| © 2024 Scholar Rock, Inc. All rights reserved. Third Quarter 2024 Business Update November 12, 2024 1 Exhibit 99.2 |
| © 2024 Scholar Rock, Inc. All rights reserved. Forward-Looking Statements This presentation contains "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding Scholar Rock’s future expectations, plans and prospects, including without limitation, Scholar Rock’s expectations regarding its growth, strategy, progress and timing of its clinical trials for apitegromab and SRK-181 and its preclinical programs, including SRK-439, and indication selection and development timing, including the timing of any regulatory submissions, the therapeutic potential, clinical benefits and safety of any product candidates, expectations regarding timing, success and data announcements of current ongoing preclinical and clinical trials, its cash runway, expectations regarding the achievement of important milestones, the ability of any product candidate to perform in humans in a manner consistent with earlier nonclinical, preclinical or clinical trial data, and the potential of its product candidates and proprietary platform. The use of words such as “may,” “might,” “could,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify such forward-looking statements. All such forward-looking statements are based on management's current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, without limitation, whether the results from the Phase 3 SAPPHIRE trial will be sufficient to support regulatory approval, that the full results from the Phase 3 SAPPHIRE trial may differ from the topline data, that preclinical and clinical data, including the results from the Phase 2 or Phase 3 clinical trial of apitegromab, or Part A or Part B of the Phase 1 clinical trial of SRK-181, are not predictive of, may be inconsistent with, or more favorable than, data generated from future or ongoing clinical trials of the same product candidates, including, without limitation, the Phase 2 clinical trial of apitegromab in obesity or Part B of the Phase 1 clinical trial of SRK-181; Scholar Rock’s ability to provide the financial support, resources and expertise necessary to identify and develop product candidates on the expected timeline; the data generated from Scholar Rock’s nonclinical and preclinical studies and clinical trials; information provided or decisions made by regulatory authorities; competition from third parties that are developing products for similar uses; Scholar Rock’s ability to obtain, maintain and protect its intellectual property; Scholar Rock’s dependence on third parties for development and manufacture of product candidates including, without limitation, to supply any clinical trials; and Scholar Rock’s ability to manage expenses and to obtain additional funding when needed to support its business activities and establish and maintain strategic business alliances and new business initiatives, and as well as those risks more fully discussed in the section entitled "Risk Factors" in Scholar Rock’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2024, as well as discussions of potential risks, uncertainties, and other important factors in Scholar Rock’s subsequent filings with the Securities and Exchange Commission. Any forward-looking statements represent Scholar Rock’s views only as of today and should not be relied upon as representing its views as of any subsequent date. All information in this presentation is as of the date of the presentation, and Scholar Rock undertakes no duty to update this information unless required by law. This presentation may also contain estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we compete are necessarily subject to a high degree of uncertainty and risk. Apitegromab, SRK-181, and SRK-439 are investigational drug candidates under evaluation. Apitegromab, SRK-181, and SRK-439 have not been approved for any use by the FDA or any other regulatory agency and the safety and efficacy of apitegromab, SRK-181 and SRK-439 have not been established. |
| © 2024 Scholar Rock, Inc. All rights reserved. Company Overview Jay Backstrom, M.D., MPH President & Chief Executive Officer 3 |
| © 2024 Scholar Rock, Inc. All rights reserved. 4 Company Speakers Jay Backstrom, M.D., MPH President & Chief Executive Officer Ted Myles, MBA Chief Operating Officer and Chief Financial Officer |
| © 2024 Scholar Rock, Inc. All rights reserved. Today’s Agenda 5 Topic Speaker Company Overview Jay Backstrom, President & Chief Executive Officer Ted Myles, Chief Operating Officer & Chief Financial Officer Financial Update Conclusions Jay Backstrom, President & Chief Executive Officer Q&A Session |
| © 2024 Scholar Rock, Inc. All rights reserved. Expanding the Executive Team • Beth Shafer, Ph.D. joined as Chief Business Officer Advancing apitegromab in SMA • Positive Phase 3 SAPPHIRE Study • BLA/MAA submission planned for Q1 2025 • Preparing for commercial launch EMBRAZE Ph 2 Trial continuing momentum • Enrollment completed early with topline expected in Q2 2025 SRK-439 data demonstrating value in obesity program • Updated data November 5 at Obesity Week SRK-181 data reinforcing clinical proof of concept • Additional clinical data November 9 at SITC 6 A Successful Q3, Continuing to Build on Strong 2024 Progress Advancing Corporate Strategy Beth Shafer, Ph.D., Chief Business Officer |
| © 2024 Scholar Rock, Inc. All rights reserved. Our Purpose: Create Possibilities for Those Living with Spinal Muscular Atrophy (SMA) Muscle is everything. I want to live knowing that I have the strength to take care of myself if left alone. - Lyza 7 © 2024 Scholar Rock, Inc. All rights reserved. |
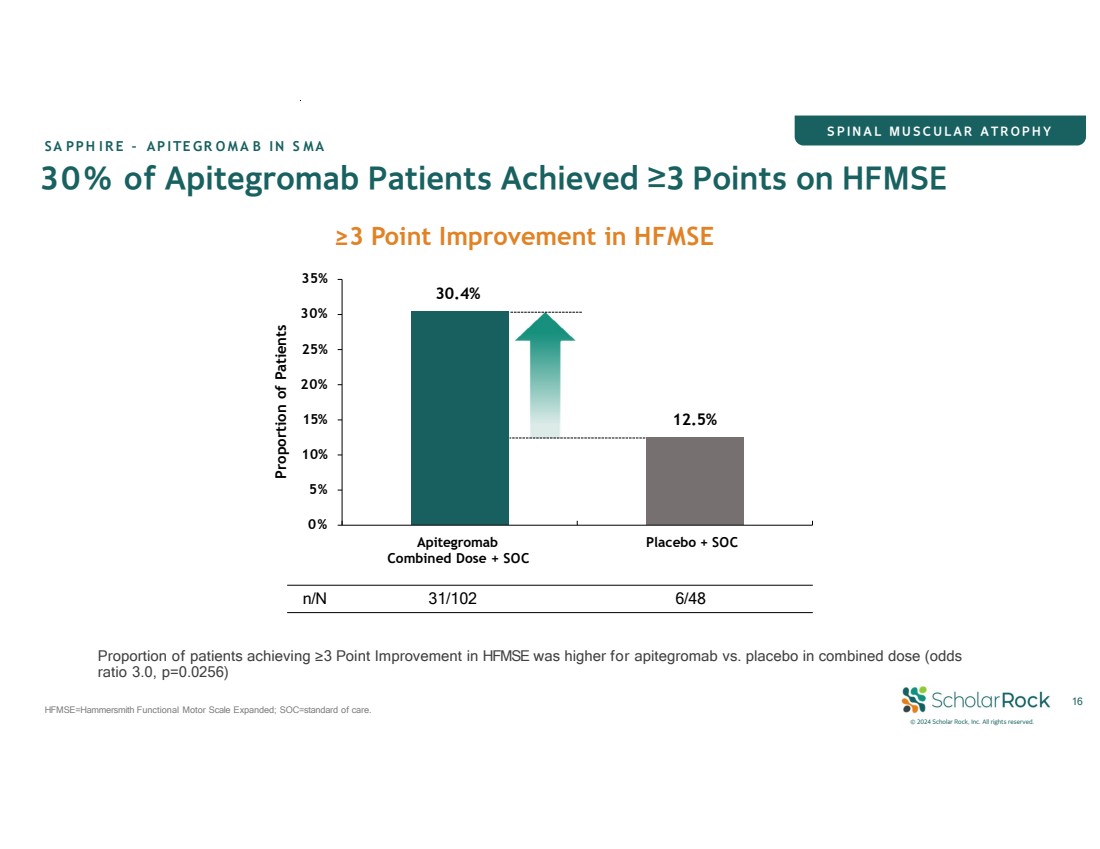
| © 2024 Scholar Rock, Inc. All rights reserved. 8 CONSISTENT clinically meaningful benefit across all age groups (2-21) FAVORABLE SAFETY profile consistent with >48 months experience in Phase 2 TOPAZ trial 30% of apitegromab patients ACHIEVED ≥3 POINT IMPROVEMENT IN HFMSE† 1.8 (p=0.0192) POINT IMPROVEMENT in HFMSE* vs. placebo MET PRIMARY ENDPOINT: Positive Phase 3 SAPPHIRE Trial: Transformative Benefit in SMA Apitegromab has the potential to alter the course of SMA * Based on apitegromab combined dose (10 mg/kg and 20 mg/kg) + SOC versus placebo + SOC † 12.5% of patients on placebo + SOC achieved a ≥3-point improvement in HFMSE SOC=Standard of care (i.e., nusinersen or risdiplam) SPINAL MUSCULAR ATROPHY SAPPHIRE – APITEGROMAB IN SMA |
| © 2024 Scholar Rock, Inc. All rights reserved. 9 SAPPHIRE – APITEGROMAB IN SMA Early-dosed (CHERISH/SHINE) Delayed-dosed (SHINE) Sham control (CHERISH) -6 -4 -2 0 2 4 6 ∆ -1.3 (SD: 9.3) From Baseline ∆ -2.3 (SD: 9.6) From Baseline ∆ -0.4 (SD: 4.9) From Baseline Study Day [Year] Change (SE) in HFMSE Early-dosed (CHERISH/SHINE) n= Delayed-dose (SHINE) n= Sham control (CHERISH) n= 84 42 42 84 41 84 41 84 40 41 83 18 42 76 24 39 83 37 83 37 79 35 74 29 75 30 54 22 61 39 0 92 [0.3] 169 [0.5] 253 [0.7] 350 [1.0] 450 [1.2] 690 [1.9] 930 [2.5] 1170 [3.2] 1410 [3.9] 1710 [4.7] 2070 [5.7] 2430 [6.7] 2790 [7.6] Time Period Similar to TOPAZ study Time Period Similar to SAPPHIRE study Finkel RS et al. “Final Safety and Efficacy Data From the SHINE Study in Participants With Infantile-Onset and Later-Onset SMA.” Presented at Cure SMA Annual Conference, July 2024 *Patient age based on those received active treatment (mean or median) 1. This information from third-party studies is provided for background purposes only and is not intended to convey or imply a comparison to the SAPPHIRE clinical trial results SMN=survival motor neuron SMN-targeted therapies prevent further degeneration of motor neurons1, yet lack any direct impact on muscle atrophy Substantial room for improvement in the current approved treatment landscape exists Despite Chronic SMN Therapy, SMA Patients Continue To Lose Function Over Time SPINAL MUSCULAR ATROPHY |
| © 2024 Scholar Rock, Inc. All rights reserved. 10 CI=Confidence Interval; EXP=Exploration Subpopulation; HFMSE=Hammersmith Functional Motor Scale Expanded; LS=Least Squares; MEP=Main Efficacy Population; SOC=standard of care. SAPPHIRE – APITEGROMAB IN SMA Early and Increasing HFMSE Improvement vs. Placebo Least Squares Mean (+/- SE) Change from Baseline in HFMSE Total Score by Visit (MITT Set) Apitegromab-treated patients improved on HFMSE, while placebo patients declined on HFMSE over 12 months -2 -1 0 1 2 Placebo + SOC (N=50) Apitegromab combined dose + SOC (N=106) 0 8 16 24 32 40 Time (weeks) 52 LS Mean Change from Baseline in HFMSE Placebo + SOC 50 50 50 48 50 49 48 Apitegromab + SOC 106 105 105 101 102 102 102 SPINAL MUSCULAR ATROPHY |
| © 2024 Scholar Rock, Inc. All rights reserved. -5 -4 -3 -2 -1 0 1 2 3 4 5 11 Improvement in HFMSE Consistent Across Doses and Age Groups SAPPHIRE – APITEGROMAB IN SMA LS Mean Difference (95% CI) [Active-Placebo] 1.8 (0.30, 3.32) 2-12 years apitegromab combined dose (10 & 20 mg/kg; n=106) vs. placebo (n=50) 1.4 (-0.34, 3.13) 2-12 years apitegromab 20 mg/kg dose (n=53) vs. placebo (n=50) 2.2 (0.49, 3.95) 2-12 years apitegromab 10 mg/kg dose (n=53) vs. placebo (n=50) 1.8 (-1.06, 4.57) 13-21 years apitegromab 20 mg/kg dose (n=22) vs. placebo (n=10) 1.8 (0.46, 3.16) Pooled 2-21 years apitegromab (n=128) vs. placebo (n=60) Favor Placebo Favor Apitegromab Change from Baseline in HFMSE Total Score at 12 Months* CI=Confidence Interval; EXP=Exploration Subpopulation; HFMSE=Hammersmith Functional Motor Scale Expanded; SOC=standard of care. *n values at 12-month endpoint SPINAL MUSCULAR ATROPHY |
| © 2024 Scholar Rock, Inc. All rights reserved. 30% of Apitegromab Patients Achieved ≥3 Points on HFMSE SA PPH IRE – APITEGR OMA B IN S MA ≥3 Point Improvement in HFMSE 30.4% 16 HFMSE=Hammersmith Functional Motor Scale Expanded; SOC=standard of care. 12.5% 10% 5% 0% 15% 30% 25% 20% 35% Apitegromab Combined Dose + SOC Placebo + SOC Proportion of Patients n/N 31/102 6/48 Proportion of patients achieving ≥3 Point Improvement in HFMSE was higher for apitegromab vs. placebo in combined dose (odds ratio 3.0, p=0.0256) SPINAL MUSCULAR ATROPHY |
| © 2024 Scholar Rock, Inc. All rights reserved. 13 Broadly representative study population Improvement seen across all age groups (2-21) Moving quickly on filing BLA and MAA in Q1 2025 * If approved by regulatory authorities Potential to be Suitable for Broad SMA Population* SPINAL MUSCULAR ATROPHY SAPPHIRE – APITEGROMAB IN SMA |
| © 2024 Scholar Rock, Inc. All rights reserved. Next Wave of Innovation: Selective Anti-Myostatin for Healthy Weight Management 14 Tests hypothesis of selective anti-myostatin in obese population POC study start* POC data readout Q2* Ph 2 proof-of-concept trial APITEGROMAB + GLP-1 Agonist 2024 2025 IND* SRK-439 + GLP-1 Receptor Agonist Novel asset for cardiometabolic indication Phase 1 trial Cardiometabolic Disorders *Expected timelines POC = Proof of Concept OBESITY |
| © 2024 Scholar Rock, Inc. All rights reserved. 15 OBESITY Preclinical data to date show strong potential to support healthier weight loss in combination with GLP-1 RA: Preservation of lean mass during GLP-1 RA-induced weight loss and improvement in fasting glucose Increase in lean mass and attenuation of fat mass regain following GLP-1 RA withdrawal Greater potency compared to an anti-ACTRII antibody Increase in lean mass and lowered fat mass gain following treatment with metformin and SRK-439 Give Us Confidence in SRK-439 Strong Scientific Validation and Promising Preclinical Evidence |
| © 2024 Scholar Rock, Inc. All rights reserved. Potential Benefits Our Solution Delivers Attractive Clinical Risk/Benefit Profile 16 • Inhibition of myostatin, a negative regulator of muscle, is known to promote muscle growth and function • Apitegromab, a selective myostatin inhibitor, has been shown in a Phase 2 Proof-of-Concept study to improve motor function • Given muscle’s important role in energy metabolism, preserving lean mass has the potential to improve durability of weight loss • Selective targeting minimizes off-target effects, potentially supporting long-term use for healthy weight management THERAPEUTIC POTENTIAL OF OUR MUS CLE-TARGETED APPROACH IN OBESIT Y Preserving muscle Improving function Improving durability Long-term safety Key Points OBESITY |
| © 2024 Scholar Rock, Inc. All rights reserved. Financial & Business Update Ted Myles Chief Operating Officer & Chief Financial Officer |
| © 2024 Scholar Rock, Inc. All rights reserved. Commercial Prep Underway • Continuing SMA stakeholder engagement and education • Ensuring an optimized treatment experience • Building team to deliver US & European launch Financial Update 18 High Sense of Urgency to Deliver for Patients Strong Balance Sheet • Cash balance as of September 30, 2024, $139 million, in addition to $324 million of net proceeds from October follow-on offering • Pro forma cash of approximately $463 million, runway into Q4 2026 Expanding Anti-Myostatin Platform • Expanding to SMA <2 with OPAL • Plans for EMBRAZE readout and SRK-439 filing in 2025 show pipeline momentum |
| © 2024 Scholar Rock, Inc. All rights reserved. Building to Achieve Commercial Success 19 Geographic Expansion • Commercialize in selected European countries* • Remaining Europe and ROW expansion through distributorships & partnerships • Commercialize in selected European countries* • Remaining Europe and ROW expansion through distributorships & partnerships Geographic Expansion • Commercialize in selected European countries* • Remaining Europe and ROW expansion through distributorships & partnerships U.S. Launch* • Payer engagement • Patient services implementation • Monthly home infusion • Efficient US customer-facing footprint (<50 FTEs) • Payer engagement • Patient services implementation • Monthly home infusion • Efficient US customer-facing footprint (<50 FTEs) U.S. Launch* • Payer engagement • Patient services implementation • Monthly home infusion • Efficient US customer-facing footprint (<50 FTEs) Building the Foundation • MSL engagement at CureSMA and MDA centers • Life Takes Muscle – first muscle-focused disease education • Continued partnership with patient advocacy groups • MSL engagement at CureSMA and MDA centers • Life Takes Muscle – first muscle-focused disease education • Continued partnership with patient advocacy groups Building the Foundation • MSL engagement at CureSMA and MDA centers • Life Takes Muscle – first muscle-focused disease education • Continued partnership with patient advocacy groups 2024 2025 2026+ * Subject to regulatory approval ROW= Rest of world |
| © 2024 Scholar Rock, Inc. All rights reserved. Path to Achieving Commercial Success in SMA 20 Clear unmet need and favorable market dynamics Competitive and attractive potential profile Engagement, patient focus & execution The right market The right medicine The right plan Apitegromab global revenue potential >$1.0B1 1 Scholar Rock internal estimates as of May 2024 |
| © 2024 Scholar Rock, Inc. All rights reserved. 21 Upcoming Planned Key Milestones * If approved by relevant health authorities • Submit FDA and EMA applications in Q1 2025 • Request priority review (FDA) and accelerated assessment (EMA) Apitegromab Regulatory Submissions • US launch in Q4 2025 and European launch to follow Apitegromab Commercial Launch in SMA* • Obesity: EMBRAZE readout expected in Q2 2025 • SMA: Under 2 study initiation planned for mid-2025 Anti-Myostatin Clinical Momentum |
| © 2024 Scholar Rock, Inc. All rights reserved. Conclusions Jay Backstrom, M.D., MPH President & Chief Executive Officer 22 |
| © 2024 Scholar Rock, Inc. All rights reserved. TGFβ=Transforming growth factor-beta. OUR MISSION To discover, develop, and deliver life-changing therapies by harnessing cutting-edge science to create new possibilities for people living with serious diseases We are a global leader in harnessing the life-changing potential of the TGFβ superfamily |
| © 2024 Scholar Rock, Inc. All rights reserved. 18 Potential to Transform Standard of Care in SMA 1.8-point improvement in HFMSE (p=0.0192) compared to placebo Patients improving on apitegromab vs. declining on placebo Favorable safety profile supports durability of treatment >48 months treatment experience in SMA1 Clear and Meaningful Improvement Well-tolerated Safety Profile Broadly representative study population Improvement across all age groups (2-21) Potential to be Suitable for Broad SMA Population* 1 Based on TOPAZ patients receiving combination therapy after 4 years of treatment. Data cutoff date: April 2024 * If approved by regulatory authorities |
| © 2024 Scholar Rock, Inc. All rights reserved. Q&A Session 25 |
| © 2024 Scholar Rock, Inc. All rights reserved. 26 Company Speakers Jay Backstrom, M.D., MPH President & Chief Executive Officer Ted Myles, MBA Chief Operating Officer and Chief Financial Officer |
| © 2023 Scholar Rock, Inc. All rights reserved. 27 Thank you! |