UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
For the fiscal year ended
or
For the transition period from __________ to __________
Commission file number:

(Exact name of registrant as specified in its charter)
| (State or Other Jurisdiction of Incorporation or Organization) | (I.R.S. Employer Identification Number) |
(Address of principal executive offices)
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a
well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the registrant is not
required to file reports pursuant to Section 13 or 15(d) of the Exchange Act. Yes ☐
Indicate by check mark whether the registrant
(1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the past 12 months
(or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements
for the past 90 days.
Indicate by check mark whether the registrant
has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405
of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| ☒ | Smaller reporting company | ||
| Emerging growth company |
If an emerging growth company, indicate by check
mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting
standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant
has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial
reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or
issued its audit report.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate by check mark whether the registrant
is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No
State the aggregate market value of voting and
non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average
bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal
quarter: $
The
number of shares of the registrant’s common stock outstanding as of March 21,
2023 was
DOCUMENTS INCORPORATED BY REFERENCE
None.
TABLE OF CONTENTS
i
CAUTIONARY NOTICE
This annual report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Those forward-looking statements include our expectations, beliefs, intentions and strategies regarding the future.
These and other factors that may affect our financial results are discussed more fully in “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in this report. Moreover, we operate in a very competitive and rapidly changing environment, and new risks emerge from time to time. It is not possible for us to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this report may not occur and actual results could differ materially and adversely from those anticipated or implied in our forward-looking statements. Although we believe that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. Moreover, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. We caution readers not to place undue reliance on any forward-looking statements. We do not undertake, and specifically disclaim any obligation, to update or revise such statements to reflect new circumstances or unanticipated events as they occur, and we urge readers to review and consider disclosures we make in this and other reports that discuss factors germane to our business. See in particular our reports on Forms 10-K, 10-Q, and 8-K subsequently filed from time to time with the Securities and Exchange Commission.
ii
RISK FACTOR SUMMARY
Our business is subject to numerous risks and uncertainties, including those described in “Risk Factors” in this Annual Report on Form 10-K. These risks include, but are not limited to the following:
| ● | We are a clinical-stage biopharmaceutical company with limited operating history. |
| ● | We have a history of significant operating losses and anticipate continued operating losses for the foreseeable future. |
| ● | We expect we will need additional financing to execute our business plan and fund operations, which additional financing may not be available on reasonable terms or at all. | |
| ● | The report of our independent registered public accounting firm for the year ended December 31, 2022 states that due to our lack of revenue from commercial operations, significant losses and need for additional capital there is substantial doubt about our ability to continue as a going concern. |
| ● | Our business model is entirely dependent on certain patent rights licensed to us from the University of Texas at Austin, and the loss of those license rights would, in all likelihood, cause our business, as presently contemplated, to fail. |
| ● | Our business model includes, in part, the licensing of our TFF Platform to other pharmaceutical companies, however technology licensing in the pharmaceutical industry is a lengthy process and subject to several risks and factors outside of our control, and we cannot forecast our ability to successfully license our technology or the length of time it may take to establish a new licensing relationship. |
| ● | Our business may be adversely affected by the COVID-19 pandemic. |
| ● | We will be completely dependent on third parties to manufacture our product candidates for clinical and commercial purposes, and the commercialization of our product candidates could be halted, delayed or made less profitable if those third parties fail to obtain manufacturing approval from the FDA or comparable foreign regulatory authorities fail to provide us with sufficient quantities of our product candidates or fail to do so at acceptable quality levels or prices. |
| ● | If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates. |
| ● | Our business operations could suffer in the event of information technology systems’ failures or security breaches. |
| ● | Our success is entirely dependent on our ability to obtain the marketing approval for our product candidates by the FDA and the regulatory authorities in foreign jurisdictions in which we intend to market our product candidates, of which there can be no assurance. |
| ● | Clinical testing is expensive, is difficult to design and implement, can take many years to complete and is uncertain as to outcome. |
| ● | Even if we receive regulatory approval for any of our product candidates, we may not be able to successfully commercialize the product and the revenue that we generate from its sales, if any, may be limited. |
| ● | Even if we obtain marketing approval for any of our product candidates, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expense. Additionally, our product candidates could be subject to labeling and other restrictions and withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our product candidates. |
| ● | Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions. |
iii
| ● | Even though we may apply for orphan drug designation for a product candidate, we may not be able to obtain orphan drug marketing exclusivity. |
| ● | Current and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain. |
| ● | Any termination or suspension of, or delays in the commencement or completion of, any necessary studies of any of our product candidates for any indications could result in increased costs to us, delay or limit our ability to generate revenue and adversely affect our commercial prospects. |
| ● | Third-party coverage and reimbursement and health care cost containment initiatives and treatment guidelines may constrain our future revenues. |
| ● | It is difficult and costly to protect our intellectual property rights, and we cannot ensure the protection of these rights. |
| ● | Our product candidates may infringe the intellectual property rights of others, which could increase our costs and delay or prevent our development and commercialization efforts. |
| ● | We may be subject to claims that we have wrongfully hired an employee from a competitor or that we or our employees have wrongfully used or disclosed alleged confidential information or trade secrets of their former employers. |
| ● | The market price of our shares may be subject to fluctuation and volatility. You could lose all or part of your investment. |
| ● | If securities or industry analysts do not continue to publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline. |
| ● | Future capital raises may dilute your ownership and/or have other adverse effects on our operations. |
| ● | If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. |
| ● | We may be at an increased risk of securities class action litigation. |
| ● | Our charter documents and Delaware law may inhibit a takeover that stockholders consider favorable. |
| ● | Our certificate of incorporation and amended and restated bylaws designate the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain litigation that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or other employees. |
iv
PART I
Item 1. Business
Background
TFF Pharmaceuticals, Inc. was formed as a Delaware corporation on January 24, 2018 for the purpose of developing and commercializing innovative drug products based on our patented Thin Film Freezing, or TFF, technology platform. Since our formation, we have focused on the development of our initial drug candidates, the establishment of strategic relationships with established pharmaceutical companies for the licensing of our TFF technology platform and the pursuit of additional working capital. We have not commenced revenue-producing operations. Unless otherwise indicated, the terms “TFF Pharmaceuticals,” “Company,” “we,” “us,” and “our” refer to TFF Pharmaceuticals, Inc. and its wholly-owned subsidiaries.
Overview
We are a clinical stage biopharmaceutical company focused on developing and commercializing innovative drug products based on our patented Thin Film Freezing, or TFF, technology platform. Based on our internal and sponsored testing and studies, we believe that our TFF platform can significantly improve the solubility of poorly water-soluble drugs, which make up approximately 40% of marketed pharmaceuticals worldwide, thereby improving the bioavailability and pharmacokinetics of those drugs. We believe that in the case of some new drugs that cannot be developed due to poor water solubility, our TFF platform has the potential to increase the pharmacokinetic effect of the drug to a level allowing for its development and commercialization. When administered as an inhaled dry powder for treatment of lung disorders, we believe the TFF platform formulations can be used to increase efficacy and minimize systemic toxicities and drug-drug interactions.
As of the date of this report, we have two product candidates in clinical trials, TFF Voriconazole Inhalation Powder, or TFF VORI, and TFF Tacrolimus Inhalation Powder, or TFF TAC. To date, we have completed one Phase 1 study in healthy volunteers and one Phase 1b study in patients with asthma exploring the safety, tolerability and pharmacokinetics of TFF VORI. As of the date of this report, a Phase 2 clinical trial of TFF VORI in patients with invasive pulmonary aspergillosis has been initiated. We have also completed one Phase 1 study in healthy volunteers examining the safety, tolerability and pharmacokinetics of TFF TAC. As of the date of this report, a Phase 2 clinical trial of TFF TAC in lung transplant patients has been initiated.
We are also actively engaged in the analysis and testing of dry powder formulations of several drugs and vaccines through parenteral, topical, ocular, pulmonary and nasal applications through feasibility studies and material transfer agreements with U.S. and international pharmaceutical companies and certain government agencies. We intend to initially focus on the development of inhaled dry powder drugs for the treatment of pulmonary diseases and conditions. While the TFF platform was designed to improve solubility of poorly water-soluble drugs generally, the researchers at University of Texas at Austin, or UT, found that the technology was particularly useful in generating dry powder particles with properties which allow for superior inhalation delivery, especially to the deep lung, which is an area of extreme interest in respiratory medicine. We believe that our TFF platform can significantly increase the number of pulmonary drug products that can be delivered directly to the lung. We intend to design our dry powder drug products for use with dry powder inhalers, which are generally considered to be the most effective and patient-friendly of all breath-actuated inhalers. We plan to focus on developing inhaled dry powder formulations of existing off-patent drugs suited for lung diseases and conditions, which we believe includes dozens of potential drug candidates, many of which have a potential market of over $1 billion.
We intend to directly pursue the development of dry powder formulations of off-patent drugs through the U.S. Food and Drug Administration’s, or FDA’s, 505(b)(2) regulatory pathway and in corresponding regulatory paths in other foreign jurisdictions. The 505(b)(2) pathway contains full reports of investigations of safety and effectiveness but at least some of the information required for approval comes from studies not conducted by or for the NDA applicant. 505(b)(2) products have the potential advantage of significantly lower development costs and shorter development timelines versus traditional new molecular entities. The clinical requirements for a 505(b)(2) drug candidate can vary widely from product to product depending primarily on whether the product candidate claims a new indication, provides for a different route of administration or claims improved safety compared to the existing approved product, and may include bioequivalence trials, limited safety and efficacy trials, or full Phase I through III trials. Unless the FDA releases a guidance document, the clinical requirement for a 505(b)(2) product candidate is typically not known until the drug sponsor has a pre-IND and an end of Phase 2 meeting with the FDA. For example, based on our meetings to date with the FDA, we believe we may need to conduct additional clinical trials beyond the current Phase 2 trials for TFF VORI and TFF TAC prior to filing for marketing approval for either product.
1
TFF TAC has been awarded orphan drug status. We also believe that in some cases our other dry powder drug products may qualify for the FDA’s orphan drug status.
We intend to commercialize our TFF platform and internally developed product candidates through the following means:
| ● | We may out-license our internally developed product candidates, such as TFF VORI and TFF TAC, or agree to jointly develop such products with a third-party pharmaceutical company; |
| ● | Upon and subject to receipt of the requisite approvals, we may directly commercialize our internally developed product candidates through a combination of our internal direct sales and third-party marketing and distribution partnerships; and |
| ● | We may pursue the licensing of our TFF platform or a joint development arrangement for a particular field of use with a third-party pharmaceutical company. |
The Problem We Address
Solubility is an issue that all drugs must address. No matter how active or potentially active a new drug is against a particular molecular target, if the drug is not available in solution at the site of action, it is most likely not a viable development candidate. Based on independent third-party studies, 40% of currently marketed drugs and at least 75% of drugs under development have poor water solubility, which can prohibit development since most pharmaceutical companies cannot or will not conduct rigorous preclinical and clinical studies on a molecule that does not have a sufficient pharmacokinetic profile due to poor water solubility. Water solubility can also be an issue for some marketed drugs. Based on independent third-party studies, only two-thirds of the drugs on the World Health Organization, or WHO, Essential Drug List were classified as high solubility. A marketed drug with poor water solubility can show performance limitations, such as incomplete or erratic absorption, poor bioavailability, and slow onset of action. Effectiveness can vary from patient to patient, and there can be a strong effect of food on drug absorption. Finally, it may be necessary to increase the dose of a poorly soluble drug to obtain the efficacy required, which can lead to adverse side effects, toxicity issues and increased costs.
In addition to water solubility issues generally, certain drugs that target lung conditions and diseases have poor solubility that prevents them from being delivered by way of inhalation, especially via a breath-actuated inhaler and can only be given orally or intravenously. Breath-actuated inhalers include dry powder inhalers, metered dose inhalers and some nebulizers. A dry powder inhaler delivers drugs in a dry powder form directly to the lungs by way of a deep, fast breath on the mouth of the inhaler. A metered dose inhaler uses propellant to push medication to the lungs. A nebulizer creates a mist that is breathed into the lungs through a mouthpiece. The dry powder inhaler is generally considered to be the most effective and convenient form of breath-actuated inhaler for all users, other than for those whose severe condition does not allow them to take a sufficiently deep breath.
We believe the primary benefit of a breath-actuated inhaler is its ability to administer a greater portion of the drug dosage directly to the target site. Dosing directly to the lungs has been shown to allow for better effect with fewer adverse events. In addition, it has been shown that dosing directly to the lungs requires a lower dose of drug, compared to delivery by oral or parenteral routes. While breath-actuated inhalers allow for a greater portion of the administered drug to reach the treatment site, which should allow for much smaller dosages compared to oral or intravenous delivery, not all drugs targeting lung conditions and diseases can be formulated for use with a breath-actuated inhaler. We believe there are dozens of off-patent drugs targeting lung conditions and diseases that are currently not eligible for delivery by way of breath-actuated inhalers, many of which have a potential market of over $1 billion. This is the market we intend to initially address through our development of dry powder drugs utilizing our TFF platform.
2
Our Thin Film Freezing Platform
Our development of dry powder drugs is enabled by technology licensed to us by the University of Texas at Austin, or UT. Researchers at UT have developed a technology employing a process called Thin Film Freezing, or TFF. While the TFF platform was designed to improve solubility of poorly water-soluble drugs generally, the researchers at UT found that the technology was particularly useful in generating dry powder particles with properties suitable for inhalation delivery, especially to the deep lung, via dry powder inhaler delivery systems, an area of extreme interest in respiratory medicine. The TFF process results in a “Brittle Matrix Particle,” which exhibits a low bulk density, high surface area, and optionally an amorphous morphology, allowing the particles to supersaturate when contacting the target site, such as lung tissue. The aerodynamic properties of the particles are such that the amount of drug deposited to the deep lung may, in some cases, reach ten times that achieved via the oral route.
The TFF process, outlined in the figures below, involves processing a drug or drugs in a solvent system, and it will often include agents designed to promote dispersion and avoid clumping and excipients to promote adhesion to the target site. The drug solution is then applied to a cryogenic substrate, such as a liquid nitrogen cooled stainless steel drum. When the drug solution contacts the cryogenic surface it vitrifies, or flash freezes, resulting in a “drug ice.” The solvent system is removed by lyophilization, resulting in Brittle Matrix Particles, shown in the photographs below, that are highly porous, large surface area, low-density particles. The process uses industry standard solvents, recognized excipients, a custom-made TFF drum and conventional process equipment.
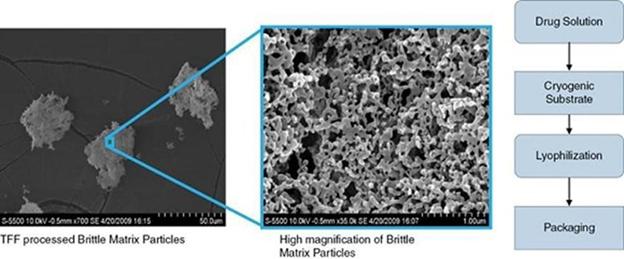
We believe our TFF platform is a breakthrough platform technology for making dry powders from drugs which previously were not candidates for the dry powder inhaler or any breath-actuated inhaler. We believe our TFF technology opens the way for direct-to-lung delivery of dozens of pharmaceuticals, including the reformulation of existing drugs into a more safe and convenient inhaled dry powder product. We believe the technology can be used with molecules of all types and works with existing and off-the-shelf dry powder inhalers without the need for any additional equipment or devices.
We believe our TFF platform presents the following high value opportunities:
| ● | Reformulation of drugs for lung conditions. Today, many drugs intended for lung conditions are only given orally or intravenously due to properties that make them ill-suited for direct delivery by inhalers. Given by these routes, typically only a small fraction of the drug reaches the lungs, and these drugs may cause unwanted and even deadly side effects. We believe that our TFF platform will for the first time allow many of these medications to be formulated into the convenient, direct-to-lung dry powder inhaler format, thereby enhancing efficacy and reducing or eliminating side effects by directly delivering the drug to the target site. |
3
| ● | Biologics. Biopharmaceuticals (or biologics) are by far the fastest growing sector in the pharmaceutical industry today. According to GlobalData, the market for biologics was valued at approximately $430 billion in 2022 and is expected to reach $720 billion by 2027. Biologics are most commonly delivered intravenously, and they can be an especially challenging class of drugs for formulation into a dry powder. We believe our TFF platform is uniquely suited to meet many of the challenges of biologic formulations, and our UT collaborators have demonstrated, via animal model testing and in vitro testing, the effectiveness of the TFF technology to produce dry powder biologics with, in some cases, up to 100% activity retained. We intend to explore dry powder forms of numerous biological drugs, including drugs intended to treat indications other than lung conditions and diseases. We are also pursuing TFF formulations of aluminum salt containing vaccines, which by virtue of providing a dry powder formulation would remove the requirement for liquid suspension and cold chain. | |
| ● | Combination Drugs. Combination drugs are products with two or more active pharmaceutical ingredients. In addition to providing for increased patient compliance with multiple medications, some drugs act synergistically and provide for superior benefit when given as a combination. However, combining pharmaceutical agents can be challenging, especially for inhalation delivery. Our TFF platform has shown the ability to produce fixed dose combinations of many agents in a manner that delivers the drugs simultaneously to the site of action in a precise amount. |
The TFF platform was invented and developed by researchers at University of Texas at Austin, or UT, led by Robert O. Williams, III, Ph.D. UT has granted to us an exclusive worldwide, royalty bearing license to the patent rights for the TFF platform in all fields of use. We continue to work with Dr. Williams and his UT team through a series of Sponsored Research Agreements, or SRAs, with UT. Our SRAs with UT are industry standard sponsored research agreements pursuant to which UT provides to us certain product formulation, characterization and evaluation services regarding potential product candidates incorporating our TFF technology in exchange for our payment of UT’s expenses and reasonable overhead. The services conducted by UT were carried out under the direction of Dr. Williams, who is the principal inventor of the TFF technology. The current SRA expires in July 2025 and is subject to renewal upon mutual agreement of the parties. The SRAs includes customary provisions concerning confidentiality, indemnification and intellectual property rights, including each party’s exclusive ownership of all intellectual property developed solely by them and the parties’ joint ownership of all intellectual property developed jointly. All patented intellectual property rights relating to the TFF technology developed solely or jointly by UT are subject to our patent license agreement with UT and are included among our licensed patent rights. Pursuant to those SRAs, Dr. Williams and his team, together with their labs and collaborators, provide expertise and initial development work, including:
| ● | the preliminary development and in vitro evaluation of our drug candidates; |
| ● | the determination of the key characteristics influencing performance of our product candidates; |
| ● | the determination of the formulation and manufacturing parameters that influence the key characteristics of our product candidates; |
| ● | supply of bulk dry powders for initial good laboratory practice, or GLP, and non-GLP toxicity studies; |
| ● | supportive stability for future GLP and GMP studies; and |
| ● | the evaluation of the in vivo performance of our product candidates in various animal models. |
In June 2022, we established our own laboratory in Austin,Texas where we undertake certain product formulation, characterization and evaluation services with regard to potential product candidates. We established our own laboratory to obtain direct ownership over all intellectual property developed within our laboratory and to address concerns on the part of our partners over potential conflicts with UT.
Our Internal Product Candidates
We intend to initially focus on the development of inhaled dry powder drugs for the treatment of pulmonary diseases and conditions. Our dry powder drug product candidates will be designed for use with dry powder inhalers, which are generally considered to be the most effective of all breath-actuated inhalers. We plan to focus on developing dry powder drugs intended for lung diseases and conditions that are off-patent, which we believe includes dozens of potential drug candidates, many of which have a potential market of over $1 billion. As of the date of this report, we have identified and are focusing on two initial drug candidates and with each we are in the early stages of clinical development.
4
TFF Voriconazole Inhalation Powder, TFF VORI – For the Treatment of Invasive Pulmonary Aspergillosis
We have developed an inhaled dry powder formulation of voriconazole, or TFF VORI, intended to treat invasive pulmonary aspergillosis, or IPA, a severe fungal pulmonary disease with an overall 84-day all-cause mortality rate of approximately 30% despite use of standard of care therapy. IPA occurs primarily in patients with severe immunodeficiency, such as bone marrow and solid organ transplant recipients, and patients with chemotherapy-induced immunodeficiency, hematologic malignancy, or HIV. To date, the approved antifungals used to treat IPA have been delivered orally or intravenously, where doses required to achieve efficacy have been associated with systemic toxicities and drug-drug interaction issues, which places a premium on any formulation that can improve the drugs’ efficacy and/or safety and tolerability. Due to the nature of these drugs, it has not been possible to make formulations for breath-actuated inhalers that might maximize lung concentration while limiting side effects.
Voriconazole is an off-patent, first-line drug for the treatment of IPA. We believe TFF VORI represents an opportunity for the treatment of IPA, which has the potential to put the drug exactly where it is needed (viz., the lung) while minimizing off target toxic effects. Voriconazole is currently marketed in Australia, Canada, Europe and the U.S. as VFEND, and is available in several strengths and presentations for oral delivery or IV infusion. As of the date of this report, the Clinical Practice Guidelines released by the Infectious Diseases Society of America recommend voriconazole as first-line monotherapy for IPA. However, since the registration of VFEND in Europe and the U.S. in 2002, several studies have examined the exposure-response relationship with voriconazole. Those studies have identified a relationship between low voriconazole exposure and higher rates of treatment failure and high voriconazole exposure and higher propensity for neurotoxicity. Studies have also shown that voriconazole delivered orally or intravenously is associated with a high degree of exposure variability. In the case of oral delivery, the high degree of variability can be partly explained by the effect of food as high-fat meals decrease maximum concentrations by 34 to 58%. In addition, voriconazole when delivered orally or intravenously has been shown to have many serious adverse reactions, including hepatic toxicity, arrhythmias and QT prolongation, infusion related reactions, visual disturbances, severe cutaneous adverse reactions, photosensitivity and renal toxicity. Hepatic toxicity, arrythmias and severe cutaneous adverse reactions have been associated with fatalities. These studies confirm that when administered orally or intravenously, voriconazole provides a narrow therapeutic window between treatment success and unacceptable treatment toxicity.
We believe TFF VORI could be used for the treatment or prophylaxis of IPA and would benefit patients by providing the drug at the site of invasive fungal infections, while reducing or eliminating the potential serious side effects and fatal toxicities associated with oral and parenteral voriconazole. We believe the potential enhanced efficacy and/or improved safety and tolerability offered by TFF VORI may decrease the rate of voriconazole treatment failures and the need for later line therapies with their associated toxicities. We also believe that the administration of TFF VORI directly to the lungs will remove the variability in exposures due to the effects of food. In addition, animal and in vitro studies have shown that our TFF prepared dry powder formulation will improve the solubility of voriconazole compared to oral or intravenous delivery. We believe that the combination of improved solubility and direct-to-lung administration of TFF VORI will increase exposures in the lung while decreasing systemic exposures and minimizing systemic toxicities and drug-drug interactions.
To date, we have completed Good-Laboratory Practices (GLP) repeat-dose toxicity studies in rats (a 28-day inhalation toxicity study) and dogs (14-day, 13-week and 26-week inhalation toxicity studies). After a pre-IND meeting and gaining FDA agreement on the 505(b)(2) regulatory pathway, we filed an IND and initiated and completed a Phase 1 study in healthy volunteers and a Phase 1b study in patients with asthma to assess the safety, tolerability and pharmacokinetics of TFF VORI. As of the date of this report, a Phase 2 clinical trial of TFF VORI in patients with invasive pulmonary aspergillosis is underway. Future studies will be planned based on emerging data from this Phase 2 study.
5
TFF Tacrolimus Inhalation Powder, TFF TAC — For Prevention of Lung Transplant Rejection
We have developed TFF TAC, a dry powder formulation of tacrolimus, an immunosuppressive drug used in transplant medicine. Prograf (tacrolimus) is currently the first line calcineurin inhibitor used in the maintenance regimen to prevent rejection after lung transplantation despite its many significant systemic toxicities.
According to product labeling and prescribing information for Prograf, serious and otherwise important adverse drug reactions associated with Prograf include lymphoma and other malignancies, serious infections, new onset diabetes after transplant, nephrotoxicity, neurotoxicity, hyperkalemia, hypertension, anaphylactic reaction after injection, myocardial hypertrophy, pure red cell aplasia, and thrombotic microangiopathy, including hemolytic uremic syndrome and thrombotic thrombocytopenic purpura. Of particular concern is nephrotoxicity, which was reported in approximately 52% of kidney transplantation patients and in 40% and 36% of liver transplantation patients receiving Prograf in the U.S. and European randomized trials, respectively, and in 59% of heart transplantation patients in a European randomized trial.
Tacrolimus is an off-patent drug and we have developed TFF TAC to be used with a dry powder inhaler. Because our dry powder version would provide for a high local lung concentration, it is expected that the oral doses of tacrolimus can be weaned to minimize systemic toxicities while maintaining local lung immune suppression to prevent rejection. We believe our drug candidate may have a high likelihood of success in competing in the immunosuppressant market for lung and heart/lung transplants. TFF TAC has been awarded orphan drug status.
To date, we have completed GLP repeat-dose toxicity studies in rats (3-day and 28-day inhalation toxicity studies) and cynomolgus monkeys (28-day and 26-week inhalation toxicity studies). After a pre-IND meeting with the FDA, we gained FDA agreement on the 505(b)(2) regulatory pathway. We have since completed a Phase 1 study in healthy volunteers to assess the safety, tolerability and pharmacokinetics of TFF TAC. As of the date of this report, a Phase 2 clinical trial of TFF TAC in patients in lung transplant patients is underway. Future studies will be planned based on emerging data from this Phase 2 study.
Other Potential Dry Powder Products
Our business model is to develop proprietary innovative drug product candidates that offer functional or commercial advantages, or both, to currently available alternatives. In our initial evaluation of the market, we have identified a number of potential drug candidates that show promise upon initial assessment, for three of which we have conducted meaningful development activities, including dry powder formulations of:
Vaccines. Vaccines containing aluminum salts make up approximately 35% of all vaccines. Aluminum salts are incorporated into many vaccine formulations as an adjuvant, which is a substance added to vaccines to enhance the immune response of vaccinated individuals. A major limitation with these vaccines is that they are fragile and to maintain their efficacy they must be formulated as liquid suspensions and kept in a cold chain (2–8°C) during transport and storage, which is burdensome and expensive. Also, exposure of the liquid vaccines to either ambient or freezing temperatures will cause a loss of efficacy, including particle aggregation in the case of freezing. Alternatives to cold chain have been examined, including the introduction of stabilizing agents in vaccines to prevent aggregation during freezing and the application of novel freezing and drying techniques; however, we believe that to date none of these techniques have led to an acceptable alternative to cold chain.
We have conducted characterization analyses of certain TFF formulated aluminum salt containing vaccines. Our evaluations suggest that aluminum salt containing vaccines can be successfully converted from liquid suspension into dry powder using our TFF platform and that the dry powder can later be reconstituted at the time of use without causing particle aggregation or a decrease in immunogenicity. In addition, the dry powder vaccine did not aggregate after repeated dry-freezing-and-thawing. We believe that the TFF platform may be used to formulate new vaccines, or to reformulate existing vaccines, that are adjuvanted with aluminum salts into dry vaccine powder without an appreciable decline in immunogenicity.
6
We have engaged pharmaceutical companies in the vaccine space in discussions concerning a potential joint development of TFF formulated aluminum salt containing vaccines. However, we do not intend to pursue the development of our dry powder formulation of aluminum salt containing vaccines beyond performance characterization and efficacy data through early animal testing until such time, if ever, as we obtain a development partner. There can be no assurance, however, that our early testing and development will lead to a commercial dry powder formulation of aluminum salt containing vaccines.
Niclosamide. On August 12, 2020, we entered into a licensing and collaboration agreement with UNION Therapeutics A/S in which UNION acquired an option to obtain a worldwide exclusive license for the TFF technology formulated niclosamide. In the first quarter of 2022, we completed a Phase 1 clinical trial of TFF Niclosamide. We and UNION Therapeutics have not further progressed TFF Niclosamide pending the parties’ further review of the Phase 1 results, animal data, and anti-viral market opportunities. We have conducted independent market research to assess the additional therapeutic and market opportunities for TFF Niclosamide in other serious viral infections.
Augmenta monoclonal antibodies (mAbs). On November 1, 2020, we entered into a joint development and collaboration agreement with Augmenta Bioworks, Inc. pursuant to which the parties agreed to collaborate on the joint development of novel commercial products incorporating Augmenta’s human-derived mAbs for potential COVID-19 therapeutics. Both companies collaborated to conduct pre-clinical evaluations and successfully formulated the active pharmaceutical ingredient. Based on the pre-clinical results in the Omicron COVID-19 variant, however we have suspended further development at this time.
Other Potential Product Candidates. We have identified a number of additional promising drug candidates for dry powder formulation. Many of these potential drug candidates are off-patent drugs for which we would directly pursue the development of a dry powder formulation through the FDA’s 505(b)(2) regulatory pathway. We have not commenced meaningful development activities for any of these product candidates at this time and there can be no assurance that we will pursue any of the product candidates below.
| Candidate | Intervention | Indication | ||
| Rapamycin | Acute Treatment | Lymphangioleiomyomatosis | ||
| Alpha-1-antitrypsin | Chronic Treatment | Alpha-1antitrypsin deficiency | ||
| GM-CSF (filgrastim) | Treatment | Autoimmune pulmonary alveolar proteinosis | ||
| Treprostinil | Treatment | Pulmonary Arterial Hypertension | ||
| Pembrolizumab (Keytruda) | Acute Treatment | Cancer: Non–Small Cell Lung Cancer, Liver, brain, melanoma, metastatic | ||
| Cisplatin | Acute Treatment | Lung or esophageal cancer | ||
| Gemcitabine | Acute Treatment | Lung or esophageal cancer | ||
| Isoniazid/Rifampicin | Acute Treatment | Tuberculosis | ||
| Amphotericin B | Acute Treatment | Antifungal | ||
| Palivizumab | Prophylaxis | Tuberculosis | ||
| Ciprofloxacin | Acute Treatment | Infection | ||
| Tobramycin | Acute Treatment | Infection | ||
| Azithromycin | Acute Treatment | Infection | ||
| Calcium channel blockers | Acute Treatment | Raynaud’s disease | ||
| Sumatriptin | Acute Treatment | Migraine | ||
| Stem cells | Lung remodeling | Pneumococcal pneumonia; cardiomyopathy |
We believe that our TFF technology provides a diverse and effective way to develop solutions for lung specific disorders. Many potentially beneficial drugs for lung diseases and disorders are unable to be dosed in high enough concentrations to provide therapeutic benefit to the lung due to the systemic nature (oral or IV dosing) of the drug leading to systemic toxicities before the drug reaches therapeutic levels in the lung. We believe our TFF platform has the potential to take these difficult to formulate drugs and develop products to be delivered directly to the lung for treatment of lung disorders. This direct dosing to the lung may reduce plasma levels and has the potential to increase efficacy while reducing side effects.
7
Our Intended Regulatory Pathway
The 505(b)(2) pathway is intended for molecules that have been previously approved by the FDA or have already been proven to be safe and effective. A 505(b)(2) product reformulates the known molecule in a new strength or dosage form, or for a new route of administration. 505(b)(2) products have the advantage of potentially significantly lower development costs and shorter development timelines versus traditional new molecular entities. We intend to maximize our use of the 505(b)(2) pathway for our current product candidates.
A 505(b)(2) new drug application, or NDA, is an application that contains full reports of investigations of safety and effectiveness, but where at least some of the information required for approval comes from studies not conducted by or for the applicant. This alternate regulatory pathway enables the applicant to rely, in part, on the FDA’s findings of safety and efficacy for an existing product, or published literature, in support of its application. A 505(b)(2) product candidate might rely on the clinical studies or literature of a previously FDA-approved drug or rely on the literature and physician usage of an FDA-unapproved drug. The clinical requirements for a 505(b)(2) drug candidate can vary widely from product to product depending primarily on whether the product candidate claims a new indication or claims improved safety compared to the existing approved product, and may include bioequivalence trials, limited safety and efficacy trials, or full Phase I through III trials. Unless the FDA releases a guidance document, the clinical requirement for a new product candidate is typically not known until the drug sponsor has obtained FDA feedback. We believe there is a significant opportunity to pursue dry powder formulations of off-patent drugs using the 505(b)(2) regulatory pathway.
Because our 505(b)(2) dry powder drug candidates will represent a new formulation of an existing drug, we will need to obtain FDA approval of the TFF prepared drug candidate before we can begin commercialization. However, because we begin our formulation with a drug that has previously received FDA approval in another form, we believe that in most cases we should qualify for the FDA’s 505(b)(2) regulatory pathway, which potentially will take less time and investment than the standard FDA approval process.
TFF TAC has been awarded orphan drug status. We also believe that in some cases our other dry powder drug products may qualify for the FDA’s orphan drug status. Under the Orphan Drug Act, the FDA may grant orphan designation to a drug intended to treat a rare disease or condition generally affecting fewer than 200,000 individuals in the United States, or in other limited cases. Orphan drug designation provides for seven years of exclusivity, independent of patent protection, to the company that brings a particular orphan drug to market. In addition, companies developing orphan drugs are eligible for certain incentives, including tax credits for qualified clinical testing. Furthermore, an NDA for a product that has received orphan drug designation is not subject to a prescription drug user fee unless the application includes an indication other than the rare disease or condition for which the drug was designated.
Manufacturing
We have entered into short-term contract manufacturing agreements with Societal CDMO, CoreRx, Inc., Catalent Pharma Solutions, and Experic, LLC for their provision of certain product testing, development and preclinical and clinical manufacturing services for our TFF VORI and TFF TAC product candidates. Our agreements with Societal CDMO, CoreRx, Catalent, and Experic include customary provisions concerning confidentiality, indemnification and intellectual property rights, including our exclusive ownership of all intellectual property developed severally or jointly relating to our TFF technology. We have not entered into agreements with any contract manufacturers for commercial supply, however, we believe that Societal CDMO, CoreRx, Catalent, and Experic, among several other manufacturers, have the experience and the capacity to serve as a commercial contract manufacturer. We believe we will be able to engage a commercial contract manufacturer for our product candidates in a timely manner at competitive pricing.
Each of CoreRx’s, Societal CDMO’s, Catalent’s, and Experic’s facilities and services are conducted in accordance with the FDA’s current good manufacturing practices, or cGMPs, regulations. Pursuant to the agreements with CoreRx, Societal CDMO, Catalent, and Experic, they will support clinical supplies and provide release and stability testing of the respective TFF drug product candidate. Specific tasks will include:
| ● | Engineering review and TFF technology installation; |
| ● | Familiarization with TFF technology, including powder processing and handling; |
| ● | Analytical method transfer, development, and validation; |
8
| ● | Conducting process development trials and short-term supportive stability analysis; |
| ● | Scale-up and demonstration batches of the product candidate; |
| ● | Manufacture and analytical characterization of materials to support toxicology studies, both placebo and active; |
| ● | Process train qualification for cGMP manufacturing; |
| ● | Manufacturing and release of cGMP batches for clinical trials; and |
| ● | Conducting formal stability study under the guidelines of International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, or ICH. |
Licenses and Intellectual Property Rights
We hold rights to our TFF technology pursuant to a patent license agreement with the University of Texas at Austin, or UT. UT is the owner of 141 U.S. and international patents and patent applications with claims covering the TFF platform. Pursuant to the patent license agreement, we hold an exclusive worldwide, royalty bearing license to the rights to all current and future patents held by UT relating to the TFF technology, including any divisionals, continuations and extensions, in all fields of use. The patent license agreement also provides us with a non-exclusive license to all know-how related to the TFF technology. We have also filed four US and foreign patent applications relating to certain elements of the thin film freezing platform.
We are required to pay royalties to UT in the amount of 2% of net sales received by us from the sale of products covered by the licensed patent rights. We will also be required to make certain milestone payments to UT in connection with the certain regulatory submissions and approvals and pay fees in connection with any assignments or sublicenses, including:
| ● | $50,000 upon each approval of an IND for the first indication of each product candidate; | |
| ● | $100,000 upon submission of a final Phase II report (or a foreign equivalent) on the first product candidate; | |
| ● | $250,000 upon submission of a final Phase III report (or a foreign equivalent) on the first product candidate; | |
| ● | $500,000 upon regulatory approval in the U.S. (or a foreign equivalent) on the first product candidate; and | |
| ● | $500,000 upon regulatory approval in the U.S. (or a foreign equivalent) on the second product candidate or on the second indication of the first product candidate. Pursuant to the UT patent license agreement, UT has agreed to consult with us concerning the development and implementation of a strategy for the prosecution and maintenance of the licensed patent rights, including any infringement of the licensed patents rights by third parties. However, UT has retained control and final decision-making authority over such matters. We are responsible for the payment of all fees and expenses involved in the prosecution and maintenance of the licensed patent rights and are obligated to negotiate in good faith with UT over the funding and allocation of any recovery involved in any patent infringement action brought to enforce the licensed patent rights, which are presently scheduled to expire over a period of time commencing in 2023 and ending in 2035. The term of the UT patent license agreement is co-terminus with the licensed patent rights. However, UT has the right to terminate the patent license agreement, or any part of the licensed patent rights or field of use, in the event of our breach of any provision of the patent license agreement that remains uncured after UT’s written notice of breach and an applicable cure period or in the event we initiate any proceeding to challenge the validity or scope of the licensed patent rights. The agreement also contains customary representations, warranties, covenants and indemnities by the parties. |
In addition to the licensed patent rights, we also rely on our trade secrets, know-how and continuing technological innovation to develop and maintain our proprietary position. We will vigorously defend our intellectual property to preserve our rights and gain the benefit of our technological investments.
9
Government Regulations and Funding
Pharmaceutical companies are subject to extensive regulation by foreign, federal, state and local agencies, such as the U.S. FDA, and various similar agencies in most countries worldwide. The manufacture, distribution, marketing and sale of pharmaceutical products are subject to government regulation in the U.S. and various foreign countries. Additionally, in the U.S., we must follow rules and regulations established by the FDA requiring the presentation of data indicating that our product candidates are safe and efficacious and are manufactured in accordance with cGMP regulations. If we do not comply with applicable requirements, we may be fined, the government may refuse to approve our marketing applications or allow us to manufacture or market our product candidates, and we may be criminally prosecuted. We, our manufacturers and clinical research organizations, may also be subject to regulations under other foreign, federal, state and local laws, including, but not limited to, the U.S. Occupational Safety and Health Act, the Resource Conservation and Recovery Act, the Clean Air Act and import, export and customs regulations as well as the laws and regulations of other countries. The U.S. government has increased its enforcement activity regarding illegal marketing practices domestically and internationally. As a result, pharmaceutical companies must ensure their compliance with the Foreign Corrupt Practices Act and federal healthcare fraud and abuse laws, including the False Claims Act.
These regulatory requirements impact our operations and differ from one country to another, so that securing the applicable regulatory approvals of one country does not imply the approval of another country. The approval procedures involve high costs and are manpower intensive, usually extend over many years and require highly skilled and professional resources.
FDA Market Approval Process
The steps usually required to be taken before a new drug may be marketed in the U.S. generally include:
| ● | completion of pre-clinical laboratory and animal testing; |
| ● | completion of required chemistry, manufacturing and controls testing; |
| ● | the submission to the FDA of an IND, which must be evaluated and found acceptable by the FDA before human clinical trials may commence; |
| ● | performance of adequate and well-controlled human clinical trials to establish the safety, pharmacokinetics and efficacy of the proposed drug for its intended use; |
| ● | submission and approval of an NDA; |
| ● | successful pre-approval inspection of the manufacturer and analytical testing facilities; and |
| ● | agreement with FDA of the label language, including the prescribing information insert. |
Clinical studies are conducted under protocols detailing, among other things, the objectives of the study, what types of patients may enter the study, schedules of tests and procedures, drugs, dosages, and length of study, as well as the parameters to be used in monitoring safety, and the efficacy criteria to be evaluated. A protocol for each clinical study and any subsequent protocol amendments must be submitted to the FDA as part of the IND process.
Clinical trials are usually conducted in three phases. Phase I clinical trials are normally conducted in small groups of healthy volunteers to assess safety and tolerability of various dosing regimens and pharmacokinetics. After a safe dose has been established, in Phase II clinical trials the drug is administered to small populations of sick patients to look for initial signs of efficacy via dose ranging studies in treating the targeted disease or condition and to continue to assess safety and the effective doses to be studied in larger trials in Phase III. In the case of vaccines, the participants are healthy and the signs of efficacy can be obtained in early Phase I, therefore this Phase is defined as Phase I/II. Phase III clinical trials are usually multi-center, double-blind controlled trials in hundreds or even thousands of subjects at various sites to assess as fully as possible both the safety and effectiveness of the drug.
10
Clinical trials must be conducted in accordance with the FDA’s good clinical practice, or GCP, requirements. The FDA may order the temporary or permanent discontinuation of a clinical study at any time or impose other sanctions if it believes that the clinical study is not being conducted in accordance with FDA requirements or that the participants are being exposed to an unacceptable health risk. An institutional review board, or IRB, generally must approve the clinical trial design and patient informed consent at study sites that the IRB oversees and also may halt a study, either temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions. Additionally, some clinical studies are overseen by an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board or committee. This group recommends whether or not a trial may move forward at designated check points based on access to certain data from the study. The clinical study sponsor may also suspend or terminate a clinical trial based on evolving business objectives and/or competitive climate.
As a product candidate moves through the clinical testing phases, manufacturing processes are further defined, refined, controlled and validated. The level of control and validation required by the FDA increases as clinical studies progress. We and the third-party manufacturers on which we rely for the manufacture of our product candidates and their respective components (including the API) are subject to requirements that drugs be manufactured, packaged and labeled in conformity with cGMPs. To comply with cGMP requirements, manufacturers must continue to spend time, money and effort to meet requirements relating to personnel, facilities, equipment, production and process, labeling and packaging, quality control, recordkeeping and other requirements.
Assuming completion of all required testing in accordance with all applicable regulatory requirements, detailed information on the product candidate is submitted to the FDA in the form of an NDA, requesting approval to market the product for one or more indications, together with payment of a user fee, unless waived. An NDA includes all relevant data available from pertinent nonclinical and clinical studies, including negative or ambiguous results as well as positive findings, together with detailed information on the chemistry, manufacture, controls and proposed labeling, among other things. To support marketing approval, the data submitted must be sufficient in quality and quantity to establish the safety and efficacy of the product candidate for its intended use to the satisfaction of the FDA. The FDA also conducts a pre-approval inspection of the manufacturer and laboratory prior to approval of the NDA.
If an NDA submission is accepted for filing, the FDA begins an in-depth review of the NDA. Under the Prescription Drug User Fee Act, or PDUFA, the FDA’s goal is to complete its initial review and respond to the applicant within ten months of submission, unless the application relates to an unmet medical need, or is for a serious or life-threatening indication, in which case the goal may be within six months of NDA submission. However, PDUFA goal dates are not legal mandates and the FDA response often occurs several months beyond the original PDUFA goal date. Further, the review process and the target response date under PDUFA may be extended if the FDA requests or the NDA sponsor otherwise provides additional information or clarification regarding information already provided in the NDA. The NDA review process can, accordingly, be very lengthy. During its review of an NDA, the FDA may refer the application to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it typically follows such recommendations. Data from clinical studies are not always conclusive and the FDA and/or any advisory committee it appoints may interpret data differently than the applicant.
After the FDA evaluates the NDA and inspects manufacturing facilities where the drug product and/or its API will be produced and tested, it will either approve commercial marketing of the drug product with prescribing information for specific indications or issue a complete response letter indicating that the application is not ready for approval and stating the conditions that must be met in order to secure approval of the NDA. If the complete response letter requires additional data and the applicant subsequently submits that data, the FDA nevertheless may ultimately decide that the NDA does not satisfy its criteria for approval. The FDA could also approve the NDA with a Risk Evaluation and Mitigation Strategies, or REMS, plan to mitigate risks, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling, development of adequate controls and specifications, or a commitment to conduct post-marketing testing. Such post-marketing testing may include Phase IV clinical trials and surveillance to further assess and monitor the product’s safety and efficacy after approval. Regulatory approval of products for serious or life-threatening indications may require that participants in clinical studies be followed for long periods to determine the overall survival benefit of the drug.
11
If the FDA approves one of our product candidates, we will be required to comply with a number of post-approval regulatory requirements. We would be required to report, among other things, certain adverse reactions and production problems to the FDA, provide updated safety and efficacy information and comply with requirements concerning advertising and promotional labeling for any of our product candidates. Also, quality control and manufacturing procedures must continue to conform to cGMPs after approval, and the FDA periodically inspects manufacturing facilities to assess compliance with cGMPs, which imposes extensive procedural, substantive and record keeping requirements. If we seek to make certain changes to an approved product, such as certain manufacturing changes, we may need FDA review and approval before the change can be implemented.
While physicians may use products for indications that have not been approved by the FDA, we may not label or promote the product for an indication that has not been approved. Securing FDA approval for new indications is similar to the process for approval of the original indication and requires, among other things, submitting data from adequate and well-controlled studies that demonstrate the product’s safety and efficacy in the new indication. Even if such studies are conducted, the FDA may not approve any change in a timely fashion, or at all.
The FDA may also require post-marketing testing, or Phase IV testing, as well as risk minimization action plans and surveillance to monitor the effects of an approved product or place conditions or an approval that could otherwise restrict the distribution or use of the product.
Section 505(b)(2) New Drug Applications
We intend to submit applications for both of our lead therapeutic candidates via the 505(b)(2) regulatory pathway. As an alternate path for FDA approval of new indications or new formulations of previously-approved products, a company may file a Section 505(b)(2) NDA, instead of a “stand-alone” or “full” NDA. Section 505(b)(2) of the FDCA was enacted as part of the Drug Price Competition and Patent Term Restoration Act of 1984, otherwise known as the Hatch-Waxman Amendments. Section 505(b)(2) permits the submission of an NDA where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference. Some examples of products that may be allowed to follow a 505(b)(2) path to approval are drugs that have a new dosage form, strength, route of administration, formulation or indication.
The Hatch-Waxman Amendments permit the applicant to rely upon certain published nonclinical or clinical studies conducted for an approved product or the FDA’s conclusions from prior review of such studies. The FDA may require companies to perform additional studies or measurements to support any changes from the approved product. The FDA may then approve the new product for all or some of the labeled indications for which the reference product has been approved, as well as for any new indication supported by the Section 505(b)(2) application. While references to nonclinical and clinical data not generated by the applicant or for which the applicant does not have a right of reference are allowed, all development, process, stability, qualification and validation data related to the manufacturing and quality of the new product must be included in an NDA submitted under Section 505(b)(2).
To the extent that the Section 505(b)(2) applicant is relying on the FDA’s conclusions regarding studies conducted for an already approved product, the applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA’s Approved Drug Products with Therapeutic Equivalence Evaluations, or Orange Book. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The Section 505(b)(2) application also will not be approved until any non-patent exclusivity, such as exclusivity for obtaining approval of a new chemical entity, listed in the Orange Book for the reference product has expired. If the Orange Book certifications outlined above are not accomplished, the Section 505(b)(2) applicant may invest a significant amount of time and expense in the development of its products only to be subject to significant delay and patent litigation before its products may be commercialized.
12
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug intended to treat a rare disease or condition affecting fewer than 200,000 individuals in the United States, or in other limited cases. Orphan drug designation (ODD) provides for seven years of market exclusivity, independent of patent protection, to the company with ODD that brings a particular product to market. In addition, companies developing orphan drugs are eligible for certain incentives, including tax credits for qualified clinical testing. In addition, an NDA for a product that has received orphan drug designation is not subject to a prescription drug user fee unless the application includes an indication other than the rare disease or condition for which the drug was designated.
To gain exclusivity, if a product that has orphan drug designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to the orphan drug exclusivity, which means that the FDA may not approve any other applications to market the same active moiety for the same indication for seven years, except in limited circumstances, such as another drug’s showing of clinical superiority over the drug with orphan exclusivity. Competitors, however, may receive approval of different active moieties for the same indication or obtain approval for the same active moiety for a different indication. In addition, doctors may prescribe products for off-label uses and undermine our exclusivity. Orphan drug exclusivity could block the approval of one of our product candidates for seven years if a competitor obtains approval for the same active moiety for the same indication before we do, unless we are able to demonstrate that our product is clinically superior.
We may plan to pursue orphan drug designation and exclusivity for some of our product candidates in the United States, European Union, and other geographies of interest for specific products. We cannot guarantee that we will obtain orphan drug designation for any products in any jurisdiction. Even if we are able to obtain orphan drug designation for a product, we cannot be sure that such product will be approved, that we will be able to obtain orphan drug exclusivity upon approval, if ever, or that we will be able to maintain any exclusivity that is granted.
Continuing Regulation
After a drug is approved for marketing and enters the marketplace, numerous regulatory requirements continue to apply. These include, but are not limited to:
| ● | the FDA’s cGMP regulations require manufacturers, including third party manufacturers, to follow stringent requirements for the methods, facilities and controls used in manufacturing, processing and packing of a drug product; |
| ● | labeling regulations and the FDA prohibitions against the promotion of drugs for unapproved uses (known as off-label uses), as well as requirements to provide adequate information on both risks and benefits during promotion of the drug; |
| ● | approval of product modifications or use of a drug for an indication other than approved in an NDA; |
| ● | adverse drug experience regulations, which require us to report information on adverse events during pre-market testing and post-approval safety reporting; |
| ● | NDA quarterly reporting for the first three years, then annual reporting thereafter, of changes in chemistry, manufacturing and control or CMC, labeling, clinical studies and findings, and toxicology studies from the data submitted in the NDA; |
| ● | post-market testing and surveillance requirements, including Phase IV trials, when necessary to protect the public health or to provide additional safety and effectiveness data for the drug; and |
| ● | the FDA’s recall authority, whereby it can ask, or under certain conditions order, drug manufacturers to recall from the market a product that is in violation of governing laws and regulation. After a drug receives approval, any modification in conditions of use, active ingredient(s), route of administration, dosage form, strength or bioavailability, will require a new approval, for which it may be possible to submit a 505(b)(2), accompanied by additional clinical data necessary to demonstrate the safety and effectiveness of the product with the proposed changes. Additional clinical studies may be required for proposed changes. |
13
Other U.S. Healthcare Laws and Compliance Requirements
For products distributed in the United States, we will also be subject to additional healthcare regulation and enforcement by the federal government and the states in which we conduct our business. Applicable federal and state healthcare laws and regulations include the following:
| ● | The federal healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order, or recommendation of, any good or service, for which payment may be made under federal healthcare programs such as Medicare and Medicaid; |
| ● | The Ethics in Patient Referrals Act, commonly referred to as the Stark Law, and its corresponding regulations, prohibit physicians from referring patients for designated health services (including outpatient drugs) reimbursed under the Medicare or Medicaid programs to entities with which the physicians or their immediate family members have a financial relationship or an ownership interest, subject to narrow regulatory exceptions, and prohibits those entities from submitting claims to Medicare or Medicaid for payment of items or services provided to a referred beneficiary; |
| ● | The federal False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government claims for payment that are false or fraudulent or making a false statement to avoid, decrease, or conceal an obligation to pay money to the federal government; |
| ● | Health Insurance Portability and Accountability Act of 1996, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information. This statute also prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items, or services; and |
| ● | Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government. |
Reimbursement
Sales of our product candidates in the United States may depend, in part, on the extent to which the costs of the product candidates will be covered by third-party payers, such as government health programs, commercial insurance and managed health care organizations. These third-party payers are increasingly challenging the prices charged for medical products and services. Additionally, the containment of health care costs has become a priority of federal and state governments, and the prices of drugs have been a focus in this effort. The United States government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. If these third-party payers do not consider our product candidates to be cost-effective compared to other available therapies, they may not cover our product candidates after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our product candidates on a profitable basis.
14
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, imposes new requirements for the distribution and pricing of prescription drugs for Medicare beneficiaries and includes a major expansion of the prescription drug benefit under Medicare Part D. Under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities which will provide coverage of outpatient prescription drugs. Part D plans include both stand-alone prescription drug benefit plans and prescription drug coverage as a supplement to Medicare Advantage plans. Unlike Medicare Parts A and B, Part D coverage is not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. However, Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, though not necessarily all the drugs in each category or class. Any formulary used by a Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee. Government payment for some of the costs of prescription drugs may increase demand for product candidates for which we receive marketing approval. However, any negotiated prices for our product candidates covered by a Part D prescription drug plan will likely be lower than the prices we might otherwise obtain. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payers often follow Medicare coverage policy and payment limitations in setting their own payment rates. Any reduction in payment that results from the MMA may result in a similar reduction in payments from non-governmental payers.
On February 17, 2009, the American Recovery and Reinvestment Act of 2009 was signed into law. This law provides funding for the federal government to compare the effectiveness of different treatments for the same illness. A plan for the research will be developed by the Department of Health and Human Services, the Agency for Healthcare Research and Quality and the National Institutes of Health, and periodic reports on the status of the research and related expenditures will be made to Congress. Although the results of the comparative effectiveness studies are not intended to mandate coverage policies for public or private payers, it is not clear how such a result could be avoided and what if any effect the research will have on the sales of our product candidates, if any such product or the condition that it is intended to treat is the subject of a study. It is also possible that comparative effectiveness research demonstrating benefits in a competitor’s product could adversely affect the sales of our product candidates. Decreases in third-party reimbursement for our product candidates or a decision by a third-party payer to not cover our product candidates could reduce physician usage of the product candidates and have a material adverse effect on our sales, results of operations and financial condition.
Employees and Human Capital Resources
As of the date of this report, we have 15 employees, including our executive officers, and several consultants providing technical, financial and general administrative services.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and new employees, advisors and consultants. The principal purposes of our equity incentive plans are to attract, retain and reward personnel through the granting of stock-based compensation awards, in order to increase stockholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives.
Available Information
Our website is located at www.tffpharma.com. The information on or accessible through our website is not part of this annual report on Form 10-K. A copy of this annual report on Form 10-K is located at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC also maintains an internet site that contains reports and other information regarding our filings at www.sec.gov.
15
Item 1A. Risk Factors
Investing in our common stock involves a high degree of risk. Before purchasing our common stock, you should read and consider carefully the following risk factors as well as all other information contained in this report, including our financial statements and the related notes. Each of these risk factors, either alone or taken together, could adversely affect our business, operating results and financial condition, as well as adversely affect the value of an investment in our common stock. There may be additional risks that we do not presently know of or that we currently believe are immaterial, which could also impair our business and financial position. If any of the events described below were to occur, our financial condition, our ability to access capital resources, our results of operations and/or our future growth prospects could be materially and adversely affected and the market price of our common stock could decline. As a result, you could lose some or all of any investment you may make in our common stock.
Risks Related to Our Business
We are a clinical-stage biopharmaceutical company with limited operating history. We are a biopharmaceutical company, newly-formed in January 2018, and have limited operating history. We have not commenced revenue-producing operations. In 2021, we completed Phase I human clinical trials for our TFF VORI and TFF TAC product candidates and as of the date of this report we have Phase 2 clinical trials underway for both product candidates. To date, our operations have otherwise consisted of preliminary research and development, drug formulation and characterization and testing of our initial product candidates. Our limited operating history makes it difficult for potential investors to evaluate our technology or prospective operations. As a development stage biopharmaceutical company, we are subject to all the risks inherent in the organization, financing, expenditures, complications and delays involved with a new business. Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in the early stages of development, especially clinical-stage biopharmaceutical companies such as ours. Potential investors should carefully consider the risks and uncertainties that a company with a limited operating history will face. In particular, potential investors should consider that we may be unable to:
| ● | successfully implement or execute our business plan, or ensure that our business plan is sound; |
| ● | successfully complete pre-clinical and clinical trials and obtain regulatory approval for the marketing of our product candidates; |
| ● | successfully demonstrate a favorable differentiation between our dry powder candidates and the current products on the market; |
| ● | our ability to commercially license our TFF platform to other pharmaceuticals companies; |
| ● | successfully contract for the manufacture of our clinical drug products and establish a commercial drug supply; |
| ● | secure market exclusivity and/or adequate intellectual property protection for our product candidates; |
| ● | attract and retain an experienced management and advisory team; and |
| ● | raise sufficient funds in the capital markets to effectuate our business plan, including product and clinical development, regulatory approval and commercialization for our product candidates. |
Investors should evaluate an investment in us in light of the uncertainties encountered by developing companies in a competitive environment. There can be no assurance that our efforts will be successful or that we will ultimately be able to attain profitability. If we cannot successfully execute any one of the foregoing, our business may not succeed and your investment will be adversely affected. You must be prepared to lose all of your investment.
We have a history of significant operating losses and anticipate continued operating losses for the foreseeable future. For the fiscal years ended December 31, 2022 and 2021, we incurred a net loss of $31.8 million and $31.0 million, respectively. As of December 31, 2022, we had an accumulated deficit of $97.1 million. We expect to continue to incur substantial expenses without any corresponding revenues unless and until we are able to obtain regulatory approval and successfully commercialize at least one of our product candidates or enter into one or more commercial license agreements for our TFF platform. However, there can be no assurance we will be able to obtain regulatory approval for any of our product candidates or enter into a commercial license. Even if we are able to obtain regulatory approval and subsequently commercialize our product candidates or successfully license our TFF platform, there can be no assurance that we will generate significant revenues or ever achieve profitability.
16
We expect to have significant research, regulatory and development expenses as we advance our product candidates towards commercialization. As a result, we expect to incur substantial losses for the foreseeable future, and these losses will be increasing. We are uncertain when or if we will be able to achieve or sustain profitability. If we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. Failure to become and remain profitable may impair our ability to sustain operations and adversely affect our business and our ability to raise capital. If we are unable to generate positive cash flow within a reasonable period of time, we may be unable to further pursue our business plan or continue operations, in which case you may lose your entire investment.
We expect we will need additional financing to execute our business plan and fund operations, which additional financing may not be available on reasonable terms or at all. As of December 31, 2022, we had total assets of approximately $24.1 million and working capital of approximately $17.9 million. As of December 31, 2022, our liquidity included approximately $16.6 million of cash and cash equivalents. As of the date of this report, we believe that we will need additional capital to fund our operations over the twelve months from the date of this report. We intend to seek additional funds through various financing sources, including the sale of our equity and debt securities, licensing fees for our technology and co-development and joint ventures with industry partners, with a preference towards licensing fees for our technology and co-development and joint ventures with industry partners. In addition, we will consider alternatives to our current business plan that may enable to us to achieve revenue producing operations and meaningful commercial success with a smaller amount of capital. However, there can be no guarantees that such funds will be available on commercially reasonable terms, if at all. If such financing is not available on satisfactory terms, we may be unable to further pursue our business plan and we may be unable to continue operations, in which case you may lose your entire investment.
The report of our independent registered public accounting firm for the year ended December 31, 2022 states that due to our lack of revenue from commercial operations, significant losses and need for additional capital there is substantial doubt about our ability to continue as a going concern.
Our business model is entirely dependent on certain patent rights licensed to us from the University of Texas at Austin, and the loss of those license rights would, in all likelihood, cause our business, as presently contemplated, to fail. We hold an exclusive worldwide, royalty bearing license to the patent rights for the TFF platform in all fields of use granted by the University of Texas at Austin, or UT. Our current business model, which focuses exclusively on the development of drugs using the TFF technology, is based entirely on the availability of the patent rights licensed to us by UT under the patent license agreement. The patent license agreement requires us to pay royalties and milestone payments and conform to a variety of covenants and agreements, and in the event of our breach of the agreement, UT may elect to terminate the agreement. As of the date of this report, we believe we are in compliance with the patent license agreement and consider our relationship with UT to be excellent. However, in the event of our breach of the patent license agreement for any reason, and our inability to cure such breach within any cure period or obtain a waiver from UT, we could lose the patent license agreement, which would result in our loss of all rights to the TFF technology.
Our business model includes the licensing of our TFF Platform to other pharmaceutical companies, however technology licensing in the pharmaceutical industry is a lengthy process and subject to several risks and factors outside of our control, and we cannot forecast our ability to successfully license our technology or the length of time it takes to establish a new licensing relationship. Our business model includes the joint development of dry powder formulations of proprietary drugs owned or licensed by other pharmaceutical companies. As of the date of this report, we are at various stages of feasibility studies of new chemical entities with multiple U.S. and international pharmaceutical companies. Our involvement with these pharmaceuticals companies typically begins with our formulation of dry powder versions of one or more proprietary drugs owned by the pharmaceutical company, followed by a period of feasibility testing and evaluation of the dry powder formulations by our potential licensee. Assuming the feasibility study is successful, and our dry powder formulation appears to provide the expected benefits, our ability to convert the successful test into a commercial license of our TFF platform is dependent on a number of risks and factors, many of which are outside our control, including:
| ● | the rate of adoption and incorporation of new technologies, including our TFF platform by members of the pharmaceutical industry generally; |
| ● | our potential licensee’s internal evaluation of the economic benefits of marketing a dry powder version of a drug that may be currently marketed by the potential licensee, regardless of the benefits or advantages of the dry powder version; |
| ● | our potential licensee’s internal budgetary and product development issues, including their ability to commit the capital and human resources towards the development and of the dry powder product candidate; |
| ● | our potential licensee’s willingness to accept our requirements for upfront fees and ongoing royalties; and |
| ● | the other risks relating to the adoption of our TFF platform discussed through this “Risk Factor” section. |
In addition, we believe that in many cases our potential licensee engages with us in the early-stage feasibility testing as part of their evaluation of multiple drug and drug delivery options and prior to making any decision or commitment to the development of a dry powder version of their proprietary drug product. Consequently, even if our TFF platform is successful in early feasibility studies, our potential licensee may decide, for reasons unrelated to the performance of our TFF platform, not to enter into a license agreement with us. Therefore, we are unable to predict the degree to which our proposed licensing model will be successful.
17
Our business may be adversely affected by the recent COVID-19 outbreak. In December 2019, a novel strain of coronavirus, COVID-19, was reported to have surfaced in Wuhan, China. In January 2020, this coronavirus spread to other countries, including the United States, and efforts to contain the spread of COVID-19 have included lock-downs and self-isolation procedures, which have, at times. significantly limited business operations and restricted internal and external meetings. As of the date of this report, the COVID-19 pandemic has had a relatively insignificant impact on our operations. During 2020, we experienced a temporary suspension of dosing in the Phase I clinical trial for TFF TAC due to the COVID-19 pandemic and the pandemic has otherwise caused minor slowing in the timing of certain non-clinical and clinical activities by us and our collaborators and service providers during 2020 and 2021. However, the COVID-19 pandemic has not caused us to forego, abandon or materially delay any proposed activities. While we believe we have been able to effectively manage the disruption caused by the COVID-19 pandemic to date, there can be no assurance that our operations, including the development of our drug candidates, will not be disrupted or materially adversely affected in the future by the COVID-19 pandemic or an epidemic or outbreak of an infectious disease like the outbreak of COVID-19. Further, the outbreak and any preventative or protective actions that our customers may take in respect of COVID-19 may result in a period of disruption to other work in progress. Our customers’ businesses could be disrupted, and our future costs and potential revenues and technology evaluations could be negatively affected. Any resulting financial impact cannot be reasonably estimated at this time but may materially affect our business and financial condition. The extent to which COVID-19 impacts our results will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others.
We currently have no sales and marketing organization. If we are unable to establish satisfactory sales and marketing capabilities or secure a third-party sales and marketing relationship, we may not be able to successfully commercialize any of our product candidates. At present, we have no sales or marketing personnel. Upon and subject to initial receipt of the requisite regulatory approvals for one or more of our drug products, we intend to commercialize our drug products through a combination of our internal direct sales force, third-party marketing and distribution relationships. In some cases, such as involving the development of combination drugs or the development of dry powder formulations of patented drugs, we intend to pursue the licensing of our TFF technology or enter into a joint development arrangement. If we are not successful in recruiting sales and marketing personnel and building a sales and marketing infrastructure or entering into appropriate collaboration arrangements with third parties, we will have difficulty successfully commercializing our product candidates, which would adversely affect our business, operating results and financial condition.
Even if we enter into third-party marketing and distribution arrangements, we may have limited or no control over the sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily on the success of the efforts of these third parties. In terms of establishing a sales and marketing infrastructure, we will have to compete with established and well-funded pharmaceutical and biotechnology companies to recruit, hire, train and retain sales and marketing personnel. Factors that may inhibit our efforts to build an internal sales organization or enter into collaboration arrangements with third parties include:
| ● | our inability to recruit and retain adequate numbers of effective sales and marketing personnel; |
| ● | the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to prescribe any of our product candidates; |
| ● | the lack of complementary products to be offered by sales personnel, which may put us at a competitive disadvantage relative to companies with more extensive product lines; and |
| ● | unforeseen costs and expenses associated with creating an internal sales and marketing organization. |
We will be completely dependent on third parties to manufacture our product candidates, and the commercialization of our product candidates could be halted, delayed or made less profitable if those third parties fail to obtain manufacturing approval from the FDA or comparable foreign regulatory authorities fail to provide us with sufficient quantities of our product candidates or fail to do so at acceptable quality levels or prices. We do not currently have, nor do we plan to acquire, the capability or infrastructure to manufacture our drug candidates for use in our clinical trials or for commercial sales, if any. As a result, we will be obligated to rely on contract manufacturers, if and when any of our product candidates are approved for commercialization. We have entered into short-term contract manufacturing agreements with IriSys, Inc., CoreRx, Inc. and Experic for their provision of certain product testing, development and clinical manufacturing services for our TFF VORI and TFF TAC product candidates, respectively, and we are currently in discussion with several contract manufacturers for the commercial supply of any drug candidates we are able to bring to market. However, we have not entered into agreements with any contract manufacturers for commercial supply and may not be able to engage contract manufacturers for commercial supply of any of our product candidates on favorable terms to us, or at all, should the need arise.
18
The facilities used by our current and future contract manufacturers to manufacture our product candidates must be approved by the FDA or comparable foreign regulatory authorities. Such approvals are subject to inspections that will be conducted after we submit a New Drug Application, or NDA, or Biologics License Application, or BLA, to the FDA or their equivalents to other relevant regulatory authorities. We will not control the manufacturing process of our product candidates, and will be completely dependent on our contract manufacturing partners for compliance with Current Good Manufacturing Practices, or cGMPs, for manufacture of both active drug substances and finished drug products. These cGMP regulations cover all aspects of the manufacturing, testing, quality control, storage, distribution and record keeping relating to our product candidates. If our contract manufacturers do not successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or others, we will not be able to secure or maintain regulatory approval for product made at their manufacturing facilities. If the FDA or a comparable foreign regulatory authority does not approve these facilities for the manufacture of our product candidates or if it withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, manufacture, obtain regulatory approval for or market our product candidates, if approved. Likewise, we could be negatively impacted if any of our contract manufacturers elect to discontinue their business relationship with us.
Our contract manufacturers will be subject to ongoing periodic unannounced inspections by the FDA and corresponding state and foreign agencies for compliance with cGMPs and similar regulatory requirements. We will not have control over our contract manufacturers’ compliance with these regulations and standards. Failure by any of our contract manufacturers to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, failure to grant approval to market any of our product candidates, delays, suspensions or withdrawals of approvals, inability to supply product, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect our business. In addition, we will not have control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. Failure by our contract manufacturers to comply with or maintain any of these standards could adversely affect our ability to develop, manufacture, obtain regulatory approval for or market any of our product candidates, if approved.
If, for any reason, these third parties are unable or unwilling to perform we may not be able to locate alternative manufacturers or formulators or enter into favorable agreements with them and we cannot be certain that any such third parties will have the manufacturing capacity to meet future requirements. If these manufacturers or any alternate manufacturer of finished drug product experiences any significant difficulties in its respective manufacturing processes for our active pharmaceutical ingredients, or APIs, or finished products or should cease doing business with us for any reason, we could experience significant interruptions in the supply of any of our product candidates or may not be able to create a supply of our product candidates at all. Were we to encounter manufacturing difficulties, our ability to produce a sufficient supply of any of our product candidates might be negatively affected. Our inability to coordinate the efforts of our third-party manufacturing partners, or the lack of capacity available at our third-party manufacturing partners, could impair our ability to supply any of our product candidates at required levels. Because of the significant regulatory requirements that we would need to satisfy in order to qualify a new bulk drug substance or finished product manufacturer, if we face these or other difficulties with our then current manufacturing partners, we could experience significant interruptions in the supply of any of our product candidates if we decided to transfer the manufacture of any of our product candidates to one or more alternative manufacturers in an effort to deal with such difficulties.
Any manufacturing problem or the loss of a contract manufacturer could be disruptive to our operations and result in development delays and lost sales. Additionally, we will rely on third parties to supply the raw materials needed to manufacture our product candidates. Any such reliance on suppliers may involve several risks, including a potential inability to obtain critical materials and reduced control over production costs, delivery schedules, reliability and quality. Any unanticipated disruption to the operation of one of our contract manufacturers caused by problems with suppliers could delay shipment of any of our product candidates, increase our cost of goods sold and result in lost sales.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates. We will face a potential risk of product liability as a result of the clinical testing of our product candidates and will face an even greater risk of such liability if we commercialize any of our product candidates. For example, we may be sued if any product we develop, including any of our product candidates, or any materials that we use in our product candidates allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability and a breach of warranties. In the U.S., claims could also be asserted against us under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. Even successful defense of these claims would require us to employ significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| ● | decreased demand for any of our product candidates or any future products that we may develop; |
| ● | injury to our reputation; |
| ● | failure to obtain regulatory approval for our product candidates; |
| ● | withdrawal of participants in our clinical trials; |
| ● | costs associated with our defense of the related litigation; |
19
| ● | a diversion of our management’s time and our resources; |
| ● | substantial monetary awards to trial participants or patients; |
| ● | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| ● | the inability to commercialize some or all of our product candidates; and |
| ● | a decline in the value of our stock. |
As of the date of this report, we have procured insurance coverage for our human clinical trials, which we consider adequate for our current level of clinical testing and development, however we do not carry product liability insurance. We intend to obtain product liability insurance at the time we commence commercial sale of our initial product. Our inability to obtain and retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop. Although we will endeavor to obtain and maintain such insurance in coverage amounts we deem adequate, any claim that may be brought against us could result in a court judgment or settlement in an amount that is not covered, in whole or in part, by our insurance or that is in excess of the limits of our insurance coverage. Our insurance policies would also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. As a result, we may have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts.
Our business operations could suffer in the event of information technology systems’ failures or security breaches. While we believe that we have implemented adequate security measures within our internal information technology and networking systems, our information technology systems may be subject to security breaches, damages from computer viruses, natural disasters, terrorism, and telecommunication failures. Any system failure or security breach could cause interruptions in our operations in addition to the possibility of losing proprietary information and trade secrets. To the extent that any disruption or security breach results in inappropriate disclosure of our confidential information, our competitive position may be adversely affected and we may incur liability or additional costs to remedy the damages caused by these disruptions or security breaches.
Sales of counterfeit versions of our product candidates, as well as unauthorized sales of our product candidates, may have adverse effects on our revenues, business, results of operations and damage our brand and reputation. Our product candidates may become subject to competition from counterfeit pharmaceutical products, which are pharmaceutical products sold under the same or very similar brand names and/or having a similar appearance to genuine products, but which are sold without proper licenses or approvals. Such products divert sales from genuine products, often are of lower cost and quality (having different ingredients or formulations, for example), and have the potential to damage the reputation for quality and effectiveness of the genuine product. Obtaining regulatory approval for our product candidates is a complex and lengthy process. If during the period while the regulatory approval is pending illegal sales of counterfeit products begin, consumers may buy such counterfeit products, which could have an adverse impact on our revenues, business and results of operations. In addition, if illegal sales of counterfeits result in adverse side effects to consumers, we may be associated with any negative publicity resulting from such incidents. Although pharmaceutical regulation, control and enforcement systems throughout the world have been increasingly active in policing counterfeit pharmaceuticals, we may not be able to prevent third parties from manufacturing, selling or purporting to sell counterfeit products competing with our product candidates. Such sales may also be occurring without our knowledge. The existence and any increase in production or sales of counterfeit products or unauthorized sales could negatively impact our revenues, brand reputation, business and results of operations.
Risks Related to Product Regulation
Our success is entirely dependent on our ability to obtain the marketing approval for our product candidates by the FDA and the regulatory authorities in foreign jurisdictions in which we intend to market our product candidates, of which there can be no assurance. We are not permitted to market our product candidates as prescription pharmaceutical products in the United States until we receive approval of an NDA from the FDA, or in any foreign countries until we receive the requisite approval from such countries. In the United States, the FDA generally requires the completion of clinical trials of each drug to establish its safety and efficacy and extensive pharmaceutical development to ensure its quality before an NDA is approved. Of the large number of drugs in development, only a small percentage result in the submission of an NDA to the FDA and even fewer are eventually approved for commercialization. As of the date of this report, we have not submitted an NDA to the FDA or comparable applications to other regulatory authorities for any of our product candidates.
20
Because our initial dry powder drug candidates, TFF VORI and TFF TAC, are established drugs that are off-patent, we have gained FDA agreement on the 505(b)(2) regulatory pathway for these product candidates. We believe that our initial drug product candidates will qualify for FDA approval through the FDA’s 505(b)(2) regulatory pathway and through corresponding regulatory paths in other foreign jurisdictions. The clinical requirements for a 505(b)(2) drug candidate can vary widely from product to product depending primarily on whether the product candidate claims a new indication, provides for a different route of administration, or claims improved safety compared to the existing approved product, and may include bioequivalence trials, limited safety and efficacy trials, or full Phase I through III trials. To the extent we claim that our drug product candidates target a new indication or offer improved safety compared to the existing approved products, and it is our present expectation that we will do so in many cases, it is likely that we will be required to conduct additional clinical trials, potentially including a full Phase I through Phase III development program, in order to obtain marketing approval.
Our business model is to pursue the development of off-patent drugs for which we would directly pursue the development of a dry powder formulation through the FDA’s 505(b)(2) regulatory pathway; however, not all of our product candidates will target off-patent drugs and, at least in the case of a dry powder formulation of CBD, our product candidate may not be a drug. We do not expect any dry powder formulation of a CBD drug product to be off-patent and our proposed dry powder formulation of aluminum salt vaccines may not be off-patent. We also expect that our dry powder formulation of a CBD drug product will likely require a full NDA; however, a non-pharmaceutical CBD dry powder formulation may not require FDA approval. We expect that our dry powder formulation of aluminum salt vaccines will require a biological license application, or BLA, which is very similar to a full NDA through the FDA’s 505(b)(1) regulatory pathway.
Our success depends on our receipt of the regulatory approvals described above, and the issuance of such regulatory approvals is uncertain and subject to a number of risks, including the following:
| ● | the results of toxicology studies may not support the filing of an IND for our product candidates; |
| ● | the FDA or comparable foreign regulatory authorities or Institutional Review Boards, or IRB, may disagree with the design or implementation of our clinical trials; |
| ● | we may not be able to provide acceptable evidence of our product candidates’ safety and efficacy; |
| ● | the results of our clinical trials may not be satisfactory or may not meet the level of statistical or clinical significance required by the FDA, European Medicines Agency, or EMA, or other regulatory agencies for us to receive marketing approval for any of our product candidates; |
| ● | the dosing of our product candidates in a particular clinical trial may not be at an optimal level; |
| ● | patients in our clinical trials may suffer adverse effects for reasons that may or may not be related to our product candidates; |
| ● | the data collected from clinical trials may not be sufficient to support the submission of an NDA, BLA or other submission or to obtain regulatory approval in the United States or elsewhere; |
| ● | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
| ● | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval of our product candidates. |
21
The process of obtaining regulatory approvals is expensive, often takes many years, if approval is obtained at all, and can vary substantially based upon, among other things, the type, complexity and novelty of the product candidates involved, the jurisdiction in which regulatory approval is sought and the substantial discretion of the regulatory authorities. Changes in regulatory approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for a submitted product application may cause delays in the approval or rejection of an application. Regulatory approval obtained in one jurisdiction does not necessarily mean that a product candidate will receive regulatory approval in all jurisdictions in which we may seek approval, but the failure to obtain approval in one jurisdiction may negatively impact our ability to seek approval in a different jurisdiction. Failure to obtain regulatory approval for our product candidates for the foregoing, or any other reasons, will prevent us from commercializing our product candidates, and our ability to generate revenue will be materially impaired.
Clinical testing is expensive, is difficult to design and implement, can take many years to complete and is uncertain as to outcome. Our business model depends entirely on the successful development, regulatory approval and commercialization of our product candidates, which may never occur. In 2020 and 2021, we completed Phase I human clinical trials for our TFF VORI and TFF TAC product candidates, and in 2022 we initiated Phase 2 clinical trials for both product candidates. However, there can be no assurance that our Phase 2 clinical trials will be successful or that we will continue clinical development TFF VORI and TFF TAC in support of an approval from the FDA or comparable foreign regulatory authorities for any indication. We note that most product candidates never reach the clinical development stage and even those that do commence clinical development have only a small chance of successfully completing clinical development and gaining regulatory approval. Success in early phases of pre-clinical and clinical trials does not ensure that later clinical trials will be successful, and interim results of a clinical trial do not necessarily predict final results. A failure of one or more of our clinical trials for TFF VORI and TFF TAC can occur at any stage of testing. We may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates. Therefore, our business currently depends entirely on the successful development, regulatory approval and commercialization of our product candidates, which may never occur.
Even if we receive regulatory approval for any of our product candidates, we may not be able to successfully commercialize the product and the revenue that we generate from its sales, if any, may be limited. If approved for marketing, the commercial success of our product candidates will depend upon each product’s acceptance by the medical community, including physicians, patients and health care payors. The degree of market acceptance for any of our product candidates will depend on a number of factors, including:
| ● | demonstration of clinical safety and efficacy; |
| ● | relative convenience, dosing burden and ease of administration; |
| ● | the prevalence and severity of any adverse effects; |
| ● | the willingness of physicians to prescribe our product candidates, and the target patient population to try new therapies; |
| ● | efficacy of our product candidates compared to competing products; |
| ● | the introduction of any new products that may in the future become available targeting indications for which our product candidates may be approved; |
| ● | new procedures or therapies that may reduce the incidences of any of the indications in which our product candidates may show utility; |
22
| ● | pricing and cost-effectiveness; |
| ● | the inclusion or omission of our product candidates in applicable therapeutic and vaccine guidelines; |
| ● | the effectiveness of our own or any future collaborators’ sales and marketing strategies; |
| ● | limitations or warnings contained in approved labeling from regulatory authorities; |
| ● | our ability to obtain and maintain sufficient third-party coverage or reimbursement from government health care programs, including Medicare and Medicaid, private health insurers and other third-party payors or to receive the necessary pricing approvals from government bodies regulating the pricing and usage of therapeutics; and |
| ● | the willingness of patients to pay out-of-pocket in the absence of third-party coverage or reimbursement or government pricing approvals. |
If any of our product candidates are approved, but do not achieve an adequate level of acceptance by physicians, health care payors, and patients, we may not generate sufficient revenue and we may not be able to achieve or sustain profitability. Our efforts to educate the medical community and third-party payors on the benefits of our product candidates may require significant resources and may never be successful.
In addition, even if we obtain regulatory approvals, the timing or scope of any approvals may prohibit or reduce our ability to commercialize our product candidates successfully. For example, if the approval process takes too long, we may miss market opportunities and give other companies the ability to develop competing products or establish market dominance. Any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render our product candidates not commercially viable. For example, regulatory authorities may approve any of our product candidates for fewer or more limited indications than we request, may not approve the price we intend to charge for any of our product candidates, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve any of our product candidates with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that indication. Further, the FDA or comparable foreign regulatory authorities may place conditions on approvals or require risk management plans or a Risk Evaluation and Mitigation Strategy, or REMS, to assure the safe use of the drug. Moreover, product approvals may be withdrawn for non-compliance with regulatory standards or if problems occur following the initial marketing of the product. Any of the foregoing scenarios could materially harm the commercial success of our product candidates.
Even if we obtain marketing approval for any of our product candidates, we will be subject to ongoing obligations and continued regulatory review, which may result in significant additional expense. Additionally, our product candidates could be subject to labeling and other restrictions and withdrawal from the market and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our product candidates. Even if we obtain regulatory approval for any of our product candidates for an indication, the FDA or foreign equivalent may still impose significant restrictions on their indicated uses or marketing or the conditions of approval, or impose ongoing requirements for potentially costly and time-consuming post-approval studies, including Phase IV clinical trials, and post-market surveillance to monitor safety and efficacy. Our product candidates will also be subject to ongoing regulatory requirements governing the manufacturing, labeling, packaging, storage, distribution, safety surveillance, advertising, promotion, recordkeeping and reporting of adverse events and other post-market information. These requirements include registration with the FDA, as well as continued compliance with current Good Clinical Practices regulations, or cGCPs, for any clinical trials that we conduct post-approval. In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with current cGMPs, requirements relating to quality control, quality assurance and corresponding maintenance of records and documents.
The FDA has the authority to require a REMS as part of an NDA or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria or requiring patient testing, monitoring and/or enrollment in a registry.
23
With respect to sales and marketing activities related to our product candidates, advertising and promotional materials must comply with FDA rules in addition to other applicable federal, state and local laws in the United States and similar legal requirements in other countries. In the United States, the distribution of product samples to physicians must comply with the requirements of the U.S. Prescription Drug Marketing Act. Application holders must obtain FDA approval for product and manufacturing changes, depending on the nature of the change. We may also be subject, directly or indirectly through our customers and partners, to various fraud and abuse laws, including, without limitation, the U.S. Anti-Kickback Statute, U.S. False Claims Act, and similar state laws, which impact, among other things, our proposed sales, marketing, and scientific/educational grant programs. If we participate in the U.S. Medicaid Drug Rebate Program, the Federal Supply Schedule of the U.S. Department of Veterans Affairs, or other government drug programs, we will be subject to complex laws and regulations regarding reporting and payment obligations. All of these activities are also potentially subject to U.S. federal and state consumer protection and unfair competition laws. Similar requirements exist in many of these areas in other countries.
In addition, if any of our product candidates are approved for a particular indication, our product labeling, advertising and promotion would be subject to regulatory requirements and continuing regulatory review. The FDA strictly regulates the promotional claims that may be made about prescription products. In particular, a product may not be promoted for uses that are not approved by the FDA as reflected in the product’s approved labeling. If we receive marketing approval for our product candidates, physicians may nevertheless legally prescribe our products to their patients in a manner that is inconsistent with the approved label. If we are found to have promoted such off-label uses, we may become subject to significant liability and government fines. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant sanctions. The federal government has levied large civil and criminal fines against companies for alleged improper promotion and has enjoined several companies from engaging in off-label promotion. The FDA has also requested that companies enter into consent decrees of permanent injunctions under which specified promotional conduct is changed or curtailed. If we or a regulatory agency discover previously unknown problems with a product candidate, such as adverse events of unanticipated severity or frequency, problems with the facility where the product is manufactured, or we or our manufacturers fail to comply with applicable regulatory requirements, we may be subject to the following administrative or judicial sanctions:
| ● | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| ● | issuance of warning letters or untitled letters; |
| ● | clinical holds; |
| ● | injunctions or the imposition of civil or criminal penalties or monetary fines; |
| ● | suspension or withdrawal of regulatory approval; |
| ● | suspension of any ongoing clinical trials; |
24
| ● | refusal to approve pending applications or supplements to approved applications filed by us, or suspension or revocation of product license approvals; |
| ● | suspension or imposition of restrictions on operations, including costly new manufacturing requirements; or |
| ● | product seizure or detention or refusal to permit the import or export of product. |
The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and generate revenue. Adverse regulatory action, whether pre- or post-approval, can also potentially lead to product liability claims and increase our product liability exposure.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions. Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, but a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from those in the United States, including additional preclinical studies or clinical trials, as clinical studies conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval.
Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our product candidates in certain countries. If we fail to comply with the regulatory requirements in international markets and/ or to receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our product candidates will be harmed.
Even though we may apply for orphan drug designation for a product candidate, we may not be able to obtain orphan drug marketing exclusivity. We believe that in some cases our dry powder drug products may qualify for the FDA’s orphan drug status. There is no guarantee that the FDA will grant any future application for orphan drug designation for any of our product candidates, which would make us ineligible for the additional exclusivity and other benefits of orphan drug designation.
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug available in the United States for this type of disease or condition will be recovered from sales of the product. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten the duration of regulatory review and approval process. In addition to the potential period of exclusivity, orphan designation makes a company eligible for grant funding of up to $400,000 per year for four years to defray costs of clinical trial expenses, tax credits for clinical research expenses and potential exemption from the FDA application user fee.
If a product that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan drug exclusivity, which means the FDA may not approve any other applications to market the same drug for the same indication for seven years, except in limited circumstances, such as (i) the drug’s orphan designation is revoked; (ii) its marketing approval is withdrawn; (iii) the orphan exclusivity holder consents to the approval of another applicant’s product; (iv) the orphan exclusivity holder is unable to assure the availability of a sufficient quantity of drug; or (v) a showing of clinical superiority to the product with orphan exclusivity by a competitor product. If a drug designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan drug exclusivity. There can be no assurance that we will receive orphan drug designation for any of our product candidates in the indications for which we think they might qualify, if we elect to seek such applications.
25
Current and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and affect the prices we may obtain. In the United States and some foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval for our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell our product candidates. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We do not know whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
In the United States, the Medicare Modernization Act, or MMA, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sales prices for drugs. In addition, this legislation authorized Medicare Part D prescription drug plans to use formularies where they can limit the number of drugs that will be covered in any therapeutic class. As a result of this legislation and the expansion of federal coverage of drug products, we expect that there will be additional pressure to contain and reduce costs. These cost reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for our product candidates and could seriously harm our business. While the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates, and any reduction in reimbursement that results from the MMA may result in a similar reduction in payments from private payors.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010 or, collectively, the Health Care Reform Law, is a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. The Health Care Reform Law revised the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, the law imposed a significant annual fee on companies that manufacture or import branded prescription drug products.
The Health Care Reform Law remains subject to legislative efforts to repeal, modify or delay the implementation of the law. If the Health Care Reform Law is repealed or modified, or if implementation of certain aspects of the Health Care Reform Law are delayed, such repeal, modification or delay may materially adversely impact our business, strategies, prospects, operating results or financial condition. We are unable to predict the full impact of any repeal, modification or delay in the implementation of the Health Care Reform Law on us at this time. Due to the substantial regulatory changes that will need to be implemented by Centers for Medicare & Medicaid Services, or CMS, and others, and the numerous processes required to implement these reforms, we cannot predict which healthcare initiatives will be implemented at the federal or state level, the timing of any such reforms, or the effect such reforms or any other future legislation or regulation will have on our business.
In addition, other legislative changes have been proposed and adopted in the United States since the Health Care Reform Law was enacted. We expect that additional federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, and in turn could significantly reduce the projected value of certain development projects and reduce or eliminate our profitability.
Any termination or suspension of, or delays in the commencement or completion of, any necessary studies of any of our product candidates for any indications could result in increased costs to us, delay or limit our ability to generate revenue and adversely affect our commercial prospects. The commencement and completion of clinical studies can be delayed for a number of reasons, including delays related to:
| ● | the FDA or a comparable foreign regulatory authority failing to grant permission to proceed and placing the clinical study on hold; |
| ● | subjects for clinical testing failing to enroll or remain enrolled in our trials at the rate we expect; |
26
| ● | a facility manufacturing any of our product candidates being ordered by the FDA or other government or regulatory authorities to temporarily or permanently shut down due to violations of cGMP requirements or other applicable requirements, or cross-contaminations of product candidates in the manufacturing process; |
| ● | any changes to our manufacturing process that may be necessary or desired; |
| ● | subjects choosing an alternative treatment for the indications for which we are developing our product candidates, or participating in competing clinical studies; |
| ● | subjects experiencing severe or unexpected drug-related adverse effects; |
| ● | reports from clinical testing on similar technologies and products raising safety and/or efficacy concerns; |
| ● | third-party clinical investigators losing their license or permits necessary to perform our clinical trials, not performing our clinical trials on our anticipated schedule or employing methods consistent with the clinical trial protocol, cGMP requirements, or other third parties not performing data collection and analysis in a timely or accurate manner; |
| ● | inspections of clinical study sites by the FDA, comparable foreign regulatory authorities, or IRBs finding regulatory violations that require us to undertake corrective action, result in suspension or termination of one or more sites or the imposition of a clinical hold on the entire study, or that prohibit us from using some or all of the data in support of our marketing applications; |
| ● | third-party contractors becoming debarred or suspended or otherwise penalized by the FDA or other government or regulatory authorities for violations of regulatory requirements, in which case we may need to find a substitute contractor, and we may not be able to use some or any of the data produced by such contractors in support of our marketing applications; |
| ● | one or more IRBs refusing to approve, suspending or terminating the study at an investigational site, precluding enrollment of additional subjects, or withdrawing its approval of the trial; reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| ● | deviations of the clinical sites from trial protocols or dropping out of a trial; |
| ● | adding new clinical trial sites; |
| ● | the inability of the CRO to execute any clinical trials for any reason; and |
| ● | government or regulatory delays or “clinical holds” requiring suspension or termination of a trial. |
Product development costs for any of our product candidates will increase if we have delays in testing or approval or if we need to perform more or larger clinical studies than planned. Additionally, changes in regulatory requirements and policies may occur and we may need to amend study protocols to reflect these changes. Amendments may require us to resubmit our study protocols to the FDA, comparable foreign regulatory authorities, and IRBs for reexamination, which may impact the costs, timing or successful completion of that study. If we experience delays in completion of, or if we, the FDA or other regulatory authorities, the IRB, or other reviewing entities, or any of our clinical study sites suspend or terminate any of our clinical studies of any of our product candidates, its commercial prospects may be materially harmed and our ability to generate product revenues will be delayed. Any delays in completing our clinical trials will increase our costs, slow down our development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences may harm our business, financial condition and prospects significantly. In addition, many of the factors that cause, or lead to, termination or suspension of, or a delay in the commencement or completion of, clinical studies may also ultimately lead to the denial of regulatory approval of our product candidates. In addition, if one or more clinical studies are delayed, our competitors may be able to bring competing products to market before we do, and the commercial viability of any of our affected product candidates could be significantly reduced.
27
Third-party coverage and reimbursement and health care cost containment initiatives and treatment guidelines may constrain our future revenues. Our ability to successfully market our product candidates will depend in part on the level of reimbursement that government health administration authorities, private health coverage insurers and other organizations provide for the cost of our product candidates and related treatments. Countries in which any of our product candidates are sold through reimbursement schemes under national health insurance programs frequently require that manufacturers and sellers of pharmaceutical products obtain governmental approval of initial prices and any subsequent price increases. In certain countries, including the United States, government-funded and private medical care plans can exert significant indirect pressure on prices. We may not be able to sell our product candidates profitably if adequate prices are not approved or coverage and reimbursement is unavailable or limited in scope. Increasingly, third-party payors attempt to contain health care costs in ways that are likely to impact our development of products including:
| ● | failing to approve or challenging the prices charged for health care products; |
| ● | introducing reimportation schemes from lower priced jurisdictions; |
| ● | limiting both coverage and the amount of reimbursement for new therapeutic products; |
| ● | denying or limiting coverage for products that are approved by the regulatory agencies but are considered to be experimental or investigational by third-party payors; and |
| ● | refusing to provide coverage when an approved product is used in a way that has not received regulatory marketing approval. |
Risks Relating to Our Intellectual Property Rights
We are dependent on rights to certain technologies licensed to us. We do not have complete control over these technologies and any loss of our rights to them could prevent us from selling our product candidates. As noted above, our business model is entirely dependent on certain patent rights licensed to us by the University of Texas at Austin, or UT. See, “Risk Factors — Risks Relating to Our Business — Our business model is entirely dependent on certain patent rights licensed to us from the University of Texas at Austin, and the loss of those license rights would, in all likelihood, cause our business, as presently contemplated, to fail.” Because we will hold those rights as a licensee, we have limited control over certain important aspects of those patent rights. Pursuant to the patent license agreement, UT has reserved the right to control all decisions concerning the prosecution and maintenance of all U.S. and foreign patents, as well as all decisions concerning the enforcement of any actions against potential infringers of the patent rights. We believe that UT shares a common interest in these matters with us, and UT has agreed to consult with us on the prosecution and enforcement of possible infringement claims as well as other matters for which UT has retained control. However, there can be no assurance that UT will agree with our views as to how best to prosecute, maintain and defend the patent rights subject to the patent license agreement.
It is difficult and costly to protect our intellectual property rights, and we cannot ensure the protection of these rights. Our commercial success will depend, in part, on our ability to successfully defend the patent rights subject to our patent license agreement with UT against third-party challenges and successfully enforcing these patent rights against third party competitors. The patent positions of pharmaceutical companies can be highly uncertain and involve complex legal, scientific and factual questions for which important legal principles remain unresolved. Changes in either the patent laws or in interpretations of patent laws may diminish the value of our intellectual property. Accordingly, we cannot predict the breadth of claims that may be allowable or enforceable in the patent applications subject to the UT patent license agreement. The patents and patent applications relating to our TFF platform and related technologies may be challenged, invalidated or circumvented by third parties and might not protect us against competitors with similar products or technologies.
28
The degree of future protection afforded by the patent rights licensed to us is uncertain because legal means afford only limited protection and may not adequately protect our rights, permit us to gain or keep our competitive advantage, or provide us with any competitive advantage at all. We cannot be certain that any patent application owned by a third party will not have priority over patent applications in which we hold license rights or that we will not be involved in interference, opposition or invalidity proceedings before United States or foreign patent offices.
Additionally, if UT were to initiate legal proceedings against a third party to enforce a patent covering any of our product candidates, the defendant could counterclaim that such patent is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge include alleged failures to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement. Grounds for unenforceability assertions include allegations that someone connected with prosecution of the patent withheld relevant information from the United States Patent and Trademark Office, or the U.S. PTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review and equivalent proceedings in foreign jurisdictions, e.g. opposition proceedings. Such proceedings could result in revocation or amendment of UT’s patents in such a way that they no longer cover our product candidates or competitive products. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to validity, for example, we cannot be certain that there is no invalidating prior art, of which UT and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on any of our product candidates. Such a loss of patent protection would have a material adverse impact on our business.
In the future, we may rely on know-how and trade secrets to protect technology, especially in cases in which we believe patent protection is not appropriate or obtainable. However, know-how and trade secrets are difficult to protect. While we intend to require employees, academic collaborators, consultants and other contractors to enter into confidentiality agreements, we may not be able to adequately protect our trade secrets or other proprietary or licensed information. Typically, research collaborators and scientific advisors have rights to publish data and information in which we may have rights. Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts are sometimes less willing to protect trade secrets than patents. Moreover, our competitors may independently develop equivalent knowledge, methods and know-how.
If we fail to obtain or maintain patent protection or trade secret protection for our product candidates or our technologies, third parties could use our proprietary information, which could impair our ability to compete in the market and adversely affect our ability to generate revenues and attain profitability.
Our product candidates may infringe the intellectual property rights of others, which could increase our costs and delay or prevent our development and commercialization efforts. Our success depends in part on avoiding infringement of the proprietary technologies of others. The pharmaceutical industry has been characterized by frequent litigation regarding patent and other intellectual property rights. Identification of third-party patent rights that may be relevant to our proprietary technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. Additionally, because patent applications are maintained in secrecy until the application is published, we may be unaware of third-party patents that may be infringed by commercialization of any of our product candidates or any future product candidate. There may be certain issued patents and patent applications claiming subject matter that we may be required to license in order to research, develop or commercialize any of our product candidates, and we do not know if such patents and patent applications would be available to license on commercially reasonable terms, or at all. Any claims of patent infringement asserted by third parties would be time-consuming and may:
| ● | result in costly litigation; |
| ● | divert the time and attention of our technical personnel and management; |
29
| ● | prevent us from commercializing a product until the asserted patent expires or is held finally invalid or not infringed in a court of law; |
| ● | require us to cease or modify our use of the technology and/or develop non-infringing technology; or |
| ● | require us to enter into royalty or licensing agreements. |
Third parties may hold proprietary rights that could prevent any of our product candidates from being marketed. Any patent-related legal action against us claiming damages and seeking to enjoin commercial activities relating to any of our product candidates or our processes could subject us to potential liability for damages and require us to obtain a license to continue to manufacture or market any of our product candidates or any future product candidates. We cannot predict whether we would prevail in any such actions or that any license required under any of these patents would be made available on commercially acceptable terms, if at all. In addition, we cannot be sure that we could redesign our product candidates or any future product candidates or processes to avoid infringement, if necessary. Accordingly, an adverse determination in a judicial or administrative proceeding, or the failure to obtain necessary licenses, could prevent us from developing and commercializing any of our product candidates or a future product candidate, which could harm our business, financial condition and operating results.
We expect that there are other companies, including major pharmaceutical companies, working in the areas competitive to our product candidates which either has resulted, or may result, in the filing of patent applications that may be deemed related to our activities. If we were to challenge the validity of these or any issued United States patent in court, we would need to overcome a statutory presumption of validity that attaches to every issued United States patent. This means that, in order to prevail, we would have to present clear and convincing evidence as to the invalidity of the patent’s claims. If we were to challenge the validity of these or any issued United States patent in an administrative trial before the Patent Trial and Appeal Board in the U.S. PTO, we would have to prove that the claims are unpatentable by a preponderance of the evidence. There is no assurance that a jury and/or court would find in our favor on questions of infringement, validity or enforceability. Even if we are successful, litigation could result in substantial costs and be a distraction to management.
We may be subject to claims that we have wrongfully hired an employee from a competitor or that we or our employees have wrongfully used or disclosed alleged confidential information or trade secrets of their former employers. As is commonplace in our industry, we will employ individuals who were previously employed at other pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject in the future to claims that our employees or prospective employees are subject to a continuing obligation to their former employers (such as non-competition or non-solicitation obligations) or claims that our employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
30
Risks Related to Owning Our Common Stock
The market price of our shares may be subject to fluctuation and volatility. You could lose all or part of your investment. The market price of our common stock is subject to wide fluctuations in response to various factors, some of which are beyond our control. Since shares of our common stock were sold in our initial public offering in October 2019 at a price of $5.00 per share, the reported high and low sales prices of our common stock have ranged from $0.62 to $21.14 through March 21, 2023. The market price of our shares on the NASDAQ Global Market may fluctuate as a result of a number of factors, some of which are beyond our control, including, but not limited to:
| ● | actual or anticipated variations in our and our competitors’ results of operations and financial condition; |
| ● | market acceptance of our product candidates; |
| ● | changes in earnings estimates or recommendations by securities analysts, if our shares are covered by analysts; |
| ● | development of technological innovations or new competitive products by others; |
| ● | announcements of technological innovations or new products by us; |
| ● | publication of the results of preclinical or clinical trials for our product candidates; |
| ● | failure by us to achieve a publicly announced milestone; |
| ● | delays between our expenditures to develop and market new or enhanced products and the generation of sales from those products; |
| ● | developments concerning intellectual property rights, including our involvement in litigation brought by or against us; |
| ● | regulatory developments and the decisions of regulatory authorities as to the approval or rejection of new or modified products; |
| ● | changes in the amounts that we spend to develop, acquire or license new products, technologies or businesses; |
| ● | changes in our expenditures to promote our product candidates; |
| ● | our sale or proposed sale, or the sale by our significant stockholders, of our shares or other securities in the future; |
| ● | changes in key personnel; |
| ● | success or failure of our research and development projects or those of our competitors; |
| ● | the trading volume of our shares; and |
general economic and market conditions and other factors, including factors unrelated to our operating performance.
We have received a notice of delisting or failure to satisfy a continued listing rule from the Nasdaq. On March 2, 2023, we received a notice of delisting from the Nasdaq Stock Market, LLC. The notice stated that we had fallen below compliance with respect to the continued listing standard set forth in Rule 5450(a)(1) of the Nasdaq Listing Rules because the closing bid price of our common stock over the previous 30 consecutive trading-day period had fallen below $1.00 per share.
31
Pursuant to the notice and Rule 5810(c)(3)(A) of the Nasdaq Listing Rules, we have 180 days from the date of the notice, or until August 29, 2023, to regain compliance with the minimum bid price requirement in Rule 5450(a)(1) by achieving a closing bid price for our common stock of at least $1.00 per share over a minimum of 10 consecutive business days. If we do not regain compliance with Rule 5450(a)(1) during the initial 180-day period, we may be eligible for additional time to regain compliance, subject to our transfer to the Nasdaq Capital Market and compliance with the Nasdaq’s continued listing requirement for market value of publicly held shares and all other initial listing standards for The Nasdaq Capital Market, with the exception of the bid price requirement, and our provision of certain undertakings to the Nasdaq. However, there can be no assurance that we will be afforded additional time to regain compliance with the minimum bid price requirement following the initial 180-day period. If we are unable to regain compliance with Nasdaq Listing Rule 5450(a)(2) in a timely manner, the Nasdaq will commence suspension and delisting procedures.
These factors and any corresponding price fluctuations may materially and adversely affect the market price of our shares and result in substantial losses being incurred by our investors. In the past, following periods of market volatility, public company stockholders have often instituted securities class action litigation. If we were involved in securities litigation, it could impose a substantial cost upon us and divert the resources and attention of our management from our business.
If securities or industry analysts do not continue to publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline. The trading market for our common stock depends in part on the research and reports that securities or industry analysts publish about us or our business. If industry analysts cease coverage of us, the trading price for our common stock would be negatively affected. If one or more of the analysts who cover us downgrade our common stock or publish inaccurate or unfavorable research about our business, our common stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, demand for our common stock could decrease, which might cause our common stock price and trading volume to decline. In addition, independent industry analysts may provide reviews of our product candidates and our TFF platform’s capabilities, as well as those of our competitors, and perception of our offerings in the marketplace may be significantly influenced by these reviews. We have no control over what these industry analysts report, and because industry analysts may influence current and potential customers, our brand could be harmed if they do not provide a positive review of our products and platform capabilities or view us as a market leader.
Future capital raises may dilute your ownership and/or have other adverse effects on our operations. If we raise additional capital by issuing equity securities, our existing stockholders’ percentage ownership will be reduced and these stockholders may experience substantial dilution. If we raise additional funds by issuing debt securities, these debt securities would have rights senior to those of our common stock and the terms of the debt securities issued could impose significant restrictions on our operations, including liens on our assets. If we raise additional funds through collaborations and licensing arrangements, we may be required to relinquish some rights to our intellectual property or candidate products, or to grant licenses on terms that are not favorable to us.
We are an “emerging growth company” under the JOBS Act of 2012 and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors. We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including, but not limited to:
| ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
| ● | reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; |
32
| ● | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments; and |
| ● | extended transition periods available for complying with new or revised accounting standards. |
We have chosen to take advantage of all of the benefits available under the JOBS Act, including the exemptions discussed above. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
We will remain an “emerging growth company” for up to five years, although we will lose that status sooner if our revenues exceed $1.07 billion, if we issue more than $1 billion in non-convertible debt in a three year period, or if the market value of our common stock that is held by non-affiliates exceeds $700 million as of June 30 in any future year.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. We are required to provide a report on management’s assessment of our internal control over financial reporting. Once we are neither an emerging growth company nor a non-accelerated filed, we will be required to obtain an attestation from our independent registered public accounting firm on our internal control report. Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act, or the subsequent testing by our independent registered public accounting firm when required, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retrospective changes to our financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common shares. There is also a risk that neither we nor our independent registered public accounting firm (when applicable in the future) will be able to conclude within the prescribed timeframe that internal controls over financial reporting is effective as required by Section 404. As a result, investors could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
We have not paid dividends in the past and have no immediate plans to pay dividends. We plan to reinvest all of our earnings, to the extent we have earnings, to cover operating costs and otherwise become and remain competitive. We do not plan to pay any cash dividends with respect to our securities in the foreseeable future. We cannot assure you that we would, at any time, generate sufficient surplus cash that would be available for distribution to the holders of our common stock as a dividend. Therefore, you should not expect to receive cash dividends on our common stock.
We may be at an increased risk of securities class action litigation. Historically, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biotechnology and pharmaceutical companies have experienced significant stock price volatility in recent years. If we were to be sued, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
Our charter documents and Delaware law may inhibit a takeover that stockholders consider favorable. The provisions of our second amended and restated certificate of incorporation, or Certificate, and amended and restated bylaws and applicable provisions of Delaware law may delay or discourage transactions involving an actual or potential change in control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to be in their best interests. The provisions in our Certificate and amended and restated bylaws:
| ● | limit who may call stockholder meetings; |
| ● | do not provide for cumulative voting rights; and |
| ● | provide that all board vacancies may be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum. |
33
In addition, Section 203 of the Delaware General Corporation Law may limit our ability to engage in any business combination with a person who beneficially owns 15% or more of our outstanding voting stock unless certain conditions are satisfied. This restriction lasts for a period of three years following the share acquisition. These provisions may have the effect of entrenching our management team and may deprive you of the opportunity to sell your shares to potential acquirers at a premium over prevailing prices. This potential inability to obtain a control premium could reduce the price of our common stock.
Our Certificate and amended and restated bylaws designate the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain litigation that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or other employees. Provisions in our Certificate and amended and restated bylaws provide that the Court of Chancery of the State of Delaware will, to the fullest extent permitted by law, be the sole and exclusive forum for:
| ● | any derivative action or proceeding brought on our behalf; |
| ● | any action asserting a claim of breach of a fiduciary duty owed to us or our stockholders by any of our directors, officers or other employees; |
| ● | any action asserting a claim against us or any of our directors, officers or other employees arising pursuant to any provision of Delaware law or our charter documents; or |
| ● | any action asserting a claim against us or any of our directors, officers or other employees governed by the internal affairs doctrine, but excluding actions to enforce a duty or liability created by the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. |
These exclusive forum provisions do not apply to claims under the Securities Act or the Exchange Act. These exclusive forums provisions, however, do provide that if no state court located in the State of Delaware has jurisdiction, the federal district court for the District of Delaware shall be the exclusive forum. By becoming a stockholder in our company, you will be deemed to have notice of and have consented to the provisions of our Certificate and amended and restated bylaws related to choice of forum, but will not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder. The choice of forum provisions in our Certificate and amended and restated bylaws may limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or any of our directors, officers or other employees, which may discourage lawsuits with respect to such claims. Alternatively, if a court were to find the choice of forum provision contained in our Certificate and amended and restated bylaws to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations and financial condition.
Item 1B. Unresolved Staff Comments
Not applicable.
Item 2. Properties
We lease approximately 1,000 square feet of office space in Fort Worth, Texas at the rate of $4,000 per month. The lease has no term and is on a month-to-month basis. We also lease 1,500 square feet of office space in Doylestown, Pennsylvania. The lease agreement is for one year and expires October 31, 2023, subject to our option to renew for an additional year. The monthly lease rate is $3,090. We lease approximately 3,750 square feet of lab space in Austin, Texas at a current rate of $7,163 per month. The lease agreement is for three years and expires on May 31, 2025. The lease has an additional three-year option for renewal.
Item 3. Legal Proceedings
As of the date of this report, there are no legal proceedings to which we or our properties are subject. We may be involved, from time to time, in legal proceedings and claims arising in the ordinary course of its business. Such matters are subject to many uncertainties and outcomes and are not predictable with assurance.
Item 4. Mine Safety Disclosures
Not applicable.
34
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Repurchases of Equity Securities
Market Information
Our common stock has traded on the NASDAQ Stock Market under the symbol “TFFP.”
Holders of Record
As of March 21, 2023, there were six holders of record of our common stock.
Dividend Policy
We have never declared or paid cash dividends on our common stock. We presently intend to retain earnings to finance the operation and expansion of our business.
Equity Compensation Plan Information
We have adopted the TFF Pharmaceuticals, Inc. 2018 Stock Incentive Plan (“2018 Plan”) providing for the grant of non-qualified stock options and incentive stock options to purchase shares of our common stock and for the grant of restricted and unrestricted share grants. We reserved 3,284,480 shares of our common stock under the 2018 Plan. All officers, directors, employees and consultants to our company are eligible to participate under the 2018 Plan. The purpose of the 2018 Plan is to provide eligible participants with an opportunity to acquire an ownership interest in our company.
In September 2021, we adopted the TFF Pharmaceuticals, Inc. 2021 Stock Incentive Plan (“2021 Plan”), which was also approved by our stockholders at our annual meeting of stockholders held on November 4, 2021. The 2021 Plan provides for the grant of non-qualified stock options and incentive stock options to purchase shares of our common stock, the grant of restricted and unrestricted share awards and grant of restricted stock units. We reserved 4,200,000 shares of our common stock under the 2021 Plan. All of our employees and any subsidiary employees (including officers and directors who are also employees), as well as all of our nonemployee directors and other consultants, advisors and other persons who provide services to us will be eligible to receive incentive awards under the 2021 Plan.
The following table sets forth certain information as of December 31, 2022 about our stock plans under which our equity securities are authorized for issuance.
| Plan Category | (a) Number of Securities to be Issued Upon Exercise of Outstanding Options | (b) Weighted- Average Exercise Price of Outstanding Options | (c) Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected In Column (a)) | |||||||||
| Equity compensation plans approved by security holders | 2,909,057 | $ | 5.96 | 1,773,522 | ||||||||
| Equity compensation plans not approved by security holders | — | — | — | |||||||||
| Total | 2,909,057 | $ | 5.96 | 1,773,522 | ||||||||
Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 6. Reserved
35
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
General
We were formed as a Delaware corporation on January 24, 2018 for the purpose of developing and commercializing innovative drug products based on our patented Thin Film Freezing, or TFF, technology platform”. Since our formation, we have focused on the development of our initial drug candidates, the establishment of strategic relationships with established pharmaceutical companies for the licensing of our TFF technology platform and the pursuit of additional working capital. We have not commenced revenue-producing operations.
Since our organization in 2018, we have engaged in the following financing transactions:
Series A Preferred Stock Placements. In March 2018, we conducted a private placement of 5,662,000 shares of our Series A preferred stock, at an offering price of $2.50 per share, for the gross proceeds of approximately $14.2 million, and in May 2019 we conducted a private placement of 3,268,000 shares of our Series A preferred stock, at an offering price of $2.50 per share, for the gross proceeds of approximately $8.2 million. The shares of our Series A preferred stock accumulated dividends at the rate of 6% per annum. The shares of Series A preferred stock, including all accrued but unpaid dividends on the Series A preferred stock, which totaled $1,603,709, automatically converted into 9,571,692 shares of our common stock concurrent with the completion of our initial public offering at the conversion price of $2.50.
Initial Public Offering. On October 25, 2019, we conducted an initial public offering of 4,400,000 shares of common stock at a public offering price of $5.00 per share. After the payment of underwriter discounts and offering expenses, and after giving effect to the underwriters’ exercise of its overallotment option on November 20, 2019 to purchase an additional 479,300 shares of our common stock at the offering price of $5.00 per share, we received net proceeds of approximately $21.8 million.
August 2020 Private Placement. On August 13, 2020, we conducted a private placement of 3,048,654 shares of common stock, at a purchase price per share of $8.50, for aggregate gross proceeds of approximately $25,914,000, before deducting selling commissions and other offering expenses. After deducting the placement agent commissions and offering expenses, we received net proceeds of approximately $24,280,000.
March 2021 Public Offering. On March 30, 2021, we conducted a public offering of 2,140,000 shares of common stock, at a purchase price per share of $14.00, for aggregate gross proceeds of approximately $30,000,000, before deducting underwriter discounts and offering expenses. After deducting the underwriter discounts and offering expenses, we received net proceeds of approximately $28,015,000.
ATM Offering. On June 10, 2022, we entered into an Open Market Sale Agreement with Jefferies LLC, as agent, under which we may offer and sell, from time to time at our sole discretion, shares of our common stock having an aggregate offering price of up to $35.0 million in an “at-the-market” offering, to or through the agent. From July 2022 through September 30, 2022, we sold 104,011 shares of our common stock at average price of $5.96 per share resulting in net proceeds of approximately $405,000, after deducting sales agent commissions and offering expenses.
November 2022 Public Offering. In November 2022, we completed a public offering, selling 9,282,609 shares of common stock and warrants to purchase up to 4,641,305 shares of common stock at an offering price of $1.15 per share. We received gross proceeds of approximately $10,675,000. In addition, we granted the underwriter a 45-day option to purchase an additional 15% of the number of shares of common stock and warrants at the public offering price, less underwriting discounts and commissions. The option was exercised in November 2022 and the underwriter purchased an additional 1,392,392 shares of common stock and warrants to purchase up to 696,196 shares of common stock and we received additional gross proceeds of approximately $1,601,251. We received net proceeds of $11,235,626, after deducting underwriting discounts and offering-related expenses.
36
Results of Operations
We were formed in January 2018 and have not commenced revenue-producing operations. To date, our operations have consisted of the development and early-stage testing, Phase 1 human clinical trials of our initial product candidates and the current Phase 2 clinical trials of our TFF VORI and TFF TAC. In connection with our organization on January 24, 2018, we entered into a Contribution and Subscription Agreement with Lung Therapeutics, Inc., or LTI, our former parent, pursuant to which we agreed to acquire from LTI certain of LTI’s non-core intellectual property rights and other assets, or the Acquired Assets, all of which relate to our Thin Film Freezing technology. We closed on the acquisition of the Acquired Assets concurrent with the close of the initial Series A preferred stock financing in March 2018.
In December 2019, we established a wholly owned Australian subsidiary, TFF Pharmaceuticals Australia Pty Ltd. in order to conduct clinical research.
As of the date of this report, the COVID-19 pandemic has had a relatively insignificant impact on our operations. During 2020, we experienced a temporary suspension of dosing in the Phase I clinical trial for our TFF TAC due to the COVID-19 pandemic and the pandemic has otherwise caused minor slowing in the timing of certain non-clinical and clinical activities by us and our collaborators and service providers during 2020 and the first quarter of 2021. However, the COVID-19 pandemic has not caused us to forego, abandon or materially delay any proposed activities. While we believe we have been able to effectively manage the disruption caused by the COVID-19 pandemic to date, there can be no assurance that our operations, including the development of our drug candidates, will not be disrupted or materially adversely affected in the future by the COVID-19 pandemic or an epidemic or outbreak of an infectious disease like the outbreak of COVID-19.
The following table summarizes our results of operations with respect to the items set forth below for the fiscal years ended December 31, 2022 and December 31, 2021 together with the percentage change for those items.
| Years ended December 31, | ||||||||||||||||
| 2022 | 2021 | Increase (Decrease) | Change | |||||||||||||
| Grant revenue | $ | 495,805 | $ | 88,161 | $ | 407,644 | 462 | % | ||||||||
| Research and development expense | $ | 18,496,340 | $ | 21,300,865 | $ | (2,804,525 | ) | (13 | )% | |||||||
| General and administrative expense | 13,796,255 | 10,573,954 | 3,222,301 | 30 | % | |||||||||||
| Total operating expense | $ | 32,292,595 | $ | 31,874,819 | $ | 417,776 | 1 | % | ||||||||
We have entered into feasibility and material transfer agreements with third parties that provide us with funds in return for certain research and development activities. During the years ended December 31, 2022 and 2021, we recognized $495,805 and $88,161, respectively, of grant revenue.
Research and development expense was as follows for the years indicated:
| Years ended December 31, | ||||||||||||||||
| 2022 | 2021 | Increase (Decrease) | Change | |||||||||||||
| Manufacturing | $ | 5,871,707 | $ | 9,217,872 | $ | (3,346,165 | ) | (36 | )% | |||||||
| Clinical | 7,002,307 | 4,465,281 | 2,537,025 | 57 | % | |||||||||||
| Preclinical | 507,551 | 3,788,942 | (3,281,391 | ) | (87 | )% | ||||||||||
| Payroll and related | 1,384,306 | 670,926 | 713,380 | 106 | % | |||||||||||
| Stock-based compensation | 908,710 | 459,492 | 449,218 | 98 | % | |||||||||||
| Lab | 588,860 | 227,126 | 361,734 | 159 | % | |||||||||||
| Depreciation | 346,244 | 111,453 | 234,791 | 211 | % | |||||||||||
| Sponsored research | 593,347 | 848,708 | (255,361 | ) | (30 | )% | ||||||||||
| CMC | 422,274 | 580,641 | (158,367 | ) | (27 | )% | ||||||||||
| Other | 871,034 | 930,424 | (59,390 | ) | (6 | )% | ||||||||||
| Total research and development expense | $ | 18,496,340 | $ | 21,300,865 | $ | (2,804,525 | ) | (13 | )% | |||||||
37
General and administrative expense was as follows for the years indicated:
| Years ended December 31, | ||||||||||||||||
| 2022 | 2021 | Increase (Decrease) | Change | |||||||||||||
| Consulting | $ | 1,339,791 | $ | 1,315,123 | $ | 24,668 | 2 | % | ||||||||
| Insurance | 2,407,503 | 999,262 | 1,408,241 | 141 | % | |||||||||||
| Office expenses | 449,056 | 266,045 | 183,011 | 69 | % | |||||||||||
| Patent | 345,251 | 291,809 | 53,442 | 18 | % | |||||||||||
| Payroll and related | 2,213,507 | 1,943,896 | 269,611 | 14 | % | |||||||||||
| Professional fees | 2,130,657 | 1,324,842 | 805,815 | 61 | % | |||||||||||
| Marketing | 1,207,569 | 1,002,333 | 205,236 | 20 | % | |||||||||||
| Stock-based compensation | 3,343,122 | 3,088,876 | 254,246 | 8 | % | |||||||||||
| Other | 359,799 | 341,768 | 18,031 | 5 | % | |||||||||||
| Total general and administrative expense | $ | 13,796,255 | $ | 10,573,954 | $ | 3,222,301 | 30 | % | ||||||||
The following table summarizes our other income and interest income for the years ended December 31, 2022 and December 31, 2021 together with the percentage change for those items.
| Years ended December 31, | ||||||||||||||||
| 2022 | 2021 | Increase (Decrease) | Change | |||||||||||||
| Other income | $ | — | $ | 696,714 | $ | (696,714 | ) | (100 | )% | |||||||
| Interest income | $ | 26,728 | $ | 51,232 | $ | (24,504 | ) | (48 | )% | |||||||
Other income in 2021 consists of $652,877 of refundable Australian research and development incentive program payments for expenditures incurred during 2020 and $43,836 received from the U.S. Internal Revenue Service related to research and development tax credits for expenditures incurred during 2020. Interest income decreased during fiscal 2022 due to lower balances in interest-bearing accounts.
We incurred a net loss of $31.8 million and $31.0 million for the fiscal years ended December 31, 2022 and 2021, respectively.
Financial Condition
As of December 31, 2022, we had total assets of approximately $24.1 million and working capital of approximately $17.9 million. As of December 31, 2022, our liquidity included approximately $16.6 million of cash and cash equivalents. On June 10, 2022, we entered into an Open Market Sale Agreement with Jefferies LLC, as agent, under which we may offer and sell, from time to time at our sole discretion, shares of our common stock having an aggregate offering price of up to $35.0 million in an “at-the-market” or ATM offering, to or through the agent, in which we sold shares of our common stock for net proceeds of $405,000 during 2022. In November 2022, we sold shares of our common stock and warrants to purchase shares of common stock for net proceeds of $11.2 million in an underwritten public offering. As of the date of this report, we will need additional capital to fund our operations over the 12 months following the date of this report. We intend to seek additional funding through various financing sources, including the sale of our equity and/or debt securities, and/or licensing fees for our technology and co-development and joint ventures with industry partners. We believe that our current cash and cash equivalents, and our access to capital through the sale of our equity securities, including the ATM offering, are sufficient to fund our present plan of operations for the next 12 months from the date of filing of these consolidated financial statements. In addition, we will consider alternatives to our current business plan that may enable us to achieve our product development goals with a smaller amount of capital. However, there can be no guarantees that such funds, including any potential funds through the sale of our equity securities, including our ATM offering, will be available on commercially reasonable terms, if at all. If such financing is not available on satisfactory terms, we may be unable to further pursue our business plan and we may be unable to continue operations, in which case you may lose your entire investment.
The report of our independent registered public accounting firm for the year ended December 31, 2022 states that due to our lack of revenue from commercial operations, significant losses and need for additional capital there is substantial doubt about our ability to continue as a going concern.
38
Cash Flows
The following table sets forth a summary of our cash flows for the years ended December 31, 2022 and 2021:
| 2022 | 2021 | |||||||
| Net cash used in operating activities | $ | (27,342,160 | ) | $ | (29,556,971 | ) | ||
| Cash used in investing activities | (1,551,326 | ) | (868,505 | ) | ||||
| Cash flows provided by financing activities | 11,751,003 | 28,884,984 | ||||||
| Effect of exchange rate changes | (39,874 | ) | 34,359 | |||||
| Net change in cash and cash equivalents | $ | (17,182,357 | ) | $ | (1,506,133 | ) | ||
The decrease in cash used in operating activities is primarily a result of changes in operating assets and liabilities. The investing activity is related to purchases of property and equipment. The financing activity for 2022 primarily consists of the November 2022 public offering and proceeds from the ATM offering. The financing activity for 2021 primarily consists of the March 2021 public offering and proceeds from exercises of stock options and warrants.
Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in accordance with accounting principles generally accepted in the United States of America, or GAAP. The preparation of our consolidated financial statements and related disclosures requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, costs and expenses in our consolidated financial statements. We base our estimates on historical experience, known trends and events and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. We evaluate our estimates and assumptions on an ongoing basis. Our actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are described in more detail in Note 3 to our consolidated financial statements included herein, we believe that the following accounting policies are those most critical to the judgments and estimates used in the preparation of our consolidated financial statements.
Stock-Based Compensation
We compute stock-based compensation in accordance with authoritative guidance. We use the Black-Scholes-Merton option-pricing model to determine the fair value of its stock options. The Black-Scholes-Merton option-pricing model includes various assumptions, including the fair market value of our common stock, expected life of stock options, the expected volatility and the expected risk-free interest rate, among others. These assumptions reflect our best estimates, but they involve inherent uncertainties based on market conditions generally outside our control.
As a result, if other assumptions had been used, stock-based compensation cost, as determined in accordance with authoritative guidance, could have been materially impacted. Furthermore, if we use different assumptions on future grants, stock-based compensation cost could be materially affected in future periods.
For grants of our common stock, we use the closing stock price on the date of grant as the fair value of the common stock.
39
Research and Development Expenses
In accordance with authoritative guidance, we charge research and development costs to operations as incurred. Research and development expenses consist of personnel costs for the design, development, testing and enhancement of our technology, and certain other allocated costs, such as depreciation and other facilities related expenditures.
Collaborative Arrangements
We consider the nature and contractual terms of arrangements and assesses whether an arrangement involves a joint operating activity pursuant to which we are an active participant and exposed to significant risks and rewards dependent on the commercial success of the activity. If we are an active participant and exposed to significant risks and rewards dependent on the commercial success of the activity, we account for such arrangement as a collaborative arrangement.
For collaborative arrangements where a collaborative partner is not a customer for certain research and development activities, we account for payments received for the reimbursement of research and development costs as a contra-expense in the period such expenses are incurred. This reflects the joint risk sharing nature of these activities within a collaborative arrangement. We classify payments owed or receivables recorded as other current liabilities or prepaid expenses and other current assets.
If payments from the collaborative partner to us represent consideration from a customer in exchange for distinct goods and services provided, then we account for those payments within the scope of ASC 606, Revenue from Contracts with Customers.
Research and Development Tax Incentive
We are eligible to obtain a cash refund from the Australian Taxation Office for eligible research and development expenditures under the Australian R&D Tax Incentive Program (the “Australian Tax Incentive”). The Company recognizes the Australian Tax Incentive when there is reasonable assurance that the cash refund will be received, the relevant expenditure has been incurred, and the consideration can be reliably measured.
As we have determined that it has reasonable assurance that we will receive the cash refund for eligible research and development expenditures, we record the Australian Tax Incentive as a reduction to research and development expenses as the Australian Tax Incentive is not dependent on us generating future taxable income, our ongoing tax status, or tax position. At each period end, management estimates the refundable tax offset available to us based on available information at the time.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Not applicable.
40
Item 8. Financial Statements and Supplementary Data
Index to Consolidated Financial Statements
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of
TFF Pharmaceuticals, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of TFF Pharmaceuticals, Inc. (the “Company”) as of December 31, 2022 and 2021, the related consolidated statements of operations and comprehensive loss, stockholders’ equity and cash flows for each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 2, the Company has not generated revenue from commercial operations since inception, has incurred significant losses and needs to raise additional capital to fund its operations. These conditions raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Marcum llp
We have served as the Company’s auditor since 2018.
March 31, 2023
F-2
TFF PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2022 | 2021 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Receivable due from collaboration agreement | ||||||||
| Research and development tax incentive receivable | ||||||||
| Prepaid assets and other current assets | ||||||||
| Total current assets | ||||||||
| Operating lease right-of use asset, net | ||||||||
| Property and equipment, net | ||||||||
| Other assets | ||||||||
| Note receivable - Augmenta | ||||||||
| Total assets | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued compensation | ||||||||
| Deferred research grant revenue | ||||||||
| Current portion of operating lease liability | ||||||||
| Total current liabilities | ||||||||
| Operating lease liability, net of current portion | ||||||||
| Total liabilities | ||||||||
| Commitments and contingencies (see Note 4) | ||||||||
| Stockholders’ equity: | ||||||||
| Common stock; $ | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated other comprehensive loss | ( | ) | ( | ) | ||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total stockholders’ equity | ||||||||
| Total liabilities and stockholders’ equity | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-3
TFF PHARMACEUTICALS, INC.
CONSOLIDATED
STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| CONSOLIDATED STATEMENTS OF OPERATIONS | ||||||||
| Grant revenue | $ | $ | ||||||
| Operating expenses: | ||||||||
| Research and development | ||||||||
| General and administrative | ||||||||
| Total operating expenses | ||||||||
| Loss from operations | ( | ) | ( | ) | ||||
| Other income: | ||||||||
| Other income | - | |||||||
| Interest income | ||||||||
| Total other income | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| $ | ( | ) | $ | ( | ) | |||
| CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Other comprehensive loss: | ||||||||
| Foreign currency translation adjustments | ( | ) | ||||||
| Comprehensive loss | $ | ( | ) | $ | ( | ) | ||
The accompanying notes are an integral part of these consolidated financial statements.
F-4
TFF PHARMACEUTICALS, INC.
CONSOLIDATED
STATEMENTS OF STOCKHOLDERS’ EQUITY
FOR THE YEARS ENDED DECEMBER 31, 2022 AND 2021
| Common Stock | Additional Paid in | Accumulated Other Comprehensive | Accumulated | Total Stockholders’ | ||||||||||||||||||||
| Shares | Amount | Capital | Loss | Deficit | Equity | |||||||||||||||||||
| Balance, January 1, 2021 | $ | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||
| Sale of common stock, net of offering costs | ||||||||||||||||||||||||
| Issuance of common stock for stock option exercises | ||||||||||||||||||||||||
| Issuance of common stock for warrant exercises | ||||||||||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||
| Foreign currency translation adjustment | - | |||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||
| Balance, December 31, 2021 | ( | ) | ( | ) | ||||||||||||||||||||
| Sales of common stock through the at-the-market offering, net of offering costs | - | - | ||||||||||||||||||||||
| Sales of common stock and warrants through public offering, net of offering costs | - | - | ||||||||||||||||||||||
| Issuance of common stock for stock option exercises | ||||||||||||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||||||
| Foreign currency translation adjustment | - | ( | ) | ( | ) | |||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||
| Balance, December 31, 2022 | $ | $ | $ | ( | ) | $ | ( | ) | $ | |||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-5
TFF PHARMACEUTICALS, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustment to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock-based compensation | ||||||||
| Depreciation and amortization | ||||||||
| Changes in operating assets and liabilities: | ||||||||
| Receivable due from collaboration agreement | ( | ) | ( | ) | ||||
| Research and development tax incentive receivable | ( | ) | ||||||
| Prepaid assets and other current assets | ( | ) | ||||||
| Accounts payable | ( | ) | ||||||
| Accrued compensation | ( | ) | ||||||
| Deferred revenue | ||||||||
| Operating lease obligation | ( | ) | ||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchases of property and equipment | ( | ) | ( | ) | ||||
| Net cash used in investing activities | ( | ) | ( | ) | ||||
| Cash flows from financing activities: | ||||||||
| Net proceeds from issuances of common stock | ||||||||
| Net proceeds from issuances of common stock and warrants | ||||||||
| Proceeds from issuance of common stock for stock option exercises | ||||||||
| Proceeds from issuance of common stock for warrant exercises | ||||||||
| Net cash provided by financing activities | ||||||||
| Effect of exchange rate changes on cash and cash equivalents | ( | ) | ||||||
| Net change in cash and cash equivalents | ( | ) | ( | ) | ||||
| Cash and cash equivalents at beginning of year | ||||||||
| Cash and cash equivalents at end of year | $ | $ | ||||||
| Supplemental disclosure of non-cash investing and financing activities: | ||||||||
| Cashless exercise of warrants | $ | $ | ||||||
| ROU asset obtained for new operating lease | $ | $ | ||||||
| Conversion of collaboration receivable to note receivable | $ | $ | ||||||
| Purchases of equipment included in accounts payable | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-6
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
NOTE 1 – ORGANIZATION AND DESCRIPTION OF BUSINESS
TFF Pharmaceuticals, Inc. (the “Company”) was incorporated in the State of Delaware on January 24, 2018. The Company’s initial focus is on the development of inhaled dry powder drugs to enhance the treatment of pulmonary diseases and conditions. In December 2019, the Company established a wholly owned Australian subsidiary, TFF Pharmaceuticals Australia Pty Ltd (“TFF Australia”), in order to conduct clinical research. TFF Pharmaceuticals, Inc., along with TFF Australia, are collectively referred to as the “Company”. The Company is in the development stage and is devoting substantially all of its efforts toward technology research and development and the human clinical trials of its initial product candidates.
March 2021 Public Offering
On
March 30, 2021, the Company completed a public offering (“March 2021 Offering”), selling
ATM Offering
On
June 10, 2022, the Company entered into an Open Market Sale Agreement with Jefferies LLC, as agent, under which the Company may offer
and sell, from time to time at its sole discretion, shares of its common stock having an aggregate offering price of up to $
November 2022 Public Offering
In
November 2022, the Company completed a public offering (“November 2022 Offering”), selling
NOTE 2 – GOING CONCERN AND MANAGEMENT’S PLANS
The accompanying consolidated financial statements have been prepared under the assumption the Company will continue to operate as a going concern, which contemplates the realization of assets and the settlement of liabilities in the normal course of business. The consolidated financial statements do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts of liabilities that may result from uncertainty related to the Company’s ability to continue as a going concern.
For the years ended December 31, 2022 and 2021,
the Company reported a net loss of $
Management believes that the Company does not have sufficient capital resources to sustain operations through at least the next twelve months from the date of this filing. Additionally, in view of the Company’s expectation to incur significant losses for the foreseeable future it will be required to raise additional capital resources in order to fund its operations, although the availability of, and the Company’s access to such resources, is not assured. Accordingly, management believes that there is substantial doubt regarding the Company’s ability to continue operating as a going concern through at least the next twelve months from the date of this filing
F-7
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The Company’s consolidated financial statements are presented in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and the rules and regulations of the Securities and Exchange Commission (“SEC”) and reflect the financial position, results of operations and cash flows for all periods presented.
Principles of Consolidation
The consolidated financial statements include the accounts of TFF Pharmaceuticals, Inc. and its wholly owned subsidiary, TFF Australia. All material intercompany accounts and transactions have been eliminated in consolidation.
Foreign Currency
The currency of TFF Australia, the Company’s international subsidiary, is in Australian dollars. Foreign currency denominated assets and liabilities are translated into U.S. dollars using the exchange rates in effect at each balance sheet date. Results of operations and cash flows are translated using the average exchange rates throughout the period. The effect of exchange rate fluctuations on translation of assets and liabilities is included as a separate component of stockholders’ equity in accumulated other comprehensive income (loss).
Geographic Concentrations
The
Company conducts business in the U.S. and Australia. As of December 31, 2022 and 2021, the Company maintained
Cash and Cash Equivalents
The
Company maintains its operating accounts in financial institutions in the U.S. and in Australia. The balances are insured up to specified
limits. The Company’s cash is maintained in checking accounts and money market funds with maturities of less than three months
when purchased, which are readily convertible to known amounts of cash, and which in the opinion of management are subject to insignificant
risk of loss in value. As of December 31, 2022 and 2021, the Company had cash in Australia of AUD$
Property and Equipment, net
Property
and equipment are stated at cost less accumulated depreciation and amortization. The Company calculates depreciation using the straight-line
method over the estimated useful lives of the assets, which range from
F-8
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
Leases
At
the inception of an arrangement, the Company determines whether an arrangement is or contains a lease based on the facts and circumstances
present in the arrangement. An arrangement is or contains a lease if the arrangement conveys the right to control the use of an identified
asset for a period of time in exchange for consideration. Leases with a term greater than
Operating lease liabilities and their corresponding right-of-use assets are recorded based on the present value of lease payments over the expected remaining lease term. Certain adjustments to the right-of-use asset may be required for items such as prepaid or accrued rent. The interest rate implicit in the Company’s leases is typically not readily determinable. As a result, the Company utilizes its incremental borrowing rate, which reflects the fixed rate at which the Company could borrow on a collateralized basis the amount of the lease payments in the same currency, for a similar term, in a similar economic environment.
Fair Value of Financial Instruments
Authoritative guidance requires disclosure of the fair value of financial instruments. The Company’s financial instruments consist of cash and cash equivalents and accounts payable, the carrying amounts of which approximate their estimated fair values primarily due to the short-term nature of the instruments or based on information obtained from market sources and management estimates. The Company measures the fair value of certain of its financial assets and liabilities on a recurring basis. A fair value hierarchy is used to rank the quality and reliability of the information used to determine fair values. Financial assets and liabilities carried at fair value which is not equivalent to cost will be classified and disclosed in one of the following three categories:
Level 1 — Quoted prices (unadjusted) in active markets for identical assets and liabilities.
Level 2 — Inputs other than Level 1 that are observable, either directly or indirectly, such as unadjusted quoted prices for similar assets and liabilities, unadjusted quoted prices in the markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 — Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
Income Taxes
In accordance with authoritative guidance, deferred tax assets and liabilities are recorded for temporary differences between the financial reporting and tax bases of assets and liabilities using the current enacted tax rate expected to be in effect when the differences are expected to reverse. A valuation allowance is recorded on deferred tax assets unless realization is considered more likely than not.
The Company evaluates its tax positions taken or expected to be taken in the course of preparing the Company’s tax returns to determine whether the tax positions are “more-likely-than-not” of being sustained by the applicable tax authority. Tax positions not deemed to meet the “more-likely-than-not” threshold are not recorded as a tax benefit or expense in the current year. The Company recognizes interest and penalties, if any, related to uncertain tax positions in interest expense. No interest and penalties related to uncertain tax positions were accrued at either December 31, 2022 or 2021.
The Company follows authoritative guidance which requires the evaluation of existing tax positions. The Company files in the federal and various state jurisdictions. Management has analyzed all open tax years, as defined by the statute of limitations, for all major jurisdictions. Open tax years are those that are open for examination by taxing authorities. The Company’s tax years since its incorporation in 2019 and forward are subject to examination by tax authorities due to the carryforward of unutilized net operating losses and research and development credits.
F-9
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
Revenue Recognition
The Company has entered into feasibility and material transfer agreements (“Feasibility Agreements”) with third parties that provide the Company with funds in return for certain research and development activities. Revenue from the Feasibility Agreements is recognized in the period during which the related qualifying services are rendered and costs are incurred, provided that the applicable conditions under the Feasibility Agreements have been met.
The Feasibility Agreements are on a best-effort basis and do not require scientific achievement as a performance obligation. All fees received under the Feasibility Agreements are non-refundable. The costs associated with the Feasibility Agreements are expensed as incurred and are reflected as a component of research and development expense in the accompanying consolidated statements of operations.
Funds
received from the Feasibility Agreements are recorded as revenue as the Company is the principal participant in the arrangement because
the activities under the Feasibility Agreements are part of the Company’s development programs. In those instances where the Company
first receives consideration in advance of providing underlying services, the Company classifies such consideration as deferred revenue
until (or as) the Company provides the underlying services. In those instances where the Company first provides the underlying services
prior to its receipt of consideration, the Company records a grant receivable. During the years ended December 31, 2022 and 2021, the
Company rendered the related services and recognized revenue and research and development expenses of $
Collaborative Arrangements
The Company considers the nature and contractual terms of arrangements and assesses whether an arrangement involves a joint operating activity pursuant to which the Company is an active participant and is exposed to significant risks and rewards dependent on the commercial success of the activity. If the Company is an active participant and is exposed to significant risks and rewards dependent on the commercial success of the activity, the Company accounts for such arrangement as a collaborative arrangement under Accounting Standards Codification (“ASC”) 808, Collaborative Arrangements. ASC 808 describes arrangements within its scope and considerations surrounding presentation and disclosure, with recognition matters subjected to other authoritative guidance, in certain cases by analogy.
For arrangements determined to be within the scope of ASC 808 where a collaborative partner is not a customer for certain research and development activities, the Company accounts for payments received for the reimbursement of research and development costs as a contra-expense in the period such expenses are incurred. This reflects the joint risk sharing nature of these activities within a collaborative arrangement. The Company classifies payments owed or receivables recorded as other current liabilities or prepaid expenses and other current assets, respectively, in the Company’s consolidated balance sheets. Please refer to Note 5, “Joint Development Agreement” for additional details regarding the Company’s joint development agreement (“JDA”) with Augmenta Bioworks, Inc. (“Augmenta”).
If payments from the collaborative partner to the Company represent consideration from a customer in exchange for distinct goods and services provided, then the Company accounts for those payments within the scope of ASC 606, Revenue from Contracts with Customers. The Company does not currently have any collaborative arrangements that are accounted for under ASC 606.
Research and Development Expenses
In accordance with authoritative guidance, the Company charges research and development costs to operations as incurred. Research and development expenses consist of personnel costs for the design, development, testing and enhancement of the Company’s technology, and certain other allocated costs, such as depreciation and other facilities related expenditures.
Research and Development Tax Incentive
The Company is eligible to obtain a cash refund from the Australian Taxation Office for eligible research and development expenditures under the Australian R&D Tax Incentive Program (the “Australian Tax Incentive”). The Company recognizes the Australian Tax Incentive when there is reasonable assurance that the cash refund will be received, the relevant expenditure has been incurred, and the consideration can be reliably measured. During the year ended December 31, 2021, the Company received its first cash refund under the Australian Tax Incentive, which was for expenditures incurred during 2020. Therefore, the Company recorded amounts received, or that it expects to receive, for expenditures incurred during 2020 as other income in the consolidated statements of operations.
F-10
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
As
the Company has determined that it has reasonable assurance that it will receive the cash refund for eligible research and development
expenditures, beginning with expenditures incurred during the year ended December 31, 2021, the Company records the Australian Tax Incentive
as a reduction to research and development expenses as the Australian Tax Incentive is not dependent on the Company generating future
taxable income, the Company’s ongoing tax status, or tax position. At each period end, management estimates the refundable tax
offset available to the Company based on available information at the time. This percentage of eligible research and development expenses
reimbursable under the Australian Tax Incentive is
The
research and development incentive receivable represents an amount due in connection with the Australian Tax Incentive and from the IRS.
The Company has recorded a research and development tax incentive receivable of $
Basic and Diluted Earnings per Common Share
Basic net loss per common share is calculated by dividing the net loss by the weighted-average number of common shares outstanding for the period. Diluted net loss per share is computed by dividing the net loss by the weighted-average number of common shares and dilutive share equivalents outstanding for the period, determined using the treasury-stock and if-converted methods. Since the Company has had net losses for all periods presented, all potentially dilutive securities are anti-dilutive.
For the years ended December 31, 2022 and 2021, the Company had the following potential common stock equivalents outstanding which were not included in the calculation of diluted net loss per common share because inclusion thereof would be anti-dilutive:
| Years
Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Stock Options | ||||||||
| Warrants | ||||||||
Use of Estimates
The preparation of consolidated financial statements in conformity with GAAP requires the Company’s management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting period. Significant estimates include the fair value of stock-based compensation, valuation allowance against deferred tax assets and related disclosures. Actual results could differ from those estimates.
Common Stock Warrants
The Company classifies as equity any warrants that (i) require physical settlement or net-share settlement or (ii) provide the Company with a choice of net-cash settlement or settlement in its own shares (physical settlement or net-share settlement). The Company classifies as assets or liabilities any contracts that (i) require net-cash settlement (including a requirement to net cash settle the contract if an event occurs and if that event is outside the Company’s control), (ii) gives the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement) or (iii) that contain reset provisions that do not qualify for the scope exception. The Company assesses classification of its common stock warrants and other freestanding derivatives at each reporting date to determine whether a change in classification between assets and liabilities is required. The Company’s freestanding derivatives consist of warrants to purchase common stock that were issued in connection with services provided to the Company and the November 2022 Offering. The Company evaluated these warrants to assess their proper classification and determined that the common stock warrants meet the criteria for equity classification in the consolidated balance sheet. The warrants issued for services provided to the Company are measured at fair value, which the Company determines using the Black-Scholes-Merton option-pricing model.
F-11
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
Stock-Based Compensation
The Company computes stock-based compensation in accordance with authoritative guidance. The Company uses the Black-Scholes-Merton option-pricing model to determine the fair value of its stock options. The Black-Scholes-Merton option-pricing model includes various assumptions, including the fair market value of the common stock of the Company, expected life of stock options, the expected volatility and the expected risk-free interest rate, among others. These assumptions reflect the Company’s best estimates, but they involve inherent uncertainties based on market conditions generally outside the control of the Company.
As a result, if other assumptions had been used, stock-based compensation cost, as determined in accordance with authoritative guidance, could have been materially impacted. Furthermore, if the Company uses different assumptions on future grants, stock-based compensation cost could be materially affected in future periods.
Risks and Uncertainties
In December 2019, COVID-19, a novel strain of coronavirus, was first identified in China. In March 2020, the World Health Organization categorized COVID-19 as a pandemic, and the virus has spread to over 100 countries, including the United States. The impact of this pandemic has been and will likely continue to be extensive in many aspects of society, which has resulted in and will likely continue to result in significant disruptions to the global economy, as well as businesses and capital markets around the world.
Potential impacts to the Company’s business include, but are not limited to, temporary closures of facilities of its vendors, disruptions or restrictions on its employees’ ability to travel, disruptions to or delays in ongoing laboratory experiments, preclinical studies, clinical trials, third-party manufacturing supply and other operations, the supply of comparator products, the potential diversion of healthcare resources and staff away from the conduct of clinical trials to focus on pandemic concerns, interruptions or delays in the operations of the U.S. Food and Drug Administration or other regulatory authorities, and the Company’s ability to raise capital and conduct business development activities.
The Company has experienced COVID-19 related delays in its Phase 2 clinical trials for TFF Voriconazole Inhalation Powder (“TFF VORI”) and TFF Tacrolimus Inhalation Powder (“TFF TAC”). While the Company believes it will be able to effectively manage the delays, there can be no assurance that its operations, including the development of its drug candidates, will not be disrupted or materially adversely affected in the future by the COVID-19 pandemic or an epidemic or outbreak of an infectious disease like the outbreak of COVID-19.
Recent Accounting Standards
In August 2020, the Financial Accounting Standards Board issued Accounting Standards Update (“ASU”) No. 2020-06, Debt - Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging -Contracts in Entity’ Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’ Own Equity (“ASU 2020-06”), which simplifies accounting for convertible instruments by removing major separation models required under current GAAP. The ASU also removes certain settlement conditions that are required for equity-linked contracts to qualify for the derivative scope exception, and it simplifies the diluted earnings per share calculation in certain areas. The provisions of ASU 2020-06 are applicable for fiscal years beginning after December 15, 2023, with early adoption permitted no earlier than fiscal years beginning after December 15, 2020. The Company is currently evaluating the impact of ASU 2020- 06 on its consolidated financial statements.
The Company’s management does not believe that any other recently issued, but not yet effective, accounting standards if currently adopted would have a material effect on the consolidated financial statements.
NOTE 4 - COMMITMENTS AND CONTINGENCIES
Operating Leases
In
October 2018, the Company entered into a lease agreement for office space in Doylestown, Pennsylvania. The lease commenced on October
15, 2018 and expires on October 31, 2023, as amended. The lease has an additional one-year option for renewal, and the base rent is $
F-12
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
Supplemental balance sheet information related to leases was as follows:
| December 31, 2022 | ||||
| Operating leases: | ||||
| Operating lease right-of-use assets | $ | |||
| Operating lease liability - current portion | $ | |||
| Operating lease liability - long-term portion | ||||
| Total operating lease liabilities | $ | |||
Supplemental lease expense related to leases was as follows:
For The Years Ended December 31, | ||||||||||
| Lease | Statement of Operations Classification | 2022 | 2021 | |||||||
| Operating lease cost | Research and development | $ | $ | |||||||
| Short-term lease cost | Research and development | |||||||||
| Short-term lease cost | General and administrative | |||||||||
| Total lease expense | $ | $ | ||||||||
Other information related to operating leases:
| December 31, 2022 | ||||
| Weighted-average remaining lease term | ||||
| Weighted-average discount rate | % | |||
Supplemental cash flow information related to operating leases was as follows:
For
The Years Ended | ||||||||
| 2022 | 2021 | |||||||
| Cash paid for operating lease liabilities | $ | $ | ||||||
Approximate future minimum lease payments under non-cancellable leases (including short-term leases) are as follows:
| Fiscal Year Ending December 31, | ||||
| 2023 | $ | |||
| 2024 | ||||
| 2025 | ||||
| Total minimum lease payments | ||||
| Less: Imputed interest | ( | ) | ||
| Total | $ | |||
F-13
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
Legal
The Company may be involved, from time to time, in legal proceedings and claims arising in the ordinary course of its business. Such matters are subject to many uncertainties and outcomes and are not predictable with assurance. While management believes that such matters are currently insignificant, matters arising in the ordinary course of business for which the Company is or could become involved in litigation may have a material adverse effect on its business and financial condition. To the Company’s knowledge, neither the Company nor any of its properties are subject to any pending legal proceedings.
NOTE 5 – LICENSE AND AGREEMENTS
In
July 2015, the University of Texas at Austin (“UT”) granted to the Company’s former parent, LTI, an exclusive worldwide,
royalty bearing license to the patent rights for the TFF platform in all fields of use, other than vaccines for which LTI received a
non-exclusive worldwide, royalty bearing license to the patent rights for the TFF platform. In March 2018, LTI completed an assignment
to the Company all of its interest to the TFF platform, including the patent license agreement with UT, at which time the Company paid
UT an assignment fee of $
In
May 2018, the Company entered into a master services agreement and associated individual study contracts with ITR Canada, Inc. (“ITR”)
to provide initial contract pre-clinical research and development services for the Company’s drug product candidates.
In April 2019, the Company entered into a master services agreement
with Societal CDMO (formally known as Irisys, LLC) to provide contract manufacturing services for one of the Company’s drug product
candidates, TFF VORI. The Company had a credit due in connection with this agreement of approximately $
In
January 2020, TFF Australia entered into a master consultancy agreement with Novotech (Australia) Pty Ltd. (formally known as Clinical
Network Services Pty Ltd.)
F-14
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
In May 2020, TFF Australia entered into an amended clinical trial
research agreement with Nucleus Network Pty Ltd. to provide a Phase I study of one of the Company’s drug candidates, TFF TAC. The
accounts payable due in connection with this agreement was approximately $
On
August 12, 2020, the Company entered into a licensing and collaboration agreement with UNION therapeutics A/S in which UNION acquired
an option to obtain a worldwide exclusive license for the TFF technology in combination with niclosamide. Pursuant to the terms of the
license agreement, UNION can exercise its option to obtain the license within 45 days after the complete data has been received by UNION
from investigator-initiated trials. Upon exercise of the option, UNION shall be responsible to pay all expenses incurred in the development
of any licensed product. The Company will be eligible to receive milestone payments upon the achievement of certain milestones in the
development the licensed products, based on completion of clinical trials, pre-marketing approvals and/or the receipt of at least $
In
January 2021, the Company entered into a master services agreement with Experic to provide contract manufacturing services for one of
the Company’s drug product candidates, TFF VORI. The accounts payable
due in connection with this agreement was approximately $
In
January 2022, the Company entered into a Letter of Intent with Synteract, Inc. to provide contract research and development services,
which was replaced by a Master Services Agreement entered into in May 2022, for one of the Company’s drug product candidates, TFF
VORI. The accounts payable due in connection with this agreement was approximately
$
Joint Development Agreement
On November 2, 2020, the Company and Augmenta entered into the JDA pursuant to which the Company and Augmenta (collectively the “Parties”) agreed to work jointly to develop one or more novel commercial products incorporating Augmenta’s human derived monoclonal antibody for the treatment of patients with COVID-19 and the Company’s patented Thin Film Freezing technology platform. Each party retains full ownership over its existing assets.
The
Parties will share development costs with each party funding its fifty-percent-share at specified times. In the event that one of the
Parties fails to make its pro rata share payment, the other party may terminate the JDA. In lieu of terminating the JDA, the non-defaulting
party may elect to continue the JDA by paying the delinquent amount and each party’s pro rata share of the JDA will automatically
adjust by the amount paid. In addition, in the event Augmenta experienced a default on its required payment, Augmenta had the one-time
right to elect to require the Company to purchase Augmenta’s interest in the JDA (“Put Right”) for a one-time fee of
$
F-15
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
The JDA is within the scope of ASC 808 as the Company and Augmenta are both active participants in the research and development activities and are exposed to significant risks and rewards that are dependent on commercial success of the activities of the arrangement. The research and development activities are a unit of account under the scope of ASC 808 and are not promises to a customer under the scope of ASC 606.
The
Company records its portion of the research and development expenses as the related expenses are incurred. All payments received or amounts
due from Augmenta for reimbursement of shared costs are accounted for as an offset to research and development expense. During the years
ended December 31, 2022 and 2021, the Company recorded research and development expenses of $
Effective January 1, 2023, the Company and Augmenta entered into a convertible note purchase agreement (“Augmenta Note”) in which the receivable due from Augmenta was converted into a convertible note receivable (see Note 10). The Augmenta Note satisfies Augmenta’s requirement to fund its fifty-percent-share of the development costs under the JDA. In addition, the Company and Augmenta agreed to suspend the development work under the JDA. The Augmenta Note has a maturity date of January 1, 2026; therefore, the Company has reflected the amount due under the Augmenta Note as a long-term note receivable as of December 31, 2022.
NOTE 6 – STOCKHOLDERS’ EQUITY
Common Stock
March 2021 Offering
On
March 30, 2021, the Company completed the March 2021 Offering, selling
ATM Offering
From
July 2022 through September 30, 2022, the Company sold
November 2022 Public Offering
In
November 2022, the Company completed the November 2022 Offering, selling
Stock Option Exercises
During
the year ended December 31, 2021,
During
the year ended December 31, 2022,
F-16
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
Warrant Exercises
During
the year ended December 31, 2021,
During
the year ended December 31, 2021,
NOTE 7 – WARRANTS
On
February 1, 2021, the Company issued a
In determining the fair value for warrants, the expected life of the Company’s warrants was determined using the contractual life. The methodology in determining all other inputs to calculate the fair value utilizing the Black-Scholes-Merton option pricing model is the same as the stock option methodology described in Note 8 for stock options.
In
connection with the November 2022 Offering, the Company issued warrants to purchase
A summary of warrant activity for the years ended December 31, 2022 and 2021 is as follows:
| Number
of Shares | Range
of Exercise Prices | Weighted- Average Exercise Prices | Weighted- Average Remaining Life | |||||||||||||
| Outstanding at January 1, 2021 | $ | $ | ||||||||||||||
| Issued | ||||||||||||||||
| Exercised | ( | ) | ||||||||||||||
| Outstanding at December 31, 2021 | ||||||||||||||||
| Issued | ||||||||||||||||
| Exercised | ||||||||||||||||
| Outstanding at December 31, 2022 | $ | $ | ||||||||||||||
The
warrants outstanding at December 31, 2022 had an aggregate intrinsic value of approximately $
F-17
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
NOTE 8 – STOCK BASED COMPENSATION
In
January 2018,
In
September 2021,
The following table summarizes the stock-based compensation expense recorded in the Company’s results of operations during the years ended December 31, 2022 and 2021 for stock options and warrants:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Research and development | $ | $ | ||||||
| General and administrative | ||||||||
| $ | $ | |||||||
As
of December 31, 2022, there was approximately $
The Company records compensation expense for employee and nonemployee awards with graded vesting using the straight-line method. The Company recognizes compensation expense over the requisite service period applicable to each individual award, which generally equals the vesting term. The Company estimates the fair value of each option award using the Black-Scholes-Merton option pricing model. Forfeitures are recognized when realized.
The Company estimated the fair value of employee and nonemployee stock options using the Black-Scholes option pricing model. The fair value of stock options issued was estimated using the following assumptions:
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Weighted average exercise price | $ | $ | ||||||
| Weighted average grant date fair value | $ | $ | ||||||
| Assumptions | ||||||||
| Expected volatility | % | % | ||||||
| Expected term (in years) | ||||||||
| Risk-free interest rate | % | % | ||||||
| Expected dividend yield | % | % | ||||||
The risk-free interest rate was obtained from U.S. Treasury rates for the applicable periods. The Company’s expected volatility was based upon the historical volatility for industry peers and used an average of those volatilities. The expected life of the Company’s options was determined using the simplified method as a result of limited historical data regarding the Company’s activity for employee awards and the contractual term for nonemployee awards. The dividend yield considers that the Company has not historically paid dividends, and does not expect to pay dividends in the foreseeable future. The Company uses the closing stock price on the date of grant as the fair value of the common stock.
F-18
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
The following table summarizes stock option activity during the years ended December 31, 2022 and 2021:
Number of Shares | Weighted- Average Exercise Prices | Weighted- Average Remaining Contractual Term (In Years) | Intrinsic Value | |||||||||||||
| Outstanding at January 1, 2021 | $ | $ | ||||||||||||||
| Granted | ||||||||||||||||
| Exercised | ( | ) | ||||||||||||||
| Outstanding at December 31, 2021 | $ | $ | ||||||||||||||
| Granted | ||||||||||||||||
| Exercised | ( | ) | ||||||||||||||
| Exercised | ( | ) | ||||||||||||||
| Outstanding at December 31, 2022 | $ | $ | ||||||||||||||
| Exercisable at December 31, 2022 | $ | $ | ||||||||||||||
Option Modifications
Effective
March 21, 2022, one of the members of the Company’s board of directors, Dr. Brian Windsor, resigned. As part of his resignation
from the board of directors, modifications were made to Dr. Windsor’s vested and non-vested stock option awards including acceleration
of certain non-vested option awards and the extension of the post-termination exercise period of certain stock option awards. During
the year ended December 31, 2022, in accordance with ASC Topic 718, Compensation-Stock Compensation, the Company recorded a one-time,
non-cash incremental compensation expense net of the required reversal of previously recognized compensation attributed to non-vested
shares in the amount of approximately $
Effective
December 4, 2022, the Company’s CEO, Glenn Mattes, resigned. As part of his resignation, modifications were made to certain of
Mr. Mattes’ vested stock option awards to extend the post-termination exercise period of these stock option awards. During the
year ended December 31, 2022, in accordance with ASC 718, the Company recorded a one-time, non-cash incremental compensation expense
in the amount of approximately $
NOTE 9 – INCOME TAXES
The Company had no income tax expense due to operating losses incurred for the years ended December 31, 2022 and 2021. The Company accounts for income taxes in accordance with ASC 740, which requires that the tax benefit of net operating losses, temporary differences and credit carryforwards be recorded as an asset to the extent that management assesses that realization is “more likely than not.” Realization of the future tax benefits is dependent on the Company’s ability to generate sufficient taxable income within the carryforward period. Because of the Company’s recent history of operating losses, management believes that recognition of the deferred tax assets arising from the above-mentioned future tax benefits is currently not likely to be realized and, accordingly, has provided a full valuation allowance.
F-19
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
The Company’s income tax expense for the years ended December 31, 2022 and 2021 are summarized below:
| December 31, | ||||||||
| 2022 | 2021 | |||||||
| Current: | ||||||||
| Federal | $ | $ | ||||||
| State | ||||||||
| Foreign | ||||||||
| Total current | $ | $ | ||||||
| Deferred: | ||||||||
| Federal | $ | ( | ) | $ | ( | ) | ||
| State | ||||||||
| Foreign | ( | ) | ||||||
| Change in valuation allowance | ||||||||
| Total deferred | ||||||||
| Income tax provision (benefit) | $ | $ | ||||||
The Company’s deferred tax assets are as follows:
| December 31, | ||||||||
| 2022 | 2021 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforwards | $ | $ | ||||||
| Research and development tax credit | ||||||||
| Section 174 amortization | - | |||||||
| Intangibles | ||||||||
| Stock compensation | ||||||||
| Accruals and other | ( | ) | - | |||||
| Total deferred tax assets | ||||||||
| Valuation allowances | ( | ) | ( | ) | ||||
| Net deferred tax assets | $ | $ | ||||||
The effective tax rate of the Company’s provision (benefit) for income taxes differs from the federal statutory rate as follows:
| December 31, | ||||||||
| 2022 | 2021 | |||||||
| Statutory rate | % | % | ||||||
| State rate | % | % | ||||||
| Foreign | ( | )% | ( | )% | ||||
| Permanent book/tax differences | ( | )% | ( | )% | ||||
| Research and development credit | % | % | ||||||
| Changes in valuation allowance | ( | )% | ( | )% | ||||
| Total | ||||||||
As
of December 31, 2022 and 2021, the Company had gross federal income tax net operating loss (“NOL”) carryforwards of $
F-20
TFF PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
For The Years Ended December 31, 2022 and 2021
Utilization of U.S. net operating losses and tax credit carryforwards may be limited by “ownership change” rules, as defined in Sections 382 and 383 of the Code. Similar rules may apply under state tax laws. The Company has not conducted a study to-date to assess whether a limitation would apply under Sections 382 and 383 of the Code as and when it starts utilizing its net operating losses and tax credits. The Company will continue to monitor activities in the future. In the event the Company previously experienced an ownership change, or should experience an ownership change in the future, the amount of net operating losses and research and development credit carryovers available in any taxable year could be limited and may expire unutilized.
The
CARES Act was signed into law on March 27, 2020 as a response to the economic challenges facing U.S. businesses caused by the COVID-19
global pandemic. The CARES Act allowed net operating loss incurred in 2018-2020 to be carried back
The Inflation Reduction Act (“IRA”) was enacted on August 16, 2022. The IRA introduced new provisions
including a
Due
to the uncertainty surrounding the realization of the benefits of its deferred assets, including NOL carryforwards, the Company has provided
a
The
Company accounts for uncertain tax positions in accordance with the provisions of ASC 740, Income Taxes. When uncertain tax positions
exist, the Company recognizes the tax benefit of tax positions to the extent that the benefit will more likely than not be realized.
The determination as to whether the tax benefit will more likely than not be realized is based upon the technical merits of the tax position
as well as consideration of the available facts and circumstances. As of December 31, 2022, the Company had a reserve for uncertain tax
positions of $
A reconciliation of the change in the unrecognized tax positions for the year ended December 31, 2022 is as follows:
| Federal and State | ||||
| Balance at December 31, 2021 | $ | |||
| Additions for tax positions related to current year | ||||
| Decreases for tax positions related to prior years | ||||
| Balance at December 31, 2022 | $ | |||
NOTE 10 – SUBSEQUENT EVENTS
The Company has performed an evaluation of events occurring subsequent to December 31, 2022 through the filing date of this Annual Report. Based on its evaluation, nothing other than the events described below need to be disclosed.
Effective
January 1, 2023, the Company and Augmenta entered into the Augmenta Note in which a receivable due from Augmenta in connection with the
JDA was converted into a convertible note receivable (see Note 5). Under the terms of the Augmenta Note, Augmenta agreed to pay the principal
amount of $
F-21
The Company has the following optional conversion rights under the Augmenta Note:
| ● | The Company may convert, at any time and at its option, all outstanding principal and accrued and unpaid interest into shares of Augmenta common stock at a price per share equal to an amount obtained by dividing $ |
| ● | If Augmenta completes a private placement sale of its preferred stock in the amount less than $ |
In addition, the outstanding principal and accrued and unpaid interest under the Augmenta Note will automatically convert in the following scenarios:
| ● | If Augmenta completes a financing with gross proceeds of at least $ |
| ● | If Augmenta completes an underwritten public offering with gross proceeds of at least $ |
| ● | If a change of control occurs prior to the payment in full of the principal amount of the Augmenta Note, then the Company will be paid all outstanding principal and accrued and unpaid interest, plus a premium of |
F-22
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
Not applicable.
Item 9A. Controls and Procedures
(a) Evaluation of Disclosure Controls and Procedures.
Our management, with the participation of our chief executive officer and chief financial officer evaluated the effectiveness of our disclosure controls and procedures pursuant to Rule 13a-15(e) under the Exchange Act. Based upon that evaluation, our management, including our chief executive officer and chief financial officer, concluded that our disclosure controls and procedures were effective as of December 31, 2022 in ensuring all material information required to be filed has been made known in a timely manner.
(b) Changes in internal control over financial reporting.
There were no changes to our internal control over financial reporting, as defined in Rule 13a-15(f) under the Exchange Act that occurred during the quarter ended December 31, 2022 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
(c) Management’s report on internal controls over financial reporting.
Our management is responsible for establishing and maintaining adequate internal controls over financial reporting, as defined under Rule 13a-15(f) under the Exchange Act. Our management has assessed the effectiveness of our internal controls over financial reporting as of December 31, 2022 based on the framework established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 Framework) (“COSO”). Our internal control system was designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. An internal control material weakness is a significant deficiency, or aggregation of deficiencies, that does not reduce to a relatively low level the risk that material misstatements in financial statements will be prevented or detected on a timely basis by employees in the normal course of their work. Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2022, and based on that evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2022.
This report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to the rules of the Securities and Exchange Commission that permit us to provide only management’s report in this Annual Report.
Item 9B. Other Information
Not applicable.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections
Not applicable.
41
PART III
Item 10. Directors, Executive Officers and Corporate Governance
The following sets forth information regarding the current executive officers and directors of the Company as of March 24, 2022.
|
Name |
Age | Position | ||
| Harlan Weisman, M.D. | 70 | President, Chief Executive Officer and Vice-Chairman of the Board | ||
| Kirk Coleman | 50 | Chief Financial Officer | ||
| Christopher Cano | 52 | Chief Operating Officer and Vice President of Business Development | ||
| Zamaneh Mikhak, M.D. | 58 | Chief Medical Officer | ||
| Aaron Fletcher, Ph.D. (a), (b), (c) | 42 | Chairman of the Board, Independent Director | ||
| Brandi Roberts (a), (c) | 49 | Independent Director | ||
| Robert S. Mills (c) | 70 | Independent Director | ||
| Stephen C. Rocamboli (a), (b) | 51 | Independent Director |
| (a) | Member of the Audit Committee of our Board. |
| (b) | Member of the Compensation Committee of our Board. |
| (c) | Member of the Nominating and Corporate Governance Committee of our Board. |
Harlan Weisman, M.D. has served as our President and Chief Executive Officer since December 2022, and as a member of our Board since December 2018. Since 2012, Dr. Weisman has also been Managing Director of And-One Consulting, LLC, which is engaged in the business of advising medical product companies, investment firms, and government and non-government healthcare organizations in formulating and implementing strategies for driving innovation in healthcare products and services. Since 2014, Dr. Weisman has also served as Executive Chairman of the Board of 3Dbio Therapeutics, a company using 3D bioprinting technology to develop whole tissue implants that fully integrate into the body. Dr. Weisman was co-founder, Chairman and Chief Executive Officer of Flame Biosciences, Inc. a clinical stage company focused on the research, development and commercialization of transformative therapies for cancer, from January 2020 to January 2022. From February 2016 through 2019, Dr. Weisman served as co-founder and Chief Scientific Officer for Mycrobiomics, a company developing counseling and educational material to help consumers to understand the microbiome and improve their health and well-being. Between December 2012 and December 2013, Dr. Weisman was Chairman and Chief Executive Officer of Coronado Biosciences, a biopharmaceutical company developing novel immunotherapies for autoimmune diseases and cancer. Between 2012 and 2019, Dr. Weisman served on the Board of Directors of ControlRad, Inc, a medical device company developing technology to reduce radiation exposure during fluoroscopic procedures. Dr. Weisman also served on the Board of Directors of Caelum Biosciences, Inc. from 2019 until its acquisition by AstraZeneca in 2021. Since 2012, Dr. Weisman has also been a senior advisor to CRG, an investment management firm making structured debt and equity investments in healthcare companies. Since 2016, Dr. Weisman has been a venture advisor to the Israel Biotech Fund, which invests and develops clinical-stage biotechnology companies based in Israel. From 2010 to 2016, Dr. Weisman served on the Board of Governors of the Patient Centered Outcomes Research Institute, established by the U.S. Congress as part of the Patient Protection and Affordable Care Act of 2010. Dr. Weisman was the Chief Science and Technology Officer of the Johnson & Johnson Medical Devices and Diagnostics Group from 2006 to 2012 and served as Chairman of the J&J Worldwide R&D Council. Dr. Weisman was Company Group Chairman of J&J Pharmaceutical Research & Development from 2004 to 2006.
We believe that Dr. Weisman’s significant education and experience as a senior executive officer in the field of healthcare qualifies him to serve on our Board.
Kirk Coleman has served as our Chief Financial Officer since January 2018. Since 2012, Mr. Coleman also served as an executive officer of Steelhead Capital Management, LLC and Bios Partners, LP, a venture capital firm focused on investment in early-stage and growth-stage biotech and medical device companies. From 1998 to 2008, Mr. Coleman was Treasurer for EFO Holdings, LP, a family office. Mr. Coleman has over 20 years of experience in venture capital investments. Mr. Coleman received a BBA in Accounting from Texas Christian University in 1995.
42
Christopher Cano has served our Chief Operating Officer and Vice President of Business Development since September 24, 2020, and previously served as our Director of Business Development since December 1, 2018. Prior to joining the Company, Mr. Cano served as the Vice President of Business Development at Aqua Pharmaceuticals, LLC, an Almirall company. Prior to Aqua Pharmaceuticals, Mr. Cano was the Head of Business Development at Duchesnay USA, Inc. and held a number of other business development roles at Noven Pharmaceuticals, Inc., a Hitsamitsu company, Agile Therapeutics, Liberty Medical, Nucryst Pharmaceuticals, and Barrier Therapeutics. Mr. Cano has served as the founder and Managing Partner of C2 Strategic Solutions, LLC, a consulting firm providing business development and licensing services to life science companies since January 2011. Mr. Cano holds a bachelor’s degree in finance from Villanova University and a master’s degree in business management from Rider University.
Zamaneh Mikhak, M.D. has served as our Chief Medial officer since January 2023. Dr. Mikhak is a physician-scientist Board Certified in Allergy and Immunology with over 23 years of clinical experience and 18 years of basic and translational research experience. Her industry experience spans across big pharma and small biotech, including biologics and small molecules in rare and common diseases in multiple therapeutic areas. Dr. Mikhak most recently served as Senior Vice President, Head of Clinical Development for Cogent Biosciences, where she oversaw the clinical development function across the company’s major clinical programs. Prior to Cogent, she served as Vice President, Clinical Development at Boston Pharmaceuticals where she led the strategy and clinical development of Avizakimab and executed a global Phase 2 study in systemic lupus erythematosus during the pandemic. Previously, as Senior Director at Kiniksa Pharmaceuticals, Dr. Mikhak advanced Vixarelimab from the preclinical stage into Phase 2 studies in seven indications in approximately two years and generated data towards a successful IPO. Prior to joining Kiniksa, she served as Translation Medicine Lead for early-stage programs across the portfolio at Sanofi Genzyme and led a program in food allergy. Prior to her industry experience, Dr. Mikhak spent over 20 years in clinical practice. From 2006-2014, she practiced medicine at Massachusetts General Hospital, where she provided care to patients with a variety of atopic and immunologic diseases. During her tenure with Boston Children’s Hospital, she established the Healthy Link Asthma Education Program that identified and treated approximately 300 patients with high-risk asthma. Dr. Mikhak was an Assistant Professor at Harvard Medical School and conducted basic and translational research as an NIH funded Principal Investigator. She is the lead author on numerous high-profile scientific publications. Dr. Mikhak is Board Certified in Allergy and Immunology. She completed her Fellowship in Allergy and Immunology at Boston Children’s Hospital and completed her Residency in Pediatrics at Children’s National Medical Center in Washington, D.C. She received her M.D. from the University of Pennsylvania School of Medicine and her B.A. in Biology with distinction in Cell Biology from Boston University.
Aaron Fletcher, Ph.D. has served as a member of our Board since January 2018 and has served as the Chairman of the Board since December 2018. Since 2012, Dr. Fletcher has served as founder and President of Bios Research, a financial services firm that provides public equity research in the healthcare industry tailored to institutional firms and large family offices. Since 2014, Dr. Fletcher has also served as Managing Partner of Bios Partners, LP, a venture capital firm focused on investment in early-stage and growth-stage biotech and medical device companies. Dr. Fletcher also serves as a director of LTI, Cue Biopharma, Inc (Nasdaq: CUE), Actuate Therapeutics and CogRx Therapeutics. Dr. Fletcher holds a Ph.D. in Biochemistry from Colorado State University and serves as a visiting professor at Dallas Baptist University. Dr. Fletcher has worked as an independent consultant for the biotech/healthcare equity industry for over ten years.
We believe that Dr. Fletcher’s significant experience and knowledge of the pharmaceutical industry as a research analyst, venture investor and academic qualifies him to serve on our Board.
Brandi Roberts has served as a member of our Board since March 25, 2022. Ms. Roberts has more than 25 years of public accounting and finance experience, including 22 years at publicly traded pharmaceutical, medical technology, and life science companies. Ms. Roberts has served as the Chief Financial Officer of Longboard Pharmaceuticals, Inc., a publicly traded clinical stage biopharmaceutical company, since January 2021. Previously, Ms. Roberts served as Chief Financial Officer of Lineage Cell Therapeutics, Inc., a publicly traded clinical-stage biotechnology company, from January 2019 to January 2021. Ms. Roberts served as Chief Financial Officer of REVA Medical, Inc., a medical device company, from August 2017 to January 2019. Subsequently, Reva filed a prepackaged voluntary Chapter 11 bankruptcy petition on January 14, 2020 and emerged from bankruptcy protection in United States effective February 26, 2020. Ms. Roberts previously served as Chief Financial Officer of Mast Therapeutics, Inc., a publicly traded biopharmaceutical company, from January 2013 to April 2017, and as its Senior Vice President, Finance, from March 2011 to January 2013. Previously, she held senior positions at Alphatec Spine, Inc., Artes Medical, Inc., Stratagene Corporation, and Pfizer, Inc. Ms. Roberts currently serves as Chair of the Southwest Chapter of the Association of Bioscience Financial Officers and has served on the Board of Temple Therapeutics BV since November 2019. Ms. Roberts is a certified public accountant with the State of California and received her B.S. degree in business administration from the University of Arizona and her M.B.A. from the University of San Diego.
43
We believe that Ms. Roberts’ significant accounting and finance background, including her significant experience as a chief financial officer of biopharmaceutical companies, qualifies her to serve on our Board.
Robert S. Mills has served as a member of our Board since January 2018. Mr. Mills also served as our President and Chief Executive Officer from January 2018 to May 1, 2018, and also served as the Executive Chairman of our Board from January 2018 to December 2018. Mr. Mills has served as the founder and President of RSM Consulting, LLC since January 1, 2015 and as the chairman of the board of directors of LTI since May 7, 2015. From August 2011 to December 2014, Mr. Mills was President and Chief Executive Officer of SPL Pharmaceuticals, the leading manufacturer of heparin and pancreatin, until its sale to a Chinese pharmaceutical company. Mr. Mills also served as a member of the board of directors of SPL Pharmaceuticals from 2011 to 2014. From May 2010 to February 2011, Mr. Mills served as President and as a member of the board of directors of Qualitest Pharmaceuticals, which was acquired by Endo Pharmaceuticals for $1.2 billion. From 2006 to 2010, Mr. Mills served as President and Chief Operating/Executive Officer and as a member of the board of directors of Columbia Laboratories, Inc., which has since been renamed Juniper Pharmaceuticals, Inc. (Nasdaq: CBRX). Mr. Mills was recognized as a finalist for Entrepreneur of the Year for New Jersey in 2009 by Ernst and Young. Mr. Mills holds a B.S. Degree from Grove City College and numerous graduate business credits from Temple University.
We believe that Mr. Mills’ significant experience as chief executive officer in various pharmaceutical companies and his service on several other boards, including the board of LTI, qualifies him to serve on our Board.
Stephen C. Rocamboli has served as a member of our Board since December 2018. Mr. Rocamboli has served as Chief Executive Officer of Perla Therapeutics, Inc., a developer of antibody therapeutics for the treatment of cancer, since July 2020. Mr. Rocamboli has served as Chief Business Officer, General Counsel and Corporate Secretary of Advantagene, Inc., d/b/a Candel Therapeutics, a privately held immune-oncology company based in Needham, Massachusetts, between April 2015 and May 2020. Between 2010 and April 2015, Mr. Rocamboli served as general partner of Integrin Partners, LLC, a consulting firm providing corporate development and strategic transaction advisory and general counsel services to life science companies, investors and entrepreneurs. Between 2010 and 2012, Mr. Rocamboli also served as partner of Beijing International Group, an international affiliate of Integrin Partners. Between 2014 and 2015, Mr. Rocamboli also served as Special Counsel to Wyrick Robbins Yates & Ponton, LLP, focusing on life sciences transactions. Between 2008 and 2018, Mr. Rocamboli was a co-founder and served as President of Pear Tree Pharmaceuticals, a development stage pharmaceutical company focused on the development and commercialization of innovative pharmaceuticals that address the unique unmet needs of aging women and women with breast cancer until its sale to Daré Bioscience, Inc. Prior to joining Pear Tree, Mr. Rocamboli was Senior Managing Director and General Counsel of Paramount BioCapital and its affiliated companies between 2004 and 2007, and was Deputy General Counsel of Paramount from 1999 to 2004. During his tenure at Paramount he was also Partner at Orion Biomedical Fund. Mr. Rocamboli has served as a member of the board of directors of several public and private life sciences companies, including Foresight Biotherapeutics (sold to Shire Pharmaceuticals in 2015) and currently serves as a member of the board of directors of two privately held life sciences companies in New York. Mr. Rocamboli received his B.A. degree from The State University of New York at Albany and his J.D. from Fordham University School of Law.
We believe that Mr. Rocamboli’s significant experience and knowledge of the pharmaceutical industry as a counsel and entrepreneur, and his service on other corporate boards, qualifies him to serve on our Board.
44
Corporate Governance
Audit Committee
Our Audit Committee consists of Brandi Roberts, Stephen Rocamboli and Aaron Fletcher, with Ms. Roberts serving as Chairperson. The composition of our Audit Committee meets the requirements for independence under current Nasdaq Stock Market listing standards and Rule 10A-3 under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Each member of our Audit Committee meets the financial literacy requirements of the Nasdaq Stock Market listing standards. Ms. Roberts is an audit committee financial expert within the meaning of Item 407(d) of Regulation S-K under the Securities Act of 1933, as amended (“Securities Act”).
Compensation Committee Interlocks and Insider Participation
None of our independent directors, Aaron Fletcher, Ph.D., Brandi Roberts, Robert S. Mills or, Stephen C. Rocamboli, is currently or has been at any time one of our officers or employees, except for Mr. Mills’ service as an interim executive officer from January 2018 to December 2018. None of our executive officers currently serves, or has served during the last year, as a member of the board or compensation committee of any entity that has one or more executive officers serving as a member of our Board.
Code of Conduct
We have adopted a code of conduct for all employees, including the chief executive officer, principal financial officer and principal accounting officer or controller, and/or persons performing similar functions, which is available on our website, under the link http://ir.tffpharma.com/corporate-governance.
Item 11. Executive Compensation
Officer Compensation
The following table sets forth the compensation awarded to or earned by our chief executive officer and our two other highest paid executive officers for the years ended December 31, 2022 and 2021. In reviewing the table, please note that Glenn Mattes served as our President and Chief Executive Officer throughout 2021 and 2022 up through December 4, 2022 and Harlan Weisman, M.D. was appointed to serve as our President and Chief Executive Officer on December 4, 2022.
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Option Awards ($) | Total | |||||||||||||
| Glenn Mattes, | 2022 | $ | 475,000 | $ | -- | $ | -- | $ | 475,000 | |||||||||
| Former CEO | 2021 | $ | 450,000 | $ | 146,250 | $ | -- | $ | 596,250 | |||||||||
| Harlan Weisman, | 2022 | $ | 41,955 | $ | -- | $ | -- | $ | 41,955 | |||||||||
| CEO | 2021 | $ | -- | $ | -- | $ | -- | $ | -- | |||||||||
| Kirk Coleman, | 2022 | $ | 309,750 | $ | 72,470 | $ | -- | $ | 382,220 | |||||||||
| CFO | 2021 | $ | 300,000 | $ | 78,500 | $ | -- | $ | 378,500 | |||||||||
| Christopher Cano, | 2022 | $ | 333,125 | $ | 20,500 | $ | -- | $ | 353,625 | |||||||||
| COO and Business Director | 2021 | $ | 325,000 | $ | 62,250 | $ | 276,920 | $ | 664,170 | |||||||||
45
The dollar amounts in the Option Awards columns above reflect the values of options as of the grant date for the years ended December 31, 2022 and 2021, in accordance with ASC 718, Compensation-Stock Compensation and, therefore, do not necessarily reflect actual benefits received by the individuals. Assumptions used in the calculation of these amounts are included in Note 8 to our audited consolidated financial statements.
Narrative Disclosure to Summary Compensation Table
Mattes Employment Agreement
We entered into an agreement with Mr. Mattes dated April 23, 2018. Mr. Mattes served as our President and Chief Executive Officer pursuant to that agreement until December 20, 2018, at which time we entered into a superseding agreement with Mr. Mattes described below. We paid Mr. Mattes at the rate of $25,000 per month under the April 2018 agreement. The April 2018 agreement contained customary provisions relating to intellectual property assignment, confidentiality and indemnification.
We also entered into an executive employment agreement dated December 20, 2018 with Mr. Mattes, which became effective, and replaced and superseded the April 2018 agreement, upon the close of our initial public offering in October 2019. Pursuant to Mr. Mattes’ executive employment agreement, he continued to serve as our President and Chief Executive Officer. Pursuant to the December 2018 employment agreement, we agreed to pay Mr. Mattes at the rate of $33,333 per month commencing upon the close of the IPO, and on May 14, 2020 we amended Mr. Mattes’ employment agreement to increase his salary to $37,500 effective as of June 1, 2020. Mr. Mattes was eligible to receive a bonus of up to 50% of his base salary, commencing with calendar year 2019, based on performance parameters set by our Board, and was also eligible for participation in our incentive compensation plans. Mr. Mattes received a partial bonus of $146,250 for 2021. Mr. Mattes’ executive employment agreement entitled him to reasonable and customary health insurance and other benefits, at our expense, and a severance payment in the amount of 12 months of his base salary in the event of his termination by us without cause or his resignation for good reason, as such terms were defined in the executive employment agreement. Mr. Mattes’ executive employment agreement is an “at will” agreement subject to termination by either party at any time and for any reason. The agreement contained customary provisions relating to intellectual property assignment, confidentiality and indemnification.
In connection with Mr. Mattes’ resignation in December 2022, we entered into a Separation Agreement and General Release with him pursuant to which:
| ● | Mr. Mattes has agreed to provide certain transition services and cooperation, as requested by us, for a period of seven months from the date of his resignation; |
| ● | We agreed to continue payment of Mr. Mattes’ monthly salary for a period of seven months, instead of the 12 months provided for under Mr. Mattes’ employment agreement; |
| ● | We agreed to extend for a period of two years from the date of Mr. Mattes’ resignation options held by Mr. Mattes to purchase up to 450,000 shares of our common stock of the Company, which otherwise would have expired on the one-year anniversary of Mr. Mattes’ resignation: and |
| ● | Mr. Mattes provided us and our affiliates with a general release along with customary covenants of nondisparagement and nonsolicitation. |
Weisman Employment Agreement
In connection with his appointment as President and Chief Executive Officer, we entered into an executive employment agreement with Dr. Weisman pursuant to which we have agreed to pay him an annual salary of $550,000, plus a performance-based bonus of up to 50% of Dr. Weisman’s then-current salary based on performance metrics to be determined by the Board and a severance payment in the amount of 12 months of his base salary and a pro-rata target bonus for the year of termination in the event of his termination by us without cause or his resignation for good reason, as such terms are defined in the executive employment agreement. Dr. Weisman and his family are entitled to medical benefits, at the Company’s expense, on par with those offered to the other senior executive officers of the Company. Dr. Weisman is otherwise eligible to participate in all other benefit plans offered to the senior executive officers of the Company.
46
In connection the appointment of Dr. Weisman, we granted Dr. Weisman options to purchase up to 1,792,450 shares of our common stock over a ten-year period at an exercise price of $1.19 per share. The options shall vest during the period of Dr, Weisman’s service as chief executive officer of the Company as follows: options to purchase 298,750 shares of common stock shall vest and be immediately exercisable upon the six-month anniversary of the date of grant and options to purchase 49,790 shares of common stock shall vest and become exercisable in 30 monthly installments commencing on the seven-month anniversary of the date of grant. In the event of our termination of Dr. Weisman without cause or his resignation for good reason, as such terms are defined in his executive employment agreement, the options shall continue to vest for 12 months following his termination or resignation and all such options shall expire on the two-year anniversary of his termination or resignation. The options are granted under the Company’s 2021 Stock Incentive Plan and the termination of the options are subject to the Plan except as set forth above.
Coleman Employment Agreement
We have entered into an executive employment agreement dated February 15, 2019 with Mr. Coleman pursuant to which he serves as our Chief Financial Officer. Initially, we compensated Mr. Coleman under the employment agreement at the rate of $16,666 per month, which was amended as of December 1, 2019 to increase Mr. Coleman’s salary to $21,666 per month, and further amended on September 24, 2020 to increase Mr. Coleman’s salary to $25,000 per month. On June 7, 2022, we further amended Mr. Coleman’s employment agreement to increase his salary to $26,500 per month effective June 16, 2022.
Mr. Coleman is eligible to receive a bonus of up to 30% of his base salary, commencing with calendar year 2019, based on performance parameters set by our Board, and is also eligible for participation in our incentive compensation plans. Mr. Coleman received a partial bonus of $78,500 for 2021 and a partial bonus of $72,470 for 2022. Mr. Coleman’s employment agreement entitles him to reasonable and customary health insurance and other benefits, at our expense, and a severance payment in the amount of 12 months of his base salary in the event of his termination by us without cause or his resignation for good reason, as such terms are defined in the executive employment agreement. Mr. Coleman’s employment agreement is an “at will” agreement subject to termination by either party at any time and for any reason. The agreement contains customary provisions relating to intellectual property assignment, confidentiality and indemnification.
Cano Employment Agreement
We have entered into an executive employment agreement dated December 18, 2019, which was later amended and restated as of September 24, 2020, with Mr. Cano pursuant to which he serves as our Chief Operating Officer and Vice President of Business Development. From December 2018 to September 2020, Mr. Cano was compensated for his services at the rate of $20,834 per month. Effective as of September 24, 2020, we amended Mr. Cano’s employment agreement to increase his salary to $27,084 per month. On June 7, 2022, we further amended Mr. Cano’s employment agreement to increase his salary to $28,334 effective June 16, 2022. Mr. Cano is entitled to receive a commission of 1% of net proceeds received by the Company, up to a maximum of $1,000,000 per calendar year, from sublicenses of patent rights, provided that with respect to any net proceeds from sublicenses for which the Company is obligated to pay a third-party a sales commission, Mr. Cano’s commission rate will be 0.5% of such net proceeds. Mr. Cano is eligible to receive an annual bonus of 20% of his base salary for meeting key performance requirements, quotas, and assigned objectives determined annually by the Board. Mr. Cano received a partial bonus of $62,250 for 2021 and a partial bonus of $20,500 for 2022.
Pursuant to the employment agreement, Mr. Cano is eligible to participate in all benefits, plans, and programs, which are now, or may hereafter be, available to other executive employees of the Company. Mr. Cano’s employment agreement contains standard provisions concerning noncompetition, nondisclosure and indemnification.
In the event Mr. Cano’s employment with the Company is terminated by the Company without cause, or Mr. Cano resigns for good reason, the Company shall pay Mr. Cano, in addition to all other amounts then due and payable, twelve (12) additional monthly installments of his base salary, less statutory deductions and withholdings.
The employment agreements with our executive officers were unanimously approved by our full Board. No officer or employee of our Company was involved in the Board’s deliberation over the employment agreements of our executive officers, other Glenn Mattes, our chief executive officer.
47
Potential Payments upon Termination
As noted above, the officer employment agreements entitle each officer to reasonable and customary health insurance and other benefits, at our expense, and a severance payment based on their then annual salary and related benefits in the event of our termination of their employment without cause or their resignation for good reason.
If a qualifying involuntary termination had occurred on December 31, 2022, our executive officers would have been eligible to receive the following amounts:
| Name | Type of Payment | Termination of Employment ($) | Change in Control | |||||||
| Harlan Weisman | Cash Severance | $ | 550,000 | $ | – | |||||
| Equity Acceleration | $ | – | $ | – | ||||||
| Kirk Coleman | Cash Severance | $ | 318,000 | $ | – | |||||
| Equity Acceleration | $ | – | $ | – | ||||||
| Christopher Cano | Cash Severance | $ | 340,000 | $ | – | |||||
| Equity Acceleration | $ | – | $ | – | ||||||
Outstanding Equity Awards at December 31, 2022
Set forth below is information concerning the equity awards held by our named executive officers as of December 31, 2022.
| Option Awards | ||||||||||||||
| Name | Number
of Securities Underlying Unexercised Options (#) Exercisable | Number
of Securities Underlying Unexercised Options (#) Unexercisable1 | Option Exercise Price ($) | Option Expiration Date | ||||||||||
| Harlan Weisman | 92,102 | – | $ | 2.50 | 09/26/2028 | |||||||||
| 32,845 | 10,949 | $ | 5.00 | 10/29/2029 | ||||||||||
| 2,717 | 906 | $ | 5.00 | 11/19/2029 | ||||||||||
| 11,250 | 8,750 | $ | 13.65 | 08/24/2030 | ||||||||||
| -- | 1,792,450 | $ | 1.19 | 12/03/2032 | ||||||||||
| Kirk Coleman | 91,250 | 18,750 | $ | 2.50 | 04/11/2029 | |||||||||
| 53,972 | 17,991 | $ | 5.00 | 10/28/2029 | ||||||||||
| 4,400 | 1,480 | $ | 5.00 | 11/28/2029 | ||||||||||
| 37,500 | 12,500 | $ | 5.16 | 12/19/2029 | ||||||||||
| 22,500 | 17,500 | $ | 13.65 | 08/23/2030 | ||||||||||
| Christopher Cano | 7,968 | 532 | $ | 2.50 | 02/01/2029 | |||||||||
| 22,500 | 7,500 | $ | 5.16 | 12/19/2029 | ||||||||||
| 18,750 | 11,250 | $ | 5.81 | 06/24/2030 | ||||||||||
| 5,625 | 4,375 | $ | 13.65 | 08/23/2030 | ||||||||||
| 44,156 | 34,344 | $ | 14.06 | 09/10/2030 | ||||||||||
| 13,437 | 29,563 | $ | 7.93 | 09/21/2031 | ||||||||||
With regard to unexercisable options, 25% of the option award vests and first becomes exercisable on the first anniversary of the date of grant, with the remaining 75% of the option award vesting in 12 equal quarterly installments thereafter.
48
Director Compensation
Set forth below is a summary of the compensation we paid to our non-executive directors during the year ended December 31, 2022. In reviewing the table, please note:
| ● | Brandi Roberts joined our Board in March 2022; |
| ● | Messrs. Windsor, Fairbairn and Thurman resigned from our Board in March 2022, August 2022 and September 2022, respectively; and |
| ● | Harlan Weisman served as non-executive director until December 4, 2022, at which time he was appointed President and Chief Executive Officer and he was no longer entitled to director fees. The table below reflects only his director fees. Please “Officer Compensation” above for a summary of his executive compensation in 2022. |
| Name | Fees Earned or Paid in Cash ($) | Option Awards ($) | All Other Compensation ($) | Total ($) | ||||||||||||
| Aaron Fletcher, Ph.D. | $ | 35,000 | $ | 33,475 | $ | – | $ | 68,475 | ||||||||
| Robert S. Mills | $ | 35,000 | $ | 33,475 | $ | 100,000 | (1) | $ | 168,475 | |||||||
| Brandi Roberts | $ | 28,278 | $ | 507,312 | (2) | $ | – | $ | 535,590 | |||||||
| Stephen Rocamboli | $ | 40,000 | $ | 33,475 | $ | – | $ | 73,475 | ||||||||
| Harlan Weisman, M.D. | $ | 37,065 | $ | – | $ | – | $ | 37,065 | ||||||||
| Randy Thurman | $ | 30,000 | $ | – | $ | – | $ | 30,000 | ||||||||
| Malcolm Fairbairn | $ | 21,358 | $ | – | $ | – | $ | 21,358 | ||||||||
| Brian Windsor | $ | 8,750 | $ | – | $ | – | $ | 8,750 | ||||||||
| (1) | Represents our payment of consulting fees to Mr. Mills during 2022. |
| (2) | Includes an initialoption grant, in connection with Ms. Roberts’ appointment to the Board, to purchase 95,000 shares of common stock at an exercise price of $6.90 per share. |
The dollar amounts in the Option Awards columns above reflect the values of options as of the grant date for the years ended December 31, 2022, in accordance with ASC 718, Compensation-Stock Compensation and, therefore, do not necessarily reflect actual benefits received by the individuals. Assumptions used in the calculation of these amounts are included in Note 8 to our audited consolidated financial statements.
We do not compensate any of our executive directors for their service as a director. During 2022, our non-executive director compensation policy provided for our payment of a quarterly $8,750 cash retainer to our non-employee directors, plus an additional $1,250 per quarter for serving as a chairman of any committee of the Board.
In December 2022, our Board, on the recommendation of the compensation committee of the Board, approved the following compensation policy for our non-executive directors commencing with the 2023 calendar year:
| ● | An annual Board retainer of $65,000 for the Chairperson of the Board and an annual retainer of $35,000 for all other non-executive directors; | |
| ● | An annual grant of non-qualified stock options under the 2021 Plan to purchase .075% of the issued and outstanding shares of our common stock as of the date of grant on a fully diluted basis, all such options to have a ten-year term and vest on the one-year anniversary of the date of grant; | |
| ● | Upon appointment to the Board, an initial grant of non-qualified stock options under the Plan to purchase .1875% of the issued and outstanding shares of our common stock as of the date of grant on a fully diluted basis, all such options to have a ten-year term and vest in 36 equal monthly installments, with a 12 month cliff; | |
| ● | An annual retainer of $10,000 for the chairs of the compensation committee and nominating and corporate governance committee and an annual retainer of $15,000 for the chair of the audit committee; and | |
| ● | An annual retainer of $5,000 for the other members of the compensation committee and nominating and corporate governance committee and an annual retainer of $7,500 for the other members of the audit committee. |
We also reimburse our independent directors for their reasonable expenses incurred in connection with attending meetings of our Board. From time to time, we engage our executive directors to provide consulting services on our behalf and, as disclosed below, during 2021 we engaged Robert S. Mills to provide to us certain consulting services in the area of manufacturing and operations.
49
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth certain information regarding the beneficial ownership of our common stock as of March 15, 2023 by:
| ● | each person who is known by us to be the beneficial owner of more than five percent (5%) of our issued and outstanding shares of common stock; |
| ● | each of our directors, director nominees and named executive officers; and |
| ● | all directors, director nominees and executive officers as a group. |
The beneficial ownership of each person was calculated based on 36,193,085 common shares issued and outstanding as of March 15, 2023. The SEC has defined “beneficial ownership” to mean more than ownership in the usual sense. For example, a person has beneficial ownership of a share not only if he owns it, but also if he has the power (solely or shared) to vote, sell or otherwise dispose of the share. Beneficial ownership also includes the number of shares that a person has the right to acquire within 60 days, pursuant to the exercise of options or warrants or the conversion of notes, debentures or other indebtedness. Two or more persons might count as beneficial owners of the same share. Unless otherwise indicated, the address for each reporting person is 1751 River Run, Suite 400, Fort Worth, Texas 76107.
| Name of Director, Executive Officer or Director Nominees | Number of Shares | Percentage Owned | ||||||
| Harlan Weisman, M.D. | 360,389 | (1) | 1.0 | % | ||||
| Kirk Coleman | 259,077 | (2) | * | |||||
| Christopher Cano | 124,937 | (3) | * | |||||
| Aaron Fletcher, Ph.D. | 928,344 | (4) | 3.0 | % | ||||
| Robert S. Mills | 150,649 | (5) | * | |||||
| Stephen Rocamboli | 104,318 | (6) | * | |||||
| Brandi Roberts | 23,750 | (7) | * | |||||
| Directors, nominees and executive officers as a group | 1,966,464 | 5.0 | % | |||||
| * | Less than 1%. |
| Name and Address of 5% + Holders | Number
of Shares | Percentage Owned | ||||||
| Double Black Diamond
Offshore Ltd. 2100 McKinney Avenue, Suite 1900 Dallas, Texas 75201 | 1,842,000 | 5.0 | % | |||||
| Laurence
W. Lytton 467 Central Park West New York, NY 10025 | 3,125,722 | 9.0 | % | |||||
| (1) | Includes 145,774 shares issuable upon exercise of currently exercisable options. |
| (2) | Includes 243,402 shares issuable upon exercise of currently exercisable options. |
| (3) | Includes 124,937 shares issuable upon exercise of currently exercisable options. |
| (4) | Includes 107,455 shares issuable upon exercise of currently exercisable options and warrants held by Dr. Fletcher and an entity affiliated with Dr. Fletcher. |
| (5) | Includes 134,669 shares issuable upon exercise of currently exercisable options. |
| (6) | Includes 88,138 shares issuable upon exercise of currently exercisable options. |
| (7) | Includes 23,750 shares issuable upon exercise of currently exercisable options. |
50
Item 13. Certain Relationships and Related Transactions, and Director Independence
Related Party Transactions
Since January 1, 2021, we have not entered into any transactions where the amount exceeded the lesser of $120,000 or one percent (1%) of the average of our total assets as of December 31, 2021 and 2020 with any of our directors, officers, beneficial owners of five percent or more of our common shares, any immediate family members of the foregoing or entities of which any of the foregoing are also officers or directors or in which they have a material financial interest, other than the compensatory arrangements with our executive officers and directors described elsewhere in this report.
We have adopted a policy that any transactions with directors, officers, beneficial owners of five percent or more of our common stock, any immediate family members of the foregoing or entities of which any of the foregoing are also officers or directors or in which they have a financial interest, will only be on terms consistent with industry standards and approved by a majority of the disinterested directors of our Board.
Director Independence
Our Board may establish the authorized number of directors from time to time by resolution. Our Board presently consists of five (5) authorized members. Generally, under the listing requirements and rules of the Nasdaq Stock Market, independent directors must comprise a majority of a listed company’s board of directors. Our Board has undertaken a review of its composition, the composition of its committees and the independence of each director. Our Board has determined that, other than Dr. Weisman, by virtue of his executive officer position, none of our director nominees has a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each is “independent” as that term is defined under the applicable rules and regulations of the SEC and the listing requirements and rules of the Nasdaq Stock Market. In making this determination, our Board considered the current and prior relationships that each nonemployee director nominee has with our Company and all other facts and circumstances our Board deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each nonemployee director nominee. Accordingly, a majority of our directors are independent, as required under applicable Nasdaq Stock Market rules, as of the date of this report.
Item 14. Principal Accountant Fees and Services
Fees Incurred for Services by Principal Accountant
The following table sets forth the aggregate fees billed to us for services rendered to us for the years ended December 31, 2022 and 2021 by our independent registered public accounting firm, Marcum LLP.
| 2022 | 2021 | |||||||
| Audit Fees (A) | $ | 150,329 | $ | 143,800 | ||||
| Audit - Related Fees | – | – | ||||||
| Tax Fees | 20,085 | 14,935 | ||||||
| $ | 170,414 | $ | 158,735 | |||||
| (A) | The audit fees consisted of fees for the audit of our financial statements, the review of the interim financial statements included in our quarterly reports on Form 10-Q, and other professional services provided in connection with the statutory and regulatory filings or engagements and capital market financings. |
Pre-Approval Policies and Procedures
The Audit Committee has responsibility for selecting, appointing, evaluating, compensating, retaining and overseeing the work of the independent registered public accounting firm. In recognition of this responsibility, the Audit Committee has established policies and procedures in its charter regarding pre-approval of any audit and non-audit service provided to the Company by the independent registered public accounting firm and the fees and terms thereof.
The Audit Committee considered the compatibility of the provision of other services by its registered public accountant with the maintenance of their independence. The Audit Committee approved all audit services provided by Marcum LLP in 2022 and 2021. Except for certain corporate tax compliance services, Marcum LLP did not perform any non-audit services in 2022 or 2021.
51
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a) Financial statements
Reference is made to the Index and Financial Statements under Item 8 in Part II hereof where these documents are listed.
(b) Financial statement schedules
Financial statement schedules are either not required or the required information is included in the consolidated financial statements or notes thereto filed under Item 8 in Part II hereof.
(c) Exhibits
The exhibits to this Annual Report on Form 10-K are set forth below. The exhibit index indicates each management contract or compensatory plan or arrangement required to be filed as an exhibit.
|
Number |
Exhibit Description | Method of Filing | ||
| 3.1 | Second Amended and Restated Certificate of Incorporation of the Registrant | Incorporated by reference from the Registrant’s Registration Statement on Form S-1 filed on August 20, 2019. | ||
| 3.2 | Certificate of Amendment to Second Amended and Restated Certificate of Incorporation of the Registrant | Filed electronically herewith. | ||
| 4.1 | Specimen Certificate representing shares of common stock of Registrant | Incorporated by reference from the Registrant’s Registration Statement on Form S-1 filed on September 27, 2019. | ||
| 4.2 | Warrant dated October 29, 2019 issued to National Securities Corporation | Incorporated by reference from the Registrant’s Annual Report on Form 10-K filed on March 27, 2020. | ||
| 4.3 | Warrant dated November 20, 2019 issued to National Securities Corporation | Incorporated by reference from the Registrant’s Annual Report on Form 10-K filed on March 27, 2020. | ||
| 4.4 | Description of Capital Stock | Incorporated by reference from the Registrant’s Annual Report on Form 10-K filed on March 10, 2021 | ||
| 4.5 | Form of Warrant issued to investors in November 2022 Follow-On Offering | Filed electronically herewith | ||
| 10.1 | Patent License Agreement dated July 8, 2015 between Lung Therapeutics, Inc. and The University of Texas at Austin | Incorporated by reference from the Registrant’s Registration Statement on Form S-1 filed on August 20, 2019. | ||
| 10.2* | TFF Pharmaceuticals, Inc. 2018 Stock Incentive Plan | Incorporated by reference from the Registrant’s Registration Statement on Form S-1 filed on August 20, 2019. | ||
| 10.3* | Amended and Restated Consulting Agreement dated December 20, 2018 between Robert Mills and the Registrant | Incorporated by reference from the Registrant’s Registration Statement on Form S-1 filed on August 20, 2019. |
52
| 10.4* | Executive Employment Agreement dated December 20, 2018 between Glenn Mattes and the Registrant | Incorporated by reference from the Registrant’s Registration Statement on Form S-1 filed on August 20, 2019. | ||
| 10.5 | Amendment No. 1 to Patent License Agreement dated November 30, 2018 between the Registrant and The University of Texas at Austin | Incorporated by reference from the Registrant’s Registration Statement on Form S-1 filed on August 20, 2019. | ||
| 10.6* | Employment Agreement dated February 15, 2019, by and between the Registrant and Kirk Coleman | Incorporated by reference from the Registrant’s Registration Statement on Form S-1 filed on August 20, 2019. | ||
| 10.7 | Form of Registration Rights Agreement dated August 10, 2020 between the Company and investors named therein | Incorporated by reference from the Registrant’s Current Report on Form 8-K filed on August 11, 2020 | ||
| 10.8* | Amendment No. 1 Dated May 14, 2020 to Executive Employment Agreement Between Glenn Mattes and Registrant | Incorporated by reference from the Registrant’s Quarterly Report on Form 10-Q filed on August 13, 2020. | ||
| 10.9* | Amended and Restated Employment Agreement dated September 24, 2020 by and between the Registrant and Christopher Cano | Incorporated by reference from the Registrant’s Annual Report on Form 10-K filed on March 10, 2021. | ||
| 10.10* | TFF Pharmaceuticals, Inc. 2021 Stock Incentive Plan | Incorporated by reference from the Registrant’s Definitive Proxy Statement filed on September 23, 2021 | ||
| 10.11 | Executive Employment Agreement Between Zamaneh Mikhak, M.D. and Registrant | Filed electronically herewith | ||
| 10.12 | Executive Employment Agreement Between Harlan Weisman, M.D. and Registrant | Filed electronically herewith | ||
| 21.1 | List of Subsidiaries | Incorporated by reference from the Registrant’s Annual Report on Form 10-K filed on March 27, 2020. | ||
| 23.1 | Consent of Marcum LLP | Filed electronically herewith | ||
| 31.1 | Certification under Section 302 of the Sarbanes-Oxley Act of 2002. | Filed electronically herewith. | ||
| 31.2 | Certification under Section 302 of the Sarbanes-Oxley Act of 2002. | Filed electronically herewith. | ||
| 32.1 | Certifications Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, 18 U.S.C. Section 1350. | Filed electronically herewith. | ||
| 101.INS | Inline XBRL Instance Document. | Filed electronically herewith | ||
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document. | Filed electronically herewith | ||
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document. | Filed electronically herewith | ||
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document. | Filed electronically herewith | ||
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document. | Filed electronically herewith | ||
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document. | Filed electronically herewith | ||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). | Filed electronically herewith |
| * | Indicates management compensatory plan, contract or arrangement. |
Item 16. Form 10-K Summary
Not provided.
53
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this annual report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
| TFF PHARMACEUTICALS, INC. | ||
| Date: March 31, 2023 | By: | /s/ Harlan Weisman |
| Harlan Weisman, | ||
| Chief Executive Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Harlan Weisman | Chief Executive Officer and Director | March 31, 2023 | ||
| Harlan Weisman | (Principal Executive Officer) | |||
| /s/ Kirk Coleman | Chief Financial Officer | March 31, 2023 | ||
| Kirk Coleman | (Principal Financial and Accounting Officer) | |||
| /s/ Aaron Fletcher | Chairman of the Board | March 31, 2023 | ||
| Aaron Fletcher, Ph. D | ||||
| /s/ Robert S. Mills, Jr. | Director | March 31, 2023 | ||
| Robert S. Mills, Jr. | ||||
| /s/ Stephen Rocamboli | Director | March 31, 2023 | ||
| Stephen Rocamboli | ||||
| /s/ Brandi Roberts | Director | March 31, 2023 | ||
| Brandi Roberts |
54