UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported):
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction | (Commission | (I.R.S. Employer |
| of incorporation) | File Number) | Identification No.) |
| (Address
of principal executive offices) |
(Zip Code) |
Registrant’s telephone number, including area
code: (
Not Applicable
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) | |
| Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) | |
| Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) | |
| Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant
has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant
to Section 13(a) of the Exchange Act.
Item 7.01 Regulation FD Disclosure.
On June 24, 2024, Entrada Therapeutics, Inc. (the “Company”) issued a press release providing an update on ENTR-601-44, its most advanced product candidate being developed for patients with Duchenne muscular dystrophy (“DMD”). The press release is furnished as Exhibit 99.1 to this Current Report on Form 8-K.
The information in Item 7.01 of this Current Report on Form 8-K (including Exhibit 99.1) shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
Item 8.01. Other Events.
Update on ENTR-601-44
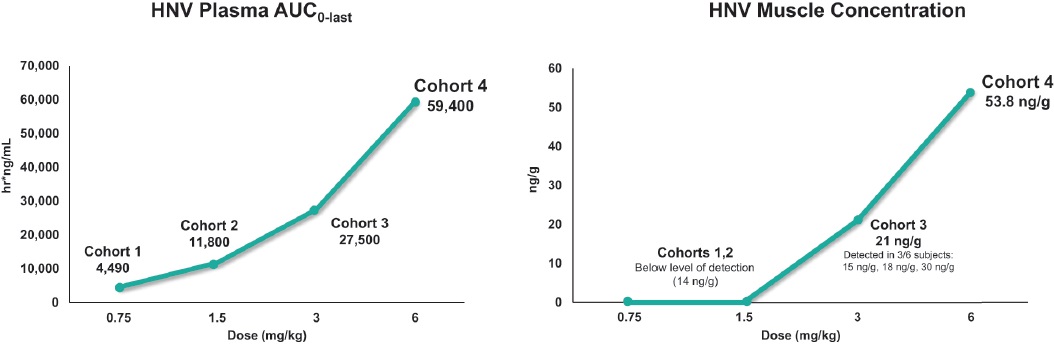
On June 24, 2024, the Company announced positive preliminary data from its Phase 1 clinical trial of ENTR-601-44, its most advanced product candidate being developed for patients with DMD. The data announced is based upon data collected to date, is aggregated based upon the placebo and study drug groups and does not include urinary biomarkers for its 6 mg/kg cohort.
The primary objective of the Company’s Phase 1 clinical trial was to evaluate the safety and tolerability of a single dose of ENTR-601-44. The trial also evaluated pharmacokinetics and target engagement, as measured by exon skipping in the skeletal muscle. The trial included a total of 32 healthy male volunteers across four cohorts, with each cohort consisting of six participants receiving ENTR-601-44 and two participants receiving a placebo control. The doses administered across the cohorts were 0.75 mg/kg, 1.5 mg/kg, 3 mg/kg (“Cohort 3”) and 6 mg/kg (“Cohort 4”).
Safety and Tolerability
There were no serious adverse events, no drug-related adverse events, and no clinically significant changes or trends noted in vital signs, electrocardiograms (“ECGs”), physical exams or laboratory assessments observed in the trial. More specifically, there were no severe treatment emergent adverse events (“TEAEs”) and no TEAEs leading to discontinuation reported in the study in any cohort. In addition, there were no clinically significant changes from baseline values observed in renal function-related findings (BUN, Cr, CysC, Mg, eGFR) or in creatine kinase. Further, no clinically significant lab values in hematology, blood chemistry and urinalysis were observed, no changes in urinary biomarkers through the first three cohorts (does not include urinary biomarker data from Cohort 4), no clinically significant changes from baseline recordings noted on ECGs for all four cohorts, and no clinically significant changes from baseline recordings noted on vitals and physical exams for all four cohorts. Three TEAEs were reported from Cohort 4, all of which were resolved by Day 28. Two of these were mild headaches and the other was a moderate toothache. Two were assessed as not related to study drug and the other was assessed as unlikely related. There were 22 TEAEs reported across all cohorts. All 22 TEAEs were assessed as mild or moderate in severity, with 20 assessed as not related to study drug and two assessed as unlikely related to study drug. Headache was the most commonly reported TEAE. Among the seven reported TEAEs of headache, two were assessed as moderate and not related to study drug and five were assessed as mild. The table below summarizes the number of participants reporting TEAEs by cohort.
Treatment Emergent Adverse Events
| Event Category | Number of Participants Experiencing an Event | |||||||||||||||
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Grand Total | ||||||||||||
| n=8 | n=8 | n=8 | n=8 | N=32 | ||||||||||||
| n (%) | n (%) | n (%) | n (%) | n (%) | ||||||||||||
| Serious Adverse Event (SAE) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||||||
| Treatment-Emergent Adverse Event (TEAE) | 6 (75.0) | 3 (37.5) | 3 (37.5) | 3 (37.5) | 15 (47.0) | |||||||||||
| SAE leading to death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||||||
| SAE leading to study drug discontinuation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||||||
| TEAE leading to death | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||||||
| TEAE leading to study drug discontinuation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||||||||||
Pharmacokinetics and Pharmacodynamics
Target engagement as measured by exon skipping on a ng/g of tissue adjusted basis was observed with muscle concentration detected in all six subjects in Cohort 4 (mean of 53.8 ng/g, range 40 ng/g-73.5 ng/g) as summarized below.
Pharmacokinetics

Dose dependent increases of active PMO were observed in the study and mean target engagement as measured by exon skipping was 0.44% (range 0.3-0.65%). Exon skipping was statistically significant when compared to the placebo control (p<0.005) in Cohort 4. The charts below summarize the muscle concentration and exon skipping observed in the trial.
Muscle Concentration and Exon Skipping
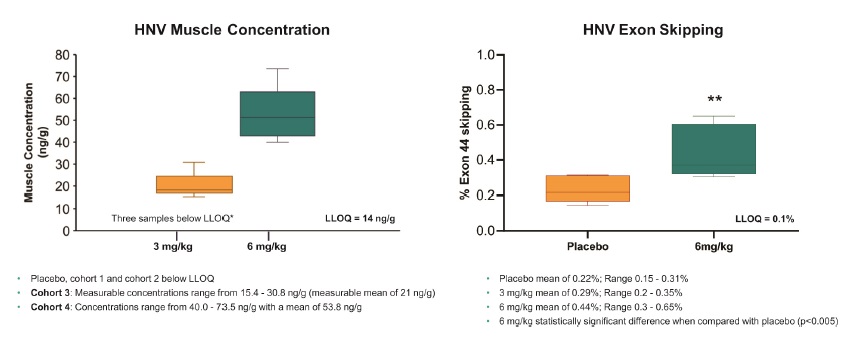
*LLOQ: Lower limit of quantitation.
Dose dependent increases observed in muscle concentration of active PMO were based upon three out of six patients in Cohort 3 with measurable PMO concentrations ranging from 15.4 to 30.8 ng/g (measurable mean of 21 ng/g) and four out of six patients in Cohort 4 with measurable PMO concentrations ranging from 40.0 to 73.5 ng/g with a mean of 53.8 ng/g and a geometric mean of 52.4 ng/g. Muscle concentration and exon skipping were measured via needle biopsy collected approximately 72 hours post infusion. Mean exon skipping was 0.22% ± 0.067 in participants who received placebo, 0.29% ± 0.068 in Cohort 3 and 0.43% ± 0.14 in Cohort 4.
Based on the positive preliminary data from the Phase 1 clinical trial, the Company plans to submit regulatory applications in the fourth quarter of 2024 to initiate separate global Phase 2 clinical trials for ENTR-601-44 in patients with DMD who are exon 44 skipping amenable and for ENTR-601-45 in patients with DMD who are exon 45 skipping amenable, subject to regulatory feedback. In addition, the Company plans to submit regulatory applications in 2025 to initiate a global Phase 2 clinical trial for its third Duchenne candidate, ENTR-601-50, in patients who are exon 50 skipping amenable, subject to regulatory feedback.
Forward-Looking Statements
This Current Report on Form 8-K contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this Current Report on Form 8-K, including statements regarding the Company’s strategy, future operations, prospects and plans, objectives of management, the validation and differentiation of the Company's approach and its ability to provide a potential treatment for patients, the translatability of the preliminary ENTR-601-44-101 data to the complete data set, expectations regarding the starting dose for the Company’s planned Phase 2 clinical trial for ENTR-601-44, expectations regarding significant accumulation of exon skipping and dystrophin production in patients, expectations regarding improvement in functional outcomes for patients after multiple doses of ENTR-601-44, expectations regarding the importance of endosomal escape to therapeutic index optimization, expectations regarding the timing of regulatory filings for the planned Phase 2 clinical trials for ENTR-601-44 and ENTR-601-45 in the fourth quarter of 2024, and ENTR-601-50 in 2025, the ability to recruit for and complete a global Phase 2 trial for ENTR-601-44, ENTR-601-45 and ENTR-601-50, the potential of the Company’s EEV product candidates, including the potential for ENTR-601-44 to be a transformative treatment option and EEV platform, and the continued development and advancement of ENTR-601-44, ENTR-601-45 and ENTR-601-50 for the treatment of Duchenne and the partnered product VX-670 for the treatment of myotonic dystrophy type 1, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “might,” “objective,” “ongoing,” “plan,” “predict,” “project,” “potential,” “should,” or “would,” or the negative of these terms, or other comparable terminology are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including: uncertainties inherent in the identification and development of product candidates, including the conduct of research activities and the initiation and completion of preclinical studies and clinical trials; uncertainties as to the availability and timing of results from preclinical and clinical studies; the timing of and the Company’s ability to submit and obtain regulatory clearance and initiate clinical trials; whether results from preclinical studies will be predictive of the results of later preclinical studies and clinical trials; whether preliminary clinical data will be predictive of final clinical data; whether the Company’s cash resources will be sufficient to fund the Company’s foreseeable and unforeseeable operating expenses and capital expenditure requirements; as well as the risks and uncertainties identified in the Company’s filings with the Securities and Exchange Commission (SEC), including the Company’s most recent Form 10-K and in subsequent filings the Company may make with the SEC. In addition, the forward-looking statements included in this Current Report on Form 8-K represent the Company’s views as of the date of this Current Report on Form 8-K. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements at some point in the future, it specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this Current Report on Form 8-K.
| Item 9.01 | Financial Statements and Exhibits. |
| (d) | Exhibits. |
| Exhibit No. | Description | |
| 99.1 | Press Release issued by Entrada Therapeutics, Inc. on June 24, 2024. | |
| 104 | Cover Page Interactive Data File (embedded within the Inline XBRL document). |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Entrada Therapeutics, Inc. | |
| Date: June 24, 2024 | /s/ Dipal Doshi |
| Dipal Doshi | |
| Chief Executive Officer |