false2019FY0001645113--12-31P5YP6YP4YP3Y00016451132019-01-012019-12-31iso4217:USD00016451132019-06-28xbrli:shares00016451132020-02-1900016451132019-12-3100016451132018-12-3100016451132018-01-012018-12-3100016451132017-01-012017-12-31iso4217:USDxbrli:shares0001645113us-gaap:CommonStockMember2016-12-310001645113us-gaap:AdditionalPaidInCapitalMember2016-12-310001645113us-gaap:AccumulatedOtherComprehensiveIncomeMember2016-12-310001645113us-gaap:RetainedEarningsMember2016-12-3100016451132016-12-310001645113us-gaap:AdditionalPaidInCapitalMember2017-01-012017-12-310001645113us-gaap:CommonStockMember2017-01-012017-12-310001645113us-gaap:RetainedEarningsMember2017-01-012017-12-310001645113us-gaap:AccumulatedOtherComprehensiveIncomeMember2017-01-012017-12-310001645113us-gaap:CommonStockMember2017-12-310001645113us-gaap:AdditionalPaidInCapitalMember2017-12-310001645113us-gaap:AccumulatedOtherComprehensiveIncomeMember2017-12-310001645113us-gaap:RetainedEarningsMember2017-12-3100016451132017-12-310001645113us-gaap:AdditionalPaidInCapitalMember2018-01-012018-12-310001645113us-gaap:CommonStockMember2018-01-012018-12-310001645113us-gaap:RetainedEarningsMember2017-01-0100016451132017-01-010001645113us-gaap:AccumulatedOtherComprehensiveIncomeMember2018-01-012018-12-310001645113us-gaap:RetainedEarningsMember2018-01-012018-12-310001645113us-gaap:CommonStockMember2018-12-310001645113us-gaap:AdditionalPaidInCapitalMember2018-12-310001645113us-gaap:AccumulatedOtherComprehensiveIncomeMember2018-12-310001645113us-gaap:RetainedEarningsMember2018-12-310001645113us-gaap:AdditionalPaidInCapitalMember2019-01-012019-12-310001645113us-gaap:CommonStockMember2019-01-012019-12-310001645113us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-01-012019-12-310001645113us-gaap:RetainedEarningsMember2019-01-012019-12-310001645113us-gaap:CommonStockMember2019-12-310001645113us-gaap:AdditionalPaidInCapitalMember2019-12-310001645113us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-12-310001645113us-gaap:RetainedEarningsMember2019-12-31xbrli:pure0001645113srt:MinimumMembernvcr:ComputersAndLaboratoryEquipmentMember2019-01-012019-12-310001645113srt:MaximumMembernvcr:ComputersAndLaboratoryEquipmentMember2019-01-012019-12-310001645113srt:MinimumMemberus-gaap:FurnitureAndFixturesMember2019-01-012019-12-310001645113srt:MaximumMemberus-gaap:FurnitureAndFixturesMember2019-01-012019-12-310001645113us-gaap:EquipmentMember2019-01-012019-12-310001645113us-gaap:LeaseholdImprovementsMember2019-01-012019-12-310001645113srt:MinimumMembernvcr:FieldEquipmentUnderOperatingLeasesMember2019-01-012019-12-310001645113nvcr:FieldEquipmentUnderOperatingLeasesMembersrt:MaximumMember2019-01-012019-12-310001645113nvcr:FieldEquipmentUnderOperatingLeasesMember2019-01-012019-12-310001645113nvcr:FieldEquipmentUnderOperatingLeasesMember2018-01-012018-12-310001645113nvcr:FieldEquipmentUnderOperatingLeasesMember2017-01-012017-12-310001645113us-gaap:DifferenceBetweenRevenueGuidanceInEffectBeforeAndAfterTopic606Memberus-gaap:AccountingStandardsUpdate201409Member2018-01-012018-01-010001645113us-gaap:DifferenceBetweenRevenueGuidanceInEffectBeforeAndAfterTopic606Memberus-gaap:AccountingStandardsUpdate201409Member2018-01-010001645113us-gaap:ShippingAndHandlingMember2019-01-012019-12-310001645113us-gaap:ShippingAndHandlingMember2018-01-012018-12-310001645113us-gaap:ShippingAndHandlingMember2017-01-012017-12-310001645113us-gaap:RestrictedStockUnitsRSUMember2019-01-012019-12-310001645113us-gaap:EmployeeStockOptionMember2019-01-012019-12-310001645113srt:MaximumMember2019-01-012019-12-310001645113us-gaap:AccountingStandardsUpdate201602Member2019-01-012019-01-010001645113us-gaap:CashMember2019-12-310001645113us-gaap:CashMember2018-12-310001645113us-gaap:MoneyMarketFundsMember2019-12-310001645113us-gaap:MoneyMarketFundsMember2018-12-310001645113nvcr:ComputersAndLaboratoryEquipmentMember2019-12-310001645113nvcr:ComputersAndLaboratoryEquipmentMember2018-12-310001645113us-gaap:FurnitureAndFixturesMember2019-12-310001645113us-gaap:FurnitureAndFixturesMember2018-12-310001645113us-gaap:EquipmentMember2019-12-310001645113us-gaap:EquipmentMember2018-12-310001645113us-gaap:LeaseholdImprovementsMember2019-12-310001645113us-gaap:LeaseholdImprovementsMember2018-12-310001645113nvcr:FieldEquipmentMember2019-01-012019-12-310001645113nvcr:FieldEquipmentMember2018-01-012018-12-310001645113nvcr:FieldEquipmentMember2017-01-012017-12-310001645113us-gaap:DefinedBenefitPlanDebtSecurityMember2019-09-300001645113us-gaap:DefinedBenefitPlanRealEstateMember2019-09-300001645113us-gaap:DefinedBenefitPlanEquitySecuritiesMember2019-09-300001645113nvcr:OtherPlanAssetAllocationMember2019-09-3000016451132019-09-300001645113nvcr:TwentyEighteenCreditFacilityMember2018-02-070001645113us-gaap:DebtInstrumentRedemptionPeriodOneMembernvcr:TwentyEighteenCreditFacilityMember2018-02-072018-02-070001645113nvcr:TwentyEighteenCreditFacilityMemberus-gaap:DebtInstrumentRedemptionPeriodTwoMember2018-02-072018-02-070001645113nvcr:TwentyEighteenCreditFacilityMember2019-12-310001645113nvcr:TwentyEighteenCreditFacilityMember2019-01-012019-12-310001645113nvcr:TermLoanMember2018-03-310001645113nvcr:SettlementAgreementMember2018-01-012018-03-310001645113nvcr:SettlementAgreementMember2018-01-012018-12-310001645113nvcr:LicenseAndCollaborationAgreementMember2018-09-100001645113nvcr:LicenseAndCollaborationAgreementMembersrt:MaximumMember2018-09-100001645113srt:MinimumMembernvcr:LicenseAndCollaborationAgreementMemberus-gaap:LicenseMember2018-09-092018-09-100001645113nvcr:LicenseAndCollaborationAgreementMember2018-10-012018-12-310001645113nvcr:LicenseAndCollaborationAgreementMember2020-01-01us-gaap:LicenseMemberus-gaap:AccountingStandardsUpdate201409Member2019-12-310001645113nvcr:LicenseAndCollaborationAgreementMemberus-gaap:LicenseMemberus-gaap:AccountingStandardsUpdate201409Member2019-01-012019-12-310001645113nvcr:LicenseAndCollaborationAgreementMemberus-gaap:LicenseMemberus-gaap:AccountingStandardsUpdate201409Member2018-01-012018-12-310001645113us-gaap:PendingLitigationMembernvcr:PazMember2019-02-012019-02-280001645113us-gaap:ProFormaMember2017-01-012017-12-310001645113us-gaap:ScenarioAdjustmentMember2017-01-012017-12-310001645113us-gaap:StateAndLocalJurisdictionMember2019-12-310001645113us-gaap:ForeignCountryMember2019-12-310001645113us-gaap:WarrantMember2018-01-012018-12-310001645113us-gaap:WarrantMember2017-01-012017-12-310001645113nvcr:TwoThousandFifteenPlanMemberus-gaap:EmployeeStockOptionMember2019-01-012019-12-310001645113nvcr:TwoThousandFifteenPlanMemberus-gaap:RestrictedStockUnitsRSUMember2019-01-012019-12-310001645113nvcr:TwoThousandFifteenPlanMember2018-12-302019-12-310001645113nvcr:TwoThousandFifteenPlanMember2018-12-310001645113nvcr:TwoThousandFifteenPlanMember2019-12-310001645113us-gaap:CommonStockMemberus-gaap:EmployeeStockMember2016-08-012019-12-310001645113us-gaap:EmployeeStockMember2019-01-012019-12-310001645113us-gaap:EmployeeStockMember2019-12-310001645113srt:MinimumMemberus-gaap:EmployeeStockOptionMember2019-01-012019-12-310001645113srt:MaximumMemberus-gaap:EmployeeStockOptionMember2019-01-012019-12-310001645113srt:MinimumMemberus-gaap:EmployeeStockOptionMember2018-01-012018-12-310001645113srt:MaximumMemberus-gaap:EmployeeStockOptionMember2018-01-012018-12-310001645113srt:MinimumMemberus-gaap:EmployeeStockOptionMember2017-01-012017-12-310001645113srt:MaximumMemberus-gaap:EmployeeStockOptionMember2017-01-012017-12-310001645113us-gaap:EmployeeStockOptionMember2018-01-012018-12-310001645113us-gaap:EmployeeStockOptionMember2017-01-012017-12-310001645113us-gaap:EmployeeStockMember2018-01-012018-12-310001645113us-gaap:EmployeeStockMember2017-01-012017-12-310001645113srt:MinimumMemberus-gaap:EmployeeStockMember2019-01-012019-12-310001645113srt:MaximumMemberus-gaap:EmployeeStockMember2019-01-012019-12-310001645113srt:MinimumMemberus-gaap:EmployeeStockMember2018-01-012018-12-310001645113srt:MaximumMemberus-gaap:EmployeeStockMember2018-01-012018-12-310001645113srt:MinimumMemberus-gaap:EmployeeStockMember2017-01-012017-12-310001645113srt:MaximumMemberus-gaap:EmployeeStockMember2017-01-012017-12-310001645113us-gaap:RestrictedStockUnitsRSUMember2018-12-310001645113us-gaap:RestrictedStockUnitsRSUMember2019-12-310001645113nvcr:CostOfRevenueMember2019-01-012019-12-310001645113nvcr:CostOfRevenueMember2018-01-012018-12-310001645113nvcr:CostOfRevenueMember2017-01-012017-12-310001645113us-gaap:ResearchAndDevelopmentExpenseMember2019-01-012019-12-310001645113us-gaap:ResearchAndDevelopmentExpenseMember2018-01-012018-12-310001645113us-gaap:ResearchAndDevelopmentExpenseMember2017-01-012017-12-310001645113nvcr:SalesAndMarketingMember2019-01-012019-12-310001645113nvcr:SalesAndMarketingMember2018-01-012018-12-310001645113nvcr:SalesAndMarketingMember2017-01-012017-12-310001645113us-gaap:GeneralAndAdministrativeExpenseMember2019-01-012019-12-310001645113us-gaap:GeneralAndAdministrativeExpenseMember2018-01-012018-12-310001645113us-gaap:GeneralAndAdministrativeExpenseMember2017-01-012017-12-310001645113srt:MinimumMembernvcr:ExercisePriceRangeOneMember2019-01-012019-12-310001645113srt:MaximumMembernvcr:ExercisePriceRangeOneMember2019-01-012019-12-310001645113nvcr:ExercisePriceRangeOneMember2019-12-310001645113nvcr:ExercisePriceRangeOneMember2019-01-012019-12-310001645113srt:MinimumMembernvcr:ExercisePriceRangeTwoMember2019-01-012019-12-310001645113srt:MaximumMembernvcr:ExercisePriceRangeTwoMember2019-01-012019-12-310001645113nvcr:ExercisePriceRangeTwoMember2019-12-310001645113nvcr:ExercisePriceRangeTwoMember2019-01-012019-12-310001645113srt:MinimumMembernvcr:ExercisePriceRangeThreeMember2019-01-012019-12-310001645113srt:MaximumMembernvcr:ExercisePriceRangeThreeMember2019-01-012019-12-310001645113nvcr:ExercisePriceRangeThreeMember2019-12-310001645113nvcr:ExercisePriceRangeThreeMember2019-01-012019-12-310001645113srt:MinimumMembernvcr:ExercisePriceRangeFourMember2019-01-012019-12-310001645113nvcr:ExercisePriceRangeFourMembersrt:MaximumMember2019-01-012019-12-310001645113nvcr:ExercisePriceRangeFourMember2019-12-310001645113nvcr:ExercisePriceRangeFourMember2019-01-012019-12-310001645113srt:MinimumMembernvcr:ExercisePriceRangeFiveMember2019-01-012019-12-310001645113nvcr:ExercisePriceRangeFiveMembersrt:MaximumMember2019-01-012019-12-310001645113nvcr:ExercisePriceRangeFiveMember2019-12-310001645113nvcr:ExercisePriceRangeFiveMember2019-01-012019-12-310001645113srt:MinimumMembernvcr:ExercisePriceRangeSixMember2019-01-012019-12-310001645113srt:MaximumMembernvcr:ExercisePriceRangeSixMember2019-01-012019-12-310001645113nvcr:ExercisePriceRangeSixMember2019-12-310001645113nvcr:ExercisePriceRangeSixMember2019-01-012019-12-310001645113srt:MinimumMembernvcr:ExercisePriceRangeSevenMember2019-01-012019-12-310001645113srt:MaximumMembernvcr:ExercisePriceRangeSevenMember2019-01-012019-12-310001645113nvcr:ExercisePriceRangeSevenMember2019-12-310001645113nvcr:ExercisePriceRangeSevenMember2019-01-012019-12-310001645113us-gaap:EmployeeStockOptionMember2019-01-012019-12-310001645113us-gaap:EmployeeStockOptionMember2018-01-012018-12-310001645113us-gaap:EmployeeStockOptionMember2017-01-012017-12-310001645113us-gaap:RestrictedStockUnitsRSUMember2019-01-012019-12-310001645113us-gaap:RestrictedStockUnitsRSUMember2018-01-012018-12-310001645113us-gaap:RestrictedStockUnitsRSUMember2017-01-012017-12-310001645113us-gaap:WarrantMember2019-01-012019-12-310001645113us-gaap:WarrantMember2018-01-012018-12-310001645113us-gaap:WarrantMember2017-01-012017-12-310001645113country:US2019-12-310001645113country:US2018-12-310001645113country:US2017-12-310001645113country:CH2019-12-310001645113country:CH2018-12-310001645113country:CH2017-12-310001645113country:IL2019-12-310001645113country:IL2018-12-310001645113country:IL2017-12-310001645113country:DE2019-12-310001645113country:DE2018-12-310001645113country:DE2017-12-310001645113nvcr:OthersCountriesMember2019-12-310001645113nvcr:OthersCountriesMember2018-12-310001645113nvcr:OthersCountriesMember2017-12-310001645113country:US2019-01-012019-12-310001645113country:US2018-01-012018-12-310001645113country:US2017-01-012017-12-310001645113country:DE2019-01-012019-12-310001645113country:DE2018-01-012018-12-310001645113country:DE2017-01-012017-12-310001645113us-gaap:EMEAMember2019-01-012019-12-310001645113us-gaap:EMEAMember2018-01-012018-12-310001645113us-gaap:EMEAMember2017-01-012017-12-310001645113country:JP2019-01-012019-12-310001645113country:JP2018-01-012018-12-310001645113country:JP2017-01-012017-12-310001645113country:CN2019-01-012019-12-310001645113country:CN2018-01-012018-12-310001645113country:CN2017-01-012017-12-3100016451132019-10-012019-12-3100016451132019-07-012019-09-3000016451132019-04-012019-06-3000016451132019-01-012019-03-3100016451132018-10-012018-12-3100016451132018-07-012018-09-3000016451132018-04-012018-06-3000016451132018-01-012018-03-3100016451132017-10-012017-12-3100016451132017-07-012017-09-3000016451132017-04-012017-06-3000016451132017-01-012017-03-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
______________________________________________________________________
FORM 10-K
______________________________________________________________________
(Mark One)
| | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2019
or
| | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number 001-37565
______________________________________________________________________
NovoCure Limited
(Exact Name of Registrant as Specified in Its Charter)
______________________________________________________________________
| | | | | |
Jersey (State or Other Jurisdiction of Incorporation or Organization) | 98-1057807 (I.R.S. Employer Identification No.) |
No. 4 The Forum
Grenville Street
St. Helier, Jersey JE2 4UF
(Address of Principal Executive Offices, including zip code)
Registrant’s telephone number, including area code: +44 (0) 15 3475 6700
Securities registered pursuant to Section 12(b) of the Act:
| | | | | | | | | | | | | | | | | | |
| | Title of each class | | Trading Symbol(s) | | Name of each exchange on which registered | |
| Ordinary shares, no par value per share | | NVCR | | NASDAQ Global Select Market | |
Securities registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
______________________________________________________________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of "large accelerated filer," "accelerated filer," "smaller reporting company" and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| | | | | | | | | | | | | | |
| Large accelerated filer | ☒ | | Accelerated filer | ☐ |
| Non-accelerated filer | ☐ | | Smaller reporting company | ☐ |
| | | | Emerging Growth Company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the outstanding common equity of the registrant held by non-affiliates as of the last business day of the registrant’s most recently completed second fiscal quarter was $3,943,182,013.
The number of shares of the registrant’s ordinary shares outstanding as of February 19, 2020 was 99,640,549.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its 2020 annual meeting of shareholders are incorporated by reference into Items 10, 11, 12, 13, and 14 of Part III of this Form 10-K. Such definitive proxy statement will be filed with the Securities and Exchange Commission within 120 days after the end of the registrant’s fiscal year ended December 31, 2019.
TABLE OF CONTENTS
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
In addition to historical facts or statements of current condition, this report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements contained in this report are based on our current plans, expectations, hopes, beliefs, intentions or strategies concerning future developments and their impact on us. Forward-looking statements contained in this report constitute our expectations or forecasts of future events as of the date this report was filed with the Securities and Exchange Commission and are not statements of historical fact. You can identify these statements by the fact that they do not relate strictly to historical or current facts. Such statements may include words such as "anticipate," "will," "estimate," "expect," "project," "intend," "should," "plan," "believe," "hope," and other words and terms of similar meaning in connection with any discussion of, among other things, future operating or financial performance, strategic initiatives and business strategies, regulatory or competitive environments, our intellectual property and research and development related to our Tumor Treating Fields delivery systems marketed under various brand names, including "Optune," the NovoTTF-100L System ("NovoTTF-100L") and software, tools and other items to support and optimize the delivery of Tumor Treating Fields (collectively, the "Products"). In particular, these forward-looking statements include, among others, statements about:
•our research and development, clinical trial and commercialization activities and projected expenditures;
•the further commercialization of our Products for current and future indications;
•our business strategies and the expansion of our sales and marketing efforts in the United States and in other countries;
•the market acceptance of our Products for current and future indications by patients, physicians, third-party payers and others in the healthcare and scientific community;
•our plans to pursue the use of our Products for the treatment of solid tumor cancers other than glioblastoma ("GBM") and malignant pleural mesothelioma ("MPM");
•our estimates regarding revenues, expenses, capital requirements and needs for additional financing;
•our ability to obtain regulatory approvals for the use of our Products in cancers other than GBM and MPM;
•our ability to acquire from third-party suppliers the supplies needed to manufacture our Products;
•our ability to manufacture adequate supply;
•our ability to secure and maintain adequate coverage from third-party payers to reimburse us for our Products for current and future indications;
•our ability to receive payment from third-party payers for use of our Products for current and future indications;
•our ability to maintain and develop our intellectual property position;
•our ability to manage the risks associated with business disruptions caused by natural disasters, pandemics such as the COVID-19 (coronavirus) or international conflict or other disruptions outside of our control;
•our cash needs; and
•our prospects, financial condition and results of operations.
These forward-looking statements involve a number of risks and uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. Factors which may cause such differences to occur include those risks and uncertainties set forth under Part I, Item IA, Risk Factors, of this Annual Report on Form 10-K, as
well as other risks and uncertainties set forth from time to time in the reports we file with the U.S. Securities and Exchange Commission the ("SEC"). We do not intend to update publicly any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.
PART I
ITEM 1. BUSINESS
Overview
We are a global oncology company with a proprietary platform technology called Tumor Treating Fields, the use of electric fields tuned to specific frequencies to disrupt solid tumor cancer cell division. Our key priorities are to drive commercial adoption of Optune and NovoTTF-100L, our commercial Tumor Treating Fields delivery systems, and to advance clinical and product development programs intended to extend overall survival in some of the most aggressive forms of cancer.
Optune is approved by the U.S. Food and Drug Administration ("FDA") under the Premarket Approval ("PMA") pathway for the treatment of adult patients with newly diagnosed GBM in combination with temozolomide, a chemotherapy drug, and for adult patients with GBM following confirmed recurrence after chemotherapy as monotherapy treatment. We also have approval or a CE certificate to market Optune for the treatment of GBM in the European Union ("EU"), Japan and certain other countries. NovoTTF-100L is approved by the FDA under the Humanitarian Device Exemption ("HDE") pathway to treat MPM in combination with standard chemotherapies. We have submitted an application for CE certification to market NovoTTF-100L in the EU.
We market Optune in the U.S., Austria, Germany, Israel, Japan, Sweden and Switzerland, which we refer to as our "active markets," and we market NovoTTF-100L in the U.S. With respect to GBM, our sales and marketing efforts are principally focused on driving adoption with both neuro-oncologists and radiation oncologists. With respect to MPM, our commercial efforts are principally focused on generating awareness with radiation oncologists and on establishing a dialogue with third-party payers around access to NovoTTF-100L. We are expanding our commercial operations into France with an initial focus on developing key opinion leader relationships in GBM and establishing a path to reimbursement for our Products.
We believe the mechanism of action behind Tumor Treating Fields therapy may be broadly applicable to solid tumor cancers. Currently, we are conducting phase 3 pivotal trials evaluating the use of Tumor Treating Fields in non-small-cell lung cancer ("NSCLC"), brain metastases from NSCLC ("brain metastases"), pancreatic cancer and ovarian cancer. We are also conducting phase 2 pilot trials evaluating the use of Tumor Treating Fields in liver cancer and gastric cancer. We anticipate expanding our clinical pipeline over time to study the safety and efficacy of Tumor Treating Fields for additional solid tumor indications. We plan to initiate at least one additional randomized, well-controlled clinical trial in GBM in order to further advance the scientific evidence supporting the use of Optune in GBM and to gather additional information about Optune's optimal use. The first trial planned will study the potential clinical benefit of initiating Optune concurrent with radiation therapy versus following radiation therapy.
Our therapy is delivered through a medical device and we have several product development programs underway intended to improve efficacy and usability for patients. Our intellectual property portfolio contains over 180 issued patents and numerous patent applications pending worldwide. We believe we own global commercialization rights to our Products in oncology and are well-positioned to extend those rights into the future as we continue to find innovative ways to improve our Products.
In 2018, we granted Zai Lab (Shanghai) Co., Ltd. ("Zai") a license to commercialize Optune in China, Hong Kong, Macau and Taiwan ("Greater China") under a License and Collaboration Agreement (the "Zai Agreement"). The Zai Agreement also establishes a development partnership intended to accelerate the development of Tumor Treating Fields in multiple solid tumor cancer indications. For additional information, see Note 12 to the Consolidated Financial Statements.
Our ordinary shares are quoted on the NASDAQ Global Select Market under the symbol "NVCR." We were incorporated in the Bailiwick of Jersey in 2000. Our principal operations are located in the U.S., Israel and Switzerland.
Our therapy
Tumor Treating Fields is a cancer therapy that uses electric fields tuned to specific frequencies to disrupt cell division, inhibiting tumor growth and causing affected cancer cells to die. Our track record of fundamental scientific research extends across two decades and, in all of our preclinical research to date, Tumor Treating Fields has demonstrated a consistent anti-mitotic effect. In our clinical research and commercial experience to date, Tumor Treating Fields has exhibited no systemic toxicity, with mild to moderate skin irritation being the most common side
effect. Tumor Treating Fields is intended principally for use in combination with other standard-of-care cancer treatments.
Recognizing what electric fields are and how they can be utilized for medical applications is essential to understanding Tumor Treating Fields. An electric field is a field of force. Electric fields surround all charged objects. An electric field exerts forces on charged objects within it. Tumor Treating Fields uses alternating electric fields specifically tuned to target cancer cells. Once the electric fields enter the cancer cell, they attract and repel charged proteins during cancer cell division.
Tumor Treating Fields utilizes the natural electrical properties of dividing cancer cells. While many intracellular molecules are slightly polarized or neutral, some are highly polarized and are strongly affected by Tumor Treating Fields. For example, tubulin is a highly polarized cellular protein that must orient spatially to form the mitotic spindle, which segregates chromosomes into two daughter cells during mitosis. Tumor Treating Fields exerts forces on tubulin, disrupting mitotic spindle formation and causing probable cell death. Septin is another highly polarized molecule in cells that must orient spatially to form the contractile ring needed to split daughter cells during mitosis. Tumor Treating Fields exerts forces on septin, leading to improper localization of the contractile ring. This process causes membrane blebbing, a sign of cell damage that may result in cell death.
In addition to its anti-mitotic effect, Tumor Treating Fields has been shown to inhibit DNA damage repair, to induce autophagy, to reduce cell migration and invasion, to increase cell membrane permeability and disrupt the blood-brain barrier, and to induce immunogenic cell death. Research is ongoing to enhance our understanding of the multi-pronged mechanism behind Tumor Treating Fields. We provide independent researchers a preclinical laboratory bench system, known as inovitro™, and we grant funding to support basic and translational research on Tumor Treating Fields.
We believe Tumor Treating Fields causes minimal damage to healthy cells surrounding affected cancer cells. Tumor Treating Fields does not stimulate or heat tissue and targets dividing cancer cells with specific characteristics, such as size. Tumor Treating Fields is regionally delivered to the tumor site rather than systemically delivered throughout the body and, as a result, the parts of the body not covered by Tumor Treating Fields are generally not affected.
Our technology
Treatment with Tumor Treating Fields is delivered through a portable medical device. The complete delivery system, called Optune or NovoTTF-100L, includes a portable electric field generator, transducer arrays, rechargeable batteries and accessories. Sterile, single-use transducer arrays are placed directly on the skin in the region surrounding the tumor and connected to the electric field generator to deliver therapy. Transducer arrays are changed when hair growth or the hydrogel reduces array adhesion to the skin. The therapy is designed to be delivered continuously throughout the day and night, and efficacy is strongly correlated to time on therapy. If the device is not on, the patient is not being treated. The electric field generator can be run from a standard power outlet or carried with a battery in a specially designed bag that we provide to patients.
We plan to use the same field generator technology across all indications for which our Products are approved. We plan to specifically target individual solid tumor types by optimizing field generator parameters such as frequency and power output. Our transducer arrays have been developed and are in use, either commercially or clinically, for application on the head, chest and abdomen.
Through engineering efforts, we plan to continue to enhance our Products to improve efficacy and usability for patients. Our engineering efforts are primarily focused on innovations to the portable electric field generator, transducer arrays and treatment planning software. We are developing a second generation torso system, a flexible torso transducer array, a high intensity transducer array, a remote download capability for the monthly usage report and next generation transducer array layout planning software. Over time, we may have the opportunity to optimize the energy delivered to individual patients, potentially improving efficacy. Any enhancements will be subject to applicable regulatory reviews and approvals.
Our commercial business
Optune is currently marketed in our active markets for the treatment of GBM, the most common form of primary brain cancer and an aggressive disease for which there are few effective treatment options. NovoTTF-100L is currently marketed in the U.S. for the treatment of MPM, a rare cancer that has been strongly linked to asbestos
exposure. Our first commercial priority in each active market is to generate awareness of our Products and our clinical trial data.
EF-14 phase 3 pivotal clinical trial data for the treatment of newly diagnosed GBM
In 2015, we received FDA approval to market Optune for the treatment of adult patients with newly diagnosed supratentorial GBM in combination with temozolomide. The FDA approved Optune for newly diagnosed GBM based on the EF-14 trial ("EF-14"), which was a randomized, phase 3 pivotal clinical trial which compared, post radiation, Optune plus temozolomide versus temozolomide alone for the treatment of newly diagnosed GBM. The primary endpoint of the trial was progression-free survival and a powered secondary endpoint was overall survival.
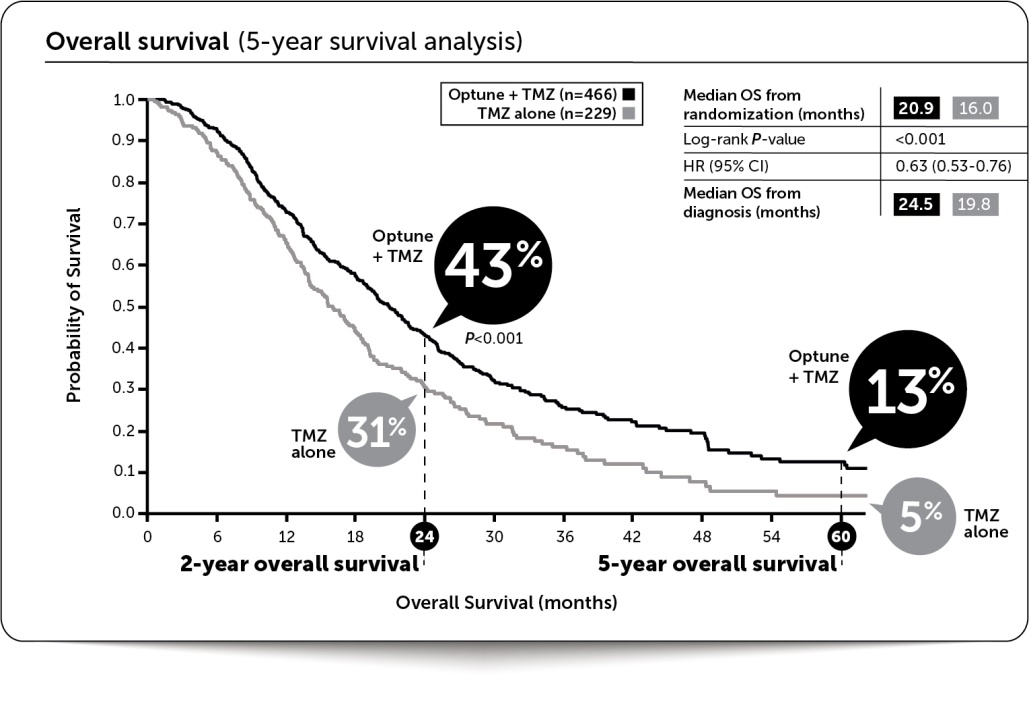
In EF-14, Optune plus temozolomide demonstrated unprecedented five-year survival results. Median overall survival was extended by nearly five months (median overall survival of 20.9 months versus 16.0 months for temozolomide alone). Median progression-free survival was extended by 2.7 months to 6.7 months for Optune plus temozolomide from 4.0 months for temozolomide alone. The final EF-14 data were published in JAMA in 2017.
The following graph presents the overall survival data in the intent-to-treat population from our five-year analysis:
The extension of progression-free and overall survival in patients receiving Optune in combination with temozolomide in EF-14 was not specific to any prognostic subgroup or tumor genetic marker and was consistent regardless of MGMT methylation status, extent of resection, age, performance status or gender. Optune was safely combined with temozolomide with no significant increase in serious adverse events compared with temozolomide alone. The most common side effect related to Optune was mild to moderate skin irritation.
Quality of life data from a pre-specified analysis of EF-14 demonstrated that patients treated with Optune and temozolomide maintained quality of life over time and across predefined daily-functioning domains. Both healthcare professionals and patients reported stable quality of life evaluation scores up to one year of Optune use. Physical, role, social, emotional and cognitive functioning for patients treated with Optune and temozolomide all remained stable and comparable with patients treated with temozolomide alone.
In 2018, the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology® (NCCN Guidelines®) for Central Nervous Systems Cancers were updated to include alternating electric fields therapy (Optune) in combination with temozolomide following standard brain radiation therapy with concurrent temozolomide as a Category 1 recommended postoperative adjuvant treatment option for patients with newly diagnosed supratentorial GBM.
A post-hoc analysis of EF-14 showed that more time on Optune predicted increased survival in GBM patients. An Optune monthly usage threshold as low as 50 percent correlated with significantly improved outcomes in patients treated with Optune together with temozolomide compared to patients treated with temozolomide alone. The greater the patients’ monthly usage of Optune, the better their outcomes. Patients who used Optune more than 90 percent
of the time (n=43) had the greatest chance of survival: a median survival of 24.9 months from randomization and a five-year probability of survival of 29.3 percent.
In 2018, a separate post-hoc analysis of EF-14 showed that higher levels of energy at the tumor bed predicted increased survival in GBM patients. Patients treated with Optune at higher energy levels (power loss densities greater than or equal to 1.1 mW/cm3; n=122) had a median overall survival of 25.2 months compared to a median overall survival of 21.0 months for patients treated with Optune at lower energy levels (power loss densities less than 1.1 mW/cm3; n=195).
In these analyses, both time on therapy and higher levels of energy (or higher power loss density) were associated with improved overall survival, independent of each other. Patients who used Optune more than 20 hours per day at higher energy levels (n=36) had a median overall survival of 37 months (95% CI 21-48 months). For Optune, dose density can be defined as time on therapy times the energy delivered, or cumulative energy
EF-11 phase 3 pivotal clinical trial data for the treatment of recurrent GBM
We initially received FDA approval for Optune in 2011 for use as a monotherapy treatment for adult patients with GBM, following confirmed recurrence after chemotherapy. The FDA approved Optune based on the EF-11 trial ("EF-11"), a randomized, phase 3 pivotal clinical trial.
EF-11 was a multi-center, active controlled clinical trial of 237 adults with recurrent GBM. Participants received either Optune as a monotherapy (n=120) or the physician’s choice of chemotherapy (n=117). Chemotherapies chosen for the active control arm included mainly bevacizumab, nitrosureas and temozolomide. The primary endpoint was superiority in overall survival. Overall survival for patients treated with Optune alone and active chemotherapy was 6.6 months and 6.0 months, respectively (p=0.27: HR = 0.86). The trial demonstrated that Optune provided clinically comparable survival with an overall better quality of life.
Twice as many EF-11 patients responded to Optune than to active chemotherapy (12 patients versus 6 patients). Three patients in the Optune alone arm had a complete response versus no patients in the active chemotherapy arm.
STELLAR phase 2 registration trial data for the treatment of MPM
In 2019, we received FDA approval via the HDE pathway to market NovoTTF-100L for the treatment of adult patients with unresectable, locally advanced or metastatic MPM concurrent with pemetrexed and platinum-based chemotherapy. The FDA approved NovoTTF-100L for MPM based on the STELLAR trial ("STELLAR"). STELLAR was a single-arm, open-label, multi-center trial designed to test the safety and efficacy of Optune in combination with pemetrexed combined with cisplatin or carboplatin in patients with unresectable, previously untreated MPM. The trial was powered to prospectively determine the overall survival in patients treated with NovoTTF-100L plus chemotherapy. Secondary endpoints included overall response rate (per mRECIST criteria), progression-free survival and safety.
STELLAR investigated safety and efficacy among 80 patients treated with NovoTTF-100L plus standard of care chemotherapy. In STELLAR, the median overall survival was 18.2 months (95% CI, 12.1-25.8 months) across all patients treated with NovoTTF-100L plus chemotherapy. The median overall survival was 21.1 months for patients with epithelioid MPM (n=53) and 12.1 months for patients with non-epithelioid MPM (n=21). 62% of patients enrolled in STELLAR who used NovoTTF-100L plus chemotherapy were still alive at one year, with 42% of patients alive at two years. The disease control rate in patients with at least one follow-up CT scan performed (n=72) was 97%. 40% of patients had a partial response, 57% had stable disease, and 3% had progressive disease. The median progression-free survival was 7.6 months (95% CI, 6.7-9.8 months).
There was no increase in serious systemic adverse events when NovoTTF-100L was added to chemotherapy. Mild-to-moderate skin irritation was the only device-related side effect with NovoTTF-100L. The STELLAR data were published in The Lancet Oncology in 2019.
Our commercial markets
We have built a commercial organization and market Optune for the treatment of GBM in the U.S., Austria, Germany, Israel, Japan, Sweden and Switzerland, which we refer to as our active markets. We have also built a commercial organization to market NovoTTF-100L for the treatment of MPM in the U.S.
In 2020, we estimate that approximately:
•15,000 people will be diagnosed with GBM or tumors that typically progress to GBM in the U.S. Of this population, we estimate that approximately 11,200 patients are candidates for treatment with Optune based upon the rate of disease progression and medical eligibility. We estimate that approximately 8,200 of eligible patients will actively seek treatment.
•4,600 people will be diagnosed with GBM or tumors that typically progress to GBM in Germany. Of this population, we estimate that approximately 3,400 patients are candidates for treatment with Optune based upon the rate of disease progression and medical eligibility. We estimate that approximately 2,500 of eligible patients will actively seek treatment.
•2,200 people will be diagnosed with GBM or tumors that typically progress to GBM in Japan. Of this population, we estimate that approximately 1,600 patients are candidates for treatment with Optune based upon the rate of disease progression and medical eligibility. We estimate that approximately 1,200 of eligible patients will actively seek treatment.
•1,600 people will be diagnosed with GBM or tumors that typically progress to GBM in our other active markets: Austria, Israel, Sweden and Switzerland. Of this population, we estimate that approximately 1,200 patients are candidates for treatment with Optune based upon the rate of disease progression and medical eligibility. We estimate that approximately 900 of eligible patients will actively seek treatment.
In 2020, we estimate that approximately 3,000 people are diagnosed with MPM in the U.S. each year. Of this population, we estimate that approximately 2,400 patients are candidates for treatment with NovoTTF-100L based upon the rate of disease progression and medical eligibility.
We believe there are many more patients who could benefit from treatment with Tumor Treating Fields than are currently on therapy. We continue to focus on increasing penetration for GBM in our active markets and on successfully expanding our MPM business in the U.S. In the future, we anticipate strategically expanding into additional geographic markets and additional indications, pending regulatory approval.
Commercial execution
Healthcare providers must undergo a certification training in order to prescribe our Products. As of December 31, 2019, we trained more than 3,200 GBM prescribers in our active markets. We believe these prescribers treat the majority of GBM patients in our active markets. We have initiated a phased launch for MPM shaped by our learnings from our GBM rollout. As of December 31, 2019, we trained 36 MPM prescribers in the U.S.
As of December 31, 2019, we had more than 80 sales force colleagues globally. With respect to the treatment of GBM, our sales and marketing efforts are principally focused on driving adoption with both neuro-oncologists and radiation oncologists. In certain countries, neurosurgeons and medical oncologists also drive adoption. With respect to the treatment of MPM, our sales and marketing efforts are principally focused on certification training and supporting the required Institutional Review Board ("IRB") approval process. Radiation oncologists are the exclusive prescribers in the U.S. for the treatment of MPM. We believe that radiation oncologists will continue to play an increasingly important role in driving adoption of our Products in both current and future indications.
We currently operate as a direct-to-patient distributor of our Products in all active markets except for Japan. In Japan, we distribute Optune through hospitals and provide patient support services under a contractual arrangement with the hospital. Once an eligible patient is identified by a certified prescriber, the healthcare provider’s office submits a prescription order form and supporting documentation to us. We employ a team of Device Support Specialists who provide technical training to the patient and any caregivers. Once treatment is initiated, we provide 24/7 technical support for patients and caregivers as well as assistance with insurance reimbursement. We also provide the healthcare provider and the patient with a monthly usage report for monitoring patient time on therapy. We believe we have the experience, expertise and infrastructure to scale our sales and marketing efforts in our active markets. In addition to our commercial organization, we believe we have established a scalable supply chain.
Billing and reimbursement
We provide our Products directly to patients following receipt of a prescription order and a signed patient service agreement (except in Japan as described above). The number of active patients on therapy is our principal revenue
driver. An active patient is a patient who is receiving treatment under a commercial prescription order as of the measurement date, including patients who may be on a temporary break from treatment and who plan to resume treatment in less than 60 days. Growth in the number of active patients is a factor of both new patient starts and treatment duration. Median treatment duration differs based upon the patient's clinical diagnosis.
We bill payers a single monthly fee for a month of therapy and we bear the financial risk of securing payment from third-party payers and patients in all markets except for Japan. We distribute our Products through hospitals in Japan with the hospitals receiving reimbursement from the government-mandated insurance program and in turn contracting with us for the equipment, supplies and services necessary to treat patients with our Product.
Currently, the monthly list price for our therapy in the U.S. is $21,000 and we have set list prices in our other active markets that are approximately equivalent to this price, subject to currency fluctuations. We typically negotiate discounts from our list price with healthcare payers, and in certain cases we accept government-mandated discounts from our list prices in order to secure reimbursement for our Products.
We continue to work with payers to expand access to Optune for patients with GBM. As of December 31, 2019, a substantial majority of Americans with private health insurance had coverage of Optune for newly diagnosed GBM and/or recurrent GBM. As of September 2019, Americans who are beneficiaries of the Medicare fee-for-service program also have coverage of Optune for newly diagnosed GBM. Our team is focused on working through the typical administrative ramp-up with Medicare to ensure that we realize the full financial benefit as soon as possible. We are actively appealing Medicare fee-for-service coverage denials up to and including the Administrative Law Judge ("ALJ") process with Centers for Medicare and Medicaid Services ("CMS").
In Germany, we bill healthcare payers for individual cases, and each case is evaluated individually on its merits and under the payer’s specific rules for such cases. In 2019, the German Institute for Quality and Efficiency in Healthcare ("IQWiG") published its rapid report concluding that, based on a review of EF-14 data, patients with newly diagnosed GBM lived longer when treated with Optune in addition to standard chemotherapy without affecting quality of life. According to the published timeline, we now expect a national reimbursement decision in Germany no later than October 2020. We will continue to bill payers for individual cases as we advance through the review process in Germany.
We have received national reimbursement for Optune in Austria, Israel, Japan and Sweden. We are pursuing national reimbursement for Optune in Switzerland.
As of December 31, 2019, the total number of contracted GBM lives was approximately 263 million in the U.S., approximately 19 million in our active EMEA markets and approximately 127 million in Japan.
We are engaged in an initial dialogue with certain payers regarding access to NovoTTF-100L for patients with MPM. We anticipate that MPM claims during initial commercialization will go through an appeal process with payers, similar to our early experience with GBM. We anticipate that our ability to gain meaningful coverage for NovoTTF-100L will be dependent on inclusion in the relevant clinical guidelines for MPM.
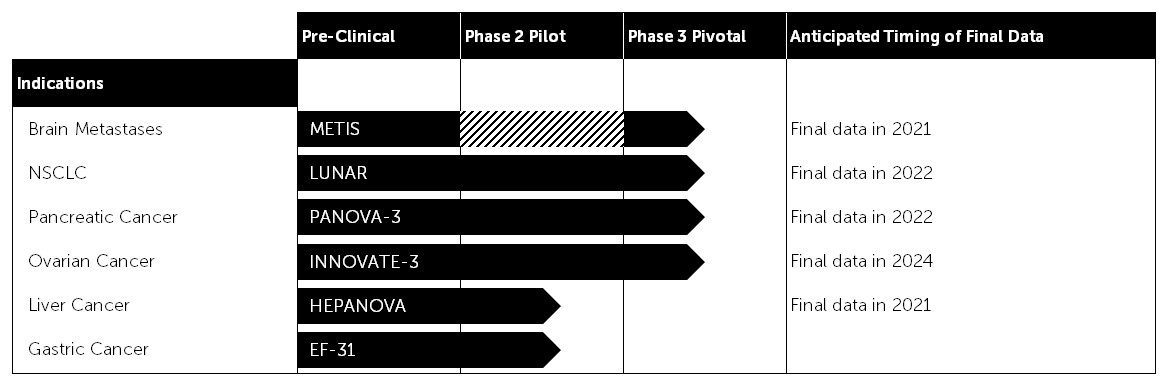
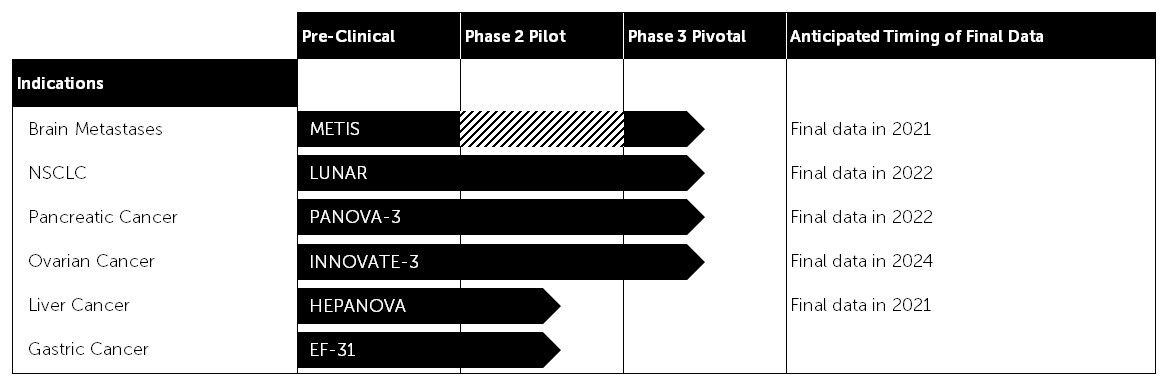
Our clinical pipeline
Based on the results of our preclinical research, we have developed a pipeline strategy to advance Tumor Treating Fields through phase 2 pilot, phase 3 pivotal trials and phase 4 post-marketing studies across multiple solid tumor types. We anticipate expanding our clinical pipeline over time to include additional solid tumor cancer indications.
Current Clinical Pipeline
The solid tumor cancers subject to our phase 2 pilot and phase 3 pivotal trials, as well as the trials themselves, are described in greater detail below.
Brain metastases
Metastatic cancer is cancer that has spread from the place where it first started to another place in the body. In metastasis, cancer cells break away from where they first formed (the primary cancer), travel through the blood or lymph system, and form new tumors (the metastatic tumors) in other parts of the body. The exact incidence of brain metastases is unknown because no national cancer registry documents brain metastases, and estimates from scientific literature vary greatly based on the study methodology applied. However, it has been estimated that up to 200,000 new cases are diagnosed in the U.S. each year. Brain metastases occur in roughly 20% of all cancer patients, and we believe that between 25 to 40% of patients with NSCLC develop brain metastases.
Brain metastases are commonly treated with a combination of surgery and radiation. Chemotherapy is often given for the primary tumor, but many chemotherapy agents do not cross the blood brain barrier and are thus ineffective in the treatment of brain metastases. When brain metastases appear, they are either surgically removed or treated with radiation using stereotactic radiosurgery ("SRS") when possible. Whole brain radiation therapy, although effective in delaying progression or recurrence of brain metastases when given either before or after SRS, is associated with neurotoxicity with a significant decline in cognitive functioning. Thus, whole brain radiation therapy is often delayed until later in the disease course and is often used as a last resort. This practice results in a window of unmet need after localized surgery and SRS are used and before whole brain radiation therapy is administered to delay or prevent the additional spread of brain metastases.
Phase 3 pivotal trial
In 2016, we enrolled the first patient in our METIS trial ("METIS"), a phase 3 pivotal trial testing the effectiveness of SRS plus Tumor Treating Fields compared to SRS alone in patients with brain metastases resulting from NSCLC. The primary endpoint of METIS is time to first intracranial progression. Secondary endpoints include, among others, time to neurocognitive failure, overall survival and radiological response rate following study treatments. The study is designed to accrue 270 patients with data analyzed 12 months after the last patient in. The majority of clinical sites were on board leading into 2019, but per site enrollment has been slower than anticipated. We are now focused on accelerating enrollment at each clinical site and are working closely with investigators to ensure they have the tools and resources needed to effectively communicate the trial protocol to as many eligible patients as possible. We anticipate data will be available in 2021.
Non-small cell lung cancer
Lung cancer is the most common cause of cancer-related death worldwide, and NSCLC accounts for approximately 85% of all lung cancers. The incidence of NSCLC is approximately 193,000 new cases annually in the U.S., approximately 400,000 new cases annually in Europe, and approximately 101,000 new cases annually in Japan.
Physicians use different combinations of surgery, radiation and pharmacological therapies to treat NSCLC, depending on the stage of the disease. Surgery, which may be curative in a subset of patients, is usually used in
early stages of the disease. Since 1991, radiation with a combination of platinum-based chemotherapy drugs has been the first line standard of care for locally advanced or metastatic NSCLC. Certain immune checkpoint inhibitors have recently been approved for the first line treatment of NSCLC and the standard of care in this setting appears to be evolving rapidly. The standard of care for second line treatment is also evolving and may include platinum-based chemotherapy for patients who received immune checkpoint inhibitors as their first line regimen, pemetrexed, docetaxel or immune checkpoint inhibitors.
Phase 2 pilot trial
In 2013, we published the results of our phase 2 pilot trial, the EF-15 trial ("EF-15"), evaluating the safety and efficacy of Tumor Treating Fields in the treatment of advanced NSCLC. EF-15 focused on the effects of treatment with Tumor Treating Fields in combination with standard of care pemetrexed chemotherapy. Results of the pemetrexed Phase 3 FDA registration trial were used as a historical control in this trial.
A total of 42 patients were recruited to the study with a minimum follow-up of six months. Efficacy results based on 41 evaluable patients showed both progression-free survival and overall survival for patients receiving Tumor Treating Fields in combination with pemetrexed increased compared to historical control data for pemetrexed alone. Median time to in-field progression in the Tumor Treating Fields-treated group was 6.5 months (compared to 2.9 months in the historical control) and median overall survival was 13.8 months (compared to 8.3 months in the historical control). Adverse events reported in this combination study were comparable to those reported with pemetrexed alone, suggesting minimal added toxicities due to Tumor Treating Fields.
Phase 3 pivotal trial
In 2017, we enrolled the first patient in our LUNAR trial ("LUNAR"), a phase 3 pivotal trial testing the effectiveness of Tumor Treating Fields in combination with immune checkpoint inhibitors or docetaxel versus immune checkpoint inhibitors or docetaxel alone for patients who progressed during or after platinum-based therapy. The primary endpoint is superior overall survival of patients treated with Tumor Treating Fields plus immune checkpoint inhibitors or docetaxel versus immune checkpoint inhibitors or docetaxel alone. We believe our protocol incorporates the evolving standard of care for second-line treatment of NSCLC.
LUNAR is designed to enroll 534 patients with data analyzed 18 months after the last patient in. The lung cancer clinical space is currently very active with substantial competition for sites and patients. We have more than 80 trial sites in North America and Europe and we intend to increase the footprint of the study by more than 50 percent to include additional clinical sites and additional countries across Eastern Europe and Asia to accelerate enrollment. We anticipate final data will be available in 2022. The protocol specifies an enrollment-driven interim analysis at 432 patients, which we anticipate will occur in the second half of 2020.
Pancreatic cancer
Pancreatic cancer is one of the most lethal cancers and is the third most frequent cause of death from cancer in the U.S. While overall cancer incidence and death rates are remaining stable or declining, the incidence and death rates for pancreatic cancer are increasing. The incidence of pancreatic cancer is approximately 51,000 new cases annually in the U.S., approximately 132,500 new cases annually in Europe, and approximately 43,000 new cases annually in Japan. Pancreatic cancer has a five-year relative survival rate in the single digits, at just 9 percent.
Physicians use different combinations of surgery, radiation and pharmacological therapies to treat pancreatic cancer, depending on the stage of the disease. For patients with locally advanced pancreatic cancer involving encasement of arteries but no extra-pancreatic disease, the standard of care is surgery followed by chemotherapy with or without radiation. Unfortunately, the majority of locally advanced cases are diagnosed once the cancer is no longer operable, generally leaving chemotherapy with or without radiation as the only treatment option.
Phase 2 pilot trial
In 2018, we published the results of our phase 2 pilot trial in advanced pancreatic adenocarcinoma, the PANOVA trial ("PANOVA"), examining Tumor Treating Fields in combination with standard of care chemotherapy.
PANOVA was a multicenter, non-randomized, open-label trial. The trial included 40 patients with locally advanced or metastatic pancreatic cancer whose tumors could not be removed surgically and who had not received chemotherapy or radiation therapy prior to the clinical trial. Patients were enrolled between 2014 and 2016 in two cohorts: The first cohort of 20 patients received Tumor Treating Fields with standard doses of gemcitabine alone.
The second cohort of 20 patients received Tumor Treating Fields with standard doses of nab-paclitaxel plus gemcitabine.
In the first cohort, efficacy results showed that progression-free survival and overall survival of patients treated with Tumor Treating Fields combined with gemcitabine were more than double those of gemcitabine-treated historical controls. Median progression-free survival in the Tumor Treating Fields-treated group was 8.3 months (compared to 3.7 months in the gemcitabine historical control), with locally advanced patients reaching a median progression-free survival of 10.3 months and patients with metastatic disease reaching a median progression-free survival of 5.7 months. The median overall survival for all patients was 14.9 months (compared to 6.7 months in the gemcitabine historical control). Median overall survival was not reached in locally advanced patients and 86% of patients were alive at end of follow up. Patients with metastatic disease experienced a median overall survival of 8.3 months. One-year survival was 55% (compared to 22% in the gemcitabine historical control). Of 11 patients with available CT scans, 5 (45%) had a partial response (compared to 7% with gemcitabine alone), 5 (45%) had stable disease, which means that the cancer is neither decreasing nor increasing in extent or severity, and 1 (10%) had progressive disease.
In the second cohort, efficacy results showed that progression-free survival and overall survival of patients treated with Tumor Treating Fields combined with nab-paclitaxel plus gemcitabine were more than double those of nab-paclitaxel plus gemcitabine-treated historical controls. Median progression-free survival in the Tumor Treating Fields-treated group was 12.7 months (compared to 5.5 months in the nab-paclitaxel plus gemcitabine historical control) and median overall survival was not yet reached. The one-year survival rate was 72% (compared to 35% in nab-paclitaxel plus gemcitabine historical control). Of the 15 patients with available CT scans, 6 (40%) had a partial response (compared to 23% with the nab-paclitaxel plus gemcitabine alone), 7 (47%) had stable disease and 2 (13%) had progressive disease.
Safety results from both cohorts suggested that Tumor Treating Fields plus first-line chemotherapies nab-paclitaxel and/or gemcitabine may be tolerable and safe in patients with advanced pancreatic cancer. Patients reported no serious adverse events related to Tumor Treating Fields.
Phase 3 pivotal trial
In 2018, we enrolled the first patient in our PANOVA-3 trial ("PANOVA-3"), a phase 3 pivotal trial testing the effectiveness of Tumor Treating Fields with nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine alone as a front-line treatment for unresectable locally advanced pancreatic cancer. The primary endpoint of PANOVA-3 is overall survival. Secondary endpoints include progression-free survival, local progression-free survival, objective response rate, one-year survival rate, quality of life, pain-free survival, resectability rate and toxicity.
The study is designed to accrue 556 patients with data analyzed 18 months after the last patient in. With 85 sites on board at the end of 2019, we have seen an increase in our enrollment rates in recent quarters. Recruitment is expected to continue to accelerate in 2020 given the anticipated international expansion of the study, which will increase the total number of sites to approximately 135 by year end. We anticipate final data will be available in 2022. The protocol specifies an enrollment-driven interim analysis at last patient in, which we anticipate will occur in 2021.
Ovarian cancer
In the U.S., ovarian cancer ranks fifth in cancer deaths among women, accounting for more deaths than any other cancer of the female reproductive system. Ovarian cancer incidence increases with age, and the median age at time of diagnosis is 63 years old. The incidence of ovarian cancer is approximately 24,500 new cases annually in the U.S., approximately 68,000 new cases annually in Europe, and approximately 11,000 new cases annually in Japan.
Physicians use different combinations of surgery and pharmacological therapies to treat ovarian cancer, depending on the stage of the disease. Surgery is usually used in early stages of the disease and is usually combined with chemotherapy, including paclitaxel and platinum-based chemotherapy. Unfortunately, the majority of patients are diagnosed at an advanced stage when the cancer has spread outside of the ovaries to include regional tissue involvement and/or metastases. Platinum-based chemotherapy remains part of the standard of care in advanced ovarian cancer, but most patients with advanced ovarian cancer will have tumor progression or, more commonly,
recurrence. Almost all patients with recurrent disease ultimately develop platinum resistance, and the prognosis for this population remains poor.
Phase 2 pilot trial
In 2018, we published the results of our phase 2 pilot trial in recurrent ovarian cancer, the INNOVATE trial ("INNOVATE"), examining Tumor Treating Fields in combination with standard of care chemotherapy. INNOVATE was a multi-center, non-randomized, open-label trial designed to test the feasibility, safety and preliminary efficacy of Tumor Treating Fields in combination with weekly paclitaxel. The paclitaxel control arm from the bevacizumab phase 3 FDA registration trial was used as a historical control in this trial.
A total of 31 patients were recruited to the study with a minimum follow-up of six months. Safety results suggested that Tumor Treating Fields in combination with weekly paclitaxel may be tolerable and safe as second-line treatment for patients with recurrent ovarian cancer. Median progression-free survival in the Tumor Treating Fields-treated group was 8.9 months (compared to 3.9 months in the paclitaxel-alone historical control), and median overall survival was not yet reached. The one-year survival rate was 61%. Efficacy results based on the 31 evaluable patients suggested more than doubling of the progression-free survival and an improvement in overall survival among patients who received Tumor Treating Fields therapy with paclitaxel compared to paclitaxel alone.
Phase 3 pivotal trial
In 2019, we enrolled the first patient in our INNOVATE-3 trial ("INNOVATE-3"), a phase 3 pivotal trial testing the effectiveness of Tumor Treating Fields with paclitaxel in patients with platinum-resistant ovarian cancer. The primary endpoint of INNOVATE-3 is overall survival. Secondary endpoints include progression-free survival, objective response rate, severity and frequency of adverse events, time to undisputable deterioration in health-related quality of life or death, and quality of life.
The European Network for Gynaecological Oncological Trial groups ("ENGOT") and The GOG Foundation, Inc. ("GOG"), third-party clinical trial networks, are collaborating with us on the trial. ENGOT and GOG were involved in the development of the trial, and the collaborations are intended to facilitate enrollment of INNOVATE-3 at leading cancer centers in Europe and the United States. The study is designed to accrue 540 patients with data analyzed 18 months after the last patient in. We are encouraged by the initial speed of enrollment, which we view as a sign of enthusiasm from the investigator community and anticipate final data in 2024. The protocol specifies an enrollment-driven interim analysis at last patient in, which we anticipate will occur in 2022.
Liver cancer
Liver cancer is a leading cause of cancer deaths worldwide and is the fifth leading cause of cancer deaths annually in the U.S. The incidence of liver cancer is approximately 38,000 new cases annually in the U.S., approximately 82,500 new cases annually in Europe, and approximately 35,500 new cases annually in Japan. The five-year survival rate with existing standards of care is less than 18%.
Hepatocellular carcinoma is the most widespread type of cancer that originates from the liver. Advanced liver cancer has spread either to the lymph nodes or to other organs and, because these cancers are widespread, they cannot be treated with surgery. The current common standard treatment for patients with advanced disease and those who progressed on loco-regional therapy is systemic therapy with sorafenib.
Phase 2 pilot trial
In 2018, we opened our HEPANOVA trial, a single-arm, phase 2 pilot clinical trial in liver cancer testing the safety and efficacy of Tumor Treating Fields in combination with sorafenib for the treatment of advanced hepatocellular cancer that are not eligible for standard local therapies or surgery. The primary endpoint is overall response rate, and secondary endpoints include progression-free and overall survival at one year. The trial is expected to enroll a total of 25 patients in multiple centers across Europe and we anticipate data will be available in 2021.
Gastric cancer
Gastric cancer is the third leading cause of cancer deaths worldwide and the second leading cause of cancer deaths in China. The incidence of gastric cancer is approximately 456,000 new cases annually in China, approximately 26,000 new cases annually in the U.S., approximately 133,000 new cases annually in Europe and 116,000 new cases annually in Japan. The five-year overall survival rate of gastric cancer is approximately 36%.
Current therapies include surgery, chemotherapy, radiotherapy and targeted therapy. A commonly used chemotherapy regimen in treating gastric cancer is XELOX, a combination of oxaliplatin and capecitabine. In patients diagnosed with advanced gastric cancer that is no longer operable, combination chemotherapy extends progression-free survival and overall survival to 3-6 months and 8-14 months, respectively.
Phase 2 pilot trial
In 2020, we opened our EF-31 trial, a single-arm, phase 2 pilot clinical trial in gastric cancer in partnership with Zai testing the safety and efficacy of Tumor Treating Fields and XELOX chemotherapy as first-line treatment for patients with unresectable gastric adenocarcinoma or gastroesophageal junction adenocarcinoma. The primary endpoint is investigator-assessed objective response rate, and secondary endpoints include progression-free and overall survival. The trial is expected to enroll a total of 28 patients in multiple centers across Greater China.
Zai License and Collaboration Agreement
In September 2018, we announced a strategic collaboration with Zai. The collaboration agreement grants Zai a license to commercialize our Products in Greater China and establishes a development partnership intended to accelerate the development of Tumor Treating Fields in multiple solid tumor cancer indications. Zai has launched Optune for the treatment of newly diagnosed GBM in Hong Kong. The Chinese regulatory authorities designated Optune as an Innovative Medical Device and have accepted the Marketing Authorization Application for the GBM application in China. Zai is pursuing a clinical trial waiver and, should a clinical trial waiver be granted, Zai intends to launch Optune in China in 2020. For additional information, see Note 12 to the Consolidated Financial Statements.
Manufacturing and supply chain
We outsource production of all of our system components to qualified partners. Disposable transducer array manufacturing, the dominant activity in our manufacturing supply chain, includes several specialized processes. Production of the durable system components follows standard electronic medical device methodologies.
We have supply agreements in place with our third-party manufacturing partners. While we currently obtain some critical materials for use in certain jurisdictions from single source suppliers, we have developed or are in the process of developing and obtaining regulatory approval for second sources for critical materials in all jurisdictions. We hold safety stocks of single source components in quantities that we believe are sufficient to protect against possible supply chain disruptions. We anticipate that the diversification of our supply chain will both ensure a continuity of supply and reduce costs.
Intellectual property
We believe we own global commercialization rights to our Products in oncology and are well-positioned to extend those rights into the future as we continue to find innovative ways to improve our Products. Our robust global patent and intellectual property portfolio consists of over 180 issued patents covering various aspects of Tumor Treating Fields and our Products. In the U.S., our patents have expected expiration dates between 2021 and 2037. We have also filed over 125 additional patent applications worldwide, including 33 new U.S. patent applications in 2019, that, if issued, may protect aspects of our platform beyond the current last-to-expire patent in the relevant market. These pending applications cover innovations relating to our transducer arrays, field generators and software platform, in addition to other topics related to Tumor Treating Fields. Our reliance on intellectual property involves certain risks, as described under the heading "Risk factors—Risks relating to intellectual property."
In addition to our patent portfolio, we further protect our intellectual property by maintaining the confidentiality of our trade secrets, know-how and other confidential information. Given the length of time and expense associated with bringing delivery systems candidates through development and regulatory approval to the market place, the healthcare industry has traditionally placed considerable importance on obtaining patent protection and maintaining trade secrets, know-how and other confidential information for significant new technologies, products and processes.
Our policy is to require each of our employees, consultants and advisors to execute a confidentiality agreement before beginning their employment, consulting or advisory relationship with us. These agreements generally provide that the individual must keep confidential and not disclose to other parties any confidential information developed or learned by the individual during the course of their relationship with us except in limited circumstances. These agreements also generally provide that we own, or the individual is required to assign to us, all inventions conceived by the individual in the course of rendering services to us. Despite measures taken to protect our intellectual
property, unauthorized parties may copy certain aspects of our products or obtain and use information that we believe is proprietary.
Pursuant to our strategic collaboration with Zai, we granted Zai a license to commercialize Tumor Treating Fields in Greater China. For additional information, see Note 12 to the Consolidated Financial Statements.
In 2015, we entered into a settlement agreement with the Technion Research and Development Foundation to resolve certain potential disputes regarding intellectual property developed by our founder and previously assigned to us. For additional information, see Note 12 to the Consolidated Financial Statements.
In 2005, we granted an exclusive license to a third party, NovoBiotic LLC, to certain of our key intellectual property for use outside the field of oncology. We are not entitled to any future revenues from this license.
Competition
The market for cancer treatments is intensely competitive, subject to rapid change and significantly affected by new product and treatment introductions and other activities of industry participants. The general bases of competition are overall effectiveness, side effect profile, cost, availability of reimbursement and general market acceptance of a product as a suitable cancer treatment.
We believe our intellectual property rights would provide an obstacle to the introduction of Tumor Treating Fields delivery systems by a competitor, and we intend to protect and enforce our intellectual property. In addition, even after the expiration of our U.S. patents, we believe that potential U.S. market entrants applying low-intensity, alternating electric fields to solid tumors in the U.S. will have to undertake their own clinical trials and regulatory submissions to prove equivalence to our Products, a necessary step in receiving regulatory approvals for a competing product.
Presently, the traditional biotechnology, pharmaceutical and medical technology industries expend significant resources in developing novel and proprietary therapies for the treatment of solid tumors, including GBM, MPM and other indications that we are currently investigating. As we work to increase market acceptance of our Products, we compete with companies commercializing or investigating other anti-cancer therapies, some of which are in clinical trials for GBM or MPM that currently specifically exclude patients who have been or are being treated with our Products. The introduction of competing therapies could materially impact our business and financial results.
Government regulation
In the United States, our Products and our operations are subject to extensive regulation by the FDA under the Federal Food, Drug, and Cosmetic Act ("FDCA"). In the EU member states where we market our Products and operate, we are currently subject to, inter alia, the Medical Device Directive ("MDD") as implemented into national legislation by the EU member states. From May 26, 2020, the MDD will be replaced and repealed by the Medical Device Regulation ("MDR"), which will apply directly in all EU member states. In Switzerland, our Products and operations are subject to, inter alia, the Medical Devices Ordinance, which implements the MDD into Swiss law. In Japan, our Products and operations are subject to regulation by the Pharmaceuticals and Medical Device Agency ("PMDA") under the Pharmaceuticals and Medical Devices Act ("PMD Act"). In addition, our Products must meet the requirements of a large and growing body of national, regional and international standards that govern the preclinical and clinical testing, manufacturing, labeling, certification, storage, recordkeeping, advertising, promotion, export and marketing and distribution, among other things, of our Products for current and future indications.
In the U.S., advertising and promotion of medical devices, in addition to being regulated by the FDA, is also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. In the EU, advertising and promotion is subject to not only the general provisions of the MDD or MDR, but also general EU advertising rules on misleading and comparative advertising and unfair commercial practices, as implemented at the EU member state level, such as the Heilmittelwerbegesetz in Germany. Promotional activities for FDA-regulated products of other companies have been the subject of government enforcement actions brought under healthcare laws and consumer protection statutes. In addition, we are required to meet analogous regulatory requirements in countries outside the U.S., which can change rapidly with relatively short notice. Competitors can also initiate litigation alleging false advertising for our promotional efforts under the Lanham Act, or under similar state laws.
Our research, development and clinical programs, as well as our manufacturing and marketing operations, are also subject to extensive regulation.
Failure by us or by our suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or other regulatory authorities, which may result in any number of regulatory enforcement actions, or civil or criminal liability.
Food and Drug Administration
The FDA regulates the development, testing, manufacturing, labeling, storage, recordkeeping, promotion, marketing, distribution and service of medical devices in the U.S. to ensure that medical products distributed domestically are safe and effective for their intended uses. In addition, the FDA regulates the export of medical devices manufactured in the U.S. to international markets and the importation of medical devices manufactured abroad. The FDA has broad post-market and regulatory enforcement powers to ensure compliance with the FDCA.
The FDA governs the following activities that we perform or that are performed on our behalf:
•product design, development and manufacture;
•product safety, testing, labeling and storage;
•record keeping procedures;
•product marketing, sales and distribution; and
•post-marketing surveillance, complaint handling, medical device reporting, reporting of deaths, serious injuries or device malfunctions and repair or recall of products.
We have registered three of our facilities with the FDA. We are subject to announced and unannounced inspections by the FDA to determine our compliance with the Quality System Regulation ("QSR") and other regulations and these inspections include the manufacturing facilities of our suppliers.
FDA’s premarket clearance and approval requirements
Unless an exemption applies, before we can commercially distribute medical devices in the U.S., we must obtain, depending on the type of device, either prior 510(k) clearance or premarket approval ("PMA") from the FDA. The FDA classifies medical devices into one of three classes. Devices deemed to pose lower risks are placed in either class I or II, which typically requires the manufacturer to submit to the FDA a premarket notification requesting permission to commercially distribute the device. This process is generally known as 510(k) clearance. Some low-risk devices are exempted from this requirement. Devices deemed by the FDA to pose the greatest risks, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously cleared 510(k) device, are placed in class III, generally requiring PMA.
Premarket approval (PMA) pathway
Optune and NovoTTF-100L are classified as Class III devices as they are deemed to be life-sustaining devices. Accordingly, we were required to receive PMA for Optune, which the FDA granted in April 2011 and October 2015 for the treatment of recurrent and newly diagnosed supratentorial GBM, respectively, in adult patients. We expect that we will be required to receive PMA for the use of our Products for future indications.
A PMA must be supported by extensive data, including from technical tests, preclinical studies and clinical trials, manufacturing information and intended labeling to demonstrate, to the FDA’s satisfaction, the safety and effectiveness of a medical device for its intended use. During the PMA review period, the FDA will typically request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will generally conduct a pre-approval inspection of the manufacturing facility or facilities to ensure compliance with the QSR. Prior to approval of the Optune PMA for the treatment of recurrent GBM, we and our critical component suppliers were each inspected by the FDA.
New PMAs or PMA supplements are required for modifications that affect the safety or effectiveness of our delivery systems, including, for example, certain types of modifications to a delivery system’s indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the
device covered by the original PMA and may not require any or as extensive clinical data as the original PMA required, or the convening of an advisory panel. The FDA requires a company to make the determination as to whether a new PMA or PMA supplement application is to be filed. If a company determines that neither a new PMA nor a PMA supplement application is required for modifications, it must nevertheless notify the FDA of these modifications in its PMA Annual Report. The FDA may review a company’s decisions when reviewing the PMA Annual Report and require the filing of an application.
As is typical with medical device companies, we have received approval for a number of post-approval PMA supplements for GBM, including for modifications to Optune’s electric field generator, transducer arrays, software, manufacturing processes and labeling. Future modifications may be considered by us as the need arises, some of which we may deem to require a PMA supplement application and others to require reporting in our PMA Annual Report.
For class III devices intended to treat disease affecting 8,000 individuals or less per year in the U.S., called Humanitarian Use Devices ("HUD"), the FDA has a separate marketing authorization pathway called the HDE. Approval basis for an HDE is a "reasonable assurance of safety" and that the probable benefit to health outweighs risk of injury from its use, which means a traditional phase 3 pivotal trial usually is not required to support approval.
In 2019, the FDA approved NovoTTF-100L for the treatment of MPM under the HDE pathway. Devices approved through an HDE application are subject to certain requirements, including specific labeling restrictions and the requirement that a facility’s IRB or Local Committee approve the use of the device before it can be distributed in that facility. In addition, there is a general prohibition on profiting from sales of devices approved under the HDE standard. As part of the approval process, we applied for an exemption from this limitation, which the FDA granted. Otherwise, HDE approved devices are generally required to follow the same requirements as PMA approved devices.
Clinical trials
Clinical trials are generally required to support approval of a PMA or HDE. Such trials generally require an Investigational Device Exemption ("IDE") approval from the FDA for a specified number of patients and study sites, unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. Clinical trials are subject to extensive monitoring, recordkeeping and reporting requirements. Clinical trials must be conducted under the oversight of an IRB for the relevant clinical trial sites and must comply with FDA regulations, including those relating to current Good Clinical Practices ("cGCPs"). To conduct a clinical trial, we also are required to obtain the patients’ informed consent in form and substance that complies with both FDA requirements and state and federal privacy and human subject protection regulations. We, the FDA or the respective IRB could suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA approval to market the product in the U.S.
Post-approval studies are also typically required as a condition of PMA to reinforce the reasonable assurance of safety and effectiveness. Such studies are conducted in the post-market setting with the approved device, often to address the long-term use of the device or other discrete questions that may have been raised based on the clinical data from the IDE clinical study. The FDA required a post-approval registry study as a condition of approval for Optune for recurrent GBM. We have completed this study and the study data have been submitted for presentation at an upcoming medical conference.
Foreign approvals and CE mark
Sales and marketing of medical devices outside of the U.S. are subject to foreign regulatory requirements that vary widely from country to country. These include the requirement to obtain CE Certification and to affix a CE mark to our medical devices in the EU. Whether or not we have obtained FDA approval, our delivery systems must be subject to conformity assessment procedure involving a notified body, a private organization designated by an EU member state to conduct conformity assessment procedures under the MDD/MDR. Apart from low risk medical devices (Class I with no measuring function and which are not sterile), where the manufacturer can issue a declaration of conformity based on a self-assessment of the conformity of its products with the Essential Requirements laid down in the MDD, a conformity assessment procedure requires the intervention of a notified body. The notified body typically audits and examines the products’ technical file, including the clinical data, and the quality system for the manufacture, design and final inspection of our devices before issuing a CE Certificate
demonstrating compliance with the relevant Essential Requirements or the quality system requirements laid down in the relevant Annexes to the MDD. Following the issuance of this CE Certificate, we can draw up a declaration of conformity and affix the CE mark to the delivery systems covered by this CE Certificate and the declaration of conformity. The time required to CE mark our delivery systems or to obtain approval from other non-U.S. authorities may be longer or shorter than that required for FDA approval. Currently, pursuant to the Mutual Recognition Agreement ("MRA") in place between the EU and Switzerland, our Products bearing a CE mark may be exported from the EU to Switzerland. However, this MRA is only valid until May 25, 2020 and if no new MRA has been agreed to by then, Switzerland will be considered a third country with respect to medical devises. In the EU, before carrying out a clinical study, the sponsor must receive a positive opinion from a local ethics committee and approval from the national competent authority in the relevant EU member states in which the clinical study will be conducted. When a clinical study relates to a CE marked medical device that will be used as part of the study according to its intended use, the approval of the national competent authorities is not required for the use of such medical device in the study. In Japan, we must obtain approvals from the MHLW to market our delivery systems. Each regulatory approval process outside of the U.S. includes all the risks associated with FDA regulation, as well as country-specific regulations.
Pervasive and continuing regulation
After a device is placed on the market, numerous regulatory requirements apply depending upon the country in which the device is being marketed. These may include:
•product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action;
•QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process for products marketed in the U.S.;
•labeling regulations and FDA and equivalent competent authority in other jurisdictions requiring promotion is truthful and non-misleading and prohibiting the promotion of products for uncleared, unapproved or off-label uses;
•approval of product modifications that affect the safety or effectiveness of one of our delivery systems that has been approved or is the subject of a CE Certificate;
•Medical Device Reporting regulations of the FDCA and medical device vigilance, which require that manufacturers comply with FDA or equivalent competent authority requirements in other jurisdictions to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur;
•post-approval restrictions or conditions, including post-approval study commitments;
•post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device;
•the FDA’s and equivalent competent authority’s recall authority, whereby they can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations;
•regulations pertaining to voluntary recalls; and
•notices of corrections or removals.
Our delivery systems could be subject to voluntary recall if we, the FDA or another applicable regulatory authority determine, for any reason, that our delivery systems pose a risk of injury or are otherwise defective. Moreover, the FDA and other applicable regulatory authorities can order a mandatory recall if there is a reasonable probability that our delivery system would cause serious adverse health consequences or death.
The FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by the FDA to determine our compliance with the QSR and other regulations, and these inspections include the manufacturing facilities of our subcontractors. We are also subject to FDA’s broad regulatory enforcement power around promotional activities. Failure by us or by our suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or other applicable regulatory authorities, which may result in sanctions, including, but not limited to:
•untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;
•unanticipated expenditures to address or defend such actions;
•customer notifications for repair, replacement and/or refunds;
•recall, detention or seizure of our delivery systems;
•operating restrictions or partial suspension or total shutdown of production;
•refusing or delaying our requests for approval of delivery system candidates or a modified version of Optune;
•withdrawal of PMA/HDE approvals or suspension, variation or withdrawal of CE Certificates that have already been granted;
•refusal to grant export approval for our delivery systems; or
•civil and/or criminal prosecution by the U.S. Department of Justice or other enforcement authorities outside of the U.S.
To date, our facility and those of our critical suppliers have been inspected by the FDA in order to obtain FDA approval of our Products. No inspectional observations were identified and no FDA Form 483s were issued following these inspections.
DME accreditation and licensing and other requirements
In the U.S., we are subject to accreditation and licensing requirements as a DME supplier in most states and must meet the supplier standards of Medicare, Medicaid and other federal healthcare programs. Certain states require that DME providers maintain an in-state location. Although we believe we are in compliance with all applicable federal and state regulations regarding accreditation and licensure requirements and similar requirements in other jurisdictions, if we are found to be noncompliant, we could lose our accreditation or licensure in such states or our supplier rights under such federal healthcare programs, which could prohibit us from selling our current or future delivery systems to patients in such state or to that federal healthcare program.
Healthcare regulatory matters
In addition to FDA restrictions on the marketing of medical devices, several other U.S. federal and state laws have been applied to restrict certain business practices in the healthcare industry and penalize unlawful conduct. These laws include the federal Anti-Kickback Statute, the federal prohibition on physician self-referrals (commonly known as the "Stark Law") and the federal False Claims Act.
The U.S. federal Anti-Kickback Statute is a criminal, intent-based statute that prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving any remuneration to induce or in return for purchasing, leasing, ordering, recommending or arranging for the purchase, lease, order or recommendation of any healthcare item or service that may be paid for, in whole or in part, by Medicare, Medicaid or another federal healthcare program. Among other arrangements, this statute has been interpreted to apply to financial arrangements between medical device manufacturers on one hand and prescribers and purchasers on the other. Although there are a number of statutory exceptions and regulatory safe harbors that protect certain common activities from prosecution under the law, the exceptions and safe harbors are drawn narrowly and practices that involve the provision of remuneration intended to induce ordering, purchasing, leasing or recommending of a medical device may be subject to scrutiny if they do not qualify for an exception or safe harbor. In some cases, our practices may not meet all of the technical elements for protection under a federal Anti-Kickback Statute exception or safe harbor. Similarly,
as a supplier, we are also subject to the federal beneficiary anti-inducement statute, which prohibits us from offering any remuneration to a beneficiary of Medicare or Medicaid that is likely to influence that beneficiary’s choice of therapy, unless an exception applies. This can include, but is not limited to, the provision of inappropriate financial assistance to purchase our Products. Recent government investigations and enforcement actions have focused on the provision of financial assistance to patients by providers and suppliers. As noted, there are established exceptions from liability, but we cannot guarantee that all of our practices will fall squarely within those exceptions.
As a DME supplier, we also are subject to the Stark law, which is a strict liability law that prohibits Medicare payments for certain "designated health services" ("DHS") including DME ordered by physicians who, personally or through an immediate family member, have an ownership interest in or a compensation arrangement with the furnishing DHS entity. The Stark law contains a number of specific exceptions that, if met, permit physicians who have certain financial relationships with a DHS entity to make referrals to that entity and for that entity to bill Medicare for such services.
The False Claims Act prohibits any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. The government has pursued numerous cases under the False Claims Act in connection with the off-label promotion of medical products and various other health care law violations.Notably, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
The majority of states also have statutes or regulations similar to the federal Anti-Kickback Statute, Stark Law and False Claims Act laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, that apply regardless of the payer (e.g., including private/commercial payors or cash-pay scenarios).
Numerous federal and state laws and regulations, including the Health Insurance Portability and Accountability Act of 1996 as amended by the Health Information Technology for Economic and Clinical Health Act ("HITECH" and collectively "HIPAA"), govern the collection, dissemination, use, security and privacy of individually identifiable health information. We believe we are in substantial compliance with such applicable laws and regulations, including HIPAA.
HIPAA also includes a number of federal criminal provisions, including for healthcare fraud and for false statements relating to healthcare matters. The healthcare fraud provision prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payers. The false statements provision prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Many states have similar healthcare fraud laws or insurance fraud laws that apply to claims for healthcare reimbursement.
Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines and imprisonment.
Legislation similar to the federal Anti-Kickback Statute, the Stark Law and False Claims Act has been adopted in foreign countries, including a number of EU member states.
In the EU, the General Data Protection Regulation ("GDPR") has applied since May 25, 2018. The GDPR harmonizes data privacy laws and rules for the processing of personal data, including patient and employee data, across the EU and repeals and replaces Directive 95/46/EC of the European Parliament and of the Council of October 24, 1995, and applicable national laws. The GDPR has added a number of strict data protection and security requirements for companies processing personal data of EU residents, including when such data is transferred outside the EU.
In the U.S., the federal Physician Payment Sunshine Act ("Sunshine Act") requires certain manufacturers of drugs, medical devices, biologicals or medical supplies that participate in U.S. federal health care programs to track and then report certain payments and transfers of value given to "Covered Recipients." The term "Covered Recipients" currently includes U.S.-licensed physicians and teaching hospitals, and, for reports submitted on or after January 1, 2022, physician assistants, nurse practitioners, clinical nurse specialists, certified nurse anesthetists, and certified nurse-midwives. The Sunshine Act requires that manufacturers collect this information on a yearly basis and then
report it to Centers for Medicare & Medicaid Services by the 90th day of each subsequent year. We have adopted policies and codes of conduct regarding our interactions with Covered Recipients and believe we are in material compliance with the Sunshine Act. However, our failure to adhere to these requirements could materially adversely impact our business and financial results. Additionally, a number of states have transparency reporting requirements similar to (and in some cases broader than) the Sunshine Act, and regulations similar to the Sunshine Act have been adopted in foreign countries including a number of EU member states.
In addition, the U.S. Foreign Corrupt Practices Act ("FCPA") prohibits corporations and individuals from engaging in certain activities to obtain or retain business outside the U.S. or to influence a person working in an official capacity in a foreign country. It is illegal to pay, offer to pay or authorize the payment of anything of value to any official of another country, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in that capacity. Legislation similar to the FCPA has been adopted in foreign countries, including a number of EU member states.
Employees
As of December 31, 2019, we had 782 employees. We believe relations with our employees are good.
Available information
Our corporate website address is www.novocure.com. Our website is an inactive textual reference and nothing on our website is incorporated by reference in this Annual Report. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to reports filed pursuant to Sections 13(a) and 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act, are available free of charge on our website as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC. These filings are also available on the SEC’s website at www.sec.gov.
We may use our website as a means of disclosing material information and for complying with our disclosure obligations under Regulation Fair Disclosure promulgated by the SEC. Accordingly, investors should monitor our website, in addition to following our press releases, SEC filings, public conference calls, webcasts and our social media accounts.
ITEM 1A. RISK FACTORS
An investment in our ordinary shares involves a high degree of risk. Investors and prospective investors should carefully consider all of the information in this Annual Report on Form 10-K, including the risks and uncertainties described below. Any of the following risks could have a material adverse effect on our business, prospects, financial condition and results of operations. In any such case, the trading price of our ordinary shares could decline, and you could lose all or part of your investment. In assessing these risks, you should also refer to the other information contained in this Annual Report on Form 10-K, including our consolidated financial statements and the related notes thereto.
Risks relating to our business and our Products
Our business and prospects depend heavily on Optune, which is currently approved only for the treatment of GBM, and NovoTTF-100L, which is approved for the treatment of MPM. If we are unable to increase sales of our Products, obtain further regulatory approvals for and further commercialize our Products for the treatment of additional indications or are significantly delayed or limited in doing so, our business and prospects will be materially harmed.
To date we have received FDA regulatory approval under the PMA pathway and certain approvals in other jurisdictions for the use of Optune for the treatment of adult patients with newly diagnosed GBM in combination with temozolomide (a form of chemotherapy) and for the treatment of adult patients with recurrent GBM as monotherapy. Optune has obtained a CE Certification, and affixed a CE mark, for the treatment of GBM in the EU. We have also received FDA approval under the HDE pathway to market NovoTTF-100L for unresectable, locally advanced or metastatic, malignant pleural mesothelioma in combination with standard chemotherapies. However, such approvals and the CE Certification, and affixing of a CE mark, of our Products, as applicable, do not guarantee future revenues for these indications. Further, until we receive FDA and analogous approval in other jurisdictions for the use of our Products for other indications, almost all of our revenues will derive from sales and royalties from sales of Optune for the treatment of newly diagnosed and recurrent GBM and NovoTTF-100L for MPM. The commercial
success of our Products and our ability to generate and maintain revenues from the sale of our Products will depend on a number of factors, including:
•our ability to obtain additional regulatory approvals for and further commercialize our Products;
•our ability to develop, obtain regulatory approval for and appropriately commercialize our Products for additional indications;
•the acceptance of our Products by patients and the healthcare community, including physicians and third-party payers (both private and governmental), as therapeutically effective and safe;
•the relative cost, safety and efficacy of alternative therapies;
•our ability to obtain and maintain sufficient coverage or reimbursement by private and governmental third-party payers;
•the ability of our third-party manufacturers to manufacture our Products in sufficient quantities with acceptable quality;
•our ability to provide marketing, distribution and customer support for our Products;
•results of future clinical studies relating to our Products or other competitor products for similar indications;
•compliance with applicable health care and cybersecurity laws and regulations;
•the maintenance of our existing regulatory approvals; and
•the consequences of any reportable adverse events involving our Products.
In addition, the promotion of our Products is limited to approved indications, which vary by geography, and the FDA label for Optune is limited in certain respects (for example, it is not approved for use in the brain stem, is not approved for use as monotherapy in newly diagnosed GBM and is limited for use by adults ages 22 and older), which may reduce the number of patients to whom it may be prescribed. Similarly, the label for NovoTTF-100L also contains certain limitations which may adversely affect adoption, including the requirement to display on all marketing materials that the efficacy of the product has not been established, a limitation of use by adults ages 22 and older only, and the absence of Phase III clinical data.
Our ability to generate future revenues will also depend on achieving regulatory approval of, and eventual commercialization of, our Products for additional indications. However, obtaining regulatory approval of our Products for additional indications is not guaranteed. Our near-term prospects are substantially dependent on our ability to obtain regulatory approvals on the timetable we have anticipated, and thereafter to further successfully commercialize our Products for additional indications. Regulatory changes or actions in which we operate or propose to operate may further affect our ability to obtain regulatory approvals on the anticipated timetable. If we are not able to receive such approvals or to further commercialize our Products, or are significantly delayed or limited in doing so, our business and prospects will be materially harmed and we may need to delay our initiatives or even significantly curtail operations.
To date, we have incurred substantial operating losses.
We were founded in 2000 and have incurred substantial operating losses to date. In assessing our prospects, you must consider the risks and difficulties frequently encountered by companies in new and rapidly evolving markets, particularly companies engaged in the development and sales of oncology products. These risks include our ability to:
•continue to develop and enhance our Products;
•obtain regulatory approval to commercialize our Products for additional indications and enhance or modify our Products;
•increase our sales, marketing and distribution organization to commercialize our Products;
•perform preclinical and clinical research, engineering research and development and clinical trials on our Products and Tumor Treating Fields;
•establish and increase awareness and acceptance of our Products;
•implement and successfully execute our business and marketing strategy;
•respond effectively to competitive pressures and developments;
•maintain, protect and expand our intellectual property portfolio;
•operate in compliance with applicable health care and cybersecurity laws and regulations;
•expand our presence in our key markets;
•attract, retain and motivate qualified personnel; and
•grow our organization to support our operations and our clinical pipeline and expand commercialization efforts.
We anticipate continuing to incur significant costs associated with commercializing our Products for approved indications including product sales, marketing, manufacturing and distribution expenses. We expect our research, development and clinical trials expenses to increase in connection with our ongoing activities and as additional indications enter late-stage clinical development. Our expenses could increase beyond expectations if, for example, we are required by the FDA, or other regulatory agencies, to change manufacturing processes for our Products, or to perform clinical, nonclinical or other types of studies in addition to those that we currently anticipate. Our revenues are dependent, in part, upon the size of the markets in the jurisdictions in which we receive regulatory approval, the accepted price for our Products and the ability to obtain reimbursement at the accepted applicable price. If the number of addressable patients is not as significant as we estimate, the indications approved by regulatory authorities are narrower than we expect or the eligible population for treatment is narrowed by competition, regulatory approvals, physician choice or treatment guidelines, we may not generate significant revenues. If we are not able to generate significant revenues, we may never be sustainably profitable.
If we do not achieve our projected research and development and commercialization goals in the timeframes we announce or expect, our results of operations would be adversely affected and we may need to raise additional capital to fund our operations.
We estimate the timing of the accomplishment of various scientific, engineering, clinical, regulatory and other goals, which we sometimes refer to as milestones. These milestones may include the commencement or completion of scientific studies and clinical trials, the submission of regulatory filings in the U.S. and other jurisdictions and the receipt of regulatory approvals in such jurisdictions. From time to time, we may publicly announce the expected timing of some of these milestones. All of these milestones are based on a variety of assumptions that may not turn out to be accurate or relevant in hindsight. For example, our key milestones include clinical development and regulatory milestones for the use of our Products to treat brain metastases, non-small cell lung cancer, pancreatic cancer and ovarian cancer. We can provide no assurance that we will achieve these milestones on our expected timetable, or at all. The actual timing of the achievement of these milestones can vary dramatically from our estimates, in many cases for reasons beyond our control, depending on numerous factors, including:
•the rate of progress, costs and results of our research and development activities and clinical trials;
•our ability to identify and engage appropriate health care professionals to conduct our clinical trials;
•the extent of competing clinical trials for the same cohort of eligible patients;
•our ability to identify and enroll patients who meet clinical trial eligibility criteria;
•the extent of scheduling conflicts with participating clinicians and clinical institutions;
•the occurrence of adverse events during clinical trials;
•the occurrence of adverse events due to a cybersecurity breach involving our Products;
•the receipt of approvals by our competitors of competing products and by us of additional indications for our Products;
•our ability to achieve coverage and reimbursement milestones with private and governmental third-party payers;
•our ability to access sufficient, reliable and cost-effective supplies of components used in the manufacture of our Products, including the batteries, transducer arrays and other materials;
•our ability to develop and maintain a compliant sales and marketing organization and/or enter into sales and marketing collaborations for our Products; and
•changes in regulations and other actions by regulators.
If we do not achieve any of these milestones in the timeframes we expect and generate sufficient revenues, and/or if we are unable to obtain sufficient additional funds through financings, the proceeds from long-term loans, strategic collaborations or the license or sale of certain of our assets on a timely basis when necessary, we may be required to reduce expenses by delaying, reducing or curtailing the development of our Products and we may need to raise additional capital to fund our operations, which we may not be able to obtain on favorable terms, if at all. If we fail to commence or complete, or experience delays in or are forced to curtail, our proposed clinical programs or otherwise fail to adhere to our projected development milestones in the timeframes we announce or expect (or within the timeframes expected by analysts or investors), or we fail to raise any required additional capital, any of such events could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline. We will need to generate significant revenues to be sustainably profitable, and we may never do so.
We may not be successful in our efforts to create a pipeline of future indications for our Products and successfully commercialize them.
We are pursuing clinical development of our Products to treat a variety of solid tumors. For these future indications, we are at varying stages of development and we generally do not have relevant regulatory approvals to market our Products for these indications. Further, we do not currently intend to pursue indications involving solid tumors of the throat or extremities, and we do not believe our Products would be efficacious for non-solid tumor cancers like lymphoma or other blood cancers.
Even if we are successful in continuing to build our pipeline, obtaining regulatory approvals and commercializing our Products for additional indications are susceptible to risks of failure, including the significant risk that the development of our Products for any potential indications will fail to demonstrate adequate efficacy or an acceptable safety profile, to gain regulatory approval and/or to become commercially viable. We cannot provide any assurance that we will be able to advance any of these additional indications through the development and commercialization process. Our research programs may initially show promise in addressing additional indications, yet fail to yield approvals or commercialization for many reasons, including the following:
•we may not be able to assemble sufficient resources to pursue clinical trials for additional indications;
•our Products may not succeed in preclinical or clinical testing for additional indications;
•our Products may, on further study be shown to have harmful side effects for other indications or other characteristics that indicate they are unlikely to be effective or otherwise do not meet applicable regulatory criteria for such indications;
•competitors may develop alternative treatments that render our Products obsolete or less attractive;
•the market for our Products may change so that the continued development of our pipeline as currently contemplated is no longer appropriate;
•we may not be able to produce our Products for current and future indications in commercial quantities at an acceptable cost, or at all;
•our Products may not meet standards set by applicable regulatory authorities to obtain approval or clearance to market our Products for additional indications; and
•our Products may not be accepted as safe, effective, convenient, cost-effective or otherwise desirable by patients, the medical community, regulatory authorities or third-party payers.
If any of these events occur, we may be forced to delay or abandon our development efforts for our anticipated pipeline, which would have a material adverse effect on our business and prospects and could potentially cause our stock price to decline and cause us to cease operations. Moreover, any of these events regarding the use of our Products in any particular indication may have a negative effect on the approval process for other indications and/or result in losing or delaying approval of our Products for other indications, which may exacerbate the harm to our business and prospects.
If we encounter difficulties enrolling patients in our clinical trials, our clinical trials could be delayed or otherwise adversely affected.
The timely completion of clinical trials depends, among other things, on our ability to enroll a sufficient number of patients who remain in the trial until its conclusion. We may experience difficulties in patient enrollment in our clinical trials for a variety of reasons, including:
•the severity of the disease under investigation;
•the limited size and nature of the patient population;
•the patient eligibility criteria defined in our protocol and other clinical trial protocols;
•the nature of the trial protocol, including the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects;
•the ability to obtain IRB approval at clinical trial locations
•clinicians’ and patients’ perceptions as to the potential advantages, disadvantages and side effects of our Products in relation to other available therapies, including any new drugs or treatments that may be approved for the indications we are pursuing;
•availability of other clinical trials that exclude use of our Products;
•the possibility or perception that enrolling in a Product’s clinical trial may limit the patient’s ability to enroll in future clinical trials for other therapies due to protocol restrictions;
•the possibility or perception that our software is not secure enough to maintain patient privacy;
•patient referral practices of physicians;
•the ability to monitor patients adequately during and after treatment;
•the availability of appropriate clinical trial investigators, support staff and proximity of patients to clinical sites;
•physicians’ or our ability to obtain and maintain patient consents; and
•the risk that patients enrolled in clinical trials will choose to withdraw from or otherwise not be able to complete a clinical trial.
Patients may be discouraged from enrolling and/or remaining enrolled in our clinical trials if the trial protocol requires them to undergo extensive follow-up to assess the safety and effectiveness of our Products, or if they
determine that the treatments received under the trial protocols are not attractive or involve unacceptable risks or discomforts. Patients may also not participate in our clinical trials if they choose to participate in contemporaneous clinical trials of competing products. In addition, the inclusion of critically ill patients in our clinical trials may result in deaths or other adverse medical events for reasons that may not be related to our Products, or, in those trials where our Products are being tested in combination with one or more other therapies, for reasons that may be attributable to the other therapies, but which can nevertheless negatively affect clinical trial results. If we have difficulty enrolling and retaining a sufficient number or diversity of patients to conduct our clinical trials as planned, we may need to delay or terminate ongoing or planned clinical trials, either of which would have an adverse effect on our business.
If we are unable to continue the development of an adequate sales and marketing organization or contract with third parties to assist us, we may not be able to successfully commercialize our Products for current and future indications.
To achieve commercial success for our Products, we must continue to compliantly develop and grow our sales and marketing organization and, as necessary, enter into sales and distribution relationships with third parties to market and sell our Products. Developing and managing a sales and marketing organization is a difficult, expensive and time consuming process. To be successful we must:
•recruit and retain adequate numbers of effective and experienced sales and marketing personnel;
•effectively train our sales personnel on the benefits and risks of our Products and healthcare compliance;
•establish and maintain successful and compliant sales, marketing and education programs that educate health care providers so they can appropriately inform their patients about our Products; and
•manage geographically disbursed sales functions and marketing campaigns.
We may not be able to successfully develop adequate sales and marketing capabilities to achieve our growth objectives. We will have to compete with other medical device, pharmaceutical and life sciences companies to recruit, hire, train and retain the sales and marketing personnel that we anticipate we will need, and the nature of our Products may make it more difficult to compete for sales and marketing personnel. In addition, because our current Products require, and we anticipate our future Products will require, physician training and education, our sales and marketing organization must grow substantially as we expand our approved indications and markets. As a consequence, our expenses associated with building up and maintaining our sales force and marketing capabilities may be disproportionate to the revenues we may be able to generate on sales of our Products.
If we are unable to establish adequate sales and marketing capabilities or successful sales and distribution relationships, we may fail to realize the full revenue potential of our Products for current and future indications, and we may not be able to achieve the necessary growth in a cost-effective manner or realize a positive return on our investment. In our current and future sales and distribution agreements with other companies, we generally do not and may not have control over the resources or degree of effort that any of these third parties may devote to our Products, and if they fail to devote sufficient time and resources to the marketing of our Products, or if their performance is substandard, it will adversely affect our revenues.
The success of our business may be dependent on the actions of our collaborative partners.
Our global business strategy includes, in part, the consummation of collaborative arrangements with companies who will support the development and commercialization of our products and technology in return for royalties on commercial sales and milestone payments for progress in clinical development, regulatory approval and sales targets. For example, we have exclusively licensed rights to commercialize our Products in the field of oncology in Greater China (the "Zai Territory") to Zai pursuant to an agreement that also establishes a development partnership for Tumor Treating Fields in multiple solid tumor indications. Zai is responsible for the development and commercialization of our Products in Greater China at its sole cost with certain assistance from us.
When we collaborate with a third party for development and commercialization of a Product in a particular territory, we can expect to relinquish some or all of the control over the future success of that Product to the third party in that territory. In addition, our collaborative partners may have the right to abandon research or development projects and terminate applicable agreements, including payment obligations, prior to or upon the expiration of the agreed-upon terms. We may not be successful in establishing or maintaining collaborative arrangements on acceptable terms or
at all, collaborative partners may terminate funding before completion of projects, our Products may not achieve the criteria for milestone payments, our collaborative arrangements may not result in successful product commercialization, our Products may not receive acceptable pricing and we may not derive any revenue from such arrangements. To the extent that we are not able to develop and maintain collaborative arrangements, we would need to devote substantial capital to undertake development and commercialization activities on our own in order to further expand our global reach, and we may be forced to limit the territories in which we commercialize our Products.
Reliance on collaborative relationships poses a number of risks, including the following:
•our collaborators may not perform their obligations as expected or in compliance with applicable laws;
•the prioritization, amount and timing of resources dedicated by our collaborators to their respective collaborations with us is not under our control;
•some Products with respect to which we collaborate may be viewed by our collaborators as competitive with their own product candidates or products;
•our collaborators may elect not to proceed with the development of Products that we believe to be promising;
•disagreements with collaborators, including disagreements over proprietary rights, contract interpretation or the preferred course of development or commercialization, might cause delays or termination of the research, development or commercialization of Products, might lead to additional responsibilities for us with respect to Products, or might result in litigation or arbitration, any of which would be time-consuming and expensive;
•acts or omissions by collaborators may create liability for us in the jurisdictions in which we operate;
•some of our collaborators might develop independently, or with others, products that could compete with our Products;
•a delay in the development or regulatory approval timelines for our Products in a licensed territory would result in a potential delay or loss of milestone payments and future royalties (if any) from the partnership; and
•if a collaboration is terminated for any reason, then we may need to establish a new development and commercialization partnership to further our Products in the relevant market. There can be no assurance that we would be able to find such a partner.
We may not be successful in achieving market acceptance of our Products by healthcare professionals, patients and/or third-party payers in the timeframes we anticipate, or at all, which could have a material adverse effect on our business, prospects, financial condition and results of operations.
Our business model is predicated on achieving market acceptance of our Products as a monotherapy or in combination with well-established cancer treatment modalities like surgery, radiation and pharmacological therapies. We may not achieve market acceptance of our Products for current or future indications in the amount of time that we have anticipated, or at all, for a number of different reasons, including the following factors:
•it may be difficult to gain broad acceptance of our Products because they are new technologies and involve a novel mechanism of action, and as such physicians may be reluctant to prescribe our Products without prior experience or additional data or training;
•physicians may be reluctant to prescribe our Products due to their perception that the clinical trials are not appropriately designed as they are, for example, unblinded;
•physicians at large academic universities and medical centers may prefer to enroll patients into clinical trials instead of prescribing our Products;
•it may be difficult to gain broad acceptance at community hospitals where the number of patients seeking cancer treatment may be more limited than at larger medical centers, and such community hospitals may not be willing to invest in the resources necessary for their physicians to become trained to use our Products, which could lead to reluctance to prescribe our Products;
•patients may be reluctant to elect to use our Products for various reasons, including a perception that the treatment is untested or difficult to use (for example, they will need to shave the areas on their bodies where the arrays are applied) or a perception that our software is not secure;
•our Products may have side effects (for example, dermatitis where the transducer arrays are placed) and our Products cannot be worn in all circumstances (for example, they cannot get wet and are difficult to wear in high temperatures); and
•the price of our Products includes a monthly fee for use of the delivery system and therefore, as the duration of the treatment course increases, the overall price will increase correspondingly, and, when used in combination with other treatments, the overall cost of treatment will be greater than using a single type of treatment.
In particular, our Products may not achieve market acceptance for current or future indications because of the following additional factors:
•achieving patient acceptance could be difficult because we are targeting devastating diseases with poor prognoses, and not all patients with potentially short lifespans are willing to comply with requirements of treatment with our Products, such as the need to use our Products for at least 18 hours per day, carrying around a device and shaving the area where the arrays are worn, and other patients may forego our Products for privacy, cosmetic, visibility or mobility reasons;
•achieving patient compliance is difficult because the recommended average daily use of our currently marketed Products is at least 18 hours a day, requiring patients to wear the delivery system nearly continuously, which to some extent restricts physical mobility because the battery must be frequently exchanged and recharged, and the patient or a caregiver must ensure that it remains continuously operable and this may also impact the pool of patients to whom physicians may be willing to prescribe our Products, as physicians may be reluctant to prescribe our Products;
•certain patients are contraindicated to using our Products due to a variety of factors, including but not limited to, those who have an active implanted medical device, those who have a skull defect, and those who are sensitive to conductive hydrogels;
•there are certain perceived limitations to our study designs or data obtained from our clinical trials;
•efficacy may also be limited in instances where patients take a break from the delivery system when experiencing skin rashes, while bathing or swimming (because our Products should not get wet), or while traveling; and
•certain adverse events reported in clinical trials by patients treated with our Products as monotherapy include medical device site reaction, headache, malaise, muscle twitching, fall and skin ulcer; additional adverse events reported in clinical trials by patients treated with our Products in combination with chemotherapies in addition to the above, were thrombocytopenia, nausea, constipation, vomiting, fatigue and other side effects consistent with treatment with chemotherapies.
In addition, even if we are successful in achieving market acceptance of our Products for GBM or MPM, we may be unsuccessful in achieving market acceptance of our Products as a treatment for other solid tumor cancers, such as brain metastases, NSCLC, pancreatic cancer, ovarian cancer and other solid tumor cancers, because certain radiation, chemotherapies and/or systemic medical therapies may become or remain the preferred standard of care for these indications.
There may be other factors that are presently unknown to us that also may negatively impact our ability to achieve market acceptance of our Products. If we do not achieve market acceptance of our Products in the timeframes we anticipate, or are unable to achieve market acceptance at all, our business, prospects, financial condition and results of operations could be materially adversely affected, and our stock price could decline.
Failure to secure and maintain adequate coverage and reimbursement from third-party payers could adversely affect acceptance of our Products and reduce our revenues.
We expect that the vast majority of our revenues will come from third-party payers either directly to us in markets where we provide our Products or plan to provide our delivery system candidates to patients or indirectly via payments made to hospitals or other entities providing our Products or which may in the future provide our delivery system candidates to patients.
In the U.S., private payers cover the largest segment of the population, with the remainder either uninsured or covered by governmental payers. We anticipate that the majority of the third-party payers outside the U.S. will be government agencies, government sponsored entities or other payers operating under significant regulatory requirements from national or regional governments.
Third-party payers may decline to reimburse for procedures, supplies or services not under coverage policies. Additionally, some third-party payers may decline to reimburse us for a particular patient even with the existence of a coverage policy. Additionally, private commercial and government payers may be permitted to consider the cost of a treatment in approving coverage or in setting payment for the treatment.
Private and government payers around the world are increasingly challenging the prices charged for medical products and services. Additionally, the containment of healthcare costs has become a priority of governments around the world. Adoption of additional price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our revenues and operating results. If third-party payers do not consider our Products or the combination of our Products with additional treatments to be cost-justified under a required cost-testing model, they may not cover our Products for their populations or, if they do, the level of payment may not be sufficient to allow us to sell our Products on a profitable basis.
Reimbursement for the treatment of patients with medical devices around the world is governed by complex mechanisms established on a national or sub-national level in each country. These mechanisms vary widely among countries and evolve constantly, reflecting the efforts of these countries to reduce public spending on healthcare. As a result, obtaining and maintaining reimbursement for the treatment of patients with medical devices has become more challenging globally. We cannot guarantee that the use of our Products will receive reimbursement approvals and cannot guarantee that our existing reimbursement approvals will be maintained in any country.
We provide financial assistance to patients in certain markets who qualify based on established financial criteria. Primarily, we provide financial assistance to patients where we have or are actively pursuing reimbursement. This financial assistance is intended to defray out-of-pocket costs for our Products for patients who begin treatment but who are unable to pay for the costs of their treatment not covered by insurance. Our costs associated with this program could increase if payers increase the cost-sharing burden of patients or we do not obtain reimbursement coverage and we elect to continue providing financial assistance in those markets. Additionally we provide charitable donations to foundations that can then provide financial assistance to those receiving health care coverage from federal or state funded programs. Enforcement actions and changes to government regulations related to manufacturer-sponsored patient assistance programs that could reduce our ability to support patients financially in the future.
Our failure to secure or maintain adequate coverage or reimbursement for our Products by third-party payers in the U.S. or in the other jurisdictions in which we market our Products could have a material adverse effect on our business, revenues and results of operations and cause our stock price to decline.
We may not be successful in securing and maintaining reimbursement codes necessary to facilitate accurate and timely billing for our Products or physician services attendant to our Products.
Third-party payers, healthcare systems, government agencies or other groups often issue reimbursement codes to facilitate billing for products and physician services used in the delivery of medicine. Within the U.S., the billing
codes most directly related to our Products are contained in the Healthcare Common Procedure Coding System ("HCPCS code set"). The HCPCS code set contains Level I codes that describe physician services, also known as Common Procedural Terminology codes ("CPT codes") and Level II codes that primarily describe products. CMS is responsible for issuing the HCPCS Level II codes. The American Medical Association issues HCPCS Level I codes.
We have secured unique HCPCS Level II codes that describe Optune and we are able to use these codes in the U.S. to bill third-party payers. Loss of these codes or any alteration in the payment attached to these codes would materially impact our operating results. We do not have a unique HCPCS Level II code for NovoTTF-100L at this time.
No CPT codes currently exist to describe physician services related to the delivery of therapy using our Products. We may not be able to secure CPT codes for physician services related to our Products based on the relatively low incidence of GBM. Our future revenues and results may be affected by the absence of CPT codes, as physicians may be less likely to prescribe the therapy when not adequately reimbursed for the time, effort, skill, practice expense and malpractice costs required to provide the therapy to patients.
Outside the U.S, we have not secured codes to describe our Products or to document physician services related to the delivery of therapy using our Products. The failure to obtain and maintain these codes could affect the future growth of our business.
There is no assurance that Medicare or the Medicare Administrative Contractors will provide, or continue to provide, coverage or adequate payment rates for our Products.
We anticipate that a significant portion of patients using our Products will be beneficiaries under the Medicare fee-for-service program. Failure to secure or maintain coverage or maintain adequate payment from Medicare would reduce our revenues and may also affect the coverage and payment decisions of other third-party payers in the U.S.
Medicare classifies Optune and NovoTTF-100L as durable medical equipment ("DME"). Medicare has the authority to issue national coverage determinations or to defer coverage decisions to its regional Medicare Administrative Contractors ("MACs"). The fact that only two MACs administer the entire DME program may negatively affect our ability to petition individual medical policy decision-makers at the MACs for coverage. The absence of a positive coverage determination or a future restriction to existing coverage from Medicare or the DME MACs would materially affect our future revenues.
Additionally, Medicare has the authority to publish the price of DME products. Medicare has published a price for Optune that falls below the median price that we have established with non-Medicare payers. Medicare may in the future publish prices for our Products that do not reflect then-current prices for our Products or Medicare may decrease existing prices published for our Products. Medicare price schedules are frequently referenced by private payers in the U.S. and around the world. Medicare's publication of prices for our Products that are below established prices of our Products could materially reduce our revenues and operating results with respect to non-Medicare payers in the U.S. and our other active markets.
Medicare has assigned the billing codes describing Optune to the DME category for products that require frequent and substantial servicing. DME items in this billing category are billed monthly and payment is not capped after a time period. If Medicare revises its payment category classifications for our Products, this action could materially reduce our revenues and operating results.
CMS requires prior authorization for certain DME items. Claims for such items that did not receive prior authorization before they were furnished to a beneficiary will be automatically denied. In the event Medicare adds one of our Products to the list of items requiring prior authorization that may reduce our ability to bill and secure payment for patients who would otherwise be covered to use our Product under the Medicare fee-for-service program.
Medicare issued guidance in 2018 that imposes a series of requirements on the MACs, including a requirement to form contractor advisory committees to review new LCDs and to subject almost all LCD revisions to a public comment period. These requirements have slowed and could continue to slow the process for LCD revisions, and may delay coverage for our Products in future indications.
The Medicare fee-for-service program has denied coverage for all claims prior to the September 1, 2019 effective date for the DME MAC LCD, which provides coverage for Optune for the treatment of newly diagnosed GBM subject to certain conditions and restrictions. We expect that Medicare will continue to deny essentially all claims that do not meet the coverage policy terms. Although we are actively appealing these coverage denials, we are unable to bill the vast majority of our existing Medicare fee-for-service patients for amounts not paid by Medicare. Therefore, we are absorbing and may continue to absorb the costs of treatment for amounts not paid by Medicare.
We appeal Medicare coverage denials through the Medicare appeals process: redetermination by a MAC, reconsideration by a Qualified Independent Contractor, hearing before an ALJ at the Office of Medicare Hearings and Appeals, review by the Medicare Appeals Council, and judicial review in U.S. District Court. Currently, there is a considerable backlog of appeals at the ALJ level and there are significant delays in the assignment of ALJ cases. Thus, we anticipate that, even if we are successful in winning our appeals, we will experience a significant delay in securing payment for Medicare patients when Medicare’s DME MACs deny coverage for patients who start therapy.
We depend on single-source suppliers for some of our components. The loss of these suppliers could prevent or delay shipments of our Products, delay our clinical trials or otherwise adversely affect our business.
In certain jurisdictions, we source some of the components of our Products from only a single vendor. If any one of these single-source suppliers were to fail to continue to provide components to us on a timely basis, or at all, our business and reputation could be harmed. Our policy is to seek and maintain second-source suppliers, but we can provide no assurance we will secure or maintain such suppliers. We are in the process of developing and obtaining regulatory approval for second sources for components in all jurisdictions. Various steps must be taken before securing these suppliers, including qualifying these suppliers in accordance with regulatory requirements, but we may never receive such approvals.
Establishing additional or replacement suppliers for any components of our Products, and obtaining any additional regulatory approvals required to add or replace suppliers, will take a substantial amount of time and could result in increased costs and impair our ability to produce our Products, which would have a material adverse effect on our business, prospects, financial condition and results of operations. We may have difficulty obtaining similar components from other suppliers that are acceptable to the FDA or other regulatory authorities, or to comply with requirements governing design and manufacturing in the EU. The risks associated with the failure of our suppliers to comply with strictly enforced regulatory requirements as described below are exacerbated by our dependence on single-source suppliers. Furthermore, since some of these suppliers are located outside of the U.S., we are subject to export laws in other jurisdictions and U.S. import and customs regulations, which complicate and could delay shipments of components to us. Changes in U.S. social, political, regulatory and economic conditions or in laws and policies governing foreign trade, manufacturing, development and investment in the territories and countries where we may develop and sell products, and any negative sentiments towards the U.S. as a result of such changes, could adversely affect our business.
If we experience any deficiency in the quality of, delay in or loss of availability of any components supplied to us by third-party suppliers, or if we have to switch to replacement suppliers, we may face additional regulatory delays and the manufacture and delivery of our Products would be interrupted for an extended period of time, which could materially adversely affect our business, prospects, financial condition and results of operations. In addition, we may be required to obtain prior regulatory approval if we use different suppliers or components. Such changes could affect our FDA regulatory approvals and the compliance of our Products with the Essential Requirements laid down in Annex I to the Medical Devices Directive and the validity of our current CE Certificates of Conformity. If we are required to obtain prior regulatory approval from the FDA or regulatory authorities in other jurisdictions or to conduct a new conformity assessment procedure and obtain new CE Certificates of Conformity in the EU to use different suppliers or components for our Products, regulatory approval or the CE Certificates of Conformity for our Products may not be received on a timely basis, or at all, which would have a material adverse effect on our business, prospects, financial condition and results of operations.
Quality control problems with respect to delivery systems and components supplied by third-party suppliers could have a material adverse effect on our reputation, our clinical trials or the commercialization
of our Products and, as a result, a material adverse effect on our business, prospects, financial condition and results of operations.
Our Products, which are manufactured by third parties, are highly technical and are required to meet exacting specifications. Any quality control problems that we experience with respect to the delivery systems and components supplied by third-party suppliers could have a material adverse effect on our reputation, our attempts to complete our clinical trials, our operating expenses or the commercialization of our Products. The failure of our suppliers to comply with strictly enforced regulatory requirements could expose us to regulatory action, including warning letters, product recalls, suspension or termination of distribution, product seizures or civil penalties. If we experience any delay or deficiency in the quality of products supplied to us by third-party suppliers, or if we have to switch to replacement suppliers, we may face additional regulatory delays and the manufacture and delivery of our Products would be interrupted for an extended period of time, which would materially adversely affect our business, prospects, financial condition and results of operations.
If the third parties on which we rely to conduct our clinical trials and to assist us with research and development do not perform as contractually required or expected, we may not be able to obtain regulatory approvals for or commercialize our Products.
We do not have the ability to independently conduct some of our preclinical and development activities and all of our clinical trials for our Products and we must rely on third parties, such as contract research organizations, medical institutions, clinical investigators, contract laboratories and collaborative partners, to conduct such trials. We and these third parties are required to comply with current good clinical practices ("cGCPs") which are regulations and guidelines enforced by the FDA under the medical device Quality System Regulation ("QSR") and comparable regulatory authorities in other jurisdictions for clinical development. We and these third parties are also required to comply with current good laboratory practices ("cGLPs") which are regulations and guidelines enforced by the FDA and comparable regulatory authorities in other jurisdictions for nonclinical laboratory studies. Regulatory authorities enforce these cGLPs and cGCPs through periodic inspections of trial sponsors, laboratories, principal investigators and trial sites. If we or any of these third parties fail to comply with applicable cGLP and cGCP regulations, the data generated in our nonclinical studies and clinical trials may be deemed unreliable and the FDA or regulatory authorities in other jurisdictions may require us to perform additional nonclinical or clinical trials before approving our approved applications. We cannot be certain that, upon inspection or review of our data, such regulatory authorities will determine that any of our nonclinical studies or clinical trials comply with the applicable cGLP or cGCP regulations.
Any third parties conducting our preclinical, clinical and other development programs are not and will not be our employees and, except for remedies available to us under our agreements with such third parties, we cannot control whether or not they devote sufficient time and resources to our ongoing preclinical, clinical and nonclinical programs. These third parties may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting nonclinical studies, clinical studies or other development activities, which could affect their performance on our behalf. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if these third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for our Products or successfully commercialize our Products on a timely basis, if at all, and our business, prospects and results of operations may be adversely affected.
Continued testing of our Products may not yield successful results and could reveal currently unknown safety hazards associated with our Products.
Our research and development programs are designed to test the safety and efficacy of our Products and Tumor Treating Fields through extensive preclinical and clinical testing. Even if our ongoing and future clinical trials are completed as planned, we cannot be certain that their results will support our claims or that the FDA and other regulatory authorities will agree with our conclusions. Success in preclinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior trials and preclinical studies. The clinical trial process may fail to demonstrate that our delivery system candidates are safe and effective for the proposed indicated uses, which could cause us to abandon a delivery system candidate and may delay development of others. It is also possible that patients enrolled in clinical trials will experience adverse side effects that have not been previously observed. In addition, our preclinical studies and
clinical trials for our delivery system candidates involve a relatively small patient population and, as a result, these studies and trials may not be indicative of future results.
We may experience numerous unforeseen events during, or as a result of, the testing process that could delay or prevent further commercialization of our Products, including the following:
•safety and efficacy results for our Products obtained in our preclinical and clinical testing may be inconclusive or may not be predictive of results obtained in future clinical trials, following long-term use or in much larger populations;
•unanticipated adverse events may occur during our clinical trials;
•the data collected from our clinical trials may not reach statistical significance due to limited sample size or otherwise not be sufficient to support FDA or other regulatory approval; and
•our Products may not produce the desired effect or may result in adverse health effects or other effects that are not currently known that may preclude additional regulatory approval or result in additional limitations to commercial use if approved.
To date, patients treated with our Products in our clinical trials have experienced treatment-related side effects, including dermatitis (including mild to moderate skin irritation) where the transducer arrays are placed, headaches, weakness, falls, fatigue, muscle twitching and skin ulcers, along with traditional side-effects associated with the chemotherapeutic agents often co-administered with our Products. There may be additional side effects observed in future clinical trials and/or through real-world experience with patients using our Products. Undesirable side effects caused by our Products could cause us or regulatory authorities to interrupt, delay or terminate clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other regulatory authorities. Results of our clinical trials could reveal a high and unacceptable severity and prevalence of side effects.
If unacceptable side effects arise in the development of our Products for future indications, we could suspend or terminate our clinical trials or the FDA or other regulatory authorities could order us to cease clinical trials or deny approval of our delivery system candidates for any or all targeted indications, narrow the approved indications for use or otherwise require restrictive product labeling or marketing or require further clinical trials, which may be time-consuming and expensive and may not produce results supporting FDA or other regulatory approval of our Products in a specific indication. Treatment-related side effects could also affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff. We expect to have to train medical personnel using our delivery system candidates to understand the side effect profiles for our clinical trials and upon any commercialization of our Products for future indications. Inadequate training in recognizing or managing the potential side effects of our Products could result in patient injury or death. Any of these occurrences may harm our business, prospects and financial condition significantly.
Any delay or termination of our clinical trials will delay the filing of submissions for regulatory approvals of our Products and ultimately our ability to commercialize our Products and generate revenues. Furthermore, we may abandon our Products for indications that we previously believed to be promising. Any of these events could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
We face competition from numerous competitors, most of whom have far greater resources than we have, which may make it more difficult for us to achieve significant market penetration and which may allow our competitors to develop additional oncology treatments to compete with our Products.
The oncology market is intensely competitive, subject to rapid change and significantly affected by new product introductions and other market activities of industry participants. Our Products primarily compete with radiation and pharmacological therapies. We may face additional competition as advancements are made in the field of anti-cancer therapies and as we enter additional oncological markets. To date, we have conducted clinical trials where our Products are used in combination with a certain subset of other anti-cancer therapies. Many of our competitors are large, well-capitalized companies with significantly greater market share and resources than we have. As a
consequence, they are able to spend more aggressively on product development, marketing, sales and other initiatives than we can. Many of these competitors could have:
•significantly greater name recognition and experience;
•established relationships with government agencies, healthcare professionals, patients and third-party payers;
•established distribution networks;
•additional product lines, and the ability to offer rebates or bundle products to offer higher discounts or more competitive pricing or other incentives to gain a competitive advantage; and
•greater financial and human resources for research and development, sales and marketing, patent litigation and/or acquisitions.
Although we believe our Products represent a treatment modality that can be used in combination with other cancer treatment modalities, our current and future competitors may at any time develop additional drugs or devices for the treatment of GBM, MPM, or other solid tumors that could be more effective from a therapeutic or cost-basis perspective than using our Products. In our currently-approved indications, if current or future competitors develops a product that proves to be superior or comparable to our Products, our revenues may decline. In addition, some of our competitors may compete by lowering the price of their cancer treatments. If these competitors’ products were to gain acceptance by healthcare professionals, patients or third-party payers, a downward pressure on prices could result. If prices were to fall, we may not be able to improve our gross margins or sales growth sufficiently to be sustainably profitable. For future indications, other companies could view us as a competitor and attempt to block our market entry or otherwise hinder our Product growth in a market.
As we expand, we may experience difficulties managing our growth.
Our anticipated growth will place a significant strain on our management and on our operational and financial resources and systems. We could face challenges inherent in efficiently managing a more complex business with an increased number of employees over large geographic distances, including the need to implement appropriate systems, policies, benefits and compliance programs. Failure to manage our growth effectively could materially adversely affect our business. Additionally, our anticipated growth will increase the demands placed on our third-party suppliers, resulting in an increased need to carefully monitor the available supply of components and services and to scale up our quality assurance programs. There is no guarantee that our suppliers will be able to support our anticipated growth. Any failure by us to manage our growth effectively could have an adverse effect on our ability to achieve our development and commercialization goals.
Because of the specialized nature of our business, the termination of relationships with our key employees, consultants and advisors may prevent us from developing our Products, conducting clinical trials, commercializing our Products and obtaining any necessary financing. Further, the inability to recruit and retain additional personnel may have an adverse effect on our ability to successfully operate our business.
We are highly dependent on the members of our executive team, the loss of whose services may adversely impact the achievement of our objectives. While we have entered into employment agreements with each of our key executives, any of them could leave our employment at any time. We do not have "key person" insurance on any of our employees. The loss of the services of one or more of our current employees might impede the achievement of our business objectives.
The competition for qualified personnel in the oncology field is intense, and we rely heavily on our ability to attract and retain qualified scientific, technical and managerial personnel. Our future success depends upon our ability to attract, retain and motivate highly skilled employees. In order to commercialize our Products successfully, we will be required to expand our workforce, particularly in the areas of research and development and clinical trials, sales and marketing and supply chain management. These activities will require the addition of new personnel and the development of additional expertise by existing management personnel. We face intense competition for qualified individuals from numerous pharmaceutical, biopharmaceutical and biotechnology companies, as well as academic and other research institutions. We may not be able to attract and retain these individuals on acceptable terms or at all. Failure to do so could materially harm our business.
Changes in tax or other laws, regulations or treaties, changes in our status under U.S. or non-U.S. laws or adverse determinations by taxing or other governmental authorities could increase our tax burden or otherwise affect our financial condition or results of operations, as well as subject our shareholders to additional taxes.
The amount of taxes we pay is subject to a variety of tax laws in the various jurisdictions in which we and our subsidiaries are organized and operate. Our domestic and international tax liabilities are dependent on the location of earnings among these various jurisdictions. Such tax liabilities could be affected by changes in tax or other laws, treaties and regulations, as well as the interpretation or enforcement thereof by tax or other governmental entities in any relevant jurisdiction. The amount we pay in tax to any particular jurisdiction depends, in part, on the correct interpretation of the tax laws in such jurisdiction, and we have made a number of determinations as to the effect of such tax laws in our particular circumstances. In some cases, the determinations we have made as to the effect of the tax laws in a particular jurisdiction depend on the continuing effectiveness of administrative rulings we have received from the tax authorities in that jurisdiction, while in other cases, our determinations are based on the reasoned judgment of our tax advisors. Although we believe that we are in compliance with the administrative rulings we have received, that the assumptions made by our tax advisors in rendering their advice remain correct, and that as a result we are in compliance with applicable tax laws in the jurisdictions where we and our subsidiaries are organized and operate, a taxing authority in any such jurisdiction may challenge our interpretation of those laws and assess us or any of our subsidiaries with additional taxes.
Additionally, from time to time, proposals can be made and legislation can be introduced to change the tax laws, regulations or interpretations thereof (possibly with retroactive effect) of various jurisdictions or limit tax treaty benefits that, if enacted, could materially increase our tax burden, increase our effective tax rate or otherwise have a material adverse impact on our financial condition and results of operations. It is possible that these changes could adversely affect our business. While we monitor proposals and other developments that would materially impact our tax burden and effective tax rate and investigate our options accordingly, we could still be subject to increased taxation on a going forward and retroactive basis no matter what action we undertake if certain legislative proposals or regulatory changes are enacted, certain tax treaties are amended and/or our interpretation of applicable tax or other laws is challenged and determined to be incorrect. Any alternative interpretations of applicable tax laws asserted by a tax authority or changes in tax laws, regulations or accounting principles that limit our ability to take advantage of tax treaties between jurisdictions, modify or eliminate the deductibility of various currently deductible payments, increase the tax burden of operating or being resident in a particular country, result in transfer pricing adjustments or otherwise require the payment of additional taxes, may have a material adverse effect on our cash flows, financial condition and results of operations
The termination or revision of any of our tax rulings or indirect tax exemptions that we have or may have in the future may have a material adverse effect on our cash flows, financial condition and results of operations.
Product liability suits, whether or not meritorious, could be brought against us due to alleged defective delivery systems or for the misuse of our Products. These suits could result in expensive and time-consuming litigation, payment of substantial damages and an increase in our insurance rates.
If our current or future delivery systems are defectively designed or manufactured, contain defective components or are misused, or if someone claims any of the foregoing, whether or not meritorious, we may become subject to substantial and costly litigation. For example, we may be sued if our Products causes or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. This may occur if our Products are misused or damaged, have a sudden failure or malfunction (including with respect to safety features) or are otherwise impaired due to wear and tear. Even absent a product liability suit, malfunctions of our Products or misuse by physicians or patients would need to be remedied swiftly in order to maintain continuous use and ensure efficacy of our Products.
Any product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the delivery system, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our Products. Even successful defense may require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
•decreased demand for our Products;
•injury to our reputation;
•withdrawal of clinical trial participants and inability to continue clinical trials;
•initiation of investigations by regulators;
•costs to defend the related litigation;
•a diversion of management’s time and our resources;
•substantial monetary awards to trial participants or patients;
•product recalls, withdrawals or labeling, marketing or promotional restrictions;
•loss of revenues;
•exhaustion of any available insurance and our capital resources;
•the inability to commercialize any delivery system candidate; and
•a decline in our share price.
Product liability claims could divert management’s attention from our core business, be expensive to defend and result in sizable damage awards against us. We may not have sufficient insurance coverage for all claims. Any product liability claims brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing continuing coverage, could harm our reputation in the industry and could reduce revenues. Product liability claims in excess of our insurance coverage would be paid out of cash reserves, if any, which could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline. Even if our agreements with our third-party manufacturers and suppliers entitle us to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
Other future litigation and regulatory actions could have a material adverse impact on the Company.
From time to time, we may be subject to litigation and other legal and regulatory proceedings relating to our business or investigations or other actions by governmental agencies, including as described in Part I, Item 3 "Legal Proceedings" of this Annual Report on Form 10-K. No assurances can be given that the results of these or new matters will be favorable to us. An adverse resolution of lawsuits, arbitrations, investigations or other proceedings or actions could have a material adverse effect on our financial condition and results of operations, including as a result of non-monetary remedies. Defending ourselves in these matters may be time-consuming, expensive and disruptive to normal business operations and may result in significant expense and a diversion of management’s time and attention from the operation of our business, which could impede our ability to achieve our business objectives. Additionally, any amount that we may be required to pay to satisfy a judgment, settlement, fine or penalty may not be covered by insurance. Subject to the Jersey Companies Law, our articles of association permit us to indemnify any director against any liability, to purchase and maintain insurance against any liability for any director and to provide any director with funds (whether by loan or otherwise) to meet expenditures incurred or to be incurred by such director in defending any criminal, regulatory or civil proceedings or in connection with an application for relief (or to enable any such director to avoid incurring such expenditure). In addition, we have entered into indemnification agreements with each of our directors and officers to indemnify them against certain liabilities and expenses arising from their being a director or officer to the maximum extent permitted by Jersey law. In the event we are required to make such payments to our directors and officers, there can be no assurance that any of these payments will not be material.
Global economic, political and industry conditions constantly change and unfavorable conditions may have a material adverse effect on our business and results of operations.
We are a global oncology company with worldwide operations. Volatile economic, political and market conditions, such as political or economic instability, majority hostilities or acts of terrorism, in the regions in which we operate may have a negative impact on our operating results and our ability to achieve our business objectives. We may not have insight into economic and political trends that could emerge and negatively affect our business. In
addition, significant or volatile changes in exchange rates between the U.S. dollar and other currencies may have a material adverse impact upon our liquidity, revenues, costs and operating results.
In particular, we have research facilities located in Israel, and certain key suppliers manufacture their goods in one physical location in Israel. Due to the high-conflict nature of this area, Israel could be subject to additional political, economic and military confines, which could result in a material adverse effect on our operations. Parties with whom we do business have sometimes declined to travel to Israel during periods of heightened unrest or tension, forcing us to make alternative arrangements when necessary. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in the agreements.
Additionally, natural disasters and pandemics, such as the COVID-19 coronavirus outbreak, could have a significant adverse effect on our business, including interruption of our commercial and clinical operations, supply chain disruption, endangerment of our personnel, fewer patient visits, increased patient drop-out rates, delays in recruitment of new patients, and other delays or losses of materials and results.
We are increasingly dependent on information technology systems and subject to privacy and security laws. Our Products and our systems and infrastructure face certain risks, including from cyber security breaches and data leakage.
We increasingly rely upon technology systems and infrastructure. Our technology systems, including our Products, are potentially vulnerable to breakdown or other interruption by fire, power loss, system malfunction, unauthorized access and other events. Likewise, data privacy breaches by employees and others with both permitted and unauthorized access to our Products and our systems may pose a risk that protected patient information ("PI") may be exposed to unauthorized persons or to the public, or may be permanently lost. The increasing use and evolution of technology, including cloud-based computing, creates additional opportunities for the unintentional dissemination of information, intentional destruction of confidential information stored in our systems or in non-encrypted portable media or storage devices. We could also experience a business interruption, information theft of confidential information, or reputational damage from industrial espionage attacks, malware or other cyber incidents, which may compromise our system infrastructure or lead to data leakage, either internally or at our third-party service providers or other business partners.
Additionally, we must comply with numerous laws and regulations governing the processing, collection, dissemination, access, use, sharing and security of PI. In the U.S., HIPAA provides data privacy and security provisions for safeguarding medical information. Additionally, states in the U.S. are enacting local privacy laws (e.g., California). In the EU, the GDPR harmonizes data privacy laws and rules on the processing of personal data, including patient and employee data, across the EU and repeals and replaces Directive 95/46/EC of the European Parliament and of the Council of October 24, 1995, and applicable national laws. The GDPR has a number of strict data protection and security requirements for companies processing data of EU residents, including when such data is transferred outside of the EU. Additionally, we need to comply with analogous privacy laws in other jurisdictions in which we operate.
While we have invested heavily in the protection of data and information technology and in related training, there can be no assurance that our efforts will prevent significant breakdowns, breaches in our systems or other cyber incidents or ensure compliance with all applicable security and privacy laws, regulations and standards, including with respect to third-party service providers that utilize sensitive personal information, including PI, on our behalf. Any such breakdown, breach, incident or failure to comply could expose us to a risk of loss of information, litigation, penalties, remediation costs and potentially significant liability to customers, employees, business partners and regulatory authorities, including, for example, under HIPAA in the United States and the GDPR in the EU, and may ultimately have a material adverse effect upon our reputation, business, operations or financial condition.
Disruption of critical information systems or material security breaches in our products may adversely affect our business and customer relations.
Information technology helps us operate efficiently, develop and commercialize our products, interface with and support our customers, maintain financial accuracy and efficiency and produce our financial statements. The size and complexity of our computer systems, and scope of our geographic reach, make us potentially vulnerable to IT system breakdowns, internal and external malicious intrusion, cyberattacks and computer viruses. Because the techniques used to obtain unauthorized access, or to sabotage systems, change frequently and generally are not
recognized until launched against a target, we may be unable to anticipate these techniques or to implement adequate preventative measures. If we do not allocate and effectively manage the resources necessary to build and sustain the proper technology infrastructure or properly manage third-party contractors who perform data management services on our behalf, then a security breach could subject us to, among other things, transaction errors, business process inefficiencies, the loss of customers, damage to our reputation, business disruptions or the loss of or damage to intellectual property. Such security breaches could expose us to a risk of loss of information, litigation, penalties, remediation costs and potentially significant liability to customers, employees, business partners and regulatory authorities, including, for example, under HIPAA in the United States and the GDPR in the EU. If our data management systems (including third party data management systems) do not effectively collect, secure, store, process and report relevant data for the operation of our business, whether due to equipment malfunction or constraints, software deficiencies, or human error, our ability to effectively plan, forecast and execute our business plan and comply with applicable laws and regulations will be impaired. Any such impairment could materially and adversely affect our financial condition and results of operations.
We develop and commercialize hardware products that rely upon software to operate properly and software products that produce treatment data and confidential patient information. Additionally, we process sensitive personal data, including patient health information, in the normal course of operating our business. While we have implemented security measures to protect our hardware and software products and systems from unauthorized access, these measures may not be effective. A security breach, whether of our products, systems or third-party hosting services we utilize, could disrupt treatments being provided by our products, disrupt access to our customers’ stored information, such as patient treatment data and health information, and could lead to the loss of, damage to or public disclosure of such data and information, including patient health information. Such an event could have serious negative consequences, including possible patient injury, regulatory action, fines, penalties and damages, reduced demand for our products, an unwillingness of customers to use our products, harm to our reputation and brand and time-consuming and expensive litigation, any of which could have a material adverse effect on our financial results.
If we were to experience a significant security breach of our information systems or data, the cost associated with the investigation, remediation and potential notification of the breach to customers, including patients, and counterparties could be material. We carry a limited amount of insurance for cybersecurity liability, and our insurance coverage may be inadequate. In the future, our insurance coverage may be expensive or not be available on acceptable terms or in sufficient amounts, if at all.
Changes in our technology could result in impairment charges in future periods.
U.S. generally accepted accounting principles ("GAAP") require annual (or more frequently if events or changes in circumstances warrant) impairment tests of goodwill, intangible assets and other long-lived assets. Generally speaking, if the carrying value of the asset is in excess of the estimated fair value of the asset, the carrying value will be adjusted to fair value through an impairment charge. Circumstances such as changes in technology or in the way an asset is being used may trigger an impairment review. Any negative perception of such a deficit could have an adverse effect on the price of our ordinary shares and could impair our ability to obtain new financing or refinance existing indebtedness.
If any of our or our suppliers’ or manufacturers’ facilities are damaged or our clinical, research and development or other business processes interrupted, our business could be seriously harmed.
We conduct our business in a limited number of facilities around the world. Damage or extended periods of interruption to our or our suppliers’ or manufacturers’ corporate, development or research facilities or delays in the transportation, import or export of finished goods or components due to fire, natural disaster, power loss, communications failure, unauthorized entry, terrorist attacks or other events could cause us to cease or delay development and/or delivery of our Products. Our internal computer systems may fail or suffer security breaches, which could result in a material disruption of our business. Our business may be seriously harmed by such delays and interruption.
Our research facilities are located in Israel, and certain key suppliers manufacture goods in physical locations in Israel. Although our facilities have not sustained any damage from such attacks, this is a high conflict area and any future attacks and resulting damage could adversely affect our operations. Our business insurance only covers certain specified events associated with war or terrorism in the Middle East, and may not cover all such events. Any losses or damages incurred by us could have a material adverse effect on our business.
We have significant debt service obligations and may incur additional indebtedness in the future, which could adversely affect our financial condition and results of operations and our ability to react to changes in our business.
We currently have $150.0 million of principal indebtedness outstanding under our Loan and Security Agreement dated as of February 7, 2018, between us, as borrower, and BioPharma Credit PLC, as lender ("2018 Credit Facility"). We may incur additional indebtedness in the future. Our existing indebtedness and any additional indebtedness we may incur could require us to divert funds identified for other purposes for debt service and impair our liquidity position.
The fact that a substantial portion of our cash flow from operations could be needed to make payments on our indebtedness could have important consequences, including the following:
•increasing our vulnerability to general adverse economic and industry conditions or increased interests rates;
•reducing the availability of our cash flow for other purposes;
•limiting our flexibility in planning for or reacting to changes in our business and the markets in which we operate, which would place us at a competitive disadvantage compared to our competitors that may have less exposure to debt;
•limiting our ability to borrow additional funds for working capital, capital expenditures and other investments; and
•failing to comply with the covenants in our debt agreements could result in all of our indebtedness becoming immediately due and payable.
Our ability to obtain necessary funds through borrowing, as well as our ability to service our indebtedness, will depend on our ability to generate cash flow from operations. Our ability to generate cash is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control. If our business does not generate sufficient cash flow from operations or if future borrowings are not available to us under our 2018 Credit Facility or otherwise in amounts sufficient to enable us to fund our liquidity needs, our financial condition and results of operations may be adversely affected. Our inability to make scheduled payments on our debt obligations in the future would require us to refinance all or a portion of our indebtedness on or before maturity, sell assets or seek additional equity investment. We may not be able to take any of such actions on a timely basis, on terms satisfactory to us or at all.
Covenants in our debt agreements restrict our operational flexibility.
Our 2018 Credit Facility contains usual and customary restrictive covenants relating to the operation of our business, including restrictions on our ability:
•to incur or guarantee additional indebtedness;
•to incur or permit to exist certain liens;
•to enter into certain sale and lease-back transactions;
•to make certain investments, loans and advances;
•to effect certain mergers, consolidations, asset sales and acquisitions;
•to pay dividends on, or redeem or repurchase, capital stock, enter into transactions with affiliates or materially change our business; and
•to repay or modify certain other agreements with respect to other material indebtedness or modify our organizational documents.
The United Kingdom’s exit from the EU could adversely impact our business, results of operations and financial condition.
On January 31, 2020, the United Kingdom ("UK") officially withdrew from the EU ("Brexit"). Brexit is subject to a transition period until December 31, 2020, which could be extended up to two years under certain conditions (the "Brexit Transition Period"). During the Brexit Transition Period, EU legislation related to, inter alia, medical devices will continue to apply in the U.K. There is uncertainty as to the scope, nature and terms of the relationship between the UK and the EU after the Brexit Transition Period. This uncertainty could adversely impact customer and investor confidence, result in additional market volatility, including in the value of the British pound and European euro, legal uncertainty and divergent national laws and regulations. At this time, we cannot predict the impact that Brexit, once the Brexit Transition Period ends, will have on our business generally and our UK and European activities more specifically, and no assurance can be given that our operating results, financial condition and prospects would not be adversely impacted by the result.
Risks relating to regulation
Our delivery system candidates must undergo rigorous preclinical and clinical testing and we must obtain regulatory approvals, which could be costly and time-consuming and subject us to unanticipated delays or prevent us from marketing any delivery systems.
Our research and development activities, as well as the manufacturing and marketing of our Products, are subject to regulation, including regulation for safety, efficacy and quality, by the FDA in the U.S. and comparable authorities in other countries. FDA regulations and the regulations of comparable regulatory authorities in other jurisdictions are wide-ranging and govern, among other things:
•the conduct of preclinical and clinical studies;
•product design, development, manufacturing and testing;
•product labeling;
•product storage and shipping;
•premarket clearance, approval and conformity assessment procedures;
•premarket clearance, approval and conformity assessment procedures for modifications introduced in marketed products;
•post-market surveillance and monitoring;
•reporting of adverse events or incidents and implementation of corrective actions, including product recalls;
•pricing and reimbursement;
•interactions with healthcare professionals;
•interactions with patients;
•information security;
•advertising and promotion; and
•product sales and distribution.
Clinical testing can be costly and take many years, and the outcome is uncertain and susceptible to varying interpretations. Moreover, success in preclinical and early clinical trials does not ensure that large-scale trials will be successful or predict final results. Acceptable results in early trials may not be replicable in later trials. A number of companies in the oncology industry have suffered significant setbacks in advanced clinical trials, even after promising results in earlier trials. Negative or inconclusive results or adverse events or incidents during a clinical
trial could cause it to be redone or terminated. In addition, failure to construct appropriate clinical trial protocols could result in the test or control group experiencing a disproportionate number of adverse events or incidents and could cause a clinical trial to be suspended, redone or terminated. We cannot be certain if or when the FDA, comparable regulatory agencies in other jurisdictions or our notified body might request additional studies, under what conditions such studies might be requested, or what the size or length of any such studies might be. The clinical trials of our delivery system candidates may not be completed on schedule, the FDA, comparable agencies in other jurisdictions or our notified body may order us to stop or modify our research, or the FDA, comparable regulatory agencies in other jurisdictions or our notified body may not ultimately approve or issue a CE Certificate for any of our delivery system candidates for commercial sale. The data collected from our clinical trials may not be sufficient to support regulatory approval in the U.S., Japan and other countries or to obtain a CE Certificate in the EU for our various future delivery system candidates. Even if we believe the data collected from our clinical trials are sufficient, the FDA, comparable regulatory bodies in other jurisdictions and our notified body have substantial discretion in the assessment and approval or conformity assessment processes and may disagree with our interpretation of the data. Our failure to adequately demonstrate the safety and efficacy of any of our delivery system candidates would delay or prevent regulatory approval in the U.S., Japan and other countries obtaining a CE Certificate (and therefore the CE marking) in the EU of our delivery system candidates, which could prevent us from being sustainably profitable.
We currently market Optune in the U.S., the EU, Switzerland, Israel and Japan. We currently market NovoTTF-100L in the U.S. only. We intend to market our Products in a number of additional international markets. In order to market our Products in any jurisdiction and for other indications or purposes, we may be required to obtain separate regulatory approvals or CE Certificates for our Products, as applicable. The requirements governing the conduct of clinical trials and manufacturing and marketing of our delivery system candidates outside the U.S. vary widely from country to country. CE Certificates and regulatory approvals in other jurisdictions may take longer to obtain than FDA approvals and can require, among other things, additional testing and different clinical trial designs. CE Certification processes and regulatory approvals in other jurisdictions include essentially all of the risks associated with the FDA approval processes. Some regulatory agencies in other jurisdictions must also approve prices of the delivery systems. Approval of a product by the FDA does not guarantee approval of the same product by the health authorities of other countries or CE marking of our Products in the EU and vice versa. In addition, changes in regulatory policy in the U.S. or in other countries for the approval or CE marking of a medical device during the period of product development and regulatory agency review or notified body review of each submitted new application may cause delays or rejections.
The MDR, which will apply from May 26, 2020, will introduce significant changes to the regulatory framework for medical devices in the EU. These changes may prevent or delay the CE Certification of our delivery system candidates or impact our ability to modify Optune on a timely basis. In particular, the lack of MDR guidance documents at EU level and the limited availability of qualified notified bodies might affect our ability to timely comply and demonstrate such compliance with the new requirements or delay the MDR CE Certification of our delivery system candidates or Optune.
We may choose to, or may be required to, suspend, repeat or terminate our clinical trials if they are not conducted in accordance with regulatory requirements, the results are negative or inconclusive or the trials are not well designed.
Clinical trials must be conducted in accordance with the FDA’s cGCPs and the equivalent laws and regulations applicable in other jurisdictions in which the clinical trials are conducted. The clinical trials are subject to oversight by the FDA, regulatory agencies in other jurisdictions, ethics committees and institutional review boards at the medical institutions where the clinical trials are conducted. In addition, clinical trials must be conducted with delivery system candidates produced under the FDA’s QSR and in accordance with the applicable regulatory requirements in the other jurisdictions in which the clinical trials are conducted. The conduct of clinical trials may require large numbers of test patients. Patient enrollment is a function of many factors, including the size of the patient population for the target indication, the proximity of patients to clinical sites, the eligibility criteria for the trial, the existence of competing clinical trials and the availability of alternative or new treatments. Clinical trials may be suspended by the FDA or by a regulatory agency in another jurisdiction at any time if the FDA or the regulatory agency finds deficiencies in the conduct of these trials or it is believed that these trials expose patients to unacceptable health risks.
We, the FDA or regulatory agencies in other jurisdictions might delay or terminate our clinical trials of a delivery system candidate for various reasons, including:
•the delivery system candidate may have unforeseen adverse side effects;
•the time required to determine whether the delivery system candidate is effective may be longer than expected;
•we may not agree with the FDA, a regulatory authority in another jurisdiction or an ethics committee regarding the protocol for the conduct of a clinical trial;
•new therapies may become the standard of care while we are conducting our clinical trials, which may require us to revise or amend our clinical trial protocols or terminate a clinical trial;
•fatalities may occur during a clinical trial due to medical problems that may or may not be related to clinical trial treatments;
•the delivery system candidate may not appear to be more effective than current therapies;
•there may be insufficient patient enrollment in the clinical trials; or
•we may not be able to produce sufficient quantities of the delivery system candidate to complete the trials.
Furthermore, the process of obtaining and maintaining regulatory approvals in the U.S. and other jurisdictions and CE Certificates in the EU for new therapeutic products is lengthy, expensive and uncertain. It can vary substantially, based on the type, complexity and novelty of the product involved. Accordingly, any of our delivery system candidates could take a significantly longer time than we expect to, or may never, gain regulatory approval or obtain CE Certificates in the EU, which could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
Healthcare reform and other legislative and regulatory changes in the U.S. and in other countries may adversely affect our business and financial results.
In response to perceived increases in healthcare costs in recent years, there have been and continue to be proposals by the U.S. federal government, state governments, regulators and third-party payers to control these costs and, more generally, to reform the U.S. healthcare system. In the U.S., the Patient Protection and Affordable Care Act (the "PPACA"), was enacted in 2010 with a goal of reducing the cost of healthcare and substantially changing the way healthcare is financed by both government and private insurers.
The current U.S. Administration and members of the U.S. Congress have stated that they will seek to modify, repeal, or otherwise invalidate all, or certain provisions of, the PPACA. For example,in December 2017, the U.S. President signed the Tax Cuts and Jobs Act, which, among numerous other actions, repealed the individual mandate of the PPACA, effective January 1, 2019. In December 2018, a federal district court in Texas ruled the individual mandate was unconstitutional and could not be severed from the PPACA. As a result, the court ruled the remaining provisions of the PPACA were also invalid, though the court declined to issue a preliminary injunction with respect to the PPACA. This litigation is still ongoing, but places great uncertainty upon the longevity and nature of the PPACA moving forward. Additionally, while the House and Senate attempted, but failed, to pass legislation to comprehensively repeal the PPACA, these efforts may be resumed. Further legislative changes to and regulatory changes under the PPACA also remain possible.
There is uncertainty with respect to the impact the U.S. Administration and any attempted legislation may have, if any, and any changes will likely take time to unfold and could have an impact on coverage and reimbursement for healthcare items and services, including our Products and our delivery system candidates. For example, the PPACA requires that health insurance plans sold to individuals and small businesses provide coverage for "essential health benefits" ("EHBs"), which are defined according to state-specific benchmark plans. The Department of Health and Human Services issued a final rule that provides states with greater flexibility in how they select their EHB-benchmark plan, including providing states with substantially more options in what they can select as an EHB-benchmark plan and allowing states to build their own set of benefits as part of their EHB-benchmark plan, subject
to certain requirements. Providing the states with this increased flexibility to define EHBs may have the effect of decreasing coverage for anti-cancer devices such as our Products.
In addition, other legislative changes have been proposed and adopted in the U.S. since the PPACA was enacted. The Budget Control Act of 2011, as amended requires Medicare payments for all items and services, including our Products and related services, to be reduced by up to 2.0% under sequestration (i.e., automatic spending reductions, calculated each year by the Office of Management and Budget). Subsequent legislation extended the 2.0% reduction, on average, to 2027 unless additional Congressional action is taken. Additional sequestration orders could also be triggered, potentially resulting in up to a 4.0% reduction in Medicare payments.
To the extent Medicare makes a negative decision regarding the fees for our Products or declines to cover our Products, our business would be subject to material damage.
.
In 2017, CMS issued a final rule that aims to streamline the Medicare appeals process and includes changes such as permitting the designation of Medicare Appeals Council decisions as precedential, expanding the Office of Medicare Hearings and Appeals’ available adjudicator pool, and simplifying proceedings when CMS or CMS contractors are involved, among others. The final rule became effective on March 20, 2017. We are monitoring the implementation of this final rule and cannot predict to what extent CMS may or may not use this final rule in denying coverage for our Products.
The process governing Medicare appeals and the significant backlog of appeals at the ALJ level was the subject of multi-year litigation. The litigation was adjudicated in November 2018 with the court ordering the Department of Health and Human Services to clear the backlog by 2022, and reach compliance with the 90-day timeline to decide ALJ cases by this time. We cannot provide any assurance that our outstanding ALJ appeals will be favorably decided, or that the Department of Health and Human Services will meet this deadline.
We believe that substantial uncertainty remains regarding the net effect of the PPACA, or its repeal and potential replacement, on our business, including uncertainty over how benefit plans purchased on exchanges will cover our Products, how the expansion or contraction of the Medicaid program will affect access to our Products, the effect of risk-sharing payment models such as Accountable Care Organizations and other value-based purchasing programs on coverage for our Products, and the effect of the general increase or decrease in Federal oversight of healthcare payers. The taxes imposed and the expansion in government’s role in the U.S. healthcare industry under the PPACA, if unchanged, may result in decreased revenues, lower reimbursements by payers for our Products and reduced medical procedure volumes, all of which could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
Finally, in the U.S., there is increased focus on drug pricing, and the U.S. President, policy officials and lawmakers have expressed a clear interest in efforts to reduce prices for drugs and biologicals, further increase transparency around prices and price increases, lower out-of-pocket costs for consumers, and decrease spending on drugs by government programs. We expect regulatory changes and continued Congressional investigations and negative media attention in the coming months with respect to drugs reimbursed by federal healthcare programs. While the current focus is on pharmaceutical products, the scrutiny and concern with respect to rising healthcare costs could have a negative impact on our operations as well.
In the future, the U.S. Congress could also pass additional healthcare laws and CMS could implement regulatory changes. Further, various healthcare reform proposals have emerged at the state level. These laws and regulations could potentially affect coverage and reimbursement for our Products and our delivery system candidates. However, we cannot predict the ultimate content, timing or effect of any future federal or state healthcare initiatives or the impact any future legislation or regulation will have on us. The national competent authorities in the EU member states, Switzerland, Israel, Japan, and other jurisdictions are increasingly active in their goal of reducing public spending on healthcare. We cannot, therefore, guarantee that the treatment of patients with our Products would be reimbursed in any particular country or, if successfully included on reimbursement lists will remain thereon.
We are subject to extensive regulation by the FDA and comparable authorities in other jurisdictions, which could restrict the sales and marketing of our Products and could cause us to incur significant costs to
maintain compliance. In addition, we may become subject to additional regulation in other jurisdictions as we increase our efforts to market and sell Optune or NovoTTF-100L and future Products outside of the U.S.
We market and sell our Products, and expect to market and sell future Products, subject to extensive regulation by the FDA and numerous other federal, state and governmental authorities in other jurisdictions. These regulations are broad and relate to, among other things, the conduct of preclinical and clinical studies, product design, development, manufacturing, labeling, testing, product storage and shipping, premarket clearance and approval, conformity assessment procedures, premarket clearance and approval of modifications introduced in marketed products, post-market surveillance and monitoring, reporting of adverse events and incidents, pricing and reimbursement, interactions with healthcare professionals, interactions with patients, information security, advertising and promotion and product sales and distribution. Although we have received FDA approval to market Optune in the U.S. for the treatment of adult patients with newly diagnosed GBM (in combination with temozolomide) and recurrent GBM and approval to market NovoTTF-100L for adults patients with MPM, we will require additional FDA approval to market our Products for other indications. We may be required to obtain approval of a new PMA, HDE or PMA supplement application for modifications made to our Products. This approval process is costly and uncertain, and it could take one to three years, or longer, from the time the application is filed with the FDA. We may make modifications in the future that we believe do not or will not require additional approvals. If the FDA disagrees, and requires new PMAs, HDEs, or PMA supplements for the modifications, we may be required to recall and to stop marketing the modified versions of our Products.
In addition, before our Products can be marketed in the EU, our Products must obtain a CE Certificate from a notified body. New intended uses of CE marked medical devices falling outside the scope of the current CE Certificate require a completely new conformity assessment before the device can be CE marked and marketed in the EU for the new intended use.
These approval processes can be expensive, lengthy and entail significant fees. The process required to gather necessary information and draw up documentation in order to obtain CE Certification of a medical device in the EU can be expensive and lengthy and its outcome can be uncertain. We may make modifications to our Products in the future that we believe do not or will not require notifications to our notified body or new conformity assessments to permit the maintenance of our current CE Certificate. If the competent authorities of the EU member states or our notified body disagree and require the conduct of a new conformity assessment, the modification of the existing CE Certificate or the issuance of a new CE Certificate, we may be required to recall or suspend the marketing of the modified versions of Optune.
In Japan, new medical devices or new therapeutic uses of medical devices falling outside the scope of the existing approval by the MHLW require a new assessment and approval for each such new device or use. Accordingly, we may be required to obtain a new approval from MHLW before we launch a modified version of our Products or the use of our Products for additional indications. Approval time frames from the MHLW vary from simple notifications to review periods of one or more years, depending on the complexity and risk level of the device. In addition, importation into Japan of medical devices is subject to "Quality Management System (QMS) Ordinance," which includes the equivalent of "Good Import" regulations in the U.S. As with any highly regulated market, significant changes in the regulatory environment could adversely affect our ability to commercialize Optune in Japan.
In the U.S. and other jurisdictions, we also are subject to numerous post-marketing regulatory requirements, which include regulations under the QSR related to the manufacturing of our Products, labeling regulations and medical device reporting regulations, which require us to report to the FDA or comparable regulatory authorities in other jurisdictions and our notified body if our Products cause or contributes to a death or serious injury, or malfunction in a way that would likely cause or contribute to a death or serious injury. In addition, these regulatory requirements may change in the future in a way that adversely affects us. If we fail to comply with present or future regulatory requirements that are applicable to us, we may be subject to enforcement action by the FDA or comparable regulatory authorities in other jurisdictions and notified bodies, which may include any of the following sanctions:
•untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;
•unanticipated expenditures to address or defend such actions;
•patient notification, or orders for repair, replacement or refunds;
•voluntary or mandatory recall, withdrawal or seizure of our current or future delivery systems;
•administrative detention by the FDA or other regulatory authority in another jurisdiction of medical devices believed to be adulterated or misbranded;
•operating restrictions, suspension or shutdown of production;
•refusal or delay of our requests for PMA or analogous approval for new intended uses for or modifications to our Products;
•refusal or delay of our requests for PMA or analogous approval of new delivery systems;
•refusal or delay in obtaining CE Certificates for new intended uses for or modifications to our Products;
•suspension, variation or withdrawal of the CE Certificates granted by our notified body in the EU;
•prohibition or restriction of Products being placed on the market;
•operating restrictions;
•suspension or withdrawal of PMA or analogous approvals that have already been granted;
•refusal to grant export approval for our Products or any delivery system candidates; or
•criminal prosecution.
The occurrence of any of these events could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
Over time, we expect to make modifications to our Products intended to improve efficacy, reduce side effects, enhance the user experience and other purposes. Modifications to our Products may require approvals of new PMAs, HDEs, or PMA supplement applications, modified or new CE Certificates and analogous regulatory approvals in other jurisdictions or even require us to cease promoting or to recall the modified versions of our Products until such clearances, approvals or modified or new CE Certificates are obtained, and the FDA, comparable regulatory authorities in other jurisdictions or our notified body may not agree with our conclusions regarding whether new approvals are required.
Any modification to a device approved through the PMA or HDE pathway that impacts the safety or effectiveness of the device requires submission to the FDA and FDA approval of a PMA supplement application or even a new PMA or HDE application, as the case may be. The FDA requires a company to make the determination as to whether a new PMA, HDE or PMA supplement application is to be filed, but the FDA may review the company’s decision. For example, in the past, we have made initial determinations that certain modifications did not require the filing of a new PMA or PMA supplement application and have notified the FDA of these changes in our PMA Annual Report, but after its review of our PMA Annual Report, the FDA requested that we submit these modifications to the FDA as a PMA supplement application. From time to time, we may make other changes to the delivery systems, software, packaging, manufacturing facilities and manufacturing processes and may submit additional PMA supplement applications for these changes. FDA may conduct a facility inspection as part of its review and approval process. In addition, it is possible that the FDA will require a human factors (user interface) study. It is also possible that the FDA may require additional clinical data. We can provide no assurance that we will receive FDA approval for these changes on a timely basis, or at all. We also may make additional changes in the future that we may determine do not require the filing of a new PMA or PMA supplement application. The FDA may not agree with our decisions regarding whether the filing of new PMAs or PMA supplement applications are required.
In addition, any substantial change introduced to a medical device or to the quality system certified by our notified body requires a new conformity assessment of the device and can lead to changes to the CE Certificates or the preparation of a new CE Certificate of Conformity. Substantial changes may include, among others, the introduction of a new intended use of the device, a change in its design or a change in the company’s suppliers. Responsibility for determination that a modification constitutes a substantial change lies with the manufacturer of the medical device. We must inform the notified body that conducted the conformity assessment of the Products we market or sell in the EU of any planned substantial changes to our quality system or changes to our Products which could,
among other things, affect compliance with the Essential Requirements laid down in Annex I to the MDD/MDR or the devices’ intended use. The notified body will then assess the changes and verify whether they affect the Product’s conformity with the Essential Requirements laid down in Annex I to the MDD/MDR or the conditions for the use of the device. If the assessment is favorable, the notified body will issue a new CE Certificate or an addendum to the existing CE Certificate attesting compliance with the Essential Requirements laid down in Annex I to the MDD/MDR. There is a risk that the competent authorities of the EU member states or our notified body may disagree with our assessment of the changes introduced to our Products. The competent authorities of the EU member states or our notified body also may come to a different conclusion than the FDA on any given product modification.
In addition, medical devices that have obtained a CE Certification under the MDD may in principle continue to be marketed under such CE Certificate until the CE Certificate expires and at the latest until May 27, 2024, provided that the manufacturer complies with the MDR’s additional requirements related to post-marketing surveillance, market surveillance, vigilance, and registration of economic operators and of devices. However, if such medical devices undergo a significant change in their design or intended use, we would need to obtain a new CE Certificate under the MDR for these devices.
If the FDA disagrees with us and requires us to submit a new PMA, HDE, or PMA supplement application for then-existing modifications and/or the competent authorities of the EU member states or our notified body disagree with our assessment of the change introduced in a product, its design or its intended use, we may be required to cease promoting or to recall the modified product until we obtain approval and/or until a new conformity assessment has been conducted in relation to the product, as applicable. In addition, we could be subject to significant regulatory fines or other penalties. Furthermore, our Products could be subject to recall if the FDA, comparable regulatory authorities in other jurisdictions, or our notified body determine, for any reason, that our Products are not safe or effective or that appropriate regulatory submissions were not made. Any recall or requirement that we seek additional approvals or clearances could result in significant delays, fines, increased costs associated with modification of a product, loss of revenues and potential operating restrictions imposed by the FDA, comparable foreign regulatory authorities in other jurisdictions, or our notified body. Delays in receipt or failure to receive approvals/certification, or the failure to comply with any other existing or future regulatory requirements, could reduce our sales, profitability and future growth prospects.
We will spend considerable time and money complying with federal, state and local rules, regulations and guidance in other jurisdictions in addition to FDA regulations, and, if we are unable to fully comply with such rules, regulations and guidance, we could face substantial penalties.
We are subject to extensive regulation by the U.S. federal government and the states and other countries in which we conduct our business. U.S. federal government healthcare laws apply when we submit a claim on behalf of a U.S. federal healthcare program beneficiary, or when a customer submits a claim for an item or service that is reimbursed under a U.S. federal government-funded healthcare program, such as Medicare or Medicaid. The laws that affect our ability to operate our business in addition to the Federal Food, Drug, and Cosmetic Act and FDA regulations include, but are not limited to, the following:
•the Federal Anti-Kickback Statute, which prohibits offering or providing remuneration of any kind with the intent to induce or reward referrals for items or services reimbursable by a federal healthcare program;
•the U.S. Federal False Claims Act and state false claims acts, which prohibit submitting false claims or causing the submission of false claims to the federal or a state government;
•Medicare laws and regulations that prescribe requirements for coverage and payment, including the conditions of participation for DME suppliers, and laws prohibiting false claims or unduly influencing selection of products for reimbursement under Medicare and Medicaid;
•healthcare fraud statutes that prohibit false statements and improper claims to any third-party payer;
•the federal physician self-referral prohibition, commonly known as the Stark law, which, absent an applicable exception, prohibits physicians from referring Medicare patients to an entity for the provision of certain designated health services (including DME) if the physician (or a member of the physician’s immediate family) has an impermissible financial relationship with that entity and prohibits the DHS entity from billing for such improperly referred services;
•the Federal Beneficiary Anti-Inducement Statute, which prohibits the offering of any remuneration to a beneficiary of Medicare or Medicaid that is likely to influence that beneficiary’s choice of provider or supplier. This can include, but is not limited to, inappropriate provision of patient services including financial assistance. Recent government investigations have focused on this particular prohibition. There are established exceptions from liability, but we cannot guarantee that all of our practices will fall squarely within those exceptions;
•similar state anti-kickback, false claims, insurance fraud and self-referral laws, which may not be limited to government-reimbursed items, as well as state laws that require us to maintain permits or licenses to distribute durable medical equipment;
•federal and state accreditation and licensing requirements applicable to DME providers and equivalent requirements in other jurisdictions;
•the U.S. Foreign Corrupt Practices Act, which can be used to prosecute companies in the U.S. for arrangements with physicians or other parties outside the U.S. if the physician or party is a government official of another country and the arrangement violates the law of that country;
•the Federal Trade Commission Act, the Lanham Act and similar federal and state laws regulating truthfulness in advertising and consumer protection;
•the federal Sunshine Act and similar state and foreign laws, which require periodic reporting of payments and other transfers of value made to U.S.-licensed physicians, teaching hospitals, and for reports submitted on or after January 1, 2022, physician assistants, nurse practitioners, clinical nurse specialists, certified nurse anesthetists, and certified nurse-midwives; and
•HIPAA in the US, the GDPR in the EU and other regional data privacy rules that require certain levels of protection of individuals’ personal data.
Similar laws exist in the EU, individual EU member states and other countries. These laws are complemented by EU or national professional codes of practices.
The laws and codes of practices applicable to us are subject to evolving interpretations. Moreover, certain U.S. federal and state laws regarding healthcare fraud and abuse and certain laws in other jurisdictions regarding interactions with healthcare professionals and patients are broad and we may be required to restrict certain of our practices to be in compliance with these laws. Healthcare fraud and abuse laws also are complex and even minor, inadvertent irregularities, or even the perception of impropriety, can potentially give rise to claims that a statute has been violated.
Any violation of these laws could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline. Similarly, if there is a change in law, regulation or administrative or judicial interpretations, we may have to change our business practices or our existing business practices could be challenged as unlawful, which likewise could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline. Fines and penalties for violations of these laws and regulations could include severe criminal and civil penalties, including, for example, significant monetary damages, exclusion from participation in the federal healthcare programs, and permanent disbarment of key employees. Any penalties, damages, fines, curtailment or restructuring of our operations would adversely affect our ability to operate our business, our prospects and our financial results. In addition, any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and damage our reputation.
In addition, although we believe that we have the required licenses, permits and accreditation to dispense our Products in the future, a regulator could find that we need to obtain additional licenses or permits. We also may be subject to mandatory reaccreditation and other requirements in order to maintain our billing privileges. Failure to satisfy those requirements could cause us to lose our privileges to bill governmental and private payers. If we are required to obtain permits or licenses that we do not already possess, we also may become subject to substantial additional regulation or incur significant expense.
To ensure compliance with Medicare, Medicaid and other regulations, federal and state governmental agencies and their agents, including DME MACs, may conduct audits of our operations to support our claims submitted for payment of items furnished to beneficiaries and health care providers. For example, CMS contracts with Recovery Audit Contractors ("RACs") on a contingency fee basis to conduct post-payment reviews to detect and correct improper payments in the Medicare fee-for-service program. The RAC program’s scope also includes managed Medicare plans and Medicaid claims. RAC denials are appealable; however, there currently are significant delays in the ALJ process, which could negatively impact our ability to appeal RAC payment denials. In addition, CMS employs various other program integrity contractors to perform post-payment audits of claims and identify overpayments, and state Medicaid agencies and other contractors have increased their review activities. Private and government-funded managed care payers may also conduct similar post-payment audits. Depending on the nature of the conduct found in such audits and whether the underlying conduct could be considered systemic, the resolution of these audits could adversely impact our revenue, financial condition and results of operations.
Further, in many instances, there can be a significant lack of clarity regarding the required documentation for the audit and audit methodology. As such, the clarity and completeness of each patient medical file, some of which is the work product of physicians not employed by us, is essential to successfully challenging any payment denials. For example, the DME MAC Supplier Manuals provide that clinical information from the "patient's medical record" is required to justify the initial and ongoing medical necessity for the provision of DME. Some DME MACs, CMS staff and government subcontractors have taken the position, that the "patient's medical record" refers not to documentation maintained by the DME supplier but instead to documentation maintained by the patient's physician, healthcare facility or other clinician, and that clinical information created by the DME supplier's personnel and confirmed by the patient's physician is not sufficient to establish medical necessity. If the physicians working with our patients do not adequately document, among other things, their diagnoses and plans of care, our risks related to audits and payment denials in general are greater.
If we, our collaborative partners, our contract manufacturers or our component suppliers fail to comply with the FDA’s QSR or equivalent regulations established in other countries, the manufacturing and distribution of our Products could be interrupted, and our Product sales and results of operations could suffer.
We, our collaborative partners, our contract manufacturers and our component suppliers are required to comply with the FDA’s QSR and the equivalent quality system requirements imposed by the laws and regulations in other jurisdictions, which are a complex regulatory framework that covers the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our Products. All aspects of our supply chain are subject to periodic inspections and audits by the FDA, notified bodies and other regulatory authorities to ensure continuing compliance. We cannot assure you that our facilities or our contract manufacturers’ or component suppliers’ facilities would pass any future quality system inspection. If our or any of our contract manufacturers’ or component suppliers’ facilities fails a quality system inspection, the manufacturing or distribution of our Products could be interrupted and our operations disrupted. Failure to take adequate and timely corrective action in response to an adverse quality system inspection could force a suspension or shutdown of our packaging and labeling operations or the manufacturing operations of our contract manufacturers, and lead to suspension, variation or withdrawal of our regulatory approvals or a recall of our Products. If any of these events occurs, we may not be able to provide our customers with our Products on a timely basis, our reputation could be harmed and we could lose customers, any or all of which could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
Our Products may in the future be subject to recalls that could harm our reputation, business and financial results.
The FDA and similar governmental authorities in other jurisdictions have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause serious injury or death. In addition, governmental bodies in other jurisdictions have the authority to require the recall of our Products in the event of material deficiencies or defects in design or manufacture. Distributors and manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us or one of our manufacturers could occur as a result of component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. The FDA requires that certain classifications of recalls be reported to the FDA within ten working days after the recall is initiated. Requirements for the reporting of product recalls to the competent authorities are imposed in other
jurisdictions in which our Products are or would be marketed in the future. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA or to the competent authorities of other countries. In the future, we may initiate voluntary recalls involving our Products that we determine do not require notification of the FDA or to other equivalent non-U.S. authorities. If the FDA or the equivalent non-U.S. authorities disagree with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA and the equivalent non-U.S. authorities could take enforcement action if we fail to report the recalls when they were conducted. Recalls of our Products would divert managerial and financial resources and could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
If our Products cause or contribute to a death or a serious injury, or malfunction in certain ways, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA Medical Device Reporting regulations and the equivalent regulations applicable in other jurisdictions in which our Products are or may be marketed in the future, medical device manufacturers are required to report to the FDA and to the equivalent non-U.S. authorities information that a device has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to death or serious injury if the malfunction of the device or one of our similar devices were to recur. If we fail to report these events to the FDA or to the equivalent authorities in other jurisdictions within the required time frames, or at all, the FDA or the equivalent authorities in other jurisdictions could take enforcement action against us. Any such adverse event involving our Products also could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.
We may be subject to fines, penalties or injunctions if we are determined to be promoting the use of our Products for unapproved or off-label uses.
Medical devices may be marketed only for the indications for which they are approved. Our promotional materials and training materials must comply with FDA regulations and other applicable laws and regulations governing the promotion of our Products in the U.S. and other jurisdictions. Currently, Optune is approved for treatment of adult patients with newly diagnosed GBM (in combination with temozolomide) and recurrent GBM in the U.S. and is approved for treatment of adult patients with GBM in Japan. In the EU and Switzerland, we have CE marked the Optune delivery system for the treatment of newly diagnosed GBM (in combination with temozolomide), recurrent GBM, and advanced NSCLC (in combination with standard-of-care chemotherapy). Optune is also approved in Israel and in Australia for the treatment of recurrent GBM and newly diagnosed GBM (in combination with temozolomide). The NovoTTF-100L System is only approved in the U.S. for the treatment of unresectable, locally advanced or metastatic MPM.
If the FDA or the competent authorities in other jurisdictions, including the EU member states, determine that our promotional materials or training constitutes promotion of an unapproved use, they could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled or warning letter, an injunction, seizure, civil fines and criminal penalties. It is also possible that authorities in other federal, state or national enforcement in other jurisdictions might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and the commercialization of our Products could be impaired.
U.S. legislative or FDA regulatory reforms may make it more difficult and costly for us to obtain regulatory approval of our delivery system candidates and to manufacture, market and distribute our Products after approval is obtained.
From time to time, legislation is drafted and introduced in the U.S. Congress that could significantly change the statutory provisions governing the regulatory approval, manufacture and marketing of regulated products or the reimbursement thereof. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our Products. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of future delivery
system candidates. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our Products. It is impossible to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be. Any change in the laws or regulations that govern the clearance and approval processes relating to our current and future delivery systems could make it more difficult and costly to obtain clearance or approval for new delivery systems, or to produce, market and distribute our Products. Significant delays in receiving clearance or approval, or the failure to receive clearance or approval for our new delivery systems would have an adverse effect on our ability to expand our business in the U.S.
We are affected by and subject to environmental laws and regulations that could be costly to comply with or that may result in costly liabilities.
We are subject to environmental laws and regulations, including those that impose various environmental controls on the manufacturing, transportation, storage, use and disposal of batteries and hazardous chemicals and other materials used in, and hazardous waste produced by, the manufacturing of our Products. We incur and expect to continue to incur costs to comply with these environmental laws and regulations. Additional or modified environmental laws and regulations, including those relating to the manufacture, transportation, storage, use and disposal of materials used to manufacture our Products or restricting disposal or transportation of batteries, may be imposed that may result in higher costs.
In addition, we cannot predict the effect that additional or modified environmental laws and regulations may have on us, our third-party suppliers of equipment, batteries and our Products or our customers. For example, we and our suppliers rely on an exemption from the European Directive 2011/65/EU relating to the restriction of the use of certain hazardous substances in electrical and electronic equipment relating to lead content in our transducer arrays. To the extent this exemption is revoked, it may have a material impact on our business and results of operations.
Safety issues concerning lithium-ion batteries could have a material adverse impact on our business.
Our Products use lithium ion batteries. On rare occasions, lithium-ion cells can rapidly release the energy they contain by venting smoke and flames in a manner that can ignite nearby materials as well as other lithium-ion cells. A failure in the lithium ion battery contained in a Product could occur, which could result in accidents, casualty or damages, and subject us to lawsuits, product recalls, or redesign efforts. In addition, we store a significant number of lithium-ion cells at our facilities. Any failure of battery cells or a safety issue or fire related to the cells could disrupt our operations. Such damage or injury could lead to adverse publicity and potentially a safety recall.
Regulations on the transportation of lithium ion batteries may affect our business.
The transportation of lithium and lithium-ion batteries is regulated worldwide. Laws regulating the transportation of batteries have been and may be enacted which could impose additional costs that could harm our ability to be profitable.
Under recommendations adopted by the International Air Transport Association ("IATA"), our batteries currently require a Class 9 designation for transportation. Our larger first generation delivery system batteries must be properly packaged and labeled in order to be shipped by air transport as cargo. Our smaller second generation delivery system batteries can be shipped without the class 9 sticker if shipped with the device but require the class 9 sticker if shipped by air separately. The larger batteries are not allowed on passenger aircraft according to the IATA standards. The smaller batteries are allowed as carry on only and cannot be checked as luggage. Consequently, we offer to ship batteries for patients who are traveling by air.
If additional restrictions are put in place that limit our ability to ship our Products by air freight or on water borne cargo, it could have an adverse effect on our supply chain, our inventory management procedures and processes and our ability to fill prescriptions and service patients in a timely manner, which could have a material adverse effect on our business, prospects, financial condition and results of operations. In addition, compliance with future worldwide or IATA approval process and regulations could require significant time and resources from our technical staff and, if redesign were necessary, could delay the introduction of new products.
Risks relating to intellectual property
If we fail to protect, sustain, further build and enforce our intellectual property rights, including to our proprietary technology, trade secrets or know how, competitors may be able to develop competing therapies.
Our success depends, in part, on our ability to obtain and maintain protection for our Products and technologies under the patent laws or other intellectual property laws of the U.S. and other countries. The standards that the U.S. Patent and Trademark Office ("USPTO") and its counterparts in other jurisdictions use to grant patents are not always applied predictably or uniformly and can change. Consequently, we cannot be certain as to whether pending patent applications will result in issued patents, and we cannot be certain as to the type and extent of patent claims that may be issued to us in the future. Any issued patents may not contain claims that will permit us to stop competitors from using similar technology.
Our current intellectual property portfolio consists of over 180 issued patents. In the U.S. the patents have expected expiration dates between 2021 and 2037 . As our patents expire we will be subject to additional risks. Patent expiration could adversely affect our ability to protect future product development and our competitors may develop and market competing products. We have also filed additional patent applications in several countries that may never be issued. Consequently, our operating results and financial position could be materially adversely affected. In addition, due to the extensive time needed to develop, test and obtain regulatory approval for our treatment therapies, any patents that protect our product candidates may expire early during commercialization. This may reduce or eliminate any market advantages that such patents may give us and harm our financial position. If we fail to develop and successfully launch new products prior to the expiration of patents for our existing products, our sales and achieving patient acceptance with respect to those products could decline significantly. We may not be able to develop and successfully launch more advanced replacement products before these and other patents expire.
Our existing and future patent portfolio also may be vulnerable to legal challenges. The standards that courts use to interpret patents are not always applied predictably or uniformly and can change, particularly as new technologies develop. On September 16, 2011, President Obama signed into law the Leahy-Smith America Invents Act ("AIA") a significant patent law reform. The AIA implements a first-inventor-to-file standard for patent approval, changes the legal standards for patentability and creates a post-grant review system. As a result of the uncertainties of patent law in general, and surrounding the interpretation of the AIA in particular, we cannot predict with certainty how much protection, if any, will be given to our patents if we attempt to enforce them and they are challenged in court. Any attempt to enforce our intellectual property rights may also be time-consuming and costly, may divert the attention of management from our business, may ultimately be unsuccessful or may result in a remedy that is not commercially valuable. Such attempts may also provoke third parties to assert claims against us or result in our intellectual property being narrowed in scope or declared to be invalid or unenforceable.
In addition, we rely on certain proprietary trade secrets, know-how and other confidential information. We have taken measures to protect our unpatented trade secrets, know-how and other confidential information, including the use of confidentiality and assignment of inventions agreements with our employees, consultants and certain contractors. It is possible, however, that these persons may breach or challenge the agreements, that our trade secrets may otherwise be misappropriated or that competitors may independently develop or otherwise discover our trade secrets. There is therefore no guarantee that we will be able to obtain, maintain and enforce the intellectual property rights that may be necessary to protect and grow our business and to provide us with a meaningful competitive advantage, and our failure to do so could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
The oncology industry is characterized by patent and other intellectual property litigation and disputes, and any litigation, dispute or claim against us may cause us to incur substantial costs, could place a significant strain on our financial resources, divert the attention of management from our business, harm our reputation and require us to remove certain delivery systems from the market.
Whether a product infringes a patent or violates other intellectual property rights involves complex legal and factual issues, the determination of which is often uncertain. Any intellectual property dispute, even a meritless or unsuccessful one, would be time consuming and expensive to defend and could result in the diversion of our management’s attention from our business and result in adverse publicity, the disruption of research and
development and marketing efforts, injury to our reputation and loss of revenues. Any of these events could negatively affect our business, prospects, financial condition and results of operations.
Third parties may assert that Tumor Treating Fields, our Products, the methods employed in the use of our Products or other activities infringe on their patents. Such claims may be made by competitors seeking to obtain a competitive advantage or by other parties, many of whom have significantly larger intellectual property portfolios than we have. Additionally, in recent years, individuals and groups have begun purchasing intellectual property assets for the purpose of making claims of infringement and attempting to extract settlements from companies like ours. The risk of infringement claims is exacerbated by the fact that there are numerous issued and pending patents relating to the treatment of cancer. Because patent applications can take many years to issue, and in many cases remain unpublished for many months after filing, there may be applications now pending of which we are unaware that may later result in issued patents that our Products may infringe. There could also be existing patents that one or more components of our Products may inadvertently infringe. As the number of competitors in the market for the treatment of cancer grows, the possibility of inadvertent patent infringement by us or a patent infringement claim against us increases. To the extent we gain greater market visibility, our risk of being subject to such claims is also likely to increase. If a third party’s patent was upheld as valid and enforceable and we were found to be infringing, we could be prevented from making, using, selling, offering to sell or importing our Products or other delivery system candidates, unless we were able to obtain a license under that patent or to redesign our systems to avoid infringement. A license may not be available at all or on terms acceptable to us, and we may not be able to redesign our Products to avoid any infringement. Modification of our Products or development of delivery system candidates to avoid infringement could require us to conduct additional clinical trials and to revise our filings with the FDA and other regulatory bodies, which would be time-consuming and expensive. If we are not successful in obtaining a license or redesigning our delivery systems, we may be unable to make, use, sell, offer to sell or import our delivery systems and our business could suffer. We may also be required to pay substantial damages and undertake remedial activities, which could cause our business to suffer.
We may also be subject to claims alleging that we infringe or violate other intellectual property rights, such as copyrights or trademarks, may have to defend against allegations that we misappropriated trade secrets, and may face claims based on competing claims of ownership of our intellectual property. The confidentiality and assignment of inventions agreements that our employees, consultants and other third parties sign may not in all cases be enforceable or sufficient to protect our intellectual property rights. In addition, we may face claims from third parties based on competing claims to ownership of our intellectual property.
We also employ individuals who were previously employed at other medical device companies, and as such we may be subject to claims that such employees have inadvertently or otherwise used or disclosed the alleged trade secrets or other proprietary information of their former employers. Any such litigation, dispute or claim could be costly to defend and could subject us to substantial damages, injunctions or other remedies, which could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
The patent rights on which we rely to protect the intellectual property underlying our Products may not be adequate, which could enable third parties to use our technology or market competing products, which would harm our continued ability to compete in the market.
Our success will depend in part on our continued ability to develop or acquire commercially valuable patent rights and to protect these rights adequately. The scope of some of our patents is limited to certain ranges. For example, some of our patents protect low-intensity (1-3 V/cm) and intermediate frequency (100-300 kHz) alternating electric fields, but do not cover intensities and frequencies for electric fields that are outside of these ranges. While intensities and frequencies of electric fields outside of these ranges have not yet proven to be effective treatment modalities, that may not be the case in the future. Our patent position is generally uncertain and involves complex legal and factual questions. The risks and uncertainties that we face with respect to our patents and other related rights include the following:
•the pending patent applications we have filed may not result in issued patents or may take longer than we expect to result in issued patents;
•the pending patent applications and patents we own may be subject to interference proceedings or similar disputes over the priority of the inventions claimed;
•the claims of any patents that are issued may not provide meaningful protection;
•we may not be able to develop additional proprietary technologies that are patentable;
•changes in patent laws or their interpretation in the U.S. and other countries (including the recently enacted AIA) could diminish the value of our patents, narrow the scope of our patent protection or adversely affect our ability to obtain new patents;
•obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by government patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements;
•other parties may challenge patents, patent claims or patent applications licensed or issued to us, and such patents, patent claims or patent applications may be narrowed or found to be invalid or unenforceable; and
•other companies may design around or expand upon technologies we have patented or developed.
We also may fail to apply for or be unable to obtain patent rights in some other countries. In addition, the legal systems of certain countries may not protect our rights to the same extent as the laws of the U.S., which could affect our ability to enforce patent rights effectively in such other countries. For a variety of reasons, we may decide not to file for patent protection for certain of our intellectual property. Our patent rights underlying Tumor Treating Fields and our Products may not be adequate, and our competitors or customers may design around our proprietary technologies or independently develop similar or alternative technologies or products that are equal or superior to ours without infringing on any of our patent rights. In addition, the patents licensed or issued to us may not provide a competitive advantage, and may be insufficient to prevent others from commercializing products similar or identical to ours. The occurrence of any of these events could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
We have limited intellectual property rights in other jurisdictions outside of our key markets and may not be able to protect our intellectual property rights throughout the world.
We have limited intellectual property rights outside of our key markets. In some countries outside the U.S., we do not have any intellectual property rights, and our intellectual property rights in other countries outside the U.S. have a different scope and strength compared to our intellectual property rights in the U.S. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the U.S. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but where enforcement rights are not as strong as those in the U.S. These products may compete with our delivery systems, and our patents or other intellectual property rights may not be effective or adequate to prevent such competition.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our delivery systems.
As is the case with other medical device companies, our success is heavily dependent on our intellectual property rights, and particularly on our patent rights. Obtaining and enforcing patents in the medical device industry involves both technological and legal complexity, and is therefore costly, time consuming and inherently uncertain. In addition, the U.S. has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents once obtained. Depending on decisions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could further negatively impact the value of our patents, narrow the scope of available patent protection or weaken the rights of patent owners.
Risks relating to our ordinary shares
The market price for our ordinary shares may be volatile, which could result in substantial losses.
The market price for our ordinary shares may be volatile and subject to wide fluctuations in response to factors such as publication of clinical studies relating to our Products, our system candidates or a competitor’s product, actual or anticipated fluctuations in our quarterly results of operations, changes in financial estimates by securities research analysts, negative publicity, studies or reports, changes in the economic performance or market valuations of other companies that operate in our industry, changes in the availability of third-party reimbursement in the U.S. or other countries, changes in governmental regulations or in the status of our regulatory approvals or applications, announcements by us or our competitors of material acquisitions, strategic partnerships, joint ventures or capital commitments, intellectual property litigation, release of transfer restrictions on our outstanding ordinary shares, and economic or political conditions in the U.S. or elsewhere.
Our ordinary shares are issued under the laws of Jersey, which may not provide the level of legal certainty and transparency afforded by incorporation in a U.S. state.
We are incorporated under the laws of the Bailiwick of Jersey, Channel Islands. Jersey legislation regarding companies is largely based on English corporate law principles. However, there can be no assurance that Jersey law will not change in the future or that it will serve to protect investors in a similar fashion afforded under corporate law principles in the U.S., which could adversely affect the rights of investors.
U.S. shareholders may not be able to enforce civil liabilities against us.
We are a Jersey entity with most of our assets located outside of the U.S. Although we have appointed an agent for service of process in the U.S. for purposes of U.S. federal securities laws, a number of our directors and executive officers and a number of directors of each of our subsidiaries are not residents of the U.S., and all or a substantial portion of the assets of such persons are located outside the U.S. As a result, it may not be possible for investors to effect service of process within the U.S. upon such persons or to enforce against them judgments obtained in U.S. courts predicated upon the civil liability provisions of the federal securities laws of the U.S.
We have been advised by our Jersey lawyers that the courts of Jersey would recognize any final and conclusive judgment under which a sum of money is payable (not being a sum payable in respect of taxes or other charges of a like nature or in respect of a fine or other penalty) obtained against us in the courts of any other territory in respect of certain enforceable obligations in accordance with the principles of private international law as applied by Jersey law (which are broadly similar to the principles accepted under English common law) and such judgment would be sufficient to form the basis of proceedings in the Jersey courts for a claim for liquidated damages in the amount of such judgment. In such proceedings, the Jersey courts would not re-hear the case on its merits save in accordance with such principles of private international law. Obligations may not necessarily be enforceable in Jersey in all circumstances or in accordance with their terms; and in particular, but without limitation: (i) any agreement purporting to provide for a payment to be made in the event of a breach of such agreement would not be enforceable to the extent that the Jersey courts were to construe such payment to be a penalty that was excessive, in that it unreasonably exceeds the maximum damages that an obligee could have suffered as a result of the breach of an obligation; (ii) the Jersey courts may refuse to give effect to any provision in an agreement that would involve the enforcement of any revenue or penal laws in other jurisdictions; and (iii) the Jersey courts may refuse to allow unjust enrichment or to give effect to any provisions of an agreement (including provisions relating to contractual interest on a judgment debt) that it considers usurious.
Our annual and quarterly results may fluctuate due to a number of factors and, as a result, could fall below investor expectations or estimates by securities research analysts, which may cause the trading price of our ordinary shares to decline.
Our revenues and results of operations are difficult to predict, and potentially may vary significantly from period to period. As a result of a number of factors, many of which are beyond our control, it is possible that results of operations for future periods may be below the expectations of public market analysts and investors, which could cause our stock price to decline. Factors that may affect our quarterly results include, but are not limited to:
•failure to obtain regulatory approval for our delivery systems;
•failure to effectively commercialize our delivery systems;
•competition; and
•changes in the laws and regulations that affect our operations.
As a result, investors should not rely on year-to-year or quarter-to-quarter comparisons of results of operations as an indication of future performance.
Substantial future sales of our ordinary shares in the public market, or the perception that such sales may occur, could cause the price of our ordinary shares to decline.
Sales of a substantial number of shares of our ordinary shares in the public market could occur at any time. These sales, or the perception in the market that holders of a large number of shares intend to sell shares, could reduce the market price of our ordinary shares stock. Our outstanding shares, including shares issuable under our equity incentive plans, may be freely sold in the public market at any time to the extent permitted by Rules 144 and 701 under the Securities Act of 1933, as amended, or the Securities Act, or to the extent such shares have already been registered under the Securities Act and are held by non-affiliates of ours.
In addition, certain of our employees, executive officers, directors and affiliated stockholders have entered or may enter into Rule 10b5-1 plans providing for sales of shares of our ordinary shares from time to time. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the employee, director or officer when entering into the plan, without further direction from the employee, officer, director or affiliated stockholder. A Rule 10b5-1 plan may be amended or terminated in some circumstances. Our employees, executive officers, directors and affiliated stockholders also may buy or sell additional shares outside of a Rule 10b5-1 plan when they are not in possession of material, nonpublic information.
Our memorandum and articles of association contain anti-takeover provisions that could adversely affect the rights of holders of our ordinary shares.
Our amended and restated memorandum and articles of association, referred to as the memorandum and articles of association, contain certain provisions that could limit the ability of third parties to acquire control of our company, including a provision that grants authority to our board of directors to issue from time to time one or more classes of preferred shares without action by our shareholders and to determine, with respect to any class of preferred shares, the terms and rights of that class. The provisions could have the effect of depriving our shareholders of the opportunity to sell their ordinary shares at a premium over the prevailing market price by discouraging third parties from seeking to obtain control of our company in a tender offer or similar transactions.
If securities or industry analysts do not publish research or publish unfavorable or inaccurate research about our business, our share price and trading volume could decline.
The trading market for our ordinary shares will continue to depend, in part, on the research and reports that securities or industry analysts publish about us or our business. We may be unable to sustain coverage by well-regarded securities and industry analysts. If either none or only a limited number of securities or industry analysts maintain coverage of our company, or if these securities or industry analysts are not widely respected within the general investment community, the trading price for our ordinary shares would be negatively impacted. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who cover us downgrade our ordinary shares or publish inaccurate or unfavorable research about our business, the price of our ordinary shares would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our ordinary shares could decrease, which might cause the price of our ordinary shares and trading volume to decline.
If we fail to maintain proper and effective internal controls, our ability to produce accurate and timely financial statements could be impaired and investors’ views of us could be harmed.
As a public company, we are required to maintain internal control over financial reporting and to report any material weaknesses in such internal controls. If we or our independent registered public accounting firm identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our
ordinary shares could decline and we could be subject to sanctions or investigations by the SEC or other regulatory authorities, which would require additional financial and management resources.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2. PROPERTIES
Our U.S. operations center is located in Portsmouth, New Hampshire, our global supply chain and European operations center is located in Root, Switzerland and our research and development operations are located in Haifa, Israel. We also lease additional office and warehouse space across North America, Europe, Israel and Japan. We believe that our current facilities are adequate for our present purposes, but we may need additional space as we continue to grow and expand our operations. We believe that suitable additional or alternative office, laboratory, and manufacturing space would be available as required in the future on commercially reasonable terms.
ITEM 3. LEGAL PROCEEDINGS
In February 2019, a civil claim was filed in the District Court in Haifa, Israel (the "Court"), by Ofir Paz ("Paz"), a former member of our Board of Directors, and EES Investments Ltd., a company wholly owned by Paz (together with Paz, "Plaintiff") against us and Prof. Yoram Palti ("Respondents"). Based on Plaintiff's recent motions described below, Plaintiff claims that he is entitled to approximately 1,200,000 ordinary shares (as adjusted for share capital splits since 2003). In May 2019, we filed a motion to dismiss the claim that is still pending. Plaintiff has also filed motions in September and December 2019 to add Asaf Danziger as a Respondent and change the basis of his claims from breach of contract to wrongful deregistration. These motions are pending. We believe that the complaint is without merit and plan to defend against this claim vigorously. We have not accrued any amounts in respect of these claims, as we believe liability is not probable and the amount of any potential liability cannot be reasonably estimated.
In addition, from time to time, we are involved in claims, proceedings, and litigation arising in the ordinary course of business. We do not believe these matters, even if adversely adjudicated or settled, would have a material adverse effect on our financial condition, results of operations or cash flows.
ITEM 4. MINE SAFETY DISCLOSURES
None.
Information about our Executive Officers
Our executive officers are elected by and serve at the discretion of our board of directors. The following table lists information about our executive officers:
| | | | | | | | | | | | | | |
| Name | | Age | | Position |
| Asaf Danziger | | 53 | | | Chief Executive Officer and Director |
| William Doyle | | 57 | | | Executive Chairman |
| Michael Ambrogi | | 56 | | | Chief Operating Officer |
| Ely Benaim | | 59 | | | Chief Medical Officer |
| Wilhelmus Groenhuysen | | 62 | | | Chief Financial Officer |
| Todd Longsworth | | 45 | | | General Counsel |
| Pritesh Shah | | 42 | | | Chief Commercial Officer |
Asaf Danziger has served as our Chief Executive Officer since 2002 and has been a director of NovoCure since 2012. From 1998 to 2002, Mr. Danziger was CEO of Cybro Medical, a subsidiary of Imagyn Medical Technologies, Inc. Mr. Danziger holds a B.Sc. in material engineering from Ben Gurion University of the Negev, Israel.
William Doyle has served as our Executive Chairman since 2016, as Chairman of the Board since 2009 and has been a director of NovoCure since 2004. Mr. Doyle has served as a director of Optinose, Inc., a commercial-stage specialty pharmaceuticals company, since 2004 and Minerva Neurosciences, Inc., a clinical-stage biopharmaceutical company, since 2017. Mr. Doyle has also been the managing director of WFD Ventures LLC, a
private venture capital firm he co-founded, since 2002 and was formerly a member of the investment team at Pershing Square Capital Management L.P., a private investment firm. Prior to 2002, Mr. Doyle was a member of Johnson & Johnson’s Medical Devices and Diagnostics Group Operating Committee and was vice president, Licensing and Acquisitions. While at Johnson & Johnson, Mr. Doyle was also chairman of the Medical Devices Research and Development Council, Worldwide president of Biosense-Webster, Inc. and a member of the board of directors of Cordis Corporation and Johnson & Johnson Development Corporation, Johnson & Johnson’s venture capital subsidiary. Mr. Doyle holds an S.B. in materials science and engineering from the Massachusetts Institute of Technology and an M.B.A. from Harvard Business School. Mr. Doyle serves on Harvard Business School’s Board of Dean’s Advisors and MIT’s Institute of Medical Engineering & Science Visiting Committee.
Michael Ambrogi has been our Chief Operating Officer since 2010 and previously served as our U.S. General Manager from 2006 to 2010. Mr. Ambrogi has overall responsibility for our ongoing operations, engineering, manufacturing, service and human resources activities worldwide. From 1991 to 2006, Mr. Ambrogi worked for Deka Research and Development Corporation, inventor Dean Kamen’s research and development firm, last serving as general manager. Mr. Ambrogi led Deka’s teams on many products including the Baxter HomeChoice peritoneal dialysis machine, the Davol Hydroflex surgical irrigation device, the Cordis Crowne Stent and the J&J IBOT 3000 mobility system. Earlier in his career, from 1988 to 1990, Mr. Ambrogi was a consultant with McKinsey & Company, a global management consultant firm. Mr. Ambrogi holds a S.B. in mechanical engineering from MIT.
Ely Benaim has been our Chief Medical Officer since 2019. Dr. Benaim previously served as Chief Medical Officer for Rexahn Pharmaceuticals from 2015 to 2019, where he was responsible for leading clinical development programs and providing strategic and clinical guidance. Prior to joining Rexahn, he was chief medical officer and senior vice president of regulatory affairs at BERG Health from 2013 to 2015. From 2011 to 2013, Dr. Benaim was senior director, clinical research and global clinical lead for Millennium Pharmaceuticals Inc./Takeda Pharmaceuticals Company. From 2007 to 2010, he was vice president, clinical affairs for Sangamo BioSciences. Dr. Benaim received his M.D. from the Universidad Central de Venazuela, Caracas, and completed his pediatric residency training at the University of South Florida. Dr. Benaim completed fellowships in pediatric oncology and bone marrow transplantation at St. Jude's Children's Research Hospital.
Wilhelmus Groenhuysen has been our Chief Financial Officer since 2012. He has served on the Board of Optinose Inc., a commercial-stage specialty pharmaceuticals company, since October 2017. From 2007 to 2011, Mr. Groenhuysen worked for Cephalon, Inc., a U.S. biopharmaceutical company, last serving as executive vice president and chief financial officer, where he had responsibility for worldwide finance, commercial operations and risk management. From 1987 to 2007, Mr. Groenhuysen worked for Philips Group in various assignments in Europe, Asia and the United States, the latest of which started in 2002 when he was promoted to chief financial officer and senior vice president of Philips Electronics North America Corporation. Mr. Groenhuysen holds a Master’s Degree in Business Economics from VU University Amsterdam and graduated as a Registered Public Controller at VU University Amsterdam.
Todd Longsworth joined Novocure in 2012 and serves as General Counsel. Mr. Longsworth worked for Cephalon, Inc., a U.S. biopharmaceutical company, from 2005 to 2012, last serving as Mergers and Acquisitions, Securities and Corporate Governance Counsel. Prior to joining Cephalon, he was an associate at WilmerHale LLP, a global law firm from 2001 to 2005. Mr. Longsworth earned his B.A. from Duke University and his J.D. from the University of Pennsylvania.
Pritesh Shah joined Novocure in November 2012 and serves as Chief Commercial Officer. Prior to joining Novocure, Mr. Shah had extensive experiences in leading oncology commercial and medical affairs functions at Roche, Genentech, Bristol-Myers Squibb, OSI Oncology and AVEO Oncology. He holds a Doctor of Pharmacy from the University of Maryland and a master’s degree in Strategic Communication and Leadership from Seton Hall University.
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Our ordinary shares are quoted on the NASDAQ Global Select Market under the symbol "NVCR."
Holders of Ordinary Shares
As of February 19, 2020, there were 99,640,549 holders of record of our ordinary shares. On February 19, 2020, the last reported sale price of our ordinary shares on the NASDAQ Global Select Market was $95.75 per share.
Dividend Policy
We have not paid any dividends on our ordinary shares since our inception and do not anticipate paying any dividends on our ordinary shares in the foreseeable future.
Performance Graph
The following performance graph is being furnished as part of this annual report and shall not be deemed "filed" with the SEC or incorporated by reference into any of our filings under the Securities Act of 1933, as amended (the "Securities Act"), or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
The following graph shows the total shareholder return of an investment of $100 in cash at market close on October 2, 2015 (the first day of trading of our ordinary shares) through December 31, 2019 for (1) our ordinary shares, (2) the Russell 2000 Index, and (3) the Nasdaq Biotechnology Index. Pursuant to applicable SEC rules, all values assume reinvestment of the full amount of all dividends; however, no dividends have been declared on our ordinary shares to date. The shareholder return shown on the graph below is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future stockholder returns.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 10/2/15 | | 12/15 | | 12/16 | | 12/17 | | 12/18 | | 12/19 |
| NovoCure Ltd | 100.00 | | | 122.32 | | | 42.94 | | | 110.50 | | | 183.15 | | | 461.00 | |
| Russell 2000 | 100.00 | | | 103.59 | | | 125.67 | | | 144.07 | | | 128.21 | | | 160.93 | |
| NASDAQ Biotechnology | 100.00 | | | 111.62 | | | 89.13 | | | 106.14 | | | 96.28 | | | 121.97 | |
Recent Sales of Unregistered Securities
From January 1, 2017 to December 31, 2019, we have issued the following securities in unregistered transactions, which include warrants and options to acquire our ordinary shares. We believe that each of the following instances was exempt from registration under the Securities Act in reliance on Regulation S under the Securities Act, under Section 4(a)(2) of the Securities Act regarding transactions not involving a public offering and under Rule 701 promulgated under the Securities Act:
| | | | | | | | | | | | | | | | | | | | |
| Issuance | | Date of sale or issuance | | Number of securities | | Consideration (U.S. dollars in thousands) |
| Exercise of Warrants | | January 1, 2017 to December 31, 2017 | | 809,636 | | | $ | 20 | |
| Exercise of Warrants | | January 1, 2018 to December 31, 2018 | | 440,960 | | | $ | 10 | |
Issuer Purchases of Equity Securities
Not applicable.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table gives information about our ordinary shares that may be issued upon the exercise of stock options and vesting of restricted stock units, as applicable, under all of our existing equity compensation plans as of December 31, 2019, including the 2003 Share Option Plan (the "2003 Plan"), the 2013 Share Option Plan (the "2013 Plan"), the 2015 Omnibus Incentive Plan (the "2015 Plan") and the Employee Share Purchase Plan (the "ESPP"). Each of the 2003 Plan, the 2013 Plan, the 2015 Plan and the ESPP has been approved by the Company’s shareholders.
Equity Compensation Plan Information
| | | | | | | | | | | | | | | | | | | | |
| Plan Category | | (a) Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants and Rights | | (b) Weighted Average Exercise Price of Outstanding Options, Warrants and Rights | | (c) Number of Securities Remaining Available for Future Issuance (Excludes Securities Reflected in Column (a)) |
| Equity compensation plans approved by shareholders | | 11,825,205 | | | $ | 21.63 | | | 19,876,607 | |
| Equity compensation plans not approved by shareholders | | — | | | — | | | — | |
| Total | | 11,825,205 | | | $ | 21.63 | | | 19,876,607 | |
ITEM 6. SELECTED FINANCIAL DATA
The following tables set forth our consolidated statements of operations data:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | | | | | |
| U.S. dollars in thousands | | 2019 | | 2018 | | 2017 | | 2016 | | 2015 |
| Net revenues | | | $ | 351,318 | | | $ | 248,069 | | | $ | 177,026 | | | $ | 82,888 | | | $ | 33,087 | |
| Cost of revenues | | | 88,606 | | | 80,048 | | | 55,609 | | | 39,870 | | | 20,610 | |
| Impairment of field equipment | | | — | | | — | | | — | | | 6,412 | | | — | |
| Gross Profit | | | 262,712 | | | 168,021 | | | 121,417 | | | 36,606 | | | 12,477 | |
| Research, development and clinical trials | | | 79,003 | | | 50,574 | | | 38,103 | | | 41,467 | | | 43,748 | |
| Sales and marketing | | | 96,675 | | | 77,663 | | | 63,528 | | | 59,449 | | | 38,861 | |
| General and administrative | | | 87,948 | | | 73,456 | | | 59,114 | | | 51,007 | | | 33,864 | |
| Total Operating expenses | | | 263,626 | | | 201,693 | | | 160,745 | | | 151,923 | | | 116,473 | |
| Operating income (loss) | | | (914) | | | (33,672) | | | (39,328) | | | (115,317) | | | (103,996) | |
| Financial expenses (income), net | | | 7,910 | | | 12,270 | | | 9,169 | | | 6,147 | | | 3,151 | |
| Income (loss) before income tax | | | (8,824) | | | (45,942) | | | (48,497) | | | (121,464) | | | (107,147) | |
| Income tax | | | (1,594) | | | 17,617 | | | 13,165 | | | 10,381 | | | 4,434 | |
| Net income (loss) | | | $ | (7,230) | | | $ | (63,559) | | | $ | (61,662) | | | $ | (131,845) | | | $ | (111,581) | |
| | | | | | | | | | |
| Basic and diluted net income (loss) per ordinary share | | | $ | (0.07) | | | $ | (0.69) | | | $ | (0.70) | | | $ | (1.54) | | | $ | (3.67) | |
Non-cash share-based compensation expense included in costs and expenses:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | | | | | |
| U.S. dollars in thousands | | 2019 | | 2018 | | 2017 | | 2016 | | 2015 |
| Cost of revenues | | $ | 2,231 | | | $ | 1,261 | | | $ | 467 | | | $ | 623 | | | $ | 174 | |
| Research, development and clinical trials | | 7,570 | | | 4,709 | | | 3,587 | | | 3,155 | | | 2,529 | |
| Sales and marketing | | 11,897 | | | 7,393 | | | 3,784 | | | 5,111 | | | 2,496 | |
| General and administrative | | 30,718 | | | 26,483 | | | 19,278 | | | 12,552 | | | 6,661 | |
| Total share-based compensation expense | | $ | 52,416 | | | $ | 39,846 | | | $ | 27,116 | | | $ | 21,441 | | | $ | 11,860 | |
Consolidated balance sheet data:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | December 31, | | | | | | | | |
| U.S. dollars in thousands | | 2019 | | 2018 | | 2017 | | 2016 | | 2015 |
| Cash and cash equivalents | | $ | 177,321 | | | $ | 140,622 | | | $ | 78,592 | | | $ | 99,780 | | | $ | 119,423 | |
| Short-term investments | | 148,769 | | | 105,256 | | | 104,719 | | | 119,854 | | | 150,001 | |
| Total assets | | 479,448 | | | 339,793 | | | 265,298 | | | 282,081 | | | 307,336 | |
| Working capital | | 353,636 | | | 256,809 | | | 194,932 | | | 224,991 | | | 265,277 | |
| Current liabilities | | 86,311 | | | 64,560 | | | 50,202 | | | 36,882 | | | 28,627 | |
| Long - term liabilities | | 175,347 | | | 162,974 | | | 101,532 | | | 102,854 | | | 27,889 | |
| Total shareholders' equity | | $ | 217,790 | | | $ | 112,259 | | | $ | 113,564 | | | $ | 142,345 | | | $ | 250,820 | |
Condensed cash flow data:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, | | | | | | | | |
| U.S. dollars in thousands | | 2019 | | 2018 | | 2017 | | 2016 | | 2015 |
| Net cash provided by (used in) operating activities | | $ | 26,620 | | | $ | (1,865) | | | $ | (33,134) | | | $ | (107,592) | | | $ | (99,884) | |
| Net cash provided by (used in) investing activities | | (51,667) | | | (5,493) | | | 8,628 | | | 12,996 | | | (115,269) | |
| Net cash provided by (used in) financing activities | | 61,681 | | | 69,369 | | | 5,168 | | | 75,124 | | | 276,989 | |
| Effect of exchange rate differences on cash and cash equivalents | | 26 | | | 27 | | | 8 | | | 10 | | | — | |
| Net increase (decrease) in cash, cash equivalents and restricted cash | | $ | 36,660 | | | $ | 62,038 | | | $ | (19,330) | | | $ | (19,462) | | | $ | 61,836 | |
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Management’s Discussion and Analysis of Financial Condition and Results of Operations ("MD&A") is intended to provide information to assist you in better understanding and evaluating our financial condition and results of operations. We encourage you to read this MD&A in conjunction with our consolidated financial statements and the notes thereto included in Part II, Item 8 of this Annual Report on Form 10-K. This discussion contains forward-looking statements that involve risks and uncertainties. Please refer to the information under the heading "Cautionary Note Regarding Forward-Looking Statements" elsewhere in this report. References to the words "we," "our," "us," and the "Company" in this report refer to NovoCure Limited, including its consolidated subsidiaries.
Overview
We are a global oncology company with a proprietary platform technology called Tumor Treating Fields, the use of electric fields tuned to specific frequencies to disrupt solid tumor cancer cell division. Our key priorities are to drive adoption of Optune and the NovoTTF-100L, our commercial Tumor Treating Fields delivery systems and to advance clinical and product development programs intended to extend overall survival in some of the most aggressive forms of cancer.
Optune is approved by the U.S. FDA under the PMA pathway for the treatment of adult patients with newly diagnosed GBM in combination with temozolomide, a chemotherapy drug, and for adult patients with GBM following confirmed recurrence after chemotherapy as monotherapy treatment. We also have approval to market Optune for the treatment of GBM in the EU, Japan and certain other countries. NovoTTF-100L is approved by the FDA under the HDE pathway to treat MPM in combination with standard chemotherapies. We have submitted an application to our notified body for CE Certification for NovoTTF-100L in the EU.
We market Optune in the U.S., Austria, Germany, Israel, Japan, Sweden and Switzerland, which we refer to as our active markets, and we market NovoTTF-100L in the U.S. With respect to the treatment of GBM, our sales and marketing efforts are principally focused on driving adoption with both neuro-oncologists and radiation oncologists. With respect to the treatment of MPM, our commercial efforts are principally focused on generating awareness with radiation oncologists and on establishing a dialogue with third-party payers around access to NovoTTF-100L. We are expanding our commercial operations into France with an initial focus on developing key opinion leader relationships in GBM and establishing a path to reimbursement for our Products.
We believe the mechanism of action behind Tumor Treating Fields therapy may be broadly applicable to solid tumor cancers. Currently, we are conducting phase 3 pivotal trials evaluating the use of Tumor Treating Fields in brain metastases, NSCLC, pancreatic cancer and ovarian cancer. We are also conducting phase 2 pilot trials evaluating the use of Tumor Treating Fields in liver cancer and gastric cancer. We plan to initiate additional randomized trials in GBM in order to further advance the scientific evidence supporting the use of Optune in GBM and to gather additional information about Optune's optimal use. We anticipate expanding our clinical pipeline over time to study the safety and efficacy of Tumor Treating Fields for additional solid tumor indications.
The table below presents the current status of the ongoing or completed clinical trials in our pipeline and anticipated timing of final data.
Our therapy is delivered through a medical device, and we have several product development programs underway intended to improve efficacy and usability for patients. We believe we have a robust patent and intellectual property portfolio, with over 180 issued patents and numerous patent applications pending worldwide. We believe we own global commercialization rights to our Products in oncology and that we are well-positioned to extend those rights into the future as we continue to find innovative ways to improve our Products.
In 2018, we granted Zai a license to commercialize Optune in Greater China under the Zai Agreement. The Zai Agreement also establishes a development partnership intended to accelerate the development of Tumor Treating Fields in multiple solid tumor indications. For additional information, see Note 12 to the Consolidated Financial Statements.
Our collaboration with Zai could be affected by the recent COVID-19 coronavirus outbreak, as travel restrictions and work curtailment may slow down patient enrollment and regulatory actions by the Chinese government. Our supply chain includes certain raw materials and component parts sourced from China, the supply of which may also be affected by the outbreak. The outbreak did not have an effect on our results of operations in 2019, however there can be no assurance that our future results would not be adversely affected, particularly if the outbreak materially impacts our other active markets.
We view our operations and manage our business in one operating segment. Our net revenues were $351.3 million for the year ended December 31, 2019, $248.1 million for the year ended December 31, 2018 and $177.0 million for the year ended December 31, 2017. Our net loss was $7.2 million for the year ended December 31, 2019, $63.6 million for the year ended December 31, 2018 and $61.7 million for the year ended December 31, 2017. As of December 31, 2019, we had an accumulated deficit of $650.9 million. Our net loss primarily resulted from costs incurred in connection with our preclinical and clinical trial programs, costs incurred to commercialize our Products and general and administrative costs necessary to operate as a global oncology business.
Commentary on Results of Operations
Net revenues
Our revenues are primarily derived from patients using our Products in our active markets. We charge for treatment with our Products on a monthly basis. Our potential net revenues per patient are determined by our ability to secure payment, the monthly fee we collect and the number of months that the patient remains on therapy.
We also receive revenues pursuant to the Zai Agreement. For additional information regarding the Zai Agreement, see Note 12 to the Consolidated Financial Statements.
Cost of revenues
We contract with third parties to manufacture our Products. Our cost of revenues is primarily comprised of the following:
•disposable transducer arrays;
•depreciation expense for the field equipment, including the electric field generator used by patients; and
•personnel and overhead costs such as facilities, freight and depreciation of property, plant and equipment associated with managing our inventory, warehousing and order fulfillment functions.
Operating expenses
Our operating expenses consist of research, development and clinical trials, sales and marketing and general and administrative expenses. Personnel costs are a significant component for each category of operating expenses and consist of wages, benefits and bonuses. Personnel costs also include share-based compensation.
Research, development and clinical trials
Our research, development and clinical trials activity is focused on advancing Tumor Treating Fields through clinical trials across multiple solid tumor types and improving the efficacy and usability of our delivery systems. Research, development and clinical trials costs, including direct and allocated expenses, are expensed as incurred and consist primarily of the following:
•personnel costs for those employees involved in our preclinical and basic research, clinical development programs, medical affairs, product development and regulatory activities;
•costs to conduct research, product development and clinical trial activity through agreements with contract research organizations and other third parties;
•manufacturing expenses associated with our Products, including durable components and disposable arrays, utilized in clinical trials and other research;
•costs associated with medical grants, publications, presentations and investigator-sponsored trials;
•professional fees related to regulatory approvals and conformity assessment procedures; and
•facilities, depreciation and other allocated expenses, which include direct and allocated expenses for rent and maintenance of facilities, depreciation of leasehold improvements and equipment and laboratory and other supplies.
The following table summarizes our research, development and clinical trial expenses by program for the years ended December 31, 2019, 2018 and 2017:
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| U.S. dollars in thousands | | 2019 | | 2018 | | 2017 |
| Preclinical and basic research | | $ | 6,874 | | | | $ | 4,132 | | | | $ | 3,339 | |
| Clinical development programs: | | | | | | | | | | | |
| METIS | | 4,699 | | | | 3,640 | | | | 2,614 | |
| LUNAR | | 7,243 | | | | 4,326 | | | | 1,929 | |
| PANOVA - 3 | | 5,368 | | | | 3,076 | | | | 495 | |
| INNOVATE - 3 | | 4,822 | | | | 510 | | | | — | |
| Additional clinical development programs | | 2,153 | | | | 1,941 | | | | 2,170 | |
| Personnel and indirect clinical development costs | | 10,856 | | | | 8,075 | | | | 7,219 | |
| Product development | | 4,944 | | | | 3,367 | | | | 3,277 | |
| Medical affairs | | 10,867 | | | | 7,714 | | | | 6,429 | |
| Other research, development and clinical trial costs (1) | | 21,177 | | | | 13,793 | | | | 10,631 | |
| Research, development and clinical trials | | $ | 79,003 | | | | $ | 50,574 | | | | $ | 38,103 | |
(1) Other research, development and clinical trial costs include regulatory affairs, quality assurance, intellectual property, product safety, allocated facilities, other overhead costs and share-based compensation.
We expect our research, development and clinical trial expenses to increase as we continue to advance clinical and product development programs intended to extend overall survival in multiple solid tumor cancers.
Sales and marketing
Sales and marketing expenses consist primarily of personnel costs, travel, marketing and promotional activities, commercial shipping and facilities costs. Over the next few years, we expect to continue to make significant expenditures associated with selling and marketing our Products, primarily in connection with continued commercialization in the United States, EU and Japan for the treatment of our approved indications.
General and administrative
General and administrative expenses consist primarily of personnel, professional fees and facilities costs. General and administrative personnel costs include our executive, finance, human resources, information technology and legal functions. These costs also include our contributions to support industry and patient groups. Our professional fees consist primarily of accounting, information technology, legal and other consulting costs. We expect that general and administrative expenses will increase to support our growth. In addition, we incur significant legal and accounting costs related to compliance with SEC rules and regulations, including the costs of achieving and maintaining compliance with Section 404 of the Sarbanes-Oxley Act of 2002 and compliance with rules of the NASDAQ Stock Market, as well as insurance, investor relations and other costs associated with being a public company.
Financial expenses, net
Financial expenses, net primarily consists of credit facility interest expense and related debt issuance costs, interest income from cash balances and short-term investments and gains (losses) from foreign currency transactions. Our reporting currency is the U.S. dollar. We have historically held substantially all of our cash balances in U.S. dollar denominated accounts to minimize the risk of translational currency exposure.
Critical accounting policies and estimates
In accordance with U.S. GAAP, in preparing our financial statements we must make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of net revenues and expenses during the reporting period. We develop and periodically change these estimates and assumptions based on historical experience and on various other factors that we believe are reasonable under the circumstances. Actual results may differ from these estimates.
The critical accounting policies requiring estimates, assumptions and judgments that we believe have the most significant impact on our consolidated financial statements are described below.
Revenue recognition
In May 2014, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2014-09, Revenue from Contracts with Customers (Topic 606) (ASU 2014-09), an updated standard on revenue recognition and issued subsequent amendments to the initial guidance in March 2016, April 2016, May 2016 and December 2016 within ASU 2016-08, 2016-10, 2016-12 and 2016-20, respectively. The Company adopted the standard effective January 1, 2018 using the modified retrospective method for all contracts. The reported results for 2018 and thereafter reflect the application of Accounting Standards Codification ("ASC") 606 guidance while the reported results for 2017 were prepared under the guidance of ASC 605, Revenue Recognition (ASC 605). The amount of revenue recognized in 2018 and 2019 reflects the consideration to which the Company expects to be entitled to receive in exchange for our Products. The adoption of this standard did not have a material impact on our financial position, results of operations or cash flows. For additional information, see Note 2(l) to the Consolidated Financial Statements.
We also receive revenues pursuant to the Zai Agreement. For additional information regarding the Zai Agreement, see Note 12 to the Consolidated Financial Statements.
Share-based compensation
Under the FASB's ASC 718, Compensation-Stock Compensation, we measure and recognize compensation expense for share options granted to our employees and directors and for our ESPP based on the fair value of the awards on the date of grant. The fair value of share options is estimated at the date of grant using the Black-Scholes option pricing model and for market condition awards we also use the Monte-Carlo simulation model. Both models requires management to apply judgment and make estimates, including:
•the expected term of the stock option award, which we calculate using the simplified method, in accordance with ASC No.718-10-S99-1 (SAB No. 110) as we have insufficient historical information regarding our stock options to provide a basis for an estimate;
•the expected share price volatility of our underlying ordinary shares, which we estimate based on the historical volatility of a representative group of publicly traded biopharmaceutical and medical technology companies with similar characteristics to us for a period matching the expected term assumption when there is not sufficient historical information for our ordinary shares;
•the risk-free interest rate, which we base on the yield curve of U.S. Treasury securities with periods commensurate with the expected term of the options being valued; and
•the expected dividend yield, which we estimate to be zero based on the fact that we have never paid cash dividends and have no present intention to pay cash dividends.
For information about our ESPP, see Note 14(b) to the Consolidated Financial Statements.
We recognize share-based compensation costs only for those shares expected to vest over the requisite vesting period of the award, which is generally the option vesting term of four years, using the accelerated method.
The table below summarizes the assumptions that were used to estimate the fair value of the options granted to employees during the periods presented:
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| Expected term (years) | | 5.50-6.00 | | 5.50-6.25 | | 5.50-6.25 |
| Expected volatility | | 55%-61% | | | 52%-55% | | | 57%-59% | |
| Risk-free interest rate | | 1.73%-2.40% | | | 2.70%-2.99% | | | 1.97%-2.23% | |
| Dividend yield | | 0.00% | | | 0.00% | | | 0.00% | |
If any of the assumptions used in the Black-Scholes option pricing model change significantly, share-based compensation for future awards may differ materially from the awards granted previously.
So long as our ordinary shares are publicly traded in a liquid market, we will rely on the daily trading price of our ordinary shares when we estimate the fair value of options granted.
We incurred share-based compensation expense of $52.4 million, $39.8 million and $27.1 million during the years ended December 31, 2019, 2018 and 2017, respectively. As of December 31, 2019, we have unrecognized compensation expense of $62.5 million, which is expected to be recognized over a weighted average period of approximately 2.51 years. We expect to continue to grant equity awards in the future, and to the extent that we do, our recognized share-based compensation expense will likely increase.
Long-lived assets
Property and equipment and field equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful life of the relevant asset. We make estimates of the useful life of our property and equipment and field equipment, based on similar assets purchased in the past and our historical experience with such similar assets, in order to determine the depreciation expense to be recorded for each reporting period.
Our field equipment consists of equipment being utilized under rental agreements accounted for on a monthly basis as an operating lease, as well as service pool equipment. Service pool equipment is equipment owned and maintained by us that is swapped for equipment that needs repair or maintenance by us while being used by a patient. We record a provision for any excess, lost or damaged equipment when warranted based on an assessment of the equipment.
We assess impairment whenever events or changes in circumstances indicate that the carrying amount of the asset is impaired or the estimated useful life is no longer appropriate. Circumstances such as changes in technology or in the way an asset is being used may trigger an impairment review. For additional information, see Notes 2(i) and 2(j) to the Consolidated Financial Statements.
Inventories
Inventories are stated at the lower of cost or net realizable value. We regularly evaluate the ability to realize the value of inventory. If the inventories are deemed damaged, if actual demand for our delivery systems declines, or if market conditions are less favorable than those projected, inventory write-offs may be required. For additional information, see Note 2(g) to the Consolidated Financial Statements.
Income taxes
As part of the process of preparing our consolidated financial statements, we are required to calculate our income taxes based on taxable income by jurisdiction. We make certain estimates and judgments in determining our income taxes, including assessment of our uncertain tax positions, for financial statement purposes. Significant changes to these estimates may result in an increase or decrease to our tax provision in the subsequent period when such a change in estimate occurs.
Uncertain tax positions are based on estimates and assumptions that have been deemed reasonable by management. Our estimates of unrecognized tax benefits and potential tax benefits may not be representative of actual outcomes.
For additional information, see Note 13 to the Consolidated Financial Statements.
Recently issued accounting pronouncements
For a description of our recently issued accounting pronouncements, see Note 2(x) to the Consolidated Financial Statements.
Results of operations
The following discussion provides an analysis of our results of operations and reasons for material changes therein for 2019 as compared to 2018. See "Results of Operations" in Part II, Item 7, Management's Discussion and
Analysis of Financial Condition and Results of Operations in the Company's 2018 Annual Report on Form 10-K, filed with the SEC on February 28, 2019, for an analysis of the 2018 results as compared to 2017.
The following table sets forth our consolidated statements of operations data:
| | | | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, | | | | |
| U.S. dollars in thousands, except share and per share data | | 2019 | | 2018 | | 2017 |
| Net revenues | | $ | 351,318 | | | $ | 248,069 | | | $ | 177,026 | |
| Cost of revenues | | 88,606 | | | 80,048 | | | 55,610 | |
| Gross profit | | 262,712 | | | 168,021 | | | 121,417 | |
| Operating costs and expenses: | | | | | | |
| Research, development and clinical trials | | 79,003 | | | 50,574 | | | 38,103 | |
| Sales and marketing | | 96,675 | | | 77,663 | | | 63,528 | |
| General and administrative | | 87,948 | | | 73,456 | | | 59,114 | |
| Total operating costs and expenses | | 263,626 | | | 201,693 | | | 160,746 | |
| Operating income (loss) | | (914) | | | (33,672) | | | (39,328) | |
| Financial expenses (income), net | | 7,910 | | | 12,270 | | | 9,169 | |
| Income (loss) before income tax | | (8,824) | | | (45,942) | | | (48,497) | |
| Income tax | | (1,594) | | | 17,617 | | | 13,165 | |
| Net income (loss) | | $ | (7,230) | | | $ | (63,559) | | | $ | (61,662) | |
| | | | | | |
| Basic and diluted net income (loss) per ordinary share | | $ | (0.07) | | | $ | (0.69) | | | $ | (0.70) | |
| | | | | | |
| Weighted average number of ordinary shares used in computing basic and diluted net income (loss) per ordinary share | | 97,237,549 | | | 91,828,043 | | | 88,546,719 | |
The following table details the share-based compensation expense included in costs and expenses:
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| U.S. dollars in thousands | | 2019 | | 2018 | | 2017 |
| Cost of revenues | | $ | 2,231 | | | $ | 1,261 | | | $ | 467 | |
| Research, development and clinical trials | | 7,570 | | | 4,709 | | | 3,587 | |
| Sales and marketing | | 11,897 | | | 7,393 | | | 3,784 | |
| General and administrative | | 30,718 | | | 26,483 | | | 19,278 | |
| Total share-based compensation expense | | $ | 52,416 | | | $ | 39,846 | | | $ | 27,116 | |
Key performance indicators
We believe certain commercial operating statistics are useful to investors in evaluating our commercial business as they help investors evaluate and compare the adoption of our Products from period to period. The number of active patients on therapy is our principal revenue driver. An "active patient" is a patient who is receiving treatment under a commercial prescription order as of the measurement date, including patients who may be on a temporary break from treatment and who plan to resume treatment in less than 60 days. Prescriptions are a leading indicator of demand. A "prescription received" is a commercial order for Optune or NovoTTF-100L that is received from a physician certified to treat patients with our Products for a patient not previously on Optune or NovoTTF-100L. Orders to renew or extend treatment are not included in this total.
The following table includes certain commercial operating statistics for and as of the end of the periods presented.
| | | | | | | | | | | | | | | | | |
| | December 31, | | | | |
| Operating statistics | 2019 | | 2018 | | 2017 |
| Active patients at period end | | | | | |
| United States | 1,952 | | | 1,637 | | | 1,320 | |
| EMEA: | | | | | |
| Germany | 493 | | | | 439 | | | | 356 | |
| Other EMEA | 272 | | | 215 | | | 156 | |
| Japan | 192 | | | 92 | | | 2 | |
| 2,909 | | | 2,383 | | | 1,834 | |
| | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| Prescriptions received in period | | | | | |
| United States | 3,833 | | | 3,741 | | | 3,102 | |
| EMEA: | | | | | | | | |
| Germany | 872 | | | 858 | | | 731 | |
| Other EMEA | 360 | | | 299 | | | 280 | |
| Japan | 306 | | | 162 | | | 6 | |
| 5,371 | | | 5,060 | | | 4,119 | |
In the U.S., there were three active MPM patients on therapy as of December 31, 2019 and four MPM prescriptions were received in the year ended December 31, 2019.
Year ended December 31, 2019 compared to year ended December 31, 2018
The following discussion provides an analysis of our results of operations and reasons for material changes therein for 2019 as compared to 2018. See "Results of Operations" in Part II, Item 7, Management's Discussion and Analysis of Financial Condition and Results of Operations in the Company's 2018 Annual Report on Form 10-K, filed with the SEC on February 28, 2019 (the "2018 Annual Report"), for analysis of the 2018 results as compared to 2017.
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | | | |
| 2019 | | 2018 | | Change | | % Change |
| Net revenues | $ | 351,318 | | | $ | 248,069 | | | $ | 103,249 | | | 42 | % |
Net revenues. Net revenues increased by $103.2 million, or 42%, to $351.3 million for the year ended December 31, 2019 from $248.1 million for the year ended December 31, 2018. This was primarily due to an increase of 526 active patients in our active markets, representing 22% growth, and an increase in the net revenues per active patient per month. The increase in net revenues per active patient per month was primarily driven by improving reimbursement approval rates in the U.S. and EMEA.
For the year ended December 31, 2019, we recognized $5.5 million in net revenues from Medicare beneficiaries billed under the newly defined coverage policy, which became effective on September 1, 2019. We continue to work through typical administrative ramp-up issues and expect our net revenues from Medicare beneficiaries to continue to increase as we gain experience processing Medicare claims.
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | | | |
| 2019 | | 2018 | | Change | | % Change |
| Cost of revenues | $ | 88,606 | | | $ | 80,048 | | | $ | 8,558 | | | 11 | % |
Cost of revenues. Our cost of revenues increased by $8.6 million, or 11%, to $88.6 million for the year ended December 31, 2019 from $80.0 million for the year ended December 31, 2018. The increase in cost of revenues was primarily due to the cost of shipping transducer arrays to a higher volume of commercial patients. Efficiency initiatives and scale drove a 12% decrease in the cost of revenues per active patient per month from $3,133 for the year ended December 31, 2018 to $2,755 for the year ended December 31 2019. Cost of revenues per active patient is calculated by dividing the cost of revenues for the quarter by the average of the active patients at the end of the prior quarter and the end of the current quarter. This quarterly figure is then divided by three to estimate the monthly cost of revenues per active patient. Gross margin was 75% for the year ended December 31, 2019 and 68% for the year ended December 31, 2018.
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | | | |
| 2019 | | 2018 | | Change | | % Change |
| Operating expenses: | | | | | | | |
| Research, development and clinical trials | $ | 79,003 | | | $ | 50,574 | | | $ | 28,429 | | | 56 | % |
| Sales and marketing | 96,675 | | | 77,663 | | | 19,012 | | | 24 | % |
| General and administrative | 87,948 | | | 73,456 | | | 14,492 | | | 20 | % |
| Total operating expenses | $ | 263,626 | | | $ | 201,693 | | | $ | 61,934 | | | 31 | % |
| | | | | | | |
Research, development and clinical trials expenses. Research, development and clinical trials expenses increased by $28.4 million, or 56%, to $79.0 million for the year ended December 31, 2019 from $50.6 million for the year ended December 31, 2018. The change is primarily due to a $13.6 million increase in clinical development expenses driven by our four ongoing phase 3 pivotal trials, a $2.7 million increase in preclinical and basic research expenses and a $12.1 million increase in costs associated with medical affairs, product development and other research, development and clinical trial expenses.
Sales and marketing expenses. Sales and marketing expenses increased by $19.0 million, or 24%, to $96.7 million for the year ended December 31, 2019 from $77.7 million for the year ended December 31, 2018. The change was primarily due to a $5.0 million increase in marketing expenses related to the launch of NovoTTF-100L and a $8.5 million increase in our personnel costs to support our growing commercial business.
General and administrative expenses. General and administrative expenses increased by $14.5 million, or 20%, to $87.9 million for the year ended December 31, 2019 from $73.5 million for the year ended December 31, 2018. The change was primarily due to an increase in personnel costs and an increase in professional services.
| | | | | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | | | |
| | 2019 | | 2018 | | Change | | % Change |
| Financial expenses (income), net | $ | 7,910 | | | $ | 12,270 | | | $ | (4,360) | | | (36) | % |
Financial expenses, net. Financial expenses, net decreased by $4.4 million, or 36%, to $7.9 million for the year ended December 31, 2019 from $12.3 million for the year ended December 31, 2018. The change was primarily due to a decrease in amortization costs. In 2018, accelerated amortization costs were triggered by the repayment of our 2015 term loan credit facility.
| | | | | | | | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | | | |
| 2019 | | 2018 | | Change | | % Change |
| Income tax | $ | (1,594) | | | $ | 17,617 | | | $ | (19,211) | | | (109) | % |
Income taxes. Income taxes decreased by $19.2 million, or 109%, resulting in a tax benefit of $1.6 million for the year ended December 31, 2019 compared to a $17.6 million expense for the year ended December 31, 2018. The change was primarily a result of amortization of intellectual property rights, research and development credits claimed in the U.S. and the tax effect of exercises of previously granted share-based compensation in the U.S.
Non-GAAP financial measures
We also measure our performance based upon a non-U.S. GAAP measurement of earnings before interest, taxes, depreciation, amortization and shared-based compensation ("Adjusted EBITDA"). We believe Adjusted EBITDA is useful to investors in evaluating our operating performance because it helps investors evaluate and compare the results of our operations from period to period by removing the impact of earnings attributable to our capital structure, tax rate and material non-cash items, specifically share-based compensation.
We calculate Adjusted EBITDA as operating income before financial expenses and income taxes, net of depreciation, amortization and share-based compensation. The following table reconciles net loss (which is the most directly comparable U.S. GAAP operating performance measure) to Adjusted EBITDA.
| | | | | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| Net income (loss) | | $ | (7,230) | | | | $ | (63,559) | | | | $ | (61,661) | |
| Add: Income tax | | (1,594) | | | | 17,617 | | | | 13,165 | |
| Add: Financial income (expenses), net | | 7,910 | | | | 12,270 | | | | 9,169 | |
| Add: Depreciation and amortization | | 8,460 | | | | 9,006 | | | | 7,677 | |
| EBITDA | | $ | 7,546 | | | | $ | (24,666) | | | | $ | (31,650) | |
| Add: Share-based compensation | | 52,416 | | | | 39,846 | | | | 27,116 | |
| Adjusted EBITDA | | $ | 59,962 | | | | $ | 15,180 | | | | $ | (4,534) | |
Adjusted EBITDA increased by $44.8 million, or 295%, to $60.0 million for the year ended December 31, 2019 from $15.2 million for the year ended December 31, 2018. This improvement in fundamental financial performance was driven by net revenue growth coupled with an ongoing commitment to disciplined management of expenses.
Liquidity and capital resources
We have incurred significant losses and cumulative negative cash flows from operations since our founding in 2000. As of December 31, 2019, we had an accumulated deficit of $650.9 million. To date, we have primarily financed our operations through the issuance and sale of equity and the proceeds from long-term loans.
At December 31, 2019, we had $177.3 million in cash and cash equivalents and $148.8 million in short-term investments. At December 31, 2019, our cash, cash equivalents and short-term investments totaled $326.1 million, an increase of $80.2 million compared to $245.9 million at December 31, 2018. The increase in our cash, cash equivalents and short-term investments was primarily due to net cash provided by operating activities and the exercise of options.
We believe our cash, cash equivalents and short-term investments as of December 31, 2019 are sufficient for our operations for at least the next 12 months based on our existing business plan and our ability to control the timing of significant expense commitments. We expect that our research, development and clinical trials expenses, sales and marketing expenses and general and administrative expenses will continue to increase over the next several years and may outpace our gross profit. As a result, we may need to raise additional capital to fund our operations.
The following summary of our cash flows for the periods indicated has been derived from our consolidated financial statements, which are included elsewhere in this Annual Report:
| | | | | | | | | | | | | | | | | | | | |
| | | Year ended December 31, | | | | |
| U.S. dollars in thousands | | 2019 | | 2018 | | 2017 |
| Net cash provided by (used in) operating activities | | $ | 26,620 | | | $ | (1,865) | | | $ | (33,134) | |
| Net cash provided by (used in) investing activities | | (51,667) | | | (5,493) | | | 8,628 | |
| Net cash provided by financing activities | | 61,681 | | | 69,369 | | | 5,168 | |
| Effect of exchange rate changes on cash and cash equivalents | | 26 | | | 27 | | | 8 | |
| Net increase (decrease) in cash, cash equivalents and restricted cash | | $ | 36,660 | | | $ | 62,038 | | | $ | (19,330) | |
Operating activities
Net cash used in operating activities primarily represents our net loss for the periods presented. Adjustments to net loss for non-cash items include share-based compensation, depreciation, amortization and asset write-downs. Operating cash flows are also impacted by changes in operating assets and liabilities, principally trade payables, deferred revenues, other payables, prepaid expenses and trade receivables.
Net cash provided by operating activities was $26.6 million for the year ended December 31, 2019 compared to $1.9 million used in the year ended December 31, 2018. Gross profit increased by $94.7 million 2019 versus 2018, fully funding incremental investments of $28.4 million in research and development and $33.5 million in sales, marketing, general and administrative expenses. The year over year increase in cash provided by in operating activities was driven primarily by a decrease in the reported net loss and an increase in the amount of non-cash shared-based compensation included in the reported net loss, offset by an increase in working capital.
For additional information, see Note 12 to the Consolidated Financial Statements.
Investing activities
Our investing activities consist primarily of capital expenditures to purchase property and equipment and field equipment, as well as investments in and redemptions of our short-term investments.
Net cash used in investing activities was $51.7 million for the year ended December 31, 2019 compared to net cash used in investing activities of $5.5 million for the year ended December 31, 2018. The year-over-year increase in cash used in investing activities is primary related to the purchase of an additional $45.0 million in short-term investments.
Financing activities
To date, our primary financing activities have been the sale of equity and the proceeds from long-term loans.
Net cash provided by financing activities was $61.7 million for the year ended December 31, 2019 compared to $69.4 million for the year ended December 31, 2018. The year-over-year decrease in cash provided by financing activities was primarily related to an increase in the exercise of options offset by the 2018 increase in the principal amount of our term loan credit facility.
Term loan credit facility
As of December 31, 2019, our material outstanding indebtedness consisted of $150.0 million of principal outstanding under our term loan credit facility (the "2018 Credit Facility"). Interest on the outstanding loan is 9% annually, payable quarterly in arrears. The 2018 Credit Facility will mature on February 7, 2023, at which time any unpaid principal and accrued unpaid interest in respect of the loan will be due and payable. We may prepay amounts outstanding under the 2018 Credit Facility in full at any time. Any prepayment (whether permitted or mandatory) is subject to a prepayment premium and/or make-whole payment. The pre-payment fee if we prepay outstanding loan amounts prior to February 7, 2021 is 2.0% and is 1.0% if made after the February 7, 2021 but prior to February 7, 2022. If we prepay outstanding loan amounts prior to August 7, 2020, we must pay a make-whole amount equal to the amount of interest that would have accrued on the amount of all principal we prepaid from the date of such prepayment through February 7, 2021. We must prepay the 2018 Credit Facility (i) in full or in part upon the entry into certain licensing arrangements and (ii) in full in the event of a change of control.
All obligations under the 2018 Credit Facility are guaranteed by certain of our domestic direct and indirect subsidiaries. In addition, the obligations under the 2018 Credit Facility were secured by a first-priority security interest in substantially all of the property and assets of, as well as the equity interests owned by, us and the other guarantors.
The 2018 Credit Facility contains customary covenants. As of December 31, 2019, we were in compliance with such covenants.
For additional information, see Note 10 to the Consolidated Financial Statements.
Contractual obligations and commitments
The following summarizes our significant contractual obligations and commitments as of December 31, 2019:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | December 31, | | | | | | | | | | | | |
| Contractual Obligations: | | 2020 | | 2021 | | 2022 | | 2023 | | 2024 | | After | | Total |
| | | (in thousands) | | | | | | | | | | | | |
| Operating leases and other loans | | $ | 5,046 | | | $ | 5,030 | | | $ | 3,823 | | | $ | 2,524 | | | $ | 1,934 | | | $ | 4,081 | | | $ | 22,438 | |
| Purchase obligations (1) | | 27,206 | | | 7,325 | | | 243 | | | — | | | — | | | 12,119 | | | 46,894 | |
Term loan credit facility (2) | | — | | | — | | | — | | | — | | | 150,000 | | | — | | | 150,000 | |
Term loan credit facility interest (2) | | $ | 13,688 | | | $ | 13,725 | | | $ | 13,688 | | | $ | 13,688 | | | $ | 1,388 | | | $ | — | | | $ | 56,177 | |
_____________________________________________
(1)Our purchase obligations include commitments with certain of our suppliers, primarily for the purchase of Product components along with other commitments to purchase goods or services in the normal course of business. We make such commitments through a combination of purchase orders, supplier contracts, and open orders based on projected demand information. These amounts include approximately $39.1 million of commitments with three major suppliers.
(2)On February 7, 2018, the Company and certain of its subsidiaries entered into a Loan and Security Agreement ("2018 Loan Agreement") with a lender pursuant to which such lender made a term loan to the Company in the principal amount of $150.0 million (the "2018 Credit Facility"). The term loan has a fixed per annum interest rate of 9.0%. For additional information, see Note 10 to the Consolidated Financial Statements.
Except as described above, there were no material changes in our commitments under contractual obligations in the year ended December 31, 2019.
The total amount of unrecognized tax expenses for uncertain tax positions was $0.1 million and $0.1 million at December 31, 2019 and December 31, 2018, respectively. Payment of these obligations would result from settlements with taxing authorities. Due to the difficulty in determining the timing of settlements, these obligations are not included in the above table. We do not expect a significant tax payment related to these obligations within the next year.
We also have employment agreements with certain employees that require the funding of a specific level of payments if certain events, such as a change in control or termination without cause, occur. In the course of normal business operations, we also have agreements with contract service providers to assist in the performance of our research and development (including clinical trials) and manufacturing activities. We could also enter into additional collaborative research, contract research, manufacturing and supplier agreements in the future, which may require up-front payments and even long-term commitments of cash.
Off-balance sheet arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements as defined under SEC rules.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are exposed to market risks in the ordinary course of our business. Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our market risk exposure is primarily a result of fluctuations in interest rates and foreign currency exchange rates. We do not hold or issue financial instruments for trading purposes. There were no material quantitative changes in our market risk exposures between the year ended December 31, 2019 and the year ended December 31, 2018.
Interest rate sensitivity
Our exposure to market risk for changes in interest rates relates primarily to our investment portfolio. Our cash, cash equivalents and short-term investment accounts as of December 31, 2019 totaled $326.1 million and consist of cash, cash equivalents and short-term investments with maturities of less than one year from the date of purchase. Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of the interest rates in the United States. However, because of the short-term nature of the instruments in our portfolio and our intent to hold instruments to maturity, a 10% change in market interest rates would not be expected to have a material impact on our financial condition or our results of operations.
Foreign currency exchange risk
Our consolidated results of operations and cash flow are subject to fluctuations due to changes in foreign currency exchange rates. All of our revenues are generated in the local currency for commercial markets. Our expenses are generally denominated in the currencies in which our operations are located, which is primarily in the United States, Switzerland, Germany, Israel and Japan. Our consolidated results of operations and cash flows are, therefore, subject to fluctuations due to changes in foreign currency exchange rates and may be adversely affected in the future due to changes in foreign exchange rates. The effect of a hypothetical 10% change in foreign currency exchange rates applicable to our business would not have a material impact on our historical consolidated financial statements. We do not hedge our foreign currency exchange risk.
ITEM 8. FINANCIAL STATEMENTS
NovoCure Limited
Index to consolidated financial statements
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of NovoCure Limited
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of NovoCure Limited and subsidiaries (the "Company") as of December 31, 2019 and 2018, the related consolidated statements of operations, comprehensive loss, changes in shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2019 and the related notes (collectively referred to as the "consolidated financial statements"). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) ("PCAOB"), the Company's internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) and our report dated February 27, 2020 expressed an unqualified opinion thereon.
Adoption of ASU No. 2014-09
As discussed in Note 2 to the consolidated financial statements, the Company changed its method of accounting for revenue in 2018 due to the adoption of Accounting Standards Update (ASU) No. 2014-09, Revenue from Contracts with Customers (Topic 606), and the related amendments.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which it relates.
Revenue recognition – Measuring variable consideration
| | | | | |
| Description of the Matter | As per the Company's consolidated statements of operations, the net revenues recognized during the fiscal year of 2019 amounted to a sum of $351.3 million, which included variable consideration estimates. As described in Note 2 to the consolidated financial statements, the transaction price is determined based on the consideration to which the Company will be entitled in exchange for providing Optune solution. The company provides certain patients with implicit price concessions, which results in variable consideration. According to historical records, the Company expects to receive, in aggregate for a given portfolio, less than the gross revenue net of explicit discounts. Auditing the Company's measurement of variable consideration involved challenging judgment because the calculation involves uncertainty and subjective management assumptions about estimates of expected price concessions. The implicit discount includes both an estimate of claims that will pay at an amount less than billed and an estimate of claims that will not pay within a given time horizon. The implicit discount adjustments to the transaction price are due to concessions, not collectability concerns driven by payer credit risk. |
| How We Addressed the Matter in Our Audit | We obtained an understanding, evaluated the design and tested the operating effectiveness of internal controls over the Company's process to calculate variable consideration, including the underlying assumptions about estimates of expected price concessions. Our audit procedures included, among others, evaluating the methodology used, analyzing the significant assumptions discussed above, and testing the accuracy and completeness of the underlying data used in management's calculation. This included testing inputs of the calculation by reconciliation of the data between the various information systems, performing independent recalculation of the Company's estimate and evaluating the historical accuracy of management's estimates by comparing such estimates to subsequent actual results. |
KOST FORER GABBAY & KASIERER
A Member of Ernst & Young Global
We have served as the Company‘s auditor since 2003.
Tel-Aviv, Israel
February 27, 2020
Report of Independent Registered Public Accounting Firm
To the board of directors and shareholders of NovoCure Limited
Opinion on Internal Control over Financial Reporting
We have audited NovoCure Limited and subsidiaries internal control over financial reporting as of December 31, 2019, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework) (the "COSO Criteria"). In our opinion, NovoCure Limited and subsidiaries (the "Company") maintained, in all material respects, effective internal control over financial reporting as of December 31, 2019, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (PCAOB), the consolidated balance sheets of the Company as of December 31, 2019 and 2018, the related consolidated statements of operations, comprehensive loss, changes in shareholders’ equity and cash flows for each of the three years in the period ended December 31, 2019 and the related notes, and our report dated February 27, 2020 expressed an unqualified opinion thereon.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects.
Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
KOST FORER GABBAY & KASIERER
A Member of Ernst & Young Global
Tel-Aviv, Israel
February 27, 2020
NovoCure Limited and subsidiaries
Consolidated balance sheets
| | | | | | | | | | | |
| December 31, | | |
| U.S. dollars in thousands | 2019 | | 2018 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 177,321 | | | $ | 140,622 | |
| Short-term investments | 148,769 | | | 105,256 | |
| Restricted cash | 2,095 | | | 2,134 | |
| Trade receivables, net | 58,859 | | | 36,523 | |
| Receivables and prepaid expenses | 29,202 | | | 14,279 | |
| Inventories | 23,701 | | | 22,555 | |
| Total current assets | 439,947 | | | 321,369 | |
| Long-term assets: | | | |
| Property and equipment, net | 9,342 | | | 8,442 | |
| Field equipment, net | 7,684 | | | 6,924 | |
| Right-of-use assets, net | 17,571 | | | — | |
| Other long-term assets | 4,904 | | | 3,058 | |
| Total long-term assets | 39,501 | | | 18,424 | |
| Total assets | $ | 479,448 | | | $ | 339,793 | |
The accompanying notes are an integral part of the consolidated financial statements.
NovoCure Limited and subsidiaries
Consolidated balance sheets
| | | | | | | | | | | |
| December 31, | | |
| U.S. dollars in thousands, except share and per share data | 2019 | | 2018 |
| Liabilities and shareholders’ equity | | | |
| Current liabilities: | | | |
| Trade payables | $ | 36,925 | | | $ | 26,708 | |
| Other payables and accrued expenses | 49,386 | | | 37,852 | |
| Total current liabilities | 86,311 | | | 64,560 | |
| Long-term liabilities: | | | |
| Long-term loan, net of discount and issuance costs | 149,424 | | | 149,268 | |
| Deferred revenues | 7,807 | | | 9,929 | |
| Long term leases | 14,140 | | | — | |
| Employee benefit liabilities | 3,754 | | | 2,683 | |
| Other long-term liabilities | 222 | | | 1,094 | |
| Total long-term liabilities | 175,347 | | | 162,974 | |
| Total liabilities | 261,658 | | | 227,534 | |
| Commitments and contingencies | | | |
| Shareholders’ equity: | | | |
| Share capital - | | | |
Ordinary shares - No par value, Unlimited shares authorized; Issued and outstanding: 99,528,435 shares and 93,254,185 shares at December 31, 2019 and December 31, 2018 respectively; | — | | | — | |
| Additional paid-in capital | 871,442 | | | 757,314 | |
| Accumulated other comprehensive loss | (2,767) | | | (1,400) | |
| Accumulated deficit | (650,885) | | | (643,655) | |
| Total shareholders’ equity | 217,790 | | | 112,259 | |
| Total liabilities and shareholders’ equity | $ | 479,448 | | | $ | 339,793 | |
The accompanying notes are an integral part of the consolidated financial statements.
NovoCure Limited and subsidiaries
Consolidated statements of operations
| | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | |
| U.S. dollars in thousands, except share and per share data | 2019 | | 2018 | | 2017 |
| Net revenues | $ | 351,318 | | | $ | 248,069 | | | $ | 177,026 | |
| Cost of revenues | 88,606 | | | 80,048 | | | 55,610 | |
| Gross profit | 262,712 | | | 168,021 | | | 121,417 | |
| | | | | |
| Operating costs and expenses: | | | | | |
| Research, development and clinical trials | 79,003 | | | 50,574 | | | 38,103 | |
| Sales and marketing | 96,675 | | | 77,663 | | | 63,528 | |
| General and administrative | 87,948 | | | 73,456 | | | 59,114 | |
| Total operating costs and expenses | 263,626 | | | 201,693 | | | 160,745 | |
| | | | | |
| Operating income (loss) | (914) | | | (33,672) | | | (39,328) | |
| Financial expenses (income), net | 7,910 | | | 12,270 | | | 9,169 | |
| | | | | |
| Income (loss) before income taxes | (8,824) | | | (45,942) | | | (48,497) | |
| Income tax | (1,594) | | | 17,617 | | | 13,165 | |
| Net income (loss) | $ | (7,230) | | | $ | (63,559) | | | $ | (61,662) | |
| | | | | |
| Basic and diluted net income (loss) per ordinary share | $ | (0.07) | | | $ | (0.69) | | | $ | (0.70) | |
Weighted average number of ordinary shares used in computing basic and diluted net income (loss) per share | 97,237,549 | | | 91,828,043 | | | 88,546,719 | |
The accompanying notes are an integral part of the consolidated financial statements.
NovoCure Limited and subsidiaries
Consolidated statements of comprehensive loss
| | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | |
| U.S. dollars in thousands | 2019 | | 2018 | | 2017 |
| Net income (loss) | $ | (7,230) | | | $ | (63,559) | | | $ | (61,662) | |
| Other comprehensive income (loss), net of tax : | | | | | |
| Change in foreign currency translation adjustments | (304) | | | 27 | | | 8 | |
| Pension benefit plan | (1,063) | | | (84) | | | 532 | |
| Total comprehensive income (loss) | $ | (8,597) | | | $ | (63,616) | | | $ | (61,122) | |
The accompanying notes are an integral part of the consolidated financial statements.
NovoCure Limited and subsidiaries
Statements of changes in shareholders’ equity
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Ordinary shares | | | | | | | | |
| U.S. dollars in thousands, except share data | | (Shares) | | Additional paid-in capital | | Accumulated other comprehensive income (loss) | | Retained earnings (accumulated deficit) | | Total shareholders’ equity |
| Balance as of December 31, 2016 | | 87,066,446 | | | $ | 664,154 | | | $ | (1,883) | | | $ | (519,926) | | | $ | 142,345 | |
| | | | | | | | | | |
| Share-based compensation to employees | | — | | | 27,116 | | | — | | | — | | | 27,116 | |
| Exercise of options and warrants | | 2,244,153 | | | 3,685 | | | — | | | — | | | 3,685 | |
| Issuance of shares in connection with employee stock purchase plan | | 167,433 | | | 1,540 | | | — | | | — | | | 1,540 | |
| Tax benefit from share-based award activity | | — | | | 670 | | | — | | | (670) | | | — | |
Other comprehensive income (loss) net of tax benefit of $68 | | — | | | — | | | 540 | | | — | | | 540 | |
| Net income (loss) | | — | | | — | | | — | | | (61,662) | | | (61,662) | |
| Balance as of December 31, 2017 | | 89,478,032 | | | 697,165 | | | (1,343) | | | (582,258) | | | 113,564 | |
| | | | | | | | | | |
| Share-based compensation to employees | | — | | | 39,846 | | | — | | | — | | | 39,846 | |
| Exercise of options and warrants | | 3,688,781 | | | 18,468 | | | — | | | — | | | 18,468 | |
| Issuance of shares in connection with employee stock purchase plan | | 87,372 | | | 1,835 | | | — | | | — | | | 1,835 | |
| Cumulative effect adjustment resulting from ASU 2016-09 adoption | | — | | | — | | | — | | | 2,162 | | | 2,162 | |
Other comprehensive income (loss) net of tax expense of $10 | | — | | | — | | | (57) | | | — | | | (57) | |
| Net income (loss) | | — | | | — | | | — | | | (63,559) | | | (63,559) | |
| Balance as of December 31, 2018 | | 93,254,185 | | | 757,314 | | | (1,400) | | | (643,655) | | | 112,259 | |
| | | | | | | | | | |
| Share-based compensation to employees | | — | | | 52,416 | | | — | | | — | | | 52,416 | |
| Exercise of options and warrants | | 6,206,884 | | | 59,245 | | | — | | | — | | | 59,245 | |
| Proceeds from issuance of shares | | 67,366 | | | 2,467 | | | — | | | — | | | 2,467 | |
Other comprehensive loss, net of tax expenses of $(145) | | — | | | — | | | (1,367) | | | — | | | (1,367) | |
| Net income (loss) | | — | | | — | | | — | | | (7,230) | | | (7,230) | |
| Balance as of December 31, 2019 | | 99,528,435 | | | $ | 871,442 | | | $ | (2,767) | | | $ | (650,885) | | | $ | 217,790 | |
The accompanying notes are an integral part of the consolidated financial statements.
NovoCure Limited and subsidiaries
Consolidated statements of cash flows
| | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | |
| U.S. dollars in thousands | 2019 | | 2018 | | 2017 |
| Cash flows from operating activities: | | | | | |
| Net income (loss) | $ | (7,230) | | | $ | (63,559) | | | $ | (61,662) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Depreciation and amortization | 8,460 | | | 9,006 | | | 7,677 | |
| Asset write-downs and impairment of field equipment | 398 | | | 407 | | | 241 | |
| Share-based compensation | 52,416 | | | 39,846 | | | 27,116 | |
| Foreign currency remeasurement loss (gain) | (917) | | | — | | | — | |
| Decrease (increase) in accounts receivables | (36,496) | | | (10,325) | | | (21,249) | |
| Amortization of discount (premium) | (2,176) | | | 1,022 | | | 252 | |
| Decrease (increase) in inventories | (1,159) | | | (529) | | | 3,524 | |
| Decrease (increase) in other long-term assets | 3,446 | | | (949) | | | (554) | |
| Increase (decrease) in accounts payables and accrued expenses | 16,883 | | | 13,713 | | | 13,310 | |
| Increase (decrease) in other long-term liabilities | (7,006) | | | 9,503 | | | (1,789) | |
| Net cash provided by (used in) operating activities | $ | 26,620 | | | $ | (1,865) | | | $ | (33,134) | |
| Cash flows from investing activities: | | | | | |
| Purchase of property, equipment and field equipment | (10,485) | | | (6,711) | | | (7,366) | |
| Proceeds from maturity of short-term investments | 420,661 | | | 255,000 | | | 120,000 | |
| Purchase of short-term investments | (461,843) | | | (253,782) | | | (104,006) | |
| Net cash provided by (used in) investing activities | $ | (51,667) | | | $ | (5,493) | | | $ | 8,628 | |
The accompanying notes are an integral part of the consolidated financial statements.
NovoCure Limited and subsidiaries
Consolidated statements of cash flows
| | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | |
| U.S. dollars in thousands | 2019 | | 2018 | | 2017 |
| Cash flows from financing activities: | | | | | |
| Proceeds from issuance of shares, net | $ | 2,467 | | | $ | 1,835 | | | $ | 1,540 | |
| Proceeds from long-term loan, net | — | | | 149,150 | | | 19 | |
| Repayment of long-term loan | (31) | | | (100,084) | | | (76) | |
| Exercise of options and warrants | 59,245 | | | 18,468 | | | 3,685 | |
| Net cash provided by financing activities | $ | 61,681 | | | $ | 69,369 | | | $ | 5,168 | |
| | | | | |
| Effect of exchange rate changes on cash, cash equivalents and restricted cash | $ | 26 | | | $ | 27 | | | $ | 8 | |
| | | | | |
| Increase (decrease) in cash, cash equivalents and restricted cash | 36,660 | | | 62,038 | | | (19,330) | |
| Cash, cash equivalents and restricted cash at the beginning of the year | 142,756 | | | 80,718 | | | 100,048 | |
| Cash, cash equivalents and restricted cash at the end of the year | $ | 179,416 | | | $ | 142,756 | | | $ | 80,718 | |
| | | | | |
| Supplemental cash flow activities: | | | | | |
| Cash paid during the year for: | | | | | |
| Income taxes | $ | 11,241 | | | $ | 20,350 | | | $ | 10,286 | |
| Interest | $ | 13,699 | | | $ | 13,334 | | | $ | 10,162 | |
| Non-cash activities in accordance with ASC-842: | | | | | |
| Right-of-use assets obtained in exchange for lease liabilities | $ | 22,943 | | | $ | — | | | $ | — | |
The accompanying notes are an integral part of the consolidated financial statements.
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 1: Organization
NovoCure Limited (including its consolidated subsidiaries, the "Company") was incorporated in the Bailiwick of Jersey and is principally engaged in the development, manufacture and commercialization of Tumor Treating Fields delivery systems, including Optune and NovoTTF-100L (our "Products"), for the treatment of solid tumor cancers. The Company has received regulatory approval from the U.S. Food and Drug Administration ("FDA") under the Premarket Approval pathway and regulatory approvals and clearances in certain other countries for Optune to treat adult patients with glioblastoma. The Company also has received FDA approval under the Humanitarian Device Exemption pathway to market NovoTTF-100L for unresectable, locally advanced or metastatic malignant pleural mesothelioma in combination with standard chemotherapies.
During the year ended December 31, 2019, the Company implemented changes to its corporate entity operating structure, including transferring certain intellectual property to its Swiss subsidiary, primarily to align corporate entities with the Company’s evolving operations and business model. See Note 13.
Note 2: Basis of presentation and significant accounting policies
The consolidated financial statements are prepared according to United States generally accepted accounting principles ("U.S. GAAP").
a. Use of estimates:
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. The Company evaluates on an ongoing basis its assumptions, including those related to contingencies, deferred taxes, tax liabilities, useful-life of field equipment, right-of-use assets and lease liabilities, pension liabilities, revenue recognition, and share-based compensation costs. The Company’s management believes that the estimates, judgment and assumptions used are reasonable based upon information available at the time they are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities at the dates of the consolidated financial statements, and the reported amounts of net revenue and expenses during the reporting period. Actual results could differ from those estimates.
b. Financial statements in U.S. dollars:
The accompanying financial statements have been prepared in U.S. dollars in thousands, except for share and per-share data.
The Company finances its operations in U.S. dollars and a substantial portion of its costs and revenues from its primary markets is incurred in U.S. dollars. As such, the Company’s management believes that the U.S. dollar is the currency of the primary economic environment in which NovoCure Limited and certain subsidiaries operate. The Company’s reporting currency is U.S. dollars.
Transactions and balances denominated in U.S. dollars are presented at their original amounts. Monetary accounts maintained in currencies other than the U.S. dollar are re-measured into dollars in accordance with Accounting Standards Codification (ASC) No. 830-10, "Foreign Currency Matters." All transaction gains and losses of the re-measurement of monetary balance sheet items are reflected in the consolidated statements of operations as financial income or expenses, as applicable.
For a subsidiary whose functional currency has been determined to be its local currency, assets and liabilities are translated at year-end exchange rates and statement of operations items are translated at average exchange rates prevailing during the year. Such translation adjustments are recorded as a separate component of accumulated other comprehensive income (loss) in shareholders' equity.
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
c. Principles of consolidation:
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. Intercompany transactions and balances, including unrealized profits from intercompany sales, have been eliminated upon consolidation.
d. Cash equivalents:
Cash equivalents are short-term, highly liquid investments that are readily convertible into cash with a maturity of three months or less at the date acquired.
e. Short-term investments and restricted cash:
1. Short-term investments:
The Company accounts for investments in debt securities in accordance with ASC 320, "Investments—Debt and Equity Securities." Management determines the appropriate classification of its investments in marketable debt securities at the time of purchase and reevaluates such determinations at each balance sheet date. For the years ended December 31, 2019 and 2018, all securities are classified as held-to-maturity since the Company has the intent and ability to hold the securities to maturity and, accordingly, debt securities are stated at amortized cost.
The amortized cost of held-to-maturity securities is adjusted for amortization of premiums and accretion of discounts to maturity and any other than temporary impairment losses. Such amortization and interest are included in the consolidated statement of operations as financial income or expenses, as appropriate.
For the three years ended December 31, 2019, no impairment losses have been identified.
2. Restricted cash:
The Company has restricted cash used as security for the use of Company credit cards, presented in short-term assets. Additionally, the Company has pledged bank deposits to cover bank guarantees related to facility rental agreements, fleet lease agreements and customs payments presented in other long-term assets (see Note 12).
f. Trade receivables:
The Company’s trade receivables balance contains billed and unbilled commercial activities. As needed, the Company records an allowance for doubtful accounts by reserving for specifically identified doubtful accounts. The Company periodically reviews its customers’ credit risk and payment history. To date, the Company has not experienced any material credit losses related to counter-party risk.
g. Inventories:
Inventories are stated at the lower of cost or net realizable value. Cost is determined using the weighted average method. The Company regularly evaluates its ability to realize the value of inventory. If the inventories are deemed damaged, if actual demand for the Company’s delivery systems deteriorates, or if market conditions are less favorable than those projected, inventory write-offs may be required.
Inventory write-offs of $310, $684, and $489, respectively, were recorded for the years ended December 31, 2019, 2018 and 2017.
h. Property and equipment:
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets at the following rates:
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
| | | | | |
| | % |
| Computers and laboratory equipment | 15 - 33 |
| Office furniture | 6 - 33 |
| Production equipment | 20 |
| Leasehold improvements | Over the shorter of the term of the lease or its useful life |
i. Field equipment:
Field equipment is stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful life of the field equipment, which was determined to be 18 to 36 months. Field equipment is equipment being utilized under service agreements, and accounted for in accordance with ASC 842 on a monthly basis as an operating lease (see Note 2(w)). The Company records a write-off provision for any excess, lost or damaged equipment when warranted based on an assessment of the equipment. Write-offs for equipment are included in cost of revenues. During the years ended December 31, 2019, 2018 and 2017, write-offs for $327, $350, and $195, respectively, were recorded (see Note 7).
j. Impairment of long-lived assets:
The Company’s long-lived assets are reviewed for impairment in accordance with ASC 360-10, "Property, Plant and Equipment," whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. Recoverability of an asset to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the asset. If such asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds its fair value. During the three years ended December 31, 2019, no impairment losses have been identified.
k. Other long-term assets:
Restricted deposits and long-term lease deposits associated with office rent and vehicles under operating leases are presented in other long-term assets.
l. Revenue recognition:
Our Products are comprised of two main components: (1) an electric field generator and (2) transducer arrays and related accessories. We retain title to the electric field generator, and the patient is provided replacement transducer arrays and technical support for the device during the term of treatment. The electric field generator and transducer arrays are always supplied and function together and are not sold on a standalone basis.
In May 2014, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2014-9, Revenue from Contracts with Customers (Topic 606) (ASU 2014-9), an updated standard on revenue recognition and issued subsequent amendments to the initial guidance in March 2016, April 2016, May 2016 and December 2016 within ASU 2016-8, 2016-10, 2016-12 and 2016-20, respectively (collectively, "ASC 606"). The core principle of the new standard is for companies to recognize revenue to depict the transfer of goods and services to patients in amounts that reflect the consideration to which the company expects to be entitled in exchange for those goods and services. In addition, the new standard requires expanded disclosures. The Company has adopted the standard effective January 1, 2018 using the modified retrospective method for all contracts. The reported results for 2018 and 2019 reflect the application of ASC 606 guidance while the reported results for 2017 were prepared under the guidance of ASC 605, Revenue Recognition (ASC 605). The amount of revenue recognized in 2018 and 2019 reflects the consideration to which the Company expects to be entitled to receive in exchange for our Products.
The Company uses the portfolio approach to apply the standard to portfolios of contracts with similar characteristics.
To recognize revenue under ASC 606, the Company applies the following five steps:
1.Identify the contract with a patient. A contract with a patient exists when (i) the Company enters into an enforceable contract with a patient that defines each party’s rights regarding delivery of and payment for a Product, (ii) the contract has commercial substance and (iii) the Company determines that collection of
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
substantially all consideration for such Product is probable based on the payer’s intent and ability to pay the promised consideration. The evidence of a contract generally consists of a prescription, a patient service agreement and the verification of the assigned payer for the contract and intention to collect.
2.Identify the performance obligations in the contract. Our contracts include the lease of the device, the supply obligation of disposable transducer arrays and technical support for the term of treatment. To the extent a contract includes multiple promised products and/or services, the Company must apply judgment to determine whether those products and/or services are capable of being distinct in the context of the contract. If these criteria are not met the promised products and/or services are accounted for as a combined performance obligation. In the Company’s case, the device, support, and disposables are provided as one inseparable package of monthly treatment for a single monthly fee. For more information, see Note 2(w).
3.Determine the transaction price. The transaction price is determined based on the consideration to which the Company will be entitled in exchange for providing a Product to the patient. To the extent the transaction price includes variable consideration, the Company estimates the amount of variable consideration that should be included in the transaction price utilizing either the expected value method or the most likely amount method depending on the nature of the variable consideration. Variable consideration is included in the transaction price if, in the Company’s judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. The Company has agreements with many payers that define explicit discounts off the gross transaction price. In addition to the explicit discounts negotiated with each payer, the Company expects to receive, in aggregate for a given portfolio, less than the gross revenue net of explicit discounts. ASC 606 requires that the Company recognize this variable consideration as an implicit discount in the billing period. The implicit discount includes both an estimate of claims that will pay at an amount less than billed and an estimate of claims that will not pay within a given time horizon. The implicit discount adjustments to the transaction price are due to concessions, not collectability concerns driven by payer credit risk.
4.Allocate the transaction price to performance obligations in the contract. If a contract contains a single performance obligation, the entire transaction price is allocated to the single performance obligation. As discussed above, there is a combined performance obligation under the Company’s contracts and, therefore, the monthly transaction price determined for the performance obligation will be recognized over time ratably over the monthly term of the treatment.
5.Recognize revenue when or as the Company satisfies a performance obligation. The Company satisfies performance obligations over time. Revenue is recognized at the time the related performance obligation is satisfied by transferring a promised service to a patient. The patient consumes the benefits of treatment on a daily basis over the monthly term. As this criterion is met, the revenues will be recognized over the monthly term. For more information, see Note 2(w).
At adoption of ASC 606 on January 1, 2018, trade receivables increased by $2,807, deferred revenues increased by $645 and the Company recorded a cumulative impact to its accumulated deficit of $2,162 in 2018. Total deferred revenues balances at December 31, 2019 and 2018 were $19,580 and $18,769, respectively, presented as short-term and long-term liabilities. Unbilled revenues include revenues recognized for therapy provided and not invoiced in the reported period, and are presented as part of accounts receivable.
Revenues are presented net of indirect taxes.
Net revenues in 2019 and 2018 also include amounts recognized pursuant to the Zai Agreement. For additional information, see Note 12.
m. Charitable care:
The Company provides treatment at no charge to patients who meet certain criteria under its charitable care policy. Because the Company does not pursue collection of amounts determined to qualify as charity, they are not reported as revenue. The Company's costs of care provided under charitable care were $2,847, $2,762 and $1,483 for the
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
years ended December 31, 2019, 2018 and 2017, respectively. These amounts were determined by applying charitable care as a percentage of gross billings to total cost of goods sold.
n. Shipping and handling costs:
The Company does not separately bill its customers for shipping and handling costs associated with shipping Products to its customers. These direct shipping and handling costs of $2,688, $2,936 and $5,322 for the years ended December 31, 2019, 2018 and 2017, respectively are included in Sales and Marketing costs.
o. Accounting for share-based compensation:
The Company accounts for share-based compensation in accordance with ASC 718, "Compensation—Stock Compensation." ASC 718 requires companies to estimate the fair value of share-based compensation awards on the date of grant using an option-pricing model. The value of the award is recognized as an expense over the requisite service periods in the Company’s consolidated statements of operations. The Company's policy is to account for forfeitures as they occur.
The Company recognizes compensation costs for the value of awards granted using the accelerated method over the requisite service period of the award, which is generally the restricted share unit vesting term of three years and option vesting term of four years, respectively.
The Company applies the Black-Scholes model as it believes it is the most appropriate fair value method for all equity awards and for the Employee Share Purchase Plan (the "ESPP"). For market condition awards, the Company also applies the Monte-Carlo simulation model. The Black-Scholes model requires a number of assumptions, of which the most significant are the share price, expected volatility and the expected award term.
The computation of expected volatility is based on actual historical share price volatility of comparable companies when there is not sufficient historical information for the Company. Expected term of options granted is calculated using the average between the vesting period and the contractual term to the expected term of the options in effect at the time of grant. The Company has historically not paid dividends and has no foreseeable plans to pay dividends and, therefore, uses an expected dividend yield of zero in the option pricing model. The risk-free interest rate is based on the yield of U.S. treasury bonds with equivalent terms.
p. Fair value of financial instruments:
The carrying amounts of cash and cash equivalents, short-term investments, restricted cash, receivables and prepaid expenses, trade receivables, trade payables and other accounts payable and accrued expenses approximate their fair value due to the short-term maturity of such instruments. Based upon the borrowing terms and conditions currently available to the Company, the carrying values of the long-term loans approximate fair value.
The Company accounts for certain assets and liabilities at fair value under ASC 820, "Fair Value Measurements and Disclosures." Fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or a liability.
The hierarchy below lists three levels of fair value based on the extent to which inputs used in measuring fair value are observable in the market. The Company categorizes each of its fair value measurements in one of these three levels based on the lowest level input that is significant to the fair value measurement in its entirety.
The three levels of inputs that may be used to measure fair value are as follows:
Level 1 - Observable inputs that reflect quoted prices (unadjusted) for identical assets or liabilities in active markets;
Level 2 - Includes other inputs that are directly or indirectly observable in the marketplace, other than quoted prices included in Level 1, such as quoted prices for similar assets or liabilities in active markets, quoted prices for identical
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
or similar assets or liabilities in markets with insufficient volume or infrequent transactions, or other inputs that are observable (model-derived valuations in which significant inputs are observable), or can be derived principally from or corroborated by observable market data; and
Level 3 - Unobservable inputs which are supported by little or no market activity.
The availability of observable inputs can vary from instrument to instrument and is affected by a wide variety of factors, including, for example, the type of instrument, the liquidity of markets and other characteristics particular to the transaction. To the extent that valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment and the instrument is categorized as Level 3.
q. Basic and diluted net loss per share:
Basic net income (loss) per share is computed based on the weighted average number of ordinary shares outstanding during each period. Diluted net income per share is computed based on the weighted average number of ordinary shares outstanding during the period, plus potential dilutive shares considered outstanding during the period, in accordance with ASC 260-10, as determined under the treasury stock method. As the inclusion of all potential dilutive shares (deriving from options, RSUs and the ESPP) outstanding would be anti-dilutive, basic and diluted net loss per ordinary share was the same for each full year presented. See Note 19 for additional information regarding the three month periods ended September 30, 2019 and December 31, 2019.
r. Income taxes:
The Company accounts for income taxes in accordance with ASC 740-10, "Income Taxes." ASC 740-10 prescribes the use of the liability method whereby deferred tax asset and liability account balances are determined based on differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company provides a valuation allowance, to reduce deferred tax assets to their estimated realizable value, if needed.
The Company established reserves for uncertain tax positions based on the evaluation of whether or not the Company’s uncertain tax position is "more likely than not" to be sustained upon examination. The Company records interest and penalties pertaining to its uncertain tax positions in the financial statements as income tax expense.
s. Concentration of risks:
Our cash, cash equivalents, short-term investments and trade receivables are potentially subject to a concentration of risk. Cash, cash equivalents and short-term investments are invested at top tier financial institutions globally. As such, these investments may be in excess of insured limitations or not insured in certain jurisdictions. Generally, these investments may be redeemed upon demand and therefore, bear minimal risk.
The Company has no off-balance sheet concentrations of credit risk such as foreign exchange contracts, option contracts or other foreign hedging arrangements.
t. Retirement, pension and severance plans:
The Company has a 401(k) retirement savings plan for its U.S. employees. Each eligible employee may elect to contribute a portion of the employee’s compensation to the plan. Company contributions to the plan are at the sole discretion of the Company's Board of Directors. Currently, the Company provides a matching contribution of 50% of the employee's contributions, up to a maximum of three percent (3%) of the employee's annual salary. The Company began making matching contributions as of January 1, 2019 and, as of December 31, 2019, the Company had made matching contributions in the amount of $978 pursuant to the plan.
The Company sponsors a defined benefit plan (the "Swiss Plan") for all its employees in Switzerland for retirement benefits, as well as benefits on death or long-term disability, whereby the employee and the Company contribute a portion of the employee's compensation to the plan. The Swiss Plan is part of a collective pension fund managed by a top tier insurance company. The Company’s exposure under the Swiss Plan is insured. The Company has financial exposure under the Swiss Plan only in the event that the insurance company does not meet its obligations. The Company accounts for this potential counterparty risk in accordance with ASC 715, "Compensation –
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Retirement Benefits" (see Note 9). The pension expense for the years ended December 31, 2019, 2018 and 2017 was $984, $882 and $1,036, respectively.
Israeli law generally requires payment of severance pay upon dismissal of an employee or upon termination of employment in certain other circumstances. The Company contributes to employee pension plans to fund its severance liabilities. According to Section 14 of Israel Severance Pay Law, the Company makes deposits on behalf of its employees with respect to the Company’s severance liability and therefore no obligation is provided for in the financial statements. Severance pay liabilities with respect to employees who are not subject to Section 14, are provided for in the financial statements based upon the number of years of service and the latest monthly salary and the related deposits are recorded as an asset based on the cash surrender value. Contributions pursuant to these obligations for the years ended December 31, 2019, 2018 and 2017 amounted to $784, $526 and $506, respectively.
u. Contingent liabilities:
The Company accounts for its contingent liabilities in accordance with ASC 450, "Contingencies." A provision is recorded when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated.
With respect to legal matters, provisions are reviewed and adjusted to reflect the impact of negotiations, estimated settlements, legal rulings, advice of legal counsel and other information and events pertaining to a particular matter.
v. Other comprehensive income (loss):
The Company accounts for comprehensive income (loss) in accordance with ASC 220, "Comprehensive Income." ASC 220 establishes standards for the reporting and display of comprehensive income (loss) and its components. Comprehensive income (loss) generally represents all changes in shareholders' equity during the period except those resulting from investments by, or distributions to, shareholders. The accumulated other comprehensive income (loss), net of taxes, relates to a pension liability and foreign currency translation adjustments.
w. Leases:
1.Lessee accounting:
On January 1, 2019, the Company adopted ASU No. 2016-02, Leases (ASC 842). The Company determines if an arrangement is a lease and the classification of that lease at inception based on: (1) whether the contract involves the use of a distinct identified asset, (2) whether the Company obtains the right to substantially all the economic benefits from the use of the asset throughout the period, and (3) whether the Company has a right to direct the use of the asset. The Company elected to not recognize a lease liability or right-of-use ("ROU") asset for leases with a term of twelve months or less. The Company also elected the practical expedient to not separate lease and non-lease components for its leases.
ROU assets represent the right to use an underlying asset for the lease term and lease liabilities represent the obligation to make minimum lease payments arising from the lease. ROU assets are initially measured at amounts, which represents the discounted present value of the lease payments over the lease, plus any initial direct costs incurred. The ROU assets are reviewed for impairment. The lease liability is initially measured at lease commencement date based on the discounted present value of minimum lease payments over the lease term. The implicit rate within the operating leases are generally not determinable; therefore, the Company uses the Incremental Borrowing Rate ("IBR") based on the information available at commencement date in determining the present value of lease payments. The Company’s IBR is estimated to approximate the interest rate on similar terms and payments and in economic environments where the leased asset is located.
Certain leases include options to extend or terminate the lease. An option to extend the lease is considered in connection with determining the ROU asset and lease liability when it is reasonably certain that the Company will exercise that option. An option to terminate is considered unless it is reasonably certain that the Company will not exercise the option.
2. Lessor accounting - Operating leases:
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
ASC 842 provides lessors with an optional practical expedient, by class of underlying asset, not to separate non-lease components from the associated lease component and, instead, to account for those components as a single component if the non-lease components otherwise would be accounted for under the new revenue guidance (ASC 606) and both of the following criteria are met:
1. The timing and pattern of transfer of the lease component and the non-lease component(s) are the same; and
2. The lease component would be classified as an operating lease if it were accounted for separately.
The Company's product supply agreements include the right to use the device (lease component), the supply obligation of disposable transducer arrays and technical support for the term of treatment (non-lease component).
If the lease component is the predominant component, the Company accounts for all revenues under such lease as a single component in accordance with the new lease accounting standard. Conversely, if the non-lease component is the predominant component, all revenues under such lease are accounted for in accordance with the revenue recognition accounting standard. The Company's operating leases qualify for the single component accounting, and the non-lease component in each of the Company's leases is predominant. Therefore, The Company accounts for all revenues from its operating leases in accordance with the revenue recognition accounting standard.
x. Recently adopted accounting pronouncements:
In 2016, the FASB issued ASU No. 2016-02, "Leases (Topic 842)", which amends the existing standards for lease accounting, requiring lessees to recognize most leases on their balance sheets. The new standard establishes a right-of-use model that requires a lessee to recognize a right-of-use asset and lease liability on the balance sheet for all leases. Leases will be classified as finance or operating. The standard is effective for interim and annual reporting periods beginning after December 15, 2018.
The provisions of ASU 2016-02 are to be applied using a modified retrospective approach. In July 2018, the FASB issued ASU No. 2018-11, "Targeted Improvements - Leases (Topic 842)" (together with ASU 2016-02, "ASC 842"). This update provides an additional (and optional) transition method to adopt the new leases standard. Under this method, an entity initially applies the new leases standard at the adoption date and recognizes a cumulative-effect adjustment to the opening balance of retained earnings in the period of adoption. Consequently, the prior comparative period’s financials will remain the same as those previously presented. The Company adopted the new standard as of January 1, 2019 and it has also elected to adopt the package of practical expedients permitted in ASC 842.
Upon implementation of ASC 842, effective January 1, 2019, the Company recorded an increase in right-of-use assets obtained in exchange for lease liabilities of $15,733 on its opening balance sheet. The standard did not have a material impact to the Company's consolidated statements of comprehensive income.
The consolidated financial statements for the year ended December 31, 2019 are presented under the new standard, while comparative years presented are not adjusted and continue to be reported in accordance with Topic 840, Leases.
y. Recently issued accounting pronouncements:
In June 2016, the FASB issued ASU 2016-13, Financial Instruments - Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. ASU 2016-13 amends the impairment model to utilize an expected loss methodology in place of the currently used incurred loss methodology, which will result in the more timely recognition of losses. ASU 2016-13 also applies to employee benefit plan accounting, with an effective date of the first quarter of fiscal 2020. The amendments in this update are effective for fiscal years beginning after December 31, 2019, including interim periods within those fiscal years. We have adopted the new standard effective January 1, 2020 and do not expect the adoption of this guidance to have a material impact on the Company's consolidated financial statements.
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
In August 2018, FASB issued ASU 2018-15, Intangibles—Goodwill and Other—Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement That Is a Service Contract. The amendments in this ASU align the requirements for capitalizing implementation costs incurred in a hosting arrangement with the requirements for capitalizing implementation costs incurred to develop or obtain internal-use software. The implementation costs incurred in a hosting arrangement that is a service contract should be presented as a prepaid asset in the balance sheet and expensed over the term of the hosting arrangement to the same line item in the statement of income as the costs related to the hosting fees. The guidance in this ASU is effective for fiscal years beginning after December 15, 2019, including interim periods within those fiscal years, and early adoption is permitted including adoption in any interim period. The amendments should be applied either retrospectively or prospectively to all implementation costs incurred after adoption. The Company is currently evaluating the impact that the standard will have, if any, on its consolidated financial statements and related disclosures.
In August 2018, the FASB issued ASU 2018-14—Compensation—Retirement Benefits—Defined Benefit Plans—General (Topic 715-20): Disclosure Framework—Changes to the Disclosure Requirements for Defined Benefit Plans. ASU 2018-14 improves disclosure requirements for employers that sponsor defined benefit pension or other postretirement plans. This standard is effective for fiscal years ending after December 15, 2020, for public business entities. Early adoption is permitted. An entity should apply the amendments in this ASU on a retrospective basis to all periods presented. The Company is currently evaluating the impact that the standard will have, if any, on its consolidated financial statements and related disclosures.
In December 2019, the FASB issued Accounting Standard Update No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (ASU 2019-12). This guidance will be effective for the Company in the first quarter of 2021 on a prospective basis, and early adoption is permitted. The Company is currently evaluating the impact of the new guidance on the Company's consolidated financial statements.
Note 3: Cash and Cash equivalents and Short-term investments
a.Cash and cash equivalents:
Cash equivalents include items almost as liquid as cash, such as certificates of deposit and time deposits with maturity periods of three months or less when purchased.
| | | | | | | | | | | |
| December 31, | | |
| 2019 | | 2018 |
| Cash | $ | 18,377 | | | $ | 9,197 | |
| Money market funds | 158,944 | | | 131,425 | |
| Total cash and cash equivalents | $ | 177,321 | | | $ | 140,622 | |
b. Short-term investments
The Company invests in marketable U.S. Treasury Bills ("T-bills") that are classified as held-to-maturity securities. The amortized cost and recorded basis of the T-bills are presented as short-term investments.
| | | | | | | | | | | |
| December 31, | | |
| 2019 | | 2018 |
| Short-term investments | $ | 148,769 | | | $ | 105,256 | |
Quoted market prices were applied to determine the fair value of cash equivalents and short-term investments, therefore they are categorized as Level 1 in accordance with ASC 820, "Fair Value Measurements and Disclosures." The estimated fair value of our short-term investments as of December 31, 2019 and 2018 was $148,738 and $105,266, respectively.
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 4: Receivables and prepaid expenses
The following table sets forth the Company’s receivables and prepaid expenses:
| | | | | | | | | | | |
| December 31, | | |
| 2019 | | 2018 |
| Advances to and receivables from suppliers | $ | 5,097 | | | $ | 4,565 | |
| Government authorities | 21,382 | | | 6,106 | |
| Prepaid expenses | 2,251 | | | 3,531 | |
| Others | 471 | | | 77 | |
| | $ | 29,202 | | | $ | 14,279 | |
Note 5: Inventories
The following table sets forth the Company’s inventories:
| | | | | | | | | | | |
| | December 31, | | |
| | 2019 | | 2018 |
| Raw materials | $ | 3,912 | | | $ | 870 | |
| Work in process | 6,482 | | | 8,667 | |
| Finished goods | 13,308 | | | 13,018 | |
| | $ | 23,701 | | | $ | 22,555 | |
Note 6: Property and equipment, net
The following table sets forth the Company’s property and equipment, net:
| | | | | | | | | | | |
| December 31, | | |
| 2019 | | 2018 |
| Cost: | | | |
| Computers and laboratory equipment | $ | 15,448 | | | $ | 12,220 | |
| Office furniture | 2,486 | | | 2,308 | |
| Production equipment | 1,237 | | | 1,228 | |
| Leasehold improvements | 4,818 | | | 4,192 | |
| Total cost | $ | 23,988 | | | $ | 19,948 | |
| Accumulated depreciation and amortization | (14,647) | | | (11,506) | |
| Depreciated cost | $ | 9,342 | | | $ | 8,442 | |
The Company capitalized software costs according to FASB's ASC 350-40, "Accounting for the Costs of Computer Software Developed or Obtained for Internal Use." Cumulative capitalization as of December 31, 2019 and 2018 was $7,199 and $6,256, respectively. Amortization of capitalized software costs for the years ended December 31, 2019, 2018 and 2017 was $1,682, $1,486 and $1,226, respectively.
Depreciation expense was $2,080, $1,967 and $1,968 for the years ended December 31, 2019, 2018 and 2017, respectively.
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 7: Field equipment, net
The following table sets forth the Company’s field equipment, net:
| | | | | | | | | | | |
| December 31, | | |
| 2019 | | 2018 |
| Field equipment | $ | 21,075 | | | $ | 17,380 | |
| Accumulated depreciation | (13,391) | | | (10,456) | |
| Field equipment, net | $ | 7,684 | | | $ | 6,924 | |
Depreciation expense was $4,631, $5,553 and $4,483 for the years ended December 31, 2019, 2018 and 2017, respectively. Write downs of $326, $350, and $195 were identified for the years ended December 31, 2019, 2018 and 2017, respectively.
Note 8: Other payables and accrued expenses
The following table sets forth the Company’s other payables and accrued expenses:
| | | | | | | | | | | |
| December 31, | | |
| | 2019 | | 2018 |
| Employees and payroll accruals | $ | 20,904 | | | $ | 16,717 | |
| Taxes payable and others | 12,011 | | | 12,263 | |
| Deferred revenues | 11,773 | | | 8,840 | |
| Other | 4,699 | | | 32 | |
| | $ | 49,386 | | | $ | 37,852 | |
Note 9: Employee benefit obligations
The Company's liability in respect of the Swiss Plan (see Note 2(t)) is the projected benefit obligation calculated using the projected unit credit method. The projected benefit obligation as of December 31, 2019 represents the actuarial present value of the estimated future payments required to settle the obligation that is attributable to employee service rendered before that date. Swiss Plan assets are recorded at fair value. Pension expense is presented in the payroll expenses in the various functions in which the employees are engaged. Actuarial gains and losses arising from differences between the actual and the expected return on the Swiss Plan assets are recognized in accumulated other comprehensive income (loss) and amortized over the requisite service period. The Swiss Plan is part of a collective pension foundation of pooled investments managed by a top tier insurance company. The Company and the employees pay retirement contributions, which are defined as a percentage of the employees’ covered salaries. Interest is credited to the employees’ account at the minimum rate provided in the Swiss Plan, which represents the Swiss Plan’s primary asset. The targeted allocation for these funds is as follows:
| | | | | | | | |
| Asset Allocation by Category as of September 30, 2019: | | Asset allocation (%) |
| Asset Category: | | |
| Debt Securities | | 36% | |
| Real Estate | | 26% | |
| Equity Securities | | 33% | |
| Others | | 5% | |
| Total | | 100% | |
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
The following table sets forth the Swiss Plan’s funded status and amounts recognized in the consolidated financial statements for the year ended December 31, 2019 and 2018:
| | | | | | | | | | | |
| December 31, | | |
| | 2019 | | 2018 |
| Change in Benefit Obligation | | | |
| Projected benefit obligation at beginning of year | $ | 12,249 | | | $ | 10,317 | |
| Interest cost | 114 | | | 64 | |
| Company service cost | 932 | | | 820 | |
| Employee contributions | 599 | | | 486 | |
| Prior service cost | — | | | — | |
| Benefits paid | (109) | | | 475 | |
| Actuarial loss | 1,900 | | | 87 | |
| Projected benefit obligation at end of year | $ | 15,685 | | | $ | 12,249 | |
| Change in Plan Assets | | | | | |
| Fair value of plan assets at beginning of year | $ | 9,936 | | | $ | 8,243 | |
| Actual return on plan assets | 1,031 | | | 3 | |
| Employer contributions | 899 | | | 729 | |
| Employee contributions | 599 | | | 486 | |
| Benefits paid | (109) | | | 475 | |
| Fair value of plan assets at end of year | $ | 12,356 | | | $ | 9,936 | |
| Funded Status at End of year | | | | | |
| Excess of obligation over assets | $ | 3,329 | | | $ | 2,313 | |
| Change in Accrued Benefit Liability | | | | | |
| Accrued benefit liability at beginning of year | $ | (2,313) | | | $ | (2,074) | |
| Company contributions made during year | 899 | | | 729 | |
| Net periodic benefit cost for year | (1,024) | | | (874) | |
| Net decrease (increase) in accumulated other comprehensive loss | (891) | | | (94) | |
| Accrued benefit liability at end of year | $ | (3,329) | | | $ | (2,313) | |
| | | | | | | | | | | |
| December 31, | | |
| | 2019 | | 2018 |
| Non-current plan assets | $ | 12,357 | | | $ | 9,936 | |
| Non-current liability | 15,686 | | | 12,249 | |
| Accrued benefit liability at end of year | $ | (3,329) | | | $ | (2,313) | |
| Projected Benefit Payments | | | | | |
| Projected year 1 | $ | 270 | | | $ | 206 | |
| Projected year 2 | 1,434 | | | 205 | |
| Projected year 3 | 255 | | | 1,158 | |
| Projected year 4 | 260 | | | 195 | |
| Projected year 5 | 571 | | | 200 | |
| Projected years 6-10 | 2,109 | | | 2,859 | |
The fair value of the plan assets is the estimated cash surrender value of the insurance contract at December 31, 2019. The level of inputs used to measure fair value was Level 2.
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
| | | | | | | | | | | |
| Year ended December 31, | | |
| | 2019 | | 2018 |
| Net Periodic Benefit Cost | | | |
| Service cost | $ | 932 | | | $ | 820 | |
| Interest cost (income) | 114 | | | 69 | |
| Expected return on plan assets | (97) | | | (54) | |
| Amortization of transition obligation | 54 | | | 65 | |
| Amortization of prior service costs | (19) | | | (18) | |
| Total net periodic benefit cost | $ | 984 | | | $ | 882 | |
| | | |
| Weighted average assumptions: | | | |
| Discount rate as of December 31 | 0.20 % | | | 0.90% | |
| Expected long-term rate of return on assets | 0.20 % | | | 0.90% | |
| Rate of compensation increase | 1.00% | | | 1.00% | |
Mortality and disability assumptions (*) | BVG 2015 GT | | BVG 2015 GT |
(*) Mortality data used for actuarial calculation.
Note 10: Long-term loan, net of discount and issuance costs
On February 7, 2018, the Company and certain of its subsidiaries entered into a Loan and Security Agreement ("2018 Loan Agreement") with BioPharma Credit PLC pursuant to which such lender made a term loan to the Company in the principal amount of $150 million (the "2018 Credit Facility"). The term loan, which was drawn in full upon execution of the 2018 Loan Agreement, bears interest at 9.0% per annum, payable quarterly in arrears. The Company used a portion of the proceeds of the 2018 Credit Facility to repay in full the Company’s obligations under its then-existing term loan credit facility and is using the remaining proceeds to fund general corporate purposes.
The 2018 Credit Facility will mature on February 7, 2023, at which time any unpaid principal and accrued unpaid interest in respect of the term loan will be due and payable. The Company may prepay the term loan, in full, at any time. The Company must prepay the term loan (i) in full or in part upon the entry into certain licensing arrangements and (ii) in full in the event of a change of control. In each case, any prepayment (whether permitted or mandatory) is subject to a prepayment premium and/or make-whole payment. The prepayment fee if the Company prepays outstanding loan amounts prior to February 7, 2021 is 2.0% and is 1.0% if made after the February 7, 2021 but prior to February 7, 2022.If the Company prepays outstanding loan amounts prior to August 7, 2020, it must pay a make-whole amount equal to the amount of interest that would have accrued on the amount of all principal that is prepaid from the date of such prepayment through February 7, 2021.
All obligations under the 2018 Credit Facility are guaranteed by the Company’s current and future direct and indirect subsidiaries. In addition, the obligations under the 2018 Credit Facility are secured by a first-priority security interest in substantially all of the property and assets of, as well as the equity interests owned by, the Company and certain of the other guarantors. The 2018 Credit Facility contains other customary covenants.
Total net issuance costs of the 2018 Credit Facility, which were $576 as of December 31, 2019, are presented net of the 2018 Credit Facility proceeds and are amortized to interest expense over the five year term of the loan using the effective interest method.
On February 7, 2018, the Company’s 2015 term loan credit facility was terminated upon the Company’s repayment in full of the term loan issued thereunder. The unamortized discount in the amount of $1,160 and issuance costs in the amount of $1,399 were fully amortized and included in the Company’s finance expenses in the first quarter of 2018.
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 11: Other long-term liabilities
| | | | | | | | | | | |
| December 31, | | |
| | 2019 | | 2018 |
| Deferred rent liability | $ | 40 | | | $ | 773 | |
| Leasehold improvements financing and other | 66 | | | 94 | |
| Unrecognized tax benefits (Note 13(e)) | 116 | | | 103 | |
| Deferred tax liability | — | | | 124 | |
| | $ | 222 | | | $ | 1,094 | |
Note 12: Commitments and contingent liabilities
a.Operating leases
The facilities of the Company are leased under various operating lease agreements for periods ending no later than 2030. The Company also has the option to extend the term of certain facility lease agreements and these are included in the calculation of right-of-use assets. The Company also leases motor vehicles under various operating leases, which expire on various dates, the latest of which is in 2022.
Under ASC 842, all leases with durations greater than 12 months, including non-cancelable operating leases, are now recognized on the balance sheet. The aggregated present value of lease agreements, net of deferred rent, is recorded as a long-term asset titled right-of-use assets. The corresponding lease liabilities are split between other payables and long-term lease liabilities.
Upon implementation of ASC 842, effective January 1, 2019, the Company recorded an increase in right-of-use assets obtained in exchange for lease obligations of $15,733 on our opening balance sheet. Future minimum lease payments under non-cancelable operating leases as of December 31, 2019, are as follows:
| | | | | |
| | December 31, |
| | 2019 |
| Future minimum lease payments: | | | |
| 2020 | | $ | 5,046 | |
| 2021 | | 5,030 | |
| 2022 | | 3,823 | |
| 2023 | | 2,524 | |
| 2024 | | 1,934 | |
| Thereafter | | 4,081 | |
| Total future minimum lease payments | | $ | 22,438 | |
| Less imputed interest | | (3,663) | |
| Net present value of future minimum lease payments | | $ | 18,775 | |
| | | |
| Current year end | | | |
| Short-term lease liabilities | | $ | 4,635 | |
| Long-term lease liabilities | | 14,140 | |
| Net present value of future minimum lease payments | | $ | 18,775 | |
| | |
| Weighted average of remaining operating lease term (years) | | 5.54 |
| | |
| Weighted average of operating lease discount rate | | 7.32 | % |
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Lease and rental expense for the years ended December 31, 2019, 2018 and 2017 was $4,607, $4,033, and $3,474, respectively.
b. Bank guarantee and pledges
As of December 31, 2019 and 2018 the Company pledged bank deposits of $1,557 and $1,143, respectively, to cover bank guarantees in respect of its leases of operating facilities and obtained guarantees by the bank for the fulfillment of the Company’s lease commitments of $1,390 and $1,299, respectively.
c. Technion Settlement Agreement
In the first quarter of 2018, the Company made the final milestone payment of $5.5 million (the "Milestone Payment") to the Technion Research and Development Foundation ("Technion") pursuant to the settlement agreement dated February 10, 2015 (the "Settlement Agreement"). Pursuant to the Settlement Agreement, and in exchange for a release of potential disputes regarding intellectual property developed by our founder and previously assigned to us, the Company was obligated to pay the Milestone Payment to Technion in the quarter following the quarter in which the Company achieved $250.0 million of cumulative net sales (as defined in the Settlement Agreement) (the "Net Sales Milestone"). The Company met the Net Sales Milestone in the fourth quarter of 2017.
d. Zai License and Collaboration Agreement
On September 10, 2018, the Company entered into a License and Collaboration Agreement (the "Zai Agreement") with Zai Lab (Shanghai) Co., Ltd. ("Zai"). Under the Zai Agreement, the Company granted Zai exclusive rights to commercialize Optune in the field of oncology in China, Hong Kong, Macau and Taiwan ("Greater China"). The Zai Agreement also established a development partnership for Optune in multiple solid tumor indications. In partial consideration for the license grant to Zai for Greater China, the Company was entitled to a non-refundable, up-front license fee in the amount of $15 million (the "License Fee"). The Zai Agreement also provides for certain development, regulatory and commercial milestone payments totaling up to $78 million. Furthermore, pursuant to the Zai Agreement, Zai will pay the Company tiered royalties at percentage rates from 10 up to the mid-teens on the net sales of the licensed products in Greater China. Zai is purchasing licensed products for commercial use exclusively from the Company at the Company’s fully burdened manufacturing cost.
Zai paid the License Fee in the fourth quarter of 2018. Net of withholding taxes, the Company received $12.7 million.
The Company recognizes revenue pursuant to the License Agreement with Zai in accordance with ASC 606, "Revenue Recognition from Customers." The License Fee, net of withholding taxes, is deferred and recognized over related six year performance period commencing September 10, 2018 ("Zai Performance Period"). The License Fee will be recognized on a straight-line basis, resulting in revenue of $2,115 and $767 for the years ended December 31, 2019 and 2018, respectively. Revenue from any future commercial milestone payments will be recognized upon the achievement of such milestones and clinical or regulatory milestone payments will be recognized in a straight line over the applicable performance period, in accordance with ASC 606. Revenue from royalty payments are recognized in accordance with ASC 606 in the period accrued.
e. Paz Litigation
In February 2019, a civil claim was filed in the District Court in Haifa, Israel (the "Court"), by Ofir Paz ("Paz"), a former member of the Company's Board of Directors, and EES Investments Ltd., a company wholly owned by Paz (together with Paz, "Plaintiff") against the Company and Prof. Yoram Palti ("Respondents"). Based on Plaintiff's recent motions described below, Plaintiff claims that he is entitled to approximately 1,200,000 ordinary shares (as adjusted for share capital splits since 2003). In May 2019, the Company filed a motion to dismiss the claim that is still pending. Plaintiff has also filed motions in September and December 2019 to add Asaf Danziger as a Respondent and change the basis of his claims from breach of contract to wrongful deregistration. These motions are pending. The Company believes that the complaint is without merit and plans to defend against this claim vigorously. The Company has not accrued any amounts in respect of these claims, as it believes liability is not probable and the amount of any potential liability cannot be reasonably estimated.
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 13: Income taxes
a. Tax provision
The provision (benefit) for income taxes from continuing operations is comprised of:
Income (loss) before income taxes:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| United States (U.S.) | $ | (87,925) | | | $ | (114,890) | | | $ | (77,654) | |
| Non-U.S. | 79,101 | | | 68,948 | | | 29,157 | |
Total income (loss) before income taxes | $ | (8,824) | | | $ | (45,942) | | | $ | (48,497) | |
Income tax:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| Current: | | | | | |
| U.S. (1) | $ | (6,143) | | | $ | 6,701 | | | $ | 8,491 | |
| Non-U.S. (2) | 4,405 | | | 10,568 | | | 5,028 | |
| Total current | $ | (1,738) | | | $ | 17,269 | | | $ | 13,519 | |
| Deferred: | | | | | | | | |
| U.S. | $ | — | | | — | | | $ | (3) | |
| Non-U.S. | 144 | | | 348 | | | (351) | |
| Total deferred | 144 | | | 348 | | | (354) | |
| Total income tax provision | $ | (1,594) | | | $ | 17,617 | | | $ | 13,165 | |
(1) This change was driven primarily by research and development credits claimed in the U.S. and deductions associated with share-based compensation.
(2) This change primarily resulted from amortization of intellectual property rights.
b. Theoretical tax
The Company's effective tax rate is affected by the tax rates in the various jurisdictions in which the Company operates. For purposes of comparability, the Company used the notional U.S. federal income tax rate of 21% for the 2019 and 2018 tax year and 35% for the 2017 tax year when presenting the Company's reconciliation of the income tax provision. A reconciliation of the provision for income taxes compared with the amounts at the notional federal statutory rate was:
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
| | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| Loss before income taxes | $ | (8,824) | | | | $ | (45,942) | | | | $ | (48,497) | |
| U.S statutory income tax rate | 21.0 | % | | | 21.0 | % | | | 35.0 | % |
| Notional U.S. federal income taxes at statutory rate | $ | (1,853) | | | | $ | (9,648) | | | | $ | (16,974) | |
| Non-deductible expenses | 741 | | | | 1,030 | | | | 3,308 | |
| Foreign taxes rate differential | (4,216) | | | | (6,000) | | | | (7,333) | |
| Change in valuation allowance (see Note 13(c)) | 244,344 | | | | 28,657 | | | | 5,742 | |
| State income taxes | (16,679) | | | | 1,957 | | | | (9,089) | |
| Change in excess tax benefit | (26,528) | | | | 2,088 | | | | 2,203 | |
| Unamortized intangible assets | (189,410) | | | | — | | | | — | |
| Research and Development Credits | (2,333) | | | | — | | | | — | |
| Other | (5,673) | | | | (326) | | | | 34,881 | |
| Unrecognized tax expense (benefit) | 13 | | | | (141) | | | | 427 | |
| Income tax | $ | (1,594) | | | | $ | 17,617 | | | | $ | 13,165 | |
| Effective tax rate | 18.1 | % | | | (38.3) | % | | | (27.1) | % |
In 2017, the Tax Cuts and Jobs Act ("TCJA") was signed into law in the U.S. Changes include, but are not limited to, a corporate tax rate decrease from 35% to 21% effective for tax years beginning after December 31, 2017. In accordance with ASC 740, the Company recorded $34.8 million of deferred tax expense in connection with the remeasurement of certain deferred tax assets and liabilities. This was fully offset by a valuation allowance. Accordingly, there was no net impact on the Company’s income tax expense for the year ended December 31, 2017. The Company’s subsidiary in the United States does not have any foreign subsidiaries and, therefore, the remaining provisions of the TCJA have no material impact on the Company's results of operations. December 22, 2018 marked the end of the measurement period for purposes of ASU 2018-05, and the Company concluded that no change was required to its initial assessment.
The table below reflects the 2017 net impact of the TCJA:
| | | | | | | | | | | | | | | | | |
| | December 31, 2017 | | | | |
| | ETR before TCJA | | US Tax Cuts & Jobs Act Impact | | Reported ETR |
| U.S statutory income taxes rate | 35.0 | % | | | — | % | | | 35.0 | % |
| Non-deductible expenses | (6.8) | | | | — | | | | (6.8) | |
| Foreign taxes rate differential | 15.1 | | | | — | | | | 15.1 | |
| Change in valuation allowance | (83.4) | | | | 71.5 | | | | (11.9) | |
| State income taxes | 12.8 | | | | 5.9 | | | | 18.7 | |
| Share based compensation | 2.0 | | | | (6.5) | | | | (4.5) | |
| Change in unrecognized taxes expense | (0.9) | | | | 0.1 | | | | (0.8) | |
| Other | (0.9) | | | | (71.0) | | | | (71.9) | |
| Effective taxes rate | (27.1) | % | | | — | % | | | (27.1) | % |
c. Deferred income tax
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets and liabilities are as follows:
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
| | | | | | | | | | | |
| December 31, | | |
| | 2019 | | 2018 |
| Deferred tax assets: | | | |
| Implicit discounts recognized under ASC 606 (see Note 2) | $ | 124,255 | | | | $ | 99,316 | |
| Net operating loss carryforwards (see Note 13(d)) | 35,267 | | | | 843 | |
| Share based compensation | 12,253 | | | | 10,886 | |
| Deferred revenue | 2,450 | | | | 1,643 | |
| Interest limitations | 4,028 | | | | — | |
| Unamortized intangible assets (1) | 176,783 | | | | — | |
| Other temporary differences | 2,359 | | | | 1,384 | |
| Total gross deferred tax assets | $ | 357,395 | | | | $ | 114,072 | |
| Less: valuation allowance | (357,012) | | | | (112,360) | |
| Total deferred tax assets | $ | 383 | | | | $ | 1,712 | |
| Deferred tax liabilities: | | | | | | |
| Fixed assets | 380 | | | | 1,427 | |
| Other liabilities | 3 | | | | — | |
| Total gross deferred tax liabilities | $ | 383 | | | | $ | 1,427 | |
| | | | | | |
| Net deferred taxes assets | $ | — | | | | $ | 285 | |
(1) The Company recorded a deferred tax asset of $189,410 related to unamortized intangible assets. Amortization of this intangible asset will be straight-line through 2029. As of December 31, 2019, the balance of this deferred tax asset was $176,783, offset by a valuation allowance of the same amount.
d. Carryforward loss:
As of December 31, 2019, the Company had $108,172 of U.S. federal net operating loss carryforwards ("NOLs") and $128,912 of U.S. state NOLs. The U.S. federal NOLs carry forward indefinitely. Also, approximately $21,524 in U.S. state NOLs carry forward indefinitely, with the remainder expiring from 2022 through 2039.
In addition, the Company had $38,603 of non-U.S NOLs as of December 31, 2019. Approximately $618 of the non-U.S. NOLs carry forward indefinitely, with the remainder expiring between 2026 and 2036.
e. Uncertain tax benefits:
A reconciliation of the beginning and ending balances of uncertain tax benefits is as follows:
| | | | | | | | | | | | | | | | | |
| | December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| Balance at beginning of the year | $ | 103 | | | | $ | 2,827 | | | | $ | 2,400 | |
| Additions (reductions) for taxes positions related current year | — | | | | (141) | | | | 55 | |
| Additions (reductions) for taxes positions related to prior years | 13 | | | | (2,583) | | | | 372 | |
| Balance at the end of the year | $ | 116 | | | | $ | 103 | | | | $ | 2,827 | |
The Company recognizes interest and penalties related to unrecognized tax benefits in tax expense. During the years ended December 31, 2019, 2018 and 2017, the Company accrued $13, $2 and $95, respectively, for interest and penalties expenses related to uncertain tax positions.
We file income tax returns in the U.S. and various state and foreign jurisdictions. We are currently not under examination by the Internal Revenue Service, and any state, local or foreign taxing jurisdictions. Additional tax years within the period 2015 to 2019 remain subject to examination by the U.S. Internal Revenue Service. Furthermore, tax years 2014 to 2019 remain subject to examination in other U.S. state and municipal jurisdictions, as well as foreign jurisdictions.
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 14: Share capital
Share capital is composed as follows:
| | | | | | | | | | | |
| Issued and outstanding Number of shares December 31, | | |
| | 2019 | | 2018 |
| Ordinary shares no par value | 99,528,435 | | | | 93,254,185 | |
a. Warrants:
As part of the Series D and E Convertible Preferred share investment agreements, the investors received warrants to purchase ordinary shares. The Company accounted for these warrants as equity instruments based on the guidance of ASC 815, "Derivatives and Hedging," ASC 480-10, "Distinguishing Liabilities from Equity," its related FASB staff positions, ASC 815-40 "Contracts in Entity’s Own Stock" and the AICPA Technical Practice Aid for accounting for preferred shares and warrants, including the roadmap for accounting for freestanding financial instruments indexed to, and potentially settled in, a company’s own stock.
In the years ended December 31, 2018 and 2017, warrants to purchase 504,225 and 1,418,711 ordinary shares, respectively, were cashlessly exercised, resulting in the issuance of 437,081 and 803,138 ordinary shares, respectively. Also, in the year ended December 31, 2018 and 2017 warrants to purchase 3,879 and 6,498 ordinary shares, respectively, with an exercise price of $3.59 per share were exercised for cash. No warrants were outstanding as of December 31, 2019 and 2018.
b. Share option plans and ESPP:
Until the IPO in October 2015, the Company maintained and granted option awards under the 2003 Share Option Plan (the "2003 Plan") and the 2013 Equity Incentive Share Option Plan (the "2013 Plan") for the Company’s officers, directors, employees and advisors. The 2003 Plan and the 2013 Plan terminated as of the IPO as to future awards, but they continue to govern option awards previously granted thereunder.
In September 2015, the Company adopted the 2015 Omnibus Incentive Plan (the "2015 Plan"). The Company’s shareholders approved the 2015 Plan in September 2015. Under the 2015 Plan, the Company can issue various types of equity compensation awards such as restricted shares, performance shares, restricted stock units ("RSUs"), performance units, long-term cash award and other share-based awards. Options granted under the 2015 Plan generally have a four-year vesting period and expire ten years after the date of grant. Options granted under the 2015 Plan that are cancelled or forfeited before expiration become available for future grants. RSUs granted under the 2015 Plan vest in equal installments over a three-year period.
On December 31, 2019, in accordance with the terms of the 2015 Plan, the number of shares available for issuance under the 2015 Plan automatically increased by 4% of the Company’s outstanding ordinary shares as of December 30, 2019. As a result, the number of shares available for issuance under the 2015 Plan increased from 27,035,515 shares to 31,015,695 shares. As of December 31, 2019, 15,826,518 ordinary shares are available for grant under the 2015 Plan.
In September 2015, the Company adopted an ESPP to encourage and enable eligible employees to acquire ownership of the Company’s ordinary shares purchased through accumulated payroll deductions on an after-tax basis. The ESPP is intended to be an "employee stock purchase plan" within the meaning of Section 423 of the Code and the provisions of the ESPP will be construed in a manner consistent with the requirements of such section. The Company began its offerings under the ESPP on August 1, 2016. The Company issued 414,559 ordinary shares for the plan periods ended through December 31, 2019.
On December 31, 2019, in accordance with the terms of the ESPP, the number of shares available for purchase by eligible employees who participate in the ESPP automatically increased by 1% of the Company’s outstanding ordinary shares outstanding on December 30, 2019. As of December 31, 2019, 4,050,089 ordinary shares are available for offering under the ESPP.
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
The fair value of share-based awards was estimated using the Black-Scholes model for all equity grants. For market condition awards, the Company also applied the Monte-Carlo simulation model, with the following underlying assumptions:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| Stock Option Plans | | | | | |
| Expected term (years) | 5.50-6.00 | | 5.50-6.25 | | 5.50-6.25 |
| Expected volatility | 55%-61% | | 52%-55% | | 57%-59% |
| Risk-free interest rate | 1.73%-2.40% | | 2.70%-2.99% | | 1.97%-2.23% |
| Dividend yield | 0.00 | % | | 0.00 | % | | 0.00 | % |
| ESPP | | | | | |
| Expected term (years) | 0.50 | | 0.50 | | 0.50 |
| Expected volatility | 44%-62% | | 45%-53% | | 76%-82% |
| Risk-free interest rate | 2.10%-2.51% | | 1.61%-2.14% | | 0.62%-1.13% |
| Dividend yield | 0.00 | % | | 0.00 | % | | 0.00 | % |
A summary of the status of the Company’s options to purchase ordinary shares as of December 31, 2019 and changes during the year ended on that date is presented below:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, 2019 | | | | |
| | Number of options | | Weighted average exercise price | | Aggregate intrinsic value |
| Outstanding at beginning of year | 14,438,215 | | | $ | 13.56 | | | |
| Granted | 1,552,987 | | | 50.45 | | | |
| Exercised | (5,466,170) | | | 10.83 | | | |
| Forfeited and cancelled | (174,222) | | | 22.11 | | | |
| Outstanding at end of year | 10,350,810 | | | $ | 20.40 | | | $ | 661,150 | |
| Exercisable options | 3,217,923 | | | $ | 15.55 | | | $ | 221,146 | |
A summary of the status of the Company’s RSUs as of December 31, 2019 and changes during the year ended on that date is presented below:
| | | | | | | | | | | | | | | | | |
| | Year ended December 31, 2019 | | | | |
| | Number of RSUs | | Weighted average grant date fair value price | | Aggregate intrinsic value |
| Unvested at beginning of year | 1,613,197 | | | $ | 14.04 | | | |
| Granted | 634,694 | | | 52.28 | | | | |
| Vested | (740,714) | | | 13.47 | | | | |
| Forfeited and cancelled | (32,782) | | | 37.84 | | | | |
| Unvested at end of year | 1,474,395 | | | $ | 30.26 | | | $ | 124,247 | |
The total equity-based compensation expense related to all of the Company’s equity-based awards recognized for the years ended December 31, 2019, 2018 and 2017, was comprised as follows:
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
| | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| Cost of revenues | $ | 2,231 | | | | $ | 1,261 | | | | $ | 467 | |
| Research, development and clinical trials | 7,570 | | | | 4,709 | | | | 3,587 | |
| Sales and marketing | 11,897 | | | | 7,393 | | | | 3,784 | |
| General and administrative | 30,718 | | | | 26,483 | | | | 19,278 | |
| Total share-based compensation expense | $ | 52,416 | | | | $ | 39,846 | | | | $ | 27,116 | |
As of December 31, 2019, unamortized share-based compensation costs amounted to $62,498 and are expected to be recognized over a weighted average period of approximately 2.51 years.
The weighted average grant date exercise price of the Company’s options granted during the years ended December 31, 2019, 2018 and 2017 were $50.45, $23.73 and $10.53 per share, respectively.
The weighted average grant date fair values of the Company’s options forfeited and cancelled during the years ended December 31, 2019, 2018 and 2017 were $22.11, $15.09 and $12.54, respectively.
The aggregate intrinsic values for the options exercised during the years ended December 31, 2019, 2018 and 2017 were $266,626, $57,813 and $17,945, respectively. The aggregate intrinsic value is calculated as the difference between the per share exercise price and the deemed fair value of the Company’s ordinary shares for each share subject to an option multiplied by the number of shares subject to options at the date of exercise. The Company deemed the fair value of the Company’s ordinary shares to be $84.27, $33.48 and $20.20 per share as of December 31, 2019, 2018, and 2017, respectively.
The options outstanding as of December 31, 2019 are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
| Exercise price | | Number of options outstanding | | Weighted average remaining contractual term | | Number of options exercisable | | Weighted average remaining contractual term |
| $ | | | | (years) | | | | (years) |
0.23 - 10.00 | | 2,256,916 | | | 6.15 | | 984,380 | | | 4.87 |
10.01 - 20.00 | | 3,205,758 | | | 6.83 | | 869,058 | | | 5.84 |
20.01 - 30.00 | | 2,933,518 | | | 7.4 | | 1,225,951 | | | 6.57 |
30.01 - 40.00 | | 424,414 | | | 8.56 | | 138,534 | | | 8.47 |
40.01 - 50.00 | | 1,347,064 | | | 9.29 | | — | | | — |
50.01 - 60.00 | | 44,380 | | | 9.39 | | — | | | — |
60.01 - 90.00 | | 138,760 | | | 9.7 | | — | | | — |
| | | 10,350,810 | | | 7.28 | | 3,217,923 | | | 5.94 |
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 15: Financial expenses, net
The following table sets forth the Company’s total financial expenses, net:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| Financial expenses: | | | | | |
| Interest expense | $ | (13,718) | | | $ | (13,491) | | | $ | (10,261) | |
| Amortization of credit facility costs | (156) | | | (2,777) | | | (1,111) | |
| Foreign currency transaction losses | (431) | | | (398) | | | — | |
| Others | (338) | | | (242) | | | (321) | |
| | $ | (14,643) | | | $ | (16,908) | | | $ | (11,693) | |
| Financial income: | | | | | | | | |
| Amortization of treasury bills premium | $ | 2,331 | | | $ | 1,986 | | | $ | 859 | |
| Foreign currency transaction gains | — | | | — | | | 549 | |
| Interest income | 4,402 | | | 2,652 | | | 1,116 | |
| | $ | 6,733 | | | $ | 4,638 | | | $ | 2,524 | |
| Total financial expenses, net | $ | (7,910) | | | $ | (12,270) | | | $ | (9,169) | |
Note 16: Basic and diluted net loss per share
The following table sets forth the computation of the Company’s basic and diluted net loss per ordinary share:
| | | | | | | | | | | | | | | | | |
| | Year ended December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| Net income (loss) attributable to ordinary shares as reported | $ | (7,230) | | | $ | (63,559) | | | $ | (61,662) | |
| Shares used in computing net income (loss) per ordinary share, basic and diluted | 97,237,549 | | | 91,828,043 | | | 88,546,719 | |
| Net income (loss) per ordinary share, basic and diluted | $ | (0.07) | | | | $ | (0.69) | | | | $ | (0.70) | |
For the full years ended December 31, 2019, 2018 and 2017, all outstanding options, restricted share units and warrants have been excluded from the calculation of the diluted net loss per share since their effect was anti-dilutive. The following table presents the potentially dilutive shares that were excluded from the computation of diluted net income (loss) per share:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | |
| 2019 | | 2018 | | 2017 |
| Options | 8,041,159 | | | | 7,744,521 | | | | 4,195,762 | |
| Restricted share units | 1,152,180 | | | | 1,238,657 | | | | 641,358 | |
| Warrants | — | | | | — | | | | 384,774 | |
| Total potential anti-dilutive shares | 9,193,339 | | | | 8,983,178 | | | | 5,221,894 | |
Note 17: Subcontractor
In certain markets and for certain key components, the Company is currently dependent upon sole source suppliers used in its delivery systems. The Company’s management believes that in most cases other suppliers could provide similar components at comparable terms. A change of suppliers which requires FDA or other regulatory approval, however, could cause a material delay in manufacturing and a possible loss of sales, which could adversely affect the Company’s operating results and financial position.
Note 18: Supplemental information
The following table presents long-lived assets by location:
| | | | | | | | | | | | | | | | | |
| December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| United States | $ | 8,896 | | | | $ | 8,289 | | | | $ | 10,372 | |
| Switzerland | 3,067 | | | | 2,513 | | | | 5,114 | |
| Israel | 2,753 | | | | 2,236 | | | | 2,081 | |
| Germany | 729 | | | | 1,054 | | | | 190 | |
| Others | 1,581 | | | | 1,274 | | | | 310 | |
| Total long-lived assets | $ | 17,026 | | | | $ | 15,366 | | | | $ | 18,067 | |
The Company’s net revenues by geographic region, based on the patient’s location are summarized as follows:
| | | | | | | | | | | | | | | | | |
| Year ended December 31, | | | | |
| | 2019 | | 2018 | | 2017 |
| United States | $ | 232,805 | | | $ | 168,414 | | | $ | 134,688 | |
| EMEA: | | | | | |
| Germany | 86,564 | | | 67,849 | | | 40,215 | |
| Other EMEA | 8,782 | | | 4,653 | | | 1,820 | |
| Japan | 17,912 | | | 6,351 | | | 303 | |
| Greater China (1) | 5,255 | | | 802 | | | — | |
| Total net revenues | $ | 351,318 | | | $ | 248,069 | | | $ | 177,026 | |
(1) Reflects revenue recognized in accordance with the Zai Agreement, pursuant to which Zai is commercializing Optune in China, Hong Kong, Macau and Taiwan (referred to in this table as "Greater China"). For additional information, see Note 12.
Note 19: Selected quarterly financial information (Unaudited)
The following table sets forth selected financial information for the Company:
| | | | | | | | | | | | | | | | | | | | | | | |
| | 2019 | | | | | | |
| | Three months ended | | | | | | |
| | December 31 | | September 30 | | June 30 | | March 31 |
| Net revenues | $ | 99,234 | | | | $ | 92,062 | | | | $ | 86,713 | | | | $ | 73,309 | |
| Gross profit | 74,448 | | | | 69,162 | | | | 65,607 | | | | 53,495 | |
| Operating income (loss) | 153 | | | | 3,855 | | | | 1,196 | | | | (6,118) | |
| Net income (loss) | 4,260 | | | | 1,930 | | | | (1,270) | | | | (12,150) | |
| Basic net income (loss) per ordinary share | $ | 0.04 | | | | $ | 0.02 | | | | $ | (0.01) | | | | $ | (0.13) | |
| Weighted average number of ordinary shares used in computing basic net income (loss) per share | 99,226,445 | | | | 98,485,519 | | | | 96,356,317 | | | | 94,811,282 | |
| Diluted net income (loss) per ordinary share | $ | 0.04 | | | | $ | 0.02 | | | | $ | (0.01) | | | | $ | (0.13) | |
| Weighted average number of ordinary shares used in computing diluted net income (loss) per share | 107,911,519 | | | | 107,604,578 | | | | 96,356,317 | | | | 94,811,282 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | 2018 | | | | | | |
| | Three months ended | | | | | | |
| | December 31 | | September 30 | | June 30 | | March 31 |
| Net revenues | $ | 69,674 | | | $ | 64,756 | | | $ | 61,514 | | | $ | 52,125 | |
| Gross profit | 46,646 | | | 45,807 | | | 41,681 | | | 33,887 | |
| Operating income (loss) | (8,664) | | | (5,246) | | | (7,085) | | | (12,677) | |
| Net income (loss) | (15,631) | | | (11,694) | | | (15,510) | | | (20,724) | |
| Basic and diluted net income (loss) per ordinary share | $ | (0.17) | | | $ | (0.13) | | | $ | (0.17) | | | $ | (0.23) | |
| Weighted average number of ordinary shares used in computing basic and diluted net income (loss) per share | 93,083,298 | | | 92,911,375 | | | 91,331,862 | | | 89,985,612 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | 2017 | | | | | | |
| | Three months ended | | | | | | |
| | December 31 | | September 30 | | June 30 | | March 31 |
| Net revenues | $ | 53,661 | | | | $ | 50,109 | | | | $ | 38,376 | | | | $ | 34,880 | |
| Gross profit | 38,021 | | | | 34,956 | | | | 25,224 | | | | 23,216 | |
| Operating income (loss) | (4,506) | | | | (5,919) | | | | (15,530) | | | | (13,373) | |
| Net income (loss) | (10,945) | | | | (11,498) | | | | (21,174) | | | | (18,045) | |
| Basic and diluted net income (loss) per ordinary share | $ | (0.12) | | | | $ | (0.13) | | | | $ | (0.24) | | | | $ | (0.21) | |
| Weighted average number of ordinary shares used in computing basic and diluted net income (loss) per share | 89,389,364 | | | | 89,125,646 | | | | 88,218,868 | | | | 87,452,983 | |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
(a) Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K. Based on that evaluation, the Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K have been designed and are functioning effectively to provide reasonable assurance that the information required to be disclosed by us in reports filed under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. We believe that a controls system, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the controls system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within a company have been detected.
(b) Management’s Annual Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d- 15(f) under the Exchange Act. Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with general accepted accounting principles. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2019. In making this assessment, it used the criteria established in Internal Control- Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Based on such assessment, management has concluded that, as of December 31, 2019, our internal control over financial reporting is effective based on those criteria.
(c) Attestation Report of the Registered Public Accounting Firm
The effectiveness of our internal control over financial reporting as of December 31, 2019, has been audited by Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global, an independent registered public accounting firm, as stated in their attestation report, which is included herein.
(d) Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting during our most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
ITEM 9B. OTHER INFORMATION
None.
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors
The information required by Item 10 is incorporated herein by reference to the information contained under the caption "Proposal 1 — Election of Directors" in our definitive proxy statement related to the 2020 annual meeting of shareholders.
Executive Officers
The information concerning our executive officers required by this Item 10 is provided under the caption "Information about our Executive Officers" in Part I hereof.
Audit Committee
The information required by this Item 10 is incorporated by reference to the information contained under the caption "Corporate Governance" in our definitive proxy statement related to the 2020 annual meeting of shareholders.
Code of Ethics
The information concerning our Code of Ethics is incorporated by reference to the information contained under the caption "Corporate Governance" in our definitive proxy statement related to the 2020 annual meeting of shareholders.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this Item 11 is incorporated by reference to the information contained under the caption "2019 Director Compensation" and "Executive Compensation" in our definitive proxy statement related to the 2020 annual meeting of shareholders.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by Item 12 regarding the ownership of our ordinary shares is incorporated by reference to the information contained under the caption "Information About Stock Ownership" in our definitive proxy statement related to the 2020 annual meeting of shareholders.
The information required by Item 12 with respect to securities authorized for issuance under our equity compensation plans is provided under the caption "Equity Compensation Plan Information" in Part II, Item 5 hereof.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by Item 13 is incorporated by reference to the information contained under the captions "Proposal 1 – Election of Directors," "Corporate Governance," and "Certain Relationships and Related Party Transactions" in our definitive proxy statement related to the 2020 annual meeting of shareholders.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by Item 14 is incorporated by reference to the information contained under the caption "Proposal 2 – Approval and Ratification of Approval and Appointment of Independent Registered Public Accounting Firm" in our definitive proxy statement related to the 2020 annual meeting of shareholders.
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a) DOCUMENTS FILED AS PART OF THIS REPORT
The following is a list of our consolidated financial statements and our subsidiaries and supplementary data included in this Annual Report on Form 10-K under Item 8 of Part II hereof:
| | | | | |
| 1. FINANCIAL STATEMENTS AND SUPPLEMENTAL DATA | |
| | |
| | Report of Independent Registered Public Accounting Firm. |
| | |
| | Consolidated Balance Sheets as of December 31, 2019, 2018 and 2017. |
| | |
| | Consolidated Statements of Operations for the years ended December 31, 2019, 2018 and 2017. |
| | |
| | Consolidated Statement of Comprehensive Loss for the years ended December 31, 2019, 2018 and 2017. |
| | |
| | Consolidated Statements of Shareholders’ Equity for the years ended December 31, 2019, 2018 and 2017. |
| | |
| | Consolidated Statements of Cash Flows for the years ended December 31, 2019, 2018 and 2017. |
| | |
| | Notes to Consolidated Financial Statements. |
| | |
| 2. FINANCIAL STATEMENT SCHEDULES | |
Schedules are omitted because they are not applicable or are not required, or because the required information is included in the consolidated financial statements or notes thereto.
(b) EXHIBITS
The following is a list of exhibits filed as part of this Annual Report on Form 10-K. Where so indicated by footnote, exhibits that were previously filed are incorporated by reference. For exhibits incorporated by reference, the location of the exhibit in the previous filing is indicated.
EXHIBIT INDEX
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Exhibit Description | | Incorporated by Reference | | | | | | Filed
Herewith |
| | | | Form | | Date | | Number | | |
| 3.1 | | | | S-1/A | | 9/21/15 | | 3.3 | | |
| 3.2 | | | | 8-K | | 6/6/18 | | 3.2 | | |
| 4.1 | | | | | | | | 4.1 | | X |
| 4.2 | | | | DRS | | 6/24/15 | | 4.2 | | |
| 4.3 | | | | DRS | | 6/24/15 | | 4.3 | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Exhibit Description | | Incorporated by Reference | | | | | | Filed
Herewith |
| | | | Form | | Date | | Number | | |
| 10.1 | | | | DRS | | 6/24/15 | | 10.1 | | |
| 10.2 | | | | 8-K | | 12/30/16 | | 10.2 | | |
| 10.3 | | | | 8-K | | 3/31/17 | | 10.1 | | |
| 10.4 | | | | 8-K | | 10/5/17 | | 10.1 | | |
| 10.5 | | | | 10-Q | | 4/26/18 | | 10.1 | | |
| 10.6 | | | | 10-Q | | 10/25/18 | | 10.2 | | |
| 10.7 | | | | DRS/A | | 8/11/15 | | 10.13 | | |
| 10.8 | | | | DRS | | 6/24/15 | | 10.3 | | |
| 10.9 | | | | DRS | | 6/24/15 | | 10.4 | | |
| 10.10 | | | | S-1/A | | 9/21/15 | | 10.5 | | |
| 10.11 | | | | S-1/A | | 9/21/15 | | 10.14 | | |
| 10.12 | | | | S-1/A | | 9/21/15 | | 10.15 | | |
| 10.13 | | | | S-1/A | | 9/21/15 | | 10.17 | | |
| 10.14 | | | | S-1/A | | 9/21/15 | | 10.18 | | |
| 10.15 | | | | 8-K | | 12/22/15 | | 10.1 | | |
| 10.16 | | | | 8-K | | 12/22/15 | | 10.2 | | |
| 10.17 | | | | 8-K | | 12/22/15 | | 10.3 | | |
| 10.18 | | | | 8-K | | 12/22/15 | | 10.4 | | |
| 10.19 | | | | 8-K | | 12/22/15 | | 10.5 | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Exhibit Description | | Incorporated by Reference | | | | | | Filed
Herewith |
| | | | Form | | Date | | Number | | |
| 10.20 | | | | 10-K | | 3/1/16 | | 10.25 | | |
| 10.21 | | | | 8-K | | 5/12/17 | | 10.1 | | |
| 10.22 | | | | 8-K | | 5/12/17 | | 10.2 | | |
| 10.23 | | | | 8-K | | 4/4/18 | | 10.1 | | |
| 10.24 | | | | 8-K | | 8/1/17 | | 99.1 | | |
| 10.25 | | | | 8-K | | 3/22/16 | | 10.1 | | |
| 10.26 | | | | 8-K | | 5/13/16 | | 10.1 | | |
| 10.27 | | | | 8-K | | 6/30/16 | | 10.1 | | |
| 10.28 | | | | 8-K | | 4/26/18 | | 10.1 | | |
| 10.29 | | | | 8-K | | 10/14/16 | | 10.1 | | |
| 10.30 | | | | 8-K | | 10/14/16 | | 10.2 | | |
| 10.31 | | | | 8-K | | 10/14/16 | | 10.3 | | |
| 10.32 | | | | 10-K | | 2/23/17 | | 10.27 | | |
| 10.33 | | | | 10-Q | | 10/25/18 | | 10.1 | | |
| 10.34 | | | | 10-K | | 2/23/17 | | 10.28 | | |
| 10.35 | | | | 10-K | | 2/23/17 | | 10.29 | | |
| 10.36 | | | | 10-K | | 2/23/17 | | 10.30 | | |
| 10.37 | | | | 10-K | | 2/23/17 | | 10.31 | | |
| 10.38 | | | | 8-K | | 4/4/18 | | 10.2 | | |
| 21 | | | | | | | | | | X |
| 23.1 | | | | | | | | | | X |
| 31.1 | | | | | | | | | | X |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Exhibit
Number | | Exhibit Description | | Incorporated by Reference | | | | | | Filed
Herewith |
| | | | Form | | Date | | Number | | |
| 31.2 | | | | | | | | | | X |
| 32.1* | | | | | | | | | | X |
| 32.2* | | | | | | | | | | X |
| 101.INS | | Inline XBRL Instance Document | | | | | | | | X |
| 101.SCH | | Inline XBRL Taxonomy Extension Schema Document | | | | | | | | X |
| 101.CAL | | Inline XBRL Taxonomy Extension Calculation Linkbase Document | | | | | | | | X |
| 101.DEF | | Inline XBRL Taxonomy Extension Definition Linkbase Document | | | | | | | | X |
| 101.LAB | | Inline XBRL Taxonomy Extension Label Linkbase Document | | | | | | | | X |
| 101.PRE | | Inline XBRL Extension Presentation Linkbase Document | | | | | | | | X |
| 104 | | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | | | | | | | | X |
| | | | | | | | |
| | |
| | |
| * | The certifications attached as Exhibits 32.1 and 32.2 that accompany this Annual Report on Form 10-K are not deemed filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of NovoCure Limited under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Form 10-K, irrespective of any general incorporation language contained in such filing. | |
| † | Confidential treatment has been granted for certain information set forth in this exhibit. Such information has been omitted and filed separately with the Securities and Exchange Commission. | |
| # | Compensation plans and arrangements for executive officers and others. | |
This Annual Report on Form 10-K includes trademarks of NovoCure Limited and other persons. All trademarks or trade names referred to herein are the property of their respective owners.
ITEM 16. FORM 10-K SUMMARY
None.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Date: February 27, 2020
| | | | | | | | | | | |
| | NovoCure Limited | |
| | | |
| | By: | /s/ Asaf Danziger |
| | | Asaf Danziger |
| | | Chief Executive Officer |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Date: | | Signature | | Title |
| | | | | |
| February 27, 2020 | | /s/ Asaf Danziger | | Chief Executive Officer and Director (Principal Executive Officer) |
| | | Asaf Danziger | | |
| | | | | |
| February 27, 2020 | | /s/ Wilhelmus Groenhuysen | | Chief Financial Officer (Principal Financial and Accounting Officer) |
| | | Wilhelmus Groenhuysen | | |
| | | | | |
| February 27, 2020 | | /s/ William F. Doyle | | Executive Chairman and Director |
| | | William F. Doyle | | |
| | | | | |
| February 27, 2020 | | /s/ Kinyip Gabriel Leung | | Director |
| | | Kinyip Gabriel Leung | | |
| | | | | |
| | | | | |
| February 27, 2020 | | /s/ Jeryl L. Hilleman | | Director |
| | | Jeryl L. Hilleman | | |
| | | | | |
| February 27, 2020 | | /s/ David T. Hung | | Director |
| | | David T. Hung | | |
| | | | | |
| February 27, 2020 | | /s/ Martin J. Madden | | Director |
| | | Martin J. Madden | | |
| | | | | |
| February 27, 2020 | | /s/ Sherilyn D. McCoy | | Director |
| | | Sherilyn D. McCoy | | |
| | | | | |
| February 27, 2020 | | /s/ Charles G. Phillips III | | Director |
| | | Charles G. Phillips III | | |
| | | | | |
| February 27, 2020 | | /s/ William A. Vernon | | Director |
| | | William A. Vernon | | |