
Exhibit 10.1 CERTAIN INFORMATION IN THIS DOCUMENT, MARKED BY [**] HAS BEEN OMITTED FROM THIS EXHIBIT BECAUSE IT IS BOTH (I) NOT MATERIAL AND (II) WOULD LIKELY BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. MASTER IN VITRO DIAGNOSTICS AGREEMENT between FOUNDATION MEDICINE, INC., ARVINAS OPERATIONS, INC. and, solely for purposes of Section 11.19 hereof, ARVINAS, INC. Dated as of June 4, 2022

Master In Vitro Diagnostics Agreement 1 of [33] CONFIDENTIAL MASTER IN VITRO DIAGNOSTICS AGREEMENT This Master In Vitro Diagnostics Agreement (this “Agreement”) is effective as of June 4, 2022 (the “Effective Date”) by and between Foundation Medicine, Inc., a Delaware corporation with its principal place of business at 150 Second Street, Cambridge, Massachusetts 02141, United States (“FMI”), Arvinas Operations, Inc., a Delaware corporation with its principal place of business at 5 Science Park, 395 Winchester Avenue, New Haven, CT 06511 (“Company”) and, solely for purposes of Section 11.19 hereof, Arvinas, Inc., a Delaware corporation with its principal place of business at 5 Science Park, 395 Winchester Avenue, New Haven, CT 06511 (“Company Parent”). FMI and Company are sometimes referred to herein individually as a “Party” and collectively as the “Parties.” RECITALS WHEREAS, the Parties desire to collaborate to enable FMI to seek and obtain Regulatory Approval for one or more FMI Assays for use with one or more Company Products, in each case, as set forth in a Statement of Work (SOW) (each capitalized term as defined below) executed by each Party under this Agreement and according to the following terms and conditions; and [**]. NOW, THEREFORE, in consideration of the premises and the mutual promises and conditions set forth herein and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties, intending to be legally bound, do hereby agree as follows: ARTICLE 1 DEFINITIONS Unless otherwise specifically provided herein, the following terms shall have the following meanings: 1.1. “Activities” means the activities to be performed by a Party under an applicable SOW. 1.2. “Affiliate” means any Person that (i) directly or indirectly controls a Party, (ii) is directly or indirectly controlled by a Party, or (iii) is controlled, directly or indirectly, by the ultimate parent company of a Party. For purposes of this definition, “control” and, with correlative meaning, the term “controlled by” means the ownership, directly or indirectly, of more than fifty percent (50%) of the voting securities or other ownership interest of an organization or otherwise having the power to govern the financial and operating policies or to appoint the management of an organization. Notwithstanding the foregoing, for purposes of this Agreement, Roche Holding Ltd, of Basel, Switzerland and Chugai Pharmaceutical Co., Ltd., of Tokyo, Japan, and their respective direct or indirect subsidiaries (including in the case of Roche Holding Ltd, Genentech, Inc., Roche Diagnostics Corporation, Roche Molecular Systems, Inc. and their respective direct and indirect subsidiaries), other than direct or indirect subsidiaries of FMI, shall not be Affiliates of FMI (and thus shall constitute Third Parties for purposes of this Agreement). 1.3. [**]. 1.4. “Analytical Validation Samples” means any Samples provided by Company or any of its Affiliates [**] For clarity, an Analytical Validation Sample may also be a Clinical Development Sample or Trial Enrollment Sample.

Master In Vitro Diagnostics Agreement 2 of [33] CONFIDENTIAL 1.5. “Analytical Validation Studies” means any and all studies directed to establishing the accuracy, reliability, reproducibility, sensitivity, specificity, or other performance of any [**]. 1.6. “Applicable Law” means applicable laws, rules and regulations, including any rules, regulations, guidance or other requirements of a Regulatory Authority, that may be in effect from time to time and applicable to a particular activity hereunder, and shall be deemed to include the applicable regulations and guidances of the FDA that constitute [**] (and, if and as appropriate under the circumstances, International Conference on Harmonization (ICH) guidance). 1.7. “Approved IVD” means, with respect to an IVD and country or territory, that such IVD has been granted Regulatory Approval for such country or territory for the intended use, including, for example, any required PMA Approval. The term “Approved IVD” is not intended, and shall not be construed, to include any IVD solely for research or investigational purposes. 1.8. “Assay Submission Package” means [**]. 1.9. “CE Marking Approval” means completion of all conformity assessment procedures required under the IVD Directive or IVD Regulation, as applicable, including obtaining any necessary certifications by a Notified Body of the conformity of an in vitro diagnostic medical device with the requirements of the IVD Directive or IVD Regulation, as applicable, and applicable harmonized standards necessary for the manufacturer of such device to affix a CE mark and place such device on the market in the EU. 1.10. “Change of Control” means a transaction occurring after the Effective Date in which a Party or any parent company of such Party: (i) sells, conveys or otherwise disposes of all or substantially all of its property or business to which this Agreement relates; or (ii)(a) merges or consolidates with any other Person (other than a wholly-owned subsidiary of such Party or such parent company) or (b) effects any other transaction or series of transactions; in each case ((a) or (b)), such that the stockholders of such Party or such parent company immediately prior thereto, in the aggregate, no longer own, directly or indirectly, beneficially or legally, at least fifty percent (50%) of the outstanding voting securities or capital stock of the surviving Person following the closing of such merger, consolidation, other transaction or series of transactions. 1.11. “CLIA” means the Clinical Laboratory Improvement Amendments of 1988, their implementing regulations and guidance or any corresponding or similar foreign laws, regulations or guidance. 1.12. “Clinical Development Samples” means Samples obtained by or on behalf of Company or any of its Affiliates from subjects enrolled in Clinical Studies of any Company Product and provided to FMI for use in connection with the Activities. [**]. 1.13. “Clinical Outcomes Data” means clinical data [**]generated in the conduct of Clinical Studies conducted by or on behalf of Company or any of its Affiliates with respect to a Company Product that are related to the safety or efficacy of such Company Product. 1.14. “Clinical Studies” means studies conducted in human subjects and required by Applicable Law or recommended by a Regulatory Authority to obtain or maintain Regulatory Approvals for a Therapeutic Product. 1.15. “Collaboration Data” means that portion of the [**].

Master In Vitro Diagnostics Agreement 3 of [33] CONFIDENTIAL 1.16. “Collaboration IP” means [**]. 1.17. “Collaboration Know-How” means Know-How that is conceived, generated or otherwise developed [**]. 1.18. “Commercialization” means any and all activities directed to the preparation for sale of, offering for sale of, or sale of a product, including activities related to marketing, promoting, distributing and importing such product, and interacting with Regulatory Authorities regarding any of the foregoing. For clarity, when used in relation to an FMI Assay or other IVD, “Commercialization” includes activities directed to the preparation for the sale of, offering for sale of, or sale of a service using such FMI Assay or other IVD, including activities related to marketing and promoting such service, and interacting with Regulatory Authorities regarding any of the foregoing. When used as a verb, “to Commercialize” and “Commercializing” mean to engage in Commercialization, and “Commercialized” has a corresponding meaning. 1.19. “Commercially Reasonable Efforts” means such efforts that are consistent with the efforts and resources normally associated with good business practice and standards [**]. 1.20. “Companion Diagnostic” means an Approved IVD that provides information essential to the safe and effective use of a corresponding Therapeutic Product or is otherwise necessary for the Regulatory Approval of a Therapeutic Product. 1.21. “Company Background IP” means [**]. 1.22. “Company Product” means a Therapeutic Product identified in an SOW for Development or Commercialization with an FMI Assay identified in such SOW. 1.23. “Complementary Diagnostic” means an Approved IVD that provides information helpful to the safe and effective use of a corresponding Therapeutic Product, but that is not a Companion Diagnostic. 1.24. “Control” means, with respect to any (i) (a) Know-How, (b) Patent or (c) other intellectual property right; (ii) Regulatory Documentation; or (iii) material, possession of the right, whether directly or indirectly and whether by ownership, license or otherwise (other than by operation of the licenses and other grants in Sections 6.2 and 6.3), to grant a license, sublicense or other right (including the right to reference Regulatory Documentation) to or under such Know-How, Patent, other intellectual property right, Regulatory Documentation or material as provided for herein without violating the terms of any agreement with any Third Party. 1.25. “Deliverables” means the work product or other deliverables a Party agrees to provide to the other Party with respect to a project, as specified in the applicable SOW. 1.26. “Development” means activities relating to the development, optimization, validation or clinical testing of any product (including any Product), including activities relating to obtaining or maintaining Regulatory Approval of such product. When used as a verb, “Develop” means to engage in Development. 1.27. “Disclosing Party” means, with respect to Confidential Information, the Party that provides or is deemed to provide such Confidential Information to the other Party. 1.28. “Drug Approval Application” means a “new drug application” as defined in the FFDCA, a “biologics license application” as defined in the FFDCA, or any corresponding foreign

Master In Vitro Diagnostics Agreement 4 of [33] CONFIDENTIAL application in any country or territory, including, with respect to the EU, a marketing authorization application filed with the EMA pursuant to the centralized approval procedure or with the applicable Regulatory Authority of a country in the EU with respect to the mutual recognition procedure or any other national approval, and any supplement to any of the foregoing. 1.29. “EMA” means the European Medicines Agency and any successor agency thereto. 1.30. “European Union” or “EU” means that certain economic, scientific and political organization of member states known as the European Union, as it may be constituted from time to time, or any successor thereto. 1.31. “Exploit” means to make, have made, import, use, sell, offer for sale or otherwise exploit, including to research, Develop, Commercialize, register, manufacture, have manufactured, hold or keep (whether for disposal or otherwise), have used, export, transport, distribute, promote, market or have sold or otherwise dispose of. For clarity, when used in relation to any IVD, “Exploit” includes the performance of any service using such IVD, and the making, having made, import, use, sale or offer for sale or other exploitation of such IVD for use in connection with such service, including the research, Development, Commercialization, registration, manufacture, having manufactured, holding or keeping (whether for disposal or otherwise), having used, exportation, transportation, distribution, promotion, marketing or having sold or otherwise disposing of such IVD for use in connection with such service. “Exploitation” means the act of Exploiting. 1.32. “FDA” means the United States Food and Drug Administration and any successor agency thereto. 1.33. “FFDCA” means the United States Federal Food, Drug, and Cosmetic Act, as amended from time to time, together with any rules, regulations and requirements promulgated thereunder (including all additions, supplements, extensions and modifications thereto). 1.34. “FMI Assay” means [**]. 1.35. “FMI Contracting Collaborator” means a Third Party with which FMI or any of its Affiliates has entered or enters into a contractual arrangement pursuant to which such Third Party collaborates with FMI in relation to, or is granted rights to Exploit, the FMI Technology Platform (or aspects thereof) or any IVD in countries or territories outside the United States. 1.36. “FMI Operational Documentation” means documents and information setting forth or associated with the internal practices, policies, procedures, and systems used by or on behalf of FMI or any of its Affiliates to conduct and [**] including all documents and information related to compliance with QSR and CLIA requirements in the United States and any corresponding or similar requirements under Applicable Law for any other country or territory. 1.37. “FMI Technology Platform” means FMI products or services for testing of specimens to identify [**] and related technologies and any improvements to any of the foregoing, in each case, existing as of the Effective Date or during the Term. 1.38. [**]. 1.39. “IND” means (i) an investigational new drug application filed with the FDA for authorization to commence Clinical Studies or any corresponding or similar application in other countries

Master In Vitro Diagnostics Agreement 5 of [33] CONFIDENTIAL or regulatory jurisdictions and (ii) all supplements and amendments that may be filed with respect to the foregoing. 1.40. “Indication” means, as specified in an applicable SOW, a disease or condition that a Company Product may be used to treat or prevent, subject to Regulatory Approval. 1.41. “IVD” or “In Vitro Diagnostic” means a product or service for in vitro testing of patient or subject specimens, or other biological materials, for use in the diagnosis or evaluation of a disease, including to identify any genomic alterations or signatures, or for the prediction or monitoring of a response to any Therapeutic Product (or other agent) or other prognostic use, whether used for research, exploratory purposes, or as a clinical diagnostic. The term “IVDs” includes “Investigation Use Only” products, “Research Use Only” products, Companion Diagnostics, Complementary Diagnostics and other IVDs. 1.42. “IVD Directive” means Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices, as amended from time to time, and as implemented in the EU member states under national law, or any statutory modification, extension or re-enactment thereof. 1.43. “IVD Regulation” means Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 210/227/EU. 1.44. “Know-How” means all tangible and intangible: information, techniques, technology, practices, trade secrets, inventions (whether patentable or not), methods, know-how, data (including [**]), results (including [**]), analytical and quality control data, descriptions, software and algorithms, in each case, of a scientific or technical nature, excluding [**] Documentation and [**] Documentation. 1.45. “Market” means, on a Company Product-by-Company Product and Indication- by-Indication basis, as specified in an applicable SOW, a country or territory for which the Parties intend to Develop and seek Regulatory Approval of an FMI Assay for use with such Company Product for such Indication to enable the Commercialization of such FMI Assay in such country or territory. 1.46. “Materials” means Samples or other biological materials, compounds, reagents and supplies that Company delivers or causes to be delivered to FMI in connection with an SOW. 1.47. “Notified Body” means an entity licensed, authorized or approved by the applicable government agency, department or other authority to assess and certify the conformity of an in vitro diagnostic medical device with the requirements of the IVD Directive or IVD Regulation, as applicable, and applicable harmonized standards. 1.48. “Patents” means: (i) all national, regional and international patents and patent applications, including provisional patent applications; (ii) all patent applications claiming priority from such patent applications, provisional applications or from an application claiming priority from either of these, including divisionals, continuations, continuations-in-part, converted provisionals and continued prosecution applications; (iii) any and all patents that have issued or in the future issue from the foregoing patent applications ((i) and (ii)), including utility models, petty patents, innovation patents and design patents and certificates of invention; (iv) any and all extensions or restorations by existing or future extension or restoration mechanisms, including revalidations, reissues, re-examinations, reviews and extensions (including any supplementary protection certificates and the like) of the foregoing patents or patent applications ((i), (ii) and (iii)); and (v) any similar rights, including so-called pipeline protection or

Master In Vitro Diagnostics Agreement 6 of [33] CONFIDENTIAL any importation, revalidation, confirmation or introduction patent or registration patent or patent of additions to any of such foregoing patent applications and patents. 1.49. “Permitted Representative” means a representative duly authorized by Company who shall be bound by written confidentiality and non-use obligations no less stringent than those set forth in this Agreement, and shall explicitly exclude any direct competitors of FMI 1.50. “Person” means an individual, sole proprietorship, partnership, limited partnership, limited liability partnership, corporation, limited liability company, business trust, joint stock company, trust, unincorporated association, joint venture or other similar entity or organization, including a government or political subdivision, department or agency of a government. 1.51. “PMA” means (i) a premarket approval application filed with the FDA or any corresponding or similar application in other countries or regulatory jurisdictions and (ii) all supplements and amendments that may be filed with respect to the foregoing. 1.52. “PMA Approval” means approval in accordance with Section 515 of the FFDCA and 21 C.F.R. Part 814 of a PMA, including approval of supplemental PMAs (including those supplemental PMAs reviewed using the ‘Real-Time Review’ process), by the FDA for a Class III device or similar approval in other countries or regulatory jurisdictions. 1.53. “Product” means, in the case of Company, any Company Product and, in the case of FMI, any FMI Assay, in each case, as designated in an applicable SOW. 1.54. “Program,” as such term is used in relation to partial termination of this Agreement, or to any consequences of any such partial termination, means, collectively, an applicable FMI Assay, Company Product, Indication and Market covered by an SOW. For clarity, an SOW may cover [**]. 1.55. “Quality System Regulation” or “QSR” means the requirements applicable to manufacturers of finished medical devices (including design control and current good manufacturing practices) pertaining to the methods used in, and the facilities and controls used for, the design, manufacture, packaging, labeling, storage, installation, and servicing of all finished devices intended for human use, as specified in 21 C.F.R. Part 820 and FDA’s guidance documents, and all successor applicable regulations and guidance documents thereto. 1.56. “Receiving Party” means, with respect to Confidential Information, the Party that receives or is deemed to receive such Confidential Information from the other Party or its agents. 1.57. “Regulatory Approval” means any and all clearances, approvals, licenses, registrations or authorizations of any Regulatory Authority necessary to commercially distribute, sell or market a product (including a Company Product or an FMI Assay) in a country or territory, including, in the case of an IVD, any required certificates of conformity from a Notified Body and the manufacturer’s formal declaration of conformity that the IVD complies with the requirements for CE marking. 1.58. “Regulatory Authority” means any applicable supra-national, federal, national, regional, state, provincial or local regulatory agencies, departments, bureaus, commissions, councils or other government entities regulating or otherwise exercising authority with respect to the Exploitation of Therapeutic Products or IVDs (including Products) in any country, regulatory jurisdiction or territory, including the FDA for the United States and the EMA for the European Union, and any Notified Body that has responsibility for the regulation of any IVD for any country, regulatory jurisdiction or territory, and any successor(s) to any of the foregoing.

Master In Vitro Diagnostics Agreement 7 of [33] CONFIDENTIAL 1.59. “Regulatory Documentation” means: all (i) applications (including all INDs, Drug Approval Applications, investigational device exemption filings, 510(k)s, de novo determinations, humanitarian device exemption filings and PMAs), registrations, licenses, authorizations and approvals (including Regulatory Approvals); and (ii) correspondence and reports submitted to or received from Regulatory Authorities (including minutes and official contact reports relating to any communications with any Regulatory Authority), including all adverse event files and complaint files; in each case ((i) and (ii)), relating to an FMI Assay or a Company Product. 1.60. “Samples” means biological materials, including human tissue and its derivatives or components (e.g., cell lines and DNA), as may be further identified in an applicable SOW, which may include [**] Samples, as applicable. 1.61. “Selected Biomarker(s)” means the one or more biomarkers identified in an applicable SOW as relevant to an FMI Assay and a Company Product. 1.62. “Senior Officer” means, with respect to FMI, its Vice President of Biopharma and Corporate Alliances and with respect to Company, its Chief Scientific Officer, or their respective designees. 1.63. “SOW” or “Statement of Work” means a plan executed by an authorized representative of both Parties under this Agreement that details a project, in the form provided as Attachment A. 1.64. “SOW Effective Date” means, with respect to an SOW, the date on which such SOW becomes effective, as set forth in such SOW. 1.65. “Statistical Analysis Plan” means any statistical analysis plan used or developed for use in performing any statistical analysis under the applicable SOW for the purpose of [**]. 1.66. “Therapeutic Product” means any product that constitutes or contains a chemical or biologic substance for the medical cure, treatment or prevention of disease. 1.67. “Third Party” means any Person other than FMI, Company and their respective Affiliates. 1.68. [**]. 1.69. “Trademark Rights” means any word, name, symbol, color, shape, designation or any combination thereof, including any trademark, service mark, trade name, corporate name, brand name, sub-brand name, trade dress, product configuration, program name, delivery form name, certification mark, collective mark, logo (including corporate logo), tagline, slogan, design or business symbol, that functions as an identifier of source or origin, whether or not registered and all statutory and common law rights therein and all registrations and applications therefor, together with all goodwill associated with, or symbolized by, any of the foregoing. 1.70. “Trial Enrollment Samples” means Samples obtained by or on behalf of Company or any of its Affiliates in connection with any Clinical Study of a Company Product and provided to FMI for testing in order to determine the eligibility of a patient for enrollment in such Clinical Study. 1.71. “United States” or “U.S.” means the United States of America and its territories and possessions (including the District of Columbia and Puerto Rico).
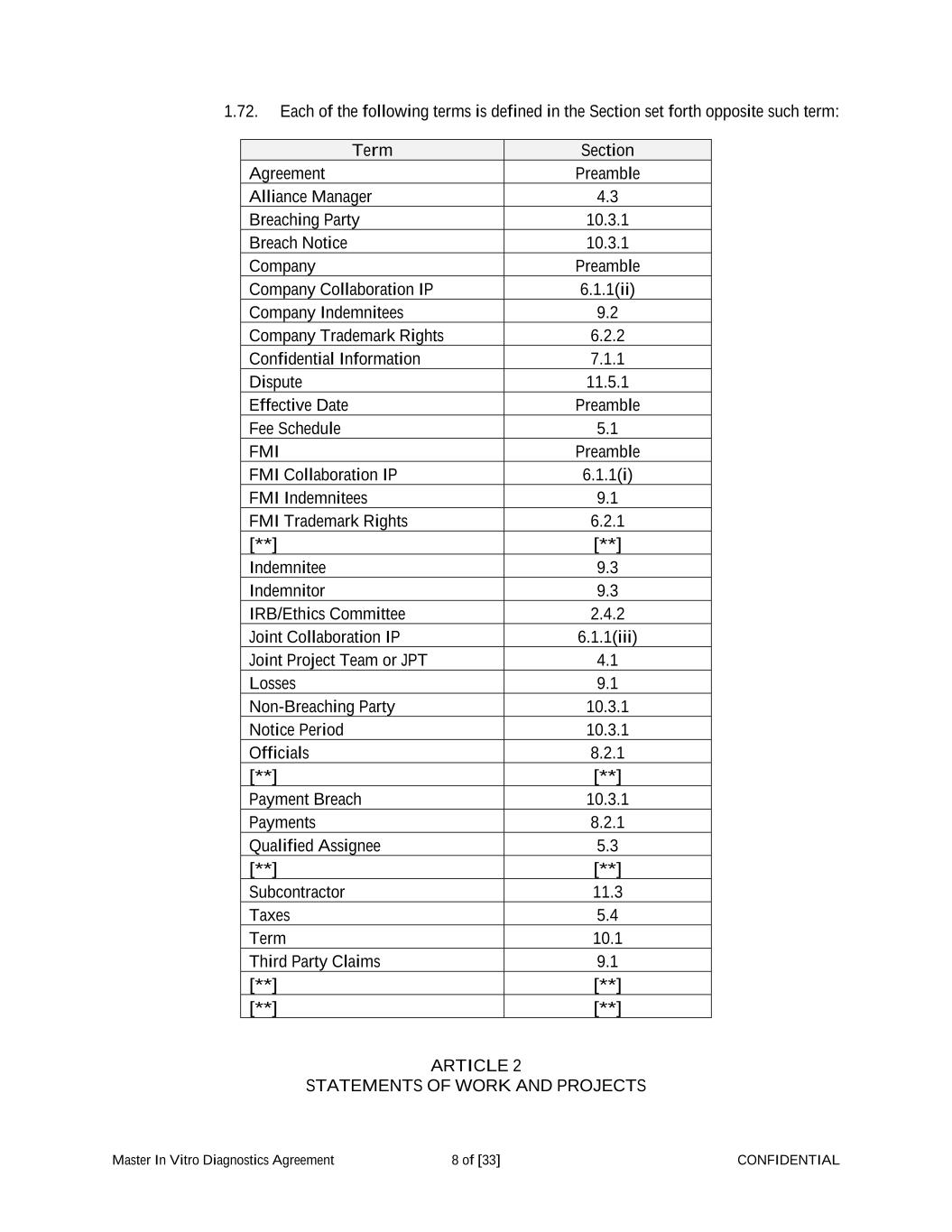
Master In Vitro Diagnostics Agreement 8 of [33] CONFIDENTIAL 1.72. Each of the following terms is defined in the Section set forth opposite such term: Term Section Agreement Preamble Alliance Manager 4.3 Breaching Party 10.3.1 Breach Notice 10.3.1 Company Preamble Company Collaboration IP 6.1.1(ii) Company Indemnitees 9.2 Company Trademark Rights 6.2.2 Confidential Information 7.1.1 Dispute 11.5.1 Effective Date Preamble Fee Schedule 5.1 FMI Preamble FMI Collaboration IP 6.1.1(i) FMI Indemnitees 9.1 FMI Trademark Rights 6.2.1 [**] [**] Indemnitee 9.3 Indemnitor 9.3 IRB/Ethics Committee 2.4.2 Joint Collaboration IP 6.1.1(iii) Joint Project Team or JPT 4.1 Losses 9.1 Non-Breaching Party 10.3.1 Notice Period 10.3.1 Officials 8.2.1 [**] [**] Payment Breach 10.3.1 Payments 8.2.1 Qualified Assignee 5.3 [**] [**] Subcontractor 11.3 Taxes 5.4 Term 10.1 Third Party Claims 9.1 [**] [**] [**] [**] ARTICLE 2 STATEMENTS OF WORK AND PROJECTS

Master In Vitro Diagnostics Agreement 9 of [33] CONFIDENTIAL 2.1. Statements of Work. 2.1.1. In General. In the event the Parties agree that a project should be conducted under this Agreement, then the Parties may negotiate, prepare and execute an SOW for such project. Upon execution of one or more SOWs, such SOW(s) shall be deemed to be incorporated into and subject to the terms and conditions of this Agreement, and this Agreement (including such SOW(s)) shall constitute the entire agreement between the Parties with respect to the subject matter of such SOW(s). If there is a conflict between the terms of this Agreement and the terms of an SOW, then the terms of this Agreement shall prevail, unless such SOW specifically and expressly supersedes this Agreement on a specific matter, in which case the terms of the SOW shall prevail, but only with respect to such SOW and such matter. Nothing herein shall create an express or implied obligation on the part of either Party to enter into any other agreement or any SOW(s). 2.1.2. Additional Markets. In the event that Company desires to Commercialize a Company Product for an Indication in a country or territory that is not a Market with respect to such Company Product and Indication, where the relevant Regulatory Authorities for such country or territory require that a Companion Diagnostic for such Company Product be available in such country or territory for any such Commercialization, Company may provide written notice to FMI of such desire and specifying such country or territory. Without limiting Section 2.1.1, the Parties shall discuss in good faith whether to amend the applicable SOW or enter into a new SOW to provide for FMI to conduct further Development of, and seek Regulatory Approval for, the applicable FMI Assay for use as a Companion Diagnostic for such Company Product for such Indication for such country or territory, and to provide for additional milestones or other payments to FMI in connection therewith. Upon the Parties entering into such amendment or new SOW, such additional country or territory will be considered a Market under the applicable SOW. 2.2. Performance of Activities. Each Party shall[**]to perform the Activities, including the provision of any [**], in each case, assigned or allocated to it under an applicable SOW. Notwithstanding the foregoing, the Parties acknowledge that each Party’s performance under each SOW [**] For clarity, as between the Parties, (i) Company shall, at its own expense, be responsible for the Development of the Company Product(s), including the conduct of the Clinical Studies for the Company Product(s)[**]. 2.3. Coordination. FMI shall keep Company reasonably informed, through the applicable JPT, of its Development Activities for each FMI Assay Developed under an SOW for use with a Company Product for each applicable Market and Indication. Company shall keep FMI reasonably informed, through the applicable JPT, of its Development activities (including any Activities) for each Company Product for which an FMI Assay is Developed under an SOW, to the extent that such Development activities could reasonably impact Development of such FMI Assay. 2.4. Materials. 2.4.1. In General. Company shall provide to FMI, free of charge, the Materials specified in each SOW. Without limitation to the foregoing, (i) Company shall provide to FMI, free of charge, any [**]. If FMI determines that any Materials provided by or on behalf of Company do not conform to their descriptions under the applicable SOW or are not suitable for the Activities under the applicable SOW, then Company shall provide new or replacement Materials. 2.4.2. Use and Disclosure. [**]. 2.4.3. Samples Requirements. Company shall ensure that all Samples transferred by or on behalf of Company to FMI under this Agreement will be or have been collected,

Master In Vitro Diagnostics Agreement 10 of [33] CONFIDENTIAL stored, handled, transported, and delivered in a manner appropriate to ensure compliance with all Applicable Law. To the extent Applicable Law requires any informed consent or other authorization for the collection or provision to FMI of any such Samples or any accompanying data, or for the use by or on behalf of FMI as permitted by this Agreement and any applicable SOW of any such Samples or accompanying data, then Company shall ensure that such informed consent or other authorization is obtained with a scope that permits such activities. With respect to any such Sample or accompanying data, (i) at FMI’s reasonable request, Company shall provide to FMI (a) a copy of any protocol for any Clinical Study pursuant to which such Sample or data was obtained and the institutional review board or other ethics committee (“IRB/Ethics Committee”) approval thereof, (b) a copy of any other necessary IRB/Ethics Committee approvals, (c) any applicable form of informed consent or other authorization and (d) a written attestation that all necessary approvals, informed consents or other authorizations have been obtained, and (ii) to the extent any amendment to any such protocol or form of informed consent or other authorization would impact the use of any FMI Assay in connection with a Clinical Study pursuant to any SOW, Company shall promptly inform FMI in writing of such amendment and provide to FMI any applicable updated versions of the items described in clauses (i)(a)-(d) above. FMI shall handle, store, use and, as applicable, transport the Samples provided to it by or on behalf of Company in a manner consistent with all Applicable Law. Company shall not, without first obtaining FMI’s prior written consent and appropriate informed consent, if applicable, deliver to FMI personally identifiable healthcare information or other data that could potentially identify a specific individual, in connection with the Samples or otherwise. Upon Company’s prompt written request following the end of the term of an SOW, FMI shall, at Company’s sole expense, return to Company any unused Samples associated with such SOW that were provided to FMI by or on behalf of Company. 2.5. Regulatory Approval of FMI Assays, Company Products and Rights of Reference. 2.5.1. FMI Assays. (i) FMI shall, at its own expense, have the sole right to prepare, obtain and maintain Regulatory Approvals for, and to conduct communications with the Regulatory Authorities regarding Regulatory Approvals for, any FMI Assay. As between the Parties, all Regulatory Documentation (including all Regulatory Approvals) generated by FMI or any of its Affiliates with respect to any FMI Assay anywhere in the world and all FMI Operational Documentation shall be owned by, and shall be the sole property and held in the name of, FMI or its designee. At FMI’s reasonable request, Company shall provide FMI with any Regulatory Documentation and other information (including Collaboration Data) in the Control and possession of Company or any of its Affiliates with respect to each Company Product as may be necessary for, or reasonably requested by, FMI or any of its Affiliates to obtain or maintain Regulatory Approvals for any FMI Assay Developed under this Agreement. Without limiting the foregoing: (a) if FMI determines that [**] then, at FMI’s request, Company shall, at its option, either [**] and (b) in the event that a Regulatory Authority requires post- PMA Approval or post-CE Marking Approval activities that are not Activities set forth in a development plan included in an applicable SOW as a condition of obtaining or maintaining PMA Approval or CE Marking Approval (as applicable) for use of any FMI Assay Developed pursuant to such SOW(s) with any Company Product for a Market and Indication, then [**]. (ii) FMI shall provide Company with an opportunity to review and comment on all regulatory filings that are [**] for use with the applicable Company Product for the applicable Indication, in each case, to the extent any such filing involves [**] such Company Product [**]

Master In Vitro Diagnostics Agreement 11 of [33] CONFIDENTIAL FMI shall [**] consider in good faith Company’s comments with respect thereto to the extent relating to such Company Product; provided that in no event shall FMI be obligated to delay the submission of any such regulatory filing due to Company’s failure to provide its comments in a timely manner. To the extent legally permissible, FMI shall provide Company with an opportunity to attend any scheduled meeting with a Regulatory Authority for the applicable Market(s) regarding obtaining Regulatory Approval of the applicable FMI Assay for use as an Approved IVD with the applicable Company Product for the applicable Indication solely to the extent such meeting includes (or is reasonably anticipated to include) a material discussion of such Company Product. At FMI’s reasonable request, Company shall participate in any such scheduled meeting. Notwithstanding anything contained in this Section 2.5 or any term or condition of this Agreement to the contrary, Company acknowledges and agrees that FMI shall have no obligation to provide or disclose to Company any documents or other materials (including Regulatory Documentation or other information) with respect to [**]. 2.5.2. Company Products. (i) Company shall, at its own expense, have the sole right to prepare, obtain and maintain Regulatory Approvals for, and to conduct communications with Regulatory Authorities regarding Regulatory Approvals for, any Company Product. As between the Parties, all Regulatory Documentation (including all Regulatory Approvals) generated by Company or any of its Affiliates with respect to any Company Product anywhere in the world shall be owned by, and shall be the sole property and held in the name of, Company or its designee. At Company’s reasonable request, FMI shall provide Company with any Regulatory Documentation and other information in the Control and possession of FMI or any of its Affiliates with respect to each FMI Assay Developed pursuant to this Agreement (including Collaboration Data) as may be necessary for, or reasonably requested by, Company or any of its Affiliates to refer to such FMI Assay in obtaining or maintaining Regulatory Approvals for any Company Product for each applicable Indication. (ii) Company shall provide FMI with an opportunity to review and comment on all regulatory filings that are required to be made to obtain Regulatory Approval of the applicable Company Product for the applicable Indication(s) and applicable Market(s) for use with the applicable FMI Assay, in each case, to the extent any such filing involves a material discussion of such FMI Assay and which discussion has not been the subject of a prior opportunity for review and comment by FMI. Company shall consider in good faith FMI’s comments with respect thereto to the extent relating to such FMI Assay; provided that in no event shall Company be obligated to delay the submission of any such regulatory filing due to FMI’s failure to provide its comments in a timely manner. To the extent legally permissible, Company shall provide FMI with an opportunity to attend any scheduled meeting with a Regulatory Authority for the applicable Market(s) regarding obtaining Regulatory Approval of the applicable Company Product for the applicable Indication solely to the extent such meeting includes (or is reasonably anticipated to include) a material discussion of an applicable FMI Assay. At Company’s reasonable request, FMI shall participate in any such scheduled meeting. 2.5.3. Rights of Reference. (i) Company shall, and shall ensure that its Affiliates shall, upon request provide FMI (and FMI’s designated Affiliates, (sub)licensees and subcontractors) with any appropriate letters or other similar documentation necessary to authorize such Person to cross-reference and rely (on a non-exclusive basis) upon the contents of Company’s or any of its Affiliate’s (or, to the extent Controlled by Company or any of its Affiliates, any of their respective (sub)licensee’s) Regulatory Documentation for any Company Product [**]. (ii) FMI shall, and shall ensure that its Affiliates shall, upon request provide Company (and Company’s designated Affiliates and (sub)licensees) with any appropriate letters

Master In Vitro Diagnostics Agreement 12 of [33] CONFIDENTIAL or other similar documentation necessary to authorize such Person to cross-reference and rely (on a non- exclusive basis) upon the contents of FMI’s or any of its Affiliate’s (or, to the extent Controlled by FMI or any of its Affiliates, any of their respective (sub)licensee’s) Regulatory Documentation for any FMI Assay Developed [**] pursuant to this Agreement [**]. 2.6. Compliance; Audits. 2.6.1. Compliance. Each of FMI and Company shall, and shall cause its Affiliates, and shall require its (sub)licensees and Subcontractors, to comply in all material respects with all Applicable Law with respect to its Development activities under this Agreement. 2.6.2. Audits. During the Term and no more than [**], on not less than [**] days’ prior written notice and during FMI’s normal business hours, Company shall have the right to audit or have audited by a Permitted Representative, solely to the extent necessary to confirm FMI’s compliance with CLIA, QSR and other Applicable Law with respect to the conduct of the Activities, [**] Notwithstanding anything to the contrary in this Section 2.6.2: (a) any audit conducted by or on behalf of Company [**] shall be deemed an audit under this Section 2.6.2[**] any audit conducted by or on behalf of Company under this Section 2.6.2 shall be deemed an audit[**]provided, however, that if Company [**] notifies FMI of any such audit [**]; and (b) if any Regulatory Authority wishes to conduct an inspection on one or more days reserved for Company to conduct, or have conducted, any audit pursuant to this Section 2.6.2, then FMI shall have the right, upon written or telephonic notice to Company and without liability, to cancel such audit and shall work with Company in good faith to reschedule such audit for one or more days that does not conflict with such inspection. 2.6.3. Permitted Representatives. To the extent Company elects to utilize the services of a Permitted Representative to perform an audit permitted by Section 2.6.2, the selection of such Permitted Representative shall be approved by FMI. Any auditor (including any Permitted Representative) shall be subject to FMI’s confidentiality, security and safety policies, and any audit shall not be unreasonably disruptive to FMI’s business operations, and shall be reasonable in scope and duration. Any information, records or other materials provided by FMI in connection with such audit as well as any report, summary or other documentation resulting from such audit shall constitute FMI’s Confidential Information. 2.7. Non-Exclusive Relationship. The Parties agree that this Agreement and any applicable SOW, and the relationship of the Parties hereunder and thereunder, are non-exclusive, including in the following non-limiting respects. [**]. ARTICLE 3 COMMERCIALIZATION 3.1. Coordination on Commercialization Activities. Unless a different time period is specified in an applicable SOW, no later than [**] months prior to the planned final submission to the applicable Regulatory Authority of an application for Regulatory Approval for an FMI Assay for use with the applicable Company Product for a given Market and Indication, the applicable JPT shall commence discussions of the high-level strategy (i) for FMI to make or cause to be made commercially available an FMI Assay for use with the Company Product for such Market and such Indication and (ii) for Company to make or cause to be made commercially available such Company Product for such Market and such

Master In Vitro Diagnostics Agreement 13 of [33] CONFIDENTIAL Indication, to the extent relevant to FMI’s activities pursuant to clause (i) of this sentence. The JPT shall serve as a forum for the Parties to discuss periodically such strategies and to coordinate such activities as appropriate from time to time or as the Parties may further agree. 3.2. FMI Obligations. Following receipt of the required Regulatory Approvals for the applicable FMI Assay for use with the applicable Company Product for the applicable Market(s) and Indication(s), [**]; provided that such obligation is not intended and shall not be construed to require FMI to engage in any promotion or marketing for any FMI Assay (for clarity, including any such promotion or marketing specific to any Company Product). 3.3. Company Obligations. Following receipt of the required Regulatory Approvals for both (i) the applicable Company Product and (ii) the applicable FMI Assay for use with such Company Product, in each case ((i) and (ii)), for the applicable Market(s) and Indication(s), [**]. 3.4. Commercialization Terms. As between the Parties, Company shall have the sole right to establish the terms of sale for, and otherwise Commercialize, any Company Product and FMI shall have the sole right to establish the terms of sale for, and otherwise Commercialize, any FMI Assay, in each case, [**]. 3.5. Statements and Compliance with Applicable Law. Each Party shall and shall cause its Affiliates and Subcontractors to, comply in all material respects with all Applicable Law with respect to the Commercialization of, in the case of FMI, any FMI Assay Developed, or constituting an Approved IVD, for use with a Company Product, and in the case of Company, any Company Product. ARTICLE 4 COLLABORATION MANAGEMENT 4.1. Joint Project Teams. Within [**] days after the effective date of each SOW, the Parties shall establish a joint project team (each, a “Joint Project Team” or “JPT”) for the project under such SOW, which shall consist of [**] representatives from each Party, each with the requisite experience and seniority to enable such representatives to make decisions on behalf of the Party such representative represents with respect to the issues falling within the jurisdiction of such JPT. For clarity, a representative of a Party may represent such Party on more than one JPT. [**]. Each Party may substitute one or more of its representatives to the JPT, or its designated chairperson, on written notice to the other Party. 4.1.1. Functions of Joint Project Teams. Each JPT shall: (i) serve as a forum for coordinating the Parties’ efforts to carry out a project under an applicable SOW and for discussing progress in relation to the same [**] (ii) discuss proposed amendments to the applicable SOW from time to time (and, for clarity, any amendments shall be effective only if approved by the Parties in writing in accordance with Section 11.8); (iii) discuss the overall strategy, including the submission plans, for obtaining and maintaining Regulatory Approval of (a) each FMI Assay for use with the applicable Company Product for the applicable Indication and Market and (b) each Company Product, to the extent relating to or likely to impact any applicable FMI Assay or Regulatory Approval thereof; (iv) discuss in good faith revised milestone events and milestone payment amounts contained in the applicable Fee Schedule to the extent such revision is provided for pursuant to Section 5.2; and (v) conduct such other responsibilities as may be assigned to each JPT hereunder or in accordance with this Agreement or an applicable SOW, or as may be mutually agreed by the Parties in writing. 4.2. General Provisions Applicable to Joint Project Teams. Each JPT shall meet as agreed by the Parties, in person [**] or by teleconference or video conference. The chairperson of the

Master In Vitro Diagnostics Agreement 14 of [33] CONFIDENTIAL applicable JPT shall be responsible for calling meetings, coordinating the creation of meeting agendas, and circulating minutes of each meeting for approval by the Parties. A meeting of a given JPT shall require the presence of at least [**] appointed by each Party. Representatives of the Parties on a JPT may attend meetings thereof either in person or by telephone, video conference or similar means in which each participant can hear what is said by, and be heard by, the other participants. Representation by proxy shall be allowed. Alliance Managers and other employees or consultants of a Party who are not representatives of the Parties on a JPT may attend meetings of such JPT; provided that such attendees (i) are subject to written obligations of confidentiality and non-use with respect to Confidential Information substantially similar to the obligations of confidentiality and non-use of the applicable Party pursuant to Article 7 (with a duration of confidentiality and non-use that is commercially reasonable under the circumstances); and (ii) in the case of non-employees of a Party, such attendance shall be subject to the consent of the other Party, which shall not be unreasonably withheld, conditioned or delayed. Each Party shall be responsible for all of its own expenses of participating in JPT meetings. 4.2.1. Decision-Making. The Parties agree that each JPT’s role shall be limited to coordination and providing a forum for discussion with regard to matters within the functions of such JPT and [**] provided that this sentence is not intended, and shall not be construed, to relieve either Party from its obligations under this Agreement. 4.2.2. Limitations on Authority. Each Party shall retain the rights, powers, and discretion granted to it under this Agreement and no such rights, powers, or discretion shall be delegated to or vested in any JPT unless such delegation or vesting of rights is expressly provided for in this Agreement or an applicable SOW, or the Parties otherwise expressly so agree in writing. No JPT shall have the power to amend, modify, or waive compliance with any provision of this Agreement or any applicable SOW. 4.3. Alliance Managers. Each Party shall appoint [**] who shall oversee contact between the Parties for all matters between meetings of the JPTs and shall have such other responsibilities as the Parties may agree in writing after the Effective Date (the “Alliance Manager”). Each Party’s Alliance Manager may be replaced at any time on written notice to the other Party. The Alliance Managers shall not have decision-making authority. For clarity, each Party’s Alliance Manager may, if elected by such Party, also serve as a representative of such Party on one or more JPTs. ARTICLE 5 PAYMENTS AND RECORDS 5.1. Fee Schedules for SOWs. Each SOW shall include a schedule of fees applicable to the project performed thereunder (each, a “Fee Schedule”), which may generally include: (i) agreed milestone payments by Company upon the achievement of corresponding milestone events set forth in such SOW and (ii) per Sample acquisition (as applicable) and testing fees for any genomic profiling activities under such SOW. 5.2. Milestones. Company shall pay to FMI the non-refundable, non-creditable milestone payments as specified in each applicable SOW upon the achievement of the corresponding milestone events and in accordance with the provisions of this Article 5. Company acknowledges and agrees that the inclusion of any milestone events with respect to any FMI Assay in an applicable SOW shall not be construed as implying that such milestone events can or will be achieved. In the event that [**] then the Parties shall discuss in good faith and adopt revised milestone events and milestone payment amounts or other fee adjustments to ensure FMI is fully compensated for the performance of Development Activities under the applicable SOW.

Master In Vitro Diagnostics Agreement 15 of [33] CONFIDENTIAL 5.3. Covenant of Company. Company covenants to FMI that, if Company assigns this Agreement to a Third Party, then, unless otherwise agreed by the Parties in writing, Company shall remain responsible for all payments hereunder; provided that if Company assigns this Agreement to a Third Party that is a Qualified Assignee in accordance with Section 11.4.1, then Company shall no longer be responsible for such payments as of the effective date of such assignment to the extent the obligation to make such payments is assumed in writing by such Qualified Assignee. “Qualified Assignee” means, [**]. 5.4. Miscellaneous. FMI shall invoice Company (i) following achievement of the applicable milestone event with respect to milestone payments and (ii) [**] with respect to other payments. Each invoice shall be submitted electronically and shall contain a statement of the milestone event achieved or other Activities performed with respect to the applicable payments [**] Company shall pay each invoice within [**] days of receipt thereof. All payments to FMI shall be remitted by deposit of United States Dollars in the requisite amount to such bank account as FMI may from time to time designate by notice to Company. Neither Party shall have the right to offset any amount owed by the other Party to such first Party under or in connection with this Agreement. All fees set forth in this Agreement are exclusive of sales and use taxes, including all applicable goods and services tax, value- added tax (VAT), local taxes, applicable duties, electronic delivery taxes, excise taxes, levies and import fees (collectively, “Taxes”). If applicable, Company shall pay any Taxes that are imposed by Applicable Law in connection with payments by Company to FMI under this Agreement. If any payment owed FMI is not paid when due, then Company shall pay interest thereon (before and after any judgment) at an annual rate (but with interest accruing on a daily basis) of [**] percent ([**]%), such interest to run from the date on which such payment became due until payment thereof in full together with such interest. ARTICLE 6 INTELLECTUAL PROPERTY 6.1. Collaboration IP. 6.1.1. Ownership and Use of Collaboration IP. Subject to the license grant in Section 6.3, as between the Parties: (i) FMI shall own all right, title and interest in and to: (a) [**]; (b) [**]; (c) [**]; and (d) [**]. (ii) Company shall own all right, title and interest in and to: (a) [**]; (b) [**]; (c) [**]; (d) [**]; and

Master In Vitro Diagnostics Agreement 16 of [33] CONFIDENTIAL (e) [**]. (iii) The Parties shall each own an equal, undivided interest in and to: (a) [**]; (b) [**]; and (c) [**]. (iv) [**]. (v) Each Party shall make reasonable efforts to promptly disclose to the other Party in writing the conception, reduction to practice, generation or other development by or on behalf of such first Party or any of its Affiliates of any invention included in the Joint Collaboration IP. FMI shall make reasonable efforts to promptly disclose to Company in writing the conception, reduction to practice, generation or other development by or on behalf of FMI or any of its Affiliates of any invention included in the Company Collaboration IP. Company shall make reasonable efforts to promptly disclose to FMI in writing the conception, reduction to practice, generation or other development by or on behalf of Company or any of its Affiliates of any invention included in the FMI Collaboration IP. Except as expressly set forth in this Section 6.1.1(v) and as required pursuant to Section 2.5.1 neither Party shall have the affirmative duty to disclose any Collaboration IP to the other Party. (vi) Each Party shall, and does hereby, assign, and shall cause its Affiliates and its and their (sub)licensees to so assign, to the other Party, without additional compensation, such right, title and interest in and to any Know-How, improvements and other inventions, as well as any intellectual property rights with respect thereto (including Patents), as is reasonably necessary to fully effect ownership as provided for in this Section 6.1.1. 6.1.2. Prosecution and Maintenance of Patents Within Collaboration IP. As between the Parties, Company shall have the sole right, but not the obligation, using counsel of its own choice, to prepare, file, prosecute and maintain Patents within the Company Collaboration IP, worldwide, and to be responsible for any related interference, re-issuance, re-examination, review, opposition proceedings and patent term extensions, in each case, at its sole cost and expense. As between the Parties, FMI shall have the sole right, but not the obligation, using counsel of its own choice, to prepare, file, prosecute and maintain Patents within the FMI Collaboration IP worldwide, and to be responsible for any related interference, re-issuance, re-examination, review, opposition proceedings and patent term extensions, in each case, at its sole cost and expense. In the case of any Patents within the Joint Collaboration IP, [**]. Notwithstanding the foregoing, the Parties shall cooperate and implement reasonable Patent filing and prosecution strategies (including filing divisionals, continuations or otherwise) so that, to the extent reasonably feasible, claims that cover the inventions constituting Company Collaboration IP, FMI Collaboration IP and Joint Collaboration IP, are pursued in mutually exclusive patent applications. 6.1.3. Enforcement and Defense of Collaboration IP. As between the Parties, Company, at its sole cost and expense and using counsel of its own choice, shall have the sole right, but not the obligation, to prosecute infringement or misappropriation of and to defend any alleged or threatened assertion of invalidity or unenforceability with respect to Company Collaboration IP. As between the Parties, FMI, at its sole cost and expense and using counsel of its own choice, shall have the sole right, but not the obligation, to prosecute infringement or misappropriation of and to defend any alleged or threatened assertion of invalidity or unenforceability with respect to FMI Collaboration IP. In the case of any Joint Collaboration IP, [**].

Master In Vitro Diagnostics Agreement 17 of [33] CONFIDENTIAL 6.2. Trademark License Grants. 6.2.1. Subject to Section 6.2.3, FMI, on behalf of itself and its Affiliates, hereby grants to Company and its Affiliates a non-exclusive, royalty-free right and license, with the right to grant sublicenses, to use the FMI Trademark Rights, if applicable, for use in performance of the Activities and to refer to any FMI Assay constituting an Approved IVD for use with each Company Product in Exploiting such Company Product, in each case, for the applicable Indication(s) and Market(s); provided, however, that any such reference shall be limited to information included in the label for such FMI Assay for the applicable Market. Company agrees that any use of the FMI Trademark Rights by Company or its Affiliates or sublicensees shall inure to the benefit of FMI. For purposes of this Agreement, “FMI Trademark Rights” means (i) any Trademark Rights used by or on behalf of FMI or any of its Affiliates in connection with the Commercialization of any FMI Assay (other than the Trademark Rights Controlled by Company and any of its Affiliates) that are Controlled by FMI or any of its Affiliates as of the Effective Date or during the Term; and (ii) the domain names Controlled by FMI or any of its Affiliates as of the Effective Date or during the Term, which domain names incorporate one or more of the Trademark Rights described in clause (i) as all or part of their URL address. 6.2.2. Subject to Section 6.2.3, Company, on behalf of itself and its Affiliates, hereby grants to FMI and its Affiliates a non-exclusive, royalty-free right and license, with the right to grant sublicenses, to use the Company Trademark Rights, if applicable, for use in performance of the Activities and in Exploiting any FMI Assay constituting an Approved IVD for use with each Company Product, in each case, for the applicable Indication(s) and Market(s); provided, however, that any reference to Company or the applicable Company Product made under such right and license shall be limited to information included in the label for such Company Product for the applicable Market. FMI agrees that any use of the Company Trademarks Rights by FMI or its Affiliates or sublicensees shall inure to the benefit of Company. For purposes of this Agreement, “Company Trademark Rights” means (i) any Trademark Rights used by or on behalf of Company or any of its Affiliates in connection with the Commercialization of the Company Product(s) (other than the Trademark Rights Controlled by FMI and any of its Affiliates) that are Controlled by Company or any of its Affiliates as of the Effective Date or during the Term; and (ii) the domain names Controlled by Company or any of its Affiliates as of the Effective Date or during the Term, which domain names incorporate one or more of the Trademark Rights described in clause (i) as all or part of their URL address. 6.2.3. Each Party and its Affiliates shall, and shall require that any sublicensee shall, [**]. 6.3. [**]. 6.4. Third Party Licenses. 6.4.1. FMI shall promptly notify Company in writing in the event [**]. 6.4.2. With regard to any [**]. 6.4.3. Except as otherwise provided in this Section 6.4, each Party shall be [**] responsible for any other [**]fees that it or any of its Affiliates may incur in performing [**]. For clarity, nothing in this Section 6.4 is intended to restrict Company from pursuing any [**] Therapeutic Product. 6.5. Restricted Data. Prior to providing any [**] notwithstanding anything to the contrary in this Agreement or any SOW, the provisions of Attachment B shall apply.

Master In Vitro Diagnostics Agreement 18 of [33] CONFIDENTIAL 6.6. Retained Rights. Neither Party grants to the other Party under this Agreement any intellectual property licenses or rights, express or implied, by estoppel or otherwise, other than those licenses or rights explicitly set forth in this Agreement. [**]. ARTICLE 7 CONFIDENTIALITY AND NON-DISCLOSURE 7.1. Confidentiality Obligations. 7.1.1. During the Term and for a period of [**] years thereafter, each Party shall and shall cause its officers, directors, employees and agents to (i) keep confidential, in a manner consistent with such Party’s treatment of its own confidential or proprietary information, but in no event less than reasonable measures, (ii) not publish or otherwise disclose, directly or indirectly, except to the extent such disclosure is expressly permitted by the terms of this Agreement or any applicable SOW, and (iii) not use, except for the purposes of fulfilling its obligations or exercising its rights under this Agreement, in each case ((i)-(iii)), any Confidential Information of the other Party. “Confidential Information” of a Party means all data, materials and information, including all Know-How and other business, financial, legal or technical information, in any form (written, oral, photographic, electronic, magnetic, or otherwise) provided by or on behalf of such Party or its Affiliate to the other Party or its Affiliate in connection with this Agreement or an applicable SOW, whether prior to, on or after the Effective Date, that is marked or otherwise identified as confidential or proprietary at the time of disclosure or that a reasonable person would, by its nature, understand to be confidential or proprietary, including all copies thereof. 7.1.2. Notwithstanding Section 7.1.1, (i) the terms of this Agreement and each SOW shall be deemed the Confidential Information of both Parties (and both Parties shall be deemed to be the Receiving Party and the Disclosing Party with respect thereto); (ii) Confidential Information constituting Know-How included in the FMI Collaboration IP shall be deemed the Confidential Information of FMI (and FMI shall be deemed to be the Disclosing Party and Company shall be deemed to be the Receiving Party with respect thereto); (iii) Confidential Information constituting Know-How included in the Company Collaboration IP shall be deemed the Confidential Information of Company (and Company shall be deemed to be the Disclosing Party and FMI shall be deemed to be the Receiving Party with respect thereto); and (iv) Confidential Information constituting Know-How included in the Joint Collaboration IP shall be deemed the Confidential Information of both Parties (and both Parties shall be deemed to be the Receiving Party and the Disclosing Party with respect thereto). 7.1.3. Notwithstanding the foregoing provisions of this Section 7.1, Section 7.1.1 shall not apply to the Receiving Party with respect to any Confidential Information of the Disclosing Party to the extent it can be established by the Receiving Party through competent evidence that such Confidential Information: (i) is or hereafter becomes publicly available through no breach of any obligation of confidentiality by the Receiving Party; (ii) is subsequently received by the Receiving Party from a Third Party who is not bound by any obligation of confidentiality with respect to such information; (iii) was in the Receiving Party’s possession prior to disclosure by the Disclosing Party without any obligation of confidentiality with respect to such information; or (iv) is independently developed by or for the Receiving Party without reference to the Disclosing Party’s Confidential Information; provided that the exceptions under clauses (iii) and (iv) shall not apply to the Receiving Party with respect to Confidential Information that a Party generates but is deemed to be the Receiving Party with respect thereto pursuant to Section 7.1.2. Notwithstanding anything to the contrary herein, the exceptions set forth in this Section 7.1.3 shall not apply with respect to the terms of this Agreement or any SOW.

Master In Vitro Diagnostics Agreement 19 of [33] CONFIDENTIAL 7.2. Permitted Disclosures. The Receiving Party may disclose Confidential Information of the Disclosing Party to the extent that such disclosure is: (i) made in response to a valid order of a court of competent jurisdiction or other supra-national, federal, national, regional, state, provincial or local governmental or regulatory body of competent jurisdiction or, if in the reasonable opinion of the Receiving Party’s legal counsel, such disclosure is otherwise required by Applicable Law, including by the U.S. Securities Exchange Commission, or by any stock exchange upon which such Party’s securities are listed or to which an application for listing has been submitted; provided, however, that the Receiving Party shall first have given notice to the Disclosing Party (to the extent permitted by Applicable Law) and given the Disclosing Party a reasonable opportunity to quash such order or to obtain a protective order or confidential treatment requiring that the Confidential Information and documents that are the subject of such order be held in confidence by such court or agency or, if disclosed, be used only for the purposes for which the order was issued; (ii) subject to the Disclosing Party’s prior written consent, not to be unreasonably withheld, conditioned or delayed, made by or on behalf of the Receiving Party to a patent authority for purposes of obtaining or enforcing Patents included in the Collaboration IP, in a manner not inconsistent with Section 6.1; (iii) made by or on behalf of the Receiving Party to potential or actual investors or acquirers as may be reasonably necessary in connection with their evaluation of such potential or actual investment or acquisition; [**] provided, however, in each case ((i)- (v)), that the Receiving Party shall take reasonable measures to assure confidential treatment of such information, to the extent such protection is available; and provided, further, that in the that case of clause (iii) or (v) such Persons shall be subject to obligations of confidentiality and non-use with respect to such Confidential Information substantially similar to the obligations of confidentiality and non-use of the Receiving Party pursuant to this Article 7 (with a duration of confidentiality and non-use that is commercially reasonable under the circumstances). 7.3. Use of Name. Except as expressly provided herein, including Section 6.2, in connection with this Agreement or any Activities hereunder, neither Party shall mention or otherwise use the name, logo or Trademark Rights of the other Party or any of its Affiliates or any of its or their (sub)licensees (or any abbreviation or adaptation thereof) in any publication, press release, marketing and promotional material or other form of publicity without the prior written approval of such other Party. The restrictions imposed by this Section 7.3 shall not prohibit either Party from (i) making any disclosure identifying the other Party to the extent required in connection with its exercise of its rights or obligations under this Agreement or any applicable SOW or (ii) making any disclosure identifying the other Party that is required by Applicable Law or the rules of a stock exchange on which the securities of the Party making such disclosure are listed (or to which an application for listing has been submitted). 7.4. Public Announcements. Neither Party shall issue any public announcement, press release or other public disclosure regarding the terms of this Agreement or the terms of any SOW without the other Party’s prior written consent, except for any such disclosure that is, in the opinion of the disclosing Party’s counsel, required by Applicable Law and with respect to which reasonable prior notice and opportunity to comment thereon is given to the other Party. 7.5. Publication. Each Party recognizes that the publication of papers regarding results of and other information regarding activities under this Agreement or any applicable SOW, including oral presentations and abstracts, may be beneficial to both Parties; provided that such publications are subject to reasonable controls to protect Confidential Information. Accordingly, each Party shall have the right to review and approve (not to be unreasonably delayed or withheld) any paper proposed for publication by the other Party, including any oral presentation or abstract, which includes Collaboration Data [**] or which otherwise includes Confidential Information of the other Party the disclosure of which in such publication is not otherwise permitted under Section 7.1 or 7.2. Notwithstanding the foregoing, (i) if a Party requests approval to publish or publicly present any Confidential Information constituting Know-How included in the Joint Collaboration IP, the other Party shall consider such request in good faith, and (ii) the publishing or presenting Party shall (a) subject to

Master In Vitro Diagnostics Agreement 20 of [33] CONFIDENTIAL clause (i) above, comply with the other Party’s request to delete from any such paper or presentation any Confidential Information of the other Party the disclosure of which in such publication is not otherwise permitted under Section 7.1 or 7.2 and (b) withhold publication of any such paper or presentation for up to [**] days after such other Party’s written request in order to permit the Parties to obtain patent protection if either Party deems it reasonably necessary. 7.6. Return of Confidential Information. Upon expiration or termination of this Agreement or an applicable SOW for any reason, either Party may request in writing and the non- requesting Party shall either, with respect to Confidential Information of the other Party to which such non-requesting Party does not retain rights under the surviving provisions of this Agreement or such SOW: (i) promptly destroy all copies of such Confidential Information in the possession or control of the non-requesting Party and confirm such destruction in writing to the requesting Party; or (ii) promptly deliver to the requesting Party, at the requesting Party’s sole cost and expense, all copies of such Confidential Information in the possession or control of the non-requesting Party. Notwithstanding the foregoing, the non-requesting Party shall be permitted to retain such Confidential Information (a) to the extent reasonably necessary or useful for purposes of performing any continuing obligations or exercising any ongoing rights hereunder or under any SOW and, in any event, a single copy of such Confidential Information for archival purposes and (b) any computer records or files containing such Confidential Information that have been created solely by such non-requesting Party’s automatic archiving and back- up procedures, to the extent created and retained in a manner consistent with such non-requesting Party’s standard archiving and back-up procedures, but not for any other uses or purposes. All Confidential Information shall continue to be subject to the terms of this Agreement for the period set forth in Section 7.1.1. ARTICLE 8 REPRESENTATIONS, WARRANTIES AND COVENANT 8.1. Representations, Warranties and Covenant. 8.1.1. Mutual Representations, Warranties and Covenant. FMI and Company each represents and warrants to the other Party, as of the Effective Date, and, in the case of any representation and warranty as it relates to an SOW, as of the applicable SOW Effective Date, and covenants to the other Party that: (i) it is a corporation duly organized, validly existing and in good standing under the laws of the jurisdiction of its organization and has all requisite power and authority, corporate or otherwise, to execute, deliver and perform this Agreement; (ii) the execution and delivery of this Agreement and the applicable SOW and the performance by it of the transactions contemplated hereby have been, or in the case of an SOW, will be as of the applicable SOW Effective Date, duly authorized by all necessary corporate action on its part; (iii) this Agreement is, and, in the case of the applicable SOW, will be as of the applicable SOW Effective Date, a legal, valid and binding obligation of such Party enforceable against it in accordance with the terms and conditions hereof and thereof, subject to the effects of bankruptcy, insolvency or other laws of general application affecting the enforcement of creditor rights, judicial principles affecting the availability of specific performance and general principles of equity (whether enforceability is considered a proceeding at law or equity); (iv) it is not under [**] any obligation, contractual or otherwise, to any Person that conflicts with the terms of this Agreement; and (v) neither it nor any of its Affiliates [**] has been debarred or is subject to debarment and neither it nor any of its Affiliates [**] will use in any capacity, in connection with the Activities to be performed under this Agreement, any Person who has been debarred pursuant to Section 306 of the FFDCA or who is the subject of a conviction described in such section. Each Party covenants that it will inform the other Party in writing promptly upon becoming aware that it or any such Person who is performing Activities hereunder is debarred or is the subject of a conviction described in Section 306 of the FFDCA, or if any action, suit, claim, investigation or legal or administrative proceeding is pending or, to the best of its or its

Master In Vitro Diagnostics Agreement 21 of [33] CONFIDENTIAL Affiliates’ knowledge, is threatened, relating to the debarment or conviction of it or any such Person performing Activities hereunder. 8.1.2. Additional Representation and Warranty and Covenant of Each Party. Each Party represents and warrants to the other Party, as of the Effective Date, and, as it relates to an SOW, as of the applicable SOW Effective Date, and covenants to the other Party that, subject to Section 11.4.2, each Party has and will at all times have all rights with respect to any [**]. 8.2. Covenants of the Parties. 8.2.1. Each Party agrees that neither it, nor anyone acting on its behalf, shall, either directly or indirectly, offer, make, or promise any payment of money or other assets in connection with this Agreement (collectively, “Payments”) to any government or political party officials, officials of international public organizations, candidates for public office, or persons acting on behalf of any of the foregoing (collectively, “Officials”) where such Payments would violate Applicable Law. 8.2.2. Each Party acknowledges that no employee of the other Party or its Affiliates shall have authority to give direction, either written or oral, relating to the making of any commitment by such first Party or its agents to any Third Party in violation of the terms of this Section 8.2. 8.3. DISCLAIMER OF WARRANTIES. NEITHER PARTY MAKES ANY REPRESENTATIONS OR GRANTS ANY WARRANTIES, EXPRESS OR IMPLIED, THAT THE OBJECTIVES OF ANY SOW CAN OR WILL BE ACHIEVED OR AS TO THE TIMING OR COST AND EXPENSE ASSOCIATED WITH THE ACHIEVEMENT OF ANY SUCH OBJECTIVES. EXCEPT FOR THE EXPRESS WARRANTIES SET FORTH IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATIONS OR GRANTS ANY WARRANTIES HEREUNDER, EXPRESS OR IMPLIED, EITHER IN FACT OR BY OPERATION OF LAW, BY STATUTE OR OTHERWISE AND EACH PARTY SPECIFICALLY DISCLAIMS HEREUNDER ANY OTHER WARRANTIES, WHETHER WRITTEN OR ORAL OR EXPRESS OR IMPLIED, INCLUDING ANY WARRANTY OF QUALITY, MERCHANTABILITY OR FITNESS FOR A PARTICULAR USE OR PURPOSE OR ANY WARRANTY AS TO THE VALIDITY OF ANY PATENTS OR THE NON- INFRINGEMENT OF ANY INTELLECTUAL PROPERTY RIGHTS OF THIRD PARTIES. ARTICLE 9 INDEMNITY 9.1. Indemnification of FMI. Company shall defend FMI, its Affiliates and its and their respective [**]; except in the cases of clause (i) and clause (ii), for any Losses to the extent arising from or occurring as a result of (a) [**]. 9.2. Indemnification of Company. FMI shall defend Company, its Affiliates, and its and their respective [**]; except in the cases of clause (i) and clause (ii), for any Losses to the extent arising from or occurring as a result of [**]. 9.3. Indemnification Procedures. A Party seeking indemnification (an “Indemnitee”) shall (i) provide the indemnifying Party (“Indemnitor”) with written notice, within a reasonable time after notice of any applicable Third Party Claim is received by Indemnitee; (ii) allow Indemnitor to have sole control of the defense or settlement of the Third Party Claim; provided, however, that Indemnitor shall not settle any Third Party Claim in a manner which may impose any obligation on or have a material adverse impact on Indemnitee without the prior written consent of the Indemnitee; and

Master In Vitro Diagnostics Agreement 22 of [33] CONFIDENTIAL (iii) provide Indemnitor with reasonable assistance, information and authority reasonably necessary to perform Indemnitor’s obligations. 9.4. LIMITATIONS OF LIABILITY. 9.4.1. SPECIAL, INDIRECT AND OTHER LOSSES. [**]. 9.4.2. GENERAL LIMITATION. [**]. ARTICLE 10 TERM AND TERMINATION 10.1. Term. The term of this Agreement (the “Term”) commences on the Effective Date and continues until terminated in accordance with Section 10.2, 10.3 or 10.4; provided, however, that each SOW shall become effective as of the applicable SOW Effective Date. 10.2. Company Termination at Will. Subject to the terms and conditions of any applicable SOW, including any termination fees provided for therein, Company may terminate this Agreement, either in its entirety or on a SOW-by-SOW or on a Program-by-Program basis, for convenience by providing written notice of its intent to terminate to FMI, in which case, such termination shall be effective [**] days after FMI’s receipt of such written notice. 10.3. Termination of this Agreement in its Entirety. 10.3.1. Material Breach. Without limiting its other rights or remedies under this Agreement, either Party (in such capacity, the “Non-Breaching Party”) may terminate this Agreement in its entirety (including all SOWs then in effect) immediately upon written notice to the other Party in the event the other Party (in such capacity, the “Breaching Party”) (i) has breached any of its material obligations under this Agreement and (ii) has failed to cure such breach within [**] days following receipt of written notice from the Non-Breaching Party of such breach (such period of time, the “Notice Period” and such written notice, with respect to any material breach under this Section 10.3.1 or Section 10.4.1, a “Breach Notice”); provided that such Notice Period shall be [**] days in the event of a failure to make any payment when due (“Payment Breach”) and provided further that, except for Payment Breaches, the Notice Period will automatically be extended for a period of time, not to exceed [**] days following delivery of the Breach Notice, in the event that (a) such breach cannot be cured within the Notice Period and (b) the Breaching Party commences actions to cure such breach within the Notice Period and thereafter diligently continues such actions. 10.3.2. Mutual Agreement. The Parties may terminate this Agreement in its entirety (including all SOWs then in effect) at any time by mutual written agreement. 10.3.3. Insolvency. If either Party: (i) files for protection under bankruptcy or insolvency laws; (ii) makes an assignment for the benefit of creditors; (iii) appoints or suffers appointment of a receiver or trustee over substantially all of its property that is not discharged within [**] days after such filing; (iv) proposes a written agreement of composition or extension of its debts; (v) proposes or is a party to any dissolution or liquidation; (vi) files a petition under any bankruptcy or insolvency act or has any such petition filed against it that is not discharged within [**] days of the filing thereof; or (vii) admits in writing its inability generally to meet its obligations as they fall due in the general course, then the other Party may terminate this Agreement in its entirety (including all SOWs then in effect) effective immediately upon written notice to such Party.

Master In Vitro Diagnostics Agreement 23 of [33] CONFIDENTIAL 10.4. Termination of this Agreement with respect to a Program. 10.4.1. Material Breach. Without limitation to Section 10.3.1, if the Breaching Party has breached any of its material obligations under this Agreement with respect to a Program, in addition to any other right or remedy the Non-Breaching Party may have, the Non-Breaching Party may terminate this Agreement with respect to such Program immediately upon written notice to the Breaching Party in the event that (i) the Non-Breaching Party has delivered a Breach Notice to the Breaching Party and (ii) the Breaching Party has failed to cure the applicable breach within the Notice Period; provided that such Notice Period shall be [**] days in the event of a Payment Breach; and provided further that, except for Payment Breaches, the Notice Period will automatically be extended for a period of time, not to exceed [**] days following delivery of the Breach Notice, in the event that (a) such breach cannot be cured within the Notice Period and (b) the Breaching Party commences actions to cure such breach within the Notice Period and thereafter diligently continues such actions. 10.4.2. Mutual Agreement. The Parties may terminate this Agreement with respect to any Program at any time by mutual written agreement. 10.4.3. Deadlock. In the event that either Party declares a deadlock with respect to a Program in accordance with Section 11.5.2, then either may terminate this Agreement with respect to such Program by providing [**] days’ prior written notice to the other Party; provided that the Parties shall continue to discuss the issues with respect to which the applicable Party declared a deadlock in good faith during such notice period; and provided, further, that such termination shall not become effective at the end of such notice period if FMI agrees to proceed without revisions to the milestone events, milestone payment amounts or other fee adjustments or the Parties otherwise reach agreement on the issue(s) with respect to which a Party declared such deadlock. 10.4.4. Inability to Obtain Regulatory Approval for an FMI Assay. In the event that an applicable Regulatory Authority provides written notice of its determination that such Regulatory Authority will not grant a Regulatory Approval for an FMI Assay for use with an applicable Company Product for an applicable Market and Indication, then, following receipt of such notice, either Party may terminate this Agreement with respect to the applicable Program by providing [**] days’ prior written notice to the other Party. 10.4.5. Inability to Obtain Regulatory Approval for a Company Product. In the event that an applicable Regulatory Authority provides written notice of its determination that such Regulatory Authority will not grant a Regulatory Approval for a Company Product for an applicable Market and Indication, then, following receipt of such notice, either Party may terminate this Agreement with respect to the applicable Program by providing [**] days’ prior written notice to the other Party. 10.4.6. Inability to Secure Third Party Licenses. [**]. 10.4.7. Other Termination Rights of FMI. (i) [**]. (ii) [**]. 10.5. Rights in Bankruptcy. All rights and licenses granted under or pursuant to this Agreement (including any SOW) by Company or FMI are and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code or any analogous provisions in any other country or jurisdiction, licenses of rights to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. The Parties agree that the Parties, as licensees of such rights under this Agreement,

Master In Vitro Diagnostics Agreement 24 of [33] CONFIDENTIAL shall retain and may fully exercise all of their rights and elections under the U.S. Bankruptcy Code or any analogous provisions in any other country or jurisdiction. 10.6. Certain Consequences of Termination. 10.6.1. Termination of Agreement in its Entirety. If this Agreement is terminated in its entirety pursuant to Section 10.2 or 10.3, then the following terms and conditions shall apply (without limitation to Section 10.8), except in the event of termination by Company pursuant to Section 10.3.1: (i) [**]; (ii) [**]; (iii) [**]; (iv) the license granted by Company to FMI in Section 6.2.2 shall survive in the case of any such termination occurring after Regulatory Approval of any FMI Assay for use as an Approved IVD with a Company Product; and (v) [**]. 10.6.2. Post-Termination Payment Obligations. (i) Payment of Amounts Due. If this Agreement is terminated in its entirety or with respect to any Program, or portion thereof, then Company shall (if applicable, with respect to the terminated Program): (a) [**]; (b) [**] (c) [**]; (d) [**]; and (e) [**]. The amounts due from Company to FMI pursuant to this Section 10.6.2 shall be paid within [**] days after FMI provides Company an invoice with respect thereto (or, with respect to clauses (a) through (e) above, by the date on which such amount is due under Section 5.4, if earlier). (ii) Termination Fees. Without limitation to any other amounts that may become payable pursuant to Section 10.6.2(i), if (a) Company terminates this Agreement in its entirety or with respect to any Program pursuant to Section 10.2 and (b) any applicable SOW provides for payment of a termination fee in connection with such termination, then Company shall pay to FMI such termination fee within [**] days after FMI provides Company an invoice therefor. 10.7. Remedies. Except as otherwise expressly provided herein, termination of this Agreement or any SOW in accordance with the provisions hereof shall not limit remedies that may otherwise be available in law or equity. 10.8. Accrued Rights; Surviving Obligations.

Master In Vitro Diagnostics Agreement 25 of [33] CONFIDENTIAL 10.8.1. Termination of this Agreement for any reason shall be without prejudice to any rights that shall have accrued to the benefit of a Party prior to such termination, including, for clarity, any payments owed to FMI in relation to the period prior to such termination, regardless of whether a termination fee is paid. Such termination shall not relieve a Party from obligations that are expressly indicated to survive the termination or expiration of this Agreement. Without limiting the foregoing, Sections 2.4.2, 2.4.3, 2.5.1(i) (first and second sentences only, except as otherwise provided in this Section 10.8.1), [**] (in each case, to the extent provided in Section 10.6.1 or 10.8.2), 2.5.1(ii) (last sentence only), 2.5.2(i) (first and second sentences only), [**], 2.7, 3.4, 5.4 (as to amounts accrued as of the effective date of termination and, mutatis mutandis, amounts owed pursuant to Section 10.6.2 or otherwise upon or after termination), 6.1, 6.2.2 (to the extent provided in Section 10.6.1 [**]), 6.2.3 (to the extent the license in Section 6.2.2 survives), 6.3 [**], and only to the extent provided in Section 10.6.1 [**]), 6.4.3, 6.5 (and Attachment B), 6.6, 8.3, 10.5, 10.6, 10.7 and this Section 10.8 and Article 1 (to the extent necessary to interpret the remaining surviving provisions), Article 7 (in case of Sections 7.1 and 7.2, for the period set forth in Section 7.1.1), Article 9 and Article 11 (for clarity, including Section 11.19) (provided, however, that Section 11.5 shall survive solely for purposes of any Dispute arising in connection with the application of Section 10.6 or this Section 10.8 or any Dispute arising prior to the effective date of termination) shall survive the termination or expiration of this Agreement for any reason. 10.8.2. If this Agreement is terminated with respect to any Program pursuant to Section 10.2 or 10.4, then following such termination, the provisions of this Agreement shall remain in effect, except that the rights and obligations of the Parties hereunder shall exclude rights and obligations to the extent directed to such Program, and such Program shall be excluded from the role and responsibilities of the JPTs; provided, however, that (a) subject to clauses (b) and (c) below, each of the provisions listed in the last sentence of Section 10.8.1 shall survive with respect to such Program (as would apply in the case of termination of this Agreement in its entirety), (b) if this Agreement is terminated with respect to such Program (or in its entirety) by Company pursuant to Section 10.2 after the filing of an application for Regulatory Approval of the applicable FMI Assay for use with the applicable Company Product for the applicable Market and Indication, then (1) [**]. 10.8.3. Except to the extent otherwise expressly provided in this Agreement, including Sections 10.6.2, 10.8.1 or 10.8.2, or in any applicable SOW, (i) in the case of any termination of this Agreement in its entirety, all SOWs shall terminate in their entirety and the provisions thereof shall not survive after the applicable termination date, and (ii) in the case of any termination of this Agreement with respect to a Program, (a) any SOW that covers such Program [**] shall terminate in its entirety and the provisions thereof shall not survive after the applicable termination date, (b) any SOW that covers such Program and one or more other Programs shall terminate [**], and (c) any SOW that does not cover such Program shall not terminate, and the provisions thereof remain in effect without modification. ARTICLE 11 MISCELLANEOUS 11.1. Force Majeure. In the event either Party shall be delayed or hindered in or prevented from the performance of any act required hereunder by reasons of strike, lockouts, labor troubles, restrictive government or judicial orders or decrees, riots, insurrection, war, acts of God, inclement weather, disease outbreak, epidemic, pandemic or other similar reason or a cause beyond such Party’s control (including, for clarity, the COVID-19 pandemic or any associated government restrictions or disruptions or shortages of labor, materials or power, communications, transportation or other similar services), then performance of such act shall be excused for the period of such delay, other than the payment of monies when due hereunder. Any timelines affected by such force majeure shall be extended for a period equal to that of the delay. Notice of the start and stop of any such force majeure shall be provided to the other Party.

Master In Vitro Diagnostics Agreement 26 of [33] CONFIDENTIAL 11.2. Export Control. This Agreement is made subject to any restrictions concerning the export of products or technical information from the United States or other countries that may be imposed on the Parties from time to time. Each Party agrees that it will not export, directly or indirectly, any technical information acquired from the other Party under this Agreement or any products using such technical information to a location or in a manner that at the time of export requires an export license or other governmental approval, without first obtaining the written consent to do so from the appropriate agency or other governmental entity in accordance with Applicable Law. 11.3. Subcontracting. Subject to the terms of this Agreement, each Party shall have the right to engage Affiliates or Third Parties (including in connection with the grant of a license to one or more Third Party (sub)licensees) to perform its obligations under this Agreement (each such Third Party, a “Subcontractor”); [**] the extent that a Party utilizes its Affiliates or engages Subcontractors after the Effective Date to perform tasks within the scope of any SOW, such Party shall ensure all such Affiliates or Subcontractors are obligated to treat the other Party’s Confidential Information as confidential based on commercially reasonable terms and conditions. [**]. 11.4. Assignment; Affiliates. 11.4.1. Except as provided in Section 11.3, neither this Agreement nor any rights or obligations hereunder shall be assignable or otherwise transferable, in whole or in part, by a Party without the prior written consent of the other Party, except that each Party shall have the right, without such consent (but subject to Section 5.3 in the case of Company), to effect such assignment or transfer, in whole but not in part: (i) to any of its Affiliates (provided, however, that under this clause (i) the assigning Party shall remain responsible to the other Party for the performance of any such assigned or transferred obligations), or (ii) to any successor in interest (whether by merger, acquisition or asset purchase) to all or substantially all of the business to which this Agreement relates including, for clarity, in the case of FMI, to any successor in interest to its business with respect to the FMI Assay(s) intended for use with any Company Product; provided, however, that the assigning or transferring Party shall provide written notice to the other Party within [**] days after such assignment or transfer. All validly assigned or transferred rights or obligations of a Party shall inure to the benefit of and be enforceable by, or be binding on and be enforceable against, as applicable, the permitted successors and assigns of such Party. Any attempted assignment or other transfer in violation of this Section 11.4.1 shall be void and of no effect. 11.4.2. The rights to Know-How or other information, materials and intellectual property: (i) controlled by a Third Party permitted assignee of a Party that were controlled by such assignee (and not such Party) immediately prior to such assignment (other than as a result of a license or other grant of rights, covenant or assignment by such Party or its Affiliates to, or for the benefit of, such Third Party); or (ii) controlled by an Affiliate of a Party that becomes an Affiliate through any direct or indirect Change of Control of such Party or parent of such Party, that were controlled by such Affiliate (and not such Party) immediately prior to such Change of Control (other than as a result of a license or other grant of rights, covenant or assignment by such Party or its other Affiliates to, or for the benefit of, such Affiliate), [**]. 11.4.3. Notwithstanding the foregoing, each Party shall have the right to perform any or all of its obligations and exercise any or all of its rights under this Agreement through any Affiliate, and, in the case of FMI, through any FMI Contracting Collaborator. 11.5. Dispute Resolution. 11.5.1. Referral to Senior Officers. Except with respect to matters reserved for the final decision-making authority of one Party or the other pursuant to Section 4.2.1 or as provided in

Master In Vitro Diagnostics Agreement 27 of [33] CONFIDENTIAL Section 11.10, if a dispute arises between the Parties in connection with or relating to this Agreement (including any SOW), or any document or instrument delivered in connection herewith (each, a “Dispute”), then either Party shall have the right to refer such Dispute to the Senior Officers for attempted resolution by good faith negotiations during a period of [**] business days. For clarity, matters reserved for the final decision-making authority of a Party pursuant to Section 4.2.1 and matters that are subject to the provisions of Section 11.10 shall not be referable to Senior Officers for resolution. 11.5.2. Resolution. Any final decision mutually agreed to in writing by the Senior Officers shall be conclusive and binding on the Parties. If the Senior Officers are unable to resolve any such Dispute within the period set forth in Section 11.5.1, then: (i) [**]; and (ii) except as provided in clause (i), with respect to any other unresolved Dispute, either Party shall be free to exercise any right of such Party to institute litigation in accordance with Section 11.6. 11.5.3. Interim Relief. Notwithstanding anything herein to the contrary, nothing contained in this Section 11.5 shall preclude either Party from seeking interim or provisional relief, including a temporary restraining order, preliminary injunction or other interim equitable relief, including concerning a Dispute, if reasonably necessary to protect the interests of such Party. This Section 11.5.3 shall be specifically enforceable. 11.6. Governing Law, Jurisdiction and Service. This Agreement shall be governed by and construed in accordance with the laws of the State of New York, excluding any conflicts or choice of law rule or principle that might otherwise refer construction or interpretation of this Agreement to the substantive law of another jurisdiction. The Parties agree to exclude the application to this Agreement of the United Nations Convention on Contracts for the International Sale of Goods. The Parties hereby irrevocably and unconditionally consent to the exclusive jurisdiction of the courts of the Southern District of New York for any action, suit or proceeding (other than appeals therefrom) arising out of or relating to this Agreement and agree to not commence any action, suit or proceeding (other than appeals therefrom) related thereto except in such courts. The Parties irrevocably and unconditionally waive their right to a jury trial. The Parties further hereby irrevocably and unconditionally waive any objection to the laying of venue of any action, suit or proceeding (other than appeals therefrom) arising out of or relating to this Agreement in the courts of the Southern District of New York and hereby further irrevocably and unconditionally waive and agree to not plead or claim in any such court that any such action, suit or proceeding brought in any such court has been brought in an inconvenient forum. Each Party further agrees that service of any process, summons, notice or document by registered mail to its address set forth in Section 11.7 shall be effective service of process for any action, suit or proceeding brought against it under this Agreement in any such court. 11.7. Notices. Any notice, request, demand, waiver, consent, approval or other communication permitted or required under this Agreement shall be in writing, shall refer specifically to this Agreement (and any applicable SOW) and shall be deemed given only if delivered by hand or sent by electronic transmission (with transmission confirmed) or by an internationally recognized overnight delivery service that maintains records of delivery, addressed to a Party at its respective address specified below or to such other address as the Party to whom notice is to be given may have provided to the other Party in accordance with this Section 11.7. Such notice shall be deemed to have been given as of the date delivered by hand or electronically transmitted (with transmission confirmed) or on the second business day (at the place of delivery) after deposit with an internationally recognized overnight delivery service. Any notice with respect to any Dispute or breach (or alleged breach) or termination of this Agreement that is delivered by electronic transmission shall be confirmed by a hard copy delivered as soon as practicable thereafter. This Section 11.7 is not intended to govern the day-to-day business communications between the Parties in performing their obligations under the terms of this Agreement or any SOW.

Master In Vitro Diagnostics Agreement 28 of [33] CONFIDENTIAL If to Company, to: Arvinas Operations, Inc. 5 Science Park 395 Winchester Avenue New Haven, CT 06511 U.S.A. Attention: Legal Department Facsimile: N/A Email: [**] If to Company Parent, to: Arvinas, Inc. 5 Science Park 395 Winchester Avenue New Haven, CT 06511 U.S.A Attention: Legal Department Facsimile: N/A Email: [**] If to FMI, to: Foundation Medicine, Inc. 10 Canal Park Cambridge, MA 02141 U.S.A. Attention: VP, Business Development Facsimile: [**] With a copy to: Foundation Medicine, Inc. 10 Canal Park Cambridge, MA 02141 U.S.A. Attention: General Counsel Email: [**] 11.8. Entire Agreement; Amendments. This Agreement, together with any and all SOWs executed hereunder and incorporated herein, and any Appendix, Attachment, Annex or Schedule hereto or thereto, sets forth and constitutes the entire agreement and understanding between the Parties with respect to the subject matter hereof, and all prior agreements, understandings, promises and representations, whether written or oral, with respect thereto, including the Mutual Confidentiality Agreement entered into by Company and FMI dated as of August 30, 2021, are superseded hereby. Each Party confirms that it is not relying on any representations or warranties of the other Party except as specifically set forth in this Agreement. No amendment, modification, release or discharge with respect to this Agreement (including any SOW) shall be binding upon the Parties unless in writing and duly executed by an authorized representative of each Party. Subject to Section 2.1.1, in the event of any inconsistencies between this Agreement and any schedules or other attachments hereto (including any SOW), the terms of this Agreement shall control. 11.9. English Language. This Agreement shall be written and executed in and all other communications under or in connection with this Agreement shall be in, the English language. Any translation into any other language shall not be an official version thereof and in the event of any conflict in interpretation between the English version and such translation, the English version shall control. 11.10. Equitable Relief. Each Party acknowledges and agrees that the restrictions set forth in Article 6 and Article 7 are reasonable and necessary to protect the legitimate interests of the other Party and that such other Party would not have entered into this Agreement in the absence of such restrictions and that any breach or threatened breach of any provision of such Articles may result in irreparable injury to such other Party for which there will be no adequate remedy at law. In the event of a breach or threatened breach of any provision of such Articles, the non-breaching Party shall be authorized and entitled to seek from any court of competent jurisdiction injunctive relief, whether preliminary or permanent and specific performance, which rights shall be cumulative and in addition to any other rights or remedies to which such non-breaching Party may be entitled in law or equity. Each Party agrees to waive any requirement that the other Party (i) post a bond or other security as a condition for obtaining any such relief or (ii) show irreparable harm, balancing of harms, consideration of the public interest or inadequacy of monetary damages as a remedy. Nothing in this Section 11.10 is intended or should be

Master In Vitro Diagnostics Agreement 29 of [33] CONFIDENTIAL construed, to limit either Party’s right to equitable relief or any other remedy for a breach of any other provision of this Agreement. 11.11. Waiver and Non-Exclusion of Remedies. Any term or condition of this Agreement (including any SOW) may be waived at any time by the Party that is entitled to the benefit thereof, but no such waiver shall be effective unless set forth in a written instrument duly executed by or on behalf of the Party waiving such term or condition. The waiver by either Party of any right under this Agreement, or of the failure to perform or of a breach by the other Party, shall not be deemed a waiver of any other right under this Agreement or of any other breach or failure by such other Party whether of a similar nature or otherwise. The rights and remedies provided herein are cumulative and do not exclude any other right or remedy provided by Applicable Law or otherwise available except as expressly set forth herein. 11.12. No Benefit to Third Parties. Except as specifically provided in Sections 9.1 and 9.2, the covenants and agreements set forth in this Agreement are for the sole benefit of the Parties and their successors and permitted assigns and they shall not be construed as conferring any rights on any other Persons. 11.13. Further Assurance. Each Party shall duly execute and deliver or cause to be duly executed and delivered, such further instruments and do and cause to be done such further acts and things, including the filing of such assignments, agreements, documents and instruments, as may be reasonably necessary or as the other Party may reasonably request in connection with this Agreement or to carry out more effectively the provisions and purposes hereof or to better assure and confirm unto such other Party its rights and remedies under this Agreement. 11.14. Relationship of the Parties. It is expressly agreed that FMI and Company shall be independent contractors and that the relationship between the Parties under this Agreement and any SOW(s) shall not constitute a partnership, joint venture or agency. Neither FMI nor Company shall have the authority to make any statements, representations or commitments of any kind, or to take any action that will be binding on the other, without the prior written consent of the other Party to do so. All persons employed by a Party shall be employees of such Party and not of the other Party, and all costs and obligations incurred by reason of any such employment shall be for the account and expense of such first Party. 11.15. References. Unless otherwise specified, (i) references in this Agreement to any Article, Section, Appendix, Attachment, Annex or Schedule shall mean references to such Article, Section, Appendix, Attachment, Annex or Schedule of this Agreement; (ii) references in any Section to any clause are references to such clause of such Section; and (iii) references to any agreement, instrument or other document in this Agreement refer to such agreement, instrument or other document as originally executed or, if subsequently amended, restated, replaced or supplemented from time to time, as so amended, replaced or supplemented and in effect at the relevant time of reference thereto. 11.16. Construction. Except where the context otherwise requires, wherever used, the singular shall include the plural, the plural the singular, the use of any gender shall be applicable to all genders and the word “or” is used in the inclusive sense (and/or). Whenever this Agreement refers to a number of days, unless otherwise specified, such number refers to calendar days. To the extent any provision of this Agreement requires for any purpose a Party’s consent or approval, such consent or approval shall not be unreasonably withheld, conditioned or delayed, unless otherwise expressly provided. The headings of this Agreement are for convenience of reference only and in no way define, describe, extend or limit the scope or intent of this Agreement or the intent of any provision contained in this Agreement. Each of the terms “including,” “include,” or “includes” as used herein shall mean including, without limiting the generality of any description preceding each such term. Any capitalized

Master In Vitro Diagnostics Agreement 30 of [33] CONFIDENTIAL term used in any SOW but not otherwise defined therein shall have the meaning provided in this Agreement. The language of this Agreement shall be deemed to be the language mutually chosen by the Parties and no rule of strict construction shall be applied against either Party. 11.17. Severability. If any one or more provisions of this Agreement shall be found to be illegal or unenforceable in any respect, the validity, legality and enforceability of the remaining provisions shall not in any way be affected or impaired thereby, provided the surviving agreement materially comports with the Parties’ original intent. The Parties agree that any such illegal or unenforceable provisions will be deemed replaced with valid and enforceable provisions that achieve, to the extent possible, the business purposes and intent of such invalid and unenforceable provisions. 11.18. Counterparts. This Agreement and any SOW(s) may be executed in counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. This Agreement and any SOW(s) may be executed by facsimile, PDF format via email or other electronically transmitted signatures and such signatures shall be deemed to bind each Party as if they were original signatures. 11.19. [**]. SIGNATURE PAGE FOLLOWS.

Signature Page to Master In Vitro Diagnostics Agreement Master In Vitro Diagnostics Agreement Page 31 of [33] CONFIDENTIAL IN WITNESS THEREOF, this Agreement has been executed by the Parties and Company Parent through their duly authorized representatives as of the Effective Date. ARVINAS OPERATIONS, INC. FOUNDATION MEDICINE, INC. By: /s/ Sean Cassidy By: /s/ John Truesdell Name: Sean Cassidy Name: John Truesdell Title: CFO Title: VP Biopharma Partnership Development ARVINAS, INC. By: /s/ Sean Cassidy Name: Sean Cassidy Title: CFO

ACTIVE/117906126.4 Master In Vitro Diagnostics Agreement Page 32 of 33 CONFIDENTIAL