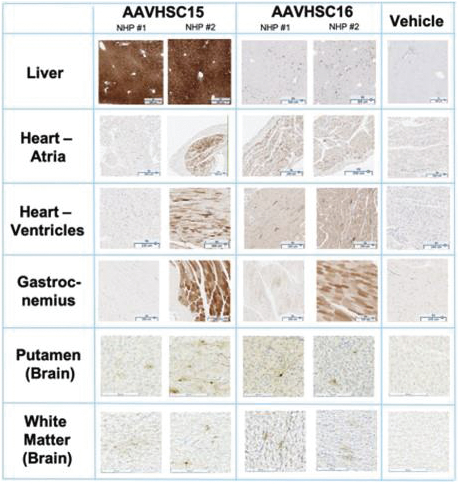
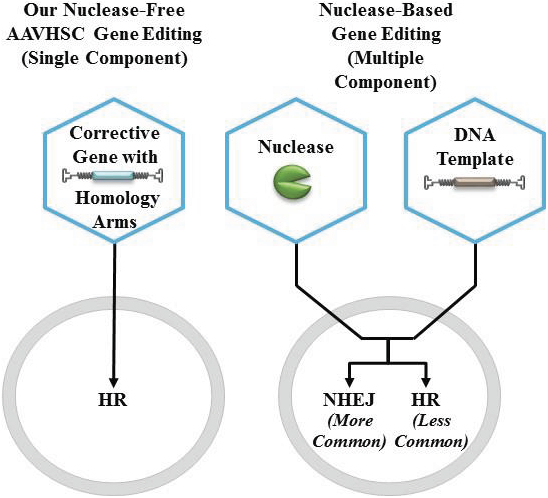
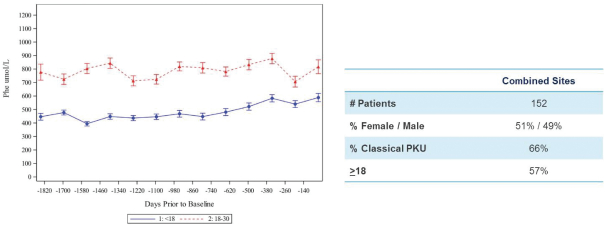
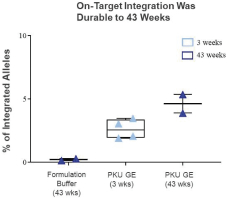
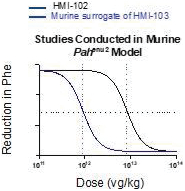
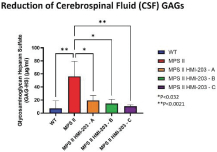
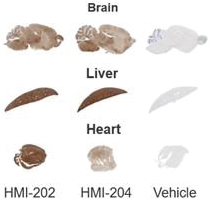
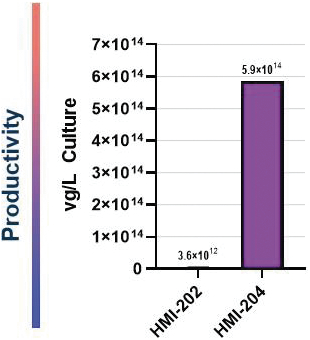
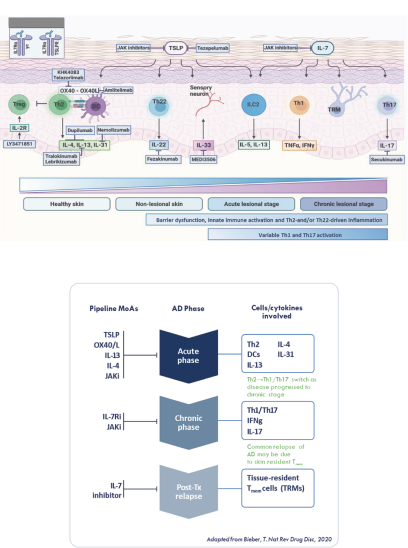
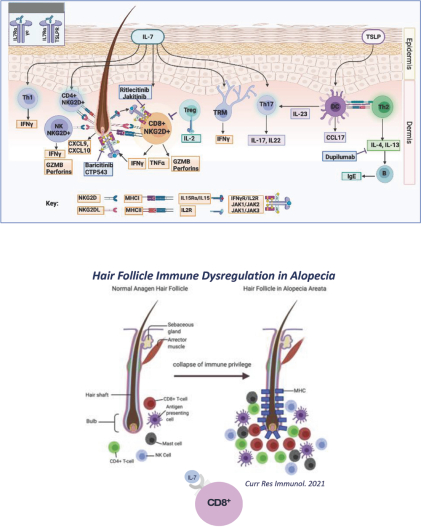
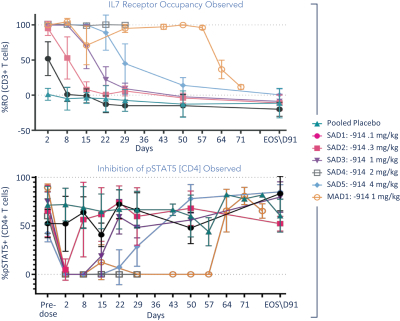
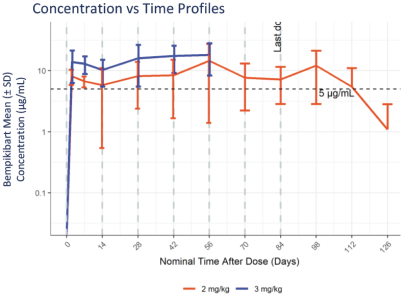
The information in this proxy statement/prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This proxy statement/prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JANUARY 26, 2024

|

|
PROPOSED MERGER
YOUR VOTE IS VERY IMPORTANT
To the Stockholders of Homology Medicines, Inc. and Q32 Bio Inc.,
Homology Medicines, Inc., a Delaware corporation, or Homology, and Q32 Bio Inc., a Delaware corporation, or Q32, entered into an Agreement and Plan of Merger, or the Merger Agreement, on November 16, 2023, pursuant to which a direct, wholly owned subsidiary of Homology, Kenobi Merger Sub, Inc., or Merger Sub, will merge with and into Q32, with Q32 surviving as a direct, wholly owned subsidiary of Homology, and the surviving corporation of the merger, which transaction is referred to herein as the Merger. Homology following the Merger is also referred to herein as the combined company.
At the time the certificate of merger is filed with and accepted by the Secretary of State of the State of Delaware, or the Effective Time, as described in more detail in the section titled “The Merger—Effective Time of the Merger” beginning on page 187 of the accompanying proxy statement/prospectus, each share of Q32 common stock (after giving effect to the conversion of each share of Q32’s preferred stock into Q32 common stock and the conversion of Q32’s convertible notes into Q32 common stock and including all such shares that are converted into Q32 common stock) will be converted into the right to receive a number of shares of Homology common stock equal to the total number of shares of Homology common stock to be issued in the Merger multiplied by the applicable Q32 stockholder’s percentage interest in Q32 as set forth in the allocation certificate to be provided by Q32, or the Allocation Certificate, and as described in more detail in the section titled “The Merger Agreement—Allocation Certificate” beginning on page 193 of the accompanying proxy statement/prospectus. The Q32 common stock that will be converted in connection with the Merger includes Q32 common stock to be issued pursuant to the subscription agreement by and among Q32 and certain parties to purchase an aggregate of $42.0 million of shares of Q32’s common stock, which transaction is referred to as the Pre-Closing Financing. If any Q32 common stock outstanding immediately prior to the Effective Time is unvested or is subject to a repurchase option or a risk of forfeiture under any applicable agreement with Q32, then the shares of Homology common stock issued in exchange for such shares of Q32 common stock will to the same extent be unvested and subject to the same repurchase option or risk of forfeiture, and such shares of Homology common stock will be marked with appropriate legends.
In connection with the Merger, each option to purchase shares of Q32 common stock outstanding as of immediately prior to the Effective Time will be assumed by Homology and converted, at the Effective Time, into an option to acquire the number of shares of Homology common stock equal to the number of shares of Q32 common stock subject to such option as of immediately prior to the Effective Time multiplied by the exchange ratio formula in the Merger Agreement and rounding that result down to the nearest whole number of shares, at a per share exercise price equal to the per share exercise price of such option immediately prior to the Effective Time multiplied by the exchange ratio formula in the Merger Agreement, rounded up to the nearest whole cent, and each unexercised warrant to purchase shares of Q32 common stock outstanding immediately prior to the Effective Time will be assumed by Homology and converted into a warrant to purchase shares of Homology’s common stock, with adjustments to the number of shares and the exercise price to reflect the exchange ratio formula in the Merger Agreement.
Each share of Homology common stock that is issued and outstanding at the Effective Time will remain issued and outstanding and such shares will be unaffected by the Merger but will be subject to a proposed reverse stock split of Homology’s issued and outstanding common stock at a ratio ranging from any whole number between 1-for-10 and 1-for-30 as determined by the Homology board of directors in its discretion, or the Reverse Stock Split, as described in the section titled “Matters Being Submitted to a Vote of Homology Stockholders—Proposal No. 3: Approval of the Amendment to the Restated Certificate of Incorporation of Homology Effecting the Reverse Stock Split” beginning on page 238 of the accompanying proxy statement/prospectus. Each