UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 20-F
(Mark One)
|
o |
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR 12(g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
|
|
or | |
|
|
|
|
x |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
|
|
|
or | |
|
|
|
|
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to |
|
|
|
|
or | |
|
|
|
|
o |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report . . . . . . . . . . . . . . . . . . .
For the transition period from to
Commission file number: 001-38639
|
111, Inc. |
|
(Exact name of Registrant as specified in its charter) |
|
|
|
N/A |
|
(Translation of Registrant’s name into English) |
|
|
|
Cayman Islands |
|
(Jurisdiction of incorporation or organization) |
|
|
|
3-5/F, No. 295 ZuChongZhi Road, Pudong New Area Shanghai, 201203 The People’s Republic of China |
|
(Address of principal executive offices) |
|
|
|
Yang Chen, Chief Financial Officer Telephone: +86 21 2053-6666 Email: chenyang@111.com.cn 3-5/F, No.295 ZuChongZhi Road, Pudong New Area Shanghai, 201203 The People’s Republic of China |
|
(Name, Telephone, Email and/or Facsimile number and Address of Company Contact Person) |
Securities registered or to be registered pursuant to Section 12(b) of the Act:
|
Title of Each Class |
|
Name of Each Exchange on Which Registered |
|
American depositary shares (one American depositary share representing two Class A ordinary shares, par value US$0.00005 per share)
|
|
The Nasdaq Stock Market LLC (The Nasdaq Global Market) |
|
Class A ordinary shares, par value US$0.00005 per share* |
|
The Nasdaq Stock Market LLC (The Nasdaq Global Market) |
* Not for trading, but only in connection with the listing on The Nasdaq Global Market of American depositary shares.
Securities registered or to be registered pursuant to Section 12(g) of the Act:
|
Not Applicable |
|
(Title of Class) |
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
|
Not Applicable |
|
(Title of Class) |
Indicate the number of outstanding shares of each of the Issuer’s classes of capital or common stock as of the close of the period covered by the annual report.
As of December 31, 2018, there were 163,088,106 ordinary shares outstanding, par value US$0.00005 per share, being the sum of 91,088,106 Class A ordinary shares and 72,000,000 Class B ordinary shares.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
o Yes x No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
o Yes x No
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
x Yes o No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
o Yes o No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer o |
|
Accelerated filer o |
|
Non-accelerated filer x |
|
Emerging growth company x |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. x
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012. Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
|
U.S. GAAP x |
|
International Financial Reporting Standards as issued |
|
Other o |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
o Item 17 o Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
o Yes x No
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court.
o Yes o No
|
1 | ||
|
2 | ||
|
|
2 | |
|
2 | ||
|
2 | ||
|
2 | ||
|
41 | ||
|
69 | ||
|
69 | ||
|
85 | ||
|
93 | ||
|
94 | ||
|
95 | ||
|
96 | ||
|
106 | ||
|
107 | ||
|
|
108 | |
|
108 | ||
|
Material Modifications to the Rights of Security Holders and Use of Proceeds |
108 | |
|
109 | ||
|
110 | ||
|
110 | ||
|
110 | ||
|
111 | ||
|
Purchases of Equity Securities by the Issuer and Affiliated Purchasers |
111 | |
|
111 | ||
|
111 | ||
|
111 | ||
|
|
111 | |
|
111 | ||
|
111 | ||
|
111 | ||
|
115 | ||
Unless otherwise indicated or the context otherwise requires, all information in this annual report reflects the following:
· “1 Clinic” refers to our internet hospital;
· “1 Drug Mall” refers to our online wholesale pharmacy;
· “1 Drugstore” refers to our online retail pharmacy;
· “ADSs” refer to American depositary shares, each of which represents two Class A ordinary shares;
· “China” or the “PRC” refers to the People’s Republic of China, excluding, for the purposes of this annual report only, Hong Kong, Macau and Taiwan;
· “GMV” refers to the total value of all orders shipped for products sold under our direct sales model, net of returns, plus the total value of all orders shipped for products sold on our marketplace by our marketplace sellers, inclusive of returns, during the specified period;
· “marketplace sellers” refer to third-party merchants on our 1 Drugstore and 1 Drug Mall, which include distributors and resellers that sell products through our online retail pharmacy or online wholesale pharmacy under our marketplace model;
· “medical professionals” refer to doctors, pharmacists and medical assistants;
· “New Retail” refers to the seamless integration of our online retail pharmacy and offline pharmacy network by leveraging our smart supply chain and cloud-based solutions to improve the efficiency throughout the value chain;
· “pharmaceutical companies” refer to manufacturers of pharmaceutical and other health and wellness products;
· “pharmacies” refer to independent pharmacies, pharmacy chains and in-house pharmacies within clinics and private hospitals;
· “RMB” or “Renminbi” refers to the legal currency of China;
· “shares” or “ordinary shares” refers to our ordinary shares comprising Class A and Class B ordinary shares, par value US$0.00005 per share;
· “SKU” refers to stock keeping unit;
· “smart supply chain” refers to a supply chain built upon a technology infrastructure that is designed to analyze massive amounts of data to facilitate the customization, productivity and efficiency needed in the New Retail era. Our smart supply chain consists of multiple components, including our fulfillment infrastructure, cloud-based inventory management and our supply chain management;
· “suppliers” refer to distributors and pharmaceutical companies from whom we source our products for our direct sales model;
· “U.S. GAAP” refers to generally accepted accounting principles in the United States;
· “US$,” “U.S. dollars,” “$,” or “dollars” refers to the legal currency of the United States; and
· “we,” “us,” “our company,” “our,” or “111” refers to 111, Inc., its subsidiaries, and, in the context of describing our operations and consolidated financial information, our variable interest entities in China.
This annual report on Form 20-F contains forward-looking statements that involve risks and uncertainties. All statements other than statements of current or historical facts are forward-looking statements. These statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements.
You can identify these forward-looking statements by terminology such as “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “likely to” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements include, but are not limited to:
· our goals and strategies;
· our future business development, financial condition and results of operations;
· expected changes in our revenues, costs or expenditures;
· our expectations regarding demand for and market acceptance of our services;
· competition in our industry; and
· government policies and regulations relating to our industry.
We would like to caution you not to place undue reliance on these forward-looking statements and you should read these statements in conjunction with the risk factors disclosed in “Item 3D. Key Information—Risk Factors.” Those risks are not exhaustive. We operate in a rapidly evolving environment. New risks emerge from time to time and it is impossible for our management to predict all risk factors, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ from those contained in any forward-looking statement. We do not undertake any obligation to update or revise the forward-looking statements except as required under applicable law.
Item 1. Identity of Directors, Senior Management and Advisers
Not applicable.
Item 2. Offer Statistics and Expected Timetable
Not applicable.
A. Selected Financial Data
The following selected consolidated statements of comprehensive loss data for the years ended December 31, 2016, 2017 and 2018 and selected consolidated balance sheet data as of December 31, 2017 and 2018 have been derived from our audited consolidated financial statements, which are included in this annual report beginning on page F-1. The following selected consolidated balance sheet data as of December 31, 2016 have been derived from our audited consolidated financial statements, which are not included in this annual report.
Our consolidated financial statements are prepared and presented in accordance with U.S. GAAP. Our historical results do not necessarily indicate results expected for any future periods. You should read the selected consolidated financial data in conjunction with our consolidated financial statements and the related notes in conjunction with “Item 5. Operating and Financial Review and Prospects” included elsewhere in this annual report.
|
|
|
For the Year Ended December 31, |
| ||||||
|
|
|
2016 |
|
2017 |
|
2018 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Selected Consolidated Statements of Comprehensive Loss Data: |
|
|
|
|
|
|
|
|
|
|
Net revenues |
|
873,837 |
|
959,486 |
|
1,785,970 |
|
259,759 |
|
|
Operating costs and expenses: |
|
|
|
|
|
|
|
|
|
|
Cost of products sold |
|
(796,230 |
) |
(868,719 |
) |
(1,681,700 |
) |
(244,592 |
) |
|
Fulfillment expenses |
|
(68,445 |
) |
(55,880 |
) |
(73,930 |
) |
(10,753 |
) |
|
Selling and marketing expenses (1) |
|
(252,829 |
) |
(190,074 |
) |
(260,040 |
) |
(37,822 |
) |
|
General and administrative expenses (1) |
|
(60,836 |
) |
(53,434 |
) |
(98,759 |
) |
(14,364 |
) |
|
Technology expenses(1) |
|
(61,767 |
) |
(48,133 |
) |
(71,248 |
) |
(10,363 |
) |
|
Other operating income (expenses), net |
|
1,990 |
|
2,732 |
|
(668 |
) |
(97 |
) |
|
Total operating costs and expenses |
|
(1,238,117 |
) |
(1,213,508 |
) |
(2,186,345 |
) |
(317,991 |
) |
|
Loss from operations |
|
(364,280 |
) |
(254,022 |
) |
(400,375 |
) |
(58,232 |
) |
|
Loss before income taxes |
|
(363,446 |
) |
(249,327 |
) |
(382,033 |
) |
(55,565 |
) |
|
Income tax expense |
|
— |
|
— |
|
(8 |
) |
(1 |
) |
|
Net loss |
|
(363,446 |
) |
(249,327 |
) |
(382,041 |
) |
(55,566 |
) |
|
Net loss attributable to noncontrolling interest |
|
765 |
|
747 |
|
1,950 |
|
285 |
|
|
Deemed dividend to Series D convertible preferred shareholders |
|
(55,281 |
) |
— |
|
— |
|
— |
|
|
Net loss attributable to ordinary shareholders |
|
(417,962 |
) |
(248,580 |
) |
(380,091 |
) |
(55,281 |
) |
(1) Share-based compensation expenses are allocated in operating expense items as follows:
|
|
|
For the Year Ended December 31, |
| ||||||
|
|
|
2016 |
|
2017 |
|
2018 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
General and administrative expenses |
|
1,846 |
|
5,176 |
|
22,477 |
|
3,269 |
|
|
Selling and marketing expenses |
|
1,382 |
|
3,674 |
|
23,561 |
|
3,427 |
|
|
Technology expenses |
|
210 |
|
1,071 |
|
5,321 |
|
774 |
|
|
Total |
|
3,438 |
|
9,921 |
|
51,359 |
|
7,470 |
|
|
|
|
As of December 31, |
| ||||||
|
|
|
2016 |
|
2017 |
|
2018 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Selected Consolidated Balance Sheets Data: |
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
373,505 |
|
167,660 |
|
853,740 |
|
124,171 |
|
|
Short-term investments |
|
266,823 |
|
293,533 |
|
252,805 |
|
36,769 |
|
|
Accounts receivable, net of allowance of doubtful accounts of nil at December 31, 2016, 2017 and 2018 |
|
28,388 |
|
20,398 |
|
28,569 |
|
4,155 |
|
|
Inventories |
|
134,734 |
|
144,056 |
|
210,836 |
|
30,665 |
|
|
Prepayments and other current assets |
|
97,359 |
|
104,818 |
|
161,147 |
|
23,438 |
|
|
Total assets |
|
941,605 |
|
763,384 |
|
1,546,418 |
|
224,917 |
|
|
Accounts payable |
|
97,983 |
|
128,140 |
|
212,258 |
|
30,872 |
|
|
Accrued expenses and other current liabilities |
|
74,170 |
|
73,018 |
|
102,261 |
|
14,873 |
|
|
Total liabilities |
|
172,153 |
|
201,158 |
|
322,654 |
|
46,928 |
|
|
Total mezzanine equity |
|
1,457,455 |
|
1,506,930 |
|
— |
|
— |
|
|
Total (deficit) equity |
|
(688,003 |
) |
(944,704 |
) |
1,223,764 |
|
177,989 |
|
B. Capitalization and Indebtedness
Not applicable.
C. Reasons for the Offer and Use of Proceeds
Not applicable.
D. Risk Factors
Risks Related to Our Business and Industry
We are subject to extensive and evolving regulatory requirements, non-compliance with which, or changes in which, may materially and adversely affect our business and prospects.
Due to the complex nature of our business, we are subject to legal and regulatory requirements of multiple industries in the PRC. These industries primarily include internet, healthcare, internet healthcare and pharmaceutical retail and wholesale industries.
Various regulatory authorities of the PRC government are empowered to promulgate and implement regulations governing broad aspects of the internet and healthcare industries. In respect of the healthcare industry, in particular, any violation of the relevant laws, rules and regulations may result in harsh penalties and, under certain circumstances, lead to criminal prosecution.
Meanwhile, the regulations of both the internet industry and its internet healthcare sector are relatively new and evolving, and their interpretation and enforcement involve significant uncertainty. As a result, under certain circumstances, it may be difficult to determine what actions or omissions would be deemed in violation of applicable laws and regulations. These uncertainties entail risks that may materially and adversely affect our business prospects. Due to the uncertainty and complexity of the regulatory environment, we cannot assure you that future laws and regulations would not render our operations non-compliant or that we would always be in full compliance with applicable laws and regulations. Compliance with future laws and regulations may require us to change our business models and practices at an undeterminable and possibly significant financial cost. These additional monetary expenditures may increase future overhead, which may, in turn, have a material adverse effect on our business, financial condition and results of operations.
Furthermore, the introduction of new services and products may require us to comply with additional, yet undetermined, laws and regulations. Compliance may require obtaining appropriate permits, licenses or certificates as well as expending additional resources to monitor developments in the relevant regulatory environment. The failure to adequately comply with these future laws and regulations may delay, or possibly prevent, some of our products or services from being offered to users, which may have a material adverse effect on our business, financial condition and results of operations.
The pharmaceutical retail and wholesale industry in China is subject to extensive government regulation and supervision as well as monitoring by various government authorities. Certain other laws, rules and regulations may affect the pricing, demand and distribution of pharmaceutical products, such as those relating to procurement, prescription and dispensing of drugs by hospitals and other medical institutions, retail pharmacy, government funding for private healthcare and medical services, and the inclusion of products in the drugs catalogs for national basic medical insurance, on-the-job injury insurance and maternity insurance promulgated by the Ministry of Human Resources and Social Security of the People’s Republic of China, or the MOHRSS. In addition, the pharmaceutical manufacturing, pharmaceutical distribution, pharmaceutical retail, healthcare services and medical device industries in China are each subject to extensive and changing government regulations and supervision. Any unfavorable regulatory changes in these industries may also increase our compliance burden and materially and adversely affect our business, profitability and prospects.
Our business, financial condition and results of operations may be materially and adversely affected if we are unable to compete effectively in the PRC general health and wellness market, and we may fail to sufficiently and promptly respond to rapid changes in government regulations, treatment of diseases and customer preferences.
The PRC general health and wellness market is highly competitive. Our key competitors include pharmaceutical retail companies including traditional offline pharmacies, and online platforms, as well as B2B platforms and traditional pharmaceutical distributors, and companies that offer internet healthcare services. These companies may have substantially greater financial, technical, research and development, marketing, distribution, retail and other resources than we do. They may also have longer operating histories, a larger customer base or broader and deeper market coverage. Furthermore, when we expand into other markets, we will face competition from new competitors, domestic or foreign, who may also enter markets where we currently operate.
In addition, many operators in the healthcare industry have consolidated in recent years to create larger healthcare enterprises with greater bargaining power, which has resulted in greater pricing pressures. If this consolidation trend continues, it could give the resulting enterprises even greater bargaining power, which may lead to further competitive pressure. New partnerships and strategic alliances in the healthcare industry also can alter market dynamics and adversely impact our businesses and competitive positioning.
The technologies that we and our competitors employ are evolving rapidly, and new developments frequently result in price competition, product obsolescence and altered market landscape. Any significant increase in competition may have a material adverse effect on our revenue and profitability as well as on our business and prospects. We cannot assure you that we will be able to continually distinguish our products and services from our competitors’, preserve and improve our relationships with various participants in the healthcare value chain, or increase or even maintain our existing market share. We may lose market share, and our financial condition and results of operations may deteriorate significantly if we fail to compete effectively.
We may not be able to manage the growth of our business and our expansion plans and operations or implement our business strategies on schedule or within our budget, or at all.
Our business has become increasingly complex in terms of both the type and scale of our operations. Any expansion may increase the complexity of our operations and place a significant strain on our managerial, technological, operational, financial and human resources. We recently launched our online wholesale business and various value-added services, including online consultation services, cloud prescription services and data services. Our current and planned personnel, systems, procedures and controls may not be adequate to support our future operations. We cannot assure you that we will be able to effectively manage our growth or to implement all these systems, procedures and control measures successfully. If we are not able to manage our growth effectively, our business and prospects may be materially and adversely affected.
We are also continually executing a number of growth initiatives, strategies and operating plans designed to enhance our business, including launching various new services utilizing the latest big data and AI technologies. The anticipated benefits from these efforts are based on assumptions that may prove to be inaccurate. Moreover, we may not be able to successfully complete these growth initiatives, strategies and operating plans and realize all of the benefits that we expect to achieve or it may be more costly to do so than we anticipate. If, for any reason, the benefits we realize are less than our estimates or the implementation of these growth initiatives, strategies and operating plans adversely affect our operations or cost more or take longer to effectuate than we expect, or if our assumptions prove inaccurate, our business, financial condition and results of operations may be materially and adversely affected.
In addition, we may seek and pursue opportunities via joint ventures or strategic partnerships for expansion from time to time, and we may face similar risks and uncertainties as listed above. Failure to properly address these risks and uncertainties may materially and adversely affect our ability to carry out acquisitions and other expansion plans, integrate and consolidate newly acquired or newly formed businesses, and realize all or any of the anticipated benefits of such expansion, which may have a material adverse effect on our business, financial condition, results of operations and prospects.
We have incurred operating losses in the past, and may not be able to achieve or maintain profitability in the future.
We began our operations in 2012 and we experienced net loss in the amount of RMB363.4 million, RMB249.3 million and RMB382.0 million (US$55.6 million) in 2016, 2017 and 2018, respectively. We expect our operating costs and expenses to increase in the future in absolute terms as we expand our operations. We may also incur additional legal, accounting, and other expenses as a public company. If our revenue does not grow at a greater rate than our expenses, we will not be able to achieve and maintain profitability. We may incur significant losses in the future for various reasons, many of which may be beyond our control. Additionally, we may encounter unforeseen expenses, operating delays, or other unknown factors that may result in losses in the future. If our cost of sales and expenses continuously exceed our revenue, our business may be materially and adversely affected and we may not be able to achieve or maintain profitability.
Our pharmaceutical retail and wholesale businesses are subject to a variety of risks, which may have a material and adverse effect on our business, financial condition and results of operations.
We are subject to certain risks in our pharmaceutical retail and wholesale businesses, including:
· inability to successfully execute effective advertising, marketing and promotional programs necessary to maintain and increase awareness of our brands, products and services;
· failure to implement effective pricing and other strategies in response to market competition;
· inability to respond to changes in demand and preferences of pharmacy customers and consumers in a timely manner;
· inability to stock an adequate supply of pharmaceutical and other health and wellness products that meet the demand of our pharmacy customers and consumers;
· overall consumer spending on healthcare in China;
· inability to obtain and maintain regulatory or governmental permits, approvals and clearances, or to pass PRC government inspections or audits; and
· the risk of, and resulting liability from, any contamination, injury or other harm caused by any use, misuse or misdiagnosis involving products distributed by us.
The occurrence of any such risks in our pharmaceutical retail and wholesale businesses may damage our overall business and reputation, and may have a material and adverse effect on our financial condition and results of operations.
Our business generates and processes a large amount of data, and the improper use or disclosure of such data could harm our reputation and have a material adverse effect on our business and prospects.
We generate and process a large amount of personal, transaction, demographic and behavioral data including medical records and other personal information. We face risks inherent in handling large volumes of data and in securing and protecting such data. In particular, we face a number of data-related challenges related to our business operations, including:
· protecting the data in and hosted on our system, including against attacks on our system by external parties or fraudulent behavior by our employees;
· addressing concerns related to privacy and sharing, safety, security and other factors; and
· complying with applicable laws, rules and regulations relating to the collection, use, disclosure or security of personal information, including any requests from regulatory and government authorities relating to such data.
Regulatory requirements regarding the protection of such data are constantly evolving and can be subject to significant change, making the extent of our responsibility in that regard uncertain. Under certain regulations, rules and measures promulgated by the Ministry of Industry and Information Technology of the People’s Republic of China, or the MIIT, since 2011, any collection and use of a user’s personal information by an internet services provider must be subject to the consent of the user, abide by the principles of legality, rationality and necessity, and be within the specified purposes, methods and scopes. The internet services provider must keep all information collected strictly confidential and is prohibited from divulging, tampering with or destroying any such information, or selling or providing such information to other parties. In particular, the Cyber Security Law of the People’s Republic of China, or the Cyber Security Law, which took effect on June 1, 2017, is formulated to maintain network security, safeguard the cyberspace sovereignty, national security and public interests, protect the lawful rights and interests of citizens, legal persons and other organizations, and further enhance personal information protection, such as through requirements on the collection, use, processing, storage and disclosure of personal information. Furthermore, in August 2018, the SCNPC promulgated the E-Commerce Law of the People’s Republic of China, or the E-Commerce Law, to regulate the e-commerce activities conducted within the territory of the PRC, which further strengthens the protection of consumers’ personal data and privacy. Since the Cyber Security Law, E-Commerce Law and relevant regulations, rules and measures are relatively new, there are uncertainties as to the interpretation and application of these laws and regulations, and it is possible that our data protection practices are or will be inconsistent with regulatory requirements. Any violation of the provisions and requirements under the Cyber Security Law, E-Commerce Law and other relevant regulations, rules and measures may subject us to warnings, fines, confiscation of illegal gains, revocation of licenses, suspension of business, shutting down of websites or even criminal liabilities. Complying with such requirements could cause us to incur substantial expenses or to alter or change our practice in a manner that could harm our business. Any systems failure or security breach or lapse that results in the unauthorized release of our user data could harm our reputation and brand and, consequently, our business, in addition to exposing us to potential legal liability.
Our privacy policies and practices concerning the collection, use and disclosure of user data are posted on our mobile app. Any failure, or perceived failure, by us to comply with our privacy policies or with any applicable regulatory requirements or privacy protection-related laws, rules and regulations could result in proceedings or actions against us by governmental entities or others. These proceedings or actions may subject us to significant penalties and negative publicity, require us to change our business model or practices, increase our costs and severely disrupt our business, which may materially and adversely affect our business, financial condition, results of operations and prospects.
Our failure to properly manage various participants in our ecosystem may materially and adversely affect our business.
We rely on various participants, including, but not limited to, pharmacies, pharmaceutical companies, marketplace sellers and medical professionals, in the provision of services and products in our ecosystem, and the success of our business depends on our ability to properly manage them.
We consider a variety of factors before entering into contractual arrangements with them. Nevertheless, we have limited control over the quality of work and performance of our ecosystem participants in their provision of services and products over our website and mobile platform or otherwise, and they may breach such contractual arrangements and subject us to claims and liabilities that may affect our business operations.
We have also implemented quality control standards and procedures to manage their work and performance in our ecosystem. However, there can be no assurance that our monitoring of their work and performance will be sufficient to ensure the quality of their work. In the event that a third party fails to meet our quality and operating standards contracted in our agreements or as required by relevant PRC laws and regulations, our operations may suffer and our business, financial condition and results of operations may be materially and adversely affected. Furthermore, because of the contractual relationships, we could be perceived as responsible for the actions of such participants and, as a result, suffer reputational damage. This may adversely affect our ability to attract new pharmacies, pharmaceutical companies, medical professionals and marketplace sellers, and to engage them as providers within our ecosystem.
Any lack of requisite approvals, licenses or permits applicable to our business, or any non-compliance with relevant laws and regulations, may have a material and adverse effect on our business, financial condition, results of operations and prospects.
Our business is subject to governmental supervision and regulation by various PRC governmental authorities including, but not limited to, the Ministry of Commerce of the People’s Republic of China, or MOFCOM, the MIIT, the National Health and Family Planning Commission of the People’s Republic of China, or the NHFPC, which was restructured and integrated into the National Health Commission of the People’s Republic of China, or the NHC, China Food and Drug Administration, or the CFDA, the State Administration for Industry and Commerce, or the SAIC, which was, together with the CFDA, integrated into the State Administration for Market Regulation, or the SAMR, the Cyberspace Administration of China, or the CAC, and the corresponding local regulatory authorities. Such government authorities promulgate and enforce laws and regulations that cover a variety of business activities that our operations concern, such as provision of internet information, online medical services, online and offline retail, sales and online operation of pharmaceutical products and medical devices, sales of food, and internet advertisement, among other things. These regulations in general regulate the entry into, the permitted scope of, as well as approvals, licenses and permits for, the relevant business activities.
In addition to obtaining necessary approvals, licenses and permits for conducting our business, we must comply with relevant laws and regulations. Our businesses, such as online and offline pharmaceutical retail and wholesale distribution and online healthcare services, are subject to various and complex laws and regulations, extensive government regulations and supervision. We may not be fully informed of all and new requirements under relevant laws and regulations in a timely manner, and even if we become aware of new requirements, due to uncertainties in their interpretations and implementation, it will be difficult for us to determine what actions or omissions would be deemed as violations of applicable laws and regulations. We may also not be able to respond to evolving laws and regulations and take appropriate action in time to adjust our business model. As a result, we may be in violation or non-compliance with such laws and regulations.
In particular, under the Administrative Measures for the Supervision and Administration of Circulation of Pharmaceuticals promulgated by the CFDA in 2007, a company is prohibited from selling prescription drugs to consumers without prescription, via internet or by post. A company in violation of such prohibitions will be instructed to rectify, given a disciplinary warning, and/or imposed an administrative penalty of no more than RMB30,000 per violation. See “Item 4. Information on the Company—B. Business Overview— Regulations— Regulations Relating to Online Operation of Drugs and Medical Devices.” In the past, we had received disciplinary warnings and administrative penalties due to certain non-compliance incidents in relation to prescription drugs sales. We have adjusted our sales model and relevant functions of our online platforms in response to such warnings and penalties. However, it remains uncertain that our sales model and online platforms as adjusted are and will be in full compliance with the relevant laws and regulations, which are evolving and subject to changes. In addition, due to the complexity of our IT system, its potential errors, or human errors, mistakes or misconduct by our offline retail pharmacies, we cannot assure you that we can fully comply with and meet the requirements under all laws and regulations related to the sale of prescription drugs. Any failure to comply with such laws and regulations could materially and adversely affects our business, results of operations, financial condition and prospects.
Due to the uncertainties in the regulatory environment of the industries in which we operate, there can be no assurance that we have obtained or applied for all the approvals, permits and licenses required for conducting our business and all activities in the PRC, or that we would be able to maintain our existing approvals, permits and licenses or obtain any new approvals, permits and licenses if required by any future laws or regulations. If we fail to obtain and maintain approvals, licenses or permits required for our business, or to comply with relevant laws and regulations, we could be subject to liabilities, fines, penalties and operational disruptions, or we could be required to modify our business model, which could materially and adversely affect our business, financial condition and results of operations.
We may become subject to product liability and medical liability claims, which could cause us to incur significant expenses and be liable for significant damages if not covered by insurance.
We are exposed to risks inherent in marketing, distributing and selling pharmaceutical and other health and wellness products and providing online healthcare and medical services in China. Claims, customer complaints or administrative penalties may arise if any of our products are deemed or proven to be unsafe, ineffective or defective, or they are found to contain illicit substances. We may also be subject to allegations of having engaged in practices such as improper filling of prescriptions, distribution of counterfeit and substandard medicines, or providing inadequate warnings or insufficient or misleading disclosures of side effects.
In addition, in the event that any use or misuse of the products we distribute results in personal injury, suicide or death, product liability claims may be brought against us for damages. If we are unable to defend ourselves against such claims, among other things, we may be subject to civil liabilities for physical injury, death or other losses caused by our products, to criminal liabilities, and to the revocation of our business licenses. In addition, we may be required to suspend sales or cease sales of the relevant products.
We face risks of medical liability claims against our in-house medical team, external doctors and us. We only carry insurance covering medical malpractice claims for our in-house doctors and some of our external doctors. Adequate professional malpractice insurance coverage may not be available to our in-house medical team, external doctors or us in the future on commercially acceptable terms, or at all.
Any claims made against us that are not fully covered by insurance could be costly to defend against, result in substantial damage awards against us and divert the attention of our management and our in-house medical team and external doctors from our operations, which could have a material adverse effect on our business, financial condition, results of operations and reputation.
Failure to maintain optimal inventory levels and assortment of products could increase our operating costs or lead to unfulfilled customer orders, either of which could have a material and adverse effect on our business, financial condition, results of operations and prospects.
We need to maintain optimal inventory levels in order to operate our pharmaceutical retail and wholesale businesses successfully and to meet the demands of pharmacy customers and consumers. We manage inventories of pharmaceutical and other health and wellness products under our direct sales model, while marketplace sellers manage inventories of their products. We are exposed to inventory risk as a result of rapid changes in product life cycles, changing consumer preferences, uncertainty of product developments and launches, manufacturer back orders and other vendor-related problems, as well as the volatile economic environment in China. We cannot assure you that we will accurately predict these trends and events and avoid over-stocking or under-stocking of products. Furthermore, demand for products could change significantly between the time when the products are ordered and the time when they are ready for delivery. When we begin to sell a new product, it is particularly difficult to forecast product demand accurately.
As our pharmaceutical retail and wholesale businesses carry a wide range of products and maintain significant inventory levels for a substantial portion of our merchandise, we may be unable to sell such inventory in sufficient quantities or during the relevant sales seasons. While we did not have any net write-downs of our inventories to their net realizable value in 2016, 2017 and 2018, inventory levels in excess of customer demand may result in inventory write-downs, expiration of products or an increase in inventory holding costs and a potential negative effect on our liquidity.
Conversely, if we underestimate customer demand, or if our suppliers fail to provide products to us in a timely manner, we may experience inventory shortages, which may, in turn, result in unfulfilled customer orders, leading to a negative impact on our customer relationships. We cannot assure you that we will be able to maintain proper inventory levels for our pharmaceutical retail and wholesale operations, and any such failure may have a material and adverse effect on our business, financial condition, results of operations and prospects.
We closely monitor the inventory levels of other products of which our marketplace sellers manage inventories. However, there can be no assurance that our monitoring and related measures will be effective in ensuring fulfillment of our customers’ orders at our online retail pharmacy and online wholesale pharmacy. Our failure to maintain proper inventory levels for our retail and wholesale businesses may have a material and adverse effect on our business, financial condition, results of operations and prospects.
Third-party logistics and delivery companies are used to fulfill and deliver orders placed on our platform. If these logistics and delivery companies fail to provide reliable delivery services, our business and prospects, as well as our financial condition and results of operations, may be materially and adversely affected.
We leverage our large-scale operations and reputation to enter into contractual arrangements with a number of third-party delivery companies to deliver our products to our pharmacy customers and consumers. We may also use third-party service providers to ship products from our fulfillment centers to delivery stations or to deliver bulky item products. Interruptions to or failures in these third parties’ delivery services could prevent the timely or proper delivery of our products to pharmacy customers and consumers. These interruptions may be due to events that are beyond our control or the control of these delivery companies, such as inclement weather, natural disasters, transportation disruptions or labor unrest. We may not be able to find alternative delivery companies to provide delivery services in a timely and reliable manner, or at all. If products are not delivered in proper condition or on a timely basis, our business and reputation could suffer.
Our self-developed technologies are complex and may contain undetected errors or may not operate properly, which could adversely affect our business, financial condition and results of operations.
Our self-developed technology platform provides our consumers and other participants in our ecosystem with the ability to conduct a variety of actions essential to the operations of our business and the delivery of our solution. Technology development is time-consuming, expensive and complex, and may involve unforeseen difficulties. We may encounter technical obstacles, and it is possible that we may discover additional problems that prevent our technologies from operating properly and consequently adversely affect our information infrastructure and other aspects of our business where our technologies are applied. If our solution does not function reliably in terms of performance, we may lose existing, or fail to attract new participants to our platform, which may damage our reputation and adversely affect our business.
Moreover, data services, supply chain management systems, and other proprietary technologies we provide to pharmacies, pharmaceutical companies and other customers are complex and those we offer may develop or contain undetected defects or errors. Material performance problems, defects or errors in our existing or new software and applications and services may arise in the future and may result from interface issues between our systems and data that we did not develop and the function of which is beyond our control or undetected in our testing. These defects and errors, and any failure by us to identify and address them, could result in loss of revenue or market share, diversion of development resources, harm to our reputation and increased service and maintenance costs. Defects or errors may discourage existing or potential customers from utilizing our solution. Correction of defects or errors could prove to be impossible or impracticable. The costs incurred in correcting any defects or errors may be substantial and could have a material adverse effect on our business, financial condition and results of operations. Defects or errors may also affect our pharmacies, pharmaceutical companies or other customers who rely on our self-developed technologies in the operation of their businesses, which may have a material adverse effect on our reputation, business, results of operations and prospects.
We may be subject to penalties or disputes against us for failure to manage the multi-institution practices of our in-house medical team and external doctors.
The practice of doctors is strictly regulated under PRC laws, rules and regulations. Doctors who practice at medical institutions must hold practicing licenses and may only practice within the scope of their licenses and at the specific medical institutions as stated in their licenses. A doctor is required to register the medical institutions at which he or she practices in his or her license. If a doctor is found practicing at a medical institution not registered in his or her license, the doctor would be subject to regulatory penalties, from warning to suspension of practice and, in the worst-case scenario, revocation of licenses. A doctor practicing in multiple institutions must apply to register or file with competent in-charge administrative authorities and can only have the right to prescribe medicine at the registered or filed practicing institution. If the doctor issues a prescription in a medical institution not registered in his or her license, the relevant medical institution would also be subject to regulatory penalties, including a fine of up to RMB5,000 and, in the worst-case scenario, revocation of the medical institution’s Practicing License for Medical Institutions.
We cannot assure you that our in-house and external doctors will complete the registration and relevant government procedures in a timely manner, or at all, or that our in-house and external doctors will not practice outside the permitted scope of their respective licenses. Our failure to properly manage the registration of our in-house doctors may subject us to administrative penalties against our medical institution, including fines, or, in the worst-case scenario, revocation of our Practicing License for Medical Institutions, any of which could materially and adversely affect our business. Meanwhile, if our in-house and external doctors are found to have deficient registration or found to be practicing beyond the scope permitted by relevant authorities, they may be disciplined and lose their practicing licenses. In the event that the multi-institution practices of our in-house and external doctors are in breach of their contractual obligations owed to other institutions, such as non-compete obligations, we may be exposed to indemnity or other legal liabilities if we are deemed to have aided in these breaches, and are therefore susceptible to legal disputes and potential damages. As a result, we may no longer be able to employ them in offering our online consultation services, which could materially and adversely affect our business. In addition, there can be no assurance that we could timely find qualified replacements on commercially reasonable terms, or at all.
We may not be able to prevent others from unauthorized use of our intellectual property, which could harm our business and competitive position.
We regard our trademarks, copyrights, patents, domain names, know-how, proprietary technologies, and similar intellectual property as critical to our success, and we rely on a combination of intellectual property laws and contractual arrangements, including confidentiality agreements with our employees and third parties, to protect our proprietary rights. Despite these measures, it is often difficult to enforce intellectual property rights in China. Even where adequate laws exist, certain procedural issues create effective obstacles to the proper enforcement of intellectual property rights. In addition, the available remedies in both court proceeding and through administrative enforcement are often inadequate to address infringement or to provide intellectual property rights holders with full compensation for the losses caused. Accordingly, we may not be able to effectively protect our intellectual property rights or to enforce confidentiality undertakings in China. In addition any of our intellectual property rights could be challenged, invalidated, circumvented or misappropriated, or such intellectual property may not be sufficient to provide us with competitive advantages. In addition, although we are not aware of any copycat websites or mobile apps that attempt to cause confusion or traffic diversion from us at the moment, we may become an attractive target to such attacks in the future because of our brand recognition.
In addition, there can be no assurance that our patent applications would be approved, that any issued patents would adequately protect our intellectual property, or that such patents would not be challenged by third parties or found by a judicial authority to be invalid or unenforceable.
We may be subject to allegations, lawsuits and administrative penalties relating to the sale, distribution, marketing and advertising of counterfeit or substandard products in our pharmaceutical retail and wholesale businesses, which may damage our brand and reputation and have a material adverse effect on our business, financial condition, results of operations and business prospects.
Certain products distributed or sold in the pharmaceutical retail and wholesale markets in China may be manufactured without proper licenses or approvals and/or fraudulently mislabeled with respect to their content and/or manufacturer. These products are generally referred to as counterfeit or substandard pharmaceutical products. The current counterfeit and substandard product regulation control and enforcement system in China is not sufficiently mature to completely eliminate the manufacturing and sales of counterfeit pharmaceutical products. Counterfeit and substandard pharmaceutical products are generally sold at lower prices than authentic pharmaceutical products, and, in some cases, are very similar in appearance to the authentic pharmaceutical products. Therefore, the presence of counterfeit products of pharmaceuticals distributed or sold by us can quickly erode our sales volumes and revenue for the relevant products.
Furthermore, counterfeit or substandard products may or may not have the same chemical composition as the authentic counterparts, which may make them less effective than the authentic ones, entirely ineffective, or more likely to cause severe adverse side effects. We may not be able to identify those counterfeit or substandard products we source from our suppliers. Any unintentional and unknowing sales of counterfeit or substandard products in our pharmaceutical distribution or retail businesses, or sales of counterfeit and substandard products by third parties illegally using our brand names, may subject us to negative publicity, fines and other administrative penalties, or even result in litigation relating to the sale, marketing and advertising of those products. Moreover, the continuing presence of counterfeit and substandard products may reinforce the negative image of distributors and retail pharmacies among consumers in general, and may severely harm the reputation and brand names of pharmaceutical companies, including ourselves. Similarly, consumers may buy counterfeit and substandard products that are in direct competition with products distributed or sold in our pharmaceutical retail and wholesale businesses, which may materially and adversely affect the sales volumes of the relevant products in our portfolio and further impact our business, financial condition, results of operations and prospects.
If we fail to provide satisfactory customer experience and continue to increase our retail and wholesale customer base, our business may be materially and adversely affected.
Our business is highly dependent on the receptiveness of our pharmacy customers and consumers to our services and products as well as their willingness to use, and to increase the frequency and extent of their utilization of, our solution. Their degree of receptiveness to our services and products depends on a number of factors, including the demonstrated accuracy and efficacy of our offerings compared to those of others, turnaround time, cost-effectiveness, convenience and marketing support. In addition, negative publicity concerning our solution or the internet healthcare market as a whole could limit market acceptance of our solution, especially that of the online consultation services. Meanwhile, there can be no assurance that our efforts and ability to demonstrate the value of our solution and the relative benefits of our services and products over those of our competitors to our pharmacy customers and consumers would be successful. If we fail to achieve an adequate level of acceptance by our pharmacy customers and consumers of our services and products, or if our solution does not drive their engagement, then our business may not develop as expected, or at all, and our business, financial condition or results of operations may be materially and adversely affected.
The success of our business also hinges on our ability to provide satisfactory customer experience, which depends on our ability to continue to deliver quality care to our users, to maintain the quality of our services and products, to source services and products that are responsive to customer demands, and to provide timely and reliable delivery, flexible payment options and satisfactory after-sales services. Such ability, in turn, depends on a variety of factors beyond our control. In particular, we rely on a number of third parties in the provision of our services and products. Their failure to provide a high-quality customer experience to our pharmacy customers and consumers may adversely affect our pharmacy customers’ and consumers’ receptiveness of, and willingness to utilize our solution, which may damage our reputation and cause us to lose pharmacy customers and consumers.
In addition, we operate a customer service center to provide assistance to our pharmacy customers and consumers. If our customer service representatives fail to provide satisfactory service, or if waiting times are too long due to high volume of inquiries from customers at peak times, our brand and customer loyalty may be adversely affected. Moreover, any negative publicity or poor feedback on our customer service may harm our brand and reputation and, in turn, cause us to lose pharmacy customers and consumers and market share.
The failure of in-house medical team and external doctors to provide adequate and proper service to consumers may have a material and adverse effect on our business, financial condition and results of operations.
Our in-house medical team, external doctors and other employees, may provide sub-standard services, mishandle sensitive information, engage in other misconduct or commit medical malpractice, which could subject us to medical liability claims. Our business, financial condition, results of operations and reputation may be materially and adversely affected if any such claims are made against us in connection with these actions that are not fully covered by insurance. See “—We may become subject to product liability and medical liability claims, which could cause us to incur significant expenses and be liable for significant damages if not covered by insurance.” With respect to external doctors, as they often work remotely, we have limited control over them as well as the quality of their online medical consultation services. There can be no assurance that our risk management procedures will be sufficient to monitor their performance and control the quality of their work. In the event that the external doctors fail to comply with the contractual obligations and applicable laws in relation to the provision of our online consultation services, our user experience could deteriorate, and we may suffer as a result of any actual or alleged misconduct by them, which could materially and adversely affect our business, financial condition, results of operations and reputation.
The failure of our marketplace sellers to control the quality of products they sell on our platform, or to make timely and accurate delivery of their products sold on our platform, may have a material and adverse effect on our business, financial condition and results of operations.
Under the direct sales model, we manage inventories in an integrated process. Under our marketplace model, many of our marketplace sellers use their own facilities to store their products and utilize their own or third-party delivery systems to deliver their products to our pharmacy customers and consumers, which makes it difficult for us to ensure that our pharmacy customers and consumers get consistent quality products and services for all products sold through our online platforms. If any marketplace seller fails to control the quality of the products that it sells on our platforms, or if it does not deliver the products or delivers them late or delivers products that are materially different from their description, or if it sells counterfeit or unlicensed products through our platforms, or if it does not possess requisite licenses or permits as required by relevant laws and regulations despite our online background check for such licenses or permits on the marketplace seller, the reputation of our retail and wholesale pharmacy and our brand may be materially and adversely affected and we could face claims and may be held liable for damages in connection with such claims.
Any disruption to the operation of our current fulfillment facilities, or to the development of our new facilities, could reduce or negatively impact sales and have a material adverse effect on our business, financial condition and results of operations.
We rely on our fulfillment centers for the continuing operation of our pharmaceutical distribution business. Natural disasters or other unanticipated catastrophic events, including power interruptions, water shortage, storms, fires, earthquakes, terrorist attacks and wars, as well as changes in governmental planning for the land underlying these facilities, could significantly impair our ability to operate our business and destroy any inventory located in these facilities. In addition, our fulfillment centers that meet the requirements of modern logistics operations for guaranteed storage safety, optimal and flexible space utilization and high operational efficiency are in short supply. We may not be able to replace these facilities and equipment in a timely manner, should any of the foregoing occur.
Furthermore, the leases for our fulfillment centers and our use thereof could be challenged by third parties or government authorities, which may cause interruptions to our business operations. Certain lessors of our leased fulfillment centers have not provided us with their property ownership certificates or any other documentation proving their right to lease those properties to us. If our lessors are not the owners of the properties and they have not obtained consents from the owners or their lessors or permits from the relevant government authorities, our leases could be invalidated and we may have to renegotiate the leases with the owners or the parties who have the right to lease the properties, and the terms of the new leases may be less favorable to us. Also, certain of our leasehold interests in leased properties have not been registered with the relevant PRC government authorities as required by PRC law, which may expose us to potential fines. Although we are not aware of any claims or actions being contemplated or initiated by government authorities, property owners or any other third parties with respect to our leasehold interests in or use of such properties, we cannot assure you that our use of such leased properties will not be challenged. In the event that our use of leased properties is successfully challenged, we may be subject to fines and forced to relocate the affected operations. We can provide no assurance that we will be able to find suitable replacement sites on terms acceptable to us on a timely basis, or at all, or that we will not be subject to material liability resulting from third parties’ challenges on our use of such properties.
Our wide variety of accepted payment methods subjects us to third-party payment processing-related risks.
We accept payments using a variety of methods, including payment on delivery, bank transfers, online payments with credit cards and debit cards issued by major banks in China, and payment through third-party online payment platforms. For certain payment methods, including credit and debit cards, we pay interchange and other fees, which may increase over time and raise our operating costs and lower our profit margins. We may also be subject to fraud and other illegal activities in connection with the various payment methods we offer, including online payment and cash-on-delivery options. We also rely on third parties to provide payment processing services. We use third-party couriers to deliver all of the orders. The delivery personnel of our third-party couriers collect payments on our behalf if our customers opt for the payment-on-delivery option, and we require the third-party couriers to remit the payment collected to us on the following day. If these companies fail to remit the payment collected to us in a timely fashion, or at all, if they become unwilling or unable to provide these services to us, or if their service quality deteriorates, our business could be disrupted. We may also be subject to fraud and other illegal activities in connection with the various payment methods we offer, including online payment and cash-on-delivery options. Although we rely on third parties to provide payment processing services, we are also subject to various rules, regulations and requirements, regulatory or otherwise, governing electronic funds transfers, which could change or be reinterpreted to make it difficult or impossible for us to comply. If we fail to comply with these rules or requirements, we may be subject to fines and higher transaction fees and lose our ability to accept credit and debit card payments from our pharmacy customers and consumers, process electronic funds transfers or facilitate other types of online payments, and our business, financial condition and results of operations could be materially and adversely affected.
Any damage to the reputation and recognition of our brand names, including negative publicity against us, may materially and adversely affect our business operations and prospects.
We depend on our reputation and brand names in many aspects of our business operations. However, we cannot assure you that we will be able to maintain a positive reputation or brand name for all of our products in the future. Our reputation and brand names may be materially and adversely affected by a number of factors, many of which are beyond our control, including:
· adverse associations with the third party-branded products we sell or which are sold in our stores or on our platform, including with respect to their efficacy or side effects;
· lawsuits and regulatory investigations against us or otherwise relating to our products or industry;
· improper or illegal conduct by our employees, retail and wholesale pharmacies and third-party promoters, that is not authorized by us; and
· adverse publicity associated with us, our products or our industry, whether founded or unfounded.
Any damage to our brand names or reputation as a result of these or other factors may cause our products to be perceived unfavorably by pharmacies, doctors, regulators and consumers and the existing and prospective employees, retail and wholesale pharmacies and third-party promoters, and our business operations and prospects could be materially and adversely affected as a result.
Our business may be materially and adversely affected by adverse news, scandals or other incidents associated with the PRC general health and wellness industry.
Incidents that reflect doubt as to the quality or safety of pharmaceutical products manufactured, distributed or sold by other participants in the PRC general health and wellness industry, particularly the internet healthcare industry, including our competitors, have been, and may continue to be, subject to widespread media attention. Such incidents may damage the reputation of not only the parties involved, but also the general health and wellness industry in general, even if such parties or incidents have no relation to us, our management, our employees, our suppliers, our distributors or our retail pharmacies. Such negative publicity may indirectly and adversely affect our reputation and business operations. In addition, incidents not related to product quality or safety, or other negative publicity or scandals implicating us or our employees, regardless of merit, may also have an adverse impact on us and our reputation and corporate image.
If our risk management and internal control system is not adequate or effective, and if it fails to detect potential risks in our business as intended, our business, financial condition and results of operations could be materially and adversely affected.
We have established our internal control system, such as an organizational framework and, policies and procedures that are designed to monitor and control potential risk areas relevant to our business operations. However, due to the inherent limitations in the design and implementation of our internal control system, our internal control system may not be sufficiently effective in identifying, managing and preventing all risks if external circumstances change substantially or extraordinary events take place.
Furthermore, our new business initiatives may give rise to additional internal control risks that are currently unknown to us, despite our efforts to anticipate such issues. If our internal control system fails to detect potential risks in our business as intended or is otherwise exposed to weaknesses and deficiencies, our business, financial condition and results of operations could be materially and adversely affected.
Our risk management and internal controls also depend on effective implementation by our employees. There can be no assurance that such implementation by our employees will always function as intended or such implementation will not involve any human errors, mistakes or intentional misconduct. If we fail to implement our policies and procedures in a timely manner, or fail to identify risks that affect our business with sufficient time to plan for contingencies for such events, our business, financial condition and results of operations could be materially and adversely affected, particularly with respect to the maintenance of our relevant approvals and licenses granted by governments.
We may experience failures in our information technology system, which could materially and adversely affect our business, financial condition and results of operations.
We depend heavily on our information technology system to manage our business processes, to record and process our operational and financial data, and to provide reliable services. We have built secure, stable and scalable IT infrastructure. However, our information technology system may fail due to natural disasters or failures of public infrastructure, our information technology infrastructure or our applications software systems that are wholly or partially beyond our control. Any material disruption to the operation of our information technology system could have a material adverse effect on our business. Our failure to address these problems could result in our inability to perform, or delays in our performance of, critical business operational functions, loss of key business data, or our failure to comply with regulatory functions, which could materially and adversely affect our business operations and customer service.
We may be held liable for information or content displayed on, retrieved from or linked to our mobile applications or website, which may materially and adversely affect our business and operating results.
In addition to our website, we also offer healthcare products and services through our mobile applications, which are regulated by the Administrative Provisions on Mobile Internet Applications Information Services, or the APP Provisions, promulgated by the CAC, on June 28, 2016 and effective on August 1, 2016. According to the APP Provisions, the providers of mobile applications shall not create, copy, publish or distribute information and content that is prohibited by laws and regulations. We have implemented internal control procedures screening the information and content on our mobile applications to ensure their compliance with the APP Provisions. However, we cannot assure that all the information or content displayed on, retrieved from or linked to our mobile applications complies with the requirements of the APP Provisions at all times. If our mobile applications were found to be violating the APP Provisions, we may be subject to administrative penalties, including warning, service suspension or removal of our mobile applications from the relevant mobile application store, which may materially and adversely affect our business and operating results.
We rely on assumptions and estimates to calculate certain key operating metrics, and inaccuracies in such metrics may harm our reputation and adversely affect our business.
Certain key operating metrics in this annual report are calculated using our internal data that have not been independently verified by third parties. While these numbers are based on what we believe to be reasonable calculations for the applicable periods of measurement, there are some challenges in measuring those metrics, such as GMV and repurchase rate. In addition, our key operating metrics are derived and calculated based on different assumptions and estimates, and you should be cautious of such assumptions and estimates when assessing our operating performance.
Our operating metrics may differ from estimates published by third parties or from similarly titled metrics used by our competitors due to differences in data availability, sources and methodology. If third parties do not perceive our user metrics to be accurate representations of our user base or user engagement, or if we discover material inaccuracies in our operating metrics, our reputation may be harmed and third parties may be less willing to allocate their resources or spending to us, which could adversely affect our business and operating results.
Some pharmaceutical products offered by us are subject to price restrictions and will continue to be subject to price competition in China, but may be pending on changes of the regulations.
Some of our pharmaceutical products were subject to government price controls in the form of fixed retail prices or retail price ceilings and periodic downward adjustments imposed by National Development and Reform Commission, or the NDRC, and other authorities. Pursuant to the Notice Regarding the Opinion on Facilitating the Pharmaceutical Pricing Reform jointly issued by the National Development and Reform Commission, or the NDRC, the NHFPC and five other PRC government agencies in May 2015, the price ceilings imposed by the PRC government on pharmaceutical products other than narcotic and Class I psychotropic drugs were lifted on June 1, 2015, and these products would be subject to a more market-based pricing system adopted by medical insurance bureaus and relevant authorities.
Even prior to the lifting of government price controls on pharmaceutical products, the prices of prescription drugs in China had been determined by the centralized tender process and the prices of OTC drugs in China had been determined by arm’s-length, commercial negotiation and market factors such as brand recognition, market competition and consumer demand. There is no assurance that the application of the more market-based pricing system will result in a higher product pricing compared to the government-controlled pricing, as competition from other retailers and wholesalers, particularly those offering the same products but with lower prices, may force us to lower our sales prices to the previous government-controlled price levels. Consequently, our profitability may suffer and our business, financial condition and results of operations may also be materially and adversely affected.
If we fail to implement and maintain an effective system of internal controls to remediate our material weakness over financial reporting, we may be unable to accurately report our results of operations, meet our reporting obligations or prevent fraud, and investor confidence and the market price of the ADSs may be materially and adversely affected.
Prior to our initial public offering in September 2018, we have been a private company with limited accounting personnel and other resources with which we address our internal control over financial reporting. In connection with the audits of our consolidated financial statements as of and for the years ended December 31, 2016, 2017 and 2018, we and our independent registered public accounting firm identified one material weakness and one significant deficiency in our internal control over financial reporting. As defined in the standards established by the U.S. Public Company Accounting Oversight Board, or PCAOB, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis.
The material weakness that has been identified relates to our lack of sufficient competent financial reporting and accounting personnel with appropriate understanding of U.S. GAAP to design and implement formal period-end financial reporting controls and procedures, to address complex U.S. GAAP technical accounting issues, and to prepare and review our consolidated financial statements and related disclosures in accordance with U.S. GAAP and financial reporting requirements set forth by the SEC.
Following the identification of the material weakness, the significant deficiency and other control deficiencies, we have taken measures and plan to continue to take measures to remedy these control deficiencies. See “Item 15. Controls and Procedures—Internal Control Over Financial Reporting.” However, the implementation of these measures may not fully address these deficiencies in our internal control over financial reporting, and we cannot conclude that they have been fully remedied. Our failure to correct these control deficiencies or our failure to discover and address any other control deficiencies could result in inaccuracies in our financial statements and impair our ability to comply with applicable financial reporting requirements and related regulatory filings on a timely basis. Moreover, ineffective internal control over financial reporting could significantly hinder our ability to prevent fraud.
We are a public company in the United States subject to the Sarbanes-Oxley Act of 2002. Section 404 of the Sarbanes-Oxley Act of 2002, will require that we include a report of management on our internal control over financial reporting in our annual report on Form 20-F beginning with our annual report for the fiscal year ending December 31, 2019. In addition, once we cease to be an “emerging growth company” as such term is defined in the JOBS Act, our independent registered public accounting firm must attest to and report on the effectiveness of our internal control over financial reporting. Our management may conclude that our internal control over financial reporting is not effective. Moreover, even if our management concludes that our internal control over financial reporting is effective, our independent registered public accounting firm, after conducting its own independent testing, may issue a report that is qualified if it is not satisfied with our internal controls or the level at which our controls are documented, designed, operated or reviewed, or if it interprets the relevant requirements differently from us. In addition, after we become a public company, our reporting obligations may place a significant strain on our management, operational and financial resources and systems for the foreseeable future. We may be unable to timely complete our evaluation testing and any required remediation.
During the course of documenting and testing our internal control procedures, in order to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act of 2002, we may identify other weaknesses and deficiencies in our internal control over financial reporting. In addition, if we fail to maintain the adequacy of our internal control over financial reporting, as these standards are modified, supplemented or amended from time to time, we may not be able to conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act of 2002. Generally, if we fail to achieve and maintain an effective internal control environment, we could suffer material misstatements in our financial statements and fail to meet our reporting obligations, which would likely cause investors to lose confidence in our reported financial information. This could in turn limit our access to capital markets, harm our results of operations, and lead to a decline in the trading price of the ADSs. Additionally, ineffective internal control over financial reporting could expose us to increased risk of fraud or misuse of corporate assets and subject us to potential delisting from the stock exchange on which we list, regulatory investigations and civil or criminal sanctions.
Competition for employees is intense, and we may not be able to attract and retain the qualified and skilled employees needed to support our business.
We believe our success depends on the efforts and talent of our employees, including medical professionals, risk management, software engineering, financial and marketing personnel. Our future success depends on our continued ability to attract, develop, motivate and retain qualified and skilled employees. Competition for highly skilled technical, risk management and financial personnel is extremely intense. We may not be able to hire and retain these personnel at compensation levels consistent with our existing compensation and salary structure. Some of the companies with which we compete for experienced employees have greater resources than we have and may be able to offer more attractive terms of employment.
In addition, we invest significant time and expenses in training our employees, which increases their value to competitors who may seek to recruit them. If we fail to retain our employees, we could incur significant expenses in hiring and training new employees, and the quality of our services and our ability to serve various participants in the pharmaceutical value chain could decline, resulting in a material adverse effect to our business.
Our business depends on the continued efforts of our senior management. If one or more of our key executives were unable or unwilling to continue in their present positions, our business may be severely disrupted.
Our future success depends heavily upon the continued services of our senior executives, key research and development personnel and key sales and marketing personnel. We rely on the expertise and experience of our founders, Dr. Gang Yu and Mr. Junling Liu, especially in areas of supply chain management and e-commerce. Our research and development team is critical to the development of proprietary technologies used by our online and offline, retail and wholesale businesses, and realization of the potential benefits of our intellectual property. In addition, success in the distribution of our products depends on the dedication and skills of our sales and marketing personnel. Accordingly, our ability to attract and retain key personnel is a critical factor in our competitiveness. Competition for these individuals could require us to offer higher compensation and other benefits in order to attract and retain them, which could increase our operating expenses and, in turn, materially and adversely affect our financial condition and results of operations. If we are unable to attract or retain the personnel required to achieve our business objectives, our business could be severely disrupted.
We do not maintain key-person insurance for members of our management team. If we lose the services of any senior management, we may not be able to identify suitable or qualified replacements, and may incur additional expenses to recruit and train new personnel, which could severely disrupt our business and prospects and prolong our expansion strategies and plans. Furthermore, if any of our executive officers joins a competitor or forms a competing company, we may lose a significant number of our existing pharmacy customers and consumers and potentially lose our substantial research and development achievements, which could have a material adverse effect on our business, financial condition, results of operations and prospects.
We may from time to time become party to litigation, other legal or administrative disputes and proceedings that may materially and adversely affect us.
In the course of our ordinary business operations, we may become a party to litigation, legal proceedings, claims, disputes or arbitration proceedings from time to time. Any ongoing litigation, legal proceedings, claims, disputes or arbitration proceedings may distract our senior management’s attention and consume our time and other resources. In addition, even if we ultimately succeed in such litigation, legal proceedings, claims, disputes or arbitration proceedings, there may be negative publicity attached to such litigation, legal proceedings, claims, disputes or arbitration proceedings, which may materially and adversely affect our reputation and brand names. In the case of an adverse verdict, we may be required to pay significant monetary damages, assume significant liabilities or suspend or terminate parts of our operations. As a result, our business, financial condition, results of operations and prospects may be materially and adversely affected.
We may not have sufficient insurance to cover our business risks.
We have obtained insurance to cover certain potential risks and liabilities, such as professional liability insurance for our doctors in connection with their provision of medical consultation services over our platform, and product liability insurance for us and our suppliers with respect to products sold in our retail pharmacy and online wholesale pharmacy through 1 Drugstore and 1 Drug Mall, respectively. However, we may not be able to acquire any insurance for certain types of risks such as business liability or service disruption insurance for all of our operations in the PRC, and our coverage may not be adequate to compensate for all losses that may occur, particularly with respect to loss of business or operations. For example, we do not maintain business interruption insurance, nor do we maintain key-man life insurance. Any business disruption, litigation, regulatory action, outbreak of epidemic disease or natural disaster could also expose us to substantial costs and diversion of resources. There can be no assurance that our insurance coverage is sufficient to prevent us from any loss or that we will be able to successfully claim our losses under our current insurance policies on a timely basis, or at all. If we incur any loss that is not covered by our insurance policies, or the compensated amount is significantly less than our actual loss, our business, financial condition and results of operations could be materially and adversely affected.
We may be subject to intellectual property infringement claims, which may be expensive to defend and may disrupt our business and operations.
We cannot be certain that our operations or any aspects of our business do not or would not infringe upon or otherwise violate trademarks, patents, copyrights or other intellectual property rights held by third parties. We may be, from time to time, or in the future, become subject to legal proceedings and claims relating to the intellectual property rights of others. In addition, there may be other third-party intellectual property that is infringed by our products, services or other aspects of our business. There could also be existing intellectual property of which we are not aware that our products may inadvertently infringe. There can be no assurance that holders of such intellectual property purportedly relating to some aspect of our technology platform or business, if any such holders exist, would not seek to enforce such intellectual property against us in the PRC or in any other jurisdictions, as applicable. Furthermore, the application and interpretation of PRC intellectual property related laws and the procedures and standards in the PRC are still evolving and are uncertain, and there can be no assurance that PRC courts or regulatory authorities would agree with our analysis. If we are found to have violated the intellectual property rights of others, we may be subject to liability for our infringement activities or may be prohibited from using such intellectual property, and we may incur licensing fees or be forced to develop alternatives of our own. In addition, we may incur significant expenses, and may be forced to divert management’s time and other resources from our business and operations to defend against these third-party infringement claims, regardless of their merits. Successful infringement or licensing claims made against us may result in significant monetary liabilities and may materially disrupt our business and operations by restricting or prohibiting our use of the intellectual property in question, which may materially and adversely affect our business, financial condition and results of operations.
Failure to renew our current leases or locate desirable alternatives for our facilities could materially and adversely affect our business.
We lease properties for our offices, offline retail pharmacies and fulfillment centers. We may not be able to successfully extend or renew such leases upon expiration of the current term on commercially reasonable terms or at all, and may therefore be forced to relocate our affected operations. This could disrupt our operations and result in significant relocation expenses, which could adversely affect our business, financial condition and results of operations. In addition, we compete with other businesses for premises at certain locations or of desirable sizes. As a result, even though we could extend or renew our leases, rental payments may significantly increase as a result of the high demand for the leased properties. In addition, we may not be able to locate desirable alternative sites for our facilities as our business continues to grow and failure in relocating our affected operations could adversely affect our business and operations.
Security breaches and attacks against our systems and network, and any potential resultant breach or failure to otherwise protect confidential and proprietary information, could damage our reputation and adversely affect our business, financial condition and results of operations.
We rely heavily on technology, particularly the internet, to provide high-quality online services. However, our technology operations are vulnerable to disruptions arising from human error, natural disasters, power failure, computer viruses, spam attacks, unauthorized access and other similar events. Disruptions to, or instability of, our technology or external technology that allows our pharmacy customers and consumers to use our online services and products could materially harm our business and reputation.
Although we have employed significant resources to develop security measures against breaches, our cybersecurity measures may not detect or prevent all attempts to compromise our systems, including distributed denial-of-service attacks, viruses, malicious software, break-ins, phishing attacks, social engineering, misconduct or sabotage by our employees, security breaches or other attacks and similar disruptions that may jeopardize the security of information stored in and transmitted by our systems or that we otherwise maintain. Breaches of our cybersecurity measures could result in unauthorized access to our systems, misappropriation of information or data, deletion or modification of user information, or a denial-of-service or other interruption to our business operations. As techniques used to obtain unauthorized access to or sabotage systems change frequently and may not be known until launched against us, we may be unable to anticipate, or implement adequate measures to protect against, these attacks. There can be no assurance that we would not in the future be subject to such attacks that may result in material damages or remediation costs. If we are unable to avert these attacks and security breaches, we could be subject to significant legal and financial liability, our reputation would be harmed and we could sustain substantial revenue loss from lost sales and customer dissatisfaction.
In addition, we may not have the resources or technical sophistication to anticipate or prevent rapidly evolving types of cyber-attacks. Cyber-attacks may target us, our users or other participants of our ecosystem, or the information infrastructure on which we depend. Actual or anticipated attacks and risks may cause us to incur significantly higher costs, including costs to deploy additional personnel and network protection technologies, train employees, and engage third-party experts and consultants. Cybersecurity breaches may harm our reputation and business, and materially and adversely affect our financial condition and results of operations.
Our failure to comply with anti-corruption laws and regulations, or effectively manage our employees, marketplace sellers and affiliates, could severely damage our reputation, and materially and adversely affect our business, financial condition, results of operations and prospects.
We are subject to risks in relation to actions taken by us, our employees, marketplace sellers or affiliates that constitute violations of the anti-corruption laws and regulations. There have been several instances of corrupt practices in the pharmaceutical industry, including, among other things, receipt of kickbacks, bribes or other illegal gains or benefits by pharmacies, hospitals and medical practitioners from manufacturers, distributors and retail pharmacies in connection with the prescription of pharmaceutical products. While we adopt strict internal procedures and work closely with relevant government agencies to ensure compliance of our business operations with relevant laws and regulations, our efforts may not be sufficient to ensure that we comply with relevant laws and regulations at all times. If we, our employees, marketplace sellers or affiliates violate these laws, rules or regulations, we could be subject to fines and/or other penalties. In the case of our retail and wholesale businesses, the products involved may be seized and our operations may be suspended. Actions by PRC regulatory authorities or the courts to provide an interpretation of PRC laws and regulations that differs from our interpretation or to adopt additional anti-bribery or anti-corruption related regulations could also require us to make changes to our operations. Our reputation, corporate image, and business operations may be materially and adversely affected if we fail to comply with these measures or become the target of any negative publicity as a result of actions taken by us, our employees, marketplace sellers or affiliates, which may in turn have a material adverse effect on our business, financial condition, results of operations and prospects.
Our delivery, return and exchange policies may materially and adversely affect our results of operations.
We have adopted shipping policies that do not necessarily pass the full cost of shipping on to our pharmacy customers and consumers. We have also adopted policies that permit the return and exchange of our products within thirty days in certain circumstances for specified reasons. We may also be required by law to adopt new or amend existing return and exchange policies from time to time. For example, pursuant to the Consumer Protection Law and relevant regulations and rules, consumers are generally entitled to return products purchased within seven days upon receipt without reason when they purchase the products from business operators on the internet with certain exception, such as pharmaceutical products. These policies subject us to additional costs and expenses which we may not recoup through increased revenue. Our ability to handle a large volume of returns is unproven. If we revise these policies to reduce our costs and expenses, our pharmacy customers and consumers may be dissatisfied, which may result in loss of existing consumers and pharmacy customers or failure to acquire new consumers and pharmacy customers at a desirable pace, which may materially and adversely affect our results of operations.
If we are subject to higher product return rates, our business, financial condition and results of operations may be materially and adversely affected.
We have established a thirty-day product return policy in certain circumstances for specified reasons. In addition, pursuant to the Consumer Protection Law, consumers are generally entitled to return purchased products within seven days upon receipt without giving any reasons when they purchase the products from business operators on the internet. Although a majority of our products may not be returned or exchanged under the Administrative Standard of Pharmaceutical Operating Quality, prohibiting returns and exchanges of pharmaceutical products except for quality reasons, if our product return rates increase or are higher than expected, our revenues and costs can be negatively impacted. Furthermore, as we cannot return some products to our suppliers pursuant to our contracts with them or if return rates for such products increase significantly, we may experience an increase in our inventory balance, inventory impairment and fulfillment cost, which may materially and adversely affect our working capital. As a result, our business, financial condition and results of operations may be materially and adversely affected.
We may not be able to conduct our marketing activities effectively, properly, or at reasonable costs, and we are subject to limitations in promoting our services and products, which will have an impact on our business operations.
We invest significant resources in a variety of different marketing and brand promotion efforts designed to enhance our brand recognition and increase sales of our services and products. However, our brand promotion and marketing activities may not be well received and may not result in the levels of sales that we anticipate. Meanwhile, marketing approaches and tools in the PRC internet healthcare market are continually evolving, which may further require us to enhance our marketing approaches and experiment with new marketing methods to keep pace with industry developments and customer preferences. Failure to refine our existing marketing approaches or to introduce new marketing approaches in a cost-effective manner could reduce our market share and materially and adversely affect our financial condition, results of operations and profitability. In addition, we are subject to certain limitations in promoting services and products. Our in-house medical team and external doctors and other relevant parties in the provision of our medical and wellness services have to comply with rules and regulations that restrict the promotion or dissemination of information about the professional healthcare services and practice provided by licensed doctors, and the publication or marketing efforts for the predominant purpose of promoting the products or services of doctors to consumers or potential consumers. Such restrictions may affect our ability to further enhance our brand recognition or secure new business opportunities in the future.
Under PRC laws and regulations, all advertisements published online containing drug names, applicable symptoms treated by such drugs (major functions) or other drug-related content, and advertisements published online containing medical device names and the applicable scope, performance, structure and composition, function and other contents relevant to medical device are subject to examination by relevant government authorities. We are prohibited from publishing advertisements of prescription drugs on our website and must ensure that any advertisement of medical treatment, drugs or medical devices does not include any assertion or guarantee as to the function and safety or any statement of curative rate and effectiveness of such medical treatment, drugs or medical devices. Any violation of advertisement-related laws and regulations may subject us to fine, or even suspension of our business or revocation of our business license. See “Item 4. Information on the Company—B. Business Overview—Regulations—Regulations Relating to Online Advertising.” Although we have implemented internal procedures to examine the content of advertisements displayed on our website, we cannot assure you that all such content meets the requirements under PRC advertising-related laws and regulations at all times. In the past, we have been required to pay penalties for advertisements displayed on our website due to non-compliance with advertising laws.
There can be no assurance that our existing practices of monitoring our information dissemination process and publication would continue to be effective and would comply fully with laws and regulations. Should there be any change in the relevant rules and regulations, or change of interpretation thereof, we, our in-house medical team, external doctors and other relevant third parties may be regarded as breaching the relevant rules and regulations and may be subject to regulatory penalties or disciplinary actions, which may materially and adversely affect our business and reputation.
We may not be able to detect or prevent fraud or other misconduct committed by our employees or third parties.
Fraud or other misconduct by our employees, such as unauthorized business transactions, bribery and breach of our internal policies and procedures, unauthorized access to or leakage of the data of our consumers and pharmacy customers, or by third parties, such as breach of law, may be difficult to detect or prevent. It could subject us to financial loss and sanctions imposed by governmental authorities while seriously damaging our reputation. This may also impair our ability to effectively attract prospective users, develop customer loyalty, obtain financing on favorable terms and conduct other business activities.
In particular, we may face risks with respect to fictitious or other fraudulent activities. There can be no assurance that the measures we have implemented to detect and reduce the occurrence of fraudulent activities would be effective in combating fraudulent transactions or improving overall satisfaction among our consumers and pharmacy customers, pharmaceutical companies and marketplace sellers. Our marketplace sellers may also engage in fictitious or “phantom” transactions with themselves or collaborators in order to artificially inflate their ratings, reputation and search results rankings. This activity may harm other third parties by enabling the perpetrating marketplace seller to be favored over legitimate ones, may harm consumers by deceiving them into believing that a marketplace seller is more reliable or trusted than that marketplace seller actually is, and result in inflated GMV from our online marketplace.
Our risk management systems, information technology systems and internal control procedures are designed to monitor our operations and overall compliance. However, we may be unable to identify non-compliance or suspicious transactions promptly, or at all. Furthermore, it is not always possible to detect and prevent fraud or other misconduct committed by our employees, ecosystem participants or other third parties, and the precautions we take to prevent and detect such activities may not be effective. Therefore, we are subject to the risk that fraud or other misconduct may have previously occurred but was undetected, or may occur in the future. This may materially and adversely affect our business, financial condition and results of operations.
We may need additional capital but may not be able to obtain it on favorable terms or at all.
We may require additional cash resources due to operating losses or future growth and development of our business, including any investments or acquisitions we may decide to pursue. If our cash resources are insufficient to satisfy our cash requirements, we may seek to issue additional equity or debt securities or obtain new or expanded credit facilities. Our ability to obtain external financing in the future is subject to a variety of uncertainties, including our future financial condition, results of operations, cash flows, share price performance, liquidity of international capital and lending markets and the PRC governmental regulations over foreign investment and the PRC healthcare industry. In addition, incurring indebtedness would subject us to increased debt service obligations and could result in operating and financing covenants that would restrict our operations. There can be no assurance that financing would be available in a timely manner or in amounts or on terms favorable to us, or at all. Any failure to raise needed funds on terms favorable to us, or at all, could severely restrict our liquidity as well as have a material adverse effect on our business, financial condition and results of operations. Moreover, any issuance of equity or equity-linked securities could result in significant dilution to our existing shareholders.
Our business could be disrupted by network interruptions.
Our business depends on the efficient and uninterrupted operation of our computer and communications systems and our entire information infrastructure is located in China. Our information infrastructure contains substantial quantities of data relating to our supply chain information, competitive pricing data and customer base, such as customer behavior, consultation records and transaction data, among other things, which enable our users to access the full range of our services and other ecosystem participants to conduct their operations efficiently and effectively over our platforms. Although we have certain precautions to address potential interruptions, such preparation may not be sufficient and we do not carry business interruption insurance. Furthermore, despite any precautions we may take, the occurrence of a natural disaster, such as an earthquake, flood or fire, or other unanticipated incidents at our information infrastructure facilities in China, including power outages, telecommunications delays or failures, break-ins to our systems or computer viruses, could result in delays or interruptions to our platform and operations as well as loss of our consumers’ and other participants’ data. Any of these events could damage our reputation, materially disrupt our ecosystem and subject us to liability and claims, which may materially and adversely affect our business, financial condition and results of operations.
An occurrence of a natural disaster, widespread health epidemic or other outbreaks could have a material adverse effect on our business, financial condition and results of operations.
Our business could be materially and adversely affected by natural disasters, such as snowstorms, earthquakes, fires or floods, the outbreak of a widespread health epidemic or other events, such as wars, acts of terrorism, environmental accidents, power shortage, labor unrest or communication interruptions. The occurrence of such a disaster or prolonged outbreak of an epidemic illness or other adverse public health developments in the PRC or elsewhere could materially disrupt our business and operations. Such events could also significantly affect our industry and cause a temporary closure of the facilities we use for our operations, which would severely disrupt our operations and have a material adverse effect on our business, financial condition and results of operations. Our operations could be disrupted if any of our employees were suspected of having any of the epidemic illnesses, since this could require us to quarantine some or all of such employees or disinfect the facilities used for our operations. In addition, our revenue and profitability could be materially reduced to the extent that a natural disaster, health epidemic or other outbreak harms the global or PRC economy in general. Our operations could also be severely disrupted if our users or other participants were affected by such natural disasters, health epidemics or other outbreaks.
Risks Related to Our Corporate Structure
If the PRC government deems that the contractual arrangements in relation to our variable interest entities and their subsidiaries do not comply with PRC regulatory restrictions on foreign investment in the relevant industries, or if these regulations or the interpretation of existing regulations change in the future, we could be subject to severe penalties or be forced to relinquish our interests in those operations.
Foreign ownership of internet-based businesses, such as provision of online information and other value-added telecommunication services, and medical institutions, are subject to restrictions under current PRC laws and regulations. For example, foreign investors are generally not allowed to own more than 50% of the equity interests in a value-added telecommunication service provider with the exception relating to e-commerce business, and any such foreign investor must have experience in providing value-added telecommunications services overseas and maintain a good track record, and the medical institution can only be established as sino-foreign equity or cooperative joint venture in accordance with the currently-effective Guidance Catalogue of Industries for Foreign Investment and other applicable laws and regulations.
We are a Cayman Islands company and our PRC subsidiary, Yao Fang, is considered a wholly foreign owned enterprise. To comply with PRC laws and regulations, we set up a series of contractual arrangements entered into among Yao Fang, our variable interest entities, and their shareholders to conduct our operations in China. For a detailed description of these contractual arrangements, see “Item 4. Information on the Company—C. Organizational Structure—Contractual Arrangements with Our Variable Interest Entities.” As a result of these contractual arrangements, we exert control over our variable interest entities and their subsidiaries and consolidate their operating results in our financial statements under U.S. GAAP. We conduct our pharmaceutical wholesale and retail business, online retail pharmacy, 1 Drugstore, pharmacopoeia and our internet hospital, 1 Clinic, via our variable interest entities, Yihao Pharmacy, Yihao Pharmaceutical Chain and Anshun Southwest Internet Hospital Co., Ltd., or Southwest Internet Hospital, respectively.
In the opinion of our PRC legal counsel, Commerce & Finance Law Offices, the ownership structures of our variable interest entities currently does not, result in any violation of the applicable PRC laws or regulations currently in effect; and the contractual arrangements among Yao Fang, our variable interest entities and their shareholders, are governed by PRC laws or regulations, and are currently valid, binding and enforceable in accordance with the applicable PRC laws or regulations currently in effect, and do not result in any violation of the applicable PRC laws or regulations currently in effect.
However, Commerce & Finance Law Offices has also advised us that there are substantial uncertainties regarding the interpretation and application of current or future PRC laws and regulations, and there can be no assurance that the PRC government will ultimately take a view that is consistent with the opinion of our PRC counsel. In January 2015, the MOFCOM published a discussion draft of the proposed Foreign Investment Law, or the Draft FIL, for public review and comments. Among other things, the Draft FIL expands the definition of foreign investment and introduces the principle of “actual control” in determining whether a company is considered a foreign-invested enterprise, or an FIE. Under the Draft FIL, variable interest entities would also be deemed as FIEs, if they are ultimately “controlled” by foreign investors, and would be subject to restrictions on foreign investments. Subsequently, in December 2018, the standing committee of the National People’s Congress reviewed a draft Foreign Investment Law, and this draft was subsequently published for public comment. In March 2019, a new draft of Foreign Investment Law was submitted to the National People’s Congress for review and was approved on March 15, 2019, which will come into effect from January 1, 2020. The approved Foreign Investment Law does not touch upon the relevant concepts and regulatory regimes that were historically suggested for the regulation of VIE structures, and thus this regulatory topic remains unclear under the Foreign Investment Law. Since the Foreign Investment Law is new, there are substantial uncertainties exist with respect to its implementation and interpretation and it is also possible that the VIE entities will be deemed as foreign invested enterprises and be subject to restrictions in the future. Such restrictions may cause interruptions to our operations, products and services and may incur additional compliance cost, which may in turn materially and adversely affect our business, financial condition and results of operations.
If our ownership structure and contractual arrangements with our variable interest entities are found to be in violation of any existing or future PRC laws or regulations, or we fail to obtain or maintain any of the required permits or approvals, the relevant governmental authorities would have broad discretion in dealing with such violation, including levying fines, confiscating our income or the income of our PRC subsidiaries, variable interest entities or their subsidiaries, revoking the business licenses and/or operating licenses of such entities, shutting down our servers or blocking our online platforms, discontinuing or placing restrictions or onerous conditions on our operations, requiring us to undergo a costly and disruptive restructuring, and taking other regulatory or enforcement actions that could be harmful to our business. Any of these actions could cause significant disruption to our business operations and severely damage our reputation, which would in turn materially and adversely affect our business, financial condition and results of operations. If any of these occurrences results in our inability to direct the activities of our variable interest entities and their subsidiaries, and/or our failure to receive economic benefits from our variable interest entities and their subsidiaries, we may not be able to consolidate their results into our consolidated financial statements in accordance with U.S. GAAP.
We rely on contractual arrangements with our variable interest entities and their shareholders, for a significant portion of our business operations, which may not be as effective as direct ownership in providing operational control.
We have relied and expect to continue to rely on contractual arrangements with our variable interest entities and their shareholders to operate our pharmaceutical wholesale and retail business, 1 Drugstore, pharmacopoeia and our internet hospital, 1 Clinic, through Yihao Pharmacy, Yihao Pharmaceutical Chain and Southwest Internet Hospital, respectively. For a description of these contractual arrangements, see “Item 4. Information on the Company—C. Organizational Structure—Contractual Arrangements with Our Variable Interest Entities.” These contractual arrangements may not be as effective as direct ownership in providing us with control over our variable interest entities and their subsidiaries. For example, our variable interest entities or their shareholders may fail to fulfill their contractual obligations with us, by, among other things, failing to maintain our website and use the domain names and trademarks in a manner as stipulated in the contractual arrangements, or taking other actions that are detrimental to our interests.
If we had direct ownership of our variable interest entities, we would be able to exercise our rights as shareholders to effect changes in their board of directors, which in turn could implement changes, subject to any applicable fiduciary obligations, at the management and operational level. However, under the current contractual arrangements, we rely on the performance by our variable interest entities and their shareholders of their obligations under the contractual arrangements to exercise control over our variable interest entities and their subsidiaries. The shareholders of our variable interest entities may not act in the best interests of our company or may not perform their obligations under these contracts. Such risks exist throughout the period in which we intend to operate certain portions of our business through the contractual arrangements with our variable interest entities and their shareholders. Although we have the right to replace any shareholder of such entities under the contractual arrangements, if any of these shareholders are uncooperative or any dispute relating to these contracts remains unresolved, we will have to enforce our rights under these contracts through the operations of PRC laws and arbitration, litigation and other legal proceedings, the outcome of which will be subject to uncertainties in the PRC legal system. Therefore, our contractual arrangements with our variable interest entities and their shareholders may not be as effective in ensuring our control over the relevant portion of our business operations as direct ownership would be.
Any failure by our variable interest entities or their respective shareholders to perform their obligations under our contractual arrangements with them would have a material adverse effect on our business.
We have entered into a series of contractual arrangements with our variable interest entities and their shareholders. For a description of these contractual arrangements, see “Item 4. Information on the Company—C. Organizational Structure—Contractual Arrangements with Our Variable Interest Entities.” If our variable interest entities or their shareholders fail to perform their respective obligations under the contractual arrangements, we may incur substantial costs and expend additional resources to enforce such arrangements. We may also have to rely on legal remedies under PRC laws, including seeking specific performance or injunctive relief, and claiming damages, which we cannot assure you will be effective under PRC laws. For example, if the shareholders of our variable interest entities were to refuse to transfer their equity interests in such entities to us or our designee when we exercise the purchase option pursuant to these contractual arrangements, or if they were otherwise to act in bad faith toward us, then we may have to take legal action to compel them to perform their contractual obligations.
All the agreements under our contractual arrangements are governed by PRC laws and provide for the resolution of disputes through arbitration in China. Accordingly, these contracts would be interpreted in accordance with PRC laws and any disputes would be resolved in accordance with PRC legal procedures. The legal system in the PRC is not as developed as in some other jurisdictions, such as the United States. As a result, uncertainties in the PRC legal system could limit our ability to enforce these contractual arrangements. Meanwhile, there are very few precedents and little formal guidance as to how contractual arrangements in the context of a variable interest entity should be interpreted or enforced under PRC laws. There remain significant uncertainties regarding the ultimate outcome of such arbitration should legal action become necessary. In addition, under PRC laws, rulings by arbitrators are final and parties cannot appeal the arbitration results in court unless such rulings are revoked or determined unenforceable by a competent court. If the losing parties fail to carry out the arbitration awards within a prescribed time limit, the prevailing parties may only enforce the arbitration awards in PRC courts through arbitration award recognition proceedings, which would require additional expenses and delay. In the event that we are unable to enforce these contractual arrangements, or if we suffer significant delay or other obstacles in the process of enforcing these contractual arrangements, we may not be able to exert effective control over our variable interest entities and their subsidiaries, and our ability to conduct our business may be negatively affected. See “—Risks Related to Doing Business in China—Uncertainties in the interpretation and enforcement of PRC laws and regulations could limit the legal protections available to us.”
The shareholders of our variable interest entities may have potential conflicts of interest with us, which may materially and adversely affect our business and financial condition.
The equity interests of our variable interest entity, Yihao Pharmacy, are held by Mr. Yue Xuan, a family member of Dr. Gang Yu, and Ms. Jing Liu, a family member of Mr. Junling Liu. These shareholders may have potential conflicts of interest with us. These shareholders may breach, or cause our variable interest entities to breach, the existing contractual arrangements, which would have a material adverse effect on our ability to effectively control our variable interest entities and their subsidiaries and receive economic benefits from them. For example, these shareholders may be able to cause our agreements with our variable interest entities to be performed in a manner adverse to us by, among other things, failing to remit payments due under the contractual arrangements to us on a timely basis. We cannot assure you that when conflicts of interest arise, any or all of these shareholders will act in the best interests of our company or such conflicts will be resolved in our favor.
Currently, we do not have any arrangements to address potential conflicts of interest between these shareholders and our company, except that we could exercise our purchase option under the exclusive option agreement with these shareholders to request them to transfer all of their equity interests in our variable interest entities to a PRC entity or individual designated by us, to the extent permitted by PRC laws. If we cannot resolve any conflict of interest or dispute between us and these shareholders, we would have to rely on legal proceedings, which could result in the disruption of our business and subject us to substantial uncertainty as to the outcome of any such legal proceedings.
Contractual arrangements in relation to our variable interest entities, may be subject to scrutiny by the PRC tax authorities and they may determine that we, or our variable interest entities and their subsidiaries, owe additional taxes, which could negatively affect our financial condition and the value of your investment.
Under applicable PRC laws and regulations, arrangements and transactions among related parties may be subject to audit or challenge by the PRC tax authorities. The PRC enterprise income tax law requires every enterprise in China to submit its annual enterprise income tax return together with a report on transactions with its related parties to the relevant tax authorities. The tax authorities may impose reasonable adjustments on taxation if they have identified any related party transactions that are inconsistent with arm’s length principles. We may face material and adverse tax consequences if the PRC tax authorities determine that the contractual arrangements among Yao Fang, our wholly owned subsidiary in China, our variable interest entities and their shareholders were not entered into on an arm’s length basis in such a way as to result in an impermissible reduction in taxes under applicable PRC laws, regulations and rules, and adjust income of our variable interest entities in the form of a transfer pricing adjustment. A transfer pricing adjustment could, among other things, result in a reduction of expense deductions recorded by our variable interest entities for PRC tax purposes, which could in turn increase their tax liabilities without reducing Yao Fang’s tax expenses. Furthermore, the PRC tax authorities may impose late payment fees and other penalties on our variable interest entities for the adjusted but unpaid taxes according to the applicable regulations. Our financial position could be materially adversely affected if our variable interest entities’ tax liabilities increase or if they are required to pay late payment fees and other penalties.
If we exercise the option to acquire equity ownership of our variable interest entities, the ownership transfer shall be approved or filed with PRC governmental authorities and subject to taxation, which may result in substantial costs.
Pursuant to the contractual arrangements, Yao Fang has the exclusive right to purchase all or any part of the equity interests in our variable interest entities from the respective shareholders for free or at a lowest price as permitted by the then applicable PRC laws. The equity transfer shall be subject to the approvals from or filings with the MOFCOM, the MIIT, the SAIC and/or their local competent branches. In addition, the equity transfer price may be subject to review and tax adjustment by the relevant tax authority. In particular, the shareholders of our variable interest entities will be subject to PRC individual or enterprise income tax on the difference between the equity transfer price and the then current registered capital of our variable interest entities and the payment, after deducting such tax, to Yao Fang may also be subject to enterprise income tax, which may result in substantial costs.
We may lose the ability to use and benefit from assets held by our variable interest entities that are material to the operation of our business if the entity goes bankrupt or becomes subject to a dissolution or liquidation proceeding.
Our variable interest entities hold certain assets that are material to the operation of our business, including, among others, intellectual properties. Under the contractual arrangements, our variable interest entities may not, and the shareholders of our variable interest entities may not cause them to, in any manner, sell, transfer, mortgage or dispose of their assets or their legal or beneficial interests in the business without our prior consent. However, in the event these shareholders breach these contractual arrangements and voluntarily liquidate our variable interest entities, or our variable interest entities declare bankruptcy and all or part of their assets become subject to liens or rights of third-party creditors, or are otherwise disposed of without our consent, we may be unable to continue some or all of our business activities, which could materially adversely affect our business, financial condition and results of operations. If our variable interest entities undergo a voluntary or involuntary liquidation proceeding, the independent third-party creditors may claim rights to some or all of these assets, thereby hindering our ability to operate our business, which could materially and adversely affect our business, financial condition and results of operations.
Risks Related to Doing Business in China
Changes in China’s economic, political or social conditions or government policies could have a material adverse effect on our business and results of operations.
Substantially all of our operations are located in China. Accordingly, our business, prospects, financial condition and results of operations may be influenced to a significant degree by political, economic and social conditions in China generally and by continued economic growth in China as a whole.
The Chinese economy differs from the economies of most developed countries in many respects, including the amount of government involvement, level of development, growth rate, control of foreign exchange and allocation of resources. Although the Chinese government has implemented measures emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets and the establishment of improved corporate governance in business enterprises, a substantial portion of productive assets in China are still owned by the government. In addition, the Chinese government continues to play a significant role in regulating industry development by imposing industrial policies. The Chinese government also exercises significant control over China’s economic growth through allocating resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies.
While the Chinese economy has experienced significant growth over the past decades, growth has been uneven, both geographically and among various sectors of the economy. The Chinese government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures may benefit the overall Chinese economy, but may have a negative effect on us. For example, our financial condition and results of operations may be adversely affected by government control over capital investments or changes in tax regulations. In addition, in the past the Chinese government has implemented certain measures, including interest rate increases, to control the pace of economic growth. These measures may cause decreased economic activity in China, and since 2012, the Chinese economy has slowed down. Any prolonged slowdown in the Chinese economy may reduce the demand for our products and services and materially and adversely affect our business and results of operations.
Uncertainties in the interpretation and enforcement of PRC laws and regulations could limit the legal protections available to us.
The PRC legal system is based on written statutes and prior court decisions have limited value as precedents. Since these laws and regulations are relatively new and the PRC legal system continues to rapidly evolve, the interpretations of many laws, regulations and rules are not always uniform and enforcement of these laws, regulations and rules involves uncertainties.
From time to time, we may have to resort to administrative and court proceedings to enforce our legal rights. However, since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of administrative and court proceedings and the level of legal protection we enjoy than in more developed legal systems. Furthermore, the PRC legal system is based in part on government policies and internal rules (some of which are not published in a timely manner or at all) that may have retroactive effect. As a result, we may not be aware of our violation of these policies and rules until sometime after the violation. Such uncertainties, including uncertainty over the scope and effect of our contractual, property (including intellectual property) and procedural rights, could materially and adversely affect our business and impede our ability to continue our operations.
We may be adversely affected by the complexity, uncertainties and changes in PRC regulation of pharmaceutical and healthcare industry and internet-related businesses, and any lack of requisite approvals, licenses or permits applicable to our business may have a material adverse effect on our business and results of operations.
Our business is subject to governmental supervision and regulation by the relevant PRC governmental authorities, including but not limited to the MOFCOM, the MIIT, the National Medical Products Administration, or the NMPA, the NHFPC and the SAMR and their counterparts. Together, these government authorities promulgate and enforce regulations that cover many aspects of the operation of pharmaceutical operation, medical and healthcare services and internet-related business, including foreign ownership of, and the licensing and permit requirements pertaining to, companies in such business. The laws and regulations related to medical and healthcare services and internet-related business are evolving rapidly, and their interpretation and enforcement involve significant uncertainties. As a result, in certain circumstances it may be difficult to determine what actions or omissions may be deemed to be in violation of applicable laws and regulations. Under PRC laws, an entity must obtain the pharmaceutical operation license from the NMPA or its counterpart for conducting pharmaceutical wholesale and retail business, the value-added telecommunication service operating licenses from the MIIT or its counterpart for either online information services or third-party e-commerce platform, and a medical institution shall obtain a practicing license of medical institutions from the NHFPC for provision of medical diagnosis and treatment services. We have made great efforts to obtain all applicable licenses and permits necessary to our main business. However, the interpretation and application of existing PRC laws, regulations and policies and possible new laws, regulations or policies relating to the pharmaceutical operation, medical and healthcare services and internet-related business have created substantial uncertainties regarding the legality of existing and future foreign investments in, and the businesses and activities of, pharmaceutical operation, medical and healthcare services and internet-related business industry in China, including our business, we cannot assure you that we have obtained all the permits or licenses required for conducting our business or will be able to maintain our existing licenses or obtain new ones. If the PRC government considers that we were operating without the proper approvals, licenses or permits or promulgates new laws and regulations that require additional approvals or licenses or imposes additional restrictions on the operation of any part of our business, it has the power, among other things, to levy fines, confiscate our income, revoke our business licenses, and require us to discontinue our relevant business or impose restrictions on the affected portion of our business. Any of these actions by the PRC government may have a material adverse effect on our business and results of operations.
We rely on dividends and other distributions on equity paid by our PRC subsidiaries to fund any cash and financing requirements we may have, and any limitation on the ability of our PRC subsidiaries to make payments to us could have a material adverse effect on our ability to conduct our business.
We are a holding company, and we rely on dividends and other distributions on equity paid by our PRC subsidiaries for our cash and financing requirements, including the funds necessary to pay dividends and other cash distributions to our shareholders and service any debt we may incur. If our PRC subsidiaries incur debt on their own behalf in the future, the instruments governing the debt may restrict their ability to pay dividends or make other distributions to us, which may restrict our ability to satisfy our liquidity requirements. In addition, since we have no direct equity interests in our variable interest entities and only collect the revenue under the contractual arrangement, the PRC tax authorities may require our PRC subsidiary, Yao Fang, to adjust its taxable income under the contractual arrangements it currently has in place with our variable interest entities and their subsidiaries, in a manner that would materially and adversely affect their ability to pay dividends and other distributions to us.
Under PRC laws and regulations, our PRC subsidiaries may pay dividends only out of their accumulated after-tax profits as determined in accordance with PRC accounting standards and regulations. In addition, Chinese domestic entities are required to set aside at least 10% of their accumulated after-tax profits each year, if any, to fund certain statutory reserve funds, until the aggregate amount of such funds reaches 50% of their registered capital. In addition, a wholly foreign-owned enterprise may allocate, at its discretion, a portion of its after-tax profits based on PRC accounting standards to staff welfare and bonus funds. These reserve funds and staff welfare and bonus funds are not distributable as cash dividends.
At the end of 2016, the People’s Bank of China, or PBOC, and the State Administration of Foreign Exchange, or SAFE, implemented a series of capital control measures, including stricter vetting procedures for China-based companies to remit foreign currency for overseas acquisitions, dividend payments and shareholder loan repayments. For instance, the PBOC issued the Circular on Further Clarification of Relevant Matters Relating to Offshore Renminbi Loans Provided by Domestic Enterprises, in November 2016, which provides that offshore Renminbi loans provided by a domestic enterprise to offshore enterprises that are its affiliates in equity shall not exceed a certain amount that is equal to the most recent audited owner’s equity multiplied by a ratio determined by the PBOC, and may constrain our PRC subsidiaries’ ability to provide offshore loans to us. The PRC government may continue to strengthen its capital controls and our PRC subsidiaries’ dividends and other distributions may be subjected to tighter scrutiny in the future. Any limitation on the ability of our PRC subsidiaries to pay dividends or make other distributions to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends, or otherwise fund and conduct our business. See also “—If we are classified as a PRC resident enterprise for PRC income tax purposes, such classification could result in unfavorable tax consequences to us and our non-PRC shareholders or ADS holders.”
PRC regulation of loans to and direct investment in PRC entities by offshore holding companies and governmental control of currency conversion may delay or prevent us from using the proceeds of our initial public offering to make loans to or make additional capital contributions to our PRC subsidiaries, which could materially and adversely affect our liquidity and our ability to fund and expand our business.
We are an offshore holding company conducting our operations in China through our PRC subsidiaries and variable interest entities. We may make loans to our PRC subsidiaries and variable interest entities subject to the approval from governmental authorities and limitation of amount, or we may make additional capital contributions to our wholly foreign-owned subsidiaries in China.
Any funds we transfer to our PRC subsidiaries, either as a shareholder loan or as an increase in registered capital, are subject to filing or registration with the relevant governmental authorities in China. According to the relevant PRC regulations on foreign-invested enterprises in China, capital contributions to our PRC subsidiaries are subject to the requirement of making necessary filings and registration with other governmental authorities in China. In addition, (a) any foreign loan procured by our PRC subsidiaries is required to be registered with SAFE, or its local branches, and (b) each of our PRC subsidiaries may not procure loans which exceed the statutory limit. Any medium or long-term loan to be provided by us to our variable interest entities must be recorded and registered by the NDRC and SAFE or its local branches. We may not complete such recording or registrations on a timely basis, if at all, with respect to future capital contributions or foreign loans by us to our PRC subsidiaries. If we fail to complete such recording or registration, our ability to use the proceeds of our initial public offering and to capitalize our PRC operations may be negatively affected, which could adversely affect our liquidity and our ability to fund and expand our business.
In 2008, SAFE promulgated the Notice of the General Affairs Department of the State Administration of Foreign Exchange on the Relevant Operating Issues concerning the Improvement of the Administration of Payment and Settlement of Foreign Currency Capital of Foreign-invested Enterprises, or Circular 142, which used to regulate the conversion by foreign-invested enterprises of foreign currency into Renminbi by restricting the usage of converted Renminbi. In March 2015, SAFE promulgated the Notice of the State Administration of Foreign Exchange on Reforming the Administrative Approach Regarding the Settlement of the Foreign Exchange Capitals of Foreign-invested Enterprises, or Circular 19. Circular 19 took effect as of June 1, 2015 and superseded Circular 142 on the same date. Circular 19 launched a nationwide reform of the administration of the settlement of the foreign exchange capitals of foreign-invested enterprises and allows foreign-invested enterprises to settle their foreign exchange capital at their discretion, but continues to prohibit foreign-invested enterprises from using the Renminbi fund converted from their foreign exchange capitals for expenditures beyond their business scopes. In June 2016, SAFE promulgated the Notice of the State Administration of Foreign Exchange on Reforming and Standardizing the Administrative Provisions on Capital Account Foreign Exchange Settlement, or Circular 16. Circular 16 continues to prohibit foreign-invested enterprises from, among other things, using the Renminbi fund converted from its foreign exchange capitals for expenditure beyond its business scope, investment and financing (except for guarantee products issued by a bank or otherwise permitted by laws), providing loans to non-affiliated enterprises or constructing or purchasing real estate not for self-use (except for real estate enterprise). Circular 19 and Circular 16 may significantly limit our ability to transfer to and use in China the net proceeds from our initial public offering, which may adversely affect our business, financial condition and results of operations.
Fluctuations in exchange rates could have a material adverse effect on our results of operations and the price of the ADSs.
The value of the Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions in China and by China’s foreign exchange policies. In July 2005, the PRC government changed its decade-old policy of pegging the value of the Renminbi to the U.S. dollar, and the Renminbi appreciated more than 20% against the U.S. dollar over the following three years. Between July 2008 and June 2010, this appreciation halted and the exchange rate between the Renminbi and the U.S. dollar remained within a narrow band. Since June 2010, Renminbi has fluctuated against the U.S. dollar, at certain times significantly and unpredictably. With the development of the foreign exchange market progressing towards interest rate liberalization and Renminbi internationalization and economic uncertainties in both China and the rest of the world, the PRC government may in the future announce further changes to the exchange rate system and we cannot assure you that the Renminbi will not appreciate or depreciate significantly in value against the U.S. dollar in the future. It is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between the Renminbi and the U.S. dollar in the future.
All of our revenue and substantially all of our costs are denominated in Renminbi and our reporting currency is Renminbi. Significant revaluation of the Renminbi may have a material and adverse effect on your investment. For example, to the extent that we need to convert U.S. dollars we received from our initial public offering into Renminbi for our operations, appreciation of the Renminbi against the U.S. dollar would reduce the Renminbi amount we would receive from the conversion. Conversely, if we decide to convert our Renminbi into U.S. dollars for the purpose of making payments for dividends on our ordinary shares or ADSs or for other business purposes, appreciation of the U.S. dollar against the Renminbi would reduce the U.S. dollar amount available to us.
Very limited hedging options are available in China to reduce our exposure to exchange rate fluctuations. To date, we have not entered into any hedging transactions in an effort to reduce our exposure to foreign currency exchange risk. While we may decide to enter into hedging transactions in the future, the availability and effectiveness of these hedges may be limited and we may not be able to hedge our exposure adequately or at all. In addition, our currency exchange losses may be magnified by PRC exchange control regulations that restrict our ability to convert Renminbi into foreign currency.
Governmental control of currency conversion may limit our ability to utilize our operating revenue effectively and affect the value of your investment.
The PRC government imposes controls on the convertibility of the Renminbi into foreign currencies and, in certain cases, the remittance of currency out of China. We receive substantially all of our operating revenue in Renminbi. Under our current corporate structure, our company in the Cayman Islands relies on dividend payments from our PRC subsidiaries to fund any cash and financing requirements we may have.
Under existing PRC foreign exchange regulations, payments of current account items, such as profit distributions and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior approval from SAFE by complying with certain procedural requirements. Therefore, our PRC subsidiary, Yao Fang, is able to pay dividends in foreign currencies to us without prior approval from SAFE, subject to the certain procedures under PRC foreign exchange regulation. But approval from or registration with appropriate government authorities is required where Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses under capital account items such as overseas investment and the repayment of loans denominated in foreign currencies.
In light of the substantial capital outflows of China in 2016 due to the weakening of Renminbi, the PRC government has imposed more restrictive foreign exchange policies and stepped up scrutiny of major outbound capital movement. More restrictions and substantial vetting processes have been put in place by SAFE to regulate cross-border transactions falling under the capital account. The PRC government may at its discretion further restrict access to foreign currencies in the future for current account transactions. If the foreign exchange control system prevents us from obtaining sufficient foreign currencies to satisfy our foreign currency demands, we may not be able to pay dividends in foreign currencies to our shareholders, including holders of our ADSs.
Failure to make adequate contributions to various employee benefit plans and withhold individual income tax on employees’ salaries as required by PRC regulations may subject us to penalties.
Companies operating in China are required to participate in various government-mandated employee benefit contribution plans, including certain social insurance, housing funds and other welfare-oriented payment obligations, and contribute to the plans in amounts equal to certain percentages of salaries, including bonuses and allowances, of our employees up to a maximum amount specified by the local government from time to time at locations where we operate our businesses. The requirement of employee benefit contribution plans has not been implemented consistently by the local governments in China given the different levels of economic development in different locations. Companies operating in China are also required to withhold individual income tax on employees’ salaries based on the actual salary of each employee upon payment. We may be subject to late fees and fines in relation to the underpaid employee benefits and under-withheld individual income tax, our financial condition and results of operations may be adversely affected.
The M&A Rules and certain other PRC regulations establish complex procedures for some acquisitions of Chinese companies by foreign investors, which could make it more difficult for us to pursue growth through acquisitions in China.
The Regulations on Mergers and Acquisitions of Domestic Companies by Foreign Investors, or the M&A Rules, adopted by six PRC regulatory agencies in 2006 and amended in 2009, and some other regulations and rules concerning mergers and acquisitions, established additional procedures and requirements that could make merger and acquisition activities by foreign investors more time-consuming and complex, including requirements in some instances that the MOFCOM be notified in advance of any change-of-control transaction in which a foreign investor takes control of a PRC domestic enterprise. Moreover, the Anti-Monopoly Law of the People’s Republic of China requires that the MOFCOM shall be notified in advance of any concentration of undertaking if certain thresholds are triggered. In addition, a regulation related to security review on mergers and acquisitions of domestic enterprise by foreign investors issued by the MOFCOM that became effective in September 2011 specifies that mergers and acquisitions by foreign investors that raise “national defense and security” concerns and mergers and acquisitions through which foreign investors may acquire de facto control over domestic enterprises that raise “national security” concerns are subject to strict review by the MOFCOM, and the rules prohibit any activities attempting to bypass a security review, including by structuring the transaction through a proxy or contractual control arrangement. In the future, we may grow our business by acquiring complementary businesses. Complying with the requirements of the above-mentioned regulations and other relevant rules to complete such transactions could be time-consuming, and any required approval processes, including obtaining approval from the MOFCOM or its local counterparts may delay or inhibit our ability to complete such transactions, which could affect our ability to expand our business or maintain our market share.
PRC regulations relating to offshore investment activities by PRC residents may limit our PRC subsidiaries’ ability to increase their registered capital or distribute profits to us or otherwise expose us or our PRC resident beneficial owners to liability and penalties under PRC law.
In October 2005, SAFE issued a circular on relevant issues relating to foreign exchange administration in fund financing and roundtrip investment by domestic residents via offshore special purpose vehicles, or Circular 75, requiring PRC residents, including individual and entities, to register with the relevant local branch of SAFE before establishing or controlling any company outside of China, referred to as an offshore special purpose company, for the purpose of raising funds from overseas to acquire or exchange assets of, or acquiring equity interest in, PRC companies held by such PRC residents.
In July 2014, the SAFE issued a circular on foreign exchange administration involved in overseas investment, financing and roundtrip investment conducted by PRC residents via offshore special purpose vehicles, or Circular 37, which replaced Circular 75 and further requires PRC residents or entities to register with SAFE or its local branch in connection with their establishment or control of an offshore entity established for the purpose of overseas investment or financing by either onshore or offshore assets or equity legally held by such PRC residents. In February 2015, SAFE released the Notice of the State Administration of Foreign Exchange on Further Simplifying and Improving the Policies of Foreign Exchange Administration Applicable to Direct Investment, or Circular 13, which further clarified that offshore individuals who have foreign identification and use their offshore assets or equity to make contributions into an offshore special purpose vehicle are not subject to the registration under Circular 37.
If our shareholders who are PRC residents or entities do not complete their registration as required, our PRC subsidiary, Yao Fang, may be prohibited from distributing their profits and proceeds from any reduction in capital, share transfer or liquidation to us, and we may be restricted in our ability to contribute additional capital to our PRC subsidiaries. Moreover, failure to comply with the SAFE registration described above could result in liability under PRC laws for evasion of applicable foreign exchange restrictions.
Since Dr. Gang Yu and Mr. Junling Liu are non-PRC citizens with foreign identification who establish and make contributions to our Cayman Islands holding company by their offshore assets, they are not subject to the foreign exchange registrations for their offshore investment, financing and roundtrip investment in accordance with Circular 75 then in effect and Circular 37.
However, we may not be informed of the identities of all PRC residents holding direct or indirect interest in our company, nor can we compel our beneficial owners to comply with the requirements of Circular 37. As a result, we cannot assure you that all of our shareholders or beneficial owners who are PRC residents or entities have complied with, and will in the future make or obtain any applicable registrations or approvals required by, Circular 37 or other PRC applicable law and regulations related to outbound investment. Failure by such shareholders or beneficial owners to comply with Circular 37, or failure by us to amend the foreign exchange registrations of our PRC subsidiaries, could subject us to fines or legal sanctions, restrict our overseas or cross-border investment activities and limit our PRC subsidiaries’ ability to make distributions or pay dividends to us or affect our ownership structure, which could adversely affect our business and prospects.
Any failure to comply with PRC regulations regarding the registration requirements for employee stock incentive plans may subject the PRC plan participants or us to fines and other legal or administrative sanctions.
Pursuant to the Notices on Issues Concerning the Foreign Exchange Administration for Domestic Individuals Participating in Stock Incentive Plan of Overseas Publicly-Listed Company, promulgated by SAFE in 2012, or SAFE Notices No. 7, PRC citizens and non-PRC citizens who reside in China for a continuous period of no less than one year who participate in any stock incentive plan of an overseas publicly listed company offered to the director, supervisor, senior management and other employees of, and any individual who has labor relationship with its domestic affiliated entities are required to register with SAFE through a domestic qualified agent, which could be a PRC subsidiary of such overseas listed company, and complete certain other procedures. In addition, an overseas entrusted institution must be retained to handle matters in connection with the exercise or sale of stock options and the purchase or sale of shares and interests. We and our directors, executive officers and other employees who are PRC citizens or who reside in the PRC for a continuous period of no less than one year and who have been granted stock options are subject to these regulations. Failure to complete the SAFE registrations for our employee incentive plans may subject them to fines and legal sanctions, and may also limit our ability to contribute additional capital into our PRC subsidiaries and limit our PRC subsidiaries’ ability to distribute dividends to us. We also face regulatory uncertainties that could restrict our ability to adopt additional incentive plans for our directors, executive officers and employees under PRC law.
In addition, the State Administration of Taxation, or SAT, has issued certain circulars concerning employee stock options and restricted shares. Under these circulars, our employees working in China who exercise stock options or are granted restricted shares will be subject to PRC individual income tax. Our PRC subsidiaries have obligations to file documents related to employee stock options or restricted shares with relevant tax authorities and to withhold individual income taxes of those employees who exercise their share options or are granted with restricted shares. If our employees fail to pay or we fail to withhold their income taxes according to relevant laws and regulations, we may face sanctions imposed by the tax authorities or other PRC governmental authorities.
U.S. regulatory bodies may be limited in their ability to conduct investigations or inspections of our operations in China.
Any disclosure of documents or information located in China by foreign agencies may be subject to jurisdiction constraints and must comply with China’s state secrecy laws, which broadly define the scope of “state secrets” to include matters involving economic interests and technologies. There is no guarantee that requests from U.S. federal or state regulators or agencies to investigate or inspect our operations will be honored by us, by entities who provide services to us or with whom we associate, without violating PRC legal requirements, especially as those entities are located in China. Furthermore, under the current PRC laws, an on-site inspection of our facilities by any of these regulators may be limited or prohibited.
If we are classified as a PRC resident enterprise for PRC income tax purposes, such classification could result in unfavorable tax consequences to us and our non-PRC shareholders or ADS holders.
Under the Enterprise Income Tax Law of the PRC, or the EIT Law, and its implementation rules, an enterprise established outside of the PRC with “de facto management body” within the PRC is considered a resident enterprise and will be subject to the enterprise income tax on its global income at the rate of 25%. The implementation rules define the term “de facto management body” as the body that exercises full and substantial control and overall management over the business, productions, personnel, accounts and properties of an enterprise. On April 22, 2009, the SAT issued a circular, known as Circular 82, which provides certain specific criteria for determining whether the “de facto management body” of a PRC-controlled enterprise that is incorporated offshore is located in China. According to Circular 82, an offshore incorporated enterprise controlled by a PRC enterprise or a PRC enterprise group will be regarded as a PRC tax resident by virtue of having its “de facto management body” in China and will be subject to PRC enterprise income tax on its global income only if all of the following conditions are met: (i) the primary location of the day-to-day operational management is in the PRC; (ii) decisions relating to the enterprise’s financial and human resource matters are made or are subject to approval by organizations or personnel in the PRC; (iii) the enterprise’s primary assets, accounting books and records, company seals, and board and shareholder resolutions, are located or maintained in the PRC; and (iv) at least 50% of voting board members or senior executives habitually reside in the PRC.
Although this circular only applies to offshore enterprises controlled by PRC enterprises or PRC enterprise groups, not those controlled by PRC individuals or foreigners, the criteria set forth in the circular may reflect the SAT’s general position on how the “de facto management body” text should be applied in determining the tax resident status of all offshore enterprises. If the PRC tax authorities determine that we should be classified as a PRC resident enterprise for PRC tax purposes, our global income will be subject to income tax at a uniform rate of 25%, which may have a material adverse effect on our financial condition and results of operations. Notwithstanding the foregoing provision, the EIT Law also provides that, if a PRC resident enterprise directly invests in another PRC resident enterprise, the dividends received by the investing PRC resident enterprise from the invested PRC resident enterprise are exempted from income tax, subject to certain conditions. However, it remains unclear how the PRC tax authorities will interpret the PRC tax resident treatment of an offshore company with indirect ownership interests in PRC resident enterprises through intermediary holding companies.
Moreover, if the PRC tax authorities determine that our company is a PRC resident enterprise for PRC enterprise income tax purposes, gains realized on the sale or other disposal of ADSs or ordinary shares may be subject to PRC tax, at a rate of 10% in the case of non-PRC enterprises, or 20% in the case of non-PRC individuals (in each case, subject to the provisions of any applicable tax treaty), if such gains are deemed to be from PRC sources. Any such tax may reduce the returns on your investment in the ADSs.
We may not be able to obtain certain benefits under the relevant tax treaty on dividends paid by our PRC subsidiaries to us through our Hong Kong subsidiary.
Pursuant to the EIT Law and its implementation rules, if a non-resident enterprise has not set up an organization or establishment in the PRC, or has set up an organization or establishment but the income derived has no actual connection with such organization or establishment, it will be subject to a withholding tax on its PRC-sourced income at a rate of 10%. Pursuant to the Arrangement between Mainland China and the Hong Kong Special Administrative Region for the Avoidance of Double Taxation and Tax Evasion on Income, or the Double Taxation Arrangement, the withholding tax rate in respect to the payment of dividends by a PRC enterprise to a Hong Kong enterprise is reduced to 5% from a standard rate of 10% if the Hong Kong enterprise directly holds at least 25% of the PRC enterprise. There are also other conditions for enjoying the reduced withholding tax rate according to other relevant tax rules and regulations.
In February 2009, SAT issued SAT Notice No. 81, pursuant to which an enterprise must be the “beneficial owner” of the relevant dividend income in order to enjoy the preferential withholding tax rates on dividend. If, however, such enterprise otherwise qualifies for such preferential withholding tax rates through any transaction or arrangement, whose main purpose is to qualify for such preferential withholding tax rates, the enterprise nevertheless cannot enjoy the preferential withholding tax rates and the competent tax authority has the power to adjust the applicable withholding tax rates if it so determines. A SAT Notice No. 9 issued by SAT that took effect in April 2018 indicated that “beneficial owner” refers to a person who has ownership and disposal rights to the income or any rights and assets arising from such income, and the tax authority has discretion to determine whether or not an enterprise is determined as a “beneficial owner.” However, since the SAT Notice No. 9 is newly issued, it remains unclear how the PRC tax authorities will implement SAT Notice No. 9 in practice and to what extent they will affect the dividend withholding tax rates for dividends distributed by our PRC subsidiaries to our Hong Kong subsidiary. If the relevant tax authority determines that our Hong Kong subsidiary is a conduit company and does not qualify as the “beneficial owner” of the dividend income it receives from our PRC subsidiaries, the higher 10% withholding tax rate will apply to such dividends.
Therefore, we cannot assure you that our determination regarding our qualification to enjoy the preferential tax treatment will not be challenged by the relevant PRC tax authority or we will be able to complete the necessary filings with the relevant PRC tax authority and enjoy the preferential withholding tax rate of 5% under the Double Taxation Arrangement with respect to dividends to be paid by our PRC subsidiaries to Yao Wang Corporation Limited, our Hong Kong subsidiary.
We face uncertainty with respect to indirect transfers of equity interests in PRC resident enterprises by their non-PRC holding companies.
We face uncertainties regarding the reporting on and consequences of previous private equity financing transactions involving the transfer and exchange of shares in our company by non-PRC resident investors.
According to the Notice on Strengthening Administration of Enterprise Income Tax for Share Transfers by Non-PRC Resident Enterprises issued by SAT on December 10, 2009, or SAT Circular 698, where a non-PRC resident enterprise transfers the equity interests in a PRC resident enterprise indirectly through a disposition of equity interests in an offshore holding company (other than the sale on a public stock market of shares of an offshore enterprise purchased on a public stock market), or an Indirect Transfer, the non-PRC resident enterprise, as the seller, may be subject to PRC enterprise income tax of up to 10% of the gains derived from the Indirect Transfer in certain circumstances.
On February 3, 2015, the SAT issued the Announcement on Several Issues Concerning the Enterprise Income Tax on Indirect Property Transfers by Non-PRC Resident Enterprises, or SAT Notice No. 7, to supersede the existing tax rules in relation to the tax treatment of the Indirect Transfer, while the other provisions of SAT Circular 698 are irrelevant to the Indirect Transfer remain in force. SAT Notice No. 7 introduces a new tax regime that is significantly different from that under a notice issued by SAT Circular 698. It extends SAT’s tax jurisdiction to capture not only the Indirect Transfer as set forth under SAT Circular 698 but also transactions involving indirect transfer of (i) real properties in China and (ii) assets of an “establishment or place” situated in China, by a non-PRC resident enterprise through a disposition of equity interests in an offshore holding company. SAT Notice No. 7 also extends the interpretation with respect to the disposition of equity interests in an offshore holding company broadly. In addition, SAT Notice No. 7 further clarifies how to assess reasonable commercial purposes and introduces safe harbors applicable to internal group restructurings. However, it also brings challenges to both offshore transferor and transferee as they are required to make self-assessments on whether an Indirect Transfer or similar transaction should be subject to PRC tax and whether they should file or withhold any tax payment accordingly. On October 17, 2017, the SAT issued a Notice Concerning Withholding Income Tax of Non-Resident Enterprise, or SAT Notice No. 37, which abolished SAT Circular 698 and certain provisions of SAT Notice No. 7 and SAT Notice No. 37 further reduced the burden of the withholding obligator, such as revocation of contract filing requirements and tax liquidation procedures, strengthened the cooperation of tax authorities in different places, and clarified the calculation of tax payable and mechanism of foreign exchange.
There is uncertainty as to the application of SAT Notice No. 7 and SAT Notice No. 37. In the event that non-PRC resident investors were involved in our private equity financing transactions and such transactions were determined by the competent tax authorities as lacking reasonable commercial purposes, we and our non-PRC resident investors may become at risk of being taxed under and SAT Notice No. 7 and SAT Notice No. 37 and may be required to expend costly resources to comply with SAT Notice No. 7 and SAT Notice No. 37, or to establish a case to be tax exempt under SAT Notice No. 7 and SAT Notice No. 37, which may cause us to incur additional costs and may have a negative impact on the value of your investment in us.
The PRC tax authorities have discretion under SAT Notice No. 7 and SAT Notice No. 37 to adjust the taxable capital gains based on the difference between the fair value of the transferred equity interests and the investment cost. We may pursue acquisitions in the future that may involve complex corporate structures. If we are deemed as a non-PRC resident enterprise under the EIT Law and if the PRC tax authorities adjust the taxable income of the transactions under SAT Notice No. 7 and SAT Notice No. 37, our income tax expenses associated with such potential acquisitions will increase, which may have an adverse effect on our financial condition and results of operations.
The audit report included in this annual report is prepared by an auditor who is not inspected by the PCAOB and, as such, our investors are deprived of the benefits of such inspection.
Our independent registered public accounting firm that issues the audit report included in our annual report filed with the U.S. Securities and Exchange Commission, or the SEC, as auditors of companies that are traded publicly in the United States and a firm registered with the PCAOB, is required by the laws of the United States to undergo regular inspections by the PCAOB to assess its compliance with the laws of the United States and professional standards. Because our auditors are located in the PRC, a jurisdiction where the PCAOB is currently unable to conduct inspections without the approval of the Chinese authorities, our auditors are not currently inspected by the PCAOB. On December 7, 2018, the SEC and the PCAOB issued a joint statement highlighting continued challenges faced by the U.S. regulators in their oversight of financial statement audits of U.S.-listed companies with significant operations in China. The joint statement reflects a heightened interest in an issue that has vexed U.S. regulators in recent years. However, it remains unclear what further actions the SEC and PCAOB will take to address the problem.
Inspections of other firms that the PCAOB has conducted outside China have identified deficiencies in those firms’ audit procedures and quality control procedures, which may be addressed as part of the inspection process to improve future audit quality. This lack of PCAOB inspections in China prevents the PCAOB from regularly evaluating our auditor’s audits and its quality control procedures. As a result, investors may be deprived of the benefits of PCAOB inspections.
The inability of the PCAOB to conduct inspections of auditors in China makes it more difficult to evaluate the effectiveness of our auditor’s audit procedures or quality control procedures as compared to auditors outside of China that are subject to PCAOB inspections. Investors may lose confidence in our reported financial information and procedures and the quality of our financial statements.
Proceedings instituted by the SEC against the “big four” PRC-based accounting firms, including our independent registered public accounting firm, could result in financial statements being determined to not be in compliance with the requirements of the Exchange Act.
In late 2012, the SEC commenced administrative proceedings under Rule 102(e) of its Rules of Practice and also under the Sarbanes-Oxley Act against the Chinese affiliates of the “big four” accounting firms (including our auditors). The Rule 102(e) proceedings initiated by the SEC relate to these firms’ inability to produce documents, including audit work papers, in response to the request of the SEC pursuant to Section 106 of the Sarbanes-Oxley Act, as the auditors located in the PRC are not in a position lawfully to produce documents directly to the SEC because of restrictions under PRC law and specific directives issued by the China Securities Regulatory Commission, or the CSRC. The issues raised by the proceedings are not specific to our auditors or to us, but affect equally all audit firms based in China and all China-based businesses with securities listed in the United States.
In January 2014, the administrative judge reached an Initial Decision that the “big four” accounting firms should be barred from practicing before the SEC for six months. Thereafter, the accounting firms filed a Petition for Review of the Initial Decision, prompting the SEC Commissioners to review the Initial Decision, determine whether there had been any violation and, if so, determine the appropriate remedy to be placed on these audit firms.
In February 2015, the Chinese affiliates of the “big four” accounting firms (including our auditors) each agreed to censure and pay a fine to the SEC to settle the dispute and avoid suspension of their ability to practice before the SEC and audit U.S. listed companies. The settlement requires the firms to follow detailed procedures and to seek to provide the SEC with access to the Chinese firms’ audit documents via the CSRC. If future document productions fail to meet the specified criteria, the SEC retains the authority to impose a variety of additional measures (e.g., imposing penalties such as suspensions, restarting the administrative proceedings).
In the event that the SEC restarts the administrative proceedings, depending upon the final outcome, listed companies in the United States with major PRC operations may find it difficult or impossible to retain auditors in respect of their operations in the PRC, which could result in financial statements being determined to not be in compliance with the requirements of the Exchange Act, and could result in delisting. Moreover, any negative news about the proceedings against these audit firms may cause investor uncertainty regarding China-based, United States-listed companies and the market price of our shares or ADSs may be adversely affected. If our independent registered public accounting firm was denied, temporarily, the ability to practice before the SEC and we were unable to timely find another registered public accounting firm to audit and issue an opinion on our financial statements, our financial statements could be determined to not be in compliance with the requirements of the Exchange Act.
Risks Related to American Depositary Shares
The market price for the ADSs may be volatile.
Since the ADSs became listed on Nasdaq on September 12, 2018, the trading price of the ADSs has ranged from US$5.60 to US$16.83. The trading prices of the ADSs are likely to be volatile and could fluctuate widely due to factors beyond our control. This may happen because of broad market and industry factors, like the performance and fluctuation in the market prices or the underperformance or deteriorating financial results of internet or other companies based in China that have listed their securities in the United States in recent years. The securities of some of these companies have experienced significant volatility since their initial public offerings, including, in some cases, substantial price declines in their trading prices. The trading performances of other Chinese companies’ securities after their offerings, including internet and e-commerce companies, may affect the attitudes of investors toward Chinese companies listed in the United States, which consequently may impact the trading performance of the ADSs, regardless of our actual operating performance. In addition, any negative news or perceptions about inadequate corporate governance practices or fraudulent accounting, corporate structure or other matters of other Chinese companies may also negatively affect the attitudes of investors towards Chinese companies in general, including us, regardless of whether we have conducted any inappropriate activities. In addition, securities markets may from time to time experience significant price and volume fluctuations that are not related to our operating performance, such as the large decline in share prices in the United States, China and other jurisdictions in late 2008, early 2009 and the second half of 2011, which may have a material adverse effect on the market price of the ADSs.
In addition to the above factors, the price and trading volume of the ADSs may be highly volatile due to multiple factors, including the following:
· regulatory developments affecting us, our consumers or our industry;
· conditions in the online healthcare industry and the public perception of the legitimacy and ethics of certain business practices of our competitors or other market players within the industry;
· announcements of studies and reports relating to the quality of our product and service offerings or those of our competitors;
· changes in the economic performance or market valuations of other online healthcare platforms;
· actual or anticipated fluctuations in our quarterly results of operations and changes or revisions of our expected results;
· changes in financial estimates by securities research analysts;
· announcements by us or our competitors of new product and service offerings, acquisitions, strategic relationships, joint ventures or capital commitments;
· additions to or departures of our senior management;
· detrimental negative publicity about us, our management or our industry;
· fluctuations of exchange rates between the Renminbi and the U.S. dollar;
· release or expiry of any transfer restrictions on our outstanding ordinary shares or the ADSs; and
· sales or perceived potential sales of additional ordinary shares or ADSs.
The trading market for the ADSs will depend in part on the research and reports that securities or industry analysts publish about us or our business. If research analysts do not establish and maintain adequate research coverage or if one or more of the analysts who cover us downgrade the ADSs or publish inaccurate or unfavorable research about our business, the market price for our ADSs would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, we could lose visibility in the financial markets, which, in turn, could cause the market price or trading volume for the ADSs to decline.
Our dual-class share structure with different voting rights will limit your ability to influence corporate matters and could discourage others from pursuing any change of control transactions that holders of our Class A ordinary shares and ADSs may view as beneficial.
We have a dual-class share structure such that our ordinary shares consists of Class A ordinary shares and Class B ordinary shares. In respect of matters requiring the votes of shareholders, holders of Class A ordinary shares will be entitled to one vote per share, while holders of Class B ordinary shares will be entitled to fifteen votes per share based on our proposed dual-class share structure. Each Class B ordinary share is convertible into one Class A ordinary share at any time by the holder thereof, while Class A ordinary shares are not convertible into Class B ordinary shares under any circumstances. Upon any sale, transfer, assignment or disposition of any Class B ordinary share by our Founders (defined in our memorandum and articles of association to mean Dr. Gang Yu and Mr. Junling Liu) or Founder Affiliate (as defined in our memorandum and articles of association) to any person who is not a “Founder Affiliate,” or upon a change of ultimate beneficial ownership of any Class B ordinary share to any person who is not a Founder Affiliate, such Class B ordinary share shall be automatically and immediately converted into one Class A ordinary share.
As of March 31, 2019, our founders, Dr. Gang Yu and Mr. Junling Liu, beneficially own all of our issued and outstanding Class B ordinary shares. These Class B ordinary shares constitute approximately 44.1% of our total issued and outstanding share capital and 92.2% of the aggregate voting power of our total issued and outstanding share capital due to the disparate voting powers associated with our dual-class share structure. See “Item 6. Directors, Senior Management and Employees—E. Share Ownership.” As a result of the dual-class share structure and the concentration of ownership, holders of Class B ordinary shares will have considerable influence over matters such as decisions regarding mergers, consolidations and the sale of all or substantially all of our assets, election of directors and other significant corporate actions. Such holders may take actions that are not in the best interest of us or our other shareholders. This concentration of ownership may discourage, delay or prevent a change in control of our company, which could have the effect of depriving our other shareholders of the opportunity to receive a premium for their shares as part of a sale of our company and may reduce the price of our ADSs. This concentrated control will limit your ability to influence corporate matters and could discourage others from pursuing any potential merger, takeover or other change of control transactions that holders of Class A ordinary shares and ADSs may view as beneficial.
The dual-class structure of our ordinary shares may adversely affect the trading market for our ADSs.
S&P Dow Jones and FTSE Russell have recently announced changes to their eligibility criteria for inclusion of shares of public companies on certain indices, including the S&P 500, to exclude companies with multiple classes of shares and companies whose public shareholders hold no more than 5% of total voting power from being added to such indices. In addition, several shareholder advisory firms have announced their opposition to the use of multiple class structures. As a result, the dual-class structure of our ordinary shares may prevent the inclusion of our ADSs representing Class A ordinary shares in such indices and may cause shareholder advisory firms to publish negative commentary about our corporate governance practices or otherwise seek to cause us to change our capital structure. Any such exclusion from indices could result in a less active trading market for our ADSs. Any actions or publications by shareholder advisory firms critical of our corporate governance practices or capital structure could also adversely affect the value of our ADSs.
Because we do not expect to pay dividends in the foreseeable future, you must rely on price appreciation of the ADSs for return on your investment.
We currently intend to retain most, if not all, of our available funds and any future earnings to fund the development and growth of our business. As a result, we do not expect to pay any cash dividends in the foreseeable future. Therefore, you should not rely on an investment in the ADSs as a source for any future dividend income.
Our board of directors has discretion as to whether to distribute dividends, subject to certain restrictions under Cayman Islands law, namely that our company may only pay dividends out of profits or share premium account; provided that in no circumstances may a dividend be paid if this would result in our company being unable to pay its debts as they fall due in the ordinary course of business. In addition, our shareholders may by ordinary resolution declare a dividend, but no dividend may exceed the amount recommended by our board of directors. Even if our board of directors decides to declare and pay dividends, the timing, amount and form of future dividends, if any, will depend on, among other things, our future results of operations and cash flow, our capital requirements and surplus, the amount of distributions, if any, received by us from our subsidiaries, our financial condition, contractual restrictions and other factors deemed relevant by our board of directors. Accordingly, the return on your investment in our ADSs will likely depend entirely upon any future price appreciation of our ADSs. There is no guarantee that our ADSs will appreciate in value in the future or even maintain the price at which you purchased the ADSs. You may not realize a return on your investment in our ADSs and you may even lose your entire investment in our ADSs.
Substantial future sales or perceived potential sales of ADSs in the public market could cause the price of the ADSs to decline.
Sales of ADSs in the public market, or the perception that these sales could occur, could cause the market price of the ADSs to decline. As of March 31, 2019, we have 163,317,328 ordinary shares outstanding, including 91,317,328 Class A ordinary shares represented by ADSs. All of our ADSs will be freely transferable without restriction or additional registration under the Securities Act of 1933, as amended, or the Securities Act. The remaining Class A ordinary shares will be available for sale subject to volume and other restrictions as applicable under Rules 144 and 701 under the Securities Act.
Certain holders of our Class A ordinary shares may cause us to register, under the Securities Act, the sale of their shares. Registration of these shares under the Securities Act would result in ADSs representing these shares becoming freely tradable without restriction under the Securities Act immediately upon the effectiveness of the registration. Sales of ADSs representing these registered shares in the public market could cause the price of the ADSs to decline.
The voting rights of holders of ADSs are limited by the terms of the deposit agreement, and you may not be able to exercise your right to direct the voting of the underlying Class A ordinary shares which are represented by your ADSs.
As a Cayman Islands exempted company, we are not obliged by the Companies Law to call shareholders’ annual general meetings. As a holder of ADSs, you will not have any direct right to attend general meetings of our shareholders or to cast any votes at such meetings. You will only be able to exercise the voting rights that attach to the underlying Class A ordinary shares represented by your ADSs indirectly by giving voting instructions to the depositary in accordance with the provisions of the deposit agreement. Under the deposit agreement, you may vote only by giving voting instructions to the depositary, as the holder of the underlying Class A ordinary shares that are represented by your ADSs. If we ask the depositary to solicit your instructions, upon receipt of your voting instructions, the depositary will endeavor to vote the underlying Class A ordinary shares in accordance with your instructions. If we do not instruct the depositary to solicit, you can still send voting instructions to the depositary, and the depositary may, but is not required, to endeavor to carry out those instructions. You will not be able to directly exercise any right to vote with respect to the underlying Class A ordinary shares unless you withdraw the shares and become the registered holder of such shares prior to the record date for the general meeting. Under our memorandum and articles of association, the minimum notice period required to be given by our company to our registered shareholders for convening a general meeting is ten calendar days. When a general meeting is convened, you may not receive sufficient advance notice to enable you to withdraw the underlying shares which are represented by your ADSs and become the registered holder of such shares prior to the record date for the general meeting to allow you to attend the general meeting or to vote directly with respect to any specific matter or resolution which is to be considered and voted upon at the general meeting. In addition, under our memorandum and articles of association, for the purposes of determining those shareholders who are entitled to attend and vote at any general meeting, our directors may close our register of members and/or fix in advance a record date for such meeting, and such closure of our register of members or the setting of such a record date may prevent you from withdrawing the underlying shares which are represented by your ADSs and becoming the registered holder of such shares prior to the record date, so that you would not be able to attend the general meeting or to vote directly. Where any matter is to be put to a vote at a general meeting, the depositary will endeavor to notify you of the upcoming vote and to deliver our voting materials to you if we ask it to. We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote the underlying shares which are represented by your ADSs. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for their manner of carrying out your voting instructions. This means that you may not be able to exercise your right to direct the voting of the underlying shares that are represented by your ADSs, and you may have no legal remedy if the underlying shares are not voted as you requested.
The depositary for our ADSs may give us a discretionary proxy to vote the Class A ordinary shares underlying your ADSs if you do not give voting instructions, which could adversely affect your interests.
Under the deposit agreement for the ADSs, if (i) we timely instruct the depositary to solicit your voting instructions, the depositary does not receive your instructions by the specified date and (ii) we confirm to the depositary that:
· we wish a discretionary proxy to be given;
· we reasonably believe there is no substantial opposition as to a matter to be voted on at the meeting; and
· a matter to be voted on at the meeting would not have a material adverse impact on shareholders,
then the depositary will give us a proxy to vote the shares represented by your ADSs. The effect of this discretionary proxy is that, if you fail to give voting instructions to the depositary as to how to vote the Class A ordinary shares underlying your ADSs at any particular shareholders’ meeting, you cannot prevent our ordinary shares underlying your ADSs from being voted at that meeting, and it may make it more difficult for shareholders to influence our management. Holders of our Class A ordinary shares are not subject to this discretionary proxy.
The deposit agreement may be amended or terminated without your consent.
We and the depositary may amend the deposit agreement, and we may initiate termination of it, without your consent. If you continue to hold your ADSs after an amendment to the deposit agreement, you agree to be bound by the deposit agreement as amended. See “Item 12. Description of Securities Other Than Equity Securities—Description of American Depositary Shares” for more information.
Your right to participate in any future rights offerings may be limited, which may cause dilution of your holdings.
We may from time to time distribute rights to our shareholders, including rights to acquire our securities. However, we cannot make such rights available to you in the United States unless we register the securities to which the rights relate under the Securities Act or an exemption from the registration requirements is available. Under the deposit agreement, the depositary will not make rights available to you unless the underlying securities to be distributed to ADS holders are either registered under the Securities Act or exempt from registration under the Securities Act. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause such a registration statement to be declared effective and we may not be able to establish a necessary exemption from registration under the Securities Act. Accordingly, you may be unable to participate in our rights offerings in the future and may experience dilution in your holdings.
You may not receive dividends or other distributions on our Class A ordinary shares and you may not receive any value for them, if it is illegal or impractical to make them available to you.
The depositary has agreed to pay to you the cash dividends or other distributions it or the custodian receives on our Class A ordinary shares or other deposited securities underlying our ADSs, after deducting its fees and expenses. You will receive these distributions in proportion to the number of Class A ordinary shares your ADSs represent. However, the depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any holders of ADSs. For example, it would be unlawful to make a distribution to a holder of ADSs if it consists of securities that require registration under the Securities Act but that are not properly registered or distributed under an applicable exemption from registration. The depositary may also determine that it is not feasible to distribute certain property through the mail. Additionally, the value of certain distributions may be less than the cost of mailing them. In these cases, the depositary may determine not to distribute such property. We have no obligation to register under U.S. securities laws any ADSs, ordinary shares, rights or other securities received through such distributions. We also have no obligation to take any other action to permit the distribution of ADSs, ordinary shares, rights or anything else to holders of ADSs. This means that you may not receive distributions we make on our Class A ordinary shares or any value for them if it is illegal or impractical for us to make them available to you. These restrictions may cause a material decline in the value of the ADSs.
Certain judgments obtained against us by our shareholders may not be enforceable.
We are an exempted company limited by shares incorporated under the laws of the Cayman Islands. We conduct substantially all of our operations in China and substantially all of our assets are located in China. In addition, a majority of our directors and executive officers reside within China, and most of the assets of these persons are located within China. As a result, it may be difficult or impossible for you to effect service of process within the United States upon these individuals, or to bring an action against us or against these individuals in the United States in the event that you believe your rights have been infringed under the U.S. federal securities laws or otherwise. Even if you are successful in bringing an action of this kind, the laws of the Cayman Islands and of the PRC may render you unable to enforce a judgment against our assets or the assets of our directors and officers. However, the deposit agreement gives you the right to submit claims against us to binding arbitration, and arbitration awards may be enforceable against us and our assets in China even when court judgments are not.
You may face difficulties in protecting your interests, and your ability to protect your rights through U.S. courts may be limited, because we are incorporated under Cayman Islands law.
We are an exempted company limited by shares incorporated under the laws of the Cayman Islands. Our corporate affairs are governed by our memorandum and articles of association, the Companies Law (2018 Revision) of the Cayman Islands and the common law of the Cayman Islands. The rights of shareholders to take action against our directors, actions by our minority shareholders and the fiduciary duties of our directors to us under Cayman Islands law are to a large extent governed by the common law of the Cayman Islands. The common law of the Cayman Islands is derived in part from comparatively limited judicial precedent in the Cayman Islands as well as from the common law of England, the decisions of whose courts are of persuasive authority, but are not binding, on a court in the Cayman Islands. The rights of our shareholders and the fiduciary duties of our directors under Cayman Islands law are not as clearly established as they would be under statutes or judicial precedent in some jurisdictions in the United States. In particular, the Cayman Islands has a less developed body of securities laws than the United States. Some U.S. states, such as Delaware, have more fully developed and judicially interpreted bodies of corporate law than the Cayman Islands. In addition, Cayman Islands companies may not have standing to initiate a shareholder derivative action in a federal court of the United States.
Shareholders of Cayman Islands exempted companies like us have no general rights under Cayman Islands law to inspect corporate records or to obtain copies of lists of shareholders of these companies. Our directors will have discretion under our memorandum and articles of association to determine whether or not, and under what conditions, our corporate records may be inspected by our shareholders, but are not obliged to make them available to our shareholders. This may make it more difficult for you to obtain the information needed to establish any facts necessary for a shareholder resolution or to solicit proxies from other shareholders in connection with a proxy contest.
As a result of all of the above, our public shareholders may have more difficulty in protecting their interests in the face of actions taken by our management, members of the board of directors or controlling shareholders than they would as public shareholders of a company incorporated in the United States.
We may need additional capital and may sell additional ADSs or other equity securities or incur indebtedness, which could result in additional dilution to our shareholders or increase our debt service obligations.
We may require additional cash resources due to changed business conditions or other future developments, including any investments or acquisitions we may decide to pursue. If our cash resources are insufficient to satisfy our cash requirements, we may seek to sell additional equity or debt securities or obtain a credit facility. The sale of additional equity securities or equity-linked debt securities could result in additional dilution to our shareholders. The incurrence of indebtedness would result in debt service obligations and could result in operating and financing covenants that would restrict our operations. We cannot assure you that financing will be available in amounts or terms acceptable to us, if at all.
Our memorandum and articles of association contains anti-takeover provisions that could discourage a third party from acquiring us and adversely affect the rights of holders of our Class A ordinary shares and the ADSs.
Our memorandum and articles of association contains certain provisions that could limit the ability of others to acquire control of our company, including a provision that grants authority to our board of directors to establish and issue from time to time one or more series of preferred shares without action by our shareholders and to determine, with respect to any series of preferred shares, the terms and rights of that series. These provisions could have the effect of depriving our shareholders and holders of the ADSs of the opportunity to sell their shares or ADSs at a premium over the prevailing market price by discouraging third parties from seeking to obtain control of our company in a tender offer or similar transactions. In addition, our dual-class structure could discourage others from pursuing any change of control transactions. See “—Our dual-class share structure with different voting rights will limit your ability to influence corporate matters and could discourage others from pursuing any change of control transactions that holders of our Class A ordinary shares and the ADSs may view as beneficial.”
Certain existing shareholders have substantial influence over our company and their interests may not be aligned with the interests of our other shareholders.
As of March 31, 2019, our directors and officers collectively owned an aggregate of 93.8% of the total voting power of our outstanding ordinary shares. As a result, they have substantial influence over our business, including significant corporate actions such as mergers, consolidations, election of directors and other significant corporate actions.
They may take actions that are not in the best interest of us or our other shareholders. This concentration of ownership may discourage, delay or prevent a change in control of our company, which could deprive our shareholders of an opportunity to receive a premium for their shares as part of a sale of our company and may reduce the price of the ADSs. These actions may be taken even if they are opposed by our other shareholders. In addition, the significant concentration of share ownership may adversely affect the trading price of the ADSs due to investors’ perception that conflicts of interest may exist or arise. For more information regarding our principal shareholders and their affiliated entities, see “Item 6. Directors, Senior Management and Employees—E. Share Ownership.”
We have granted, and may continue to grant, share incentive awards, which may result in increased share-based compensation expenses.
We adopted our 2016 share incentive plan, or the 2016 Plan, in 2016 to promote our success and the interests of our shareholders by providing a means through which we may grant equity-based incentives to attract, motivate, retain and reward certain officers, employees, directors, consultants and other eligible persons and to further link the interests of recipients with those of our shareholders generally. We adopted certain share incentive policies in December 2013 and August 2014, or the 2013 Policy and the 2014 Policy, respectively. Since the adoption of the 2016 Plan, we stopped granting awards under the 2013 Policy or the 2014 Policy, although the outstanding awards under the 2013 Policy and the 2014 Policy are still being administered under their respective policies. In August 2018, we adopted our 2018 Share Incentive Plan, or the 2018 Plan, which replaced the 2016 Plan in its entirety. Upon the effectiveness of the 2018 Plan, we no longer grant any awards under the 2016 Plan. Outstanding awards granted under the 2016 Plan will remain effective and be subject to the terms and conditions of the 2018 Plan. Under the 2016 Plan, we were authorized to grant options to purchase ordinary shares of our company. The maximum number of ordinary shares which may be issued pursuant to all awards under the 2013 Policy, the 2014 Policy and the 2016 Plan is 13,671,109. Under the 2018 Plan, the maximum number of our shares that may be issued pursuant to all awards is 13,671,109, plus an annual increase on the first day of each fiscal year during the ten-year term of the 2018 Plan commencing with the fiscal year beginning January 1, 2019, by an amount equal to the lesser of (i) 1.0% of the total number of shares issued and outstanding on the last day of the immediately preceding fiscal year, and (ii) such number of shares as may be determined by our board of directors. As of the date of this annual report, options to purchase 7,891,262 Class A ordinary shares and 60,000 restricted share units are granted and outstanding under the 2013 Policy, the 2014 Policy and the 2016 Plan, and options to purchase 1,544,697 Class A ordinary shares and 2,040,827 restricted share units are granted and outstanding under the 2018 Plan. We recognized share-based compensation expenses in the amount of RMB3.4 million, RMB9.9 million and RMB51.4 million (US$7.5 million) in 2016, 2017 and 2018, respectively. See “Item 6. Directors, Senior Management and Employees—B. Compensation.” We believe the granting of share-based compensation is of significant importance to our ability to attract, retain and incentivize key personnel and employees, and we will continue to grant share-based compensation to employees in the future. As a result, our expenses associated with share-based compensation may increase, which may have an adverse effect on our results of operations.
We are an emerging growth company within the meaning of the Securities Act and may take advantage of certain reduced reporting requirements.
We are an “emerging growth company,” as defined in the JOBS Act, and we may take advantage of certain exemptions from various requirements applicable to other public companies that are not emerging growth companies including, most significantly, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002 so long as we are an emerging growth company. As a result, if we elect not to comply with such auditor attestation requirements, our investors may not have access to certain information they may deem important.
We are a foreign private issuer within the meaning of the rules under the Exchange Act, and as such we are exempt from certain provisions applicable to U.S. domestic public companies.
Because we qualify as a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the securities rules and regulations in the United States that are applicable to U.S. domestic issuers, including:
· the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q or current reports on Form 8-K;
· the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act;
· the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and
· the selective disclosure rules by issuers of material nonpublic information under Regulation FD.
We are required to file an annual report on Form 20-F within four months of the end of each fiscal year. In addition, we intend to publish our results on a quarterly basis as press releases, distributed pursuant to the rules and regulations of the Nasdaq Global Market. Press releases relating to financial results and material events will also be furnished to the SEC on Form 6-K. However, the information we are required to file with or furnish to the SEC will be less extensive and less timely compared to that required to be filed with the SEC by U.S. domestic issuers. As a result, you may not be afforded the same protections or information that would be made available to you were you investing in a U.S. domestic issuer.
As a company incorporated in the Cayman Islands, we are permitted to adopt certain home country practices in relation to corporate governance matters that differ significantly from the Nasdaq Global Market corporate governance requirements; these practices may afford less protection to shareholders than they would enjoy if we complied fully with the Nasdaq Global Market corporate governance requirements. Currently, we do not have any immediate plans to rely on home country practice with respect to our corporate governance.
We may lose our foreign private issuer status in the future, which could result in significant additional costs and expenses.
As discussed above, we are a foreign private issuer, and therefore, we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act. The determination of foreign private issuer status is made annually on the last business day of an issuer’s most recently completed second fiscal quarter. We would lose our foreign private issuer status if, for example, more than 50% of our ordinary shares are directly or indirectly held by residents of the U.S. and we fail to meet additional requirements necessary to maintain our foreign private issuer status. In the future, if we lose our foreign private issuer status as of the last date of our second fiscal quarter, we would be required to file with the SEC periodic reports and registration statements on U.S. domestic issuer forms beginning on the following January 1, which are more detailed and extensive than the forms available to a foreign private issuer. We would also have to mandatorily comply with U.S. federal proxy requirements, and our officers, directors and principal shareholders would become subject to the short-swing profit disclosure and recovery provisions of Section 16 of the Exchange Act. In addition, we would lose our ability to rely upon exemptions from certain corporate governance requirements under the Nasdaq Global Market listing rules. As a U.S. listed public company that is not a foreign private issuer, we would incur significant additional legal, accounting and other expenses that we will not incur as a foreign private issuer, and accounting, reporting and other expenses in order to maintain a listing on a U.S. securities exchange.
There can be no assurance that we will not be classified as a passive foreign investment company for United States federal income tax purposes for any taxable year, which could subject United States investors in our ADSs or ordinary shares to significant adverse United States federal income tax consequences.
We will be a “passive foreign investment company,” or PFIC, if, in any particular taxable year, either (a) 75% or more of our gross income for such year consists of certain types of “passive” income or (b) 50% or more of the average quarterly value of our assets (as determined on the basis of fair market value) during such year produce or are held for the production of passive income (the “asset test”). Although the law in this regard is unclear, we intend to treat our variable interest entities (including their subsidiaries) as being owned by us for United States federal income tax purposes, not only because we exercise effective control over the operation of such entities but also because we are entitled to substantially all of their economic benefits, and, as a result, we consolidate their results of operations in our consolidated financial statements. Assuming that we are the owner of our variable interest entities (including their subsidiaries) for United States federal income tax purposes, and based upon our current and expected income and assets (including goodwill), we do not believe we were a PFIC for the taxable year ended December 31, 2018 and we do not presently expect to be a PFIC for the current taxable year or the foreseeable future.
While we do not expect to become a PFIC, because the value of our assets for purposes of the asset test may be determined by reference to the market price of our ADSs, fluctuations in the market price of our ADSs may cause us to become a PFIC for the current or subsequent taxable years. The determination of whether we will be or become a PFIC will also depend, in part, on the composition of our income and assets, which may be affected by how, and how quickly, we use our liquid assets. If we determine not to deploy significant amounts of cash for active purposes or if it were determined that we do not own the stock of our variable interest entities for United States federal income tax purposes, our risk of being a PFIC may substantially increase. Because there are uncertainties in the application of the relevant rules and that PFIC status is a factual determination made annually after the close of each taxable year, there can be no assurance that we will not be a PFIC for the current taxable year or any future taxable year.
If we are a PFIC in any taxable year, a U.S. holder (as defined in “Item 10. Additional Information—E. Taxation—United States Federal Income Taxation Considerations”) may incur significantly increased United States federal income tax on gain recognized on the sale or other disposition of the ADSs or ordinary shares and on the receipt of distributions on the ADSs or ordinary shares to the extent such gain or distribution is treated as an “excess distribution” under the United States federal income tax rules and such holder may be subject to burdensome reporting requirements. Further, if we are a PFIC for any year during which a U.S. holder holds ADSs or our ordinary shares, we generally will continue to be treated as a PFIC for all succeeding years during which such U.S. holder holds our ADSs or ordinary shares. For more information see “Item 10. Additional Information— E. Taxation— United States Federal Income Taxation Considerations—Passive Foreign Investment Company Considerations.”
We will incur increased costs as a result of being a public company.
As a public company, we incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, as well as rules subsequently implemented by the SEC and the Nasdaq Global Market, impose various requirements on the corporate governance practices of public companies. As a company with less than US$1.07 billion in net revenues for our last fiscal year, we qualify as an “emerging growth company” pursuant to the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 of the Sarbanes-Oxley Act of 2002 in the assessment of the emerging growth company’s internal control over financial reporting.
We expect these rules and regulations to increase our legal and financial compliance costs and to make some corporate activities more time-consuming and costly. We expect to incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404 of the Sarbanes-Oxley Act of 2002 and the other rules and regulations of the SEC. For example, as a result of becoming a public company, we will need to increase the number of independent directors and adopt policies regarding internal controls and disclosure controls and procedures. We also expect that operating as a public company will make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. In addition, we will incur additional costs associated with our public company reporting requirements. It may also be more difficult for us to find qualified persons to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these rules and regulations, and we cannot predict or estimate with any degree of certainty the amount of additional costs we may incur or the timing of such costs.
In the past, shareholders of a public company often brought securities class action suits against the company following periods of instability in the market price of that company’s securities. If we were involved in a class action suit, it could divert a significant amount of our management’s attention and other resources from our business and operations, which could harm our results of operations and require us to incur significant expenses to defend the suit. Any such class action suit, whether or not successful, could harm our reputation and restrict our ability to raise capital in the future. In addition, if a claim is successfully made against us, we may be required to pay significant damages, which could have a material adverse effect on our financial condition and results of operations.
Item 4. Information on the Company
A. History and Development of the Company
We commenced operations in October 2012 through Guangdong Yihao Pharmaceutical Chain Co., Ltd., or Yihao Pharmaceutical Chain. In January 2013, Yihao Pharmaceutical Chain established its subsidiary Shanghai Yaowang E-Commerce Co., Ltd., or Shanghai Yaowang. In May 2013, Yao Wang Holdings Ltd. was incorporated under the laws of the Cayman Islands as our offshore holding company, which changed its name to New Peak Group in June 2015, and subsequently changed its name to 111, Inc. in April 2018. In June 2013, Yao Wang Corporation Limited, or Yao Wang, was incorporated in Hong Kong as a wholly owned subsidiary of 111, Inc. Yao Fang Information Technology (Shanghai) Co., Ltd., or Yao Fang, was established in August 2013 as a wholly owned subsidiary of Yao Wang in the PRC. In September 2013, Yao Fang entered into a series of contractual agreements with Guangdong Yihao Pharmacy Co., Ltd., or Yihao Pharmacy, Yihao Pharmaceutical Chain and Shanghai Yaowang and their respective shareholders such that Yihao Pharmacy, Yihao Pharmaceutical Chain and Shanghai Yaowang were each treated as a variable interest entity of Yao Fang, and Yao Fang consolidated the financial results of Yihao Pharmacy, Yihao Pharmaceutical Chain and Shanghai Yaowang in its consolidated financial statements in accordance with U.S. GAAP.
Through Yao Fang, we obtained control over Yihao Pharmacy, Yihao Pharmaceutical Chain and Shanghai Yaowang, or collectively, our variable interest entities, based on a series of contractual arrangements. See “—C. Organizational Structure —Contractual Arrangements with Our Variable Interest Entities.”
In May 2018, Chongqing Yihao Pharmacy Co., Ltd. was established as a wholly owned subsidiary of Yihao Pharmacy in the PRC. In December 2018, Yihao Pharmacy transferred 100% equity interests in Chongqing Yihao Pharmacy Co., Ltd. to Yao Fang. In June 2018, Tianjin Yihao Pharmacy Co., Ltd. was established as a wholly owned subsidiary of Yihao Pharmacy in the PRC. In July 2018, Kunshan Yifang Pharmacy Co., Ltd. was also established as a wholly owned subsidiary of Yihao Pharmacy in the PRC.
On September 15, 2018, our ADSs commenced trading on Nasdaq under the symbol “YI.” We raised from our initial public offering approximately US$101.2 million in net proceeds (including the net proceeds generated from the offering of additional 809,555 ADSs upon the underwriters’ partial exercise of their over-allotment option), after deducting underwriting commissions and the offering expenses payable by us.
Our principal executive offices are located at 3-5/F, No.295 ZuChongZhi Road, Pudong New Area, Shanghai, the People’s Republic of China. Our telephone number at this address is +86 21 2053 6666. Our registered office in the Cayman Islands is located at the offices of Vistra (Cayman) Limited, P.O. Box 31119, Grand Pavilion Hibiscus Way, 802 West Bay Road, Grand Cayman, KY1-1205, Cayman Islands. Our agent for service of process in the United States is Puglisi & Associates, located at 850 Library Avenue, Suite 204, Newark, Delaware 19711.
B. Business Overview
In 2010, our founders launched 1 Drugstore (1药网), one of the first online retail pharmacies in China. Today, we provide hundreds of millions of consumers with better access to pharmaceutical products and medical services, directly through our online retail pharmacy and indirectly through our offline pharmacy network. In 2016, we commenced our online medical services through our internet hospital, 1 Clinic (1诊), to provide consumers with cost-effective and convenient online consultation and electronic prescription services. Our online wholesale pharmacy, 1 Drug Mall (1药城), serves as a one-stop shop for pharmacies to source a vast selection of pharmaceutical products.
Our New Retail Platform
New Retail aims to improve the efficiency of selling and buying, as well as customer experience, by integrating e-commerce, brick-and-mortar retail, and logistics with data throughout the value chain. In 2016, we began the transformation from a pure B2C business to a New Retail Platform, integrating our online retail pharmacy and offline pharmacy network by leveraging our smart supply chain and cloud-based solutions. This model allows us to collect and analyze data from a large number of transactions, which we use to continuously increase the efficiency of our smart supply chain, and the intelligence of our cloud-based solutions.
Our Ecosystem
We connect pharmacies, pharmaceutical companies, medical professionals, insurance companies and consumers in our ecosystem, and we improve the efficiency and transparency of the pharmaceutical value chain. We create value for various participants in our integrated online and offline platform in the healthcare ecosystem in China: (i) consumers who purchase pharmaceutical and other health and wellness products and seek medical services; (ii) pharmacies, including independent pharmacies, pharmacy chains and in-house pharmacies within clinics and private hospitals who purchase pharmaceutical products and interact with consumers through our platform; (iii) suppliers, such as pharmaceutical companies and distributors; (iv) marketplace sellers, who use our platform to distribute and sell their products; (v) insurance companies, which are integral to the roll out of our new closed-loop internet “healthcare + pharma” pharmacy benefits management model and (vi) medical professionals who provide healthcare services through our platform.
Our Products and Services to Consumers
Our Online Retail Pharmacy
Our online retail pharmacy is an integral part of our holistic online and offline platform. In 2010, our founders launched our online retail pharmacy, 1 Drugstore, to fulfill the healthcare needs of consumers. 1 Drugstore is currently available through our 1 Drugstore app or website. We provide consumers with a wide variety of pharmaceutical products and other merchandise, including drugs, nutritional supplements, contact lenses, medical supplies and devices, personal care products as well as baby products. We operate our online retail pharmacy under either direct sales model or the marketplace model.
Our Direct Sales Model
In our online direct sales model, we acquire products from suppliers and sell them directly to consumers. For this model, we need to manage inventories to ensure effective inventory management and may adjust inventory level based on fluctuation in supply and prices, seasonality, popularity of a particular product, and we also take into consideration the shelf life. See “—Supply Chain Management—Inventory Management.” Under this model, we also operate our independent branded storefronts in leading e-commerce platforms in China such as Tmall.com and JD.com. We pay these third-party e-commerce platforms commissions as a percentage of sales.
Our Marketplace Model
We introduced an online marketplace in 2016 to leverage our brand recognition, large and growing customer base, and proprietary technology platform. Under our marketplace model, third-party sellers offer products to consumers over our online marketplace. As of December 31, 2018, we had over 250 marketplace sellers on 1 Drugstore, including online pharmacies.
We facilitate transactions between marketplace sellers and consumers through our marketplace. We provide transaction processing and billing services on all orders on our online marketplace, while the marketplace sellers are responsible for inventory management, fulfillment and delivery. We require marketplace sellers to meet our standards for authenticity and reliability. We aim to offer consumers the same high quality customer experience regardless of the source of the products they choose.
We collect commission fees and platform usage fees from marketplace sellers according to the terms of our individual contracts with them. The commission fees are generally charged as a percentage of sales, depending on product category, among other things. We also charge a fixed annual platform usage fees to marketplace sellers for maintaining storefronts on our platform. We provide order processing services for all orders on our online marketplace.
Our Offline Retail Pharmacy
We also operate a network of offline retail pharmacies branded as “Yi Hao Pharmacy,” mainly in Guangdong province, which enables us, as required by the relevant laws and regulations, to operate our online pharmaceutical retail businesses. As of December 31, 2018, we had 12 offline retail pharmacies in Guangzhou, Shanghai, Tianjin and Kunshan. Revenue contribution from our Yi Hao Pharmacies was insignificant in 2016, 2017 and 2018.
Our Product Offerings to Consumers
We carry diverse and comprehensive products in our online retail and offline retail pharmacies. Our unique integrated retail and wholesale supply chain and inventory management combines the management of retail and wholesale SKUs. The merchandise offered by us and the marketplace sellers can be broadly classified into the following major categories:
Drugs. We display drugs, including prescription drugs and over-the counter, or OTC, drugs such as western medicines and traditional Chinese medicines.
Nutritional supplements. We display nutritional supplements, including a variety of vitamins, and dietary products.
Contact lenses. We offer a comprehensive selection of contact lenses that cover all major brands.
Medical supplies and devices. We offer a variety of general purpose medical supplies and devices such as bandages and thermometers.
Other products. Our other products include personal care products such as skin care, birth control, sexual wellness products as well as baby products.
We also sell seasonal and promotional items tailored to local consumer demand for convenience and quality. In 2017, we further expanded our product offerings by introducing more health and wellness products and smart wearable devices. We believe that offering these products increases the order size spend per visit by meeting the growing demand for one-stop shopping convenience.
Consumers can browse our products by category or scan barcodes of drugs they find in store and easily find them on our online retail pharmacy.
Pricing and Payment of Products
We offer competitive pricing to attract and retain consumers. Under the direct sales model, prices are set by us with reference to major online and offline competitors, taking into account our overall pricing strategy for different categories. We believe our prices are generally lower than those of offline pharmacy chains and independent pharmacies. We constantly monitor the prices of products offered by our competitors through our pricing intelligence system. See “—Technology and IT Infrastructure— Cloud-based Applications.” Under our marketplace model, sellers are free to set their own prices, but are encouraged to set comparable and competitive prices. We also occasionally offer significant discounts on certain products for a limited time in flash sales or other promotional activities, including our anniversary sale and “November 11 sale.” We make continuous efforts to maintain and improve an efficient cost structure and create incentives for our suppliers to provide us with competitive prices.
We provide our consumers flexible payment options for both direct sales and marketplace models. Our payment options include in-person settlement (which is required and the only option for the purchase of prescription drugs), bank transfers, online payments with credit cards and debit cards, and payment through third-party online payment platforms, such as WeChat Payment and Alipay. For fulfillment and delivery options, please see “—Supply Chain Management—Fulfillment and Delivery.”
Our Online Consultation and E-prescription Services
We strive to provide our consumers with convenient access not only to pharmaceutical products, but also to medical services. We commenced our online consultation services, through 1 Clinic, a licensed internet hospital operated by us, in 2016, to address the need for cost-effective and convenient evaluation of health and medical conditions. Our online consultation utilizes a user-friendly interface embedded in 1 Drugstore website and app designed to empower consumers to remotely access healthcare. This service covers a wide range of conditions and cases, with a primary focus on common and chronic illnesses. For conditions that require in person or further examination or laboratory testing, we generally refer our consumers to hospitals.
Consumers access our online consultations primarily through photo and text consultations, phone calls and video consultations. They can select a doctor of their choosing based on the doctor’s availability and his/her profile displayed on our platform. Our photo and text consultation sessions are offered to consumers for free. We typically charge a fixed amount of fees per consultation session for consultations through video and online assisted telephone calls, which differ based on the type of consultation used and are paid through our convenient online payment system.
The first step to utilize our services is to provide basic personal information, descriptions of conditions and any medical records or laboratory test results through our online platform. Each medical consultation session typically lasts around 5 to 15 minutes, depending on the complexity of the case. Based on the consumer’s response to inquiries on the condition at issue, the doctor provides medical recommendations, issues prescriptions, or advises the consumer to have an examination conducted at a hospital and uploads the results to our system.
As of December 31, 2018, our medical team comprised nearly 90 full-time in-house medical professionals, and over 2,000 external doctors registered on our platform.
We provide ongoing training and professional development programs to our in-house medical professionals. We conduct weekly evaluations of our in-house doctors and medical assistants in respect of quality of service, user feedback and efficiency. We have also adopted a quality control system with standardized protocols for our services performed by our in-house medical team. We contract services from external doctors who practice at reputable hospitals with significant experience and appropriate credentials. We require external doctors to register with us and to agree to our terms of use, pursuant to which they must comply with both our specified work scope and quality requirements, and the applicable rules and regulations. See “—Risk Management and Internal Control—Healthcare Service Quality and Safety.”
We offer e-prescription services to consumers as an integral part of the online consultation process, subject to our stringent compliance procedures. Each of our prescriptions is issued by qualified doctors. The e-prescription services is also available when a consumer needs to purchase a prescription drug through the offline pharmacy network. See “—Our Products and Services to Pharmacies—Our Cloud Prescription Services.”
Customer Service and Satisfaction
Providing satisfactory customer services is a high priority. Our commitment to consumers is reflected in the high service quality provided by our customer service staff and speedy fulfillment and delivery services. We have high levels of customer satisfaction, as evidenced by a customer satisfaction rate over 98.4% on 1 Drugstore in 2018.
Our Products and Services to Pharmacies
We have enabled more than 150,000 offline pharmacies to better serve their consumers, as of December 31, 2018. This network of pharmacies represents the largest virtual pharmacy network in the world in terms of the number of pharmacy stores, as of May 18, 2018, according to Frost & Sullivan. The pharmacy customers we serve include small and medium-sized retail pharmacy chains, independent pharmacies and in-house pharmacies within clinics and private hospitals, spanning across 31 provinces in China.
Our Online Wholesale Pharmacy
We provide comprehensive, intelligent and integrated distribution solutions through our online wholesale pharmacy, 1 Drug Mall, available both through our 1 Drug Mall app and website. Overall, our business involves a process of sourcing from suppliers, warehousing, processing orders and invoicing, payment collection and delivering to pharmacies. These pharmacy customers include independent pharmacies, pharmacy chains, in-house pharmacies within clinics and private hospitals, as well as certain select distributors that have both retail and wholesale businesses.
1 Drug Mall features an extensive selection of pharmaceutical and other health and wellness products sourced from pharmaceutical companies and other suppliers. A substantial majority of prescription and OTC drugs displayed on 1 Drug Mall are available under our direct sales model. Our broad and fast-growing product offerings enable us to satisfy the purchasing needs of our pharmacy customers. Meanwhile, our strong sourcing capability, coupled with our highly cost-effective distribution model, enables us to bypass traditional layers of the distribution network to provide competitive prices to our pharmacy customers.
Our Direct Sales Model
We primarily conduct our wholesale distribution business through our direct sales model, where we procure pharmaceutical products from pharmaceutical companies or distributors and sell to our pharmacy customers. As of December 31, 2018, we directly sourced from 93 pharmaceutical companies and 275 distributors. Leveraging our strong relationship with our suppliers, we offer a comprehensive selection of pharmaceutical products at market-competitive pricing. Under the direct sales model, we are responsible for the fulfillment and delivery of the products sold.
Our Marketplace Model
We also operate an online marketplace where third-party sellers can directly sell to pharmacies. These marketplace sellers primarily consist of traditional offline distributors. They leverage our platform and customer base to grow their business, while at the same time complementing our product offerings under our direct sales model. Our marketplace business model under our wholesale business is similar in many respects to our retail business, including the charging model. For a detailed discussion, please see “—Our Products and Services to Consumers—Our Marketplace Model.”
Payment, Exchange and Return
We generally require advance payment or payment-upon-delivery for purchases. For certain select pharmacy customers, we may grant a credit period of up to a month. We generally do not offer product return and exchange service unless the damages are caused by our fault.
Our Cloud-based Inventory Management Services
Most of our pharmacy customers, in particular, independent pharmacies and in-house pharmacies within clinics and private hospitals, do not have a comprehensive inventory and demand forecast system. Purchases by these customers are primarily made based on historical experience, and their inventory turnover days are generally long due to a lack of detailed, precise planning and bulk purchase patterns. In addition, their inventory level is subject to fluctuations as a result of seasonal or other factors beyond their control.
Our online wholesale pharmacy, featuring a vast selection of pharmaceutical products and speedy delivery, enables cloud-based inventory management. Pharmacy customers, rather than relying on advance but often imprecise planning, can collaborate with us for inventory visibility and on-demand offering. We simplify and streamline the procurement process and shorten the procurement cycle. Our pharmacy customers typically do not need to negotiate or enter into any purchase and sale agreement or make any purchase commitment. The purchase orders of our pharmacy customers on 1 Drug Mall are processed in real time. Typically, the order processing will take less than eight minutes, after which orders will be dispatched for delivery through our fulfillment network. The inventory on demand and just-in-time delivery offer significant benefits to our pharmacy customers. Instead of bulk purchases and maintaining large inventory, pharmacies procure their inventory with more precision, reducing their working capital needs and enabling them to quickly respond to market demand. As a result, we are able to improve the inventory turnover of our pharmacy customers.
Our Cloud Prescription Services
Pharmacies, especially independent pharmacies and small-to-medium sized pharmacy chains, often lack onsite doctors to offer prescriptions to consumers with minor ailments or chronic diseases visiting their stores. In 2018, we began to use our cloud prescription services to leverage our existing online consultation and e-prescription service platform to offer convenient online consultation services to these consumers onsite. Once consumers obtain their prescriptions from us, they will be able to purchase prescription drugs from our pharmacy customers. In return for our services, we charge our pharmacy customers a fixed amount of annual service fees. The annual service fees vary, depending on the number of online consultations performed, the number of e-prescriptions issued, and the amount of drugs purchased in connection with our services. As of December 31, 2018, we offered cloud prescription services to around 3,200 pharmacies located in 20 provinces in China. In the fourth quarter of 2018, in connection with our cloud prescription services, we performed around 3,600 online consultations and issued approximately 3,200 e-prescriptions on average per day.
Our Smart Procurement Services
Leveraging our extensive experience in inventory management and our data analytics capabilities, we launched our smart procurement services in the first quarter of 2018 to cooperate with pharmacies to collect their historical purchase orders and inventory data. We then typically analyze historical purchase patterns, the location of the pharmacy and regional supply and demand information, any epidemic status and trend, and current pricing and promotions. Through our proprietary big data analytic platform and sophisticated prediction model, we can make individualized purchase recommendations for our pharmacy customers’ review.
Given our broad reach within the pharmaceutical value chain, we believe that we will be able to detect trends in the industry and forecast demand, therefore, meeting the procurement needs of pharmacies through our customized generated purchase orders. Our smart procurement service is also capable of comparing prices across different sellers on our platform to ensure the best pricing for our pharmacy customers. As pharmacy customers gradually increase the use of our services, we believe that we can accumulate data to optimize our own supply chain management, while providing better solutions to both pharmacies and upstream suppliers with more relevant service offerings.
Customer Experience
We are committed to optimizing and achieving satisfaction of our pharmacy customers. This commitment drives every aspect of our operations, which are focused on five core components: extensive product offerings, competitive pricing, transformational online procurement processes and frequent customer engagement, as well as timely and accurate fulfillment. We also build customer loyalty and encourage pharmacies to make repurchases by actively engaging with them. After assisting our pharmacy customers to open accounts and establishing initial relationships, our on-the-ground sales force frequently liaise with these pharmacies via in-person visits, telephone calls or other social network tools to share the latest promotional information and drive repeat purchases. We also use data insight provided by digital CSO to guide our on-the-ground sales force to strength our customer acquisition and operation effectiveness.
Our Services to Pharmaceutical Companies
We source products from pharmaceutical companies and distributors, while at the same time providing them with data services and other value-added services.
Data Services
Leveraging our direct reach to many touchpoints of the healthcare and pharmaceutical value chain, we launched our data services in 2018 on a pilot basis to help our pharmaceutical companies expand their reach and gain valuable insights to the distribution channel and consumers. We compile, aggregate and analyze our detailed sales data to uncover purchase patterns and predict future purchase behaviors and demand. With our broad consumer base, we can extract valuable information from our extensive database. For a particular product, we analyze the regional and seasonal sales patterns, the amounts of orders, the frequency of purchases, any particular preference for packaging, and other factors that may affect sales. Data mining of our available data is a powerful tool to predict consumer behaviors and market trends, allowing pharmaceutical companies to make knowledge-driven decisions in their sales forecasts and budgeting. For a discussion of our pricing intelligence system, see “—Technology and IT Infrastructure—Cloud-based Applications.”
Digital Contract Sales Organization (CSO) Services
In 2018, we launched our digital CSO services and began to work with a number of pharmaceutical companies on a pilot basis. Our digital CSO services connect upstream pharmaceutical companies, midstream distributors and downstream pharmacies and pharmaceutical sales representatives, enabling real-time tracing of sales and other related intelligence and empowering pharmaceutical companies with marketing capabilities. Our digital CSO services can be accessed through different portals such as WeChat, our App and Web portal. For pharmaceutical sales representatives who are connected to CSO, we provide CRM, purchase order management and digital marketing and promotion services. For pharmacies, we provide patient education and pharmacist training. Our digital CSO services generally include activities such as brand events, online medical and product trainings and promotions at pharmacies.
Our Services to Medical Professionals
We provide medical professionals with services to enable them to better serve patients and improve service quality.
E-medical Record and Patient Management
We create and maintain, in secured electronic storage, a copy of electronic medical records for certain consumers. These e-medical records allow consumers to access past consultation history and communicate with doctors for follow-up or new consultations. Our cloud-based platform and e-medical record services also enable more efficient patient management by doctors. Doctors use our system for reviewing e-medical records with the patient’s consent.
Pharmacopoeia
Pharmacopoeia is an online medical encyclopedia that enables doctors, pharmacists and other medical professionals, especially those of our pharmacy customers, to offer trustworthy health information to end users. Its searchable database features a comprehensive range of information relating to different types of drugs, including their indications, directions and usage, side effects, precautions and interactions with other drugs. It also provides health and wellness information and tips. Medical professionals can search drugs by medical condition, drug name, symptoms or otherwise browse our homepage of pharmacopoeia.
Suppliers
We have an extensive network of suppliers, consisting primarily of pharmaceutical companies and distributors. As of December 31, 2018, we directly sourced from 93 pharmaceutical companies and 275 distributors. We believe that competitive sources are readily available for substantially all of the merchandise we carry on our platform, and we have diversified our procurement sources to obtain more favorable terms and minimize our inventory risk.
Supplier Selection
When choosing suppliers, we take into consideration, among other things, whether their products complement our overall product offering, the quality and prices of their products, market reputation, production and/or distribution capacity and the market potential of their products. Before we engage with any new supplier, we also examine their qualifications and licenses to verify that they operate their businesses in compliance with applicable laws, rules and regulations.
Our Relationship with Pharmaceutical Companies and Key Suppliers
We have dedicated teams that work closely with our top suppliers, especially pharmaceutical companies, to strengthen our relationships with them. For the same product, the price from a pharmaceutical company is generally lower than from a distributor. We aim for qualification by major pharmaceutical companies as a “tier one” distributor so as to directly source from them. As of December 31, 2018, we have obtained such qualifications from 93 pharmaceutical companies and directly source from them. We also seek to cooperate with other “tier one” distributors who may have negotiated attractive prices for particular products. Our cooperation with these suppliers allows us to expand our product offerings and procure products manufactured by pharmaceutical companies without an established relationship with us. We intend to help pharmaceutical companies expand their end user base by leveraging our network of pharmacies. We also provide them with customized channel management services, as well as data flow, operational support, marketing, user data analysis and other value-added services. Together with pharmaceutical companies, we work to develop medical know-how including academic content and product trainings.
Supply Chain Management
We combine advanced technologies and supply chain optimization techniques to integrate the front and the back end of the supply chain and optimize our inventory management. Our unique integrated retail and wholesale supply chain and inventory management allow us to share inventories among 1 Drugstore, 1 Drug Mall, and Yi Hao Pharmacy, significantly increasing our operational efficiency. Supported by our proprietary supply chain management systems, efficiently designed supply chain protocols and processes, strategically located fulfillment centers and nationwide fulfillment network, our supply chain enables inventory on demand and just-in-time delivery for our consumers. As a result of our advanced supply chain management system, we have seen tangible improvements in our own and our customers’ efficiency levels.
Supply Chain Technologies and Systems
Our supply chain management system consists of four separate subsystems supported by proprietary software that allows us to effectively collaborate with third-party service providers and interact with our consumers and pharmacy customers. All of our systems are designed to comply with Good Supply Practices (GSP) for pharmaceutical products, and connect with provincial food and drug administrations for real-time monitoring.
Warehouse Management System (WMS). We customize our proprietary warehouse management system to meet the specific needs of our pharmaceutical distribution business. Our WMS enables us to closely monitor each step of the fulfillment process from guiding inventory receiving and put-away, optimizing picking and shipping of orders and advising on inventory replenishment. Our advanced WMS software optimizes our warehouse space and employees’ time, supports paperless material handling in a digital WMS environment and automates the interaction between our employees and material handling equipment, such as conveyor belts. For example, we developed advanced algorithms to optimize picking, packing and shipping. At each fulfillment center, inventory is bar-coded and tracked through our management information system, allowing real-time monitoring of inventory levels across our fulfillment network and item tracking at each fulfillment center. Our shelf space hosts the same inventory for both our wholesale and retail businesses, while the assignment and allocation logics are designed to cater to the different requirements for fast-moving and long-tail products, optimizing fulfillment efficiency. The seamless connection with our other supply chain management modules has led to increased inventory accuracy, greater space utilization, increased warehouse productivity and improved customer service.
Transportation Management System (TMS). Our transportation management system enables full operational control and visibility from dispatch to delivery, and from invoicing to receivables collections. Our TMS is integrated with third-party accounting systems. All of these systems are customer-oriented and allow for full shipment tracking and visibility, as well as for customer shipment input.
Procurement Management System (PMS). Our procurement management system promotes transparency and compliance. It consists of various modules with different levels of authorizations to different personnel. We are in the process of developing our data platform that is fully compatible with and can connect to many of our suppliers’ ordering systems to allow seamless information exchange. We expect the new system to enhance the efficiency of various aspects of our purchase process, such as stocking and account settlement.
Order Management System (OMS). Our order management system allows us to manage inventory cost and pricing, and process orders from both pharmacy customers and consumers. It also provides us with flexible pricing and promotions to satisfy our customers’ needs. Our order management system enhances our visibility into our customers’ preferences, merchandise and supply chain, resulting in improved customer service, improved operational efficiency, enhanced management analytics and increased inventory synergies.
Inventory Management
We manage our inventory, both retail and wholesale, in an integrated manner. Our inventory, fulfillment and delivery services are centrally managed from our headquarters. Our inventory management allows our retail and wholesale businesses to access and share all of our inventory resources.
We continually seek to improve our inventory control and minimize inventory risk. We analyze historical sales data and days in inventory to establish inventory management plans. We may adjust our inventory management plans based on factors such as fluctuations in supply and prices, seasonality and sales of a particular product. Our inventory optimization model uses sophisticated algorithms to help determine when to replenish an SKU. We also perform regular spot inventory counts in our fulfillment centers. We monitor the shelf life of our pharmaceutical products by conducting periodic reviews, and either make sales promotion plans or make inventory write-downs depending on the status of the inventory.
Our inventory includes high level of stock for certain products that we consider as strategic reserves. These products are generally purchased at favorable price terms, and have a long shelf life. They also help us preempt possible industry-wide shortages.
Fulfillment and Delivery
As of December 31, 2018, our fulfillment network consists of four regional fulfillment centers strategically located in Guangzhou in Guangdong Province in Southern China, Kunshan in Jiangsu Province in Eastern China (which is within close proximity of Shanghai), Tianjin in Northern China and Chongqing in Western China. In the future, we plan to selectively establish additional fulfillment centers to improve our geographical coverage while maintaining our operational efficiency.
We leverage our large-scale operations and reputation to obtain favorable contractual terms from third-party delivery companies. To reduce the risk of reliance on any single delivery company, we typically contract with two or more regional delivery companies in each major city. We regularly monitor and review the delivery companies’ performance and their compliance with our contractual terms. In addition, we typically require the delivery companies to pay deposits or provide payment guarantees before providing services to us. We typically negotiate and enter into logistics agreements on an annual basis.
We are generally able to deliver to major cities in 23 provinces within 24 hours, and nationwide within 72 hours.
Risk Management and Internal Control
We have adopted and implemented various policies and procedures to ensure rigorous risk management and internal control, and we are dedicated to continually improving these policies and procedures.
Our risk management and internal control policies and procedures cover various aspects of our business operations such as product safety, healthcare quality and safety, regulatory risk management, government affairs and regulatory compliance.
Product Quality and Safety
We place strong emphasis on quality and safety of the products we sell on our platform. We conduct random quality inspections of products we procure, and reject the shipment if it fails to meet our quality standards. Our quality control department rigorously implements quality control procedures.
Healthcare Service Quality and Safety
We value the quality and safety of the healthcare services we provide. We strive to minimize medical risks arising from our operations. We have never received any written notice or penalty for material non-compliance or violation of healthcare service quality and safety laws or regulations, nor have we received any recommendation for improvement with respect to healthcare service quality and safety from any government authority.
The skills, competence and attitude of our in-house medical team are essential for the quality of care that our users receive. We continually monitor the risk in relation to services provided by our in-house medical team to ensure the risk management policies and procedures have been strictly followed, so as to achieve effective and efficient governance, risk and control processes.
We have adopted stringent hiring procedures for doctors, pharmacists and medical assistants, which involve in-person interviews and assessments of technical knowledge. Our in-house medical team receives regular training on relevant safety policies, standards, protocols and procedures and is required to strictly comply with them in all aspects of our operations. We conduct frequent evaluations of our in-house doctors, pharmacists and medical assistants.
For external doctors, we generally require them to provide us with their qualifications and licenses and to strictly adhere to the work scope and quality requirements specified in their service agreements in compliance with applicable legal and regulatory requirements.
For healthcare institutions to which we refer our consumers, we consider a variety of factors such as reputation, scale of business, service quality and capability, as well as their facilities. We typically require healthcare institutions who cooperate with us to maintain requisite licenses, comply with relevant laws and regulations and follow our service guidelines. We also carefully monitor feedback from our consumers on the services provided by these healthcare institutions, and take that into consideration when determining our continued cooperation with such healthcare institutions. We are not responsible for any losses to our consumers resulting from disputes or breach of obligations in relation to the provision of the relevant services.
Regulatory Compliance and Risk Management
We have a dedicated public relations department, consisting of government relations and public relations teams and with a leader who has over 10 years of experience in regulatory compliance and risk management in Fortune 500 companies. We have designed and adopted strict internal procedures to ensure compliance of our business operations with all relevant laws and regulations and have established a code of conduct to regulate employee behavior and activities. In addition, we continually review the implementation of our risk management policies and measures to ensure our policies and implementation are effective and sufficient.
We work closely with relevant government agencies that have jurisdiction over our business. We maintain frequent communications with government agencies before implementing new business initiatives or when regulatory uncertainties arise as new laws or regulations are promulgated. We actively provide our inputs on proposed regulations that are subject to public comments. We are often invited to comment on proposed regulations by relevant government authorities during the comment solicitation process.
As part of our risk management and internal control measures, we have adopted a series of internal regulations against corrupt and fraudulent activities, which include measures against receiving bribes and kickbacks, and misuse of company assets. We have anti-corruption and anti-bribery clauses in a majority of our major business contracts, and we require our suppliers and other third parties who cooperate with us to comply with relevant laws and regulations.
Data Privacy and Protection
We are committed to protecting information and privacy of our consumers and other participants on our platform. We have developed a company-wide policy on data security to preserve individual personal information and privacy. We strictly comply with laws and regulations and do not distribute or sell our users’ personal data for any purpose. We encrypt user data in network transmissions and in backend storage to ensure confidentiality. To minimize the risk of data loss, we conduct regular data backup and data recovery tests. Our database can only be accessed by certain designated and authorized personnel after assessment and approval procedures, whose actions are recorded and monitored.
Technology and IT Infrastructure
Our proprietary technology is one of our core competitive advantages. As of December 31, 2018, our technology and IT team consisted of 293 employees, including core team members with extensive experience with leading internet, online retail and e-commerce companies in China. We have built our technology platform primarily relying on proprietary software and systems that we have developed in-house. We develop and maintain various online platforms that connect the respective systems of various participants in the healthcare ecosystem, enabling them to access our services and connect with other participants in the ecosystem. As a result, they are able to conveniently share information and conduct their operations efficiently over our platform.
Data Collection, Aggregation and Analytics and Transaction Support
Our data assets are the backbone of our data analytics capabilities. We collect data under various scenarios across the entire pharmaceutical value chain. The high volumes of traffic over our platform have brought us large amounts of data, collected with the consumer’s due authorization. Our strong data mining and user behavior analytics capabilities allow us to build a comprehensive profile for each consumer. Data analytics is extensively used in various aspects of our operations.
In addition, we collect a wealth of data on our supply chain, such as cost per delivery, delivery time requirements, positive customer feedback and other similar indicators. Based on our extensive database of supply chain information, we create operational goals and insights, including optimal time by which deliveries must be made to elicit positive consumer feedback and optimal delivery routes that minimize cost per delivery. We use the data we possess to simplify supply chain management, enabling our business to operate more efficiently, giving us more visibility and control over our inventory and reducing our operational costs. For discussion of our supply chain management and related technologies, please see “—Supply Chain Management.”
Cloud-based Applications
Our platform is built on highly scalable and reliable cloud-based technology architecture that can accommodate the increasing scale and complexity of our business operations. Our IT framework includes service-oriented architecture, business intelligence, single sign-on, ERP Open API, pay component, image recognition, message-oriented middleware, task scheduling center and radio frequency identification, or RFID. Service-oriented architecture is a style of software design where services are provided to the other components by application components. ERP Open API is standardized API, or application programming interface, that is compatible with different ERP systems adopted by pharmacy customers. Message-oriented middleware is a software or hardware infrastructure that supports sending and receiving messages between distributed systems, allowing application modules to be distributed over heterogeneous platforms and reducing the complexity of developing applications that span across multiple operating systems and network protocols. We are able to rapidly enroll consumers, pharmacy customers and suppliers onto our platform and seamlessly include them in our system.
Our sophisticated CRM system enables us to effectively gather, analyze and use customer data to plan customized marketing activities. In addition, we also provide our data insights to pharmacies and marketplace sellers to help them optimize their sales and marketing strategies. Our CRM system enables us to reduce costs and increase profitability through increased customer loyalty and attention. Our online platform for doctors also has a CRM system and lays the foundation of patient management and assists the interaction between medical professionals.
Our business intelligence system provides operational analysis, sales forecasts and other application-oriented intelligent products that facilitate data-driven decision making. One such application is our pricing intelligence system, which applies data mining techniques to discover, match, extract and report on competitive pricing data to optimize our pricing strategy relative to our competition. This pricing intelligence system helps us gain a better understanding of our price position in the market and make automatic adjustments to thousands of our SKUs. We also use our pricing intelligence system to provide data services to pharmaceutical companies. See “—Our Services to Pharmaceutical Companies.”
Our IT Infrastructure
We are committed to maintaining a secure online platform. We have built a firewall that monitors and controls incoming and outgoing traffic on our platform 24/7. Once any abnormal activity is detected, our system will immediately notify our IT team and simultaneously take automatic protective and remedial measures, such as activating third-party traffic control services, to prevent any harm to our platform. We conduct periodic reviews of our technology platform identifying and correcting problems that may undermine our system security.
Our stable IT infrastructure is hosted by two separate cloud service providers. We achieve redundancy and reliability of our network through a real-time multi-layer data backup system. Our platform adopts modular architecture that consists of multiple connected components, each of which can be separately upgraded and replaced without compromising the functioning of other components. This makes our platform both highly reliable and scalable.
Our platform is scalable and can be easily expanded as data storage requirements and user visits increase. In addition, load balancing technology helps us improve distribution of workloads across multiple computing components, optimizing resource utilization and minimizing response time.
Sales and Marketing
Our marketing and promotion strategy is to build brand recognition, increase customer traffic, attract new customers, build strong customer loyalty and develop incremental revenue opportunities.
We employ a variety of methods to attract potential consumers. Generally, we expand our user base on our marketplace through search engines, social media and word-of-mouth referrals. We offer incentives to new consumers and pharmacy customers who make purchases for the first time on our platform. We also offer flash sales and brand promotion events on our website and mobile application to engage with existing consumers in an effort to increase retention and repurchases. Our principal marketing programs include advertising our company and our solutions through our mobile platform and other media.
We acquire pharmacy customers primarily through our effective on-the-ground sales operation to allow rapid expansion of our wholesale business. We have full-time employees who visit independent pharmacies, pharmacy chains and in-house pharmacies within clinics and private hospitals to promote our online wholesale pharmacy and our inventory management services. We also hire independent contractors who work for us on a commission-basis to promote our products and services to pharmacies through our “City Partners” program. We also coordinate market development and promotion efforts for our pharmacy customers, which may include flash sales, seasonal sales discounts and rebates.
Competition
We believe our business model is unique and our services encompass the entire pharmaceutical value chain. We believe there are no comparable companies that directly compete with us. However, we face intense competition in certain business segments and verticals:
· we compete against other pharmaceutical retail companies including traditional offline pharmacies and online platforms, such as Ali Health and JD.com; and
· we also face competition from numerous B2B platforms and traditional pharmaceutical distributors.
We believe that our ability to compete effectively depends on many factors, including the variety of our products, our pricing competitiveness, user experience on our platform, our technological leadership, effectiveness of our risk management, our partnership with third parties, our marketing and selling efforts and the strength and reputation of our brands.
Furthermore, as our business continues to grow rapidly, we face significant competition for highly skilled personnel, including management, engineers, product managers and risk management personnel. The success of our growth strategy depends in part on our ability to retain existing personnel and add additional highly skilled employees. We believe that our early mover advantage and leading market position help us to compete efficiently against our competitors.
Intellectual Property
We rely on a combination of copyright, trademark and trade secret laws and restrictions on disclosure to protect our intellectual property rights. We have registered 18 software copyrights with the PRC National Copyright Administration. We have 26 registered domain names, including 111.com.cn and yaoex.com. As of December 31, 2018, we had 259 registered trademarks, including our “1药网” trademark. As of December 31, 2018, we have applied for three patents with the PRC State Intellectual Property Office.
Insurance
We maintain property insurance policies covering certain equipment and other property that are essential to our business operations to safeguard against risks and unexpected events. We also provide social security insurance including pension, medical insurance, unemployment insurance, maternity insurance, on-the-job injury insurance and housing fund plans through a PRC government-mandated benefit contribution plan for our employees. We maintain product liability insurance. We also maintain professional malpractice insurance for our in-house licensed medical practitioners and some of our external doctors. We do not maintain business interruption insurance. We consider our insurance coverage to be sufficient for our business operations in China.
Regulations
This section sets forth a summary of the most significant rules and regulations that affect our business activities in China.
Regulation Relating to Foreign Investment
Investment in the PRC conducted by foreign investors and foreign-owned enterprises shall comply with the Guidance Catalogue of Industries for Foreign Investment, or the Catalogue, which was first issued in 1995 and amended from time to time. The current effective Catalogue was promulgated by the MOFCOM and the NDRC in June 2017 and became effective in July 2017, and contains specific provisions guiding market access of foreign capital and stipulates in detail the areas of entry pertaining to the categories of encouraged foreign-invested industries, restricted foreign-invested industries and prohibited foreign-invested industries. The latter two categories are included in the negative list, which was first introduced into the Catalogue in 2017, and listed, in a unified manner, the restrictive measures for the entry of foreign investment. On June 28, 2018, the MOFCOM and the NDRC jointly promulgated the Special Administrative Measures for Access of Foreign Investment (Negative List) (2018 Edition), to replace the negative list attached to the Catalogue in 2017, which took effect on July 28, 2018. Any industry not listed in the Catalogue is a permitted industry and generally open to foreign investment unless specifically prohibited or restricted by PRC laws and regulations. According to the Catalogue, value-added telecommunications services (with the proportion of foreign investment not exceeding 50%, except for e-commerce) and medical institutions (limited to sino-foreign equity joint venture or sino-foreign cooperative joint venture) are restricted for foreign investment. Yao Fang, our wholly-owned PRC subsidiary, is a foreign-invested enterprise and conducts technical services and consultation and sale of goods that falls in permitted industries for foreign investment. Wuhan Central China, our 70% owned PRC subsidiary, is an entity invested by a foreign-invested enterprise and conducts online B2B pharmaceutical e-commerce business which is classified as a type of value-added telecommunications services. It falls in the restricted foreign-invested industry but is not subject to the 50% foreign investment restriction.
In September 2016, the Standing Committee of China’s National People’s Congress, or the SCNPC, passed a decision in connection with the revision of four laws, including the trio of laws regulating foreign investment in China, which became effective in October 2016. According to this decision, establishment of a foreign-invested enterprise, or the FIE, in a sector not subject to special entry administrative measures will be simplified by going through government filing instead of a government approval process, which applies to its establishment, separation, merger or other major modifications and operation duration and extension; but the special entry administrative measures are to be separately promulgated or approved to be promulgated by the State Council. According to a notice issued by the NDRC and the MOFCOM in October 2016, the special entry administrative measures shall be applicable and implemented to the restricted foreign-invested industries, prohibited foreign-invested industries and encouraged foreign-invested industries which have requirements as to shareholding and qualifications of senior management stipulated in the then-effective Catalogue. At the same date, the MOFCOM promulgated the Provisional Filing Administrative Measures on Establishment and Modifications for Foreign Investment Enterprises, as amended in July 2017 and June 2018, which request the establishment and modifications of foreign-invested enterprises not subject to the special entry administrative measures, to be filed with the delegated commerce authorities and specify the procedures and requirements for such filing in detail.
In March 2019, a new draft of Foreign Investment Law was submitted to the National People’s Congress for review and was approved on March 15, 2019, which will come into effect from January 1, 2020 and replace the trio of laws regulating foreign investment in China. Under new Foreign Investment Law, foreign investment refers to investment activity directly or indirectly conducted by foreign natural persons, enterprises or other organizations, including the following circumstances: (i) a foreign investor establishes a foreign-funded enterprise within the territory of China, independently or jointly with any other investor; (ii) a foreign investor acquires shares, equities, property shares or any other similar rights and interests of an enterprise within the territory of China; (iii) a foreign investor makes investment to initiate a new project within the territory of China, independently or jointly with any other investor; and (iv) a foreign investor makes investment in any other way stipulated by laws, administrative regulations or provisions of the State Council. Foreign investors shall not invest in any field forbidden by the negative list for access of foreign investment and shall conform to the investment conditions stipulated under the negative list for any field restricted by the negative list. Fields not included in the negative list shall be managed under the principle that domestic investment and foreign investment shall be treated uniformly. In addition, a foreign investment information reporting system shall be established and foreign investors or foreign-funded enterprises shall submit the investment information to competent departments for commerce through the enterprise registration system and the enterprise credit information publicity system.
Our PRC subsidiary, Yao Fang, as a foreign-invested enterprise, is not subject to the special entry administrative measures and has filed with the competent commerce authority for its establishment and modification as requested. Our PRC subsidiary, Wuhan Central China, an entity invested by a foreign-invested enterprise and conducts business which falls in the restricted foreign-invested industry, has obtained the approval from and made filing with the competent commerce authority for its modifications. Our variable interest entities which are not foreign-invested enterprises are not required to be filed with commercial authorities under such measures.
In August 2006, six PRC regulatory agencies, including the MOFCOM, jointly adopted the M&A Rules, which became effective in September 2006 and were amended in 2009. The M&A Rules also establish procedures and requirements that could make certain acquisitions of PRC companies by foreign investors more time-consuming and complex, including requirements in some instances that MOFCOM be notified in advance of any change-of-control transaction in which a foreign investor takes control of a PRC domestic enterprise. In addition, according to the Notice on Establishing the Security Review System for Mergers and Acquisitions of Domestic Enterprises by Foreign Investors issued by the General Office of the State Council in February 2011, the Rules on Implementation of Security Review System for the Merger and Acquisition of Domestic Enterprises by Foreign Investors issued by the MOFCOM in August 2011, mergers and acquisitions by foreign investors that raise “national defense and security” concerns and mergers and acquisitions through which foreign investors may acquire de facto control over domestic enterprises that raise “national security” concerns are subject to strict review by the MOFCOM, and the regulations prohibit any activities attempting to bypass such security review, including by structuring the transaction through a proxy or contractual control arrangement.
Regulation Relating to Value-added Telecommunications Services
Telecommunications Regulations
The Telecommunications Regulations of the People’s Republic of China, or the Telecom Regulations, promulgated in September 2000 and amended in July 2014 and February 2016 respectively, are the primary PRC laws governing telecommunication services, and set out the general framework for the provision of telecommunication services by domestic PRC companies. The Telecom Regulations require that telecommunications service providers obtain operating licenses prior to commencing operations. The Telecom Regulations draw a distinction between “basic telecommunications services” and “value-added telecommunications services.” The catalogue of Telecommunications Business, or the Telecom Catalogue, issued as an attachment to the Telecom Regulations, identifies information services and online data and transaction processing services as value-added telecommunications services.
In July 2017, the MIIT issued the revised Measures on the Administration of Telecommunications Business Operating Permits, or the Telecom License Measures, which became effective in September 2017, to supplement the Telecom Regulations. The Telecom License Measures require that an operator of value-added telecommunications services obtain a value-added telecommunications business operating license, from the MIIT or its provincial level counterparts. The term of a license for value-added telecommunication business is five years and subject to annual inspection. Yihao Pharmaceutical Chain has obtained the Value-Added Telecommunications Services Operating License for conducing information services and online data and transaction processing services (e-commerce only), while Wuhan Central China has obtained the value-added telecommunications services operating license to conduct online data and transaction processing services (e-commerce only).
Foreign Investment in Value-Added Telecommunications
Foreign direct investment in telecommunications companies in China is also regulated by the Regulations for the Administration of Foreign-Invested Telecommunications Enterprises, or the FITE Regulations, which were issued by the State Council in December 2001 and amended in September 2008 and February 2016, respectively. The FITE Regulations stipulate that a foreign invested telecommunications enterprise in the PRC, or the FITE, must be established as a sino-foreign equity joint venture for operations in the PRC. Under the FITE Regulations and in accordance with WTO-related agreements, the foreign party investing in a FITE engaging in value-added telecommunications services may hold up to 50% of the equity interests of the FITE. In addition, the major foreign party to be the shareholder of the FITE must satisfy a number of stringent performance and operational experience requirements, including demonstrating a good track record and experience in operating a value-added telecommunications business. The FITE that meets these requirements must obtain approvals from the MIIT and the MOFCOM or its counterparts, which retain considerable discretion in granting approvals. Furthermore, the foreign party investing in e-commerce business, as a type of value-added telecommunication services, has been allowed to hold up to 100% of the equity interests of the FITE based on the Notice of the Ministry of Industry and Information Technology on Removing the Restrictions on Foreign Equity Ratios in Online Data Processing and Transaction Processing (Operating E-commerce) Business issued on in June 2015 and the current effective Telecom Catalogue.
In July 2006, the Ministry of Information Industry, which was restructured and integrated into the MIIT, promulgated the Notice of the Ministry of Information Industry on Intensifying the Administration of Foreign Investment in Value-added Telecommunications Services, or the MII Notice, which reiterates certain requirements of the FITE Regulations and strengthens the administration by the MII. Under the MII Notice, if a foreign investor intends to invest in a PRC value-added telecommunications business, the FITE must be established to apply for a telecommunications business license applicable to the business. In addition, a domestic company that holds a license for the provision of value-added telecommunications services is prohibited from leasing, transferring or selling the license to foreign investors in any form, and from providing any assistance, including providing resources, sites or facilities, to foreign investors to conduct value-added telecommunications businesses illegally in China. Trademarks and domain names that are used in the provision of value-added telecommunications services must be owned by the license holder or its shareholders. The MII Notice also requires that each value-added telecommunications services license holder have appropriate facilities for its approved business operations and to maintain such facilities in the business regions covered by its license. Yihao Pharmaceutical Chain, our variable interests entity holding the value-added telecommunications services license, owns the domain names, trademarks, and facilities which are appropriate for the telecommunication business provided by Yihao Pharmaceutical Chain.
Internet Information Services
In September 2000, the State Council promulgated the Measures for the Administration of Internet Information Services, or the ICP Measures, as amended in 2011. Under the ICP Measures, the internet information services is divided into commercial internet information services and non-commercial internet services. The operators of non-commercial internet information services must file with relevant governmental authorities and operators of commercial internet information services in China must obtain a license for internet information provision, or ICP license, from the relevant governmental authorities, and the provision of particular information services, such as news, publishing, education, healthcare, medicine and medical device, and must also comply with relevant laws and regulations and obtain the approval from competent governmental authorities.
Mobile Internet Applications Information Services
In June 2016, the CAC promulgated the APP Provisions, which became effective in August 2016. Under the APP Provisions, mobile application providers and application store service providers are prohibited from engaging in any activity that may endanger national security, disturb the social order, or infringe the legal rights of third parties, and may not produce, copy, issue or disseminate through internet mobile applications any content prohibited by laws and regulations. The APP Provisions also require application providers to procure relevant approval to provide services through such applications and require application store service providers to register with local branches of the CAC within 30 days after they start providing application store services.
Regulations Relating to Pharmaceutical Operation and Service
Pharmaceutical Operation
In September 1984, the SCNPC promulgated the Drug Administration Law of the People’s Republic of China, or the Drug Administration Law, which was amended in 2001, 2013 and 2015 respectively to regulate all entities or individuals engaging in research, manufacture, operation, use, supervision and management of drugs within the PRC. According to the Drug Administration Law, no pharmaceutical operation, including pharmaceutical wholesale and pharmaceutical retail business, is permitted without obtaining the Pharmaceutical Operation License. The Implementation Rules for the Drug Administration Law, was promulgated by the State Council in August 2002 and amended in 2016 and 2019, which emphasized the detailed implementation rules of drugs administration. The CFDA promulgated the Measures for the Administration of Pharmaceutical Operation License in February 2004 as amended in 2017, which stipulate the procedures for applying the Pharmaceutical Operation License and the requirements and qualifications for pharmaceutical wholesalers or pharmaceutical retailers with respect to their management system, personnel, facilities and etc. The valid term of the Pharmaceutical Operation License is five years and shall be renewed six months prior to its expiration date. Yihao Pharmacy, Yihao Pharmaceutical Chain and its branches and Chongqing Yihao Pharmacy Co., Ltd. have obtained the Pharmaceutical Operation License, respectively.
According to the Measures on Prescription Drugs and OTC Drugs Classification Management and the Interim Provisions on the Circulation of Prescription and OTC Drugs (Trial), which were both promulgated by the State Drug Administration, which was restructured and integrated into the CFDA, in 1999 and became effective in January 2000, drugs are divided into prescription drugs and over-the-counter drugs, or OTC drugs. For prescription drugs, the dispensing, purchase and use can only be based on the prescription issued by the certified medical practitioner or certified medical assistant practitioner. In addition, the prescription drugs can only be advertised and promoted in professional medical magazines. OTC drugs, on the other hand, are further divided into Class A and Class B and they both can be purchased and used without a prescription and promoted in public upon approval by the relevant governmental authorities. The pharmaceutical wholesale enterprises distributing prescription drugs and/or OTC drugs, as well as pharmaceutical retail enterprises selling prescription drugs and/or Class A OTC drugs are required to obtain the Pharmaceutical Operation License.
According to the Administrative Measures for the Supervision and Administration of Circulation of Pharmaceuticals, promulgated by the CFDA in January 2007 and effective in May 2007, pharmaceutical manufacture and operation enterprises and medical institutions shall be responsible for the quality of pharmaceuticals they manufacture, provide or use. The operation of prescription drugs is highly regulated under these rules. Prescription drugs may not be sold by pharmaceutical retail enterprises without valid prescriptions and an enterprise in violation of such restriction will be instructed to rectify any violation, given a disciplinary warning, and/or imposed a fine of no more than RMB1,000. In addition, a pharmaceutical manufacture or operation enterprise shall not sell prescription drugs directly to the public by post or over internet, and the enterprise in violation of such restriction shall be instructed to rectify, given a disciplinary warning, and fined the lesser of (i) two times the value of the pharmaceuticals sold and (ii) RMB 30,000. Furthermore, the Administrative Standard of Pharmaceutical Operating Quality, promulgated by the CFDA in April 2000 and amended in 2012, 2015 and 2016, respectively, and the Administrative Measures for Identification of Pharmaceutical Operating Quality Administrative Standards, promulgated by the CFDA in April 2003, the pharmaceutical operation enterprise shall take effective quality control measures over the process of procurement, storage, transportation and sale of drugs in order to ensure their quality, and is required to obtain a GSP certificate, from the relevant governmental authorities. The GSP certificate is valid for five years and shall be renewed three months prior to its expiration date upon a re-examination by the competent governmental authorities. Yihao Pharmacy, Yihao Pharmaceutical Chain and its branches and Chongqing Yihao Pharmacy Co., Ltd., each have obtained the GSP certificate.
Medical Devices Operation
According to the Regulations on the Supervision and Administration of Medical Devices, which was promulgated by the State Council in January 2000 and amended in 2014 and 2017, respectively, and the Supervision and Management Measures on Medical Devices Operation, which was promulgated by the CFDA in July 2014 and amended in 2017, business operations of medical devices are regulated based on the degree of risks involving the medical devices, which are divided into three categories. Operation of Class I medical devices does not require a license or record-filing, while operations of Class II medical devices and Class III medical devices are subject to record-filing and licensing requirements, respectively. An entity engaging in the operation of medical devices shall meet certain requirements with respect to its management system, personnel, facilities etc., and shall apply for approval to operate Class III medical devices and make record-filing with relevant governmental authority to operate Class II medical devices. The valid term of medical devices operation permit is five years. Yihao Pharmacy, Yihao Pharmaceutical Chain and its branches, and Chongqing Yihao Pharmacy Co., Ltd., have obtained the record-filing certificates for the business operations of Class II medical devices and the business permits for the business operations of Class III medical devices, which are necessary to cover their current business.
Regulations Relating to Online Operation of Drugs and Medical Devices
Internet Drug Information Service
The Administrative Measures on Internet Drug Information Service, or Internet Drug Measures, was promulgated by the CFDA in July 2004 and amended in 2017, pursuant to which the internet drug information services is to provide drug (including medical device) information services to online users, which is divided into commercial internet drug information services and non-commercial internet drug information services. The website operator that provides drugs (including medical devices) information services must obtain an Internet Drug Information Service Qualification Certificate from the competent counterpart of the CFDA. The valid term for an Internet Drug Information Service Qualification Certificate is five years and may be renewed at least six months prior to its expiration date upon a re-examination by the relevant governmental authorities. Yihao Pharmacy, Yihao Pharmaceutical Chain and Wuhan Central China have each obtained the Internet Drug Information Service Qualification Certificate to provide commercial internet drug information services.
Furthermore, as requested by Internet Drug Measures, the information relating to drugs shall be accurate and scientific in nature, and its provision shall comply with the relevant laws and regulations. No product information of stupefacient, psychotropic drugs, medicinal toxic drugs, radiopharmaceutical, detoxification drugs and pharmaceutics made by medical institutes shall be distributed on the website. In addition, advertisements relating to drugs (including medical devices) shall be approved by the NMPA or its competent counterparts.
Internet Drug Transaction Services
The Interim Provisions on the Examination and Approval of Internet Drug Transaction Services, or Interim Portions of Internet Drug Transaction, were promulgated by the CFDA in September 2005 and became effective in December 2005, and regulate transaction of drugs (including medical devices and packing materials and containers that are in direct contact with drugs) over internet, including the provision of transaction services among pharmaceutical manufacturers, pharmaceutical operation enterprises and medial institutes, the services provided by pharmaceutical manufacturers and pharmaceutical wholesale enterprises to other third parties via their own websites and services provided by pharmaceutical retail chain enterprises to individual consumers. According to Interim Portions of Internet Drug Transaction enterprises engaging in providing drug transaction services over the internet must obtain an Internet Drug Transaction Qualification Certificate. Such certificates have a term of five years and have three types: A certificate, B certificate and C certificate. They are only issued to three kinds of enterprises: (i) enterprises that provide drug transaction services to pharmaceutical manufactures, pharmaceutical operation enterprises and medical institutions, but do not participate in pharmaceutical manufacture and operation and do not own, have no property relationship or other economic interest with the administrative organizations, medical institutions or pharmaceutical manufacture and operation enterprises; (ii) pharmaceutical manufacturers and pharmaceutical wholesale enterprises that deal with other third-party enterprises via their own websites; (iii) the pharmaceutical retail chain enterprises that provide OTC drug transaction services for individual consumers via the internet. Wuhan Central China, Yihao Pharmacy and Yihao Pharmaceutical Chain have obtained the Internet Drug Transaction Qualification Certificates for A certificate, B certificate and C certificate, respectively.
However, according to the Decision on the Cancellation of the Third Batch of Items Subject to Administrative Permission by Local Governments Designated by the Central Government, promulgated by the State Council in January 2017, except for third-party platforms, all approval of internet drug transaction service enterprises implemented by counterparts of CFDA at the provincial level are cancelled. In April 2017, the General Office of the CFDA promulgated a notice on implementing the above mentioned decision, pursuant to which pharmaceutical manufacture enterprises and pharmaceutical wholesale enterprises may carry out internet drug (including medical device) transactions with other enterprises through their own websites, but shall not provide internet drug (including medical device) transaction services to individual consumers. In addition, pharmaceutical retail chain enterprises may provide internet drug (including medical device) transaction services to individual consumers, but they shall not exceed the business scope permitted by license and filings and display information of prescription drugs on related transaction webpages, or sell prescription drugs or the OTC drugs under special administrative requirements; as indicated in such decision, the CFDA will promulgate subsequently the relevant rules on supervision of internet drug (including medical device) transaction.
Furthermore, according to the Decision on the Cancellation of Various Items Subject to Administrative Permission promulgated by the State Council in September 2017, the enterprises engaging in internet drug transaction service as a third-party platform shall no longer be subject to the examination and approval of the CFDA before carrying out such business. In November 2017 the General Office of the CFDA promulgated a Notice on Strengthening the Administration and Supervision of Internet Drug and Medical Devices Transaction, which specify the approval to conduct internet drug transaction service as the third-party platform is cancelled, but enterprises carrying out internet drug (including medical) transaction services shall establish a comprehensive supervision system in general and also request local counterparts of CFDA to implement day-to-day supervision and examination with respect to qualification access examination, products inspection, storage of transaction data and legal liabilities etc.
Online Sales of Drugs and Medical Device
Under PRC laws and regulations, the drugs and medical devices are allowed to be sold online in general except the prescription drugs that cannot be sold by pharmaceutical manufacture and operating enterprise or medical institution directly to the public by post or via internet.
In November 2017, the CFDA released a draft of the Administrative Measures for Supervision and Regulation of Online Drug Sales for public consultation, which aims to regulate online drug sales in the PRC. In February 2018, the CFDA also released a draft for online drug sales for public consultation. Both drafts stipulate that sellers and online platforms engaging in this business shall obtain relevant certificates and have sufficient medical staff and operating systems to guarantee the safety and quality of the drugs. Furthermore, these entities shall make record-filing with the counterparts of CFDA at provisional level. In particular, the websites for sale of drugs to individuals are not allowed to display prescription drugs information via the internet. As of the date of this annual report, both drafts have not come into effect and there are substantial uncertainties with respect to their enactment timetable and final provisions.
In December 2017, the CFDA promulgated the Administration and Supervision Measures of Online Sales of Medical Devices, or the Online Medical Devices Sales Measures, which became effective in March 2018. According to the Online Medical Devices Sales Measures, enterprises engaged in online sales of medical devices must be medical device manufacture and operation enterprises with medical devices production licenses or operation licenses or being filed for record in accordance with laws, unless such licenses or record-filing is not required by laws and regulations, and the third-party platform for provision of online medical devices transaction services shall obtain an Internet Drug Information Services Qualification License. Either enterprises for online sales of medical devices or enterprises for provision of medical devices online transaction services shall take technical measures to ensure the data and materials of medical devices online sales are authentic, completed and retrospective, for example the records of sale information of medical devices shall be kept for two years after the valid period of the medical devices, and for no less than five years in case of no valid period, or be kept permanently in case of implanted medical devices.
Regulations Relating to Online Trading
In January 2014, the SAIC promulgated the Administrative Measures for Online Trading, or Online Trading Measures, which became effective in March 2014, to regulate all operating activities for products sale and services provision via the internet (including mobile internet). It stipulates the obligations of online products operators and services providers and certain special requirements applicable to third-party platform operators. The MOFCOM promulgated the Provisions on the Procedures for Formulating Transaction Rules of Third Party Online Retail Platforms (Trial) in December 2014, which became effective in April 2015, to guide and regulate the formulation, revision and enforcement of transaction rules by online retail third-party platforms operators. These measures impose more stringent requirements and obligations on third-party platform operators. For example, third-party platform operators are obligated to make public and file their transaction rules with MOFCOM or their respective provincial counterparts, examine and register the legal status of each third-party merchant selling products or services on their platforms and display on a prominent location on a merchant’s webpage the information stated in the merchant’s business license or a link to its business license. Where third-party platform operators also act as online distributors, these third-party platform operators must make a clear distinction between their online direct sales and sales of third-party merchant products on their third-party platforms. Furthermore, in August 2018, the SCNPC promulgated the E-Commerce Law, which took effect on January 1, 2019 and aims to regulate the e-commerce activities conducted within the territory of the PRC. According to the E-Commerce Law, e-commerce operators shall comply with the principles of voluntariness, equality, fairness, and good faith, abide by laws, observe business ethics, equally participate in market competition. It further strengthened the performing obligations of e-commerce operators regarding to the protection of consumers’ rights and interests, environmental protection, intellectual property protection, and the protection of cyberspace safety and personal information, and also emphasized the commitment by e-commerce operators over the quality of products and services.
After the issuance of Online Trading Measures, the SAIC has issued a number of guidelines and implementing rules aimed at adding greater specificity to these regulations and continues to consider and issue guidelines and implementing rules in this industry. For example, three PRC governmental authorities (the Ministry of Finance of the People’s Republic of China, or the MOF, General Administration of Customs, or the GAC, and the SAT) issued the New Cross-Border E-commerce Retail Imports Tax Notice in March 2016, which became effective in April 8, 2016 and was amended in November 2018, to regulate cross-border e-commerce trading and introduced the concept of the cross-border e-commerce retail importation goods inventory, or the Cross-Border E-Commerce Goods Inventory, which has been issued and updated by the three authorities together with other relevant authorities from time to time. Two batches of the Cross-Border E-Commerce Goods Inventory have been issued in April 2016, which have been replaced by the Cross-Border E-Commerce Goods Inventory (2018 Edition) issued in November 2018 and the Notice of Relevant Matters on Implementation of New Cross-Border E-Commerce Retail Importation Supervision and Administration Requirements issued by the GAC in May 2016 to further implement the rules. According to the Notice of Relevant Work on improving Cross-Border E-Commerce Retail Importation Supervision and Administration issued in November 2018, retail imported goods on cross-border e-commerce platforms will be temporarily treated as personal items which are not subject to stricter regulations and higher tax rates applicable to normal imported goods in 37 cross-border e-commerce trial areas.
Regulations Relating to the Medical Industry
Medical Institution
The Administrative Regulations on Medical Institutions was promulgated by the State Council in February 1994 and amended in 2016 to regulate all medical institutions, such as hospitals, health centers, sanatoriums, out-patient departments, clinics and health posts (rooms) as well as first-aid stations. The establishment of medical institutions by the entity or individual shall be subject to examination and approval of the health administrative authorities at the county level or above and medical institutions must obtain the approval certificate to establish the medical institution. Furthermore, the medical institution shall also register with competent health administrative authorities for practice and operation, and obtain the License for Practicing of Medical Institutions after examination by competent health administrative authorities. Southwest Internet Hospital has obtained a License for Practicing of Medical Institutions, and Guangdong Yihao Pharmaceutical Chain Co., Ltd. Dongshan Branch Traditional Chinese Medicine Clinic and Guangdong Yihao Pharmaceutical Chain Co., Ltd. Yuexiu Branch Traditional Chinese Medicine Clinic both have obtained Licenses for Practicing of Medical Institution only for traditional Chinese medicine and internal medicine.
Internet Medical Services
On May 1, 2009, the Ministry of Health of the People’s Republic of China, which was restructured and integrated into the NHFPC and subsequently to the NHC, promulgated the Administrative Measures on Internet Medical and Healthcare Information Services to regulate the business relating to providing online medical and healthcare information; but such measures were abolished in January 2016.
In August 2014, the NHFPC issued an opinion to promote medical institution’s remote diagnosis and treatment services. Under this opinion, the medical institutions shall possess qualified personnel, technology, facilities to carry out remote diagnosis and treatment services, and shall also satisfy certain requirements. Non-medical institutions are prohibited from providing remote diagnosis and treatment services.
In July 2015, the State Council issued a guiding opinion to promote activities of the concept of “Internet +”, including popularizing the model of online medical services to develop the medical services via internet and support medical information sharing service platform. In December 2016, the State Council promulgated the 13th Five-Year sanitation and health planning, which suggests to comprehensively implement “Internet +” Healthcare service, and encourages the establishment of regional remote medical service platform and promotes the vertical flow of high quality medical resources.
According to an official reply to a proposal put forward in the fourth meeting of the twelfth committee of the Chinese People’s Political Consultative Conference in June 2016 and released in November 2016 on the website of NHFPC, it indicates that no online diagnosis and treatment shall be allowed except for remote diagnosis and treatment; but it allows online health consultation provided by practitioners or other personnel via network platform operated by internet companies and medical consultation made by practitioners without provision of written diagnosis and prescription and other implementation of doctors’ advice. As indicated in another official reply to a recommendation made in the fifth meeting of China’s twelfth National People’s Congress issued in December 2017 by the NHFPC, the administration measures for internet diagnosis and treatment are under research and formulation, which aims to specify the business scope of internet diagnosis and treatment, information security, patient privacy protection, supervision and legal liabilities.
In April, 2018, the General Office of State Council promulgated the opinions concerning development of “internet + healthcare” that specifies that both medical institutions and qualified enterprises could set up and operate “internet hospital” to offer approved services and the prescription drugs for common and chronic disease prescribed by pharmacist could be delivered by third parties designated by pharmaceutical operating entities, which would allow the pharmaceutical enterprise to sell the prescription drugs to individual by mail. In July 2018, the NHC and the State Administration of Traditional Chinese Medicine, or the SATCM, jointly promulgated the Notice of Carrying Out In-depth Activities for the Benefit of the People regarding “Internet + Healthcare”, which further specified that after e-prescriptions have been approved by pharmacists, pharmaceutical enterprises can designate qualified third party couriers to deliver the prescription drugs.
In July 2018, the NHC and the SATCM issued the Administrative Measures for Online Diagnosis and Treatment (Trial), the Administrative Measures for Internet Hospitals (Trial), or the Internet Hospital Administrative Measures and the Administrative Standards for Remote Diagnosis (Trial). According to these measures and standards, internet hospitals including the internet hospitals used as second name of a physical medical institution and the internet hospitals established independently while relying on a physical medical institution, and are subject to access restrictions. The application for establishment of internet hospitals shall be submitted to the registration department of the physical medical institution. The Internet Hospital Administrative Measures also provide the basic standards for internet hospitals, including the requirements for diagnosis subjects, department settings, staffs, buildings and facilities, rules and regulations.
Medical Practitioners
In June 1998, the SCNPC promulgated the Law on Licensed Medical Practitioners of the People’s Republic of China, or the Licensed Medical Practitioners Law, which became effective in May 1999 and was amended in 2009. According to the Licensed Medical Practitioners Law, registered doctors can work in medical or healthcare institutions according to the registered place, category and scope of business to engage in the relevant services of medical treatment, prevention or healthcare. Person who fails to register as a doctor and obtain the practicing certificate shall not practice medicine. In February 2017, the NHFPC promulgated the Administrative Measures for the Registration of Medical Practitioners, or the Medical Practitioners Registration Measures, which became effective in April 2017 and stipulate that medical practitioners shall obtain the Practice License for Medical Practitioners to practice upon registration.
In November 2014, the NHFPC, the NDRC, the MOHRSS, the SATCM and the China Insurance Regulatory Commission jointly promulgated Several Opinions on Promoting and Standardizing Multi-Institution Practice of Medical Practitioners. According to these opinions, the clinical, dental and traditional Chinese medicine practitioners are allowed to practice in multiple places. The medical practitioners who meet certain requirements and conditions shall register with competent health administrative authorities and obtain the consent from the medical institution where he or she first practices before practicing in other places. Moreover, under the Medical Practitioners Registration Measures, a medical practitioner practicing in multiple institutions at the same place of practice shall determine one institution as his or her primary practicing institution, and apply for registration to the competent administrative authorities of health and family planning that approve the practice at such institution, and for other institutions where a medical practitioner intends to practice, he or she should apply for the record at the relevant administrative authorities of health and family planning that approve the practice of such institutions, which names should be indicated in the record. In addition, a medical practitioner intends to add the practicing institution beyond the place of practice, he or she should apply for registering such institution to the relevant administrative authorities of health and family planning authority that approve the practice of such institutions.
According to the Measures for the Administration of Prescriptions issued by the NHFPC in February 2007, a registered medical practitioner shall obtain the corresponding prescription right at the registered practice place and the registered medical practitioner shall issue prescriptions according to the relevant requirements. The prescriptions issued by a registered assistant medical practitioner shall not become effective until it is signed by a registered medical practitioner.
Regulations Relating to Food Business
General Administration on Food Operation
The Food Safety Law of the People’s Republic of China, which was effective as from June 2009 and amended by the SCNPC in April 2015 and December 2018 and became effective in December 2018, and the Implementation Regulations of the Food Safety Law of the PRC, which took effect as from July 2009 and were amended by the State Council in 2016, regulate food safety and set up a system of the supervision, monitoring and evaluation of food safety and adopt food safety standards. The State Council implements a licensing system for the food production and transaction. To engage in food production, sale or catering services, the business operator shall obtain a license in accordance with the laws. Furthermore, the State Council implements strict supervision and administration for special categories of foods such as healthcare food, special formula foods for medical purposes and infant formula.
The Administrative Measures for Food Business Licensing, promulgated by the CFDA in August 2015 and amended in 2017 regulates the food business licensing activities, strengthens the supervision and management of food business and ensures food safety. Food business operators shall obtain one Food Business License for one business venue where they engage in food business activities. The valid term of a food business license is five years. Yao Fang, Yihao Pharmacy, Yihao Pharmaceutical Chain and its branches and Chongqing Yihao Pharmacy Co., Ltd. have obtained the Food Business Licenses.
Regulations Relating to Product Quality and Consumers Protection
According to the Product Quality Law of the People’s Republic of China, which was effective as from September 1993 and amended by the SCNPC in 2000, 2009 and 2018, respectively, products for sale must satisfy relevant safety standards and sellers shall adopt measures to maintain the quality of products for sale. Sellers may not sell mix impurities or imitations into products, or substitute fake products for genuine ones, or substitute defective products for good ones or substitute substandard products for standard ones. For sellers, any violation of state or industrial standards for health and safety or other requirements may result in civil liabilities and administrative penalties, such as compensation for damages, fines, confiscation of products illegally sold and the proceeds from such sales and even revoking business license; in addition, severe violations may subject the responsible individual or enterprise to criminal liabilities.
According to the Consumers Rights and Interests Protection Law of the People’s Republic of China, or Consumers Rights and Interests Protection Law, which became effective in January 1994 and was amended by the SCNPC in 2009 and 2013 respectively, business operators should guarantee that the products and services they provide satisfy the requirements for personal or property safety, and provide consumers with authentic information about the quality, function, usage and term of validity of the products or services. The consumers whose interests have been damaged due to the products or services that they purchase or accept on the internet trading platforms may claim damages to sellers or service providers. Where the operators of the online trading platforms are unable to provide the real names, addresses and valid contact details of the sellers or service providers, the consumers may also claim damages to the providers of the online trading platforms. Operators of online trading platforms that clearly knew or should have known that sellers or service providers use their platforms to infringe upon the legitimate rights and interests of consumers but fail to take necessary measures must bear joint and several liabilities with the sellers or service providers. Moreover, if business operators deceive consumers or knowingly sell substandard or defective products, they should not only compensate consumers for their losses, but also pay additional damages equal to three times the price of the goods or services.
In January 2017, the SAIC issued the Interim Measures for No Reason Return of Online Purchased Products within Seven Days, which became effective in March 2017, further clarifying the scope of consumers’ rights to make returns without a reason, including exceptions, return procedures and online trading platform operators’ responsibility to formulate seven-day no-reason return rules and related consumer protection systems, and supervise the merchants for compliance with these rules.
Regulations Relating to Online Advertising
Foreign Investment on Advertising
The principal regulation governing foreign-invested advertising agencies in China are the Administrative Measures for Foreign Invested Advertising Enterprise, which was abolished due to the decision on Repealing the Administrative Measures for Foreign Invested Advertising Enterprise issued by SAIC in June 2015. According to the Foreign Investment Industrial Guidance Catalogue (2015 Revision), which came into effect in April 2015, foreign investors are allowed to own 100% of an advertising agency in China subject to certain qualification requirements. However, foreign investment in advertising agencies that provide online advertising services is still subject to restrictions of foreign investment in the value-added telecommunications business.
Administration on Internet Advertisement
In April 2015, the SCNPC enacted the Advertising Law of the People’s Republic of China, or the Advertising Law, which became effective in September 2015 and was amended in October 2018. The Advertising Law regulates commercial advertising activities in the PRC and sets out the obligations of advertisers, advertising operators, advertising publishers and advertising spokespeople, and prohibits any advertisement from containing any obscenity, pornography, gambling, superstition, terrorism or violence-related content. Any advertiser in violation of such requirements to advertisement content will be ordered to cease publishing such advertisements and imposed a fine ranging from RMB200,000 to RMB1,000,000; in severe circumstances, the business license of such advertiser may be revoked, and the relevant authorities may revoke the approval document for advertisement examination and refuse to accept applications submitted by such advertiser for one year. In addition, any advertising operator or advertising publisher in violation of such requirements will be imposed a fine ranging from RMB200,000 to RMB1,000,000, and the advertisement fee received will be confiscated; in severe circumstances, the business license of such advertising operator or advertising publisher may be revoked.
Except that certain prescription drugs are prohibited from advertising, the advertisement of prescription drugs can be only made on designated medical or pharmaceutical journals. Any display of prescription drugs advertisements outside the designated media channels may result in violations of such restrictions by the advertising operator, confiscation of advertising fees and a fine ranging from RMB200,000 to RMB1,000,000, or, in severe circumstances, revocation of business license. In addition, any advertisement for medical treatment, pharmaceutical or medical devices must not contain any assertion or guarantee on the function and safety, or any statement on curative rate or effectiveness of such medical treatment, pharmaceutical or medical devices, and any violation of such requirements will result in a fine equivalent to an amount up to three times the amount of the advertising fees, or a fine ranging from RMB100,000 to RMB200,000 if the advertising fees cannot be calculated or are significantly low; and in severe circumstances, a fine equivalent to the amount up to five times the amount of the advertising fees will be imposed, or a fine ranging from RMB200,000 to RMB1,000,000 if the advertising fees cannot be calculated or are significantly low. Moreover, the Advertising Law also provides that the internet information service providers must not publish advertisements related to medical treatments, drugs, medical devices or health foods in the disguised form of providing healthcare and health maintenance knowledge.
The Interim Measures for Administration of Internet Advertising, or the Internet Advertising Measures regulating the internet-based advertising activities, were adopted by the SAIC in July 2016 and became effective in September 2016. According to the Internet Advertising Measures, internet advertisers are responsible for the authenticity of the advertisements content and all online advertisements must be marked “Advertisement” so that viewers can easily identify them as such.
Pursuant to the Measures for Drug Advertisements Examination and the Measures for Medical Device Advertisement Examination which became effective in May 2007 and May 2009, and were both amended in December 2018, all advertisement containing drug names, applicable symptom to be cured by such drugs (major functions) or other drug-related content, the medical device names and the applicable scope, performance, structure and composition, function and other content relevant to medical device that are published through various media or in various forms shall be examined according to such measures, except for the advertisement merely for OTC drugs’ and medical devices’ name and publication of prescription drugs’ name at designated professional pharmaceutical magazine. The applicants for drug and medical device advertisement license numbers must be pharmaceutical or medical device manufacture enterprises or pharmaceutical or medical device operation enterprises that have obtained the consent from pharmaceutical or medical device manufacture enterprise. The valid period of drug and medical device advertisement license numbers shall be one year and the content of approved advertisement may not be altered without prior approval, otherwise a new license number shall be reapplied for the revised content of drug and medical device advertisement.
Regulations relating to Internet Information Security and Privacy Protection
PRC government authorities have enacted laws and regulations with respect to internet information security and protection of personal information from any abuse or unauthorized disclosure. Internet information in China is regulated and restricted from a national security standpoint.
In June 2017, the Cyber Security Law of the People’s Republic of China, or the Cyber Security Law, promulgated by SCNPC took effect, which is formulated to maintain the network security, safeguard the cyberspace sovereignty, national security and public interests, protect the lawful rights and interests of citizens, legal persons and other organizations, and requires that a network operator, which includes, among others, internet information services providers, take technical measures and other necessary measures to safeguard the safe and stable operation of the networks, effectively respond to the network security incidents, prevent illegal and criminal activities, and maintain the integrity, confidentiality and availability of network data. The Cyber Security Law reaffirms the basic principles and requirements set forth in other existing laws and regulations on personal information protections and strengthens the obligations and requirements of internet service providers, which include but are not limited to: (i) keeping all user information collected strictly confidential and setting up a comprehensive user information protection system; (ii) abiding by the principles of legality, rationality and necessity in the collection and use of user information and disclosure of the rules, purposes, methods and scopes of collection and use of user information; and (iii) protecting users’ personal information from being leaked, tampered with, destroyed or provided to third parties. Any violation of the provisions and requirements under the Cyber Security Law and other related regulations and rules may result in administrative liabilities such as warnings, fines, confiscation of illegal gains, revocation of licenses, suspension of business, and shutting down of websites, or, in severe cases, criminal liabilities.
In July 2013, the MIIT issued the Provisions on Protecting Personal Information of Telecommunication and Internet Users to further define the personal information of user to include user name, birthday, identification number, address, phone number, account, passcode, and others that may be used to identify the user solely in addition to other information such as location and service time of users. Furthermore, according to the interpretations issued by the Supreme People’s Court and the Supreme People’s Procuratorate in May 2017, personal information means various information recorded electronically or through other manners, which may be used to identify individuals or activities of individuals, including but not limited to the name, identification number, contact information, address, user account and passcode, property ownership and location tracking.
In November 2015, the Ninth Amendment to the Criminal Law issued by the SCNPC became effective, pursuant to which, any internet service provider that fails to comply with obligations related to internet information security administration as required by applicable laws and refuses to rectify upon order is subject to criminal penalty for (i) any large-scale dissemination of illegal information; (ii) any severe consequences due to the leakage of the user information; (iii) any serious loss of criminal evidence; or (iv) other severe circumstances. Furthermore, any individual or entity that (i) sells or distributes personal information in a manner which violates relevant regulations, or (ii) steals or illegally obtain any personal information is subject to criminal penalty in severe circumstances.
Regulations Relating to Intellectual Property
Copyright
China has adopted comprehensive legislation governing intellectual property rights, including trademarks and copyrights. China is a signatory to the primary international conventions on intellectual property rights and has been a member of the Agreement on Trade Related Aspects of Intellectual Property Rights since its accession to the WTO in December 2001.
On In September 1990, the SCNPC promulgated the Copyright Law of the People’s Republic of China, effective in June 1991 and amended in 2001 and 2010 respectively. The amended Copyright Law extends copyright protection to internet activities, products disseminated over the internet and software products. In addition, there is a voluntary registration system administered by the Copyright Protection Centre of China.
In order to further implement the Computer Software Protection Regulations, promulgated by the State Council in December 2001 and amended in 2011 and 2013 respectively, the National Copyright Administration issued Computer Software Copyright Registration Procedures in February 2002, which specify detailed procedures and requirements with respect to the registration of software copyrights.
Trademark
According to the Trademark Law of the People’s Republic of China, promulgated by the SCNPC in August 1982, and amended in 1993, 2001 and 2013 respectively, the Trademark Office of the SAIC is responsible for the registration and administration of trademarks in China. The SAIC under the State Council has established a Trademark Review and Adjudication Board for resolving trademark disputes. Registered trademarks are valid for ten years from the date the registration is approved. A registrant may apply to renew a registration within twelve months before the expiration date of the registration. If the registrant fails to apply in a timely manner, a grace period of six additional months may be granted. If the registrant fails to apply before the grace period expires, the registered trademark shall be deregistered. Renewed registrations are valid for ten years. In April 2014, the State Council issued the revised Implementation of the Trademark Law, which specified the requirements of applying for trademark registration and review.
Patent
According to the Patent Law of the People’s Republic of China promulgated by the SCNPC in 1984 and amended in 1992, 2000 and 2008, respectively, a patentable invention or a utility model must meet three criteria: novelty, inventiveness and practicability. A patent is valid for a twenty-year term for an invention and a ten-year term for a utility model or design, starting from the application date.
Domain Names
In May 2012, the China Internet Network Information Center issued the Implementing Rules for Domain Name Registration setting forth the detailed rules for registration of domain names. In August 2017, the MIIT promulgated the Administrative Measures on Internet Domain Names, or the Domain Name Measures. The Domain Name Measures regulate the registration of domain names, such as the top-level domain name “.cn”.
Regulations Relating to Foreign Exchange and Dividend Distributions
The principal regulations governing foreign currency exchange in China are the Foreign Exchange Administration Regulations, which was promulgated by the State Council in January 1996, which became effective in April 1996 and was subsequently amended in 1997 and 2008 and the Regulations on the Administration of Foreign Exchange Settlement, Sale and Payment which was promulgated by the PBOC in June 1996 and became effective in July 1996. Under these regulations, the Renminbi for current account items is freely convertible, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions, but not for capital account items, such as direct investments, loans, and investments in securities outside of the PRC, unless the prior approval of the SAFE or its local counterpart is obtained. Foreign invested enterprises are permitted to convert their after-tax dividends into foreign exchange and to remit such foreign exchange out of their foreign exchange bank accounts in the PRC.
The principal regulations governing distribution of dividends of foreign holding companies include the Foreign Investment Enterprise Law, which was amended in 2000 and 2016 respectively, and the Administrative Rules under the Foreign Investment Enterprise Law, which was amended in 2001 and 2014 respectively. Under these regulations, foreign investment enterprises in China may pay dividends only out of their accumulated profits, if any, determined in accordance with the PRC accounting standards and regulations. In addition, foreign investment enterprises in the PRC are required to allocate at least 10% of their accumulated profits each year, if any, to fund certain reserve funds unless these reserves have reached 50% of the registered capital of the enterprises. These reserves are not distributable as cash dividends. Furthermore, under the EIT Law, which became effective in January 2008, the maximum tax rate for the withholding tax imposed on dividend payments from PRC foreign invested companies to their overseas investors that are not regarded as “resident” for tax purposes is 20%. The rate was reduced to 10% under the Implementing Regulations for the EIT Law issued by the State Council. However, a lower withholding tax rate might be applied if there is a tax treaty between China and the jurisdiction of the foreign holding companies, such as tax rate of 5% in the case of Hong Kong companies that holds at least 25% of the equity interests in the foreign-invested enterprise, and certain requirements specified by PRC tax authorities are satisfied.
Regulations Relating to Stock Incentive Plans
According to the Notice of Issues Related to the Foreign Exchange Administration for Domestic Individuals Participating in Stock Incentive Plan of Overseas Listed Company, or the Share Incentive Rules, which was issued by the SAFE in February 2012 and other regulations, directors, supervisors, senior management and other employees participating in any share incentive plan of an overseas publicly-listed company who are PRC citizens or non-PRC citizens residing in China for a continuous period of not less than one year, subject to certain exceptions, are required to register with the SAFE. All such participants need to authorize a qualified PRC agent, such as a PRC subsidiary of overseas publicly-listed company to register with the SAFE and handle foreign exchange matters such as opening accounts, transferring and settlement of the relevant proceeds. The Share Incentive Rules further require an offshore agent to be designated to handle matters in connection with the exercise of share options and sale of proceeds for the participants of share incentive plans.
Failure to complete the SAFE registrations for our employee incentive plans after our listing may subject them to fines and legal sanctions, and may also limit our ability to contribute additional capital into our PRC subsidiaries and limit our PRC subsidiaries’ ability to distribute dividends to us.
Regulations Relating to Employment
The Labor Law of the People’s Republic of China, or the Labor Law, which became effective in January 1995 and was amended in 2009 and 2018, and the Employment Contract Law of the People’s Republic of China, or the Employment Contract Law, effective in January 2008 and amended in 2012, require employers to provide written contracts to their employees, restrict the use of temporary workers and aim to give employees long-term job security. Employers must pay their employees wages equal to or above local minimum wage standards, establish labor safety and workplace sanitation systems, comply with state labor rules and standards and provide employees with appropriate training on workplace safety. In September 2008, the State Council promulgated the Implementing Regulations for the PRC Employment Contract Law which became effective immediately and interprets and supplements the provisions of the Employment Contract Law.
Under the Labor Contract Law, an employer shall limit the number of dispatched workers so that they do not exceed a certain percentage of its total number of workers. In January 2014, the MOHRSS issued the Interim Provisions on Labor Dispatching, which became effective in March 2014, pursuant to which it provides that the number of dispatched workers used by an employer shall not exceed 10% of the total number of its employees.
The PRC governmental authorities have passed a variety of laws and regulations regarding social insurance and housing funds from time to time, including, among others, the Social Insurance Law of the People’s Republic of China, the Regulation of Insurance for Labor Injury, the Regulations of Insurance for Unemployment, the Provisional Insurance Measures for Maternal Employees, the Interim Administrative Provisions on Registration of Social Insurance and the Administrative Regulations on the Housing Provident Fund. Pursuant to these laws and regulations, PRC companies must make contributions at specified levels for their employees to the relevant local social insurance and housing fund authorities. Failure to comply with such laws and regulations may result in various fines and legal sanctions and supplemental contributions to the local social insurance and housing fund regulatory authorities.
C. Organizational Structure
The following diagram illustrates our corporate structure, including our principal subsidiaries, consolidated affiliated entities and subsidiaries of consolidated affiliated entities as of the date of this annual report on Form 20-F:
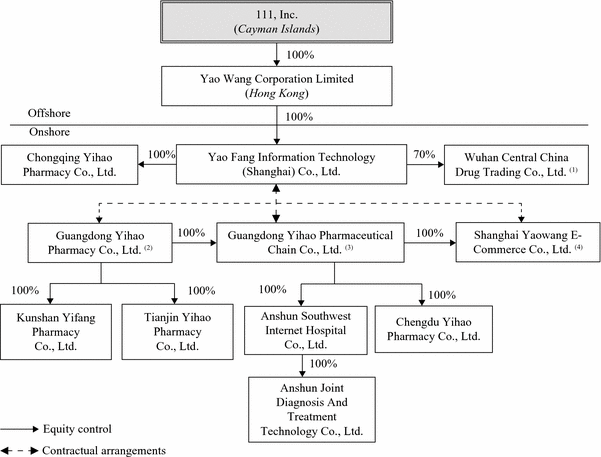
(1) The shareholders of Wuhan Central China Drug Trading Co., Ltd. include Yao Fang Information Technology (Shanghai) Co., Ltd. (70%) and Wuhan Zall Venture Capital Co., Ltd. (30%).
(2) Guangdong Yihao Pharmacy Co., Ltd. is our variable interest entity. The shareholders of Guangdong Yihao Pharmacy Co., Ltd. include Mr. Yue Xuan (50%) and Ms. Jing Liu (50%). Mr. Yue Xuan is a family member of Dr. Gang Yu, our co-founder and co-chairman. Ms. Jing Liu is a family member of Mr. Junling Liu, our co-founder, co-chairman and chief executive officer. See ‘‘—Contractual Arrangements with Our Variable Interest Entities.’’
(3) Guangdong Yihao Pharmaceutical Chain Co., Ltd. is our variable interest entity and is wholly owned by Guangdong Yihao Pharmacy Co., Ltd. See ‘‘—Contractual Arrangements with Our Variable Interest Entities.’’
(4) Shanghai Yaowang E-Commerce Co., Ltd. is our variable interest entity and is wholly owned by Guangdong Yihao Pharmaceutical Chain Co., Ltd. See ‘‘—Contractual Arrangements with Our Variable Interest Entities.’’
Contractual Arrangements with Our Variable Interest Entities
PRC laws and regulations impose restrictions on foreign ownership and investment in internet-based businesses such as provision of online information and other value-added telecommunications services. We are a Cayman Islands company and our PRC subsidiary, Yao Fang, is considered a wholly foreign owned enterprise. To comply with PRC laws and regulations, we have entered into a series of contractual arrangements, through Yao Fang, with our variable interest entities and the shareholders of our variable interest entities to obtain effective control over our variable interest entities and their subsidiaries.
We currently conduct our business through our variable interest entities and their subsidiaries based on these contractual arrangements, which allow us to:
· exercise effective control over our variable interest entities and their subsidiaries;
· receive substantially all of the economic benefits from our variable interest entities and their subsidiaries; and
· have an exclusive option to purchase all or part of the equity interest in our variable interest entities when, and to the extent, permitted by PRC law.
As a result of these contractual arrangements, we have become the primary beneficiary of our variable interest entities under U.S. GAAP. We have consolidated the financial results of our variable interest entities and their subsidiaries in our consolidated financial statements in accordance with U.S. GAAP.
The following is a summary of the currently effective contractual arrangements in relation to our wholly owned subsidiary, Yao Fang, our variable interest entities and their shareholders.
Agreements that Allow Us to Receive Economic Benefits from Our Variable Interest Entities
Exclusive Support Services Agreements. Yao Fang entered into exclusive support services agreements with each of our variable interest entities. Pursuant to these agreements, Yao Fang has the exclusive right to provide our variable interest entities with support services, including training, financial support, equipment and asset support, labor support, intellectual property support and other services relating to the day-to-day operations of our variable interest entities. Without Yao Fang’s prior written consent, our variable interest entities shall not accept any services or similar services covered by these agreements from any third party. Our variable interest entities agree to pay service fees in an amount equivalent to the balance calculated as quarterly revenue minus expenses of our variable interest entities on a quarterly basis. Yao Fang owns the intellectual property rights arising out of the services performed under these agreements. Unless Yao Fang terminates these agreements or pursuant to other provisions of these agreements, these agreements will remain effective for ten years to be automatically extended for another ten years thereafter.
Agreements that Provide Us with Effective Control over Our Variable Interest Entities
Proxy Agreement. Pursuant to the proxy agreement, each shareholder of our variable interest entities irrevocably authorizes Yao Fang to act as its attorney-in-fact to exercise all of such shareholder’s voting and other rights associated with the shareholder’s equity interest in our variable interest entities, including but not limited to, the right to attend shareholder meetings on behalf of such shareholder, the right to vote, the right to manage the variable interest entities and the right to appoint legal representatives, directors and other management. The proxy agreement remains in force for the same period as the exclusive support services agreements.
Equity Pledge Agreement. Yao Fang has entered into an equity pledge agreement with each shareholder of our variable interest entities. Pursuant to these equity pledge agreements, each shareholder of our variable interest entities has pledged all of his, her or its respective equity interest in our variable interest entities to Yao Fang to guarantee the performance by such shareholder and our variable interest entities of their respective obligations under the exclusive support services agreements, the proxy agreement, the exclusive option agreements, and payment of all accounts payable to Yao Fang from time to time. If our variable interest entities or any of their shareholders breach any obligations under these agreements, Yao Fang, as pledgee, will be entitled to dispose of the pledged equity and have priority to be compensated by the proceeds from the disposal of the pledged equity. Each of the shareholders of variable interest entities agrees that he, she or it will not dispose of the pledged equity interests, create or allow any encumbrance on the pledged equity interests, or engage in any activities that may have adverse effects on the pledger’s asset conditions without the prior written consent of Yao Fang. These equity pledge agreements will remain effective until our variable interest entities and their shareholders discharge all their respective obligations under the contractual arrangements which shall include, among other things, full payment of services fees to the WFOE under the exclusive support services agreement, and granting exclusive option to the WFOE or any third party designated by the WFOE to purchase all or part of their respective equity interests at the lowest price permitted by law under the exclusive option agreements, and repay all accounts payable to the WFOE. The equity pledge for all of our variable interest entities has been registered with local PRC authorities.
Rights and Obligations Assignment Agreement. Shuhong Yuan’s 50% equity interests in Yihao Pharmacy were transferred to Jing Liu pursuant to a share transfer agreement dated July 13, 2017. As a result, Jing Liu has become a shareholder of Yihao Pharmacy, our variable interest entity. In connection with this transaction, Jing Liu and Yue Xuan, both shareholders of our variable interest entity, Yihao Pharmacy, and Shuhong Yuan, a party to the contractual arrangements with Yihao Pharmacy, entered into the rights and obligations assignment agreement with Yao Fang and Yihao Pharmacy on July 13, 2017. Pursuant to this agreement, Shuhong Yuan assigned all of her rights and obligations under the exclusive option agreement, proxy agreement and equity pledge agreement to Jing Liu. As such, Jing Liu is deemed to have entered into and is currently bound by the contractual arrangements with this variable interest entity.
Agreements that Provide Us with the Option to Purchase the Equity Interest in Our Variable Interest Entities
Exclusive Option Agreements. Yao Fang has entered into exclusive option agreements with shareholders of our variable interest entities. Pursuant to these exclusive option agreements, the shareholders of our variable interest entities have irrevocably granted Yao Fang or any third party designated by Yao Fang an exclusive option to purchase all or part of their respective equity interests in our variable interest entities. The purchase price shall be the lowest price permitted by law. Without Yao Fang’s prior written consent, our variable interest entities shall not, among other things, supplement or amend their articles of association, increase or decrease the registered capital, sell, dispose of or set any encumbrance on their assets or revenue, enter into any material contracts, merge with any other persons or make any investments, or distribute dividends. The shareholders of our variable interest entities also jointly and severally undertake that they will not transfer, pledge or otherwise dispose of their respective equity interests in our variable interest entities to any third party or create or allow any encumbrance on their equity interests without Yao Fang’s prior written consent within the term of these agreements. These agreements will remain effective for the same period as the exclusive support services agreements.
In the opinion of Commerce & Finance Law Offices, our PRC counsel, the ownership structures of our variable interest entities, currently do not result in any violation of the applicable PRC laws or regulations currently in effect; and the agreements under the contractual arrangements among Yao Fang, our variable interest entities and their shareholders, are governed by PRC laws or regulations, and are currently valid, binding and enforceable in accordance with the applicable PRC laws or regulations currently in effect, and do not result in any violation of the applicable PRC laws or regulations currently in effect.
However, our PRC counsel, Commerce & Finance Law Offices, advised us that there are substantial uncertainties regarding the interpretation and application of current and future PRC laws, regulations and rules and there can be no assurance that the PRC government will ultimately take a view that is consistent with the opinion of our PRC counsel. In particular, in January 2015, the MOFCOM published the Draft FIL for public review and comments. Among other things, the Draft FIL, expands the definition of foreign investment and introduces the principle of “actual control” in determining whether a company is considered an FIE. Under the Draft FIL, our variable interest entities would also be deemed as FIEs, if they are ultimately “controlled” by foreign investors, and be subject to restrictions on foreign investments. However, the Draft FIL has not taken a position on what actions will be taken with respect to the existing companies controlled by foreign investors with the “variable interest entity” structure. In December 2018, the MOFCOM published the New Draft FIL. The concept of “actual control” was completely deleted from the New Draft FIL, which means variable interest entities that are ultimately controlled by foreign investors may not be subject to more strictly restriction in a short term. However, the possibility that such entities will be deemed as foreign-invested enterprise and subject to relevant restrictions in the future shall not be excluded. Accordingly, the PRC regulatory authorities may in the future take a view that is contrary to the above opinion of our PRC counsel. If the PRC government finds that the agreements that establish the structure for operating our online pharmaceutical and medical business do not comply with PRC government restrictions on foreign investment in value-added telecommunications services business, such as the internet content provision services, we could be subject to severe penalties, including being prohibited from continuing operations. See “Item 3. Key Information—D. Risk Factors—Risks Related to Our Corporate Structure” and “—Risks Related to Doing Business in China.”
A. Property, Plant and Equipment
Our corporate headquarters is located in Shanghai, China. As of December 31, 2018, we leased office space in Shanghai with an area of approximately 4,394 square meters. As of December 31, 2018, we also leased office space in Guangzhou, Wuhan, Beijing, Chengdu and Anshun with a total area of approximately 2,493 square meters. In addition, we leased properties in various locations in China for operations of Yi Hao Pharmacies, with a total area of approximately 2,288 square meters as of December 31, 2018. With respect to our fulfillment centers, we leased these facilities with a total area of approximately 36,197 square meters in Kunshan, Guangzhou, Tianjin and Chongqing as of December 31, 2018. We lease our premises from unrelated third parties under operating lease agreements. We believe that our existing facilities are generally adequate to meet our current needs, but we expect to seek additional space as needed to accommodate future growth, especially as we expand our fulfillment network and establish more fulfillment centers.
Item 4A. Unresolved Staff Comments
None.
Item 5. Operating and Financial Review and Prospects
The following discussion of our financial condition and results of operations is based upon, and should be read in conjunction with, our audited consolidated financial statements and the related notes included in this annual report on Form 20-F. This report contains forward-looking statements. See “Forward-Looking Information.” In evaluating our business, you should carefully consider the information provided under the caption “Item 3. Key Information—D. Risk Factors” in this annual report on Form 20-F. We caution you that our businesses and financial performance are subject to substantial risks and uncertainties.
A. Operating Results
Overview
Today, we provide hundreds of millions of consumers with better access to pharmaceutical products and medical services, directly through our online retail pharmacy and indirectly through our offline pharmacy network. According to Frost & Sullivan, 1 Drugstore has been the largest direct sales online pharmacy in China since 2016 in terms of GMV. In 2016, we commenced our online medical services through our internet hospital, 1 Clinic (1诊), to provide consumers with cost-effective and convenient online consultation and electronic prescription services.
We are building our core competencies in the areas of smart supply chain, cloud-based solutions, big data and medical expertise, and are reshaping the pharmaceutical value chain in China using our New Retail platform. Not only do we serve consumers directly through our online retail pharmacy, we also enabled more than 150,000 offline pharmacies to better serve their consumers as of December 31, 2018. Our online wholesale pharmacy, 1 Drug Mall (1药城), serves as a one-stop shop for pharmacies to source a vast selection of pharmaceutical products. This network of pharmacies represents the largest virtual pharmacy network in the world in terms of the total number of pharmacy stores, as of May 18, 2018, according to Frost & Sullivan.
In 2016, we began the transformation from a pure B2C business to a New Retail platform, integrating our online retail pharmacy and offline pharmacy network by leveraging our smart supply chain and cloud-based solutions. This model allows us to collect and analyze data from a large number of transactions, which we use to continuously increase the efficiency of our smart supply chain, and the intelligence of our cloud-based solutions.
We currently derive our revenues primarily from selling and distributing pharmaceutical and other health and wellness products. We also generate revenues from service modules such as marketplace vendor commissions; brand promotion, data and other marketing services for pharmaceutical companies and others.
We successfully implemented our business transformation and our revenue reached RMB959.5 million in 2017 and RMB1,786.0 million (US$259.8 million) in 2018, of which product revenues from the B2B segment reached RMB86.9 million and RMB922.8 million (US$134.2 million), respectively. Our net loss margin improved from 41.6% in 2016 to 26.0% in 2017 and further to 21.4% in 2018.
Key Factors Affecting Our Results of Operations
Our results of operations are affected by general factors driving China’s general health and wellness industry, especially pharmaceutical retail and wholesale distribution and internet healthcare industries in China.
Our business expansion and revenue growth have been and will continue to be affected by the development of the general health and wellness industry in China, which is in turn driven by increasing disposable income and healthcare spending, rising awareness of health, an aging population, increasing life expectancy, increasing penetration of mobile internet, favorable government policies and increasing coverage of medical insurance. Unfavorable changes in any of these general industry conditions could negatively affect demand for our products and services and negatively and materially affect our results of operations.
We are affected by government policies and regulations that address all aspects of our operations, including qualifications and licensing requirements for online and offline sales and distribution of pharmaceutical and other health and wellness products, online healthcare services and online hospitals, among other things. See also ‘‘Item 3. Key Information—D. Risk Factors—Risks Related to Our Business and Industry—We are subject to extensive and evolving regulatory requirements, non-compliance with which, or changes in which, may materially and adversely affect our business and prospects.’’ We have benefitted from certain recent favorable regulatory and policy changes in China, especially various policy initiatives that have promoted the distribution of pharmaceutical products. We expect that the implementation of these measures relating to the distribution of pharmaceutical products will also affect market competition and drive industry consolidation.
While our business is influenced by general factors driving the general health and wellness market in China, we believe our results of operations are more directly affected by company-specific factors, including the following major factors.
Our Ability to Attract and Retain Consumers and Pharmacies
Our net revenues are dependent on our ability to attract and retain our consumers and pharmacies.
· Consumers. Historically, our revenue growth was primarily driven by the sale of pharmaceutical and other health and wellness products through our online retail pharmacy to consumers. The average revenue per consumer grew from RMB672.6 in 2016 to RMB866.9 in 2017 and to RMB1,102 (US$160.3) in 2018. Steady and continued growth of our consumer base and their stickiness to our platform allows us to maintain a solid foundation for our business to continue to expand.
· Pharmacies. Since we launched our online wholesale pharmacy, 1 Drug Mall, in May 2017, we have significantly increased the scale of our B2B business through the sale of pharmaceutical products to pharmacies. As of December 31, 2018, we have served more than 150,000 pharmacies, compared to over 48,000 pharmacies as of December 31, 2017. We have also diversified the types of pharmacy customers we serve and have expanded our reach not only to independent pharmacies and pharmacy chains, but also to in-house pharmacies within clinics and private hospitals. We have thus far accumulated considerable offline pharmacy resources, which contributed to the increase of product revenues from the B2B segment to RMB922.8 million (US$134.2 million) in 2018. We expect to further expand our offline pharmacy market and to develop this revenue stream. Product revenues from the B2B segment contributed 9.0% and 51.6% to our total net revenues in 2017 and 2018, respectively.
We rely on a diverse array of online marketing channels to attract consumers, including using social media such as WeChat and Weibo and paid placement on major online search engines in China. With respect to growing our pharmacy customers, we rely on the effective operation of our on-the-ground sales force to promote our products and services. Our ability to continue to reach more consumers and pharmacies will affect the growth of our business and our net revenues.
Our Ability to Create Value for Participants in the Healthcare Ecosystem and Increase Monetization
We are a pioneer in developing and applying technologies to create an integrated online and offline platform in the healthcare ecosystem in China. Our results of operations depend on our ability to create value for various participants in the healthcare ecosystem and increase monetization for these participants. Consumers and pharmacies are drawn to our platform because we offer a wide selection of competitively priced pharmaceutical and other health and wellness products, as well as efficient and comprehensive services. In addition, we offer to suppliers, pharmaceutical companies, medical professionals and other participants in the ecosystem our innovative cloud-based solutions, such as data service, CSO, smart supply chain services and other value-added services. Our success depends on our ability to continuously offer attractive products and services, therefore increasing user stickiness and attracting more participants to our close-loop online and offline platform. We have also implemented various initiatives and invested significantly to ramp up our cloud-based solutions and improve our smart supply chain services. As we further enhance our technologies and IT infrastructure, we aim to create more value for these participants, increasing their engagement and connection and deepening our penetration in the healthcare ecosystem, which we anticipate will create additional monetization venues for us to drive our revenue growth.
Our Ability to Manage Our Mix of Product and Service Offerings
Our results of operations are also affected by the mix of products and services we offer. We currently derive our revenues primarily from the sale and distribution of pharmaceutical and other health and wellness products to our pharmacy customers and consumers. We also earn commissions and service fees from marketplace sellers on our online marketplace. Different products and services have different cost structures. For example, the various services we provide generally have higher fixed costs. The revenue contributions from our online direct sales model, our online marketplace model and our services have a major influence on our profitability. We intend to better manage the mix of our product and service offerings in order to improve our profitability.
Our Ability to Control Operating Costs and Expenses and Improve Efficiency
Our cost of products sold represents primarily the purchase price of products and inbound shipping charges if any, as well as inventory write-downs. In 2018, we sourced our products from over 500 suppliers, including pharmaceutical companies and distributors. As our business further grows in scale, we expect to obtain more favorable terms from suppliers, including pricing terms, credit period and volume-based rebates. In addition, we aim to create value for our suppliers, especially pharmaceutical companies, by providing an effective and transparent channel for selling large volumes of their products online and by offering them valuable data insights on market demand, customer preferences and supply chain information. We believe this value proposition will also help us deepen our relationships with suppliers, obtain favorable terms and reduce our procurement costs. Our selling and marketing expenses are a significant contributor to our operating costs and expenses, and they primarily consist of payroll, bonus and employee benefits of sales and marketing staff, advertising costs, agency fees and costs for promotional materials. In 2016, 2017 and 2018, selling and marketing expenses amounted to 28.9%, 19.8% and 14.6% of our total net revenues, respectively. We expect our selling and marketing expenses to remain substantial in absolute terms as we implement new business initiatives, such as deploying additional sales personnel to promote our 1 Drug Mall and our value-added services to pharmacies. As our business grows, we anticipate that our technology and fulfillment expenses will increase in absolute terms in the foreseeable future in light of our anticipated expansion and investment plans.
In 2018, we were able to grow our customer base organically without increasing our marketing. Our procurement capabilities strengthened with the increase in the number of our customers. As a result, our cost of products sold declined in 2018 and is expected to further decrease in the future.
We continuously seek to streamline our operations and improve our supply chain and inventory management. Controlling costs and operating expenses to achieve optimal operating efficiency is important to our success. As our business grows in scale, we expect to have significant operating leverage and realize structural cost savings.
Key Components of Results of Operations
Net Revenues
The following table sets forth the components of our net revenues by amounts and percentages of our total net revenues for the periods presented:
|
|
|
For the Year Ended December 31, |
| ||||||||||||
|
|
|
2016 |
|
2017 |
|
2018 |
| ||||||||
|
|
|
RMB |
|
% |
|
RMB |
|
% |
|
RMB |
|
US$ |
|
% |
|
|
|
|
(in thousands, except for percentages) |
| ||||||||||||
|
Product revenues |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
B2C segment product revenues |
|
870,361 |
|
99.6 |
|
862,327 |
|
89.9 |
|
847,476 |
|
123,260 |
|
47.5 |
|
|
B2B segment product revenues |
|
— |
|
— |
|
86,890 |
|
9.0 |
|
922,751 |
|
134,209 |
|
51.6 |
|
|
Total product revenues |
|
870,361 |
|
99.6 |
|
949,217 |
|
98.9 |
|
1,770,227 |
|
257,469 |
|
99.1 |
|
|
Service revenues |
|
3,476 |
|
0.4 |
|
10,269 |
|
1.1 |
|
15,743 |
|
2,290 |
|
0.9 |
|
|
Total |
|
873,837 |
|
100.0 |
|
959,486 |
|
100.0 |
|
1,785,970 |
|
259,759 |
|
100.0 |
|
Product revenues. We generate and report product revenues under our direct sales model from two reportable segments: the B2C segment and the B2B segment. Product revenues from our B2C segment are generated from the sale of pharmaceutical and other health and wellness products through 1 Drugstore and offline pharmacies to consumers. In 2016, 2017 and 2018, a substantial majority of our revenues were attributable to product revenues from the B2C segment. We also generate product revenues from the B2B segment through the sale of pharmaceutical products to pharmacies on 1 Drug Mall. We expect our product revenues, in particular, those from B2B segment, to grow significantly as we attract more pharmacies as customers. Our product revenue from the B2B business segment in 2018 reached RMB922.8 million (US$134.2 million), representing over a tenfold increase from 2017.
Service revenues. Service revenues primarily consist of marketplace (MP) service fees we charge to marketplace sellers to which we provide access to 1 Drugstore where they are able to effectively sell their products. We charge marketplace sellers commission fees equal to an agreed percentage of the sales price of the product when a sale is completed and also charge marketplace sellers an annual non-refundable up-front fee for platform usage. We refer to these fees as MP service revenue. Since we launched our MP service, it has made substantial contribution to our service revenues. Our service revenues increased significantly by 52.4% from RMB10.3 million in 2017 to RMB15.7 million (US$2.3 million) in 2018, which was primarily attributable to an increase of RMB3.6 million (US$0.5 million) in MP service revenues. We also generate service revenues by providing other ancillary services, mainly online medical consultation services. We expect our service revenues, although not a material contribution to our net revenues currently or in the near future, to grow as we expand our online marketplace and increase the service coverage of our cloud-based solutions, such cloud prescription services and data services to more pharmacies and pharmaceutical companies.
In November 2017, we started to offer a membership program to consumers for a fixed quarterly or yearly service fee. Under this program, members can enjoy additional free shipping and certain price discounts by using coupons offered exclusively pursuant to such memberships. We do not expect membership fee will contribute a significant amount to our revenue in the future.
Operating costs and expenses
The following table sets forth the components of our operating costs and expenses by amounts and percentages of total operating costs and expenses for the periods presented:
|
|
|
For the Year Ended December 31, |
| ||||||||||||
|
|
|
2016 |
|
2017 |
|
2018 |
| ||||||||
|
|
|
RMB |
|
% |
|
RMB |
|
% |
|
RMB |
|
US$ |
|
% |
|
|
|
|
(in thousands, except for percentages) |
| ||||||||||||
|
Operating costs and expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of products sold |
|
(796,230 |
) |
64.3 |
|
(868,719 |
) |
71.6 |
|
(1,681,700 |
) |
(244,592 |
) |
76.9 |
|
|
Fulfillment expenses |
|
(68,445 |
) |
5.5 |
|
(55,880 |
) |
4.6 |
|
(73,930 |
) |
(10,753 |
) |
3.4 |
|
|
Selling and marketing expenses |
|
(252,829 |
) |
20.4 |
|
(190,074 |
) |
15.6 |
|
(260,040 |
) |
(37,822 |
) |
11.9 |
|
|
General and administrative expenses |
|
(60,836 |
) |
4.9 |
|
(53,434 |
) |
4.4 |
|
(98,759 |
) |
(14,364 |
) |
4.5 |
|
|
Technology expenses |
|
(61,767 |
) |
5.0 |
|
(48,133 |
) |
4.0 |
|
(71,248 |
) |
(10,363 |
) |
3.3 |
|
|
Other operating income (expenses), net |
|
1,990 |
|
(0.1 |
) |
2,732 |
|
(0.2 |
) |
(668 |
) |
(97 |
) |
0.0 |
|
|
Total |
|
(1,238,117 |
) |
100.0 |
|
(1,213,508 |
) |
100.0 |
|
(2,186,345 |
) |
(317,991 |
) |
100.0 |
|
Cost of products sold. Cost of products sold consists of the purchase price of products and inbound shipping charges, as well as inventory write-downs, less rebates earned from vendors in the form of credits that we can apply against trade amounts owed to these vendors pursuant to binding arrangements when we complete a specified cumulative level of purchases within a specified time period. Cost of products does not include other direct costs related to costs of product sales such as shipping and handling expense, payroll and employee benefits for logistic staff, logistic centers rental expenses and depreciation expenses. Therefore, our cost of products sold may not be comparable to that of other companies, which include such expenses in their costs of products sold. We expect our cost of products sold to grow in absolute terms as our business continues to grow.
Fulfillment expenses. Fulfillment expenses primarily consist of payroll, bonus and employee benefits for logistics staff, logistics centers rental expenses, shipping and handling expenses and packaging expenses. We expect our fulfillment expenses as a percentage of our total net revenues to decrease because larger B2B orders reduce our cost as a percentage as a result of the way pricing is undertaken by the logistics business. In addition, we have implemented more cost-saving initiatives and continue to expand our fulfillment network to leverage our scale.
Selling and marketing expenses. Selling and marketing expenses primarily consist of payroll, bonus and employee benefits for sales and marketing staff, advertising costs, agency fees and costs for promotional materials. We expect our selling and marketing expenses to remain substantial in absolute terms as we implement new business initiatives, such as deploying additional sales personnel to promote our 1 Drug Mall and our value-added services to pharmacies.
General and administrative expenses. General and administrative expenses primarily consist of payroll, bonus and employee benefit costs for corporate employees, legal, finance, rental expenses, and other corporate overhead costs. We expect our general and administrative expenses to increase in absolute terms in the foreseeable future due to the anticipated growth of our business as well as accounting, insurance, investor relations and other public company costs, but to decrease as a percentage of our total net revenues as we leverage the scale of our business.
Technology expenses. Technology expenses primarily consist of payroll, bonus and employee benefits for our technology and system department staffs and expenses incurred for the development and enhancement of our websites, technology platforms and applications. We expect our technology expenses to grow in absolute terms as we expand our technology team, enhance our big data analytics capabilities and develop new features and applications to better serve various participants in the healthcare ecosystem, but to decrease as a percentage of our total net revenues as we are able to leverage the scale of our business as we continue to grow.
Results of Operations
The following table sets forth a summary of our consolidated results of operations for the periods indicated. The period-to-period comparisons of results of operations should not be relied upon as indicative of future performance. This information should be read together with our audited consolidated financial statements and related notes included elsewhere in this annual report.
|
|
|
For the Year Ended December 31, |
| ||||||||||||
|
|
|
2016 |
|
2017 |
|
2018 |
| ||||||||
|
|
|
RMB |
|
% |
|
RMB |
|
% |
|
RMB |
|
US$ |
|
% |
|
|
|
|
(in thousands) | |||||||||||||
|
Net Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product revenues |
|
870,361 |
|
99.6 |
|
949,217 |
|
98.9 |
|
1,770,227 |
|
257,469 |
|
99.1 |
|
|
Service revenues |
|
3,476 |
|
0.4 |
|
10,269 |
|
1.1 |
|
15,743 |
|
2,290 |
|
0.9 |
|
|
Total net revenues |
|
873,837 |
|
100.0 |
|
959,486 |
|
100.0 |
|
1,785,970 |
|
259,759 |
|
100.0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating costs and expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of products sold |
|
(796,230 |
) |
(91.1 |
) |
(868,719 |
) |
(90.5 |
) |
(1,681,700 |
) |
(244,592 |
) |
(94.2 |
) |
|
Fulfillment expenses |
|
(68,445 |
) |
(7.8 |
) |
(55,880 |
) |
(5.8 |
) |
(73,930 |
) |
(10,753 |
) |
(4.1 |
) |
|
Selling and marketing expenses (1) |
|
(252,829 |
) |
(28.9 |
) |
(190,074 |
) |
(19.8 |
) |
(260,040 |
) |
(37,822 |
) |
(14.6 |
) |
|
General and administrative expenses (1) |
|
(60,836 |
) |
(7.0 |
) |
(53,434 |
) |
(5.6 |
) |
(98,759 |
) |
(14,364 |
) |
(5.5 |
) |
|
Technology expenses(1) |
|
(61,767 |
) |
(7.1 |
) |
(48,133 |
) |
(5.0 |
) |
(71,248 |
) |
(10,363 |
) |
(4.0 |
) |
|
Other operating income (expenses), net |
|
1,990 |
|
0.2 |
|
2,732 |
|
0.3 |
|
(668 |
) |
(97 |
) |
(0.0 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total operating costs and expenses |
|
(1,238,117 |
) |
(141.7 |
) |
(1,213,508 |
) |
(126.4 |
) |
(2,186,345 |
) |
(317,991 |
) |
(122.4 |
) |
|
Loss from operations |
|
(364,280 |
) |
(41.7 |
) |
(254,022 |
) |
(26.4 |
) |
(400,375 |
) |
(58,232 |
) |
(22.4 |
) |
|
Interest income |
|
2,308 |
|
0.3 |
|
4,013 |
|
0.4 |
|
4,352 |
|
633 |
|
0.2 |
|
|
Interest expense |
|
(751 |
) |
(0.1 |
) |
(55 |
) |
0.0 |
|
— |
|
— |
|
— |
|
|
Foreign exchange gain (loss) |
|
2,630 |
|
0.3 |
|
(3,492 |
) |
(0.4 |
) |
2,459 |
|
358 |
|
0.1 |
|
|
Other income (loss), net |
|
(3,353 |
) |
(0.4 |
) |
4,229 |
|
0.4 |
|
11,531 |
|
1,676 |
|
0.6 |
|
|
Loss before income taxes |
|
(363,446 |
) |
(41.6 |
) |
(249,327 |
) |
(26.0 |
) |
(382,033 |
) |
(55,565 |
) |
(21.4 |
) |
|
Income tax expense |
|
— |
|
— |
|
— |
|
— |
|
(8 |
) |
(1 |
) |
(0.0 |
) |
|
Net loss |
|
(363,446 |
) |
(41.6 |
) |
(249,327 |
) |
(26.0 |
) |
(382,041 |
) |
(55,566 |
) |
(21.4 |
) |
(1) Share-based compensation expenses are allocated to operating expense line items as follows:
|
|
|
For the Year Ended December 31, | |||||||
|
|
|
2016 |
|
2017 |
|
2018 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) | |||||||
|
General and administrative expenses |
|
1,846 |
|
5,176 |
|
22,477 |
|
3,269 |
|
|
Selling and marketing expenses |
|
1,382 |
|
3,674 |
|
23,561 |
|
3,427 |
|
|
Technology expenses |
|
210 |
|
1,071 |
|
5,321 |
|
774 |
|
|
Total |
|
3,438 |
|
9,921 |
|
51,359 |
|
7,470 |
|
The Year Ended December 31, 2018 Compared to the Year Ended December 31, 2017
Net Revenues
Our net revenues increased by 86.1% from RMB959.5 million in 2017 to RMB1,786.0 million (US$259.8 million) in 2018. This increase was primarily due to the significant increase in product revenues from B2B segment . Our net revenues experienced consistent monthly growth and are significantly impacted by the annual and mid-year e-commerce festivals.
Product Revenues by Segment. Product revenues increased by 86.5% from RMB949.2 million in 2017 to RMB1,770.2 million (US$257.5 million) in 2018, due to an increase in the number of offline pharmacies through which more products were sold. We experienced a significant increase in product revenues from B2B segment, which increased by 961.9% to RMB922.8 million (US$134.2 million) from RMB86.9 million last year. Product revenues from B2C segment decreased by 1.7% to RMB847.5 (US$123.3 million) from RMB862.3 million last year.
Service revenues. Our service revenues increased by 52.4% from RMB10.3 million in 2017 to RMB15.7 million (US$2.3 million) in 2018, which was primarily attributable to the launch of our B2B MP service in 2018 and the expansion of our B2C MP services.
Segment Cost of Products Sold
Cost of products sold increased by 93.6% from RMB868.7 million in 2017 to RMB1,681.7 million (US$244.6 million) in 2018, in line with our overall revenue growth , which is primarily attributable to growth in sales and a change in revenue mix with a much higher proportion of B2B business.
Segment Profit/Loss
As a result of the foregoing, our segment profit from our B2C segment decreased by 2.2% from RMB82.2 million in 2017 to RMB80.4 million (US$11.7 million) in 2018, while our segment profit from our B2B segment was RMB8.1 million in (US$1.2 million) 2018.
Operating Costs and Expenses
Our operating costs and expenses increased by 80.2% from RMB1,213.5 million in 2017 to RMB2,186.3 million (US$318.0 million) in 2018, with increases in in the following categories of operating expenses.
Cost of Products Sold. Our cost of products sold increased by 93.6% from RMB868.8 million in 2017 to RMB1,681.7 million (US$244.6 million) in 2018, primarily due to growth in sales and a change in revenue mix with a much higher proportion of B2B business.
Fulfillment Expenses. Our fulfillment expenses increased by 32.2% from RMB55.9 million in 2017 to RMB73.9 million (US$10.8 million) in 2018, primarily as a result of growth In B2B business. Fulfillment expenses accounted for 4.1% of net revenue in 2018 as compared to 5.8% last year.
Selling and Marketing Expenses. Our selling and marketing expenses increased by 36.8% from RMB190.1 million in 2017 to RMB260.0 million (US$37.8 million) in 2018. The increase was primarily attributable to increase in the number of sales staff and expenses associated with the expansion of B2B business. Selling and marketing expenses accounted for 14.6% of net revenue in 2018 as compared to 19.8% last year.
General and Administrative Expenses. Our general and administrative expenses increased by 85.0% from RMB53.4 million in 2017 to RMB98.8 million (US$14.4 million) in 2018. The increase was primarily due to the following reasons:
a) increase in management salary by 42.3% from RMB26.0 million in 2017 to RMB37.0 million in 2018. The increase was primarily due to an increase of management headcount;
b) increase in share-based compensation by 332.7% from RMB5.2 million in 2017 to RMB22.5 million in 2018. The increase was primarily due to growth of management team; and
c) increase in consulting service by 358.3% from RMB2.4 million in 2017 to RMB11.0 million in 2018. The increase was primarily due to IPO-related consulting service.
Technology Expenses. Our technology expenses increased by 48.0% from RMB48.1 million in 2017 to RMB71.2 million (US$10.4 million) in 2018, mainly due to investments in platform and product development, including the recruitment of technology-related staffs. Technology expenses accounted for 4.0% of net revenue in 2018 as compared to 5.0% last year.
Net Loss
As a result of the foregoing, we recorded a net loss of RMB382.0 million (US$55.6 million) in 2018 and a net loss of RMB249.3 million in 2017.
The Year Ended December 31, 2017 Compared to the Year Ended December 31, 2016
Net Revenues
Our net revenues increased by 9.8% from RMB873.8 million in 2016 to RMB959.5 million in 2017. This increase was primarily due to the increases in product revenues from the B2B segment of RMB86.9 million and, to a lesser extent, service revenues. Our net revenues experienced consistent monthly growth and are significantly impacted by the annual and mid-year e-commerce festivals.
Product Revenues by Segment. Product revenues increased by 9.1% from RMB870.4 million in 2016 to RMB949.2 million in 2017, due to an increase of RMB86.9 million in product revenues from the B2B segment due to our accumulation of considerable offline pharmacy resources, offset by a decrease of RMB8.0 million in product revenues from the B2C segment. Our product revenues from the B2B segment were nil in 2016, and we launched 1 Drug Mall in 2017 and have since grown our B2B business rapidly. We expect to further expand the offline pharmacy market and develop our product revenues from the B2B segment. Our product revenues from the B2C segment decreased slightly in 2017 because we invested in the growth of our online marketplace business, which resulted in change in revenue mix between our product and service revenues.
Service revenues. Our service revenues increased significantly by 194.3% from RMB3.5 million in 2016 to RMB10.3 million in 2017, which was primarily attributable to an increase of RMB6.3 million in MP service revenues. Our MP service revenue increased as our online marketplace grew. In 2017, in addition to commission fees charged based on transaction value, we also started to charge marketplace sellers annual fixed fees for maintaining storefronts with us.
Segment Cost of Products Sold
Cost of products sold increased by 9.1% from RMB796.2 million in 2016 to RMB868.7 million in 2017, due to an increase of RMB88.6 million in cost of products sold from the B2B segment, and offset by a decrease of RMB16.1 million in cost of products sold from the B2C segment. These changes were primarily attributable to changes in procurement costs of our products, which were consistent with the growth of our business and respective changes in product revenues from the B2C segment and the B2B segment.
Segment Profit/Loss
As a result of the foregoing, our segment profit from our B2C segment increased by 10.9% from RMB74.1 million in 2016 to RMB82.2 million in 2017, while our segment loss from our B2B segment was RMB1.7 million in 2017.
Operating Costs and Expenses
Our operating costs and expenses decreased by 2.0% from RMB1,238.1 million in 2016 to RMB1,213.5 million in 2017, with an increase in cost of products sold as described in “—Segment Cost of Products Sold” and decreases in the following categories of operating expenses.
Fulfillment Expenses. Our fulfillment expenses decreased by 18.3% from RMB68.4 million in 2016 to RMB55.9 million in 2017, primarily as a result of a decrease in the number of customer orders. In order to meet the discount requirement, the average purchase amount per order increased in 2017, resulting in the decreased fulfillment expense per order as marginal fulfillment expense decreased. The decrease was partially offset by an increase in warehouse renovation amortization of approximately RMB3.0 million.
Selling and Marketing Expenses. Our selling and marketing expenses decreased by 24.8% from RMB252.8 million in 2016 to RMB190.1 million in 2017. The decrease was primarily attributable to our marketing strategy in 2017, which shifted in focus from advertising and marketing to medical conference sponsorships. Such marketing strategy enabled us to effectively reduce our marketing expenses while raising brand awareness.
General and Administrative Expenses. Our general and administrative expenses decreased by 12.2% from RMB60.8 million in 2016 to RMB53.4 million in 2017. The decrease was primarily due to the following reasons:
a) decrease in management fees by 50.0% from RMB4.8 million in 2016 to RMB2.4 million in 2017. The decrease was primarily due to a decrease of 150 personnel in 2017 as compared with 2016, thus reducing office, travel and repair expenses. Additionally, we ceased operations of our three branches in 2017, further reducing management fees;
b) decrease in decoration and repair costs by 60.6% from RMB3.3 million in 2016 to RMB1.3 million in 2017. The decrease resulted from the ceased operations of our three branches in 2017; and
c) decrease in recruitment fees by 60% from RMB2.0 million in 2016 to RMB0.8 million in 2017. The decrease was mainly attributable to the rapid expansion of our organizational restructuring in early 2016, resulting in recruitment fees for executives search.
Technology Expenses. Our technology expenses decreased by 22.2% from RMB61.8 million in 2016 to RMB48.1 million in 2017. We made certain investments in our technology and incurred upfront costs in 2016, and we made incremental improvement in 2017, which incurred less technology expense. We also adjusted headcount to improve our operational efficiency in 2017. Our reduced headcount is in line with the decrease in technology expenses.
Net Loss
As a result of the foregoing, we recorded a net loss of RMB249.3 million in 2017 and a net loss of RMB363.4 million in 2016.
Taxation
Cayman Islands
We are incorporated in the Cayman Islands. The Cayman Islands currently has no income, corporation or capital gains tax and no estate duty, inheritance tax or gift tax. The Cayman Islands does not impose a withholding tax on payments of dividends to shareholders.
Hong Kong
Our subsidiary incorporated in Hong Kong is subject to Hong Kong profit tax at a rate of 16.5%. No Hong Kong profit tax has been levied as we did not have assessable profit that was earned in or derived from the Hong Kong subsidiary during the periods presented. Hong Kong does not impose a withholding tax on dividends.
China
Enterprise Income Tax. According to the EIT Law, which was promulgated on March 16, 2007 and amended in 2017 and 2018, and its implementing regulation, an income tax rate of 25% generally applies to all enterprises incorporated in the PRC, including our PRC subsidiaries, our variable interest entities and their subsidiaries. Under the EIT Law, an enterprise established outside the PRC with “de facto management bodies” within the PRC is considered a resident enterprise for PRC enterprise income tax purposes and is generally subject to a uniform 25% enterprise income tax rate on its worldwide income. Although we do not believe that 111, Inc. or Yao Wang Corporation Limited should be considered as a PRC resident enterprise for PRC tax purposes, PRC income tax at a rate of 25% would generally be applicable to our worldwide income if we were to be considered a PRC resident enterprise.
Dividend Withholding Tax. According to the EIT Law and its implementation rules, the profits of a foreign-invested enterprise arising in 2008 and thereafter that are distributed to its immediate holding company outside the PRC are subject to withholding tax at a rate of 10%, but a lower withholding tax rate will be applied if there is a beneficial tax treaty between the PRC and the jurisdiction of the foreign holding company. A holding company in Hong Kong, for example, will be eligible, with approval of the PRC local tax authority, to be subject to a 5% withholding tax rate under the Double Taxation Arrangement, if such holding company is considered to be a non-PRC resident enterprise and holds at least 25% of the equity interests in the PRC foreign-invested enterprise distributing the dividends. However, the PRC tax authorities will review preferential tax treatment under the “substance over form” principle and grant such treatment on a case-by-case basis. Therefore, if such Hong Kong holding company is not considered to be the beneficial owner of such dividends under applicable PRC tax regulations, such dividend will remain subject to withholding tax at a rate of 10%.
Value-Added Tax. According to the Provisional Regulations of the PRC on Value-added Tax promulgated by the State council on December 13, 1993 and amended in 2008, 2016 and 2017, and the Detailed Rules for the Implementation of the Provisional Regulations of the PRC on Value-added Tax which was promulgated by the MOF, and the SAT on December 18, 2008 and became effective on January 1, 2009 and amended on October 28, 2011, all enterprises and individuals that engage in the sale of goods and services, the provision of tangible personal property leasing services or importation of goods shall pay value-added tax at different tax rates of 0%, 6%, 11% or 17% due to different business; in addition, the small-scale taxpayers shall be subject to tax rate of 3%, except as otherwise specified by the State Council. On April 4, 2018, the MOF and the SAT issued the Notice on Adjusting Value-added Tax Rate, which became effective from May 1, 2018 and stipulates that the previous tax rate of 17%, 11% for the taxable sale activities or importing goods will be adjusted to 16% and 10% respectively. Moreover, on the same date, the MOF and the SAT issued another notice to unify the criteria of small-scale value-added tax payers, which became effective from May 1, 2018. On November 16, 2011, the MOF and the SAT promulgated a Pilot Plan for Imposition of Value-Added Tax to Replace Business Tax in Shanghai, or Pilot Plan, which became effective on January 1, 2012 and stipulates that any entity in Shanghai that falls in the category of “selected modern service industries” was required to switch from being a business tax payer to become a value-added tax payer, who is permitted to offset expenses incurred in providing the relevant services it provides from the taxable income. The Pilot Plan was expanded to other regions in September 2012, and was further expanded nationwide beginning on August 1, 2013. The MOF and the SAT subsequently promulgated several circulars in December 2013, April 2014 and March 2016 to further expand the scope of services which are to be subject to value-added tax instead of business tax.
Inflation
To date, inflation in China has not materially affected our results of operations. According to the National Bureau of Statistics of China, the year-over-year percent changes in the consumer price index for December 2016, 2017 and 2018 were increases of 2.1%, 1.8% and 1.9%, respectively. Although we have not been materially affected by inflation in the past, we may be affected if China experiences higher rates of inflation in the future.
Critical Accounting Policies, Judgments and Estimates
We prepare our financial statements in conformity with U.S. GAAP, which requires us to make judgments, estimates and assumptions. We continually evaluate these estimates and assumptions based on the most recently available information, our own historical experience and various other assumptions that we believe to be reasonable under the circumstances. Since the use of estimates is an integral component of the financial reporting process, actual results could differ from our expectations as a result of changes in our estimates. Some of our accounting policies require a higher degree of judgment than others in their application and require us to make significant accounting estimates.
The following descriptions of critical accounting policies, judgments and estimates should be read in conjunction with our consolidated financial statements and other disclosures included in this annual report. When reviewing our financial statements, you should consider (i) our selection of critical accounting policies, (ii) the judgments and other uncertainties affecting the application of such policies and (iii) the sensitivity of reported results to changes in conditions and assumptions.
Inventories
Inventories, consisting of products available for sale, are stated at the lower of cost or market value. Cost of inventory is determined using the weighted average cost method. Adjustments are recorded to write down the cost of inventory to the estimated market value due to slow-moving or damaged goods, which is dependent upon factors such as historical and forecasted consumer demand, and promotional environment. Write-downs are recorded in cost of products sold in the consolidated statements of income (loss) and comprehensive income (loss).
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and impairment. The renovations, betterments and interest cost incurred during construction are capitalized. Property and equipment are depreciated at rates sufficient to write off their costs less impairment and residual value, if any, over the estimated useful lives on a straight-line basis.
Construction in progress represents leasehold improvements under construction or being installed and is stated at cost. Cost comprises original cost of property and equipment, installation, construction and other direct costs. Construction in progress is transferred to leasehold improvements and depreciation commences when the asset is ready for its intended use.
Expenditures for repairs and maintenance are expensed as incurred. Gain or loss on disposal of property and equipment, if any, is recognized in the consolidated statements of comprehensive loss as the difference between the net sales proceeds and the carrying amount of the underlying asset.
Revenue Recognition
We follow five steps to recognize revenue under ASC 606: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation.
We report revenue net of discount, business tax, value added tax and related surcharges. Our net revenue consists of product revenues and service revenues.
Product Revenues
We generate our product revenues from the sale of pharmaceutical and other health and wellness products through our online platforms and offline pharmacies to our consumers. We also generate revenues from the sale of drugs to pharmacies through 1 Drug Mall, our online wholesale pharmacy.
We utilize delivery service providers to deliver goods to our consumers and pharmacy customers. The delivery service is not considered as a separate obligation as it is an integral process for us to fulfill our promises to transfer the products. As a result, revenue is recognized at the point in time when the goods are delivered to the designated address and received by consumers and pharmacy customers.
We are entitled to return any product for incorrect delivery, packing or delivering damages or other serious quality issues. We estimate sales return based on historical experience. The amount of sales returns accrual was insignificant as of December 31, 2016, 2017 and 2018.
We voluntarily provide discount coupons through our websites during our marketing activities. These coupons are not related to prior purchases, and can only be utilized in conjunction with subsequent purchases on our platforms. These discount coupons are recorded as a reduction of revenues at the time of use.
Product revenue was recorded net of surcharges and value added tax which ranges from 0% to 17% for different kinds of products based on sales amount. Surcharges are sales related taxes representing the City Maintenance and Construction Tax and Education Surtax. We record revenue on a gross basis because we control the products before they are transferred to consumers and pharmacy customers. We have made this determination on the basis that: we are primarily responsible for fulfilling our promise to deliver the specified products to consumers and pharmacy customers, we have inventory risk before the specified products are transferred to a customer or after transfer of control to consumers and pharmacy customers, and we also have discretion in establishing the price for the specified products.
Service Revenues
Service revenues primarily consist of MP service fees we charge to marketplace sellers to which we provide access to 1 Drugstore for sales of their products. We refer these fees as MP service revenue. We have determined that we are not the principal in the arrangement as we are not responsible for fulfilling the order for the specified products we do not bear the inventory risk for the products, nor do we have the ability to establish prices. We charge marketplace sellers commission fees equal to an agreed percentage of the sales price of the product when a sale is completed and also charge marketplace sellers an annual non-refundable up-front fee for platform usage. The promise to the customer, which is the marketplace seller, is to arrange for the sale which is considered as one performance obligation. Therefore, we recognize the up-front fee and commission at the point in time when the sale is completed.
We also generate service revenues by providing other ancillary services, which include advertisement display services and an online medical consultation service. The advertisement display service revenues represent the amount we received from our advertising customers, mainly pharmaceutical companies, by displaying the advertisement of products through our LED screens installed at offline pharmacy stores and the revenue is recognized over the period of time when the advertisement is displayed. Our online medical consultation service represents the consultation services we provide with in-house full-time medical professionals and the revenue is recognized when the consultation is completed.
Since November 2017, we started to offer a quarterly or annual membership program to our consumers, who pay a non-refundable upfront amount of quarterly or monthly service fees. Members obtain rights to specific numbers of coupons which provide price discounts on future purchases, limited times of free shipping and limited medical consultations during the membership period. We allocate the fees to these performance obligations based on estimated stand-alone selling prices and recognize revenue when the goods or services are provided to consumers and coupons are redeemed, or when the coupons expire at the end of membership period.
Cost of Products Sold
Cost of products sold consists of the purchase price of products and inbound shipping charges if any. We periodically receive rebates of a specified amount of cash consideration from certain vendors in the form of credits that we can apply against trade amounts owed to vendors pursuant to a binding arrangement only if we complete a specified cumulative level of purchases within a specified time period. The rebates do not represent a payment for assets or services delivered to the vendor or a reimbursement of costs incurred by us to sell vendors’ products. We account for the rebates received from our vendors as a reduction to the price we pay for the products purchased and therefore records such amounts as a reduction of cost of products sold when recognized in the consolidated financial statements. Rebates are earned based on reaching minimum purchase thresholds within a specified period. When volume rebates can be reasonably estimated based on our experience and current forecasts, a portion of the rebate is recognized as we make progress towards the purchase threshold. Cost of products does not include other direct costs related to cost of product sales such as shipping and handling expense, payroll and employee benefits of logistics staff, logistics centers rental expenses and depreciation expenses. Therefore, our cost of products sold may not be comparable to other companies which include such expenses in their costs of products.
Share-based Compensation
Awards Granted to Employees
We grant employee share options to eligible employees and accounts for these share based awards in accordance with ASC 718 Compensation—Stock Compensation.
Employees’ share-based awards are measured at the grant date fair value of the awards and recognized as expenses a) immediately at grant date if no vesting conditions are required; or b) using the straight-line vesting method over the requisite service period, which is the vesting period. To the extent the required vesting conditions are not met, resulting in the forfeiture of the share-based awards, previously recognized compensation expense relating to those awards are reversed.
We determined, with the assistance of an independent third-party valuation firm, the fair value of the stock options granted to employees. The Black Scholes option pricing model was applied in determining the estimated fair value of the options granted to employees.
Awards Granted to Non-Employees
We have accounted for equity instruments issued to non-employees in accordance with the provisions of ASC 505, Equity-based payments to non-employees. All transactions in which goods or services are received in exchange for equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. As there is no performance commitment associated with the equity instrument issued to non-employees, we re-measure the awards using the then-current fair value at each reporting date until the measurement date, generally when the services are completed and awards are vested, and attribute the changes in those fair values over the service period by the straight-line method.
Fair Value of Ordinary Shares
Prior to our initial public offering in September 2018, we had been a private company with no quoted market prices for our ordinary shares. We therefore need to make estimates of the fair value of our ordinary shares at various dates in order to determine the fair value of our ordinary shares at the date of the grant of a share-based compensation award to our employees.
The following table sets forth the fair value of our ordinary shares estimated at different times with the assistance from an independent valuation firm. The fair value per share is based on the total number of shares taking into account of the 1-to-2 share split occurred on September 14, 2015. The valuation was performed on a retrospective basis, instead of contemporaneous basis because, at that time of valuation, our limited financial and human resources were principally focused on business development efforts.
|
Date |
|
Fair Value |
|
Discount |
|
DLOM |
|
Purpose of Valuation |
|
|
|
|
US$ |
|
|
|
|
|
|
|
|
December 31, 2013 |
|
0.12 |
|
25.0 |
% |
25.0 |
% |
Share option grant and to determine potential beneficial conversion feature in connection with the issuance of Series B convertible preferred shares |
|
|
December 31, 2014 |
|
0.38 |
|
24.0 |
% |
20.0 |
% |
Share option grant and to determine potential beneficial conversion feature in connection with the issuance of Series C convertible preferred shares |
|
|
June 30, 2015 |
|
1.14 |
|
24.0 |
% |
18.0 |
% |
Share option grant |
|
|
December 31, 2015 |
|
1.68 |
|
23.0 |
% |
15.0 |
% |
Share option grant and to determine potential beneficial conversion feature in connection with the modification of Series D and D+ convertible preferred shares |
|
|
June 30, 2016 |
|
3.67 |
|
22.0 |
% |
11.4 |
% |
Share option grant and to determine potential beneficial conversion feature in connection with the issuance of Series D and D+ convertible preferred shares |
|
|
December 31, 2016 |
|
4.60 |
|
21.0 |
% |
10.0 |
% |
Share option grant |
|
|
June 30, 2017 |
|
5.89 |
|
20.0 |
% |
10.0 |
% |
Share option grant |
|
|
December 31, 2017 |
|
7.30 |
|
20.0 |
% |
9.0 |
% |
Share option grant |
|
|
March 31, 2018 |
|
8.59 |
|
20.0 |
% |
8.6 |
% |
Share option grant |
|
|
June 30, 2018 |
|
9.51 |
|
20.0 |
% |
8.6 |
% |
Share option grant |
|
In determining the fair value of our ordinary shares, we applied the income approach/discounted cash flow analysis as the primary approach based on our projected cash flow using our best estimate as of the valuation date. The determination of the fair value of our ordinary shares requires complex and subjective judgments to be made regarding our projected financial and operating results, our unique business risks, the liquidity of our shares and our operating history and prospects at the time of valuation.
The discounted cash flow method of the income approach involves applying appropriate discount rate of the weighted average cost of capital, or WACC, to discount the forecasted future cash flows to the present value.
WACC. We calculated WACC of the business as of the valuation dates using the capital asset pricing model, or CAPM, the most commonly adopted method for estimating the required rate of return for equity. Under CAPM, WACC is determined with consideration of, the risk-free rate, systematic risk, equity market premium, size of our company, the scale of our business and our ability in achieving forecasted projections. In deriving WACC, certain publicly traded companies engaged in the healthcare distribution business were selected for reference as our guideline companies. To reflect the operating environment in China and the general sentiment in the U.S. capital markets towards the healthcare distribution business, the guideline companies were selected with consideration of the following factors: (i) the guideline companies should provide similar services, and (ii) the guideline companies should either have their principal operations in Asia Pacific region, as we operate in China, and/or are publicly listed companies in the United States as our ADSs are listed in the United States.
After considering WACC, the relative risk of the industry and the characteristics of our company, we used a discount rate of WACC of 25% as of December 31, 2013, 24% as of December 31, 2014, 24% as of June 30, 2015, 23% as of December 31, 2015, 22% as of June 30, 2016, 21% as of December 31, 2016, 20% as of June 30, 2017, 20% as of December 31, 2017, 20% as of March 31, 2018 and 20% as of June 30, 2018.
We also applied a discount for lack of marketability, or DLOM, ranging from 25% to 9%, to reflect the fact that there is no readily available and liquid market for trading shares in a closely-held company like us. When determining the DLOM, the option-pricing method and empirical studies were used. Under the option-pricing method, the cost of the put option, which can hedge the price change before the privately held shares can be sold, was considered as a basis to determine the discount for lack of marketability. This option pricing method was used because it takes into account certain company-specific factors, including the timing of our initial public offering and the volatility of the share price of the guideline companies engaged in the same industry.
After our initial public offering, we relied on the market price of our ADSs to evaluate the fair value of our ordinary shares.
Fair Value of Options
We have adopted certain share incentive policies and plan. For a detailed discussion, please see “Item 6. Directors, Senior Management and Employees—B. Compensation—Share Incentives.” Our share based compensation expense is measured based on the fair value of options as calculated under the Black Scholes model. The management is responsible for determining the fair value of options on the grant date for employees and the fair value of options on the measurement date for non-employees.
The following table presents the assumptions used to estimate the fair values of the share options granted in 2016, 2017 and 2018:
|
|
|
2016 |
|
2017 |
|
2018 |
|
Risk-free rate of return(1) |
|
0.65% ~ 1.20% |
|
1.31% ~ 1.76% |
|
2.01% ~ 2.63% |
|
Contractual life of option(2) |
|
10 years |
|
10 years |
|
10 years |
|
Estimated volatility rate(3) |
|
20% ~ 23% |
|
25% |
|
27% ~ 38% |
|
Dividend yield(4) |
|
nil |
|
nil |
|
nil |
|
Fair value per ordinary share(5) |
|
US$3.67 ~ $4.60 |
|
US$5.89 ~ $7.30 |
|
US$3.00 ~ $9.51 |
(1) We estimate risk-free interest rate based on the U.S. treasury bonds with maturity similar to the maturity of the options as of the valuation dates.
(2) Contractual life of option is the time interval between grant date and date of expiring.
(3) We estimate expected volatility by reference to the historical price volatilities of ordinary shares of several comparable companies in the same industry as the Group.
(4) We have never declared or paid any cash dividends on our capital stock, and we do not anticipate any dividend payments on our ordinary shares in the foreseeable future.
(5) The fair value of underlying ordinary share is derived as described under “—Fair Value of Ordinary Shares.”
The assumptions used in fair value recognition represent our best estimates, but these estimates involve inherent uncertainties and the application of our judgment. If factors change or different assumptions are used, the fair value could be materially different for any period. Moreover, the estimates of fair value are not intended to predict actual future events or the value that ultimately will be realized by grantees who receive share-based awards, and subsequent events are not indicative of the reasonableness of the original estimates of fair value made by us for accounting purposes.
Recent Accounting Pronouncements
A list of recently issued accounting pronouncements that are relevant to us is included in note 2 to our consolidated financial statements included elsewhere in this annual report.
B. Liquidity and Capital Resources
To date, we have financed our operations primarily through cash generated by the issuance of preferred shares in private placements and our initial public offering in 2018. As of December 31, 2016, 2017 and 2018, we had RMB373.5 million, RMB167.7 million and RMB853.7 million (US$124.2 million), respectively, in cash and cash equivalents. Our cash and cash equivalents primarily consist of cash on hand and demand deposits. In addition, as of December 31, 2016, 2017 and 2018, we had RMB266.8 million, RMB293.5 million and RMB252.8 million (US$36.8 million), respectively, in short-term investments. Our short-term investments consist of wealth management products, which are financial products with variable interest rates purchased from financial institutions with an original maturity period of less than one year. Our operating cash flow and cash are also affected by our ability to manage our inventory effectively and to enhance our overall supply chain efficiency. We believe that our supply chain and effective inventory management, which have resulted in declined inventory turnover days, support the growing scale of our business, free our valuable working capital and improve our liquidity.
On December 28, 2018, certain of our subsidiaries entered into a credit agreement with ZheShang Bank, which provides a revolving credit facility that allows us to borrow up to RMB475.0 million (US$69.1 million) for working capital purpose. Any advances drawn on the credit facility will incur interest at one-year loan prime rate plus 10% and mature within 6 months. As of December 31, 2018, no amounts have been drawn on the line of credit facility.
We believe that our current cash and cash equivalents, short-term investments and our anticipated cash flows from operations will be sufficient to meet our anticipated working capital requirements and capital expenditures for the next 12 months.
We may, however, need additional capital in the future to fund our continued operations. If we determine that our cash requirements exceed the amount of cash and cash equivalents we have on hand at the time, we may seek to issue equity or debt securities or obtain credit facilities. The issuance and sale of additional equity or the incurrence of convertible loans would result in further dilution to our shareholders. The incurrence of indebtedness would result in increased fixed obligations and could result in operating covenants that might restrict our operations. We cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all.
We expect that substantially all of our future net revenues will be denominated in Renminbi. Under existing PRC foreign exchange regulations, payments of current account items, including profit distributions, interest payments and trade and service-related foreign exchange transactions, can be made in foreign currencies without prior SAFE approval as long as certain routine procedural requirements are fulfilled. Therefore, our PRC subsidiary, Yao Fang, is allowed to pay dividends in foreign currencies to us without prior SAFE approval by following certain routine procedural requirements. However, approval from or registration with competent government authorities is required where the Renminbi is to be converted into foreign currency and remitted out of China to pay capital expenses such as the repayment of loans denominated in foreign currencies. The PRC government may at its discretion restrict access to foreign currencies for current account transactions in the future.
The following table sets forth material amounts of cash and short-term investments disaggregated by currency denomination as of December 31, 2018 in each jurisdiction in which our affiliated entities are domiciled:
|
|
|
PRC |
|
Hong Kong |
|
Cayman Islands |
|
|
|
|
(in thousands) |
| ||||
|
Cash in RMB |
|
17,809 |
|
1 |
|
— |
|
|
Cash in US$ |
|
12,291 |
|
70,990 |
|
38,300 |
|
|
Short-term investments in RMB |
|
— |
|
— |
|
— |
|
|
Short-term investments in US$ |
|
— |
|
20,163 |
|
16,606 |
|
Cash Flows
The following table sets forth a summary of our cash flows for the periods presented:
|
|
|
For the Year Ended December 31, |
| ||||||
|
|
|
2016 |
|
2017 |
|
2018 |
| ||
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
(in thousands) |
| ||||||
|
Summary Consolidated Cash Flow Data: |
|
|
|
|
|
|
|
|
|
|
Net cash used in operating activities |
|
(388,646 |
) |
(204,372 |
) |
(343,018 |
) |
(49,891 |
) |
|
Net cash provided by (used in) investing activities |
|
(267,554 |
) |
(36,125 |
) |
44,454 |
|
6,466 |
|
|
Net cash provided by financing activities |
|
148,419 |
|
49,500 |
|
972,697 |
|
141,473 |
|
|
Net increase (decrease) in cash and cash equivalents |
|
(472,451 |
) |
(205,845 |
) |
686,080 |
|
99,786 |
|
|
Cash and cash equivalents at the beginning of period |
|
845,956 |
|
373,505 |
|
167,660 |
|
24,385 |
|
|
Cash and cash equivalents at the end of period |
|
373,505 |
|
167,660 |
|
853,740 |
|
124,171 |
|
Operating Activities
Net cash used in operating activities in 2018 was RMB343.0 million (US$49.9 million) and primarily consisted of our net loss of RMB382.0 million (US$55.6 million), as adjusted for non-cash items and the effects of changes in operating assets and liabilities. Adjustment for non-cash items primarily included RMB51.4 million (US$7.5 million) of share-based compensation expenses and RMB11.2 million (US$1.6 million) of depreciation and amortization expenses, partially offset by an increase in investment income of RMB10.9 million (US$1.6 million). In 2018, the principal items accounting for the changes in operating assets and liabilities were an increase of RMB84.1 million (US$12.2 million) in accounts payable, partially offset by an increase in inventory of RMB66.8 million (US$9.8 million) and an increase in prepayments and other current assets of RMB56.3 million (US$8.2 million). The increases in accounts payable and inventory were primarily due to an increase in our inventory storage level to meet increased demands.
Net cash used in operating activities in 2017 was RMB204.4 million and primarily consisted of our net loss of RMB249.3 million, as adjusted for non-cash items and the effects of changes in operating assets and liabilities. Adjustments for non-cash items primarily included RMB14.8 million of depreciation and amortization expenses and RMB9.9 million of share-based compensation expenses. In 2017, the principal items accounting for the changes in operating assets and liabilities were an increase of RMB30.2 million in accounts payable which was primarily due to the expansion of our services and increases to our inventory storage as the business grew, partially offset by an increase in inventory of RMB9.3 million and an increase in prepayments and other current assets of RMB7.5 million. As our sales volume grew, we increased our inventory storage level and made more prepayments to secure popular pharmaceutical products.
Net cash used in operating activities in 2016 was RMB388.6 million and primarily consisted of net loss of RMB363.4 million, as adjusted for non-cash items and the effects of changes in operating assets and liabilities. Adjustments for non-cash item primarily included RMB12.1 million of depreciation and amortization expenses and RMB3.4 million of share-based compensation expenses. In 2016, the principal items accounting for the changes in operating assets and liabilities were an increase in inventory of RMB22.0 million due to newly opened offline pharmacies and an increase in prepayments and other current assets of RMB35.6 million. As our sales volume grew, we increased our inventory storage level and made more prepayments to secure popular pharmaceutical products.
Investing Activities
Net cash provided by investing activities in 2018 was RMB44.5 million (US$6.5 million), consisting primarily of proceeds from sale or maturity of short-term investments of RMB578.4 million (US$84.1 million), partially offset by purchases of short-term investments of RMB519.2 million (US$75.5 million).
Net cash used in investing activities in 2017 was RMB36.1 million, consisting primarily of purchase of short-term investments of RMB109.4 million, partially offset by proceeds from sale or maturity of short-term investments of RMB80.2 million.
Net cash used in investing activities in 2016 was RMB267.6 million, consisting primarily of the purchase of short-term investments of RMB267.6 million.
Financing Activities
Net cash provided by financing activities in 2018 was RMB972.7 million (US$141.5 million), consisting of proceeds from preferred shareholders of RMB277.8 million (US$40.4 million) and net proceeds from our initial public offering of RMB694.9 million (US$101.2 million).
Net cash provided by financing activities in 2017 was RMB49.5 million, consisting almost entirely of proceeds from preferred shareholders of RMB49.5 million.
Net cash provided by financing activities in 2016 was RMB148.4 million, consisting primarily of proceeds from preferred shareholders of RMB195.9 million, offset by debt repayment of RMB47.5 million.
Capital Expenditures
We made capital expenditures of RMB6.9 million, RMB6.9 million and RMB14.8 million (US$2.2 million) in 2016, 2017 and 2018, respectively. In these periods, our capital expenditures were primarily used for purchases of property, equipment and software. We will continue to make capital expenditures, including establishing more fulfillment centers to meet the expected growth of our business.
Holding Company Structure
111, Inc. is a holding company with no material operations of its own. We conduct our operations primarily through our PRC subsidiaries, our variable interest entities and their subsidiaries in China. As a result, 111, Inc.’s ability to pay dividends depends upon dividends paid by our PRC subsidiaries. If our existing PRC subsidiaries or any newly formed ones incur debt on their own behalf in the future, the instruments governing their debt may restrict their ability to pay dividends to us. In addition, Yao Fang, our wholly foreign owned subsidiary in China is permitted to pay dividends to us only out of their retained earnings, if any, as determined in accordance with PRC accounting standards and regulations. Under PRC law, each of our other PRC subsidiaries and our variable interest entities in China is required to set aside at least 10% of its after-tax profits each year, if any, to fund certain statutory reserve funds until such reserve funds reach 50% of their registered capital. In addition, our wholly foreign-owned subsidiaries in China may allocate a portion of their after-tax profits based on PRC accounting standards to enterprise expansion funds and staff bonus and welfare funds at their discretion, and our variable interest entities may allocate a portion of their after-tax profits based on PRC accounting standards to a surplus fund at their discretion. The statutory reserve funds and the discretionary funds are not distributable as cash dividends. Remittance of dividends by a wholly foreign-owned company out of China is subject to examination by the banks designated by SAFE. Our PRC subsidiary, Yao Fang, has not paid dividends and will not be able to pay dividends until they generate accumulated profits and meet the requirements for statutory reserve funds.
C. Research and Development, Patents and Licenses, etc.
See “Item 4. Information on the Company—B. Business Overview—Technology and IT Infrastructure” and “Item 4. Information on the Company—B. Business Overview—Intellectual Property.”
D. Trend Information
Other than as disclosed elsewhere in this annual report, we are not aware of any trends, uncertainties, demands, commitments or events for the year ended December 31, 2018 that are reasonably likely to have a material and adverse effect on our net revenues, income, profitability, liquidity or capital resources, or that would cause the disclosed financial information to be not necessarily indicative of future results of operations or financial conditions.
E. Off-Balance Sheet Commitments and Arrangements
We have not entered into any financial guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as shareholder’s equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or product development services with us.
F. Tabular Disclosure of Contractual Obligations
The following table sets forth our contractual obligations, including interest payments, as of December 31, 2018:
|
|
|
|
|
Less than 1 |
|
|
|
|
|
More than 5 |
|
|
|
|
Total |
|
year |
|
1-3 years |
|
3-5 years |
|
years |
|
|
|
|
(in RMB thousands) |
| ||||||||
|
Operating lease commitments |
|
104,904 |
|
30,443 |
|
41,818 |
|
16,134 |
|
16,509 |
|
|
Total |
|
104,904 |
|
30,443 |
|
41,818 |
|
16,134 |
|
16,509 |
|
Our operating lease commitments relate to our leases of certain offices and fulfillment centers. Our lease expenses for the years ended December 31, 2016 and 2017 and 2018 were RMB24.0 million, RMB23.9 million and RMB27.1 million (US$3.9 million), respectively.
Other than those shown above, we did not have any significant capital and other commitments, long-term obligations or guarantees as of December 31, 2018.
G. Safe Harbor
See “Forward-Looking Information” on page 2 of this annual report.
Item 6. Directors, Senior Management and Employees
A. Directors and Executive Officers
The following table sets forth information regarding our directors and executive officers as of the date of this annual report.
|
Directors and Executive Officers |
|
Age |
|
Position/Title |
|
Gang Yu |
|
59 |
|
Co-founder and Co-Chairman |
|
Junling Liu |
|
54 |
|
Co-founder, Co-Chairman and Chief Executive Officer |
|
Yang Chen |
|
48 |
|
Chief Financial Officer |
|
Harry Chi Hui |
|
55 |
|
Director |
|
Nee Chuan Teo |
|
48 |
|
Independent Director |
|
Jian Sun |
|
54 |
|
Independent Director |
|
Jun Luo |
|
51 |
|
Independent Director |
Dr. Gang Yu is our co-founder and has served as our executive chairman since 2015. Since September 2018, Dr. Yu has served as our co-chairman. Dr. Yu has over 23 years of experience in the technology sector and 14 years of experience in the e-commerce industry. He is a recipient of numerous prestigious international awards, including the 2002 Franz Edelman Management Science Achievement Award from INFORMS and the 2012 Martin K. Starr Excellence in Production and Operations Management Practice Award from POMS. Dr. Yu co-founded and served as chairman of YHD.com, a leading e-commerce company in China. Dr. Yu currently serves as an independent director on the board of Baozun, Inc. (Nasdaq: BZUN), as a director of Midea Group Co., Ltd. (SZSE: 000333) and LightInTheBox Holding Co., Ltd. (NYSE: LITB), and as the co-chairman of the board of Zall Group (02098.HK). Prior to founding YHD.com, Dr. Yu served as the vice president of Worldwide Procurement at Dell Inc. from 2006 to 2007 and the vice president of Worldwide Supply Chain at Amazon.com from 2004 to 2006. Before Amazon, Dr. Yu was the chair professor at McCombs School of Business at The University of Texas at Austin from 1989 to 2004. Dr. Yu received his bachelor’s degree in science from Wuhan University in 1982, master’s degree in physics from Cornell University in 1986 and Ph.D. degree in decision sciences from The Wharton School of the University of Pennsylvania in 1990. Dr. Yu has published 6 books and over 80 journal articles. Dr. Yu also holds three U.S. patents related to airline optimization solutions.
Mr. Junling Liu is our co-founder and has served as our chairman and chief executive officer since 2015. Since September 2018, Mr. Liu has served as our co-chairman. He co-founded and served as chief executive officer of YHD.com from 2008 to 2015. Prior to founding YHD.com, Mr. Liu served as the global vice president and president for mainland China and Hong Kong at Dell Inc. from 2006 to 2007. He also held numerous executive positions at internationally renowned technology companies such as Avaya China, Openwave Systems and Lucent Technologies Asia. Since January 2015, he has been an independent director of Autohome Inc. (NYSE: ATHM), the leading online destination for automobile consumers. Mr. Liu also serves as an independent director of Hua Medicine (02552.HK). Mr. Liu received his bachelor’s degree in education from Flinders University in Australia in 1991 and master’s degree in international business administration from Flinders University in 1998.
Mr. Yang Chen has served as our chief financial officer since February 2019. Mr. Chen served as chief financial officer of iKang Healthcare Group Inc. (Nasdaq: KANG) from April 2013 to February 2019. Prior to that, Mr. Chen was vice president of finance and strategy for Campbell Soup Asia Limited and Lee Kum Kee Sauce Group from 2008 to 2013. He has held various senior finance positions at Dumex China, Pepsi Greater China Region and Wyeth China, and started his career as an auditor at Arthur Andersen & Co. in 1992. Mr. Chen received his bachelor’s degree from the Shanghai University of Finance and Economics in 1992 and his executive master of business administration degree from the Olin School of Business at Washington University in 2004.
Mr. Harry Chi Hui has served as our director since December 2014. Mr. Hui is the founder and managing partner of ClearVue Partners, a private equity firm that invests in growth stage companies in China’s consumer sectors. Prior to founding ClearVue Partners, Mr. Hui served as chief marketing officer and president at Pepsico Investment (China) Limited from 2006 to 2010. He also served as the president of Universal Music Asia, one of the world’s largest music company, where he managed all aspects of the company’s business across Asia from 2002 to 2006. Mr. Hui received his bachelor’s degree in economics and business from University at Albany-SUNY in 1985 and master’s degree in business administration from University of Southern California in 1992.
Mr. Nee Chuan Teo has served as our independent director since September 2018. Mr. Teo is the chief financial officer of Huazhu Group Limited (formerly China Lodging Group Limited, Nasdaq: HTHT), a leading fast-growing multi-brand hotel group in China. Prior to joining Huazhu Group Limited, Mr. Teo served as chief financial officer of Rnomac International Group from 2011 to 2015, the largest Volvo construction equipment distributor in China. He also served as financial controller in Focus Media Group from 2007 to 2009. Mr. Teo received his bachelor of science in accounting and financial analysis from Warwick University, the United Kingdom. He is a chartered certified accountant in the United Kingdom and a certified public accountant in the United States and Hong Kong.
Mr. Jian Sun has served as our independent director since September 2018. Mr. Sun is an executive director and the general manager of BTG Hotels (Group) Co., Ltd. (Shanghai Stock Exchange Stock Code: 600258), a top tourism service company in China. Prior to joining BTG Hotels Group, Mr. Sun served as executive director and chief executive officer of Home Inns Group, a leading economy hotel chain in China previously listed on Nasdaq, from 2004 to 2016. From 2010 to 2014, Mr. Sun served as independent director, chairman of the compensation committee and a member of the audit committee of Mecox Lane Limited, an online platform for apparel and accessories listed on Nasdaq. Since 2014, Mr. Sun has served as an independent director and a member of the compensation committee of two companies listed on the New York Stock Exchange, including Leju Holdings Limited, a leading online-to-offline real estate services provider in China, and eHi Car Services Limited, a leading car services and car rental provider in China. Mr. Sun holds a bachelor’s degree from Shanghai Medical University in China.
Mr. Jun Luo has served as our independent director since September 2018. Mr. Luo is the co-founder and chief executive officer of Tujia & Sweetome Group, a leading short-term property rental firm in China. Prior to co-founding Tujia & Sweetome Group, Mr. Luo served as general manager of Shanghai SINA Leju and executive president at China Real Estate Information Corporation. Mr. Luo received his bachelor’s degree in accounting from Shanghai University of Finance and Economics in 1994 and master’s degree in software engineering from Beihang University in China in 2010.
B. Compensation
For the year ended December 31, 2018, we paid an aggregate of approximately RMB7.6 million (US$1.1 million) in cash and other benefits to our directors and executive officers. For share incentive grants to our officers and directors, see “—Share Incentives.” We have not set aside or accrued any amount to provide pension, retirement or other similar benefits to our executive officers and directors. Our PRC subsidiaries are required by law to make contributions equal to certain percentages of each employee’s salary for his or her pension insurance, medical insurance, unemployment insurance and other statutory benefits and a housing provident fund.
Share Incentives
Share Incentive Policies
We adopted certain share incentive policies in December 2013 and August 2014, or the 2013 Policy and the 2014 Policy, respectively, for the purpose of granting share based compensation awards to our officers, employees, directors, consultants and other eligible persons to incentivize their performance and promote the success of our business.
Share Incentive Plans
2016 Plan
We adopted our 2016 Share Incentive Plan, or the 2016 Plan, in January 2016, to promote our success and the interests of our shareholders by providing a means through which we may grant equity-based incentives to attract, motivate, retain and reward certain officers, employees, directors, consultants and other eligible persons to further link the interests of recipients with those of our shareholders generally. Since the adoption of the 2016 Plan, we stopped granting awards under the 2013 Policy or the 2014 Policy, although the outstanding awards under the 2013 Policy and the 2014 Policy are still being administered under their respective policies.
The following paragraphs summarize the terms of the 2016 Plan.
Types of Awards. The 2016 Plan permits awards of options, share appreciation rights, restricted shares and restricted share units.
Plan Administration. The 2016 Plan will be administered by our board of directors or by a committee designated by our board of directors. The committee or the full board of directors, as applicable, will determine the participants to receive awards, the type and number of awards to be granted to each participant, and the terms and conditions of each award grant.
Award Agreement. Generally, awards granted under the 2016 Plan are evidenced by an award agreement that sets forth terms, conditions and limitations for each award, which must be consistent with the plan.
Exercise Price. The plan administrator determines the exercise price for each award, which is stated in the award agreement.
Eligibility. We may grant awards only to those persons that the plan administrator determines to be eligible persons, which may include our employees, directors and consultants.
Term of the Awards. The term of each award granted under the 2016 Plan may not exceed ten years from the date of the grant.
Vesting Schedule. In general, the plan administrator determines the vesting schedule, which is set forth in the award agreement.
Acceleration of Awards upon Change in Control. The plan administrator may determine, at the time of grant or thereafter, that an award will become vested and exercisable, in full or in part, in the event that a change in control of our company occurs.
Transfer Restrictions. Awards may not be transferred in any manner by the recipient other than by will or the laws of descent and distribution, except as otherwise provided by the plan administrator.
Termination. Unless terminated earlier, the 2016 Plan has a term of fifteen years.
Under the 2013 Policy, the 2014 Policy and the 2016 Plan, the maximum aggregate number of shares which may be issued pursuant to all awards is 13,671,109 ordinary shares. As of the date of this annual report, options to purchase a total of 7,891,262 ordinary shares and 60,000 restricted share units were granted and outstanding under the 2013 Policy, the 2014 Policy and the 2016 Plan.
2018 Plan
In August 2018, we adopted our 2018 Share Incentive Plan, or the 2018 Plan, which became effective on September 15, 2018, one day after the completion of our initial public offering, replacing the 2016 Plan in its entirety. The 2018 Plan allows us to offer share-based incentive awards to employees, officers, directors and individual consultants who render services to us. The 2018 Plan permits the grant of options, restricted shares and restricted share units, or other types of awards, in the form of cash or otherwise, as approved by our board of directors or a committee thereof. The maximum number of Class A ordinary shares that may be issued pursuant to all awards under the 2018 Plan is 13,671,109, plus an annual increase on the first day of each fiscal year of our company during the ten-year term of the 2018 Plan commencing with the fiscal year beginning January 1, 2019, by an amount equal to the lesser of (i) 1.0% of the total number of shares issued and outstanding on the last day of the immediately preceding fiscal year, and (ii) such number of shares as may be determined by our board of directors. Upon the effectiveness of the 2018 Plan, we no longer grant any awards under the 2016 Plan. Outstanding awards granted under the 2016 Plan will remain effective and be subject to the terms and conditions of the 2018 Plan. As of the date of this annual report, options to purchase a total of 1,544,697 Class A ordinary shares and 2,040,827 restricted share units were granted and outstanding under the 2018 Plan. The following paragraphs summarize the terms of the 2018 Plan:
Plan Administration. Our board of directors, or a committee designated by our board of directors, will administer the plan. The plan administration committee will determine the provisions and terms and conditions of each grant.
Award Agreements. Options and other awards granted under the plan are evidenced by an award agreement that sets forth the terms, conditions and limitations for each grant, which may include the term of the award and the provisions applicable in the event the grantee’s employment or service terminates.
Exercise Price. The exercise price of an option will be determined by the plan administration committee, which may be a fixed price or a variable price related to the fair market value on the grant date of the respective option. The exercise price of granted options may be amended or adjusted in the absolute discretion of the plan administration committee without the approval of our shareholders or the recipients of the options.
Eligibility. We may grant awards to employees, directors and consultants of our company and our majority-owned subsidiaries as determined by the plan administration committee.
Vesting Schedule. In general, the plan administration committee determines the vesting schedule, which is specified in the relevant award agreement.
Acceleration of Awards upon Change in Control. If a change-of-control corporate transaction occurs, the plan administration committee may, in its sole discretion, provide for (i) all awards outstanding to terminate at a specific time in the future and give each participant the right to exercise the vested portion of such awards during a specific period of time, or (ii) the purchase of any award for an amount of cash equal to the amount that could have been attained upon the exercise of such award, or (iii) the replacement of such award with other rights or property selected by the plan administration committee in its sole discretion, or (iv) payment of award in cash based on the value of ordinary shares on the date of the change-of-control corporate transaction plus reasonable interest.
Term of the Options. The term of each option grant shall be stated in the award agreement, provided that the term shall not exceed ten years from the date of the grant.
Transfer Restrictions. Subject to certain exceptions, awards may not be transferred by the recipient.
Termination of the Plan. The 2018 Plan shall terminate in 2028, provided that our board of directors may terminate the plan at any time and for any reason.
Our Employee Share Holding Platform
In November 2014, we established Gold Prized Investment Limited, or Gold Prized, a company incorporated in the British Virgin Islands, as an offshore employee share holding platform to allow our employees in China to receive share incentives.
Gold Prized is wholly owned by Shanghai Yiyao Enterprise Management Partners, or Yiyao Partners, a limited partnership formed in the PRC and owned by Ms. Jing Liu (0.81%), a family member of Mr. Junling Liu, and Ms. Ying Song (99.19%), a family member of Dr. Gang Yu. Ms. Ying Song is the general partner while Ms. Jing Liu is the sole limited partner of Yiyao Partners.
We issued class C ordinary shares to Gold Prized, and did not grant any rights associated with the class C ordinary shares held by Gold Prized to our directors and executive officers or any other employees. We have transferred the class C ordinary shares issued to Gold Prized back to our company and have reserved those shares for the 2016 Plan. Gold Prized has since ceased to be a shareholder of our company.
The following table summarizes, as of the date of this annual report, the outstanding share incentive awards we have granted to our directors and executive officers under the 2013 Policy, the 2014 Policy, the 2016 Plan and the 2018 Plan:
|
Name |
|
Ordinary |
|
Exercise |
|
Grant Date |
|
Expiration Date |
| |
|
Yang Chen |
|
* |
|
$ |
4.47 |
|
3/31/2019 |
|
3/30/2029 |
|
|
|
|
* |
|
|
† |
2/28/2019 |
|
2/27/2029 |
| |
|
Nee Chuan Teo |
|
* |
|
|
† |
9/12/2018 |
|
9/11/2028 |
| |
|
Jian Sun |
|
* |
|
|
† |
9/12/2018 |
|
9/11/2028 |
| |
|
Jun Luo |
|
* |
|
|
† |
9/12/2018 |
|
9/11/2028 |
| |
|
All directors and executive officers as a group |
|
1,364,704 |
|
|
|
|
|
|
| |
* Less than one percent of our total outstanding shares.
† Restricted share units.
C. Board Practices
Board of Directors
Our board of directors consists of six directors. A director is not required to hold any shares in our company to qualify to serve as a director. A director who is in any way, whether directly or indirectly, interested in a contract or transaction or proposed contract or transaction with our company is required to declare the nature of his interest at a meeting of our directors. A director may vote with respect to any contract or transaction or proposed contract or transaction notwithstanding that he may be interested therein, and if he does so his vote shall be counted and he may be counted in the quorum at any meeting of our directors at which any such contract or transaction is considered. Our directors may exercise all the powers of our company to borrow money, mortgage or charge its undertaking, property and uncalled capital and to issue debentures or other securities whenever money is borrowed or as security for any debt, liability or obligation of our company or of any third party.
Committees of the Board of Directors
We have three committees under the board of directors: an audit committee, a compensation committee and a nominating and corporate governance committee. We have adopted a charter for each of the three committees. Each committee’s members and functions are described below.
Audit Committee
Our audit committee consists of Nee Chuan Teo, Jian Sun and Jun Luo, and is chaired by Nee Chuan Teo. Nee Chuan Teo, Jian Sun and Jun Luo each satisfies the “independence” requirements of Rule 5605(c)(2) of the Nasdaq Stock Market Rules and meet the independence standards under Rule 10A-3 under the Exchange Act. We have determined that Nee Chuan Teo qualifies as an “audit committee financial expert.” The audit committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The audit committee is responsible for, among other things:
· selecting the independent registered public accounting firm and pre-approving all auditing and non-auditing services permitted to be performed by the independent registered public accounting firm;
· reviewing with the independent auditors any audit problems or difficulties and management’s response;
· reviewing and approving all proposed related party transactions, as defined in Item 404 of Regulation S-K under the Securities Act;
· discussing the annual audited financial statements with management and the independent registered public accounting firm;
· reviewing major issues as to the adequacy of our internal controls and any special audit steps adopted in light of material control deficiencies;
· annually reviewing and reassessing the adequacy of our audit committee charter;
· meeting separately and periodically with management and the independent registered public accounting firm; and
· reporting regularly to the board.
Compensation Committee
Our compensation committee consists of Gang Yu, Nee Chuan Teo and Jian Sun, and is chaired by Gang Yu. Nee Chuan Teo and Jian Sun each satisfies the “independence” requirements of Rule 5605(a)(2) of the Nasdaq Stock Market Rules. The compensation committee assists the board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers. Our chief executive officer may not be present at any committee meeting during which his compensation is deliberated upon. The compensation committee is responsible for, among other things:
· reviewing the total compensation package for our executive officers and making recommendations to the board with respect to it;
· reviewing the compensation of our non-employee directors and making recommendations to the board with respect to it; and
· periodically reviewing and approving any long-term incentive compensation or equity plans, programs or similar arrangements, annual bonuses, and employee pension and welfare benefit plans.
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee consists of Junling Liu, Jian Sun and Jun Luo, and is chaired by Junling Liu. Jian Sun and Jun Luo each satisfies the “independence” requirements of Rule 5605(a)(2) of the Nasdaq Stock Market Rules. The nominating and corporate governance committee assists the board in selecting individuals qualified to become our directors and in determining the composition of the board and its committees. The nominating and corporate governance committee is responsible for, among other things:
· recommending nominees to the board for election or re-election to the board, or for appointment to fill any vacancy on the board;
· reviewing annually with the board the current composition of the board with regards to characteristics such as independence, age, skills, experience and availability of service to us;
· selecting and recommending to the board the names of directors to serve as members of the audit committee and the compensation committee, as well as of the nominating and corporate governance committee itself; and
· monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance.
Terms of Directors and Executive Officers
Our board of directors may, by the affirmative vote of a simple majority of the remaining directors present and voting at a meeting of our board, which shall include the affirmative vote of at least one Founder as long as either Founder is a director, appoint any person as a director, to fill a vacancy on the board arising from the office of any director being vacated. Our shareholders may also appoint any person to be a director by way of ordinary resolution. Our directors are not subject to a term of office and hold office until such time as they are removed from office by ordinary resolution of the shareholders. A director will cease to be a director if, among other things, the director (i) becomes bankrupt or makes any arrangement or composition with his creditors; (ii) dies or is found by our company to be or becomes of unsound mind, (iii) resigns his office by notice in writing to the company, or (iv) without special leave of absence from our board, is absent from three consecutive board meetings and our directors resolve that his office be vacated.
Our founders serve as co-chairmen of our board of directors. For so long as each of our founders is a director of our company, he shall be a co-chairman of our board of directors, until he resigns as co-chairman or ceases to be a director (in which event he shall automatically cease to be a co-chairman). If either founder ceases to be a co-chairman, the other founder shall continue as the sole chairman of our board (unless our board, with the consent of the other founder, elects and appoints another director to be another co-chairman). Upon both founders ceasing to be co-chairmen or chairman, our board shall elect and appoint the co-chairmen or chairman at their discretion.
Subject to the foregoing, our officers are elected by and serve at the discretion of our board of directors.
Employment Agreements and Indemnification Agreements
We have entered into employment agreements with our senior executive officers. Pursuant to these agreements, we are entitled to terminate a senior executive officer’s employment for cause at any time without remuneration for certain acts of the officer, such as being convicted of any criminal conduct, any act of gross or willful misconduct or any serious, willful, grossly negligent or persistent breach of any employment agreement provision, or engaging in any conduct which may make the continued employment of such officer detrimental to our company. In connection with the employment agreements, each senior executive officer agrees to hold all information, know-how and records in any way connected with the products of our company, including, without limitation, all software and computer formulas, designs, specifications, drawings, data, manuals and instructions and all customer and supplier lists, sales and financial information, business plans and forecasts, all technical solutions and the trade secrets of our company, in strict confidence perpetually. Each officer also agrees that we shall own all the intellectual property developed by such officer during his or her employment.
We have entered into indemnification agreements with each of our directors and executive officers. Under these agreements, we agree to indemnify them against certain liabilities and to reimburse them for expenses in connection with claims made by reason of their being a director or officer of our company.
D. Employees
The following table sets forth the numbers of our employees categorized by function as of December 31, 2018.
|
|
|
As of December 31, 2018 |
| ||
|
|
|
Number |
|
% of Total |
|
|
Functions: |
|
|
|
|
|
|
Wholesale pharmacy business |
|
494 |
|
36.6 |
% |
|
Retail pharmacy business |
|
316 |
|
23.4 |
% |
|
Supply chain |
|
91 |
|
6.7 |
% |
|
Procurement |
|
72 |
|
5.3 |
% |
|
Research and development and IT |
|
293 |
|
21.7 |
% |
|
General and administrative |
|
84 |
|
6.2 |
% |
|
Total |
|
1,350 |
|
100.0 |
% |
As required by laws and regulations in China, we participate in various employee social security plans that are organized by municipal and provincial governments including, among other things, pension, medical insurance, unemployment insurance, maternity insurance, on-the-job injury insurance and housing fund plans through a PRC government-mandated benefit contribution plan. We are required under PRC law to make contributions to employee benefit plans at specified percentages of the salaries, bonuses and certain allowances of our employees, up to a maximum amount specified by the local government from time to time.
We typically enter into standard employment agreements and confidentiality agreements or clauses with our senior management and core personnel. These contracts include a standard non-compete covenant that prohibits the employee from competing with us, directly or indirectly, during his or her employment and for two years after termination of his or her employment.
We maintain a good working relationship with our employees and the labor union in Guangzhou, and we have not experienced any material labor disputes.
E. Share Ownership
The following table sets forth information with respect to the beneficial ownership of our shares as of March 31, 2019 by:
· each of our current directors and executive officers; and
· each person known to us to own beneficially more than 5% of our shares.
The calculations in the table below are based on 163,317,328 ordinary shares outstanding as of March 31, 2019, comprising of (i) 91,317,328 Class A ordinary shares, and (ii) 72,000,000 Class B ordinary shares.
Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, we have included shares that the person has the right to acquire within 60 days after March 31, 2019, including through the exercise of any option, warrant or other right or the conversion of any other security. These shares, however, are not included in the computation of the percentage ownership of any other person.
|
|
|
Class A |
|
Class B |
|
Percentage of |
|
Percentage of |
|
|
|
|
Shares |
|
Shares |
|
basis† |
|
power†† |
|
|
Directors and Executive Officers:* |
|
|
|
|
|
|
|
|
|
|
Gang Yu(1)(10) |
|
— |
|
36,000,000 |
|
22.0 |
|
46.1 |
|
|
Junling Liu(2)(7) |
|
— |
|
36,000,000 |
|
22.0 |
|
46.1 |
|
|
Yang Chen |
|
— |
|
— |
|
— |
|
— |
|
|
Harry Chi Hui(3)(8) |
|
19,100,646 |
|
— |
|
11.7 |
|
1.6 |
|
|
Nee Chuan Teo(4) |
|
— |
|
— |
|
— |
|
— |
|
|
Jian Sun(5) |
|
— |
|
— |
|
— |
|
— |
|
|
Jun Luo(6) |
|
— |
|
— |
|
— |
|
— |
|
|
All directors and executive officers as a group |
|
19,100,646 |
|
72,000,000 |
|
55.8 |
|
93.8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Principal Shareholders: |
|
|
|
|
|
|
|
|
|
|
Sunny Bay Global Limited(7) |
|
— |
|
36,000,000 |
|
22.0 |
|
46.1 |
|
|
ClearVue YW Holdings, Ltd.(8) |
|
19,100,646 |
|
— |
|
11.7 |
|
1.6 |
|
|
Verlinvest Asia (HK) Limited(9) |
|
15,748,935 |
|
— |
|
9.6 |
|
1.3 |
|
|
Infinity Cosmo Limited(10) |
|
— |
|
11,494,252 |
|
7.0 |
|
14.7 |
|
|
First Pharmacia International(11) |
|
8,690,562 |
|
— |
|
5.3 |
|
0.7 |
|
Notes:
* Except for Mr. Harry Chi Hui, Mr. Nee Chuan Teo, Mr. Jian Sun and Mr. Jun Luo, the business address of our directors and executive officers is 3-5/F, No. 295 ZuChongZhi Road, Pudong New Area, Shanghai, the People’s Republic of China.
** For each person or group included in this column, percentage of aggregate voting power represents voting power based on both Class A and Class B ordinary shares held by such person or group with respect to all outstanding shares of our Class A and Class B ordinary shares as a single class. Each holder of our Class A ordinary shares is entitled to one vote per share. Each holder of our Class B ordinary shares is entitled to fifteen votes per share. Our Class B ordinary shares are convertible at any time by the holder into Class A ordinary shares on a one-for-one basis.
(1) Represents 24,505,748 Class B ordinary shares held by Dr. Gang Yu, and 11,494,252 Class B ordinary shares held by Infinity Cosmo Limited, a company incorporated in the British Virgin Islands. Infinity Cosmo Limited is controlled by Gang Yu Irrevocable Trust.
(2) Represents 36,000,000 Class B ordinary shares held by Sunny Bay Global Limited, a company incorporated in the British Virgin Islands. Sunny Bay Global Limited is wholly owned by Mr. Junling Liu.
(3) Beneficial ownership calculation is based solely on a review of a Schedule 13G filed with the SEC on February 11, 2019. Represents 19,100,646 Class A ordinary shares held by ClearVue YW Holdings, Ltd. ClearVue YW Holdings, Ltd. is wholly owned by ClearVue Partners, L.P., a Cayman Islands exempted limited partnership. ClearVue Partners GP, L.P., a Cayman exempted limited partnership, is the general partner of ClearVue Partners, L.P. ClearVue Partners Ltd., a Cayman Islands exempted company, is the general partner of ClearVue Partners GP, L.P. Mr. Harry Chi Hui owns 60% of the equity interest in ClearVue Partners Ltd. Mr. Hui disclaims beneficial ownership with respect to the shares held by ClearVue YW Holdings, Ltd., except to the extent of his pecuniary interest therein. The business address of Mr. Harry Chi Hui is Unit 902, No.1717, West Nanjing Road, Shanghai, China.
(4) The business address of Mr. Nee Chuan Teo is 2266, HongQiao Road, Shanghai, China.
(5) The business address of Mr. Jian Sun is 124 Caobao Road, Xuhui District, Shanghai, China.
(6) The business address of Mr. Jun Luo is Room 101, K Unit, No. 8 Liuying Road, Hongkou District, Shanghai, China.
(7) Represents 36,000,000 Class B ordinary shares held by Sunny Bay Global Limited, a company incorporated in the British Virgin Islands. Sunny Bay Global Limited is wholly owned by Mr. Junling Liu. The registered office address of Sunny Bay Global Limited is Vistra Corporate Services Centre, Wickhams Cay II, Road Town, Tortola, VG1110, British Virgin Islands.
(8) Beneficial ownership calculation is based solely on a review of a Schedule 13G filed with the SEC on February 11, 2019. Represents 19,100,646 Class A ordinary shares directly held by ClearVue YW Holdings, Ltd. ClearVue YW Holdings, Ltd. is wholly owned by ClearVue Partners, L.P., a Cayman Islands exempted limited partnership. ClearVue Partners GP, L.P., a Cayman exempted limited partnership, is the general partner of ClearVue Partners, L.P. ClearVue Partners Ltd., a Cayman Islands exempted company, is the general partner of ClearVue Partners GP, L.P. Mr. Harry Chi Hui owns 60% of the equity interest in ClearVue Partners Ltd. The registered office address of ClearVue YW Holdings, Ltd. is Harneys Services (Cayman) Limited, 4th Floor, Harbour Place, 103 South Church Street, George Town, P.O. Box 10240, Grand Cayman KY1-1002, Cayman Islands.
(9) Beneficial ownership calculation is based solely on a review of a Schedule 13G filed with the SEC on January 18, 2019. Represents 15,748,935 Class A ordinary shares held by Verlinvest Asia (HK) Limited, a limited company incorporated in Hong Kong. Verlinvest Asia (HK) Limited is wholly owned by Verlinvest SA, a company incorporated in Belgium. Verlinvest SA is 88% owned by Verlinvest Group SA, a company incorporated in Belgium, and 12% owned by a group of affiliated entities. Verlinvest Group SA is 90% owned by Vedihold SA, a company incorporated in Luxembourg, and 10% owned by DLF Participations SCA, a company incorporated in Luxembourg. Vedihold SA is 38% owned by Vedipar SA, a company incorporated in Luxembourg. No individual owns, directly or indirectly, 10% or more of the remaining 62% shares in Vedihold SA. No individual owns, directly or indirectly, 10% or more shares in Vedipar SA. The registered office address of Verlinvest Asia (HK) Limited is 31/F., 148 Electric Road, North Point, Hong Kong.
(10) Represents 11,494,252 Class B ordinary shares held by Infinity Cosmo Limited, a company incorporated in the British Virgin Islands. Infinity Cosmo Limited is controlled by Gang Yu Irrevocable Trust, a trust managed by Zedra Asia Limited, as the trustee. Dr. Gang Yu is the settlor of the Gang Yu Irrevocable Trust, and Dr. Yu’s family members are the trust’s beneficiaries. Under the terms of this trust, a family member of Gang Yu has the power to direct the trustee with respect to the disposal of, and the exercise of any voting and other rights attached to, the shares held by Infinity Cosmo Limited in our company. The registered office address of Infinity Cosmo Limited is Sertus Chambers, P.O. Box 905, Quastisky Building, Road Town, Tortola, British Virgin Islands.
(11) Beneficial ownership calculation is based solely on a review of a Schedule 13G filed with the SEC on February 14, 2019. Represents 8,690,562 Class A ordinary shares directly held by First Pharmacia International, a Cayman Islands exempted company. First Pharmacia International is held by BVCF III L.P. (92.38%) and BVCF III-A L.P. (7.62%), each a Cayman Islands exempted limited partnership. The registered office address of First Pharmacia International is Walkers Corporate Limited, Cayman Corporate Centre, 27 Hospital Road, George Town, Grand Cayman, KY1-9008, Cayman Islands.
Our ordinary shares are divided into Class A ordinary shares and Class B ordinary shares. Holders of Class A ordinary shares are entitled to one vote per share, while holders of Class B ordinary shares are entitled to ten votes per share. We issued Class A ordinary shares represented by the ADSs in our initial public offering.
To our knowledge, As of March 31, 2019, a total of 20,986,967 ordinary shares, representing approximately 12.9% of our total outstanding ordinary shares, were held by two record shareholders in the United States, including The Bank of New York Mellon, the depositary of our ADS program. The number of beneficial owners of our ADSs in the United States is likely to be much larger than the number of record holders of our ordinary shares in the United States.
Except for the above, we are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
Item 7. Major Shareholders and Related Party Transactions
A. Major Shareholders
Please refer to “Item 6. Directors, Senior Management and Employees—E. Share Ownership.”
B. Related Party Transactions
Contractual Arrangements with Our Variable Interest Entities and Their Shareholders
PRC laws and regulations currently restrict foreign ownership and investment in value-added telecommunications services in China. As a result, we operate our relevant business through our variable interest entities and their subsidiaries based on a series of contractual arrangements. For a description of these contractual arrangements, see “Item 4. Information on the Company—C. Organizational Structure—Contractual Arrangements with Our Variable Interest Entities.”
Shareholders Agreement
We entered into our tenth amended and restated shareholders agreement on June 19, 2018 with our then-existing shareholders. The shareholders agreement provides for board representation rights to certain shareholders, which terminated upon our initial public offering. The shareholders agreement also provides for certain information and inspection rights, board observer rights, preferential rights, including right of participation, right of first refusal, co-sale rights and redemption rights. All information and inspection rights and preferential rights terminated upon our initial public offering.
Registration Rights
Pursuant to our current shareholders agreement, we have granted certain registration rights to our shareholders. Set forth below is a description of the registration rights granted under the agreement.
Demand Registration Rights. At any time after six months following a QIPO as defined in the shareholders agreement, holders of at least 25% of our registrable securities have the right to demand in writing that we file a registration statement covering the registration of their registrable securities. We have the right to defer filing of a registration statement for a period of not more than 90 days if our board of directors determines in good faith that filing of a registration statement in the near future will be materially detrimental to us and our shareholders, but we cannot exercise the deferral right more than once during any twelve-month period. We are not obligated to effect more than two demand registrations. Further, if the registrable securities are offered by means of an underwritten offering, and the underwriters advise us in writing that marketing factors require a limitation of the number of share to be underwritten, the underwriters may reduce as required and allocate the shares to be included in the registration statement among holders of our registrable securities on a pro rata basis, subject to certain limitations.
Piggyback Registration Rights. If we propose to file a registration statement for a public offering of our securities, we must offer holders of our registrable securities an opportunity to be included in such registration. If the underwriters determine in good faith that marketing factors require a limitation of the number of shares to be underwritten, the registrable securities shall allocate first to us, second, to each holder of our registrable securities requesting inclusion of their registrable securities pursuant to the piggyback registration, on a pro rata basis, and third, to other holders of our securities.
Form F-3 or Form S-3 Registration Rights. After our initial public offering, we shall use our best efforts to qualify for registration on Form F-3 or Form S-3. Holders of 10% or more of our registrable securities may request us in writing to file a registration statement on Form F-3 or Form S-3 if we qualify for registration on such forms, subject to certain limitations. We have the right to defer filing for a period of not more than 60 days if our board of directors determines in good faith that effecting registration at such time would be materially detrimental to us and our shareholders, but we cannot exercise the deferral right more than once during any twelve-month period, and we may not register any of our other shares during such 60-day period. The holders of our registrable securities are entitled to an unlimited number of registrations on Form F-3 or Form S-3. We, however, are not obligated to effect such registration if we have effected two such registrations within any twelve-month period.
Expenses of Registration. We will bear all registration expenses, other than underwriting discounts and selling commissions applicable to the sale of registrable securities, incurred in connection with registrations, filings or qualification pursuant to the shareholders agreement. We will not be required to pay for any expenses of any registration proceeding begun pursuant to demand registration rights, unless subject to certain exception, if the registration request is subsequently withdrawn at the request of a majority of the holders of the registrable securities to be registered.
Termination of Obligations. We have no obligation to effect any demand, piggyback or Form F-3 or Form S-3 registration upon the earlier of (i) the fifth anniversary from the date of closing of a QIPO, (ii) a Trade Sale as defined in the shareholders agreement, and (iii) with respect to any holder of our registrable securities, the date following a QIPO on which such holder holds less than 1% of our total outstanding share capital.
Employment Agreements and Indemnification Agreements
See “Item 6—Directors, Senior Management and Employees—C. Board Practices”
Share Incentive Plans
See “Item 6. Directors, Senior Management and Employees—B. Compensation”
Transactions with Other Related Parties
In September 2018, we purchased electronic equipment for RMB157,000 (US$22,835) from Zhejiang Youzhan Information Technology Co., Ltd., an entity controlled by our chief operating officers. No similar purchases were made in 2016 and 2017.
C. Interests of Experts and Counsel
Not applicable.
A. Consolidated Statements and Other Financial Information
We have appended consolidated financial statements filed as part of this annual report.
Legal Proceedings
We are currently not a party to any material legal or administrative proceedings. We may from time to time be subject to various legal or administrative claims and proceedings arising in the ordinary course of business. Litigation or any other legal or administrative proceeding, regardless of the outcome, is likely to result in substantial cost and diversion of our resources, including our management’s time and attention.
Dividend Policy
We have not previously declared or paid cash dividends and we have no plan to declare or pay any dividends in the near future on our shares. We currently intend to retain most, if not all, of our available funds and any future earnings to operate and expand our business.
We are a holding company incorporated in the Cayman Islands. We rely principally on dividends from our PRC subsidiaries for our cash requirements, including any payment of dividends to our shareholders. PRC regulations may restrict the ability of our PRC subsidiaries to pay dividends to us. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—We rely on dividends and other distributions on equity paid by our PRC subsidiaries to fund any cash and financing requirements we may have, and any limitation on the ability of our PRC subsidiaries to make payments to us could have a material adverse effect on our ability to conduct our business.”
Our board of directors has discretion as to whether to distribute dividends, subject to certain restrictions under Cayman Islands law, namely that our company may only pay dividends either out of profits or share premium account, and provided always that in no circumstances may a dividend be paid if this would result in our company being unable to pay its debts as they fall due in the ordinary course of business. In addition, our shareholders may by ordinary resolution declare a dividend, but no dividend may exceed the amount recommended by our board of directors. Even if our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant. If we pay any dividends on our ordinary shares, we will pay those dividends which are payable in respect of the Class A ordinary shares underlying ADSs to the depositary, as the registered holder of such Class A ordinary shares, and the depositary will then pay such amounts to ADS holders in proportion to the Class A ordinary shares underlying the ADSs held by such ADS holders, subject to the terms of the deposit agreement, including the fees and expenses payable thereunder. See “Item 12. Description of Securities Other Than Equity Securities—D. American Depositary Shares.” Cash dividends on our ordinary shares, if any, will be paid in U.S. dollars.
B. Significant Changes
Except as disclosed elsewhere in this annual report, we have not experienced any significant changes since the date of our audited consolidated financial statements included in this annual report.
A. Offering and Listing Details
Our ADSs, each representing two Class A ordinary shares, have been listed on The Nasdaq Global Market under the symbol “YI” since September 12, 2018.
B. Plan of Distribution
Not applicable.
C. Markets
The ADSs have been listed on Nasdaq since September 12, 2018 under the symbol “YI.”
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
Item 10. Additional Information
A. Share Capital
Not applicable.
B. Memorandum and Articles of Association
We are a Cayman Islands exempted company and our affairs are governed by our memorandum and articles of association and the Companies Law (2018 Revision) of the Cayman Islands, referred to as the Companies Law below, and the common law of the Cayman Islands. The following are summaries of material provisions of our twelfth amended and restated memorandum and articles of association, as well as the Companies Law insofar as they relate to the material terms of our ordinary shares.
Registered Office and Objects
Our registered office in the Cayman Islands is located at the offices of Vistra (Cayman) Limited, P.O. Box 31119, Grand Pavilion Hibiscus Way, 802 West Bay Road, Grand Cayman, KY1-1205, Cayman Islands. Our agent for service of process in the United States is Puglisi & Associates. The objects of our company are unrestricted and we have the full power and authority to carry out any object not prohibited by the law of the Cayman Islands.
Board of Directors
See “Item 6. Directors, Senior Management and Employees—C. Board Practices—Board of Directors.”
Ordinary Shares
General
Our ordinary shares are divided into Class A ordinary shares and Class B ordinary shares. Holders of our Class A ordinary shares and Class B ordinary shares will have the same rights except for voting and conversion rights, as described below. All of our outstanding ordinary shares are fully paid and non-assessable. Certificates representing the ordinary shares are issued in registered form. Our ordinary shares are issued in registered form and are issued when registered in our register of members. Our shareholders who are non-residents of the Cayman Islands may freely hold and vote their ordinary shares. Our company will issue only non-negotiable shares, and will not issue bearer or negotiable shares.
Conversion
Each Class B ordinary share may be converted into one Class A ordinary share at any time at the option of the holder thereof, while Class A ordinary shares cannot be converted into Class B ordinary shares under any circumstances.
Upon any sale, transfer, assignment or disposition of any Class B ordinary share by our Founders (defined in our memorandum and articles of association to mean Dr. Gang Yu and Mr. Junling Liu) or Founder Affiliate (as defined in our memorandum and articles of association) to any person who is not a ‘‘Founder Affiliate,’’ or upon a change of ultimate beneficial ownership of any Class B ordinary share to any person who is not a Founder Affiliate, such Class B ordinary share shall be automatically and immediately converted into one Class A ordinary share. However, the creation of any pledge, charge, encumbrance or other third party right on any Class B ordinary share to secure a holder’s contractual or legal obligations shall not be deemed as a sale, transfer, assignment or disposition unless and until any such pledge, charge, encumbrance or other third party right is enforced and results in the third party holding legal title to the relevant Class B ordinary shares, in which case all the related Class B ordinary shares shall be automatically converted into the same number of Class A ordinary shares.
Furthermore, if at any time the Founders and the Founder Affiliates collectively own less than 5% of the total number of the issued and outstanding shares of our company, all of the issued and outstanding Class B ordinary shares shall be automatically converted into the same number of Class A ordinary shares.
Register of Members
Under Cayman Islands law, we must keep a register of members and there should be entered therein:
· the names and addresses of the members, a statement of the shares held by each member, and of the amount paid or agreed to be considered as paid, on the shares of each member;
· the date on which the name of any person was entered on the register as a member; and
· the date on which any person ceased to be a member.
Under Cayman Islands law, the register of members of our company is prima facie evidence of the matters set out therein (i.e., the register of members will raise a presumption of fact on the matters referred to above unless rebutted) and a member registered in the register of members is deemed as a matter of Cayman Islands law to have legal title to the shares as set against its name in the register of members. Once our register of members has been updated, the shareholders recorded in the register of members should be deemed to have legal title to the shares set against their name.
If the name of any person is incorrectly entered in or omitted from our register of members, or if there is any default or unnecessary delay in entering on the register the fact of any person having ceased to be a member of our company, the person or member aggrieved (or any member of our company or our company itself) may apply to the Cayman Islands Grand Court for an order that the register be rectified, and the Court may either refuse such application or it may, if satisfied of the justice of the case, make an order for the rectification of the register.
Dividends
The holders of our ordinary shares are entitled to such dividends as may be declared by our board of directors or shareholders in a general meeting (provided always that dividends may be declared and paid only out of funds legally available therefor, namely out of either profit or our share premium account, and provided further that a dividend may not be paid if this would result in our company being unable to pay its debts as they fall due in the ordinary course of business). Our shareholders may by ordinary resolution declare a dividend, but no dividend may exceed the amount recommended by our board of directors.
Voting Rights
Holders of ordinary shares have the right to receive notice of, attend, speak and vote at general meetings of our company. Holders of Class A ordinary shares and Class B ordinary shares shall, at all times, vote together as one class on all matters submitted to a vote by the members at any general meeting of our company. Each Class A ordinary share shall entitle the holder thereof to one vote on all matters subject to the vote at general meetings of our company, and each Class B ordinary share shall entitle the holder thereof to fifteen votes on all matters subject to the vote at general meetings of our company. Voting at any meeting of shareholders is by show of hands unless a poll is demanded. A poll may be demanded by the chairman of such meeting or any one shareholder present in person or by proxy.
Maples and Calder (Hong Kong) LLP, our counsel as to Cayman Islands law, has advised that such voting structure is in compliance with current Cayman Islands law as in general terms, a company and its shareholders are free to provide in the articles of association for such rights as they consider appropriate, subject to such rights not being contrary to any provision of the Companies Law and not inconsistent with common law. Maples and Calder (Hong Kong) LLP has confirmed that the inclusion in our memorandum and articles of association of provisions giving weighted voting rights to specific classes of shareholders generally or to specific classes of shareholders on specific resolutions is not prohibited by the Companies Law. Further, weighted voting provisions have been held to be valid as a matter of English common law and therefore it is expected that such would be upheld by a Cayman Islands court.
An ordinary resolution to be passed by the shareholders requires the affirmative vote of a simple majority of the votes attached to the ordinary shares cast by those shareholders who are present in person or by proxy at a general meeting, while a special resolution requires the affirmative vote of no less than two-thirds of the votes attached to the ordinary shares cast by those shareholders who are present in person or by proxy at a general meeting. Both ordinary resolutions and special resolutions may also be passed by a unanimous written resolution signed by all the shareholders of our company, as permitted by the Companies Law and our memorandum and articles of association. A special resolution will be required for important matters such as a change of name or making changes to our memorandum and articles of association.
Transfer of Ordinary Shares
Any of our shareholders may transfer all or any of his or her ordinary shares by an instrument of transfer in the usual or common form or any other form approved by our board of directors. However, our board of directors may, in its absolute discretion, decline to register any transfer of any ordinary share which is not fully paid up or on which our company has a lien. Our board of directors may also decline to register any transfer of any ordinary share unless:
· the instrument of transfer is lodged with us, accompanied by the certificate for the ordinary shares to which it relates and such other evidence as our board of directors may reasonably require to show the right of the transferor to make the transfer;
· the instrument of transfer is in respect of only one class of shares;
· the instrument of transfer is properly stamped, if required;
· any fee related to the transfer has been paid to us; and
· in the case of a transfer to joint holders, the transfer is not to more than four joint holders.
If our directors refuse to register a transfer, they are required, within three calendar months after the date on which the instrument of transfer was lodged, to send to each of the transferor and the transferee notice of such refusal. The registration of transfers of shares may, on ten calendar days’ notice being given by advertisement in such one or more newspapers, by electronic means or by any other means in accordance with the relevant code, rules and regulations of the Nasdaq Global Market, be suspended and the register of members closed at such times and for such periods (not exceeding thirty calendar days in any calendar year) as our directors may determine.
Liquidation
On a return of capital on winding up or otherwise (other than on conversion, redemption or purchase of ordinary shares), assets available for distribution among the holders of ordinary shares will be distributed among the holders of the ordinary shares on a pro rata basis. If our assets available for distribution are insufficient to repay all of the paid-up capital, the assets will be distributed so that the losses are borne by our shareholders proportionately. We are a ‘‘limited liability’’ company registered under the Companies Law, and under the Companies Law, the liability of our members is limited to the amount, if any, unpaid on the shares respectively held by them. Our memorandum and articles of association contains a declaration that the liability of our members is so limited.
Calls on Ordinary Shares and Forfeiture of Ordinary Shares
Our board of directors may from time to time make calls upon shareholders for any amounts unpaid on their ordinary shares. The ordinary shares that have been called upon and remain unpaid are subject to forfeiture.
Redemption, Repurchase and Surrender of Ordinary Shares
We may issue shares on terms that such shares are subject to redemption, at our option or at the option of the holders thereof, on such terms and in such manner as may be determined, before the issue of such shares, by our board of directors or by a special resolution of our shareholders. Our company may also repurchase any of our shares provided that the manner and terms of such purchase have been approved by our board of directors or by an ordinary resolution of our shareholders or are otherwise authorized by our memorandum and articles of association. Under the Companies Law, the redemption or repurchase of any share may be paid out of our company’s profits or out of the proceeds of a fresh issue of shares made for the purpose of such redemption or repurchase, or out of capital (including share premium account and capital redemption reserve) if our company can, immediately following such payment, pay its debts as they fall due in the ordinary course of business. In addition, under the Companies Law no such share may be redeemed or repurchased (a) unless it is fully paid up, (b) if such redemption or repurchase would result in there being no shares outstanding, or (c) if the company has commenced liquidation. In addition, our company may accept the surrender of any fully paid share for no consideration.
Variations of Rights of Shares
If at any time, our share capital is divided into different classes of shares, all or any of the rights attached to any class of shares may, subject to any rights or restrictions for the time being attached to any class, only be materially adversely varied with the consent in writing of the holders of two-thirds of the issued shares of that class or with the sanction of a special resolution passed at a separate meeting of the holders of shares of that class. The rights conferred upon the holders of the shares of any class issued with preferred or other rights will not, subject to any rights or restrictions for the time being attached to the shares of that class, be deemed to be materially adversely varied by, inter alia, the creation, allotment or issue of further shares ranking pari passu with or subsequent to them or the redemption or purchase of any shares of any class by our company. The rights of the holders of shares shall not be deemed to be materially adversely varied by the creation or issue of shares with preferred or other rights including, without limitation, the creation of shares with enhanced or weighted voting rights.
General Meetings of Shareholders and Shareholder Proposals
As a Cayman Islands exempted company, we are not obliged by the Companies Law to call shareholders’ annual general meetings.
Shareholders’ annual general meetings and any other general meetings of our shareholders may be convened by the chairman of our board of directors, or any co-chairman of our board of directors, or by a majority of our board of directors. Advance notice of at least ten calendar days is required for the convening of our annual general shareholders’ meeting and any other general meeting of our shareholders. A quorum required for a general meeting of shareholders consists of one or more shareholders present in person or by proxy (or, if a corporation or other non-natural person, by its duly authorized representative), holding shares which carry in aggregate not less than one-third of the total number of votes attaching to all issued and outstanding shares in our company which are entitled to vote at the meeting.
Cayman Islands law provides shareholders with only limited rights to requisition a general meeting, and does not provide shareholders with any right to put any proposal before a general meeting. However, these rights may be provided in a company’s articles of association. Our memorandum and articles of association allow any shareholders holding shares which carry in aggregate not less than one-third of the total number of votes attaching to all issued and outstanding shares in our company, that carry the right to vote at general meetings of our company to requisition an extraordinary general meeting of our shareholders, in which case our directors are obliged to call such meeting and to put the resolutions so requisitioned to a vote at such meeting; however, our memorandum and articles of association do not provide our shareholders with any right to put any proposals before annual general meetings or extraordinary general meetings not called by such shareholders.
Election and Removal of Directors
Our memorandum and articles of association provide that, unless otherwise determined by our company in general meeting, our board will consist of not less than three directors. There are no provisions relating to retirement of directors upon reaching any age limit. Our board of directors may, by the affirmative vote of a simple majority of the remaining directors present and voting at a meeting of our board, which shall include the affirmative vote of at least one Founder as long as either Founder is a director, appoint any person as a director, to fill a vacancy on the board arising from the office of any director being vacated. In the event of a vacancy arising from the office of an independent director being vacated, our board may only appoint another independent director to fill such vacancy.
Our shareholders may also appoint any person to be a director by way of ordinary resolution. A director may be removed with or without cause by ordinary resolution. A vacancy on the board created by the removal of a director by ordinary resolution of our shareholders may be filled by an ordinary resolution or by the affirmative vote of a simple majority of the remaining directors present and voting at a meeting of our board. The notice of any meeting at which a resolution to remove a director shall be proposed or voted upon must contain a statement of the intention to remove that director and such notice must be served on that director not less than ten calendar days before the meeting. Such director is entitled to attend the meeting and be heard on the motion for his removal. In addition, a director will cease to be a director if, among other things, the director (i) becomes bankrupt or makes any arrangement or composition with his creditors, (ii) dies or is found by our company to be or becomes of unsound mind, (iii) resigns his office by notice in writing to the company, or (iv) without special leave of absence from our board, is absent from three consecutive board meetings and our directors resolve that his office be vacated.
Chairman and Co-chairmen of our Board
For so long as each of our founders is a director of our company, he shall be a co-chairman of our board of directors, until he resigns as co-chairman or ceases to be a director (in which event he shall automatically cease to be a co-chairman). If either founder ceases to be a co-chairman, the other founder shall continue as the sole chairman of our board (unless our board, with the consent of the other founder, elects and appoints another director to be another co-chairman). Upon both founders ceasing to be co-chairmen or chairman, our board shall elect and appoint the chairman or co-chairmen at their discretion.
Proceedings of Board of Directors
Our memorandum and articles of association provide that our business is to be managed and conducted by our board of directors. The quorum necessary for board meetings may be fixed by the board and, unless so fixed at another number, will be a majority of the directors. Our memorandum and articles of association provide that the board may from time to time at its discretion exercise all powers of our company to raise or borrow money, to mortgage or charge all or any part of the undertaking, property and assets (present and future) and uncalled capital of our company and issue debentures, bonds and other securities of our company, whether outright or as collateral security for any debt, liability or obligation of our company or of any third party.
Inspection of Books and Records
Holders of our ordinary shares have no general right under Cayman Islands law to inspect or obtain copies of our list of shareholders or our corporate records. However, we intend to provide our shareholders with annual audited financial statements.
Changes in Capital
Our shareholders may from time to time by ordinary resolution:
· increase our share capital by such sum, to be divided into shares of such amount, as the resolution shall prescribe;
· consolidate and divide all or any of our share capital into shares of a larger amount than our existing shares;
· sub-divide our existing shares, or any of them into shares of a smaller amount than that fixed by our memorandum of association; or
· cancel any shares which, at the date of the passing of the resolution, have not been taken or agreed to be taken by any person and diminish the amount of our share capital by the amount of the shares so cancelled.
Our shareholders may by special resolution, subject to confirmation by the Grand Court of the Cayman Islands on an application by our company for an order confirming such reduction, reduce our share capital or any capital redemption reserve in any manner permitted by law.
Exempted Company
We are an exempted company incorporated with limited liability under the Companies Law. The Companies Law distinguishes between ordinary resident companies and exempted companies. Any company that is registered in the Cayman Islands but conducts business mainly outside of the Cayman Islands may apply to be registered as an exempted company. The requirements for an exempted company are essentially the same as for an ordinary company except for the exemptions and privileges listed below:
· an exempted company does not have to file an annual return of its shareholders with the Registrar of Companies of the Cayman Islands;
· an exempted company’s register of members is not required to be open to inspection;
· an exempted company does not have to hold an annual general meeting;
· an exempted company may issue no par value, negotiable or bearer shares;
· an exempted company may obtain an undertaking against the imposition of any future taxation (such undertakings are usually given for 20 years in the first instance);
· an exempted company may register by way of continuation in another jurisdiction and be deregistered in the Cayman Islands;
· an exempted company may register as a limited duration company; and
· an exempted company may register as a segregated portfolio company.
“Limited liability” means that the liability of each shareholder is limited to the amount unpaid by the shareholder on that shareholder’s shares of the company (except in exceptional circumstances, such as involving fraud, the establishment of an agency relationship or an illegal or improper purpose or other circumstances in which a court may be prepared to pierce or lift the corporate veil).
We are subject to reporting and other informational requirements of the Exchange Act, as applicable to foreign private issuers. Except as otherwise disclosed in this annual report, we currently comply with the Nasdaq Global Market rules in lieu of following home country practice.
C. Material Contracts
We have not entered into any material contracts other than in the ordinary course of business and other than those described in “Item 4. Information on the Company” or elsewhere in this annual report on Form 20-F.
D. Exchange Controls
See “Item 4. Information on the Company—B. Business Overview—Regulations—Regulations Relating to Foreign Exchange and Dividend Distributions.”
E. Taxation
The following summary of material Cayman Islands, PRC and U.S. federal income tax consequences of an investment in ADSs or ordinary shares is based upon laws and relevant interpretations thereof in effect as of the date of this annual report, all of which are subject to change. This summary does not deal with all possible tax consequences relating to an investment in ADSs or ordinary shares, such as the tax consequences under state, local and other tax laws.
Cayman Islands Taxation
The Cayman Islands currently levies no taxes on individuals or corporations based upon profits, income, gains or appreciation and there is no taxation in the nature of inheritance tax or estate duty. There are no other taxes likely to be material to investors levied by the government of the Cayman Islands except for stamp duties which may be applicable on instruments executed in, or, after execution, brought within the jurisdiction of the Cayman Islands. The Cayman Islands is not party to any double tax treaties which are applicable to any payments made by our company. There are no exchange control regulations or currency restrictions in the Cayman Islands.
Payments of dividends and capital in respect of ordinary shares and ADSs will not be subject to taxation in the Cayman Islands and no withholding will be required on the payment of a dividend or capital to any holder of ordinary shares or ADSs, nor will gains derived from the disposal of ordinary shares or ADSs be subject to Cayman Islands income or corporation tax.
No stamp duty is payable in respect of the issue of our ordinary shares or on an instrument of transfer in respect of our ordinary shares.
People’s Republic of China Taxation
Although we are incorporated in the Cayman Islands, we may be treated as a PRC resident enterprise for PRC tax purposes under the EIT Law. The EIT Law provides that an enterprise established outside the PRC but whose “de facto management body” is located in the PRC is treated as a PRC resident enterprise for PRC tax purposes. The implementing rules of the EIT Law merely define the location of the “de facto management body” as “organizational body which effectively manages and controls the production and business operation, personnel, accounting, properties and other aspects of operations of an enterprise.” Based on a review of the facts and circumstances, we do not believe that 111, Inc. or Yao Wang Corporation Limited should be considered a PRC resident enterprise for PRC tax purposes. However, there is limited guidance and implementation history of the Enterprise Income Tax Law. If 111, Inc. were to be considered a PRC resident enterprise, then PRC income tax at a rate of 10% would generally be applicable to any gain realized on the transfer of our ADSs or ordinary shares by investors that are “non-resident enterprises” of the PRC and to any interest or dividends payable by us to such investors. See “Item 3. Key Information—D. Risk Factors—Risks Related to Doing Business in China—If we are classified as a PRC resident enterprise for PRC income tax purposes, such classification could result in unfavorable tax consequences to us and our non-PRC shareholders or ADS holders.”
Furthermore, pursuant to the EIT Law and its implementation rules, if a non-resident enterprise has not set up an organization or establishment in the PRC, or has set up an organization or establishment but the income derived has no actual connection with such organization or establishment, it will be subject to a withholding tax on its PRC-sourced income at a rate of 10%. On February 3, 2015, the SAT issued the Announcement on Several Issues Concerning the Enterprise Income Tax on Indirect Property Transfers by Non-PRC Resident Enterprises, pursuant to which the indirect transfer of assets of an “establishment or place” situated in China, by a non-PRC resident enterprise through a disposition of equity interests in an offshore holding company may also be treated as a transfer of PRC taxable assets and, as a result, the gain derived from this indirect transfer by a non-PRC enterprise shareholder (other than the sale at public stock market of shares that purchased by an offshore enterprise in public stock market) may be subject to PRC enterprise income tax at a rate of 10%. Therefore, the deposition of ADSs or ordinary shares acquired not at public stock market by investors in private transaction may subject to withholding tax rate at a rate of 10%.
United States Federal Income Taxation Considerations
The following discussion is a summary of United States federal income tax considerations generally applicable to the ownership and disposition of our ADSs or ordinary shares by a U.S. holder (as defined below) that holds our ADSs or ordinary shares as “capital assets” (generally, property held for investment) under the United States Internal Revenue Code of 1986, as amended (the “Code”). This discussion is based upon existing United States federal income tax law, which is subject to differing interpretations and may be changed, possibly with retroactive effect. No ruling has been sought from the Internal Revenue Service (the “IRS”) with respect to any United States federal income tax consequences described below, and there can be no assurance that the IRS or a court will not take a contrary position. This discussion does not address all aspects of United States federal income taxation that may be important to particular investors in light of their individual circumstances, including investors subject to special tax rules (for example, certain financial institutions, insurance companies, broker-dealers, traders in securities that have elected the mark-to-market method of accounting for their securities, partnerships and their partners, regulated investment companies, real estate investment trusts, and tax-exempt organizations (including private foundations)), investors who are not U.S. holders, investors who own (directly, indirectly, or constructively) 10% or more of our stock (by vote or value), investors that will hold their ADSs or ordinary shares as part of a straddle, hedge, conversion, constructive sale, or other integrated transaction for United States federal income tax purposes, investors required to accelerate the recognition of any item of gross income with respect to our ADSs or ordinary shares as a result of such income being recognized on an applicable financial statement, or investors that have a functional currency other than the United States dollar, all of whom may be subject to tax rules that differ significantly from those summarized below. In addition, this discussion does not discuss any non-United States, alternative minimum tax, state, or local tax or any non-income tax (such as the U.S. federal gift or estate tax) considerations, or the Medicare tax on net investment income. Each U.S. holder is urged to consult its tax advisor regarding the United States federal, state, local, and non-United States income and other tax considerations of an investment in our ADSs or ordinary shares.
General
For purposes of this discussion, a “U.S. holder” is a beneficial owner of our ADSs or ordinary shares that is, for United States federal income tax purposes, (i) an individual who is a citizen or resident of the United States, (ii) a corporation (or other entity treated as a corporation for United States federal income tax purposes) created in, or organized under the laws of, the United States or any state thereof or the District of Columbia, (iii) an estate the income of which is subject to United States federal income taxation regardless of its source, or (iv) a trust (A) the administration of which is subject to the primary supervision of a United States court and which has one or more United States persons who have the authority to control all substantial decisions of the trust or (B) that has otherwise elected to be treated as a United States person under the Code or applicable United States Treasury regulations.
If a partnership (or other entity or arrangement treated as a partnership for United States federal income tax purposes) is a beneficial owner of our ADSs or ordinary shares, the tax treatment of a partner in the partnership will generally depend upon the status of the partner and the activities of the partnership. Partnerships holding our ADSs or ordinary shares and partners in such partnerships are urged to consult their tax advisors as to the particular United States federal income tax consequences of an investment in our ADSs or ordinary shares.
For United States federal income tax purposes, it is generally expected that a U.S. holder of ADSs will be treated as the beneficial owner of the underlying shares represented by the ADSs. The remainder of this discussion assumes that a U.S. holder of our ADSs will be treated as the beneficial owner of the underlying shares represented by the ADSs. Accordingly, deposits or withdrawals of ordinary shares for ADSs will generally not be subject to United States federal income tax.
Passive Foreign Investment Company Considerations
A non-United States corporation, such as our company, will be a “passive foreign investment company,” or PFIC, for United States federal income tax purposes, if, in any particular taxable year, either (i) 75% or more of its gross income for such year consists of certain types of “passive” income or (ii) 50% or more of the average quarterly value of its assets (as determined on the basis of fair market value) during such year produce or are held for the production of passive income. For this purpose, cash and assets readily convertible to cash are categorized as a passive asset and the company’s unbooked intangibles associated with active business activities may generally be classified as active assets.
Passive income generally includes, among other things, dividends, interest, rents, royalties, and gains from the disposition of passive assets. We will be treated as owning a proportionate share of the assets and income of any other corporation in which we own, directly or indirectly, at least 25% (by value) of the stock. Although the law in this regard is unclear, we intend to treat our variable interest entities (including their subsidiaries) as being owned by us for United States federal income tax purposes, and we treat it that way, not only because we exercise effective control over the operation of such entities but also because we are entitled to substantially all of their economic benefits, and, as a result, we consolidate their results of operations in our consolidated financial statements. If it were determined, however, that we are not the owner of our variable interest entities (including their subsidiaries) for United States federal income tax purposes, we may be treated as a PFIC for the current taxable year and any subsequent taxable year. Assuming that we are the owner of our variable interest entities (including their subsidiaries) for United States federal income tax purposes, and based upon our current and expected income and assets, we do not believe we were a PFIC for the taxable year ended December 31, 2018 and we do not presently expect to be a PFIC for the current taxable year or the foreseeable future.
While we do not expect to be or become a PFIC in the current or future taxable years, the determination of PFIC status will depend, in part, upon the composition of our income and assets, and the continued existence of our goodwill at that time. Fluctuations in the market price of our ADSs may cause us to become a PFIC for the current or subsequent taxable years because the value of our assets for the purpose of the asset test, including goodwill and other unbooked intangibles, may be determined by reference to the market value of our ADSs from time to time which may be volatile.
In addition, the composition of our income and assets will be affected by how, and how quickly, we use our liquid assets. If we determine not to deploy significant amounts of cash for active purposes or if we were treated as not owning our variable interest entities (including their subsidiaries) for United States federal income tax purposes, our risk of being classified as a PFIC may substantially increase. Because our PFIC status for any taxable year is a factual determination that can be made only after the close of a taxable year, there can be no assurance that we will not be a PFIC for the current taxable year or any future taxable year. If we are a PFIC for any year during which a U.S. holder holds our ADSs or ordinary shares, we generally will continue to be treated as a PFIC for all succeeding years during which such U.S. holder holds our ADSs or ordinary shares.
The discussion below under “Dividends” and “Sale or Other Disposition of ADSs or Ordinary Shares” is written on the basis that we will not be or become a PFIC for United States federal income tax purposes. The United States federal income tax rules that apply if we are a PFIC for the current taxable year or any subsequent taxable year are generally discussed below under “Passive Foreign Investment Company Rules.”
Dividends
Subject to the PFIC rules discussed below, any cash distributions (including the amount of any tax withheld) paid on our ADSs or ordinary shares out of our current or accumulated earnings and profits, as determined under United States federal income tax principles, will generally be includible in the gross income of a U.S. holder as dividend income on the day actually or constructively received by the U.S. holder, in the case of ordinary shares, or by the depositary, in the case of ADSs. Because we do not intend to determine our earnings and profits on the basis of United States federal income tax principles, any distribution paid will generally be reported as a dividend for United States federal income tax purposes. Dividends received on our ADSs or ordinary shares will not be eligible for the dividends received deduction allowed to corporations.
Individuals and other non-corporate U.S. holders will generally be subject to tax at the lower capital gain tax rate applicable to “qualified dividend income,” provided that certain conditions are satisfied, including that (1) our ADSs are readily tradable on an established securities market in the United States, or, in the event that we are deemed to be a PRC resident enterprise under the PRC tax law, we are eligible for the benefit of the United States-PRC income tax treaty, (2) we are neither a PFIC nor treated as such with respect to a U.S. holder (as discussed below) for the taxable year in which the dividend is paid and the preceding taxable year, and (3) certain holding period requirements are met. We expect our ADSs will be considered to be readily tradable on the Nasdaq Global Market, which is an established securities market in the United States. There can be no assurance that our ADSs will continue to be considered readily tradable on an established securities market in later years. Since we do not expect that our ordinary shares will be listed on established securities markets, we do not believe that dividends that we pay on our ordinary shares that are not backed by ADSs currently meet the conditions required for the reduced tax rate. However, in the event we are deemed to be a resident enterprise under the PRC Enterprise Income Tax Law, we may be eligible for the benefits of the United States-PRC income tax treaty (which the U.S. Treasury Department has determined is satisfactory for this purpose) and in that case, we would be treated as a qualified foreign corporation with respect to dividends paid on our ordinary shares as well as our ADSs. Each non-corporate U.S. holder is advised to consult its tax advisors regarding the availability of the reduced tax rate applicable to qualified dividend income for any dividends we pay with respect to our ADSs or ordinary shares.
Dividends will generally be treated as income from foreign sources for United States foreign tax credit purposes and will generally constitute passive category income. In the event that we are deemed to be a PRC “resident enterprise” under the Enterprise Income Tax Law, a U.S. holder may be subject to PRC withholding taxes on dividends paid on our ADSs or ordinary shares. See “Item 10. Additional Information—E. Taxation—People’s Republic of China Taxation.” In that case, a U.S. holder may be eligible, subject to a number of complex limitations, to claim a foreign tax credit in respect of any foreign withholding taxes imposed on dividends received on ADSs or ordinary shares. A U.S. holder who does not elect to claim a foreign tax credit for foreign tax withheld may instead claim a deduction, for United States federal income tax purposes, in respect of such withholdings, but only for a year in which such U.S. holder elects to do so for all creditable foreign income taxes. The rules governing the foreign tax credit are complex. U.S. holders are advised to consult their tax advisors regarding the availability of the foreign tax credit under their particular circumstances.
Sale or Other Disposition of ADSs or Ordinary Shares
Subject to the PFIC rules discussed below, a U.S. holder will generally recognize capital gain or loss upon the sale or other disposition of ADSs or ordinary shares in an amount equal to the difference between the amount realized upon the disposition and the U.S. holder’s adjusted tax basis in such ADSs or ordinary shares. Any capital gain or loss will be long-term if the ADSs or ordinary shares have been held for more than one year and will generally be United States source gain or loss for United States foreign tax credit purposes. Long-term capital gain of individuals and other non-corporate U.S. holders is generally eligible for a reduced rate of taxation. The deductibility of a capital loss may be subject to limitations.
In the event that we are treated as a PRC “resident enterprise” under the Enterprise Income Tax Law and gain from the disposition of the ADSs or ordinary shares is subject to tax in the PRC, a U.S. holder that is eligible for the benefits of the income tax treaty between the United States and the PRC may elect to treat the gain as PRC source income. If a U.S. holder is not eligible for the benefits of the income tax treaty or fails to make the election to treat any gain as foreign source, then such U.S. holder may not be able to use the foreign tax credit arising from any PRC tax imposed on the disposition of the ADSs or ordinary shares unless such credit can be applied (subject to applicable limitations) against U.S. federal income tax due on other income derived from foreign sources in the same income category (generally, the passive category). U.S. holders are advised to consult their tax advisors regarding the tax consequences if a foreign tax is imposed on a disposition of our ADSs or ordinary shares, including the availability of the foreign tax credit under their particular circumstances and the election to treat any gain as PRC source.
Passive Foreign Investment Company Rules
If we are a PFIC for any taxable year during which a U.S. holder holds our ADSs or Class A ordinary shares, and unless the U.S. holder makes a mark-to-market election (as described below), the U.S. holder will generally be subject to special tax rules that have a penalizing effect, regardless of whether we remain a PFIC, for subsequent taxable years, on (i) any excess distribution that we make to the U.S. holder (which generally means any distribution paid during a taxable year to a U.S. holder that is greater than 125% of the average annual distributions paid in the three preceding taxable years or, if shorter, the U.S. holder’s holding period for the ADSs or Class A ordinary shares), and (ii) any gain realized on the sale or other disposition, including, under certain circumstances, a pledge, of ADSs or ordinary shares. Under the PFIC rules:
· such excess distribution and/or gain will be allocated ratably over the U.S. holder’s holding period for the ADSs or Class A ordinary shares;
· such amount allocated to the current taxable year and any taxable years in the U.S. holder’s holding period prior to the first taxable year in which we are a PFIC, or pre-PFIC year, will be taxable as ordinary income;
· such amount allocated to each prior taxable year, other than a pre-PFIC year, will be subject to tax at the highest tax rate in effect for that year; and
· an interest charge generally applicable to underpayments of tax will be imposed on the tax attributable to each prior taxable year, other than a pre-PFIC year.
If we are a PFIC for any taxable year during which a U.S. holder holds our ADSs or Class A ordinary shares and any of our non-United States subsidiaries is also a PFIC, such U.S. holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC for purposes of the application of these rules. U.S. holders are advised to consult their tax advisors regarding the application of the PFIC rules to any of our subsidiaries.
As an alternative to the foregoing rules, a U.S. holder of “marketable stock” in a PFIC may make a mark-to-market election with respect to our ADSs. The mark-to-market election is available only for stock that is regularly traded on a national securities exchange that is registered with the SEC, or on a foreign exchange or market that the IRS determines is a qualified exchange that has rules sufficient to ensure that the market price represents a legitimate and sound fair market value. Our ADSs are listed on the Nasdaq Global Market, which is an established securities market in the United States. Consequently, if our ADSs continue to be listed on the Nasdaq Global Market and are regularly traded, we expect that the mark-to-market election would be available to a U.S. Holder that holds our ADSs were we to be or become a PFIC. Our ADSs are expected to qualify as being regularly traded, but no assurance may be given in this regard.
Because a mark-to-market election cannot be made for any lower-tier PFICs that a PFIC may own, a U.S. holder who makes a mark-to-market election with respect to our ADSs will generally continue to be subject to the general PFIC rules with respect to such U.S. holder’s indirect interest in any investments held by us that are treated as an equity interest in a PFIC for United States federal income tax purposes. If a mark-to-market election is made, the U.S. holder will generally (i) include as ordinary income for each taxable year that we are a PFIC the excess, if any, of the fair market value of ADSs held at the end of the taxable year over the adjusted tax basis of such ADSs and (ii) deduct as an ordinary loss the excess, if any, of the adjusted tax basis of the ADSs over the fair market value of such ADSs held at the end of the taxable year, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. The U.S. holder’s adjusted tax basis in the ADSs would be adjusted to reflect any income or loss resulting from the mark-to-market election. If a U.S. holder makes an effective mark-to-market election, in each year that we are a PFIC any gain recognized upon the sale or other disposition of the ADSs will be treated as ordinary income and loss will be treated as ordinary loss, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. If a U.S. holder makes a mark-to-market election it will be effective for the taxable year for which the election is made and all subsequent taxable years unless the ADSs are no longer regularly traded on a qualified exchange or the IRS consents to the revocation of the election. It should also be noted that it is intended that only the ADSs and not the ordinary shares are listed on the Nasdaq Global Market. Consequently, if a U.S. holder holds ordinary shares that are not represented by ADSs, such holder generally will not be eligible to make a mark-to-market election if we are or were to become a PFIC.
If a U.S. holder makes a mark-to-market election in respect of a PFIC and such corporation ceases to be a PFIC, the U.S. holder will not be required to take into account the mark-to-market gain or loss described above during any period that such corporation is not a PFIC.
We do not intend to provide information necessary for U.S. holders to make qualified electing fund elections, which, if available, would result in tax treatment different from (and generally less adverse than) the general tax treatment for PFICs described above.
If a U.S. holder owns our ADSs or ordinary shares during any taxable year that we are a PFIC, such holder would generally be required to file an annual IRS Form 8621. Each U.S. holder is advised to consult its tax advisors regarding the potential tax consequences to such holder if we are or become a PFIC, including the possibility of making a mark-to-market election.
B. Dividends and Paying Agents
Not applicable.
C. Statement by Experts
Not applicable.
D. Documents on Display
We are subject to periodic reporting and other informational requirements of the Exchange Act as applicable to foreign private issuers, and are required to file reports and other information with the SEC. Specifically, we are required to file annually an annual report on Form 20-F within four months after the end of each fiscal year, which is December 31. All information filed with the SEC can be obtained over the internet at the SEC’s website at www.sec.gov or inspected and copied at the public reference facilities maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. You can request copies of documents, upon payment of a duplicating fee, by writing to the SEC. As a foreign private issuer, we are exempt from the rules under the Exchange Act prescribing the furnishing and content of quarterly reports and proxy statements, and officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
We will furnish the Bank of New York Mellon, the depositary of the ADSs, with our annual reports, which will include a review of operations and annual audited consolidated financial statements prepared in conformity with U.S. GAAP, and all notices of shareholders’ meetings and other reports and communications that are made generally available to our shareholders. The depositary will make such notices, reports and communications available to holders of ADSs and, upon our request, will mail to all record holders of ADSs the information contained in any notice of a shareholders’ meeting received by the depositary from us.
E. Subsidiary Information
Not applicable.
Item 11. Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
Our exposure to interest rate risk primarily relates to the interest income generated by excess cash, which is mostly held in interest-bearing bank deposits and financial instruments. These interest-bearing bank deposits and financial instruments carry a degree of interest rate risk. We have not been exposed to material risks due to changes in interest rates, and we have not used any derivative financial instruments to manage our interest risk exposure. However, our future interest income may fall short of expectations due to changes in market interest rates.
Foreign Exchange Risk
All of our net revenues and substantially all of our expenses are denominated in Renminbi. Our exposure to foreign exchange risk primarily relates to cash and cash equivalent denominated in U.S. dollars. We do not believe that we currently have any significant direct foreign exchange risk and have not used any derivative financial instruments to hedge exposure to such risk. Although our exposure to foreign exchange risks should be limited in general, the value of your investment in our ADSs will be affected by the exchange rate between U.S. dollar and Renminbi because the value of our business is effectively denominated in Renminbi, while our ADSs will be traded in U.S. dollars.
The value of Renminbi against the U.S. dollar and other currencies may fluctuate and is affected by, among other things, changes in political and economic conditions and the foreign exchange policy adopted by the PRC government. On July 21, 2005, the PRC government changed its policy of pegging the value of the Renminbi to the U.S. dollar. Following the removal of the U.S. dollar peg, the Renminbi appreciated more than 20% against the U.S. dollar over the following three years. Between July 2008 and June 2010, this appreciation halted and the exchange rate between the Renminbi and the U.S. dollar remained within a narrow band. Since June 2010, the PRC government has allowed the Renminbi to appreciate slowly against the U.S. dollar again, and it has appreciated more than 10% since June 2010. On August 11, 2015, the People’s Bank of China announced plans to improve the central parity rate of the Renminbi against the U.S. dollar by authorizing market-makers to provide parity to the China Foreign Exchange Trading Center operated by the People’s Bank of China with reference to the interbank foreign exchange market closing rate of the previous day, the supply and demand for foreign currencies as well as changes in exchange rates of major international currencies. Effective from October 1, 2016, the International Monetary Fund added Renminbi to its Special Drawing Rights currency basket. Such change and additional future changes may increase volatility in the trading value of the Renminbi against foreign currencies. The PRC government may adopt further reforms of its exchange rate system, including making the Renminbi freely convertible in the future. Accordingly, it is difficult to predict how market forces or PRC or U.S. government policy may impact the exchange rate between Renminbi and the U.S. dollar in the future.
To the extent that we need to convert U.S. dollars into Renminbi for our operations, appreciation of Renminbi against the U.S. dollar would reduce the Renminbi amount we receive from the conversion. Conversely, if we decide to convert Renminbi into U.S. dollars for the purpose of making payments for dividends on our ordinary shares or ADSs, servicing our outstanding debt, or for other business purposes, appreciation of the U.S. dollar against the Renminbi would reduce the U.S. dollar amounts available to us.
As of December 31, 2018, we had Renminbi-denominated cash and cash equivalents of RMB17.8 million (US$2.6 million). A 10% depreciation of Renminbi against the U.S. dollar based on the foreign exchange rate on December 31, 2018 would result in a decrease of US$0.2 million in cash and cash equivalents. A 10% appreciation of Renminbi against the U.S. dollar based on the foreign exchange rate on December 31, 2018 would result in an increase of US$0.3 million in cash and cash equivalents.
Item 12. Description of Securities Other Than Equity Securities
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
D. American Depositary Shares
Fees and Charges Our ADS Holders May Have to Pay
The Bank of New York Mellon, as depositary, will register and deliver American Depositary shares, also referred to as ADSs. Each ADS will represent two ordinary shares (or a right to receive two ordinary shares) deposited with The Hong Kong and Shanghai Banking Corporation Limited, as custodian for the depositary in Hong Kong. Each ADS will also represent any other securities, cash or other property which may be held by the depositary. The deposited shares together with any other securities, cash or other property held by the depositary are referred to as the deposited securities. The depositary’s office at which the ADSs will be administered is located at 240 Greenwich Street, New York, New York 10286.
|
Persons depositing or withdrawing shares or ADS |
|
For: |
|
$5.00 (or less) per 100 ADSs (or portion of 100 ADSs) |
|
Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property
Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
|
|
|
|
|
$0.05 (or less) per ADS |
|
Any cash distribution to ADS holders |
|
|
|
|
|
A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs |
|
Distribution of securities distributed to holders of deposited securities (including rights) that are distributed by the depositary to ADS holders |
|
|
|
|
|
$0.05 (or less) per ADS per calendar year |
|
Depositary services |
|
|
|
|
|
Registration or transfer fees |
|
Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares |
|
|
|
|
|
Expenses of the depositary |
|
Cable and facsimile transmissions (when expressly provided in the deposit agreement)
Converting foreign currency to U.S. dollars |
|
|
|
|
|
Taxes and other governmental charges the depositary or the custodian has to pay on any ADSs or shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes |
|
As necessary |
|
|
|
|
|
Any charges incurred by the depositary or its agents for servicing the deposited securities |
|
As necessary |
Fees and Other Payments Made by the Depositary to Us
The depositary has agreed to reimburse us annually for our expenses incurred in connection with investor relationship programs and any other program related to our ADS facility and the travel expense of our key personnel in connection with such programs. The depositary has also agreed to provide additional payments to us based on the applicable performance indicators relating to our ADS facility. There are limits on the amount of expenses for which the depositary will reimburse us, but the amount of reimbursement available to us is not necessarily tied to the amount of fees the depositary collects from investors. In 2018, we received approximately US$1.6 million (after tax) reimbursement from the depositary for our expenses incurred in connection with investor relationship programs related to the ADS facility and the travel expense of our key personnel in connection with such programs.
Item 13. Defaults, Dividend Arrearages and Delinquencies
None.
Item 14. Material Modifications to the Rights of Security Holders and Use of Proceeds
Material Modifications to the Rights of Security Holders
See “Item 10. Additional Information—B. Memorandum and Articles of Association—Ordinary Shares” for a description of the rights of securities holders, which remain unchanged.
Use of Proceeds
The following “Use of Proceeds” information relates to the registration statement on Form F-1, as amended (File Number: 333-226849) in relation to our initial public offering of 7,175,000 ADSs representing 14,350,000 of our Class A ordinary shares, and the underwriters’ partial exercise of their option to purchase from us 809,555 additional ADSs representing 1,619,110 Class A ordinary shares, at an initial offering price of $14.00 per ADS. J.P. Morgan Securities LLC, Citigroup Global Markets Inc. and China International Capital Corporation Hong Kong Securities Limited are the representatives of the underwriters.
As a result of our initial public offering, we raised an aggregate of approximately US$101.2 million in net proceeds, after deducting underwriting commissions and the offering expenses payable by us. For the period from September 12, 2018, the date that the F-1 Registration Statement was declared effective by the SEC, to the date of this annual report, we had not used the net proceeds from our initial public offering.
We still intend to use the proceeds from our initial public offering, as disclosed in our registration statements on Form F-1, for (i) general corporate purposes, including investment in product development, sales and marketing activities, technology infrastructure, capital expenditures, improvement of corporate facilities and other general and administrative matters, and (ii) acquisition of, or investment in, technologies, solutions or business that complement our business.
Item 15. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer and chief financial officer, has performed an evaluation of the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report, as required by Rule 13a-15(b) under the Exchange Act.
Based upon that evaluation, our management has concluded that, due to the material weakness described below, as of December 31, 2018, our disclosure controls and procedures were not effective in ensuring that the information required to be disclosed by us in the reports that we file and furnish under the Exchange Act was recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and that the information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to our management, as appropriate, to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
This annual report does not include a report of management’s assessment regarding internal control over financial reporting or an attestation report by our independent registered public accounting firm due to a transition period established by rules of the SEC for newly public companies.
Internal Control over Financial Reporting
Prior to our initial public offering in September 2018, we were a private company with limited accounting personnel and other resources with which to address our internal controls. Our independent registered public accounting firm has not conducted an audit of our internal control over financial reporting. In the course of auditing our consolidated financial statements as of and for the year ended December 31, 2017, we and our independent registered public accounting firm identified one material weakness and one significant deficiency in our internal control over financial reporting. As defined in the standards established by the U.S. Public Company Accounting Oversight Board, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis.
The material weakness that has been identified relates to our lack of sufficient competent financial reporting and accounting personnel with appropriate understanding of U.S. GAAP to design and implement formal period-end financial reporting controls and procedures, to address complex U.S. GAAP technical accounting issues, and to prepare and review our consolidated financial statements and related disclosures in accordance with U.S. GAAP and financial reporting requirements set forth by the SEC.
Following the identification of the material weakness, the significant deficiency, and other control deficiencies, we have taken measures and plan to continue to take measures to remedy these control deficiencies. We have hired additional qualified financial and accounting staff with working experience of U.S. GAAP and SEC reporting requirements, including our chief financial officer. In addition, we have established a comprehensive policy and procedure manual, to allow early detection, prevention and resolution of potential compliance issues. We intend to conduct regular and continuous U.S. GAAP accounting and financial reporting programs and to send our financial staff to attend external U.S. GAAP training courses. We are in the process of implementing new financial software to improve visibility of data, journal entries and closing and reporting process controls. Furthermore, we will continue to further expedite and streamline our reporting process and develop our compliance process. We also intend to hire additional resources to strengthen the financial reporting function and set up a financial and system control framework. However, we cannot assure you that all these measures will be sufficient to remediate material weaknesses in time, or at all. See “Item 3. Key Information—D. Risk Factors—Risks Related to Our Business and Industry—If we fail to implement and maintain an effective system of internal controls to remediate our material weakness over financial reporting, we may be unable to accurately report our results of operations, meet our reporting obligations or prevent fraud, and investor confidence and the market price of our ADSs may be materially and adversely affected.”
As a company with less than US$1.07 billion in revenue for our last fiscal year, we qualify as an “emerging growth company” pursuant to the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other requirements that are otherwise applicable generally to public companies. These provisions include exemption from the auditor attestation requirement under Section 404 of the Sarbanes-Oxley Act of 2002 in the assessment of the emerging growth company’s internal control over financial reporting.
Changes in Internal Control over Financial Reporting
Other than as described above, there were no changes in our internal controls over financial reporting that occurred during the period covered by this annual report on Form 20-F that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 16A. Audit Committee Financial Expert
Our board of directors has determined that Mr. Nee Chuan Teo, an independent director (under the standards set forth in Nasdaq Stock Market Rule 5605(c)(2) and Rule 10A-3 under the Exchange Act) and chairman of our audit committee, is an audit committee financial expert.
Our board of directors adopted a code of business conduct and ethics that applies to our directors, officers, employees and advisors in August 2018. We have posted a copy of our code of business conduct and ethics on our website at http://ir.111.com.cn.
Item 16C. Principal Accountant Fees and Services
The following table sets forth the aggregate fees by categories specified below in connection with certain professional services rendered by Deloitte Touche Tohmatsu Certified Public Accountants LLP, our principal external auditors, for the periods indicated.
|
|
|
For the year ended December 31, |
| |||
|
|
|
2017 |
|
2018 |
| |
|
|
|
(in thousands) |
| |||
|
Audit fees and audit-related fees (1) |
|
— |
|
US$ |
1,671 |
|
(1) “Audit fees and audit-related fees” means the aggregate fees billed in each of the fiscal years listed for professional services rendered by our principal auditors for the audit or review of our annual or quarterly financial statements, fees for assurance services rendered in connection with our IPO in 2018.
The policy of our audit committee or our board of directors is to pre-approve all audit and non-audit services provided by Deloitte Touche Tohmatsu Certified Public Accountants LLP, including audit services, tax services and other services as described above.
Item 16D. Exemptions from the Listing Standards for Audit Committees
Not applicable.
Item 16E. Purchases of Equity Securities by the Issuer and Affiliated Purchasers
None.
Item 16F. Change in Registrant’s Certifying Accountant
Not applicable.
Item 16G. Corporate Governance
As a Cayman Islands exempted company listed on NASDAQ, we are subject to the Nasdaq corporate governance listing standards. However, NASDAQ rules permit a foreign private issuer like us to follow the corporate governance practices of its home country. Certain corporate governance practices in the Cayman Islands, which is our home country, may differ significantly from the Nasdaq corporate governance listing standards. Currently, we do not plan to rely on home country exemption for corporate governance matters. However, if we choose to follow home country practice in the future, our shareholders may be afforded less protection than they otherwise would under the Nasdaq corporate governance listing standards applicable to U.S. domestic issuers. See “Item 3. Key Information—D. Risk Factors— Risks Related to the American Depositary Shares—We are a foreign private issuer within the meaning of the rules under the Exchange Act, and as such we are exempt from certain provisions applicable to U.S. domestic public companies.”
Item 16H. Mine Safety Disclosure
Not applicable.
We have elected to provide financial statements pursuant to Item 18.
The consolidated financial statements of 111, Inc., its subsidiaries and its consolidated affiliated entities are included at the end of this annual report.
|
Exhibit |
|
Description of Document |
|
1.1 |
|
|
|
|
|
|
|
2.1 |
|
|
|
|
|
|
|
2.2 |
|
|
|
|
|
|
|
2.3 |
|
|
Exhibit |
|
Description of Document |
|
4.1 |
|
|
|
|
|
|
|
4.2 |
|
|
|
|
|
|
|
4.3 |
|
|
|
|
|
|
|
4.4 |
|
|
|
|
|
|
|
4.5 |
|
|
|
|
|
|
|
4.6 |
|
|
|
|
|
|
|
4.7 |
|
|
|
|
|
|
|
4.8 |
|
|
|
|
|
|
|
4.9 |
|
|
|
|
|
|
|
4.10 |
|
|
|
|
|
|
|
4.11 |
|
|
|
|
|
|
|
4.12 |
|
|
|
|
|
|
|
4.13 |
|
|
Exhibit |
|
Description of Document |
|
4.14 |
|
|
|
|
|
|
|
4.15 |
|
|
|
|
|
|
|
4.16 |
|
|
|
|
|
|
|
4.17 |
|
|
|
|
|
|
|
4.18 |
|
|
|
|
|
|
|
4.19 |
|
|
|
|
|
|
|
4.20 |
|
|
|
|
|
|
|
4.21 |
|
|
|
|
|
|
|
4.22 |
|
|
|
|
|
|
|
8.1* |
|
List of Principal Subsidiaries and Consolidated Affiliated Entities |
|
|
|
|
|
11.1 |
|
|
|
|
|
|
|
12.1* |
|
|
Exhibit |
|
Description of Document |
|
12.2* |
|
|
|
|
|
|
|
13.1** |
|
|
|
|
|
|
|
13.2** |
|
|
|
|
|
|
|
15.1* |
|
|
|
|
|
|
|
15.2* |
|
|
|
|
|
|
|
15.3* |
|
Consent of Deloitte Touche Tohmatsu Certified Public Accountants LLP |
|
|
|
|
|
101.INS* |
|
XBRL Instance Document |
|
|
|
|
|
101.SCH* |
|
XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
101.CAL* |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
101.DEF* |
|
XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
|
|
101.LAB* |
|
XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
101.PRE* |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
* Filed herewith
** Furnished herewith
The registrant hereby certifies that it meets all of the requirements for filing its annual report on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
|
|
111, Inc. | |
|
|
| |
|
|
| |
|
|
By: |
/s/ Junling Liu |
|
|
Name: Junling Liu | |
|
|
Title: Chief Executive Officer and Co-Chairman of the Board | |
|
|
| |
|
Date: April 10, 2019 |
| |
111, INC
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
TO SHAREHOLDERS AND THE BOARD OF DIRECTORS OF
111, INC.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of 111, Inc. (the “Company”), its subsidiaries, variable interest entities (“VIEs”) and VIE’s subsidiaries (collectively referred to as the “Group”) as of December 31, 2018 and 2017, and the related consolidated statements of comprehensive loss, shareholders’ (deficit) equity, and cash flows for each of the three years in the period ended December 31, 2018 and the related notes and financial statement schedule included as Schedule I (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Group as of December 31, 2018 and 2017, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2018, in conformity with accounting principles generally accepted in the United States of America.
Convenience Translation
Our audits also comprehended the translation of Renminbi amounts into United States dollar amounts and, in our opinion, such translation has been made in conformity with the basis stated in Note 2(ad). Such United States dollar amounts are presented solely for the convenience of readers in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Group’s management. Our responsibility is to express an opinion on the Group’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Group is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Group’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/Deloitte Touche Tohmatsu Certified Public Accountants LLP
Shanghai, China
April 9, 2019
We have served as the Group’s auditor since 2018.
111, INC.
(Amounts in thousands, except for share and per share data)
|
|
|
|
|
As of December 31, |
| ||||
|
|
|
Note |
|
2017 |
|
2018 |
|
2018 |
|
|
|
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
ASSETS |
|
|
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
|
|
167,660 |
|
853,740 |
|
124,171 |
|
|
Short-term investments |
|
3 |
|
293,533 |
|
252,805 |
|
36,769 |
|
|
Accounts receivable, net of allowance of doubtful accounts of nil as of December 31, 2017 and 2018 |
|
|
|
20,398 |
|
28,569 |
|
4,155 |
|
|
Inventories |
|
4 |
|
144,056 |
|
210,836 |
|
30,665 |
|
|
Prepayments and other current assets |
|
5 |
|
104,818 |
|
161,147 |
|
23,438 |
|
|
Total current assets |
|
|
|
730,465 |
|
1,507,097 |
|
219,198 |
|
|
Property and equipment |
|
6 |
|
17,028 |
|
20,302 |
|
2,953 |
|
|
Intangible assets |
|
7 |
|
4,751 |
|
4,503 |
|
655 |
|
|
Long-term investments |
|
8 |
|
11,140 |
|
11,140 |
|
1,620 |
|
|
Other Non-current Asset |
|
9 |
|
— |
|
3,376 |
|
491 |
|
|
Total assets |
|
|
|
763,384 |
|
1,546,418 |
|
224,917 |
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND EQUITY |
|
|
|
|
|
|
|
|
|
|
Current liabilities including amounts of the consolidated VIE without recourse to the Company (Note 2(b)): |
|
|
|
|
|
|
|
|
|
|
Accounts payable |
|
|
|
128,140 |
|
212,258 |
|
30,872 |
|
|
Accrued expenses and other current liabilities |
|
10 |
|
73,018 |
|
102,261 |
|
14,873 |
|
|
Total current liabilities |
|
|
|
201,158 |
|
314,519 |
|
45,745 |
|
|
Other Non-current liabilities |
|
9 |
|
— |
|
8,135 |
|
1,183 |
|
|
Total liabilities |
|
|
|
201,158 |
|
322,654 |
|
46,928 |
|
|
Commitments and contingencies (Note 20) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
MEZZANINE EQUITY |
|
|
|
|
|
|
|
|
|
|
Series A convertible preferred shares, $0.00005 par value; 4,200,000 and nil shares authorized, issued, and outstanding as of December 31, 2017 and 2018, respectively |
|
11 |
|
12,922 |
|
— |
|
— |
|
|
Series B convertible preferred shares, $0.00005 par value; 11,396,178 and nil shares authorized, issued, and outstanding as of December 31, 2017 and 2018, respectively |
|
11 |
|
57,980 |
|
— |
|
— |
|
|
Series C convertible preferred shares, $0.00005 par value; 31,739,234 and nil shares authorized, issued, and outstanding as of December 31, 2017 and 2018, respectively |
|
11 |
|
450,324 |
|
— |
|
— |
|
|
Series D convertible preferred shares, $0.00005 par value; 27,783,584 and nil shares authorized, issued, and outstanding as of December 31, 2017 and 2018, respectively |
|
11 |
|
1,263,523 |
|
— |
|
— |
|
|
Subscription receivable of Series D convertible preferred shares |
|
11 |
|
(277,819 |
) |
— |
|
— |
|
|
Total Mezzanine equity |
|
|
|
1,506,930 |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
SHAREHOLDERS’ (DEFICIT) EQUITY |
|
|
|
|
|
|
|
|
|
|
Ordinary shares Class A ($0.00005 par value per share; 72,000,000 and 800,000,000 shares authorized, 72,000,000 and 91,088,106 shares issued and outstanding as of December 31, 2017 and 2018, respectively) |
|
12 |
|
25 |
|
29 |
|
4 |
|
|
Ordinary shares Class B ($0.00005 par value per share; 839,209,895 and 72,000,000 shares authorized, nil and 72,000,000 issued and outstanding as of December 31, 2017 and 2018, respectively) |
|
12 |
|
— |
|
25 |
|
4 |
|
|
Ordinary shares Class C ($0.00005 par value per share; 13,671,109 and nil shares authorized, 1,607,901 and nil shares issued, nil shares outstanding as of December 31, 2017 and 2018, respectively) |
|
12 |
|
0 |
|
— |
|
— |
|
|
Subscription receivable |
|
|
|
(2,200 |
) |
— |
|
— |
|
|
Additional paid-in capital |
|
|
|
12,121 |
|
2,540,878 |
|
369,555 |
|
|
Accumulated deficit |
|
|
|
(1,003,638 |
) |
(1,383,729 |
) |
(201,255 |
) |
|
Accumulated other comprehensive income |
|
|
|
47,550 |
|
67,073 |
|
9,755 |
|
|
Total shareholders’ (deficit) equity |
|
|
|
(946,142 |
) |
1,224,276 |
|
178,063 |
|
|
Non-controlling interest |
|
|
|
1,438 |
|
(512 |
) |
(74 |
) |
|
Total (deficit) equity |
|
|
|
(944,704 |
) |
1,223,764 |
|
177,989 |
|
|
Total liabilities, mezzanine equity and (deficit) equity |
|
|
|
763,384 |
|
1,546,418 |
|
224,917 |
|
The accompanying notes are an integral part of these consolidated financial statements.
111, INC.
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(Amounts in thousands, except for share and per share data)
|
|
|
|
|
Years Ended December 31, |
| ||||||
|
|
|
Note |
|
2016 |
|
2017 |
|
2018 |
|
2018 |
|
|
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
Net revenues |
|
13 |
|
873,837 |
|
959,486 |
|
1,785,970 |
|
259,759 |
|
|
Operating costs and expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Cost of products sold |
|
|
|
(796,230 |
) |
(868,719 |
) |
(1,681,700 |
) |
(244,592 |
) |
|
Fulfillment expenses |
|
|
|
(68,445 |
) |
(55,880 |
) |
(73,930 |
) |
(10,753 |
) |
|
Selling and marketing expenses |
|
|
|
(252,829 |
) |
(190,074 |
) |
(260,040 |
) |
(37,822 |
) |
|
General and administrative expenses |
|
|
|
(60,836 |
) |
(53,434 |
) |
(98,759 |
) |
(14,364 |
) |
|
Technology expenses |
|
|
|
(61,767 |
) |
(48,133 |
) |
(71,248 |
) |
(10,363 |
) |
|
Other operating income (expenses), net |
|
|
|
1,990 |
|
2,732 |
|
(668 |
) |
(97 |
) |
|
Total operating costs and expenses |
|
|
|
(1,238,117 |
) |
(1,213,508 |
) |
(2,186,345 |
) |
(317,991 |
) |
|
Loss from operations |
|
|
|
(364,280 |
) |
(254,022 |
) |
(400,375 |
) |
(58,232 |
) |
|
Interest income |
|
|
|
2,308 |
|
4,013 |
|
4,352 |
|
633 |
|
|
Interest expense |
|
|
|
(751 |
) |
(55 |
) |
— |
|
— |
|
|
Foreign exchange gain (loss) |
|
|
|
2,630 |
|
(3,492 |
) |
2,459 |
|
358 |
|
|
Other (loss) income, net |
|
|
|
(3,353 |
) |
4,229 |
|
11,531 |
|
1,676 |
|
|
Loss before income taxes |
|
|
|
(363,446 |
) |
(249,327 |
) |
(382,033 |
) |
(55,565 |
) |
|
Income tax expense |
|
16 |
|
— |
|
— |
|
(8 |
) |
(1 |
) |
|
Net loss |
|
|
|
(363,446 |
) |
(249,327 |
) |
(382,041 |
) |
(55,566 |
) |
|
Net loss attributable to non-controlling interest |
|
|
|
765 |
|
747 |
|
1,950 |
|
285 |
|
|
Deemed dividend on Series D convertible preferred shares |
|
|
|
(55,281 |
) |
— |
|
— |
|
— |
|
|
Net loss attributable to ordinary shareholders |
|
|
|
(417,962 |
) |
(248,580 |
) |
(380,091 |
) |
(55,281 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income (loss) |
|
|
|
|
|
|
|
|
|
|
|
|
Unrealized gains of available-for-sales securities, net of tax of nil for 2016, 2017 and 2018 |
|
|
|
1,415 |
|
5,181 |
|
8,734 |
|
1,270 |
|
|
Realized gains of available-for-sale securities, net of tax |
|
|
|
— |
|
(1,154 |
) |
(10,869 |
) |
(1,581 |
) |
|
Foreign currency translation adjustments |
|
|
|
39,832 |
|
(21,347 |
) |
21,658 |
|
3,150 |
|
|
Comprehensive loss |
|
|
|
(376,715 |
) |
(265,900 |
) |
(360,568 |
) |
(52,442 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss per share: |
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
15 |
|
(5.81 |
) |
(3.45 |
) |
(3.82 |
) |
(0.56 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average number of shares used in computation of loss per share: |
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
15 |
|
72,000,000 |
|
72,000,000 |
|
99,451,210 |
|
99,451,210 |
|
The accompanying notes are an integral part of these consolidated financial statements.
111, INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ (DEFICIT) EQUITY
(Amounts in thousands, except for share data)
|
|
|
Ordinary Shares |
|
Ordinary Shares |
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
| ||||
|
|
|
Class A |
|
Class B |
|
Additional Paid- |
|
Subscription |
|
Accumulated |
|
Comprehensive |
|
Non-controlling |
|
Total (Deficit) |
| ||||
|
|
|
Share |
|
Amount |
|
Share |
|
Amount |
|
in Capital |
|
receivables |
|
deficit |
|
Income (Loss) |
|
Interest |
|
Equity |
|
|
|
|
|
|
RMB |
|
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
RMB |
|
RMB |
|
RMB |
|
|
Balance at January 1, 2016 |
|
72,000,000 |
|
25 |
|
— |
|
— |
|
7,803 |
|
(2,225 |
) |
(346,137 |
) |
23,623 |
|
2,950 |
|
(313,961 |
) |
|
Share-based compensation |
|
— |
|
— |
|
— |
|
— |
|
3,438 |
|
— |
|
— |
|
— |
|
— |
|
3,438 |
|
|
Net loss |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(362,681 |
) |
— |
|
(765 |
) |
(363,446 |
) |
|
Deemed dividend on Series D convertible preferred shares |
|
— |
|
— |
|
— |
|
— |
|
(9,041 |
) |
— |
|
(46,240 |
) |
— |
|
— |
|
(55,281 |
) |
|
Unrealized gains of available-for-sales securities, net of tax |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
1,415 |
|
— |
|
1,415 |
|
|
Foreign currency translation |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
39,832 |
|
— |
|
39,832 |
|
|
Balance at January 1, 2017 |
|
72,000,000 |
|
25 |
|
— |
|
— |
|
2,200 |
|
(2,225 |
) |
(755,058 |
) |
64,870 |
|
2,185 |
|
(688,003 |
) |
|
Receipts of subscription receivables from shareholders |
|
— |
|
— |
|
— |
|
— |
|
— |
|
25 |
|
— |
|
— |
|
— |
|
25 |
|
|
Share-based compensation |
|
— |
|
— |
|
— |
|
— |
|
9,921 |
|
— |
|
— |
|
— |
|
— |
|
9,921 |
|
|
Net loss |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(248,580 |
) |
— |
|
(747 |
) |
(249,327 |
) |
|
Unrealized gains of available-for-sales securities, net of tax |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
5,181 |
|
— |
|
5,181 |
|
|
Reclassification of realized gains, net of tax |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(1,154 |
) |
— |
|
(1,154 |
) |
|
Foreign currency translation |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(21,347 |
) |
— |
|
(21,347 |
) |
|
Balance at December 31, 2017 |
|
72,000,000 |
|
25 |
|
— |
|
— |
|
12,121 |
|
(2,200 |
) |
(1,003,638 |
) |
47,550 |
|
1,438 |
|
(944,704 |
) |
|
Share surrendered for cancellation (Note 12) |
|
— |
|
— |
|
— |
|
— |
|
(2,200 |
) |
2,200 |
|
— |
|
— |
|
— |
|
— |
|
|
Share-based compensation |
|
— |
|
— |
|
— |
|
— |
|
51,359 |
|
— |
|
— |
|
— |
|
— |
|
51,359 |
|
|
Re-designation of Class A ordinary shares into Class B ordinary shares (Note 12) |
|
(72,000,000 |
) |
(25 |
) |
72,000,000 |
|
25 |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
|
Issuance of ordinary shares upon initial public offering (“IPO”), net of issuance costs of RMB19,134 |
|
15,969,110 |
|
5 |
|
— |
|
— |
|
694,873 |
|
— |
|
— |
|
— |
|
— |
|
694,878 |
|
|
Conversion of preferred shares into Class A ordinary shares upon IPO |
|
75,118,996 |
|
24 |
|
— |
|
— |
|
1,784,725 |
|
— |
|
— |
|
— |
|
— |
|
1,784,749 |
|
|
Net loss |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(380,091 |
) |
— |
|
(1,950 |
) |
(382,041 |
) |
|
Unrealized gains of available-for-sales securities, net of tax |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
8,734 |
|
— |
|
8,734 |
|
|
Reclassification of realized gains, net of tax |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
(10,869 |
) |
— |
|
(10,869 |
) |
|
Foreign currency translation |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
21,658 |
|
— |
|
21,658 |
|
|
Balance at December 31, 2018 |
|
91,088,106 |
|
29 |
|
72,000,000 |
|
25 |
|
2,540,878 |
|
— |
|
(1,383,729 |
) |
67,073 |
|
(512 |
) |
1,223,764 |
|
The accompanying notes are an integral part of these consolidated financial statements.
111, INC
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts in thousands)
|
|
|
|
|
Years Ended December 31, |
| ||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
2018 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating activities: |
|
|
|
|
|
|
|
|
|
|
Net loss |
|
(363,446 |
) |
(249,327 |
) |
(382,041 |
) |
(55,566 |
) |
|
Adjustments to reconcile net income to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
Share-based compensation |
|
3,438 |
|
9,921 |
|
51,359 |
|
7,470 |
|
|
Depreciation and amortization |
|
12,060 |
|
14,820 |
|
11,266 |
|
1,639 |
|
|
Loss (gain) on disposal of property and equipment |
|
22 |
|
(10 |
) |
1,110 |
|
161 |
|
|
Investment Income |
|
— |
|
— |
|
(10,869 |
) |
(1,581 |
) |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
Accounts receivable |
|
1,977 |
|
7,990 |
|
(8,171 |
) |
(1,188 |
) |
|
Inventories |
|
(22,028 |
) |
(9,322 |
) |
(66,780 |
) |
(9,713 |
) |
|
Prepayments and other current assets |
|
(35,555 |
) |
(7,459 |
) |
(56,329 |
) |
(8,193 |
) |
|
Other non-current assets |
|
— |
|
— |
|
(3,376 |
) |
(491 |
) |
|
Accounts payable |
|
8,935 |
|
30,157 |
|
84,118 |
|
12,234 |
|
|
Accrued expenses and other current liabilities |
|
5,951 |
|
(1,142 |
) |
28,560 |
|
4,154 |
|
|
Other non-current liabilities |
|
— |
|
— |
|
8,135 |
|
1,183 |
|
|
Net cash used in operating activities |
|
(388,646 |
) |
(204,372 |
) |
(343,018 |
) |
(49,891 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Investing activities: |
|
|
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
(5,346 |
) |
(6,798 |
) |
(14,443 |
) |
(2,101 |
) |
|
Purchases of intangible assets |
|
(1,579 |
) |
(62 |
) |
(376 |
) |
(55 |
) |
|
Purchase of long-term investments |
|
— |
|
(140 |
) |
— |
|
— |
|
|
Purchase of short-term investments |
|
(267,576 |
) |
(109,380 |
) |
(519,187 |
) |
(75,513 |
) |
|
Proceeds from sale or maturity of short-term investments |
|
6,670 |
|
80,198 |
|
578,359 |
|
84,120 |
|
|
Proceeds from disposition of property and equipment, net |
|
277 |
|
57 |
|
101 |
|
15 |
|
|
Net cash provided by (used in) investing activities |
|
(267,554 |
) |
(36,125 |
) |
44,454 |
|
6,466 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Financing activities: |
|
|
|
|
|
|
|
|
|
|
Proceeds from ordinary shareholders |
|
1 |
|
25 |
|
— |
|
— |
|
|
Proceeds from IPO, net of issuance cost |
|
— |
|
— |
|
694,878 |
|
101,066 |
|
|
Proceeds from preferred shareholders |
|
195,918 |
|
49,475 |
|
277,819 |
|
40,407 |
|
|
Debt repayments |
|
(47,500 |
) |
— |
|
— |
|
— |
|
|
Net cash provided by financing activities |
|
148,419 |
|
49,500 |
|
972,697 |
|
141,473 |
|
|
Effect of exchange rate changes on cash and cash equivalents |
|
35,330 |
|
(14,848 |
) |
11,947 |
|
1,738 |
|
|
Net (decrease) increase in cash and cash equivalents |
|
(472,451 |
) |
(205,845 |
) |
686,080 |
|
99,786 |
|
|
Cash and cash equivalents at the beginning of the year |
|
845,956 |
|
373,505 |
|
167,660 |
|
24,385 |
|
|
Cash and cash equivalents at the end of the year |
|
373,505 |
|
167,660 |
|
853,740 |
|
124,171 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental disclosure of cash flow information: |
|
|
|
|
|
|
|
|
|
|
Interest paid |
|
751 |
|
— |
|
— |
|
— |
|
|
Income tax paid |
|
— |
|
— |
|
8 |
|
1 |
|
|
Supplemental disclosures of non-cash investing and financing activities: |
|
|
|
|
|
|
|
|
|
|
Change in fair value of available-for-sale investments |
|
1,415 |
|
5,181 |
|
8,734 |
|
1,270 |
|
|
Purchases of property and equipment included in payables |
|
174 |
|
164 |
|
848 |
|
123 |
|
The accompanying notes are an integral part of these consolidated financial statements.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
1. ORGANIZATION AND PRINCIPAL ACTIVITIES
111, Inc. (the “Company”), was incorporated under the laws of the Cayman Islands in May 2013. The Company, through its subsidiaries, variable interest entities (“VIEs”) and VIE’s subsidiaries (collectively, the “Group”), operates an integrated online and offline platform in the healthcare ecosystem in China, whereby the Group is principally engaged in the sales of medical and wellness products through online retail and wholesale pharmacies and offline retail pharmacies, as well as provision of certain value-added services, such as online consultation services and e-prescription services to consumers in the People’s Republic of China (the “PRC”).
The Group started to offer services in October 2012 through Guangdong Yihao Pharmacy Co., Ltd (“Yihao Pharmacy”), a consolidated VIE incorporated in the PRC and its subsidiaries which were acquired and controlled by the nominees of Dr. Gang Yu and Mr. Junling Liu (collectively, the “Founders”) with each holding a 50% equity interest.
In May 2013, the Company was incorporated by the Founders through their immediate family members, each maintaining identical ownership interests in Yihao Pharmacy. In September 2013, the Company, through its wholly owned subsidiary in PRC, entered into a series of contractual arrangements with Yihao Pharmacy and its nominee shareholders (see Note 2(b)) for a description of the VIE arrangements pursuant to which the Company and its subsidiary were established as the primary beneficiary of Yihao Pharmacy). As a result of these transactions entered into to accomplish the reorganization, there was no change in the economic ownership of the shareholders given Yihao Pharmacy and the Company had the same beneficial shareholders and identical interests prior to and after the reorganization, and as such, the reorganization lacked economic substance. Therefore, the Company accounted for these transactions akin to a reorganization of entities under common control. The reorganization was necessary to comply with the PRC law and regulations which restrict foreign ownership of companies engaged in providing internet content distribution services. In June 2016, the shareholding rights of the Company were transferred from the immediate family members to the Founders.
|
Name of subsidiaries |
|
Date of establishment |
|
Place of |
|
Percentage of |
|
Principal activities |
|
Yao Wang Corporation Limited (“Yao Wang”) |
|
June 4, 2013 |
|
Hong Kong |
|
100% |
|
Investment holding |
|
Yaofang Information Technology (Shanghai) Co., Ltd (“Yaofang” or “WFOE”) |
|
August 12, 2013 |
|
Shanghai |
|
100% |
|
Warehousing, logistics, research and development, and consulting |
|
Guangdong Yihao Pharmacy Co., Ltd. (“Yihao Pharmacy”) |
|
March 7, 2003 |
|
Guangdong |
|
VIE |
|
Warehousing, logistics and procurement |
|
Guangdong Yihao Pharmaceutical Chain Co., Ltd. (“Yihao Pharmaceutical Chain”) |
|
November 1, 2001 |
|
Guangdong |
|
VIE |
|
Retail |
|
Shanghai Yaowang E-commerce Co., Ltd. (“Shanghai Yaowang”) |
|
January 15, 2013 |
|
Shanghai |
|
VIE |
|
Electronic Commerce |
|
Chengdu Yihao Pharmacy Co., Ltd. (“Chengdu Yihao Pharmacy”) |
|
August 22, 2017 |
|
Chengdu |
|
VIE’s subsidiary |
|
Retail |
|
Anshun Southwest Internet Hospital Co., Ltd (“Southwest Internet Hospital” ) |
|
July 5, 2016 |
|
Anshun |
|
VIE’s subsidiary |
|
Internet hospital business |
|
Anshun Joint Diagnosis And Treatment Technology Co., Ltd (“Anshun Technology” ) |
|
February 8, 2017 |
|
Anshun |
|
VIE’s subsidiary |
|
Internet hospital business |
|
Wuhan Central China Drug Trading Co., Ltd. (“Wuhan Huazhong”) |
|
August 5, 2015 |
|
Wuhan |
|
70% |
|
Software development and information technology support |
|
Chongqing Yihao Pharmacy Co., Ltd.(“Chongqing Yihao Pharmacy”) |
|
May 18, 2018 |
|
Chongqing |
|
WFOE’s subsidiary |
|
Warehousing and logistics |
|
Tianjin Yihao Pharmacy Co., Ltd. (“Tianjin Yihao Pharmacy”) |
|
June 20, 2018 |
|
Tianjin |
|
VIE’s subsidiary |
|
Warehousing and logistics |
|
Kunshan Yifang Pharmacy Co., Ltd. (“Kunshan Yifang Pharmacy”) |
|
July 30, 2018 |
|
Kunshan |
|
VIE’s subsidiary |
|
Warehousing and logistics |
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES
(a) Basis of presentation
The consolidated financial statements of the Group have been prepared in accordance with the accounting principles generally accepted in the United States of America (“US GAAP”).
(b) Basis of consolidation
The consolidated financial statements include the financial statements of the Company, its subsidiaries, VIEs and VIE’s subsidiaries for which the Company is the primary beneficiary. All intercompany transactions, balances and unrealized profit and losses have been eliminated upon consolidation.
The Group evaluates the need to consolidate certain variable interest entities in which equity investors do not have the characteristics of a controlling financial interest or do not have sufficient equity at risk for the entity to finance its activities without additional subordinated financial support or the entity is structured with disproportionate voting rights, and substantially all of the activities are conducted on behalf of an investor with disproportionately few voting rights.
The Group is deemed as the primary beneficiary of and consolidates variable interest entities when the Group has the power to direct the activities that most significantly impact the economic success of the entities and effectively assumes the obligation to absorb losses or has the rights to receive benefits that are potentially significant to the entities.
As a foreign-invested company engaged in Internet-based businesses, the Group is subject to significant restrictions under current PRC laws and regulations, specifically the Company and its PRC subsidiary, Yao Fang, as a wholly foreign owned enterprise (“WFOE”), are both restricted from holding the licenses that are necessary for the online operation in China. To comply with these restrictions, the Company conducts the online operations principally through Yihao Pharmacy. Yihao Pharmacy holds the licenses necessary to conduct the internet-related operations of 1 Drugstore and 1 Drug Mall in China.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(b) Basis of consolidation (Continued)
Since the Company does not have any equity interests in Yihao Pharmacy, in order to exercise effective control over its operations, the Company, through its wholly owned subsidiary, the WFOE, entered into a series of contractual arrangements with Yihao Pharmacy and its shareholders, pursuant to which the Company is entitled to receive effectively all economic benefits generated from Yihao Pharmacy shareholders’ equity interests in it. Details of the key agreements entered into between the WFOE, Yihao Pharmacy and each of its two individual shareholders nominated by the Founders (“Nominees”) in September 2013 are as follows:
The agreements that provide the Company effective control over the VIE include:
Exclusive Option Agreement: Under the exclusive option agreement, the Nominees granted an irrevocable assets and equity option to WFOE, that entitles WFOE or its designated entity or individual to acquire all or a portion of the assets owned by Yihao Pharmacy and its subsidiaries and all the equity interests held by nominees in Yihao Pharmacy and its subsidiaries at its sole discretion, at zero price or the lowest price permitted under PRC laws then in effect. The option may be exercised by WFOE or its designee. The exclusive option agreement remains effective for the same period as the exclusive support service agreement.
Proxy Agreement: Under the shareholder voting right proxy agreement, the Nominees irrevocably grant any person designated by WFOE the power to exercise all voting rights. This Agreement may not be terminated without the consent of WFOE, which may unilaterally terminate the agreement, by giving a thirty (30) day prior written notice to the Nominees. The proxy agreement remains in force for the same period as the exclusive support services agreement.
The agreements that transfer economic benefits to the Company include:
Equity Pledge Agreement: Under the equity pledge agreement, all of the equity interest in Yihao Pharmacy were pledged to WFOE to guarantee the performance of the obligations of Yihao Pharmacy and Nominees under the exclusive support services agreement, the proxy agreement, the exclusive option agreement, and repayment of all accounts payable to WFOE from time to time. If the Nominees or Yihao Pharmacy breach their respective contractual obligations, WFOE, as pledgee, will be entitled to certain rights, including the right to dispose the pledged equity interests. Pursuant to the equity pledge agreement, the Nominees shall not transfer, assign or otherwise create any new encumbrance on their respective equity interest in Yihao Pharmacy without the prior written consent of WFOE. The equity pledge right enjoyed by WFOE will expire when the Nominees and Yihao Pharmacy have fully performed their respective contractual obligations, including but not limited to pay services fees to the WFOE under the exclusive support services agreement, authorize the WFOE to act as its attorney-in fact to exercise shareholders’ rights of Nominees under the proxy agreements, grant exclusive option to the WFOE or any third party designated by WFOE to purchase all or part of their respective equity interests at the lowest price permitted by law under the exclusive option agreements, and repay all accounts payable to WFOE.
Exclusive Support Service Agreement: Pursuant to the exclusive support service agreement, WFOE provides Yihao Pharmacy with a series of technical support services and is entitled to receive related fees. This agreement shall be in full force and effective until Yihao Pharmacy’s valid operation term as stated on business license expires. During the term of this agreement, WFOE shall be the exclusive provider of the services. Yihao Pharmacy shall not seek or accept similar services from other providers without the prior written approval of WFOE. The agreement will remain effective for ten years and will be automatically extended for another ten years thereafter, unless WFOE terminates the agreement or it is terminated in advance pursuant to other provisions of the agreement such as bankruptcy of one party or one party’s failure to perform its obligation for more than six consecutive months due to a force majeure event.
Similar contractual agreements were also entered into by WFOE, Yihao Pharmaceutical Chain and Yao Wang, and their respective shareholders in September 2013.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(b) Basis of consolidation (Continued)
US GAAP provides guidance on the identification of VIE and financial reporting for entities over which control is achieved through means other than voting interests. The Group evaluates each of its interests in an entity to determine whether or not the investee is a VIE and, if so, whether the Group is the primary beneficiary of such VIE. In determining whether the Group is the primary beneficiary, the Group considers if the Group (1) has power to direct the activities that most significantly affect the economic performance of the VIE, and (2) receives the economic benefits of the VIE that could be significant to the VIE. If deemed the primary beneficiary, the Group consolidates the VIE.
The irrevocable power of attorney has conveyed all shareholder rights held by the VIEs’ shareholders to WFOE, including the right to appoint board members who nominate the general managers of the VIEs to conduct day-to-day management of the VIEs’ businesses, and to approve significant transactions of the VIEs. In addition, the exclusive option agreements provide WFOE with a substantive kick-out right of the VIEs shareholders through an exclusive option to purchase all or any part of the shareholders’ equity interest in the VIEs at zero price or the lowest price permitted under PRC laws then in effect. In addition, through the exclusive support services agreements, the Company established the right to receive benefits from the VIEs that could potentially be significant to the VIEs, and through the equity pledge agreement, the Company has, in substance, an obligation to absorb losses of the VIEs that could potentially be significant to the VIEs.
Risks in relation to the VIE structure
The Group believes that the VIE arrangements are in compliance with PRC law and are legally enforceable. However, there are certain risks related to the VIE arrangements, which include but are not limited to the following:
· If the Group’s ownership structure is found to be in violation of any existing or future PRC laws or regulations, the relevant governmental authorities, including the China Securities Regulatory Commission, would have broad discretion in dealing with such violation, including levying fines, confiscating its income or the income of the WFOE, Yao Fang, revoking the business licenses or operating licenses of the WFOE, shutting down the Group’s servers or blocking the Group’s websites, discontinuing or placing restrictions or onerous conditions on the Group’s operations, requiring the Group to undergo a costly and disruptive restructuring, restricting or prohibiting the Group’s use of various funding to finance its business and operations in China, and taking other regulatory or enforcement actions that could be harmful to the Group’s business;
· The Group relies on contractual arrangements with the VIEs and their equity holders for a majority of its PRC operations, which may not be as effective as direct ownership in providing operational control;
· The Group may have to incur significant cost to enforce, or may not be able to effectively enforce, the contractual arrangements with the VIEs and their equity holders in the event of a breach or non-compliance by the VIEs or their equity holders;
· Under the contractual arrangements with the VIEs and their shareholders, (a) the Company may replace any such individual as a shareholder of the VIEs at the Company’s discretion, and (b) each of two individuals has executed a power of attorney to appoint the WFOE or its designated third party to vote on their behalf and exercise shareholder rights of the VIE. However, the Company cannot assure that these individuals will act in the best interests of the Company should any conflicts of interest arise, or that any conflicts of interest will be resolved in the Company’s favor. These individuals may breach or cause the VIE to breach the existing contractual arrangements. If the Company cannot resolve any conflicts of interest or disputes between the Company and any of these individuals, the Company would have to rely on legal proceedings, which may be expensive, time-consuming and disruptive to its operations. There is also substantial uncertainty as to the outcome of any such legal proceedings.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(b) Basis of consolidation (Continued)
The following amounts and balances of the VIEs were included in the Group’s consolidated financial statements after the elimination of intercompany balances and transactions:
|
|
|
As of December 31, |
| ||
|
|
|
2017 |
|
2018 |
|
|
Current Assets: |
|
|
|
|
|
|
Cash and cash equivalents |
|
20,414 |
|
8,527 |
|
|
Short-term investments |
|
26,000 |
|
— |
|
|
Accounts receivable |
|
20,398 |
|
28,351 |
|
|
Inventories |
|
143,564 |
|
210,285 |
|
|
Prepayments and other current assets |
|
79,648 |
|
133,655 |
|
|
Total current assets |
|
290,024 |
|
380,818 |
|
|
Property and equipment |
|
5,059 |
|
9,962 |
|
|
Intangible assets, net |
|
360 |
|
382 |
|
|
Long-term investments |
|
11,140 |
|
11,140 |
|
|
Other non-current assets |
|
— |
|
1,446 |
|
|
Total assets |
|
306,583 |
|
403,748 |
|
|
|
|
|
|
|
|
|
Current Liabilities: |
|
|
|
|
|
|
Accounts payable |
|
(127,965 |
) |
(211,954 |
) |
|
Accrued expenses and other current liabilities |
|
(34,124 |
) |
(50,356 |
) |
|
Total liabilities |
|
(162,089 |
) |
(262,310 |
) |
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(b) Basis of consolidation (Continued)
|
|
|
Years Ended December 31, |
| ||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
|
Net revenues |
|
872,661 |
|
959,153 |
|
1,785,757 |
|
|
Total cost and expenses |
|
(865,419 |
) |
(930,567 |
) |
(1,809,656 |
) |
|
Net income (loss) |
|
7,242 |
|
28,586 |
|
(23,899 |
) |
|
|
|
Years Ended December 31, |
| ||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
|
Net cash used in operating activities |
|
(148,886 |
) |
(124,409 |
) |
(112,425 |
) |
|
Net cash used in investing activities |
|
(473 |
) |
(54 |
) |
(7,308 |
) |
|
Net cash provided by financing activities |
|
— |
|
— |
|
— |
|
The VIEs contributed approximately 99% of the Group’s consolidated revenues for each of the years ended December 31, 2016, 2017 and 2018. As of December 31, 2017 and 2018, the VIEs accounted for an aggregate of approximately 40% and 26%, respectively, of the consolidated total assets, and approximately 81% and 81%, respectively, of the consolidated total liabilities. Assets not associated with the VIEs mainly consisted of cash and cash equivalents and short-term investments.
Since September 2013, WFOE started paying advertising fees and marketing fees to external suppliers for the VIEs and recharges all or portion of these expenses to the VIEs at cost given that VIEs are in a loss position. The advertising fees and marketing fees charged by WFOE were RMB 119,684, 162,844 and 79,742 for the years ended December 31, 2016, 2017 and 2018, respectively.
There are no terms in any arrangements, considering both explicit arrangements and implicit variable interests that require the Company or its subsidiaries to provide financial support to the VIEs. However, if the VIEs were ever to need financial support, the Company or its subsidiaries may, at its option and subject to statutory limits and restrictions, provide financial support to its VIEs through loans to the shareholders of the VIEs or entrustment loans to the VIEs.
The Group believes that there are no assets held in the consolidated VIE that can be used only to settle obligations of the VIEs, except for registered capital and the PRC statutory reserves. As the consolidated VIE is incorporated as a limited liability company under the PRC Company Law, creditors of the VIEs do not have recourse to the general credit of the Company for any of the liabilities of the consolidated VIE.
Relevant PRC laws and regulations restrict the VIEs from transferring a portion of their net assets, equivalent to the balance of their statutory reserve and their share capital, to the Company in the form of loans and advances or cash dividends.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(c) Use of estimates
The preparation of the consolidated financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the period. Areas where management uses subjective judgment include estimating inventory write-down, collectability of receivables, the useful lives of long-lived assets, assessing the impairment of long-term investments and long-lived assets, valuation of ordinary share, share-based compensation expenses, recoverability of deferred tax assets, sales return and the fair value of the financial instruments. Management bases the estimates on historical experience and various other assumptions that are believed to be reasonable, the results of which form the basis for making judgments about the carrying values of assets and liabilities. Actual results could differ from these estimates.
(d) Cash and cash equivalents
Cash and cash equivalents consist of cash on hand and demand deposits, which are unrestricted as to withdrawal and use, and which have original maturities of three months or less when purchased.
(e) Short-term investments
Short-term investments include wealth management products, which are certain financial products with variable interest rates purchased from certain financial institutions with an original maturity period of less than one year. The Group classifies the wealth management products as “available-for-sale” debt securities. These investments are recorded at fair market value with the unrealized gains or losses recorded in accumulated other comprehensive income (loss) as a component of shareholders’ (deficit) equity. The assessment of impairment of short-term investments is based on whether the decline in fair value is other-than-temporary. The Group assesses its available-for-sale debt securities for other-than-temporary impairment by considering factors including, but not limited to, its ability and intent to hold the individual security, severity of the impairment, expected duration of the impairment and forecasted recovery of fair values. If the Group determines a decline in fair value is other-than-temporary, the cost basis of the individual security is written down to fair value as a new cost basis and the amount of the write-down is accounted for as a realized loss charged in the consolidated statement of income and comprehensive income. The fair values of the investments would not be adjusted for subsequent recoveries in fair values. There was no impairment on available-for-sale debt securities for the years ended December 31, 2016, 2017 and 2018.
(f) Accounts receivable, net
Accounts receivable mainly consists of amounts receivable from product delivery service providers and payment processing service providers, which are recognized and carried at the original invoice amount less an allowance for doubtful accounts. The Group establishes an allowance for doubtful accounts primarily based on the age of the receivables and factors surrounding the credit risk of specific customers.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(g) Inventories
Inventories, consisting of products available for sale, are stated at the lower of cost or market value. Cost of inventory is determined using the weighted average cost method. Adjustments are recorded to write down the cost of inventory to the estimated market value due to slow-moving or damaged products, which is dependent upon factors such as historical and forecasted consumer demand, and promotional environment. Write-downs are recorded in cost of products sold in the consolidated statements of comprehensive loss.
(h) Property and equipment
Property and equipment are stated at cost less accumulated depreciation and impairment. The renovations, betterments and interest cost incurred during construction are capitalized. Property and equipment are depreciated at their costs less impairment and residual value, if any, over the estimated useful lives on a straight-line basis. The estimated useful lives are as follows:
|
Leasehold improvements |
|
Shorter of the lease term or their estimated useful lives |
|
Furniture, fixtures and equipment |
|
3 years |
|
Electronic equipment |
|
3 years |
|
Vehicles |
|
5 years |
Construction in progress represents leasehold improvements under construction or being installed and is stated at cost. Cost comprises original cost of property and equipment, installation, construction and other direct costs. Construction in progress is transferred to leasehold improvements and depreciation commences when the asset is ready for its intended use.
Expenditures for repairs and maintenance are expensed as incurred. Gain or loss on disposal of property and equipment, if any, is recognized in the consolidated statements of comprehensive loss as the difference between the net sales proceeds and the carrying amount of the underlying asset. There was no interest cost capitalized during the years ended December 31, 2016, 2017 and 2018.
(i) Intangible assets
Intangible assets mainly consist of externally purchased software which are amortized over an estimated useful life of ten years on a straight-line basis.
(j) Impairment of long-lived assets
Long-lived assets are evaluated for impairment whenever events or changes in circumstances (such as a significant adverse change to market conditions that will impact the future use of the assets) indicate that the carrying value of an asset may not be fully recoverable. When these events occur, the Group evaluates the impairment for the long-lived assets by comparing the carrying value of the assets to an estimate of future undiscounted cash flows expected to be generated from the use of the assets and their eventual disposition. If the sum of the expected future undiscounted cash flows is less than the carrying value of the assets, the Group recognizes an impairment loss based on the excess of the carrying value of the assets over the fair value of the assets. For the years ended December 31, 2016, 2017 and 2018, there was no impairment of the Group’s long-lived assets.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(k) Long-term investments
The Group measures its equity securities without a readily determinable fair value at its cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer. As of December 31, 2018 and 2017, long-term investments were RMB11,140 with no changes in value recognized.
The Group is required to perform an impairment assessment of its investments whenever events or changes in business circumstances indicate that the carrying value of the investment may not be fully recoverable. An impairment loss is recognized in the consolidated statements of comprehensive loss equal to the excess of the investment’s cost over its fair value when the impairment is deemed other-than-temporary. No impairment was recorded for the Group’s long-term investments for the years ended December 31, 2016, 2017 and 2018.
(l) Revenue recognition
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-09, “Revenue from Contracts with Customers (“ASC 606”). This standard replaced existing revenue recognition rules with a comprehensive revenue measurement and recognition standard and expanded disclosure requirements. The ASU also includes guidance regarding the accounting for contract acquisition costs, which includes sales commissions. The Group has early adopted ASC 606 and all subsequent ASUs that modified ASC 606 on January 1, 2017 using the full retrospective method which requires the Group to present its financial statements for all periods as if Topic 606 had been applied to all prior periods.
The Group follows five steps for its revenue recognition under ASC 606:
· Step 1: Identify the contract (s) with a customer
· Step 2: Identify the performance obligations in the contract
· Step 3: Determine the transaction price
· Step 4: Allocate the transaction price to the performance obligations in the contract
· Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(l) Revenue recognition (Continued)
The Group’s revenue is reported net of discount, value added tax and related surcharges. The primary sources of the Group’s revenues are as follows:
Product Revenues
The Group recognizes revenues from the sale of medicines, healthcare products and other wellness merchandise through its online platforms, including its internet website 1 Drugstore, cellular phone application and its offline pharmacies to its consumers (the “B2C Business”). The Group also generates revenues from the sale of medicines to its pharmacy customers through the online platform 1 Drug Mall (the “B2B Business”).
Under both B2C Business and B2B Business, revenues from product sales are recognized at the point in time when the delivery is made and when title and risk of loss transfers to the consumers and pharmacy customers. Revenues are measured as the amount of consideration the Group expects to receive in exchange for transferring products to consumers and pharmacy customers (“transaction price”). To the extent that the transaction price includes variable consideration, the Group estimates the amount of variable consideration that should be included in the transaction price utilizing the most likely amount method. Variable consideration is included in the transaction price if, in the Group’s judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. The Group provides the right of return in circumstances when there is packing or delivery damage or other quality problems identified within 30 days which is considered to be a form of variable consideration. The Group estimates sales returns based on historical experience and based on such, the amount of sales returns accrual was insignificant as of December 31, 2017 and 2018.
The Group voluntarily provides discount coupons through its websites during its marketing activities. These coupons are not related to prior purchases, and can only be utilized in conjunction with subsequent purchases on the Group’s platforms. The coupons are recorded as a reduction of revenue at the time of use.
Under both B2B and B2C Businesses the Group utilizes delivery service providers to deliver products to its consumers and pharmacy customers (“shipping activities”) but the delivery service is not considered as a separate obligation as the shipping activities are performed before the consumers and pharmacy customers obtain control of the products. Therefore, shipping activities are not considered a separate promised service to the consumers and pharmacy customers, but rather are activities to fulfill the Group’s promise to transfer the products and are recorded as fulfillment expenses.
Product revenues are recorded net of surcharges and value added tax (“VAT”) ranging from 0% to 17% for different kinds of products based on the sales amount. Surcharges are sales related taxes representing the City Maintenance and Construction Tax and Education Surtax. The Group records revenues on a gross basis because the Group controls the products before they are transferred to the consumers and pharmacy customers determined on the basis that: (1) the Group is primarily responsible for fulfilling its promise to deliver the specified products to consumers and pharmacy customers; (2) the Group has inventory risk before the specified products are transferred to a consumers and pharmacy customers or after transfer of control to the consumers and pharmacy customers, and (3) the Group has discretion in establishing the price for the specified products.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(l) Revenue recognition (Continued)
Service revenues
Service revenues primarily consist of fees charged to third-party marketplace sellers for whom the Group acts as an agent to facilitate the marketplace sellers’ online sales of their products through the online platform 1 Drugstore, which is referred to as marketplace service (“MP”) revenue. The Group has determined it is not the principal in the arrangement as it is not responsible to fulfill the order for the specified products, it does not bear the inventory risk for the products, nor does it have the ability to establish prices. The Group charges the marketplace sellers commission fees equal to an agreed percentage of the sales price of the product when a sale is completed and also charges market place sellers an annual non-refundable up-front fee for platform usage. The promise to the customer, which is the marketplace seller, is to arrange for the sale which is considered as one performance obligation. Therefore, the Group recognizes the up-front fee and commission at the point in time when the sale is completed.
The Group also provides other ancillary services, which include advertisement display services and an online medical consultation service. The advertisement display service revenues represent amounts the Group receives from its customers, mainly pharmaceutical companies, by displaying the advertisement of products through the Group’s LED screens installed at offline pharmacy stores and are recognized over the period of time when the advertisement is displayed. The online medical consultation service represents the consultation services the Group provides with in-house full-time medical professionals and the revenue is recognized when the consultation is completed.
Beginning in November 2017, the Group started to offer a quarterly or annual membership program to its consumers, who pay a non-refundable upfront fee in exchange for specified price discounts on future purchases, limited times of free shipping, and limited times of medical consultation during the membership period. The Group allocates the fee to these performance obligations based on estimated stand-alone selling prices and recognizes revenue when the goods or services are provided to consumers and coupons are redeemed or when the coupons expire at the end of membership period.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(m) Cost of products sold
Cost of products sold consists of the purchase price of products and inbound shipping charges. The Group periodically receives rebates from certain vendors in the form of credits that the Group can apply against trade amounts owed to vendors pursuant to a binding arrangement only if the Group completes a specified cumulative level of purchases within a specified time period. The rebates do not represent a payment for assets or services delivered to the vendor or a reimbursement of costs incurred by the Group to sell vendors’ products. The Group accounts for the rebates received from its vendors as a reduction to the price the Group pays for the products purchased and therefore records such amounts as a reduction of cost of products sold when recognized in the consolidated financial statements. Rebates are earned based on reaching minimum purchase thresholds within a specified period, typically on a fiscal quarterly or annual basis. Cost of products does not include other direct costs related to cost of product sales such as shipping and handling expense, payroll and benefits of logistics staff, logistics centers rental expenses and depreciation expenses. Therefore, the Group’s cost of products sold may not be comparable to other companies which include such expenses in their cost of products.
(n) Fulfillment expenses
Fulfillment expenses primarily consist of payroll, bonus and benefits of logistics staff, logistics centers rental expenses, shipping and handling expenses, and packaging expenses.
(o) Selling and marketing expenses
Selling and marketing expenses primarily consist of payroll, bonus and benefits of sales and marketing staff, advertising costs, agency fees and costs for promotional materials.
Advertising expenses are charged to the statements of comprehensive loss in the period incurred. The amounts of advertising expenses incurred were RMB125,692, RMB62,749 and RMB30,221 for the years ended December 31, 2016, 2017 and 2018, respectively.
(p) Technology expenses
Technology expenses primarily consist of payroll, bonus and benefits of the staff in the technology and system department incurred for development and enhancement to the Group’s websites and platform applications.
For internal and external use software, the Group expenses all costs incurred for the preliminary project stage and post implementation-operation stage of development, and costs associated with repair or maintenance of the existing platform. Costs incurred in the application development stage are capitalized and amortized over the estimated useful life. The amount of the Group’s technology expenses qualifying for capitalization has been insignificant, and as a result, all development costs incurred for development of internal used software have been expensed as incurred.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(q) General and administrative expenses
General and administrative expenses primarily consist of payroll, bonus and benefit costs for corporate employees, legal, finance, rental expenses and other corporate overhead costs.
(r) Government grants
Government grants represent rewards provided by the relevant PRC government authorities to the Group for tax refunds and support for investment in certain local districts, which are typically granted based on the amount of investments the Group made as well as income generated by the Group in such districts. Such subsidies allow the Group full discretion to utilize the funds and are used by the Group for general corporate purposes. Normally, the Group does not receive written confirmation from local governments indicating the approval of the cash subsidy before cash is received, and therefore cash subsidies are recognized when received and when all the conditions for their receipts have been satisfied. Government grants recognized were RMB1,993, RMB3,282 and RMB2,166 for the years ended December 31, 2016, 2017 and 2018, respectively, which were recorded in other operating income (expenses), net.
(s) Income Taxes
Current income taxes are provided for in accordance with the laws of the relevant taxing authorities. As part of the process of preparing financial statements, the Group is required to estimate its income taxes in each of the jurisdictions in which it operates. The Group accounts for income taxes using the liability method. Under this method, deferred income taxes are recognized for tax consequences in future years of differences between the tax bases of assets and liabilities and their reported amounts in the financial statements at each year-end and tax loss carry forwards. Deferred tax assets and liabilities are measured using enacted tax rates applicable for the differences that are expected to affect taxable income. Deferred tax assets are reduced by a valuation allowance when, based upon the weight of available evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized.
(t) Value added taxes
The Group’s PRC subsidiaries are subject to VAT at rates ranged from 0% to 17% on proceeds received from customers, and are entitled to a deduction for VAT already paid or borne on the products purchased by them. The VAT balance is recorded in other current assets or other current liabilities on the consolidated balance sheets.
(u) Operating leases as lessee
Leases, including leases of offices and warehouses, where substantially all the rewards and risks of ownership of assets remain with the lessors are accounted for as operating leases. Payments made under operating leases are recognized as an expense on a straight-line basis over the lease term. The Group had no capital leases for any of the years stated herein.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(v) Comprehensive income (loss)
Comprehensive income (loss) is defined to include all changes in equity except those resulting from investments by owners and distributions to owners. During the periods presented, comprehensive income (loss) is reported in the consolidated statements of comprehensive loss, and other comprehensive loss includes foreign currency translation adjustments and fair value change of available-for-sale securities.
(w) Foreign currency translation
The reporting currency of the Group is the Renminbi (“RMB”). The functional currency of the Company and Yao Wang is the United States dollar (“US dollar”). The functional currency of all the other significant subsidiaries and the variable interest entities is RMB. The determination of the respective functional currency is based on the criteria of Accounting Standard Codification (“ASC”) 830, Foreign Currency Matters.
Monetary assets and liabilities denominated in currencies other than the applicable functional currencies are translated into the functional currencies at the prevailing rates of exchange at the balance sheet date. Nonmonetary assets and liabilities are remeasured into the applicable functional currencies at historical exchange rates. Transactions in currencies other than the applicable functional currencies during the year are converted into the functional currencies at the applicable rates of exchange prevailing at the transaction dates. Transaction gains and losses are recognized in the consolidated statements of comprehensive loss.
Assets and liabilities are translated from each entity’s functional currency to the reporting currency at the exchange rate on the balance sheet date. Equity amounts are translated at historical exchange rates, and revenues, expenses, gains and losses are translated using the average rate for the year. Translation adjustments are reported as cumulative translation adjustments and are shown as a separate component of accumulated other comprehensive loss in the consolidated statements of shareholders’ (deficit) equity.
(x) Concentration of credit risk
Financial instruments that potentially expose the Group to concentration of credit risk consist primarily of cash and cash equivalents, short-term investments, accounts receivable and prepayments. The Group places its cash and cash equivalents and short-term investments with financial institutions with high-credit ratings and quality. Accounts receivable mainly consist of amounts receivable from product delivery service providers and payment processing service providers, which are all with good collection history. There are no significant concentrations of credit risk. With respect to prepayments, the Group performs on-going credit evaluations of the financial condition of these suppliers.
Concentration of customers
There were no customers individually representing 10% or more of revenues for the years ended December 31, 2016, 2017 and 2018.
There were no customers individually representing 10% or more of accounts receivable as of December 31, 2017 and 2018.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(x) Concentration of credit risk (Continued)
Concentration of suppliers
The following supplier accounted for 10% or more of purchases for the years ended December 31, 2016, 2017 and 2018:
|
|
|
Years Ended December 31, |
| ||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
|
Product purchases: |
|
|
|
|
|
|
|
|
A |
|
12.4 |
% |
14.1 |
% |
13.9 |
% |
|
B |
|
* |
|
* |
|
12.7 |
% |
The following suppliers accounted for 10% or more of balances of accounts payable as of December 31, 2017 and 2018:
|
|
|
2017 |
|
2018 |
|
|
Accounts payable: |
|
|
|
|
|
|
A |
|
16.0 |
% |
18.9 |
% |
* Less than 10%.
Foreign currency risk
Renminbi (“RMB”) is not a freely convertible currency. The State Administration of Foreign Exchange, under the authority of the People’s Bank of China, controls the conversion of RMB into foreign currencies. The value of RMB is subject to changes in central government policies and to international economic and political developments affecting supply and demand in the China Foreign Exchange Trading System market. The cash and cash equivalents of the Group included aggregated amounts of RMB 56,947and RMB 17,810, which were denominated in RMB, as of December 31, 2017 and 2018, respectively.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(y) Fair value
Fair value is defined as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Group considers the principal or most advantageous market in which it would transact and it considers assumptions that market participants would use when pricing the asset or liability.
The established fair value hierarchy requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. The three levels of inputs may be used to measure fair value include:
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices included within Level 1 that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
When available, the Group uses quoted market prices to determine the fair value of an asset or liability. If quoted market prices are not available, the Group measures fair value using valuation techniques that use, when possible, current market-based or independently sourced market parameters, such as interest rates.
The estimated fair value of the Group’s financial instruments of which the inputs used to value are classified as Level 2 and are not reported at fair value, including cash and cash equivalents, accounts receivable, other current assets, accounts payable, other current liabilities, approximates their carrying value due to their short-term nature.
Since January 1, 2018, the Group adopted the ASU 2016-01, Financial Instruments—Overall (Subtopic 825-10). Under the new ASC, entities no longer use the cost method of accounting as it was applied before and the new ASC requires equity investments (except those accounted for under the equity method of accounting or those that result in consolidation of the investee) to be measured at fair value with changes in fair value recognized in net income. However, a company can elect to measure equity investments that do not have readily determinable fair values at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer (the “measurement alternative”). After management’s assessment of each of the equity investments described in Note 8, management concluded that investments do not have readily determinable fair values, and elects the measurement alternative.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(z) Share-based compensation
Awards Granted to Employees
The Group grants share options to eligible employees and accounts for these share based awards in accordance with ASC 718 Compensation-Stock Compensation.
Employees’ share-based awards are measured at the grant date fair value of the awards and recognized as expenses a) immediately at grant date if no vesting conditions are required; or b) using straight-line vesting method over the requisite service period, which is the vesting period. To the extent the required vesting conditions are not met resulting in the forfeiture of the share-based awards, previously recognized compensation expense relating to those awards are reversed.
Prior to the IPO of the Company, with the assistance of an independent third party valuation firm, determined the fair value of the stock options granted to employees. The Black Scholes option pricing model was applied in determining the estimated fair value of the options granted to employees.
After the IPO of the Company, in determining the fair value of the share options and Ordinary Share Units, the closing market price of the underlying shares on the grant date is applied.
Awards Granted to Non-Employees
The Group has accounted for equity instruments issued to non-employees in accordance with the provisions of ASC 505, Equity-based payments to nonemployees. All transactions in which goods or services are received in exchange for equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. As there is no performance commitment associated with the equity instrument issued to non-employees, the Group remeasures the awards using the then-current fair value at each reporting date until the measurement date, generally when the services are completed and awards are vested, and attributes the changes in those fair values over the service period by straight-line method.
(aa) Earnings (loss) per share
Basic earnings (loss) per ordinary share is computed by dividing net income (loss) attributable to ordinary shareholders by weighted average number of ordinary shares outstanding during the period.
The Group’s convertible preferred shares are participating securities as the preferred shares participate in undistributed earnings on an as-if-converted basis. Accordingly, the Group uses the two-class method whereby undistributed net income is allocated on a pro rata basis to each participating share to the extent that each class may share income for the period. Undistributed net loss is not allocated to preferred shares because they are not contractually obligated to participate in the loss allocated to the ordinary shares.
Diluted earnings (loss) per ordinary share reflects the potential dilution that could occur if securities were exercised or converted into ordinary shares. The Group has convertible preferred shares and stock options, which could potentially dilute basic earnings per share in the future. To calculate the number of shares for diluted income per share, the effect of the convertible preferred shares is computed using the as-if-converted method; the effect of the stock options is computed using the treasury stock method.
In September 2018, the Company’s shareholders voted in favor of a proposal to adopt a dual-class share structure, pursuant to which the Company’s authorized share capital were reclassified and redesigned into Class A ordinary shares and Class B ordinary shares (Note 12). Both Class A ordinary shares and Class B ordinary shares are entitled to the same dividend right, as such, this dual class share structure has no impacts to the earnings per share calculation. Basic earnings per share and diluted earnings per share are the same for each Class A ordinary shares and Class B ordinary shares.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(aa) Earnings (loss) per share (Continued)
Upon the consummation of the Company’s IPO in September 2018, the convertible redeemable preferred shares were converted into Class A ordinary shares. The two-class method of computing earnings per share ceased to apply on such conversion date.
(ab) Segment reporting
In accordance with ASC 280, Segment Reporting, the Group’s chief operating decision maker (“CODM”) has been identified as the Co-Chairmen and Chief Executive Officer, who reviews the segment information when making decisions about allocating resources and assessing performance of the Group. The Group organized its operations into two segments: B2C segment and B2B segment. There are no internal revenue transactions between the reportable segments. The Group does not distinguish expenses between segments in its internal reporting, and reports expenses by nature as a whole. Furthermore, the Group’s CODM is not provided with asset information by segment. As such, no asset information by segment is presented. The following tables summarize the Group’s product revenues and segment profit/(loss) generated by its segments.
|
|
|
Years Ended December 31, |
| ||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
|
B2C segment |
|
|
|
|
|
|
|
|
Product revenues |
|
870,361 |
|
862,327 |
|
847,476 |
|
|
Cost of products sold* |
|
(796,230 |
) |
(780,137 |
) |
(767,073 |
) |
|
Segment profit for B2C Business |
|
74,131 |
|
82,190 |
|
80,403 |
|
|
|
|
|
|
|
|
|
|
|
B2B segment |
|
|
|
|
|
|
|
|
Product revenues |
|
— |
|
86,890 |
|
922,751 |
|
|
Cost of products sold* |
|
— |
|
(88,582 |
) |
(914,627 |
) |
|
Segment (loss) profit for B2B Model |
|
— |
|
(1,692 |
) |
8,124 |
|
|
|
|
|
|
|
|
|
|
|
Total segment profit |
|
74,131 |
|
80,498 |
|
88,527 |
|
*For segment reporting purpose, purchase rebate is allocated to B2C segment and B2B segment primarily based on the amount of cost of products sold for each segment. Cost of products sold does not include other direct costs related to cost of product sales such as shipping and handling expense, payroll and benefits of logistic staff, logistic centers rental expenses and depreciation expenses, which are recorded in the fulfillment expenses.
As the Group operates in the PRC and all of the Group’s long-lived assets are located in the PRC, no geographical segments are presented.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(ab) Segment reporting (Continued)
The following is the reconciliation of reportable segment revenues to the Group’s consolidated revenue:
|
|
|
Years Ended December 31, |
| ||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
|
|
|
|
|
|
|
|
|
|
Total revenues for reportable segments |
|
870,361 |
|
949,217 |
|
1,770,227 |
|
|
Service revenues |
|
3,476 |
|
10,269 |
|
15,743 |
|
|
|
|
|
|
|
|
|
|
|
Total consolidated revenues |
|
873,837 |
|
959,486 |
|
1,785,970 |
|
The following is a reconciliation of the reportable segments’ measures of profit or loss to the Group’s consolidated loss before income taxes:
|
|
|
Years Ended December 31, |
| ||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
|
|
|
|
|
|
|
|
|
|
Total profit for reportable segments |
|
74,131 |
|
80,498 |
|
88,527 |
|
|
Unallocated amounts: |
|
|
|
|
|
|
|
|
Service Revenues |
|
3,476 |
|
10,269 |
|
15,743 |
|
|
Fulfillment expenses |
|
(68,445 |
) |
(55,880 |
) |
(73,930 |
) |
|
Selling and marketing expenses |
|
(252,829 |
) |
(190,074 |
) |
(260,040 |
) |
|
General and administrative expenses |
|
(60,836 |
) |
(53,434 |
) |
(98,759 |
) |
|
Technology expenses |
|
(61,767 |
) |
(48,133 |
) |
(71,248 |
) |
|
Other operating income (expenses), net |
|
1,990 |
|
2,732 |
|
(668 |
) |
|
Interest income |
|
2,308 |
|
4,013 |
|
4,352 |
|
|
Interest expense |
|
(751 |
) |
(55 |
) |
— |
|
|
Foreign exchange gain (loss) |
|
2,630 |
|
(3,492 |
) |
2,459 |
|
|
Other income (loss),net |
|
(3,353 |
) |
4,229 |
|
11,531 |
|
|
Loss before income tax |
|
(363,446 |
) |
(249,327 |
) |
(382,033 |
) |
Revenues from different product groups and services are as follows:
|
|
|
Years Ended December 31, |
| ||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
|
|
|
|
|
|
|
|
|
|
Product Revenues |
|
870,361 |
|
949,217 |
|
1,770,227 |
|
|
Drugs |
|
541,318 |
|
649,341 |
|
1,489,917 |
|
|
Nutritional supplements |
|
110,295 |
|
123,214 |
|
190,425 |
|
|
Contact lenses |
|
115,547 |
|
107,275 |
|
47,295 |
|
|
Medical supplies and devices |
|
68,819 |
|
49,414 |
|
26,563 |
|
|
Other products |
|
34,382 |
|
19,973 |
|
16,027 |
|
|
Service Revenues |
|
3,476 |
|
10,269 |
|
15,743 |
|
|
MP Service |
|
2,472 |
|
8,767 |
|
12,375 |
|
|
Other Services |
|
1,004 |
|
1,502 |
|
3,368 |
|
|
Total |
|
873,837 |
|
959,486 |
|
1,785,970 |
|
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(ac) Recently issued accounting pronouncements
New Accounting Pronouncements Recently Adopted
In October 2016, the FASB issued ASU 2016-16, Income Taxes (Topic 740): Intra-Entity Transfers of Assets Other Than Inventory, which removes the prohibition in ASC 740 against the immediate recognition of the current and deferred income tax effects of intra-entity transfers of assets other than inventory. The ASU, which is part of the Board’s simplification initiative, is intended to reduce the complexity of US GAAP and diversity in practice related to the tax consequences of certain types of intra-entity asset transfers, particularly those involving intellectual property. For public business entities, the ASU is effective for annual periods beginning after December 15, 2017, and interim periods within those annual periods. The ASU requires a modified retrospective method of adoption. The Group adopted this standard in January 2018. This standard did not have a significant impact on its consolidated financial statements.
In May 2017, the FASB issued ASU 2017-09, Compensation—Stock Compensation (Topic 718): Scope of Modification Accounting. The guidance provides clarity and reduces diversity in practice and cost and complexity when accounting for a change to the terms or conditions of a share-based payment award. The amendments in this update are effective for all entities for annual periods, and interim periods within those annual periods, beginning after December 15, 2017. The ASU requires a prospective method of adoption. Early adoption is permitted. As such, the Group adopted this standard in January 2018. This standard did not have a significant impact on its consolidated financial statements.
New Accounting Pronouncements Not Yet Adopted
In June 2018, the FASB issued ASU 2018-07, Compensation—Stock Compensation (Topic 718):—Improvements to Nonemployee Share-Based Payment Accounting. ASU 2018-07 simplifies the accounting for nonemployee share-based payment transactions for acquiring goods and services from nonemployees. The amendments in this update are effective for public business entities for fiscal years beginning after December 15, 2018, including interim periods within that fiscal year. Early adoption is permitted, but no earlier than an entity’s adoption date of Topic 606.The Group does not expect the adoption of this ASU will have a significant impact on its consolidated financial statements.
In August 2018, the FASB issued ASU 2018-13, Disclosure framework—changes to the disclosure requirements for fair value measurement. The amendments in ASU 2018-13 eliminate the requirements to disclose the amount and reasons for transfers between Level 1 and Level 2 of the fair value hierarchy, valuation processes for Level 3 fair value measurements, and policy for timing of transfers between levels. ASU 2018-13 also provides clarification in the measurement uncertainty disclosure by explaining that the disclosure is to communicate information about the uncertainty in measurement as of the reporting date. In addition, ASU 2018-13 added the following requirements: changes in unrealized gains and losses for the period included in other comprehensive income for recurring Level 3 fair value measurements held at the end of the reporting period; and range and weighted average of significant unobservable inputs used in Level 3 fair value measurements. Finally, ASU 2018-13 updated language to further encourage entities to apply materiality when considering de minimus for disclosure requirements. The guidance will be applied retrospectively for fiscal years beginning after December 15, 2019, and interim periods within those fiscal years, with the exception of amendments to changes in unrealized gains and losses, the range and weighted average of significant unobservable inputs used for Level 3 fair value measurements, and the narrative description of measurement uncertainty which will be applied prospectively. Early adoption is permitted. The Group does not expect the adoption of this ASU will have a significant impact on its consolidated financial statements.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
2. SUMMARY OF PRINCIPAL ACCOUNTING POLICIES (Continued)
(ac) Recently issued accounting pronouncements (Continued)
In February 2016, the FASB released ASU 2016-02, Leases, which requires lessees to record lease contracts on the balance sheet by recognizing a right-of-use asset and lease liability with certain practical expedients available. The accounting for lessors remains largely unchanged. In July 2018, the FASB released Accounting Standards Update No. 2018-11 (“ASU 2018-11”), Leases (Topic 842): Targeted Improvements, providing entities with an additional optional transition method. The provisions of ASU 2016-02, and all related ASUs, are effective for interim periods and fiscal years beginning after December 15, 2018, with early adoption permitted. The Group expects to adopt ASU 2016-02 utilizing the optional transition approach allowed under ASU 2018-11 and applying the package of practical expedients beginning January 1, 2019. This option allows entities to initially apply the new leases standard at the adoption date and recognize a cumulative-effect adjustment to the opening balance of retained earnings in the period of adoption. By applying ASU 2016-02 at the adoption date, as opposed to at the beginning of the earliest period presented, the Group’s reporting for periods prior to January 1, 2019 will continue to be reported in accordance with Leases (Topic 840). The Group are still assessing the potential impact that ASU 2016-02 will have on the financial statements and disclosures, but the Group currently anticipates recognizing right-of-use lease assets in the range of RMB57 million to RMB70 million and related lease liabilities in the range of RMB56 million to RMB69 million for operating leases. The Group does not believe the standard will materially affect its consolidated statements of comprehensive loss or consolidated statements of cash flows.
(ad) Convenience translation
Translations of balances in the consolidated balance sheets, consolidated statements of comprehensive loss, and consolidated statements of cash flows from RMB into US dollar as of and for the year ended December 31, 2018 are solely for the convenience of the readers and were calculated at the rate of 6.8755, representing the noon buying rate set forth in the H.10 statistical release of the U.S. Federal Reserve Board on December 31, 2018. No representation is made that the RMB amounts could have been, or could be, converted, realized or settled into US dollar at that rate on December 31, 2018, or at any other rate.
3. SHORT-TERM INVESTMENTS
Short-term investments as of December 31, 2017 and 2018 were as follows:
|
|
|
As of December 31, |
| ||
|
|
|
2017 |
|
2018 |
|
|
Wealth Management Products |
|
293,533 |
|
252,805 |
|
The Group classifies the wealth management products as “available-for-sale” debt securities which are recorded at fair value. For the years ended December 31, 2016, 2017 and 2018, the Group recorded RMB1,415, RMB5,181 and RMB8,734 increase in fair value of these available-for-sale debt securities, net of tax, in other comprehensive income (loss), respectively, and nil, RMB1,154 and RMB10,869 of realized gains transferred from other comprehensive income to other income (loss) when the security was sold. No impairment charges were recorded for the years ended December 31, 2016, 2017 and 2018, respectively.
4. INVENTORIES
Inventories as of December 31, 2017 and 2018 were as follows:
|
|
|
As of December 31, |
| ||
|
|
|
2017 |
|
2018 |
|
|
Products |
|
144,056 |
|
210,836 |
|
No write-down has been made to the inventories as of December 31, 2017 and 2018.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
5. PREPAYMENT AND OTHER CURRENT ASSETS
Prepayment and other current assets, as of December 31, 2017 and 2018 were as follows:
|
|
|
As of December 31, |
| ||
|
|
|
2017 |
|
2018 |
|
|
Value added tax recoverable |
|
49,980 |
|
70,908 |
|
|
Rebate receivable from suppliers |
|
25,098 |
|
58,827 |
|
|
Deposits (Note) |
|
19,477 |
|
11,602 |
|
|
Advance to suppliers |
|
4,202 |
|
3,315 |
|
|
Interest Receivable |
|
— |
|
2,124 |
|
|
Prepaid IT & Insurance expense |
|
1,892 |
|
5,034 |
|
|
Others |
|
4,169 |
|
9,337 |
|
|
Total |
|
104,818 |
|
161,147 |
|
Note: Deposits consist of amounts paid to certain vendors for advertising and rentals.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
6. PROPERTY AND EQUIPMENT
Property and equipment consists of the following:
|
|
|
As of December 31, |
| ||
|
|
|
2017 |
|
2018 |
|
|
Cost: |
|
|
|
|
|
|
Leasehold improvements |
|
26,269 |
|
34,147 |
|
|
Electronic equipment |
|
16,059 |
|
18,730 |
|
|
Furniture, fixtures and equipment |
|
5,346 |
|
8,566 |
|
|
Vehicles |
|
549 |
|
595 |
|
|
|
|
48,223 |
|
62,038 |
|
|
Less: Accumulated depreciation |
|
(31,877 |
) |
(41,736 |
) |
|
|
|
16,346 |
|
20,302 |
|
|
Construction in progress |
|
682 |
|
— |
|
|
Property and equipment, net |
|
17,028 |
|
20,302 |
|
Depreciation expense was RMB11,362, RMB14,203 and RMB10,643 for the years ended December 31, 2016, 2017 and 2018, respectively.
7. INTANGIBLE ASSETS
Intangible assets consist of the following:
|
|
|
As of December 31, |
| ||
|
|
|
2017 |
|
2018 |
|
|
|
|
|
|
|
|
|
Purchased software |
|
6,555 |
|
6,926 |
|
|
Less: Accumulated amortization |
|
(1,804 |
) |
(2,423 |
) |
|
Total |
|
4,751 |
|
4,503 |
|
Amortization expense of intangible assets for the years ended December 31, 2016, 2017 and 2018 amounted to RMB698, RMB617 and RMB623, respectively. Estimated amortization expenses of the existing intangible assets for each of the five years ending December 31, 2023 and thereafter is RMB665, RMB664, RMB664, RMB664, RMB658 and RMB1,188, respectively.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
8. LONG-TERM INVESTMENTS
Long-term investments as of December 31, 2017 and 2018 were as follows:
|
|
|
As of December 31, |
| ||
|
|
|
2017 |
|
2018 |
|
|
Equity securities without a readily determinable fair value: |
|
|
|
|
|
|
Xixi |
|
11,000 |
|
11,000 |
|
|
Longyan Huiyuan |
|
140 |
|
140 |
|
|
Total |
|
11,140 |
|
11,140 |
|
In June 2015, the Group purchased 5.21% equity interest in Shanghai Xixi Maternal and Baby Care Service Co., Ltd. (“Xixi”) at the consideration of RMB11,000. In September 2017, the Group purchased 1% equity interest in Longyan Huiyuan Pharmacy Co., Ltd. (“Longyan Huiyuan”) at the consideration of RMB140. The Group measures its equity securities without a readily determinable fair value at its cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issuer.
9. OTHER NON-CURRENT ASSETS AND LIABILITIES
|
|
|
As of December 31, |
| ||
|
|
|
2017 |
|
2018 |
|
|
Other Non-current assets: |
|
|
|
|
|
|
Long term deposits related to leased warehouses and office space |
|
— |
|
3,376 |
|
|
Total Other Non-current Assets |
|
— |
|
3,376 |
|
|
Other Non-current Liabilities: |
|
|
|
|
|
|
ADR Reimbursement (note) |
|
— |
|
8,135 |
|
|
Total Other Non-current Liabilities: |
|
— |
|
8,135 |
|
Note: According to the American Depositary Receipts (the “ADR”) arrangements signed on August 9, 2018, the Group has the right to receive reimbursements as a return for using Depositary Bank’s services, subject to the compliance by the Group with the terms of the agreement. The Group performed a detailed assessment of the requirements and recognizes the reimbursements it is expected to be entitled to over the five-year contract term. RMB 663 was recorded in other income for the year ended December 31, 2018. RMB 8,135 was recorded in other non-current liabilities as of December 31, 2018 and RMB 2,200 was recorded in accrued expenses and other current liabilities for the current portion.
10. ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES
Accrued expenses and other current liabilities as of December 31, 2017 and 2018 were as follows:
|
|
|
As of December 31, |
| ||
|
|
|
2017 |
|
2018 |
|
|
Accrued advertising expense |
|
13,348 |
|
10,492 |
|
|
Salary and welfare payables |
|
12,312 |
|
25,042 |
|
|
Accrued rental expenses |
|
8,020 |
|
8,891 |
|
|
Accrued delivery service fees |
|
1,682 |
|
7,153 |
|
|
Payable to marketplace sellers (note) |
|
8,262 |
|
10,510 |
|
|
Deposits from marketplace sellers |
|
6,293 |
|
6,015 |
|
|
Advance from customers |
|
11,722 |
|
15,489 |
|
|
Tax Payables |
|
1,454 |
|
2,466 |
|
|
Others |
|
9,925 |
|
16,203 |
|
|
Total |
|
73,018 |
|
102,261 |
|
Note: Amounts relate to cash collected on behalf of marketplace sellers for products sold through the Group’s online platform.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
11. CONVERTIBLE PREFERRED SHARES
Upon the completion of the Company’s IPO in September, 2018, all of the outstanding Series A, B, C and D convertible preferred shares were converted into 75,118,996 ordinary shares. The history of the issuance of the preferred shares is as following:
In September 2013, the Company issued 2,100,000 Series A convertible preferred shares with an issuance price of US$1 per share to a group of investors for a cash consideration of US$2,100 (equivalent of RMB12,922).
In December 2013, the Company issued 5,698,089 Series B convertible preferred shares with an issuance price of US$1.6627 per share for a cash consideration of US$9,474 (equivalent to RMB57,980).
In December 2014, the Company issued 15,869,617 Series C convertible preferred shares with an issuance price of US$4.6027 per share for a cash consideration of US$73,043 (equivalent to RMB450,909), net of issuance cost of US$90 (equivalent to RMB585).
In September 2015, the Company split each of the issued and unissued ordinary shares and preferred shares into two shares, and the authorized preferred shares consisted of 4,200,000 Series A preferred shares, 11,396,178 Series B preferred shares, and 31,739,234 Series C preferred shares.
From November 2015 to January 2016, the Company issued 26,598,954 Series D and D+ (collectively, “Series D”) convertible preferred shares with an issuance price of US$7.0679 per share for a cash consideration of US$188,000 (equivalent to RMB1,208,242). Unreceived consideration of US$50,000 (equivalent to RMB327,294) and US$42,700 (equivalent to RMB277,819) from Series D convertible preferred shareholders was recorded as subscription receivable as of December 31, 2016 and 2017, respectively.
In June 2016, the Company issued additional 1,184,630 Series D preferred shares to Series D preferred shareholders at a price of US$0.00005 per share to compensate for the dilution caused by the additional issuance and reservation for share incentive plan. As a result, the issuance price of Series D preferred shares decreased from US$7.0679 to US$6.7665 per share. The Company accounted for this transaction as a modification to Series D preferred shares and the difference between the fair value of Series D preferred shares immediately before and after the amendment as determined by the Company with the assistance of an independent valuation firm was recognized as a deemed dividend in the amount of RMB55,281 as a compensation to Series D preferred shareholders.
The key terms of the Series A, B, C and D preferred shares (collectively, “Preferred Shares”) were as follows:
Conversion rights
Each holder of Preferred Shares shall have the right, at such holder’s sole discretion, to convert all or any portion of the Preferred Shares into ordinary shares on a one-for-one basis at any time. The initial conversion price is the issuance price of Preferred Shares, subject to adjustment in the event of (1) stock splits, share combinations, share dividends and distribution, recapitalizations and similar events, and (2) issuance of new securities at a price per share less than the conversion price in effect on the date of or immediately prior to such issuance. In that case, the conversion price shall be reduced concurrently to the subscription price of such issuance.
11. CONVERTIBLE PREFERRED SHARES (Continued)
The Preferred Shares will be automatically converted into ordinary shares at the then applicable conversion price upon the earlier of (1) the closing of a Qualified Initial Public Offering (defined as a firm underwritten public offering of the ordinary shares of the Company in NASDAQ, NYSE, a recognized stock exchange in China or HK Stock Exchange, that has been registered under the United States Securities Act of 1933 or any other applicable laws, as amended from time to time, including any successor statutes, with an implied pre-offering valuation of the Company of at least US$2,000,000), or (2) the date specified by written consent or agreement of a majority of holders of Preferred Shares of each series.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
Voting rights
Holders of Class A ordinary shares, Class B ordinary shares and Class C ordinary shares shall at all times vote together as one class on all resolutions submitted to a vote by the shareholders. Each Class A ordinary share shall be entitled to twenty (20) votes on all matters subject to vote at general meetings of the Company and each Class B ordinary share, Class C ordinary share and Preferred Share shall be entitled to one (1) vote on all matters subject to vote at general meetings of the Company,
Dividends
There were no preferential dividend rights mentioned for preferred shareholders in the agreements.
Liquidation preference
In the event of any liquidation, dissolution or winding up of the Company, either voluntary or involuntary, the holders of Series D, C, B and A Preferred Shares are entitled to receive, prior to any distribution to the holders of ordinary shares, an amount equal to the Issue Price of each series of Preferred Shares as adjusted for share dividends, splits, combinations, recapitalizations or similar events, and plus all accrued or declared but unpaid dividends thereon (the “Preference Amount”).
In the event insufficient funds are available to pay in full the Preference Amount in respect of each preferred shareholders, the sequence of liquidation right of all series of Preferred Shares was as follows: (1) the holders of the Series D Preferred Shares; (2) the holders of the Series C Preferred Shares; (3) the holders of the Series B Preferred Shares; and (4) the holders of the Series A Preferred Share. After the full liquidation Preference Amount on all issued and outstanding Preferred Shares has been paid, any remaining funds or assets of the Company legally available for distribution to shareholders shall be distributed pro rata among the holders of the Preferred Shares (on an as-converted basis) together with the holders of the ordinary shares.
A liquidation event includes, (i) any liquidation, dissolution or winding up of the Company, whether voluntary or involuntary; (ii) any sale, transfer, license, lease or other disposition of all or substantially all of the assets of the Group to a third party unaffiliated with any member of the Group; or (iii) merger or consolidation, scheme of arrangement or other similar transaction (including, without limitation, an acquisition by way of a share purchase) in which a majority of the outstanding voting power of the Company is transferred.
11. CONVERTIBLE PREFERRED SHARES (Continued)
The Company classified the Preferred Shares as mezzanine equity as these convertible preferred shares are redeemable upon the occurrence of a conditional event (i.e. a liquidation event). The holders of the Preferred Shares have a liquidation preference and will not receive the same form of consideration upon the occurrence of the conditional event as the ordinary shareholders would. The holders of Preferred Shares have the ability to convert the instrument into the Company’s ordinary shares. The conversion option of the convertible preferred shares do not qualify for bifurcation accounting because the conversion option is clearly and closely related to the host instrument and the underlying ordinary shares are not publicly traded nor readily convertible into cash.
The Group has determined that there was no beneficial conversion feature (“BCF”) attributable to the Preferred Shares, as the effective conversion price was not less than the fair value of the ordinary shares on the respective commitment date. The Group re-evaluates whether additional BCF is required to be recorded upon the modification to the effective conversion price of the Preferred Shares and determined that there was no BCF.
12. ORDINARY SHARES
As of December 31, 2017, the authorized shares consist of 72,000,000 Class A ordinary shares, 839,209,895 Class B ordinary shares, and 13,671,109 Class C ordinary shares. The Class A ordinary shares are issuable only to the Founders. The Class B ordinary shares are issuable to preferred shareholders upon conversion of the preferred shares. The Class C ordinary shares are issuable to option holders upon exercise of the share options. Each Class A ordinary Share is entitled to twenty (20) votes while each Class B or Class C ordinary share or preferred share is entitled to one vote on all matters subject to vote at general meetings of the Company.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
In 2015 and 2016, the Group issued total of 1,607,901 Class C Ordinary Shares to Gold Prized Investment Limited (‘‘Gold Prized’’) to establish a reserve pool for future issuance of equity share incentive to the Group’s employees. While these ordinary shares were legally issued to Gold Prized, the voting rights and associated economic rights remained with the Group. As such, none of these ordinary shares were considered to be granted under the incentive plan, and the Company accounted for these shares as issued but not outstanding. In June 2018, Gold Prized irrevocably surrendered these 1,607,901 Class C ordinary shares (“Surrendered Shares”) registered under its name to the Group resulting in the cancellation of the related subscription receivable of RMB2,200, which has no effect on the Group’s total (deficit) equity amount. The Surrendered Shares were cancelled with effect from June 2018 and the Company reserved those shares in the authorized share capital to be issued pursuant to the Plan.
In September 2018, with the effective of the revised Articles of Association, the Company’s authorized share capital was changed to US$50 divided into 1,000,000,000 shares comprising (i) 800,000,000 Class A ordinary shares of a par value of US$0.00005 each, (ii) 72,000,000 Class B ordinary shares of a par value of US$0.00005 each and (iii) 128,000,000 shares of a par value of US$0.00005 each of such class or classes as Company’s board of directors may determine. All 72,000,000 issued and outstanding Class A ordinary shares beneficially owned by Dr. Gang Yu and Mr. Junling Liu were re-designated as Class B ordinary shares, and all other issued and outstanding shares were re-designated as Class A ordinary shares. Each Class A ordinary share entitles the holder to one vote, and each Class B ordinary share entitles the holder to fifteen votes on all matters subject to the vote at general meetings of the Company. .
In September 2018, 75,118,996 preferred shares were converted into Class A ordinary shares and the Company issued 15,969,110 Class A ordinary shares with the completion of the IPO.
13. NET REVENUE
Disaggregation of revenues
All of the Group’s revenues for the years ended December 31, 2016, 2017 and 2018 were generated within the PRC. The following table illustrates the disaggregation of the Group’s revenue streams by type of customers and nature of services the Group offered:
|
|
|
Years Ended December 31, |
| ||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
|
Product Revenues |
|
870,361 |
|
949,217 |
|
1,770,227 |
|
|
B2C Business |
|
870,361 |
|
862,327 |
|
847,476 |
|
|
B2B Business |
|
— |
|
86,890 |
|
922,751 |
|
|
Service Revenues |
|
3,476 |
|
10,269 |
|
15,743 |
|
|
MP Service |
|
2,472 |
|
8,767 |
|
12,375 |
|
|
Other Services |
|
1,004 |
|
1,502 |
|
3,368 |
|
|
Total |
|
873,837 |
|
959,486 |
|
1,785,970 |
|
Contract balance
The typical contract term of MP service is no more than one year and the remaining unsatisfied performance obligation as of December 31, 2017 and 2018 was insignificant.
In some arrangements from which product revenue is generated, the Group receives advance payments from consumers and pharmacy customers before the product is delivered, which is recorded as advance from customers included in the accrued expenses and other current liabilities on the consolidated balance sheet. The movements of the Group’s accounts receivable and advances from customers are as follows:
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
|
|
|
Accounts |
|
Advances from |
|
|
Opening Balance as of January 1, 2017 |
|
28,388 |
|
6,015 |
|
|
(Decrease)/increase, net |
|
(7,990 |
) |
5,707 |
|
|
Ending Balance as of December 31, 2017 |
|
20,398 |
|
11,722 |
|
|
Increase/(decrease), net |
|
8,171 |
|
3,767 |
|
|
Ending Balance as of December 31, 2018 |
|
28,569 |
|
15,489 |
|
Revenue amounted RMB6,015 and RMB11,722 were recognized in the years ended December 31, 2017 and 2018, respectively that were included in the balance of advance from customers at the beginning of the each year.
14. SHARE-BASED COMPENSATION
Employee Share options:
During the years ended December 31, 2016, 2017 and 2018, options to purchase 1,801,900, 2,303,900 shares and 5,296,204 shares respectively, were granted to the Group’s employees. The weighted-average grant-date exercise price of the options granted to employees in 2016, 2017 and 2018 was US$1.73, US$1.84 and US$ 2.17 per share, respectively. The options granted have a contractual term of 10 years and generally vest over a four-year period, with two typical vesting schedules: (1) 40% of the awards vesting one year after the grant date, with the remaining 60% of the awards vesting evenly on an annual basis over the 3 years thereafter; or (2) 25% of the awards vesting on the anniversary of the grant date each year.
The Black Scholes model was applied in determining the estimated fair value of the options granted. The model requires the input of highly subjective assumptions. The following table presents the assumptions used to estimate the fair values of the share options granted for the years ended December 31, 2016, 2017 and 2018:
|
|
|
2016 |
|
2017 |
|
2018 |
|
Risk-free rate of return |
|
0.65%~1.20% |
|
1.31%~1.76% |
|
2.01%~2.63% |
|
Contractual life of option |
|
10 years |
|
10 years |
|
10 years |
|
Estimated volatility rate |
|
20%~23% |
|
25% |
|
27%~38% |
|
Dividend yield |
|
Nil |
|
Nil |
|
Nil |
|
Fair value per ordinary share |
|
US$3.67~$4.60 |
|
US$5.89~$7.30 |
|
US$3.00~$9.51 |
The weighted-average grant-date fair value of the options granted in 2016, 2017 and 2018 is US$1.93, US$3.98 and US$5.69 per share, respectively.
A summary of employee option activity under the Plan during the years ended December 31, 2017 and 2018 is presented below:
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
|
|
|
Number of |
|
Weighted |
|
Weighted |
|
Aggregate |
|
|
|
|
|
|
US$ |
|
Years |
|
US$ |
|
|
Outstanding at January 1, 2016 |
|
2,005,000 |
|
0.43 |
|
|
|
|
|
|
Granted |
|
1,801,900 |
|
1.73 |
|
|
|
|
|
|
Forfeited |
|
(345,250 |
) |
1.51 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at January 1, 2017 |
|
3,461,650 |
|
1.00 |
|
8.46 |
|
3,843 |
|
|
Granted |
|
2,303,900 |
|
1.84 |
|
|
|
|
|
|
Forfeited |
|
(1,866,225 |
) |
1.56 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2017 |
|
3,899,325 |
|
1.22 |
|
7.98 |
|
7,970 |
|
|
Granted |
|
5,296,204 |
|
2.17 |
|
|
|
|
|
|
Forfeited |
|
(304,900 |
) |
1.96 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2018 |
|
8,890,629 |
|
1.76 |
|
8.28 |
|
36,888 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Vested and Exercisable as of December 31, 2018 |
|
2,399,663 |
|
0.79 |
|
6.21 |
|
2,743 |
|
|
Vested or expected to vest as of December 31, 2018 |
|
8,890,629 |
|
1.76 |
|
8.28 |
|
36,888 |
|
14. SHARE-BASED COMPENSATION (Continued)
Non- Employee Share options:
At January 1, 2016, options to purchase 1,035,962 shares were outstanding with weighted average exercise price of US$ 0.98 and 995,962 options have vested. During the years ended December 31, 2016, 2017 and 2018, options to purchase 50,000 shares, 25,625 shares and 35,000 shares respectively, were issued to the individual advisors who are non-employees of the Group, all with an exercise price of US$1.99 and 40,000 options were forfeited. The options were issued in payment for their consultation services which was expected to be performed over 4 years from the date of issue. As services are performed, 25% of the awards vest on the anniversary of the grant date each year. The estimated fair value of the awards were determined using the Black Scholes model and assumptions disclosed above. As of December 31, 2018, 1,106,587 options were outstanding of which, 1,039,087 have vested and exercisable and the remainder are expected to vest.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
Share-based compensation for all share options:
The Group recorded share based compensation expense of RMB3,438, RMB9,921 and RMB51,359 for the years ended December 31, 2016, 2017 and 2018, respectively, which were classified in the accompanying consolidated statements of operations as follows:
|
|
|
Years Ended December 31, |
| ||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
|
General and administrative expenses |
|
1,846 |
|
5,176 |
|
22,477 |
|
|
Selling and marketing expenses |
|
1,382 |
|
3,674 |
|
23,561 |
|
|
Technology and content expenses |
|
210 |
|
1,071 |
|
5,321 |
|
|
Total |
|
3,438 |
|
9,921 |
|
51,359 |
|
As of December 31, 2018, there was RMB 183,086 of total unrecognized compensation expense related to unvested share options. That cost is expected to be recognized over a weighted-average period of 2.7 years.
15. LOSS PER SHARE
The following table sets forth the computation of basic and diluted loss per share for the years indicated:
|
|
|
Years Ended December 31, |
| ||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to ordinary shareholders |
|
(417,962 |
) |
(248,580 |
) |
(380,091 |
) |
|
Weighted average number of ordinary shares-basic and diluted |
|
72,000,000 |
|
72,000,000 |
|
99,451,210 |
|
|
|
|
|
|
|
|
|
|
|
Net loss per share-basic and diluted |
|
(5.81 |
) |
(3.45 |
) |
(3.82 |
) |
The Group has determined that its convertible Preferred Shares are participating securities as the Preferred Shares participate in undistributed earnings on an as-if-converted basis. The holders of the Preferred Shares are entitled to receive dividends on a pro rata basis, as if their shares had been converted into ordinary shares. Accordingly, the Group uses the two-class method of computing net earnings per share, for ordinary and Preferred Shares according to participation rights in undistributed earnings. However, undistributed net loss is only allocated to ordinary shareholders because holders of Preferred Shares are not contractually obligated to share losses.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
15. LOSS PER SHARE (Continued)
As a result of the Group’s net loss for the three years ended December 31, 2016, 2017 and 2018, Series A, B, C and D Preferred Shares and share options outstanding in the respective periods were excluded from the calculation of diluted loss per share as their inclusion would have been anti-dilutive.
|
|
|
Years Ended December 31, |
| ||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
|
Series A Preferred Shares |
|
4,200,000 |
|
4,200,000 |
|
— |
|
|
Series B Preferred Shares |
|
11,396,178 |
|
11,396,178 |
|
— |
|
|
Series C Preferred Shares |
|
31,739,234 |
|
31,739,234 |
|
— |
|
|
Series D Preferred Shares |
|
27,783,584 |
|
27,783,584 |
|
— |
|
|
Shares options |
|
4,547,612 |
|
5,010,912 |
|
9,997,216 |
|
16. INCOME TAXES
Cayman Islands
Under the current laws of the Cayman Islands, the Company is not subject to tax on income or capital gain.
Hong Kong
Yao Wang is subject to Hong Kong profit tax at a rate of 16.5%. No Hong Kong profit tax has been provided as the Group has not had assessable profit that was earned in or derived from Hong Kong during the years presented.
PRC
Under the Law of the People’s Republic of China on Enterprise Income Tax (“EIT Law”), domestically-owned enterprises and foreign-invested enterprises are subject to a uniform tax rate of 25%.
Except a subsidiary that is subject to RMB8 income taxes, there is no provision for income taxes because the Company and all of its owned subsidiaries are in cumulative loss positions for all the periods presented.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
16. INCOME TAXES (Continued)
A reconciliation between the effective income tax rate and the PRC statutory income tax rate is as follows:
|
|
|
Years Ended December 31, |
| ||
|
|
|
2017 |
|
2018 |
|
|
PRC statutory tax rate |
|
25 |
% |
25 |
% |
|
Tax effect of other expenses that are not deductible in determining taxable profit |
|
(3 |
)% |
(9 |
)% |
|
|
|
|
|
|
|
|
Effect of change in valuation allowance |
|
(22 |
)% |
(16 |
)% |
|
Effective tax rate |
|
0 |
% |
0 |
% |
The principal components of the Group’s deferred income tax assets and liabilities as of December 31, 2017 and 2018 are as follows:
|
|
|
As of December 31, |
| ||
|
|
|
2017 |
|
2018 |
|
|
Deferred tax assets: |
|
|
|
|
|
|
Net loss carryforward |
|
201,614 |
|
256,850 |
|
|
Deductible advertising expense |
|
14,786 |
|
17,964 |
|
|
Accrued expenses and payroll payable |
|
8,985 |
|
12,399 |
|
|
Others |
|
11 |
|
88 |
|
|
Valuation allowance |
|
(225,396 |
) |
(287,301 |
) |
|
Total deferred tax assets |
|
— |
|
— |
|
|
Deferred tax liabilities: |
|
|
|
|
|
|
Total deferred tax liabilities |
|
— |
|
— |
|
As of December 31, 2017 and 2018, valuation allowance of RMB225,396 and RMB287,301 was provided, respectively. The Group considers positive and negative evidence to determine whether some portion or all of the deferred tax assets will more likely than not be realized. This assessment considers, among other matters, the nature, frequency and severity of recent losses, forecasts of future profitability, the duration of statutory carryforward periods, the Group’s experience with tax attributes expiring unused and tax planning alternatives. Valuation allowances have been established for deferred tax assets based on a more likely than not threshold. The Group’s ability to realize deferred tax assets depends on its ability to generate sufficient taxable income within the carryforward periods provided for in the tax law.
As of December 31, 2018, the Group had tax loss carryforwards of RMB 1,027,400 which will expire between 2019 and 2023 if not used.
The Group determines whether or not a tax position is “more-likely-than-not” of being sustained upon audit based solely on the technical merits of the position. The Group does not anticipate any significant changes to its liability for unrecognized tax benefits within the next 12 months.
According to the PRC Tax Administration and Collection Law, the statute of limitations is three years if the underpayment of income taxes is due to computational errors made by the taxpayer. The statute of limitations will be extended to five years under special circumstances, which are not clearly defined, but an underpayment of income tax liability exceeding RMB100 is specifically listed as a special circumstance. In the case of a transfer pricing related adjustment, the statute of limitations is ten years. There is no statute of limitations in the case of tax evasion. The Group’s PRC subsidiaries are therefore subject to examination by the PRC tax authorities from 2013 through 2018 on non-transfer pricing matters, and from 2009 through 2018 on transfer pricing matters.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
17. RELATED PARTY TRANSACTIONS
The table below sets forth the related party and its relationship with the Group
|
Name of related party |
|
Relationship with the Group |
|
Zhejiang Youzhan Information Technology Co., Ltd. |
|
Entity controlled by Chief Operating Officers of the Group |
In September 2018, the Group purchased electronic equipment RMB157 from Zhejiang Youzhan Information Technology Co., Ltd., and there were no similar purchases occurred in 2017 and 2016.
The following table presents amounts owed from related parties as of December 31, 2017 and 2018:
|
|
|
Years Ended December 31, |
| ||
|
|
|
2017 |
|
2018 |
|
|
Accrued expenses and other current liabilities |
|
— |
|
157 |
|
18. MAINLAND CHINA CONTRIBUTION PLAN
Full time employees of the Group in the PRC participate in a government-mandated defined contribution plan pursuant to which certain pension benefits, medical care, unemployment insurance, employee housing fund and other welfare benefits are provided to employees. PRC labor regulations require the Group to accrue for these benefits based on a certain percentage of the employees’ salaries. The total contribution for such employee benefits were RMB36,305, RMB31,500 and RMB44,598 for the years ended December 31, 2016, 2017 and 2018, respectively. The Group has no ongoing obligation to its employees subsequent to its contributions to the PRC plan.
19. RESTRICTED NET ASSETS
Pursuant to laws applicable to entities incorporated in the PRC, the subsidiaries of the Group in the PRC must make appropriations from after-tax profit to non-distributable reserve funds. These reserve funds include one or more of the following: (i) a general reserve, (ii) an enterprise expansion fund and (iii) a staff bonus and welfare fund. Subject to certain cumulative limits, the general reserve fund requires annual appropriation of 10% of after tax profit (as determined under accounting principles generally accepted in the PRC at each year-end) until the accumulative amount of such reserve fund reaches 50% of their registered capital; the other fund appropriations are at the subsidiaries’ discretion. These reserve funds can only be used for the specific purposes of offsetting future losses, enterprise expansion and staff bonus and welfare and are not distributable as cash dividends. During the years ended December 31, 2016 2017 and 2018, no appropriation to statutory reserves was made because the PRC subsidiaries had substantial losses during such periods. In addition, due to restrictions on the distribution of share capital from the Company’s PRC subsidiaries, the PRC subsidiaries share capital of RMB 1,152,821 and RMB 1,531,183 at December 31, 2017 and 2018 is considered restricted, which are not available for distribution to the Company by its PRC subsidiaries in the form of dividends, loans or advances.
111, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2016, 2017 and 2018
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
20. COMMITMENTS AND CONTINGENCIES
(a) Operating lease commitments
The Group has entered into lease agreements for certain offices and warehouses which it operates. Such leases are classified as operating leases. The Group’s lease expenses for the years ended December 31, 2016, 2017 and 2018 were RMB24,038, RMB23,871 and RMB27,089 respectively.
Future minimum lease payments under non-cancellable operating lease agreements at December 31, 2018 were as follows:
|
Years Ending December 31, |
|
|
|
|
2019 |
|
30,443 |
|
|
2020 |
|
27,496 |
|
|
2021 |
|
14,322 |
|
|
2022 |
|
8,299 |
|
|
2023 |
|
7,835 |
|
|
Thereafter |
|
16,509 |
|
|
Total |
|
104,904 |
|
(b) Contingencies
The Group is subject to periodic legal or administrative proceedings in the ordinary course of its business. The Group does not believe that any currently pending legal or administrative proceeding to which the Group is a party will have a material adverse effect on the financial statements.
21. CREDIT FACILITY
On December 28, 2018, certain subsidiaries of the Group entered into a credit agreement with ZheShang Bank to provide a revolving credit facility that allows the Company to borrow up to RMB 475,000 for working capital purpose. Any advances drawn on the credit facility will incur interest at one-year loan prime rate plus 10% and mature within 6 months. As of December 31, 2018, no amounts have been drawn on the line of credit facility.
ADDITIONAL FINANCIAL INFORMATION — FINANCIAL STATEMENTS SCHEDULE I
111, INC.
FINANCIAL INFORMATION FOR PARENT COMPANY
BALANCE SHEETS
(Amounts in thousands, except for share data and per share data, unless otherwise stated)
|
|
|
2017 |
|
2018 |
|
2018 |
|
|
|
|
RMB |
|
RMB |
|
US$ |
|
|
|
|
|
|
|
|
(Note 2 (ad)) |
|
|
ASSETS |
|
|
|
|
|
|
|
|
Current assets: |
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
1,954 |
|
262,859 |
|
38,231 |
|
|
Short-term investments |
|
132,880 |
|
115,815 |
|
16,845 |
|
|
Prepayments and other current assets |
|
34,131 |
|
538,543 |
|
78,328 |
|
|
Total current assets |
|
168,965 |
|
917,217 |
|
133,404 |
|
|
Long-term investments |
|
391,823 |
|
317,478 |
|
46,174 |
|
|
Total assets |
|
560,788 |
|
1,234,695 |
|
179,578 |
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND EQUITY |
|
|
|
|
|
|
|
|
Other current liabilities |
|
— |
|
2,284 |
|
332 |
|
|
Other non-current liabilities |
|
— |
|
8,135 |
|
1,183 |
|
|
Total liabilities |
|
— |
|
10,419 |
|
1,515 |
|
|
|
|
|
|
|
|
|
|
|
MEZZANINE EQUITY |
|
|
|
|
|
|
|
|
Series A convertible preferred shares, $0.00005 par value; 4,200,000 and nil shares authorized, issued, and outstanding as of December 31, 2017 and 2018, respectively |
|
12,922 |
|
— |
|
— |
|
|
Series B convertible preferred shares, $0.00005 par value; 11,396,178 and nil shares authorized, issued, and outstanding as of December 31, 2017 and 2018, respectively |
|
57,980 |
|
— |
|
— |
|
|
Series C convertible preferred shares, $0.00005 par value; 31,739,234 and nil shares authorized, issued, and outstanding as of December 31, 2017 and 2018, respectively |
|
450,324 |
|
— |
|
— |
|
|
Series D convertible preferred shares, $0.00005 par value; 27,783,584 and nil shares authorized, issued, and outstanding as of December 31, 2017 and 2018, respectively |
|
1,263,523 |
|
— |
|
— |
|
|
Subscription receivable of Series D convertible Preferred Shares |
|
(277,819 |
) |
— |
|
— |
|
|
Total Mezzanine equity |
|
1,506,930 |
|
— |
|
— |
|
|
|
|
|
|
|
|
|
|
|
SHAREHOLDERS’ (DEFICIT) EQUITY |
|
|
|
|
|
|
|
|
Ordinary shares Class A ($0.00005 par value per share; 72,000,000 and 800,000,000 shares authorized, 72,000,000 and 91,088,106 shares issued and outstanding as of December 31, 2017 and 2018, respectively) |
|
25 |
|
29 |
|
4 |
|
|
Ordinary shares Class B ($0.00005 par value per share; 839,209,895 and 72,000,000 shares authorized, nil and 72,000,000 issued and outstanding as of December 31, 2017 and 2018, respectively) |
|
0 |
|
25 |
|
4 |
|
|
Ordinary shares Class C ($0.00005 par value per share; 13,671,109 and nil shares authorized, 1,607,901 and nil shares issued, , nil shares outstanding as of December 31, 2017 and 2018, respectively) |
|
0 |
|
— |
|
— |
|
|
Subscription receivable |
|
(2,200 |
) |
— |
|
— |
|
|
Additional paid-in capital |
|
12,121 |
|
2,540,878 |
|
369,555 |
|
|
Accumulated deficit |
|
(1,003,638 |
) |
(1,383,729 |
) |
(201,255 |
) |
|
Accumulated other comprehensive income |
|
47,550 |
|
67,073 |
|
9,755 |
|
|
Total shareholders’ (deficit) equity |
|
(946,142 |
) |
1,224,276 |
|
178,063 |
|
|
Total liabilities, mezzanine equity and (deficit) equity |
|
560,788 |
|
1,234,695 |
|
179,578 |
|
ADDITIONAL FINANCIAL INFORMATION — FINANCIAL STATEMENTS SCHEDULE I
111, INC.
FINANCIAL INFORMATION FOR PARENT COMPANY
STATEMENTS OF COMPREHENSIVE LOSS
(Amounts in thousands, unless otherwise stated)
|
|
|
Years Ended December 31, |
| ||||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
2018 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ (Note 2 (ad)) |
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|
General and administrative expenses |
|
(1,244 |
) |
(834 |
) |
(2,145 |
) |
(312 |
) |
|
Interest income (expense), net |
|
— |
|
— |
|
371 |
|
54 |
|
|
Other operating income (expense), net |
|
— |
|
— |
|
31 |
|
5 |
|
|
Other income |
|
— |
|
— |
|
3,253 |
|
473 |
|
|
(loss) Income before tax |
|
(1,244 |
) |
(834 |
) |
1,510 |
|
220 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of loss of subsidiaries and VIEs |
|
(361,437 |
) |
(247,746 |
) |
(381,601 |
) |
(55,501 |
) |
|
Net loss |
|
(362,681 |
) |
(248,580 |
) |
(380,091 |
) |
(55,281 |
) |
|
Deemed dividend on Series D convertible preferred shares |
|
(55,281 |
) |
— |
|
— |
|
— |
|
|
Net loss attributable to ordinary shareholders |
|
(417,962 |
) |
(248,580 |
) |
(380,091 |
) |
(55,281 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income (loss) |
|
|
|
|
|
|
|
|
|
|
Unrealized securities holding gains (loss), net of tax of nil for 2017 and 2018 |
|
234 |
|
2,196 |
|
(286 |
) |
(42 |
) |
|
Realized securities holding (gains), net of tax of nil for 2017 and 2018 |
|
— |
|
— |
|
(399 |
) |
(58 |
) |
|
Foreign currency translation adjustments, net of tax of nil for 2017 and 2018 |
|
39,832 |
|
(21,347 |
) |
21,658 |
|
3,150 |
|
|
Unrealized securities holding gains of subsidiaries and VIEs, net of tax of nil for 2017 and 2018 |
|
1,181 |
|
1,831 |
|
9,020 |
|
1,312 |
|
|
Realized securities holding (gains) of subsidiaries and VIEs, net of tax of nil for 2017 and 2018 |
|
— |
|
— |
|
(10,470 |
) |
(1,523 |
) |
|
Comprehensive loss |
|
(376,715 |
) |
(265,900 |
) |
(360,568 |
) |
(52,442 |
) |
ADDITIONAL FINANCIAL INFORMATION — FINANCIAL STATEMENTS SCHEDULE I
111, INC.
FINANCIAL INFORMATION FOR PARENT COMPANY
STATEMENTS OF CASH FLOWS
(Amounts in thousands, unless otherwise stated)
|
|
|
Years Ended December 31, |
| ||||||
|
|
|
2016 |
|
2017 |
|
2018 |
|
2018 |
|
|
|
|
RMB |
|
RMB |
|
RMB |
|
US$ |
|
|
Operating activities: |
|
|
|
|
|
|
|
|
|
|
Net loss |
|
(362,681 |
) |
(248,580 |
) |
(380,091 |
) |
(55,281 |
) |
|
Adjustments to reconcile net income to net cash (used in) provided by operating activities: |
|
|
|
|
|
|
|
|
|
|
Share of loss of subsidiaries and VIEs |
|
361,437 |
|
247,746 |
|
381,601 |
|
55,501 |
|
|
Other current liabilities |
|
— |
|
— |
|
2,284 |
|
332 |
|
|
Other non-current liabilities |
|
|
|
|
|
8,135 |
|
1,183 |
|
|
Investment income |
|
|
|
|
|
(2,591 |
) |
(377 |
) |
|
Net Cash (used in) provided by operating activities |
|
(1,244 |
) |
(834 |
) |
9,338 |
|
1,358 |
|
|
Investing activities: |
|
|
|
|
|
|
|
|
|
|
Purchase of long-term investments |
|
(531,374 |
) |
— |
|
(821,962 |
) |
(119,549 |
) |
|
Purchase of short-term investments |
|
(138,974 |
) |
— |
|
— |
|
— |
|
|
Proceeds from sale or maturity of short-term investments |
|
— |
|
— |
|
25,160 |
|
3,659 |
|
|
Net cash used in investing activities |
|
(670,348 |
) |
— |
|
(796,802 |
) |
(115,890 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Financing activities: |
|
|
|
|
|
|
|
|
|
|
Proceeds from ordinary shareholders |
|
1 |
|
25 |
|
— |
|
— |
|
|
Proceeds from IPO, net of issuance cost |
|
— |
|
— |
|
694,878 |
|
101,066 |
|
|
Proceeds of preferred shareholders |
|
12,956 |
|
— |
|
277,819 |
|
40,407 |
|
|
Net cash provided by financing activities |
|
12,957 |
|
25 |
|
972,697 |
|
141,473 |
|
|
Effect of exchange rate changes on cash and cash equivalents |
|
22,970 |
|
(147 |
) |
75,672 |
|
11,006 |
|
|
Net (decrease)/increase in cash and cash equivalents |
|
(635,665 |
) |
(956 |
) |
260,905 |
|
37,947 |
|
|
Cash and cash equivalents at the beginning of the year |
|
638,575 |
|
2,910 |
|
1,954 |
|
284 |
|
|
Cash and cash equivalents at the end of the year |
|
2,910 |
|
1,954 |
|
262,859 |
|
38,231 |
|
ADDITIONAL FINANCIAL INFORMATION — FINANCIAL STATEMENTS SCHEDULE I
111, INC.
FINANCIAL INFORMATION FOR PARENT COMPANY
Note to Schedule I
Schedule I has been provided pursuant to the requirements of Rule 12-04(a) and 5-04-(c) of Regulation S-X, which require condensed financial information as to the financial position, change in financial position and results of operations of a parent company as of the same dates and for the same periods for which audited consolidated financial statements have been presented when the restricted net assets of consolidated subsidiaries exceed 25 percent of consolidated net assets as of the end of the most recently completed fiscal year.
The condensed financial information has been prepared using the same accounting policies as set out in the accompanying consolidated financial statements except that the equity method has been used to account for investments in its subsidiaries. Such investments in subsidiaries are presented on the balance sheets as investment in subsidiaries and the profit of the subsidiaries is presented as income in investment in subsidiaries.
Certain information and footnote disclosures normally included in financial statements prepared in accordance with accounting principles generally accepted in the United States of America have been condensed or omitted. The footnote disclosures contain supplemental information relating to the operations of the Company and, as such, these statements should be read in conjunction with the notes to the accompanying consolidated financial statements.
As of December 31, 2018, there are no material contingencies, mandatory dividend, significant provisions for long-term obligations or guarantees of the Company, except for those which have separately disclosed in the consolidated financial statements.