Filed Pursuant to Rule 424(b)(4)
Registration No. 333-281892
4,020,000 Shares of Common Stock
5,070,910 Pre-Funded Warrants to Purchase 5,070,910 Shares of Common Stock
18,181,820 Common Warrants to Purchase 18,181,820 Shares of Common Stock
23,252,730 Shares of Common Stock underlying the Pre-Funded Warrants and Common Warrants

This is a reasonable best efforts offering of up to 4,020,000 shares (the “shares”) of our common stock, par value $0.001 per share ("common stock") together with 18,181,820 common warrants to purchase 18,181,820 shares of common stock at a combined offering price of $0.33 per share and common warrants. Each share of common stock is being offered together with two common warrants, each to purchase one share of common stock. The common warrants will be exercisable beginning on the effective date of such stockholder approval as may be required by the applicable rules and regulations of the Nasdaq Capital Market (or any successor entity) to permit the exercise of the common warrants (“Stockholder Approval”) at an exercise price of $0.33 per share and will expire five years from the date of Stockholder Approval. In the event that we are unable to obtain any required Stockholder Approval, the common warrants will not be exercisable and therefore have no value. In addition, the common warrants will include a provision that resets their exercise price in the event of a share split, share dividend, share combination or other such event (the "Share Combination Event"), of our common stock, to a price equal to the lower of (i) the then current exercise price and (ii) lowest volume weighted average price ("VWAP") during the period commencing five consecutive trading days immediately preceding and ending immediately after the five consecutive trading days beginning on the date we effect a Share Combination Event. The shares of common stock and common warrants will be separately issued. This prospectus also covers the shares of common stock issuable from time to time upon the exercise of the common warrants.
We are also offering 5,070,910 pre-funded warrants to those purchasers, whose purchase of shares of common stock in this offering would result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock following the consummation of this offering in lieu of the shares of our common stock that would result in ownership in excess of 4.99% (or, at the election of the purchaser, 9.99%). Each pre-funded warrant will be exercisable for one share of common stock at an exercise price of $0.0001 per share. Each pre-funded warrant is being offered together with the same two common warrants, each to purchase one share of common stock described above being offered with each share of common stock. The purchase price of each pre-funded warrant will equal the combined offering price per share of common stock and common warrants being sold in this offering, less the $0.0001 per share exercise price of each such pre-funded warrant. Each pre-funded warrant will be exercisable upon issuance and will expire when exercised in full. The pre-funded warrants and common warrants will be separately issued. For each pre-funded warrant that we sell, the number of shares of common stock that we are selling will be decreased on a one-for-one basis. This prospectus also covers the shares of common stock issuable from time to time upon the exercise of the pre-funded warrants.
There is no established public trading market for the pre-funded warrants or common warrants, and we do not expect a market to develop. We do not intend to apply for listing of the pre-funded warrants or common warrants on any securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the pre-funded warrants and common warrants will be limited.
Roth Capital Partners, LLC, or the placement agent, acted as our exclusive placement agent in connection with this offering. The placement agent has agreed to use its reasonable best efforts to arrange for the sale of the securities offered by this prospectus. The placement agent is not purchasing or selling any of the securities we are
offering and the placement agent is not required to arrange the purchase or sale of any specific number or dollar amount of securities. We have agreed to pay to the placement agent the placement agent fees set forth in the table below, which assumes that we sell all of the securities offered by this prospectus. There is no arrangement for funds to be received in escrow, trust or similar arrangement. There is no minimum number of shares of securities or minimum aggregate amount of proceeds that is a condition for this offering to close. Because there is no escrow account and no minimum number of securities or amount of proceeds, investors could be in a position where they have invested in us, but we have not raised sufficient proceeds in this offering to adequately fund the intended uses of the proceeds as described in this prospectus. We will bear all costs associated with the offering. See “Plan of Distribution” on page 24 of this prospectus for more information regarding these arrangements. This offering will terminate no later than October 15, 2024, unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that date.
Our common stock is listed on the Nasdaq Capital Market under the symbol “APVO.” On September 16, 2024, the last reported sale price of our common stock on the Nasdaq Capital Market was $0.33 per share. All share, common warrant and pre-funded warrant numbers are based on a combined offering price of $0.33 per share or pre-funded warrant, as applicable, and common warrants.
You should read this prospectus, together with additional information described under the headings “Incorporation of Certain Information By Reference” and “Where You Can Find More Information,” carefully before you invest in any of our securities.
Investing in our securities involves a high degree of risk. See the section entitled “Risk Factors” beginning on page 7 of this prospectus and in the documents incorporated by reference into this prospectus for a discussion of risks that should be considered in connection with an investment in our securities.
|
|
Per Share and Common Warrant |
|
|
Per Pre-Funded Warrant and Common Warrant |
|
|
Total |
|
|||
Offering price |
|
$ |
0.3300 |
|
|
$ |
0.3299 |
|
|
$ |
2,999,493.21 |
|
Placement Agent fees(1) |
|
$ |
0.0231 |
|
|
$ |
0.0231 |
|
|
$ |
209,964.52 |
|
Proceeds to us, before expenses(2) |
|
$ |
0.3069 |
|
|
$ |
0.3068 |
|
|
$ |
2,789,528.68 |
|
________________________
(1)
We have agreed to pay the placement agent a cash placement commission equal to 7% of the aggregate proceeds from this offering subject to a partial adjustment in the event certain investors participate. We have also agreed to reimburse the Placement Agent for certain expenses incurred in connection with this offering. See “Plan of Distribution” for additional information about the compensation payable to the placement agent.
(2)
Because there is no minimum number of securities or amount of proceeds required as a condition to closing in this offering, the actual offering amount, placement agent fees, and proceeds to us, if any, are not presently determinable and may be substantially less than the total maximum offering amounts set forth above. We estimate the total expenses of this offering payable by us, excluding the placement agent fee, will be approximately $265,000.
The delivery of the shares of common stock and any pre-funded warrants and common warrants to purchasers is expected to be made no later than October 15, 2024.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Roth Capital Partners
The date of this prospectus is September 16, 2024.
TABLE OF CONTENTS
|
Page |
i |
|
ii |
|
1 |
|
5 |
|
7 |
|
11 |
|
12 |
|
13 |
|
14 |
|
20 |
|
24 |
|
27 |
|
27 |
|
28 |
|
28 |
ABOUT THIS PROSPECTUS
We incorporate by reference important information into this prospectus. You may obtain the information incorporated by reference without charge by following the instructions under “Where You Can Find More Information.” You should carefully read this prospectus as well as additional information described under “Incorporation of Certain Information by Reference,” before deciding to invest in our securities.
We have not, and the placement agent has not, authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses prepared by or on behalf of us or to which we have referred you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the securities offered hereby, and only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus or in any applicable free writing prospectus is current only as of its date, regardless of its time of delivery or any sale of our securities. Our business, financial condition, results of operations and prospects may have changed since that date.
The information incorporated by reference or provided in this prospectus contains statistical data and estimates, including those relating to market size and competitive position of the markets in which we participate, that we obtained from our own internal estimates and research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Industry publications, studies and surveys generally state that they have been obtained from sources believed to be reliable. While we believe our internal company research is reliable and the definitions of our market and industry are appropriate, neither this research nor these definitions have been verified by any independent source.
For investors outside the United States: We have not, and the placement agent has not, done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities and the distribution of this prospectus outside the United States.
This prospectus and the information incorporated by reference into this prospectus contain references to our trademarks and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus and the information incorporated by reference into this prospectus, including logos, artwork, and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other company.
i
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, and any documents we incorporate by reference, contain certain forward-looking statements that involve substantial risks and uncertainties. All statements contained in this prospectus and any documents we incorporate by reference, other than statements of historical facts, are forward-looking statements including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
The words “anticipate”, “believe”, “estimate”, “expect”, “intend”, “may”, “plan”, “predict”, “project”, “target”, “potential”, “will”, “would”, “could”, “should”, “continue” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among other things, statements about:
•
our ability to continue as a going concern;
•
our failure to maintain compliance with the Nasdaq Capital Market's ("Nasdaq") continued listing requirements could result in the delisting of our common stock;
•
our plans to develop and commercialize our drug candidates;
•
our ability to become profitable;
•
our estimates regarding expenses, future revenue, capital requirements and needs for additional financing;
•
our ability to maintain and establish collaborations or obtain additional funding;
•
our ability to obtain regulatory approval of current and future drug candidates;
•
our expectations regarding our ability to fund operating expenses and capital expenditure requirements with our existing cash and cash equivalents, and future expenses and expenditures;
•
our ability to secure sufficient funding and alternative source of funding to support when needed and on terms favorable to us to support our business objective, product development, other operations or commercialization efforts;
•
the success of our clinical development activities, clinical trials and research and development programs;
•
our ability to retain key employees, consultants and advisors;
•
our ability to obtain, maintain, protect and enforce sufficient intellectual property rights for our candidates and technology;
•
our anticipated strategies and our ability to manage our business operations effectively;
•
the impact of legislative, regulatory or policy changes;
•
the possibility that we may be adversely impacted by other economic, business, and/or competitive factors; and
•
other risks and uncertainties, including those listed in the "Risk Factors" section of this prospectus and the documents incorporated by reference herein.
These forward-looking statements are only predictions and we may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, so you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our business, financial condition and operating results. We have included important factors in the cautionary statements included in this prospectus that could cause actual future results or events to differ materially from the forward-looking statements
ii
that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
You should read this prospectus with the understanding that our actual future results may be materially different from what we expect. We do not assume any obligation to update any forward-looking statements whether as a result of new information, future events or otherwise, except as required by applicable law.
iii
PROSPECTUS SUMMARY
This summary highlights information contained elsewhere in this prospectus. This summary does not contain all of the information that you should consider before deciding to invest in our securities. You should read this entire prospectus carefully, including the “Risk Factors” section in this prospectus and under similar captions in the documents incorporated by reference into this prospectus. In this prospectus, unless otherwise stated or the context otherwise requires, references to the terms “APVO,” “the Company,” “we,” “us” and “our” refer to Aptevo Therapeutics Inc., together with its subsidiaries, unless the context otherwise requires. This prospectus and the information incorporated herein by reference include trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated by reference into this prospectus and the information incorporated herein by reference are the property of their respective owners.
Business Overview
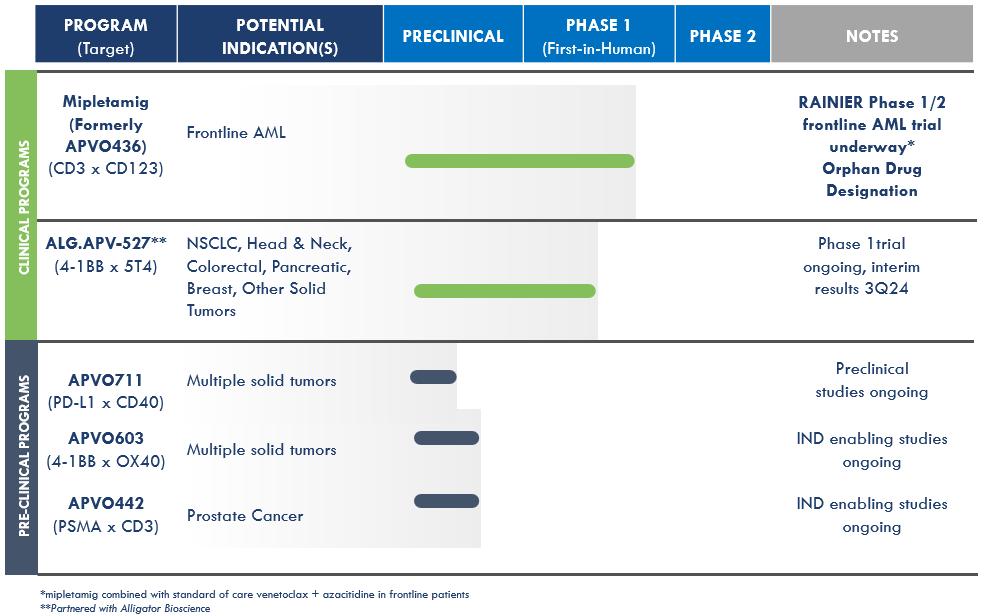
We are a clinical-stage, research and development biotechnology company focused on developing novel immunotherapy candidates for the treatment of different forms of cancer. We have developed two versatile and enabling platform technologies for rational design of precision immune modulatory drugs and have two clinical candidates and three preclinical candidates currently in development. Clinical candidate mipletamig (formerly APVO436) is a CD3xCD123 T-cell engager currently being clinically evaluated in the RAINIER trail, part one of a Phase 1b/2 program initiated in August 2024 for the treatment of acute myelogenous leukemia (AML). Clinical candidate ALG.APV-527 targets 4-1BB (co-stimulatory receptor) and 5T4 (tumor antigen). The compound is designed to reactivate antigen-primed T-cells to specifically kill tumor cells and is currently being evaluated for the treatment of multiple solid tumor types.
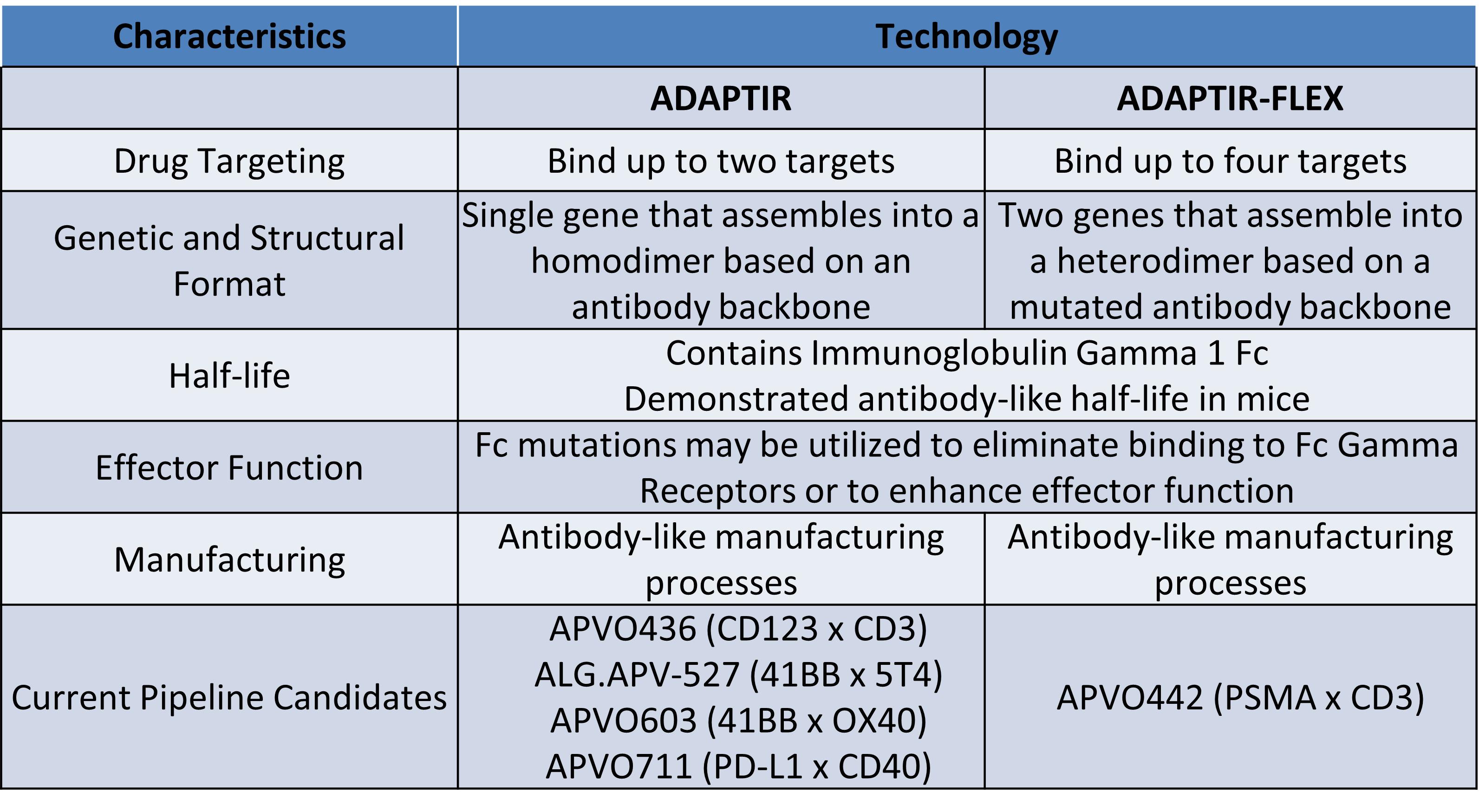
Preclinical candidates, APVO603 and APVO711, were also developed using our ADAPTIR™ modular protein technology platform. Our preclinical candidate APVO442 was developed using our ADAPTIR-FLEX™ modular protein technology platform.
Our ADAPTIR and ADAPTIR-FLEX platforms are designed to generate monospecific, bispecific, and multi-specific antibody candidates capable of enhancing the human immune system against cancer cells. ADAPTIR and ADAPTIR-FLEX are both modular platforms, which gives us the flexibility to potentially generate immunotherapeutic candidates with a variety of mechanisms of action. This flexibility in design allows us to generate novel therapeutic candidates that may provide effective strategies against difficult to treat, as well as advanced forms of cancer. We have successfully designed and constructed numerous investigational-stage product candidates based on our ADAPTIR platform. The ADAPTIR platform technology is designed to generate monospecific and bispecific immunotherapeutic proteins that specifically bind to one or more targets, for example, bispecific therapeutic molecules, which may have structural and functional advantages over monoclonal antibodies. The structural differences of ADAPTIR molecules over monoclonal antibodies allow for the development of ADAPTIR immunotherapies that are designed to engage immune effector cells and disease targets to produce signaling responses that modulate the immune system to kill tumor cells.
We believe we are skilled at candidate generation, validation, and subsequent preclinical and clinical development using the ADAPTIR platform and the ADAPTIR-FLEX platform to generate bispecific and multi-specific candidates or other candidates to our platform capabilities. We have developed a preclinical candidate based on the ADAPTIR-FLEX platform which is advancing in our pipeline. We are developing our ADAPTIR and ADAPTIR-FLEX molecules using our protein engineering, preclinical development, process development, and clinical development capabilities.
Our Strategy
We seek to grow our business by, among other things:
Advancing our lead clinical blood cancer candidate, mipletamig, through clinical development to evaluate its therapeutic potential alone and in combination with other therapies. Based on the positive results from our Phase 1 dose escalation and Phase 1b dose expansion studies, we initiated RANIER in August 2024 as part of our ongoing program to evaluate mipletamig in combination with venetoclax + azacitidine for frontline patients with acute myeloid leukemia (AML). RAINIER will be conducted in two parts, first with a Phase 1b frontline AML study followed by a Phase 2 study.
1
Advancing our lead solid tumor candidate, ALG.APV-527, developed in partnership with Alligator Bioscience AB (Alligator), further in the clinic. Aptevo and Alligator continue to investigate ALG.APV-527 for the treatment of multiple solid tumor types with 5T4-tumor expressing antigens. This drug candidate is in an ongoing first-in-human Phase I clinical trial that started in the first quarter of 2023. ALG.APV-527 targets the 4-1BB co-stimulatory receptor (on T lymphocytes and NK cells) and 5T4 (solid tumor antigen) and is designed to promote anti-tumor immunity. Aptevo believes this compound has the potential to be clinically important because 4-1BB can stimulate the immune cells (tumor-specific T-cells and NK cells) involved in tumor control, making 4-1BB a particularly compelling target for cancer immunotherapy.
Continued development and advancement of our preclinical candidates, APVO603 (targeting 4-1BB (CD137) and OX40 (CD134), both members of the TNF-receptor family), APVO442 (targeting Prostate Specific Membrane Antigen (PSMA), a tumor antigen that is highly expressed on prostate cancer cells and CD3), and APVO711 (an anti-PD-L1 x anti-CD40 compound). We continue to advance APVO711, APVO603 and APVO442 through preclinical and IND-enabling studies. In January 2023, we filed a provisional patent with the U.S. Patent and Trademark Office (USPTO) pertaining to APVO711. In January 2024, the provisional patent was amended to include new preclinical data and a patent application under the Patent Cooperation Treaty ("PCT") was filed pertaining to APVO711, which has the potential to treat a range of solid malignancies such as head and neck cancer. APVO711 is a dual mechanism bispecific antibody candidate that is designed to provide synergistic stimulation of CD40 on antigen presenting cells while simultaneously blocking the PD-1/PD-L1 inhibitory pathway to potentially promote a robust anti-tumor response. Preclinical studies are planned to further evaluate the mechanism of action and efficacy of APVO711.
Development of novel bispecific and multi-specific proteins for the treatment of cancer using our ADAPTIR and ADAPTIR-FLEX platforms. We have expertise in molecular and cellular biology, immunology, oncology, pharmacology, translational sciences, antibody engineering and the development of protein therapeutics. This includes target validation, preclinical proof of concept, cell line development, protein purification, bioassay and process development and analytical characterization. We focus on product development using our ADAPTIR and ADAPTIR-FLEX platforms. We plan to generate additional monospecific, bispecific, and multi-specific protein immunotherapies for development, potentially with other collaborative partners, to exploit the potential of the ADAPTIR and ADAPTIR-FLEX platforms. We will select novel candidates that have the potential to demonstrate proof of concept early in development. We expect to continue to expand the ADAPTIR and ADAPTIR-FLEX product pipelines to address areas of unmet medical need. Bispecific therapeutics are increasingly recognized as potent anti-cancer agents. Eleven new bispecific agents have been approved for use by the FDA in the last three years and there is a total of 125 bispecific drug candidates currently in development. We believe our candidates in development and our future molecules derived from our ADAPTIR and ADAPTIR-FLEX platforms will be highly competitive in the market as they are rationally designed for safety and tolerability as well as efficacy.
Establishing collaborative partnerships to broaden our pipeline and provide funding for research and development. We intend to pursue collaborations with other biotechnology and pharmaceutical companies, academia, and non-governmental organizations to advance our product portfolio.
2
Product Candidates and Platform Technology
Product Portfolio
Our current product candidate pipeline is summarized in the table below:
Platform Technologies
3
Recent Developments
Warrant Re-Price
In connection with this offering, we also agreed to amend certain existing warrants that were previously issued on (i) November 9, 2023 to purchase up to 362,900 shares of our common stock and have an exercise price of $0.515 per share, (ii) April 15, 2024 to purchase up to 4,444,446 shares of our common stock and have an exercise price of $0.515 per share and (iii) July 1, 2024 to purchase up to 7,015,428 shares of our common stock and have an exercise price of $0.515 per share (collectively, the “Existing Warrants”), such that effective upon the closing of this offering, the Existing Warrants will be amended to have a reduced exercise price equal to $0.33 per share and include the same exercise price adjustments as the common warrants issued in connection with this offering, as described more fully under the heading “Description of Securities we are Offering” beginning on page 20 of this prospectus. The exercise of the repriced Existing Warrants will be subject to the Stockholder Approval along with the common warrants issued in this offering.
Phase 1 ALG.APV-527 Monotherapy Trial Data
On September 16, 2024, we and Alligator Bioscience AB announced positive interim data from the dose escalation phase of the Phase 1 trial evaluating ALG.APV-527 for the treatment of solid tumors likely to express the tumor antigen 5T4. The results, which include clinical activity, safety, tolerability outcomes, pharmacokinetics and pharmacodynamics were presented in a poster session on Saturday, September 14, 2024, at the European Society for Medical Oncology Annual Congress in Barcelona, Spain.
Smaller Reporting Company
Additionally, we are a “smaller reporting company” as defined in Rule 10(f)(1) of Regulation S-K. To the extent we qualify as a smaller reporting company, we may continue to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not smaller reporting companies, including, among other things, providing only two years of audited financial statements and we are also permitted to elect to incorporate by reference information filed after the effective date of the S-1 registration statement of which this prospectus forms a part. We will remain a smaller reporting company until the last day of the fiscal year in which (1) the market value of our shares of Common Stock held by non-affiliates exceeds $250 million as of the prior June 30, or (2) our annual revenues exceeded $100 million during such completed fiscal year and the market value of our shares of Common Stock held by non-affiliates exceeds $700 million as of the prior June 30.
Corporate Information
On August 6, 2015, Emergent BioSolutions Inc. (“Emergent”), announced a plan to separate into two independent publicly traded companies. To accomplish this separation, Emergent created Aptevo Therapeutics Inc. (“Aptevo”), to be the parent company for the development-based biotechnology business focused on novel oncology and hematology therapeutics. Aptevo was incorporated in Delaware in February 2016 as a wholly owned subsidiary of Emergent. To effect the separation, Emergent made a pro rata distribution of Aptevo’s Common Stock to Emergent’s stockholders on August 1, 2016.
Our Common Stock currently trades on the Nasdaq under the symbol “APVO.” Our primary executive offices are located at 2401 4th Avenue, Suite 1050, Seattle, Washington and our telephone number is (206) 838-0500. Our website address is www.aptevotherapeutics.com. The information contained in, or that can be accessed through, our website is not a part of or incorporated by reference in this prospectus, and you should not consider it part of this prospectus or of any prospectus supplement. We have included our website address in this prospectus solely as an inactive textual reference.
4
THE OFFERING
Common Stock to be Offered |
|
4,020,000 shares.
|
Pre-funded Warrants to be Offered |
|
We are also offering to certain purchasers whose purchase of shares of common stock in this offering would otherwise result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock immediately following the consummation of this offering, the opportunity to purchase, if such purchasers so choose, up to 5,070,910 pre-funded warrants to purchase shares of common stock, in lieu of shares of common stock that would otherwise result in any such purchaser’s beneficial ownership exceeding 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding common stock. Each pre-funded warrant will be exercisable for one share of our common stock. The purchase price of each pre-funded warrant and accompanying common warrants will equal the price at which the share of common stock and accompanying common warrants are being sold in this offering, minus $0.0001, and the exercise price of each pre-funded warrant will be $0.0001 per share. The pre-funded warrants will be exercisable immediately and may be exercised at any time until all of the pre-funded warrants are exercised in full. This offering also relates to the shares of common stock issuable upon exercise of any pre-funded warrants sold in this offering. For each pre-funded warrant we sell, the number of shares of common stock we are offering will be decreased on a one-for-one basis. Because we will issue two common warrants, each to purchase one share of common stock for each share of our common stock and for each pre-funded warrant to purchase one share of our common stock sold in this offering, the number of common warrants sold in this offering will not change as a result of a change in the mix of the shares of our common stock and pre-funded warrants sold.
|
Common Warrants to be Offered |
|
Common warrants to purchase up to 18,181,820 shares of our common stock. Each common warrant has an exercise price of $0.33 per share of common stock. Common warrants will become exercisable beginning on the date of the Stockholder Approval and will expire five years from the date of Stockholder Approval.
The shares of common stock and pre-funded warrants, and the accompanying common warrant, as the case may be, can only be purchased together in this offering but will be issued separately and will be immediately separable upon issuance. This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the common warrants.
|
Common Stock Outstanding Before this Offering
|
|
9,421,174 shares. |
Common Stock to be Outstanding Immediately After this Offering
|
|
18,512,084 shares, (assuming full exercise of the pre-funded warrants and assuming no exercise of the common warrants). |
Use of Proceeds |
|
We estimate that the net proceeds from this offering will be approximately $2.5 million, excluding the proceeds, if any, from the cash exercise of the common warrants in this offering. We currently intend to use the net proceeds from this offering for working capital and general corporate purposes, including the further development of our product candidates. See “Use of Proceeds” for additional information.
|
Risk Factors |
|
An investment in our securities involves a high degree of risk. See “Risk Factors” beginning on page 7 of this prospectus and the other information included and incorporated by reference in |
5
|
|
this prospectus for a discussion of the risk factors you should carefully consider before deciding to invest in our securities.
|
Nasdaq Symbol |
|
Our common stock is listed on the Nasdaq Capital Market under the symbol “APVO.” There is no established public trading market for the pre-funded warrants or common warrants, and we do not expect a market to develop. In addition, we do not intend to apply to list the pre-funded warrants or common warrants on any national securities exchange or other nationally recognized trading system. Without an active trading market, the liquidity of the pre-funded warrants and common warrants will be limited. |
The above discussion is based on 9,421,174 shares of our common stock outstanding as of September 16, 2024, assumes no sale of pre-funded warrants and excludes, as of that date, the following:
•
9,624 shares of Common Stock issuable upon the exercise of stock options outstanding at a weighted average exercise price of $557.31 per share;
•
69,458 shares of Common Stock issuable upon the vesting of outstanding restricted stock units at a weighted average fair value per unit of $6.68 per share;
•
101,766 shares of Common Stock reserved for future grants of equity-based awards under our equity incentive plans;
•
1,316 and 1,316 shares of Common Stock issuable upon the exercise of Series A and Series B common warrants, respectively, at an exercise price of $27.28 per share;
•
8,762 and 8,762 shares of Common Stock issuable upon the exercise of Series A-1 and Series A-2 common warrants, respectively, at an exercise price of $10.25;
•
8,762 and 8,762 shares of Common Stock issuable upon the exercise of Series B-1 and Series B-2 common warrants, respectively, at an exercise price of $10.25;
•
133,332 shares of Common Stock issuable upon the exercise of common warrants at an exercise price of $1.35; and
•
17,997,853 shares of Common Stock issuable upon the exercise of common warrants at an exercise price of $0.515.
6
RISK FACTORS
An investment in our securities involves a high degree of risk. Before deciding whether to purchase our securities, including the shares of common stock offered by this prospectus, you should carefully consider the risks and uncertainties described under “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, any subsequent Quarterly Report on Form 10-Q and our other filings with the SEC, all of which are incorporated by reference herein. If any of these risks actually occur, our business, financial condition and results of operations could be materially and adversely affected and we may not be able to achieve our goals, the value of our securities could decline and you could lose some or all of your investment. Additional risks not presently known to us or that we currently believe are immaterial may also significantly impair our business operations. If any of these risks occur, our business, results of operations or financial condition and prospects could be harmed. In that event, the market price of our common stock and the value of the warrants could decline, and you could lose all or part of your investment.
Risks Related to This Offering
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds, including for any of the purposes described in the section of this prospectus entitled “Use of Proceeds.” You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the net proceeds are being used appropriately. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our securities to decline and delay the development of our product candidates. Pending the application of these funds, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
You will experience immediate and substantial dilution in the net tangible book value per share of the common stock you purchase. You may also experience future dilution as a result of future equity offerings.
The price per share, together with the number of shares of our common stock we propose to issue and ultimately will issue if this offering is completed, may result in an immediate decrease in the market price of our common stock. Our historical net tangible book value as of July 31, 2024 was $2.5 million, or approximately $0.26 per share of our common stock. After giving effect to the 9,090,910 shares of our common stock or the exercise of the pre-funded warrants to be sold in this offering at a combined offering price of $0.33 per share and common warrants, our as adjusted net tangible book value as of July 31, 2024 would have been $5.0 million, or approximately $0.27 per share of our common stock. This represents an immediate increase in the net tangible book value of $0.01 per share of our common stock to our existing stockholders and an immediate dilution in net tangible book value of approximately $0.06 per share of our common stock to new investors, representing the difference between the combined offering price and our as adjusted net tangible book value as of July 31, 2024, after giving effect to this offering, and the combined offering price per share and common warrants.
In addition, in order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share in this offering. In the event that the outstanding options or warrants are exercised or settled, or that we make additional issuances of common stock or other convertible or exchangeable securities, you could experience additional dilution. We cannot assure you that we will be able to sell shares or other securities in any other offering at a price per share that is equal to or greater than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders, including investors who purchase shares of common stock in this offering. The price per share at which we sell additional shares of our common stock or securities convertible into common stock in future transactions, may be higher or lower than the price per share in this offering. As a result, purchasers of the shares we sell, as well as our existing stockholders, will experience significant dilution if we sell at prices significantly below the price at which they invested.
7
There is no public market for the common warrants or pre-funded warrants being offered by us in this offering.
There is no established public trading market for the common warrants or the pre-funded warrants, and we do not expect a market to develop. In addition, we do not intend to apply to list the common warrants or pre-funded warrants on any national securities exchange or other nationally recognized trading system. Without an active market, the liquidity of the common warrants and pre-funded warrants will be limited.
The common warrants are not exercisable until Stockholder Approval and may not have any value.
Under Nasdaq listing rules, the common warrants are not exercisable without Stockholder Approval for the issuance of shares issuable upon exercise of the common warrants. While we intend to use reasonable best efforts to seek Stockholder Approval for issuances of shares of common stock issuable upon exercise of the common warrants, there is no guarantee that the Stockholder Approval will ever be obtained. The common warrants will be exercisable commencing on the date Stockholder Approval is obtained, if at all, at an initial exercise price per share of $0.33. In the event that the price of a share of our common stock does not exceed the exercise price of the common warrants during the period when the common warrants are exercisable, the common warrants may not have any value. If we are unable to obtain the Stockholder Approval, the common warrants will not be exercisable and therefore would have no value.
In addition, we may incur substantial cost, and management may devote substantial time and attention, in attempting to obtain Stockholder Approval of the issuance of shares of common stock upon exercise of the common warrants issued in this offering.
The common warrants and pre-funded warrants are speculative in nature.
The common warrants and pre-funded warrants offered hereby do not confer any rights of share of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at a fixed price. Specifically, only following receipt of the Stockholder Approval, holders of the common warrants may acquire the shares of common stock issuable upon exercise of such warrants at an exercise price of $0.33 per share of common stock, and holders of the pre-funded warrants may acquire the shares of common stock issuable upon exercise of such warrants at an exercise price of $0.0001 per share of common stock. Moreover, following this offering, the market value of the common warrants and pre-funded warrants is uncertain and there can be no assurance that the market value of the common warrants or pre-funded warrants will equal or exceed their respective offering prices. There can be no assurance that the market price of the shares of common stock will ever equal or exceed the exercise price of the common warrants or pre-funded warrants, and consequently, whether it will ever be profitable for holders of common warrants to exercise the common warrants or for holders of the pre-funded warrants to exercise the pre-funded warrants.
Holders of the warrants offered hereby will have no rights as common stockholders with respect to the shares of our common stock underlying the warrants until such holders exercise their warrants and acquire our common stock, except as otherwise provided in the warrants.
Until holders of the common warrants and the pre-funded warrants acquire shares of our common stock upon exercise thereof, such holders will have no rights with respect to the shares of our common stock underlying such warrants, except to the extent that holders of such warrants will have certain rights to participate in distributions or dividends paid on our common stock as set forth in the warrants. Upon exercise of the common warrants and the pre-funded warrants, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
This is a reasonable best efforts offering, no minimum amount of securities is required to be sold, and we may not raise the amount of capital we believe is required for our business plans, including our near-term business plans.
The placement agent has agreed to use its reasonable best efforts to solicit offers to purchase the securities in this offering. The placement agent has no obligation to buy any of the securities from us or to arrange for the purchase or sale of any specific number or dollar amount of the securities. There is no required minimum number of securities that must be sold as a condition to completion of this offering. Because there is no minimum offering amount required as a condition to the closing of this offering, the actual offering amount, placement agent fees and proceeds to us are not presently determinable and may be substantially less than the maximum amounts set forth above. We may sell fewer than all of the securities offered hereby, which may significantly reduce the amount of proceeds received by us,
8
and investors in this offering will not receive a refund in the event that we do not sell an amount of securities sufficient to support our continued operations, including our near-term continued operations. Thus, we may not raise the amount of capital we believe is required for our operations in the short-term and may need to raise additional funds, which may not be available or available on terms acceptable to us.
Purchasers who purchase our securities in this offering pursuant to a securities purchase agreement may have rights not available to purchasers that purchase without the benefit of a securities purchase agreement.
In addition to rights and remedies available to all purchasers in this offering under federal securities and state law, the purchasers that enter into a securities purchase agreement will also be able to bring claims of breach of contract against us. The ability to pursue a claim for breach of contract provides those investors with the means to enforce the covenants uniquely available to them under the securities purchase agreement including, but not limited to: (i) timely delivery of securities; (ii) agreement to not enter into any financings for 60 days from closing; and (iii) indemnification for breach of contract.
Resales of our common stock in the public market during this offering by our stockholders may cause the market price of our common stock to fall.
Sales of a substantial number of shares of our common stock could occur at any time. The issuance of new shares of our common stock could result in resales of our common stock by our current stockholders concerned about the potential ownership dilution of their holdings. In turn, these resales could have the effect of depressing the market price for our common stock.
Our common stock may be at risk for delisting from the Nasdaq Capital Market in the future if we do not maintain compliance with Nasdaq’s continued listing requirements. Delisting could adversely affect the liquidity of our common stock and the market price of our common stock could decrease.
Our common stock is currently listed on Nasdaq. Nasdaq has minimum requirements that a company must meet in order to remain listed on Nasdaq, including corporate governance standards and a requirement that we maintain a minimum closing bid price of $1.00 per share.
On June 25, 2024, the Company received a letter from Nasdaq notifying the Company that, for the last 30 consecutive business days, the bid price of the Company’s common stock had closed below $1.00 per share, the minimum closing bid price required by the continued listing requirements of Nasdaq Listing Rule 5550(a)(2) (the “Bid Price Requirement”).
Nasdaq’s letter has no immediate impact on the listing of the Company’s common stock, which will continue to be listed and traded on Nasdaq, subject to the Company’s compliance with the other continued listing requirements. Nasdaq’s letter provides the Company 180 calendar days, or until December 23, 2024 (the "Compliance Date"), to regain compliance with the Bid Price Requirement. To regain compliance with the Bid Price Requirement, the closing bid price of the Company’s common stock must be at least $1.00 per share for a minimum of ten consecutive business days before the Compliance Date. The Company may be eligible for an additional 180-day period to regain compliance if the Company meets all other listing standards of Nasdaq, with the exception of the bid price requirement, and provides written notice to Nasdaq of its intention to cure the deficiency during the second compliance period, by effecting a reverse stock split, if necessary. However, Nasdaq Listing Rule 5810(c)(3)(A)(iv) states that any listed company that fails to meet the Bid-Price Requirement after effecting one or more reverse stock splits over the prior two-year period with a cumulative ratio of 250 shares or more to one, then the company is not eligible for a compliance period to regain compliance and would be subject to delisting by Nasdaq. If during any compliance period, a company's security has a closing bid price of $0.10 or less for ten consecutive trading days, the Company's security would be subject to delisting immediately. In addition, under proposed Nasdaq rules, if the price of the Company's common stock fails to satisfy the Bid Price Requirement within one year of the Company's previous 1-for-44 reverse stock split effectuated on March 5, 2024, then the Company's common stock would be subject to delisting by Nasdaq without any opportunity for a cure period. In the event the Company fails to regain compliance, the Company would have the right to a hearing before the Nasdaq Listing Qualifications Panel (the "Panel"). There can be no assurance that, if the Company receives a delisting notice and appeals the delisting determination by the Panel, such appeal would be successful.
9
The Company intends to take all reasonable measures available to regain compliance under the Nasdaq Listing Rules and remain listed on Nasdaq, including by effecting a reverse stock split.
In the future, if we fail to maintain such minimum requirements and a final determination is made by Nasdaq that our common stock must be delisted, the liquidity of our common stock would be adversely affected and the market price of our common stock could decrease. In addition, if delisted, we would no longer be subject to Nasdaq rules, including rules requiring us to have a certain number of independent directors and to meet other corporate governance standards. Our failure to be listed on Nasdaq or another established securities market would have a material adverse effect on the value of your investment in us.
If our common stock is not listed on Nasdaq or another national exchange, the trading price of our common stock is below $5.00 per share and we have net tangible assets of $6,000,000 or less, the open-market trading of our common stock will be subject to the “penny stock” rules promulgated under the Securities Exchange Act of 1934, as amended. If our shares become subject to the “penny stock” rules, broker-dealers may find it difficult to effectuate customer transactions and trading activity in our securities may be adversely affected.
This offering may cause the trading price of our common stock to decrease.
The price per share, together with the number of shares of common stock we propose to issue and ultimately will issue if this offering is completed, may result in an immediate decrease in the market price of our common stock. This decrease may continue after the completion of this offering.
10
USE OF PROCEEDS
We estimate that the net proceeds from the offering will be approximately $2.5 million, after deducting the placement agent fees and estimated offering expenses payable by us, assuming no sale of any fixed combinations of pre-funded warrants and warrants offered hereunder. We may only receive additional proceeds from the exercise of the common warrants issuable in connection with this offering if the Stockholder Approval is obtained and, if such common warrants are exercised in full for cash, the estimated net proceeds will increase to $5.5 million.
We currently intend to use the net proceeds from this offering for working capital to fund our clinical programs and general corporate purposes, including the further development of our product candidates. This expected use of proceeds from this offering represents our intentions based upon our current plans and prevailing business conditions, which could change in the future as our plans and prevailing business conditions evolve. The amounts and timing of our use of proceeds will vary depending on a number of factors, including the amount of cash generated or used by our operations. As a result, we will retain broad discretion in the allocation of the net proceeds of this offering.
11
CAPITALIZATION
The following table presents a summary of our cash and cash equivalents and capitalization as of July 31, 2024:
•
on an actual basis; and
•
on an as adjusted basis to reflect the issuance and sale of common stock and accompanying common warrants in this offering, after deducting placement agent fees and estimated offering expenses payable by us. The as adjusted basis assumes no Pre-Funded warrants are sold in this offering and excludes the proceeds, if any, from the exercise of any Common Warrants issued in this offering.
The unaudited as adjusted information below is prepared for illustrative purposes only and our capitalization following the completion of this offering will be adjusted based on the actual offering price and other terms of this offering determined at pricing. You should read the following table in conjunction with Management’s Discussion and Analysis of Financial Condition and Results of Operations and the historical financial statements and related notes in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2023 and our Quarterly Report on Form 10-Q for the period ended June 30, 2024, incorporated herein by reference.
|
|
As of July 31, 2024 |
|
|||||
(in thousands) |
|
Actual |
|
|
As adjusted |
|
||
Cash and cash equivalents |
|
$ |
7,554 |
|
|
$ |
10,079 |
|
Common stock: $0.001 par value; 500,000,000 shares authorized; 9,420,489 shares issued and outstanding, actual; 18,511,399 shares issued and outstanding, pro forma as adjusted |
|
|
74 |
|
|
|
83 |
|
Additional paid-in capital |
|
|
242,905 |
|
|
|
245,421 |
|
Accumulated deficit |
|
|
(237,971 |
) |
|
|
(237,971 |
) |
Total stockholders' equity |
|
$ |
5,008 |
|
|
$ |
7,533 |
|
The number of shares of our Common Stock outstanding before and after this offering is based on 9,420,489 shares of our common stock outstanding as of July 31, 2024, assumes no sale of pre-funded warrants and excludes, as of that date, the following:
•
9,656 shares of Common Stock issuable upon the exercise of stock options outstanding at a weighted average exercise price of $559.05 per share;
•
70,205 shares of Common Stock issuable upon the vesting of outstanding restricted stock units at a weighted average fair value per unit of $8.51 per share;
•
101,686 shares of Common Stock reserved for future grants of equity-based awards under our equity incentive plans;
•
1,316 and 1,316 shares of Common Stock issuable upon the exercise of Series A and Series B common warrants, respectively, at an exercise price of $27.28 per share;
•
8,762 and 8,762 shares of Common Stock issuable upon the exercise of Series A-1 and Series A-2 common warrants, respectively, at an exercise price of $10.25;
•
8,762 and 8,762 shares of Common Stock issuable upon the exercise of Series B-1 and Series B-2 common warrants, respectively, at an exercise price of $10.25;
•
133,332 shares of Common Stock issuable upon the exercise of common warrants at an exercise price of $1.35; and
•
17,997,853 shares of Common Stock issuable upon the exercise of common warrants at an exercise price of $0.515.
12
DILUTION
If you purchase shares of our common stock, your interest will be diluted immediately to the extent of the difference between the offering price per share you will pay in this offering and the as adjusted net tangible book value per share of our common stock after this offering. Net tangible book value per share represents our total tangible assets less total liabilities, divided by the number of shares of our common stock outstanding.
As of July 31, 2024, our net tangible book value was $2.5 million, or $0.26 per share of Common Stock.
After giving effect to the foregoing pro forma adjustments and the sale by us of 9,090,910 shares of common stock (or pre-funded warrants in lieu of) and accompanying common warrants at a combined offering price of $0.33 per share and $0.0001 per pre-funded warrant and accompanying common warrants, and after deducting the placement agent fees and estimated offering expenses payable by us, our as adjusted net tangible book value as of July 31, 2024, would have been $5.0 million, or $0.27 per share. This represents an immediate increase in as adjusted net tangible book value of approximately $0.01 per share to our existing stockholders, and an immediate dilution of $0.06 per share to purchasers of shares in this offering, as illustrated in the following table:
Combined offering price per share and common warrants |
|
$ |
0.33 |
|
Net tangible book value per share as of July 31, 2024 |
|
$ |
0.26 |
|
Net increase in net tangible book value per share attributable to existing shareholders |
|
$ |
0.01 |
|
As adjusted net tangible book value per share after this offering |
|
$ |
0.27 |
|
Dilution in net tangible book value per share to new investors in the offering |
|
$ |
(0.06 |
) |
The above discussion is based on 9,420,489 shares of our common stock outstanding as of July 31, 2024, assumes no sale of pre-funded warrants and excludes, as of that date, the following:
•
9,656 shares of Common Stock issuable upon the exercise of stock options outstanding at a weighted average exercise price of $559.05 per share;
•
70,205 shares of Common Stock issuable upon the vesting of outstanding restricted stock units at a weighted average fair value per unit of $8.51 per share;
•
101,686 shares of Common Stock reserved for future grants of equity-based awards under our equity incentive plans;
•
1,316 and 1,316 shares of Common Stock issuable upon the exercise of Series A and Series B common warrants, respectively, at an exercise price of $27.28 per share;
•
8,762 and 8,762 shares of Common Stock issuable upon the exercise of Series A-1 and Series A-2 common warrants, respectively, at an exercise price of $10.25;
•
8,762 and 8,762 shares of Common Stock issuable upon the exercise of Series B-1 and Series B-2 common warrants, respectively, at an exercise price of $10.25;
•
133,332 shares of Common Stock issuable upon the exercise of common warrants at an exercise price of $1.35; and
•
17,997,853 shares of Common Stock issuable upon the exercise of common warrants at an exercise price of $0.515.
13
DESCRIPTION OF CAPITAL STOCK
The following summary of the rights of our capital stock is not complete and is subject to and qualified in its entirety by reference to our Charter and Bylaws, copies of which are filed as exhibits to our Annual Report on Form 10-K for the year ended December 31, 2023, filed with the SEC on March 5, 2024, and the Certificate of Designations and forms of securities, copies of which are filed as exhibits to the registration statement of which this prospectus forms a part , which are incorporated by reference herein.
As of the date of this prospectus, our certificate of incorporation, authorizes us to issue up to 500,000,000 shares of Common Stock, $0.001 par value per share, and 15,000,000 shares of preferred stock, $0.001 par value per share. Our Common Stock is registered under Section 12(b) of the Exchange Act and is listed on the Nasdaq under the trading symbol “APVO.” As of September 16, 2024, 9,421,174 shares of Common Stock were outstanding and no shares of preferred stock were outstanding.
The following summary describes the material terms of our capital stock. The summary is qualified in its entirety by reference to our certificate of incorporation and our bylaws.
Authorized Shares
Our authorized Shares consists of 500,000,000 shares of Common Stock and 15,000,000 shares of preferred stock, $0.001 par value per share (the “Preferred Stock”). Our Common Stock is registered under Section 12(b) of the Exchange Act and is listed on the Nasdaq under the trading symbol “APVO.”
Common Stock
Voting Rights
Each holder of our Common Stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, including the election of directors. Under our amended and restated certificate of incorporation and amended and restated bylaws, our stockholders do not have cumulative voting rights. Because of this, the holders of a majority of the shares of Common Stock entitled to vote in any election of directors can elect all of the directors standing for election, if they should so choose.
Dividends
Subject to preferences that may be applicable to any then-outstanding shares of preferred stock, holders of Common Stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by our board of directors out of legally available funds.
Liquidation
In the event of our liquidation, dissolution or winding up, holders of Common Stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any then-outstanding shares of preferred stock.
Rights and Preferences
Each share of Common Stock includes an associated right pursuant to and as set forth in the Rights Agreement that we entered into with Broadridge Corporate Issuer Solutions, Inc. on November 8, 2020 (the “rights agreement”). Each right initially represents the right to purchase from us one one-thousandth of a share of our Series A Junior Participating Preferred Stock, par value $0.001 per share. This right is not exercisable until the occurrence of certain events specified in such rights agreement. The value attributable to these rights, if any, is reflected in the value of our Common Stock. The rights agreement and the rights granted thereunder will expire upon the earliest to occur of (i) the date on which all of such rights are redeemed, (ii) the date on which such rights are exchanged, and (iii) the close of business on November 4, 2024.
Fully Paid and Nonassessable.
All of our outstanding shares of Common Stock are fully paid and nonassessable.
14
Preferred Stock
Pursuant to our amended and restated certificate of incorporation, our board of directors has the authority, without further action by our stockholders, to designate up to 15,000,000 shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting, or the designation of, such series, any or all of which may be greater than the rights of common stock.
The Delaware General Corporation Law (“DGCL”) provides that the holders of preferred stock will have the right to vote separately as a class on any proposal involving fundamental changes in the rights of holders of that preferred stock. This right is in addition to any voting rights that may be provided for in the applicable certificate of designation.
Warrants
Series A and Series B Common Warrants 2023 - As of September 16, 2024, we have issued and outstanding 1,316 Series A and 1,316 Series B common warrants to purchase shares of our Common Stock at an exercise price of $27.28 per share and 41,239 Series A common warrants outstanding with an amended exercise price or $0.515 per share. The Series A common warrants and Series B common warrants are immediately exercisable and expire in August 2028 and February 2025, respectively. The exercise price and the number of shares of Common Stock purchasable upon the exercise of the warrants are subject to adjustment upon the occurrence of specific events, including sales of additional shares of Common Stock, stock dividends, stock splits, reclassifications and combinations of our Common Stock. If, at any time warrants are outstanding, any fundamental transaction occurs, as described in the warrants, the successor entity must assume the obligations to the warrant holders. Additionally, in the event of a fundamental transaction, the holders of the warrants will be entitled to receive upon exercise of the warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the warrants immediately prior to such fundamental transaction. Holders of the warrants do not have the rights or privileges of holders of our common shares, including any voting rights, until they exercise their warrants, with exceptions for participation in rights offerings or extraordinary distributions.
New Series A and Series B Common Warrants - November 2023 - On November 9, 2023, we entered into a warrant Inducement Agreement (“Inducement Agreement”) with certain holders of our existing Series A common warrants and Series B common warrants (together, the "Existing Warrants") to purchase shares of common stock. Pursuant to the terms of the Inducement Agreement, the holders agreed to exercise for cash their Existing Warrants to purchase up to an aggregate of 363,930 shares of common stock at an exercise price of $10.25 during the period from the date of the Inducement Agreement until December 8, 2023. In consideration of the holder’s agreement to exercise the Existing Warrants, we agreed to issue new Series A and new Series B common warrants (together, the "New Warrants"), to purchase a number of shares of common stock equal to 200% of the number of shares of common stock issued upon exercise of the Existing Warrants. Holders exercised 140,726 Existing Series A and 181,965 Existing Series B Warrants and the Company received $3.3 million in gross proceeds. As of September 16, 2024, we have 17,524 New Series A and 17,524 New Series B Common Warrants outstanding from our November 2023 warrant inducement with an exercise price of $10.25 per share and 263,928 New Series A and 346,406 New Series B common warrants with an amended exercise price of $0.515 per share. If the New Warrants are exercised, we may receive up to an additional $0.7 million in gross proceeds.
Common Warrants April 2024 - As of September 16, 2024, we have issued and outstanding common warrants to purchase 133,332 shares of our Common Stock at an exercise price of $1.35 per share issued as part of our April 2024 public offering (the "April 2024 Warrants") and 6,666,668 common warrants outstanding with an amended exercise price of $0.515 per share. The April 2024 Warrants are immediately exercisable and expire on April 10, 2029. The exercise price and the number of shares of Common Stock purchasable upon the exercise of the April 2024 Warrants are subject to adjustment upon the occurrence of specific events, including stock dividends, stock splits, reclassifications and combinations of our Common Stock. If, at any time the April 2024 Warrants are outstanding and a fundamental transaction, as described in the April 2024 Warrant, and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting
15
power represented by our outstanding common stock, occurs the holders of the April 2024 Warrants will be entitled to receive upon exercise of the April 2024 Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the April 2024 Warrants immediately prior to such fundamental transaction, other than one in which a successor entity that is a publicly traded corporation (whose stock is quoted or listed for trading on a national securities exchange, including, but not limited to, the New York Stock Exchange, the NYSE American, the Nasdaq Global Select Market, the Nasdaq Global Market or the Nasdaq Capital Market) assumes the common warrant such that the warrant shall be exercisable for the publicly traded common stock of such successor entity. Additionally, as more fully described in the common warrants, at the option of the holders of the common warrants the holder can receive consideration in the same type and form of an amount equal to the Black Scholes value (as defined in the common warrant) of such unexercised common warrants on the date of consummation of such transaction. Except as otherwise provided in the April 2024 Warrants or by virtue of the holder’s ownership of shares of our common stock, such holder of April 2024 Warrants does not have the rights or privileges of a holder of our common stock, including any voting rights, until such holder exercises such holder’s April 2024 Warrants. The April 2024 Warrants provide that the holders of the April 2024 Warrants have the right to participate in distributions or dividends paid on our shares of common stock.
Common Warrants - July 2024 - As of September 16, 2024, we have issued and outstanding common warrants to purchase up to 10,679,612 shares of our Common Stock at an exercise price of $0.515 per share issued as part of our July 2024 registered direct offering (the "July 2024 Warrants"). The July 2024 Warrants became exercisable upon approval by stockholders on August 6, 2024, and expire on August 6, 2029. The exercise price of the July 2024 Warrants is subject to adjustment for stock splits and similar capital transactions and is subject to repricing in the event of a share split, share dividend, share combination or other such event as described in the July 2024 Warrants. If, at any time the July 2024 Warrants are outstanding and a fundamental transaction, as described in the July 2024 Warrant, and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock occurs, the holders of the July 2024 Warrants will be entitled to receive upon exercise of the July 2024 Warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the July 2024 Warrants immediately prior to such fundamental transaction, other than one in which a successor entity that is a publicly traded corporation (whose stock is quoted or listed for trading on a national securities exchange, including, but not limited to, the New York Stock Exchange, the NYSE American, the Nasdaq Global Select Market, the Nasdaq Global Market or the Nasdaq Capital Market) assumes the common warrant such that the warrant shall be exercisable for the publicly traded common stock of such successor entity. Additionally, as more fully described in the common warrants, at the option of the holders of the common warrants, the holder can receive consideration in the same type and form of an amount equal to the Black Scholes value (as defined in the common warrant) of such unexercised common warrants on the date of consummation of such transaction. Except as otherwise provided in the July 2024 Warrants or by virtue of the holder’s ownership of shares of our common stock, such holder of July 2024 Warrants does not have the rights or privileges of a holder of our common stock, including any voting rights, until such holder exercises such holder’s July 2024 Warrants. The July 2024 Warrants provide that the holders of the July 2024 Warrants have the right to participate in distributions or dividends paid on our shares of common stock.
Stockholder Registration Rights
Certain holders of shares of our Common Stock are entitled to certain rights with respect to registration of such shares under the Securities Act. These shares are referred to as registrable securities. The holders of these registrable securities possess registration rights described in additional detail below pursuant to the terms of a registration rights agreement.
The registration of shares of our Common Stock pursuant to the exercise of registration rights described below would enable the holders to trade these shares without restriction under the Securities Act when the applicable registration statement is declared effective. We will pay the registration expenses, other than underwriting discounts, selling commissions and stock transfer taxes, of the shares registered pursuant to the demand and piggyback registration rights described below.
Generally, in an underwritten offering, the managing underwriter, if any, has the right, subject to specified conditions, to limit the number of shares the holders may include.
16
Demand Registration Rights
The holders of the registrable securities are entitled to certain demand registration rights. At any time, the holders of at least 20% of the registrable securities, on not more than two occasions, may request that we register all or a portion of their shares, subject to certain specified exceptions. Such request for registration must cover securities the aggregate offering price of which, before payment of underwriting discounts and commissions, exceeds $10,000,000.
Piggyback Registration Rights
In connection with the filing of the registration statement of which this prospectus forms a part, the holders of the registrable securities were entitled to, and the necessary percentage of holders waived, their rights to notice of such filing and to include their shares of registrable securities in the registration statement of which this prospectus forms a part. If we propose to register for offer and sale any of our securities under the Securities Act in a future offering, either for our own account or for the account of other security holders, the holders of these shares will be entitled to certain “piggyback” registration rights allowing them to include their shares in such registration, subject to certain marketing and other limitations. As a result, whenever we propose to file a registration statement under the Securities Act, including a registration statement on Form S-3 as discussed below, other than with respect to a demand registration or a registration statement on Forms S-4 or S-8 or related to stock issued upon conversion of debt securities, the holders of these shares are entitled to notice of the registration and have the right, subject to limitations that the underwriters may impose on the number of shares included in the registration, to include their shares in the registration.
Lincoln Park Registration Rights
On February 16, 2022, we entered into a Purchase Agreement ("2022 Purchase Agreement") and a Registration Rights Agreement with Lincoln Park (the "Registration Rights Agreement"). The 2022 Purchase Agreement and Registration Rights Agreement replaced the Purchase Agreement and Registration Rights Agreement with Lincoln Park that we entered into on December 20, 2018. Under the 2022 Purchase Agreement, Lincoln Park committed to purchase up to $35.0 million of our common stock over a 36-month period commencing after the satisfaction of certain conditions, which are within our control, as set forth in the 2022 Purchase Agreement. The purchase price per share will be based on prevailing market prices; provided, however, that the prevailing market price is not below $1.00. We agreed to and issued 2,256 shares of our common stock to Lincoln Park for no cash consideration as an initial fee for its commitment to purchase shares of our common stock under the 2022 Purchase Agreement.
Certain Anti-Takeover Provisions of Our Certificate of Incorporation, Our Bylaws, the DGCL and our Rights Plan
Delaware Law
We are subject to Section 203 of the DGCL, which prohibits a Delaware corporation from engaging in any business combination with any interested stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
•
before such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder;
•
completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction began, excluding for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder) those shares owned (1) by persons who are directors and also officers and (2) employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
•
on or after such date, the business combination is approved by the board of directors and authorized at an annual or special meeting of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder.
In general, Section 203 defines a “business combination” to include the following:
17
•
any merger or consolidation involving the corporation and the interested stockholder;
•
any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested stockholder;
•
subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder;
•
any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or
•
the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits by or through the corporation.
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the person’s affiliates and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Staggered Board; Removal of Directors. Our amended and restated certificate of incorporation provides for our board of directors to be divided into three classes with staggered three-year terms. Only one class of directors is elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Because our stockholders do not have cumulative voting rights, stockholders holding a majority of the shares of Common Stock outstanding are able to elect all of our directors. Our certificate of incorporation and our bylaws also provide that directors may be removed by the stockholders only for cause upon the vote of 75% of our outstanding Common Stock. Furthermore, the authorized number of directors may be changed only by resolution of the board of directors, and vacancies and newly created directorships on the board of directors may, except as otherwise required by law or determined by the board, only be filled by a majority vote of the directors then serving on the board, even though less than a quorum.
Stockholder Action by Written Consent. Our amended and restated certificate of incorporation and amended and restated bylaws also provide that all stockholder actions must be effected at a duly called meeting of stockholders and eliminates the right of stockholders to act by written consent without a meeting. Our amended and restated bylaws also provide that only our chairman of the board, chief executive officer or the board of directors pursuant to a resolution adopted by a majority of the total number of authorized directors may call a special meeting of stockholders.
Requirements for Advance Notification of Stockholder Nominations, Proposals and Amendments. Our amended and restated bylaws also provide that stockholders seeking to present proposals before a meeting of stockholders to nominate candidates for election as directors at a meeting of stockholders must provide timely advance notice in writing, and specify requirements as to the form and content of a stockholder’s notice. Our certificate of incorporation and bylaws provide that the stockholders cannot amend many of the provisions described above except by a vote of 75% or more of our outstanding Common Stock.
Shareholder Rights Plan. On November 8, 2020, our board of directors adopted a rights plan pursuant to our rights agreement. The rights plan works by causing substantial dilution to any person or group that acquires beneficial ownership of ten percent (10%) or more of our Common Stock without the approval of our board of directors. As a result, the overall effect of the rights plan and the issuance of the rights pursuant to the rights plan may be to render more difficult or discourage a merger, tender or exchange offer or other business combination involving the Company that is not approved by our board of directors. The rights plan is not intended to interfere with any merger, tender or exchange offer or other business combination approved by our board of directors. The rights plan also does not prevent our board of directors from considering any offer that it considers to be in the best interest of our stockholders.
These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to reduce our vulnerability to hostile takeovers and to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and may have the effect of delaying changes in our control or management. As a consequence, these provisions may also inhibit fluctuations in the market price of our stock that could result from actual or rumored takeover attempts. We believe that the benefits of these provisions, including increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure our company, outweigh the disadvantages of discouraging takeover proposals, because negotiation of takeover proposals could result in an improvement of their terms.
18
On November 2, 2023, Aptevo Therapeutics Inc. entered into Amendment No. 3 to the Rights Agreement, dated as of November 8, 2020, between the Company and Broadridge Corporate Issuer Solutions, Inc., as Rights Agent, as amended. The Amendment extends the definition of “Final Expiration Date” (as defined in the Rights Agreement) and certain related language in the Rights Agreement to November 4, 2024. The Amendment also changes the definition of “Purchase Price” (as defined in the Rights Agreement) and certain related language in the Rights Agreement to $2.02 per one one-thousandth of a Preferred Share.
Transfer Agent and Registrar
The transfer agent and registrar for our Common Stock is Broadridge, which can be contacted at 51 Mercedes Way, Edgewood, NY 11717, shareholder@broadridge.com, or +1 (720) 378-5591. The transfer agent for any series of preferred stock that we may offer under this prospectus will be named and described in the prospectus supplement for that series.
Listing on the Nasdaq Capital Market
Our Common Stock is listed on the Nasdaq Capital Market under the symbol “APVO.”
19
DESCRIPTION OF SECURITIES WE ARE OFFERING
We are offering up to 9,090,910 shares of our common stock (or pre-funded warrants in lieu of) and up to 18,181,820 common warrants to purchase up to 18,181,820 shares of common stock. Each share of common stock is being offered together with two common warrants, each to purchase one share of common stock. We are also offering pre-funded warrants to those purchasers whose purchase of shares of common stock in this offering would result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the purchaser, 9.99%) of our outstanding shares of common stock following the consummation of this offering in lieu of the shares of common stocks that would result in such excess ownership. Each pre-funded warrant will be exercisable for one share of common stock. Each pre-funded warrant is being offered together with two common warrants, each to purchase one share of common stock. No warrant for fractional shares of common stock will be issued, rather warrants will be issued only for whole shares of common stock. We are also registering the shares of common stock issuable from time to time upon exercise of the pre-funded warrants and common warrants offered hereby.
Common Stock
The material terms and provisions of our common stock are described under the caption “Description of Capital Stock” in this prospectus and are incorporated herein by reference.
Common Warrants
General
The following is a summary of certain terms and provisions of the common warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the common warrant, the form of which will be filed as an exhibit to the Form 8-K filed in connection with this offering. Prospective investors should carefully review the terms and provisions of the form of common warrant for a complete description of the terms and conditions of the common warrants.
Stockholder Approval
Under Nasdaq listing rules, the common warrants are not exercisable without Stockholder Approval. We have agreed to hold a special meeting of the stockholders, within ninety (90) days following the closing of this offering, in order to seek such Stockholder Approval as may be required by the applicable rules and regulations of the Nasdaq Capital Market (or any successor entity) from our stockholders in order to permit the exercise of the common warrants. We cannot assure you that we will be able to obtain Stockholder Approval. In the event that we are unable to obtain the Stockholder Approval, the common warrants will not be exercisable and therefore have no value. We have agreed to use reasonable best efforts to hold a special meeting of stockholders (which may also be at the annual meeting of stockholders) at the earliest practicable date after the date hereof, but in no event later than ninety days after the closing of the offering, in order to obtain Stockholder Approval. If the Company does not obtain Stockholder Approval with respect to the terms of the common warrants at the first special meeting of the stockholders, the Company shall call a meeting every sixty (60) days thereafter to seek such Stockholder Approval until the date on which Stockholder Approval is obtained or the Warrants are no longer outstanding.
Duration, Exercise Price and Form
The Company is offering common warrants to purchase up to an aggregate of 18,181,820 shares of our common stock. Each common warrant may be exercised, in cash or by a cashless exercise at the election of the holder at any time following the date of Stockholder Approval and from time to time thereafter through and including the five year anniversary of the initial exercise date. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price. Subject to the rules and regulations of the applicable trading market, the Company may at any time during the term of the common warrant reduce the then current exercise price to any amount and for any period of time deemed appropriate by the board of directors of the Company. The common warrants will be issued separately from the common stock and will be held separately immediately thereafter. Two common warrants, each to purchase one share of our common stock will be issued for every share of common
20
stock or pre-funded warrant to purchase one share purchased in this offering. The common warrants will be issued in certificated form only.
Exercisability
The common warrants are not exercisable without first obtaining the Stockholder Approval. Assuming the Stockholder Approval is obtained, the common warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of such holder’s common warrants to the extent that the holder would own more than 4.99% of the outstanding common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding stock after exercising the holder’s common warrants up to 9.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the common warrants.
Cashless Exercise
If, at the time a holder exercises its common warrants, a registration statement registering the issuance of the shares of common stock underlying the common warrants under the Securities Act is not then effective or available for the issuance of such shares, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the common warrant.
Fundamental Transactions
In the event of a fundamental transaction, as described in the form of common warrant, and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the common warrants will be entitled to receive upon exercise of the common warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the common warrants immediately prior to such fundamental transaction, other than one in which a successor entity that is a publicly traded corporation (whose stock is quoted or listed for trading on a national securities exchange, including, but not limited to, the New York Stock Exchange, the NYSE American, the Nasdaq Global Select Market, the Nasdaq Global Market or the Nasdaq Capital Market) assumes the common warrant such that the warrant shall be exercisable for the publicly traded common stock of such successor entity. Additionally, as more fully described in the common warrants, at the option of the holders of the common warrants the holder can receive consideration in the same type and form of an amount equal to the Black Scholes value (as defined in the common warrant) of such unexercised common warrants on the date of consummation of such transaction.
Exercise Price Adjustments
If at any time on or after the date of issuance there occurs any share split, share dividend, share combination recapitalization or other similar transaction involving our common stock and the lowest daily volume weighted average price during the period commencing five consecutive trading days immediately preceding and ending immediately after the five consecutive trading days beginning on the date of such Share Combination Event, is less than the exercise price of the common warrants then in effect, then the exercise price of the common warrants will be reduced to the lowest daily volume weighted average price during such period. Notwithstanding the foregoing, if one or more Share Combination Events occur prior to the Stockholder Approval being obtained and a reduction of the exercise price did not occur, once the Shareholder Approval is obtained, the exercise price will automatically be reduced to equal the lowest price with respect to any Share Combination Event that occurred prior to the Stockholder Approval being obtained.
If while the common warrants are outstanding, the Company issues or sells, or is deemed to have issued or sold, any common stock and/or common stock equivalents other than in connection with certain exempt issuances, with a purchase price per share less than the exercise price in effect immediately prior to such issuance or sale or
21
deemed issuance or sale, then immediately after such issuance or sale or deemed issuance or sale, the exercise price then in effect will be reduced to an amount equal to the new issuance price.
Transferability
Subject to applicable laws, a common warrant may be transferred at the option of the holder upon surrender of the common warrant to us together with the appropriate instruments of transfer.
Fractional Shares
No fractional shares of common stock will be issued upon the exercise of the common warrants. Rather, the number of shares of common stock to be issued will, at the Company's election, either be rounded up to the next whole share or we will pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Trading Market
There is no established trading market for the common warrants, and we do not expect an active trading market to develop. We do not intend to apply to list the common warrants on any securities exchange or other trading market. Without a trading market, the liquidity of the common warrants will be extremely limited.
Right as a Stockholder
Except as otherwise provided in the common warrants or by virtue of the holder’s ownership of shares of our common stock, such holder of common warrants does not have the rights or privileges of a holder of our common stock, including any voting rights until such holder exercises such holder’s common warrants. The common warrants will provide that the holders of the common warrants have the right to participate in distributions or dividends paid on our shares of common stock.
Waivers and Amendments
The common warrant may be modified or amended or the provisions of the common warrant waived with our and the holder’s written consent.
Pre-funded Warrants
General
The following summary of certain terms and provisions of the pre-funded warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the pre-funded warrant, the form of which will be filed as an exhibit to the registration statement of which this prospectus forms a part. Prospective investors should carefully review the terms and provisions of the form of pre-funded warrant for a complete description of the terms and conditions of the pre-funded warrants.
Duration, Exercise Price and Form
Each pre-funded warrant offered hereby will have an initial exercise price per share of common stock equal to $0.0001. The pre-funded warrants will be immediately exercisable and will expire when exercised in full. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of share dividends, share splits, reorganizations or similar events affecting our shares of common stock and the exercise price. Subject to the rules and regulations of the applicable trading market, we may at any time during the term of the pre-funded warrant, subject to the prior written consent of the holders, reduce the then current exercise price to any amount and for any period of time deemed appropriate by our board of directors. The pre-funded warrants will be issued in certificated form only.
Exercisability
The pre-funded warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of the pre-funded warrant to the extent that the holder would own more than 4.99% of the outstanding shares of common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of beneficial ownership of outstanding shares after exercising the holder’s pre-funded warrants up to 9.99% of the number of our shares of common stock
22
outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the pre-funded warrants. Purchasers of pre-funded warrants in this offering may also elect prior to the issuance of the pre-funded warrants to have the initial exercise limitation set at 9.99% of our outstanding shares of common stock.
Cashless Exercise
If, at the time a holder exercises its pre-funded warrants, a registration statement registering the issuance of the shares of common stock underlying the pre-funded warrants under the Securities Act is not then effective or available, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the pre-funded warrants.
Fractional Shares
No fractional shares of common stock will be issued upon the exercise of the pre-funded warrants. Rather, the number of shares of common stock to be issued will be, at the Company's election, either rounded up to the next whole share or we will pay a cash adjustment in respect of such final fraction in an amount equal to such fraction multiplied by the exercise price.
Transferability
Subject to applicable laws, a pre-funded warrant may be transferred at the option of the holder upon surrender of the pre-funded warrants to us together with the appropriate instruments of transfer.
Trading Market
There is no trading market available for the pre-funded warrants on any securities exchange or nationally recognized trading system, and we do not expect a trading market to develop. We do not intend to list the pre-funded warrants on any securities exchange or nationally recognized trading market. Without a trading market, the liquidity of the pre-funded warrants will be extremely limited. The shares of common stock issuable upon exercise of the pre-funded warrants are currently traded on the Nasdaq Capital Market.
Right as a Shareholder
Except as otherwise provided in the pre-funded warrants or by virtue of such holder’s ownership of shares of common stock, the holders of the pre-funded warrants do not have the rights or privileges of holders of our shares of common stock, including any voting rights, until they exercise their pre-funded warrants. The pre-funded warrants will provide that the holders of the pre-funded warrants have the right to participate in distributions or dividends paid on our shares of common stock.
Fundamental Transaction
In the event of a fundamental transaction, as described in the pre-funded warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the pre-funded warrants will be entitled to receive upon exercise of the pre-funded warrants the kind and amount of securities, cash or other property that the holders would have received had they exercised the pre-funded warrants immediately prior to such fundamental transaction, other than one in which a successor entity that is a publicly traded corporation (whose stock is quoted or listed for trading on a national securities exchange, including, but not limited to, the New York Stock Exchange, the NYSE American, the Nasdaq Global Select Market, the Nasdaq Global Market or the Nasdaq Capital Market) assumes the common warrant such that the warrant shall be exercisable for the publicly traded common stock of such successor entity.
Waivers and Amendments
The pre-funded warrant may be modified or amended or the provisions of the pre-funded warrant waived with our and the holder’s written consent.
23
PLAN OF DISTRIBUTION
We engaged Roth Capital Partners, LLC (“Roth” or the “placement agent”), to act as our exclusive placement agent to solicit offers to purchase the securities offered by this prospectus on a reasonable best efforts basis. Roth is not purchasing or selling any securities, nor are they required to arrange for the purchase and sale of any specific number or dollar amount of securities, other than to use their “reasonable best efforts” to arrange for the sale of the securities by us. Therefore, we may not sell the entire amount of securities being offered. There is no minimum amount of proceeds that is a condition to closing of this offering. The placement agent does not guarantee that it will be able to raise new capital in this offering. The terms of this offering were subject to market conditions and negotiations between us and prospective investors in consultation with the placement agent. The placement agent will have no authority to bind us. This offering will terminate no later than October 15, 2024, unless we decide to terminate the offering (which we may do at any time in our discretion) prior to that date. We will have one closing for all the securities purchased in this offering. The combined offering price per share (or pre-funded warrant) and accompanying common warrants will be fixed for the duration of this offering. Roth may engage one or more sub-placement agents or selected dealers to assist with the offering.
We will enter into a securities purchase agreement directly with the institutional investors, at the investor’s option, who purchase our securities in this offering. Investors who do not enter into a securities purchase agreement shall rely solely on this prospectus in connection with the purchase of our securities in this offering.
Placement Agent Fees and Expenses
The following table shows the per share and accompanying common warrants, and per pre-funded and accompanying common warrants, and total placement agent fees we will pay in connection with the sale of the securities in this offering.
|
|
Per Share and Common Warrant |
|
|
Per Pre-Funded Warrant and Common Warrant |
|
|
Total |
|
|||
Offering price |
|
$ |
0.3300 |
|
|
$ |
0.3299 |
|
|
$ |
2,999,493.21 |
|
Placement Agent fees(1) |
|
$ |
0.0231 |
|
|
$ |
0.0231 |
|
|
$ |
209,964.52 |
|
Proceeds to us, before expenses(2) |
|
$ |
0.3069 |
|
|
$ |
0.3068 |
|
|
$ |
2,789,528.68 |
|
1.
We have agreed to pay to the placement agent a cash fee equal to 7% of the aggregate gross proceeds raised in this offering subject to a partial adjustment in the event certain investors participate. Because there is no minimum offering amount required as a condition to closing in this offering, the actual aggregate cash placement fee, if any, is not presently determinable and may be substantially less than the maximum amount set forth above.
2.
We have also agreed to reimburse the Placement Agent at closing for legal and other expenses incurred by the placement agent in connection with this offering in an amount equal to $100,000.
We estimate that the total expenses of the offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding placement agent fees, will be approximately $265,000, all of which are payable by us. This figure includes the placement agent’s accountable expenses, including, but not limited to, legal fees for placement agent’s legal counsel, that we have agreed to pay at the closing of the offering up to an aggregate expense reimbursement of $100,000.
Tail
In the event this offering does not close we have also agreed to pay the Placement Agent a tail fee equal to the cash compensation in this offering, subject to certain exceptions, if any investor, who the Placement Agent conducted discussions with on behalf of the Company during the 90 days following its engagement, or if any of the separately agreed upon investors, provides us with capital in any offering of the Company’s securities during the six month period following expiration or termination of our engagement of the Placement Agent.
24
Right of First Refusal
We have granted a right of first refusal to the placement agent pursuant to which it has the right to act as
the exclusive book-running manager, underwriter or placement agent, as applicable, if we decide to raise capital through a public offering or private placement or any other capital-raising financing of equity, equity-linked or debt securities at any time prior to December 31, 2024.
Other Relationships
The placement agent may, from time to time, engage in transactions with or perform services for us in the ordinary course of its business and may continue to receive compensation from us for such services.
Determination of Offering Price
The combined offering price per share and common warrants and the combined offering price per pre-funded warrant and common warrants we are offering and the exercise prices and other terms of the warrants were negotiated between us and the investors, in consultation with the placement agent based on the trading of our common stock prior to this offering, among other things. Other factors considered in determining the offering prices of the securities we are offering and the exercise prices and other terms of the warrants include the history and prospects of our company, the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment of our management, general conditions of the securities markets at the time of the offering and such other factors as were deemed relevant.
Lock-Up Agreements
Each of our officers and directors have agreed to be subject to a lock-up period of 90 days following the date of this prospectus. This means that, during the applicable lock-up period, they may not offer for sale, contract to sell, or sell any shares of our common stock or any securities convertible into, or exercisable or exchangeable for, shares of our common stock subject to certain customary exceptions. In addition, we have agreed to not issue any shares of common stock or securities exercisable or convertible into shares of common stock for a period of 60 days following the closing date of this offering, subject to certain exceptions, or enter into an agreement to issue securities at a future determined price, for a period of 90 days following the closing date of this offering.
Transfer Agent and Registrar
Broadridge Financial Solutions, Inc. is the transfer agent and registrar for our common stock, which can be contacted at 51 Mercedes Way, Edgewood, NY 11717, shareholder@broadridge.com, or +1 (720) 378-5591.
The Nasdaq Capital Market Listing
Our common stock is currently listed on the Nasdaq Stock Market LLC under the symbol “APVO.” On September 16, 2024, the reported closing price per share of our common stock was $0.33. There is no established public trading market for the common warrants or pre-funded warrants, and we do not expect such markets to develop. In addition, we do not intend to apply for a listing of the common warrants or pre-funded warrants on any national securities exchange or other nationally recognized trading system.
Indemnification
We have agreed to indemnify the placement agent against certain liabilities, including certain liabilities arising under the Securities Act, or to contribute to payments that the placement agent may be required to make for these liabilities.
25
Regulation M
The placement agent may be deemed to be an underwriter within the meaning of Section 2(a)(11) of the Securities Act and any fees received by it and any profit realized on the sale of the securities by it while acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. The placement agent will be required to comply with the requirements of the Securities Act and the Exchange Act of 1934, as amended (the “Exchange Act”), including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of our securities by the placement agent. Under these rules and regulations, the placement agent may not (i) engage in any stabilization activity in connection with our securities; and (ii) bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act, until they have completed their participation in the distribution.
Electronic Distribution
A prospectus in electronic format may be made available on a website maintained by the placement agent and the placement agent may distribute prospectuses electronically. Other than the prospectus in electronic format, the information on these websites is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or the placement agent and should not be relied upon by investors.
26
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate by reference” the information we file with it, which means that we can disclose important information to you by referring you to those documents instead of having to repeat the information in this prospectus. The information incorporated by reference is considered to be part of this prospectus, and because we are a smaller reporting company, later information that we file with the SEC will automatically update and supersede this information. We incorporate by reference the documents listed below and any future filings (including those made after the initial filing of the registration statement of which this prospectus is a part and prior to the effectiveness of such registration statement) we will make with the SEC under Sections 13(a), 13(c), 14, or 15(d) of the Exchange Act until the termination of the offering of the shares covered by this prospectus (other than information furnished under Item 2.02 or Item 7.01 of Form 8-K):
•
our Annual Report on Form 10-K for the year ended December 31, 2023 filed with the SEC on March 5, 2024;
•
our Quarterly Report on Form 10-Q for the quarter ended March 31 and June 30, 2024, filed with the SEC on May 8, 2024, and August 8, 2024, respectively;
•
our definitive proxy statement on Schedule 14A, filed with the SEC on April 23, 2024, and July 15, 2024;
•
our Current Report on Form 8-K filed with the SEC on February 6, 2024, March 5, 2024, March 7, 2024, April 15, 2024, May 8, 2024, June 10, 2024; July 1, 2024, July 1, 2024, August 7, 2024, and September 16, 2024; and
•
the description of our Common Stock contained in Exhibit 4.8 to our Annual Report on Form 10-K for the year ended December 31, 2023 filed with the SEC on March 5, 2024, including any amendment or report filed for the purpose of updating such description.
As a smaller reporting company, we also are incorporating by reference any future information filed (rather than furnished) by us with the SEC under Section 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, after the date of the initial filing of the registration statement of which this prospectus is a part and before the effective date of the registration statement and after the date of this prospectus until the termination of the offering. Any statements contained in a previously filed document incorporated by reference into this prospectus is deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus, or in a subsequently filed document also incorporated by reference herein, modifies or supersedes that statement.
We will provide to each person, including any beneficial owner, to whom a prospectus is delivered, at no cost, upon written or oral request, a copy of any or all of the reports or documents that have been incorporated by reference in the prospectus contained in the registration statement but not delivered with the prospectus. You should direct requests for documents to:
Aptevo Therapeutics Inc.
2401 4th Avenue, Suite 1050
Seattle, WA 98121
Attn: General Counsel
(206) 838-0500
This prospectus is part of a registration statement we filed with the SEC. That registration statement and the exhibits filed along with the registration statement contain more information about us and the shares in this offering. Because information about documents referred to in this prospectus is not always complete, you should read the full documents which are filed as exhibits to the registration statement. You may read and copy the full registration statement and its exhibits at the SEC’s website.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus is part of a registration statement we filed with the SEC. This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities we are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. You should rely only on the
27
information contained in this prospectus or incorporated by reference into this prospectus. We have not authorized anyone else to provide you with different information. We are not making an offer of these securities in any jurisdiction where the offer is not permitted. You should assume that the information contained in this prospectus, or any document incorporated by reference in this prospectus, is accurate only as of the date of those respective documents, regardless of the time of delivery of this prospectus or any sale of our securities.
We are subject to the informational requirements of the Exchange Act and in accordance therewith we file annual, quarterly, and other reports, proxy statements and other information with the Commission under the Exchange Act. Such reports, proxy statements and other information, including the Registration Statement, and exhibits and schedules thereto, are available to the public through the Commission’s website at www.sec.gov.
We make available free of charge on or through our website our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, as soon as reasonably practicable after we electronically file such material with or otherwise furnish it to the Commission. The registration statement and the documents referred to under “Incorporation of Certain Information by Reference” are also available on our website, https://aptevotherapeutics.gcs-web.com/financial-reports/sec-filings.
We have not incorporated by reference into this prospectus the information on our website, and you should not consider it to be a part of this prospectus.
LEGAL MATTERS
The validity of the securities offered hereby will be passed upon for us by Paul Hastings LLP, Washington, DC. The placement agent is being represented by Ellenoff Grossman & Schole LLP, New York, NY.
EXPERTS
Our consolidated financial statements as of December 31, 2023 and 2022, and for the years then ended, incorporated in this prospectus by reference and included in our Annual Report on Form 10-K for the year ended December 31, 2023, have been audited by Moss Adams LLP, an independent registered public accounting firm, as stated in their report (which report expresses an unqualified opinion and includes an explanatory paragraph related to a going concern uncertainty), which is incorporated herein by reference. Such consolidated financial statements are incorporated by reference in reliance upon the report of such firm given their authority as experts in accounting and auditing.
28
4,020,000 Shares of Common Stock
5,070,910 Pre-Funded Warrants to Purchase 5,070,910 Shares of Common Stock
18,181,820 Common Warrants to Purchase 18,181,820 Shares of Common Stock
23,252,730 Shares of Common Stock underlying the Pre-Funded Warrants and Common Warrants

PROSPECTUS
Roth Capital Partners
The date of this prospectus is September 16, 2024.
29