As filed with the Securities and Exchange Commission on June 3, 2024
Registration No. 333-__________________________
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
| Catheter Precision, Inc. |
| (Exact name of registrant as specified in its charter) |
| Delaware |
| 3841 |
| 38-3661826 |
| (State or other jurisdiction of incorporation or organization) |
| (Primary Standard Industrial Classification Code Number) |
| (I.R.S. Employer Identification Number) |
1670 Highway 160 West, Suite 205
Fort Mill, SC 29708
973-691-2000
(Address, including zip code, and telephone number, including area code of registrant’s principal executive offices)
David Jenkins
Executive Chairman of the Board
and Chief Executive Officer
Catheter Precision, Inc.
1670 Highway 160 West, Suite 205
Fort Mill, SC 29708
973-691-2000
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| B. Joseph Alley, Jr., Esq. Arnall Golden Gregory LLP Suite 2100 171 17th Street NW Atlanta, Georgia 30363-1031 (404) 873-8500 |
| Ivan K. Blumenthal, Esq. Daniel Bagliebter, Esq. Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. 919 Third Avenue New York, New York 10022 (212) 935-3000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ |
|
|
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act ☐
The registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or on such date as the Securities and Exchange Commission, acting pursuant to Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities, and it is not soliciting offers to buy these securities in any jurisdiction where such offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED June 3, 2024
PRELIMINARY PROSPECTUS

______ Shares of Common Stock
Pre-Funded Warrants to Purchase _____ Shares of Common Stock
Up to ________ Shares of Common Stock Issuable Upon Exercise of Pre-Funded Warrants
Underwriter Warrants to Purchase up to ______ Shares of Common Stock
Up to ________ Shares of Common Stock Issuable Upon Exercise of Underwriter Warrants
This is a firm commitment public offering of ____ shares of common stock (the “Shares”), par value $0.0001 per share, of Catheter Precision, Inc., a Delaware corporation (the “Company”), at an assumed public offering price of $____ per share, based on the last sale price of our common stock as reported on NYSE American on _____, 2024. We also are offering warrants (the “Pre-Funded Warrants”) to purchase up to _____ Shares to certain investors, if any, whose purchase of Shares in this offering would otherwise result in such purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock immediately following the consummation of this offering, in lieu of Shares that would otherwise result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock. The purchase price of each Pre-Funded Warrant is equal to the offering price at which a Share of common stock is sold in this offering, minus $0.0001, and each Pre-Funded Warrant will be exercisable for one share of common stock at an exercise price of $0.0001. This prospectus also relates to the offering of the Shares issuable upon exercise of these Pre-Funded Warrants. For each Pre-Funded Warrant that we sell, the number of Shares of common stock that we are selling will be decreased on a one-for-one basis. The assumed public offering price used throughout this prospectus has been included for illustration purposes only. The actual offering price may differ materially from the assumed price used in the prospectus and will be determined by negotiations between us and the underwriters and may not be indicative of prices of the actual offering price.
Our common stock is listed on NYSE American under the symbol “VTAK.” There is no established public trading market for the Pre-Funded Warrants, and we do not expect a market to develop. We do not intend to list the Pre-Funded Warrants on NYSE American or any other national securities exchange or automated quotation system.
Certain of our existing stockholders have indicated an interest in purchasing an aggregate of up to $[ ] of Common Stock in this offering at the public offering price per Share of common stock and on the same terms as other purchasers in this offering. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters may determine to sell more, fewer or no Shares of common stock in this offering to any of these stockholders, or any of these stockholders may determine to purchase more, fewer or no Shares of common stock in this offering. The underwriters will receive the same underwriting discount on any Shares of common stock purchased by these stockholders as they will on any other securities sold to the public in this offering.
You should carefully read this prospectus and any amendments or supplements accompanying this prospectus, together with any documents incorporated by reference herein or therein, before you make your investment decision.
Investing in our securities involves a high degree of risk. See “Risk Factors” on page 18 of this prospectus and in the documents incorporated by reference in this prospectus, as updated by any applicable prospectus supplement, and other future filings we make with the Securities and Exchange Commission that are incorporated by reference into this prospectus, for a discussion of the factors you should consider carefully before deciding to purchase our securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
|
| Per Share |
|
| Per Pre-Funded Warrant |
|
| Total |
| |||
| Public offering price |
| $ |
|
| $ |
|
| $ |
| |||
| Underwriting discounts and commissions(1) |
| $ |
|
| $ |
|
| $ |
| |||
| Proceeds, before expenses, to us (2) |
| $ |
|
| $ |
|
| $ |
| |||
| (1) | Represents underwriting discounts equal to ___% per Share or Pre-Funded Warrant, as applicable. |
| (2) | We have also agreed to pay the underwriters a management fee equal to 1% of the aggregate gross proceeds received from the sale of the securities in the transaction. In addition, we have agreed to reimburse the underwriters for certain expenses and issue the underwriters warrants the ("Underwriter Warrants”) to purchase 6% of the total number of Shares sold in this offering, including Shares underlying Pre-Funded Warrants, at an exercise price equal to 155% of the public offering price of the Shares sold in this offering. See “Underwriting” on page [ ] for additional information regarding underwriting compensation. |
We have granted the underwriters a 45-day option to purchase up to additional Shares of our common stock at the public offering price, less underwriting discounts and commissions.
The underwriters expect to deliver the Shares and Pre-Funded Warrants against payment on or about ____________________, 2024.
Ladenburg Thalmann
The date of this prospectus is ____________, 2024.
TABLE OF CONTENTS
|
|
| 1 |
| |
|
|
| 2 |
| |
|
|
| 14 |
| |
|
|
| 18 |
| |
|
|
| 24 |
| |
| MARKET FOR COMMON STOCK |
|
|
| |
|
|
| 25 |
| |
| DESCRIPTION OF PRE-FUNDED WARRANTS |
|
|
| |
|
|
| 26 |
| |
|
|
| 27 |
| |
|
|
| 31 |
| |
|
|
| 35 |
| |
|
|
| 35 |
| |
|
|
| 35 |
| |
|
|
| 36 |
|
We urge you to read carefully this prospectus, together with the information incorporated herein by reference as described under the heading “Where You Can Find Additional Information” before buying any of the securities being offered.
You should rely only on the information contained or incorporated by reference in this prospectus or in any free writing prospectus. We and the underwriter have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. This prospectus may only be used where it is legal to offer and sell Shares of our common stock and other securities. If it is against the law in any jurisdiction to make an offer to sell these Shares and other securities, or to solicit an offer from someone to buy these Shares and/or other securities, then this prospectus does not apply to any person in that jurisdiction, and no offer or solicitation is made by this prospectus to any such person. You should assume that the information appearing in this prospectus or in any applicable free writing prospectus is accurate only as of the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or of any sale of common stock or other securities. Our business, financial condition, results of operations and prospects may have changed since such date. Information contained on our website is not a part of this prospectus.
A prospectus supplement may add to, update or change the information contained in this prospectus. You should read both this prospectus and any applicable prospectus supplements together with additional information described below under the heading “Where You Can Find Additional Information.”
This prospectus may contain references to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that the applicable licensor will not assert, to the fullest extent under applicable law, its rights to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
| 1 |
|
This summary highlights important features of this offering and the information contained elsewhere in or incorporated by reference into this prospectus. Because this is only a summary, it does not contain all of the information that you should consider before investing in our securities. You should carefully read this entire prospectus and any applicable prospectus supplement, including the information contained under the heading “Risk Factors” and all other information included or incorporated by reference into this prospectus and any applicable prospectus supplement in their entirety before you invest in our securities.
Unless otherwise stated, all references in this prospectus to “we,” “us,” “our,” the “Company,” “Catheter Precision” and similar designations refer to Catheter Precision, Inc. (formerly Ra Medical Systems, Inc.) and all entities included in our financial statements.
Company Overview
We (together with our consolidated operating subsidiary, the “Company” or “Catheter”) were incorporated in California on September 4, 2002, and reincorporated in Delaware in July 2018. The Company was initially formed to develop, commercialize and market an excimer laser-based platform for use in the treatment of vascular and dermatological immune-mediated inflammatory diseases, including the DABRA product line.
On January 9, 2023, the Company merged with Catheter Precision, Inc., or “Old Catheter”, a privately-held Delaware corporation (the “Merger”), and the business of Old Catheter became a wholly owned subsidiary of the Company, which today is our only operating subsidiary. Prior to the Merger with Old Catheter, we operated under the name Ra Medical Systems, Inc. Following the Merger, we discontinued the Company’s legacy lines of business and the use of any of its DABRA-related assets. For further information about these historical lines of business, see “Item 1. Business” of the Company’s Form 10-K for the fiscal year ended December 31, 2021. Since the Merger, we have shifted the focus of our operations to Old Catheter’s product lines, and effective August 17, 2023, we changed our name to Catheter Precision, Inc. Accordingly, our current activities primarily relate to Old Catheter’s historical business which comprises the design, manufacture and sale of new and innovative medical technologies focused in the field of cardiac electrophysiology, or “EP.”
Our two primary products include the VIVO System and LockeT. The VIVO System, which is an acronym for View into Ventricular Onset System (“VIVO” or “VIVO System”), is a non-invasive imaging system that offers 3D cardiac mapping to help with localizing the sites of origin of idiopathic ventricular arrhythmias in patients with structurally normal hearts prior to EP procedures.
Our newest product, LockeT, is a suture retention device indicated for wound healing by distributing suture tension over a larger area in the patient in conjunction with a figure of eight suture closure. LockeT is intended to temporarily secure sutures and aid clinicians in locating and removing sutures efficiently.
Our product portfolio also includes the Amigo® Remote Catheter System, or Amigo, a robotic arm that serves as a catheter control device. Prior to 2018, Old Catheter marketed Amigo. We own the intellectual property related to Amigo, and this product is under consideration for future research and development of a generation 2 product.
Company Information
Our principal executive offices are located at 1670 Highway 160 West, Suite 205, Fort Mill, SC 29708, and our telephone number is 973-691-2000. Our corporate website address is ir.catheterprecision.com. Our website and the information contained on, or that can be accessed through, the website will not be deemed to be incorporated by reference in, and is not considered part of, this filing. You should not rely on any such information in making your decision whether to purchase our common stock. We make available free of charge through our website, at ir.catheterprecision.com, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus.
|
| 2 |
| Electrophysiology Market Overview
The EP Market is a rapid growing segment in healthcare and includes well known medical devices such as pacemakers, electrocardiogram (ECG) systems, and cardiac catheters and lesser-known products such as intracardiac mapping systems and fluoroscopy systems (similar to x-ray in real time). The EP market includes large medical device companies such as Medtronic, Plc., Abbott Laboratories, Biosense-Webster (J&J) and Boston Scientific Corp. and is estimated to be $15.1 billion by 2028 (CAGR of 13.0%). Population growth, increasing rates of heart disease and the rising cost of healthcare are driving growth in the EP markets.
The catheter ablation market was larger than $3.5 billion in 2022 and is estimated to grow to $14.5 billion by 2032 (13.5% CAGR).
The exact number of ventricular ablations performed each year is not well documented. However, in the last 10 years, ventricular ablation has become a fast-growing treatment option due to updated treatment guidelines, improved technology and raising incidence rates. The ventricular ablation market is expected to grow at a rate of 14.5% CAGR through 2032. Over a ten-year period, one study in Australia demonstrated a growth as high as 18% of ventricular tachycardia. Of note, this surpassed the growth rate of Atrial Fibrillation (12.7%) which has historically been the largest incidence of cardiac arrhythmias.
The Heart Rhythm Society, or HRS, Expert Consensus Statement on Catheter Ablation of Ventricular Arrhythmias, published in May 2019 recommends catheter ablation in preference to anti arrhythmic drugs or in the situation where anti arrhythmic therapy has failed or is not tolerated. The guidelines also recommend ablation for reducing recurrent VT and implantable cardioverter-defibrillator shocks.
Existing Treatments and Methods for Catheter Ablations
Traditionally, the first line of treatment for cardiac arrhythmias is medication. Unfortunately, this is not a permanent fix, and most patients eventually need a catheter ablation.
Catheter Ablation Procedure Overview
An electrophysiologist stands next to the patient’s bed near the patient’s groin. A catheter or catheters are inserted into the femoral vein (located at the groin) and navigated into the right side of the heart. Depending on the type of arrhythmia, the catheter is inserted into the atrium or the ventricle. Once inserted, a diagnostic catheter is used in conjunction with an invasive (traditional) mapping system to create a map/model of the patient’s heart. This allows the physician to see the individual patient’s cardiac structures and size. Once the map is created, the physician begins to “pace map.” This process requires the physician to move the catheter from spot to spot to determine the electrical conduction at different areas to determine if the tissue in that area is responsible for the arrhythmia. Once the area is located, the physician will provide a form of energy (radiofrequency, cryo, etc.) to ablate the tissue in that spot.
Treatment Challenges for Ventricular Arrhythmias
Ablation locations within the ventricle are very difficult to identify. Often, patients are highly symptomatic (dizzy, breathing difficulties, etc.) but the arrhythmia is infrequent. When this happens, it is hard to predict when the patient will be having an “active” arrhythmia. Because of this, the physician may not be able to identify the location even when using medication to induce the arrhythmia. Without confirmation during invasive mapping, the patient is removed from the electrophysiology lab without the ablation procedure being performed and the patient is required to return at a later date and try again for a successful outcome.
Even when a patient has frequent ventricular arrhythmias, the process of pace-mapping often takes 4 - 5 hours to identify the location for ablation, which can increase the likelihood of patient complications due to the extended time under anesthesia. Lastly, many patients with untreated ventricular arrhythmias cannot tolerate anesthesia well, thus invasive mapping that takes a long time is not an option for them.
|
| 3 |
| Treatment Challenges for Atrial Arrhythmias
Catheter ablation for atrial arrhythmias is more standardized and “advanced” than for ventricular ablations, thus less pace mapping is required. Instead, a procedure called Pulmonary Vein Isolation (“PVI”) is performed for atrial fibrillation, and a single line is ablated for atrial flutter. In pulmonary vein isolation, tiny scars are created in the left upper chamber of the heart in the area where the four lung (pulmonary) veins connect.
Despite steady improvement in the tools available to perform effective procedures, there is clear study evidence that catheter based atrial fibrillation treatment technology can become more effective. According to a study entitled “Long Term Outcomes of Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta- Analysis” published in the Journal of American Heart Association on March 18, 2013, which looked at multiple individual studies covering over 6,000 patients, “single procedure freedom from atrial fibrillation at long term follow up was 53.1%.” The same study found “with multiple procedures performed, the long-term success rate was 79.8%.” Ineffective treatment may result in patients undergoing two or more EP procedures to achieve relief from atrial fibrillation at an estimated cost in the range of $20,000 or more per procedure.
Specific reasons have not been proven for the lower success rate of initial ablation procedures. However, there is growing evidence that better results occur if the treating EP physician is able to make better lesions by maintaining stable contact force of the catheter against the heart wall, thereby reliably delivering the energy required to eliminate the abnormal rhythms. Variation in catheter contact force occurs as the physician attempts to manually position and hold the catheter tip in a stable position during cases lasting 2 to 3 hours in order to perform typically over 100 ablations of the cardiac anatomy.
Large multi-national medical device companies, such as Medtronic, Inc., Boston Scientific Corp., Abbott Laboratories, St. Jude Medical, Inc. (acquired by Abbott Laboratories in 2017) and the Biosense Webster division of Johnson & Johnson, among others, continue to invest heavily to develop and introduce new devices and technologies to improve patient outcomes. Included among these are force-sensing catheters, including the Biosense SmartTouch TM catheter, which provide a continuous readout of the contact force between the catheter and the heart wall. Our Vivo System is focused on the controlled delivery of these catheter technologies to enhance both the performance of ablation procedures and the ease and safety for the physicians who perform them.
A recent peer-reviewed multicenter study sponsored by Biosense Webster, entitled “Paroxysmal AF Catheter Ablation with a Contact Force Sensing Catheter” published in 2014 found that catheter ablation success rates can be as high as 80% when the physician is able to maintain stable contact force within investigator selected working ranges. “When the CF (contact force) employed was between investigator selected working ranges > 80% of the time during therapy, outcomes were 4.25 times more likely to be successful.” Further, “stable CF during radiofrequency application increases the likelihood of twelve-month success.” However, it should be noted that, using manually controlled methods, the physicians in the study could only maintain optimal tissue contact in less than 30% of the patients studied.
In addition, another study, sponsored by St. Jude Medical, Inc. (acquired by Abbott Laboratories in 2017) and published in 2015 showed similar findings using their recently FDA-approved contact-force sensing catheter, TOCCASTAR. In the TOCCASTAR study, 85.5% of ablation procedure patients were free of atrial fibrillation at one year after the procedure when optimal catheter tip contact force was maintained, versus only 67.7% when non-optimal contact force was achieved.
Our Products
Our products VIVO and LockeT are used in connection with catheter ablation procedures by (in the case of VIVO) providing pre-procedure, non-invasive mapping of arrhythmias and (in the case of LockeT) ensuring efficient hemostasis in conjunction with a figure-of-eight suture, and temporarily securing sutures at the access site. We also believe that LockeT may be useful in connection with other structural heart procedures that require femoral closure, such as procedures for pacemakers, heart valves, heart valve repair, and left atrial appendage devices. |
| 4 |
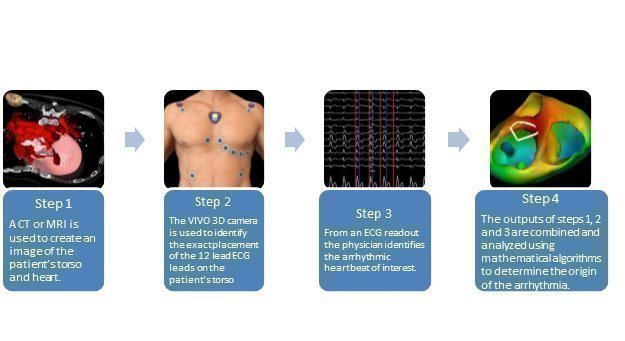
| VIVO™ System
VIVO is an FDA-cleared and CE marked product that utilizes non-invasive inputs to locate the origin of ventricular arrhythmias. VIVO has been used in more than 1,000 procedures in leading U.S. and European hospitals under a limited commercial launch that commenced in the third quarter of 2021. A full-scale commercial launch commenced in Q1 2023 in conjunction with the expansion of a direct sales force in the US.
VIVO is a non-invasive imaging system that offers 3D cardiac mapping to help with localizing the sites of origin of idiopathic ventricular arrhythmias in patients with structurally normal hearts prior to electrophysiology procedures. The VIVO system has achieved a CE Mark allowing it to be commercialized in the European Union and has been placed at several hospitals in Europe. FDA 510(k) Clearance in the United States was received in June 2019.
The VIVO software is provided on an off the shelf laptop, and the system includes a 3D camera. In addition, the system can only be used with a disposable component, the VIVO Positioning Patches, which are required for each procedure.
| ||||||
|
|
|
|
|
|
|
|
|
The VIVO software contains proprietary algorithms that are based on standard EP principles. However, the accuracy of the algorithms is improved because it does not use generalized assumptions and instead, uses patient specific information. VIVO uses standard clinical inputs such as a CT or MRI and a 12 lead ECG, both of which are routinely gathered for most EP procedures, allowing VIVO to seamlessly integrate into the workflow. A 3D photograph is obtained of the patient’s torso after the ECG leads are in place and all of these clinical inputs are combined to generate a 3D map of the patient’s heart with a location of the earliest onset of the ventricular arrhythmia. | ||||||
| 5 |
| VIVO Workflow
|
| 6 |
| Clinical Use and Studies
To date, VIVO has been used in more than 1,000 procedures, by more than 30 physicians in 10 countries. Initial clinical work was completed with the first-generation software, which resulted in FDA 510(k) Clearance in June 2019.
The U.S. multi-center study enrolled 51 patients from 5 centers. Of note, the Principal Investigator and center to have the highest enrollment was Johns Hopkins University in Baltimore, Maryland. This study was conducted to evaluate the accuracy of VIVO as compared to invasive mapping systems (current prevailing method for determining arrhythmia origins). VIVO met all study endpoints and correctly matched the predicted arrhythmia origin in 44/44 patients (100%; primary endpoint) and correctly matched paced sites in 225/226 locations (99.56%; secondary endpoint). In some instances, this study showed that VIVO has better predictability for arrhythmia origin than a physician’s manual review of a 12 lead ECG.
While conducting the initial clinical study for FDA submission, we developed generation 2 in parallel with a goal to have this version complete and ready to submit upon 510(k) clearance of generation 1. We successfully achieved this goal and received CE Mark and FDA 510(k) Clearance for generation 2 in 2020.
Additional clinical work has occurred with generation 2. Until recently, this data has been single center, physician-initiated research and has resulted in peer reviewed clinical science at electrophysiology conferences and in journals.
Three physicians, at different centers, in the UK conducted a feasibility study for Stereotactic Ablative Radiotherapy, or SABR, and published their data on nine patients. SABR is an ablation technique utilizing non-invasive methods akin to proton therapy for cancer treatment. To do a complete non-invasive ablation, accurately predicting the ablation location non-invasively is key to procedural success, and VIVO was utilized for this purpose. Non-invasive ablation is a new technique and requires additional data, but it is showing promise and has generated excitement within the EP community. If accepted for wide-spread treatment, this would allow for previously un-ablatable patients to receive lifesaving treatments.
In February 2023, a study from the Royal Brompton Hospital was published. This study enrolled 15 patients with 24 VTs (ventricular contractions) and PVCs (premature ventricular contractions). VIVO accurately identified VT and PVC origin in 23/24 (96%) and sub-localized in 100% of subjects. Acute success was achieved in 100% of cases. Standard ECG algorithms, conducted by 3 physicians in blind trials, only identified the correct chamber in 50-88% of the patients and sub-localized within the right ventricular outflow tract (septum v free wall) in 37 – 58% of subjects. Of note, six patients had previously attended for nine attempted ablations collectively, which were either unsuccessful or aborted owing to lack of spontaneously occurring clinical PVCs. One patient had previously reported for four separate attempts without PVCs and ablations were aborted, but collection of a single beat allowed VIVO to create an analysis map and provide the physician with information to complete the ablation for all these patients. In addition, this study showed a 27% reduction in procedure time when using VIVO as compared to a historical cohort. This study concluded that VIVO can accurately identify arrhythmia origin with an accuracy that is superior to that of established ECG algorithms.
In April 2022, one physician from the Netherlands presented an abstract at EHRA (European Heart Rhythm Association), focused on using VIVO as a way to screen patients prior to the ablation procedure. This study of 15 patients concludes that using VIVO pre-procedurally may enable the physician to determine procedure success rates and prevent unnecessary ablation procedures. This data will need to be further studied in larger numbers but determining success in advance of the procedure would improve ablation therapy, which has a high failure rate and thus requires additional ablation procedures.
In October 2021 the first patient was enrolled in the VIVO EU Registry. This registry aims to gather data about how VIVO is used in real-world settings, outside of a rigorous clinical study. The registry will enroll 125 patients across Europe and the UK and collect information about different workflows and applications for VIVO. Enrollment of 125 patients was completed in June 2023. The study requires 12-month follow-up and data collection is planned for completion in Q3 2024. This data serves multiple purposes including fulfilling European regulatory requirements for on-going data collection, publication of multi-center data, and future development of studies and improvements to the VIVO technology. |
| 7 |
| Currently, there is an ongoing physician-initiated study at Coventry hospital in the UK. This study will enroll 50 patients with Re-entrant Ventricular Tachycardia. These patients have hearts that are not structurally normal and scarred tissue is present in the ventricle. This data will be used for publication and to support an FDA submission to expand the current labeling of the existing product.
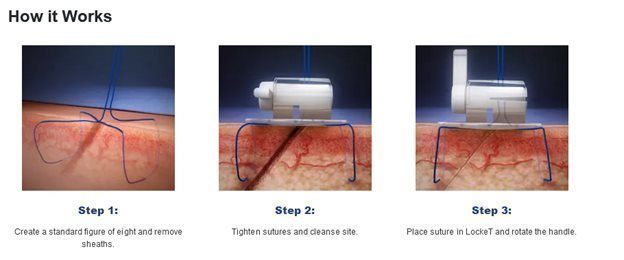
LockeT
As catheters are put into the body, they are put through the skin and into a blood vessel. After the procedure is completed the catheter is removed, and each access site must be closed and bleeding stopped (hemostasis). Ablation for atrial fibrillation (“AFIB”) creates up to four different perforations, each one requiring closure. LockeT is a suture retention device for use as part of the closure process. It is designed to be used in conjunction with a “figure of eight” suture. Each LockeT device can assist with the closure of two perforations, and therefore up to two devices are expected to be used for each AFIB ablation.
We believe LockeT offers a cost-effective solution for access site closure, with multiple features we believe clinicians will find attractive. It is transparent, which allows for easier monitoring of the site, and is designed for a wide range of catheter sizes. It utilizes a crank to provide pressure to the site and provide more efficient hemostasis (i.e., to stop the bleeding from the access site). LockeT simplifies the closure process, making it easy to monitor, adjust and remove as needed.
LockeT is a sterile, Class I product that we registered with the FDA in February 2023, at which time we began initial shipments for product evaluations. In May 2023, Catheter began the process to seek CE Mark approval for LockeT, and we are currently expecting to receive this approval in the second half of 2024. Once we receive CE Mark approval, initial international shipments to distributors may begin. LockeT is indicated for wound healing by distributing suture tension over a larger area in the patient in conjunction with a figure of eight suture closure and is intended to temporarily secure sutures and aid clinicians in locating and removing sutures efficiently. |
| 8 |
| Clinical studies for LockeT began during 2023. The three phases of the current studies are planned to show the product’s effectiveness and benefits, including faster wound closure, earlier ambulation, potentially leading to early hospital discharge, and lower costs for the healthcare provider and/or insurance payor. This data is intended to provide crucial data for marketing and to expand our indications for use with the FDA.
The Phase I - First in Man Feasibility Study was completed in 2023 and showed the device works for its intended purpose, that there were no safety events and gathered initial data to support Phase II submission to Institutional Review Board (IRB). The results were submitted to the Journal of American Academy of Cardiologists in January 2024.
Phase II received IRB approval in late 2023 and is anticipated to be completed by the end of our 2024 third quarter. This phase will compare manual compression (standard of care) to LockeT in a one-to-one randomized study of up to 110 patients and assess improved time to hemostasis and ambulation when using LockeT versus manual compression.
Phase III IRB approval is in process and will compare LockeT to one or more competitive products in a one-to-one randomized study of 100 patients and will include cost comparisons and assess risk of hematoma when using LockeT versus those competitive products. We anticipate this phase to be completed in early 2025.
We are still in the early phases of our roll out for LockeT, which is now under evaluation at several U.S. medical centers. As we build out our sales network, we are focused on placing the product for evaluation at more centers throughout the U.S. During the second quarter of 2024, the product received its first approval for future use by a U.S. medical center, which has submitted several purchase orders to date. Although there are competing devices in the marketplace that assist with vascular closure, we believe that LockeT, which is easy to use, offers unique advantages that will lead to ready adoption in the marketplace.
Our Strategy
Our goal is to become a leading medical imaging and device company in the field of cardiac electrophysiology, and we are dedicated to developing and delivering electrophysiology products to provide patients, hospitals, and physicians with novel technologies and solutions to improve the lives of patients with cardiac arrhythmias. We aim to establish VIVO and LockeT as integral tools used by cardiac electrophysiologists during and following ablation treatment of ventricular arrhythmias, by reducing procedure time and patient complications and increasing procedural efficiencies and success.
Customers
Our primary customers are hospitals providing cardiac electrophysiology lab procedures. We believe there are 2,000 to 3,000 EP labs in the U.S. and a similar number of labs outside of the U.S. performing approximately 600,000 ablation procedures annually. During fiscal 2023, we had two individual customers that represented approximately 32% and 20% of our total revenues, respectively, and four customers (including the two just described) that in the aggregate represented approximately 72% of our total revenues.
Sales and Marketing
Today, we use a mix of distribution partners (Europe), independent sales agents (U.S.) specializing in EP products, and direct employees providing clinical support and product specialization. In the U.S., LockeT and VIVO, including the VIVO System and patches, are currently sold by direct employees who call on electrophysiologists, lab staff and hospital administrators. This sales team qualifies appropriate prospective customers, and with support from our direct clinical specialists they conduct product demonstrations, and support customer training and case usage. In Europe, our products are sold through distributors, supported by two full-time contracted employees. |
| 9 |
| We also have co-marketing and spot distribution agreements with Stereotaxis, Inc., that allow for the promotion of VIVO by Stereotaxis to customers who may benefit from VIVO at certain hospitals using a Stereotaxis Robotic Magnetic Navigation System, in exchange for a commission of 45% of any revenue generated from VIVO at these robotic hospitals; the 45% payments will continue beyond the initial sale, if any.
We continue to hire additional clinical support and direct sales representation as we continue the buildout of our sales network for both VIVO and LockeT. We focus on sales staff who are experienced in the electrophysiology field and are able to identify and target prospective customers to educate, and demonstrate our products, leading to adoption and purchase of our technology. We will continue to use direct clinical specialists to provide training and ongoing clinical support.
In the future, we intend to market our products in the U.S. and certain international markets using a combination of a direct sales force and independent distributors. This requires us to make a significant investment building our U.S. commercial infrastructure and sales force and in recruiting and training our sales representatives and clinical specialists for U.S. commercialization of VIVO. This is a lengthy process that requires recruiting appropriate sales representatives, establishing a commercial infrastructure in the United States, and training our sales representatives, and will require significant ongoing investment by us. Following initial training, our sales representatives typically require lead time in the field to grow their network of accounts, coordinate their sales efforts with each hospital’s capital budgeting and acquisition cycle and produce sales results. Successfully recruiting and training a sufficient number of productive sales representatives is required to achieve growth at the rate we desire.
Outside the U.S., we will continue to foster additional key partner relationships with distributors who will market, sell and support its products.
In addition, we believe there are opportunities to offer additional complementary products through our sales and marketing channels that would enhance the productivity of our sales force and provide additional scale to revenue, better covering fixed operating costs.
Manufacturing and Availability of Raw Materials
VIVO manufacturing, inventory and product fulfillment is housed in our approximate 2,000 square feet facility in Fort Mill, South Carolina. This facility currently has one full-time employee who oversees manufacturing, quality objectives, and order fulfillment. The VIVO system includes VIVO software, loaded onto an off-the-shelf laptop, which we equip with a 3D camera. We purchase laptops and cameras that have been manufactured by third parties. Disposable VIVO Positioning Patches are also required for use of the system, and the manufacture of the patches is outsourced. We also outsource updating and troubleshooting of the software, as needed, to a third-party software engineering company from time to time. LockeT manufacturing, inventory and product fulfillment has been subcontracted to the company that is also providing research and development of the product.
LockeT is manufactured by a third party, Zien Medical Technologies, located in Salt Lake City, Utah. Zien is responsible for procuring product, packaging, assembly and sterilization of LockeT. Once sterilization is complete Catheter Precision reviews the Device History Report (DHR) and approves the product before it is placed into inventory.
Competition
The medical device industry is highly competitive, subject to rapid change and significantly affected by new product introductions and other activities of industry participants. We face potential competition from major medical device companies worldwide, many of which have longer, more established operating histories, and significantly greater financial, technical, marketing, sales, distribution, and other resources. Our overall competitive position is dependent upon a number of factors, including product performance and reliability, manufacturing cost, and customer support. Our primary competitors in the cardiac electrophysiology space include known medical devices such as pacemakers, electrocardiogram, or ECG, systems and cardiac catheters, but also laboratory equipment such as intracardiac mapping systems and fluoroscopy systems (similar to x-ray in real time). The EP market includes large medical device companies such as Medtronic, Plc., Abbott Laboratories, Biosense-Webster (J&J) and Boston Scientific Corp. LockeT’s direct competitors include Abbott’s Perclose device, Haemonetic’s VASCADE device and Inari Medical’s FlowStasis device. |
| 10 |
| Reimbursement
At this time, there is no reimbursement for VIVO or LockeT. Ablation procedures are reimbursed using one current procedural technology, or CPT, code, which varies depending on the type and complexity of the procedure. The range of reimbursement for ablations varies within regions but can be as much as $20,000 or more.
We currently intend, in the future, to hire a reimbursement specialist to guide us through the process of obtaining a CPT code specifically for VIVO. Although a new Category III CPT codes is approved and available starting July 1, 2024, Category III codes, which are temporary, do not have a payment rate established, and payment is at the discretion of payors; further, payors generally require a high level of clinical data through long-term patient studies to demonstrate that a treatment produces favorable results in a cost-effective manner relative to other treatments, in order to be willing to provide reimbursement based on Category III codes. Successful execution of our current commercialization and build out for VIVO will be needed in order to move Category III codes to permanent Category I codes.
Research and Development
The major focus of our research and development team is to leverage our existing technology platform for new applications and improvements to our existing applications, including multiple engineering efforts to improve our current products. Future research and development efforts will involve continued enhancements to and cost reductions for VIVO and LockeT. We will also explore the development of other products that can be derived from our core technology platform and intellectual property. Our research and development team works together with our commercial team to set development priorities based on communicated customer needs. The feedback received from our customers is reviewed and evaluated for incorporation into new products. Our research and development function has been outsourced to a third-party provider.
In the future we intend to develop a generation 3 of VIVO. This version would have expanded indications to include ischemic heart disease and improve usability by the hospital staff. It would also contain more automaticity, potentially reducing our need for clinical support.
Resources Material to Our Business
Patents and Proprietary Technology
Patents
We have a number of patents covering our intellectual property, both in the U.S., as well in a number of international countries. We consider the U.S. to be the most important market for our products, and hence, the most important country for the filing of patents. Any foreign filings are merely replicates of the U.S. filings. For the U.S., we have the following patent positions for the different product areas: | ||
|
|
|
|
|
| · | VIVO – We have two U.S. patents granted on the original VIVO concept, which have been licensed from a third party. We consider the primary component to be the ideas around utilizing a 3D camera to identify the exact location of the body surface electrodes. These two patents expire in 2038. An additional two applications have been granted, which disclosed ideas around merging of the heart models to other heart images and expire in 2038 and 2040. An additional three applications were published, all filed in 2021, covering the idea of determining the thickness of the wall of the ventricle, covering the concept of the rendering of a heart model and likely outcomes of an EP procedure. An additional application was filed in September 2023 and is not yet published. |
|
|
|
|
|
| · | LockeT – Suture Retention Device - We have four published U.S. patent applications. These cover the basic concept, methods of use and the design of the conceived device. |
|
|
|
|
|
| · | AMIGO – We have twenty issued U.S. patents. The first patent, filed in 2006 and expiring in 2031, covers the basic idea, with a three way motor, a remote control, a sled device, and a docking station for a catheter. The more detailed ideas behind the original concept were covered in three patents filed between 2011 and 2013 and expiring in 2026. Additional concepts and methods were filed with six patents between 2010 and 2013, with expirations between 2029 and 2031. We consider the most relevant of the intellectual property to be the guiding track with opposing flexible guides to hold the catheter stable as it is advanced, the form and function of the controller handle, and the introducer interface of the arm to the introducer. An additional ten patents, filed between 2013 and 2017, and expiring in 2034 to 2037, are patents covering ideas not used in the original commercial device, but potential ideas for future embodiments. |
| 11 |
| License and Other Agreements
PEACS, NV Software and Technology License Agreement
On May 1, 2016, we entered into a certain Software and Technology License Agreement with PEACS, NV, a Netherlands company, or the License Agreement, for the exclusive worldwide license of the underlying technology to its VIVO product, including intellectual property rights and patent applications pertaining thereto. The license was for use of the technology for the field of use defined as “the localization of the origin of cardiac activation for the electrophysiology treatment and/or detection of cardiac arrhythmias.” The License Agreement called for us to pay for the prosecution and maintenance of patents to protect the technology.
In May 2021, the License Agreement was modified to modify the field of use to specifically exclude the use of clinical applications for the implanting of atrial or ventricular pacemakers, including bi-ventricular pacemakers.
LockeT Royalty Agreements
We have acquired the rights to Locket pursuant to certain assignment agreements, including an assignment and royalty agreement (the “Assignment and Royalty Agreement”) with one of the co-inventors. Pursuant to the Assignment and Royalty Agreement, we agreed to pay a royalty fee of 5% on net sales up to $1 million. Thereafter, if a patent for the LockeT device is obtained from the U.S. Patent and Trademark Office, we will pay a royalty fee of 2% of net sales up to a total of $10 million in royalties. However, no further royalty payments will be due after December 31, 2033, or after the expiration, cancelation or abandonment of the patents that are the subject of the agreement, whichever is earlier. In addition, at the time of the Merger, additional royalty rights with respect to the LockeT device were granted to certain holders, or the Noteholders, of Old Catheter’s outstanding convertible promissory notes in exchange for forgiveness of the interest that had accrued under those notes but remained unpaid, pursuant to the terms of certain Debt Settlement Agreements. The Debt Settlement Agreements provided for the Noteholders to receive, in the aggregate, approximately 12% of the net sales, if any, of the LockeT device, commencing upon the first commercial sale through December 31, 2035.
Trademarks
We own or have rights to trademarks that we use in connection with the operation of our business. We own or have rights to trademarks for Ra Medical Systems and Catheter Precision and their logos, as well as other trademarks such as AMIGO. In February 2024 we filed a trademark for LockeT.
Trade Secrets
We also have relied upon trade secrets, know-how and technological innovation, and may in the future rely upon licensing opportunities, to develop and maintain its competitive position. We have protected our proprietary rights through a variety of methods, including confidentiality agreements and proprietary information agreements with suppliers, employees, consultants and others who may have access to proprietary information.
Government Regulations
Governmental authorities in the U.S. (at the federal, state, and local levels) and abroad extensively regulate, among other things, the research and development, testing, manufacture, quality control, clinical research, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing, and export and import of products such as those we market and are developing. See our Form 10-K for the fiscal year ended December 31, 2023, for more information about the impact on our business from these and other pertinent regulations. |
| 12 |
| Segment Information
We operate our business as one segment which includes all activities related to the marketing, sales and development of medical technologies focused in the field of cardiac EP. The chief operating decision-maker reviews the operating results on an aggregate basis and manages the operations as a single operating segment.
Employees
As of May 28, 2024, we had a total of 19 employees, including 19 full-time employees, which includes finance and administrative, sales and marketing and clinical professionals. We also have retained a total of four persons as independent contractors. We are planning to increase our sales force in support of product launches but currently have no other plans to increase our staff.
Recent Developments
Interim Financing
On May 30, 2024, we borrowed $500,000 from David Jenkins, our Chairman of the Board and Chief Executive Officer, pursuant to a promissory note dated May 30, 2024 in order to fund our short-term liquidity needs. The borrowed amount bears interest at the rate of 8% per annum, and all outstanding principal and interest under the note will be due and payable on August 30, 2024. We expect to repay this loan using proceeds from this offering. See “Use of Proceeds.”
Proposed Reverse Stock Split
Our stockholders will vote on a proposed reverse stock split at our July 3, 2024 annual meeting of stockholders. If approved by our stockholders, the reverse stock split proposal would permit, but would not require, the Board to effect a reverse stock split of our Common Stock issued and outstanding or held in treasury by a ratio of not less than 1-for-5 and not more than 1-for-15, with the exact ratio to be set at a whole number within this range as determined by the Board, or a duly authorized committee thereof, in its sole discretion. The reverse stock split, if effected, would affect all of our holders of common stock uniformly, including Shares issued in this offering. See our definitive proxy statement on Schedule 14A filed with the Securities and Exchange Commission on May 16, 2024, which has been incorporated by reference herein, for additional information.
Proposed Reduction in Authorized Shares
Our Amended and Restated Certificate of Incorporation, as amended to date, currently authorizes the issuance of 310 million shares of capital stock, consisting of 300 million shares of common stock and 10 million shares of preferred stock. At our July 3, 2024 annual meeting of stockholders, our stockholders will vote on a proposal to reduce our authorized common stock, either (i) in the event the reverse stock split is approved and effected, from 300 million shares to 30 million shares, with authorized preferred stock remaining at 10 million shares; or (ii) in the event the reverse stock split is not approved and effected, from 300 million shares to 100 million shares, with authorized preferred stock remaining at 10 million shares.
Corporate Information
Our principal executive offices are located at 1670 Highway 160 West, Suite 205, Fort Mill, South Carolina 29708. Our telephone number is (973) 691-2000. Our corporate website address is www.catheterprecision.com. Information contained on, or that can be accessed through, our website is not incorporated by reference into this document, and you should not consider information on our website to be part of this document. |
| 13 |
|
|
|
| Common stock offered | _____ Shares of common stock. |
|
|
|
| Common stock outstanding prior to this Offering | 7,573,403 Shares of common stock. |
|
|
|
| Common stock to be outstanding immediately after this Offering (assuming all offered Shares are sold) | _____ Shares of common stock (____ shares if the underwriter exercises its option to cover over-allotments, if any), excluding Pre-Funded Warrants, if any. |
|
|
|
| Pre-Funded Warrants offered | We are also offering to those purchasers, if any, whose purchase of Shares in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock immediately following the consummation of this offering, the opportunity to purchase, if such purchasers so choose, [ ] Pre-Funded Warrants, in lieu of Shares that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (at an exercise price of $0.0001 per Pre-Funded Warrant) of our outstanding shares of common stock. The purchase price of each Pre-Funded Warrant will equal the public offering price at which Warrants are being sold to the public in this offering, minus $0.0001. See “Description of Securities We Are Offering—Pre-Funded Warrants.” This prospectus also relates to the offering of the Shares issuable upon exercise of the Pre-Funded Warrants. |
|
|
|
| Over-allotment option
| We have granted the underwriter an option for a period of 45 days from the date of this prospectus to purchase up to an additional __ Shares of common stock, at a purchase price per additional Share equal to the public offering price per Share, less the underwriting discount. |
| 14 |
| Underwriter Warrants | We have agreed to issue to the underwriters warrants, or the Underwriter Warrants, to purchase up to 6% of the total number of Shares sold in this offering, including Shares underlying Pre-Funded Warrants, or [ ] Shares of common stock, assuming the exercise of the over-allotment option in full, as a portion of the compensation payable to the underwriters in connection with this offering. The Underwriter Warrants will be immediately exercisable upon issuance at an exercise price equal to 155% of the public offering price of the Shares sold in this offering, expire on the fifth anniversary of the commencement of sales in this offering, and are otherwise in substantially similar form to the Pre-Funded Warrants issued in the offering. The Underwriter Warrants and the Shares of common stock underlying the Underwriter Warrants are being registered on the registration statement of which this prospectus is a part. See “Underwriting” on page [ ] of this prospectus. |
|
|
|
| Lock-up Agreements | We have agreed with the underwriters not to sell additional equity securities for a period of 90 days after the effective date of this Offering. Our directors and officers have agreed with the underwriters not to offer for sale, sell, contract to sell, pledge or otherwise dispose of any of their Shares of our common stock or securities convertible into our common stock, subject to certain exceptions, for a period of 90 days after the date of this prospectus, which restriction may be waived in the discretion of the underwriter. |
|
|
|
| Use of proceeds | Assuming all offered Shares are sold, either in the form of Shares or Pre-Funded Warrants, we estimate that the net proceeds from this offering will be approximately $ million, assuming a public offering price of $_______, based on the last sale price of our common stock as reported on NYSE American on , 2024 or approximately $ million if the underwriter exercises its over-allotment option in full, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
We intend to use the net proceeds from this offering, together with other available funds, to support our operations, including for clinical trials, for working capital and for other general corporate purposes, including the payment of accrued liabilities and the repayment of interim financing from our Chairman of the Board and Chief Executive Officer. See “Prospectus Summary—Recent Developments—Interim Financing” and “Use of Proceeds.” |
| 15 |
| Risk factors | Investing in our securities involves a high degree of risk. You should read the “Risk Factors” section included in this prospectus, on page 18, and the risk factors incorporated by reference in this prospectus for a discussion of factors to consider carefully before deciding to invest in Shares of our common stock or Pre-Funded Warrants.
|
| Indications of Interest | Certain of our existing stockholders have indicated an interest in purchasing an aggregate of up to $[ ] of Common Stock in this offering at the public offering price per Share of common stock and on the same terms as other purchasers in this offering. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters may determine to sell more, fewer or no Shares of common stock in this offering to any of these stockholders, or any of these stockholders may determine to purchase more, fewer or no Shares of common stock in this offering. The underwriters will receive the same underwriting discount on any Shares of common stock purchased by these stockholders as they will on any other securities sold to the public in this offering.
|
| 16 |
| NYSE American symbol | “VTAK”. | |
| The information above is based on 7,573,403 Shares of our common stock outstanding as of June 3, 2024, assumes no exercise of the underwriter’s over-allotment option and no exercise of the Pre-Funded Warrants issued pursuant to this offering, and also does not include as of such date, the following: | ||
|
|
|
|
|
| · | 989,593 Shares of common stock issuable upon the exercise of outstanding options to purchase shares of common stock issued to directors, employees and consultants at a weighted average exercise price of $1.7589 per share, 210,842 shares of which are currently exercisable; |
|
|
|
|
|
| · | 11,042,137 shares of common stock issuable upon the exercise of outstanding warrants to purchase Shares of common stock at a weighted average exercise price of $5.31 per share, all of which are currently exercisable, subject to applicable beneficial ownership blockers; |
|
|
|
|
|
| · | 2,313,956 shares of common stock issuable upon conversion of outstanding Series A Convertible Preferred Stock, all of which are currently convertible; and |
|
|
|
|
|
| · | 12,656,011 Shares of common stock issuable upon conversion of outstanding Shares of convertible Series X Preferred Stock, none of which are currently convertible. |
|
|
|
|
| Except as otherwise indicated herein, all information in this prospectus reflects or assumes no exercise of the underwriter’s option to purchase up to an additional [ ] Shares of our common stock to cover over-allotments, if any. | ||
| 17 |
Investing in our securities involves a high degree of risk. Before deciding whether to purchase any of the securities being registered pursuant to the registration statement of which this prospectus is a part, you should carefully consider the Risk Factors described below as well as the risks and uncertainties discussed under “Special Note Regarding Forward-Looking Statements” below and elsewhere in this prospectus, and the risk factors set forth under “Risk Factors” in our previous SEC filings, all of which are incorporated by reference into this prospectus:
|
| · | our most recent Annual Report on Form 10-K, |
|
|
|
|
|
| · | our most recent Quarterly Report on Form 10-Q filed subsequent to such filing, and |
|
|
|
|
|
| · | discussions of potential risks, uncertainties, and other important factors in our subsequent filings with the SEC. |
The Risk Factors set forth in this Prospectus and in the filings described above may be amended, supplemented or superseded from time to time by other reports and/or prospectus supplements we file with the SEC in the future, and you should carefully consider any such additional or modified risk factors and other information provided in any such future filings that may be available after the date of this prospectus before making your investment decision.
If any of the risks set forth in this Prospectus and/or in the filings described above actually occur, it may materially harm our business, financial condition, liquidity and results of operations. As a result, the market price of our securities could decline, and/or the available secondary market for our securities may diminish or become non-existent, and you could lose all or part of your investment or lose liquidity in the Shares. The risks and uncertainties we describe in this prospectus and in the documents incorporated by reference herein are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe are immaterial could materially adversely affect our business, operating results and financial condition, as well as adversely affect the value of an investment in our securities, and the occurrence of any of these risks might cause you to lose all or part of your investment.
Risks Relating to This Offering
We have broad discretion to determine how to use the proceeds raised in this offering, and we may not use the proceeds effectively.
Our management will have broad discretion over the use of proceeds from this offering, and we could spend the proceeds from this offering in ways with which you may not agree or that do not yield a favorable return. We intend to use the net proceeds from this offering to support our operations, including for clinical trials, for working capital and for other general corporate purposes, including the payment of accrued expenses, including repayment of interim financing from our Chairman of the Board and Chief Executive Officer. See “Prospectus Summary—Recent Developments—Interim Financing.” If we do not invest or apply the proceeds of this offering in ways that improve our operating results, we may fail to achieve expected financial results, which could cause our stock price to decline.
You will experience immediate and substantial dilution when you purchase securities in this offering.
You will incur immediate and substantial dilution as a result of this offering. After giving effect to the assumed sale by us of ______ Shares of our common stock in this offering at the public offering price of $____ per share of common stock, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, investors in this offering will suffer an immediate dilution of $____ per share. Furthermore, if the underwriters exercise their option to purchase additional Shares of common stock and/or warrants to purchase Shares of common stock, you will experience further dilution.
If we issue additional common stock, or securities convertible into or exchangeable or exercisable for common stock, our stockholders, including investors who purchase Shares of common stock in this offering, may experience additional dilution, and any such issuances may result in downward pressure on the price of our common stock. We may not be able to sell Shares or other securities in any other offering at a price per share that is equal to or greater than the price per share paid by investors in this offering, and investors purchasing Shares or other securities in the future could have rights superior to existing stockholders. See the section entitled “Dilution” below for a more detailed discussion of the dilution you will incur if you purchase common stock in this offering.
| 18 |
The issuance of additional equity securities may negatively impact the trading price of our common stock.
We have issued equity securities in the past, will issue equity securities in this offering and expect to continue to issue equity securities to finance our activities in the future. In addition, outstanding options and warrants to purchase our common stock may be exercised and additional options and warrants may be issued, resulting in the issuance of additional Shares of common stock. The issuance by us of additional equity securities would result in dilution to our stockholders, and even the perception that such an issuance may occur could have a negative impact on the trading price of our common stock.
A substantial number of Shares of our common stock and/or Pre-Funded Warrants may be sold in this offering, which could cause the price of our common stock to decline.
In this offering, we seek to sell _____ Shares of common stock, representing approximately ____% of our outstanding common stock as of June 3, 2024, or _____ Pre-Funded Warrants in lieu of Shares that would otherwise result in such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock. This sale and any future sales of a substantial number of Shares of our common stock in the public market, or the perception that such sales may occur, could adversely affect the price of our common stock. We cannot predict the effect, if any, that market sales of those Shares of common stock or the availability of those Shares of common stock for sale will have on the market price of our common stock.
A significant number of additional Shares of our common stock may be issued upon the conversion of existing securities, including the Series A and Series X Preferred Stock and outstanding options and warrants, which issuances would substantially dilute existing stockholders and may depress the market price of our common stock.
As of June 3, 2024, there were 7,573,403 Shares of common stock outstanding, plus 27,001,697 Shares underlying preferred stock, warrants, and options. See “Description of Capital Stock,” filed as Exhibit 4.2 to our Form 10-K for fiscal year ended December 31, 2023. The issuance of such Shares of common stock would substantially dilute the proportionate ownership and voting power of existing security holders, and their issuance, or the possibility of their issuance, may depress the market price of our common stock.
Future issuances of preferred stock may adversely affect the market price for our common stock.
Additional issuances and sales of preferred stock, or the perception that such issuances and sales could occur, may cause prevailing market prices for our common stock to decline and may adversely affect our ability to raise additional capital in the financial markets at times and prices favorable to us.
There is no public market for the Pre-Funded Warrants being offered in this offering.
There is no public trading market for the Pre-Funded Warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the Pre-Funded Warrants on any securities exchange or nationally recognized trading system, including NYSE American. Without an active market, the liquidity of the Pre-Funded Warrants will be limited.
Holders of Pre-Funded Warrants purchased in this offering will have no rights as holders of our common stock with respect to the Shares underlying such Pre-Funded Warrants until such holders exercise their Pre-Funded Warrants and acquire our common stock.
Until holders of Pre-Funded Warrants acquire Shares of our common stock upon exercise of the Pre-Funded Warrants, holders of Pre-Funded Warrants will have no rights with respect to the Shares of our common stock underlying such Pre-Funded Warrants including with respect to dividends and voting rights. Upon exercise of the Pre-Funded Warrants, the holders will be entitled to exercise the rights of a holder of our common stock only as to matters for which the record date occurs after the exercise date.
| 19 |
Significant holders or beneficial holders of our common stock may not be permitted to exercise Pre-Funded Warrants that they hold.
A holder of a Pre-Funded Warrant will not be entitled to exercise any portion of any Pre-Funded Warrant which, upon giving effect to such exercise, would cause (i) the aggregate number of Shares of our common stock beneficially owned by the holder (together with its affiliates) to exceed 4.99% (or, at the election of the holder, 9.99%) of the number of Shares of our common stock outstanding immediately after giving effect to the exercise, or (ii) the combined voting power of our securities beneficially owned by the holder (together with its affiliates) to exceed 4.99% (or, at the election of the holder, 9.99%) of the combined voting power of all of our securities then outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded Warrants. As a result, you may not be able to exercise your Pre-Funded Warrants for Shares of our common stock at a time when it would be financially beneficial for you to do so. In such circumstance you could seek to sell your Pre-Funded Warrants to realize value, but you may be unable to do so in the absence of an established trading market for the Pre-Funded Warrants.
As noted above, you are urged to review, in addition to the foregoing, important risk factors contained in our periodic reports filed with the SEC and incorporated herein by reference, which describe in more detail risk factors pertaining to our business and our common stock including but not limited to the following:
Risks Related to Our Financial Position and Need for Additional Capital
|
| · | We will be required to raise additional funds to finance our operations and continue as a going concern; We may not be able to do so when necessary, and/or the terms of any financings may not be advantageous to us. |
|
|
|
|
|
| · | Our business has a history of losses, will incur additional losses, and may never achieve profitability. |
Risks Related to Our Internal Controls
|
| · | We have identified material weaknesses in our internal control over financial reporting. These material weaknesses could adversely affect our ability to report our results of operations and financial condition accurately and in a timely manner. |
|
|
|
|
|
| · | Compliance with Sarbanes-Oxley Act Section 404 could have a material adverse impact on our business. |
Risks Related to Our Business and Products
Product Liability Risks Related to our Vivo and LockeT Products
We may incur material losses and costs as a result of product liability claims that may be brought against us and recalls, which may adversely affect our results of operations and financial condition. Furthermore, as a medical device company, we face an inherent risk of damage to our reputation if one or more of our products are, or are alleged to be, defective.
Our business exposes us to potential product liability risks that are inherent in the design, manufacture and marketing of our products. In particular, our medical device products are often used in connection with or subsequent to surgical and intensive care settings with seriously ill patients. For example, our LockeT product is designed to be applied to a sutured wound on the human body for varying periods of time, and component failures, lack of appropriate sterility, manufacturing flaws, design defects or inadequate disclosure of product-related risks with respect to these or other products we manufacture or sell could result in an unsafe condition or injury to, or death of, the patient. Further, with respect to our LockeT product, we have outsourced manufacturing to a third-party and therefore face additional risk regarding the quality of that manufacturing. As a result, we face an inherent risk of monetary liability and damage to our reputation if one or more of our products are, or are alleged to be, defective. Although we carry product liability insurance, we may be exposed to product liability claims in the event that our products actually or allegedly fail to perform as expected or the use of our products results, or is alleged to result, in bodily injury and/or property damage. The outcome of litigation, particularly any class-action lawsuits, is difficult to quantify. Plaintiffs often seek recovery of very large or indeterminate amounts, including punitive damages. The magnitude of the potential losses relating to these lawsuits may remain unknown for substantial periods of time and the cost to defend against any such litigation may be significant. Accordingly, we could experience material product liability losses in the future and incur significant costs to defend these claims.
| 20 |
In addition, if any of our products are, or are alleged to be, defective, we may voluntarily participate, or be required by applicable regulators, to participate in a recall of that product if the defect or the alleged defect relates to safety. In the event of a recall, we may experience lost sales and be exposed to individual or class-action litigation claims and reputational risk. Product liability and recall costs may have a material adverse effect on our business, financial condition and results of operations.
Additional Business and Product Risks
|
| · | We will not be able to reach profitability unless we are able to achieve our product expansion and growth goals; our VIVO launch plans require significant investment in infrastructure and sales representatives. |
|
|
|
|
|
| · | Our research and development and commercialization efforts may depend on entering into agreements with corporate collaborators. |
|
|
|
|
|
| · | We have entered into joint marketing agreements with respect to our products, and may enter into additional joint marketing agreements, that will reduce our revenues from product sales. |
|
|
|
|
|
| · | Royalty agreements with respect to LockeT, the surgical vessel closing pressure device, will reduce any future profits from this product. |
|
|
|
|
|
| · | If we experience significant disruptions in our information technology systems, our business may be adversely affected. |
|
|
|
|
|
| · | Litigation and other legal proceedings may adversely affect our business. |
|
|
|
|
|
| · | If we make acquisitions or divestitures, we could encounter difficulties that harm our business. |
|
|
|
|
|
| · | Failure to attract and retain sufficient qualified personnel could also impede our growth. |
|
|
|
|
|
| · | Our revenues may depend on our customers’ receipt of adequate reimbursement from private insurers and government sponsored healthcare programs. |
|
|
|
|
|
| · | We may be unable to compete successfully with companies in our highly competitive industry, many of whom have substantially greater resources than we do. |
|
|
|
|
|
| · | Our future operating results depend upon our ability to obtain components in sufficient quantities on commercially reasonable terms or according to schedules, prices, quality and volumes that are acceptable to us, and suppliers may fail to deliver components, or we may be unable to manage these components effectively or obtain these components on such terms. |
|
|
|
|
|
| · | If hospitals, physicians and patients do not accept our current and future products or if the market for indications for which any product candidate is approved is smaller than expected, we may be unable to generate significant revenue, if any. |
|
|
|
|
|
| · | The recent coronavirus outbreak (“COVID-19”) adversely affected our financial condition and results of operations and we cannot provide any certainty as to whether there will be future impacts from COVID-19 or another pandemic. |
|
|
|
|
|
| · | A variety of risks associated with marketing our products internationally could materially adversely affect our business. |
| 21 |
|
| · | The impact of the military conflicts in Ukraine and Israel, and the actions that have been and could be taken by other countries, including new and stricter sanctions and actions taken in response to such sanctions, have affected, and may continue to affect, our business and results of operations, including our supply chain. |
|
|
|
|
|
| · | If the third parties on which we rely for the conduct of our clinical trials and results do not perform our clinical trial activities in accordance with good clinical practices and related regulatory requirements, we may be unable to obtain regulatory approval for or commercialize our product candidates. |
|
|
|
|
|
| · | We may be adversely affected by product liability claims, unfavorable court decisions or legal settlements. |
|
|
|
|
|
| · | Our ability to use our net operating loss carryforwards may be limited. |
|
|
|
|
|
| · | We may have to make milestone payments under the Settlement Agreement we entered into with the Department of Justice (“DOJ”). |
Risks Related to Government Regulation and our Industry
|
| · | We are subject to pervasive and continuing regulation by the FDA and other regulatory agencies. Our products may be subject to additional recalls, revocations or suspensions after receiving FDA or foreign approval or clearance, which could divert managerial and financial resources, harm our reputation, and adversely affect our business. |
|
|
|
|
|
| · | Changes in trade policies among the U.S. and other countries, in particular the imposition of new or higher tariffs, could place pressure on our average selling prices as our customers seek to offset the impact of increased tariffs on their own products. Increased tariffs or the imposition of other barriers to international trade could have a material adverse effect on our revenues and operating results. |
|
|
|
|
|
| · | Product clearances and approvals can often be denied or significantly delayed. |
|
|
|
|
|
| · | Although we have obtained regulatory clearance for our VIVO and LockeT products in the U.S. and certain non-U.S. jurisdictions, our business plans include expanding uses for our products, which will require additional clearances; and even after clearance is obtained, our products remain subject to extensive regulatory scrutiny. |
|
|
|
|
|
| · | If we or our suppliers fail to comply with the FDA’s Quality System Regulation, or QSR, or any applicable state equivalent, our operations could be interrupted, and our potential product sales and operating results could suffer. |
|
|
|
|
|
| · | Our products may be subject to additional recalls, revocations or suspensions after receiving FDA or foreign approval or clearance, which could divert managerial and financial resources, harm our reputation, and adversely affect our business. |
|
|
|
|
|
| · | If any of our products cause or contribute to a death or a serious injury, or malfunction in certain ways, we will be required to report under applicable medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions. |
|
|
|
|
|
| · | Healthcare reform initiatives and other administrative and legislative proposals may adversely affect our business, financial condition, results of operations and cash flows in our key markets. |
| 22 |
Risks Related to our Intellectual Property
|
| · | If we are unable to obtain and maintain patent protection for our products, our competitors could develop and commercialize products and technology similar or identical to ours, and our ability to successfully commercialize our existing products and any products we may develop, and our technology may be adversely affected. |
Risks Related to Ownership of Our Common Stock
|
| · | The price of our stock has been and may continue to be volatile, which could result in substantial losses for investors. Further, an active, liquid and orderly trading market for our common stock may not be sustained and we do not know what the market price of our common stock will be, and as a result it may be difficult for you to sell your Shares of our common stock. |
|
|
|
|
|
| · | The ownership of our common stock is highly concentrated, and may become more so in the near future, which may prevent you and other stockholders from influencing significant corporate decisions and may result in conflicts of interest that could cause the company stock price to decline. |
|
|
|
|
|
| · | We are a smaller reporting company, and we cannot be certain if the reduced reporting requirements applicable to smaller reporting companies will make our common stock less attractive to investors. |
|
|
|
|
|
| · | Future sales and issuances of a substantial number of shares of our common stock or rights to purchase common stock by our stockholders in the public market could result in additional dilution of the percentage ownership of our stockholders and cause our stock price to fall. |
|
|
|
|
|
| · | Anti-takeover provisions under our charter documents and Delaware law could delay or prevent a change of control which could limit the market price of our common stock and may prevent or frustrate attempts by our stockholders to replace or remove members of our board of directors or our current management and may adversely affect the market price of our common stock. |
|
|
|
|
|
| · | Our certificate of incorporation provides that the Court of Chancery of the State of Delaware and the federal district courts of the U.S. are the exclusive forums for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees. |
|
|
|
|
|
| · | We are subject to the continued listing requirements of the NYSE American. If we are unable to comply with such requirements, our common stock would be delisted from the NYSE American, which would limit investors’ ability to effect transactions in our common stock and subject us to additional trading restrictions. |
|
|
|
|
|
| · | We have not paid dividends in the past and have no immediate plans to pay dividends. |
| 23 |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, any applicable prospectus supplement or free writing prospectus and our SEC filings that are incorporated by reference into this prospectus and any applicable prospectus supplement or free writing prospectus contain or incorporate by reference “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), Section 21E of the Exchange Act and the Private Securities Litigation Reform Act of 1995, and such statements are subject to the “safe harbor” created by those sections.
Forward-looking statements are not historical facts, but rather are based on current expectations, estimates, assumptions, and projections about the business and future financial results of the medical device industry, and other legal, regulatory and economic developments. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “intend,” “should,” “could,” “would,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “predict,” “potential,” “continue,” “likely,” and similar expressions (including their use in the negative) intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
These statements relate to future events or our future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Forward-looking statements include, but are not limited to, statements about competition from larger and more established companies in our markets, our ability to successfully grow our business and legislative, regulatory and economic developments, including changing business conditions in the industries in which we operate and the economy in general, as well as financial performance, expectations with respect to our business and product development, including VIVO, LockeT and Amigo; litigation outcomes; the listing status of our common stock; and existing and/or prospective customers.
Our forward-looking statements involve risks and uncertainties, including those described in our SEC filings and incorporated herein by reference herein, and the other risks set forth in “Risk Factors.” These risks and uncertainties may cause results to differ materially from the plans, expectations, predictions or projections set forth in (or underlying statements set forth in) this prospectus and our other SEC filings and public statements.
In addition, our forward-looking statements are based on current plans, estimates and projections, which are subject to change based on shifting circumstances, and therefore, you are cautioned not to place undue reliance on them. These statements may discuss goals, intentions, plans and/or expectations as to future plans, trends, events, results of operations or financial condition, listed status of our common stock or other matters, all of which are based on current beliefs of our management, as well as assumptions made by, and information currently available to, management.
Forward-looking statements contained in this prospectus and in our other SEC filings speak only as of the date on which the statements were made and are not guarantees of future performance. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future, and we have no intention to do so.
No forward-looking statement can be guaranteed, and our actual results could differ materially from those projected or discussed in our forward-looking statements for many reasons, including the risks and uncertainties described above and those discussed in our SEC filings and incorporated herein by reference as described under “Risk Factors.” Given these risks and uncertainties, readers should not place undue reliance on our forward-looking statements and should carefully consider such risks and uncertainties, as well as additional risks and uncertainties that may be described in other documents filed by us from time to time with the SEC, including any prospectus supplements we may file after the date of this prospectus. See “Where You Can Find Additional Information” beginning on page __ of this prospectus.
| 24 |
We have not paid dividends in the past, and we currently intend to retain future earnings, if any, and all currently available funds for use in the operation of our business and do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our Board of Directors after considering our financial condition, results of operations, capital requirements, business prospects and other factors the Board of Directors deems relevant, and subject to the restrictions contained in our current or future financing instruments.
| 25 |
We estimate the net proceeds from the sale of Shares of common stock and Pre-Funded Warrants to purchase Shares of common stock that we are offering will be approximately $___ million, after deducting estimated underwriting discounts and commissions and our estimated offering expenses, assuming no exercise of the underwriter’s over-allotment option. We will receive nominal proceeds, if any, upon exercise of the Pre-Funded Warrants.
We intend to use the net proceeds from this offering, together with other available funds, to support our operations, including for clinical trials, for working capital and for other general corporate purposes, including the payment of accrued liabilities and the repayment of interim financing from our Chairman of the Board and Chief Executive Officer. See “Prospectus Summary—Recent Developments—Interim Financing.” Other than principal of $500,000 plus accrued interest at 8% per annum to be repaid to our Chairman of the Board and Chief Executive Officer, we have not yet determined the amount of net proceeds to be used specifically for any of the foregoing purposes.
Pending use of the proceeds as described above, we intend to invest the net proceeds of this offering in short-term, interest-bearing, investment-grade securities or certificates of deposit.
The amounts and timing of our actual expenditures will depend on numerous factors, including the progress of our clinical trials, as well as the amount of cash used in our operations. We may find it necessary or advisable to use the net proceeds for other purposes, and we will have broad discretion in the application of the net proceeds and investors will be relying on the judgment of our management regarding the application of the net proceeds from this offering.
Based upon our historical and anticipated future growth and our financial needs, we may engage in additional financings of a character and amount that we determine as the need arises. We may raise additional capital through additional public or private financings, the incurrence of debt and other available sources.
| 26 |
If you invest in our securities, your ownership interest will be diluted to the extent of the difference between the public offering price per share of our common stock or Pre-Funded Warrant and the as adjusted net tangible book value per share of our common stock or Pre-Funded Warrant immediately after this offering. Net tangible book value per share is calculated by subtracting our total liabilities from our total tangible assets, which is total assets less intangible assets, and dividing this amount by the number of shares of common stock outstanding.
As of March 31, 2024, we had a historical net tangible book (deficit) of ($7,283,000) or ($.9617) per share of common stock, based on the number of Shares of common stock outstanding at March 31, 2024. Our historical net tangible book value per share is the amount of our total tangible assets less our total liabilities at March 31, 2024, divided by the number of Shares of common stock outstanding at March 31, 2024.
After giving effect to the sale of Shares of common stock in this offering at an assumed public offering price of $ per share, which is based upon the last reported sale price of our common stock on NYSE American on , 2024, and the sale, but not the exercise, of the Pre-Funded Warrants to purchase Shares of our common stock at the public offering price of $ per Pre-Funded Warrant (which equals the public offering price of the common stock less the $0.0001 per share exercise price of each Pre-Funded Warrant), and after deducting underwriting discounts and commissions and estimated offering expenses payable by us, and excluding the proceeds to us, if any, from the exercise of the Pre-Funded Warrants issued pursuant to this offering, our as adjusted net tangible book value per share as of March 31, 2024 was $ , or $ per share of common stock. This represents an immediate increase in adjusted net tangible book value of $ per share to existing stockholders and immediate dilution of $ per share to new investors purchasing Shares of common stock in this offering at the public offering price.
The following table illustrates this dilution on a per share basis:
| Assumed public offering price per share |
|
|
|
| $ |
| ||
| Historical net tangible book value per share as of March 31, 2024 |
| ($.9617) |
|
|
|
|
| |
| As adjusted net tangible book value per share immediately after this offering |
| $ |
|
|
|
|
| |
| Dilution per share to new investors in this offering |
|
|
|
|
| $ |
|
|
The information discussed above is illustrative only, and the dilution information following this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
A $__ increase (decrease) in the assumed public offering price of $ per share, which is based upon the last reported sale price of our common stock on NYSE American on , 2024, would increase (decrease) our as adjusted net tangible book value after this offering to $ per share and the dilution to new investors purchasing common stock in this offering to $ per share, assuming that the number of Shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. An increase of Shares in the number of Shares offered by us, as set forth on the cover page of this prospectus, would increase our as adjusted net tangible book value after this offering to $ per share and decrease the dilution to new investors purchasing common stock in this offering to $ per share, assuming no change in the assumed public offering price per share and after deducting underwriting discounts and commissions and estimated offering expenses payable by us. A decrease of Shares in the number of Shares offered by us would decrease the as adjusted net tangible book value after this offering to $ per share and increase the dilution to new investors purchasing common stock in this offering to $ per share, assuming no change in the assumed public offering price per share and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriter exercises their over-allotment option to purchase additional Shares in full, the as adjusted net tangible book value per share after giving effect to the offering would be $ per share. This represents an increase in as adjusted net tangible book value of $ per share to existing stockholders and dilution in as adjusted net tangible book value of $ per share to new investors.
| 27 |
The above discussion and table do not take into account further dilution to investors purchasing our common stock in this offering that could occur upon the exercise of outstanding options and warrants having a per share exercise price less than the public offering price per share in this offering. To the extent that outstanding options or warrants outstanding as of June 3, 2024 are exercised or other Shares are issued, investors purchasing our common stock in this offering will experience further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe that we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of our common stock, including through the sale of securities convertible into or exchangeable or exercisable for common stock, the issuance of these securities could result in further dilution to our stockholders, including investors purchasing our common stock in this offering.
Investors who purchase common stock upon the exercise of the Pre-Funded Warrants offered hereby may experience dilution depending on our net tangible book value at the time of exercise.
The information above is based on 7,573,403 Shares of our common stock outstanding as of June 3, 2024, assumes no exercise of the underwriter’s over-allotment option and no exercise of the Pre-Funded Warrants issued pursuant to this offering, and also does not include as of such date, the following:
|
| · | 989,593 Shares of common stock issuable upon the exercise of outstanding options to purchase shares of common stock issued to directors, employees and consultants at a weighted average exercise price of $1.7589 per share, 210,842 shares of which are currently exercisable; |
|
| · | 11,042,137 shares of common stock issuable upon the exercise of outstanding warrants to purchase Shares of common stock at a weighted average exercise price of $5.31 per share, all of which are currently exercisable, subject to applicable beneficial ownership blockers; |
|
| · | 2,313,956 shares of common stock issuable upon conversion of outstanding Series A Convertible Preferred Stock, all of which are currently convertible; and |
|
| · | 12,656,011 Shares of common stock issuable upon conversion of outstanding Shares of convertible Series X Preferred Stock, none of which are currently convertible. |
| 28 |
DESCRIPTION OF SECURITIES WE ARE OFFERING
The following description summarizes certain terms of our capital stock, certain provisions of our certificate of incorporation and bylaws and certain terms of the Pre-Funded Warrants included in this offering. This summary does not purport to be complete and is qualified in its entirety by the provisions of our certificate of incorporation and bylaws and the provisions of the Pre-Funded Warrants, copies of which are filed with the SEC as exhibits to the Registration Statement on Form S-1 of which this prospectus forms a part, and to the applicable provisions of Delaware law.
Our authorized capital stock consists of 310,000,000 shares of capital stock, of which 300,000,000 shares are designated as common stock, $0.0001 par value per share, and 10,000,000 shares are designated as preferred stock, $0.0001 par value per share; however, our stockholders will vote on proposals to effect a reverse stock split and to reduce our authorized common stock at our 2024 Annual Meeting of Stockholders to be held on July 3, 2024. See “Summary—Recent Developments.” Our board of directors is authorized, without stockholder approval, except as required by the listing standards of the NYSE American, to issue shares of our preferred stock. As of June 3, 2024, there were 7,573,403 shares of common stock issued and outstanding and outstanding options to purchase up to 989,593 shares of our common stock. There were 113 holders of record of our common stock. As of June 3, 2024, there were 3,703 shares of our convertible Series A preferred stock outstanding, which are convertible into up to 2,313,956 shares of our common stock, and 12,656.011 shares of our convertible Series X preferred stock outstanding, which are convertible into up to 12,656,011 shares of our common stock.
As of June 3, 2024, 11,042,137 shares of our common stock were issuable upon exercise of outstanding warrants to purchase shares of our common stock, at a weighted average exercise price of $5.31 per share. The terms of the outstanding warrants are more specifically described in the Description of Capital Stock filed as Exhibit 4.2 to our Annual Report on Form 10-K for the year ended December 31, 2023, which is incorporated by reference herein.
Pre-Funded Warrants
The following is a brief summary of certain terms and conditions of the Pre-Funded Warrants being offered by us. The following description is subject in all respects to the provisions contained in the Pre-Funded Warrants.
Form
The Pre-Funded Warrants will be issued as individual warrant agreements to each individual purchaser of a warrant.
Term
The Pre-Funded Warrants do not expire.
Exercisability
The Pre-Funded Warrants are exercisable at any time after their original issuance. The Pre-Funded Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice and by payment in full of the exercise price in immediately available funds for the number of Shares of our common stock purchased upon such exercise. As an alternative to payment of the exercise price in immediately available funds, the holder may elect to exercise the Pre-Funded Warrant through a cashless exercise, in which case the holder would receive upon such exercise the net number of Shares of our common stock determined according to the formula set forth in the Pre-Funded Warrant. No fractional Shares of our common stock will be issued in connection with the exercise of a Pre-Funded Warrant. In lieu of fractional Shares, we will pay the holder an amount in cash equal to the fractional amount multiplied by the last closing trading price of our common stock on the exercise date.
| 29 |
Exercise Limitations
Under the Pre-Funded Warrants, we may not effect the exercise of any Pre-Funded Warrant, and a holder will not be entitled to exercise any portion of any Pre-Funded Warrant, which, upon giving effect to such exercise, would cause (i) the aggregate number of Shares of our common stock beneficially owned by the holder (together with its affiliates) to exceed 4.99% (or, at the election of the holder, 9.99%) of the number of Shares of our common stock outstanding immediately after giving effect to the exercise, or (ii) the combined voting power of our securities beneficially owned by the holder (together with its affiliates) to exceed 4.99% (or, at the election of the holder, 9.99%) of the combined voting power of all of our securities then outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-Funded Warrants.
Exercise Price
The exercise price per whole share of our common stock issuable upon the exercise of the Pre-Funded Warrants is $0.0001 per share of our common stock. The exercise price of the Pre-Funded Warrants and the number of Shares of our common stock issuable upon exercise of the Pre-Funded Warrants is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders. The exercise price will not be adjusted below the par value of our common stock.
Transferability
Subject to applicable laws, the Pre-Funded Warrants may be offered for sale, sold, transferred or assigned without our consent. The Pre-Funded Warrants will be held in definitive form by the purchasers. The ownership of the Pre-Funded Warrants and any transfers of the Pre-Funded Warrants will be registered in a warrant register maintained by us or our transfer agent.
Exchange Listing
We do not plan on applying to list the Pre-Funded Warrants on NYSE American, any other national securities exchange or any other nationally recognized trading system.
Fundamental Transactions
In the event of a fundamental transaction, as described in the Pre-Funded Warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, upon consummation of such a fundamental transaction, the holders of the Pre-Funded Warrants will be entitled to receive upon exercise of the Pre-Funded Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the Pre-Funded Warrants immediately prior to such fundamental transaction without regard to any limitations on exercise contained in the Pre-Funded Warrants.
No Rights as a Stockholder
Except by virtue of such holder’s ownership of Shares, the holder of a Pre-Funded Warrant does not have the rights or privileges of a holder of our common stock, including any voting rights or the rights to receive dividends, until the holder exercises the Pre-Funded Warrant.
| 30 |
We are offering the securities described in this prospectus through the underwriter named below. We have entered into an underwriting agreement dated _________, 2024, with Ladenburg Thalmann & Co. Inc. as the underwriter in this offering. Subject to the terms and conditions of the underwriting agreement, the underwriter has agreed to purchase the number of our securities set forth opposite its name below.
| Underwriters | Number of Shares of Common Stock | Number of Pre-Funded Warrants | ||
| Ladenburg Thalmann & Co. Inc. |
|
| ||
| Total |
|
|
A copy of the underwriting agreement has been filed as an exhibit to the registration statement of which this prospectus is part.
We have been advised by the underwriter that it proposes to offer the Shares of common stock and Pre-Funded Warrants directly to the public at the public offering price set forth on the cover page of this prospectus. Any securities sold by the underwriter to securities dealers will be sold at the public offering price less a selling concession not in excess of $______ per share of common stock and Pre-Funded Warrant.
The underwriting agreement provides that the underwriters’ obligation to purchase the securities we are offering is subject to conditions contained in the underwriting agreement, including, among others, the continued accuracy of representations and warranties made by us in the underwriting agreement, delivery of legal opinions, and the absence of any material changes in our assets, business or prospects after the date of this prospectus.
No action has been taken by us or the underwriter that would permit a public offering of the securities in any jurisdiction outside the United States where action for that purpose is required. None of our securities included in this offering may be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sales of any of the securities offering hereby be distributed or published in any jurisdiction except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who receive this prospectus are advised to inform themselves about and to observe any restrictions relating to this offering of securities and the distribution of this prospectus. This prospectus is neither an offer to sell nor a solicitation of any offer to buy the securities in any jurisdiction where that would not be permitted or legal.
The underwriter has advised us that they do not intend to confirm sales to any account over which they exercise discretionary authority. Certain of our existing stockholders have indicated an interest in purchasing an aggregate of up to $[ ] of Common Stock in this offering at the public offering price per Share of common stock and on the same terms as other purchasers in this offering. However, because indications of interest are not binding agreements or commitments to purchase, the underwriters may determine to sell more, fewer or no Shares of common stock in this offering to any of these stockholders, or any of these stockholders may determine to purchase more, fewer or no Shares of common stock in this offering. The underwriters will receive the same underwriting discount on any Shares of common stock purchased by these stockholders as they will on any other securities sold to the public in this offering.
| 31 |
Underwriting Discount and Expenses
The following table summarizes the underwriting discount and commission to be paid to the underwriter by us.
|
|
| Per Share (1) |
|
| Per Pre-Funded Warrant (1) |
|
| Total Without Over-Allotment |
|
| Total With Full Over-Allotment |
| ||||
| Public offering price |
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||
| Underwriting discounts and commissions to be paid to underwriters by us(2)(3) |
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||
| Proceeds, before expenses, to us |
| $ |
|
| $ |
|
| $ |
|
| $ |
| ||||
__________________
(1) The public offering price and underwriting discount corresponds, in respect of the securities, to a public offering price per share of common stock of $____ ($_____ net of the underwriting discount).
(2) We have also agreed to pay the underwriters a management fee equal to 1% of the aggregate gross proceeds received from the sale of the securities in the transaction. In addition, we have agreed to reimburse the underwriters for certain expenses and issue the underwriters warrants to purchase 6% of the total number of Shares sold in this offering (the "Underwriter Warrants”), including Shares underlying Pre-Funded Warrants, at an exercise price equal to 155% of the public offering price of the Shares sold in this offering.
(3) We have granted a 45-day option to the underwriters to purchase up to ________ additional Shares of common stock at the public offering price per share of common stock set forth above less the underwriting discounts and commissions solely to cover over-allotments, if any.
We estimate the total expenses payable by us for this offering to be approximately $_________, which amount includes (i) the underwriting discount of $__________, (ii) reimbursement of the accountable expenses of the underwriters, including the legal fees of the representative, in an amount not to exceed $________ for pre-closing expenses plus $______ for closing expenses and (iii) other estimated company expenses of approximately $_________ which includes legal, accounting, and printing costs and various fees associated with the registration and listing of our Shares.
Over-allotment Option
We have granted to the underwriters an option exercisable not later than 45 days after the date of this prospectus to purchase up to an additional __________ Shares of common stock at the public offering price per share of common stock set forth on the cover page hereto less the underwriting discounts and commissions. The underwriters may exercise the option solely to cover overallotments, if any, made in connection with this offering. If any additional Shares of common stock are purchased, the underwriters will offer these Shares on the same terms as those on which the other securities are being offered.
Underwriters’ Warrants
We have also agreed to issue to the representative of the underwriters a warrant to purchase a number of Shares of common stock equal to 6% of the total number of Shares of common stock sold in this offering, including Shares underlying Pre-Funded Warrants, at an exercise price equal to 155% of the public offering price of the Shares of common stock sold in this offering. The underwriters’ warrant will be exercisable commencing on the date of issuance and until the fifth anniversary of the commencement of sales of this offering.
Right of First Refusal
We have granted to Ladenburg Thalmann & Co. Inc. the right of first refusal which continues through December 31, 2025 to act as sole bookrunner, exclusive placement agent or exclusive sales agent in connection with any financing of the Company, subject to certain conditions.
| 32 |
Tail
We have also agreed to pay the underwriter a tail fee equal to the cash and Pre-Funded Warrant compensation in this offering, if any investor, who was contacted or introduced to us by the underwriter during the term of its engagement, provides us with capital in any public or private offering or other financing or capital raising transaction during the twelve(12) month period following expiration or termination of our engagement of the underwriter or, if earlier, three months after specified personnel cease to be an employee of the underwriter, provided, however, that the Company has the right to terminate its engagement of the underwriter for cause in compliance with FINRA Rule 5110(g)(5) (B)(i), which termination for cause eliminates the Company’s obligations with respect to the tail.
Electronic Distribution
A prospectus in electronic format may be made available on the website maintained by the underwriter or selling group members, if any, participating in the offering. The representative may allocate a number of shares to the selling group members, if any, for sale to their online brokerage account holders. Any such allocations for online distributions will be made on the same basis as other allocations.
Listing
Our shares of common stock are listed on NYSE American under the symbol “VTAK.” We do not intend to list the Pre-Funded Warrants on NYSE American or any other national securities exchange or automated quotation system.
Lock-up Agreements
Our officers, directors, each of their respective affiliates and associated partners have agreed with the underwriter to be subject to a lock-up period of 90 days following the date of this prospectus. This means that, subject to certain exceptions, during the applicable lock-up period, such persons may not offer for sale, contract to sell, sell, distribute, grant any option, right or warrant to purchase, pledge, hypothecate or otherwise dispose of, directly or indirectly, any Shares of our common stock or any securities convertible into, or exercisable or exchangeable for, Shares of our common stock. Certain limited transfers are permitted during the lock-up period if the transferee agrees to these lock-up restrictions. We have also agreed, in the underwriting agreement, to similar lock-up restrictions on the issuance and sale of our securities for 90 days following the closing of this offering, although we will be permitted to issue stock options or stock awards to directors, officers and employees under our existing plans. Ladenburg Thalmann & Co. Inc. may, in their sole discretion and without notice, waive the terms of any of these lock-up agreements.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Equiniti Trust Company, LLC (formerly known as American Stock Transfer and Trust Company).
Determination of Offering Price
The public offering price of the securities offered by this prospectus was determined by negotiation between us and the underwriter. Among the factors that were considered in determining the public offering price:
|
| · | our history and our prospects; |
|
| · | the industry in which we operate; |
|
| · | our past and present operating results; |
|
| · | the previous experience of our executive officers; and |
|
| · | the general condition of the securities markets at the time of this offering. |
The public offering price stated on the cover page of this prospectus should not be considered an indication of the actual value of the Shares of common stock and/or Pre-Funded Warrants sold in this offering. That price is subject to change as a result of market conditions and other factors and we cannot assure you that the Shares of common stock and/or Pre-Funded Warrants sold in this offering can be resold at or above the public offering price.
| 33 |
Stabilization, Short Positions and Penalty Bids
The underwriters may engage in syndicate covering transactions stabilizing transactions and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of our common stock:
|
| · | Syndicate covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover syndicate short positions. Such a naked short position would be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in the offering. |
|
|
|
|
|
| · | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specific maximum. |
|
|
|
|
|
| · | Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These syndicate covering transactions, stabilizing transactions, and penalty bids may have the effect of raising or maintaining the market prices of our securities or preventing or retarding a decline in the market prices of our securities. As a result the price of our common stock may be higher than the price that might otherwise exist in the open market. Neither we nor the underwriter make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected on NYSE American, in the over-the-counter market or on any other trading market and, if commenced, may be discontinued at any time.
In connection with this offering, the underwriter also may engage in passive market making transactions in our common stock in accordance with Regulation M during a period before the commencement of offers or sales of Shares of our common stock in this offering and extending through the completion of the distribution. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for that security.
However, if all independent bids are lowered below the passive market maker’s bid that bid must then be lowered when specific purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither we nor the underwriter make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the prices of our securities. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that any transactions, once commenced will not be discontinued without notice.
Indemnification
We have agreed to indemnify the underwriter against certain liabilities, including certain liabilities arising under the Securities Act, or to contribute to payments that the underwriter may be required to make for these liabilities.
| 34 |
Arnall Golden Gregory LLP will pass upon the validity of the Shares of common stock and Pre-Funded Warrants being offered hereby. Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., New York, New York is acting as counsel to the underwriter in connection with this offering.
The financial statements of Catheter Precision, Inc., as of December 31, 2023 and for the year then ended, which have been incorporated by reference in this prospectus by reference to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, have been audited by WithumSmith+Brown, PC, the Company’s independent registered public accounting firm, as set forth in their report thereon. Such financial statements are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
The financial statements of Catheter Precision, Inc., as of December 31, 2022 and for the year then ended, which have been incorporated by reference in this prospectus by reference to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023, have been audited by Haskell & White LLP, the Company’s previous independent registered public accounting firm, as set forth in their report thereon. Such financial statements are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We file annual, quarterly and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov.
We make available, free of charge, through our investor relations website, our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, statements of changes in beneficial ownership of securities and amendments to those reports and statements as soon as reasonably practicable after they are filed with the SEC. The address for our website ir.catheterprecision.com. The contents on our website are not part of this prospectus, and the reference to our website does not constitute incorporation by reference into this prospectus of the information contained at that site.
This prospectus is part of a registration statement we filed with the SEC. This prospectus omits some information contained in the registration statement in accordance with SEC rules and regulations. You should review the information and exhibits in the registration statement for further information about us and our securities. Statements in this prospectus concerning any document we filed as an exhibit to the registration statement or that we otherwise filed with the SEC are not intended to be comprehensive and are qualified by reference to these filings. You should review the complete document to evaluate these statements. You can obtain a copy of the registration statement from the SEC’s website.
| 35 |
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” into this prospectus the information we file with the SEC. This means that we can disclose important information to you by referring you to those documents. Any statement contained in a document incorporated by reference in this prospectus shall be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained herein, or in any subsequently filed document, which also is incorporated by reference herein, modifies or supersedes such earlier statement. Any such statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
We hereby incorporate by reference into this prospectus the following documents that we have filed with the SEC under the Exchange Act (other than current reports on Form 8-K, or portions thereof, furnished under Items 2.02 or 7.01 of Form 8-K):
|
| · | our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on April 1, 2024; |
|
|
|
|
|
| · | our Quarterly Report on Form 10-Q for the quarter ended March 31, 2024, filed with the SEC on May 6, 2024; |
|
|
|
|
|
| · | our Current Reports on Form 8-K and amendments thereto, if any, filed with the SEC on January 4, 2024, January 25, 2024, April 3, 2024, May 21, 2024, and June 3, 2024; and |
|
|
|
|
|
| · | our 2024 definitive proxy statement filed with the SEC on May 16, 2024. |
All documents that we file with the SEC pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act (other than current reports on Form 8-K, or portions thereof, furnished under Items 2.02 or 7.01 of Form 8-K) (i) after the initial filing date of the registration statement of which this prospectus forms a part and prior to the effectiveness of such registration statement and (ii) after the date of this prospectus, and prior to the termination of the offering shall be deemed to be incorporated by reference in this prospectus from the date of filing of the documents, unless we specifically provide otherwise. Information that we file with the SEC will automatically update and may replace information previously filed with the SEC. To the extent that any information contained in any current report on Form 8-K or any exhibit thereto, was or is furnished to, rather than filed with the SEC, such information or exhibit is specifically not incorporated by reference.
Upon written or oral request made to us at the address or telephone number below, we will, at no cost to the requester, provide to each person, including any beneficial owner, to whom this prospectus is delivered, a copy of any or all of the information that has been incorporated by reference in this prospectus (other than an exhibit to a filing, unless that exhibit is specifically incorporated by reference into that filing), but not delivered with this prospectus. You may also access this information on our website at catheterprecision.com by viewing the “SEC Filings” subsection of the “Investors” menu. No additional information on our website is deemed to be part of or incorporated by reference into this prospectus. We have included our website address in this prospectus solely as an inactive textual reference.
Catheter Precision, Inc.
1670 Highway 160 West, Suite 205
Fort Mill, SC 29708
973-691-2000
| 36 |

______ Shares of Common Stock
Pre-Funded Warrants to Purchase _____ Shares of Common Stock
Up to ________ Shares of Common Stock Issuable Upon Exercise of Pre-Funded Warrants
Underwriter Warrants to Purchase up to ______ Shares of Common Stock
Up to ________ Shares of Common Stock Issuable Upon Exercise of Underwriter Warrants
Ladenburg Thalmann
Preliminary Prospectus
____________, 2024
| 37 |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the fees and expenses incurred or expected to be incurred by us in connection with the issuance and distribution of the securities being registered hereby, other than underwriting discounts and commissions. Except for the SEC registration fee, all amounts are estimates.
| SEC registration fee |
| $ | 807 |
|
| Finra filing fee |
|
|
|
|
| Legal fees and expenses |
|
| 175,000 |
|
| Accounting fees and expenses |
|
| 65,000 |
|
| Printing fees and engraving expenses |
|
| 2,000 |
|
| Miscellaneous expenses |
|
| 3,000 |
|
|
|
|
|
|
|
| Total |
| $ |
|
|
Item 14. Indemnification of Directors and Officers
Our amended and restated certificate of incorporation and amended and restated bylaws contain provisions that eliminate the personal liability of our directors and executive officers for monetary damages for breach of their fiduciary duties as directors or officers.
Section 145 of the Delaware General Corporation Law provides that a corporation may indemnify any person made a party to an action by reason of the fact that he or she was a director, executive officer, employee or agent of the corporation or is or was serving at the request of a corporation against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with such action if he or she acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of an action by or in right of the corporation, no indemnification may generally be made in respect of any claim as to which such person is adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery or other adjudicating court determines that, despite the adjudication of liability but in view of all of the circumstances of the case, such person is fairly and reasonably entitled to indemnity for such expenses which the Court of Chancery or such other court shall deem proper.
In addition, as permitted by Section 145 of the Delaware General Corporation Law, our amended and restated certificate of incorporation and amended and restated bylaws provide that:
We shall indemnify our directors and officers for serving us in those capacities or for serving other business enterprises at our request, to the fullest extent permitted by Delaware law. Delaware law provides that a corporation may indemnify such person if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of us and, with respect to any criminal proceeding, had no reasonable cause to believe such person’s conduct was unlawful.
We may, in our discretion, indemnify employees and agents in those circumstances where indemnification is permitted by applicable law.
We are required to advance expenses, as incurred, to its directors and officers in connection with defending a proceeding, except that such director or officer shall undertake to repay such advances if it is ultimately determined that such person is not entitled to indemnification.
| 38 |
We will not be obligated pursuant to the amended and restated bylaws to indemnify a person with respect to proceedings initiated by that person, except with respect to proceedings authorized by our board of directors or brought to enforce a right to indemnification.
The rights conferred in the amended and restated certificate of incorporation and amended and restated bylaws are not exclusive, and we are authorized to enter into indemnification agreements with its directors, officers, employees, and agents and to obtain insurance to indemnify such persons.
We may not retroactively amend the bylaw provisions to reduce our indemnification obligations to directors, officers, employees, and agents.
We have historically entered into indemnification agreements with our directors and executive officers, in addition to the indemnification provided for in our amended and restated certificate of incorporation and amended and restated bylaws, and we may also do so in the future. The indemnification agreements and our amended and restated certificate of incorporation and amended and restated bylaws require us to indemnify our directors and officers to the fullest extent permitted by Delaware law.
We have purchased and currently intend to maintain insurance on behalf of each and any person who is or was a director or officer of us against any loss arising from any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusions.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us, we have been informed that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Item 15. Recent Sales of Unregistered Securities
In the three years preceding the filing of this Registration Statement, the Registrant has not issued any unregistered securities, except as set forth below (information below has been adjusted for our 1 for 50 reverse stock split effected on September 30, 2022):
On July 22, 2022, we reduced the exercise price of all Series A Common Stock Purchase Warrants and Series B Common Stock Purchase Warrants, or the Existing Warrants, that were issued in our February 2022 underwritten public offering from $25.00 per share to $14.00, or the Warrant Repricing. Following the Warrant Repricing, we entered into warrant inducement offer letters, or the Inducement Letters, with certain investors to immediately exercise up to approximately 444,000 of the Existing Warrants held by such investors, or the Inducement Offer. In consideration for exercising the Existing Warrants, pursuant to the terms of the Inducement Letters, we offered to issue to the investors a new Series C Common Stock Purchase Warrant, or the Series C Warrant, if the investor exercised a Series A Warrant or a new Series D Common Stock Purchase Warrant, or the Series D Warrant, and together with the Series C Warrants, if the investor exercised a Series B Warrant, in each case, to purchase up to a number of Shares of common stock equal to 100% of the number of Shares of common stock issued pursuant to the immediate exercise of the corresponding Series A Warrants and Series B Warrants, as applicable. We received aggregate gross proceeds of approximately $6.2 million from the exercise of the Series A Warrants, which resulted in the issuance of an aggregate of approximately 444,000 Shares of common stock, together with the corresponding issuance of Series C Warrants exercisable for approximately 444,000 Shares of common stock. None of the Series B Warrants were exercised pursuant to the Inducement Offer, and no Series D Warrants were issued. The Series C Warrants have an exercise price of $14.00 and a term of five years. The Series C Warrants and the Shares underlying the Series C Warrants were issued in a private placement pursuant to Section 4(a)(2) of the Securities Act based on representations made by the Series A Warrant holders. The Shares issued upon exercise of the Series A Warrants were registered in our February 2022 underwritten public offering. While the Shares underlying the Series C Warrants were registered for resale following the Inducement Offer, the Series C Warrants themselves have not been registered.
| 39 |
On January 9, 2023, the Registrant completed its acquisition of Catheter Precision, Inc., a privately-held Delaware corporation (“Old Catheter”), pursuant to an Amended and Restated Agreement and Plan of Merger (the “Merger Agreement”), by and among the Registrant, Old Catheter, Rapid Merger Sub 1, Inc., a newly-created wholly-owned subsidiary of the Registrant (“First Merger Sub”), and Rapid Merger Sub 2, LLC, a newly-created wholly owned subsidiary of the Registrant (“Second Merger Sub” and together with First Merger Sub, the “Merger Subs”), entered into on January 9, 2023, pursuant to which the First Merger Sub merged with and into Old Catheter, with Old Catheter being the surviving corporation (the “First Merger Surviving Registrant”) and a wholly-owned subsidiary of the Registrant (the “First Effective Time”), and then, immediately following the First Effective Time, and as part of the same overall transaction, the First Merger Surviving Registrant merged with and into the Second Merger Sub (the “Second Effective Time”), with the Second Merger Sub being the surviving limited liability registrant (the “Second Merger Surviving Registrant”) (such transactions collectively, the “Merger,” with the Registrant following the Merger being referred to herein as the “Post-Merger Combined Registrant”). The Merger Agreement amends and restates in its entirety the Agreement and Plan of Merger (the “Original Agreement”) entered into between the parties to the Original Agreement on September 9, 2022. Immediately upon the First Effective Time, each share of common stock of Old Catheter, par value $0.001 (“Catheter Common Stock”) issued and outstanding immediately prior to the First Effective Time (subject to certain exclusions set forth in the Merger Agreement) was converted into the right to receive a number of shares of a new class of the Registrant’s preferred stock, designated Series X Convertible Preferred Stock, par value $0.0001 per share (the “Series X Preferred Stock”), calculated in the manner described below.
Each share of Old Catheter Common Stock previously outstanding was converted into a number of shares of Series X Preferred Stock equal to approximately 0.6705 (the “Exchange Ratio”), divided by one thousand (1,000). In addition, the principal amount owing under certain of Catheter’s convertible promissory notes (the “Converted Catheter Notes”), representing an aggregate principal amount of $25,215,000, pursuant to certain Debt Settlement Agreements (as defined in the Merger Agreement) converted into shares of Series X Preferred Stock, at a conversion price of $3.20 per one one-thousandth of a share of Series X Preferred Stock. In exchange for the forgiveness of the interest accrued but remaining unpaid under the Converted Catheter Notes, under the terms of the Debt Settlement Agreements the holders of Converted Catheter Notes also received certain royalty rights which equal, in aggregate, 11.82% per year on Net Sales (as defined in the Merger Agreement), if any, of Old Catheter’s LockeT vessel closure device, which is currently under development. All outstanding options to purchase Old Catheter Common Stock (“Catheter Options”) were assumed and converted, at the First Effective Time, into options to purchase, in the aggregate, approximately 753,699 Shares of the Registrant’s Common Stock, representing a conversion at the Exchange Ratio. Each share of Series X Preferred Stock was contingently convertible into one thousand (1,000) Shares of Registrant Common Stock, subject to certain requirements, and has no voting rights.
The shares of Series X Preferred Stock issued in the Merger were allocated as follows:
1. The total number of Shares of Registrant Common Stock allocated to all Old Catheter stakeholders (including stockholders, debtholders and option holders) was approximately 15,403,291 Shares. After subtracting approximately 753,699 Shares of Registrant Common Stock allocated to underlie assumed Old Catheter options (which was calculated by multiplying the Exchange Ratio by the number of options assumed), the number of Shares of Registrant Common Stock allocated to Old Catheter debtholders and stockholders, in the aggregate, was approximately 14,649,592. This number was divided by 1000, to arrive at a total of approximately 14,649.592 shares of Series X Preferred Stock issuable to Old Catheter debtholders and stockholders.
2. The $25,215,000 principal amount of Converted Catheter Notes converted, pursuant to the terms of the Debt Settlement Agreements, into approximately 7,879.689 shares of Series X Preferred Stock at a conversion price (the “Conversion Price”) of $3.20 for each one one-thousandth of a share of Series X Preferred Stock, which was equal to 80% of the $4.00 per share price at which certain Registrant warrants were repriced, and certain of the repriced warrants were exercised, in the Warrant Repricing (as defined below), which closed concurrently with the Merger and is described in more detail below.
3. After deducting the total number of shares of Series X Preferred Stock to be issued to holders of the Converted Notes, approximately 6,769.903 shares of Series X Preferred Stock were issued to Old Catheter stockholders.
The Shares of Registrant Common Stock and preferred stock, and the Registrant options and the Shares of Registrant Common Stock underlying them, that were offered and/or issued in the Merger were offered and sold in transactions exempt from registration under the Securities Act, in reliance on Section 4(a)(2) thereof, because the offer and sale of such securities did not involve a “public offering” as defined in Section 4(a)(2) of the Securities Act, and other applicable requirements were met, based on representations made or deemed to be made by the Old Catheter shareholders.
| 40 |
On January 9, 2023, the Registrant reduced the exercise price of certain existing warrants of the Registrant (“Additional Existing Warrants”) exercisable for 331,608 Shares of Registrant Common Stock, which were held by Armistice Master Fund Ltd. (“Armistice”), from exercise prices ranging from $14.00 to $526.50 per share to $4.00 per share (the “Warrant Repricing”). In connection with the Warrant Repricing, the Registrant entered into a warrant inducement offer letter (the “Inducement Letter”) with Armistice pursuant to which it would exercise up to all of the 331,608 Additional Existing Warrants (the “Inducement Offer). In consideration for exercising the Additional Existing Warrants, pursuant to the terms of the Inducement Letter, the Registrant issued to Armistice a new Series E Common Stock Purchase Warrant (the “Series E Warrant”), to purchase up to a number of Shares of common stock equal to 100% of the number of Shares of common stock issued pursuant to the exercise of the Additional Existing Warrants. The Series E Warrant has an exercise price of $4.00 and a term of five years. The Additional Existing Warrants were exercised in full, and the Registrant received aggregate gross proceeds of approximately $1.3 million from the exercise of the Additional Existing Warrants, resulting in the issuance of up to an aggregate of approximately 331,608 Shares of common stock.
The Series E Warrants and the Shares underlying the Series E Warrants were issued in a private placement pursuant to Section 4(a)(2) of the Securities Act, based on representations made by Armistice.
On January 9, 2023, the Registrant also entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) for a private placement (the “Private Placement”) with Armistice. Pursuant to the Securities Purchase Agreement, Armistice agreed to purchase (a) Class A Units at a price that was the lower of $3.00 per unit and 90% of the 5 day volume weighted average closing price of the Registrant’s Common Stock immediately prior to obtainment of the approval of the Registrant’s stockholders of conversion of the PIPE Preferred Stock and PIPE Warrants (as each are defined below), each consisting of one share of Common Stock, one Series F Common Stock Purchase Warrant (“Series F Warrant”) and one Series G Common Stock Purchase Warrant (“Series G Warrant” and together with the Series F Warrants, the “PIPE Warrants”), and (b) Class B Units at a price of $1,000.00 per unit, each consisting of one share of a new series of the Registrant’s preferred stock, designated as Series A Convertible Preferred Stock, par value $0.0001 (the “PIPE Preferred Stock”), and one Series F Warrant and one Series G Warrant for each share of Registrant Common Stock underlying the PIPE Preferred Stock (each share of which is convertible into a number of Shares of Registrant common stock equal to $1,000 divided by the lower of $3.00 and 90% of the 5 day volume weighted average closing price of the Registrant’s Common Stock immediately prior to obtainment of the approval of the Registrant’s stockholders of conversion of the PIPE Preferred Stock and PIPE Warrants (the “Preferred Conversion Price”)), for an aggregate purchase price of approximately $8.0 million. The allocation between Class A and Class B units was to be determined by Armistice prior to their issuance. The closing under the Securities Purchase Agreement and the sale and issuance of the Class A Units and Class B Units (and the issuance of any underlying Common Stock) was subject to the approval of the Registrant’s stockholders.
The PIPE Warrants are exercisable at an exercise price of $3.00 per share, subject to adjustments as provided under the terms of the PIPE Warrants and subject to certain beneficial ownership blockers. The Series F Warrants have a term of two years from the date stockholder approval was obtained, and the Series G Warrants have a term of six years from the date that stockholder approval was obtained. Shares of PIPE Preferred Stock are convertible at any time at the option of the holder into Shares of Registrant Common Stock at the Preferred Conversion Price. The conversion price is subject to adjustment in the case of stock splits, stock dividends, combinations of shares and similar recapitalization transactions and is subject to certain beneficial ownership blockers.
Pursuant to the Securities Purchase Agreement, on March 23, 2023, following stockholder approval obtained on March 21, 2023, in consideration of approximately $8.0 million in cash, the Registrant issued to Armistice Class A units consisting of one common share, one Series F and one Series G warrant at a purchase price of $1.60029 per unit. In lieu of Class A units, for beneficial ownership purposes, as elected by Armistice, the Registrant also issued Class B units at the same price per unit consisting of convertible preferred stock convertible into approximately 625 Shares of common stock per share of preferred, one Series F and one Series G warrant. Each Class B Unit contained an amount of Series A Preferred Stock that was convertible into one share of common stock. A total of 497,908 Shares of common stock, 7,203 convertible Series A preferred shares convertible into 4,501,060 common Shares, 4,999,093 Series F and 4,999,093 Series G warrants were issued in the private placement.
| 41 |
The securities offered and issued pursuant to the Securities Purchase Agreement and in the private placement on March 23, 2023 and described above were offered in a private placement under Section 4(a)(2) of the Securities Act, and/or Rule 506(b) of Regulation D promulgated thereunder, based on the representations made by Armistice in the Securities Purchase Agreement.
Pursuant to the terms of the Amended and Restated Agreement and Plan of Merger entered into between the Registrant and Old Catheter on January 9, 2023, following approval of the Registrant’s stockholders on March 21, 2023, approximately 1,974.905 shares of the Registrant’s Series X Preferred Stock converted into approximately 1,974,905 Shares of Registrant Common Stock at 5 pm Eastern time on March 23, 2023. No consideration or commissions were paid in connection with the conversion. The remaining approximately 12,656.011 shares of Series X Preferred Stock may convert into approximately 12,656,011 Shares of Common Stock on or after July 9, 2024, provided that the Registrant meets the initial listing requirements of a national stock exchange or has been delisted from NYSE American.
The securities issued were exempt under Section 3(a)(9) of the Act, as no commission or other remuneration was paid for soliciting the conversion.
On July 5, 2023, the Registrant issued 1,093,552 Shares of its Common Stock to Armistice in connection with the conversion of 1,750 shares of its outstanding Series A Convertible Preferred Stock. The Shares were issued in connection with two separate conversions of 875 shares of Series A Convertible Preferred Stock into 546,776 Shares of common stock that occurred on July 3, 2023. Each share of Series A Convertible Preferred Stock is convertible into approximately 625 Shares of common stock. The Common Stock was issued pursuant to the exemption contained in Section 3(a)(9) of the Securities Act, which applies to transactions in which a security is exchanged by an issuer with its existing security holders exclusively where no commission or other remuneration is paid or given directly or indirectly for soliciting such exchange.
On July 24, 2023, the Registrant issued 546,776 Shares of its Common Stock to Armistice in connection with the conversion of 875 shares of its outstanding Series A Convertible Preferred Stock. The conversion occurred on July 24, 2023. Each share of Series A Convertible Preferred Stock is convertible into approximately 625 Shares of common stock. The Common Stock was issued pursuant to the exemption contained in Section 3(a)(9) of the Securities Act, which applies to transactions in which a security is exchanged by an issuer with its existing security holders exclusively where no commission or other remuneration is paid or given directly or indirectly for soliciting such exchange.
On January 24, 2024, we issued 546,776 Shares of its common stock in connection with the conversion of 875 shares of our outstanding Series A Convertible Preferred Stock. The conversion occurred on January 23, 2024. Each share of Series A Convertible Preferred Stock is convertible into approximately 625 Shares of common stock. The common stock was issued pursuant to the exemption contained in Section 3(a)(9) of the Securities Act, which applies to transactions in which a security is exchanged by an issuer with its existing security holders exclusively where no commission or other remuneration is paid or given directly or indirectly for soliciting such exchange. The Shares issued have been registered for resale on an effective registration statement on Form S-1.
On May 1, 2024, we issued an award of non-plan options to purchase 250,000 Shares of Company common stock as an inducement grant to Marie-Claude Jacques, our Chief Commercial Officer. The options have an exercise price of $0.5321 per share, vest annually over five years and have a term of 10 years. The options were granted pursuant to the exemption contained in Section 4(a)(2) of the Securities Act.
Item 16. Exhibits and Financial Statement Schedules
(a) The following exhibits are filed as part of this registration statement.
| 42 |
EXHIBIT INDEX
|
|
|
|
| Incorporated by Reference | ||||||
| Exhibit Number |
| Description |
| Form |
| File No. |
| Exhibit |
| Filing Date |
|
|
|
|
|
|
|
|
|
|
|
|
| 1.1* |
| Form of Underwriting Agreement |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 2.1 |
| 1/13/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
| Amended and Restated Certificate of Incorporation of the Registrant. |
| 8-K |
| 001-38677 |
| 3.1 |
| 10/1/2018 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Certificate of Amendment to Amended and Restated Certificate of Incorporation of the Registrant. |
| 8-K |
| 001-38677 |
| 3.1 |
| 11/17/2020 | |
|
|
|
|
|
|
|
|
|
|
| |
|
| Certificate of Amendment to Amended and Restated Certificate of Incorporation of the Registrant. |
| 8-K |
| 001-38677 |
| 3.1 |
| 9/20/2022 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Certificate of Designation of Series X Convertible Preferred Stock. |
| 8-K |
| 001-38677 |
| 3.1 |
| 1/13/2023 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 3.2 |
| 1/13/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 3.1 |
| 8/4/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 3.2 |
| 10/1/2018 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 3.1 |
| 8/17/2022 | ||
|
|
|
|
|
|
|
|
|
|
|
|
| 4.1 |
| [omitted] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4.2 |
| [omitted] |
|
|
|
|
|
|
|
|
| 43 |
|
|
| 8-K |
| 001-38677 |
| 4.1 |
| 5/22/2020 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 4.2 |
| 5/22/2020 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 4.3 |
| 5/22/2020 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-1 |
| 333-239887 |
| 4.3 |
| 7/16/2020 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-1 |
| 333-239887 |
| 4.4 |
| 7/16/2020 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-1 |
| 333-239887 |
| 4.5 |
| 7/16/2020 | ||
|
|
|
|
|
|
|
|
|
|
|
|
| 4.9 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-1/A |
| 333-262195 |
| 4.9 |
| 2/3/2022 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 4.1 |
| 7/22/2022 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 4.4 |
| 2/9/2022 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-Q |
| 001-38677 |
| 4.7 |
| 8/15/2022 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 4.1 |
| 1/13/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 4.2 |
| 1/13/2023 |
| 44 |
|
|
| 8-K |
| 001-38677 |
| 4.3 |
| 1/13/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
| 4.16* |
| Form of Pre-Funded Warrant offered hereby |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 4.17* |
| Form of Underwriters’ Warrant issued hereunder |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 5.1* |
| Opinion of Arnall Golden Gregory LLP |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.1 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2018 Form of Indemnification Agreement between the Registrant and directors and executive officers. |
| S-1 |
| 333-226191 |
| 10.2 |
| 8/24/2018 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Ra Medical Systems, Inc. 2018 Stock Compensation Plan and Forms of Award Agreement thereunder. |
| S-1 |
| 333-226191 |
| 10.3 |
| 7/16/2018 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 99.1 |
| 10/13/2020 | ||
|
|
|
|
|
|
|
|
|
|
|
|
| 10.5 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.6 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.7 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.8 |
| [omitted] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.9 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 10.11 |
| 4/16/2020 |
| 45 |
|
|
| 8-K |
| 001-38677 |
| 10.6 |
| 1/13/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 10.1 |
| 1/19/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| S-1 |
| 333-237701 |
| 10.15 |
| 4/16/2020 | ||
|
|
|
|
|
|
|
|
|
|
|
|
| 10.12 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.13 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.14 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-K |
| 001-38677 |
| 10.19 |
| 3/17/2021 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-K |
| 001-38677 |
| 10.20 |
| 3/17/2021 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
| Notice of Suspension of Corporate Integrity Agreement, dated January 11, 2023. |
| 10-K |
| 001-38677 |
| 10.16.1 |
| 3/28/2023 | |
|
|
|
|
|
|
|
|
|
|
|
|
| 10.17 |
| [omitted.] |
|
|
|
|
|
|
|
|
| 46 |
| 10.18 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.19 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.20 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.21 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10.22 |
| [omitted.] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 10.1 |
| 7/22/2022 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 10.4 |
| 1/13/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 3.2 |
| 1/13/2023 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| - Ex. B to January 2023 SPA (form of Registration Rights Agreement). |
| 8-K |
| 001-38677 |
| 10.5 |
| 1/13/2023 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 4.2 |
| 1/13/2023 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 4.3 |
| 1/13/2023 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 10.5 |
| 1/13/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 10.3 |
| 1/13/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-K |
| 001-38677 |
| 10.27.1 |
| 3/28/2023 |
| 47 |
|
|
| 10-K |
| 001-38677 |
| 10.27.2 |
| 3/28/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-K |
| 001-38677 |
| 10.27.3 |
| 3/28/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-K |
| 001-38677 |
| 10.28 |
| 3/28/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
| Assignment and Agreement from Auston Locke in relation to LockeT dated July 15, 2022 |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Assignment and Agreement from David A. Jenkins in relation to LockeT dated January 24, 2023 |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 10.1 |
| 6/3/2024 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-K |
| 001-38677 |
| 10.29 |
| 3/28/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
| Extension Agreement dated January 11, 2022 to the Stereotaxis Marketing Agreement. |
| 10-K |
| 001-38677 |
| 10.29.1 |
| 3/28/2023 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Addendum One dated May 27, 2022 to the Stereotaxis Marketing Agreement. |
| 10-K |
| 001-38677 |
| 10.29.2 |
| 3/28/2023 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-K |
| 001-38677 |
| 10.30.1 |
| 3/28/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
| Consulting Agreement dated February 1, 2018, with Patricia Kennedy. |
| 10-K |
| 001-38677 |
| 10.31 |
| 3/28/2023 |
| 48 |
|
|
| 10-K |
| 001-38677 |
| 10.31.1 |
| 3/28/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| DEF 14A |
| 001-38677 |
| Annex C |
| 05/25/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-K |
| 001-38677 |
| 10.31.3 |
| 04/01/2024 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2023 Form of Nonstatutory Stock Option Agreement Under 2023 Equity Incentive Plan |
| 10-K |
| 001-38677 |
| 10.31.4 |
| 04/01/2024 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
| 2023 Form of Incentive Stock Option Agreement Under 2023 Equity Incentive Plan |
| 10-K |
| 001-38677 |
| 10.31.5 |
| 04/01/2024 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 10-K |
| 001-38677 |
| 10.31.6 |
| 04/01/2024 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
| Non-plan Stock Option Award granted May 1, 2024, to Marie-Claude Jacques |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Software and Technology License Agreement dated May 1, 2016, with Peacs BV. |
| 10-K |
| 001-38677 |
| 10.32 |
| 3/28/2023 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Settlement and Amendment Agreement dated May 24, 2021 with Peacs BV. |
| 10-K |
| 001-38677 |
| 10.32.1 |
| 3/28/2023 | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 10.2 |
| 6/3/2024 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 8-K |
| 001-38677 |
| 16.1 |
| 6/26/2023 |
| 49 |
|
|
| 10-K |
| 001-38677 |
| 21.1 |
| 3/28/2023 | ||
|
|
|
|
|
|
|
|
|
|
|
|
|
| Consent of Withum Smith+Brown, PC, Independent Registered Public Accounting Firm. |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Consent of Haskell & White LLP, Independent Registered Public Accounting Firm. |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| 23.3* |
| Consent of Arnall Golden Gregory LLP (included in Exhibit 5.1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||
|
|
|
|
|
|
|
|
|
|
|
|
|
| Power of Attorney (contained on signature page to this Registration Statement). |
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| * | To be filed by amendment |
| ** | Schedules and exhibits have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The Company undertakes to furnish supplemental copies of any of the omitted schedules and exhibits upon request by the U.S. Securities and Exchange Commission. |
| *** | Filed herewith. |
| + | Indicates a management contract or compensatory plan. |
(b) There are no financial statement schedules provided because the information called for is either not required or is shown either in the financial statements or the notes thereto.
| 50 |
Item 17. Undertakings
(a) The undersigned registrant hereby undertakes:
(1) That, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report pursuant to section 13(a) or section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(2) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
(3) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(4) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| 51 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Park City, State of Utah, on June 3, 2024.
|
| CATHETER PRECISION, INC. |
| |
|
|
|
|
|
|
| By: | /s/ David A. Jenkins |
|
|
| David A. Jenkins |
| |
|
| Executive Chairman of the Board and Chief Executive Officer |
|
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints David Jenkins and Margrit Thomassen, and each of them acting individually, as his or her true and lawful attorneys-in-fact and agent, with full power of each to act alone, with full powers of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this registration statement (including post-effective amendments and any related registration statements filed pursuant to Rule 462 and otherwise), and to file the same with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully for all intents and purposes as he or she might or could do in person, hereby ratifying and confirming that all said attorneys-in-fact and agents, or any of them or their substitute or resubstitute, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
| Title |
| Date |
|
|
|
|
|
|
| /s/ David A. Jenkins |
|
|
| June 3, 2024 |
| David A. Jenkins |
| Director, Executive Chairman of the Board and Chief Executive Officer (Principal Executive Officer) |
|
|
|
|
|
|
|
|
| /s/ Margrit Thomassen |
|
|
| June 3, 2024 |
| Margrit Thomassen |
| Interim Chief Financial Officer and Secretary (Principal Financial and Accounting Officer) |
|
|
|
|
|
|
|
|
| /s/ James J. Caruso |
|
|
|
|
| James J. Caruso |
| Director |
| June 3, 2024 |
|
|
|
|
|
|
| /s/ Martin Colombatto |
|
|
| June 3, 2024 |
| Martin Colombatto |
| Director |
|
|
|
|
|
|
|
|
| /s/ John P. Francis |
|
|
| June 3, 2024 |
| John P. Francis |
| Director |
|
|
| 52 |