date. There are no minimum sales commitments under the agreement.
In April 2020, we entered into a distribution agreement with All County Health Care Inc. (or All County), a Medicare certified home health company specializes in the provision of quality home-based healthcare services. Under the agreement, All County will distribute our Prizma in the United States and will offer customers certain additional Prizma related services. The agreement does not provide for a term. In addition, there are no minimum sales commitments under the agreement.
GRS Marketing Services Agreement
On September 30, 2020, we entered into a media and marketing service agreement (which we refer to as the GRS Agreement) with GRS, an affiliate of Guthy-Renker, LLC. Guthy-Renker is one of the largest and most respected direct marketing companies in the world. Since 1988, Guthy-Renker has discovered and developed dozens of consumer products in the beauty, skincare, and wellness categories.
Pursuant to the GRS Agreement, GRS will, for a three-year term, exclusively oversee all television production, radio creative and social media for our company in the United States, including our Prizma mobile medical monitor and other consumer products and services. GRS will manage all television advertising, social and radio media buying, based on approved monthly budgets.
In consideration of GRS’ marketing advisory and creative services, we shall pay to GRS a low five-digit monthly retainer and a low single digit percentage gross sales commission on all of our U.S. sales of consumer products and services, but excluding IDTF revenue. We have also agreed to issue to GRS the GRS Warrant, which is a nine year warrant exercisable for 526,338 Ordinary Shares (after giving effect to adjustments including the November 2022 Reverse Split). The GRS Warrant vested in two equal tranches, the first immediately (with such warrant having an exercise price of A$4.5 (approximately $3.15) and the second on the first anniversary of the execution of the GRS Agreement (with such warrant to have an exercise price the lesser of a fifty percent discount to the price to the public in our initial public offering or fifty percent discount to the price of our Ordinary Shares on the date of vesting).
Under the GRS Agreement, if we terminate the GRS Agreement within twelve months from signing a definitive agreement, then we will be restricted from using or buying any television, radio, digital or social media advertising in the United States for twelve months following the date of termination. Such restriction will not apply if we choose to accelerate the vesting of the GRS Warrant. In addition, such restriction will not apply if we and GRS fulfill their respective obligations under the GRS Agreement through the end of the three-year term.
We have suspended our obligations under the GRS Agreement due to a failure by GRS to fulfill its obligations. We have begun to evaluate our options for legal recourse against GRS to obtain fulfilment of GRS’s obligations or to receive amounts that were previously paid by us to GRS.
UnityPoint Health Pilot
On April 16, 2021, we were notified that the Cardiovascular Services arm of UnityPoint Health Methodist (or UnityPoint) in Peoria, Illinois was entering into a pilot program with us, for using our Prizma device on cardiac patients. We were advised that the pilot will initially include 500 patients, who will be monitored remotely to detect abnormal heart rate and conditions associated with a decrease in the oxygenation of the lungs such as worsening hear failure. As part of the pilot, our monitoring call centers will provide constant reporting and will use our device to detect any cases of emergent need for cardiac care. It is intended that in the event that an abnormality will be detected, the patient will be seen by a cardiology provider at UnityPoint’s Peoria clinic, potentially preventing unnecessary visits to the emergency room and hospitalization.
54
Heartbuds Collaboration
On November 30, 2021, we entered into a joint development, licensing and distribution agreement with Heartbuds AK, LLC. Heartbuds is a medical device company that developed a medical device used with a smart phone to enable doctors and others to listen to and record a patient’s heart and lungs (or HB1).
Pursuant to the joint development agreement, we and Heartbuds will jointly develop a newer, enhanced model or generation of the HB1 to be included with the sale and distribution of our Prisma (or HB2). According to the agreement, Heartbuds has the right to develop, promote, market, license, sell and distribute the HB1, the HB2 and any other newer models and generations thereafter (or collectively the HB Devices), independent of the Prisma.
In consideration for the licensing rights and distribution, we will pay to Heartbuds the following royalties with respect to each subscription, lease, sale or other similar transaction involving the Prisma that includes any HB Device: (i) a recurring $0.25 per month royalty for each lease or subscription; and (ii) a one-time royalty of $2.50 for each sale. In return, Heartbuds will pay us the following royalties with respect to each subscription, lease, sale or other similar transaction of an HB Device that does not include a Prisma unit and is with a party that is unaffiliated with our marketing or sales: (i) a recuring monthly royalty of 5% of lease or subscription fees for each lease or subscription and (ii) a one-time royalty of 5% of the sales price for each sale. In addition, on the date of the agreement, we provided Heartbuds with the following additional consideration: (i) 3,265 of our Ordinary Shares, and (ii) $1,000,000 in options to acquire our Ordinary Shares at an exercise price of $114.45 per share. From the date that the HB2 will be approved by the U.S. FDA until the last day of the 18-calendar month thereafter, the options shall vest on a pro rata basis based on the actual number of devices that Heartbuds will sell to be calculated relative to the agreed target of 20,000 devices.
Both we and Heartbuds may terminate the agreement if the U.S. FDA has not approved the HB2 on or before the third anniversary date of the agreement.
Implementation of Our Strategy in China
Since May 2018, we have been in contact with Dongtai City Internet Hospital (or the Dongtai Hospital) on several occasions. The Dongtai Hospital provides patients with various remote health services including online medical consultation, health monitoring, extension of prescriptions and more. The Dongtai Hospital has purchased 100 Prizma devices to be used as part of a pilot for their Citizen Health System Program which is aimed at connecting the clinics located in villages, district hospitals and the City Third Grade Class-A hospital together through the internet.
The Prizma devices purchased by the Dongtai Hospital will be used by nursing homes, allowing the collection of residents’ vital signs data without having them to visit a medical institution, and storing the data in the database system. The sharing of data will enable efficiency in monitoring and treatment, and will allow doctors from the lower level hospitals to consult with doctors form the higher-level hospitals at any time.
During the outbreak of COVID-19, we received positive feedback from the Dongtai Hospital team that indicated that our Prizma device may provide an efficient solution that fits their need to monitor patients’ body temperature and blood oxygen remotely. Commercialization of the project is on hold and dependent upon NMPA approval being granted.
We have commenced the process of penetrating the Chinese market. To this end, we:
| ● | submitted the NMPA files; and | |
| ● | are in process NMPA approval process for the Extended Holter Patch System. |
55
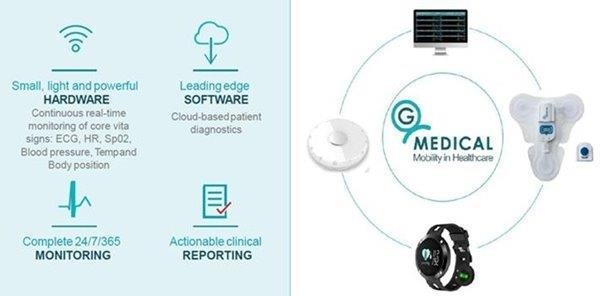
Our Home Testing Kits Business
In summer 2022, we took a strategic decision to divert to more comprehensive home testing solutions as an expansion of our patient services and as part of our vision to move towards a home-based health care system. On July 13, 2022, we announced that out wholly owned subsidiary, G Medical Health and Wellness, Inc., has developed seven different at-home tests. In addition, on October 6, 2022, we introduced a Monkeypox consumer home health test kit, along with 23 additional new direct to consumer home collection kits with 24 to 48 hours results, all of which are expected to be available to consumers during the third quarter of 2023 through popular big-box retailers, pharmacies and online. The tests are developed for testing health issues related to hormones, sexual transmitted disease, nutrition, colon cancer, food sensitivity, and allergies, with results going directly to the user within days. The prices for our at-home testing kits will range from $49 to $259.
On June 27, 2022, we entered into a wholesale sales consulting agreement with Ideaology 360, LLC (“Ideaology 360”) to engage Ideaology 360 to serve as our exclusive wholesale sales representative in the United States to assist us with, among other things, developing and implementing our overall strategic plan for online and retail sales of our at-home test kits, developing marketing and advertising campaigns to create awareness and drive sales of our at-home test kits as well as educating a national sales broker team about our at-home test kits. The term of our agreement with Ideaology 360 is one year and automatically renewable for successive one year terms, unless either of us provides the other party with 60 days written notice prior to the end of the relevant term. Ideaology 360 will be paid a monthly fee plus sales commission for its services to us.
Our COVID-19 Testing and Laboratories Services
In December 2021, we launched our COVID-19 testing business in the State of California, providing four kinds of diagnostic tests –Rapid Antigen, A/B Flu, PCR and Antibody tests and operated in six locations performing point-of-care tests in communities in communities in Southern and Northern California. The volume of COVID-19 testing has decreased significantly since April 2022. Given the decrease in COVID-19 cases, in the second half of fiscal year 2022 we decided to discontinue our COVID-19 related business activities. We have written off inventory and fixed assets related to our COVID-19 business for the fiscal year ended December 31, 2022.
Our Monitoring Services
Our monitoring services in the United States focus on two main verticals:
| ● | IDTF Monitoring Services (B2B): CPT based services; and | |
| ● | Private Monitoring Services (B2B, B2C and B2B2C): services provided by a different entity, independent to the IDTF |
56
Independent Diagnostic Testing Facility (IDTF) Monitoring Services
Our provision of IDTF monitoring services is comprised of arrhythmia monitoring services for patients (including MCT, extended Holter and CEM), and use our Prizma device’s RPM of vital signs on a daily basis and generating reports that allow physicians to track their patients’ health condition.
The graphic below represents the workflow being done by our medical monitoring service centers, from the moment a single patient is enrolled in the service until a summary medical report is delivered to the prescribing physician.
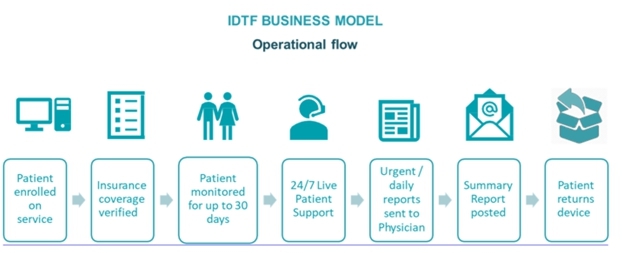
An IDTF can sell services as a provider to physicians, hospitals and patients. All services are provided according to physician’s prescription done through the enrollment form on our proprietary website. After receiving an enrollment form, we will verify that the patient has an insurance coverage for the service requested by the physician and then will provide the patient, directly or through a physician, with the monitoring device. The patient will wear the monitoring device during the total service monitoring time, and we will monitor and support the patient and send medical notifications to the patient’s physician, according to the type of service provided. Once service is completed, we will send the physician an end-of-session report with a summary of all relevant information detected during the service period. We will also send the bill out to the relevant insurance company and collect the money according to the specific CPT code and monitoring services that were provided.
Under the IDTF umbrella, we offer four types of services for remote cardiac monitoring:
| ● | MCT: a 24/7 remote cardiac monitoring and transmission in near-real time of specific arrhythmias recognized by the algorithm. This service can be provided to patients for up to 30 days and is reimbursed for up to twice a year per patient. The reimbursement under CPT code 93229 will range from $400 to $1,000 depending on the insurance company, locality, and coverage. Our MCT product’s marketing name is the Spider. | |
| ● | Extended Holter (AECG): cardiac recording 24/7 for more than 48 hours and up to 14 days. Analysis is done off-line when the device is returned to the physician’s office and data is uploaded. This service can be reimbursed multiple times a year per patient. Reimbursement under CPT codes 93243 and 93247 will range from $36 to $300 depending on the insurance company, locality and coverage. Our AECG product’s marketing name is the Gpatch. |
57
| ● | Cardiac Event Monitor (CEM): cardiac monitoring and recording only when the patient has an arrhythmia. This service is available for up to 30 days and reimbursed per patient multiple times a year. Reimbursement under CPT code 93271 will range from $150 to $250 depending on the insurance company, locality and coverage. | |
| ● | 24/48 Hours Holter Monitoring: cardiac recording for up to 24-48 hours only. This service can be provided per patient, multiple times per year. Reimbursement under CPT code 93226 will range from $38 to $55 depending on the insurance company, locality and coverage. |
We entered the U.S. arrhythmia monitoring services industry through our December 2017 acquisition of CardioStaff, an IDTF based in Austin, Texas. The IDTF provides physicians and hospitals with 24/7 remote cardiac monitoring services which utilize our Holter, extended Holter, MCT and event monitoring devices. CardioStaff was rebranded as GMedDx and is expected to serve as a platform for introducing our innovative suite of clinical-grade products into outpatient settings, physician practices, hospitals and senior care facilities in the United States.
In November 2018, we acquired a second IDTF, Telerhythmics, based in Memphis, Tennessee. Telerhythmics operates mainly across the Southeastern United States, and provides hospitals and physicians with cardiac monitoring services including MCT, Holter and event monitoring. In addition to its traditional activities, Telerhythmics will utilize the Prizma device for RPM services.
We have entered into approximately 140 commercial payor agreements across local, regional and national markets as well as an agreement with CMS, which provides health coverage to more than 100 million people.
In May 2019, we entered into PPAs with Prime Health Services, Inc and Ancillary Care Services, Inc. The PPAs have further and significantly increased our footprint in the healthcare delivery system of cardiac monitoring and provide more exposure to our future patient base and third-party payer populations.
We have been approved and CPT-coded with more than 150 healthcare insurance providers to be reimbursed by our services.
Private Monitoring Services
In addition to IDTF monitoring services, we will provide private monitoring services, utilizing our Prizma device. Under the private service umbrella, we offer three main lines of services:
| ● | Prizma OTC: sales of the Prizma device to consumers with a user portal service which will allow them to save their medical tests and create their own medical information reports and share such reports remotely or when visiting their physicians. | |
| ● | Prizma Concierge Medicine Services: a 24/7 call center service that allows subscribers to speak directly with a physician regarding the collected data. | |
| ● | Prizma Care Facility/Nursing Home Services: a 24/7 service that assists care facilities and nursing homes to track diagnostic information for patients and residents. |
Manufacturing
Our Prizma device and Extended Holter Patch System are expected to be manufactured by a high quality third party in China, which has all the applicable regulatory approvals. The third party also has manufacturing sites in the United States. Currently, we use contract manufacturers in Israel to meet our manufacturing requirements.
Our Home Collection Kits are expected to be manufactured by a high quality third party in China, which has all the applicable regulatory approvals in order to distribute the products in the US.
58
Competition and Competitive Advantages
The monitoring services industry is very competitive and characterized by rapidly advancing technologies with a strong emphasis on proprietary products and software. We recognize that our competitive success will depend upon constant investments in innovative, pioneering technological solutions. We believe that many of our competitors only offer one narrow scope of capabilities and are not perceived as convenient for all monitoring scenarios, or have not received regulatory approvals for product enhancements. We believe that our competitive advantages include:
| ● | Strong research and development capabilities: our management team is led by individuals with over 25 years of experience in developing mobile embedded medical sensors and software; |
| ● | Existing regulatory approvals: currently, we have the CE mark for our Prizma device and Extended Holter Patch System, U.S. FDA clearance for our Prizma device, and OTC authorization for our Prizma device and Extended Holter Patch System based on an EUA policy (QT prolongation syndrome in hospitals). We have also been granted Australian regulatory approval by the TGA for our Prizma device and Extended Holter Patch System. We received registration with the Italian Health Ministry’s database of medical products and were granted our Permit License by the Taiwan FDA for our Prizma device. We are also preparing our application to the Chinese NMPA for our Prizma device; | |
| ● | Go-to-market strategy: our management has proven their ability to execute our go-to-market strategy as described below, with over 25 years of medical device development and commercialization experience in the United States, China, parts of Europe, Australia, South Africa, Japan, the Asia Pacific region and Brazil; | |
| ● | A one-stop multi-function and multi-account platform: with extensive experience in developing mobile embedded medical sensors, we offer multi-function devices with quick upgrades, more add-ins and multi-accounts for all family members; | |
| ● | An extensive ecosystem with greatest data monetization potential: we provide companies with medical grade solutions, efficient healthcare delivery and potential for collaboration, and enable consumers to access real-time monitoring, accurate medical data and a resource sharing platform. |
United States Competitive Review
Our arrhythmia monitoring service primary competitors in the U.S. include iRhythm Technologies, Inc. (Nasdaq: IRTC), and Philips Healthcare. These companies have either developed or acquired patch-based mobile cardiac monitors. iRhythm Technologies, Inc. became a public company in late 2016 with their proprietary ZIO patch, a single lead ECG wearable that is worn for up to 14 days. Several small start-ups are also trying to compete in the cardiac monitoring space, either with an MCT device or a patch-based service.
Other competitors are companies that sell standard Holter monitors and analysis systems including GE Healthcare, Philips Healthcare, Mortara Instrument, Inc., and Welch Allyn Holdings, Inc. (now part of Hill-Rom Holdings, Inc.).
We believe that the principal competitive strength of our arrhythmia monitoring service derives from the combination of several factors including: a range of monitoring modalities to meet a variety of patients’ needs; multi-lead ECG configurations that provide greater diagnostic yields; quality of our clinical staff to accurately detect and identify arrhythmias; clear and comprehensive reports for physician interpretation; contracted rates with third-party payors; government reimbursement for our products and services; experience, knowledge and availability of our account representative and customer support services; flexible workflow protocols to address account methodologies; and our relationships with physicians, hospitals, insurers, and other third-party payors.
59
Our competitors in the mHealth space include, among others, AliveCor (ECG only), Qardia (more geared to fitness) and Tytocare (home care device with various attachments for examining ears, throat, heart, lungs, abdomen, skin, and capturing heart rate and temperature data).
In the mHealth space, we may compete directly or indirectly with companies such as Teledoc Health, Inc., DarioHealth Corp. and Itamar Medical, Inc., however, we believe that our Prizma clinical grade solution has several differentiators that elevate its market position and our competitive advantage, including multiple measurements on one application (ECG, temperature, Sp02, stress analysis, etc.) and an easy to use platform which provides more value to a greater range of markets (consumer, medical clinics, chronic care).
China Competition Review
The graphic below represents the primary companies and their respective products that compete, or have the potential to compete, with our products and services in the Chinese market:
| Product | Tests | Regulatory Approval | Comments | |||
| Products of Company A | Body temperature, blood pressure and glucose level measurement | New models of blood pressure monitors completed clinical trials | Various product types | |||
| Products of Company B | 24 hours of continuous accurate temperature measurement and high temperature alarm; Accurate monitoring of blood oxygen, heartbeat, blood circulation; Intelligent management of blood pressure | One product is in registration application for Certificate for Medical Device; another product obtained the Medical Device Registration Certificate | Various product types | |||
| Products of Company C | Multiple diabetes indicators such as glucose level, blood lipids, glycated hemoglobin, uric acid, etc. | One product received valid Medical Device Registration Certificate; another product is applying for Medical Device Registration Certificate | Single product line for diabetes monitoring and related chronic disease detection | |||
| Products of Company D | Changes in blood oxygen and body temperature monitoring, blood pressure measurement, sleep improvement | In the type examination stage | Various devices with different functions | |||
| Products of Company E | Blood pressure measurement, heart rate monitoring | Have obtained the second-class Medical Device Registration Certificate | Limited tests | |||
| Product of Company F |
Heart rate measurement; Designed for users to track daily activities including distance, calories burned, sleep quality, steps, and time |
No regulatory approvals | Not for clinical use |
In China, we believe that our competitive advantage lies in our technology, products and services which offer health care providers and patients an all-inclusive solution for remote health monitoring, as opposed to only one narrow scope of capabilities. Thus, we believe that due to our strong technology and our experience and know-how in monitoring patients remotely, G Medical China is well positioned to gain a significant market share in China.
60
Intellectual Property
We have made significant investments in the development of our patent portfolio to protect our technologies and programs, and we intend to continue to do so. Our intellectual property portfolio consists of seven granted patents as well as five patent applications which are still pending approval by the USPTO.
Patents
The following table sets forth our patent portfolio as of the date of this annual report:
| Patent Name | Jurisdiction | Product | Type of Protection | Expiration Date | ||||
| Systems and Methods for Vital Signs Monitoring with Ear Piece | United States | VSMS | Utility Patent | February 28, 2036 | ||||
| Jacket for Medical Module | China | Prizma | Utility Model | January 30, 2028 | ||||
| Health Monitoring Device that Includes A Compact Oximeter | China | Prizma | Utility Model | January 30, 2028 | ||||
| Jacket for Medical Module Methods and Systems for Vital Signs | United States | Prizma | Utility Patent | February 14, 2039 | ||||
| Methods and Systems For Vital Signs Monitoring with Ear Piece | United States | VSMS | Utility Patent | February 28, 2036 | ||||
| Method and System for Locating a Defibrillator | United States | VSMS | Utility Patent | August 7, 2039 | ||||
| Method, Device and System for Non-Invasively Monitoring Physiological Parameters | United States | VSMS, Prizma | Utility Patent | February 8, 2036 | ||||
| Health Monitoring Devices that Includes A Compact Oximeter(1) | United States | Prizma | Utility Patent | May 15, 2040 | ||||
| A Robust Medical Device and Method(2) | United States | Prizma | Utility Patent | October 27, 2040 | ||||
| Coating Electrodes of Medical Devices(2) | United States | Prizma | Utility Patent | February 8, 2042 | ||||
| Method and System for Obtaining Physical Condition That Lead to a Defibrillator Countershock(2) | United States | VSMS | Utility Patent | September 25, 2027 | ||||
| Methods and Systems For Vital Signs Monitoring with Ear Piece(2) | United States | VSMS | Utility Patent | February 28, 2036 |
| (1) | Patent allowed. |
| (2) | Patent pending. |
On January 30, 2023, we announced that we received a patent issue notification from the USPTO for a patent, which was issued on February 7, 2023, called “Method and System for Vital Signs Monitoring with Earpiece”, which covers the vital signs monitoring system that we are developing using multiple sensing mechanisms to be used on different body locations. This vital sign monitoring system will potentially provide physicians more versatility in patients’ monitoring and would simplify in and out-patients’ monitoring.
61
Other Intellectual Property
On August 4, 2016, our subsidiary, G Medical Israel, and Mennen Medical Ltd. (or Mennen) (a company incorporated in Israel), entered into a software licensing agreement pursuant to which we were granted a worldwide, perpetual, irrevocable (other than in case of breach), royalty-free, non-exclusive license to use the arrhythmia software and high risk detection application (including all existing preprocessing) and respiration module manufactured and developed by Mennen (including subject to certain conditions, any updates, upgrades, modification and customizations, if requested by us, which shall be priced separately) and to incorporate and integrate the arrhythmia software and high risk detection application with our ECG wireless devices. Pursuant to the terms of the software licensing agreement, Mennen was paid $110,000.
Trademarks
We own one U.S. trademark and one U.S. trademark application:
| ● | PRIZMA - US trademark 87815896 allowed April 9, 2019 | |
| ● | PRIZMA-CARE - US trademark application filed December 22, 2021 serial number 97/184615 |
Trade Secrets and Know-How
We also rely on trade secrets, know-how, and continuing innovation to develop and maintain our competitive position. We cannot be certain that patents will be granted with respect to any of our pending patent applications or with respect to any patent applications filed by us in the future, nor can we be sure that any of our existing patents or any patents granted to us in the future will be commercially useful in protecting our technology.
Our success depends, in part, on an intellectual property portfolio that supports future revenue streams and erects barriers to our competitors. We are maintaining and building our patent portfolio through filing new patent applications, prosecuting existing applications, and licensing and acquiring new patents and patent applications.
Despite these measures, any of our intellectual property and proprietary rights could be challenged, invalidated, circumvented, infringed or misappropriated. Intellectual property and proprietary rights may not be sufficient to permit us to take advantage of current market trends or otherwise to provide competitive one. For more information, see “Item 3.D. Risk Factors-Risks Related to our Intellectual Property.”
Government Regulation
The principal markets that we have targeted for our medical devices are the United States, the EU, Australia, New Zealand and China. The following is an overview of the regulatory regimes in these jurisdictions.
General Overview of United States Medical Device Regulation
Under Section 201(h) of the Federal Food, Drug, and Cosmetic Act (or the FDC Act), a medical device is an article, which, among other things, is intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment or prevention of disease, in man or other animals.
Medical devices sold in the United States are subject to varying levels of regulatory control, the most comprehensive of which requires that a clinical trial may need to be conducted before a device receives clearance for commercial distribution.
Our current devices are classified as medical devices and are subject to regulation by numerous agencies and legislative bodies, including the U.S. FDA, and its foreign counterparts.
U.S. FDA regulations govern product design and development, pre-clinical and clinical testing, manufacturing, labeling, storage, pre-market clearance or approval, advertising and promotion, sales, distribution, device recalls and other similar matters.
62
Specifically, the U.S. FDA classifies medical devices into one of three classes:
| ● | Class I devices are relatively simple and can be manufactured and distributed in the United States with general controls; |
| ● | Class II devices are more complex and require greater scrutiny, with most Class II devices requiring regulatory clearance by the U.S. FDA before being distributed in the United States; or |
| ● | Class III devices are new and frequently help sustain life or are high-risk devices and usually approved through pre-market approval (or PMA). |
Summary of the Medical Device Distribution Process in the United States
Unless an exemption applies, medical devices commercially distributed in the United States require a 510(k) clearance, or a 510(k)+ “de-novo” clearance, or PMA from the U.S. FDA. Our current devices fall into the classification of a Class II device.
510(k) Clearance Process. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use or indications for use, requires a new 510(k) clearance or could even require a premarket application approval. The U.S. FDA requires each manufacturer to assess whether the proposed changes to the medical device are substantial or not; if the change is not substantial, then the manufacturer can issue an internal Letter to File (also called Memo to File) exempting the device from a new 510(k) clearance or a supplement submission, however the U.S. FDA has the right to review any such assessment. If the U.S. FDA disagrees with the determination, then the agency may retroactively require the manufacturer to seek 510(k) clearance. The U.S. FDA can also require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or premarket application approval is obtained.
De Novo Classification. If the U.S. FDA denies 510(k) clearance of a device because it is novel and because an adequate predicate device does not exist (resulting in a determination of non- substantial equivalence), then the “de novo classification” procedure can be invoked based upon reasonable assurance that the device is safe and effective for its intended use. This procedure approximates the level of scrutiny in the 510(k) process but may add several months to the clearance process. If the U.S. FDA approves the “de novo” request, then the device is permitted to enter commercial distribution in the same manner as if 510(k) clearance had been granted.
PMA Process. After the U.S. FDA approves a Class III medical device via the PMA route, a new premarket application or premarket application supplement is required in the event of a modification to the device, its labeling or its manufacturing process. The premarket application approval pathway is much more costly, lengthy and uncertain; it generally takes from one to three years or longer.
Coronavirus Disease 2019 (COVID-19) Emergency Use Authorizations for Medical Devices
On February 4, 2020, the U.S. Secretary of the Department of Health and Human Services (HHS) determined, pursuant to section 564 of the FDC, that there was a significant potential for a public health emergency that has a significant potential to affect national security or the health and security of United States citizens living abroad and that involves COVID-19. The virus which causes the illness COVID-19was named SARS-CoV-2. Based on the EUA policy, which remained in force during the public health emergency related to COVID-19, the U.S. FDA granted us OTC authorization for our Prizma device and Extended Holter Patch. On May 11, 2023, the COVID-19 public health emergency ended.
63
Laboratory Registration, Accreditation and Licensing Requirements
We are subject to federal and state regulation of laboratory licensure. Congress passed the CLIA, which establishes quality standards for all laboratory testing designed to ensure the accuracy, reliability and timeliness of patient test results. Under CLIA, a laboratory is any facility that performs laboratory testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease or the impairment or assessment of health. CLIA requires that we hold a certificate applicable to the type of laboratory examinations we perform and that we comply with various standards with respect to personnel qualifications, facility administration, proficiency testing, quality control and assurance and inspections. Laboratories must register and list their tests with Centers for Medicare & Medicaid Services, or CMS, the agency that oversees CLIA, and CLIA compliance and certification is a prerequisite to be eligible to bill government payors and many private payors for our tests. We are subject to survey and inspection every two years to assess compliance with CLIA’s program standards, and we are subject to additional unannounced inspections. Laboratories performing high complexity testing are required to meet more stringent requirements than laboratories performing less complex tests. In addition, a laboratory like that is certified as “high complexity” under CLIA may obtain analyte specific reagents, which are used as the basis for diagnostic tests that are developed and validated for use in examinations the laboratory performs itself known as LDTs.
In addition, we may in the future elect to participate in the accreditation program of CAP. CMS has deemed CAP standards to be equally or more stringent than CLIA regulations and has approved CAP as a recognized accrediting organization. Inspection by CAP is performed in lieu of inspection by CMS for CAP-accredited laboratories.
General Overview of European and Non-European Medical Device Regulation
Regulatory approval and sales of medical devices outside the United States are subject to foreign regulatory requirements that may vary widely from country to country. These laws and regulations range from simple product registration requirements in some countries to complex clearance and production controls in others. As a result, the processes and time periods required to obtain foreign marketing clearance may be longer or shorter than those necessary to obtain U.S. FDA clearance.
Commercialization of medical devices in Europe is regulated by the EU. The EU presently requires that all medical products bear the CE mark, an international symbol of adherence to quality assurance standards and demonstrated clinical effectiveness. Compliance with the Medical Device Directive (or MDD), or the Active Implantable Medical Device Directive, or the In Vitro Diagnostic Medical Device Directive, as audited by an EEA Notified Body and certified by a recognized European Competent Authority, permits the manufacturer to affix the CE mark on its products. If the medical device is classified as a Class I device per the MDD, and is not provided sterile, then the manufacturer can affix the CE mark to its Class I devices by means of a self-declaration only, without the need for approval by the EEA Notified Body.
On October 1, 2016, we obtained ISO 13485 certification for our quality management system. In addition, in September 2017 we received CE mark certification for our Prizma device, and in November 2017 we received CE mark certification for the Extended Holter Patch System. CE certification allows the devices to be marketed and sold in all the member states of the EEA as well as in certain other countries worldwide. (The CE certification ended on January 9, 2022. From May 2022 we will have to comply with the Medical Device Regulation and not Medical Device Directive.)
In addition, in November 2017, we obtained regulatory clearance (TGA) to market the Prizma device in Australia. In April 2020, we received Taiwan FDA approval to market the Prizma device.
To the extent that in the future we seek to market our products outside of countries in the EEC, we may be required to comply with the applicable regulatory requirements in each such country. Such regulatory requirements vary by country and may be tedious. As a result, no assurance can be given that we will be able to satisfy the regulatory requirements to market or sell our products in any such country.
Registration of Medical Devices in China
Registration of medical devices in China is handled and approved by the NMPA. In order to apply for NMPA registration for a medical device, a non-Chinese manufacturer is required to appoint a local Chinese agent who will coordinate the NMPA device registration and assist in determining the classification of the medical device in China, using NMPA Order No. 15 and the NMPA’s classification database. Class II and III device manufacturers should also identify predicates and determine the clinical data requirements for their device and how to satisfy them. Our products fall under Class II (non-invasive) devices.
64
Registration in China may be a longer process than registration in other countries, as the NMPA does usually not recognize a clinical trial or device testing performed outside of China. As a result, a clinical trial and/or device testing is usually required to be performed or repeated in China. In addition, currently all regulatory documentation needs to be submitted in Chinese.
In February 2018, our subsidiary Guangzhou Yimei Innovative Medical Science and Technology Co., Ltd. was granted acceptance to the Green Channel expedited Guangdong Provincial NMPA regulatory approval process for the Prizma medial smartphone case. The special review and approval procedures for innovative medical devices ensures the safety and effectiveness of products and services for the Chinese market.
Clinical Studies in the United States
Even when a clinical study has an approved Investigational Device Exemption from the U.S. FDA under significant risk determination, and has been approved by an Institutional Review Board under non-significant risk determination and/or has been approved by a local or regional Ethics Committee, the study is subject to factors beyond a manufacturer’s control, including, but not limited to the fact that the institutional review board at a given clinical site might not approve the study, might decline to renew approval (required annually), or might suspend or terminate the study before the study has been completed. There is no assurance that a clinical study at any given site will progress as anticipated; the interim results of a study may not be satisfactory leading the sponsor or others to terminate the study, there may be an insufficient number of patients who qualify for the study or who agree to participate in the study, or the investigator at the site may have priorities other than the study. Also, there can be no assurance that the clinical study will provide sufficient evidence to assure the U.S. FDA that the product is safe, effective and performs as intended as a prerequisite for granting market clearance.
Post-Clearance Matters
Even if the U.S. FDA or other similar non-U.S. regulatory agencies clears or approves a medical device for use, the regulatory agency may limit the intended uses of the device in such a way that manufacturing and distributing the device may not be commercially feasible. After clearance or approval to market is given, the U.S. FDA and similar foreign regulatory agencies, upon the occurrence of certain events, are authorized under various circumstances to withdraw the clearance or approval or require changes to a device, its manufacturing process or its labeling or additional proof that regulatory requirements have been met.
In the United States, a manufacturer of a device approved through the premarket approval application process is not permitted to make changes to the device which affects its safety or effectiveness without first submitting a supplement application to its premarket approval application and obtaining U.S. FDA clearance for that supplement. In some instances, the U.S. FDA may require a clinical trial to support a supplement application.
A manufacturer of a device cleared through a 510(k) submission or a 510(k)+ “de-novo” submission must submit another premarket notification if it intends to make a change or modification in the device that could significantly affect the safety or effectiveness of the device, such as a significant change or modification in design, material, chemical composition, energy source or manufacturing process.
Any change in the intended uses of a premarket approval application device or a 510(k) device requires an approval supplement or cleared premarket notification. Exported devices are subject to the regulatory requirements of each country to which the device is exported, as well as certain U.S. FDA export requirements.
65
Mobile Medical Applications Guidance in the United States
On February 9, 2015, the U.S. FDA issued final guidance for developers of mobile medical applications, or apps, which are software programs that run on mobile communication devices and perform the same functions as traditional medical devices. The guidance outlines the U.S. FDA’s tailored approach to mobile apps. The U.S. FDA plans to exercise enforcement discretion (meaning it may choose not to enforce all requirements under the FDC Act) for the majority of mobile apps, as they pose minimal risk to consumers. The U.S. FDA currently plans to focus its regulatory oversight on a subset of mobile medical apps that present a greater risk to patients if they do not work as intended, such as mobile medical apps that:
| ● | are intended to be used as an accessory to a regulated medical device - for example, an application that allows a health care professional to make a specific diagnosis by viewing a medical image from a picture archiving and communication system on a smart mobile device or a mobile tablet; or |
| ● | transform a mobile platform into a regulated medical device - for example, an application that turns a smart mobile device into an ECG machine to detect abnormal heart rhythms or determine if a patient is experiencing a heart attack. |
While some features of the Prizma device are classified by the U.S. FDA as medical functions and require regulatory clearance by the U.S. FDA, other features may be classified as wellness functions following the guidelines set out in U.S. FDA Guidance document, “Multiple Function Products,” dated April 27, 2018 (draft guidance at this stage).
Ongoing Regulation by the U.S. FDA
Even after a device receives clearance or approval and is placed on the market, numerous regulatory requirements apply. These include:
| ● | establishment registration and device listing; |
| ● | quality system regulation, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all phases of the product life-cycle; |
| ● | labeling regulations and U.S. FDA prohibitions against the promotion of products for uncleared, unapproved or “off-label” uses, and other requirements related to promotional activities; |
| ● | medical device reporting regulations, which require that manufacturers report to the U.S. FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur; |
| ● | corrections and removals reporting regulations, which require that manufacturers report to the U.S. FDA field corrections and product recalls or removals if undertaken to reduce a health risk posed by the device or to remedy a violation of the FDC Act that may present a health risk; and |
| ● | post-market surveillance regulations, which apply when necessary to protect the health of the general public or to provide additional safety and effectiveness data for the device. |
Failure to comply with applicable regulatory requirements can result in enforcement action by the U.S. FDA, which may include any of the following sanctions: fines, injunctions, civil or criminal penalties, recall or seizure of our current or future products, operating restrictions, partial suspension or total shutdown of production, refusing our request for 510(k) clearance or PMA approval of new products, rescinding previously granted 510(k) clearances or withdrawing previously granted PMA approvals.
We may be subject to announced and unannounced inspections by the U.S. FDA, and these inspections may include the manufacturing facilities of our subcontractors. If, as a result of these inspections, the U.S. FDA determines that our or our subcontractors’ equipment, facilities, laboratories or processes do not comply with applicable U.S. FDA regulations and conditions of product clearance, the U.S. FDA may seek civil, criminal or administrative sanctions and/or remedies against us, including the suspension of our manufacturing and selling operations.
66
Ongoing Regulation by International Regulators
International sales of medical devices are subject to foreign government regulations, which may vary substantially from country to country.
In order to maintain the right to affix the CE mark to market medical devices in the EEA, the notified body needs to perform an annual surveillance audit of a company’s premises and, if needed, also of the premises of critical subcontractors of the manufacturer.
Additionally, the EU Directives dictate the following requirements:
| ● | vigilance system, which requires the manufacturer to immediately notify the relevant competent authority when a company product has been involved in an incident that (i) led to death, a serious injury, or serious deterioration in the state of health of a patient, user or other, third-party; or (ii) may have led to death, serious injury or serious deterioration in the state of health of a patient, user or other, third-party; and |
| ● | post-market surveillance including a documented procedure to review experience gained from medical devices on the market and to implement any necessary corrective action that would be commensurate with the nature and risks involved with the given product. |
Failure to comply with applicable regulatory requirements can result in enforcement action by the regulatory agency, which may include any of the following sanctions: fines, injunctions, civil or criminal penalties, recall or seizure of our current or future products, shutting down all or certain of our services, operating restrictions, partial suspension or total shutdown of production, refusing our request for renewing clearance and/or registration of our products or granting clearance/registration for new products.
State Licensure Requirements
Several U.S. states require that Durable Medical Equipment (or DME) providers should be licensed in order to sell products to patients in that state. Certain of these states require that DME providers maintain an in-state location. If these rules are determined to be applicable to us and if we were found to be noncompliant, we could lose our licensure in that state, which could prohibit us from selling our current or future products to patients in that state.
Federal Anti-Kickback and Self-Referral Laws
The U.S. Federal Anti-Kickback Statute prohibits the knowing and willful offer, payment, solicitation or receipt of any form of remuneration in return for, or to induce the:
| ● | referral of a person; |
| ● | furnishing or arranging for the furnishing of items or services reimbursable under Medicare, Medicaid or other governmental programs; or |
| ● | purchase, lease, or order of, or the arrangement or recommendation of the purchasing, leasing, or ordering of any item or service reimbursable under Medicare, Medicaid or other governmental programs. |
To the extent we are required to comply with these regulations, it is possible that regulatory agencies could allege that we have not complied, which could subject us to certain sanctions. Noncompliance with the federal anti-kickback legislation can result in exclusion from Medicare, Medicaid or other governmental programs, restrictions on our ability to operate in certain jurisdictions, as well as civil and criminal penalties, any of which could have an adverse effect on our business and results of operations.
67
Federal law also includes a provision commonly known as the “Stark Law,” which prohibits a physician from referring Medicare or Medicaid patients to an entity providing “designated health services,” including a company that furnishes durable medical equipment, in which the physician has an ownership or investment interest or with which the physician has entered into a compensation arrangement. Violation of the Stark Law could result in denial of payment, disgorgement of reimbursements received under a noncompliant arrangement, civil penalties, and exclusion from Medicare, Medicaid or other governmental programs.
Federal False Claims Act (or the FCA)
The FCA provides, in part, that the federal government may bring a lawsuit against any person whom it believes has knowingly presented, or caused to be presented, a false or fraudulent request for payment from the federal government, or who has made a false statement or used a false record to get a claim approved. In addition, amendments in 1986 to the FCA have made it easier for private parties to bring “qui tam” whistleblower lawsuits against companies. Penalties include civil penalties ranging from $11,463 to $22,927 for each false claim, subject to yearly inflation adjustment, plus three times the amount of damages that the federal government sustained because of the act of that person.
Civil Monetary Penalties Law
The U.S. Federal Civil Monetary Penalties Law prohibits the offering or transferring of remuneration to a Medicare or Medicaid beneficiary that the person knows or should know is likely to influence the beneficiary’s selection of a particular supplier of Medicare or Medicaid payable items or services. Noncompliance can result in civil monetary penalties of up to $10,000, subject to inflation adjustment, for each wrongful act, assessment of three times the amount claimed for each item or service and exclusion from the Federal healthcare programs.
State Fraud and Abuse Provisions
Many states have also adopted some form of anti-kickback and anti-referral laws and false claims acts. A determination of liability under such laws could result in fines and penalties and restrictions on our ability to operate in these jurisdictions.
Administrative Simplification of the Health Insurance Portability and Accountability Act of 1996
HIPAA mandated the adoption of standards for the exchange of electronic health information in an effort to encourage overall administrative simplification and enhance the effectiveness and efficiency of the healthcare industry. Ensuring privacy and security of patient information is one of the key factors driving the legislation.
Clinical Trials
Clinical trials are generally required to support a premarket approval application and are sometimes required for 510(k) clearance, CE marking and NMPA approval.
We have undertaken clinical trials and performance tests in Israel and China in order to validate the accuracy, quality of measurements and usability of our products and services. The results from the trials and performance tests have already been used to support the regulatory submissions for CE marking for the Prizma device. In the future, we intend to use clinical trial data with respect to obtaining the CE mark for our VSMS and for NMPA approval in relation to the Prizma device and Vital Signs System. Results from the clinical trials are not required by us to obtain U.S. FDA clearance through the 510(k) process.
Israel Clinical Trial
We completed clinical evaluation and performance tests in Israel, which consisted of measuring temperature, ECG, oxygen saturation, stress level and heart rate using the Prizma device and Vital Signs System.
A total of 32 patients were recruited for the trial during their hospitalization period at the Assaf Harofeh University Medical Center in Israel. During the trial, measurements were taken three times a day from each participant throughout the duration of hospitalization (10 days). These measurements were taken using our devices, and in parallel were taken with the hospital’s approved equipment as a benchmark.
68
The results of the trial demonstrate that the measurements taken with our Prizma device and Vital Signs System fall within NMPA approval and CE “pass” criteria, meaning that the results taken from our devices fell within the acceptance criteria set out by the relevant technical standards for both safety and accuracy in each of the measurement parameters.
China Clinical Trial
We completed a clinical trial at a NMPA and U.S. FDA certified laboratory in China measuring oxygen saturation (Spo2). The purpose of the trial was to compare the quality and accuracy of the Prizma device to the laboratory’s approved equipment as a benchmark.
A total of 16 patients were recruited for the trial who fell within the testing criteria, which required healthy subjects capable of undergoing controlled hypoxemia (low oxygen saturation). During the trial, participants were subjected to controlled decreases of oxygen saturation in their blood, with 24 separate samples taken from each participant to measure the oxygen saturation using the Prizma device. The trial was conducted by taking a blood test from patients to measure their oxygen saturation, while also taking measurements of oxygen saturation using the Prizma device and a reference gold standard device approved by the NMPA and U.S. FDA that is currently used in hospitals to measure oxygen saturation.
The results of the trial demonstrate that the Prizma oxygen saturation measurements fall within the standards “pass” criteria in relation to the measurement taken from the laboratory’s standard oxygen saturation measurement devices, meaning that the mean deviation from the reference device did not exceed 1.3%. The results from this trial were part of our application for NMPA approval.
Future Clinical Trial
We are starting a clinical trial in Israel to test the adhesive quality and signal absorption performance of the VSMS Extended Holter Patch. The trial is being conducted by a well-known dermatologist and a team of investigators and will involve 30 recruits.
C. Organizational Structure
We have 13 wholly-owned subsidiaries: G Medical Innovations Ltd., G Medical Innovations USA Inc., G Medical Health and Wellness, Inc, G Medical Health and Wellness Lab, Inc., G Medical Innovations MK Ltd., G Medical Innovations Asia Limited, G Medical Diagnostic Services, Inc, G Medical Mobile Health Solutions, Inc, Telerhythmics LLC, G Medical Tests and Services, Inc, G Medical Lab Services, Inc., G Medical Innovations UK Ltd., and Guangzhou Yimei Innovative Medical Science and Technology Co., Ltd.
In addition, G Medical Tests and Services Inc. holds 80% of G Medical Lab Services, Inc. G Medical Innovations Asia Limited wholly-owns two subsidiaries: G Medical Innovations UK Ltd and 70% of Guangzhou Yimei Innovative Medical Science and Technology Co., Ltd.
69
A diagram of our organizational structure as of the date of this annual report is below:
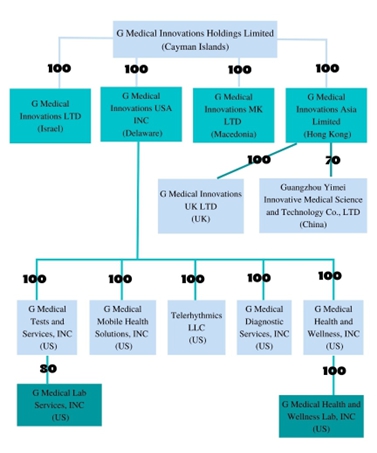
D. Property, Plant and Equipment
Our corporate headquarters is located at 5 Oppenheimer St., Rehovot 7670105, Israel, where we currently occupy approximately 3,229 square feet. We lease our facilities and our lease ends on December 31, 2023. Our current monthly rent payment is NIS 20,074 (approximately $5,900). In the United States, our primary facility is located at 12708 Riata Vista Cir Ste A-103, Austin, TX 78727, where we currently occupy approximately 3,202 square feet. We lease our facilities, and the monthly rent payment is $6,537. Our lease ends on April 30, 2026.
We consider that our current office space is sufficient to meet our anticipated needs for the foreseeable future and is suitable for the conduct of our business.
ITEM 4A. UNRESOLVED STAFF COMMENTS
Not applicable.
70
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
A. Operating Results.
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the related notes included elsewhere in this annual report on Form 20-F. The discussion below contains forward-looking statements that are based upon our current expectations and are subject to uncertainty and changes in circumstances. Actual results may differ materially from these expectations due to inaccurate assumptions and known or unknown risks and uncertainties, including those identified in “Cautionary Note Regarding Forward-Looking Statements” and under “Risk Factors” elsewhere in this annual report on Form 20-F. Our discussion and analysis for the year ended December 31, 2021 compared to the year ended December 31, 2020 can be found in our annual report on Form 20-F for the fiscal year ended December 31, 2021, filed with the SEC on April 29, 2022.
Overview
We are a mHealth and digital health company that develops and markets clinical and consumer medical-grade health monitoring solutions and offers end-to-end support for remote monitoring and telemedicine projects. With extensive experience in the field of medical devices, digital health and patients monitoring, we are committed to raising the global level of healthcare by empowering caregivers and patients to better monitor, manage and improve clinical and personal health outcomes. We believe that we are at the forefront of the digital health revolution in developing the next generation of mobile technologies and services that are designed to empower consumers, patients, and providers to better monitor, manage and improve clinical and personal health outcomes, especially for those who suffer from CVD, pulmonary disease and diabetes. Our business and future revenue stream can be broken down into two business areas: monitoring services and home collection kits for lab testing.
Monitoring Services
We possess innovative MCT monitors purchased from third parties that utilize sophisticated Deep Neural Network based Artificial Intelligence (AI) backend ECG processing software—Cardiologs, which we use as a powerful secondary analysis tool once the monitoring session ends. This dual analysis approach is not offered by most of our competitors. Further, we have two CMS-approved IDTFs, each staffed with experienced clinical staff and billing teams, nationwide insurance contracts, and a full portfolio of diagnostic monitoring tools, including the G-Patch devices for extended holter (AECG), the Spider device for mobile cardiac telemetry (MCT), and the Prizma device for remote patient monitoring (RPM).
As medicine and technology become integrated, we believe we are well positioned to capture market share and grow in this business area. Our product line consists of our Prizma medical device, a clinical grade device that can transform a smartphone into a medical monitoring device, enabling both healthcare providers and individuals to monitor, manage and share a wide range of vital signs and biometric indicators. In addition, we are developing a Wireless Vital Signs Monitoring System, which is expected to provide full, continuous, and real time monitoring of a wide range of vital signs and biometrics. The monitoring services include IDTF monitoring services and private monitoring services.
Home Collection Kits for Lab Testing
In the second half of 2022, we took a strategic decision to divert to more comprehensive home testing solutions as an expansion of our patient services and as part of our vision to move towards a home-based health care system. On July 13, 2022, we announced that our wholly owned subsidiary, G Medical Health and Wellness, Inc., has developed seven different at-home tests to collect samples of blood, saliva, stool, urine or a vaginal swab each of which we expect will be available to be purchased privately by the user in a retail store or online. Once purchased, the user will be able to collect the sample in the privacy and comfort of his or her home and send the sample to our CLIA certified lab for analysis. Once it arrives at the lab, the sample will be analyzed, and results typically will be available to the customer within days in our secured and HIPAA compliant portal. In addition, on October 6, 2022, we introduced a Monkeypox consumer home health test kit, along with 23 additional new direct to consumer home collection kits with 24 to 48 hours results, all of which are expected to be available to consumers the third quarter of 2023 through popular big-box retailers, pharmacies and online. The collection kits are developed for testing health issues related to hormones, sexual transmitted disease, nutrition, colon cancer, food sensitivity, and allergies, with results going directly to the user within days. The entire data, from both vital signs and laboratory tests, is collected, and managed under one database and give the user the ability to create, manage and control their EMR, with the ability to retrieve and share it with third parties anywhere and anytime.
71
Our management team is led by individuals with over 25 years of experience in developing mobile embedded medical sensors and software, algorithms and ambulatory medical devices, and with extensive experience filing for, and obtaining approval from, the FDA for medical devices, including devices approved when the members of our management team were employed at other companies. Our management has proven their ability to execute our go-to-market strategy as described below, with over 25 years of medical device development and commercialization experience in the United States, China, Europe, Australia, South Africa, Japan, the Asia Pacific region and Brazil.
Results of Operations
Revenues and Cost of Revenues
Our total revenue consists of services and sale of products and our cost of revenues consists of cost of services and cost of products. Currently, vast majority of our business activity is in the USA. As such, most of our revenues are from services and Current Procedural Terminology (or CPT) reimbursements from our medical call centers (IDTF). Those includes revenues from our cardiac monitoring services as MCT, CEM, Extended Holters and Holters services.
The following table discloses the breakdown of revenues and costs of revenues:
| Year Ended December 31, | ||||||||
| U.S. dollars in thousands, except share and per share data | 2022 | 2021 | ||||||
| Revenues | ||||||||
| Services | 4,404 | 4,911 | ||||||
| Products | 16 | 50 | ||||||
| Total revenues | 4,420 | 4,961 | ||||||
| Cost of revenues | ||||||||
| Cost of services | 3,523 | 3,386 | ||||||
| Cost of sales of products | 113 | 66 | ||||||
| Total cost of revenues | 3,636 | 3,452 | ||||||
| Gross profit (loss) | 784 | 1,509 | ||||||
Operating Expenses
Our current operating expenses consist of two components - research and development expenses, and selling, general and administrative expenses.
Our research and development expenses consist primarily of salaries and related personnel expenses, subcontractor’s expenses and other related research and development expenses.
Selling, general and administrative expenses consist primarily of salaries and related expenses, share based compensation, professional service fees for accounting, legal and bookkeeping, facilities, travel expenses and other general and administrative expenses.
72
Comparison of the Year Ended December 31, 2022 and the Year Ended December 31, 2021
The table below provides our results of operations for the years ended December 31, 2022 and 2021.
| Year Ended December 31, | ||||||||
| U.S. dollars in thousands | 2022 | 2021 | ||||||
| Revenues | ||||||||
| Services | 4,404 | 4,911 | ||||||
| Products | 16 | 50 | ||||||
| Total revenues | 4,420 | 4,961 | ||||||
| Cost of revenues | ||||||||
| Cost of services | 3,523 | 3,386 | ||||||
| Cost of sales of products | 113 | 66 | ||||||
| Total cost of revenues | 3,636 | 3,452 | ||||||
| Gross profit (loss) | 784 | 1,509 | ||||||
| Operating expenses: | ||||||||
| Research and development expenses | 2,292 | 1,680 | ||||||
| Selling, general and administrative expenses | 20,605 | 10,797 | ||||||
| Operating loss | 22,113 | 10,968 | ||||||
| Financial income (expenses), net | 8,171 | (3,622 | ) | |||||
| Loss before taxes on income | 13,942 | 14,590 | ||||||
| Income tax expense (benefit) | 16 | (3 | ) | |||||
| Loss for the year from continuing operation | 13,958 | 14,587 | ||||||
| Loss from discontinued operations | 11,108 | 301 | ||||||
| Total loss for the year | 25,066 | 14,888 | ||||||
Revenues and Cost of Revenues
Our total revenues for the year ended December 31, 2022 amounted to $4,420 thousand, which consists primarily of services (approximately 99% of total revenues), representing a decrease of $541 thousand or 11%, compared to $4,961 thousand for the year ended December 31, 2021.
Our cost of revenues for the year ended December 31, 2022 amounted to $3,636 thousand, which consists primarily of cost of services (96% of cost of revenues), representing an increase of $184 thousand, or 5.3%, compared to $3,452 thousand for the year ended December 31, 2021. The increase of cost of revenues was mainly related to an increase in payroll and related expenses.
Research and Development Expenses
Our research and development expenses for the year ended December 31, 2022 amounted to $2,292 thousand representing an increase of $612 thousand, or 36%, compared to $1,680 thousand, for the year ended December 31, 2021. The increase was primarily attributable to increase in subcontractors’ expenses, payroll and related expenses.
Selling, General and Administrative Expenses
Our selling, general and administrative expenses for the year ended December 31, 2022 amounted to $20,605 thousand, representing an increase of $9,808 thousand, or 91%, compared to $10,797 thousand for the year ended December 31, 2021. The increase was primarily attributable to an increase in (i) share-based compensation expenses; (ii) shares issuance costs; and (iii) payroll and related expenses in 2022 compared to 2021.
73
Operating loss
As a result of the foregoing, our operating loss for the year ended December 31, 2022 amounted to $22,113 thousand compared to an operating loss of $10,968 thousand, for the year ended December 31, 2021, an increase of $11,145 thousand or 102%. The increase reflects the increase in selling, general and administrative expenses, and the increase in research and development expenses for 2022 compared to 2021.
Financial Expense and Income
Financial expense and income consist of interest, bank fees, revaluation of the derivative liability and exchange rate differences.
We recognized net financial income for the year ended December 31, 2022 of $8,171 thousand compared to net financial expenses of $3,622 thousand for the year ended December 31, 2021. The increase was primarily attributable to increase in financials expenses resulted from derivative liabilities – warrants revaluation.
Loss for the year from continuing operations
As a result of the foregoing, our total loss from continuing operations for the year ended December 31, 2022 was $13,958 thousand, compared to $14,587 thousand for the year ended December 31, 2021, a decrease of $629 thousand, or 4%.
B. Liquidity and Capital Resources
As of December 31, 2022, we had $295 thousand in cash and cash equivalents.
We expect that our sources of liquidity for the remainder of 2023 will be fund raisings and a commitment from our Chief Executive Officer (who is our major shareholder) to continue and support our ongoing operation for the foreseeable future (if other sources of funding are not available to us and under certain conditions).
The table below presents our cash flows for the periods indicated:
| Year Ended December 31, | ||||||||
| U.S. dollars in thousands | 2022 | 2021 | ||||||
| Operating activities | (18,479 | ) | (11,494 | ) | ||||
| Investing activities | (2,123 | ) | (17 | ) | ||||
| Financing activities | 14,863 | 17,267 | ||||||
| Net increase (decrease) in cash and cash equivalents | (5,739 | ) | 5,756 | |||||
Cash Flows Used in Operating Activities
Net cash used in operating activities was $18,479 thousand, during the year ended December 31, 2022, compared to net cash used in operating activities of $11,494 thousand during the year ended December 31, 2021, primarily resulted from (i) an increase in trade payables in the amount of $2,323 thousand during 2022 compared to decrease in trade payables of $1,736 thousand during 2021, (ii) an increase in net loss during 2022 compared to 2021 of $10,178 thousand, (iii) an increase in equity-based compensation expenses of $11,840 thousand compared to 2021, (iv) a decrease in derivatives in the amount of $10,232 thousand during 2022 compared to decrease in derivatives of $311 thousand during 2021, and (v) a decrease in fair value of convertible loans in the amount of $1,321 thousand during 2022 compared to increase in fair value of convertible securities of $3,677 thousand during 2021.
74
Cash Flows Used in Investing Activities
Net cash used in investing activities was $2,123 thousand during the year ended December 31, 2022, compared to net cash used in investing activities of $17 thousand during the year ended December 31, 2021, primarily resulted from purchase of property, plant and equipment of $2,121 thousand during 2022 compared to $550 thousand during 2021.
Cash Flows Provided by Financing Activities
Net cash provided by financing activities was $14,863 thousand for the year ended December 31, 2022, compared to net cash provided by financing activities of $17,267 thousand for the year ended December 31, 2021. The decrease in net cash provided by financing activities was primarily resulted from (i) issuance of shares and warrants in the amount of $3,442 thousand during 2022 compared to issuance of shares and warrants in the amount of $13,417 thousand during 2021, (ii) issuance of convertible securities and warrants in the amount of $14,851 thousand during 2022 compared to $5,750 thousand during 2021 and (iii) repayment of convertible securities and financial liability of $5,921 thousand during 2022 compared to$536 thousand during 2021.
Current Outlook
To date, we have not achieved profitability and have sustained net losses in every fiscal year since our inception, and we have financed our operations primarily through proceeds from issuance of our Ordinary Shares and warrants, loans from our controlling shareholder and other convertible debenture. We expect to generate revenues from our existing business activities and incur costs of revenues related to those activities.
We expect to generate revenues from the sale of our products and other revenues in the future. However, we do not expect these revenues to support all of our operations in the near future. We expect our expenses to increase in connection with our activities, particularly as we continue the development of our products, and continue our commercialization efforts. Accordingly, we expect that we will require substantial additional funding in connection with the growth of our operations, continuing our research and development activity, commercializing our products and to proceed with pilot projects that partly will have to be financed by us in order to penetrate relevant markets. Until we can generate significant recurring revenues and achieve profitability, we will need to seek additional sources of funds through the sale of additional equity securities, debt or other securities. However, there is no assurance that we will be successful accomplishing these plans. Any required additional capital, whether forecasted or not, may not be available on reasonable terms, or at all. If we are unable to obtain additional financing or are unsuccessful in commercializing our products and securing sufficient funding, we may be required to reduce activities, curtail or even cease operations. We expect to continue incurring losses and negative cash flows from operations until our products reach profitability.
Financial Arrangements
In April 2020, we entered into a loan agreement (or the PPP Loan) with Bank of America, NA pursuant to the Paycheck Protection Program under the Coronavirus Aid, Relief, and Economic Security Act (the Cares Act). The PPP Loan provided us with $873,487 and required no collateral or personal guarantees. We applied for Forgiveness for the PPP Loan and on April 3, 2021, we received approval for a full forgiveness from the SBA and the loan was fully paid from SBA to Bank of America. We have used all of the $873,487, extended to us under the PPP Loan, for business-related purposes, such as to retain workers and maintain payroll, make lease and utility payments.
75
In August 2020, we secured firm commitments from institutional and professional investors to raise A$5,000,000 through the issue of 31,746 fully paid Ordinary Shares in our company at an issue price of A$157 per Ordinary Share (or the Placement). We engaged MST Financial as sole lead manager and bookrunner to the Placement. On August 13, 2020, we issued the 31,746 fully paid Ordinary Shares to the investors pursuant to the Placement.
On September 27, 2020, ASX resolved to remove our company from the Official List of ASX following the approval by our shareholders of such voluntary delisting on September 21, 2020 in accordance with ASX Listing Rule 17.11. On October 22, 2020, our Ordinary Shares were delisted from the ASX.
On December 21, 2020, we entered into a transaction (or the December 2020 CLA Transaction), whereby we entered into a securities purchase agreement, collectively with the documents ancillary thereto, including convertible debentures and warrants to purchase our Ordinary Shares, with Alpha Capital Anstalt (or Alpha), pursuant to which we obtained a convertible loan in an aggregate amount of $350,000, against issuance of convertible debentures (or the December 2020 Financing Debentures), and warrants to purchase 2,277 Ordinary Shares (or the December 2020 Financing Warrants). The December 2020 Financing Debentures had a six-month term from issuance and bared interest at 10% per annum. The December 2020 Financing Debentures were convertible into our Ordinary Shares at a conversion price equal to 80% of the public offering price per share in our initial public offering. The foregoing debentures and interest have been repaid in full.
Alpha was also granted a 12-month participation right in a future financing equal to 50% of the subsequent financing. Alpha was also provided a right to purchase $150,000 of additional debentures on the same terms for a period of six months from the date of the December 2020 CLA Transaction. On February 17, 2021, Alpha exercised the foregoing right to purchase $150,000, against issuance of additional convertible debentures (or the February 2021 Financing Debentures) and warrants to purchase 976 Ordinary Shares (or the February 2021 Financing Warrants), on the same terms as the December 2020 CLA Transaction. The foregoing debentures and interest have been repaid in full.
The December 2020 Financing Warrants and the February 2021 Financing Warrants had an exercise price per share of $1.50 per share. The December 2020 Financing Warrants and the February 2021 Financing Warrants have a five year term and will be exercisable for cash or on a cashless basis if no registration statement is available for resale of the Ordinary Shares issuable upon exercise of the December 2020 Financing Warrants and the February 2021 Financing Warrants.
On April 7, 2021, we entered into a transaction (or the April CLA Transaction), whereby we entered into a securities purchase agreement, collectively with the documents ancillary thereto, including convertible debentures and warrants to purchase our Ordinary Shares, with Jonathan B. Rubini, pursuant to which we obtained a convertible loan in an aggregate amount of $600,000, against issuance of convertible debentures (or the April 2021 Financing Debentures) and warrants to purchase 3,903 Ordinary Shares (or the April 2021 Financing Warrants). The April 2021 Financing Debentures have a six-month term from issuance and bear interest at 10% per annum until October 7, 2021. The April 2021 Financing Debentures are convertible into our Ordinary Shares at a conversion price equal to $140. As of October 7, 2021 the interest was increased to 12% per annum until April 2022 and then the interest rate shall increase to 16% per annum until a repayment date of October 7, 2023. In October 2022, we agreed inter alia, to amend the 10% convertible debenture, originally dated April 7, 2021, as amended and restated on June 1, 2022, such that the applicable interest rate is 18% per annum with effect from October 1, 2022 until the extended maturity date of October 7, 2023 and to amend the conversion price adjustment date to October 7, 2023. On April 18, 2023, we repaid in full the principal and accrued interest of the April 2021 Convertible Debentures, which amounted to $759,576.
76
The April 2021 Financing Warrants have an exercise price per share equal to the per share price of our Ordinary Shares in our next equity financing of at least $10,000,000, including without limitation, an initial public offering, subject to standard adjustments. The April 2021 Financing Warrants have a five-year term and will be exercisable for cash or on a cashless basis if no registration statement is available for resale of the Ordinary Shares issuable upon exercise of the April 2021 Financing Warrants. Jonathan B. Rubini was also granted a 12-month participation right in a future financing equal to 50% of the subsequent financing.
In June 2021, we completed our underwritten initial public offering of 85,714 units, each consisting of one Ordinary Share and one warrant to purchase one Ordinary Share, at a combined public offering price of $175 per unit for aggregate gross proceeds of approximately $15.0 million, prior to deducting underwriting discounts, commissions, and other offering expenses. In addition, and together with the closing of the offering, the underwriter partially exercised its over-allotment offering with respect to warrants to purchase 12,858 Ordinary Shares. Such warrants were issued at an offering price of $0.35 and have an exercise price of $218.75.
In December 2021, we entered into a securities purchase agreement relating to the purchase and sale of a senior convertible note (or the December 2021 Note) for gross proceeds of $5,000,000 (or the Lind Purchase Agreement) with Lind Global Fund II LP (or Lind Global). The Lind Purchase Agreement and December 2021 Note provides for, among other things, the issuance of the December 2021 Note with a $5,800,000 face value, with a 24-month maturity, and a fixed conversion price of $122.5 per share (or December 2021 Note Conversion Price) of the Company’s Ordinary Shares. We are required to make principal payments in 20 equal monthly installments commencing 120 days after funding (or the December 2021 Note Repayment). At our discretion, the December 2021 Note Repayments can be made in: (i) cash; (ii) Ordinary Shares (after Ordinary Shares are registered) (or the Repayment Shares); or a combination of both. Repayment Shares will be priced at 90% of the average of the five lowest daily volume weighted average price (VWAPs) during the 20 trading days prior to the payment date (or the December 2021 Note Repayment Price). We have the right to buy-back the outstanding face value of the December 2021 Note at any time with no penalty (or the Buy-Back Right). Should We exercise our Buy-Back Right, Lind will have the option to convert up to 25% of the face value of the December 2021 Note at the lesser of the Conversion Price or Repayment Price. Additionally, the December 2021 Note ranks senior to other of the Company debt.
Further, pursuant to the Lind Purchase Agreement, we issued to Lind a warrant to purchase up to 32,766 Ordinary Shares (or the December 2021 Warrant). The December 2021 Warrant may be exercisable with cash payment for 60 months with an exercise price of $122.5 per Ordinary Share and may be exercised on a cashless basis at any time after the earlier of (a) 120 days following the issuing date or (b) in the event that a registration statement covering the underlying Ordinary Shares is not deemed effective. In addition, at any time prior to December 1, 2022, subject to the mutual agreement the parties may carry out a second closing for an additional $5,000,000.
In February 2022, we repaid $2,541,000 to Lind, representing $2,420,000 of the face value of the December 2021 Note, pursuant to the Lind Purchase Agreement, which required us to repay 20% of the gross proceeds received by us from the transaction contemplated by the January 2022 SPA described below, plus a 5% premium.
In January 2022, we entered into a definitive securities purchase agreement (or the January 2022 SPA) with an institutional investor for the issuance, in a private placement, of an aggregate of 68,571 Ordinary Shares par value $3.15 per share, or pre-funded warrants in lieu thereof (or each, a January 2022 Pre-Funded Warrant) and warrants (or the January 2022 Ordinary Warrants) to purchase up to an aggregate of 68,572 Ordinary Shares, at a purchase price of $175.00 per Ordinary Share (or Pre-Funded Warrant) and associated warrant (or the January 2022 Offering). The January 2022 Ordinary Warrants have an exercise price of $175.00 per share, were exercisable immediately upon issuance and have a term of five years. The Pre-Funded Warrants, and the associated Ordinary Warrant, were sold at a price of $175.00 each, including the Pre-Funded Warrant exercise price of $0.0035 per Ordinary Share. The Pre-Funded Warrants are exercisable at any time after the date of issuance upon payment of the exercise price. The Company also entered into an agreement (or the January 2022 Placement Agency Agreement) with A.G.P./Alliance Global Partners (or A.G.P.), as sole placement agent, dated January 30, 2020, pursuant to which A.G.P. agreed to serve as the placement agent for the Company in connection with the Offering. The Company agreed to pay the Placement Agent a cash placement fee equal to 7% of the gross proceeds received in the Offering. In addition, the Company agreed to issue A.G.P. warrants (or the January 2022 Placement Agent Warrants) to purchase 3,435 Ordinary Shares, or 5% of the Ordinary Shares (or Pre-Funded Warrant equivalents) issued in the Offering. The Placement Agent Warrants are exercisable six months after their issuance, are exercisable for five years from their initial exercisability and have an exercise price of $175.00 per share.
77
In February 2022, we entered into a definitive securities purchase agreement with Lind Global, which exercised its right of participation in the January 2022 SPA (or the Lind SPA), for the issuance, in a private placement, of an aggregate of 571 Ordinary Shares par value $3.15 per share and Ordinary Warrants to purchase up to an aggregate of 572 Ordinary Shares, at a purchase price of $175.00 per Ordinary Share and associated warrant. The terms of the Lind SPA are substantially similar to the terms of the January 2022 SPA, and the terms of the Ordinary Warrants issued pursuant to the Lind SPA are substantially similar to the terms of the warrants issued pursuant to the January 2022 SPA.
In April 2022, we entered into a definitive securities purchase agreement (or the April 2022 SPA) with an institutional investor for the issuance, in a private placement, of an aggregate of 142,857 Ordinary Shares par value $3.15 per share, or pre-funded warrants in lieu thereof (or each, an April 2022 Pre-Funded Warrant) and warrants (or the April 2022 Ordinary Warrants) to purchase up to an aggregate of 178,572 Ordinary Shares, at a purchase price of $52.50 per Ordinary Share (or the April 2022 Pre-Funded Warrant) and associated warrant (or the April 2022 Offering). The April 2022 Ordinary Warrants have an exercise price of $52.50 per share, were exercisable immediately upon issuance and have a term of five years. The April 2022 Pre-Funded Warrants, and the associated April 2022 Ordinary Warrant, were at a price of $52.50 each, including the April 2022 Pre-Funded Warrant exercise price of $0.0035 per Ordinary Share. The April 2022 Pre-Funded Warrants are exercisable at any time after the date of issuance upon payment of the exercise price. The Company also entered into an agreement (or the April 2022 Placement Agency Agreement) with A.G.P, as sole placement agent, dated April 18, 2022, pursuant to which the Placement Agent agreed to serve as the placement agent for the Company in connection with the April 2022 Offering. The Company agreed to pay the Placement Agent a cash placement fee equal to 7% of the gross proceeds received in the April Offering. In addition, the Company agreed to issue the Placement Agent warrants (or the April 2022 Placement Agent Warrants) to purchase 7,150 Ordinary Shares, or 5% of the Ordinary Shares (or Pre-Funded Warrant equivalents) issued in the April 2022 Offering. The April 2022 Placement Agent Warrants were exercisable six months after their issuance, are exercisable for five years from their initial exercisability and have an exercise price of $52.50 per share.
In addition, we entered into an amendment (or the Warrant Amendment) with the holder of our Ordinary Warrants to purchase up to an aggregate of 68,572 Ordinary Shares, with a purchase price of $175 per Ordinary Share pursuant to the January 2022 SPA. The Warrant Amendment modified the purchase price per Ordinary Share of the Ordinary Warrants to $52.50.
In April 2022, we entered into a definitive securities purchase agreement with Lind Global, which exercised its right of participation in the April 2022 SPA (or the April 2022 Lind SPA) for the issuance, in a private placement, of an aggregate of 9,523 Ordinary Shares par value $3.15 per share and Ordinary Warrants to purchase up to an aggregate of 11,905 Ordinary Shares, at a purchase price of $52.50 per Ordinary Share and associated warrant, for gross proceeds of approximately $500,000. The terms of the April 2022 Lind SPA are substantially similar to the terms of the April 2022 SPA, and the terms of the Ordinary Warrants issued pursuant to the April 2022 Lind SPA are substantially similar to the terms of the warrants issued pursuant to the April 2022 SPA. Also, certain warrants to purchase an aggregate of 572 Ordinary Shares of the Company that were issued to Lind Global in February 2022 have been amended to have a reduced exercise price of $52.50 per Ordinary Share.
In addition, on April 20, 2022, we repaid Lind Global $3,380,000 as repayment in full of that certain senior convertible note dated December 15, 2021.
On April 28, 2022, Dr. Yacov Geva signed an undertaking whereby Dr. Geva committed to finance the Company’s operations for a period of 12 months so long as he is the Company’s controlling shareholder, and/or the Company cannot be financed externally from any other sources, or until a sum of $10 million be received by the Company for its operations during 2022. In consideration therefor, the Company agreed to issued Dr. Geva 57,142 Ordinary Shares of the Company and an additional warrant to purchase 57,143 Ordinary Shares with an exercise price of $1.24 (the closing price of our Ordinary Shares on April 27, 2022).
78
On July 18, 2022, Armistice Capital Fund Ltd. entered into a letter agreement with us (the “Letter Agreement”). Under the terms of the Letter Agreement, in consideration for Armistice Capital Fund Ltd. agreeing to exercise $2.0 million of its existing warrants, which equals warrants to purchase 68,027 of Ordinary Shares, at a reduced exercise price of $29.4 (the “Existing Warrant Exercise”), we agreed to issue a new ordinary warrant for 264,150 warrant shares (the “New Warrant”), equal to the aggregate of (a) 85,034 warrant shares (125% of the 68,027 Ordinary Shares issued as a result of the Existing Warrant Exercise) and (b) the balance of 179,116 warrants held by Armistice Capital Fund Ltd. in the Company whose exercise price was reduced to $32.55. The 179,116 then outstanding warrants held by Armistice Capital Fund Ltd. were cancelled. The New Warrant was initially exercisable commencing 6 months following the date of the issuance thereof and has a term of exercise until April 20, 2028, and an exercise price equal to $32.55. In connection with facilitating the Existing Warrant Exercise and the issuance of the New Warrant, we and Armistice Capital Fund Ltd. also entered into an Amendment Agreement to that certain securities purchase agreement dated April 18, 2022 by and among the Company and Armistice Capital Fund Ltd. (the “Amendment Agreement”). As a result of such amendment, the exercise price of 45,242 ordinary warrants held by Lind Global Fund II LP was reduced to an exercise price equal to $32.55 and have a term of exercise until April 30, 2028.
On October 20, 2022, we entered into an agreement with Jonathan B. Rubini (the “Investor”) in connection with a private placement investment for 79,365 Ordinary Shares and warrants to purchase 79,366 Ordinary Shares with price of $6.30 per share and associated warrant, for aggregate consideration of $500,000. The warrants are exercisable at any time beginning 30 days after issuance with a term of five years from issuance. In connection with this private placement investment, the Investor and the Company agreed, inter alia, to amend the applicable interest rate and conversion price adjustment date of the April 2021 Financing Debentures.
On September 16, 2022, we entered into a sales agreement (the “Sales Agreement”) with A.G.P./Alliance Global Partners (the “Agent”) pursuant to which we could offer and sell, from time to time, our Ordinary Shares, through the Agent in an “at-the-market” offering, as defined in Rule 415(a)(4) promulgated under the Securities Act, for an aggregate offering price of up to $3.0 million. The Agent was entitled to compensation at a commission rate of 3.0% of the gross sales price per share sold pursuant to the terms of the Sales Agreement. As of January 15, 2023, sales of our Ordinary Shares under the Sales Agreement were completed and we have received net proceeds of approximately $2.9 million and have completed this “at-the-market” offering.
On October 6, 2022, Dr. Yacov Geva, committed to finance our operations for the next 12 months and until November 30, 2023, provided and as long as he continues to be a controlling shareholder and/or the Company cannot be financed externally from any other sources and/or until a sum of $10 million be received by the Company for its operations this year, whichever is earlier. In exchange, for providing this commitment, Dr. Geva was issued 71,428 Ordinary Shares and 71,429 warrants to purchase Ordinary Shares (cashless) at an exercise price of $7.70 as of the day of giving this commitment. On January 30, 2023, our Chief Executive Officer, Dr. Yacov Geva, extended his commitment to finance our operations up to February 28, 2024, which, on March 24, 2023, was further extended to April 30, 2024. On May 15, 2023, Dr. Yacov Geva extended his commitment to finance our operations up to May 30, 2024 provided and as long as the Company cannot be financed externally from any other sources and/or until a sum of $10 million be received by the Company for its operations for the year, whichever is earlier.
On December 29, 2022, the Board of Directors approved a loan agreement, dated December 21, 2022 (the “Loan Agreement”), between the Company and Dr. Geva. Under the terms of the Loan Agreement, the total amount of the loan provided by Dr. Geva to the Company is $999,552, with an annual interest rate of 12%. The following installments have been made under the terms of the Loan Agreement and advanced to the Company: $198,582 on October 9, 2022; $85,106 on October 11, 2022; $252,596 on November 10, 2022; $175,000 on December 1, 2022 (which is attributed to waived compensation to which Dr. Geva was entitled as Chief Executive Officer of the Company); and $288,268 on December 20, 2022. In addition, (i) Dr. Geva was granted 515,233 Ordinary Shares of the Company and (ii) Dr. Geva was granted 515,233 warrants to purchase Ordinary Shares of the Company with an exercise price of $1.94 per Ordinary Share.
On April 4, 2023, we closed an underwritten public offering of 5,470,000 Ordinary Shares and 6,530,000 pre-funded warrants to purchase Ordinary Shares (or each, an April 2023 Pre-Funded Warrant), each sold to the public at a price of $0.80 (inclusive of the exercise price of $0.001 of each April 2023 Pre-Funded Warrant) for aggregate gross proceeds of approximately $9,600,000 million, before deducting underwriting discounts and offering expenses. In addition, we granted the underwriters a 45-day option to purchase up to an additional 1,800,000 Ordinary Shares and/or April 2023 Pre-Funded Warrants to cover over-allotments at the public offering price, less the underwriting discount.
79
C. Research and development, patents and licenses, etc.
For a description of our research and development programs and the amounts that we have incurred over the last two years pursuant to those programs, please see “Item 5. Operating and Financial Review and Prospects— A. Operating Results—Operating Expenses— Research and Development Expenses” and “Item 5. Operating and Financial Review and Prospects— A. Operating Results— Comparison of the year ended December 31, 2022 to the year ended December 31, 2021— Research and Development Expenses.”
D. Trend Information
On February 16, 2023, we received a letter from the listing qualifications department staff of the Nasdaq notifying us that Nasdaq had determined to delist our Ordinary Shares and tradeable warrants from Nasdaq based on (i) our non-compliance with the minimum $2,500,000 stockholders’ equity requirement for continued listing set forth in Nasdaq Listing Rule 5550(b) and (ii) providing the Staff a submission of information that contained material misrepresentations, in violation of Nasdaq Listing Rule 5250(a)(1), in relation to our extraordinary general meeting of shareholders, which was adjourned on February 9, 2023 and ultimately held and concluded on February 16, 2023. We originally believed and communicated to the Staff that we had met the quorum requirement on February 9, 2023 and believed that an adjournment would not be necessary. However, the meeting was adjourned due to lack of the required quorum present to open and conduct the meeting, and on February 16, 2023, a quorum was present and our shareholders voted upon and approved all agenda items at the meeting.
On February 23, 2023, we requested a hearing before the Panel, which stays any delisting or suspension action pending the hearing and the expiration of any additional extension period granted by the Panel following the hearing.
On May 11, 2023, the Panel granted the request for continued listing on Nasdaq subject to the requirement that we, by no later than August 4, 2023 provide the Panel with unaudited interim financial statements for the second quarter of 2023 and demonstrate to the Panel that we continue to meet the equity requirement. Upon review of our interim financial statements for the second quarter of 2023, the Panel will decide if we demonstrated an ability to maintain compliance with the continued listing requirements on a long-term basis. In addition, from May 11, 2023 until the end of the Exception Period, the Panel reserves the right to reconsider the terms of this exception based on any event, condition or circumstance that exists or develops that would, in the opinion of the Panel, make the continued listing of our securities on Nasdaq inadvisable or unwarranted. Until the end of the Exception Period, we are required to provide prompt notification to the Panel of any significant events that may affect our compliance with Nasdaq requirements. See also “Risk Factors—Risks Related to The Ownership of our Ordinary Shares—Failure to meet Nasdaq’s continued listing requirements could result in the delisting of our Ordinary Shares, negatively impact the price of our Ordinary Shares and negatively impact our ability to raise additional capital”.
E. Critical Accounting Estimates
We describe our significant accounting policies more fully in Note 2 to our consolidated financial statements included elsewhere in this annual report. We believe that the accounting policy described in Note 2 is critical in order to fully understand and evaluate our financial condition and results of operations.
We prepare our consolidated financial statements in accordance with IFRS. At the time of the preparation of the consolidated financial statements, our management is required to use estimates, evaluations and assumptions which affect the application of the accounting policy and the amounts reported for assets, obligations, income and expenses. Any estimates and assumptions are continually reviewed. The changes to the accounting estimates are credited during the period in which the change to the estimate is made.
80
Share-based compensation
The consolidated entity has a share-based remuneration scheme for employees and other service providers, including options and performance rights. The fair value of options was estimated by using the Black Scholes and Monte-Carlo simulation approach model. The simulation model was designed to model the company’s equity value over time.
The abovementioned valuation models were designed to take into account the unique terms and conditions of the different classes of performance rights and/or options, as well as our capital structure and the volatility of our assets, on the date of grant, based on certain assumptions.
Those conditions are described in the share-based compensation Note 14 to our consolidated financial statements included elsewhere in this annual report. The fair value of the equity settled options granted is charged to statement of comprehensive income over the vesting period of each tranche and the credit is recorded to equity, based on the consolidated entity’s estimate of shares that will eventually vest.
Goodwill impairment testing
We review goodwill impairment once a year or more frequently if an event or change of circumstances indicate that there is an impairment. As of December 31, 2022, we did not have any goodwill in our consolidated financial statements.
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and Senior Management
The following table sets forth certain information relating to our directors and senior management as of May 15, 2023. Unless otherwise stated, the address for our directors and senior management is at the Company’s registered address c/o 5 Oppenheimer, Rehovot 7670105, Israel.
| Name | Age | Position | ||
| Dr. Kenneth R. Melani (1)(2)(3)(6) | 70 | Chairman of the Board of Directors | ||
| Dr. Yacov Geva (6) | 73 | President and Chief Executive Officer, Director | ||
| Igor Bluvstein | 42 | Chief Financial Officer | ||
| Benny Tal | 64 | Vice President Research and Development Corporate | ||
| Oded Shahar | 63 | Senior Vice President Mergers and Acquisitions Corporate | ||
| Dror Nuriel-Roth | 48 | Executive Vice President U.S. Operations | ||
| Dr. Yehoshua (Shuki) Gleitman (1)(3)(6) | 74 | Director | ||
| Prof. Zeev Rotstein (2)(3)(5) | 73 | Director | ||
| Urs Wettstein (1)(2)(3)(5) | 68 | Director and Chairman of the Audit and Risk Committee | ||
| Chanan Epstein (3) | 70 | Director |
| (1) | Member of the Nomination and Remuneration Committee |
| (2) | Member of the Audit and Risk Committee |
| (3) | Independent Director (as defined under Nasdaq Stock Market Rules) |
| (4) | Member of Class I with a term ending at the 2024 annual general meeting of shareholders to be held in 2025 |
| (5) | Member of Class II with a term ending at the 2022 annual general meeting of shareholders to be held in 2023 |
| (6) | Member of Class III with a term ending at the 2023 annual general meeting of shareholders to be held in 2024 |
81
Dr. Kenneth R. Melani, Chairman of the Board of Directors
Dr. Kenneth R. Melani has served on our board of directors as Chairman since August 2014. Dr. Melani has over 30 years of experience in the U.S. healthcare industry, providing service as a provider, supplier and insurer. In April 2016, Dr. Melani founded Velocity Fund Partners LP and has since served as its managing partner. Dr. Melani has been the president and principal owner of KRM Group since 2012. From 2013 to 2014, he was the chairman of the board of directors of LifeWatch AG (previously, SIX: LIFE) (formerly Card Guard AG and Card Guard Scientific Survival Ltd.). Prior to that, he spent 23 years at Highmark Inc. (formerly Blue Cross of Western Pennsylvania), where he served in various capacities, including president and chief executive officer for nine years. He serves on the board of directors of EdLogics (since 2014), and since 2016, he has served as the chairman of the board of directors of DermalBiomics, Periovance, and SkinJect. Dr. Melani holds a B.A. in Chemistry from Washington and Jefferson College (summa cum laude) and an M.D. from Wake Forest University School of Medicine. Dr. Melani is board certified in internal medicine.
Dr. Yacov Geva, President and Chief Executive Officer, Director
Dr. Yacov Geva has served as our President and Chief Executive Officer. A well-known pioneer in the industry of medical technologies and RPM services. As the founder of LifeWatch AG (former Card Guard AG and Card Guard Scientific Survival Ltd.) he successfully led the company to its initial public offering. From 1989 to 2014, Dr. Geva was a member and the Chairman of the Board of Directors and Corporate Chief Executive Officer of LifeWatch AG. During 1979 to 1989, Dr. Geva served as a Chief Mechanical Engineer with Vishay Israel - a subsidiary of Vishay Intertechnology, USA. Dr. Geva holds a B.Sc. in Mechanical and Nuclear Engineering from the Technion-Israeli Institute of Technology, a Ph.D. (with honors) in Business Administration from the International School of Management, Paris and an honorary doctorate from Oxford Brookes University. Dr. Geva is also a senior member of the royal society of medicine in the UK.
Igor Bluvstein, Chief Financial Officer
Mr. Igor Bluvstein has served as our Chief Financial Officer since July 31, 2022. Mr. Bluvstein has over fifteen years of experience working in financial leadership positions in the e-commerce, biotechnology, petrochemical, and medical cannabis industries. From 2020 through 2022, Mr. Bluvstein served as the Chief Financial Officer and Director at MDD Group of Companies. From 2016 to 2020, he served as Regional Chief Financial Officer at Frutarom Industries Ltd. (acquired by International Flavors & Fragrances Inc. (NYSE: IFF)). From 2015 until 2016, he served as the Chief Financial Officer of an e-commerce retailer. From 2011 until 2015, Mr. Bluvstein served as a Financial Controller at Mirland Development Corporation PLC. From 2008 through 2011, he served as a senior auditor at Ernst & Young Global Limited. Mr. Bluvstein holds a Bachelor of Arts in accounting and economics from the Open University in Israel. He is a Certified Public Accountant (CPA).
Benny Tal, Vice President Research and Development Corporate and Chief Technology Officer Corporate
Mr. Benny Tal has served as our Senior Vice President Research and Development since December 2017 and our Chief Technology Officer since 2021. From 2014 to 2017, Mr. Tal was the research and development manager of Spectronix Ltd. Prior to that, from 2000 to 2014, Mr. Tal served in several electronics engineering and operations positions in LifeWatch Services, Inc. Mr. Tal has is an experienced research and development manager with a demonstrated history of working in the research industry. Mr. Tal holds a B.Sc. in electrical engineering and M.B.A from Ben-Gurion University, Israel.
82
Oded Shahar, Senior Vice President Mergers and Acquisitions Corporate
Mr. Oded Shahar has served as our Senior Vice President Mergers and Acquisitions since July 2017. Mr. Shahar has over 25 years of international business and banking experience. He held senior executive positions including head of the Israeli branch of Crédit Agricole Corporate and Investment Bank following hands-on experience in investment banking in Paris and private banking in Switzerland. From 2004 to 2007, Mr. Shahar was the Senior Country Officer in Israel of one of the top ten global banks. From 2007 to 2017, Mr. Shahar was the Senior Partner in one of Israel’s leading law firms. Mr. Shahar is qualified as a lawyer and as a Notary and is a member of the Israel Bar Association. His fields of expertise are banking and international investments, mergers & acquisitions and international contracts.
Dror Nuriel-Roth, EVP U.S. Operations
Ms. Dror Nuriel-Roth has served as the Executive Vice President of U.S. Operations since January 2019. Ms. Nuriel-Roth is focused on managing all operations aspects, including sales, clinical, logistics, reimbursements, customer service and managed care in our facilities in Memphis, Chicago and Austin. Ms. Nuriel-Roth brings years of experience in operations management and project management in the healthcare industry. Since 2011, Ms. Nuriel-Roth has served in various capacities at LifeWatch Services Inc., including senior vice president of operations from November 2016 to November 2017, vice president of business operations from May 2014 to November 2016 and director of reimbursement from June 2011 to May 2014. Ms. Nuriel-Roth holds a Bachelor of Business Administration in Economy and Financing and Master of Business Administration in Economy and Marketing from the Interdisciplinary Center in Hertzelia, Israel.
Dr. Yehoshua (Shuki) Gleitman, Director
Dr. Shuki Gleitman has served on our board of directors since February 2017. Dr. Gleitman has served as the chairman of the Guangzhou Israel Biotech Fund since 2016, chairman of the board of directors of Capital Point Group since 2006, a board member and chairman of the audit and financial committees of Elbit Systems (Nasdaq, TASE: ESLT) from 2010 to March 2020, chairman of the YoYa Group since 2014, senior advisor to the World Bank (national policy for innovation) since 2001 and senior strategy advisor to Serbia Innovation Fund since 2014 and chair of Georgia tech investment committee. Prior to holding those positions, from 1992 to 1997, Dr. Gleitman was the Chief Scientist and Director General of Israel’s Ministry of Industry and Trade, where he managed all of the Israeli Government technological program and was responsible for allocating over $1.5 billion in grants in the framework of promoting research and development activities in the Israeli high-tech industry. Dr. Gleitman also served as the chief executive officer of Ampal Investment Group (Nasdaq: AMPL), in which he led a $330 million joint venture with Motorola Israel, founding Mirs Communications Ltd., Israel’s fourth largest cellular operator. Dr Gleitman is the Honorary Consul General of Singapore in Israel (since 1999) and a member of the board of government of HUJ. Dr. Gleitman holds a Ph.D. (with distinction), M.Sc. (with distinction) and B.Sc. in Physical Chemistry, from the Hebrew University of Jerusalem.
Prof. Zeev Rotstein, Director
Prof. Zeev Rotstein has served on our board of directors since March 2019. Prof. Rotstein had served as the director general of Hadassah Medical Organization between February 2016 and August 2021, and as an Associate Clinical Professor at the Hebrew University of Jerusalem and, prior to this appointment, he was the Sackler School director general of Medicine Chaim Sheba Medical Center at Tel Hashomer and was an Associate Clinical Professor at Tel Aviv University. Prof. Rotstein holds the degree of Doctor of Science, Honoris Causa from the University of Nicosia and is an Honorary Fellow of the Interdisciplinary Center, Herzliya. Prof. Rotstein has acted as an expert consultant in the construction of several medical facilities throughout the world including Centro Medico La Paz, Equatorial Guinea (major referral hospital in Equatorial Guinea), the Lagoon Hospital, Accra, Ghana and currently the Hadassah Skolkovo, Moscow (a branch of Hadassah Medical Cluster Oncological Centre and Polyclinic of Skolkovo, Moscow Organization). During his extensive career, Prof. Rotstein served as treasurer at the State’s Physician Organization, chairman of the World Fellowship of the Israel Medical Association (I.M.A.), and member of the Editorial Board of Associations des Médécins Israelites de France. Prof. Rotstein served as a committee member of the I.M.A. Scientific Committee. Most recently Prof. Rotstein served as Chairman of Israel’s Drug Basket Committee for 2020, one of the most important positions in the Israeli health system. Prof Rotstein is also a board member in NETO-MELINDA, a public company in Tel Aviv Stock Exchange. Prof. Rotstein holds a Phan M.D. in Cardiology from the Sackler School of Medicine at the Tel- Aviv University, and an M.B.A. (cum laude) from the Leon Recanati Graduate School of Business Administration at the Tel- Aviv University, and has held fellowships at the New York Department of Health, Tufts University, and Johns Hopkins Medical Centre School of Hygiene and Public Health.
83
Urs Wettstein, Director
Mr. Urs Wettstein has served on our board of directors since February 2017. Mr. Wettstein has been an advisor and investor in numerous pre-IPO investments since. From 2001 to 2014, he served as non-executive vice chairman of the board of directors of LifeWatch AG (previously, SIX: LIFE). From 1983 to 2007, he managed and operated his own accounting, auditing and tax consultancy firm that he founded in Zurich, Switzerland. Mr. Wettstein is a Swiss Certified Public Accountant.
Chanan Epstein, Director
Mr. Chanan Epstein has served on our board of directors since June 2021. Mr. Epstein is a senior technology and telecom executive with substantial experience in domestic and international markets. Since 2000, he has served as a Senior Vice President at Amdocs (Nasdaq: DOX), a leading software and service provider to telecom and media companies, and he began his service at Amdocs in 1995. At Amdocs, Mr. Epstein is responsible for developing and maintaining key customer relationships worldwide. In this role, he is also instrumental in driving forward strategic deals, leveraging his CEO/CXO relationships across the telecom industry in North America and Asia. Prior to joining Amdocs, from 1974 to 1991, Mr. Epstein served in the Israeli Air Force. Ultimately attaining the rank of Colonel, he oversaw research and development of Command, Control, Communications and Intelligence operational systems, as well as avionics software. During his military career, Mr. Epstein spent several years in the U.S. leading key strategic ventures between the U.S. and Israeli Air Forces, specifically heading up the F-16 avionics software project at General Dynamics. Mr. Epstein sits on the board of a number of private and public companies, including MobileSmith Health (Nasdaq: MOS), RFCode and Copilot. He is also an active technology investor and a mentor to numerous executives. Mr. Epstein received his BA in mathematics and computer science from Bar Ilan University in Israel, as well as participated (partially completed) in the master program of computer science at Weizmann institute in Israel.
Arrangements for Election of Directors and Members of Management
There are no arrangements or understandings with major shareholders, customers, suppliers or others pursuant to which any of our executive management or our directors were selected.
B. Compensation
Under Cayman Islands law, we are not required to disclose compensation paid to our senior management on an individual basis and we have not otherwise publicly disclosed this information elsewhere. The following table presents in the aggregate all compensation we paid, or will need to pay, to all of our directors and senior management as a group for the year ended December 31, 2022. The table does not include any amounts we paid to reimburse any of such persons for costs incurred in providing us with services during this period.
All amounts reported in the tables below reflect the cost to us, in thousands of U.S. dollars, for the period ended December 31, 2022.
| Salary, bonuses and Related Benefits | Pension, Retirement and Other Similar Benefits | Share Based Compensation(1) | ||||||||||
| All directors and senior management as a group, consisting of 12 persons | $ | 2,058 | $ | 110 | $ | 5,647 | ||||||
| (1) | Represents the value of the entire grant calculated as of the grant date |
84
Employment Agreements with Executive Officers
We, and through certain of our subsidiaries, have entered into written employment or consulting agreements with each of our executive officers. All of these agreements contain customary provisions regarding noncompetition, confidentiality of information and assignment of inventions. However, the enforceability of the noncompetition provisions may be limited under applicable law. In addition, in accordance with our Amended and Restated Memorandum and Articles of Association, we have entered into agreements with each executive officer and director pursuant to which we have agreed to indemnify each of them up to a certain amount, and to the extent that these liabilities are not covered by directors and officers insurance. See “Item 7.B. Related Party Transactions” for additional information.
For a description of the terms of our options and option plan, see “Item 6.E. Share Ownership—Equity Incentive Plan.”
Directors’ Service Contracts
The terms of the appointment of our executive and non-executive directors are agreed upon and set out in writing at the time of their respective appointment in Director Appointment Letters. Dr. Yacov Geva, an executive director, is not a party to a Director Appointment Letter and receives no compensation for his service as a director. The Director Appointment Letters set out the key terms and conditions of the director’s appointment, including their duties, rights and responsibilities, time commitment and the board of directors’ expectations regarding involvement with committees of the board of directors. The Director Appointment Letters do not provide for benefits upon the conclusion of their respective service. After the election of our directors at our 2017 annual general meeting of shareholders, held in February 2017, the Director Appointment Letters provided that the Chairman of the board of directors and each of our other directors shall receive annual base compensation of A$100,000 (approximately $72,000) and A$40,000 (approximately $29,000), respectively. In May 2018, our board of directors approved the deferral of the compensation owed under the Director Appointment Letters. See “Item 7.B. Related Party Transactions” for additional information.
Non-Executive Director Remuneration
Our Amended and Restated Memorandum and Articles of Association specify that the maximum aggregate remuneration of Non-Executive Directors shall be A$350,000 (approximately $252,000). With the exception of Dr. Yacov Geva, all of our directors are Non-Executive Directors whose compensation is within the limits of our Amended and Restated Memorandum and Articles of Association. In January 2022, our Board of Directors resolved that the monthly base salary of Dr. Geva as our Chief Executive Officer will be $100,000, which was effective from February 2022.
Foreign Private Issuer Status and Voluntary Compliance with Certain Nasdaq Requirements
The Sarbanes-Oxley Act, as well as related rules subsequently implemented by the SEC, require “foreign private issuers,” such as us, to comply with various corporate governance practices. In addition, following the listing of the Ordinary Shares on the Nasdaq Capital Market, we will be required to comply with the Nasdaq Stock Market Rules. Under those rules, we may elect to follow certain corporate governance practices permitted under the Cayman Islands Companies Act in lieu of compliance with corresponding corporate governance requirements otherwise imposed by the Nasdaq Stock Market Rules for U.S. domestic registrants.
85
In accordance with Cayman law and practice and subject to the exemption set forth in Rule 5615 of the Nasdaq Stock Market Rules, we have elected to follow the provisions of the Cayman Islands Companies Act rather than the Nasdaq Stock Market Rules, with respect to the following requirements:
| ● | Quorum. While the Nasdaq Stock Market Rules require that the quorum for purposes of any meeting of the holders of a listed company’s common voting stock, as specified in the company’s bylaws, be no less than 33 1/3% of the company’s issued and outstanding common voting stock, under Cayman Islands Companies Act there is no minimum attendance threshold for general meetings of shareholders to be quorate. Our Amended and Restated Memorandum and Articles of Association provide that twenty five percent (25%) of our issued and outstanding shares present, in person or by proxy or a duly appointed representative, who are entitled to vote on the business to be transacted, shall constitute a quorum for a general meeting. However, the quorum set forth in our Amended and Restated Memorandum and Articles of Association with respect to an adjourned meeting consists of at least one shareholder present in person or by proxy. |
| ● | Shareholder approval. While the Nasdaq Stock Market Rules require that issuers obtain shareholder approval prior to the issuance of securities in connection with certain acquisitions, private placements of securities, or the establishment or amendment of certain stock option, purchase or other compensation plans, under the Cayman Islands Companies Act, there is no requirement for shareholder approval of share issuances (or the terms on which shares may be issued) and no statutory pre-emption rights apply. Under our Amended and Restated Memorandum and Articles of Association, our board of directors is authorized to issue shares (or to grant warrants, options or other rights to acquire shares) subject to the restriction that the number of shares in issue may not exceed our authorized share capital set forth in our Amended and Restated Memorandum and Articles of Association and generally subject to the Cayman Islands Companies Act and our Amended and Restated Memorandum and Articles of Association. |
However, we have voluntarily elected to adhere to the Nasdaq Stock Market Rules and not the Cayman Islands Companies Act notwithstanding our status as a foreign private issuer, with respect to the requirements of:
| ● | maintaining a board of directors with a majority of “independent” directors and having those directors meet regularly without other members present; |
| ● | maintaining a compensation committee of our board of directors (which is our Nomination and Remuneration Committee) comprised solely of independent directors and governed by a committee charter; and |
| ● | having director nominees be selected or recommended for selection by either a majority of our independent directors or a nominations committee comprised solely of independent directors, which will be undertaken by our Nomination and Remuneration Committee. |
C. Board Practices
Committees of the Board of Directors
Our board of directors has established two standing committees, the audit and risk committee and the nomination and remuneration committee.
86
Audit and Risk Committee
Our board of directors has appointed an audit and risk committee, which assists our board of directors in monitoring and reviewing any matters of significance affecting financial reporting and compliance. Our audit and risk committee, acting pursuant to a written charter, is comprised of Mr. Urs Wettstein, Mr. Zeev Rotstein and Mr. Kenneth Melani. The chairman of this committee is Mr. Urs Wettstein. Our board of directors is primarily responsible for the oversight of our risk management and internal compliance and control framework.
Our board of directors has adopted an audit and risk committee charter, which sets forth, among others, the responsibilities of the audit and risk committee. Our board of directors intends to adopt an audit (and risk) committee charter to be effective upon the listing of the Ordinary Shares on Nasdaq setting forth, among others, the responsibilities of the audit (and risk) committee consistent with the rules of the SEC and Nasdaq Listings Rules, including, among others, the following:
| ● | oversight of our independent registered public accounting firm and recommending the engagement, compensation or termination of engagement of our independent registered public accounting firm to the board of directors; |
| ● | recommending the terms of audit and non-audit services provided by the independent registered public accounting firm for pre-approval by our board of directors; and | |
| ● | reviewing and monitoring, if applicable, legal matters with significant impact, finding of regulatory authorities’ findings, receive reports regarding irregularities and legal compliance, acting according to “whistleblower policy” and recommend to our board of directors if so required. |
Nasdaq Stock Market Requirements for Audit Committee
Under the Nasdaq Stock Market Rules, we are required to maintain an audit committee consisting of at least three members, all of whom are independent and are financially literate and one of whom has accounting or related financial management expertise.
Our audit and risk committee will be comprised of Mr. Urs Wettstein, Mr. Zeev Rotstein and Mr. Kenneth Melani. All of the members of our audit and risk committee are “independent,” as such term is defined under Nasdaq Stock Market Rules. All members of our audit and risk committee meet the requirements for financial literacy under the Nasdaq Stock Market Rules.
Nomination and Remuneration Committee
Cayman Islands law does not impose specific requirements on the establishment of a nomination and remuneration committee or nominating process.
However, our board of directors has appointed a nomination and remuneration committee, which assists our board of directors in monitoring and reviewing any matters of significance affecting the remuneration of our board of directors and our employees and any matters of significance affecting the composition of our board of directors and our management. Our nomination and remuneration committee, acting pursuant to written charters, and as a single committee, is comprised of Mr. Urs Wettstein, Mr. Shuki Gleitman and Mr. Kenneth Melani. The chairman of this committee is Mr. Kenneth Melani.
Our nomination and remuneration committee follows compensation committee membership and charter requirements and the director nomination requirements prescribed under the Nasdaq Stock Market Rules.
The charters adopted by our board of directors set forth the responsibilities of the nomination and remuneration committee in its capacity in nomination and remuneration oversight duties, respectively.
87
The responsibilities of the nomination and remuneration committee include, with respect to nomination oversight, among others, the following:
| ● | maintaining our board of directors’ appropriate mix of skills and experience to be an effective decision-making body; and | |
| ● | ensuring that our board of directors is comprised of directors who contribute to our successful management and discharge their duties having regards for the law and the highest standards of corporate governance. |
The responsibilities of the nomination and remuneration committee include, with respect to compensation oversight, among others, the following:
| ● |
review the on-going appropriateness and relevant of the executive remuneration policy and other executive benefit programs;
| |
| ● | ensure that remuneration policies fairly and responsibly reward executives having regards to our performance, our performance as a company, the performance of the executive and prevailing remuneration expectations in the market. | |
| ● | oversee and advise on remuneration of executive directors; | |
| ● | review and approve the design of any executive incentive plans; | |
| ● | review the impact of any proposed changes in accounting policies on the financial statements; and | |
| ● | review our quarterly, half yearly and annual results. |
Fiduciary Duties of Directors and Officers
As a matter of Cayman Islands law, the duties of a director primarily derive from common law, the Cayman Islands Companies Act, and our Amended and Restated Memorandum and Articles of Association. Under common law principles that will be applied by the Cayman Islands courts, directors have fiduciary duties to a company including: (i) the duty to act honestly and in good faith in what he or she considers are the best interests of the company (generally meaning the interests of the shareholders as a whole); (ii) the duty of loyalty and to avoid actual or potential conflicts of interest arising between his or her duties to the company and his or her personal interest; (iii) a duty to exercise his or her powers as a director under the Cayman Islands Companies Act and the articles of association of the company only for the purposes for which they are conferred and not for a collateral or improper purpose; (iv) a duty not to fetter his or her discretion as a director; and (v) a duty of care, diligence and skill.
Appointment and Removal of Directors
Appointment and removal by shareholders
In accordance with our Amended and Restated Memorandum and Articles of Association, the Company may by ordinary resolution at an annual general meeting (and not at any other general meeting) appoint a person who is willing to act to be a director either to fill a vacancy or as an addition to the existing directors, provided that:
| ● | any such appointment would not cause the total number of directors to exceed any maximum number applying to us; and |
| ● | no person other than a director seeking re-election shall be eligible for appointment by ordinary resolution unless the person or some shareholder intending to propose his or her nomination has, at least 30 business days before the meeting at which his or her proposed appointment is to be considered, left at our registered office a notice in writing duly signed by the nominee giving his or her consent to the nomination and signifying his or her candidature for the office or the intention of the shareholder to propose the person. Notice of every candidature for election as a director will be given to each shareholder with or as part of the notice of the annual general meeting at which the election is to be proposed. |
Shareholders shall not be entitled to requisition a general meeting to propose the appointment or election of a director.
88
In accordance with our Amended and Restated Memorandum and Articles of Association, the Company may by special resolution remove any Director before the expiration of his period of office, but without prejudice to any claim for damages which he may have for breach of any contract of service between him and the Company.
Appointment by Directors
Under our Amended and Restated Memorandum and Articles of Association, our board of directors has the power to appoint at any time any person who is willing to act as a director, either to fill a vacancy or as an additional director (subject to any requirements as to minimum or maximum number of directors then applying to us). Any director so appointed is required to retire at the next annual general meeting after such appointment and shall be eligible to stand for re-election as a director at such meeting.
Rotational Retirement of Directors
Our Amended and Restated Memorandum and Articles of Association provide for a split of the board of directors into three classes with staggered three-year terms. At each annual general meeting of our shareholders, the election or re-election of directors following the expiration of the term of office of the directors of that class of directors will be for a term of office that expires on the third annual general meeting following such election or re-election, such that each year the term of office of only one class of directors will expire. A director who retires by rotation at an annual general meeting may, if willing, be reappointed by ordinary resolution.
Size of the Board and Board Vacancies
Our Amended and Restated Memorandum and Articles of Association provide that, unless determined otherwise by special resolution, there shall be a minimum of two directors and a maximum of seven directors.
Conflicts of Interest
Cayman Islands law restricts transactions between a company and its directors unless there are provisions in the articles of association which provide a mechanism to alleviate possible conflicts of interest. Under our Amended and Restated Memorandum and Articles of Association, a director must disclose the nature and extent of his or her interest in any matter, transaction or arrangement, and following such disclosure the interested director may vote in respect of any matter, transaction or arrangement in which he or she is interested. The interested director shall be counted in the quorum at such meeting and the resolution may be passed by a majority of the directors present at the meeting.
Indemnification of Directors and Executive Officers and Limitation of Liability
The Cayman Islands Companies Act does not limit the extent to which a company’s articles of association may provide for indemnification of directors and officers, except to the extent that it may be held by the Cayman Islands courts to be contrary to public policy, such as to provide indemnification against civil fraud or the consequences of committing a crime. Under our Amended and Restated Memorandum and Articles of Association provide that, to the maximum extent permitted by law, every current and former director and officer (excluding an auditor) is entitled to be indemnified out of our assets against any liability, action, proceeding, claim, demand, costs, damages or expenses, including legal expenses, which such indemnified person may incur in that capacity unless such liability arose as a result of the actual fraud or willful default.
A Cayman Islands company may also purchase insurance for directors and certain other officers against liability incurred as a result of any negligence, default, breach of duty or breach of trust in relation to the company. We expect to maintain director’s and officer’s liability insurance covering our (and G Medical China’s) directors and officers with respect to general civil liability, including liabilities under the Securities Act, which he or she may incur in his or her capacity as such. We have entered into indemnification agreements with all of our directors and officers and our corporate secretary. Each such indemnification agreement provides the office holder with indemnification permitted under applicable law and up to a certain amount, and to the extent that these liabilities are not covered by directors and officers insurance.
89
Approval of Related Party Transactions under Cayman Islands Law
Although Cayman Islands law does not regulate transactions between a company and its significant shareholders, it does provide that the board of directors is required to comply with fiduciary duties which they owe to the company under Cayman Islands law, including the duty to ensure that, in their opinion, only such transactions entered into are in good faith in the best interests of the company are entered into for a proper corporate purpose and not with the effect of perpetrating a fraud on the minority shareholders. In addition, in the event that any payment obligation, transfer of property or grant of charge thereon is made to a related party that is also a creditor at a time when the company is insolvent, the Cayman Islands Companies Act provides that such transfer is deemed to be a preference and therefore is invalid if it occurred within six months immediately preceding the commencement of a liquidation.
D. Employees.
As of December 31, 2022, we had 10 members of senior management (including our Chief Executive Officer), all of which are engaged on a full-time basis, and one (Oded Shahar, our Senior Vice President Mergers and Acquisitions) is engaged as an independent contractor. In addition, we have 61 employees located in the United States, 9 employees located in Israel and an aggregate of 4 full time employees located in China and Macedonia.
As of December 31, 2021, we had 10 members of senior management (including our Chief Executive Officer), all of which are engaged on a full-time basis, and one (Oded Shahar, our Senior Vice President Mergers and Acquisitions) is engaged as an independent contractor. In addition, we have 72 employees located in the United States, 7 employees located in Israel and an aggregate of 3 full time employees located in China and Macedonia.
As of December 31, 2020, we had 10 members of senior management (including our Chief Executive Officer), all of which are engaged on a full-time basis, and one (Oded Shahar, our Senior Vice President Mergers and Acquisitions) is engaged as an independent contractor. In addition, we have 51 employees located in the United States, 8 employees located in Israel and an aggregate of 7 full time employees located in China and Macedonia.
None of our employees is represented by labor unions or covered by collective bargaining agreements. However, in Israel, we are subject to certain Israeli labor laws, regulations and national labor court precedent rulings, as well as certain provisions of collective bargaining agreements applicable to us by virtue of extension orders issued in accordance with relevant labor laws by the Israeli Ministry of Economy and which apply such agreement provisions to our employees even though they are not part of a union that has signed a collective bargaining agreement.
All of our employment and consulting agreements include employees’ and consultants’ undertakings with respect to non-competition and assignment to us of intellectual property rights developed in the course of employment and confidentiality. The enforceability of such provisions is limited by Israeli law.
E. Share Ownership.
See “Item 7.A. Major Shareholders” below.
Equity Incentive Plan
We maintain one equity incentive plan - the Global Equity Incentive Plan. As of the date of this annual report, the number of Ordinary Shares reserved for the exercise of options granted under the Global Plan was 31,663.
90
Our Global Plan was adopted by our board of directors in December 2016, and became effective immediately thereafter, and will expire in December 2026. Our and our subsidiaries’ employees, directors, officers, and service providers, including those who are our controlling shareholder are eligible to participate in this plan and receive awards of options, share appreciation rights (or SARs), restricted shares, restricted share units (or RSUs), and any other share-based grant, referred to as, individually or collectively, the Awards.
Our Global Plan is administered by our board of directors and the terms of grants, including exercise price, method of payment, vesting schedule, acceleration of vesting and the other matters necessary in the administration of the Global Plan. As a default, our Global Plan provides that upon termination of employment for any reason, other than in the event of death, retirement, disability or cause, all unvested options and SARs will expire and all vested options and SARS will generally be exercisable for 90 days following such termination, subject to the terms of the Global Equity Plan and the governing option agreement. Notwithstanding the foregoing, in the event that employment is terminated for misconduct or if an option or SAR holder engaged in misconduct after the date of the termination of their employment with us, all options and SARs granted to such person, whether vested or unvested, shall immediately expire.
Upon termination of employment due to death or disability, all the vested options and SARs at the time of termination of employment, will generally be exercisable for either six or 12 months, respectively, or such shorter or longer period as determined by the plan administrator, our board of directors, subject to the terms of the Global Equity Plan. Unless otherwise set forth in an Award agreement, or in an applicable sub-plan, upon termination of employment due to retirement, all the vested options and SARs at the time of termination of employment, will be exercisable.
Our Global Equity Plan provides that upon termination of employment for any reason, other than in the event of death, all RSUs vested in accordance with an RSU agreement, shall entitle the holder of such vested RSUs to payment (whether in Ordinary Shares, cash, or otherwise, as determined by our board of directors). Upon termination of employment due to death, we shall, upon request and payment of the aggregate purchase price therefor, issue or transfer the Ordinary Shares related to the RSUs, which have vested at the time of the termination of employment due to death, within six months following the termination of employment due to death.
We also have two sub-plans to the Global Plan, one for Israeli residents (or the Israeli Sub-Plan) and the other for U.S. citizens or residents (or the U.S. Sub-Plan).
Under the Israeli Sub-Plan, Awards issued under the Global Equity Plan to eligible Israeli employees, officers, directors, and service providers would qualify for provisions of Section 102(b)(2) or (3) of the Israeli Income Tax Ordinance of 1961 (New Version) (or the Tax Ordinance). Pursuant to such Section 102(b)(2) or (3), as the case may be, qualifying Awards issued under the Global Equity Plan and shares issued upon exercise of such Awards are, in accordance with the Israeli Plan, held in trust and registered in the name of a trustee selected by the board of directors. The trustee may not release these options or shares to the holders thereof for two years from the date of the registration of the options in the name of the trustee. Under Section 102, any tax payable by an employee from the grant or exercise of the options is deferred until the transfer of the options or Ordinary Shares by the trustee to the employee or upon the sale of the options or Ordinary Shares, and gains may qualify to be taxed as capital gains at a rate equal to 25%, subject to compliance with specified conditions. Our Israeli non-employee service providers and controlling shareholders may only be granted options under Section 3(9) of the Tax Ordinance, which does not provide for similar tax benefits. The Global Equity Plan also permits granting options to Israeli grantees who do not qualify under Section 102(b)(2) or (3).
Under the U.S. Sub-Plan, Awards issued under the Global Equity Plan to eligible U.S. employees, officers, directors, and service providers shall be treated as either nonqualified share options or incentive share options (which may be issued only to our employees and only after the U.S. Sub-Plan is approved in accordance with Section 422(b)(1) of the Internal Revenue Code of 1986 (or the Code)). In December 2016 and April 2019, our board of directors and shareholders, respectively, adopted the U.S. Sub-Plan for U.S. persons.
91
Performance Rights
In the last three years we have granted 154,153 performance rights classified into ten classes to certain of our officers, directors, employees and service providers as incentive securities. In July 2020 and in January 2022, 158 Class D performance rights and 28,575 Class E performance were vested and converted into Ordinary Shares, respectively. In January 2023, 8,289 from these performance rights were forfeited
The performance rights convert into Ordinary Shares on a 1:1 basis, upon the occurrence of the following vesting milestones for each class of performance rights:
| ● | Class A performance rights vests upon achieving a market capitalization of greater than $100,000,000, which will be calculated based on the Company’s 20-day VWAP of Ordinary Shares on the ASX (adjusted by the AUD/USD exchange rate quoted on the Reserve Bank of Australia prior to the last trading day pursuant to which the Company’s VWAP of Shares is being calculated) or the Company’s closing market price on a trading day on Nasdaq (or the Conversion Price) multiplied by the total issued Ordinary Shares; |
| ● | Class B performance right vests upon achieving a market capitalization of greater than $150,000,000 which will be calculated based on the Conversion Price multiplied by the total issued Ordinary Shares; |
| ● | Class C performance right vests upon achieving a market capitalization of greater than $200,000,000, which will be calculated based on the Conversion Price multiplied by the total issued Ordinary Shares; and |
| ● | Class D performance rights vests upon achieving a market capitalization of greater than $250,000,000, which will be calculated based on the Conversion Price multiplied by the total issued Ordinary Shares. |
In January 2022, our board approved the grant of an aggregate of 132,868 performance rights to certain of our officers, employees, directors and service provider as incentive securities. The performance rights convert into Ordinary Shares on a 1:1 basis, upon the occurrence of the following vesting milestones for each class of performance rights:
| ● | 28,575 Class E performance rights vests upon achieving a market capitalization of greater than $75,000,000. On January 19, 2022, this performance milestone was achieved and 28,575 Class E performance rights were converted to Ordinary Shares; |
| ● | 9,524 Class F performance right vests upon achieving a market capitalization of greater than $100,000,000; |
| ● | 9,524 Class G performance right vests upon achieving a market capitalization of greater than $125,000,000; | |
| ● | 9,527 Class H performance right vests upon achieving a market capitalization of greater than $150,000,000; |
| ● | 37,863 Class I performance right vests upon achieving a market capitalization of greater than $175,000,000 and |
| ● | 37,855 Class J performance right vests upon achieving a market capitalization of greater than $200,000,000. |
In general, when we achieve a milestone, we must notify the holder of the performance rights, in writing, that the relevant milestone has been satisfied. Thereafter, at the election of the holder of such performance rights, within a period of three months following the satisfaction of the milestone, each performance right shall vest and convert into one of our Ordinary Shares for no consideration.
All shares issued upon the vesting of the performance rights will, upon their conversion, rank pari passu in all respects with the other Ordinary Shares. The performance rights are not listed on any exchange. Performance rights shall be adjusted in the events of the issuance of bonus shares are issued pro-rata to shareholders or in the event that our issued share capital is reorganized. A performance right does not confer upon its holder an entitlement to vote or receive dividends.
92
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major Shareholders
The following table sets forth information regarding beneficial ownership of our Ordinary Shares as of the date of this annual report by:
| ● | each person, or group of affiliated persons, known to us to be the beneficial owner of more than 5% of our issued and outstanding Ordinary Shares; | |
| ● | each of our directors and executive officers; and | |
| ● | all of our current directors and executive officers as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC, and includes voting or investment power with respect to Ordinary Shares. Ordinary Shares issuable under share options or warrants that are exercisable within 60 days after May 15, 2023, are deemed issued and outstanding for the purpose of computing the percentage ownership of the person holding the options or warrants but are not deemed issued and outstanding for the purpose of computing the percentage ownership of any other person.
We are not controlled by another corporation, by any foreign government or by any natural or legal persons except as set forth herein, and here are no arrangements known to us which would result in a change in control of our company at a subsequent date. Except as indicated in footnotes to this table, we believe that the shareholders named in this table have sole voting and investment power with respect to all shares shown to be beneficially owned by them, based on information provided to us by such shareholders. Unless otherwise noted below, each beneficial owner’s address is: c/o G Medical Innovations Holdings Ltd., P.O. Box 10008, Willow House, Cricket Square, Grand Cayman, KY1-1001, Cayman Islands.
| No. of Shares
Beneficially Owned | Percentage
Owned | |||||||
| Holders of more than 5% of our voting securities: | ||||||||
| Dr. Yacov Geva (**)(2) | 2,719,734 | 18.6 | % | |||||
| Directors and executive officers who are not 5% holders: | ||||||||
| Dr. Kenneth R. Melani (**)(3) | 27,204 | * | % | |||||
| Igor Bluvstein | - | - | % | |||||
| Benny Tal (4) | 4,026 | * | % | |||||
| Dror Muriel – Rot (5) | 5,343 | * | % | |||||
| Oded Shahar (6) | 7,818 | * | % | |||||
| Chanan Epstein (**)(7) | 1,041 | * | % | |||||
| Dr. Yehoshua (Shuki) Gleitman (**)(8) | 7,173 | * | % | |||||
| Prof. Zeev Rotstein (**)(9) | 6,041 | * | % | |||||
| Urs Wettstein (**)(10) | 6,424 | * | % | |||||
| All directors and executive officers as a group (10 persons) | 2,784,804 | 19 | % | |||||
| (*) | Less than one percent (1%). |
| (**) | Indicates director of the Company. |
| (1) | The percentages shown are based 13,971,492 Ordinary Shares (assumes the exercise in full of pre-funded warrants) issued and outstanding as of May 15, 2023, and Ordinary Shares issuable under share options or warrants that are exercisable within 60 days after such date. |
| (2) | Includes (i) 2,034,252 Ordinary Shares, and (ii) options to purchase 4,171, 3,576, 5,358 and 28,572 Ordinary Shares that are exercisable within 60 days after May 15, 2023, at an exercise price of $114.45, $73.5 $69.3 and $22,75 per share, respectively (iii) warrants to purchase 71,429, 57,143 and 515,233 Ordinary Shares that are exercisable within 60 days after May 15, 2023, at an exercise price of $7.7, $43.4 and $1.94 per share, respectively. |
93
| (3) | Includes (i) 5,888 Ordinary Shares, and (ii) options to purchase 1,669 , 2,146 , 3,215 and 14,286 Ordinary Shares that are exercisable within 60 days after May 15, 2023, at exercise prices of $114.45, $73.5 ,$69.3 and $22,75 per share respectively. |
| (4) | Includes (i) 1,650 Ordinary Shares, and (ii) options to purchase 49, 1,253 and 1,074 Ordinary Shares that are exercisable within 60 days May 15, 2023, at an exercise price of $689.85, $114.45 and $73.5 per share, respectively. |
| (5) | Includes (i) 1,587 Ordinary Shares, and (ii) options to purchase 1,253 and 1,074 Ordinary Shares that are exercisable within 60 days after May 15, 2023, at an exercise price of $114.45 and $73.5 per share, respectively. |
| (6) | Includes (i) 2,276 Ordinary Shares, and (ii) options to purchase 1,253, 1,431 and 2,858 Ordinary Shares that are exercisable within 60 days after May 15, 2023, at an exercise price of $114.45, $73.5 and $22.75 per share, respectively. |
| (7) | Includes (i) 206 Ordinary Shares, and (ii) options to purchase 835 Ordinary Shares that are exercisable within 60 days after May 15, 2023, at an exercise price of $114.45. |
| (8) | Includes (i) 1,872 Ordinary Shares, and (ii) options to purchase 835, 1,608 and 2,858 Ordinary Shares that are exercisable within 60 days after May 15, 2023, at an exercise price of $114.45, $69.3 and $22.75 per share, respectively. |
| (9) | Includes (i) 1,990 Ordinary Shares, and (ii) options to purchase 835, 1,073 and 2,143 Ordinary Shares that are exercisable within 60 days after May 15, 2023, at an exercise price of $114.45, $69.3 and $22.75 per share, respectively. |
| (10) | Includes (i) 1,658 Ordinary Shares, and (ii) options to purchase 835, 1,073 and 2,858 Ordinary Shares that are exercisable within 60 days after May 15, 2023 at an exercise price of $114.45, $69.3 and $22.75 per share, respectively. |
Changes in Percentage Ownership by Major Shareholders
In May 2017, we consummated an initial public offering of A$13.5 million (approximately $9 million) on the ASX. Prior to the offering, our President and Chief Executive Officer, Dr. Geva, held approximately 91% of our issued and outstanding share capital, and following the public offering, Dr. Geva held approximately 57% of our issued and outstanding share capital. On October 6, 2022, in exchange for providing a commitment to finance the Company’s operations for the next 12 months and until November 30, 2023, subject to certain conditions, we issued Dr. Geva 71,428 Ordinary Shares and 71,429 warrants to purchase Ordinary Shares. On December 28, 2022, in connection with the Loan Agreement, Dr. Geva was issued 515,233 Ordinary Shares of the Company. As of the date of this annual report, Dr. Geva holds beneficially owns 14.6% of our Ordinary Shares.
On October 20, 2022, we entered into an agreement with Jonathan B. Rubini (the “Investor”) in connection with a private placement investment for 79,365 Ordinary Shares and warrants to purchase 79,366 Ordinary Shares with price of $6.30 per share and associated warrant, for aggregate consideration of $500,000. The warrants are exercisable at any time beginning 30 days after issuance with a term of five years from issuance. Following this private placement investment, the Investor held approximately 1% of our Ordinary Shares.
Record Holders
Based upon a review of the information provided to us by our transfer agent, as of May 15, 2023, there were a total of 6,685 holders of record of our shares, of which 2,034 record holders hold 8,757,263 of our Ordinary Shares, or approximately 68.76% of our issued and outstanding share capital, and had a registered address in the United States. These numbers are not representative of the number of beneficial holders of our shares nor is it representative of where such beneficial holders reside, since many of these shares were held of record by brokers or other nominees.
94
We are not controlled by another corporation, by any foreign government or by any natural or legal persons except as set forth herein, and there are no arrangements known to us which would result in a change in control of our company at a subsequent date.
B. Related Party Transactions
The following is a description of the material terms of those transactions with related parties to which we are party and which were in effect since January 1, 2020.
Employment Agreements
We have entered into written employment and service agreements with each of our executive officers and have executed Director Appointment Letters with each member of our board of directors, with the exception of Dr. Yacov Geva. All employment or consulting agreements with our executive officers contain customary provisions regarding noncompetition, confidentiality of information and assignment of inventions. However, the enforceability of the noncompetition provisions may be limited under applicable law. In addition, we have entered into agreements with each executive officer and director pursuant to which we have agreed to indemnify each of them up to a certain amount and to the extent that these liabilities are not covered by directors and officers insurance. Members of our senior management are eligible for bonuses each year. Our Director Appointment Letters contain confidentiality clauses and entitle each director to annual cash compensation. See “Item 6.B—Employment Agreements with Executive Officers” and “—Directors’ Service Contracts.”
Options
Since our inception, we have granted options to purchase our Ordinary Shares to our officers and certain of our directors. We describe our option plans under “Management-Equity Incentive Plan.”
Performance Rights
Since May 2017, we have granted performance rights to certain of our officers and directors and certain consultants whereupon achievement of certain milestones, the holder of the performance rights may exercise their rights to receive such number of our Ordinary Shares represented by the performance rights. We describe our performance rights under “Management-Equity Incentive Plan-performance rights.”
Shareholder Loans
Dr. Yacov Geva, our President, Chief Executive Officer, director, and major shareholder has provided certain interest and non-interest bearing loans to us, as disclosed below.
On March 19, 2020, our shareholders approved the conversion of an additional amount of $5 million of the amount outstanding under the 2018 Credit Line into 29,631 Ordinary Shares. On July 23, 2020, we issued to Dr. Geva 14,939 Ordinary Shares as consideration for the conversion of $1.95 million owed to Dr. Geva pursuant to the 2016 Credit Line and 2018 Credit Line. In July 2020, an additional amount of $1.95 million of the amount outstanding under the 2018 Credit Line was converted into 14,939 Ordinary Shares and the remaining outstanding amount was paid in cash.
The Company and Dr. Geva agreed to convert an additional amount of US$2,000,000 of the Amount Outstanding (being A$2,906,554.28, based on the AUD:USD exchange rate quoted by the Reserve Bank of Australia on May 17, 2019 of $0.6881) (Additional Conversion Amount). The price at which the Additional Conversion Amount converted equivalent to the issue price per share under the Company’s proposed public offering in the United States, such price to be no less than A$630 (approximately $434). The number of shares that the Company issued to Dr. Geva in respect to the Additional Conversion Amount was 4,613 Shares.
95
In addition, in April 2021 (effective January 1,2021) Dr. Geva extended a loan to us in the amount of $267,000, which bared interest at a fixed rate of 15%. The loan and interest were repaid in full in July 2021.
On April 28, 2022, Dr. Yacov Geva signed an undertaking whereby Dr. Geva committed to finance the Company’s operations for a period of 12 months so long as he is the Company’s controlling shareholder, and/or the Company cannot be financed externally from any other sources, or until a sum of $10 million be received by the Company for its operations during 2022. In consideration therefor, the Company agreed to issued Dr. Geva 57,142 Ordinary Shares and an additional warrant to purchase 57,143 Ordinary Shares with an exercise price equal of $1.24 (the closing price of the Company’s Ordinary Shares on April 27, 2022).
On October 6, 2022, Dr. Yacov Geva, committed to finance our operations for the next 12 months and until November 30, 2023, provided and as long as he continues to be a controlling shareholder and/or the Company cannot be financed externally from any other sources and/or until a sum of $10 million be received by the Company for its operations this year, whichever is earlier. In exchange, for providing this commitment, Dr. Geva was issued 71,428 Ordinary Shares and 71,429 warrants to purchase Ordinary Shares (cashless) at an exercise price of $7.70 as of the day of giving this commitment.
On December 29, 2022, the Board of Directors approved a loan agreement, dated December 21, 2022 (the “Loan Agreement”), between the Company and Dr. Geva. Under the terms of the Loan Agreement, the total amount of the loan provided by Dr. Geva to the Company is $999,552, with an annual interest rate of 12%. The following installments have been made under the terms of the Loan Agreement and advanced to the Company: $198,582 on October 9, 2022; $85,106 on October 11, 2022; $252,596 on November 10, 2022; $175,000 on December 1, 2022 (which is attributed to waived compensation to which Dr. Geva was entitled as Chief Executive Officer of the Company); and $288,268 on December 20, 2022. In addition, (i) Dr. Geva was granted 515,233 Ordinary Shares of the Company and (ii) Dr. Geva will be granted 515,223 warrants to purchase Ordinary Shares of the Company with an exercise price of $1.94 per Ordinary Share, conditioned upon the approval of our Capital Reduction by the Grand Court of the Cayman Islands.
On January 30, 2023, our Chief Executive Officer, Dr. Yacov Geva, extended his commitment to finance our operations up to February 28, 2024, which, on March 24, 2023, was further extended to April 30, 2024. On May 15, 2023, Dr. Yacov Geva extended his commitment to finance our operations up to May 30, 2024 provided and as long as the Company cannot be financed externally from any other sources and/or until a sum of $10 million be received by the Company for its operations for the year, whichever is earlier.
Services Agreement with Dr. Brendan De Kauwe
On February 29, 2020, we entered into a services agreement with our former director, Dr. Brendan de Kauwe, whereby we receive business services from Dr. de Kauwe. Pursuant to the agreement, Dr. de Kauwe will be entitled to a monthly payment of $10,000. In addition, Dr. de Kauwe will also be entitled to commission, in an amount equal to five percent of the direct revenue actually paid to us by third parties presented to us by Dr. de Kauwe. In November 2020, the services agreement with Dr. de Kauwe was terminated. On February 27, 2023, we received notice from Dr. de Kauwe which stated his resignation as a member of our board of directors effective on February 1, 2023.
Lease Agreement
We entered into a lease agreement dated February 2019, through our Israeli subsidiary, with Ad Marom Assets and Initiation Ltd. (or Ad Marom), a company controlled by our controlling shareholder, Dr. Yacov Geva. This lease agreement was to come into effect no later than January 2022, since the facility remains under construction. According to the agreement, we were to pay Ad Marom a monthly payment of NIS 61,560 (approximately $17,000) in consideration for approximately 11,044 square feet, for a period of 60 months from time of commencement of the lease. In May 2021, we canceled the lease agreement with no further commitments from either party.
96
C. Interests of Experts and Counsel
Not applicable.
ITEM 8. FINANCIAL INFORMATION.
A. Consolidated Statements and Other Financial Information.
See “Item 18. Financial Statements.”
Legal Proceedings
From time to time, we may be involved in various claims and legal proceedings relating to claims arising out of our operations. As of the date of this annual report, we are not currently a party to any legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
Dividends
We have never declared or paid cash dividends to our shareholders. We currently intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination to pay dividends will be made at the discretion of our board of directors and will depend on various factors, including applicable laws, our results of operations, financial condition, future prospects and any other factors deemed relevant by our board of directors.
Under the Cayman Islands Companies Act and our Amended and Restated Memorandum and Articles of Association, a Cayman Islands company may pay a dividend out of its realized or unrealized profit or share premium account, but a dividend may not be paid if this would result in the company being unable to pay its debts as they fall due in the ordinary course of business. According to our Amended and Restated Memorandum and Articles of Association, dividends can be declared and paid out of funds lawfully available to us. Dividends may be declared and paid in cash or in kind (including paid up share capital or securities in another corporate body). Dividends, if any, would be paid in proportion to the number of Ordinary Shares a shareholder holds. Any dividend unclaimed after a period of three years from the date the dividend became due for payment shall be forfeited and shall revert to us.
B. Significant Changes
Other than as otherwise described in this annual report on Form 20-F and as set forth below, no significant change has occurred in our operations since the date of our consolidated financial statements included in this annual report on Form 20-F.
97
ITEM 9. THE OFFER AND LISTING
A. Offer and Listing Details
Our Ordinary Shares are listed on the Nasdaq Capital Market under the symbol “GMVD”.
B. Plan of Distribution
Not applicable.
C. Markets
Our Ordinary Shares are listed on the Nasdaq Capital Market.
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
Copies of our Memorandum of Association and Amended and Restated Articles of Association are attached as Exhibits 1.1 and 1.2 to this annual report, respectively. Other than as disclosed below, the information called for by this Item is set forth in Exhibit 2.1 to this annual report and is incorporated by reference into this annual report.
C. Material Contracts
Except as set forth below, we have not entered into any material contract within the two years prior to the date of this annual report on Form 20-F, other than contracts entered into in the ordinary course of business, or as otherwise described herein in “Item 4.A. History and Development of the Company”, “Item 4.B. Business Overview”, “Item 5C. Liquidity and Capital Resources”, “Item 7A. Major Shareholders” or “Item 7B. Related Party Transactions” above.
D. Exchange Controls
Under Cayman Islands law, non-residents of the Cayman Islands may freely hold, vote and transfer ordinary shares in the same manner as Cayman Islands residents, subject to the provisions of the Companies Law and our amended and restated memorandum and articles of association. There is no exchange control legislation in the Cayman Islands or any laws or regulations which affect the remittance of dividends, interest or other payments to non-resident holders of our securities.
E. Taxation.
The following description is not intended to constitute a complete analysis of all tax consequences relating to the acquisition, ownership and disposition of our Ordinary Shares. You should consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign, or other taxing jurisdiction.
98
Cayman Islands Taxation
Prospective investors should consult their professional advisers on the possible tax consequences of buying, holding or selling any Ordinary Shares under the laws of their country of citizenship, residence or domicile.
The following is a discussion on certain Cayman Islands income tax consequences of an investment in the Ordinary Shares. The discussion is a general summary of present law, which is subject to prospective and retroactive change. It is not intended as tax advice, does not consider any investor’s particular circumstances, and does not consider tax consequences other than those arising under Cayman Islands law.
No stamp duty, capital duty, registration or other issue or documentary taxes are payable in the Cayman Islands on the creation, issuance or delivery of the Ordinary Shares. The Cayman Islands currently have no form of income, corporate or capital gains tax and no estate duty, inheritance tax or gift tax. There are currently no Cayman Islands’ taxes or duties of any nature on gains realized on a sale, exchange, conversion, transfer or redemption of the Ordinary Shares. Payments of dividends and capital in respect of the Ordinary Shares will not be subject to taxation in the Cayman Islands and no withholding will be required on the payment of interest and principal or a dividend or capital to any holder of the Ordinary Shares, nor will gains derived from the disposal of the Ordinary Shares be subject to Cayman Islands income or corporation tax as the Cayman Islands currently have no form of income or corporation taxes.
There is no income tax treaty or convention currently in effect between the United States and the Cayman Islands.
We are incorporated under the laws of the Cayman Islands as an exempted company with limited liability and, as such, may apply for an undertaking from the Governor of the Cayman Islands that no law enacted in the Cayman Islands during the period of 20 years from the date of the undertaking imposing any tax to be levied on profits, income, gains or appreciation shall apply to us or our operations and no such tax or any tax in the nature of estate duty or inheritance tax shall be payable (directly or by way of withholding) on the Ordinary Shares, debentures or other obligations of ours.
Material U.S. Federal Income Tax Consequences
THE FOLLOWING SUMMARY IS INCLUDED HEREIN FOR GENERAL INFORMATION AND IS NOT INTENDED TO BE, AND SHOULD NOT BE CONSIDERED TO BE, LEGAL OR TAX ADVICE. EACH U.S. HOLDER SHOULD CONSULT WITH HIS OR HER OWN TAX ADVISOR AS TO THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND SALE OF ORDINARY SHARES, INCLUDING THE EFFECTS OF APPLICABLE STATE, LOCAL, FOREIGN OR OTHER TAX LAWS AND POSSIBLE CHANGES IN THE TAX LAWS.
Subject to the limitations described in the next two paragraphs, the following discussion summarizes the material U.S. federal income tax consequences to a “U.S. Holder” arising from the purchase, ownership and sale of the Ordinary Shares. For this purpose, a “U.S. Holder” is a holder of Ordinary Shares that is: (1) an individual citizen or resident of the United States, including an alien individual who is a lawful permanent resident of the United States or meets the substantial presence residency test under U.S. federal income tax laws; (2) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) or a partnership (other than a partnership that is not treated as a U.S. person under any applicable U.S. Treasury regulations) created or organized under the laws of the United States or the District of Columbia or any political subdivision thereof; (3) an estate, the income of which is includable in gross income for U.S. federal income tax purposes regardless of its source; (4) a trust if a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust; or (5) a trust that has a valid election in effect to be treated as a U.S. person to the extent provided in U.S. Treasury regulations.
This summary is for general information purposes only and does not purport to be a comprehensive description of all of the U.S. federal income tax considerations that may be relevant to a decision to purchase our Ordinary Shares. This summary generally considers only U.S. Holders that will own our Ordinary Shares as capital assets. Except to the limited extent discussed below, this summary does not consider the U.S. federal tax consequences to a person that is not a U.S. Holder, nor does it describe the rules applicable to determine a taxpayer’s status as a U.S. Holder. This summary is based on the provisions of the Code, final, temporary and proposed U.S. Treasury regulations promulgated thereunder, administrative and judicial interpretations thereof, and the United States-Israel Income Tax Treaty, all as in effect as of the date hereof and all of which are subject to change, possibly on a retroactive basis, and all of which are open to differing interpretations. We will not seek a ruling from the Internal Revenue Service (or IRS), with regard to the U.S. federal income tax treatment of an investment in our Ordinary Shares by U.S. Holders and, therefore, can provide no assurances that the IRS will agree with the conclusions set forth below.
99
This discussion does not address all of the aspects of U.S. federal income taxation that may be relevant to a particular U.S. holder based on such holder’s particular circumstances and in particular does not discuss any estate, gift, generation-skipping transfer, state, local, excise or foreign tax considerations. In addition, this discussion does not address the U.S. federal income tax treatment of a U.S. Holder who is: (1) a bank, life insurance company, regulated investment company, or other financial institution or “financial services entity,” (2) a broker or dealer in securities or foreign currency; (3) a person who acquired our Ordinary Shares in connection with employment or other performance of services; (4) a U.S. Holder that is subject to the U.S. alternative minimum tax; (5) a U.S. Holder that holds our Ordinary Shares as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction or other risk-reduction transaction for U.S. federal income tax purposes; (6) a tax-exempt entity; (7) real estate investment trusts or grantor trusts; (8) a U.S. Holder that expatriates out of the United States or a former long-term resident of the United States; or (9) a person having a functional currency other than the U.S. dollar. This discussion does not address the U.S. federal income tax treatment of a U.S. Holder that owns, directly or constructively, at any time, Ordinary Shares representing 10% or more of the shares of our company. Additionally, the U.S. federal income tax treatment of partnerships (or other pass-through entities) or persons who hold Ordinary Shares through a partnership or other pass-through entity are not addressed.
Each prospective investor is advised to consult his or her own tax adviser for the specific tax consequences to that investor of purchasing, holding or disposing of our Ordinary Shares, including the effects of applicable state, local, foreign or other tax laws and possible changes in the tax laws.
Taxation of Dividends Paid on Ordinary Shares
We do not intend to pay dividends in the foreseeable future. In the event that we do pay dividends, and subject to the discussion under the heading “Passive Foreign Investment Companies” below and the discussion of “qualified dividend income” below, a U.S. Holder, other than certain U.S. Holders that are U.S. corporations, will be required to include in gross income as ordinary income the amount of any distribution paid on the Ordinary Shares (including the amount of any Israeli tax withheld on the date of the distribution), to the extent that such distribution does not exceed our current and accumulated earnings and profits, as determined for U.S. federal income tax purposes. The amount of a distribution which exceeds our earnings and profits will be treated first as a non-taxable return of capital, reducing the U.S. Holder’s tax basis for the Ordinary Shares to the extent thereof, and then capital gain. We do not expect to maintain calculations of our earnings and profits under U.S. federal income tax principles and, therefore, U.S. Holders should expect that the entire amount of any distribution generally will be reported as dividend income.
In general, preferential tax rates for “qualified dividend income” and long-term capital gains are applicable for U.S. Holders that are individuals, estates or trusts. For this purpose, “qualified dividend income” means, inter alia, dividends received from a “qualified foreign corporation.” A “qualified foreign corporation” is a if our Ordinary Shares are readily tradable on the Nasdaq Capital Market or another established securities market in the United States. Dividends will not qualify for the preferential rate if we are treated, in the year the dividend is paid or in the prior year, as a PFIC, as described below under “Passive Foreign Investment Companies.” A U.S. Holder will not be entitled to the preferential rate: (1) if the U.S. Holder has not held our Ordinary Shares for at least 61 days of the 121 day period beginning on the date which is 60 days before the ex-dividend date, or (2) to the extent the U.S. Holder is under an obligation to make related payments with respect to positions in substantially similar or related property. Any days during which the U.S. Holder has diminished its risk of loss on our Ordinary Shares are not counted towards meeting the 61-day holding period. Finally, U.S. Holders who elect to treat the dividend income as “investment income” pursuant to Section 163(d)(4) of the Code will not be eligible for the preferential rate of taxation.
Dividends paid with respect to our ordinary shares will be treated as foreign source income, which may be relevant in calculating the holder’s foreign tax credit limitation. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends that we distribute generally should constitute “passive category income,” or, in the case of certain U.S. Holders, “general category income.” A foreign tax credit for foreign taxes imposed on distributions may be denied if holders do not satisfy certain minimum holding period requirements. The rules relating to the determination of the foreign tax credit are complex, and U.S. Holders should consult their tax advisor to determine whether and to what extent such holder will be entitled to this credit.
100
Taxation of the Sale, Exchange or other Disposition of Ordinary Shares
Except as provided under the PFIC rules described below under “Passive Foreign Investment Companies,” upon the sale, exchange or other disposition of our Ordinary Shares, a U.S. Holder will recognize capital gain or loss in an amount equal to the difference between such U.S. Holder’s tax basis for the Ordinary Shares, determined in U.S. dollars, and the U.S. dollar value of the amount realized on the disposition (or its U.S. dollar equivalent determined by reference to the spot rate of exchange on the date of disposition, if the amount realized is denominated in a foreign currency). The gain or loss realized on the sale, exchange or other disposition of Ordinary Shares will be long-term capital gain or loss if the U.S. Holder has a holding period of more than one year at the time of the disposition. Individuals who recognize long-term capital gains may be taxed on such gains at reduced rates of tax. The deduction of capital losses is subject to various limitations.
Passive Foreign Investment Companies
Special U.S. federal income tax laws apply to U.S. taxpayers who own shares of a corporation that is a PFIC. We will be treated as a PFIC for U.S. federal income tax purposes for any taxable year that either:
| ● | 75% or more of our gross income (including our pro rata share of gross income for any company, in which we are considered to own 25% or more of the shares by value), in a taxable year is passive; or |
| ● | At least 50% of our assets generally determined on the basis of a quarterly average and based upon fair market value (including our pro rata share of the assets of any company in which we are considered to own 25% or more of the shares by value) are held for the production of, or produce, passive income. |
For this purpose, passive income generally consists of dividends, interest, rents, royalties, gains from disposition of passive assets and gain from commodities and securities transactions. Cash is treated as generating passive income.
We believe that we will not be a PFIC for the current taxable year and do not expect to become a PFIC in the foreseeable future. The tests for determining PFIC status are applied annually, and it is difficult to make accurate projections of future income and assets which are relevant to this determination. In addition, our PFIC status may depend in part on the market value of our Ordinary Shares. Accordingly, there can be no assurance that we currently are not or will not become a PFIC.
If we currently are or become a PFIC, each U.S. Holder who has not elected to mark the shares to market (as discussed below), would, upon receipt of certain “excess distributions” by us and upon disposition of our Ordinary Shares at a gain: (1) have such excess distribution or gain allocated ratably over the U.S. Holder’s holding period for the Ordinary Shares, as the case may be; (2) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (3) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. Distributions received by a U.S. Holder in a taxable year that are greater than 125% of the average annual distributions received during the shorter of the three preceding taxable years or the U.S. Holder’s holding period for the ordinary shares will be treated as excess distributions. In addition, when shares of a PFIC are acquired by reason of death from a decedent that was a U.S. Holder, the tax basis of such shares would not receive a step-up to fair market value as of the date of the decedent’s death, but instead would be equal to the decedent’s basis if lower, unless all gain were recognized by the decedent. Indirect investments in a PFIC may also be subject to these special U.S. federal income tax rules.
101
The PFIC rules described above would not apply to a U.S. Holder who makes a QEF election for all taxable years that such U.S. Holder has held the Ordinary Shares while we are a PFIC, provided that we comply with specified reporting requirements. Instead, each U.S. Holder who has made such a QEF election is required for each taxable year that we are a PFIC to include in income such U.S. Holder’s pro rata share of our ordinary earnings as ordinary income and such U.S. Holder’s pro rata share of our net capital gains as long-term capital gain, regardless of whether we make any distributions of such earnings or gain. In general, a QEF election is effective only if we make available certain required information. The QEF election is made on a shareholder-by-shareholder basis and generally may be revoked only with the consent of the IRS. We do not intend to notify U.S. Holders if we believe we will be treated as a PFIC for any tax year. In addition, we do not intend to furnish U.S. Holders annually with information needed in order to complete IRS Form 8621 and to make and maintain a valid QEF election for any year in which we or any of our subsidiaries are a PFIC. Therefore, the QEF election will not be available with respect to our Ordinary Shares.
In addition, the PFIC rules described above would not apply if we were a PFIC and a U.S. Holder made a mark-to-market election. A U.S. Holder of our Ordinary Shares which are regularly traded on a qualifying exchange, including the Nasdaq Capital Market, can elect to mark the Ordinary Shares to market annually, recognizing as ordinary income or loss each year an amount equal to the difference as of the close of the taxable year between the fair market value of the Ordinary Shares and the U.S. Holder’s adjusted tax basis in the Ordinary Shares. Losses are allowed only to the extent of net mark-to-market gain previously included income by the U.S. Holder under the election for prior taxable years.
U.S. Holders who hold our Ordinary Shares during a period when we are a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC. U.S. Holders are strongly urged to consult their tax advisors about the PFIC rules.
Tax on Net Investment Income
U.S. Holders who are individuals, estates or trusts will generally be required to pay a 3.8% Medicare tax on their net investment income (including dividends on and gains from the sale or other disposition of our Ordinary Shares), or in the case of estates and trusts on their net investment income that is not distributed to beneficiaries of the estate or trust. In each case, the 3.8% Medicare tax applies only to the extent the U.S. Holder’s total adjusted income exceeds applicable thresholds.
Information Reporting and Withholding
A U.S. Holder may be subject to backup withholding at a rate of 24% with respect to cash dividends and proceeds from a disposition of Ordinary Shares. In general, backup withholding will apply only if a U.S. Holder fails to comply with specified identification procedures. Backup withholding will not apply with respect to payments made to designated exempt recipients, such as corporations and tax-exempt organizations. Backup withholding is not an additional tax and may be claimed as a credit against the U.S. federal income tax liability of a U.S. Holder, provided that the required information is timely furnished to the IRS.
Certain U.S. Holders with interests in “specified foreign financial assets” (including, among other assets, our Ordinary Shares, unless such Ordinary Shares are held on such U.S. Holder’s behalf through a financial institution) may be required to file an information report with the IRS if the aggregate value of all such assets exceeds $50,000 on the last day of the taxable year or $75,000 at any time during the taxable year (or such higher dollar amount as may be prescribed by applicable IRS guidance); and may be required to file a Report of Foreign Bank and Financial Accounts, if the aggregate value of the foreign financial accounts exceeds $10,000 at any time during the calendar year. You should consult your own tax advisor as to the possible obligation to file such information report.
F. Dividends and Paying Agents
Not applicable.
102
G. Statement by Experts
Not applicable.
H. Documents on Display
We are subject to certain information reporting requirements of the Exchange Act, applicable to foreign private issuers and under those requirements will file reports with the SEC. The SEC maintains an internet site at http://www.sec.gov that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. Our filings with the SEC will also be available to the public through the SEC’s website at www.sec.gov.
As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders will be exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as U.S. domestic companies whose securities are registered under the Exchange Act. However, we will file with the SEC, within 120 days after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm, and may submit to the SEC, on a Form 6-K, unaudited quarterly financial information.
We maintain a corporate website https://gmedinnovations.com/. Information contained on, or that can be accessed through, our website and the other websites referenced above do not constitute a part of this annual report on Form 20-F. We have included these website addresses in this annual report on Form 20-F solely as inactive textual references.
I. Subsidiary Information.
Not applicable.
J. Annual Report to Security Holders.
Not applicable.
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risk in the ordinary course of our business. Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our market risk exposure is primarily a result of fluctuations in foreign currency exchange rates and interest rates.
Quantitative and Qualitative Disclosures about Market Risk
We are exposed to market risks in the ordinary course of our business. Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our current investment policy is to invest available cash in bank deposits with banks that have a credit rating of at least A-minus. Accordingly, some of our cash and cash equivalents is held in deposits that bear interest. Given the current low rates of interest we receive, we will not be adversely affected if such rates are reduced. Our market risk exposure is primarily a result of U.S. dollar/NIS and U.S. dollar/RMB exchange rates.
103
Foreign Currency Exchange Risk
Our functional and reporting currency is the U.S. dollar. Our foreign currency exposures give rise to market risk associated with exchange rate movements of the NIS, mainly against the U.S. dollar. A material portion of our expenses consist principally of payments in NIS made to employees, subcontractors and consultants for clinical trials, other research and development activities, and purchase of new equipment. A material portion of our research and development is conducted through collaboration agreements denominated in U.S. dollars, and therefore our net research and development expenses are subject to significant foreign currency risk. If the NIS fluctuates significantly against either the U.S. dollar, it may have a negative impact on our results of operations.
To date, we have not entered into any hedging arrangements with respect to foreign currency risk or other derivative financial instruments. In the future, we may enter into currency hedging transactions to decrease the risk of financial exposure from fluctuations in the operating currencies. These measures, however, may not adequately protect us from the material adverse effects of such fluctuations.
Interest Rate Risk
At present, our investments consist primarily of cash and cash equivalents in short-term deposits. The primary objective of our investment activities is to preserve our capital to fund our operations. Our investments are exposed to market risk due to fluctuation in interest rates, which may affect our interest income and the fair market value of our investments, if any. We manage this exposure by performing ongoing evaluations of our investments. Due to the short-term maturities, if any, of our investments to date, their carrying value has always approximated their fair value. We believe that our exposure to interest rate risk is not significant and a 1% change in market interest rates would not have a material impact on our assets.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A. Debt Securities.
Not applicable.
B. Warrants and rights.
Not applicable.
C. Other Securities.
Not applicable.
D. American Depositary Shares
Not applicable.
104
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
None.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
In June 2021, the Company closed its initial public offering of 3,000,000 units, each unit consisting of one ordinary share and one warrant to purchase one ordinary share of the Company for gross proceeds of approximately $15,000 before deducting underwriting discounts and commissions and other offering-related expenses in the amount of $2,150. The net proceeds from the offering have been used as described in the final prospectus filed with the SEC on June 28, 2021, under File No. 333-253852.
ITEM 15. CONTROLS AND PROCEDURES
(a) Disclosure Controls and Procedures
Our disclosure controls and procedures are designed to provide reasonable assurance of achieving the desired control objectives. Our management, including our Chief Executive Officer and Chief Financial Officer, recognizes that any control system, no matter how well designed and operated, is based upon certain judgments and assumptions and cannot provide absolute assurance that its objectives will be met. Similarly, an evaluation of controls cannot provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been detected.
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(d) and 15d-15(e) under the Exchange Act) as of December 31, 2022, or the Evaluation Date. Based on such evaluation, those officers have concluded that, as of the Evaluation Date, our disclosure controls and procedures are effective in recording, processing, summarizing and reporting, on a timely basis, information required to be included in periodic filings under the Exchange Act and that such information is accumulated and communicated to management, including our principal executive and financial officers, as appropriate to allow timely decisions regarding required disclosure.
(b) Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) under the Exchange Act. Our management conducted an assessment of the effectiveness of our internal control over financial reporting as of December 31, 2022, based on the criteria set forth in Internal Control-Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on that assessment, our management concluded that our internal control over financial reporting was not effective as of December 31, 2022, due to insufficient number of financial reporting personnel with an appropriate level of knowledge, experience, and training in application of IFRS and SEC rules and regulations commensurate with our reporting requirements, 15 deficiencies identified and a large number of controls that were not performed.
As defined in Regulation 12b-2 under the Securities Exchange Act, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis.
We have taken action toward remediating these material weaknesses by providing enhanced training to existing financial and accounting employees on related IFRS issues. In addition, to remediate these material weaknesses, we are implementing measures and developing, communicating, and implementing an accounting policy manual for our financial reporting personnel for recurring transactions, period-end closing processes and policy relating to segregation of duties.
Although we believe there has been some progress in remediating this weakness, the implementation of these initiatives may not fully address any material weakness or other deficiencies that we may have in our internal control over financial reporting.
(c) Attestation Report of the Registered Public Accounting Firm
This annual report does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting due to a transition period established by rules of the SEC for newly public companies.
105
(d) Changes in Internal Control over Financial Reporting
During the year ended December 31, 2022, there were no changes in our internal control over financial reporting that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
(e) Limitations on Effectiveness of Controls and Procedures
The effectiveness of any system of internal control over financial reporting, including ours, is subject to inherent limitations, including the exercise of judgment in designing, implementing, operating, and evaluating the controls and procedures, and the inability to eliminate misconduct completely. Accordingly, any system of internal control over financial reporting, including ours, no matter how well designed and operated, can only provide reasonable, not absolute assurances. In addition, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. We intend to continue to monitor and upgrade our internal controls as necessary or appropriate for our business, but there can be no assurance that such improvements will be sufficient to provide us with effective internal control over financial reporting.
ITEM 16. RESERVED
ITEM 16A. AUDIT AND RISK COMMITTEE FINANCIAL EXPERT
Our board of directors has appointed an audit and risk committee, which assists our board of directors in monitoring and reviewing any matters of significance affecting financial reporting and compliance. Our audit and risk committee, acting pursuant to a written charter, is comprised of Mr. Urs Wettstein, Mr. Zeev Rotstein and Mr. Kenneth Melani. The chairman of this committee is Mr. Urs Wettstein, who serves as the financial expert of the committee. Our board of directors is primarily responsible for the oversight of our risk management and internal compliance and control framework.
ITEM 16B. CODE OF ETHICS
Our board of directors has adopted a Code of Ethics applicable to all of our directors and employees, including our Chief Executive Officer, Chief Financial Officer, controller or principal accounting officer, or other persons performing similar functions, which is a “code of ethics” as defined in Item 16B of Form 20-F promulgated by the SEC. The full text of the Code of Ethics is posted on our website at www.gmedinnovations.com. Information contained on, or that can be accessed through, our website does not constitute a part of this a part of this annual report on Form 20-F and is not incorporated by reference herein. If we make any amendment to the Code of Ethics or grant any waivers, including any implicit waiver, from a provision of the Code of Ethics, we will disclose the nature of such amendment or waiver on our website to the extent required by the rules and regulations of the SEC. We have not granted any waivers under our Code of Business Conduct and Ethics.
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table provides information regarding fees paid by us to Ziv Haft and/or other member firms of BDO for all services, including audit services, for the years ended December 31, 2022 and 2021:
| 2022 | 2021 | |||||||
| (USD in thousands) | ||||||||
| Audit fees (1) | 169,000 | 169,000 | ||||||
| Audit-Related Fees | - | - | ||||||
| Tax fees (2) | - | - | ||||||
| All other fees | - | - | ||||||
| Total | 169,000 | 169,000 | ||||||
| (1) | The audit fees for the years ended December 31, 2022 and 2021 includes professional services rendered in connection with the audit of our annual consolidated financial statements and the review of our consolidated interim financial statements, statutory audits of the Company and its subsidiaries, issuance of consents and assistance with review of documents filed with the SEC. |
| (2) | Tax fees for the years ended December 31, 2022 and 2021 were for services related to tax advice, including assistance with tax audit. |
106
Pre-Approval of Auditors’ Compensation
Our audit and risk committee has a pre-approval policy for the engagement of our independent registered public accounting firm to perform certain audit and non-audit services. Pursuant to this policy, which is designed to assure that such engagements do not impair the independence of our auditors, the audit and risk committee pre-approves annually a catalog of specific audit and non-audit services in the categories of audit services, audit-related services and tax services that may be performed by our independent registered public accounting firm. If a type of service, that is to be provided by our auditors, has not received such general pre-approval, it will require specific pre-approval by our audit and risk committee. The policy prohibits retention of the independent registered public accounting firm to perform the prohibited non-audit functions defined in applicable SEC rules.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES
Not applicable.
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
In May 2022, our board of directors authorized a share repurchase plan (or the Repurchase Plan), allowing the Company to invest up to $1,000,000 to repurchase our Ordinary Shares from time to time, in open market transactions, and/or in privately negotiated transactions or in any other legally permissible ways, depending on market conditions, share price, trading volume and other factors. Such repurchases will be made in accordance with applicable U.S. securities laws and regulations, under the Exchange Act, and other applicable law, and are subject to the approval of the Israeli court, which was granted in September 2022. Under the Repurchase Plan, we may repurchase all or a portion of the authorized repurchase amount. The Repurchase Plan does not obligate us to repurchase any specific number of the ordinary shares and may be suspended or terminated at any time at management’s discretion. As of May 15, 2023, 5,699 shares have been repurchased, at an average price per share of $21.18, under the Repurchase Plan.
ITEM 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT
Not applicable.
107
ITEM 16G. CORPORATE GOVERNANCE
The Sarbanes-Oxley Act, as well as related rules subsequently implemented by the SEC, require “foreign private issuers,” such as us, to comply with various corporate governance practices. In addition, following the listing of the Ordinary Shares on the Nasdaq Capital Market, we will be required to comply with the Nasdaq Stock Market Rules. Under those rules, we may elect to follow certain corporate governance practices permitted under the Cayman Islands Companies Act in lieu of compliance with corresponding corporate governance requirements otherwise imposed by the Nasdaq Stock Market Rules for U.S. domestic registrants.
In accordance with Cayman law and practice and subject to the exemption set forth in Rule 5615 of the Nasdaq Stock Market Rules, we have elected to follow the provisions of the Cayman Islands Companies Act rather than the Nasdaq Stock Market Rules, with respect to the following requirements:
| ● | Quorum. While the Nasdaq Stock Market Rules require that the quorum for purposes of any meeting of the holders of a listed company’s common voting stock, as specified in the company’s bylaws, be no less than 33 1/3% of the company’s issued and outstanding common voting stock, under Cayman Islands Companies Act there is no minimum attendance threshold for general meetings of shareholders to be quorate. Our Post-Reduction Amended and Restated Memorandum and Articles of Association provide that twenty five percent (25%) of our issued and outstanding shares present, in person or by proxy or a duly appointed representative, who are entitled to vote on the business to be transacted, shall constitute a quorum for a general meeting. However, the quorum set forth in our Amended and Restated Memorandum and Articles of Association with respect to an adjourned meeting consists of at least one shareholder present in person or by proxy. For additional information see “Description of Share Capital and Governing Documents—Material Differences in Corporate Law.” |
| ● | Shareholder approval. While the Nasdaq Stock Market Rules require that issuers obtain shareholder approval prior to the issuance of securities in connection with certain acquisitions, private placements of securities, or the establishment or amendment of certain stock option, purchase or other compensation plans, under the Cayman Islands Companies Act, there is no requirement for shareholder approval of share issuances (or the terms on which shares may be issued) and no statutory pre-emption rights apply. Under our Amended and Restated Memorandum and Articles of Association, our board of directors is authorized to issue shares (or to grant warrants, options or other rights to acquire shares) subject to the restriction that the number of shares in issue may not exceed our authorized share capital set forth in our Amended and Restated Memorandum and Articles of Association and generally subject to the Cayman Islands Companies Act and our Amended and Restated Memorandum and Articles of Association. |
However, we have voluntarily elected to adhere to the Nasdaq Stock Market Rules and not the Cayman Islands Companies Act notwithstanding our status as a foreign private issuer, with respect to the requirements of:
| ● | maintaining a board of directors with a majority of “independent” directors and having those directors meet regularly without other members present; |
| ● | maintaining a compensation committee of our board of directors (which is our Nomination and Remuneration Committee) comprised solely of independent directors and governed by a committee charter; and |
| ● | having director nominees be selected or recommended for selection by either a majority of our independent directors or a nominations committee comprised solely of independent directors, which will be undertaken by our Nomination and Remuneration Committee. |
ITEM 16H. MINE SAFETY DISCLOSURE
Not applicable.
ITEM 16I. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
108
PART III
ITEM 17. FINANCIAL STATEMENTS
We have elected to provide financial statements and related information pursuant to Item 18.
ITEM 18. FINANCIAL STATEMENTS
The consolidated financial statements and the related notes required by this Item are included in this annual report on Form 20-F beginning on page F-1.
ITEM 19. EXHIBITS
109
| * | Filed herewith. |
| & | Furnished herewith |
| ^ | Certain confidential information contained in this exhibit, has been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K, because it (i) is not material and (ii) would be competitively harmful if publicly disclosed. |
110
SIGNATURES
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on Form 20-F filed on its behalf.
| G MEDICAL INNOVATIONS HOLDINGS LTD. | ||
| Date: May 16, 2023 | By: | /s/ Yacov Geva |
| Yacov Geva | ||
| President, CEO and Director | ||
111
G MEDICAL INNOVATIONS HOLDINGS LTD.
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2022
GMEDICAL INNOVATIONS HOLDINGS LTD.
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2022
TABLE OF CONTENTS
_______________________
________________
____________
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To The Board of Directors and Stockholders of G Medical Innovations Holdings Ltd.
Rehovot, Israel.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statements of financial position of G Medical Innovations Holdings Ltd. and its subsidiaries (the “Company”) as of December 31, 2022 and 2021, the related consolidated statements of comprehensive loss, changes in shareholders’ deficit, and cash flows for each of the three years in the period ended December 31, 2022, and the related notes (collectively, the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2022, in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
| /s/ | |
| Certified Public Accountants (Isr.) | |
| BDO Member Firm |
We have served as the Company’s auditor since 2015.
May 16, 2023
F-2
G MEDICAL INNOVATIONS HOLDINGS LTD.
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
| December 31, 2022 | December 31, 2021 | |||||||||
| Note | US$ in thousands | |||||||||
| ASSETS | ||||||||||
| CURRENT ASSETS: | ||||||||||
| Cash and cash equivalents | ||||||||||
| Restricted deposit | ||||||||||
| Inventory, net | 4 | |||||||||
| Trade receivables, net | 5 | |||||||||
| Other accounts receivable | 6 | |||||||||
| Loan to major shareholder, net | 10 | |||||||||
| Total current assets | ||||||||||
| NON-CURRENT ASSETS: | ||||||||||
| Other accounts receivable | 6 | |||||||||
| Property, plant and equipment, net | 7,14 | |||||||||
| Total non-current assets | ||||||||||
| Total assets | ||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-3
G MEDICAL INNOVATIONS HOLDINGS LTD.
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
| December 31, 2022 | December 31, 2021 | |||||||||||
| Note | US$ in thousands | |||||||||||
| LIABILITIES AND SHAREHOLDERS’ DEFICIT | ||||||||||||
| CURRENT LIABILITIES: | ||||||||||||
| Short term loan and current portion of long-term loans | 11 | |||||||||||
| Trade payables | ||||||||||||
| Other accounts payable | 9 | |||||||||||
| Financial liability at fair value | 8 | |||||||||||
| Short term convertible securities at fair value | 8,23 | |||||||||||
| Convertible securities | 8,23 | |||||||||||
| Derivative liabilities at fair value – warrants | 8,23 | |||||||||||
| Loan from major shareholder at fair value | 10 | |||||||||||
| Short term portion of lease liability | 14 | |||||||||||
| Total current liabilities | ||||||||||||
| NON-CURRENT LIABILITIES: | ||||||||||||
| Long term convertible securities at fair value | 8,23 | |||||||||||
| Long term loans | 11 | |||||||||||
| Long term lease liability | 14 | |||||||||||
| Total non-current liabilities | ||||||||||||
| SHAREHOLDERS’ DEFICIT: | 13 | |||||||||||
| Ordinary Shares; $ | ||||||||||||
| Other reserve | ||||||||||||
| Translation reserve | ||||||||||||
| Additional paid in capital | ||||||||||||
| Treasury shares | ( | ) | ||||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||||||
| G Medical innovations holdings ltd. Shareholders’ deficit | ( | ) | ( | ) | ||||||||
| Non-controlling interest | ||||||||||||
| Total shareholders’ deficit | ( | ) | ( | ) | ||||||||
| TOTAL LIABILITIES AND SHAREHOLDERS’ DEFICIT | ||||||||||||
| (*) |
| May 15, 2023 | ||||||
| Date of approval of the financial statements | Igor Bluvstein Chief Financial Officer | Dr. Yacov Geva President and Chief Executive Officer | Dr. Kenneth R. Melani Chairman of the Board of Directors |
The accompanying notes are an integral part of the consolidated financial statements.
F-4
G MEDICAL INNOVATIONS HOLDINGS LTD.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
| Year ended December 31, 2022 |
Year ended December 31, 2021 |
Year ended December 31, 2020 |
||||||||||||
| Note | US$ in thousands (except for share data) | |||||||||||||
| Revenue: | 2.S | |||||||||||||
| Products | ||||||||||||||
| Services | ||||||||||||||
| Total revenue | ||||||||||||||
| Cost of revenue: | ||||||||||||||
| Cost of sales of products | ||||||||||||||
| Cost of services | 15 | |||||||||||||
| Total cost of revenue | ||||||||||||||
| Gross profit (loss) | ||||||||||||||
| Operating expenses: | ||||||||||||||
| Research and development expenses | 16 | |||||||||||||
| Selling, general and administrative expenses | 17.A | |||||||||||||
| Operating loss | ||||||||||||||
| Financial income | ||||||||||||||
| Financial expense | ||||||||||||||
| Financial income (expense), net | 17.B | ( |
) | ( |
) | |||||||||
| Loss before tax | ||||||||||||||
| Income tax expense (benefit) | 19 | ( |
) | ( |
) | |||||||||
| Loss for the year from continuing operations | ||||||||||||||
| Loss from discontinued operations | 22 | - | ||||||||||||
| Total Loss for the year | ||||||||||||||
| Loss for the year attributed to: | ||||||||||||||
| Non-controlling interests | ||||||||||||||
| G Medical innovations holdings ltd. shareholders’ | ||||||||||||||
| Loss from discontinued operations | 22 | |||||||||||||
| G Medical innovations holdings ltd. shareholders’ | ||||||||||||||
| $ | ( |
) | $ | *( |
) | $ | *( |
) | ||||||
| $ | ( |
) | $ | ( |
) | |||||||||
| 18 | $ | ( |
) | $ | *( |
) | $ | *( |
) | |||||
| Weighted average ordinary shares outstanding: | * |
* |
||||||||||||
| * |
The accompanying notes are an integral part of the consolidated financial statements.
F-5
G MEDICAL INNOVATIONS HOLDINGS LTD.
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
| G Medical Innovations Holdings Ltd. Shareholders’ Deficit | ||||||||||||||||||||||||||||||||||||
| Share capital | Other reserve | Translation reserve | Additional paid in capital | Accumulated deficit | Treasury Shares | Total | Non-controlling Interest | Total shareholders’ deficit | ||||||||||||||||||||||||||||
| US$ in thousands | ||||||||||||||||||||||||||||||||||||
| Balance at December 31, 2021 | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
| Changes during the year: | ||||||||||||||||||||||||||||||||||||
| Issuance of ordinary shares, net | ||||||||||||||||||||||||||||||||||||
| Issuance of ordinary shares in respect of warrants and pre-funded warrants exercise | ||||||||||||||||||||||||||||||||||||
| Share-based compensation | ||||||||||||||||||||||||||||||||||||
| Issuance of ordinary shares in return of loan from major shareholder | ( | ) | ||||||||||||||||||||||||||||||||||
| Treasury shares | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||||
| Comprehensive loss for the period: | ||||||||||||||||||||||||||||||||||||
| Net comprehensive loss | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||
| Balance at December 31, 2022 | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-6
G MEDICAL INNOVATIONS HOLDINGS LTD.
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ DEFICIT
| G Medical Innovations Holdings Ltd. Shareholders’ Deficit | ||||||||||||||||||||||||||||||||
| Share capital | Other reserve | Translation reserve | Additional paid in capital | Accumulated deficit | Total | Non-controlling Interest | Total shareholders’ deficit | |||||||||||||||||||||||||
| US$ in thousands | ||||||||||||||||||||||||||||||||
| Balance at January 1, 2020 | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||
| Changes during the year: | ||||||||||||||||||||||||||||||||
| Issuance of ordinary shares, net | ||||||||||||||||||||||||||||||||
| Share based compensation | ||||||||||||||||||||||||||||||||
| Conversion of financial liability to shares | ||||||||||||||||||||||||||||||||
| Conversion of loans from major shareholder into shares | ||||||||||||||||||||||||||||||||
| Comprehensive loss for the period: | ||||||||||||||||||||||||||||||||
| Net comprehensive loss | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||
| Balance at December 31, 2020 | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||
| Changes during the year: | ||||||||||||||||||||||||||||||||
| Issuance of ordinary shares and warrants, net | ||||||||||||||||||||||||||||||||
| Share based compensation | ||||||||||||||||||||||||||||||||
| Conversion of loans into shares | ||||||||||||||||||||||||||||||||
| Comprehensive loss for the period: | ||||||||||||||||||||||||||||||||
| Net comprehensive loss | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||
| Balance at December 31, 2021 | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-7
G MEDICAL INNOVATIONS HOLDINGS LTD.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year ended December 31, 2022 | Year ended December 31, 2021 | Year ended December 31, 2020 | ||||||||||
| US$ in thousands | ||||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss for the year | ( | ) | ( | ) | ( | ) | ||||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation and amortization | ||||||||||||
| Loss from inventory write off | ||||||||||||
| Impairment of goodwill | ||||||||||||
| Change in fair value of derivatives | ( | ) | ( | ) | ( | ) | ||||||
| Change in fair value of financial liability | ||||||||||||
| Accrued interest on convertible security | ||||||||||||
| Revaluation of restricted deposit | ||||||||||||
| Share based compensation | ||||||||||||
| Forgiveness of PPP loan | ( | ) | ||||||||||
| Accrued interest of long-term loans | ||||||||||||
| Changes in deferred taxes | ( | ) | ( | ) | ||||||||
| Loss from disposal equipment | ||||||||||||
| Change in fair value of Convertible loans | ( | ) | ||||||||||
| Decrease (increase) in trade receivable, net | ( | ) | ( | ) | ||||||||
| Increase in other accounts receivable | ( | ) | ( | ) | ||||||||
| Increase (decrease) in inventories | ( | ) | ( | ) | ||||||||
| Increase (decrease) in trade payables | ( | ) | ||||||||||
| Increase (decrease) in other accounts payable | ( | ) | ||||||||||
| Accrued interest on loans from major shareholder | - | |||||||||||
| Capital loss from sale of fixed assets | - | |||||||||||
| Financial expenses, net | ( | ) | ||||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ( | ) | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||||||
| Purchase of property, plant and equipment | ( | ) | ( | ) | ( | ) | ||||||
| Purchase of other assets | ( | ) | ||||||||||
| Proceeds from sales of property, plant and equipment | - | |||||||||||
| Change in restricted deposit | ( | ) | ||||||||||
| Net cash used in Investing activities | ( | ) | ( | ) | ( | ) | ||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||
| Issuance of shares and warrants, net | ||||||||||||
| Issuance of ordinary shares in respect of warrants and pre-funded warrants exercise, net | ||||||||||||
| Receipts (repayment) of loan from major shareholder, net | ( | ) | ||||||||||
| Issuance of convertible securities and warrants | ||||||||||||
| Receipts of long-term loans from bank | ||||||||||||
| Principal paid on lease liabilities | ( | ) | ( | ) | ( | ) | ||||||
| Repayment of convertible securities and financial liability | ( | ) | ( | ) | ( | ) | ||||||
| Changes in short term bank credit | ( | ) | ||||||||||
| Purchase of Treasury Shares | ( | ) | ||||||||||
| Repayment of loans | ( | ) | ( | ) | ( | ) | ||||||
| Net cash provided by financing activities | ||||||||||||
| Increase in cash and cash equivalents | ( | ) | ||||||||||
| Effects of exchange rate changes on cash and cash equivalents | ||||||||||||
| Cash and cash equivalents at beginning of the year | ||||||||||||
| Cash and cash equivalents at the end of the year | ||||||||||||
| * |
F-8
G MEDICAL INNOVATIONS HOLDINGS LTD.
CONSOLIDATED STATEMENTS OF CASH FLOWS
APPENDIX A - AMOUNTS PAID DURING THE YEAR FOR:
| Year ended December 31, 2022 | Year ended December 31, 2021 | Year ended December 31, 2020 | ||||||||||
| US$ in thousands | ||||||||||||
| Interest | ||||||||||||
| Tax | ||||||||||||
APPENDIX B – NON-CASH ACTIVITIES:
| Year ended December 31, 2022 | Year ended December 31, 2021 | Year ended December 31, 2020 | ||||||||||
| US$ in thousands | ||||||||||||
| Conversion of convertible loan and loans into shares and warrants | - | |||||||||||
| Share issuance related to exercise of warrants and Pre-funded warrants | ||||||||||||
| Convertible securities - classification into financial liability at fair value | ||||||||||||
| Conversion of loan from major shareholder into shares | ||||||||||||
| Recognition of right of use assets and lease liabilities | ||||||||||||
| Convertible securities - classification into convertible financial liability at amortized cost | ||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-9
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 1 - DESCRIPTION OF BUSINESS:
Overview:
G Medical Innovations Holdings Ltd. (“G Medical” and together with its subsidiaries, the “Company”) was incorporated in October 2014 under Cayman Islands law. G Medical’s registered address is P.O. Box 10008, Willow House, Cricket Square, Grand Cayman, KY1-1001, Cayman Islands.
In May 2017, the Company was admitted to the official list on the Australian Stock Exchange (“ASX”) under the symbol GMV. In October 2020, the Company voluntarily delisted itself from the official list on the ASX.
In June 2021,
The Company is an early commercial stage healthcare company engaged in the development of next generation mobile health (or mHealth) and telemedicine solutions, and monitoring service platforms. The Company believes that it is at the forefront of the digital health revolution in developing the next generation of mobile technologies and services that are designed to empower consumers, patients and providers to better monitor, manage and improve clinical and personal health outcomes, especially for those who suffer from cardiovascular disease (“CVD”), pulmonary disease and diabetes. In addition, in December 2021 the Company started a new business activity of COVID-19 testing, in the State of California, providing four kinds of diagnostic tests –Rapid Antigen, A/B Flu, PCR and Antibody tests which has been discontinued during the second half of 2022 (see Note 22).
In July 2022 the Company started a new operations of Direct-To-Customer health testing kits health issues ranging from food sensitivity, indoor/outdoor allergies, HPV, thyroid functioning, testosterone levels, and the hemoglobin A1C test, with results going directly to the user within days. This operation is still in an early stage and no revenue has been recorded in 2022.
The accompanying consolidated financial statements have been prepared
assuming that the Company will continue as a going concern. The Company incurred a net loss of $
These consolidated financial statements of the Company were authorized for issue by the Board of Directors on May 15, 2023.
F-10
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES:
The principal accounting policies adopted in the preparation of the consolidated financial statements are set out below. The policies have been consistently applied to all the years presented, unless otherwise stated.
| A. | Basis of preparation |
These consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (“IASB”). The consolidated financial statements have been prepared under the historical cost convention except for certain financial liabilities, which are measured at fair value until conversion. The Company has elected to present the consolidated statement of comprehensive loss using the function of expense method.
| B. | Basis of consolidation |
Where the Company has control over an investee, it is classified as a subsidiary. The Company controls an investee if all three of the following elements are present: power over the investee, exposure to variable returns from the investee, and the ability of the investor to use its power to affect those variable returns. Control is reassessed whenever facts and circumstances indicate that there may be a change in any of these elements of control. The consolidated financial statements present the results of the Company and its subsidiaries as if they formed a single entity. Intercompany transactions and balances between group companies are therefore eliminated in full.
| C. | Organizational Structure |
The consolidated financial statements of the Company include the accounts of the Company and the following subsidiaries:
| Entity name | State incorporated | Percent ownership | ||
| G Medical Innovations Holdings Ltd. | ||||
| G Medical Innovations Ltd. | ||||
| G Medical Innovations Asia Ltd. | ||||
| G Medical Innovations UK Ltd.* | ||||
| Guangzhou Yimei Innovative Medical Science and Technology Co., Ltd. | ||||
| G Medical Innovations MK Ltd. | ||||
| G Medical Innovations USA Inc. | ||||
| G Medical Diagnostic Services, Inc. (Formerly CardioStaff Diagnostic Services Inc) | ||||
| Telerhythmics, LLC | ||||
| G Medical Tests and Services, Inc** | ||||
| G Medical Lab Services, Inc** | ||||
| G Medical Mobile Health Solution, Inc | ||||
| G Medical Health and Wellness, Inc*** | ||||
| G Medical Health and Wellness Lab, Inc*** |
| * |
| ** |
| *** |
F-11
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (CONT.):
| C. | Organizational Structure (cont.) |
The consolidated financial statements incorporate the results of business combinations using the acquisition method. In the statement of financial position, the acquirer’s identifiable assets, liabilities and contingent liabilities are initially recognized at their fair values at the acquisition date. The results of acquired operations are included in the consolidated statement of comprehensive loss from the date on which control is obtained. They are deconsolidated from the date on which control ceases. The goodwill represents the excess of the costs of a business combination over the interest in the fair value of identifiable assets, liabilities and contingent liabilities acquired. Cost of a business combination are comprised of the fair values of assets given, liabilities assumed and equity instruments issued. Any costs of acquisition are charged to profit or loss. The Company recognizes any non-controlling interest in its acquisitions on an acquisition-by-acquisition basis, either at fair value or at the non-controlling interest’s proportionate share of the recognized amounts of acquirer’s identifiable net assets.
| D. | Transaction with Non-controlling interests |
Transactions with non-controlling interests that do not result in loss of control is accounted for as equity transactions – that is, as transactions with the owners in their capacity as owners. The difference between fair value of any consideration paid and the relevant share acquired of the carrying value of net assets of the subsidiary is recorded in equity. Gains or losses on disposals to non-controlling interests are also recorded in equity.
| E. | Use of estimates and assumptions in the preparation of the consolidated financial statements |
The preparation of consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities including estimates for transactions with the major shareholder, disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenues and expenses during the reporting periods. By their nature, these estimates are subject to measurement uncertainty and are reviewed periodically and adjustments, if necessary, are made in the year which they are identified. Actual results could differ from those estimates.
The Company uses estimates that affect the reported amounts regarding derivative liabilities – warrants, convertible securities and share based compensation (See also Note 3).
| F. | Non-controlling interests |
Total comprehensive loss of non-wholly owned subsidiaries is attributed to owners of the parent and to the non-controlling interests in proportion to their relative ownership interests.
| G. | Foreign currency |
The consolidated financial statements are prepared in U.S. Dollars (the functional currency). Transactions and balances in foreign currencies are converted into U.S. Dollars in accordance with the principles set forth by International Accounting Standard (IAS) 21 “The Effects of Changes in Foreign Exchange Rates”. Accordingly, transactions and balances have been converted as follows:
| ● | Monetary assets and liabilities – at the rate of exchange applicable at the consolidated statements of financial position date. |
| ● | Exchange gains and losses from the aforementioned conversion are recognized in the statement of comprehensive loss. |
| ● | Expense items – at exchange rates applicable as of the date of recognition of those items. |
| ● | Non-monetary items are converted at the rate of exchange used to convert the related consolidated statements of financial position items, i.e., at the time of the transaction. |
F-12
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (CONT.):
| H. | Cash and cash equivalents |
Cash equivalents are considered by the Company to be highly liquid investments, including, short-term deposits with banks and the maturity of which do not exceed three months at the time of deposit, and which are not restricted.
| I. | Restricted deposit |
Restricted deposit is considered by the Company to be deposits with banks which are used mainly as a security for guarantees provided against payable payments in advance.
| J. | Research and development |
Research costs are expensed as incurred. Development expenditures on an individual project are recognized as an intangible asset when the Company can demonstrate:
| ● | The product is technically and commercially feasible. |
| ● | The Company intend to complete the product so that it will be available for use or sale. |
| ● | The Company has the ability to use the product or sell it. |
| ● | The Company has the technical, financial and other resources to complete the development and to use or sell the product. |
| ● | The Company can demonstrate the probability that the product will generate future economic benefits. |
| ● | The Company is able to measure reliability the expenditure attributable to the product during the development. |
During the years 2022 and 2021 the Company did not meet the above criteria therefore all the development costs have been recognized as expenses.
| K. | Leases |
The Company applied the following practical expedients when applying IFRS 16 (January 1, 2019) to leases previously classified as operating leases:
| ● | Applied a single discount rate to a portfolio of leases with reasonably similar characteristics. |
| ● | Applied the exemption not to recognize right-of-use assets and liabilities for leases with less than 12 months of lease term remaining as of the date of initial application and do not contain a purchase option. |
| ● | Applied the practical expedient provided by the standard to recognize right-of-use assets equal to the lease liability upon initial application. |
Under IFRS 16, the Company recognizes right-of-use assets and lease liabilities for most leases.
Right-of-use assets:
The Company recognizes right-of-use assets at the commencement date of the lease (i.e., the date the underlying asset is available for use). Right-of-use assets are measured at cost, less any accumulated depreciation and any accumulated impairment losses, and adjusted for any re-measurement of lease liabilities. The cost of right-of-use assets comprises the amount of the initial measurement of the lease liability; lease payments made at or before the commencement date less any lease incentives received; and initial direct costs incurred. The recognized right-of-use assets are depreciated on a straight-line basis over the shorter of its estimated useful life and the lease term. Right-of-use assets are subject to impairment. The right-of-use assets are presented within property, plant and equipment.
F-13
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (CONT.):
| K. | Leases (cont.) |
Lease liabilities:
At the commencement date of the lease, the Company recognizes lease liabilities measured at the present value of lease payments to be made over the lease term. The lease payments include fixed payments (including in substance fixed payments) less any lease incentives receivable, variable lease payments that depend on an index or a rate, and amounts expected to be paid under residual value guarantees. The lease payments also include the exercise price of a purchase option that is reasonably certain to be exercised by the Company and payments of penalties for terminating a lease, if the lease term reflects the Company exercising the option to terminate. The variable lease payments that do not depend on an index or a rate are recognized as expense in the period on which the event or condition that triggers the payment occurs.
Lease term:
The term of a lease is determined as the non-cancellable period for which a lessee has the right to use an underlying asset, together with periods covered by an option to extend the lease if the lessee is reasonably certain to exercise that option periods.
| L. | Earnings per share |
Basic and diluted earnings per share is calculated as net loss attributable to the shareholders of the Company, divided by the weighted average number of ordinary shares in circulation during the year.
| M. | Government grant |
Government grants are not recognized before there is reasonable assurance that the Company would comply with the conditions attached and that the grants would be accepted.
When entitlement to a government grant is created as compensation for expenses or losses already incurred or in order to provide immediate support to the Company without any future reference costs, the Company recognized the grant in profit or loss during the period in which entitlement to the grant was created.
In cases other than the above, government grants have been recognized in profit or loss on a systematic basis over periods that the Company recognizes costs that are referred to as expenses for which the grants are intended to provide compensation.
Grants relating to the expense were
recorded in the statement of comprehensive loss less the related expenses. In April 2020, under the Coronavirus Aid, Relief, and Economic
Security Act (the “CARES Act”) in the United States the U.S subsidiary signed an agreement with Small Business Administration
(“SBA”) receive a loan according to the Paycheck Protection Program (“PPP”) in the amount of approximately $
F-14
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (CONT.):
| N. | Provisions |
Provisions are recognized when the Company has a legal or constructive obligation, as a result of past events, for which it is probable that an outflow of economic benefits will result and that outflow can be reliably measured. Provisions are measured using the best estimate of the amounts required to settle the obligation at the end of the reporting period.
| O. | Fair value measurement |
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value measurement is based on the presumption that the transaction to sell the asset or transfer the liability takes place either:
| 1. | In the principal market for the asset or liability, or |
| 2. | In the absence of a principal market, in the most advantageous market for the asset or liability. |
The principal or the most advantageous market must be accessible to the Company. The fair value of an asset or a liability is measured using the assumptions that market participants would use when pricing the asset or liability, assuming that market participants act in their economic best interest. A fair value measurement of a non-financial asset takes into account a market participant’s ability to generate economic benefits by using the asset in its highest and best use or by selling it to another market participant that would use the asset in its highest and best use.
The Company uses valuation techniques that are appropriate in the circumstances and for which sufficient data are available to measure fair value, maximizing the use of relevant observable inputs and minimizing the use of unobservable inputs.
Classification of fair value hierarchy
The financial instruments presented in the statement of financial position at fair value are grouped into classes with similar characteristics using the following fair value hierarchy which is determined based on the source of input used in measuring fair value:
| Level 1 | - | Quoted prices (unadjusted) in active markets for identical assets or liabilities. |
| Level 2 | - | Inputs other than quoted prices included within Level 1 that are observable either directly or indirectly. |
| Level 3 | - | Inputs that are not based on observable market data (valuation techniques which use inputs that are not based on observable market data). |
F-15
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (CONT.):
| P. | Financial instruments |
Financial assets
The Company classifies its financial assets into the following category, based on the business model for managing the financial asset and its contractual cash flow characteristics. The Company’s accounting policy for the relevant category is as follows:
Amortized cost: These assets arise principally from the services rendered to customers (e.g. trade receivables), but also incorporate other types of financial assets where the objective is to hold these assets in order to collect contractual cash flows and the contractual cash flows are solely payments of principal and interest. They are initially recognized at fair value plus transaction costs that are directly attributable to their acquisition or issue and are subsequently carried at amortized cost using the effective interest rate method, less provision for impairment. Impairment provisions for trade receivables are recognized based on the simplified approach within IFRS 9 using a provision matrix in the determination of the lifetime expected credit losses. During this process the probability of the non-payment of the trade receivables is assessed. This probability is then multiplied by the amount of the expected loss arising from default to determine the lifetime expected credit loss for the trade receivables. For trade receivables, which are reported net, such provisions are recorded in a separate provision account with the loss being recognized within general and administrative expenses in the consolidated statements of comprehensive income. On assessment that the trade receivable will not be collectable, the gross carrying value of the asset is written off against the associated provision.
Financial liabilities
The Company’s accounting policy for its financial liabilities is as follows:
Fair value through profit or loss: This category comprises of convertible securities and warrants which are carried in the consolidated statement of financial position at fair value with changes in fair value recognized in the consolidated statement of comprehensive income. The treatment of the changes in the credit risk of those items were designated for being recognized in other comprehensive income. Amortized cost: Other financial liabilities include bank borrowings, loans from banks, trade payables, loans from major shareholders, leases and financial liability are initially recognized at fair value less any transaction costs directly attributable to the issue of the instrument. Such liabilities are subsequently measured at amortized cost using the effective interest method, which ensures that any interest expense over the period is at a constant interest rate on the balance of the liability carried in the statement of financial position. Interest expense in this context includes initial transaction costs, as well as any interest or coupon payable while the liability is outstanding.
De-recognition
Financial assets - The Company derecognizes a financial asset when the contractual rights to the cash flows from the financial asset expire or it transfers the rights to receive the contractual cash flows.
Financial Liabilities - The Company derecognizes a financial liability when its contractual obligations are discharged or cancelled or expire.
F-16
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (CONT.):
| P. | Financial instruments (cont.) |
Impairment of financial assets
Expected credit losses (“ECL”) and their measurement:
In order to manage the credit risks associated with customer receivables, the Company aims to secure certain financial guarantees prior to entering into business relationship with its customers.
To this end, the Company has developed a four-level matrix, which is based on past experience and historical data along with projections of the future into consideration, in order to group the ECL:
| 1. | Receivable from sale of products – prepayment by credit card on the Company’s website. |
| 2. | Receivables from Medicare and Medicaid Services (“CMS”) – reimbursement, which the Company receives per the relevant Current Procedural Terminology (“CPT”) code rate for the services rendered to the patient covered by CMS. |
| 3. | Receivables from contracted third-party payors – the Company has negotiated amounts for its monitoring services provided to patients covered by commercial healthcare insurance carriers. |
| 4. | Receivables from non-contracted payors - non-contracted commercial and government insurance carriers often reimburse out of network rates provided for under the relevant CPT codes on a case rate basis. The transaction price is based on an average of the Company’s historical collection experience, and it is reviewed quarterly. |
ECL are measured as the unbiased probability-weighted present value of all cash shortfalls over the expected life of each financial asset. For receivables from services, ECL are mainly calculated with a statistical model using three major risk parameters: probability of default, loss given default, and exposure at default. The estimation of these risk parameters incorporates all available relevant information, not only historical and current loss data, but also reasonable and supportable forward-looking information reflected by the future expectation factors. This information includes macroeconomic factors (e.g., gross domestic product growth, unemployment rate, cost performance index) and forecasts of future economic conditions. For receivables from services, these forecasts are performed using a scenario analysis (base case, adverse, and optimistic scenarios).
The expected credit loss for customer receivables is measured using the simplified method in accordance with IFRS 9, which requires estimation of the life-time expected credit loss for trade receivables.
As of December 31, 2022 and December
31, 2021, ECL for trade and other account receivables were $
Definition of default, including reasons for selecting the definition
For the contracted and CMS portfolios, the Company has historical experience of collecting substantially most of the negotiated contractual rates and determined at contract inception that these customers, and or their related third-party payor that pays the Company on their behalf, have the intention and ability to pay the promised consideration. As such, the Company is not providing an implicit price concession but, rather, have chosen to accept the risk of default, and adjustments to the transaction price are recorded as bad debt expense. For non-contracted portfolios, the Company is providing an implicit price concession because the Company does not have a contract with the underlying payor, the result of which requires us to estimate transaction price based on historical cash collections utilizing the expected value method. Subsequent adjustments to the transaction price are recorded as an adjustment to revenue and not as an expense.
F-17
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (CONT.):
| P. | Financial instruments (cont.) |
Write-off policy
The Company writes off its financial assets if any of the following occur:
| ● | Inability to locate the debtor. |
| ● | Discharge of the debt in a bankruptcy. |
| ● | It is determined that the efforts to collect the debt are no longer cost effective given the size of receivable. |
| ● | Open debts over 18 months. |
The collections department must comply with the collection efforts outlined in the policy to collect on delinquent customer accounts before any write-offs are made.
| Q. | Impairment of non-financial assets |
Goodwill and other intangible assets that have an indefinite useful life are not subject to amortization and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be impaired. Other non-financial assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. An impairment loss is recognized for the amount by which the asset’s carrying amount exceeds its recoverable amount. Recoverable amount is the higher of an asset’s fair value less costs of disposal and value-in-use.
The value-in-use is the present value of the estimated future cash flows relating to the asset using a pre-tax discount rate specific to the asset or cash-generating unit to which the asset belongs. Assets that do not have independent cash flows are grouped together to form a cash-generating unit. A cash-generating unit is the smallest group of assets that independently generates cash flow and whose cash flow is largely independent of the cash flows generated by other assets.
| R. | Property, plant, and equipment |
Items of property, plant, and equipment are initially recognized at cost. Cost includes directly attributable costs and the estimated present value of any future costs of dismantling and removing items. Depreciation is computed by the straight-line method, based on the estimated useful lives of the assets, as follows:
| Estimated useful lives | ||
| Computers and electronic equipment | ||
| Furniture and equipment | ||
| Vehicles | ||
| Leasehold Improvement | ||
| Lab equipment |
F-18
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (CONT.):
| S. | Revenue recognition |
Service revenue
The Company’s revenue is generated primarily from providing cardiac monitoring services. Revenue is recognized when the Company satisfies a performance obligation by transferring service to a customer, and collectability of the contract consideration is probable. The Company’s revenue is measured based on consideration specified in the contract with each customer. Revenue is only recognized if it is highly probable that a subsequent change in its estimate would not result in a significant revenue reversal. The Company provides cardiac services using four types of monitors: Mobile Cardiac Telemetry (“MCT”), Event Extended Holter, Holter, and Prizma device’s RPM of vital signs. The Company’s services consist of the delivery of reports containing analysis of data captured by the physical device to the prescribing physician and the revenue is recognized upon the delivery of the customer’s report.
Billings for services reimbursed by third party payers, including Medicare and Medicaid, are recorded as revenue net of contractual allowances.
Contractual allowances are estimated based on historical collections by CPT code (Current Procedural Terminology) for specific payers or class of payers and represent the difference between the list price (the billing rate) and the reimbursement rate for each payer.
All of the Company’ Service Revenue are generated from service provided through an independent diagnostic testing facility model which allows the Company to bill Medicare, Medicaid, or one of the third-party healthcare insurers directly for services provided. The Company also receives reimbursement directly from patients through co-pays and self-pay arrangements.
A summary of the payment arrangements with payers is as follows:
| ● | CMS (Centers for Medicare & Medicaid Services) - the Company receive reimbursement per the relevant CPT (Current Procedural Terminology) code rate for the services rendered to the patient covered by CMS. |
| ● | Contracted third-party payers – the Company has negotiated amounts for its monitoring services provided to patients covered by commercial healthcare insurance carriers. |
| ● | Non-contracted payers - non-contracted commercial and government insurance carriers often reimburse out of network rates provided for under the relevant CPT codes on a case rate basis. The transaction price is based on an average of the Company’s historical collection experience, and it is reviewed quarterly. For the contracted and CMS portfolios the Company has historical experience of collecting most of the negotiated contractual rates and determined at contract inception that these customers, and or their related third-party payer that pays the Company on their behalf, have the intention and ability to pay the promised consideration. As such, the Company is not providing an implicit price concession but, rather, have chosen to accept the risk of default, and adjustments to the transaction price are recorded as bad debt expense. For non-contracted portfolios, the Company is providing an implicit price concession because the Company does not have a contract with the underlying payer, the result of which requires us to estimate transaction price based on historical cash collections utilizing the expected value method. Subsequent adjustments to the transaction price are recorded as an adjustment to revenue and not as bad debt expense. |
Covid-19 services
In December 2021 the Company started a new business activity of COVID-19 testing in the State of California, providing its COVID-19 testing services. There are two groups of payors from which the Company seeks payment for testing services provided to patients:
US federal government’s Health Resources & Services Administration program (“HRSA”). and Insurance companies.
F-19
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (CONT.):
| S. | Revenue recognition (cont.) |
US federal government’s Health Resources & Services Administration program (“HRSA”)
On March 22, 2022, the HRSA program stopped accepting claims for Covid-19 testing and treatment due to lack of sufficient funds. Despite requests from the Acting Director of the Office of Management and Budget and the White House Coordinator for Covid-19 Response for additional emergency funding for the uninsured program, emergency funding has not been allocated to the HRSA uninsured program as of the financial statement issuance date. If funding for the HRSA program is reinstituted in the future, the Company will submit eligible claims for reimbursement to HRSA.
For the twelve months ended December 31, 2022, the Company recognized
revenue in the amount of approximately $
Insurance companies - the Company has not yet recognized revenue due to the remaining uncertainty concerning the existence of enforceable contracts with the insurance companies in respect of collectability as it lacks sufficient experience with private insurance companies.
For accounting purposes, since the Company did not meet the revenue recognition criteria in accordance with IFRS 15 the Company will recognize revenue on cash basis.
Sale of devices
Sales of products consist of revenue from the sale of Prizma Medical Smartphone Case as well as future sale of Kits. The Company recognizes revenue at the amount to which it expects to be entitled when control of the products or services is transferred to its customers. Control is generally transferred when the Company has a present right to payment and title and the significant risks and rewards of ownership of products are transferred to its customers. There is limited judgement needed in identifying the point control passes: once physical delivery of the products to the agreed location has occurred, the Company no longer has physical possession, the Company usually will have a present right to payment (as a single payment on delivery) and retains none of the significant risks and rewards of the goods in question.
For most of the Company’s products sales, control transfers when products are shipped.
| T. | New standards, interpretations and amendments not yet effective. |
There are several standards, amendments to standards, and interpretations, which have been issued by the IASB that are effective in future accounting periods that the Company has decided not to adopt early.
F-20
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 2 - SIGNIFICANT ACCOUNTING POLICIES (CONT.):
| T. | New standards, interpretations and amendments not yet effective (cont.) |
The Company has not early adopted any standard, interpretation or amendment that has been issued but is not yet effective. There are a number of standards, amendments to standards, and interpretations which have been issued by the IASB that are effective in future accounting periods that the Group has decided not to adopt early.
The following amendments are effective for the period beginning January 1, 2023:
| ● | Disclosure of Accounting Policies (Amendments to IAS 1 and IFRS Practice Statement 2); |
| ● | Definition of Accounting Estimates (Amendments to IAS 8); and |
| ● | Deferred Tax Related to Assets and Liabilities arising from a Single Transaction (Amendments to IAS 12). |
The following amendments are effective for the period beginning January 1, 2024:
| ● | IFRS 16 Leases (Amendment – Liability in a Sale and Leaseback) |
| ● | IAS 1 Presentation of Financial Statements (Amendment – Classification of Liabilities as Current or Non-current) |
| ● | IAS 1 Presentation of Financial Statements (Amendment – Non-current Liabilities with Covenants) |
The Company is currently assessing the impact of these new accounting standards and amendments.
The Company does not expect any other standards issued by the IASB, but not yet effective, to have a material impact on the Company.
| U. | Inventories |
Inventories are initially recognized at cost, and subsequently at the lower of cost and net realizable value. Cost comprises all costs of purchase, costs of conversion and other costs incurred in bringing the inventories to their present location and condition. Weighted average cost is used to determine the cost of ordinarily interchangeable items. A provision is made to reduce excess and obsolete inventories to net realizable value.
| V. | Post-employment benefits |
One of the Company subsidiaries, has a post-employment benefits plan, The plan is financed by contributions to insurance companies and classified as defined contribution plans. The Company has contributed for all of its employee’s contribution plans pursuant to Section 14 to the Severance Pay Law which the Company pays fixed contributions and will have no legal or constructive obligation to pay further contributions if the fund does not hold sufficient amounts to pay all employee benefits relating to employee service in the current and prior periods.
| W. | Transactions with shareholders |
A loan received from the controlling shareholder is recorded on first recognition in the financial statements of the Company as a liability according to its fair value. The difference between the amount of the loan received or given and its fair value on the date of recognition for the first time is recognized in equity. After first recognition, the loan is presented in the financial statements of the Company in accordance with the effective interest method.
F-21
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 3 - CRITICAL ACCOUNTING ESTIMATES AND JUDGEMENTS:
Share based compensation
The Company measures the share-based expense and the cost of equity-settled transactions with employees by reference to the fair value of the equity instruments at the date at which they are granted. The measurement of the fair value of the share-based expense with service providers measured at the day that the service was provided. The fair value is determined using an accepted options pricing model (Black Scholes model and Monte-Carlo). The model is based on share price, grant date and on assumptions regarding expected volatility, expected life of the options, expected dividend, and a no risk interest rate. As for granted options which are settled in equity instruments, the fair value of the options at the grant date is charged to the statement of comprehensive loss over the vesting period. Non-market vesting conditions are taken into account by adjusting the number of equity instruments expected to vest at each reporting date so that, ultimately, the cumulative amount recognized over the vesting period is based on the number of options that eventually vest. Non-vesting conditions and market vesting conditions are factored into the fair value of the options granted. As long as all other vesting conditions are satisfied, a charge is made irrespective of whether the market vesting conditions are satisfied.
Share based compensation (cont.)
For Financial instruments recognized in Fair Value – See note 2)P).
Financial liabilities at fair value
The fair value of financial liabilities at fair value was estimated by using a Black Scholes model and Monte-Carlo simulation approach, which was aimed to model the value of the Company’s assets over time. The simulation approach was designed to take into account the terms and conditions financial liability, as well as the capital structure of the Company and the volatility of its assets. The valuation was performed based on management’s assumptions and projections.
Expected credit losses (“ECL”)
See accounting policy in Note 2.P.
NOTE 4 - INVENTORY:
| December 31, 2022 | December 31, 2021 | |||||||
| Raw materials | ||||||||
| Finished goods | ||||||||
| Total | ||||||||
The Company recorded an inventory write off in
the amount of $
F-22
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 5 – TRADE RECEIVABLES, NET:
| December 31, 2022 | December 31, 2021 | |||||||
| Customers | ||||||||
| Less expected credit loss | ( | ) | ( | ) | ||||
| Total | ||||||||
NOTE 6 - OTHER ACCOUNTS RECEIVABLE:
| December 31, 2022 | December 31, 2021 | |||||||
| Prepaid expenses | ||||||||
| Institutions | ||||||||
| Advances to suppliers | ||||||||
| other | ||||||||
| Total other accounts receivable | ||||||||
| Less long-term portion of prepaid expenses | ( | ) | ( | ) | ||||
| Total current other accounts receivable | ||||||||
NOTE 7 - PROPERTY, PLANT AND EQUIPMENT, NET:
| Computers and electronic equipment | Furniture and equipment |
Vehicles |
Leasehold | Right of use assets * | Total | |||||||||||||||||||
| Cost: | ||||||||||||||||||||||||
| Balance at January 1, 2021 | ||||||||||||||||||||||||
| Additions in 2021 | - | - | ||||||||||||||||||||||
| Disposals in 2021 | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||
| Balance at December 31, 2021 | ||||||||||||||||||||||||
| Accumulated depreciation: | ||||||||||||||||||||||||
| As of January 1, 2021 | ||||||||||||||||||||||||
| Additions | ||||||||||||||||||||||||
| Disposals | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||
| As of December 31, 2021 | ||||||||||||||||||||||||
| Net Book Value: | ||||||||||||||||||||||||
| As of December 31, 2021 | ||||||||||||||||||||||||
| * |
F-23
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 7 - PROPERTY, PLANT AND EQUIPMENT, NET (CONT.):
| Computers and electronic equipment | Furniture and equipment |
Vehicles |
Leasehold | Right of use assets | Total | |||||||||||||||||||
| Cost: | ||||||||||||||||||||||||
| As of January 1, 2022 | ||||||||||||||||||||||||
| Additions | ||||||||||||||||||||||||
| Disposals | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||
| As of December 31, 2022 | ||||||||||||||||||||||||
| Accumulated depreciation: | ||||||||||||||||||||||||
| As of January 1, 2022 | ||||||||||||||||||||||||
| Additions | ||||||||||||||||||||||||
| Disposals | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||||||
| As of December 31, 2022 | ||||||||||||||||||||||||
| Net Book Value: | ||||||||||||||||||||||||
| As of December 31, 2022 | ||||||||||||||||||||||||
| * | See | also Note 14 – Leases |
NOTE 8 - CONVERTIBLE SECURITIES AND FINANCIAL LIABILITY AT FAIR VALUE:
| A. |
For
lead manager services, the Company granted a warrant to purchase an aggregate of
The Convertible Securities Warrants were classified as a derivative financial liability and are re-measured each reporting date, with changes in fair value recognized in finance expense (income), net, since the exercise price is denominated in AUD and the functional currency of the Company is the USD.
The Company designated upon initial recognition that the Convertible Securities be measured at fair value through profit or loss. The transaction costs for lead manager services were recorded through profit or loss and equity proportionately among the fair value of the issued securities (notes, Convertible Securities, warrants, and ordinary shares).
As of December 31, 2020, the Company had fulfilled and repaid all its obligations under this Convertible Securities agreement.
As
of December 31, 2022 and 2021, the fair value amount of those warrants was $
F-24
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 8 - CONVERTIBLE SECURITIES AND FINANCIAL LIABILITY AT FAIR VALUE (CONT.):
| B. | On December 21, 2020, the
Company entered into a CLA transaction (the “CLA Transaction”), whereby the Company entered into a securities purchase agreement,
including convertible debentures and warrants to purchase the Company’s ordinary shares, with Alpha Capital Anstalt (“Alpha”),
pursuant to which the Company obtained a convertible loan in an aggregate amount of $ |
The December 2020 Financing Warrants have an exercise price per share equal to the per share price of the Company’s ordinary shares in the Nasdaq IPO in June, subject to standard adjustments and have a five-year term.
Alpha
was also provided a right to purchase $
In 2020, the convertible debentures, as well as the warrants and the right to purchase additional convertible debentures were classified as a derivative financial liability.
During October 2021 the company repaid the two convertible debentures to Alpha along with the accrued interest due.
As
of December 31, 2022 and 2021, the fair value amount of those warrants was $
| C. | In April 2021, the Company entered into a securities purchase
agreement, including convertible debentures and warrants to purchase the Company’s ordinary shares, with Jonathan B. Rubini (“Rubini”),
pursuant to which the Company obtained a convertible loan in an aggregate amount of $ |
In June 2022, the
Company and Rubini signed an amended and restated convertible debenture which amended the terms of the original convertible debenture
agreement signed on April 7, 2021. According to the amended and restated agreement the Company promised to pay Rubini the principal sum
of $
In October
2022, the Company and Rubini signed an additional amended and restated convertible debenture which amended the terms of the amended
and restated convertible debenture agreement by extending the repayment date of principal and interest. signed June 2022. According
to the amended and restated agreement the Company promised to pay Rubini the principal sum of $
Since the amendments
of this convertible debenture were considered to be non-substantial, the Company accounted for it as an adjustment to the existing liability.
The amended convertible debenture is now measured at amortized cost using the effective interest method and as of December 31, 2022,
the total amount of this convertible debenture liability was $
This convertible debenture was fully repaid in April 2023.
F-25
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 8 - CONVERTIBLE SECURITIES AND FINANCIAL LIABILITY AT FAIR VALUE (CONT.):
| D. | In December 2021, the Company entered into a Convertible
Loan Agreement (“Lind CLA Agreement”) transaction (the “December 2021 Note”) whereby the Company entered into
a securities purchase agreement relating to the purchase and sale of a senior convertible note for gross proceeds of US$ |
At the Company discretion, the repayments can be made in: (i) cash; (ii) ordinary shares (after ordinary shares are registered) (or the Repayment Shares); or a combination of both. Repayment Shares will be priced at 90% of the average of the five lowest daily VWAPs (Volume Weighted Average Price).
Additionally, the
December 2021 Note ranks senior to other of the Company debt. Further, the Lind CLA Agreement provides that Lind will also receive a
common shares purchase warrant to purchase up to
The Company has
repaid in full the convertible loan in two instalments, an amount of $
As of December 31,
2021, the fair value amount of this convertible note was $
Following the April
18, 2022, securities purchase agreement the warrants to purchase an aggregate of
| E. | In January 2022, the Company entered into a definitive securities
purchase agreement with Armistice Capital Fund Ltd (“Armistice”) for the issuance, in a private placement, of an aggregate
of |
The Ordinary Warrants
have an exercise price of $
The Ordinary Warrants and the pre-funded warrants were classified as a derivative financial liability.
F-26
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 8 - CONVERTIBLE SECURITIES AND FINANCIAL LIABILITY AT FAIR VALUE (CONT.):
| E. | (cont.) |
The initial fair
value of the Ordinary Warrants and of the pre-funded warrants at issuance was $
In July 2022, this warrants and other warrants which were issued to Armistice were canceled and new warrants were issued, see below in paragraph G.
The Company remeasured the pre-funded warrants fair value at March
25, 2022 (date of exercise) as $
All of those pre-funded
warrants were exercised on March 25, 2022, and
As a result of the
above, Lind Global Fund II LP (“Lind Global”) exercised its right of participation, and the Company entered into a definitive
securities purchase agreement with Lind Global (the “Lind SPA”) for the issuance, in a private placement, of an aggregate
of
The terms of the Lind SPA are substantially similar to the terms of the aforementioned securities purchase agreement, and the terms of the Ordinary Warrants issued pursuant to the Lind SPA are substantially similar to the terms of the warrants issued pursuant to the aforementioned securities purchase agreement.
The warrants were
classified as a derivative financial liability and the initial fair value of the warrants at issuance was $
In July 2022 this warrants and other warrants which were issued to Lind Global were canceled and new warrants were issued, see below in paragraph H.
In April 2022,
the Company entered into a definitive securities purchase agreement with Armistice Capital Fund Ltd (“Armistice”) for the
issuance, in a private placement, of an aggregate of
The gross proceeds
to the Company from this private placement were $
The Ordinary Warrants
have an exercise price of $
The Ordinary Warrants
and the pre-funded warrants were classified as a derivative financial liability. The initial fair value of the Ordinary Warrants and
of the pre-funded warrants at issuance was $
In July 2022 this warrants and other warrants which were issued to Armistice were canceled and new warrants were issued, see below in paragraph G.
All of the pre-funded
warrants were exercised on June 17, 2022 and
F-27
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 8 - CONVERTIBLE SECURITIES AND FINANCIAL LIABILITY AT FAIR VALUE (CONT.):
| F. | In April 2022, the Company entered into an Amendment with
the holder, Armistice , of its warrants, as mentioned above in paragraph (E), to purchase up to an aggregate of |
In July 2022 the
holder exercised its rights under the Warrants to purchase
As a result of the
Prior SPA as mentioned above, on April 20, 2022, Lind Global Fund II LP (“Lind
Global”) exercised its right of participation, and the Company entered into a definitive securities purchase agreement with Lind
Global (the “Lind SPA”) for the issuance, in a private placement, of an aggregate of
The terms of the
Lind SPA are substantially similar to the terms of the aforementioned securities purchase agreement, and the terms of the Ordinary Warrants
issued pursuant to the Lind SPA are substantially similar to the terms of the warrants issued pursuant to the aforementioned securities
purchase agreement. Also, all the warrants to purchase an aggregate of
| G. | In July 2022, the Company issued a warrant to purchase Ordinary Share to Armistice to purchase up to an
aggregate of |
This Warrant was issued further to that certain Securities Purchase Agreement dated as of July 18, 2022 and replaced those certain Ordinary Share Purchase Warrants issued to the holder in February 2022 and April 2022.
The Warrant was classified as a derivative financial liability. The
initial fair value of the Warrant at issuance was $
| H. | In July 2022, the Company issued an Ordinary Share Purchase
Warrant to Lind to purchase up to an aggregate of |
The Warrant was classified as a derivative financial liability. The
initial fair value of the Warrant at issuance was $
As of December 31, 2022 the fair value measurement of the warrants
was determined using the Black Scholes model. a Monte-Carlo simulation model. The main assumptions used in the valuation model were:
(1) risk free rate
F-28
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 8 - CONVERTIBLE SECURITIES AND FINANCIAL LIABILITY AT FAIR VALUE (CONT.):
| I. | In October 2022, the Company
entered into an agreement with Jonathan B. Rubini, or the Investor, in connection with a private placement investment for |
The Warrant was classified as a derivative financial liability. The
initial fair value of the warrant at issuance was $
| J. | The warrants in this Note were classified as a derivative financial liability, since, among the rest, the exercise price might differ in some conditions. |
NOTE 9 - OTHER ACCOUNTS PAYABLE:
| December 31, 2022 | December 31, 2021 | |||||||
| Employees and authorities | ||||||||
| Contingent liability | ||||||||
| Others | ||||||||
| Total | ||||||||
NOTE 10 - MAJORE SHAREHOLDER BALANCE:
On December 29, 2022, the board of directors of
G Medical Innovations Holdings Ltd. (the “Company”) approved a loan agreement, dated December 21, 2022 (the “Loan Agreement”),
between the Company and Dr. Yacov Geva, the Company’s Chief Executive Officer and a major shareholder (the “Major Shareholder”).
Under the terms of the Loan Agreement, the total amount of the loan provided by the Lender to the Company is $
The Company allocated the debt and equity instruments
based the fair value of the debt with residual value for the equity. As of December 31, 2022, the total value of the loan was $
During 2022 the Major Shareholder has drawn advances towards future
due amounts. The Company recorded these amounts with an accrued interest rate of
NOTE 11 - LONG TERM LOANS:
| Linked to | Interest rate | December 31, 2022 | December 31, 2021 | |||||||||
| Long term loans | ||||||||||||
| Long term loans | ||||||||||||
| Less- Current portion | ( | ) | ( | ) | ||||||||
| Total | ||||||||||||
| * |
F-29
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 11 - LONG TERM LOANS (CONT.):
| A. | Upon G Medical Diagnostic Services Inc (“CardioStaff”)
acquisition, additional long- term loans were added to the Company. The loans bear interest of between |
As of
December 31, 2022 and December 31, 2021, the total amount of those US loans was $
| B. | Reconciliation of the changes in liabilities for which cash flows have been, or will be classified as financing activities in the statement of cash flows: |
| Loans | ||||
| As of January 1, 2022 | ||||
| Changes from financing cash flows: | ||||
| Repayment of loans | ( | ) | ||
| Total changes from financing cash flows | ||||
| Accrued interest of long-term loans | ||||
| As of December 31, 2022 | ||||
| Loans | ||||
| As of January 1, 2021 | ||||
| Changes from financing cash flows: | ||||
| Receipts of long-term loans | ||||
| Conversion loans to shares | ( | ) | ||
| Repayment of loans | ( | ) | ||
| Total changes from financing cash flows | ( | ) | ||
| Accrued interest of long-term loans | ||||
| As of December 31, 2021 | ||||
NOTE 12 - COMMITMENTS AND CONTINGENCIES:
The Israeli subsidiary’s’ (G Medical Innovations Ltd) entire assets and rights were pledged as a floating charge to secure bank borrowings.
NOTE 13 - SHAREHOLDERS’ DEFICIT:
| A. | The ordinary shares in the Company entitle their holders the right to receive notice to participate and vote in general meetings of the Company and the right to receive dividends, if and when declared. |
| Number of shares | ||||||||||||||||
| December 31, 2022 | December 31, 2021* | |||||||||||||||
| Authorized | Issued and outstanding | Authorized | Issued and outstanding | |||||||||||||
| Ordinary shares of $ | ||||||||||||||||
| Warrants** | ||||||||||||||||
| * |
| (**) | The Warrants will be exercisable
at a price equal to $ |
F-30
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 13 - SHAREHOLDERS’ DEFICIT (CONT.):
| B. | On June 29, 2021, the Company closed its initial public offering
(the “IPO”) of |
The Company also granted an option
to the underwriter to purchase up to
The IPO warrants are immediately
exercisable on the date of the issuance at an exercise price of $
In addition, and upon the consummation
of the Company’s initial public offering, the Company issued
The Company issued
| C. | Reverse stock split: |
On November 15, 2022, the Company’s
shareholders approved, a 35-for-1 consolidation (hereinafter referred to as a reverse stock split of 35:1) of the Company’s ordinary
shares pursuant to which holders of the Company’s ordinary shares received one ordinary share for every 35 ordinary share held.
| D. | On December 3, 2021, the Company entered into a development and distribution agreement with Heartbuds AK LLC. Following this agreement, the Company issued |
| E. | During the year ended December 31, 2022 the Company issued |
| F. | The warrants that were granted to financial advisors and consultants during 2022 and 2021 are as follows: |
| Amount | Exercise price | Expiration date | ||||||
| A$ | ||||||||
| A$ | ||||||||
| A$ | ||||||||
| A$ | ||||||||
| A$ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
| $ | ||||||||
F-31
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 13 - SHAREHOLDERS’ DEFICIT (CONT.):
| G. | Options, warrants and shares granted to employees and service providers: |
| 1. |
| 2. | In July 2021, the Company signed an agreement with a service
provider to provide consulting services. As part of the consideration, on April 8, 2022, the Company issued to the service provider |
| 3. | In June 2022, the Company issued warrants to purchase |
| 4. | In January 2017, the Board of Directors approved a Global
Equity Incentive Plan (the “Plan”). The Plan will expire in December 2026. As of the December 31, 2022, the number of ordinary
shares reserved for the exercise of options granted under the Plan is |
| 5. | The Company and the Company’s subsidiaries employees, directors, officers, and service providers, including major shareholder are eligible to participate in this Plan and receive awards of options, share appreciation rights (“SARs”), restricted shares, restricted share units (“RSUs”), and any other share-based grant, referred to as, individually or collectively. A summary of the status of the Company’s option plan granted to employees as of December 31, 2022, and changes during the relevant period ended on that date is presented below: |
| Year ended December 31, 2022 | Year ended December 31, 2021* | Year ended December 31, 2020* | ||||||||||||||||||||||
| Number of options | Weighted average Exercise price | Number of options | Weighted average Exercise price | Number of options | Weighted average Exercise price | |||||||||||||||||||
| Outstanding at beginning of year | $ | $ | $ | |||||||||||||||||||||
| Exercised | ( | ) | $ | ( | ) | $ | ||||||||||||||||||
| Granted | $ | $ | ||||||||||||||||||||||
| Forfeited and cancelled | ( | ) | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||
| Outstanding at end of year | $ | $ | $ | |||||||||||||||||||||
| Exercisable options | $ | $ | $ | |||||||||||||||||||||
| * |
F-32
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 13 - SHAREHOLDERS’ DEFICIT (CONT.):
| G. | Options, warrants and shares granted to employees and service providers (Cont.): |
| 5. | (cont.) |
The options to employees outstanding as of December 31, 2022, are comprised, as follows:
| Exercise price | Outstanding as of December 31, 2022 |
Weighted average remaining contractual term |
Exercisable as of December 31, 2022 |
Weighted average remaining contractual term |
||||||||||||||
| (years) | (years) | |||||||||||||||||
| - | ||||||||||||||||||
| 6. | On September 5, 2021, the Board of Directors approved to
issue a total of |
| 7. | On November 15, 2021, the Board of Directors approved to
issue a total of |
| 8. | On December 23, 2021, the Board of Directors approved to
issue a total of |
| 9. | On June 16, 2022, the Board of Directors approved to issue
a total of |
| 10. | On November 30, 2021, the Company entered a joint development, licensing, and distribution agreement with Heartbuds AK, LLC. Pursuant to the joint development agreement, the company and Heartbuds will jointly develop a newer, enhanced model or generation of Heartbuds product to be included with the sale and distribution of the company’s Prisma devise (the “HB2”). |
On the date of the agreement, the Company
issued Heartbuds
From the date that the HB2 is approved
by the FDA until the last day of the 18-calendar month thereafter the warrants shall vest on a pro rata basis based on the actual number
of devices that Heartbuds will sell to be calculated relative to the agreed target of
| 11. | In December 2021, |
All the options and shares granted during 2021 to employees and service providers were valued using a Black Scholes model based, which is designed to model the Company’s equity value over time. The main assumptions used were: (1) risk-free rate: 0.78-1.27%; (2) volatility: 50%-60%; and (3) time until expiration: 5 years.
During 2022 all the
options and shares granted to employees and service providers were valued using a Black Scholes model based, which is designed to model
the Company’s equity value over time. The main assumptions used were: (1) risk-free rate:
F-33
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 13 - SHAREHOLDERS’ DEFICIT (CONT.):
| G. | Options, warrants and shares granted to employees and service providers (Cont.): |
| 12. | Performance rights: |
During July 2020
the Company granted
The performance rights are convertible into ordinary shares of the Company on a 1:1 basis, upon the occurrence of the following vesting milestones for each class of performance rights:
| ■ | Class A incentive performance right – |
| i. | The Company’s 20-day VWAP of ordinary shares of the Company on the ASX (adjusted by the AUD/USD exchange rate quoted on the Reserve Bank of Australia prior to the last trading day pursuant to which the Company’s VWAP of ordinary shares is being calculated); or |
| ii. | If applicable, the Company’s closing market price on a trading day on Nasdaq, (Conversion Price) multiplied by the total issued share capital of the Company. |
| ■ | Class B incentive performance right – |
| ■ | Class C incentive performance right – |
| ■ | Class D incentive performance right – |
All the above incentive performance rights were valued using a Monte-Carlo
based risk-neutral valuation model, which is designed to model the Company’s equity value over time.
In January 2022,
the Company granted four series of performance shares in a total amount of
In January 2022,
the Company’s market value reached $
On January 19, 2022,
the Company granted two additional series of performance shares in a total amount of
In 2022, 2021 and
2020 the Company recorded an expense related to options performance shares and shares granted at the amount of $
F-34
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 13 - SHAREHOLDERS’ DEFICIT (CONT.):
| H. | Share repurchase program |
On May 20, 2022,
the Company announced that its board of directors authorized a share repurchase program to acquire up to $
| I. | Exercise of ordinary shares purchase warrants |
On July 18, 2022
In connection with the agreement with Armistice the exercise price of 45,242 Ordinary Warrants held by Lind Global will be reduced to an exercise price equal to $32.55 and have a term of exercise until April 30, 2028. (See also note 8(H)).
| J. | Private placement |
On October 20, 2022,
G Medical Innovations Holdings Ltd. (the “Company”) entered into an agreement with Rubini in connection with a private placement
investment for
The initial fair value of the Ordinary Warrants at issuance was $
| K. | “At The Market” offering |
In September 2022
As of December 31, 2022 the Company has sold 104,113 of its Ordinary shares through the ATM for a net consideration of $367.
| L. | Capital Commitment Agreement with GEM: |
In November 2019,
the Company entered into the Capital Commitment Agreement with GEM Yield Fund LLC SCS and GEM Yield Bahamas Ltd (“GEM”) (the “Capital Commitment Agreement”). The Capital Commitment Agreement secures a capital commitment of up to approximately A$
| M. | The Convertible Securities Warrants were classified as a derivative financial liability and measured with changes in fair value recognized in finance expense (income), net. |
F-35
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 13 - SHAREHOLDERS’ DEFICIT (CONT.):
| N. | During 2022 the Company issued |
The December 2022 and 2021 measurement was applied using a Monte -Carlo simulation model for Lind. The Black Scholes model was applied for all others below and the key parameters used were as follows:
| Fair Value as at December 31, 2022 | Risk free rate | Volatility of assets | Expected Term | Expected dividend yield | ||||||||||||||
| Armistice warrants | % | % | % | |||||||||||||||
| Rubini warrants | % | % | % | |||||||||||||||
| Lind warrants | % | % | % | |||||||||||||||
| Alpha Capital, MEF and GEM warrants | % | % | % | |||||||||||||||
| Total derivative liabilities -warrants | ||||||||||||||||||
| (*) |
| Fair Value as at December 31, 2021 * |
Risk free rate |
Volatility of assets |
Expected Term |
Expected dividend yield | ||||||||||||||
| Convertible Securities Warrants and GEM Warrants | % | % | % | |||||||||||||||
| Rubini warrants | % | % | % | |||||||||||||||
| Alpha Capital warrants | % | % | % | |||||||||||||||
| Lind warrants | % | % | % | |||||||||||||||
| Total derivative liabilities -warrants | ||||||||||||||||||
NOTE 14 - LEASES:
The Company has lease contracts for office facilities
and motor vehicles used in its operations. Leases of office facilities generally have lease terms between
The Company also has certain leases of office facilities with lease terms of 12 months or less. The Company applies the ’short-term lease’ recognition exemption for these leases.
During the year the Company entered into two new lease agreements in Newhall and Irvine, California, both for a period of three years, that had been presented as part of a right-of-use asset – property, plant and equipment.
Set out below are the carrying amounts of right-of-use assets recognized and the movements during the period:
Office facilities | Motor vehicles |
Total | ||||||||||
| At January 1, 2022 | ||||||||||||
| Additions | ||||||||||||
| Cancellations | ( | ) | ( | ) | ||||||||
| Depreciation expense | ( | ) | ( | ) | ( | ) | ||||||
| As at December 31, 2022 | ||||||||||||
F-36
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 14 - LEASES (CONT.):
Set out below are the carrying amounts of lease liabilities and the movements during the period:
| 2022 | ||||
| At January 1, 2022 | ||||
| Additions | ||||
| Cancellations | ( | ) | ||
| Payments | ( | ) | ||
| Linked exchange rate | ( | ) | ||
| As at December 31, 2022 | ||||
| Current | ||||
| Non-current | ||||
The following are the amounts recognized in profit or loss:
| 2022 | ||||
| Depreciation expense of right-of-use assets | ||||
| Interest expense on lease liabilities | ||||
| Total amount recognized in profit or loss | ||||
The Company had total cash outflows for leases
of $
NOTE 15 – COST OF SERVICES:
| Year ended | Year ended | Year ended | ||||||||||
| December 31, 2022 | December 31, 2021 | December 31, 2020 | ||||||||||
| Payroll and related | ||||||||||||
| Depreciation and amortization | ||||||||||||
| Subcontractors | ||||||||||||
| Freight | ||||||||||||
| Others | ||||||||||||
| Total | ||||||||||||
NOTE 16 - RESEARCH AND DEVELOPMENT EXPENSES:
| Year ended | Year ended | Year ended | ||||||||||
| December 31, 2022 | December 31, 2021 | December 31, 2020 | ||||||||||
| Payroll and related | ||||||||||||
| Subcontractors and materials | ||||||||||||
| Share based compensation | ||||||||||||
| Depreciation and amortization | ||||||||||||
| Patents | ( | ) | ||||||||||
| Travel expenses | ||||||||||||
| Others | ||||||||||||
| Total | ||||||||||||
F-37
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 17.A - SELLING, GENERAL AND ADMINISTRATIVE EXPENSES:
| Year ended | Year ended | Year ended | ||||||||||
| December 31, 2022 | December 31, 2021 | December 31, 2020 | ||||||||||
| Payroll and related | ||||||||||||
| Professional services | ||||||||||||
| Expected credit loss | ||||||||||||
| Convertible transaction costs | ||||||||||||
| Shares issuance costs | ||||||||||||
| Contingent liabilities | ||||||||||||
| Depreciation and amortization | ||||||||||||
| Insurance | ||||||||||||
| Share based compensation | ||||||||||||
| Rent and office maintenance | ||||||||||||
| Travel expenses | ||||||||||||
| Others | ||||||||||||
| Total | ||||||||||||
NOTE 17.B – FINANCIAL INCOME (EXPENSES), NET
| Year ended | Year ended | Year ended | ||||||||||
| December 31, 2022 | December 31, 2021 | December 31, 2020 | ||||||||||
| Share based compensation expenses | ||||||||||||
| Other Financial expenses | ||||||||||||
| Total Financial expenses | ||||||||||||
| Gain due to change in fair value of derivative liability and warrants | ||||||||||||
| Other Financial income | ||||||||||||
| Total Financial income | ||||||||||||
| Financial income (expense), net | ( | ) | ( | ) | ||||||||
NOTE 18 – LOSS PER SHARE:
Loss per share have been calculated using the weighted average number of shares in issue during the relevant financial periods, the weighted average number of equity shares in issue and loss for the period as follows:
Year ended | Year ended | Year ended | ||||||||||
| G Medical innovations holdings ltd. shareholders' loss from continuing operations | ( | ) | ( | ) | ( | ) | ||||||
| G Medical innovations holdings ltd. shareholders' loss from discontinued operations | ( | ) | ( | ) | ||||||||
| Total loss for the year attributable to shareholders | ( | ) | ( | ) | ( | ) | ||||||
| Weighted average number of ordinary shares | ||||||||||||
| $ | ( | ) | $ | *( | ) | $ | *( | ) | ||||
| $ | ( | ) | $ | ( | ) | |||||||
| Total basic and diluted loss per share | $ | ( | ) | $ | ( | ) | $ | ( | ) | |||
| * |
F-38
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 19 - TAX ON INCOME:
| A. | Taxes on income: |
Cayman Islands:
The Company has incorporated in the Cayman Islands and under the local current laws; the Company is not subject to corporate income tax.
Israel:
Israeli
corporate tax rate is
United States of America:
The U.S. subsidiary incorporated in 2017 and is subject to local corporate tax in the United States. As of December 31, 2022, the U.S. subsidiary has not received a final tax assessment.
| B. | Reconciliation between the theoretical tax on the pre-tax income and the tax expense (benefit): |
| Year ended December 31, 2022 |
Year ended December 31, 2021 |
Year ended December 31, 2020 |
||||||||||
| Loss before income tax | ||||||||||||
| Statutory tax rate | % | % | % | |||||||||
| Income tax at the statutory tax rate | ||||||||||||
| Expenses not recognized for tax purposes | - | |||||||||||
| Recondition of deferred tax asset with were not recognized on prior periods | ( |
) | ( |
) | ||||||||
| Income tax benefit | ( |
) | ( |
) | ||||||||
| C. | Income tax expense (benefit): |
| Year ended | Year ended | Year ended | ||||||||||
| December 31, 2022 | December 31, 2021 | December 31, 2020 | ||||||||||
| Current | ||||||||||||
| Deferred taxes, net | ( | ) | ( | ) | ||||||||
| ( | ) | ( | ) | |||||||||
| D. | Net losses carry forwards: |
As of December 31, 2022, the Israeli
Company has estimated carry forward tax losses of approximately ,732. The USA Subsidiaries have estimated carry forward tax losses
of approximately $
F-39
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 20 - RELATED PARTIES:
The following transactions arose with related parties:
| Transaction | Year ended December 31, 2022 |
Year ended December 31, 2021 |
Year ended December 31, 2020 |
|||||||||
| Short term employee benefits * | ||||||||||||
| Social benefits costs | ||||||||||||
| Share based compensation) Employees) | ||||||||||||
| Share based compensation) Directors) | ||||||||||||
| * |
Liabilities to related parties:
| Name | December 31, 2022 | December 31, 2021 | December 31, 2020 | |||||||||
| Key management personnel | ||||||||||||
| Loans from major shareholder | ||||||||||||
In April 2022 the Company’s
CEO, Dr. Yacov Geva, committed to finance the Company’s operations for the next 12 months until the end of April 2023 provided and as
long as the Company’s CEO continues to be a controlling shareholder and/or the Company cannot be financed externally from any other
sources and/or until a sum of $
In exchange, for providing the required security, the Company allotted
to the CEO
On October 6, 2022, the
major shareholder of the Company, committed to finance the Company’s operations for the next 12 months until November 30, 2023 provided
and as long as the major shareholder continue to be a controlling shareholder and/or the Company cannot be financed externally from any
other sources and/or until a sum of $
The total fair value of the shares
and the warrants, as measured on issuance date, amounted to $
See note 24.D regarding re extending for the major shareholder of the Company commitment to finance the Company.
On December 29, 2022, our Board of
Directors approved a loan agreement, dated December 21, 2022 (the “Loan Agreement”), between the Company and Dr. Geva. Under
the terms of the loan agreement, the total amount of the loan provided by Dr. Geva to the Company is $
The Company accounting allocated the debt and equity instruments based on the fair value of the debt with residual value for the equity.
F-40
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 21 - SEGMENT REPORTING:
The Company identified the Company’s CEO as its chief operating decision maker (“CODM”).
As the Company’s CODM, the CEO receives information on a segregated basis (for review on a regular basis) for each business unit, i.e. services, products and kits. The consolidated financial statements present the statements of comprehensive loss revenues from each segment on a standalone basis as well as cost of sale of each segment – i.e. there are no transactions between segments. The information as presented in the consolidated financial statements is essentially the same information provided to the CODM and the same information regarding decisions about allocating resources.
The Company accounts for its segment information in accordance with IFRS 8 “Segment Reporting” which establishes annual and interim reporting standards for operating segments of a Company based on the Company’s internal accounting methods.
Operating segments are based upon its internal organization structure,
the manner in which our operations are managed and the availability of separate financial information. In 2022,
Products: Development, manufacture and marketing of wireless diagnostic equipment for the medical industry and consumer market.
Patient Services: Cardiac monitoring services of MCT, Event, Holter, Extended Holter and Pacemaker
Covid -19 testing: three kinds of diagnostic tests – (i) Rapid Antigen + A/B Flu Combo test, (ii) PCR test and (iii) Antibody test.
Kits – direct-To-Customer health testing kits health issues ranging from food sensitivity, indoor/outdoor allergies, HPV, thyroid functioning, testosterone levels, and the hemoglobin A1C test, with results going directly to the user within days.
During the second half of the year 2022 the Company took two strategic decisions:
1) The Company decided to discontinue the Covid-19 activity and this segment is reported as a discontinued operation in the financial statements for the year 2022. See also Note 22.
2) The Company decided to divert to more comprehensive home testing solutions as an expansion of its patient services and as part of its vision to move towards a home-based health care system.
For the year ended December 31, 2022:
Products | Kits | Patient Services | Total | |||||||||||||
| Revenues from external customers | - | |||||||||||||||
| Segment loss | ||||||||||||||||
| Unallocated G&A expenses | ||||||||||||||||
| Finance income, net | ||||||||||||||||
| Loss before income taxes | ||||||||||||||||
In the discontinued operation the revenues from external customers was $
F-41
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 21 - SEGMENT REPORTING (CONT.):
For the year ended December 31, 2021:
| Products | Patient Services | Total | ||||||||||
| Revenues from external customers | ||||||||||||
| Segment loss | ||||||||||||
| Unallocated G&A expenses | ||||||||||||
| Finance expenses, net | ||||||||||||
| Loss before income taxes from continuing operations | ||||||||||||
In the discontinued operation
the revenues from external customers was $
For the year ended December 31, 2020:
| Products | Patient Services | Total | ||||||||||
| Revenues from external customers | ||||||||||||
| Segment loss | ||||||||||||
| Unallocated G&A expenses | ||||||||||||
| Finance expenses, net | ||||||||||||
| Loss before income taxes from continuing operations | ||||||||||||
NOTE 22 – DISOCNTINUED OPERATION:
During the first half of 2022, the Company operated six locations performing point-of-care tests in communities in Southern and Northern California. The volume of COVID-19 testing has decreased significantly since April 2022. Given the decrease in COVID-19 cases, in the second half of fiscal year 2022 the Company took a strategic decision to discontinue its COVID-19 related business activities.
The consolidated statements of comprehensive loss included a net loss from discontinued operations in the amount of $
Below are the results of the discontinued segment’s activity for the years ended December 31, 2022 and 2021:
| December 31, 2022 | December 31, 2021 | |||||||
| Revenue | ||||||||
| Cost of revenue | ||||||||
| Gross loss | ||||||||
| Selling, general and administrative expenses | ||||||||
| Other expense | ||||||||
| Loss for the year | ||||||||
Below are the cash flow amounts relating to the discontinued segment for the years ended December 31, 2022 and 2021:
| December 31, 2022 | December 31, 2021 | |||||||
| Loss for the year | ||||||||
| Operating activities | ||||||||
| Investment activities | ( | ) | ( | ) | ||||
| Financing activities | ( | ) | ( | ) | ||||
| Net cash provided by (used in) discontinued operations | ||||||||
F-42
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 23 - FINANCIAL INSTRUMENTS AND RISK MANAGEMENT:
The Company is exposed to a variety of financial risks, which results from its financing, operating and investing activities. The objective of financial risk management is to contain, where appropriate, exposures in these financial risks to limit any negative impact on the Company’s financial performance and position.
The Company’s financial instruments are its cash and cash equivalents, restricted deposit, trade receivables, bank loans and short-term bank credit, trade payables, loans from major shareholder, convertible securities, derivative liabilities and financial liability at fair value. The main purpose of these financial instruments is to finance the Company’s operation. The Company actively measures, monitors and manages its financial risk exposures by various functions pursuant to the segregation of duties and principals. The risks arising from the Company’s financial instruments are mainly credit risk, currency risk and liquidity risk. The risk management policies employed by the Company to manage these risks are discussed below.
Credit risk:
Credit risk arises when a failure by counterparties to discharge their obligations could reduce the amount of future cash inflows from financial assets on hand at the balance sheet date. The Company closely monitors the activities of its counterparties and controls the access to its intellectual property which enables it to ensure the prompt collection of customers’ balances. The Company’s main financial assets are cash and cash equivalents as well as restricted deposit and trade receivables that represent the Company’s maximum exposure to credit risk in connection with its financial assets.
The carrying amount of financial assets represents the maximum credit exposure. The maximum exposure to credit risk at the reporting date was:
| December 31, 2022 | December 31, 2021* | |||||||
| Cash and Cash Equivalents | ||||||||
| Restricted deposit | ||||||||
| Trade receivables | ||||||||
| Loan to major shareholder, net | - | |||||||
| Total | ||||||||
Currency risk:
Currency risk is the risk that the value of financial instruments will fluctuate due to changes in foreign exchange rates. Currency risk arises when future commercial transactions and recognized assets and liabilities are denominated in a currency that is not the Company’s functional currency. The Company is exposed to foreign exchange risk arising from various currency exposures primarily with respect to the New Israeli Shekel, the RMB and the AUD. The Company’s policy is not to enter into any currency hedging transactions. The carrying amounts of the Company’s foreign currency denominated monetary assets and monetary liabilities at the reporting date are as follows:
| Assets | December 31, 2022 | |||||||||||||||
| NIS | AUD | RMB | Total | |||||||||||||
| Cash and cash equivalents | ||||||||||||||||
| Restricted deposit | ||||||||||||||||
| Loan to major shareholder, net | ||||||||||||||||
| Accounts receivable other | ||||||||||||||||
Liabilities | ||||||||||||||||
| NIS | AUD | RMB | Total | |||||||||||||
| Trade and other payables | ||||||||||||||||
| Loans | ||||||||||||||||
Loan from major shareholder | ||||||||||||||||
| Obligation under operating leases | ||||||||||||||||
| Derivative liabilities | ||||||||||||||||
| Net | ( | ) | ( | ) | ( | ) | ||||||||||
F-43
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 23 - FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (CONT.):
Currency risk (Cont.):
| Assets | December 31, 2021 | |||||||||||||||
| NIS | AUD | RMB | Total | |||||||||||||
| Cash and cash equivalents | ||||||||||||||||
| Restricted deposit | ||||||||||||||||
| Liabilities | ||||||||||||||||
| NIS | AUD | RMB | Total | |||||||||||||
| Trade and other payables | ||||||||||||||||
| Loans | ||||||||||||||||
| Derivative liabilities | ||||||||||||||||
| Net | ( | ) | ( | ) | ( | ) | ||||||||||
A 10% strengthening of the United States Dollar
against the following currencies would have increased (decreased) equity and the income statement by the amounts shown below. This analysis
assumes that all other variables, in particular interest rates, remain constant. For a
| December 31, 2022 | December 31, 2021 | |||||||
| Linked to NIS | ( | ) | ( | ) | ||||
| % | % | |||||||
| ( | ) | ( | ) | |||||
| Linked to AUD | ( | ) | ( | ) | ||||
| % | % | |||||||
| ( | ) | ( | ) | |||||
| Linked to RMB | ||||||||
| % | % | |||||||
Liquidity risks:
Liquidity risk is the risk that arises when the maturity of assets and the maturity of liabilities do not match. An unmatched position potentially enhances profitability but can also increase the risk of loss. The Company has procedures with the object of minimizing such loss by maintaining sufficient cash and other highly liquid current assets and by having available an adequate amount of committed credit facilities.
The following tables detail the Company’s remaining contractual maturity for its financial liabilities. The tables have been drawn up based on the undiscounted cash flows of financial liabilities based on the earliest date on which the Company can be required to pay.
| December 31, 2022 | December 31, 2021 | |||||||
| Trade payables | ||||||||
| Loans (see also note 11) | ||||||||
| Financial liability at fair value | ||||||||
| Convertible Securities (see also note 8) | ||||||||
| Derivative liabilities - warrants (see also note 13N) | ||||||||
Loan from major shareholder | ||||||||
| Lease liabilities (see also note 14) | ||||||||
| Total | ||||||||
F-44
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 23 - FINANCIAL INSTRUMENTS AND RISK MANAGEMENT (CONT.):
Fair value of financial instrument:
| Fair value measurements using input type | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| As of December 31, 2022 | ||||||||||||||||
| Derivative liabilities – warrants | ( | ) | ( | ) | ||||||||||||
| Convertible securities | ( | ) | ( | ) | ||||||||||||
| Total | ( | ) | ( | ) | ||||||||||||
| Fair value measurements using input type | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| As of December 31, 2021 | ||||||||||||||||
| Derivative liabilities – warrants | ( |
( |
||||||||||||||
| Convertible securities | ( |
( |
||||||||||||||
| Total | ( |
( |
||||||||||||||
At December 31, 2022, the fair value measurement
of the warrants in the table above is related to Alpha, Rubini, Lind and Armistice was estimated using the Black Scholes model, based
on a variety of significant unobservable inputs and thus represents a level 3 measurement within the fair value hierarchy including:
risk-free interest rate in the range of
At December 31, 2022, the fair value measurement,
of the Rubini convertible debenture was estimated using the Monte Carlo simulation analysis based on a variety of significant unobservable
inputs and thus represents a level 3 measurement within the fair value hierarchy. The key inputs that were used in the valuation were:
risk-free interest rate of
At December 31, 2021, the fair value measurement
of the warrants in the table above is related to Alpha and Rubini, was estimated using the Black Scholes model, based on a variety of
significant unobservable inputs and thus represents a level 3 measurement within the fair value hierarchy including: risk-free interest
rate of
At December 31, 2021, the fair value measurement,
of the Lind convertible debentures and warrant was estimated using the Monte Carlo simulation analysis based on a variety of significant
unobservable inputs and thus represents a level 3 measurement within the fair value hierarchy. The key inputs that were used in the valuation
were: risk-free interest rate of
Derivative liability | ||||
| Derivative liability - warrants as of January 1, 2021 | ( | ) | ||
| Issuance of financial instruments | ( | ) | ||
| Gain due to change in fair value of derivative liability | ||||
| Derivative liability - warrants as of December 31, 2021 | ( | ) | ||
| Issuance of financial instruments | ( | ) | ||
| Exercise of warrants | ||||
| Gain due to change in fair value of derivative liability | ||||
| Derivative liability - warrants as of December 31, 2022 | ( | ) | ||
| Convertible Securities | ||||
| Convertible securities as of January 1, 2021 | ( | ) | ||
| Issuance of convertible securities | ( | ) | ||
| Payments of convertible securities | ||||
| Conversion of convertible securities to regular loan | ||||
| Loss due to change in fair value of convertible securities | ( | ) | ||
| Convertible securities as of December 31, 2021 | ( | ) | ||
| Payments of convertible securities | ||||
| Gain due to change in fair value of convertible securities | ||||
| Convertible securities as of December 31, 2022 | ||||
F-45
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 24 - SUBSEQUENT EVENTS:
| A. | Nasdaq staff listing determination: |
On February 17, 2023, the Company announced that on February 16, 2023, the Company received notice from the Listing Qualifications Department (the “Staff”) of The Nasdaq Stock Market LLC (“Nasdaq”) that, based upon the Company’s non-compliance with the stockholders’ equity requirement for continued listing on The Nasdaq Capital Market, as set forth in Nasdaq Listing Rule 5550(b) (the “Equity Rule”), the Company is subject to delisting from Nasdaq unless the Company timely requests a hearing before a Nasdaq Hearings Panel (the “Panel”). Accordingly, the Company has requested a hearing before the Panel, which will stay any delisting or suspension action pending the hearing and the expiration of any additional extension period granted by the Panel following the hearing. The Hearing was held on April 13, 2023.
On May 11, 2023, the Panel granted the request for continued listing on Nasdaq subject to the requirement that the Company, by no later than August 4, 2023 (or the Exception Period), provide the Panel with unaudited interim financial statements for the second quarter of 2023 and demonstrate to the Panel that the Company continue to meet the equity requirement. Upon review of Company’s interim financial statements for the second quarter of 2023, the Panel will decide if the Company demonstrated an ability to maintain compliance with the continued listing requirements on a long-term basis. In addition, from May 11, 2023 until the end of the Exception Period, the Panel reserves the right to reconsider the terms of this exception based on any event, condition or circumstance that exists or develops that would, in the opinion of the Panel, make the continued listing of the Company’s securities on Nasdaq inadvisable or unwarranted. Until the end of the Exception Period, the Company required to provide prompt notification to the Panel of any significant events that may affect the Company’s compliance with Nasdaq requirements.
| B. | At-The-Market offering (“ATM”): |
As described in Note 14(K) above,
in September 2022 the Company entered into a sales agreement with its sales agent pursuant to which the Company may offer and sell, from
time to time, to or through the Sales Agent as agent or principal, ordinary shares, par value $
In January 2023 the Company has sold
additional
| C. | Share capital reduction: |
Under Cayman Islands law, we may not issue Ordinary Shares at a price under the par value for our Ordinary Shares except in accordance with the provisions of the Companies Act (as revised) of the Cayman Islands (which, amongst other things, requires a sanction of the Grand Court of the Cayman Islands). Our Amended and Restated Memorandum and Articles of Association permit us to reduce our share capital in any way, including by reducing the par value of our issued share capital, cancelling any paid-up share capital which is lost or unrepresented by available assets, and extinguishing or reducing the liability of any of our shares, by way of special resolution and by order from the Grand Court of the Cayman Islands confirming such reduction.
On December 23, 2022, the Company
had an extraordinary general meeting of shareholders to effect a reduction of its share capital and adopt a new Amended and Restated
Memorandum and Articles of Association, which would become effective simultaneously with the Capital Reduction becoming effective
(the “Post-Reduction Amended and Restated Memorandum and Articles of Association”). All resolutions proposed, as
described herein, were passed. Namely, shareholders approved to reduce the issued share capital of the Company by the cancellation
of $
Following the meeting, it became apparent that the notice calling the general meeting on December 23, 2022 was only posted to shareholders on December 19, 2022 and shareholders may not have received sufficient notice of that meeting. In order for there to be sufficient notice under our Amended and Restated Memorandum and Articles of Association, there needs to be five days’ clear notice of the meeting. The result of this procedural irregularity is that the adjourned meeting on December 28, 2022 and the resolutions passed at that meeting are considered to have a defect. To cure the irregularity, on January 24, 2023, the Company called a further general meeting for our shareholders to reconsider and, if thought fit, re-approve the matters set out in the December 28, 2022 meeting and certain other matters (including amendments to our Amended and Restated Memorandum and Articles of Association). This meeting was scheduled for February 9, 2023 and, on such date, the meeting was adjourned to February 16, 2023 due a lack of an effective quorum. The adjourned meeting was convened on February 16, 2023, a quorum was present and our shareholders voted upon and approved all agenda items.
F-46
G MEDICAL INNOVATIONS HOLDINGS LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
(US$ in thousands)
NOTE 24 - SUBSEQUENT EVENTS (CONT.):
| C. | Share capital reduction (cont.) |
On March 6, 2023, we filed our application to seek an order of the Grand Court of the Cayman Islands for confirmation and approval of the Capital Reduction, which was approved by the Grand Court of the Cayman Islands on March 23, 2023, with no conditions attached. The Capital Reduction will therefore become effective upon registration by the Registrar of Companies of the Cayman Islands of (1) the order of the Grand Court of the Cayman Islands confirming the Capital Reduction and (2) the minutes approved by the Grand Court of the Cayman Islands containing the particulars required under the Companies Act (as revised) of the Cayman Islands (together the “Capital Reduction Documents”). On March 24, 2023, we filed the Capital Reduction Documents with the Registrar of Companies of the Cayman Islands and the Capital Reduction became effective.
| D. | Major shareholder commitment |
On May 15, 2023, the major shareholder and Chief Executive Officer
of the Company, extended his commitment to finance the Company up to May 30, 2024, provided and as long as, the Company cannot be finance
externally from any other sources and/ or until a sum of $
| E. | Public offering |
On April 3, 2023 the Company completed a public offering of
As part of the Public Offering the
Company’s CEO, Dr. Yacov Geva purchased
F-47