Exhibit 99.1
Preliminary and Subject to Completion, dated December 21, 2015
PRELIMINARY INFORMATION STATEMENT
ONCOCYTE CORPORATION
Common Stock, no par value
This information statement is being furnished in connection with the distribution of shares of common stock, no par value, of OncoCyte Corporation (“OncoCyte”) to holders of common shares, no par value, of BioTime, Inc. (the “Distribution”).
OncoCyte is currently a 76.39 % owned subsidiary of BioTime and is engaged in the business of developing laboratory tests for the diagnosis of cancer. Following the Distribution, we will continue to be a majority owned subsidiary of BioTime but our common stock will be publicly traded and we will continue to build our own management team.
You will receive one share of OncoCyte common stock for every 20 BioTime common shares you hold as of the close of business on December 21, 2015, the “record date” of the Distribution. If you sell your BioTime common shares after the record date and before the date of the Distribution, you also will be selling your right to receive shares of OncoCyte common stock in the Distribution. The Distribution will be made in book-entry form. We expect the Distribution to occur on December 31, 2015. In this information statement we sometimes refer to the shares of OncoCyte common stock to be distributed to BioTime shareholders in the Distribution as “Distribution Shares.”
No shareholder approval of the Distribution by BioTime shareholders is required or sought and you are not being asked for a proxy to vote on the Distribution, and you are requested not to send us or BioTime a proxy.
BioTime shareholders will not be required to pay for the Distribution Shares they receive in the Distribution or to surrender or exchange BioTime common shares in order to receive their Distribution Shares or to take any other action in connection with the Distribution.
There is no current trading market for OncoCyte common stock. However, we have applied to list OncoCyte common stock on the NYSE MKT under the symbol OCX. If our listing application is not approved, we plan to arrange for the trading of our common stock on the OTC Bulletin Board no later than the completion of the Distribution.
In reviewing this information statement, you should carefully consider the matters described under “Risk Factors” for a discussion of certain factors that should be considered by recipients of our common stock. The Distribution will not qualify as a tax free reorganization for U.S. federal income tax purposes.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this information statement is truthful or complete. Any representation to the contrary is a criminal offense.
This information statement does not constitute an offer to sell or the solicitation of an offer to buy any securities.
The date of this information statement is December , 2015.
TABLE OF CONTENTS
| |
Page
|
|
Information Statement Summary
|
4
|
| |
|
|
Questions and Answers About OncoCyte and the Distribution
|
8
|
| |
|
|
Risk Factors
|
12
|
| |
|
|
The Distribution
|
27
|
| |
|
|
Market for Our Common Equity
|
30
|
| |
|
|
Dividend Policy
|
30
|
| |
|
|
Capitalization
|
30
|
| |
|
|
Summary and Selected Financial Data
|
31
|
| |
|
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
32
|
| |
|
|
Business
|
40
|
| |
|
|
Management
|
65
|
| |
|
|
Executive Compensation
|
72
|
| |
|
|
Security Ownership by Certain Beneficial Owners and Management
|
77
|
| |
|
|
Certain Federal Income Tax Considerations
|
81
|
| |
|
|
Shares Eligible For Future Sale
|
84
|
| |
|
|
Description of Securities
|
86
|
| |
|
|
Index to Audited Financial Statements
|
F-1
|
| |
|
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
| |
|
|
Index to Unaudited Condensed Interim Financial Statements
|
F-23
|
This information statement is being furnished solely to provide information to BioTime shareholders who will receive shares of our common stock in the Distribution. It is not and is not to be construed as an inducement or encouragement to buy or sell any of our securities or any securities of BioTime. This information statement describes our business, our relationship with BioTime and how the Distribution affects BioTime and its shareholders, and provides other information to assist you in evaluating the benefits and risks of holding or disposing of our common stock that you will receive in the Distribution. You should be aware of certain risks relating to the Distribution, our business, and ownership of our common stock, which are described under the heading “Risk Factors.”
You should not assume that the information contained in this information statement is accurate as of any date other than the date set forth on the cover. Changes to the information contained in this information statement may occur after that date, and we undertake no obligation to update the information, except in the normal course of our public disclosure obligations and practices.
You should rely only on the information contained in this information statement. We have not, and BioTime has not, authorized any other person to provide you with information different from, or in addition to, that contained in this information statement. The information contained in this information statement is accurate only as of its date, regardless of the time of the delivery of Distribution Shares.
FOR RESIDENTS OF THE PHILIPPINES:
THESE SECURITIES ARE BEING OFFERED OR SOLD PURSUANT TO AN EXEMPT TRANSACTION UNDER SECTION 10.1(c) OF THE PHILIPPINES SECURITIES REGULATION CODE.
THE SECURITIES BEING OFFERED OR SOLD HAVE NOT BEEN REGISTERED WITH THE PHILIPPINES SECURITIES AND EXCHANGE COMMISSION UNDER THE SECURITIES REGULATION CODE. ANY FURTHER OFFER OR SALE THEREOF IS SUBJECT TO REGISTRATION REQUIREMENTS UNDER THE CODE UNLESS SUCH OFFER OR SALE QUALIFIES AS AN EXEMPT TRANSACTION
Industry and Market Data
This information statement contains market data and industry forecasts that were obtained from industry publications, third party market research and publicly available information. These publications generally state that the information contained therein has been obtained from sources believed to be reliable. While we believe that the information from these publications is reliable, we have not independently verified such information.
This information statement also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. We obtained the industry and market data in this information statement from our own research as well as from industry and general publications, surveys and studies conducted by third parties, some of which may not be publicly available. Such data involves a number of assumptions and limitations and contains projections and estimates of the future performance of the industries in which we operate that are subject to a high degree of uncertainty. We caution you not to give undue weight to such projections, assumptions and estimates.
REVERSE STOCK SPLIT
On November 18, 2015, OncoCyte effected a 1-for-2 reverse stock split of its common stock. All references to common stock, warrants, contingently issuable common stock warrants, and options to purchase common stock, and all per share data and related information, including the price at which shares of common stock have been sold or may be issued, have been retroactively adjusted, where applicable, in this Information Statement to reflect the reverse stock split of OncoCyte common stock as if it had occurred at the beginning of the earliest period presented.
INFORMATION STATEMENT SUMMARY
This summary provides an overview of selected information contained elsewhere in this information statement or in our registration statement on Form 10 in their entirety, including the information discussed under “Risk Factors” and our financial statements and the related notes thereto included elsewhere in this information statement.
OncoCyte Corporation
Unless otherwise indicated herein, the terms “we,” “our,” or “us,” refer to OncoCyte Corporation.
Overview
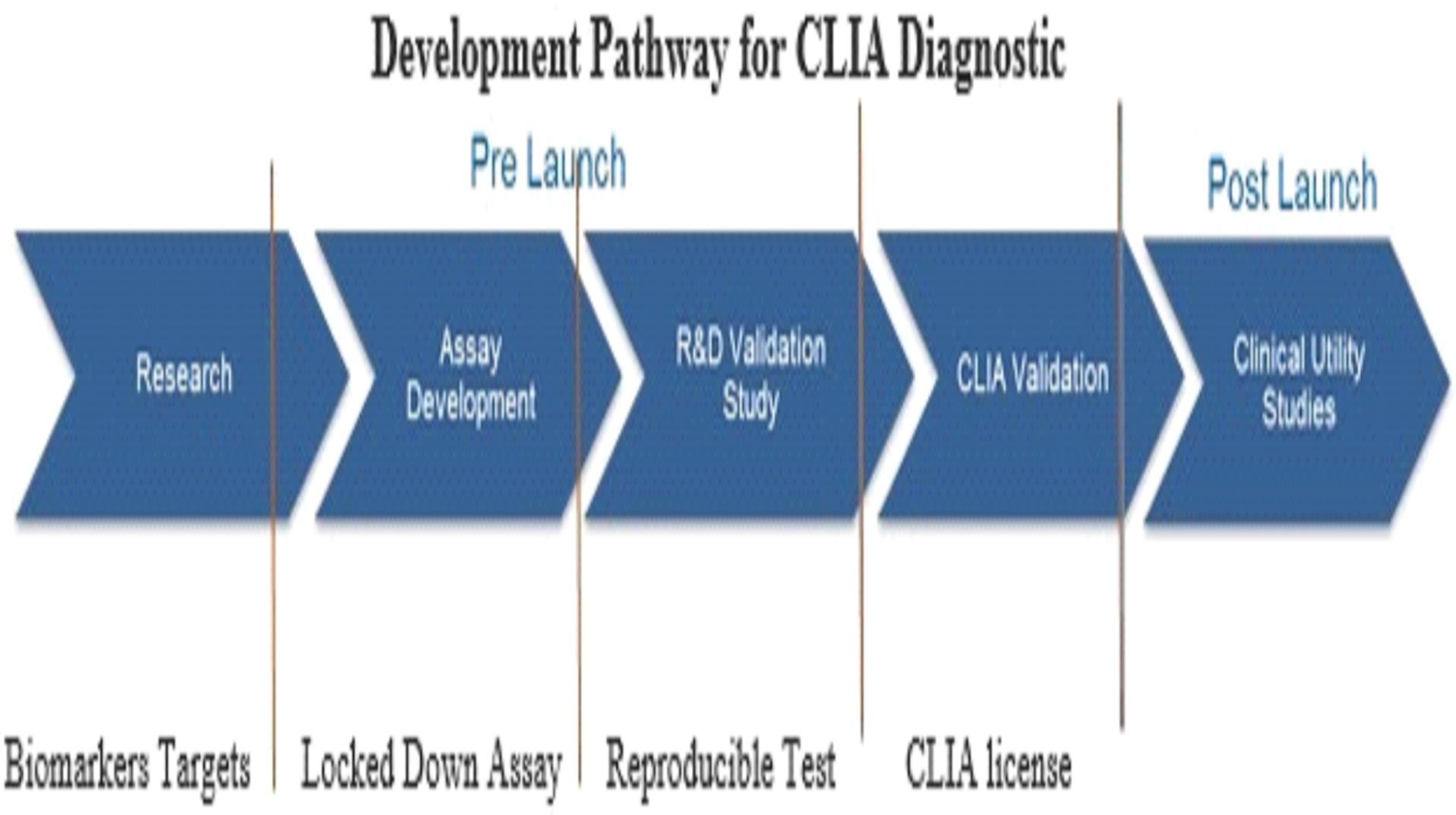
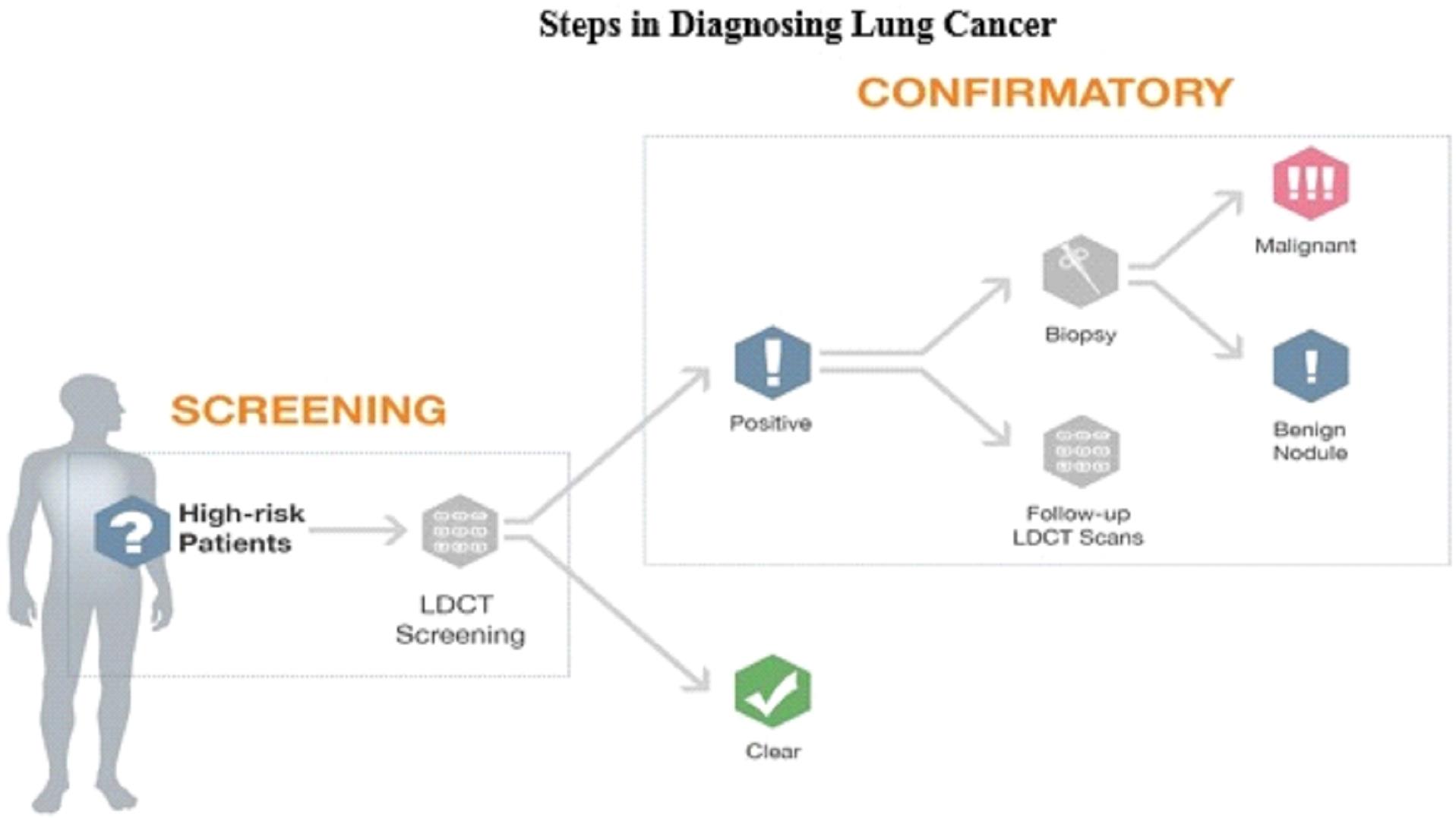
Our mission is to develop highly accurate, easy to administer, non-invasive liquid biopsy diagnostic tests in areas of high unmet need in oncology. Our initial focus will be confirmatory diagnostics that are used in conjunction with imaging to confirm initial diagnoses. In addition, we will be developing screening diagnostics as potential replacements for screening imaging procedures that do not meet the needs of patients, health care providers or payers. For some indications, we will also be pursuing the probability of recurrence of a specific cancer through the development of prognostics; or companion diagnostics that help a physician determine which therapy is the optimal treatment for the patient.
Our initial liquid biopsy diagnostic tests will be confirmatory diagnostics and are being developed to reduce false positive results associated with current diagnostic techniques. These new diagnostic tests are intended to:
| |
·
|
Improve health outcomes through early diagnoses and better prognostic capabilities;
|
|
·
|
Reduce the cost of care through the avoidance of more costly diagnostic procedures, including invasive biopsy and cystoscopic procedures; and
|
|
·
|
Improve the quality of life for cancer patients by reducing the anxiety associated with non-definitive diagnoses.
|
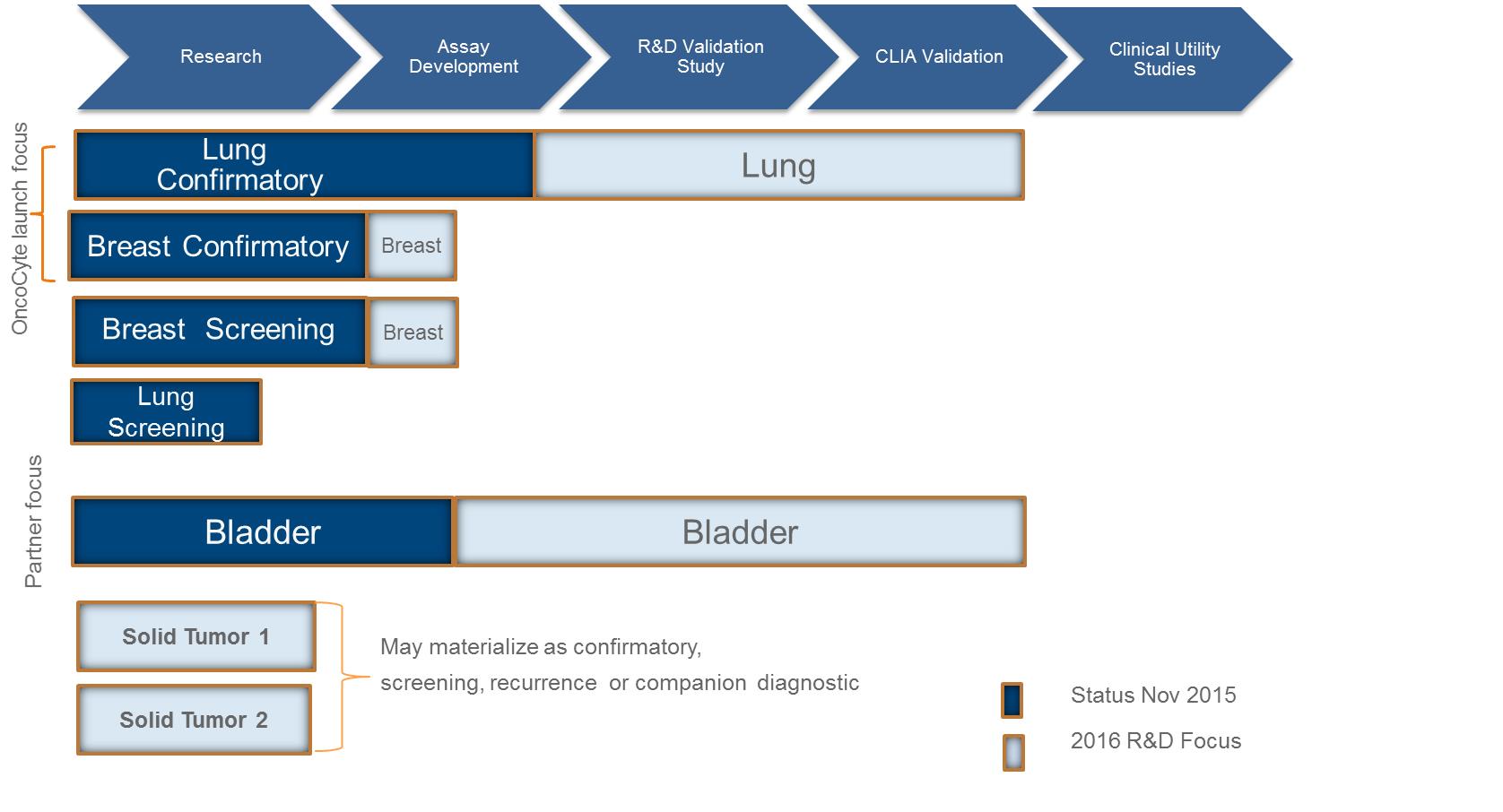
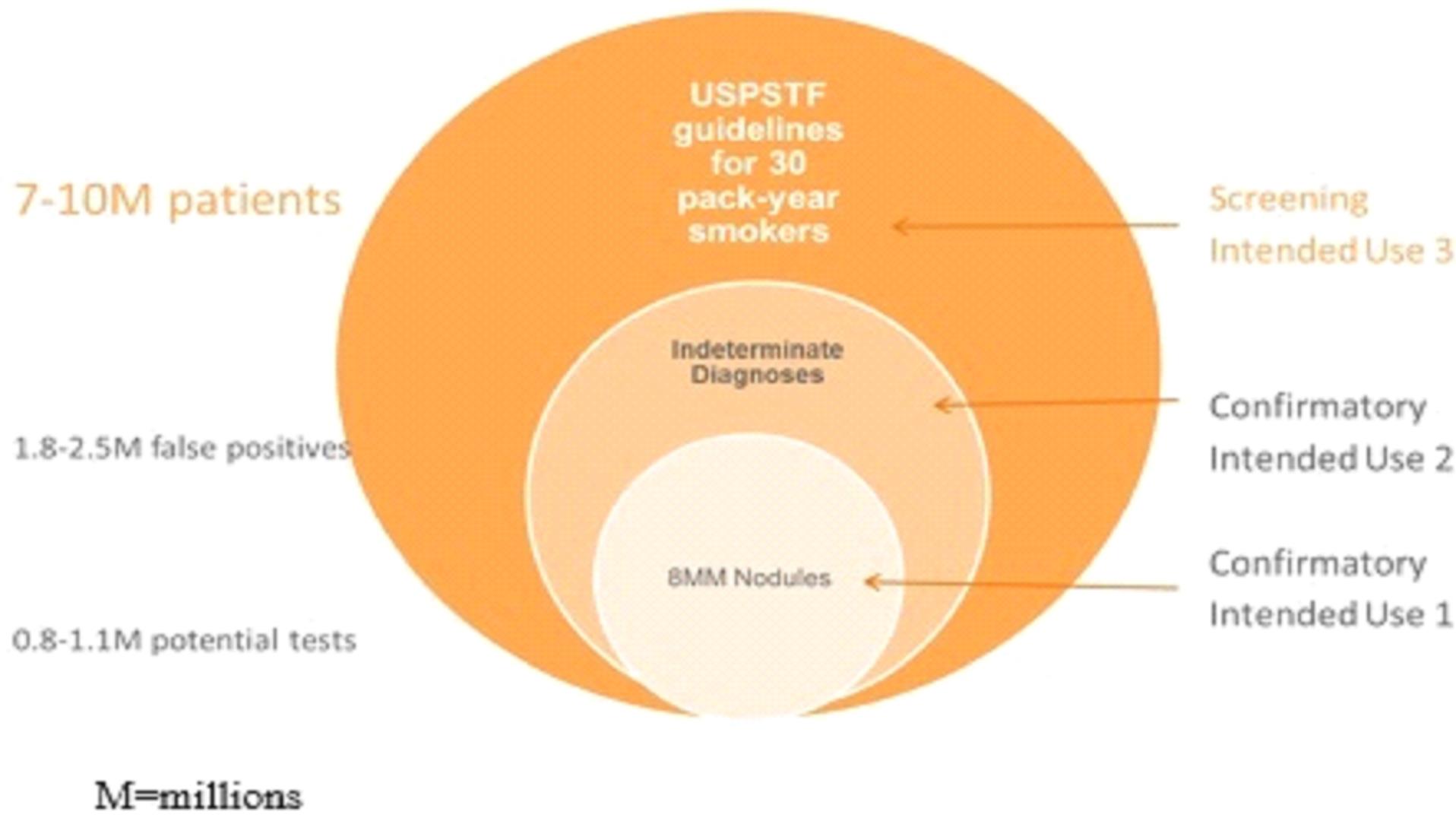
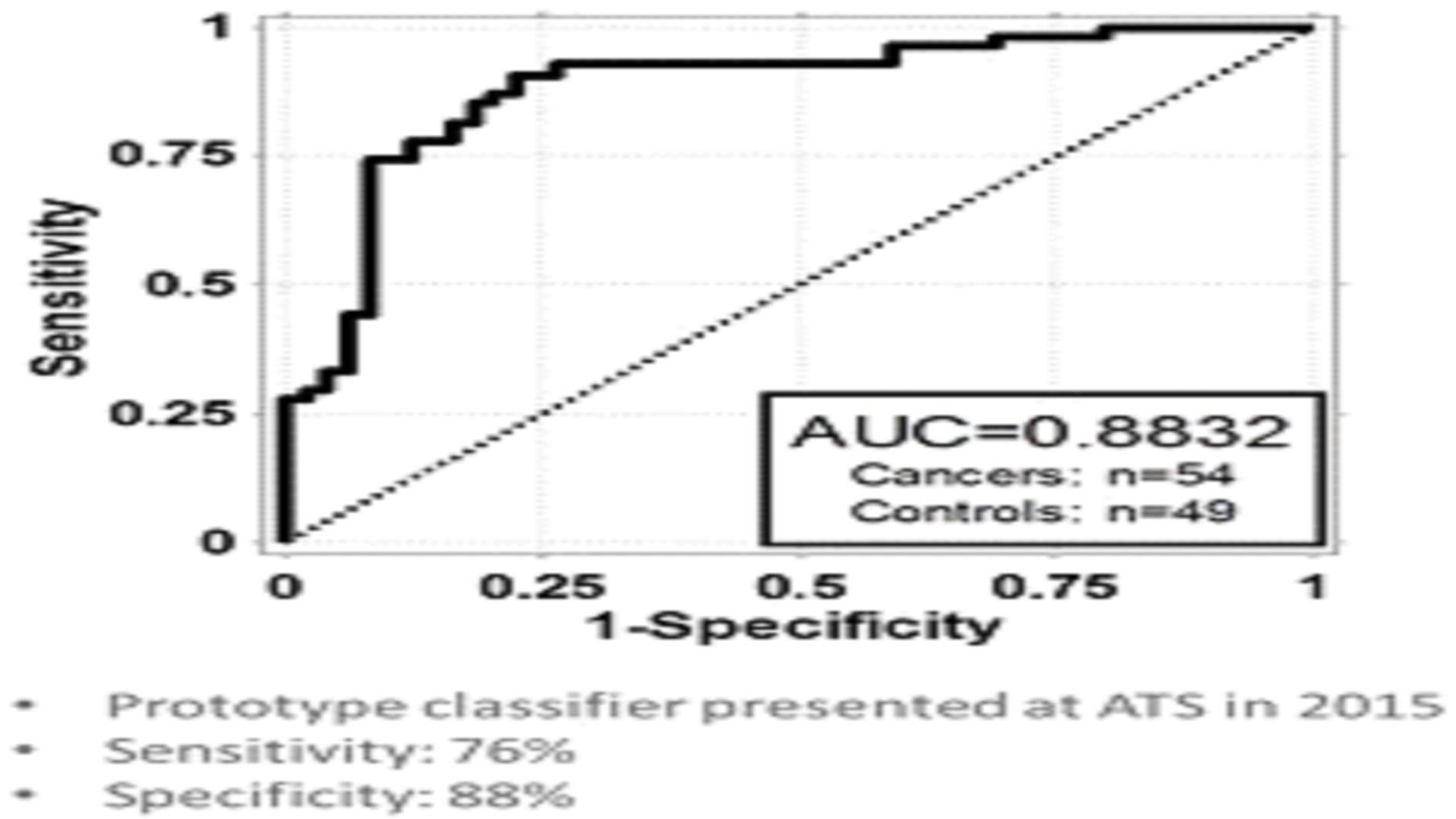
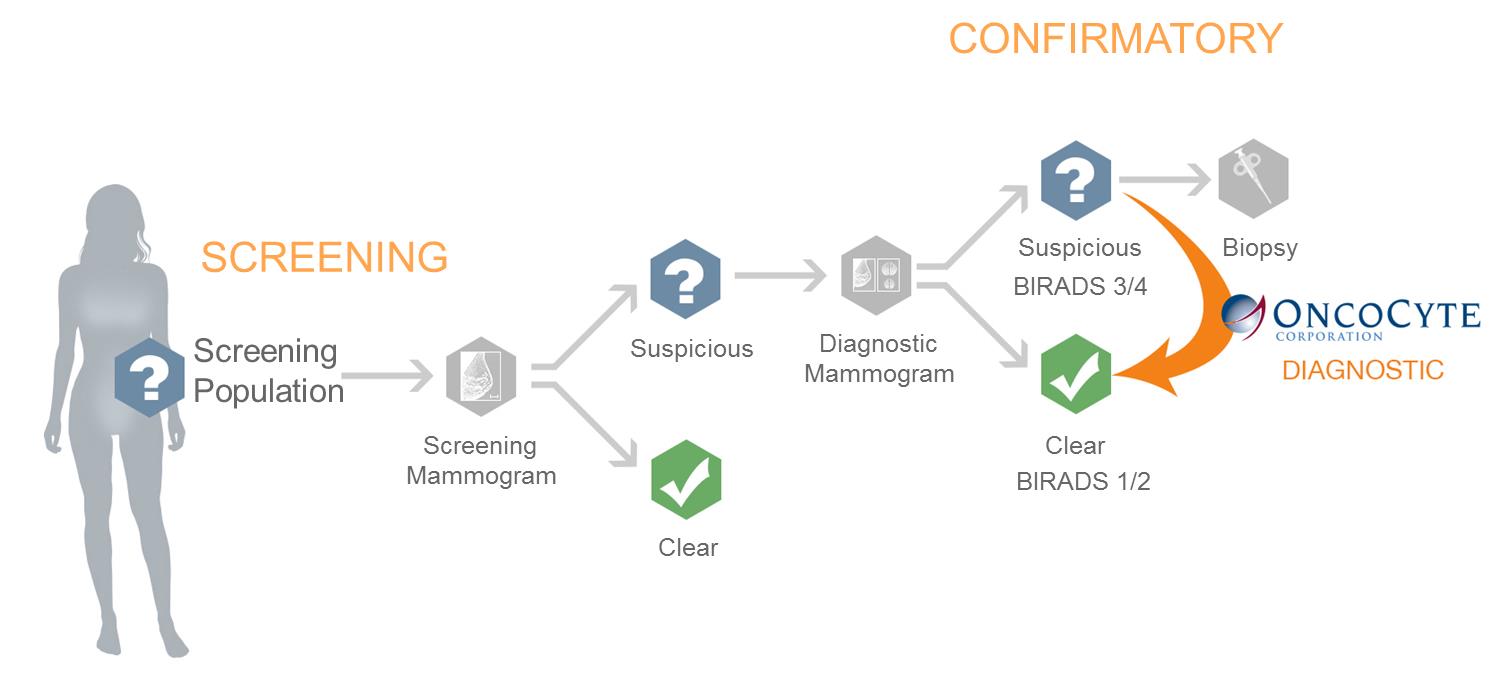
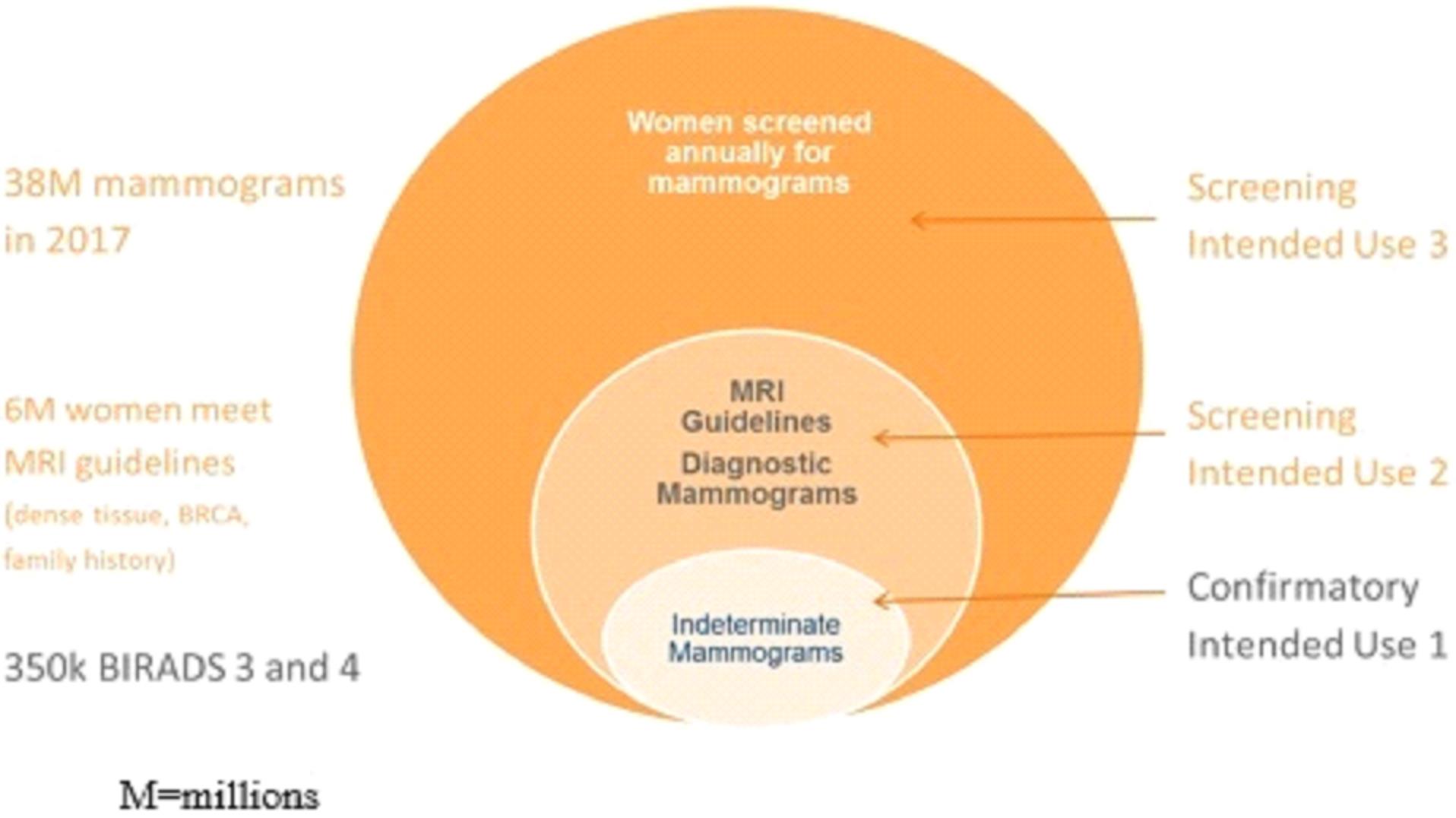
We are currently working on diagnostic tests for three types of cancer: lung cancer, breast cancer, and bladder cancer. Additionally we have early stage diagnostic research programs for other solid tumor cancers in our research and development pipeline.
Business Strategy
From our inception in late 2009 through March 2011, we focused our efforts on the development of embryonic stem cell-derived cancer therapies. In April 2011, we initiated development of molecular cancer diagnostics utilizing a discovery platform that focuses on identifying genetic markers broadly expressed in numerous types of cancer. The shift in focus from stem cell-derived cancer therapies to molecular cancer diagnostic was driven by a development focus on a unique scientific approach, the growing need for better cancer diagnostic protocols and a window of opportunity created by guidelines for lung and breast cancer screening. The diagnostic markers we have discovered thus far could address unmet needs in cancer diagnostic indications that have a strong potential to generate short- to mid-term revenues, resulting in a risk-balanced diagnostic test development strategy.
Our current development strategy for cancer diagnostic tests is to evaluate and validate specific diagnostics using methods of detecting proteins, messenger RNA (“mRNA”) or micro RNA (“miRNA”) approach based on unmet medical need, market size and ease of use. We believe that this approach allows us to have a broader look into the genetic markers that differentially express in cancer. Our development strategy will be matched to our market planning strategy to determine which:
|
·
|
Diagnostic tests to prioritize in our development program;
|
|
·
|
Diagnostic tests we should market ourselves;
|
|
·
|
Diagnostic tests we should co-market through an alliance with one or more other companies; and
|
|
·
|
Diagnostic tests we should out-license to third parties for development and/or commercialization.
|
For the near term, we plan to devote most of our financial resources to the development and commercialization of our initial laboratory diagnostic tests for certain types of cancer. While diagnostics are presently our primary focus, we may devote a portion of our resources to cancer therapeutic development based on our current technology, which pertains to homing peptides and the derivation of vascular cells engineered to deliver a toxic payload to the developing blood vessels of a malignant tumor to destroy the tumor, or based on any proof of concept and early stage clinical results that may emerge from our diagnostic work, including with our proprietary biomarkers such as Collagen Type X (“COLX”). The extent of our work in the cancer therapeutics field will depend in part on the financial resources available to us, whether from revenues from the development and commercialization of cancer diagnostics, or from funds obtained through capital transactions. Because the development of cancer therapeutics will be a longer term and more capital intensive project than diagnostic test development, in lieu of completing the development of therapeutics products ourselves, we may seek to license out the development of potential cancer therapeutics to biopharmaceutical companies focused on therapeutic development and commercialization.
Additional Information
OncoCyte Corporation is a majority-owned subsidiary of BioTime, Inc. We were incorporated in 2009 in the state of California as a subsidiary of BioTime. Our principal executive offices are located at 1301 Harbor Bay Parkway, Alameda, California 94502. Our telephone number is (510) 521-3390.
We are an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012. We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act of 1933, as amended (the “Securities Act”); (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission, or the SEC. We refer to the Jumpstart Our Business Startups Act of 2012 herein as the “JOBS Act,” and references herein to “emerging growth company” shall have the meaning associated with it in the JOBS Act.
As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable, in general, to public companies that are not emerging growth companies. These provisions include:
|
·
|
Reduced disclosure about our executive compensation arrangements;
|
|
·
|
No non-binding shareholder advisory votes on executive compensation or golden parachute arrangements;
|
|
·
|
Exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting; and
|
|
·
|
Reduced disclosure of financial information in this registration statement, including two years of audited financial information and two years of selected financial information.
|
We may take advantage of these exemptions for up to five years or such earlier time that we are no longer an emerging growth company
Summary of the Distribution
The following is a summary of the terms of the Distribution. See “The Distribution” for a more detailed description of the matters described below.
|
Distributing company
|
|
BioTime, Inc.
|
| |
|
|
|
Company whose shares are to be distributed
|
|
OncoCyte Corporation
|
| |
|
|
|
Distribution ratio
|
|
Each holder of BioTime common shares will receive a dividend of one share of OncoCyte common stock for every 20 BioTime common shares held on the record date.
|
| |
|
|
|
Securities to be distributed
|
|
Approximately 4,744,707 shares of OncoCyte common stock, which will constitute approximately 18.69% of the OncoCyte common stock outstanding immediately after the Distribution. The number of shares that BioTime will distribute to its shareholders will be reduced to the extent that cash payments are made in lieu of the issuance of fractional shares of OncoCyte common stock, as described below. Also, the shares of OncoCyte common stock distributed to OncoCyte on account of the 619,706 BioTime common shares that OncoCyte owns will revert to the status of authorized but unissued shares upon receipt by OncoCyte and will no longer be outstanding. See “Description of Capital Stock—Common Stock.”
|
| |
|
|
|
Proposed Trading Symbol
|
|
OCX |
| |
|
|
|
Fractional shares
|
|
BioTime will not distribute any fractional shares of OncoCyte common stock to its shareholders. Instead, the distribution agent will aggregate fractional shares into whole shares, sell the whole shares in the open market at prevailing market prices and distribute the aggregate net cash proceeds of the sales pro rata to each BioTime shareholder (other than OncoCyte) who otherwise would have been entitled to receive a fractional share in the Distribution. Recipients of cash in lieu of fractional shares will not be entitled to any interest on the amounts of payment made in lieu of fractional shares.
|
| |
|
|
|
Record date
|
|
The record date is the close of business on December 21, 2015. Only holders of BioTime common shares as of the close of business on the record date will be entitled to receive OncoCyte common stock in the Distribution.
|
| |
|
|
|
Distribution Date
|
|
The Distribution Date will be on or about December 31, 2015.
|
| |
|
|
|
Relationship between OncoCyte and BioTime after the Distribution
|
|
After the Distribution, BioTime will continue to own, directly or through a subsidiary, approximately 14,866,888 shares of OncoCyte common stock, which will represent approximately 58.55% of the OncoCyte common stock outstanding after the Distribution. Accordingly, OncoCyte will remain a majority-owned subsidiary of BioTime immediately after the Distribution. BioTime will continue to provide OncoCyte with use of office and laboratory facilities and equipment; laboratory and office supplies; utility services to the extent the same are provided to the shared office and laboratory facilities; information technology support; human resources; technology licensing (including technology licensed from third parties) and other limited services consistent with past practices under an existing Shared Facilities Agreement. The companies may also enter into other agreements providing for the allocation of tax benefits, employee matters and liabilities arising from periods prior to the Distribution, and other aspects of the relationship between the two companies and other BioTime subsidiaries. |
|
Management of OncoCyte
|
|
OncoCyte has a board of directors (the “Board of Directors”) consisting of seven directors, three of whom qualify as “independent” directors under the rules of the NYSE MKT, and four of whom do not qualify as “independent” because they are officers of OncoCyte or BioTime or are non-independent directors of BioTime. OncoCyte will have its own executive officers, although its Chief Financial Officer will also serve as Chief Financial Officer of BioTime. See “Management.”
|
| |
|
|
|
Dividend policy
|
|
We do not plan on paying any cash dividends on our common stock in the immediate future. Instead, we will retain any income we may earn to finance our business operations. All decisions regarding the declaration and payment of dividends will be evaluated from time to time in light of our financial condition, earnings, growth prospects, other uses of cash, funding requirements, applicable law and other factors our Board of Directors deems relevant. See the section entitled “Dividend Policy.”
|
| |
|
|
|
Risk factors
|
|
You should carefully consider the matters discussed under the section entitled “Risk Factors.”
|
| |
|
|
|
Amendment or Cancellation of the Distribution
|
|
BioTime may, in its sole discretion: (a) terminate the Distribution prior to delivery of the Distribution Shares to BioTime shareholders; (b) change the Distribution Date for the Distribution to a later date; (c) change the record date prior to the Distribution of the Distribution Shares to BioTime shareholders; or (d) amend or modify the terms of the Distribution. If BioTime determines to terminate the Distribution, changes the Distribution Date or the record date, or amends or modifies the terms of the Distribution, BioTime and OncoCyte will issue a press release and each will file a Current Report on Form 8-K and OncoCyte will provide a supplement to this Information Statement disclosing the applicable changes.
|
QUESTIONS AND ANSWERS ABOUT ONCOCYTE AND THE DISTRIBUTION
Q: Why am I receiving this information statement?
A: BioTime is delivering this information statement to you because you were a holder of BioTime common shares on the record date for the Distribution.
Q: What is the Distribution?
A: The Distribution is the distribution of one share of OncoCyte common stock for every 20 common shares of BioTime that were outstanding on the record date. No action is required for you to participate in the Distribution. After the Distribution, BioTime shareholders other than BioTime subsidiaries will receive in the Distribution, approximately 17.8% of the shares of OncoCyte common stock that will be outstanding immediately upon the completion of the Distribution, while BioTime will continue to own, directly or through a subsidiary, approximately 58.55% of the outstanding OncoCyte shares. Certain current minority shareholders of OncoCyte will own the balance of the OncoCyte shares, and will acquire additional OncoCyte shares through the Distribution to the extent that they own BioTime common shares on the record date.
Q: What will I receive in the Distribution?
A: In the Distribution, BioTime shareholders will receive one share of OncoCyte common stock for every 20 BioTime common shares they own as of the record date for the Distribution. No fractional shares will be issued. Those BioTime shareholders who would otherwise be entitled to receive fractional shares will receive cash in lieu of fractional shares. For example, a BioTime shareholder who holds 110 BioTime common shares as of the record date will, after the Distribution, (i) continue to hold 110 BioTime common shares and (ii) receive 5 shares of OncoCyte common stock and cash in lieu of fractional shares. Immediately after the Distribution, BioTime shareholders will still own their BioTime common shares and they will still own an interest in BioTime’s current businesses, but they will own that interest as two separate stock investments rather than as a single investment.
Q: What is OncoCyte?
A: We are an existing majority-owned subsidiary of BioTime engaged in the business of developing laboratory tests for the diagnosis of cancer.
Q: Why is BioTime distributing OncoCyte stock to BioTime shareholders?
A: BioTime believes that creating a separate scientific and management team for OncoCyte and fostering public ownership of OncoCyte common stock will better enable OncoCyte to focus on maximizing opportunities for its cancer diagnosis business, to hire and retain scientists and managers in the future, and to access the capital markets to obtain the financing that OncoCyte will need in the long run to fund its research and product development programs, to establish a clinical testing laboratory for its diagnostic tests, and to commercialize its diagnostic tests. Both BioTime and OncoCyte believe that the Distribution will present the opportunity for enhanced performance of both BioTime and OncoCyte, and BioTime believes that the Distribution will also enhance the value of the OncoCyte common stock that BioTime continues to own, thus providing additional value to BioTime shareholders.
BioTime’s board of directors has determined that the Distribution is in the best interests of BioTime and its shareholders. The following potential benefits were considered by BioTime’s board of directors in making the determination to effect the Distribution:
|
·
|
allowing each company to separately pursue the business strategies that best suit its long-term interests;
|
|
·
|
creating separate companies that have different financial characteristics, which may appeal to different investor bases and allow for clarity on valuation of the respective businesses;
|
|
·
|
creating opportunities to more efficiently finance ongoing operations, including product development and clinical trials of new diagnostic products, establishing a clinical laboratory, and commercializing products;
|
|
·
|
creating opportunities to more efficiently finance acquisitions;
|
|
·
|
allowing each company to establish an expense structure appropriate for its business and size; and
|
|
·
|
creating effective management and employee incentives tied to each company’s performance.
|
For a further explanation of the reasons for the Distribution and more information about our business, see “The Distribution—Reasons for the Distribution” and “Business.”
Q: What is the record date for the Distribution?
A: The record date is December 21, 2015, and the determination of BioTime shares ownership for the purposes of the Distribution will be determined as of 5:00 p.m., New York City Time, on that date.
Q: When will the Distribution occur?
A: Shares of OncoCyte common stock will be distributed on or about December 31, 2015.
Q: Can BioTime decide to cancel or delay the Distribution?
A: Yes. The Distribution is conditioned upon satisfaction or waiver of certain conditions. See “The Distribution—Distribution Conditions and Termination.” BioTime also has the right to postpone or terminate the Distribution even if all of these conditions are met, if at any time BioTime’s board of directors determines, in its sole discretion that completing the Distribution would not be in the best interest of BioTime and its shareholders.
Q: What will happen to the listing of BioTime common shares?
A: Nothing. BioTime common shares will continue to be traded on the NYSE MKT and TASE under the symbol BTX.
Q: Will the Distribution affect the market price of my BioTime common shares?
A: The immediate impact of the Distribution on the market price of BioTime common shares cannot be determined. On the one hand, the establishment of an independent market value for OncoCyte common stock could enhance the value of the OncoCyte stock that BioTime will continue to own after the Distribution. On the other hand, the price of BioTime common shares could decline in view of the fact that BioTime will own a smaller portion of OncoCyte. Accordingly, the combined trading prices of BioTime common shares and OncoCyte common stock after Distribution Date may be less than or greater than the trading price of BioTime common shares prior to the Distribution. Until the market has fully analyzed the relative values of BioTime and OncoCyte after the Distribution, and a trading market for OncoCyte common stock is established, the price of both BioTime common shares and OncoCyte common stock may fluctuate significantly.
Q: What does a BioTime shareholder need to do now?
A: BioTime shareholders do not need to take any action to participate in the Distribution. The approval of the BioTime shareholders is not required or sought to effect the Distribution and BioTime shareholders have no appraisal rights in connection with the Distribution. BioTime is not seeking a proxy from any shareholders and you are requested not to send BioTime or us a proxy. BioTime shareholders will not be required to pay anything for the shares of OncoCyte common stock distributed in the Distribution or to surrender any BioTime common shares. BioTime shareholders should not send their BioTime share certificates to BioTime, OncoCyte or the distribution agent and transfer agent. BioTime shareholders will automatically receive their shares of OncoCyte common stock when the Distribution is effected and will receive cash for any fractional shares. After the Distribution, the certificates and book-entry interests representing your BioTime common shares will continue to represent interests in the BioTime businesses following the Distribution, excluding only the portion of OncoCyte distributed to BioTime shareholders. The book-entry interests representing OncoCyte common stock that BioTime shareholders receive in the Distribution will represent an equity interest in OncoCyte.
Q: Are there risks to owning OncoCyte common stock?
A: Yes. Our business is subject to both general and specific risks relating to our operations. In addition, there will be market risks associated with the ownership of OncoCyte common stock. See “Risk Factors.”
Q: What are the U.S. federal income tax consequences of the Distribution to BioTime shareholders?
A: We expect that, if the Distribution occurs during 2015, BioTime will not have overall accumulated earnings and profits or current earnings and profits (each determined for U.S. federal income tax purposes) for 2015 after giving effect to any gain realized by BioTime as result of the Distribution. If BioTime does not have either (a) overall accumulated earnings and profits or (b) current earnings and profits for the taxable year that includes the Distribution, then:
| |
·
|
if the fair market value of the shares of OncoCyte common stock received does not exceed the BioTime shareholder’s basis in the shareholder’s BioTime shares, the shareholder would recognize no taxable gain as a result of the Distribution, and would be deemed to have received a return of capital that would reduce the shareholder’s basis in the shareholder’s BioTime shares by the fair market value of the shares of common stock received; and
|
| |
·
|
if the fair market value of the shares of common stock distributed to a BioTime shareholder exceeds the BioTime shareholder’s basis in the shareholder’s BioTime shares, then the excess would be taxable as gain from the sale or exchange of property that may be taxed as a long-term or short-term capital gain depending upon the shareholder’s holding period in the BioTime shares.
|
If BioTime has overall accumulated earnings and profits or current earnings and profits for the taxable year that includes the Distribution (each as determined for U.S. federal income tax purposes), including any earnings and profits resulting from the Distribution, then:
|
·
|
the Distribution would be taxed as a dividend to a BioTime shareholder to the extent of the lesser of the shareholder’s allocable share of BioTime’s earnings and profits and the fair market value of the shares of OncoCyte common stock received by the shareholder in the Distribution; and
|
|
·
|
if the fair market value of the shares of OncoCyte common stock received by a BioTime shareholder exceeds the shareholder’s allocable share of BioTime's earnings and profits, the excess would be a return of capital that will reduce the shareholder’s basis in the shareholder’s BioTime shares by that excess and, to the extent it exceeds the BioTime shareholder’s basis in the shareholder’s BioTime shares, would be taxable as gain from the sale or exchange of property that may be taxed as a long-term or short-term capital gain depending upon the shareholder’s holding period in the BioTime shares.
|
Additional matters concerning the U.S. federal income tax consequences of the Distribution are summarized in the section of this information statement entitled “Tax Matters-Material U.S. Federal Income Tax Consequences of the Distribution.” You should consult your own tax advisor as to the particular consequences of the Distribution to you.
Q: What if I want to sell my BioTime common shares or my OncoCyte common stock?
A: You should consult with your own financial advisors, such as your stockbroker, bank or tax advisor. BioTime and OncoCyte do not make any recommendations on the purchase, retention, or sale of BioTime common shares or OncoCyte common stock. If you do decide to sell any shares, you should make sure your stockbroker, bank or other nominee understands whether you want to sell your BioTime stock or your OncoCyte stock after it is distributed, or both.
Q: Where will I be able to trade shares of my OncoCyte common stock?
A: Currently there is no public market for OncoCyte common stock. We have applied to list OncoCyte common stock on the NYSE MKT under the symbol OCX. If our listing application is not approved, we plan to arrange to have OncoCyte common stock traded on the OTC Bulletin Board. Trading in shares of OncoCyte common stock may begin on a “when-issued” basis on or shortly before the Distribution Date, and “regular way” trading will begin on the first trading day following the Distribution Date. If trading does begin on a “when-issued” basis, you may purchase or sell OncoCyte common stock after that time, but your transaction will not settle until after the Distribution Date. On the first trading day following the Distribution Date, “when-issued” trading in respect of OncoCyte common stock will end and “regular way” trading will begin. We cannot predict the trading prices for OncoCyte common stock before or after the Distribution Date.
Q: Where can BioTime shareholders get more information?
A: Before the Distribution, if you have any questions relating to the Distribution, you should contact:
BioTime, Inc.
1301 Harbor Bay Parkway, Suite 100
Alameda, CA 94502
Attention: Dan Lawrence
Telephone: (510) 521-3390
After the Distribution, if you have any questions relating to OncoCyte common stock, you should contact:
OncoCyte Corporation
1301 Harbor Bay Parkway, Suite 100
Alameda, CA 94502
Attention: Dan Lawrence
Telephone: (510) 521-3390
Q: Who will be the distribution agent, transfer agent and registrar for OncoCyte common stock?
A: The distribution agent, transfer agent and registrar for OncoCyte common stock will be American Stock Transfer & Trust Company, LLC.
RISK FACTORS
Our business is subject to various risks, including those described below. You should consider the following risk factors, together with all of the other information included in this information statement, which could materially adversely affect our proposed operations, our business prospects, and financial condition, and the value of an investment in our business. There may be other factors that are not mentioned here or of which we are not presently aware that could also affect our business operations and prospects.
Risks Related to Our Business Operations
We are a development stage company and have incurred operating losses since inception and we do not know if we will attain profitability
Since our inception in September 2009, we have incurred operating losses and negative cash flow and we expect to continue to incur losses and negative cash flow in the future. Our net losses for the nine months ended September 30, 2015 and the years ended December 31, 2014 and 2013 were approximately $5.2 million, $5.0 million, and $3.5 million, respectively, and we had an accumulated deficit of approximately $20.6 million, $15.4 million and $10.4 million as of September 30, 2015, and December 31, 2014 and 2013, respectively. Since inception, we have financed our operations through the sale of our common stock to our current shareholders, loans from BioTime and BioTime affiliates, and sale of BioTime common shares that we hold as available-for-sale securities. Although BioTime may continue to provide administrative support to us on a reimbursable basis, there is no assurance that BioTime will provide future financing. There is no assurance that we will be able to obtain any additional financing that we may need after the completion of the Distribution, or that any such financing that may become available will be on terms that are favorable to us and our shareholders. Ultimately, our ability to generate sufficient operating revenue to earn a profit depends upon our success in developing and marketing or licensing our diagnostic tests and technology.
We will spend a substantial amount of our capital on research and development but we might not succeed in developing diagnostic tests and technologies that are useful in medicine
|
·
|
We are attempting to develop new medical diagnostic tests and technologies. The main focus of our business is on diagnostic tests for cancer. Our diagnostic tests are being developed through the use of blood and urine samples obtained in prospective and retrospective clinical trials involving humans, but none of our diagnostic tests have been used in medicine to diagnose cancer. Our technologies many not prove to be sufficiently efficacious to use in the diagnosis of cancer.
|
|
·
|
Some of our research could also have applications in new cancer therapeutics. None of our experimental therapeutic technologies have been applied in human medicine and have only been used in laboratory studies in vitro.
|
|
·
|
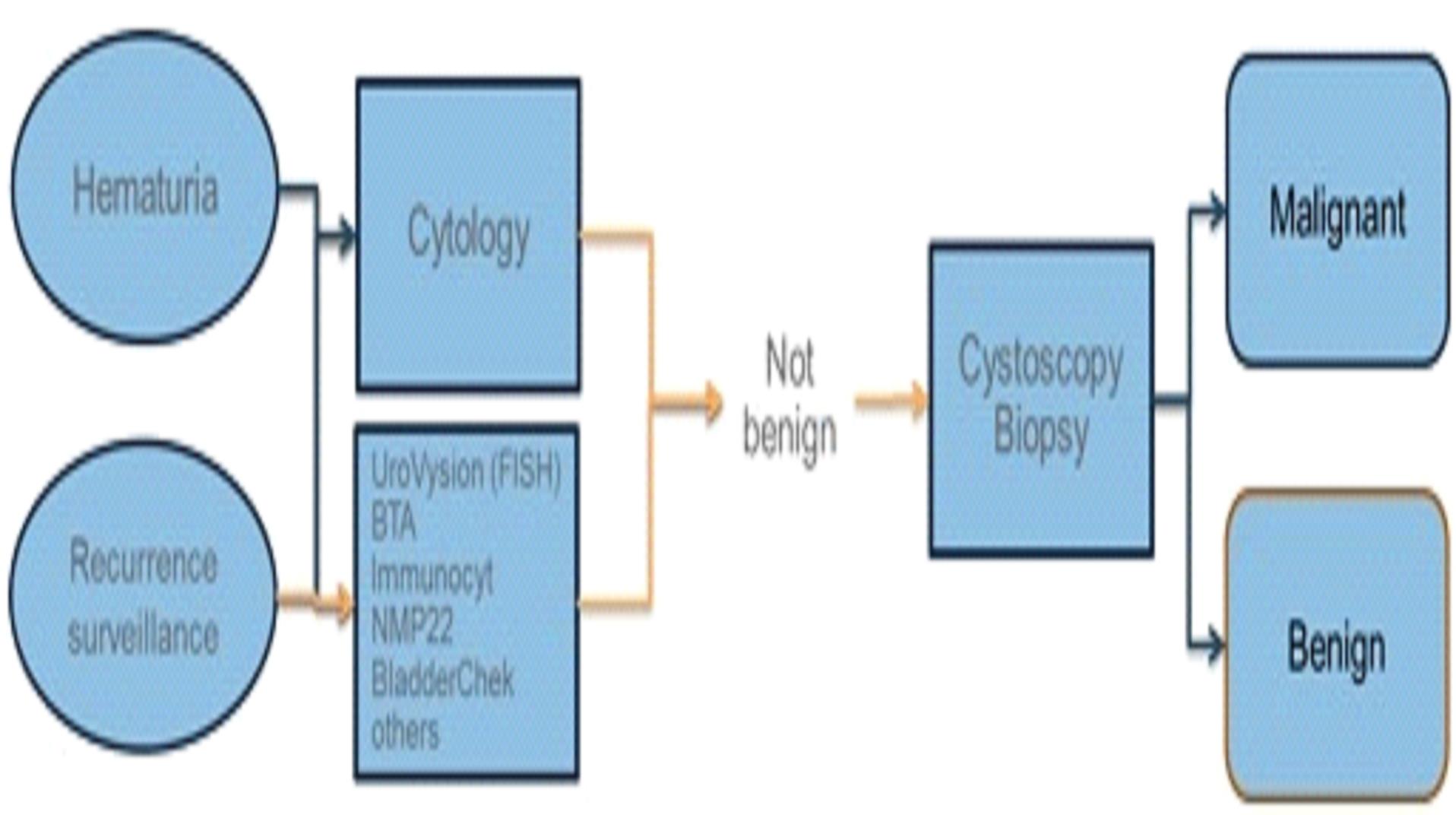
The experimentation we are doing is costly, time consuming, and uncertain as to its results. We incurred research and development expenses amounting to approximately $3.1 million, $4.0 million, and $2.9 million during the nine months ended September 30, 2015, and years ended December 31, 2014 and 2013, respectively. Since 2011, most of our research has been devoted to the development of our lead diagnostic tests to detect lung cancer, breast cancer, and bladder cancer.
|
|
·
|
If we are successful in developing a new technology or diagnostic test, refinement of the new technology or diagnostic test and definition of the practical applications and limitations of the technology or diagnostic test may take years and require the expenditure of large sums of money.
|
We do not currently have any diagnostic tests on the market and have not yet generated any revenues from operations
|
·
|
We need to successfully develop and market or license the diagnostic tests that we are developing in order to earn revenues in sufficient amounts to meet our operating expenses.
|
|
·
|
Without diagnostic test sales or licensing fee revenues, we will not be able to operate at a profit, and we will not be able to cover our operating expenses without raising additional capital.
|
|
·
|
Should we be able to successfully develop and market our diagnostic tests we may not be able to receive reimbursement for them from payers, such as health insurance companies, health maintenance organizations and Medicare, or any reimbursement that we receive may be lower than we anticipate.
|
Sales of any diagnostic tests that we may develop could be adversely impacted by the reluctance of physicians to adopt the use of our tests and the availability of competing diagnostic tests
|
·
|
Physicians and hospitals may be reluctant to try a new diagnostic test due to the high degree of risk associated with the application of new technologies and diagnostic test in the field of human medicine, especially if the new test differs from the current standard of care for detecting cancer in patients.
|
|
·
|
Competing tests for the initial diagnosis, reoccurrence diagnosis and optimal treatment of cancer are being manufactured and marketed by established companies and by other smaller biotechnology companies.
|
|
·
|
Currently there are two diagnostic tests for lung cancer and multiple diagnostic tests for bladder cancer on the market. There is one diagnostic product for breast cancer that has been approved in Europe. In order to compete with other diagnostic tests, particularly any that sell at lower prices, our diagnostic tests will have to provide medically significant advantages or be more cost effective.
|
|
·
|
There also is a risk that our competitors may succeed in developing safer, more accurate or more cost effective diagnostic tests that could render our diagnostic tests and technologies obsolete or noncompetitive
|
It is likely that we will need to issue additional equity or debt securities in order to raise additional capital needed to pay our operating expenses
|
·
|
We plan to continue to incur substantial research and development expenses and we anticipate that we will be incurring significant sales and marketing costs as we develop and commercialize our diagnostic test candidates. We may need to raise additional capital to pay operating expenses until we are able to generate sufficient revenues from diagnostic test sales, royalties, and license fees, and we may need to sell additional equity or debt securities to meet those capital needs.
|
|
·
|
Sales of additional equity securities by us could result in the dilution of the interests of our shareholders.
|
We need to obtain a license to certain technology in order to complete the development of our diagnostic test for lung cancer
The research and development of our lung cancer diagnostic test has been conducted primarily by The Wistar Institute of Anatomy and Biology (“Wistar”) pursuant to a Sponsored Research Agreement, as amended (the “SRA”) with us, and Wistar owns core intellectual property rights relating to that lung cancer diagnostic test. Under the SRA, we have an option to license from Wistar the data, methods, techniques, processes, and other technical information (“Wistar Inventions”) related to molecular diagnostics for lung cancer developed or discovered by the principal investigator or anyone working under her direction in the performance of the sponsored research. We have exercised our options to license certain Wistar Inventions and we and Wistar are negotiating the terms of definitive license agreements with Wistar. However, there is no assurance that we will reach agreement with Wistar on the terms of any definitive license agreements, or that the terms of any license agreements that we may enter into will be favorable to us from a commercial point of view. If we fail to reach agreement with Wistar for licenses to use any Wistar Inventions, we may not be able to complete the development of our lung cancer diagnostic test. Consequently, we may need to commence, at our expense, new research and development work without the right to use the Wistar Inventions, or, if feasible, license technology from a third party.
If we fail to meet our obligations under license agreements, we may lose our rights to key technologies on which our business depends
Our business will depend on several critical technologies that we plan to license from Wistar for our lung cancer diagnostic test. We expect that the license agreements will impose obligations on us, including payment obligations and obligations to pursue development and commercialization of diagnostic tests under the licensed patents or technology. If Wistar believes that we have failed to meet our obligations under a license agreement, Wistar could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, a loss of the licensed rights. During the period of any such litigation our ability to carry out the development and commercialization of potential diagnostic tests, and our ability to raise any capital that we might then need, could be significantly and negatively affected. If our license rights were restricted or ultimately lost, we would not be able to continue to use the licensed technology in our business.
We do not yet have a certified diagnostic laboratory for use in conducting cancer diagnostic tests
We need to lease a facility, construct and equip a diagnostic laboratory, hire a staff to operate the laboratory, and obtain federal and state certification or licensing of the laboratory for use in conducting cancer diagnostic tests. We do not know how long it will take to locate and lease a suitable facility and to build and obtain the required certifications and licenses for the laboratory. Once a suitable facility is located and leased, we will need to expend a substantial part of our cash on hand and management resources to complete construction, equipping, and staffing of the laboratory.
We have limited marketing and sales resources and no distribution resources for the commercialization of any diagnostic tests that we might successfully develop
If we are successful in developing marketable diagnostic tests, we will need to build our own marketing and sales capability, which would require the investment of significant financial and management resources to recruit, train, and manage a sales force.
Our business could be adversely affected if we lose the services of the key personnel upon whom we depend
Our diagnostics program is directed primarily by our Vice President of Research, Dr. Karen Chapman. Our commercial activities are directed primarily by our Chief Executive Officer William Annett and our Vice President of Marketing, Dr. Kristine C. Mechem. The loss of Dr. Chapman, Mr. Annett, or Dr. Mechem could have a material adverse effect on our business.
Our business and operations could suffer in the event of system failures
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such events could cause interruption of our operations. For example, the loss of data for our diagnostic test candidates could result in delays in our regulatory filings and development efforts and significantly increase our costs. To the extent that any disruption or security breach was to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of our diagnostic test candidates could be delayed.
Failure of our internal control over financial reporting could harm our business and financial results
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting for external purposes in accordance with accounting principles generally accepted in the U.S. Internal control over financial reporting includes maintaining records that in reasonable detail accurately and fairly reflect our transactions; providing reasonable assurance that transactions are recorded as necessary for preparation of our financial statements; providing reasonable assurance that receipts and expenditures of our assets are made in accordance with management authorization; and providing reasonable assurance that unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements would be prevented or detected on a timely basis. Because of its inherent limitations, internal control over financial reporting is not intended to provide absolute assurance that a misstatement of our financial statements would be prevented or detected. Our growth and entry into new diagnostic tests, technologies and markets will place significant additional pressure on our system of internal control over financial reporting. Any failure to maintain an effective system of internal control over financial reporting could limit our ability to report our financial results accurately and timely or to detect and prevent fraud.
We will initially rely in part on financial systems maintained by BioTime and upon services provided by BioTime personnel. BioTime will allocate certain expenses among itself, us, and BioTime’s other subsidiaries, which creates a risk that the allocations may not accurately reflect the benefit of an expenditure or use of financial or other resources by us, BioTime as our parent company, and the BioTime subsidiaries among which the allocations are made.
Risks Related to Our Industry
We will face certain risks arising from regulatory, legal, and economic factors that affect our business and the business of other companies engaged in the development and marketing of diagnostic tests for human diseases. Because we are a small company without revenues and with limited capital resources, we may be less able to bear the financial impact of these risks than larger companies that have substantial income and available capital.
We will need to obtain regulatory approval of our diagnostic test candidates and laboratory facilities
We will need to receive certification for our diagnostic laboratory under the Clinical Laboratory Improvements Amendment (“CLIA”), and we will need to obtain United States Food and Drug Administration (“FDA”) and other regulatory approvals for any in vitro diagnostics (“IVDs”) that we may develop, in order to market those diagnostic tests. The need to obtain regulatory approval to market a new diagnostic test means that:
|
·
|
The diagnostic tests that we may develop cannot be sold until the Centers for Medicare and Medicaid Services (the “CMS”) or the FDA, and corresponding foreign regulatory authorities approve the laboratory tests or the IVDs for medical use.
|
|
·
|
We will have to obtain a CLIA certificate of registration license for our laboratory for the manufacture and use of diagnostic tests and as part of the submission, our laboratory will be inspected.
|
|
·
|
In addition to meeting federal regulatory requirements, each state has its own laboratory certification and inspection requirements for a CLIA laboratory that must be met in order to sell diagnostic tests in the state.
|
|
·
|
We will have to conduct expensive and time consuming clinical trials of new diagnostic tests. The full cost of conducting and completing clinical trials necessary to obtain FDA approval of IVD tests or CLIA certification of a new laboratory diagnostic test or for gaining reimbursement from health insurance companies, health maintenance organizations, Medicare, and other third party payers cannot be presently determined but could exceed our current financial resources.
|
|
·
|
Data obtained from preclinical and clinical studies is susceptible to varying interpretations that could delay, limit or prevent regulatory agency approvals. Delays or denials of the regulatory approvals may be encountered as a result of changes in regulatory agency policy, regulations, or laws.
|
|
·
|
A diagnostic test that is approved may be subject to restrictions on use.
|
|
·
|
The FDA can withdraw approval of an FDA regulated product if problems arise
|
|
·
|
CLIA licensed laboratories can lose their licenses if problems arise during a periodic inspection.
|
The FDA may impose additional regulations for laboratory developed tests such as the ones we are developing
The FDA issued two draft guidance documents that set forth a proposed risk-based regulatory framework that would apply varying levels of FDA oversight to laboratory developed tests (“LDTs”) such as those we are developing. If the FDA implements new regulatory measures:
|
·
|
We may be required to obtain pre-market clearance or approval before selling our diagnostic tests;
|
|
·
|
As a result of required FDA pre-market review, our tests may not be cleared or approved on a timely basis, if at all;
|
|
·
|
FDA labeling requirements may limit our claims about our diagnostic tests, which may have a negative effect on orders from physicians;
|
|
·
|
The regulatory approval process may involve, among other things, successfully completing additional clinical trials and making a 510(k) submission, or filing a pre-market approval application with the FDA; and,
|
|
·
|
If regulatory actions affect any of the reagents we obtain from suppliers and use in conducting our tests, our business could be adversely affected in the form of increased costs of testing or delays, limits or prohibitions on the purchase of reagents necessary to perform our testing.
|
If the FDA regulates LDTs and requires that we seek pre-market approval, there is no assurance that we will be able to comply with FDA requirements.
It may take two years or more to conduct the clinical studies and trials necessary to obtain pre-market approval from the FDA. Even if our clinical trials are completed as planned, we cannot be certain that the results will support our test claims or that the FDA will agree with our conclusions regarding our test results. Success in early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior clinical trials and studies. If we are required to conduct pre-market clinical trials, delays in the commencement or completion of clinical testing could significantly increase our test development costs and delay commercialization. Many of the factors that may cause or lead to a delay in the commencement or completion of clinical trials may also ultimately lead to delay or denial of regulatory clearance or approval. The clinical trial process may fail to demonstrate that our tests are effective for the proposed indicated uses, which could cause us to abandon a test candidate and may delay development of other tests.
Clinical trial failures can occur at any stage of the testing and we may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent commercialization of our current or future diagnostic tests
Clinical trial failures or delays can occur at any stage of the trials, and may be directly or indirectly caused by a variety of factors, including but not limited to:
|
·
|
Delays in securing clinical investigators or trial sites for our clinical trials;
|
|
·
|
Delays in obtaining Institutional Review Board and other regulatory approvals to commence a clinical trial;
|
|
·
|
Slower than anticipated rates of patient recruitment and enrollment, or failing to reach the targeted number of patients due to competition for patients from other trials;
|
|
·
|
Limited or no availability of coverage, reimbursement and adequate payment from health maintenance organizations and other third party payers for the use of our diagnostic test candidates in our clinical trials;
|
|
·
|
Negative or inconclusive results from clinical trials;
|
|
·
|
Approval and introduction of new diagnostic or changes in standards of practice or regulatory guidance that render our clinical trial endpoints or the targeting of our proposed indications obsolete;
|
|
·
|
Inability to monitor patients adequately during or after treatment or problems with investigator or patient compliance with the trial protocols;
|
|
·
|
Inability to replicate in large controlled studies safety and efficacy data obtained from a limited number of patients in uncontrolled trials; and
|
|
·
|
Inability or unwillingness of medical investigators to follow our clinical protocols.
|
We will depend on Medicare and a limited number of private payers for a significant portion of our revenues, and our revenues could decline if these payers fail to provide timely and adequate payment for our diagnostic tests
We expect that a substantial portion of the patients for whom we will perform diagnostic tests will have Medicare as their primary medical insurance. Even if our planned tests are otherwise successful, reimbursement for the Medicare-covered portions of our planned tests might not, without Medicare reimbursement, produce sufficient revenues to enable us to reach profitability and achieve our other commercial objectives.
Medicare and other third-party payers may change their coverage policies or cancel future contracts with us at any time; review and adjust the rate of reimbursement; or stop paying for our tests altogether, which would reduce our total revenues. Payers have increased their efforts to control the cost, utilization, and delivery of health care services, and have undertaken measures to reduce payment rates for and decrease utilization of clinical laboratory testing. Because of the cost-trimming trends, any third-party payers that will cover and provide reimbursement for our diagnostic tests may suspend, revoke or discontinue coverage at any time, or may reduce the reimbursement rates payable to us. Any such action could have a negative impact on our revenues, which may have a material adverse effect on our financial condition, results of operations and cash flows.
Changes in healthcare laws and policies may have a material adverse effect on our financial condition, results of operations and cash flows
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act (collectively “ACA”) substantially changed the way health care is financed by both governmental and private insurers. Among the ACA’s key changes, the ACA reduced payment rates under the Medicare Clinical Laboratory Fee Schedule and established an Independent Payment Advisory Board to reduce the per capita rate of growth in Medicare spending if spending exceeds a target growth rate. Such provisions may negatively impact payment rates for our diagnostic tests.
The Protecting Access to Medicare Act of 2014 (“PAMA”) significantly altered the payment methodology under the Clinical Laboratory Fee Schedule that determines Medicare coverage for laboratory tests. Under PAMA, clinical laboratories are required to report test payment data for each Medicare-covered clinical diagnostic laboratory test and beginning in 2017, the Medicare payment rate for each clinical diagnostic laboratory test will be equal to the weighted median amount for the test from the most recent data collection period.
Congress has proposed on several occasions to impose a 20% coinsurance payment requirement on patients for clinical laboratory tests reimbursed under the Medicare Clinical Laboratory Fee Schedule, which would require us to bill patients for these amounts. In the event that Congress were to ever enact such legislation, the cost of billing and collecting for our tests could often exceed the amount actually received from the patient.
On September 25, 2015, CMS released preliminary determinations for the calendar year 2016 for the Medicare Clinical Laboratory Fee Schedule for some test codes, including some for oncology diagnostics, as had been anticipated. These preliminary determinations were based on a cross walk approach rather than a gap-fill approach. A cross walk approach matches a new code for a diagnostic against existing codes to determine the appropriate payment rate; while a gap-fill approach looks at local pricing patterns, including charges for the tests and any discounts on charges and payments determined by other payers. At this point it is not clear what methodology CMS may use in their determinations for future diagnostics.
Beginning January 1, 2017, Medicare payment for any new advanced diagnostic test will be based on the list price or charge. After the test is commercially available for two quarters, the laboratory will be required to report payment and volume information and that data will be used to set payment for the test for the following year.
|
·
|
If data shows that the list price was greater than 130% of the payment using established methodology (a weighted median), CMS will recoup the difference from the laboratory through a payment claw back.
|
|
·
|
Payment will be updated annually based on the weighted median of commercial payer reimbursement.
|
We cannot predict whether future health care initiatives will be implemented at the federal or state level, or how any future legislation or regulation may affect us. The expansion of government’s role in the U.S. health care industry as a result of the ACA, and changes to the reimbursement amounts paid by Medicare and other payers for diagnostic tests may have a materially adverse effect on our business, financial condition, results of operations and cash flows.
Because of certain Medicare billing policies, we may not receive complete reimbursement for tests provided to Medicare patients
Medicare has coverage policies that can be national or regional in scope. Coverage means that the test or assay is approved as a benefit for Medicare beneficiaries. If there is no coverage, neither the supplier nor any other party, such as a diagnostic laboratory, may receive reimbursement from Medicare for the service. Regional policies are directed by Medicare’s regional Medicare Administrative Contractors (“MACs”). Reimbursement for our diagnostic testing may be negatively impacted by California MAC’s policies.
Long payment cycles of Medicare, Medicaid and/or other third-party payors, or other payment delays, could hurt our cash flows and increase our need for working capital
Medicare and Medicaid have complex billing and documentation requirements that we will have to satisfy in order to receive payment. Failure to comply with these requirements and other laws applicable to billing may result in, among other things, non-payment, refunds, exclusion from government healthcare programs, and civil or criminal liabilities, any of which may have a material adverse effect on our revenues and earnings. Similarly, the failure of private health insurers or other private third-party payers to properly process our payment claims in a timely manner could delay our receipt of payment for our diagnostic tests and services, which may have a material adverse effect on our cash flows.
Private health insurance company policies may deny coverage or limit the amount they will reimburse us for the performance of our diagnostic tests
Patients who are not covered by Medicare will generally rely on health insurance provided by private health insurance companies. If we are considered a “non-contracted provider” by a third-party payer, that payer may not reimburse patients for diagnostic tests performed by us or doctors within the payer’s network of covered physicians may not use our services to perform diagnostic tests for their patients. As a result we may need to enter into contracts with health insurance companies or other private payers to provide diagnostic tests to their insured patients at specified rates of reimbursement which may be lower than the rates we might otherwise collect.
We may be required to comply with federal and state laws governing the privacy of health information, and any failure to comply with these laws could result in material criminal and civil penalties
The Health Insurance Portability and Accountability Act (“HIPAA”) sets forth security regulations that establish administrative, physical and technical standards for maintaining the confidentiality, integrity and availability of Protected Health Information in electronic form. We also may be required to comply with state laws that are more stringent than HIPAA or that provide individuals with greater rights with respect to the privacy or security of, and access to, their health care records. The Health Information Technology for Economic and Clinical Health Act (“HITECH”) established certain health information security breach notification obligations that require covered entities to notify each individual whose “protected health information” is breached.
We may incur significant compliance costs related to HIPAA and HITECH privacy regulations and varying state privacy regulations and varying state privacy and security laws. Given the complexity of HIPAA and HITECH and their overlap with state privacy and security laws, and the fact that these laws are rapidly evolving and are subject to changing and potentially conflicting interpretation, our ability to comply with the HIPAA, HITECH and state privacy requirements is uncertain and the costs of compliance are significant. The costs of complying with any changes to the HIPAA, HITECH and state privacy restrictions may have a negative impact on our operations. Noncompliance could subject us to criminal penalties, civil sanctions and significant monetary penalties as well as reputational damage.
We are subject to federal and state healthcare fraud and abuse laws and regulations and could face substantial penalties if we are unable to fully comply with such laws
We are subject to health care fraud and abuse regulation and enforcement by both the federal government and the states in which we conduct our business. These health care laws and regulations include the following:
|
·
|
The federal Anti-Kickback Statute;
|
|
·
|
The federal physician self-referral prohibition, commonly known as the Stark Law;
|
|
·
|
The federal false claims and civil monetary penalties laws;
|
|
·
|
The federal Physician Payment Sunshine Act requirements under the ACA; and
|
|
·
|
State law equivalents of each of the federal laws enumerated above.
|
Any action brought against us for violation of these laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of these laws and regulations, we may be subject to any applicable penalty associated with the violation, including, among others, administrative, civil and criminal penalties, damages and fines, and/or exclusion from participation in Medicare, Medicaid programs, including the California Medical Assistance Program (Medi-Cal—the California Medicaid program) or other state or federal health care programs. Additionally, we could be required to refund payments received by us, and we could be required to curtail or cease our operations.
Risks Related to Intellectual Property
If we are unable to obtain and enforce patents and to protect our trade secrets, others could use our technology to compete with us, which could limit opportunities for us to generate revenues by licensing our technology and selling diagnostic tests
|
·
|
Our success will depend in part on our ability to obtain and enforce patents and maintain trade secrets in the United States and in other countries. If we are unsuccessful in obtaining and enforcing patents, our competitors could use our technology and create diagnostic tests that compete with our diagnostic tests, without paying license fees or royalties to us.
|
|
·
|
The preparation, filing, and prosecution of patent applications can be costly and time consuming. Our limited financial resources may not permit us to pursue patent protection of all of our technology and diagnostic tests throughout the world.
|
|
·
|
Even if we are able to obtain issued patents covering our technology or diagnostic tests, we may have to incur substantial legal fees and other expenses to enforce our patent rights in order to protect our technology and diagnostic tests from infringing uses. We may not have the financial resources to finance the litigation required to preserve our patent and trade secret rights.
|
|
·
|
The Supreme Court decisions in Mayo Collaborative Services v. Prometheus Laboratories, Inc. and Association for Molecular Pathology v. Myriad Genetics may adversely impact our ability to obtain patent protection for some or all of our diagnostic tests, which use certain gene markers to indicate the presence of certain cancers. The claims in the contested patents that were the subject of the Supreme Court decision in Mayo Collaborative Services v. Prometheus Laboratories, Inc. were directed to measuring the serum level of a drug metabolite and adjusting the dosing regimen of the drug based on the metabolite level. The Supreme Court said that a patent claim that merely claimed a mathematical correlation between the blood levels of a drug metabolite and the best dosage of the drug was not patentable subject matter because it did no more than recite a correlation that occurs in nature. In Association for Molecular Pathology v. Myriad Genetics, the Supreme Court ruled that the discovery of the precise location and sequence of certain genes, mutations of which can dramatically increase the risk of breast and ovarian cancer, was not patentable. Knowledge of the gene location and sequences was used to determine the genes’ typical nucleotide sequence, which, in turn, enabled the development of medical tests useful for detecting mutations in these genes in a particular patient to assess the patient’s cancer risk. But the mere discovery of an important and useful gene did not render the genes patentable as a new composition of matter. The holdings in Mayo Collaborative Services v. Prometheus Laboratories, Inc. and Association for Molecular Pathology v. Myriad Genetics may limit our ability to obtain patent protection on diagnostic methods that merely recite a correlation between a naturally occurring event and a diagnostic outcome associated with that event. |
There is no certainty that our pending or future patent applications will result in the issuance of patents
We have filed patent applications for technology that we have developed, and we may obtain licenses for patent applications covering genes that we or our partners have discovered, that we believe will be useful in producing new diagnostic tests. We may also file additional new patent applications in the future seeking patent protection for new technology or diagnostics tests or products that we develop ourselves or jointly with others. However, there is no assurance that any of our licensed patent applications, or any patent applications that we have filed or that we may file in the future in the United States or abroad, will result in the issuance of patents.
The process of applying for and obtaining patents can be expensive and slow
|
·
|
The preparation and filing of patent applications, and the maintenance of patents that are issued, may require substantial time and money.
|
|
·
|
A patent interference proceeding may be instituted with the U.S. Patent and Trademark Office (the “USPTO”) when more than one person files a patent application covering the same technology, or if someone wishes to challenge the validity of an issued patent. At the completion of the interference proceeding, the USPTO will determine which competing applicant is entitled to the patent, or whether an issued patent is valid. Patent interference proceedings are complex, highly contested legal proceedings, and the USPTO’s decision is subject to appeal. This means that if an interference proceeding arises with respect to any of our patent applications, we may experience significant expenses and delay in obtaining a patent, and if the outcome of the proceeding is unfavorable to us, the patent could be issued to a competitor rather than to us
|
|
·
|
A derivation proceeding may be instituted by the USPTO or an inventor alleging that a patent or application was derived from the work of another inventor.
|
|
·
|
Post Grant Review under the new America Invents Act will make available opposition-like proceedings in the United States. As with the USPTO interference proceedings, Post Grant Review proceedings will be very expensive to contest and can result in significant delays in obtaining patent protection or can result in a denial of a patent application.
|
|
·
|
Oppositions to the issuance of patents may be filed under European patent law and the patent laws of certain other countries. As with USPTO interference proceedings, these foreign proceedings can be very expensive to contest and can result in significant delays in obtaining a patent or can result in a denial of a patent application.
|
Our patents may not protect our diagnostic tests from competition
|
·
|
We might not be able to obtain any patents beyond the bladder cancer marker patent that has been issued by the USPTO, and any patents that we do obtain might not be comprehensive enough to provide us with meaningful patent protection.
|
|
·
|
There will always be a risk that our competitors might be able to successfully challenge the validity or enforceability of any patent issued to us.
|
|
·
|
In addition to interference proceedings, the USPTO can reexamine issued patents at the request of a third party. Our patents may be subject to inter partes review (replacing the reexamination proceeding), a proceeding in which a third party can challenge the validity of one of our patents to have the patent invalidated. This means that patents owned or licensed by us may be subject to reexamination and may be lost if the outcome of the reexamination is unfavorable to us.
|
We may be subject to patent infringement claims that could be costly to defend, which may limit our ability to use disputed technologies, and which could prevent us from pursuing research and development or commercialization of some of our diagnostic tests, require us to pay licensing fees to have freedom to operate and/or result in monetary damages or other liability for us
The success of our business depends significantly on our ability to operate without infringing patents and other proprietary rights of others. If the technology that we use infringes a patent held by others, we could be sued for monetary damages by the patent holder or its licensee, or we could be prevented from continuing research, development, and commercialization of diagnostic tests that rely on that technology, unless we are able to obtain a license to use the patent. The cost and availability of a license to a patent cannot be predicted, and the likelihood of obtaining a license at an acceptable cost would be lower if the patent holder or any of its licensees is using the patent to develop or market a diagnostic tests with which our diagnostic test would compete. If we could not obtain a necessary license, we would need to develop or obtain rights to alternative technologies, which could prove costly and could cause delays in diagnostic test development, or we could be forced to discontinue the development or marketing of any diagnostic tests that were developed using the technology covered by the patent.
Risks Related to Our Relationship with BioTime
We are a subsidiary of BioTime, and accordingly our business is substantially controlled by BioTime
Prior to the Distribution, BioTime owned approximately 76.39% of our issued and outstanding shares of common stock, and after the Distribution will continue to own approximately 58.55% of our outstanding shares of common stock. This means that BioTime will have the voting power, through its ownership of shares of our common stock, to elect our entire Board of Directors and to control our management.
BioTime could cause corporate actions to be taken even if the interests of BioTime conflict with the interests of our other shareholders. This concentration of voting power could have the effect of deterring or preventing a change in control that might be beneficial to our other shareholders.
As the majority shareholder, BioTime will have the voting power to approve or disapprove any matter of corporate transaction presented to our shareholders for approval, including but not limited to:
|
·
|
Any amendment of our articles of incorporation or bylaws;
|
|
·
|
Any merger or consolidation of us with another company;
|
|
·
|
Any recapitalization or reorganization of our capital stock;
|
|
·
|
Any sale of assets or purchase of assets; or
|
|
·
|
A corporate dissolution or a plan of liquidation of our business.
|
We will initially rely upon BioTime for certain services and resources
Although we plan to have our own research facilities in the near future, our own scientific personnel, and many critical management personnel, we will initially rely on BioTime to provide certain management and administrative services, including patent prosecution, certain legal services, accounting, financial management, and controls over financial accounting and reporting. We have entered into a Shared Facilities and Services Agreement (“Shared Facilities Agreement”) with BioTime under which we have agreed to bear costs allocated to us by BioTime for the use of BioTime human resources and for services and materials provided for our benefit by BioTime. We will pay BioTime 105% of its costs of providing personnel and services to us, and for any use of its facilities by us, including an allocation of general overhead based on that use. We may also share the services of some research personnel with BioTime.
If BioTime’s human resources and facilities are not sufficient to serve both BioTime’s needs and ours, we will have to hire additional personnel of our own, either on a full-time or part-time basis, as employees or as consultants, and the cost of doing so could be greater than the costs that would be allocated to us by BioTime. Also, any new personnel that we may need to hire may not be as familiar with our business or operations as BioTime’s personnel, which means that we would incur the expense and inefficiencies related to training new employees or consultants.
Three of our directors are officers or directors of BioTime
Three of the seven members of our Board of Directors are also officers or directors of BioTime and one of our directors is our Chief Executive Officer. This commonality of directors means that we will not have a Board of Directors making business decisions on our behalf independent from BioTime and our management. Even those of our directors who do not serve on the BioTime Board of Directors will be elected to our Board of Directors by BioTime, and they may be removed from our Board of Directors by BioTime, as the majority shareholder.
Conflicts of interest may arise from our relationship with BioTime
Our relationship with BioTime could give rise to certain conflicts of interest that could have an impact on our research and development programs, business opportunities, and operations generally.
|
·
|
Even if we utilize different technologies than BioTime or its other subsidiaries, we could find ourselves in competition with them for research scientists, financing and other resources, licensing, manufacturing, and distribution arrangements, and for customers if we and BioTime or another BioTime subsidiary both bring diagnostic tests to market.
|
|
·
|
Because we are a subsidiary of BioTime, BioTime could prevent us from engaging in research and development programs, investments, business ventures, or agreements to develop, license, or acquire diagnostic tests or technologies that would or might compete with those owned, licensed, or under development by BioTime or any of its other subsidiaries.
|
|
·
|
BioTime and its other subsidiaries will engage for their own accounts in research and product development programs, investments, and business ventures, and we will not be entitled to participate or to receive an interest in those programs, investments, or business ventures. BioTime and its other subsidiaries will not be obligated to present any particular research and development, investment, or business opportunity to us, even if the opportunity would be within the scope of our research and development plans or programs, business objectives, or investment policies. These opportunities may include, for example, opportunities to acquire businesses or assets, including but not limited to patents and other intellectual property that could be used by us or by BioTime or by any of BioTime’s other subsidiaries. Our respective boards of directors will have to determine which company should pursue those opportunities, taking into account relevant facts and circumstances at the time, such as the financial and other resources of the companies available to acquire and utilize the opportunity, and the best “fit” between the opportunity and the business and research and development programs of the companies. However, since BioTime will have the ultimate power to elect the members of our Board of Directors, BioTime may have the ultimate say in decision making with respect to the allocation of opportunities. |
|
·
|
If we enter into any patent or technology license or sublicense, or any other agreement with BioTime or with another BioTime subsidiary, the BioTime companies that are parties to the agreement may have a conflict of interest in determining how and when they should enforce their rights under the agreement if the other BioTime company that is a party were to default or otherwise fail to perform any of its obligations under the agreement.
|
|
·
|
One of our significant assets as of December 10, 2015 is 619,706 BioTime common shares that we acquired from BioTime in exchange for shares of our common stock. We expect to sell the BioTime common shares from time to time, or to pledge those shares as collateral for loans, to raise capital to finance our operations. Because a sale of those shares could have a depressing effect on the market value of BioTime common shares, BioTime will have a continuing interest in the number of shares we sell, the prices at which we sell the shares, and time and manner in which the shares are sold. Further, we may need to sell or find it desirable to sell BioTime common shares at the same time as BioTime, or other BioTime subsidiaries that hold BioTime common shares, also desire to sell some of their BioTime common shares. Concurrent sales of BioTime common shares by us, BioTime, or other BioTime subsidiaries could have a depressing effect on the market price of the BioTime common shares, lowering the price at which we and they are able to sell BioTime common shares and resulting in lower net proceeds from the sales. We plan to coordinate any future sales of our BioTime common shares with BioTime and its other subsidiaries in order to provide an orderly and controlled process for raising capital through the sale of BioTime shares. This will include an agreement as to the number of shares to be sold, the time period or “market window” for selling shares, the use of a common securities broker-dealer, and a fair allocation of net sales based on average sales prices during any trading day on which we and they sell BioTime shares. |
|
·
|
Each conflict of interest will be resolved by our respective boards of directors in keeping with their fiduciary duties and such policies as they may implement from time to time. However, the terms and conditions of patent and technology licenses and other agreements between us and BioTime or other BioTime subsidiaries will not be negotiated on an arm’s-length basis due to BioTime’s ownership of a controlling interest in us and due to the commonality of directors serving on our respective boards of directors.
|
Risks Related to Our Dependence on Third Parties
There is a limited number of manufacturers of molecular diagnostic equipment and related chemical reagents necessary for the provision of our diagnostic tests
In order to develop molecular diagnostics and to provision our diagnostic tests, we will need to acquire certain analytic equipment. There are only a few manufacturers of the equipment we will need and the chemical reagents that are required for use with a particular manufacturer’s equipment will be available only from that equipment manufacturer. If the manufacturer of the equipment we acquire discontinues operation or if we experience supply or quality issues with their equipment or reagents, it may become necessary for us to acquire different analytic equipment, which would require additional experiments to ensure reproducibility of our test results using the new equipment. As a result, we may be unable to provide our diagnostic tests for a period of time.
If we fail to enter into and maintain successful strategic alliances for diagnostic tests that we elect to co-develop, co-market, or out-license, we may have to reduce or delay our diagnostic test development or increase our expenditures
In order to facilitate the development, manufacture and commercialization of our diagnostic tests we may enter into strategic alliances with pharmaceutical companies or other industry participants to advance our programs and enable us to maintain our financial and operational capacity. We will face significant competition in seeking appropriate alliances. We may not be able to negotiate alliances on acceptable terms, if at all. If we fail to create and maintain suitable alliances, we may have to limit the size or scope of, or delay, one or more of our product development or research programs, or we will have to increase our expenditures and will need to obtain additional funding, which may be unavailable or available only on unfavorable terms.
If we are able to enter into development and marketing arrangements with pharmaceutical or medical device companies for our diagnostic tests, we may license product development, manufacturing, and marketing rights to the pharmaceutical or medical device company or to a joint venture company formed with the pharmaceutical or medical device company. Under such arrangements we might receive only a royalty on sales of the diagnostic tests developed or an equity interest in a joint venture company that develops the diagnostic test. As a result, our revenues from the sale of those diagnostic tests may be substantially less than the amount of revenues and gross profits that we might receive if we were to develop, manufacture, and market the diagnostic tests ourselves.
We may become dependent on possible future collaborations to develop and commercialize many of our diagnostic test candidates and to provide the manufacturing, regulatory compliance, sales, marketing and distribution capabilities required for the success of our business
We may enter into various kinds of collaborative research and development, manufacturing, and diagnostic test marketing agreements to develop and commercialize our diagnostic tests. Any future milestone payments and cost reimbursements from collaboration agreements could provide an important source of financing for our research and development programs, thereby facilitating the application of our technology to the development and commercialization of our diagnostic tests, but there are risks associated with entering into collaboration arrangements.
There is a risk that we could become dependent upon one or more collaborative arrangements for diagnostic test development or manufacturing or as a source of revenues from the sale of any diagnostic tests that may be developed by us alone or through one of the collaborative arrangements. A collaborative arrangement upon which we might depend might be terminated by our collaboration partner or they might determine not to actively pursue the development or commercialization of our diagnostic tests. A collaboration partner also may not be precluded from independently pursuing competing diagnostic tests or technologies.
There is a risk that a collaboration partner might fail to perform its obligations under the collaborative arrangements or may be slow in performing its obligations. In addition, a collaboration partner may experience financial difficulties at any time that could prevent it from having available funds to contribute to the collaboration. If a collaboration partner fails to conduct its diagnostic test development, manufacturing, commercialization, regulatory compliance, sales and marketing or distribution activities successfully and in a timely manner, or if it terminates or materially modifies its agreements with us, the development and commercialization of one or more diagnostic test candidates could be delayed, curtailed or terminated because we may not have sufficient financial resources or capabilities to continue diagnostic test development, manufacturing, and commercialization on our own.
Risks Pertaining to Our Common Stock
Ownership of our common stock will entail certain risks associated with the absence of a previous trading market for our common stock, volatility of prices for our shares, and the fact that we do not pay dividends.
There is presently no public market for our common stock and there is no assurance that a market will develop and be sustained
There is presently no public market for our common stock or any other OncoCyte securities. There can be no assurance that an active market for our common stock will materialize or, if a market does develop, that it will be sustained.
Additional shares of our common stock will become eligible for public sale, and sales of those shares could create downward pressure on the trading price of our common stock
After the Distribution, BioTime, directly and through a subsidiary, will continue to hold approximately 14,866,888 shares of OncoCyte common stock and four other OncoCyte shareholders will own a total of 6,003,000 shares of OncoCyte common stock that were outstanding prior to the Distribution. Under Securities and Exchange Commission Rule 144, those shares will become eligible to be publicly sold in compliance with the provisions of Rule 144 after the shares have been beneficially owned for at least one year, or for at least six months if the shares are sold after we have been subject to the reporting requirements of Section 13 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), for a period of 90 days and have filed all reports required under the Exchange Act, other than form 8-K. Based on the date of issue, we expect that 4,500,000 shares will become eligible for sale under Rule 144 immediately after the Distribution.
We have agreed to register for sale under the Securities Act 15,256,312 shares of common stock that we sold to certain investors, including BioTime, without registration under the Securities Act. We have agreed to file a registration statement covering those shares promptly after the date on which we first become eligible to register those securities on Form S-3. Under the rules for the use of Form S-3, 12 calendar months after the date of this Information Statement we will first become eligible to register securities on Form S-3.
Sales of OncoCyte common stock under Rule 144 or through an effective registration statement under the Securities Act could create downward pressure on the trading price of our common stock.
Because we are engaged in the development of medical diagnostic tests, the price of our stock may rise and fall rapidly
The market price of our common stock, like that of the shares of many biotechnology companies, may be highly volatile. The price of our common stock may rise or fall rapidly as a result of a number of factors, including:
|
·
|
Sales or potential sales of substantial amounts of our common stock;
|
|
·
|
Results of preclinical testing or clinical trials of our diagnostic test candidates or those of our competitors;
|
|
·
|
Announcements about us or about our competitors, including clinical trial results, regulatory approvals, new diagnostic test introductions and commercial results;
|
|
·
|
The cost of our development programs;
|
|
·
|
The success of competitive diagnostic tests or technologies;
|
|
·
|
Litigation and other developments relating to our issued patents or patent applications or other proprietary rights or those of our competitors;
|
|
·
|
Conditions in the diagnostic, pharmaceutical or biotechnology industries;
|
|
·
|
Actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;
|
|
·
|
Variations in our financial results or those of companies that are perceived to be similar to us, including the failure of our earnings to meet analysts’ expectations;
|
|
·
|
General economic, industry and market conditions; and
|
|
·
|
Changes in payer coverage and or reimbursement.
|
Many of these factors are beyond our control. The stock markets in general, and the market for pharmaceutical and biotechnological companies in particular, have been experiencing extreme price and volume fluctuations which have affected the market price of the equity securities without regard to the operating performance of the issuing companies. Broad market fluctuations, as well as industry factors and general economic and political conditions, may adversely affect the market price of our common stock.
Because we do not pay dividends, our stock may not be a suitable investment for anyone who needs to earn dividend income
We do not pay cash dividends on our common stock. For the foreseeable future we anticipate that any earnings generated in our business will be used to finance the growth of our business and will not be paid out as dividends to our shareholders. This means that our stock may not be a suitable investment for anyone who needs to earn income from their investments.
Securities analysts may not initiate coverage or continue to cover our common stock, and this may have a negative impact on the market price of our shares
If a trading market for our common stock develops, the market will depend, in part, on the research and reports that securities analysts publish about our business and our common stock. We do not have any control over these analysts. There is no guarantee that securities analysts will cover our common stock. If securities analysts do not cover our common stock, the lack of research coverage may adversely affect the market price of those shares. If securities analysts do cover our shares, they could issue reports or recommendations that are unfavorable to the price of our shares, and they could downgrade a previously favorable report or recommendation, and in either case our share price could decline as a result of the report. If one or more of these analysts ceases to cover our shares or fails to publish regular reports on our business, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
You may experience dilution of your ownership interests if we issue additional shares of common stock or preferred stock
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present shareholders. We are currently authorized to issue an aggregate of 55,000,000 shares of capital stock consisting of 50,00,000 shares of common stock and 5,000,000 “blank check” shares of preferred stock. Upon completion of the Distribution, we expect that there will be approximately 25,422,000 shares of common stock outstanding, 1,500,000 shares of common stock that may become issuable upon issuance of contingently issuable warrants at $0.02 per share, (see Note 6 to the Unaudited Interim Condensed Financial Statements), and 2,235,000 shares of common stock reserved for issuance upon the exercise of options under our employee stock option plan. No shares of preferred stock are presently outstanding.
We may issue additional common stock or other securities that are convertible into or exercisable for common stock in order to raise additional capital, or in connection with hiring or retaining employees or consultants, or in connection with future acquisitions of licenses to technology or rights to acquire diagnostic tests in connection with future business acquisitions, or for other business purposes. The future issuance of any such additional common stock or other securities may create downward pressure on the trading price of our common stock.
We may also issue preferred stock having rights, preferences, and privileges senior to the rights of our common stock with respect to dividends, rights to share in distributions of our assets if we liquidate our company, or voting rights. Any preferred stock may also be convertible into common stock on terms that would be dilutive to holders of common stock.
Unless our common stock is approved for listing on a national securities exchange it will be subject to the so-called “penny stock” rules that impose restrictive sales practice requirements
If we are unable to obtain approval from a national securities exchange to list our common stock, those shares could become subject to the so-called “penny stock” rules if the shares have a market value of less than $5.00 per share. The SEC has adopted regulations that define a penny stock to include any stock that has a market price of less than $5.00 per share, subject to certain exceptions, including an exception for stock traded on a national securities exchange. The SEC regulations impose restrictive sales practice requirements on broker-dealers who sell penny stocks to persons other than established customers and accredited investors. An accredited investor generally is a person whose individual annual income exceeded $200,000, or whose joint annual income with a spouse exceeded $300,000 during the past two years and who expects their annual income to exceed the applicable level during the current year, or a person with net worth in excess of $1.0 million, not including the value of the investor’s principal residence and excluding mortgage debt secured by the investor’s principal residence up to the estimated fair market value of the home, except that any mortgage debt incurred by the investor within 60 days prior to the date of the transaction shall not be excluded from the determination of the investor’s net worth unless the mortgage debt was incurred to acquire the residence. For transactions covered by this rule, the broker-dealer must make a special suitability determination for the purchaser and must have received the purchaser’s written consent to the transaction prior to sale. This means that if we are unable to list our common stock on a national securities exchange, the ability of shareholders to sell their OncoCyte common stock in the secondary market could be adversely affected.
If a transaction involving a penny stock is not exempt from the SEC’s rule, a broker-dealer must deliver a disclosure schedule relating to the penny stock market to each investor prior to a transaction. The broker-dealer also must disclose the commissions payable to both the broker-dealer and its registered representative, current quotations for the penny stock, and, if the broker-dealer is the sole market-maker, the broker-dealer must disclose this fact and the broker-dealer’s presumed control over the market. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the customer’s account and information on the limited market in penny stocks.
We are an “emerging growth company,” and may elect to comply with reduced public company reporting requirements applicable to emerging growth companies, which could make our common stock less attractive to investors
We are an “emerging growth company,” as defined in the JOBS Act, and we may to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies” including reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an “emerging growth company.” We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.0 billion or more; (ii) the fifth anniversary of the completion of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
We will incur costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives
As a public reporting company, we will incur significant legal, accounting and other expenses. The Sarbanes-Oxley Act of 2002 and rules subsequently implemented by the SEC, have imposed various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will entail significant legal and financial compliance costs and will make some activities more time consuming and costly. For example, we expect that these rules and regulations may make it difficult and expensive for us to obtain director and officer liability insurance, and we may be required to accept low policy limits and coverage.
THE DISTRIBUTION
General
BioTime presently operates a substantial portion of its stem cell and regenerative medicine business through subsidiaries, some of which are wholly-owned, and some, like OncoCyte, are majority-owned by BioTime but have minority shareholders. BioTime currently owns 76.39% of the outstanding shares of OncoCyte common stock, and the remaining 23.61% of the OncoCyte shares outstanding are owned by three other shareholders. OncoCyte also has its own stock option plan under which it has granted options to purchase common stock to certain officers, directors, employees and consultants, some of whom are also officers, directors, employees or consultants of BioTime.
On December 10, 2015, the BioTime board of directors formally approved the Distribution to each holder of record of BioTime common shares at the close of business on December 21, 2015 in the ratio of one share of OncoCyte common stock for every 20 BioTime common shares held.
Reasons for the Distribution
After considering how to:
|
·
|
best provide management and employee incentives tied to OncoCyte’s growth and financial performance in order to facilitate the hiring and retention of high quality managers, research scientists, and other employees;
|
|
·
|
best position OncoCyte to finance its future capital needs for its operations;
|
|
·
|
best position OncoCyte to finance business and technology acquisitions; and
|
|
·
|
maximize value for BioTime shareholders,
|
In order to finance OncoCyte’s ongoing working capital needs, including for research and development and general administrative expenses, BioTime has had to contribute capital to OncoCyte. The source of that equity capital has been the sale of BioTime common shares. While OncoCyte has relied on and benefitted from such financing, the sale of BioTime common shares has diluted BioTime shareholders’ overall interests in BioTime and its other operating subsidiaries. The Distribution will give OncoCyte direct access to capital markets, so that OncoCyte may no longer need to rely on BioTime to obtain necessary financing to fund its operations. After the Distribution, OncoCyte’s financing activities in the capital markets may be dilutive to shareholders of OncoCyte who do not participate in the financing, but any dilutive effect on the shareholders of BioTime will be confined to any dilution of BioTime’s equity interest in OncoCyte, and will not dilute BioTime shareholders’ equity interest in other aspects of BioTime’s business.
In addition to providing OncoCyte access to the capital markets to obtain the financing, BioTime believes that creating a separate management team for OncoCyte and fostering public ownership of OncoCyte common stock will better enable OncoCyte to focus on maximizing opportunities for its cancer diagnosis business, to establish a clinical testing laboratory for its diagnostic tests, and to commercialize its diagnostic tests. The Distribution will facilitate deeper understanding by investors of the different businesses of BioTime and OncoCyte, allowing investors to more transparently value the merits, performance, and future prospects of each company, which could increase overall shareholder value.
The Distribution will also provide enhanced liquidity to holders of BioTime common shares, who after the Distribution will hold two separate publicly traded securities that they may seek to retain or monetize. Investors will have a more targeted investment opportunity by having equity in two separate companies with different investment and business characteristics, including opportunities for growth, capital structure, business model, and financial returns. BioTime also believes that the Distribution may also enhance the value of the OncoCyte common stock that BioTime continues to own, thus providing additional value to BioTime shareholders. Neither BioTime nor OncoCyte can assure that, following the Distribution, any of these benefits will be realized to the extent anticipated or at all.
BioTime’s board of directors also considered a number of other factors in evaluating the Distribution, including:
|
·
|
the one-time and on-going expenditures and financial costs of the Distribution to both BioTime and OncoCyte;
|
|
·
|
the possibility that the Distribution may affect the financial strength of BioTime or its subsidiaries;
|
|
·
|
the potential tax consequences to BioTime, BioTime shareholders and OncoCyte; and
|
|
·
|
the risk that the combined trading prices of OncoCyte common stock and BioTime common shares after the Distribution may be lower than the trading price of BioTime’s common shares before the Distribution.
|
BioTime’s board of directors concluded, however, that the potential benefits of the Distribution outweigh these factors and that the Distribution is appropriate and advisable for BioTime and its shareholders at this time.
Manner of Effecting the Distribution
The Distribution will be made on the Distribution Date, which is expected to be December 31, 2015. As a result of the Distribution, each BioTime shareholder will receive one share of OncoCyte common stock for every 20 BioTime common shares that the shareholder owns. No fractional shares will be issued. Those BioTime shareholders who would otherwise be entitled to receive fractional shares will receive cash in lieu of fractional shares. In order to be entitled to receive OncoCyte shares in the Distribution, BioTime shareholders must be shareholders at the close of business of the NYSE MKT on the record date, which is December 21, 2015. The distribution of the shares of OncoCyte common stock will be made in book-entry form. Each Distribution Share that is distributed will be validly issued, fully paid and nonassessable and free of preemptive rights. See “Description of Securities.”
BioTime shareholders will not be required to pay for the Distribution Shares of OncoCyte that they receive in the Distribution and will not need to surrender or exchange BioTime common share certificates in order to receive their OncoCyte stock, or to take any other action in connection with the Distribution. No vote of BioTime shareholders is required or sought in connection with the Distribution and BioTime shareholders have no appraisal rights in connection with the Distribution.
As of December 10, 2015, OncoCyte owned 619,706 BioTime common shares. Any OncoCyte common stock distributed to OncoCyte with respect to the BioTime common shares that it owns will automatically revert to the status of authorized but unissued shares and will not be outstanding. However, OncoCyte will continue to own the same number of BioTime common shares.
Cash in lieu of fractional shares
The distribution agent will not deliver any fractional shares of OncoCyte common stock in connection with the Distribution. Instead, the distribution agent will aggregate fractional shares into whole shares, sell the whole shares in the open market at prevailing market prices, and distribute the aggregate cash proceeds of the sales, net of brokerage fees and other costs, pro rata to each holder of BioTime common shares who would otherwise be entitled to receive a fractional share in the Distribution. Those cash payments will be made to the holders in the same accounts in which the underlying shares are held. If a BioTime shareholder physically holds BioTime stock certificates, that shareholder’s check for any cash that he or she may be entitled to receive instead of fractional shares will be included together with the account statement in the mailing that the distribution agent expects to send out on or after the Distribution Date.
BioTime, OncoCyte and the distribution agent cannot guarantee any minimum sale price for the fractional shares of OncoCyte common stock. Neither BioTime nor OncoCyte will pay any interest on the proceeds from the sale of fractional shares.
Distribution Conditions and Termination
We expect that the Distribution will be effective on the Distribution Date, December 31, 2015, provided that, among other things:
|
·
|
the SEC has declared effective our Form 10, of which this information statement is a part, under the Exchange Act;
|
|
·
|
OncoCyte and BioTime have received all material licenses, permits, estoppels, consents, approvals, authorizations, qualifications and orders of governmental authorities and third parties as are necessary for consummation of the Distribution; and
|
|
·
|
no action, proceeding or investigation shall have been instituted or threatened before any court or administrative body to restrain, enjoin or otherwise prevent the consummation of the Distribution, and no restraining order or injunction issued by any court of competent jurisdiction.
|
The fulfillment of the foregoing conditions will not create any obligation on BioTime’s part to effect the Distribution, and the board of directors of BioTime has reserved the right to amend, modify or abandon the Distribution at any time prior to the Distribution Date. The board of directors of BioTime may also waive any of these conditions.
In addition, BioTime has the right not to complete the Distribution if, at any time, the BioTime board of directors determines, in its sole discretion, that the Distribution is not in the best interests of BioTime and its shareholders. If BioTime determines to terminate the Distribution, changes the Distribution Date or the record date, or amends or modifies the terms of the Distribution, BioTime and OncoCyte will issue a press release and each will file a Current Report on Form 8-K and OncoCyte will provide a supplement to this Information Statement disclosing the applicable changes.
Reason for Furnishing this Information Statement
This information statement is being furnished solely to provide information to BioTime shareholders who will receive shares of OncoCyte common stock in the Distribution. It is not and is not to be construed as an inducement or encouragement to buy or sell any securities. We believe that the information contained in this information statement is accurate as of the date set forth on the cover. Changes may occur after that date and neither OncoCyte nor BioTime undertakes any obligation to update the information except in the normal course of our respective public disclosure obligations.
MARKET FOR OUR COMMON EQUITY
There is presently no market for our common stock and there is no assurance that a market will developed or be sustained. We have applied to list OncoCyte common stock on the NYSE MKT under the symbol OCX. If our listing application is not approved, we plan to arrange for the trading of our common stock on the OTC Bulletin Board no later than the completion of the Distribution. If our common stock initially trades on the OTC Bulletin Board, we intend to apply to list our common stock on a national securities exchange if and when we are able to meet the applicable listing standards of an exchange, but there is no assurance that any listing application that we may file will be approved by the exchange.
DIVIDEND POLICY
We have never declared any cash dividends on our common stock. For the foreseeable future we anticipate that any earnings generated in our business will be used to finance the growth of our business and will not be paid out as dividends to our shareholders.
CAPITALIZATION
The following table sets forth our cash and cash equivalents and capitalization as of September 30, 2015 and December 31, 2014. You should read this table in conjunction with the sections entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Description of Securities” and our financial statements and related notes included elsewhere in this information statement (in thousands).
| |
|
September 30,
2015
(unaudited)
|
|
|
December 31,
2014
|
|
| |
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
9,410
|
|
|
$
|
257
|
|
|
Preferred stock, no par value, 5,000 shares authorized; none issued and outstanding
|
|
$
|
-
|
|
|
|
-
|
|
|
Common stock, no par value, 50,000 shares authorized; 23,914 and 18,200 shares issued and outstanding at September 30, 2015 and December 31, 2014, respectively
|
|
|
30,979
|
|
|
|
15,147
|
|
|
Accumulated other comprehensive loss on available-for-sale securities
|
|
|
(1,442
|
)
|
|
|
(822
|
)
|
|
Accumulated deficit
|
|
|
(20,595
|
)
|
|
|
(15,399
|
)
|
|
Total stockholders’ equity (deficit)
|
|
|
8,942
|
|
|
|
(1,074
|
)
|
| |
|
|
|
|
|
|
|
|
|
Total capitalization
|
|
$
|
8,942
|
|
|
$
|
(1,074
|
)
|
SUMMARY AND SELECTED FINANCIAL DATA
The following table summarizes the relevant financial data for our business and should be read in conjunction with the sections entitled “Risk Factors,” “Capitalization” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and with our financial statements and related notes, all included elsewhere in this information statement (in thousands).
| |
|
Nine Months Ended September 30,
(unaudited)
|
|
|
Year Ended December 31,
|
|
| |
|
2015
|
|
|
2014
|
|
|
2014
|
|
|
2013
|
|
|
OPERATING EXPENSES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
$
|
3,098
|
|
|
$
|
2,837
|
|
|
$
|
3,962
|
|
|
$
|
2,943
|
|
|
General and administrative
|
|
|
2,081
|
|
|
|
638
|
|
|
|
1,011
|
|
|
|
552
|
|
|
Total operating expenses
|
|
|
5,179
|
|
|
|
3,475
|
|
|
|
4,973
|
|
|
|
3,495
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(5,179
|
)
|
|
|
(3,475
|
)
|
|
|
(4,973
|
)
|
|
|
(3,495
|
)
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER EXPENSES, NET
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net
|
|
|
16
|
|
|
|
2
|
|
|
|
2
|
|
|
|
-
|
|
|
Other expense, net
|
|
|
1
|
|
|
|
2
|
|
|
|
11
|
|
|
|
-
|
|
|
Total other expense, net
|
|
|
17
|
|
|
|
4
|
|
|
|
13
|
|
|
|
-
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET LOSS
|
|
$
|
(5,196
|
)
|
|
$
|
(3,479
|
)
|
|
$
|
(4,986
|
)
|
|
$
|
(3,495
|
)
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per common share
|
|
$
|
(0.26
|
)
|
|
$
|
(0.19
|
)
|
|
$
|
(0.27
|
)
|
|
$
|
(0.19
|
)
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding used to compute net loss per common share, basic and diluted
|
|
|
19,803
|
|
|
|
18,200
|
|
|
|
18,200
|
|
|
|
18,200
|
|
| |
|
September 30,
2015
(unaudited)
|
|
December 31, 2014
|
|
Balance Sheet Data:
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
9,410
|
|
|
$
|
257
|
|
|
Available-for-sale securities, at fair value
|
|
|
2,598
|
|
|
|
3,280
|
|
|
Intangible assets, net
|
|
|
1,290
|
|
|
|
1,472
|
|
|
Total assets
|
|
|
14,298
|
|
|
|
6,356
|
|
|
Total liabilities
|
|
|
5,356
|
|
|
|
7,430
|
|
|
Total stockholders’ equity (deficit)
|
|
$
|
8,942
|
|
|
$
|
(1,074
|
)
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following Management’s Discussion and Analysis of Financial Condition and Results of Operations is intended to provide information necessary to understand our unaudited interim condensed financial statements for the nine months ended September 30, 2015 and 2014, and our audited financial statements for the years ended December 31, 2014 and 2013, and highlight certain other information which, in the opinion of management, will enhance a reader's understanding of our financial condition, changes in financial condition and results of operations. These historical financial statements may not be indicative of our future performance. This Management's Discussion and Analysis of Financial Condition and Results of Operations contains a number of forward-looking statements, all of which are based on our current expectations and could be affected by the uncertainties and risks described throughout this filing, particularly in “Risk Factors.”
Emerging Growth Company Status
The Jumpstart our Business Startups Act of 2012 (“JOBS Act”) permits an "emerging growth company" such as OncoCyte to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. However, we will comply with newly adopted or revised accounting standards when they become applicable to public companies because our financial statements will be consolidated with those of BioTime, which is not an emerging growth company under the JOBS Act and is therefore not permitted to delay the adoption of new or revised accounting standards that become applicable to public companies. This election under the JOBS Act to not delay the adoption of new or revised accounting standards is irrevocable.
Management’s Discussion and Analysis of Financial Condition and Results of Operations.
We were incorporated during September 2009. Our operations have included planning and launching research and diagnostic test development programs in house and with partners, pursuing patents, and conducting clinical trials.
The inherent uncertainties of developing new diagnostic tests for medical use make it impossible to predict the amount of time and expense that will be required to complete the development and commence commercialization of new diagnostic tests. There is no assurance that we will be successful in developing new technology or diagnostic tests, or that any technology or diagnostic tests that we may develop will be proven safe and effective in diagnosis of cancer in humans, or will be successfully commercialized.
We believe we have sufficient capital to carry out our current research and development plan at least through September 30, 2016. We may need to obtain additional financing in order to continue our operations after this date. Our determination as to when we will seek new financing and the amount of financing that we will need will be based on our evaluation of the progress we make in our research and development program, any changes to or the expansion of the scope and focus of our research, and our projection of future costs. See “Liquidity and Capital Resources” for a discussion of our available capital resources, our potential need for future financing, and possible sources of capital.
Critical Accounting Policies
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts in our financial statements and related notes. Our significant accounting policies are described in Note 2 to our financial statements included elsewhere in this information statement. We have identified below our critical accounting policies and estimates that we believe require the greatest amount of judgment. On an ongoing basis, we evaluate our estimates that are subject to significant judgment including those related to the allocation of direct and indirect expenses, useful lives associated with long-lived assets, loss contingencies, valuation allowances related to deferred income taxes, and assumptions used to value stock-based awards, convertible debt, conversion features, warrants or other equity instruments. Actual results could differ materially from those estimates. On an ongoing basis, we evaluate our estimates compared to historical experience and trends, which form the basis for making judgments about the carrying value of assets and liabilities. To the extent that there are material differences between our estimates and our actual results, our future financial statement presentation, financial condition, results of operations and cash flows will be affected.
We believe the assumptions and estimates associated with the following have the greatest potential impact on our financial statements.
Related party transactions - Shared Facilities and Services Agreement
As more fully described in Note 4 to our financial statements, to the extent we do not employ our own human resources for operations, our parent company, BioTime, or BioTime commonly controlled and consolidated subsidiaries provide certain employees for administrative or operational services, as necessary, for our benefit, under the Shared Facilities Agreement. Accordingly, BioTime allocates expenses such as salaries and payroll related expenses incurred and paid on behalf of OncoCyte based on the amount of time that particular employees devote to our affairs. Other expenses such as legal, accounting, marketing, travel, and entertainment expenses are allocated to us to the extent that those expenses are incurred by or on behalf of OncoCyte. BioTime also allocates certain overhead expenses such as insurance, internet and telephone expenses based on a percentage determined by management. These allocations are made based upon activity-based allocation drivers such as time spent, percentage of square feet of office or laboratory space used, and percentage of personnel devoted to our operations or management. Management evaluates the appropriateness of the percentage allocations on a quarterly basis and believes that this basis for allocation is reasonable.
Stock-based compensation
We recognize compensation expense related to employee option grants and restricted stock units, if any, in accordance with FASB ASC 718, Compensation – Stock Compensation (“ASC 718”).
We estimate the fair value of employee stock-based payment awards on the grant-date and recognize the resulting fair value, net of estimated forfeitures, over the requisite service period. We use the Black-Scholes option pricing model for estimating the fair value of options granted under our stock option plan. The fair value of restricted stock units, if any, is determined based on the value of the underlying common stock. We have elected to treat stock-based payment awards with graded vesting schedules and time-based service conditions as a single award and recognize stock-based compensation on a straight-line basis, net of estimated forfeitures, over the requisite service period.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718 and FASB ASC 505-50, Equity-Based Payments to Non-Employees (“ASC 505-50”). Stock option awards issued to non-employees, consisting principally of BioTime or BioTime commonly controlled and consolidated subsidiaries’ employees performing services for us, are accounted for at fair value using the Black-Scholes option pricing model. We believe that the fair value of the stock options is more reliably measured than the fair value of services received. We record compensation expense based on the then-current fair values of the stock options at each financial reporting date. Compensation recorded during the service period is adjusted in subsequent periods for changes in the stock options' fair value until the earlier of the date at which the non-employee’s performance is complete or a performance commitment is reached, which is generally when the stock option award vests. Compensation expense for non-employee grants is recorded on a straight-line basis in the statements of operations.
The Black-Scholes option pricing model requires us to make certain assumptions including the fair value of the underlying common stock, the expected term, the expected volatility, the risk-free interest rate and the dividend yield.
The fair value of the shares of our common stock underlying the stock options has historically been determined by our Board of Directors. Because there has been no public market for our common stock, our Board of Directors has determined the fair value of our common stock at the time of the grant of options by considering a number of objective and subjective factors including contemporaneous sales of our common stock to investors, valuation of comparable companies, operating and financial performance and general and industry-specific economic outlook, among other factors. The fair value our common stock will be determined by our Board of Directors until such time as our common stock is listed on an established stock exchange or national market system. We determined the fair value in accordance with applicable elements of the practice aid issued by the American Institute of Certified Public Accountants titled Valuation of Privately Held Company Equity Securities Issued As Compensation.
The expected term of employee stock options represents the weighted-average period that the stock options are expected to remain outstanding. We estimate the expected term of options granted based upon the “simplified method” provided under Staff Accounting Bulletin, Topic 14 (“SAB Topic 14”).
Because our common stock has no publicly traded history, we estimate the expected volatility of the awards from the historical volatility of selected public companies within the biotechnology industry with comparable characteristics to us, including similarity in size, lines of business, market capitalization, revenue and financial leverage. We determined the expected volatility assumption using the frequency of daily historical prices of comparable public company’s common stock for a period equal to the expected term of the options. We periodically assess the comparable companies and other relevant factors used to measure expected volatility for future stock option grants.
The risk-free interest rate assumption is based upon observed interest rates on the United States government securities appropriate for the expected term of our stock options.
The dividend yield assumption is based on our history and expectation of dividend payouts. We have never declared or paid any cash dividends on our common stock, and we do not anticipate paying any cash dividends in the foreseeable future.
The assumptions that were used to calculate the grant date fair value of our employee and non-employee stock option grants for the years ended December 31, 2014 and 2013 were as follows.
| |
|
2014(1)
|
|
2013
|
|
|
Expected life (in years)
|
|
- |
|
|
4.13 |
|
|
|
Risk-free interest rates
|
|
- |
% |
|
1.28 |
%
|
|
|
Volatility
|
|
- |
%
|
|
70.68 |
%
|
|
|
Dividend yield
|
|
- |
%
|
|
0.00 |
%
|
|
(1) No stock options were granted in 2014.
The assumptions that were used to calculate the grant date fair value of our employee and non-employee stock option grants for the nine months ended September 30, 2015 and 2014 were as follows.
| |
|
2015
|
|
2014(1)
|
|
|
Expected life (in years)
|
|
6.89 |
|
|
- |
|
|
|
Risk-free interest rates
|
|
1.81 |
%
|
|
- |
%
|
|
|
Volatility
|
|
74.25 |
%
|
|
- |
%
|
|
|
Dividend yield
|
|
0.00 |
%
|
|
- |
%
|
|
(1) No stock options were granted in 2014.
Stock-based compensation expense is recognized based on awards that are ultimately expected to vest, and as a result, the amount has been reduced by estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures are estimated based on our historical experience and future expectations.
The determination of stock-based compensation is inherently uncertain and subjective and involves the application of valuation models and assumptions requiring the use of judgment. If we had made different assumptions, our stock-based compensation expense for all periods presented may have been significantly different.
Accounting for BioTime Shares
We account for the BioTime shares we hold as available-for-sale equity securities in accordance with ASC 320-10-25, Investments – Debt and Equity Securities, as the shares have a readily determinable fair value quoted on the NYSE MKT and are held principally for future working capital purposes, as necessary. These shares are measured at fair value and reported as current assets on the balance sheet based on the closing trading price of the security as of the date being presented. Unrealized holding gains and losses due to changes to the fair value of these shares are excluded from the statements of operations and reported in equity as part of other comprehensive income or loss, net of income taxes, until realized. Realized gains and losses for shares sold are reclassified out of accumulated other comprehensive income or loss and included in equity, as an increase or decrease to common stock equity consistent with, and pursuant to, ASC 805-50, transactions between entities under common control.
Fair Value Measurements
We account for fair value measurements in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 820, Fair Value Measurements (“ASC 820”). ASC 820 establishes a single authoritative definition of fair value, sets out a framework for measuring fair value and expands on required disclosures about fair value measurement. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are the following:
Level 1 – Quoted prices in active markets for identical assets and liabilities.
Level 2 – Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted market prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
In determining fair value, we utilize valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible as well as considers counterparty credit risk in its assessment of fair value. For the periods presented, we had no financial assets or liabilities recorded at fair value on a recurring basis, except for cash equivalents consistent of money market funds and the available-for-sale securities of BioTime common stock held by us described above. These assets are measured at fair value using the period-end quoted market prices as a Level 1 input.
The carrying amounts of cash equivalents, prepaid expenses and other current assets, amounts due to BioTime and other affiliates, accounts payable, accrued expenses and other current liabilities approximate fair values because of the short-term nature of these items.
The fair value of our contingently issuable common stock warrants is estimated using a Black-Scholes valuation model, using Level 3 inputs, then adjusted for the probability of actually issuing those warrants to arrive at the probability-adjusted fair value. Since the contingently issuable warrants are equity classified and considered issued for accounting purposes as of June 30, 2015, no further remeasurement of the fair value of the warrants was necessary in subsequent periods for financial statement reporting purposes, regardless of changes in the probability of issuance or if the warrants are actually issued.
We have elected not to measure the related party convertible promissory note payable (disclosed in Note 5 to our unaudited interim condensed financial statements) at fair value, with the changes in fair value recognized in the statements of operations each period in accordance with ASC 815-15, Derivatives and Hedging – Embedded Derivatives. The debt conversion feature can only be physically settled by delivery of our common stock for converted principal and interest. The debt conversion feature does not meet the definition of an embedded derivative requiring bifurcation under the provisions of ASC 815-10 since, among other factors, we are not permitted to cash settle, and BioTime cannot demand a cash settlement, in the event of a conversion and there is no net settlement available outside the debt agreement since the shares of our common stock underlying the conversion are not readily convertible into cash. The debt instrument does not fall within any of the three categories of liabilities defined in the scope of ASC 480-10, Distinguishing Liabilities from Equity. Accordingly, we account for the convertible debt instrument in accordance with ASC 470-20, Debt with Conversion and Other Options. On the date the convertible debt was issued, the conversion price was equal to the fair value of our common stock underlying the conversion and no beneficial conversion feature was present based on the guidance of ASC 470-20.
The issuance date fair value of the related party convertible debt is determined using a hybrid instrument valuation model by applying a market valuation method for the debt instrument using a risk-adjusted interest rate similar to “CC” and “D” rated junk bonds and, a Black-Sholes option pricing model for the conversion feature. The debt instrument and the conversion feature issuance date fair values are determined using Level 3 inputs since both the risk adjustment made to the interest rate for the debt and the principal assumptions made in the option pricing model for the conversion feature are specific to OncoCyte and considered to be significant unobservable key inputs.
Due to the proximity of the May 8, 2015 issuance date and the September 30, 2015 balance sheet presented in this information statement, the carrying value of the related party convertible debt approximates its fair value as of September 30, 2015.
Long-lived intangible assets
Long-lived intangible assets, primarily consisting of acquired patents, patent applications, and licenses to use certain patents are stated at acquired cost, less accumulated amortization. Amortization expense is computed using the straight-line method over the estimated useful lives of the assets over a period of 10 years.
Impairment of long-lived assets
We assess the impairment of long-lived assets, which consist primarily of long-lived intangible assets, furniture and equipment, whenever events or changes in circumstances indicate that such assets might be impaired and the carrying value may not be recoverable. If events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable and the expected undiscounted future cash flows attributable to the asset are less than the carrying amount of the asset, an impairment loss equal to the excess of the asset’s carrying value over its fair value is recorded. To date, there have been no such impairment losses.
Income taxes
We have filed a standalone U.S. federal income tax return since our inception. For California purposes, our activity for 2013 and 2014 has been included in BioTime’s California Combined tax return. The provision for income taxes has been determined as if we had filed separate tax returns for the periods presented. Accordingly, our effective tax rate in future years could vary from our historical effective tax rates depending on our future legal structure and related tax elections. The historical deferred tax assets, including the operating losses and credit carryforwards generated by us, will remain with us. We account for income taxes in accordance with GAAP, which prescribes the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. Our judgments regarding future taxable income may change over time due to changes in market conditions, changes in tax laws, tax planning strategies or other factors. If our assumptions and consequently our estimates change in the future, the valuation allowance may be increased or decreased, which may have a material impact on our statements of operations.
The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. We will recognize accrued interest and penalties, if any, related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of the financial statements periods presented herein. We are not aware of any uncertain tax positions that could result in significant additional payments, accruals, or other material deviation for the periods presented herein. We are currently unaware of any tax issues under review.
Research and development expenses
Research and development expenses consist of personnel costs and related benefits, including stock-based compensation, and expenses for outside consultants. These expenses include both direct and allocated or indirect overhead costs. Research and development costs are expensed as incurred.
General and administrative expenses
Our general and administrative expenses relate primarily to compensation and related benefits, including stock-based compensation, for executive and corporate personnel, including direct and allocated costs from BioTime; professional and consulting fees; direct and indirect allocated overhead.
Results of Operations
Comparison of Nine Months Ended September 30, 2015 and 2014
The following tables show our operating expenses for the nine months ended September 30, 2015 and 2014 (in thousands).
| |
|
Nine Months Ended
September 30,
|
|
|
|
|
|
|
|
| |
|
2015
|
|
2014
|
|
$ Increase
|
|
% Increase
|
|
|
Research and development expenses
|
|
$
|
3,098
|
|
|
$
|
2,837
|
|
|
$
|
+261
|
|
|
|
+9.2
|
% |
|
|
General and administrative expenses
|
|
|
2,081
|
|
|
|
638
|
|
|
|
+1,443
|
|
|
|
+226.2
|
% |
|
Research and development expenses
Research and development expenses for the nine months ended September 30, 2015 increased to $3.1 million from $2.8 million for the same period in 2014. Research and development expenses for the nine months ended September 30, 2015 reflect the following expense increases: consulting fees and outside research and service costs primarily related to regulatory and clinical trials of our diagnostic tests increased by $474,000. This was in part offset by a $214,000 decrease in and patent, license, and trademark related fees.
We expect to continue to incur a significant amount of research and development expenses.
General and administrative expenses
General and administrative expenses for the nine months ended September 30, 2015 increased to $2.1 million from $638,000 for the same nine month period in 2014. General and administrative expenses for the nine months ended September 30, 2015 reflect the following expense increases: $512,000 of stock based compensation expenses to employees and consultants allocated to general and administrative expenses largely attributed to an increase in staffing, including newly hired executives; $216,000 of accounting and audit related expenses; $185,000 of general consulting expenses; $110,000 of salaries and payroll related expenses allocated to general and administrative expenses; $97,000 of legal expenses; $92,000 of investor and public relations related expenses; and $91,000 of recruiting expenses.
Comparison of the Year Ended December 31, 2014 and Year Ended December 31, 2013
The following tables show our operating expenses for the year ended December 31, 2014 and 2013 (in thousands).
| |
|
Year Ended
December 31,
|
|
|
|
|
|
|
|
|
|
| |
|
2014
|
|
|
2013
|
|
|
$ Increase
|
|
|
% Increase
|
|
|
Research and development expenses
|
|
$
|
3,962
|
|
|
|
$
|
2,943
|
|
|
|
$
|
+1,019
|
|
|
|
|
+35
|
% |
|
|
General and administrative expenses
|
|
|
1,011
|
|
|
|
|
552
|
|
|
|
|
+459
|
|
|
|
|
+83
|
% |
|
The following table shows the approximate amounts and percentages of our total research and development expenses of $4.0 million and $2.9 million allocated to our primary research and development projects during the years ended December 31, 2014 and 2013, respectively (in thousands).
| |
|
Amount(1)
|
|
|
Percent
|
|
|
Program
|
|
2014
|
|
|
2013
|
|
|
2014
|
|
|
2013
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General
|
|
$
|
1,621
|
|
|
|
$
|
2,852
|
|
|
|
|
40.9
|
% |
|
|
|
96.9
|
% |
|
|
Bladder Cancer Confirmatory Diagnostic
|
|
$
|
1,143
|
|
|
|
$
|
27
|
|
|
|
|
28.9
|
% |
|
|
|
0.9
|
% |
|
|
Breast Cancer Confirmatory Diagnostic
|
|
$
|
1,057
|
|
|
|
$
|
49
|
|
|
|
|
26.7
|
% |
|
|
|
1.7
|
% |
|
|
Lung Cancer Confirmatory Diagnostic
|
|
$
|
76
|
|
|
|
$
|
-
|
|
|
|
|
1.9
|
% |
|
|
|
-
|
% |
|
|
COLX
|
|
$
|
65
|
|
|
|
$
|
15
|
|
|
|
|
1.6
|
% |
|
|
|
0.5
|
% |
|
|
Total
|
|
$
|
3,962
|
|
|
|
$
|
2,943
|
|
|
|
$
|
100.0
|
% |
|
|
$
|
100.0
|
% |
|
|
(1) |
Amount also includes certain general research and development expenses, such as laboratory supplies, laboratory expenses, rent allocated, and insurance allocated to research and development expenses, incurred directly by BioTime on behalf of OncoCyte and allocated to OncoCyte under the Shared Facilities Agreement. |
General and administrative expenses
General and administrative expenses for the year ended December 31, 2014 increased to $1.0 million from $552,000 for the same period in 2013. General and administrative expenses for the year ended December 31, 2014 reflect the following expense increases: $173,000 of salaries and payroll related expenses allocated to general and administrative expenses and $229,000 in salaries and related expenses for accounting services and building maintenance allocated from BioTime based on fixed rates evaluated on a quarterly basis by BioTime management and salaries for other services allocated based on monthly timesheets.
Income taxes
As of September 30, 2015, we had net operating loss carryforwards of approximately $18.1 million for U.S. federal income tax purposes, which expire through 2035. As of December 31, 2014, we had net operating loss carryforwards of approximately $13.9 million for U.S. federal income tax purposes, which expire through 2034. Due to our losses incurred for all periods presented, we did not record any provision or benefit for income taxes. OncoCyte’s federal research and development credit carryforwards as of September 30, 2015 will expire through 2035.
During 2014, OncoCyte sold 406,756 BioTime common shares in open market transactions which resulted in a taxable gain of approximately $1.3 million. This taxable gain was fully offset by current operating losses, thus resulting in no income taxes due from the sale. During the first nine months of 2015, OncoCyte sold 13,356 BioTime common shares in open market transactions which resulted in a taxable gain of approximately $44,000. This taxable gain is expected to be fully offset by current operating losses, thus resulting in no income taxes due from the sale.
At September 30, 2015 and December 31, 2014, OncoCyte has recorded a deferred tax liability of $0.9 million and $1.1 million, respectively, resulting from the difference in the tax basis of BioTime shares held by OncoCyte as compared to the basis of such shares reported on OncoCyte’s balance sheet.
A valuation allowance is provided when it is more likely than not that some portion of the deferred tax assets will not be realized. OncoCyte established a full valuation allowance for all periods presented due to the uncertainty of realizing future tax benefits from its net operating loss carryforwards and other deferred tax assets.
Liquidity and Capital Resources
At September 30, 2015, we had $9.4 million of cash and cash equivalents and held BioTime available-for-sale securities valued at $2.6 million. It is likely that we will need to obtain additional debt or equity capital in order to finance our operations. If equity or debt financing is necessary, we cannot assure that such financing will be available on favorable terms, if at all. Since inception, we have financed our operations through the sale of our common stock to our current shareholders, loans from BioTime and BioTime affiliated entities, and the sale of BioTime available-for-sale securities. The amount of revenue that may be earned through the licensing and sale of our diagnostic tests and technology, if any revenue is earned at all, the timing of the receipt of diagnostic test sales revenues, license fees, and royalty payments, if any at all, and the future availability and terms of equity or debt financing, are uncertain.
Based on cash and other liquid assets currently on hand and projected rates of expenditure we believe that we will be able to fund our ongoing operations through December 31, 2016, including costs related to research and development, general and administrative, commercial planning and market development, and development and licensing of a CLIA certified laboratory. If results of the development efforts in the lung or bladder diagnostic products are successful to the point where we believe that a commercial product can be launched successfully then additional capital of up to $5.0 million may be required during 2016 for building out a sales and marketing team and launching the product. Dependent on results of any product commercialization and ongoing sales, additional capital might be required in 2017 or beyond to develop and launch other products, for working capital, and for other expenses.
The unavailability or inadequacy of financing or revenues to meet future capital needs could force us to modify, curtail, delay, or suspend some or all aspects of our planned operations. Sales of additional equity securities could result in the dilution of the interests of our shareholders.
Cash used in operations
During the nine months ended September 30, 2015 and the year ended December 31, 2014, our total research and development expenditures were $3.1 million and $4.0 million, respectively, and our general and administrative expenditures were $2.1 million and $1.0 million, respectively. Net loss for the nine months ended September 30, 2015 and the year ended December 31, 2014 amounted to $5.2 million and $5.0 million, respectively. Net cash used in operating activities during these periods amounted to $2.5 million and $1.2 million, respectively, primarily due to the net loss generated during the periods offset by changes in working capital.
Cash flows provided by investing activities
During the nine months ended September 30, 2015 and the year ended December 31, 2014, net cash provided by investing activities were $33,000 and $1.3 million principally from the sales of 13,356 and 406,756 BioTime common shares in the open market for $44,000 and $1.3 million in cash proceeds, respectively.
Cash provided by financing activities
During the nine months ended September 30, 2015 we received $3.3 million in cash from the sale of 1,500,000 shares of our common stock to two existing shareholders and $8.3 million in cash from the sale of 2,710,857 shares of our common stock to BioTime.
Contractual obligations
We had no contractual obligations as of September 30, 2015, with the exception of a Shared Facilities Agreement with BioTime under which we reimburse BioTime for a portion of the rent and other expenses of leasing our office and laboratory facility in Alameda, California, and for BioTime’s cost of providing us with the use of laboratory and office equipment and supplies, utilities, and personnel. BioTime’s lease expires on February 29, 2016. We are presently being allocated from BioTime, 10% of certain general and administrative overhead expenses and 25% of certain general research and development expenses, which include BioTime’s insurance, general office and laboratory supplies, shipping and postage, internet and telephone expenses, certain office equipment repair and maintenance expenses, and base monthly rent and other costs arising under the lease of the laboratory and office facility that we share with BioTime. Our share of the base rent expense recognized by BioTime for the office and laboratory facility during the nine months ended September 30, 2015 was approximately $13,000 per month. Salaries and related expenses for accounting services and building maintenance are allocated based on a fixed percentage evaluated by BioTime management on a quarterly basis and adjusted based on the level of activity in each quarter. Salaries for any other services are allocated based on monthly timesheets.
Off-Balance Sheet Arrangements
As of September 30, 2015 and as of December 31, 2014, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of SEC Regulation S-K.
Quantitative and Qualitative Disclosures about Market Risk
Foreign Currency Exchange Risk
We are not presently exposed in a significant degree to foreign exchange currency risks because we are not conducting international business at this time, and we do not engage in foreign currency hedging activities. If we engage in international transactions, we will need to translate foreign currencies into U.S. dollars for reporting purposes, and currency fluctuations could have an impact on our financial results.
Credit Risk
We place some of our cash in U.S. banks and invest most of our cash in money market funds. Deposits with banks may temporarily exceed the amount of insurance provided on such deposits. We will monitor the cash balances in the accounts and adjust the cash balances as appropriate, but if the amount of a deposit at any time exceeds the federally insured amount at a bank, the uninsured portion of the deposit could be lost, in whole or in part, if the bank were to fail. Our investments in money market funds are not insured or guaranteed by the United States government or any of its agencies.
Interest Rate Risk
We invest most of our cash in money market funds. The primary objective of our investments will be to preserve principal and liquidity while earning a return on our invested capital, without incurring significant risks. Our future investment income is not guaranteed and may fall short of expectations due to changes in prevailing interest rates, or we may suffer losses in principal if the net asset value of a money market fund falls below $1 per share.
BUSINESS
Overview
Our mission is to develop highly accurate, simple to administer non-invasive liquid biopsy diagnostic tests in areas of high unmet need in oncology. Our initial focus will be confirmatory diagnostics that are used in conjunction with imaging to confirm initial diagnoses. In addition, we will be developing screening diagnostics as potential replacements for screening imaging procedures that do not meet the needs of patients, health care providers or payers. For some indications, we will also be pursuing the development of prognostics that predict the probability of recurrence of a specific cancer, or companion diagnostics that help a physician determine which therapy is the optimal treatment for the patients.
Our initial liquid biopsy diagnostic tests will be confirmatory diagnostics and are being developed to reduce false positives associated with the current diagnostic protocols. These new diagnostic tests are intended to:
|
·
|
Improve health outcomes through early diagnoses and better prognostic capabilities;
|
|
·
|
Reduce the cost of care through the avoidance of more costly diagnostic procedures, including invasive biopsy and cystoscopic procedures; and
|
|
·
|
Improve the quality of life for cancer patients by reducing the anxiety associated with non-definitive diagnoses.
|
We are currently working on diagnostic tests for three types of cancer: lung cancer, breast cancer, and bladder cancer. Additionally we have early stage diagnostic research programs for other solid tumor cancers in our research and development pipeline.
Diagnostic Tests
Our research has demonstrated that many of the same genes associated with the normal growth of embryonic stem cells are abnormally reactivated by cancer cells. Under this premise, we have established a proprietary gene expression dataset using mRNA and miRNA microarray technology. Our dataset contains expression levels of over 47,000 genes from over 600 unique samples, representing both normal and cancerous tissues and cell lines, including multiple human embryonic stem cell lines. This broad, bioinformatics-based approach has allowed us to identify numerous genes abnormally activated in cancer or tumor cells. Many of these genes have not been previously associated with cancer. Moreover, expression of a large subset of these genes is found across numerous cancer types, such as cancers of the lung, breast, bladder, colon, and ovaries, suggesting that these genes may control fundamental processes during cancer growth and progression. This gene expression data presents numerous diagnostic test opportunities, such as tests designed to:
|
· |
Screen patient for the presence of cancer; |
|
· |
Confirm an indeterminate result from a screening procedure for cancer such as mammogram Breast Imaging Reporting and Data System (“BI-RADS”) 3 or 4 – defined as nodules that are probably not cancerous or have suspicious abnormality; Low Dose Computed Tomography (“LDCT”) scan positive result, or indeterminate cytology; |
|
· |
Determine which treatment courses have highest chances for producing a favorable response in individual patients; and |
|
· |
Monitor for recurrence of a patient’s cancer |
Our scientists subsequently determined that the patterns of the proteins produced from a subset of these genes could be detected in the blood or urine of cancer patients, but not in the blood or urine of healthy people. The technology that we are using is called liquid biopsy where instead of taking a sample from tumor tissue, liquid biopsies examine the DNA shed by tumors or cancer cells in the blood or urine.
The percentage of times that the test correctly identified people as cancer-free, which defines the test's specificity, was higher than that of commonly used tests or procedures, such as, cytology, LDCT screening, mammograms and MRIs. This finding, combined with initial evidence that prospective screening diagnostic tests utilizing blood or urine may be useful for diagnosing a broad range of cancer types, led us to prioritize the development of diagnostic tests to detect the presence of various human cancers.
Based on substantial unmet needs, large markets, and data generated thus far from patient serum or urine screening, we are focusing our efforts on biomarkers associated with lung, breast and bladder cancers, and especially on detectable amounts of several cancer-associated biomarkers in patients with early-stage disease. The apparent high correlation of certain combinations of biomarkers in lung, breast and bladder cancer has identified these three diseases as promising initial targets.
The relative ease of administering a liquid biopsy diagnostic and cost savings due to the elimination of more costly and invasive biopsy procedures, we believe will make liquid biopsy diagnostic tests useful as routine tests that could be performed in men and women of any age and at any desired frequency to detect lung, breast and bladder cancer. If successfully developed, our tests will initially reduce diagnosis uncertainty and eliminate unnecessary down-stream procedures resulting from indeterminate LDCT, cytology or mammogram tests.
We intend to initially develop and market a lung cancer diagnostic test in the United States before seeking regulatory approvals required to market the diagnostic test in other countries. The test developed will be a blood screening test for cancer markers, which will be regulated under CLIA as an LDT. We may also pursue IVD filings through the FDA or through the European Directive on IVDs.
We plan to start the process to establish a laboratory in early 2016, including ordering the equipment and hiring the personnel, for which we will apply for CLIA registration and certification.
Types of Diagnostic Use
Once we have completed development of a liquid biopsy diagnostic test, we may commence marketing that diagnostic test for one or more specific kinds of use which relate to the kind of diagnostic evaluation that a physician is performing for a patient. Our diagnostics may take one or more of four different types of use depending on the type of cancer and the performance of the diagnostic. These intended uses include:
|
· |
Confirmatory diagnostics – confirmatory diagnostics are used in conjunction with a current standard of care screening procedure. For example, our lung confirmatory diagnostic would be used in conjunction with LDCT to confirm a suspicious result by yielding a secondary suspicious versus benign result. In the case of the benign results, patients would not need additional invasive procedures to determine the presence of cancer. In the case of the suspicious results, additional procedures would be highly warranted; |
|
· |
Screening diagnostics – screening diagnostics would replace or be used as an alternative to existing screening procedures. A screener diagnostic for breast cancer could be used as an alternative to MRIs for women with a family history of breast cancer, BRCA mutations or dense breast tissue. This test could become part of a routine annual or other periodic physical examination; |
|
· |
Recurrence diagnostics also known as prognostics – are used for patients who had previously been diagnosed with cancer but are currently in remission. In the case of our bladder diagnostic, the test could be used in lieu of a painful, costly cystoscopy to confirm whether the cancer has returned. This test could become part of the follow-up of bladder cancer patients; and |
|
· |
Companion diagnostics – used by physicians to help determine an optimal therapy for a specific patient. An example of this would be HER2+ and Herceptin. |
Oncology Diagnostic Tests Progress to Date
We first announced the development of our confirmatory and screening diagnostics during December 2011 and have achieved several key advances since then, including:
|
· |
Evaluated over 50 potential cancer biomarkers discovered by OncoCyte and BioTime using antibody-based ELISA technology in blood serum samples from a proprietary sample bank derived from over 600 donors, including patients with cancers of the breast, colon, and pancreas, as well as healthy volunteers; |
|
· |
Selected seven of the newly discovered serum markers demonstrated to be significantly elevated in cancer patients as compared to healthy controls; |
|
· |
Initiated plans for the commercial development and manufacture of monoclonal antibodies to these markers for potential inclusion in diagnostic test kits; |
|
· |
Completed experiments characterizing COL10A1, one of the seven priority cancer biomarkers discovered using OncoCyte’s proprietary cancer microarray dataset, and publication of a manuscript summarizing this work in the peer-reviewed journal Future Oncology, which demonstrates the localization of the marker in breast cancer but not in healthy breast tissue; |
|
· |
Extended sponsor research agreement with Wistar continuing the development of a lung confirmatory diagnostic; |
|
· |
Presented of preliminary findings at American Thoracic Society in May 2015 showing a ROC score of 0.88 for their mRNA and miRNA classifier; |
|
· |
Initiated a first and second clinical study collecting urine samples from patients undergoing cystoscopies to support development of confirmatory and recurrence diagnostics for bladder cancer; |
|
· |
Presented preliminary findings at American Association for Cancer Research in April 2015 showing a ROC score of 0.91; |
|
· |
Initiated a clinical study in 2014 collecting blood samples from patients undergoing mammography for the detection of breast cancer; |
|
· |
Developed a preliminary classifier diagnostic based on number of mRNA biomarkers; and |
|
· |
Have submitted 13 U.S. patents pending and 38 international patents issued or pending, with claims covering use of various cancer markers in the diagnosis and/or prognosis of various cancers. |
The Development Pathway
Our liquid biopsy diagnostic tests for cancer will each go through four stages of development prior to commercialization: the research stage; assay development; validation studies; and CLIA validation. Clinical utility studies will also be conducted after commencement of the marketing of a diagnostic test.
The first stage of the development of a CLIA LDT is the research stage. In the research stage of a molecular diagnostic, biological markers are analyzed to determine if specific markers are differentially expressed in certain diseases. We are developing blood and urine tests that differentiate malignant patient samples from benign patient samples by looking at differences in the amount of specific mRNA and miRNA expressed in whole blood or urine. The objective of this phase of the development process is to delineate promising biomarkers.
In the second stage, assay development, the best performing mRNA and miRNA biomarkers are combined into an assay. The optimal combination of biomarkers that are to be utilized in the final diagnostic are determined through bioinformatics and machine learning software strategies, and assay/marker reliability and usability. The end result of this stage is a “locked down” assay.
The locked down assay is first engineered and tested for reliability, reproducibility, accuracy, precision and stability in series of research and development studies, which we referred to as R&D validation studies, that result in the validation of the assay. In these R&D validation studies, blinded samples are run through the assay to confirm that the results reported by the assay are consistent across an appropriate range of real world, day to day variables including operating temperature variances and sample differences.
After the completion of the R&D validation study, studies and analysis are run in the CLIA laboratory – the laboratory where the diagnostic will be performed after commercial launch – in order to confirm the reliability of the diagnostic test and the full test system in the clinical environment. The CLIA validation phase confirms that the diagnostic test being used routinely in the clinical oncology market meets the appropriate regulatory and clinical standards. Successful completion of this stage results in the finalization and lockdown of the commercial diagnostic test system. At this point, the laboratory undergoes the inspection and certification process, which allows the marketing of the diagnostic in specific states or countries.
The final phase of the diagnostic pathway occurs after the final diagnostic test has been launched and consists of carrying out one or more clinical utility studies. These studies are important for obtaining coverage and reimbursement by payers such as Medicare, Medicaid, third party commercial insurers, health maintenance organizations (“HMOs”), and large corporations that self-insure. Clinical utility studies analyze the healthcare economics associated with a diagnostic test, and in particular whether it results in overall patient benefits and decreased expenditures for the healthcare system. These studies track the progress of patients who have had the diagnostic test administered; for those patients where the diagnostic test has ruled out the possibility of a disease, downstream procedures are tracked to see if physician behavior has changed. The results of this phase may be published in peer review journals and are generally compiled in dossiers to share with managed care groups, including both public and commercial payers. Obtaining positive results in clinical utility studies is very important in obtaining positive coverage and reimbursement decisions by payers.
For example, in our first product candidate - the lung confirmatory diagnostic - patients who have received a suspicious finding in a low dose computed tomography (LDCT) screening will be tested with our diagnostic. During our post marketing clinical utility studies, we will be tracking patients with a benign result to see if any unnecessary downstream procedures (bronchoscopy or surgical biopsy) are still performed. In other words, we will track whether our diagnostic tests prevent unnecessary procedures and reduce the overall cost of diagnosing lung cancer, or whether they are used in addition to downstream procedures, and thereby increase overall costs.
Our three most advanced diagnostic tests (lung, breast and bladder cancer tests) are in the assay development stage. We anticipate that our lung cancer diagnostic test will move into the R&D validation stage in 2016 and finish the CLIA validation stage by the end of 2016 but there can be no assurance that the development of that diagnostic test will advance in that time frame.
OncoCyte Product Pipeline
Lung Cancer Diagnostic Test
Current Standard of Care
The current standard of care for diagnosing lung cancer in high risk patients is LDCT scanning which became part of the current standard of care with the United States Preventive Services Task force (“USPSTF”) guidelines recommending annual LDCTs for patients at high risk for lung cancer. The USPSTF was created in 1984 as an independent, volunteer panel of national experts in prevention and evidence-based medicine. The USPSTF works to improve the health of all Americans by making evidence-based recommendations about clinical preventive services such as screenings, counseling services, and preventive medications.
The guidelines, released in December of 2013, recommend annual LDCT scans for all Americans aged 55 to 80 years old who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years. A 30 pack-year smoking history is defined as the number of cigarette packs smoked per day times the number of years smoked. A 30 pack-year patient would include the following types of patients:
|
· |
Person who has smoked a pack a day (20 cigarettes) for 30 years; |
|
· |
Person who has smoked 15 cigarettes a day for 40 years; and/or |
|
· |
Person who has smoked 40 cigarettes a day for 15 years. |
These guidelines were driven by a need to improve the standard of care for diagnosing lung cancer. Currently, the survival rate for lung cancer is very low – only 17% of people are still alive five years after a lung cancer diagnosis. These low survival rates result in one of the highest mortality rates for lung cancer, which is projected to kill 158,000 Americans in 2015.
Moreover, the survival rate, unlike many other types of cancer, has not increased significantly in the last 30 years. The low probability of surviving lung cancer significantly affected the late diagnosis – with more than half of all patients diagnosed after the point that the cancer has spread. USPSTF guidelines were developed to increase the probability of detecting lung cancer in earlier stages, which can significantly improve the survival rates.
Lung Cancer Survival Rates by Stage
However, the earlier detection of lung cancer will not come without risks. LDCTs are highly sensitive imaging procedures and they result in many false positives. Clinical studies have shown that 26% of LDCTs are indeterminate of which 96% are shown to be false positives. This results in patients being referred for risky downstream procedures including bronchoscopies, needle biopsies and surgery. These invasive procedures have been shown to result in morbidity and mortality including:
|
· |
0.5 to 1% mortality and |
|
· |
4-20% major complications. |
Source: Evaluation of Individuals with Pulmonary Nodules: When is it Lung Cancer? Chest 2013 May:143 (Suppl):e83-e120.
Market for Lung Cancer Diagnostic Tests
Lung cancer is a primary cause of cancer-related death, in part because there is no effective diagnostic test to screen patients for lung cancer at an early stage.
USPSTF guidelines, which recommend LDCT scans for patients at high risk for lung cancer, may impact up to 10 million Americans who fit the criteria of 30 pack-year smokers. Research has shown that 26% of patients will have suspicious LDCT results, and around 96% of those indeterminate results will be false positives which could result in as many as 2 million unnecessary lung biopsy procedures.
We will initially focus on patients with indeterminate diagnoses of larger nodules over 8 millimeters, which is shown as Intended Use 1 in the graph below. This market is estimated to include between 800,000 and 1.1 million patients annually. We intend to expand the use of our lung cancer diagnostic into Intended Use 2 which targets patients with smaller nodules, who currently are put into a wait and hold pattern. These patients are scheduled for repeated LDCTs, risking the increase radiation exposure and incurring incremental costs to determine whether the nodule is growing. Finally, we will work on a diagnostic that could be used as a screening diagnostic and potentially replace LDCTs for the 7-10 million patients who meet the USPSTF guidelines for high risk, which is represented as Intended Use 3 in the following graph.
Market Opportunity for Lung Diagnostics
Clinical Trials
We are collaborating with Wistar to develop the confirmatory lung cancer diagnostic test and in a large, multi-site clinical study evaluating the blood-based lung cancer diagnostic test. This collaboration involves the development of a prototype assay and a clinical study with over 2,000 blood samples obtained at six clinical sites from patients with a high-risk profile for development of lung cancer. Enrollment has continued since 2014 with all sites meeting or exceeding their collection goals. Additionally, we recently started recruiting additional patients through clinical trials that we are sponsoring directly.
Large clinical trials are needed to produce patient subsamples that ensure the development of a highly reliable, accurate diagnostic test. In the case of the lung cancer trials, samples are being collected from patients who are at risk for lung cancer, based on having positive or suspicious results from LDCT screening, and who have undergone biopsies to determine the pathology results. Additionally, we will be collecting samples from patients who used alternative screening procedures such as chest x-rays and who were referred for biopsies.
Wistar investigators are currently assessing gene expression patterns in blood cells of patients with malignant lung disease and patients with non-malignant lung disease. Preliminary analysis of patient data from this study was completed during the first quarter of 2015 and preliminary findings from the research showed a sensitivity of 76% and a specificity of 88%. Sensitivity refers to the probability of detecting the presence of the disease accurately; while specificity refers to the probability of accurately predicting not having the disease.
The lung confirmatory diagnostic is currently in the assay development stage. We anticipate that the assay will move into the R&D validation study stage in early 2016 and complete the CLIA validation stage in late 2016 but there can be no assurance that the development of that diagnostic test will advance in that time frame.
Data concerning the OncoCyte/Wistar preliminary lung assay performance with initial biomarkers and classifiers was presented at the American Thoracic Society in May of 2015. The OncoCyte/Wistar preliminary lung assay had a false positive rate of only 12%. In comparison, National Lung Screening Test results reported in the New England Journal of Medicine (August 2011) showed that LDCTs have a very high false positive rate of approximately 96%.
Wistar ATS Presentation
Sensitivity is the probability of detecting the presence of the disease accurately. A sensitivity of 76% means that three out of four cancers were detected. Specificity is the probability of accurately predicting not having the disease. In this case, approximately 9 out of 10 people without the disease were predicted. A false positive rate is a function of specificity and is equal to 1 minus specificity or 12% in this case.
As part of our SRA with Wistar, we have an option to exclusively license any inventions, discoveries or technology developed by Wistar, or by us using Wistar technology, in the course of the collaborative research. We have exercised certain of these exclusive options and are negotiating license agreements with Wistar.
In addition to the trials with Wistar, under an agreement with Cornell University we have obtained blood samples from lung cancer patients and from cancer-free patients for use in determining levels of tumor-associated gene expression in these samples. The results of these analyses supplemented the results of our study with Wistar.
Breast Cancer Diagnostic Tests
Current Standard of Care
The early detection of cancer is associated with improved outcomes for patients. Mammography has been widely used since the 1970s for breast cancer screening in asymptomatic women; in 2013 over 38 million screening mammograms were performed in the US alone. Current US National Cancer Institute (“NCI”) guidelines recommend screening mammograms every one to two years in women 40 years and older, while the American Cancer Society and the National Comprehensive Cancer Network both recommend screening mammography every year starting at age 40. However in November of 2009, USPSTF revised their screening recommendations increasing the age to 50 and length of time between screenings from annual to biennial. This was partially driven by the concerns around false positives. Approximately 10% of women are recalled from screening mammography for further testing and approximately 95% of those women’s test results end up as false positives. Over the course of 10 years of screening, one out of every two women will experience a false positive with 7% to 17% of those women having unnecessary biopsies.
At the same time, mammogram screening in women aged 40 to 74 has been associated with relative reduction in breast cancer mortality of 15% to 20%. However, the NCI estimates that approximately 20% of all breast cancers are not detected by mammography during screening. These false negatives or missed diagnoses, together with the false positives or over diagnoses, indicate a strong unmet need for a breast cancer screening test with superior specificity and sensitivity when compared to standard screening mammography.
Additionally guidelines recommend MRI screening for approximately 6 million women who either have a family history, a BRCA gene mutation or dense breast tissue, since mammograms have been shown to miss cases of cancer in patients that meet these profiles.
Breast Cancer Screening Protocol
OncoCyte is developing a confirmatory diagnostic test that could be used with women who have an indeterminate mammogram result (BI-RADS 3 or 4). In the case of a mammogram BI-RADS 3 score, repeat imaging is recommended, which means that women may have to schedule another mammogram or they may be referred to a more costly MRI procedure. In the case of a mammogram BI-RADS 4 score, women are often referred for a biopsy. Our breast confirmatory diagnostic could be incorporated into breast screening protocols to confirm whether women with BI-RADS 3 or 4 scores need to undergo additional costly imaging or an invasive biopsy.
The early detection of cancer and its precursors is associated with improved outcomes for patients. Mammography has been widely used since the 1970s for breast cancer screening in asymptomatic women; in 2013 over 38 million screening mammograms were performed in the U.S. alone. Current US National Cancer Institute (“NCI”) guidelines recommend screening mammograms every one to two years in women 40 years and older, while the American Cancer Society and the National Comprehensive Cancer Network both recommend screening mammography every year starting at age 40. However in November of 2009, USPSTF revised their screening recommendations and increased the age to 50 years of age and biennial versus annual screening. This was partially driven by the concerns around false positives. Mammogram screening in women aged 40 to 74 has been associated with relative reduction in breast cancer mortality of 15% to 20%. However, the NCI estimates that approximately 20% of all breast cancers are not detected by mammography during annual screening, which indicates a strong unmet need for a breast-cancer screening test with superior specificity and sensitivity when compared to standard screening mammography. In addition, approximately 10% of women are recalled from screening mammography for further testing and approximately 95% of these women’s test results end up as false positives. Over the course of 10 years of screening, one out of every two women will experience a false positive with 7% to 17% of these women having unnecessary biopsies.
Additionally guidelines recommend MRI screening for approximately 6 million women who either have a family history, a BRCA gene mutation or dense breast tissue, since mammograms have been shown to miss cases of cancer in patients that meet these profiles.
Market Opportunity
Each year approximately 350,000 women have mammograms that are suspicious and many of these women are sent on to biopsies. This is the focus of our initial research for our breast cancer confirmatory diagnostic as shown in the following graph. We are planning to expand our research efforts to include the second intended use – women who meet the guidelines for MRIs. There are over 6 million women in the U.S. for whom the guidelines recommend both a mammogram and a MRI yearly.
We plan to expand the use of our diagnostic in the future to meet the needs for a better breast cancer screening diagnostic, which could impact up to 38 million women each year. Research over the last 25 years has shown that large numbers of women are having unnecessary biopsies resulting in estimates of $4 billion a year being spent on false positives (Health Affairs, 34, no.4 (2015):576-583).
Market Opportunity for Breast Cancer Diagnostic Tests
Breast Cancer Diagnostic Clinical Trials
We have tested the performance of our cancer markers in detecting breast cancer in a set of 134 samples from patients with confirmed cases of breast cancer. The outcome of this initial experiment led us to start prospective clinical trials during the first quarter of 2014 with our sponsorship of a 600 patient study at Scottsdale Medical Imaging Laboratories. As of July 2015, we had collected over 900 patient blood samples. Data from this and ongoing studies will be used to support an initial use of the breast cancer diagnostic test by radiologists to aid in determining the malignancy potential of suspicious mammography findings, and by oncologists as a tool for recurrence surveillance in breast-cancer survivors. We anticipate that we will complete enrollment by the third quarter of 2016. As part of the initial collaboration, we have retained all rights to develop and market our proprietary breast cancer diagnostic tests.
Bladder Cancer Diagnostic Tests
Current Standard of Care
The current standard of care for bladder cancer diagnosis is cytology and cystoscopies. Urine cytology is a test to look for abnormal cells in a patient’s urine. Urine cytology is used along with other tests and procedures to diagnose urinary tract cancers. Cystoscopy is a procedure that allows a doctor to examine the lining of the bladder and the urethra, tube that carries urine out of the body. A hollow tube called a cystoscope, equipped with a lens, is inserted into the patient’s urethra and slowly advanced into the bladder. Increasingly over the years, cystoscopies have been used in conjunction with cytology which has resulted in increasing costs for the detection of bladder cancer.
Current Bladder Diagnostic Protocol
Market Opportunity
Bladder cancer has the highest lifetime treatment costs per patient of all cancers. The high recurrence rate and ongoing invasive monitoring requirement are the key contributors to the economic and human toll of this disease.
Urothelial carcinoma constitutes more than 90% of bladder cancers in the Americas, Europe and Asia. Although most patients with bladder cancer can be treated with organ-sparing chemotherapy, UC has a relapse rate of nearly 70% and can progress to invasive, metastatic, and lethal disease. The regular surveillance and treatment of recurrent disease from the time of diagnosis for the remainder of a patient’s life makes urothelial Carcinoma the most costly malignancy on a per patient basis. The problem is amplified because the two standard methods for surveillance - microscopic assessment of urinary cytology specimens and bladder cystoscopy – which possess significant limitations with respect to both performance and cost. Although urine cytology does have a very high positive predictive value and low false positive rate, it has a low negative predictive value and a high indeterminate rate. Patients who have indeterminate urine cytology results commonly undergo cystoscopy, which is painful, time consuming, costly, and unnecessary in many cases since a neoplasm is often not present. In urothelial carcinoma, as in virtually all other cancers, earlier and more accurate diagnosis, including diagnosis of disease recurrence, is generally associated with better outcomes and lower cost.
Overall markets for bladder cancer diagnostics are large and growing. Based on National Cancer Institute statistics released in 2012, it was estimated that in 2013 over 72,000 new cases of bladder cancer would occur in the United States and a total of over 550,000 men and women alive would have a history of bladder cancer and be subject to recurrence surveillance testing using cystoscopy or urine cytology. Additionally, another 3 million patients present yearly with hematuria (blood in urine), an early symptom of bladder cancer and 500,000 patients have indeterminate cytology findings. These three patient profiles: indeterminate cytology, hematuria and surveillance, could result in a potential market opportunity of approximately 4.5 million tests yearly.
Sending urine specimens to us for analysis using our diagnostic tests instead of performing a cystoscopy procedure would be a significant departure from the current standard of care in the diagnosis of bladder cancer. Urologists may be reluctant or unwilling to change their practices and utilize our diagnostic test for bladder cancer even if our test is proven to have a high rate of accuracy in detecting the presence or absence of cancer.
The potential resistance of urologists to adopt the use of our bladder cancer diagnostic test, means that marketing that test could require a substantial effort by a sales force meeting with urologists. Due to this concern and our limited financial and marketing resources, we may seek to enter into an agreement with a larger company that has greater marketing resources for the marketing of our bladder cancer test. We may license out both completion of development and marketing to another company, retaining rights to receive a royalty on sales and possibly some sales related milestone payments, or we may complete the development of the test and seek to co-market the test with another company in an arrangement that might provide for a sharing of marketing costs and revenues. There is no assurance that we will be successful in entering into a licensing or co-marketing arrangement or that a licensee or co-marketing partner will succeed in marketing our bladder cancer diagnostic test. If we enter into a license or co-marketing agreement, our revenues from the sale of our bladder cancer diagnostic test may be substantially less than the amount of revenues and gross profits that we might receive if we were to market that diagnostic test ourselves.
Bladder Cancer Diagnostic Test Clinical Trials
As part of our clinical development of a urine-based bladder cancer diagnostic test, we initiated a clinical trial in January 2014 that has been expanded to a multi-site trial. The trial will involve up to 1,200 patient samples obtained from at least nine large urology clinics located throughout the United States. Patient enrollment as of December 10, 2015 is approximately 92% complete with almost 1,100 samples in house. The clinical trial is designed to expand the potential use of our bladder cancer test beyond pathology laboratories and into urologic practices at the point of cystoscopy. The goal of the current clinical trial is to compare the performance of our bladder cancer markers to the performance of cystoscopy. Investigators in the trial are collecting urine samples from patients undergoing cystoscopy for the diagnosis of either primary or recurrent bladder cancer. Cystoscopy and biopsy results will be compared with the results of our proprietary diagnostic test panel in determining the overall performance of our classifier and markers.
The clinical trial should be completed by the end of 2015 and we have locked down the assay and are anticipating the completion of the R&D validation study stage and the CLIA validation by midyear 2016.
In May of 2015, we presented preliminary findings of our bladder research at the American Association of Cancer Research. Preliminary findings showed a sensitivity of 90% and a specificity of 83%. Sensitivity is the probability of detecting the presence of the disease accurately. A sensitivity of 90% means that 9 out of 10 cancers were detected. Specificity is the probability of accurately predicting not having the disease. We expect that the inclusion of additional markers will improve the diagnostics sensitivity and specificity further.
Future Diagnostic Development Milestones
Over the next two years, our goal is to achieve the following milestones relating to the development and commercialization of our cancer diagnostic tests:
|
· |
Licensing Wistar Inventions; |
|
· |
Out-licensing or co-marketing partnership for our bladder cancer confirmatory and recurrence diagnostic; |
|
· |
Completion of a prospective patient study for analytical validation of lung and breast cancer diagnostics; |
|
· |
Analytical validation of the lung and breast confirmatory diagnostics – analytical validation is a series of experiments with the same samples to ensure that the test results can be replicated under different circumstances; |
|
· |
Establish CLIA laboratory and obtain certificate of registration, certificate of compliance and inspection for all 50 states; |
|
· |
Launch of a confirmatory diagnostic test for lung cancer; |
|
· |
Proof of concept for a screening diagnostic test for lung cancer; and |
|
· |
Proof of concept for a screening diagnostic and/or confirmatory diagnostic test for breast cancer. |
Achieving the milestones will require expanding our research team to include diagnostic development personnel as well as sufficient clinical trial samples to conduct all the validation tests required.
Technology for Diagnostic Tests
In our liquid biopsy tests for lung, breast and bladder cancer, we are using the same general strategy for the identification of mRNA and miRNA biomarkers and are developing a gene expression classifier to interpret the differential gene expression or miRNA expression and yield a malignant versus benign determination. Ultimately our research may rely on only one type of biomarker in a specific indication. In the case of lung cancer, our test will be developed on mRNA biomarkers only. In the case of breast cancer, we are studying the use of both RNA markers and monoclonal antibodies directed to proteins, potentially including COLX, as a blood-based test assaying concentrations of the proteins and/or RNA markers.
Blood samples are collected by venipuncture into tubes and total RNA is isolated. mRNA biomarkers were identified using microarray equipment. The best performing mRNA biomarkers will be transferred to the commercial platform we will use in our CLIA laboratory. Differentially expressed miRNAs will be identified by screening the human V3 miRNA panel or alternative RNA detection methods. The optimal combination and final panel of mRNA and miRNA biomarkers together with potential protein-based assays will be determined using bioinformatics and machine learning strategies. The optimal classifier will be developed that yields the best discrimination between malignant and benign. The performance of the final biomarker panel and classifier will be tested on an independent set of samples to determine performance characteristics.
For bladder cancer, we are developing a urine test for use in recurrence screening and hematuria. The bladder cancer diagnostic is based on differential mRNA expression in urine samples. mRNA biomarkers were identified using microarray and top biomarkers were transferred to the commercial platform. A streamlined assay was developed that uses crude urine sediment lysates rather than purified RNA, eliminating the need for RNA isolation and amplification. The optimal classifier will be developed that yields the best discrimination between malignant and benign. The performance of the final biomarker panel and classifier will be tested on an independent set of samples to determine performance characteristics.
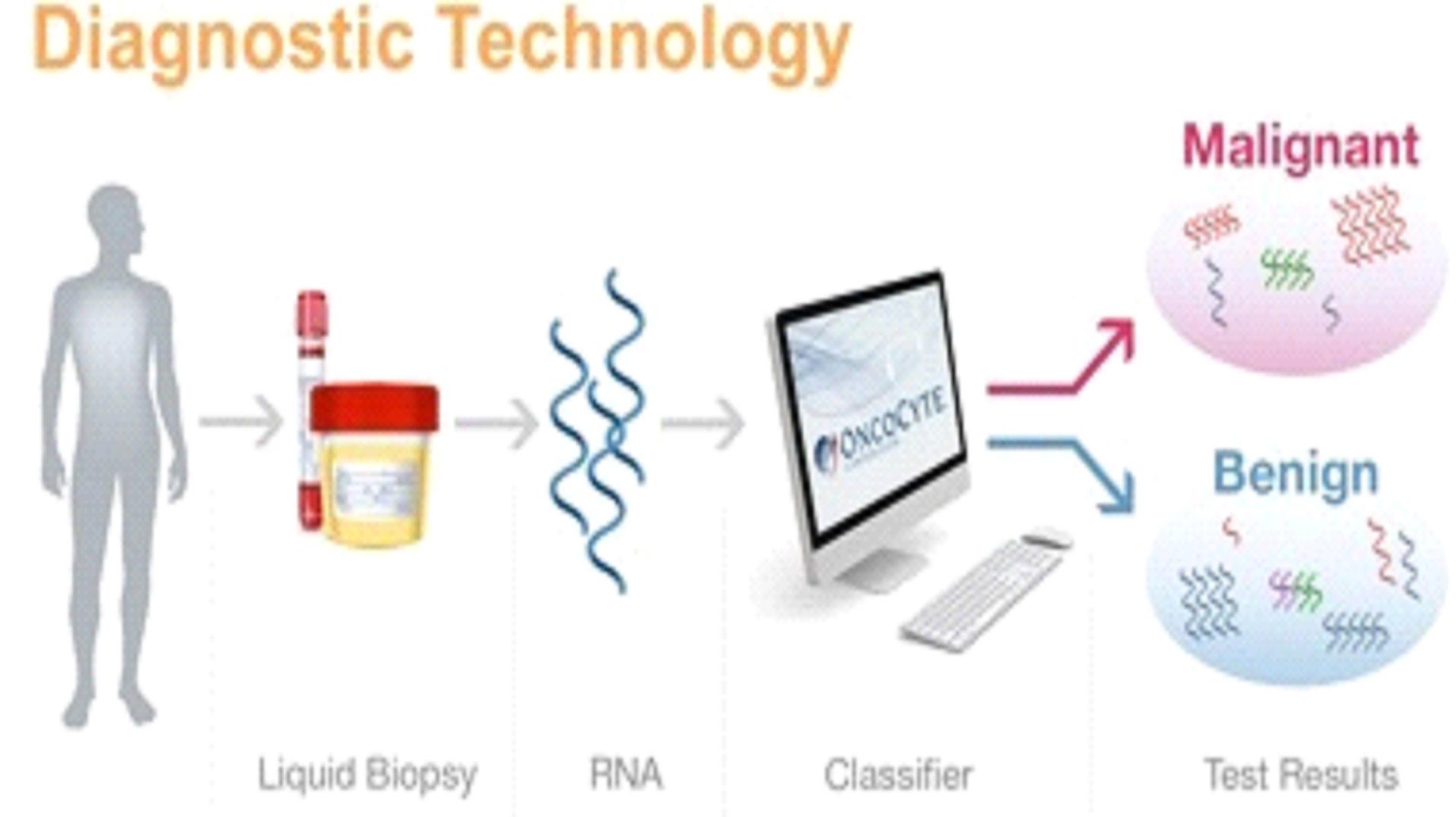
Biomarkers are important to the diagnosis of cancer in that their presence or absence in a specific patient sample drives the sensitivity and specificity scores of a molecular diagnostic. For example if a specific mRNA is only seen expressed in patients with cancer, it can be used to help make a malignant call on that sample. The use of biomarkers with a classifier can help ensure that the sensitivity score, which is a measure of correcting identifying the disease is sufficiently high to reduce false positives, ensuring that patients with the disease are correctly diagnosed. At the same time, biomarkers can be used to hone the specificity measure, which is a measure of correctly identifying patients without the disease, which reduces the number of patients who are unnecessary referred to biopsy.
Sponsored Research Agreement with The Wistar Institute of Anatomy and Biology
We have entered into a SRA, with Wistar pursuant to which Wistar investigators are conducting a multi-center patient study in which they are assessing gene expression patterns in blood cells of patients with malignant versus non-malignant lung disease. We have agreed to provide funding for the research that Wistar is conducting for us under the SRA. Under the SRA, we have an option to obtain a worldwide, exclusive, royalty bearing license, with the right to sublicense, to use the data, methods, techniques, processes, and Wistar Inventions related to molecular diagnostics for lung cancer developed or discovered by the principal investigator or anyone working under her direction in the performance of the sponsored research.
In addition to the funding we provided to Wistar through September 30, 2015 for research under the SRA, we have agreed to provide an additional $751,310 of funding for additional research. We will also pay certain costs related to the filing, prosecution, and maintenance of patents related to Wistar Inventions for which we have exercised our option to negotiate a license. The amount of those payments is not presently determinable. If the negotiating period for obtaining a license from Wistar expires without the execution of a license agreement, our obligation to pay those patent costs will terminate.
We have agreed to indemnify Wistar, certain related persons, and the principal investigator against liabilities, damages, losses and expenses due to claims by any third party which result or arise out of the SRA, the options to license Wistar Inventions, or any licenses to Wistar Inventions.
The SRA will terminate on July 16, 2016 or upon the earlier completion of the sponsored research. The SRA may be terminated at an earlier date (a) by a party if the other party breaches the terms of the SRA and fails to timely cure the breach, (b) by Wistar in the event of certain insolvency or bankruptcy proceedings or similar events pertaining to us, or (c) by either party for any reason, subject to a specified period of prior written notice. Wistar will be entitled to retain payments made prior to the early termination of the SRA to the extent of its reasonable costs of work performed prior to the termination, plus the costs of noncancellable commitments incurred by Wistar prior to the notice of termination.
We have exercised our options to license certain Wistar Inventions and we and Wistar are negotiating the terms of definitive license agreements. However, there is no assurance that we will reach agreement with Wistar on the terms of any definitive license agreements, or that the terms of any license agreements that we may enter into will be favorable to us from a commercial point of view. The SRA provides for a specified negotiating period, after which our option to license Wistar Inventions will expire. If we fail to reach agreement with Wistar for licenses to use the existing Wistar Inventions or for any additional Wistar Inventions that may arise from the research and development work they are performing for us, we may not be able to complete the development of our lung cancer diagnostic test or we may need to commence, at our expense, new research and development work without the right to use the Wistar Inventions, or, if feasible, license technology from a third party.
Manufacturing
Facilities Required
Under a Shared Facilities Agreement with BioTime, we have use of laboratory and office space at BioTime’s facility in Alameda, California. BioTime is planning to relocate its headquarters and as part of that relocation, OncoCyte expects to have access to a space sufficient for its CLIA laboratory.
Raw Materials
The processing of our diagnostics will use commercially available reagents that are sourced by a well-known manufacturer of molecular diagnostic analyzers, prep stations and reagents that has been in business for over 10 years. Although we do not believe that we will encounter sourcing issues for these supplies, if an interruption in supply were to occur, we might need to find a different source of supply of both the reagents and the analytic equipment that we will be using in our CLIA laboratory. An interruption in supply of reagents could cause us to suspend or limit laboratory operations, and a change in analytic equipment could require us to re-establish various testing procedures, which also could disrupt our operations.
Marketing
Following CLIA certification for our laboratory and diagnostic tests, we will market our diagnostic tests directly to health care providers working in the areas of lung cancer and in other areas of cancer where we will be developing molecular diagnostics. These health care providers will collect blood samples or send patients to laboratories to have blood or urine samples collected. These blood samples, also referred to as liquid biopsies, will be sent to our CLIA laboratory in California, either by the health care provider or the laboratory, where the sample will be run through an assay and a gene expression classifier to determine a binary result, either benign or suspicious. That result will be presented to the physician ordering the procedure in a standardized report.
We will ramp up these sales and marketing teams over the next year. Over time, we will continue to grow our sales, market access and marketing organizations to increase the awareness and utilization of our diagnostic test and to prepare for additional diagnostic test launches.
Patents and Trade Secrets
We rely primarily on patents and contractual obligations with employees and third parties to protect our proprietary rights. We have sought, and intend to continue to seek, appropriate patent protection for important and strategic components of our proprietary technologies by filing patent applications in the U.S. and certain foreign countries. There can be no assurance that any of our patents will guarantee protection or market exclusivity for our diagnostic tests and diagnostic test candidates. We may also use license agreements both to access technologies developed by other companies and universities and to convey certain intellectual property rights to others. Our financial success will be dependent in part on our ability to obtain commercially valuable patent claims and to protect our intellectual property rights and to operate without infringing upon the proprietary rights of others. As of August 14, 2015, we have 13 U.S. patents pending and 38 international patents issued or pending to protect our proprietary technologies.
Patents and Expiration Dates
The patents we hold and those for which we have an exclusive option to license from Wistar have been issued in certain key countries and will expire at various times.
OncoCyte Diagnostic Patent Portfolio
|
Patent
family
|
Indication
|
Jurisdictions filed at
(current status pending
unless indicated otherwise)
|
Expiry date
|
Claims
(PCT or US pending)
|
Types of claims
supported by
specification
|
|
ONCO-040
|
Breast
|
United States
|
May 6, 2033
|
Composition of matter; use or process
|
Composition of matter; use or process
|
|
ONCO-052
|
Breast
|
United States
Australia
Canada
China
EPO
Hong Kong
Japan
Korea
|
Aug 16, 2032
|
Use or process
|
Composition of matter; use or process
|
|
ONCO-05O
|
Bladder
|
United States
Australia – ISSUED
Australia divisional
Canada
EPO
Hong Kong
|
Jun 22, 2032
|
Composition of matter; use or process
Australia issued claims – use or process
|
Composition of matter; use or process
|
|
ONCO-056
|
Bladder
|
United States
Australia
Canada
China
EPO
Hong Kong
Japan
Korea
|
Nov 15, 2032
|
Composition of matter; use or process
|
Composition of matter; use or process
|
|
ONCO-060
|
Bladder
|
PCT application filed July 22, 2014 – national stage applications TBD
|
July 22, 2034
|
Composition of matter; use or process
|
Composition of matter; use or process
|
|
ONCO-065
|
Bladder
|
United States - Provisional
|
Jan 28, 2036
|
Composition of matter; use or process
|
Composition of matter; use or process
|
|
WST 1 (1)
|
Lung
|
United States – ISSUED
United States Continuation
Canada
EPO – national patents ISSUED in France, Germany, Spain, United Kingdom
India
|
Dec 5, 2028
|
US issued: Composition of matter
US, Canada, India pending: Composition of matter; use or process
EPO: Composition of matter; use or process
|
Composition of matter; use or process
|
|
WST 2 (1)
|
Lung
|
United States –Provisional
|
May 19, 2036
|
Composition of matter; use or process
|
Composition of matter; use or process
|
|
ONCO-053
|
Pancreas
|
United States
|
Jan 14, 2033
|
Composition of matter; use or process
|
Composition of matter; use or process
|
|
ONCO-054
|
Thyroid
|
United States
|
Jan 11, 2033
|
Composition of matter; use or process
|
Composition of matter; use or process
|
|
ONCO-057
|
Ovarian
|
United States
|
Oct 3, 2032
|
Composition of matter; use or process
|
Composition of matter; use or process
|
|
ONCO-058
|
Colon
|
United States
Australia
Canada
China
EPO
Hong Kong
Japan
Korea
|
Aug 31, 2032
|
Composition of matter; use or process
|
Composition of matter; use or process
|
|
ONCO-051
|
Pan
|
United States
Australia
Canada
EPO
Hong Kong
|
Jun 22, 2032
|
Use or process
|
Composition of matter; use or process
|
|
ONCO-059
|
Pan
|
United States
Australia
Canada
China
EPO
Hong Kong
Japan
Korea
|
Aug 31, 2032
|
Composition of matter; use or process
|
Composition of matter; use or process
|
|
ONCO-064
|
Pan
|
PCT application filed July 23, 2014 – national stage applications TBD
|
July 23, 2034
|
Composition of matter; use or process
|
Composition of matter; use or process
|
(1) Owned by Wistar
All patents and patent applications listed in the above table, with the exception of Wistar patents and patent applications (WST 1 and WST 2), are owned by OncoCyte. OncoCyte has an exclusive option to license the Wistar patents and the terms of a license agreement are currently being negotiated. All patents and patent applications provide support for both composition of matter and process or use claims. In the instances the currently pending claims are directed to either a method or composition of matter, the election is due to an issued restriction requirement and we intend to pursue the non-elected subject matter in a continuation application or applications. All patent expiration dates are provided. The U.S. dates are exclusive of any potential Patent Term Adjustment, which is not applicable in the other jurisdictions.
General Risks Related to Obtaining and Enforcing Patent Protection
Our patents and patent applications are directed to compositions of matter, formulations, methods of use and/or methods of manufacturing. The patent positions of pharmaceutical and biotechnology companies, including ours, are generally uncertain and involve complex legal and factual questions. Our business could be negatively impacted by any of the following:
|
· |
The claims of any patents that are issued may not provide meaningful protection, may not provide a basis for commercially viable diagnostic tests or may not provide us with any competitive advantages; |
|
· |
Our patents may be challenged by third parties; |
|
· |
Others may have patents that relate to our technology or business that may prevent us from marketing our diagnostic test candidates unless we are able to obtain a license to those patents; |
|
· |
Patent applications to which we have rights may not result in issued patents; and/or |
|
· |
We may not be successful in developing additional proprietary technologies that are patentable. |
In addition, others may independently develop similar or alternative technologies, duplicate any of our technologies and, if patents are licensed or issued to us, design around the patented technologies licensed to or developed by us. Moreover, we could incur substantial costs in litigation if we have to defend ourselves in patent lawsuits brought by third parties or if we initiate such lawsuits
The United States Supreme Court’s decisions in Mayo Collaborative Services v. Prometheus Laboratories, Inc. and Association for Molecular Pathology v. Myriad Genetics may limit our ability to obtain patent protection on diagnostic methods that merely recite a correlation between a naturally occurring event and a diagnostic outcome associated with that event. Our cancer diagnostic tests are based on the presence of certain genetic markers for a variety of cancers. In Mayo Collaborative Services v. Prometheus Laboratories, Inc., the Supreme Court ruled that patent protection is not available for the use of a mathematical correlation of the presence of a well-known naturally occurring metabolite as a means of determining proper drug dosage. The claims in the contested patents that were the subject of that decision were directed to measuring the serum level of a drug metabolite and adjusting the dosing regimen of the drug based on the metabolite level. The Supreme Court said that a patent claim that merely claimed a correlation between the blood levels of a drug metabolite and the best dosage of the drug was not patentable subject matter because it did no more than recite a correlation that occurs in nature.
In Association for Molecular Pathology v. Myriad Genetics, the Supreme Court ruled that the discovery of the precise location and sequence of certain genes, mutations of which can dramatically increase the risk of breast and ovarian cancer, was not patentable. Knowledge of the gene location and sequences was used to determine the genes’ typical nucleotide sequence, which, in turn, enabled the development of medical tests useful for detecting mutations in these genes in a particular patient to assess the patient’s cancer risk. But the mere discovery of an important and useful gene did not render the genes patentable as a new composition of matter.
The USPTO has issued interim guidelines in light of the Supreme Court decisions indicating that process claims having a natural principle as a limiting step will be evaluated to determine if the claim includes additional steps that practically apply the natural principle such that the claim amounts to significantly more than the natural principle itself. Because the diagnostic tests that we are developing combine an innovative methodology with newly discovered compositions of matter, we are hopeful that this Supreme Court decision will not preclude the availability of patent protection for our diagnostic tests.
There is a risk that any patent applications that we file and any patents that we hold or later obtain could be challenged by third parties and declared invalid or infringing of third party claims. A patent interference proceeding may be instituted with the USPTO when more than one person files a patent application covering the same technology, or if someone wishes to challenge the validity of an issued patent filed before March 16, 2013. At the completion of the interference proceeding, the USPTO will determine which competing applicant is entitled to the patent, or whether an issued patent is valid. Patent interference proceedings are complex, highly contested legal proceedings, and the USPTO’s decision is subject to appeal. This means that if an interference proceeding arises with respect to any of our patent applications, we may experience significant expenses and delay in obtaining a patent, and if the outcome of the proceeding is unfavorable to us, the patent could be issued to a competitor rather than to us. In addition to interference proceedings, the USPTO can reexamine issued patents at the request of a third party seeking to have the patent invalidated. After March 16, 2013 an inter partes review proceeding will allow third parties to challenge the validity of an issued patent where there is a reasonable likelihood of invalidity. This means that patents owned or licensed by us may be subject to re-examination and may be lost if the outcome of the re-examination is unfavorable to us.
Post Grant Review under the America Invents Act makes available opposition-like proceedings in the United States. As with the USPTO interference proceedings, Post Grant Review proceedings will be very expensive to contest and can result in significant delays in obtaining patent protection or can result in a denial of a patent application. Also, a derivation proceeding may be instituted by the USPTO or an inventor alleging that a patent or application was derived from the work of another inventor.
Oppositions to the issuance of patents may be filed under European patent law and the patent laws of certain other countries. As with the USPTO interference proceedings, these foreign proceedings can be very expensive to contest and can result in significant delays in obtaining a patent or can result in a denial of a patent application.
The enforcement of patent rights often requires litigation against third party infringers, and such litigation can be costly to pursue. Even if we succeed in having new patents issued or in defending any challenge to issued patents, there is no assurance that our patents will be comprehensive enough to provide us with meaningful patent protection against our competitors.
In addition to patents, we rely on trade secrets, know-how, and continuing technological advancement to maintain our competitive position. We have entered into intellectual property, invention, and non-disclosure agreements with our employees, and it is our practice to enter into confidentiality agreements with our consultants. There can be no assurance, however, that these measures will prevent the unauthorized disclosure or use of our trade secrets and know-how, or that others may not independently develop similar trade secrets and know-how or obtain access to our trade secrets, know-how, or proprietary technology.
Competition
We face substantial competition in our business, and that competition is likely to intensify further as new diagnostic tests and technologies reach the market. Superior new diagnostic tests are likely to sell for higher prices and generate higher profit margins once acceptance by the medical community is achieved. Those companies that are successful in introducing new diagnostic tests and technologies to the market first may gain significant economic advantages over their competitors in the establishment of a customer base and track record for the performance of their diagnostic tests and technologies. Such companies will also benefit from revenues from sales that could be used to strengthen their research and development, production, and marketing resources. All companies engaged in the medical products industry face the risk of obsolescence of their products and technologies as more advanced or cost effective products and technologies are developed by their competitors. As the industry matures, companies will compete based upon the performance and cost effectiveness of their products.
The cancer diagnostic test industry is characterized by rapidly evolving technology and intense competition. Our competitors include major multinational diagnostic companies and specialty biotechnology companies. Many of these companies are well-established and possess technical, research and development, financial, and sales and marketing resources significantly greater than ours. In addition, smaller biotech companies may form strategic collaborations, partnerships, and other types of joint ventures with larger, well established industry competitors that afford these companies’ potential research and development and commercialization advantages. Academic institutions, governmental agencies, and other public and private research organizations are also conducting and financing research activities which may produce diagnostic tests directly competitive to those we are developing.
Molecular diagnostic competitors in the category of our first planned diagnostic test launch (lung confirmatory) include two currently marketed diagnostic tests. Xpresys Lung was launched in late 2013 by Integrated Diagnostics and the company has recently announced coverage by major payers. The other currently marketed diagnostic test is Early CDT lung, which was launched in 2012 by a European diagnostics company OncImmune Ltd. OncImmune has sold its U.S. assets to a CLIA laboratory, operating under the name OncImmune USA, LLC. Additionally, we anticipate competition from Exact Sciences Corp, Gensignia Life Sciences, Inc. and Veracyte, Inc. which have diagnostic tests in the pipeline. Gensignia announced the certification of its CLIA lab in October of this year.
In addition to molecular diagnostics, an imaging competitor has a diagnostic test that may compete directly against our confirmatory lung diagnostic test. VisionGate, Inc. has a sputum (spit) test that is read by their proprietary Cell-CT ® system, which takes an optical CT scan of individual cells to generate 3D images of each cell.
Third-Party Payer Reimbursement
Billing, Coverage, and Reimbursement for Diagnostic tests
Revenues from our clinical laboratory testing will be derived from several different sources. Depending on the billing arrangement, the instruction of the ordering physician and applicable law, parties that may reimburse us for our services include:
|
· |
Third-party payers that provide coverage to the patient, such as an insurance company, a managed care organization or a governmental payer program; |
|
· |
Physicians or other authorized parties, such as hospitals or independent laboratories, that order the testing service or otherwise refer the testing services to us; or |
|
· |
Patients in cases where the patient has no insurance, has insurance that partially covers the testing, or owes a co-payment, co-insurance or deductible amount. |
Medicare
We expect that a substantial portion of the patients for whom we may perform diagnostic tests will have Medicare as their primary medical insurance. We cannot assure that, without Medicare reimbursement our planned tests will produce sufficient revenues to enable us to reach profitability and achieve our other commercial objectives.
Clinical diagnostic laboratory tests are generally reimbursed under the Medicare Clinical Laboratory Fee Schedule. Reimbursement under the Medicare program for the diagnostic tests that we will offer is based on the Medicare Clinical Laboratory Fee Schedule, which is subject to geographic adjustments and is updated annually.
Medicare payment amounts are established for each Current Procedural Terminology (“CPT”) code. CPT codes are the main data code set used by physicians, hospitals, laboratories and other health care professionals to report separately- payable clinical laboratory services for reimbursement purposes. The CPT coding system is maintained and updated on an annual basis by the American Medical Association (“AMA”). Each year, new laboratory test codes are added to the fee schedules and corresponding fees are developed in response to a public comment process. We will request a unique CPT code from the AMA for our diagnostic test. Any updates and changes in CPT coding and reimbursement methods could impact our revenues. The introduction of new codes by Centers for Medicare and Medicaid Services (“CMS”) in combination with other actions with regard to pricing could result in lower reimbursements to us than those we may anticipate, or could result in a reduction in the payments that we may receive, for our tests and could make it more difficult to obtain coverage from Medicare or other payers. There can be no guarantees that Medicare and other payers will establish positive or adequate coverage policies or reimbursement rates.
In addition, under the Clinical Laboratory Fee Schedule, Medicare also sets a cap on the amount that it will pay for any individual test. This cap, usually referred to as the National Limitation Amount, is set at a percentage of the median of all the contractor fee schedule amounts for each billing code.
Medicare has coverage policies that can be national or regional in scope. Coverage means that the test is approved as a benefit for Medicare beneficiaries. If there is no coverage, neither we nor any other party, such as a reference laboratory, may receive reimbursement from Medicare for the service.
Legislative and Regulatory Changes Impacting Medicare Reimbursements
From time to time, Congress has revised the Medicare statute and the formulas it establishes for both the Medicare Clinical Laboratory Fee Schedule. The payment amounts under the Medicare fee schedules are important because they not only will determine our reimbursement under Medicare, but those payment amounts are also often used as a basis for payment amounts set by other governmental and private third-party payers. For example, state Medicaid programs are prohibited from paying more than the Medicare fee schedule limit for clinical laboratory services furnished to Medicaid recipients.
Under the statutory formula for Medicare Clinical Laboratory Fee Schedule amounts, increases are made annually based on the Consumer Price Index for All Urban Consumers as of June 30 for the previous twelve-month period. The Affordable Care Act (“ACA”) has, among other things, imposed cuts to the Medicare reimbursement for clinical laboratories. The ACA replaced the 0.5% cut enacted by the Medicare Improvements for Patients and Providers Act with a “productivity adjustment” that will reduce the Consumer Price Index update in payments for clinical laboratory tests. The ACA includes a 1.75% reduction in the CPI update for clinical laboratories for the years 2011 through 2015. The Middle Class Tax Relief and Job Creation Act of 2012 (“MCTRJCA”), enacted in 2012, mandated an additional change in reimbursement for clinical laboratory service programs. This legislation required CMS to reduce the Medicare Clinical Laboratory Fee Schedule by 2% in 2013, which in turn has served as a base for subsequent years. As a consequence of the changes required by ACA and MCTRJCA, payment for clinical laboratory services has gone down in recent years.
Under the Protecting Access to Medicare Act of 2014 (“PAMA”), which was signed to law in April 2014, there will be major changes to the payment formula under the Medicare Clinical Laboratory Fee Schedule. Beginning January 1, 2016, each clinical laboratory must report laboratory test payment data for each Medicare-covered clinical diagnostic laboratory test that it furnishes during a time period to be defined by future regulations. The reported data must include the payment rate reflecting all discounts, rebates, coupons and other price concessions and the volume of each test that was paid by each private payer, such as health insurance issuers, group health plans, Medicare Advantage plans and Medicaid managed care organizations.
PAMA has the potential to significantly impact the way that laboratory tests are reimbursed by Medicare. Reimbursement rates for advanced diagnostic tests will initially be based on list prices or charges and then will be pegged to the average price paid by commercial third party payers such as health insurance companies. Diagnostics in this category must meet one of the following criteria:
|
· |
Analysis of multiple biomarkers of DNA, RNA or proteins combined with a unique algorithm to yield a single patient-specific result; |
|
· |
Cleared or approved by the FDA; or |
|
· |
Meets other similar criteria established by the Secretary of Health and Human Services. |
Beginning January 1, 2017 Medicare payment for any new advanced diagnostic test will be based on the list price/charge. After the test is commercially available for two quarters, the laboratory will be required to report payment and volume information and this data will be used to set payment for the test for the following year.
|
· |
If data shows that the list price was greater than 130% of the payment using established methodology, generally a weighted median, CMS will recoup the difference from the laboratory through a payment claw back. |
|
· |
Payment will be updated annually based on the weighted median of commercial payer reimbursement. |
Congress has proposed on several occasions to impose a 20% coinsurance charge on patients for clinical laboratory tests reimbursed under the Medicare Clinical Laboratory Fee Schedule, which would require us to bill patients for these amounts. Because of the relatively low reimbursement for many clinical laboratory tests, in the event that Congress were to ever enact such legislation, the cost of billing and collecting for these services would often exceed the amount actually received from the patient and effectively increase our costs of billing and collecting.
On September 25, 2015, CMS released preliminary determinations for the calendar year 2016 for the Medicare Clinical Laboratory Fee Schedule for some test codes, including some for oncology diagnostics, as had been anticipated. These preliminary determinations were based on a cross walk approach rather than a gap-fill approach. A cross walk approach matches a new code for a diagnostic against existing codes to determine the appropriate payment rate; while a gap-fill approach looks at local pricing patterns, including charges for the tests and any discounts on charges and payments determined by other payers. At this point it is not clear what methodology CMS may use in their determinations for future diagnostics.
Some Medicare claims may be subject to policies issued by the former and current Medicare Administrative Contractor (“MAC”) for California. CMS relies on a network of MACs to process Medicare claims, and MACs serve as the primary operational contact between the Medicare Fee-For-Service program, and approximately 1.5 million health care providers enrolled in the program. Palmetto GBA, acting on behalf of many MACs, issued a Local Coverage Determination that affects coverage, coding and billing of many molecular diagnostic tests. Under this Local Coverage Determination, Palmetto GBA stated that it would not cover any molecular diagnostic tests unless the test is expressly included in a National Coverage Determination issued by CMS or a Local Coverage Determination or coverage article issued by Palmetto GBA. Denial of coverage for our diagnostic tests by Palmetto GBA or its successor MAC, Noridian Healthcare Solutions, or reimbursement at inadequate levels, would have a material adverse impact on our business and on market acceptance our planned diagnostic tests.
Private and Governmental Third Party Payers
Where there is a private or governmental third-party payer coverage policy in place, we will bill the payer and the patient in accordance with the established policy. Where there is no coverage policy in place, we will pursue reimbursement on a case-by-case basis. Our efforts in obtaining reimbursement based on individual claims, including pursuing appeals or reconsiderations of claims denials, could take a substantial amount of time, and bills may not be paid for many months, if at all. Furthermore, if a third-party payer denies coverage after final appeal, payment may not be received at all.
Reimbursement rates paid by private third-party payers can vary based on whether the provider is considered to be an “in-network” provider, a participating provider, a covered provider, an “out-of-network” provider or a non-participating provider. These definitions can vary among payers. An in-network provider usually has a contract with the payor or benefits provider. This contract governs, among other things, service-level agreements and reimbursement rates. In certain instances an insurance company may negotiate an in-network rate for our testing. An in-network provider may have rates that are lower per test than those that are out-of-network, and that rate can vary widely. Rates vary based on the payor, the testing type and often the specifics of the patient’s insurance plan. If a laboratory agrees to contract as an in-network provider, it generally expects to receive quicker payment and access to additional covered patients. However, it is likely that we will initially be considered an “out-of-network” or non-participating provider by payers who cover the vast majority of lives until such time that we can negotiate contracts with these payers.
We cannot predict whether, or under what circumstances, payers will reimburse for all components of our tests. Full or partial denial of coverage by payers, or reimbursement at inadequate levels, would have a material adverse impact on our business and on market acceptance of our tests.
Direct Billing Laws and Other State Law Restrictions on Billing for Laboratory Services
Laws and regulations in certain states prohibit laboratories from billing physicians or other purchasers for testing that they order. Some states may allow laboratories to bill physicians directly but may prohibit the physician and, in some cases, other purchasers from charging more than the purchase price for the services, or may allow only for the recovery of acquisition costs, or may require disclosure of certain information on the invoice. In some cases, and if not prohibited by law or regulation, we may bill physicians, hospitals and other laboratories directly for the services that they order. An increase in the number of states that impose similar restrictions could adversely affect us by encouraging physicians to perform laboratory services in-house or by causing physicians to refer services to other laboratories that are not subject to the same restrictions.
Government Regulation
Clinical Laboratory Improvement Amendments of 1988 and State Regulation
As a provider of laboratory testing on human specimens for the purpose of disease diagnosis, prevention, or treatment, we will be required to hold certain federal, state and local licenses, certifications and permits to conduct our business. In 1988, Congress enacted CLIA, which established quality standards for all laboratories providing testing to ensure the accuracy, reliability and timeliness of patient test results regardless of where the test was performed. Our laboratory will obtain a CLIA certificate of accreditation. We also will be required to meet certain laboratory licensing and other requirements under state laws. Our laboratory will hold the required licenses from the applicable state agencies in the states in which we operate or from which we receive blood or urine samples for testing.
Under CLIA, a laboratory is defined as any facility which performs laboratory testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease, or the impairment of, or assessment of health of human beings. CLIA requires that we hold a certificate applicable to the complexity of the categories of testing we perform and that we comply with certain standards. CLIA further regulates virtually all clinical laboratories by requiring that they comply with various operational, personnel, facilities administration, quality and proficiency testing requirements intended to ensure that their clinical laboratory testing services are accurate, reliable and timely. CLIA certification is also a prerequisite to be eligible to be reimbursed for services provided to state and federal health care program beneficiaries. CLIA is user-fee funded. Therefore, all costs of administering the program must be covered by the regulated facilities, including certification and survey costs.
Under the CLIA, laboratory licensing requires a site inspection, review of standard operating procedures and verification that diagnostic results can be reproduced reliably across a number of different conditions. Before submitting for a license, extensive clinical testing, which is typically done in two phases, must be undertaken at a hospital or medical center to demonstrate optimal use, safety, and efficacy of the test in diagnosing a specific condition. Each clinical study is conducted under the auspices of an IRB that will consider, among other things, ethical factors, the safety of human subjects, and the possible liability of the institution.
Clinical studies are generally conducted in two phases. The first phase is analytical validation which is done in the research laboratory and involves the replication of consistent results for the same sample across a spectrum of different conditions. Once the analytical validation is completed, the assay moves into clinical validation. In clinical validation tests are run to confirm that consistent results for the same sample can be obtained in the actual laboratory that will conduct the commercial tests.
We will be subject to regular surveys and inspections to assess compliance with program standards. Laboratories performing high complexity testing are required to meet more stringent requirements than laboratories performing less complex tests.
CLIA and FDA Regulation of Diagnostic Tests
Our diagnostic tests will likely be classified as LDTs and consequently be governed under the CLIA regulations, as administered by CMS, as well as by applicable state laws. Historically, the FDA has exercised enforcement restraint with respect to most LDTs and has not required laboratories that offer LDTs to comply with FDA requirements for medical devices, such as registration, device listing, quality systems regulations, premarket clearance or premarket approval, and post-market controls. In recent years, however, the FDA has stated it intends to end its policy of enforcement restraint and begin regulating certain LDTs as medical devices. On October 3, 2014, the FDA issued two draft guidance documents, entitled “Framework for Regulatory Oversight of Laboratory Developed Tests (LDTs)” and “FDA Notification and Medical Device Reporting for Laboratory Developed Tests (LDTs)”, respectively, that set forth a proposed risk-based regulatory framework that would apply varying levels of FDA oversight to LDTs. The FDA has indicated that it does not intend to modify its policy of enforcement restraint until the draft guidance documents are finalized. It is unclear at this time when, or if, the draft guidance documents will be finalized, and even then, the new regulatory requirements are proposed to be phased-in consistent with the schedule set forth in the guidance, which may happen in as little as 12 months after the draft guidance is finalized for certain high-priority LDTs. Nevertheless, the FDA may decide to regulate certain LDTs on a case-by-case basis at any time.
Failure to comply with applicable FDA regulatory requirements may trigger a range of enforcement actions by the FDA, including warning letters, civil monetary penalties, injunctions, criminal prosecution, recall or seizure, operating restrictions, partial suspension or total shutdown of operations, and denial of or challenges to applications for clearance or approval, as well as significant adverse publicity. We cannot predict the ultimate form or impact of any such FDA guidance and the potential effect on our diagnostic test services.
We cannot provide any assurance that FDA regulation, including premarket review, will not be required in the future for our diagnostic tests, whether through additional guidance or regulations issued by the FDA, new enforcement policies adopted by the FDA or new legislation enacted by Congress. It is possible that proposed legislation discussed above or other new legislation could be enacted into law, or new regulations or guidance could be issued by the FDA. Such new legislation may result in new or increased regulatory requirements for us to continue to offer our diagnostic tests or to develop and introduce new tests or services.
If premarket review, including approval, is required, our business could be negatively affected until such review is completed and clearance to market or approval is obtained. If we are selling any of our diagnostic tests when new FDA approval requirements are implemented, we may be required to suspend sales until we obtain premarket clearance or approval. If our diagnostic tests are allowed to remain on the market but there is uncertainty about the legal status of those tests, if we are required by the FDA to label them investigational, or if labeling claims the FDA allows us to make are limited, order levels for the use of our tests may decline and reimbursement may be adversely affected.
New regulations could also require, among other things, additional clinical studies and submission of a premarket notification or filing a PMA application with the FDA. For example, LDTs with the same intended use as a cleared or approved companion diagnostic are defined in FDA’s draft guidance as “high-risk LDTs (Class III medical devices)” for which premarket review would be required. This may include the use of our LDTs for screening patients for cancer. See the discussion of FDA regulation of medical devices below under In Vitro Diagnostics (“IVDs”). If premarket review is required by the FDA, there can be no assurance that our diagnostic tests will be cleared or approved on a timely basis, if at all, nor can there any be assurance that labeling claims allowed by the FDA will be consistent with our intended claims or will be adequate to support continued adoption of and reimbursement for our tests. Compliance with FDA regulations would increase the cost of conducting our business, and subject us to heightened requirements of the FDA and penalties for failure to comply with these requirements. We may also decide voluntarily to pursue FDA premarket review of our diagnostic tests if we determine that doing so would be appropriate.
California State Laboratory Licensing
In addition to federal certification requirements of laboratories under CLIA, licensure will be required and maintained under California law for the San Francisco Bay Area based laboratory that we plan to establish. Such laws include standards for the day-to-day operation of a clinical reference laboratory, including the training and skills required of personnel and quality control. In addition, California laws mandate proficiency testing, which involves testing of specimens that have been specifically prepared for the laboratory.
We will not be permitted to perform diagnostic tests at our California CLIA laboratory until it is certified by the state, and if after certification our laboratory falls out of compliance with California standards, the California Department of Health Services (“DHS”) may suspend, restrict or revoke our license to operate our laboratory, assess substantial civil money penalties, or impose specific corrective action plans. Any such actions could materially affect our business.
Other States’ Laboratory Licensing
Some states require licensure of out-of-state laboratories that accept specimens from those states. Our laboratories will need to pass various state inspections in order to get licensed to provide LDTs in each of state that requires licensure. In addition to the inspection requirements of the other states, Pennsylvania, Florida and Maryland have laws that require a certificate of compliance, and New York has its own special inspection requirements that must be met, in order to market our diagnostics in those states or to perform diagnostic tests on specimens received from patients residing in those states.
In Vitro Diagnostics
In the future, we may elect to develop IVDs, which are regulated by the FDA as medical devices. Medical devices marketed in the United States are subject to the regulatory controls under CLIA, the Federal Food, Drug, and Cosmetic Act, and regulations adopted by the FDA. Some requirements, known as premarket requirements, apply to medical devices before they are marketed, and other requirements, known as post-market requirements, apply to medical devices after they are marketed.
The particular premarket requirements that must be met to market a medical device in the United States will depend on the classification of the device under FDA regulations. Medical devices are categorized into one of three classes, based on the degree of risk they present. Devices that pose the lowest risk are designated as Class I devices, devices that pose moderate risk are designated as Class II devices and are subject to general controls and special controls, and the devices that pose the highest risk are designated as Class III devices and are subject to general controls and premarket approval.
A premarket submission to the FDA will be required for some Class I products, most Class II, and all Class III devices. Most Class I and some Class II devices are exempt from premarket submission requirements. Some Class I and most Class II devices may only be marketed after a 510(k) premarket notification, while a more extensive PMA or Premarket Approval is required to market Class III devices.
Unless and until the FDA’s draft guidance documents are finalized and the resulting new regulatory requirements are phased in our initial confirmatory diagnostics will not require FDA filing before launch. Since the tests are being developed as LDTs, the regulatory pathway that we will be following is the CLIA certification and inspection pathway. If the new requirements are phased in or if we elect to develop IVDs, our future screenings diagnostics may require a 510(k) submission or a PMA. In a 510(k) submission, the device sponsor must demonstrate that the new device is “substantially equivalent” to a predicate device in terms of intended use, technological characteristics, and performance testing. A 510(k) requires demonstration of substantial equivalence to another device that is legally marketed in the United States. Substantial equivalence means that the new device is at least as safe and effective as the predicate. A device is substantially equivalent if, in comparison to a predicate it (a) has the same intended use as the predicate; and has the same technological characteristics as the predicate; or (b) has the same intended use as the predicate; and has different technological characteristics and the information submitted to FDA; does not raise new questions of safety and effectiveness; and is demonstrated to be at least as safe and effective as the legally marketed predicate device.
A claim of substantial equivalence does not mean the new and predicate devices must be identical. Substantial equivalence is established with respect to intended use, design, energy used or delivered, materials, chemical composition, manufacturing process, performance, safety, effectiveness, labeling, biocompatibility, standards, and other characteristics. A device may not be marketed in the United States until the submitter receives a letter declaring the device substantially equivalent. If the FDA determines that a device is not substantially equivalent, the applicant may resubmit another 510(k) with new data, or request a Class I or II designation through the FDA’s de novo process which allows a new device without a valid predicate to be classified into Class I or II if it meets certain criteria, or file a reclassification petition, or submit a PMA.
A new 510(k) submission is required for changes or modifications to an existing approved device, where the modifications could significantly affect the safety or effectiveness of the device or the device is to be marketed for a new or different indication for use.
A PMA for Class III devices is the most stringent type of premarket submission. Before the FDA approves a PMA, the sponsor must provide valid scientific evidence demonstrating reasonable assurance of safety and effectiveness for the device’s intended use.
Submission of an application is no guarantee that the CMS or FDA will find it complete and accept it for filing. If an application is accepted for filing or licensing, following the CMS or FDA’s review, the CMS or FDA may grant marketing approval, request additional information or deny the application if it determines that the application does not provide an adequate basis for approval.
Health Insurance Portability and Accountability Act
Under HIPAA, the Department of Health and Human Services (“HHS”) has issued regulations to protect the privacy and security of protected health information used or disclosed by health care providers, such as us. HIPAA also regulates standardization of data content, codes and formats used in health care transactions and standardization of identifiers for health plans and providers. Penalties for violations of HIPAA regulations include civil and criminal penalties.
CMS and the Office of Civil Rights issued a final rule in February 2014 to amend both the HIPAA and CLIA regulations. The final rule amended the HIPAA privacy rule to remove the CLIA laboratory exceptions, and as a result, HIPAA-covered laboratories are now required to provide individuals, upon request, with access to their completed test reports. Under the new rules CLIA laboratories and CLIA-exempt laboratories may provide copies of a patient’s completed test reports that, using the laboratory’s authentication process, can be identified as belonging to that patient. These changes to the CLIA regulations and the HIPAA Privacy Rule provide individuals with a greater ability to access their health information, empowering them to take a more active role in managing their health and health care. CLIA laboratories must create and maintain policies, procedures, and other documentation necessary to inform patients of the right to access laboratory test reports and how to exercise that right.
We intend to fully comply with these regulations. The requirements under these regulations may periodically change and could have an effect on our business operations if compliance becomes substantially more costly than under current requirements.
New laws governing privacy may also be adopted in the future. We will take steps to comply with health information privacy requirements of which we are aware and to with which we must comply. However, we can provide no assurance that we will remain in compliance with diverse privacy requirements in all of the jurisdictions in which we do business. Failure to comply with privacy requirements could result in civil or criminal penalties, which could have a materially adverse effect on our business.
Physician Referral Prohibitions
Under a federal law directed at “self-referral,” commonly known as the Stark Law, there are prohibitions, with certain exceptions, on Medicare and Medicaid payments for laboratory tests referred by physicians who personally, or through a family member, have a “financial relationship”—including an investment or ownership interest or a compensation arrangement—with the clinical laboratory performing the tests. Several Stark Law exceptions are relevant to arrangements involving clinical laboratories, including: (1) fair market value compensation for the provision of items or services; (2) payments by physicians to a laboratory for clinical laboratory services; (3) certain space and equipment rental arrangements that satisfy certain requirements, and (4) personal services arrangements that satisfy certain requirements. The laboratory cannot submit claims to the Medicare Part B program for services furnished in violation of the Stark Law, and Medicaid reimbursements may be at risk as well. Penalties for violating the Stark Law include the return of funds received for all prohibited referrals, fines, civil monetary penalties and possible exclusion from the federal health care programs. Many states have comparable laws that are not limited to Medicare and Medicaid referrals.
Corporate Practice of Medicine
A number of states, including California, do not allow business corporations to employ physicians to provide professional services. This prohibition against the “corporate practice of medicine” is aimed at preventing corporations such as us from exercising control over the medical judgments or decisions of physicians. The state licensure statutes and regulations and agency and court decisions that enumerate the specific corporate practice rules vary considerably from state to state and are enforced by both the courts and regulatory authorities, each with broad discretion. If regulatory authorities or other parties in any jurisdiction successfully assert that we are engaged in the unauthorized corporate practice of medicine, we could be required to restructure our contractual and other arrangements. In addition, violation of these laws may result in sanctions imposed against us and/or the professional through licensure proceedings, and we could be subject to civil and criminal penalties that could result in exclusion from state and federal health care programs.
Federal and State Fraud and Abuse Laws
A variety of federal and state laws prohibit fraud and abuse. These laws are interpreted broadly and enforced aggressively by various state and federal agencies, including CMS, the Department of Justice, the Office of Inspector General for HHS, and various state agencies. In addition, the Medicare and Medicaid programs increasingly use a variety of contractors to review claims data and to identify improper payments as well as fraud and abuse. These contractors include Recovery Audit Contractors, Medicaid Integrity Contractors and Zone Program Integrity Contractors. In addition, CMS conducts Comprehensive Error Rate Testing audits, the purpose of which is to detect improper Medicare payments. Any overpayments identified must be repaid unless a favorable decision is obtained on appeal. In some cases, these overpayments can be used as the basis for an extrapolation, by which the error rate is applied to a larger universe of claims, and which can result in even higher repayments.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting, receiving, or providing remuneration, directly or indirectly, to induce or in return for either the referral of an individual, or the furnishing, recommending, or arranging for the purchase, lease or order of any health care item or service reimbursable, in whole or in part, under a federal health care program. The definition of “remuneration” has been broadly interpreted to include anything of value, including gifts, discounts, credit arrangements, payments of cash, ownership interests and providing anything at less than its fair market value. Recognizing that the Anti- Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements within the health care industry, the Office of Inspector General for HHS has issued a series of regulatory “safe harbors.” These safe harbor regulations set forth certain requirements that, if met, will assure immunity from prosecution under the federal Anti-Kickback Statute. Although full compliance with these provisions ensures against prosecution under the federal Anti-Kickback Statute, the failure of a transaction or arrangement to fit within a specific safe harbor does not necessarily mean that the transaction or arrangement is illegal or that prosecution under the federal Anti-Kickback Statute will be pursued. For further discussion of the impact of federal and state health care fraud and abuse laws and regulations on our business, see the section entitled “Risk Factors.”
HIPAA also created new federal crimes, including health care fraud and false statements relating to health care matters. The health care fraud statute prohibits knowingly and willfully executing a scheme to defraud any health care benefit program, including private third-party payers. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from federal health care programs, such as the Medicare and Medicaid programs. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. A violation of this statute is a felony and may result in fines, imprisonment or exclusion from federal health care programs.
Many states have laws similar to the federal laws described above and the state laws may be broader in scope and may apply regardless of payor.
Other Regulatory Requirements
Our laboratory will be subject to federal, state and local regulations relating to the handling and disposal of regulated medical waste, hazardous waste and biohazardous waste, including chemical, biological agents and compounds, blood samples and other human tissue. Typically, we will use outside vendors who are contractually obligated to comply with applicable laws and regulations to dispose of such waste. These vendors will be licensed or otherwise qualified to handle and dispose of such waste.
The Occupational Safety and Health Administration has established extensive requirements relating to workplace safety for health care employers, including requirements to develop and implement programs to protect workers from exposure to blood-borne pathogens by preventing or minimizing any exposure through needle stick or similar penetrating injuries.
Employees
As of December 10, 2015, we employed seven persons on a full-time basis and one person on a part-time basis. Three full-time employees hold Ph.D.s in one or more fields of science.
Legal Proceedings
From time to time, we may be involved in routine litigation incidental to the conduct of our business. We are not presently a party to any legal proceedings.
MANAGEMENT
Directors
The names and ages of our directors are:
William Annett, 61, joined OncoCyte as Chief Executive Officer in June 2015. Prior to becoming our Chief Executive Officer, Mr. Annett was a Managing Director at Accenture from 2011 to 2014, where he founded, built, and headed Accenture’s West Coast Life Sciences practice with sales, marketing, and delivery responsibilities for the entire territory. His clients included most of the major biotech and pharmaceutical companies in the western United States. At Genentech, from 2003 until 2011, Mr. Annett led the Commercial Strategy group and managed large operational projects with several hundred team members. He also directed the Project Finance function for research and development, which supported all development pipeline products with more than 200 clinical trials. In 2001 Mr. Annett founded and until 2003 served as CEO of Corra Life Sciences, a prenatal diagnostics company, which worked with a consortium of universities to develop blood tests for the major diseases of pregnancy. Mr. Annett also previously served as Chief Executive Officer of BioFX Laboratories, Inc. from 1999 to 2000. Early in his career, Mr. Annett also founded a consumer products company, which he led for six years as Chief Executive Officer. During his tenure, the company became publicly traded on NASDAQ and was then acquired. Mr. Annett holds an MBA from the Harvard Business School.
Andrew Arno, 56, joined our Board of Directors in June 2015 and has 30 years of experience working with emerging growth companies. He is currently Vice Chairman of “The Special Equities Group” at Chardan Capital Markets, LLC, a privately held investment banking firm, and from June 2013 until July 2015 served as Managing Director of Emerging Growth Equities, an investment bank, and Vice President of Sabr, Inc., a family investment group. He was previously President of LOMUSA Limited, an investment banking firm. From 2009 to 2012, Mr. Arno served as Vice Chairman and Chief Marketing Officer of Unterberg Capital, LLC, an investment advisory firm that he co-founded. He was also Vice Chairman and Head of Equity Capital Markets of Merriman Capital LLC, an investment banking firm, and served on the board of the parent company, Merriman Holdings, Inc. Mr. Arno currently serves on the board of Smith Micro Software, Inc. and Asterias Biotherapeutics, Inc.
Mr. Arno brings over 30 years’ experience handling a wide range of corporate and financial matters and his background as an investment banker and strategic advisor to emerging growth companies qualifies him to serve on our Board of Directors.
Alfred D. Kingsley, 72, joined the Board of Directors during September 2009 and became Chairman of the Board during December 2010. Mr. Kingsley is also the Chairman of the Board of Directors of BioTime. Mr. Kingsley has been general partner of Greenway Partners, L.P., a private investment firm, and President of Greenbelt Corp., a business consulting firm, since 1993. Greenbelt Corp. served as BioTime’s financial advisor from 1998 until June 30, 2009. Mr. Kingsley was Senior Vice-President of Icahn and Company and its affiliated entities for more than 25 years. Mr. Kingsley is a director of our subsidiary Asterias Biotherapeutics, Inc. Mr. Kingsley holds a BS degree in economics from the Wharton School of the University of Pennsylvania, and a J.D. degree and LLM in taxation from New York University Law School.
Mr. Kingsley’s long career in corporate finance and mergers and acquisitions includes substantial experience in helping companies to improve their management and corporate governance, and to restructure their operations in order to add value for shareholders. Mr. Kingsley was instrumental in structuring our equity financings, and in the acquisition of the assets of CTI.
Andrew J. Last, 56, joined the Board of Directors during December 2015. Mr. Last has been Executive Vice President and Chief Operating Officer of Affymetrix since 2010. Before joining Affymetrix, Mr. Last served as Vice President, Global and Strategic Marketing of BD Biosciences and as General Manager of Pharmingen from 2004 to 2010. From 2002 to 2004, Mr. Last held management positions at Applied Biosystems, Inc., including as Vice President and General Manager from 2003-2004 and Vice President of Marking 2002-2003. Earlier in his career, he served in a variety of management positions at other companies, including Incyte Genomics and Monsanto. Mr. Last holds Ph. D. and MS degrees with specialization in Agrochemical Chemicals and Bio-Aeronautics, respectively, from Cranfield University, and a BS degree in Biological Sciences from the University of Leicester in the United Kingdom.
Aditya Mohanty, 49, joined the Board of Directors during April 2015. Mr. Mohanty was appointed Co-Chief Executive Officer of BioTime during October 2015, after serving as BioTime‘s Chief Operating Officer since December 2014. Mr. Mohanty previously served in a number of executive positions at Shire plc, including as President/Head Regenerative Medicine from 2013 to 2014, as Senior Vice President, Business and Technical Operations from 2012 to 2013, as Global Franchise Head MPS from 2010 to 2012, and as Vice President of Operations/Product General Manager from 2005 to 2012. Shire plc is a biotechnology company focused on research, development and commercialization of novel biological products for rare diseases. Mr. Mohanty was VP of Manufacturing and Operations at Transkaryotic Therapies, Inc. from 2002 to 2005 when it was acquired by Shire. Before joining Transkaryotic Therapies, Mr. Mohanty held a number of management positions at Baxter Healthcare Corporation, Bioscience Division from 1990 to 2002. Mr. Mohanty received an MBA degree from Saint Mary’s College, an MS in Chemical Engineering from Clarkson University, and a B. Tech in Chemical Engineering from REC Trichy, in India.
Cavan Redmond, 54, joined our Board of Directors in August of 2015. Since 2014, Mr. Redmond has served as Partner for Zarsy, LLC. Mr. Redmond served as Chief Executive Officer of WebMD from May 2012 until May 2013. From August 2011 until May 2012, Mr. Redmond served as Group President, Animal Health, Consumer Healthcare and Corporate Strategy of Pfizer Inc., a pharmaceutical company. He served as Pfizer’s Group President, Animal Health, Consumer Healthcare, Capsugel and Corporate Strategy from December 2010 until August 2011 and as its Senior Vice President and Group President, Pfizer Diversified Businesses from October 2009 until December 2010. Prior to Pfizer’s acquisition of Wyeth, a pharmaceutical company, Mr. Redmond served as President, Wyeth Consumer Healthcare and Animal Health Business from May 2009 until October 2009. Before that, he held the positions of President, Wyeth Consumer Healthcare from December 2007 until May 2009 and Executive Vice President and General Manager, BioPharma, Wyeth Pharmaceuticals from 2003 until December 2007.
Michael D. West, Ph.D., 62, has served on the Board of Directors since September 2009 and served as our Chief Executive Officer from our inception until January 2011. Dr. West was appointed Chief Executive Officer of BioTime during October 2007 and became Co-Chief Executive Officer during October 2015. Dr. West has served on BioTime’s Board of Directors since 2002. Dr. West is also Vice President of Technology Integration and a director of Asterias Biotherapeutics, Inc. Prior to becoming Chief Executive Officer of BioTime in October 2007, Dr. West served as Chief Executive Officer, President, and Chief Scientific Officer of Acata, Inc., a company engaged in developing human stem cell technology for use in regenerative medicine. Dr. West also founded Geron Corporation, and from 1990 to 1998 he was a Director and Vice-President of Geron, where he initiated and managed programs in telomerase diagnostics, oligonucleotide-based telomerase inhibition as anti-tumor therapy, and the cloning and use of telomerase in telomerase-mediated therapy wherein telomerase is utilized to immortalize human cells. From 1995 to 1998 Dr. West organized and managed the research between Geron and its academic collaborators James Thomson and John Gearhart that led to the first isolation of human embryonic stem and human embryonic germ cells. Dr. West received a B.S. Degree from Rensselaer Polytechnic Institute in 1976, an M.S. Degree in Biology from Andrews University in 1982, and a Ph.D. from Baylor College of Medicine in 1989 concentrating on the biology of cellular aging.
Dr. West is an internationally renowned pioneer and expert in stem cell research, and has extensive academic and business experience in age-related degenerative diseases, telomerase molecular biology, and human embryonic stem cell research and development. Dr. West brings to our Board of Directors the proven ability to conceive of and manage innovative research and development programs that have made scientifically significant discoveries in the field of human embryonic stem cells, and the ability to build companies focused on the great potential of regenerative medicine.
Director Independence
Our Board of Directors has determined that Andrew Arno, Andrew Last, and Cavan Redmond qualify as “independent” in accordance with Section 803(A) of the NYSE MKT Company Guide. The members of our Audit Committee also meet the independence standards under Section 803(B)(2) of the NYSE MKT Company Guide and Section 10A-3 under the Securities Exchange Act of 1934, as amended. In determining the independence of directors, our Board of Directors considered relationships and transactions during 2015 and during the past three fiscal years between each director or any member of the director’s immediate family, on the one hand, and our company and our affiliates, on the other hand. The purpose of this review was to determine whether any such relationships or transactions were inconsistent with a determination that the director is independent. The only compensation or remuneration that we provide to our independent directors is compensation as non-employee directors. None of these directors, nor any of the members of their families, have participated in any transactions with us that would disqualify them as “independent” directors under the standard described above.
William Annett, Michael D. West, and Alfred D. Kingsley do not qualify as “independent” because we presently or during the past three years have compensated them as executive officers, and Dr. West and Mr. Mohanty are full-time employees of BioTime, our controlling shareholder.
Controlled Company Exception
After the completion of the Distribution, BioTime will continue to beneficially own more than 50% of our common stock and voting power. As a result, we will be a “controlled company” within the meaning of the NYSE MKT corporate governance standards. Under the NYSE MKT corporate governance standards, a company of which more than 50% of the voting power is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance standards, including (1) the requirement that a majority of the board of directors consist of independent directors, (2) the requirement that the company have a compensation committee that is composed entirely of independent directors, and (3) the requirement that the company have a nominating and corporate governance committee that is composed entirely of independent directors. Following the Distribution, assuming that we are approved for listing on the NYSE MKT, we plan to utilize these exemptions. As a result, following this offering, we will not have a majority of independent directors on our Board of Directors, and we will not have a nominating and corporate governance committee that is composed entirely of independent directors. Accordingly, you will not have the same protections afforded to stockholders of companies that are subject to all of the NYSE MKT corporate governance requirements. In the event that we cease to be a “controlled company,” we will be required to comply with these provisions within the transition periods specified in the NYSE MKT corporate governance rules. Nevertheless, under the NYSE MKT corporate governance standards, we will still be required to have an Audit Committee composed of at least three independent directors with a written charter addressing the Audit Committee’s purpose and responsibilities. Also, we have elected to designate a Compensation Committee comprised of two directors who qualify as “independent” in accordance with Section 803(A) and Section 805(c) of the NYSE MKT Company Guide, though we will not be required by the NYSE MKT to maintain the Compensation Committee so long as we are a controlled company.
Committees
Audit Committee
The Board of Directors has an Audit Committee that was formed during September 2015. The members of the Audit Committee are Andrew Arno, Andrew Last, and Cavan Redmond each of whom qualifies as being “independent” under Section 8.03(A) and 8.03(B) of the NYSE MKT Company Guide and under Rule 10A-3 of the Exchange Act. Mr. Arno is the Chairman of the Audit Committee. The purpose of the Audit Committee is to recommend the engagement of our independent registered public accountants, to review their performance and the plan, scope, and results of the audit, and to review and approve the fees we pay to our independent registered public accountants. The Audit Committee also will review our accounting and financial reporting procedures and controls, and all transactions between us and our executive officers, directors, and shareholders who beneficially own 5% or more of any class of our voting securities. We have adopted a written charter for our Audit Committee and plan to post the charter on our website.
Compensation Committee
The Board of Directors has a Compensation Committee that was formed during September 2015. The members of the Compensation Committee are Andrew Arno and Cavan Redmond, both of whom qualify as “independent” in accordance with Section 803(A) and Section 805(c) of the NYSE MKT Company Guide. The Compensation Committee will determine or recommend to the Board of Directors the terms and amount of executive compensation and grants of options and other awards to key employees, consultants, and independent contractors under our Stock Option Plan. We have adopted a written charter for our Compensation Committee and plan to post the charter on our website.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that will apply to all of our employees, officers and directors, and such other our personnel as may be designated by the Chairman of our Audit Committee. We plan to post a copy of the code on our website and to disclose any amendments to the code, or any waivers of its requirements, on our website.
Compensation of Directors
Directors and members of committees of the Board of Directors who are our employees are entitled to receive compensation as employees but are not compensated for serving as directors or attending meetings of the Board of Directors or committees of the Board. All directors are entitled to reimbursements for their out-of-pocket expenses incurred in attending meetings of the Board of Directors or committees of the Board of Directors.
Non-employee directors, other than the Chairman of the Board of Directors, will receive an annual fee of $15,000 in cash, plus $1,000 for each regular or special meeting of the Board of Directors attended or $500 for each meeting attended by telephone conference call, and options to purchase 10,000 shares of common stock under our 2010 Stock Option.
The annual fee of cash will be paid, and the stock options granted will vest and become exercisable, in four equal quarterly installments, provided that the non-employee director remains a director on the last day of the applicable quarter. The options will expire if not exercised five years from the date of grant.
Directors who serve on the Audit Committee or the Compensation Committee shall receive, in addition to other fees payable to them as directors, the following annual fees:
|
· |
Audit Committee Chairman: $10,000 |
|
· |
Audit Committee Member other than Chairman: $7,000 |
|
· |
Compensation Committee Chairman: $7,500 |
|
· |
Compensation Committee Member other than Chairman: $5,000 |
Members of Audit Committee and Compensation Committee will also receive $1,000 for each committee meeting attended in person, and $500 for each committee meeting attended by telephone.
None of our current directors or any former directors received any compensation for serving on our Board of Directors during the fiscal year ended December 31, 2014.
Executive Officers
William Annett, Karen Chapman, Alfred D. Kingsley, Kristine C. Mechem and Russell Skibsted are our only executive officers. Karen Chapman is the wife of Michael West, a member of our Board of Directors.
Karen B. Chapman has been Vice President of Research since March 2015. Dr. Chapman served as Director of Bioinformatics at OncoCyte from April 2011 to March 2015 leading the biomarker discovery group. She has focused her business career on biotechnology and medical applications of genetic technologies. While a scientist at Geron Corporation of Menlo Park, California, from 1994 to 1997, Dr. Chapman participated in the cloning of human telomerase and has been issued approximately 25 patents. Dr. Chapman was a co-founder of Origen Therapeutics, a company focused on the manufacture of therapeutic proteins in transgenic animal systems. She was also Senior Scientist and Associate Director of Business Development at Acata Inc. where she managed research efforts in the epigenetic and telomere status of embryonic stem cells and business contract negotiations. Dr. Chapman received her Ph.D. from Johns Hopkins University School of Medicine in 1991.
Kristine C. Mechem joined OncoCyte Corporation as Vice President of Marketing in August 2015 after serving as a commercialization consultant assisting in the areas of portfolio management, launch planning and forecasting from April 2015 until joining OncoCyte. Dr. Mechem served as a Director of Business Insights and Analytics at Abbott Diabetes Care, from 2011 until 2014, leading a team of analysts and forecasters supporting the business planning process and developing monthly key performance indicators for its senior management. Dr. Mechem brings to OncoCyte extensive experience within the biotechnology and diagnostics industry. Dr. Mechem’s leadership and industry experience includes: marketing, planning and analytic roles. Her experience spans from smaller early stage diagnostic companies such as Corra Life Sciences, to larger biopharma companies like Abbott Laboratories and Genentech. At Corra Life Sciences, a prenatal diagnostics company, she was a co-founder and served as Vice President of Business Development and Marketing from2006 until 2007. Dr. Mechem provided business development and marketing support working to secure co-development partners and license intellectual property from three prominent universities. At Genentech from 2009 until 2010, Dr. Mechem supported the portfolio management and led the business planning process for a $400 million annual revenue product. Her increasing roles of responsibility included long range planning, commercial opportunity assessments, and target product profile market research. Dr. Mechem holds a Ph.D. from the University of Chicago, where she was a National Institute of Child Health and Human Development (NICHD) fellow. She also completed the Stanford Business School Executive Program in Strategy and Organization.
Russell Skibsted was appointed as our Chief Financial Officer and as BioTime’s Chief Financial Officer during November 2015. Mr. Skibsted comes to OncoCyte and BioTime from Proove Biosciences, Inc. where he had served as Chief Financial Officer since 2014. From 2013 to 2014 Mr. Skibsted was Managing Director and Chief Financial Officer of RSL Ventures, where he provided financial consulting services to public and private companies in the life sciences sector. Mr. Skibsted served as Senior Vice President, Chief Financial Officer and Secretary of Aeolus Pharmaceuticals, a publicly traded biopharma company, from 2010 to 2013, and was Senior Vice President and Chief Business Officer of Spectrum Pharmaceuticals, a publicly traded, biopharmaceutical company, from 2006 to 2009. Previously, from 2004 to 2006, Mr. Skibsted served as Chief Financial Officer of Hana Biosciences, and from 2000 to 2004 he served as Chief Financial Officer and Portfolio Management Partner of Asset Management Company, a venture capital firm. Mr. Skibsted holds a B.A. in economics from Claremont McKenna College and an MBA from the Stanford Graduate School of Business.
Other Key Management Team Members
Lyssa Friedman joined OncoCyte as a consultant in April 2014 and was named Vice President of Clinical and Regulatory Affairs in September 2015. Ms. Friedman started her career as a registered nurse specializing in oncology, and has led clinical research operations teams for more than 15 years. Ms. Friedman was a founding team member at Veracyte, Inc. and worked there from 2008 to 2014, where she executed a 49-site, 4000-subject clinical validation study that resulted in the launch of the Afirma ® Gene Expression Classifier. She later oversaw clinical utility studies that contributed to positive coverage decision from the Centers for Medicare and Medicaid Services and major U.S private insurers. Ms. Friedman is an author of about a dozen peer-reviewed publications and is a frequent speaker on diagnostic development, clinical and regulatory affairs, and reimbursement. Ms. Friedman received her masters in Public Administration from the University of San Francisco.
Advisory Board Members
In addition to our employees, OncoCyte has formed an advisory board of experts in bioinformatics and diagnostics development.
|
Name
|
Functional Expertise
|
Experience
|
|
Ljubomir Buturovic
|
Bioinformatics and Algorithm development
|
CEO and Co-founder Clinica Persona
VP and Chief Scientist, Pathwork Diagnostics,
Director Bioinformatics Inctye Corp, SFSU Adjunct Professor
Support for a number of FDA approved diagnostics
|
|
Lyndal Hesterberg
|
Product Development
|
Senior Advisor Carmenta Biosciences, CTO Crescendo Biosciences
|
|
Steven Rosenberg
|
Genomics Diagnostics Discovery and Development
|
CXO CardioDx, CSO CareDx, Chiron Research Fellow
|
Indemnification of Directors and Officers
Section 317 of the California Corporations Code permits indemnification of directors, officers, employees and other agents of corporations under certain conditions and subject to certain limitations. In addition, Section 204(a)(10) of the California Corporations Code permits a corporation to provide, in its articles of incorporation, that directors shall not have liability to the corporation or its shareholders for monetary damages for breach of fiduciary duty, subject to certain prescribed exceptions. Article Five of our Articles of Incorporation contains provisions for the indemnification of directors, officers, employees and other agents within the limitations permitted by Section 317 and for the limitation on the personal liability of directors permitted by Section 204(b)(10), subject to the exceptions required thereby.
Under Article VI of our bylaws, any person who is or was one of our directors or officers, employees, or other agents, or is or was serving at our request as a director, officer, employee, or agent of another foreign or domestic corporation, partnership, joint venture, trust, or other enterprise, will be considered an “agent” entitled to indemnification against expenses arising under certain proceedings. To the maximum extent permitted by the California General Corporation Law, we will indemnify any person who was or is a party, or is threatened to be made a party, to any proceeding (other than an action by or in the right of OncoCyte) by reason of the fact that the person is or was an agent of OncoCyte, against expenses, judgments, fines, settlements, and other amounts actually and reasonably incurred in connection with the proceeding if that person acted in good faith and in a manner that person reasonably believed to be in the best interests of OncoCyte and, in the case of a criminal proceeding, had no reasonable cause to believe his conduct of was unlawful. The termination of any proceeding by judgment, order, settlement, conviction, or upon a plea of nolo contendere or its equivalent shall not, of itself, create a presumption that the agent did not act in good faith and in a manner which agent reasonably believed to be in the best interests of OncoCyte or that the agent had reasonable cause to believe that his conduct was unlawful.
We will also indemnify any agents any who was or is a party or is threatened to be made a party, to any threatened, pending or completed action by or in the right of OncoCyte to procure a judgment in its favor by reason of the fact that person is or was an agent of OncoCyte, against expenses actually and reasonably incurred by the agent in connection with the defense or settlement of that action if the agent acted in good faith, in a manner that the agent believed to be in the best interests of OncoCyte and with such care, including reasonable inquiry, as an ordinarily prudent person in a like position would use under similar circumstances. No indemnification shall be made:
|
· |
In respect of any claim, issue or matter as to which that the agent is adjudged to be liable to OncoCyte in the performance of that person's duty to OncoCyte, unless and only to the extent that the court in which that action was brought shall determine upon application that, in view of all the circumstances of the case, that the agent is fairly and reasonably entitled to indemnity for the expenses which the court shall determine; |
|
· |
Of amounts paid in settling or otherwise disposing of a threatened or pending action, with or without court approval; or |
|
· |
Of expenses incurred in defending a threatened or pending action which is settled or otherwise disposed of without court approval. |
To the extent that an agent of OncoCyte is successful on the merits in defense of any proceeding for which the agent is entitled to indemnification under our bylaws, the agent will be indemnified against expenses of the defense actually and reasonably incurred.
Except as with respect to a successful defense on the merits in a proceeding, indemnification will be provided under our bylaws only if authorized in the specific case on a determination that indemnification of the agent is proper in the circumstances because the agent has met the applicable standard of conduct set forth in our bylaws, by:
|
· |
A majority vote of a quorum consisting of directors who are not parties to the proceeding; |
|
· |
Approval by the affirmative vote of a majority of the shares of OncoCyte entitled to vote represented at a duly held meeting at which a quorum is present or by the written consent of holders of a majority of the outstanding shares entitled to vote. For this purpose, the shares owned by the person to be indemnified shall not be considered outstanding or entitled to vote thereon; or |
|
· |
The court in which the proceeding is or was pending, on application made by OncoCyte or the agent or the attorney or other person rendering services in connection with the defense, whether or not such application by the agent, attorney, or other person is opposed by OncoCyte. |
Expenses incurred in defending any proceeding may be advanced by OncoCyte before the final disposition of the proceeding on receipt of an undertaking by or on behalf of the agent to repay the amount of the advance unless it shall be determined ultimately that the agent is entitled to be indemnified as authorized in our bylaws.
We will not provide indemnification or advance expenses on behalf of an agent in any circumstance where it appears:
|
· |
That it would be inconsistent with a provision of our articles of incorporation, a resolution of the shareholders, or an agreement in effect at the time of the accrual of the alleged cause of action asserted in the proceeding in which the expenses were incurred or other amounts were paid, which prohibits or otherwise limits indemnification; or |
|
· |
That it would be inconsistent with any condition expressly imposed by a court in approving a settlement. |
EXECUTIVE COMPENSATION
Compensation Committee Interlocks and Insider Participation in Compensation Decisions
We did not have a Compensation Committee prior to September 2015, as we are a subsidiary of BioTime. The members of our newly organized Compensation Committee are Andrew Arno and Cavan Redmond, both of whom qualify as “independent” in accordance with Section 803(A) and Section 805(c) of the NYSE MKT Company Guide. Neither Mr. Arno nor Mr. Redmond is a current or former officer or employee of OncoCyte or BioTime or any of BioTime’s other subsidiaries, nor did either of them have any relationship with OncoCyte, BioTime or any of BioTime’s other subsidiaries requiring disclosure in this information statement under Item 404 of SEC Regulation S-K.
Prior to the formation of our Compensation Committee, executive compensation was determined by our Board of Directors, subject to approval by the Board of Directors or Compensation Committee of BioTime in the case of any OncoCyte officer or director who was also an officer or director of BioTime.
During last fiscal year, none of our executive officers served as (a) a member of the compensation committee (or other board committee performing equivalent functions or, in the absence of any such committee, the entire board of directors) of another entity, one of whose executive officers serves on our Compensation Committee, (b) a director of another entity, one of whose executive officers serves on our Compensation Committee, or (c) a member of the compensation committee (or other board committee performing equivalent functions or, in the absence of any such committee, the entire board of directors) of another entity, one of whose executive officers served on our Board of Directors, except that certain of our executive officers, including Michael D. West, Robert W. Peabody, and Alfred D. Kingsley, served on the boards of directors or as executive officers of BioTime and as directors and executive officers of one or more of BioTime’s other subsidiaries, and in their capacity as directors of OncoCyte and other BioTime subsidiaries each of them determined the compensation of other executive officers of OncoCyte and those BioTime subsidiaries, though none of them voted on matters pertaining to their own personal compensation.
Except for grants of stock options, our executive officers who were also executive officers of BioTime did not receive any direct compensation from us during 2014 and 2013 and during the first nine months of 2015. Instead, those executive officers and directors were compensated by BioTime in their respective capacities. BioTime allocated to us as an expense a portion of the compensation paid to our executive officers.
Executive Employment Agreements
We have entered into Employment Agreements with our Chief Executive Officer William Annett, our Vice President of Marketing Kristine Mechem, and our Vice President of Research Karen Chapman. Mr. Skibsted has an Employment Agreement with BioTime, and BioTime allocates a portion of his compensation to us as an allocated cost under the Shared Facilities Agreement.
Pursuant to his Employment Agreement, Mr. Annett will receive a base annual salary of $320,000. He is eligible to receive annual cash incentive bonus awards determined by the Board of Directors, with a target bonus of not less than 35% of his base salary, based on his achievement of specific, objectively determinable, performance goals at target levels for the year. If the specified performance goals are achieved at maximum levels, the amount of the annual bonus will be up to 150% of Mr. Annett’s base salary as determined by the Board of Directors in its sole discretion.
Mr. Annett’s employment agreement contains provisions entitling him to severance benefits under certain circumstances. If we terminate Mr. Annett’s employment without “cause” or if he resigns for “good reason” as those terms are defined in his employment agreement, he will be entitled to receive as a severance benefit six months base salary, a pro rates portion of the target bonus for the year, payable on the date that annual bonuses would otherwise be payable to executives, and any unvested stock options that would have vested during the six months following termination of his employment (the “Severance Period”) will vest, and the period during which his vested options may be exercised will be extended to earlier of the date twelve months after termination of his employment or the expiration date of the option. If Mr. Annett’s employment is terminated without “cause” or if he resigns for “good reason” within twelve months following a “Change of Control,” he will be entitled to twelve months base salary and the Severance Period will be twelve months rather than six months. In addition, if Mr. Annett elects continued health insurance coverage under the Consolidated Omnibus Budget Reconciliation Act (“COBRA”) we will reimburse him for or we will directly pay the premiums for that coverage until the earlier of the end of the Severance Period or the date on which he receives equivalent health care insurance in connection with new employment. In order to receive the severance benefits, Mr. Annett must execute a general release of all claims against us and must return all our property in his possession.
“Change of Control” means (A) the acquisition of our voting securities by a person or an Affiliated Group entitling the holder to elect a majority of our directors; provided, that an increase in the amount of voting securities held by a person or Affiliated Group who on the date of the Employment Agreement beneficially owned (as defined in Section 13(d) of the Exchange Act, and the regulations thereunder) more than 10% of our voting securities shall not constitute a Change of Control; and provided, further, that an acquisition of voting securities by one or more persons acting as an underwriter in connection with a sale or distribution of voting securities shall not constitute a Change of Control, (B) the sale of all or substantially all of our assets; or (C) a merger or consolidation in which we merge or consolidate into another corporation or entity in which our shareholders immediately before the merger or consolidation do not own, in the aggregate, voting securities of the surviving corporation or entity (or the ultimate parent of the surviving corporation or entity) entitling them, in the aggregate (and without regard to whether they constitute an Affiliated Group) to elect a majority of the directors or persons holding similar powers of the surviving corporation or entity (or the ultimate parent of the surviving corporation or entity). A Change of Control shall not be deemed to have occurred if all of the persons acquiring our voting securities or assets, or merging or consolidating with us, are one or more of our direct or indirect subsidiaries or parent corporations. “Affiliated Group” means (A) a person and one or more other persons in control of, controlled by, or under common control with, such person; and (B) two or more persons who, by written agreement among them, act in concert to acquire voting securities entitling them to elect a majority of our directors. “Person” includes both people and entities.
Mr. Annett’s Employment Agreement also provides that if any of the payments to him would constitute “parachute payments” under applicable provisions of the Internal Revenue Code and would be subject to excise tax, we will use our best efforts to obtain shareholder approval of the payment, or Mr. Annett may elect to accept a reduced amount that would not be subject to the excise tax on parachute payments.
Pursuant to her Employment Agreement, Ms. Mechem will receive a base annual salary of $200,000. She is eligible to receive annual cash incentive bonus awards determined by the Board of Directors, with a target bonus of not less than 30% of her base salary, based on her achievement of objectively determinable performance goals for the year. If we terminate Ms. Mechem’s employment without “cause” as defined in her Employment Agreement, she will be entitled to receive, as a severance benefit, payment of three months base salary if she has been employed by us for one year or less, or six months base salary, if she has been employed by us for more than one year. The severance compensation may be paid in a lump sum or, at our election, in installments consistent with the payment of her salary while employed by us. In order to receive the severance benefits, Ms. Mechem must execute a general release of all claims against us and must return all our property in her possession.
Pursuant to her Employment Agreement, Ms. Chapman’s base annual salary has been set by the Board of Directors at $200,000. She is eligible to receive annual bonus awards as may be approved by the Board of Directors in its discretion, based on her achievement of goals or milestones set by the Board of Directors. If we terminate Ms. Chapman’s employment without “cause” as defined in her Employment Agreement, she will be entitled to receive, as a severance benefit, payment of three months base salary. The severance compensation may be paid in a lump sum or, at our election, in installments consistent with the payment of her salary while employed by us. In order to receive the severance benefits, Ms. Chapman must execute a general release of all claims against us and must return all our property in her possession.
The following tables show certain information relating to the compensation of our former Chief Executive Officer. There were no other executive officers whose compensation exceeded $100,000 during the fiscal year ended December 31, 2014. Dr. Wagner is referred to as a “Named Executive Officer.” William Annett became our current Chief Executive Officer during June 2015 and did not receive compensation as an officer or employee during the last fiscal year.
SUMMARY COMPENSATION TABLE
|
Name and principal position
|
|
Year
|
|
Salary
|
|
|
Bonus
|
|
|
Option
Awards (1)
|
|
|
All other
compensation
|
|
|
Total
|
|
|
Joseph P. Wagner
|
|
2014
|
|
$267,800
|
|
|
$10,000
|
|
|
$—
|
|
|
$12,614(2)
|
|
|
$290,414
|
|
|
Chief Executive Officer
|
|
2013
|
|
$260,000
|
|
|
$10,000
|
|
|
$—
|
|
|
$12,533(2)
|
|
|
$282,533
|
|
|
(1) |
No options were granted to Dr. Wagner during the years ended December 2014 and 2013. |
|
(2) |
Entirely represents employer contributions to Dr. Wagner’s 401(k) plan. Dr. Wagner served as our Chief Executive Officer until May 29, 2015. |
Stock Options Outstanding at Year End
The following table summarizes certain information concerning stock options granted by us under our 2010 Stock Option Plan, and held as of December 31, 2014 by our former Chief Executive Officer, who is our only Named Executive Officer:
| OUTSTANDING EQUITY AWARDS AT YEAR-END |
|
|
Option Awards
|
|
|
Name
|
|
Number of Securities Underlying Unexercised Options Exercisable
|
|
|
Number of Securities Underlying Unexercised Options Unexercisable
|
|
|
Option Exercise Price
|
|
Option Expiration Date
|
|
|
Joseph P. Wagner
|
|
391,666(1)
|
|
|
8,333
|
|
|
$1.34
|
|
January 30, 2018
|
|
|
(1) |
These options became exercisable at the rate of 8,333 shares per month while Dr. Wagner was our employee. Dr. Wagner served as our Chief Executive Officer until May 29, 2015 and exercised 3,000 of his vested options in August 2015. The remaining unvested and vested options expired or were forfeited as a result of his resignation. |
Stock Option Grants to Executive Officers During 2015
The following tables show certain information relating to the grant of stock options to our current executive officers during 2015, including options granted to Mr. Annett and Ms. Mechem under the terms of their respective Employment Agreements. No options were granted to any of our executive officers during 2014.
2015 GRANTS OF PLAN-BASED AWARDS
|
Name
|
|
Number of Securities Underlying Options
|
|
|
Exercise Price of Option Awards ($/share) (1)
|
|
|
William Annett(1)
|
|
|
605,000
|
(2) |
|
|
$
|
2.20
|
|
|
|
Karen Chapman
|
|
|
100,000
|
(3) |
|
|
$
|
2.20
|
|
|
|
Alfred D. Kingsley
|
|
|
75,000
|
(3) |
|
|
$
|
2.20
|
|
|
|
Kristine Mechem
|
|
|
100,000
|
(3) |
|
|
$
|
3.16
|
|
|
|
(1) |
In addition to the options shown in this table, Mr. Annett holds vested options to purchase 5,000 shares of our common stock at an exercise price of $2.20 per share that were granted to him upon his appointment to our Board of Directors as a non-employee director during January 2015. |
|
(2) |
Options to purchase 5,000 shares were fully vested on the date of grant. For the remaining 600,000 options, 25% will vest and thereby become exercisable one year from the date of grant, and the balance will vest in equal 36 monthly installments commencing on the first anniversary date of the grant, based upon Mr. Annett’s continued service as an employee or officer of OncoCyte. |
|
(3) |
25% of these options will vest and thereby become exercisable one year from the date of grant, and the balance will vest in equal 36 monthly installments commencing on the first anniversary date of the grant. |
Stock Option Plan
We have adopted an Employee Stock Option Plan (the “Plan”) pursuant to which we may grant options to purchase, or we may sell as restricted stock, up to a total of 4,000,000 shares of common stock. The following summary of the Plan is qualified in all respects by reference to the full text of the Plan, a copy of which is has been filed as an exhibit to our registration statement on Form 10.
Administration of the Plan
The Plan is administered by our Board of Directors, which recommends to the Board of Directors which of our officers, directors, employees, consultants, and independent contractors should be awarded options or restricted stock, the number of shares subject to the options granted or shares of restricted stock to be sold, the exercise price of the options or purchase price of restricted stock, and certain other terms and conditions of the options and restricted stock. As permitted by the Plan, the Board of Directors has delegated administration of the Plan to the Compensation Committee.
No options may be granted under the Plan more than ten years after the date the Plan was adopted by the Board of Directors, and no options granted under the Plan may be exercised after the expiration of ten years from the date of grant.
Terms of the Options
Options granted under the Plan may be either “incentive stock options” within the meaning of Section 422(b) of the Internal Revenue Code of 1986, as amended (the “Code”), or non-qualified stock options. Incentive stock options may be granted only to our employees and employees any parent or subsidiary company. The exercise price of incentive stock options granted under the Plan must be equal to not less than 85% of the fair market of our common stock on the date the option is granted. In the case of an optionee who, at the time of grant, owns more than 10% of the combined voting power of all classes of our capital stock, the exercise price of any incentive stock option must be at least 110% of the fair market value of the common stock on the grant date, and the term of the option may be no longer than five years. The aggregate fair market value of the common stock (determined as of the grant date of the option) with respect to which incentive stock options become exercisable for the first time by an optionee in any calendar year may not exceed $100,000.
The options’ exercise price may be payable in cash or in shares of common stock having a fair market value equal to the exercise price, or in a combination of cash and common stock.
Options granted under the Plan are nontransferable (except by will or the laws of descent and distribution) and may vest upon the satisfaction of reasonable conditions determined by the Board of Directors or Compensation Committee. Incentive stock options may be exercised only during employment or within three months after termination of employment, subject to certain exceptions in the event of the death or disability of the optionee, provided, that the Board of Directors or Compensation Committee may waive this provision.
Certain Adjustments to Number of Shares and Exercise Price
The number of shares of common stock covered by the Plan, and the number of shares of common stock and the exercise price per share of each outstanding option, shall be proportionately adjusted for any increase or decrease in the number of issued and outstanding shares of common stock resulting from a subdivision or consolidation of shares or the payment of a stock dividend, or any other increase or decrease in the number of issued and outstanding shares of common stock effected without receipt of consideration by us.
Corporate Reorganization or Liquidation
In the event of the dissolution or liquidation of OncoCyte, or in the event of a reorganization, merger, or consolidation of OncoCyte as a result of which OncoCyte common stock is changed into or exchanged for cash or property or securities not issued by us, or upon a sale of substantially all of our property to, or the acquisition of stock representing more than eighty percent 80% of the voting power of our capital stock then outstanding by, another corporation or person, the Plan and all options granted under the Plan shall terminate, unless provision can be made in writing in connection with such transaction for either the continuance of the Plan and/or for the assumption of options granted under the Plan, or the substitution for such options by options covering the stock of a successor corporation, or a parent or a subsidiary of a successor corporation, with appropriate adjustments as to the number and kind of shares and prices.
Restricted Stock Sales
In lieu of granting options, we may enter into restricted stock purchase agreements with employees under which they may purchase common stock subject to certain vesting and repurchase restrictions. We have the right to repurchase unvested shares at the shareholder’s cost upon the occurrence of specified events, such as termination of employment. The price at which shares may be sold under restricted stock purchase agreements will be not less than 85% of fair market value, or 100% of fair market value in the case of stock sold to a person who owns capital stock representing more than 10% of the combined voting power of all classes of our capital stock. We may permit employees or consultants, but not executive officers or directors, who purchase stock under restricted stock purchase agreements to pay for their shares by delivering a promissory note that is secured by a pledge of their shares.
Other Compensation Plans
We do not have any pension plans, defined benefit plans, or non-qualified deferred compensation plans.
Risk Considerations and Recoupment Policies
The Compensation Committee of our Board of Directors will consider, in establishing and reviewing our executive compensation programs, whether the program encourages unnecessary or excessive risk taking. Our executive compensation arrangements include a fixed salary that provides a steady income so that executives do not feel pressured to focus exclusively on stock price performance or short term financial targets to the detriment of our long-term operational and strategic objectives. We may supplement fixed salaries with discretionary bonus awards based on the executive’s performance as well as ours, and bonus awards based on our receipt of research grant funding. The stock options that we have granted to our executive officers under the Plan vest over four years, assuring that the executives take a long-term perspective in viewing their equity ownership.
Because we have not adopted compensation plans, or made incentive awards, based on quantified financial performance measures, we have not adopted specific policies regarding the adjustment or recovery of awards or payments if the relevant performance measures are restated or otherwise adjusted in a manner that would reduce the size of an award or payment. We may adopt such policies, however, if we adopt incentive compensation plans or grant incentive bonuses based on financial performance measures.
Tax Considerations
Section 162(m) of the Internal Revenue Code places a $1.0 million limit on the amount of compensation that a company can deduct in any one year for compensation paid to its chief executive officer and the three most highly-compensated executive officers employed by the company at the end of the year, other than the company’s chief financial officer. The $1.0 million deduction limit does not apply to compensation that is performance-based and provided under a shareholder-approved plan. We have never awarded cash compensation, in the form of salary and bonuses, in excess of the $1.0 million limit. Our stock option awards are designed to qualify for tax deductibility. Notwithstanding the foregoing, we may elect to pay compensation to executive officers that may not be fully deductible if we believe that is necessary to attract, retain and reward high-performing executives.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Principal Shareholders
The following table sets forth information concerning beneficial ownership of our common stock by each shareholder known by us to be the beneficial owner of 5% or more of our common stock based on known beneficial ownership of OncoCyte common stock as of December 10, 2015, and on an estimated pro forma basis giving effect to the Distribution. We are not aware of any holder of BioTime common shares who will become a beneficial owner of 5% or more of our common stock as result of receiving shares in the Distribution.
|
Shareholder
|
|
Number of Shares Before Distribution
|
|
|
Percent of Total
|
|
|
Number of Shares After Distribution
|
|
|
Percent of Total
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BioTime, Inc.
1301 Harbor Bay Parkway, Suite 100
Alameda, California 94502
|
|
|
19,418,952
|
|
|
|
|
76.39
|
% |
|
|
|
14,866,888 |
(1)
|
|
|
|
58.55
|
% |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
George Karfunkel
126 East 56th Street/15th Floor
New York, New York 10022
|
|
|
3,500,000
|
|
|
|
|
13.77
|
% |
|
|
|
3,659,860 |
|
|
|
|
14.41 |
% |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bernard Karfunkel
c/o The Sabr Group
126 East 56th Street/15th Floor
New York, New York 10022
|
|
|
2,500,000
|
|
|
|
|
9.83
|
% |
|
|
|
2,536,666 |
|
|
|
|
9.99 |
% |
|
|
(1)
|
Includes 192,644 shares issuable to BioTime’s subsidiary Asterias Biotherapeutics, Inc.
|
Security Ownership of Management
The following table sets forth information concerning beneficial ownership of our common stock by each member of our Board of Directors, our executive officers, and all of our executive officers and directors as a group as of December 10, 2015, and on an estimated pro forma basis giving effect to the Distribution.
|
|
|
Number of
Shares
Before
Distribution
|
|
|
Percent
of Total
|
|
|
Number of
Shares After
Distribution(1)
|
|
|
Percent
of
Total
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
William Annett(2)
|
|
|
10,000
|
|
|
|
*
|
|
|
|
10,000
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Michael D. West(3)
|
|
|
506,250
|
|
|
|
2.0
|
%
|
|
|
593,848
|
|
|
|
2.3
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Alfred D. Kingsley(4)
|
|
|
143,750
|
|
|
|
*
|
|
|
|
534,765
|
|
|
|
2.1
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Russell Skibsted
|
|
|
-
|
|
|
|
*
|
|
|
|
-
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Karen B. Chapman(5)
|
|
|
506,250
|
|
|
|
2.0
|
%
|
|
|
593,848
|
|
|
|
2.3
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Kristine C. Mechem(6)
|
|
|
-
|
|
|
|
*
|
|
|
|
-
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Andrew Arno(7)
|
|
|
5,000
|
|
|
|
*
|
|
|
|
5,000
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cavan Redmond(8)
|
|
|
5,000
|
|
|
|
*
|
|
|
|
5,000
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Andrew J. Last(9)
|
|
|
2,500
|
|
|
|
*
|
|
|
|
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aditya Mohanty
|
|
|
-
|
|
|
|
*
|
|
|
|
-
|
|
|
|
*
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All executive officers and directors as a group (10 persons)(10)
|
|
|
672,500
|
|
|
|
2.6
|
%
|
|
|
1,107,314
|
|
|
|
4.2
|
%
|
|
(1) |
Includes shares beneficially owned as of December 10, 2015 and Distribution Shares that the named executive or director will receive in the Distribution on account of the BioTime common shares they own. |
|
(2) |
Includes 10,000 shares that may be acquired through the exercise of stock options that are presently exercisable. Excludes 600,000 shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
|
(3) |
Includes 250,000 shares that may be acquired by Dr. West and 200,000 shares that may be acquired by Karen Chapman through the exercise of stock options that are presently exercisable, and 31,250 shares that may be acquired by Dr. West and 25,000 shares that may be acquired by Karen Chapman upon the exercise of certain stock options that may become exercisable within 60 days. Excludes 93,750 shares that may be acquired upon the exercise of certain stock options held by Dr. West and 75,000 shares that may be acquired upon the exercise of certain stock options held by Dr. Chapman that are not presently exercisable and that will not become exercisable within 60 days. Dr. West and Dr. Chapman are husband and wife. |
|
(4) |
Includes 125,000 shares that may be acquired through the exercise of stock options that are presently exercisable and 18,750 shares that may be acquired upon the exercise of certain stock options that may become exercisable within 60 days. Excludes 56,250 shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
|
(5) |
Includes 200,000 shares that may be acquired by Dr. Chapman and 250,000 shares that may be acquired by Michael D. West through the exercise of stock options that are presently exercisable, and 25,000 shares that may be acquired by Dr. Chapman and 31,250 shares that may be acquired by Dr. West upon the exercise of certain stock options that may become exercisable within 60 days. Excludes 75,000 shares that may be acquired by Dr. Chapman and 93,750 shares that may be acquired by Dr. West, upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. Dr. West and Dr. Chapman are husband and wife. |
|
(6) |
Excludes 100,000 shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
|
(7) |
Includes 2,500 shares that may be acquired through the sale of options that are presently exercisable and 2,500 shares that may be acquired upon the exercise of certain stock options that may become exercisable within 60 days. Excludes 5,000 shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
|
(8) |
Includes 2,500 shares that may be acquired through the sale of options that are presently exercisable and 2,500 shares that may be acquired upon the exercise of certain stock options that may become exercisable within 60 days. Excludes 5,000 shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
|
(9) |
Includes 2,500 shares that may be acquired upon the exercise of certain stock options that may become exercisable within 60 days |
|
(10) |
Includes 672,500 shares that may be acquired upon the exercise of certain stock options that are presently exercisable or that may become exercisable within 60 days. Excludes 942,500 shares that may be acquired upon the exercise of certain stock options that are not presently exercisable and that will not become exercisable within 60 days. |
Certain Relationships and Related Party Transactions
In May 2015, we entered into Subscription Agreements with George Karfunkel and Bernard Karfunkel (the “Investors”) and BioTime (the “Subscription Agreements”). Under the Subscription Agreements, we sold 1,500,000 shares of our common stock for $3.3 million in cash, including 500,000 shares sold to George Karfunkel, and 1,000,000 shares sold to Bernard Karfunkel. Concurrently, BioTime purchased 1,500,000 shares of our stock in exchange for the cancelation of $3.3 million of our indebtedness owed to BioTime, and we delivered to BioTime a convertible promissory note (the “Note”) with regard to an additional $3.3 million of our outstanding indebtedness to BioTime. The Note bore interest at the rate of 1% per annum and was payable on November 30, 2016. Originally, BioTime had the right to convert the principal amount of the Note plus accrued interest into shares of our common stock at a conversion price of $2.20 per share commencing on a future date. On November 19, 2015, the Note was amended to permit BioTime to convert the Note into shares of our common stock at any time prior to payment in full of the principal balance and accrued interest and on November 20, 2015 BioTime converted the Note into 1,508,095 shares of our common stock.
In June 2015, after the sale of stock under the Subscription Agreements was completed, we entered into a second agreement with the Investors. Under the second agreement, the Investors agreed that if on or before June 30, 2016 we conduct another rights offering to our shareholders at a pre-offer valuation of at least $40.0 million, the Investors will purchase shares in that offering with an aggregate purchase price equal to the lesser of (a) a percentage of total amount of capital which we then seek to raise in the rights offer and in any concurrent offering to third parties equal to the Investors’ aggregate pro rata share of outstanding OncoCyte common stock on the record date for the rights offering, determined on a fully diluted basis, and (b) $3.0 million, or such lesser amount requested by us. Under the second agreement, we agreed that if shares of our common stock are not publicly traded on any stock exchange or over the counter market by January 15, 2016, we will issue to the Investors, warrants to purchase, in the aggregate, 1,500,000 shares of common stock at an exercise price of $0.02 per share. If issued, the warrants will expire on December 31, 2016.
The Investors also agreed that, for a period of one year from the date of the second agreement, neither of them shall invest or engage, directly or indirectly, whether as a partner, equity holder, lender, principal, agent, affiliate, consultant or otherwise, in any business anywhere in the world that develops products for the diagnosis and treatment of cancer or otherwise competes with us in any way; provided, however, that the passive ownership of less than 5% of the outstanding stock of any publicly-traded corporation will not be deemed, solely by reason thereof, to be in violation of that agreement.
During September 2015, we entered into Subscription Agreement with BioTime pursuant to which BioTime purchased 2,710,857 shares of our common stock for $8,349,440 in cash.
The foregoing offers and sales of our common stock were made in reliance upon the exemption from registration under the Securities Act pursuant to Section 4(a)(2) thereof and Regulation D thereunder.
We have entered into a Registration Rights Agreement, as amended, pursuant to which we have agreed to register for sale under the Securities Act, at our expense, shares of common stock sold to the Investors and BioTime. We have agreed to file a registration statement following a written request for registration from any holder or group of holders of not less than 25% of the shares covered by the Registration Rights Agreement, but not earlier than one year after we completes an initial public offering of our common stock registered under the Securities Act (an “IPO”). If, after we complete an IPO, we propose to register any of our securities for sale under the Securities Act, other than with respect to a subscription rights offer to our shareholders or registration statements on Form S-8, the holders of registration rights may include their shares in our registration statement, to the extent that their shares are not eligible for sale under SEC Rule 144 and subject to certain other limitations. We and the holders of the registration rights have agreed to indemnify each other from certain liabilities in connection the registration, offer, and sale of securities under a registration statement, including liabilities arising under the Securities Act.
In August 2015 our former Chief Executive Officer, Joseph Wagner, exercised options to purchase 3,000 shares of common stock for $4,020 in cash. The sale was made in reliance upon the exemption from registration under the Securities Act pursuant to Rule 701.
Shared Facilities Agreement and Relationship with BioTime
Since inception, BioTime has provided us with the use of office and laboratory facilities, laboratory and office equipment and supplies, utilities, insurance, and the services of its employees and contractors, for which we have reimbursed BioTime, either through cash payments, shares of our common stock, or delivering convertible promissory notes. As of September 30, 2015, we had approximately $437,000 due to BioTime.
We have entered into a Shared Facilities Agreement with BioTime through which BioTime will continue to provide us with the use of its facilities, equipment and supplies, utilities, and personnel at its cost plus 5%. However, to date BioTime has not charged us the 5% markup.
BioTime is not required to hire any additional personnel or to acquire any additional equipment or supplies for our use. We expect to hire our own personnel and to acquire our own equipment and supplies for our own exclusive use as the need arises.
The Shared Facilities Agreement will remain in effect from year to year, unless either party gives the other party written notice stating that this agreement shall terminate on December 31 of that year, or unless the agreement is otherwise terminated under another provision of the agreement.
Either party may terminate the Shared Facilities Agreement immediately upon the occurrence of a default by the other party. A default will be deemed to have occurred if a party (i) fails to pay any sum due under the Shared Facilities Agreement, or fails to perform any other obligation under the agreement, and the failure continues for a period of 5 days after written notice from the party seeking to terminate the agreement; (ii) becomes the subject of any order for relief in a proceeding under any Debtor Relief Law; (iii) becomes unable to pay, or admits in writing the party’s inability to pay, its debts as they mature; (iv) makes an assignment for the benefit of creditors; (v) applies for or consents to the appointment of any receiver, trustee, custodian, conservator, liquidator, rehabilitation, or similar officer for the party or for all or any part of the party’s property or assets, or any such officer is appointed for such party or any part of its assets without the party’s consent and such appointment is not dismissed or discharged within 60 calendar days; (vi) institutes or consents to any proceeding under any Debtor Relief Law with respect to the party or all or any part of the party’s property or assets, (vii) becomes subject to any proceeding under any Debtor Relief Law without the consent of the party if such case or proceeding continues undismissed or unstayed for 60 calendar days; or (viii) dissolves or liquidates or takes any action to dissolve or liquidate. As used in the Shared Facilities Agreement, the term Debtor Relief Law means the Bankruptcy Code of the United States of America, as amended, or any other similar debtor relief law affecting the rights of creditors generally.
Under the Shared Facilities Agreement, we have agreed to defend, indemnify, and hold harmless BioTime, BioTime’s shareholders, directors, officers, employees, and agents against and from any and all claims arising from our use of BioTime’s office and laboratory facilities, and from any of our work or other activities there, including all activities, work, and services performed by BioTime employees, contractors, and agents for us. The scope of our indemnification obligations also includes any and all claims arising from any breach or default on our part in the performance of any of our obligations under the terms of the Shared Facilities Agreement, or arising from any act or omission (including, but not limited to negligent acts or omissions) of us or any of our officers, agents, employees, contractors, guests, or invitees acting in that capacity. We are also assuming all risk of damage to property or injury to persons in, upon, or about the BioTime’s office and laboratory facilities, from any cause other than BioTime’s willful malfeasance or sole gross negligence. BioTime will not be liable to us for any loss or damages of any kind caused by, arising from, or in connection with (i) the performance of services by BioTime personnel for us, or the failure of any BioTime employee, contractor, or agent to perform any services for us, or (ii) any delay, error, or omission by any BioTime employee, contractor, or agent in the performance of services for us, except to the extent the loss or damage is the result of fraud, gross negligence or willful misconduct by a BioTime employee, contractor, or agent.
Approval by the Board of Directors
All of the transactions described above were reviewed directly by the Board of Directors, and the Board of Directors determined whether to approve or withhold approval of each transaction. The Board of Directors applied such criteria as it determined to be appropriate in connection with its evaluation of each proposed transaction on a transaction by transaction basis, and did not have any written guidelines governing the Board of Directors’ exercise of its discretion. The directors considered such factors as they deemed relevant to the particular transaction.
Related Person Transaction Policy
During November 2015, we adopted a Related Person Transaction Policy that applies to transactions exceeding $120,000 in which any of our officers, directors, beneficial owners of more than 5% of our common shares, or any member of their immediate family, has a direct or indirect material interest, determined in accordance with the policy (a “Related Person Transaction”). A Related Person Transaction must be reported to our outside legal counsel and our Chief Financial Officer, and will be subject to review and approval by our Audit Committee prior to effectiveness or consummation, to the extent practical. In addition, any Related Person Transaction that is ongoing in nature will be reviewed by the Audit Committee annually to ensure that the transaction has been conducted in accordance with any previous approval and that all required disclosures regarding the transaction are made.
The Audit Committee will review all relevant information available to it about a Related Person Transaction. The Audit Committee may approve or ratify the Related Person Transaction only if the Audit Committee determines that, under all of the circumstances, the transaction is in, or is not in conflict with, our best interests. The Audit Committee may, in its sole discretion, impose such conditions as it deems appropriate on us or the Related Person in connection with approval of the Related Person Transaction.
As appropriate for the circumstances, the Audit Committee will review and consider:
|
• |
the interest of the officer, director, beneficial owner of more than 5% of our common shares, or any member of their immediate family (“Related Person”) in the Related Person Transaction; |
|
• |
the approximate dollar value of the amount involved in the Related Person Transaction; |
|
• |
the approximate dollar value of the amount of the Related Person’s interest in the transaction without regard to the amount of any profit or loss; |
|
• |
whether the transaction was undertaken in the ordinary course of our business; |
|
• |
whether the transaction with the Related Person is proposed to be, or was, entered into on terms no less favorable to us than terms that could have been reached with an unrelated third party; |
|
• |
the purpose of, and the potential benefits to the transaction to us; and |
|
• |
any other information regarding the Related Person Transaction or the Related Person in the context of the proposed transaction that would be material to investors in light of the circumstances of the particular transaction. |
A copy of our Related Person Transaction Policy will be posted on our website at www.oncocyte.com.
CERTAIN FEDERAL INCOME TAX CONSIDERATIONS
Material U.S. Federal Income Tax Consequences of the Distribution
The following discussion of applicable U.S. federal income tax law is a general discussion only that focuses exclusively on the U.S. federal income tax consequences of the Distribution. This summary is based on the United States Internal Revenue Code of 1986, as amended (the “Code”), the Treasury Regulations promulgated thereunder and judicial and administrative interpretations thereof, in each case as in effect and available as of the date of this prospectus and all of which are subject to change at any time, possibly with retroactive effect. Any such change could affect the tax consequences described below.
This summary does not discuss all tax considerations that may be relevant to shareholders in light of their particular circumstances, nor does it address the consequences to shareholders subject to special treatment under the U.S. federal income tax laws, such as tax-exempt entities, non-resident alien individuals, foreign entities, foreign trusts and estates and beneficiaries thereof, insurance companies and dealers in securities. In addition, this summary does not address any state, local or foreign tax consequences.
The following discussion does not purport to address the U.S. federal income tax position of any individual taxpayer, and does not include any matters of state or local tax laws or regulations, or any matters of any tax laws or regulations of any country other than the United States of America. The following discussion is not and should not be construed to be tax advice to any BioTime shareholder or any recipient of any OncoCyte common stock.
This discussion is limited to recipients of OncoCyte common stock in the Distribution that are “U.S. Holders” (as defined below) and who hold BioTime common shares as capital assets.
A U.S. Holder is a person or entity that is, for U.S. federal income tax purposes:
|
· |
an individual who is a citizen or a resident of the United States; |
|
· |
a corporation, or other entity taxable as a corporation for U.S. federal income tax purposes, created or organized under the laws of the United States or any state thereof or the District of Columbia; |
|
· |
an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or |
|
· |
a trust, if (i) a court within the United States is able to exercise primary jurisdiction over its administration and one or more United States persons have the authority to control all of its substantial decisions, or (ii) in the case of a trust that was treated as a domestic trust under the law in effect before 1997, a valid election is in place under applicable Treasury Regulations. |
|
· |
a trust, if (i) a court within the United States is able to exercise primary jurisdiction over its administration and one or more United States persons have the authority to control all of its substantial decisions, or (ii) in the case of a trust that was treated as a domestic trust under the law in effect before 1997, a valid election is in place under applicable Treasury Regulations. |
If a recipient of OncoCyte common stock in the Distribution is a partnership for U.S. federal income tax purposes, the tax treatment of a partner in that partnership will generally depend upon the status of the partner and the activities of the partnership. If you are a partner of such a partnership, you should consult your tax advisor regarding the tax consequences of the Distribution.
All BioTime shareholders should consult their own tax advisors concerning the specific tax consequences of the Distribution to them in light of their particular circumstances. This summary is not intended to be, nor should it be construed to be, legal or tax advice to any BioTime shareholder or recipient of OncoCyte common stock
BioTime is distributing the Distribution Shares to its shareholders pro rata, other than with respect to fractional shares and with respect to shareholders residing in Excluded Jurisdictions. No fractional shares will be distributed. Any fractional shares of common stock will instead be aggregated and sold and the net cash proceeds will be distributed in lieu thereof.
BioTime should recognize gain (but not loss) on the Distribution in an amount equal to the excess of the fair market value of the Distribution Shares distributed over BioTime’s tax basis in those shares (which, in general, would equal BioTime’s tax basis in the assets contributed to OncoCyte in exchange for the shares of OncoCyte common stock). We expect that, if the Distribution occurs during 2015, BioTime will not have overall accumulated earnings and profits or current earnings and profits for 2015 after giving effect to any gain realized by BioTime as result of the Distribution. If BioTime does not have either (a) overall accumulated earnings and profits or (b) current earnings and profits for the taxable year that includes the Distribution (each as determined for U.S. federal income tax purposes), then:
|
· |
if the fair market value of the shares of OncoCyte common stock received does not exceed the BioTime shareholder’s basis in the shareholder’s BioTime shares, the shareholder would recognize no taxable gain as a result of the Distribution, and would be deemed to have received a return of capital that would reduce the shareholder’s basis in the shareholder’s BioTime shares by the fair market value of the shares of common stock received; and |
|
· |
if the fair market value of the shares of common stock distributed to a BioTime shareholder exceeds the BioTime shareholder’s basis in the shareholder’s BioTime shares, then the excess would be taxable as gain from the sale or exchange of property that may be taxed as a long-term or short-term capital gain depending upon the shareholder’s holding period in the BioTime shares. |
If BioTime has overall accumulated earnings and profits or current earnings and profits for the taxable year that includes the Distribution (each as determined for U.S. federal income tax purposes), including any earnings and profits resulting from the Distribution, then:
|
· |
the Distribution would be taxed as a dividend to a BioTime shareholder to the extent of the lesser of the shareholder’s allocable share of BioTime’s earnings and profits and the fair market value of the shares of OncoCyte common stock received by the shareholder in the Distribution; and |
|
· |
if the fair market value of the shares of OncoCyte common stock received by a BioTime shareholder exceeds the shareholder’s allocable share of BioTime's earnings and profits, the excess would be a return of capital that will reduce the shareholder’s basis in the shareholder’s BioTime shares by that excess and, to the extent it exceeds the BioTime shareholder’s basis in the shareholder’s BioTime shares, would be taxable as gain from the sale or exchange of property that may be taxed as a long-term or short-term capital gain depending upon the shareholder’s holding period in the BioTime shares |
In addition, a BioTime shareholder’s basis in the shares of OncoCyte common stock should be equal to the fair market value of the OncoCyte shares on date of the Distribution, and the shareholder’s holding period for those shares should begin on the day following the Distribution.
Backup Withholding and Information Reporting
Generally, we must report annually to the IRS the amount of dividends paid to the shareholder, the shareholder’s name and address, and the amount of tax withheld, if any. A similar report will be sent to the shareholder. Pursuant to applicable income tax treaties or other agreements, the IRS may make these reports available to tax authorities in the shareholder’s country of residence.
Payments of dividends on our shares of common stock or payments of proceeds from the disposition of our shares of common stock made to the shareholder may be subject to additional information reporting and backup withholding at a current rate of 28% unless the shareholder:
|
· |
furnishes a correct taxpayer identification number, certify that the shareholder is not subject to backup withholding on the Form W-9 or successor form and otherwise complies with all the applicable requirements of the backup withholding rules; or |
|
· |
establishes an exemption, for example, by properly certifying non-U.S. status on a Form W-8BEN or another appropriate version of IRS Form W-8; provided, however, backup withholding and information reporting may apply if either we or our paying agent has actual knowledge, or reason to know, that the shareholder is a U.S. person. |
Backup withholding is not an additional tax; rather, the U.S. income tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, a refund or credit may generally be obtained from the IRS, provided that the required information is furnished to the IRS in a timely manner.
Sale of Shares of OncoCyte Common Stock
In general, gain or loss will be recognized upon a sale of shares of OncoCyte common stock. The sale of shares of common stock will cause gain or loss to be recognized only with respect to the shares sold. The amount of such gain or loss will be the difference between the amount received in exchange for the shares sold and the holder's adjusted basis in the shares sold. The holder's tax basis in a share of common stock purchased through the exercise of rights will be the subscription price paid. The holder's tax basis in a share of common stock received from BioTime through the Distribution will be determined in the manner described above.
Such gain or loss will generally be treated as capital gain or loss if the shares of common stock sold were held as a capital asset. The gain or loss on the sale of shares of common stock will be long term capital gain or loss if shares of common stock were held for more than one year, and short term capital gain or loss if the shares of common stock had been held for one year or less. The holding period for OncoCyte common stock received from BioTime in the Distribution will commence on December 31, 2015. To the extent applicable, the straddle rules, summarized below, will likely defer the commencement of a holder's holding period for this purpose, resulting in short term capital gain or loss.
SHARES ELIGIBLE FOR FUTURE SALE
There presently is no public market for our common stock or the rights, or any of our other securities. We cannot predict the effect, if any, that market sales of shares of our common stock, or the availability of those shares for sale, will have on the market price of our common stock prevailing from time to time. Future sales of any series of our common stock in the public market, or the availability of such shares for sale in the public market, could cause the prevailing market price of our common stock to fall could adversely affect our ability to raise equity capital in the future.
Following the completion of the Distribution and based on the number of shares of our capital stock outstanding as of December 10, 2015, we will have a total of 25,390,967 shares of our common stock outstanding. All of the 4,744,707 Distribution Shares (other than those received by us on account of the BioTime common shares we hold) will be freely tradable, except that any shares received by our affiliates, as that term is defined in Rule 144 under the Securities Act, may only be sold in compliance with the Rule 144 limitations described below. The shares of our common stock that we receive from BioTime in the Distribution on account of the BioTime common shares we hold will cease to be outstanding upon issuance to us and will be restored to the status of authorized but unissued shares.
The remaining 20,646,260 outstanding shares of common stock will be, and shares subject to outstanding stock options will upon issuance be, deemed “restricted securities” as defined in Rule 144 under the Securities Act. Restricted securities may be sold in the public market only if they are registered, or if they qualify for an exemption from registration under Rule 144 or Rule 701, under the Securities Act, which rules are summarized below.
Rule 144
In general, under Rule 144 as currently in effect, once we have been subject to the public company reporting requirements of Section 13 or Section 15(d) of the Exchange Act for at least 90 days, a person who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during the 90 days preceding a sale and who has beneficially owned the shares of our common stock proposed to be sold for at least six months is entitled to sell those shares without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then that person would be entitled to sell those shares without complying with any of the requirements of Rule 144.
In general, under Rule 144, as currently in effect, our affiliates or persons selling shares of our common stock on behalf of our affiliates will be entitled to sell, within any three-month period, a number of shares that does not exceed the greater of:
|
· |
1% of the number of shares of our capital stock then outstanding, which will equal 253,909 shares immediately after this offering; or |
|
· |
the average weekly trading volume of our common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to that sale. |
Sales under Rule 144 by our affiliates or persons selling shares of our common stock on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of our capital stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of our company during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. Rule 701 also permits affiliates of our company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required to wait until 90 days after the date of this information statement before selling those shares pursuant to Rule 701.
Registration Rights
We have agreed to register for sale under the Securities Act 15,256,312 shares of common stock that we sold to certain investors without registration under the Securities Act. We have agreed to file a registration statement covering those shares following a written request for registration from any holder or group of holders of not less than 25% of the shares covered by the Registration Rights Agreement, but not earlier than one year after we completes an IPO. See “Security Ownership of Certain Beneficial Owners and Management – Certain Relationships and Related Party Transactions.”
Form S-8 Registration Statement
We intend to file a registration statement on Form S-8 under the Securities Act promptly after the completion of this offering to register shares of our common stock subject to options outstanding, as well as common stock reserved for future issuance, under our Stock Option Plan. The registration statement on Form S-8 is expected to become effective immediately upon filing, and shares of our common stock covered by the registration statement will then become eligible for sale in the public market, subject to the Rule 144 limitations applicable to affiliates and vesting restrictions. See “Executive Compensation—Stock Option Plan” for a description of our Stock Option Plan.
The following table shows certain information concerning the options outstanding and available for issuance under all of our compensation plans and agreements as of December 31, 2014:
|
Plan Category
|
|
Number of Shares to be Issued Upon Exercise of Outstanding Options, Warrants, and Rights
|
|
|
Weighted Average Exercise Price of the Outstanding Options, Warrants, and Rights
|
|
|
Number of Shares Remaining Available for Future Issuance Under Equity Compensation Plans
|
|
|
OncoCyte Equity Compensation Plans Approved by Shareholders
|
|
|
1,361,250
|
|
|
|
$
|
1.52
|
|
|
|
|
638,750
|
|
|
Additional information concerning our stock option plan and the stock options of our subsidiaries may be found in Note 6 to the Financial Statements, including the reverse stock split information in Note 1.
DESCRIPTION OF SECURITIES
Common Stock
Our Articles of Incorporation currently authorize the issuance of up to 50,000,000 shares of common stock, no par value, of which 25,421,952 shares were outstanding at December 10, 2015. As of December 10, 2015, there were five holders of the common stock, but following the completion of the Distribution we expect that there will be approximately 14,053 holders of our common stock based on the share position listings of BioTime as of November 30, 2015. Each holder of record of common stock is entitled to one vote for each outstanding share owned, on every matter properly submitted to the stockholders for their vote.
Subject to the dividend rights of holders of any of the preferred stock that may be issued from time to time, holders of common stock are entitled to any dividend declared by the Board of Directors out of funds legally available for that purpose. We have not paid any cash dividends on our common stock, and it is unlikely that any cash dividends will be declared or paid on our common stock in the foreseeable future. Instead, we plan to retain our cash for use in financing our future operations and growth.
Subject to the prior payment of the liquidation preference to holders of any preferred stock that may be issued, holders of common stock are entitled to receive on a pro rata basis all of our remaining assets available for distribution to the holders of common stock in the event of the liquidation, dissolution, or winding up of our operations. Holders of common stock do not have any preemptive rights to become subscribers or purchasers of additional shares of any class of our capital stock.
Transfer Agent
The transfer agent and registrar for the common stock is American Stock Transfer and Trust Company, LLC, 6201 15th Avenue, Brooklyn, New York 11219.
Preferred Stock
Our Articles of Incorporation currently authorize the issuance of up to 5,000,000 shares of preferred stock, no par value. We may issue preferred stock in one or more series, at any time, with such rights, preferences, privileges and restrictions as the Board of Directors may determine, all without further action of our shareholders. Any series of preferred stock which may be authorized by the Board of Directors in the future may be senior to and have greater rights and preferences than the common stock. There are no shares of preferred stock presently outstanding and we have no present plan, arrangement, or commitment to issue any preferred stock.
ONCOCYTE CORPORATION
INDEX TO AUDITED FINANCIAL STATEMENTS
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
Balance Sheets as of December 31, 2014 and 2013
|
F-3
|
|
Statements of Operations for the Years Ended December 31, 2014 and 2013
|
F-4
|
|
Statements of Comprehensive Loss for the Years Ended December 31, 2014 and 2013
|
F-5
|
|
Statements of Stockholders’ Equity (Deficit) for the Years Ended December 31, 2014 and 2013
|
F-6
|
|
Statements of Cash Flows for the Years Ended December 31, 2014 and 2013
|
F-7
|
|
Notes to the Financial Statements
|
F-8
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
OncoCyte Corporation
We have audited the accompanying balance sheets of OncoCyte Corporation as of December 31, 2014 and 2013 and the related statements of operations, comprehensive loss, stockholders’ equity (deficit), and cash flows for each of the two years in the period ended December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of OncoCyte Corporation at December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2014, in conformity with accounting principles generally accepted in the United States of America.
/s/ OUM & CO. LLP
San Francisco, California
October 1, 2015, except for Note 9, as to which the date is December 21, 2015
ONCOCYTE CORPORATION
BALANCE SHEETS
(In thousands)
| |
|
December 31,
|
|
| |
|
2014
|
|
|
2013
|
|
|
ASSETS
|
|
|
|
|
|
|
|
CURRENT ASSETS
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
257
|
|
|
$
|
101
|
|
|
Available-for-sale securities, at fair value
|
|
|
3,280
|
|
|
|
4,630
|
|
|
Prepaid expenses and other current assets
|
|
|
114
|
|
|
|
120
|
|
|
Total current assets
|
|
|
3,651
|
|
|
|
4,851
|
|
| |
|
|
|
|
|
|
|
|
|
NONCURRENT ASSETS
|
|
|
|
|
|
|
|
|
|
Intangible assets, net
|
|
|
1,472
|
|
|
|
1,714
|
|
|
Equipment and furniture, net
|
|
|
118
|
|
|
|
148
|
|
|
Deferred tax assets
|
|
|
1,115
|
|
|
|
1,574
|
|
| |
|
|
|
|
|
|
|
|
|
TOTAL ASSETS
|
|
$
|
6,356
|
|
|
$
|
8,287
|
|
| |
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES
|
|
|
|
|
|
|
|
|
|
Amount due to parent, BioTime
|
|
$
|
5,735
|
|
|
$
|
2,912
|
|
|
Amount due to affiliates
|
|
|
154
|
|
|
|
51
|
|
|
Accounts payable
|
|
|
144
|
|
|
|
103
|
|
|
Accrued expenses and other current liabilities
|
|
|
282
|
|
|
|
32
|
|
|
Deferred tax liabilities
|
|
|
1,115
|
|
|
|
1,574
|
|
|
Total current liabilities
|
|
|
7,430
|
|
|
|
4,672
|
|
| |
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES
|
|
|
7,430
|
|
|
|
4,672
|
|
| |
|
|
|
|
|
|
|
|
|
Commitments and contingencies (see Note 8)
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
Preferred stock, no par value, 5,000 shares authorized; none issued and outstanding
|
|
|
-
|
|
|
|
-
|
|
|
Common stock, no par value, 50,000 shares authorized; 18,200 shares issued and outstanding at December 31, 2014 and 2013
|
|
|
15,147
|
|
|
|
15,398
|
|
|
Accumulated other comprehensive loss on available-for-sale securities
|
|
|
(822
|
)
|
|
|
(1,370
|
)
|
|
Accumulated deficit
|
|
|
(15,399
|
)
|
|
|
(10,413
|
)
|
|
Total stockholders’ equity (deficit)
|
|
|
(1,074
|
)
|
|
|
3,615
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
$
|
6,356
|
|
|
$
|
8,287
|
|
The accompanying notes are an integral part of these financial statements.
ONCOCYTE CORPORATION
STATEMENTS OF OPERATIONS
(In thousands, except per share data)
| |
|
Year Ended December 31,
|
|
| |
|
2014
|
|
|
2013
|
|
| |
|
|
|
|
|
|
|
OPERATING EXPENSES
|
|
|
|
|
|
|
|
Research and development
|
|
$
|
3,962
|
|
|
$
|
2,943
|
|
|
General and administrative
|
|
|
1,011
|
|
|
|
552
|
|
|
Total operating expenses
|
|
|
4,973
|
|
|
|
3,495
|
|
| |
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(4,973
|
)
|
|
|
(3,495
|
)
|
| |
|
|
|
|
|
|
|
|
|
OTHER EXPENSES, NET
|
|
|
|
|
|
|
|
|
|
Interest expense, net
|
|
|
2
|
|
|
|
-
|
|
|
Other expenses, net
|
|
|
11
|
|
|
|
-
|
|
|
Total other expenses, net
|
|
|
13
|
|
|
|
-
|
|
| |
|
|
|
|
|
|
|
|
|
NET LOSS
|
|
$
|
(4,986
|
)
|
|
$
|
(3,495
|
)
|
| |
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per common share
|
|
$
|
(0.27
|
)
|
|
$
|
(0.19
|
)
|
| |
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding used to compute net loss per common share, basic and diluted
|
|
|
18,200
|
|
|
|
18,200
|
|
The accompanying notes are an integral part of these financial statements.
ONCOCYTE CORPORATION
STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
| |
|
Year Ended December 31,
|
|
| |
|
2014
|
|
|
2013
|
|
| |
|
|
|
|
|
|
|
NET LOSS
|
|
$
|
(4,986
|
)
|
|
$
|
(3,495
|
)
|
| |
|
|
|
|
|
|
|
|
|
Other comprehensive loss, net of tax:
|
|
|
|
|
|
|
|
|
|
Transfer of realized loss into equity on sale of BioTime shares
|
|
|
569
|
|
|
|
|
|
|
Unrealized (loss) gain on BioTime shares held as available-for-sale securities, net
|
|
|
(21
|
)
|
|
|
592
|
|
| |
|
|
|
|
|
|
|
|
|
COMPREHENSIVE LOSS
|
|
$
|
(4,438
|
)
|
|
$
|
(2,903
|
)
|
The accompanying notes are an integral part of these financial statements.
ONCOCYTE CORPORATION
STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(In thousands)
| |
|
Common Stock
|
|
|
Accumulated
Other
Comprehensive
|
|
|
Accumulated
|
|
|
Stockholders’
Equity
|
|
| |
|
Shares
|
|
|
Amount
|
|
|
Loss
|
|
|
Deficit
|
|
|
(Deficit)
|
|
|
BALANCE AT JANUARY 1, 2013
|
|
|
18,200
|
|
|
$
|
15,072
|
|
|
$
|
(1,962
|
)
|
|
$
|
(6,918
|
)
|
|
$
|
6,192
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(3,495
|
)
|
|
|
(3,495
|
)
|
|
Unrealized gain on available-for-sale securities
|
|
|
-
|
|
|
|
-
|
|
|
|
592
|
|
|
|
-
|
|
|
|
592
|
|
|
Stock-based compensation
|
|
|
-
|
|
|
|
326
|
|
|
|
-
|
|
|
|
-
|
|
|
|
326
|
|
|
BALANCE AT DECEMBER 31, 2013
|
|
|
18,200
|
|
|
|
15,398
|
|
|
|
(1,370
|
)
|
|
|
(10,413
|
)
|
|
|
3,615
|
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(4,986
|
)
|
|
|
(4,986
|
)
|
|
Unrealized loss on available-for-sale securities
|
|
|
-
|
|
|
|
-
|
|
|
|
(21
|
)
|
|
|
-
|
|
|
|
(21
|
)
|
|
Stock-based compensation
|
|
|
-
|
|
|
|
318
|
|
|
|
-
|
|
|
|
-
|
|
|
|
318
|
|
|
Transfer of realized loss into equity from sale of BioTime shares
|
|
|
-
|
|
|
|
(569
|
)
|
|
|
569
|
|
|
|
-
|
|
|
|
-
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BALANCE AT DECEMBER 31, 2014
|
|
|
18,200
|
|
|
$
|
15,147
|
|
|
$
|
(822
|
)
|
|
$
|
(15,399
|
)
|
|
$
|
(1,074
|
)
|
The accompanying notes are an integral part of these financial statements.
ONCOCYTE CORPORATION
STATEMENTS OF CASH FLOWS
(In thousands)
| |
|
Year Ended December 31
|
|
| |
|
2014
|
|
|
2013
|
|
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(4,986
|
)
|
|
$
|
(3,495
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation expense
|
|
|
39
|
|
|
|
35
|
|
|
Amortization of intangible assets
|
|
|
242
|
|
|
|
242
|
|
|
Stock-based compensation
|
|
|
318
|
|
|
|
326
|
|
| |
|
|
|
|
|
|
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Amount due to parent, BioTime Inc.
|
|
|
2,823
|
|
|
|
2,136
|
|
|
Amount due to affiliates
|
|
|
103
|
|
|
|
72
|
|
|
Prepaid expenses and other current assets
|
|
|
6
|
|
|
|
(74
|
)
|
|
Accounts payable and accrued liabilities
|
|
|
291
|
|
|
|
48
|
|
| |
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(1,164
|
)
|
|
|
(710
|
)
|
| |
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Purchase of equipment
|
|
|
(9
|
)
|
|
|
(25
|
)
|
|
Proceeds from sale of BioTime shares
|
|
|
1,329
|
|
|
|
-
|
|
| |
|
|
|
|
|
|
|
|
|
Cash provided by (used in) investing activities
|
|
|
1,320
|
|
|
|
(25
|
)
|
| |
|
|
|
|
|
|
|
|
|
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS
|
|
|
156
|
|
|
|
(735
|
)
|
|
CASH AND CASH EQUIVALENTS:
|
|
|
|
|
|
|
|
|
|
At beginning of the period
|
|
|
101
|
|
|
|
836
|
|
|
At end of the period
|
|
$
|
257
|
|
|
$
|
101
|
|
| |
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL SCHEDULE OF NONCASH FINANCING AND INVESTING ACTIVITIES
|
|
|
|
|
|
|
|
|
|
Unrealized (loss) gain on BioTime available-for-sale securities
|
|
$
|
(21
|
)
|
|
$
|
592
|
|
|
Transfer of realized loss into equity on sale of BioTime shares
|
|
|
569
|
|
|
|
-
|
|
The accompanying notes are an integral part of these financial statements.
ONCOCYTE CORPORATION
NOTES TO FINANCIAL STATEMENTS
1. Organization, Description of the Business and Liquidity
OncoCyte Corporation (“OncoCyte”) was incorporated in 2009 in the state of California as a majority-owned subsidiary of Bio Time, Inc. (“BioTime”), a publicly traded biotechnology company focused in the field of regenerative medicine. From inception through March 2011, OncoCyte focused substantially all of its efforts on the development of embryonic stem cell-derived cancer therapies. In April 2011, OncoCyte initiated development of molecular cancer diagnostics utilizing a discovery platform that focuses on identifying genetic markers broadly expressed in numerous types of cancer. OncoCyte is presently focusing its efforts on developing diagnostic tests for use in detecting a variety of cancers including breast, bladder, and lung cancers.
Liquidity
For all periods presented, OncoCyte had generated no revenues. Since inception, OncoCyte has financed its operations through the sale of its common stock to its current shareholders, including BioTime, loans from its parent company, BioTime and other BioTime affiliates, and sale of BioTime common shares that OncoCyte held as available-for-sale securities (see Note 5). OncoCyte has incurred operating losses and negative cash flows since inception, and had an accumulated deficit of $15.4 million and $10.4 million as December 31, 2014 and 2013, respectively.
OncoCyte plans to continue to invest significant resources in research and development in the field of molecular cancer diagnostics. OncoCyte expects to continue to incur operating losses and negative cash flows. As of December 31, 2014, OncoCyte had $257,000 in cash and cash equivalents and held BioTime shares available-for-sale, valued at $3.3 million, which OncoCyte may use for working capital purposes, as necessary (see Note 9).
2. Summary of Significant Accounting Policies
Basis of presentation
The financial statements presented herein have been prepared on a separate, stand-alone basis. The financial statements are presented in accordance with U.S. generally accepted accounting principles (“GAAP”). BioTime has consolidated the results of OncoCyte into BioTime’s consolidated results based on BioTime’s ability to control OncoCyte’s operating and financial decisions and policies through the majority ownership of OncoCyte common stock throughout the periods presented. BioTime owned 75.3% of the outstanding common stock of OncoCyte at December 31, 2014 and 2013.
To the extent OncoCyte does not have its own employees or human resources for its operations, BioTime or BioTime commonly controlled and consolidated subsidiaries provide certain employees for administrative or operational services, as necessary, for the benefit of OncoCyte (see Note 4). Accordingly, BioTime allocates expenses such as salaries and payroll related expenses incurred and paid on behalf of OncoCyte based on the amount of time that particular employees devote to OncoCyte affairs. Other expenses such as legal, accounting, marketing, travel, and entertainment expenses are allocated to OncoCyte to the extent that those expenses are incurred by or on behalf of OncoCyte. BioTime also allocates certain overhead expenses such as insurance, internet and telephone expenses based on a percentage determined by management. These allocations are made based upon activity-based allocation drivers such as time spent, percentage of square feet of office or laboratory space used, and percentage of personnel devoted to OncoCyte’s operations or management. Management evaluates the appropriateness of the percentage allocations on a quarterly basis and believes that this basis for allocation is reasonable.
As further discussed in Notes 4 and 6, in connection with the services performed by employees of BioTime, or employees of other BioTime commonly controlled and consolidated subsidiaries within the BioTime group of affiliated entities, OncoCyte grants stock options to those employees performing services for OncoCyte and records stock-based compensation expense in the accompanying statements of operations for these services performed in the periods presented.
Reverse stock split
On November 18, 2015 OncoCyte effected a one-for-two reverse stock split of its common stock and a proportional adjustment to the conversion ratio for the related party convertible debt (see Note 9).
All share, per-share and related information including the price at which shares of common stock have been sold or may be issued, have been retroactively adjusted, in these financial statements and accompanying footnotes, where applicable, to reflect the impact of the reverse stock split including an adjustment to the related party debt conversion ratio and conversion prices.
Use of estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, management evaluates estimates which are subject to significant judgment, including those related to the allocation of direct and indirect expenses, useful lives associated with long-lived intangible assets, equipment and furniture, loss contingencies, valuation allowances related to deferred income taxes, and assumptions used to value stock-based awards, debt or other equity instruments. Actual results could differ materially from those estimates.
Fair value measurements
OncoCyte accounts for fair value measurements in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 820, Fair Value Measurements (“ASC 820”). ASC 820 establishes a single authoritative definition of fair value, sets out a framework for measuring fair value and expands on required disclosures about fair value measurement. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. ASC 820 describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are the following:
Level 1 – Quoted prices in active markets for identical assets and liabilities.
Level 2 – Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted market prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
In determining fair value, OncoCyte utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible, and also considers counterparty credit risk in its assessment of fair value. For the periods presented, OncoCyte has no financial assets or liabilities recorded at fair value on a recurring basis, except for cash and cash equivalents consisting of money market funds and the available-for-sale securities of BioTime common stock held by OncoCyte described below. These assets are measured at fair value using the period-end quoted market prices as a Level 1 input.
The carrying amounts of cash equivalents, prepaid expenses and other current assets, amounts due to BioTime and other affiliates, accounts payable, accrued expenses and other current liabilities approximate fair values because of the short-term nature of these items.
Cash and cash equivalents
Cash equivalents typically consist of highly liquid investments, with maturities of three months or less when purchased. At December 31, 2014 and 2013, OncoCyte's cash balances totaled $257,000 and $101,000, respectively, and consist entirely of bank account deposits and amounts held in money market funds.
Financial instruments that potentially subject OncoCyte to credit risk consist principally of cash and cash equivalents. OncoCyte, at times, maintains cash and cash equivalent balances at financial institutions in excess of amounts insured by United States government agencies. OncoCyte places its cash and cash equivalents with high credit quality financial institutions.
Accounting for BioTime shares
OncoCyte accounts for the BioTime shares it holds as available-for-sale equity securities in accordance with ASC 320-10-25, Investments – Debt and Equity Securities, as the shares have a readily determinable fair value quoted on the NYSE MKT and are held principally for sale to meet future working capital needs. These shares are measured at fair value and reported as current assets on the balance sheet based on the closing trading price of the security as of the date being presented. Unrealized holding gains and losses are excluded from the statements of operations and reported in equity as part of other comprehensive income or loss, net of income taxes, until realized. Realized gains and losses for shares sold are reclassified out of accumulated other comprehensive income or loss and included in equity, as an increase or decrease to equity in common stock consistent with, and pursuant to, ASC 805-50, transactions between entities under common control.
In 2014, OncoCyte sold 406,756 shares of BioTime common stock in the open market for $1.3 million in cash proceeds to be used for working capital purposes. The sale resulted in a $569,000 realized loss, which was reclassified out of accumulated other comprehensive loss and recorded as a decrease to common stock equity on the dates of sale.
As of December 31, 2014, OncoCyte holds 879,418 shares of BioTime common stock available-for-sale with a fair market value of $3.3 million.
Long-lived intangible assets
Long-lived intangible assets, primarily consisting of acquired patents, patent applications, and licenses to use certain patents are stated at acquired cost, less accumulated amortization (see Note 3). Amortization expense is computed using the straight-line method over the estimated useful lives of the assets over a period of 10 years.
Equipment and furniture
Equipment and furniture are stated at cost, less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, generally over a period of 36 to 120 months. Maintenance and repairs are expensed as incurred whereas significant renewals and betterments are capitalized. When assets are retired or otherwise disposed of, the cost and the related accumulated depreciation are removed from the respective accounts and any resulting gain or loss is reflected in OncoCyte’s results of operations.
Impairment of long-lived assets
OncoCyte assesses the impairment of long-lived assets, which consist primarily of long-lived intangible assets, furniture and equipment, whenever events or changes in circumstances indicate that such assets might be impaired and the carrying value may not be recoverable. If events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable and the expected undiscounted future cash flows attributable to the asset are less than the carrying amount of the asset, an impairment loss equal to the excess of the asset’s carrying value over its fair value is recorded. Through 2014, there have been no such impairment losses.
Income taxes
OncoCyte has filed a standalone U.S. federal income tax return since its inception. For California purposes, OncoCyte’s activity for 2013 and 2014 has been included in BioTime’s California Combined tax return. The provision for income taxes has been determined as if OncoCyte had filed separate tax returns for the periods presented. Accordingly, the effective tax rate of OncoCyte in future years could vary from its historical effective tax rates depending on the future legal structure of OncoCyte and related tax elections. The historical deferred tax assets, including the operating losses and credit carryforwards generated by OncoCyte, will remain with OncoCyte. OncoCyte accounts for income taxes in accordance with GAAP, which prescribes the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. OncoCyte’s judgments regarding future taxable income may change over time due to changes in market conditions, changes in tax laws, tax planning strategies or other factors. If OncoCyte’s assumptions and consequently its estimates change in the future, the valuation allowance may be increased or decreased, which may have a material impact on OncoCyte’s statements of operations.
The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. OncoCyte will recognize accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of December 31, 2014 and 2013. OncoCyte is not aware of any uncertain tax positions that could result in significant additional payments, accruals, or other material deviation for the years ended December 31, 2014 and 2013. OncoCyte is currently unaware of any tax issues under review.
Research and development expenses
Research and development expenses consist primarily of personnel costs and related benefits, including stock-based compensation, and expenses for outside consultants. These expenses include both direct and allocated or indirect overhead costs (see Note 4). Research and development costs are expensed as incurred.
General and administrative expenses
OncoCyte’s general and administrative expenses relate primarily to compensation and related benefits, including stock-based compensation, for executive and corporate personnel, including direct costs and costs allocated by BioTime; professional and consulting fees; direct overhead and indirect overhead allocated by BioTime (see Note 4).
Stock-based compensation
OncoCyte recognizes compensation expense related to employee option grants and restricted stock grants, if any, in accordance with FASB ASC 718, Compensation – Stock Compensation (“ASC 718”).
OncoCyte estimates the fair value of employee stock-based payment awards on the grant-date and recognizes the resulting fair value, net of estimated forfeitures, over the requisite service period. OncoCyte uses the Black-Scholes option pricing model for estimating the fair value of options granted under OncoCyte’s Stock Option Plan. The fair value of each restricted stock grant, if any, is determined based on the value of the common stock granted or sold. OncoCyte has elected to treat stock-based payment awards with graded vesting schedules and time-based service conditions as a single award and recognizes stock-based compensation on a straight-line basis, net of estimated forfeitures, over the requisite service period.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718 and FASB ASC 505-50, Equity-Based Payments to Non-Employees (“ASC 505-50”). Stock option awards issued to non-employees, consisting principally of employees of BioTime or employees of BioTime commonly controlled and consolidated subsidiaries who perform services for OncoCyte, are accounted for at fair value using the Black-Scholes option pricing model. Management believes that the fair value of the stock options is more reliably measured than the fair value of services received. OncoCyte records compensation expense based on the then-current fair values of the stock options at each financial reporting date. Compensation recorded during the service period is adjusted in subsequent periods for changes in the fair value of the stock options until the earlier of the date at which the non-employee’s performance is complete or a performance commitment is reached, which is generally when the stock option award vests. Compensation expense for non-employee grants is recorded on a straight-line basis in the statements of operations.
The Black-Scholes option pricing model requires OncoCyte to make certain assumptions including the fair value of the underlying common stock, the expected term, the expected volatility, the risk-free interest rate and the dividend yield (see Note 6).
The fair value of the shares of common stock underlying the stock options has historically been determined by the Board of Directors. Because there has been no public market for OncoCyte’s common stock, the Board of Directors has determined the fair value of the common stock at the time of the grant of options by considering a number of objective and subjective factors including contemporaneous sales of common stock to investors, valuation of comparable companies, operating and financial performance and general and industry-specific economic outlook, amongst other factors. The fair value of the underlying common stock will be determined by the Board of Directors until such time as OncoCyte’s common stock is traded on an established stock exchange or national market system. The fair value was determined in accordance with applicable elements of the practice aid issued by the American Institute of Certified Public Accountants titled Valuation of Privately Held Company Equity Securities Issued As Compensation.
The expected term of employee stock options represents the weighted-average period that the stock options are expected to remain outstanding. OncoCyte estimates the expected term of options granted based upon the “simplified method” provided under Staff Accounting Bulletin, Topic 14, or SAB Topic 14.
Because OncoCyte’s common stock has no publicly traded history, OncoCyte estimates the expected volatility of the awards from the historical volatility of selected public companies within the biotechnology industry with comparable characteristics to OncoCyte, including similarity in size, lines of business, market capitalization, revenue and financial leverage. OncoCyte determined the expected volatility assumption using the frequency of daily historical prices of comparable public company’s common stock for a period equal to the expected term of the options. OncoCyte periodically assesses the comparable companies and other relevant factors used to measure expected volatility for future stock option grants.
The risk-free interest rate assumption is based upon observed interest rates on the United States government securities appropriate for the expected term of OncoCyte’s stock options.
The dividend yield assumption is based on OncoCyte’s history and expectation of dividend payouts. OncoCyte has never declared or paid any cash dividends on its common stock, and OncoCyte does not anticipate paying any cash dividends in the foreseeable future.
Net loss per common share
Basic net loss per common share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding for the period. Diluted net loss per share reflects the weighted-average number of shares of common stock outstanding plus the potential effect of dilutive securities or contracts which are convertible to common stock, such as options and warrants (using the treasury stock method) and shares issuable in future periods, except in cases where the effect would be anti-dilutive. Because OncoCyte reported net losses for all periods presented, all potentially dilutive common stock are antidilutive for those periods.
The computations of basic and diluted net loss per common share for the years ended December 31, 2014 and 2013 are as follows (in thousands, except per share amounts):
| |
|
2014
|
|
2013
|
|
Net loss
|
|
$
|
(4,986
|
)
|
|
$
|
(3,495
|
)
|
|
Weighted average common shares outstanding – basic and diluted
|
|
|
18,200
|
|
|
|
18,200
|
|
|
Net loss per common share – basic and diluted
|
|
$
|
(0.27
|
)
|
|
$
|
(0.19
|
)
|
The following common stock equivalents were excluded from the computation of diluted net loss per common share of common stock for the for the years ended December 31, 2014 and 2013 because including them would have been antidilutive (in thousands):
| |
|
2014
|
|
|
2013
|
|
|
Stock options under Stock Option Plan
|
|
1,361
|
|
|
1,375
|
|
Segments
OncoCyte’s executive management team, as a group, represents the entity’s chief operating decision makers. To date, OncoCyte’s executive management team has viewed OncoCyte’s operations as one segment that includes, the research and development of diagnostic tests for the detection of cancer. As a result, the financial information disclosed materially represents all of the financial information related to OncoCyte’s sole operating segment.
Recent accounting pronouncements
In June 2014, the FASB issued ASU No. 2014-10, “Development Stage Entities” (Topic 915) – Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation. ASU 2014-10 eliminates the concept of a development stage entity (“DSE”) from GAAP and the current incremental reporting requirements for a DSE, including inception-to-date information, will no longer apply. The standard also clarifies that the disclosures in Topic 275, Risks and Uncertainties, apply to entities for which planned principal operations have not yet commenced. ASU 2014-10 is effective for annual periods beginning after December 15, 2014, and interim periods therein. Earlier adoption is permitted. OncoCyte early adopted ASU 2014-10 beginning January 1, 2013, and for interim periods thereafter.
In June 2014, the FASB issued ASU No. 2014-12 “Compensation – Stock Compensation” (Topic 718). The ASU provides guidance for accounting for share-based payments when the terms of an award provide that a performance target could be achieved after the requisite service period. That is the case when an employee is eligible to retire or otherwise terminate employment before the end of the period in which a performance target (for example, profitability target) could be achieved and still be eligible to vest in the award if and when the performance target is achieved. The ASU requires a performance target that effects vesting and that could be achieved after the requisite service period be treated as a performance condition. Compensation cost should be recognized in the period in which it becomes probable that such performance condition would be achieved and should represent the compensation cost attributable to the periods for which the requisite service has already been rendered. For public business entities, the ASU is effective for annual reporting periods beginning after December 15, 2015, and interim periods therein. Early application is permitted. OncoCyte is in the process of evaluating the impact, if any, of adoption of the ASU on its financial statements.
In August 2014, the FASB issued ASU No. 2014-15 “Presentation of Financial Statements – Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern ” requiring management to evaluate on a regular basis whether any conditions or events have arisen that could raise substantial doubt about the entity’s ability to continue as a going concern. The guidance 1) provides a definition for the term “substantial doubt,” 2) requires an evaluation every reporting period, interim periods included, 3) provides principles for considering the mitigating effect of management’s plans to alleviate the substantial doubt, 4) requires certain disclosures if the substantial doubt is alleviated as a result of management’s plans, 5) requires an express statement, as well as other disclosures, if the substantial doubt is not alleviated, and 6) requires an assessment period of one year from the date the financial statements are issued. The standard is effective for OncoCyte’s reporting year ending December 31, 2016, and interim periods thereafter. Early adoption is permitted. OncoCyte is in the process of evaluating the impact, if any, of adoption of the ASU on its financial statements.
3. Selected Balance Sheet Components
Prepaid expenses and other current assets
At December 31, 2014 and 2013, prepaid expenses and other current assets were comprised of the following (in thousands):
| |
|
2014
|
|
|
2013
|
|
|
Prepaid license fees
|
|
$
|
111
|
|
|
|
$
|
120
|
|
|
|
Other prepaid expenses and current asset
|
|
|
3
|
|
|
|
|
-
|
|
|
|
Prepaid expenses and other current assets
|
|
$
|
114
|
|
|
|
$
|
120
|
|
|
Accrued expenses and other current liabilities
At December 31, 2014 and 2013, accrued expenses and other current liabilities were comprised of the following (in thousands):
| |
|
2014
|
|
|
2013
|
|
|
Accrued bonuses and payroll related expenses
|
|
$
|
147
|
|
|
|
$
|
-
|
|
|
|
Other accrued expenses
|
|
|
135
|
|
|
|
|
32
|
|
|
|
Accrued expenses and other current liabilities
|
|
$
|
282
|
|
|
|
$
|
32
|
|
|
Intangible assets, net
In 2011, OncoCyte, through its parent, BioTime, acquired substantially all of the assets of Cell Targeting, Inc. (“CTI”), a company that was engaged in cancer therapy. The assets acquired consist primarily of patents, patent applications, and licenses to use certain patents. OncoCyte issued 261,959 shares of BioTime common stock to CTI with a market value of $2.3 million and paid CTI $250,000 in cash to acquire the assets. The asset purchase was accounted for as a business combination under the acquisition method of accounting. OncoCyte amortizes intangible assets over their useful lives estimated to be 10 year at the date of the acquisition.
At December 31, 2014 and 2013, intangible assets were comprised of the following (in thousands):
| |
|
2014
|
|
|
2013
|
|
|
Intangible assets
|
|
$
|
2,419
|
|
|
|
$
|
2,419
|
|
|
|
Accumulated amortization
|
|
|
(947
|
) |
|
|
|
(705
|
) |
|
|
Intangible assets, net
|
|
$
|
1,472
|
|
|
|
$
|
1,714
|
|
|
Amortization expense amounted to approximately $242,000 annually.
Equipment and furniture, net
At December 31, 2014 and 2013, equipment and furniture were comprised of the following (in thousands):
| |
|
2014
|
|
|
2013
|
|
|
Equipment and furniture
|
|
$
|
251
|
|
|
|
$
|
242
|
|
|
|
Accumulated depreciation
|
|
|
(133
|
)
|
|
|
|
(94
|
)
|
|
|
Equipment and furniture, net
|
|
$
|
118
|
|
|
|
$
|
148
|
|
|
Depreciation expense amounted to approximately $39,000 and $35,000 for the years ended December 31, 2014 and 2013, respectively.
4. Related Party Transactions
Shared Facilities and Service Agreement
On October 8, 2009, OncoCyte and BioTime executed a Shared Facilities and Services Agreement (“Shared Facilities Agreement”). Under the terms of the Shared Facilities Agreement, BioTime will allow OncoCyte to use its premises and equipment located at Alameda, California for the sole purpose of conducting business. BioTime will also provide accounting, billing, bookkeeping, payroll, treasury, payment of accounts payable, and other similar administrative services to OncoCyte. BioTime may also provide the services of attorneys, accountants, and other professionals who may also provide professional services to BioTime and its other subsidiaries. BioTime will also provide OncoCyte with the services of its laboratory and research personnel, including BioTime employees and contractors, for the performance of research and development work for OncoCyte at the premises.
BioTime charges OncoCyte a Use Fee for services received and usage of facilities, equipment, and supplies. For each billing period, BioTime prorates and allocates costs incurred, as applicable, to OncoCyte, such costs include services of Bio Time employees, equipment, insurance, lease, professional, software, supplies and utilities. Allocation depends on key cost drivers including actual documented use, square footage of facilities used, time spent, costs incurred by or for OncoCyte, or upon proportionate usage by BioTime and OncoCyte, as reasonably estimated by BioTime (collectively “Use Fees”). BioTime, at its discretion, has the right to charge OncoCyte a 5% markup on such allocated costs although BioTime has not elected to charge this markup since the inception of the Shared Facilities Agreement. The allocated cost of BioTime employees and contractors who provide services is based upon records maintained of the number of hours of such personnel devoted to the performance of services.
The Use Fee is determined and invoiced to OncoCyte on a quarterly basis for each calendar quarter of each calendar year. If the Shared Facilities Agreement terminates prior to the last day of a billing period, the Use Fee will be determined for the number of days in the billing period elapsed prior to the termination of the Shared Facilities Agreement. Each invoice will be payable in full by OncoCyte within 30 days after receipt. Any invoice, or portion thereof, not paid in full when due will bear interest at the rate of 15% per annum until paid, unless the failure to make a payment is due to any inaction or delay in making a payment by BioTime employees from OncoCyte funds available for such purpose, rather than from the unavailability of sufficient funds legally available for payment or from an act, omission, or delay by any employee or agent of OncoCyte. To date BioTime has not charged OncoCyte any interest.
In addition to the Use Fees, OncoCyte will reimburse BioTime for any out of pocket costs incurred by BioTime for the purchase of office supplies, laboratory supplies, and other goods and materials and services for the account or use of OncoCyte, provided that invoices documenting such costs are delivered to OncoCyte with each invoice for the Use Fee. Furthermore, BioTime will have no obligation to purchase or acquire any office supplies or other goods and materials or any services for OncoCyte, and if any such supplies, goods, materials or services are obtained for OncoCyte, BioTime may arrange for the suppliers thereof to invoice OncoCyte directly.
The Shared Facilities Agreement will remain in effect from year each year, unless either party gives the other party written notice stating that the Shared Facilities Agreement will terminate on December 31 of that year, or unless the agreement otherwise terminated under another provision of the agreement.
In aggregate, BioTime allocated and charged such Use Fees to OncoCyte approximating $344,000 and $93,000 included in general and administrative expenses, and $552,000 and $194,000 included in research and development expenses included in the statements of operations during the years ended December 31, 2014 and 2013, respectively.
As of December 31, 2014 and 2013, OncoCyte had $5.9 million and $3.0 million outstanding and payable to BioTime and affiliates included in current liabilities in connection with the costs incurred under the Shared Facilities Agreement. Since these amounts are due and payable in 30 days of being invoiced, the payables are classified as current liabilities for all periods presented.
5. Shareholders’ Equity
Preferred Stock
OncoCyte is authorized to issue up to 5,000,000 shares of no par value preferred stock. To date, no preferred shares are issued and outstanding.
Common Stock
OncoCyte has up to 50,000,000 shares of no par value common stock authorized. The holders of OncoCyte’s common stock are entitled to receive ratably dividends when, as, and if declared by the Board of Directors out of funds legally available. Upon liquidation, dissolution, or winding up, the holders of OncoCyte common stock are entitled to receive ratably the net assets available after the payment of all debts and other liabilities and subject to the prior rights of OncoCyte outstanding preferred shares, if any.
The holders of common stock are entitled to one vote for each share held on all matters submitted to a vote of OncoCyte stockholders. The holders of common stock have no preemptive, subscription, or redemption rights. The outstanding shares of common stock are fully paid and non-assessable.
In 2009, OncoCyte issued 8,500,000 shares of common stock to BioTime for $8,500 in cash in connection with OncoCyte incorporation and initial capitalization. In 2009, OncoCyte raised $4.0 million in cash through the sale of 3,000,000 shares of common stock to other investors.
In January 2011, OncoCyte issued 1,700,000 shares of its common stock to BioTime in exchange for 261,959 BioTime common shares with a market value of $2.3 million, and $250,000 in cash, which OncoCyte used as aggregate consideration for the acquisition of the assets of Cell Targeting, Inc. (see Note 3).
In August 2011, OncoCyte raised $3.0 million in cash through the sale of 1,500,000 shares of common stock to other investors and OncoCyte raised an additional $1.0 million in cash through the sale of 500,000 shares of OncoCyte common stock to BioTime. BioTime also acquired an additional 3,000,000 shares of OncoCyte common stock in exchange for 1,286,174 BioTime common shares having a market value of $6.0 million on the date of the share purchase.
6. Stock-based Compensation
Stock Option Plan
In August 2010, BioTime, as our majority shareholder, approved the 2010 Stock Option Plan (the “Plan”) under which OncoCyte reserved 2,000,000 shares of common stock for the grant of stock options or the sale of restricted stock. The Plan also permits OncoCyte to issue such other securities as its Board of Directors or the Compensation Committee administering the Plan may determine.
No options may be granted under the Plan more than ten years after the date upon which the Plan was adopted by the Board of Directors, and no options granted under the Plan may be exercised after the expiration of ten years from the date of grant. Under the Plan, options to purchase common stock may be granted to employees, directors and certain consultants at exercise prices not less than the fair market value of common stock at date of grant, subject to certain limited exceptions for options granted in substitution of other options. Options may be fully exercisable immediately, or may be exercisable according to a schedule or conditions specified by the Board of Directors or the Compensation Committee. Generally, OncoCyte stock options have service related vesting conditions based on the continued performance of services for OncoCyte. The Plan also permits OncoCyte to award restricted stock for services rendered or to sell common stock to employees subject to vesting provisions under restricted stock agreements that provide for forfeiture of unvested shares upon the occurrence of specified events. OncoCyte may permit employees or consultants, but not officers or directors, who purchase stock under restricted stock purchase agreements, to pay for their shares by delivering a promissory note that is secured by a pledge of their shares. To date, only stock options have been issued under the Plan.
As discussed in Note 4, in connection with the services performed by employees of BioTime, or employees of other BioTime commonly controlled and consolidated subsidiaries within the BioTime group of affiliated entities, OncoCyte grants stock options to those employees performing services for OncoCyte and records stock-based compensation expense in the accompanying statements of operations for these services performed in the periods presented.
Stock Options
Options granted under the Plan may be either “incentive stock options” within the meaning of Section 422(b) of the Internal Revenue Code of 1986, as amended (the “Code”), or non-qualified stock options. Incentive stock options may be granted only to OncoCyte employees and employees of its subsidiaries, if any. The exercise price of stock options granted under the Plan must be equal to the fair market of OncoCyte common stock on the date the option is granted. In the case of an optionee who, at the time of grant, owns more than 10% of the combined voting power of all classes of OncoCyte stock, the exercise price of any incentive stock option must be at least 110% of the fair market value of the common stock on the grant date, and the term of the option may be no longer than five years. The aggregate fair market value of OncoCyte common stock (determined as of the grant date of the option) with respect to which incentive stock options become exercisable for the first time by an optionee in any calendar year may not exceed $100,000.
The options’ exercise price may be payable in cash or in common stock having a fair market value equal to the exercise price, or in a combination of cash and common stock, or other legal consideration for the issuance of stock as the Board of Directors or Compensation Committee may approve.
Incentive stock options granted under the Plan are nontransferable except by will or the laws of descent and distribution and may be exercised only during employment or within three months after termination of such employment, subject to certain exceptions in the event of the death or disability of the optionee.
Options other than incentive stock options under the Code are also nontransferable except by will or the laws of descent and distribution, except to the extent that the Board of Directors or Committee permits the optionee to transfer an option to a family member, a trust for family members, or other persons approved by the Board of Directors or Committee in its discretion.
Generally options will be exercisable only while the optionee remains an employee, director or consultant, or during a specific period thereafter as approved by the Board of Directors or Committee, but in the case of the termination of an employee, director, or consultant’s services due to death or disability, the period for exercising a vested option shall be extended to the earlier of 12 months after termination or the expiration date of the option.
The number of shares of common stock covered by the Plan, and the number of shares of common stock and the exercise price per share of each outstanding option, shall be proportionately adjusted for any increase or decrease in the number of issued and outstanding shares of common stock resulting from a subdivision or consolidation of shares or the payment of a stock dividend, or any other increase or decrease in the number of issued and outstanding shares of common stock effected without receipt of consideration by OncoCyte.
Options Granted
As of December 31, 2014, 638,750 remained available for grants under the Plan.
A summary of OncoCyte stock option activity under the Plan and related information follows (in thousands except weighted average exercise price):
|
Option
|
|
Available for
Grant
|
|
|
Number of
Shares
|
|
|
Weighted
Average
Exercise
Price
|
|
|
Outstanding at January 1, 2013
|
|
|
639
|
|
|
|
|
1,361
|
|
|
|
$
|
1.50
|
|
|
|
Granted
|
|
|
(45
|
) |
|
|
|
45
|
|
|
|
|
3.00
|
|
|
|
Forfeited or cancelled
|
|
|
31
|
|
|
|
|
(31
|
) |
|
|
|
2.00
|
|
|
|
Outstanding at December 31, 2013
|
|
|
625
|
|
|
|
|
1,375
|
|
|
|
$
|
1.52
|
|
|
|
Forfeited or cancelled
|
|
|
13
|
|
|
|
|
(13
|
) |
|
|
|
2.00
|
|
|
|
Outstanding at December 31, 2014
|
|
|
638
|
|
|
|
|
1,362
|
|
|
|
$
|
1.52
|
|
|
|
Exercisable at December 31, 2014
|
|
|
|
|
|
|
|
1,276
|
|
|
|
$
|
1.50
|
|
|
There were no exercises of stock options during the years ended December 31, 2014 and 2013.
Total proceeds if all options granted and outstanding as of December 31, 2014 were exercised would be approximately $2.1 million.
At December 31, 2014 and 2013, OncoCyte had approximately $87,000 and $416,000 of total unrecognized compensation expense, net of estimated forfeitures, related to the Plan that will be recognized over a weighted-average period of approximately 0.91 and 1.38 years, respectively.
OncoCyte recorded stock-based compensation expense in the following categories on the accompanying statements of operations for the years ended December 31, 2014 and 2013 (in thousands):
| |
|
2014
|
|
|
2013
|
|
|
Research and development
|
|
$
|
177
|
|
|
|
$
|
183
|
|
|
|
General and administrative
|
|
|
141
|
|
|
|
|
143
|
|
|
|
Total stock-based compensation expense
|
|
$
|
318
|
|
|
|
$
|
326
|
|
|
The assumptions that were used to calculate the grant date fair value of OncoCyte’s employee and non-employee stock option grants for the years ended December 31, 2014 and 2013 were as follows.
| |
|
2014(1)
|
|
|
2013
|
|
|
Expected life (in years)
|
|
|
-
|
|
|
|
|
4.13
|
|
|
|
Risk-free interest rates
|
|
|
-
|
% |
|
|
|
1.28
|
% |
|
|
Volatility
|
|
|
-
|
% |
|
|
|
70.68
|
% |
|
|
Dividend yield
|
|
|
-
|
% |
|
|
|
0.00
|
% |
|
| |
(1)
|
No stock options were granted in 2014.
|
Stock-based compensation expense is recognized based on awards that are ultimately expected to vest, and as a result, the amount has been reduced by estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures are estimated based on OncoCyte’s historical experience and future expectations.
The determination of stock-based compensation is inherently uncertain and subjective and involves the application of valuation models and assumptions requiring the use of judgment. If OncoCyte had made different assumptions, its stock-based compensation expense, and net loss for years ended December 31, 2014 and 2013, may have been significantly different.
There was no net income tax benefit recognized in the statements of operations for stock-based compensation expense for non-qualified stock options, as OncoCyte fully offsets net deferred tax assets with a valuation allowance (see Note 7). In addition, OncoCyte does not recognize deferred income taxes for incentive stock option compensation expense, and records a tax deduction only when a disqualified disposition has occurred.
7. Income Taxes
OncoCyte has filed a standalone U.S. federal income tax return since its inception. For California purposes, OncoCyte’s activity for 2013 and 2014 has been included in BioTime’s California Combined tax return. The provision for income taxes has been determined as if OncoCyte had filed separate tax returns for the periods presented. Accordingly, the effective tax rate of OncoCyte in future years could vary from its historical effective tax rates depending on the future legal structure of OncoCyte and related tax elections. The deferred tax assets, including the operating loss and credit carryforwards, generated by OncoCyte, will remain with OncoCyte.
The primary components of the net deferred tax assets and liabilities at December 31, 2014 and 2013 were as follows (in thousands):
| |
|
2014
|
|
|
2013
|
|
|
Current deferred tax assets (liabilities):
|
|
|
|
|
|
|
|
|
|
Available-for-sale securities
|
|
$
|
(1,115
|
) |
|
|
$
|
(1,574
|
) |
|
|
Total current deferred tax liabilities
|
|
$
|
(1,115
|
) |
|
|
$
|
(1,574
|
) |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Non-current deferred tax assets (liabilities):
|
|
|
|
|
|
|
|
|
|
|
|
Net operating loss carryforwards
|
|
$
|
4,879
|
|
|
|
$
|
4,021
|
|
|
|
Research and development credit carryforwards
|
|
|
1,030
|
|
|
|
|
832
|
|
|
|
Patents and fixed assets
|
|
|
141
|
|
|
|
|
57
|
|
|
|
Stock-based compensation
|
|
|
24
|
|
|
|
|
21
|
|
|
|
Valuation Allowance
|
|
|
(4,959
|
) |
|
|
|
(3,357
|
) |
|
|
Total non-current deferred tax assets
|
|
$
|
1,115
|
|
|
|
$
|
1,574
|
|
|
Due to losses incurred for all periods presented, OncoCyte did not record any provision or benefit for income taxes.
Income taxes differed from the amounts computed by applying the U.S. federal income tax of 34% to pretax losses from operations as a result of the following:
| |
|
2014
|
|
|
2013
|
|
|
Computed tax benefit at federal statutory rate
|
|
|
34
|
% |
|
|
|
34
|
% |
|
|
Permanent differences
|
|
|
(2
|
%) |
|
|
|
(3
|
%) |
|
|
Change in valuation allowance
|
|
|
(34
|
%) |
|
|
|
(34
|
%) |
|
|
Research and development credits
|
|
|
2
|
% |
|
|
|
3
|
% |
|
| |
|
|
0
|
% |
|
|
|
0
|
% |
|
As of December 31, 2014, OncoCyte has net operating loss carryforwards of approximately $13.9 million for U.S. federal income tax purposes, which expire through 2034. As of December 31, 2013, OncoCyte has net operating loss carryforwards of approximately $11.4 million for U.S. federal income tax purposes, which expire through 2033. OncoCyte’s federal research and development credit carryforwards of $502,000 as of December 31, 2014 and $405,000 as of December 31, 2013, will expire through 2034 and 2033, respectively. The state research and development credit carryforwards of $528,000 as of December 31, 2014 and $427,000 as of December 31, 2013 do not expire.
During 2014, OncoCyte sold 406,756 BioTime common shares in open market transactions which resulted in a taxable gain of approximately $1.3 million. This taxable gain was fully offset by current operating losses, thus resulting in no income taxes due from the sale. As of December 31, 2014, OncoCyte recorded a $1.1 million deferred tax liability resulting from the difference in the tax basis of BioTime shares held as compared to the basis of such shares reported on OncoCyte’s balance sheet (see Note 2).
A valuation allowance is provided when it is more likely than not that some portion of the deferred tax assets will not be realized. OncoCyte established a full valuation allowance for all periods presented due to the uncertainty of realizing future tax benefits from its net operating loss carryforwards and other deferred tax assets. The change in the valuation allowance was $1.6 million and $1.1 million for the years ended December 31, 2014 and 2013, respectively.
Internal Revenue Code Section 382 places a limitation (“Section 382 Limitation”) on the amount of taxable income that can be offset by net operating loss (“NOL”) carryforwards after a change in control (generally greater than 50% change in ownership within a three-year period) of a loss corporation. California has similar rules. Generally, after a control change, a loss corporation cannot deduct NOL carryforwards in excess of the Section 382 Limitation. Due to these “change in ownership” provisions, utilization of the NOL and tax credit carryforwards may be subject to an annual limitation regarding their utilization against taxable income in future periods. There has not been a change in ownership for any of the periods presented.
OncoCyte may be subject to potential income tax examination by U.S. federal or states authorities. These potential examinations may include inquiries regarding the timing and amount of deductions, and compliance with U.S. federal and state tax laws. In general, OncoCyte is no longer subject to tax examination by major taxing authorities for years before 2010. Although the statute is closed for purposes of assessing additional income and tax in those years, the taxing authorities may still make adjustments to the net operating loss and credit carryforwards used in open years. Any potential examinations may include inquiries regarding the timing and amount of deductions, and compliance with U.S. federal and state tax laws.
8. Commitments and Contingencies
OncoCyte had no commitments other than those under the Shared Facilities and Services Agreement described in Note 4. The minimum fixed payments due under the Shared Facilities Agreement are approximately $15,000 per month.
Tax Filings
OncoCyte tax filings are subject to audit by taxing authorities in jurisdictions where it conducts business. These audits may result in assessments of additional taxes that are subsequently resolved with the authorities or potentially through the courts. Management believes OncoCyte has adequately provided for any ultimate amounts that are likely to result from these audits; however, final assessments, if any, could be significantly different than the amounts recorded in the financial statements.
Litigation – General
OncoCyte is subject to various claims and contingencies in the ordinary course of its business, including those related to litigation, business transactions, employee-related matters, and others. When OncoCyte is aware of a claim or potential claim, it assesses the likelihood of any loss or exposure. If it is probable that a loss will result and the amount of the loss can be reasonably estimated, OncoCyte will record a liability for the loss. If the loss is not probable or the amount of the loss cannot be reasonably estimated, OncoCyte discloses the claim if the likelihood of a potential loss is reasonably possible and the amount involved could be material. OncoCyte is not aware of any claims likely to have a material adverse effect on its financial condition or results of operations.
Employment Contracts
OncoCyte has entered into employment contracts with certain executive officers. Under the provisions of the contracts, OncoCyte may be required to incur severance obligations for matters relating to changes in control, as defined, and involuntary terminations. At December 31, 2014, total potential severance obligations in connection with the termination of employment contracts approximated $170,000 for termination without cause and for termination due to a change in control.
Indemnification
In the normal course of business, OncoCyte may provide indemnifications of varying scope under OncoCyte’s agreements with other companies or consultants, typically OncoCyte’s clinical research organizations, investigators, clinical sites, suppliers and others. Pursuant to these agreements, OncoCyte will generally agree to indemnify, hold harmless, and reimburse the indemnified parties for losses and expenses suffered or incurred by the indemnified parties arising from claims of third parties in connection with the use or testing of OncoCyte’s diagnostic tests. Indemnification provisions could also cover third party infringement claims with respect to patent rights, copyrights, or other intellectual property pertaining to OncoCyte’s diagnostic tests. The term of these indemnification agreements will generally continue in effect after the termination or expiration of the particular research, development, services, or license agreement to which they relate. The potential future payments OncoCyte could be required to make under these indemnification agreements will generally not be subject to any specified maximum amount. Historically, OncoCyte have not been subject to any claims or demands for indemnification. OncoCyte also maintain various liability insurance policies that limit OncoCyte’s exposure. As a result, OncoCyte believe the fair value of these indemnification agreements is minimal. Accordingly, OncoCyte have not recorded any liabilities for these agreements as of December 31, 2014 and 2013.
9. Subsequent Events
In May 2015, OncoCyte entered into Subscription Agreements with two of its investors and BioTime (the “Subscription Agreements”). Under the Subscription Agreements, OncoCyte sold 1,500,000 shares of its common stock for $3.3 million in cash to two of its shareholders (the “Investors”), including 500,000 shares sold to George Karfunkel and 1,000,000 shares sold to Bernard Karfunkel, each of whom is a beneficial owner of more than 5% of the outstanding common shares of OncoCyte. Concurrently, BioTime purchased 1,500,000 shares of OncoCyte common stock in exchange for the cancelation of $3.3 million of indebtedness owed to BioTime by OncoCyte, and OncoCyte delivered to BioTime a convertible promissory note (the “Note”) for an additional $3.3 million of OncoCyte’s indebtedness to BioTime. The Note bears interest at the rate of 1% per annum and will mature and be payable on November 30, 2016. BioTime will have the right to convert the principal amount of the Note plus accrued interest into shares of OncoCyte common stock at a conversion price of $2.20 per share commencing on the earliest of November 8, 2016, or six months after OncoCyte completes an initial underwritten public offering of its common stock, or upon the occurrence of an “Event of Default” as defined in the Note. An Event of Default includes a failure of OncoCyte to pay any amount due on the Note or the commencement of bankruptcy proceedings by or against OncoCyte or the occurrence of certain insolvency related events, the dissolution or liquidation of OncoCyte, or any material breach or default by OncoCyte under any loan agreement, promissory note, or other instrument evidencing indebtedness payable to a third party. The conversion price is subject to pro rata adjustment in the event of a stock split, combination, reclassification, or similar event.
In June 2015, after the sale of stock under the Subscription Agreements were completed, OncoCyte and the Investors entered into a second agreement. Under the second agreement, the Investors agreed that if on or before June 30, 2016 OncoCyte conducts another rights offering to its shareholders at a pre-offer valuation of at least $40.0 million the Investors will purchase shares in that offering with an aggregate purchase price equal to the lesser of (a) a percentage of total amount of capital which OncoCyte then seeks to raise in the rights offer and in any concurrent offering to third parties equal to the Investors’ aggregate pro rata share of the outstanding OncoCyte common stock on the record date for the rights offering, determined on a fully diluted basis, and (b) $3.0 million, or such lesser amount requested by OncoCyte. Under the second agreement, OncoCyte agreed that if shares of OncoCyte common stock are not publicly traded on any stock exchange or over the counter market by January 15, 2016, OncoCyte will issue to the Investors, warrants to purchase, in the aggregate, 1,500,000 shares of OncoCyte common stock at an exercise price of $0.02 per share. If issued, the warrants will expire on December 31, 2016.
The Investors also agreed that, for a period of one year from the date of the second agreement, neither of them shall invest or engage, directly or indirectly, whether as a partner, equity holder, lender, principal, agent, affiliate, consultant or otherwise, in any business anywhere in the world that develops products for the diagnosis and treatment of cancer or otherwise competes with OncoCyte in any way; provided, however, that the passive ownership of less than 5% of the outstanding stock of any publicly-traded corporation will not be deemed, solely by reason thereof, to be in violation of that agreement.
In January 2015, OncoCyte amended the Plan by making an additional 2,000,000 shares of its common stock available for the grant of stock option and restricted stock awards and OncoCyte granted options to purchase 642,500 shares at an exercise price of $2.20 per share. In June 2015, OncoCyte granted options to purchase 610,000 shares at an exercise price of $2.20 per share. In August 2015, OncoCyte granted options to purchase 150,000 shares at an exercise price of $3.06 per share and on September 9, 2015, OncoCyte granted options to purchase 20,000 shares at an exercise price of $3.16 per share.
In June 2015, OncoCyte entered into a new employment agreement with its Chief Executive Officer, under which he will receive an annual salary of $320,000, 605,000 options to purchase OncoCyte common shares, 25% of which shall vest one year from the date of grant, and the balance in equal 36 monthly installments, and an additional 5,000 options that were fully vested on the date of grant. All of the stock options have an exercise price of $2.20 per share and expire 10 years from the date of grant. Under the employment agreement, the executive is eligible to earn an annual cash incentive bonus award determined by the Board of Directors in respect of each fiscal year during the executive’s employment, with a target bonus equal to no less than 35% of base salary for achievement of the specified performance goals at target levels for the applicable calendar year. The annual bonus payable is based upon the level of achievement of objectively determinable company and individual performance goals for the applicable calendar year, as determined by the Board of Directors in consultation with the executive. If the specified performance goals for the applicable calendar year are achieved at maximum levels, the annual bonus payable can be up to 150% of the annual base salary, as determined by the Board of Directors, in its sole discretion.
In August 2015, OncoCyte entered into a new employment agreement with its Vice President of Marketing, under which the executive will receive an annual salary of $200,000 and 100,000 options to purchase OncoCyte common shares, 25% of which shall vest one year from the date of grant, and the balance in equal 36 monthly installments. The stock options have an exercise price of $3.06 per share and expire 10 years from the date of grant. Under the employment agreement, the executive is eligible to earn an annual cash incentive bonus award determined by the Board of Directors in respect of each calendar year during the executive’s employment, with a target bonus equal to no less than 30% of base salary for achievement of the specified performance goals at target levels for the applicable calendar year. The annual bonus payable is based upon the level of achievement of objectively determinable company and individual performance goals for the applicable calendar year, as determined by the Board of Directors in consultation with the executive.
On September 17, 2015, OncoCyte sold 13,356 shares of BioTime stock in the open market for gross cash proceeds of approximately $44,000.
During September 2015, OncoCyte entered into a Subscription Agreement with BioTime pursuant to which BioTime purchased 2,710,857 shares of OncoCyte common stock for $8.3 million in cash, as part of a subscription offer made to all OncoCyte shareholders on a pro rata basis. The other OncoCyte shareholders had the right to purchase a total of 974,447 shares of OncoCyte common stock at the same price paid by BioTime, until November 16, 2015, when the right expired unexercised.
On October 1, 2015, OncoCyte sold 246,356 shares of BioTime stock to certain institutional investors under a negotiated transaction for gross cash proceeds of approximately $771,000.
On November 18, 2015, OncoCyte effected a one-for-two reverse stock split of its common stock and a proportional adjustment to the conversion ratio for the related party convertible debt.
On November 19, 2015, the Note described in Note 5 was amended to permit BioTime to convert the Note into shares of OncoCyte common stock at any time prior to payment in full of the principal balance and accrued interest, and on November 20, 2015, BioTime converted the Note into 1,508,095 shares of OncoCyte common stock.
OncoCyte has evaluated subsequent events and transactions for potential recognition or disclosure in the financial statements through December 21, 2015, the day the financial statements were available for issuance.
ONCOCYTE CORPORATION
INDEX TO UNAUDITED CONDENSED INTERIM FINANCIAL STATEMENTS
|
Condensed Balance Sheets as of September 30, 2015 (unaudited) and December 31, 2014
|
F-24
|
|
Condensed Statements of Operations for the Nine Months Ended September 30, 2015 (unaudited) and 2014 (unaudited)
|
F-25
|
|
Condensed Statements of Comprehensive Loss for the Nine Months Ended September 30, 2015 (unaudited) and 2014 (unaudited)
|
F-26
|
|
Condensed Statements of Stockholders’ Equity (Deficit) for the Nine Months Ended September 30, 2015 (unaudited)
|
F-27
|
|
Condensed Statements of Cash Flows for the Nine Months Ended September 30, 2015 (unaudited) and 2014 (unaudited)
|
F-28
|
|
Notes to the Condensed Financial Statements (unaudited)
|
F-29
|
ONCOCYTE CORPORATION
CONDENSED BALANCE SHEETS
(In thousands)
| |
|
September 30, 2015
(unaudited)
|
|
|
December 31, 2014
(Note 1)
|
|
|
ASSETS
|
|
|
|
|
|
|
|
CURRENT ASSETS
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
9,410
|
|
|
$
|
257
|
|
|
Available-for-sale securities, at fair value
|
|
|
2,598
|
|
|
|
3,280
|
|
|
Prepaid expenses and other current assets
|
|
|
20
|
|
|
|
114
|
|
|
Total current assets
|
|
|
12,028
|
|
|
|
3,651
|
|
| |
|
|
|
|
|
|
|
|
|
NONCURRENT ASSETS
|
|
|
|
|
|
|
|
|
|
Intangible assets, net
|
|
|
1,290
|
|
|
|
1,472
|
|
|
Equipment and furniture, net
|
|
|
97
|
|
|
|
118
|
|
|
Deferred tax assets
|
|
|
883
|
|
|
|
1,115
|
|
| |
|
|
|
|
|
|
|
|
|
TOTAL ASSETS
|
|
$
|
14,298
|
|
|
$
|
6,356
|
|
| |
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES
|
|
|
|
|
|
|
|
|
|
Amount due to parent, BioTime
|
|
$
|
437
|
|
|
$
|
5,735
|
|
|
Amount due to affiliates
|
|
|
35
|
|
|
|
154
|
|
|
Accounts payable
|
|
|
282
|
|
|
|
144
|
|
|
Accrued expenses and other current liabilities
|
|
|
419
|
|
|
|
282
|
|
|
Deferred tax liabilities
|
|
|
883
|
|
|
|
1,115
|
|
|
Total current liabilities
|
|
|
2,056
|
|
|
|
7,430
|
|
|
Convertible note payable, related party
|
|
|
3,300
|
|
|
|
-
|
|
|
TOTAL LIABILITIES
|
|
|
5,356
|
|
|
|
7,430
|
|
| |
|
|
|
|
|
|
|
|
|
Commitments and contingencies (see Note 9)
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
|
|
|
|
|
|
|
|
Preferred stock, no par value, 5,000 shares authorized; none issued and outstanding
|
|
|
-
|
|
|
|
-
|
|
|
Common stock, no par value, 50,000 shares authorized; 23,914 and 18,200 shares issued and outstanding at September 30, 2015 (unaudited) and December 31, 2014, respectively
|
|
|
30,979
|
|
|
|
15,147
|
|
|
Accumulated other comprehensive loss on available-for-sale securities
|
|
|
(1,442
|
)
|
|
|
(822
|
)
|
|
Accumulated deficit
|
|
|
(20,595
|
)
|
|
|
(15,399
|
)
|
|
Total stockholders’ equity (deficit)
|
|
|
8,942
|
|
|
|
(1,074
|
)
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT)
|
|
$
|
14,298
|
|
|
$
|
6,356
|
|
The accompanying notes are an integral part of these unaudited condensed financial statements.
ONCOCYTE CORPORATION
CONDENSED STATEMENTS OF OPERATIONS
(In thousands, except per share data)
(unaudited)
| |
|
Nine Months Ended September 30,
|
|
| |
|
2015
|
|
|
2014
|
|
| |
|
|
|
|
|
|
|
OPERATING EXPENSES
|
|
|
|
|
|
|
|
Research and development
|
|
$
|
3,098
|
|
|
$
|
2,837
|
|
|
General and administrative
|
|
|
2,081
|
|
|
|
638
|
|
|
Total operating expenses
|
|
|
5,179
|
|
|
|
3,475
|
|
| |
|
|
|
|
|
|
|
|
|
Loss from operations
|
|
|
(5,179
|
)
|
|
|
(3,475
|
)
|
| |
|
|
|
|
|
|
|
|
|
OTHER EXPENSES, NET
|
|
|
|
|
|
|
|
|
|
Interest expense, net
|
|
|
16
|
|
|
|
2
|
|
|
Other expenses, net
|
|
|
1
|
|
|
|
2
|
|
|
Total other expenses, net
|
|
|
17
|
|
|
|
4
|
|
| |
|
|
|
|
|
|
|
|
|
NET LOSS
|
|
$
|
(5,196
|
)
|
|
$
|
(3,479
|
)
|
| |
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per common share
|
|
$
|
(0.26
|
)
|
|
$
|
(0.19
|
)
|
| |
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding used to compute net loss per common share, basic and diluted
|
|
|
19,803
|
|
|
|
18,200
|
|
The accompanying notes are an integral part of these unaudited condensed financial statements.
ONCOCYTE CORPORATION
CONDENSED STATEMENTS OF COMPREHENSIVE LOSS
(In thousands)
(unaudited)
| |
|
Nine Months Ended September 30,
|
|
| |
|
2015
|
|
|
2014
|
|
| |
|
|
|
|
|
|
|
NET LOSS
|
|
$
|
(5,196
|
)
|
|
$
|
(3,479
|
)
|
| |
|
|
|
|
|
|
|
|
|
Other comprehensive loss, net of tax
|
|
|
|
|
|
|
|
|
|
Transfer of realized loss into equity on sale of BioTime shares
|
|
|
18
|
|
|
|
73
|
|
|
Unrealized loss on BioTime shares held as available-for-sale securities, net
|
|
|
(638
|
)
|
|
|
(533
|
)
|
| |
|
|
|
|
|
|
|
|
|
COMPREHENSIVE LOSS
|
|
$
|
(5,816
|
)
|
|
$
|
(3,939
|
)
|
The accompanying notes are an integral part of these unaudited condensed financial statements.
ONCOCYTE CORPORATION
CONDENSED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
(In thousands)
(unaudited)
| |
|
Common Stock
|
|
|
Accumulated
Other
Comprehensive
|
|
|
Accumulated
|
|
|
Stockholders’ Equity
|
|
| |
|
Shares
|
|
|
Amount
|
|
|
Loss
|
|
|
Deficit
|
|
|
(Deficit)
|
|
|
BALANCE AT JANUARY 1, 2015
|
|
|
18,200
|
|
|
$
|
15,147
|
|
|
$
|
(822
|
)
|
|
$
|
(15,399
|
)
|
|
$
|
(1,074
|
)
|
|
Net loss
|
|
|
-
|
|
|
|
-
|
|
|
|
-
|
|
|
|
(5,196
|
)
|
|
|
(5,196
|
)
|
|
Issuance of common stock for cash to investors
|
|
|
1,500
|
|
|
|
3,300
|
|
|
|
-
|
|
|
|
-
|
|
|
|
3,300
|
|
|
Issuance of common stock for partial payment of related party amounts due to BioTime
|
|
|
1,500
|
|
|
|
3,300
|
|
|
|
-
|
|
|
|
-
|
|
|
|
3,300
|
|
|
Issuance of common stock for cash to BioTime
|
|
|
2,711
|
|
|
|
8,350
|
|
|
|
-
|
|
|
|
-
|
|
|
|
8,350
|
|
|
Exercise of options
|
|
|
3
|
|
|
|
4
|
|
|
|
-
|
|
|
|
-
|
|
|
|
4
|
|
|
Transfer of realized loss into equity from sale of BioTime shares
|
|
|
-
|
|
|
|
(18
|
)
|
|
|
18
|
|
|
|
-
|
|
|
|
-
|
|
|
Unrealized loss on available-for-sale securities
|
|
|
-
|
|
|
|
-
|
|
|
|
(638
|
)
|
|
|
-
|
|
|
|
(638
|
)
|
|
Stock-based compensation
|
|
|
-
|
|
|
|
831
|
|
|
|
-
|
|
|
|
-
|
|
|
|
831
|
|
|
Contingently issuable warrants to investors
|
|
|
-
|
|
|
|
65
|
|
|
|
-
|
|
|
|
-
|
|
|
|
65
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
BALANCE AT SEPTEMBER 30, 2015
|
|
|
23,914
|
|
|
$
|
30,979
|
|
|
$
|
(1,442
|
)
|
|
$
|
(20,595
|
)
|
|
$
|
8,942
|
|
The accompanying notes are an integral part of these unaudited condensed financial statements.
ONCOCYTE CORPORATION
CONDENSED STATEMENTS OF CASH FLOWS
(In thousands)
(unaudited)
| |
|
Nine Months Ended September 30,
|
|
| |
|
2015
|
|
|
2014
|
|
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
|
|
|
|
|
|
|
Net loss
|
|
$
|
(5,196
|
)
|
|
$
|
(3,479
|
)
|
|
Adjustments to reconcile net loss to net cash used in operating activities:
|
|
|
|
|
|
|
|
|
|
Depreciation expense
|
|
|
32
|
|
|
|
29
|
|
|
Amortization of intangible assets
|
|
|
181
|
|
|
|
181
|
|
|
Stock-based compensation
|
|
|
831
|
|
|
|
164
|
|
|
Contingently issuable warrant expense to investors
|
|
|
65
|
|
|
|
-
|
|
|
Changes in operating assets and liabilities:
|
|
|
|
|
|
|
|
|
|
Amount due to parent, BioTime
|
|
|
1,290
|
|
|
|
2,531
|
|
|
Amount due to affiliates
|
|
|
(119
|
)
|
|
|
77
|
|
|
Prepaid expenses and other current assets
|
|
|
94
|
|
|
|
65
|
|
|
Accounts payable and accrued liabilities
|
|
|
275
|
|
|
|
78
|
|
|
Accrued interest on related party convertible debt
|
|
|
13
|
|
|
|
-
|
|
| |
|
|
|
|
|
|
|
|
|
Net cash used in operating activities
|
|
|
(2,534
|
)
|
|
|
(354
|
)
|
| |
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Purchase of equipment
|
|
|
(11
|
)
|
|
|
(13
|
)
|
|
Proceeds from sale of BioTime shares
|
|
|
44
|
|
|
|
329
|
|
| |
|
|
|
|
|
|
|
|
|
Net cash provided by investing activities
|
|
|
33
|
|
|
|
316
|
|
| |
|
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
|
|
|
|
|
|
|
|
|
Proceeds from issuance of common stock
|
|
|
11,650
|
|
|
|
-
|
|
|
Proceeds from exercise of stock options
|
|
|
4
|
|
|
|
-
|
|
| |
|
|
|
|
|
|
|
|
|
Net cash provided by financing activities
|
|
|
11,654
|
|
|
|
-
|
|
| |
|
|
|
|
|
|
|
|
|
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS
|
|
|
9,153
|
|
|
|
(38
|
)
|
|
CASH AND CASH EQUIVALENTS:
|
|
|
|
|
|
|
|
|
|
At beginning of the period
|
|
|
257
|
|
|
|
101
|
|
|
At end of the period
|
|
$
|
9,410
|
|
|
$
|
63
|
|
The accompanying notes are an integral part of these unaudited condensed financial statements.
ONCOCYTE CORPORATION
NOTES TO CONDENSED FINANCIAL STATEMENTS
1. Organization, Description of the Business and Liquidity
OncoCyte Corporation (“OncoCyte”) was incorporated in 2009 in the state of California as a majority-owned subsidiary of Bio Time, Inc. (“BioTime”), a publicly traded biotechnology company focused in the field of regenerative medicine. From inception through March 2011, OncoCyte focused substantially all of its efforts on the development of embryonic stem cell-derived cancer therapies. In April 2011, OncoCyte initiated development of molecular cancer diagnostics utilizing a discovery platform that focuses on identifying genetic markers broadly expressed in numerous types of cancer. OncoCyte is presently focusing its efforts on developing diagnostic tests for use in detecting a variety of cancers including breast, bladder, and lung cancers.
Liquidity
For all periods presented, OncoCyte had generated no revenues. Since inception, OncoCyte has financed its operations through the sale of its common stock to its current shareholders, including BioTime, loans from its parent company, BioTime and other BioTime affiliates, and sale of BioTime common shares OncoCyte holds as available-for-sale securities (see Note 5). OncoCyte has incurred operating losses and negative cash flows since inception, and had an accumulated deficit of $20.6 million (unaudited) and $15.4 million as September 30, 2015 and December 31, 2014, respectively.
OncoCyte plans to continue to invest significant resources in research and development in the field of molecular cancer diagnostics. OncoCyte expects to continue to incur operating losses and negative cash flows. As of September 30, 2015, OncoCyte had $9.4 million in cash and cash equivalents and held BioTime shares available-for-sale, valued at $2.6 million (unaudited), which OncoCyte may use for working capital purposes, as necessary (see Note 10).
2. Summary of Significant Accounting Policies
Basis of presentation
The condensed financial statements presented herein have been prepared on a separate, stand-alone basis. The condensed financial statements are presented in accordance with U.S. generally accepted accounting principles (“GAAP”). BioTime has consolidated the results of OncoCyte into BioTime’s consolidated results based on BioTime’s ability to control OncoCyte’s operating and financial decisions and policies through the majority ownership of OncoCyte common stock throughout the periods presented. BioTime owned 74.9% (unaudited) of the outstanding common stock of OncoCyte at September 30, 2015.
To the extent OncoCyte does not have its own employees or human resources for its operations, BioTime or BioTime commonly controlled and consolidated subsidiaries provide certain employees for administrative or operational services, as necessary, for the benefit of OncoCyte (see Note 4). Accordingly, BioTime allocates expenses such as salaries and payroll related expenses incurred and paid on behalf of OncoCyte based on the amount of time that particular employees devote to OncoCyte affairs. Other expenses such as legal, accounting, marketing, travel, and entertainment expenses are allocated to OncoCyte to the extent that those expenses are incurred by or on behalf of OncoCyte. BioTime also allocates certain overhead expenses such as insurance, internet and telephone expenses based on a percentage determined by management. These allocations are made based upon activity-based allocation drivers such as time spent, percentage of square feet of office or laboratory space used, and percentage of personnel devoted to OncoCyte’s operations or management. Management evaluates the appropriateness of the percentage allocations on a quarterly basis and believes that this basis for allocation is reasonable.
As further discussed in Notes 4 and 7, in connection with the services performed by employees of BioTime, or employees of other BioTime commonly controlled and consolidated subsidiaries within the BioTime group of affiliated entities, OncoCyte grants stock options to those employees performing services for OncoCyte and records stock-based compensation expense in the accompanying statements of operations for these services performed in the periods presented.
Reverse stock split
On November 18, 2015, OncoCyte effected a one-for-two reverse stock split of its common stock and a proportional adjustment to the conversion ratio for the related party convertible debt (see Note 10).
All share, per-share and related information including the price at which shares of common stock have been sold or may be issued, have been retroactively adjusted, in these financial statements and accompanying footnotes, where applicable, to reflect the impact of the reverse stock split including an adjustment to the related party debt conversion ratio and conversion prices.
Unaudited interim financial information
The accompanying interim balance sheet as of September 30, 2015, the statements of operations and of cash flows for the nine months ended September 30, 2015 and 2014, the statement of stockholders’ equity (deficit) for the nine months ended September 30, 2015 and financial information disclosed in these notes to the condensed financial statements related to the nine months ended September 30, 2015 and 2014 are unaudited. These unaudited interim financial statements have been prepared in accordance with GAAP. In the opinion of the OncoCyte’s management, the unaudited interim financial statements have been prepared on the same basis as the audited financial statements and include all adjustments, which include only normal recurring adjustments, necessary for a fair statement of OncoCyte’s statement of financial position as of September 30, 2015 and its results of operations and its cash flows for the nine months ended September 30, 2015 and 2014. The results for the nine months ended September 30, 2015 are not necessarily indicative of the results expected for any other interim period or for the full year. These interim financial statements and the financial information disclosed in these notes to the financial statements should be read in conjunction with the annual audited financial statements and notes thereto for the years ended December 31, 2014 and 2013 included in this information statement.
Use of estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, management evaluates estimates which are subject to significant judgment including those related to the allocation of direct and indirect expenses, useful lives associated with long-lived intangible assets, equipment and furniture, loss contingencies, valuation allowances related to deferred income taxes, and assumptions used to value stock-based awards, convertible debt, conversion features, warrants or other equity instruments. Actual results could differ materially from those estimates.
Fair value measurements
OncoCyte accounts for fair value measurements in accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 820, Fair Value Measurements (“ASC 820”). ASC 820 describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value, which are the following:
Level 1 – Quoted prices in active markets for identical assets and liabilities.
Level 2 – Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted market prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 – Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
In determining fair value, OncoCyte utilizes valuation techniques that maximize the use of observable inputs and minimize the use of unobservable inputs to the extent possible as well as considers counterparty credit risk in its assessment of fair value. For the periods presented, OncoCyte has no financial assets or liabilities recorded at fair value on a recurring basis, except for cash equivalents consisting of money market funds and the available-for-sale securities of BioTime common stock held by OncoCyte described in this Note 2. These assets are measured at fair value using the period-end quoted market prices as a level 1 input.
The carrying amounts of cash equivalents, prepaid expenses and other current assets, amounts due to BioTime and other affiliates, accounts payable, accrued expenses and other current liabilities approximate fair values because of the short-term nature of these items.
The fair value of OncoCyte’s contingently issuable common stock warrants (see Note 6) is estimated using a Black-Scholes valuation model, using level 3 inputs, then adjusted for the probability of actually issuing those warrants to arrive at the probability-adjusted fair value. Since the contingently issuable warrants are equity classified and considered issued for accounting purposes as of June 30, 2015 (unaudited), no further remeasurement of the warrants’ fair value was made in subsequent periods for financial statement reporting purposes.
OncoCyte has elected not to measure the related party convertible promissory note payable (disclosed in Note 5) at fair value, with the changes in fair value recognized in the statements of operations each period in accordance with ASC 815-15, Derivatives and Hedging – Embedded Derivatives. The debt’s conversion feature can only be physically settled by delivery of common stock for converted principal and interest. The conversion feature does not meet the definition of an embedded derivative requiring bifurcation under the provisions of ASC 815-10 since, among other factors, OncoCyte is not permitted to, and BioTime cannot demand, a cash settlement in the event of a conversion and there is no net settlement available outside the debt agreement since the shares of common stock of OncoCyte underlying the conversion are not readily convertible into cash. The debt instrument does not fall within any of the three categories of liabilities defined in the scope of ASC 480-10, Distinguishing Liabilities from Equity. Accordingly, OncoCyte accounts for the convertible debt instrument in accordance with ASC 470-20, Debt with Conversion and Other Options. On the date the convertible debt was issued, the conversion price was equal to the fair value of the common stock underlying the conversion and no beneficial conversion feature was present based on the guidance of ASC 470-20 (see Note 5).
The issuance date fair value of the related party convertible debt is determined using a hybrid instrument valuation model by applying a market valuation method for the debt instrument using a risk-adjusted interest rate similar to “CC” and “D” rated junk bonds and, a Black-Sholes option pricing model for the conversion feature. The debt instrument and the conversion feature issuance date fair values are determined using level 3 inputs since both the risk adjustment made to the interest rate for the debt and the principal assumptions made in the option pricing model for the conversion feature are specific to OncoCyte and considered to be significant unobservable key inputs (see Note 5).
Due to the proximity of the May 8, 2015 issuance date and the September 30, 2015 balance sheet presented herein, the carrying value of the convertible debt approximates its fair value as of September 30, 2015 (unaudited) (see Note 5).
Cash and cash equivalents
Cash equivalents typically consist of highly liquid investments, with maturities of three months or less when purchased. At September 30, 2015 and December 31, 2014, OncoCyte’s cash balances totaled approximately $9.4 million (unaudited) and $257,000, respectively, and consist entirely of bank account deposits and amounts held in money market funds.
Financial instruments that potentially subject OncoCyte to credit risk consist principally of cash and cash equivalents. OncoCyte, at times, maintains cash and cash equivalents balances at financial institutions in excess of amounts insured by United States government agencies. OncoCyte places its cash and cash equivalents with high credit quality financial institutions.
Accounting for BioTime shares
OncoCyte accounts for the BioTime shares it holds as available-for-sale equity securities in accordance with ASC 320-10-25, Investments – Debt and Equity Securities, as the shares have a readily determinable fair value quoted on the NYSE MKT and are held principally for sale for future working capital purposes, as necessary. These shares are measured at fair value and reported as current assets on the balance sheet based on the closing trading price of the security as of the date being presented. Unrealized holding gains and losses are excluded from the statements of operations and reported in equity as part of other comprehensive income or loss, net of income taxes, until realized. Realized gains and losses for shares sold are reclassified out of accumulated other comprehensive income or loss and included in equity, as an increase or decrease to common stock equity consistent with, and pursuant to, ASC 805-50, transactions between entities under common control.
During the nine months ended September 30, 2015, OncoCyte sold 13,356 shares of BioTime stock in the open market for $44,000 (unaudited) in cash proceeds to be used for working capital purposes. The sale resulted in realized loss (unaudited) of $18,000, which was transferred out of accumulated other comprehensive loss and recorded as a decrease to common stock equity on the date of sale. During the nine months ended September 30, 2014, OncoCyte sold 86,156 shares of BioTime stock in the open market for $329,000 (unaudited) in cash proceeds to be used for working capital purposes. The sale resulted in a $73,000 realized loss (unaudited), which was reclassified out of accumulated other comprehensive loss and recorded as a decrease to common stock equity on the date of sale.
As of September 30, 2015, OncoCyte held 866,062 shares of BioTime common stock available-for-sale with a fair value of $2.6 million (unaudited).
Long-lived intangible assets
Long-lived intangible assets, primarily consisting of acquired patents, patent applications, and licenses to use certain patents are stated at acquired cost, less accumulated amortization (see Note 3). Amortization expense is computed using the straight-line method over the estimated useful lives of the assets over a period of 10 years.
Equipment and furniture
Equipment and furniture are stated at cost, less accumulated depreciation. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, generally over a period of 36 to 120 months. Maintenance and repairs are expensed as incurred whereas significant renewals and betterments are capitalized. When assets are retired or otherwise disposed of, the cost and the related accumulated depreciation are removed from the respective accounts and any resulting gain or loss is reflected in OncoCyte’s results of operations.
Impairment of long-lived assets
OncoCyte assesses the impairment of long-lived assets, which consist primarily of long-lived intangible assets, furniture and equipment, whenever events or changes in circumstances indicate that such assets might be impaired and the carrying value may not be recoverable. If events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable and the expected undiscounted future cash flows attributable to the asset are less than the carrying amount of the asset, an impairment loss equal to the excess of the asset’s carrying value over its fair value is recorded. To date, there have been no such impairment losses.
Income taxes
OncoCyte has filed a standalone U.S. federal income tax return since its inception. For California purposes, OncoCyte’s activity for 2013 and 2014 has been included in BioTime’s California Combined tax return. The provision for income taxes has been determined as if OncoCyte had filed separate tax returns for the periods presented. Accordingly, the effective tax rate of OncoCyte in future years could vary from its historical effective tax rates depending on the future legal structure of OncoCyte and related tax elections. The historical deferred tax assets, including the operating losses and credit carryforwards generated by OncoCyte, will remain with OncoCyte. OncoCyte accounts for income taxes in accordance with GAAP, which prescribes the use of the asset and liability method, whereby deferred tax asset or liability account balances are calculated at the balance sheet date using current tax laws and rates in effect. Valuation allowances are established when necessary to reduce deferred tax assets when it is more likely than not that a portion or all of the deferred tax assets will not be realized. OncoCyte’s judgments regarding future taxable income may change over time due to changes in market conditions, changes in tax laws, tax planning strategies or other factors. If OncoCyte's assumptions and consequently its estimates change in the future, the valuation allowance may be increased or decreased, which may have a material impact on OncoCyte’s statements of operations.
The guidance also prescribes a recognition threshold and a measurement attribute for the financial statement recognition and measurement of tax positions taken or expected to be taken in a tax return. For those benefits to be recognized, a tax position must be more-likely-than-not sustainable upon examination by taxing authorities. OncoCyte will recognize accrued interest and penalties related to unrecognized tax benefits as income tax expense. No amounts were accrued for the payment of interest and penalties as of September 30, 2015 (unaudited) and December 31, 2014. OncoCyte is not aware of any uncertain tax positions that could result in significant additional payments, accruals, or other material deviation for the nine months period ended September 30, 2015 and the year ended December 31, 2014. OncoCyte is currently unaware of any tax issues under review.
Research and development expenses
Research and development expenses consist primarily of personnel costs and related benefits, including stock-based compensation, and expenses for outside consultants. These expenses include both direct and allocated or indirect overhead costs (see Note 4). Research and development costs are expensed as incurred.
General and administrative expenses
OncoCyte’s general and administrative expenses relate primarily to compensation and related benefits, including stock-based compensation, for executive and corporate personnel, including direct costs and costs allocated by BioTime; professional and consulting fees; direct and indirect allocated overhead (see Note 4).
Stock-based compensation
OncoCyte recognizes compensation expense related to employee option grants and restricted stock grants, if any, in accordance with FASB ASC 718, Compensation – Stock Compensation (“ASC 718”).
OncoCyte estimates the fair value of employee stock-based payment awards on the grant-date and recognizes the resulting fair value, net of estimated forfeitures, over the requisite service period. OncoCyte uses the Black-Scholes option pricing model for estimating the fair value of options granted under OncoCyte’s Stock Option Plan. The fair value of each restricted stock grant, if any, is determined based on the value of the common stock granted or sold. OncoCyte has elected to treat stock-based payment awards with graded vesting schedules and time-based service conditions as a single award and recognizes stock-based compensation on a straight-line basis, net of estimated forfeitures, over the requisite service period.
Compensation expense for non-employee stock-based awards is recognized in accordance with ASC 718 and FASB ASC 505-50, Equity-Based Payments to Non-Employees (“ASC 505-50”). Stock option awards issued to non-employees, consisting principally employees of BioTime or BioTime commonly controlled and consolidated subsidiaries performing services for OncoCyte, are accounted for at fair value using the Black-Scholes option pricing model. Management believes that the fair value of the stock options is more reliably measured than the fair value of services received. OncoCyte records compensation expense based on the then-current fair values of the stock options at each financial reporting date. Compensation recorded during the service period is adjusted in subsequent periods for changes in the fair value of the stock options until the earlier of the date at which the non-employee’s performance is complete or a performance commitment is reached, which is generally when the stock option award vests. Compensation expense for non-employee grants is recorded on a straight-line basis in the statements of operations.
Net loss per common share
Basic net loss per common share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding for the period. Diluted net loss per common share reflects the weighted-average number of shares of common stock outstanding plus the potential effect of dilutive securities or contracts which are convertible to common stock, such as options and warrants (using the treasury stock method) and related party convertible debt (using the if converted method) and shares issuable in future periods, except in cases where the effect would be anti-dilutive. Because OncoCyte reported net losses for all periods presented, all potential contractual issuances of common stock are antidilutive for those periods.
The computations of basic and diluted net loss per common share are as follows (in thousands, except per share amounts):
| |
|
Nine Months Ended September 30,
(unaudited)
|
|
| |
|
2015
|
|
|
2014
|
|
|
Net loss
|
|
$
|
(5,196
|
) |
|
|
$
|
(3,479
|
) |
|
|
Weighted average common shares outstanding – basic and diluted
|
|
|
19,803
|
|
|
|
|
18,200
|
|
|
|
Net loss per common share – basic and diluted
|
|
$
|
(0.26
|
) |
|
|
$
|
(0.19
|
) |
|
The following common stock equivalents were excluded from the computation of diluted net loss per share of common stock for the periods presented because including them would have been antidilutive (in thousands):
| |
|
Nine Months Ended September 30,
(unaudited)
|
|
| |
|
2015
|
|
|
2014
|
|
|
Stock options granted and outstanding under Stock Option Plan
|
|
|
2,235
|
|
|
|
|
1,362
|
|
|
|
Related party convertible debt (if converted shares)
|
|
|
1,500
|
|
|
|
|
-
|
|
|
|
Contingently issuable warrants to shareholders
|
|
|
1,500
|
|
|
|
|
-
|
|
|
|
Total
|
|
|
5,235
|
|
|
|
|
1,362
|
|
|
Segments
OncoCyte’s executive management team, as a group, represents the entity’s chief operating decision makers. To date, OncoCyte’s executive management team has viewed OncoCyte’s operations as one segment that includes the research and development of diagnostic tests for the detection of cancer. As a result, the financial information disclosed materially represents all of the financial information related to OncoCyte’s sole operating segment.
Recent accounting pronouncements
There are no recently issued accounting standards which are not yet effective which OncoCyte believes would materially impact the financial statements.
3. Selected Balance Sheet Components
Prepaid expenses and other current assets
At September 30, 2015 and December 31, 2014, prepaid expenses and other current assets were comprised of the following (in thousands):
| |
|
September 30, 2015
(unaudited)
|
|
|
December 31,
2014
|
|
|
Prepaid license fees
|
|
$
|
20
|
|
|
|
$
|
111
|
|
|
|
Other prepaid expenses
|
|
|
-
|
|
|
|
|
3
|
|
|
|
Prepaid expenses and other current assets
|
|
$
|
20
|
|
|
|
$
|
114
|
|
|
Accrued expenses and other current liabilities
At September 30, 2015 and December 31, 2014, accrued expenses and other current liabilities were comprised of the following (in thousands):
| |
|
September 30,
2015
(unaudited)
|
|
|
December 31,
2014
|
|
|
Accrued bonuses and payroll related expenses
|
|
$
|
110
|
|
|
|
$
|
147
|
|
|
|
Other accrued expenses
|
|
|
309
|
|
|
|
|
135
|
|
|
|
Accrued expenses and other current liabilities
|
|
$
|
419
|
|
|
|
$
|
282
|
|
|
Intangible assets, net
In 2011, OncoCyte, thorough its parent, BioTime, acquired substantially all of the assets of Cell Targeting, Inc. (“CTI”), a company that was engaged in research in cancer therapy. The assets acquired consist primarily of patents, patent applications, and licenses to use certain patents. OncoCyte issued 261,959 shares of BioTime common stock to CTI with a market value of $2.3 million and paid CTI $250,000 in cash to acquire the assets. The asset purchase was accounted for as a business combination under the acquisition method of accounting. OncoCyte amortizes intangible assets over their useful lives estimated to be 10 years at the date of the acquisition.
At September 30, 2015 and December 31, 2014, intangible assets were comprised of the following (in thousands):
| |
|
September 30,
2015
(unaudited)
|
|
|
December 31,
2014
|
|
|
Intangible assets
|
|
$
|
2,419
|
|
|
|
$
|
2,419
|
|
|
|
Accumulated amortization
|
|
|
(1,129
|
) |
|
|
|
(947
|
) |
|
|
Intangible assets, net
|
|
$
|
1,290
|
|
|
|
$
|
1,472
|
|
|
Amortization expense amounted to approximately $181,000 (unaudited) for the nine months ended September 30, 2015 and 2014.
Equipment and furniture, net
At September 30, 2015 and December 31, 2014, equipment and furniture were comprised of the following (in thousands):
| |
|
September 30,
2015
(unaudited)
|
|
|
December 31,
2014
|
|
|
Equipment and furniture
|
|
$
|
261
|
|
|
|
$
|
251
|
|
|
|
Accumulated depreciation
|
|
|
(164
|
) |
|
|
|
(133
|
) |
|
|
Equipment and furniture, net
|
|
$
|
97
|
|
|
|
$
|
118
|
|
|
Depreciation expense amounted to approximately $32,000 (unaudited) and $29,000 (unaudited) for the nine months ended September 30, 2015 and 2014, respectively.
4. Related Party Transactions
Shared Facilities and Service Agreement
In aggregate, BioTime allocated and charged such Use Fees to OncoCyte approximating $379,000 and $242,000 included in general and administrative expenses, and $435,000 and $423,000 included in research and development expenses included in the statements of operations during the nine months ended September 30, 2015 and 2014, respectively.
As of September 30, 2015 and December 31, 2014, OncoCyte had $472,000 (unaudited) and $5.9 million outstanding and payable to BioTime and affiliates included in current liabilities in connection with the costs incurred under the Shared Facilities and Services Agreement (the “Shared Facilities Agreement”). Since these amounts are due and payable in 30 days of being invoiced, the payables are classified as current liabilities for all periods presented.
5. Related Party Convertible Promissory Note Payable
In May 2015, OncoCyte entered into Subscription Agreements with BioTime and two other existing shareholders (see Note 6). In connection with the Subscription Agreements, BioTime purchased 1,500,000 shares of OncoCyte common stock in exchange for the cancelation of $3.3 million of indebtedness owed to BioTime by OncoCyte, and OncoCyte delivered to BioTime a convertible promissory note (the “Note”) for an additional $3.3 million of OncoCyte’s indebtedness to BioTime. The cancelation of the aggregate $6.6 million of indebtedness owed to BioTime was an extinguishment of debt under the provisions of ASC 470-50, Debt Modification and Extinguishment. Based on a valuation performed by OncoCyte, the issuance date fair value of the Note was $3.3 million (see Note 2) and, the fair value of the OncoCyte common stock on the date of the exchange was $3.3 million. Accordingly, no gain or loss resulted from the debt extinguishment.
The Note has a stated interest rate of 1% per annum. Interest on the Note is due and payable on January 2 of each year until the maturity date and any accrued but unpaid interest, along with principal, is due on the maturity date. The Note is due and payable on November 30, 2016, if not converted sooner, and can be prepaid sooner only with the written consent of BioTime.
The Note is convertible into 1,500,000 shares of OncoCyte common stock at a conversion price of $2.20 per share (see Note 2). The conversion price is subject to pro rata adjustment in the event of a stock split, combination, reclassification, or similar event. BioTime, at its election, can convert the Note into common stock on or after the first to occur of (i) November 8, 2016, (ii) the date that is six months after the first closing date on which OncoCyte completes a firm commitment underwritten initial public offering of its common stock under the Securities Act of 1933, as amended, and (iii) the occurrence of an Event of Default, as defined in the Note. An Event of Default includes OncoCyte’s failure to pay any amount due on the Note or the commencement of bankruptcy proceedings by or against OncoCyte or the occurrence of certain insolvency related events, OncoCyte’s corporate dissolution or liquidation, or any material breach or default by OncoCyte under any loan agreement, promissory note, or other instrument evidencing indebtedness payable to a third party.
6. Shareholders’ Equity
Common Stock
During September 2015, OncoCyte entered into a Subscription Agreement with BioTime pursuant to which BioTime purchased 2,710,857 shares of OncoCyte common stock for $8.3 million in cash, as part of a subscription offer made to all OncoCyte shareholders on a pro rata basis. The other OncoCyte shareholders had the right to purchase a total of 974,447 shares of OncoCyte common stock at the same price paid by BioTime, until November 16, 2015, when the right expired unexercised.
In May 2015, OncoCyte entered into Subscription Agreements with Bio Time and two other existing shareholders (“Investors”). Under the Subscription Agreements, OncoCyte issued 1,500,000 shares of common stock to the Investors for $3.3 million cash, or $2.20 per share. Concurrently, BioTime purchased 1,500,000 shares of OncoCyte common stock in exchange for the cancelation of $3.3 million of indebtedness owed to BioTime by OncoCyte (see Note 5).
Contingently Issuable Common Stock Warrants
In June 2015, after the sale of stock under the Subscription Agreements was completed, OncoCyte and the Investors entered into a second agreement. Under the second agreement, the Investors agreed that if on or before June 30, 2016 OncoCyte conducts another rights offering to its shareholders at a pre-offer valuation of at least $40.0 million the Investors will purchase shares in that offering with an aggregate purchase price equal to the lesser of (a) a percentage of total amount of capital which OncoCyte then seeks to raise in the rights offer and in any concurrent offering to third parties equal to the Investors’ aggregate pro rata share of the outstanding OncoCyte common stock on the record date for the rights offering, determined on a fully diluted basis, and (b) $3.0 million, or such lesser amount requested by OncoCyte. Under the second agreement, OncoCyte agreed that if shares of OncoCyte common stock are not publicly traded on any stock exchange or over the counter market by January 15, 2016, OncoCyte will issue to the Investors, warrants to purchase, in the aggregate, 1,500,000 shares of OncoCyte common stock at an exercise price of $0.02 per share. If issued, the warrants will expire on December 31, 2016.
The Investors also agreed that, for a period of one year from the date of the second agreement, neither of them shall invest or engage, directly or indirectly, whether as a partner, equity holder, lender, principal, agent, affiliate, consultant or otherwise, in any business anywhere in the world that develops products for the diagnosis and treatment of cancer or otherwise competes with OncoCyte in any way; provided, however, that the passive ownership of less than 5% of the outstanding stock of any publicly-traded corporation will not be deemed, solely by reason thereof, to be in violation of that agreement.
For accounting purposes, these contingently issuable common stock warrants, under the second agreement described above, are considered to be issued as an equity classified instrument. OncoCyte estimated the issue date fair value of the warrants using a Black-Scholes valuation model and management believes that there is a low probability of not satisfying the contingency and having to issue the warrants. Accordingly, the probability-adjusted, fair value of the warrants was $65,400 (unaudited) on the issuance date and recognized as a general and administrative expense, with a corresponding increase to common stock equity, during the nine months ended September 30, 2015 (see Note 2). However, for purposes of determining the weighted-average number of shares of common stock outstanding to calculate basic net loss per share, the shares underlying the warrants are not considered issued and outstanding since, as of, and for the nine months ended September 30, 2015, not all conditions of actually issuing the warrants had been satisfied as required under ASC 260-10-45, Computation of Basic Earnings per Share, since the shares are contingently issuable shares as defined by ASC 260-10-45.
7. Stock-based Compensation
Options Granted
As of September 30, 2015, there were 1,762,000 shares available for grant under the Plan.
A summary of OncoCyte stock option activity under the Plan and related information follows (in thousands, except weighted average exercise price):
|
Options
|
|
Available for
Grant
|
|
|
Number of
Shares
|
|
|
Weighted
Average
Exercise
Price
|
|
|
Outstanding at January 1, 2015
|
|
|
638
|
|
|
|
|
1,362
|
|
|
|
$
|
1.52
|
|
|
|
Increase in option pool
|
|
|
2,000
|
|
|
|
|
-
|
|
|
|
|
-
|
|
|
|
Granted
|
|
|
(1,438
|
) |
|
|
|
1,438
|
|
|
|
|
2.20
|
|
|
|
Exercised
|
|
|
|
|
|
|
|
(3
|
) |
|
|
|
1.34
|
|
|
|
Forfeited or cancelled
|
|
|
562
|
|
|
|
|
(562
|
) |
|
|
|
1.58
|
|
|
|
Outstanding at September 30, 2015 (unaudited)
|
|
|
1,762
|
|
|
|
|
2,235
|
|
|
|
$
|
1.94
|
|
|
|
Exercisable at September 30, 2015 (unaudited)
|
|
|
|
|
|
|
|
952
|
|
|
|
$
|
1.60 |
|
|
A summary of unvested option activity under the Plan is presented below (in thousands, except weighted average grant-date fair value):
|
Options
|
|
Number of
Shares
|
|
|
Weighted-
Average Grant-
Date Fair
Value
|
|
|
Unvested at January 1, 2015
|
|
|
85
|
|
|
|
$
|
2.00
|
|
|
|
Granted
|
|
|
1,437
|
|
|
|
|
2.20
|
|
|
|
Forfeited
|
|
|
(160
|
) |
|
|
|
2.20
|
|
|
|
Vested
|
|
|
(79
|
) |
|
|
|
2.00
|
|
|
|
Unvested at September 30, 2015 (unaudited)
|
|
|
1,283
|
|
|
|
$
|
2.20
|
|
|
The weighted-average grant-date fair value of options granted during the nine months ended September 30, 2015 was $1.78 per share. There were 3,000 options exercised during the nine months ended September 30, 2015 for total proceeds of $4,000. There were no options granted or exercised during the nine months ended September 30, 2014.
As of September 30, 2015, there was $1.9 million of total unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the Plans. That cost is expected to be recognized over a weighted-average period of 3.4 years.
OncoCyte recorded stock-based compensation expense in the following categories in the accompanying statements of operations (in thousands):
| |
|
Nine Months Ended September 30,
(unaudited)
|
|
| |
|
2015
|
|
|
2014
|
|
|
Research and development
|
|
$
|
238
|
|
|
|
$
|
94
|
|
|
|
General and administrative
|
|
|
593
|
|
|
|
|
70
|
|
|
|
Total stock-based compensation expense
|
|
$
|
831
|
|
|
|
$
|
164
|
|
|
The Black-Scholes option pricing model requires OncoCyte to make certain assumptions including the fair value of the underlying common stock, the expected term, the expected volatility, the risk-free interest rate and the dividend yield.
The fair value of the shares of common stock underlying the stock options has historically been determined by the Board of Directors. Because there has been no public market for OncoCyte’s common stock, the Board of Directors has determined the fair value of the common stock at the time of the grant of options by considering a number of objective and subjective factors including contemporaneous sales of common stock to investors, valuation of comparable companies, operating and financial performance and general and industry-specific economic outlook, among other factors. The fair value of the underlying common stock will be determined by the Board of Directors until such time as OncoCyte’s common stock is traded on an established stock exchange or national market system. The fair value was determined in accordance with applicable elements of the practice aid issued by the American Institute of Certified Public Accountants titled Valuation of Privately Held Company Equity Securities Issued As Compensation.
The expected term of employee stock options represents the weighted-average period that the stock options are expected to remain outstanding. OncoCyte estimates the expected term of options granted based upon the “simplified method” provided under Staff Accounting Bulletin, Topic 14, or SAB Topic 14.
Because OncoCyte’s common stock has no publicly traded history, OncoCyte estimates the expected volatility of the awards from the historical volatility of selected public companies within the biotechnology industry with comparable characteristics to OncoCyte, including similarity in size, lines of business, market capitalization, revenue and financial leverage. OncoCyte determined the expected volatility assumption using the frequency of daily historical prices of comparable public company’s common stock for a period equal to the expected term of the options. OncoCyte periodically assesses the comparable companies and other relevant factors used to measure expected volatility for future stock option grants.
The risk-free interest rate assumption is based upon observed interest rates on the United States government securities appropriate for the expected term of OncoCyte’s stock options.
The dividend yield assumption is based on OncoCyte’s history and expectation of dividend payouts. OncoCyte has never declared or paid any cash dividends on its common stock, and OncoCyte does not anticipate paying any cash dividends in the foreseeable future.
The assumptions that were used to calculate the grant date fair value of OncoCyte’s employee and non-employee stock option grants for the nine months ended September 30, 2015 and 2014 were as follows.
| |
|
Nine Months Ended September
30,
(unaudited)
|
|
| |
|
2015
|
|
|
2014(1)
|
|
|
Expected life (in years)
|
|
|
6.89
|
|
|
|
|
-
|
|
|
|
Risk-free interest rates
|
|
|
1.81
|
% |
|
|
|
-
|
% |
|
|
Volatility
|
|
|
74.25
|
% |
|
|
|
-
|
% |
|
|
Dividend yield
|
|
|
0.00
|
% |
|
|
|
-
|
% |
|
| |
(1)
|
No stock options were granted in 2014.
|
Stock-based compensation expense is recognized based on awards that are ultimately expected to vest, and as a result, the amount has been reduced by estimated forfeitures. Forfeitures are estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Forfeitures are estimated based on OncoCyte’s historical experience and future expectations.
The determination of stock-based compensation is inherently uncertain and subjective and involves the application of valuation models and assumptions requiring the use of judgment. If OncoCyte had made different assumptions, its stock-based compensation expense, and net loss for the nine months ended September 30, 2015 and 2014, may have been significantly different.
There was no net income tax benefit recognized in the statements of operations for stock-based compensation expense for non-qualified stock options as OncoCyte fully offsets net deferred tax assets with a valuation allowance (see Note 8). In addition, OncoCyte does not recognize deferred income taxes for incentive stock option compensation expense, and records a tax deduction only when a disqualified disposition has occurred.
8. Income Taxes
OncoCyte has filed a standalone U.S. federal income tax return since its inception. For California purposes, OncoCyte’s activity for 2013 and 2014 has been included in BioTime’s California Combined tax return. The provision for income taxes has been determined as if OncoCyte had filed separate tax returns for the periods presented. Accordingly, the effective tax rate of OncoCyte in future years could vary from its historical effective tax rates depending on the future legal structure of OncoCyte and related tax elections. The deferred tax assets, including the operating loss and credit carryforwards, generated by OncoCyte, will remain with OncoCyte.
The primary components of the net deferred tax assets and liabilities at September 30, 2015 and December 31, 2014 were as follows (in thousands):
| |
|
September 30,
2015
(unaudited)
|
|
|
December 31,
2014
|
|
|
Current deferred tax assets (liabilities):
|
|
|
|
|
|
|
|
|
|
Available-for-sale securities
|
|
$
|
(883
|
) |
|
|
$
|
(1,115
|
) |
|
|
Total current deferred tax liabilities
|
|
$
|
(883
|
) |
|
|
$
|
(1,115
|
) |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Non-current deferred tax assets (liabilities):
|
|
|
|
|
|
|
|
|
|
|
|
Net operating loss carryforwards
|
|
$
|
6,290
|
|
|
|
$
|
4,879
|
|
|
|
Research and development credit carryforwards
|
|
|
1,140
|
|
|
|
|
1,030
|
|
|
|
Patent and fixed assets
|
|
|
176
|
|
|
|
|
141
|
|
|
|
Stock-based compensation
|
|
|
476
|
|
|
|
|
24
|
|
|
|
Valuation allowance
|
|
|
(7,199
|
) |
|
|
|
(4,959
|
) |
|
|
Total non-current deferred tax assets
|
|
$
|
883
|
|
|
|
$
|
1,115
|
|
|
Income taxes differed from the amounts computed by applying the U.S. federal income tax of 34% to pretax losses from operations as a result of the following:
| |
|
Nine Months Ended September 30,
|
|
| |
|
2015
|
|
|
2014
|
|
|
Computed tax benefit at federal statutory rate
|
|
|
34
|
% |
|
|
|
34
|
% |
|
|
Permanent differences
|
|
|
2
|
% |
|
|
|
(5
|
%) |
|
|
Change in valuation allowance
|
|
|
(36
|
%) |
|
|
|
(31
|
%) |
|
|
Research and development credits
|
|
|
0
|
% |
|
|
|
2
|
% |
|
| |
|
|
-
|
|
|
|
|
-
|
|
|
As of September 30, 2015, OncoCyte has net operating loss carryforwards of approximately $18.1 million for federal tax purposes, which expire through 2035. As of December 31, 2014, OncoCyte has net operating loss carryforwards of approximately $13.9 million for federal tax purposes, which expire through 2034. OncoCyte’s federal research and development credit carryforwards of $502,000 as of September 30, 2015 and December 31, 2014 will expire through 2034. Congress has not reinstated the research and development credit for federal purpose for 2015, so the federal research and development credit is unchanged from December 31, 2014. The state research and development credit carryforwards of $638,000 as of September 30, 2015 and $528,000 as of December 31, 2014 do not expire.
During 2014, OncoCyte sold 406,756 BioTime common shares in open market transactions which resulted in a taxable gain of approximately $1.3 million. This taxable gain was fully offset by current operating losses, thus resulting in no income taxes due from the sale. During the first nine months of 2015, OncoCyte sold 13,356 BioTime common shares in open market transactions which resulted in a taxable gain of approximately $44,000. This taxable gain expected to be fully offset by current operating losses, thus resulting in no income taxes due from the sale. OncoCyte has recorded a deferred tax liability of $0.9 million and $1.1 million at September 30, 2015 and December 31, 2014, respectively, resulting from the difference in the tax basis of BioTime shares held by OncoCyte as compared to the basis of such shares reported on OncoCyte’s balance sheet.
A valuation allowance is provided when it is more likely than not that some portion of the deferred tax assets will not be realized. OncoCyte established a full valuation allowance for all periods presented due to the uncertainty of realizing future tax benefits from its net operating loss carryforwards and other deferred tax assets.
Internal Revenue Code Section 382 places a limitation (“Section 382 Limitation”) on the amount of taxable income that can be offset by net operating loss (“NOL”) carryforwards after a change in control (generally greater than 50% change in ownership within a three-year period) of a loss corporation. California has similar rules. Generally, after a control change, a loss corporation cannot deduct NOL carryforwards in excess of the Section 382 Limitation. Due to these “change in ownership” provisions, utilization of the NOL and tax credit carryforwards may be subject to an annual limitation regarding their utilization against taxable income in future periods. There has not been a change in ownership for any of the periods presented.
OncoCyte may be subject to potential income tax examination by U.S. federal or U.S. states’ authorities. These potential examinations may include inquiries regarding the timing and amount of deductions, and compliance with U.S. federal and state tax laws. In general, OncoCyte is no longer subject to tax examination by major taxing authorities for years before 2010. Although the statute is closed for purposes of assessing additional income and tax in those years, the taxing authorities may still make adjustments to the net operating loss and credit carryforwards used in open years. Therefore the statute should be considered open as it relates to the net operating loss and credit carryforwards. Any potential examinations may include inquiries regarding the timing and amount of deductions, and compliance with U.S. federal and state tax laws.
9. Commitments and Contingencies
OncoCyte had no commitments other than those under the Shared Facilities Agreement described in Note 4. The minimum fixed payments due under the Shared Facilities Agreement are approximately $13,000 per month.
Tax Filings
OncoCyte tax filings are subject to audit by taxing authorities in jurisdictions where it conducts business. These audits may result in assessments of additional taxes that are subsequently resolved with the authorities or potentially through the courts. Management believes OncoCyte has adequately provided for any ultimate amounts that are likely to result from these audits; however, final assessments, if any, could be significantly different than the amounts recorded in the financial statements.
Litigation – General
OncoCyte is subject to various claims and contingencies in the ordinary course of its business, including those related to litigation, business transactions, employee-related matters, and others. When OncoCyte is aware of a claim or potential claim, it assesses the likelihood of any loss or exposure. If it is probable that a loss will result and the amount of the loss can be reasonably estimated, OncoCyte will record a liability for the loss. If the loss is not probable or the amount of the loss cannot be reasonably estimated, OncoCyte discloses the claim if the likelihood of a potential loss is reasonably possible and the amount involved could be material. OncoCyte is not aware of any claims likely to have a material adverse effect on its financial condition or results of operations.
Employment Contracts
In August 2015, OncoCyte entered into a new employment agreement with its Vice President of Marketing, under which the executive will receive an annual salary of $200,000 and 100,000 options to purchase OncoCyte common shares, 25% of which shall vest one year from the date of grant, and the balance in equal 36 monthly installments. The stock options have an exercise price of $3.06 per share and expire 10 years from the date of grant. Under the employment agreement, the executive is eligible to earn an annual cash incentive bonus award determined by the Board of Directors in respect of each calendar year during the executive’s employment, with a target bonus equal to no less than 30% of base salary for achievement of the specified performance goals at target levels for the applicable calendar year. The annual bonus payable is based upon the level of achievement of objectively determinable company and individual performance goals for the applicable calendar year, as determined by the Board of Directors in consultation with the executive.
In June 2015, OncoCyte entered into a new employment agreement with its Chief Executive Officer, under which the executive will receive an annual salary of $320,000, 605,000 options to purchase OncoCyte common shares, 25% of which shall vest one year from the date of grant, and the balance in equal 36 monthly installments, and an additional 5,000 options that were fully vested on the date of grant. All of the stock options have an exercise price of $2.20 per share and expire 10 years from the date of grant. Under the employment agreement, the executive is eligible to earn an annual cash incentive bonus award determined by the Board of Directors in respect of each fiscal year during the executive’s employment, with a target bonus equal to no less than 35% of base salary for achievement of the specified performance goals at target levels for the applicable calendar year. The annual bonus payable is based upon the level of achievement of objectively determinable company and individual performance goals for the applicable calendar year, as determined by the Board of Directors in consultation with the executive. If the specified performance goals for the applicable calendar year are achieved at maximum levels, the annual bonus payable can be up to 150% of the annual base salary, as determined by the Board of Directors, in its sole discretion.
OncoCyte has entered into employment contracts with certain executive officers. Under the provisions of the contracts, OncoCyte may be required to incur severance obligations for matters relating to changes in control, as defined, and involuntary terminations. At September 30, 2015, total potential severance obligations in connection with the termination of employment contracts approximated $303,000 for termination without cause and $397,000 for termination due to a change in control.
Indemnification
In the normal course of business, OncoCyte may provide indemnifications of varying scope under OncoCyte’s agreements with other companies or consultants, typically OncoCyte’s clinical research organizations, investigators, clinical sites, suppliers and others. Pursuant to these agreements, OncoCyte will generally agree to indemnify, hold harmless, and reimburse the indemnified parties for losses and expenses suffered or incurred by the indemnified parties arising from claims of third parties in connection with the use or testing of OncoCyte’s diagnostic tests. Indemnification provisions could also cover third party infringement claims with respect to patent rights, copyrights, or other intellectual property pertaining to OncoCyte’s diagnostic tests. The term of these indemnification agreements will generally continue in effect after the termination or expiration of the particular research, development, services, or license agreement to which they relate. The potential future payments OncoCyte could be required to make under these indemnification agreements will generally not be subject to any specified maximum amount. Historically, OncoCyte have not been subject to any claims or demands for indemnification. OncoCyte also maintains various liability insurance policies that limit OncoCyte’s exposure. As a result, OncoCyte believes the fair value of these indemnification agreements is minimal. Accordingly, OncoCyte has not recorded any liabilities for these agreements as of September 30, 2015 and December 31, 2014.
10. Subsequent Events
On October 1, 2015, OncoCyte sold 246,356 shares of BioTime stock to certain institutional investors under a negotiated transaction for gross cash proceeds of approximately $771,000.
On November 18, 2015, OncoCyte effected a one-for-two reverse stock split of its common stock and a proportional adjustment to the conversion ratio for the related party convertible debt and conversion prices.
On November 19, 2015, the Note described in Note 5 was amended to permit BioTime to convert the Note into shares of OncoCyte common stock at any time prior to payment in full of the principal balance and accrued interest, and on November 20, 2015 BioTime converted the Note into 1,508,095 shares of OncoCyte common stock.
OncoCyte has evaluated subsequent events and transactions for potential recognition or disclosure in the financial statements through December 21, 2015, the day the financial statements were available for issuance.