Exhibit 99.1

CORPORATE PRESENTATION DECEMBER 2019 1 1

2 DISCLAIMER 2 This Presentation is for informational purposes only and does not constitute an offer to sell, a solicitation of an offer to buy , or a recommendation to purchase any equity, debt or other financial instruments of CannBioRx Life Sciences Corp. (“CannBioRx”) or KBL Merger Corp. IV (“KBL”) or any of CannBioRx’s or KBL’s affiliates’ securities. This Prese nta tion has been prepared to assist interested parties in making their own evaluation with respect to the proposed business combination of CannBioRx and KBL and for no other purpose. The information contained herein does not pu rpo rt to be all - inclusive. The data contained herein is derived from various internal and external sources. No representation is made as to the reasonableness of the assumptions made within or the accuracy or comple ten ess of any projections or any other information contained herein. Any data on past performance or projections contained herein is no indication as to future performance. CannBioRx and KBL assume no obligation to update the information in this Presentation. Forward - Looking Statements This Presentation includes “forward - looking statements” within the meaning of U.S. federal securities laws. Words such as “expec t,” “estimate,” “project,” “budget,” “forecast,” “anticipate,” “intend,” “plan,” “may,” “will,” “could,” “should,” “believes,” “predicts,” “potential,” “continue” and similar expressions are intended to identify such forw ard - looking statements. These forward - looking statements involve significant risks and uncertainties that could cause the actual results to differ materially from the expected results and, consequently, you should not rely on the se forward - looking statements as predictions of future events. These forward - looking statements and factors that may cause such differences include, without limitation, statements relating to the timing and completion of the proposed business combination; KBL’s continued listing on the Nasdaq Stock Market until closing of the proposed business combination; expectations regarding the capitalization, resources and ownership structure of th e combined company; the inability to recognize the anticipated benefits of the proposed business combination, which may be affected by, among other things, the amount of cash available following redemptions by KBL st ockholders; the ability to meet the Nasdaq Stock Market's listing standards following the consummation of the transactions contemplated by the proposed business combination; costs related to the proposed business co mbi nation; expectations with respect to future performance, growth and anticipated acquisitions; ability to recognize the anticipated benefits of the proposed business combination; CannBioRx's ability to exec ute its plans to develop and market new drug products and the timing and costs of these development programs; CannBioRx's estimates of the size of the markets for its potential drug products; potential litigation inv olving KBL or CannBioRx or the validity or enforceability of CannBioRx's intellectual property; global economic conditions; geopolitical events and regulatory changes; access to additional financing; and other risks and u nce rtainties indicated from time to time in filings with the Securities and Exchange Commission (the “SEC”). Other factors include the possibility that the proposed business combination does not close, including due to the fai lur e to receive required security holder approvals, or the failure of other closing conditions. The foregoing list of factors is not exclusive. Additional information concerning these and other risk factors is contained in KB L’s most recent filings with the SEC and will be contained in the proxy statement/prospectus to be filed as result of the transactions described above. All subsequent written and oral forward - looking statements concerning KBL or Cann BioRx, the transactions described herein or other matters and attributable to KBL or CannBioRx or any person acting on their behalf are expressly qualified in their entirety by the cautionary statements above. Readers ar e c autioned not to place undue reliance upon any forward - looking statements, which speak only as of the date made. None of KBL or CannBioRx undertake or accept any obligation or undertaking to release publicly any updates or rev isions to any forward - looking statement to reflect any change in their expectations or any change in events, conditions or circumstances on which any such statement is based. Additional Information and Where to Find It KBL has filed a registration statement on Form S - 4, which includes a preliminary proxy statement/prospectus for KBL’s stockholde rs, with the SEC. KBL’s definitive proxy statement/prospectus will be mailed to KBL’s stockholders that do not opt to receive the document electronically. KBL and CannBioRx urge investors, stockholders and other in terested persons to read the preliminary proxy statement/prospectus, as well as other documents that will be filed with the SEC, because these documents will contain important information about the proposed busi nes s combination transaction. Such persons can also read KBL’s Annual Report on Form 10 - K for the fiscal year ended December 31, 2018, for a description of the security holdings of its officers and directors and their r esp ective interests as security holders in the consummation of the proposed business combination transaction. KBL’s definitive proxy statement/prospectus, which is included in the registration statement, will be mailed to sto ckholders of KBL as of a record date to be established. KBL’s stockholders will also be able to obtain a copy of such documents, without charge, by directing a request to: KBL Merger Corp. IV, 150 West 56 th Street, Suite 5901, New York, NY 10019; e - mail: admin@kblvc.com . These documents can also be obtained, without charge, at the SEC’s website ( http://www.sec.gov ). Participants in the Solicitation KBL and its directors and executive officers, may be deemed to be participants in the solicitation of proxies for the special me eting of KBL’s stockholders to be held to approve the proposed transactions in connection with the business combination. Information regarding the persons who may, under the rules of the SEC, be deemed participants in the so lic itation of KBL’s stockholders in connection with the proposed transactions are set forth in the preliminary proxy statement/prospectus included in the registration statement that was filed with the SEC on November 12, 201 9. You can find information about KBL’s executive officers and directors in its Annual Report on Form 10 - K for the fiscal year ended December 31, 2018, which was filed with the SEC on April 1, 2019. You can obtain free copies of these documents from KBL using the contact information above. Disclaimer This Presentation is not a proxy statement or solicitation of a proxy, consent or authorization with respect to any securitie s o r in respect of the proposed transaction and shall not constitute an offer to sell or a solicitation of an offer to buy the securities of KBL and CannBioRx, nor shall there be any sale of any such securities in any state or jurisdic tio n in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction. No offer of securities shall be made except by means of a prospectus me eting the requirements of Section 10 of the Securities Act of 1933, as amended.

INFLAMMATION DRIVES THE WORLD’S BIGGEST DISEASES The experienced and world - renowned scientific team are proven leaders in this field. • Sir Marc Feldmann: Centocor sold to J&J for $4.9 B USD • Lawrence Steinman: Tysabri sold to Royalty Pharma for $2.85 B USD • Jonathan Rothbard: Amylin sold to AstraZeneca and Bristol - Myers Squibb for $7 B USD • Raphael Mechoulam: Identified major phytocannabinoids (tetrahydrocannabinol (THC), cannabidiol (CBD) etc.) and discovered the endocannabinoid system Prof Sir Marc Feldmann in his office with Gairdner Award, European Inventor of Year, and Lasker Award. Dr Lawrence Steinman signing National Academy of Science register. Prof Raphael Mechoulam receiving Honorary Doctorate in Madrid. 3

4 CANNBIORX HIGHLIGHTS 4 4 • Scientific team and founders are pioneers with proven track record in drug discovery from the University of Oxford, Hebrew University and Stanford University • Developing three families of novel drugs addressing significant market opportunities in inflammation, fibrosis and pain: - Fibrosis & Anti - TNF - Synthetic CBD Analogs ( SCAs ) - a 7nAChR • Multiple programs in synchronized stages of development combined with IP portfolio reduces risk • Numerous near - term inflection points for anti - TNF programs: one program late stage 2b/3 trial, two additional clinical programs projected to start Q4 2020 • Initial clinical anti - TNF clinical trials funded by investments and grants (UK and Dutch) . • *Regulatory approvals obtained from the UK Medicines and Healthcare Products Regulatory Agency (MHRA) and the Dutch Centrale Commissie Mensgebonden Onderzoek (CCMO) and the relevant accredited ethics committees to perform clinical trials in the UK and The Netherlands for anti - TNF products. • Three anti - inflammatory therapeutic programs potentially used in combination * No meetings have been held with, and no applications or requests for approval have been submitted to the FDA for any products at this time.

5 Mr George Hornig COO & Acting CFO Dr Marlene Krauss CEO CO - FOUNDERS AND MANAGEMENT 5 Dr Jonathan Rothbard CSO Prof Jagdeep Nanchahal CMO Prof Raphael Mechoulam Founder, CBRx Prof Sir Marc Feldmann Co - Chairman Prof Lawrence Steinman Co - Chairman • Pioneer of anti - TNF therapy, world’s biggest drug class ($40B USD pa) • Numerous accolades including Crafoord and Lasker Awards, fellow of the Royal Society • Godfather of cannabinoid chemistry; discovered the body’s endocannabinoid system • Recipient of Israel Exact Sciences Prize, member of Israel Academy of Science and Humanities • Discovered role of integrins, led to Natalizumab, highly effective treatment for MS and IBD • Member of National Academy of Sciences, many accolades including Charcot Prize; founder of Centocor (sold to J&J for $4.9B USD) • Harvard Business School, Harvard Medical School, Cornell University • CEO of 4 SPACs and 3 VC funds; invested more than $1B USD in early to mid - stage companies • Harvard College, Harvard Business School, Harvard Law School • Considerable banking experience, in high - level positions for Deutsche Bank and Credit Suisse • Stanford University, broad experience in small molecule development • Founder of 5 biotech companies; Amylin sold to AstraZeneca and BMS for $5.3B USD • Surgeon - scientist, leading 2b/3 trial funded by Wellcome Trust and UK Dept. of Health • Member of the Royal College of Surgeons; discovered new treatments for fibrosis Incredible discoveries in scientific fields; successful business leaders

6 FIBROSIS & ANTI - TNF (CLINICAL STAGE)* SYNTHETIC CBD ANALOGS (SCAs) a 7nAChR TECHNOLOGIES Repurposing of anti - TNF for major unmet needs, other patented drugs Novel non - psychoactive synthetic CBD analogs Novel α7 nAChR agonists TARGETED DISEASES NEAR TERM • Early stage Dupuytren’s disease (DD) • Frozen Shoulder • Post Operative Delirium/Cognitive Deficit (POCD) FURTHER OUT • Non - Alcoholic Steatohepatitis (NASH) • Arthritis • Pain/Inflammation • Smoking cessation induced Ulcerative Colitis (UC) initially • Other inflammatory indications will be targeted after results in UC COMPETITIVE ADVANTAGE • DD : no treatment for early disease • Frozen Shoulder: local steroid only for short term pain relief, does not modulate long - term disease activity • POCD : No treatment available • Novel, >99.5% pure, • Robust batch to batch consistency (non - botanical) • Developing advanced formulation for increased bioavailability • Orally available • Potentially as effective as biologics (like anti - TNF) • Proven lack of toxicity STAGE • DD : Phase 2b/3 in early DD, results Q1/2 2021 • Frozen Shoulder : Initiate Phase 2 trials Q4 2020 • POCD : Initiate Phase 3 trials Q4 2020 • NASH : Preclinical studies to begin Q2 2020 • Preclinical – lead SCAs and formulations being identified • Trials planned in arthritis and pain • Preclinical – optimizing new compounds based on safe a 7nAchR agonists INTELLECTUAL PROPERTY • Patents issued for treatment of DD & POCD with anti - TNF • Additional patents issued (anti - IL - 33) or pending in localized and systemic fibrosis and delivery systems • Patent issued for Cyclohexenyl compounds , compositions and uses thereof • Patents pending & to be filed • Three patents issued, one patent pending OVERVIEW:CANNBIORX DEVELOPMENT PROGRAMS 6 *Regulatory approvals obtained from the MHRA and CCMO and the relevant accredited ethics committees to perform clinical trials in the UK and The Netherlands. No meetings have been held with, and no applications or requests for approval have been submitted to the FDA for any products at this time.

α7nAChR • Smoking cessation induced ulcerative colitis SCAs • Chronic pain • Early arthritis PLATFORM INDICATION PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Fibrosis & Anti - TNF * • Dupuytren’s contracture • Frozen shoulder • PO C D • NASH Est. start Q1 2023 Est. start Q3 2022 Est. start Q2 2020 Est. start Q4 2020 Est. start Q4 2020 ongoing ongoing ongoing ongoing THREE PLATFORM TECHNOLOGIES TARGETING MULTIPLE INDICATIONS 7 Est. start Q3 2021 7 *Regulatory approvals obtained from the MHRA and CCMO and the relevant accredited ethics committees to perform clinical trial s i n the UK and The Netherlands. No meetings have been held with, and no applications or requests for approval have been submitted to the FD A f or any products at this time.

FIBROSIS & ANTI - TNF PROGRAM O xf o r d CLINICAL STAGE LEAD PROGRAM Targeting common diseases - facilitates trials and potential sales 8 Led by Profs Jagdeep Nanchahal & Sir Marc Feldmann, Oxford Dr Glenn Larsen, USA Clinical trials supported by Prof Sallie Lamb, UK

FIBROSIS & ANTI - TNF PL ATFORM Developing targeted therapies for: » Early Stage Dupuytren’s disease (DD) - patent issued; Phase 2b/3 results expected Q1/2 2021* » Frozen shoulder - patent issued; clinical trials projected in Q4 2020 » Post operative cognitive decline (POCD) - patent issued, clinical trials projected in Q4 2020 » Liver fibrosis (NASH) - initial laboratory studies done with Celgene - BMS on human tissue; preclinical studies to begin in Q2 2020 9 DD FROZEN SHOULDER POCD NASH *Approval only from MHRA/CCMO and relevant accredited ethics committees.

10 UNIQUE COMPETITIVE ADVANTAGE # 1 • Working with disease experts and key opinion leaders, who are highly motivated to improve patient standard of care • No payment for trial patients required in the UK/EU • Easier recruitment. Access to large registries of patients/diseases • Staff costs can be covered by academic grants ( Wellcome Trust, NIHR) • Established reputation and prestige in conducting clinical trials across academic and clinical networks 1 • Skilled at writing protocols compliant with regulatory authorities, seeking approvals, accessing patient registries, establishing good working relations with trial centers and coordination of patient recruitment and data collection across multiple sites, industry standard statistical analysis of blinded data, report submission…. • Well practiced in publishing trials in peer reviewed clinical journals COST EFFECTIVE, TIME EFFICIENT, ACADEMIC LED CLINICAL TRIALS PERFORMED IN UK 1 https://www.ndorms.ox.ac.uk/octru Prof Sallie Lamb Prof Jagdeep Nanchahal

11 UNIQUE COMPETITIVE ADVANTAGE # 2 Studies in DD lead the way for novel approach to develop clinical programs in other fibrotic diseases: • All fibrosis preceded by inflammation, involves myofibroblasts • Our targets identified in patient myofibroblasts and associated immune cells • Using human tissue from DD nodules • Tissue and cells from most fibrotic diseases not readily accessible as diagnosed late • Competitors use animals or late stage cells in culture, neither reflect human disease • Novel therapeutics testable, assessed by molecular changes • Same approach will be applied to target discovery in NASH 11 NOVEL USE OF HUMAN DISEASE TISSUE TO IDENTIFY NEW TARGETS IN FIBROSIS

12 TNFR2 cell membrane extracellular intracellular Anti - TNF *Use - of patent issued for DD, patent pending for unique delivery system, current program TNF Anti - TNFR2 mab * patent pending, future program TNF Wnt activation leading to FIBROSIS (↑ expression of a - SMA, Col1A1 genes etc ) UNEXPECTED DISCOVERY – TNF/TNFR2 signaling activates Wnt pathway and transcription of fibrosis genes. Verjee…Nanchahal (2013) CBRx drugs block TNF induced activation of pro - fibrotic pathways to reduce fibrosis Unraveling the signaling pathways promoting fibrosis in Dupuytren’s disease reveals TNF as a therapeutic target Liaquat S. Verjee a , Jennifer S.N. Verhoekx a,b , James K. K. Chan a , Thomas Krausgruber a , Vicky Nicolaidou a , David Izadi a , Dominique Davidson c , Marc Feldmann a, 1 , Kim S. Midwood a , and Jagdeep Nanchahal a, 1 a Kennedy Institute of Rheumatology, University of Oxford, London W6 8LH, United Kingdom b Department of Plastic and Rescontructive Surgery, Erasmus Medical Centre, 3015, Rotterdam, The Netherlands c Department of Plastic Surgery, St John’s Hospital, Livingston EH54 6PP, United Kingdom RATIONALE FOR TNF BLOCKADE IN FIBROSIS
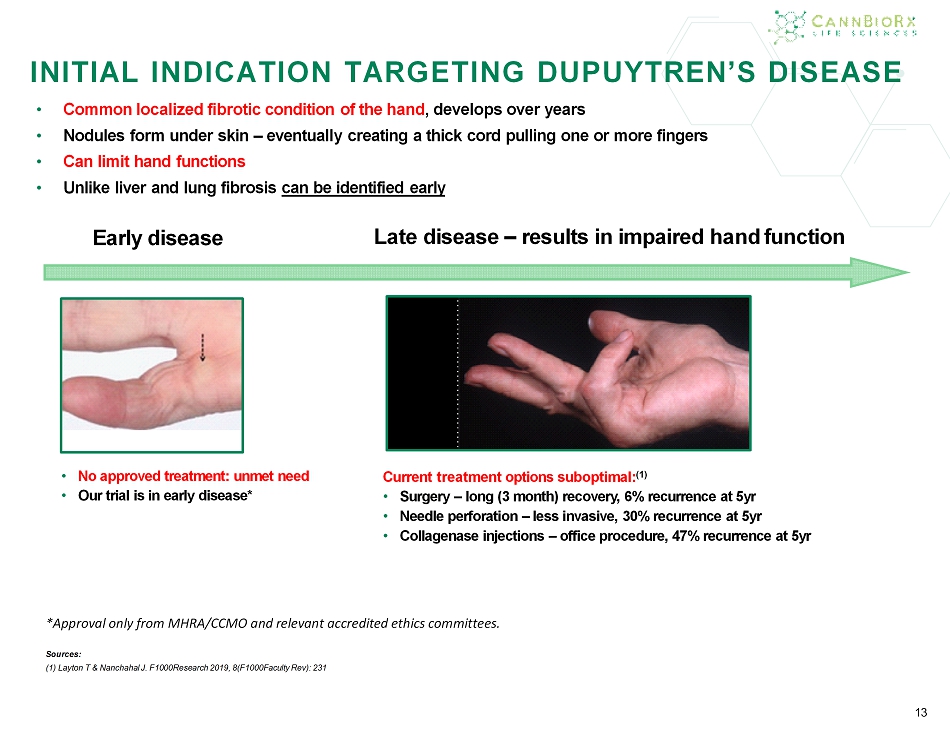
13 INITIAL INDICATION TARGETING DUPUY TREN’S DISEASE • C ommon localized fibrotic condition of the hand , develops over years • Nodules form under skin – eventually creating a thick cord pulling one or more fingers • Can limit hand functions • Unlike liver and lung fibrosis can be identified early Early disease 13 Late disease – results in impaired hand function • No approved treatment : unmet need • Our trial is in early disease * Current treatment options suboptimal: (1) • S urgery – long (3 month) recovery, 6% recurrence at 5yr • N eedle perforation – less invasive, 30% recurrence at 5yr • C ollagenase injections – office procedure, 47% recurrence at 5yr *Approval only from MHRA / CCMO and relevant accredited ethics committees. Sources: (1) Layton T & Nanchahal J. F1000Research 2019, 8(F1000Faculty Rev): 231

Anti - Tumour Necrosis Factor Therapy for Dupuytren’s Disease: A Randomised Dose Response Proof of Concept Phase 2a Clinical Trial Jagdeep Nanchahal 23,* , Catherine Ball 23 , Dominique Davidson 24 , Lynn Williams 23 , William Sones 25 , Fiona E McCann 23 , Marisa Cabrita 23 , Jennifer Swettenham 23 , Neil J. Cahoon 24 , Bethan Copsey 25 , E. Anne Francis 23 , Peter C. Taylor 23 , Joanna Black 25 , Vicki S. Barber 25 , Susan Dutton 25 , Marc Feldmann 23 , Sarah E. Lamb 25 PHASE 2A COMPLETED : 40 MG ( in 0.4ML) ADALIMUMAB IS EFFECTIVE - The first trial of any targeted therapy in early DD* • Dose ranging with 28 patients. • 40 mg in 0.4ml - effective dose. • Funded by HICF (Wellcome Trust + Dept of Health) and CannBioRx 14 EBioMedicine 33 (2018) 282 - 288 TRIAL OVERVIEW Adalimumab injected directly into the nodule Demonstrated efficacy at high concentration & dose 15mg in 0.3ml (1.60 “ 0.67) 40mg in 0.4ml Placebo (1.51 “ 0.65) 35mg in 0.7ml* (1.44 “ 0.48) (1.09 “ 0.89) *Leakage observed from site injection due to large volume 5.0 4.0 3.0 2.0 1.0 0.0 Placebo 15mg 35mg 40mg a dalimumab Treatment p=0.024 p=0.006 p<0.001 1250 1000 750 500 250 0.0 Placebo 15mg 35mg 40mg a dalimumab p=0.019 *Approval only from MHRA/CCMO and relevant accredited ethics committees.

15 • Randomized blinded trial in patients with early DD injected with optimal dose adalimumab * • Every 3 months for 1 year (4 injections), following for a total of 18 months • Outcome measures include nodule hardness, size and disease progression • Randomized 181 patients across 3 sites in UK and the Netherlands • FULLY ENROLLED, FULLY PAID FOR • All UK patients have received final injection • Results expected Q1/2 2021 PHASE 2b/3 TRIAL FULLY ENROLLED – LOCAL ADALIMUMAB IN EARLY DD CannBioRx clinical trial 2b/3 - Nanchahal J et al, 2017 Wellcome Open Research, 2:37 Trial sites - Oxford, Edinburgh, Groningen *Approval only from MHRA/CCMO and relevant accredited ethics committees.

16 Estimated future worldwide market for Dupuytren’s >$1B per year (1) All current treatments for Dupuytren's are for LATE stage disease • 4% of the EU & US population suffer from Dupuytren’s disease ( 1,2) • Assume ~25% of these (1% total) are symptomatic & require treatment (3) Potential patients in the U.S. (1% of 315M) = 3M patients • Conservatively assume 25% of symptomatic patients get treatment (4) US 3M x 25% EU (Assume 50% of US) ~ 0.8M pa ~ 0.4M patients 1. Hindocha S, McGrouther DA, Bayat A (2009) Hand (NY) 4(3):256 - 69. 2. Lanting et al. (2014) PRS 133: 593 - 603 3. Nanchahal J, et al. (2017) Wellcome Open Res 2:37., 4. Nanchahal J, personal communication L ARGE MARKET OPPORTUNIT Y FOR EARLY DUPUY TREN'S DISEASE

ADDITIONAL INDICATIONS: 17 • Over 300,000 hip fractures each y ea r in the U.S. alone 1 • Strong pre - clinical and clinical evidence for anti - TNF as preventative therapy • Patent claims granted, patent is licensed from Kennedy Trust, UK • Phase 3 multi - centre trial of pre - operative anti - TNF in hip fracture surgery planned to initiate by Q4 2020, single dose administered just prior to surgery, to be complete in 4 years • Grant application submitted to EME (Efficacy and Mechanism Evaluation) board of National Institute of Health Research (NIHR) • Trial to be executed in the UK, and therefore protocol will be approved by MHRA and Research Ethics Committee. Will seek advice from the FDA when appropriate • Working with Prof Matt Costa, trauma surgeon - previously recruited 20,000 patients to hip fracture trials Post Operative Delirium/Cognitive Deficit (POCD) • Direct entry to phase 3 permissible: • Anti - TNF has a well defined safety profile, dosing understood • Have access to patients for large scale trial required for sufficient powering 1 https:// www.cdc.gov / homeandrecreationalsafety /falls/ adulthipfx.html

18 • Mice subjected to surgery (open tibial fracture) experience a rapid increase in plasma TNF levels (A) - not caused by anesthesia alone (B) • Administration of pre - operative anti - TNF reduces freezing behavior, indicative of contextual fear memory, characteristic of cognitive decline (C) • Surgery in humans triggers TNF release, and is associated with reduced brain activity cognitive decline 1 - 2 B C Plasma TNF increases after surgery Surgery impaired fear memory is restored with pre - op anti - TNF EVIDENCE THAT TNF PLAYS A ROLE IN POCD 1 Clark IA, Vissel B. Front Neurosci (2018) 12:257. 2 Alam A et al, EBioMedicine (2018) 37:547 - 556 Forsberg A et al, Annals of Neurology (2017) 81:4, 572 - 582 Plasma TNF increases after surgery and correlates with post operative delirium In mice In man Generated patent, licensed from Kennedy Inst. for treatment of POCD with anti - TNF Kazmierski J et al, International Psychogeriatrics (2014), 26:5, 845 – 855

ADDITIONAL FIBROTIC INDICATIONS CONTINUED : • Painful inflammatory condition that progresses to scarring, limiting movement • Affects 9% of the of the population aged 25 - 64yr, more common in diabetics 1 • Only treatment for early stage is local steroid injection for short term relief • Phase 2 clinical trials planned for local injection of anti - TNF, initiates Q 4 2020 • Trial protocol completed and grant application submitted to NIHR (National Institute of Health Research) in collaboration with leading experts who pioneered delivery of large trials in frozen shoulder • Will obtain MHRA and Research Ethics Committee approval (clinical trial to be conducted in the UK) and will seek FDA advice when appropriate • Long - term damage characterized by the replacement of normal liver tissue by scar tissue • Most commonly caused by non - alcoholic fatty liver disease (NAFLD) , which affects ~30% of the US population 2 • ~2% of patients with non - alcoholic fatty liver disease and 15 - 20% with non - alcoholic steatohepatitis (NASH) progress to cirrhosis 3 • No approved therapeutic for NASH • Lab program in collaboration with Celgene - BMS for target discovery using human liver samples • Optimised process for acquiring fresh human liver tissue from clinical network 19 1 Walker - Bone K et al (2004) Arthritis Rheum 51(4):642 - 651 2 Rinella ME & Sanyal AJ (2016) Nat Rev Gastroenterol Hepatol 13(4):196 - 205 3 Ibid. Frozen Shoulder Fibrosis of the liver

NEXT GENERATION THERAPEUTICS: ANTI - TNFR2 & ANTI - IL - 33 INHIBITORS 20 20 Soucre for Diagram Above: Izadi D et al. Sci. Adv. 2019; 5 : eaay0370 4 December 2019 – supp data Dupuytren’s disease fibrotic nodules comprise myofibroblasts and immune cells (macrophages and mast cells mostly) Proposed mechanism: 1. Myofibroblasts secrete IL - 33 2. IL - 33 signals through ST2 receptor on mast cells and macrophages 3. Triggers production of TNF 4. TNF drives differentiation and activation of myofibroblasts PUTATIVE THERAPEUTIC INTERVENTIONS 1. Anti - TNF (in Phase 2b/3 trial with approval only from MHRA / CCMO and relevant accredited ethics committees) 2. Anti - IL - 33 and/or anti - TNFR2 (next generation) » Double pronged approach, blocking production of TNF and downstream signaling Patents filed for anti - TNFR2 and anti - IL - 33 Claims in USA granted for IL - 33, others pending

FIBROSIS & ANTI - TNF CLINICAL DEVELOPMENT PLAN * 2 0 20 2 0 21 2 0 2 2 2 0 23 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Anti - TNF DD phase 2b/3 trial 21 Ph 2b results P h 3 results Q4 2026 POCD results Frozen Shoulder (FS) Preparation POCD Preparation Approval to do trial** Anti - TNF FS phase 2 trial Anti - TNF POCD phase 3 trial Approval to do trial** Therapeutic development Ta rget identified Phase 3 Results *Based on current proposals. **Regulatory approvals obtained from the MHRA and CCMO and the relevant accredited ethics committees to perform clinical tria ls in the UK and The Netherlands. No meetings have been held with, and no applications or requests for approval have been submitted to the FDA fo r a ny products at this time. Proceed to registration or further phase 3 trial if required Target discovery in human liver fibrosis

SYNTHETIC CBD ANALOGS (SCAs) 22 Jerusalem, Israel Led by Profs R Mechoulam, R Gallily, A Domb, A Hoffman – HU, Jerusalem Prof Sir Marc Feldmann, Prof R Williams, Dr L Topping – Oxford Clinical trials supported by Prof Sallie Lamb, UK

Synthetic CBD analogs (SCAs) for unmet needs in pain and inflammation 23 • Safe & non - psychoactive • Formulated to offer improved oral bioavailability (> three - fold) • Rigorously tested in clinical trials for inflammatory pain (efficacy and dosing) • Granted market approval by FDA, EMA and others • A real alternative to unregulated consumption of medical marijuana or OTC CBD (no clinical evidence, not FDA approved, unreliable composition, unpredictable dosing and safety ….) Regulatory landscape Only two drugs approved , both from GW Pharma, both cannabis derived: 1. Epidiolex - oral CBD for treatment of rare childhood epilepsies – Lennox Gastaut and Dravet Syndrome, FDA and EMA approved, - recently recommended by National Institute for Health and Care Excellence) NICE in UK for National Health Service (NHS) reimbursement 2. Sativex ( nabiximols ) – THC:CBD (1:1) oromucosal spray for multiple sclerosis (MS) related spasticity, approved by EMA but not by FDA. » Points to opportunities across Europe and UK for uptake of medical cannabinoids 1 » FDA warns about unregulated consumption of CBD 2,3 » More trials needed to establish safety 1 “ GW Pharma Shares Rise ” https://www.marketwatch.com/story/gw - pharma - shares - rise - after - uk - recommends - nhs - reimburse - its - cbd - based - epilepsy - drug - 2019 - 11 - 11 2 “CBD has the potential to harm you” https://www.marketwatch.com/story/cbd - has - the - potential - to - harm - you - fda - warns - consumers - 2019 - 11 - 25 3 “FDA warns 15 companies” https://www.fda.gov/news - events/press - announcements/fda - warns - 15 - companies - illegally - selling - various - products - containing - cannabi diol - agency - details Developing proprietary compounds which aim to be: CBRx SCAs are pure, not derived from cannabis, offer more efficient & controlled oral dosing, easy to administer, positioned for treatment of residual pain in inflammatory arthritis and other – not competing with GW products which are plant derived!

• Non - psychoactive CBD analogs (SCAs) are anti - inflammatory, and elicit analgesic effects • Studied by Mechoulam, Gallily, Feldmann since 1998 ( Malfait et al, PNAS 2000) PLATFORM DESCRIPTION * Patented drug we licensed from HU, but expect to discover superior drugs from ongoing research ** CBD derivative, patent being filed , agreement with Domb & HU completed † not filed yet OUR DRUGS 1. HU - 436* 2. Domb patent 1** 3. Mechoulam patent 2 † 4. Mechoulam patent 3 & others † HOW DOES IT WORK? OUR PRODUCTS: WHY MAN - MADE? • CBD signals through multiple GPCR receptors, eg CB2R, TRPV - 1, 5HT1 α , GPR55, GPR18 and others • Anti - inflammatory, analgesic and anxiolytic properties NON - PSYCHOACTIVE SCAs • Scientifically formulated analogs of CBD (SCAs) have been synthesized and patented , new formulations under analysis • Analysed in animal models of inflammation and pain • High purity (>99.5%) • CBD from plants are typically ≤ 98% pure, contain THC, minor cannabinoids, terpenes, flavonoids etc. • Consistent across batches , more favourable for obtaining regulatory approval 24

× Variable composition, potency, and may contain undesirable contaminants x We will use SYNTHETIC >99.5% pure SCAs 25 • CBD and CBD - PNL administered orally to rats & plasma l evels assessed over time • CBD - PNL >3x absorption compared to CBD alone • CBD - PNL safe and well tolerated • Additional methods to improve absorption are being patented under a recently completed agreement with HU Problems with MM / OTC CBD Our Solution × Side effects can be triggered by THC (e.g. psychosis) × Little clinical data from approved drugs exist (outside of epilepsy) to determine dosing × Variable uptake and low absorption (~4 - 9%) due to lipophilic properties of CBD / CBD - like x We will use synthetic CBD Analogs (SCAs) – no THC x P lanning blinded clinical trials initially in musculoskeletal pain and arthritis x Developing novel, patented ProNanoLipospheres (PNL) which enhance bioavailability (opposite graph shows improvements offered by CBD - PNL vs CBD IN PEG) PROBLEMS OF MEDICAL MARIJUANA (MM) & OTC CBD THAT CANNBIORX SOLVES FDA position – Nov 25, 2019 1 “There are many unanswered questions about the science, safety, and quality of products containing CBD. Some products are bei ng marketed with unproven medical claims and could be produced with unsafe manufacturing practices”. "The agency is committed to supporting the development of new drugs, including cannabis and cannabis - derived drugs, through the investigational new drug and drug approval process…”. 1 “ What you need to know” https://www.fda.gov/consumers/consumer - updates/what - you - need - know - and - what - were - working - find - out - about - products - containing - canna bis - or - cannabis 2 “GW Pharma shares rise” https://www.marketwatch.com/story/gw - pharma - shares - rise - after - uk - recommends - nhs - reimburse - its - cbd - based - epilepsy - drug - 2019 - 11 - 11 3 Cherniakov I, et al. (2017) European J of Pharm. Sci 109:21 - 30 • With the only cannabinoid FDA approved drug, GW Pharma shares have gained 15% in 2019, outperforming the broader cannabis sector 2 . • FDA warnings have been issued to 15 companies in US illegally selling CBD products with unproven medical claims. • CBRx will develop SCAs for treatment of well - defined clinical indications, adhering fully to regulatory approved procedures for obtaining marketing authorisation. 3

CBD - A SUPERIOR TREATMENT FOR ARTHRITIS CBD reduces inflammation in knee arthritis – unpublished new data PROBLEM • Very early arthritis, pain & swelling is not effectively treated clinically • Nonsteroidals do not help, can increase TNF 1 • Existing therapies are suboptimal: • Methotrexate has side effects patients dislike • Anti - TNF is costly and use restricted / delayed by NICE (National Institute of Clinical Evidence, in UK) • Early rheumatoid or psoriatic arthritis is badly treated: delays mean the " window of opportunity " for best results is missed SOLUTION • For v ery early arthritis: novel SCAs being developed • Effective anti - inflammatory (better than NSAIDs) • Effective analgesic • For e arly established RA, PsA : SCA will be tried in combination (offers additional patentability) • Trials will be performed by Oxford rheumatologists and trial experts Oral CBD is effective in mouse model of RA A. M. Malfait , R. Mechoulam, M. Feldmann, R. Gallily PNAS 2000 ;97:17:9561 - 9566 From the first clinical signs of arthritis, mice were given CBD orally , at the following doses: 50 mg/kg ( ᶭ ), 25mg/kg ( Ÿ ), or 10 mg/kg (o). CBD was administered intraperitoneally to mice with zymosan induced arthritis in the left knee. Inflammation intensity is marked by colour scale shown on right, using a fluorescent reporter of cathepsin. CBD (5 - 25 mg/kg) attenuates local inflammation in a dose dependent manner. 26 Zymosan induced arthritis in left knee 1 Page TH …… & Feldmann M (2010), J Immunol. 15;185(6):3694 - 701

SCA PIPELINE * 2020 2021 2022 2023 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Development of l ead SCA Phase 1 Phase 2a/b Indication tbc R e s ul t s 27 R e s u l t s SCA pipeline Phase 2a/b in early arthritis Therapeutic development of SCA 2 Preclinical development of SCA 2 Indication tbc *Based on current proposals. No regulatory approvals sought from appropriate authorities at this time.

α7 n ACh R PROGRAM 2 8 California, USA Prof L Steinman & Dr J Rothbard Supported by Evotec, US 28

• Decade of research on immune suppression in multiple sclerosis led to realization of the importance of the a 7 subunit of nicotinic Acetylcholine Receptor ( nAChR ) • a 7 nAChR also a central factor in evolutionarily ancient neural circuit to control of inflammation 1&2 • Large pharma identified a 7 as a pharmaceutical target for Alzheimer’s disease and schizophrenia • multiple specific agonists developed • all shown to be safe, but did not meet milestones in human clinical trials • strategic goal of CannBioRx to repurpose drugs for inflammation WHY DEVELOP a 7 AGONISTS FOR INFLAMMATION? 29 1 Rothbard JB, Rothbard JJ, Soares L, Fathman CG, and Steinman L. Identification of a common immune regulatory pathway induced by small heat shock proteins, amyloid fibrils, and nicotine. Proc Natl Acad Sci U S A. 2018 115:7081 - 7086. 2 Tracey KJ. Reflex control of immunity. Nat Rev Immunol. (2009) 9:418 – 28

3 0 CHEMISTRY • Synthesize patentable, orally bioavailable α7 agonist • Chemically modify known agonist to retain/improve anti - inflammatory activity • Retain CNS penetration Bioassays • Inhibition of TNFα & IL6 secretion after stimulation with LPS in cell lines and primary cells • Stimulation of Ca+2 flux in α7 transfected HEK293 cells • qPCR measurement of inhibition of a set of proinflammatory cytokines and transcriptional activators • Inhibition of inflammation and secretion of proinflammatory cytokines in vivo in murine models of inflammation a 7 AGONIST DEVELOPMENT STRATEGY 30

3 1 Significant Unmet Need Existing Therapies Are Sub - Optimal Existing Therapy Issues Anti - inflammatory drugs (5 - aminosalicylates, corticosteroids) × capability to induce remission is quite low × known deleterious side effects of steroids Immunosuppressants × long - term administration of thiopurine may correlate with an increased risk of developing lymphoma × cyclosporine leads to kidney damage Infliximab (anti - TNF) × serious adverse events, such as opportunistic infections, including tuberculosis, as well as congestive heart failure in cardiopathic patients • Nicotine binds a 7 and is a known immune suppressive • a subgroup of patients who cease smoking subsequently acquire ulcerative colitis • Treatment of these patients with a 7 agonist has a high probability of therapeutic success (can be viewed as nicotine replacement therapy without issues of addiction) SELECTION OF CLINICAL INDICATION 31

3 2 Faster Time to Market Lower Development Costs Targeted Clinical Trial Novel Therapeutic Target Significant Unmet Need Repurposing drugs previously proven safe (targeted Alzheimer’s & Schizophrenia) Drugs stimulate vagal nerve, leading to localized anti - inflammatory response, similar to nicotine’s MoA Existing therapies have occasional serious adverse effects and target the disease’s pathogenesis further downstream First clinical trial targeting patients who ceased smoking and developed ulcerative colitis Nicotine consumption protects against ulcerative colitis, lupus, and schizophrenia a 7 nAChR PLATFORM, A NOVEL THERAPEUTIC PLATFORM FOR UC - essential receptor in the neural circuit controlled by the vagus nerve 32

3 3 *Based on current proposals. No regulatory approvals obtained from appropriate authorities at this time. 2020 2021 2022 2023 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Pre - clinical efficacy α7nAChR pipeline Lead identified Lead optimisation Clinical development For smoking cessation induced UC a 7nAChR PIPELINE* 33

34 CONCLUSION 34 • Scientific team and founders are pioneers with proven track record in drug discovery from the University of Oxford, Hebrew University and Stanford University • Developing three families of novel drugs addressing significant market opportunities in inflammation, fibrosis and pain: - Fibrosis & Anti - TNF - Synthetic CBD Analogs ( SCAs ) - a 7nAChR • Multiple programs in synchronized stages of development combined with IP portfolio reduces risk • Numerous near - term inflection points for anti - TNF programs: one program late stage 2b/3 trial, two additional clinical programs projected to start Q4 2020 • Initial clinical anti - TNF clinical trials funded by investments and grants (UK and Dutch) . • *Regulatory approvals obtained from the UK Medicines and Healthcare Products Regulatory Agency (MHRA) and the Dutch Centrale Commissie Mensgebonden Onderzoek (CCMO) and the relevant accredited ethics committees to perform clinical trials in the UK and The Netherlands for anti - TNF products. • Three anti - inflammatory therapeutic programs potentially used in combination * No meetings have been held with, and no applications or requests for approval have been submitted to the FDA for any products at this time.

CANNBIORX LIFE SCIENCES CORP Registered Address 1201 Orange Street Suite 600 Wilmington, DE 19801 USA 35

36 APPENDIX

FIBROSIS PROGRAM PATENTS ( Pg 1of 3) METHOD OF TREATING EARLY STAGE DUPUYTREN'S DISEASE Country Application No. C&D Docket No. Status Australia 2017248273 8554 - A - PCT - AU Application pending in Australian Patent Office Canada 3,020,327 8554 - A - PCT - CA Application pending in Canadian Patent Office Europe 17779836.0 8554 - A - PCT - EPO Application pending in European Patent Office U.S. 16/089,234 8554 - A - PCT - US Application pending in U.S. Patent Office METHOD OF TREATING A LOCALIZED FIBROTIC DISORDER USING AN IL - 33 ANTAGONIST Country Application No. C&D Docket No. Status Australia 2016226414 87158 - A - PCT - AU Application pending in Australian Patent Office Canada 2,978,449 87158 - A - PCT - CA Application pending in Canadian Patent Office Europe 16759325.0 87158 - A - PCT - EPO Application pending in European Patent Office Hong Kong 18107063.7 87158 - A - PCT - EPO - Hong Kong Application pending in Hong Kong Patent Office U.S. 15/555,027 87158 - A - PCT - US Notice of Allowance issued August 1, 2019. METHOD OF TREATING A LOCALIZED FIBROTIC DISORDER USING A TNF RECEPTOR 2 ANTAGONIST Country Application No. C&D Docket No. Status Australia 2016226415 87158 - B - PCT - AU Application pending in Australian Patent Office Canada 2,978,431 87158 - B - PCT - CA Application pending in Canadian Patent Office Europe 16759326.8 87158 - B - PCT - EPO Application pending in European Patent Office Hong Kong 18107062.8 87158 - B - PCT - EPO - Hong Kong Application pending in Hong Kong Patent Office U.S. 15/555,030 87158 - B - PCT - US Application pending in U.S. Patent Office , filed August 31, 2017 37

FIBROSIS PROGRAM PATENTS TREATMENT FOR DUPUYTREN’S DISEASE Country Application No. C&D Docket No. Status Australia 2011322482 90330 - A - PCT - AU Australian Patent No. 2011322482, granted July 20 , 2017 Australia 2017204267 90330 - AZ - PCT - AU Australian Patent No. 2017204267, granted September 19, 2019 Canada 2,847,197 90330 - A - PCT - CA Application pending in Canadian Patent Office Europe 11779628.4 90330 - A - PCT - EPO The European patent is valid or being validated in: Austria (Patent No. E - 1075071) Belgium (Patent No. 2362446) Denmark Finland (Patent No. 2362446) France (Patent No. 2362446) Germany (Patent No. 2362446) Iceland (Patent No. 2362446) Ireland (Patent No. 2362446 - IE) Italy (Appl. No. 502019000019925) Netherlands (Patent No. 2362446) Norway (Patent No. 2362446) Spain (Appl. No. 300310256) Sweden (Patent No. 2362446) Switzerland/Liechtenstein (Patent No. 2362446) and United Kingdom (Patent No. 2362446) Japan 2013 - 535462 90330 - A - PCT - JP Japanese Patent No. 6004494, granted September 16, 2016 U.S. 13/882,262 90330 - A - PCT - US U.S. Patent No. 9,138,458, granted September 22, 2015 U.S. 14/852,442 90330 - AA - PCT - US U.S. Patent No. 10273296 , granted April 30, 2019 38 FIBROSIS PROGRAM PATENTS ( Pg 2 of 3)

FIBROSIS PROGRAM PATENTS METHOD OF TREATING LOCALIZED FIBROTIC DISORDERS USING AN IL - 33/TNF BISPECIFIC ANTIBODY Country Application No. C&D Docket No. Status U.S. 16/328,979 87158 - F - PCT - US Filed February 27, 2019. Europe 17924768.9 87158 - F - PCT - EPO Filed April 1, 2019. METHOD OF TREATING SYSTEMIC FIBROTIC DISORDERS USING AN IL - 33/TNF BISPECIFIC ANTIBODY Country Application No. C&D Docket No. Status U.S. 16/329,013 87158 - G - PCT - US Filed February 27, 2019. Europe 17847574.5 87158 - G - PCT - EPO Filed April 1, 2019. 39 FIBROSIS PROGRAM PATENTS (Table 3 of 3) POCD PATENT METHOD FOR TREATMENT OF POST OPERATIVE COGNITIVE DYSFUNCTION Country Application No. C&D Docket No. Status U.S. 15/063,775 Granted April 12, 2016. Europe Japan

SCA PROGRAM PATENTS Cyclohexenyl compounds, compositions comprising them and uses thereof Country Application No. Status Activity expected Comments US2 10239848 Granted Maintenance due by September 21, 2022 Claims directed to a method for treating obesity or a disease/disorder associated therewith by administration of a compound of the formula as defined in claim 1 US3 16/274,107 Pending Claims directed to a method for treating pain or associated condition or symptom by administration of a compound of the formula as defined in claim 1 IL 248256 Granted Renewal due by April 21, 2021 Claims directed to a pharmaceutical composition for treatment of (i) obesity or a disease/disorder associated therewith ; or (ii) abnormal food consumption or body weight, or a condition or symptom associated therewith, comprising a compound of the formula 1 as defined in claim 1 EP 15726740.2 Pending OA/Allowance may be expected CN 2015820978 Pending OA/Allowance may be expected Decision on grant expected shortly (as of 25 Aug, 2019 ) CA 2944837 Pending Filed Jul 10, 2019 STATUS OF PATENT APPLICATIONS UPDATED Oct 09 , 2019 CANN - 001 – Cyclohexenyl compounds, compositions comprising them and uses thereof (HU436 & HU435) Applicant: Yissum research development company of the Hebrew University of Jerusalem Ltd. Inventors: Ruth Gallily, Raphael Mechoulam, Aviva Breuer Priority: US Provisional Application No. 61/981,997, filed April 21, 2014 (expired) International Filing Dates: April 21, 2015 OA = Office Action 40

α 7 n ACh R PROGRAM PATENTS 41 Country Application No. Priority Date Description US Application US20180333451A1 B - 1a lymphocyte and/or macrophage targeting and activation to treat medical conditions with inflammatory or autoimmune components Japan 2018 - 526196 2015 - 11 - 18 Canada 3004908 US Grant US8835391B2 2006 - 12 - 11 Alpha B - crystallin as a therapy for multiple sclerosis US Grant US7875589B2 2006 - 12 - 11 Alpha B - crystallin as a therapy for rheumatoid arthritis US Grant US8771689B2 2006 - 12 - 11 Alpha B - crystallin as a therapy for ischemia or inflammation