Exhibit 99.1
Oncology Leadership in Pretargeted Radioimmunotherapy Platform and Antibody - based Therapies January 2024

Disclaimer Statements in this presentation about future expectations, plans and prospects, as well as any other statements regarding matters that are not historical facts, constitute “forward - looking statements” within the meaning of Section 27 A of the Securities Act of 1933 and Section 21 E of the Securities Exchange Act of 1934 . Such statements include, but are not limited to, statements about the Company’s growth prospects and expectations related thereto ; expectations with respect to the Company’s financial expectations, including the Company’s 2023 operating expense, cash burn and DANYELZA net product revenue guidance, and the Company’s estimated cash runway and sufficiency of cash resources and related assumptions ; the Company’s ability to deliver value ; expectations with respect to the achievement of milestones and the timing thereof ; implied and express statements regarding the future of the Company’s business, including with respect to expansion and its goals ; the Company’s plans and strategies, development, commercialization and product distribution plans, including potential partnerships ; expectations with respect to the Company’s products and product candidates, including potential territory and label expansion of DANYELZA and the potential market opportunity related thereto and potential benefits thereof, and the potential of the SADA Technology and potential benefits and applications and the timing thereof ; SADA’s potential to be an industry game - changer ; expectations with respect to current and future clinical and pre - clinical studies and the Company’s and its partners’ research and development programs, including with respect to timing and results ; expectations related to the timing of the initiation and completion of regulatory submissions ; additional product candidates and technologies ; expectations regarding collaborations or strategic partnerships and the potential benefits thereof ; expectations related to the use of cash and cash equivalents, and the need for, timing and amount of any future financing transaction ; expectations with respect to the Company’s future financial performance ; and other statements that are not historical facts . Words such as ‘‘anticipate,’’ ‘‘believe,’’ “contemplate,” ‘‘continue,’’ ‘‘could,’’ ‘‘estimate,’’ ‘‘expect,’’ “hope,” ‘‘intend,’’ ‘‘may,’’ ‘‘might,’’ ‘‘plan,’’ ‘‘potential,’’ ‘‘predict,’’ ‘‘project,’’ ‘‘should,’’ ‘‘target,’’ “will”, ‘‘would’’, “guidance,” and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words . The Company’s product candidates and related technologies are novel approaches to cancer treatment that present significant challenges . Actual results may differ materially from those indicated by such forward - looking statements as a result of various factors, including but not limited to : risks associated with the Company’s financial condition and need for additional capital ; the risks that actual results of the Company’s restructuring plan and revised business plan will not be as expected ; risks associated with the Company’s development work ; cost and success of the Company’s product development activities and clinical trials ; the risks of delay in the timing of the Company’s regulatory submissions or failure to receive approval of its drug candidates ; the risks related to commercializing any approved pharmaceutical product including the rate and degree of market acceptance of product candidates ; development of sales and marketing capabilities and risks associated with failure to obtain sufficient reimbursement for products ; the risks related to the Company’s dependence on third parties including for conduct of clinical testing and product manufacture ; the Company’s ability to enter into new partnerships and to maintain existing partnerships ; the risks related to government regulation ; risks related to market size and approval, risks associated with protection of the Company’s intellectual property rights ; risks related to employee matters and managing growth ; risks related to the Company’s common stock ; risks associated with macroeconomic conditions, including the conflict between Russia and Ukraine and sanctions related thereto, the state of war between Israel and Hamas and the related risk of a larger regional conflict, inflation, increased interest rates, uncertain global credit and capital markets and disruptions in banking systems ; the completion of financial closing procedures, final audit adjustments and other developments that may arise that would cause the Company’s expectations with respect to the Company’s 2023 guidance to differ, perhaps materially, from the financial results that will be reflected in the Company’s audited consolidated financial statements for the fiscal year ended December 31 , 2023 ; and other risks and uncertainties affecting the Company including those described in the “Risk Factors” section included in the Company’s Annual Report on Form 10 - K for the fiscal year ended December 31 , 2022 , the Company’s Quarterly Report on Form 10 - Q for the quarter ended September 30 , 2023 and future filings and reports by the Company . Any forward - looking statements contained in this press release speak only as of the date hereof, and the Company undertakes no obligation to update any forward - looking statement, whether as a result of new information, future events or otherwise . This presentation includes statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties or us . Industry publications and third - party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information . All of the market data used in this presentation involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates . While we believe these industry publications and third - party research, surveys and studies are reliable, we have not independently verified such data . The industry in which we operate is subject to a high degree of uncertainty, change and risk due to a variety of factors, which could cause results to differ materially from those expressed in the estimates made by the independent parties and by us . 2
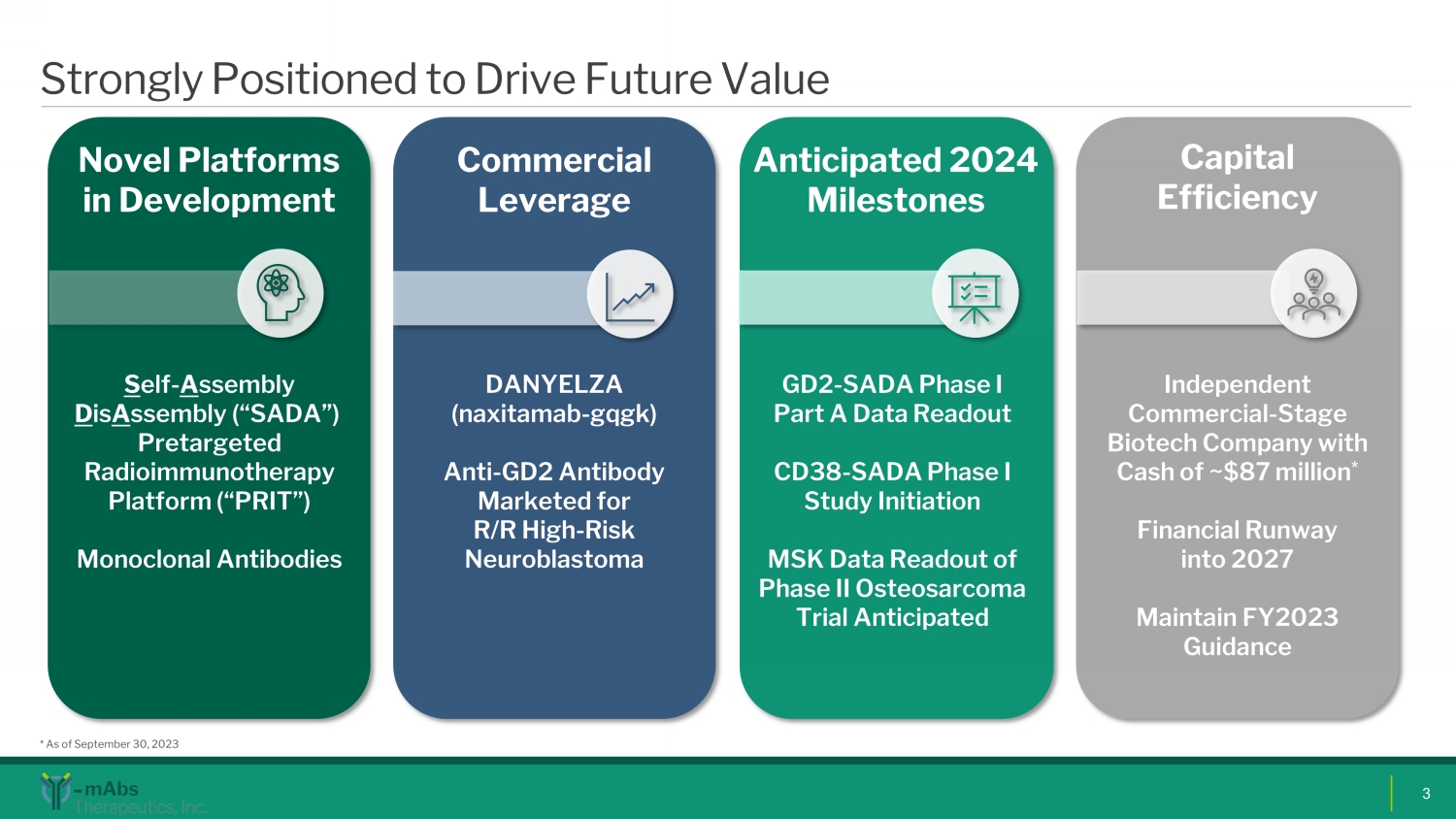
Strongly Positioned to Drive Future Value 3 Novel Platforms in Development S elf - A ssembly D is A ssembly (“SADA”) Pretargeted Radioimmunotherapy Platform (“PRIT ”) Monoclonal Antibodies GD2 - SADA Phase I Part A Data Readout CD38 - SADA Phase I Study Initiation MSK Data Readout of Phase II Osteosarcoma Trial Anticipated Anticipated 2024 Milestones Commercial Leverage DANYELZA ( naxitamab - gqgk ) Anti - GD2 Antibody Marketed for R/R High - Risk Neuroblastoma Capital Efficiency Independent Commercial - S tage Biotech C ompany with Cash of ~$87 million * Financial Runway into 2027 Maintain FY2023 Guidance * As of September 30, 2023

Advancing Focused Pipeline with Multiple Value - Added Catalysts Ahead 4 Study Therapeutic Area Preclinical Phase I Phase II/Pivotal Approved Trial Sponsor Status Lead Programs 201 Relapsed/Refractory High - Risk Neuroblastoma (Pediatric) U.S. FDA approved Naxitamab - gqgk (Anti - GD2) 12 - 230 Relapsed/Refractory High - Risk Neuroblastoma (Pediatric) U.S. FDA approved BCC018 Front - Line Induction in High - Risk Neuroblastoma (Pediatric) Randomized trial expected Q2 2024 15 - 096 Relapsed Second - Line Osteosarcoma Expected MSK data readout in Q4 2024 17 - 251 Chemoimmunotherapy for Relapsed/ Refractory High - Risk Neuroblastoma Study completed SADA (Radioimmunotherapy) 1001 GD2 - SADA: Solid Tumors (SCLC, Malignant Melanoma, Sarcoma) First cohorts treated 1201 CD38 - SADA: Non - Hodgkin Lymphoma Expected Phase I initiation in 2024 Early Programs SADA (Radioimmunotherapy) GD2 - SADA: Neuroblastoma Expected IND filing in 2024 HER2 - SADA Expected IND filing in 2025 B7H3 - SADA Expected IND filing in 2025 DANYELZA ( naxitamab - gqgk ) Confirmatory Trial DANYELZA ( naxitamab - gqgk )

5 Novel SADA Pretargeted Radioimmunotherapy Technology Platform
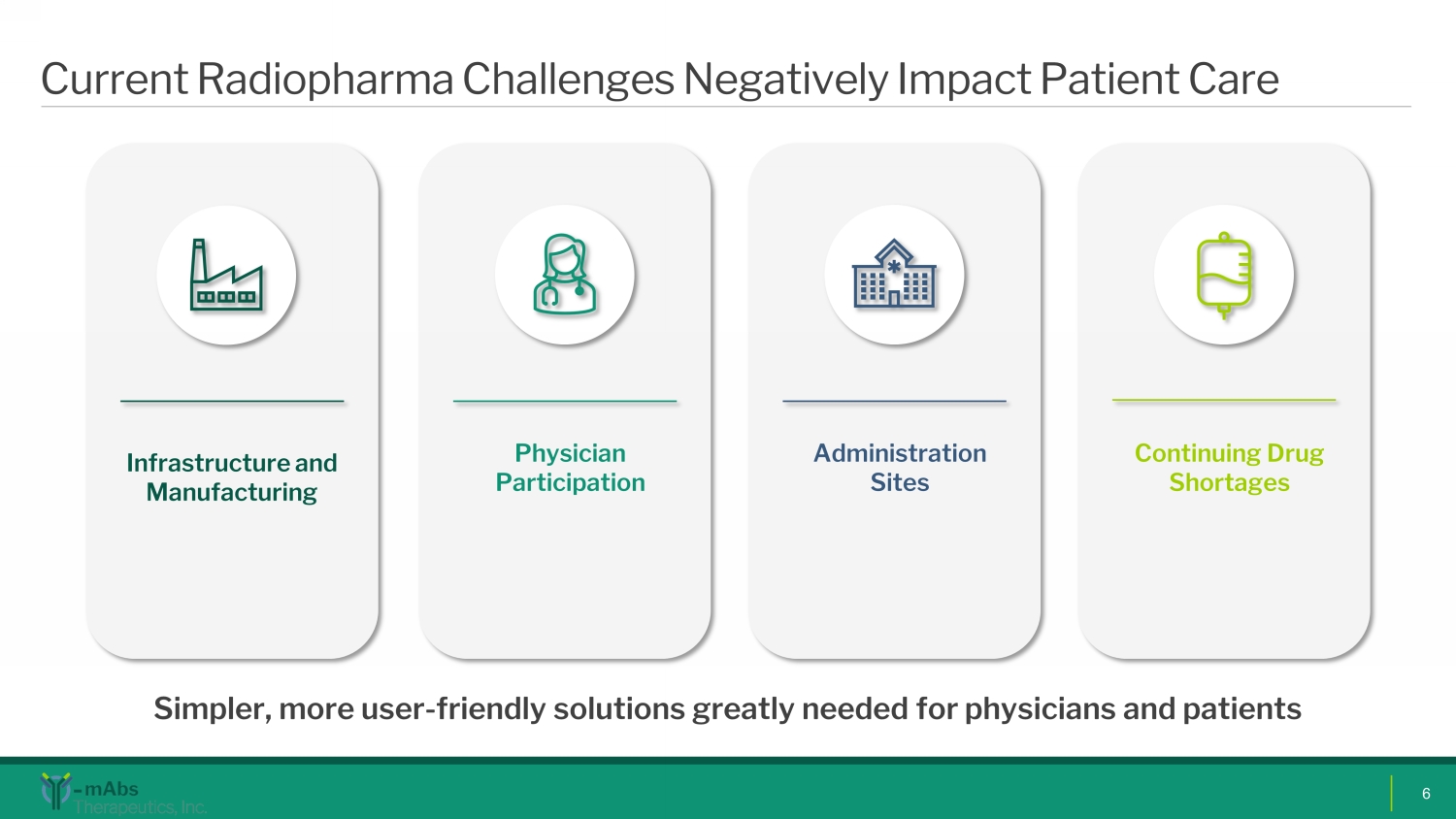
Current Radiopharma Challenges Negatively Impact Patient Care 6 Infrastructure and Manufacturing Physician Participation Administration Sites Continuing Drug Shortages Simpler, more user - friendly solutions greatly needed for physicians and patients
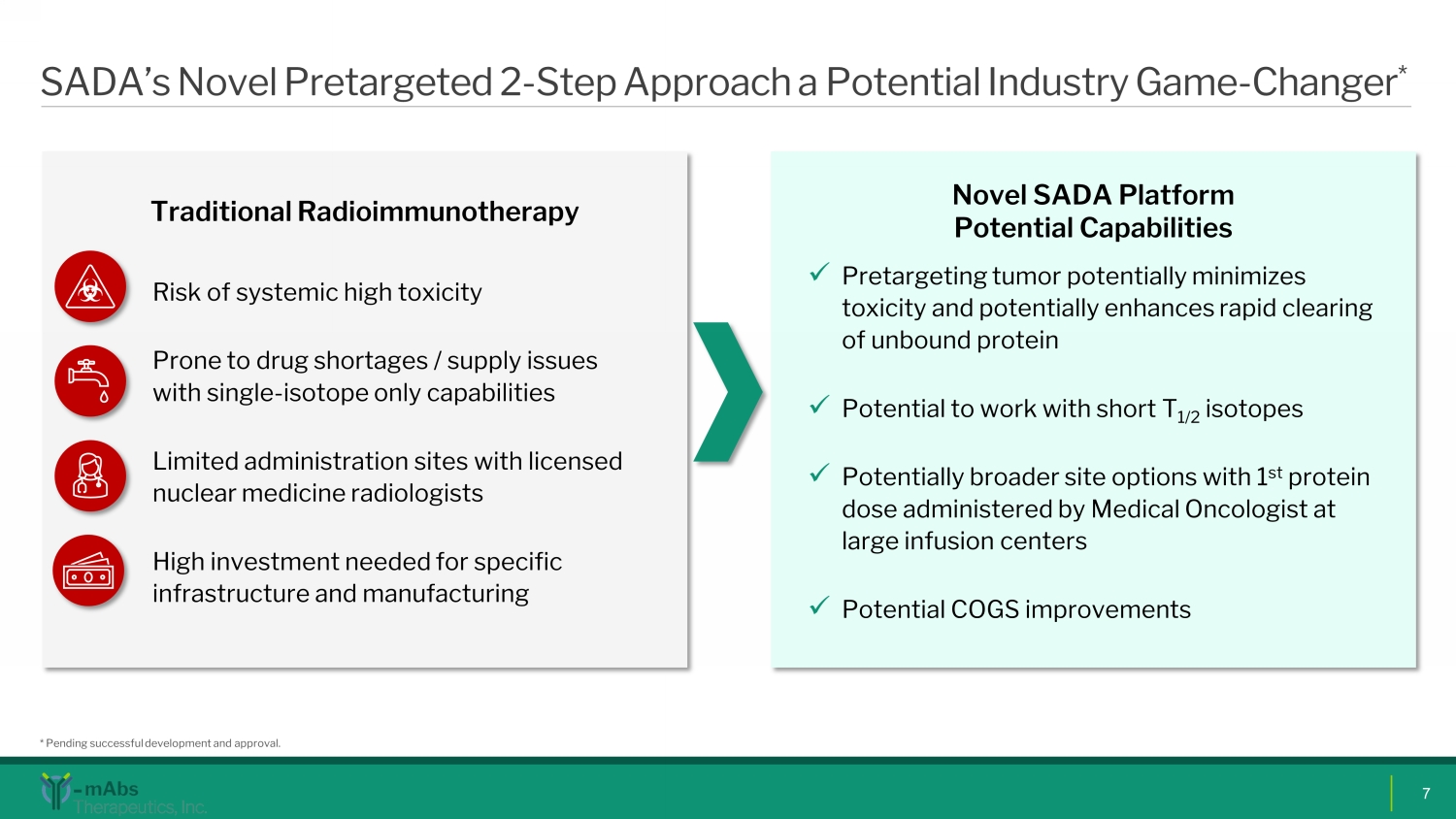
SADA’s Novel Pretargeted 2 - Step Approach a Potential Industry Game - Changer * 7 Novel SADA Platform Potential Capabilities x Pretargeting tumor potentially minimizes toxicity and potentially enhances rapid clearing of unbound protein x Potential to work with short T 1/2 isotopes x Potentially broader site options with 1 st protein dose administered by Medical Oncologist at large infusion centers x Potential COGS improvements Traditional Radioimmunotherapy Risk of systemic high toxicity Prone to drug shortages / supply issues with single - isotope only capabilities Limited administration sites with licensed nuclear medicine radiologists High investment needed for specific infrastructure and manufacturing * Pending successful development and approval.
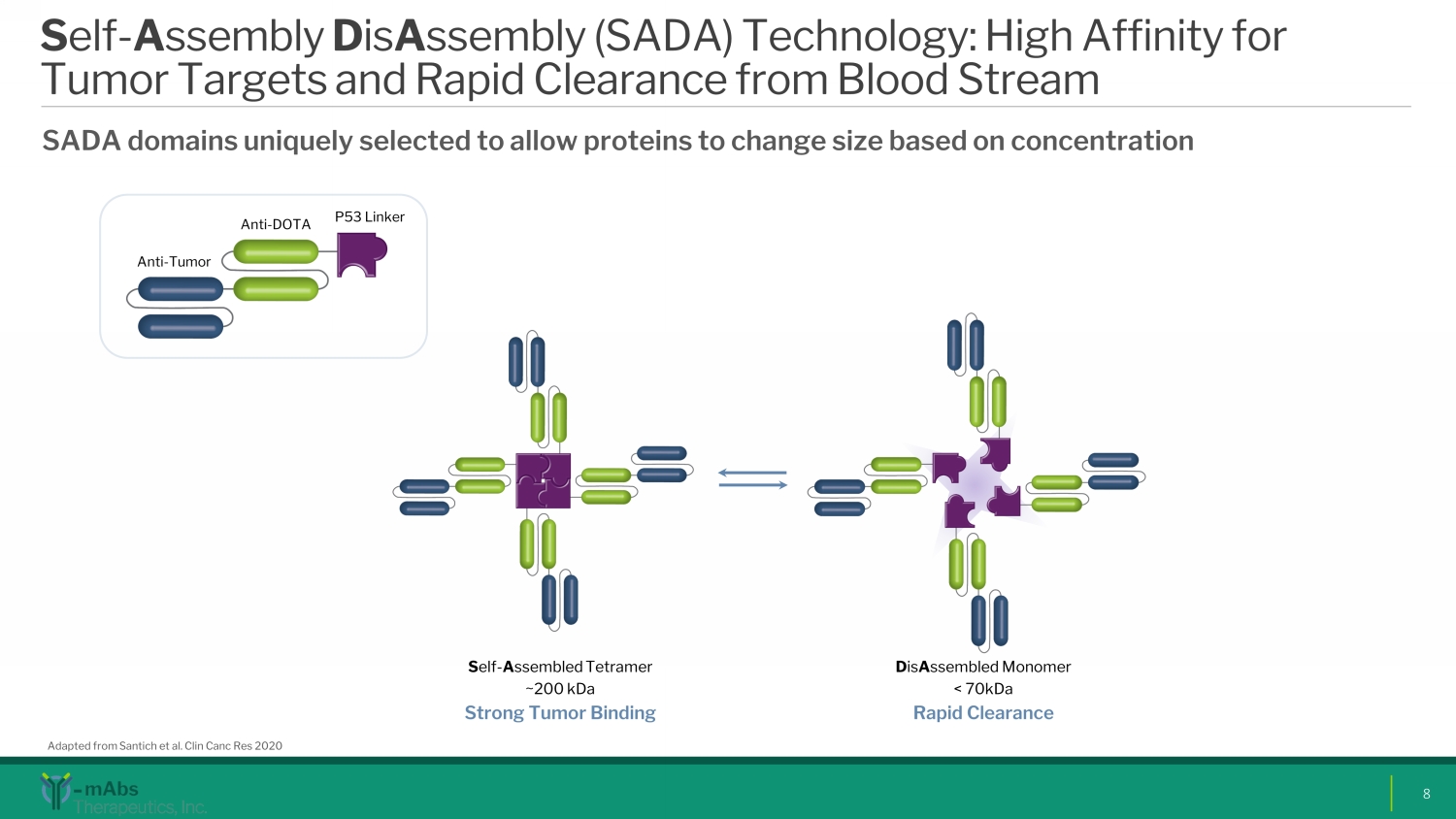
S elf - A ssembly D is A ssembly (SADA) Technology: High Affinity for Tumor Targets and Rapid Clearance from Blood Stream SADA domains uniquely selected to allow proteins to change size based on concentration Adapted from Santich et al. Clin Canc Res 2020 S elf - A ssembled Tetramer ~200 kDa Strong Tumor B inding D is A ssembled Monomer < 70kDa Rapid Clearance Anti - Tumor Anti - DOTA P53 Linker 8 SK0
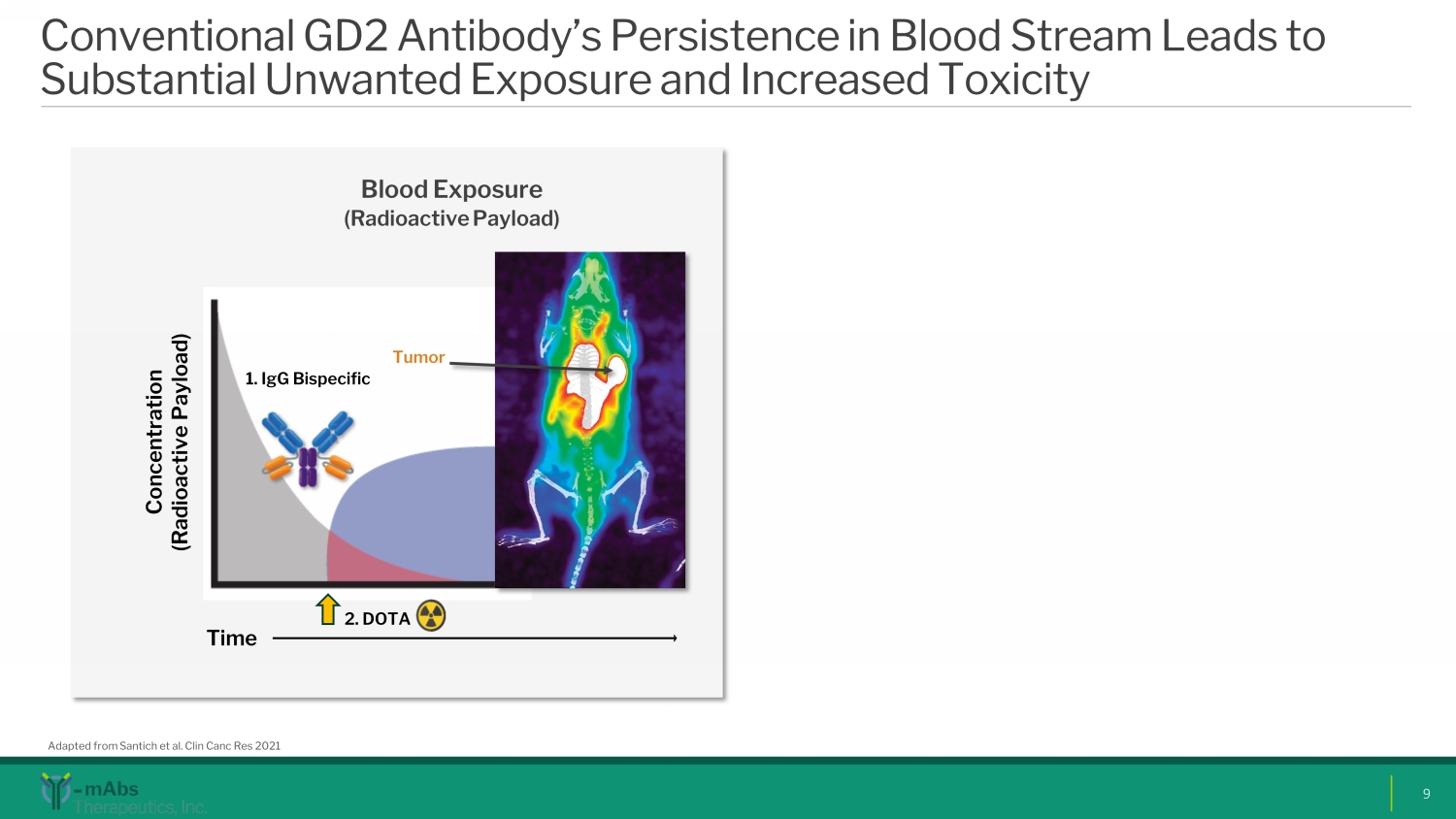
Conventional GD2 Antibody’s Persistence in Blood Stream Leads to Substantial Unwanted Exposure and Increased Toxicity 9 Adapted from Santich et al. Clin Canc Res 2021 Blood E xposure (Radioactive P ayload ) 1. IgG Bispecific 2. DOTA Time Concentration (Radioactive Payload) Tumor
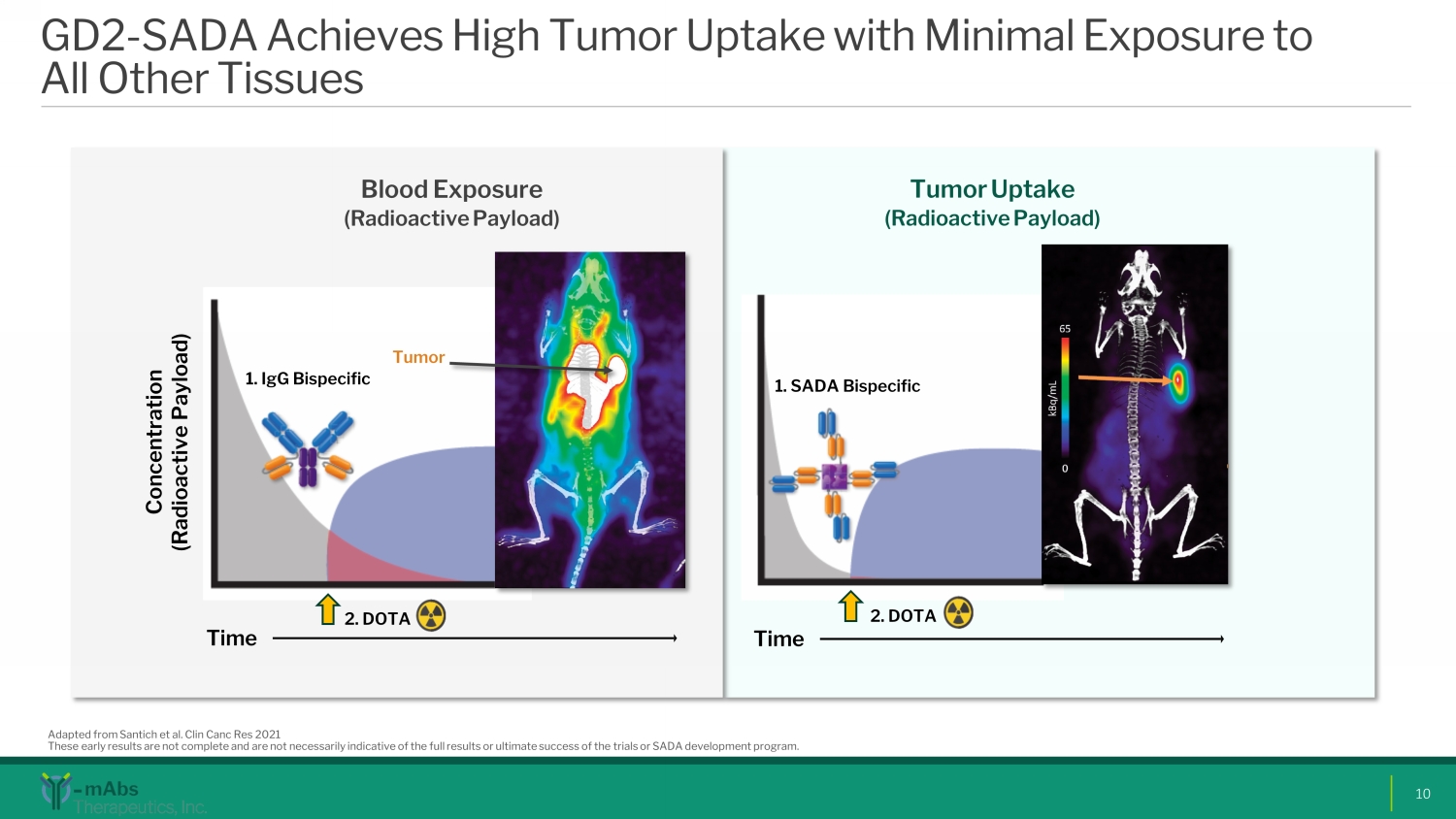
GD2 - SADA Achieves High Tumor Uptake with Minimal Exposure to All Other Tissues 10 Blood E xposure (Radioactive Payload) 1. IgG Bispecific 2. DOTA 1. SADA Bispecific 2. DOTA Concentration (Radioactive Payload) Tumor Tumor U ptake (Radioactive P ayload ) Adapted from Santich et al. Clin Canc Res 2021 These early results are not complete and are not necessarily indicative of the full results or ultimate success of the trials or SADA development program. Time Time
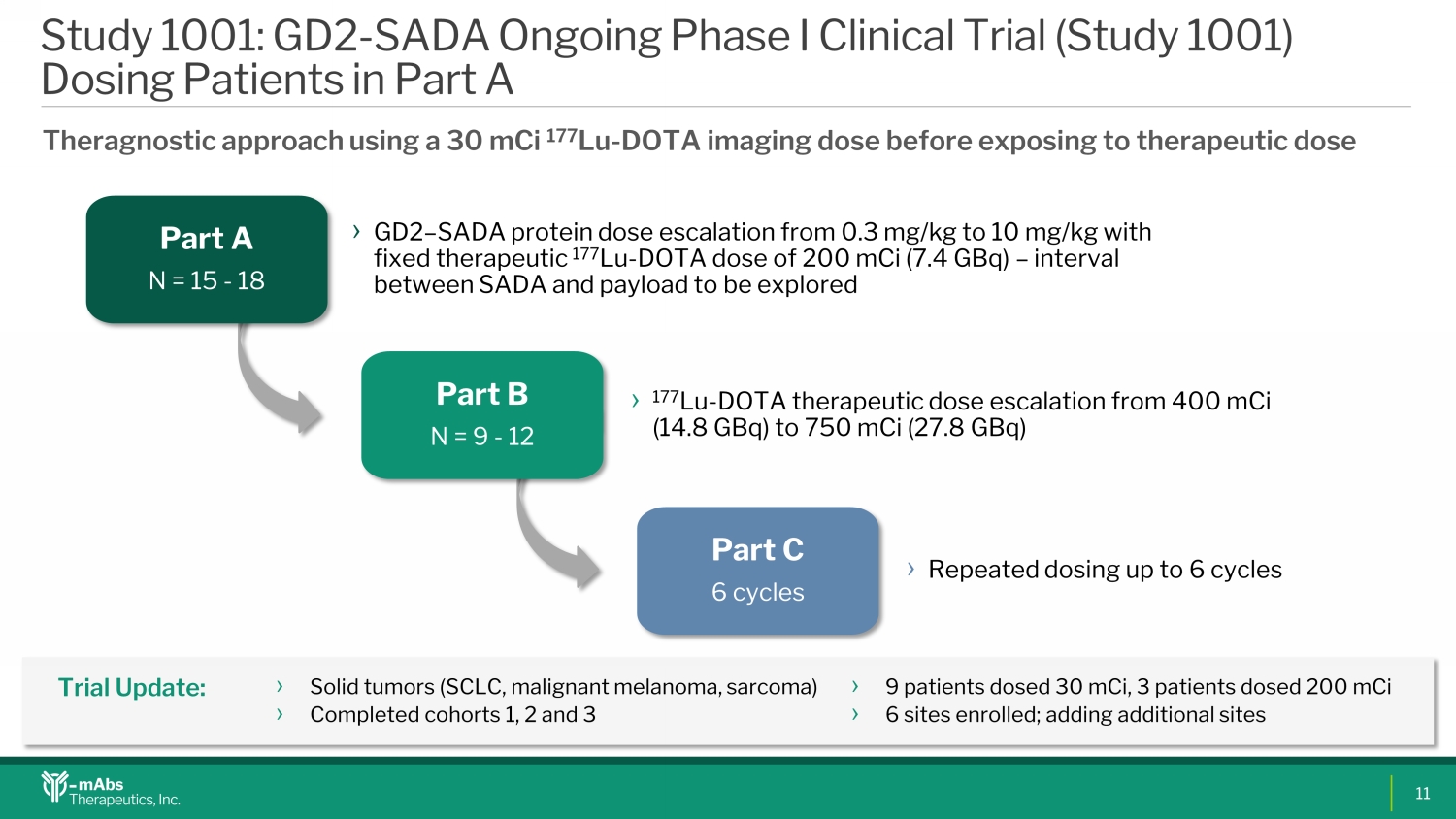
Part A N = 15 - 18 › GD2 – SADA protein dose escalation from 0.3 mg/kg to 10 mg/kg with fixed therapeutic 177 Lu - DOTA dose of 200 mCi (7.4 GBq) – interval between SADA and payload to be explored Part B N = 9 - 12 › 177 Lu - DOTA therapeutic dose escalation from 400 mCi (14.8 GBq) to 750 mCi (27.8 GBq) Part C 6 cycles › Repeated dosing up to 6 cycles Study 1001: GD2 - SADA Ongoing Phase I Clinical Trial (Study 1001) Dosing Patients in Part A Theragnostic approach using a 30 mCi 177 Lu - DOTA imaging dose before exposing to therapeutic dose › Solid tumors (SCLC, malignant melanoma, sarcoma) › Completed cohorts 1, 2 and 3 › 9 patients dosed 30 mCi , 3 patients dosed 200 mCi › 6 sites enrolled; adding additional sites Trial Update: 11 SK0
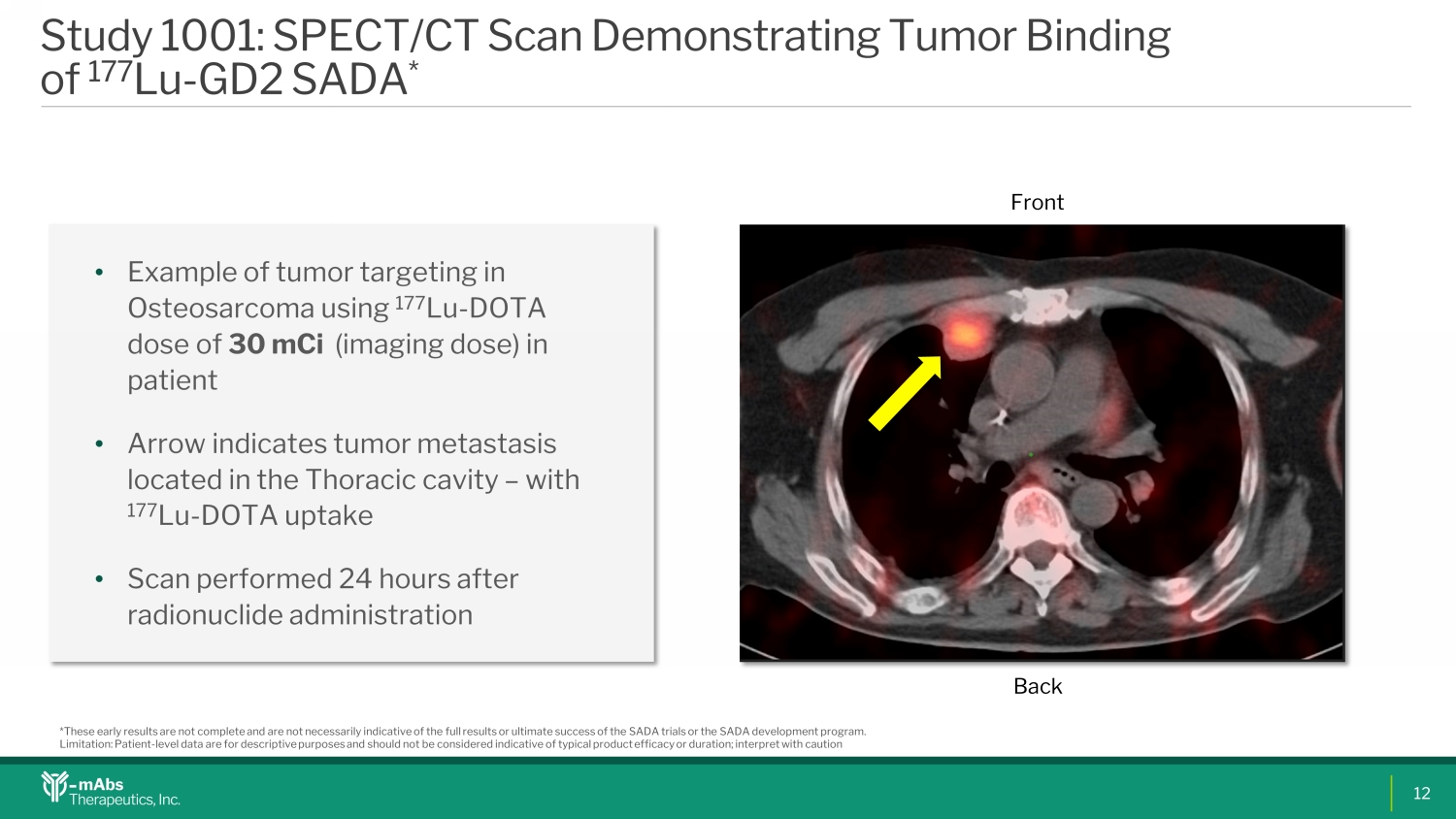
Study 1001: SPECT/CT Scan Demonstrating Tumor Binding of 177 Lu - GD2 SADA * • Example of tumor targeting in Osteosarcoma using 177 Lu - DOTA dose of 30 mCi (imaging dose) in patient • Arrow indicates tumor metastasis located in the Thoracic cavity – with 177 Lu - DOTA uptake • Scan performed 24 hours after radionuclide administration *These early results are not complete and are not necessarily indicative of the full results or ultimate success of the SADA tri als or the SADA development program. Limitation: Patient - level data are for descriptive purposes and should not be considered indicative of typical product efficacy or duration; interpret with caution Front Back 12
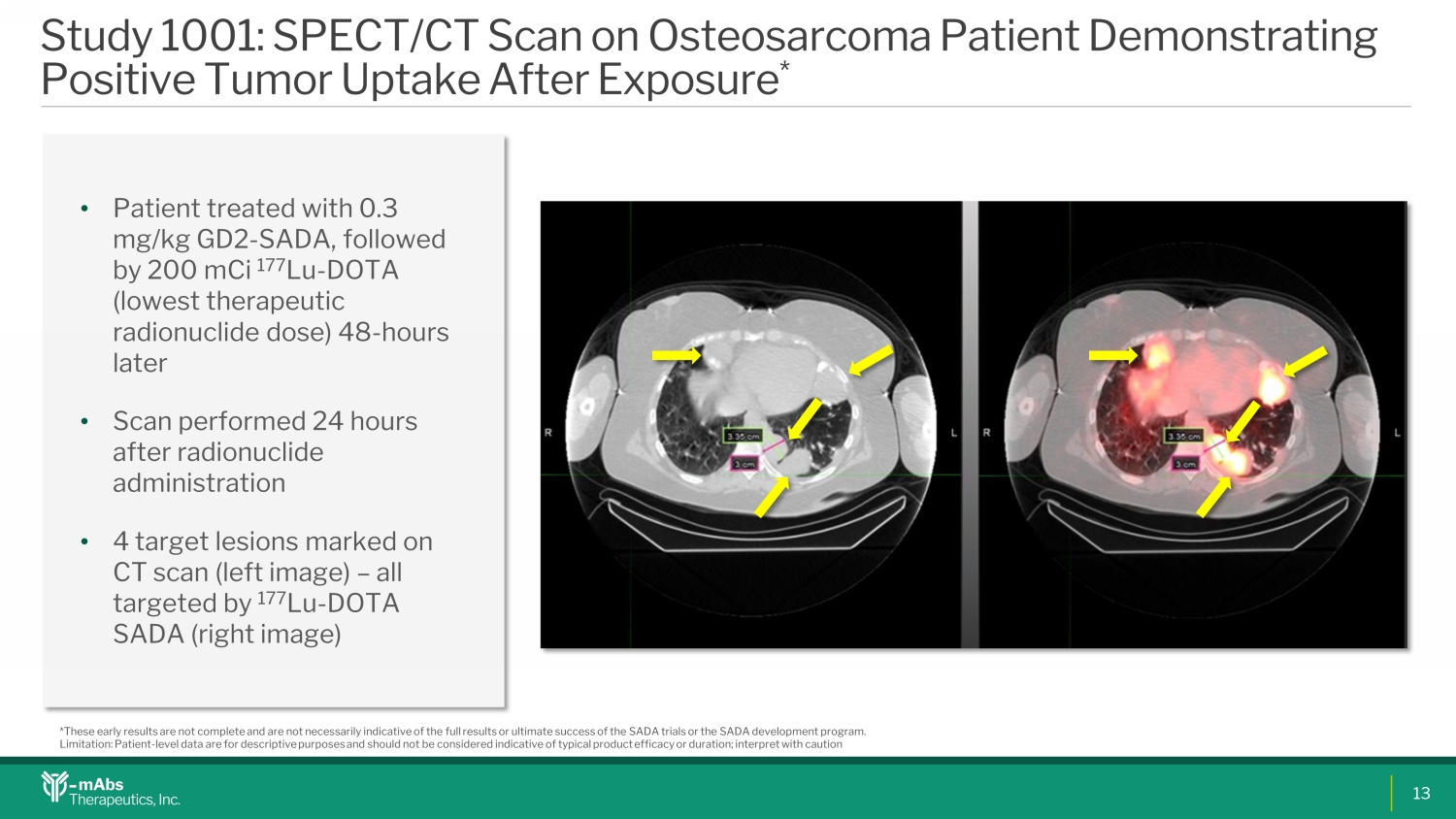
Study 1001: SPECT/CT Scan on Osteosarcoma Patient Demonstrating Positive Tumor Uptake After Exposure * • Patient treated with 0.3 mg/kg GD2 - SADA, followed by 200 mCi 177 Lu - DOTA (lowest therapeutic radionuclide dose) 48 - hours later • Scan performed 24 hours after radionuclide administration • 4 target lesions marked on CT scan (left image) – all targeted by 177 Lu - DOTA SADA (right image) *These early results are not complete and are not necessarily indicative of the full results or ultimate success of the SADA tri als or the SADA development program. Limitation: Patient - level data are for descriptive purposes and should not be considered indicative of typical product efficacy or duration; interpret with caution 13 CD0CD1

Ongoing GD2 - SADA Phase I Trial: Initial PK Data * 14 Non - QC data 0 20 40 60 80 100 120 140 GD2 - SADA conc Hours *These early results are not complete and are not necessarily indicative of the full results or ultimate success of the SADA tri als or the SADA development program. Limitation: Patient - level data are for descriptive purposes and should not be considered indicative of typical product efficacy or duration; interpret with caution 1.0 mg/kg protein dose 0.3 mg/kg protein dose

15 Novel SADA Platform Potentially Provides Simplicity and Enhanced Precision for Physicians and Patients * › Additional early - stage programs include: › HER2 - SADA › B7H3 - SADA Ongoing GD2 - SADA Phase I Trial (Study 1001) › Evidence of tumor update › No DLTs or pain observed to date › Demonstrated PoC that GD2 - SADA targets and binds to tumor in humans › Potential to shift radio - immunotherapy treatment paradigm for patients and physicians with simplicity and enhanced precision of novel SADA platform CD38 - SADA Phase I Trial (Study 1201) › IND cleared by U.S. FDA › First - in - human in patients with R/R non - Hodgkin Lymphoma › Trial initiation expected in Q2 2024 *These early results are not complete and are not necessarily indicative of the full results or ultimate success of the SADA tri als or the SADA development program. SL0
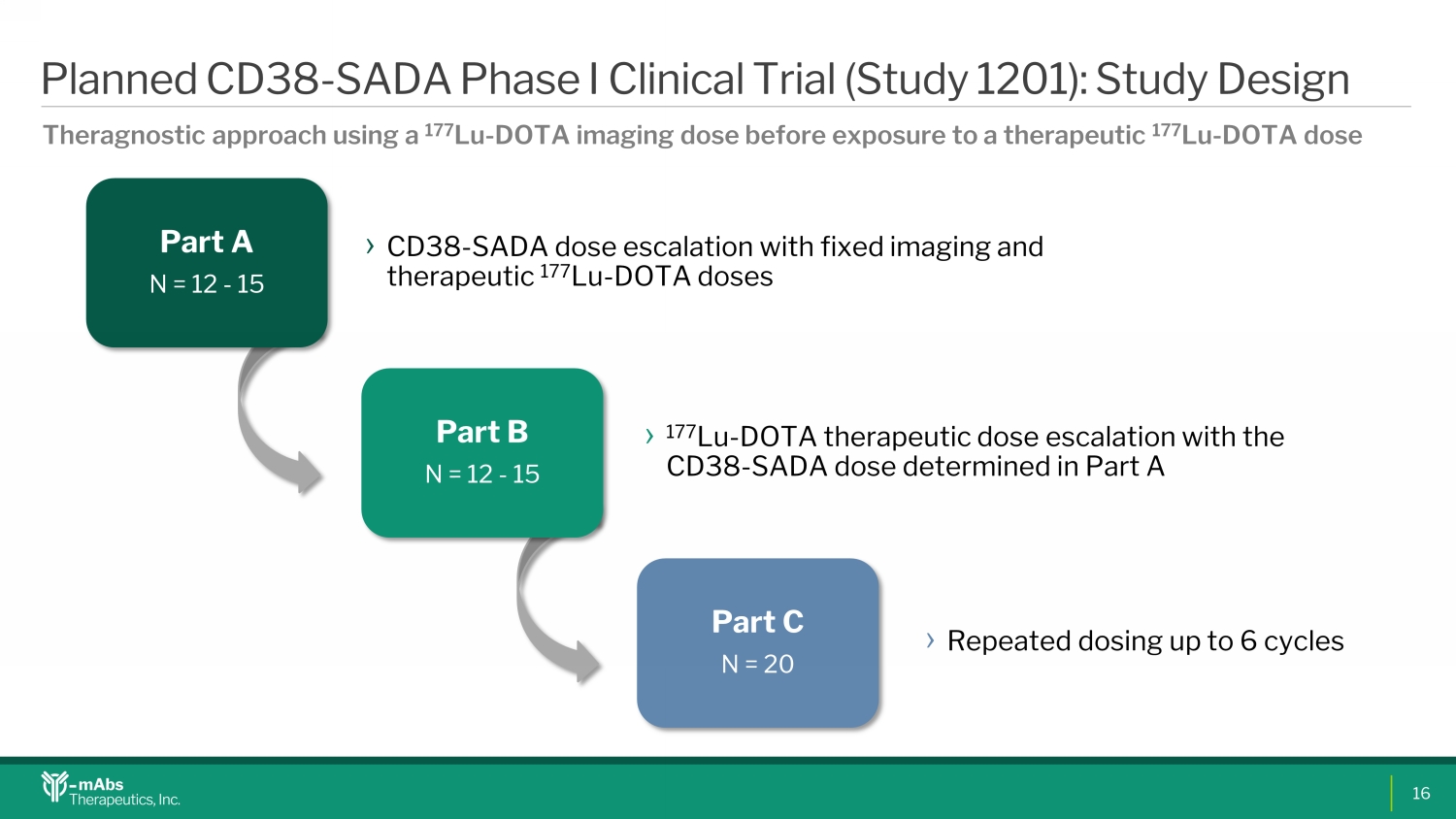
Part A N = 12 - 15 › CD38 - SADA dose escalation with fixed imaging and therapeutic 177 Lu - DOTA doses Part B N = 12 - 15 › 177 Lu - DOTA therapeutic dose escalation with the CD38 - SADA dose determined in Part A Part C N = 20 › Repeated dosing up to 6 cycles Planned CD38 - SADA Phase I Clinical Trial (Study 1201): Study Design Theragnostic approach using a 177 Lu - DOTA imaging dose before exposure to a therapeutic 177 Lu - DOTA dose 16

17 Commercial Progress DANYELZA® ( naxitamab - gqgk ): GD2 Antibody for R/R High - Risk Neuroblastoma
Solid Drivers of Market Uptake • New DANYELZA campaign rolled out in Q4 2023 • 167 new patient starts to date since 2021 launch • Increasing share of U.S. anti - GD2 market with 17% ** DANYELZA: Only FDA - Approved Medicine for R/R NB Patients 18 Neuroblastoma • NB forms in certain types of nerve tissue, most frequently starting from adrenal glands; can also develop in the neck, chest, abdomen or spine • NB is the most common cancer in infants FDA Approval for R/R Neuroblastoma (NB) • Differentiated therapy: › Humanized antibody › Rapid infusion, modest toxicity › Administered in outpatient treatment setting • U.S. addressable market: › 2L NB: 300 patients Global Commercial Launch Performance • Q3 2023 net sales of $20.0 million • 59 sites across the U.S. have utilized DANYELZA * • Ex - U.S. commercial ramp progressing in China; Strong EU demand through WEP • Additional regulatory approvals in LATAM * As of January 4, 2024 ** YTD through September 30, 2023 This indication is approved under accelerated approval. Continued approval for this indication may be contingent upon verific ati on and description of clinical benefit in a confirmatory trial(s). VR0 SK1 SK2
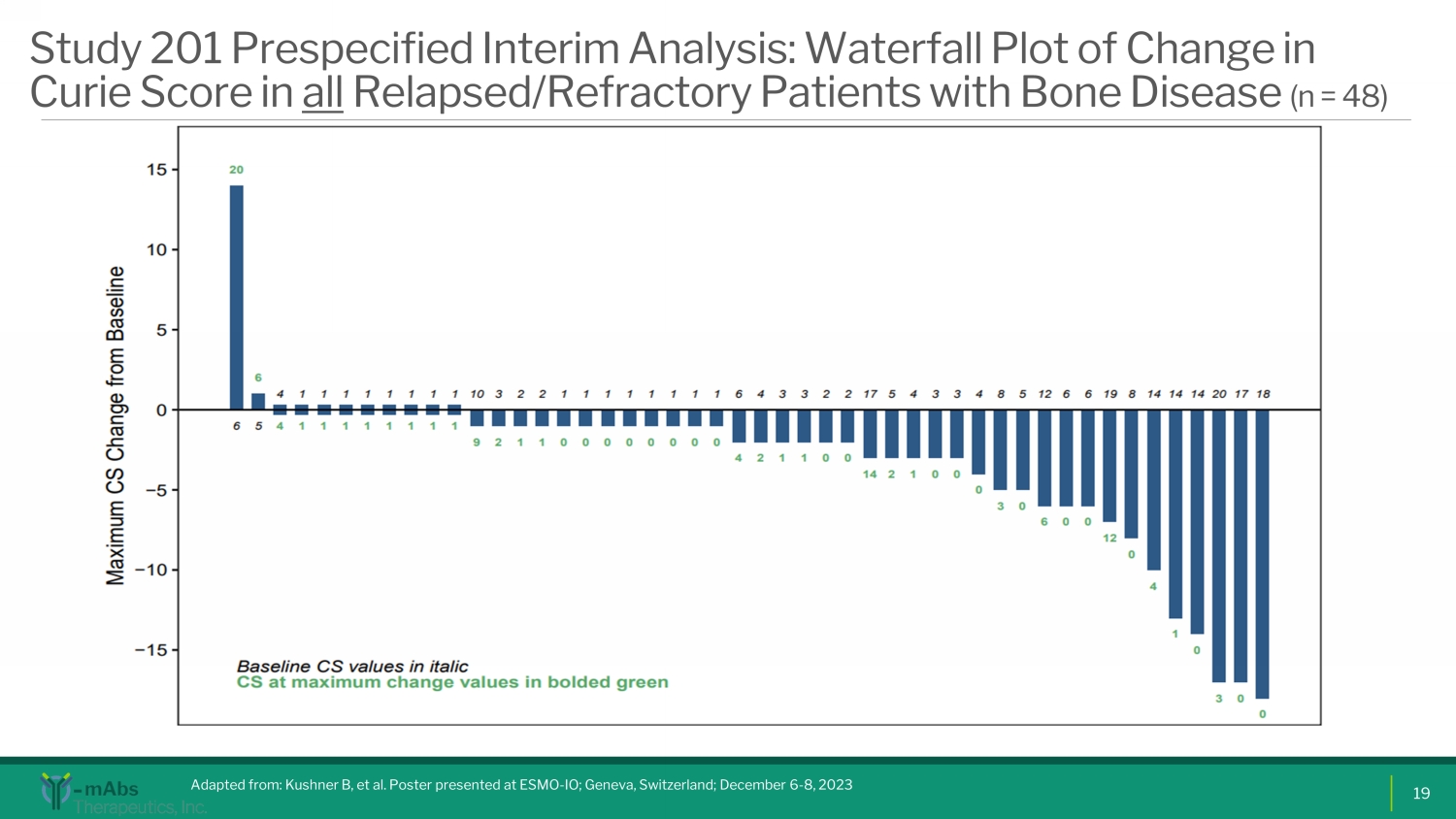
Study 201 Prespecified Interim Analysis: Waterfall Plot of Change in Curie Score in all Relapsed/Refractory Patients with Bone Disease (n = 48) Adapted from: Kushner B, et al. Poster presented at ESMO - IO; Geneva, Switzerland; December 6 - 8, 2023 19
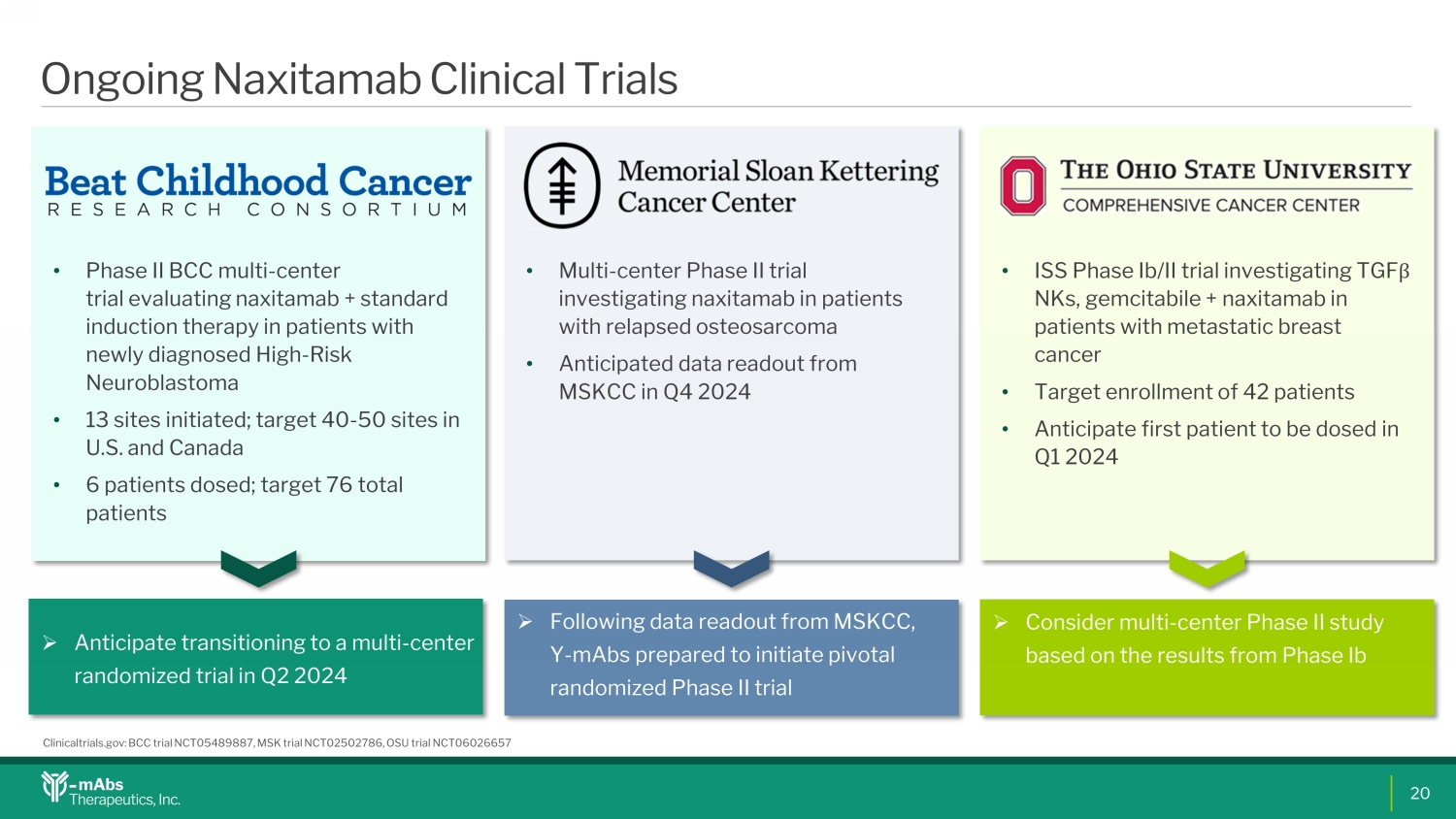
Ongoing Naxitamab Clinical Trials • Phase II BCC multi - center trial evaluating naxitamab + standard induction therapy in patients with newly diagnosed High - Risk Neuroblastoma • 13 sites initiated; target 40 - 50 sites in U.S. and Canada • 6 patients dosed; target 76 total patients • Multi - center Phase II trial investigating naxitamab in patients with relapsed osteosarcoma • Anticipated data readout from MSKCC in Q4 2024 » Anticipate transitioning to a multi - center randomized trial in Q2 2024 » Following data readout from MSKCC, Y - mAbs prepared to initiate pivotal randomized Phase II trial • ISS Phase Ib /II trial investigating TGF β NKs, gemcitabile + naxitamab in patients with metastatic breast cancer • Target enrollment of 42 patients • Anticipate first patient to be dosed in Q1 2024 » Consider multi - center Phase II study based on the results from Phase Ib Clinicaltrials.gov: BCC trial NCT05489887, MSK trial NCT02502786, OSU trial NCT06026657 20VR0VR1
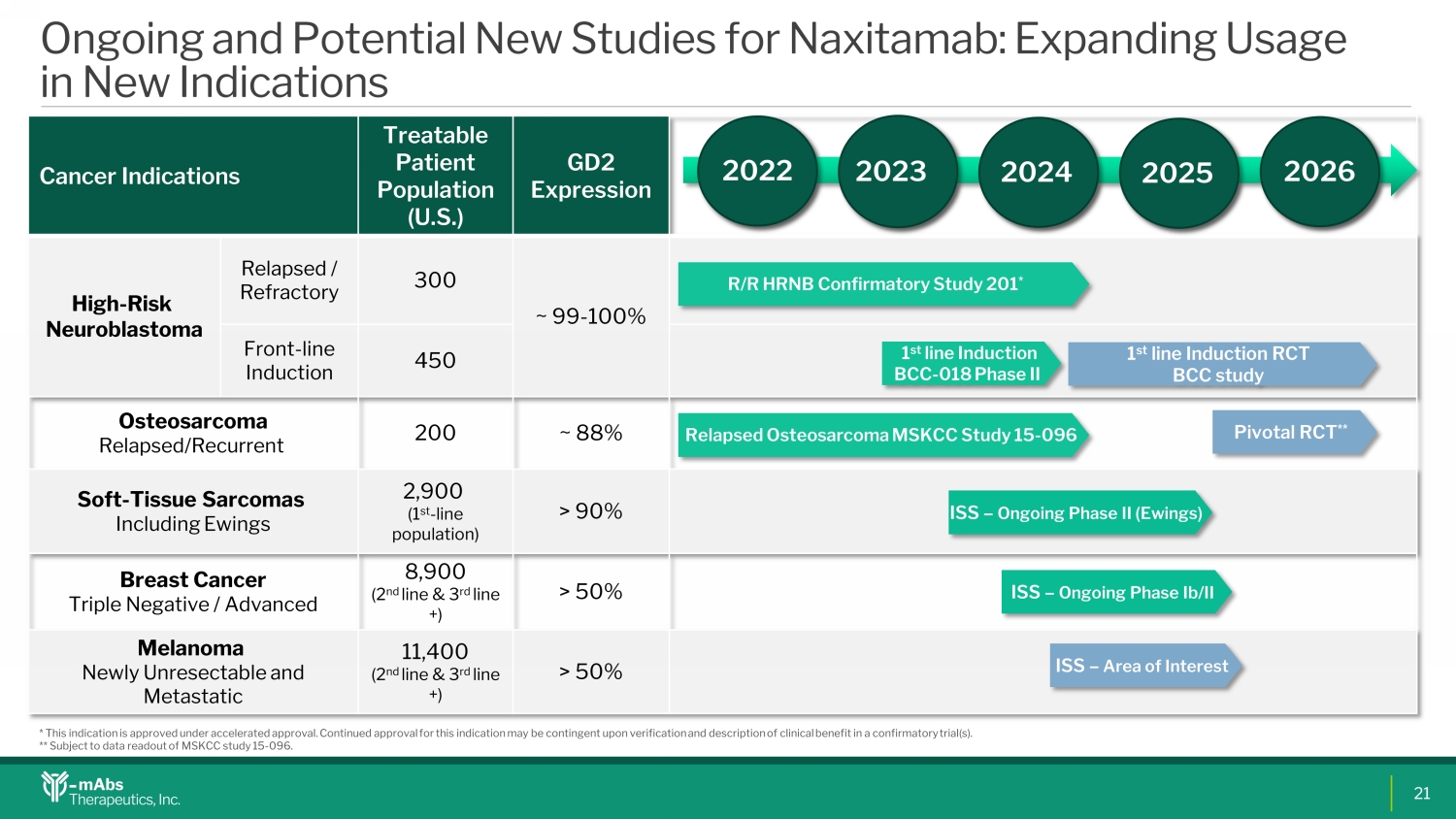
Ongoing and Potential New Studies for Naxitamab : Expanding Usage in New Indications Cancer Indications T reatable Patient Population (U.S.) GD2 E xpression High - Risk Neuroblastoma Relapsed / Refractory 300 ~ 99 - 100% Front - line Induction 450 Osteosarcoma Relapsed/ R ecurrent 200 ~ 88% Soft - T issue S arcomas Including Ewings 2 , 900 ( 1 st - line population) > 90% Breast Cancer Triple N egative / Advanced 8 , 900 ( 2 nd line & 3 r d line +) > 50% Melanoma N ewly Unresectable and Metastatic 11 , 400 ( 2 nd line & 3 r d line +) > 50% 202 2 202 3 202 4 202 5 202 6 R/R HRNB Confirmatory Study 201 * Relapsed Osteosarcoma MSKCC Study 15 - 096 Pivotal RCT ** I SS – Ongoing Phase II ( Ewings ) I SS – Ongoing Phase Ib /II I SS – Area of Interest * This indication is approved under accelerated approval. Continued approval for this indication may be contingent upon verif ica tion and description of clinical benefit in a confirmatory trial(s). ** Subject to data readout of MSKCC study 15 - 096. 1 st line Induction BCC - 018 Phase II 1 st line Induction RCT BCC study 21VR0VR1VR2
22 DANYELZA Addresses Significant Unmet Needs in R/R High - Risk NB with Expansion Potential Across Broader Patient Populations U.S. commercialization in high - risk NB. Launch in China by SciClone; LATAM partner Adium ; EU access via WEP Studies 12 - 230 and 201 formed primary basis of approval in November 2020. Reached 100 patients in Study 201 Multiple potential advantages over other GD2 targeting antibody - based therapies: Modest toxicity, shorter infusion time, ability to be administered in outpatient setting Granted ODD and BTD. Frontline study ongoing

23 Company Takeaways
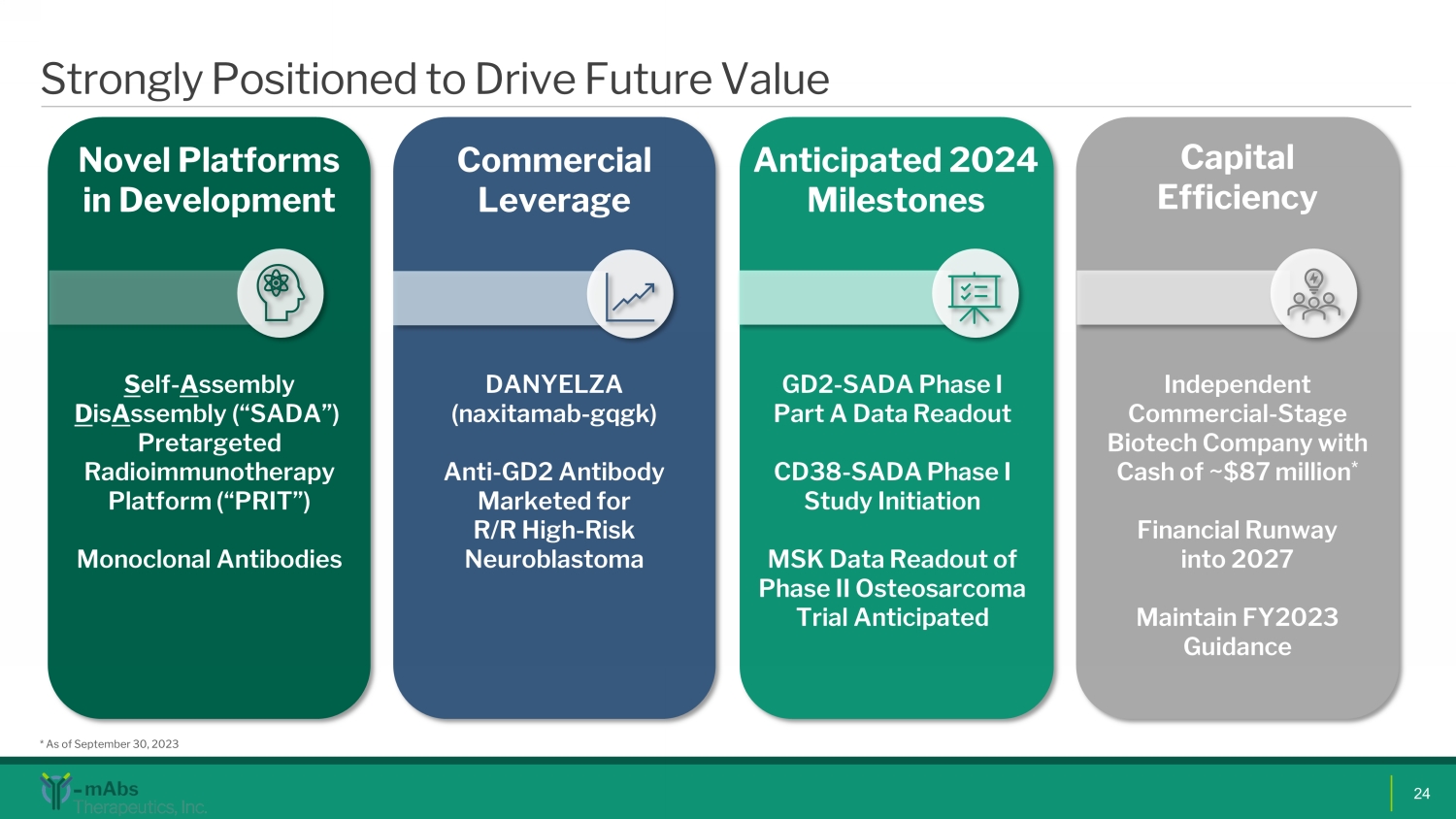
Strongly Positioned to Drive Future Value 24 Novel Platforms in Development S elf - A ssembly D is A ssembly (“SADA”) Pretargeted Radioimmunotherapy Platform (“PRIT ”) Monoclonal Antibodies GD2 - SADA Phase I Part A Data Readout CD38 - SADA Phase I Study Initiation MSK Data Readout of Phase II Osteosarcoma Trial Anticipated Anticipated 2024 Milestones Commercial Leverage DANYELZA ( naxitamab - gqgk ) Anti - GD2 Antibody Marketed for R/R High - Risk Neuroblastoma Capital Efficiency Independent Commercial - S tage Biotech C ompany with Cash of ~$87 million * Financial Runway into 2027 Maintain FY2023 Guidance * As of September 30, 2023

THANK YOU