Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 1-K
ANNUAL REPORT
ANNUAL REPORT PURSUANT TO REGULATION A OF THE SECURITIES ACT OF 1933
For the fiscal year ended December 31, 2018
LunaDNA, LLC
(Exact name of registrant as specified in its charter)
Commission File Number: 024-10903
| Delaware | 82-0631362 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 4110 Campus Point Court San Diego, CA (Address of principal executive offices) |
92121 (Zip Code) | |
(858) 299-4669
Registrant’s telephone number, including area code
Limited Liability Company Interests
(Title of each class of securities issued pursuant to Regulation A)
Table of Contents
| 3 | ||||
| 4 | ||||
| Management’s Discussion and Analysis of Financial Condition and Results of Operations |
17 | |||
| 18 | ||||
| Security Ownership of Management and Certain Securityholders |
21 | |||
| 22 | ||||
| 22 | ||||
| 22 | ||||
| 35 |
-2-
Table of Contents
Part II.
STATEMENT REGARDING FORWARD-LOOKING INFORMATION
We make statements in this Annual Report on Form 1-K (“Annual Report”) that are forward-looking statements within the meaning of the federal securities laws. These forward-looking statements contain information about our expectations, beliefs or intentions regarding our business, financial condition, results of operations, strategies or prospects, and other similar matters. These forward-looking statements are based on management’s current expectations and assumptions about future events, which are inherently subject to uncertainties, risks and changes in circumstances that are difficult to predict. These statements may be identified by words such as “expects,” “plans,” “projects,” “will,” “may,” “anticipates,” “believes,” “should,” “intends,” “estimates,” and other words of similar meaning.
Many factors could cause actual results to differ materially from those in forward-looking statements. Unknown or unpredictable factors that could also adversely affect our business, financial condition and results of operations may arise from time to time. The forward-looking statements discussed in this report may not prove to be accurate. Accordingly, you should not place undue reliance on these forward-looking statements, which only reflect the views of management as of the date of this Annual Report. We undertake no obligation to update or revise forward-looking statements to reflect changed assumptions, the occurrence of unanticipated events or changes to future operating results or expectations, except as required by law.
-3-
Table of Contents
| Item 1. | Business |
Our Company
LunaDNA, LLC, which we refer to as LunaDNA, our Company, we, us or our, is building the world’s first and largest human health database that is owned by a community comprised of its members, which we refer to as the Database. Our Database is comprised of self-contributed genomic, phenotypic, medical, health and related data satisfying our requirements, which we refer to as Member Data. Through community participation, we aim to create a dynamic, secure, and longitudinal Database along with a supporting ecosystem geared towards the improvement of human health. By making the Database available, with our members’ consent, to researchers, we aim to facilitate discoveries which lead to new treatments, increased actionability, and greater predictive power of genomic information for disease and wellness applications. The personal health impact, societal health benefits, and economic value that can be created through clearer associations between genomics and health outcomes can be realized in a myriad of ways, including accelerating toward an era of precision medicine and preventative healthcare.
We are a limited liability company managed by LunaPBC, Inc., a Delaware public benefit corporation, which we refer to as our Manager. We issue unit-denominated common limited liability company interests to our members, which we refer to as shares. Unlike the holders of common stock in a corporation, our shareholders have very limited rights, as described in further detail under “Securities Being Offered” on page 36 of our offering circular dated December 3, 2018, which we refer to as the Offering Circular, and have no right to control our operations. We believe that keeping our platform attractive will include, but will not be limited to, managing our Company in a manner that maximizes funds for distributions to our members no less frequent than annually. However, we do not guarantee the frequency or amount distributions to our members, and as discussed at “Our Limited Liability Company Operating Agreement—Distributions and Dividends” on page 42 of our Offering Circular, our Manager has control over the frequency and amount of distributions.
On December 5, 2018, we commenced principal operations and admitted a person other than our Manager as a member. As of April 26, 2019, we have issued 19,068 shares, with an additional 6,580 shares earned but not yet issued.
Recent Developments – Genetic Alliance Partnership
In January 2019, our Manager entered into a Master Agreement, or the Agreement, with Genetic Alliance, or GA, a non-profit organization, in order to merge GA’s Platform for Engaging Everyone Responsibly engagement platform, or PEER, with our Database, once the Database IP contains PEER equivalent functionality. The initial term of the Agreement ends on the fifth anniversary of the date on which both parties agree in writing that the Database IP substantially contains PEER equivalent functionality. We currently estimate that the term will start by the end of 2019.
PEER is an award-winning technology solution for collecting health data directly from individuals. In 2015, PEER was honored by the White House as one of nine leading platforms in the advancement of precision medicine. PEER gives individuals complete control over how their PEER data is shared for research. Organizations and communities interested in using PEER to collect individual health data for a specific purpose do so by creating customized registries. Currently, PEER is used by 45 disease communities representing approximately 50,000 individual participants. The GA network includes more than 1,200 disease-specific advocacy organizations, as well as reaches over 10,000 universities, private companies, government agencies, and public policy organizations.
Pursuant to the Agreement, our Manager granted a non-exclusive, non-transferable license to GA to use the Database IP that contains PEER equivalent functionality for so long as the Agreement is in effect. Additionally, GA granted our Manager a non-exclusive license to use the PEER application and PEER data during the term of the Agreement, subject to various restrictions.
Pursuant to the Agreement, GA is transitioning stewardship of PEER data to our Manager. GA and our Manager hope to facilitate the ultimate use of a more functional integrated database, which includes both our Database and PEER data, by researchers or other third-party users. The Agreement also calls for GA and our Manager to negotiate a value sharing arrangement with GA in connection with success generated through the efforts of GA. Such efforts of GA may include ongoing enrollment, support, and sustaining of GA partners and GA’s efforts with PEER participants to increase engagement in research studies. We currently contemplate that certain expenses of hosting the PEER data will be the responsibility of our company after March 31, 2020 under our Management Agreement.
PEER participants will also be able to join the LunaDNA community and be eligible to receive shares when contributing their Member Data to the Database, subject to local regulations or laws, such as those governing securities, data rights, or data privacy.
The Agreement may be terminated by either party for convenience upon 18 months’ advance written notice or sooner upon material, uncured default.
Implementation of the Agreement is subject to technical and operational uncertainty, and the goal of merging of our Database with the PEER database may not happen within the contemplated time frame, or at all.
Genomics Opportunity
The application of genomics is relevant at many points during an individual’s life (see Figure 1). We envision a future where everyone’s genomic information is individually owned and referenced at many stages of life—from planning a baby, to
-4-
Table of Contents
determining the medication that is best suited to the individual’s unique biology, through managing disease and maximizing wellness. Scientific and medical research has made clear that the information encoded in our genome will be able to help individuals—as patients and personal health advocates—understand inherent health strengths and provide insights into possible health risk factors, equipping individuals and their health practitioners to approach preventative healthcare strategies and lifestyle decisions more informatively. Genomic data can also provide an assessment on the impact of lifestyle on an individual’s biology.

Figure 1. Timeline of Sequencing Applications in Medicine from Pre-Womb to Tomb
Despite advances in genomics technology that make genome sequencing more accessible than ever from a cost perspective (see Figure 2), modern science, research and medicine are still far from broad and lifelong adoption of genomic information for many reasons, including information complexity, reimbursement of genomics by payers, and lack of common frameworks around data interpretation, usage, and management. One of the most powerful challenges to the genomics trajectory and impact opportunity, especially in the healthcare system, is that genomic information is largely regarded as not actionable or predictive enough due to limited scientific research. To unlock the power of the genome and its potential for discovery, science, research, and medicine, we must create a new platform for research.
Genomics data is now plentiful, as described in “Leveraging Intersecting Trends” below. The true issue is data aggregation and organization to enable discovery, which is why we are building the Database.
-5-
Table of Contents
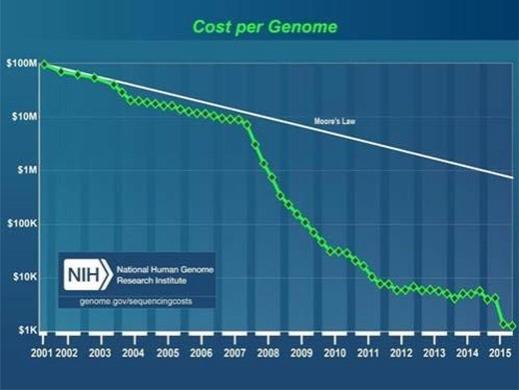
Figure 2. Cost per ‘draft’ whole human genome sequence, National Human Genome Research Institute
Unlocking the Potential for Discovery
The Database aims to address four primary issues that have hindered genomics research:
| 1. | The scale and scope of discovery datasets have been insufficient for discovery and broad applicability of discoveries to the widest population. Researchers require more samples, more data types (DNA, phenotype, health history, lifestyle, environment, nutrition), and greater diversity (gender, ethnicity, age, socioeconomic). |
| 2. | The data in databases have lacked a harmonized structure; they cannot be aggregated for calibrated and reproducible discoveries. |
| 3. | Data is siloed and will likely remain isolated, despite calls to share data. Most institutional incentives and business models are to retain data because that’s what their business or laboratory was established to do and what their members and stakeholders expect them to do; moreover, in many instances, consent was not granted from the individual to release their data for research. |
| 4. | People have been treated as specimen sources and not holders of tremendous value for medical research. Genomic research has been disease-centric as opposed to being people-centric. People care about their holistic health, which includes both prevention (to maximize wellness) and treatment (during sickness). The current health industry rewards only disease treatments. We believe that people hold valuable health information and should be treated as research partners and recognized and rewarded for their contributions to our mission. |
People-Centric Model
When enabled, we feel that individuals will seek involvement as research partners with the opportunity to fight disease, especially if they are managing a chronic condition. In addition to the need to accelerate our understanding and treatments of common complex disease, approximately 7,000 different types of rare disease and disorders afflict 30 million people in the United States that remain a mystery. Similar to the United States, Europe has approximately 30 million people living with rare diseases, and an estimated 350 million people worldwide suffer from rare diseases. Healthy people also bring tremendous research value, not only as controls in disease study, but also as study subjects to understand how they have avoided disease.
-6-
Table of Contents
We are aiming to unlock the power of the genome by unlocking the potential for discovery with the largest aggregation of genomic and health data ever assembled. By engaging individuals proactively and responsibly, our goal is to facilitate purpose-driven deep engagement that will lead to an information-rich, active, and longitudinal data community. Through a silo-free, people-centered effort, we aim to achieve the scale and scope to enable research for a wide range of diseases, both common and rare, as well as increase our understanding of healthy states. We believe a flexible platform of magnitude architected with smart contract capability and technical extensibility to ingest data associated with new monitors of health states (e.g., wearables) will have the statistical power to reveal the genomic underpinnings of many diseases and also to detect associations between nutritional, environmental, or other exposures to health outcomes.
Market Background
The Genomics Era
On June 26, 2000, in the East Wing of the White House, United States President William J. Clinton and British Prime Minister Tony Blair announced the successful completion of the first whole human genome sequence. This groundbreaking scientific achievement, called the Human Genome Project, encompassed over 10 years and cost $2.7 billion to complete. Sequencing a human genome heralded a new era for understanding and treating human disease based on the fundamental building blocks of life – DNA. With the sequence of the human genome in hand, the next step was to identify the genetic variants that increase the risk for common diseases like cancer and diabetes. The vision was that if the underpinnings of disease could be decoded, then precision or personalized medicine would be possible.
The State of Genomic Discovery
Genomics is the study of the function and the evolution of genomes. In humans, this typically refers to the 23 pairs of chromosomes and mitochondrial DNA that make up the full complement of DNA present in every cell. Many hereditary diseases, such as Huntington’s Disease, as well as other conditions, can be traced back to specific gene mutations observable in the DNA code. To date, genomic research studies have identified genetic causes of hundreds of traits and diseases, including breast cancer, high cholesterol, rheumatoid arthritis, schizophrenia, height, atrial fibrillation, and responses to various medications. These studies not only provide diagnostic value for families and individuals but, moreover, provide meaningful insights into gene function and disease mechanisms that enable better drug design and targeted treatments.
During the last decade, genome-wide association studies (GWAS) that utilize common variants in our genome, instead of analyzing the whole genome, have emerged as the primary method of discovering genetic variants associated with complex traits and disease. The GWAS approach was utilized primarily due to economics. The measurement of hundreds of thousands of common variants in our genome was more than one-thousand times less costly than acquiring 3.3 billion bases in whole genome sequencing studies. Unlike traditional linkage mapping approaches, which are based on analyzing patterns of disease inheritance in families, GWAS is based on the observation that common polymorphic genetic markers that are close to a causative disease allele are often statistically associated with disease status in large cohorts of unrelated individuals.
A major strength of GWAS is its ability to locate causative genetic variants with fine-scale resolution. However, GWAS requires obtaining and analyzing data from large numbers of samples. In many cases, data from tens of thousands of individuals are required to achieve adequate statistical power.
Although GWAS studies have successfully identified thousands of common genomic variants that contribute to diseases, each variant rarely accounts for more than a small fraction of disease causation. The full genome, including rare genomic variants detected through direct DNA sequencing, the microbiome, the epigenome, and other environmental factors are together thought to explain the vast majority of disease impacting human health. Detailed genome sequencing of millions of individuals will be required to fully understand genetic contributions to disease and health. While large amounts of genomic and other data are freely available from public databases such as the National Center for Biotechnology Information (NCBI), in general, such data have been of little interest to the pharmaceutical industry due to the high variation in data quality and standards of data encoding. To meet these criteria, pharmaceutical companies typically rely on data collected in-house.
In summary, the causes of many genetic diseases remain stubbornly hidden despite advances in technology to read whole genomes of individuals cost-effectively. We envision that the scope, scale, and harmonized data architecture of the Database will help reveal genotype-phenotype associations that otherwise could not be found due to lack of statistical power and/or data interoperability.
-7-
Table of Contents
Precision Medicine
Precision medicine proposes to invert the healthcare framework by recognizing that each patient is biologically unique. Rather than clinical trials to determine whether a therapy is safe and effective for most of the population before it is available to all of the population, personalized medicine applies technology to big data to investigate whether therapies will be effective for that specific patient and, equally important, if it will not be effective and potentially harmful for that patient.
This distinction is important because diseases are unique to the individual. Diseases manifest and progress differently in different people, and treatments that are effective for one person may fail altogether for another. The promise of precision medicine is that patients will respond to targeted therapies and avoid the all too common, ineffective, costly, and often damaging treatment regimen. The cost of “imprecise” medicine has been well documented (see Figure 3).

Figure 3. Personalized medicine: Time for one-person trials
-8-
Table of Contents
Our DNA represents a “barcode” that is individually unique and can be leveraged in precision medicine and health. Futurists project that everyone will have genome information as a resource, on file and actionable, before they get sick so that it can be leveraged to maximize the health strengths that individuals naturally possess, while avoiding an individual’s inherent health weakness through lifestyle decisions. Precision medicine will increasingly leverage advances in big data to analyze large amounts of genomic data and apply the understanding gained to individual diseases and treatment. As stated, for centuries, the engine of medicine has been the clinical trial that poses the central question, “what is effective for most people?”. Genomic data and the technology developed to compute, analyze, and understand these data is increasingly regarded as the engine of medicine going forward.
We believe that the research enabled by our Database will drive the development and application of more genome-guided therapeutics, ensuring that the right medicine is given to the right patient at the right time.
The DNA-Aware and Data-Engaged Consumer
The popularity of the direct-to-consumer (DTC) genetic testing market segment signals an ever-expanding paradigm shift among consumers who are seeking more individualized health insights and greater control over their own healthcare.
Since the 1980s, consumers have pushed for access to their laboratory results, but access became slow to evolve due to concerns by doctors and regulators that consumers may try to self-diagnose without understanding the complexity of the data. With advances in genetic testing technology at accessible cost points and the mainstream nature of personalized medicine, DTC laboratory testing is becoming increasingly popular—attributed in part to actress Angelina Jolie’s op-ed in The New York Times in May 2013 called ‘My Medical Choice,’ which documents her medical decisions based on BRCA1 gene mutations and family history of cancer. Likewise and following suit, consumers are becoming health hobbyists and self-quantifiers, taking individualized healthcare into their own hands. Consumers have become medical consumers as well as patients. This has created a shift in the doctor/patient relationship as individuals have become more knowledgeable about their own health, view themselves as unique biologically in a one-sizefits all healthcare system, and want more control over their personal information and treatment decisions.
Almost 20 years since the Human Genome Project, consumer-directed genomic testing has become practically routine with over 12 million individuals purchasing genetic tests ranging from entertaining applications in genealogy and wellness to genetic profiling of tumors to provide targeted treatment guidance. A January 2018 report from market research firm Kalorama Information estimates that the consumer market for genetic health testing alone could nearly triple from about $99 million in 2017 to $310 million in 2022.
As technological advances continue to drive down the costs of genome sequencing, from approximately $1,000 per genome today to $100 or less within the next few years, there will be an increasing availability of this highly valuable health data. Several trends are continuing to shape the DTC market including the growing demand for maximizing wellness, early disease detection and diagnosis, personalized medicine, importance of disease monitoring, and expanded digital monitoring and sensing technologies. In addition, consumer-directed but physician mediated genomic tests are emerging with companies like Veritas Genetics partnering with healthcare systems like the Mayo Clinic.
Privacy, Security, and Trust
Arguably, no data is as personal as an individual’s own genome—the essential blueprint of each individual’s life, and, to an extent, each individual’s family. Privacy concerns are paramount in the design of any biomedical study involving human participants and especially genomics. These concerns arise from the many potential abuses of personal genetic and medical data, including denial of healthcare services due to genetic predispositions, racial discrimination, and disclosure of intimate familial relationships such as non-paternity.
In current practice, privacy is typically protected by concealing the identities of study participants, while certain types of de-identified data are shared freely. Standard data security controls are often sufficient for protecting identity data itself, but in many cases the freely-shared component remains vulnerable to misuse. For example, advances in re-identification techniques have made it possible to infer surnames from certain types of genetic data. For this reason, genomic data and pre-defined aspects of one’s personal information are not shared publicly by data aggregation efforts, even when de-identified.
There are a variety of reasons people participate in biomedical studies. Some reasons may be personal, such as the desire to know one’s ancestry and disease predispositions. Other motivations may be broader or more altruistic, such as the desire to
-9-
Table of Contents
improve human health and society. In all cases, there must exist a level of trust between the research participant and the investigators that they are pursuing a shared goal. Unfortunately, failure of researchers to maintain the trust of study participants can have lasting negative effects on science as a whole.
A notable example is the 2010 legal battle between the Havasupai tribe and Arizona State University that ensued after researchers used genetic data collected from tribe members to study sensitive topics outside the perceived scope of the project (type 2 diabetes), such as inbreeding, demographic history, and schizophrenia. Further eroding public trust in research activities is the fact that many pharmaceutical and biotech companies forgo an open, collaborative approach to research and development for strategic reasons because they estimate its benefits are outweighed by legal, regulatory, and intellectual-property risks. The perceived lack of transparency and sense of common purpose often discourages study participants from providing broad consent to use their data in these cases. Trust remains a significant factor in individuals both consenting to studies and providing important health and medical data.
Privacy, security, and trust are core pillars of the Database and are reflected in our team, mission, transparency, and the technology we are using to ensure the best possible management and maintenance of information. It is important to note that we, unlike others who are brokering sale of individuals’ data, will be marketing discovery based on de-identified metadata.
Research Participant Consent
Nearly all medical research requires some form of informed consent by, or on behalf of, the research participant. In this process, the individual enrolling in the study provides their voluntary agreement to participate in the research, and understands the risks associated with their participation. Sometimes the field of the informed consent is very narrow, such as in clinical trials for pharmaceutical companies interested in deep, focused studies of a particular biological function or disease. In other cases, the consent can be broad, enabling future exploratory studies into research questions that are yet to be defined. Occasionally, data collected as part of a research study can be shared and/or re-examined by other investigators for a secondary study. In practical terms, this variability means the usefulness of a collection of data sets is circumscribed by the subset with the narrowest terms of consent. This presents a clear scalability problem and limits the utility of historical datasets, if the individuals comprising the data are unavailable to provide a more broad informed consent.
Our Manager has endeavored to ensure our informed consent process is clear and effective.
Drug Development & Data Monetization
One of the primary use cases for mining genomic databases is the opportunity to identify new drug targets. Rational drug design leverages biological understanding to develop therapies targeted at disease pathways and mechanisms of action, and can be applied to both common and rare diseases. By understanding the genetics of disease and the role mutated genes play in the cell, drug developers can pursue a more “rational” design approach. This promise of genomic insights to drive better drug development motivated the purchase of deCODE Genetics by drug developer Amgen in 2012 and at least a dozen pharmaceutical company partnership deals with consumer genetics company 23andMe, which reportedly holds genomic data on approximately four million customers.
A recent example of how rare genetic mutations can lead to understanding of the biology underlying common diseases and lead to cures for the broader population came from the study of a small sample of patients with familial hypercholesterolemia (FH). FH is suspected when LDL-cholesterol is above 190 mg/dL in adults and above 160 mg/dL in children without cholesterol-lowering treatment and poses a life-long risk of severe cardiovascular disease. Based on these family studies, researchers discovered a monogenic form of FH that is due to severe mutations in one of three genes: LDLR, APOB, or PCSK9. This observation led to the development of monoclonal antibodies to lower LDL by blocking the PCSK9 gene, and has now been commercialized by five different drug companies offering the therapy to lower cholesterol in the general population, not just those with FH.
Currently, an estimated 90 percent of potential medicines entering clinical trials fail to demonstrate the necessary efficacy and safety, and never reach patients. Many of these failures are due to an incomplete understanding of the link between the biological target of a drug and human disease. By contrast, medicines developed with human genetic evidence have had substantially higher success rates and patient care has benefited. In the spirit of more rational drug design guided by genomic information, Regeneron Pharmaceuticals reportedly brought together AbbVie, Alnylam Pharmaceuticals, AstraZeneca, Biogen, and Pfizer to each commit $10 million to enable genomic sequencing of the UK Biobank of 500,000 samples. These deals demonstrate a growing interest in access to genomic information for discovering drug targets, repositioning drugs, and
-10-
Table of Contents
better understanding the genetic underpinnings of disease. Our model enables smaller participants to make discoveries and provide an alternative source of data for “big pharma.”
Data Challenges
Unfortunately, much of the genomic data and phenotype data collected by commercial genomics companies, laboratories, and pharmaceutical companies remains siloed and inaccessible to the research and medical communities. In some cases, the reason is inefficient database design or poor data management practices. The pharmaceutical industry lags behind other sectors in several indicators of digital maturity. More often, however, is the strategic decision to attempt to extract value from data themselves, hampering meaningful data sharing across organizational boundaries. Additionally and importantly, the revenues of their data monetization strategies have never been shared with those who contributed the data. Often discovery companies go to great lengths and expense to ensure data provenance, completeness, and integrity, and hence these institutions attribute a much lower value to data from other entities.
Many pharmaceutical companies have begun integrating patient data from apps, wearable devices, and electronic medical records (EMRs) to improve healthcare and make discoveries about disease. Technology companies are also entering this market, such as IBM with its Watson Health program, and Apple with its HealthKit platform. Given these trends, it seems unlikely to us that the pace of future research will be limited by information technology problems, but rather hindered by corporate self-interest to keep the data private.
Another major challenge is the current state of EMRs. While adoption of EMRs is almost at 100 percent in the United States, in this new health economy, effective implementation of EMRs is still in the early stages. The standards deployed in terms of how they are used and the medical terminology adopted varies greatly from institution to institution. Standards for medical nomenclature and inter-relationship, or ontology, can vary greatly and, in some cases, health care providers still rely on the comments section of the patient record to record important information. Historic clinical records are often simply PDF files or pictures of hand written medical histories. Ingesting, curating, and harmonizing this information to the quality required for identifying links between our genome and our lifestyle to disease remains a major task and challenge.
By aligning with a broad base of members who individually have access to their data generated from these previously siloed sourced, we plan to collect, aggregate and make available to researchers a much broader range of data then available from the current siloed sources.
Our Solution
Leveraging Intersecting Trends
The two intersecting trends of inexpensive and accessible personal DNA testing and nearly frictionless transaction capacity create our opportunity. As discussed above in the section “—Market Background—The DNA-Aware and Data-Engaged Consumer” above, individual DNA testing is going mainstream, led by consumer-friendly companies like 23andMe, Ancestry.com, MyHeritage, and National Geographic in partnership with Helix, a partially owned subsidiary of Illumina. For example, Ancestry.com announced they sold 1.5 million DNA test kits between Black Friday and Cyber Monday in 2017. Approximately 12 million people reportedly have genotyping or sequencing data purchased through DTC genetic testing companies.
In addition to DTC products, there are new and emerging opportunities for individuals to receive their DNA information that can be contributed to us. Consumer-directed and physician-mediated wellness offerings are growing in popularity as individuals look to work with a healthcare provider to maximize their wellness. For example, the Mayo Clinic offers preventative whole genome sequencing through Veritas Genetics. Large-scale population health projects like the United States National Institute of Health’s All of Us Research Program will accelerate individual’s access to their genomic information. We also plan to engage with disease foundations who have very engaged patient communities and funding for genomics research, but who typically lack the interest and skills to stand up and manage a genomics database.
Together, these forces create the ideal time and place to develop a shared, secure, and member-controlled medical research data platform.
Broad Data Collection & Engagement
To date, obtaining large volumes of high-quality biological, health, and lifestyle data has been a major challenge in the medical research field. By being independent and agnostic to DNA analysis technology platform and brand, we can gather
-11-
Table of Contents
data from multiple sources without conflict of interest. A member’s genomic information can be acquired by companies that help him or her learn about himself or herself, while also being shared in the Database in support of disease discovery. There is no need to choose between these two options.
As part of our solution, new members can join our community and receive shares by contributing validated genomic information and providing consent for such data to be used anonymously in population-wide disease research. A wide range of genomic data types will be accepted, including genotyping, exome, and whole genome data files. In addition to the genomic data, we will collect high-quality health, medical, and environmental data from new and existing members. These data can come from EMRs, surveys on diet and exercise, health history, and data from biometric/wearable devices.
Given the high variation in data quality throughout the industry, we will take certain measures to validate the data submitted by our members and potential members to assure a high quality of data in our Database. Such measures will include requiring members to provide supporting information along with submissions, employing spam-blocking techniques, cross-checking data, assessing overlap and generally confirming that data is submitted in accordance with our terms for such data type.
Recognition of the importance of these combined datasets is demonstrated in the launch of large-scale projects including:
| • | Geisinger Health System’s MyCode Community Health Initiative including genome sequence data from 250,000 individuals, coupled to health records; |
| • | Apple’s Health app including heart rate, activity, sleep, and nutrition data; |
| • | Biometric data such as that collected by implanted and radio frequency-enabled pacemakers or glucose monitors; |
| • | Regeneron-UK BioBank partnership including genome sequence data from 500,000 individuals, coupled to health records and a wide range of phenotypes collected by UK BioBank; and |
| • | The National Institute of Health’s All of Us Research Program including environmental, wearable (e.g., FitBit), and genomic data on one million United States residents. |
The data collected in these projects are often accessible to the data owners (i.e., the patient/participant), but are often primarily captured in siloed databases and infrequently shared with the broader research and medical communities. Our solution is compatible with these efforts in that we are requesting that our members share their data with us on a non-exclusive basis.
One of our main goals is to encourage participants to remain active and engaged in the research process over time, providing regular data contributions and coming back to learn about how their data is being used. Crowdsourced funding platforms such as Patreon have been successful at building long-term relationships between artists and art patrons by providing a means to share art on a regular weekly or daily schedule, and see art in early creative stages. Such a relationship between scientists and study participants could greatly benefit the scientific creative process as well.
Platform Description
We are building our “community-owned” Database by providing our members share ownership in our Database as consideration for contributing personal genomic and phenotypic data, such as medical, health and health-related data for medical research, as described in “Securities Being Offered—Consideration” beginning on page 37 of the Offering Circular.
Our Database will earn income through the sale of access to its de-identified metadata and research findings to our customers in the research and medical industries.
Information inputs into our Database include self-contributed information, medical data, and DTC testing product data files. Our Database is extensible such that future data inputs (e.g., wearables) can be added. The collective de-identified data creates a metadata resource for nonprofit and for-profit research to be conducted with the help of enabling informatics and artificial intelligence resources. As value is derived from the access to and discoveries from our Database, a portion of that value flows back to the community via their share ownership.
Research Queries
We make de-identified Member Data in an aggregated, indexed, or otherwise analyzed fashion available to third-parties for their research use in ways that are designed to avoid the potential for learning the identity of the person or persons who contributed a particular item or set of Member Data. This population-level research may have various purposes, including the
-12-
Table of Contents
advancement of genomic science, identifying links between human genomes and disease, and other commercial applications, such as determining opportunities for targeted research that would seek your voluntary participation (e.g. how many members share a particular genotype and phenotype).
Targeted Research
We may use individual Member Data to determine whether you may be eligible to participate in targeted research and to then seek your voluntary participation in the targeted research. For example, if a third-party pharmaceutical company is interested in doing research on individuals who may have a particular genotypic, genomic, or phenotypic profile, we may query our Database to determine the subset of members (based on their de-identified Database record number) that have the relevant profile for participation in the study, and then initiate an anonymous invitation to matching members to request their participation in the study.
We may receive compensation from researchers for identifying potential members for targeted research. Initially, our Manager will negotiate with these researchers to provide them introductory access to members and our Manager may be compensated by such researchers for such services without sharing such compensation with our Company or any members. The research sponsors may thereafter negotiate directly with our members, and any such members who elect to participate in any such targeted research may be compensated directly by such researchers. Any such compensation to members will be determined entirely through private agreements between such members and the applicable researchers.
Community Ownership
By making our Database community-owned, our members become participants in and beneficiaries of the project, encouraging new members to join and members to actively participate and continue contributing their data to us for additional shares. As a member deposits more genomic, phenotypic, biometric (e.g., wearables) and other valuable Member Data in the Database, that member’s ownership stake in our Database increases. When medical or research organizations pay us to run queries against the Database, our profits from these transactions will enable dividends to our Database members, proportionately to the number of shares held and therefore the value of data each member contributed.
We believe that as our Database grows, it will become increasingly valuable to the medical and research industries. Unlike other companies currently marketing DTC test kits, proceeds generated by selling query access to our Database, such as to pharmaceutical companies, will inure in part to the benefit of the member community. Members will always retain the ability to withdraw their Member Data from our Database by terminating their consent with respect to their Member Data or purging selected Member Data.
Community ownership addresses many of the challenges that exist due to: prevalence of data silos; lack of trust in commercial entities monetizing an individual’s data; lack of trust in research activities that go dark once information is provided; and lack of single data standards hindering large-scale biomedical research studies.
We will update members on studies being performed and results that accrue. It is anticipated that a primary motivation for many members will be to support the greater good through scientific discovery. We aim to encourage this type of participation through regular communications in order to build trust in the management of our Database and its contributions to science.
Extensibility of the Database
The first focus of our Database is driving associations between genomic information and health outcomes, as well as determining social determinants of health. However, as science advances and other “omic technologies” (which are primarily aimed at the universal detection of genes (genomics), mRNA (transcriptomics), proteins (proteomics) and metabolites (metabolomics)) in a specific biological sample (see Figure 4), such as gut microbiome or proteomics become less expensive and more accessible to patients and consumers, our Database will be scalable and capable of incorporating these other data types to further researchers’ ability to understand and digitize the medical and health essence of a human being.
-13-
Table of Contents

Figure 4. Geographic information system (GIS) of a human being.
The ability to digitize the medical essence of a human being is predicated on the integration of multi-scale data, akin to a digital map, which consists of superimposed layers of data such as street, traffic and satellite views. For a human being, these layers include demographics and the social graph, biosensors to capture the individual’s physiome, imaging to depict the anatomy (often along with physiologic data), and the biology from the various omics (genome-DNA sequence, transcriptome, proteome, metabolome, microbiome, and epigenome). In addition to all these layers, there is one’s important environmental exposure data, known as the “exposome.”
Blockchain Technology
To provide our community of members with transparency into our operations, including providing a tamper-evident record of the transactions described below, we are evaluating the feasibility of using the blockchain to store select information about several types of transaction related to our company. Implementation of a blockchain for these functions entails various risks, and we may for that or various other reasons abandon these efforts. At the present time and if feasible, we plan to store the following record types in our blockchain, which we currently store using only conventional database technology.
| • | Members Joining or Withdrawing: When a new member first joints our community by contributing Member Data to our Database, or if an existing member withdraws from our Company, we would record that fact together with an anonymized identifier for that member in the blockchain. Member identities will be anonymized by using a hashed or numerical identifier or similar de-identifying measure to represent each member. |
| • | Member Data Contribution or Withdrawal: When a new or existing member contributes new Member Data to our Database, or an existing Member has us purge some or all of his or her Member Data from our Database, we would record the type of data submitted or purged, the number of shares issued to the member (in case of a contribution) or redeemed by us or the member (in case of a purge), and the member’s de-identified identifier discussed in the preceding bullet point. |
| • | Database Queries: When a third party conducts a query against our Database, we would add the occurrence of the query and the anonymized identity of the third party to the blockchain. In some cases we may add a description of the query as well. The blockchain will not include any results of queries. |
We may in the future add additional data types to our blockchain to create greater transparency to our community, subject to our privacy and security policies.
-14-
Table of Contents
The largest advantage of blockchain technology over exclusive reliance on our current conventional database technology is that we intend to implement our blockchain solution using an independent, decentralized network of servers, such as the Ethereum (or similar) network, to improve public verifiability. The decentralized network operates by having multiple nodes validate any changes to the blockchain, such as the addition of a new transaction, and a change is accepted to the blockchain only if a majority of nodes agree that the transaction is valid. Independence means that independent third parties will control the nodes validating transaction and updating the blockchain. In our intended implementation, the network would not delete old transactions; instead, each new transaction would be added to the existing blockchain, enabling members to have a full transaction history.
While blockchain technology is relatively new, we anticipate that the advantages of this approach would be twofold. First, every member or potential investor would have assurances that every block of data retrieved from the blockchain – i.e., every transaction –is uncorrupted and unaltered since its initial recording. Second, each member or potential investor can trace how the blockchain has been appended over time and validate that our current reports to members, including the member’s share holdings and the total outstanding shares, are accurate.
In the event we implement our blockchain, we also intend to develop a tool which will enable anybody to review the data stored in the blockchain. Moreover, since we intend to make our blockchain publicly accessible on the Ethereum (or similar) network, any third party, including any member or potential investor, would be able to develop their own tools, or employ existing compatible tools provided by third parties, to retrieve the blockchain data.
Our Unique Advantages
Public Service Vision
Our Company was founded with a vision of public service at least as important as potential financial remuneration. Trust in us by our members is paramount. We recognize our members are voluntarily placing some of their most private health data in our Database, and we understand we will need to work hard to earn and maintain the trust that informs that contribution. Our operating agreement authorizes our Manager, a public benefit corporation, to make decisions that prioritizes public welfare. However, as demonstrated by other public benefit corporations, such as Patagonia and Warby Parker, what is in the interest of the public can also show significant financial returns in the long run.
Public benefit corporations are a relatively new class of corporations that are intended to produce a public benefit and to operate in a responsible and sustainable manner. Under Delaware law, public benefit corporations are required to identify in their certificate of incorporation the public benefit or benefits they will promote and their directors have a duty to manage the affairs of the corporation in a manner that balances the pecuniary interests of the stockholders, the best interests of those materially affected by the corporation’s conduct, and the specific public benefit or public benefits identified in the public benefit corporation’s certificate of incorporation. Public benefit corporations organized in Delaware are also required to assess their benefit performance internally and to disclose publicly at least biennially a report detailing their success in meeting their benefit objectives.
Our Manager’s public benefit, as provided in its certificate of incorporation, is to create and maintain a community-owned genomic and phenotypic database that is designed to solve humankind’s most important medical challenges.
Given the large number of rare, chronic, and overlooked diseases, and the huge volume of genomics data being generated, it is critical that genomics data be made available to researchers to support the public good. Traditionally, this has been the domain of government institutions such as the National Institutes of Health and various non-profit foundations. However, foundations, while supportive of data sharing, are often not in the position to build the required technology to create the searchable container, or database, nor are they in a position to be responsible for sustaining the data and safeguarding the privacy and security of the data.
First Class Team in the Epicenter of Genomics
Our Manager’s executive team is comprised of experienced leaders in science and genomics, engineering, economics, and large-scale consumer engagement platforms. See “Directors and Officers” beginning on page 18.
-15-
Table of Contents
San Diego, the Capital of Genomics
We are proud to be headquartered in San Diego, where biotech and high tech, especially as it relates to genomics, are part of our economic and community fabric. As the number one most patent intensive genomics market in the United States, San Diego is leading the charge in a new era of healthcare. Personalized medicine and technology are taking precedence, with local genomics companies, research institutions, and universities at the forefront. Other highlights from the San Diego Regional Economic Development Corporation 2017 report include:
| • | Leadership: San Diego is poised to continue its leadership in the field of precision medicine. With more than 115 genomics-related firms, San Diego has companies that handle every aspect of the genomics value-chain, from sampling and sequencing (e.g., Illumina, Thermo Fisher Scientific) to analysis and interpretation (e.g., AltheaDX, Human Longevity, Inc.) to clinical applications (e.g., Celgene, Arcturus Therapeutics), creating a complete ecosystem. Additionally, San Diego conducts the fundamental scientific research, due in part to the concentration of research institutes, that form the basis for many global genomics therapies and interventions. |
| • | Capital: While San Diego is home to just one percent of the United States population, it received 22 percent – $292 million – of the venture capital funding in genomics in 2016. Continually, San Diego’s numerous nonprofit research institutes command a large share of federal funding (e.g., NIH). In fact, San Diego received $3.2 million federal contract dollars in 2016 – more than any other United States region. |
| • | Talent: San Diego produces more genomics-ready graduates, relative to the size of its workforce, than any other United States region. With nearly 2,000 average genomics-related degrees (biochemistry, cognitive science, and bioinformatics) conferred per year, San Diego’s genomics companies benefit from the preparatory work of the region’s top academic institutions. In that vein, it is projected that the local talent pool for key genomics occupations will grow by an additional 10 percent by 2021. |
Government Regulation
Health Insurance Portability and Accountability Act of 1996 (HIPAA)
We are not subject to the Health Insurance Portability and Accountability Act of 1996, as amended, commonly known as HIPPA, because we are not a “covered entity” for purposes of that law. Accordingly, the privacy and other protections afforded to patients by HIPAA do not apply to us.
European General Data Protection Regulation (GDPR)
The European General Data Protection Regulation, commonly referred to as the GDPR, went into effect in May 2018. The GDPR increases privacy rights for individuals in Europe, extends the scope of responsibilities for data controllers and data processors and imposes increased requirements and potential penalties on companies offering goods or services to individuals who are located in Europe or monitoring the behavior of such individuals (including by companies based outside of Europe). Noncompliance can result in penalties of up to the greater of €20 million, or 4% of global company revenues. While we are currently accepting only members who reside in the United States, and hence are currently not subject to the requirements of the GDPR, we have structured our policies and procedures to comply with the GDPR.
Employees
We have no full time employees. We are managed by our Manager. Currently, our Manager has 12 full-time and one part-time employee.
Legal Proceedings
We know of no existing or pending legal proceedings against us or our Manager, nor are we or our Manager involved as a plaintiff in any proceeding or pending litigation.
-16-
Table of Contents
| Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
Overview
We were organized as a limited liability company under the laws of the State of Delaware on April 23, 2018. We are managed by LunaPBC, Inc., a Delaware public benefit corporation, which we refer to as our Manager. We have entered into a management services agreement, which we refer to as the Management Agreement, with our Manager to provide business, operational and financial management services to us. The rights, duties and powers of our Manager are governed by the terms of the Management Agreement and the Company operating agreement, which we refer to as our Operating Agreement. We seek to build the world’s first and largest human health database of Member Data, which we refer to as our Database, that is owned by a community comprised of its members. Our Database is comprised of various types of genomic and phenotypic data, such as medical, health and health-related data.
Operating Results
During the period from April 23, 2018 (inception) to December 4, 2018, our Manager was our sole member. As such, we have accounted for the expenses paid on our behalf by our Manager as our expenses with a corresponding credit to members’ share capital, in accordance with the guidance in Staff Accounting Bulletin Topic 5T – Accounting for expenses or liabilities paid by principal stockholder(s) (“SAB Topic 5T”).
At December 4, 2018, the expenses paid on our behalf by our Manager total $532,173. These expenses primarily consisted of all fees, costs and expenses incurred in connection with our organization and in connection with the initial offer and sale of our shares, including legal and accounting fees.
Our Manager automatically resigned as, and ceased to be, a member on December 5, 2018 when the first contributor of data to LunaDNA was admitted as a member. From December 5, 2018, we do not believe that SAB Topic 5T continues to apply and have ceased recording as our expenses those expenses that our Manager incurred on our behalf after our Manager resigned as a member and for which our Manager does not have a contractual right of reimbursement. Our Manager is obligated to cover our expenses without reimbursement until March 30, 2020 pursuant to our Management Agreement.
Liquidity and Capital Resources
To date, all of our expenses have been covered by our Manager. Our Manager is obligated to cover our expenses without reimbursement until March 30, 2020 pursuant to our Management Agreement. As discussed in Operating Results above, we have accounted for the expenses paid on our behalf by our Manager as our expenses with a corresponding credit to member’s share capital, in accordance with the guidance in SAB Topic 5T. From December 5, 2018, we have ceased recording as our expenses those expenses that our Manager incurred on our behalf after our Manager resigned as a member and for which our Manager does not have a contractual right of reimbursement.
In the future, we will be obligated to promptly reimburse our Manager for all our expenses advanced by our Manager, in accordance with our Management Agreement (which the Manager may modify unilaterally). Our dependence on our Manager for funding of these expenses raises substantial doubt about our ability to continue as a going concern.
Our Manager’s capacity to fund all of our organizational expenses and to fund our operational expenses and the development expenses through the time when we generate significant revenues from operations is dependent on our Manager’s existing cash resources and its ability to obtain additional capital financing from investors sufficient to meet our needs and the needs of our Manager’s other operations. Our Manager has funded its operations and our operations primarily by sales of equity securities. Our Manager believes that it will be able to raise capital that, combined with its existing cash resources, will be sufficient to fund all of our organizational expenses and to fund our operational expenses and the development expenses through the time when we generate significant revenues, at which time our revenues may be used to pay both our operational expenses and the management fee to our Manager (which management fee may then fund further development expenses). However, there can be no assurance that our Manager will be successful in its fundraising efforts. If our Manager is not successful in its intended fundraising efforts, our Manager may be required to delay various planned expenditures for development and marketing of our Database, which delays could materially adversely delay generation of revenues and potentially jeopardize our ability to continue as a going concern.
We may not generate operating revenue for an indeterminate period of time, until third parties begin to pay us for services related to our Database. Upon generation of revenue, we will be obligated to pay a management fee to our Manager.
Plan of Operations
We commenced principal operations on December 5, 2018 when the first member data contribution was accepted and the first shares were issued to a member contributing data. We have not generated any revenues to date. Our only activities from April 23, 2018 (inception) through December 4, 2018 were organizational activities and those necessary to prepare for the offering of our shares. We may not generate operating revenue for an indeterminate period of time until third parties begin
-17-
Table of Contents
to pay us for services related to the Database. We expect to incur increased expenses as a result of being a public company (for legal, financial reporting, accounting and auditing compliance). We will rely on our Manager to fund our plan of operations for the next twelve months.
| Item 3. | Directors and Officers |
We do not have any directors, officers, or significant employees. We are managed by our Manager, and we sometimes refer to our Manager’s officers and directors as our officers and directors and as our management. Neither our Manager, nor our Manager’s directors, is elected by our members, and neither will be subject to re-election by our members in the future. Our Manager may not be removed by our members for any reason.
Our Manager has a board of directors consisting of three directors. The following are the directors, key officers and significant employees of our Manager, who by virtue of their collective ownership of our Manager, control our Manager. No other person or entity controls our Manager.
| Name |
Position with Manager |
Age | Term of Office |
Approximate Hours per Week for Part-Time Employees |
||||||||
| Robert Kain | Chief Executive Officer and Director | 57 | Since October 2017 | |||||||||
| Dawn Barry | President and Director | 44 | Since January 2018 | |||||||||
| Scott Kahn, Ph.D. | Chief Information Officer | 59 | Since January 2018 | 30 Hours | ||||||||
| David Lewis | Chief Financial Officer, Treasurer, Secretary and Director | 47 | Since October 2017 | |||||||||
| Debora Thompson | Vice President, Strategic and Business Ops | 45 | Since March 2018 | |||||||||
| Kenneth Bloom James White |
VP & Chief Architect VP, Commercial |
|
41 42 |
|
Since April 2018 Since April 2019 |
|||||||
Robert Kain has been LunaPBC’s Chief Executive Officer (Principal Executive Officer) since October 2017. Bob is a renowned pioneer in genomics and a co-founder of LunaPBC. Bob joined Illumina pre-IPO in 1999 and retired in 2014 as the Chief Engineering Officer. At Illumina, Bob led the invention of the modern, high-throughput genome sequencer that brought the cost from millions of dollars per genome down to less than $1,000 U.S. dollars. Bob is lead inventor on 28 United States patents that led to the breakthroughs that have revolutionized genome sequencing. Prior to joining Illumina, Bob was the Director of the Microarray Business Unit at Molecular Dynamics. He is also on the Scientific Advisory Boards of Dovetail Genomics, Singular Genomics, and Edenroc Biosecurity, and is the co-founder and acting chief executive officer of Revere Biosensor, a private company. Additionally, he is the co-founder and chairman of the board of a successful health & fitness business, Mesa Rim Climbing and Fitness Center, with multiple locations in San Diego and Reno, Nevada. Bob received a BS in Physics from San Diego State University and an MBA from Saint Mary’s College of California.
Dawn Barry has been LunaPBC’s President since January 2018. Dawn is an esteemed thought leader and veteran of the genomics industry and a co-founder of LunaPBC. She has given TED talks and is a frequent guest speaker on personalized medicine and genomics. Dawn served as the Vice President of Applied Genomics at Illumina, Inc through January 2018. Dawn integrated market development strategies with product and business model innovation to accelerate the application of genomics in medicine and personal healthcare. Dawn joined Illumina in 2005. Prior to Illumina, Dawn spent seven years at Genaissance Pharmaceuticals, one of the first genomics startups focused on individualized medicine and DNA-based diagnostic testing. Dawn was named San Diego Business Journal’s 2017 Business Woman of the Year and was a speaker at TEDxSanDiego 2016. She holds a BS in biology from the University of Vermont and a MBA from the University of Connecticut School of Business.
Scott Kahn, Ph.D., has been LunaPBC’s Chief Information Officer since January 2018. Scott leads LunaPBC’s information and data strategy and information security. Scott joined Illumina in 2005 as its first Chief Information Officer and also served as its VP of Commercial and Enterprise Informatics, where he led all corporate information services, software development, and bioinformatics. Prior to Illumina, Scott was the Chief Science Officer and General Manager of Life Sciences at Accelrys (currently Biovia/Dassault Systems) responsible for 65 percent of the company’s business. Scott received his PhD in Theoretical Organic Chemistry and was an Assistant Professor of Chemistry at the University of Illinois Urbana-Champaign.
-18-
Table of Contents
David Lewis has been LunaPBC’s Chief Financial Officer, Treasurer and Secretary since October 2017. David is a co-founder of LunaPBC and leads the company’s strategic funding and investment initiatives. A successful life science, equity and credit investor, David’s background is in investing in public and private companies on behalf of institutional investors, family offices, and high-net worth individuals. David started his career in investment banking at Lehman Brothers in 1994, then left banking to pursue several analyst and portfolio manager roles at CitiGroup, First Manhattan, and J Goldman & Co. In 2006 David Co-founded Ganley Investments with the financial backing of a large multinational family office, and served as its Chief Investment Officer. He holds a Bachelor of Business Science with honors in Accounting and Finance from the University of Cape Town, South Africa.
Debora Thompson has been LunaPBC’s Vice President, Strategic and Business Operations, since March 2018. Debora joined LunaPBC in March 2018 to spearhead strategic planning and execution of the LunaPBC infrastructure development. Prior to that, she spent over 10 years at Illumina leading projects and teams to accelerate genotyping and next-generation sequencing into applied and clinical markets. She served as chief of staff to both the new and emerging markets and applied genomics business units. Her passion and commitment to improving healthcare through discovery is anchored by a background in clinical molecular biology research and almost 20 years in biotech. She holds an MS from the University of Central Florida.
Kenneth (Kirby) Bloom has been LunaPBC’s Chief Architect since April 2018. In that role, Kirby is primarily responsible for architecting and developing the engagement and discovery platform that invites, aggregates, and organizes Member Data; establishes members as shareholders; and enables medical research across the de-identified aggregated Member Data. Prior to this role, Kirby was Head of Software & Informatics at Illumina, Inc. where he led the ideation and development of several sample-to-answer software solutions for Illumina’s sequencing and microarray platforms serving forensics, transplant diagnostics, food and agriculture, microbiology, and clinical segments. He began working at Illumina in 2007. With almost 20 years of experience in software design, engineering and databases, Kirby specializes in user centric interfaces and experiences and highly scalable, distributed cloud architectures and data engineering. He holds a BS in Management Information Systems from Texas Tech University and is currently working towards his Masters in Information & Data Science at University of California, Berkeley.
James White has been LunaPBC’s Vice President, Member Engagement & Experience since April 2019. James joined LunaPBC to reinforce and exemplify the member-first mission through people-centered design. He is an advocate of technology democratization to advance health discovery and human well-being. As an agency leader with over twenty years of brand strategy, consumer advertising and experience design, James has built effective infrastructure for shareholders, consumers and brands. James has worked with companies such as Adidas, GlaxoSmithKline and Qualcomm to create a formula to measure return on meaningful experience. He now will apply his methodologies to shape the Luna member experience. James holds a Joint Honours Degree, BSc. Psychology & Human Physiology from the University of Birmingham, UK.
Family Relationships
There are no family relationships among any of our officers or directors.
Potential Conflicts of Interest
Since we do not have an audit or compensation committee comprised of independent persons, the functions that would have been performed by such committees are performed by our Manager. Thus, there is a potential conflict of interest in that our Manager has the authority to determine issues concerning its own and its management’s compensation and other audit issues that may affect management decisions.
Involvement in Certain Legal Proceedings
In the last five years,
| • | no petition under the federal bankruptcy laws or any state insolvency law has been filed by or against, nor has a receiver, fiscal agent or similar officer been appointed by a court for the business or property of, any director of executive officer of our Manager, or any partnership in which he or she was general partner at or within two years before the time of such filing, or any corporation or business association of which he or she was an executive officer at or within two years before the time of such filing, and |
-19-
Table of Contents
| • | no director of executive officer of our Manager has been convicted in a criminal proceeding (excluding traffic violations and other minor offenses). |
Compensation of our Manager
For information regarding the compensation of our Manager, please see “Compensation of our Manager” on page 32
of our Offering Circular.
-20-
Table of Contents
| Item 4. | Security Ownership of Management and Certain Securityholders |
The following table sets forth the approximate beneficial ownership of our shares as of April 26, 2019 for each director, key officer and significant employee of our Manager and for the directors, key officers and significant employees of our Manager as a group. As of such date, 25,648 shares were issued or earned. No person or group holds more than 10% of our shares.
| Name of Beneficial Owner (1) (2) | Number of Shares Beneficially Owned |
Percent of All Shares |
||||||
| Robert Kain |
54 | * | ||||||
|
|
|
|
|
|||||
| Dawn Barry |
4 | * | ||||||
|
|
|
|
|
|||||
| Scott Kahn, Ph.D. |
64 | * | ||||||
|
|
|
|
|
|||||
| David Lewis |
54 | * | ||||||
|
|
|
|
|
|||||
| Debora Thompson |
54 | * | ||||||
|
|
|
|
|
|||||
| Kenneth Bloom |
52 | * | ||||||
|
|
|
|
|
|||||
| James White |
2 | * | ||||||
|
|
|
|
|
|||||
| All directors and executive officers of our Manager as a group (7 persons) |
284 | 1.1 | % | |||||
|
|
|
|
|
|||||
| * | Represents less than 1% of our outstanding common shares. | |||
|
(1) |
Under SEC rules, a person is deemed to be a “beneficial owner” of a security if that person has or shares “voting power,” which includes the power to dispose of or to direct the disposition of such security. A person also is deemed to be a beneficial owner of any securities which that person has a right to acquire within 60 days. Under these rules, more than one person may be deemed to be a beneficial owner of the same securities and a person may be deemed to be a beneficial owner of securities as to which he or she has no economic or pecuniary interest. | |||
|
(2) |
Each listed beneficial owner has an address in care of our principal executive offices at 4110 Campus Point Court, San Diego, CA 92130. | |||
-21-
Table of Contents
| Item 5. | Interest of Management and Others in Certain Transactions |
Please see “Interest of Management and Others in Certain Transactions and Conflicts of Interest” on page 34 of our Offering Circular.
During the period from April 23, 2018 (inception) to December 4, 2018, our Manager was our sole member. As such, we have accounted for the expenses paid on our behalf by our Manager as our expenses with a corresponding credit to paid in capital, in accordance with the guidance in SAB Topic 5T.
At December 4, 2018, the expenses paid on our behalf by our Manager totaled $532,173. These expenses primarily consisted of all fees, costs and expenses incurred in connection with our organization and in connection with the initial offer and sale of our shares, including legal and accounting fees.
Our Manager automatically resigned as, and ceased to be, a member on December 5, 2018 when the first contributor of data to LunaDNA was admitted as a member. From December 5, 2018, we do not believe that SAB Topic 5T continues to apply and have ceased recording as our expenses those expenses that our Manager incurred on our behalf after our Manager resigned as a member and for which our Manager does not have a contractual right of reimbursement. Our Manager is obligated to cover our expenses without reimbursement until March 30, 2020 pursuant to our Management Agreement.
| Item 6. | Other Information |
None.
| Item 7. | Financial Statements |
| Page | ||||
| 23 | ||||
| 24 | ||||
| 25 | ||||
| 26 | ||||
| 27 | ||||
| 28 | ||||
-22-
Table of Contents
To the Members
LunaDNA LLC
Solana Beach, CA
Report on the Financial Statement
We have audited the accompanying financial statements of LunaDNA, LLC, a Delaware limited liability company, which comprise the balance sheet as of December 31, 2018, and the related statement of operations, statement of changes in members’ capital, and statement of cash flows for the period since inception (April 23, 2018) through December 31, 2018, and the related notes to the financial statements.
Management’s Responsibility for the Financial Statement
Management is responsible for the preparation and fair presentation of these financial statements in accordance with accounting principles generally accepted in the United States of America; this includes the design, implementation and maintenance of internal control relevant to the preparation and fair presentation of the financial statement that is free from material misstatement, whether due to fraud or error.
Auditors’ Responsibility
Our responsibility is to express an opinion on these financial statements based on our audit. We conducted our audit in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the financial statements. The procedures selected depend on the auditors’ judgment, including the assessment of the risks of material misstatement of the financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to LunaDNA, LLC’s preparation and fair presentation of the financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of LunaDNA, LLC’s internal control. Accordingly, we express no such opinion. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of significant accounting estimates made by management, as well as evaluating the overall presentation of the financial statements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the financial statements referred to above presents fairly, in all material respects, the financial position of LunaDNA, LLC as of December 31, 2018 and the results of its operations and its cash flows for the period since inception (April 23, 2018) through December 31, 2018 in accordance with accounting principles generally accepted in the United States of America.
Matter of Emphasis
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has incurred losses and is dependent on additional financing to fund operations. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans regarding those matters are also described in Note 2 to the financial statements. The financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that may result from the outcome of this uncertainty.
/s/ Mayer Hoffman McCann P.C.
San Diego, California
April 30, 2019
-23-
Table of Contents
LunaDNA, LLC
Balance Sheet
| Assets |
||||
| Intangible Assets — Member Data |
$ | 540 | ||
|
|
|
|||
| Total Assets |
$ | 540 | ||
|
|
|
|||
| Liabilities and Members’ Capital |
||||
| Liabilities |
||||
| Due to Members |
$ | 150 | ||
|
|
|
|||
| Total Liabilities |
$ | 150 | ||
|
|
|
|||
| Members’ Capital |
||||
| Members’ Share Capital |
390 | |||
|
|
|
|||
| Total Members’ Capital |
$ | 390 | ||
|
|
|
|||
|
|
|
|||
| Total Liabilities and Members’ Capital |
$ | 540 | ||
|
|
|
|||
The accompanying notes are an integral part of these financial statements.
-24-
Table of Contents
LunaDNA, LLC
For the period from April 23, 2018 (inception) through December 31, 2018
| Expenses |
||||
| General and Administrative |
$ | 532,173 | ||
|
|
|
|||
| Total Expenses |
$ | 532,173 | ||
|
|
|
|||
| Net Loss |
$ | (532,173) | ||
|
|
|
|||
The accompanying notes are an integral part of these financial statements.
-25-
Table of Contents
LunaDNA, LLC
Statement of Changes in Members’ Capital
For the period from April 23, 2018 (inception) through December 31, 2018
| Members’ | ||||
| Share Capital | ||||
| Balance as of April 23, 2018 |
$ | - | ||
| Contributions |
532,563 | |||
| Net Loss |
(532,173) | |||
|
|
|
|||
| Balance as of December 31, 2018 |
$ | 390 | ||
|
|
|
|||
The accompanying notes are an integral part of these financial statements.
-26-
Table of Contents
LunaDNA, LLC
For the period from April 23, 2018 (inception) through December 31, 2018
| Cash Flows From Operating Activities |
||||
| Net Loss |
$ | (532,173) | ||
|
|
|
|||
| Net Cash used in Operating Activities |
(532,173) | |||
|
|
|
|||
| Cash Flows from Financing Activities |
||||
| Contributions by Manager paid directly to vendors |
532,173 | |||
|
|
|
|||
| Net cash provided by Financing Activities |
532,713 | |||
|
|
|
|||
| Net Increase (Decrease) in Cash and Cash Equivalents |
- | |||
| Cash and Cash Equivalents, Beginning of Period |
- | |||
|
|
|
|||
| Cash and Cash Equivalents, End of Period |
$ | - | ||
|
|
|
|||
| Supplemental disclosure of non-cash items: |
||||
| Shares issued for member data |
$ | 390 | ||
| Shares to be issued for member data |
$ | 150 | ||
The accompanying notes are an integral part of these financial statements.
-27-
Table of Contents
LunaDNA, LLC
NOTES TO THE FINANCIAL STATEMENTS
As of December 31, 2018
1. Organization and Purpose
LunaDNA, LLC (the “Company”), a Delaware limited liability company, was formed on April 23, 2018. The Company was formed to build, operate, maintain, query and otherwise deal with databases comprised of member data that is licensed to the Company (collectively, the “Database”). The Company is building a human health database that is owned by a community comprised of its members which is referred to as the Database. The Database is comprised of self-contributed genomic, phenotypic, medical, health and related data satisfying our requirements, which is referred to as Member Data. The Company commenced principal operations on December 5, 2018 but has not generated revenue as of December 31, 2018.
The Company has entered into a management services agreement (the “Management Agreement”) with LunaPBC, Inc. (the “Manager”), a Delaware public benefit corporation, to provide business, operational and financial management services to the Company. The rights, duties and powers of the Manager are governed by the Management Agreement and Company operating agreement (“Operating Agreement”). The Manager, acting alone, has the power and authority to act for and bind the Company. The Management Agreement may not be terminated by the Company and can be terminated by the Manager with thirty days’ written notice.
2. Going Concern
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company is a business that has not generated any revenues as of April 30, 2019.
The Company has no plans to raise capital to fund its operations, and it is therefore dependent on the Manager for all Organizational Expenses (defined in Note 10 – Expenses) and presently dependent on the Manager for all Operational Expenses (defined in Note 10 – Expenses). The Company is also dependent on the Manager for all costs associated with development, improvement and maintenance of the Database (“Development Expenses”). The Manager is not entitled to reimbursement for Organizational Expenses. The Manager is not entitled to reimbursement for Operational Expenses paid by the Manager that are or would have been, but for the provisions of the Management Agreement denying such reimbursement, accrued by the Company on or before March 31, 2020 (see Note 10 – Expenses). The Manager will be entitled to reimbursement for Operational Expenses accrued by the Company after March 31, 2020. The Manager is not entitled to reimbursement for Development Expenses. While the Manager intends to continue to fund the Company’s expenses for the next 12 months and beyond, the Manager has not committed to the Company to fund any particular amounts for any of these purposes and may not have the financial resources to do so. The Company’s dependence on the Manager for funding of these expense, losses incurred and lack of funds raise substantial doubt about the Company’s ability to continue as a going concern.
The Manager’s ability to fund all of the Organizational Expenses and to fund Operational Expenses and Development Expenses through the time when the Company generates significant revenues from operations is dependent on the Manager’s existing cash resources and the ability of the Manager to obtain additional capital financing from investors sufficient to meet the needs of the Company and the Manager’s other operations. The Manager has funded its operations and the Company’s operations primarily by sales of equity securities. The Manager believes that it will be able to raise capital that, combined with its existing cash resources, will be sufficient to fund all of the Organizational Expenses and to fund Operational Expenses and Development Expenses through the time when the Company generates significant revenues, at which time revenues earned by the Company may be used to pay both Operational Expenses and the management fee to the Manager (which management fee may then fund further Development Expenses). However, there can be no assurance that the Manager will be successful in its fundraising efforts. If the Manager is not successful in its intended fundraising efforts, the Company will be required to delay various planned expenditures for development of the Database, which delays could adversely delay generation of revenues and potentially jeopardize the Company’s ability to continue as a going concern.
3. Summary of Significant Accounting Policies
The following is a summary of significant accounting policies applied by the Company in preparation of its financial statements. The policies are in accordance with the accounting principles generally accepted in the United States of America (“GAAP”) and are presented in U.S. dollars.
Use of Estimates - The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets
-28-
Table of Contents
and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Income Taxes – The Company has elected to be taxed as a corporation and uses the liability method of accounting for income taxes as set forth in ASC 740, Income Taxes. Under the liability method, deferred taxes are determined based on the temporary differences between the financial statement and tax basis of assets and liabilities using tax rates expected to be in effect during the years in which the basis differences reverse. A valuation allowance is recorded when it is unlikely that the deferred tax assets will be realized. The Company assesses its income tax positions and records tax benefits for all years subject to examination based upon our evaluation of the facts, circumstances and information available at the reporting date. In accordance with ASC 740-10, for those tax positions where there is a greater than 50% likelihood that a tax benefit will be sustained, the Company’s policy is to record the largest amount of tax benefit that is more likely than not to be realized upon ultimate settlement with a taxing authority that has full knowledge of all relevant information. For those income tax positions where there is less than 50% likelihood that a tax benefit will be sustained, no tax benefit will be recognized in the financial statements. The Company has evaluated its income tax positions and has determined that it does not have any uncertain tax positions. The Company will recognize interest and penalties related to any uncertain tax positions through its income tax expense. The Company may in the future become subject to federal, state, and local income taxation though it has not been since its inception. The Company is not presently subject to any income tax audit in any taxing jurisdiction.
Recent Accounting Pronouncements – Management does not believe that any recently issued, but not yet effective, accounting standards could have a material effect on the accompanying balance sheet. As new accounting pronouncements are issued, the Company will adopt those that are applicable under the circumstances.
4. Member Data
The Company established the value for the different types of Member Data, which was used to determine the number of shares issued in exchange for the contribution of such Member Data, based on the fair value as determined by an accepted standard. In using an accepted standard, the Company noted the absence of bona fide sales of similarly limited and revocable Member Data rights made within a reasonable time. The Company used three independent, market-based valuation methods to determine the fair value of Member Data received in exchange for the Company’s shares. The valuation of Member Data involved first estimating the fair value of Genotyping by DNA Genome-Wide Microarray or by Sequencing data, which is referred to as a GT file. Other genomic contributions were then valued in relation to the fair value of a GT file.
Each valuation method applied was based on observable, third-party market transaction data using either the market approach valuation technique or the income approach valuation technique. The market approach is a valuation technique that uses prices and other relevant information generated by market transactions involving identical or comparable (that is, similar) assets, liabilities, or a group of assets and liabilities, such as a business. The market approach bases the value measurement on what other similar unrelated enterprises or comparable transactions indicate the value to be. Specifically, investments by unrelated parties in comparable equity securities (or assets) of the subject enterprise or transactions in comparable equity securities (or assets) of comparable enterprises are evaluated. Financial and nonfinancial metrics may be used in conjunction with the market approach to estimate the fair value of the privately issued securities of the subject enterprise. The income approach uses valuation techniques that convert future amounts (for example, cash flows or income and expenses) to a single current amount (that is, discounted). The fair value measurement is estimated on the basis of the value indicated by current market expectations about those future amounts. The income approach obtains its conceptual support from its basic assumption that value emanates from expectations of future income and cash flows.
Two of the Company’s valuation methods were based off of the market approach and yielded values of $3.75 and $3.33 per GT file. The Company’s third valuation method was based off of the income approach and yielded a value of $3.35 per GT file. The Company took the arithmetic mean of the three methods to arrive at an estimated fair value of $3.48 for a GT file. The Company determined to issue 50 shares for each GT file, resulting in an estimate of the fair value of each share at $0.069, which was rounded to $0.07 per share. The Company established 50 shares per GT file to yield a baseline value per share (that is, $0.07) that enables it to value more precisely other Member Data types as a fraction or multiple of the value of the GT File.
-29-
Table of Contents
5. Fair Value Measurements
The Company uses a three-tier fair value hierarchy to prioritize the inputs used in the Company’s fair value measurements. These tiers include: Level 1, defined as observable inputs such as quoted prices in active markets for identical assets; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions.
While the Company has valued the member data based on certain observable, third-party market transaction data (see Note 4), the Company has classified member data within Level 3 of the fair value hierarchy because each valuation method also utilizes significant unobservable inputs. For the two market approach methods, significant unobservable inputs include the average number of times a consumer would license his or her GT file, the percentage of information that a mid-price electronic health record (EHR) would contain when compared to the total information that the Company would be able to obtain from a highly engaged member, and the percentage of information a GT file of a member would represent compared to the total value of all contributions by that member. For the income approach method, significant unobservable inputs include the projected number of members, the number of studies/queries per member, the associated revenues for the first seven years after qualification, the estimated growth rate in earnings per member, the discount rate, and the long-term sustainable annual growth rate.
The following table provides a reconciliation of assets measured at fair value using significant unobservable inputs (Level 3) for the period ended December 31, 2018:
| Level 3 Assets |
||||
| Balance at April 23, 2018 |
$ | - | ||
| Additions to Member Data |
540 | |||
|
|
|
|||
| Balance at December 31, 2018 |
$ | 540 | ||
6. Members’ Capital
Pursuant to the Operating Agreement, the Company is authorized to issue an unlimited amount of unit-denominated common limited liability company interests, which it refers to as shares. Pursuant to its offering circular, filed with the Securities and Exchange Commission (the “SEC”) pursuant to Rule 252(g)(2) on December 3, 2018, the Company may issue up to 714,285,714 shares for an aggregate, maximum gross dollar offering of $50,000,000 in an offering (the “Offering”) qualified under Regulation A under the Securities Act of 1933, as amended (the “Securities Act”). The shares will not be offered for cash but in exchange for genomic, phenotypic, medical, health and related data satisfying our requirements, which the Company refers to as Member Data. The aggregate offering price or aggregate sales for our shares is based on the fair value of Member Data as established by an accepted standard. At December 31, 2018 the Company has issued 5,566 shares at a par value of $0.07 per share and 2,146 shares that have been earned but not yet issued.
The shares are non-transferable, except as may be required by law, and are not listed for trading on any exchange or automated quotation system. The members do not have any voting, consent or management rights relating to the management and operation of the Company, except to appoint a liquidator upon the dissolution of our Company if the Company does not have a Manager at that time.
A member may resign as a member for all purposes at any time by redeeming all of its shares. A member may, at any time and from time to time, elect to terminate the Company’s license to use the Member Data he or she contributed to the Company by written notice to the Company made in accordance with the then current Member Data policies, in which case the Shares issued in exchange for the Company’s original rights to such Member Data will be redeemed. A member will automatically cease to be a member for all purposes immediately upon such member ceasing to own of record any shares.
From April 23, 2018 to December 4, 2018 the Manager was the sole member of the Company. In accordance with our operating agreement, the Manager automatically resigned as, and ceased to be, a member upon another person being admitted as a member of the Company. Accordingly, the Company accounts for the expenses paid on its behalf by
-30-
Table of Contents
the Manager while the Manager is the sole member as expenses of the Company with a corresponding credit to Members’ Share Capital, in accordance with the guidance in SAB Topic 5T. On December 5, 2018, the Company commenced principal operations and admitted a person other than the Manager as a member. As a result, the Manager automatically resigned as a member. Once an investor became a member, the sole relationship between the Company and the Manager is the contractual services relationship that exists pursuant to the Operating Agreement and the Management Agreement and the manager provisions of the Delaware Limited Liability Company Act (Chapter 18 of Title 6 of the Delaware Code), and the Company does not believe that the guidance in SAB Topic 5T continues to apply on or after December 5, 2018. Accordingly, the Company has not recorded as expenses of the Company those expenses that the Manager incurred after the Manager was no longer a member and for which the Manager does not have a contractual right of reimbursement. As of December 4, 2018, the expenses paid on behalf of the Company by the Manager total $532,173.
Contributions
An individual may be admitted as a member and issued shares by contributing Member Data, as defined in the Operating Agreement, to the Company in accordance with the Company’s Member Data policies, subject to the Manager accepting such contribution. Members have no obligation to contribute funds to the capital of the Company or to make additional contributions. A member may, in such member’s discretion, make additional contributions of Member Data in return for additional shares from time to time in accordance with the Company’s Member Data policies, subject to the Manager accepting such contribution.
Distributions
The Manager intends for the Company to distribute (i) distributable cash from operations (but excluding proceeds from the sale of all or substantially all of the Company’s assets) for each annual period no less frequently than once per annum; provided that if the estimated distribution per share is not expected to exceed $0.02, as adjusted appropriately for any share dividend, share split, combination or other similar recapitalization with respect to the Company’s equity, the Manager may elect to not make a distribution in such annual period, and (ii) proceeds, if any, realized by or available to the Company (after deducting therefrom an amount for addition to a reserve for contingencies, working capital, and the payment of unreserved or unfunded Company obligations, such amounts to be established by the Manager in its discretion) from the sale of all or substantially all of the Company’s assets within thirty days following the receipt by the Company of such proceeds. Upon a member’s resignation or termination as a member or partial redemption of shares, the member will no longer be entitled to any further distributions with respect to the Shares redeemed, irrespective of whether distributable cash was available for distribution at or prior to such time.
7. Company Operating Agreement
The Manager was the sole initial member. The Manager automatically resigned as, and ceased to be, a member upon the admittance of another person as a member of the Company. In the event the Company would otherwise have no member, the Manager will automatically be admitted as a member, but will again automatically resign upon the subsequent admission of another person as member of the Company as provided in the immediately preceding sentence. In its capacity as initial member, the Manager had no obligation to make any contribution to, or pay any liabilities of, the Company, and had no right or entitlement to any distributions from the Company.
8. Related Party Transactions
The Company anticipates conducting various transactions with the Manager, a related party, including but not limited to those specified in the Management Agreement (see Note 10). The Company and the Manager are under common control. The Company will follow the accounting and disclosure requirements under ASC 850-10-50 with regards to any related party transactions and relationships.
9. Due to Members
At December 31, 2018, 2,146 shares have been earned but not yet issued. These shares have been recorded as a liability of $150 on the balance sheet.
-31-
Table of Contents
10. Management Agreement
Management Fee
The Company is obligated to pay to the Manager a management fee (the “Management Fee”) calculated as the sum of (i) 50% of the Net Revenues (defined below) of the Company; plus (ii) the amount of all Non-Profit Revenue (defined below) of the Company.
“Net Revenues” means (i) all revenue (excluding Non-Profit Revenue) recognized by the Company from (a) discovery activities that derive value from the content contained in the Database and (b) license fees, royalties and other revenue derived from intellectual property acquired through collaborations with third parties with respect to Member Data, less (ii) Operational Expenses. For this purpose, Operational Expenses do not include Organizational Expenses, Operational Expenses borne by the Manager (See Note 10 – Expenses) or the Management Fee. See also Note 10 –Manager Operations Separate from the Company, concerning activities that the Manager may conduct that do not generate revenue for the Company and are therefore excluded from Net Revenues.
“Non-Profit Revenue” means all revenues recognized by the Company from transactions with corporations, trusts, unincorporated association, or other types of organizations (“Non-Profit Organizations”) that (i) are exempt from federal income tax under section 501(c)(3) of the Internal Revenue Code of 1986, as amended, (ii) have applied in good faith for a determination of such exemption from the Internal Revenue Service, or (iii) would be eligible to be so exempt, in the reasonable opinion of the Manager, if operated in the United States. Examples of Non-Profit Organizations include disease foundations, research organizations, and organizations for the benefit of minority or economically disadvantaged groups. License fees, royalties and other revenues earned from Collaboration IP (as defined below) generated through collaboration with a Non-Profit Organization are not Non-Profit Revenue and will be included in Net Revenues, even if such license fees, royalties and other revenues are paid by Non-Profit Organizations.
The Manager will determine the Management Fee with respect to Net Revenues and Non-Profit Revenue for each fiscal quarter. The Management Fee is payable within ten (10) days following the closing of the Company’s books for each quarter, to the extent of available cash in the Company. To the extent sufficient cash is not available at a payment date, the unpaid portion of the Management Fee will accrue and be paid promptly following the Company’s receipt of cash sufficient to pay the amount in arrears.
Manager Operations Separate from the Company
The Company will earn revenues from data discovery activities that derive value from the content contained in the Database, such as (i) providing to third parties including but not limited to pharmaceutical and biotechnology discovery companies (collectively, “Customers”), access to the Database for population-level research, and (ii) making available to Customers, directly or through the Manager, contact information for Members who have elected to allow the sharing of such contact information. The Management Agreement indicates that certain activities are not data discovery activities and permits the Manager in its sole discretion to choose to conduct such activities for its own or for its affiliate’s account or for Customers without accounting to the Company for the revenues derived from such activities. Excluded activities are:
• Communication and member engagement services provided to Customers following a data discovery activity that identifies or provides contact information of members;
• assisting Customers in collecting longitudinal data of identified members that is at the time of initial collection outside the Database;
• the offer or sale of value added goods and services to members, whether by the Manager, an affiliate of the Manager or a third party offering goods or services that may be of interest to members;
• professional services such as scientific consultation or project management; and
• any and all other activities of the Manager that do not derive direct value from the content of the Database.
Database Development and Intellectual Property License
Pursuant to the Management Agreement, the Manager develops and improves the Database at the Manager’s sole expense. The Manager will own all intellectual property, including U.S. and foreign patents and trade secrets, conceived or discovered by the Manager, solely or jointly, related to the Database (collectively, “Database IP”). Database IP does not include intellectual property rights the Company acquires from collaborations with third parties that obtain access to the Database (“Collaboration IP”), which Collaboration IP will be owned by the Company.
-32-
Table of Contents
The Company enjoys a non-exclusive license for the Database IP, which license will continue in perpetuity in the event of termination of the Management Agreement, the Manager ceasing to serve as manager of the Company or the dissolution of the Manager.
Other Intellectual Property
The Manager owns certain trademarks, trade dress, logos, internet domain names, websites and associated software and social media accounts created for the Company. The Company has an exclusive license to all such properties but the Manager has reserved a non-exclusive right to use such properties in connection with its operations that are separate from those of the Company.
Expenses
The Manager is responsible for, and not entitled to reimbursement for, all fees, costs or expenses incurred by it on behalf of the Company in connection with organizing and managing the Company and in connection with the initial offer and sale of the Shares, including printing, travel, filing fees, marketing expenses, legal and accounting fees, and similar fees incurred in connection with the investigation, evaluation, registration, qualification, issuance and sale thereof, such as costs incurred in qualifying for the exemption from registration pursuant to Regulation A under the Securities Act with the SEC, including any post-qualification amendments or supplements to the initial Regulation A offering statement (collectively, the “Organizational Expenses”).
The Company is obligated to reimburse the Manager promptly for all Operational Expenses (defined below) advanced by the Manager. Notwithstanding the foregoing, the Manager is not entitled to reimbursement for Operational Expenses paid by the Manager if such Operational Expenses are or would have been, but for the provisions of the Management Agreement denying reimbursement, accrued by the Company under generally accepted accounting principles on or before March 31, 2020. “Operational Expenses” are all costs and expenses related to the Company’s operations, but excluding the Organizational Expenses, costs to develop Database IP and the Manager’s overhead and compensation related expenses, including compensation and expenses of the officers, directors, employees, auditors, attorneys and other agents of the Manager and fees and expenses for administrative, bookkeeping, clerical and related support services, office space and facilities, utilities, telephone and email of the Manager, all of which are the responsibility of the Manager.
11. Income Taxes
The Company accounts for income taxes under the asset and liability method. Deferred income taxes reflect the temporary differences between assets and liabilities recognized for financial reporting purposes and amounts recognized for income tax reporting purposes, net operating loss carryforwards, and other tax credits measured by applying currently enacted tax laws. Valuation allowances are provided when necessary to reduce deferred tax assets to an amount that is more likely than not to be realized.
The Company has evaluated the available evidence supporting the realization of its gross deferred tax assets including the amount and timing of future taxable income and has determined that it is more likely than not that the $32,000 deferred tax assets will not be fully realized and has established a valuation allowance against those assets as of December 31, 2018.
At December 31, 2018, the Company has federal and state (California) net operating loss carryforwards of approximately $1,000 each which are available to offset future taxable income. The federal carryforwards are carried forward indefinitely and the state carryforwards will expire by December 31, 2038. In addition, net operating loss carryforwards may be limited in the event of changes in ownership as defined by Internal Revenue Code Section 382.
The Financial Accounting Standards Board provides guidance regarding how uncertain tax positions that have been taken or are expected to be taken on a company’s tax return should be recognized, measured, presented, and disclosed in the consolidated financial statements. The Company believes that it has not taken any significant uncertain tax positions. Generally, the tax returns and amount of taxable income of the Company are subject to examination by federal and state taxing authorities during the three and four-year period, respectively, prior to the period covered by the consolidated financial statements.
-33-
Table of Contents
12. Subsequent Events
The Company has evaluated subsequent events through April 30, 2019, the date these financial statements were available to be issued.
As of April 30, 2019, the Company has issued an additional 13,502 shares, with a further 4,434 shares earned but not yet issued.
On April 29, 2019, the Company and the Manager entered into an amendment to the Management Agreement, which amendment was effective as of April 23, 2018. The amendment clarified and conformed the definition of “Database IP” (see Note 10—Database Development and Intellectual Property License). The amendment had no adverse effect on the rights or obligations of the Company under the Management Agreement.
In January 2019, the Manager entered into a Master Agreement (the “GA Agreement”), with Genetic Alliance (“GA”), a non-profit organization, in order to merge GA’s Platform for Engaging Everyone Responsibly (“PEER”) engagement platform with the Database, once the Database IP contains PEER equivalent functionality. The initial term of the GA Agreement ends on the fifth anniversary of the date on which both parties agree in writing that the Database IP substantially contains PEER equivalent functionality. The Company currently estimates that the term will start by the end of 2019.
Pursuant to the GA Agreement, the Manager granted a non-exclusive, non-transferable license to GA to use the Database IP that contains PEER equivalent functionality for so long as the GA Agreement is in effect. Additionally, GA granted the Manager a non-exclusive license to use the PEER application and PEER data during the term of the GA Agreement, subject to various restrictions.
The Agreement also calls for GA and our Manager to negotiate a value sharing arrangement with GA in connection with success generated through the efforts of GA. The Company currently contemplates that certain expenses of hosting the PEER data will be the responsibility of the Company after March 31, 2020 under the Management Agreement.
The GA Agreement may be terminated by either party for convenience upon 18 months’ advance written notice or sooner upon material, uncured default.
-34-
Table of Contents
| Item 8. | Exhibits |
The following exhibits are filed as part of this Annual Report:
* Filed herewith.
-35-
Table of Contents
SIGNATURES
Pursuant to the requirements of Regulation A, this issuer has duly caused this Annual report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Diego, State of California, on April 30, 2019.
| LunaDNA, LLC | ||
| By: | LunaPBC, Inc. | |
| Its Manager | ||
| By: | /s/ Robert Kain | |
| Robert Kain Chief Executive Officer | ||
Pursuant to the requirements of Regulation A, this Annual Report has been signed below by the following persons on behalf of the issuer in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Robert Kain |
Chief Executive Officer and Director of LunaPBC, Inc. (Principal Executive Officer) | April 30, 2019 | ||
| Robert Kain | ||||
| /s/ David Lewis |
Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) and Director of LunaPBC, Inc. | April 30, 2019 | ||
| David Lewis | ||||
| /s/ Dawn Barry |
President and Director of LunaPBC, Inc. | April 30, 2019 | ||
| Dawn Barry | ||||
-36-