
BioHarvest Sciences Inc. Management’s Discussion and Analysis For the three and six months ended June 30, 2024 (Expressed in U.S. dollars)
|
INTRODUCTION
The following Management’s Discussion and Analysis (“MD&A”) for BioHarvest Sciences Inc., together with its wholly owned subsidiaries (“BioHarvest Sciences” or the “Company”) prepared as of August 29, 2024, has been prepared in accordance with International Financial Reporting Standards (“IFRS”) as issued by the International Accounting Standard Board (IASB). All amounts (other than per share amounts) are stated in U.S dollars rounded to the nearest thousand, unless otherwise indicated.
The following information should be read in conjunction with the unaudited interim condensed consolidated financial statements for the three and six months ended June 30, 2024, and the related notes to those financial statements.
Statements in this report that are not historical facts are forward-looking statements involving known and unknown risks and uncertainties, which could cause actual results to vary considerably from these statements. Readers are cautioned not to put undue reliance on forward-looking statements.
The Company is publicly traded on the Canadian Securities Exchange under the symbol BHSC, on the OTC under the symbol CNVCF and on the Frankfurt Stock Exchange under the symbol 8MV, the Munich Stock Exchange under the symbol “8MV”, the Stuttgart Stock Exchange under the symbol “CA09076J1084.SG” and the Tradegate Exchange under the symbol “8MV”.
Continuous disclosure materials are available on our website at www.bioharvest.com. This additional information is not incorporated into this Management’s Discussion and Analysis and does not constitute a part of this Management’s Discussion and Analysis.
1
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This MD&A contains certain information that may constitute “forward-looking information” and “forward-looking statements” (collectively, “forward-looking statements”) which are based upon the Company’s current internal expectations, estimates, projections, assumptions and beliefs. Such statements can be identified by the use of forward-looking terminology such as “expect,” “likely”, “may,” “will,” “should,” “intend,” or “anticipate”, “potential”, “proposed”, “estimate” and other similar words, including negative and grammatical variations thereof, or statements that certain events or conditions “may” or “will” happen, or by discussions of strategy. Forward-looking statements include estimates, plans, expectations, opinions, forecasts, projections, targets, guidance, or other statements that are not statements of fact. The forward-looking statements included in this MD&A are made only as of the date of this MD&A. Forward-looking statements in this MD&A may include, but are not limited to, statements with respect to: a) licensing risks; b) regulatory risks; c) change in laws, regulations and guidelines; d) market risks; e) expansion of facilities; f) history of net losses; and g) competition. Certain of the forward-looking statements and forward-looking information and other information contained herein concerning the, nutraceutical, pharmaceutical and cosmeceutical industries, the general expectations of the Company concerning these industries and concerning the Company are based on estimates prepared by the Company using data from publicly available governmental sources, from market research and industry analysis and on assumptions based on data and knowledge of these industries, which the Company believes to be reasonable. The Company is not aware of any misstatement regarding any industry or government data presented herein. Although the Company believes that the expectations reflected in such forward-looking statements are reasonable, it can give no assurance that such expectations will prove to have been correct. The Company’s forward-looking statements are expressly qualified in their entirety by this cautionary statement. In particular, but without limiting the foregoing, disclosure in this MD&A under “Nature of the Business and Overview of Operations” as well as statements regarding the Company’s objectives, plans and goals, including future operating results and economic performance may make reference to or involve forward-looking statements. A number of factors could cause actual events, performance or results to differ materially from what is projected in the forward-looking statements. See “Risk and Uncertainties” for further details. The purpose of forward-looking statements is to provide the reader with a description of management’s expectations, and such forward-looking statements may not be appropriate for any other purpose. You should not place undue reliance on forward- looking statements contained in this MD&A. The Company undertakes no obligation to update or revise any forward-looking statements.
2
GOING CONCERN
The Company has incurred losses from operations since its inception. As of June 30, 2024, the Company has an Accumulated Deficit of $90,773 thousands. The Company generated negative cash flows from operating activities of $2,536 thousands and a loss in the amount of $7,268 thousands for the six months ended June 30, 2024. As of the date of the issuance of the unaudited interim condensed consolidated financial statements for the three and six Months ended June 30, 2024, the Company has not yet commenced generating sufficient sales to fund its operations, and therefore depends on fundraising from new and existing investors to finance its activities. These factors raise material uncertainties that may cast significant doubt about the Company’s ability to continue as a going concern. The Company’s plans to fund near term anticipated activities based on proceeds from capital fund raising and future revenues.
The unaudited interim condensed consolidated financial statements for the three and six months ended June 30, 2024, do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
3
NATURE OF BUSINESS AND OVERVIEW OF OPERATIONS
1.Summary
BioHarvest Sciences Inc. (the “Company” or “BioHarvest Sciences”) was incorporated under the Business Corporations Act of British Columbia on April 19, 2013.
2.Corporate Structure
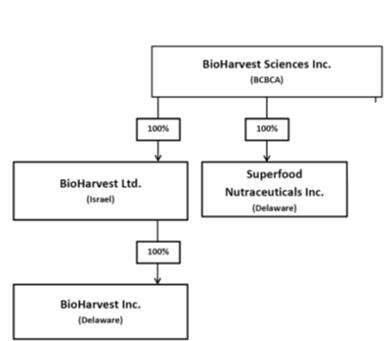
3.Overview of the business
The Company is a biotechnology company that has developed the Botanical Synthesis Platform Technology, which enables the Company to grow, in bioreactors at an industrial scale, the active and beneficial ingredients in certain fruits and plants without the need to grow the plant itself. The Botanical Synthesis Platform Technology is a non-genetically modified organism platform that can produce plant cells with higher concentrations of active ingredients (as compared to those that are produced naturally), as well as high levels of solubility and bio-availability. The Botanical Synthesis Platform Technology is economical, ensures consistency and avoids the negative environmental impacts associated with traditional agriculture by providing consistent product production, a year-round production cycle and products that are devoid of sugar, calories and contaminants, such as pesticides, heavy metals and residues.
4
The Company is currently focused on utilizing the Botanical Synthesis Platform Technology to develop the next generation of science-based and clinically proven health solutions through its two (2) business units:
1.The Products Business Unit, comprising:
(a)Nutraceuticals: Research, development, manufacturing, marketing and sales of science-based health and wellness nutraceutical solutions which are manufactured and sold as dietary supplements and/or functional food (capsules, powders, chews and other delivery mechanisms such as coffee, teas and electrolyte-enhanced beverages); and
(b)Cosmeceuticals: Research and development for future manufacturing, marketing and sales of science-based health and cosmeceutical solutions which are to be developed, manufactured and sold as cosmetic products; and
2.The CDMO Services Business Unit comprising a CDMO that offers customers from the pharmaceuticals, cosmeceuticals, nutraceuticals and nutrition industries, through an end-to-end service agreement, the development and manufacturing of specific plant-based active molecules.
Products Business Unit Activities:
I.Nutraceuticals
The Company is engaged in the research and development of science-based health and wellness solutions for the nutraceutical industry. The Company’s first product entry into this market is a polyphenol/anti-oxidant superfruit product called VINIA®, which is a red grape powder that supplies the benefits of red wine consumption but without the sugar, calories and alcohol found in wine.
VINIA® is made of red grape (Vitis vinifera) cells grown in the Company’s proprietary bioreactor facility. VINIA® is a fine, dry pink-purple powder containing a matrix of polyphenols (with a high concentration of piceid resveratrol) in their natural state (as can be found in red wine) that has additive and synergistic benefits. One of the main active ingredients in VINIA® is piceid resveratrol, maintaining the quality and inherent benefits present in nature without any solvent extraction or genetic modification. VINIA® is soluble when integrated with various liquids or cosmetics.
The Company has invested over $80 million, primarily in R&D activities, to support the business. This investment has enabled the Company to develop a disruptive technology platform which mirrors nature and allows it to efficiently produce plant cells that are identical to those originally sourced from the parent plant, ensuring optimal bio-availability and efficacy of the secondary metabolites.
In terms of manufacturing capacity, the Company has established a 20 ton manufacturing facility and commenced implementation of the required technology and process improvements to drive significant cost reduction through economies of scale. This facility received Good Manufacturing Practice (GMP) approvals from the Israeli Ministry of Health in October 2021 as well as key ISO certifications. The Company completed the biological technology transfer to the new manufacturing facility in March 2022 and has commenced actively scaling up its manufacturing of
5
VINIA® red grape cells at this new facility. This enables the Company to better meet the increasing demand for VINIA ® which is driven from the US market as a result of the Company’s marketing activities. The Company continued to focus significant resources in the second quarter of 2024 to increase its bioreactor capacity levels for each bioreactor by 22% to improve capacity levels and operational efficiency. In addition, in April, 2024, the Company validated the successful growing of its red grape cells in a bioreactor with an increased further capacity of 35%. The Company started to commence operationalizing this larger bioreactor size in August and will complete the scale up of all its Bioreactors to this size by the end of year. In addition, the Company has successfully secured additional 3rd party drying capacity to be able to cope with growing quarter on quarter demand levels. The Company expects this to positively impact capacity levels and gross profit delivery in the 2nd half of 2024.
In the second quarter of 2024, VINIA® revenues increased by 119% versus the comparable period in the previous year. This was a major demonstration of the Company’s ability to scale its VINIA® business using its Botanical Synthesis technology. Importantly, as a result of the aggressive scaling of the business and management’s focus on driving efficiencies where possible across the value chain, the Company continues to improve gross profit margin levels of the Products business unit, realizing a gross profit margin increase to 52% during the second quarter of 2024 as compared to 40% during the comparable period in the previous year. Gross margin levels of the Products business unit in the second quarter of 2024 exceeded the 50% threshold. Management continues to focus on accelerating revenue momentum and improving gross profit margins as well as marketing efficiencies.
The Company has a well-developed innovation pipeline in its Products business unit. Over the next 12 months, the Company plans to introduce a number of new products under the VINIA® brand as well as additional cell-based products utilizing its Botanical Synthesis technology. The pipeline of products planned, based on VINIA®, includes the launching of consumer products in major multibillion dollar categories including a hot tea beverages products line and electrolyte enhanced beverage line. The Company has experienced a very successful initial launch of its Keurig compatible coffee pods. Since launch in December 2023 up until the date of this MD&A, the Company has sold more than $878,000 of VINIA Superfood Bloodflow Coffee.
II.Cosmeceuticals
Since Q1 2023, the Company has spent significant resources investigating the opportunities that exist for its Red Grape Cell molecules in the growing beauty and cosmetics skincare market. The skin care market in the United States is worth approximately US$23 billion as of 2023, and the Company believes that consumers are searching for new natural and natural origin molecules to better address their skin care needs.
In Q1 2023, the Company conducted a small-scale skin care assessment in Seoul, South Korea (the “Skin Care Assessment”), which received positive anecdotal feedback from all participants regarding their various skin ailments, such as atopic dermatosis, psoriasis, facial redness and folliculitis, after using VINIA®.
Based on the results of the Skin Care Assessment, the Company is developing a clinical study in the United States to assess the efficacy of VINIA® as both a dietary supplement and a topical cosmetic solution on skin health promotion, with a view to launching a VINIA® topical solution product (the “VINIA® Topical Solution”) in the United States by the end of 2025.
6
CDMO Services Business Unit
In Q1 2024, the Company announced the launch of its CDMO Services Business Unit, including its entry into two (2) development agreements to develop complex molecules.
This CDMO Services Business Unit allows pharmaceutical, cosmeceutical, nutraceutical and nutrition industry companies the opportunity to partner with the Company to utilize the Botanical Synthesis Platform Technology through a CDMO contracting model. The Botanical Synthesis Platform Technology enables the development and manufacturing of patentable plant-based small molecules, complex molecules and unique compositions, which include both small and complex molecules. The Botanical Synthesis Platform Technology can develop complex molecules, otherwise known as biologics, which have a number of unique advantages, including lower costs of development and manufacturing, a faster speed of development and non-immunogenic properties that enhance safety. As a result of these advantages, the Company has decided to name these unique plant-derived complex molecules BIOLOGICS+. BIOLOGICS+ will help address unmet needs in the health industry across pharmaceutical, nutraceutical, cosmeceutical and nutrition verticals.
The Company currently has a number of customers in its short-term pipeline and expects to sign a number of additional contracts by the end of 2024 with customers from the pharmaceutical, nutraceutical, cosmeceutical and food ingredients/nutrition industries.
Environmental, Social and Governance Reporting:
On September 2021 the Company announced the publication of its inaugural Environmental, Social, and Governance (ESG) Report, detailing the Company’s performance and ongoing commitment to creating a sustainable future. The report is aligned with the United Nations Sustainable Development Goals and the reporting requirements of the Task Force on Climate-Related Financial Disclosures and the Sustainability Accounting Standards Board.
On September 6, 2022, Business Intelligence Group awarded the Company its prestigious Sustainability Leadership Award. The award recognizes the sustainability impact of the Company’s Botanical Synthesis platform technology, which enables industrial production of plant metabolites without growing the plant itself. The Company received the award with other industry thought leaders such as AstraZeneca, Agilent, and Honeywell.
Significant Developments
To better understand the Company’s financial results, it is important to gain an appreciation of the significant events, transactions and activities that occurred during or have affected the period under review up to and including the date of this MD&A.
1.During the six-month period ended June 30, 2024, the Company issued 2,940,882 common shares as a result of conversion of Convertible Loans.
2.During the six-month period ended June 30, 2024, the Company issued 106,132 common shares as a result of exercise of options.
7
3.During the six-month period ended June 30, 2024, the Company issued 1,359,216 Early Conversion Warrants to purchase shares of the Company at $7.77. 1,178,501 warrants will expire on October 30, 2025, and 180,715 warrants will expire on December 22, 2025.
4.During the six-month period ended June 30, 2024, the Company issued 603,904 units at price of $7.17 per unit. Each unit consists of one common share of the Company and one quarter (1/4) of one $7.68 warrant and one quarter (1/4) of one $11.52 warrant. Each whole $7.68 warrant will entitle the holder to purchase one common share and is exercisable for a period of 6 months. Each whole $11.52 warrant will entitle the holder to purchase one common share and is exercisable for a period of 18 months.
5.On May 27, 2024, the Company’s shareholders approved a 35-for-1 share consolidation (hereinafter referred to as the Share Consolidation) of the Company’s common shares pursuant to which the holders of the Company’s common shares received one common share for every 35 common shares held. The 35:1 Share Consolidation was approved by the Canadian Securities Exchange and is effective from June 3, 2024. All common shares (issued and unissued) were consolidated on the basis that every 35 common shares of no-par value were consolidated into 1 common share of no-par value.
Selected Information
| Three-month period ended | Six-month period ended | ||
| June 30, | June 30, | ||
| 2024 | 2023 | 2024 | 2023 |
| USD in thousands | |||
Revenues | 6,027 | 2,750 | 11,371 | 4,913 |
Net loss and comprehensive loss | 687 | 2,850 | 7,268 | 3,602 |
Basic and diluted loss per share (*) | (0.04) | (0.21) | (0.48) | (0.27) |
(*)Restated for giving effect to the reverse stock split (see also ‘Significant Developments’ in section 5)
| As at June 30, | |
| 2024 | 2023 |
| USD in thousands | |
|
|
|
Total Assets | 27,014 | 15,002 |
Total current liabilities | 8,938 | 12,890 |
Total non-current liabilities | 11,374 | 3,789 |
8
Three-month period ended June 30, 2024, compared to the three-month period ended June 30, 2023:
Revenues, of which 99% relate to the Products Business Unit of the Company, were $6,027 thousands for the three months ended June 30, 2024, as compared to $2,750 thousands during the same period in the prior year. The increase in the second quarter of 2024 is a result of the Company’s significant scaling of its business-to-consumer and medical practitioner focused e-commerce strategy.
Cost of revenues were $2,925 thousands for the three months ended June 30, 2024, as compared to $1,644 thousands during the same period in the prior year. The increase is due to growth in production, demand and sales during the period.
Gross margins were 52% for the three months ended June 30, 2024, as compared to 40% during the same period in the prior year. The increase in gross margins was a result of the Company’s continuing focus on cost reduction and production scaling.
Research and development expenses were $1,088 thousands for the three months ended June 30, 2024, as compared to $812 thousands during the same period in the prior year. The change is mainly due to an increase in wages and salaries (related to the CDMO services business unit) as well as professional fees and depreciation and amortization to support both segments.
Sales and marketing expenses, which relate mainly to the Products Business Unit, were $2,812 thousands for the three months ended June 30, 2024, as compared to $1,851 thousands during the same period in the prior year. The change is due to the higher marketing expenditure and wages and salaries required to support sales growth.
General and administrative expenses decreased to $978 thousands for the three months ended June 30, 2024, as compared to $1,318 thousands during the same period in the prior year. The decrease was driven by the allocation of wages and salaries of several employees to research and development to support the new CDMO services business unit. General and administrative expenses are incurred to support both of our business segments.
Finance expenses, were $378 thousands for the three months ended June 30, 2024, as compared to 159 thousands during the same period in the prior year. The increase is primarily the result of issuance of warrants. Finance expenses are incurred to support both of our business segments.
Finance income was $1,467 thousands for the three months ended June 30, 2024, as compared to $184 thousands during the same period in the prior year. The increase is primarily the result of fair value adjustments applicable to the Company’s convertible loan recorded in the three-month period ended June 30, 2024. Finance income is incurred to support both of our business segments.
9
Six-month period ended June 30, 2024, compared to the six-month period ended June 30, 2023:
Revenues, of which 99% relate to the Products Business Unit of the Company, were $11,371 thousands for the six months ended June 30, 2024, as compared to $4,913 thousands during the same period in the prior year. The increase in the first half of 2024 is a result of the Company’s significant scaling of its business-to-consumer and medical practitioner focused e-commerce strategy.
Cost of revenues were $5,266 thousands for the six months ended June 30, 2024, as compared to $3,015 thousands during the same period in the prior year. The increase is due to an increase in demand and sales, with a corresponding increase in production, during the period.
Gross margins were 54% for the six months ended June 30, 2024, as compared to 39% during the same period in the prior year. The increase in gross margins was a result of the Company’s focus on cost reduction and production scaling.
Research and development expenses were $2,122 thousands for the six months ended June 30, 2024, as compared to $1,423 thousands during the same period in the prior year. The change is mainly due to an increase in wages and salaries, primarily to support the new CDMO Services Business Unit, as well as professional fees, raw materials, share-based compensation and depreciation and amortization to support both segments.
Sales and marketing expenses, which relates mainly the Products Business Unit, were $5,376 thousands for the six months ended June 30, 2024, as compared to $3,692 thousands during the same period in the prior year. The change is due to the higher marketing expenditure and wages and salaries required to support sales growth.
General and administrative expenses decreased to $1,807 thousands for the six months ended June 30, 2024, as compared to $2,193 thousands during the same period in the prior year. The decrease was driven by the allocation of wages and salaries of several employees to research and development to support the new CDMO Services Business Unit. General and administrative expenses are incurred to support both of our business segments.
Finance expenses were $4,117 thousands for the six months ended June 30, 2024, as compared to $302 thousands during the same period in the prior year. The increase is primarily the result of fair value adjustments applicable to the Company’s convertible loans and issuance of warrants. Finance expenses are incurred to support both of our business segments.
Finance income weas $49 thousands for the six months ended June 30, 2024, as compared to $2,110 thousands during the same period in the prior year. The decrease is primarily the result of fair value adjustments applicable to the Company’s convertible loan recorded in the six-month period ended June 30, 2023. Finance income is incurred to support both of our business segments.
10
Summary of Quarterly Results
The following represents the summarized quarterly financial results for the past eight quarters:
| Three Months ended | |||
| June 30, 2024 | March 31, 2024 | December 31, 2023 | September 30, 2023 |
| USD in thousands | |||
Revenues | 6,027 | 5,344 | 4,520 | 3,239 |
687 | 6,581 | 7,235 | 1,727 | |
Net loss | 687 | 6,581 | 7,235 | 1,727 |
Net loss per share (*) | (0.04) | (0.48) | (0.53) | (0.13) |
|
| |||
| Three Months ended | |||
| June 30, 2023 | March 31, 2023 | December 31, 2022 | September 30, 2022 |
| USD in thousands | |||
Revenues | 2,750 | 2,163 | 2,444 | 1,517 |
Net loss before income taxes | 2,850 | 752 | 2,806 | 3,924 |
Net loss | 2,850 | 752 | 2,806 | 3,924 |
Net loss per share (*) | (0.21) | (0.06) | (0.21) | (0.30) |
(*)Restated for giving effect to the reverse stock split (see also ‘Significant Developments’ in section 5)
Financial instruments and risk management
The Company is exposed to a variety of financial risks, which results from its financing, operating and investing activities. The objective of financial risk management is to contain, where appropriate, exposures to these financial risks to limit any negative impact on the Company’s financial performance and position. The Company’s financial instruments are its Cash and cash equivalents, Restricted cash, Trade accounts receivable, Other accounts receivable, Trade accounts payable, Other accounts payable and Liability to Agricultural Research Organization. The main purpose of these financial instruments is to raise finance for the Company’s operation. The Company actively measures, monitors and manages its financial risk exposures by various functions, including the segregation of duties and the application of financial control principals. The risks arising from the Company’s financial instruments are mainly currency risk and liquidity risk. The Company has no interest rate risk as the balances exposure to interest is minimal. The risk management policies employed by the Company to manage these risks are discussed below.
Foreign currency risk
Foreign exchange risk arises when the Company enters into transactions denominated in a currency other than its functional currency. The Company is exposed to currency risk to the extent that there is a mismatch between the currency in which it is denominated and the respective functional currency of the company. The currencies in which some transactions are primarily denominated are CAD, US dollars and NIS. The Company’s policy is not to enter into any economic hedging transactions to neutralize the effects of foreign currency fluctuations.
11
Liquidity and Capital resources
The unaudited interim condensed consolidated financial statements for the three and six months ended June 30, 2024, have been prepared on a going concern basis whereby the Company is assumed to be able to realize its assets and discharge its liabilities in the normal course of operations. The unaudited interim condensed consolidated financial statements do not reflect adjustments that would be necessary if the going concern assumption were not appropriate. If the going concern assumption was not appropriate for the unaudited interim condensed consolidated financial statements, then adjustments of a material nature would be necessary in the carrying value of assets such as property and equipment, liabilities, the reported expenses, and the balance sheet classifications used. Management continues to pursue financing opportunities for the Company to ensure that it will have sufficient cash to carry out its planned programs beyond the next year.
At June 30, 2024, the Company had cash and cash equivalents of $5,168 thousands (June 30, 2023, $1,812 thousands). The Company had current assets of $9,255 thousands (June 30, 2023, $4,891 thousands) and current liabilities of $8,938 thousands (June 30, 2023, $12,890 thousands).
At June 30, 2024, the Company had net working capital of positive $317 thousands (June 30, 2023, negative $7,999 thousands).
During the six months ended June 30, 2024, the Company’s overall position of cash and cash equivalents increase by $187 thousands (June 30, 2023, increased by $76 thousands). This change in cash and cash equivalents held can be attributed to the following:
·The Company’s net cash used in operating activities during the six months ended June 30, 2024, was $2,536 thousands as compared to net cash used of $4,533 thousands for the six months ended June 30, 2023. The amount is primarily a result of the losses incurred in the operations of the Company.
·The Company’s net cash used in investing activities during the six months ended June 30, 2024, was $2,155 thousands as compared to net cash used of $743 thousands for the six months ended June 30, 2023. The amounts used primarily to the purchase of property and equipment.
·The Company’s net cash provided by financing activities during the six months ended June 30, 2024, was $4,490 thousands as compared to net cash provided by financing activities of $5,364 thousands for the six months ended June 30, 2023.
Since the Company will not be able to generate cash from its operations in the foreseeable future, the Company will have to rely on the issuance of shares or the exercise of options, warrants and loans to fund ongoing operations and investment. The ability of the Company to raise capital will depend on market conditions and it may not be possible for the Company to issue shares on acceptable terms or at all.
Off Balance Sheet Agreements
The Company has not entered into any material off-balance sheet arrangements such as guarantee contracts, contingent interests in assets transferred to unconsolidated entities, derivative financial obligations or arrangements with respect to any obligations under a variable interest equity arrangement.
12
Transactions with Related Parties
The Company’s key management personnel have the authority and responsibility for overseeing, planning, directing, and controlling the activities of the Company. Key management personnel include members of the Board of Directors, the Chief Executive Officer and the Chief Financial Officer.
Compensation earned by key management for the three- and six-months period ended June 30, 2024, and June 30, 2023, was as follows:
Related party transactions:
| Three months ended June 30, 2024 | Six months ended June 30, 2024 | Three months ended June 30, 2023 | Six months ended June 30, 2023 |
Compensation of key management personnel of the Company: |
|
|
|
|
CEO management fees | 87 | 204 | 115 | 238 |
Chairman management fees | 99 | 231 | 365 | 433 |
CFO management fees | 8 | 15 | 8 | 15 |
Share based payment to CEO | - | - | 2 | 11 |
Share based payment to Chairman | - | - | 61 | 175 |
Other related party transactions: |
|
|
|
|
Share based payments | 9 | 11 | 3 | 7 |
Related party balances:
| As at June 30, 2024 | As at December 31, 2023 |
Due to CEO | 29 | 29 |
Critical Accounting Estimates and Judgements
The preparation of unaudited interim condensed consolidated financial statements requires management to make judgments, estimates and assumptions that affect the application of policies and reported amounts of assets and liabilities, and revenue and expenses.
The estimates and associated assumptions are based on historical experience and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis of making the judgments about carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis.
The key assumptions concerning the future and other key sources of estimation uncertainty at the reporting date that have a significant risk of causing a material adjustment to the carrying amounts
13
of assets and liabilities within the next financial period, are described below. The Company based its assumptions and estimates on parameters available when the Unaudited Interim Condensed Consolidated Financial Statements were prepared. Existing circumstances and assumptions about future developments, however, may change due to market changes or circumstances arising that are beyond the control of the Company. Such changes are reflected in the assumptions when they occur.
1.Liability to Agricultural Research Organization:
The Company measures the liability to the Agricultural Research Organization, each period, based on discounted cash flows derived from the Company’s future anticipated revenues. The discount rate reflects the market rate.
2.Determining the transaction price and amounts allocated to the performance obligations:
In transactions with customers that include variable consideration, the Company assesses, based on past experience, business forecasts and current economic conditions, whether it is highly probable that a significant reversal in the amount of revenue recognized will not occur when the uncertainty associated with the variable consideration is subsequently resolved. In determining the transaction price for each contract with a customer, the Company considers the effect of the right of return.
The Company also assesses for each transaction with variable consideration the approach that will best reflect the amount of the consideration to which the Company will be entitled, using either the “expected value” method or the “most likely amount” method.
Common Share Data
As at the date of this MD&A, the Company had the following securities issued and outstanding:
Type of Security | Number Outstanding |
Common shares | 17,327,716 |
Stock options | 1,796,688 |
Warrants | 2,252,903 |
RSU | 20,000 |
Investor Relations Contracts
There are no investor relations contacts outstanding.
Contractual Obligations
The Company has no contractual obligations that have not been disclosed.
Risks and Uncertainties
Global Economic Uncertainty. The Company’s ability to raise capital is subject to the risk of adverse changes in the market value of the Company’s share price. Periods of macroeconomic weakness or recession and heightened market volatility caused by adverse geopolitical
14
developments could increase these risks, potentially resulting in adverse impacts on the Company’s ability to raise further capital on favorable terms. The impact of geopolitical tension, such as the conflict in the Middle East, a deterioration in the bilateral relationship between the US and China or an escalation in conflict between Russia and Ukraine, including any resulting sanctions, export controls or other restrictive actions that may be imposed by the US and/or other countries against governmental or other entities in, for example, Russia, also could lead to disruption, instability and volatility in global trade patterns, which may in turn impact the Company’s ability to source necessary raw materials and other inputs for manufacturing or the Company’s ability to close new revenue generating orders.
On October 7, 2023, an attack was launched against Israel by Hamas (a terror organization) which thrust Israel into a state of war (hereinafter: “The state of war”) in Israel and in Gaza strip. The company is continuing with its operations both in Israel and globally, as the state of war had no material impact on its operations or business result. While none of the Company’s facilities or infrastructure have been damaged since the war broke out on October 7, 2023, the import and export of goods may experience disruptions in and out of Israel as a result of such military conflict. The Company currently relies on drying locations in Northern Israel, in areas which may be at greater risk for disruption as a result of such military conflict and is in the process of examining and evaluating other potential drying alternatives and growing inventory levels in the United States to mitigate the risk of disruption. The Company continues to assess the effects of the state of war on its Consolidated Financial Statements and business
Market Risks. The Company’s securities trade on public markets and the trading value thereof is determined by the evaluations, perceptions and sentiments of both individual investors and the investment community taken as a whole. Such evaluations, perceptions and sentiments are subject to change, both in short-term time horizons and long-term time horizons. An adverse change in investor evaluations, perceptions and sentiments could have a material adverse outcome on the Company and its securities.
Financing Risks. The Company will be dependent on raising capital through a combination of debt and/or equity offerings. There can be no assurance that the capital markets will remain favorable in the future, and/or that the Company will be able to raise the financing needed to continue its business at favorable terms, or at all. Restrictions on the Company’s ability to finance could have a material adverse outcome on the Company and its securities.
Share Price Volatility and Price Fluctuations. In recent years, the securities markets in Canada have experienced a high level of price and volume volatility, and the market prices of securities of many corporations have experienced wide fluctuations which have not necessarily been related to the operating performance, underlying asset values or prospects of such companies.
Key Personnel Risks. The Company’s efforts are dependent to a large degree on the skills and experience of certain of its key personnel, including the board of directors. The Company does not maintain “key man” insurance policies on these individuals. Should the availability of these persons’ skills and experience be in any way reduced or curtailed, this could have a material adverse outcome on the Company and its securities.
General Business Risk and Liability. Given the nature of the Company’s business, it may from time to time be subject to claims or complaints from investors or others in the normal course of business. The legal risk facing the Company, its directors, officers and employees in this respect
15
includes potential liability for violations of securities laws, breach of fiduciary duty or misuse of investors’ funds. Some violations of securities laws and breach of fiduciary duty could result in civil liability, fines, sanctions or the suspension or revocation of the Company’s right to carry on its existing business. The Company may incur significant costs in connection with such potential liabilities.
Competition. There is the potential that the Company will face intense competition from other companies, some of which can be expected to have more financial resources, industry, manufacturing and marketing experience than the Company. Additionally, there is potential that the industry will undergo consolidation, creating larger companies that may have increased geographic scope and other economies of scale. Increased competition between larger, better-financed competitors with geographic or other structural advantages could materially and adversely affect the business, financial condition and results of operations of the Company. To remain competitive, the Company will require a continued level of investment in research and development, marketing, sales and client support. The Company may not have sufficient resources to maintain research and development, marketing, sales and client support efforts on a competitive basis which could materially and adversely affect the business, financial condition and results of operations of the Company.
Reliance on Key Business Inputs. The Company’s business is dependent on a number of key inputs and their related costs including raw materials and suppliers related to its growing operations as well as electricity, water, and other utilities. Any significant interruption or negative change in the availability or economics of the supply chain for key inputs could materially impact the business, financial condition and operating results of the Company. Any liability to secure required supplies and services or to do so on appropriate terms could also have a materially adverse impact on the business, financial condition, and operating results of the Company.
Potential product recalls. Manufacturers and distributers of products are sometimes subjected to the recall or return of their products for a variety of reasons, including product defects, such as contamination, unintended harmful side effects or interactions with other substances, packing safety and inadequate or inaccurate labeling disclosers. If the Company’s product is recalled due to an alleged product defect or for any other reason, the Company could be required to incur the unexpected expenses of the recall and any legal proceedings that might arise in connection with the recall.
The Company may lose a significant number of sales and may not be able to replace those sales at an acceptable margin or at all. In addition, a product recall may require significant management attention.
Although the Company had detailed procedures in place for testing finished products, there can be no assurance that any quality, potency or contamination problem will be detected in time to avoid unforeseen product recalls, regulatory action or lawsuit. Additionally, if one of the Company’s products was subject to recall, the image of the Company could be harmed. A recall for any one of the foregoing reasons could lead to decreased demand for the Company’s products and could have a material adverse effect on the results of operations and financial condition of the Company.
History of Net Losses; Accumulated Deficit; Lack of Revenue from Operations. The Company has incurred net losses to date. The Company may continue to incur losses. There is no certainty that the Company will operate profitably or provide a return on investment in the future.
16
Uninsurable risks. The Company may become subject to liability for events against which it cannot insure or against which it may elect not to insure. Such events could result in substantial damage to property and personal injury. The payment of any such liabilities may have a material, adverse effect on the Company’s financial position.
No History of Dividends. Since incorporation, the Company has not paid any cash or other dividends on its common stock and does not expect to pay such dividends in the foreseeable future, as all available funds will be invested primarily to finance the Company’s operations. The Company will need to achieve profitability prior to any dividends being declared.
Additional information related to the Company, is available for viewing on SEDAR+ at www.sedarplus.ca. This additional information is not incorporated into this Management’s Discussion and Analysis and does not constitute a part of this Management’s Discussion and Analysis.
17