| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| |
||
(Address of principal executive offices) |
(Zip Code) | |
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
☒ |
Accelerated filer |
☐ | ||||
Non-accelerated filer |
☐ |
Smaller reporting company |
||||
Emerging growth company |
||||||
Page |
||||||
| PART I |
||||||
| Item 1. |
4 |
|||||
| Item 1A. |
51 |
|||||
| Item 1B. |
107 |
|||||
| Item 2. |
107 |
|||||
| Item 3. |
107 |
|||||
| Item 4. |
107 |
|||||
| PART II |
||||||
| Item 5. |
108 |
|||||
| Item 6. |
108 |
|||||
| Item 7. |
109 |
|||||
| Item 7A. |
130 |
|||||
| Item 8. |
130 |
|||||
| Item 9. |
130 |
|||||
| Item 9A. |
130 |
|||||
| Item 9B. |
131 |
|||||
| PART III |
||||||
| Item 10. |
132 |
|||||
| Item 11. |
132 |
|||||
| Item 12. |
132 |
|||||
| Item 13. |
132 |
|||||
| Item 14. |
132 |
|||||
| PART IV |
||||||
| Item 15. |
133 |
|||||
| Item 16. |
136 |
|||||
| • | the impacts of the COVID-19 pandemic; |
| • | the initiation, timing, progress and results of our current and future preclinical studies and clinical trials and our research and development programs; |
| • | our estimates regarding expenses, future revenue, capital requirements and need for additional financing; |
| • | our expectations regarding our ability to fund our operating expenses and capital expenditure requirements with our cash, cash equivalents and investments and the period in which we expect that such cash, cash equivalents and investments will enable us to fund such operating expenses and capital expenditure requirements; |
| • | our plans to develop our product candidates; |
| • | the timing of and our ability to submit applications for, obtain and maintain regulatory approvals for our product candidates; |
| • | the potential advantages of our product candidates; |
| • | the rate and degree of market acceptance and clinical utility of our product candidates; |
| • | our estimates regarding the potential market opportunity for our product candidates; |
| • | our commercialization, marketing and manufacturing capabilities and strategy; |
| • | our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates; |
| • | our ability to identify additional products, product candidates or technologies with significant commercial potential that are consistent with our commercial objectives; |
| • | the impact of government laws and regulations; |
| • | our competitive position; |
| • | developments relating to our competitors and our industry; and |
| • | our ability to establish collaborations or obtain additional funding. |
| • | The COVID-19 pandemic has, and may continue to, adversely disrupt our operations, including our ability to complete our ongoing clinical trials, and may have other adverse effects on our business and operations. The pandemic has caused substantial disruption in the financial markets and economies worldwide, both of which could result in adverse effects on our business, operations and ability to raise capital. |
| • | We and Sanofi Pasteur Inc., the vaccines global business unit of Sanofi S.A., may not successfully develop on an expedited timeframe an mRNA vaccine against a novel strain of coronavirus named SARS-CoV-2, COVID-19. |
| • | The manufacture, scale, validation and production of our potential SARS-CoV-2 |
| • | We have incurred significant losses since inception. We expect to incur losses for at least the next several years and may never achieve or maintain profitability. As of December 31, 2020, we had an accumulated deficit of $413.3 million. |
| • | Our approach to the discovery and development of product candidates based on mRNA is unproven, and we do not know whether we will be able to successfully develop any products. |
| • | We are dependent on the success of MRT5005 for patients with cystic fibrosis. If we are unable to complete satisfactorily the clinical development of, obtain marketing approval for or successfully commercialize MRT5005, our business would be substantially harmed. |
| • | Clinical drug development is a lengthy and expensive process with uncertain timelines and uncertain outcomes. |
| • | We may not have sufficient quantities of our product candidates at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts. |
| • | If we are unable to obtain and maintain patent protection for our products and technology, our competitors could develop and commercialize products and technology similar or identical to ours, and our ability to successfully commercialize our products and technology may be adversely affected. |
| • | If we fail to comply with our obligations under our licenses to develop and commercialize our product candidates, we could lose such intellectual property rights or owe damages to the licensor of such intellectual property. |
| • | If we are ultimately unable to obtain regulatory approval for our product candidates under the U.S Food and Drug Administration, the European Medicines Agency and other regulatory authorities, our business will be substantially harmed. |
| • | We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do. |
| • | restores gene expression without entering the cell nucleus or changing the genome; |
| • | enables the treatment of diseases that were previously undruggable by using the cell’s own machinery to produce fully functional proteins; |
| • | has drug-like properties that are familiar to health care providers, including the potential to repeat and adjust dosing in a chronic setting; and |
| • | permits rapid development from target gene selection to product candidate. |

| • | Rapidly advance MRT5005 through clinical development as a first-in-class non-amenable to currently approved CFTR modulators with the potential to pursue future clinical development in all patients with CF, regardless of the specific genetic mutation. |
| • | Pursue the development of mRNA infectious disease vaccines through our collaboration with Sanofi, including vaccines against influenza and SARS-CoV-2. |
| • | Focus primarily on identifying product candidates for additional pulmonary diseases caused by insufficient protein production through use of our lung delivery platform. |
| • | Develop additional product candidates that address diseases with significant unmet medical need in which the production of a desirable protein or the degradation of an unwanted protein can have a therapeutic effect or that could be applied to produce therapeutic antibodies and vaccines in areas such as infectious disease and other diseases. |
| • | Continue to develop next-generation delivery technologies for further applications in lung and liver diseases, as well as in vaccine applications. |
| • | Explore opportunities to collaborate where potential partners may add strategic value to our platform or programs. |
| • | Develop deep and active relationships with patient advocacy groups in order to better understand the needs of patients to optimize our treatment approaches and also to identify patients that could potentially benefit from our MRT product candidates, if approved. |
| • | Seek strategic acquisitions or in-licenses of technology or assets that may complement our proprietary MRT platform and programs. |
| • | Aggressively strengthen and protect our intellectual property and scientific and technical know-how. |
| • | Maintain the flexibility to develop and potentially commercialize products ourselves. |

| • | Stability |
| • | Immunogenicity |
| • | Delivery off-target toxicity and degradation. |
| • | Manufacture of mRNA Products non-immunogenic therapeutic mRNA in quantities sufficient to support clinical trials and commercial production. |

| • | Enhanced Stability |
| • | Targeting Lower Immunogenicity |
| • | Tissue-Specific Delivery off-target toxicity and unwanted stimulation of the immune system. |
| • | Scalable and Flexible Manufacturing |
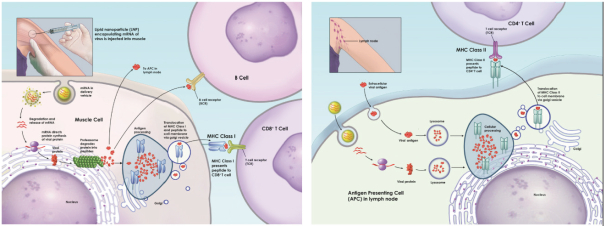

| • | preclinical testing including laboratory tests, animal studies and formulation studies, which must be performed in accordance with the FDA’s good laboratory practice, or GLP, regulations and standards; |
| • | submission to the FDA of an IND for human clinical testing, which must become effective before human clinical trials may begin; |
| • | approval by an independent institutional review board, or IRB, representing each clinical site before each clinical trial may be initiated; |
| • | performance of adequate and well-controlled human clinical trials to establish the safety, potency, purity and efficacy of the product candidate for each proposed indication, in accordance with current good clinical practices, or GCP; |
| • | preparation and submission to the FDA of a biologics license application, or BLA, for a biologic product which includes not only the results of the clinical trials, but also, detailed information on the chemistry, manufacture and quality controls for the product candidate and proposed labelling for one or more proposed indication(s); |
| • | review of the product candidate by an FDA advisory committee, where appropriate or if applicable; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities, including those of third parties, at which the product candidate or components thereof are manufactured to assess compliance with cGMP requirements and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; |
| • | satisfactory completion of any FDA audits of the non-clinical and clinical trial sites to assure compliance with GCP and the integrity of clinical data in support of the BLA; |
| • | payment of user fees and securing FDA licensure of the BLA to allow marketing of the new biologic product; and |
| • | compliance with any post-approval requirements, including the potential requirement to implement a risk evaluation and mitigation strategy, or REMS, and the potential requirement to conduct any post-approval studies required by the FDA. |
| • | restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls; |
| • | fines, warning letters or holds on post-approval clinical trials; |
| • | refusal of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of product license approvals; |
| • | product seizure or detention, or refusal to permit the import or export of products; or |
| • | injunctions or the imposition of civil or criminal penalties. |
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, paying, receiving or providing remuneration, directly or |
| indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal health care program such as Medicare and Medicaid; |
| • | the federal civil and criminal false claims laws, including the federal civil False Claims Act, and civil monetary penalties laws, which prohibit individuals or entities from, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false, fictitious or fraudulent or knowingly making, using or causing to made or used a false record or statement to avoid, decrease or conceal an obligation to pay money to the federal government. |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal laws that prohibit, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any health care benefit program or making false statements relating to health care matters; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and their respective implementing regulations, including the Final Omnibus Rule published in January 2013, which impose obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| • | the Foreign Corrupt Practices Act, or FCPA, which prohibits companies and their intermediaries from making, or offering or promising to make improper payments to non-U.S. officials for the purpose of obtaining or retaining business or otherwise seeking favorable treatment; |
| • | the federal false statements statute, which prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for health care benefits, items or services; |
| • | the federal transparency requirements known as the federal Physician Payments Sunshine Act, under the Patient Protection and Affordable Care Act, as amended by the Health Care Education Reconciliation Act, or the Affordable Care Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies to report annually to the Centers for Medicare & Medicaid Services, or CMS, within the United States Department of Health and Human Services, information related to payments and other transfers of value made by that entity to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members; and |
| • | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to health care items or services that are reimbursed by non-government third-party payors, including private insurers. |
| • | an annual, nondeductible fee on any entity that manufactures or imports certain branded prescription drug agents or biologic agents, which is apportioned among these entities according to their market share in certain government health care programs; |
| • | an increase in the rebates a manufacturer must pay under the Medicaid Drug Rebate Program to 23.1% and 13% of the average manufacturer price for branded and generic drugs, respectively; |
| • | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale |
| • | extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations, unless the drug is subject to discounts under the 340B drug discount program; |
| • | a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for drugs that are inhaled, infused, instilled, implanted or injected; |
| • | expansion of eligibility criteria for Medicaid programs by, among other things, allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for certain individuals with income at or below 133% of the federal poverty level, thereby potentially increasing manufacturers’ Medicaid rebate liability; |
| • | expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; |
| • | new requirements under the federal Physician Payments Sunshine Act for drug manufacturers to report information related to payments and other transfers of value made to physicians and teaching hospitals as well as ownership or investment interests held by physicians and their immediate family members; |
| • | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; |
| • | creation of the Independent Payment Advisory Board, which, if and when impaneled, will have authority to recommend certain changes to the Medicare program that could result in reduced payments for prescription drugs; and |
| • | establishment of a Center for Medicare and Medicaid Innovation at CMS to test innovative payment and service delivery models to lower Medicare and Medicaid spending, potentially including prescription drug spending. |
| • | the applicant must complete an identified program of studies within a time period specified by the competent authority, the results of which form the basis of a reassessment of the benefit/risk profile; |
| • | the medicinal product in question may be supplied on medical prescription only and may in certain cases be administered only under strict medical supervision, possibly in a hospital and in the case of a radiopharmaceutical, by an authorized person; and |
| • | the package leaflet and any medical information must draw the attention of the medical practitioner to the fact that the particulars available concerning the medicinal product in question are as yet inadequate in certain specified respects. |
| • | Compliance with the EU’s stringent pharmacovigilance or safety reporting rules must be ensured. These rules can impose post-authorization studies and additional monitoring obligations. |
| • | The manufacturing of authorized medicinal products, for which a separate manufacturer’s license is mandatory, must also be conducted in strict compliance with the applicable EU laws, regulations and guidance, including Directive 2001/83/EC, Directive 2003/94/EC, Regulation (EC) No 726/2004 and the European Commission Guidelines for Good Manufacturing Practice. These requirements include compliance with EU cGMP standards when manufacturing medicinal products and active pharmaceutical ingredients, including the manufacture of active pharmaceutical ingredients outside of the EU with the intention to import the active pharmaceutical ingredients into the EU. |
| • | The marketing and promotion of authorized drugs, including industry-sponsored continuing medical education and advertising directed toward the prescribers of drugs and/or the general public, are strictly |
| regulated in the EU notably under Directive 2001/83EC, as amended, and EU Member State laws. Direct-to-consumer |
| • | We provide competitive pay and compensation packages, including stock-based compensation awards and cash-based performance awards. |
| • | We provide a robust professional development program and career development program, where we strive to continually improve performance and promote professional competencies, including a Building Opportunities for Leadership and Development program and mentorship programs. |
| • | We offer numerous wellness programs to support all of our employees’ physical, mental and financial well-being, which we have significantly expanded during the COVID-19 pandemic, including fitness reimbursements and mindfulness programs to manage anxiety, stress, sleep and overall well-being, as well as a student loan assistance program. |
Item 1A. |
Risk Factors. |
| • | diversion of healthcare resources away from the conduct of our clinical trials in order to focus on pandemic concerns, including the availability of necessary materials, the attention of physicians serving as our clinical trial investigators, access to hospitals serving as our clinical trial sites, and availability of hospital staff supporting the conduct of our clinical trials; |
| • | potential interruptions in global shipping affecting the transport of clinical trial materials, such as investigational drug product, patient samples, and other supplies used in our clinical trials; |
| • | the impact of further limitations on travel that could interrupt key clinical trial activities, such as clinical trial site initiations and monitoring activities, travel by our employees, contractors or patients to clinical trial sites, or the ability of employees at any of our contract manufacturers or contract research organizations to report to work, any of which could delay or adversely impact the conduct or progress of our clinical trials for MRT5005 and other research and manufacturing activities, and limit the amount of clinical data we will be able to report; |
| • | any future interruption of, or delays in receiving, supplies of clinical trial material from our contract manufacturing organizations due to staffing shortages, production slowdowns or stoppages, or disruptions in delivery systems; |
| • | availability of future capacity at our contract manufacturers to produce sufficient drug substance and drug product to meet forecasted clinical trial demand if any of these manufacturers elect or are required to divert attention or resources to the manufacture of other pharmaceutical products; |
| • | delays in ongoing laboratory experiments and operations if we are required to further reduce the number of employees in our laboratories, or if the contract research organizations, or CROs, we have retained to supplement our internal research efforts are unable to perform as anticipated, whether due to capacity constraints, staffing shortages, or otherwise; and |
| • | business disruptions caused by potential workplace closures and an increased reliance on employees working from home, challenges in recruiting employees required to execute on our research and development plans, cybersecurity and data accessibility issues, and communication or transit disruptions, any of which could adversely impact our business operations and delay necessary interactions among our employees and between our company and the third parties upon which we rely. |
| • | continue the clinical development of MRT5005; |
| • | continue the development of mRNA vaccine candidates against infectious diseases, including MRT5500, the lead vaccine candidate against SARS-CoV-2; |
| • | leverage our programs to advance our other product candidates into preclinical and clinical development; |
| • | seek regulatory approvals for any product candidates that successfully complete clinical trials; |
| • | seek to discover and develop additional product candidates; |
| • | expand our manufacturing, operational, financial and management systems; |
| • | increase personnel, including personnel to support our research, clinical development, manufacturing and commercialization efforts and our operations as a public company; |
| • | maintain, expand and protect our intellectual property portfolio; |
| • | acquire or in-license other product candidates and technologies; and |
| • | incur additional legal, accounting and other expenses in operating as a public company; and |
| • | establish a sales, marketing, medical affairs and distribution infrastructure to commercialize any product candidates for which we may obtain marketing approval and intend to commercialize on our own or jointly. |
| • | completing preclinical and clinical development of our product candidates and identifying and developing new product candidates; |
| • | seeking and obtaining marketing approvals for any of our product candidates; |
| • | launching and commercializing product candidates for which we obtain marketing approval by establishing a sales force, marketing, medical affairs and distribution infrastructure or, alternatively, collaborating with a commercialization partner; |
| • | achieving formulary status in hospitals and adequate coverage and reimbursement by government and third-party payors for our product candidates; |
| • | establishing and maintaining supply and manufacturing relationships with third parties that can provide adequate, in both amount and quality, products and services to support clinical development and the market demand for our product candidates, if approved; |
| • | obtaining market acceptance of our product candidates as viable treatment options; |
| • | addressing any competing technological and market developments; |
| • | negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter and performing our obligations in such collaborations; |
| • | maintaining, protecting and expanding our portfolio of intellectual property rights, including patents, trade secrets and know-how; |
| • | defending against third-party interference or infringement claims, if any; and |
| • | attracting, hiring and retaining qualified personnel. |
| • | the impacts of the COVID-19 pandemic and our response to it; |
| • | the scope, progress, results and costs of researching and developing our product candidates, and conducting preclinical studies and clinical trials; |
| • | the success of our collaboration with Sanofi; |
| • | the costs, timing and outcome of regulatory review of our product candidates; |
| • | the costs of future activities, including product sales, medical affairs, marketing, manufacturing and distribution, for any of our product candidates for which we receive marketing approval; |
| • | the costs of manufacturing commercial-grade products and sufficient inventory to support commercial launch; |
| • | the ability to receive additional non-dilutive funding, including grants from organizations and foundations; |
| • | the revenue, if any, received from commercial sale of our products, should any of our product candidates receive marketing approval; |
| • | the cost and timing of hiring new employees to support our continued growth; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; |
| • | our ability to establish and maintain collaborations on favorable terms, if at all; |
| • | the extent to which we acquire or in-license other product candidates and technologies; and |
| • | the timing, receipt and amount of sales of, or milestone payments related to or royalties on, our current or future product candidates, if any. |
| • | successful patient enrollment in and completion of clinical trials; |
| • | a safety, tolerability and efficacy profile that is satisfactory to the FDA, EMA or other regulatory authorities for marketing approval; |
| • | timely receipt of marketing approvals from applicable regulatory authorities; |
| • | the extent of any required post-marketing approval commitments to applicable regulatory authorities; |
| • | establishment and maintenance of arrangements with third-party manufacturers for both clinical and any future commercial manufacturing; |
| • | adequate ongoing availability of raw materials and drug product for clinical development and any commercial sales; |
| • | obtaining and maintaining patent, trade secret protection and regulatory exclusivity, both in the United States and internationally; |
| • | protection of our rights in our intellectual property portfolio; |
| • | successful launch of commercial sales following any marketing approval; |
| • | a continued acceptable safety profile following any marketing approval; |
| • | commercial acceptance by hospitals, the patient community, the medical community and third-party payors; |
| • | the availability of coverage and adequate reimbursement from third-party payors; |
| • | the performance of our future collaborators, if any; and |
| • | our ability to compete with other therapies. |
| • | delays in reaching a consensus with regulatory authorities or collaborators on trial design; |
| • | delays in reaching agreement on acceptable terms with CROs and clinical trial sites; |
| • | delays in opening clinical trial sites or obtaining required institutional review board or independent ethics committee approval at each clinical trial site; |
| • | delays in recruiting suitable subjects or a sufficient number of subjects to participate in our clinical trials; |
| • | imposition of a clinical hold by regulatory authorities, including upon submission of an IND, or as a result of a serious adverse event or after an inspection of our clinical trial operations or trial sites; |
| • | failure by us, any CROs we engage, clinical investigators or any other third parties to adhere to clinical trial requirements; |
| • | failure to perform the clinical trial in accordance with good clinical practices, or GCP, or applicable regulatory requirements in the European Union, the United States, or other countries; |
| • | delays in the testing, validation, manufacturing and delivery of our product candidates to the clinical sites, including delays by third parties with whom we have contracted to perform certain of those functions; |
| • | delays or failures in demonstrating the comparability of product manufactured at one facility or with one process to product manufactured at another facility or with another process, including clinical trials to demonstrate such comparability; |
| • | delays in having patients complete participation in a trial or return for post-treatment follow-up; |
| • | clinical trial sites or subjects dropping out of a trial; |
| • | selection of clinical endpoints that require prolonged periods of clinical observation or analysis of the resulting data; |
| • | occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits; |
| • | occurrence of serious adverse events in trials of the same class of agents conducted by other sponsors; and |
| • | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols. |
| • | inability to generate sufficient preclinical or other in vivo in vitro |
| • | delays in reaching a consensus with regulatory agencies on study design. |
| • | coordination between us, CROs and any future collaborators in our efforts to enroll and administer the clinical trial; |
| • | size of the patient population and process for identifying patients; |
| • | design of the trial protocol; |
| • | eligibility and exclusion criteria; |
| • | perceived risks and benefits of the product candidate under study; |
| • | availability of competing commercially available therapies and other competing product candidates’ clinical trials; |
| • | time of year in which the trial is initiated or conducted; |
| • | variations in the seasonal incidence of the target indication; |
| • | severity of the disease under investigation; |
| • | ability to obtain and maintain subject consent; |
| • | ability to enroll and treat patients in a timely manner; |
| • | risk that enrolled subjects will drop out before completion of the trial; |
| • | proximity and availability of clinical trial sites for prospective patients; |
| • | patient referral practices of physicians; and |
| • | ability to monitor subjects adequately during and after treatment. |
| • | be delayed in obtaining marketing approval for our product candidates, if at all; |
| • | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| • | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; |
| • | be subject to changes in the way the product is administered; |
| • | be required to perform additional clinical trials to support approval or be subject to additional post-marketing testing requirements; |
| • | have regulatory authorities withdraw, or suspend, their approval of the product or impose restrictions on its distribution in the form of a modified risk evaluation and mitigation strategy, or REMS; |
| • | be subject to the addition of labeling statements, such as contraindications or warnings, including a black box warning; |
| • | be sued; or |
| • | experience damage to our reputation. |
| • | clinical practice patterns and standards of care that vary widely among countries; |
| • | non-U.S. regulatory authority requirements that could restrict or limit our ability to conduct our clinical trials; |
| • | administrative burdens of conducting clinical trials under multiple non-U.S. regulatory authority schema; |
| • | foreign exchange fluctuations; and |
| • | diminished protection of intellectual property in some countries. |
| • | Sanofi has significant discretion in determining the efforts and resources that it will apply to our collaboration; |
| • | Sanofi may not perform its obligations as expected; |
| • | The clinical trials conducted as part of our collaboration with Sanofi may not be successful; |
| • | Sanofi may not pursue development and/or commercialization of any vaccine candidates that achieve regulatory approval or may elect not to continue or renew development or commercialization programs based on clinical trial results, changes in Sanofi’s strategic focus or available funding or external factors, such as an acquisition, that divert resources or create competing priorities; |
| • | Sanofi has final decision-making authority for conducting clinical trials, and this may result in Sanofi delaying clinical trials, providing insufficient funding for clinical trials, stopping a clinical trial or abandoning a vaccine candidate, repeating or conducting new clinical trials or requiring a new formulation of a vaccine candidate for clinical testing; |
| • | We may not have access to, or may be restricted from disclosing, certain information regarding vaccine candidates being developed or commercialized under our collaboration with Sanofi and, consequently, may have limited ability to inform our stockholders about the status of such vaccine candidates; |
| • | Sanofi has an existing collaboration with GlaxoSmithKline plc to develop a SARS-CoV-2 |
| • | Sanofi may view vaccine candidates developed in collaboration with us as competitive with their own product candidates or products, which may cause Sanofi to cease to devote resources to the commercialization of our vaccine candidates; |
| • | Sanofi may not commit sufficient resources to the marketing and distribution of any such of our vaccine candidates that achieve regulatory approval; |
| • | Disagreements with Sanofi, including disagreements over proprietary rights, contract interpretation or the preferred course of development of any of our vaccine candidates, may cause delays or termination of the research, development, manufacture or commercialization of such vaccine candidates, may lead to additional responsibilities for us with respect to such vaccine candidates or may result in litigation or arbitration, any of which would be time-consuming and expensive. Moreover, in certain circumstances, there could be a misalignment between our contractual obligations to Sanofi and any upstream contractual obligations we may owe to our licensors or other third parties; |
| • | Sanofi may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation. For example, Sanofi has the first right to enforce or defend certain of our intellectual property rights under our collaboration with respect to products in Licensed Fields, and although we may have the right to assume the enforcement and defense of such intellectual property rights if Sanofi does not, our ability to do so may be compromised by Sanofi’s actions; |
| • | Disputes may arise with respect to the ownership of intellectual property developed pursuant to our collaboration with Sanofi; |
| • | Sanofi may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability; and |
| • | Sanofi may terminate our collaboration for convenience after a specified notice period and, if terminated, we could be required to raise additional capital to pursue further development or commercialization of the applicable vaccine candidates. |
| • | reliance on the third party for regulatory compliance and quality assurance; |
| • | the possible breach of the manufacturing agreement by the third party; |
| • | the possible misappropriation of our proprietary information, including our trade secrets and know-how; and |
| • | the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us. |
| • | a covered benefit under the applicable health plan; |
| • | safe, effective and medically necessary; |
| • | appropriate for the specific patient population; |
| • | cost-effective; and |
| • | neither experimental nor investigational. |
| • | the efficacy and safety of such product candidates as demonstrated in clinical trials; |
| • | the potential and perceived advantages of our product candidates over other treatments; |
| • | the cost-effectiveness of treatment relative to alternative treatments; |
| • | the clinical indications for which the product candidate is approved by the FDA, the EMA or other regulatory body; |
| • | the willingness of physicians to prescribe new therapies over the existing standard of care and future new therapies; |
| • | the willingness of the target patient population to try new therapies; |
| • | the prevalence and severity of any side effects; |
| • | product labeling or product insert requirements of the FDA, EMA or other regulatory authorities, including any limitations or warnings contained in a product’s approved labeling, including any black box warning; |
| • | relative convenience and ease of administration; |
| • | our ability to educate the medical community and third-party payors about the benefit of our product candidates; |
| • | the strength of marketing and distribution support; |
| • | the timing of market introduction of competitive products; |
| • | any restrictions on the use of our products together with other medications; |
| • | publicity concerning our products or competing products and treatments; and |
| • | sufficient third-party payor insurance coverage and adequate reimbursement. |
| • | different regulatory requirements for approval of drugs and biologics in foreign countries; |
| • | reduced protection for intellectual property rights; |
| • | unexpected changes in tariffs, trade barriers and regulatory requirements; |
| • | economic weakness, including inflation, or political instability in foreign economies and markets; |
| • | different pricing and reimbursement regimes; |
| • | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| • | foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
| • | workforce uncertainty in countries where labor unrest is more common than in the United States; |
| • | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| • | business interruptions resulting from geopolitical actions, including war and terrorism or natural disasters, including earthquakes, typhoons, floods and fires. |
| • | decreased demand for any product candidates that we may develop; |
| • | loss of revenue; |
| • | substantial monetary awards to trial participants or patients; |
| • | significant time and costs to defend the related litigation; |
| • | withdrawal of clinical trial participants; |
| • | the inability to commercialize any product candidates that we may develop; and |
| • | injury to our reputation and significant negative media attention. |
| • | comply with FDA regulations or the regulations applicable in the European Union and other jurisdictions; |
| • | provide accurate information to the FDA, the EMA and other regulatory authorities; |
| • | comply with health care fraud and abuse laws and regulations in the United States and abroad; |
| • | comply with the U.S. Foreign Corrupt Practices Act, or FCPA, or other anti-corruption laws and regulations; |
| • | comply with U.S. federal securities laws relating to trading in our common stock; |
| • | report financial information or data accurately; or |
| • | disclose unauthorized activities to us. |
| • | the scope of rights granted under the license agreement and other interpretation-related issues; |
| • | the extent to which our technology and processes infringe the intellectual property of the licensor that is not subject to the licensing agreement; |
| • | the sublicensing of patent and other rights under any collaborative development relationships; |
| • | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| • | the inventorship or ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
| • | the priority of invention of patented technology. |
| • | others may be able to make products that are similar to our product candidates but that are not covered by the claims of the patents that we own or license or may own in the future; |
| • | we, or any partners or collaborators, might not have been the first to make the inventions covered by the issued patent or pending patent application that we license or may own in the future; |
| • | we, or any partners or collaborators, might not have been the first to file patent applications covering certain of our or their inventions; |
| • | others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our owned or licensed intellectual property rights; |
| • | it is possible that our pending licensed patent applications or those that we may own in the future will not lead to issued patents; |
| • | issued patents that we hold rights to may be held invalid or unenforceable, including as a result of legal challenges by our competitors; |
| • | our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets; |
| • | we may not develop additional proprietary technologies that are patentable; |
| • | the patents of others may have an adverse effect on our business; and |
| • | we may choose not to file a patent for certain trade secrets or know-how, and a third party may subsequently file a patent covering such intellectual property. |
| • | the FDA may disagree with the design or implementation of our clinical trials; |
| • | we may be unable to demonstrate to the satisfaction of the FDA that a product candidate is safe, pure and potent or effective for its proposed indication; |
| • | results of clinical trials may not meet the level of statistical significance required by the FDA for approval; |
| • | we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| • | the FDA may disagree with our interpretation of data from preclinical studies or clinical trials; |
| • | data collected from clinical trials of our product candidates may not be sufficient to support the submission of a BLA to the FDA or other submission or to obtain regulatory approval in the United States; |
| • | the FDA may find deficiencies with or fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical and commercial supplies; and |
| • | the approval policies or regulations of the FDA may significantly change in a manner rendering our clinical data insufficient for approval. |
| • | restrictions on such products, manufacturers or manufacturing processes; |
| • | restrictions on the labeling or marketing of a product; |
| • | restrictions on product distribution or use; |
| • | requirements to conduct post-marketing studies or clinical trials; |
| • | warning letters or untitled letters; |
| • | withdrawal of the products from the market; |
| • | refusal to approve pending applications or supplements to approved applications that we submit; |
| • | recall of products; |
| • | restrictions on coverage by third-party payors; |
| • | fines, restitution or disgorgement of profits or revenue; |
| • | suspension or withdrawal of marketing approvals, including license revocation; |
| • | refusal to permit the import or export of products; |
| • | product seizure; and |
| • | injunctions or the imposition of civil or criminal penalties. |
| • | Anti-Kickback Statute |
| • | False Claims Act per-claim penalties; |
| • | HIPAA |
| • | HIPAA and HITECH |
| • | Transparency Requirements |
| • | Analogous State and Foreign Laws |
| • | an annual, non-deductible fee on any entity that manufactures or imports specified branded prescription drugs and biologic products; |
| • | an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program; |
| • | expansion of federal health care fraud and abuse laws, including the civil False Claims Act and the federal Anti-Kickback Statute, new government investigative powers and enhanced penalties for noncompliance; |
| • | a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale |
| • | extension of manufacturers’ Medicaid rebate liability; |
| • | expansion of eligibility criteria for Medicaid programs; |
| • | expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program; |
| • | new requirements to report certain financial arrangements with physicians and teaching hospitals; |
| • | a new requirement to annually report drug samples that manufacturers and distributors provide to physicians; and |
| • | a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. |
| • | results of preclinical studies and clinical trials of our product candidates or those of our competitors; |
| • | public announcements about the scientific community’s evolving understanding of the COVID-19 pandemic and the potential effectiveness of vaccines, treatments, public health measures and other approaches to addressing the disease; |
| • | the success of competitive products or technologies; |
| • | commencement or termination of collaborations; |
| • | regulatory or legal developments in the United States and other countries; |
| • | developments or disputes concerning patent applications, issued patents or other proprietary rights; |
| • | the recruitment or departure of key personnel; |
| • | the level of expenses related to any of our product candidates or clinical development programs; |
| • | the results of our efforts to discover, develop, acquire or in-license additional product candidates; |
| • | actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts; |
| • | variations in our financial results or those of companies that are perceived to be similar to us; |
| • | changes in the structure of health care payment systems; |
| • | market conditions in the pharmaceutical and biotechnology sectors; |
| • | the entry into significant acquisitions, strategic partnerships or divestitures by us or our competitors; |
| • | significant sales of our common stock, including sales by our directors, executive officers or 5% stockholders; |
| • | general economic, industry and market conditions, such as the impact of the COVID-19 pandemic on our industry and market conditions; and |
| • | the other factors described in this “Risk Factors” section. |
| • | establish a classified board of directors such that not all members of the board are elected at one time; |
| • | allow the authorized number of our directors to be changed only by resolution of our board of directors; |
| • | limit the manner in which stockholders can remove directors from the board; |
| • | establish advance notice requirements for stockholder proposals that can be acted on at stockholder meetings and nominations to our board of directors; |
| • | require that stockholder actions must be effected at a duly called stockholder meeting and prohibit actions by our stockholders by written consent; |
| • | limit who may call stockholder meetings; |
| • | authorize our board of directors to issue preferred stock without stockholder approval, which could be used to institute a stockholder rights plan, or so-called “poison pill,” that would work to dilute the stock ownership of a potential hostile acquirer, effectively preventing acquisitions that have not been approved by our board of directors; and |
| • | require the approval of the holders of at least 75% of the votes that all our stockholders would be entitled to cast to amend or repeal certain provisions of our certificate of incorporation or bylaws. |
| • | employee-related expenses, including salaries, related benefits and stock-based compensation expense for employees engaged in research and development functions; |
| • | expenses incurred in connection with the preclinical and clinical development of our product candidates, including under agreements with third parties, such as consultants and contract research organizations, or CROs; |
| • | the cost of manufacturing drug products for use in our preclinical studies and clinical trials, including under agreements with third parties, such as consultants and contract manufacturing organizations, or CMOs; |
| • | laboratory supplies; |
| • | facilities, depreciation and other expenses, which include direct or allocated expenses for rent and maintenance of facilities and insurance; |
| • | costs to fulfill our obligations under our collaboration with Sanofi; |
| • | costs related to compliance with regulatory requirements; and |
| • | payments made under third-party licensing agreements. |
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
(in thousands) |
||||||||
| Vaccine program |
$ | 30,576 | $ | 1,930 | ||||
| MRT5005 program |
16,219 | 30,203 | ||||||
| Discovery program |
12,740 | 8,123 | ||||||
| MRT5201 program |
— | 6,226 | ||||||
| Oligonucleotide program |
— | 202 | ||||||
| Unallocated research and development expenses |
50,094 | 29,685 | ||||||
| |
|
|
|
|||||
| Total research and development expenses |
$ | 109,629 | $ | 76,369 | ||||
| |
|
|
|
|||||
| • | the timing and progress of preclinical and clinical development activities, including delays resulting from the COVID-19 pandemic; |
| • | the number and scope of preclinical and clinical programs we decide to pursue; |
| • | our ability to maintain our current research and development programs and to establish new ones; |
| • | establishing an appropriate safety profile with investigational new drug, or IND, enabling studies; |
| • | successful patient enrollment in, and the initiation and completion of, clinical trials; |
| • | the successful completion of clinical trials with safety, tolerability and efficacy profiles that are satisfactory to the FDA or any comparable foreign regulatory authority; |
| • | the receipt of regulatory approvals from applicable regulatory authorities; |
| • | the timing, receipt and terms of any marketing approvals from applicable regulatory authorities; |
| • | the success of our collaboration with Sanofi; |
| • | our ability to establish new licensing or collaboration arrangements; |
| • | the performance of our future collaborators, if any; |
| • | establishing commercial manufacturing capabilities or making arrangements with third-party manufacturers; |
| • | development and timely delivery of commercial-grade drug formulations that can be used in our clinical trials and for commercial launch; |
| • | obtaining, maintaining, defending and enforcing patent claims and other intellectual property rights; |
| • | launching commercial sales of our product candidates, if approved, whether alone or in collaboration with others; and |
| • | maintaining a continued acceptable safety profile of the product candidates following approval. |
Year Ended December 31, |
Change |
|||||||||||
2020 |
2019 |
|||||||||||
(in thousands) |
||||||||||||
| Collaboration revenue |
$ | 138,811 | $ | 7,804 | $ | 131,007 | ||||||
| Operating expenses: |
||||||||||||
| Research and development |
109,629 | 76,369 | 33,260 | |||||||||
| General and administrative |
35,922 | 28,632 | 7,290 | |||||||||
| Change in fair value of contingent consideration |
48,575 | 13 | 48,562 | |||||||||
| Impairment of intangible asset |
— | 18,559 | (18,559 | ) | ||||||||
| |
|
|
|
|
|
|||||||
| Total operating expenses |
194,126 | 123,573 | 70,553 | |||||||||
| |
|
|
|
|
|
|||||||
| Loss from operations |
(55,315 | ) | (115,769 | ) | 60,454 | |||||||
| Other income, net |
1,528 | 1,990 | (462 | ) | ||||||||
| |
|
|
|
|
|
|||||||
| Loss before benefit from income taxes |
(53,787 | ) | (113,779 | ) | 59,992 | |||||||
| Benefit from income taxes |
— | 486 | (486 | ) | ||||||||
| |
|
|
|
|
|
|||||||
| Net loss |
$ | (53,787 | ) | $ | (113,293 | ) | $ | 59,506 | ||||
| |
|
|
|
|
|
|||||||
Year Ended December 31, |
Change |
|||||||||||
2020 |
2019 |
|||||||||||
(in thousands) |
||||||||||||
| Direct external research and development expenses by program: |
||||||||||||
| Vaccine program |
$ | 30,576 | $ | 1,930 | $ | 28,646 | ||||||
| MRT5005 program |
16,219 | 30,203 | (13,984 | ) | ||||||||
| Discovery program |
12,740 | 8,123 | 4,617 | |||||||||
| MRT5201 program |
— | 6,226 | (6,226 | ) | ||||||||
| Oligonucleotide program |
— | 202 | (202 | ) | ||||||||
| Unallocated research and development expenses: |
||||||||||||
| Personnel related (including stock-based compensation) |
27,242 | 18,652 | 8,590 | |||||||||
| Other |
22,852 | 11,033 | 11,819 | |||||||||
| |
|
|
|
|
|
|||||||
| Total research and development expenses |
$ | 109,629 | $ | 76,369 | $ | 33,260 | ||||||
| |
|
|
|
|
|
|||||||
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
(in thousands) |
||||||||
| Net cash provided by (used in) operating activities |
$ | 236,316 | $ | (82,162 | ) | |||
| Net cash used in investing activities |
(215,122 | ) | (18,186 | ) | ||||
| Net cash provided by financing activities |
240,129 | 129,654 | ||||||
| |
|
|
|
|||||
| Net increase in cash, cash equivalents and restricted cash |
$ | 261,323 | $ | 29,306 | ||||
| |
|
|
|
|||||
| • | continue the clinical development of MRT5005; |
| • | continue the development of mRNA vaccine candidates against infectious diseases, including MRT5500, the lead vaccine candidate against SARS-CoV-2; |
| • | leverage our programs to advance our other product candidates into preclinical and clinical development; |
| • | seek regulatory approvals for any product candidates that successfully complete clinical trials; |
| • | seek to discover and develop additional product candidates; |
| • | expand our manufacturing, operational, financial and management systems; |
| • | increase personnel, including personnel to support our research, clinical development, manufacturing and commercialization efforts and our operations as a public company; |
| • | maintain, expand and protect our intellectual property portfolio; |
| • | acquire or in-license other product candidates and technologies; |
| • | incur additional legal, accounting and other expenses in operating as a public company; and |
| • | establish a sales, marketing, medical affairs and distribution infrastructure to commercialize any product candidates for which we may obtain marketing approval and intend to commercialize on our own or jointly. |
| • | the impacts of the COVID-19 pandemic and our response to it; |
| • | the scope, progress, results and costs of researching and developing our product candidates, and conducting preclinical studies and clinical trials; |
| • | the success of our collaboration with Sanofi; |
| • | the costs, timing and outcome of regulatory review of our product candidates; |
| • | the costs of future activities, including product sales, medical affairs, marketing, manufacturing and distribution, for any of our product candidates for which we receive marketing approval; |
| • | the costs of manufacturing commercial-grade products and sufficient inventory to support commercial launch; |
| • | the ability to receive additional non-dilutive funding, including grants from organizations and foundations; |
| • | the revenue, if any, received from commercial sale of our products, should any of our product candidates receive marketing approval; |
| • | the cost and timing of hiring new employees to support our continued growth; |
| • | the costs of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending intellectual property-related claims; |
| • | the ability to establish and maintain collaborations on favorable terms, if at all; |
| • | the extent to which we acquire or in-license other product candidates and technologies; and |
| • | the timing, receipt and amount of sales of, or milestone payments related to or royalties on, our current or future product candidates, if any. |
| • | vendors in connection with preclinical development activities; |
| • | CROs and investigative sites in connection with preclinical studies and clinical trials; and |
| • | CMOs in connection with the production of preclinical and clinical trial materials. |
F-2 |
||||
F-6 |
||||
F-7 |
||||
F-8 |
||||
F-9 |
||||
F-10 |
||||
F-11 |
Incorporation by Reference |
||||||||||
Exhibit Number |
Description |
Form |
Date of Filing |
Exhibit Number |
Filed Herewith | |||||
| 32.1 | Certification of Principal Executive Officer and Principal Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 | X | ||||||||
| 101.INS | Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document | |||||||||
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document | |||||||||
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document | |||||||||
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | |||||||||
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document | |||||||||
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document | |||||||||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL with applicable taxonomy extension information contained in Exhibits 101) | |||||||||
| * | Indicates management contract or compensatory plan |
| ^ | SEC File No. 333-225368 |
| ^^ | SEC File No. 001-38550 |
| + | Confidential treatment has been granted as to certain portions, which portions have been omitted and filed separately with the U.S. Securities and Exchange Commission. |
| † | Pursuant to Item 601(b)(2) of Regulation S-K, the Registrant agrees to furnish supplementally a copy of any omitted schedule or exhibit to the Asset Purchase Agreement to the Securities and Exchange Commission upon request. |
| # | Portions of this exhibit have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K. |
TRANSLATE BIO, INC. | ||||
| Date: March 1, 2021 | By: |
/s/ Ronald C. Renaud, Jr. | ||
Ronald C. Renaud, Jr. | ||||
President and Chief Executive Officer | ||||
| Signature |
Title |
Date | ||
| /s/ Ronald C. Renaud, Jr. Ronald C. Renaud, Jr. |
President and Chief Executive Officer, Director (Principal Executive Officer and Principal Financial Officer) | March 1, 2021 | ||
| /s/ Robert D. Prentiss Robert D. Prentiss |
Vice President, Corporate Controller and Treasurer (Principal Accounting Officer) | March 1, 2021 | ||
| /s/ Daniel S. Lynch Daniel S. Lynch |
Chairman of the Board | March 1, 2021 | ||
| /s/ Daniella Beckman Daniella Beckman |
Director | March 1, 2021 | ||
| /s/ George Demetri, M.D. George Demetri, M.D. |
Director | March 1, 2021 | ||
| /s/ Jean-François Formela, M.D. Jean-François Formela, M.D. |
Director | March 1, 2021 | ||
| /s/ Owen Hughes Owen Hughes |
Director | March 1, 2021 | ||
| /s/ Robert J. Meyer, M.D. Robert J. Meyer, M.D. |
Director | March 1, 2021 | ||
| /s/ Robert M. Plenge, M.D., Ph.D. Robert M. Plenge, M.D., Ph.D. |
Director | March 1, 2021 | ||
Page |
||||
F-2 |
||||
F-6 |
||||
F-7 |
||||
F-8 |
||||
F-9 |
||||
F-10 |
||||
F-11 |
||||
December 31, 2020 |
December 31, 2019 |
|||||||
Assets |
||||||||
Current assets: |
||||||||
Cash and cash equivalents |
$ | $ | ||||||
Investments |
||||||||
Collaboration receivables |
||||||||
Prepaid expenses and other current assets |
||||||||
Restricted cash |
||||||||
Total current assets |
||||||||
Property and equipment, net |
||||||||
Right-of-use |
||||||||
Goodwill |
||||||||
Intangible assets, net |
||||||||
Other assets |
||||||||
Total assets |
$ | $ | ||||||
Liabilities and Stockholders’ Equity |
||||||||
Current liabilities: |
||||||||
Accounts payable |
$ | $ | ||||||
Accrued expenses |
||||||||
Current portion of deferred revenue |
||||||||
Current portion of operating lease liability |
||||||||
Total current liabilities |
||||||||
Contingent consideration |
||||||||
Deferred revenue, net of current portion |
||||||||
Operating lease liability, net of current portion |
||||||||
Total liabilities |
||||||||
Commitments and contingencies (Notes 3 and 13) |
||||||||
Stockholders’ equity: |
||||||||
Preferred stock, $ s hares issued and outstanding as of December 31, 2020 and 2019 |
||||||||
Common stock, $ |
||||||||
Additional paid-in capital |
||||||||
Accumulated deficit |
( |
) | ( |
) | ||||
Accumulated other comprehensive income |
— | |||||||
Total stockholders’ equity |
||||||||
Total liabilities and stockholders’ equity |
$ | $ | ||||||
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
| Collaboration revenue |
$ | $ | ||||||
| Operating expenses: |
||||||||
| Research and development |
||||||||
| General and administrative |
||||||||
| Change in fair value of contingent consideration |
||||||||
| Impairment of intangible asset |
— | |||||||
| |
|
|
|
|||||
| Total operating expenses |
||||||||
| |
|
|
|
|||||
| Loss from operations |
( |
) | ( |
) | ||||
| Other income, net |
||||||||
| |
|
|
|
|||||
| Loss before benefit from income taxes |
( |
) | ( |
) | ||||
| Benefit from income taxes |
— | |||||||
| |
|
|
|
|||||
| Net loss |
$ | ( |
) | $ | ( |
) | ||
| |
|
|
|
|||||
| Net loss per share—basic and diluted |
$ | ( |
) | $ | ( |
) | ||
| |
|
|
|
|||||
| Weighted average common shares outstanding—basic and diluted |
||||||||
| |
|
|
|
|||||
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
| Net loss |
$ | ( |
) | $ | ( |
) | ||
| Other comprehensive loss: |
||||||||
| Unrealized gains (losses) on available-for-sale |
( |
) | ||||||
| |
|
|
|
|||||
| Comprehensive loss |
$ | ( |
) | $ | ( |
) | ||
| |
|
|
|
|||||
| Common Stock |
Additional Paid-in Capital |
Accumulated Deficit |
Accumulated Other Comprehensive Income |
Total Stockholders’ Equity |
||||||||||||||||||||
Shares |
Amount |
|||||||||||||||||||||||
| Balances at December 31, 2018 |
$ | $ | $ | ( |
) | $ | $ | |||||||||||||||||
| Issuance of common stock in connection with private placement, net of placement agent fees and offering costs |
— | — | ||||||||||||||||||||||
| Issuance of common stock in connection with public offering, net of underwriting discounts and commissions and offering costs |
— | — | ||||||||||||||||||||||
| Issuance of common stock in connection with a former employee letter agreement |
— | — | — | |||||||||||||||||||||
| Forfeited restricted common stock |
( |
) | — | ( |
) | — | — | ( |
) | |||||||||||||||
| Exercise of stock options |
— | — | — | |||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | ||||||||||||||||||||
| Unrealized gains on available-for-sale |
— | — | — | — | ||||||||||||||||||||
| Net loss |
— | — | — | ( |
) | — | ( |
) | ||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balances at December 31, 2019 |
( |
) | ||||||||||||||||||||||
| Issuance of common stock in connection with public offerings, net of underwriting discounts and commissions and offering costs |
— | — | ||||||||||||||||||||||
| Issuance of common stock in connection with Securities Purchase Agreement |
— | — | ||||||||||||||||||||||
| Exercise of stock options |
— | — | ||||||||||||||||||||||
| Issuance of common stock under employee stock purchase plan |
— | — | — | |||||||||||||||||||||
| Stock-based compensation expense |
— | — | — | — | ||||||||||||||||||||
| Unrealized losses on available-for-sale |
— | — | — | — | ( |
) | ( |
) | ||||||||||||||||
| Net loss |
— | — | — | ( |
) | — | ( |
) | ||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balances at December 31, 2020 |
$ | $ | $ | ( |
) | $ | — | $ | ||||||||||||||||
| |
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
| Cash flows from operating activities: |
||||||||
| Net loss |
$ | ( |
) | $ | ( |
) | ||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
||||||||
| Depreciation and amortization expense |
||||||||
| Stock-based compensation expense |
||||||||
| Impairment of intangible asset |
— | |||||||
| Change in fair value of contingent consideration |
||||||||
| Deferred income tax benefit |
— | ( |
) | |||||
| Changes in operating assets and liabilities: |
||||||||
| Collaboration receivables |
( |
) | ( |
) | ||||
| Prepaid expenses and other assets |
( |
) | ( |
) | ||||
| Right-of-use |
||||||||
| Long-term prepaid rent |
( |
) | ( |
) | ||||
| Accounts payable |
( |
) | ||||||
| Accrued expenses |
||||||||
| Lease liability |
( |
) | ( |
) | ||||
| Deferred revenue |
( |
) | ||||||
| |
|
|
|
|||||
| Net cash provided by (used in) operating activities |
( |
) | ||||||
| |
|
|
|
|||||
| Cash flows from investing activities: |
||||||||
| Purchases of investments |
( |
) | ( |
) | ||||
| Sales and maturities of investments |
||||||||
| Purchases of property and equipment |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Net cash used in investing activities |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Cash flows from financing activities: |
||||||||
| Proceeds from public offerings, net of underwriting discounts and commissions |
||||||||
| Payments of public offering costs |
( |
) | ( |
) | ||||
| Proceeds from Securities Purchase Agreement |
— | |||||||
| Proceeds from private placement, net of placement agent fees |
— | |||||||
| Payments of private placement offering costs |
— | ( |
) | |||||
| Proceeds from option exercises |
||||||||
| Proceeds from issuance of common stock under employee stock purchase plan |
— | |||||||
| |
|
|
|
|||||
| Net cash provided by financing activities |
||||||||
| |
|
|
|
|||||
| Net increase in cash, cash equivalents and restricted cash: |
||||||||
| Cash, cash equivalents and restricted cash at beginning of period |
||||||||
| |
|
|
|
|||||
| Cash, cash equivalents and restricted cash at end of period |
$ | $ | ||||||
| |
|
|
|
|||||
| Cash, cash equivalents and restricted cash at end of period: |
||||||||
| Cash and cash equivalents |
$ | $ | ||||||
| Restricted cash |
||||||||
| |
|
|
|
|||||
| Total cash, cash equivalents and restricted cash at end of period |
$ | $ | ||||||
| |
|
|
|
|||||
| Supplemental disclosure of non-cash investing and financing activities: |
||||||||
| Purchases of property and equipment included in accounts payable and accrued expenses |
$ | $ | ||||||
| Issuance of common stock in connection with a former employee letter agreement |
$ | — | $ | |||||
Estimated Useful Life | ||
Laboratory equipment |
5 years | |
Computer equipment |
3 years | |
Office equipment |
5 years | |
Leasehold improvements |
Shorter of lease term or 10 years |
• |
Level 1—Quoted prices in active markets for identical assets or liabilities. |
• |
Level 2—Observable inputs (other than Level 1 quoted prices), such as quoted prices in active markets for similar assets or liabilities, quoted prices in markets that are not active for identical or similar assets or liabilities, or other inputs that are observable or can be corroborated by observable market data. |
• |
Level 3—Unobservable inputs that are supported by little or no market activity that are significant to determining the fair value of the assets or liabilities, including pricing models, discounted cash flow methodologies and similar techniques. |
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
Collaboration revenue |
$ | $ | ||||||
December 31, 2020 |
December 31, 2019 |
|||||||
Contract liabilities |
||||||||
Deferred revenue |
$ | $ | ||||||
December 31, 2020 |
||||||||||||||||||||
Estimated Life |
Gross Carrying Amount |
Accumulated Amortization |
Impairment Charge |
Net Carrying Amount |
||||||||||||||||
(In thousands) |
||||||||||||||||||||
Definite-lived intangible assets: |
||||||||||||||||||||
MRT |
$ | $ | ( |
) | $ | $ | ||||||||||||||
Indefinite-lived intangible assets: |
||||||||||||||||||||
IPR&D - CF |
Indefinite | |||||||||||||||||||
Total intangible assets, net |
$ | $ | ( |
) | $ | $ | ||||||||||||||
December 31, 2019 |
||||||||||||||||||||
Estimated Life |
Gross Carrying Amount |
Accumulated Amortization |
Impairment Charge |
Net Carrying Amount |
||||||||||||||||
(In thousands) |
||||||||||||||||||||
Definite-lived intangible assets: |
||||||||||||||||||||
MRT |
$ | $ | ( |
) | $ | — | $ | |||||||||||||
Indefinite-lived intangible assets: |
||||||||||||||||||||
IPR&D - CF |
Indefinite | — | — | |||||||||||||||||
IPR&D - OTC |
Indefinite | — | ( |
) | — | |||||||||||||||
Total intangible assets, net |
$ | $ | ( |
) | $ | ( |
) | $ | ||||||||||||
Fair Value Measurements as of December 31, 2020 Using: |
||||||||||||||||
Level 1 |
Level 2 |
Level 3 |
Total |
|||||||||||||
Assets: |
||||||||||||||||
Money market funds |
$ | $ | $ | $ | ||||||||||||
U.S. treasuries |
||||||||||||||||
U.S. government agency bonds |
||||||||||||||||
| $ | $ | $ | $ | |||||||||||||
Liabilities: |
||||||||||||||||
Contingent consideration |
$ | $ | $ | $ | ||||||||||||
| $ | $ | $ | $ | |||||||||||||
Fair Value Measurements as of December 31, 2019 Using: |
||||||||||||||||
Level 1 |
Level 2 |
Level 3 |
Total |
|||||||||||||
Assets: |
||||||||||||||||
Money market funds |
$ | — | $ | $ | — | $ | ||||||||||
U.S. government agency bonds |
— | — | ||||||||||||||
| $ | — | $ | $ | — | $ | |||||||||||
Liabilities: |
||||||||||||||||
Contingent consideration |
$ | — | $ | — | $ | $ | ||||||||||
| $ | — | $ | — | $ | $ | |||||||||||
December 31, 2020 |
December 31, 2019 |
|||||||||||||||
Amortized Cost |
Fair Value |
Amortized Cost |
Fair Value |
|||||||||||||
Due within one year |
$ |
$ |
$ |
$ |
||||||||||||
Due after one year through two years |
— |
— |
||||||||||||||
Total available-for-sale |
$ |
$ |
$ |
$ |
||||||||||||
Unobservable Inputs |
Fair Value at |
|||||||||||
Projected Year of Payment |
December 31, 2020 |
December 31, 2019 |
||||||||||
Earnout payments |
$ | |||||||||||
Milestone payments |
||||||||||||
| $ | $ | |||||||||||
Fair Value |
||||
Balance as of December 31, 2019 |
$ | |||
Increase in fair value of contingent consideration |
||||
Balance as of December 31, 2020 |
$ | |||
December 31, |
December 31, |
|||||||
2020 |
2019 |
|||||||
Laboratory equipment |
$ | $ | ||||||
Computer equipment |
||||||||
Office equipment |
||||||||
Leasehold improvements |
||||||||
Construction in progress |
||||||||
Less: Accumulated depreciation and amortization |
( |
) | ( |
) | ||||
| $ | $ | |||||||
December 31, |
December 31, |
|||||||
2020 |
2019 |
|||||||
| Accrued employee compensation and benefits |
$ | $ | ||||||
| Accrued external research and development expenses |
||||||||
| Accrued consultant and professional fees |
||||||||
| Other |
||||||||
| |
|
|
|
|||||
| $ | $ | |||||||
| |
|
|
|
|||||
Number of Shares |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term |
Intrinsic Value |
|||||||||||||
(in years) |
||||||||||||||||
| Outstanding as of December 31, 2019 |
$ | $ | ||||||||||||||
| Granted |
$ | |||||||||||||||
| Exercised |
( |
) | $ | |||||||||||||
| Forfeited |
( |
) | $ | |||||||||||||
| |
|
|||||||||||||||
| Outstanding as of December 31, 2020 |
$ | $ | ||||||||||||||
| |
|
|||||||||||||||
| Exercisable as of December 31, 2020 |
$ | $ | ||||||||||||||
| Vested and expected to vest as of December 31, 2020 |
$ | $ | ||||||||||||||
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
| Risk-free interest rate |
% | % | ||||||
| Expected term (in years) |
||||||||
| Expected volatility |
% | % | ||||||
| Expected dividend yield |
% | % | ||||||
Number of Shares |
Weighted Average Grant-Date Fair Value |
|||||||
| Unvested restricted common stock outstanding as of December 31, 2019 |
$ | |||||||
| Forfeited restricted common stock |
$ | |||||||
| Vested restricted common stock |
( |
) | $ | |||||
| |
|
|||||||
| Unvested restricted common stock outstanding as of December 31, 2020 |
$ | |||||||
| |
|
|||||||
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
| Research and development expenses |
$ | $ | ||||||
| General and administrative expenses |
||||||||
| |
|
|
|
|||||
| $ | $ | |||||||
| |
|
|
|
|||||
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
| U.S. federal statutory income tax rate |
( |
)% |
( |
)% | ||||
| State income taxes, net of federal benefit |
( |
) |
( |
) | ||||
| Research and development tax credits and orphan drug credit |
( |
) |
( |
) | ||||
| Stock-based compensation |
( |
) |
||||||
| Other permanent differences |
||||||||
| Change in deferred tax asset valuation allowance |
||||||||
| Officers compensation |
|
|
|
|
|
|
|
|
| NOL and tax credit expiration under 382 |
|
|
|
|
|
|
|
|
| Other |
( |
) | ||||||
| |
|
|
|
|||||
| Effective income tax rate |
% | ( |
)% | |||||
| |
|
|
|
|||||
December 31, |
||||||||
2020 |
2019 |
|||||||
| Deferred tax assets: |
||||||||
| Net operating loss carryforwards |
$ | $ | ||||||
| Research and development tax credit and orphan drug credit carryforwards |
||||||||
| Intangibles |
||||||||
| Deferred revenue |
||||||||
| Depreciation and amortization |
||||||||
| Accrued expenses and other |
||||||||
| |
|
|
|
|||||
| Total deferred tax assets |
||||||||
| |
|
|
|
|||||
| Valuation allowance |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Net deferred tax assets |
$ | $ | ||||||
| |
|
|
|
|||||
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
| Valuation allowance at beginning of year |
$ | ( |
) | $ | ( |
) | ||
| Increases recorded to income tax provision |
( |
) | ( |
) | ||||
| |
|
|
|
|||||
| Valuation allowance at end of year |
$ | ( |
) | $ | ( |
) | ||
| |
|
|
|
|||||
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
| Numerator: |
||||||||
| Net loss |
$ | ( |
) | $ | ( |
) | ||
| Denominator: |
||||||||
| Weighted average common shares outstanding—basic and diluted |
||||||||
| |
|
|
|
|||||
| Net loss per share—basic and diluted |
$ | ( |
) | $ | ( |
) | ||
| |
|
|
|
|||||
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
| Options to purchase common stock |
||||||||
| Unvested restricted common stock |
— | |||||||
| |
|
|
|
|||||
| |
|
|
|
|||||
Year Ended December 31, |
||||||||
2020 |
2019 |
|||||||
Lease cost |
||||||||
Operating lease cost |
$ | $ | ||||||
Total lease cost |
$ | $ | ||||||
Other information |
||||||||
Operating cash flows from operating leases |
$ | $ | ||||||
Operating lease liabilities arising from obtaining right-of-use |
$ | — | ||||||
Weighted-average remaining lease term |
||||||||
Weighted-average discount rate |
% | % | ||||||
December 31, 2020 |
||||
2021 |
$ | |||
2022 |
||||
2023 |
||||
2024 |
||||
2025 |
||||
2026 and thereafter |
||||
Total future minimum lease payments |
||||
Less: imputed interest |
( |
) | ||
Present value of lease liabilities |
$ | |||
December 31, 2019 |
||||
2020 |
$ | |||
2021 |
||||
2022 |
||||
2023 |
||||
2024 |
||||
2025 and thereafter |
||||
Total future minimum lease payments |
||||
Less: imputed interest |
( |
) | ||
Present value of lease liabilities |
$ | |||
2020 |
||||||||||||||||||||
First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
Total |
||||||||||||||||
| Collaboration revenue |
$ | $ | $ | $ | $ | |||||||||||||||
| Total operating expenses |
||||||||||||||||||||
| Income (loss) from operations |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||||||
| Net income (loss) |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||||||
| Net income (loss) per share applicable to common |
$ | ( |
) | $ | ( |
) | $ | $ | ( |
) | $ | ( |
) | |||||||
| Net income (loss) per share applicable to common stockholders—diluted |
$ | ( |
) | $ | ( |
) | $ | $ | ( |
) | $ | ( |
) | |||||||
2019 |
||||||||||||||||||||
First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
Total |
||||||||||||||||
| Collaboration revenue |
$ | $ | $ | $ | $ | |||||||||||||||
| Total operating expenses |
||||||||||||||||||||
| Loss from operations |
( |
) | ( |
) | ( |
) | ( |
) | ( |
) | ||||||||||
| Net loss |
( |
) | ( |
) | ( |
) | ( |
) | ( |
) | ||||||||||
| Net loss per share applicable to common stockholders—basic and diluted |
$ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | $ | ( |
) | |||||