000169853012/312021Q3false00016985302021-01-012021-09-30xbrli:shares00016985302021-11-12iso4217:USD00016985302021-09-3000016985302020-12-31iso4217:USDxbrli:shares00016985302021-07-012021-09-3000016985302020-07-012020-09-3000016985302020-01-012020-09-300001698530us-gaap:CommonStockMember2020-12-310001698530us-gaap:AdditionalPaidInCapitalMember2020-12-310001698530us-gaap:RetainedEarningsMember2020-12-310001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-12-310001698530us-gaap:CommonStockMember2021-01-012021-03-310001698530us-gaap:AdditionalPaidInCapitalMember2021-01-012021-03-3100016985302021-01-012021-03-310001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-01-012021-03-310001698530us-gaap:RetainedEarningsMember2021-01-012021-03-310001698530us-gaap:CommonStockMember2021-03-310001698530us-gaap:AdditionalPaidInCapitalMember2021-03-310001698530us-gaap:RetainedEarningsMember2021-03-310001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-03-3100016985302021-03-310001698530us-gaap:AdditionalPaidInCapitalMember2021-04-012021-06-3000016985302021-04-012021-06-300001698530us-gaap:CommonStockMember2021-04-012021-06-300001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-04-012021-06-300001698530us-gaap:RetainedEarningsMember2021-04-012021-06-300001698530us-gaap:CommonStockMember2021-06-300001698530us-gaap:AdditionalPaidInCapitalMember2021-06-300001698530us-gaap:RetainedEarningsMember2021-06-300001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-06-3000016985302021-06-300001698530us-gaap:AdditionalPaidInCapitalMember2021-07-012021-09-300001698530us-gaap:CommonStockMember2021-07-012021-09-300001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-07-012021-09-300001698530us-gaap:RetainedEarningsMember2021-07-012021-09-300001698530us-gaap:CommonStockMember2021-09-300001698530us-gaap:AdditionalPaidInCapitalMember2021-09-300001698530us-gaap:RetainedEarningsMember2021-09-300001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-09-300001698530us-gaap:CommonStockMember2019-12-310001698530us-gaap:AdditionalPaidInCapitalMember2019-12-310001698530us-gaap:RetainedEarningsMember2019-12-310001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-12-3100016985302019-12-310001698530us-gaap:AdditionalPaidInCapitalMember2020-01-012020-03-3100016985302020-01-012020-03-310001698530us-gaap:CommonStockMember2020-01-012020-03-310001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-01-012020-03-310001698530us-gaap:RetainedEarningsMember2020-01-012020-03-310001698530us-gaap:CommonStockMember2020-03-310001698530us-gaap:AdditionalPaidInCapitalMember2020-03-310001698530us-gaap:RetainedEarningsMember2020-03-310001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-03-3100016985302020-03-310001698530us-gaap:CommonStockMember2020-04-012020-06-300001698530us-gaap:AdditionalPaidInCapitalMember2020-04-012020-06-3000016985302020-04-012020-06-300001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-04-012020-06-300001698530us-gaap:RetainedEarningsMember2020-04-012020-06-300001698530us-gaap:CommonStockMember2020-06-300001698530us-gaap:AdditionalPaidInCapitalMember2020-06-300001698530us-gaap:RetainedEarningsMember2020-06-300001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-06-3000016985302020-06-300001698530us-gaap:AdditionalPaidInCapitalMember2020-07-012020-09-300001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-07-012020-09-300001698530us-gaap:RetainedEarningsMember2020-07-012020-09-300001698530us-gaap:CommonStockMember2020-09-300001698530us-gaap:AdditionalPaidInCapitalMember2020-09-300001698530us-gaap:RetainedEarningsMember2020-09-300001698530us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-09-3000016985302020-09-300001698530us-gaap:CollaborativeArrangementMemberxcur:AbbVieCollaborationMember2021-07-012021-09-30xbrli:pure0001698530xcur:COVID19Member2021-07-012021-09-30xcur:agreement0001698530xcur:IpsenCollaborationMemberus-gaap:CollaborativeArrangementMember2021-07-302021-07-300001698530xcur:IpsenCollaborationMemberus-gaap:CollaborativeArrangementMemberxcur:ExercisePeriodOneMember2021-07-302021-07-300001698530xcur:IpsenCollaborationMemberxcur:ExercisePeriodTwoMemberus-gaap:CollaborativeArrangementMember2021-07-302021-07-300001698530xcur:IpsenCollaborationMemberus-gaap:CollaborativeArrangementMember2021-07-300001698530xcur:IpsenCollaborationMemberxcur:IpsenHDMember2021-09-300001698530xcur:IpsenCollaborationMemberxcur:IpsenASMember2021-09-300001698530xcur:IpsenCollaborationMemberus-gaap:CollaborativeArrangementMember2021-01-012021-09-300001698530xcur:IpsenCollaborationMemberus-gaap:CollaborativeArrangementMember2021-07-012021-09-300001698530xcur:IpsenCollaborationMemberus-gaap:CollaborativeArrangementMember2021-09-300001698530xcur:IpsenCollaborationMembersrt:MinimumMemberus-gaap:CollaborativeArrangementMember2021-01-012021-09-300001698530xcur:IpsenCollaborationMemberus-gaap:CollaborativeArrangementMembersrt:MaximumMember2021-01-012021-09-300001698530us-gaap:CollaborativeArrangementMemberxcur:AbbVieCollaborationMember2019-11-132019-11-130001698530xcur:DevelopmentandCommercializationLicenseMemberus-gaap:CollaborativeArrangementMemberxcur:AbbVieCollaborationMember2019-11-132019-11-130001698530us-gaap:CollaborativeArrangementMembersrt:MaximumMemberxcur:AbbVieCollaborationMember2019-11-132019-11-130001698530us-gaap:CollaborativeArrangementMemberxcur:AbbVieCollaborationMember2021-01-012021-09-300001698530us-gaap:CollaborativeArrangementMemberxcur:AbbVieCollaborationMember2020-07-012020-09-300001698530us-gaap:CollaborativeArrangementMemberxcur:AbbVieCollaborationMember2020-01-012020-09-300001698530us-gaap:CollaborativeArrangementMemberxcur:AbbVieCollaborationMember2021-09-300001698530srt:MinimumMemberus-gaap:CollaborativeArrangementMemberxcur:AbbVieCollaborationMember2021-01-012021-09-300001698530us-gaap:CollaborativeArrangementMembersrt:MaximumMemberxcur:AbbVieCollaborationMember2021-01-012021-09-300001698530us-gaap:CollaborativeArrangementMemberxcur:AbbVieCollaborationMember2020-12-310001698530xcur:DermelixLicenseAgreementMemberus-gaap:CollaborativeArrangementMember2019-02-172019-02-170001698530xcur:DermelixLicenseAgreementMemberus-gaap:CollaborativeArrangementMember2019-12-310001698530xcur:DermelixLicenseAgreementMemberus-gaap:CollaborativeArrangementMember2021-01-012021-09-300001698530xcur:DermelixLicenseAgreementMemberus-gaap:CollaborativeArrangementMember2021-07-012021-09-300001698530xcur:DermelixLicenseAgreementMemberus-gaap:CollaborativeArrangementMember2020-07-012020-09-300001698530xcur:DermelixLicenseAgreementMemberus-gaap:CollaborativeArrangementMember2020-01-012020-09-300001698530xcur:IpsenCollaborationMember2020-12-310001698530xcur:IpsenCollaborationMember2021-01-012021-09-300001698530xcur:IpsenCollaborationMember2021-09-300001698530xcur:AbbVieCollaborationMember2020-12-310001698530xcur:AbbVieCollaborationMember2021-01-012021-09-300001698530xcur:AbbVieCollaborationMember2021-09-300001698530us-gaap:EquipmentMember2021-09-300001698530us-gaap:EquipmentMember2020-12-310001698530us-gaap:LeaseholdImprovementsMember2021-09-300001698530us-gaap:LeaseholdImprovementsMember2020-12-310001698530xcur:ComputersandSoftwareMember2021-09-300001698530xcur:ComputersandSoftwareMember2020-12-310001698530us-gaap:FurnitureAndFixturesMember2021-09-300001698530us-gaap:FurnitureAndFixturesMember2020-12-310001698530us-gaap:ConstructionInProgressMember2021-09-300001698530us-gaap:ConstructionInProgressMember2020-12-310001698530us-gaap:ShortTermInvestmentsMember2021-09-300001698530us-gaap:CorporateDebtSecuritiesMember2021-09-300001698530us-gaap:USGovernmentAgenciesDebtSecuritiesMember2021-09-300001698530us-gaap:ShortTermInvestmentsMember2020-12-310001698530us-gaap:CorporateDebtSecuritiesMember2020-12-310001698530us-gaap:USTreasurySecuritiesMember2020-12-310001698530us-gaap:USGovernmentAgenciesDebtSecuritiesMember2020-12-310001698530us-gaap:SecuredDebtMemberxcur:MidCapCreditAgreementMember2020-09-250001698530us-gaap:SecuredDebtMemberxcur:MidCapCreditAgreementTranche1Member2020-09-252020-09-250001698530us-gaap:SecuredDebtMemberxcur:MidCapCreditAgreementTranche2Member2021-09-300001698530us-gaap:SecuredDebtMemberxcur:MidCapCreditAgreementMember2020-09-252020-09-25xcur:installmentPayment0001698530us-gaap:SecuredDebtMemberxcur:MidCapCreditAgreementMembersrt:ScenarioForecastMember2022-10-010001698530us-gaap:SecuredDebtMemberxcur:MidCapCreditAgreementMemberus-gaap:DebtInstrumentRedemptionPeriodOneMember2021-09-300001698530us-gaap:SecuredDebtMemberxcur:MidCapCreditAgreementMemberus-gaap:DebtInstrumentRedemptionPeriodTwoMember2021-09-3000016985302020-09-252020-09-250001698530us-gaap:SecuredDebtMemberxcur:MidCapCreditAgreementTranche1Member2020-09-250001698530us-gaap:SecuredDebtMemberxcur:MidCapCreditAgreementMember2021-01-012021-09-300001698530us-gaap:SecuredDebtMemberxcur:MidCapCreditAgreementTranche2Member2020-09-250001698530us-gaap:SecuredDebtMemberxcur:MidCapCreditAgreementTranche2Member2021-07-012021-09-300001698530us-gaap:SecuredDebtMemberxcur:MidCapCreditAgreementTranche2Member2021-01-012021-09-300001698530us-gaap:LoansPayableMemberus-gaap:SecuredDebtMemberxcur:HerculesTechnologyGrowthCapitalMember2020-03-022020-03-02utr:sqft0001698530xcur:ChicagoMember2020-07-012020-07-010001698530xcur:ChicagoMember2020-07-01xcur:extensionPeriodxcur:votePerShare0001698530us-gaap:CommonStockMember2021-09-300001698530us-gaap:CommonStockMember2020-12-310001698530us-gaap:CommonStockMember2021-03-270001698530us-gaap:CommonStockMember2021-04-280001698530us-gaap:CommonStockMember2021-05-030001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-12-310001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-01-012021-03-310001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-03-310001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-04-012021-06-300001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-06-300001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-07-012021-09-300001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2021-09-300001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2019-12-310001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-01-012020-03-310001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-03-310001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-04-012020-06-300001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-06-300001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-07-012020-09-300001698530us-gaap:AccumulatedNetUnrealizedInvestmentGainLossMember2020-09-300001698530xcur:ExicureInc.2017EquityIncentivePlanMember2021-01-012021-09-300001698530xcur:ExicureInc.2017EquityIncentivePlanMember2021-09-300001698530xcur:ExicureInc.2017EquityIncentivePlanMemberus-gaap:EmployeeStockOptionMember2021-01-012021-01-010001698530srt:ChiefFinancialOfficerMember2021-05-012021-05-310001698530srt:ChiefFinancialOfficerMember2021-09-300001698530us-gaap:EmployeeStockMember2017-09-300001698530us-gaap:EmployeeStockMember2017-09-012017-09-300001698530us-gaap:EmployeeStockMember2021-05-142021-05-140001698530us-gaap:EmployeeStockMember2021-01-012021-01-010001698530us-gaap:EmployeeStockMember2020-01-012020-01-010001698530us-gaap:EmployeeStockMember2021-09-300001698530us-gaap:ResearchAndDevelopmentExpenseMember2021-07-012021-09-300001698530us-gaap:ResearchAndDevelopmentExpenseMember2020-07-012020-09-300001698530us-gaap:ResearchAndDevelopmentExpenseMember2021-01-012021-09-300001698530us-gaap:ResearchAndDevelopmentExpenseMember2020-01-012020-09-300001698530us-gaap:GeneralAndAdministrativeExpenseMember2021-07-012021-09-300001698530us-gaap:GeneralAndAdministrativeExpenseMember2020-07-012020-09-300001698530us-gaap:GeneralAndAdministrativeExpenseMember2021-01-012021-09-300001698530us-gaap:GeneralAndAdministrativeExpenseMember2020-01-012020-09-300001698530srt:MinimumMember2021-01-012021-09-300001698530srt:MaximumMember2021-01-012021-09-300001698530srt:MinimumMember2020-01-012020-09-300001698530srt:MaximumMember2020-01-012020-09-300001698530srt:WeightedAverageMember2021-01-012021-09-300001698530srt:WeightedAverageMember2020-01-012020-09-300001698530srt:MinimumMember2021-09-300001698530srt:MaximumMember2021-09-300001698530srt:WeightedAverageMember2021-09-300001698530srt:MinimumMember2020-09-300001698530srt:MaximumMember2020-09-300001698530srt:WeightedAverageMember2020-09-3000016985302020-01-012020-12-310001698530us-gaap:RestrictedStockUnitsRSUMember2020-12-310001698530us-gaap:RestrictedStockUnitsRSUMember2021-01-012021-09-300001698530us-gaap:RestrictedStockUnitsRSUMember2021-09-300001698530us-gaap:EmployeeStockOptionMember2021-01-012021-09-300001698530us-gaap:EmployeeStockOptionMember2020-01-012020-09-300001698530us-gaap:RestrictedStockUnitsRSUMember2021-01-012021-09-300001698530us-gaap:RestrictedStockUnitsRSUMember2020-01-012020-09-300001698530us-gaap:WarrantMember2021-01-012021-09-300001698530us-gaap:WarrantMember2020-01-012020-09-300001698530us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:FairValueInputsLevel1Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2021-09-300001698530us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CorporateDebtSecuritiesMember2021-09-300001698530us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CorporateDebtSecuritiesMember2021-09-300001698530us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CorporateDebtSecuritiesMember2021-09-300001698530us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel3Member2021-09-300001698530us-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:FairValueInputsLevel1Memberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:FairValueInputsLevel2Memberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2021-09-300001698530us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:CommercialPaperMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:CommercialPaperMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2021-09-300001698530us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CorporateDebtSecuritiesMember2021-09-300001698530us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CorporateDebtSecuritiesMember2021-09-300001698530us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CorporateDebtSecuritiesMember2021-09-300001698530us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel3Member2021-09-300001698530us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2021-09-300001698530us-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2021-09-300001698530us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2021-09-300001698530us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:FairValueInputsLevel1Memberus-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:MoneyMarketFundsMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2020-12-310001698530us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:CommercialPaperMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:CommercialPaperMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:CommercialPaperMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2020-12-310001698530us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CorporateDebtSecuritiesMember2020-12-310001698530us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CorporateDebtSecuritiesMember2020-12-310001698530us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:CorporateDebtSecuritiesMember2020-12-310001698530us-gaap:FairValueMeasurementsRecurringMemberus-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel3Member2020-12-310001698530us-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:FairValueInputsLevel1Memberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:FairValueInputsLevel2Memberus-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:USTreasurySecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2020-12-310001698530us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2020-12-310001698530us-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:FairValueInputsLevel1Memberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:FairValueInputsLevel2Memberus-gaap:FairValueMeasurementsRecurringMember2020-12-310001698530us-gaap:FairValueMeasurementsRecurringMemberus-gaap:FairValueInputsLevel3Member2020-12-310001698530xcur:WarrantLiabilityMemberus-gaap:CommonStockMember2020-12-310001698530xcur:WarrantLiabilityMemberus-gaap:CommonStockMember2021-01-012021-09-300001698530xcur:WarrantLiabilityMemberus-gaap:CommonStockMember2021-09-300001698530xcur:ConsultingServicesMembersrt:DirectorMember2020-01-012020-09-300001698530xcur:ConsultingServicesMembersrt:DirectorMember2021-01-012021-09-30
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
______________________________________
FORM 10-Q
______________________________________
☒ QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the quarterly period ended September 30, 2021
or
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number: 001-39011
______________________________________
EXICURE, INC.
(Exact name of registrant as specified in its charter)
_____________________________________
| | | | | | | | |
Delaware | | 81-5333008 |
(State or other jurisdiction of incorporation or organization) | | (IRS Employer Identification No.) |
2430 N. Halsted St.
Chicago, IL 60614
(Address of principal executive offices)
Registrant’s telephone number, including area code (847) 673-1700
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | |
| Title of each class | | Trading symbol(s) | | Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share | | XCUR | | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | |
Large accelerated filer | ☐ | | Accelerated filer | ☐ |
Non-accelerated filer | ☒ | | Smaller reporting company | ☒ |
| | | Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.☒
Indicate by check mark whether the registrant is a shell company (as defined by Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of November 12, 2021, there were 88,120,951 shares of the registrant’s common stock, par value $0.0001 per share, outstanding.
EXICURE, INC.
QUARTERLY REPORT ON FORM 10-Q
TABLE OF CONTENTS
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q, including the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” contains express or implied “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. All statements other than statements of historical fact contained in this Quarterly Report on Form 10-Q are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “could,” “will,” “would,” “should,” “expect,” “plan,”, “anticipate,” “believe,” “estimate,” “intend,” “predict,” “seek,” “contemplate,” “project,” “continue,” “potential,” “ongoing” or the negative of these terms or other comparable terminology. Forward-looking statements also include the assumptions underlying or relating to such statements.
Although we believe that the expectations reflected in the forward-looking statements contained herein are reasonable, such expectations or any of the forward-looking statements may prove to be incorrect and actual results could differ materially from those projected or assumed in the forward-looking statements. Our future financial condition and results of operations, as well as any forward-looking statements, are subject to inherent risks and uncertainties, including, but not limited to, the risk factors described in the “Risk Factor Summary” below and set forth in Part II, Item 1A “Risk Factors” below and for the reasons described elsewhere in this Quarterly Report on Form 10-Q. All forward-looking statements and reasons why results may differ included in this report are made as of the date hereof and we do not intend to update any forward-looking statements except as required by law or applicable regulations. These forward-looking statements include, but are not limited to, statements concerning the following:
•our ability to raise substantial additional capital to fund our planned operations in the near term and to continue as a going concern;
•our expectations regarding the impact of the ongoing COVID-19 pandemic including the expected duration of disruption and immediate and long-term delays, interruptions or other adverse effects to clinical trials, patient enrollment and clinical activation, delays in regulatory review, preclinical research and development, or R&D, collaboration and partnership programs, manufacturing and supply chain interruptions, adverse effects on healthcare systems and disruption of the global economy overall, and the overall impact of the COVID-19 pandemic on our business, financial condition and results of operations;
•our estimates of expenses, use of cash, timing of future cash needs, ongoing losses, future revenue, including our estimates used to determine revenue recognition in connection with our collaboration agreements, and capital requirements, including our expectations relating to our needs for additional financing;
•the initiation, timing, progress and results of our current and future preclinical studies, clinical trials, collaboration and partnership programs, including our ongoing clinical trials and any planned clinical trials for XCUR-FXN, cavrotolimod (AST-008) or any of our other product candidates, and the R&D programs we are pursuing or may pursue;
•our ability to advance our product candidates into, and successfully complete, clinical trials;
•the timing and likelihood of regulatory filings for our current and future product candidates including any Investigational New Drug, or IND, application, Investigational Medicinal Product Dossier, or IMPD, Clinical Trial Application, or CTA, New Drug Application, or NDA, or other regulatory submissions;
•our ability to obtain and maintain regulatory approval of our product candidates in the indications for which we plan to develop them, and any related restrictions, limitations or warnings in the label of an approved drug or therapy;
•the size and growth potential of the markets for our product candidates, if approved, and the rate and degree of market acceptance of our product candidates, including reimbursement that may be received from payors;
•the diversion of healthcare resources away from the conduct of clinical trials as a result of the ongoing COVID-19 pandemic, including the diversion of hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our clinical trials;
•the interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel, quarantines or social distancing protocols imposed or recommended by federal or state governments, employers and others or voluntarily adopted in connection with the ongoing COVID-19 pandemic;
•our dependence on current and future collaborators for advancement of therapeutic candidates pursuant to the terms of such collaborations, including ability to obtain and maintain regulatory approval and commercialization, if approved;
•the status of clinical trials, development timelines and discussions with regulatory authorities related to product candidates under development by us and our collaborators;
•our receipt and timing of any milestone payments or royalties under any current or future research collaboration and license agreements or arrangements;
•our ability to identify and develop therapeutic candidates for treatment of additional disease indications;
•the rate and degree of market acceptance of any approved therapeutic candidates;
•the commercialization of any approved therapeutic candidates;
•the implementation of our business model and strategic plans for our business, technologies and therapeutic candidates;
•our ability to obtain additional funds for our operations;
•our ability to obtain and maintain intellectual property protection for our technologies and therapeutic candidates and our ability to operate our business without infringing the intellectual property rights of others;
•our reliance on third parties to conduct our preclinical studies and clinical trials;
•our reliance on third party supply and manufacturing partners to supply the materials and components for, and manufacture, our R&D, preclinical and clinical trial supplies;
•our expectations regarding our ability to attract and retain qualified key management and technical personnel;
•our expectations regarding the time during which we will be an emerging growth company under the Jumpstart Our Business Startups Act of 2012;
•the impact of government laws and regulations as well as developments relating to our competitors or our industry; and
•other factors that may impact our financial and clinical results.
These statements relate to future events or our future operational or financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by these forward-looking statements. Factors that may cause actual results to differ materially from current expectations include,
among other things, those listed in Part II, Item 1A of this Quarterly Report on Form 10-Q under the section titled “Risk Factors” and elsewhere in this Quarterly Report on Form 10-Q.
Any forward-looking statement in this Quarterly Report on Form 10-Q reflects our current view with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our business, results of operations, industry and future growth. Given these uncertainties, you should not place undue reliance on these forward-looking statements. No forward-looking statement is a guarantee of future performance. You should read this Quarterly Report on Form 10-Q and the documents that we reference herein and have filed with the SEC as exhibits thereto completely and with the understanding that our actual future results may be materially different from any future results expressed or implied by these forward-looking statements. Except as required by law, we assume no, and specifically decline any, obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
This Quarterly Report on Form 10-Q also contains or may contain estimates, projections and other information concerning our industry, our business and the markets for certain therapeutics, including data regarding the estimated size of those markets, their projected growth rates and the incidence of certain medical conditions. Information that is based on estimates, forecasts, projections or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Unless otherwise expressly stated, we obtained these industry, business, market and other data from reports, research surveys, studies and similar data prepared by third parties, industry, medical and general publications, government data and similar sources. In some cases, we do not expressly refer to the sources from which these data are derived.
Except where the context otherwise requires, in this Quarterly Report on Form 10-Q, the “Company,” “Exicure,” “we,” “us” and “our” refers to Exicure, Inc., a Delaware corporation, and, where appropriate, its subsidiary.
SUMMARY RISK FACTORS
Investing in common stock involves numerous risks, including the risks described in “Item 1A. Risk Factors” of this Quarterly Report on Form 10-Q. Below are some of these risks, any one of which could materially adversely affect our business, financial condition, results of operations and prospects.
•Our consolidated financial statements have been prepared assuming that we will continue as a going concern.
•We are a clinical-stage biotechnology company with a history of losses. We expect to continue to incur significant losses for the foreseeable future and may never achieve or maintain profitability, which could result in a decline in the market value of our common stock.
•Our approach to the discovery and development of innovative therapeutic treatments based on our technology is unproven and may not result in marketable products.
•Our therapeutic candidates are in early stages of development and may fail in development or suffer delays that materially and adversely affect their commercial viability.
•Product development involves a lengthy and expensive process with an uncertain outcome, and results of earlier preclinical studies and clinical trials may not be predictive of future clinical trial results.
•We will need substantial additional funds to advance the development of our therapeutic candidates, and we cannot guarantee that we will have sufficient funds available in the future to develop and commercialize our current or future therapeutic candidates.
•Our business could be adversely affected by the effects of health epidemics, including the global COVID‑19 pandemic, in regions where we or third parties on which we rely have business operations and at our clinical trial sites, as well as the business or operations of our contract research organizations, or CROs, or other third parties with whom we conduct business.
•If we continue to experience delays or difficulties in the enrollment of patients in clinical trials, our receipt of necessary regulatory approvals could be further delayed or prevented.
•Our quarterly operating results may fluctuate significantly or may fall below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline.
•We may not successfully engage in strategic transactions, including any additional collaborations we seek, which could adversely affect our ability to develop and commercialize product candidates, impact our cash position, increase our expense and present significant distractions to our management.
•If third parties on which we depend to conduct our preclinical studies and clinical trials do not perform as contractually required, fail to satisfy regulatory or legal requirements, or miss expected deadlines, our development program could be delayed with materially adverse effects on our business, financial condition, results of operations and prospects.
•Because we rely on third-party manufacturing and supply partners, our supply of research and development, preclinical studies and clinical trial materials may become limited or interrupted or may not be of satisfactory quantity or quality.
•We face competition from entities that have developed or may develop therapeutic candidates for our target disease indications, including companies developing novel treatments and technology platforms based on modalities and technology similar to ours. If these companies develop technologies, including delivery technologies, or therapeutic candidates more rapidly than we do or their technologies are more effective, our ability to develop and successfully commercialize therapeutic candidates may be adversely affected.
•The market may not be receptive to our therapeutic candidates based on a novel therapeutic modality, and we may not generate any future revenue from the sale or licensing of therapeutic candidates.
•Any inability to attract and retain qualified key management and technical personnel would impair our ability to implement our business plan.
•We currently license patent rights from Northwestern University and may in the future license patent rights from third-party owners or licensees. If Northwestern University or such other owners or licensees do not properly or successfully obtain, maintain or enforce the patents underlying such licenses, or if they retain or license to others any competing rights, our competitive position and business prospects may be adversely affected.
•Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
PART I - FINANCIAL INFORMATION
Item 1. Financial Statements.
EXICURE, INC.
UNAUDITED CONDENSED CONSOLIDATED BALANCE SHEETS
(in thousands, except share and per share data)
| | | | | | | | | | | |
| September 30,
2021 | | December 31,
2020 |
| | | |
| ASSETS | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 51,885 | | | $ | 33,262 | |
| Short-term investments | 8,953 | | | 48,818 | |
| Accounts receivable | — | | | 11 | |
| | | |
| Prepaid expenses and other assets | 3,309 | | | 4,231 | |
| Total current assets | 64,147 | | | 86,322 | |
| Property and equipment, net | 4,171 | | | 4,123 | |
| Right-of-use asset | 8,117 | | | 8,606 | |
| Other noncurrent assets | 1,465 | | | 1,393 | |
| Total assets | $ | 77,900 | | | $ | 100,444 | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY | | | |
| Current liabilities: | | | |
| | | |
| Accounts payable | $ | 2,485 | | | $ | 1,866 | |
| Accrued expenses and other current liabilities | 5,480 | | | 3,525 | |
| Deferred revenue, current | 14,015 | | | 8,343 | |
| Total current liabilities | 21,980 | | | 13,734 | |
| Long-term debt, net | 16,801 | | | 16,589 | |
| | | |
| Deferred revenue, noncurrent | 16,929 | | | — | |
| Lease liability, noncurrent | 7,551 | | | 7,959 | |
| Other noncurrent liabilities | 656 | | | 656 | |
| | | |
| Total liabilities | $ | 63,917 | | | $ | 38,938 | |
| | | |
| Stockholders’ equity: | | | |
Preferred stock, $0.0001 par value per share; 10,000,000 shares authorized, no shares issued and outstanding, September 30, 2021 and December 31, 2020 | — | | | — | |
Common stock, $0.0001 par value per share; 200,000,000 shares authorized, 88,108,543 issued and outstanding, September 30, 2021; 87,651,352 issued and outstanding, December 31, 2020 | 9 | | | 9 | |
| Additional paid-in capital | 170,217 | | | 167,379 | |
| Accumulated other comprehensive (loss) income | (1) | | | 83 | |
| Accumulated deficit | (156,242) | | | (105,965) | |
| Total stockholders' equity | 13,983 | | | 61,506 | |
| Total liabilities and stockholders’ equity | $ | 77,900 | | | $ | 100,444 | |
See accompanying notes to the unaudited condensed consolidated financial statements.
EXICURE, INC.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
September 30, | | Nine Months Ended
September 30, |
| 2021 | | 2020 | | 2021 | | 2020 |
| Revenue: | | | | | | | |
| Collaboration revenue | $ | (3,677) | | | $ | 2,443 | | | $ | (2,601) | | | $ | 16,473 | |
| Total revenue | (3,677) | | | 2,443 | | | (2,601) | | | 16,473 | |
| Operating expenses: | | | | | | | |
| Research and development expense | 16,457 | | | 9,139 | | | 37,562 | | | 22,222 | |
| General and administrative expense | 2,947 | | | 2,424 | | | 8,937 | | | 7,227 | |
| Total operating expenses | 19,404 | | | 11,563 | | | 46,499 | | | 29,449 | |
| Operating loss | (23,081) | | | (9,120) | | | (49,100) | | | (12,976) | |
| Other (expense) income, net: | | | | | | | |
| Dividend income | 2 | | | 2 | | | 5 | | | 45 | |
| Interest income | 8 | | | 205 | | | 139 | | | 832 | |
| Interest expense | (455) | | | (27) | | | (1,314) | | | (155) | |
| Other (expense) income, net | (5) | | | 118 | | | (7) | | | 271 | |
| Total other (expense) income, net | (450) | | | 298 | | | (1,177) | | | 993 | |
| Net loss before provision for income taxes | (23,531) | | | (8,822) | | | (50,277) | | | (11,983) | |
| Provision for income taxes | — | | | — | | | — | | | — | |
| Net loss | $ | (23,531) | | | $ | (8,822) | | | $ | (50,277) | | | $ | (11,983) | |
| | | | | | | |
| Basic and diluted loss per common share | $ | (0.27) | | | $ | (0.10) | | | $ | (0.57) | | | $ | (0.14) | |
| | | | | | | |
| | | | | | | |
| Weighted-average basic and diluted common shares outstanding | 88,105,066 | | | 87,227,136 | | | 88,001,222 | | | 87,160,520 | |
| | | | | | | |
See accompanying notes to the unaudited condensed consolidated financial statements.
EXICURE, INC.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE (LOSS) INCOME
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
September 30, | | Nine Months Ended
September 30, |
| 2021 | | 2020 | | 2021 | | 2020 |
| Net loss | $ | (23,531) | | | $ | (8,822) | | | $ | (50,277) | | | $ | (11,983) | |
| Other comprehensive (loss) income, net of taxes | | | | | | | |
| Unrealized (losses) gains on available for sale securities, net of tax | (6) | | | (138) | | | (84) | | | 227 | |
| Other comprehensive (loss) income | (6) | | | (138) | | | (84) | | | 227 | |
| Comprehensive loss | $ | (23,537) | | | $ | (8,960) | | | $ | (50,361) | | | $ | (11,756) | |
See accompanying notes to the unaudited condensed consolidated financial statements.
EXICURE, INC.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(in thousands, except shares)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | | | | | | | |
| Shares | | $ | | Additional Paid-in- Capital | | Accumulated Deficit | | Accumulated Other Comprehensive Income (Loss) | | Total Stockholders' Equity |
| Balance at December 31, 2020 | 87,651,352 | | | $ | 9 | | | $ | 167,379 | | | $ | (105,965) | | | $ | 83 | | | $ | 61,506 | |
| Exercise of options | 342,246 | | | — | | | 546 | | | — | | | — | | | 546 | |
| Equity-based compensation | — | | | — | | | 517 | | | — | | | — | | | 517 | |
| | | | | | | | | | | |
| Other comprehensive loss, net | — | | | — | | | — | | | — | | | (51) | | | (51) | |
| Net loss | — | | | — | | | — | | | (12,477) | | | — | | | (12,477) | |
| Balance at March 31, 2021 | 87,993,598 | | | $ | 9 | | | $ | 168,442 | | | $ | (118,442) | | | $ | 32 | | | $ | 50,041 | |
| Equity-based compensation | — | | | — | | | 764 | | | — | | | — | | | 764 | |
| Issuance of common stock-ESPP | 103,096 | | | — | | | 131 | | | — | | | — | | | 131 | |
| Other comprehensive loss, net | — | | | — | | | — | | | — | | | (27) | | | (27) | |
| Net loss | — | | | — | | | — | | | (14,269) | | | — | | | (14,269) | |
| Balance at June 30, 2021 | 88,096,694 | | | $ | 9 | | | $ | 169,337 | | | $ | (132,711) | | | $ | 5 | | | $ | 36,640 | |
| Equity-based compensation | — | | | — | | | 889 | | | — | | | — | | | 889 | |
| Vesting of restricted stock units and related repurchases | 11,849 | | | — | | | (9) | | | — | | | — | | | (9) | |
| Other comprehensive loss, net | — | | | — | | | — | | | — | | | (6) | | | (6) | |
| Net loss | — | | | — | | | — | | | (23,531) | | | — | | | (23,531) | |
| Balance at September 30, 2021 | 88,108,543 | | | $ | 9 | | | $ | 170,217 | | | $ | (156,242) | | | $ | (1) | | | $ | 13,983 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
EXICURE, INC.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(in thousands, except shares)
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Common Stock | | | | | | | | |
| Shares | | $ | | Additional Paid-in- Capital | | Accumulated Deficit | | Accumulated Other Comprehensive Income (Loss) | | Total Stockholders’ Equity |
| Balance at December 31, 2019 | 86,069,263 | | | $ | 9 | | | $ | 162,062 | | | $ | (81,297) | | | $ | (27) | | | $ | 80,747 | |
| | | | | | | | | | | |
| Equity-based compensation | — | | | — | | | 441 | | | — | | | — | | | 441 | |
| Issuance of common stock | 1,081,184 | | | — | | | 2,766 | | | — | | | — | | | 2,766 | |
| Other comprehensive income, net | — | | | — | | | — | | | — | | | 10 | | | 10 | |
| Net income | — | | | — | | | — | | | 1,150 | | | — | | | 1,150 | |
| Balance at March 31, 2020 | 87,150,447 | | | $ | 9 | | | $ | 165,269 | | | $ | (80,147) | | | $ | (17) | | | $ | 85,114 | |
| Exercise of options | 55,899 | | | — | | | 50 | | | — | | | — | | | 50 | |
| Equity-based compensation | — | | | — | | | 544 | | | — | | | — | | | 544 | |
| Other comprehensive income, net | — | | | — | | | — | | | — | | | 355 | | | 355 | |
| Net loss | — | | | — | | | — | | | (4,311) | | | — | | | (4,311) | |
| Balance at June 30, 2020 | 87,206,346 | | | $ | 9 | | | $ | 165,863 | | | $ | (84,458) | | | $ | 338 | | | $ | 81,752 | |
| Exercise of options | 22,240 | | | — | | | $ | 19 | | | $ | — | | | $ | — | | | $ | 19 | |
| Equity-based compensation | — | | | — | | | $ | 617 | | | $ | — | | | $ | — | | | $ | 617 | |
| Other comprehensive loss, net | — | | | — | | | $ | — | | | $ | — | | | $ | (138) | | | $ | (138) | |
| Net loss | — | | | — | | | — | | | (8,822) | | | — | | | (8,822) | |
| Balance at September 30, 2020 | 87,228,586 | | | $ | 9 | | | $ | 166,499 | | | $ | (93,280) | | | $ | 200 | | | $ | 73,428 | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See accompanying notes to the unaudited condensed consolidated financial statements.
EXICURE, INC.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| | | | | | | | | | | |
| Nine Months Ended September 30, |
| 2021 | | 2020 |
| Cash flows from operating activities: | | | |
| Net loss | $ | (50,277) | | | $ | (11,983) | |
| Adjustments to reconcile net loss to cash used in operating activities: | | | |
| Depreciation and amortization | 829 | | | 494 | |
| | | |
| Amortization of right-of-use asset | 489 | | | 518 | |
| Equity-based compensation | 2,170 | | | 1,603 | |
| Amortization of long-term debt issuance costs and fees | 213 | | | 39 | |
| Amortization of investments | 183 | | | 202 | |
| Other | 6 | | | 67 | |
| Change in fair value of warrant liabilities | (15) | | | (343) | |
| Changes in operating assets and liabilities: | | | |
| Accounts receivable | 11 | | | (10) | |
| Prepaid expenses and other current assets | 922 | | | (1,473) | |
| Other noncurrent assets | (72) | | | (181) | |
| Accounts payable | 629 | | | 535 | |
| Accrued expenses | 1,697 | | | (461) | |
| Deferred revenue | 22,601 | | | (16,351) | |
| Other liabilities | (408) | | | (119) | |
| Net cash used in operating activities | (21,022) | | | (27,463) | |
| Cash flows from investing activities: | | | |
| Purchase of available for sale securities | (6,497) | | | (47,349) | |
| Proceeds from sale or maturity of available for sale securities | 46,097 | | | 47,093 | |
| Capital expenditures | (623) | | | (3,074) | |
| Net cash provided by (used in) investing activities | 38,977 | | | (3,330) | |
| Cash flows from financing activities: | | | |
| Proceeds from common stock offering | — | | | 2,973 | |
| Payment of common stock financing costs | — | | | (207) | |
| Proceeds from long-term borrowing | — | | | 17,500 | |
| Payment of long-term debt fees and issuance costs | — | | | (344) | |
| Repayment of long-term debt | — | | | (4,999) | |
| Proceeds from issuance of employee stock purchase plan | 131 | | | — | |
| Proceeds from exercise of common stock options | 546 | | | 69 | |
| Payments for minimum statutory tax withholding related to net share settlement of equity awards | (9) | | | — | |
| Net cash provided by financing activities | 668 | | | 14,992 | |
| | | |
| Net increase (decrease) in cash, cash equivalents, and restricted cash | 18,623 | | | (15,801) | |
| Cash, cash equivalents, and restricted cash - beginning of period | 34,462 | | | 48,460 | |
| Cash, cash equivalents, and restricted cash - end of period | $ | 53,085 | | | $ | 32,659 | |
EXICURE, INC.
UNAUDITED CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS (continued)
(in thousands)
| | | | | | | | | | | |
| Nine Months Ended September 30, |
| 2021 | | 2020 |
| Supplemental disclosure of cash flow information: | | | |
| Non-cash operating activities: | | | |
| Right-of-use asset acquired through operating leases | $ | — | | | $ | 8,147 | |
| Non-cash investing activities: | | | |
| Capital expenditures (accounts payable and accrued expenses) | $ | 303 | | | $ | 13 | |
| Non-cash financing activities: | | | |
| Debt fees (accrued expense and other noncurrent liabilities) | $ | — | | | $ | 834 | |
The following table provides a reconciliation of cash, cash equivalents, and restricted cash reported within the unaudited condensed consolidated balance sheets that sum to the total of the amounts shown in the unaudited condensed consolidated statements of cash flows:
| | | | | | | | | | | |
| September 30,
2021 | | December 31,
2020 |
| Cash and cash equivalents | $ | 51,885 | | | $ | 33,262 | |
| Restricted cash included in other noncurrent assets | 1,200 | | | 1,200 | |
| Total cash, cash equivalents, and restricted cash shown in the unaudited condensed consolidated statements of cash flows | $ | 53,085 | | | $ | 34,462 | |
See accompanying notes to the unaudited condensed consolidated financial statements.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
1. Description of Business and Basis of Presentation
Description of Business
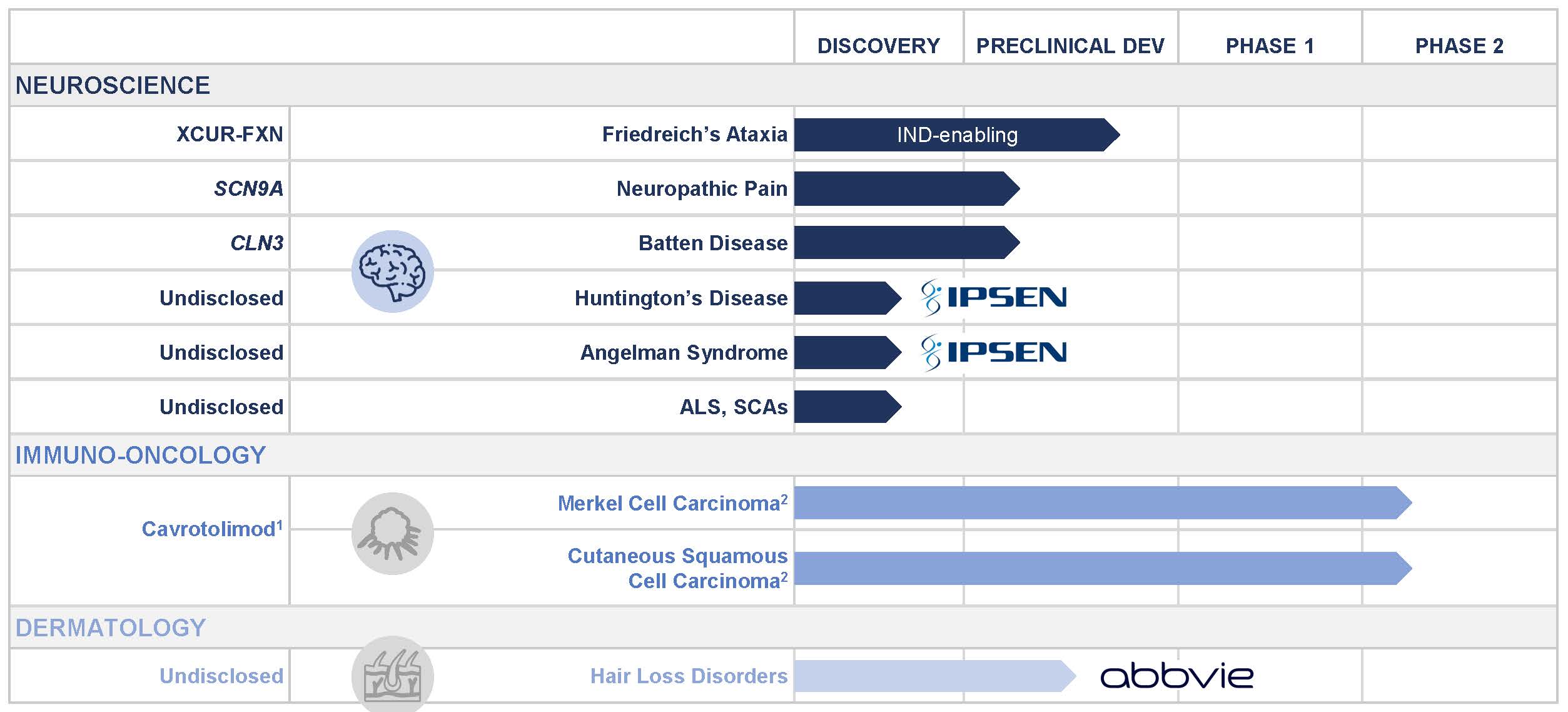
Exicure, Inc. (the “Company”) is a clinical-stage biotechnology company developing therapeutics for neurology, immuno-oncology, inflammatory diseases, and other genetic disorders based on its proprietary Spherical Nucleic Acid, or SNA™, technology. The Company believes that its proprietary SNA architecture has distinct chemical and biological properties that may provide advantages over other nucleic acid therapeutics and may have therapeutic potential to target diseases not typically addressed with other nucleic acid therapeutics. The Company is in preclinical development of XCUR-FXN, a lipid-nanoparticle SNA-based therapeutic candidate, for the intrathecal treatment of Friedreich’s ataxia (“FA”). The Company’s therapeutic candidate cavrotolimod (AST-008) is in a Phase 1b/2 clinical trial in patients with advanced solid tumors.
The Company believes one of the key strengths of its proprietary SNAs is that they have the potential for increased cellular uptake compared to conventional linear oligonucleotides and as a result the potential to achieve higher efficacy at the same doses of oligonucleotide administered. The Company has shown in clinical and preclinical studies that SNAs may have therapeutic potential in immuno-oncology and dermatology. In addition, the Company has shown in preclinical studies that SNAs may have therapeutic potential in neurology, ophthalmology, pulmonology, and gastroenterology. Accordingly, the Company has expanded its pipeline into neurology, for which investigational new drug application (“IND”)-enabling activities are ongoing for XCUR-FXN, and is concluding early stage research activities in ophthalmology, pulmonology, and gastroenterology.
Throughout these unaudited condensed consolidated financial statements, the terms the “Company,” “Exicure,” “we,” “us,” and “our” refer to Exicure, Inc. and where appropriate, its wholly owned subsidiary, Exicure Operating Company. Exicure Operating Company holds all material assets and conducts all business activities and operations of Exicure, Inc.
Basis of Presentation
The accompanying unaudited condensed consolidated financial statements as of September 30, 2021 and December 31, 2020, and for the nine months ended September 30, 2021 and 2020, have been presented in conformity with generally accepted accounting principles in the United States (“GAAP”).
Principles of Consolidation
The accompanying unaudited condensed consolidated financial statements include the accounts of Exicure and its wholly owned subsidiary, Exicure Operating Company. All intercompany transactions and accounts are eliminated in consolidation.
Significant Risks and Uncertainties
As discussed in Note 3, Collaborative Research and License Agreements, revenue recognized under the AbbVie Collaboration Agreement (as defined in Note 3, Collaborative Research and License Agreements) for the three and nine months ended September 30, 2021 reflects the cumulative catchup adjustment (reduction) of revenue of $(4,480) recorded in connection with a change in estimate that occurred during the third quarter of 2021. The Company currently estimates significant additional efforts will be required to satisfy the performance obligation under the AbbVie Collaboration Agreement. These increased estimated efforts in connection with the change in workplan resulted in less progress occurring relative to the increased estimate of total project hours to complete the research services during the three and nine months ended September 30, 2021 as compared to the amount of revenue recognized at both June 30, 2021 and December 31, 2020, which led to revenue reversals in each respective period. Due to uncertainties inherent in the estimation process, it is at least reasonably possible that estimated efforts required to complete the research services under the AbbVie Collaboration Agreement will be further revised in the
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
near-term which may result in additional adjustments (reductions of revenue) in future periods. As discussed in Note 2, Significant Accounting Policies — Revenue Recognition, determining the estimate of total project hours used to recognize revenue for our collaboration agreements requires significant judgment and any changes to those estimates may have a significant impact on the amount and timing of revenue recognition for the Ipsen Collaboration Agreement or the AbbVie Collaboration Agreement (each, as defined in Note 3, Collaborative Research and License Agreements) in future periods.
COVID-19 Risks and Uncertainties
In response to the ongoing COVID-19 pandemic, the Company has taken and continues to take active measures designed to address and mitigate the impact of the COVID-19 pandemic on its business, such as remote working policies, facilitating management’s routine communication to address employee and business concerns and providing frequent updates to the Company’s board of directors (the “Board”). During the third quarter of 2021 and through the date of this report, the Company continues to operate under COVID-19 social distancing guidelines and has generally operated with approximately 100% of its R&D staff on-site. The Company’s office and general and administrative team is also working on-site approximately 50% of the time. The Company’s preclinical development program in Friedreich’s ataxia (“FA”) is ongoing and it began IND-enabling studies for XCUR-FXN in late 2020. The Company also continues research and development activities with its collaboration partners, Ipsen Biopharm Limited (“Ipsen”) and AbbVie Inc. (“AbbVie”). However, if the COVID-19 pandemic continues to persist for an extended period of time, the Company could experience further significant disruptions to its clinical and preclinical development timelines, which would adversely affect its business, financial condition, results of operations and growth prospects.
The Company believes that the effects of the COVID-19 pandemic or its impact contributed to delays that began during the third quarter of 2020 and continued into the second half of 2021 in its enrollment plans and clinical trial site start-ups for the Phase 2 dose expansion phase of the Phase 1b/2 clinical trial for its cavrotolimod (AST-008) clinical program. As a result of these delays, pending further delays or any additional unanticipated effects of the COVID-19 pandemic on its clinical development plans, the Company now expects to report overall response rate (“ORR”) results of cavrotolimod (AST-008) in the second half of 2022 as opposed to the previously reported first half of 2022. In the third quarter of 2020, the Company implemented, and continues to employ, additional measures to increase the enrollment of patients, including frequent interaction with its clinical trial sites currently open as well as increasing the number of clinical trial sites that potentially are activated for this trial so that the Company may continue to enroll patients as initially planned. However, any additional delays may require the Company to further lengthen its clinical development timeline for cavrotolimod (AST-008). The extent to which the COVID-19 pandemic or its impact or effects may continue to impact the Company’s business, its clinical development and regulatory efforts, its corporate development objectives and the value of and market for its common stock will depend on future developments that are highly uncertain and cannot be predicted with confidence at this time, such as the ultimate duration or spread of the COVID-19 pandemic, its impact and effects, the possibility of additional periods of increases or spikes in the number of COVID-19 cases, the introduction and spread of new variants of the virus, travel restrictions, quarantines, social distancing, phased re-openings and business closure requirements in the United States and other countries, and the effectiveness of actions taken globally to contain and treat the disease including, without limitation, the effectiveness and timing of vaccination initiatives in the United States and worldwide. The global economic slowdown, the overall disruption of global healthcare systems and the other risks and uncertainties associated with the COVID-19 pandemic could have a material adverse effect on the Company’s business, financial condition, results of operations and growth prospects.
In addition, the Company is subject to other challenges and risks specific to its business and its ability to execute on its business plan and strategy, as well as risks and uncertainties common to companies in the biotechnology industry with research and development operations, including, without limitation, risks and uncertainties associated with: obtaining regulatory approval of its product candidates; delays or problems in obtaining clinical supply, loss of single source suppliers or failure to comply with manufacturing regulations; identifying, acquiring or in-licensing additional products or product candidates; product development and the
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
inherent uncertainty of clinical success; and the challenges of protecting and enhancing its intellectual property rights; and the challenges of complying with applicable regulatory requirements. In addition, to the extent the ongoing COVID-19 pandemic adversely affects the Company’s business and results of operations, it may also have the effect of heightening many of the other risks and uncertainties.
Going Concern
At each reporting period, the Company evaluates whether there are conditions or events that raise substantial doubt about the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued. The Company is required to make certain additional disclosures if it concludes substantial doubt exists and it is not alleviated by the Company’s plans or when its plans alleviate substantial doubt about the Company’s ability to continue as a going concern.
As of September 30, 2021, the Company has generated an accumulated deficit of $175,079 since inception and expects to incur significant expenses and negative cash flows for the foreseeable future. Based on the Company’s current operating plans and existing working capital at September 30, 2021, it is uncertain whether our current liquidity is sufficient to fund operations over the next twelve months from the date of the issuance of these condensed consolidated financial statements. As a result, there is substantial doubt about our ability to continue as a going concern. The Company has no committed sources of additional capital at this time and substantial additional financing will be needed by the Company to fund its operations.
Management believes that it will be able to obtain additional funding through equity or debt financings, collaboration agreements, strategic partnerships and licensing arrangements, or other arrangements, such as its “at the market offering” program pursuant to its equity distribution agreement with BMO Capital Markets Corp., to fund its current operations and business strategy. However, there can be no assurance that such additional financing will be available and, if available, can be obtained on terms acceptable to the Company. If the Company is unable to obtain such additional financing, the Company could be forced to delay, reduce or eliminate its research and development programs or clinical efforts, which could adversely affect its business prospects, or the Company may be unable to continue operations. The Company has historically principally raised capital through the public offerings of its securities. However, the COVID-19 pandemic continues to rapidly evolve and has already resulted in a significant disruption of global financial markets. If the disruption continues to persist and deepens, the Company could experience an inability to access additional capital, which could negatively affect its ability to raise capital and ultimately, its operations, liquidity and ability to continue as a going concern.
The accompanying unaudited condensed consolidated interim financial statements have been prepared as though the Company will continue as a going concern, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
Unaudited Interim Financial Information
The accompanying interim condensed consolidated balance sheet as of September 30, 2021, the interim condensed consolidated statements of operations for the three and nine months ended September 30, 2021 and 2020, the interim condensed consolidated statements of comprehensive (loss) income for the three and nine months ended September 30, 2021 and 2020, the interim condensed consolidated statements of changes in stockholders’ equity for the three and nine months ended September 30, 2021 and 2020, and the interim condensed consolidated statements of cash flows for the nine months ended September 30, 2021 and 2020, are unaudited. The interim unaudited condensed consolidated financial statements have been prepared on the same basis as the annual audited financial statements; and in the opinion of management, reflect all adjustments, which include only normal recurring adjustments necessary for the fair statement of the Company’s financial position as of September 30, 2021, the results of its operations for the three and nine months ended September 30, 2021 and 2020, and the results of its cash flows for the nine months ended September 30, 2021 and 2020. The financial data and other information disclosed in
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
these notes related to the three and nine months ended September 30, 2021 and 2020 are unaudited. The results for the three and nine months ended September 30, 2021 are not necessarily indicative of results to be expected for the year ending December 31, 2021, or any other interim periods, or any future year or period.
Use of Estimates
The preparation of the financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Management bases its estimates on certain assumptions which it believes are reasonable in the circumstances and while actual results could differ from those estimates, management does not believe that any change in those assumptions in the near term would have a significant effect on the Company’s financial position, results of operations or cash flows. Actual results in future periods could differ from those estimates.
2. Significant Accounting Policies
The Company’s significant accounting policies are disclosed in the audited consolidated financial statements and the notes thereto, which are included in the in the Company’s Annual Report on Form 10-K for the year ended December 31, 2020 filed with the U.S. Securities and Exchange Commission (“SEC”) on March 11, 2021. Since the date of those audited consolidated financial statements, there have been no material changes to the Company’s significant accounting policies, except as noted below:
Revenue recognition
Effective January 1, 2018, we adopted the provisions of Accounting Standards Codification, or ASC 606, Revenue from Contracts with Customers using the modified retrospective method for all contracts not completed as of the date of adoption.
Under ASC 606, we recognize revenue when our customer obtains control of promised goods or services, in an amount that reflects the consideration which we expect to receive in exchange for those goods or services. To determine revenue recognition for arrangements that are within the scope of ASC 606, we perform the following five steps:
1.Identify the contract with the customer. A contract with a customer exists when (i) we enter into an enforceable contract with a customer that defines each party’s rights and obligations regarding the goods or services to be transferred and identifies the related payment terms, (ii) the contract has commercial substance, and (iii) we determine that collection of substantially all consideration for goods and services that are transferred is probable based on the customer’s intent and ability to pay the promised consideration. We apply judgment in determining the customer’s intent and ability to pay, which is based on a variety of factors including the customer’s historical payment experience, or in the case of a new customer, published credit and financial information pertaining to the customer.
2.Identify the performance obligations in the contract. Performance obligations promised in a contract are identified based on the goods and services that will be transferred to the customer that are both capable of being distinct, whereby the customer can benefit from the good or service either on its own or together with other available resources, and are distinct in the context of the contract, whereby the transfer of the good or service is separately identifiable from other promises in the contract. To the extent a contract includes multiple promised goods and services, we must apply judgment to determine whether promised goods and services are both capable of being distinct and distinct in the context of the contract. If these criteria are not met, the promised goods and services are accounted for as a combined performance obligation.
3.Determine the transaction price. The transaction price is determined based on the consideration to which we will be entitled in exchange for transferring goods and services to the customer. To the extent the transaction price includes variable consideration, we estimate the amount of variable consideration that
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
should be included in the transaction price utilizing either the expected value method or the most likely amount method, depending on the nature of the variable consideration. Variable consideration is included in the transaction price if, in our judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. Any estimates, including the effect of the constraint on variable consideration, are evaluated at each reporting period for any changes. Determining the transaction price requires significant judgment.
4.Allocate the transaction price to performance obligations in the contract. If the contract contains a single performance obligation, the entire transaction price is allocated to the single performance obligation. However, if a series of distinct services that are substantially the same qualifies as a single performance obligation in a contract with variable consideration, we must determine if the variable consideration is attributable to the entire contract or to a specific part of the contract. Contracts that contain multiple performance obligations require an allocation of the transaction price to each performance obligation on a relative standalone selling price basis unless the transaction price is variable and meets the criteria to be allocated entirely to a performance obligation or to a distinct service that forms part of a single performance obligation. The consideration to be received is allocated among the separate performance obligations based on relative standalone selling prices.
5.Recognize revenue when or as the Company satisfies a performance obligation. We satisfy performance obligations either over time or at a point in time. Revenue is recognized over time if either (i) the customer simultaneously receives and consumes the benefits provided by the entity’s performance, (ii) the entity’s performance creates or enhances an asset that the customer controls as the asset is created or enhanced, or (iii) the entity’s performance does not create an asset with an alternative use to the entity and the entity has an enforceable right to payment for performance completed to date. If the entity does not satisfy a performance obligation over time, the related performance obligation is satisfied at a point in time by transferring the control of a promised good or service to a customer. Examples of control are using the asset to produce goods or services, enhance the value of other assets, or settle liabilities, and holding or selling the asset.
Revenue allocated to performance obligations relating to provision of research and development activities is recognized as the performance obligations are satisfied using an input method to measure progress, based on an estimate of the percentage of completion of the project based on the actual hours incurred on the project as a percentage of the total expected project hours. The determination of the percentage of completion requires management to estimate the total expected project hours. A detailed estimate of the total expected project hours is re-assessed every reporting period based on the latest project plan and discussions with project teams. If a change in facts or circumstances occurs, the estimate will be adjusted and the revenue will be recognized based on the revised estimate. The difference between the cumulative revenue recognized based on the previous estimate and the revenue recognized based on the revised estimate would be recognized as an adjustment to revenue in the period in which the change in estimate occurs. Determining the estimate of total project hours requires significant judgment and may have a significant impact on the amount and timing of revenue recognition. For example, as discussed in Note 3, Collaborative Research and License Agreements, revenue recognized under the AbbVie Collaboration Agreement (as defined in Note 3, Collaborative Research and License Agreements) for the three and nine months ended September 30, 2021 reflects the cumulative catchup adjustment (reduction) of revenue of $(4,480) recorded in connection with a change in estimate that occurred during the third quarter of 2021.
Licenses of intellectual property: If the license to our intellectual property is determined to be distinct from the other performance obligations identified in the arrangement, we recognize revenues from consideration allocated to the license when the license is transferred to the customer and the customer is able to use and benefit from the licenses. For licenses that are combined with other promises, we utilize judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
revenue. We evaluate the measure of progress each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition.
Milestone payments: At the inception of each arrangement that includes development milestone payments, we evaluate the probability of reaching the milestones and estimates the amount to be included in the transaction price using the most likely amount method. If it is probable that a significant revenue reversal would not occur in the future, the associated milestone value is included in the transaction price. Milestone payments that are not within our control or the licensee, such as regulatory approvals, are not considered probable of being achieved until those approvals are received and therefore revenue recognized is constrained as management is unable to assert that a reversal of revenue would not be possible. The transaction price is then allocated to each performance obligation on a relative standalone selling price basis, for which we recognize revenue as or when the performance obligations under the contract are satisfied. At the end of each subsequent reporting period, we re-evaluate the probability of achievement of such development milestones and any related constraint, and if necessary, adjusts its estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect collaboration revenues and earnings in the period of adjustment. To date, we have not recognized any milestone payment revenue from any of its collaboration agreements.
Royalties: For arrangements that include sales-based royalties, including milestone payments based on levels of sales, and the license is deemed to be the predominant item to which the royalties relate, we recognize revenue at the later of (i) when the related sales occur, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied). To date, we have not recognized any royalty revenue resulting from any of its collaboration agreements.
Recently Adopted Accounting Pronouncements
None.
Recent Accounting Pronouncements Not Yet Adopted
Financial Instruments - Credit Losses
In June 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) ASU 2016-13, Measurement of Credit Losses on Financial Instruments (“ASU 2016-13”). ASU 2016-13 is a new standard intended to improve reporting requirements specific to loans, receivables and other financial instruments. ASU 2016-13 requires that credit losses on financial assets measured at amortized cost be determined using an expected loss model, instead of the current incurred loss model, and requires that credit losses related to available-for-sale debt securities be recorded through an allowance for credit losses and limited to the amount by which carrying value exceeds fair value. ASU 2016-13 also requires enhanced disclosure of credit risk associated with financial assets. The effective date of ASU 2016-13 was deferred by ASU 2019-10, Financial Instruments—Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases (Topic 842)—Effective Dates to the annual period beginning after December 15, 2022 for companies that (i) meet the definition of an SEC filer and (ii) are eligible as “smaller reporting companies” as such term is defined by the SEC, with early adoption permitted. The Company is currently assessing the impact of adoption of ASU 2016-13 to its consolidated financial statements.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
3. Collaborative Research and License Agreements
Ipsen Collaboration Agreement
Summary of Agreement
On July 30, 2021 (the “Ipsen Effective Date”), the Company entered into a Collaboration, Option and License Agreement with Ipsen (the “Ipsen Collaboration Agreement”). Pursuant to the Ipsen Collaboration Agreement, the Company granted to Ipsen exclusive access and options to license SNA-based therapeutics arising from two collaboration programs related to the treatment of Huntington’s disease and Angelman syndrome (each, an “Ipsen Collaboration Program”), respectively. Each such license (obtained in connection with the exercise of an Ipsen Option, as defined and discussed further below) would grant to Ipsen exclusive, royalty-bearing, sublicensable, worldwide rights to develop, manufacture, use and commercialize such SNA therapeutics. Upon written notice to the Company, Ipsen may exercise its option during the corresponding collaboration program’s applicable option exercise period, (each, an “Ipsen Option Exercise Period”).
As of the Ipsen Effective Date, the Company and Ipsen have agreed upon a development plan for each Ipsen Collaboration Program that describes the development activities and timelines required to advance each such Ipsen Collaboration Program through its first IND filing (each, an “Ipsen Development Plan”). The activities described in the Ipsen Development Plans are conducted under the supervision of the Ipsen Joint Steering Committee (the “Ipsen JSC”) consisting of three members from each of the Company and Ipsen. Under the terms of the Ipsen Collaboration Agreement, the Company will use commercially reasonable efforts to conduct discovery and development in two collaboration programs for Huntington’s disease (the “HD Program”) and Angelman syndrome (the “AS Program”) (the “Ipsen Development Activities”) respectively. The Company shall be solely responsible for all costs and expenses of conducting each Ipsen Collaboration Program through the selection of SNA therapeutic candidates for further development (“Ipsen Selection”), and Ipsen shall be responsible for all costs and expenses of all activities that are necessary to enable the first filing of an IND for each proposed product candidate. In the event that Ipsen exercises an option, Ipsen will be responsible for further development from the license effective date and commercialization of the corresponding licensed product.
Following the completion of all Ipsen Development Activities for the Ipsen Selection (the “Ipsen First R&D Term Activities”), the Company is required to deliver to Ipsen a report that describes the results of the Ipsen First R&D Term Activities and identifies at least one SNA-based compound that satisfies certain criteria for such Ipsen Collaboration Program as determined by the Ipsen JSC (the “Ipsen First Option Data Package”). Following the delivery of the Ipsen First Option Data Package for an Ipsen Collaboration Program, Ipsen will have the ability for a defined period of time (the “Ipsen First Option Exercise Period”) to exercise an option (each a “First Ipsen Option”) to obtain worldwide rights and license to the Company’s SNA technology and the Company’s interest in joint collaboration technology to make, have made, import, use, sell or offer for sale any product (each an “Ipsen Licensed Product”) that results from such Ipsen Collaboration Program during the term of the Ipsen Collaboration Agreement.
In the event Ipsen (i) does not exercise the First Ipsen Option with respect to an Ipsen Collaboration Program, (ii) the Ipsen Collaboration Agreement has not expired or been terminated with respect to such Ipsen Collaboration Program, and (iii) Ipsen agrees to fully fund additional research activities for an Ipsen Collaboration Program through IND filing, the Company will be responsible for research and development activities for such Ipsen Collaboration Program through IND filing (the “Ipsen Second R&D Term Activities”). Following the completion of the Ipsen Second R&D Term Activities, the Company is required to deliver to Ipsen a report that describes the results of the Ipsen Second R&D Term Activities (the “Ipsen Second Option Data Package”). Following the delivery of the Ipsen Second Option Data Package for an Ipsen Collaboration Program, Ipsen will have the ability for a defined period of time (the “Ipsen Second Option Exercise Period”) to exercise an option (each a “Second Ipsen Option” and together with the First Ipsen Option, the “Ipsen Options”) to obtain worldwide rights and license to the Company’s SNA technology and the Company’s interest in joint collaboration technology to make, have made, import, use, sell or offer for sale any Ipsen Licensed Product” that results from such Ipsen Collaboration Program during the term of the Ipsen Collaboration Agreement.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
After Ipsen’s exercise of an Ipsen Option for an Ipsen Collaboration Program, the Company shall supply to Ipsen the licensed SNAs under current Good Manufacturing Practice, at the Company’s manufacturing cost pursuant to a clinical supply agreement to be negotiated by the Company and Ipsen in good faith following the Ipsen Effective Date and executed within twelve (12) months after the Ipsen Effective Date (the “Ipsen Supply Agreement”). The Ipsen Supply Agreement will provide for the transfer by the Company to Ipsen of all documents and information, and the provision by the Company of technical assistance and support, for Ipsen to manufacture or have manufactured by a third party contractor engaged by Ipsen the applicable licensed SNA to the extent it is intended to be actually used in the development and manufacture of the applicable licensed products.
Under the terms of the Ipsen Collaboration Agreement, the Company received an upfront payment of $20,000 (the “Ipsen Upfront Payment”). If Ipsen exercises a First Ipsen Option, Ipsen is required to pay the Company the First Ipsen Option exercise fee of $10,000 for each Ipsen Collaboration Program. If Ipsen exercises a Second Ipsen Option, Ipsen is required to pay the Company the Second Ipsen Option exercise fee of $25,000 for each Ipsen Collaboration Program.
Ipsen will pay a pre-clinical milestone payment of $5,000 for each Ipsen Collaboration Program upon achievement of such milestone regardless of whether an Ipsen Option is exercised. In addition to the option exercise fees and the pre-clinical milestones described above, if Ipsen exercises an Ipsen Option for an Ipsen Collaboration Program, development and regulatory milestones will be payable for that program upon the initiation of certain clinical trials and the filing for processing by the United States Food and Drug Administration (“FDA”) in the United States and by two additional regulators outside the United States of a marketing application for review, per the Ipsen Collaboration Program, with an aggregate total of up to $180,000 if both Ipsen Options are exercised. Commercial milestones will be payable for that Ipsen Collaboration Program upon first commercial sale of a licensed product in certain jurisdictions and the achievement of specified aggregate sales thresholds for all licensed products from that program, with an aggregate total of up to $762,000 if both Ipsen Options are exercised. In the event a therapeutic candidate subject to the Ipsen Collaboration Agreement results in commercial sales, the Company is eligible to receive tiered royalties at percentages ranging from the mid-single digits to the mid-teens on future net product sales of such commercialized therapeutic candidates. A percentage of the aforementioned payments will be due to Northwestern University upon receipt, pursuant to the terms of the Company’s existing license agreements with Northwestern University (see Note 13, Commitment and Contingencies, for more information on the Northwestern University License Agreements (as defined below)). In connection with the receipt of the Ipsen Upfront Payment, the Company paid a $3,000 license fee to Northwestern University under the terms of the Northwestern License Agreements.
The Company’s obligations to conduct activities defined in the Ipsen Development Plan under the Ipsen Collaboration Agreement commenced on July 30, 2021 and continues, unless earlier terminated, until (a) the expiration of the later-to-expire Ipsen Option Exercise Period, if Ipsen does not exercise either Ipsen Option, or (b) the expiration of the last-to-expire royalty term for any licensed product in such country on a licensed product-by-licensed product and country-by-country basis, if Ipsen exercises one or both Ipsen Options. Upon expiration of the royalty term with respect to a particular licensed product in a country, the license for such product in such country will convert to a fully-paid, irrevocable and perpetual license.
The Ipsen Collaboration Agreement also contains customary provisions for termination by either party, including by Ipsen for any or no reason in its entirety upon (90) days prior written notice and including in the event of breach of the Ipsen Collaboration Agreement, subject to cure, and by the Company upon a challenge of the licensed patents, subject, in certain cases, to customary reversion rights. Upon termination of the Ipsen Collaboration Agreement by the Company for Ipsen’s breach or bankruptcy, all licenses granted by the Company to Ipsen will terminate.
The Ipsen Collaboration Agreement includes customary representations and warranties on behalf of both the Company and Ipsen. The Ipsen Collaboration Agreement also provides for customary mutual indemnities. In addition, the Ipsen Collaboration Agreement imposes certain exclusivity obligations on Ipsen and the Company,
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
respectively, with respect to the development, use, manufacture and commercialization of oligonucleotide-based therapeutic targeting the same targets in the collaboration programs and/or certain other specific targets.
Either party may assign the Ipsen Collaboration Agreement or delegate its obligations to an affiliate or to a successor to substantially all of the business to which the Ipsen Collaboration Agreement relates without the consent of the other party.
Accounting Analysis
The Company concluded that Ipsen is a customer in this arrangement, and as such the arrangement falls within the scope of the revenue recognition guidance. Under the Ipsen Collaboration Agreement, the Company has identified two performance obligations, as follows: (1) the performance obligation related to the HD Program that includes (i) the Ipsen First R&D Term Activities related to the HD Program (the “Ipsen HD Program R&D Services”), (ii) Ipsen JSC services related to the HD Program during the Ipsen First Term (the “Ipsen HD Program JSC Services”), and (iii) activities related to the negotiation of the Ipsen Supply Agreement within twelve months of the Ipsen Effective Date; and (2) the performance obligation related to the AS Program that includes (i) the Ipsen First R&D Term Activities related to the AS Program (the “Ipsen AS Program R&D Services”), (ii) Ipsen JSC services related to the AS Program during the Ipsen First Term (the “Ipsen AS Program JSC Services”), (iii) and activities related to the negotiation of the Ipsen Supply Agreement within twelve months of the Ipsen Effective Date. The Company has concluded that the Ipsen HD Program R&D Services and the Ipsen AS Program R&D Services are not distinct from the Ipsen HD Program JSC Services and the Ipsen AS Program JSC Services, respectively. The Company has also concluded that the Ipsen HD Program JSC Services and the Ipsen AS Program JSC Services are not distinct from the activities related to entering the Ipsen Supply Agreements for each respective program. The Ipsen JSC provides oversight and management of the overall Ipsen Collaboration Agreement, and the members of the Ipsen JSC from the Company have specialized industry knowledge, particularly as it relates to SNA technology. The Ipsen JSC is meant to facilitate the early stage research being performed and coordinate the activities of both the Company and Ipsen. Further, the Ipsen JSC services are critical to the ongoing evaluation of the Ipsen Collaboration Programs and the drafting and evaluation of the Ipsen First Option Data Package. The Ipsen JSC will also provide oversight and management of the activities to enter into the Ipsen Supply Agreement. Accordingly, the Company’s participation on the Ipsen JSC is essential to Ipsen receiving value from the Ipsen HD Program R&D Services and the Ipsen AS Program R&D Services, and as such, (i) the Ipsen HD Program JSC Services, along with the Ipsen HD Program R&D Services and the activities related to entering the Ipsen Supply Agreement within twelve months of the Ipsen Effective Date for that program are considered a single performance obligation (the “Ipsen HD Program Services”) and (ii) the Ipsen AS Program JSC Services along with the Ipsen AS Program R&D Services and the activities related to entering the Ipsen Supply Agreement within twelve months of the Ipsen Effective Date for that program are considered a single performance obligation (the “Ipsen AS Program Services”).
As of the Ipsen Effective Date, the total transaction price was determined to be $20,000, consisting solely of the Ipsen Upfront Payment. The Company also utilized the most likely amount method to estimate any development and regulatory milestone payments to be received. As of the Ipsen Effective Date, there were no milestones included in the transaction price. The pre-clinical, development, regulatory, and commercial milestones were fully constrained due to the significant uncertainties surrounding such payments. The Company considered the stage of development and the risks associated with the remaining development required to achieve the milestone, as well as whether the achievement of the milestone is outside the control of the Company or Ipsen. The Company has determined that any commercial milestones and sales-based royalties will be recognized when the related sales occur and therefore, they have also been excluded from the transaction price. The Company will re-evaluate the transaction price at the end of each reporting period and as uncertain events are resolved or other changes in circumstances occur. As of September 30, 2021, the Company determined that any pre-clinical, development, regulatory, or commercial milestones continue to be constrained and therefore the related milestone payments continue to be excluded from the transaction price at September 30, 2021.
The Company allocated the total transaction price to each of the two identified performance obligations under the Ipsen Collaboration Agreement based on an expected cost plus a margin approach, as follows: $10,793 of the
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
transaction price allocated to the Ipsen HD Program Services and $9,207 of the transaction price allocated to the Ipsen AS Program Services.
The Company will recognize revenue related to each of Ipsen HD Program Services and the Ipsen AS Program Services as those performance obligations are satisfied using an input method to measure progress for each of those performance obligations. The Company believes the input method that most accurately depicts the measure of progress is the actual hours incurred to date relative to projected hours to complete the activities for the Ipsen HD Program Services and the Ipsen AS Program Services.
During the three and nine months ended September 30, 2021, the Company recognized revenue under the Ipsen Collaboration Agreement of approximately $803. As of September 30, 2021 , there was $19,197 of deferred revenue related to the Ipsen Collaboration Agreement, of which is $7,945 is classified as current and $11,252 is classified as noncurrent on the unaudited condensed consolidated balance sheet. Deferred revenue under the Ipsen Collaboration Agreement will be recognized as revenue in future periods as the Company satisfies its obligations under the Ipsen Collaboration Agreement, which the Company currently estimates to be over the next 33 to 42 months.
AbbVie Collaboration Agreement
Summary of Agreement
On November 13, 2019 (the “AbbVie Effective Date”), the Company entered into a Collaboration, Option and License Agreement (the “AbbVie Collaboration Agreement”), with a wholly-owned subsidiary of Allergan plc, Allergan. On May 8, 2020, Allergan plc, including Allergan was acquired by AbbVie. Pursuant to the AbbVie Collaboration Agreement, the Company granted to AbbVie exclusive access and options to license SNA-based therapeutics arising from two collaboration programs related to the treatment of hair loss disorders (each, an “AbbVie Collaboration Program”). Under each such license (obtained in connection with the exercise of an AbbVie Option, as defined and discussed further below), the Company would grant to AbbVie exclusive, royalty-bearing, sublicensable, nontransferable, worldwide rights to develop, manufacture, use and commercialize such SNA therapeutics. Under the AbbVie Collaboration Agreement, the Company will use commercially reasonable efforts to conduct the AbbVie Collaboration Programs, each focused on one or more hair loss disorders to discover one or more SNA products that are directed to, bind to or inhibit one or more specific AbbVie Collaboration Program targets.
As of the AbbVie Effective Date, the Company and AbbVie have agreed upon a development plan for each AbbVie Collaboration Program that describes the development activities and timelines required to advance such AbbVie Collaboration Program through its first IND filing (each, an “AbbVie Development Plan”). The activities described in the AbbVie Development Plan are conducted under the supervision of the AbbVie Joint Development Committee (the “AbbVie JDC”) consisting of three members from each of the Company and AbbVie. The Company is primarily responsible for performing early-stage discovery and preclinical activities (the “AbbVie Collaboration Program Initial Development Activities”) set forth in the AbbVie Development Plan for each AbbVie Collaboration Program and will be solely responsible for all costs and expenses related to the AbbVie Collaboration Program Initial Development Activities. AbbVie may elect, in its sole discretion and at its sole cost and expense, to conduct formulation assessment and in vivo testing as set forth in an AbbVie Development Plan.
During the third quarter of 2021, the AbbVie JDC revised the AbbVie Initial Development Plan for each AbbVie Collaboration Program. In connection with the revised workplan for each AbbVie Collaboration Program, the Company expects to perform additional early stage discovery and preclinical activities to those set forth in the AbbVie Development Plan. As such, the Company currently estimates significant additional efforts will be required to satisfy the performance obligation under the AbbVie Collaboration Agreement due to the revised workplan for each AbbVie Collaboration Program.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
Following the completion of all AbbVie Initial Development Activities, the Company is required to deliver to AbbVie a report that describes the results of the AbbVie Initial Development Activities and identifies at least one SNA-based compound that satisfies certain criteria for such AbbVie Collaboration Program as determined by the AbbVie JDC (the “AbbVie Initial Development Report”). Following the delivery of the AbbVie Initial Development Report for an AbbVie Collaboration Program, AbbVie will have the ability for a defined period of time (the “AbbVie Initial Option Exercise Period”) to exercise an option (each an “AbbVie Option”) to obtain worldwide rights and license to the Company’s SNA technology and the Company’s interest in joint collaboration technology to make, have made, import, use, sell or offer for sale any product (each an “AbbVie Licensed Product”) that results from such AbbVie Collaboration Program during the term of the AbbVie Collaboration Agreement.
At AbbVie’s sole option, AbbVie may extend the AbbVie Initial Option Exercise Period (the “AbbVie Option Extension”) and require the Company to perform IND-enabling activities described in the AbbVie Development Plan (the “AbbVie IND-Enabling Activities”), subject to the payment of additional consideration (“AbbVie Extension Exercise”). If AbbVie exercises the AbbVie Option Extension, the Company would be responsible for conducting the AbbVie IND-Enabling Activities and would be solely responsible for all costs and expenses associated with such activities. Upon completion of the AbbVie IND-Enabling Activities, the Company is required to deliver a report that describes the results of the AbbVie IND-Enabling Activities (the “AbbVie IND-Enabling Activities Data Package”) to AbbVie. Following the delivery of AbbVie IND-Enabling Activities Data Package, AbbVie will have the ability for a defined period of time (the “AbbVie Extended Option Exercise Period”) to exercise an AbbVie Option with respect to such AbbVie Collaboration Program. After the exercise of an AbbVie Option with respect to an AbbVie Collaboration Program, AbbVie will be responsible for all development, manufacturing and commercialization activities, and costs and expense associated with such activities in connection with AbbVie Licensed Products arising from such AbbVie Collaboration Program.
The Company’s obligation to conduct the activities defined in the AbbVie Development Plan under the AbbVie Collaboration Agreement commenced on November 13, 2019 and continues until the earlier of (i) the date AbbVie exercises an AbbVie Option, (ii) the date AbbVie abandons an AbbVie Collaboration Program and foregoes its AbbVie Option to that AbbVie Collaboration Program, or (iii) the fifth anniversary of the AbbVie Effective Date (the “AbbVie Research Term”). If the AbbVie Initial Option Exercise Period or AbbVie Extended Option Exercise Period is still in effect for an AbbVie Collaboration Program or if the Company has not delivered a complete AbbVie Initial Development Report or, if AbbVie made an AbbVie Extension Exercise for an AbbVie Collaboration Program, a complete AbbVie IND-Enabling Activities Data Package for such AbbVie Collaboration Program, as determined by the AbbVie JDC, then the AbbVie Research Term will automatically extend by one-year increments until such obligation is satisfied, but in no event past the seventh anniversary of the AbbVie Effective Date.
Under the terms of the AbbVie Collaboration Agreement, the Company received a $25,000 upfront, non-refundable, non-creditable cash payment (the “AbbVie Upfront Payment”) related to the Company’s research and development costs for conducting the AbbVie Development Plan for two AbbVie Collaboration Programs, each focused on one or more targets, and certain options to obtain exclusive, worldwide licenses under certain intellectual property rights owned or controlled by the Company to develop, manufacture and commercialize certain products resulting from each such AbbVie Collaboration Programs. The option exercise fee during the AbbVie Initial Option Exercise Period is $10,000 per AbbVie Collaboration Program. If AbbVie elects to extend the AbbVie Initial Option Exercise Period, AbbVie is required to pay an additional fee of $10,000. If AbbVie elects to exercise its option during the AbbVie Extended Option Exercise Period, AbbVie must pay the Company the option exercise fee of $15,000.
Following the exercise by AbbVie of an AbbVie Option with respect to an AbbVie Collaboration Program, AbbVie would be required to make certain milestone payments to the Company upon the achievement of specified development, product approval and launch, and commercial events, on an AbbVie Licensed Product by AbbVie Licensed Product basis. On an AbbVie Licensed Product by AbbVie Licensed Product basis, for the first AbbVie Licensed Product to achieve the associated milestone event, the Company is eligible to receive up to an aggregate of $55,000 for development milestone payments and $132,500 for product approval and launch milestone payments.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
The Company is also eligible for up to $175,000 in sales milestone payments on an AbbVie Collaboration Program by AbbVie Collaboration Program basis, associated with aggregate worldwide sales. Certain product approval milestones are subject to certain reductions under specified circumstances, including for payments required to be made by AbbVie to obtain certain third-party intellectual property rights.
In addition, to the extent there is any AbbVie Licensed Product, the Company would be entitled to receive tiered royalty payments of mid-single digits to the mid-teens percentage on future net worldwide product sales of such AbbVie Licensed Products, subject to certain reductions under specified circumstances. Royalties are due on a AbbVie Licensed Product by AbbVie Licensed Product and country by country basis from the date of the first commercial sale of each AbbVie Licensed Product in a country until the latest to occur of: (i) the expiration date in such country of the last to expire valid claim within the licensed intellectual property covering the manufacture, use or sale of such AbbVie Licensed Product in such country, (ii) the tenth anniversary of the first commercial sale of such AbbVie Licensed Product in such country, and (iii) the expiration of regulatory exclusivity for such AbbVie Licensed Product in such country.
AbbVie may terminate the AbbVie Collaboration Agreement for any reason or no reason, either in its entirety or on an AbbVie Collaboration Program by AbbVie Collaboration Program basis, at any time on 90 days’ prior written notice to the Company. Unless earlier terminated, the term of the AbbVie Collaboration Agreement shall continue until (i) if both AbbVie Option Exercise Periods expire without AbbVie exercising either AbbVie Option, the expiration of the later to expire AbbVie Option Exercise Period, and (ii) if either or both AbbVie Options are exercised on an AbbVie Licensed Product by AbbVie Licensed Product and country-by-country basis, the expiration of the royalty term for such AbbVie Licensed Product in such country. Either party may terminate the AbbVie Collaboration Agreement if the other party has materially breached or defaulted in the performance of any of its material obligations and such breach or default continues after the specified cure period.
Termination of the AbbVie Collaboration Agreement for any reason will not release either party from any liability which, at the time of such termination, has already accrued to the other party or which is attributable to a period prior to such termination. In addition, termination of the AbbVie Collaboration Agreement will not preclude either party from pursuing any rights and remedies it may have under the agreement or at law or in equity with respect to any breach of the AbbVie Collaboration Agreement. If either party terminates the AbbVie Collaboration Agreement, the license and rights granted to AbbVie with respect to the terminated AbbVie Collaboration Program or AbbVie License Product shall terminate.
Accounting Analysis
The Company concluded that AbbVie is a customer in this arrangement, and as such the arrangement falls within the scope of the revenue recognition guidance. Under the AbbVie Collaboration Agreement, the Company has identified a single performance obligation that includes (i) the research and development activities during the AbbVie Research Term (the “AbbVie R&D Services”), and (ii) AbbVie Joint Development Committee services during the AbbVie Research Term (the “AbbVie JDC Services”). The Company has concluded that the AbbVie R&D Services is not distinct from the AbbVie JDC Services during the AbbVie Research Term. The AbbVie JDC provides oversight and management of the overall AbbVie Collaboration Agreement, and the members of the AbbVie JDC from the Company have specialized industry knowledge, particularly as it relates to SNA technology. The AbbVie JDC is meant to facilitate the early-stage research being performed and coordinate the activities of both the Company and AbbVie. Further, the AbbVie JDC services are critical to the ongoing evaluation of an AbbVie Collaboration Program and the drafting and evaluation of the AbbVie Initial Development Report and the AbbVie IND-Enabling Data Package. Accordingly, the Company’s participation on the AbbVie JDC is essential to AbbVie receiving value from the AbbVie R&D Services and as such, the AbbVie JDC Services along with the AbbVie R&D Services are considered one performance obligation (the “AbbVie Collaboration Program Services”). In addition, the Company has concluded that the option to purchase two development and commercialization licenses is considered a marketing offer as the options did not provide any discounts or other rights that would be considered a material right in the arrangement, and thus, not a performance obligation at the onset of the agreement. The consideration for these options will be accounted for when they are exercised.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
As of the AbbVie Effective Date, the total transaction price was determined to be $25,000, consisting solely of the AbbVie Upfront Payment. The Company also utilized the most likely amount method to estimate any development and regulatory milestone payments to be received. As of the AbbVie Effective Date, there were no milestones included in the transaction price. The milestones were fully constrained due to the significant uncertainties surrounding such payments. The Company considered the stage of development and the risks associated with the remaining development required to achieve the milestone, as well as whether the achievement of the milestone is outside the control of the Company or AbbVie. The Company has determined that any commercial milestones and sales-based royalties will be recognized when the related sales occur and therefore they have also been excluded from the transaction price. The Company will re-evaluate the transaction price at the end of each reporting period and as uncertain events are resolved or other changes in circumstances occur. As of September 30, 2021, the Company determined that any development, regulatory or commercial milestones continue to be constrained and therefore the related milestone payments continue to be excluded from the transaction price at September 30, 2021.
The Company will recognize revenue related to the AbbVie Collaboration Program Services as the performance obligation is satisfied using an input method to measure progress. The Company believes the input method that most accurately depicts the measure of progress is the actual hours incurred to date relative to projected hours to complete the research service.
As discussed above, during the third quarter of 2021, the AbbVie JDC revised the AbbVie Initial Development Plan for each AbbVie Collaboration Program. As a result, the Company has increased its estimate of total hours to complete the research services, requiring an adjustment to cumulative revenue recognized (considered a change in estimate pursuant to ASC 606) as of September 30, 2021. As such, during the third quarter of 2021, the Company recorded a cumulative catchup adjustment (reduction) of revenue of $(4,480).
During the three and nine months ended September 30, 2021, the Company recognized revenue under the AbbVie Collaboration Agreement of approximately $(4,480) and $(3,404), respectively, reflecting the cumulative catchup adjustment (reduction) of revenue of $(4,480) discussed above due to a change in estimate. The Company currently estimates significant additional efforts will be required to satisfy the performance obligation under the AbbVie Collaboration Agreement due to the revised workplan for each AbbVie Collaboration Program. These increased estimated efforts in connection with the change in workplan resulted in less progress occurring relative to the increased estimate of total project hours to complete the research services during the three and nine months ended September 30, 2021 as compared to the amount of revenue recognized at both June 30, 2021 and December 31, 2020, which led to revenue reversals in each respective period. During the three and nine months ended September 30, 2020, the Company recognized revenue under the AbbVie Collaboration Agreement of approximately $2,402 and $16,351, respectively. As of September 30, 2021, there was $11,747 of deferred revenue related to the AbbVie Collaboration Agreement, of which is $6,070 is classified as current and $5,677 is classified as noncurrent on the unaudited condensed consolidated balance sheet. Deferred revenue under the AbbVie Collaboration Agreement will be recognized as revenue in future periods as the Company satisfies its obligations under the AbbVie Collaboration Agreement, which the Company currently estimates to be over the next 24 to 27 months. As of December 31, 2020, there was $8,343 of deferred revenue related to the AbbVie Collaboration Agreement, which was classified as current on the unaudited condensed consolidated balance sheet based on the estimated contract completion date at that time.
Dermelix Collaboration Agreement
On February 17, 2019, Exicure entered into a License and Development Agreement (the “Dermelix Collaboration Agreement”) with Dermelix, LLC d/b/a Dermelix Biotherapeutics (“Dermelix”). Under the terms of the Dermelix Collaboration Agreement, Exicure received an upfront payment of $1,000, to be applied against the initial $1,000 of the Company’s development expenses.
The Company initially recorded the upfront payment of $1,000 as deferred revenue related to its wholly unsatisfied performance obligation and reduced this balance to zero during 2019 by recognizing revenue as services
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
were provided. The Company recognized no revenue under the Dermelix Collaboration Agreement during the three and nine months ended September 30, 2021. The Company recognized $41 and $122 of revenue during the three and nine months ended September 30, 2020 which reflected reimbursement by Dermelix for additional costs incurred by Exicure for early-stage development costs beyond the initial $1,000 upfront payment.
Summary of Contract Liabilities
Up-front payments are recorded as deferred revenue upon receipt or when due until such time as the Company satisfies its performance obligations under these arrangements.
The following table presents changes in the balances of the Company’s contract liabilities (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Deferred Revenue Balance at January 1, 2021 | | Additions | | Revenue
(Recognized) Reversed | | Deferred Revenue Balance at September 30, 2021 |
| Ipsen Collaboration Agreement | | $ | — | | | $ | 20,000 | | | $ | (803) | | | $ | 19,197 | |
| AbbVie Collaboration Agreement | | $ | 8,343 | | | $ | — | | | $ | 3,404 | | | $ | 11,747 | |
| Total | | $ | 8,343 | | | $ | 20,000 | | | $ | 2,601 | | | $ | 30,944 | |
4. Supplemental Balance Sheet Information
Prepaid expenses and other current assets
| | | | | | | | | | | |
| September 30, 2021 | | December 31, 2020 |
| Prepaid clinical, contract research and manufacturing costs | $ | 2,133 | | | $ | 2,336 | |
| Interest receivable | 27 | | | 161 | |
| Prepaid insurance | 40 | | | 694 | |
| Other | 1,109 | | | 1,040 | |
| Prepaid expenses and other current assets | $ | 3,309 | | | $ | 4,231 | |
Property and equipment, net
| | | | | | | | | | | |
| September 30, 2021 | | December 31, 2020 |
| Scientific equipment | $ | 6,136 | | | $ | 5,476 | |
| Leasehold improvements | — | | | 192 | |
| Computers and software | 60 | | | 46 | |
| Furniture and fixtures | 30 | | | 30 | |
| Construction in process | 318 | | | 119 | |
| Property and equipment, gross | 6,544 | | | 5,863 | |
| Less: accumulated depreciation | (2,373) | | | (1,740) | |
| Property and equipment, net | $ | 4,171 | | | $ | 4,123 | |
Depreciation and amortization expense was $283 and $159 for the three months ended September 30, 2021 and 2020, respectively, and $829 and $494 for the nine months ended September 30, 2021 and 2020, respectively.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
Accrued expenses and other current liabilities
| | | | | | | | | | | |
| September 30, 2021 | | December 31, 2020 |
| Accrued clinical, contract research and manufacturing costs | $ | 2,741 | | | $ | 1,372 | |
| Accrued payroll-related expenses | 1,388 | | | 1,158 | |
| | | |
| | | |
| Lease liability, current | 440 | | | 223 | |
| Accrued other expenses | 911 | | | 772 | |
| Accrued expenses and other current liabilities | $ | 5,480 | | | $ | 3,525 | |
5. Investments
As of September 30, 2021 and December 31, 2020, the Company primarily invested its excess cash in debt instruments of corporations, the U.S. Treasury, financial institutions, and U.S. government agencies with strong credit ratings and an investment grade rating at or above a long-term rating of Aa3/AA- and a short-term rating of P1/A1. The Company has established guidelines relative to diversification and maturities that maintain safety and liquidity. The Company periodically reviews and modifies these guidelines to maximize trends in yields and interest rates without compromising safety and liquidity.
The following table summarizes the contract maturity of the available-for-sale securities the Company held as of September 30, 2021:
| | | | | |
| One year or less | 100 | % |
| After one year but within two years | — | % |
| Total | 100 | % |
All of the Company’s available-for-sale securities are available to the Company for use in its current operations. As a result, the Company categorizes all of these securities as current assets even though the stated maturity of some individual securities may be one year or more beyond the balance sheet date.
The amortized cost, gross unrealized holding gains, gross unrealized holding losses and fair value of cash equivalents and available-for-sale securities by type of security at September 30, 2021 and December 31, 2020 were as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| September 30, 2021 |
| Amortized Costs | | Gross Unrealized Holding Gains | | Gross Unrealized Holding Losses | | Fair Value |
| Commercial paper | $ | 6,498 | | | $ | — | | | $ | (1) | | | $ | 6,497 | |
| Corporate notes/bonds | 1,205 | | | — | | | — | | | 1,205 | |
| | | | | | | |
| U.S. Government agency securities | 1,251 | | | — | | | — | | | 1,251 | |
| $ | 8,954 | | | $ | — | | | $ | (1) | | | $ | 8,953 | |
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2020 |
| Amortized Costs | | Gross Unrealized Holding Gains | | Gross Unrealized Holding Losses | | Fair Value |
| Commercial paper | $ | 15,992 | | | $ | 2 | | | $ | (3) | | | $ | 15,991 | |
| Corporate notes/bonds | 29,227 | | | 72 | | | (3) | | | 29,296 | |
| U.S. Treasuries | 2,251 | | | 2 | | | — | | | 2,253 | |
| U.S. Government agency securities | 1,265 | | | 13 | | | — | | | 1,278 | |
| $ | 48,735 | | | $ | 89 | | | $ | (6) | | | $ | 48,818 | |
6. Debt
MidCap Credit Agreement
On September 30, 2021, the Company entered into an amendment (“Amendment No. 3”) to the Company’s Credit and Security Agreement, dated as of September 25, 2020, as amended on October 21, 2020 and July 30, 2021, with MidCap Financial Trust, as agent (“MidCap”), and the lenders party thereto from time to time (as amended by Amendment No. 3, the “MidCap Credit Agreement”).
The MidCap Credit Agreement provides for a secured term loan facility in an aggregate principal amount of up to $25,000 (the “MidCap Credit Facility”). The Company borrowed the first advance of $17,500 (“Tranche 1”) on September 25, 2020 (the “Closing Date”). Amendment No. 3 extends the availability of the second advance of $7,500 (“Tranche 2”) under the MidCap Credit Agreement from September 30, 2021 to December 31, 2021, subject to the Company’s satisfaction of certain applicable funding conditions as described in Amendment No. 3, including the Company’s issuance of equity interests, subject to certain limitations, resulting in receipt by the Company of unrestricted net cash proceeds of at least $20,000, and the contribution by the Company, and receipt by Exicure Operating Company, of such cash proceeds to the capital of Exicure Operating Company.
Tranche 1 and if borrowed, Tranche 2, each bear interest at a floating rate equal to 6.25% per annum, plus the greater of (i) 1.50% or (ii) one-month LIBOR. Interest on each loan advance is due and payable monthly in arrears. Principal on each loan advance is payable in 36 equal monthly installments beginning October 1, 2022 until paid in full on October 1, 2025 (the “Maturity Date”). Prepayments of the loans under the MidCap Credit Agreement, in whole or in part, will be subject to early termination fees in an amount equal to 3.0% of principal prepaid if prepayment occurs on or prior to the first anniversary of the Closing Date and 1.0% of principal prepaid if prepayment occurs after the first anniversary of the Closing Date and prior to the maturity date. In connection with execution of the MidCap Credit Agreement, the Company paid MidCap a $125 origination fee.
At the Maturity Date or on any earlier date on which all amounts advanced to the Company become due and payable in full, or are otherwise paid in full, the Company is required to pay an exit fee equal to 3.75% of the principal amount of all loans advanced to the Company under the MidCap Credit Agreement. Upon the advance of Tranche 1, the Company accrued $656 for the related exit fee.
The Company’s obligations under the MidCap Credit Agreement are secured by a security interest in substantially all of its assets, excluding intellectual property (which is subject to a negative pledge). Additionally, the Company’s future subsidiaries, if any, may be required to become co-borrowers or guarantors under the MidCap Credit Agreement.
The MidCap Credit Agreement contains customary affirmative covenants and customary negative covenants limiting the Company’s ability and the ability of the Company’s subsidiaries, if any, to, among other things, dispose of assets, undergo a change in control, merge or consolidate, make acquisitions, incur debt, incur liens, pay dividends, repurchase stock and make investments, in each case subject to certain exceptions.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
The MidCap Credit Agreement also contains customary events of default relating to, among other things, payment defaults, breaches of covenants, a material adverse change, delisting of the Company’s common stock, bankruptcy and insolvency, cross defaults with certain material indebtedness and certain material contracts, judgments, and inaccuracies of representations and warranties. Upon an event of default, the agent and the lenders may declare all or a portion of the Company’s outstanding obligations to be immediately due and payable and exercise other rights and remedies provided for under the agreement. During the existence of an event of default, interest on the obligations could be increased by 2.0%.
Total proceeds, net of fees and issuance costs, borrowed under Tranche 1 were $16,512. Fees and issuance costs of $332, as well as fees of $656 that are payable to MidCap at maturity of Tranche 1, are recorded as a reduction to the carrying amount of long-term debt on the Company’s balance sheet and will be amortized to interest expense through the maturity date of October 1, 2025 using the effective interest method. Fees and issuance costs of $73 attributed to the amount available to be borrowed under Tranche 2 were paid and recorded as deferred financing costs (other assets) and will be (i) recorded as a reduction in the carrying amount of long-term debt in future periods if amounts are borrowed under Tranche 2 or (ii) will be amortized and recorded to interest expense if amounts are not borrowed under Tranche 2. For the three and nine months ended September 30, 2021, the Company recognized $36 and $73, respectively, as interest expense and no balance of the deferred financing costs remains as of September 30, 2021.
As of September 30, 2021, the aggregate carrying value of the Company’s long-term debt is $16,801.
As of September 30, 2021, the principal maturities of the Company’s long-term debt were as follows:
| | | | | | | | |
| | September 30, 2021 |
| 2021 (remaining three months) | | $ | — | |
| 2022 | | 1,459 | |
| 2023 | | 5,833 | |
| 2024 | | 5,833 | |
| 2025 | | 4,375 | |
| Principal balance outstanding | | $ | 17,500 | |
| less: unamortized discount and debt issuance costs | | (699) | |
| Long-term debt | | $ | 16,801 | |
| Current portion | | — | |
| Noncurrent portion | | $ | 16,801 | |
| | |
| | |
The Company paid interest on the MidCap Credit Agreement of $344 and $1,041 during the three and nine months ended September 30, 2021, respectively. The Company paid interest on the Hercules Loan Agreement (as discussed below) of $0 and $142 during the three and nine months ended September 30, 2020, respectively.
Hercules Loan Agreement
On March 2, 2020, pursuant to the terms of the loan agreement with Hercules Technology Growth Capital (“Hercules”) and subsequent amendments thereto (the “Hercules Loan Agreement”), the Company repaid all remaining outstanding obligations under the Hercules Loan Agreement as of the maturity date, including the outstanding principal balance of $4,999 and the end of term fee of $100.
7. Leases
The Company’s lease arrangements at September 30, 2021 consist of (i) a lease for office and laboratory space at its headquarters in Chicago, Illinois that commenced in July 2020 (the “Chicago Lease”), (ii) a lease for office
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
space at a multi-tenant facility in Cambridge, Massachusetts that commenced in March 2019 and is cancelable at any time (the “Cambridge Lease”), and (iii) leases for office equipment (the “Office Equipment Leases”). Each of these leases are classified as operating leases.
Due to the nature of the Cambridge Lease, the Company determined that this lease represented a short-term lease with an initial term of less than twelve months and, as such, the Cambridge Lease is not recorded on the balance sheet and related lease costs are recognized in the statement of operations as they are incurred. The Company has also elected to not record the Office Equipment Leases on the balance sheet since related payment amounts and lease costs are insignificant. Lease costs for the Office Equipment Leases are recognized in the statement of operations on a straight-line basis over the lease term.
The Company’s lease arrangement for office and laboratory space at its former headquarters in Skokie, Illinois ended in February 2021 in accordance with the terms of that lease arrangement.
Chicago Lease
The Company has approximately thirty thousand square feet of office and laboratory space in Chicago, Illinois (the “Chicago Lease”). The original term (the “Original Term”) of the Chicago Lease is 10 years, commencing on July 1, 2020 (the “Commencement Date”), which is the date the premises were ready for occupancy under the terms of the Chicago Lease. The Company has options to extend the term of the Chicago Lease for two additional successive periods of five years each (the “Extension Periods”) at the then prevailing effective market rental rate.
The initial annual base rent during the Original Term is approximately $1,113 for the first 12-month period of the Original Term, payable in monthly installments beginning on the Commencement Date. Base rent thereafter is subject to annual increases of 3%, for an aggregate amount of $12,761 over the Original Term. The Company must also pay its proportionate share of certain operating expenses and taxes for each calendar year during the term. During the first 12-month period of the Original Term, the base rent and the Company’s proportionate share of operating expenses and taxes were subject to certain abatements.
In connection with the Chicago Lease, the Company will maintain a letter of credit for the benefit of the landlord in an initial amount of $1,200, which amount is subject to reduction over time. Upon execution of the Chicago Lease, the Company paid to the landlord the first installment of base rent and the estimated monthly amount of its pro rata share of taxes and its pro rata share of operating expenses in the aggregate amount of $87 which amount had been adjusted for the abatement as set forth in the lease agreement. The Company also paid the landlord a net amount of $697 toward tenant improvements.
As part of the agreement for the Chicago Lease, the Company is required to maintain a standby letter of credit during the term of the lease, currently in the amount of $1,200 and subject to reduction over time, which is secured by a restricted certificate of deposit account and presented within other noncurrent assets on the Company’s unaudited condensed consolidated balance sheet at September 30, 2021.
The Company recognized a right of use asset of $8,931 and a lease liability of $8,147 on the Commencement Date. Because the rate implicit in the Chicago Lease is not readily determinable, the Company used its incremental borrowing rate of 8.3% on the Commencement Date to determine the present value of the lease payments over the Original Term. The incremental borrowing rate represents an estimate of the interest rate the Company would incur at lease commencement to borrow an amount equal to the lease payments on a collateralized basis over the term of a lease. As of September 30, 2021, the Company determined it is not reasonably certain that the renewal option would be exercised.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
Information related to the Company’s operating lease asset and related operating lease liabilities were as follows: | | | | | | | | |
| | September 30, 2021 |
| Weighted-average remaining lease term | | 8.8 years |
| Weighted-average discount rate | | 8.3 | % |
| | |
| | |
The following table summarizes lease costs in the Company’s unaudited condensed consolidated statement of operations:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
September 30, | | Nine Months Ended
September 30, |
| 2021 | | 2020 | | 2021 | | 2020 |
| Operating lease costs | $ | 326 | | | $ | 333 | | | $ | 980 | | | $ | 712 | |
| Short term lease costs | 32 | | | 29 | | | 94 | | | 96 | |
| Variable lease costs | 231 | | | 172 | | | 803 | | | 322 | |
| Total lease costs | $ | 589 | | | $ | 534 | | | $ | 1,877 | | | $ | 1,130 | |
The Company made cash payments for operating leases of $1,721 and $1,717 during the nine months ended September 30, 2021 and 2020, respectively.
Maturities of the Company’s lease liability as of September 30, 2021 were as follows:
| | | | | | | | |
| Years Ending December 31, | | Operating Leases |
| 2021 (remaining three months) | | $ | 191 | |
| 2022 | | 1,163 | |
| 2023 | | 1,198 | |
| 2024 | | 1,235 | |
| 2025 | | 1,272 | |
| 2026 | | 1,310 | |
| Thereafter | | 4,897 | |
| Total | | $ | 11,266 | |
| Less: imputed interest | | (3,275) | |
| Total lease liability | | $ | 7,991 | |
| | |
| Current operating lease liability | | $ | 440 | |
| Noncurrent operating lease liability | | 7,551 | |
| Total lease liability | | $ | 7,991 | |
| | |
| | |
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
8. Stockholders’ Equity
Preferred Stock
As of September 30, 2021 and December 31, 2020, the Company had 10,000,000 shares of preferred stock, par value $0.0001 authorized and no shares issued and outstanding.
Common Stock
As of September 30, 2021 and December 31, 2020, the Company had authorized 200,000,000 shares of common stock, par value $0.0001. As of September 30, 2021 and December 31, 2020, the Company had 88,108,543 shares and 87,651,352 shares issued and outstanding, respectively.
The holders of shares of the Company’s common stock are entitled to one vote per share on all matters to be voted upon by the Company’s stockholders and there are no cumulative rights. Subject to preferences that may be applicable to any outstanding preferred stock, the holders of shares of the Company’s common stock are entitled to receive ratably any dividends that may be declared from time to time by the Board out of funds legally available for that purpose. In the event of the Company’s liquidation, dissolution or winding up, the holders of shares of the Company’s common stock are entitled to share ratably in all assets remaining after payment of liabilities, subject to prior distribution rights of preferred stock then outstanding. The Company’s common stock has no preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to the Company’s common stock. The outstanding shares of the Company’s common stock are fully paid and non-assessable.
Common Stock Warrants
As of September 30, 2021, no warrants were outstanding. As of December 31, 2020, warrants to purchase 413,320 shares of common stock at a price of $3.00 per share were outstanding. The warrants expired unexercised as follows: 163,174 warrants expired on March 27, 2021, 132,884 expired on April 28, 2021, and 117,262 expired on May 3, 2021. The warrants were classified as a liability which was remeasured each period at fair value. See Note 12, Fair Value Measurements for more information on the fair value of the common stock warrant liability.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
Accumulated Other Comprehensive Income (Loss)
The following table summarizes the changes in each component of accumulated other comprehensive income (loss), net of tax, for 2021:
| | | | | | | | | | | | | | |
| | Unrealized gains (losses) on short-term investments | | Total |
| Balance at December 31, 2020 | | $ | 83 | | | $ | 83 | |
| Other comprehensive loss before reclassifications | | (51) | | | (51) | |
| Net current period other comprehensive loss | | (51) | | | (51) | |
| Balance at March 31, 2021 | | $ | 32 | | | $ | 32 | |
| Other comprehensive income (loss) before reclassifications | | (27) | | | (27) | |
| | | | |
| Net current period other comprehensive income | | (27) | | | (27) | |
| Balance at June 30, 2021 | | $ | 5 | | | $ | 5 | |
| Other comprehensive income (loss) before reclassifications | | (5) | | | (5) | |
| Net losses (gains) reclassified from accumulated other comprehensive loss | | (1) | | | (1) | |
| Net current period other comprehensive income | | (6) | | | (6) | |
| Balance at September 30, 2021 | | $ | (1) | | | $ | (1) | |
| | | | |
| | | | |
| | | | |
The net gain reclassified from accumulated other comprehensive loss during the three months ended September 30, 2021 resulted from available-for-sale securities that were called prior to maturity and no gains or losses were recognized for the three and nine months ended September 30, 2021. The basis on which the cost of the securities was determined was specific identification. Proceeds related to these sales were $4,000.
The following table summarizes the changes in each component of accumulated other comprehensive income (loss), net of tax, for 2020:
| | | | | | | | | | | | | | |
| | Unrealized gains (losses) on short-term investments | | Total |
| Balance at December 31, 2019 | | $ | (27) | | | $ | (27) | |
| Other comprehensive income before reclassifications | | 10 | | | 10 | |
| Net current period other comprehensive income | | 10 | | | 10 | |
| Balance at March 31, 2020 | | $ | (17) | | | $ | (17) | |
| Other comprehensive income (loss) before reclassifications | | 352 | | | 352 | |
| Net losses (gains) reclassified from accumulated other comprehensive loss | | 3 | | | 3 | |
| Net current period other comprehensive income | | 355 | | | 355 | |
| Balance at June 30, 2020 | | $ | 338 | | | $ | 338 | |
| Other comprehensive income (loss) before reclassifications | | (138) | | | (138) | |
| Net current period other comprehensive income | | (138) | | | (138) | |
| Balance at September 30, 2020 | | $ | 200 | | | $ | 200 | |
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
The net loss reclassified from accumulated other comprehensive loss during the nine months ended September 30, 2020 resulted from sales of available-for-sale securities prior to maturity. The gross realized gains and gross realized losses of these sales were $23 and $6, respectively, for the three and nine months ended September 30, 2020. The basis on which the cost of the securities was determined was specific identification. Proceeds related to these sales were $9,404.
9. Equity-Based Compensation
2017 Equity Incentive Plan
Awards that may be awarded under the 2017 Equity Incentive Plan include non-qualified and incentive stock options, stock appreciation rights, bonus shares, restricted stock, restricted stock units, performance units and cash-based awards. The number of shares of common stock reserved for issuance under the 2017 Equity Incentive Plan automatically increases on January 1 of each year, beginning on January 1, 2020, by the lesser of (i) 4,600,000 shares, (ii) 5% of the total number of shares of our capital stock outstanding on December 31 of the preceding calendar year, or (iii) a lesser number of shares determined by the Compensation Committee of the Board (the “Compensation Committee”). The Compensation Committee made the determination to increase the share reserve for the 2017 Equity Incentive Plan by 4,382,567 shares, effective January 1, 2021.
As of September 30, 2021, the aggregate number of equity awards available for grant under the 2017 Equity Incentive Plan was 2,558,218.
Inducement Grant
In May 2021, the Company granted stock options to purchase up to 600,000 shares of common stock as a material inducement to Brian C. Bock, the Company’s Chief Financial Officer, to enter into employment with the Company (the “Inducement Grant”). The Inducement Grant, which was made pursuant to a stand-alone nonstatutory stock option agreement (the “Inducement Award Agreement”), was approved by the Compensation Committee, was awarded in accordance with Nasdaq Listing Rule 5635(c)(4) and outside of the Company’s 2017 Equity Incentive Plan and is subject to the terms and conditions of the Inducement Award Agreement. As such, any shares underlying the Inducement Grant are not, upon forfeiture, cancellation or expiration, returned to a pool of shares reserved for future issuance. As of September 30, 2021, stock options to purchase up to 600,000 shares of common stock remained outstanding under the Inducement Grant.
Employee Stock Purchase Plan
The 2017 Employee Stock Purchase Plan (the “ESPP”) was adopted by the Board in September 2017 and approved by the Company’s stockholders in September 2017. Through the ESPP, eligible employees may authorize payroll deductions of up to 15% of their compensation to purchase common stock. The maximum number of shares that an employee may purchase on any exercise date in an offer period will be the smaller of (i) 7,500 shares or (ii) such number of shares as has a fair market value (determined as of the offering date for such offer period) equal to $25,000 within one calendar year minus the fair market value of any other shares of common stock that are attributed to such calendar year. The purchase price per share at each purchase date is equal to 85% of the lower of (i) the closing market price per share of Exicure common stock on the employee’s offering date or (ii) the closing market price per share of Exicure common stock on the exercise date. Each offering period is approximately six-months in duration and the first offering period began on November 16, 2020 and ended on May 14, 2021. In connection with the end of the first offering period on May 14, 2021, the Company issued 103,096 shares of common stock that were purchased under the ESPP.
The ESPP provides that the number of shares reserved and available for issuance will automatically increase each January 1, beginning on January 1, 2018 and each January 1 thereafter through January 1, 2027, by the least of (i) 300,000 shares; (ii) 0.3% of the outstanding shares of common stock on the last day of the immediately preceding calendar year; or (iii) a lesser number of shares determined by the Board. On January 1, 2021 and 2020, the number
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
of shares of common stock available for issuance under the ESPP increased by 258,207 shares and 262,954 shares, respectively. As of September 30, 2021, there were 1,100,791 shares available for issuance under the ESPP.
Equity-based compensation expense is classified in the statements of operations as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
September 30, | | Nine Months Ended
September 30, |
| 2021 | | 2020 | | 2021 | | 2020 |
| Research and development expense | $ | 427 | | | $ | 247 | | | $ | 1,045 | | | $ | 607 | |
| General and administrative expense | 462 | | | 371 | | | 1,125 | | | 996 | |
| $ | 889 | | | $ | 618 | | | $ | 2,170 | | | $ | 1,603 | |
Unamortized equity-based compensation expense at September 30, 2021 was $7,820, which is expected to be amortized over a weighted-average period of 3.0 years.
The Company utilizes the Black-Scholes option-pricing model to determine the fair value of common stock option grants. The Black-Scholes option-pricing model was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. The model also requires the input of highly subjective assumptions. In addition to an assumption on the expected term of the option grants as discussed below, application of the Black-Scholes model requires additional inputs for which we have assumed the values described in the table below:
| | | | | | | | | | | |
| Nine Months Ended
September 30, |
| 2021 | | 2020 |
| Expected term | 5.0 to 6.1 years | | 5.2 to 6.1 years |
| Risk-free interest rate | 0.36% to 1.12%; weighted avg. 1.05% | | 0.31% to 1.68%; weighted avg. 0.61% |
| Expected volatility | 81.7% to 83.6%; weighted avg. 82.6% | | 81.0% to 85.8%; weighted avg. 83.8% |
| Forfeiture rate | 5 | % | | 5 | % |
| Expected dividend yield | — | % | | — | % |
The expected term is based upon the “simplified method” as described in Staff Accounting Bulletin Topic 14.D.2. Currently, the Company does not have sufficient experience to provide a reasonable estimate of an expected term of its common stock options. The Company will continue to use the “simplified method” until there is sufficient experience to provide a more reasonable estimate in conformance with ASC 718-10-30-25 through 30-26. The risk-free interest rate assumptions were based on the U.S. Treasury bond rate appropriate for the expected term in effect at the time of grant. The expected volatility is based on calculated enterprise value volatilities for publicly traded companies in the same industry and general stage of development. The estimated forfeiture rates were based on historical experience for similar classes of employees. The dividend yield was based on expected dividends at the time of grant.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
The fair value of the underlying common stock and the exercise price for the common stock options granted during the nine months ended September 30, 2021 and 2020 are summarized in the table below:
| | | | | | | | | | | |
| Common Stock Options Granted During Period Ended: | Fair Value of Underlying Common Stock | | Exercise Price of Common Stock Option |
Nine months ended September 30, 2021 | $1.15 to $2.57; weighted avg. $1.87 | | $1.15 to $2.57; weighted avg. $1.87 |
| Nine months ended September 30, 2020 | $1.19 to $2.80: weighted avg. $1.91 | | $1.19 to $2.80: weighted avg. $1.94 |
The weighted-average grant date fair value of common stock options granted in the nine months ended September 30, 2021 and 2020 was $1.30 and $1.33 per common stock option, respectively.
A summary of common stock option activity as of the periods indicated is as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Options | | Weighted-Average Exercise Price | | Weighted-Average Remaining Contractual Term (years) | | Aggregate Intrinsic Value (thousands) |
| Outstanding - December 31, 2020 | 7,227,296 | | | $ | 2.24 | | | 6.8 | | $ | 1,719 | |
| Granted | 4,427,586 | | | 1.87 | | | | | |
| Exercised | (342,246) | | | 1.60 | | | | | |
| Forfeited | (252,681) | | | 2.38 | | | | | |
| Outstanding - September 30, 2021 | 11,059,955 | | | $ | 2.11 | | | 7.5 | | $ | 397 | |
| Exercisable - September 30, 2021 | 5,207,872 | | | $ | 2.27 | | | 5.7 | | $ | 389 | |
Vested and Expected to Vest -
September 30, 2021 | 10,630,872 | | | $ | 2.11 | | | 7.5 | | $ | 396 | |
The aggregate intrinsic value of common stock options exercised during the nine months ended September 30, 2021 and 2020 was $243 and $74, respectively.
A summary of restricted stock unit activity of the periods indicated is as follows:
| | | | | | | | | | | |
| Restricted Stock Units | | Weighted-Average Grant Date Fair Value |
| Unvested balance - December 31, 2020 | — | | | $ | — | |
| Granted | 575,000 | | | 1.58 | |
| Vested | (17,875) | | | 1.90 | |
| Forfeited | (10,500) | | | 1.15 | |
| Unvested balance - September 30, 2021 | 546,625 | | | $ | 1.57 | |
The grant date fair value of restricted stock units is based on the Company’s closing stock price at the date of grant. At vesting, each outstanding restricted stock unit will be exchanged for one share of the Company’s common stock. The restricted stock units granted during the 2021 period generally vest evenly on a quarterly basis over a period of 4 years in exchange for continued service provided by the restricted stock unit recipient during that vesting period.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
10. Income Taxes
The Company incurred a pretax loss in each of the three and nine months ended September 30, 2021 and 2020, which consists entirely of loss in the United States and resulted in no provision for income tax expense during the periods then ended. The effective tax rate is 0% in each of the three and nine months ended September 30, 2021 and 2020 because the Company has generated tax losses and has provided a full valuation allowance against its deferred tax assets.
11. Loss Per Common Share
Basic loss per common share is calculated by dividing net loss by the weighted-average number of shares of common stock outstanding during the period. Diluted loss per common share is calculated using the treasury share method by giving effect to all potentially dilutive securities that were outstanding. Potentially dilutive options, restricted stock units and warrants to purchase common stock that were outstanding during the periods presented were excluded from the diluted loss per share calculation for the periods presented because such shares had an anti-dilutive effect due to the net loss reported in those periods. Therefore, basic and diluted loss per common share is the same for each of the three and nine months ended September 30, 2021 and 2020.
The following is the computation of loss per common share for the three and nine months ended September 30, 2021 and 2020:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
September 30, | | Nine Months Ended
September 30, |
| 2021 | | 2020 | | 2021 | | 2020 |
| Net loss | $ | (23,531) | | | $ | (8,822) | | | $ | (50,277) | | | $ | (11,983) | |
| | | | | | | |
| Weighted-average basic and diluted common shares outstanding | 88,105,066 | | | 87,227,136 | | | 88,001,222 | | | 87,160,520 | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| Loss per share - basic and diluted | $ | (0.27) | | | $ | (0.10) | | | $ | (0.57) | | | $ | (0.14) | |
| | | | | | | |
The outstanding securities presented below were excluded from the calculation of loss per common share, for the periods presented, because such securities would have been anti-dilutive due to the Company’s loss per share during that period:
| | | | | | | | | | | |
| As of September 30, |
| 2021 | | 2020 |
| Options to purchase common stock | 11,059,955 | | | 7,739,781 | |
| Restricted stock units | 546,625 | | | — | |
| Warrants to purchase common stock | — | | | 413,320 | |
12. Fair Value Measurements
ASC Topic 820, Fair Value Measurement, establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value, as follows: Level 1 Inputs - unadjusted quoted prices in active markets for identical assets or liabilities accessible to the reporting entity at the measurement date; Level 2 Inputs - other than quoted prices included in Level 1 inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the asset or liability; and Level 3 Inputs - unobservable inputs for the asset or liability used to measure fair value to the extent that observable inputs are not available, thereby allowing for situations in which there is little, if any, market activity for the asset or liability at measurement date.
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
Assets measured at fair value on a recurring basis as of September 30, 2021 are as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Total | | Level 1 | | Level 2 | | Level 3 |
| Assets | | | | | | | |
| Cash equivalents: | | | | | | | |
| Money market funds | $ | 27,641 | | | $ | 27,641 | | | $ | — | | | $ | — | |
| Corporate notes/bonds | 2,000 | | | — | | | 2,000 | | | — | |
| U.S. Treasuries | 9,999 | | | — | | | 9,999 | | | — | |
| | | | | | | |
| Short-term investments: | | | | | | | |
| Commercial paper | 6,497 | | | — | | | 6,497 | | | — | |
| Corporate notes/bonds | 1,205 | | | — | | | 1,205 | | | — | |
| | | | | | | |
| U.S. government agency securities | 1,251 | | | — | | | 1,251 | | | — | |
| Total financial assets | $ | 48,593 | | | $ | 27,641 | | | $ | 20,952 | | | $ | — | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
| | | | | | | |
Assets and liabilities measured at fair value on a recurring basis as of December 31, 2020 are as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Total | | Level 1 | | Level 2 | | Level 3 |
| Assets | | | | | | | |
| Cash equivalents: | | | | | | | |
| Money market funds | $ | 24,586 | | | $ | 24,586 | | | $ | — | | | $ | — | |
| Short-term investments: | | | | | | | |
| Commercial paper | 15,991 | | | — | | | 15,991 | | | — | |
| Corporate notes/bonds | 29,296 | | | — | | | 29,296 | | | — | |
| U.S. Treasuries | 2,253 | | | — | | | 2,253 | | | — | |
| U.S. government agency securities | 1,278 | | | — | | | 1,278 | | | — | |
| Total financial assets | $ | 73,404 | | | $ | 24,586 | | | $ | 48,818 | | | $ | — | |
| | | | | | | |
| Liabilities | | | | | | | |
| Common stock warrant liability | $ | 15 | | | $ | — | | | $ | — | | | $ | 15 | |
| Total financial liabilities | $ | 15 | | | $ | — | | | $ | — | | | $ | 15 | |
The Company uses the market approach and Level 1 and Level 2 inputs to value its cash equivalents and Level 2 inputs to value its short-term investments. The Company’s long-term debt bore interest at the prevailing market rates for instruments with similar characteristics and, accordingly, the carrying value for this instrument also approximates its fair value and the financial measurement is also classified within Level 2 of the fair value hierarchy.
As of September 30, 2021, the Company’s common stock warrant liability was $0 and no warrants were outstanding (refer to Note 8, Stockholders’ Equity, for more information). The following is a reconciliation of the Company’s liabilities measured at fair value on a recurring basis using unobservable inputs (Level 3) for the three months ended September 30, 2021:
EXICURE, INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
(in thousands, except share and per share data)
| | | | | |
| | Fair Value Measurements Using Significant Unobservable Inputs (Level 3) |
| Common Stock Warrant Liability |
| Balance at December 31, 2020 | $ | 15 | |
| Additions | — | |
| Gain included in other (expense) income, net | (15) | |
Balance at September 30, 2021 | $ | — | |
13. Commitments and Contingencies
Leases
Refer to Note 7, Leases, for a discussion of the commitments associated with the Company’s lease agreements.
Northwestern University License Agreements
On December 12, 2011, (1) AuraSense, LLC, the Company’s former parent, assigned to the Company all of its worldwide rights and interests under AuraSense, LLC’s 2009 license agreement with Northwestern University (“NU”) in the field of the use of nanoparticles, nanotechnology, microtechnology or nanomaterial-based constructs as therapeutics or accompanying therapeutics as a means of delivery, but expressly excluding diagnostics (the “assigned field”); (2) in accordance with the terms and conditions of this assignment, the Company assumed all liabilities and obligations of AuraSense, LLC as set forth in its license agreement in the assigned field; and (3) in order to secure this assignment and the patent rights from NU, the Company agreed (i) to pay NU an annual license fee, which may be credited against any royalties due to NU in the same year, (ii) to reimburse NU for expenses associated with the prosecution and maintenance of the license patent rights, (iii) to pay NU royalties based on any net revenue generated by the Company’s sale or transfer of any licensed product, (iv) to pay NU, in the event the Company grants a sublicense under the licensed patent rights, the greater of a percentage of all sublicensee royalties or a percentage of any net revenue generated by a sublicensee’s sale or transfer of any licensed product, and (v) to pay NU a percentage of all other sublicense payments received by the Company. In August 2015, the Company entered into a restated license agreement with NU (the “Restated License Agreement”). In February 2016, the Company obtained exclusive license as to NU’s rights in certain SNA technology it jointly owns with NU (the “Co-owned Technology License”). The Company’s license to NU’s rights is limited to the assigned field, however the Company has no such limitation as to its own rights in this jointly owned technology. In June 2016, the Company entered into an exclusive license with NU to obtain worldwide rights to certain inhibitors of glucosylceramide synthase and their use in wound healing in diabetes (the “Wound Healing License”). The Company’s rights and obligations in the Co-owned Technology License and the Wound Healing License agreements are substantially the same as in the Restated License Agreement from August 2015 (collectively referred to as “the Northwestern University License Agreements”). All pending patent applications under the Wound Healing License have been abandoned and the Wound Healing License has been terminated. As of September 30, 2021, the Company has paid to NU an aggregate of $11,413 in consideration of each of the obligations described above.
14. Related-Party Transactions
The Company received consulting services from, and paid fees to, one of its co-founders who is not an employee but, through April 30, 2021, served as a member of the Board. The Company recognized $75 as an expense in each of the nine months ended September 30, 2021 and 2020 in connection with these consulting services in the accompanying unaudited condensed consolidated statement of operations. The consulting agreement with this co-founder and former Board member expired on September 30, 2021 under the terms of the agreement and was not renewed.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following information should be read in conjunction with our unaudited condensed consolidated financial statements and the notes thereto included in this Quarterly Report on Form 10-Q and the audited financial information and the notes thereto included in our Annual Report on Form 10-K for the year ended December 31, 2020, which was filed with the Securities and Exchange Commission, or SEC, on March 11, 2021. This Quarterly Report on Form 10-Q contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, that involve significant risks and uncertainties. Our actual results, performance or experience could differ materially from what is indicated by any forward-looking statement due to various important factors, risks, uncertainties, assumptions and other factors including, but not limited to, those identified in this Quarterly Report on Form 10-Q and those set forth under the section titled “Risk Factors” in Part II, Item 1A of this Quarterly Report on Form 10-Q and in our other SEC filings. Such factors may be amplified by the COVID-19 pandemic and its current or future impact on our business and the global economy.
Overview
We are a clinical-stage biotechnology company developing therapeutics for neurology, immuno-oncology, inflammatory diseases, and other genetic disorders based on our proprietary Spherical Nucleic Acid, or SNA™, technology. We believe that our proprietary SNA architecture has distinct chemical and biological properties that may provide advantages over other nucleic acid therapeutics and may have therapeutic potential to target diseases not typically addressed with other nucleic acid therapeutics. We are in preclinical development of XCUR-FXN, a lipid-nanoparticle SNA-based therapeutic candidate, for the intrathecal treatment of Friedreich’s ataxia, or FA. Our therapeutic candidate cavrotolimod (AST-008) is in a Phase 1b/2 clinical trial in patients with advanced solid tumors.
We believe one of the key strengths of our proprietary SNAs is that they have the potential for increased cellular uptake compared to conventional linear oligonucleotides and as a result the potential to achieve higher efficacy at the same doses of oligonucleotide administered. We have shown in clinical and preclinical studies that SNAs may have therapeutic potential in immuno-oncology and dermatology. In addition, we have shown in preclinical studies that SNAs may have therapeutic potential in neurology, ophthalmology, pulmonology, and gastroenterology. As a consequence, we have expanded our pipeline into neurology, for which IND-enabling activities are ongoing for XCUR-FXN, and are conducting early stage research activities in ophthalmology, pulmonology, and gastroenterology.
The table below sets forth the current status of development of our SNA therapeutic candidates.
___________
(1) Cavrotolimod is a TLR9 agonist.
(2) In combination with checkpoint inhibitors.
Operating, financing, and cash flow considerations
Since our inception in 2011, we have devoted substantial resources to the research and development of SNAs and the protection and enhancement of our intellectual property. We have no products approved for sale and have primarily funded our operations through sales of our securities and collaborations. Through September 30, 2021, we have raised gross proceeds of $190.1 million from the sale of common stock and preferred stock. We have also received $56.0 million in upfront payments from collaborations, including an upfront payment of $20.0 million we received in August 2021 in connection with the Ipsen Collaboration Agreement and an upfront payment of $25.0 million we received in November 2019 in connection with the AbbVie Collaboration Agreement. On September 25, 2020, we also borrowed $17.5 million under the terms of a credit and security agreement with MidCap Financial Trust (as described further below). As of September 30, 2021, our cash, cash equivalents, short-term investments, and restricted cash were $62.0 million.
Since our inception, we have incurred significant operating losses. As of September 30, 2021, we have generated an accumulated deficit of $175.1 million. Substantially all of our operating losses resulted from expenses incurred in connection with our research programs and from general and administrative costs associated with our operations.
We expect to continue to incur losses for the foreseeable future. Our net losses may fluctuate significantly from quarter to quarter and year to year. We anticipate that our expenses will increase substantially as we:
•continue research and development of XCUR-FXN and other neurological therapeutic candidates and advance preclinical product candidates into clinical development;
•continue to advance the ongoing Phase 1b/2 clinical development for cavrotolimod (AST-008) for immuno-oncology applications;
•advance our SNA platform with our current and prospective suitable collaboration partners;
•initiate research and development, preclinical studies and clinical trials for any additional therapeutic candidates that we may pursue in the future;
•advance other therapeutic candidates through preclinical and clinical development;
•increase our research and development activities to enhance our technology platform;
•continue to manufacture increasing quantities of drug substance and drug product material for use in preclinical studies and clinical trials;
•seek regulatory approval for our therapeutic candidates that successfully complete clinical trials;
•maintain, expand and protect our intellectual property portfolio;
•acquire or in-license other approved drugs, drug candidates or technologies;
•hire additional operational, financial and management information systems and personnel, including personnel to support our product development and planned future commercialization efforts; and
•incur additional costs associated with operating as a public company.
We have not generated any revenue from commercial drug sales nor do we expect to generate substantial revenue from product sales unless or until we successfully complete development and obtain regulatory approval of and commercialize one or more of our therapeutic candidates. We do not anticipate generating revenue from drug sales for the next several years, if ever. If we obtain regulatory approval for any of our therapeutic candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and
distribution. Other sources of revenue could include a combination of research and development payments, license fees and other upfront payments, milestone payments, and royalties in connection with our current and any future collaborations and licenses. Until such time, if ever, that we generate revenue from whatever source, we expect to finance our cash needs through a combination of public or private equity offerings, debt financings and research collaboration and license agreements. We may be unable to raise capital or enter into such other arrangements when needed or on favorable terms. Our failure to raise capital or enter into such other arrangements as and when needed would have a negative impact on our financial condition and our ability to develop our therapeutic candidates.
Recent Developments
As previously reported, on November 9, 2021, the Audit Committee of our Board of Directors was notified of a claim made by a former Company senior researcher regarding alleged improprieties that researcher claims to have committed with respect to our XCUR-FXN preclinical program for the treatment of Friedreich’s ataxia. The Audit Committee has retained external counsel to conduct an internal investigation of the claim. We are currently unable to predict the timing or outcome of the investigation.
Therapeutic Development Program Updates
XCUR-FXN, Friedreich’s ataxia
We are developing XCUR-FXN, an SNA-based therapeutic candidate for the treatment of FA. We remain committed to maintaining our development plans and to pursuing our business strategy in the best interests of our stockholders as well as the patients we look to serve; however, we acknowledge that, at this point in time, we are unable to determine the potential impact of the asserted claim referenced above on our research and development activities or the timing of completion of our current research and development of our XCUR-FXN preclinical program for the treatment of FA, as the investigation of the asserted claim referenced above remains ongoing.
Cavrotolimod (AST-008)
We are currently evaluating cavrotolimod (AST-008) in a Phase 1b/2 clinical trial in patients with advanced solid tumors. As of November 4, 2021, we had 25 clinical trial sites activated for enrollment and 2 additional sites pending activation. We expect to open approximately 27 sites in total for the Phase 2 stage of the clinical trial. We anticipate all sites will be activated by the first half of 2022. As of November 4, 2021, we have dosed 37 patients with 32 mg of cavrotolimod (AST-008) in the Phase 2 portion of the clinical trial, including the primary and exploratory cohorts. Including the six patients dosed with 32 mg of cavrotolimod (AST-008) in the Phase 1b portion of the clinical trial, a total of 43 patients have been dosed with 32 mg of cavrotolimod (AST-008) through November 4, 2021. As of November 4, 2021, four of the 43 patients dosed with 32 mg of cavrotolimod (AST-008) have experienced serious adverse events, or SAEs, assessed as related to cavrotolimod by clinical trial investigators. All of these patients were dosed in the Phase 2 stage of the clinical trial. The treatment-related SAEs experienced by these four patients were hypotension (n=2), flu-like symptoms (n=1), injection-related reaction (n=1), and injection site reaction (n=1). None of the 14 patients dosed in the Phase 1b portion of the clinical trial with doses of cavrotolimod (AST-008) less than 32 mg experienced a treatment-related SAE. In summary, as of November 4, 2021, in total, 4 of 57 patients treated with cavrotolimod (AST-008) have experienced a treatment-related SAE.
We believe the impact of the ongoing COVID-19 pandemic continues to contribute to delays that began during the third quarter of 2020 and continued into the second half of 2021 in our enrollment plans and clinical trial site start-ups for the Phase 2 dose expansion phase of the Phase 1b/2 clinical trial for its cavrotolimod (AST-008) clinical program. As a result of these delays, pending further delays or any additional unanticipated effects of the COVID-19 pandemic on our clinical development plans, we now expect to report ORR results of cavrotolimod (AST-008) in the second half of 2022 rather than the first half of 2022. We will also continue to seek opportunities to advance this program through strategic collaborations or partnerships with the goal of maximizing stockholder value of the program and the full potential of our pipeline.
Update Regarding AbbVie Collaboration
We previously granted to AbbVie, Inc., or AbbVie, exclusive access and options to license SNA-based therapeutics arising from two collaboration programs we are conducting related to the treatment of hair loss disorders. During the third quarter of 2021, the joint development committee for the AbbVie collaboration made the decision to revise the initial development plan for both of the AbbVie collaboration programs. Due to these revised workplans, we expect significant additional efforts will be required to satisfy the performance obligation under our Collaboration, Option and License Agreement with AbbVie, or the AbbVie Collaboration Agreement, as we conduct additional early-stage discovery and preclinical activities than those originally set forth in the respective initial development plan. These increased estimated efforts in connection with the change in workplan resulted in less progress occurring relative to the increased estimate of total project hours to complete the research services during the three and nine months ended September 30, 2021 as compared to the amount of revenue recognized at both June 30, 2021 and December 31, 2020, which led to revenue reversals in each respective period. As such, we recorded a cumulative catchup adjustment (reduction) of revenue of $(4.5) million during the three and nine months ended September 30, 2021. Refer to Note 3, Collaborative Research and License Agreements, of the accompanying unaudited condensed consolidated financial statements for more information regarding the AbbVie Collaboration Agreement.
COVID-19 Business Update
As the global spread of the COVID-19 pandemic continues to affect our economy and our industry, we continue to monitor closely the developments and continue to take active measures to protect the health of our employees and their families, our communities, as well as our clinical trial investigators, patients, and caregivers. On June 11, 2021, Illinois moved into “Phase 5” of the Restore Illinois Plan, which is intended to permit businesses to operate at full capacity and permits fully vaccinated individuals to resume activities without wearing a mask except where required by federal, state, local, tribal, or territorial laws, rules and regulations, including local business and workplace guidance. As of November 12, 2021, Illinois remains in “Phase 5” of the Restore Illinois Plan.
Supply chain
We are working closely with our third-party manufacturers and other partners to manage our supply chain activities and mitigate potential disruptions as a result of the COVID-19 pandemic. We have observed minor delays in receipt of key chemicals, reagents and materials as certain manufacturers have had supply disruptions related to the COVID-19 pandemic. During the second half of 2021, we have experienced delays in the receipt of components needed for the manufacture of GMP drug product of cavrotolimod (AST-008) and XCUR-FXN. We are actively exploring substitutions to, and alternative sources for, these components to minimize any potential delays in the manufacture of these GMP drug products. Delays in the manufacturing of GMP drug product could adversely impact our clinical development activities for those programs. If the COVID-19 pandemic continues to persist for an extended period of time and impacts essential distribution systems such as FedEx and postal delivery, we could experience future disruptions to our supply chain and operations and associated delays in the manufacturing and our clinical supply, which would adversely impact our preclinical and clinical development activities.
Clinical operations
We believe the ongoing COVID-19 pandemic has affected our Phase 1b/2 clinical trial of cavrotolimod (AST-008). Namely, the COVID-19 pandemic or its impact has contributed to delays that began during the third quarter of 2020 and continued into the second half of 2021 in our enrollment plans and clinical trial site start-ups for the Phase 2 dose expansion phase of the clinical trial. As a result of these delays, pending further delays or any additional unanticipated effects of the COVID-19 pandemic on our clinical development plans, we now expect to report ORR results of cavrotolimod (AST-008) in the second half of 2022 rather than the first half of 2022. Beginning in third quarter of 2020, we implemented, and continue to employ, additional measures to increase the enrollment of patients, including frequent interaction with our clinical trial sites currently open as well as increasing the number of clinical trial sites that potentially are activated for this trial so that we may continue to enroll patients as initially planned. However, any additional delays may require us to further lengthen our clinical development
timeline for cavrotolimod (AST-008) and could potentially delay our proposed clinical development timeline for XCUR-FXN.
We remain committed to maintaining our development plans for cavrotolimod (AST-008) and XCUR-FXN and continue to monitor and manage the rapidly evolving situation of the effects of the pandemic. We have taken and continue to take measures to implement remote and virtual approaches, including remote patient monitoring where possible, to maintain patient safety and trial continuity and to preserve study integrity. Should the COVID-19 pandemic or its impact or effects continue, our ability to maintain patient enrollment and our clinical development timeline could continue to be negatively impacted. We could also see an impact on our ability to supply study drug, report trial results, or interact with regulators, ethics committees or other important agencies due to limitations in regulatory authority employee resources or otherwise. In addition, we rely on contract research organizations or other third parties to assist us with clinical trials, and we cannot guarantee that they will continue to perform their contractual duties in a timely and satisfactory manner as a result of the COVID-19 pandemic. As the COVID-19 pandemic continues to persist for an extended period of time, we continue to be impacted and could experience additional delays in patient enrollment for our Phase 2 stage of the Phase 1b/2 clinical trial of cavrotolimod (AST-008). Any significant disruptions to our clinical development timelines would further delay our anticipated timeline for results and adversely affect our business, financial condition, results of operations and growth prospects.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the revenue and expenses incurred during the reported periods. We base our estimates on historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not apparent from other sources. Changes in estimates are reflected in reported results for the period in which they become known. Actual results may differ from these estimates under different assumptions or conditions, including uncertainty in the current economic environment due to the ongoing COVID-19 pandemic.
Our critical accounting policies require the most significant judgments and estimates in the preparation of our consolidated financial statements. There have been no significant changes to our critical accounting policies from those which were discussed in our Annual Report on Form 10-K for the year ended December 31, 2020, except as discussed in Note 2, Significant Accounting Policies, in the accompanying notes to the unaudited condensed consolidated financial statements related to revenue recognition.
Recently adopted accounting pronouncements
None.
Recent accounting pronouncements not yet adopted
Refer to Note 2, Significant Accounting Policies, of the accompanying unaudited condensed consolidated financial statements for a description of recently accounting pronouncements not yet updated.
Results of Operations
Comparison of the Three Months Ended September 30, 2021 and 2020
The following table summarizes the results of our operations for the three months ended September 30, 2021 and 2020:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
September 30, | | | | |
| (dollars in thousands) | 2021 | | 2020 | | Change |
| Revenue: | | | | | | | |
| Collaboration revenue | $ | (3,677) | | | $ | 2,443 | | | $ | (6,120) | | | (251) | % |
| Total revenue | (3,677) | | | 2,443 | | | (6,120) | | | (251) | % |
| Operating expenses: | | | | | | | |
| Research and development expense | 16,457 | | | 9,139 | | | 7,318 | | | 80 | % |
| General and administrative expense | 2,947 | | | 2,424 | | | 523 | | | 22 | % |
| Total operating expenses | 19,404 | | | 11,563 | | | 7,841 | | | 68 | % |
| Operating loss | (23,081) | | | (9,120) | | | (13,961) | | | 153 | % |
| Other (expense) income, net: | | | | | | | |
| Dividend income | 2 | | | 2 | | | — | | | — | % |
| Interest income | 8 | | | 205 | | | (197) | | | (96) | % |
| Interest expense | (455) | | | (27) | | | (428) | | | 1,585 | % |
| Other (expense) income, net | (5) | | | 118 | | | (123) | | | n/m |
| Total other (expense) income, net | (450) | | | 298 | | | (748) | | | (251) | % |
| Net loss before provision for income taxes | (23,531) | | | (8,822) | | | (14,709) | | | 167 | % |
| Provision for income taxes | — | | | — | | | — | | | — | % |
| Net loss | $ | (23,531) | | | $ | (8,822) | | | $ | (14,709) | | | 167 | % |
Revenue
The following table summarizes our revenue earned during the periods indicated:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
September 30, | | |
| (dollars in thousands) | 2021 | | 2020 | | Change |
| Collaboration revenue: | | | | | | | |
AbbVie Collaboration Agreement | $ | (4,480) | | | $ | 2,402 | | | $ | (6,882) | | | (287) | % |
Ipsen Collaboration Agreement | 803 | | | — | | | 803 | | | n/m |
| Dermelix Collaboration Agreement | — | | | 41 | | | (41) | | | (100) | % |
| Total collaboration revenue | $ | (3,677) | | | $ | 2,443 | | | $ | (6,120) | | | (251) | % |
| Total revenue | $ | (3,677) | | | $ | 2,443 | | | $ | (6,120) | | | (251) | % |
Collaboration revenue was $(3.7) million during the three months ended September 30, 2021, reflecting a decrease of $6.1 million, or 251%, from collaboration revenue of $2.4 million for the three months ended September 30, 2020. The decrease in collaboration revenue of $6.1 million is mostly due to a decrease in revenue related to the AbbVie Collaboration Agreement of $6.9 million partially offset by revenue related to the Ipsen Collaboration Agreement of $0.8 million.
As discussed further in Note 3, Collaborative Research and License Agreements, of the accompanying unaudited condensed consolidated financial statements, revenue recognized under the AbbVie Collaboration Agreement for the three months ended September 30, 2021 reflects the cumulative catchup adjustment (reduction) of revenue of $(4.5) million in connection with the change in estimate that resulted from a change in workplan during the third quarter of 2021. We currently estimate significant additional efforts will be required to satisfy the performance obligation under the AbbVie Collaboration Agreement. These increased estimated efforts in connection with the change in workplan resulted in less progress occurring relative to the increased estimate of total project hours to complete the research services during the three and nine months ended September 30, 2021 and compared to the amount of revenue recognized at both June 30, 2021 and December 31, 2020, which led to revenue reversals in each respective period. As of September 30, 2021, deferred revenue under the AbbVie Collaboration Agreement was $11.7 million and is expected to be recognized as revenue over the next 24 to 27 months as we satisfy our obligations under the AbbVie Collaboration Agreement. In August 2021, we received an upfront payment of $20.0 million in connection with the Ipsen Collaboration Agreement for which revenue has been deferred and will be recognized as revenue in future periods as we satisfy our obligations under the Ipsen Collaboration Agreement. At September 30, 2021, deferred revenue under the Ipsen Collaboration Agreement was $19.2 million and is expected to be recognized as revenue over the next 33 to 42 months as we satisfy our obligations under the Ipsen Collaboration Agreement.
Refer to Note 3, Collaborative Research and License Agreements, of the accompanying unaudited condensed consolidated financial statements for more information regarding revenue recognition for the AbbVie Collaboration Agreement and Ipsen Collaboration Agreement.
We do not expect to generate any product revenue for the foreseeable future. However, future revenue may include amounts attributable to partnership activities including, a combination of research and development payments, license fees and other upfront payments, milestone payments, product sales and royalties, and reimbursement of certain research and development expenses, in connection with the AbbVie Collaboration Agreement, the Ipsen Collaboration Agreement, the Dermelix Collaboration Agreement or any future collaboration and licenses.
Research and development expense
The following table summarizes our research and development expenses incurred during the periods indicated:
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
September 30, | | |
| (dollars in thousands) | 2021 | | 2020 | | Change |
| Clinical development programs expense | $ | 6,394 | | | $ | 2,173 | | | $ | 4,221 | | | 194 | % |
| Platform and discovery-related expense | 5,636 | | | 4,055 | | | 1,581 | | | 39 | % |
| Employee-related expense | 3,432 | | | 2,112 | | | 1,320 | | | 63 | % |
| Facilities, depreciation, and other expenses | 995 | | | 799 | | | 196 | | | 25 | % |
| Total research and development expense | $ | 16,457 | | | $ | 9,139 | | | $ | 7,318 | | | 80 | % |
| | | | | | | |
| Full time employees | 65 | | | 48 | | | 17 | | | |
Research and development expense was $16.5 million for the three months ended September 30, 2021, reflecting an increase of $7.3 million, or 80%, from research and development expense of $9.1 million for the three months ended September 30, 2020. Since September 30, 2020, we have increased our headcount in research and development from 48 to 65 as of September 30, 2021. The increase in research and development expense for the three months ended September 30, 2021 of $7.3 million reflects this increased headcount and the related increase in research and development activities, to include increased clinical trial activities. More specifically, the increase in research and development expense for the three months ended September 30, 2021 of $7.3 million was primarily due to a net increase in costs related to our clinical development programs of $4.2 million, higher platform and discovery-related expense of $1.6 million, and higher employee-related expenses of $1.3 million.
The net increase in clinical development programs expense for the three months ended September 30, 2021 of $4.2 million was primarily due to manufacturing and toxicology study costs in connection with IND-enabling and Phase 1 clinical trial preparation activities for XCUR-FXN, in addition to higher clinical trial costs in connection with our Phase 1b/2 clinical trial for cavrotolimod (AST-008), partially offset by lower manufacturing costs for cavrotolimod (AST-008).
The increase in platform and discovery-related expense of $1.6 million was mostly due to the license fee paid to Northwestern University of $3.0 million in connection with the receipt of the upfront payment of $20.0 million from Ipsen, partially offset by lower costs for materials, reagents, supplies, and contract research organizations.
The increase in employee-related expense for the three months ended September 30, 2021 of $1.3 million was due to higher compensation and related costs in connection with the net increase in headcount during the period presented as well as certain salary increases in 2021 for existing employees.
We expect our research and development expenses to continue to increase during 2021 as compared to 2020 as we broaden our pipeline of SNA-based therapeutic candidates, continue investment into our clinical development programs, and further develop our SNA technology platform.
General and administrative expense
| | | | | | | | | | | | | | | | | | | | | | | |
| Three Months Ended
September 30, | | |
| (dollars in thousands) | 2021 | | 2020 | | Change |
| General and administrative expense | $ | 2,947 | | | $ | 2,424 | | | 523 | | | 22 | % |
| Full time employees | 12 | | | 9 | | | 3 | | | |
General and administrative expense was $2.9 million for the three months ended September 30, 2021, representing an increase of $0.5 million, or 22%, from $2.4 million for the three months ended September 30, 2020. The increase for the three months ended September 30, 2021 was mostly due to higher compensation and related costs in connection with salary increases in 2021 and an increase in headcount, as well as higher legal costs.
Interest income
The decrease in interest income of $0.2 million for the three months ended September 30, 2021 was primarily the result of lower average balances invested in available for sale securities during the three months ended September 30, 2021 as compared to the prior-year period.
Interest expense
The increase in interest expense of $0.4 million for the three months ended September 30, 2021 was the result of a higher average debt balance during the three months ended September 30, 2021 as compared to the prior-year period.
Comparison of the Nine Months Ended September 30, 2021 and 2020
The following table summarizes the results of our operations for the nine months ended September 30, 2021 and 2020:
| | | | | | | | | | | | | | | | | | | | | | | |
| Nine Months Ended
September 30, | | | | |
| (dollars in thousands) | 2021 | | 2020 | | Change |
| Revenue: | | | | | | | |
| Collaboration revenue | $ | (2,601) | | | $ | 16,473 | | | $ | (19,074) | | | (116) | % |
| Total revenue | (2,601) | | | 16,473 | | | (19,074) | | | (116) | % |
| Operating expenses: | | | | | | | |
| Research and development expense | 37,562 | | | 22,222 | | | 15,340 | | | 69 | % |
| General and administrative expense | 8,937 | | | 7,227 | | | 1,710 | | | 24 | % |
| Total operating expenses | 46,499 | | | 29,449 | | | 17,050 | | | 58 | % |
| Operating loss | (49,100) | | | (12,976) | | | (36,124) | | | 278 | % |
| Other (expense) income, net: | | | | | | | |
| Dividend income | 5 | | | 45 | | | (40) | | | (89) | % |
| Interest income | 139 | | | 832 | | | (693) | | | (83) | % |
| Interest expense | (1,314) | | | (155) | | | (1,159) | | | 748 | % |
| Other (expense) income, net | (7) | | | 271 | | | (278) | | | n/m |
| Total other (expense) income, net | (1,177) | | | 993 | | | (2,170) | | | (219) | % |
| Net loss before provision for income taxes | (50,277) | | | (11,983) | | | (38,294) | | | 320 | % |
| Provision for income taxes | — | | | — | | | — | | | — | % |
| Net loss | $ | (50,277) | | | $ | (11,983) | | | $ | (38,294) | | | 320 | % |
Revenue
The following table summarizes our revenue earned during the periods indicated:
| | | | | | | | | | | | | | | | | | | | | | | |
| Nine Months Ended
September 30, | | |
| (dollars in thousands) | 2021 | | 2020 | | Change |
| Collaboration revenue: | | | | | | | |
AbbVie Collaboration Agreement | $ | (3,404) | | | $ | 16,351 | | | $ | (19,755) | | | (121) | % |
Ipsen Collaboration Agreement | 803 | | | — | | | 803 | | | n/m |
| Dermelix Collaboration Agreement | — | | | 122 | | | (122) | | | (100) | % |
| Total collaboration revenue | $ | (2,601) | | | $ | 16,473 | | | $ | (19,074) | | | (116) | % |
| Total revenue | $ | (2,601) | | | $ | 16,473 | | | $ | (19,074) | | | (116) | % |
Collaboration revenue was $(2.6) million during the nine months ended September 30, 2021, reflecting a decrease of $19.1 million, or 116%, from collaboration revenue of $16.5 million for the nine months ended September 30, 2020. The decrease in collaboration revenue of $19.1 million was mostly due to a decrease in revenue related to the AbbVie Collaboration Agreement of $19.8 million partially offset by revenue related to the Ipsen Collaboration Agreement of $0.8 million. As discussed further above in the section titled “Comparison of the Three Months Ended September 30, 2021 and 2020,” revenue related to the AbbVie Collaboration Agreement for
the nine months ended September 30, 2021 reflects the cumulative catchup adjustment (reduction) of revenue of $(4.5) million in connection with the change in estimate during the third quarter of 2021.
Refer to Note 3, Collaborative Research and License Agreements, of the accompanying unaudited condensed consolidated financial statements for more information regarding revenue recognition for the AbbVie Collaboration Agreement and Ipsen Collaboration Agreement.
We do not expect to generate any product revenue for the foreseeable future. However, future revenue may include amounts attributable to partnership activities including, a combination of research and development payments, license fees and other upfront payments, milestone payments, product sales and royalties, and reimbursement of certain research and development expenses, in connection with the AbbVie Collaboration Agreement, the Ipsen Collaboration Agreement, the Dermelix Collaboration Agreement or any future collaboration and licenses.
Research and development expense
The following table summarizes our research and development expenses incurred during the periods indicated:
| | | | | | | | | | | | | | | | | | | | | | | |
| Nine Months Ended
September 30, | | |
| (dollars in thousands) | 2021 | | 2020 | | Change |
| Clinical development programs expense | $ | 13,199 | | | $ | 5,438 | | | $ | 7,761 | | | 143 | % |
| Platform and discovery-related expense | 11,922 | | | 9,620 | | | 2,302 | | | 24 | % |
| Employee-related expense | 9,392 | | | 5,379 | | | 4,013 | | | 75 | % |
| Facilities, depreciation, and other expenses | 3,049 | | | 1,785 | | | 1,264 | | | 71 | % |
| Total research and development expense | $ | 37,562 | | | $ | 22,222 | | | $ | 15,340 | | | 69 | % |
| | | | | | | |
| Full time employees | 65 | | | 48 | | | 17 | | | |
Research and development expense was $37.6 million for the nine months ended September 30, 2021, reflecting an increase of $15.3 million, or 69%, from research and development expense of $22.2 million for the nine months ended September 30, 2020. Since September 30, 2020, we have increased our headcount in research and development from 48 to 65 at September 30, 2021. The increase in research and development expense for the nine months ended September 30, 2021 of $15.3 million reflects this increased headcount and the related increase in research and development activities, to include increased clinical trial activities. More specifically, the increase in research and development expense for the nine months ended September 30, 2021 of $15.3 million was primarily due to a net increase in costs related to our clinical development programs of $7.8 million, higher employee-related expenses of $4.0 million, higher platform and discovery-related expense of $2.3 million, and higher facilities, depreciation, and other expenses of $1.3 million.
The net increase in clinical development programs expense for the nine months ended September 30, 2021 of $7.8 million was primarily due to manufacturing and toxicology study costs in connection with IND-enabling and Phase 1 clinical trial preparation activities for XCUR-FXN, in addition to higher clinical trial costs in connection with our Phase 1b/2 clinical trial for cavrotolimod (AST-008), partially offset by lower manufacturing costs for cavrotolimod (AST-008).
The increase in platform and discovery-related expense of $2.3 million was mostly due to the license fee paid to Northwestern University of $3.0 million in connection with the receipt of the upfront payment of $20.0 million from Ipsen, partially offset by lower costs for materials and reagents.
The increase in employee-related expense for the nine months ended September 30, 2021 of $4.0 million was due to higher compensation and related costs in connection with the net increase in headcount during the period presented as well as certain salary increases in 2021 for existing employees.
The increase in facilities, depreciation, and other expenses for the nine months ended September 30, 2021 of $1.3 million was mostly due to higher lease costs related to our Chicago lease that commenced on July 1, 2020 as well as higher depreciation expense in connection with the acquisition of additional scientific equipment that were placed in service since the prior-year period.
We expect our research and development expenses to continue to increase during 2021 as compared to 2020 as we broaden our pipeline of SNA-based therapeutic candidates, continue investment into our clinical development programs, and further develop our SNA technology platform.
General and administrative expense
| | | | | | | | | | | | | | | | | | | | | | | |
| Nine Months Ended
September 30, | | |
| (dollars in thousands) | 2021 | | 2020 | | Change |
| General and administrative expense | $ | 8,937 | | | $ | 7,227 | | | 1,710 | | | 24 | % |
| Full time employees | 12 | | | 9 | | | 3 | | | |
General and administrative expense was $8.9 million for the nine months ended September 30, 2021, representing an increase of $1.7 million, or 24%, from $7.2 million for the nine months ended September 30, 2020. The increase for the nine months ended September 30, 2021 is mostly due to higher compensation and related costs mostly due to salary increases in 2021 and an increase in headcount, recruiting costs, higher consulting and legal costs, and higher D&O insurance premium costs. This increase was partially offset by lower investor relations and franchise tax costs.
Interest income
The decrease in interest income of $0.7 million for the nine months ended September 30, 2021 was primarily the result of lower average balances invested in available for sale securities during the nine months ended September 30, 2021 as compared to the prior-year period.
Interest expense
The increase in interest expense of $1.2 million for the nine months ended September 30, 2021 was the result of a higher average debt balance during the nine months ended September 30, 2021 as compared to the prior-year period.
Liquidity and Capital Resources
Since our inception, we have incurred significant operating losses. We have generated limited revenue to date from our collaboration agreements. We have not yet commercialized any of our product candidates, which are in various phases of preclinical development and clinical trials; and we do not expect to generate revenue from sales of any product for several years, if at all. We have funded our operations to date with proceeds received from equity financings and payments received in connection with collaboration agreements.
As of September 30, 2021, our cash, cash equivalents, short-term investments, and restricted cash were $62.0 million. As of September 30, 2021, we have generated an accumulated deficit of $175.1 million since inception and expect to incur significant expenses and negative cash flows for the foreseeable future. Based on our current operating plans and existing working capital at September 30, 2021, it is uncertain whether our current liquidity is sufficient to fund operations over the next twelve months from the date of the issuance of the accompanying condensed consolidated financial statements. As a result, there is substantial doubt about our ability to continue as a going concern. We have no committed sources of additional capital at this time and substantial additional financing will be needed by us to fund our operations. Management believes that we will be able to obtain additional funding through equity or debt financings, collaboration agreements, strategic partnerships and licensing arrangements, or other arrangements, such as our “at the market offering” program pursuant to our equity distribution agreement with BMO Capital Markets Corp., or BMO, to fund our current operations and business strategy. However, there can be no assurance that such additional financing will be available and, if available, can be obtained on terms acceptable to
us. If we are unable to obtain such additional financing, we could be forced to delay, reduce or eliminate its research and development programs or clinical efforts, which could adversely affect its business prospects, or we may be unable to continue operations. We have historically principally raised capital through the sale of our securities. However, the COVID-19 pandemic continues to rapidly evolve and has already resulted in a significant disruption of global financial markets. If the disruption continues to persist and deepens, we could experience an inability to access additional capital, which could in the future negatively affect our ability to raise capital and ultimately, our operations.
MidCap Facility
On September 30, 2021, we entered into an amendment, or Amendment No. 3, to our Credit and Security Agreement, dated as of September 25, 2020, as amended on October 21, 2020 and July 30, 2021, with MidCap Financial Trust, as agent, or MidCap, and the lenders party thereto from time to time, or as amended by Amendment No. 3, the MidCap Credit Agreement.
The MidCap Credit Agreement provides for a secured term loan facility in an aggregate principal amount of up to $25.0 million, or the MidCap Credit Facility. We borrowed the first advance of $17.5 million, or Tranche 1, on September 25, 2020, or the Closing Date. Amendment No. 3 extends the availability of the second advance of $7.5 million, or Tranche 2, under the MidCap Credit Agreement from September 30, 2021 to December 31, 2021, subject to our satisfaction of certain applicable funding conditions as described in Amendment No. 3, including our issuance of equity interests, subject to certain limitations, resulting in receipt by us of unrestricted net cash proceeds of at least $20.0 million, and the contribution by us, and receipt by Exicure Operating Company, of such cash proceeds to the capital of Exicure Operating Company.
Tranche 1 and if borrowed, Tranche 2, each bear interest at a floating rate equal to 6.25% per annum, plus the greater of (i) 1.50% or (ii) one-month LIBOR. Interest on each loan advance is due and payable monthly in arrears. Principal on each loan advance is payable in 36 equal monthly installments beginning October 1, 2022 until paid in full on October 1, 2025, or the Maturity Date. Prepayments of the loans under the MidCap Credit Agreement, in whole or in part, will be subject to early termination fees in an amount equal to 3.0% of principal prepaid if prepayment occurs on or prior to the first anniversary of the Closing Date and 1.0% of principal prepaid if prepayment occurs after the first anniversary of the Closing Date and prior to the maturity date. In connection with execution of the MidCap Credit Agreement, we paid MidCap a $125,000 origination fee.
At the Maturity Date or on any earlier date on which all amounts advanced to us become due and payable in full, or are otherwise paid in full, we are required to pay an exit fee equal to 3.75% of the principal amount of all loans advanced to us under the MidCap Credit Agreement.
Our obligations under the MidCap Credit Agreement are secured by a security interest in substantially all of our assets, excluding intellectual property (which is subject to a negative pledge). Additionally, our future subsidiaries, if any, may be required to become co-borrowers or guarantors under the MidCap Credit Agreement.
The MidCap Credit Agreement contains customary affirmative covenants and customary negative covenants limiting our ability and the ability of our subsidiaries, if any, to, among other things, dispose of assets, undergo a change in control, merge or consolidate, make acquisitions, incur debt, incur liens, pay dividends, repurchase stock and make investments, in each case subject to certain exceptions.
The MidCap Credit Agreement also contains customary events of default relating to, among other things, payment defaults, breaches of covenants, a material adverse change, delisting of our common stock, bankruptcy and insolvency, cross defaults with certain material indebtedness and certain material contracts, judgments, and inaccuracies of representations and warranties. Upon an event of default, the agent and the lenders may declare all or a portion of our outstanding obligations to be immediately due and payable and exercise other rights and remedies provided for under the MidCap Credit Agreement. During the existence of an event of default, interest on the obligations could be increased by 2.0%.
At-the-Market Facility Program
In December 2020, we entered into an equity distribution agreement with BMO under which we may offer and sell in “at the market offerings” (as defined in Rule 415(a)(4) of the Securities Act of 1933, as amended) from time to time at our sole discretion, shares of our common stock having an aggregate offering price of up to $50.0 million through BMO acting as our distribution agent pursuant to a prospectus supplement and a shelf registration statement on Form S-3 that was declared effective by the SEC on January 7, 2021. Through September 30, 2021, we have not sold any shares under the equity distribution agreement.
Cash Flows
The following table shows a summary of our cash flows for the nine months ended September 30, 2021 and 2020:
| | | | | | | | | | | | | | |
| | Nine Months Ended
September 30, |
| (in thousands) | | 2021 | | 2020 |
| | (unaudited) |
| Net cash used in operating activities | | $ | (21,022) | | | $ | (27,463) | |
| Net cash provided by (used in) investing activities | | 38,977 | | | (3,330) | |
| Net cash provided by financing activities | | 668 | | | 14,992 | |
| Net increase (decrease) in cash, cash equivalents, and restricted cash | | $ | 18,623 | | | $ | (15,801) | |
Operating activities
Net cash used in operating activities was $21.0 million and $27.5 million for the nine months ended September 30, 2021 and 2020, respectively. The decrease in cash used in operating activities for the nine months ended September 30, 2021 of $6.4 million was primarily due to the receipt of the upfront payment of $20.0 million from Ipsen in connection with the Ipsen Collaboration Agreement, partially offset by higher cash used for working capital and the license fee paid to Northwestern University of $3.0 million in connection with receipt of the Ipsen Upfront Payment.
Investing activities
Net cash provided by (used in) investing activities was $39.0 million and $(3.3) million for the nine months ended September 30, 2021 and 2020, respectively. The increase in cash provided by investing activities of $42.3 million was primarily due to higher proceeds from the maturity, net of purchases, of available-for-sale securities, as well as a decrease in the purchase of scientific equipment of $2.5 million.
Financing activities
Net cash provided by financing activities of $0.7 million for nine months ended September 30, 2021 is due to the proceeds received from the exercise of stock options and the issuance of common stock in connection with our employee stock purchase plan.
Net cash provided by financing activities of $15.0 million for the nine months ended September 30, 2020 was mostly due to the net proceeds we received of $17.3 million during the period in connection with the MidCap Credit Agreement (as discussed above), as well as the net proceeds received from the sale of shares of our common stock in the amount of $2.8 million pursuant to the partial exercise of the option to purchase additional shares by the underwriters from our December 2019 financing, partially offset by the repayment of the remaining outstanding obligations under the Hercules Loan Agreement in the amount of $5.0 million upon the loan’s maturity.
Funding Requirements
We expect that our primary uses of capital will continue to be third-party clinical and research and development services, compensation and related expenses, laboratory and related supplies, legal and other regulatory expenses and general overhead costs. Because of the numerous risks and uncertainties associated with research, development and commercialization of therapeutic candidates, we are unable to estimate the exact amount of our working capital requirements. Our future capital requirements are difficult to forecast and will depend on many factors, including:
•the terms and timing of any other collaboration, licensing and other arrangements that we may establish;
•the initiation, progress, timing and completion of preclinical studies and clinical trials for our potential therapeutic candidates;
•the effects of health epidemics, including the ongoing COVID-19 pandemic, on our operations or the business or operations of our contract research organizations, or CROs, or other third parties with whom we conduct business;
•the number and characteristics of therapeutic candidates that we pursue;
•the progress, costs and results of our preclinical studies and clinical trials;
•the outcome, timing and cost of regulatory approvals;
•delays that may be caused by changing regulatory requirements;
•the cost and timing of hiring new employees to support our continued growth;
•unknown legal, administrative, regulatory, accounting, and information technology costs as well as additional costs associated with operating as a public company;
•the costs involved in filing and prosecuting patent applications and enforcing and defending patent claims;
•the costs of filing and prosecuting intellectual property rights and enforcing and defending any intellectual property-related claims;
•the costs and timing of procuring clinical and commercial supplies of our therapeutic candidates;
•the extent to which we acquire or in-license other therapeutic candidates and technologies; and
•the extent to which we acquire or invest in other businesses, therapeutic candidates or technologies.
Based on our current operating plans and existing working capital at September 30, 2021, it is uncertain whether our current liquidity is sufficient to fund operations over the next twelve months from the date of the issuance of the accompanying condensed consolidated financial statements. As a result, there is substantial doubt about our ability to continue as a going concern. We have no committed sources of additional capital at this time and substantial additional financing will be needed by us to fund our operations. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our capital resources sooner than we expect.
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances, and marketing, distribution or licensing arrangements with third parties. The COVID-19 pandemic continues to evolve and has resulted in a significant disruption of global financial markets. If the disruption continues to persist and deepens, we could experience an inability to access additional capital, which could in the future negatively affect our operations.
To the extent that we raise additional capital through future equity financings, the ownership interest of our stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our existing common stockholders. If we raise additional funds through the issuance of debt securities, these securities could contain covenants that would restrict our operations. If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, reduce or eliminate our product development efforts or future commercialization efforts, or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Contractual Obligations and Commitments
There have been no material changes to our contractual obligations and commitments from those described in our Annual Report on Form 10-K for the year ended December 31, 2020.
Off-balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements, as defined in the rules and regulations of the SEC.
JOBS Act
We are an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. Under the JOBS Act, an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. Thus, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies.
In addition, as an emerging growth company, we will not be required to provide an auditor’s attestation report on our internal control over financial reporting in future annual reports on Form 10-K as otherwise required by Section 404(b) of the Sarbanes-Oxley Act of 2002, or Sarbanes-Oxley Act. Our compliance with Section 404 of the Sarbanes-Oxley Act first became subject to management’s assessment regarding internal control over financial reporting in connection with the filing of our Annual Report on Form 10-K for the fiscal year ending December 31, 2018, and we will not be required to have an independent registered public accounting firm attest to the effectiveness of our internal control over financial reporting until the filing of our first Annual Report on Form 10-K after we lose emerging growth company status, which may not be until the 2023 Annual Report on Form 10-K.
In addition, we are also a smaller reporting company as defined in the Securities Exchange Act of 1934, as amended, or Exchange Act. We may continue to be a smaller reporting company even after we are no longer an emerging growth company. We may take advantage of certain of the scaled disclosures available to smaller reporting companies and will be able to take advantage of these scaled disclosures for so long as (i) our voting and non-voting common stock held by non-affiliates is less than $250.0 million measured on the last business day of our second fiscal quarter or (ii) our annual revenue is less than $100.0 million during the most recently completed fiscal year and our voting and non-voting common stock held by non-affiliates is less than $700.0 million measured on the last business day of our second fiscal quarter.
Item 3. Quantitative and Qualitative Disclosures about Market Risk.
As a smaller reporting company, as defined by Rule 12b-2 of the Exchange Act and in Item 10(f)(1) of Regulation S-K, we are electing scaled disclosure reporting obligations and therefore are not required to provide the information requested by this Item.
Item 4. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our periodic and current reports that we file with the SEC under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our principal executive officer and principal financial officer, as appropriate, to allow timely decisions regarding required disclosure. Management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving their objectives and management necessarily applies its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As of the end of the period covered by this Quarterly Report on Form 10-Q, we carried out an evaluation, under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Exchange Act Rule 13a-15. Based upon, and as of the date of, this evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were effective. Accordingly, management believes that the financial statements included in this report fairly present in all material respects our financial condition, results of operations and cash flows for the periods presented.
Changes in Internal Control over Financial Reporting
We continuously seek to improve the efficiency and effectiveness of our internal controls. There was no change in our internal control over financial reporting identified in connection with the evaluation required by Rule 13a-15(d) and 15d-15(d) of the Exchange Act during the fiscal quarter ended September 30, 2021 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
PART II – OTHER INFORMATION
Item 1. Legal Proceedings.
From time to time, we may be subject to legal proceedings. We are not currently a party to or aware of any proceedings that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or results of operations.
Item 1A. Risk Factors.
In addition to other information contained in this Quarterly Report on Form 10-Q, the following risks should be considered in evaluating our business and future prospects and an investment in our common stock. The risks and uncertainties described below are not the only ones we face. If any of the following risks and uncertainties develops into actual events, our business, financial condition, results of operations and cash flows could be materially adversely affected. In that case, the price of our common stock could decline and you may lose all or part of your investment.
Risks Related to Our Business
Our consolidated financial statements have been prepared assuming that we will continue as a going concern.
We have incurred recurring losses from operations since inception which raises substantial doubt about our ability to continue as a going concern. If we are unable to obtain sufficient funding, our business, prospects, financial condition and results of operations will be materially and adversely affected, and we may be unable to continue as a going concern. If we are unable to continue as a going concern, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our audited financial statements, and it is likely that investors will lose all or a part of their investment. In addition, if there remains substantial doubt about our ability to continue as a going concern, investors or other financing sources may be unwilling to provide additional funding to us on commercially reasonable terms, or at all.
We are a clinical-stage biotechnology company with a history of losses. We expect to continue to incur significant losses for the foreseeable future and may never achieve or maintain profitability, which could result in a decline in the market value of our common stock.
We are a biotechnology company developing therapeutics for immuno-oncology, genetic disorders and other indications based on our proprietary SNA technology. Since our inception in June 2011, we have devoted our resources to the development of SNA technology. We have had significant operating losses since our inception. As of September 30, 2021, we have generated an accumulated deficit of $175.1 million. For the nine months ended September 30, 2021 and 2020, our net loss was $50.3 million and $12.0 million, respectively. Substantially all of our losses have resulted from expenses incurred in connection with our research programs and from general and administrative costs associated with our operations. Our technology and therapeutic candidates are in early stages of development, and we are subject to the risks of failure inherent in the development of therapeutic candidates based on novel technologies.
We have not generated, and do not expect to generate, any product revenue for the foreseeable future, and we expect to continue to incur significant operating losses for the foreseeable future due to the cost of research and development, preclinical studies, clinical trials, and the regulatory approval process for therapeutic candidates. The amount of future losses is uncertain. Our ability to achieve profitability, if ever, will depend on, among other things, us, or any current or future collaborators, successfully developing therapeutic candidates, obtaining regulatory approvals to market and commercialize therapeutic candidates, manufacturing any approved products on commercially reasonable terms, establishing a sales and marketing organization or suitable third-party alternatives for any approved product and raising sufficient funds to finance business activities. If we, or any current or future collaborators, are unable to develop and commercialize one or more of our therapeutic candidates or if sales revenue from any therapeutic candidate that receives approval is insufficient, we will not achieve profitability, which could have a material adverse effect on our business, financial condition, results of operations and prospects.
Management has identified certain conditions or events, which, considered in the aggregate, raise substantial doubt about our ability to continue as a going concern, including the risk that we will be unable to raise adequate additional capital to fund our operations through at least the 12 months following the filing date of this Quarterly Report. Substantial doubt about our ability to continue as a going concern may create negative reactions to the price of our common stock. If we are unable to raise capital, we may be required to delay, reduce the scope of or eliminate research and development programs, or obtain funds through arrangements with collaborators or others that may require us to relinquish rights to certain drug candidates that we might otherwise seek to develop or commercialize independently. In addition, if we are unable to continue as a going concern, we may have to liquidate our assets and may receive less than the value at which those assets are carried on our financial statements, and it is likely that investors will lose all or a part of their investment. Further, the perception that we may be unable to continue as a going concern may impede our ability to pursue strategic opportunities or operate our business due to concerns regarding our ability to discharge our contractual obligations.
Our approach to the discovery and development of innovative therapeutic treatments based on our technology is unproven and may not result in marketable products.
We plan to develop a pipeline of therapeutic candidates based on our proprietary SNA technology. We believe that therapeutic candidates identified with our therapeutic discovery technology may offer an improved therapeutic approach compared to small molecules and antibodies, as well as several advantages over linear oligonucleotide-based therapeutics. However, the scientific research that forms the basis of our efforts to develop therapeutic candidates based on our SNA technology and the identification and optimization of SNA-based therapeutic candidates is relatively new. Further, the scientific evidence to support the feasibility of developing therapeutic treatments based on SNA technology is both preliminary and limited.
Therapeutic candidates based on SNA technology have not been extensively tested in humans, and a number of clinical trials conducted by other companies using oligonucleotide technologies have not been successful. We may discover that the SNA-based therapeutic candidates do not possess certain properties required for therapeutic treatment to be effective, such as the ability to remain stable in the human body for the period of time required for the therapeutic candidate to reach the target tissue or the ability to cross the cell membrane and enter into cells within the target tissue for effective delivery. We currently have only limited data, and no conclusive evidence, to suggest that we can introduce these necessary drug-like properties into SNA-based therapeutic candidate. We may spend substantial funds attempting to introduce these properties and may never succeed in doing so. In addition, therapeutic candidates based on SNA technology may demonstrate different chemical and pharmacological properties in patients than they do in laboratory studies. Even if therapeutic candidates have successful results in animal studies, they may not demonstrate the same chemical and pharmacological properties in humans and may interact with human biological systems in unforeseen, ineffective or harmful ways. As a result, we may never succeed in developing a marketable therapeutic, we may not become profitable and the value of our common stock would decline.
Further, the U.S. Food and Drug Administration, or FDA, and equivalent foreign regulatory authorities have limited experience with SNA-based therapeutics. No regulatory authority has granted approval to any person or entity, including us, to market and commercialize SNA-based therapeutics, which may increase the complexity, uncertainty and length of the regulatory approval process for our therapeutic candidates. We and any current or future collaborators may never receive approval to market and commercialize any therapeutic candidate. Even if we or a future collaborator obtain regulatory approval, the approval may be for disease indications or patient populations that are not as broad as we intended or desired or may require labeling that includes significant use or distribution restrictions or safety warnings. We or a future collaborator may be required to perform additional or unanticipated clinical trials to obtain approval or be subject to post-marketing testing requirements to maintain regulatory approval. If our SNA technology proves to be ineffective, unsafe or commercially unviable, our technology and pipeline would have little, if any, value, which could have a material adverse effect on our business, financial condition, results of operations and prospects.
Our therapeutic candidates are in early stages of development and may fail in development or suffer delays that materially and adversely affect their commercial viability.
We have no therapeutics on the market and all of our therapeutic candidates are in early stages of development. Our ability to achieve and sustain profitability depends on obtaining regulatory approvals, including an institutional review board, or IRB, approval to conduct clinical trials at particular sites for, and successfully commercializing, our therapeutic candidates, either alone or with third parties. Before obtaining regulatory approval for the commercial distribution of our therapeutic candidates, we or an existing or a future collaborator must conduct extensive preclinical studies and clinical trials to demonstrate the safety and efficacy in humans of our therapeutic candidates. Preclinical studies and clinical trials are expensive, difficult to design and implement, can take many years to complete and are uncertain as to outcome. The start or end of a clinical trial is often delayed or halted due to changing regulatory requirements, manufacturing challenges, required clinical trial administrative actions, slower than anticipated patient enrollment, changing standards of care, availability or prevalence of use of a comparative therapeutic or required prior therapy, clinical outcomes or financial constraints. For instance, delays or difficulties in patient enrollment or difficulties in retaining trial participants can result in increased costs, longer development times or termination of a clinical trial. Clinical trials of a new therapeutic candidate require the enrollment of a sufficient number of patients, including patients who are suffering from the disease the therapeutic candidate is intended to treat and who meet other eligibility criteria. Rates of patient enrollment are affected by many factors, including the size of the patient population, the eligibility criteria for the clinical trial, the age and condition of the patients, the stage and severity of disease, the nature of the protocol, the proximity of patients to clinical sites, the availability of effective treatments for the relevant disease, and COVID-19-related developments including the extent to which they may interact with any of the foregoing factors. For example, the continuing effects of the COVID-19 pandemic contributed to delays that began in the third quarter of 2020 and continued into the second half
of 2021 in our enrollment plans and clinical trial site start-ups for the Phase 2 dose expansion phase of the Phase 1b/2 clinical trial for our cavrotolimod (AST-008) clinical program, and, as a result, we now expect to report ORR results of cavrotolimod (AST-008) in the second half of 2022 rather than the first half of 2022, pending further delays or any additional unanticipated effects of the COVID-19 pandemic on our clinical development plans.
A therapeutic candidate can unexpectedly fail at any stage of preclinical and clinical development. The historical failure rate for therapeutic candidates is high due to scientific feasibility, safety, efficacy, changing standards of medical care and other variables. The results from preclinical studies or early clinical trials of a therapeutic candidate may not predict the results that will be obtained in later phase clinical trials of the therapeutic candidate. We, the FDA, an IRB, an independent ethics committee, or other applicable regulatory authorities may suspend clinical trials of a therapeutic candidate at any time for various reasons, including a finding that subjects participating in such trials are being exposed to unreasonable and significant risk of illness or injury. Similarly, an IRB or ethics committee may suspend a clinical trial at a particular trial site. We may not have the financial resources to continue development of, or to enter into collaborations for, a therapeutic candidate if we experience any problems or other unforeseen events that delay or prevent regulatory approval of, or our ability to commercialize, therapeutic candidates, including:
•negative or inconclusive results from our clinical trials or the clinical trials of others for therapeutic candidates similar to ours, leading to a decision or requirement to conduct additional preclinical testing or clinical trials or abandon a program;
•therapeutic-related side effects experienced by participants in our clinical trials or by individuals using therapeutics similar to our therapeutic candidates;
•delays in submitting INDs or clinical trial applications, or CTAs, or comparable foreign applications or delays or failure in obtaining the necessary approvals from regulators or IRBs or ethics committees to commence a clinical trial, or a suspension or termination of a clinical trial once commenced;
•conditions imposed by the FDA or comparable foreign authorities, such as the European Medicines Agency, or EMA, or European Union national competent authorities, regarding the scope or design of our clinical trials;
•further delays in enrolling research subjects in clinical trials;
•high drop-out rates of research subjects;
•inadequate supply or quality of therapeutic candidate components or materials or other supplies necessary for the conduct of our clinical trials;
•greater than anticipated clinical trial costs;
•poor effectiveness of our therapeutic candidates during clinical trials;
•unfavorable FDA or other regulatory agency inspection and review of a clinical trial site;
•failure of our third-party contractors or investigators to comply with regulatory requirements or otherwise meet their contractual obligations in a timely manner, or at all;
•delays and changes in regulatory requirements, policy and guidelines, including the imposition of additional regulatory oversight around clinical testing generally or with respect to our technology in particular, especially in light of the novelty of our therapeutic candidates;
•varying interpretations of data by the FDA and similar foreign regulatory agencies; or
•refusal of the FDA to accept data from clinical trials conducted outside the United States, or acceptance of these data subject to certain conditions by the FDA.
Product development involves a lengthy and expensive process with an uncertain outcome, and results of earlier preclinical studies and clinical trials may not be predictive of future clinical trial results.
Clinical testing is expensive and generally takes many years to complete, and the outcome is inherently uncertain. Failure can occur at any time and at any stage during the clinical trial process. The results of preclinical studies and early clinical trials of our therapeutic candidates may not be predictive of the result of any subsequent clinical trials. Therapeutic candidates that have shown promising results in early stage clinical trials may still suffer significant setbacks in subsequent clinical trials. We will have to conduct trials in our proposed indications to verify the results obtained to
date and to support any regulatory submissions for further clinical development. A number of companies in the biopharmaceutical industry have suffered significant setbacks in clinical trials due to lack of efficacy or adverse safety profiles despite promising results in earlier clinical trials. Moreover, clinical data is often susceptible to varying interpretations and analyses. We do not know whether Phase 1, Phase 2, Phase 3, or other clinical trials we may conduct will demonstrate consistent or adequate efficacy and safety with respect to the proposed indication for use sufficient to receive regulatory approval or market our therapeutic candidates. If we experience delays in the completion of, or termination of, any clinical trial of our therapeutic candidates, the commercial prospects of our therapeutic candidates may be harmed, and our ability to generate product revenues from any of these therapeutic candidates will be delayed. In addition, any delays in completing clinical trials will increase our costs, slow down our therapeutic candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Any of these occurrences could materially and adversely affect our business, financial condition, results of operations or prospects.
Additionally, some of the clinical trials we conduct may be open-label in study design and may be conducted at a limited number of clinical sites on a limited number of patients. An “open-label” clinical trial is one where both the patient and investigator know whether the patient is receiving the investigational product candidate or either an existing approved drug or placebo. Most typically, open-label clinical trials test only the investigational product candidate and sometimes may do so at different dose levels. Open-label clinical trials are subject to various limitations that may exaggerate any therapeutic effect as patients in open-label clinical trials are aware when they are receiving treatment. Open-label clinical trials may be subject to a “patient bias” where patients perceive their symptoms to have improved merely due to their awareness of receiving an experimental treatment. Moreover, patients selected for early clinical studies often include the most severe sufferers and their symptoms may have been bound to improve notwithstanding the new treatment. In addition, open-label clinical trials may be subject to an “investigator bias” where those assessing and reviewing the physiological outcomes of the clinical trials are aware of which patients have received treatment and may interpret the information of the treated group more favorably given this knowledge. Given that our planned open-label Phase 1b/2 clinical trial of cavrotolimod (AST-008) includes an open-label dosing design, the results from this clinical trial may not be predictive of future clinical trial results with this or other product candidates for which we conduct an open-label clinical trial when studied in a controlled environment with a placebo or active control.
A fast track designation by the FDA may not actually lead to a faster development or regulatory review or approval process and does not increase the likelihood that our therapeutic candidates will receive marketing approval.
If a drug is intended for the treatment of a serious or life-threatening condition and the drug demonstrates the potential to address unmet medical needs for this condition, the drug sponsor may apply for FDA fast track designation. The FDA has broad discretion whether to grant this designation. We have obtained fast track designations from the FDA for (i) cavrotolimod in combination with anti-PD-1 or anti-PD-L1 therapy for the treatment of patients with locally advanced or metastatic MCC, refractory to prior anti-PD-(L)1 blockade and (ii) cavrotolimod in combination with anti-PD-1 therapy for the treatment of patients with locally advanced or metastatic CSCC, refractory to prior anti-PD-1 blockade. However, we may not experience a faster development process, review or approval compared to conventional FDA procedures. The FDA may withdraw our fast track designation if the FDA believes that the designation is no longer supported by data from our clinical development program. Our fast track designation does not guarantee that we will qualify for or be able to take advantage of the FDA’s expedited review procedures. Many drugs that have received fast track designation have failed to obtain marketing approval.
We will need substantial additional funds to advance the development of our therapeutic candidates, and we cannot guarantee that we will have sufficient funds available in the future to develop and commercialize our current or future therapeutic candidates.
If our existing therapeutic candidates or our future therapeutic candidates enter and advance through preclinical studies and clinical trials, we will need substantial additional funds to expand our development, regulatory, manufacturing, marketing, and sales capabilities or contract with other organizations to provide these capabilities for us. We have used substantial funds to develop our therapeutic candidates and will require significant funds to conduct further research and development and preclinical studies and clinical trials of our therapeutic candidates, to seek regulatory approvals for our therapeutic candidates and to manufacture and market products, if any, that are approved for commercial sale. As of September 30, 2021, we had $53.1 million in cash, cash equivalents, and restricted cash and $9.0 million in short-term investments. Based on our current operating plans and existing working capital at September 30, 2021, it is uncertain whether our current liquidity is sufficient to fund operations over the next twelve months from the date of the issuance of the accompanying condensed consolidated financial statements. As a result, there is substantial doubt about our ability to continue as a going concern. We have no committed sources of additional capital at this time and substantial additional financing will be needed by us to fund our operations. Our future capital requirements and the period for which
we expect our existing resources to support our operations may vary significantly from what we expect. Our monthly spending levels vary based on new and ongoing development and corporate activities. Since the length of time and activities associated with successful development of our therapeutic candidates is highly uncertain, we are unable to estimate the actual funds we will require for development and any approved marketing and commercialization activities. To execute our business plan, we will need, among other things:
•to obtain the human and financial resources necessary to develop, test, obtain regulatory approval for, manufacture and market our therapeutic candidates;
•to build and maintain a strong intellectual property portfolio and avoid infringing the intellectual property of third parties;
•to establish and maintain successful licenses, collaborations and alliances;
•to satisfy the requirements of clinical trial protocols, including patient enrollment;
•to establish and demonstrate the clinical efficacy and safety of our therapeutic candidates;
•to obtain regulatory approvals;
•to manage our spending as costs and expenses increase due to preclinical studies and clinical trials, regulatory approvals, and commercialization;
•to obtain additional capital to support and expand our operations; and
•to market our products to achieve acceptance and use by the medical community in general.
If we are unable to obtain funding on a timely basis or on acceptable terms, we may have to delay, reduce or terminate our research and development programs and preclinical studies or clinical trials, if any, limit strategic opportunities or undergo reductions in our workforce or other corporate restructuring activities. We also could be required to seek funds through arrangements with collaborators or others that may require us to relinquish rights to some of our technology or therapeutic candidates that we would otherwise pursue on our own. We do not expect to realize revenue from product sales, milestone payments or royalties in the foreseeable future, if at all. Our revenue sources are, and will remain, extremely limited unless and until our therapeutic candidates are clinically tested, approved for commercialization and successfully marketed. To date, we have primarily financed our operations through the sale of equity securities, payments received in connection with our collaboration, option, and license agreement with AbbVie Inc., or AbbVie, our collaboration, option and license agreement with Ipsen Biopharm Limited, or Ipsen, our research collaboration, license, and option agreement with Purdue Pharma L.P., or Purdue, our license and development agreement with Dermelix LLC, or Dermelix, or as a primary contractor or as a subcontractor on government grants, proceeds from our credit and security agreement with MidCap Financial Trust, or MidCap, and proceeds from our loan agreement with Hercules Technology Growth Capital, or Hercules. We will be required to seek additional funding in the future and intend to do so through either collaborations, public or private equity offerings or debt financings, credit or loan facilities or a combination of one or more of these funding sources. Our ability to raise additional funds will depend on financial, economic and other factors, many of which are beyond our control. Additional funds may not be available to us on acceptable terms, or at all, and our ability to raise additional capital may be adversely impacted by potential worsening global economic conditions and disruptions to and volatility in the credit and financial markets in the United States and worldwide resulting from the ongoing COVID-19 pandemic. If we raise additional funds by issuing equity securities or by sales pursuant to our “at the market” program, our stockholders will suffer dilution and the terms of any financing may adversely affect the rights of our stockholders. In addition, as a condition to providing additional funds to us, future investors may demand, and may be granted, rights superior to those of existing stockholders. Debt financing, if available, may involve restrictive covenants limiting our flexibility in conducting future business activities, and, in the event of insolvency, debt holders would be repaid before holders of equity securities received any distribution of corporate assets.
Our business could be adversely affected by the effects of health epidemics, including the global COVID‑19 pandemic, in regions where we or third parties on which we rely have business operations and at our clinical trial sites, as well as the business or operations of our CROs or other third parties with whom we conduct business.
The outbreak of the novel strain of coronavirus, SARS-CoV-2, which causes coronavirus disease 2019, or COVID-19, continues to negatively impact the global economy. Our business and operations could be adversely affected by the effects of health epidemics, including the ongoing COVID-19 pandemic, on our business activities performed by us or by third parties with whom we conduct business, including our third party manufacturers, contract research organizations, or CROs, shippers and others. Such effects could be more pronounced in regions where we have
concentrations of clinical trial sites or other business operations. Our company headquarters is located in Chicago, Illinois, our CROs are located globally, and our substance and drug product manufacturers are located in the United States and Europe.
On June 11, 2021, Illinois moved into “Phase 5” of the Restore Illinois Plan, which is intended to permit businesses to operate at full capacity and permits fully vaccinated individuals to resume activities without wearing a mask except where required by federal, state, local, tribal, or territorial laws, rules and regulations, including local business and workplace guidance. As of November 12, 2021, Illinois remains in “Phase 5” of the Restore Illinois Plan. Certain jurisdictions have begun re-opening only to return to restrictions in the face of increases in new COVID-19 cases. The extent of and timing for lifting of government restrictions remains uncertain as the COVID-19 pandemic continues to evolve. There is no guarantee that prior or new restrictions will not be reinstated in response to the continued spread of COVID-19 or the introduction and spread of new variants of SARS-CoV-2.
In response to current and prior public health directives and orders and to ensure the safety and wellbeing of our employees, we have implemented work-from-home policies to support the community efforts to reduce the transmission of COVID-19 and protect employees, complying with guidance from federal, state/provincial or municipal government and health authorities. We implemented a number of measures to ensure employee safety and business continuity. Under social distancing guidelines for COVID-19, we were typically operating with less than 50% of our R&D staff on-site at any one time through June 30, 2020. As of July 1, 2020, we took occupancy of approximately 30,000 square feet of laboratory and office space in our new headquarters in Chicago, Illinois. Since then, we have operated under COVID-19 social distancing guidelines and have generally operated with approximately 100% of our R&D staff on-site. Our office and general and administrative team continues to generally work on site approximately 50% of the time. We are managing laboratory staffing and taking other appropriate managerial actions to maintain progress on our preclinical and collaboration programs. Business travel has been restricted and we continue to take measures to secure our research and development project activities, while work in laboratories and facilities has been organized to reduce risk of COVID-19 transmission.
The regions in which we operate are currently being affected by COVID-19 and have become subject to additional government-imposed mitigation measures to prevent the ongoing spread of COVID-19. Further, timely enrollment in our clinical trials is dependent upon clinical trial sites which may be adversely affected by the COVID-19 pandemic or its impact or effects. During the third quarter of 2020 and through September 30, 2021, we have observed delays in our enrollment plans for the Phase 2 dose expansion phase of the Phase 1b/2 clinical trial for cavrotolimod (AST-008). We believe the effects of the COVID-19 pandemic or its impact have contributed to such delays. As a result, we lengthened our clinical development timeline for cavrotolimod (AST-008) and, pending further enrollment delays or any unanticipated effects of the COVID-19 pandemic on our clinical development plans, expect to report ORR results in the second half of 2022 rather than by the first half of 2022. Quarantines, shelter-in-place, safer-at-home, social distancing requirements and similar government orders, business shutdowns and closures, phased re-openings or the perception that such orders, shutdowns or other restrictions on the conduct of business operations could continue to occur, related to COVID-19 or other infectious diseases could impact personnel at third-party manufacturing facilities and CROs in the United States and other countries, or the availability or cost of materials, which would disrupt our supply chain. Additionally, our clinical trials may involve immunocompromised patients who are at higher risk for COVID-19 and who are therefore more likely to avoid hospitals or other high risk areas.
The effects of the executive orders and our work-from-home policies may negatively impact productivity, disrupt our business and delay our clinical programs and timelines (for example, our timelines for any of our product candidates), the magnitude of which will continue to depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course.
As a result of the COVID-19 outbreak, or similar pandemics, we may experience further disruptions that could severely impact our business, clinical trials and preclinical studies, including:
•further delays or difficulties in enrolling or maintaining patients in our clinical trials, including patients who may not be able to comply with clinical trial protocols if quarantines, shelter-in-place or safer-at-home restrictions, or social distancing practices or requirements, business shutdowns and closures, among other similar requirements or government orders, continue to impede patient movement or interrupt healthcare services;
•increased rates of patients withdrawing from our clinical trials following enrollment as a result of contracting COVID-19, being forced to quarantine, or being unable to visit clinical trial locations;
•delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff who may have heightened exposure to COVID-19 or experience additional restrictions by their institutions, city, or state;
•delays or disruptions in non-clinical experiments and investigational new drug application-enabling good laboratory practice standard toxicology studies due to unforeseen circumstances in supply chain;
•diversion or prioritization of healthcare resources away from the conduct of clinical trials and towards the COVID-19 pandemic, including the diversion of hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our clinical trials, and because, who, as healthcare providers, may have heightened exposure to COVID-19 and adversely impact our clinical trial operations;
•interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others;
•interruption of our key clinical trial activities, such as clinical assessments at pre-specified time points during the trial and clinical trial site data monitoring, due to limitations on travel imposed or recommended by governmental entities, employers and others or interruption of clinical trial subject visits and study procedures (particularly any procedures that may be deemed non-essential), which may impact the integrity of subject data and clinical study endpoints;
•interruption or delays in the operations of the FDA, European Medicines Agency, or EMA, and comparable foreign regulatory agencies or their refusal to accept data from clinical trials in affected geographies, which may impact approval timelines;
•delays in necessary interactions with local regulators, ethics committees and other important agencies and contractors due to limitations in employee resources or forced furlough of government employees; and
•limitations in employee resources that would otherwise be focused on the conduct of our clinical trials, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people.
For our clinical trials that we might conduct at sites outside the United States, in addition to the risks listed above, we may also experience the following adverse impacts, particularly in countries which are experiencing heightened impact from the COVID-19 pandemic:
•delays in receiving approval from local regulatory authorities to initiate our planned clinical trials;
•delays in clinical sites receiving the supplies and materials needed to conduct our clinical trials;
•interruption in global shipping that may affect the transport of clinical trial materials, such as investigational drug product and comparator drugs used in our clinical trials;
•changes in local regulations as part of a response to the ongoing COVID-19 pandemic which may require us to change the ways in which our clinical trials are conducted, which may result in unexpected costs, or to discontinue the clinical trials altogether;
•delays in necessary interactions with local regulators, ethics committees and other important agencies and contractors due to limitations in employee resources or forced furlough of government employees; and
•the refusal of the FDA to accept data from clinical trials in these affected geographies.
The spread of COVID-19, which continues to cause broad global impact, may materially affect us economically. The trading price for our shares as well as the trading prices of other biopharmaceutical companies, as well as the broader equity and debt markets overall, have been highly volatile as a result of the COVID-19 pandemic and the resulting impact on U.S. economic activities. Although the potential economic impact brought by, and the duration or subsequent reoccurrence of, the COVID-19 pandemic may be difficult to assess or predict, a widespread and prolonged pandemic could continue to result in significant disruption of global financial markets, reducing our ability to access capital, which could in the future negatively affect our liquidity. In addition, even after the COVID-19 pandemic has subsided, a recession or market correction that has occurred or may occur in the future because of the COVID-19 could materially affect our business and the value of our common stock.
The global outbreak of COVID-19 continues to rapidly evolve. The extent to which the COVID-19 pandemic or a similar pandemic will impact our business, preclinical studies and clinical trials will depend on future developments, which are highly uncertain and cannot be predicted with confidence, such as the ultimate geographic spread of the disease, the duration and severity of the outbreak, the possibility of additional periods of increases or spikes in the number of COVID-19 cases, the introduction and spread of new variants of the virus, limitations on our ability to conduct our business in the ordinary course, any reopening plans and additional closures, travel restrictions and social distancing in the United States and other countries, business closures or business disruptions for us, our third party contractors and the
effectiveness of actions taken in the United States and other countries to contain and treat the disease, including, without limitation, the effectiveness and timing of vaccination initiatives in the United States and worldwide. The ultimate impact of the COVID-19 pandemic or a similar health pandemic is highly uncertain and subject to change; we continue to monitor the COVID-19 situation closely.
If we continue to experience delays or difficulties in the enrollment of patients in clinical trials, our receipt of necessary regulatory approvals could be further delayed or prevented.
We may not be able to initiate or continue conducting clinical trials for our product candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA or similar regulatory authorities outside the United States. Some of our competitors have ongoing clinical trials for product candidates that treat the same indications as our product candidates, and patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors’ product candidates. Patient enrollment is affected by other factors including:
•the size and nature of the patient population;
•the severity of the disease under investigation;
•the eligibility criteria for, and design of, the trial in question;
•the perceived risks and benefits of the product candidate under study;
•competition in recruiting and enrolling patients in clinical trials;
•the efforts to facilitate timely enrollment in clinical trials;
•the patient referral practices of physicians;
•the ability to monitor patients adequately during and after treatment;
•the proximity and availability of clinical trial sites for prospective patients; and
•delays or difficulties due to the COVID-19 pandemic or its impact or effects.
Our inability to enroll a sufficient number of patients for our clinical trials would result in significant delays and could require us to abandon one or more clinical trials altogether. We may encounter further difficulties and/or delays in completing our planned enrollments. Enrollment delays in our clinical trials may result in increased development costs for our product candidates, or the inability to complete development of our product candidates, which would cause the value of our company to decline, limit our ability to obtain additional financing, and materially impair our ability to generate revenues. For example, the continuing effects of the COVID-19 pandemic contributed to delays that began in the third quarter of 2020 and continued into the second half of 2021 in our enrollment plans and clinical trial site start-ups for the Phase 2 dose expansion phase of the Phase 1b/2 clinical trial for our cavrotolimod (AST-008) clinical program, and, as a result, we now expect to report ORR results of cavrotolimod (AST-008) in the second half of 2022 rather than the first half of 2022, pending further delays or any additional unanticipated effects of the COVID-19 pandemic on our clinical development plans.
Our quarterly operating results may fluctuate significantly or may fall below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline.
We expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
•variations in the level of expense related to our therapeutic candidates or future development programs;
•results of clinical trials, or the addition or termination of clinical trials or funding support by us, or a future collaborator or licensing partner;
•our execution of any collaboration, licensing or similar arrangement, and the timing of payments we may make or receive under such existing or future arrangements or the termination or modification of any such existing or future arrangements;
•changes in estimates in total project hours in connection with the revenue recognition related to our collaboration agreements that may result in a significant adjustment of non-cash revenue;
•any intellectual property infringement lawsuit or opposition, interference or cancellation proceeding in which we may become involved;
•additions and departures of key personnel;
•strategic decisions by us or our competitors, such as acquisitions, divestitures, spin-offs, joint ventures, strategic investments or changes in business strategy;
•whether or not any of our therapeutic candidates receives regulatory approval, market acceptance and demand for such therapeutic candidates;
•regulatory developments affecting our therapeutic candidates or those of our competitors; and
•changes in general economic, industry, political and market conditions, including, but not limited to, the ongoing impact of the COVID-19 pandemic.
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially.
We may not successfully engage in strategic transactions, including any additional collaborations we seek, which could adversely affect our ability to develop and commercialize product candidates, impact our cash position, increase our expense and present significant distractions to our management.
From time to time, we may consider strategic transactions, such as collaborations, acquisitions of companies, asset purchases and out- or in-licensing of product candidates or technologies. In particular, in addition to our current arrangements with Ipsen, which began in July 2021, AbbVie, which began in November 2019, and Dermelix, which began in February 2019, and Purdue, with which there no active therapeutic candidates in development and which has not indicated any further interest in development, we will evaluate and, if strategically attractive, seek to enter into additional collaborations, including with major biotechnology or pharmaceutical companies. For example, on July 30, 2021, we entered into a collaboration, option, and licensing agreement with Ipsen. The competition for collaborators is intense, and the negotiation process is time-consuming and complex. Any new collaboration may be on terms that are not optimal for us, and we may be unable to maintain any new or existing collaboration if, for example, development or approval of a therapeutic candidate is delayed, sales of an approved product do not meet expectations or the collaborator terminates the collaboration. Any such collaboration, or other strategic transaction, may require us to incur non-recurring or other charges, increase our near- and long-term expenditures and pose significant integration or implementation challenges or disrupt our management or business. These transactions entail numerous operational and financial risks, including exposure to unknown liabilities, disruption of our business and diversion of our management’s time and attention in order to manage a collaboration or develop acquired products, product candidates or technologies, incurrence of substantial debt or dilutive issuances of equity securities to pay transaction consideration or costs, higher than expected collaboration, acquisition or integration costs, write-downs of assets or goodwill or impairment charges, increased amortization expenses, difficulty and cost in facilitating the collaboration or combining the operations and personnel of any acquired business, impairment of relationships with key suppliers, manufacturers or customers of any acquired business due to changes in management and ownership and the inability to retain key employees of any acquired business. Accordingly, although there can be no assurance that we will undertake or successfully complete any transactions of the nature described above, any transactions that we do complete may be subject to the foregoing or other risks and have a material adverse effect on our business, results of operations, financial condition and prospects. Conversely, any failure to enter any collaboration or other strategic transaction that would be beneficial to us could delay the development and potential commercialization of our product candidates and have a negative impact on the competitiveness of any product candidate that reaches market.
If third parties on which we depend to conduct our preclinical studies and clinical trials do not perform as contractually required, fail to satisfy regulatory or legal requirements, or miss expected deadlines, our development program could be delayed with materially adverse effects on our business, financial condition, results of operations and prospects.
We rely on third-party clinical investigators, CROs, clinical data management organizations and consultants to design, conduct, supervise and monitor preclinical studies and clinical trials for our therapeutic candidates. Because we rely on third parties and do not have the ability to conduct preclinical studies or clinical trials independently, we have less control over the timing, quality and other aspects of preclinical studies and clinical trials than we would if we conducted them on our own. These investigators, CROs and consultants are not our employees and we have limited control over the amount of time and resources that they dedicate to our programs. These third parties may have contractual relationships with other entities, some of which may be our competitors, which may draw time and resources away from our programs. The third parties with which we contract might not be diligent, careful or timely in conducting our preclinical studies or clinical trials, resulting in the preclinical studies or clinical trials being delayed or unsuccessful.
If we cannot contract with acceptable third parties on commercially reasonable terms, or at all, or if these third parties do not carry out their contractual duties, satisfy legal and regulatory requirements for the conduct of preclinical studies or clinical trials or meet expected deadlines, our clinical development programs could be delayed and otherwise adversely affected. In all events, we are responsible for ensuring that each of our preclinical studies and clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. The FDA requires preclinical studies to be conducted in accordance with applicable good laboratory practices, or GLPs, and clinical trials to be conducted in accordance with applicable FDA regulations and good clinical practices, or GCPs, including requirements for conducting, recording and reporting the results of preclinical studies and clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of clinical trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. Any adverse development or delay in our preclinical studies or clinical trials could have a material adverse effect on our business, financial condition, results of operations and prospects.
In addition, the operations of our CROs may be constrained or disrupted by the COVID-19 pandemic. Clinical site closure and other activities that require visits to clinical sites, have been and may continue to be delayed due to prioritization of hospital resources toward the COVID-19 pandemic or concerns among patients about participating in clinical trials during a pandemic. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our preclinical studies or clinical trials in accordance with regulatory requirements or our stated protocols, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates. As a result, our results of operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
If any of our relationships with these third-party CROs terminate, we may not be able to enter into arrangements with alternative CROs or to do so on commercially reasonable terms or in a timely manner. Switching or adding additional CROs involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new CRO commences work. As a result, delays could occur, which could compromise our ability to meet our desired development timelines. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
Because we rely on third-party manufacturing and supply partners, our supply of research and development, preclinical studies and clinical trial materials may become limited or interrupted or may not be of satisfactory quantity or quality.
We rely on third-party partners to manufacture and supply the materials and components for our research and development, preclinical study and clinical trial supplies. We do not own manufacturing facilities or supply sources for such components and materials. Our manufacturing requirements include oligonucleotides and lipids. We procure our nonclinical toxicology and clinical development materials from a limited number of suppliers on a purchase order basis. There can be no assurance that our supply of research and development, preclinical study and clinical trial therapeutic candidates and other materials will not be limited, interrupted, restricted in certain geographic regions or of satisfactory quality or continue to be available at acceptable prices. In particular, any replacement of our drug product manufacturers could require significant effort and expertise because there may be a limited number of qualified replacements.
The manufacturing process for a therapeutic candidate is subject to oversight by the FDA and foreign regulatory authorities. Suppliers and manufacturers must meet applicable manufacturing requirements and undergo rigorous facility and process validation tests required by regulatory authorities in order to comply with regulatory requirements, such as current good manufacturing practices, or cGMPs. In the event that any of our suppliers or manufacturers fails to comply with such requirements or to perform its obligations to us in relation to quality, timing or otherwise, or if our supply of components or other materials becomes limited or interrupted for other reasons, we may be forced to manufacture the materials ourselves, for which we currently do not have the capabilities or resources, or enter into an agreement with another third-party, which we may not be able to do on reasonable terms, if at all. In some cases, the technical skills or technology required to manufacture our therapeutic candidates may be unique or proprietary to the original manufacturer and we may have difficulty, or there may be contractual restrictions prohibiting us from, transferring such skills or technology to another third-party and a feasible alternative may not exist. These factors would increase our reliance on such manufacturer or require us to obtain a license from such manufacturer in order to have another third-party manufacture our therapeutic candidates. If we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop therapeutic candidates in a timely manner or within budget.
We expect to continue to rely on third-party manufacturers if we receive regulatory approval for any therapeutic candidate. To the extent that we have existing, or enter into future, manufacturing arrangements with third parties, we will depend on these third parties to perform their obligations in a timely manner consistent with contractual and regulatory requirements, including those related to quality control and assurance. If we are unable to obtain or maintain third-party manufacturing for therapeutic candidates, or to do so on commercially reasonable terms, we may not be able to develop and commercialize our therapeutic candidates successfully. Our or a third-party’s failure to execute on our manufacturing requirements and comply with cGMPs could adversely affect our business in a number of ways, including:
•an inability to initiate or continue preclinical studies or clinical trials of our therapeutic candidates under development;
•delay in submitting regulatory applications, or receiving regulatory approvals, for therapeutic candidates;
•loss of the cooperation of a future collaborator;
•subjecting manufacturing facilities of our therapeutic candidates to additional inspections by regulatory authorities;
•requirements to cease distribution or to recall batches of our therapeutic candidates; and
•in the event of approval to market and commercialize a therapeutic candidate, an inability to meet commercial demands for our therapeutics.
If our relationships with our manufacturers, suppliers or other vendors are terminated or scaled back as a result of the COVID-19 pandemic or other health epidemics or pandemics, we may not be able to enter into arrangements with alternative suppliers or vendors or do so on commercially reasonable terms or in a timely manner. Further, if the COVID-19 pandemic continues to persist for an extended period of time and impacts essential distribution systems such as FedEx and postal delivery, we could experience disruptions to our supply chain and operations, and associated delays in the manufacturing and supply of our product candidates. Switching or adding additional suppliers or vendors involves substantial cost and requires management time and focus. In addition, there is a natural transition period when a new supplier or vendor commences work. As a result, delays may occur, which could adversely impact our ability to meet our desired clinical development and any future commercialization timelines. Although we carefully manage our relationships with our suppliers and vendors, there can be no assurance that we will not encounter challenges or delays in the future or that these delays or challenges will not harm our business.
We face competition from entities that have developed or may develop therapeutic candidates for our target disease indications, including companies developing novel treatments and technology platforms based on modalities and technology similar to ours. If these companies develop technologies, including delivery technologies, or therapeutic candidates more rapidly than we do or their technologies are more effective, our ability to develop and successfully commercialize therapeutic candidates may be adversely affected.
The development and commercialization of therapeutic candidates is highly competitive. We compete with a number of multinational pharmaceutical companies and specialized biotechnology companies, as well as technology being developed at universities and other research institutions. Our competitors have developed, are developing or will develop
therapeutic candidates and processes competitive with our therapeutic candidates. Competitive therapeutic treatments include those that have already been approved and accepted by the medical community and any new treatments that enter the market. We believe that a significant number of therapeutics are currently under development, and may become commercially available in the future, for the treatment of conditions for which we may try to develop therapeutic candidates. There is intense and rapidly evolving competition in the biotechnology, pharmaceutical and oligonucleotide therapeutics fields. While we believe that our SNA technology, its associated intellectual property and our scientific and technical know-how give us a competitive advantage in this space, competition from many sources remains. Our competitors include larger and better-funded pharmaceutical, biotechnology and oligonucleotide therapeutics companies. Moreover, we also compete with current and future therapeutics developed at universities and other research institutions.
We are aware of several companies that are developing oligonucleotide delivery platforms and oligonucleotide-based therapeutics. These competitors include Ionis Pharmaceuticals, Inc., Alnylam Pharmaceuticals, Inc., Dicerna Pharmaceuticals, Inc., Arbutus Biopharma Corp., Wave Life Sciences Ltd., Arrowhead Pharmaceuticals, Inc., ProQR Therapeutics N.V., Stoke Therapeutics, Inc., Neubase Therapeutics, Inc., Idera Pharmaceuticals, Inc., Avidity Biosciences, Checkmate Pharmaceuticals, Inc., Dyne Therapeutics, Inc., Atalanta Therapeutics, Inc., PepGen, Inc. and others. These and other competitors compete with us in recruiting scientific and managerial talent, and for funding from pharmaceutical companies.
Our success will partially depend on our ability to develop and protect therapeutics that are safer and more effective than competing therapeutics. Our commercial opportunity and success will be reduced or eliminated if competing therapeutics are safer, more effective, or less expensive than the therapeutics we develop.
If our therapeutic candidates are approved for the indications we are currently pursuing, they will compete with a range of therapeutic treatments that are either in development or currently marketed. A number of therapeutics for treating psoriasis and cancers are on the market or in clinical development. For the treatment of psoriasis, marketed therapies range from small molecules like topical steroids to biologics, such as AbbVie’s adalimumab. In addition, numerous compounds are in clinical development for psoriasis treatment. With respect to immunogenic cancers such as melanoma, the most common treatments are chemotherapeutic compounds, radiation therapy and now immunotherapeutic antibodies such as ipilimumab, atezolizumab, pembrolizumab and others.
Many of our competitors have significantly greater financial, technical, manufacturing, marketing, sales and supply resources or experience than we do. If we successfully obtain approval for any therapeutic candidate, we will face competition based on many different factors, including the safety and effectiveness of our therapeutics, the ease with which our therapeutics can be administered and the extent to which patients accept relatively new routes of administration, the timing and scope of regulatory approvals for these therapeutics, the availability and cost of manufacturing, marketing and sales capabilities, price, reimbursement coverage and patent position. Competing therapeutics could present superior treatment alternatives, including by being more effective, safer, less expensive or marketed and sold more effectively than any therapeutics we may develop. Competitive therapeutics may make any therapeutics we develop obsolete or noncompetitive before we recover the expense of developing and commercializing our therapeutic candidates. Such competitors could also recruit our employees, which could negatively impact our level of expertise and our ability to execute our business plan.
The market may not be receptive to our therapeutic candidates based on a novel therapeutic modality, and we may not generate any future revenue from the sale or licensing of therapeutic candidates.
Even if approval is obtained for a therapeutic candidate, we may not generate or sustain revenue from sales of the product due to factors such as whether the product can be sold at a competitive cost and otherwise accepted in the market. The therapeutic candidates that we are developing are based on our SNA technology. Market participants with significant influence over acceptance of new treatments, such as physicians and third-party payors, may not adopt a treatment based on SNA technology, and we may not be able to convince the medical community and third-party payors to accept and use, or to provide favorable reimbursement for any therapeutic candidates developed by us or any current or future collaborators. Market acceptance of our therapeutic candidates will depend on, among other factors:
•the timing of our receipt of any marketing and commercialization approvals;
•the terms of any approvals and the countries in which approvals are obtained;
•the safety and efficacy of our therapeutic candidates;
•the prevalence and severity of any adverse side effects associated with our therapeutic candidates;
•limitations or warnings contained in any labeling approved by the FDA or other regulatory authority;
•relative convenience and ease of administration of our therapeutic candidates;
•the willingness of patients to accept any new methods of administration;
•the success of our physician education programs;
•the availability of adequate government and third-party payor reimbursement;
•the pricing of our products, particularly as compared to alternative treatments; and
•availability of alternative effective treatments for indications our therapeutic candidates are intended to treat and the relative risks, benefits and costs of those treatments.
With our focus on SNAs, these risks may increase to the extent the space becomes more competitive or less favored in the commercial marketplace. Additional risks apply in relation to any disease indications we may pursue which are classified as rare diseases and allow for orphan drug designation by regulatory agencies in major commercial markets, such as the U.S., Europe and Japan. Because of the small patient population for a rare disease, if pricing is not approved or accepted in the market at an appropriate level for an approved product with orphan drug designation, such therapeutic may not generate enough revenue to offset costs of development, manufacturing, marketing and commercialization despite any benefits received from the orphan drug designation, such as market exclusivity, assistance in clinical trial design or a reduction in user fees or tax credits related to development expense. Market size is also a variable in disease indications not classified as rare. Our estimates regarding potential market size for any indication may be materially different from what we discover to exist at the time we commence commercialization, if any, for a therapeutic, which could result in significant changes in our business plan and have a material adverse effect on our business, financial condition, results of operations and prospects.
If a therapeutic candidate that has orphan drug designation subsequently receives the first FDA approval for the indication for which it has such designation, the therapeutic candidate is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same therapeutic candidate for the same indication, except in very limited circumstances, for seven years. Orphan drug exclusivity, however, could also block the approval of one of our therapeutic candidates for seven years if a competitor obtains approval of the same therapeutic candidate as defined by the FDA or if our therapeutic candidate is determined to be contained within the competitor’s therapeutic candidate for the same indication or disease.
As in the U.S., we may apply for designation of a therapeutic candidate as an orphan drug for the treatment of a specific indication in the European Union before the application for marketing authorization is made. Sponsors of orphan drugs in the European Union can enjoy economic and marketing benefits, including up to ten years of market exclusivity for the approved indication. During such period, marketing applications for similar medicinal products will not be accepted, unless certain exceptions apply. In the EU, a “similar medicinal product” is a medicinal product containing a similar active substance or substances as contained in a currently authorized orphan medicinal product, and which is intended for the same therapeutic indication.
Even if we, or any future collaborators, obtain orphan drug designation for a therapeutic candidate, such as we obtained in March 2021 for cavrotolimod for the treatment of patients with Merkel cell carcinoma, that designation may not effectively protect the therapeutic candidate from competition because different drugs can be approved for the same condition. Even after an orphan drug is approved, the FDA can subsequently approve the same drug for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective, or makes a major contribution to patient care.
Any inability to attract and retain qualified key management and technical personnel would impair our ability to implement our business plan.
Our success largely depends on the continued service of key management and other specialized personnel, including David A. Giljohann, Ph.D., our Chief Executive Officer, Brian C. Bock, our Chief Financial Officer, Matthias G. Schroff, Ph.D., our Chief Operating Officer, Douglas E. Feltner, M.D., our Chief Medical Officer, and Elias D. Papadimas, our Chief Accounting Officer. The loss of one or more members of our management team or other key employees or advisors could delay our research and development programs or clinical trials and materially harm our business, financial condition, results of operations and prospects. The relationships that our key managers have cultivated within our industry make us particularly dependent upon their continued employment with us. We are dependent on the continued service of
our technical personnel because of the highly technical nature of our therapeutic candidates and our technology and the specialized nature of the regulatory approval process. Because our management team and key employees are not obligated to provide us with continued service, they could terminate their employment with us at any time without penalty. We do not maintain key person life insurance policies on any of our management team members or key employees. Our future success will depend in large part on our continued ability to attract and retain highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing, manufacturing, governmental regulation and commercialization. We face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations.
We expect to continue to increase the size of our organization, and we may experience difficulties in managing growth.
As of September 30, 2021, we had 77 employees. As our development and commercialization plans and strategies develop, and as we further develop as a public company, we expect to need additional managerial, operational, financial and other personnel, including personnel to support our product development and planned future commercialization efforts. Future growth will impose significant added responsibilities on members of management, including:
•identifying, recruiting, integrating, maintaining and motivating additional employees;
•managing our internal development efforts effectively, including the clinical, FDA and EMA review processes for our product candidates; and
•improving our operational, financial and management controls, reporting systems and procedures.
Our future financial performance and our ability to commercialize our product candidates will depend, in part, on our ability to effectively manage any future growth, and our management may also have to divert a disproportionate amount of its attention away from day-to-day activities in order to devote a substantial amount of time to managing these growth activities.
If we are not able to effectively expand our organization by hiring new employees, we may not be able to successfully implement the tasks necessary to further develop and commercialize our product candidates and, accordingly, may not achieve our research, development and commercialization goals.
If any of our therapeutic candidates are approved for marketing and commercialization and we are unable to develop sales, marketing and distribution capabilities on our own or enter into agreements with third parties to perform these functions on acceptable terms, we will be unable to successfully commercialize any such future therapeutics.
We currently have no sales, marketing or distribution capabilities or experience. If any of our therapeutic candidates is approved, we will need to develop internal sales, marketing and distribution capabilities to appropriately commercialize such therapeutics, which would be expensive and time-consuming, or enter into collaborations with third parties to perform these services. If we decide to market our approved therapeutics directly, we will need to commit significant financial and managerial resources to develop a marketing and sales force with technical expertise and supporting distribution, administration and compliance capabilities. If we rely on third parties with such capabilities to market our approved therapeutics or decide to co-promote therapeutics with collaborators, we will need to establish and maintain compliant marketing and distribution arrangements with third parties, and there can be no assurance that we will be able to enter into such arrangements on acceptable terms or at all. In entering into third-party marketing or distribution arrangements, any revenue we receive will depend upon the efforts of the third parties and there can be no assurance that such third parties will establish adequate sales and distribution capabilities or be successful in gaining market acceptance of any approved therapeutic. If we are not successful in commercializing any therapeutic approved in the future, either on our own or through third parties, our business, financial condition, results of operations and prospects could be materially and adversely affected.
If we fail to comply with U.S. or foreign regulatory requirements, regulatory authorities could withhold marketing or commercialization approvals, limit or withdraw any marketing or commercialization approvals we may receive and subject us to other penalties that could materially harm our business.
We and our therapeutic candidates, as well as our suppliers, contract manufacturers, distributors, and contract testing laboratories are subject to extensive regulation by governmental authorities in the European Union, the U.S., and other countries, with the regulations differing from country to country.
If we or current or future collaborators, manufacturers or service providers fail to comply with applicable requirements, these regulatory authorities could refuse to issue necessary approvals for marketing and commercialization.
Even if we receive marketing and commercialization approval of a therapeutic candidate, we and our third-party service providers will be subject to continuing regulatory requirements, including a broad array of regulations related to establishment registration and product listing, manufacturing processes, risk management measures, quality and pharmacovigilance systems, pre- and post-approval clinical data, labeling, advertising and promotional activities for such therapeutic, record keeping, distribution, and import and export of therapeutics for any therapeutic for which we obtain marketing approval. We are required to submit safety and other post market information and reports and are subject to continuing regulatory review, including in relation to adverse patient experiences with the therapeutic and clinical results that are reported after a therapeutic is made commercially available, both in the U.S. and any foreign jurisdiction in which we seek regulatory approval. The FDA and certain foreign regulatory authorities, such as the EMA, have significant post-market authority, including the authority to require labeling changes based on new safety information and to require post-market studies or clinical trials to evaluate safety risks related to the use of a therapeutic or to require withdrawal of the therapeutic from the market. The FDA also has the authority to require a Risk Evaluation and Mitigation Strategies, or REMS, plan either before or after approval, which may impose further requirements or restrictions on the distribution or use of an approved therapeutic. The EMA now routinely requires risk management plans, or RMPs, as part of the marketing authorization application process, and such plans must be continually modified and updated throughout the lifetime of the product as new information becomes available. In addition, for nationally authorized medicinal products, the relevant governmental authority of any European Union member state can request an RMP whenever there is a concern about the risk/benefit balance of the product.
The manufacturer and manufacturing facilities we use to make a future therapeutic, if any, will also be subject to periodic review and inspection by the FDA and other regulatory agencies, including for continued compliance with cGMP requirements. The discovery of any new or previously unknown problems with our third-party manufacturers, manufacturing processes or facilities may result in restrictions on the therapeutic, manufacturer or facility, including withdrawal of the therapeutic from the market. If we rely on third-party manufacturers, we will not have control over compliance with applicable rules and regulations by such manufacturers. Any product promotion and advertising will also be subject to regulatory requirements and continuing regulatory review. Although a physician may prescribe products for off-label use since the FDA and other regulatory agencies do not regulate a physician’s choice of drug treatment made in the physician’s independent medical judgment, they do restrict promotional communications from companies or their sales force with respect to off-label uses of products for which marketing clearance has not been issued. Companies may only share truthful and not misleading information that is otherwise consistent with a product’s FDA approved labeling. If we or our future collaborators, manufacturers or service providers fail to comply with applicable continuing regulatory requirements in the U.S. or foreign jurisdictions in which we seek to market our therapeutics, we or they may be subject to, among other things, fines, warning and untitled letters, clinical holds, delay or refusal by the FDA or foreign regulatory authorities to approve pending applications or supplements to approved applications, suspension, refusal to renew or withdrawal of regulatory approval, recalls, seizures or administrative detention of products, refusal to permit the import or export of therapeutics, operating restrictions, inability to participate in government programs including Medicare and Medicaid, and total or partial suspension of production or distribution, injunction, restitution, disgorgement, debarment, civil and criminal penalties and criminal prosecution.
Price controls imposed in foreign markets may adversely affect our future profitability.
In some countries, particularly member states of the European Union, the pricing of prescription drugs is subject to governmental control at the national level, and in some cases also at the regional level. In these countries, pricing negotiations with governmental authorities can take considerable time after receipt of marketing approval for a therapeutic. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments may further complicate pricing and reimbursement negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various European Union member states and parallel distribution, or arbitrage between low-priced and high-priced member states, can further reduce prices. In some countries, we or current or future collaborators may be required to conduct a clinical trial or other studies that compare the cost-effectiveness of our SNA therapeutic candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If reimbursement of any therapeutic candidate approved for marketing is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business, financial condition, results of operations or prospects could be adversely affected.
Our business entails a significant risk of product liability and our inability to obtain sufficient insurance coverage could have a material adverse effect on our business, financial condition, results of operations or prospects.
Our business exposes us to significant product liability risks inherent in the development, testing, manufacturing and marketing of therapeutic treatments. Product liability claims could delay or prevent completion of our development programs. If we succeed in marketing therapeutics, such claims could result in an investigation by certain regulatory authorities, such as the FDA or foreign regulatory authorities, of the safety and effectiveness of our therapeutics, our manufacturing processes and facilities or our marketing programs and potentially a recall of our therapeutics or more serious enforcement action, limitations on the approved indications for which they may be used or suspension or withdrawal of approvals. Regardless of the merits or eventual outcome, liability claims may also result in decreased demand for our therapeutics, injury to our reputation, costs to defend the related litigation, a diversion of management’s time and our resources, substantial monetary awards to trial participants or patients and a decline in our stock price. We currently have product liability insurance that we believe is appropriate for our stage of development and may need to obtain higher levels of product liability insurance prior to marketing any of our therapeutic candidates. Any insurance we have or may obtain may not provide sufficient coverage against potential liabilities. Furthermore, clinical trial and product liability insurance is becoming increasingly expensive. As a result, we may be unable to obtain sufficient insurance at a reasonable cost to protect us against losses caused by product liability claims that could have a material adverse effect on our business.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements which could have an adverse effect on our business.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include, but is not limited to, intentional failures to comply with FDA, the Centers for Medicare & Medicaid Services, or CMS, the Department of Health and Human Services, or HHS, Office of Inspector General, or OIG, or other agency regulations, applicable laws, regulations, guidance or codes of conduct set by foreign governmental authorities or self-regulatory industry organizations, or provide accurate information to any governmental authorities, such as the FDA, comply with manufacturing standards we may establish, comply with federal and state healthcare fraud and abuse laws and regulations, report financial information or data accurately or disclose unauthorized activities to us. For example, on November 9, 2021, the Audit Committee of our Board of Directors was notified of a claim made by a former Company senior researcher regarding alleged improprieties that researcher claims to have committed with respect to our XCUR-FXN preclinical program for the treatment of Friedreich’s ataxia. The Audit Committee has retained external counsel to conduct an internal investigation of the claim. We are currently unable to predict the timing or outcome of the investigation. We are unable to determine the potential impact of the asserted claim on our research and development activities or the timing of completion of our current research and development of our XCUR-FXN preclinical program for the treatment of FA, as the investigation of the asserted claim remains ongoing. In connection with the ongoing investigation, securities class actions and other lawsuits may be filed against us, certain current and former directors, and certain current and former officers. Any future investigations or lawsuits may also adversely affect our business, financial condition, results of operations and cash flows.
In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws, regulations, guidance and codes of conduct intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws, regulations, guidance and codes of conduct may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements.
Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions, including, fines, debarment, or disqualification of those employees from participation in certain government-regulated activities, and serious harm to our reputation. This could include violations of the U.S. federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, other U.S. federal and state law, and requirements of non-U.S. jurisdictions, including the European Union Data Protection Directive.
It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws, regulations, guidance or codes of conduct. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including exclusion from participation in the U.S. federal healthcare programs, the imposition of significant fines or other sanctions.
Compliance with governmental regulations regarding the treatment of animals used in research could increase our operating costs, which would adversely affect the commercialization of our technology.
The Animal Welfare Act, or AWA, is the federal law that covers the treatment of certain animals used in research. Currently, the AWA imposes a wide variety of specific regulations that govern the humane handling, care, treatment and transportation of certain animals by producers and users of research animals, most notably relating to personnel, facilities, sanitation, cage size, and feeding, watering and shipping conditions. Third parties with whom we contract are subject to registration, inspections and reporting requirements under the AWA. Furthermore, some states have their own regulations, including general anti-cruelty legislation, which establish certain standards in handling animals. Comparable rules, regulations, and or obligations exist in many foreign jurisdictions. If we or our contractors fail to comply with regulations concerning the treatment of animals used in research, we may be subject to fines and penalties and adverse publicity, and our operations could be adversely affected.
Our internal computer systems, or those of our CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our therapeutic development programs.
Despite the implementation of security measures, our internal computer systems and those of our CROs and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such events could cause interruptions of our operations. For instance, the loss of preclinical study or clinical trial data involving our therapeutic candidates could result in delays in our development and regulatory filing efforts and significantly increase our costs. In addition, theft or other exposure of data may interfere with our ability to protect our intellectual property, trade secrets, and other information critical to our operations. We can provide no assurances that certain sensitive and proprietary information relating to one or more of our therapeutic candidates has not been, or will not in the future be, compromised. Although we have invested resources to enhance the security of our computer systems, there can be no assurances we will not experience additional unauthorized intrusions into our computer systems, or those of our CROs and other contractors and consultants, that we will successfully detect future unauthorized intrusions in a timely manner, or that future unauthorized intrusions will not result in material adverse effects on our financial condition, reputation, or business prospects. Payments related to the elimination of ransomware may materially affect our financial condition and results of operations.
Certain data breaches must also be reported to affected individuals and the government, and in some cases to the media, under provisions of HIPAA, as amended by HITECH, other U.S. federal and state law, and requirements of non-U.S. jurisdictions, including the European Union Data Protection Directive. Financial penalties may also apply in some data breaches where noncompliance with the applicable law is identified.
To the extent that any disruption or security breach were to result in a loss of, or damage to, our data, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of our therapeutic candidates could be delayed.
If we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
Our research, development and manufacturing involve the use of hazardous materials and various chemicals. We maintain quantities of various flammable and toxic chemicals in our facilities in Chicago, Illinois that are required for our research, development and manufacturing activities. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. We believe our procedures for storing, handling and disposing these materials in our Chicago facilities comply with the relevant guidelines of Chicago, the state of Illinois, and the Occupational Safety and Health Administration of the U.S. Department of Labor. Although we believe that our safety procedures for handling and disposing of these materials comply with the standards mandated by applicable regulations, the risk of accidental contamination or injury from these materials cannot be eliminated. If an accident occurs, we could be held liable for resulting damages, which could be substantial. We are also subject to numerous environmental, health and workplace safety laws and regulations, including those governing laboratory procedures, exposure to blood-borne pathogens and the handling of animals and biohazardous materials. Although we maintain workers’ compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of these materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological or hazardous materials. Additional federal, state and local laws and regulations affecting our operations may be adopted in the future. We may incur substantial costs to comply with, and substantial fines or penalties if we violate, any of these laws or regulations.
Our information technology systems could face serious disruptions that could adversely affect our business.
Our information technology and other internal infrastructure systems, including corporate firewalls, servers, leased lines and connection to the Internet, face the risk of systemic failure that could disrupt our operations. A significant disruption in the availability of our information technology and other internal infrastructure systems could cause interruptions and delays in our research and development work.
Increasing scrutiny and changing expectations from customers, regulators, investors, and other stakeholders with respect to our environmental, social and governance practices may impose additional costs on us or expose us to new or additional risks.
Companies are facing increasing scrutiny from customers, regulators, investors, and other stakeholders related to their environmental, social and governance practices. Investor advocacy groups, investment funds and influential investors are also increasingly focused on these practices, especially as they relate to the environment, health and safety, supply chain management, diversity and human rights. Failure to adapt to or comply with regulatory requirements or investor or stakeholder expectations and standards could negatively impact our reputation and the price of our ordinary shares.
Any of the factors mentioned above, or the perception that we or our suppliers, or contract manufacturers or collaborators have not responded appropriately to the growing concern for such issues, regardless of whether we are legally required to do so, may damage our reputation and have a material adverse effect on our business, financial condition, results of operations cash flows and/or ordinary share price.
Natural disasters or other unexpected events may disrupt our operations, adversely affect our results of operations and financial condition, and may not be covered by insurance.
The occurrence of one or more unexpected events, including fires, tornadoes, tsunamis, hurricanes, earthquakes, floods, and other forms of severe hazards in the United States or in other countries in which we or our suppliers or manufacturers operate or are located could adversely affect our operations and financial performance. These types of unexpected events could result in physical damage to and complete or partial closure of one or more of the manufacturing facilities operated by our contract manufacturers, or the temporary or long-term disruption in the supply of products, and/or disruption of our ability to deliver products to customers. Further, the long-term effects of climate change on general economic conditions and the pharmaceutical manufacturing and distribution industry in particular are unclear, and changes in the supply, demand or available sources of energy and the regulatory and other costs associated with energy production and delivery may affect the availability or cost of goods and services, including natural resources, necessary to run our businesses. Existing insurance arrangements may not provide protection for the costs that may arise from such events, particularly if such events are catastrophic in nature or occur in combination. Any long-term disruption in our ability to service our customers from one or more distribution centers or outsourcing facilities could have a material adverse effect on our operations, our business, results of operations and stock price.
Our current operations are concentrated in one location and any events affecting this location may have material adverse consequences.
Our current operations are located in our facilities situated in Chicago, Illinois. Any unplanned event, such as flood, fire, explosion, earthquake, extreme weather condition, medical epidemics, power shortage, telecommunication failure or other natural or man-made accidents or incidents that result in us being unable to fully utilize the facilities, may have a material adverse effect on our ability to operate our business, particularly on a daily basis, and have significant negative consequences on our financial and operating conditions. Loss of access to these facilities may result in increased costs, delays in the development of our therapeutic candidates or interruption of our business operations. As part of our risk management policy, we maintain insurance coverage at levels that we believe are appropriate for our business. However, in the event of an accident or incident at these facilities, we cannot assure you that the amounts of insurance will be sufficient to satisfy any damages and losses. If our facilities are unable to operate because of an accident or incident or for any other reason, even for a short period of time, any or all of our research and development programs may be harmed. Any business interruption may have a material adverse effect on our business, financial position, results of operations and prospects.
The investment of our cash, cash equivalents and fixed income marketable securities is subject to risks which may cause losses and affect the liquidity of these investments.
As of September 30, 2021, we had $53.1 million in cash, cash equivalents, and restricted cash and $9.0 million in short-term investments. We historically have invested excess cash in certificates of deposit or money market mutual
funds that invest in securities issued or guaranteed by the U.S. government or U.S. government agencies, floating rate and variable rate demand notes of U.S. and foreign corporations, and commercial paper. During the fourth quarter of 2019, we have made direct purchases, and expect to continue to make direct purchases of, U.S. government or U.S. government agency securities, floating rate and variable rate demand notes of U.S. and foreign corporations, and commercial paper. These investments are subject to general credit, liquidity, market and interest rate risks, including potential future impacts similar to the impact of U.S. sub-prime mortgage defaults that have affected various sectors of the financial markets and caused credit and liquidity issues. We may realize losses in the fair value of these investments, an inability to access cash in these investments for a potentially meaningful period, or a complete loss of these investments, which would have a negative effect on our financial statements.
In addition, should our investments cease paying or reduce the amount of interest paid to us, our interest income would suffer. The market risks associated with our investment portfolio may have an adverse effect on our results of operations, liquidity and financial condition.
We have incurred significant losses since our inception and expect to incur continued losses in the future. We must obtain additional funds to finance our operations and to remain a going concern.
Our recurring losses from operations raise substantial doubt about our ability to continue as a going concern. As a result, we included an explanatory paragraph in our consolidated financial statements for the period ended September 30, 2021 with respect to this uncertainty. Our ability to continue as a going concern will require us to obtain additional funding. Based on our current operating plans and existing working capital at September 30, 2021, it is uncertain whether our current liquidity is sufficient to fund operations over the next twelve months from the date of the issuance of the accompanying condensed consolidated financial statements. As a result, there is substantial doubt about our ability to continue as a going concern. We have no committed sources of additional capital at this time and substantial additional financing will be needed by us to fund our operations. We have based these estimates, however, on assumptions that may prove to be wrong, and we could spend our available financial resources much faster than we currently expect and need to raise additional funds sooner than we anticipate. The perception of our ability to continue as a going concern may make it more difficult for us to obtain financing for the continuation of our operations and could result in the loss of confidence by investors, suppliers and employees. If we are unable to raise capital when needed or on acceptable terms, we would be forced to delay, reduce or eliminate our research and development programs and commercialization efforts.
If we fail to maintain proper and effective internal controls, our ability to produce accurate financial statements on a timely basis could be impaired.
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, the Sarbanes-Oxley Act of 2002, or Sarbanes-Oxley Act, and the rules and regulations of The Nasdaq Capital Market. Pursuant to Section 404 of the Sarbanes-Oxley Act, or Section 404, we are now required to perform system and process evaluation and testing of our internal control over financial reporting to allow our management to report on the effectiveness of our internal control over financial reporting. However, while we remain an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm.
During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. Further, we may in the future discover weaknesses in our system of internal financial and accounting controls and procedures that could result in a material misstatement of our financial statements. Moreover, our internal controls over financial reporting will not prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud will be detected. Moreover, we are aware that the remote working arrangements implemented in connection with the COVID-19 pandemic potentially present new areas of risk, and we continue to monitor carefully any impact to our internal controls and procedures.
If we are unable to assert that our internal control over financial reporting is effective, investors could lose confidence in the reliability of our financial statements, the market price of our stock could decline and we could be subject to sanctions or investigations by The Nasdaq Capital Market, the SEC or other regulatory authorities.
Risks Related to Intellectual Property
If we are not able to obtain and enforce patent protection for our technology or therapeutic candidates, development and commercialization of our therapeutic candidates may be adversely affected.
Our success depends in part on our ability to obtain and maintain patents and other forms of intellectual property rights, including in-licenses of intellectual property rights of others, for our therapeutic candidates, methods used to manufacture our therapeutic candidates and methods for treating patients using our therapeutic candidates, as well as our ability to preserve our trade secrets, to prevent third parties from infringing upon our proprietary rights and to operate without infringing upon the proprietary rights of others. As of September 30, 2021, our patent portfolio consists of over 90 issued patents and allowed patent applications and 110 pending patent applications. We may not be able to apply for patents on certain aspects of our therapeutic candidates in a timely fashion or at all. Our existing issued and granted patents and any future patents we obtain may not be sufficiently broad to prevent others from using our technology or from developing competing therapeutics and technology. There is no guarantee that any of our pending patent applications will result in issued or granted patents, that any of our issued or granted patents will not later be found to be invalid or unenforceable or that any issued or granted patents will include claims that are sufficiently broad to cover our therapeutic candidates or to provide meaningful protection from our competitors. Moreover, the patent position of pharmaceutical and biotechnology companies can be highly uncertain because it involves complex legal and factual questions. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our current and future proprietary technology and therapeutic candidates are covered by valid and enforceable patents or are effectively maintained as trade secrets. If third parties disclose or misappropriate our proprietary rights, it may materially and adversely impact our position in the market.
The U.S. Patent and Trademark Office, or USPTO, and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case. The standards applied by the USPTO and foreign patent offices in granting patents are not always applied uniformly or predictably. For example, there is no uniform worldwide policy regarding patentable subject matter or the scope of claims allowable in biotechnology and pharmaceutical patents. As such, we do not know the degree of future protection that we will have on our proprietary therapeutics and technology. While we will endeavor to try to protect our therapeutic candidates with intellectual property rights such as patents, as appropriate, the process of obtaining patents is time-consuming, expensive and sometimes unpredictable.
In addition, there are numerous recent changes to the patent laws and proposed changes to the rules of the USPTO, which may have a significant impact on our ability to protect our technology and enforce our intellectual property rights. For example, the Leahy-Smith America Invents Act enacted in 2011 involves significant changes in patent legislation. The U.S. Supreme Court has ruled on several patent cases in recent years, some of which cases either narrow the scope of patent protection available in certain circumstances or weaken the rights of patent owners in certain situations. The 2013 decision by the Supreme Court in Association for Molecular Pathology v. Myriad Genetics, Inc. precludes a claim to a nucleic acid having a stated nucleotide sequence that is identical to a sequence found in nature and unmodified. We currently are not aware of an immediate impact of this decision on our patents or patent applications because we are developing oligonucleotide therapeutics which contain modifications that we believe are not found in nature. However, this decision has yet to be clearly interpreted by courts and by the USPTO. We cannot assure you that the interpretations of this decision or subsequent rulings will not adversely impact our patents or patent applications. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that may weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
Once granted, patents may remain open to opposition, interference, re-examination, post-grant review, inter partes review, nullification or derivation action in court or before patent offices or similar proceedings for a given period after allowance or grant, during which time third parties can raise objections against such initial grant. In the course of such proceedings, which may continue for a protracted period of time, the patent owner may be compelled to limit the scope of the allowed or granted claims thus attacked, or may lose the allowed or granted claims altogether. In addition, there can be no assurance that:
•Others will not or may not be able to make, use or sell compounds that are the same as or similar to our therapeutic candidates but that are not covered by the claims of the patents that we own or license.
•We or our licensors, or any current or future collaborators, are the first to make the inventions covered by each of our issued patents and pending patent applications that we own or license.
•We or our licensors, or any current or future collaborators, are the first to file patent applications covering certain aspects of our inventions.
•Others will not independently develop similar or alternative technologies or duplicate any of our technology without infringing our intellectual property rights.
•A third-party will not challenge our patents and, if challenged, a court may not hold that our patents are valid, enforceable and infringed.
•Any issued patents that we own or have licensed will provide us with any competitive advantages, or will not be challenged by third parties.
•We will develop additional proprietary technologies that are patentable.
•The patents of others will not have an adverse effect on our business.
•Our competitors will not conduct research and development activities in countries where we lack enforceable patent rights and then use the information learned from such activities to develop competitive therapeutics for sale in our major commercial markets.
Patent term may be inadequate to protect our competitive position on our future therapeutics for an adequate amount of time.
Given the amount of time required for the development, testing and regulatory review of new therapeutic candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. We expect to seek extensions of patent terms in the U.S. and, if available, in other countries where we have patents covering our product candidates. In the U.S., the Drug Price Competition and Patent Term Restoration Act of 1984 permits a patent term extension of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, the patent term extension or restoration cannot extend the remaining term of a patent beyond a total of 14 years from the approval date of the product candidate. Only one patent applicable to an approved product candidate is eligible for the extension and the application for extension must be made prior to expiration of the patent. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we intend to apply for restorations of patent term for some of our currently owned or licensed patents to add patent life beyond their current expiration dates. However, the applicable authorities, including the FDA and the USPTO in the U.S., and any equivalent regulatory authority in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. If this occurs, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and preclinical data and launch their product earlier than might otherwise be the case.
We currently license patent rights from Northwestern University and may in the future license patent rights from other third-party owners or licensees. If Northwestern University or such other owners or licensees do not properly or successfully obtain, maintain or enforce the patents underlying such licenses, or if they retain or license to others any competing rights, our competitive position and business prospects may be adversely affected.
We do, and will continue to, rely on intellectual property rights licensed from third parties to protect our technology. We are a party to a number of licenses that give us rights to third-party intellectual property that is necessary or useful for our business. In particular, we have a license from Northwestern University, which provides us the exclusive worldwide right under certain patents and patent applications owned by Northwestern University to exploit therapeutics and processes using nanoparticles, nanotechnology, microtechnology and nanomaterial-based constructs as therapeutics or accompanying therapeutics as a means of administration. We may also license additional third-party intellectual property in the future. Our success will depend in part on the ability of our licensors to obtain, maintain and enforce patent protection for our licensed intellectual property, and in particular, for those patents to which we have secured exclusive rights. Our licensors may not successfully prosecute the patent applications licensed to us. Even if patents issue or are granted, our licensors may fail to maintain these patents, may determine not to pursue litigation against other companies that are infringing these patents, or may pursue litigation less aggressively than we would. Further, we may not obtain exclusive rights, which would allow for third parties to develop competing therapeutics. Without protection for, or exclusive rights to, the intellectual property we license, other companies might be able to offer substantially identical
therapeutics for sale, which could adversely affect our competitive business position and harm our business prospects. In addition, the U.S. government has certain rights to the inventions covered by the patent rights licensed to us by third parties and Northwestern University, as an academic research and medical center, has reserved the right to practice the patent rights it has licensed to us (i) for research, teaching and/or other educationally related purposes (including the right to distribute materials for such purposes) and (ii) for use in the field of diagnostics (including theradiagnostics) and in any field other than the field of use licensed to us.
We currently have Collaboration, Option and License Agreements with Ipsen and Allergan Pharmaceuticals International Limited, or Allergan, and may in the future have additional Collaboration, Option and License Agreements with other third-parties. If Ipsen or Allergan, or such other licensees do not properly or successfully collaborate to research and develop SNAs, our competitive position and business prospects may be adversely affected.
We do, and will continue to, rely on collaboration with third parties to develop our technology. We are a party to a number of licenses that provide rights to a third-party that is necessary or useful for our business. In particular, we have a Collaboration, Option and License Agreement with Ipsen, to collaborate together to research and develop SNAs. We also have a Collaboration, Option and License Agreement with Allergan, to collaborate together to research, develop, and manufacture new nucleic acid therapeutics focusing on certain hair loss disorders.
We may also collaborate with additional third-parties in the future. Our success will depend in part on the ability of our licensees to research and develop SNAs based on our existing intellectual property. Without successful collaboration to research and develop SNAs, other companies might be able to offer substantially identical therapeutics for sale, which could adversely affect our competitive business position and harm our business prospects.
Other companies or organizations may challenge our or our licensors’ patent rights or may assert patent rights that prevent us from developing and commercializing our therapeutic candidates.
Oligonucleotide and SNA-based therapeutics are a relatively new scientific field. We have obtained grants and issuances of SNA therapeutic patents and have licensed many of these patents from a third-party on an exclusive basis for therapeutics applications. The issued patents and pending patent applications in the U.S. and in key markets around the world that we own or license claim many different methods, compositions and processes relating to the discovery, development, manufacture and commercialization of SNA therapeutics. Specifically, we own and have licensed a portfolio of patents, patent applications and other intellectual property covering SNA compositions of matter as well as their methods of use.
As the field of SNA therapeutics matures, patent applications are being processed by national patent offices around the world. There is uncertainty about which patents will issue, and, if they do, as to when, to whom, and with what claims. In addition, third parties may attempt to invalidate our intellectual property rights. Even if our rights are not directly challenged, disputes could lead to the weakening of our intellectual property rights. Our defense against any attempt by third parties to circumvent or invalidate our intellectual property rights could be costly to us, could require significant time and attention of our management and could have a material adverse effect on our business and our ability to successfully compete.
There are many issued and pending patents that claim aspects of oligonucleotide chemistry and modifications that we may need to apply to our SNA therapeutic candidates. There are also many issued patents that claim targeting genes or portions of genes that may be relevant for SNA therapeutics we wish to develop. Thus, it is possible that one or more organizations will hold patent rights to which we will need a license. If those organizations refuse to grant us a license to such patent rights on reasonable terms, we may not be able to market therapeutics or perform research and development or other activities covered by these patents.
We may be unable to protect our intellectual property rights throughout the world.
Obtaining a valid and enforceable issued or granted patent covering our technology in the U.S. and worldwide can be extremely costly. In jurisdictions where we have not obtained patent protection, competitors may use our technology to develop their own therapeutics and, further, may export otherwise infringing therapeutics to territories where we have patent protection, but where it is more difficult to enforce a patent as compared to the U.S. Competitor therapeutics may compete with our future therapeutics in jurisdictions where we do not have issued or granted patents or where our issued or granted patent claims or other intellectual property rights are not sufficient to prevent competitor activities in these jurisdictions. The legal systems of certain countries, particularly certain developing countries, make it difficult to enforce patents and such countries may not recognize other types of intellectual property protection, particularly that relating to biotechnology and pharmaceuticals. This could make it difficult for us to prevent the infringement of our patents or
marketing of competing therapeutics in violation of our proprietary rights generally in certain jurisdictions. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
We generally file a provisional patent application first, also known as a priority filing, at the USPTO. An international application under the Patent Cooperation Treaty, or PCT, is usually filed within twelve months after the priority filing. Based on the PCT filing, national and regional patent applications may be filed in the U.S., European Union, Japan, Australia and Canada and, depending on the individual case, also in any or all of, inter alia, China, India, South Korea, and Mexico. We have so far not filed for patent protection in all national and regional jurisdictions where such protection may be available. In addition, we may decide to abandon national and regional patent applications before grant or after grant by nonpayment of maintenance fees for the resulting patent. Finally, the grant proceeding of each national or regional patent is an independent proceeding which may lead to situations in which applications might in some jurisdictions be refused by the relevant registration authorities, while granted by others. It is also quite common that depending on the country, various scopes of patent protection may be granted on the same therapeutic candidate or technology.
The laws of some jurisdictions do not protect intellectual property rights to the same extent as the laws in the U.S., and many companies have encountered significant difficulties in protecting and defending such rights in such jurisdictions. If we or our licensors encounter difficulties in protecting, or are otherwise precluded from effectively protecting, the intellectual property rights important for our business in such jurisdictions, the value of these rights may be diminished and we may face additional competition from others in those jurisdictions. Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors are forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position in the relevant jurisdiction may be impaired and our business and results of operations may be adversely affected.
We or our licensors, or any current or future strategic partners, may become subject to third-party claims or litigation alleging infringement of patents or other proprietary rights or seeking to invalidate patents or other proprietary rights, and we may need to resort to litigation to protect or enforce our patents or other proprietary rights, all of which could be costly, time consuming, delay or prevent the development and commercialization of our therapeutic candidates, or put our patents and other proprietary rights at risk.
We or our licensors, or any current or future strategic partners, may be subject to third-party claims for infringement or misappropriation of patent or other proprietary rights. We are generally obligated under our license agreements to indemnify and hold harmless our licensors for damages arising from intellectual property infringement by us. If we or our licensors, or any current or future strategic partners, are found to infringe a third-party patent or other intellectual property rights, we could be required to pay damages, potentially including treble damages, if we are found to have willfully infringed. In addition, we or our licensors, or any current or future strategic partners, may choose to seek, or be required to seek, a license from a third-party, which may not be available on acceptable terms, if at all. Even if a license can be obtained on acceptable terms, the rights may be non-exclusive, which could give our competitors access to the same technology or intellectual property rights licensed to us. If we fail to obtain a required license, we or any current or future collaborator may be unable to effectively market therapeutic candidates based on our technology, which could limit our ability to generate revenue or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations. In addition, we may find it necessary to pursue claims or initiate lawsuits to protect or enforce our patent or other intellectual property rights. The cost to us in defending or initiating any litigation or other proceeding relating to patent or other proprietary rights, even if resolved in our favor, could be substantial, and litigation would divert our management’s attention. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could delay our research and development efforts and limit our ability to continue our operations.
If we were to initiate legal proceedings against a third-party to enforce a patent covering one of our therapeutics or our technology, the defendant could counterclaim that our patent is invalid or unenforceable. In patent litigation in the U.S., defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior
art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we would lose at least part, and perhaps all, of the patent protection on one or more of our therapeutics or certain aspects of our technology. Such a loss of patent protection could have a material adverse impact on our business. Patents and other intellectual property rights also will not protect our technology if competitors design around our protected technology without legally infringing our patents or other intellectual property rights.
It is also possible that we have failed to identify relevant third-party patents or applications. For example, U.S. applications filed before November 29, 2000 and certain U.S. applications filed after that date that will not be filed outside the U.S. remain confidential until patents issue. Patent applications in the U.S. and elsewhere are published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our therapeutics or technology could have been filed by others without our knowledge. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our SNA technology, our therapeutics or the use of our therapeutics. Third-party intellectual property right holders may also actively bring infringement claims against us. We cannot guarantee that we will be able to successfully settle or otherwise resolve such infringement claims. If we are unable to successfully settle future claims on terms acceptable to us, we may be required to engage in or continue costly, unpredictable and time-consuming litigation and may be prevented from or experience substantial delays in marketing our therapeutics. If we fail in any such dispute, in addition to being forced to pay damages, we may be temporarily or permanently prohibited from commercializing any of our therapeutic candidates that are held to be infringing. We might, if possible, also be forced to redesign therapeutic candidates so that we no longer infringe the third-party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business.
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may also be subject to claims that former employees or other third parties have an ownership interest in our patents or other intellectual property. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and distraction to management and other employees.
If we fail to comply with our obligations under any license, collaboration or other agreements, we may be required to pay damages and could lose intellectual property rights that are necessary for developing and protecting our therapeutic candidates or we could lose certain rights to grant sublicenses.
Our current licenses impose, and any future licenses we enter into are likely to impose, various development, commercialization, funding, milestone, royalty, diligence, sublicensing, insurance, patent prosecution and enforcement, and other obligations on us. If we breach any of these obligations, or use the intellectual property licensed to us in an unauthorized manner, we may be required to pay damages and the licensor may have the right to terminate the license, which could result in us being unable to develop, manufacture and sell therapeutics that are covered by the licensed technology or could enable a competitor to gain access to the licensed technology. Moreover, our licensors may own or control intellectual property that has not been licensed to us and, as a result, we may be subject to claims, regardless of their merit, that we are infringing or otherwise violating the licensor’s rights in such unlicensed intellectual property. In addition, while we cannot currently determine the amount of the royalty obligations we would be required to pay on sales of future therapeutics, if any, the amounts may be significant. The amount of our future royalty obligations will depend on the technology and intellectual property we use in therapeutics that we successfully develop and commercialize, if any. Therefore, even if we successfully develop and commercialize therapeutics, we may be unable to achieve or maintain profitability.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patent protection for certain aspects of our therapeutic candidates, we also consider trade secrets, including confidential and unpatented know-how important to the maintenance of our competitive position. We protect trade secrets and confidential and unpatented know-how, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to such knowledge, such as our employees, corporate collaborators, outside scientific collaborators, CROs, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants that
obligate them to maintain confidentiality and assign their inventions to us. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts in the U.S. and certain foreign jurisdictions are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
Under the terms of the Northwestern University License Agreements, Northwestern University could publish research findings relating to the patent rights licensed to us by Northwestern University, which could have a material adverse effect on our business.
We are also subject both in the U.S. and outside the U.S. to various regulatory schemes regarding requests for the information we provide to regulatory authorities, which may include, in whole or in part, trade secrets or confidential commercial information. While we are likely to be notified in advance of any disclosure of such information and would likely object to such disclosure, there can be no assurance that our challenge to the request would be successful.
We may be subject to claims that we or our employees or consultants have wrongfully used or disclosed alleged trade secrets of our employees’ or consultants’ former employers or their clients. These claims may be costly to defend and if we do not successfully do so, we may be required to pay monetary damages and may lose valuable intellectual property rights or personnel.
Many of our employees were previously employed at universities or pharmaceutical or biotechnology companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could hamper our ability to commercialize, or prevent us from commercializing, our therapeutic candidates, which could severely harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names or may be forced to stop using these names, which we need for name recognition by potential partners or customers in our markets of interest. If we are unable to establish name recognition based on our trademarks and trade names, we may not be able to compete effectively and our business may be adversely affected.
Third parties may independently develop similar or superior technology.
There can be no assurance that others will not independently develop, or have not already developed, similar or more advanced technologies than our technology; or that others will not design around, or have not already designed around, aspects of our technology and/or our trade secrets developed therefrom. If third parties develop technology similar or superior to our technology, or they successfully design around our current or future technology, our competitive position, business prospects, and results of operations could be materially and adversely affected.
The intellectual property which we have licensed from Northwestern University was discovered through government funded programs and thus may be subject to federal regulations such as “march-in” rights, certain reporting requirements, and a preference for U.S. industry. Compliance with such regulations may limit our exclusive rights, subject us to expenditure of resources with respect to reporting requirements, and limit our ability to contract with non-U.S. manufacturers.
We have licensed certain intellectual property from Northwestern University pursuant to the Northwestern University License Agreements. The Northwestern University License Agreements indicate that the rights licensed to us by Northwestern University are subject to the obligations to and the rights of the U.S. government, including those set forth in the Bayh-Dole Act of 1980, or Bayh-Dole Act. As a result, the U.S. government may have certain rights to intellectual property embodied in our current or future therapeutics based on the licensed Northwestern University intellectual
property. These U.S. government rights in certain inventions developed under a government-funded program include a non-exclusive, non-transferable, irrevocable worldwide license to use inventions for any governmental purpose. In addition, the U.S. government has the right to require us to grant exclusive, partially exclusive, or nonexclusive licenses to any of these inventions to a third-party if it determines that: (i) adequate steps have not been taken to commercialize the invention; (ii) government action is necessary to meet public health or safety needs; or (iii) government action is necessary to meet requirements for public use under federal regulations, also referred to as “march-in rights.” While the U.S. government has sparingly used, and to our knowledge never successfully exercised, such march-in rights, any exercise of the march-in rights by the U.S. government could harm our competitive position, business, financial condition, results of operations, and prospects. If the U.S. government exercises such march-in rights, we may receive compensation that is deemed reasonable by the U.S. government in its sole discretion, which may be less than what we might be able to obtain in the open market. Intellectual property generated under a government funded program is also subject to certain reporting requirements, compliance with which may require us to expend substantial resources.
In addition, the U.S. government requires that any therapeutics embodying any invention generated through the use of U.S. government funding be manufactured substantially in the U.S. The manufacturing preference requirement can be waived if the owner of the intellectual property can show that reasonable but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would be likely to manufacture substantially in the United States or that under the circumstances domestic manufacture is not commercially feasible. This preference for U.S. manufacturers may limit our ability to contract with non-U.S. therapeutic manufacturers for therapeutics covered by such intellectual property.
Risks Related to Government Regulation
We may be unable to obtain U.S. or foreign regulatory approval and, as a result, unable to commercialize our therapeutic candidates.
Our therapeutic candidates are subject to extensive governmental regulations relating to, among other things, research, testing, development, manufacturing, safety, efficacy, approval, recordkeeping, reporting, labeling, storage, packaging, advertising and promotion, pricing, marketing, sampling, and distribution of therapeutics. Rigorous preclinical testing and clinical trials and an extensive regulatory approval process are required to be successfully completed in the U.S. and in many foreign jurisdictions before a new therapeutic can be marketed. Satisfaction of these and other regulatory requirements is costly, time consuming, uncertain and subject to unanticipated delays. It is possible that none of the therapeutic candidates we may develop will obtain the regulatory approvals necessary for us or any current or future collaborators to begin selling them.
We have very limited experience in conducting and managing the clinical trials necessary to obtain regulatory approvals, including approval by the FDA as well as foreign regulatory authorities, such as the EMA and European Union national competent authorities. The time required to obtain FDA and foreign regulatory approvals is unpredictable but typically takes many years following the commencement of clinical trials, depending upon the type, complexity and novelty of the therapeutic candidate. The standards that the FDA and its foreign counterparts use when regulating us are not always applied predictably or uniformly and can change. Any analysis we perform of data from preclinical and clinical activities is subject to confirmation and interpretation by regulatory authorities, which could delay, limit or prevent regulatory approval. We may also encounter unexpected delays or increased costs due to new government regulations, for example, from future legislation or administrative action, or from changes in the policy of the FDA or foreign regulatory authorities during the period of therapeutic development, clinical trials and regulatory review by the FDA or foreign regulatory authorities. It is impossible to predict whether legislative changes will be enacted, or whether FDA or foreign laws, regulations, guidance or interpretations will be changed, or what the impact of such changes, if any, may be. In addition, unfavorable changes in our industry or the global economy, including as a result of the COVID-19 pandemic, could contribute to some of the events listed above and further impact our ability to progress our clinical trials, submit for marketing approval or commercialize our product candidates, if approved, as planned.
Because the therapeutics we are developing may represent a new class of therapeutic, the FDA and its foreign counterparts have not yet established any definitive policies, practices or guidelines in relation to these therapeutics. While we believe the therapeutic candidates that we are currently developing are regulated as new drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, the FDA could decide to regulate them or other therapeutics we may develop as biologics under the Public Health Service Act. The lack of policies, practices or guidelines may hinder or slow review by the FDA or foreign regulatory authorities of any regulatory filings that we may submit. Moreover, the FDA may respond to these submissions by defining requirements we may not have anticipated. Such responses could lead to significant delays in the clinical development of our therapeutic candidates.
Any delay or failure in obtaining required approvals could have a material adverse effect on our ability to generate revenues from the particular therapeutic candidate for which we are seeking approval. Furthermore, any regulatory approval to market a therapeutic may be subject to limitations on the approved uses for which we may market the therapeutic or the labeling or other restrictions. Regulatory authorities also may impose requirements for costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the therapeutic. In addition, the FDA has the authority to require a REMS plan as part of a NDA or a Biologics License Application, or BLA, or after approval, which may impose further requirements or restrictions on the distribution or use of an approved drug or biologic, such as limiting prescribing to certain physicians or medical centers that have undergone specialized training, limiting treatment to patients who meet certain safe-use criteria and requiring treated patients to enroll in a registry. These limitations and restrictions may limit the size of the market for the therapeutic and affect coverage and reimbursement by third-party payors.
We are also subject to numerous foreign regulatory requirements governing, among other things, the conduct of clinical trials, manufacturing and marketing authorization, pricing and third-party reimbursement. The foreign regulatory approval process varies among countries and may include all of the risks associated with FDA approval described above as well as risks attributable to the satisfaction of local regulations in foreign jurisdictions. Moreover, the time required to obtain approval may differ from that required to obtain FDA approval. Approval by the FDA does not ensure approval by regulatory authorities outside the U.S. and vice versa.
Certain of our therapeutic candidates may require companion diagnostics in certain indications. Failure to successfully develop, validate and obtain regulatory clearance or approval for such tests could harm our product development strategy or prevent us from realizing the full commercial potential of our therapeutic candidates.
Certain of our therapeutic candidates may require companion diagnostics to identify appropriate patients for those therapeutic candidates in certain indications. Companion diagnostics are subject to regulation by the FDA and comparable foreign regulatory authorities as a medical device and may require separate regulatory authorization prior to commercialization. We may rely on third parties for the design, development, testing and manufacturing of these companion diagnostics, the application for and receipt of any required regulatory authorization, and the commercial supply of these companion diagnostics. If these parties are unable to successfully develop companion diagnostics for these therapeutic candidates, or experience delays in doing so, the development of our therapeutic candidates may be adversely affected and we may not be able to obtain marketing authorization for these therapeutic candidates. Furthermore, our ability to market and sell, as well as the commercial success, of any of our therapeutic candidates that require a companion diagnostic will be tied to, and dependent upon, the receipt of required regulatory authorization and the continued ability of such third parties to make the companion diagnostic commercially available on reasonable terms in the relevant geographies. Any failure to develop, validate, obtain and maintain marketing authorization for a companion diagnostic and supply such companion diagnostic will harm our business, results of operations and financial condition.
If we or current or future collaborators, manufacturers or service providers fail to comply with healthcare laws and regulations, we or they could be subject to enforcement actions, which could affect our ability to develop, market and sell our therapeutics and may harm our reputation.
Although we do not currently have any products on the market, our current and future business operations may subject us to additional healthcare statutory and regulatory requirements and enforcement by the federal, state and foreign governments of the jurisdictions in which we conduct our business. Healthcare providers, including physicians and third-party payors play a primary role in the recommendation and prescription of any therapeutic candidates for which we obtain marketing approval. Our arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we research, market, sell or distribute our therapeutic candidates for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations, include, but are not limited to, the following:
•the U.S. federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from soliciting, receiving, offering or providing remuneration (including any kickback, bribe, or certain rebate), directly or indirectly, to induce either the referral of an individual for a healthcare item or service, or the purchasing or ordering of an item or service, for which payment may be made, in whole or in part, under a federal healthcare program, such as Medicare or Medicaid. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand, and prescribers, purchasers and formulary managers, among others, on the other. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
•the U.S. federal False Claims Act, which imposes criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government. For example, pharmaceutical companies have been prosecuted under the False Claims Act in connection with their alleged off-label promotion of drugs, purportedly concealing price concessions in the pricing information submitted to the government for government price reporting purposes, and allegedly providing free product to customers with the expectation that the customers would bill federal health care programs for the product. In addition, the government may assert that a claim including items and services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act;
•federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers;
•federal price reporting laws, which require manufacturers to calculate and report complex pricing metrics to government programs, where such reported prices may be used in the calculation of reimbursement and/or discounts on approved products;
•HIPAA includes a fraud and abuse provision sometimes referred to as the HIPAA All-Payor Fraud Law, which imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program (i.e., not just federal healthcare programs), or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services; similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation;
•HIPAA, as amended by the HITECH, and its implementing regulations, which impose obligations on certain covered entity healthcare providers, health plans, and healthcare clearinghouses and their business associates that perform certain services involving the use or disclosure of individually identifiable health information as well as their covered subcontractors, including mandatory contractual terms, with respect to safeguarding the privacy, security, and transmission of individually identifiable health information, and require notification to affected individuals and regulatory authorities of certain breaches of security of individually identifiable health information. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil actions;
•the federal Physician Payments Sunshine Act and the implementing regulations, also referred to as “Open Payments,” issued under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the ACA, which require that certain manufacturers of pharmaceutical and biological drugs reimbursable under Medicare, Medicaid, or the Children’s Health Insurance Program report to CMS all consulting fees, travel reimbursements, research grants, and other payments, transfers of value or gifts made to U.S.-licensed physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and U.S. teaching hospitals with limited exceptions, as well as ownership and investment interests held by physicians and their immediate family members. Effective January 1, 2022, these reporting obligations will extend to include transfers of value and payments made during the previous year to physician assistants, nurse practitioners, clinical nurse specialists, anesthesiologist assistants, certified registered nurse anesthetists and certified nurse midwives; and
•analogous state laws and regulations, such as, state anti-kickback and false claims laws potentially applicable to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring drug manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures, state and local laws that require the registration of pharmaceutical sales representatives, and state laws governing the privacy and security of personal data (including personal health information) in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts; and state transparency laws that require the reporting of certain pricing information; among other state laws.
Ensuring that our future business arrangements with third-parties comply with applicable healthcare laws and regulations could involve substantial costs. If our operations are found to be in violation of any such requirements, we may be subject to penalties, including significant administrative, civil and/or criminal penalties, monetary damages,
disgorgement, fines, imprisonment, additional integrity reporting requirements and regulatory oversight, the curtailment or restructuring of our operations, or exclusion from participation in government contracting, healthcare reimbursement or other government programs, including Medicare and Medicaid, any of which could adversely affect our financial results. Although an effective compliance program can mitigate the risk of investigation and prosecution for violations of these laws, these risks cannot be entirely eliminated. Any action against us for an alleged or suspected violation could cause us to incur significant legal expenses and could divert our management’s attention from the operation of our business, even if our defense is successful. In addition, achieving and sustaining compliance with applicable laws and regulations may be costly to us in terms of money, time and resources.
If we or current or future collaborators, manufacturers or service providers fail to comply with applicable federal, state or foreign laws or regulations, we could be subject to significant penalties and enforcement actions, which could affect our ability to develop, market and sell our therapeutics successfully and could harm our reputation and lead to reduced acceptance of our therapeutics by the market. These penalties and enforcement actions include, among others:
•adverse regulatory inspection findings;
•warning or untitled letters;
•voluntary product recalls or public notification or medical product safety alerts to healthcare professionals;
•restrictions on, or prohibitions against, marketing our therapeutics;
•restrictions on, or prohibitions against, importation or exportation of our therapeutics;
•suspension of review or refusal to approve pending applications or supplements to approved applications;
•exclusion from participation in government-funded healthcare programs;
•exclusion from eligibility for the award of government contracts for our therapeutics;
•integrity oversight and reporting obligations;
•FDA debarment;
•suspension or withdrawal of therapeutic approvals;
•seizures or administrative detention of therapeutics;
•injunctions; and
•civil and criminal penalties and fines.
Any therapeutics we develop may become subject to unfavorable pricing regulations, third-party coverage and reimbursement practices or healthcare reform initiatives, thereby harming our business.
The regulations that govern marketing approvals, pricing and reimbursement for new therapeutics vary widely from country to country. Some countries require approval of the sale price of a therapeutic before it can be marketed. In many countries, the pricing review period begins after marketing or therapeutic licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. Although we intend to monitor these regulations, our programs are currently in the early stages of development and we will not be able to assess the impact of price regulations for a number of years. As a result, we might obtain regulatory approval for a therapeutic in a particular country, but then be subject to price regulations that delay our commercial launch of the therapeutic and negatively impact the revenues we are able to generate from the sale of the therapeutic in that country.
Patients who are prescribed therapeutics for the treatment of their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their prescription drugs. There is significant uncertainty related to third-party payor coverage and reimbursement of newly approved therapeutics. Our ability to commercialize any therapeutics successfully also will depend in part on the extent to which coverage and reimbursement for these therapeutics and related treatments will be available from government health administration authorities, private health insurers and other organizations. However, there may be significant delays in obtaining coverage for newly-approved therapeutics. Moreover, eligibility for coverage does not necessarily signify that a therapeutic will be reimbursed in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution costs. Also, interim payments
for new therapeutics, if applicable, may be insufficient to cover our costs and may not be made permanent. Thus, even if we succeed in bringing one or more therapeutics to the market, these therapeutics may not be considered cost-effective, and the amount reimbursed for any therapeutics may be insufficient to allow us to sell our therapeutics on a competitive basis. Because our programs are in the early stages of development, we are unable at this time to determine their cost effectiveness or the likely level or method of reimbursement. Increasingly, the third-party payors who reimburse patients or healthcare providers, such as government and private insurance plans, are seeking greater upfront discounts, additional rebates and other concessions to reduce the prices for therapeutics. If the price we are able to charge for any therapeutics we develop, or the reimbursement provided for such therapeutics, is inadequate in light of our development and other costs, our return on investment could be adversely affected.
We currently expect that some therapeutics we develop may need to be administered under the supervision of a physician on an outpatient basis. Under currently applicable U.S. law, certain therapeutics that are not usually self-administered (including injectable therapeutics) may be eligible for coverage under Medicare through Medicare Part B. Medicare Part B is part of original Medicare, the federal health care program that provides health care benefits to the aged and disabled, and covers outpatient services and supplies, including certain pharmaceutical products that are medically necessary to treat a beneficiary’s health condition. Specifically, Medicare Part B coverage may be available for eligible beneficiaries when the following, among other requirements, have been satisfied:
•the product is reasonable and necessary for the diagnosis or treatment of the illness or injury for which the product is administered according to accepted standards of medical practice;
•the product is typically furnished incident to a physician’s services;
•the product has been approved by the FDA.
Under the Medicaid Drug Rebate Statute, a manufacturer must participate in the Medicaid Drug Rebate Program in order to receive payment for its covered outpatient drugs under Medicare Part B (the Medicare program that generally covers physician-administered, outpatient drugs). In addition, manufacturers who participate in the Medicaid Drug Rebate Program are also required to (1) sign the Pharmaceutical Pricing Agreement and participate in the 340B Drug Pricing Program, and (2) sign the VA Master Agreement for inclusion of the manufacturer’s drugs on the Federal Supply Schedule, or FSS. The Medicaid Drug Rebate Program requires pharmaceutical manufacturers to enter into and have in effect a national rebate agreement with the Secretary of HHS as a condition for states to receive federal matching funds for the manufacturer’s outpatient drugs furnished to Medicaid patients. Under the 340B Drug Pricing Program, the manufacturer must extend discounts to entities eligible to participate in the program. Average prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of therapeutics from countries where they may be sold at lower prices than in the U.S. Self-administered therapeutics are typically reimbursed under Medicare Part D, and therapeutics that are administered in an inpatient hospital setting are typically reimbursed under Medicare Part A under a bundled payment. On March 11, 2021, President Biden signed the American Rescue Plan Act of 2021 into law, which eliminates the statutory Medicaid drug rebate cap for single source and innovator multiple source drugs, beginning January 1, 2024. It is difficult for us to predict how Medicare coverage and reimbursement policies will be applied to our therapeutics in the future and coverage and reimbursement under different federal healthcare programs are not always consistent. Medicare reimbursement rates may also reflect budgetary constraints placed on the Medicare program.
Commercial third-party payors often rely upon Medicare coverage policies and payment limitations in setting their own reimbursement rates. Our inability to promptly obtain coverage, and adequate reimbursement from both government-funded and private payors for new therapeutics we develop and for which we obtain regulatory approval could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our financial condition.
In addition, companion diagnostic tests require coverage and reimbursement separate and apart from the coverage and reimbursement for their companion pharmaceutical or biological products. Similar challenges to obtaining coverage and reimbursement, applicable to pharmaceutical or biological products, will apply to companion diagnostics. Additionally, if any companion diagnostic provider is unable to obtain reimbursement or is inadequately reimbursed, that may limit the availability of such companion diagnostic, which would negatively impact prescriptions for our product candidates, if approved.
We believe that the efforts of governments and third-party payors to contain or reduce the cost of healthcare, and specifically, therapeutics, and legislative and regulatory proposals to broaden the availability of healthcare will continue to affect the business and financial condition of pharmaceutical and biotechnology companies. A number of legislative and
regulatory changes in the healthcare system in the U.S. and other major healthcare markets have been proposed. These developments could, directly or indirectly, affect our ability to sell our therapeutics, if approved, at a favorable price.
For example, in the U.S., in 2010, the U.S. Congress passed the ACA, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of health spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional policy reforms.
Provisions of the ACA addressing coverage and reimbursement of pharmaceutical products that may be of importance to our potential therapeutic candidates include the following:
•Increases to pharmaceutical manufacturer rebate liability under the Medicaid Drug Rebate Program due to an increase in the minimum basic Medicaid rebate on most branded prescription drugs and the application of Medicaid rebate liability to drugs used in risk-based Medicaid managed care plans.
•The expansion of the 340B Drug Pricing Program to require discounts for “covered outpatient drugs” sold to certain children’s hospitals, critical access hospitals, freestanding cancer hospitals, rural referral centers, and sole community hospitals.
•Requirements imposed on pharmaceutical companies to offer discounts on brand-name drugs to patients who fall within the Medicare Part D coverage gap, commonly referred to as the “Donut Hole.” In February 2018, Congress passed the Bipartisan Budget Act of 2018, which, effective as of 2019, increased the discount to be paid by pharmaceutical companies from 50% to 70% of a brand-name drug’s negotiated price and added biosimilars to the coverage gap discount program.
•Requirements imposed on pharmaceutical companies to pay an annual non-tax-deductible fee to the federal government based on each company’s market share of prior year total sales of branded drugs to certain federal healthcare programs, such as Medicare, Medicaid, Department of Veterans Affairs, and Department of Defense. Since we currently expect our branded pharmaceutical sales to constitute a small portion of the total federal healthcare program pharmaceutical market, we do not currently expect this annual assessment to have a material impact on our financial condition.
•For therapeutic candidates classified as biologics, marketing approval for a follow-on biologic therapeutic may not become effective until 12 years after the date on which the reference innovator biologic therapeutic was first licensed by the FDA, with a possible six-month extension for pediatric therapeutics. After this exclusivity ends, it may be possible for biosimilar manufacturers to enter the market, which is likely to reduce the pricing for such therapeutics and could affect our profitability if our therapeutics are classified as biologics.
Separately, pursuant to certain health reform legislation and related initiatives, CMS is working with various healthcare providers to develop, refine, and implement Accountable Care Organizations, or ACOs, and other innovative models of care for Medicare and Medicaid beneficiaries, including the Bundled Payments for Care Improvement Initiative, the Financial Alignment Initiative Demonstration, and other models. The continued development and expansion of ACOs and other innovative models of care will have an uncertain impact on any future reimbursement we may receive for approved therapeutics administered by such organizations.
There have been judicial, administrative, executive, and legislative challenges to certain aspects of the ACA. For example, on June 17, 2021 the U.S. Supreme Court dismissed a challenge on procedural grounds that argued the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. Thus, the ACA will remain in effect in its current form. Further, prior to the U.S. Supreme Court ruling, on January 28, 2021, President Biden issued an executive order that initiated a special enrollment period for purposes of obtaining health insurance coverage through the ACA marketplace, which began on February 15, 2021 and remained open through August 15, 2021. The executive order also instructed certain governmental agencies to review and reconsider their existing policies and rules that limit access to healthcare, including among others, reexamining Medicaid demonstration projects and waiver programs that include work requirements, and policies that create unnecessary barriers to obtaining access to health insurance coverage through Medicaid or the ACA. It is possible that the ACA will be subject to judicial or Congressional challenges in the future. In addition, Congress is considering additional health reform measures as part of the budget reconciliation process. It is unclear how any such challenges and the healthcare reform measures of the Biden administration will impact the ACA and our business.
There has been increasing legislative and enforcement interest in the United States with respect to drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed federal and state
legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. At the federal level, the Trump administration used several means to propose or implement drug pricing reform, including through federal budget proposals, executive orders and policy initiatives. For example, on July 24, 2020 and September 13, 2020, the Trump administration announced several executive orders related to prescription drug pricing that seek to implement several of the administration’s proposals. As a result, the FDA released a final rule on September 24, 2020, effective November 30, 2020, providing guidance for states to build and submit importation plans for drugs from Canada. Further, on November 20, 2020, HHS finalized a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Medicare Part D, either directly or through pharmacy benefit managers, unless the price reduction is required by law. The implementation of the rule has been delayed by the Biden administration from January 1, 2022 to January 1, 2023 in response to ongoing litigation. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a new safe harbor for certain fixed fee arrangements between pharmacy benefit managers and manufacturers, the implementation of which have also been delayed until January 1, 2023. On November 20, 2020, CMS issued an interim final rule implementing the Trump administration’s Most Favored Nation, or MFN, executive order, which would tie Medicare Part B payments for certain physician-administered drugs to the lowest price paid in other economically advanced countries, effective January 1, 2021. As a result of litigation challenging the MFN model, on August 10, 2021, CMS published a proposed rule that seeks to rescind the MFN model interim final rule. Further, in July 2021, the Biden administration released an executive order that included multiple provisions aimed at prescription drugs. In response to Biden’s executive order, on September 9, 2021, HHS released a Comprehensive Plan for Addressing High Drug Prices that outlines principles for drug pricing reform. The plan sets out a variety of potential legislative policies that Congress could pursue as well as potential administrative actions HHS can take to advance these principles. No legislation or administrative actions have been finalized to implement these principles.
In addition, individual states have also become increasingly active in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, to encourage importation from other countries and bulk purchasing.
In the European Union, similar political, economic and regulatory developments may affect our ability to profitably commercialize our therapeutic candidates, if approved. In addition to continuing pressure on prices and cost containment measures, legislative developments at the European Union or member state level may result in significant additional requirements or obstacles that may increase our operating costs. The delivery of healthcare in the European Union, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than European Union, law and policy. National governments and health service providers have different priorities and approaches to the delivery of health care and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most European Union member states have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing European Union and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our therapeutic candidates, restrict or regulate post-approval activities and affect our ability to commercialize our therapeutic candidates, if approved. In markets outside of the United States and European Union, reimbursement and healthcare payment systems vary significantly by country, and many countries have instituted price ceilings on specific products and therapies.
Further, it is possible that additional governmental action is taken in response to the COVID-19 pandemic. We cannot predict the ultimate content, timing or effect of any healthcare reform legislation or the impact of potential legislation on us. If we or any third parties we may engage are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we or such third parties are not able to maintain regulatory compliance, our current or any future therapeutic candidates we may develop may lose any regulatory approval that may have been obtained and we may not achieve or sustain profitability.
We face potential liability related to the privacy and security of health information we obtain from clinical trials sponsored by us.
Most healthcare providers, including research institutions from which we obtain patient health information, are subject to privacy and security regulations promulgated under HIPAA, as amended by the HITECH. We are not currently classified as a covered entity or business associate under HIPAA and thus are not directly subject to its requirements or penalties. However, any person may be prosecuted under HIPAA’s criminal provisions either directly or under aiding-and-abetting or conspiracy principles. Consequently, depending on the facts and circumstances, we could face substantial penalties if we receive or use individually identifiable health information from a HIPAA-covered healthcare provider or
research institution or business associate that has not satisfied HIPAA’s requirements for disclosure of individually identifiable health information. In addition, we may maintain sensitive personally identifiable information, including health information, that we receive throughout the clinical trial process, in the course of our research collaborations, and directly from individuals (or their healthcare providers) who enroll in our patient assistance programs. As such, we may be subject to state laws requiring notification of affected individuals and state regulators in the event of a breach of personal information, which is a broader class of information than the health information protected by HIPAA.
Furthermore, certain health privacy laws, data security laws, data breach notification laws, consumer protection laws and genetic testing laws may apply directly to our operations and/or those of our collaborators and may impose restrictions on our collection, use and dissemination of individuals’ health information. Patients about whom we or our collaborators obtain health information, as well as the providers who share this information with us, may have statutory or contractual rights that limit our ability to use and disclose the information. We may be required to expend significant capital and other resources to ensure ongoing compliance with applicable privacy and data security laws. Claims that we have violated individuals’ privacy rights or breached our contractual obligations, even if we are not found liable, could be expensive and time- consuming to defend and could result in adverse publicity that could harm our business.
If we or third-party manufacturers, CROs or other contractors or consultants fail to comply with applicable federal, state or local regulatory requirements, we could be subject to a range of regulatory actions that could affect our or our contractors’ ability to develop and commercialize our therapeutic candidates and could harm or prevent sales of any affected therapeutics that we are able to commercialize, or could substantially increase the costs and expenses of developing, commercializing and marketing our therapeutics. Any threatened or actual government enforcement action could also generate adverse publicity and require that we devote substantial resources that could otherwise be used in other aspects of our business. Increasing use of social media could give rise to liability, breaches of data security or reputational damage.
We are subject to European data protection laws, including the European Union’s General Data Protection Regulation 2016/679, or GDPR. If we fail to comply with existing or future data protection regulations, our business, financial condition, results of operations and prospects may be materially adversely affected.
By virtue of our clinical trial activities in the United Kingdom and Europe, we are subject to European data protection laws, including the GDPR. The GDPR which came into effect on May 25, 2018, establishes new requirements applicable to the processing of personal data (i.e., data which identifies an individual or from which an individual is identifiable), affords new data protection rights to individuals (e.g., the right to erasure of personal data) and imposes penalties for serious breaches of up to 4% annual worldwide turnover or €20 million, whichever is greater. Individuals (e.g., study subjects) also have a right to compensation for financial or non-financial losses (e.g., distress). There may be circumstances under which a failure to comply with the GDPR, or the exercise of individual rights under the GDPR, would limit our ability to utilize clinical trial data collected on certain subjects. The GDPR imposes additional responsibility and liability in relation to our processing of personal data. This may be onerous and we may be unsuccessful in implementing all measures required by data protection authorities or courts in interpretation of the GDPR, which may materially adversely affect our business, financial condition, results of operations and prospects.
Our ability to obtain services, reimbursement or funding from the federal government may be impacted by possible reductions in federal spending.
U.S. federal government agencies currently face potentially significant spending reductions. The Budget Control Act of 2011, or BCA, established a Joint Select Committee on Deficit Reduction, which was tasked with achieving a reduction in the federal debt level of at least $1.2 trillion. That committee did not draft a proposal by the BCA’s deadline. As a result, automatic cuts, referred to as sequestration, in various federal programs were scheduled to take place. This includes reductions to Medicare payments to providers of 2% per fiscal year, that began in April 2013, and, due to subsequent legislative amendments, will remain in effect through 2030 unless additional Congressional action is taken. COVID-19 relief legislation, including the Coronavirus Aid, Relief and Economic Security Act, or CARES Act, which was signed into law in March 2020 , among other things, suspended the 2% Medicare sequester from May 1, 2020 through December 31, 2021.
These reductions may also impact the ability of relevant agencies to timely review and approve therapeutic research and development, manufacturing, and marketing activities, which may delay our ability to develop, market, and sell any therapeutics we may develop.
If any of our therapeutic candidates receives marketing approval and we or others later identify undesirable side effects caused by the therapeutic candidate, our ability to market and derive revenue from the therapeutic candidates could be compromised.
In the event that any of our therapeutic candidates receive regulatory approval and we or others identify undesirable side effects, adverse events or other problems caused by one of our therapeutics, any of the following adverse events could occur, which could result in the loss of significant revenue to us and materially and adversely affect our results of operations and business:
•regulatory authorities may withdraw their approval of the therapeutic or seize the therapeutic;
•we may need to recall the therapeutic or change the way the therapeutic is administered to patients;
•additional restrictions may be imposed on the marketing of the particular therapeutic or the manufacturing processes for the therapeutic or any component thereof;
•we may be subject to fines, restitution or disgorgement of profits or revenues, injunctions, or the imposition of civil penalties or criminal prosecution;
•regulatory authorities may require the addition of labeling statements, such as a “black box” warning or a contraindication;
•regulatory authorities may require us to implement a REMS, or to conduct post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the therapeutic;
•we may be required to create a medication guide outlining the risks of such side effects for distribution to patients;
•we could be sued and held liable for harm caused to patients;
•the therapeutic may become less competitive; and
•our reputation may suffer.
Risks Related to Ownership of Our Common Stock
The market price of our common stock has been, and is likely to continue to be, highly volatile, and you may not be able to resell your shares at or above the price you paid for them.
Since July 31, 2019, our stock price has been volatile and it is likely that the trading price of our common stock will continue to be volatile. As a result of this volatility, investors may not be able to sell their common stock at or above the price paid for the shares. The market price for our common stock may be influenced by a variety of factors, including the other risks described in this section titled “Risk Factors” and the following:
•the success of competitive therapeutics or technologies;
•results of our preclinical studies and clinical trials of our therapeutic candidates, or those of our competitors, or any current or future collaborators;
•regulatory or legal developments in the United States and other countries, especially changes in laws or regulations applicable to our therapeutics;
•introductions and announcements of new therapeutics by us, our future commercialization partners, or our competitors, and the timing of these introductions or announcements;
•actions taken by regulatory agencies with respect to our therapeutics, clinical studies, manufacturing process or sales and marketing terms;
•actual or anticipated variations in our financial results or those of companies that are perceived to be similar to us;
•the success of our efforts to acquire or in-license additional technologies, therapeutics or therapeutic candidates;
•developments concerning any current or future collaborations, including but not limited to those with our sources of manufacturing supply and our commercialization partners;
•announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments;
•developments or disputes concerning patents or other proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our therapeutics;
•our ability or inability to raise additional capital and the terms on which we raise it;
•the recruitment or departure of key personnel;
•changes in the structure of healthcare payment systems;
•market conditions in the pharmaceutical and biotechnology sectors;
•actual or anticipated changes in earnings estimates or changes in stock market analyst recommendations regarding our common stock, other comparable companies or our industry generally;
•our failure or the failure of our competitors to meet analysts’ projections or guidance that we or our competitors may give to the market;
•fluctuations in the valuation of companies perceived by investors to be comparable to us;
•announcement and expectation of additional financing efforts;
•speculation in the press or investment community;
•trading volume of our common stock and overall fluctuations in U.S. equity markets, including as a result of the COVID-19 pandemic;
•sales of our common stock by us or our stockholders;
•the concentrated ownership of our common stock;
•changes in accounting principles;
•terrorist acts, acts of war or periods of widespread civil unrest;
•natural disasters and other calamities; and
•general economic, industry, political and market conditions, including, but not limited to, the ongoing impact of the COVID-19 pandemic.
In addition, the stock markets in general, and the markets for pharmaceutical and biotechnology stocks in particular, have experienced extreme volatility that has been often unrelated to the operating performance of the issuer. These broad market and industry factors, such as those related to the COVID-19 pandemic, may seriously harm the market price of our common stock, regardless of our operating performance.
Raising additional funds by issuing securities may cause dilution to existing stockholders and raising funds through lending and licensing arrangements may restrict our operations or require us to relinquish proprietary rights.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, grants and license and development agreements in connection with any collaborations. We do not have any committed external source of funds. To the extent that we raise additional capital through the issuance of shares or other securities convertible into shares, our stockholders will be diluted. Future issuances of our common stock or other equity securities, or the perception that such sales may occur, could adversely affect the prevailing market price of our common stock and impair our ability to raise capital through future offerings of equity or equity-linked securities. For example, on December 23, 2019, we completed the sale of 10,000,000 shares of our common stock in the December 2019 Offering and on August 2, 2019 we completed the sale of 31,625,000 shares of our common stock in the August 2019 Offering. The issuance of shares in both the December 2019 Offering and August 2019 Offering were pursuant to a shelf registration statement on Form S-3 that was declared effective by the SEC on July 24, 2019. The shelf registration statement allows us to sell from time-to-time up to $125.0 million of common stock, preferred stock, debt securities, warrants, or units comprised of any combination of these securities, for our own account in one or more offerings; the remaining amount available under this shelf registration after the December 2019 Offering
(inclusive of the exercise of the underwriters’ option in January 2020 to purchase additional shares at the public offering price in connection with the December 2019 Offering) is approximately $31.3 million. Further, in December 2020 we entered into an equity distribution agreement with BMO providing for the sale of up to $50.0 million of our common stock from time to time in “at the market offerings” (as defined in Rule 415(a)(4) of the Securities Act of 1933, as amended) pursuant to a prospectus supplement and a shelf registration statement on Form S-3 that was declared effective by the SEC on January 7, 2021. The issuance of the shares pursuant to the December 2019 Offering and the August 2019 Offering and/or the resale of a substantial number of shares of our common stock in the public market or the sale of any shares of our common stock under the equity distribution agreement could adversely affect the market price for our common stock and make it more difficult for you to sell shares of our common stock at times and prices that you feel are appropriate. Adverse market and price pressures that may result from the December 2019 Offering or the August 2019 Offering or an offering pursuant to the shelf registration statement or such ‘at the market’ offerings may continue for an extended period of time and continued negative pressure on the market price of our common stock could have a material adverse effect on our ability to raise additional equity capital.
Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or grant licenses on terms that may not be favorable to us. Any debt financing that we enter into may involve covenants that restrict our operations. These restrictive covenants may include limitations on additional borrowing and specific restrictions on the use of our assets as well as prohibitions on our ability to create liens, pay dividends, redeem our stock or make investments. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
We are an “emerging growth company” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including (1) not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, (2) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and (3) exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. In addition, as an emerging growth company, we are only required to provide two years of audited financial statements and two years of selected financial data. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700.0 million as of any June 30 before that time or if we have total annual gross revenue of $1.07 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31, or if we issue more than $1.0 billion in non-convertible debt during any three-year period before that time, in which case we would no longer be an emerging growth company immediately. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company” which would allow us to take advantage of many of the same exemptions from disclosure requirements including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our share price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
Based on the beneficial ownership of our common stock as of September 30, 2021, our executive officers and directors, together with holders of five percent or more of our outstanding common stock and their respective affiliates, will beneficially own approximately 42% of our outstanding common stock. As a result, these stockholders, if acting together, will continue to have significant influence over the outcome of corporate actions requiring stockholder approval, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets and any other significant corporate transaction. The interests of these stockholders may not be the same as or may even conflict with your interests. For example, these stockholders could delay or prevent a change of control of our Company, even if such a change of control would benefit our other stockholders, which could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of our company or our assets and might affect the prevailing market price of our common stock. The significant concentration of stock ownership may adversely affect the trading price of our common stock due to investors’ perception that conflicts of interest may exist or arise.
Anti-takeover provisions in our charter documents and under the General Corporation Law of the State of Delaware could make an acquisition of us more difficult and may prevent attempts by our stockholders to replace or remove our management.
Provisions in our amended and restated certificate of incorporation and our bylaws may delay or prevent an acquisition of us or a change in our management. These provisions include a classified board of directors, a prohibition on actions by written consent of our stockholders, and the ability of the Board to issue preferred stock without stockholder approval. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, or DGCL, which prohibits stockholders owning in excess of 15% of the outstanding combined organization voting stock from merging or combining with the combined organization. Although we believe these provisions collectively will provide for an opportunity to receive higher bids by requiring potential acquirers to negotiate with our Board, they would apply even if the offer may be considered beneficial by some stockholders. In addition, these provisions may frustrate or prevent any attempts by our stockholders to replace or remove then-current management by making it more difficult for stockholders to replace members of the Board, which is responsible for appointing the members of management.
Anti-takeover provisions in our charter documents could discourage, delay or prevent a change in control of us and may affect the trading price of our common stock.
Our corporate documents and the DGCL contain provisions that may enable our Board to resist a change in control of us even if a change in control were to be considered favorable by our stockholders. These provisions:
•stagger the terms of our Board and require 66 and 2/3% stockholder voting to remove directors, who may only be removed for cause;
•authorize our Board to issue “blank check” preferred stock and to determine the rights and preferences of those shares, which may be senior to our common stock, without prior stockholder approval;
•establish advance notice requirements for nominating directors and proposing matters to be voted on by stockholders at stockholders’ meetings;
•prohibit our stockholders from calling a special meeting and prohibit stockholders from acting by written consent;
•require 66 and 2/3% stockholder voting to effect certain amendments to our certificate of incorporation and bylaws; and
•prohibit cumulative voting in the election of directors, which limits the ability of minority stockholders to elect director candidates.
These provisions could discourage, delay or prevent a transaction involving a change in control of us. These provisions could also discourage proxy contests and make it more difficult for stockholders to elect directors of their choosing and cause us to take other corporate actions our stockholders desire.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
Our amended and restated certificate of incorporation designates the Court of Chancery of the State of Delaware as the sole and exclusive forum for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, employees or agents.
Our amended and restated certificate of incorporation provides that, unless we consent in writing to an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for any of the following types of actions or proceedings under Delaware statutory or common law: derivative action or proceeding brought on our behalf, any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers, employees or agents to us or our stockholders, any action asserting a claim arising pursuant to any provision of the DGCL, our amended and restated certificate of incorporation or our amended and restated bylaws or any action asserting a claim that is governed by the internal affairs doctrine, in each case subject to the Court of Chancery having personal jurisdiction over the indispensable parties named as defendants therein. This provision would not apply to suits brought to enforce a duty or liability created by the Securities Exchange Act of 1934, as amended, or any other claims for which a court or forum other than the Court of Chancery has exclusive jurisdiction or for which the Court of Chancery does not have subject matter jurisdiction. Furthermore, Section 22 of the Securities Act of 1933, as amended, or the Securities Act, creates concurrent jurisdiction for federal and state courts over all Securities Act actions. Accordingly, both state and federal courts have jurisdiction to entertain such claims. Our amended and restated certificate of incorporation also provides that any person purchasing or otherwise acquiring any interest in any shares of our common stock shall be deemed to have notice of and to have consented to this provision of our amended and restated certificate of incorporation.
This choice of forum provision may limit our stockholders’ ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, employees or agents, which may discourage such lawsuits against us and our directors, officers, employees and agents even though an action, if successful, might benefit our stockholders. Stockholders who do bring a claim in the Court of Chancery could face additional litigation costs in pursuing any such claim, particularly if they do not reside in or near Delaware. The Court of Chancery may also reach different judgments or results than would other courts, including courts where a stockholder considering an action may be located or would otherwise choose to bring the action, and such judgments or results may be more favorable to us than to our stockholders. If a court were to find this exclusive forum provision in our amended and restated certificate of incorporation to be inapplicable or unenforceable in any action, we may incur further significant additional costs associated with resolving the dispute in other jurisdictions, all of which could have a material adverse effect on our business, financial condition or results of operations.
Changes in tax laws or regulations that are applied adversely to us or our customers may have a material adverse effect on our business, cash flow, financial condition or results of operations.
New income, sales, use or other tax laws, statutes, rules, regulations or ordinances could be enacted at any time, which could adversely affect our business operations and financial performance. Further, existing tax laws, statutes, rules, regulations or ordinances could be interpreted, changed, modified or applied adversely to us. For example, legislation enacted in 2017, informally titled the Tax Cuts and Jobs Act, or the Tax Act, enacted many significant changes to the U.S. tax laws. Future guidance from the Internal Revenue Service and other tax authorities with respect to the Tax Act may affect us, and certain aspects of the Tax Act could be repealed or modified in future legislation. For example, the CARES Act modified certain provisions of the Tax Act. In addition, it is uncertain if and to what extent various states will conform to the Tax Act, the CARES Act, or any newly enacted federal tax legislation. Changes in corporate tax rates, the realization of net deferred tax assets relating to our operations, the taxation of foreign earnings, and the deductibility of expenses under the Tax Act or future reform legislation could have a material impact on the value of our deferred tax assets, could result in significant one-time charges, and could increase our future U.S. tax expense.
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
We have incurred substantial losses during our history and do not expect to become profitable in the near future and we may never achieve profitability. Our net operating loss, or NOL, carryforwards generated in tax years beginning on or before December 31, 2017, are only permitted to be carried forward for 20 years under applicable U.S. tax law. Under the
Tax Act, as modified by the CARES Act, our federal NOLs generated in tax years beginning after December 31, 2017, may be carried forward indefinitely, but the deductibility of such federal NOLs in tax years beginning after December 31, 2020, is limited to 80% of taxable income. It is uncertain if and to what extent various states will conform to the Tax Act, or the CARES Act. In addition, under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change,” generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period, the corporation’s ability to use its pre-change NOL carryforwards, and other pre-change tax attributes (such as research tax credits) to offset its post-change income or taxes may be limited. We have experienced ownership changes in the past. In addition, we may experience ownership changes in the future as a result of subsequent shifts in our stock ownership, some of which are outside of our control. As a result, if we earn net taxable income, our ability to use our pre-change NOL carryforwards to offset U.S. federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us. In addition, at the state level, there may be periods during which the use of NOLs is suspended or otherwise limited, which could accelerate or permanently increase state taxes owed.
Risks Related to our Indebtedness
Our indebtedness could adversely affect our business, financial condition and competitive position.
As of September 30, 2021, we had $17.5 million of indebtedness outstanding, consisting of our borrowing under our MidCap Credit Facility.
In order to service this indebtedness and any additional indebtedness we may incur in the future, we need to generate cash. Our ability to generate cash is subject, to a certain extent, to our ability to successfully execute our business strategy, as well as general economic, financial, competitive, regulatory and other factors beyond our control. We cannot assure you that our business will be able to generate sufficient cash flow from operations or that future borrowings or other financing will be available to us in an amount sufficient to enable us to service our indebtedness and fund our other liquidity needs. To the extent we are required to use our cash flow from operations or the proceeds of any future financing to service our indebtedness instead of funding working capital, capital expenditures or other general corporate purposes, we will be less able to plan for, or react to, changes in our business, industry and in the economy generally. This will place us at a competitive disadvantage compared to our competitors that have less indebtedness.
In addition, the MidCap Credit Agreement contains, and any agreements evidencing or governing other future indebtedness may contain, certain restrictive covenants that limit our ability, among other things, to engage in certain activities that are in our long-term best interests, including our ability to:
•incur additional indebtedness;
•create or incur liens;
•engage in certain fundamental changes, including mergers or consolidations;
•make investments, loans, advances, guarantees and acquisitions;
•sell or transfer assets;
•pay dividends and distributions and repurchase capital stock;
•engage in certain transactions with affiliates;
•enter into negative pledge clauses and clauses restricting subsidiary distributions;
•modify the terms of material documents.
While we have not previously breached and are not in breach of any of these covenants, there can be no guarantee that we will not breach these covenants in the future.
Our ability to comply with these covenants and restrictions may be affected by events and factors beyond our control. Our failure to comply with any of these covenants or restrictions could result in an event of default under our MidCap Credit Facility. This would permit the lending banks under such facilities to take certain actions, including terminating all outstanding commitments and declaring all amounts due under our credit agreement, such as outstanding principal and accrued and unpaid interest thereon, to be immediately due and payable. In addition, the lenders would have the right to proceed against the collateral we have granted to them, which includes substantially all of our assets. The occurrence of any of these events could have a material adverse effect on our business, financial condition and results of operations.
We may not be able to secure additional financing on favorable terms, or at all, to meet our future capital needs.
In the future, we may require additional capital to respond to business opportunities, challenges, acquisitions or unforeseen circumstances, and may determine to engage in equity or debt financings or enter into credit facilities or refinance existing indebtedness for other reasons. We may not be able to timely secure additional debt or equity financing on favorable terms, or at all. As discussed above, the credit agreements governing the MidCap Credit Facility contain restrictive covenants that limit our ability to incur additional indebtedness and engage in other capital-raising activities. Any debt financing obtained by us in the future could involve covenants that further restrict our capital raising activities and other financial and operational matters, which may make it more difficult for us to operate our business, obtain additional capital and pursue business opportunities, including potential acquisitions. Furthermore, if we raise additional funds through the issuance of equity or convertible debt or other equity-linked securities or through the sale of our common stock pursuant to our “at the market” program, our existing stockholders could suffer significant dilution. If we are unable to obtain adequate financing or financing on terms satisfactory to us, when we require it, our ability to continue to grow or support our business and to respond to business challenges could be significantly limited.
General Risk Factors
FINRA sales practice requirements may limit a stockholder’s ability to buy and sell our stock.
The Financial Industry Regulatory Authority, or FINRA, has adopted rules requiring that, in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative or low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA has indicated its belief that there is a high probability that speculative or low-priced securities will not be suitable for at least some customers. If these FINRA requirements are applicable to us or our securities, they may make it more difficult for broker-dealers to recommend that at least some of their customers buy our common stock, which may limit the ability of our stockholders to buy and sell our common stock and could have an adverse effect on the market for and price of our common stock.
If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. Our research coverage by securities and industry analysts is currently limited. In addition, because we did not become a reporting company by conducting an underwritten initial public offering of our common stock, security analysts of brokerage firms may not provide wider coverage of our Company. In addition, investment banks may be less likely to agree to underwrite secondary offerings on our behalf than they might if we became a public reporting company by means of an underwritten initial public offering, because they may be less familiar with our Company as a result of more limited coverage by analysts and the media, and because we became public at an early stage in our development. The failure to receive wider research coverage or support in the market for our shares will have an adverse effect on our ability to develop a liquid market for our common stock and the trading price for our stock would be negatively impacted.
In the event we obtain wider securities or industry analyst coverage, if any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our target studies and operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
None.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. Mine Safety Disclosures.
Not applicable.
Item 5. Other Information.
On November 9, 2021, the Audit Committee of the Board of Directors of the Company was notified of a claim made by a former Company senior researcher regarding alleged improprieties that researcher claims to have committed with respect to the Company’s XCUR-FXN preclinical program for the treatment of Friedreich’s ataxia. The Audit Committee has retained external counsel to conduct an internal investigation of the claim. The Company is currently unable to predict the timing or outcome of the investigation.
Item 6. Exhibits
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | Incorporated by Reference |
Exhibit No. | | Exhibit Description | | Form | | Exhibit No. | | Filing Date | | File No. |
| | | | | | | | | | |
| 3.1 | |
| | 8-K | | 3.2 | | 10/02/17 | | 000-55764 |
| | | | | | | | | | |
| 3.2 | | | | 10-K | | 3.3 | | 3/11/21 | | 001-39011 |
| | | | | | | | | | |
| 3.3 | | | | 8-K | | 3.4 | | 10/02/17 | | 000-55764 |
| | | | | | | | | | |
| 10.1 | | | | 10-Q | | 10.4 | | 8/12/21 | | 001-39011 |
| | | | | | | | | | |
| 10.2 | | | | 8-K | | 10.1 | | 10/06/21 | | 001-39011 |
| | | | | | | | | | |
| 10.3 † * | | | | | | | | | | |
| | | | | | | | | | |
| 10.4 * | | | | | | | | | | |
| | | | | | | | | | |
| 10.5 † * | | | | | | | | | | |
| | | | | | | | | | |
| 31.1* | | | | | | | | | | |
| | | | | | | | | | |
| 31.2* | | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 32.1** | | | | | | | | | | |
| | | | | | | | | | |
| 101.INS* | | Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | | | | | | | | |
| | | | | | | | | | |
| 101.SCH* | | Inline XBRL Taxonomy Extension Schema Document | | | | | | | | |
| | | | | | | | | | |
| 101.CAL* | | Inline XBRL Taxonomy Extension Calculation Linkbase Document | | | | | | | | |
| | | | | | | | | | |
| 101.DEF* | | Inline XBRL Taxonomy Extension Definition Linkbase Document | | | | | | | | |
| | | | | | | | | | |
| 101.LAB* | | Inline XBRL Taxonomy Extension Label Linkbase Document | | | | | | | | |
| | | | | | | | | | |
| 101.PRE* | | Inline XBRL Taxonomy Extension Presentation Linkbase Document | | | | | | | | |
| | | | | | | | | | |
| 104* | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | | | | | | | | |
† Certain portions of the exhibit (indicated by asterisks) have been omitted pursuant to Rule 601(b)(10) because it is both (i) not material to investors and (ii) information that the Company treats as private or confidential.
* Filed herewith.
** The certification attached as Exhibit 32.1 that accompanies this Quarterly Report on Form 10-Q is not deemed filed with the SEC and is not to be incorporated by reference into any filing of Exicure, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of such Form 10-Q), irrespective of any general incorporation language contained in such filing.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Date: November 19, 2021
| | | | | |
| EXICURE, INC. |
| |
| By: | /s/ Brian C. Bock |
| Brian C. Bock |
| Chief Financial Officer |
| ( Principal Financial Officer) |
| |
| By: | /s/ Elias D. Papadimas |
| Elias D. Papadimas |
| Chief Accounting Officer |
| ( Principal Accounting Officer) |