
As filed with the U.S. Securities and Exchange Commission on August 24, 2023.
Registration No. 333-239951
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(IRS Employer Identification No.) |
Taman
Desa,
(Address of principal executive offices, including zip code)
Registrant’s phone number, including area code
+
How Kok Choong
Chief Executive Officer
1645 Village Center Circle, Suite 17
Las Vegas, Nevada
United States, 89134
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Lawrence S. Venick, Esq. Loeb & Loeb LLP 2206-19 Jardine House 1 Connaught Place Central, Hong Kong SAR Tel: +852.3923.1111 |
Louis Taubman, Esq. Guillaume de Sampigny, Esq. Hunter Taubman Fischer & Li LLC 950 Third Avenue, 19th Floor
New York, NY 10022 Tel: 212-530-2210 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: [X]
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated Filer ☐ | Accelerated Filer ☐ | |
| (Do not check if a smaller reporting company) | Smaller
Reporting Company
|
|
Emerging
Growth Company
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the U.S. Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
EXPLANATORY NOTE
This Registration Statement contains two prospectuses, as set forth below.
| ● | Public Offering Prospectus. A prospectus to be used for the public offering of 1,100,000 shares of common stock of the Registrant (the “Public Offering Prospectus”) through the underwriter named on the cover page of the Public Offering Prospectus. |
| ● | Resale Prospectus. A prospectus to be used for the resale by the selling stockholders set forth therein of 30,169,516 shares of common stock of the Registrant (the “Resale Prospectus”). |
The Resale Prospectus is substantively identical to the Public Offering Prospectus, except for the following principal points:
| ● | they contain different outside and inside front covers and back covers; |
| ● | they contain different Offering sections in the Prospectus Summary section beginning on page 3; |
| ● | they contain different Use of Proceeds sections on page 22; |
| ● | a Selling Stockholder section is included in the Resale Prospectus; |
| ● | a Selling Stockholder Plan of Distribution is inserted; and |
| ● | the Legal Matters section in the Resale Prospectus on page 101 deletes the reference to counsel for the underwriter. |
The Registrant has included in this Registration Statement a set of alternate pages after the back cover page of the Public Offering Prospectus (the “Alternate Pages”) to reflect the foregoing differences in the Resale Prospectus as compared to the Public Offering Prospectus. The Public Offering Prospectus will exclude the Alternate Pages and will be used for the public offering by the Registrant. The Resale Prospectus will be substantively identical to the Public Offering Prospectus except for the addition or substitution of the Alternate Pages and will be used for the resale offering by the selling stockholders.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the U.S. Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION, DATED AUGUST 24, 2023 |
AGAPE ATP CORPORATION

1,100,000 of Shares of Common Stock
This is a firm commitment initial public offering of 1,100,000 of our shares of common stock, $0.0001 par value per share. We anticipate that the initial public offering price of our shares will be between US$5.50 and US$6.50 per share. The Underwriter is obligated to take and pay for all of the shares if any such shares are taken. We have granted the Underwriter a 15% over-allotment option, exercisable one or more times in whole or in part, to purchase up to 165,000 additional common stock from us at the public offering price, less the underwriting discounts, for 45 days from the closing of this offering to cover over-allotments, if any. If the Underwriter exercises the option in full, the total underwriting discounts payable will be $657,800, and the total proceeds to us, before expenses, will be $7,564,700.
Our common stock currently is quoted on the OTC Markets – Pink Sheets, operated by OTC Markets Group, under the symbol “AATP.” The last reported sale price of our common stock on the OTC Markets – Pink Sheets on May 11, 2023 was $6.00 per share.
We have applied to list our common stock on the NASDAQ Capital Market (“NASDAQ”) under the symbol “ATPC”. There can be no assurance that our application will be approved. The closing of this offering is contingent upon the successful listing of our common stock on the Nasdaq Capital Market.
Investing in our common stock is highly speculative and involves a significant degree of risk. See “Risk Factors” beginning on page 8 of this prospectus for a discussion of information that should be considered before making a decision to purchase our common stock.
Neither the U.S. Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Price to Public | Underwriting Discount(1) | Proceeds to us (before expenses) | ||||||||||
| Per Share of Common Stock | $ | 6.50 | $ | 0.52 | $ | 5.98 | ||||||
| Total(2) | $ | 7,150,000 | $ | 657,800 | $ | 7,564,700 | ||||||
| (1) | See “Underwriting” for additional disclosure regarding underwriting compensation payable by us. |
| (2) | Assuming over-allotment option granted to the Underwriter is exercised in full. We have granted the Underwriter a 15% over-allotment option, exercisable one or more times in whole or in part, to purchase up to 165,000 additional common stock from us at the public offering price, less the underwriting discounts, for 45 days from the closing of this offering to cover over-allotments, if any. See “Underwriting” for more information. |
Delivery of the shares of common stock is expected to be made on or about , 2023.

The date of this prospectus is , 2023.
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus or contained in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We have not, and the Underwriter has not, authorized anyone to provide you with information that is different from that contained in such prospectuses. We are offering to sell shares of our common stock, and seeking offers to buy shares of our common stock, only in jurisdictions where such offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
In this prospectus, we rely on and refer to information and statistics regarding our industry. We obtained this statistical, market and other industry data and forecasts from publicly available information. While we believe that the statistical data, market data and other industry data and forecasts are reliable, we have not independently verified the data.
For investors outside of the United States: neither we nor the Underwriter have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
| 2 |
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus. Because this is only a summary, it does not contain all of the information that may be important to you. You should read this entire prospectus and should consider, among other things, the matters set forth under “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, and our consolidated financial statements and related notes thereto appearing elsewhere in this prospectus before making your investment decision.
Overview
Agape ATP Corporation provides health solution advisory services to its clients. We primarily focus our efforts on attracting customers in Malaysia. We have an advisory services center called the “ATP Zeta Health Program”, which is a health program designed to effectively prevent diseases caused by polluted environments, unhealthy dietary intake and unhealthy lifestyles, and the promotion of health. The program aims to promote improved health and longevity through a combination of modern health supplements, proper nutrition and advice from skilled nutritionists and/or dieticians. For the six months ended June 30, 2023 and 2022, our revenue was approximately $0.7 million and $0.8 million, respectively, and our gross profit was approximately $0.4 million and $0.6 million, respectively. For the years ended December 31, 2022 and 2021, our revenue was approximately $1.9 million and $1.0 million, respectively, and our gross profit was approximately $1.2 million and $0.7 million, respectively.
In order to strengthen the Company’s supply chain, on May 8, 2020, the Company acquired approximately 99.99% of Agape Superior Living Sdn Bhd, a Malaysia company (“ASL”), with the goal of securing an established network marketing sales channel that has been established in Malaysia for the past 18 years. ASL has been offering the Company’s ATP Zeta Health Program as part of its product lineup. As such, the acquisition creates synergy in the Company’s operation by boosting the Company’s retail and marketing capabilities. The acquired subsidiary allows the Company to fulfill its mission of “helping people to create health and wealth” by providing a financially rewarding business opportunity to distributors and quality products to distributors and customers who seek a healthy lifestyle.
The Company deems creating public awareness on wellness and wellbeing lifestyle as essential to enhance the provision of its health solution advisory services; and therefore, on September 11, 2020, incorporated Wellness ATP International Holdings Sdn, Bhd. (“WATP”). Upon its establishment, WATP started collaborating with ASL to carry out various wellness programs.
On November 11, 2021, Agape ATP Corporation (Labuan) formed a joint-venture entity, DSY Wellness International Sdn. Bhd. (“DSY Wellness”) with Mr. Steve Yap following which Agape ATP Corporation (Labuan) owns 60% of the equity interest of DSY Wellness, to pursue the business of providing complementary health therapies. The establishment of DSY Wellness is a further expansion of our business into the health and wellness industry. Mr. Steve Yap owns 33 proprietary formulas (the “Proprietary Formulas”) for treating non-communicable disease and the Company is currently in discussion with Mr. Steve Yap to bring the Proprietary Formulas into the company for joint commercialization. Mr. Steve Yap also has existing clients receiving traditional complimentary medicine or “TCM” in Indonesia and China.
Our Products
We offer three series of programs which consist of different services and products: ATP Zeta Health Program, ÉNERGÉTIQUE and BEAUNIQUE.
Our ATP Zeta Health Program is a health program designed to promote health and general wellbeing designed to prevent health diseases caused by polluted environments, unhealthy dietary intake and unhealthy lifestyles. The program aims to promote improved health and longevity through a combination of modern health supplements, proper nutrition and advice from skilled dieticians as well as trained members and distributors.
Our ÉNERGÉTIQUE series aims to provide a total dermal solution for a healthy skin beginning from the cellular level. The series is comprised of the Energy Mask series, Hyaluronic Acid Serum and Mousse Facial Cleanser.
Our BEAUNIQUE product series focuses on the research of our diet’s impact on modifying gene expressions in order to address genetic variations and deliver a nutrigenomic solution for every individual.
The newly established subsidiary DSY Wellness is a further expansion of our business into the health and wellness industry and aims to pursue the business of providing traditional and complementary health therapies.
Our Strategies
We intend to pursue the following strategies in order to further develop and expand our business:
| ● | Expand our product range in each of our ATP Zeta Health Program, ÉNERGÉTIQUE and BEAUNIQUE series; | |
| ● | Further penetrate existing markets; | |
| ● | Deepen our relationship with existing distributors and members; |
| 3 |
| ● | Further investment into information technology such as the establishment of an e-commerce platform; | |
| ● | Expand into other geographies outside of Malaysia; and | |
| ● | Pursue growth through acquisitions of other health and wellness service provides. |
Our Competitive Strengths
We believe the following competitive strengths contribute to our success and differentiate us from our competitors:
| ● | Well established reputation; | |
| ● | Well-established product portfolio; | |
| ● | Large, highly-motivated distributor base, supported by a successful training methodology; | |
| ● | Scalable business model; and | |
| ● | Founder-led and deeply experienced management team. |
Our Challenges
Our ability to realize our mission and execute our strategies is subject to risks and uncertainties, including those relating to our ability to:
| ● | Respond to a highly competitive market; | |
| ● | Respond to concentration risk of heavy reliance on our largest supplier for the supply of products; | |
| ● | Maintain quality product and value; | |
| ● | Create brand influence; | |
| ● | Expand our product offerings; and | |
| ● | Expand our business in Malaysia and globally. |
Please see “Risk Factors” and other information included in this prospectus for a discussion of these and other risks and uncertainties that we face.
Risk Factors
An investment in our common stock involves a high degree of risk. You should consider and read carefully all of the risks and uncertainties described in “Risk Factors” beginning on page 8, together with all of the other information contained in this prospectus, including our consolidated financial statements and related notes thereto appearing elsewhere in this prospectus, before investing in our common stock. These risks could materially affect our business, financial condition and results of operations and cause the trading price of our common stock to decline. You could lose part or all of your investment. You should bear in mind, in reviewing this prospectus, that past experience is no indication of future performance. You should read “Special Note Regarding Forward-Looking Statements” for a discussion of what types of statements are forward-looking statements, as well as the significance of such statements in the context of this prospectus.
Corporate Information
Our principal executive offices are located at 1705 – 1708, Level 17, Tower 2, Faber Towers, Jalan Desa Bahagia, Taman Desa, Kuala Lumpur, Malaysia (Post Code: 58100). Our telephone number at this address is +(60) 327325716. Our registered office in Nevada is located at 1645 Village Center Circle, Suite 170, Las Vegas, Nevada, United States, 89134.
Our website is http://agapeatpgroup.com/. The information contained on our website or any third-party websites is not a part of this prospectus.
| 4 |
Corporate Structure
The following diagram illustrates our corporate structure as of the date of this prospectus and upon closing of this offering:
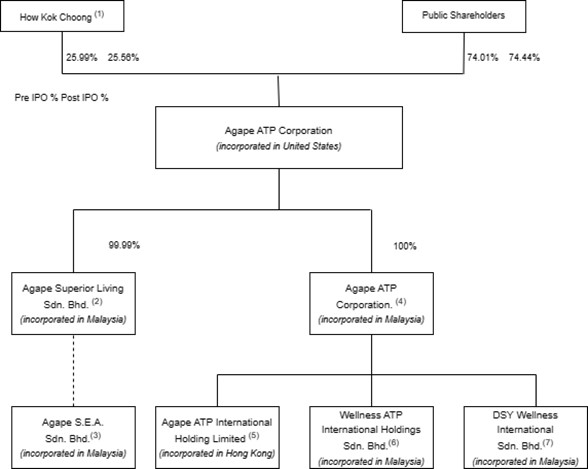
*As of the date of this prospectus.
** Upon closing of this offering and assuming no exercise of the Underwriter’s Warrants and full exercise of the over-allotment option.
Note:
| 1. | Represent 19,597,500 shares of common stock held by How Kok Choong as of the date of this prospectus and immediately after this offering, representing 25.99% and 25.56% of the common stock outstanding, respectively. |
| 2. | Agape Superior Living Sdn. Bhd. was incorporated in Kuala Lumpur, Malaysia on August 8, 2003. The remaining 0.01% was collectively held by Lim Ah Yew@Lim Soo Yew, Lor Keat Yoon and Teng Woei Wei (wife of How Kok Choong). |
| 3. | Agape S.E.A. Sdn. Bhd. was incorporated in Kuala Lumpur, Malaysia on March 4, 2004. 100% of the company’s business is transacted with Agape Superior Living Sdn. Bhd.. The company is considered a VIE of Agape Superior Living Sdn. Bhd. as the latter is the primary beneficiary since it has the following characteristics: |
| a. | The power to direct the activities of the VIE that most significantly impact the VIE’s economic performance; and | |
| b. | The obligation to absorb losses of the VIE that could potentially be significant to the VIE or the right to receive benefits from the VIE that could potentially be significant to the VIE. |
However, Agape S.E.A.’s impact to our consolidated financial statements constitutes less than 1% of our total consolidated assets and Agape S.E.A. did not contribute any revenues for us as of December 31, 2020.
| 4. | Agape ATP Corporation was incorporated in Labuan, Malaysia on March 6, 2017. |
| 5. | Agape ATP International Holding Limited was incorporated in Hong Kong on June 1, 2017. |
| 6. | Wellness ATP International Holdings Sdn. Bhd. was incorporated in Kuala Lumpur, Malaysia on September 11, 2020. |
| 7. | DSY Wellness International Sdn. Bhd. was incorporated in Kuala Lumpur, Malaysia on November 11, 2021, as a joint-venture entity between Agape ATP Corporation (Labuan) and Mr. Steve Yap. |
Conventions That Apply to This Prospectus
Unless otherwise indicated or the context otherwise requires, references in this prospectus to:
| ● | “dollar,” “USD,” “US$,” or “$” are to U.S. dollars; | |
| ● | “RM” and “Ringgit” are to the legal currency of Malaysia; and | |
| ● | “we,” “us,” “Company,” “Agape”, “Agape ATP” and “our” are to Agape ATP Corporation, the Nevada holding company, and its subsidiaries, and its consolidated affiliated entities. | |
| ● | “ASL” are to Agape Superior Living Sdn Bhd, a Malaysia company and a 99.99% owned subsidiary of Agape ATP; |
| 5 |
The Offering
| Offering Price | We currently estimate that the initial public offering price will be between US$5.50 and US$6.50 per share | |
| Common stock offered by us | 1,100,000 of shares of common stock (or 1,265,000 shares of common stock if the Underwriter exercises its over-allotment option in full) on a firm commitment basis. | |
| Common stock outstanding prior to this offering |
75,452,012 shares of common stock.
| |
| Common stock outstanding immediately after this offering |
76,552,012 shares of common stock, assuming the sale of all the shares offered in this prospectus, 76,717,012 shares of common stock if the underwriter exercise the over-allotment in full.
| |
| Gross proceeds to us, net of underwriting discount but before expenses: |
$7,564,700 assuming no exercise of the underwriter warrant and full exercise of the over-allotment option.
| |
| Over-allotment option: | We have granted to the Underwriter a 15% over-allotment option, exercisable within 45 days from the closing of this offering, to purchase up to an aggregate of 165,000 additional shares of common stock. | |
| Underwriter Warrant | We have agreed to grant to the Underwriter a warrant covering a number shares of common stock equal to 7% of the shares of common stock sold by the Underwriter in this public offering (the “Underwriter Warrant”). The Underwriter Warrant will be exercisable upon issuance and will expire on the fifth year anniversary of the date of the commencement of sales of this offering. The Underwriter Warrant and the underlying securities may not be sold, transferred, assigned, pledged, or hypothecated, or the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days beginning on the date of commencement of sales of this offering (in accordance with FINRA Rule 5110), except that they may be assigned, in whole or in part, to any officer or partner of the Underwriter, and to members of the syndicate or selling group and their respective officers or partners. The Underwriter Warrant will be exercisable at a price equal to 110% of the initial public offering price per share set forth on the cover price of this prospectus. | |
| Use of proceeds |
We plan to use the net proceeds of this offering primarily for general corporate purposes. For more information on the use of proceeds, see “Use of Proceeds” on page 22. | |
| Lock-up | We and each of, our officers, directors, and 10% or more stockholders, have agreed with the underwriters, subject to certain exceptions, not to sell, transfer or otherwise dispose of any shares of common stock or similar securities for a period of 180 days after the date of this prospectus. See “Shares Eligible for Future Sale” and “Underwriting” for more information. | |
| Trading Market |
Our common stock currently is quoted on the OTC Markets – Pink Sheets under the symbol “AATP.” We have applied to list our common stock on the Nasdaq Capital Market under the new symbol “ATPC”. At this time, Nasdaq has not yet approved our application to list our common stock. The closing of this offering is conditioned upon Nasdaq’s final approval of our listing application, and there is no guarantee or assurance that our common stock will be approved for listing on Nasdaq.
| |
| Concentration of Ownership |
Prior to this offering, our executive officers and directors beneficially own, in the aggregate, approximately 25.99% of the outstanding shares of our common stock, which will become approximately 25.56% upon completion of this offering assuming the sale of all the shares offered in this prospectus, no exercise of the Underwriter’s Warrants and full exercise of the over-allotment option.
| |
|
Trading Symbol
|
“AATP” | |
| Risk factors | You should read the “Risk Factors” section of this prospectus for a discussion of factors to consider carefully before deciding to invest in shares of our common stock. |
| 6 |
Summary Consolidated Financial Data
AGAPE ATP CORPORATION
The following tables summarize our historical consolidated financial data. We have derived the historical consolidated statements of operations data for the three and six months ended June 30, 2023 and 2022 from our unaudited condensed consolidated financial statements, and for the years ended December 31, 2022 and 2021 from our audited consolidated financial statements included elsewhere in this prospectus. The following summary consolidated financial data should be read in conjunction with the respective section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our condensed consolidated financial statements and related notes, and consolidated financial statements and related notes included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that may be expected in the future, and our results for any interim period are not necessarily indicative of the results to be expected for a full fiscal year.
Consolidated Statements of Operations Data for the:
| Three Months Ended June 30, | ||||||||
| 2023 | 2022 | |||||||
| Revenue | $ | 303,935 | $ | 396,707 | ||||
| Net loss | $ | (379,449 | ) | $ | (404,344 | ) | ||
| Net loss per share – (basic and diluted) | $ | (0.00 | ) | $ | (0.01 | ) | ||
| Six Months Ended June 30, | ||||||||
| 2023 | 2022 | |||||||
| Revenue | $ | 684,703 | $ | 805,667 | ||||
| Net loss | $ | (813,524 | ) | $ | (702,790 | ) | ||
| Net loss per share – (basic and diluted) | $ | (0.01 | ) | $ | (0.01 | ) | ||
| Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Revenue | $ | 1,856,564 | $ | 1,016,962 | ||||
| Net loss | $ | (1,666,079 | ) | $ | (2,524,680 | ) | ||
| Net loss per share – (basic and diluted) | $ | (0.02 | ) | $ | (0.01 | ) | ||
Consolidated Balance Sheet Data as of:
| As of | ||||||||||||
June 30, 2023 | December 31, 2022 | December 31, 2021 | ||||||||||
| Total assets | $ | 1,905,283 | $ | 2,791,749 | $ | 4,724,535 | ||||||
| Total liabilities | $ | 1,154,629 | $ | 1,229,295 | $ | 1,411,899 | ||||||
| 7 |
RISK FACTORS
Any investment in our securities involves a high degree of risk. You should carefully consider the risks described below, which we believe represent certain of the material risks to our business, together with the information contained elsewhere in this prospectus, before you make a decision to invest in our shares of common stock. Please note that the risks highlighted here are not the only ones that we may face. For example, additional risks presently unknown to us or that we currently consider immaterial or unlikely to occur could also impair our operations. If any of the following events occur or any additional risks presently unknown to us actually occur, our business, financial condition and operating results may be materially adversely affected. In that event, the trading price of our securities could decline and you could lose all or part of your investment.
Risks Related to Our Business and Industry
Our business and reputation may be affected by product liability claims, litigation, customer complaints, product tampering, food safety issues, food-borne illnesses, health threats, quality control concerns or adverse publicity relating to our products. Product liability insurance of our supplier may not cover our liability sufficiently or at all.
Like other consumer product manufacturers, the sale of our products involves an inherent risk of our products being found to be unfit for consumption or cause illness. Products may be rendered unfit for consumption due to raw materials or product contamination or degeneration, presence of microbials, illegal tampering of products by unauthorized third parties or other problems arising during the various stages of the procurement, production, transportation and storage processes. The occurrence of such problems may result in customer complaints, fines, penalties or adverse publicity causing serious damage to our reputation and brand, as well as product liability claims, other legal disputes and loss of revenues. Under certain circumstances, we may be required to recall our products. Even if a situation does not necessitate a product recall, we cannot assure you that product liability claims or other legal disputes will not be asserted against us as a result. Product liability insurance of our supplier may not cover our liability sufficiently or at all and will not cover liability that arises out of our default such as mishandling, poor storage condition and/or contamination of the products by us. As a result, a product liability or other judgment against us, or a product recall, could have a material adverse effect on our business, financial condition or results of operations.
Our business is susceptible to food-borne illnesses. We cannot assure you that we are able to effectively prevent all diseases or illnesses caused by our products or contamination of our products. Furthermore, our reliance on third-party product suppliers means that food-borne illness incidents could be caused by our suppliers outside of our control. New illnesses may develop in the future, or diseases with long incubation periods could arise that could give rise to claims or allegations on a retroactive basis. Reports in the media of instances of food-borne illnesses or health threats of our products or any of their major ingredients could adversely and significantly affect our sales, and have significant negative impact on our results of operations. This risk exists even if it were later determined that the illness or health threat in fact was not caused by our products.
In addition, adverse publicity about health and safety concerns, whether unfounded or not, may discourage consumers from buying our products. Even if a product liability claim is unsuccessful or is not fully pursued, the negative publicity surrounding any assertion that our products caused personal injury or illness could adversely affect our reputation and our corporate and brand image. If consumers were to lose confidence in our brand and reputation, we could suffer long-term or even permanent declines in our sales and results of operation. The amount of negative news, customers complaints and claims against us may also be very costly and may divert our management’s attention from our business operation.
We operate in a highly competitive market. If we do not compete effectively, our prospects, operating results, and financial condition could be materially and adversely affected.
The health and wellness market in Malaysia is a mature and a highly competitive market, with companies offering a variety of competitive products and services. We expect competition in our market to intensify in the future as new and existing competitors introduce new or enhanced products and services that are potentially more competitive than our products and services. The health and wellness market has a multitude of participants in the domestic market, including, but not limited, to retail health supplement providers, pharmaceutical companies, and network marketing company which supply health supplement products, such as Elken Group, USANA Group, NHF Group, Young Living, Jeunesse Global Holdings LLC, USA, Shaklee Corporation, VASAYO LLC, Amway Corporation, Sami Direct, Kyäni, Inc., Melaleuca, Inc.
We believe many of our competitors and potential competitors may have significant competitive advantages, including but not limited to, longer operating histories, ability to leverage their sales efforts and marketing expenditures across a broader portfolio of products and services, larger and broader customer bases, more established relationships with a larger number of suppliers, greater brand recognition, ability to leverage stores which they may operate, and greater financial, research and development, marketing, distribution, and other capabilities and resources than we do. Our competitors and potential competitors may also be able to develop products and services that are equal or more superior to ours, achieve greater market acceptance of their products and services, and increase sales by utilizing different distribution channels than we do. Some of our competitors may aggressively discount their products in order to gain market share, which could result in pricing pressures, reduced profit margins, lost market share, or a failure to grow market share for us. If we are not able to compete effectively against our current or potential competitors, our prospects, operating results, and financial condition could be materially and adversely affected.
We are exposed to concentration risk of heavy reliance on our three largest suppliers for the supply of our products, and any shortage of, or delay in, the supply may significantly impact on our business and results of operation.
For the six months ended June 30, 2023, we purchased $116,269, $65,338 and $33,507 from three of our major suppliers, one of them being a related party, which represented approximately 47.2%, 26.5% and 13.6%, respectively, of our total purchases for such period. For the year ended December 31, 2022, we purchased $198,376, $82,434 and $79,365 from three of our major suppliers, one of them being a related party, which represented approximately 53.3%, 22.1% and 21.3%, respectively, of our total purchases for such period. For the year ended December 31, 2021, we purchased $28,969 and $27,707 from two of our major suppliers, represented approximately 47.3% and 45.2%, respectively, of our total purchases. Our business, financial condition and operating results depend on the continuous supply of products from our major suppliers and our continuous supplier-customer relationships with them. Our heavy reliance on our major suppliers for the supply of our products will have a significant impact on our business and results of operation in the event of any shortage of, or delay in the supply.
We currently do not have long term supply agreements with our three largest suppliers for the six months ended June 30, 2023, and we typically make ad hoc purchases through submission of purchase order forms. There is no assurance that our major suppliers will continue to supply their products in the quantities and timeframes required by us to meet the needs of our customers or comply with their supply agreements with us. Our product supply may also be disrupted by potential labor disputes, strike action, natural disasters or other accidents, epidemic and pandemic affecting the supplier. If our major suppliers do not supply products to us in a timely manner or in sufficient quantities, our business, financial condition and operating results may be materially and adversely affected.
| 8 |
Furthermore, in the event of any delay in delivery of the products to us, our cash flow or working capital may be materially and adversely affected as a result of the corresponding delay in delivery of our products to our customers, and hence the delay in our receipt of payment from our customers.
Our major suppliers may change their existing sales or marketing strategy in respect of the products supplied to us by changing their export strategy, reducing its sales or production volume or changing its selling prices. Consequently, there are no assurances that our major suppliers will not appoint other dealers or distributors which may compete with us in the market where we operate. Furthermore, any significant increase in the selling prices of the products which we source from our suppliers will increase our costs and may adversely affect our profit margin if we are not able to pass the increased costs on to our customers.
There are no assurances that there will be no deterioration in our relationships with our major suppliers which could affect our ability to secure sufficient supply of products for our business. In the event that our major suppliers change their sales or marketing strategy or otherwise appoint other dealers or distributors who may compete with us, our business, financial condition and operating results may be materially and adversely affected.
We could be adversely affected by a change in consumer preferences, perception and spending habits and failure to develop or enrich our product offering or gain market acceptance of our new products could have a negative effect on our business.
The market we operate is subject to changes in consumer preference, perception and spending habits. Our performance depends significantly on factors which may affect the level and pattern of consumer spending in the market we operate. Such factors include consumer preference, consumer confidence, consumer income and consumer perception of the safety and quality of our products. Media coverage regarding the safety or quality of, or diet or health issues relating to, our products or the raw materials, ingredients or processes involved in their manufacturing, may damage consumer confidence in our products. A general decline in the consumption of our products could occur as a result of change in consumer preference, perception and spending habits at any time.
Any failure to adapt our product offering to respond to such changes may result in a decrease in our sales if such changes are related to certain of our products. Any changes in consumer preference could result in lower sales of our products, put pressure on pricing or lead to increased levels of selling and promotional expenses. In any event a decrease in customer demand on our products may also result in lower sales and slow down the consumption of our inventory to a low inventory turnover level. Any of these changes could result in a material adverse effect on our business, financial conditions or results of operations.
The success of our products depends on a number of factors including our ability to accurately anticipate changes in market demand and consumer preferences, our ability to differentiate the quality of our products from those of our competitors, and the effectiveness of our marketing and advertising campaigns for our products. We may not be successful in identifying trends in consumer preferences and developing products that respond to such trends in a timely manner. We also may not be able to effectively promote our products by our marketing and advertising campaigns and gain market acceptance. If our products fail to gain market acceptance, are restricted by regulatory requirements, or have quality problems, we may not be able to fully recover our costs and expenses incurred in our operation, and our business prospects, financial condition or results of operations may be materially and adversely affected.
If we fail to maintain quality products and value, our sales are likely to be negatively affected.
Our success depends on the safety and quality of products that we obtain from our suppliers for our customers. Our future customers will identify our brand name with a certain level of quality and value. If we cannot meet this perceived value or level of quality, we may be negatively affected and our operating results may suffer. In addition, any failure on the part of our suppliers to maintain the quality of their products, will in turn substantially harm the results of our business operations, potentially forcing us to identify other suppliers or alter our business strategy significantly.
If we are unable to create brand influence, we may not be able to maintain current or attract new users and customers for our products.
Our operational and financial performance is highly dependent on the strength of our brand. We believe brand familiarity and preference will continue to have a significant role in winning customers as the decision to buy our products and services. In order to further expand our customer base, we may need to substantially increase our marketing expenditures to enhance brand awareness through various online and offline means. Moreover, negative coverage in the media of our company could threaten the perception of our brand, and we cannot assure you that we will be able to defuse negative press coverage about our company to the satisfaction of our investors, customers and suppliers. If we are unable to defuse negative press coverage about our company, our brand may suffer in the marketplace, our operational and financial performance may be negatively impacted and the price of our shares may decline.
Currently, we sell our products, with or without customization, under our brand name “ATP”, to domestic customers in Malaysia and to overseas customers. However, if our competitors initiate a lawsuit against us for infringing their trademark, we may be forced to adopt a new brand name for our products. As a result, we may incur additional marketing cost to raise awareness of such new brand name. We may also be ordered to pay a significant amount of damages, and our business, results of operations and financial condition could be materially and adversely affected.
| 9 |
We may be unable to protect our intellectual property rights.
We rely on intellectual property laws in Malaysia and other jurisdictions to protect our trademarks. We are the registered owner of two trademarks. We have recently applied to register an additional three trademarks in Malaysia. We cannot assure you that counterfeiting or imitation of our products will not occur in the future or, if it does occur, that we will be able to address the problem in a timely and effective manner. Any occurrence of counterfeiting or imitation of our products or other infringement of our intellectual property rights could negatively affect our brand and our reputation, which in turn adversely affects the results of our operations.
Litigation to prosecute infringement of our intellectual property rights could be costly and lengthy and will divert our managerial and financial resources. We will have to bear costs of the intellectual property litigation and may be unable to recover such costs from our opposite parties. Protracted litigation could also result in our customers deferring or limiting their purchase or use of or products until such litigation is resolved. The occurrence of any of the foregoing will have a material adverse effect on our business, financial condition and results of operations.
We may incur losses resulting from product liability claims or product recalls or adverse publicity relating to our products.
We may incur losses resulting from product liability claims with respect to our products supplied by our supplier. We may face claims or liabilities which may arise if there exist any defects in quality of these products or any of these products are deemed or proven to be unsafe, defective or contaminated. In the event that the use or misuse of any product distributed by us results in personal injury or death, product liability and/or indemnity claims may be brought against us, in addition to our product recalls, and the relevant regulatory authorities in the market we operate may close down some of our related operations and take administrative actions against us. If we experience any business disruption and litigation, we may incur additional costs and have to divert our management’s attention and resources on such matters, which may adversely affect our business, financial condition and results of operations.
If we are unable to successfully develop and timely introduce new products or services or enhance existing products or services, our business, financial condition and results of operations may be materially and adversely affected.
We must continually source, develop and introduce new products and services as well as improve and enhance our existing products and services to maintain or increase our sales. The success of new or enhanced products or services may depend on a number of factors including, anticipating and effectively addressing user preferences and demand, the success of our sales and marketing efforts, effective forecasting and management of products and services demands, purchase commitments, and the quality of or defects in our products. The risk of not meeting our customers’ preferences and demands through our products and services may result in a shift in market shares, as customers instead choose products and services offered by our competitors. This may result in lower sales revenue, materially and adversely affecting our business, financial condition and results of operations.
We may not be able to manage the growth of our business and our expansion plans and operations or implement our business strategies on schedule or within our budget, or at all.
We are continually executing a number of growth initiatives, strategies and operating plans designed to enhance our business. In 2023, we plan to increase our revenue stream from health solution advisory services from our “ATP Zeta Health Program”, “ENERGETIQUE” and “BEAUNIQUE” series to align with our growth strategies. The company expects the initiative to materialize in the second half of 2023, despite competition from other health and wellness brands, and challenge in recruiting due to expanding employment market. Any expansion may increase the complexity of our operations and place a significant strain on our managerial, operational, financial and human resources. Our current and planned personnel, systems, procedures and controls may not be adequate to support our future operations. We cannot assure you that we will be able to effectively manage our growth or to implement all these systems, procedures and control measures successfully. Furthermore, the anticipated benefits from these growth initiatives, strategies and operating plans are based on assumptions that may prove to be inaccurate. Moreover, we may not be able to successfully complete these growth initiatives, strategies and operating plans and realize all of the benefits that we expect to achieve or it may be more costly to do so than we anticipate. If, for any reason, we are not able to manage our growth effectively, the benefits we realize are less than our estimates or the implementation of these growth initiatives, strategies and operating plans adversely affects our operations or costs more or takes longer to effectuate than we expect, and/or if our assumptions prove to be inaccurate, our business and prospects may be materially and adversely affected.
In addition, we may seek and pursue opportunities through joint ventures or strategic partnerships for expansion from time to time, and we may face similar risks and uncertainties as listed above. Failure to properly address these risks and uncertainties may materially and adversely affect our ability to carry out acquisitions and other expansion plans, integrate and consolidate newly acquired or newly formed businesses, and realize all or any of the anticipated benefits of such expansion, which may have a material adverse effect on our business, financial condition, results of operations and prospects.
We have a limited operating history in the Malaysia health and wellness industry, which makes it difficult to evaluate our future prospects.
We launched our ATP Zeta Super Health Program business in June 2016, the same month in which our Company was incorporated, followed by our ENERGETIQUE” and “BEAUNIQUE” series in July 2018 and March 2019, respectively, and thus, we have a limited operating history. We have limited experience in most aspects of our business operation, such as sourcing products for and offering advisory services on all the three programs. As our business develops and as we respond to competition, we may continue to introduce new product and services offerings and make adjustments to our existing product line and services and to our business operation in general. Any significant change to our business model that does not achieve expected results may have a material and adverse impact on our financial condition and results of operations. It is therefore difficult to effectively assess our future prospects.
The Malaysia health and wellness industry may not develop as expected. Prospective retail and corporate customers may not be familiar with the development of the market and may have difficulties distinguishing our products from those of our competitors. Convincing prospective customers or distributors of the value of our products or services is important to the success of our business. The risk of failing to convince potential customers or distributors to purchase products or services from us may result in the failure of our business plan. Many customers or distributors may not be interested in purchasing products and services we sell because there is no certainty that our business will succeed.
You should consider our business and prospects in light of the risks and challenges we encounter or may encounter given the rapidly evolving market in which we operate and our limited operating history. These risks and challenges include our ability to, among other things:
| ● | manage our future growth; | |
| ● | increase the utilization of our products by existing and new customers; | |
| ● | maintain and enhance our relationships with customers and distributors; | |
| ● | improve our operational efficiency; | |
| ● | attract, retain and motivate talented employees; | |
| ● | cope with economic fluctuations; | |
| ● | navigate the evolving regulatory environment; and | |
| ● | defend ourselves against legal and regulatory actions. |
Our historical growth rates may not be indicative of our future growth. If we are unable to manage the growth and increased complexity of our business, fail to control our costs and expenses, or fail to execute our strategies effectively, our business and business prospects may be materially and adversely affected.
Our historical growth rates may not be indicative of our future growth, and we may not be able to generate similar growth rates in future periods. Our revenue growth may slow, or our total revenues may decline for a number of possible reasons, including change in consumers’ preferences, changes in regulations and government policies, increasing competition, emergence of alternative business models, and general economic conditions.
| 10 |
Our total revenues decreased by approximately 15.0% from approximately 0.8 million for the six months ended June 30, 2022 to approximately 0.7 million for the six months ended June 30, 2023. Our total revenues increased by approximately 82.6% from approximately 1.0 million for the year ended December 31, 2021 to approximately 1.9 million for the year ended December 31, 2022. Our gross profits decreased by approximately 28.0% from approximately $0.6 million for the six months ended June 30, 2022 to approximately $0.4 million for the six months ended June 30, 2023.
If our growth rate declines, investors’ perceptions of our business and business prospects may be materially and adversely affected and the market price of our shares could decline.
Our lack of insurance could expose us to significant costs and business disruption.
The health and wellness industry in Malaysia is a mature market. We currently do not have any product liability or disruption insurance to cover our operations in Malaysia or overseas, which, based on public information available to us relating to Malaysia-based health and wellness companies, is consistent with customary industry practice in Malaysia. We have determined that the costs of insuring for these risks and the difficulties associated with acquiring such insurance on commercially reasonable terms make it impractical for us to have such insurance. If we suffer any losses, damages or liabilities in the course of our business operations, we may not have adequate insurance coverage to provide sufficient funds to cover any such losses, damages or product claim liabilities. Therefore, there may be instances when we will sustain losses, damages and liabilities because of our lack of insurance coverage, which may in turn materially and adversely affect our financial condition and results of operations.
A decline in general economic condition could lead to reduced consumer demand and could negatively impact our business operation and financial condition, which in turn could have a material adverse effect on our business, financial condition and results of operations.
Our operating and financial performance may be adversely affected by a variety of factors that influence the general economy. Consumer spending habits, including spending for health related products and services we sell, are affected by, among other things, prevailing economic conditions, levels of unemployment, salaries and wage rates, prevailing interest rates, income tax rates and policies, consumer confidence and consumer perception of economic conditions. In addition, consumer purchasing patterns may be influenced by consumers’ disposable income. In the event of an economic slowdown, consumer spending habits could be adversely affected and we could experience lower net sales than expected on a quarterly or annual basis which could have a material adverse effect on our business, financial condition and results of operations.
We operate in a heavily regulated industry.
Our business is principally regulated by various laws and regulations in the market we operate, such as in Malaysia the Food Act 1983 (ACT 281) and Regulations, Control of Drugs and Cosmetics Regulations 1984 mandate authorization from the Food Safety and Quality Division and National Pharmaceutical Regulatory Agency of the Ministry of Health for our Company’s products to be sold in the country. Various registrations, certificates and/or licenses for the conduct of our business are required under the above laws, which also contain provisions for requirements on the storage, labelling, advertising and importation of some of our products.
Based on our experience, some of the laws and regulations of the place where we operate our business are subject to amendments, uncertainty in interpretation and administrative actions from time to time. Therefore, we cannot assure you that, for the implementation of our business plans and the introduction of any new product, we will be able to obtain all the necessary registrations, certificates and/or licenses. Any failure to comply with the above laws and regulations may give rise to fines, administrative penalties and/or prosecution against us, which may adversely affect our reputation, financial condition or results of operation.
| 11 |
We may be adversely affected by the performance of third-party contractors.
We engaged third-party contractors to carry out logistics services. We endeavor to engage third-party companies with a strong reputation and track record, high performance reliability and adequate financial resources. However, any such third-party contractor may still fail to provide satisfactory logistics services at the level of quality or within the timeframe required by us or our customers. While we generally require our logistics contractors to fully reimburse us for any losses arising from delay in delivery or non-delivery, our results of operation and financial condition may be adversely affected if any of the losses are not borne by them. If the performance of any third-party contractor is not satisfactory, we may need to replace such contractor or take other remedial actions, which could adversely affect the cost structure and delivery schedule of our products and services and thus have a negative impact on our reputation, financial position and business operations. In addition, as we expand our business into overseas markets, there may be a shortage of third-party contractors that meet our quality standards and other selection criteria in such locations and, as a result, we may not be able to engage a sufficient number of high-quality third-party contractors in a timely manner, which may adversely affect our delivery schedules and delivery costs and hence our business, results of operations and financial conditions.
We may need additional capital, and financing may not be available on terms acceptable to us, or at all.
There is no guarantee that in the future we will generate enough profits to support our business. Although we believe that our anticipated cash flows from operating activities together with cash on hand will be sufficient to meet our anticipated working capital requirements and capital expenditures in the ordinary course of business for the next twelve months, we cannot assure you this will be the case. We may need additional cash resources in the future if we experience changes in business conditions or other developments. We may also need additional cash resources in the future if we find and wish to pursue opportunities for investment, acquisition, capital expenditure or similar actions. If we determine that our cash requirements exceed the amount of cash and cash equivalents we have on hand at the time, we may seek to issue equity or debt securities or obtain credit facilities. The issuance and sale of additional equity would result in further dilution to our stockholders. The incurrence of indebtedness would result in increased fixed obligations and could result in operating covenants that would restrict our operations. We cannot assure you that financing will be available in amounts or on terms acceptable to us, if at all.
Adverse developments in our existing areas of operation could adversely impact our results of business, results of operations and financial condition.
Our operations are focused on utilizing our sales efforts which are principally located in Malaysia. As a result, our results of operations, cash flows and financial condition depend upon the demand for our products in Malaysia. Due to the lack of broad diversification in industry type and geographic location, adverse developments in our current segment of the industry, or our existing areas of operation, could have a significantly greater impact on our business, results of operations and financial condition than if our operations were more diversified.
Our internal controls may be inadequate, which could cause our financial reporting to be unreliable and lead to misinformation being disseminated to the public.
Our management is responsible for establishing and maintaining adequate internal control over our financial reporting. As defined in Exchange Act Rule 13a-15(f), internal control over financial reporting is a process designed by, or under the supervision of, the principal executive and principal financial officer and effected by the board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that: pertain to the maintenance of records in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and/or directors of the Company; and provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements.
| 12 |
In connection with the audit of our consolidated financial statements as of December 31, 2022 and the review of our unaudited condensed consolidated financial statements as of June 30, 2023, we identified three “material weaknesses”, and other control deficiencies including significant deficiencies in our internal control over financial reporting. A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses identified related to the Company were: (i) insufficient full-time personnel with appropriate levels of accounting knowledge and experience to monitor the daily recording of transactions, address complex U.S. GAAP accounting issues and to prepare and review financial statements and related disclosures under U.S. GAAP; (ii) lack of a functional internal audit department or personnel that monitors the consistencies of the preventive internal control procedures and lack of adequate policies and procedures in internal audit function to ensure that the Company’s policies and procedures have been carried out as planned; (iii) lack of proper IT policies and procedures developed for system change management, user access management, backup management and service organization management.
The plans for the Company’s implementation of remediation of the material weaknesses are as follows:
| (a) | With respect to Material Weakness i, the Company has employed a full-time Chief Financial Officer (“CFO”), Mr. Lee Kam Fan, with relevant U.S. GAAP and SEC reporting experience and qualifications since January 12, 2021. | |
| (b) | With respect to Material Weakness ii, we intend to form an internal audit function and have plans to hire internal auditors to strengthen our overall governance. All internal auditors will be independent of our operations and will report directly to the audit committee. We intended to form the internal audit function within the next 12 months. | |
| (c) | With respect to Material Weakness iii, we intend to hire several dedicated personnel for different roles such as AP, OS and DB. This procedure would improve the segregation of duties in AP, OS and DB. Any changes to the system will require approval by a higher level of IT management. We expect to adopt the above measures within the next 12 months. |
As a public company, we will become subject to the Sarbanes-Oxley Act of 2002. Section 404 of the Sarbanes-Oxley Act, or SOX 404, will require that we include a report from management on the effectiveness of our internal control over financial reporting in our annual report on Form 10-K and in our quarterly report on Form 10-Q if we are qualified as an accelerated filer. Our management may conclude that our internal control over financial reporting is not effective. Moreover, even if our management concludes that our internal control over financial reporting is effective, our independent registered public accounting firm, after conducting its own independent testing, may issue a report that is qualified if it is not satisfied with our internal controls or the level at which our controls are documented, designed, operated or reviewed, or if it interprets the relevant requirements differently from us. In addition, after we become a public company, our reporting obligations may place a significant strain on our management, operational and financial resources and systems for the foreseeable future. We may be unable to timely complete our evaluation testing and any required remediation.
During the course of documenting and testing our internal control procedures, in order to satisfy the requirements of SOX 404, we may identify other weaknesses and deficiencies in our internal control over financial reporting. In addition, if we fail to maintain the adequacy of our internal control over financial reporting, as these standards are modified, supplemented or amended from time to time, we may not be able to conclude on an ongoing basis that we have effective internal control over financial reporting in accordance with SOX 404. If we fail to achieve and maintain an effective internal control environment, we could suffer material misstatements in our financial statements and fail to meet our reporting obligations, which would likely cause investors to lose confidence in our reported financial information. This could in turn limit our access to capital markets, harm our results of operations, and lead to a decline in the trading price of our shares. Additionally, ineffective internal control over financial reporting could expose us to increased risk of fraud or misuse of corporate assets and subject us to potential delisting from the stock exchange on which we list, regulatory investigations and civil or criminal sanctions. We may also be required to restate our financial statements from prior periods.
| 13 |
Legal disputes or proceedings could expose us to liability, divert our management’s attention and negatively impact our reputation.
We may at times be involved in potential legal disputes or proceedings during the ordinary course of business operations relating to product or other types of liability, employees’ claims, labor disputes or contract disputes that could have a material and adverse effect on our reputation, operation and financial condition. If we become involved in material or protracted legal proceedings or other legal disputes in the future, the outcome of such proceedings could be uncertain and could result in settlements or outcomes which materially and adversely affect our financial condition. In addition, any litigation or legal proceedings could incur substantial legal expenses as well as significant time and attention of our management, diverting their attention from our business and operations.
Our failure to comply with anti-corruption laws and regulations, or effectively manage our employees, customers and business partners, could severely damage our reputation, and materially and adversely affect our business, financial condition, results of operations and prospects.
We are subject to risks in relation to actions taken by us, our employees, third-party customers or third-party suppliers that constitute violations of the anti-corruption laws and regulations. While we adopt strict internal procedures and work closely with relevant government agencies to ensure compliance of our business operations with relevant laws and regulations, our efforts may not be sufficient to ensure that we comply with relevant laws and regulations at all times. If we, our employees, third-party customers or third-party suppliers violate these laws, rules or regulations, we could be subject to fines and/or other penalties. Actions by Malaysia regulatory authorities or the courts to provide an alternative interpretation of the laws and regulations or to adopt additional anti-bribery or anti-corruption related regulations could also require us to make changes to our operations. Our reputation, corporate image, and business operations may be materially and adversely affected if we fail to comply with these measures or become the target of any negative publicity as a result of actions taken by us, our employees, third-party customers or third-party suppliers.
An overall decline in the health of the economy and other factors impacting consumer spending, such as natural disasters, outbreak of viruses, illnesses, infectious diseases, contagions and the occurrence of unforeseen epidemics may affect consumer purchases, reduce demand for our products and materially harm our business, results of operations and financial condition.
Our business depends on consumer demand for our products and, consequently, is sensitive to a number of factors that influence consumer confidence and spending, including but not limited to, general current and future economic and political conditions, consumer disposable income, recession and fears of recession, unemployment, minimum wages, availability of consumer credit, consumer debt levels, interest rates, tax rates and policies, inflation, war and fears of war, inclement weather, natural disasters, terrorism, active shooter situations, outbreak of viruses, illnesses, infectious diseases, contagions and the occurrence of unforeseen epidemics (including the outbreak of the coronavirus and its potential impact on our financial results) and consumer perceptions of personal well-being and security. For example, in recent years, there have been outbreaks of epidemics in various countries, including Malaysia. Recently, there was an outbreak of a novel strain of coronavirus (COVID-19), which has spread rapidly to many parts of the world, including Malaysia. In March 2020, the World Health Organization declared the COVID-19 a pandemic. The epidemic has resulted in intermittent quarantines, travel restrictions, and the temporary closure of stores and facilities in Malaysia.
Substantially all of our revenues are concentrated in Malaysia. Consequently, our results of operations were adversely affected as a result of the implementation of Movement Control Order (MCO) by the Malaysian government. The impact on the company as a result of the MCO includes:
| ● | temporary closure of offices and travel restrictions prevented the company and our distributors from organizing offline events, which in turn stalled our marketing effort; | |
| ● | temporary suspension of product supplies to our distributors and members due supply chain disruption as our suppliers and logistics providers faced disruption and delay in their operation; and |
| 14 |
| ● | the COVID-19 outbreak has resulted in a decline in overall economic environment, which in turn lower the spending power of the consumer and consequently, the revenue of the company. |
Because of the uncertainty surrounding the COVID-19 outbreak, the financial impact related to the outbreak of and response to the coronavirus cannot be reasonably estimated at this time. There is no guarantee that our total revenues will grow or remain at the similar level year over year in the fiscal year 2023. We may have to record downward adjustments or impairment in the fair value of investments in the fiscal year 2023, if conditions have not been significantly improved and global stock markets have not recovered from recent declines.
In general, our business could be adversely affected by the effects of epidemics, pandemic or, including, but not limited to, the COVID-19, avian influenza, severe acute respiratory syndrome (SARS), the influenza A virus, Ebola virus, severe weather conditions such as flood or hazardous air pollution, or other outbreaks. In response to an epidemic, severe weather conditions, or other outbreaks, government and other organizations may adopt regulations and policies that could lead to severe disruption to our daily operations, including temporary closure of our offices and other facilities. These severe conditions may cause us and/or our partners to make internal adjustments, including but not limited to, temporarily closing down business, limiting business hours, and setting restrictions on travel and/or visits with clients and partners for a prolonged period of time. Various impact arising from a severe condition may cause business disruption, resulting in material, adverse impact to our financial condition and results of operations.
We face risks related to health epidemics, severe weather conditions and other outbreaks.
In recent years, there have been outbreaks of epidemics in various countries, including Malaysia. Recently, there was an outbreak of a novel strain of coronavirus (COVID-19), which has spread rapidly to many parts of the world, including Malaysia. In March 2020, the World Health Organization declared the COVID-19 a pandemic. The epidemic has resulted in quarantines, travel restrictions, and the temporary closure of stores and facilities in Malaysia for prolong periods.
Substantially all of our revenues are concentrated in Malaysia. Consequently, our results of operations will likely be adversely, and may be materially, affected, to the extent that the COVID-19 or any other epidemic harms the Malaysia and global economy in general. Any potential impact to our results will depend on, to a large extent, future developments and new information that may emerge regarding the duration and severity of the COVID-19 and the actions taken by government authorities and other entities to contain the COVID-19 or treat its impact, almost all of which are beyond our control. Potential impacts include, but are not limited to, the following:
| ● | temporary closure of offices, travel restrictions, financial impact of our customers or suspension supplies may negatively affect, and could continue to negatively affect, the demand for our products; | |
| ● | our customer may require additional time to pay us or fail to pay us at all, which could significantly increase the amount of accounts receivable and require us to record additional allowances for doubtful accounts. We may have to provide significant sales incentives to our sole customer during the outbreak, which may in turn materially adversely affect our financial condition and operating results; | |
| ● | any disruption of our supply chain, logistics providers or customers could adversely impact our business and results of operations, including causing us or our suppliers to cease manufacturing for a period of time or materially delay delivery to our customers, which may also lead to loss of our customers; and | |
| ● | the global stock markets have experienced, and may continue to experience, significant decline from the COVID-19 outbreak and the marketable securities that we have invested in could be materially adversely affected, which may lead to significant impairment in the fair values of our investments and in turn materially adversely affect our financial condition and operating results. |
Fluctuations in foreign currency exchange rates could have a material adverse effect on our financial results.
We earn revenues, pay expenses, own assets and incur liabilities in countries using currencies other than the U.S. dollar, including Australian Dollars, Malaysian Ringgit and Hong Kong Dollars. Since our consolidated financial statements are presented in U.S. dollars, we must translate revenues, income and expenses, as well as assets and liabilities, into U.S. dollars at exchange rates in effect during or at the end of each reporting period. Therefore, increases or decreases in the value of the U.S. dollar against other currencies affect our net operating revenues, operating income and the value of balance sheet items denominated in foreign currencies. We cannot assure you that fluctuations in foreign currency exchange rates, particularly the strengthening or weakening of the U.S. dollar against major currencies would not materially affect our financial results.
Our business depends on the continued contributions made by Dr. How Kok Choong, as our founder, chief executive officer, chief operating officer, chairman of the board of Directors, Director and secretary, the loss of who may result in a severe impediment to our business.,
Our
success is dependent upon the continued contributions made by our CEO and President, Dr. How Kok Choong. We rely on his expertise
in business operations when we are developing our business. We have no “Key Man” insurance to cover the resulting losses
in the event that any of our officer or directors should die or resign.
If Dr. How Kok Choong cannot serve the Company or is no longer willing to do so, the Company may not be able to find alternatives in a timely manner or at all. This would likely result in a severe damage to our business operations and would have an adverse material impact on our financial position and operating results. To continue as a viable operation, the Company may have to recruit and train replacement personnel at a higher cost. Additionally, if Dr. How Kok Choong joins our competitors or develops similar businesses that are in competition with our Company, our business may also be negatively impacted.
Our future success depends on our ability to attract and retain qualified long-term staff to fill management, technology, sales, marketing, and customer services positions. We have a great need for qualified talent, but we may not be successful in attracting, hiring, developing, and retaining the talent required for our success.
| 15 |
If we are not able to achieve our overall long-term growth objectives, the value of an investment in our Company could be negatively affected.
We have established and publicly announced certain long-term growth objectives. These objectives were based on, among other things, our evaluation of our growth prospects, which are generally driven by the sales potential of many product types, some of which are more profitable than others, and on an assessment of the potential price and product mix. There can be no assurance that we will realize the sales potential and the price and product mix necessary to achieve our long-term growth objectives.
We may incur losses resulting from product liability claims or product recalls or adverse publicity relating to our products.
We may incur losses resulting from product liability claims with respect to our products supplied by our suppliers. We may face claims or liabilities which may arise if there exist any defects in quality of these products or any of these products are deemed or proven to be unsafe, defective or contaminated. In the event that the use or misuse of any product distributed by us results in personal injury or death, product liability and/or indemnity claims may be brought against us, in addition to our product recalls, and the relevant regulatory authorities in the market we operate may close down some of our related operations and take administrative actions against us. If we experience any business disruption and litigation, we may incur additional costs and have to divert our management’s attention and resources on such matters, which may materially and adversely affect our business, financial condition and results of operations.
We had previously relied on the variable interest entity, Agape S.E.A. Sdn Bhd, in Malaysia for our business operations, which may not be as effective in providing operational control or enabling us to derive economic benefits as through ownership of controlling equity interests. While we no longer rely on Agape S.E.A. Sdn Bhd for our operations, we may do so in the future.
Agape S.E.A. Sdn Bhd’s equity at risk was insufficient to finance its business activities and it provided all of the Company’s purchases during the fiscal years ended December 31, 2020 and 2019. For the six months ended June 30, 2023, Agape S.E.A. Sdn Bhd did not provide any purchase to the Company. As a result, it is considered to be a variable interest entity (“VIE”) and the Company is the primary beneficiary since it has both of the following characteristics, (a) the power to direct the activities of the VIE that most significantly impact the VIE’s economic performance; and (b) the obligation to absorb losses of the VIE that could potentially be significant to the VIE or the right to receive benefits from the VIE that could potentially be significant to the VIE. However, the Company no longer relied on the VIE after the fiscal year ended December 31, 2020. For the years ended December 31, 2022 and 2021, Agape S.E.A. Sdn Bhd did not provide any purchase to the Company. In addition, Agape S.E.A.’s impact to our consolidated financial statements constitutes less than 1% of our total consolidated assets. While the Company have not made any purchases from the VIE for the six month ended June 30, 2023, we may expect to continue to rely on ASL’s beneficiary ownership structure with Agape S.E.A. to operate our business. If we fail to continue our beneficiary ownership structure with Agape S.E.A. in the future, it could have a material adverse effect on our financial condition and results of operations.
| 16 |
Risks Related to Doing Business in Malaysia
Developments in the social, political, regulatory and economic environment in Malaysia may have a material adverse impact on us.
Our business, prospects, financial condition and results of operations may be adversely affected by social, political, regulatory and economic developments in Malaysia. Such political and economic uncertainties include, but are not limited to, the risks of war, terrorism, nationalism, nullification of contract, changes in interest rates, imposition of capital controls and methods of taxation.
Negative developments in Malaysia’s socio-political environment may adversely affect our business, financial condition, results of operations and prospects. The Malaysian economy registered modest growth of approximately 8.7% and 3.1% in 2022 and 2021 respectively, according to the Department of Statistics Malaysia. Although the overall Malaysian economic environment (in which we predominantly operate) appears to be positive, there can be no assurance that this will continue to prevail in the future. Economic growth is determined by countless factors, and it is extremely difficult to predict with any level of absolute certainty.
Furthermore, on March 11, 2020, the World Health Organization or WHO declared the corona virus or COVID-19 a pandemic. To help counter the transmission of COVID-19, from March 18, 2020 to April 26, 2022, the government of Malaysia initiated (i) Movement control orders (“MCO”). The MCO had resulted in quarantines, travel restrictions, and the temporary closure of stores and facilities in Malaysia. (ii) Conditional Movement Control Order (“CMCO”) where most business sectors were allowed to operate under strict rules and Standard Operating Procedures mandated by the government of Malaysia. (iii) Recovery Movement Control Order (“RMCO”). At the height of the pandemic, on January 12, 2021, the Malaysian government even declared a state of emergency nationwide to combat COVID-19. On April 27, 2022, the Malaysian government announced the country had entered into the endemic phase with further easing of restrictions. We are witnessing the adverse impact on the purchasing power of consumers in Malaysia, where our products are mainly sold as a direct result of the prolonged pandemic. As such, the extent to which the coronavirus may continue to adversely impact the Malaysian economy is uncertain. In the event that the Malaysia economy suffers, demand for our products may diminish, which would in turn result in our profitability. This could in turn result in a substantial need for restructuring of our business objectives and could result in a partial or entire loss of an investment in our Company.
We are subject to foreign exchange control policies in Malaysia.
The ability of our subsidiaries to pay dividends or make other payments to us may be restricted by the foreign exchange control policies in the countries where we operate. For example, there are foreign exchange policies in Malaysia which support the monitoring of capital flows into and out of the country in order to preserve its financial and economic stability. The foreign exchange policies are administered by the Foreign Exchange Administration, an arm of Bank Negara Malaysia (“BNM”), the central bank of Malaysia. The foreign exchange policies monitor and regulate both residents and non-residents. Under the current Foreign Exchange Administration rules issued by BNM, non-residents are free to repatriate any amount of funds from Malaysia in foreign currency other than the currency of Israel at any time (subject to limited exceptions), including capital, divestment proceeds, profits, dividends, rental, fees and interest arising from investment in Malaysia, subject to any withholding tax. In the event BNM or any other country where we operate introduces any restrictions in the future, we may be affected in our ability to repatriate dividends or other payments from our subsidiaries in Malaysia or in such other countries. Since we are a holding company and rely principally on dividends and other payments from our subsidiaries for our cash requirements, any restrictions on such dividends or other payments could materially and adversely affect our liquidity, financial condition and results of operations.
Economic, market and political developments in the countries where we operate could have a material and adverse effect on our business.
As with all organizations that seek to reduce business risks via geographical expansion, the economic, market and political conditions in other countries, particularly emerging market conditions in Southeast Asia, could have an influence on our business. Any widespread global financial instability or a significant loss of investor confidence in emerging market economies may materially and adversely affect our business, financial condition, results of operations, prospects or reputation.
Examples of such external factors or conditions that are outside our control include, but are not limited to the following:
| ● | general economic, political and social conditions in Southeast Asian markets; |
| 17 |
| ● | consumer spending patterns in our key markets; | |
| ● | currency and interest rate fluctuations; | |
| ● | international events and circumstances such as wars, terrorist attacks, natural disasters and political instability; and | |
| ● | changes in legal regimes and governmental regulations, such as licensing and approvals, taxation, duties and tariffs, in key markets and abroad. |
For example, the global financial markets experienced significant disruptions in 2008 and the United States, Europe and other economies went into recession. The recovery from the lows of 2008 and 2009 was uneven and the global economy has continued to face new challenges. There is considerable uncertainty over the long-term effects of the expansionary monetary and fiscal policies that have been adopted by the central banks and financial authorities of some of the world’s leading economies, including the United States. For example, in 2013, the Federal Reserve Bank in the United States announced the tapering of its bond-buying program which led to a high degree of volatility in equity markets and substantial devaluations in the currencies of many emerging economies, including markets where we operate. Economic conditions in the countries where we operate might be sensitive to global economic conditions, as well as changes in domestic economic and political policies and the expected or perceived overall economic growth rate in emerging markets. We are witnessing the adverse impact on the purchasing power of consumers in Malaysia, where our products are mainly sold, as a direct result of these worldwide developments.
Management and Governance Risks
Risks Related to our Common Stock and this Offering
Volatility in our shares price may subject us to securities litigation.
The market for our shares may have, when compared to seasoned issuers, significant price volatility and we expect that our share price may continue to be more volatile than that of a seasoned issuer for the indefinite future. In the past, plaintiffs have often initiated securities class action litigation against a company following periods of volatility in the market price of its securities. We may, in the future, be the target of similar litigation. Securities litigation could result in substantial costs and liabilities and could divert management’s attention and resources.
We may never be able to pay dividends and are unlikely to do so.
To date, we have not paid, nor do we intend to pay in the foreseeable future, dividends on our common stock, even if we become profitable. Earnings, if any, are expected to be used to advance our activities and for working capital and general corporate purposes, rather than to make distributions to stockholders. Since we are not in a financial position to pay dividends on our common stock and future dividends are not presently being contemplated, investors are advised that return on investment in our common stock is restricted to an appreciation in the share price. The potential or likelihood of an increase in share price is uncertain.
| 18 |
In addition, under Nevada law, we may only pay dividends subject to our ability to service our debts as they become due and provided that our assets will exceed our liabilities after the dividend. Our ability to pay dividends will therefore depend on our ability to generate sufficient profits. Furthermore, because of the various rules applicable to our operations in Malaysia and the regulations on foreign investments as well as the applicable tax law, we may be subject to further limitations on our ability to declare and pay dividends to our stockholders.
Stockholders may be diluted significantly through our efforts to obtain financing and satisfy obligations through the issuance of securities.
Wherever possible, our board of directors will attempt to use non-cash consideration to satisfy obligations. In many instances, we believe that the non-cash consideration will consist of shares of our common stock, warrants to purchase shares of our common stock or other securities. In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our stockholders. We are authorized to issue an aggregate of 1,000,000,000 shares of common stock and 200,000,000 shares of preferred stock. We may issue additional shares of common stock or other securities that are convertible into or exercisable for our common stock in connection with hiring or retaining employees, future acquisitions, future sales of our securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of our common stock may create downward pressure on the trading price of the common stock. We expect we will need to raise additional capital in the near future to meet our working capital needs, and there can be no assurance that we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with these capital raising efforts, including at a price (or exercise prices) below the price you paid for your stock.
We are a “smaller reporting company,” and we cannot be certain if the reduced disclosure requirements applicable to smaller reporting companies will make our common stock less attractive to investors.
We are currently a “smaller reporting company”, meaning that we are not an investment company, an asset- backed issuer, or a majority-owned subsidiary of a parent company that is not a smaller reporting company and annual revenues of less than $50.0 million during the most recently completed fiscal year. In the event that we are still considered a “smaller reporting company,” at such time as we cease being an “emerging growth company,” we will be required to provide additional disclosure in our SEC filings. However, similar to an “emerging growth companies”, “smaller reporting companies” are able to provide simplified executive compensation disclosures in their filings; are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting; and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. Decreased disclosures in our SEC filings due to our status as a “smaller reporting company” may make it harder for investors to analyze our results of operations and financial prospects.
| 19 |
The offering price of our shares of common stock offered in the Resale Prospectus Resale is fixed.
The selling stockholders of the Resale Prospectus will offer and sell their shares of common stock being offered under the Resale Prospectus at $6.50 per share for the duration of the offering or until the shares are listed on a national securities exchange at which time the shares offered under the Resale Prospectus may be sold at prevailing market prices or privately negotiated prices or in transactions that are not in the public market. We have applied to list our common stock on the NASDAQ Capital Market (“NASDAQ”) under the symbol “ATPC.” No assurance can be given that our application will be approved. The closing of this offering is contingent upon the successful listing of our common stock on the Nasdaq Capital Market.
We plan to list our common stock on NASDAQ. We may not be able to maintain our listing on NASDAQ which could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions.
We have applied to list our common stock on the NASDAQ Capital Market (“NASDAQ”) under the symbol “ATPC”. Even if our common stock is approved to be listed on NASDAQ, we cannot assure you that our common stock will continue to be listed on NASDAQ in the future. In order to continue listing our securities on NASDAQ, we must maintain certain financial, distribution and share price levels. Moreover, we must comply with certain listing standards regarding the independence of our board of directors and members of our audit committee. We intend to fully comply with these requirements, but we may not continue to be able to meet these requirements in the future.
If NASDAQ delists our securities from trading on its exchange and we are not able to list our securities on another national securities exchange, we expect our securities could be quoted on an over-the-counter market. If this were to occur, we could face significant material adverse consequences, including:
| ● | a limited availability of market quotations for our securities; | |
| ● | reduced liquidity for our securities; | |
| ● | a determination that our common stock is a “penny stock” which will require brokers trading in our common stock to adhere to more stringent rules and possibly result in a reduced level of trading activity in the secondary trading market for our securities; | |
| ● | a limited amount of news and analyst coverage; and | |
| ● | a decreased ability to issue additional securities or obtain additional financing in the future. |
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as “covered securities.” Because we expect that our common stock will be listed on NASDAQ, such securities will be covered securities. Although the states are preempted from regulating the sale of our securities, the federal statute does allow the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. Furthermore, if we were no longer listed on NASDAQ, our securities would not be covered securities and we would be subject to regulations in each state in which we offer our securities.
The price of our common stock may rapidly fluctuate or may decline regardless of our operating performance, resulting in substantial losses for investors.
The trading price of our common stock following this offering may be subject to instances of extreme stock price run-ups followed by rapid price declines and stock price volatility unrelated to both our actual and expected operating performance and financial condition or prospects, making it difficult for prospective investors to assess the rapidly changing value of our stock. Further, the trading price of our common stock following this offering is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control, including limited trading volume, actual or anticipated fluctuations in our results of operations; the financial projections we may provide to the public, any changes in these projections or our failure to meet these projections; failure of securities analysts to initiate or maintain coverage of our Company, changes in financial estimates or ratings by any securities analysts who follow our Company or our failure to meet these estimates or the expectations of investors; announcements by us or our competitors of significant innovations, acquisitions, strategic partnerships, joint ventures, operating results or capital commitments; changes in operating performance and stock market valuations of other companies in our industry; price and volume fluctuations in the overall stock market, including as a result of trends in the economy as a whole; changes in our Board or management; sales of large blocks of our common stock, including sales by our executive officers, directors and significant stockholders; lawsuits threatened or filed against us; changes in laws or regulations applicable to our business; the expiration of lock-up agreements; changes in our capital structure, such as future issuances of debt or equity securities; short sales, hedging and other derivative transactions involving our capital stock; general economic and geopolitical conditions, including the current or anticipated impact of military conflict and related sanctions imposed on Russia by the United States and other countries due to Russia’s recent invasion of Ukraine; and the other factors described in this section of the prospectus captioned “Risk Factors.”
Certain recent initial public offerings of companies with relatively small public floats have experienced extreme volatility that was seemingly unrelated to the underlying performance of the respective company. Our common stock may potentially experience rapid and substantial price volatility, which may make it difficult for prospective investors to assess the value of our common stock.
In addition to the risks addressed above under “the price of our common stock may rapidly fluctuate or may decline regardless of our operating performance, resulting in substantial losses for investors,” our common stock may be subject to rapid and substantial price volatility. We may experience extreme stock price volatility unrelated to our actual or expected operating performance, financial condition or prospects, making it difficult for prospective investors to assess the rapidly changing value of our common stock. Recently, there have been instances of extreme stock price run-ups followed by rapid price declines and strong stock price volatility with a number of recent initial public offerings, especially among companies with relatively smaller public floats. As a relatively small-capitalization company, we may experience greater stock price volatility, extreme price run-ups, lower trading volume and less liquidity than large-capitalization companies. In particular, our common stock may be subject to rapid and substantial price volatility, low volumes of trades and large spreads in bid and ask prices. Such volatility, including any stock-run up, may be unrelated to our actual or expected operating performance, financial condition or prospects, making it difficult for prospective investors to assess the rapidly changing value of our common stock.
In addition, if the trading volumes of our common stock are low, persons buying or selling in relatively small quantities may easily influence prices of our common stock. This low volume of trades could also cause the price of our common stock to fluctuate greatly, with large percentage changes in price occurring in any trading day session. Holders of our common stock may also not be able to readily liquidate their investment or may be forced to sell at depressed prices due to low volume trading. Broad market fluctuations and general economic and political conditions may also adversely affect the market price of our common stock. As a result of this volatility, investors may experience losses on their investment in our common stock. A decline in the market price of our common stock also could adversely affect our ability to issue additional common stock or other securities and our ability to obtain additional financing in the future. No assurance can be given that an active market in our common stock will develop or be sustained. If an active market does not develop, holders of our common stock may be unable to readily sell the shares they hold or may not be able to sell their shares at all.
| 20 |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements, including, without limitation, in the sections captioned “Risk Factors”, “Management’s Discussion and Analysis of Financial Condition and Plan of Operations”, and “Business”. Known and unknown risks, uncertainties and other factors, including those listed under “Risk Factors,” may cause our actual results, performance or achievements to be materially different from those expressed or implied by the forward-looking statements.
You can identify some of these forward-looking statements by words or phrases such as “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “is/are likely to,” “potential,” “continue” or other similar expressions. We have based these forward-looking statements largely on our current expectations and projections about future events that we believe may affect our financial condition, results of operations, business strategy and financial needs. These forward-looking statements include statements relating to:
| ● | Our goals and strategies; | |
| ● | Our future business development, financial conditions and results of operations; | |
| ● | Our expectations regarding demand for and market acceptance of our products and services; | |
| ● | Our ability to attract and retain management; | |
| ● | Our ability to raise capital when needed and on acceptable terms and conditions; | |
| ● | The intensity of competition; | |
| ● | General economic conditions; | |
| ● | Changes in regulations; | |
| ● | Relevant government policies and regulations relating to our industry; | |
| ● | Whether the market for healthcare services continues to grow, and, if it does, the pace at which it may grow; | |
| ● | Our ability to compete against large competitors in a rapidly changing market; and | |
| ● | Our ability to comply with the continued listing standards on the exchange or trading market on which our common stock is listed for trading; and | |
| ● | The impact of COVID-19 on business environment and consumer preference. |
These forward-looking statements involve various risks and uncertainties. Although we believe that our expectations expressed in these forward-looking statements are reasonable, our expectations may later be found to be incorrect. Our actual results could be materially different from our expectations. Important risks and factors that could cause our actual results to be materially different from our expectations are generally set forth in “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Business” and other sections in this prospectus. You should thoroughly read this prospectus and the documents that we refer to with the understanding that our actual future results may be materially different from and worse than what we expect. We qualify all of our forward-looking statements by these cautionary statements.
This prospectus contains certain data and information that we obtained from private publications. Statistical data in these publications also include projections based on a number of assumptions. Our industry may not grow at the rate projected by market data, or at all. Failure of this market to grow at the projected rate may have a material and adverse effect on our business and the market price of our common stock. In addition, the rapidly changing nature of the health and wellness industry results in significant uncertainties for any projections or estimates relating to the growth prospects or future condition of our market. Furthermore, if any one or more of the assumptions underlying the market data are later found to be incorrect, actual results may differ from the projections based on these assumptions. You should not place undue reliance on these forward-looking statements.
The forward-looking statements made in this prospectus relate only to events or information as of the date on which the statements are made in this prospectus. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this prospectus and the documents that we refer to in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect.
| 21 |
USE OF PROCEEDS
We estimate that we will receive net proceeds from this offering of approximately $6.26 million, or approximately $7.24 million if the Underwriter exercises its over-allotment option in full, after deducting underwriting discounts, non-accountable expense allowance and the estimated offering expenses payable by us.
The primary purposes of this offering are to create a public market of our shares for the benefit of all stockholders, retain talented employees, and obtain additional capital. We plan to use the net proceeds of this offering as follows:
| ● | approximately 15% for strengthening sales and marketing of our products, services and branding, including further development and promotion of our e-trading platform; | |
| ● | approximately 40% for research and development (“R&D”) and technological development, including further research on enhancements of components in our current product and service offerings and the construction of a e-trading platform; | |
| ● | approximately 20% for expanding operations into ASEAN and US markets, including expansion of our market share in Indonesia, Singapore and Thailand, collaborations with US companies in terms of product R&D and expansion of e-commerce operations to target US consumers; | |
| ● | approximately 20% for future vertical and horizontal integrations, including strategic collaborations, mergers & acquisitions of insurance and health care service providers. We will further invest into production resources allowing us to produce our own products, ensuring supply and quality while reducing costs. In relation to the mergers and acquisitions, it will be mainly for our development as a comprehensive wellness ecosystem company. We have identified targets companies matching the following criteria: (i) with profitability and customer base comprising customers from the health care industry; and (ii) with established knowledge base of empirical/holistic skills, knowledge and technologies which are applicable to transform our company into a wellness ecosystem company such as skin care, cosmetic bio lab production, wellness center or complementary medical therapies for chronic health problems, manufacturers of water filtration system, etc.; and | |
| ● | the remainder for working capital and general corporate purposes, including legal, accounting and other professional fees associated with becoming a public company, general and administrative expenses associated with increased operations, and recruitment of talent associated with increase operations. |
The amounts and timing of our actual expenditures will depend on numerous factors, including the factors described under “Risk Factors.” The foregoing represents our current intentions based upon our present plans and business conditions to use and allocate the net proceeds of this offering. Our management, however, will have significant flexibility and discretion to apply the net proceeds of this offering. If an unforeseen event occurs or business conditions change, we may use the proceeds of this offering differently than as described in this prospectus.
| 22 |
DIVIDEND POLICY
We have never declared or paid any cash dividends on our common stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. We do not intend to pay cash dividends to holders of our common stock in the foreseeable future.
| 23 |
CAPITALIZATION
The following table describes our cash and our capitalization as of June 30, 2023:
| ● | on an actual basis; and | |
| ● | on an as adjusted basis to reflect our receipt of the net proceeds from this offering after deducting the underwriting discounts, non-accountable expense allowance and estimated offering expenses payable by us. |
The as adjusted information below is illustrative only and our capitalization following the completion of this offering is subject to adjustment based on the public offering price of our common stock and other terms of this offering determined at pricing. In addition, except for the last column in the first table below, the tables below assume that the Underwriter over-allotment option has not been exercised. You should read this capitalization table together with our consolidated financial statements and the related notes appearing elsewhere in this prospectus and the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section and other financial information included elsewhere in this prospectus.
| Actual | Pro Forma Adjusted for IPO(1) (2) | Pro Forma Adjusted for IPO including Over- allotment(3) | ||||||||||
| Equity: | ||||||||||||
| Preferred stock, $0.0001 par value; 200,000,000 shares authorized; no shares issued and outstanding, actual and as adjusted | $ | - | $ | - | $ | - | ||||||
| Common stock, $0.0001 par value; 1,000,000,000 shares authorized; 75,452,012, 76,552,012 and 76,717,012 shares issued and outstanding – actual, pro forma adjusted for IPO and pro forma adjusted for IPO including over-allotment | 7,545 | 7,655 | 7,672 | |||||||||
| Additional paid in capital | 6,470,716 | 12,208,272 | 13,184,230 | |||||||||
| Accumulated deficit | (5,746,112 | ) | (5,746,112 | ) | (5,746,112 | ) | ||||||
| Accumulated other comprehensive income | 11,612 | 11,612 | 11,612 | |||||||||
| Non-controlling interests | 6,893 | 6,893 | 6,893 | |||||||||
| Total stockholders’ equity | $ | 750,654 | $ | 6,488,320 | $ | 7,464,295 | ||||||
(1) Gives effect to the sale of common stock at a public offering price of $6.50 per share and to reflect the application of the proceeds after deducting our estimated offering expenses.
(2) Pro forma adjusted for IPO additional paid in capital reflects the net proceeds we expect to receive, after deducting the Underwriter discount of 8%, non-accountable expense allowance of 1% and other expenses (all the accountable expenses). We expect to receive net proceeds of $6,259,728 ($7,150,000 offering, less underwriting fee of $572,000 non-accountable expenses of $71,500 and other offering expenses of $768,834, including $522,062 which has already been paid by the Company). For an itemization of an estimation of the total offering expenses, see “Item 13. Other Expenses of issuance and Distribution” beginning on page II-1 of this prospectus.
(3) Pro forma adjusted for IPO additional paid in capital including the Underwriter’s over-allotment option reflects the net proceeds we expect to receive after the under exercise the over-allotment option in full and after deducting the underwriting discount of 8%, non-accountable expense allowance of 1% and other expenses (all the accountable expenses). We expect to receive net proceeds of $7,235,703 ($8,222,500 offering, less underwriting fee of $657,800, non-accountable expenses of $82,225 and other offering expenses of $768,834, including $522,062 which has already been paid by the Company). For an itemization of an estimation of the total offering expenses, see “Item 13. Other Expenses of issuance and Distribution” beginning on page II-1 of this prospectus.
| 24 |
DILUTION
If you invest in our common stock in this offering, your ownership interest will be diluted immediately to the extent of the difference between the public offering price per share of our common stock and the as adjusted net tangible book value per share of our common stock immediately after this offering.
Dilution results from the fact that the per share offering price is substantially in excess of the book value per share of common stock attributable to the existing stockholders for our presently outstanding shares of common stock. Net tangible book value per share is determined by dividing our total tangible assets less our total liabilities by the number of shares of our common stock outstanding. Our historical net tangible book value as of June 30, 2023, was $(82,699) or $(0.00) per share.
Our post offering as adjusted net tangible book value, which gives effect to receipt of the net proceeds from the offering and issuance of additional shares in the offering but does not take into consideration any other changes in our net tangible book value after June 30, 2023, will be approximately $7,153,004 or approximately $0.09 per share. This would result in dilution to investors in this offering of approximately $6.41 per share or approximately 98.57% from the assumed offering price of $6.50 per share. Net tangible book value per share would increase to the benefit of present stockholders by $0.09 per share attributable to the purchase of the shares by investors in this offering.
The following table sets forth the estimated net tangible book value per share after the offering and the dilution to persons purchasing shares.
| Offering(1) | Full Over- allotment Post-offering(2) |
|||||||
| Assumed offering price per common stock | $ | 6.50 | $ | 6.50 | ||||
| Net tangible book value per common stock as of June 30, 2023 | $ | (0.00 | ) | $ | (0.00 | ) | ||
| Increase in net tangible book value per share after this offering | $ | 0.08 | $ | 0.09 | ||||
| Net tangible book value per common stock after the offering | $ | 0.08 | $ | 0.09 | ||||
| Dilution per common stock to new investors | $ | 6.42 | $ | 6.41 | ||||
| Dilution per common stock to new investors (%) | 98.76 | % | 98.57 | % | ||||
| (1) | Assumes gross proceeds from offering of 1,100,000 shares of common stock. |
| (2) | Assumes gross proceeds from offering of 1,265,000 shares of common stock, if over-allotment option is exercised in full. |
The following chart illustrates our pro forma proportionate ownership, upon completion of the offering, by present stockholders and investors in this offering, compared to the relative amounts paid by each. The charts reflect payment by present stockholders as of the date the consideration was received and by investors in this offering at the offering price without deduction of the estimated underwriting discount, non-accountable expense allowance and our estimated offering expenses. The charts further assume no changes in net tangible book value other than those resulting from the offering.
| Shares Purchased | Total Consideration | Average Price | ||||||||||||||||||
| Number | Percentage | Amount | Percentage | Per Share | ||||||||||||||||
| New investors(1) | 1,100,000 | 1.44 | % | $ | 7,150,000 | 52.46 | % | $ | 6.50 | |||||||||||
| Existing stockholders | 75,452,012 | 98.56 | % | $ | 6,478,261 | 47.54 | % | $ | 0.09 | |||||||||||
| Total | 76,552,012 | 100.00 | % | $ | 13,628,261 | 100.00 | % | $ | 0.18 | |||||||||||
| (1) | Assuming the offering is fully subscribed. |
| 25 |
SELECTED CONSOLIDATED FINANCIAL DATA
AGAPE ATP CORPORATION
The following table presents selected consolidated financial data for the periods and at the dates indicated. The selected condensed consolidated statements of operations data for the three and six months ended June 30, 2023 and 2022, and the selected condensed consolidated balance sheet data as of June 30, 2023 and December 31, 2022 have been derived from our condensed consolidated financial statements, included elsewhere in this prospectus. The selected consolidated statements of operations data for the years ended December 31, 2022 and 2021, and the selected consolidated balance sheet data as of December 31, 2022 and 2021 have been derived from our consolidated financial statements, included elsewhere in this prospectus. Our historical results for any prior period are not necessarily indicative of results to be expected in any future period, and our results for any interim period are not necessarily indicative of the results expected for a full fiscal year.
You should read the following financial information together with the information under “Agape ATP Corporation Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes included elsewhere in this prospectus.
Consolidated Statements of Operations Data:
| For the Three Months Ended June 30, | ||||||||
| 2023 | 2022 | |||||||
| Revenue | $ | 303,935 | $ | 396,707 | ||||
| Cost of revenue | (107,931 | ) | (109,383 | ) | ||||
| Gross profit | 196,004 | 287,324 | ||||||
| Selling, general and administrative expenses | (555,537 | ) | (593,507 | ) | ||||
| Loss from operations | (359,533 | ) | (306,183 | ) | ||||
| Other expenses, net | (22,355 | ) | (97,769 | ) | ||||
| Benefit of (Provision for) income taxes | 2,439 | (392 | ) | |||||
| Net loss | (379,449 | ) | (404,344 | ) | ||||
| Net (loss) income attributable to non-controlling interests | (4,763 | ) | 10,556 | |||||
| Net loss attributable to Agape ATP Corporation | $ | (374,686 | ) | $ | (414,900 | ) | ||
| Net loss | $ | (379,449 | ) | $ | (404,344 | ) | ||
| Other comprehensive income (loss) | 269 | (61,156 | ) | |||||
| Comprehensive loss | $ | (379,180 | ) | $ | (465,500 | ) | ||
| Less: Comprehensive loss attributable to non-controlling interests | (5,401 | ) | (271 | ) | ||||
| Comprehensive loss attributable to Agape ATP Corporation | $ | (373,779 | ) | $ | (465,229 | ) | ||
| Loss per share – (basic and diluted) | $ | (0.00 | ) | $ | (0.01 | ) | ||
| Weighted average number of common shares outstanding (basic and diluted) | 75,452,012 | 75,452,012 | ||||||
| For
the Six Months Ended June 30, |
||||||||
| 2023 | 2022 | |||||||
| Revenue | $ | 684,703 | $ | 805,667 | ||||
| Cost of revenue | (236,289 | ) | (182,814 | ) | ||||
| Gross profit | 448,414 | 622,853 | ||||||
| Selling, general and administrative expenses | (1,261,831 | ) | (1,201,268 | ) | ||||
| Loss from operations | (813,417 | ) | (578,415 | ) | ||||
| Other expenses, net | (6,762 | ) | (115,695 | ) | ||||
| Benefit of (Provision for) income taxes | 6,655 | (8,680 | ) | |||||
| Net loss | (813,524 | ) | (702,790 | ) | ||||
| Net (loss) income attributable to non-controlling interests | (12,998 | ) | 11,207 | |||||
| Net loss attributable to Agape ATP Corporation | $ | (800,526 | ) | $ | (713,997 | ) | ||
| Net loss | $ | (813,524 | ) | $ | (702,790 | ) | ||
| Other comprehensive income (loss) | 2,346 | (73,179 | ) | |||||
| Comprehensive loss | $ | (811,178 | ) | $ | (775,969 | ) | ||
| Less: Comprehensive loss attributable to non-controlling interests | (13,619 | ) | (270 | ) | ||||
| Comprehensive loss attributable to Agape ATP Corporation | $ | (797,559 | ) | $ | (775,699 | ) | ||
| Loss per share – (basic and diluted) | $ | (0.01 | ) | $ | (0.01 | ) | ||
| Weighted average number of common shares outstanding (basic and diluted) | 75,452,012 | 100,397,696 | ||||||
| For the Years Ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Revenue | $ | 1,856,564 | $ | 1,016,962 | ||||
| Cost of revenue | (666,042 | ) | (297,333 | ) | ||||
| Gross profit | 1,190,522 | 719,629 | ||||||
| Selling, general and administrative expenses | (2,723,788 | ) | (2,578,197 | ) | ||||
| Loss from operations | (1,533,266 | ) | (1,858,568 | ) | ||||
| Other expenses, net | (136,868 | ) | (529,045 | ) | ||||
| Benefit of (Provision) for income taxes | 4,055 | (137,067 | ) | |||||
| Net loss | (1,666,079 | ) | (2,524,680 | ) | ||||
| Net (income) loss attributable to non-controlling interests | (20,820 | ) | 436 | |||||
| Net loss attributable to Agape ATP Corporation | $ | (1,686,899 | ) | $ | (2,524,244 | ) | ||
| Net loss | $ | (1,666,079 | ) | $ | (2,524,680 | ) | ||
| Other comprehensive loss | (84,132 | ) | (87,615 | ) | ||||
| Comprehensive loss | $ | (1,750,211 | ) | $ | (2,612,295 | ) | ||
| Less: Comprehensive income (loss) attributable to non-controlling interests | 20,849 | (433 | ) | |||||
| Comprehensive loss attributable to Agape ATP Corporation | $ | (1,771,060 | ) | $ | (2,611,862 | ) | ||
| Net loss per share – (basic and diluted) | $ | (0.02 | ) | $ | (0.01 | ) | ||
| Weighted average number of common shares outstanding (basic and diluted) | 87,822,337 | 376,216,452 | ||||||
Consolidated Balance Sheets Data:
| As of | ||||||||||||
| June
30, 2023 |
December
31, 2022 |
December
31, 2021 |
||||||||||
| Current assets | $ | 941,436 | $ | 2,028,534 | $ | 3,912,122 | ||||||
| Total assets | $ | 1,905,283 | $ | 2,791,749 | $ | 4,724,535 | ||||||
| Current liabilities | $ | 968,329 | $ | 1,229,295 | $ | 1,312,841 | ||||||
| Total liabilities | $ | 1,154,629 | $ | 1,229,295 | $ | 1,411,899 | ||||||
| Total equity | $ | 750,654 | $ | 1,562,454 | $ | 3,312,636 | ||||||
| 26 |
AGAPE ATP CORPORATION
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITIONS AND
RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with the section headed “Selected Consolidated Financial and Operating Data” and our consolidated financial statements and the related notes included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Company Overview
The Company and its subsidiaries are principally engaged in the health and wellness industry. The principal activity of the Company is to supply high-quality health and wellness products, including supplements to assist in cell metabolism, detoxification, blood circulation, anti-aging and products designed to improve the overall health system of the human body and various wellness programs.
Agape ATP Corporation, a Nevada corporation (“the Company”) was incorporated under the laws of the State of Nevada on June 1, 2016.
Agape ATP Corporation operates through its subsidiaries, namely, Agape ATP Corporation, a company incorporated in Labuan, Malaysia, and Agape Superior Living Sdn. Bhd. (“ASL”), a company incorporated in Malaysia.
Agape ATP Corporation is an investment holding company that holds 100% of the equity interest in Agape ATP International Holding Limited, a company incorporated in Hong Kong.
On May 8, 2020, the Company entered into a Share Exchange Agreement with Dr. How Kok Choong, CEO and director of the Company to acquire 9,590,596 ordinary shares, no par value, equivalent to approximately 99.99% of the equity interest in ASL.
On September 11, 2020, the Company incorporated WATP, a wholly owned subsidiary under the laws of Malaysia, to pursue the business of promoting wellness and wellbeing lifestyle of the community by providing services that includes online editorials, programs, events and campaigns on how to achieve positive wellness and lifestyle. On September 15, 2020, WATP entered into a business collaboration agreement with ASL to carry out certain wellness programs.
On November 11, 2021, Agape ATP Corporation (Labuan) formed a joint-venture entity, DSY Wellness International Sdn. Bhd. (“DSY Wellness”) with Mr. Steve Yap. to pursue the business of providing complementary health therapies. Agape ATP Corporation (Labuan) owns 60% of the equity interests in DSY Wellness.
Agape ATP Corporation is a company that provides health and wellness products and health solution advisory services to our clients. The Company primarily focuses its efforts on attracting customers in Malaysia. Its advisory services center on the “ATP Zeta Health Program”, which is a health program designed to prevent diseases caused by polluted environments, unhealthy dietary intake and unhealthy lifestyles, and the promotion of health. The program aims to promote improved health and longevity through a combination of modern medicine, proper nutrition and advice from skilled nutritionists and/or dieticians.
| 27 |
In order to strengthen the Company’s supply chain, on May 8, 2020, the Company successfully acquired approximately 99.99% of ASL, with the goal of securing an established network marketing sales channel that has been established in Malaysia for the past 18 years. ASL has been offering the Company’s ATP Zeta Health Program as part of its product lineup. As such, the acquisition creates synergy in the Company’s operation by boosting the Company’s retail and marketing capabilities. The acquired subsidiary allows the Company to fulfill its mission of “helping people to create health and wealth” by providing a financially rewarding business opportunity to distributors and quality products to distributors and customers who seek a healthy lifestyle.
Via ASL, the Company offers three series of programs which consist of different services and products: ATP Zeta Health Program, ÉNERGÉTIQUE and BEAUNIQUE.
The ATP Zeta Health Program is a health program designed to promote health and general wellbeing designed to prevent health diseases caused by polluted environments, unhealthy dietary intake and unhealthy lifestyles. The program aims to promote improved health and longevity through a combination of modern health supplements, proper nutrition and advice from skilled dieticians as well as trained members and distributors.
The ÉNERGÉTIQUE series aims to provide a total dermal solution for a healthy skin beginning from the cellular level. The series is comprised of the Energy Mask series, Hyaluronic Acid Serum and Mousse Facial Cleanser.
The BEAUNIQUE product series focuses on the research of our diet’s impact on modifying gene expressions in order to address genetic variations and deliver a nutrigenomic solution for every individual.
The Company deems creating public awareness on wellness and wellbeing lifestyle as essential to enhance the provision of its health solution advisory services; and therefore incorporated WATP in September 2020. Upon its establishment, WATP started collaborating with ASL to carry out various wellness programs.
To further its reach in the Health and Wellness Industry, on November 11, 2021, Agape ATP Corporation (Labuan) formed a joint-venture entity, DSY Wellness International Sdn. Bhd. (“DSY Wellness”) with Mr. Steve Yap to pursue the business of providing complementary health therapies. Agape ATP Corporation (Labuan) owns 60% of the equity interests in DSY Wellness.
Results of Operation
For the three months ended June 30, 2023 and 2022
Revenue
We generated revenue of $303,935, which comprised revenue from the Company’s network marketing business of $90,662 (approximately 29.8%); and revenue from the Company’s operations in the provision of complementary health therapies of $213,273 (approximately 70.2%) for the three months ended June 30, 2023 as compared to $396,707, which comprised revenue from the Company’s network marketing business of $184,534 (approximately 46.5%); and revenue from the Company’s operations in the provision of complementary health therapies of $212,173 (approximately 53.5%) for the three months ended June 30, 2022. Revenue from the Company’s network marketing business decreased significantly by $93,872, or approximately 50.9%. Revenue from the Company’s operations in the provision of complementary health therapies increased marginally by $1,100, or approximately 0.5%. Total revenue decreased by $92,772, or approximately 23.4%. The decrease was predominately due to the anticipated poor performance from the Company’s network marketing business. The Company is in the process of introducing a new range of products for its networking marketing business. Distributors and members curtailed the purchases of the Company’s existing products, awaiting the Company’s new products range. The launch of the Company’s new products was delayed and is expected to take place in Q2 2024.
Cost of Revenue
Cost of revenue for the three months ended June 30, 2023 amounted to $107,931 as compared to $109,383 for the three months ended June 30, 2022, a marginal decrease of $1,452, or approximately 1.3%. Cost of revenue did not decrease in tandem with decrease in revenue for the period due to the varying gross profit margins associated with the provision of complementary health therapies.
Cost of revenue typically comprise of freight-in, cost of goods purchased, packing materials and services acquired.
| 28 |
Gross Profit
Gross profit for the three months ended June 30, 2023 amounted to $196,004 and represented a gross margin of approximately 64.5% as compared to $287,324 for the three months ended June 30, 2022, equivalent to a gross margin of approximately 72.4%. The decrease in gross margin was predominantly due to lower gross margin associated with the provision of complementary health therapies as compared to the Company’s network marketing business.
Operating Expenses
Our operating expenses consist of selling expenses, commission expenses and G&A expenses (as defined below). Total operating expenses were $555,537 for the three months ended June 30, 2023, increased by $37,970 or approximately 6.4% from $593,507 for the three months ended June 30, 2022.
Selling expenses
Selling expenses for the three months ended June 30, 2023 amounted to $64,126 as compared to $79,587 for the three months ended June 30, 2022, a decrease of $15,461, or approximately 19.4%. The Company’s selling expenses are comprised of salaries and benefits expenses which represented approximately 70% to 85% of total selling expenses, credit card processing fees and promotional expenses.
Commission expenses
Commission expenses were $21,942 and $62,557 for the three months ended June 30, 2023 and 2022, respectively. The decrease in commission expenses was in line with the decrease in revenue.
General and administrative expenses (“G&A Expenses”)
G&A expenses for the three months ended June 30, 2023 amounted to $469,469, as compared to $451,363 for the three months ended June 30, 2022, an increase of $18,106, or approximately 4.0%. The Company’s G&A expenses are comprised of salaries and benefits expenses, rental expenses, professional expenses and depreciation expenses.
Other Expenses, Net
For the three months ended June 30, 2023, we recorded an amount of $22,355 as other expenses, net, as compared to $97,769 other expenses, net, for the three months ended June 30, 2022, which represented a decrease of $75,414, or approximately 77.1%.
The net other expenses of $22,355 incurred during the three months ended June 30, 2023 comprised foreign currency exchange loss of $33,700, unrealized holding gain on marketable securities of $3,790, other income, net of $4,134, interest income of $1,634 and gain on disposal of property and equipment of $1,787. The net other expenses of $97,769 incurred during the three months ended June 30, 2022 comprised foreign currency exchange loss of $67,417, unrealized holding loss on marketable securities of $35,219, other income, net of $1,341 and interest income of $3,526.
Benefit of (Provision for) Income Taxes
The Company recorded benefit of income taxes of $2,439 and provision for income taxes of $392 for the three months ended June 30, 2023 and 2022, respectively. Both the benefit of income taxes as well as the provision for income taxes were in respect of the Company’s operations in Malaysia.
| 29 |
Net Loss
Net loss decreased by $24,895 from net loss of $404,344 for the three months ended June 30, 2022 to net loss of $379,449 for the three months ended June 30, 2023, mainly due to the reasons discussed above.
For the six months ended June 30, 2023 and 2022
Revenue
We generated revenue of $684,703, which is comprised of revenue from the Company’s network marketing business of $229,521 (approximately 33.5%); and revenue from the Company’s operations in the provision of complementary health therapies of $455,182 (approximately 66.5%) for the six months ended June 30, 2023 as compared to $805,667, which is comprised of revenue from the Company’s network marketing business of $558,340 (approximately 69.3%); and revenue from the Company’s operations in the provision of complementary health therapies of $247,327 (approximately 30.7%) for the six months ended June 30, 2022. Revenue from the Company’s network marketing business decreased significantly by $329,819, or approximately 58.9%. Revenue from the Company’s operations in the provision of complementary health therapies increased significantly by $207,855, or approximately 84.0%. Total revenue decreased by $120,964, or approximately 15.0%. The decrease was predominately due to the anticipated poor performance from the Company’s network marketing business. The Company is in the process of introducing a whole new range of products for its networking marketing business. Distributors and members curtailed the purchases of the Company’s existing products, awaiting the Company’s new products range. The shortfall in revenue of the Company’s network marketing business was significantly compensated by the significant increase in revenue in the Company’s operations in the provision of complementary health therapies. This new division which commenced in February 2022 is witnessing modest growth.
Cost of Revenue
Cost of revenue for the six months ended June 30, 2023 amounted to $236,289 as compared to $182,814 for the six months ended June 30, 2022, an increase of $53,475, or approximately 29.3%. The increase was due to the varying gross profit margins in the Company’s operations in the provision of complementary health therapies.
Cost of revenue typically comprise of freight-in, cost of goods purchased, packing materials and services acquired.
Gross Profit
Gross profit for the six months ended June 30, 2023, amounted to $448,414, which represented a gross margin of approximately 65.5% as compared to $622,853 for the six months ended June 30, 2022, equivalent to a gross margin of approximately 77.3%. The decrease in gross margin was predominantly due to lower gross margin associated with the provision of complementary health therapies as compared to the Company’s network marketing business.
| 30 |
Operating Expenses
Our operating expenses consist of selling expenses, commission expenses, general and administrative expenses. Total operating expenses were $1,261,831 for the six months ended June 30, 2023, increased by $60,563 or approximately 5.0% from $1,201,268 for the six months ended June 30, 2022.
Selling expenses
Selling expenses for the six months ended June 30, 2023 amounted to $140,224 as compared to $194,198 for the six months ended June 30, 2022, a decrease of $53,974, or approximately 27.8%, predominantly due to decrease in promotional expenses. Promotional activities on the Company’s network marketing business were scaled back during the time lag between phasing out of the old products range awaiting the upcoming new range of products. The Company’s selling expenses are typically comprised of salaries and benefits expenses which represented approximately 70% to 85% of total selling expenses, credit card processing fees and promotional expenses. There was also a small reduction in the number of headcounts in the recent six months ended June 30, 2023.
Commission expenses
Commission expenses were $55,884 and $176,666 for the six months ended June 30, 2023 and 2022, respectively. The decrease in commission expenses was in line with the decrease in revenue.
General and administrative expenses (“G&A expenses”)
G&A expenses for the six months ended June 30, 2023 amounted to $1,065,723, as compared to $830,404 for the six months ended June 30, 2022, an increase of $235,319, or approximately 28.3%. The increase in G&A expenses was mainly due to G&A expenses associated with the provision of complementary health therapies and expenses incurred by the Company on its on-going uplisting exercise to Nasdaq. The Company’s G&A expenses are typically comprised of salaries and benefits expenses, rental expenses, professional expenses and depreciation expenses.
Other Expenses, Net
For the six months ended June 30, 2023, we recorded an amount of $6,762 as other expenses, net, as compared to $115,695 other expenses, net, for the six months ended June 30, 2022, represented a decrease of $108,933 or approximately 94.2%.
The net other expenses of $6,762 incurred during the six months ended June 30, 2023 comprised foreign currency exchange loss of $34,576, unrealized holding gain on marketable securities of $8,710, other income, net of $12,500, interest income of $4,817 and gain on disposal of property and equipment of $1,787. The net other expenses of $115,695 incurred during the six months ended June 30, 2022 comprised foreign currency exchange loss of $83,883, unrealized holding loss on marketable securities of $52,889, other income, net of $12,826 and interest income of $8,251.
Benefit of (Provision for) Income Taxes
The Company recorded benefit of income taxes of $6,655 and provision for income taxes of $8,680 for the six months ended June 30, 2023 and 2022, respectively. Both the benefit of income taxes as well as the provision for income taxes were in respect of the Company’s operations in Malaysia.
Net Loss
Net loss increased by $110,734 from net loss of $702,790 for the six months ended June 30, 2022 to net loss of $813,524 for the six months ended June 30, 2023, mainly due to reasons as discussed above.
Liquidity and Capital Resources
Malaysia, where the operations of the Company predominantly reside, officially transitioned to the endemic phase of COVID-19 effective April 1, 2022.
Substantially all of our revenues are concentrated in Malaysia. Consequently, our results of operations will likely be adversely, and may be materially, affected, to the extent that the COVID-19 or any other epidemic harms the Malaysia and global economy in general. Any potential impact to our results will depend on, to a large extent, future developments and new information that may emerge regarding the duration and severity of the COVID-19 and the actions taken by government authorities and other entities to contain the COVID-19 or treat its impact, almost all of which are beyond our control. Potential impacts include, but are not limited to, the following:
| ● | temporary closure of offices, travel restrictions, disruption or suspension of supplies, our customers may be negatively impacted financially resulting in which the demand for our products may be adversely affected; | |
| ● | we may have to provide significant sales incentives to our customers during the outbreak, which may in turn materially adversely affect our financial condition and operating results; and | |
| ● | any disruption of our supply chain, logistics providers or customers could adversely impact our business and results of operations, including causing us or our suppliers to cease manufacturing for a period of time or materially delay delivery to our customers, which may also lead to loss of our customers. |
| 31 |
There could still be supply chain disruptions. From our experiences operating under the much-restricted COVID-19 environment, we have modified our methods of operations, to build in sufficient buffer in the management of inventories. We may experience slight delay in products delivery lead time but barring unforeseen circumstances, the setback should be temporary.
We are currently operating primarily in Malaysia and anticipate expanding into the Asian markets in the future, with a particular focus, at least initially, on expanding into Thailand, Indonesia and Taiwan. We will explore expansion via e-commerce. Now that most nations are living alongside COVID-19, we will re-assess our plans to set up offices in the countries in which we operate to better service our customers.
There is no guarantee that the Company’s total revenues will grow or remain at similar levels year over year in 2023 and beyond.
As of June 30, 2023, we had working capital deficit of $26,893, consisting of cash and cash in bank of $332,431 and time deposits of $321,323 as compared to working capital of $799,239 consisting of cash and cash in bank of $523,619 and time deposits of $914,811 as of December 31, 2022. The Company had a net loss of $813,524 for the six months ended June 30, 2023 and accumulated deficits of $5,746,112 as of June 30, 2023 as compared to net loss of $1,666,079 for the year ended December 31, 2022 and accumulated deficits of $4,945,586 as of December 31, 2022.
In assessing our liquidity and going concern, management is projecting that the company’s revenue will revert to pre-pandemic level by Q2 2024, generating sufficient cash therefrom to cover our operating expenses. The timeline is subject to the availability of talents for sales and marketing and product development personnel as the recruitment market has become increasingly competitive post COVID-19 as many companies are ramping up to increase their workforce.
If our Company is unable to generate sufficient cash flow within the normal operating cycle of a twelve-month period to pay for its future payment obligations, we may have to consider supplementing our available sources of funds through the following sources, however, there is no assurance that management will be successful in its plans:
| ● | Equity financing from the Company’s second listing on NASDAQ to support its working capital and future growth; | |
| ● | Other available sources of financing (including debt) from banks and other financial institutions; and | |
| ● | Financial support and credit guarantee commitments from the Company’s related parties. |
Based on the above measures, management is of the opinion that the Company will probably not have sufficient funds to meet its working capital requirements and debt obligations as they become due one year from the filing date of these unaudited condensed consolidated financial statements..
The following summarizes the key components of our cash flows for the six months ended June 30, 2023 and 2022:
| For the six months ended June 30, | ||||||||
| 2023 | 2022 | |||||||
| Net cash used in operating activities | $ | (742,175 | ) | $ | (495,727 | ) | ||
| Net cash used in investing activities | (6,499 | ) | (750 | ) | ||||
| Net cash used in financing activities | (22,861 | ) | (178,926 | ) | ||||
| Effect of exchange rate on cash and cash equivalents | (13,141 | ) | (115,008 | ) | ||||
| Net change in cash and cash equivalents | $ | (784,676 | ) | $ | (790,411 | ) | ||
| 32 |
Operating activities
Net cash used in operating activities for the six months ended June 30, 2023 was $742,175, comprised of net loss of $813,524, increase in accounts receivables of $2,751, increase in amount due from related parties of $209, increase in inventories of $23,013, decrease in accounts payable – related parties of $4,758, decrease in customer deposits of $31,655, decrease in other payables and accrued liabilities of $228,812, payment of operating lease liabilities of $79,551, decrease in other payable –related parties of $3,457, the non-cash expenses on unrealized holding gain on marketable securities of $8,710, deferred tax benefit of $6,655, offset by the non-cash depreciation and amortization expense of $39,264, amortization of operating right-of-use assets of $78,333, decrease in prepaid taxes of $238,064, decrease in prepayments and deposits of $80,651 and increase in accounts payable of $24,608.
Net cash used in operating activities for the six months ended June 30, 2022 was $495,727 and were mainly comprised of the net loss of $702,790, increase in accounts receivables of $214, decrease in customer deposits of $175,936, payment of operating lease liabilities of $75,200 and decrease in other payables and accrued liabilities (including related party) of $164,056. The net cash used in operating activities was mainly offset by the non-cash depreciation and amortization expense of $37,558, amortization of operating right-of-use assets of $75,241, unrealized holding loss on marketable securities of $52,889, decrease in amount due from related parties of $2,201, the decrease in inventories of $28,790, refund in prepaid taxes of $296,219, decrease in prepayments and deposits of $102,099, increase in accounts payable (including related party) of $22,211 and increase in income tax payables of $5,261.
Investing activities
Net cash used in investing activities for the six months ended June 30, 2023 was $6,499, which was in respect of purchase of equipment.
Net cash used in investing activities for the six months ended June 30, 2022 was $750, which was in respect of purchase of equipment.
Financing activities
Deferred offering cost made up the entire net cash used in financing activities for the six months ended June 30, 2023 and 2022 of $22,861 and $178,926, respectively.
Credit Facilities
We do not have any credit facilities or other access to bank credit.
Off-Balance Sheet Arrangements
As of June 30, 2023, we have no significant off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in our financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to our stockholders.
Critical Accounting Estimates
The preparation of unaudited condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the unaudited condensed consolidated financial statements and the reported amounts of revenues and expenses during the periods presented. Significant accounting estimates reflected in the Company’s unaudited condensed consolidated financial statements include allowance for inventories obsolescence, impairment of long-lived assets and allowance for deferred tax assets. Following are the methods and assumptions used in determining our estimates.
Estimated allowance for inventories obsolescence
Management reviews inventory on hand for estimated obsolescence or unmarketable items, as compared to future demand requirements and the shelf life of the various products. Based on the review, the Company records inventory write-downs, when necessary, when costs exceed expected net realizable value. The Company did not recognize any inventory write-downs nor inventory write-off for the six months ended June 30, 2023 and 2022.
Impairment of long-lived assets
Operating right-of-use assets and property and equipment are stated at costs less accumulated depreciation and impairment, if any. In determining whether an asset is impaired, the Company has to exercise judgment and make estimation, particularly in assessing: (1) whether an event has occurred or any indicators that may affect the asset value; (2) whether the carrying value of an asset is not recoverable that is its carrying amount exceeds the amount of expected undiscounted future cash flows result from the use of the asset. Once it is established that impairment has occurred, the amount of impairment expense is determined as the difference between the carrying value of the asset and its estimated fair value based on a discounted cash flows approach.
As of June 30, 2023 and December 31, 2022, the carrying amounts of operating right-of-use assets and property and equipment amounted to $284,670 and $105,674 (December 31, 2022: $81,133 and $142,149), respectively. No impairment losses on operating right-of-use assets and property and equipment were recognized as of June 30, 2023 and 2022.
| 33 |
Allowance for deferred tax assets
The Company conducts much of its business activities in Malaysia and Hong Kong and is subject to tax in each of these jurisdictions. Significant estimates are required in determining the provision for income taxes. There are many transactions and calculations for which the ultimate tax determination is uncertain during the ordinary course of business. Where the final tax outcome of these matters is different from the amounts that were initially recorded, such differences will impact the income tax and deferred tax provisions in the period in which such determination is made.
Deferred tax assets relating to certain temporary differences and tax losses are recognized as management considers it is probable that future taxable profit will be available against which the temporary differences or tax losses can be utilized. Where the expectation is different from the original estimate, such differences will impact the recognition of deferred taxation assets and taxation in the periods in which such estimate is changed.
Critical Accounting Policies
Revenue recognition
The Company adopted Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers (ASC Topic 606). The core principle underlying the revenue recognition of this ASU allows the Company to recognize – revenue that represents the transfer of goods and services to customers in an amount that reflects the consideration to which the Company expects to be entitled in such exchange. This will require the Company to identify contractual performance obligations and determine whether revenue should be recognized at a point in time or over time, based on when control of goods and services transfers to a customer. The Company’s revenue streams are recognized at a point in time for the Company’s sale of health and wellness products.
The ASU requires the use of a new five-step model to recognize revenue from customer contracts. The five-step model requires that the Company (i) identify the contract with the customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, including variable consideration to the extent that it is probable that a significant future reversal will not occur, (iv) allocate the transaction price to the respective performance obligations in the contract, and (v) recognize revenue when (or as) the Company satisfies the performance obligation.
The Company accounts for a contract with a customer when the contract is committed in writing, the rights of the parties, including payment terms, are identified, the contract has commercial substance and consideration is probable of substantially collection.
Sales of Health and Wellness products
- Performance obligations satisfied at a point in time
The Company derives its revenues from sales contracts with its customers with revenues being recognized when control of the health and wellness products are transferred to its customer at the Company’s office or shipment of the goods. The revenue is recorded net of estimated discounts and return allowances. Products are given 60 days for returns or exchanges from the date of purchase. Historically, there were insignificant sales returns.
Under the Company’s network marketing business, the Company issues product coupons to members and distributors when these customers made purchases above certain thresholds set by the Company. Depending on the type of product coupons issued, the coupons carry varying values and can be used by the customers for reduction in the transaction price of product purchases within the coupon validity period. The value of the product coupons issued is recorded as a reduction of the Company’s revenue account upon issuance; the corresponding amount credited to the customer deposits account. Amounts in customer deposits will be reversed when the coupons are used. The Company’s coupons have a validity period of between six and twelve months. If the Company’s customers did not utilize the coupons after the validity period, the Company would recognize the forfeiture of the originated sales value of the coupons as net revenues.
Provision of Health and Wellness services
- Performance obligations satisfied at a point in time
The Company carries out its Wellness program, where the Company’s products are bundled with health screening test and a health camp program. The health screening test and the health camp programs are considered as separate performance obligations. The promises to deliver the health screening test report and the attendance at the health camp are separately identifiable, which are evidenced by the fact that the Company provides separate services of delivering the health screening test report and allowing admission of the customers to attend the health camp. The Company derives its revenues from sales contracts with its customers with revenues being recognized when the test reports are completed and delivered to its customers during the consultation section in person. The Company also separately derives its revenues from sales contracts with its customers with revenues being recognized when the health camp program was completed in the final day of the health camp.
| 34 |
Fair value of financial instruments
The accounting standard regarding fair value of financial instruments and related fair value measurements defines financial instruments and requires disclosure of the fair value of financial instruments held by the Company.
The accounting standards define fair value, establish a three-level valuation hierarchy for disclosures of fair value measurement and enhance disclosure requirements for fair value measures. The three levels are defined as follow:
| ● | Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. | |
| ● | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments. | |
| ● | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value. |
Financial instruments included in current assets and current liabilities are reported in the consolidated balance sheets at face value or cost, which approximate fair value because of the short period of time between the origination of such instruments and their expected realization and their current market rates of interest.
Accounting Standards Adopted in 2023
On January 1, 2023, the Company adopted Accounting Standards Update (“ASU”) 2016-13, Financial Instruments – Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments (“ASC 326”). This standard replaced the incurred loss methodology with an expected loss methodology that is referred to as the current expected credit loss (“CECL”) methodology.
In addition, CECL made changes to the accounting for available-for-sale debt securities. One such change is to require credit losses to be presented as an allowance rather than as a write-down on available-for-sale debt securities if management does not intend to sell and does not believe that it is more likely than not, they will be required to sell.
As ASU 2016-13 has been adopted from January 1, 2023, the Company adopted ASU 2019-05, Financial instruments – Credit Losses (Topic 326) Targeted Transition Relief at the same time.
The AUS 2019-05 provides entities that have certain instruments with an option to irrevocably elect the fair value option applied on an instrument-by-instrument basis for eligible instruments. The fair value option election does not apply to held-to-maturity debt securities.
The adoption of these ASUs did not have a material impact on the unaudited condensed consolidated financial statements for the six months ended and as at June 30, 2023.
Recent accounting pronouncements
The Company has reviewed all recently issued, but not yet effective, considers the applicability and impact of all accounting standards updates (“ASUs”). Management periodically reviews new accounting standards that are issued.
In March 2023, the FASB issued ASU No. 2023-01, “Leases (Topic 842) Common Control Arrangements”. This ASU provides guidance in ASC Topic 842 that leasehold improvements associated with common control leases should be (i) amortized by the lessee over the useful life of the leasehold improvements to the common control group, regardless of the lease term, as long as the lessee controls the use of the underlying asset through a lease, and (ii) accounted for as a transfer between entities under common control through an adjustment to equity if and when the lessee no longer controls the use of the underlying asset. The ASU 2023-01 is effective for reporting periods beginning after December 15, 2023. Early adoption is permitted for both interim and annual financial statements that have not yet been issued. When adopted in an interim period, it must be adopted from the beginning of the year that includes that interim period. The Company is currently evaluating the impact of this ASU may have on its unaudited condensed consolidated financial statements.
Except for the above-mentioned pronouncements, there are no other new recent issued accounting standards that will have a material impact on the consolidated financial position, statements of operations and cash flows.
For the years ended December 31, 2022 and 2021
Revenue
We generated revenue of $1,856,564, which comprised revenue from the Company’s network marketing business of $1,141,307 (approximately 61.5%); and revenue from the Company’s operations in the provision of complementary health therapies of $715,257 (approximately 38.5%) for the year ended December 31, 2022 as compared to $1,016,962, which amount was solely attributable to revenue from the Company’s network marketing business for the year ended December 31, 2021. Revenue from the Company’s network marketing business increased marginally by $124,345 or approximately 12.2%. Total revenue increased by a $839,602 or approximately 82.6%. The increase was predominately due to: (i) the recovery from COVID-19 in Malaysia after April 2022. The Company made progress in revenue generating as Malaysia, where the Company’s operations predominantly reside, has moved to a COVID-19 endemic phase with minimal restrictions on businesses and people movements in the country; and (ii) the Company’s operations in the provision of complementary health therapies since February 2022.
Cost of Revenue
Cost of revenue for the year ended December 31, 2022 amounted to $666,042 as compared to $297,333 for the year ended December 31, 2021, representing an increase of $368,709 or approximately 124.0%. The increase was in line with the increase in revenue as explained in the above.
Cost of revenue typically comprise of freight-in, cost of goods purchased, packing materials and services acquired.
| 35 |
Gross Profit
Gross profit for the year ended December 31, 2022 amounted to $1,190,522, represented a gross margin of, approximately 64.1%, as compared to $719,629 for the year ended December 31, 2021, which was equivalent to a gross margin of approximately 70.8%. The decrease in gross margin was predominantly due to a lower gross margin associated with the provision of complementary health therapies as compared to the Company’s network marketing business; and promotional activities undertaken by the Company to increase revenue from its network marketing business.
Operating Expenses
Our operating expenses consist of selling expenses, commission expenses, general and administrative expenses and provision for doubtful accounts.
Selling expenses
Selling expenses for the year ended December 31, 2022 amounted to $361,414 as compared to $394,682 for the year ended December 31, 2021, a decrease of $33,268 or approximately 8.4%. The Company’s selling expenses typically comprise salaries and benefits expenses, credit card processing fees and promotional expenses. The decrease in selling expenses was predominantly due to decrease in salaries and benefits expenses.
Commission expenses
Commission expenses were $405,351 and $316,267 for the years ended December 31, 2022 and 2021, respectively, representing an increase of $89,084 or approximately 28.2%. The increase in commission expenses was in line with the increase in revenue.
General and administrative expenses (“G&A expenses”)
G&A expenses for the year ended December 31, 2022 amounted to $1,957,023, as compared to $1,745,734 for the year ended December 31, 2021, representing an increase of $211,289, or approximately 12.1%. The increase in G&A expenses was mainly due to G&A expenses associated with the provision of complementary health therapies. The Company’s G&A expenses typically comprise of salaries and benefits expenses, rental expenses, professional expenses and depreciation expenses.
Provision for doubtful accounts
Provision for doubtful accounts were $0 and $121,514 for the years ended December 31, 2022 and 2021 respectively. The provision for doubtful accounts brought forward was in respect of prepayments to a supplier. As the Company’s attempts to recover the prepayments were futile, the entire provision for doubtful accounts of $121,514 was written-off during the year ended December 31, 2022.
Other (Expenses) Income
For the year ended December 31, 2022, we recorded an amount of $136,868 as other expenses, net as compared to $529,045 other expenses, net for the year ended December 31, 2021, representing a significant decrease of $392,177, or approximately 74.1%, in other expenses, net. The net other expenses of $136,868 incurred during the year ended December 31, 2022 comprised of other expenses of $79,539, interest income of $16,190 and unrealized holding loss on marketable securities of $73,519. The net other expenses of $529,405 incurred during the year ended December 31, 2021 comprised of other expense of $68,323, interest income of $25,570, unrealized holding loss on marketable securities of $505,231 and dividend income from marketable securities of $18,939. The significant decrease of other expenses, net was mainly due to the decrease of unrealized holding loss on marketable securities as a result of market price changes during the year of those investments held by the Company.
Benefit of (Provision for) Income Taxes
We had benefits of income taxes of $4,055 and provision for income taxes of $137,067 for the years ended December 31, 2022 and 2021, respectively. During the year ended December 31, 2022, our operations in Malaysia recorded benefits of income taxes due to overprovision of taxes in prior years.
During the year ended December 31, 2021, we had provision for income taxes of $114,862 due to certain permanent items required for the income taxes provision in Malaysia jurisdiction after our Malaysia local tax audit and had provision for income taxes of $22,205 on U.S. GILTI taxes provision.
| 36 |
Net Loss
We generated a net loss of $1,666,079 for the year ended December 31, 2022, as compared to $2,524,680 for the year ended December 31, 2021, a decrease of $858,601 or approximately 34.0%, predominately due to reasons as discussed above.
Liquidity and Capital Resources
Malaysia, where the operations of the Company predominantly reside, officially transitioned to the endemic phase of COVID-19 effective April 1, 2022. Restrictions on businesses and people are minimal.
Meanwhile, the government continues to encourage inoculation for those between the ages of 5 to 11 years and its adolescent group which comprised those between the ages 12 and 17. Adults who have been fully vaccinated, i.e. received two doses of the COVID-19 vaccine are encouraged to take booster shots.
Substantially all of our revenues are concentrated in Malaysia. Consequently, our results of operations will likely be adversely, and may be materially, affected, to the extent that the COVID-19 or any other epidemic harms the Malaysia and global economy in general. Any potential impact to our results will depend on, to a large extent, future developments and new information that may emerge regarding the duration and severity of the COVID-19 and the actions taken by government authorities and other entities to contain the COVID-19 or treat its impact, almost all of which are beyond our control. Potential impacts include, but are not limited to, the following:
| ● | temporary closure of offices, travel restrictions, disruption or suspension of supplies, our customers may be negatively impacted financially resulting in which the demand for our products may be adversely affected ; | |
| ● | we may have to provide significant sales incentives to our customers during the outbreak, which may in turn materially adversely affect our financial condition and operating results ; and | |
| ● | any disruption of our supply chain, logistics providers or customers could adversely impact our business and results of operations, including causing us or our suppliers to cease manufacturing for a period of time or materially delay delivery to our customers, which may also lead to loss of our customers. |
From our experiences operating under the much-restricted COVID-19 environment, we have modified our methods of operations, to build in sufficient buffer in the management of inventories. We may experience slight delay in products delivery lead time but barring unforeseen circumstances, the setback should be temporary.
We are currently operating primarily in Malaysia and anticipate expanding into the Asian markets in the future, with a particular focus, at least initially, on expanding into Thailand, Indonesia and Taiwan. We will explore expansion via e-commerce. Now that most nations are living alongside COVID-19, we will re-assess our plans to set up offices in the countries in which we operate to better service our customers.
Because of the uncertainty surrounding the COVID-19 outbreak, the financial impact related to the outbreak of and response to the COVID-19 cannot be reasonably estimated at this time. There is no guarantee that the Company’s total revenues will grow or remain at similar levels year over year in 2023 and beyond.
As of December 31, 2022, we had working capital of $799,239 consisting of cash and cash in bank of $523,619 and time deposits of $914,811 as compared to working capital of $2,599,281 consisting of cash and cash in bank of $622,501 and time deposits of $1,975,347 as of December 31, 2021. The Company had a net loss of $1,666,079 for the year ended December 31, 2022 and accumulated deficits of $4,945,586 as of December 31, 2022 as compared to net loss of $2,524,680 for the year ended December 31, 2021 and accumulated deficits of $3,258,687 as of December 31, 2021.
In assessing our liquidity and going concern, management is projecting that the company’s revenue will revert to pre-pandemic level, generating sufficient cash therefrom to cover our operating expenses.
| 37 |
If our Company is unable to generate sufficient cash flow within the normal operating cycle of a twelve-month period to pay for its future payment obligations, we may have to consider supplementing our available sources of funds through the following sources:
| ● | other available sources of financing from Malaysia banks and other financial institutions; and | |
| ● | financial support from our related parties and shareholders. |
Based on the above initiatives, management is of the opinion that the Company shall have sufficient funds to meet its working capital requirements and debt obligations as they become due in the foreseeable future twelve months from the date of issuance of this Annual Report. However, there is no assurance that management will be successful in its plans.
The following summarizes the key components of our cash flows for the years ended December 31, 2022 and 2021:
For the years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Net cash used in operating activities | $ | (811,683 | ) | $ | (845,842 | ) | ||
| Net cash used in investing activities | (32,119 | ) | (3,959 | ) | ||||
| Net cash used in financing activities | (234,466 | ) | (19,061 | ) | ||||
| Effect of exchange rate on cash and cash equivalents | (81,150 | ) | (50,890 | ) | ||||
| Net change in cash and cash equivalents | $ | (1,159,418 | ) | $ | (919,752 | ) | ||
Operating activities
Net cash used in operating activities for the year ended December 31, 2022 was $811,683 and were mainly comprised of the net loss of $1,666,079, the non-cash deferred tax benefit of $14,751, the increase in accounts receivables of $2,824, the increase in amount due from related parties of $3,786, the payment of operating lease liabilities of $145,197 and the decrease in other payables (including related parties) and accrued liabilities of $115,085. The net cash used in operating activities was mainly offset by the non-cash depreciation and amortization expense of $73,876, amortization of operating right-of-use assets of $144,064, the unrealized holding loss on marketable securities of $73,519, inventory write-downs of $5,307, the decrease in inventories of $343,483, the refund in prepaid taxes of $263,404, the decrease in prepayments and deposits of $89,113, the increase in accounts payable (including related parties) of $41,422, increase in customer deposits of $94,877 and increase in income tax payables of $6,974.
Net cash used in operating activities for the year ended December 31, 2021 was $845,842 and were mainly comprised of the net loss of $2,524,680, dividend income from marketable securities of $18,939, the increase in prepayments and deposits of $128,363, and the payment of operating lease liabilities of $138,143. The net cash used in operating activities was mainly offset by the non-cash depreciation and amortization expense of $77,758, amortization of operating right-of-use assets of $139,451, the unrealized holding loss on marketable securities of $505,231, the non-cash deferred tax expense of $10,127, inventories write-down of $36,241, provision for doubtful accounts of $121,514, the decrease of accounts receivables of $167,566, the decrease in inventories of $192,713, the refund in prepaid taxes of $430,062, the increase in customer deposits of $52,981, the increase in income tax payables of $3,988, and the increase in other payables and accrued liabilities of $226,651.
Investing activities
Net cash used in investing activities for the year ended December 31, 2022 was $32,119, the amount entirely for the purchase of equipment and intangible assets.
Net cash used in investing activities for the year ended December 31, 2021 was $3,959, the amount entirely for the purchase of equipment.
Financing activities
Net cash used in financing activities for the year ended December 31, 2022 was $234,466, the amount entirely payment of deferred offering cost.
| 38 |
Net cash used in financing activities for the year ended December 31, 2021 was $19,061, mainly comprised of payment of deferred offering cost of $15,210 and advances to related parties of $3,851.
Credit Facilities
We do not have any credit facilities or other access to bank credit.
Off-Balance Sheet Arrangements
As of December 31, 2022, we have no significant off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in our financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to our stockholders.
Critical Accounting Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the consolidated financial statements and the reported amounts of revenues and expenses during the periods presented. Significant accounting estimates reflected in the Company’s consolidated financial statements include allowance for inventories obsolescence, impairment of long-lived assets, allowance for deferred tax assets. Following are the methods and assumptions used in determining our estimates.
Estimated allowance for inventories obsolescence
Management reviews inventory on hand for estimated obsolescence or unmarketable items, as compared to future demand requirements and the shelf life of the various products. Based on the review, the Company records inventory write-downs, when necessary, when costs exceed expected net realizable value. For the years ended December 31, 2022 and 2021, the Company recognize an inventory write-downs of $5,307 and $36,241 respectively.
Impairment of long-lived assets
Operating right-of-use assets and property, plant and equipment are stated at costs less accumulated depreciation and impairment, if any. In determining whether an asset is impaired, the Company has to exercise judgment and make estimation, particularly in assessing: (1) whether an event has occurred or any indicators that may affect the asset value; (2) whether the carrying value of an asset can be supported by the recoverable amount, in the case of value in use, the present value of future cash flows which are estimating the recoverable amounts including cash flow projections and an appropriate discount rate. Changing the assumptions and estimates, including the discount rates or the growth rate in the cash flow projections, could materially affect the net present value used in the impairment test.
As of December 31, 2022 and 2021, the carrying amounts of operating right-of-use assets and property, plant and equipment amounted to $81,133 and $142,149 (December 31, 2021: $237,718 and $215,799), respectively. No impairment losses on operating right-of-use assets and property, plant and equipment were recognized as of December 31, 2022 and 2021.
Allowance for deferred tax asset
The Company conducts much of its business activities in Malaysia and Hong Kong and is subject to tax in each of these jurisdictions. Significant estimates are required in determining the provision for income taxes. There are many transactions and calculations for which the ultimate tax determination is uncertain during the ordinary course of business. Where the final tax outcome of these matters is different from the amounts that were initially recorded, such differences will impact the income tax and deferred tax provisions in the period in which such determination is made.
Deferred tax assets relating to certain temporary differences and tax losses are recognized as management considers it is probable that future taxable profit will be available against which the temporary differences or tax losses can be utilized. Where the expectation is different from the original estimate, such differences will impact the recognition of deferred taxation assets and taxation in the periods in which such estimate is changed.
| 39 |
Critical Accounting Policies
Revenue recognition
The Company adopted Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers (ASC Topic 606). The core principle underlying the revenue recognition of this ASU allows the Company to recognize – revenue that represents the transfer of goods and services to customers in an amount that reflects the consideration to which the Company expects to be entitled in such exchange. This will require the Company to identify contractual performance obligations and determine whether revenue should be recognized at a point in time or over time, based on when control of goods and services transfers to a customer. The Company’s revenue streams are recognized at a point in time for the Company’s sale of health and wellness products.
The ASU requires the use of a new five-step model to recognize revenue from customer contracts. The five-step model requires that the Company (i) identify the contract with the customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, including variable consideration to the extent that it is probable that a significant future reversal will not occur, (iv) allocate the transaction price to the respective performance obligations in the contract, and (v) recognize revenue when (or as) the Company satisfies the performance obligation.
The Company accounts for a contract with a customer when the contract is committed in writing, the rights of the parties, including payment terms, are identified, the contract has commercial substance and consideration is probable of substantially collection.
Sales of Health and Wellness products
- Performance obligations satisfied at a point in time
The Company derives its revenues from sales contracts with its customers with revenues being recognized when control of the health and wellness products are transferred to its customer at the Company’s office or shipment of the goods. The revenue is recorded net of estimated discounts and return allowances. Products are given 60 days for returns or exchanges from the date of purchase. Historically, there were insignificant sales returns.
Under the Company’s network marketing business, the Company issues product coupons to members and distributors when these customers made purchases above certain thresholds set by the Company. Depending on the type of product coupons issued, the coupons carry varying values and can be used by the customers for reduction in the transaction price of product purchases within the coupon validity period. The value of the product coupons issued is recorded as a reduction of the Company’s revenue account upon issuance; the corresponding amount credited to the customer deposits account. Amounts in customer deposits will be reversed when the coupons are used. The Company’s coupons have a validity period of between six and twelve months. If the Company’s customers did not utilize the coupons after the validity period, the Company would recognize the forfeiture of the originated sales value of the coupons as net revenues.
| 40 |
Provision of Health and Wellness services
- Performance obligations satisfied at a point in time
The Company carries out its Wellness program, where the Company’s products are bundled with health screening test and a health camp program. The health screening test and the health camp programs are considered as separate performance obligations. The promises to deliver the health screening test report and the attendance at the health camp are separately identifiable, which are evidenced by the fact that the Company provides separate services of delivering the health screening test report and allowing admission of the customers to attend the health camp. The Company derives its revenues from sales contracts with its customers with revenues being recognized when the test reports are completed and delivered to its customers during the consultation section in person. The Company also separately derives its revenues from sales contracts with its customers with revenues being recognized when the health camp program was completed in the final day of the health camp.
Fair value of financial instruments
The accounting standard regarding fair value of financial instruments and related fair value measurements defines financial instruments and requires disclosure of the fair value of financial instruments held by the Company.
The accounting standards define fair value, establish a three-level valuation hierarchy for disclosures of fair value measurement and enhance disclosure requirements for fair value measures. The three levels are defined as follow:
| ● | Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. | |
| ● | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments. | |
| ● | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value. |
Financial instruments included in current assets and current liabilities are reported in the consolidated balance sheets at face value or cost, which approximate fair value because of the short period of time between the origination of such instruments and their expected realization and their current market rates of interest.
Recent accounting pronouncements
The Company has reviewed all recently issued, but not yet effective, considers the applicability and impact of all accounting standards updates (“ASUs”). Management periodically reviews new accounting standards that are issued.
In November 2019, the FASB issued ASU No. 2019-10, which to update the effective date of ASU No. 2016-13 for private companies, not-for-profit organizations and certain smaller reporting companies applying for credit losses, leases, and hedging standard. The new effective date for these preparers is for fiscal years beginning after December 15, 2022. ASU 2019-05 is effective for the Company for annual and interim reporting periods beginning January 1, 2023 as the Company is qualified as a smaller reporting company. The Company is currently evaluating the impact ASUs 2016-13 and 2019-05 may have on its consolidated financial statements.
Except for the above-mentioned pronouncements, there are no new recent issued accounting standards that will have a material impact on the consolidated financial position, statements of operations and cash flows.
| 41 |
BUSINESS
Overview
We are a provider of health and wellness products and advisory services in the Malaysian market. We pursue our mission of helping people to create health and wealth by providing quality products to distributors and customers who seek a healthy lifestyle. We believe the quality of our products coupled with the effectiveness of our distribution network have been the primary reasons for our success and will allow us to pursue future business expansion. In order to further our supply chain, on May 8, 2020, we acquired 99.99% of Agape Superior Living Sdn Bhd, with the goal of securing an established network marketing sales channel that has been in existence in Malaysia for the past 18 years. On September 11, 2020, the Company incorporated Wellness ATP International Holdings Sdn. Bhd., a wholly owned subsidiary in Malaysia, with the aim to pursue the business of promoting wellness and wellbeing lifestyle of the community through the provision of services including online editorials, programs, events and campaigns on how to achieve positive wellness and lifestyle. On September 15, 2020, Wellness ATP International Holdings Sdn. Bhd. Entered into a business collaboration agreement with ASL to carry out certain wellness programs.
We currently offer three series of products: ATP Zeta Health Program, ÉNERGÉTIQUE and BEAUNIQUE. Our ATP Zeta Health Program is a health program designed to assist in the elimination of various diseases caused by environmental pollutants, unhealthy dietary intake and unhealthy lifestyles. The program aims to promote improved health and longevity through a combination of modern health supplements, proper nutrition and advice from skilled dieticians. Our ÉNERGÉTIQUE series aims to provide a total dermal solution for healthy skin beginning from the cellular level. The series is comprised of the Energy Mask series, Hyaluronic Acid and Mousse Facial Cleanser. Our BEAUNIQUE product series focuses on the research of our diet’s impact on modifying gene expressions to address genetic variations and deliver a personalized nutrigenomic solution for every individual.
On November 11, 2021, Agape ATP Corporation (Labuan) formed a joint-venture entity, DSY Wellness International Sdn. Bhd. (“DSY Wellness”) with Mr. Steve Yap, following which Agape ATP Corporation (Labuan) owns 60% of the equity interests in DSY Wellness, to pursue the business of providing complementary health therapies. The establishment of DSY Wellness is a further expansion of our business into the health and wellness industry. Mr. Steve Yap owns 33 proprietary formulas (the “Proprietary Formulas”) for treating non-communicable disease and the Company is currently in discussion with Mr. Steve Yap to bring the Proprietary Formulas into the company for joint commercialization. Mr. Steve Yap also has existing clients receiving traditional complimentary medicine or “TCM” in Indonesia and China.
Industry and Market Opportunities
Increasing demand in Dietary Supplement products in the Association of Southeast Asian Nations (“ASEAN”) region.
ASEAN markets have seen increasing demand for dietary supplement products since 2016 and we believe will continue to do for the foreseeable future. We believe that the ASEAN market for health supplements hold great potential for growth. According to a report published by Zion Market Research entitled “Dietary Supplements Market by Ingredients (Botanicals, Vitamins, Minerals, Amino Acids, Enzymes) for Additional Supplements, Medicinal Supplements, and Sports Nutrition Applications – Global Industry Perspective, Comprehensive Analysis and Forecast, 2020 – 2028” issued in March 2023, it is estimated that while the global dietary supplements market stood at US$191.1 billion in 2020, it is set to reach US$307.8 billion by 2028, representing a compounded annual growth rate (CAGR) of 5.9% between 2021-2028.
Source: Zion Market Research
According to an article published by Janio, a cross-border platform providing integrated logistics solutions to e-commerce brands, logistics service providers and marketplaces in Southeast Asia, in December 2019, the nutritional and dietary supplements in the ASEAN region are being prioritized by individuals in order to maintain a balanced diet and lifestyle. (Source: https://www.janio.asia/resources/articles/health-supplement-trends-in-southeast-asia-and-how-cross-border-ecommerce-plays-a-role) Consumers believe that they can make up for certain vitamin deficiencies or dietary deficiencies by consuming nutritional and dietary supplements. Many consumers also use nutritional and dietary supplements for cosmetics purposes, namely, skincare, hair strengthening, and fat burning, particularly in countries such as Malaysia and Vietnam.
For example, in Indonesia, the nutritional and dietary supplements market has recorded strong growth due to changes in consumers’ lifestyle habits and increasing awareness of preventive health measures. This is prevalent among the middle-class that has grown from approximately 37.7% of the population in 2003 to approximately 50% the population in 2020. Similarly, according to Siam Commercial Bank (SCB) Economic Intelligence Center, the prospects for the dietary supplements market in Thailand has been growing since 2015 and is predicted to grow at an average of approximately 7% per year until 2030. (source: https://www.scbeic.com/en/detail/file/product/3218/emyjfdtqlc/Note_EN_Supplement_20170209.pdf) In the Philippines, a stable economy has also been contributing to increased financial capability and the desire of Filipino consumers to improve both their mental and physical health through supplements. Conversely, Malaysia is ranked as the top country within ASEAN for both obesity and diabetes. Obesity and diabetes have been connected to heart disease and hypertension. As a result, consumers in Malaysia are increasingly aware of such potential health issues associated with eating habits and have become more proactive in searching for consumer health products to prevent such chronic diseases like diabetes and hypertension.
| 42 |
Continued Growth in the Skincare products in regions such as Asia-Pacific.
We believe that skincare products will remain highly lucrative with further potential for growth. According to Euromonitor, a global strategic market research company based in London, Asia generates approximately 51% of the world’s skin care sales, surpassing Western Europe and North America. In June 2023, Fortune Business Insights, a global market study and consulting services provider, puts the Asia-Pacific skincare sector as the largest market in the world. (source: https://www.fortunebusinessinsights.com/skin-care-market-102544) According to them, the global skincare market was valued at approximately US$104.24. billion in 2022, with the Asia-Pacific region accounted for US$54 billion, or 51.8%.The global skincare market is expected to grow from approximately US$109.71 billion to US$167.22 billion by 2030, representing a compound annual growth rate (“CAGR”) of approximately 6.21%. In terms of volume, according to Fortune Business Insights, the Asia-Pacific region is expected to grow from approximately 8.4 billion units in 2019 to approximately 9.5 billion units in 2024, representing a CAGR of approximately 2.5%.
Growth Drivers in the ASEAN Region
We believe that the market for the health and wellness industry will continue to see rapid growth, in part due to the rising wealth in the ASEAN region resulting in increased purchase power. According to an article by the Boston Consulting Group (BCG) published in May 2023, the trade in ASEAN grew by 33% between 2017 to 2021, outpaced the global trade at 24% during this period. BCG also forecasts exports from ASEAN will surge by 90% to US$3.2 trillion annually by 2031, and ASEAN’s combined GDP is projected to grow by a CAGR of 4.6% till 2030, becoming the world’s fourth largest market if viewed as single economy. (source: https://www.bcg.com/publications/2023/asean-free-trade-advantage-to-power-ahead) ASEAN countries have established six Free Trade Agreements with seven of the region’s main trading partners – Australia and New Zealand, China, India, South Korea, Japan, and Hong Kong. The Comprehensive and Progressive Agreement for Trans-Pacific Partnership (CPTPP) is one of the largest Free Trade Agreements in the world and accounts for almost 13.5% of global GDP. The agreement brings together Australia, Brunei Darussalam, Canada, Chile, Japan, Malaysia, Mexico, New Zealand, Peru, Singapore, and Vietnam, offering these countries investment access and free trade. For example, a study by the World Bank in 2018 pointed out that, conservatively, CPTPP will increase Vietnam’s GDP by 1.1% by 2030. (source: https://www.worldbank.org/en/news/press-release/2018/03/09/cptpp-brings-vietnam-direct-economic-benefits-and-stimulate-domestic-reforms-wb-report-says#:~:text=%E2%80%9CEven%20under%20conservative%20assumptions%2C%20the,Bank%20Country%20Director%20for%20V.) Furthermore, in January 2022 the Regional Comprehensive Economic Partnership (RCEP) took effect and the bloc accounts for roughly 30% of the world GDP. According to a report by Asian Development Bank, RCEP is expected to increase the income of member economies by 0.6% while adding US$245 billion to the regional economy. (source: https://blogs.adb.org/blog/three-areas-where-rcep-may-help-region-s-post-pandemic-recovery) Economic wealth has allowed for social development with more than 100 million people estimated to have joined ASEAN’s workforce over the past 20 years and another 59 million people are projected to be added by 2030. (source: https://www.sydney.edu.au/sydney-southeast-asia-centre/news/5-facts-about-asean.html#:~:text=More%20than%20100%20million%20people,third%2Dlargest%20labour%20force%20worldwide.) We believe a direct advantage of such economic growth is a fast-emerging middle class that will be attracted to our company and its products.
We believe that generally unhealthy lifestyles in the ASEAN population continues to form a basis for the growth in the health and wellness industry in the region. In a MarketWatch article citing Astute Analytica in June 2023, it was noted that the ASEAN Nutritonal Supplement market size is set to reach US$14.85 billion by 2031 from US$7.37 billion representing a CAGR of 8.40% during the forecast period. Increasing prevalence of lifestyle-induced disorders, or Non-Communicable Diseases (NCDs), such as diabetes and cancer, will be a key factor driving food supplements market growth. (source: https://www.marketwatch.com/press-release/asean-nutritional-supplements-market-research-uncovering-insights-for-strategic-decision-making-forecast-2023-to-2031-2023-06-08) Estimates computed by the World Health Organization (WHO) state that close to 8 million people die every year in Southeast Asia due to NCDs, amounting to 55% of the total deaths in the region in a given year. According to the WHO, four risk factors tobacco, alcohol, lack of exercise, and poor diets are primarily responsible for the spread of NCDs in the region. For instance, the WHO found that at least 25% of boys in Malaysia and Thailand are obese and a large number of school children across Southeast Asia are largely physically inactive. Such conditions will contribute to a growth in demand for dietary supplement products, in order to promote a healthier lifestyle.
A rapidly aging population in the ASEAN region also promotes the need for preventive measures to mitigate against rising healthcare costs. An International Monetary Fund publication in April 2017 found that in East Asia, the population is projected to be the world’s fastest-aging region with its old-age dependency ratio roughly tripling by current rends by 2050. According to an article by Fortune Business Insights published in April 2020, the aging population in Southeast Asia has led to a significant rise in incidences of lifestyle-related diseases. As a result, healthcare costs will inevitably rise, leading to increased demand in the use of supplements in preventing deteriorative health conditions.
| 43 |
Industry Challenges
In spite of its high growth, the health and wellness industry also faces certain challenges. We believe the following are some key challenges to the industry:
| ● | Price sensitivity - health and wellness products such as dietary supplements and skincare products typically have premium prices which may only be affordable to certain segments of the population. Price conscious consumers with low purchasing power may not be able to purchase such products. | |
| ● | Consumer awareness – although COVID-19 has driven the increased consumption of immunity-related health supplements, consumer awareness on the benefits of dietary supplements may be relatively low in ASEAN region, leading to slower update of products. According to an article published by Nutri Avenue in November 2022, the consumption areas of global dietary supplements are mainly distributed in countries and regions like the United States, the EU, Canada, Australia, and Japan, as those countries have the highest penetration rate of nutritional supplements. (source: https://www.nutriavenue.com/key-country-dietary-supplement-market-2022/) |
Competitive Landscape
The health and wellness industry in ASEAN remains highly competitive and fragmented. Key entry barriers of the industry include the following:
| ● | Capital requirements – Market participants are required to possess sufficient amount of capital and human resources to sustain their businesses, particularly the product research and development (R&D) process, daily operation costs and maintaining personnel knowledgeable in the products such as dietary consultants. | |
| ● | Reputation and relationship with suppliers and customers – In general, current market participants have already established an extensive business network with their upstream product suppliers, as well as established reputable products that attract customers. New market entrants without a prior supply chain and reputable products may find it difficult to build credible relationships with other suppliers or gain the trust of customers. |
Our Strategies
We intend to pursue the following strategies to further develop and expand our business:
Expand the product range in each of our ATP Zeta Health Program, ÉNERGÉTIQUE and BEAUNIQUE series
We intend to continue to expand the product range in each of our product lines, namely, ATP Zeta Health Program, ÉNERGÉTIQUE and BEAUNIQUE series.
Further Penetrate Existing Markets.
We believe that there are several opportunities to further penetrate our existing markets. For example, besides offering dietary products and services through our ATP Zeta Health Program, we have also expanded our products and services to include beauty and wellness products via the introduction of ÉNERGÉTIQUE and BEAUNIQUE series in July 2018 and March 2019, respectively, with the goal of diversifying our product offerings and catering to broader market demands. Currently we maintain three sales branches in different locations in Malaysia, namely, Kuala Lumpur, Johor Bahru and Ipoh, and appointed three stockists, to whom the Company can assign its products, at two other locations in Malaysia to further cater to our distributors, members and customers.
Currently, the Company’s distributors and members mainly consists of the ethnic Chinese community. As a multi-racial country, the majority of the population in Malaysia is comprised of Bumiputera, which is comprised of Malays and other indigenous peoples, accounting for 62% of the entire Malaysia population, with an estimated population at approximately 33.9 million (as compared to the ethnic Chinese community which accounts for less than 22.8%). (source: https://www.igi-global.com/dictionary/innovation-culture-as-a-mediator-between-specific-human-capital-and-innovation-performance-among-bumiputera-smes-in-malaysia/58789). We believe there is opportunity for further penetration of our products into other ethnicity group within the existing Malaysian market.
As we further grow our business, we may further expand our local sales centers to additional locations with the aim to further distribute our products and appeal to local demand.
On November 11, 2021, Agape ATP Corporation (Labuan) formed a joint-venture entity, DSY Wellness with Mr. Steve Yap following which Agape ATP Corporation (Labuan) owns 60% of the equity interests in DSY Wellness, to pursue the business of providing complementary health therapies. The establishment of DSY Wellness is a further expansion of our business into the health and wellness industry, with target customers including patients with chronic health disorder, chronic health issues and non-communicable diseases. We will also promote the prevention of chronic health disorder through lifestyle and nutrition programs supported by the health therapies of DSY Wellness.
| 44 |
Deepening our Relationships with Existing Members.
We offer membership and distributor discounts on all our product offerings. Customers are able to become lifetime members by paying a one-time membership fee with the purchase of specific products. Members who reach a predetermined amount of purchases per year are automatically promoted to become a distributor who also enjoys bonuses for products that they sell to other customers, as well as bonuses from the collective performance of their network group. As our members and distributors recognize the value of our platform and the quality of our products, they typically purchase additional products utilizing their membership and discount entitlements. Our sales strategy is focused on expanding our revenue per member. We believe there is significant opportunity for existing members to become distributers, as well as for distributors to further recruit new distributors under their network.
Further Investment into Information Technology such the Establishment of an E-Commerce platform.
In 2019, we embarked upon a strategic initiative to sell our products on an existing Malaysian e-trading platform to increase the efficiency of our supply chain, to better support and service our distributors and members, and to establish a global reach.
In 2022, we launched our e-trading website (https://www.agapeatp.com/index.php/en/) to provide better customer experience. We will continue to actively promote our e-trading platform for online recruitment of new members by existing distributors, as well as to provide direct sales to customers.
Once the e-trading platform has provided tangible results in the Malaysia market, we intend to expand the platform to other geographic markets in order to duplicate its success.
In line with the current popularity of using social influencers to boost product demand, the Company is also exploring the future appointment of key social influencers with significant numbers of followers as ambassadors for the Company’s products.
Geographic Expansion.
A key component of our strategy is to enter into and expand into new markets with similar cultures and a high demand for health and nutrition products. For example, the majority of our product information sessions and training seminars for distributors are currently conducted in Mandarin, which is the common language spoken amongst the majority of our distributors. We intend to invest into other Asian markets such as Taiwan, where Mandarin is also widely used and understood, allowing for the seamless transition in distributor training and membership recruitment.
With a view to facilitate geographical expansion, our future e-trading platform will also increase efficiency of our supply chain to better support and service our distributors and members as well as to provide online recruitment of new members by existing distributors and to provide direct sales to customers.
In 2022, we had launched our e-trading website (https://www.agapeatp.com/index.php/en/) to entice and provide better customer experience. We will continue to actively promote our e-trading platform for online recruitment of new members by existing distributors, as well as to provide direct sales to customers. Upon our e-trading platform having reached tangible results in the Malaysia market, we intend to expand the platform to other geographic markets in order to duplicate its success.
Pursue Growth Through the Acquisition of Other Health and Wellness Services Providers
The health and wellness industry is highly fragmented, and we intends to pursue growth through the acquisitions of services providers in the ASEAN with a strong brand presence in their area of operations. In reviewing potential targets, we identified target companies matching the following criterial (i) with profitability and customer base comprising customers from health care industry; and (ii) with established knowledge base of empirical/holistic skills, knowledge and technologies which are applicable to transform our company into a wellness ecosystem company such as skin care, cosmetic biological lab production, wellness center or complementary medicine therapies for chronic health problems, manufacturers of water filtration system, etc.
Our Competitive Strengths
We believe the following competitive strengths contribute to our success and differentiate us from our competitors:
Well Established Reputation.
We have a well-established reputation in the Malaysia market, where our newly acquired subsidiary, Agape Superior Living Sdn Bhd has been operating as a reputable provider of our ATP Zeta Health Program for over 18 years.
Well-Established Product Portfolio.
We are committed to building our brand, and distributor and customer loyalty by providing quality health-oriented and wellness products. We have no expenditures or expenses relating to research and development of our products as all of our products are acquired from unrelated third parties and rebranded by us. Due to the high costs associated with research and development of nutrition and health products, we do not maintain any facilities to produce our products. We leverage our team of in-house nutritional consultants with rich experience gained in the area of nutritionist work, in collaborating with our customers and clients to understand the health and wellness market via a process of consultative review. We then communicate our findings and proposals to third-party suppliers to improve formulations, and to bring about new products for distributors and members who are ready to market to end-users.
| 45 |
Large, Highly-Motivated Distributor Base, Supported By Successful Training Methodology.
We had over 128,652 members, including 56,462 distributors, as of June 30, 2023. Because we believe the direct sales model is the most effective way to sell our products, we devote significant resources and management attention to assist our distributors in recruiting and retaining our members. We provide our distributors with successful training methodology, which includes meetings, workshops and activities to create social connections among distributors to develop proficiency of knowledge, confidence and skills to build recruitment strength. We structured our compensation system to encourage distributors to remain active in the business and to build a distributor network of their own, which can serve to increase their income and to increase our product sales. In order to encourage entrepreneurship within our distributors, we also maintain six service centers, including three operated by our stockists, to better service our distributors and members.
Scalable Business Model.
Our business model enables us to grow our business with minimal investment in our infrastructure and other fixed costs. We do not require a company-employed sales force to market and sell our products. As a result, we do not incur direct incremental cost to add a new distributor. Our distributor compensation varies directly with sales. In addition, our distributors bear the majority of our consumer marketing expenses. Furthermore, we can readily increase inventory and distribution of our products as a result of our partnerships with our third-party suppliers.
Deeply Experienced Founder-led Management Team.
Our founder, Dr. How Kok Choong, has led our company for over 18 years. In Malaysia, Dr. How Kok Choong was recognized by the Junior Chamber Malaysia (JCM) as an Outstanding Young Malaysian 2003, and was awarded the title of Justice of Peace of Malaysia since 2005. Dr. How received the Outstanding Asian Community Contribution Award in 2011, Malaysia Top Team 50 Enterprise Award in 2011 and 2016, The Contributor Award (Medical and Health Research) in 2012, “Man of The Year” in Worldwide Excellence Award in 2015, “Man of The Year” in McMillan Global Award in 2016, The Distinguished Asia Pacific Outstanding Entrepreneur Lifetime Achievement Award in 2019, World Outstanding Chinese Entrepreneur Lifetime Award in 2019 and Certified Professional Trainer of The International Professional Managers Association in 2019. In 2023, Dr. How was awarded the Global Business Icon Leadership Lifetime Achievement Award by KSI Strategic Institute for Asia Pacific in Kiala Lumpur, Malaysia. In the same year, he was awarded the ASEAN Outstanding Entrepreneur Lifetime Achievement Award in Jakarta, Indonesia.
Mr. Lee Kam Fan, Andrew has served as our chief financial officer since January 2021. Prior to joining the Company in January 2021, Mr. Lee had approximately 38 years of accounting and finance related experience. Since July 2014, Mr. Lee has been the proprietor of Andrew Lee & Company, a Certified Public Accountants Firm in Hong Kong that provides audit and taxation services. Mr. Lee is an associate member of the Institute of Chartered Accountants in England and Wales since April 2019, a certified public accountant (practicing) of the Hong Kong Institute of Certified Public Accountants since May 2010, a fellow member of the Association of International Accountants since December 2006, and an associate member and certified tax advisor of the Tax Institute of Hong Kong since July 2010. Mr. Lee received his bachelor’s degree in business administration at the Open University of Hong Kong in June 2004 and his master’s degree in professional accounting from the Hong Kong Polytechnic University in November 2010.
Mr. Steve Yap serves as the director since 2021 and is responsible for the operation of DSY Wellness International Sdn Bhd. Mr. Steve Yap is a well-respected figure in the field of nutritional, metabolic and anti-ageing medicine in Malaysia, and is a certified nutritional practitioner. Mr. Steve Yap is committed to developing and promoting self-empowering health preventive programs for the public to cope with chronic health disorders. Over the years, Mr. Steve Yap has served in various technical committees within the Ministry of Health Malaysia. Currently, he is a member of the traditional & complementary medicine council, which involved in the drafting of the Traditional and Complementary Medicine (T&CM) Act 2016 in Malaysia. Mr. Steve Yap is also president for the Association of Nutritional Medicine Practitioners. With Mr. Steve Yap’s involvement, we believe he will significantly enhance the technical capability of the company and accelerate the development of the group in the field of wellness.
Collectively, our senior leadership team has extensive management skills, accounting, financial and nutritional knowledge and expertise.
Product Overview
We offer three series of products: (i) ATP Zeta Health Program, (ii) ÉNERGÉTIQUE and (iii) BEAUNIQUE.
Our ATP Zeta Health Program is a health program designed to promote health and general wellbeing, as well as to prevent diseases caused by polluted environments, unhealthy dietary intake and unhealthy lifestyles. At its core, the ATP Zeta Super Health Program is focused upon biological energy, Adenosine Triphosphate (ATP), at the cellular level. The stimulation of ATP production at the cellular level can increase an individual’s metabolic rate in order to promote and maintain normal and healthy functioning of the body’s systems. Our program emphasizes nutrient absorption through the membrane ion channel in order to provide complete and balanced nutrients to improve cellular health. Thus, ATP Zeta Super Health Program provides ionized and high zeta potential (high bioavailability) nutrients to enhance the absorption at the cellular level.
Our ÉNERGÉTIQUE product series is comprised of the ÉNERGÉTIQUE Mask series, Hyaluronic Acid Serum and Mousse Facial Cleanser.
The ÉNERGÉTIQUE Mask series is formulated with triple action natural ingredients and advanced technology. The innovative combination of award-winning liposome encapsulating the customized fast acting essence, produces micro-particle liposome which, when combined with collagen peptide Tencel film, creates a formulation designed to benefit the skin at the cellular level. The ÉNERGÉTIQUE series aims to provide a total dermal solution for healthy skin beginning at the cellular level. There are three types of face masks in the ÉNERGÉTIQUE Mask Series, each addressing a specific skin condition Med-Hydration, Med-Whitening and Med-Firming. The ÉNERGÉTIQUE Mask Series has clinically shown deep penetration of liposomal essence into deep skin layers within 5 minutes of application, in order to deliver immediate, deep-reaching and long-lasting benefits of skin hydration, whitening, and firming.
| 46 |
The ÉNERGÉTIQUE Hyaluronic Acid Serum is formulated with four functional hyaluronic acids and a unique peptide. It is a scientifically advanced and intensive quintuple action serum designed to promote skin hydration, reparation and regeneration to enhance skin viscoelasticity for improved skin firmness.
The ÉNERGÉTIQUE Mousse Facial Cleanser is formulated with the mildest surface-active agents available on the market. It takes the form of a unique mousse like-foam that delivers a comfortable and soft feeling to the skin during and after use without compromising the moisturizing level and viscoelastic properties of the skin. Its PH-balanced formula is suitable for all skin types for an effortless cleansing routine.
Our BEAUNIQUE product series focuses on the research of our diet’s impact on modifying gene expressions in order to address genetic variations and deliver a personalized nutrigenomic solution for every individual.
ATP Zeta Health Program
The following is a list of our ATP Zeta Health Program products:
ATP1s Survivor Select
ATP1s Survivor Select contains various essential nutrients required by the human body to maintain normal metabolism, which includes production of biological energy (ATP). Effective production of ATP enhances both physical and mental health and helps the body build resistance to diseases.

ATP2 Energized Mineral Concentrate
ATP2 is a nutritional supplement made from the finest plant substances and also is a proprietary formulation of a super-energized colloidal concentrate developed from a dibase solution. Its formula supports and enhances nutritional biochemical activities.
| 47 |

ATP3 Ionized Cal-Mag
ATP3 Ionized Cal-Mag is a specialized calcium and magnesium minerals supplement that is designed to transform into an ionic form completely before entering the body. This is compatible to the cellular ion channel theory, that all cellular metabolisms are dependent on ionic transmission, in order to achieve the highest absorption rate. This product was tested for its nanoparticle by the National Measurement Institute of Australian Government, with proven content of nanosized calcium and magnesium that has better absorption and bio-availability.

| 48 |
ATP4 Omega Blend
ATP4 Omega Blend is a proprietary oil blend that is rich in undamaged polyunsaturated essential fatty acid, which is fully extracted from plant-based ingredients. It provides a bio-effective balance of both essential fatty acids, Omega 3 and Omega 6 which are the important structural components of cell membranes that cannot be synthesized by humans.

ATP5 BetaMaxx
ATP5 BetaMaxx is derived from the cell wall of premium food-grade baker’s yeast and is a medical breakthrough result of more than 50 years of intensive research and studies by scientists and physicians. This product combines the immunostimulatory properties of molecularly structured beta 1-3, 1-6-D-glucan with other immunomodulating compounds that work to make ATP5 a unique and effective natural product.

| 49 |
AGN-Vege Fruit Fiber
AGN-Vege Fruit Fiber is a special nutrition-based formula for intestines and the stomach. It consists of four essential components for gastrointestinal health effects - fiber, probiotic the “friendly bacteria,”, prebiotic fructooligosaccharides (FOS) and digestive enzymes.

AGP1-Iron
AGP1-Iron is the purest and most advanced Colloidal Iron that is sourced from the remains of an ancient rainforest which contains the most active plant-based element from nature. The colloidal nanosized iron provides high zeta potential that promotes better absorption and cellular iron uptake through the ion channel.
| 50 |

YFA-Young Formula
YFA-Young Formula is a 100% natural unique formula, a combination of amino acid, vitamins, and minerals. It is an anti-aging and youthful maintenance supplement. It stimulates the pituitary gland to release endocrine hormones such as human growth hormone (HGH) to stimulate synergies, thus achieving the efficacy of anti-ageing through the promotion of cells vitality and strengthening of organ functionality.

| 51 |

BEAUNIQUE Mito+ and Mitogize
We discontinued ATP Regal Mitogize on October 1, 2019. In its stead, an enhanced formula, the BEAUNIQUE Mito+ was introduced in November 2019. As a strong antioxidant drink with great flavor and taste, the preeminence of BEAUNIQUE Mito+ is its ability to further protect and stimulate mitochondria (the membrane-bound organelles which produces energy for cells) in cellular energy (ATP) production with the added advantage of fewer total sugars and calories. The new formula is comprised of 11 food groups, including potent mangosteen skin extract. Backed by advanced scientific research and tested on 88 nutrigenomic profiles, the new formulation revealed enhanced antioxidant properties. 96.34% DPPH Radical Scavenging activity, an approximate 22% increase compared to Mitogize.
DSY Wellness
On November 11, 2021, Agape ATP Corporation (Labuan) formed a joint-venture entity, DSY Wellness with Mr. Steve Yap following which Agape ATP Corporation (Labuan) owns 60% of the equity interest, to pursue the business of providing complementary health therapies. The establishment of DSY Wellness is a further expansion of our business into the health and wellness industry. DSY Wellness offers health consultancy and advice, as well as nutritional supplements at medical dosages, as prescribed by in-house nutritional practitioners.
| 52 |
ORYC-Organic Youth Care Cleansing Bar
ORYC-Organic Youth Care Cleansing Bar is a natural, organic cleansing soap for skin. It contains pure Australian-accredited natural and organic plant oils acting as a high quality and natural skin lubricant. It maintains the softness of the skin while promoting skin beauty and radiance.

ÉNERGÉTIQUE
The following is a list of our ÉNERGÉTIQUE products:
N°1 Med-Hydration
Formulated with a Sea Grape (Caulerpa lentillifera) extract, the N°1 Med-Hydration enhances skin moisture and luminosity. This treatment effectively improves the moisture content of the inner skin layer and rejuvenate the skin barrier function in order to avoid moisture loss.

| 53 |
N°2 Med-Whitening
Formulated with Peach Blossom Stem Cell Extract, N°2 Med-Whitening has clinically shown its efficacy in inhibiting the melanin synthesis, down-regulating the melanin synthesis gene, boosting skin moisture level and protecting skin against UV radiation.

N°3 Med-Firming
Formulated with the Djulis (Chenopodium formosanum Koidz) Seed Extract, the native cereal plant in Taiwan is traditionally called “ruby of cereals.” The formulation is clinically proven to be effective in stimulation of collagen secretion and anti-advances glycation end-products (AGEs) reducing the glycation of skin collagen, providing protection and maintenance of the basal skin collagen production.
| 54 |

BEAUNIQUE
The Company’s BEAUNIQUE product series focuses on the research of our diet’s impact on modifying gene expressions in order to address genetic variations and deliver personalized nutrigenomic solutions for every individual.
Trim+
Trim+ is the first product launched under this series, which utilizes advanced technology to extract active ingredients in foods. Trim+ has been scientifically proven to be effective in inhibiting the activities of carbohydrates digestive enzymes, which results in a reduction of the breakdown and absorption of sugars.

| 55 |
ÉNERGÉTIQUE
On November 3, 2019, the Company expanded its beauty products under the ÉNERGÉTIQUE series, to include beauty essentials of the skincare routine, i.e. the ÉNERGÉTIQUE Hyaluronic Acid Serum and ÉNERGÉTIQUE Mousse Facial Cleanser. These products have extended the ÉNERGÉTIQUE brand vision in offering a total dermal solution for a healthy skin beginning from the cellular level.
ÉNERGÉTIQUE Hyaluronic Acid (HA) Serum
Formulated with four functional hyaluronic acids and a unique peptide, this scientifically advanced and intensive quintuple action serum has been proven to deliver 5Rs dermal benefits. Filled in an innovative yet convenient and hygienics syringe packaging, this HA serum also ensures consumer benefits for every skin type.

| 56 |
ÉNERGÉTIQUE Mousse Facial Cleanser
Formulated with mild surface-active agents available on the market, this facial cleanser is designed to deliver a distinct cleansing benefits to consumers. The unique mousse like-foam delivers a comfortable and soft feeling of the skin during and after use without compromising the moisturizing level and viscoelastic properties of the skin.

Our Business Model
We believe that the direct-selling channel is ideally suited to market our products, because sales of health solution and personal care products are strengthened by ongoing personal contact between retail consumers and distributors. This personal contact may enhance consumers’ nutritional and health education and motivate consumers to begin and maintain wellness and weight management programs. In addition, by using our products themselves, distributors can provide first-hand testimonials of product effectiveness, which can serve as a powerful sales tool.
| 57 |
We are focused on building and maintaining our distributor network by offering financially rewarding and flexible career opportunities through the sale of quality, innovative products to health conscious consumers. We believe the income opportunity provided by our bonus program appeals to a broad cross-section of our members, particularly those seeking to supplement family income, start a home business or pursue entrepreneurial, full and part-time, employment opportunities. Our distributors, who are all independent third parties, profit from selling our products and also earning bonuses through performance of their network group, the establishment of their own network group and the performance of distributors recruited under their own network group. Top performing distributors with their own physical stores may also become stockists of the company, whereby they enjoy benefits such as maintaining a certain amount of the Company’s inventory in their store premises, with the requirement that all product sales are monitored through our centralized stock tracking system and accounted back to us. The stockists have the option of returning or exchanging any unsold inventory consigned to them.
We enable distributors to maximize their potential by providing a broad array of motivational, educational and support services. We motivate our distributors through our performance-based compensation plan, product-training seminars, workshops and participation in routine promotional activities.
We are committed to providing professionally designed educational training materials that our distributors can use to enhance recruitment and to maximize their sales. We conduct several training sessions per year to motivate our distributors. These training events teach our distributors not only how to develop invaluable business-building and leadership skills, but also how to differentiate our products with their consumers, including information sessions presented by in-house nutritional consultants.
Our corporate-sponsored training events provide a forum for distributors, who otherwise operate independently, to share ideas with us and each other. In addition we are also developing an e-marketing and e-trading platform allowing for marketing and trading of products to members, as well as online recruitment of new members and to provide direct sales to customers.
We are committed to providing our distributors with quality products to help them increase sales and recruit additional distributors. We leverage our team of in-house nutritional consultants with rich experience gained in the area of nutrition, in collaborating with our customers and clients to understand the health and wellness market via a process of consultative review. This review team is headed by the Head of Product Development. We then communicate our findings and proposals to third-party suppliers to improve formulations, to bring about new products for distributors and members who are ready to market to end-users.
We place a strong emphasis on the science of nutrition. We have obtained the appropriate authorizations from the Food Safety and Quality Division, and/or registered with the National Pharmaceutical Regulatory Agency (NPRA) of the Ministry of Health, Malaysia for our products. Whenever products are purchased for inventory replenishment, samples are randomly selected from every batch for testing at laboratories registered with the Ministry of Health Malaysia.
Our Customers
General
We provide health and wellness products and advisory services to health-conscious customers in the Malaysian market. Such customers are able to enjoy membership discounts across all our products by becoming members.
Our distributors enjoy further discounts on all of our products. Besides our three sales branches that are located in Kuala Lumpur, Johor Bahru and Ipoh, our products are also distributed to customers and members by our network of three stockists who are also independent distributors, whose store premises are located in two other locations in Malaysia.
We believe that our products are particularly well-suited for direct distribution because the sale of health and nutrition products are strengthened by ongoing personal contact between retail customers and distributors. We believe our continued commitment to sourcing quality science-based products will enhance our ability to attract new customer, as well as increase the productivity and retention of our distributors.
| 58 |
Structure of the membership program
Our customers are able to become lifetime members by paying a one-time membership fee with the purchase of specific products. Doing so allows the customer to enjoy membership discounts on all our products.
Members who accumulate a predetermined number of purchases are automatically promoted to become a distributor of the Company. Other than helping distributors achieve physical health and wellness through the use of our products, we offer our distributors, who are independent third parties, bonuses based on various performance factors. Distributors are required to maintain a predetermined number of purchases per year in order to maintain their distributor status.
Top performing distributors with their own physical stores may also become stockists of the Company, whereby they enjoy benefits such as maintaining a certain amount of the Company’s inventory in their store premises. The stockists shall account to the Company for all products sales from their store premises as monitored through the Company’s centralized stock tracking system. The stockists shall have the option to either return or exchange the Company’s inventory consigned to them that are unsold.
The following table sets forth the number of members and distributors at the dates indicated:
| Number of Distributors | Number of Members | Total Number of Distributors and Members | ||||||||||
| As at June 30, 2023 | 56,462 | 72,190 | 128,652 | |||||||||
Distributors’ and members’ earnings
Distributors and members earn profits from the sales of our products to customers. Distributors enjoy additional discounts compared to members, allowing them to earn higher direct profits through the differences in pricing when selling products they bought at distributors’ prices which are more favorable than member’s prices to customers.
Members are encouraged to build their respective network group. Members are promoted to distributors if they manage to recruit the requisite number of members; and the network group is able to achieve set sales targets. Other than preferential distributor pricing for the purchase of the Company’s products, distributors enjoy bonuses from the collective performance of their network group. There are several levels of distributors depending on the size and the collective sales performance of their respective network group. Each level affords bonus benefits in a different form in ascending order. A higher-level distributor will be compensated with higher returns in the form of bonus entitlements.
Distributors and members motivation and training
We believe that motivation, inspiration and training are key elements in the success of sales via network group marketing. Together with our distributors and members, we have established a consistent schedule of gatherings to support those needs. We conduct several training sessions per year to educate and motivate our distributors and members. The training sessions are typically presented by in-house staff with suitable background in nutrition, in order to provide key nutrition information about our products, as well as providing workshops to promote presentation skills to attending participants.
Our Suppliers
Currently, all of our products are acquired from unrelated third parties located in Australia, the United States, Germany and Malaysia, and rebranded by us. Due to the high costs associated with research and development of nutrition and health products, we do not maintain any facilities to produce our products. We have no expenditures or expenses relating to research and development of our product. We leverage our team of in-house nutritional consultants with rich experience gained in the area of nutritionist work, in collaborating with our customers and clients to understand the health and wellness market via a process of consultative review. We then communicate our findings and proposals to third-party suppliers to improve formulations and to bring about new products for distributors and members who are ready to market to end-users.
Up to the year ended December 31, 2020, we purchased from Agape S.E.A. Sdn Bhd, one of our largest suppliers, through the SEA Supply Agreement. For more information, please see “The SEA Supply Agreement” below. For the year ended December 31, 2022, we purchased from our three largest suppliers through purchase order forms which included customary terms including unit price, quantity, total price of the orders, and order lead times. We did not enter into any long-term supply agreements with our major suppliers for the six months ended June 30, 2023.
| 59 |
The SEA Supply Agreement
Agape S.E.A Sdn Bhd is a dietary supplement company founded in Malaysia. We originally entered into the SEA Supply Agreement with Agape S.E.A. Sdn Bhd, which was one of our largest suppliers at the time, in May 2018. Under the SEA Supply Agreement we purchased dietary supplement products and skincare products from Agape S.E.A.
However, the Company no longer relied on the VIE after the fiscal year ended December 31, 2020. For the six months ended June 30, 2023, and the years ended December 31, 2022 and 2021, we did not purchase any products from Agape S.E.A. Sdn Bhd.
The following summarizes the major terms of the SEA Supply Agreement:
| Sales of Goods: | The agreement stipulates the type of goods sold, transported and delivered, with a minimum quantity per order between 5,000 to 10,000 units per order. | |
| Purchase price: | The agreement stipulates that the Company shall place order for goods using a purchase order. The purchase prices under the SEA Supply Agreement are based on and in accordance with each purchase order. Agape S.E.A shall be responsible for all taxes in connection with the purchase of goods under the SEA Supply Agreement. | |
| Payment: | Payment for goods is due within seven days of the date of the Agape S.E.A’s invoice, which date will not be before the date of delivery of goods. | |
| Delivery: | The delivery date and delivery destination of each purchase shall be determined by both parties in a purchase order. Agape S.E.A. shall deliver the goods in accordance with the terms and conditions specified separately in each purchase order, including without limitation the quantity and delivery date. The Company is responsible for freight insurance arising from shipment to a single delivery destination. For destinations outside of Malaysia, the Company is also responsible for freight, freight insurance, tariffs and custom clearance fees. | |
| Risk of Loss: | Title to and risk of loss of the goods shall pass to the Company upon shipment of the goods. | |
| Right of Inspection | The Company shall be allowed to examine the goods once received and shall do so within fourteen days after the receipt of the goods. In the event the Company discovers any damages, shortages or other non-conformance of the goods, the Company shall notify Agape S.E.A within fourteen days specifying the basis of the claim. In the event of non-conformance, the Company has the following options: | |
| -retuning the goods for a replacement at Agape S.E.A’s expense;-returning the goods at Agape S.E.A’s expense for a credit of the full purchase price on future transactions; or-returning the goods at Agape S.E.A’s expense for a full refund of the purchase price. | ||
| Warranties: | The buyer acknowledges that it has not relied on, and that Agape S.E.A has not made any representations or warranties with respect to the quality or condition of the goods, and is purchasing the goods on an “as is” basis. | |
| Security Interest: | The Company grants Agape S.E.A a security interest in the goods, until the Company has paid the seller in full for the goods. |
| 60 |
| Seller Representations and Warranties: | Agape S.E.A warrants that the goods are free, and at the time of delivery will be free, from any security interest or other liens or encumbrances, and there are no outstanding titles or claims of title hostile to the rights of Agape S.E.A in the goods. | |
| Limitation of Liability: | Agape S.E.A will not be liable for any indirect, special, consequential or punitive damages (including lost profits) arising out of or relating to this agreement or the transactions contemplated by it contemplates. | |
| Assignment: | Neither party may assign any of its rights or delegate any performance under the agreement, except with prior consent from the other party | |
| Governing Law: | The terms of the agreement shall be governed by and construed in accordance with the laws of England. | |
| Breach/ Termination: | Each party has an obligation to notify the other party of any breach, and where the breach is rectifiable, the breaching party has 21 days from the date of notification of its breach to rectify. |
Quality Control
At present, our products are predominately sold in Malaysia. As the contents and combination of the main ingredients in our ATP Zeta Health Program and BEAUNIQUE series are categorized as health food rather than medicines or drugs, all of our products require authorization from the Food Safety and Quality Division of the Ministry of Health, Malaysia according to the Food Act 1983 (ACT 281) & Regulations in order to be sold in the country. Accordingly, we have obtained the appropriate authorizations from the Food Safety and Quality Division of the Ministry of Health, Malaysia for all products in our ATP Zeta Health Program and BEAUNIQUE series.
Our ÉNERGÉTIQUE series is regulated under the Control of Drugs and Cosmetics Regulations 1984, the Ministry of Health, Malaysia. We have also obtained the appropriate authorizations for distribution and sale of the products.
Inventory
The Company operates a central warehouse at its head office in Kuala Lumpur, Malaysia, which typically maintains an inventory reserve of up to 6 months per product. Inventory is transferred to the Company’s sales branches via ordering through the Company’s centralized stock tracking system. Stockists of the Company are required to have physical stores, and enjoys the benefit of being able to store certain amount of inventory in their stores for convenience. The stockists shall account to the Company for all products sales from their store premises as monitored through the Company’s centralized stock tracking system. The stockists shall have the option to either return or exchange the Company’s inventory consigned to them that are unsold.
Seasonality
The Company’s business is generally not subject to any seasonality factors.
Warranty
Our products include a customer satisfaction guarantee. Under this guarantee, within 90 days of purchase, any customer who is not satisfied with our product for any reason may return it or any unused portion of it to the distributor from whom it was purchased for a full refund from the Company or credit toward the purchase of another product.
Historically, product returns have not been significant.
| 61 |
E-commerce system
In order to facilitate our continued growth and to support distributor activities, we continually invest and upgrade our platforms. In 2019, we invested in an initiative to establish e-commerce through the setup of e-trading of our products on an existing Malaysian e-commerce trading platform. Our e-trading initiative will be actively promoted for online recruitment of new members by existing distributors and to provide direct sales to customers. Once the E-trading platform has provided tangible results in the Malaysia market, we intend to expand the platform to other geographic markets in order to duplicate its success. We also intend to approach online social influencers as part of our marketing strategy to promote our products and our e-commerce platform.
Intellectual Property
We consider trademarks, patents and copyrights to protect our intellectual property rights critical to our success. We are the registered owner of five registered trademarks and with 1 trademark pending registration in Malaysia. We have one applied to register an additional one trademarks in Malaysia. We are also the registered owner of five domain names, namely “agapeatpgroup.com”, “agapeatpcorporation.com”, “atpsummit.com”, “agapeatpgroup.my” and “agapeatpgroup.com.my.”
| Category | Registration Number | Trade Marks Logo | Ownership | Country | Effective Date and Duration | |||||
| Trademark | 06010456 |
[Class 30] |
Agape Superior Living Sdn. Bhd. | Malaysia | May 20, 2016 For 10 Years | |||||
| Trademark | 2017005364 |  |
Agape ATP Corporation | Malaysia | May
05, 2017 For 10 Years | |||||
| [Class 35] | ||||||||||
| Trademark | 2019023588 | 
[Class 3] |
Agape ATP Corporation | Malaysia | July 03, 2019 For 10 Years | |||||
| Trademark | 2019023590 |  |
Agape ATP Corporation | Malaysia | July 03, 2019 For 10 Years | |||||
| [Class 5] | ||||||||||
| Trademark | 2019023589 |  |
Agape ATP Corporation | Malaysia | July
03, 2019 For 10 Years | |||||
| [Class 16] | ||||||||||
| Trademark | 2019036886 |  |
Agape Superior Living Sdn. Bhd. | Malaysia | June
03, 2019 For 10 Years | |||||
| [Class 5] |
Employees
As at June 30, 2023 we had 30 employees (excluding our Directors). The following table sets forth the number of employees by function:
| Function | Number of employees | |||
| Senior Management | 1 | |||
| Business Development Department | 2 | |||
| Finance Department | 5 | |||
| Human Resources Department | 5 | |||
| Operations Department | 8 | |||
| Product Development Department | 3 | |||
| Marketing Department | 3 | |||
| Corporate Affairs Department | 3 | |||
| Total | 30 | |||
Properties
We currently lease 6 properties ranging from approximately 2,500 to 11,900 square feet in Kuala Lumpur, Johor Bahru and Ipoh which primarily carry out the functions of a staff accommodation, warehouse, office, service centers and sales branches in different regions of Malaysia.
Insurance
The Employees’ Social Security Act, 1969, Malaysia mandates employers and employees to make a monthly contribution to the Social Security Organisation, Malaysia, (“SOCSO”) for any employee who is employed for wages paid under a contract of service or apprenticeship with an employer for the purpose of providing social security protection to employees and their dependents against occupational injuries, including industrial accident, accident during emergency at the employers’ premises, occupational diseases and commuting accidents. Depending on the monthly wages earned by the employee, employers shall cause to be deducted from the respective employee’s wages, amounts that ranges between RM0.10 to RM19.75 for monthly wages between RM30 to RM4,000. The employers’ contribution correspond to the said rates are between RM0.4 to RM69.05. Rates applicable to both the employee and employer are fixed at the maximum rate of RM19.75 and RM69.05 respectively. Employees who have attained 60 years of age are not required to contribute to the scheme. The employer’s responsibility towards this group shall be at a reduced rate which ranges between MYR0.30 to RM49.40 for the said wage band.
Other than SOCSO, effective January 1, 2018, employees and employers in the private sector are mandated to contribute to an employment insurance system, (“EIS”) under the Employment Insurance System Act, 2017. Both the employee and employer shall contribute at an equal rate at 0.2% of the employee’s wages under the scheme, subject to a maximum monthly wage rate of RM4,000. No further contribution to the scheme is required from the employee or the employer for employees who have attained 60 years of age; and employees aged 57 and above who have no prior contributions are exempted.
We do not have any third-party liability insurance to cover claims in respect of personal injury or property or environmental damage arising from accidents on our property or relating to our operations. Such insurance is not mandatory according to the laws and regulations of Malaysia. We typically do not require our distributors to purchase insurance regarding their operations. We believe this practice is consistent with customary industry standards.
Legal Proceeding
We are not subjected to nor engaged in any litigation, arbitration or claim of material importance, and no litigation, arbitration or claim of material importance is known to us to be pending or threatened by or against our Company that would have a material adverse effect on our Company’s results of operations or financial condition.
| 62 |
REGULATIONS
This section sets forth a summary of the most significant rules and regulations that affect our business activities in Malaysia or the rights of our stockholders to receive dividends and other distributions from us.
Regulations Related to Health and Wellness
The Food Act 1983 (Act 281) and the Food Regulations 1985
The primary legislations governing the various aspects of food safety and quality control in Malaysia are (i) the Food Act 1983 (Act 281) (“the 1983 Act”); and (ii) the Food Regulations 1985 (“the 1985 Regulations”), both under the purview of the Food Safety and Quality Division (FSQD) of the Ministry of Health, Malaysia. The ministry also oversees the implementation and enforcement of the legislations. The objective of the 1983 Act is to ensure that the public is protected from health hazards and fraud in the preparation, sale and use of foods and for matters incidental thereto or connected therewith.
The 1983 Act and the 1985 Regulations are applicable to all foods sold in the country either locally produced or imported, covers a broad spectrum from compositional standards to food additives, nutrient supplements, contaminants, packages and containers, food labelling, procedure for taking samples, food irradiation, and penalty.
The 1983 Act strictly prohibits food adulteration, food containing substances injurious to health and food unfit for human consumption. The legislation also ensures that consumer gets the right information from product labels; and that claims on food labels are legitimate.
Food as defined under the 1983 Act, includes every article manufactured, sold or represented for use as food or drink for human consumption or which enters into or is used in the composition, preparation, preservation, of any food or drink and includes confectionery, chewing substances and any ingredient of such food, drink, confectionery or chewing substances. This includes food for special dietary use for persons with specific diseases, disorders or medical conditions, and food which contain quantities of added nutrients allowable under the 1983 Act and the 1985 Regulations.
The general requirements on product labelling for food on sale provided under the 1985 Regulations are as follows:
| (i) | All labels shall be durably marked on the material of the package or on material firmly attached to the package. |
| (ii) | All text should be in Bahasa Malaysia, i.e. the official language of Malaysia, if the food is produced, prepared or packaged in Malaysia. If the food is imported, all text should be in Bahasa Malaysia or English. In either case, translation into other languages may be included. |
| (iii) | Important particulars required on product labels are: |
| ● | A description of the food containing the common name of its principal ingredients. In the case of mixed and blended food, the appropriate description that the contents are mixed or blended. Where the food contains beef or pork, or its derivatives, or lard, a statement to that effect. Alcohol where presence in the food should be clearly marked in capital bold-faced lettering of a non-serif character not smaller than 6 point, in the form, “CONTAINS ALCOHOL”. Where the food consists of two or more ingredients, other than water, food additives and added nutrient, the appropriate designation of each of those ingredients in descending order of proportion by weight, and wherever required by the 198s Regulations, a declaration of the proportion of such ingredient. Any ingredients known to cause hypersensitivity shall be declared on the label. | |
| ● | Quantity of the food package. |
| 63 |
| ● | The name and address of the manufacturer and packer, or the owner of the rights of manufacture or packing or the agent of any of them, for food manufactured or packed in Malaysia; and the additional information of the name and address of the importer in Malaysia and country of origin for imported food. | |
| ● | Depending on its composition, words such as “genetically modified (name of ingredient)”, “produced from genetically modified (name of ingredient)”, or “gene derived from (common name of such animal”) shall appear on the label. | |
| ● | Marked with the expiry date or the date of minimum durability of that food. | |
| ● | If the validity of date marking of food is dependent on its storage, then the storage direction of that food shall also be required on its label. |
Further, based on the Guide to Nutrition Labelling and Claims, the nutritional information that must be declared on a product label are energy, protein, carbohydrate and fat. In addition, total sugars must also be declared for ready-to-drink beverages. Information on energy value is to be expressed as kcal (kilocalories) per 100 g or per 100 ml of the food or per package if the package contains only a single portion. In addition, the energy value should also be given for each serving of the food as quantified on the label. Besides kcal, energy value may also be expressed as kilojoule (kJ). The amount of protein, carbohydrate and fat should be expressed as g per 100 g or per 100 ml of the food or per package if the package contains only a single portion. In addition, the amount of these nutrients in the food should also be given for each serving of the food as quantified on the label.
Other than the mandatory nutrients, other nutrients may also be displayed on the nutrition label. These include vitamins and minerals, dietary fibre, sodium, cholesterol, fatty acids, amino acid, nucleotide and other food components.
Both ATP Zeta Health Program and BEAUNIQUE product series are regulated under the 1983 Act and the 1985 Regulation. ASL, the product owner of these product series are subject to 1983 Act and the 1985 Regulation.
Traditional and Complementary Medicine (T&CM) Act 2016 [Act 775]
The Traditional and Complementary Medicine (T&CM) Act 2016 [Act 775] (the “TCM Act”) is an act to provide for the establishment of the T&CM Council to regulate the T&CM services in Malaysia and to provide for matters connected therewith. The TCM Act received Royal Assent on 2 March 2016 and was published in the Federal Government Gazette on 10 March 2016.
The enforcement of the TCM Act is implemented in phases. Phase 1 begun operation on August 1, 2016 with the establishment of the T&CM Council, identification of recognized practice areas, setting up the registration criteria for recognized practice areas, designation of practitioner body under section 42 of the TCM Act and enforcement of the various sections under Phase 1 in the TCM Act.
Phase 2 of the TCM Act begun operation on March 1, 2021 and include the registration of T&CM practitioners in recognized practice areas with the T&CM Council, the enforcement of various sections under the TCM Act and the operation of T&CM Regulations 2021. The transitional period of Phase 2 of the TCM Act began on March 1, 2021 and will last until February 29, 2024.
The business activities of DSY Wellness International Sdn Bhd, our complementary health therapies are regulated under TCM Act. DSY Wellness International Sdn Bhd complementary health therapies are subject to TCM Act.
Control of Drugs and Cosmetics Regulations 1984
The Malaysian government enacted the Control of Drugs and Cosmetics Regulations 1984 (“the 1984 Regulations”) to regulate the manufacture, sell, supply, import, possess or administer of cosmetics. The authority that oversee the 1984 Regulations is the National Pharmaceutical Regulatory Agency (“NPRA”) under the Ministry of Health, Malaysia. All cosmetics industry players who intend to manufacture or import any cosmetic, must apply the notification of cosmetics (“NOC”) through NPRA.
Pursuant to Regulation 18A of the 1984 Regulations, cosmetics cannot be manufactured or sold if:
| (i) | The cosmetic has not been notified with the NPRA; |
| (ii) | The person is a not person who has been designated to place the notified cosmetics in the market; |
| (iii) | The cosmetic is a notified cosmetic but it has been mixed with poison (as defined by the Poisons Act 1952); |
| (iv) | The notified cosmetic has been mixed with a registered product; |
| (v) | The cosmetic is labelled with another name other than the name notified by the Director of Pharmaceutical Services; |
| (vi) | The cosmetic has been labelled in a way that does not comply with any directives/guidelines issued by the Director of Pharmaceutical Services; |
| (vii) | The cosmetic’s notification has been cancelled by the Director of Pharmaceutical Services; or |
| (viii) | The cosmetic is labelled with words, symbols or safety features that claim to be true but is otherwise. |
ÉNERGÉTIQUE product series are regulated under the 1984 regulation. ASL, the product owner of these product series is subject to the 1984 Regulations and relevant regulation.
| 64 |
Regulations Related to Consumer Protection
Consumer Protection Act 1999 (Act 599)
The principal law for consumer protection in Malaysia is the Consumer Protection Act 1999 (Act 599) (“the 1999 Act”). The 1999 Act establishes various consumer protection mechanisms in Malaysia, and bridge gaps that may occur in other major laws, which may be inadequate in protecting consumers. The government agency which is primarily responsible for policy-making and law enforcement on consumer protection in Malaysia is the Ministry of Domestic Trade and Consumer Affairs (MDTCA). The MDTCA is also responsible for receiving consumer complaints and acts as a secretariat to the National Consumer Advisory Council (NCAC) – an institution established by the Minister of Domestic Trade and Consumer Affairs to advise him on any relevant consumer issues and the implementation of the 1999 Act.
The 1999 Act has undergone several amendments since its enactment to cover various emerging issues relating to consumers, including the inclusion unfair contract terms, inclusion of credit sale agreements of goods and the most recent amendment on July 23, 2019 related to Tribunal for Consumer Claims Malaysia. Amendments to this Act are to increase the jurisdiction limit of claim hearing from RM25,000.00 to RM50,000.00 and the increase of maximum penalty for non-compliance with the Tribunal’s award.
The 1999 Act covers almost every aspects of consumer protection; ranging from misleading and deceptive conducts, false representation and unfair practices; safety of goods and services; unfair contract terms; guarantees in respect of the supply of goods and services; and product liability; to the establishment, structure and functions of the National Consumer Advisory Council; the Committee on Advertisement; the Tribunals for Consumer Claims; and other matters related to enforcement, offences, remedies, and compensation.
All series products produced by us in Malaysia are subject to Consumer Protection Act 1999 (Act 599).
Direct Sales and Anti-Pyramid Scheme Act 1993 (Act 500) and Regulations.
In Malaysia, network marketing is regulated by the Direct Sales and Anti-Pyramid Scheme Act 1993 (Act 500) (“the 1993 Act) and Regulations. The 1993 Act provides for the licensing of persons carrying on direct sales business, for the regulation of direct selling, for prohibiting pyramid scheme or arrangement, chain distribution scheme or arrangement, or any similar scheme or arrangement, and for other matters connected therewith. The implementation and enforcement of the 1993 Act is governed by the Ministry of Domestic Trade and Consumer Affairs.
Under the 1993 Act, subject to section 14 and 42 no person shall carry on any direct sales business unless it is a company incorporated under the Companies Act 1965 and holds a valid licence granted under Section 6. The Controller may grant licence under Section 6 of the 1993 Act with conditions and licensee shall comply with the any conditions of the licence imposed by the Controller. By virtue of Section 8 of the 1983 Act, the Controller has the power to revoke a licence granted if he is satisfied that there are grounds on which his power to revoke a licence is exercisable under subsection 8(1). In lieu of revocation of licence, the Controller may restrict the licence by:
| (a) | Imposing limits on the duration of the licence; |
| (b) | Imposing conditions as he thinks desirable or expedient for the protection of the purchasers; or |
| (c) | Imposing both limits and conditions on the licence. |
| 65 |
We have the responsibility to ensure that our marketing plan is in compliance with the Direct Sales (Scheme and Conduct) Regulations 2001, not promoting pyramid scheme and have the following characteristics:
| (a) | In the presentation of the direct sales scheme, a person who carries on any direct sales business shall not mislead participants by overemphasizing on disproportionately high bonus or bonus payout. Each participant shall be provided with sales kit that includes the marketing plan and code of conduct of the company. |
| (b) | Any person who carries on a direct sales business shall provide an incentive based on the volume or quantity of goods or services sold or distributed by each participant and not based on recruitment of persons into the scheme. |
| (c) | Participants not to purchase goods or services in an unreasonable amount. Each participant is required to purchase goods or service in an amount that can be expected to be resold or consumed within a reasonable period of time. |
Regulations Related to Intellectual Property Rights
Intellectual property system in Malaysia is administered by the Intellectual Property Corporation of Malaysia (MyIPO), an agency under the Ministry of Domestic Trade and Consumer Affairs.
Trademarks Act 2019 (Act 815)
The Trademarks Act 2019 (Act 815) (“the 2019 Act”) officially came into force in Malaysia on 27 December 2019. The Act repealed the Trade Marks Act 1976 and is seen as opportune in enabling Malaysia to adhere not only to commercial demands and sophistication of the current era, but also to international standards and procedures. The Trademarks Regulations 2019 is also now in force having been gazetted in the Government Gazette on 27 December 2019.
Malaysia is also a member of various trademark-related treating, including:
| (i) | Protocol relating to the Madrid Agreement concerning the International Registration of Marks since 27 December 2019; |
| (ii) | Nice Agreement concerning the International Classification of Goods and Services since 28 September 2007; |
| (iii) | Paris Convention for the Protection of Industrial Property since 1 January 1989; and |
| (iv) | Agreement on Trade-related Aspects of Intellectual Property Rights (TRIPS) since 1 January 1995. |
The 2019 Act provides that any person who claims to be the bona fide proprietor of a trademark may apply for the registration of the trademark if:
| (i) | the person is using or intends to use the trademark in the course of trade; or |
| (ii) | the person has authorised or intends to authorise another person to use the trademark in the course of trade. |
The 2019 Act has also expanded the types of trademark recognized for registration to be more than just word, logo, numbers, name. signature, letter and to include shape of goods or their packaging, colour, sound, scent, hologram, positioning marks and sequence of motion of any combination thereof; provided that they must be signs capable of being represented graphically.
In general, Malaysia provides for protection for both registered and unregistered trademarks. Unregistered trademarks are protected under common law rights, particularly in the tort of passing off. In fact even during the examination of trademark, the Registrar shall refuse, under relative grounds of Section 24(4) of the 2019 Act, to register it if the mark’s use in Malaysia is prevented by virtue of any rule of law protecting an unregistered trademark or other sign used in the course of trade including the law of passing of.
The scope of trademark infringement and its exemptions has been substantially expanded by the 2019 Act. There could now be infringement even in the use of a similar mark on similar goods or services (as opposed to being identical). Liability will stick to secondary users who know or have reasons to believe that such use is without authorization of the trademark proprietor.
The 2019 Act and relevant regulation are applicable own our brand, word, logo, numbers, name. signature, letter and to include shape of goods or their packaging, color, sound, scent, hologram, positioning marks and/or sequence of motion of any combination.
Copyright Act 1987 (Act 332)
Copyright protection in Malaysia is governed by the Copyright Act 1987 (Act 332) (“the 1987 Act) which provides comprehensive protection for copyrightable works. The 1987 Act outlines the nature of works eligible for copyright (which includes computer software), the scope of protection, and the manner in which the protection is accorded. A unique feature of the 1987 Act is the inclusion of provisions for enforcing the Act, which include such powers to enter premises suspected of having infringing copies and to search and seize infringing copies and contrivances. Malaysia is a signatory of the Berne Convention. Foreign works of non-Berne member countries are also protected if they are made in Malaysia and are published in Malaysia within thirty days of their first publication in the country of origin.
| 66 |
Unlike trademarks, designs and patents (other intellectual property rights), there is no specific system of registration for copyright in Malaysia. Although copyright is a non-registrable right in Malaysia and enjoys automatic protection, ownership of copyright is difficult to establish. As such, proper documentation can be prepared to prove ownership. Copyright owners can claim ownership by way of a Statutory Declaration or by filing a Voluntary Notification at the MyIPO.
The definition of a literary work now includes table or compilations “whether or not expressed in words, figures or symbols and whether or not in a visible form”. The owner of copyright in a work including a derivative work, will have the exclusive right to control “the transmission of a work through wire or wireless means to the public, including the making available of a work to the public in such a way that members of the public may access the work from a place and at a time individually chosen by them”.
It is also an infringement of copyright to circumvent any effective technological measures aimed at restricting access to works, removal or alteration of any electronic rights management information without authority, or distribution, importation for distribution or communication to the public, without authority, works or copies of works in respect of which electronic rights management information has been removed or altered without authority.
The 1987 Act and the relevant regulations are benefited us which we are eligible to claim ownership by compiling proper documentation to prove ownership via a Statutory Declaration or by filing a Voluntary Notification at the MyIPO.
Regulations Related to Employment and Social Security
Employment Act 1955 (Act 265)
The Employment Act 1955 (Act 265) (“the 1955 Act) is the primary legislation on labour matters in Malaysia. The 1955 Act provides for minimum work requirements and benefits of employment, such as maximum working hours, overtime entitlement, leave entitlement, maternity protection and termination benefits. The 1955 Act applies only to employees earning a monthly wages of not more than RM2,000.00 or to employees, irrespective of their monthly wages, who are engaged in manual labour, including artisan or apprentice, or who are engaged in the operation of maintenance of mechanically propelled vehicles operated for the transport of passengers or goods or for commercial purposes, or who supervise or oversee other employees engaged in manual labour or who are engaged in any capacity in any vessel registered in Malaysia or who are engaged as domestic servant.
Children and Young Persons (Employment) Act 1966 (Act 350)
Children and Young Person (Employment) Act 1996 (Act 350) (“the 1996 Act”) prohibits children from working near hazardous and poisonous material. The 1996 Act defines a “child” is a person who is under the age of fifteen years and a “young person” is a person who is fifteen or older, but below the age of eighteen years. The 1996 Act goes on to provide the minimum working hours for a child and young person. Further, under 1996 Act no child or young person shall be, or be required or permitted to be, engaged in any employment contrary to the provisions of the Factories and Machinery Act 1967 (Act 139), the Occupational Safety and Health Act 1994 (Act 514) or the Electricity Supply Act 1990 (Act 447) or in any employment requiring him to work underground. Any person contravening the provisions under the 1996 Act shall be guilty of an offense and shall be liable on conviction to imprisonment of not exceeding 2 years or to fine not exceeding RM50,000 or to both; and for repeat offenders, shall be liable on conviction to imprisonment of not exceeding 5 years or to fine not exceeding RM100,000 or to both.
Employees’ Provident Fund Act 1991 (Act 452)
The Employees’ Provident Fund Act 1991 (Act 452) (“the 1991 Act”) imposes the statutory obligations on employers and employees to make contribution towards the Employees Provident Fund, which is essentially a fund established as a scheme of savings for employees’ retirement and the management of savings for the retirement purposes. Under the 1991 Act, any employer who fails to pay the necessary contributions shall be liable to imprisonment for a term not exceeding three years or to a fine not exceeding ten thousand ringgit or to both.
| 67 |
Employee’ Social Security Act 1969 (Act 4)
The Employee’s Social Security Act 1969 (Act 4) (“the 1969 Act’) was implemented to provide protection for employees and their families against economic and social distress in situations where the employees sustain injury or death. The schemes of social security under the 1969 Act are administered by Social Security Organization (“SOCSO”) and are financed by compulsory contributions made by the employers and the employees. Under the 1969 Act, any person who fails to make contribution shall be all be punishable with imprisonment for a term which may extend to two years, or with fine not exceeding ten thousand ringgit, or with both.
Employment Insurance System Act 2017 (Act 800)
SOCSO reached a milestone when the Employment Insurance System Act 2017 (Act 800) was introduced and enforced from 28 December 2017 with the aim to provide protection and assist workers who have lost employment through two (2) main components namely, the Employment Insurance and Active Labour Market Policies. The Employment Insurance System (EIS) provides protection to workers who have lost their employment through income replacement, reskilling and upskilling training to enhance their employability as well as employment services so that they can secure other suitable jobs fast.
Our all employees which under definition of the Employment Act 1955 (Act 265) (“the 1955 Act) are subject to the provision of Employees’ Provident Fund Act 1991 (Act 452), Employee’ Social Security Act 1969 (Act 4), and Employment Insurance System Act 2017 (Act 800).
Regulation Related to Taxation
Income Tax Act 1967 (Act 53)
The Income Tax Act 1967 (Act 53) (“the 1967 Act”) imposes a tax, known as income tax, for each year of assessment upon the income accruing in or derived from Malaysia, or received in Malaysia from other countries. A company is a tax resident in Malaysia if its management or control is exercised in Malaysia and generally, the place where directors’ meetings are held concerning management and control of the company are considered in determining where the management and control of the company is exercised.
Under the 1967 Act, any person who makes an incorrect tax return by omitting or understating income or gives incorrect information affecting chargeability to tax otherwise than in good faith shall be guilty of an offence and shall upon conviction be liable to a fine not less than RM1,000.00 and not more than RM10,000.00 and shall pay a special penalty of double the amount of tax which had been undercharged.
The Company which is incorporated under Companies Act 2016 (Act 777) is subject to Income Tax Act The Company which is incorporated under Companies Act 2016 (Act 777) is subject to Income Tax Act.
Regulation Related to Foreign Exchange Control
Financial Services Act 2013 (Act 758)
The Financial Services Act 2013 (Act 758) provides regulation and supervision of financial institutions, payment systems and other relevant entities and the oversight of the money market and foreign exchange market to promote financial stability and for related, consequential or incidental matters.
Pursuant to the Foreign Exchange Administration Rules, a resident entity with domestic ringgit is only allowed to invest abroad up to RM50 million per calendar year (“the Maximum Foreign Investment”). For the avoidance of doubt, the limit of such Maximum Foreign Investment applies to the resident entities within the group of companies.
Notwithstanding the above, the Foreign Exchange Administration Rules allows non-residents to remit out divestment proceeds, profits, dividends or any income arising from investments in Malaysia. Repatriation, however, must be made in foreign currency.
As such, if our operating subsidiaries intend to invest exceeding the Maximum Foreign Investment, we are required to seek approval from the controller of Foreign Exchange, Central Bank of Malaysia
Regulation Related to Competition Law
Competition Act 2010 (Act 712)
In Malaysia, under the Competition Act 2010 (Act 712) (“the 2010 Act), such provisions may be considered to be anti-competitive if they are found to significantly prevent, restrict or distort competition in any market for goods or services. The 2010 Act is regulated by the Malaysia Competition Commission (“MyCC”), an independent body established under the Competition Commission Act 2010 (Act 713) to enforce the 2010 Act. The Competition Commission Act 2010 empowers MyCC to carry out functions such as implement and enforce the provisions of the 2010 Act, issue guidelines in relation to the implementation and enforcement of the competition laws, act as advocate for competition matters; carry out general studies in relation to issues connected with competition in the Malaysian economy or particular sectors of the Malaysian economy; inform and educate the public regarding the ways in which competition may benefit consumers in and the economy of Malaysia.
| 68 |
The 2010 Act prohibits horizontal or vertical agreements between enterprises that either the object or effect of significantly preventing, restricting or distorting competition in Malaysia. This is referred to as “Chapter One Prohibition”. MyCC has indicated in its “Guidelines on Chapter 1 Prohibition” that in general, anti-competitive agreements will not be considered “significant” if:
| (i) | the parties to the agreement are competitors who are in the same market and their combined market share of the relevant market does not exceed 20%’ or |
| (ii) | the parties to the agreement are not competitors and their individual market share in relevant market is not more than 25%. |
Further, the 2010 Act also prohibits enterprises from abusing their “dominant position” in a market. This is referred to as the “Chapter Two Prohibition”. The term “dominant position “refers to one or more enterprises possessing such significant power in a market that they are able to adjust prices, outputs, or trading terms without effective constraint from competitors or potential competitors. There are no specific thresholds for abuse of a dominant position However, the following are the types of abuses prohibited under the 2010 Act; (i) predatory behaviour (for example, margin squeeze, and predatory pricing); (ii) refusal to supply; (iii) buying up scarce supply; and (iv) limiting output.
Pursuant to MyCC “Guidelines on Chapter 2 Prohibition”, market share above 60% would be indicative that an enterprise is dominant. Nevertheless, market share shall not by itself be regarded as conclusive of dominance and other factors will be taken into account is assessing whether an enterprise is dominant.
In there is any infringement with the 2010 Act, MyCC may (i) require that the infringement be ceased immediately; (ii) specify steps which are required to be taken by the infringing enterprise(s) to bring the infringement to an end; (iii) impose financial penalties which could, for example, be 10% of the worldwide turnover of the relevant enterprise over the period during which an infringement occurred; or (iv) take any number of other actions, including imposing sanctions and penalties, as they deem appropriate.
We shall ensure there is any infringement with the 2010 Act, which we shall not:
(a) Be the parties to the agreement are competitors who are in the same market and their combined market share of the relevant market exceed 20%; or
(b) Be the parties to the agreement are not competitors and their individual market share in relevant market is more than 25%.
Regulation Related to Establishment, Operation and Management of Malaysia Subsidiaries
Companies Act 2016 (Act 777)
The Companies Act 2016 (Act 777) (“the 2016 Act”) stipulates that a company must be registered with the Companies Commission Malaysia in order to engage in any business activity. Under the 2016 Act, a company shall have - (a) a name; (b) one or more members, having limited or unlimited liability for the obligations of the company; (c) in the case of a company limited by shares, one or more shares; and (d) one or more directors. With the liberalization in Malaysia equity policy, foreign companies/investors generally could hold 100% equity in majority industries except for strategic sectors of national interest such as water, telecommunications, ports, and energy. For every industry, there are specific sector regulations issued by the relevant governmental departments. These include regulations that could impose restrictions on the foreign ownership of equity of a company, require higher paid up capital requirements and also prior regulatory approval before the commencement of business operations. However, limits on foreign ownership do remain in place across many sectors such as telecommunications, oil & gas, tourism, wholesale and retail distributive trade, and financial services. A corporation is a “wholly-owned subsidiary” of another corporation if it has no members except— (a) that other corporation or its nominee; or (b) a wholly-owned subsidiary of that other corporation or its nominee. Private companies require a minimum of one director. A director shall ordinarily reside in Malaysia by having a principal place of residence in Malaysia.
Pursuant to the 2016 Act, appointment of an auditor is mandatory. However, the Registrar may exempt private companies from appointing an auditor where the Company is dormant, a zero-revenue company or a threshold-qualified company. Companies that elect to be exempted from audit must still lodge unaudited financial statements and the required statutory certificates with the Registrar of Companies. Since the coming into effect of the 2016 Act, private companies are no longer obligated to convene annual general meetings. However, stockholders have the right to request for the directors of the company to convene a general meeting. This right is however subject to the requirements in Section 311 of the 2016 Act.
For all companies incorporated in Malaysia (except in Labuan, Malaysia) are subject to the Companies Act 2016 (Act 777).
| 69 |
MANAGEMENT
Directors and Executive Officers
The following table sets forth information regarding our executive officers and directors as of the date of this prospectus:
| Directors and Executive Officers (Last Name, First Name) | Age | Position/ Title | ||
| How Kok Choong | 59 | Chief Executive Officer, President, Director, Chief Operating Officer, Chairman of the board of Directors and Secretary | ||
Lee Kam Fan Andrew |
61 | Chief Financial Officer | ||
Ramesh Ruben Louis |
45 | Independent Director Nominee | ||
Vong John Hing |
69 | Independent Director Nominee | ||
| Chee Chin Aik | 43 | Independent Director Nominee | ||
| * | Each of Mr. Ramesh Ruben Louis, Dr. John Hing Vong and Mr. Chee Chin Aik has accepted our appointment to be our independent director, effective upon the SEC’s declaration of effectiveness of our registration statement on Form S-1, of which this prospectus is a part. |
Dr. How Kok Choong is our founder and serves as our Chief Executive Officer, President, Director Chief Operating Officer, Chairman of the Board of Directors and Secretary since 2016. Dr. How is primarily responsible for overall development and business strategies, financial, administrative and human resources affairs of the Company. Dr. How has more than 20 years of experience in the senior management roles in the health and wellness industry. From 1987 to 2016, Dr. How was with the San Hin Group of Companies and his last position held was the group chief executive officer for the group. Since August 2003, Dr. How began to work for AGAPE Superior Living International Group as the global president and continues to hold this position. Further, since September 2009, Dr. How has worked for TH3 Holdings Sdn Bhd as president. Dr. How obtained a master’s degree and a doctorate degree in Business Administrative from Newport University, USA in December 1997 and December 2000, respectively. In Malaysia, Dr. How Kok Choong was recognized by the Junior Chamber Malaysia (JCM) as an Outstanding Young Malaysian 2003, and was awarded the title of Justice of Peace of Malaysia since 2005. Dr. How Kok Choong received the Outstanding Asian Community Contribution Award in 2011, Malaysia Top Team 50 Enterprise Award in 2011 and 2016, The Contributor Award (Medical and Health Research) in 2012, “Man of The Year” in Worldwide Excellence Award in 2015, “Man of The Year” in McMillan Global Award in 2016, The Distinguished Asia Pacific Outstanding Entrepreneur Lifetime Achievement Award in 2019, World Outstanding Chinese Entrepreneur Lifetime Award in 2019 and Certified Professional Trainer of The International Professional Managers Association in 2019.
Mr. Lee Kam Fan, Andrew serves as our chief financial officer of the Company. Prior to joining the Company in January 2021, Mr. Lee has approximately 38 years of accounting and finance related experience. Since July 2014, Mr. Lee has been the proprietor of Andrew Lee & Company. Since June 2010, Mr. Lee served as an adjunct lecturer of the HKICPA Processional Examinations Preparatory Programme at HKU Space. From January 2011 to October 2015, Mr. Lee served as the managing director at ANSA CPA Limited. From September 2010 to October 2012, Mr. Lee served as an independent non-executive director at Sunrise (China) Technology Group Limited (currently referred to as KOALA Financial Group Limited (Hong Kong stock code: 08226)). From March 2006 to April 2017, Mr. Lee was in cooperation with Friedman LLP to oversee financial statements are prepared in accordance with U.S. GAAP. From October 2000 to December 2010, Mr. Lee served as an audit manager and subsequently a partner at Clodick & Company. From April 1998 to September 2000, Mr. Lee served as a director at Nitwell Business Services Limited. From August 1994 to April 1998, Mr. Lee was an assistant audit manager at Cheng, Kwok & Chang. From July 1990 to July 1994, Mr. Lee served as an accountant at K.C. Manufacturing Company. From April 1989 to July 1990, Mr. Lee served as an accountant at Haldane, Midgley & Booth. From January 1987 to April 1989, Mr. Lee served as an audit senior at RSM Nelson Wheeler. From October 1985 to December 1986, Mr. Lee served as an audit assistant at Andrew Ma & Company. From April 1983 to September 1985, Mr. Lee served as an audit Clerk at Anthony Y.T. Tse & Company. Mr. Lee is an associate member of the Institute of Chartered Accountants in England and Wales since April 2019, a certified public accountant (practicing) of the Hong Kong Institute of Certified Public Accountants since May 2010, a fellow member of the Association of International Accountants since December 2006, and an associate member and certified tax advisor of the Tax Institute of Hong Kong since July 2010. Mr. Lee received his bachelor’s degree in business administration at the Open University of Hong Kong (currently referred to as Hong Kong Metropolitan University) in June 2004 and his master’s degree in professional accounting from the Hong Kong Polytechnic University in November 2010.
Mr. Ramesh Ruben Louis, PhD will serve as our independent director upon effectiveness of the registration statement of which this prospectus forms a part. Prior to joining the Company, Mr. Louis, PhD has approximately 25 years of accounting and finance related experience. Since January 2011, Mr. Louis, PhD has been an executive director and principal consultant of Assurance Threesixty Consulting. Since November 2009, Mr. Louis, PhD has been a professional freelance trainer and consultant at My Learning Training Resources, where he conducted various training courses including training for MIA, ACCA, CPA Australia ISCA Singapore. From May 2006 to October 2009, Mr. Louis, PhD was an executive director at Anuarul Azizan Chew Group, where he was involved in internal audit, risk management and review/assessment of internal controls assignments of various organisations including public listed companies in Malaysia. From 2000 to 2006, Mr. Louis, PhD worked in BDO Binder, where he worked in areas including corporate finance and assurance advisory, his last role being assistant audit manager. From 1997 to 1998, Mr. Louis, PhD was an audit assistant at Arthur Andersen & Co. Mr. Louis, PhD graduated with a bachelor of accounting from the National University of Malaysia in 2000, and graduated with a master of business administration from the University of Strathclyde, United Kingdom in 2012. Mr. Louis, PhD obtained a doctorate of philosophy from the University of Malaya in September, 2021. Mr. Louis, PhD became a member of CPA Malaysia in 2005, a member of the Institute of Internal Auditors Malaysia in 2010 and a member of the Association of Chartered Certified Accountants in 2011. Mr. Louis, PhD is also a director of Greenpro Capital Corp., Seatech Ventures Corp. and Fortune Valley Treasures, Inc.
Dr. Vong John Hing, PhD will serve as our independent director upon effectiveness of the registration statement of which this prospectus forms a part. Prior to joining the Company, Dr. Vong, PhD has over 44 years of fintech and education experience. Dr. Vong, Phd is currently the lead of sustainable finance at ClimateWorks Australia since September 2021, a non-executive independent council member of Regional Bank since June 2013, and a senior technical specialist of the United Nations since May 2003. Dr. Vong, PhD has been a senior technical specialist at Asian Development Bank from January 2019 to February 2022, and a senior technical consultant at World Bank Group from September 2006 to June 2021. Dr. Vong, PhD has been a professor at the National University of Singapore from May 2015 to April 2017, foundation director of the Fintech Academy at the Singapore Management University from June 2013 to December 2014 and associate professor at James Cook University Singapore from February 2012 to June 2013. From October 2008 to October 2011, Dr. Vong, PhD was a deputy chief executive officer at Sacombank in Vietnam. In 2002 Dr. Vong, PhD was a senior consultant at PAGF- DFAT Australia in the Philippines. From February 1999 to February 2001, Dr. Vong, PhD was a team leader at Deloitte Australia. From 1994 to 1998 Dr. Vong, PhD was a regional director- lecturer of the Massachusetts Institute Technology and Nanyang Fellows Program at Nanyang Technological University. From May 1978 to August 1993, Dr. Vong, PhD was a senior executive at HSBC Holdings plc in various offices in Australia and Asia. Dr. Vong, PhD completed the public disputes program in advanced negotiation at MIT-Harvard University Consensus Building Institute in 2006. Dr. Vong, PhD graduated with a BA in economics at Birmingham City University, and a MBA in economics strategy and finance at University of Bradford. He received his PhD from the University of Bradford in MIS business intelligence. Dr. Vong, PhD is currently a member of CPA Australia.
Mr. Chee Chin Aik will serve as our independent director upon effectiveness of the registration statement of which this prospectus forms a part. Prior to joining the Company, Mr. Chee has approximately 20 years of finance related experience. Mr. Chee is currently the head of South East Asia, cash and non-cash equity sales, global markets at HSBC Singapore. From October 2020 to May 2022, Mr. Chee was an executive director, head of Malaysia equity distribution and board member of JPMorgan Chase & Co. Mr. Chee has also worked at Credit Suisse for various periods, as director in institutional equity sales for Malaysia and Singapore from January 2019 to September 2020, as vice president in institutional equity sales in Singapore from January 2015 to December 2018, and associate in institutional equity sales in Singapore from April 2013 to December 2014. From January 2009 to August 2011, Mr. Chee worked as analyst and trader in equity derivatives and proprietary trading at Aminvestment Bank Berhad in Malaysia. From October 2007 to September 2008, Mr. Chee worked as equity research analyst at Credit Lyonnais Securities Asia Pacific Markets in Malaysia. From October 2006 to July 2007, Mr. Chee worked as associate in fixed income currencies and commodities at Goldman Sachs in USA. From July 2004 to October 2006, Mr. Chee worked as analyst for fixed income, corporate advisory division at Lehman Brothers Holdings Inc in USA. From Jun 2002 to July 2004, Mr. Chee worked as associate in financial regulatory and cycle examination group at National Association of Securities Dealers (FINRA) in USA. Mr. Chee graduated with a bachelor of arts from Franklin & Marshall College and MBA in finance from the University of Chicago Booth School of Business.
| 70 |
Employment Agreements
We have entered into employment agreements with all of our executive officers. Under these agreements, each of our executive officers is employed for a specified time period. We may terminate employment for cause, at any time, without advance notice or remuneration, for certain acts of the executive officer, such as conviction or plea of guilty to a felony or any crime involving moral turpitude, negligent or dishonest acts to our detriment, or misconduct or a failure to perform agreed duties. We may also terminate an executive officer’s employment without cause upon advance written notice or payment in-lieu of notice. In such case of termination by us, we will provide severance payments to the executive officer as expressly required by applicable law of the jurisdiction where the executive officer is based. The executive officer may resign at any time upon advance written notice.
Each executive officer has agreed to hold, both during and after the termination or expiry of his or her employment agreement, in strict confidence and not to use, except as required in the performance of his or her duties in connection with the employment or pursuant to applicable law, any of our confidential information or trade secrets, any confidential information or trade secrets of our clients or prospective clients, or the confidential or proprietary information of any third party received by us and for which we have confidential obligations.
Terms of Directors and Officers
Our officers are elected by and serve at the discretion of the board of directors and the stockholders voting by ordinary resolution.
Compensation of Directors and Executive Officers
For the years ended December 31, 2022 and 2021, we paid an aggregate of approximately $289,957 and $275,210, respectively, in cash and benefits to our executive officers. We do not have a share incentive program to provide for grants of awards to our directors and executive officers. We have not set aside or accrued any amount to provide pension, retirement or other similar benefits to our executive officers and directors. We have no service contracts with any of our directors providing for benefits upon termination of employment.
Board of Directors and Committees
Our board of directors will consist of four directors, including three independent directors. We will establish three committees under the board of directors immediately upon the effectiveness of our registration statement on Form S-1, of which this prospectus is a part: an audit committee, a compensation committee and a nominating and corporate governance committee. We intend to adopt and approve a charter for each of the three committees prior to consummation of this offering. Each of the committees of the board of directors shall have the composition and responsibilities described below.
Audit Committee
Mr. Louis, PhD, Dr. Vong, PhD, and Mr. Chee will be the members of our Audit Committee where Mr. Louis, PhD shall serve as the chairman. All proposed members of our Audit Committee will satisfy the independence standards promulgated by the SEC and by NASDAQ as such standards apply specifically to members of audit committees.
| 71 |
We intend to adopt and approve a charter for the Audit Committee prior to consummation of this offering. In accordance with our Audit Committee’s Charter, our Audit Committee shall perform several functions, including:
| ● | evaluate the independence and performance of, and assess the qualifications of, our independent auditor, and engage such independent auditor; | |
| ● | approve the plan and fees for the annual audit, quarterly reviews, tax and other audit-related services, and approve in advance any non-audit service to be provided by the independent auditor; | |
| ● | monitor the independence of the independent auditor and the rotation of partners of the independent auditor on our engagement team as required by law; | |
| ● | reviewing our financial statements and our management’s discussion and analysis of financial condition and results of operations to be included in our annual and quarterly reports to be filed with the SEC; | |
| ● | oversee all aspects our systems of internal accounting control and corporate governance functions on behalf of the board; | |
| ● | review and approve in advance any proposed related-party transactions and report to the full board of directors on any approved transactions; and | |
| ● | provide oversight assistance in connection with legal, ethical and risk management compliance programs established by management and the board of directors, including Sarbanes-Oxley Act implementation, and make recommendations to the board of directors regarding corporate governance issues and policy decisions. |
It is determined that Mr. Louis, PhD possesses accounting or related financial management experience that qualifies him as an “audit committee financial expert” as defined by the rules and regulations of the SEC.
Compensation Committee
Mr. Louis, PhD, Dr. Vong, PhD and Mr. Chee will be the members of our Compensation Committee where Dr. Vong, PhD shall be the chairman. All proposed members of our Compensation Committee will be qualified as independent under the current definition promulgated by NASDAQ. We intend to adopt and approve a charter for the Compensation Committee prior to consummation of this offering. In accordance with the Compensation Committee’s Charter, the Compensation Committee shall be responsible for overseeing and making recommendations to the board of directors regarding the salaries and other compensation of our executive officers and general employees and providing assistance and recommendations with respect to our compensation policies and practices.
Nominating and Governance Committee
Mr. Louis, PhD, Dr. Vong, PhD, and Mr. Chee will be the members of our Nominating and Governance Committee where Mr. Chee shall serve as the chairman. All proposed members of our Nominating and Governance Committee will be qualified as independent under the current definition promulgated by NASDAQ. The board of directors intends to adopt and approve a charter for the Nominating and Governance Committee prior to consummation of this offering. In accordance with the Nominating and Governance Committee’s Charter, the Nominating and Corporate Governance Committee shall be responsible for identifying and proposing new potential director nominees to the board of directors for consideration and reviewing our corporate governance policies.
Director Independence
Our board of directors reviewed the materiality of any relationship that each of our proposed directors has with us, either directly or indirectly. Based on this review, it is determined that Mr. Louis, PhD, Dr. Vong, PhD and Mr. Chee will be “independent directors” as defined by NASDAQ. In addition, as required by Nasdaq rules, our board of directors has made a subjective determination as to each independent director that no relationships exist, which, in the opinion of our board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In making these determinations, our board of directors reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and relationships as they may relate to us and our management.
| 72 |
Family Relationships
There is no family relationship among any of our directors or executive officers.
Board Leadership Structure and Role in Risk Oversight
Our Board of Directors, or the Board, is primarily responsible for overseeing our risk management processes on behalf of our company. The Board receives and reviews periodic reports from management, auditors, legal counsel, and others, as considered appropriate regarding our company’s assessment of risks. In addition, the Board focuses on the most significant risks facing our company and our company’s general risk management strategy, and also ensures that risks undertaken by our company are consistent with the board’s appetite for risk. While the Board oversees our company’s risk management, management is responsible for day-to-day risk management processes. We believe this division of responsibilities is the most effective approach for addressing the risks facing our company and that our board leadership structure supports this approach.
Compensation Committee Interlocks and Insider Participation
None of our executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers on our board of directors or compensation committee.
Code of Ethics
We have a code of ethics that applies to all of our employees, including our principal executive officer, principal financial officer and principal accounting officer, and the Board. A copy of this code is available in our employee handbook and under the “About Us – Code of Conduct” section of our website at www.agapeatpgroup.com. In addition, we intend to post on our website all disclosures that are required by law or the listing standards of our applicable trading market concerning any amendments to, or waivers from, any provision of the code. The reference to our website address does not constitute incorporation by reference of the information contained at or available through our website, and you should not consider it to be a part of this prospectus.
| 73 |
Involvement in Certain Legal Proceedings
To our knowledge, our directors and executive officers have not been involved in any of the following events during the past ten years:
| ● | any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; | |
| ● | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); | |
| ● | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities; | |
| ● | being found by a court of competent jurisdiction in a civil action, the SEC or the Commodity Futures Trading Commission to have violated a Federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; | |
| ● | being subject of, or a party to, any Federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any Federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or | |
| ● | being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
| 74 |
EXECUTIVE AND DIRECTOR COMPENSATION
Executive Officers’ Compensation
The following table sets forth information concerning the annual and long-term compensation earned by or paid to our Chief Executive Officer and to other persons who served as executive officers as at fiscal years ended December 31, 2022 and 2021, or the named executive officers, for services as executive officers for the said fiscal year.
Summary Compensation Table
| Name
and Principal Position |
Fiscal
Year |
Salary | Stock
Award |
Option
Awards |
Non-Equity
Incentive Plan Compensation |
Change
in Pension Value and Non- Qualified Deferred Compensation Earnings |
All
Other Compensation |
Total | |||||||||||||||||
| ($) | ($) | ($) | ($) | ($) | ($) | ($) | |||||||||||||||||||
| How Kok Choong | 2022 | 289,957 |
— | — | — | — | — |
289,957 |
|||||||||||||||||
Chief Executive Officer, President, Director, Chief Operating Officer, Chairman of the board of Directors and Secretary |
2021 | 275,210 |
— | — | — | — | — |
275,210 |
|||||||||||||||||
Lee Kam Fan Andrew |
2022 | 46,440 | — | — | — | — | — | 46,440 | |||||||||||||||||
| Chief Financial Officer | 2021 | 46,988 | — | — | — | — | — | 46,988 | |||||||||||||||||
| 75 |
Employment Agreements
How Kok Choong
Dr. How currently devotes approximately 90% per week of his time to manage the affairs of the Company. He has agreed to work with no remuneration nor drawn any (i) bonus; (ii) stock compensation; (iii) option awards; (iv) non-equity incentive plan compensation; (v) non-qualified deferred compensation earnings; and (vi) any other compensations until such time as the Company receives significant revenues necessary to provide management salaries. At this time, we cannot accurately estimate when significant revenues will occur to implement this compensation, or what the amount of the compensation will be.
Lee Kam Fan Andrew
On January 12, 2021, we entered into an Executive Employment Agreement with Mr. Lee Kan Fan Andrew, our Chief Financial Officer. Pursuant to the agreement, Mr. Lee is employed as our Chief Financial Officer. During the term of his employment, Mr. Lee will be entitled to a base salary at the annualized rate of $3,870. Pursuant to the agreement, Mr. Lee may be terminated for “cause” as defined in the agreement and Mr. Lee may resign upon the provision of a prior notice in writing not less than one (1) months to the Company or payment in lieu of notice at any time. In the event Mr. Lee is terminated without cause, we will be required to pay Mr. Lee all accrued salary, reimbursement for all business expenses. In the event Mr. Lee is terminated with cause, dies or is disabled, we will be required to pay Mr. Lee all accrued salary. Under the agreement Mr. Lee is subject to confidentiality restrictions.
Incentive Bonus
The Board may grant incentive bonuses to our executive officer and/or future executive officers in its sole discretion, if the Board of Directors believes such bonuses are in the Company’s best interest, after analyzing our current business objectives and growth, if any, and the amount of revenue we are able to generate each month, which revenue is a direct result of the actions and ability of such executives.
Option Exercises and Stock Vested
We have not granted any stock options to our executive officers since our incorporation.
Long-term, Stock Based Compensation
In order to attract, retain and motivate executive talent necessary to support the Company’s long-term business strategy we may award our executive and any future executives with long-term, stock-based compensation in the future, at the sole discretion of our Board of Directors, which we do not currently have any immediate plans to award. We have not granted any stock options to our executive officers since our incorporation.
No Pension Benefits
We do not maintain any plan that provide for payments or other benefits to our executive officers at, following or in connection with retirement and including, without limitation, any tax-qualified defined benefit plans or supplemental executive retirement plans.
| 76 |
No Nonqualified Deferred Compensation
We do not maintain any defined contribution or other plan that provides for the deferral of compensation on a basis that is not tax-qualified.
Director Compensation
| Name (Last name, First name) | Fees Earned or Paid in Cash $ | Stock Awards $ | Option Awards $ | Non-equity Incentive Plan Compensation $ | Change in Pension Value and Non-Qualified Deferred Compensation Earnings | All Other Compensation $ | Total $* | |||||||||||||||||||||
| How Kok Choong | 289,957 | — | — | — | — | — | 289,957 | |||||||||||||||||||||
Ramesh Ruben Louis | — | — | — | — | — | — | — | |||||||||||||||||||||
| John Hing Vong | — | — | — | — | — | — | — | |||||||||||||||||||||
| Chee Chin Aik | — | — | — | — | — | — | — |
Mr. Ramesh Ruben Louis was appointed to the Board of Directors of our company to serve as independent director. On March 30, 2022, Mr. Louis, PhD entered into an agreement pursuant to which he will serve as an independent director of the Company upon SEC’s declaration of effectiveness of our registration statement on Form S-1. During the term of the agreement, Mr. Louis will be entitled to director fees of US$21,600 per annum. Pursuant to the agreement, Mr. Louis may be terminated for “cause” as defined in the agreement and Mr. Louis may resign upon the provision of a prior notice in writing not less than 14 days to the Company.
Professor Dr. John Hing Vong was appointed to the Board of Directors of our company to serve as independent director. On September 12, 2022, Dr. Vong, PhD entered into an agreement pursuant to which he will serve as an independent director of the Company upon SEC’s declaration of effectiveness of our registration statement on Form S-1. During the term of the agreement, Dr. Vong, PhD will be entitled to director fees of US$21,600 per annum. Pursuant to the agreement, Dr. Vong, PhD may be terminated for “cause” as defined in the agreement and Dr. Vong, PhD may resign upon the provision of a prior notice in writing not less than 14 days to the Company.
Chee Chin Aik was appointed to the Board of Directors of our company to serve as independent director. On November 30, 2022, Mr. Chee entered into an agreement pursuant to which he will serve as an independent director of the Company upon SEC’s declaration of effectiveness of our registration statement on Form S-1. During the term of the agreement, Mr. Chee will be entitled to director fees of US$21,600 per annum. Pursuant to the agreement, Mr. Chee may be terminated for “cause” as defined in the agreement and Mr. Chee may resign upon the provision of a prior notice in writing not less than 14 days to the Company.
| 77 |
CERTAIN RELATIONSHIPS AND RELATED-PARTY TRANSACTIONS
SEC rules require us to disclose any transaction since the beginning of our last fiscal year or any currently proposed transaction in which we are a participant in which the amount involved exceeded or will exceed $120,000 and in which any related person has or will have a direct or indirect material interest. A related person is any executive officer, director, nominee for director, or holder of 5% or more of our common stock, or an immediate family member of any of those persons.
On May 8, 2020, the Company acquired approximately 99.99% of the issued share capital of Agape Superior Living Sdn Bhd from Dr. How Kok Choong. Dr. How received an aggregate consideration of $1,714,003, which was determined based on the net asset carrying value of ASL as at March 31, 2020. The aggregate consideration was satisfied by (i) the offset of the consideration whereby the Company has a loan receivable of $656,495 as of March 31, 2020 due from Dr. How; and (ii) the allotment and issue of the common stock of the Company. The Company allotted and issued 162,694 shares of the Company’s common stock, each with a par value $0.0001, representing approximately 0.0432% of the total issued and outstanding shares in the Company after the issuance of the shares, which was valued at $1,057,508 based on the closing price of $6.50 of the Company as quoted on the OTC Market on March 31, 2020.
On July 1, 2020, the Company and Dr. How Kok Choong agreed to amend the Share Exchange agreement and enter into a supplemental agreement share exchange agreement (the “Supplemental Share Exchange Agreement”). In accordance with Supplemental Share Exchange Agreement, Dr. How received an aggregate consideration of $1,804,046, which was determined based on the net asset carrying value of ASL as at March 31, 2020. The aggregate consideration shall be satisfied by (i) the offset of the consideration whereby the Company has a loan receivable of $656,495 as of March 31, 2020 due from Dr. How; and (ii) the allotment and issuance of common stock of the Company. The Company allotted and issued 176,547 shares of the Company’s common stock, par value $0.0001 (the “Shares”), representing approximately 0.0469% of the total issued and outstanding shares in the Company after the issuance of the Shares, which is valued at $1,147,551 based on the closing price of $6.50 of the Company as quoted on the OTC Market on March 31, 2020.
On February 1, 2021, Dr. How Kok Choong, our CEO and director, was appointed as the non-executive Chairman of Vettons. Vettons Sdn Bhd (“Vettons”) is an e-commerce company through which ASL conducts some of its distribution activities to its members. As of December 31, 2020, the Company has accounts receivable of $172,757 from Vettons, representing 100% of our accounts receivable.
In December 2021, there were share forfeiture agreements (the “Share Forfeiture Agreements”) between the Company and (i) HKC Talent Limited; (ii) various stockholders of the Company (the “Forfeiting Stockholders”), pursuant to which:
(i) HKC Talent Limited had agreed to forfeiture of 41,750,000 shares of common stock of the Company, and
(ii) the Forfeiting Stockholders had agreed to forfeiture, in aggregate, 44,242,000 shares of common stock of the Company. Included in (ii) is 11,242,000 shares forfeited from HKC Holdings Sdn. Bhd, a company in which Dr. How Kok Choong, is a stockholder. As a result, the outstanding shares was reduced by 85,992,000 shares of common stock.
On January 20, 2022, a share forfeiture agreement (the “Share Forfeiture Agreement”) was entered between the Company and Dr. How Kok Choong, pursuant to which Dr. How agreed to forfeit 215,008,035 shares of common stock of the Company.
*HKC Holdings Sdn Bhd is owned and controlled by How Kok Choong who is our executive officer and director. As such, HKC Holdings Sdn Bhd. is regarded a related party.
With regards to all of the above transactions we claim an exemption from registration afforded by Section 4a(2) and/or Regulation S of the Securities Act of 1933, as amended (“Regulation S”) due to the fact that the issuance of stock was made to non-U.S. persons (as defined under Rule 902 section (k)(2)(i) of Regulation S), pursuant an offshore transactions, and no directed selling efforts were made in the United States by the issuer, a distributor, any of their respective affiliates, or any person acting on behalf of any of the foregoing.
| 78 |
The Company’s related party list and relationship are as follows:
| Related parties | Relationships | |
| Agape ATP (Asia) Limited | Dr. How Kok Choong, the CEO and director of the Company is also the sole shareholder and director of Agape ATP (Asia) Limited. | |
| DSY Wellness & Longevity Center Sdn Bhd | Mr. Steve Yap, a director of DSY Wellness International Sdn Bhd, is also a director of DSY Wellness & Longevity Center Sdn Bhd. | |
| CTA Nutriceuticals (Asia) Sdn Bhd | The directors and shareholders of CTA are related parties to Mr. Steve Yap, a director of DSY International Wellness Sdn Bhd | |
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | The directors and shareholders of Welltech are related parties to Mr. Steve Yap, a director of DSY Wellness International Sdn Bhd | |
| TH3 Technology Sdn Bhd | Dr. How Kok Choong, the CEO and director of the Company is also a director of TH3 Technology Sdn Bhd. | |
| Redboy Picture Sdn Bhd | Dr. How Kok Choong, the CEO and director of the Company is also a director of Redboy Picture Sdn Bhd. | |
| Ando Design Sdn Bhd (“Ando”) | Dr. How Kok Choong, the CEO and director of the Company is also a director of Ando Design Sdn Bhd. |
Related party balances as of June 30, 2023 and December 31, 2022 are as per table below:
Related party balances
Amount due from related parties
| As of | ||||||||||||
| Name of Related Party | Relationship | Nature | June 30, 2023 | December 31, 2022 | ||||||||
| TH3 Technology Sdn Bhd (“TH3”) | Mr. How Kok Choong, the CEO and director of the Company is also a director of TH3 | Prepayment of IT expenses | $ | 1,178 | $ | 1,273 | ||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | The directors and shareholders of Welltech are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd | Deposits for products purchases | - | 9,261 | ||||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd is also a director of DSYWLC | Expenses paid for DSYWLC | 239 | - | ||||||||
| Total | $ | 1,417 | $ | 10,534 | ||||||||
Accounts payable – related parties
| As of | ||||||||||||
| Name of Related Party | Relationship | Nature | June 30, 2023 | December 31, 2022 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | The directors and shareholders of CTA are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY International Wellness Sdn Bhd | Purchases of products for the provision of complementary health therapies | $ | 19,601 | $ | 25,387 | ||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | The directors and shareholders of Welltech are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd | Purchases of beauty products | 4 | 224 | ||||||||
| Total | $ | 19,605 | $ | 25,611 | ||||||||
| 79 |
Other payable - related parties
| As of | ||||||||||||
| Name of Related Party | Relationship | Nature | June 30, 2023 | December 31, 2022 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | The directors and shareholders of CTA are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY International Wellness Sdn Bhd | Purchase of products for general use | $ | 391 | $ | 2,149 | ||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | The directors and shareholders of Welltech are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd | Purchase of products for general use | 316 | 2,147 | ||||||||
| Mr. How Kok Choong | Mr. How Kok Choong, the CEO and director of the Company | Commission expense | 323 | 584 | ||||||||
| Total | $ | 1,030 | $ | 4,880 | ||||||||
Related party transactions
Purchases
| For the three months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | The directors and shareholders of CTA are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY International Wellness Sdn Bhd | Purchases of products for the provision of complementary health therapies | $ | 55,614 | $ | 63,142 | ||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | The directors and shareholders of Welltech are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd | Purchases of beauty products | 212 | 395 | ||||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd is also a director of DSYWLC | Purchases of products for the provision of complementary health therapies | - | 127 | ||||||||
| Total | $ | 55,826 | $ | 63,664 | ||||||||
| 80 |
| For the six months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | The directors and shareholders of CTA are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY International Wellness Sdn Bhd | Purchases of products for the provision of complementary health therapies | $ | 116,269 | $ | 74,909 | ||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | The directors and shareholders of Welltech are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd | Purchases of beauty products | 17,761 | 395 | ||||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd is also a director of DSYWLC | Purchases of products for the provision of complementary health therapies | - | 127 | ||||||||
| Total | $ | 134,030 | $ | 75,431 | ||||||||
Other Purchases
| For the three months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | The directors and shareholders of CTA are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY International Wellness Sdn Bhd | Purchase of products for general use | $ | 1,291 | $ | - | ||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | The directors and shareholders of Welltech are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd | Purchase of products for general use | 1,151 | 69 | ||||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd is also a director of DSYWLC | Purchase of products for general use | 212 | - | ||||||||
| Total | $ | 2,654 | $ | 69 | ||||||||
| 81 |
Other purchases
| For the six months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | The directors and shareholders of CTA are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY International Wellness Sdn Bhd | Purchase of products for general use | $ | 2,233 | $ | - | ||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | The directors and shareholders of Welltech are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd | Purchase of products for general use | 3,407 | 69 | ||||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd is also a director of DSYWLC | Purchase of products for general use | 280 | - | ||||||||
| Total | $ | 5,920 | $ | 69 | ||||||||
Commission
| For the three months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| Mr. How Kok Choong | Mr. How Kok Choong, the CEO and director of the Company | Commission expense | $ | 1,626 | $ | 2,443 | ||||||
| Total | $ | 1,626 | $ | 2,443 | ||||||||
Commission
| For the six months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| Mr. How Kok Choong | Mr. How Kok Choong, the CEO and director of the Company | Commission expense | $ | 3,538 | $ | 5,148 | ||||||
| Total | $ | 3,538 | $ | 5,148 | ||||||||
| 82 |
Other Income
| For the three months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| Ando Design Sdn Bhd (“Ando”) | Mr. How Kok Choong, the CEO and director of the Company is also a director of Ando | Rental income | $ | 670 | $ | - | ||||||
| Redboy Picture Sdn Bhd (“Redboy”) | Mr. How Kok Choong, the CEO and director of the Company is also a director of Redboy | Rental income | 2,010 | - | ||||||||
| TH3 Technology Sdn Bhd (“TH3”) | Mr. How Kok Choong, the CEO and director of the Company is also a director of TH3 | Rental income | 67 | - | ||||||||
| Total | $ | 2,747 | $ | - | ||||||||
| For the six months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| Ando Design Sdn Bhd (“Ando”) | Mr. How Kok Choong, the CEO and director of the Company is also a director of Ando | Rental income | $ | 1,340 | $ | - | ||||||
| Redboy Picture Sdn Bhd (“Redboy”) | Mr. How Kok Choong, the CEO and director of the Company is also a director of Redboy | Rental income | 4,021 | - | ||||||||
| TH3 Technology Sdn Bhd (“TH3”) | Mr. How Kok Choong, the CEO and director of the Company is also a director of TH3 | Rental income | 67 | - | ||||||||
| Total | $ | 5,428 | $ | - | ||||||||
Other expenses
| For the three months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| TH3 Technology Sdn Bhd (“TH3”) | Mr. How Kok Choong, the CEO and director of the Company is also a director of TH3 | IT support services fee | $ | 13,647 | $ | - | ||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd is also a director of DSYWLC | Office rental expense | 8,254 | 5,602 | ||||||||
| Total | $ | 21,901 | $ | 5,602 | ||||||||
| 83 |
| For the six months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| TH3 Technology Sdn Bhd (“TH3”) | Mr. How Kok Choong, the CEO and director of the Company is also a director of TH3 | IT support services fee | $ | 27,718 | $ | - | ||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd is also a director of DSYWLC | Office rental expense | 16,363 | 11,203 | ||||||||
| Total | $ | 44,081 | $ | 11,203 | ||||||||
Related party balances as of December 31, 2022 and 2021 are as per table below:
Amount due from related parties
| As of December 31, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2022 | 2021 | ||||||||
| TH3 Technology Sdn Bhd (“TH3”) | Mr. How Kok Choong, the CEO and director of the Company is also a director of TH3 | Prepayment of IT expenses | $ | 1,273 | $ | - | ||||||
| DSY Beauty Sdn Bhd (“DSY Beauty”) | The directors and shareholders of DSY Beauty are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd | Deposits for products purchases | 9,261 | - | ||||||||
| Agape ATP (Asia) Limited (“AATP Asia”) | Mr. How Kok Choong, the CEO and director of the Company is also the sole shareholder and director of AATP Asia | Expenses paid for AATP Asia | - | 2,214 | ||||||||
| Hostastay Sdn Bhd. “Hostastay” | Mr. How Kok Choong, the CEO and director of the Company is also the director of Hostastay. Mr. How Kok Choong ceased to be the director of Hostastay as of April 21, 2021 | Sublease rent due from Hostastay | - | 4,790 | ||||||||
| Total | $ | 10,534 | $ | 7,004 | ||||||||
| 84 |
Accounts payable – related parties
| As of December 31, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2022 | 2021 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | The directors and shareholders of CTA are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY International Wellness Sdn Bhd | Purchases of products for the provision of complementary health therapies | $ | 25,387 | $ | - | ||||||
| DSY Beauty Sdn Bhd (“DSY Beauty”) | The directors and shareholders of DSY Beauty are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd | Purchases of beauty products | 224 | - | ||||||||
| Total | $ | 25,611 | $ | - | ||||||||
Other payable – related parties
| As of December 31, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2022 | 2021 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | The directors and shareholders of CTA are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY International Wellness Sdn Bhd | Purchase of products for general use | $ | 2,149 | $ | - | ||||||
| DSY Beauty Sdn Bhd (“DSY Beauty”) | The directors and shareholders of DSY Beauty are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd | Purchase of products for general use | 2,147 | - | ||||||||
| Mr. How Kok Choong | Mr. How Kok Choong, the CEO and director of the Company | Commission expense | 584 | - | ||||||||
| Total | $ | 4,880 | $ | - | ||||||||
| 85 |
Related party transactions for years ended December 31, 2022 and 2021, are as per table below:
Purchases
For the years ended December 31, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2022 | 2021 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | The directors and shareholders of CTA are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY International Wellness Sdn Bhd | Purchases of products for the provision of complementary health therapies | $ | 198,376 | $ | - | ||||||
| DSY Beauty Sdn Bhd (“DSY Beauty”) | The directors and shareholders of DSY Beauty are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd | Purchases of beauty products | 3,975 | 718 | ||||||||
| DSY Wellness & Longevity Center Sdn Bhd (“DSYWLC”) | Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd is also a director of DSYWLC. | Purchases of products for the provision of complementary health therapies | 124 | - | ||||||||
| Total | $ | 202,475 | $ | 718 | ||||||||
Other income
For the years ended December 31, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2022 | 2021 | ||||||||
| Hostastay Sdn Bhd. | Mr. How Kok Choong, the CEO and director of the Company is also the director of Hostastay. Mr. How Kok Choong ceased to be the director of Hostastay as of April 21, 2021 | Sublease rental income due from Hostastay | $ | - | $ | 4,345 | ||||||
| Total | $ | - | $ | 4,345 | ||||||||
Other purchases
For the years ended December 31, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2022 | 2021 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | The directors and shareholders of CTA are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY International Wellness Sdn Bhd | Purchases of products for the provision of complementary health therapies | $ | 5,431 | $ | - | ||||||
| DSY Beauty Sdn Bhd (“DSY Beauty”) | The directors and shareholders of DSY Beauty are related parties to Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd | Purchases of beauty products | 6,888 | - | ||||||||
| DSY Wellness & Longevity Center Sdn Bhd (“DSYWLC”) | Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd is also a director of DSYWLC. | Purchases of products for the provision of complementary health therapies | 4 | - | ||||||||
| Total | $ | 12,323 | $ | - | ||||||||
| 86 |
Commission expense
| For the years ended December 31, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2022 | 2021 | ||||||||
| Mr. How Kok Choong | Mr. How Kok Choong, the CEO and director of the Company | Commission expense | $ | 16,590 | $ | 12,758 | ||||||
| Total | $ | 16,590 | $ | 12,758 | ||||||||
Other expenses
| For the years ended December 31, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2022 | 2021 | ||||||||
| TH3 Technology Sdn Bhd (“TH3”) | Mr. How Kok Choong, the CEO and director of the Company is also a director of TH3 | IT support services fee | $ | 56,450 | $ | - | ||||||
| Redboy Picture Sdn Bhd (“Redboy”) | Mr. How Kok Choong, the CEO and director of the Company is also the director of Redboy | Sponsorship fee | 22,686 | 718 | ||||||||
| DSY Wellness & Longevity Center Sdn Bhd (“DSYWLC”) | Mr. Yap Foo Ching (Steve Yap), a director of DSY Wellness International Sdn Bhd is also a director of DSYWLC. | Office rental expenses | 21,779 | - | ||||||||
| Total | $ | 100,915 | $ | 718 | ||||||||
| 87 |
PRINCIPAL STOCKHOLDERS
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. In accordance with SEC rules, shares of our common stock which may be acquired upon exercise of stock options or warrants which are currently exercisable or which become exercisable within 60 days of the date of the applicable table below are deemed beneficially owned by the holders of such options and warrants and are deemed outstanding for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage of ownership of any other person. Subject to community property laws, where applicable, the persons or entities named in the tables below have sole voting and investment power with respect to all shares of our common stock indicated as beneficially owned by them.
The following table sets forth certain information, as of the date of this prospectus, with respect to the beneficial ownership of the outstanding common stock by (i) any holder of more than five (5%) percent; (ii) each of our executive officers and directors; and (iii) our directors and executive officers as a group. Except as otherwise indicated, each of the stockholders listed below has sole voting and investment power over the shares beneficially owned.
| Name of Beneficial Owner (1) | Common Stock Beneficially Owned | Percentage of Common Stock (1) | Voting Shares of Preferred Stock | Preferred Stock Voting Percentage Beneficially Owned | Total Voting Percentage Beneficially Owned | |||||||||||||||
| How Kok Choong, Chief Executive Officer, Chief Operating Officer, Chairman of the board of Directors, Secretary, and Director;(2) (3) | 19,608,998 | 25.99 | % | - | - | 25.99 | % | |||||||||||||
| Lee Kam Fan Andrew(4) | - | - | - | - | - | |||||||||||||||
| All officers and directors as a group | 19,608,998 | 25.99 | % | - | - | 25.99 | % | |||||||||||||
| 5% or more holders: | ||||||||||||||||||||
| HKC Talent Limited | 8,245,000 | 10.93 | % | - | - | 10.93 | % | |||||||||||||
| (1) | Applicable percentage ownership is based on 75,452,012 shares of common stock issued and outstanding and 200,000,000 preferred shares authorized but none were issued and outstanding as of the date of this prospectus. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. |
| (2) | The address of How Kok Choong is c/o Agape ATP Corporation, 1705 – 1708, Level 17, Tower 2, Faber Towers, Jalan Desa Bahagia, Taman Desa, 58100 Kuala Lumpur, Malaysia. |
| (3) | HKC Holdings Sdn Bhd is owned and controlled by How Kok Choong who is our chief executive officer, chief operating officer, chairman of the board of Directors, Director and secretary. |
| (4) | The address of Lee Kam Fan Andrew is Flat F, 17th floor, Poyang Mansion, Taikoo Shing, Hong Kong. |
| 88 |
DESCRIPTION OF CAPITAL STOCK
We have authorized capital stock consisting of 1,000,000,000 shares of common stock, par value $0.0001 per share, and 200,000,000 shares of preferred stock, par value $0.0001 per share. As of June 30, 2023, we had 75,452,012 shares of common stock issued and outstanding and no shares of preferred stock issued and outstanding.
Common Stock
All outstanding shares of common stock are of the same class and have equal rights and attributes. The holders of common stock are entitled to one vote per share on all matters submitted to a vote of stockholders of the company. All stockholders are entitled to share equally in dividends, if any, as may be declared from time to time by the Board of Directors out of funds legally available. In the event of liquidation, the holders of common stock are entitled to share ratably in all assets remaining after payment of all liabilities. The stockholders do not have cumulative or pre-emptive rights.
Preferred Stock
Our Certificate of Incorporation authorizes the issuance of up to 200,000,000 shares of preferred stock with designations, rights and preferences determined from time to time by our Board of Directors. Accordingly, our Board of Directors is empowered, without stockholder approval, to issue preferred stock with dividend, liquidation, conversion, voting, or other rights which could adversely affect the voting power or other rights of the holders of the common stock. In the event of issuance, the preferred stock could be utilized, under certain circumstances, as a method of discouraging, delaying or preventing a change in control of our company, which is sometimes referred to in corporate parlance as a “poison pill”.
Options and Restricted Stock
As of June 30, 2023, other than the securities described above, we do not have any outstanding options or restricted stock.
Other Convertible Securities
As of June 30, 2023, other than the securities described above, we do not have any outstanding convertible securities.
Securities Authorized for Issuance under Equity Compensation Plans
We have not adopted any compensatory or benefit plans for future issuances of our securities.
Market for Common Equity and Related Stockholder Matters
Our common stock is presently quoted on the OTC Markets – Pink Sheets under the symbol “AATP”. Although there is currently a bid and offer quotation for the common stock, such bid and offer are for limited and insignificant number of shares. The last sale price recorded was $6.00 per share on May 11, 2023. Because trading has been sporadic and irregular, there is no established public trading market for our common stock.
We plan to apply to list our common stock on the NASDAQ as soon as practical. No assurance can be given that our application will be approved by the NASDAQ. If our Common Stock is listed on the NASDAQ, we will be subject to continued listing requirements and corporate governance standards of NASDAQ. We expect the compliance with these new rules and regulations to significantly increase our legal, accounting and financial compliance costs.
As of the date of this prospectus, there are approximately 1,313 holders of record of our common stock.
Transfer Agent
The stock transfer agent for our securities is Vstock Transfer, LLC, 18 Lafayette Place, Woodmere, New York 11598 and telephone number is +1 (212) 828-8436.
| 89 |
SHARES ELIGIBLE FOR FUTURE SALE
Immediately prior to this offering, there was little to no trading activity in our common stock. Future sales of substantial amounts of common stock in the public market, or the perception that such sales may occur, could adversely affect the market price of our common stock.
All shares sold in this offering will be freely tradable without restriction or further registration under the Securities Act, except for any shares purchased by our “affiliates,” as that term is defined in Rule 144 under the Securities Act, whose sales would be subject to the Rule 144 resale restrictions described below, other than the holding period requirement.
Restricted securities are eligible for public sale only if they are registered under the Securities Act or if they qualify for an exemption from registration under Rules 144 or 701 under the Securities Act, described below. Restricted securities may also be sold outside of the United States to non-U.S. persons in accordance with Rule 904 of Regulation S.
Lock-Up
For further details on the lock-up agreements, see the section entitled “Underwriting – Lock-Up Agreements.”
Rule 144
Some of our stockholders will be forced to hold their shares of our common stock for at least a six-month period before they are eligible to sell those shares, and even after that six-month period, sales may not be made under Rule 144 promulgated under the Securities Act unless we and such stockholders are in compliance with other requirements of Rule 144.
In general, Rule 144 provides that (i) any of our non-affiliates that has held restricted common stock for at least six months is thereafter entitled to sell its restricted stock freely and without restriction, provided that we remain compliant and current with our SEC reporting obligations, and (ii) any of our affiliates, which includes our directors, executive officers and other person in control of us, that has held restricted common stock for at least six months is thereafter entitled to sell its restricted stock subject to the following restrictions: (a) we are compliant and current with our SEC reporting obligations, (b) certain manner of sale provisions are satisfied, (c) a Form 144 is filed with the SEC, and (d) certain volume limitations are satisfied, which limit the sale of shares within any three-month period to a number of shares that does not exceed the greater of 1% of the total number of outstanding shares. A person who has ceased to be an affiliate at least three months immediately preceding the sale and who has owned such shares of common stock for at least one year is entitled to sell the shares under Rule 144 without regard to any of the limitations described above.
Rule 701
In general, Securities Act Rule 701 allows a stockholder who purchased shares of capital stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of ours during the immediately preceding 90 days to sell those shares in reliance upon Securities Act Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. All holders of Rule 701 shares, however, are required to wait until ninety (90) days after the date of this prospectus before selling shares pursuant to Rule 701.
Regulation S
Regulation S provides generally that sales made in offshore transactions are not subject to the registration or prospectus-delivery requirements of the Securities Act.
| 90 |
TAXATION
Malaysia Taxation
The following discussion is a summary of the more relevant taxes that are applicable to our Malaysian subsidiaries with regards to transactions that they may enter into with a foreign holding company, i.e. AATP. It excludes specifically all Malaysian taxes that our Malaysian subsidiaries are subject to arising from their respective business activities in Malaysia such as income tax, various types of taxes imposable on transactions entered into in the course of conducting their business activities and taxes on capital gains. Generally, there is no taxes on capital gains in Malaysia except for real property gains tax (“RPGT”) which is a tax on gains arising from the disposal of real property or shares in real property companies (“RPC”). Neither subject affects our Malaysian subsidiaries as none of them were engaged in activities in the said areas.
The type of transactions that Malaysian subsidiaries typically enter into with their foreign holding company (that is not attributable to a business carried on in Malaysia by the foreign holding company) are royalties, interest or service fees. With respect to such income, the tax liability of the foreign holding company, it being a non-resident will be settled by way of withholding tax (“WHT”) deducted by the paying entity, i.e. the Malaysian subsidiary. The following are WHT rates that apply as per the double taxation agreement (“DTA”) exists between the United States of America and Malaysia: (Royalty: 10%, Interest: 15%, Dividends: 0%, Income other than royalty and interest: 10%)
Payments of the above types of income to non-residents (except for dividends) are subject to WHT which is due and payable to the Inland Revenue Board (IRB) within one month after paying or crediting such payments. There is no WHT on dividends paid by Malaysian companies.
Tax administration
Transfer pricing
Transfer pricing (TP) legislation
The basis for determining proper compensation is, almost universally, the arm’s length principle which has also been accepted by the Inland Revenue Board (“IRB”).
The arm’s length principle was incorporated into Section 140A of the Malaysian Income Tax Act 1967. It allows the Director General Inland Revenue (“DGIR”) to adjust any transfer prices between related parties in Malaysia which, in the view of the DGIR, do not meet the arm’s length standard.
What constitutes “arm’s length” is not defined in the Income Tax Act 1967. Consequently, the IRB has issued the TP Rules 2012 and the revised TP Guidelines 2012 to give guidance on the arm’s length standard that is acceptable to the IRB. The TP Rules and Guidelines seek to provide guidance on the application of the law on controlled transactions, the acceptable methodologies as provided in the rules and administrative requirements including the types of records and documentation expected from taxpayers involved in TP arrangements.
Advance pricing arrangements (APA)
Companies are allowed to apply for APAS from the DGIR. The objective of establishing APAS is to provide an avenue for taxpayers to obtain certainty upfront that their related party transactions meet the arm’s length standard. The IRB has issued the APA Rules 2012 and APA Guidelines 2012 to give guidance on the matter.
Statute of limitation for TP adjustments
The statute of limitation is seven (7) years from the expiration of an assessment year (“YA”) for raising an assessment or additional assessment for that YA in respect of TP adjustments for a transaction entered into between associated persons not at arm’s length.
| 91 |
Country-by-Country Reporting
The Malaysian Country-by-Country Rules require a Malaysian multinational corporation (“MNC”) group with total consolidated group revenue of RM3 billion and above in the financial year (“FY”) preceding the reporting FY (i.e. FY commencing on or after 1 January 2017) to prepare and submit the Country-by-Country Report to the IRB no later than 12 months after the close of each FY.
Malaysian entities of foreign MNC groups will generally not be required to prepare and file Country-by-Country Reports as the obligation to file will be with the ultimate holding company in the jurisdiction it is tax resident in, However, a notification to the IRB may be required.
United States Federal Income Taxation
The following discussion is a summary of the material U.S. federal income tax consequences to Non-U.S. Holders (as defined below) of the purchase, ownership and disposition of our common stock issued pursuant to this prospectus, but does not purport to be a complete analysis of all potential tax effects. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local or non-U.S. tax laws are not discussed. This discussion is based on the U.S. Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative pronouncements of the U.S. Internal Revenue Service (the “IRS”), in each case in effect as of the date of this prospectus. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect a Non-U.S. Holder. We have not sought and will not seek any rulings from the IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a contrary position to that discussed below regarding the tax consequences of the purchase, ownership and disposition of our common stock.
This discussion is limited to Non-U.S. Holders that hold our common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a Non-U.S. Holder’s particular circumstances, including the impact of the Medicare contribution tax on net investment income. In addition, it does not address consequences relevant to Non-U.S. Holders subject to special rules, including, without limitation:
| ● | U.S. expatriates and former citizens or long-term residents of the United States; | |
| ● | persons subject to the alternative minimum tax; | |
| ● | persons holding our common stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; | |
| ● | banks, insurance companies, and other financial institutions; | |
| ● | brokers, dealers or traders in securities; | |
| ● | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; | |
| ● | partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); | |
| ● | tax-exempt organizations or governmental organizations; | |
| ● | persons deemed to sell our common stock under the constructive sale provisions of the Code; | |
| ● | persons who hold or receive our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; and | |
| ● | tax-qualified retirement plans. |
If an entity treated as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of a partner in the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding our common stock and the partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences to them.
| 92 |
INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
Definition of a Non-U.S. Holder
For purposes of this discussion, a “Non-U.S. Holder” is any beneficial owner of our common stock that is neither a “U.S. person” nor an entity treated as a partnership for U.S. federal income tax purposes. A U.S. person is any person that, for U.S. federal income tax purposes, is or is treated as any of the following:
| ● | an individual who is a citizen or resident of the United States; | |
| ● | a corporation created or organized under the laws of the United States, any state thereof or the District of Columbia; | |
| ● | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or | |
| ● | a trust that (1) is subject to the primary supervision of a U.S. court and all substantial decisions of which are subject to the control of one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code), or (2) has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
Distributions
As described in the section entitled “Dividend Policy,” we do not anticipate paying any cash dividends on our common stock in the foreseeable future. However, if we do make distributions of cash or property on our common stock, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will constitute a return of capital and first be applied against and reduce a Non-U.S. Holder’s adjusted tax basis in its common stock, but not below zero. Any excess will be treated as capital gain and will be treated as described below under “—Sale or Other Taxable Disposition.”
Subject to the discussion below on effectively connected income, dividends paid to a Non-U.S. Holder will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends (or such lower rate specified by an applicable income tax treaty, provided the Non-U.S. Holder furnishes a valid IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) certifying qualification for the lower treaty rate). A Non-U.S. Holder that does not timely furnish the required documentation, but that qualifies for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. Holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
If dividends paid to a Non-U.S. Holder are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the United States to which such dividends are attributable), the Non-U.S. Holder will be exempt from the U.S. federal withholding tax described above. To claim the exemption, the Non-U.S. Holder must furnish to the applicable withholding agent a valid IRS Form W-8ECI, certifying that the dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States.
Any such effectively connected dividends will be subject to U.S. federal income tax on a net income basis at the regular graduated rates. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on such effectively connected dividends, as adjusted for certain items. Non-U.S. Holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
| 93 |
Sale or Other Taxable Disposition
A Non-U.S. Holder will not be subject to U.S. federal income tax on any gain realized upon the sale or other taxable disposition of our common stock unless:
| ● | the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the United States to which such gain is attributable); | |
| ● | the Non-U.S. Holder is a non-resident alien individual present in the United States for 183 days or more during the taxable year of the disposition and certain other requirements are met; or | |
| ● | our common stock constitutes a U.S. real property interest (“USRPI”) by reason of our status as a U.S. real property holding corporation (“USRPHC”) for U.S. federal income tax purposes. |
Gain described in the first bullet point above generally will be subject to U.S. federal income tax on a net income basis at the regular graduated rates. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on such effectively connected gain, as adjusted for certain items.
Gain described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty), which may be offset by certain U.S. source capital losses of the Non-U.S. Holder (even though the individual is not considered a resident of the United States), provided the Non-U.S. Holder has timely filed U.S. federal income tax returns with respect to such losses.
With respect to the third bullet point above, we believe we currently are not, and do not anticipate becoming, a USRPHC. Because the determination of whether we are a USRPHC depends, however, on the fair market value of our USRPIs relative to the fair market value of our non-U.S. real property interests and our other business assets, there can be no assurance we currently are not a USRPHC or will not become one in the future. Even if we are or were to become a USRPHC, gain arising from the sale or other taxable disposition by a Non-U.S. Holder of our common stock will not be subject to U.S. federal income tax if our common stock is “regularly traded,” as defined by applicable Treasury Regulations, on an established securities market, and such Non-U.S. Holder owned, actually and constructively, 5% or less of our common stock throughout the shorter of the five-year period ending on the date of the sale or other taxable disposition or the Non-U.S. Holder’s holding period.
Non-U.S. Holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
Information Reporting and Backup Withholding
Payments of dividends on our common stock will not be subject to backup withholding, provided the applicable withholding agent does not have actual knowledge or reason to know the holder is a United States person and the holder either certifies its non-U.S. status, such as by furnishing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI, or otherwise establishes an exemption. However, information returns are required to be filed with the IRS in connection with any dividends on our common stock paid to the Non-U.S. Holder, regardless of whether any tax was actually withheld. In addition, proceeds of the sale or other taxable disposition of our common stock within the United States or conducted through certain U.S.-related brokers generally will not be subject to backup withholding or information reporting, if the applicable withholding agent receives the certification described above and does not have actual knowledge or reason to know that such holder is a United States person, or the holder otherwise establishes an exemption. Proceeds of a disposition of our common stock conducted through a non-U.S. office of a non-U.S. broker that does not have certain enumerated relationships with the United States generally will not be subject to backup withholding or information reporting.
| 94 |
Copies of information returns that are filed with the IRS may also be made available under the provisions of an applicable treaty or agreement to the tax authorities of the country in which the Non-U.S. Holder resides or is established.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a Non-U.S. Holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
Additional Withholding Tax on Payments Made to Foreign Accounts
Withholding taxes may be imposed under Sections 1471 to 1474 of the Code (such Sections commonly referred to as the Foreign Account Tax Compliance Act, or “FATCA”) on certain types of payments made to non-U.S. financial institutions and certain other non-U.S. entities. Specifically, a 30% withholding tax may be imposed on dividends on, or gross proceeds from the sale or other disposition of, our common stock paid to a “foreign financial institution” or a “non-financial foreign entity” (each as defined in the Code), unless (1) the foreign financial institution undertakes certain diligence and reporting obligations, (2) the non-financial foreign entity either certifies it does not have any “substantial United States owners” (as defined in the Code) or furnishes identifying information regarding each substantial United States owner, or (3) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules. If the payee is a foreign financial institution and is subject to the diligence and reporting requirements in (1) above, it must enter into an agreement with the U.S. Department of the Treasury requiring, among other things, that it undertake to identify accounts held by certain “specified United States persons” or “United States-owned foreign entities” (each as defined in the Code), annually report certain information about such accounts, and withhold 30% on certain payments to non-compliant foreign financial institutions and certain other account holders. Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules.
Under the applicable Treasury Regulations and administrative guidance, withholding under FATCA generally applies to payments of dividends on our common stock, and will apply to payments of gross proceeds from the sale or other disposition of such stock on or after January 1, 2019.
Prospective investors should consult their tax advisors regarding the potential application of withholding under FATCA to their investment in our common stock.
| 95 |
UNDERWRITING
Under the terms and subject to the conditions of an underwriting agreement dated the date of this prospectus, the Underwriter named below, for whom Network 1 Financial Securities, Inc. is acting as the underwriter, have severally agreed to purchase, and we have agreed to sell to them, the number of shares of common stock at the initial public offering price, less the underwriting discount, as set forth on the cover page of this prospectus and as indicated below:
| Underwriter | Number of Shares | |||
Network 1 Financial Securities, Inc | 1,100,000 | |||
| Total | 1,100,000 | |||
The Underwriter is offering the shares subject to acceptance of the shares from us and subject to prior sale. The underwriting agreement provides that the obligations of the Underwriter to pay for and accept delivery of the shares of common stock offered by this prospectus are subject to the approval of certain legal matters by its counsel and to other conditions. The Underwriter is obligated to take and pay for all of the shares of common stock offered by this prospectus if any such shares are taken. However, the Underwriter is not required to take or pay for the shares covered by the Underwriter’s option to purchase additional shares described below.
We have granted to the Underwriter an option, exercisable for forty-five (45) days from the closing of this offering, to purchase up to 165,000 additional shares of common stock at the initial public offering price listed on the cover page of this prospectus, less underwriting discount. The Underwriter may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with this offering. To the extent the option is exercised, the Underwriter will become obligated, subject to certain conditions, to purchase about the same percentage of the additional shares as the number listed next to the underwriter’s name in the preceding table bears to the total number of shares listed next to the names of the Underwriter in the preceding table.
The Underwriter will offer the shares to the public at the initial public offering price set forth on the cover of this prospectus and to selected dealers at the initial public offering price less a selling concession not in excess of $ per share. After this offering, the initial public offering price, concession and reallowance to dealers may be reduced by the Underwriter. No change in those terms will change the amount of proceeds to be received by us as set forth on the cover of this prospectus. The securities are offered by the Underwriter as stated herein, subject to receipt and acceptance by them and subject to their right to reject any order in whole or in part.
As of the date of this prospectus, Network 1 Financial Securities, Inc. and affiliates of Network 1 Financial Securities, Inc. beneficially own 2,000,000 shares of our Common Stock, which represent in the aggregate less than 10% of our issued and outstanding shares of Common Stock and therefore Network 1 Financial Securities, Inc. is not deemed to have a “conflict of interest” within the meaning of FINRA Rule 5121.
Discount and Expenses
If we complete this offering, then on the closing date, we will pay the Underwriter a discount of 8% of the value of the shares of common stock sold in this offering for the investors introduced by the Underwriter, and 6% of the aggregate gross proceeds of this offering of the common stock for the investors introduced by the Company. We have also agreed to pay the Underwriter an additional non-accountable expense allowance, equal to one percent (1%) of the gross proceed received by us from the sale of our shares of common stock.
The following table summarizes the compensation and estimated expenses we will pay in the offering. Such amounts are shown assuming both no exercise and full exercise of the Underwriter’s over-allotment option.
| No Exercise of Over-allotment Option | Exercise of Over-allotment Option | |||||||||||||||
| Per Share | Total | Per Share | Total | |||||||||||||
| Maximum public offering price | $ | 6.50 | $ | 7,150,000 | $ | 6.50 | $ | 8,222,500 | ||||||||
| Underwriting discount (8%) | $ | 0.52 | $ | 572,000 | $ | 0.52 | $ | 657,800 | ||||||||
| Proceeds, before expenses, to us | $ | 5.98 | $ | 6,578,000 | $ | 5.98 | $ | 7,564,700 | ||||||||
We have also agreed to reimburse the Underwriter for all of its reasonable out-of-pocket accountable expenses, including reasonable fees and expenses of its legal counsel, roadshow expenses, offering materials, background check fee, cost associated with closing volumes and commemorative mementos in an aggregate amount not to exceed $209,500 in connection with the offering. As of the date of this prospectus, the Company has advanced $50,000 to the Underwriter to cover its out-of-pocket expenses (the “Advance”); the Advance will be reimbursed to the Company to the extent not actually incurred in compliance with FINRA Rule 5110(g)(4)(A).
We expect our total expenses for this offering to be $768,834, exclusive of the above discount. If we complete this offering, then on the closing date, we will issue shares to investors.
We have agreed to indemnify the Underwriter against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the Underwriter may be required to make in respect of those liabilities.
In connection with this offering, the Underwriter or certain of the securities dealers may distribute prospectuses electronically. No forms of prospectus other than printed prospectuses and electronically distributed prospectuses that are printable in Adobe PDF format will be used in connection with this offering.
| 96 |
Underwriter Warrant
We have also agreed to grant to the Underwriter a warrant covering a number shares of common stock equal to 7% of the common stock sold by the Underwriter in this public offering. The Underwriter Warrant will be exercisable from the date of issuance and will expire on the fifth year anniversary of the date of the commencement of sales in this offering. The Underwriter Warrant will be exercisable at a price equal to 110% of the initial public offering price. The Underwriter Warrant shall not be redeemable or cancellable. We will register the shares underlying the Underwriter Warrant and file all necessary undertakings in connection therewith. The Underwriter Warrant and the underlying securities may not be not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days beginning on the date of commencement of sales of this offering (in accordance with FINRA Rule 5110), except that they may be assigned, in whole or in part, to any officer or partner of the Underwriter, and to members of the syndicate or selling group and their respective officers or partners. The Underwriter Warrants may be exercised as to all or a lesser number of shares, will provide for cashless exercise and will contain provisions for one demand registration and unlimited “piggyback” registration rights at our expense with a duration of more than five years and seven years, respectively, from the commencement of sales of this offering. We have registered the Underwriter Warrant and the shares underlying the Underwriter Warrant in this offering.
Right of First Negotiation
In addition, we have agreed to grant to the Underwriter, upon the closing of this offering, a right of first negotiation to co-manage any public underwriting or private placement of debt or equity securities (excluding (i) shares issued under any compensation or stock option plan approved by the stockholders of the Company, (ii) shares issued in payment of the consideration for an acquisition or as part of strategic partnerships and transactions and (iii) conventional banking arrangements and commercial debt financing) of the Company or any subsidiary or successor of the Company, receiving the right to underwrite or place a number of the securities to be sold having an aggregate purchase price equal to a minimum of the aggregate purchase price of the Ordinary Shares being sold in this offering, until twelve (12) months after the effective date of the registration statement of which this prospectus forms a part.
Lock-Up Agreements
We and each of, our officers, directors, and 10% or more stockholders, have agreed not to register, offer, sell, contract to sell or grant (except for private transfers and in such case only with the express requirement that such shares continue to be subject to the same lock-up) any of our shares of common stock or any securities convertible into or exercisable or exchangeable for our shares of common stock or any warrants to purchase our shares of common stock (including, without limitation, securities of our company which may be deemed to be beneficially owned by such individuals in accordance with the rules and regulations of the Securities and Exchange Commission and securities which may be issued upon the exercise of a stock option or warrant) for a period of 180 days after the closing date of this offering. Upon the expiration of these lock-up agreements, additional shares of common stock will be available for sale in the public market.
Market and Pricing Considerations
Prior to this offering, our common stock was quoted on the OTC Markets – Pink Sheets, and there was a limited public market for our common stock. The public offering price was determined based upon the price at which our common stock was quoted on the OTC Markets – Pink Sheets, as well as by negotiations between us and the Underwriter. Among the factors considered in determining the initial public offering price are the future prospects of our company and our industry in general, our sales, earnings and certain other financial and operating information in recent periods, and the price-earnings ratios, market prices of securities and certain financial and operating information of companies engaged in activities similar to those of our company.
An active trading market for our common stock may not develop. It is possible that after this offering the shares of common stock will not trade in the public market at or above the initial offering price.
| 97 |
Application for Listing on the NASDAQ Capital Market
Our common stock currently is quoted on the OTC Markets – Pink Sheets under the symbol “AATP.” We have applied to list our common stock on the Nasdaq Capital Market under the new symbol “ATPC”. At this time, Nasdaq has not yet approved our application to list our common stock. The closing of this offering is conditioned upon Nasdaq’s final approval of our listing application, and there is no guarantee or assurance that our common stock will be approved for listing on Nasdaq.
Price Stabilization, Short Positions and Penalty Bids
In order to facilitate the offering of our common stock, the Underwriter may engage in transactions that stabilize, maintain or otherwise affect the price of our common stock. These activities may raise or maintain the market price of our common stock above independent market levels or prevent or retard a decline in the market price of our common stock. The Underwriter is not required to engage in these activities, and may end any of these activities at any time. We and the Underwriter have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
Foreign Regulatory Restrictions on Purchase of our Shares
We have not taken any action to permit a public offering of our shares outside the United States or to permit the possession or distribution of this prospectus outside the United States. People outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to this offering of our shares and the distribution of this prospectus outside the United States.
Notice to Prospective Investors in the European Economic Area
In relation to each member state of the European Economic Area, no offer of shares which are the subject of the offering has been, or will be made to the public in that Member State, other than under the following exemptions under the Prospectus Directive:
| (a) | to any legal entity which is a qualified investor as defined in the Prospectus Directive; | |
| (b) | to fewer than 150 natural or legal persons (other than qualified investors as defined in the Prospectus Directive), subject to obtaining the prior consent of the Underwriter for any such offer; or | |
| (c) | in any other circumstances falling within Article 3(2) of the Prospectus Directive, |
provided that no such offer of shares referred to in (a) to (c) above shall result in a requirement for the Company or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive, or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
Each person located in a Member State to whom any offer of shares is made or who receives any communication in respect of an offer of shares, or who initially acquires any shares will be deemed to have represented, warranted, acknowledged and agreed to and with the Underwriter and the Company that (1) it is a “qualified investor” within the meaning of the law in that Member State implementing Article 2(1)(e) of the Prospectus Directive; and (2) in the case of any shares acquired by it as a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, the shares acquired by it in the offer have not been acquired on behalf of, nor have they been acquired with a view to their offer or resale to, persons in any Member State other than qualified investors, as that term is defined in the Prospectus Directive, or in circumstances in which the prior consent of the Underwriter has been given to the offer or resale; or where shares have been acquired by it on behalf of persons in any Member State other than qualified investors, the offer of those shares to it is not treated under the Prospectus Directive as having been made to such persons.
| 98 |
The Company, the Underwriter and their respective affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgments and agreements.
This prospectus has been prepared on the basis that any offer of shares in any Member State will be made pursuant to an exemption under the Prospectus Directive from the requirement to publish a prospectus for offers of shares. Accordingly any person making or intending to make an offer in that Member State of shares which are the subject of the offering contemplated in this prospectus may only do so in circumstances in which no obligation arises for the Company or the Underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Directive in relation to such offer. Neither the Company nor the Underwriter have authorized, nor do they authorize, the making of any offer of shares in circumstances in which an obligation arises for the Company or the Underwriter to publish a prospectus for such offer.
For the purposes of this provision, the expression an “offer of shares to the public” in relation to any common stock in any Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the common stock to be offered so as to enable an investor to decide to purchase or subscribe the common stock, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression “Prospectus Directive” means Directive 2003/71/EC (as amended) and includes any relevant implementing measure in each Member State.
The above selling restriction is in addition to any other selling restrictions set out below.
Notice to Prospective Investors in Hong Kong
The securities have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies (Winding Up and Miscellaneous Provisions) Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the securities has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to securities which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of Non-CIS Securities may not be circulated or distributed, nor may the Non-CIS Securities be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”), (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275, of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
| 99 |
Where the Non-CIS Securities are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| (a) | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or | |
| (b) | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, |
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the Non-CIS Securities pursuant to an offer made under Section 275 of the SFA except:
| (a) | to an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA; | |
| (b) | where no consideration is or will be given for the transfer; | |
| (c) | where the transfer is by operation of law; | |
| (d) | as specified in Section 276(7) of the SFA; or | |
| (e) | as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore. |
| 100 |
LEGAL MATTERS
Certain legal matters with respect to the validity of the shares of common stock offered hereby and U.S. federal securities law will be passed upon for us by Loeb & Loeb LLP, New York, New York. Legal matters as to Malaysia law will be passed upon for us by Lee & Poh Partnership. Loeb & Loeb, LLP may rely upon Lee & Poh Partnership. with respect to matters governed by Malaysian law. Hunter Taubman Fischer & Li LLC is acting as U.S. securities counsel for the Underwriter. Lee & Poh Partnership is acting as Malaysia counsel for the Company.
EXPERTS
The consolidated financial statements for the years ended December 31, 2022 and 2021, included in this Registration Statement have been so included in reliance on the reports of Marcum Asia CPAs LLP and Friedman LLP, independent registered public accounting firms, given on the authority of said firm in auditing and accounting. The office of Marcum Asia CPAs LLP is located at 7 Pennsylvania Plaza Suite 830, New York, NY 10001. The office of Friedman LLP is located at One Liberty Plaza, 165 Broadway 21st Floor, New York, NY 10006.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the common stock offered by this prospectus. This prospectus, which is part of the registration statement, omits certain information, exhibits, schedules and undertakings set forth in the registration statement. For further information pertaining to us and our common stock, reference is made to the registration statement and the exhibits and schedules to the registration statement. Statements contained in this prospectus as to the contents or provisions of any documents referred to in this prospectus are not necessarily complete, and in each instance where a copy of the document has been filed as an exhibit to the registration statement, reference is made to the exhibit for a more complete description of the matters involved.
Registration statements and certain other filings made with the SEC electronically are publicly available through the SEC’s web site at http://www.sec.gov. The registration statement, including all exhibits and amendments thereto, has been filed electronically with the SEC.
We are subject to the information and periodic reporting requirements of the Exchange Act and, accordingly, we file annual reports containing financial statements audited by an independent registered public accounting firm, quarterly reports containing unaudited financial data, current reports and other reports and information with the SEC. You may inspect and copy each of our periodic reports, proxy statements and other information at the SEC’s public reference room, and at the web site of the SEC referred to above.
| 101 |
AGAPE ATP CORPORATION
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Page | ||
| Unaudited Condensed Consolidated Financial Statements | ||
| Unaudited Condensed Consolidated Balance Sheets | F-2 | |
| Unaudited Condensed Consolidated Statements of Operations and Comprehensive Loss | F-3 | |
| Unaudited Condensed Consolidated Statements of Changes in Stockholders’ Equity | F-4 | |
| Unaudited Condensed Consolidated Statements of Cash Flows | F-5 | |
| Notes to Unaudited Condensed Consolidated Financial Statements | F-6 |
| Page | ||
| Consolidated Financial Statements | ||
| Report of Independent Registered Public Accounting Firm (PCAOB ID: 5395) | F-35 | |
| Report of Independent Registered Public Accounting Firm (PCAOB ID: 711) | F-36 | |
| Consolidated Balance Sheets | F-37 | |
| Consolidated Statements of Operations and Comprehensive Loss | F-38 | |
| Consolidated Statements of Changes in Stockholders’ Equity | F-39 | |
| Consolidated Statements of Cash Flows | F-40 | |
| Notes to Consolidated Financial Statements | F-41 |
| F-1 |
AGAPE ATP CORPORATION
CONDENSED CONSOLIDATED BALANCE SHEETS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
| As of | ||||||||
June 30, 2023 | December 31, 2022 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents (Included $ | $ | $ | ||||||
| Accounts receivable | ||||||||
| Amount due from related parties | ||||||||
| Inventories | ||||||||
| Prepaid taxes (Included $ | ||||||||
| Prepayments and deposits (Included $ | ||||||||
| Total Current Assets | ||||||||
| OTHER ASSETS | ||||||||
| Property and equipment, net | ||||||||
| Intangible assets, net | ||||||||
| Operating right-of-use assets | ||||||||
| Investment in marketable securities | ||||||||
| Deferred offering costs | ||||||||
| Deferred tax assets | ||||||||
| Total other assets | ||||||||
| TOTAL ASSETS | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable | $ | $ | ||||||
| Accounts payable – related parties | ||||||||
| Customer deposits | ||||||||
| Operating lease liabilities | ||||||||
| Other payables and accrued liabilities ($ | ||||||||
| Other payable – related parties | ||||||||
| Income tax payable | ||||||||
| Total Current Liabilities | ||||||||
| NON-CURRENT LIABILITIES | ||||||||
| Operating lease liabilities | ||||||||
| Total Non-current Liabilities | ||||||||
| TOTAL LIABILITIES | $ | $ | ||||||
| STOCKHOLDERS’ EQUITY | ||||||||
| Preferred stock, $ par value; shares authorized; issued and outstanding | ||||||||
| Common Stock, par value $; shares authorized, shares issued and outstanding as of June 30, 2023 and December 31, 2022, respectively. | ||||||||
| Additional paid in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Accumulated other comprehensive income | ||||||||
| TOTAL AGAPE CORPORATION STOCKHOLDERS’ EQUITY | ||||||||
| NON-CONTROLLING INTERESTS | ||||||||
| TOTAL EQUITY | ||||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | $ | $ | ||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
| F-2 |
AGAPE ATP CORPORATION
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
| For the three months ended | For the six months ended | |||||||||||||||
| June 30, | June 30, | |||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||
| REVENUE | $ | $ | $ | $ | ||||||||||||
| COST OF REVENUE | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| GROSS PROFIT | ||||||||||||||||
| SELLING | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| COMMISSION | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| GENERAL AND ADMINISTRATIVE | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| TOTAL OPERATING EXPENSES | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| LOSS FROM OPERATIONS | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| OTHER (EXPENSES) INCOME | ||||||||||||||||
| Other income, net | ||||||||||||||||
| Interest income | ||||||||||||||||
| Unrealized holding gain (loss) on marketable securities | ( | ) | ( | ) | ||||||||||||
| Gain on disposal of property and equipment | ||||||||||||||||
| Exchange loss, net | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| TOTAL OTHER EXPENSES, NET | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| LOSS BEFORE INCOME TAXES | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| BENEFIT OF (PROVISION FOR) INCOME TAXES | ( | ) | ( | ) | ||||||||||||
| NET LOSS | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| LESS: NET (LOSS) INCOME ATTRIBUTABLE TO NON-CONTROLLING INTERESTS | ( | ) | ( | ) | ||||||||||||
| NET LOSS ATTRIBUTABLE TO AGAPE ATP CORPORATION | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| NET LOSS | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| OTHER COMPREHENSIVE INCOME (LOSS) | ||||||||||||||||
| Foreign currency translation adjustment | ( | ) | ( | ) | ||||||||||||
| TOTAL COMPREHENSIVE LOSS | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Less: Comprehensive loss attributable to non-controlling interests | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| COMPREHENSIVE LOSS ATTRIBUTABLE TO AGAPE ATP CORPORATION | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| LOSS PER SHARE | ||||||||||||||||
| Basic and diluted | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING | ||||||||||||||||
| Basic and diluted | ||||||||||||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
| F-3 |
AGAPE ATP CORPORATION
CONDENSED CONSOLIDATED STATEMENTS OF
CHANGES IN STOCKHOLDERS’ EQUITY
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
| COMMON STOCK | ADDITIONAL | ACCUMULATED OTHER | NON- | TOTAL | ||||||||||||||||||||||||
Number of shares | Par value | PAID IN CAPITAL | ACCUMULATED DEFICIT | COMPREHENSIVE INCOME | CONTROLLING INTERESTS | STOCKHOLDERS’ EQUITY | ||||||||||||||||||||||
| Balance as of December 31, 2021 | $ | $ | $ | ( | ) | $ | $ | ( | ) | $ | ||||||||||||||||||
| Forfeiture of common stock | ( | ) | ( | ) | ||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation adjustment | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Balance as of March 31, 2022 | $ | $ | $ | ( | ) | $ | $ | $ | ||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation adjustment | - | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||
| Balance as of June 30, 2022 | $ | $ | $ | ( | ) | $ | $ | $ | ||||||||||||||||||||
| COMMON STOCK | ADDITIONAL | ACCUMULATED OTHER |
NON- | TOTAL | ||||||||||||||||||||||||
| Number
of shares |
Par value |
PAID
IN CAPITAL |
ACCUMULATED DEFICIT |
COMPREHENSIVE INCOME |
CONTROLLING INTERESTS |
STOCKHOLDERS’ EQUITY |
||||||||||||||||||||||
| Balance as of December 31, 2022 | $ | |
$ | $ | ( |
) | $ | $ | $ | |||||||||||||||||||
| Net loss | - | ( |
) | ( |
) | ( |
) | |||||||||||||||||||||
| Foreign currency translation adjustment | - | |||||||||||||||||||||||||||
| Balance as of March 31, 2023 | $ | $ | $ | ( |
) | $ | $ | $ | ||||||||||||||||||||
| Net loss | - | ( |
) | ( |
) | ( |
) | |||||||||||||||||||||
| Foreign currency translation adjustment | - | ( |
) | ( |
) | |||||||||||||||||||||||
| Balance as of June 30, 2023 | $ | $ | $ | ( |
) | $ | $ | $ | ||||||||||||||||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
| F-4 |
AGAPE ATP CORPORATION
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”)
For the six months ended June 30, | ||||||||
| 2023 | 2022 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | ||||||||
| Amortization | ||||||||
| Amortization of operating right-of-use assets | ||||||||
| Unrealized holding (gain) loss on marketable securities | ( | ) | ||||||
| Deferred tax (benefit) provision | ( | ) | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivables | ( | ) | ( | ) | ||||
| Amount due from related parties | ( | ) | ||||||
| Inventories | ( | ) | ||||||
| Prepaid taxes | ||||||||
| Prepayments and deposits | ||||||||
| Accounts payable | ||||||||
| Accounts payable –related parties | ( | ) | ||||||
| Customer deposits | ( | ) | ( | ) | ||||
| Operating lease liabilities | ( | ) | ( | ) | ||||
| Other payables and accrued liabilities | ( | ) | ( | ) | ||||
| Other payable – related parties | ( | ) | ||||||
| Income tax payable | ||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of equipment | ( | ) | ( | ) | ||||
| Net cash used in investing activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Deferred offering costs | ( | ) | ( | ) | ||||
| Net cash used in financing activities | ( | ) | ( | ) | ||||
| EFFECT OF EXCHANGE RATE ON CASH AND CASH EQUIVALENTS | ( | ) | ( | ) | ||||
| DECREASE IN CASH AND CASH EQUIVALENTS | ( | ) | ( | ) | ||||
| CASH AND CASH EQUIVALENTS, beginning of period | ||||||||
| CASH AND CASH EQUIVALENTS, end of period | $ | $ | ||||||
| SUPPLEMENTAL CASH FLOWS INFORMATION | ||||||||
| Income taxes paid | $ | $ | ||||||
| SUPPLEMENTAL NON-CASH FLOWS INFORMATION | ||||||||
| Increase in right-of-use assets and lease liabilities due to lease renewal | $ | $ | ||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
| F-5 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
1. ORGANIZATION AND BUSINESS BACKGROUND
Agape ATP Corporation, a Nevada corporation (“the Company”) was incorporated under the laws of the State of Nevada on June 1, 2016.
Agape ATP Corporation operates through its subsidiaries, namely, Agape ATP Corporation (“AATP LB”), a company incorporated in Labuan, Malaysia, and Agape Superior Living Sdn. Bhd. (“ASL”), a company incorporated in Malaysia.
Agape
ATP Corporation, incorporated in Labuan, Malaysia, is an investment holding company with
On
May 8, 2020, the Company entered into a Share Exchange Agreement with Mr. How Kok Choong, CEO and director of the Company to acquire
ordinary shares, no par value, equivalent to approximately
Agape Superior Living Sdn. Bhd. is a limited company incorporated on August 8, 2003, under the laws of Malaysia.
On September 11, 2020, the Company incorporated Wellness ATP International Holdings Sdn, Bhd. (“WATP”), a wholly owned subsidiary under the laws of Malaysia, to pursue the business of promoting wellness and wellbeing lifestyle of the community by providing services that includes online editorials, programs, events and campaigns on how to achieve positive wellness and lifestyle.
On
November 11, 2021, Agape ATP Corporation (Labuan) formed a joint-venture entity, DSY Wellness International Sdn. Bhd. (“DSY Wellness”)
with an independent third party which Agape ATP Corporation (Labuan) owns
The Company and its subsidiaries are principally engaged in the Health and Wellness Industry. The principal activity of the Company is to supply high-quality health and wellness products, including supplements to assist in cell metabolism, detoxification, blood circulation, anti-aging and products designed to improve the overall health system of the human body and various wellness programs.
The accompanying consolidated financial statements reflect the activities of the Company, AATP LB, AATP HK, WATP, ASL and its variable interest entity (“VIE”), Agape S.E.A. Sdn. Bhd. (“SEA”) (See Note 3), and DSY Wellness.
Details of the Company’s subsidiaries:
| Subsidiary company name | Place and date of incorporation | Particulars of issued capital | Principal activities | Proportional of ownership interest and voting power held | ||||||||
| 1. | March 6, 2017 | % | ||||||||||
| 2. | June 1, 2017 | % | ||||||||||
| 3. | August 8, 2003 | % | ||||||||||
| 4. | March 4, 2004 | VIE | ||||||||||
| 5. | September 11, 2020 | % | ||||||||||
| 6. | November 11, 2021 | % | ||||||||||
| F-6 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
1. ORGANIZATION AND BUSINESS BACKGROUND (Continued)
Business Overview
Agape ATP Corporation is a company that provides health and wellness products and health solution advisory services to our clients. The Company primarily focus its efforts on attracting customers in Malaysia. Its advisory services center on the “ATP Zeta Health Program”, which is a health program designed to effectively prevent diseases caused by polluted environments, unhealthy dietary intake and unhealthy lifestyles, and promotion of health. The program aims to promote improved health and longevity in our clients through a combination of modern medicine, proper nutrition and advice from skilled nutritionists and/or dieticians.
In
order to strengthen the Company’s supply chain, on May 8, 2020, the Company has successfully acquired approximately
Via ASL, the Company offers three series of programs which consist of different services and products: ATP Zeta Health Program, ÉNERGÉTIQUE and BEAUNIQUE.
The ATP Zeta Health Program is a health program designed to promote health and general wellbeing designed to prevent health diseases caused by polluted environments, unhealthy dietary intake and unhealthy lifestyles. The program aims to promote improved health and longevity through a combination of modern health supplements, proper nutrition and advice from skilled dieticians as well as trained members and distributors.
The ÉNERGÉTIQUE series aims to provide a total dermal solution for a healthy skin beginning from the cellular level. The series is comprised of the Energy Mask series, Hyaluronic Acid Serum and Mousse Facial Cleanser.
The BEAUNIQUE product series focuses on the research of our diet’s impact on modifying gene expressions in order to address genetic variations and deliver a nutrigenomic solution for every individual.
The Company deems creating public awareness on wellness and wellbeing lifestyle as essential to enhance the provision of its health solution advisory services; and therefore, incorporated WATP. Upon its establishment, WATP started collaborating with ASL to carry out various wellness programs.
To
further its reach in the Health and Wellness Industry, on November 11, 2021, Agape ATP Corporation (Labuan) formed a joint-venture entity,
DSY Wellness International Sdn. Bhd. (“DSY Wellness”) with an independent third party which Agape ATP Corporation (Labuan)
owns
| F-7 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
2. GOING CONCERN
In assessing the Company’s liquidity, the Company monitors and analyzes its cash on-hand and its operating and capital expenditure commitments. The Company’s liquidity needs are to meet its working capital requirements, operating expenses and capital expenditure obligations. Equity financing is used to supplement working capital requirements of the Company.
The
Company’s management has considered whether there is substantial doubt about its ability to continue as a going concern due to
(1) the net loss of $
Management has determined there is substantial doubt about its ability to continue as a going concern. If the Company is unable to generate significant revenue, the Company may be required to curtail or cease its operations. Management is trying to alleviate the going concern risk through the following sources:
| ● | Equity financing from the Company’s second listing on NASDAQ to support its working capital and future growth; |
| ● | Other available sources of financing (including debt) from banks and other financial institutions; and |
| ● | Financial support and credit guarantee commitments from the Company’s related parties. |
Based on the above measures, management is of the opinion that the Company will probably not have sufficient funds to meet its working capital requirements and debt obligations as they become due one year from the filing date of these unaudited condensed consolidated financial statements.
There is no assurance that the Company will be successful in implementing the foregoing plans or that additional financing will be available to the Company on commercially reasonable terms, or at all. There are a number of factors that could potentially arise that could undermine the Company’s plans, such as (i) undue delay in the Company’s current pursuit in seeking second listing on NASDAQ, (ii) the slow rate in which the Company’s distributors and members re-ignite their sales activities, (iii) changes in the demand for the Company’s products and services due to the diminishing effect on the purchasing power of the public in general, as a result of the COVID-19 pandemic, and (iv) if the Company’s new business in the provision of complementary health therapies fail to grow in the manner and at the rate as planned. The Company’s inability to secure needed financing when required could require material changes to the Company’s business plans and could have a material adverse effect on the Company’s viability and results of operations.
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation
The accompanying interim unaudited condensed consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
The interim unaudited financial information as of June 30, 2023 and for the three and six months ended June 30, 2023 and 2022 have been prepared without audit, pursuant to the rules and regulations of the SEC and pursuant to Regulation S-X. Certain information and footnote disclosures, which are normally included in annual consolidated financial statements prepared in accordance with U. S. GAAP, have been omitted pursuant to those rules and regulations. The interim unaudited financial information should be read in conjunction with the audited consolidated financial statements and the notes thereto, included in the Form 10-K for the fiscal year ended December 31, 2022, which was filed with the SEC on March 31, 2023.
In the opinion of management, all adjustments (including normal recurring adjustments) necessary to present a fair statement of the Company’s unaudited financial position as of June 30, 2023, its unaudited results of operations for the three and six months ended June 30, 2023 and 2022, and its unaudited cash flows for the six months ended June 30, 2023 and 2022, as applicable, have been made. The unaudited interim results of operations are not necessarily indicative of the operating results for the full fiscal year or any future periods.
The unaudited condensed consolidated financial statements include the financial statements of the Company, its subsidiaries and its variable interest entity (“VIE”) over which the Company exercises control and, where applicable, entities for which the Company has a controlling financial interest or is the primary beneficiary. All transactions and balances among the Company, its subsidiaries and its VIE have been eliminated upon consolidation.
Principles of consolidation
Subsidiaries are those entities in which the Company, directly or indirectly, controls more than one half of the voting power; or has the power to govern the financial and operating policies, to appoint or remove the majority of the members of the board of directors, or to cast a majority of votes at the meeting of directors.
A VIE is an entity that has either a total equity investment that is insufficient to permit the entity to finance its activities without additional subordinated financial support, or whose equity investors lack the characteristics of a controlling financial interest, such as through voting rights, right to receive the expected residual returns of the entity or obligation to absorb the expected losses of the entity. The variable interest holder, if any, that has a controlling financial interest in a VIE is deemed to be the primary beneficiary and must consolidate the VIE. As of and for the three and six months ended June 30, 2023, SEA, the only VIE of the Company has no significant operations.
Use of estimates
The preparation of unaudited condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the unaudited condensed consolidated financial statements and the reported amounts of revenues and expenses during the periods presented. Significant accounting estimates reflected in the Company’s unaudited condensed consolidated financial statements include allowance for inventories obsolescence, impairment of long-lived assets and allowance for deferred tax assets. Actual results could differ from these estimates.
Cash and cash equivalents
Cash and cash equivalents represent cash on hand, time deposits placed with banks or other financial institutions and all highly liquid investments with an original maturity of three months or less.
| F-8 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Accounts receivable
Accounts receivable are recorded at the invoiced amount less an allowance for any uncollectible accounts and do not bear interest, which are due on credit term. Management reviews the adequacy of the allowance for doubtful accounts on an ongoing basis, using historical collection trends and aging of receivables. Management also periodically evaluates individual customer’s financial condition, credit history, and the current economic conditions to make adjustments in the allowance when it is considered necessary. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company’s management continues to evaluate the reasonableness of the valuation allowance policy and update it if necessary. As of June 30, 2023 and December 31, 2022, allowance of doubtful accounts was recorded.
Inventories
Inventories consist of finished goods and are stated at the lower of cost or net realizable value using the first-in first-out method. Management reviews inventory on hand for estimated obsolescence or unmarketable items, as compared to future demand requirements and the shelf life of the various products. Based on the review, the Company records inventory write-downs, when necessary, when costs exceed expected net realizable value. For the three and six months ended June 30, 2023 and 2022, the Company did not recognize any inventory write-downs nor write-off.
Prepaid taxes
Prepaid taxes include prepaid income taxes that will either be refunded or utilized to offset future income tax.
Prepayments and deposits
Prepayments
and deposits are mainly cash deposited or advanced to suppliers for future inventory purchases or service providers for future services.
This amount is refundable and bears no interest. For any prepayments and deposits determined by management that such advances will not
be in receipts of inventories, services, or refundable, the Company will recognize an allowance account to reserve such balances. Management
reviews its prepayments and deposits on a regular basis to determine if the allowance is adequate, and adjusts the allowance when necessary.
Delinquent account balances are written-off against allowance for doubtful accounts after management has determined that the likelihood
of collection is not probable. The Company’s management continues to evaluate the reasonableness of the allowance policy and update
it if necessary. There was allowance for doubtful accounts recorded nor doubtful accounts written-off during the three and six months
ended June 30, 2023 and 2022. There was
Property and equipment, net
Property and equipment are stated at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets with no residual value. The estimated useful lives are as follows:
| F-9 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
| Useful Life | ||
| Computer and office equipment | ||
| Furniture & fixtures | ||
| Leasehold improvements | ||
| Vehicle |
The cost and related accumulated depreciation of assets sold or otherwise retired are eliminated from the accounts and any gain or loss is included in the unaudited condensed consolidated statements of operations and comprehensive loss. Expenditures for maintenance and repairs are charged to earnings as incurred, while additions, renewals and betterments, which are expected to extend the useful life of assets, are capitalized. The Company also re-evaluates the periods of depreciation to determine whether subsequent events and circumstances warrant revised estimates of useful lives.
Intangible assets, net
Intangible assets, net, are stated at cost, less accumulated amortization. Amortization expense is recognized on the straight-line basis over the estimated useful lives of the assets as follows:
| Classification | Useful Life | |
| Computer software |
Impairment for long-lived assets
Long-lived
assets, including property and equipment, and intangible assets with finite lives are reviewed for impairment whenever events or changes
in circumstances (such as a significant adverse change to market conditions that will impact the future use of the assets) indicate that
the carrying value of an asset may not be recoverable. The Company assesses the recoverability of the assets based on the undiscounted
future cash flows the assets are expected to generate and recognize an impairment loss when estimated undiscounted future cash flows
expected to result from the use of the asset plus net proceeds expected from disposition of the asset, if any, are less than the carrying
value of the asset. If an impairment is identified, the Company would reduce the carrying amount of the asset to its estimated fair value
based on a discounted cash flows approach or, when available and appropriate, to comparable market values. As of June 30, 2023 and December
31, 2022,
Deferred offering costs
Deferred offering costs represents costs associated with the Company’s current offering which will be netted against the proceeds from the Company’s proposed offering for uplisting.
Investment in marketable equity securities
The Company follows the provisions of ASU 2016-01, Financial Instruments – Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities. Investments in marketable equity securities (non-current) are reported at fair value with changes in fair value recognized in the Company’s unaudited condensed consolidated statements of operations and comprehensive loss in the caption of “unrealized holding gain (loss) on marketable securities” in each reporting period.
| F-10 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Investment in non-marketable equity securities
The Company follows the provisions of ASU 2016-01, Financial Instruments – Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities. Due to the Company’s non-marketable equity securities (non-current) does not qualify for the practical expedient to estimate fair value in accordance with ASC 820-10-35-59, the Company has selected to record its investments in non-marketable equity securities (non-current) at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issue.
At each reporting period, the Company will make a qualitative assessment considering impairment indicators to evaluate whether the investment is impaired. The qualitative assessment indicators include, but are not limited to: (1) A significant deterioration in the earnings performance, credit rating, asset quality, or business prospects of the investee; (ii) A significant adverse change in the regulatory, economic, or technological environment of the investee; (iii) A significant adverse change in the general market condition of either the geographical area or the industry in which the investee operates; (iv) A bona fide offer to purchase, an offer by the investee to sell, or a completed auction process for the same or similar investment for an amount less than the carrying amount of that investment; and (v) Factors that raise significant concerns about the investee’s ability to continue as a going concern, such as negative cash flows from operations, working capital deficiencies, or noncompliance with statutory capital requirements or debt covenants. If the qualitative assessment indicators indicated that the non-marketable equity securities (non-current) is deemed to be impaired, the Company would recognize the impairment loss equal to the difference between the fair value of the investment and its carrying amount.
Customer deposits
Customer deposits represent amounts advanced by customers on product orders and unapplied unexpired coupons. Customer deposits are reduced when the related sale is recognized in accordance with the Company’s revenue recognition policy.
Revenue recognition
The Company adopted Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers (ASC Topic 606). The core principle underlying the revenue recognition of this ASU allows the Company to recognize - revenue that represents the transfer of goods and services to customers in an amount that reflects the consideration to which the Company expects to be entitled in such exchange. This will require the Company to identify contractual performance obligations and determine whether revenue should be recognized at a point in time or over time, based on when control of goods and services transfers to a customer. The Company’s revenue streams are recognized at a point in time for the Company’s sale of health and wellness products.
The ASU requires the use of a new five-step model to recognize revenue from customer contracts. The five-step model requires that the Company (i) identify the contract with the customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, including variable consideration to the extent that it is probable that a significant future reversal will not occur, (iv) allocate the transaction price to the respective performance obligations in the contract, and (v) recognize revenue when (or as) the Company satisfies the performance obligation.
The Company accounts for a contract with a customer when the contract is committed in writing, the rights of the parties, including payment terms, are identified, the contract has commercial substance and consideration is probable of substantially collection.
| F-11 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Sales of Health and Wellness products
- Performance obligations satisfied at a point in time
The Company derives its revenues from sales contracts with its customers with revenues being recognized when control of the health and wellness products are transferred to its customer at the Company’s office or shipment of the goods. The revenue is recorded net of estimated discounts and return allowances. Products are given 60 days for returns or exchanges from the date of purchase. Historically, there were insignificant sales returns.
Under the Company’s network marketing business, the Company issues product coupons to members and distributors when these customers made purchases above certain thresholds set by the Company. Depending on the type of product coupons issued, the coupons carry varying values and can be used by the customers for reduction in the transaction price of product purchases within the coupon validity period. The value of the product coupons issued is recorded as a reduction of the Company’s revenue account upon issuance; the corresponding amount credited to the customer deposits account. Amounts in customer deposits will be reversed when the coupons are used. The Company’s coupons have a validity period of between six and twelve months. If the Company’s customers did not utilize the coupons after the validity period, the Company would recognize the forfeiture of the originated sales value of the coupons as net revenues.
For
the three months ended June 30, 2023 and 2022, the Company recognized $
The
Company had contracts for the sales of health and wellness products amounting to $
Sales of products for the provision of complementary health therapies
Products for the provision of complementary health therapies are predominantly Chinese herbs in different forms, processed or otherwise, for prescriptions for treating non-communicable diseases.
Provision of Health and Wellness services
- Performance obligations satisfied at a point in time
The Company carries out its Wellness program, where the Company’s products are bundled with health screening test and a health camp program. The health screening test and the health camp programs are considered as separate performance obligations. The promises to deliver the health screening test report and the attendance at the health camp are separately identifiable, which are evidenced by the fact that the Company provides separate services of delivering the health screening test report and allowing admission of the customers to attend the health camp. The Company derives its revenues from sales contracts with its customers with revenues being recognized when the test reports are completed and delivered to its customers during the consultation session in person.
The
Company also separately derives its revenues from sales contracts with its customers with revenues being recognized when the health camp
program was completed in the final day of the health camp. For the three months ended June 30, 2023 and 2022, revenues from health and
wellness services were $
| F-12 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Disaggregated information of revenues by products are as follows:
| For the three months ended | For the six months ended | |||||||||||||||
| June 30, | June 30, | |||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||
| Survivor Select | $ | $ | $ | $ | ||||||||||||
| Ionized Cal-Mag | ||||||||||||||||
| Omega Blend | ||||||||||||||||
| Beta Maxx | ||||||||||||||||
| Iron | ||||||||||||||||
| Young Formula | ||||||||||||||||
| ATPR Mito+ | ||||||||||||||||
| Hyaluronic Acid Serum | ||||||||||||||||
| Mousse Facial Cleanser | ||||||||||||||||
| Trim+ | ||||||||||||||||
| LIVO 5 | ||||||||||||||||
| Others – Products for the provision of complementary health therapies | ||||||||||||||||
| Others | ||||||||||||||||
| Total revenues - products | ||||||||||||||||
| Health and Wellness services | ||||||||||||||||
| Total revenues - products and services | $ | $ | $ | $ | ||||||||||||
Cost of revenue
Cost
of revenue comprised freight-in, the purchase cost of manufactured goods for sale to customers and
products for the provision of complementary health therapies. For the three and six months ended June 30, 2023, cost of revenue were
$
Shipping and handling
Shipping
and handling charges amounted to $
Advertising costs
There
were
| F-13 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Commission expenses
Commission
expenses are the Company’s most significant expenses. As with all companies in the network marketing industry, the Company’s
sales channel is external to the Company. The Company’s “external sales force” is stratified into two levels based
on priority recruitment. First, there are sales distributors. Second, all members recruited by a sales distributor, directly or indirectly,
are referred to as “sales network members”. The Company pays commission to every sales distributor based on purchases made
by its sales network members which includes the independent direct sales members. Top performing distributors with their own physical
stores may also become stockists of the Company, whereby they enjoy benefits such as maintaining a certain amount of the Company’s
inventory on their store premises. The stockists shall account to the Company for all products sales from their store premises as monitored
through the Company’s centralized stock tracking system. The Company pays a separate commission to stockists based on revenue generated
from the stockists’ physical stores. Commission expenses amounted to $
Defined contribution plan
The
full-time employees of the Company are entitled to the government mandated defined contribution plan. The Company is required to accrue
and pay for these benefits based on certain percentages of the employees’ respective salaries, subject to certain ceilings, in
accordance with the relevant government regulations, and make cash contributions to the government mandated defined contribution plan.
Total expenses for the plans were $
The related contribution plans include:
| - | Social
Security Organization (“SOSCO”) – | |
| - | Employees
Provident Fund (“EPF”) –based on employee’s monthly salary, | |
| - | Employment
Insurance System (“EIS”) – | |
| - | Human
Resource Development Fund (“HRDF”) – |
Income taxes
The Company accounts for income taxes in accordance with U.S. GAAP for income taxes. The charge for taxation is based on the results for the fiscal year as adjusted for items, which are non-assessable or disallowed. It is calculated using tax rates that have been enacted or substantively enacted by the balance sheet date.
Deferred taxes is accounted for using the asset and liability method in respect of temporary differences arising from differences between the carrying amount of assets and liabilities in the consolidated financial statements and the corresponding tax basis used in the computation of assessable tax profit. In principle, deferred tax liabilities are recognized for all taxable temporary differences. Deferred tax assets are recognized to the extent that it is probable that taxable profit will be available against which deductible temporary differences can be utilized. Deferred tax is calculated using tax rates that are expected to apply to the period when the asset is realized or the liability is settled.
| F-14 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Deferred tax is charged or credited in the income statement, except when it is related to items credited or charged directly to equity, in which case the deferred tax is also dealt with in equity. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Current income taxes are provided for in accordance with the laws of the relevant taxing authorities.
An
uncertain tax position is recognized as a benefit only if it is “more likely than not” that the tax position would be sustained
in a tax examination, with a tax examination being presumed to occur. The amount recognized is the largest amount of tax benefit that
is
The Company conducts much of its business activities in Hong Kong and Malaysia and is subject to tax in each of these jurisdictions. As a result of its business activities, the Company will file separate tax returns that are subject to examination by the foreign tax authorities.
Comprehensive income (loss)
Comprehensive income (loss) consists of two components, net income (loss) and other comprehensive income (loss). Net income (loss) refers to revenue, expenses, gains and losses that under GAAP are recorded as an element of stockholders’ equity but are excluded from net income. Other comprehensive income (loss) consists of a foreign currency translation adjustment resulting from the Company not using the U.S. dollar as its functional currencies.
Non-controlling interest
The Company computes earnings (loss) per share (“EPS”) in accordance with ASC 260, “Earnings per Share”. ASC 260 requires companies to present basic and diluted EPS. Basic EPS is measured as net income (loss) divided by the weighted average ordinary share outstanding for the period. Diluted EPS presents the dilutive effect on a per share basis of the potential common stocks (e.g., convertible securities, options and warrants) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential common stocks that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS. For the three and six months ended June 30, 2023 and 2022, there were dilutive shares.
| F-15 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Foreign currencies translation and transaction
Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency using the applicable exchange rates at the balance sheet dates. The resulting exchange differences are recorded in the consolidated statements of operations and comprehensive loss.
The reporting currency of the Company is United States Dollars (“US$”) and the accompanying financial statements have been expressed in US$. The Company’s subsidiary in Labuan maintains its books and record in United States Dollars (“US$”) albeit its functional currency being the primary currency of the economic environment in which the entity operates, which is the Malaysian Ringgit (“MYR” or “RM”). The Company’s subsidiary in Hong Kong maintains its books and record in Hong Kong Dollars (“HK$”), similar to its functional currency. The Company’s subsidiary and VIE in Malaysia conducts its businesses and maintains its books and record in the local currency, Malaysian Ringgit (“MYR” or “RM”), as its functional currency.
In general, for consolidation purposes, assets and liabilities of its subsidiaries whose functional currency is not US$ are translated into US$, in accordance with ASC Topic 830-30, “Translation of Financial Statement”, using the exchange rate on the balance sheet date. Revenues and expenses are translated at average rates prevailing during the period. The gains and losses resulting from translation of financial statements of foreign subsidiary are recorded as a separate component of accumulated other comprehensive income within the statements of stockholders’ equity. Cash flows are also translated at average translation rates for the periods, therefore, amounts reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheets.
Translation of foreign currencies into US$1 have been made at the following exchange rates for the respective periods:
| As of | ||||||||
| June 30, 2023 | December 31, 2022 | |||||||
| Period-end MYR : US$1 exchange rate | ||||||||
| Period-end HKD : US$1 exchange rate | ||||||||
For the three months ended June 30, | For the six months ended June 30, | |||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||
| Period-average MYR : US$1 exchange rate | ||||||||||||||||
| Period-average HKD : US$1 exchange rate | ||||||||||||||||
| F-16 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Related parties
Parties, which can be a corporation or individual, are considered to be related if the Company has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operating decisions. Companies are also considered to be related if they are subject to common control or common significant influence.
Fair value of financial instruments
The accounting standard regarding fair value of financial instruments and related fair value measurements defines financial instruments and requires disclosure of the fair value of financial instruments held by the Company.
The accounting standards define fair value, establish a three-level valuation hierarchy for disclosures of fair value measurement and enhance disclosure requirements for fair value measures. The three levels are defined as follow:
| ● | Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. | |
| ● | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments. | |
| ● | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value. |
Financial instruments included in current assets and current liabilities are reported in the consolidated balance sheets at face value or cost, which approximate fair value because of the short period of time between the origination of such instruments and their expected realization and their current market rates of interest.
Leases
The Company adopted ASU 2016-02, “Leases” (Topic 842), and elected the practical expedients that does not require the Company to reassess: (1) whether any expired or existing contracts are, or contain, leases, (2) lease classification for any expired or existing leases and (3) initial direct costs for any expired or existing leases. For lease terms of twelve months or fewer, a lessee is permitted to make an accounting policy election not to recognize lease assets and liabilities. The Company also adopts the practical expedient that allows lessees to treat the lease and non-lease components of a lease as a single lease component. Some of the Company’s leases include one or more options to renew, which is typically at the Company’s sole discretion. The Company regularly evaluates the renewal options, and, when it is reasonably certain of exercise, it will include the renewal period in its lease term. New lease modifications result in re-measurement of the right of use (“ROU”) assets and lease liabilities. Operating ROU assets and lease liabilities are recognized at the commencement date, based on the present value of lease payments over the lease term. Since the implicit rate for the Company’s leases is not readily determinable, the Company use its incremental borrowing rate based on the information available at the commencement date in determining the present value of lease payments. The incremental borrowing rate is the rate of interest that the Company would have to pay to borrow, on a collateralized basis, an amount equal to the lease payments, in a similar economic environment and over a similar term.
| F-17 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
3. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Lease terms used to calculate the present value of lease payments generally do not include any options to extend, renew, or terminate the lease, as the Company does not have reasonable certainty at lease inception that these options will be exercised. The Company generally considers the economic life of its operating lease ROU assets to be comparable to the useful life of similar owned assets. The Company has elected the short-term lease exception, therefore operating lease ROU assets and liabilities do not include leases with a lease term of twelve months or less. Its leases generally do not provide a residual guarantee. The operating lease ROU asset also excludes lease incentives. Lease expense is recognized on a straight-line basis over the lease term.
The Company reviews the impairment of its ROU assets consistent with the approach applied for its other long-lived assets. The Company reviews the recoverability of its long-lived assets when events or changes in circumstances occur that indicate that the carrying value of the asset may not be recoverable. The assessment of possible impairment is based on its ability to recover the carrying value of the asset from the expected undiscounted future pre-tax cash flows of the related operations. The Company has elected to include the carrying amount of operating lease liabilities in any tested asset group and includes the associated operating lease payments in the undiscounted future pre-tax cash flows.
Recent accounting pronouncements
The Company has reviewed all recently issued, but not yet effective, considers the applicability and impact of all accounting standards updates (“ASUs”). Management periodically reviews new accounting standards that are issued.
In March 2023, the FASB issued ASU No. 2023-01, “Leases (Topic 842) Common Control Arrangements”. This ASU provides guidance in ASC Topic 842 that leasehold improvements associated with common control leases should be (i) amortized by the lessee over the useful life of the leasehold improvements to the common control group, regardless of the lease term, as long as the lessee controls the use of the underlying asset through a lease, and (ii) accounted for as a transfer between entities under common control through an adjustment to equity if and when the lessee no longer controls the use of the underlying asset. The ASU 2023-01 is effective for reporting periods beginning after December 15, 2023. Early adoption is permitted for both interim and annual financial statements that have not yet been issued. When adopted in an interim period, it must be adopted from the beginning of the year that includes that interim period. The Company is currently evaluating the impact of this ASU may have on its unaudited condensed consolidated financial statements.
Except for the above-mentioned pronouncements, there are no other new recent issued accounting standards that will have a material impact on the consolidated financial position, statements of operations and cash flows.
Recently adopted Accounting Pronouncements
In November 2019, the FASB issued ASU No. 2019-10, which to update the effective date of ASU No. 2016-13 for private companies, not-for-profit organizations and certain smaller reporting companies applying for credit losses, leases, and hedging standard. The new effective date for these preparers is for fiscal years beginning after December 15, 2022. ASU 2019-05 is effective for the Company for annual and interim reporting periods beginning January 1, 2023 as the Company is qualified as a smaller reporting company. The Company has accordingly adopted ASUs 2016-13 and 2019-05 in the preparation of its unaudited condensed consolidated financial statements. The adoption of the accounting standards has no material impact on the unaudited condensed consolidated financial statements for the three and six months ended June 30, 2023.
4. VARIABLE INTEREST ENTITY (“VIE”)
SEA
is a trading company incorporated on March 4, 2004, under the laws of Malaysia. SEA provided majority of ASL’s purchases. Its equity
at risk was insufficient to finance its activities and
| a. | The power to direct the activities of the VIE that most significantly impact the VIE’s economic performance; and | |
| b. | The obligation to absorb losses of the VIE that could potentially be significant to the VIE or the right to receive benefits from the VIE that could potentially be significant to the VIE. |
Accordingly, the accounts of SEA is consolidated in the accompanying financial statements.
| F-18 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
4. VARIABLE INTEREST ENTITY (“VIE”) (Continued)
The carrying amount of the VIE’s assets and liabilities were as follows:
| As of | ||||||||
| June 30, 2023 | December 31, 2022 | |||||||
| Current assets | $ | $ | ||||||
| Current liabilities | ( | ) | ( | ) | ||||
| Net deficit | $ | ( | ) | $ | ( | ) | ||
| As of | ||||||||
| June 30, 2023 | December 31, 2022 | |||||||
| Current assets: | ||||||||
| Cash | $ | $ | ||||||
| Prepaid taxes | ||||||||
| Prepayment and deposits | ||||||||
| Total current assets | $ | $ | ||||||
| Current liabilities: | ||||||||
| Accounts payable – intercompany | $ | $ | ||||||
| Other payables and accrued liabilities | ||||||||
| Total current liabilities | $ | $ | ||||||
| Net deficit | $ | ( | ) | $ | ( | ) | ||
The summarized operating results of the VIE’s are as follows:
| For the three months ended June 30, | For the six months ended June 30, | |||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||
| Operating revenues | $ | $ | $ | $ | ||||||||||||
| Gross profit | $ | $ | $ | $ | ||||||||||||
| Profit (Loss) from operations | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | ||||||
| Net loss | $ | ( | ) | $ | $ | ( | ) | $ | ( | ) | ||||||
| F-19 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
5. CASH AND CASH EQUIVALENTS
As
of June 30, 2023 and December 31, 2022 the Company has $
6. ACCOUNTS RECEIVABLE
| As of | ||||||||
| June 30, 2023 | December 31, 2022 | |||||||
| Accounts receivable | $ | $ | ||||||
| Allowance for doubtful accounts | ||||||||
| Total accounts receivable | $ | $ | ||||||
7. INVENTORIES
Inventories consist of the following:
| As of | ||||||||
| June 30, 2023 | December 31, 2022 | |||||||
| Finished goods | $ | $ | ||||||
There were inventory write-downs nor write-off for the three and six months ended June 30, 2023 and 2022, respectively.
8. PREPAYMENTS AND DEPOSITS
| As of | ||||||||
| June 30, 2023 | December 31, 2022 | |||||||
| Receivables from sales distributors | $ | $ | ||||||
| Deposits to suppliers | ||||||||
| Subtotal | ||||||||
| Less: Allowance for doubtful accounts | ||||||||
| Total | $ | $ | ||||||
| F-20 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
8. PREPAYMENTS AND DEPOSITS (Continued)
Movements of allowance for doubtful accounts are as follows:
| For the six months ended June 30, 2023 | For the year ended December 31, 2022 | |||||||
| Beginning balance | $ | $ | ||||||
| Addition | ||||||||
| Write off | ( | ) | ||||||
| Exchange rate effect | ( | ) | ||||||
| Ending balance | $ | $ | ||||||
9. PROPERTY AND EQUIPMENT, NET
Property and equipment, net consist of the following:
| As of | ||||||||
| June 30, 2023 | December 31, 2022 | |||||||
| Computer and office equipment | $ | $ | ||||||
| Furniture & fixtures | ||||||||
| Leasehold improvements | ||||||||
| Vehicle | ||||||||
| Subtotal | ||||||||
| Less: accumulated depreciation | ( | ) | ( | ) | ||||
| Total | $ | $ | ||||||
Depreciation
expense for the three months ended June 30, 2023 and 2022 amounted to $
10. INTANGIBLE ASSETS, NET
Intangible assets, net, consist of the following:
| As of | ||||||||
| June 30, 2023 | December 31, 2022 | |||||||
| Computer software | $ | $ | ||||||
| Less: accumulated amortization | ( | ) | ( | ) | ||||
| Total | $ | $ | ||||||
Amortization
expense for the three months ended June 30, 2023 and 2022 amounted to $
| F-21 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
11. INVESTMENT IN MARKETABLE SECURITIES
| (i) | On
May 17, 2018, the Company purchased shares of common stock in Greenpro Capital Corp. for $ | |
| (ii) | On
July 30, 2018, the Company disposed shares of common stock in Greenpro Capital Corp. for $ | |
| (iii) | On
October 16, 2018, the Company purchased shares of common stock in Greenpro Capital Corp. for $ | |
| (iv) | On
July 19, 2022, Greenpro Capital Corp. filed a certificate of change with the Secretary of State of Nevada to effect a reverse split
of | |
| (v) | On
November 3, 2020, the Company received dividend of shares of common stock in DSwiss, Inc. for $ | |
| (vi) | On
December 9, 2020, the Company received dividend of shares of common stock in DSwiss, Inc. for $ | |
| (vii) | On
September 27, 2021, the Company received dividend of shares of common stock in SEATech Ventures Corp. for $ | |
| (viii) | On
April 3, 2019, the Company purchased a |
| As of | ||||||||
| June 30, 2023 | December 31, 2022 | |||||||
| Cost of investment | $ | $ | ||||||
| Transfer from non-marketable security | ||||||||
| Dividend income from Greenpro Capital Corp. | ||||||||
| Unrealized holding gain (loss) | ( | ) | ||||||
| Exchange rate effect | ( | ) | ( | ) | ||||
| Investment in marketable securities | $ | $ | ||||||
12. INVESTMENT IN NON-MARKETABLE SECURITIES
On
April 3, 2019, the Company purchased a
| As of | ||||||||
| Phoenix Plus Corporation | June 30, 2023 | December 31, 2022 | ||||||
| Cost of investment | $ | $ | ||||||
| Less: Transfer to investment in marketable securities | ( | ) | ||||||
| Investment in non-marketable securities | $ | $ | ||||||
| F-22 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
13. CUSTOMER DEPOSITS
| As of | ||||||||
| June 30, 2023 | December 31, 2022 | |||||||
| Customer deposits | $ | $ | ||||||
| Unexpired product coupons | ||||||||
| Total | $ | $ | ||||||
Customer deposits represent amounts advanced by customers on product orders and unexpired product coupons issued to the Company’s members and distributors of its network marketing business.
14. OTHER PAYABLES AND ACCRUED LIABILITIES
| As of | ||||||||
| June 30, 2023 | December 31, 2022 | |||||||
| Professional fees | $ | $ | ||||||
| Promotion expenses | ||||||||
| Payroll | ||||||||
| Amounts held in eWallets (a) | ||||||||
| Tax penalty | ||||||||
| Others | ||||||||
| Total | $ | $ | ||||||
| (a) |
15. RELATED PARTY BALANCES AND TRANSACTIONS
Related party balances
Amount due from related parties
| As of | ||||||||||||
| Name of Related Party | Relationship | Nature | June 30, 2023 | December 31, 2022 | ||||||||
| TH3 Technology Sdn Bhd (“TH3”) | $ | $ | ||||||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | ||||||||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | ||||||||||||
| Total | $ | $ | ||||||||||
Accounts payable – related parties
| As of | ||||||||||||
| Name of Related Party | Relationship | Nature | June 30, 2023 | December 31, 2022 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | $ | $ | ||||||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | ||||||||||||
| Total | $ | $ | ||||||||||
| F-23 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
15. RELATED PARTY BALANCES AND TRANSACTIONS (Continued)
Related party balances
Other payable - related parties
| As of | ||||||||||||
| Name of Related Party | Relationship | Nature | June 30, 2023 | December 31, 2022 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | $ | $ | ||||||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | ||||||||||||
| Mr. How Kok Choong | ||||||||||||
| Total | $ | $ | ||||||||||
Related party transactions
Purchases
| For the three months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | $ | $ | ||||||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | ||||||||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | ||||||||||||
| Total | $ | $ | ||||||||||
| F-24 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
15. RELATED PARTY BALANCES AND TRANSACTIONS (Continued)
Related party transactions
| For the six months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | $ | $ | ||||||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | ||||||||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | ||||||||||||
| Total | $ | $ | ||||||||||
Other Purchases
| For the three months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | $ | $ | ||||||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | ||||||||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | ||||||||||||
| Total | $ | $ | ||||||||||
| F-25 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
15. RELATED PARTY BALANCES AND TRANSACTIONS (Continued)
Related party transactions
Other purchases
| For the six months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | $ | $ | ||||||||||
| SY Welltech Sdn Bhd (“Welltech”) (formerly known as DSY Beauty Sdn Bhd) | ||||||||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | ||||||||||||
| Total | $ | $ | ||||||||||
Commission
| For the three months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| Mr. How Kok Choong | $ | $ | ||||||||||
| Total | $ | $ | ||||||||||
| F-26 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
15. RELATED PARTY BALANCES AND TRANSACTIONS (Continued)
Related party transactions
Commission
| For the six months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| Mr. How Kok Choong | $ | $ | ||||||||||
| Total | $ | $ | ||||||||||
Other Income
| For the three months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| Ando Design Sdn Bhd (“Ando”) | $ | $ | ||||||||||
| Redboy Picture Sdn Bhd (“Redboy”) | ||||||||||||
| TH3 Technology Sdn Bhd (“TH3”) | ||||||||||||
| Total | $ | $ | ||||||||||
| For the six months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| Ando Design Sdn Bhd (“Ando”) | $ | $ | ||||||||||
| Redboy Picture Sdn Bhd (“Redboy”) | ||||||||||||
| TH3 Technology Sdn Bhd (“TH3”) | ||||||||||||
| Total | $ | $ | ||||||||||
| F-27 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
15. RELATED PARTY BALANCES AND TRANSACTIONS (Continued)
Related party transactions
Other expenses
| For the three months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| TH3 Technology Sdn Bhd (“TH3”) | $ | $ | ||||||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | ||||||||||||
| Total | $ | $ | ||||||||||
| For the six months ended June 30, | ||||||||||||
| Name of Related Party | Relationship | Nature | 2023 | 2022 | ||||||||
| TH3 Technology Sdn Bhd (“TH3”) | $ | $ | ||||||||||
| DSY Wellness and Longevity Center Sdn Bhd (“DSYWLC”) | ||||||||||||
| Total | $ | $ | ||||||||||
16. STOCKHOLDERS’ EQUITY
Preferred stock
As of June 30, 2023 and December 31, 2022, there were preferred stocks authorized but were issued and outstanding.
Common stock
As of June 30, 2023 and December 31, 2022, there were common stocks authorized; and shares issued and outstanding.
A share forfeiture agreement (the “Share Forfeiture Agreement”) dated January 20, 2022, between the Company and Mr. How Kok Choong, the CEO and director of the Company, pursuant to which Mr. How Kok Choong agreed to forfeit shares of common stock of the Company. As a result, the outstanding shares was reduced by shares of common stock.
There were stock options, warrants or other potentially dilutive securities outstanding as of June 30, 2023 and December 31, 2022.
| F-28 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
17. NON-CONTROLLING INTEREST
The Company’s non-controlling interest consists of the following:
| As of | ||||||||
| June 30, 2023 | December 31, 2022 | |||||||
| DSY Wellness: | ||||||||
| Paid-in capital | $ | $ | ||||||
| Retained earnings | ||||||||
| Accumulated other comprehensive (loss) income | ( | ) | ||||||
| ASL | ||||||||
| Total | $ | $ | ||||||
18. INCOME TAXES
The United States and foreign components of income (loss) before income taxes were comprised of the following:
| For the three months ended June 30, | For the six months ended June 30, | |||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||
| Tax jurisdictions from: | ||||||||||||||||
| Local – United States | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| Foreign – Malaysia | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Foreign – Hong Kong | ( | ) | ( | ) | ||||||||||||
| Loss before income tax | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
The benefit of (provision for) income taxes consisted of the following:
| For the three months ended June 30, | For the six months ended June 30, | |||||||||||||||
| 2023 | 2022 | 2023 | 2022 | |||||||||||||
| Current: | ||||||||||||||||
| - Local | $ | $ | $ | $ | ||||||||||||
| - Foreign | ( | ) | ( | ) | ||||||||||||
| Deferred: | ||||||||||||||||
| - Local | ||||||||||||||||
| - Foreign | ||||||||||||||||
| Benefit of (Provision for) income tax | $ | $ | ( | ) | $ | $ | ( | ) | ||||||||
The effective tax rate in the periods presented is the result of the mix of income earned in various tax jurisdictions that apply a broad range of income tax rates. The Company and its subsidiary that operate in various countries: United States, Malaysia (including Labuan) and Hong Kong that are subject to taxes in the jurisdictions in which they operate, as follows:
| F-29 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
18. INCOME TAXES (Continued)
United States of America
Agape
ATP Corporation was incorporated in the State of Nevada and is subject to the tax laws of the United States of America with a corporate
tax rate of
For the three and six months ended June 30, 2023 and 2022, the Company’s foreign subsidiaries did not generate any income that are subject to Subpart F tax and GILTI tax.
As
of June 30, 2023 and December 31, 2022, the operations in the United States of America incurred approximately $
Malaysia
Agape
ATP Corporation, Agape Superior Living Sdn Bhd, Agape S.E.A Sdn Bhd., Wellness ATP International Holdings Sdn Bhd. and DSY Wellness International
Sdn. Bhd. are governed by the income taxes laws of Malaysia and the income taxes provision in respect of operations in Malaysia is calculated
at the applicable tax rates on the taxable income for the periods based on existing legislation, interpretations and practices in respect
thereof. Under the Income Tax Act of Malaysia, enterprises that incorporated in Malaysia are usually subject to a unified 24% enterprise
income taxes rate while preferential tax rates, tax holidays and even tax exemption may be granted on case-by-case basis.
As
of June 30, 2023 and December 31, 2022, the operations in Malaysia incurred approximately $
Hong Kong
Agape
ATP International Holding (HK) Limited is subject to Hong Kong Profits Tax, which is charged at the statutory income rate of
| F-30 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
18. INCOME TAXES (Continued)
The following table sets forth the significant components of the aggregate deferred tax assets of the Company:
| As of | ||||||||
June 30, 2023 | December 31, 2022 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carry forwards in U.S. | $ | $ | ||||||
| Net operating loss carry forwards in Malaysia | ||||||||
| Less: valuation allowance | ( | ) | ( | ) | ||||
| Deferred tax assets, net | $ | $ | ||||||
Uncertain tax positions
The Company evaluates each uncertain tax position (including the potential application of interest and penalties) based on the technical merits, and measure the unrecognized benefits associated with the tax positions. As of June 30, 2023 and December 31, 2022, the Company did not have any significant unrecognized uncertain tax positions. The Company did not incur any interest and penalties tax for the three and six months ended June 30, 2023 and 2022.
19. CONCENTRATIONS OF RISKS
(a) Major customers
For
the three months ended June 30, 2023 and 2022, no customer accounted for
As
of June 30, 2023, two individual customers accounted for approximately
(b) Major vendors
For
the three months ended June 30, 2023, three vendors accounted for approximately
For
the six months ended June 30, 2023, the same three vendors accounted for approximately
As
of June 30, 2023, two vendors accounted for approximately
CTA
Nutriceuticals (Asia) Sdn Bhd, a related company, accounted for approximately
| F-31 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
19. CONCENTRATIONS OF RISKS (Continued)
(c) Commission Expenses to Sales Distributors and Stockists
One
sales distributor accounted for
For
the six months ended June 30, 2023, one sales distributor accounted for
(d) Credit risk
Financial
instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash. As of June 30,
2023 and December 31, 2022, $
Financial instruments that are potentially subject to credit risk consist principally of accounts receivable. The Company believes the concentration of credit risk in its account receivable is substantially mitigated by its ongoing credit evaluation process and relatively short collection terms. The Company does not generally require collateral from customers. The Company evaluates the need for an allowance for doubtful accounts based upon factors surrounding the credit risk of specific customers, historical trends and other information. Historically, the Company did not have any bad debt on its account receivable.
(e) Exchange rate risk
The Company cannot guarantee that the current exchange rate will remain steady; therefore, there is a possibility that the Company could post the same amount of profit for two comparable periods and because of the fluctuating exchange rate actually post higher or lower profit depending on exchange rate of RM and HK$ converted to US$ on that date. The exchange rate could fluctuate depending on changes in political and economic environments without notice.
20. COMMITMENTS AND CONTINGENCIES
Lease commitments
On
April 1, 2020, the Company adopted ASC 842 for ASL’s office space lease and sales and training center as the lease commencement
date upon the acquisition of ASL. The Company recognized lease liabilities of approximately $
On
May 31, 2021, the Company entered into two separate two-year leases extension with the modified lease expiring May 31, 2023 for its office
space and expiring
On
October 1, 2021, the Company entered into a lease for an apartment to serve as staff accommodation. The Company recognized
lease liabilities of approximately $
On
November 1, 2021, the Company entered into a two-years lease for a branch office and operating centre. The Company recognized lease liabilities
of approximately $
Amortization
of operating right-of-use assets for the three months ended June 30, 2023 and 2022 were $
As
of June 30, 2023, the weighted remaining term of the lease is approximately
| F-32 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
20. COMMITMENTS AND CONTINGENCIES (Continued)
The five-year maturity of the Company’s operating lease liabilities is as follow:
| Twelve Months Ending June 30, | Operating lease liabilities | |||
| 2024 | $ | |||
| 2025 | ||||
| 2026 | ||||
| Thereafter | ||||
| Total lease payments | ||||
| Less: interest | ( | ) | ||
| Present value of lease liabilities | $ | |||
The
Company also leases one office and operation center, and one shophouse with an expiring term of twelve months or less, which were classified
as operation leases. Since the lease terms for these leases were twelve months or less, a lessee is permitted to elect not to recognize
lease assets and liabilities. The Company has elected not to recognize lease assets and liabilities on these leases. As of June 30, 2023,
the Company’s commitment for minimum lease payment under these operating leases within the next twelve months were $
Rent
expense for the three months ended June 30, 2023 and 2022 was $
Contingencies
Legal
From time to time, the Company is party to certain legal proceedings, as well as certain asserted and un-asserted claims. Amounts accrued, as well as the total amount of reasonably possible losses with respect to such matters, individually and in the aggregate, are not deemed to be material to the consolidated financial statements.
| F-33 |
AGAPE ATP CORPORATION
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
(Currency expressed in United States Dollars (“US$”), except for number of shares)
20. COMMITMENTS AND CONTINGENCIES (Continued)
COVID-19
Malaysia, where the operations of the Company predominantly reside, officially transitioned to the endemic phase of COVID-19 effective April 1, 2022. Restrictions on businesses and people are minimal. Meanwhile, the government continues to encourage inoculation for those between the ages of 5 and 11 years and its adolescent group which comprised those between the ages 12 and 17. Adults who have been fully vaccinated, i.e. received two doses of the COVID-19 vaccine are encouraged to take booster shots.
Substantially all of our revenues are concentrated in Malaysia. Consequently, our results of operations will likely be adversely, and may be materially, affected, to the extent that the COVID-19 or any other epidemic harms the Malaysia and global economy in general. Any potential impact to our results will depend on, to a large extent, future developments and new information that may emerge regarding the duration and severity of the COVID-19 and the actions taken by government authorities and other entities to contain the COVID-19 or treat its impact, almost all of which are beyond our control. Potential impacts include, but are not limited to, the following:
| ● | temporary closure of offices, travel restrictions, disruption or suspension of supplies, our customers may be negatively impacted financially resulting in which the demand for our products may be adversely affected; | |
| ● | we may have to provide significant sales incentives to our customers during the outbreak, which may in turn materially adversely affect our financial condition and operating results; and | |
| ● | any disruption of our supply chain, logistics providers or customers could adversely impact our business and results of operations, including causing us or our suppliers to cease manufacturing for a period of time or materially delay delivery to our customers, which may also lead to loss of our customers. |
Because of the uncertainty surrounding the COVID-19 outbreak, the financial impact related to the outbreak of and response to the COVID-19 cannot be reasonably estimated at this time. There is no guarantee that the Company’s total revenues will grow or remain at similar levels year over year in 2023 and beyond.
21. SUBSEQUENT EVENTS
The Company has evaluated subsequent events through the date of issuance of this unaudited condensed consolidated financial statements, and does not identify any events with material financial impact on the Company’s unaudited condensed consolidated financial statements.
| F-34 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Agape ATP Corporation
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Agape ATP Corporation (the “Company”) as of December 31, 2022, the related consolidated statement of operation, comprehensive loss, stockholders’ equity and cash flows for the year then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
Critical Audit Matters
Critical audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) relate to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgments. We determined that there are no critical audit matters.
/s/ Marcum Asia CPAs LLP
Marcum Asia CPAs LLP
We have served as the Company’s auditor since 2019 (such date takes into account the acquisition of certain assets of Friedman LLP by Marcum Asia CPAs LLP effective September 1, 2022)
New
York, New York
March 31, 2023
| F-35 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Agape ATP Corporation
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheet of Agape ATP Corporation (the “Company”) as of December 31, 2021, and the related consolidated statement of operations and comprehensive loss, changes in stockholders’ equity and cash flow for the year ended December 31, 2021, and the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2021, and the result of its operations and its cash flow for the year ended December 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audit provide a reasonable basis for our opinion.
/s/ Friedman LLP
We have served as the Company’s auditor since 2019 through 2022
New York, New York
March 28, 2022

| F-36 |
AGAPE ATP CORPORATION
CONSOLIDATED BALANCE SHEETS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash and cash equivalents (Included $ | $ | $ | ||||||
| Accounts receivable | ||||||||
| Amount due from related parties | ||||||||
| Inventories | ||||||||
| Prepaid taxes (Included $ | ||||||||
| Prepayments and deposits | ||||||||
| Total Current Assets | ||||||||
| OTHER ASSETS | ||||||||
| Property and equipment, net | ||||||||
| Intangible assets, net | ||||||||
| Operating right-of-use assets | ||||||||
| Investment in marketable securities | ||||||||
| Investment in non-marketable securities | ||||||||
| Deferred offering costs | ||||||||
| Total other assets | ||||||||
| TOTAL ASSETS | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable | $ | $ | ||||||
| Accounts payable – related parties | ||||||||
| Customer deposits | ||||||||
| Operating lease liabilities | ||||||||
| Other payables and accrued liabilities ($ | ||||||||
| Other payable – related parties | ||||||||
| Income tax payable | ||||||||
| Total Current Liabilities | ||||||||
| NON-CURRENT LIABILITIES | ||||||||
| Operating lease liabilities | $ | $ | ||||||
| Deferred tax liabilities | ||||||||
| Total Non-current Liabilities | ||||||||
| TOTAL LIABILITIES | $ | $ | ||||||
| STOCKHOLDERS’ EQUITY | ||||||||
| Preferred stock, $ par value; shares authorized; issued and outstanding | ||||||||
| Common Stock, par value $; shares authorized, and shares issued and outstanding as of December 31, 2022 and 2021, respectively. | ||||||||
| Additional paid in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Accumulated other comprehensive income | ||||||||
| TOTAL AGAPE CORPORATION STOCKHOLDERS’ EQUITY | ||||||||
| NON-CONTROLLING INTERESTS | ( | ) | ||||||
| TOTAL EQUITY | ||||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-37 |
AGAPE ATP CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
| For the years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| REVENUE | $ | $ | ||||||
| COST OF REVENUE | ( | ) | ( | ) | ||||
| GROSS PROFIT | ||||||||
| SELLING | ( | ) | ( | ) | ||||
| COMMISSION | ( | ) | ( | ) | ||||
| GENERAL AND ADMINISTRATIVE | ( | ) | ( | ) | ||||
| PROVISION FOR DOUBTFUL ACCOUNTS | ( | ) | ||||||
| TOTAL OPERATING EXPENSES | ( | ) | ( | ) | ||||
| LOSS FROM OPERATIONS | ( | ) | ( | ) | ||||
| OTHER INCOME (EXPENSES) | ||||||||
| Other expenses, net | ( | ) | ( | ) | ||||
| Interest income | ||||||||
| Unrealized holding loss on marketable securities | ( | ) | ( | ) | ||||
| Dividend income from marketable securities | ||||||||
| TOTAL OTHER EXPENSES, NET | ( | ) | ( | ) | ||||
| LOSS BEFORE INCOME TAXES | ( | ) | ( | ) | ||||
| BENEFIT OF (PROVISION FOR) INCOME TAXES | ( | ) | ||||||
| NET LOSS | ( | ) | ( | ) | ||||
| NET (INCOME) LOSS ATTRIBUTABLE TO NON-CONTROLLING INTERESTS | ( | ) | ||||||
| NET LOSS ATTRIBUTABLE TO AGAPE ATP CORPORATION | $ | ( | ) | $ | ( | ) | ||
| NET LOSS | $ | ( | ) | $ | ( | ) | ||
| OTHER COMPREHENSIVE LOSS | ||||||||
| Foreign currency translation adjustment | ( | ) | ( | ) | ||||
| TOTAL COMPREHENSIVE LOSS | ( | ) | ( | ) | ||||
| Less: Comprehensive income (loss) attributable to non-controlling interests | ( | ) | ||||||
| COMPREHENSIVE LOSS ATTRIBUTABLE TO AGAPE ATP CORPORATION | $ | ( | ) | $ | ( | ) | ||
| LOSS PER SHARE | ||||||||
| Basic and diluted | $ | ) | $ | ) | ||||
| WEIGHTED AVERAGE NUMBER OF COMMON SHARES OUTSTANDING | ||||||||
| Basic and diluted | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-38 |
AGAPE ATP CORPORATION
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(Currency expressed in United States Dollars (“US$”), except for number of shares)
| COMMON STOCK | ADDITIONAL | ACCUMULATED OTHER | NON- | TOTAL | ||||||||||||||||||||||||
| Number of shares | Par value | PAID IN CAPITAL | ACCUMULATED DEFICIT | COMPREHENSIVE INCOME | CONTROLLING INTERESTS | STOCKHOLDERS’ EQUITY | ||||||||||||||||||||||
| Balance as of December 31, 2020 | $ | $ | $ | ( | ) | $ | $ | $ | ||||||||||||||||||||
| Forfeiture of common stock | ( | ) | ( | ) | ||||||||||||||||||||||||
| Contributions from non-controlling interest shareholders | - | |||||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | ( | ) | |||||||||||||||||||||
| Foreign currency translation adjustment | - | - | - | - | (87,618 | ) | 3 | (87,615 | ) | |||||||||||||||||||
| Balance as of December 31, 2021 | ( | ) | ( | ) | ||||||||||||||||||||||||
| Forfeiture of common stock | ( | ) | ( | ) | ||||||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Foreign currency translation adjustment | - | ( | ) | ( | ) | |||||||||||||||||||||||
| Balance as of December 31, 2022 | $ | $ | $ | ( | ) | $ | $ | $ | ||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-39 |
AGAPE ATP CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Currency expressed in United States Dollars (“US$”)
| For the years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | ||||||||
| Amortization | ||||||||
| Amortization of operating right-of-use assets | ||||||||
| Unrealized holding loss on marketable securities | ||||||||
| Dividend income from marketable securities | ( | ) | ||||||
| Deferred tax (benefit) expense | ( | ) | ||||||
| Inventory write-down | ||||||||
| Provision for doubtful accounts | ||||||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivables | ( | ) | ||||||
| Amount due from related parties | ( | ) | ||||||
| Inventories | ||||||||
| Prepaid taxes | ||||||||
| Prepayments and deposits | ( | ) | ||||||
| Accounts payable | ||||||||
| Accounts payable – related parties | ||||||||
| Customer deposits | ||||||||
| Operating lease liabilities | ( | ) | ( | ) | ||||
| Other payables and accrued liabilities | ( | ) | ||||||
| Other payables – related parties | ||||||||
| Income tax payables | ||||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Purchase of equipment | ( | ) | ( | ) | ||||
| Purchase of intangible assets | ( | ) | ||||||
| Net cash used in investing activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Deferred offering costs | ( | ) | ( | ) | ||||
| Advances to related parties | ( | ) | ||||||
| Net cash used in financing activities | ( | ) | ( | ) | ||||
| EFFECT OF EXCHANGE RATE ON CASH AND CASH EQUIVALENTS | ( | ) | ( | ) | ||||
| DECREASE IN CASH AND CASH EQUIVALENTS | ( | ) | ( | ) | ||||
| CASH AND CASH EQUIVALENTS, beginning of year | ||||||||
| CASH AND CASH EQUIVALENTS, end of year | $ | $ | ||||||
| SUPPLEMENTAL CASH FLOWS INFORMATION | ||||||||
| Income taxes paid | $ | $ | ||||||
| SUPPLEMENTAL NON-CASH FLOWS INFORMATION | ||||||||
| Changes in right-of-use assets and lease liabilities due to lease modifications | $ | $ | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-40 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
1. ORGANIZATION AND BUSINESS BACKGROUND
Agape ATP Corporation, a Nevada corporation (“the Company”) was incorporated under the laws of the State of Nevada on June 1, 2016.
Agape ATP Corporation operates through its subsidiaries, namely, Agape ATP Corporation (“AATP LB”), a company incorporated in Labuan, Malaysia, and Agape Superior Living Sdn. Bhd. (“ASL”), a company incorporated in Malaysia.
Agape
ATP Corporation, incorporated in Labuan, Malaysia, is an investment holding company with
On
May 8, 2020, the Company entered into a Share Exchange Agreement with Mr. How Kok Choong, CEO and director of the Company to acquire
ordinary shares, no par value, equivalent to approximately
Agape Superior Living Sdn. Bhd. is a limited company incorporated on August 8, 2003, under the laws of Malaysia.
On September 11, 2020, the Company incorporated Wellness ATP International Holdings Sdn, Bhd. (“WATP”), a wholly owned subsidiary under the laws of Malaysia, to pursue the business of promoting wellness and wellbeing lifestyle of the community by providing services that includes online editorials, programs, events and campaigns on how to achieve positive wellness and lifestyle.
On
November 11, 2021, Agape ATP Corporation (Labuan) formed a joint-venture entity, DSY Wellness International Sdn. Bhd. (“DSY Wellness”)
with an independent third party which Agape ATP Corporation (Labuan) owns
The Company and its subsidiaries are principally engaged in the Health and Wellness Industry. The principal activity of the Company is to supply high-quality health and wellness products, including supplements to assist in cell metabolism, detoxification, blood circulation, anti-aging and products designed to improve the overall health system of the human body and various wellness programs.
The accompanying consolidated financial statements reflect the activities of the Company, AATP LB, AATP HK, WATP, ASL and the variable interest entity (“VIE”), Agape S.E.A. Sdn. Bhd. (“SEA”) (See Note 3), and DSY Wellness.
Details of the Company’s subsidiaries:
| Subsidiary company name | Place and date of incorporation | Particulars of issued capital | Principal activities | Proportional of ownership interest and voting power held | |||||||
| 1. | March 6, 2017 | % | |||||||||
| 2. | June 1, 2017 | % | |||||||||
| 3. | August 8, 2003 | % | |||||||||
| 4. | March 4, 2004 | VIE | |||||||||
| 5. | September 11, 2020 | % | |||||||||
| 6. | November 11, 2021 | % | |||||||||
| F-41 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
1. ORGANIZATION AND BUSINESS BACKGROUND (CONT’D)
Business Overview
Agape ATP Corporation is a company that provides health and wellness products and health solution advisory services to our clients. The Company primarily focus its efforts on attracting customers in Malaysia. Its advisory services center on the “ATP Zeta Health Program”, which is a health program designed to effectively prevent diseases caused by polluted environments, unhealthy dietary intake and unhealthy lifestyles, and promotion of health. The program aims to promote improved health and longevity in our clients through a combination of modern medicine, proper nutrition and advice from skilled nutritionists and/or dieticians.
In
order to strengthen the Company’s supply chain, on May 8, 2020, the Company successfully acquired approximately
Via ASL, the Company offers three series of programs which consist of different services and products: ATP Zeta Health Program, ÉNERGÉTIQUE and BEAUNIQUE.
The ATP Zeta Health Program is a health program designed to promote health and general wellbeing designed to prevent health diseases caused by polluted environments, unhealthy dietary intake and unhealthy lifestyles. The program aims to promote improved health and longevity through a combination of modern health supplements, proper nutrition and advice from skilled dieticians as well as trained members and distributors.
The ÉNERGÉTIQUE series aims to provide a total dermal solution for a healthy skin beginning from the cellular level. The series is comprised of the Energy Mask series, Hyaluronic Acid Serum and Mousse Facial Cleanser.
The BEAUNIQUE product series focuses on the research of our diet’s impact on modifying gene expressions in order to address genetic variations and deliver a nutrigenomic solution for every individual.
The Company deems creating public awareness on wellness and wellbeing lifestyle as essential to enhance the provision of its health solution advisory services; and therefore, incorporated WATP. Upon its establishment, WATP started collaborating with ASL to carry out various wellness programs.
To
further its reach in the Health and Wellness Industry, on November 11, 2021, Agape ATP Corporation (Labuan) formed a joint-venture entity,
DSY Wellness International Sdn. Bhd. (“DSY Wellness”) with an independent third party which Agape ATP Corporation (Labuan)
owns
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for information pursuant to the rules and regulations of the Securities Exchange Commission (“SEC”).
The consolidated financial statements include the financial statements of the Company, its subsidiaries and the VIE over which the Company exercises control and, where applicable, entities for which the Company has a controlling financial interest or is the primary beneficiary. All transactions and balances among the Company, its subsidiaries and the VIE have been eliminated upon consolidation.
| F-42 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT’D)
Principles of consolidation
Subsidiaries are those entities in which the Company, directly or indirectly, controls more than one half of the voting power; or has the power to govern the financial and operating policies, to appoint or remove the majority of the members of the board of directors, or to cast a majority of votes at the meeting of directors.
A VIE is an entity that has either a total equity investment that is insufficient to permit the entity to finance its activities without additional subordinated financial support, or whose equity investors lack the characteristics of a controlling financial interest, such as through voting rights, right to receive the expected residual returns of the entity or obligation to absorb the expected losses of the entity. The variable interest holder, if any, that has a controlling financial interest in a VIE is deemed to be the primary beneficiary and must consolidate the VIE. As of and for the year ended December 31, 2022, SEA, the only VIE of the Company has no significant operations.
Use of estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities as of the date of the consolidated financial statements and the reported amounts of revenues and expenses during the periods presented. Significant accounting estimates reflected in the Company’s consolidated financial statements include allowance for inventories obsolescence, impairment of long-lived assets, allowance for deferred tax assets. Actual results could differ from these estimates.
Cash and cash equivalents
Cash and cash equivalents represent cash on hand, time deposits placed with banks or other financial institutions and all highly liquid investments with an original maturity of three months or less.
Accounts receivable
Accounts receivable are recorded at the invoiced amount less an allowance for any uncollectible accounts and do not bear interest, which are due on credit term. Management reviews the adequacy of the allowance for doubtful accounts on an ongoing basis, using historical collection trends and aging of receivables. Management also periodically evaluates individual customer’s financial condition, credit history, and the current economic conditions to make adjustments in the allowance when it is considered necessary. Account balances are charged off against the allowance after all means of collection have been exhausted and the potential for recovery is considered remote. The Company’s management continues to evaluate the reasonableness of the valuation allowance policy and update it if necessary. As of December 31, 2022 and 2021, allowance of doubtful accounts was recorded.
| F-43 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT’D)
Inventories
Inventories consist of finished goods and are stated at the lower of cost or net realizable value using the first-in first-out method. Management reviews inventory on hand for estimated obsolescence or unmarketable items, as compared to future demand requirements and the shelf life of the various products. Based on the review, the Company records inventory write-downs, when necessary, when costs exceed expected net realizable value.
For
the years ended December 31, 2022 and 2021, the Company recognized $
Prepaid taxes
Prepaid taxes include prepaid income taxes that will either be refunded or utilized to offset future income tax.
Prepayments and deposits
Prepayments
and deposits are mainly cash deposited or advanced to suppliers for future inventory purchases or service providers for future
services. This amount is refundable and bears no interest. For any prepayments and deposits determined by management that such
advances will not be in receipts of inventories, services, or refundable, the Company will recognize an allowance account to reserve
such balances. Management reviews its prepayments and deposits on a regular basis to determine if the allowance is adequate, and
adjusts the allowance when necessary. Delinquent account balances are written-off against allowance for doubtful accounts after
management has determined that the likelihood of collection is not probable. The Company’s management continues to evaluate
the reasonableness of the allowance policy and update it if necessary. For the years ended December 31, 2022 and 2021, the Company
wrote-off allowance for doubtful accounts of $
Property and equipment, net
Property and equipment are stated at cost less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets with no residual value. The estimated useful lives are as follows:
| Useful Life | ||
| Computer and office equipment | ||
| Furniture & fixtures | ||
| Leasehold improvements | ||
| Vehicle |
The cost and related accumulated depreciation of assets sold or otherwise retired are eliminated from the accounts and any gain or loss is included in the consolidated statements of income and comprehensive income. Expenditures for maintenance and repairs are charged to earnings as incurred, while additions, renewals and betterments, which are expected to extend the useful life of assets, are capitalized. The Company also re-evaluates the periods of depreciation to determine whether subsequent events and circumstances warrant revised estimates of useful lives.
| F-44 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT’D)
Intangible assets, net
Intangible assets, net, are stated at cost, less accumulated amortization. Amortization expense is recognized on the straight-line basis over the estimated useful lives of the assets as follows:
| Classification | Useful Life | |
| Computer software |
Impairment for long-lived assets
Long-lived
assets, including property and equipment, and intangible assets with finite lives are reviewed for impairment whenever events or changes
in circumstances (such as a significant adverse change to market conditions that will impact the future use of the assets) indicate that
the carrying value of an asset may not be recoverable. The Company assesses the recoverability of the assets based on the undiscounted
future cash flows the assets are expected to generate and recognize an impairment loss when estimated undiscounted future cash flows
expected to result from the use of the asset plus net proceeds expected from disposition of the asset, if any, are less than the carrying
value of the asset. If an impairment is identified, the Company would reduce the carrying amount of the asset to its estimated fair value
based on a discounted cash flows approach or, when available and appropriate, to comparable market values. As of December 31, 2022 and
2021,
Deferred offering costs
Deferred offering costs represents costs associated with the Company’s current offering which will be netted against the proceeds from the Company’s current offering.
Investment in marketable equity securities
The Company follows the provisions of ASU 2016-01, Financial Instruments – Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities. Investments in marketable equity securities (non-current) are reported at fair value with changes in fair value recognized in the Company’s consolidated statements of operations and comprehensive income (loss) in the caption of “unrealized holding gain loss on marketable securities” in each reporting period.
Investment in non-marketable equity securities
The Company follows the provisions of ASU 2016-01, Financial Instruments – Overall (Subtopic 825-10): Recognition and Measurement of Financial Assets and Financial Liabilities. Due to the Company’s non-marketable equity securities (non-current) does not qualify for the practical expedient to estimate fair value in accordance with ASC 820-10-35-59, the Company has selected to record its investments in non-marketable equity securities (non-current) at cost minus impairment, if any, plus or minus changes resulting from observable price changes in orderly transactions for the identical or a similar investment of the same issue.
At each reporting period, the Company will make a qualitative assessment considering impairment indicators to evaluate whether the investment is impaired. The qualitative assessment indicators include, but are not limited to: (1) A significant deterioration in the earnings performance, credit rating, asset quality, or business prospects of the investee; (ii) A significant adverse change in the regulatory, economic, or technological environment of the investee; (iii) A significant adverse change in the general market condition of either the geographical area or the industry in which the investee operates; (iv) A bona fide offer to purchase, an offer by the investee to sell, or a completed auction process for the same or similar investment for an amount less than the carrying amount of that investment; and (v) Factors that raise significant concerns about the investee’s ability to continue as a going concern, such as negative cash flows from operations, working capital deficiencies, or noncompliance with statutory capital requirements or debt covenants. If the qualitative assessment indicators indicated that the non-marketable equity securities (non-current) is deemed to be impaired, the Company would recognize the impairment loss equal to the difference between the fair value of the investment and its carrying amount.
| F-45 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT’D)
Customer deposits
Customer deposits represent amounts advanced by customers on product orders and unapplied unexpired coupons. Customer deposits are reduced when the related sale is recognized in accordance with the Company’s revenue recognition policy.
Revenue recognition
The Company adopted Accounting Standards Update (“ASU”) 2014-09, Revenue from Contracts with Customers (ASC Topic 606). The core principle underlying the revenue recognition of this ASU allows the Company to recognize - revenue that represents the transfer of goods and services to customers in an amount that reflects the consideration to which the Company expects to be entitled in such exchange. This will require the Company to identify contractual performance obligations and determine whether revenue should be recognized at a point in time or over time, based on when control of goods and services transfers to a customer. The Company’s revenue streams are recognized at a point in time for the Company’s sale of health and wellness products.
The ASU requires the use of a new five-step model to recognize revenue from customer contracts. The five-step model requires that the Company (i) identify the contract with the customer, (ii) identify the performance obligations in the contract, (iii) determine the transaction price, including variable consideration to the extent that it is probable that a significant future reversal will not occur, (iv) allocate the transaction price to the respective performance obligations in the contract, and (v) recognize revenue when (or as) the Company satisfies the performance obligation.
The Company accounts for a contract with a customer when the contract is committed in writing, the rights of the parties, including payment terms, are identified, the contract has commercial substance and consideration is probable of substantially collection.
Sales of Health and Wellness products
- Performance obligations satisfied at a point in time
The Company derives its revenues from sales contracts with its customers with revenues being recognized when control of the health and wellness products are transferred to its customer at the Company’s office or shipment of the goods. The revenue is recorded net of estimated discounts and return allowances. Products are given 60 days for returns or exchanges from the date of purchase. Historically, there were insignificant sales returns.
Under the Company’s network marketing business, the Company issues
product coupons to members and distributors when these customers made purchases above certain thresholds set by the Company. Depending
on the type of product coupons issued, the coupons carry varying values and can be used by the customers for reduction in the transaction
price of product purchases within the coupon validity period. The value of the product coupons issued is recorded as a reduction of the
Company’s revenue account upon issuance; the corresponding amount credited to the customer deposits account. Amounts in customer
deposits will be reversed when the coupons are used. The Company’s coupons have a validity period of between six and twelve months.
If the Company’s customers did not utilize the coupons after the validity period, the Company would recognize the forfeiture of
the originated sales value of the coupons as net revenues. For the years ended December 31, 2022 and 2021, the Company
recognized $
As
of December 31, 2022, the Company had contracts for the sales of health and wellness products amounting to $
Sales of products for the provision of complementary health therapies
Products for the provision of complementary health therapies are predominantly Chinese herbs in different forms, processed or otherwise, for prescriptions for treating non-communicable diseases.
| F-46 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT’D)
Provision of Health and Wellness services
- Performance obligations satisfied at a point in time
The Company carries out its Wellness program, where the Company’s products are bundled with health screening test and a health camp program. The health screening test and the health camp programs are considered as separate performance obligations. The promises to deliver the health screening test report and the attendance at the health camp are separately identifiable, which are evidenced by the fact that the Company provides separate services of delivering the health screening test report and allowing admission of the customers to attend the health camp. The Company derives its revenues from sales contracts with its customers with revenues being recognized when the test reports are completed and delivered to its customers during the consultation section in person.
The
Company also separately derives its revenues from sales contracts with its customers with revenues being recognized when the health
camp program is completed in the final day of the health camp. For the years ended December 31, 2022 and 2021, revenues from
providing health and wellness services are $
Disaggregated information of revenues by products and services are as follows:
| For the years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Survivor Select | $ | $ | ||||||
| Energized Mineral Concentrate | ||||||||
| Ionized Cal-Mag | ||||||||
| Omega Blend | ||||||||
| BetaMaxx | ||||||||
| Vege Fruit Fiber | ||||||||
| Iron | ||||||||
| Young Formula | ||||||||
| Organic Youth Care Cleansing Bar | ||||||||
| ATPR Mito+ | ||||||||
| Lipomask | ||||||||
| Energetique | ||||||||
| Trim+ | ||||||||
| Others – Products for the provision of complementary health therapies | ||||||||
| Others | ||||||||
| Total revenues – products | ||||||||
| Health and Wellness services | ||||||||
| Total revenues – products and services | $ | $ | ||||||
| F-47 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT’D)
Cost of revenue
Cost
of revenue comprised freight-in, the purchase cost of manufactured goods for sale to customers and
products for the provision of complementary health therapies. Cost of revenue amounted to $
Shipping and handling
Shipping
and handling charges amounted to $
Advertising costs
Advertising
costs amounted to $
Commission expenses
Commission
expenses are the Company’s most significant expenses. As with all companies in the network marketing industry, the Company’s
sales channel is external to the Company. The Company’s “external sales force” is stratified into two levels based
on priority recruitment. First, there are sales distributors. Second, all members recruited by a sales distributor, directly or indirectly,
are referred to as “sales network members”. The Company pays commission to every sales distributor based on purchases made
by its sales network members which includes the independent direct sales members. Top performing distributors with their own physical
stores may also become stockists of the Company, whereby they enjoy benefits such as maintaining a certain amount of the Company’s
inventory on their store premises. The stockists shall account to the Company for all products sales from their store premises as monitored
through the Company’s centralized stock tracking system. The Company pays a separate commission to stockists based on revenue generated
from the stockists’ physical stores. Commission expenses amounted to $
Defined contribution plan
The
full-time employees of the Company are entitled to the government mandated defined contribution plan. The Company is required to accrue
and pay for these benefits based on certain percentages of the employees’ respective salaries, subject to certain ceilings, in
accordance with the relevant government regulations, and make cash contributions to the government mandated defined contribution plan.
Total expenses for the plans were $
The related contribution plans include:
| - | Social
Security Organization (“SOSCO”) – | |
| - | Employees
Provident Fund (“EPF”) –based on employee’s monthly salary, | |
| - | Employment
Insurance System (“EIS”) – | |
| - | Human
Resource Development Fund (“HRDF”) – |
| F-48 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT’D)
Income taxes
The Company accounts for income taxes in accordance with U.S. GAAP for income taxes. The charge for taxation is based on the results for the fiscal year as adjusted for items, which are non-assessable or disallowed. It is calculated using tax rates that have been enacted or substantively enacted by the balance sheet date.
Deferred taxes is accounted for using the asset and liability method in respect of temporary differences arising from differences between the carrying amount of assets and liabilities in the consolidated financial statements and the corresponding tax basis used in the computation of assessable tax profit. In principle, deferred tax liabilities are recognized for all taxable temporary differences. Deferred tax assets are recognized to the extent that it is probable that taxable profit will be available against which deductible temporary differences can be utilized. Deferred tax is calculated using tax rates that are expected to apply to the period when the asset is realized or the liability is settled. Deferred tax is charged or credited in the income statement, except when it is related to items credited or charged directly to equity, in which case the deferred tax is also dealt with in equity. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Current income taxes are provided for in accordance with the laws of the relevant taxing authorities.
An
uncertain tax position is recognized as a benefit only if it is “more likely than not” that the tax position would be sustained
in a tax examination, with a tax examination being presumed to occur. The amount recognized is the largest amount of tax benefit that
is
The Company conducts much of its business activities in Hong Kong and Malaysia and is subject to tax in each of their jurisdictions. As a result of its business activities, the Company will file separate tax returns that are subject to examination by the foreign tax authorities.
Comprehensive income (loss)
Comprehensive income (loss) consists of two components, net income (loss) and other comprehensive income (loss). Net income (loss) refers to revenue, expenses, gains and losses that under GAAP are recorded as an element of stockholders’ equity but are excluded from net income. Other comprehensive income (loss) consists of a foreign currency translation adjustment resulting from the Company not using the U.S. dollar as its functional currencies.
Non-controlling interest
| F-49 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT’D)
The Company computes earnings (loss) per share (“EPS”) in accordance with ASC 260, “Earnings per Share”. ASC 260 requires companies to present basic and diluted EPS. Basic EPS is measured as net income divided by the weighted average shares of common stock outstanding for the period. Diluted EPS presents the dilutive effect on a per share basis of the potential shares (e.g., convertible securities, options and warrants) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS. For the years ended December 31, 2022 and 2021, there were dilutive shares.
Foreign currencies translation and transaction
Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency using the applicable exchange rates at the balance sheet dates. The resulting exchange differences are recorded in the consolidated statements of operations and comprehensive income (loss).
The reporting currency of the Company is United States Dollars (“US$”) and the accompanying financial statements have been expressed in US$. The Company’s subsidiary in Labuan maintains its books and record in United States Dollars (“US$”) albeit its functional currency being the primary currency of the economic environment in which the entity operates, which is the Malaysian Ringgit (“MYR” or “RM”). The Company’s subsidiary in Hong Kong maintains its books and record in Hong Kong Dollars (“HK$”), similar to its functional currency. The Company’s subsidiary and VIE in Malaysia conducts its businesses and maintains its books and record in the local currency, Malaysian Ringgit (“MYR” or “RM”), as its functional currency.
In general, for consolidation purposes, assets and liabilities of its subsidiaries whose functional currency is not US$ are translated into US$, in accordance with ASC Topic 830-30, “Translation of Financial Statement”, using the exchange rate on the balance sheet date. Revenues and expenses are translated at average rates prevailing during the period. The gains and losses resulting from translation of financial statements of foreign subsidiary are recorded as a separate component of accumulated other comprehensive income within the statements of stockholders’ equity. Cash flows are also translated at average translation rates for the periods, therefore, amounts reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the consolidated balance sheets.
Translation of foreign currencies into US$1 have been made at the following exchange rates for the respective periods:
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Period-end MYR : US$1 exchange rate | ||||||||
| Period-end HKD : US$1 exchange rate | ||||||||
| For the years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Period-average MYR : US$1 exchange rate | ||||||||
| Period-average HKD : US$1 exchange rate | ||||||||
| F-50 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT’D)
Related parties
Parties, which can be a corporation or individual, are considered to be related if the Company has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making financial and operating decisions. Companies are also considered to be related if they are subject to common control or common significant influence.
Fair value of financial instruments
The accounting standard regarding fair value of financial instruments and related fair value measurements defines financial instruments and requires disclosure of the fair value of financial instruments held by the Company.
The accounting standards define fair value, establish a three-level valuation hierarchy for disclosures of fair value measurement and enhance disclosure requirements for fair value measures. The three levels are defined as follow:
| ● | Level 1 inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. | |
| ● | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the assets or liability, either directly or indirectly, for substantially the full term of the financial instruments. | |
| ● | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value. |
Financial instruments included in current assets and current liabilities are reported in the consolidated balance sheets at face value or cost, which approximate fair value because of the short period of time between the origination of such instruments and their expected realization and their current market rates of interest.
Leases
The Company adopted ASU 2016-02, “Leases” (Topic 842), and elected the practical expedients that does not require the Company to reassess: (1) whether any expired or existing contracts are, or contain, leases, (2) lease classification for any expired or existing leases and (3) initial direct costs for any expired or existing leases. For lease terms of twelve months or fewer, a lessee is permitted to make an accounting policy election not to recognize lease assets and liabilities. The Company also adopts the practical expedient that allows lessees to treat the lease and non-lease components of a lease as a single lease component. Some of the Company’s leases include one or more options to renew, which is typically at the Company’s sole discretion. The Company regularly evaluates the renewal options, and, when it is reasonably certain of exercise, it will include the renewal period in its lease term. New lease modifications result in re-measurement of the right of use (“ROU”) assets and lease liabilities. Operating ROU assets and lease liabilities are recognized at the commencement date, based on the present value of lease payments over the lease term. Since the implicit rate for the Company’s leases is not readily determinable, the Company use its incremental borrowing rate based on the information available at the commencement date in determining the present value of lease payments. The incremental borrowing rate is the rate of interest that the Company would have to pay to borrow, on a collateralized basis, an amount equal to the lease payments, in a similar economic environment and over a similar term.
| F-51 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT’D)
Lease terms used to calculate the present value of lease payments generally do not include any options to extend, renew, or terminate the lease, as the Company does not have reasonable certainty at lease inception that these options will be exercised. The Company generally considers the economic life of its operating lease ROU assets to be comparable to the useful life of similar owned assets. The Company has elected the short-term lease exception, therefore operating lease ROU assets and liabilities do not include leases with a lease term of twelve months or less. Its leases generally do not provide a residual guarantee. The operating lease ROU asset also excludes lease incentives. Lease expense is recognized on a straight-line basis over the lease term.
The Company reviews the impairment of its ROU assets consistent with the approach applied for its other long-lived assets. The Company reviews the recoverability of its long-lived assets when events or changes in circumstances occur that indicate that the carrying value of the asset may not be recoverable. The assessment of possible impairment is based on its ability to recover the carrying value of the asset from the expected undiscounted future pre-tax cash flows of the related operations. The Company has elected to include the carrying amount of operating lease liabilities in any tested asset group and includes the associated operating lease payments in the undiscounted future pre-tax cash flows.
Recent accounting pronouncements
The Company has reviewed all recently issued, but not yet effective, considers the applicability and impact of all accounting standards updates (“ASUs”). Management periodically reviews new accounting standards that are issued.
In November 2019, the FASB issued ASU No. 2019-10, which to update the effective date of ASU No. 2016-13 for private companies, not-for-profit organizations and certain smaller reporting companies applying for credit losses, leases, and hedging standard. The new effective date for these preparers is for fiscal years beginning after December 15, 2022. ASU 2019-05 is effective for the Company for annual and interim reporting periods beginning January 1, 2023 as the Company is qualified as a smaller reporting company. The Company is currently evaluating the impact ASUs 2016-13 and 2019-05 may have on its consolidated financial statements.
Except for the above-mentioned pronouncements, there are no new recent issued accounting standards that will have a material impact on the consolidated financial position, statements of operations and cash flows.
3. VARIABLE INTEREST ENTITY (“VIE”)
SEA
is a trading company incorporated on March 4, 2004, under the laws of Malaysia. SEA provided majority of ASL’s purchases. Its equity
at risk was insufficient to finance its activities and
| a. | The power to direct the activities of the VIE that most significantly impact the VIE’s economic performance; and | |
| b. | The obligation to absorb losses of the VIE that could potentially be significant to the VIE or the right to receive benefits from the VIE that could potentially be significant to the VIE. |
Accordingly, the accounts of SEA is consolidated in the accompanying financial statements.
| F-52 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
3. VARIABLE INTEREST ENTITY (“VIE”) (CONT’D)
The carrying amount of the VIE’s assets and liabilities were as follows:
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Current assets | $ | $ | ||||||
| Current liabilities | ( | ) | ( | ) | ||||
| Net deficit | $ | ( | ) | $ | ( | ) | ||
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Current assets: | ||||||||
| Cash | $ | $ | ||||||
| Prepaid taxes | ||||||||
| Total current assets | $ | $ | ||||||
| Current liabilities: | ||||||||
| Accounts payable – intercompany | $ | $ | ||||||
| Other payables and accrued liabilities | ||||||||
| Total current liabilities | $ | $ | ||||||
| Net deficit | $ | ( | ) | $ | ( | ) | ||
The summarized operating results of the VIE’s are as follows:
| For the years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Operating revenues | $ | $ | ||||||
| Gross profit | $ | $ | ||||||
| Loss from operations | $ | ( | ) | $ | ( | ) | ||
| Net loss | $ | ( | ) | $ | ( | ) | ||
4. CASH AND CASH EQUIVALENTS
As
of December 31, 2022 and 2021 the Company had $
| F-53 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
5. ACCOUNTS RECEIVABLE
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Accounts receivable | $ | $ | ||||||
| Allowance for doubtful accounts | ||||||||
| Total | $ | $ | ||||||
6. INVENTORIES
Inventories consist of the following:
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Finished goods | $ | $ | ||||||
For
the years ended December 31, 2022 and 2021, the Company recognized $
7. PREPAYMENTS AND DEPOSITS
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Receivables from sales distributors | $ | $ | ||||||
| Deposits to suppliers | ||||||||
| Subtotal | ||||||||
| Less: Provision for doubtful accounts | ( | ) | ||||||
| Total | $ | $ | ||||||
Movements of allowance for doubtful accounts are as follows:
| For the years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Beginning balance | $ | $ | ||||||
| Addition | ||||||||
| Write off | ( | ) | ||||||
| Exchange rate effect | ( | ) | ( | ) | ||||
| Ending balance | $ | $ | ||||||
| F-54 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
8. PROPERTY AND EQUIPMENT, NET
Property and equipment, net consist of the following:
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Computer and office equipment | $ | $ | ||||||
| Furniture & fixtures | ||||||||
| Leasehold improvements | ||||||||
| Vehicle | ||||||||
| Subtotal | ||||||||
| Less: accumulated depreciation | ( | ) | ( | ) | ||||
| Total | $ | $ | ||||||
Depreciation
expense for the years ended December 31, 2022 and 2021 amounted to $
9. INTANGIBLE ASSETS, NET
Intangible assets, net, consist of the following:
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Computer software | $ | $ | ||||||
| Less: accumulated amortization | ( | ) | ( | ) | ||||
| Total | $ | $ | ||||||
Amortization
expense for the years ended December 31, 2022 and 2021 amounted to $
10. INVESTMENT IN MARKETABLE SECURITIES
| (i) | On
May 17, 2018, the Company purchased shares of common stock in Greenpro Capital Corp. for $ | |
| (ii) | On
July 30, 2018, the Company disposed shares of common stock in Greenpro Capital Corp. for $ | |
| (iii) | On
October 16, 2018, the Company purchased shares of common stock in Greenpro Capital Corp. for $ | |
| (iv) | On
July 19, 2022, Greenpro Capital Corp. filed a certificate of change with the Secretary of State of Nevada to effect a reverse split
of | |
| (v) | On
November 3, 2020, the Company received dividend of shares of common stock in DSwiss, Inc. for $ | |
| (vi) | On
December 9, 2020, the Company received dividend of shares of common stock in DSwiss, Inc. for $ | |
| (vii) | On
September 27, 2021, the Company received dividend of shares of common stock in SEATech Ventures Corp. for $ | |
| (viii) | On
April 3, 2019, the Company purchased a |
| F-55 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
10. INVESTMENT IN MARKETABLE SECURITIES (CONT’D)
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Cost of investment | $ | $ | ||||||
| Transfer from non-marketable security | ||||||||
| Dividend income from Greenpro Capital Corp. | ||||||||
| Unrealized holding loss | ( | ) | ( | ) | ||||
| Exchange rate effect | ( | ) | ( | ) | ||||
| Investment in marketable securities | $ | $ | ||||||
11. INVESTMENT IN NON-MARKETABLE SECURITIES
On
April 3, 2019, the Company purchased a
| As of December 31, | ||||||||
| Phoenix Plus Corporation | 2022 | 2021 | ||||||
| Cost of investment | $ | $ | ||||||
| Less: Transfer to investment in marketable securities | ( | ) | ||||||
| Investment in non-marketable securities | ||||||||
12. CUSTOMER DEPOSITS
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Customer deposits | $ | $ | ||||||
| Unexpired product coupons | ||||||||
| Total | $ | $ | ||||||
Customer deposits represent amounts advanced by customers on product orders and unexpired product coupons issued to the Company’s members and distributors of its network marketing business.
13. OTHER PAYABLES AND ACCRUED LIABILITIES
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Professional fees | $ | $ | ||||||
| Promotion expenses | ||||||||
| Payroll | ||||||||
| Amounts held in eWallets | ||||||||
| Tax penalty | ||||||||
| Others | ||||||||
| Total | $ | $ | ||||||
The
Company requires all members and distributors of its network marketing business to maintain an electronic wallet (eWallet) account with
the Company.
| F-56 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
14. RELATED PARTY BALANCES AND TRANSACTIONS
Related party balances
Amount due from related parties
| Name of Related | As of December 31, | |||||||||||
| Party | Relationship | Nature | 2022 | 2021 | ||||||||
| TH3 Technology Sdn Bhd (“TH3”) | Prepayment of IT expenses | $ | $ | |||||||||
| DSY Beauty Sdn Bhd (“DSY Beauty”) | Deposits for products purchases | |||||||||||
| Agape ATP (Asia) Limited (“AATP Asia”) | ||||||||||||
| Hostastay Sdn. Bhd. “Hostastay” | ||||||||||||
| Total | $ | $ | ||||||||||
Accounts payable – related parties
| Name of Related | As of December 31, | |||||||||||
| Party | Relationship | Nature | 2022 | 2021 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | $ | $ | ||||||||||
| DSY Beauty Sdn Bhd (“DSY Beauty”) | ||||||||||||
| Total | $ | $ | ||||||||||
| F-57 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
14. RELATED PARTY BALANCES AND TRANSACTIONS (CONT’D)
Related party balances
Other payable - related parties
| Name of Related | As of December 31, | |||||||||||
| Party | Relationship | Nature | 2022 | 2021 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | $ | $ | ||||||||||
| DSY Beauty Sdn Bhd (“DSY Beauty”) | ||||||||||||
| Mr. How Kok Choong | ||||||||||||
| Total | $ | $ | ||||||||||
Related party transactions
Purchases
| Name of Related | For the years ended December 31, | |||||||||||
| Party | Relationship | Nature | 2022 | 2021 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) | $ | $ | ||||||||||
| DSY Beauty Sdn Bhd (“DSY Beauty”) | ||||||||||||
| DSY Wellness & Longevity Center Sdn Bhd (“DSYWLC”) | ||||||||||||
| Total | $ | $ | ||||||||||
| F-58 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
14. RELATED PARTY BALANCES AND TRANSACTIONS (CONT’D)
Related party transactions
Other income
| Name of Related | For the years ended December 31, | |||||||||||
| Party | Relationship | Nature | 2022 | 2021 | ||||||||
| Hostastay Sdn. Bhd. | $ | $ | ||||||||||
| Total | $ | $ | ||||||||||
Other purchases
| Name of Related | For the years ended December 31, | |||||||||||
| Party | Relationship | Nature | 2022 | 2021 | ||||||||
| CTA Nutriceuticals (Asia) Sdn Bhd (“CTA”) |
| $ | $ | |||||||||
| DSY Beauty Sdn Bhd (“DSY Beauty”) | ||||||||||||
| DSY Wellness & Longevity Center Sdn Bhd (“DSYWLC”) | ||||||||||||
| Total | $ | $ | ||||||||||
| F-59 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
14. RELATED PARTY BALANCES AND TRANSACTIONS (CONT’D)
Related party transactions
Commission expense
| Name of Related | For the years ended December 31, | |||||||||||
| Party | Relationship | Nature | 2022 | 2021 | ||||||||
| Mr. How Kok Choong | $ | $ | ||||||||||
| Total | $ | $ | ||||||||||
Other expenses
| Name of Related | For the years ended December 31, | |||||||||||
| Party | Relationship | Nature | 2022 | 2021 | ||||||||
| TH3 Technology Sdn Bhd (“TH3”) | $ | $ | ||||||||||
| Redboy Picture Sdn Bhd (“Redboy”) | ||||||||||||
| DSY Wellness & Longevity Center Sdn Bhd (“DSYWLC”) | ||||||||||||
| Total | $ | $ | ||||||||||
| F-60 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
15. STOCKHOLDERS’ EQUITY
Preferred stock
As of December 31, 2022 and 2021, there were preferred stocks authorized but were issued and outstanding.
Common stock
As of December 31, 2022 and 2021, there were common stocks authorized, and shares issued and outstanding, respectively.
In December 2021, there were share forfeiture agreements (the “Share Forfeiture Agreements”) between the Company and (i) HKC Talent Limited; (ii) various shareholders of the Company (the “Forfeiting Shareholders”), pursuant to which: (i) HKC Talent Limited had agreed to forfeiture of shares of common stock of the Company, and (ii) the Forfeiting Shareholders had agreed to forfeiture, in aggregate, shares of common stock of the Company. Included in (ii) is shares forfeited from HKC Holdings Sdn. Bhd, a company in which Mr. How Kok Choong, the CEO and director of the Company, is a shareholder. As a result, the outstanding shares was reduced by shares of common stock.
A share forfeiture agreement (the “Share Forfeiture Agreement”) dated January 20, 2022, between the Company and Mr. How Kok Choong, the CEO and director of the Company, pursuant to which Mr. How Kok Choong agreed to forfeit shares of common stock of the Company. As a result, the outstanding shares was reduced by shares of common stock.
There were stock options, warrants or other potentially dilutive securities outstanding as of December 31, 2022 and 2021.
16. NON-CONTROLLING INTEREST
The Company’s non-controlling interest consists of the following:
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| DSY Wellness: | ||||||||
| Paid-in capital | $ | $ | ||||||
| Accumulated surplus (deficit) | ( | ) | ||||||
| Accumulated other comprehensive income | ||||||||
| ( | ) | |||||||
| ASL | ||||||||
| Total | $ | $ | ( | ) | ||||
| F-61 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
17. INCOME TAXES
The United States and foreign components of loss before income taxes were comprised of the following:
| For the years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Tax jurisdictions from: | ||||||||
| Local – United States | $ | ( | ) | $ | ( | ) | ||
| Foreign – Malaysia | ( | ) | ( | ) | ||||
| Foreign – Hong Kong | ( | ) | ( | ) | ||||
| Loss before income tax | $ | ( | ) | $ | ( | ) | ||
The benefit of (provision for) income taxes consisted of the following:
| For the years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| Current: | ||||||||
| - Local | $ | $ | ( | ) | ||||
| - Foreign | ( | ) | ( | ) | ||||
| Deferred: | ||||||||
| - Local | ||||||||
| - Foreign | ( | ) | ||||||
| Benefit of (Provision for) income taxes | $ | $ | ( | ) | ||||
The effective tax rate in the periods presented is the result of the mix of income earned in various tax jurisdictions that apply a broad range of income tax rates. The Company and its subsidiary that operate in various countries: United States, Malaysia (including Labuan) and Hong Kong that are subject to taxes in the jurisdictions in which they operate, are as follows:
United States of America
Agape
ATP Corporation was incorporated in the State of Nevada and is subject to the tax laws of the United States of America with a corporate
tax rate of
For the year ended December 31, 2022 and 2021, the Company’s foreign subsidiaries did not generate any income that were subject to Subpart F tax and GILTI tax.
| F-62 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
17. INCOME TAXES (CONT’D)
As
of December 31, 2022 and 2021, the operations in the United States of America incurred approximately $
For
the year ended December 31, 2021, the Company re-visited its fiscal 2020 U.S. income taxes and determined its stock dividend from Greenpro
Capital Corp as a result of its Spin-off of DSwiss Inc.’s shares in 2020 were subject to Subpart F and GILTI taxes. As a result,
the Company incurred additional income taxes expenses of $
Malaysia
Changes
to the Labuan Business Activity Tax Act (LBATA) 1990 which was gazetted and came into operation on January 1, 2019 mandate companies
incorporated in Labuan to satisfy the “substantial activity requirements” to qualify for the preferential tax rate of
Agape
Superior Living Sdn Bhd, Agape S.E.A Sdn Bhd and Wellness ATP International Holdings Sdn Bhd. are governed by the income taxes laws of
Malaysia and the income taxes provision in respect of operations in Malaysia is calculated at the applicable tax rates on the taxable
income for the periods based on existing legislation, interpretations and practices in respect thereof. Under the Income Tax Act of Malaysia,
enterprises that incorporated in Malaysia are usually subject to a unified 24% enterprise income taxes rate while preferential tax rates,
tax holidays and even tax exemption may be granted on case-by-case basis.
As
of December 31, 2022 and 2021, the operations in the Malaysia incurred approximately $
Hong Kong
Agape
ATP International Holding (HK) Limited is subject to Hong Kong Profits Tax, which is charged at the statutory income rate of
| F-63 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
17. INCOME TAXES (CONT’D)
The following table reconciles the local (United States) statutory rates to the Company’s effective tax rate for the periods indicated below:
| For the years ended December 31, | ||||||||
| 2022 | 2021 | |||||||
| U.S. statutory rate | % | % | ||||||
| Valuation allowance | ( | )% | ( | )% | ||||
| Differential tax rate in Malaysia | % | % | ||||||
| Permanent difference | %(1) | ( | )%(2) | |||||
| Effective tax rate | % | ( | )% | |||||
| (1) | The amount comprised: | ||
| - | |||
| (2) | The amount comprised: | ||
| - | |||
| - | |||
The following table sets forth the significant components of the aggregate deferred tax assets of the Company as of:
| As of December 31, | ||||||||
| 2022 | 2021 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carry forwards in U.S. | $ | $ | ||||||
| Net operating loss carry forwards in Malaysia | ||||||||
| Less: valuation allowance | ( | ) | ( | ) | ||||
| Deferred tax liabilities: | ||||||||
| Depreciation | ( | ) | ||||||
| Deferred tax liabilities, net | $ | $ | ( | ) | ||||
Uncertain tax positions
The Company evaluates each uncertain tax position (including the potential application of interest and penalties) based on the technical merits, and measure the unrecognized benefits associated with the tax positions. As of December 31, 2022 and 2021, the Company did not have any significant unrecognized uncertain tax positions. The Company did not incur interest and penalties tax for the years ended December 31, 2022 and 2021.
| F-64 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
18. CONCENTRATIONS OF RISKS
(a) Major customers
For
the years ended December 31, 2022 and 2021, no customer accounted for
As
of December 31, 2022, five individual customers accounted for approximately
(b) Major vendors
For
the year ended December 31, 2022, three vendors accounted for approximately
Via a dividend distribution, the Company acquired shares of common stock of DSwiss, Inc., represents approximately
As
of December 31, 2022, three vendors accounted for approximately
(c) Commission Expenses to Sales Distributors and Stockists
For
the year ended December 31, 2022, one sales distributor accounted for approximately
(d) Credit risk
Financial
instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash. As of December
31, 2022 and 2021, $
Financial instruments that are potentially subject to credit risk consist principally of accounts receivable. The Company believes the concentration of credit risk in its account receivable is substantially mitigated by its ongoing credit evaluation process and relatively short collection terms. The Company does not generally require collateral from customers. The Company evaluates the need for an allowance for doubtful accounts based upon factors surrounding the credit risk of specific customers, historical trends and other information. Historically, the Company did not have any bad debt on its account receivable.
(e) Exchange rate risk
The Company cannot guarantee that the current exchange rate will remain steady; therefore, there is a possibility that the Company could post the same amount of profit for two comparable periods and because of the fluctuating exchange rate actually post higher or lower profit depending on exchange rate of RM and HK$ converted to US$ on that date. The exchange rate could fluctuate depending on changes in political and economic environments without notice.
| F-65 |
AGAPE ATP CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Currency expressed in United States Dollars (“US$”), except for number of shares)
19. COMMITMENTS AND CONTINGENCIES
Lease commitments
On
April 1, 2020, the Company adopted ASC 842 for ASL’s office space lease and sales and training center as the lease commencement
date upon the acquisition of ASL. The Company recognized lease liabilities of approximately $
On
May 31, 2021, the Company entered into two separate two-year leases extension with the modified lease expiring
On October 1, 2021, the Company entered into a
lease for an apartment to serve as staff accommodation. The Company recognized lease liabilities of approximately $
On November 1, 2021, the Company entered into a lease for a branch
office and operating centre. The Company recognized lease liabilities of approximately $
As
of December 31, 2022, the weighted remaining term of the lease is approximately
The five-year maturity of the Company’s operating lease liabilities is as follow:
| Twelve Months Ending December 31, | Operating lease liabilities | |||
| 2023 | $ | |||
| Thereafter | ||||
| Total lease payments | ||||
| Less: interest | ( | ) | ||
| Present value of lease liabilities | $ | |||
The
Company also leased one office and operation center, and one shophouse with an expiring term of twelve months or less, which were classified
as operation leases. Since the lease terms for these leases were twelve months or less, a lessee is permitted to elect not to recognize
lease assets and liabilities. The Company has elected not to recognize lease assets and liabilities on these leases. As of December 31,
2022, the Company’s commitment for minimum lease payment under these operating leases within the next twelve months were $
Rent
expense for the years ended December 31, 2022 and 2021 was $
Contingencies
Legal
From time to time, the Company is party to certain legal proceedings, as well as certain asserted and un-asserted claims. Amounts accrued, as well as the total amount of reasonably possible losses with respect to such matters, individually and in the aggregate, are not deemed to be material to the consolidated financial statements.
COVID-19
Malaysia, where the operations of the Company predominantly reside, officially transitioned to the endemic phase of COVID-19 effective April 1, 2022. Restrictions on businesses and people are minimal. Meanwhile, the government continues to encourage inoculation for those between the ages of 5 and 11 years and its adolescent group which comprised those between the ages 12 and 17. Adults who have been fully vaccinated, i.e. received two doses of the COVID-19 vaccine are encouraged to take booster shots.
Substantially all of our revenues are concentrated in Malaysia. Consequently, our results of operations will likely be adversely, and may be materially, affected, to the extent that the COVID-19 or any other epidemic harms the Malaysia and global economy in general. Any potential impact to our results will depend on, to a large extent, future developments and new information that may emerge regarding the duration and severity of the COVID-19 and the actions taken by government authorities and other entities to contain the COVID-19 or treat its impact, almost all of which are beyond our control. Potential impacts include, but are not limited to, the following:
| ● | temporary closure of offices, travel restrictions, disruption or suspension of supplies, our customers may be negatively impacted financially resulting in which the demand for our products may be adversely affected; | |
| ● | we may have to provide significant sales incentives to our customers during the outbreak, which may in turn materially adversely affect our financial condition and operating results; and | |
| ● | any disruption of our supply chain, logistics providers or customers could adversely impact our business and results of operations, including causing us or our suppliers to cease manufacturing for a period of time or materially delay delivery to our customers, which may also lead to loss of our customers. |
Because of the uncertainty surrounding the COVID-19 outbreak, the financial impact related to the outbreak of and response to the COVID-19 cannot be reasonably estimated at this time. There is no guarantee that the Company’s total revenues will grow or remain at similar levels year over year in 2023 and beyond.
20. SUBSEQUENT EVENTS
The Company has evaluated subsequent events through the date of issuance of this consolidated financial statements, and does not identify any events with material financial impact on the Company’s consolidated financial statements.
| F-66 |
AGAPE ATP CORPORATION

1,100,000 Shares of Common Stock
PROSPECTUS
Network 1 Financial Securities, Inc.
You should rely only on the information contained in this prospectus. No dealer, salesperson or other person is authorized to give information that is not contained in this prospectus. This prospectus is not an offer to sell nor is it seeking an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. The information contained in this prospectus is correct only as of the date of this prospectus, regardless of the time of the delivery of this prospectus or the sale of these securities.
Until , 2023, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriter with respect to their unsold subscriptions.
The date of this prospectus is , 2023
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED ________, 2023
PRELIMINARY PROSPECTUS
Agape ATP Corporation

30,169,516 Shares of Common Stock
This prospectus relates to the resale of 30,169,516 shares of our common stock by the selling stockholders named in this prospectus. The selling stockholders will offer and sell their shares of common stock being offered under this prospectus at $6.50 per share on the OTC Markets – Pink Sheets under the symbol “AATP” or in private transactions for the duration of this offering or until the shares are listed on a national securities exchange at which time the shares may be sold at prevailing market prices or privately negotiated prices or in transactions that are not in the public market. We have applied to list our common stock on the NASDAQ Capital Market (“NASDAQ”) under the symbol “ATPC”. No assurance can be given that our application will be approved. The closing of this offering is contingent upon the successful listing of our common stock on the Nasdaq Capital Market.
We are a reporting company under Section 15(d) of the Securities Exchange Act of 1934, as amended. Our common stock is currently quoted on the OTC Markets – Pink Sheets, operated by OTC Markets Group, under the symbol “AATP.” The last reported sale price of our common stock on the OTC Markets – Pink Sheets on May 11, 2023 was $6.00 per share. We urge prospective purchasers of our Common Stock to obtain current information about the market prices of our Common Stock. There is a limited public trading market for our common stock.
Investing in our securities involves risks. You should carefully consider the risk factors beginning on page 8 of this prospectus and set forth in the documents incorporated by reference herein before making any decision to invest in our securities.
Neither the U.S. Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this registration statement. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2023
| SS-1 |
THE OFFERING
| Common stock offered by us: | 0 shares | ||
| Common Stock offered by the selling stockholders | 30,169,516 shares | ||
| Common stock to be outstanding prior to this offering: | 75,452,012 shares of common stock | ||
| Common stock to be outstanding after the offering: | 76,552,012 shares (1) | ||
| Use of proceeds: | We will not receive any of the proceeds from the sale of the common stock by the selling stockholders named in this prospectus. |
(1) Assumes the issuance by us of our common stock pursuant to the public offering prospectus filed contemporaneously herewith, no exercise of the underwriters’ over-allotment option and all of our common stock to be sold by the selling stockholders pursuant to the Resale Prospectus filed contemporaneously herewith are sold.
| SS-2 |
USE OF PROCEEDS
We will not receive any of the proceeds from the sale of the shares of common stock by the selling stockholders.
| SS-3 |
SELLING STOCKHOLDERS
The following table sets forth the names of the selling stockholders, the number of shares of common stock owned by each selling stockholder immediately prior to the date of this prospectus and the number of shares to be offered by the selling stockholder pursuant to this prospectus. The table also provides information regarding the beneficial ownership of our common stock by the Selling Stockholder as adjusted to reflect the assumed sale of all of the shares offered under this prospectus.
Percentage of beneficial ownership before this offering is based on 75,452,012 shares of our common stock outstanding as June 30, 2023. Beneficial ownership is based on information furnished by the selling stockholders. Unless otherwise indicated and subject to community property laws where applicable, the selling stockholder named in the following table has, to our knowledge, sole voting and investment power with respect to the shares beneficially owned by him.
None of the selling stockholders has had any position, office or other material relationship within past three years with the Company. None of the selling stockholders is a broker dealer or an affiliate of a broker dealer. None of the selling stockholders has an agreement or understanding to distribute any of the shares being registered. Each selling stockholder may offer for sale from time to time any or all of the shares, subject to the lock up agreements described in the “Selling Stockholder Plan of Distribution.” The table below assumes that the selling stockholders will sell all of the shares offered for sale hereby. A selling stockholder is under no obligation to sell any shares pursuant to this prospectus.
| Name
of Selling Stockholder |
Share
Beneficially Owned Prior to Offering |
Maximum
Number of Shares to be Sold |
Number
of Shares Owned after Offering |
Percentage
Ownership After Offering (%) |
||||||||||||
| HKC TALENT LIMITED | 8,245,000 | 3,300,000 | 4,945,000 | 6.42 | ||||||||||||
| SHIUH CHOENG OOI | 490,000 | 490,000 | - | 0.00 | ||||||||||||
| KWAI FAH MOOK | 451,000 | 451,000 | - | 0.00 | ||||||||||||
| LEE MEE TAN | 441,000 | 441,000 | - | 0.00 | ||||||||||||
| YEE YEN CHAI | 400,000 | 400,000 | - | 0.00 | ||||||||||||
| SHEUE YUEN BEH | 375,000 | 375,000 | - | 0.00 | ||||||||||||
| BEE KIM SER | 350,000 | 350,000 | - | 0.00 | ||||||||||||
| FOOK SENG YONG | 350,000 | 350,500 | - | 0.00 | ||||||||||||
| LAY YEN KENG | 342,500 | 342,500 | - | 0.00 | ||||||||||||
| WENG ONN CHOO | 330,000 | 330,000 | - | 0.00 | ||||||||||||
| SIAW WEI TEH | 317,500 | 317,500 | - | 0.00 | ||||||||||||
| KOCK LEONG SIOW | 317,000 | 317,000 | - | 0.00 | ||||||||||||
| LAI KHUANG TANG | 311,000 | 311,000 | - | 0.00 | ||||||||||||
| SIEW GIM LAW | 310,000 | 310,000 | - | 0.00 | ||||||||||||
| POI CHIN WONG | 310,000 | 310,000 | - | 0.00 | ||||||||||||
| YIT KEONG CHIN | 310,000 | 310,000 | - | 0.00 | ||||||||||||
| YEW SENG TAN | 300,000 | 300,000 | - | 0.00 | ||||||||||||
| YONG LEONG KENG | 285,000 | 285,000 | - | 0.00 | ||||||||||||
| MEI MEI KOR | 280,000 | 280,000 | - | 0.00 | ||||||||||||
| WAI PENG SEW | 265,000 | 265,000 | - | 0.00 | ||||||||||||
| MUN FONG CHEAH | 250,000 | 250,000 | - | 0.00 | ||||||||||||
| WEI TONG CHEE | 250,000 | 250,000 | - | 0.00 | ||||||||||||
| PEK FEN LEONG | 245,000 | 245,000 | - | 0.00 | ||||||||||||
| TZY THENG OOI | 245,000 | 245,000 | - | 0.00 | ||||||||||||
| LI CHENG SOOI | 243,000 | 243,000 | - | 0.00 | ||||||||||||
| SOON PENG LEONG | 240,000 | 240,000 | - | 0.00 | ||||||||||||
| KIM YOON YONG | 235,000 | 235,000 | - | 0.00 | ||||||||||||
| LEE ENG TAN | 235,000 | 235,000 | - | 0.00 | ||||||||||||
| KING HONG KEE | 230,000 | 230,000 | - | 0.00 | ||||||||||||
| BEE KIM LAU | 230,000 | 230,000 | - | 0.00 | ||||||||||||
| EWE BOEY TEH | 222,500 | 222,500 | - | 0.00 | ||||||||||||
| SOONG FEI YONG | 216,000 | 216,000 | - | 0.00 | ||||||||||||
| LAY HIONG CHAN | 215,000 | 215,000 | - | 0.00 | ||||||||||||
| WAI HOONG LAM | 215,000 | 215,000 | - | 0.00 | ||||||||||||
| HENG HONG GAN | 210,000 | 210,000 | - | 0.00 | ||||||||||||
| LAY SAM LEE | 207,000 | 207,000 | - | 0.00 | ||||||||||||
| MEE CHON CHAI | 205,000 | 205,000 | - | 0.00 | ||||||||||||
| CHIUN YIN LAI | 204,000 | 204,000 | - | 0.00 | ||||||||||||
| BRANDON KHAI LI YEO | 203,154 | 203,154 | - | 0.00 | ||||||||||||
| JIN YOONG LIONG | 200,000 | 200,000 | - | 0.00 | ||||||||||||
| YAW CHONG LAW | 200,000 | 200,000 | - | 0.00 | ||||||||||||
| YAW HONG LAW | 200,000 | 200,000 | - | 0.00 | ||||||||||||
| YE BEI SIM | 200,000 | 200,000 | - | 0.00 | ||||||||||||
| CHUN FAI YAT | 200,000 | 200,000 | - | 0.00 | ||||||||||||
| SIAW BOON TEH | 200,000 | 200,000 | ||||||||||||||
| SS-4 |
| HOONG LING CHING | 200,000 | 200,000 | - | 0.00 | ||||||||||||
| JESSICA CHIA WEN SIM | 200,000 | 200,000 | - | 0.00 | ||||||||||||
| CHOY KIEN ONG | 197,000 | 197,000 | - | 0.00 | ||||||||||||
| SHI CHENG CHIN | 156,000 | 156,000 | - | 0.00 | ||||||||||||
| SIEW KIM TEOH | 150,000 | 150,000 | - | 0.00 | ||||||||||||
| DAMON TESTAVERDE | 150,000 | 150,000 | - | 0.00 | ||||||||||||
| SAU KHUAN CHOY | 135,000 | 135,000 | - | 0.00 | ||||||||||||
| LEE LI PING | 131,913 | 131,913 | - | 0.00 | ||||||||||||
| LAY CHING TAN | 130,000 | 130,000 | - | 0.00 | ||||||||||||
| BAK HAA HOW | 130,000 | 130,000 | - | 0.00 | ||||||||||||
| YOKE WAH LEONG | 115,000 | 115,000 | - | 0.00 | ||||||||||||
| SING HOE KOR | 110,000 | 110,000 | - | 0.00 | ||||||||||||
| SUI YEE TEE | 107,000 | 107,000 | - | 0.00 | ||||||||||||
| YOKE MOOI YONG | 106,000 | 106,000 | - | 0.00 | ||||||||||||
| YAT LONG LEE | 106,000 | 106,000 | - | 0.00 | ||||||||||||
| PAY FAH LYE | 105,000 | 105,000 | - | 0.00 | ||||||||||||
| KAH HOU LUM | 105,000 | 105,000 | - | 0.00 | ||||||||||||
| CHOY LING WONG | 105,000 | 105,000 | - | 0.00 | ||||||||||||
| KAH MEI WONG | 102,000 | 102,000 | - | 0.00 | ||||||||||||
| PHOENIX 16 SDN BHD | 100,200 | 100,200 | - | 0.00 | ||||||||||||
| JIAN CHENG LOO | 100,000 | 100,000 | - | 0.00 | ||||||||||||
| SWEE PEI NGU | 100,000 | 100,000 | - | 0.00 | ||||||||||||
| JUN HENG CHEN | 100,000 | 100,000 | - | 0.00 | ||||||||||||
| POH ONN ONG | 100,000 | 100,000 | - | 0.00 | ||||||||||||
| WOOI THONG OOI | 100,000 | 100,000 | - | 0.00 | ||||||||||||
| JIN FOONG LIM | 100,000 | 100,000 | - | 0.00 | ||||||||||||
| LEAN HOOI LEE | 100,000 | 100,000 | - | 0.00 | ||||||||||||
| WOON CHEN HO | 100,000 | 100,000 | - | 0.00 | ||||||||||||
| YAT HENG CHAN | 100,000 | 100,000 | - | 0.00 | ||||||||||||
| YUEN LOY CHONG | 100,000 | 100,000 | - | 0.00 | ||||||||||||
| YONG ZHEN NING SHERMAN | 100,000 | 100,000 | - | 0.00 | ||||||||||||
| TONG KIM LOOI | 100,000 | 100,000 | - | 0.00 | ||||||||||||
| YOKE LIM TANG | 97,000 | 97,000 | - | 0.00 | ||||||||||||
| FOO CHOO AU | 95,000 | 95,000 | - | 0.00 | ||||||||||||
| CHEE KEONG SOO | 88,000 | 88,000 | - | 0.00 | ||||||||||||
| JIN XUAN HOLDINGS SDN BHD | 87,500 | 87,500 | - | 0.00 | ||||||||||||
| EE CHIET YAP | 85,000 | 85,000 | - | 0.00 | ||||||||||||
| KWONG SENG CHAN | 82,000 | 82,000 | - | 0.00 | ||||||||||||
| SWEE TIAN GOH | 80,000 | 80,000 | - | 0.00 | ||||||||||||
| SIOK MING LAM | 80,000 | 80,000 | - | 0.00 | ||||||||||||
| POH YIN SEK | 80,000 | 80,000 | - | 0.00 | ||||||||||||
| SAN SANG MENG | 76,000 | 76,000 | - | 0.00 | ||||||||||||
| SIEW MOI HOO | 75,000 | 75,000 | - | 0.00 | ||||||||||||
| TENG WOEI SHY | 75,000 | 75,000 | - | 0.00 | ||||||||||||
| PUA SUAT CHOO | 72,000 | 72,000 | - | 0.00 | ||||||||||||
| CHUN KEAT QUAH | 71,000 | 71,000 | - | 0.00 | ||||||||||||
| MAN MAN WONG | 70,000 | 70,000 | - | 0.00 | ||||||||||||
| SIEW WAH TAN | 70,000 | 70,000 | - | 0.00 | ||||||||||||
| KIAH LING TAN | 70,000 | 70,000 | - | 0.00 | ||||||||||||
| LEONG CHOY LOH | 70,000 | 70,000 | - | 0.00 | ||||||||||||
| JOOI PENG ENG | 65,000 | 65,000 | - | 0.00 | ||||||||||||
| SAU KUAN YEE | 61,000 | 61,000 | - | 0.00 | ||||||||||||
| YUEN PENG CHAN | 60,000 | 60,000 | - | 0.00 |
| SS-5 |
| YUIN MIN LAI | 60,000 | 60,000 | - | 0.00 | ||||||||||||
| JENNY PON | 60,000 | 60,000 | - | 0.00 | ||||||||||||
| LEE ZI HAN | 60,000 | 60,000 | - | 0.00 | ||||||||||||
| TAK PEER EU | 60,000 | 60,000 | - | 0.00 | ||||||||||||
| KWOK HUNG TSE | 59,000 | 59,000 | - | 0.00 | ||||||||||||
| LOON HUA PHANG @ DAVID PHANG | 55,000 | 55,000 | - | 0.00 | ||||||||||||
| HUEY WEN LIM | 52,000 | 52,000 | - | 0.00 | ||||||||||||
| TENG PIN KHOO | 52,000 | 52,000 | - | 0.00 | ||||||||||||
| LOU FOOK TAK | 52,000 | 52,000 | - | 0.00 | ||||||||||||
| CEDE & CO | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| YET LIEN LIM | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| SHENG CHEE LIM | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| TET EAN HEW | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| THIAN CHAI KHOO | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| CHUAN SANG CHAI | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| CHONG KEAT ONG | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| LAWRENCE CHOON YIAP LAY | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| WAI LING SEW | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| TEH LEE SUAN | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| HEW LI YOONG | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| TAN SWEE LI | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| HEW TET MIN | 50,000 | 50,000 | - | 0.00 | ||||||||||||
| MEI YIN LEONG | 45,000 | 45,000 | - | 0.00 | ||||||||||||
| ANG ONG | 45,000 | 45,000 | - | 0.00 | ||||||||||||
| SAU KAY YEE | 45,000 | 45,000 | - | 0.00 | ||||||||||||
| HONG WENG LUM | 45,000 | 45,000 | - | 0.00 | ||||||||||||
| ONG CHOO KEAT | 44,000 | 44,000 | - | 0.00 | ||||||||||||
| HUECK CAPITAL SDN BHD | 43,718 | 43,718 | - | 0.00 | ||||||||||||
| HEW YUEN HOAY | 43,000 | 43,000 | - | 0.00 | ||||||||||||
| WEI CHOONG CHONG | 42,500 | 42,500 | - | 0.00 | ||||||||||||
| WENG CHOON FAN | 42,000 | 42,000 | - | 0.00 | ||||||||||||
| SOON LEE | 41,000 | 41,000 | - | 0.00 | ||||||||||||
| KUM LOON WONG | 40,000 | 40,000 | - | 0.00 | ||||||||||||
| YAP HOONG YANG | 40,000 | 40,000 | - | 0.00 | ||||||||||||
| LEONG SAN FATT | 40,000 | 40,000 | - | 0.00 | ||||||||||||
| PUI YEE LEE | 40,000 | 40,000 | - | 0.00 | ||||||||||||
| LOW LEE SUN | 40,000 | 40,000 | - | 0.00 | ||||||||||||
| WANG CHONG | 39,000 | 39,000 | - | 0.00 | ||||||||||||
| WEI LEONG CHONG | 37,500 | 37,500 | - | 0.00 | ||||||||||||
| SOO TENG TAN | 37,000 | 37,000 | - | 0.00 | ||||||||||||
| SAW BEE YAN | 37,000 | 37,000 | - | 0.00 | ||||||||||||
| FONG YOKE KAM | 36,000 | 36,000 | - | 0.00 | ||||||||||||
| GUAN SENG TAN | 35,000 | 35,000 | - | 0.00 | ||||||||||||
| CHOON YEAN OOI | 35,000 | 35,000 | - | 0.00 | ||||||||||||
| HUI SHUANG KHOO | 35,000 | 35,000 | - | 0.00 | ||||||||||||
| KUM LIN LAI | 35,000 | 35,000 | - | 0.00 | ||||||||||||
| SHIN SHIAW KOR | 35,000 | 35,000 | - | 0.00 | ||||||||||||
| CHOON KEAT LEE | 35,000 | 35,000 | - | 0.00 | ||||||||||||
| KHAY PENG KHOO | 35,000 | 35,000 | - | 0.00 | ||||||||||||
| LUM KAH KEONG | 35,000 | 35,000 | - | 0.00 | ||||||||||||
| GOH KUEN HOONG | 34,000 | 34,000 | - | 0.00 | ||||||||||||
| LAI YEE YING | 32,210 | 32,210 | - | 0.00 | ||||||||||||
| VERN LIM GOH | 30,500 | 30,500 | - | 0.00 |
| SS-6 |
| GAN PUI KUAN | 30,023 | 30,023 | - | 0.00 | ||||||||||||
| CHIN WEI LIM | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| KAM LEONG KONG | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| YIK LOONG ONG | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| SEE SENG LEE | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| JING LIU | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| BEE CHEW TOH | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| YEOW CHOONG WONG | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| AH HONG QUAH | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| SER BEE HWEE | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| SWE LAN MOK | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| LOH AH KEEN | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| BOH CHIN YIN | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| CHAN MENG CHERK | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| CHAN WAI CHON | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| CHAN YOKE YENG | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| DANG PEK SOON | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| KHOO CHI TUAN | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| KHOR CHIN LING | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| LAM JEN THOU | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| LAU HUI YAN | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| LAW TIEN SUNG | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| THYE ZEN SAN | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| LEE SEE FUI | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| LEE YEN FEI | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| LIEW SOOK MEI | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| LIM CHIN KHENG | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| LOO WEN YI | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| NG SWEE PENG | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| NGO CHANG LAI | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| OH SIEW YIEN | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| WONG MUN LENG | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| YONG KHONG WEE | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| WANG HUN | 30,000 | 30,000 | - | 0.00 | ||||||||||||
| MING YEAN LEE | 29,500 | 29,500 | - | 0.00 | ||||||||||||
| LUM PUI LENG | 29,000 | 29,000 | - | 0.00 | ||||||||||||
| LIT CHEAN KHOO | 28,000 | 28,000 | - | 0.00 | ||||||||||||
| AIRLIFT EXPRESS SDN BHD | 27,910 | 27,910 | - | 0.00 | ||||||||||||
| KHOR CHOON WEI | 27,846 | 27,846 | - | 0.00 | ||||||||||||
| LAM SEX TIAN | 27,417 | 27,417 | - | 0.00 | ||||||||||||
| SEE WAN TANG | 26,000 | 26,000 | - | 0.00 | ||||||||||||
| LAW YEN SHI | 25,700 | 25,700 | - | 0.00 | ||||||||||||
| SHOO TAI HEA | 25,000 | 25,000 | - | 0.00 | ||||||||||||
| TAN HOCK ENG | 25,000 | 25,000 | - | 0.00 | ||||||||||||
| WON SIN THEN | 25,000 | 25,000 | - | 0.00 | ||||||||||||
| SU ING LEE | 25,000 | 25,000 | - | 0.00 | ||||||||||||
| KAM FOONG LEONG | 25,000 | 25,000 | - | 0.00 | ||||||||||||
| CHIN CHYANG CHUA | 25,000 | 25,000 | - | 0.00 | ||||||||||||
| MUN HENG CHEN | 25,000 | 25,000 | - | 0.00 | ||||||||||||
| LENG LAI LEE | 25,000 | 25,000 | - | 0.00 | ||||||||||||
| CHIANG WEI PING | 25,000 | 25,000 | - | 0.00 | ||||||||||||
| YUAN YU YA | 24,500 | 24,500 | - | 0.00 | ||||||||||||
| JUN XIANG KHOO | 24,000 | 24,000 | - | 0.00 | ||||||||||||
| YU HSIN NI | 23,200 | 23,200 | - | 0.00 | ||||||||||||
| BOY MING CHONG @ SWEE FONG CHONG | 23,000 | 23,000 | - | 0.00 |
| SS-7 |
| CHEW LIM LAY BENG | 22,544 | 22,544 | - | 0.00 | ||||||||||||
| PHAIK NGOH CHUAH | 22,000 | 22,000 | - | 0.00 | ||||||||||||
| KONG KENG YIT | 22,000 | 22,000 | - | 0.00 | ||||||||||||
| TEH LEE LEEN | 22,000 | 22,000 | - | 0.00 | ||||||||||||
| YEE ZHENG YIP | 21,740 | 21,740 | - | 0.00 | ||||||||||||
| BENG SHEAN ONG | 21,000 | 21,000 | - | 0.00 | ||||||||||||
| LIM KIM SWEE | 21,000 | 21,000 | - | 0.00 | ||||||||||||
| KONG NYOONG LEE | 21,000 | 21,000 | - | 0.00 | ||||||||||||
| YU WEI VEN | 20,414 | 20,414 | - | 0.00 | ||||||||||||
| GEE KHENG TEOH | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| FUNG CHING CHIN | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| MIN FUN LEE | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| POOI FOON TAN | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| SIN WEI GAN | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| POW LEONG YONG | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| HA NGOW ONG | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| LAI HAR KOW | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| THAIM MENG KHOO | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| AH MOOI HU | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| CHUN HAO QUAH | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| PIK HOON HO | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| POH KUEN SEK | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| POH MEI SEK | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| AMAR FAROUQ BIN KAMARUDDIN | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| TAN BOO PAR | 20,000 | 20,000 | - | 0.00 | ||||||||||||
| LIM SIEW LEAN | 19,021 | 19,021 | - | 0.00 | ||||||||||||
| YAP LING HUI | 19,000 | 19,000 | - | 0.00 | ||||||||||||
| QUEK MEE SIM | 19,000 | 19,000 | - | 0.00 | ||||||||||||
| LEE CHONG CHOW | 19,000 | 19,000 | - | 0.00 | ||||||||||||
| KAU YAN TUCK | 18,105 | 18,105 | - | 0.00 | ||||||||||||
| KHOO TANG ENG | 18,000 | 18,000 | - | 0.00 | ||||||||||||
| MEI NGOR BEH | 17,000 | 17,000 | - | 0.00 | ||||||||||||
| CHEONG CHUI LENG | 17,000 | 17,000 | - | 0.00 | ||||||||||||
| CHOW CHIN FONG | 16,576 | 16,576 | - | 0.00 | ||||||||||||
| TENG HOOI GOH | 16,000 | 16,000 | - | 0.00 | ||||||||||||
| AH NIN HU | 16,000 | 16,000 | - | 0.00 | ||||||||||||
| YIT MING LEE | 16,000 | 16,000 | - | 0.00 | ||||||||||||
| CHEW WEN FRY | 16,000 | 16,000 | - | 0.00 | ||||||||||||
| LEE CHEN WEI | 16,000 | 16,000 | - | 0.00 | ||||||||||||
| CHONG YOKE CHOO | 16,000 | 16,000 | - | 0.00 | ||||||||||||
| TAI CHING LOON | 15,979 | 15,979 | - | 0.00 | ||||||||||||
| YU WEI JOO | 15,586 | 15,586 | - | 0.00 | ||||||||||||
| YEOH LAY TENG | 15,152 | 15,152 | - | 0.00 | ||||||||||||
| FOOK KIT THAM | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| SIEW MEI MOO | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| TEOW THEAM ANG | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| YET SEE THOO | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| TIONG CHING TEE | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| KOK WENG YEE | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| LOO MI TANG | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| SOW CHOY YEE | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| YING CHEANG LAI | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| MOOI LIM | 15,000 | 15,000 | - | 0.00 |
| SS-8 |
| PING WAI LAM | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| GOOI AN TAN | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| KAM YAU LEONG | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| CHAU JINN YEE | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| FE LIX LIM | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| KAR MUN LIM | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| LAY PIN LEE | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| HSIEH HSIU-CHU | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| SIEW HEOH LIM | 15,000 | 15,000 | - | 0.00 | ||||||||||||
| CHEN TEAK HUAT | 14,707 | 14,707 | - | 0.00 | ||||||||||||
| AI LING PANG | 14,000 | 14,000 | - | 0.00 | ||||||||||||
| ONG SOH CHING | 14,000 | 14,000 | - | 0.00 | ||||||||||||
| BEH YEN THING | 14,000 | 14,000 | - | 0.00 | ||||||||||||
| CHAN KOK HONG | 14,000 | 14,000 | - | 0.00 | ||||||||||||
| TAN PEE LEE | 14,000 | 14,000 | - | 0.00 | ||||||||||||
| CHEN MEE CHING | 13,891 | 13,891 | - | 0.00 | ||||||||||||
| BEE CHENG LAW | 13,000 | 13,000 | - | 0.00 | ||||||||||||
| AH MAY LAU | 13,000 | 13,000 | - | 0.00 | ||||||||||||
| BENG HEOK TAN | 12,500 | 12,500 | - | 0.00 | ||||||||||||
| BERNARD TATT LENG LEE | 12,500 | 12,500 | - | 0.00 | ||||||||||||
| CHEW NGEE@CHOW YEE | 12,000 | 12,000 | - | 0.00 | ||||||||||||
| LEE LING TING | 12,000 | 12,000 | - | 0.00 | ||||||||||||
| PUEA FANG ENG | 12,000 | 12,000 | - | 0.00 | ||||||||||||
| HUI XIAN HENG | 12,000 | 12,000 | - | 0.00 | ||||||||||||
| TEK CHIN HEW | 12,000 | 12,000 | - | 0.00 | ||||||||||||
| CHAI VOON FEI | 12,000 | 12,000 | - | 0.00 | ||||||||||||
| GOH JIA YING | 12,000 | 12,000 | - | 0.00 | ||||||||||||
| HENG SENG CHONG | 12,000 | 12,000 | - | 0.00 | ||||||||||||
| WONG HANG CHEI | 12,000 | 12,000 | - | 0.00 | ||||||||||||
| TEE KIM HUAT | 12,000 | 12,000 | - | 0.00 | ||||||||||||
| LEE SONG YEOW | 12,000 | 12,000 | - | 0.00 | ||||||||||||
| NG KWAN-U | 11,305 | 11,305 | - | 0.00 | ||||||||||||
| LEE SIEW TSUNG | 11,000 | 11,000 | - | 0.00 | ||||||||||||
| CHIOH SEOK KIM | 11,000 | 11,000 | - | 0.00 | ||||||||||||
| GOH AIK CHUAN | 11,000 | 11,000 | - | 0.00 | ||||||||||||
| YIP SWEE LIAN | 11,000 | 11,000 | - | 0.00 | ||||||||||||
| WAN SOOK MEE | 11,000 | 11,000 | - | 0.00 | ||||||||||||
| HONG MUI YONG | 10,500 | 10,500 | - | 0.00 | ||||||||||||
| CHEN TEIK FOOI | 10,001 | 10,001 | - | 0.00 | ||||||||||||
| LIM AH BOEY | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| HENG CHOY LAI | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| IVY GAIK HWA LIM | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| WEI LIN MOO | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| YEE FIEH CHIN | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| AI NING TING | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| WEI HWA LIM | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| SEE WERN GOH | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| SZE YEE CHUAR | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| TUAN NEO SEET @ SIEW KWAN TAY | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| VOON SEONG FOO | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| WAH CHYE LIM | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| FOOK EE CHAN | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| GAIK FOON LAU | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| GUN HENG CHAN | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| KIAN LEONG WONG | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| XIN YI KHOO | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| YEAP TUAN ONG | 10,000 | 10,000 | - | 0.00 |
| SS-9 |
| YEN CHIN OOI | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| YONG HUI LAW | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| TEK KHION HEW | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| KAM MOI MONG | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| SIEW CHOO TIONG | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| FUI LIN CHONG | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| CHANG YUNG HOO | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| CHEE KONG SOO | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| CHEE LOI CHENG | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| YUN CHYN HO | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| AH CHONG LOH | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| SUJANI GUNTORO | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| WAI WAH LEE | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| CHUN SHU OOI | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| KAH YI MOO | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| KIAH LOON TAN | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| KIM HUAT LEE | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| LEE LEE TAN | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| LEE YONG DEANG | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| MUN YEE CHIN | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| SIENG ONG YAP | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| SOH JIA MUN | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| SIEW FOONG SUM | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| TIO HOCK LAI | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| CHEW LAY BEE | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| KENG YONG SENG | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| LEE LE TAN | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| NAI CHOON SIANG | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| NG HOY BAN | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| ONG EWE HIN | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| YEE SAU CHOY | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| TANG LAI KHUANG | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| LAM CHIAN KOK | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| CHUNG TU KENG | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| CHUNG WOOI HEN | 10,000 | 10,000 | - | 0.00 | ||||||||||||
| KUAN YING WONG | 9,500 | 9,500 | - | 0.00 | ||||||||||||
| TANG PEI SAN | 9,250 | 9,250 | - | 0.00 | ||||||||||||
| CHRISTINA LIN LEAN TAN | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| MEI YIN LEE | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| HUAN HING CHAN | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| KEE SIONG CHIN | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| SEE HUI KHOR | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| WONG TAI | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| LAI YUIN PIN | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| NGOI KAM HEONG | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| ONG SEOK GNOH | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| RUONAN KOH | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| LEE CHEE HOOI | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| LIM CHEE WAH | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| ONG SIEW CHONG @ | 9,000 | 9,000 | - | 0.00 | ||||||||||||
| ENG KHENG LAU | 8,500 | 8,500 | - | 0.00 | ||||||||||||
| CHEN HOOY LEE | 8,320 | 8,320 | - | 0.00 | ||||||||||||
| TAN MING FU | 8,000 | 8,000 | - | 0.00 | ||||||||||||
| LIEW KENG TECK | 8,000 | 8,000 | - | 0.00 | ||||||||||||
| ONG ENG YEW | 8,000 | 8,000 | - | 0.00 |
| SS-10 |
| MAH POOI YAN | 8,000 | 8,000 | - | 0.00 | ||||||||||||
| TEH MEI KUIN | 8,000 | 8,000 | - | 0.00 | ||||||||||||
| ONG BENG SHEAN | 8,000 | 8,000 | - | 0.00 | ||||||||||||
| TERRENCE TAN HUAT HIN | 8,000 | 8,000 | - | 0.00 | ||||||||||||
| TAN KIM LYE | 8,000 | 8,000 | - | 0.00 | ||||||||||||
| HAU WAN HOCK | 8,000 | 8,000 | - | 0.00 | ||||||||||||
| SHI YNG LOH | 7,500 | 7,500 | - | 0.00 | ||||||||||||
| KENG SUAN BEH | 7,500 | 7,500 | - | 0.00 | ||||||||||||
| SHED HEONG LOW | 7,500 | 7,500 | - | 0.00 | ||||||||||||
| KIM YONG KHOO | 7,500 | 7,500 | - | 0.00 | ||||||||||||
| AIK HONG HO | 7,500 | 7,500 | - | 0.00 | ||||||||||||
| KIM SENG WONG | 7,500 | 7,500 | - | 0.00 | ||||||||||||
| SIEW HING NG | 7,500 | 7,500 | - | 0.00 | ||||||||||||
| PANG ENG MAY | 7,273 | 7,273 | - | 0.00 | ||||||||||||
| HONG SENG LIM | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| WAI YEE LEE | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| CHOI SIM TEOH | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| YOKE MUN SEK | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| YIT FONG CHAN | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| YE CHENG CHIN | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| SENG CHONG NG | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| PUA PUA ENG | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| POH MEE HOR | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| YOONG YEW PHANG | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| BEH KEAN THYE | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| BEH YEN SUN | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| CHIAM AH SIEW | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| CHONG YEONG KON | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| CHOW CHIA SIN | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| CHU SHIH KAN | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| CHUA YAH LI | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| HENG YIANG CHURN | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| KENG SHI YUN | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| KHOR CHEE GUAN | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| KHOR YING YING | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| LAM KIM SING | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| LAU HIN REEI | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| LEE SEU WEE | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| LIM HOCK KENG | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| NG SIEW BOON | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| NGAN SIEW BUN | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| ONG SWEE SUNG | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| SHIEW BEH BEE | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| SIM POH SUAN | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| SOON HEOH KEE | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| TAN SOH YEOK | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| YEE CHOW HOI | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| LEE CHEE HOONG | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| TSANG TUNG | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| HUANG XIUYING | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| TING-YEN KUO | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| WONG KAM WENG | 7,000 | 7,000 | - | 0.00 | ||||||||||||
| CHRISTOPHER JOHN BAPTIST | 6,060 | 6,060 | - | 0.00 | ||||||||||||
| CHAN BOON LIANG | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| YOON SUN HOO | 6,000 | 6,000 | - | 0.00 |
| SS-11 |
| YEE WON CHIN | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| MOOI SEE KHOR | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| LIM CHIEW WEI | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| CHEONG CHEE SENG | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| HIEW OI CHOO | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| KUAY SEIONG LEE | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| LAM CHEE MIN | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| LEE TIAN CHIAT | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| NG PAN LIAN | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| ONG XIN MIN | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| PHUAH CHIN CHYE | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| GOH LYE CHOO | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| SIVA SHANMUGAM A/L DHANAPALU | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| CHU MIN-CHANG | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| EU TAK PEER | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| SHIM PHUI SAN | 6,000 | 6,000 | - | 0.00 | ||||||||||||
| NG HOOI HAR | 5,636 | 5,636 | - | 0.00 | ||||||||||||
| MICHAEL ALEXON TAY KAI FOONG | 5,520 | 5,520 | - | 0.00 | ||||||||||||
| THUM XUE SHENG | 5,500 | 5,500 | - | 0.00 | ||||||||||||
| CHIA CHOON MIN | 5,500 | 5,500 | - | 0.00 | ||||||||||||
| LEE YING HO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PEI NGIN NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| REN LI CHAI | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHUN YEEN OOI | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| FOONG YEE LAW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CZEE TING LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SHAN SHAN LING | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW WAN TANG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| WOOI LIANG LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| AH HONG TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| AH PENG YEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| AH SUAN HUNG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| ALAN YAP @ LEE CHERN YAP | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| BEE KIAN WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW LING LEW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW MOI KOK | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW PENG TANG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW YEN TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIN LAI CHANG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIN YAU CHEAH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SING KIAT KOR | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIOK CHIN YEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIT MOOI LEONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIWE LEY LIAU | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SOON LING CHENG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| STEPHEN KOK HIN LEONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SU LING TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW KUAN CHEN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW LAY LAW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SUI CHENG CHAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YUN HIN LOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| ABDUL MU’IZZ BIN ABD KAHAR | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHEONG KEE TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HAN KHUAN HONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| JUN GIAP LAU | 5,000 | 5,000 | - | 0.00 |
| SS-12 |
| KIM SOON LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MEI FAH YAP | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PENG LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PUI YAN GAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW MEI YEOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YEN PIN BEH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| ZHI WEI TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHANG BOON BEH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| BOON AI HENG @ BOON AI ONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| BOON HONG ANG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHUO JVIN TEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| DARREN KHAI XU YEO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LI LEAN LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SENG CHAI ONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SOO LENG NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| TAN CHAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| TECK WEE TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YEE CHIN WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SWEE CHIN LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SWEE WAN LAM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| TAI CHU LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| TECK ONN LEONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| TECK SEONG WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| TECK VOON WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| TENG CHEN MOO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| TENG PONG MOO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| TIONG WAN TEY | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| WAI LIENG NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| WEI KIAT TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| WEI LING NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| ZHI WEN LOW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| AH MENG TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| AIK KUAN CHEAH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| ANNE GAIK LIN LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| ASIA AN KHONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| BENG TECK HEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHAU SOON TIONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHEE NENG YONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHIN WOO KHOR | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHING MEI WAI | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHOON HEE HAR | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHUI CHIN LOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHUI NGO KEONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| DORIS HONG GEK TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| ENG CHAI LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| ENG ENG TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| ENG HOO CHOO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| FAN HON CHONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HENG TING CHI | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HEOK KENG ENG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HOAY NEE KHOO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HONG CHU CHIA | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HOOI JING TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HUEY PING TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KAM LAI WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KENG HUA HOO | 5,000 | 5,000 | - | 0.00 |
| SS-13 |
| KENG WOH LEONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KHENG SIANG LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KHOW CHING CHEN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KOOI HIANG OOI | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LEE LEE NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LEE SIANG LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MEI FONG LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MENG YEP CHAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MOI HWA LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| XIN HUI KHOO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YAH LING TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YAU KWANG CH’NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YEE CHEN OH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YEE SIANG ONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| WEI YEE SOO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YEOK HONG LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| WENG KONG CHOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YEOK KIM KONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YEW CHUNG LAU | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YIK SING ONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YIN FHAN CHOY | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YIN PENG SIN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YOKE FAR WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| NGIT KUAI CHEONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PEI HEE TEH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PENG LEONG TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PUI YEE YAP | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| RHUN YAN LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SAW LING QUAH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SEE YONG LOW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SEET LEE YONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SEWU WAH TEOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW ENG KWAH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW FONG LOW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW KEEN CHOK | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW LING TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SOCK KIEW LING | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SOK KEAN TIAH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SOO KHENG TEOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SOOI SEAN LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SWEE YIN CHONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| WAI SEE NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| WEI KEAT KOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| WEI LIH CHONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| WENG FUI CHAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| WENG HOE LAU | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YEE TENG LOW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YEW HENG KOEY | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YIIK YEK WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YIP HING WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YOKE LAN CHOW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YU LEAN LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YONG MENG TEO | 5,000 | 5,000 | - | 0.00 |
| SS-14 |
| YONG QUAN LAW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YUEN LIAN CHEN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YUEN WAH CHONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| AH WAH TING | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHAN THONG LOW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHONG KEOW CHAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HONG YEAN LUM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KOK TONG MOK | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LAI NGO EU | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LAY PENG KUOK | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LI WEI LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LI YEE LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MOOI MOOI HEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PUI FONG CHONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| BING BOON CHONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| BOON KWAN YEAP | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| BOON SIN QUAH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHAI YIN SIOW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHAO WEY QUAH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHEE HONG ANG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHEE RAI LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHIA YEE TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHIN CHEW SO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHIN SENG TEOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHING HWA WOO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHING WEI TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SENG LOY CHEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW SENG LOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YAN YAT HAH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHOOI NAI NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHOW HELEN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHU LENG CHAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHUEN YUONG CHEW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHUN KIT SOO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHUN YEW YEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| ERIC SZE HERN CHEN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| FOON YIN CHOONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| GUAT LEE TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HAM FAH CHIN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HAW LEONG LING | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HAW SYUAN CHAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HIONG LANG LING | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HO CHENG CHAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HOCK CHOON LING | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HOCK GUAN YEOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HOCK MING TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HOU TING CHONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HUI FANG TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HUI LING ONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| JACKIE CHOOI LIN LING | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| JIANG WEN NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| JOO LIANG YEK | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHOI CHOI WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIOK YING HEN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| THAI LIM NGAI | 5,000 | 5,000 | - | 0.00 |
| SS-15 |
| AH THYE SUM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KAM HONG YONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SAN WAH LING @ SIN WAH LIN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YIN MAI WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CYNTHIA SHING YEH CHE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| FRANCIS @ PERNG LI YONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KEE BING WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KEE HIAN WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LEE HUANG NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| OOI LING ONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| ROYCE KAO TZIAT YEOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| TIAM LAI TAN@ YOKE FOONG CHAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHUN YING WU | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| NAN ANN LOW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SENG DIONG TIANG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| DENNIS SOON CHIN LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PEAK CHOO LOW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| JOSEPH KAI WEI KOR | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KAI CHEONG LOKE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KAR MIN SOO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KAR YAN KONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KAR YEE KONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KEA CHAI LING | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KEAN HOONG WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KEAN MING WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KEAT KOK YEOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KENG LENG CHAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KHAI WAI LOW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KIN PAO KONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KING YONG KEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KOK KEONG PEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KOK SHOONG NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KOOI LEAN OOI | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KOOI SIM TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KUN SENG CHEW | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KYE SWEN KHOR | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LAY KIM KUOK | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LEAN KEONG TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LEE CHEN DEO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LEE HOW CHIN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LEE WEN WOO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LEE YEE CHEONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LENG LENG GOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LEONG SENG WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LIAN HUI TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MEE GOKE TANG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHIEW TEE WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SHIN JOWL TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LIM HONG CHAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LIP TATT YAP | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LIZA CHIEW WEI WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LUIS R DUTAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MEE CHAN WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MEE CHOO BEH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MEI HAR TEE | 5,000 | 5,000 | - | 0.00 |
| SS-16 |
| MEI LING TEN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MENG KWAN LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MEOW NING ANG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MICHELLE KUAN WEI SHIA | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MING YONG LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MOOI SUAN BEH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PAK LING LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PEI XIAN CHAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PEIK HOON TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| POH TEE KHOO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| POH YEE ONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| POOI LING PANG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PRASERT SAE LOI @ BOON KEAN LOI | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PU YUN LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| REN HAO PANG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SAW PHAIK CHEAH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SEE FONG YAP | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SEEN HENG LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SEK FOO LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SENG YI NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SHEUE NIE TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEN CHUEN TEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW HONG TEO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| JOO HENG LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YEE JING WONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| BENJAMIN ENG KEEN ONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| NAI FOO YEAP | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| MEI LING TEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SIEW YOKE TAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SAU PING PAT LAI | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHUN LOONG TEOH | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| RENE CAMPOS | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LIP CHIN HO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| BEE HONG ANG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| FU KANG CHOR | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LIN LIN LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SENG SEONG U | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| BENG SENG SOOI | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SING KIONG TING | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| AH CHU TING @ KOK CHENG TING | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| JOGINDER KAUR A/P GIAN SINGH @ KINA S | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KIM FOONG LEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHANG CHIANG JU | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHEW SOON HOCK | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HOOI HAR NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| HOOI HOE NG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHANG CHONG CHIEK | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHEAH LAY JAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHIA NOI KEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHONG BENG GEOK | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| FOO AIK SEN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| GOH LI LING | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| KHOR KENG BENG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LEE LI SEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LIONG KHIM YUN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| NG KONG SOON | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| NGAN SIEW CHIN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| TAN KAR YIAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| PANG AH SENG | 5,000 | 5,000 | - | 0.00 |
| SS-17 |
| TANG LOO SEE | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| TOH LAN KIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YAN LAI KUAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHIN HEONG CHIA | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| JEFFREY ANAK LUKE JELY | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHONG SWEE YIN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YA YOKE CHUAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHIA GET KIANG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHOY SAU KHUAN | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YAW LEE ENG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| CHIN WEI LIM | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| LEE YING HO | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| YONG POW LEONG | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| SHIM YEW KEI | 5,000 | 5,000 | - | 0.00 | ||||||||||||
| NGU GOH HUI | 4,500 | 4,500 | - | 0.00 | ||||||||||||
| TAN BOON YEE | 4,500 | 4,500 | - | 0.00 | ||||||||||||
| HOW KHOON MENG | 4,500 | 4,500 | - | 0.00 | ||||||||||||
| HSU YUAN, HSIAN-WAN | 4,300 | 4,300 | - | 0.00 | ||||||||||||
| KUO TING-YEN | 4,300 | 4,300 | - | 0.00 | ||||||||||||
| LIN SIEW MEI | 4,187 | 4,187 | - | 0.00 | ||||||||||||
| MEI CHING JOO | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LUM LEE CHIN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| YAP SENG FUNG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| GOH YIN SOON | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHIN FA HOY | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHIA SIN HOCK | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| ANG CHYE HOON | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHAI KAR YAN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHEAH CHEW LEI | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHEONG LING | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHEW LIM LAY BENG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHIEW POH IM | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHIN KAM SOON | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHIN KIM MEEI | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHIN SIEW CHING | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHIN SIN MOOI | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHOO CHIEH TA | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHOONG MOOI MOOI | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHUAH SIONG TENG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CYNTHIA LOH MEI OON | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| GAN AH TOOH | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| GOH SIEW BIN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| HIEW HUEI PANG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| HOO MEI SING | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| IVY LOW CHIEW NI | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| KHOR KOON HOE | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| KOH CHEE YONG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| KOR KOK KEONG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LAM AH CHONG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LEE CHOON JUAN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LEE SIEW YING | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LEE WAI SENG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LIEW KIAM KHEN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LIEW KIM NYEAN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LIM BING LING | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LIM HONG CHOONG | 4,000 | 4,000 | - | 0.00 |
| SS-18 |
| LIM OON SIN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LIM SOK PENG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LING YOON CHANG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LOH CHUI CHUI | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LOK YUH TENG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LOW WEY LIANG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| NG BOON GHEE | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| NG KIEW HEONG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| NG SENG FANG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| OH KAH LEE | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| OH SANG SIK | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| ONG SHUE LING | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| OOI KEAN HONG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| PAN KOK KEE | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| PHANG PEI LIN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| PUTT YOKE POI | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| QUAH LAY HOOI | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| SUM LYE YENG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| TAI CHOO YOKE | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| TAN CHIN KHEONG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| TAN CHOON CHUI | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| TAN LIAN WEI | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| TAN LIE HAU | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| TANG SIANK CHIN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| TEOH MENG PIN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| TUNG MAY YOKE | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| WONG MEE KAM | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| WONG MEI NGOH | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| YEM HUI KIM | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHANG MEI HUI | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| TEO KUET TZE | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| TAN KIM AN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| KONG MEI KENG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHAN POH YIN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| HING PUI LEE | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LEE CHOW YOONG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LIM SEOW FON | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LIM YEOK LEAN | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHIN KEE SIONG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| CHEAH SIN CHYE | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LEE WAI CHENG | 4,000 | 4,000 | - | 0.00 | ||||||||||||
| LIM SEN HUAI | 3,985 | 3,985 | - | 0.00 | ||||||||||||
| PHOENIX PLUS HOLDING SDN BHD | 3,500 | 3,500 | - | 0.00 | ||||||||||||
| TAN PEI LENG | 3,500 | 3,500 | - | 0.00 | ||||||||||||
| CHU SOO LING | 3,500 | 3,500 | - | 0.00 | ||||||||||||
| HO YEEK MING | 3,500 | 3,500 | - | 0.00 | ||||||||||||
| NG HAEN JIAN | 3,500 | 3,500 | - | 0.00 | ||||||||||||
| THUM KUM WYE | 3,500 | 3,500 | - | 0.00 | ||||||||||||
| TING SING CHUNG | 3,500 | 3,500 | - | 0.00 | ||||||||||||
| ONG YEE LYN | 3,080 | 3,080 | - | 0.00 | ||||||||||||
| THIAN KAH TUCK | 3,000 | 3,000 | - | 0.00 | ||||||||||||
| YAP GEOK HWA | 3,000 | 3,000 | - | 0.00 | ||||||||||||
| SUET MOOI TAN | 3,000 | 3,000 | - | 0.00 | ||||||||||||
| TAN KIM FEN | 3,000 | 3,000 | - | 0.00 | ||||||||||||
| LEE KIM SIONG | 3,000 | 3,000 | - | 0.00 | ||||||||||||
| SIEW SEAN KOR | 3,000 | 3,000 | - | 0.00 |
| SS-19 |
| LIEW TONG HON | 3,000 | 3,000 | - | 0.00 | ||||||||||||
| CHIA MING MING | 3,000 | 3,000 | - | 0.00 | ||||||||||||
| KONG GAIK CHENG | 3,000 | 3,000 | - | 0.00 | ||||||||||||
| LOONG YAN SANG | 3,000 | 3,000 | - | 0.00 | ||||||||||||
| TAY JUN JIE | 3,000 | 3,000 | - | 0.00 | ||||||||||||
| FRANCIS PERNG LI YONG | 3,000 | 3,000 | - | 0.00 | ||||||||||||
| LAI MEI FANG | 3,000 | 3,000 | - | 0.00 | ||||||||||||
| SIEW SIN TAN | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| CHOON HAN LEE | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| SOCK HWA LIM | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| THIAM CHAI LEE | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| TZE SHIAN OOI | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| KOW FOONG LEE | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| WOAN SHIN TOH | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| YI SHENG YONG | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| MOOI CHOO LEE | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| CHAN FATT WONG | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| CHOI HAR CHAN | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| KAM TAI YANG | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| KOON SIM NG | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| CHEE FUN TENG | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| CHEE WAI JAN | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| CHEE YUEN SIAW | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| CHIN YEE OON | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| JOO WEI TAN | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| KAR LING MOY | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| LIH LING SOOI | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| LIH WEN SOOI | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| LING YEOK TAN | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| MOO TAN TEH | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| CHEN JUNG KET | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| BAK CHOO TAN | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| LIM CHAI LENG | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| TAY YEK CHIEW | 2,500 | 2,500 | - | 0.00 | ||||||||||||
| NG LEAN HUAT | 2,200 | 2,200 | - | 0.00 | ||||||||||||
| LEE AI PIN | 2,200 | 2,200 | - | 0.00 | ||||||||||||
| TENG AH CHOY | 2,200 | 2,200 | - | 0.00 | ||||||||||||
| MARGARET LIM | 2,200 | 2,200 | - | 0.00 | ||||||||||||
| LEE HOAY SAN | 2,020 | 2,020 | - | 0.00 | ||||||||||||
| WONG KOK CHUEN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YEW YEE CIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NG KOK LEONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LOH SOON YONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN TEIK MENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| AZAM MUDZAFFAR BIN OTHMAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHAI LEE WAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KUAN YOKE FONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KONG MUN KIT | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ANG HOCK SIANG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NIUN LILY | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| BEH LAY POH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIW PIK CHIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YEE THIAM KHENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| SIN LEK PHENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHONG NYOK MOY | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN BENG LOOI | 2,000 | 2,000 | - | 0.00 |
| SS-20 |
| YANG CHEE HOAY | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TEH SIEW BEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| SIM WEI BEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHEY YEN SIEW | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HENG KUNG SIAH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIEW MIN LONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YEE TUCK KEIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YONG WEI SING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| MOO SWEE LIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HO SOOK KEAM | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NURUL SAFA LIM BINTI ABDULLAH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| PHUAH KEE HUAH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YEE LEE CHIA | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ONG KAH KI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WOO CHI KIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHAN FOOK SENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE ZHAO ING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YONG SIANG CHIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG LAN CHING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHONG EE THIAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NG HOCK LOO | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE SEE WAI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHONG YOKE MUI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TIMOTHY JIM ENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| OH ANG SANG & OH ENG SANG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHO YANG YANG BILLY | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| EWE BOON KOOI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KUMUTHAM A/P RAJOO | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YUEN QING NIAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HENG SWEE LEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HUANG XU XIANG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LOH YUEN WAH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| GOH CHIEW SIA | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| POH SAIK PIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ANG BEE HWA | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ANG SOON HENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ANG SUE KHOON | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| AW MOI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| BAH CHANG XUN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CARMEN YONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHAI FATT ANN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHAN LAI FONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHAN POH SIM | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHAN SI YUAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHAN WAH SENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHAN WENG KWONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHAN YEN FATT | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHANG CHEW KIT | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHANG HUI YEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHARLES CHEE VUI KHONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHEAH MEAR KAI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHEONG KAH LIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHEONG KIN FONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHEW LI QIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHIA CHIN BONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHIA LI HUEY | 2,000 | 2,000 | - | 0.00 |
| SS-21 |
| CHIN CHEE KHUEN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHIN FOO YOONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHIN KAR LING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHIN KIEW KWONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHOK YUN TAI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHONG MEI HONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHONG MOK CHAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHONG SIEW HEONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHONG SOON PENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHONG WEN YANN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHOR KIM HOONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHOY KOK CHOONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHUA SEE HOCK | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHUA TING TING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHUAH SEAK HWA | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHUAH YONG XUANG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| CHUN LEAN SAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| DAVID NG PEI SHENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| DWEE WAI HA | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| EDDIE NORMAN VAZ | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ENG BEE YONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ENG CHUONG SHYUAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ERIN PUNG XIU YI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| FIONA TAN EI HWA | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| FONG TING HOOI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| FOO NYEN FOH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| FOO TUCK HENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| FOONG YOKE CHENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| FREDDIE NG CHUN KIT | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| GAN KAI LING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| GAN WEI JIEH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| GOH POH TIANG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HAH CHEE KEONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HAH KIN KEONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HAH LING HUI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HAH SHIAU HUI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HAW KAH HEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HELEN SHIM | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HIEW TEIK VOOI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HO HWEE GEOK | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HO LAI KHUAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HOE HOCK KEAT | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HOO CHING CHING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| JOANNE CH’NG SUE IMM | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KALIMUTHU A/L GOVINDASAMY | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KENG SING HUAT | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KENNETH NEO TERK CHERN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KHOO JIN CHAO | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KHOO KENG GIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KHOO LAY CHENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KHOO TENG CHEONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KHOO WEI HAU | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KHOO YUIH CHYUN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KHOR CHEE BOAY | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KOH SIEW PING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KOK KWAI SUN | 2,000 | 2,000 | - | 0.00 |
| SS-22 |
| KOK WAI HIEN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KONG CHUN HERNG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KONG DING WEI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| KU YING CHYE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LAI WENG KIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LAM MEI KUEN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LAM WAN JOE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LAU KOK CHOY | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LAW PENG MOOI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LAWRENCE TANG ZHI QIAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEAN SZE LU | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE BEE PHENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE BOON LONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE CHAI THUAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE CHEE HIAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE CHIN KHOON | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE CHOW LIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE FOO YEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE KIM GUAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE SOOK CHENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE WAI LING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE YAP HUNG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE YEAP KEONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE YEOW THAI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEONG MEI YING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEONG PUI KEONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEONG YAO CHUAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEOW XUE YING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIEW CHEE HOWE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIEW CHING MOAY | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIEW KEE BOON | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIEW KEN KIEW | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIEW KHIN SIANG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIEW YEAN KIM | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIM CHENG KOOK | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIM GUAN JIE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIM KAR PIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIM KOK SWEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIM MEA CHIAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIM MUI GEIK | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIM SIEW MOOI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIM SWEE MIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIM TENG TENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIM YOKE KHUAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LIONG LENG TAI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LO TING KUAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LOH NAN CHOON | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LOH SIEW LING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LOH YE WAH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LOO MEI YONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LOO YOOK PIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LOOI SUET HUI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LOUIE XIN RU KIM | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LOW CHIN KEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| MAK WAI YEN | 2,000 | 2,000 | - | 0.00 |
| SS-23 |
| MOO AI NAH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NG CHOR KUAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NG KENG LEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NG LEE MENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NG LING LONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NG MUN AIK | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NG MUN SAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NG WAI KEANG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NG WUAN SEAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NGAN SIEOW HUI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NGAN WAN CHIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NYEU HOOI PING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| OH KENG YEANG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ONG BENG CHOO | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ONG BOON PEI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ONG CHING CHUAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ONG SAW CHOO | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| ONG YUN PING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| OO AI MEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| OOI BOON SEONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| OOI BOON SHIEH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| OOI CHARD SENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| OOI CHEONG HEAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| OOI GUAT HUA | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HO LEE LEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| OOI HEOI SAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| OOI LEAN CHOO | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| OOI PEIK HOON | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| PARVEEN KAUR A/P AWTAR SINGH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| PHUN CHOONG PHOOI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| PIONG KOK CHONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| PONG POT NYONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| PUA CHEE KUAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| SAY HUEY SHIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| SEAK LAI GOON | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| SHU MEI CHIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| SOI MOI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| SUBAGARAN A/L LETCHUMANAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| SUBRAMANIAM A/L A MUTHUKARAPAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAI CHOON YIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAM ING HAUR | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN AEE @ TAN BOON SOON | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN CHUN YONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN HIANG BOON | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN HUI SIEW | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN LEONG SENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN PEE YING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN PEK YANG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN PENG TENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN SIO FUI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN SOO NGOH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN SZE MIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN TECK SENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TANG KING HEOK | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TANG YONG SENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TEH SOON LAI | 2,000 | 2,000 | - | 0.00 |
| SS-24 |
| TENG SZE CHEW | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TEOH SOAY ANG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TEONG AH MAI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TEY EE LAI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| THEN FOO SING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| THONG MEE MIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TING AAI HONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TIONG SIEW MING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TNEH HUN NGEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TOH MUN YEW | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WEE CHOY TENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WEE HON CHUNG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WEE TEO WEI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG BOON TONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG CHOY THAI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG HUN CHIAT | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG LAI HWA | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG MUN FONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG SEET WAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG SIEW MOH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG SWEE YEN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG YEE YING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG YOON MOOI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG YOON YIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YAN LAI FONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YANG HUI SHEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YAP CHIN LEONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YAP ENG KEONG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YAP ZHEN YU | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YAU CHEW HUN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YEAP HOCK HIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YEONG YIP HING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YONG MEI CHI | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YONG WENG CHAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YONG WENG KAM | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE CHONG SENG | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| HO YOKE FOON | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TING YEE PING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| MOHAMAD ANUAR BIN SAIDIN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| YIP YUEN TAT | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NG CHAU JEUN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| NG AI REEN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| THAM WEI PING | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEOW YIT WOON | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEE WAI YEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| BOON KIM SWEE | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| SOO PHAIK CHEOK | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TAN CHIN POH | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| TOH WEI LUR | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| WONG NG CHOON | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| LEONG KAH MUN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| JOANNE CHEAH YEE SWAN | 2,000 | 2,000 | - | 0.00 | ||||||||||||
| PHANG KHAR WEI | 1,983 | 1,983 | - | 0.00 | ||||||||||||
| CLARE W TULUS | 1,900 | 1,900 | - | 0.00 | ||||||||||||
| TAN YIH MEAN | 1,820 | 1,820 | - | 0.00 | ||||||||||||
| KOH HEE LIAN | 1,500 | 1,500 | - | 0.00 | ||||||||||||
| LIEW YONG HUAT | 1,500 | 1,500 | - | 0.00 | ||||||||||||
| KOR HUR CHEN | 1,500 | 1,500 | - | 0.00 | ||||||||||||
| TAN KEAN WIN | 1,500 | 1,500 | - | 0.00 | ||||||||||||
| LIM KOK SEONG | 1,500 | 1,500 | - | 0.00 | ||||||||||||
| KAM YUEN YEE | 1,320 | 1,320 | - | 0.00 | ||||||||||||
| YONG SIT FONG | 1,100 | 1,100 | - | 0.00 |
| SS-25 |
| TRICIA KONG YUN FUN | 1,100 | 1,100 | - | 0.00 | ||||||||||||
| CHIN SU SIM | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| HOONG LI KUO | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| LEE BEE CHENG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| CHUA BOON BOON | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| LEE CHONG SOON | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| NGAN BEE KIAW | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| KHO KHENG SIONG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| THEN SHWU WON | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| JEN HUI TEO | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| KOK HOONG CHEU | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| SHERN KWOK LIM | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| CHAI HWEE HU | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| LIM POH CHOON | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| FONG SIEW MOI | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| LEE CHUN WEI | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| YEE TUCK YEIN | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| TEH ENG LOON | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| HENG LEE KUAN | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| NG HUE BOON | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| CHIN WHY LEONG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| CHEOK SOOK PENG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| TEOH AI MUI | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| NG LAI CHOON | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| CHANG HUI CHIN | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| NG LEE LEE | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| KHAW HWEE YONG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| LEE BEE HONG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| WAN CHEN CHOK | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| CHAN KAH JUNE | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| CHEW SIEW ENG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| SU WEI SIONG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| TAN POH ENG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| LAI YEW HONG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| YEE TUCK LOONG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| LEONG KANG WEI | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| ANG CHAN OOI | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| CHIA YIT MEI | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| KHAW GUAT HOON | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| LAI YEIN HONG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| LOW YEONG KEONG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| NG KHENG HANG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| NGU CHANG YUAN | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| TAM WEI YEE | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| TAN YONG CHEN | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| NG GUAN HWA | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| YEOH CHE CHIAN | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| PEONG SIOW FONG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| TAN CHEE WEI | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| KOAY SEE BENG | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| LOO AH MOY | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| LAU LAY PING | 1,000 | 1,000 | - | 0.00 | ||||||||||||
| HO AIK HONG | 800 | 800 | - | 0.00 | ||||||||||||
| GOOI MING SHENG | 700 | 700 | - | 0.00 | ||||||||||||
| KOH KIM SAI | 700 | 700 | - | 0.00 | ||||||||||||
| WON NGAR TENG | 500 | 500 | - | 0.00 | ||||||||||||
| CHONG ZHI YING | 350 | 350 | - | 0.00 | ||||||||||||
| LING CHEK PING | 350 | 350 | - | 0.00 | ||||||||||||
| SEAH AH HEANG | 350 | 350 | - | 0.00 | ||||||||||||
| Total | 35,114,516 | 30,169,516 | 4,945,000 | 6.48 |
(1) Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, securities that are currently convertible or exercisable into shares of our common stock, or convertible or exercisable into shares of our common stock within 60 days of the date hereof are deemed outstanding. Such shares, however, are not deemed outstanding for the purposes of computing the percentage ownership of any other person. Except as indicated in the footnotes to the following table, each stockholder named in the table has sole voting and investment power with respect to the shares set forth opposite such stockholder’s name.
| SS-26 |
SELLING STOCKHOLDERS PLAN OF DISTRIBUTION
Our common stock is presently quoted on the OTC Markets – Pink Sheets under the symbol “AATP”. Although there is currently a bid and offer quotation for the common stock, such bid and offer are for limited and insignificant number of shares. The last sale price recorded was $6.00 per share on May 11, 2023. Because trading has been sporadic and irregular, there is no established public trading market for our common stock.
The selling stockholders will offer and sell their shares of common stock being offered under this prospectus at $6.50 per share on the OTC Markets – Pink Sheets under the symbol “AATP” or in private transactions for the duration of this offering or until the shares are listed on a national securities exchange at which time the selling stockholders and any of their pledgees, donees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of common stock being offered under this prospectus on any stock exchange, market or trading facility on which shares of our common stock are traded or in private transactions. We have applied to list our common stock on the NASDAQ Capital Market (“NASDAQ”) under the symbol “ATPC”. No assurance can be given that our application will be approved.
The selling stockholders may use any one or more of the following methods when disposing of shares:
| ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; | |
| ● | block trades in which the broker-dealer will attempt to sell the shares as agent but may position; and resell a portion of the block as principal to facilitate the transaction; | |
| ● | purchases by a broker-dealer as principal and resales by the broker-dealer for its account; | |
| ● | an exchange distribution in accordance with the rules of the applicable exchange; | |
| ● | privately negotiated transactions; | |
| ● | to cover short sales made after the date that the registration statement of which this prospectus is a part is declared effective by the SEC; | |
| ● | broker-dealers may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share; | |
| ● | a combination of any of these methods of sale; and | |
| ● | any other method permitted pursuant to applicable law. |
The shares may also be sold under Rule 144 under the Securities Act of 1933, as amended, if available for a selling stockholder, rather than under this prospectus. The selling stockholders have the sole and absolute discretion not to accept any purchase offer or make any sale of shares if they deem the purchase price to be unsatisfactory at any particular time.
The selling stockholders may pledge their shares to their brokers under the margin provisions of customer agreements. If a selling stockholder defaults on a margin loan, the broker may, from time to time, offer and sell the pledged shares.
Broker-dealers engaged by the selling stockholders may arrange for other broker-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling stockholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated, which commissions as to a particular broker or dealer may be in excess of customary commissions to the extent permitted by applicable law.
If sales of shares offered under this prospectus are made to broker-dealers as principals, we would be required to file a post-effective amendment to the registration statement of which this prospectus is a part. In the post-effective amendment, we would be required to disclose the names of any participating broker-dealers and the compensation arrangements relating to such sales.
The selling stockholders and any broker-dealers or agents that are involved in selling the shares offered under this prospectus may be deemed to be “underwriters” within the meaning of the Securities Act in connection with these sales. Commissions received by these broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting discount under the Securities Act. Any broker-dealers or agents that are deemed to be underwriters may not sell shares offered under this prospectus unless and until we set forth the names of the underwriters and the material details of their underwriting arrangements in a supplement to this prospectus or, if required, in a replacement prospectus included in a post-effective amendment to the registration statement of which this prospectus is a part.
The selling stockholders and any other persons participating in the sale or distribution of the shares offered under this prospectus will be subject to applicable provisions of the Exchange Act, and the rules and regulations under that act, including Regulation M. These provisions may restrict activities of, and limit the timing of purchases and sales of any of the shares by, the selling stockholders or any other person. Furthermore, under Regulation M, persons engaged in a distribution of securities are prohibited from simultaneously engaging in market making and other activities with respect to those securities for a specified period of time prior to the commencement of such distributions, subject to specified exceptions or exemptions. All of these limitations may affect the marketability of the shares.
| SS-27 |
Rule 2710 requires members firms to satisfy the filing requirements of Rule 2710 in connection with the resale, on behalf of selling stockholders, of the securities on a principal or agency basis. NASD Notice to Members 88-101 states that in the event a Selling Stockholder intends to sell any of the shares registered for resale in this prospectus through a member of FINRA participating in a distribution of our securities, such member is responsible for insuring that a timely filing, if required, is first made with the Corporate Finance Department of FINRA and disclosing to FINRA the following:
| ● | it intends to take possession of the registered securities or to facilitate the transfer of such certificates; | |
| ● | the complete details of how the selling stockholders’ shares are and will be held, including location of the particular accounts; | |
| ● | whether the member firm or any direct or indirect affiliates thereof have entered into, will facilitate or otherwise participate in any type of payment transaction with the selling stockholders, including details regarding any such transactions; and | |
| ● | in the event any of the securities offered by the selling stockholders are sold, transferred, assigned or hypothecated by any Selling Stockholder in a transaction that directly or indirectly involves a member firm of FINRA or any affiliates thereof, that prior to or at the time of said transaction the member firm will timely file all relevant documents with respect to such transaction(s) with the Corporate Finance Department of FINRA for review. |
No FINRA member firm may receive compensation in excess of that allowable under FINRA rules, including Rule 2710, in connection with the resale of the securities by the selling stockholders, which total compensation may not exceed 8%.
If any of the shares of common stock offered for sale pursuant to this prospectus are transferred other than pursuant to a sale under this prospectus, then subsequent holders could not use this prospectus until a post-effective amendment or prospectus supplement is filed, naming such holders. We offer no assurance as to whether any of the selling stockholders will sell all or any portion of the shares offered under this prospectus.
We have agreed to pay all fees and expenses we incur incident to the registration of the shares being offered under this prospectus. However, each selling stockholder and purchaser is responsible for paying any discount, and similar selling expenses they incur.
We and the selling stockholders have agreed to indemnify one another against certain losses, damages and liabilities arising in connection with this prospectus, including liabilities under the Securities Act.
| SS-28 |
LEGAL MATTERS
Certain legal matters with respect to U.S. federal securities law will be passed upon for us by Loeb & Loeb LLP, New York, New York. Legal matters with respect to the legality of the shares of common stock offered hereby will be passed upon for us by Sherman & Howard L.L.C. Legal matters as to Malaysia law will be passed upon for us by Lee & Poh Partnership. Loeb & Loeb, LLP may rely upon Lee & Poh Partnership. with respect to matters governed by Malaysian law. Lee & Poh Partnership is acting as Malaysia counsel for the Company.
| SS-29 |
AGAPE ATP CORPORATION
30,169,516 Shares of Common Stock
PROSPECTUS
You should rely only on the information contained in this prospectus. No dealer, salesperson or other person is authorized to give information that is not contained in this prospectus. This prospectus is not an offer to sell nor is it seeking an offer to buy these securities in any jurisdiction where the offer or sale is not permitted. The information contained in this prospectus is correct only as of the date of this prospectus, regardless of the time of the delivery of this prospectus or the sale of these securities.
Until , 2023, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriter with respect to their unsold subscriptions.
The date of this prospectus is , 2023
| SS-30 |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
Set forth below is an estimate (except for SEC registration and FINRA filing fees, which are actual) of the approximate amount of the types of fees and expenses listed below that were paid or are payable by us in connection with the issuance and distribution of the shares of common stock to be registered by this registration statement.
| Item | Amount
to be paid | |||
| SEC registration fee | $ | 31,747 | ||
| FINRA filing fee | 63,300 | |||
| Nasdaq listing fee | 75,000 | |||
| Legal fees and expenses | 275,000 | |||
| Accounting fees and expenses | 85,000 | |||
| U.S. GAAP Consulting | 79,288 | |||
| Transfer agent fees and expenses | 5,000 | |||
| Underwriter expense reimbursement | 144,500 | |||
| Printing expenses | 7,500 | |||
| Miscellaneous expenses | 2,500 | |||
| Total | $ | 768,834 | ||
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS
The Company’s directors and executive officers are indemnified as provided by the Nevada Revised Statutes and its Bylaws. These provisions state that the Company’s directors may cause the Company to indemnify a director or former director against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, actually and reasonably incurred by him as a result of him acting as a director. The indemnification of costs can include an amount paid to settle an action or satisfy a judgment. Such indemnification is at the discretion of the Company’s board of directors and is subject to the Securities and Exchange Commission’s policy regarding indemnification.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, or otherwise, The Company has been advised that in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable.
ITEM 15. RECENT SALE OF UNREGISTERED SECURITIES
No underwriter were involved in the issuance of the securities noted below. All of the securities issued below were deemed to be exempt from registration under the Securities Act in reliance upon Regulation S for offerings made outside of the United States.
| ● | On April 5, 2017, the Company acquired Agape ATP Corporation, a company incorporated in Labuan, Malaysia. | |
| ● | On April 10, 2017, the Company issued 245,000,000 and 70,000,000 shares of common stock to Dr. How Kok Choong and HKC Holdings Sdn Bhd respectively, each with a par value of $0.0001 per share, for total additional working capital of $31,500. HKC Holdings Sdn Bhd is owned and controlled by Dr. How Kok Choong who is our chief executive officer, chief operating officer, chairman of the board of Directors, Director and secretary. | |
| ● | On May 8, 2020, the Company acquired approximately 99.99% of the issued share capital of Agape Superior Living Sdn Bhd, a company incorporated in Malaysia from Dr. How Kok Choong. |
| II-1 |
ITEM 16. EXHIBITS AND FINANCIAL STATEMENTS
Exhibits
See the Exhibit Index attached to this registration statement, which is incorporated by reference herein.
ITEM 17. UNDERTAKINGS
The undersigned registrant hereby undertakes to:
(1) File, during any period in which offers or sells are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii) To include material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; provided, however, that paragraphs (1)(i), (1)(ii) and (1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the Registrant pursuant to Section 13 and Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
| II-2 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in Kuala Lumpur, Malaysia, on August 24, 2023.
| AGAPE ATP CORPORATION | ||
| By: | /s/ How Kok Choong | |
| Name: | How Kok Choong | |
| Title: | Director, Chairman of the Board of Directors, Chief Executive Officer, Chief Operating Officer and Secretary (Principal Executive Officer) | |
POWER OF ATTORNEY
Each person whose signature appears below constitutes and appoints each of How Kok Choong and Lee Kam Fan Andrew as attorneys-in-fact with full power of substitution for him or her in any and all capacities to do any and all acts and all things and to execute any and all instruments which said attorney and agent may deem necessary or desirable to enable the registrant to comply with the Securities Act of 1933, as amended (the “Securities Act”), and any rules, regulations and requirements of the Securities and Exchange Commission thereunder, in connection with the registration under the Securities Act of shares of common stock of the registrant (the “Shares”), including, without limitation, the power and authority to sign the name of each of the undersigned in the capacities indicated below to the Registration Statement on Form S-1 (the “Registration Statement”) to be filed with the Securities and Exchange Commission with respect to such Shares, to any and all amendments or supplements to such Registration Statement, whether such amendments or supplements are filed before or after the effective date of such Registration Statement, to any related Registration Statement filed pursuant to Rule 462(b) under the Securities Act, and to any and all instruments or documents filed as part of or in connection with such Registration Statement or any and all amendments thereto, whether such amendments are filed before or after the effective date of such Registration Statement; and each of the undersigned hereby ratifies and confirms all that such attorney and agent shall do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ How Kok Choong | Chief Executive Officer, Chief Operating Officer, Director, Chairman of the Board of Directors and Secretary | August 24, 2023 | ||
| How Kok Choong | (Principal Executive Officer) | |||
| /s/ Lee Kam Fan Andrew | Chief Financial Officer | August 24, 2023 | ||
Lee Kam Fan Andrew |
(Principal Financial and Accounting Officer) |
| II-3 |
EXHIBIT INDEX
| * | To be filed by amendment. |
| ** | Previously filled |
| *** | Filed herewith |
| II-4 |