Exhibit 99.1

argenx Reports Third Quarter 2023 Financial Results and Provides Business Update
- $329 million in third quarter global net product sales
- On track to submit VYVGART® Hytrulo sBLA for CIDP by year-end 2023
- Results from the ADVANCE-IV study published in The Lancet
- Management to host conference call today at 1:30 pm CET (8:30 am ET)
October 31, 2023
Amsterdam, the Netherlands – argenx SE (Euronext & Nasdaq: ARGX), a global immunology company committed to improving the lives of people suffering from severe autoimmune diseases, today announced its third quarter 2023 financial results and provided a business update and outlook for the remainder of the year.
“We continue to prioritize patient impact with VYVGART and VYVGART Hytrulo, broadening our two gMG products into earlier treatment lines and new geographies. VYVGART has now been used in thousands of patients over multiple treatment years, and its unique clinical profile has built patient trust and physician confidence in the brand,” said Tim Van Hauwermeiren, Chief Executive Officer of argenx. “There is a significant opportunity before us to transform autoimmunity across multiple indications with VYVGART. Based on the successful ADHERE trial, we are ready to file the sBLA by the end of 2023 to bring our first-in-class FcRn blocker to CIDP patients as quickly as possible. We are also on track with two near-term pivotal readouts and an ambitious plan forward over the coming years as we continue to execute and drive innovation within our FcRn portfolio and across immunology more broadly.”
THIRD QUARTER 2023 AND RECENT BUSINESS UPDATE
VYVGART Expansion
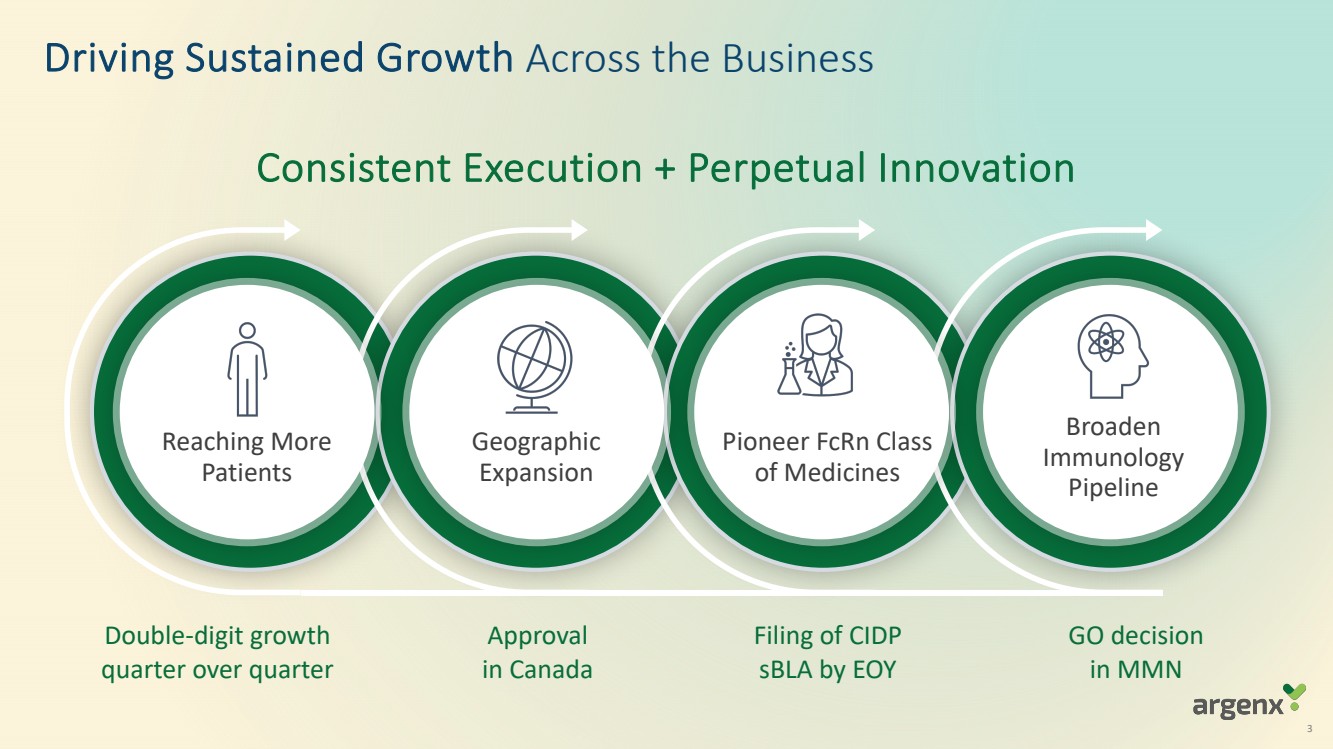
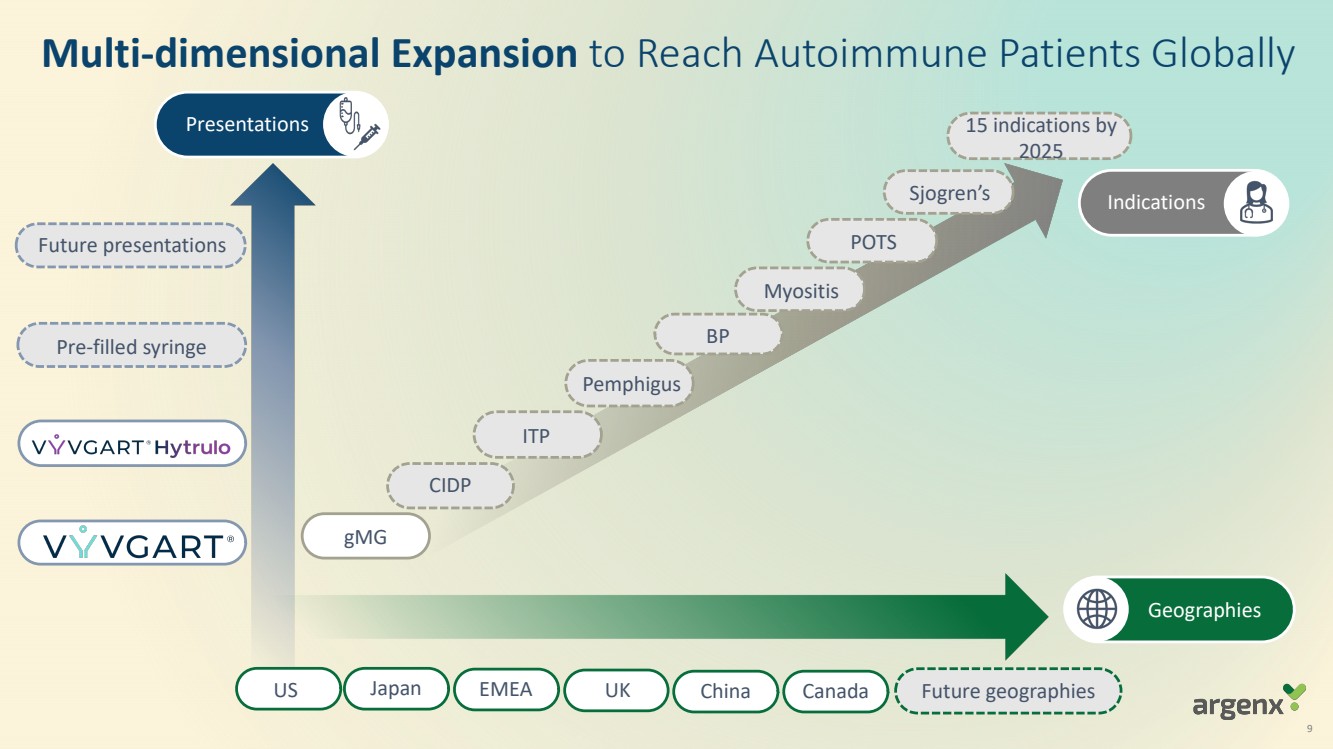
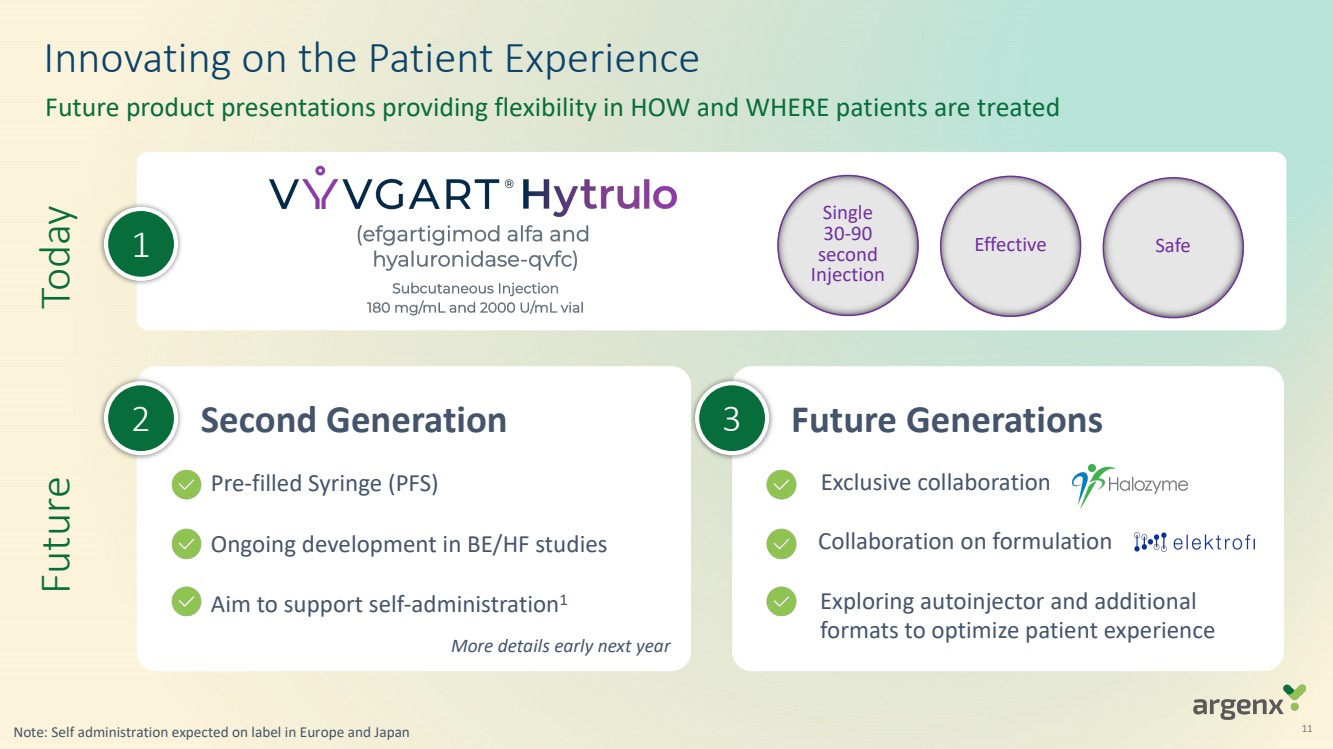
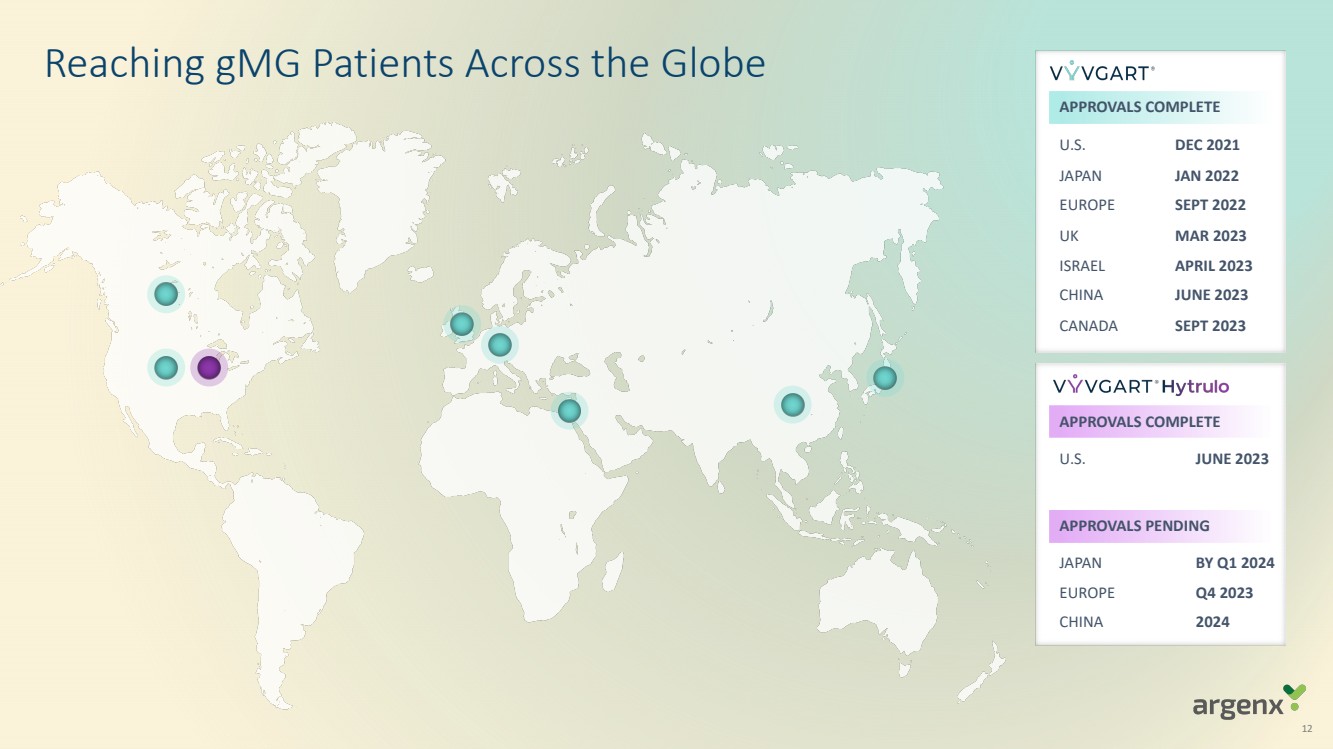
VYVGART® is a first-in-class antibody fragment targeting the neonatal Fc receptor (FcRn) and is now approved globally in seven countries or regions (U.S., Japan, EU, UK, Israel, China, Canada) for generalized myasthenia gravis (gMG). VYVGART Hytrulo (subcutaneous (SC) injection) was approved in the U.S. in June 2023. argenx is planning for multi-dimensional expansion to reach more patients with gMG and other severe autoimmune diseases through additional global regulatory approvals.
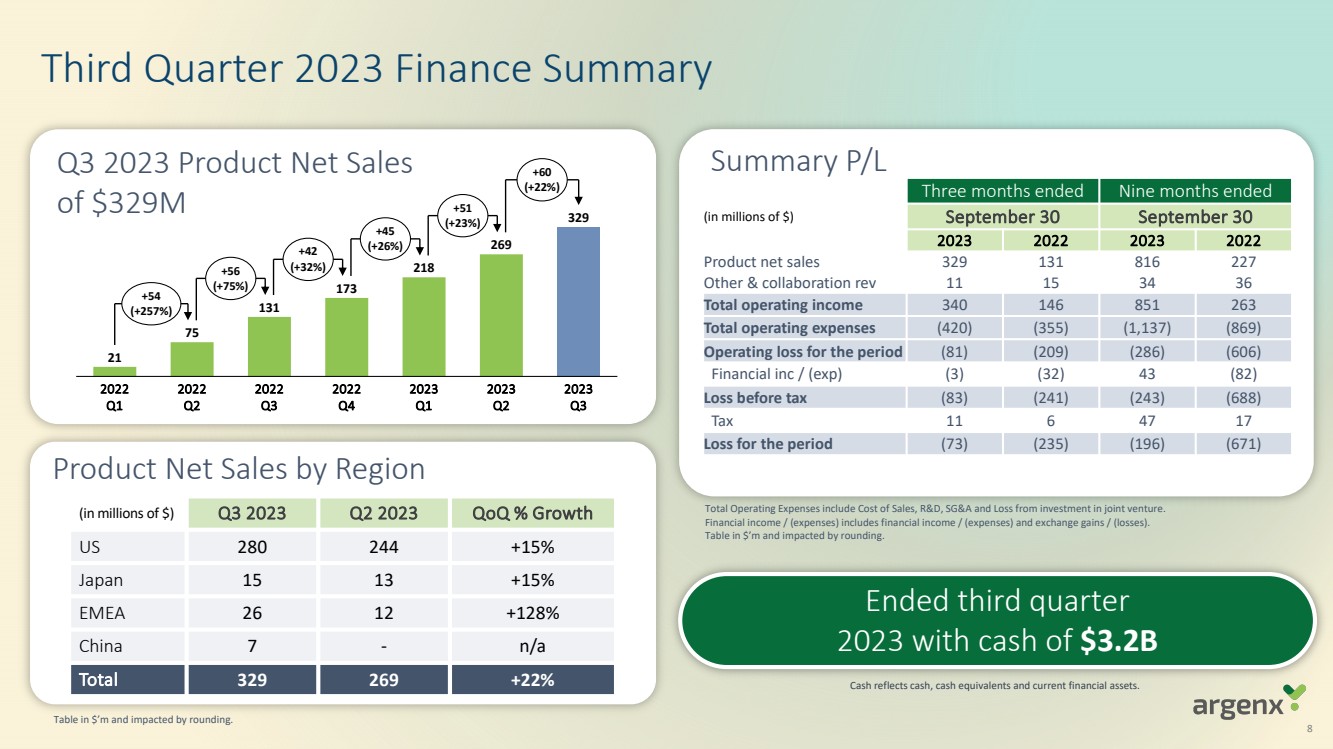
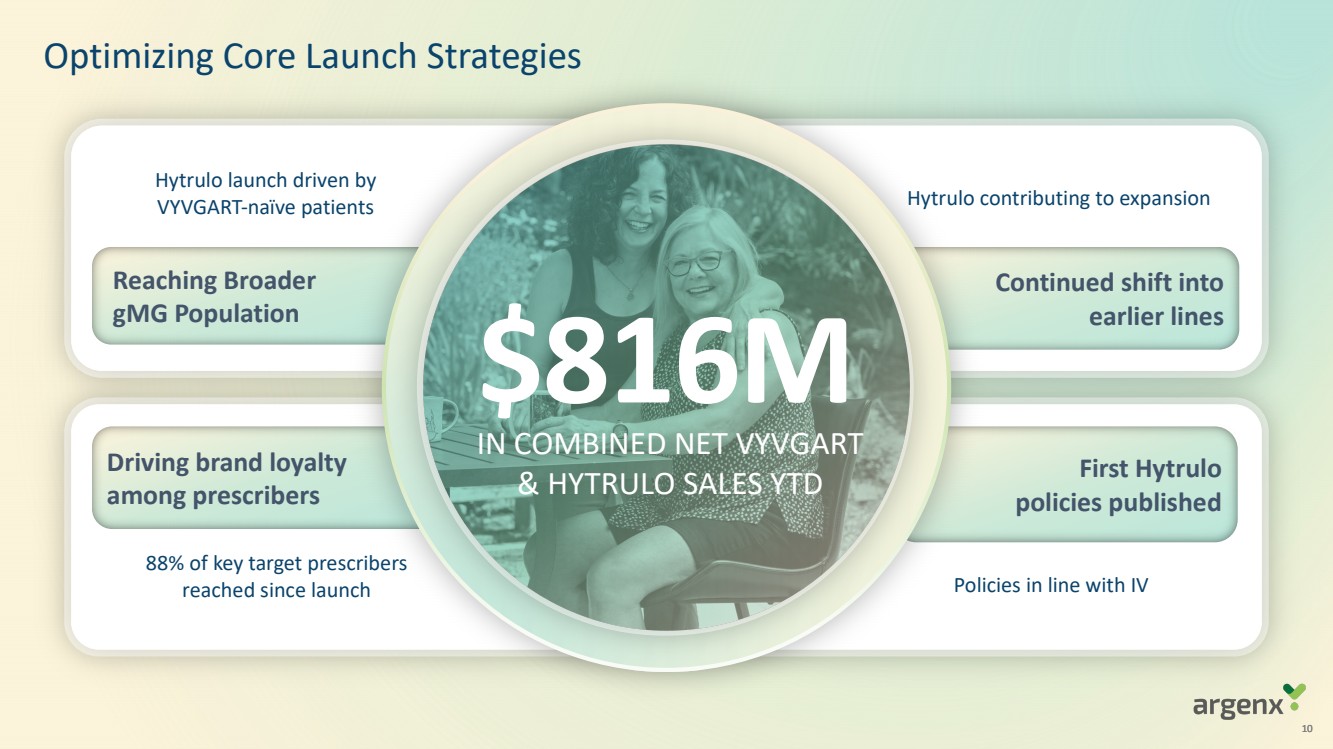
| · | Generated global net product revenues (inclusive of both VYVGART and VYVGART Hytrulo) of $329 million in the third quarter of 2023 |
| · | Health Canada approved VYVGART on September 21, 2023, marking the seventh global approval for gMG |

| · | European Commission (EC) approval of SC efgartigimod for gMG expected in fourth quarter of 2023 following positive recommendation from Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) |
| · | Japan approval decision regarding SC efgartigimod for gMG expected by first quarter of 2024 |
| · | Japan marketing authorization application (MAA) filed for VYVGART for primary immune thrombocytopenia (ITP); approval decision expected in first quarter of 2024 |
| · | U.S. supplemental Biologics License Application (sBLA) for VYVGART Hytrulo in chronic inflammatory demyelinating polyneuropathy (CIDP) on track to be filed by end of 2023 |
| · | China approval decision regarding SC efgartigimod for gMG expected by end of 2024 through partnership with Zai Lab |
Efgartigimod Research and Development
argenx is solidifying its leadership in FcRn blockade and demonstrating the broad potential of efgartigimod by advancing its clinical development programs of IgG-mediated autoimmune diseases. By 2025, efgartigimod is expected to be approved, in regulatory review or in development in 15 severe autoimmune diseases
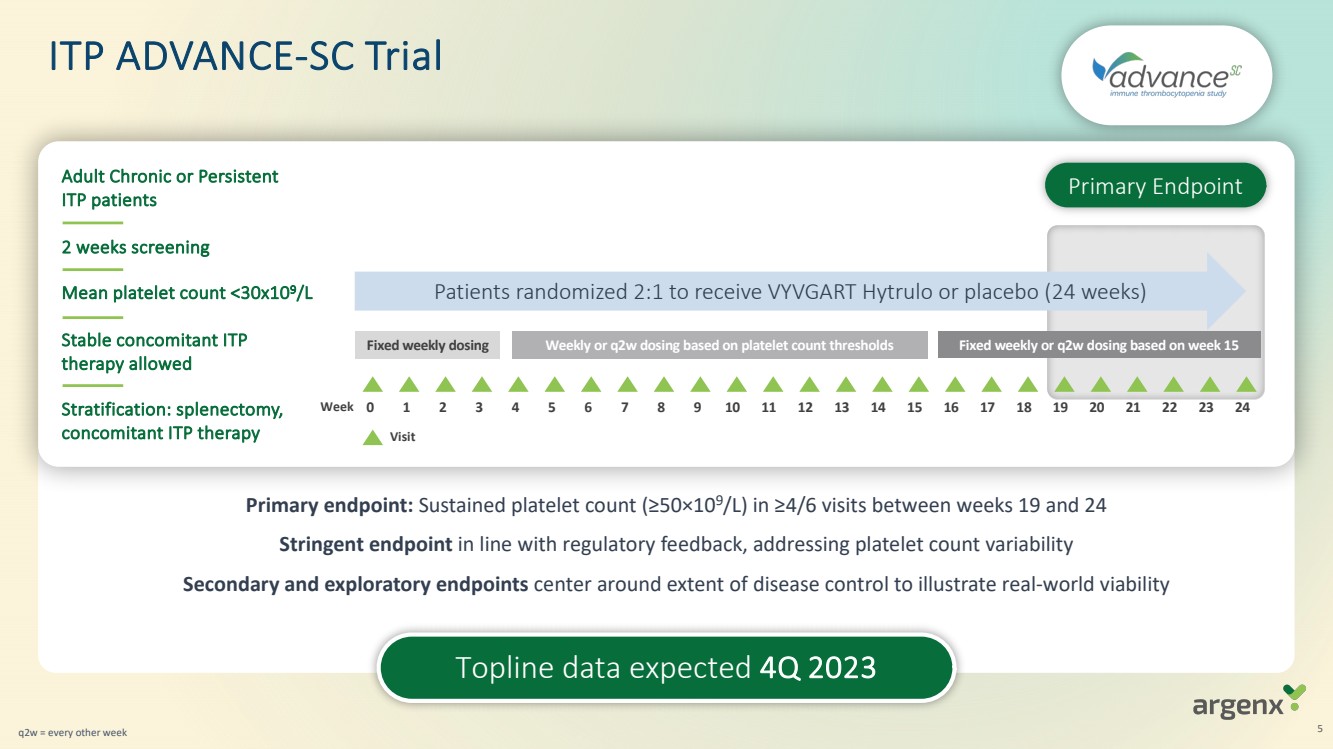
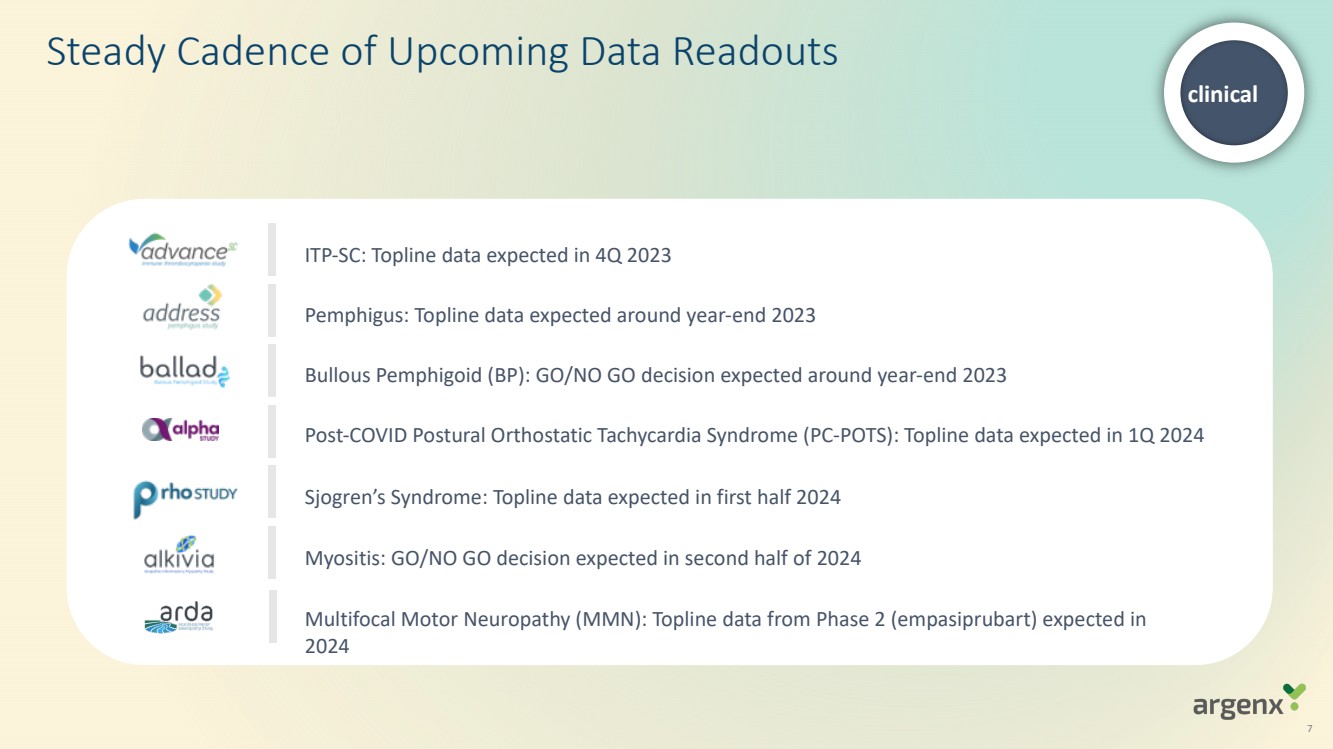
| · | Topline data from ADVANCE-SC (ITP) expected in fourth quarter of 2023; results from ADVANCE-IV study were published in The Lancet in September 2023 |
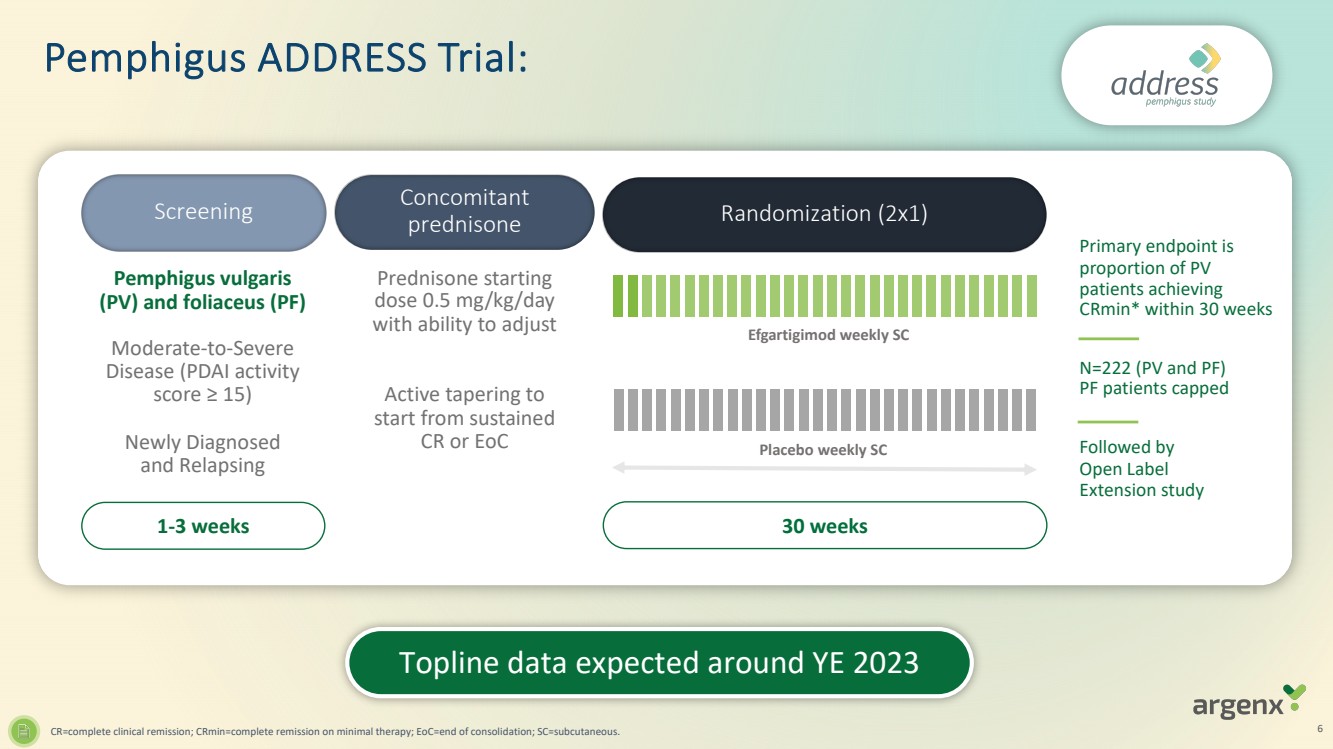
| · | Topline data from ADDRESS (pemphigus) and GO/NO GO decision from BALLAD (bullous pemphigoid) both expected around year-end 2023 |
| · | GO/NO GO decision expected from ALKIVIA (myositis) in second half of 2024 |
| · | Topline data from ALPHA (post-COVID postural orthostatic tachycardia syndrome (PC-POTS)) expected in first quarter of 2024 and RHO (Sjogren’s syndrome) in first half of 2024 |
Pipeline Progress
argenx is advancing a robust portfolio of innovative clinical programs, including empasiprubart (C2 inhibitor) and ARGX-119 (muscle-specific kinase (MuSK) agonist). Both programs have the potential to be first-in-class opportunities for multiple severe indications.
| · | Topline data from Phase 2 ARDA study of empasiprubart (ARGX-117) in multifocal motor neuropathy (MMN) expected in 2024 |
| · | Phase 1 study of ARGX-119 ongoing in healthy volunteers; subsequent Phase 1b trial planned to assess early signal detection in patients with congenital myasthenic syndrome (CMS) and amyotrophic lateral sclerosis (ALS) |
Immunology Innovation Program
argenx continues to invest in its discovery engine, the Immunology Innovation Program, to foster a robust innovation ecosystem and drive early-stage pipeline growth. argenx expects to nominate one new pipeline candidate in 2023.

THIRD QUARTER 2023 FINANCIAL RESULTS
argenx SE
UNAUDITED CONDENSED CONSOLIDATED INTERIM STATEMENTS OF PROFIT OR LOSS
| Three
Months Ended September 30, | Nine
Months Ended September 30, | |||||||||||||||
| (in thousands of $ except for shares and EPS) | 2023 | 2022 | 2023 | 2022 | ||||||||||||
| Product net sales | $ | 329,097 | $ | 131,329 | $ | 816,432 | $ | 227,325 | ||||||||
| Collaboration revenue | 692 | 6,652 | 3,047 | 9,262 | ||||||||||||
| Other operating income | 10,050 | 8,508 | 31,275 | 26,565 | ||||||||||||
| Total operating income | $ | 339,839 | $ | 146,489 | $ | 850,754 | $ | 263,152 | ||||||||
| Cost of sales | $ | (35,999 | ) | $ | (10,264 | ) | $ | (78,358 | ) | $ | (16,646 | ) | ||||
| Research and development expenses | (191,755 | ) | (236,681 | ) | (553,119 | ) | (515,568 | ) | ||||||||
| Selling, general and administrative expenses | (191,930 | ) | (108,181 | ) | (503,079 | ) | (336,845 | ) | ||||||||
| Loss from investment in joint venture | (743 | ) | - | (2,623 | ) | - | ||||||||||
| Total operating expenses | (420,427 | ) | (355,126 | ) | (1,137,179 | ) | (869,059 | ) | ||||||||
| Operating loss | $ | (80,588 | ) | $ | (208,637 | ) | $ | (286,425 | ) | $ | (605,907 | ) | ||||
| Financial income | $ | 30,049 | $ | 8,007 | $ | 67,078 | $ | 13,740 | ||||||||
| Financial expense | (231 | ) | (785 | ) | (626 | ) | (2,916 | ) | ||||||||
| Exchange gains/(losses) | (32,509 | ) | (39,609 | ) | (23,345 | ) | (92,991 | ) | ||||||||
| Loss for the period before taxes | $ | (83,279 | ) | $ | (241,024 | ) | $ | (243,318 | ) | $ | (688,074 | ) | ||||
| Income tax (expense)/benefit | $ | 10,637 | $ | 5,982 | $ | 47,437 | $ | 17,096 | ||||||||
| Loss for the period | $ | (72,642 | ) | $ | (235,042 | ) | $ | (195,881 | ) | $ | (670,978 | ) | ||||
| Loss for the year attributable to: | ||||||||||||||||
| Owners of the parent | $ | (72,642 | ) | $ | (235,042 | ) | $ | (195,881 | ) | $ | (670,978 | ) | ||||
| Weighted average number of shares outstanding | 58,128,233 | 55,203,655 | 56,512,254 | 54,049,119 | ||||||||||||
| Basis and diluted loss per share (in $) | (1.25 | ) | (4.26 | ) | (3.47 | ) | (12.41 | ) | ||||||||
| Net increase/(decrease) in cash, cash equivalents and current financial assets compared to year-end 2022 and 2021 | $ | 993,035 | $ | 48,813 | ||||||||||||
| Cash and cash equivalents and current financial assets at the end of the period | $ | 3,185,583 | $ | 2,385,541 | ||||||||||||

DETAILS OF THE FINANCIAL RESULTS
Total operating income for the third quarter and year-to-date in 2023 was $339.8 million and $850.8 million, respectively, compared to $146.5 million and $263.2 million for the same periods in 2022, and mainly consists of:
| · | Product net sales of VYVGART for the three months ended and nine months ended September 30, 2023, were $329.1 million and $816.4 million, compared to $131.3 million and $227.3 million for the same periods in 2022. |
| · | Other operating income for the third quarter and year-to-date in 2023 was $10.1 million and $31.3 million, respectively, compared to $8.5 million, and $26.6 million for the same periods in 2022. The other operating income for the three and nine months ended September 30, 2023, primarily relates to research and development tax incentives and payroll tax rebates. Other income also includes $0.7 million in royalty revenue from VYVGART sales in China. |
Total operating expenses for the third quarter and year-to-date in 2023 were $420.4 million and $1,137.2 million, respectively, compared to $335.1 million and $869.1 million for the same periods in 2022, and mainly consists of:
| · | Cost of sales for the third quarter and year-to-date in 2023 was $36.0 million and $78.4 million, respectively, compared to $10.3 million and $16.6 million for the same periods in 2022. The cost of sales was recognized with respect to the sale of VYVGART and VYVGART Hytrulo. |
| · | Research and development expenses for the third quarter and year-to-date in 2023 were $191.8 million and $553.1 million, respectively, compared to $236.7 million and $515.6 million for the same periods in 2022. The research and development expenses mainly relate to external research and development expenses and personnel expenses incurred in the clinical development of efgartigimod in various indications and the expansion of other clinical and preclinical pipeline candidates. |
| · | Selling, general and administrative expenses for the third quarter and year-to-date in 2023 were $191.9 million and $503.1 million, respectively, compared to $108.2 million and $336.8 million for the same periods in 2022. The selling, general and administrative expenses mainly relate to professional and marketing fees linked to the commercialization of VYVGART and VYVGART Hytrulo in the U.S., EU and Japan, and personnel expenses. |
Financial income for the third quarter and year-to-date in 2023 was $30.0 million and $67.1 million, respectively, compared to $8.0 million and $13.7 million for the same periods in 2022. The increase in financial income is mainly due to an increase in interest income on current financial assets and cash and cash equivalents attributable to higher interest rates.
Exchange losses for the third quarter and year-to-date in 2023 were $32.5 million and $23.3 million respectively, compared to $39.6 million and $93.0 million of exchange losses for the same periods in 2022. Exchange gains/losses are mainly attributable to unrealized exchange rate gains or losses on the cash, cash equivalents and current financial assets position in Euro.
Income tax for the third quarter and year-to-date in 2023 was $10.6 million and $47.4 million of tax benefit, respectively, compared to $6.0 million and $17.1 million of tax benefit for the same periods in 2022. Tax benefit for the nine months ended September 30, 2023, consists of $23.8 million of income tax expense and $71.3 million of deferred tax income, compared to $15.0 million of income tax expense and $32.1 million of deferred tax income for the comparable prior period.

Net loss for the three and nine-month periods ended September 30, 2023, was $72.6 million and $195.9 million, respectively, compared to $235.0 million and $671.0 million over the prior year periods. On a per weighted average share basis, the net loss was $3.47 and $12.41 for the nine months ended September 30, 2023 and 2022, respectively.
Cash, cash equivalents and current financial assets totalled $3.2 billion as of September 30, 2023, compared to $2.2 billion as of December 31, 2022. The increase in cash and cash equivalents and current financial assets resulted primarily from the closing of a global offering of shares, including a U.S. offering, which resulted in the receipt of $1.2 billion in net proceeds in July 2023, partially offset by net cash flows used in operating activities.
EXPECTED 2024 FINANCIAL CALENDAR
| · | February 29, 2024: FY 2023 financial results and business update |
| · | May 9, 2024: Q1 2024 financial results and business update |
| · | July 25, 2024: Q2 2024 financial results and business update |
| · | October 24, 2024: Q3 2024 financial results and business update |
CONFERENCE CALL DETAILS
The third quarter 2023 financial results and business update will be discussed during a conference call and webcast presentation today at 1:30 pm CET/8:30 am ET. A webcast of the live call may be accessed on the Investors section of the argenx website at argenx.com/investors. A replay of the webcast will be available on the argenx website.
Dial-in numbers:
Please dial in 15 minutes prior to the live call.
| Belgium | 32 800 50 201 |
| France | 33 800 943355 |
| Netherlands | 31 20 795 1090 |
| United Kingdom | 44 800 358 0970 |
| United States | 1 888 415 4250 |
| Japan | 81 3 4578 9081 |
| Switzerland | 41 43 210 11 32 |
About argenx
argenx is a global immunology company committed to improving the lives of people suffering from severe autoimmune diseases. Partnering with leading academic researchers through its Immunology Innovation Program (IIP), argenx aims to translate immunology breakthroughs into a world-class portfolio of novel antibody-based medicines. argenx developed and is commercializing the first approved neonatal Fc receptor (FcRn) blocker in the U.S., Japan, Israel, the EU, the UK, China and Canada. The Company is evaluating efgartigimod in multiple serious autoimmune diseases and advancing several earlier stage experimental medicines within its therapeutic franchises. For more information, visit www.argenx.com and follow us on LinkedIn, Twitter, and Instagram.

For further information, please contact:
Media:
Erin Murphy
emurphy@argenx.com
Investors:
Alexandra Roy (US)
aroy@argenx.com
Lynn Elton (EU)
lelton@argenx.com
Forward-looking Statements
The contents of this announcement include statements that are, or may be deemed to be, “forward-looking statements.” These forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “hope,” “estimates,” “anticipates,” “expects,” “intends,” “may,” “will,” or “should” and include statements argenx makes regarding its plans to execute and drive innovation within its FcRn portfolio and across immunology; its plans for multi-dimensional expansion to reach more patients with gMG and other autoimmune diseases through additional global regulatory approvals; advancement of, and anticipated clinical development, data readouts and regulatory milestones and plans, including the (1) expected EC approval of SC efgartigimod for gMG in the fourth quarter of 2023, (2) expected approval decision regarding SC efgartigimod for gMG in Japan by the first quarter of 2024, (3) expected MAA for VYVGART for primary ITP approval decision in Japan in the first quarter of 2024, (4) expected filing of the sBLA for VYVGART Hytrulo in CIDP by the end of 2023, (5) expected approval decision regarding SC efgartigimod for gMG in China by end of 2024 through its partnership with Zai Lab; (6) expected topline data from ITP in the fourth quarter of 2023, (7) expected topline data from ADDRESS and the GO/NO GO decision from BALLAD around year-end 2023, (8) expected GO/NO GO decision from ALKIVIA in the second half of 2024, (9) expected topline dtaa from ALPHA in the first quarter of 2024 and RHO in the first half of 2024, (10) expected topline data from Phase 2 ARDA study of ARGX-117 in MMN in 2024, (11) planned Phase 1b trial to assess early signal detection in patients with CMS and ALS and (12) planned nomination of a new pipeline candidate in 2023; continued investment in its Immunology Innovation Program to foster a robust innovation ecosystem and drive early-stage pipeline growth; and 2023 business and financial outlook and related plans, the timeline of future releases of financial results and business updates. By their nature, forward-looking statements involve risks and uncertainties and readers are cautioned that any such forward-looking statements are not guarantees of future performance. argenx’s actual results may differ materially from those predicted by the forward-looking statements as a result of various important factors, including inflation and deflation and the corresponding fluctuations in interest rate; regional instability and conflicts, such as the conflict between Russia and Ukraine, argenx’s expectations regarding the inherent uncertainties associated with competitive developments, preclinical and clinical trial and product development activities and regulatory approval requirements; argenx’s reliance on collaborations with third parties; estimating the commercial potential of argenx’s product candidates; argenx’s ability to obtain and maintain protection of intellectual property for its technologies and drugs; argenx’s limited operating history; and argenx’s ability to obtain additional funding for operations and to complete the development and commercialization of its product candidates. A further list and description of these risks, uncertainties and other risks can be found in argenx’s U.S. Securities and Exchange Commission (SEC) filings and reports, including in argenx’s most recent annual report on Form 20-F filed with the SEC as well as subsequent filings and reports filed by argenx with the SEC. Given these uncertainties, the reader is advised not to place any undue reliance on such forward-looking statements. These forward-looking statements speak only as of the date of publication of this document. argenx undertakes no obligation to publicly update or revise the information in this press release, including any forward-looking statements, except as may be required by law.