Use these links to rapidly review the document
Prospectus
TABLE OF CONTENTS
Filed Pursuant to Rule 424(b)(5)
Registration No. 333-214197
PROSPECTUS SUPPLEMENT
(To prospectus dated November 9, 2016)

Nabriva Therapeutics AG
Rights Offering for up to 588,127 Common Shares
Including Common Shares Represented by American Depositary Shares
Offering of American Depositary Shares Representing Common Shares
Not Subscribed for in the Rights Offering
We are offering to our common shareholders rights to subscribe for new common shares and, through The Bank of New York Mellon, our depositary and the ADS rights agent, are offering to holders of American Depositary Shares, or ADSs, non-transferable rights to subscribe for new ADSs pursuant to a rights offering. Except to the extent otherwise provided under Austrian law, the common share rights will be non-transferable. We expect to issue up to 588,127 common shares in this offering, including common shares represented by ADSs. Each ADS represents one tenth (1/10) of a common share, nominal value €1.00 per share.
Offering to Holders of ADSs
Holders of ADSs will receive 0.276 ADS rights for each ADS owned of record on November 29, 2016. One ADS right will entitle an ADS holder to subscribe for and purchase one new ADS at the U.S. dollar equivalent of €4.014 per ADS. Based on a euro-to-U.S. dollar exchange rate of €1.00 to $1.0588, we estimate that subscription price at $4.25 per ADS. To subscribe for new ADSs, a holder of ADS rights must pay to The Bank of New York Mellon $4.68 per ADS so subscribed, which represents 110% of the estimated subscription price to account for currency conversion expense, ADS issuance fees payable to the depositary and potential fluctuations in the exchange rate between the euro and the U.S. dollar. On or about December 13, 2016, the ADS rights agent will determine the actual U.S. dollar ADS subscription price by converting the euro subscription price into U.S. dollars at an exchange rate assigned by it on that date. Fractional ADS rights will not be issued, and ADS right entitlements will be reduced to the next smaller whole number of ADS rights. The ADS rights will expire at 5:00 p.m. (New York City time) on December 12, 2016. See "Description of the Offering—Offering to Holders of ADSs."
Offering to Holders of Common Shares
Holders of common shares will have the common share right to subscribe for and purchase 0.276 new common shares, at a subscription price of €40.14 per new common share, for each common share owned of record on November 29, 2016, which we refer to as the common share subscription ratio. No fractional common shares will be issued and common share right entitlements will be reduced to the next smaller whole number of common shares. Rights to subscribe for new common shares will expire at 5:00 p.m. (Vienna time) on December 14, 2016. See "Description of the Offering—Offering to Holders of Common Shares."
Potential Offering of ADSs Representing Unsubscribed Common Shares
If any new common shares are not subscribed for pursuant to the common share rights and ADS rights described above, following the expiration of the offering of such common share rights and ADS rights, we may, at the discretion of Cantor Fitzgerald & Co., based on market conditions and demand, enter into an underwriting agreement pursuant to which Cantor Fitzgerald & Co. would agree to subscribe for and purchase up to all of the unsubscribed common shares at a purchase price of €40.14 per common share for purposes of resale of ADSs representing the unsubscribed common shares. However, entry into an underwriting agreement for all or a portion of the unsubscribed shares remains at the discretion of Cantor Fitzgerald & Co. and Cantor Fitzgerald & Co. is not obligated to purchase all or any of the unsubscribed common shares. In the event that Cantor Fitzgerald & Co. and we do enter into an underwriting agreement for unsubscribed shares, Cantor Fitzgerald & Co. may sell ADSs at variable prices, which may be more or less than the purchase price.
In addition, certain of our existing principal shareholders and their affiliated entities, holding approximately 39.7% of our outstanding share capital, have indicated an interest in purchasing up to an aggregate of approximately $17.5 million of the securities we are offering. However, indications of interest are not binding agreements or commitments to subscribe and purchase. These shareholders may determine to subscribe for and purchase fewer common shares and/or ADSs than they indicate an interest in subscribing for and purchasing or not to subscribe for any common shares and/or ADSs in the rights offering or in the potential offering of ADSs representing any unsubscribed common shares. In addition, because these investors are only entitled to subscribe for and purchase their pro rata portion of any new common shares or ADSs offered as part of the offerings of ADS rights and common share rights, any purchases above this pro rata amount would occur through the potential offering of ADSs representing any unsubscribed shares. Following the expiration of the offering of ADS rights and common share rights, Cantor Fitzgerald & Co. may determine not to proceed with the potential offering of ADSs representing unsubscribed common shares or may determine to sell fewer ADSs representing unsubscribed common shares to any of these shareholders or not to sell any such ADSs to these shareholders.
We estimate that we will receive gross proceeds from this offering of approximately $25.0 million, assuming the (i) full exercise of subscription rights for the common shares and ADSs offered in this offering and (ii) issuance and sale of an aggregate of 588,127 common shares, including the sale of 114,658 common shares at the subscription price of €40.14 per new common share and 4,734,690 ADSs, representing 473,469 common shares, at the estimated subscription price of $4.25 per new ADS, assuming a euro-to-U.S. dollar exchange rate of €1.00 to $1.0588. In consideration for services provided to us in connection with the offering of ADS rights and common share rights, we have agreed to pay Cantor Fitzgerald & Co. a financial advisory fee equal to 8.0% of the U.S. dollar gross proceeds raised from this offering (including proceeds from the sale of ADSs in the potential offering of ADSs representing unsubscribed common shares). We expect that our aggregate expenses in connection with this offering, including estimated fees and offering expenses payable by us, will be approximately $4.1 million. As a result, we estimate that the net proceeds to us from this offering, based on the assumptions above, would be approximately $20.9 million. See "Use of Proceeds."
The ADSs are listed on the NASDAQ Global Market under the symbol "NBRV." On November 28, 2016, the last reported sale price of the ADSs on the NASDAQ Global Market was $4.25 per ADS. Our common shares are not listed on any national securities market or exchange. The ADS rights and, except to the extent otherwise provided under Austrian law, common share rights will not be transferable and will not be listed on any national securities market or exchange.
For information regarding this offering, please contact Georgeson LLC, the information agent, toll free at (866) 278-8941 or by e-mail at Nabriva@georgeson.com from 9:00 a.m. to 9:00 p.m., New York City time, Monday to Friday.
We are an "emerging growth company" as defined by the Jumpstart Our Business Startups Act of 2012 and, as such, we have elected to rely on certain reduced public company disclosure requirements.
Investing in the common shares or ADSs involves a high degree of risk. Please read "Risk Factors" beginning on page S-12.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
Delivery of the common shares and ADSs is expected to be made on or about December 19, 2016.
Cantor Fitzgerald & Co.
November 29, 2016
TABLE OF CONTENTS
Prospectus Supplement
S-i
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is in two parts. The first part is this prospectus supplement, which describes the specific terms of this offering and also adds to and updates information contained in the accompanying prospectus and the documents incorporated by reference herein. The second part, the accompanying prospectus, provides more general information. Generally, when we refer to this prospectus, we are referring to both parts of this document combined. To the extent there is a conflict between the information contained in this prospectus supplement and the information contained in the accompanying prospectus or any document incorporated by reference therein filed prior to the date of this prospectus supplement, you should rely on the information in this prospectus supplement; provided that if any statement in one of these documents is inconsistent with a statement in another document having a later date—for example, a document incorporated by reference in the accompanying prospectus—the statement in the document having the later date modifies or supersedes the earlier statement.
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to any document that is incorporated by reference herein were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
We have not and Cantor Fitzgerald & Co. has not authorized anyone to provide any information other than that contained or incorporated by reference in this prospectus supplement, in the accompanying prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. We and Cantor Fitzgerald & Co. take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you.
This prospectus supplement and the accompanying prospectus do not constitute an offer to sell, or a solicitation of an offer to purchase, the securities offered by this prospectus supplement and the accompanying prospectus in any jurisdiction to or from any person to whom or from whom it is unlawful to make such offer or solicitation of an offer in such jurisdiction. You should assume that the information appearing in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and in any free writing prospectus that we have authorized for use in connection with this offering is accurate only as of the date of those respective documents. It is important for you to read and consider all of the information contained in this prospectus supplement and in the accompanying prospectus, including the documents incorporated by reference herein and therein, in making your investment decision. You should also read and consider the information in the documents to which we have referred you in the sections entitled "Where You Can Find Additional Information" and "Incorporation by Reference" in this prospectus supplement and in the accompanying prospectus.
Other than in the United States, no action has been taken by us or Cantor Fitzgerald & Co. that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
S-ii
Unless the context specifically indicates otherwise, references in this prospectus supplement to "Nabriva Therapeutics AG," "Nabriva," "we," "our," "ours," "us," "our company" or similar terms refer to Nabriva Therapeutics AG together with its subsidiaries. The trademarks, trade names and service marks appearing in this prospectus are the property of their respective owners.
We present our consolidated financial statements in euros. All references in this prospectus to "$" are to U.S. dollars and all references to "€" are to euros. Solely for convenience and unless otherwise indicated, certain euro amounts have been translated into U.S. dollars at the rate of €1.00 to $1.1161, the exchange rate at the European Central Bank on September 30, 2016. These translations should not be considered representations that any such amounts have been, could have been or could be converted into U.S. dollars at that or any other exchange rate as of that or any other date.
S-iii
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein contain forward-looking statements that involve substantial risks and uncertainties. All statements contained in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein, other than statements of historical fact, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "predict," "project," "target," "potential," "will," "would," "could," "should," "continue," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The forward-looking statements in this prospectus supplement include, among other things, statements about:
- •
- the timing and conduct of our clinical trials of our lead product candidate, lefamulin, including statements regarding the timing and
completion of the trials, and the period during which the results of the trials will become available;
- •
- our expectations regarding how far into the future our cash on hand, together with the net proceeds from this offering, will fund our ongoing
operations;
- •
- our expectation to reach 60% enrollment for our first Phase 3 clinical trial of lefamulin for community-acquired bacterial pneumonia, or
CABP, by the end of 2016 and complete an interim data analysis in early 2017;
- •
- the timing of and our ability to submit applications for, obtain and maintain marketing approval of lefamulin;
- •
- the potential receipt of revenues from future sales of lefamulin;
- •
- our plans to pursue development of lefamulin for additional indications other than CABP;
- •
- our plans to pursue research and development of other product candidates;
- •
- our ability to establish and maintain arrangements for manufacture of our product candidates;
- •
- our sales, marketing and distribution capabilities and strategy;
- •
- our ability to successfully commercialize lefamulin and our other product candidates;
- •
- the potential advantages of lefamulin and our other product candidates;
- •
- our estimates regarding the market opportunities for lefamulin and our other product candidates;
- •
- the rate and degree of market acceptance and clinical benefit of lefamulin and our other product candidates;
- •
- our ability to establish and maintain collaborations;
- •
- our ability to acquire or in-license additional products, product candidates and technologies;
- •
- our current belief that it is more likely than in past years that as of December 31, 2016 we will be classified as a passive foreign
investment company, or PFIC, and our current intention to make available the information necessary to permit a U.S. holder to make a valid "QEF election";
- •
- our loss of "foreign private issuer" status as of January 1, 2017;
- •
- our future intellectual property position;
S-iv
- •
- our estimates regarding future expenses, capital requirements and needs for additional financing;
- •
- our ability to effectively manage our anticipated growth;
- •
- our ability to attract and retain qualified employees and key personnel;
- •
- our expected use of proceeds from this offering; and
- •
- other risks and uncertainties, including those described in the "Risk Factors" section of this prospectus supplement.
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this prospectus supplement, the accompanying prospectus and the information incorporated by reference herein and therein, particularly in the "Risk Factors" section of this prospectus supplement, that we believe could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
This prospectus supplement, the accompanying prospectus and the information incorporated by reference herein and therein include statistical and other industry and market data that we obtained from industry publications and research, surveys and studies conducted by third parties. Industry publications and third-party research, surveys and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information.
S-v
This summary highlights selected information contained elsewhere in this prospectus supplement, the accompanying prospectus and in the documents we incorporate by reference herein and therein. This summary does not contain all of the information you should consider before buying the ADSs or our common shares. You should read the entire prospectus supplement and the accompanying prospectus carefully, especially the risks described in "Risk Factors," in this prospectus supplement, along with our financial statements and the related notes and the other information incorporated by reference in this prospectus supplement and the accompanying prospectus, before deciding to invest in the ADSs or our common shares.
Our Company
We are a clinical stage biopharmaceutical company engaged in the research and development of novel anti-infective agents to treat serious infections, with a focus on the pleuromutilin class of antibiotics. We are developing our lead product candidate, lefamulin, to be the first pleuromutilin antibiotic available for systemic administration in humans. We are developing both intravenous, or IV, and oral formulations of lefamulin for the treatment of community-acquired bacterial pneumonia, or CABP, and intend to develop lefamulin for additional indications other than pneumonia. We have completed a Phase 2 clinical trial of lefamulin for acute bacterial skin and skin structure infections, or ABSSSI. Based on the clinical results of lefamulin for ABSSSI, as well as its rapid tissue distribution, including substantial penetration into lung tissue and fluids, we initiated two international, pivotal Phase 3 clinical trials of lefamulin for the treatment of moderate to severe CABP.
We initiated the first of these trials in September 2015 and initiated the second trial in April 2016. These are the first clinical trials we have conducted with lefamulin for the treatment of CABP. Both trials are designed to follow draft guidance published by the U.S. Food and Drug Administration, or FDA, for the development of drugs for CABP and guidance from the European Medicines Agency, or EMA, for the development of antibacterial agents. Based on our estimates regarding patient enrollment, we expect to have top-line data available for both trials in the second half of 2017. If the results of these trials are favorable, including achievement of the primary efficacy endpoints of the trials, we expect to submit applications for marketing approval for lefamulin for the treatment of CABP in both the United States and Europe in 2018. We believe that lefamulin is well suited for use as a first-line empiric monotherapy for the treatment of CABP because of its novel mechanism of action, spectrum of activity, including against multi-drug resistant pathogens, achievement of substantial drug concentrations in lung tissue and fluids, availability as both an IV and oral formulation and favorable safety and tolerability profile.
The FDA has designated each of the IV and oral formulations of lefamulin as a qualified infectious disease product, or QIDP, which provides for the extension of statutory exclusivity periods in the United States for an additional five years upon FDA approval of the product for the treatment of CABP, and granted fast track designation to these formulations of lefamulin. Fast track designation is granted by the FDA to facilitate the development and expedite the review of drugs that treat serious conditions and fill an unmet medical need. The fast track designation for the IV and oral formulations of lefamulin will allow for more frequent interactions with the FDA, the opportunity for a rolling review of any new drug application, or NDA, we submit and eligibility for priority review and a shortening of the FDA's goal for taking action on a marketing application from ten months to six months.
We believe that pleuromutilin antibiotics can help address the major public health threat posed by bacterial resistance, which the World Health Organization, or WHO, characterized in 2010 as one of the three greatest threats to human health. Increasing resistance to antibiotics used to treat CABP is a growing concern and has become an issue in selecting the appropriate initial antibiotic treatment prior
S-1
to determining the specific microbiological cause of the infection, referred to as empiric treatment. For example, the U.S. Centers for Disease Control and Prevention, or CDC, has classified Streptococcus pneumoniae, the most common respiratory pathogen, as a serious threat to human health as a result of increasing resistance to currently available antibiotics. In addition, the CDC recently reported on the growing evidence of widespread resistance to macrolides, widely used antibiotics that disrupt bacterial protein synthesis, in Mycoplasma pneumoniae, a common cause of CABP that is associated with significant morbidity and mortality. Furthermore, Staphylococcus aureus, including methicillin-resistant S. aureus, or MRSA, which has also been designated as a serious threat to human health by the CDC, has emerged as a more common cause of CABP in some regions of the world, and a possible pathogen to be covered with empiric therapy. In recognition of the growing need for the development of new antibiotics, recent regulatory changes, including priority review and regulatory guidance enabling smaller clinical trials, have led to renewed interest from the pharmaceutical industry in anti-infective development. For example, the Food and Drug Administration Safety and Innovation Act became law in 2012 and included the Generating Antibiotic Incentives Now Act, or the GAIN Act, which provides incentives, including access to expedited FDA review for approval, fast track designation and five years of potential data exclusivity extension for the development of new QIDPs.
As a result of increasing resistance to antibiotics and the wide array of potential pathogens that cause CABP, the current standard of care for hospitalized patients with CABP usually involves first-line empiric treatment with a combination of antibiotics to address all likely bacterial pathogens or monotherapy with a fluoroquinolone antibiotic. Combination therapy presents the logistical challenge of administering multiple drugs with different dosing regimens and increases the risk of drug-drug interactions and the potential for serious side effects. Fluoroquinolones are associated with safety and tolerability concerns and are typically administered in combination with other antibiotics if community-acquired MRSA is suspected. In addition, many currently available antibiotic therapies are only available for IV administration and are prescribed for seven to 14 days, meaning continued treatment requires prolonged hospitalization or a switch to a different antibiotic administered orally, with the attendant risk that the patient might respond differently.
Pleuromutilins are semi-synthetic compounds derived from a naturally occurring antibiotic and inhibit bacterial growth by binding to a specific site on the bacterial ribosome that is responsible for bacterial protein synthesis. We have developed an understanding of how to optimize characteristics of the pleuromutilin class, such as antimicrobial spectrum, potency, absorption following oral administration and tolerability, which in turn led to our selection and development of lefamulin, our lead product candidate. We have completed a Phase 2 clinical trial for ABSSSI in which the IV formulation of lefamulin achieved a high cure rate against multi-drug resistant Gram-positive bacteria, including MRSA. In addition, in preclinical studies, lefamulin showed potent antibacterial activity against a variety of Gram-positive bacteria, Gram-negative bacteria and atypical bacteria, including multi-drug resistant strains. The preclinical studies and clinical trials we have conducted to date suggest that lefamulin's novel mechanism of action is responsible for the lack of cross resistance observed with other antibiotic classes and may result in slow development of bacterial resistance to lefamulin over time. As a result of the favorable safety and tolerability profile we have observed in our clinical trials to date, we believe lefamulin has the potential to present fewer complications relative to the use of current therapies. Based on our research, we also believe that the availability of both IV and oral formulations of lefamulin, and an option to switch to oral treatment, could reduce the length of a patient's hospital stay and the overall cost of care.
We have evaluated lefamulin in more than 440 patients and subjects in seventeen completed Phase 1 clinical trials and a Phase 2 clinical trial in ABSSSI. In our Phase 1 clinical trials, we have characterized the clinical pharmacology of the IV formulation of lefamulin and shown oral bioavailability of a tablet formulation of lefamulin with rapid tissue distribution, including substantial penetration into lung tissue and fluids. In our Phase 2 clinical trial evaluating the safety and efficacy of
S-2
two different doses of the IV formulation of lefamulin administered over five to 14 days compared to the antibiotic vancomycin in patients with ABSSSI, the clinical success rate at test of cure, or TOC, for lefamulin was similar to that of vancomycin. Lefamulin has been well tolerated in all our clinical trials to date when administered by IV and oral routes. The frequency of adverse events that we observed in our Phase 2 clinical trial in ABSSSI was similar for patients treated with IV lefamulin and patients treated with vancomycin.
Based on the clinical results of lefamulin for the treatment of ABSSSI, as well as its rapid tissue distribution, including substantial penetration into lung tissue and fluids, we are evaluating lefamulin for the treatment of moderate to severe CABP in two international Phase 3 clinical trials. We are initially pursuing the development of lefamulin for CABP because of the limited development of new antibiotic classes for this indication over the past 15 years, our belief that there exists a significant unmet medical need for a first-line empiric monotherapy that addresses the growing development and spread of bacterial resistance, as well as recently clarified FDA guidance regarding the approval pathway. We initiated the first of these trials in September 2015 and the second trial in April 2016. We are also characterizing the clinical pharmacology of lefamulin in several additional Phase 1 clinical trials.
We plan to pursue a number of additional opportunities for lefamulin, including a development program for use in pediatric patients and potentially for the treatment of ABSSSI. In addition, as an antibiotic with potent activity against a wide variety of multi-drug resistant pathogens, including MRSA, we plan to explore development of lefamulin in other indications, including ventilator-associated bacterial pneumonia, or VABP, hospital-acquired bacterial pneumonia, or HABP, sexually transmitted infections, or STIs, osteomyelitis and prosthetic joint infections. Through our research and development efforts, we have also identified a topical pleuromutilin product candidate, BC-7013, which has completed a Phase 1 clinical trial.
We own exclusive, worldwide rights to lefamulin. Lefamulin is protected by issued patents in the United States, Europe and Japan covering composition of matter, which are scheduled to expire no earlier than 2028. We also have been granted patents for lefamulin relating to process and pharmaceutical crystalline salt forms in the United States, which are scheduled to expire no earlier than 2031. In addition, we own a family of pending patent applications directed to pharmaceutical compositions of lefamulin, which if issued would be scheduled to expire no earlier than 2036.
Our Strategy
Our goal is to become a fully integrated biopharmaceutical company focused on the research, development and commercialization of novel anti-infective products. The key elements of our strategy to achieve this goal are:
- •
- Complete Phase 3 clinical development of lefamulin for
CABP. We are devoting a significant portion of our financial resources and business efforts to completing the clinical development of lefamulin
for the treatment of CABP. We initiated two international Phase 3 clinical trials of lefamulin for the treatment of moderate to severe CABP. We initiated the first of these trials in September
2015 and the second trial in the April 2016. Based on our estimates regarding patient enrollment, we expect to have top-line data available for both trials in the second half of 2017. If the results
of these trials are favorable, including achievement of the primary efficacy endpoints of the trials, we expect to submit applications for marketing approval for lefamulin for the treatment of CABP in
both the United States and Europe in 2018.
- •
- Maximize the commercial potential of lefamulin for CABP. We own exclusive, worldwide rights to lefamulin. We expect that our initial target patient population for lefamulin will consist of patients with moderate to severe CABP. If lefamulin receives marketing approval from the FDA for the treatment of CABP, we plan to commercialize it in the United States with our own
S-3
- •
- Pursue the continued development of lefamulin in additional
indications. We plan to pursue the continued development of lefamulin for indications in addition to CABP. For example, we intend to further
pursue the development of lefamulin for use in pediatric patients and potentially for the treatment of ABSSSI. In addition, we are evaluating whether to pursue studies of lefamulin in patients with
VABP or HABP. We believe that lefamulin's product profile also provides the opportunity to expand to other indications beyond pneumonia. For example, investigation of the tolerability of higher single
doses of lefamulin could also support use of lefamulin for the treatment of STIs. In addition, we plan to explore longer duration of treatment with lefamulin to support development of a treatment for
osteomyelitis and prosthetic joint infections. We believe that lefamulin would be differentiated from other treatment options for each of these potential uses because of lefamulin's novel mechanism of
action, spectrum of activity, including activity against multi-drug resistant pathogens, achievement of substantial concentrations in relevant tissues, availability as both an IV and oral formulation
and favorable safety and tolerability profile.
- •
- Advance the development of other pleuromutilin product candidates and possibly compounds in other
classes. We are currently focused on developing additional pleuromutilin product candidates through our deep understanding of this class of
antibiotics. Our product candidate BC-7013 has completed a Phase 1 clinical trial. We believe that this pleuromutilin compound is well suited for the topical treatment of a variety of
Gram-positive infections, including uncomplicated skin and skin structure infections, or uSSSIs. In addition, other topical pleuromutilins from our research program have shown potent in vitro activity
against Clostridium difficile and Helicobacter
pylori. We also are actively pursuing an in-house discovery program to sustain and expand our pipeline with additional product candidates. Furthermore, we own diverse libraries
of compounds in other antibacterial classes, such as ß-lactams and acremonic acids, which are a potential basis for the discovery and development of novel antibacterial agents.
- •
- Evaluate business development opportunities and potential collaborations. We plan to evaluate the merits of entering into collaboration agreements with other pharmaceutical or biotechnology companies that may contribute to our ability to efficiently advance our product candidates, build our product pipeline and concurrently advance a range of research and development programs. Potential collaborations may provide us with funding and access to the scientific, development, regulatory and commercial capabilities of the collaborators. We also plan to encourage local and international government entities and non-government organizations to provide additional funding and support for our development programs. We may expand our product pipeline through opportunistically in-licensing or acquiring the rights to complementary products, product candidates and technologies for the treatment of a range of infectious diseases.
targeted hospital sales and marketing organization that we plan to establish. We believe that we will be able to effectively communicate lefamulin's differentiating characteristics and key attributes to clinicians and hospital pharmacies with the goal of establishing favorable formulary status for lefamulin. If lefamulin receives marketing approval outside the United States for the treatment of CABP, we expect to utilize a variety of types of collaboration, distribution and other marketing arrangements with one or more third parties to commercialize lefamulin in such markets. We also plan to develop a formulation of lefamulin appropriate for pediatric use for CABP.
S-4
Our Product Development Pipeline
The following table summarizes the indications for which we are developing our product candidates and the status of development.
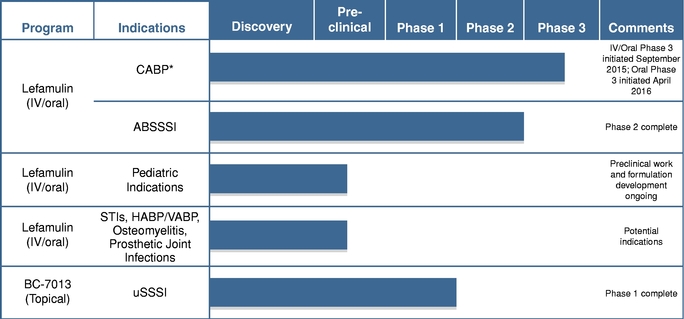
- *
- We have initiated two international Phase 3 clinical trials of lefamulin for the treatment of moderate to severe CABP. However, we have not previously conducted any clinical trials of lefamulin specifically for CABP. Our completed Phase 2 clinical trial evaluated lefamulin in patients with ABSSSI. We have obtained input from the FDA and select European authorities, including reaching agreement with the FDA on a Special Protocol Assessment, or SPA, regarding the study design of our first Phase 3 clinical trial, in anticipation of submitting applications for marketing approval for lefamulin for the treatment of CABP in both the United States and Europe in 2018, if both Phase 3 trials are successful.
Our Corporate Information
We were incorporated in October 2005 in Austria under the name Samisa Beteiligungsverwaltungs GmbH, a limited liability company organized under Austrian law, as a spin-off from Sandoz GmbH. In February 2006, we changed our name to Nabriva Therapeutics Forschungs GmbH and commenced operations. In 2007, we transformed into a stock corporation (Aktiengesellschaft) under the name Nabriva Therapeutics AG. We are incorporated under the laws of the Republic of Austria and registered at the Commercial Register of the Commercial Court of Vienna. Our executive offices are located at Leberstrasse 20, 1110 Vienna, Austria, and our telephone number is +43 (0)1 740 930. Our U.S. operations are conducted by our wholly-owned subsidiary, Nabriva Therapeutics US, Inc., a Delaware corporation established in August 2014 and located at 1000 Continental Drive, Suite 600, King of Prussia, PA 19406. Our American Depositary Shares have traded on the NASDAQ Global Market under the symbol "NBRV" since our initial public offering in the United States in September 2015.
Our website address is www.nabriva.com. The information contained on, or that can be accessed from, our website does not form part of this prospectus supplement. Our agent for service of process in the United States is CT Corporation System, 111 Eighth Avenue, New York, New York 10011.
S-5
Implications of Being an Emerging Growth Company
As a company with less than $1 billion in revenue during our last fiscal year, we qualify as an "emerging growth company" as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. These provisions include:
- •
- an exemption from compliance with the auditor attestation requirement of Section 404 of the Sarbanes-Oxley Act of 2002 on the design and
effectiveness of our internal controls over financial reporting;
- •
- an exemption from compliance with any requirement that the Public Company Accounting Oversight Board may adopt regarding mandatory audit firm
rotation or a supplement to the auditor's report providing additional information about the audit and the financial statements;
- •
- reduced disclosure about the company's executive compensation arrangements; and
- •
- exemptions from the requirements to obtain a non-binding advisory vote on executive compensation or a shareholder approval of any golden parachute arrangements.
We may take advantage of these provisions until December 31, 2020 or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company upon the earlier to occur of: the last day of the fiscal year in which we have more than $1 billion in annual revenues; the date we qualify as a "large accelerated filer," with at least more than $700 million in market value of our share capital held by non-affiliates; or the issuance by us of more than $1 billion of non-convertible debt over a three-year period. We may choose to take advantage of some, but not all, of the available benefits under the JOBS Act. We have taken advantage of some reduced reporting burdens in this prospectus supplement. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This provision allows an emerging growth company to delay the adoption of some accounting standards until those standards would otherwise apply to private companies. Since we currently report under International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB, we have irrevocably elected not to avail ourselves of delayed adoption of new or revised accounting standards and, therefore, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required by the IASB. Furthermore, beginning on January 1, 2017, when we expect to prepare our financial statements in accordance with generally accepted accounting principles in the United States, or U.S. GAAP, rather than IFRS, we will also adopt new or revised U.S. GAAP accounting standards on the relevant dates on which adoption of such standards is required for other public companies that are not emerging growth companies.
Implications of Being a Foreign Private Issuer
While we are a foreign private issuer, we are exempt from compliance with certain laws and regulations of the U.S. Securities and Exchange Commission, or SEC, and certain regulations of The NASDAQ Stock Market, including the proxy rules, the short-swing profits recapture rules and certain governance requirements, such as independent director oversight of the nomination of directors and executive compensation. In addition, we are not required to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as U.S. companies registered under the Securities Exchange Act of 1934, as amended. We determined that, as of June 30, 2016, we no longer qualified as a "foreign private issuer" under the rules and regulations of the SEC. As a result, beginning on January 1, 2017, we will no longer be entitled to "foreign private issuers" exemptions.
S-6
This summary highlights certain information contained elsewhere in this prospectus supplement. This summary does not contain all of the information that you should consider before deciding to purchase our securities. We urge you to read the entire prospectus supplement and the accompanying prospectus carefully, including the "Risk Factors" and "Special Note Regarding Forward-Looking Statements" sections, along with our consolidated financial statements and the notes to those financial statements.
Offering to Holders of ADSs |
||
ADS rights offering |
ADS holders will receive 0.276 ADS rights for each ADS owned on the ADS record date. One ADS right will entitle an ADS holder to subscribe for and purchase one new ADS. Fractional ADS rights will not be issued, and ADS right entitlements will be reduced to the next smaller whole number of ADS rights. |
|
ADS record date |
5:00 p.m. (New York City time) on November 29, 2016. |
|
ADS rights exercise period |
From November 30, 2016 through 5:00 p.m. (New York City time) on December 12, 2016. |
|
ADS trading price |
On November 28, 2016, the last reported sale price of the ADSs on the NASDAQ Global Market was $4.25 per ADS. |
|
ADS subscription price |
The estimated subscription price is $4.25 per ADS, which is the subscription price of €40.14 per common share for one tenth (1/10) of a common share translated into U.S. dollars at the rate of €1.00 to $1.0588, the exchange rate at the European Central Bank on November 28, 2016. On or about December 13, 2016, the ADS rights agent will determine the actual U.S. dollar ADS subscription price by converting the euro subscription price into U.S. dollars at an exchange rate assigned by it on that date. |
|
|
The ADS rights agent may convert currency itself or through any of its affiliates and, in those cases, acts as principal for its own account and not as an agent, fiduciary or broker on behalf of any other person and earns revenue, including, without limitation, fees and spreads that it will retain for its own account. The spread is the difference between the exchange rate assigned to the currency conversion made by the ADS rights agent and the rate that agent or its affiliate receives in an offsetting foreign currency trade. The ADS rights agent makes no representation that the exchange rate used or obtained in any currency conversion will be the most favorable rate that could be obtained at the time or that the method by which that rate will be determined will be the most favorable to ADS holders, except that the ADS rights agent will agree to act without gross negligence or willful misconduct. |
|
ADS subscription ratio |
0.276 ADS rights for each ADS held on the ADS record date. |
S-7
Estimated ADS subscription payment |
In order to exercise your ADS rights, you must pay to the ADS rights agent the ADS subscription payment of $4.68 per ADS, which represents 110% of the estimated subscription price to account for potential fluctuations in the exchange rate between the euro and the U.S. dollar, currency conversion expenses and an ADS issuance fee of the depositary of $0.05 per new ADS. |
|
|
If the actual U.S. dollar ADS subscription price (plus the ADS issuance fee and the currency conversion expense) is less than the ADS subscription payment, the ADS rights agent will refund such excess amount to the subscribing ADS holder without interest. However, if the amount of the ADS subscription payment you paid to the ADS rights agent is, for any reason, including due to currency exchange rate fluctuations, insufficient to pay the subscription price in euro plus conversion expenses and ADS issuance fees for all of the ADSs you are subscribing for, the ADS rights agent will require you to pay the deficiency as a condition to receiving your new ADSs, or will instruct the depositary to subscribe on your behalf for only the number of whole ADSs that can be subscribed for with the amount you have paid and the ADS rights agent will refund to you as soon as practicable the excess amount without interest. |
|
Procedure for exercising ADS rights |
If you hold ADSs directly, you may exercise your ADS rights during the ADS rights exercise period by delivering a properly completed and signed ADS subscription form and full payment of the ADS subscription payment for the new ADSs to the ADS rights agent, to be received prior to 5:00 p.m. (New York City time) on December 12, 2016. |
|
|
If you hold ADSs through a broker or other securities intermediary and wish to exercise your ADS rights, you should contact your securities intermediary and instruct it to subscribe on your behalf through the automated system of The Depository Trust Company, or DTC, prior to 5:00 p.m. (New York City time) on December 12, 2016, also referred to as the ADS rights expiration time. Your intermediary will charge the applicable ADS subscription payment to your account. Your broker or other securities intermediary may set a cutoff date and time to receive instructions that is earlier than the ADS rights expiration time stated above. You should contact your securities intermediary to determine the cutoff date and time that applies to you. |
|
|
We provide more details on how to exercise ADS rights under "Description of the Offering—Offering to Holders of ADSs." |
|
Transferability |
Rights to purchase ADSs in the ADS rights offering are not transferrable. |
S-8
Exercise of ADS rights irrevocable |
The exercise of ADS rights is irrevocable and may not be cancelled or modified. |
|
Unexercised rights |
If you do not exercise your ADS rights within the ADS rights exercise period, they will expire and have no further value. |
|
Depositary and ADS rights agent |
The Bank of New York Mellon. |
|
Listing |
The ADSs are listed on the NASDAQ Global Market under the symbol "NBRV." |
|
Delivery of new ADSs |
The depositary will deliver new ADSs subscribed for in the rights offering as soon as practicable after confirmation of receipt of the underlying new common shares by the depositary's custodian, which is expected to be on or about December 19, 2016. |
|
ADS issuance fee |
Subscribing holders will be charged an ADS issuance fee of $0.05 per new ADS issued, payable to the depositary. The ADS rights agent will deduct the ADS issuance fee from the ADS subscription payment in respect of each holder's subscription. |
|
New ADSs |
Each ADS represents one tenth (1/10) of a common share, nominal value €1.00 per share. |
|
|
The depositary will hold the common shares underlying the ADSs. ADS holders will have rights as provided in the deposit agreement. ADS holders may surrender their ADSs and withdraw the underlying common shares. The depositary will charge ADS holders fees for, among other acts, any surrender of ADSs for the purpose of withdrawal. We and the depositary may amend or terminate the deposit agreement without your consent. If you continue to hold your ADSs, you agree to be bound by the terms of the deposit agreement as then in effect. |
|
|
To better understand the terms of the ADSs, you should carefully read "Description of American Depositary Shares" in the accompanying prospectus. You should also read the deposit agreement, which is an exhibit to the registration statement of which this prospectus supplement forms a part. |
|
Information agent |
For information regarding this offering, please contact Georgeson LLC, the information agent, toll free at (866) 278-8941 or by e-mail at Nabriva@georgeson.com from 9:00 a.m. to 9:00 p.m., New York City time, Monday to Friday. |
|
For additional information regarding the rights offering to holders of ADSs, see "Description of the Offering—Offering to Holders of ADSs," which also contains a summary timetable containing important dates relating to the ADS rights offering. |
||
S-9
Offering to Holders of Common Shares |
|
|
Common share rights offering |
Holders of common shares will have the common share right to subscribe for and purchase 0.276 new common shares, at a subscription price of €40.14 per new common share, for each common share held on the common share record date, which we refer to as the common share subscription ratio. No fractional common shares will be issued and common share right entitlements will be reduced to the next smaller whole number of common shares. |
|
Common share record date |
November 29, 2016. |
|
Common share rights exercise period |
From November 30, 2016 through 5:00 p.m. (Vienna time) on December 14, 2016. |
|
Common share subscription price |
€40.14 per common share. |
|
Common share subscription ratio |
0.276 new common shares for each common share held on the common share record date. |
|
Procedure for exercising common share rights |
You may exercise your common share rights by delivering to us, at our executive offices at Leberstrasse 20, 1110 Vienna, Austria, a properly completed subscription form before 5:00 p.m. (Vienna time) on December 14, 2016. To validly subscribe for new common shares pursuant to your common share rights, we must receive the subscription price for your new common shares in full before 5:00 p.m. (Vienna time) on December 14, 2016. |
|
Transferability |
Rights to purchase common shares in the common share rights offering, except to the extent otherwise provided under Austrian law, will not be transferrable. |
|
Exercise of common share rights irrevocable |
The exercise of common share rights is irrevocable and may not be cancelled or modified. |
|
Unexercised rights |
If you do not exercise your common share rights within the common share rights exercise period, they will expire and have no further value. |
|
Listing |
The common shares and the common share rights are not listed on any national securities market or exchange. |
|
Delivery of new common shares |
We expect to deliver the new common shares subscribed in this rights offering on or about December 19, 2016. |
|
Information agent |
For information regarding this offering, please contact Georgeson LLC, the information agent, toll free at (866) 278-8941 or by e-mail at Nabriva@georgeson.com from 9:00 a.m. to 9:00 p.m., New York City time, Monday to Friday. |
S-10
For additional information regarding the rights offering to holders of our common shares, see "Description of Offering—Offering to Holders of Common Shares, " which also contains a summary timetable containing important dates relating to the common share rights offering. |
||
Tax Considerations |
|
|
Tax Considerations |
Although a final determination regarding our passive foreign investment company, or PFIC, status for our tax year ending December 31, 2016 cannot be made until our year-end results are known, it currently appears that there is a higher likelihood than in prior years that we may be a PFIC for the 2016 tax year. If we are characterized as a PFIC, our U.S. securityholders may suffer adverse tax consequences. See "Taxation—Taxation in the United States—Taxation of Rights Shares and ADSs—Passive Foreign Investment Company Considerations" for more information." |
|
Potential Offering of ADSs Represented by Unsubscribed Common Shares |
|
|
Potential Offering of ADSs Represented by Unsubscribed Common Shares |
If any new common shares are not subscribed for pursuant to the common share rights and ADS rights described in this prospectus supplement, following the expiration of the offering of such common share rights and ADS rights, we may, at the discretion of Cantor Fitzgerald & Co., based on market conditions and demand, enter into an underwriting agreement pursuant to which Cantor Fitzgerald & Co. would agree to subscribe for and purchase up to all of the unsubscribed common shares at a purchase price of €40.14 per common share for purposes of resale of ADSs representing the unsubscribed common shares. However, entry into an underwriting agreement for all or a portion of the unsubscribed shares remains at the discretion of Cantor Fitzgerald & Co., and Cantor Fitzgerald & Co. is not obligated to purchase all or any of the unsubscribed common shares. In the event that Cantor Fitzgerald & Co. and we do enter into an underwriting agreement for unsubscribed shares, Cantor Fitzgerald & Co. may sell ADSs at variable prices, which may be more or less than the purchase price. |
|
S-11
Investing in the ADSs or common shares involves a high degree of risk. In addition to the other information contained in this prospectus supplement, the accompanying prospectus and in the documents we incorporate by reference herein and therein, you should carefully consider the risks discussed below before making a decision about investing in the ADSs or the common shares. The risks and uncertainties discussed below are not the only ones facing us. Additional risks and uncertainties not presently known to us, or that we currently see as immaterial, may also harm our business. If any of these risks occur, our business, financial condition, results of operations and future growth prospects could be materially and adversely affected. In these circumstances, you may lose all or part of your investment.
Risks Related to Our Financial Position and Need for Additional Capital
We have incurred significant losses since our inception. We expect to incur losses for at least the next several years and may never generate profits from operations or maintain profitability.
Since inception, we have incurred significant operating losses. Our net loss was €35.4 million for the nine months ended September 30, 2016, €28.7 million for the year ended December 31, 2015 and €13.4 million for the year ended December 31, 2014. We generated a net profit of €11.2 million for the year ended December 31, 2013, primarily from the recognition of non-recurring income of €20.9 million as a result of the repurchase of a $25.0 million loan for €1.00 in connection with the termination by Forest Laboratories Inc., or Forest, of a stock purchase agreement and a decision by Forest not to exercise a related right to acquire us. As of September 30, 2016, we had accumulated losses of €161.0 million. To date, we have financed our operations primarily through the sale of our equity securities including our initial public offering of American Depositary Shares, or ADSs, and private placements of our common shares, convertible loans and research and development support from governmental grants and loans. We have devoted substantially all of our efforts to research and development, including clinical trials. We have not completed development of any drugs. We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. The net losses we incur may fluctuate significantly from quarter to quarter and year to year.
We anticipate that our expenses will increase substantially as we progress our two international Phase 3 clinical trials of our lead product candidate, lefamulin, for the treatment of community-acquired bacterial pneumonia, or CABP. We initiated the first of these trials in September 2015 and the second trial in April 2016. If the results of these two trials are favorable, including achievement of the primary efficacy endpoints of the trials, we expect to submit applications for marketing approval for lefamulin for the treatment of CABP in both the United States and Europe in 2018. We also plan to further characterize the clinical pharmacology of lefamulin. If we obtain marketing approval of lefamulin for CABP or another indication, we also expect to incur significant sales, marketing, distribution and manufacturing expenses.
In addition, our expenses will increase if and as we:
- •
- initiate or continue the research and development of lefamulin for additional indications and of our other product candidates;
- •
- seek to discover and develop additional product candidates;
- •
- seek marketing approval for any product candidates that successfully complete clinical development;
- •
- ultimately establish a sales, marketing and distribution infrastructure and scale up manufacturing capabilities to commercialize any product
candidates for which we receive marketing approval;
- •
- in-license or acquire other products, product candidates or technologies;
S-12
- •
- maintain, expand and protect our intellectual property portfolio;
- •
- expand our physical presence in the United States; and
- •
- add operational, financial and management information systems and personnel, including personnel to support our product development, our operations as a public company and our planned future commercialization efforts.
Our ability to generate profits from operations and remain profitable depends on our ability to successfully develop and commercialize drugs that generate significant revenue. Based on our current plans, we do not expect to generate significant revenue unless and until we obtain marketing approval for, and commercialize, lefamulin. We do not expect to obtain marketing approval before 2018, if at all. This will require us to be successful in a range of challenging activities, including:
- •
- completing enrollment for our Phase 3 clinical trails of lefamulin for the treatment of CABP and completing both trials as and when we
expect;
- •
- obtaining favorable results from our Phase 3 clinical trials of lefamulin for the treatment of CABP;
- •
- subject to obtaining favorable results from our Phase 3 clinical trials, applying for and obtaining marketing approval for lefamulin;
- •
- establishing sales, marketing and distribution capabilities to effectively market and sell lefamulin in the United States;
- •
- establishing collaboration, distribution or other marketing arrangements with third parties to commercialize lefamulin in markets outside the
United States;
- •
- protecting our rights to our intellectual property portfolio related to lefamulin;
- •
- contracting for the manufacture of and obtaining commercial quantities of lefamulin; and
- •
- negotiating and securing adequate reimbursement from third-party payors for lefamulin.
We may never succeed in these activities and, even if we do, may never generate revenues that are significant enough to generate profits from operations. Even if we do generate profits from operations, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to generate profits from operations and remain profitable would decrease the value of our company and could impair our ability to raise capital, expand our business, maintain our research and development efforts, diversify our product offerings or continue our operations. A decline in the value of our company could also cause our shareholders to lose all or part of their investment.
We will need substantial additional funding. If we are unable to raise capital when needed or on acceptable terms, we could be forced to delay, reduce or eliminate our product development programs or commercialization efforts.
We expect our research and development and other expenses to increase substantially in connection with our ongoing activities, particularly as we continue the development of and potentially seek marketing approval for lefamulin and, possibly, other product candidates and continue our research activities. Our expenses will increase if we suffer any delays in our Phase 3 clinical program for lefamulin for CABP, including delays in enrollment of patients. If we obtain marketing approval for lefamulin or any other product candidate that we develop, we expect to incur significant commercialization expenses related to product sales, marketing, distribution and manufacturing. Furthermore, we expect to continue to incur additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we could
S-13
be forced to delay, reduce or eliminate our research and development programs or any future commercialization efforts.
If the gross proceeds from this offering are $20.0 million or more, we estimate that such funds, after deducting estimated fees and offering expenses payable by us, together with our existing cash, cash equivalents, marketable securities and term deposits, will be sufficient to enable us to fund our operating expenses and capital expenditure requirements at least into the second quarter of 2018. If the gross proceeds from this offering are $5.0 million or more, we estimate that such funds, after deducting estimated fees and offering expenses payable by us, together with our existing cash, cash equivalents, marketable securities and term deposits, will be sufficient to enable us to fund our operating expenses and capital expenditure requirements at least into the first quarter of 2018. If the gross proceeds from this offering are less than such amounts, our capital resources may not be sufficient to enable us to fund our operating expenses and capital expenditure requirements for the time periods indicated. We have based these estimates on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect. These estimates assume, among other things, that we do not obtain any additional funding through grants and clinical trial support, collaboration agreements or debt financings.
Our future capital requirements will depend on many factors, including:
- •
- the progress, costs and results of our ongoing Phase 3 clinical trials of lefamulin;
- •
- the costs and timing of process development and manufacturing scale-up activities associated with lefamulin;
- •
- the costs, timing and outcome of regulatory review of lefamulin;
- •
- the costs of commercialization activities for lefamulin if we receive, or expect to receive, marketing approval, including the costs and timing
of establishing product sales, marketing, distribution and outsourced manufacturing capabilities;
- •
- subject to receipt of marketing approval, revenue received from commercial sales of lefamulin;
- •
- the costs of developing lefamulin for the treatment of additional indications;
- •
- our ability to establish collaborations on favorable terms, if at all;
- •
- the scope, progress, results and costs of product development of BC-7013 and any other product candidates that we may develop;
- •
- the extent to which we in-license or acquire rights to other products, product candidates or technologies;
- •
- the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending
against intellectual property-related claims;
- •
- the continued availability of Austrian governmental grants;
- •
- the rate of the expansion of our physical presence in the United States; and
- •
- the costs of operating as a public company in the United States.
Conducting clinical trials is a time consuming, expensive and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. Our commercial revenues, if any, will be derived from sales of lefamulin or any other products that we successfully develop, none of which we expect to be commercially available for several years, if at all. In addition, if approved, lefamulin or any other product candidate that we develop, in-license or acquire may not achieve commercial success. Accordingly, we will need to obtain substantial additional financing to achieve our business objectives.
S-14
Adequate additional financing may not be available to us on acceptable terms, or at all. In addition, we may seek additional capital due to favorable market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans.
Raising additional capital may cause dilution to our security holders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, and funding from local and international government entities and non-government organizations in the disease areas addressed by our product candidates and marketing, distribution or licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a security holder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. In addition, debt service obligations under any debt financings may limit the availability of our cash for other purposes, and we may be unable to make interest payments or repay the principal of such debt financings when due.
If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Our limited operating history may make it difficult for you to evaluate the success of our business to date and to assess our future viability.
Our operations to date have been limited to organizing and staffing our company, developing and securing our technology, raising capital and undertaking preclinical studies and clinical trials of our product candidates. We have not yet demonstrated our ability to successfully complete development of any product candidates, obtain marketing approvals, manufacture a commercial scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization. Consequently, any predictions you make about our future success or viability may not be as accurate as they could be if we had a longer operating history.
In addition, as a new business, we may encounter unforeseen expenses, difficulties, complications, delays and other known and unknown factors. We will need to transition from a company with a research and development focus to a company capable of supporting commercial activities. We may not be successful in such a transition.
We have relied on, and expect to continue to rely on, certain government grants and funding from the Austrian government. Should these funds cease to be available, or our eligibility be reduced, or if we are required to repay any of these funds, this could impact our ongoing need for funding and the timeframes within which we currently expect additional funding will be required.
As a company that carries out extensive research and development activities, we benefit from the Austrian research and development support regime, under which we are eligible to receive a research premium from the Austrian government equal to 12% (10%, in the case of fiscal years prior to 2016) of a specified research and development cost base. Qualifying expenditures largely comprise research
S-15
and development activities conducted in Austria, however, the research premium is also available for certain related third-party expenses with additional limitations. We received research premiums of €3.8 million for the year ended December 31, 2015 and €1.0 million for the year ended December 31, 2014. For the year ended December 31, 2013, we received research premiums of €1.4 million, which included research premium adjustments of €0.5 million for the years ended December 31, 2012 and 2011. We anticipate that our qualifying expenditures will continue to increase as a result of our ongoing Phase 3 clinical trials of lefamulin. However, as we increase our personnel and expand our business outside of Austria, we may not be able to continue to claim research premiums to the same extent as we have in previous years, as some research and development activities may no longer be considered to occur in Austria. As research premiums that have been paid out already may be audited by the tax authorities, there is a risk that parts of the submitted cost base may not be considered as eligible and therefore repayments may have to be made.
Risks Related to Product Development and Commercialization
We depend heavily on the success of our lead product candidate, lefamulin, which we are developing for CABP and other indications. If we are unable to complete our Phase 3 clinical program for lefamulin for CABP as and when expected and obtain marketing approvals for lefamulin, or if thereafter we fail to commercialize lefamulin or experience significant delays in doing so, our business will be materially harmed.
We have invested a significant portion of our efforts and financial resources in the development of lefamulin. There remains a significant risk that we will fail to successfully develop lefamulin for CABP or any other indication. We do not expect to have top-line data from our Phase 3 clinical program for lefamulin for the treatment of CABP available until the second half of 2017. The timing of the availability of such top-line data and the completion of our Phase 3 clinical program is dependent, in part, on our ability to locate and enroll a sufficient number of eligible patients in our Phase 3 clinical program on a timely basis. A significant delay in enrollment would result in delays to our development timeline and additional development costs beyond what we have budgeted. If we ultimately obtain favorable results from our Phase 3 clinical program for lefamulin for CABP, we do not expect to submit applications for marketing approval for lefamulin for this indication until 2018.
Our ability to generate product revenues, which may not occur for several years, if ever, will depend heavily on our obtaining marketing approval for and commercializing lefamulin. The success of lefamulin will depend on a number of factors, including the following:
- •
- completing our ongoing Phase 3 clinical trials as and when expected;
- •
- obtaining favorable results from clinical trials;
- •
- making arrangements with third-party manufacturers for commercial supply and receiving regulatory approval of our manufacturing processes and
our third-party manufacturers' facilities from applicable regulatory authorities;
- •
- receipt of marketing approvals from applicable regulatory authorities for lefamulin for the treatment of CABP;
- •
- launching commercial sales of lefamulin, if and when approved, whether alone or in collaboration with third parties;
- •
- acceptance of lefamulin, if and when approved, by patients, the medical community and third-party payors;
- •
- effectively competing with other therapies;
- •
- maintaining a continued acceptable safety profile of lefamulin following approval;
- •
- obtaining and maintaining patent and trade secret protection and regulatory exclusivity; and
- •
- protecting our rights in our intellectual property portfolio.
S-16
Successful development of lefamulin for the treatment of additional indications, if any, or for use in other patient populations and our ability, if it is approved, to broaden the label for lefamulin will depend on similar factors.
If we do not achieve one or more of these factors in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize lefamulin for CABP or for any additional indications, which would materially harm our business.
If clinical trials of lefamulin or any of our other product candidates fail to demonstrate safety and efficacy to the satisfaction of the U.S. Food and Drug Administration, or FDA, regulatory authorities in the European Union, or other regulatory authorities or do not otherwise produce favorable results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of lefamulin or any other product candidate.
Before obtaining marketing approval from regulatory authorities for the sale of any product candidate, we must complete preclinical development and early clinical trials, including Phase 1 clinical trials, in addition to extensive later-stage Phase 3 clinical trials, to demonstrate the safety and efficacy of our product candidates in humans. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of testing. The outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. The design of a clinical trial can determine whether its results will support approval of a product, and flaws in the design of a clinical trial may not become apparent until the clinical trial is well advanced or completed. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their products.
We have not completed any clinical trials of lefamulin specifically for CABP. Our completed Phase 2 clinical trial evaluated lefamulin in patients with acute bacterial skin and skin structure infections, or ABSSSI. Our Phase 1 clinical trials evaluated lefamulin in healthy subjects to obtain tolerance data and to understand the absorption and distribution of lefamulin in the blood and target tissues, evaluate the metabolism and elimination route of lefamulin and obtain safety and tolerability data to help predict safe and effective doses of lefamulin for the treatment of patients. In addition, we are using a different intravenous, or IV, formulation of lefamulin for our Phase 3 clinical trials for CABP than we used in our Phase 2 clinical trial for ABSSSI. We have only evaluated this new IV formulation of lefamulin, a sterile saline solution buffered by a citrate salt, in Phase 1 clinical trials. Because of these and other factors, the results of our completed clinical trials may not predict success in our Phase 3 clinical trials of lefamulin for CABP. Although we believe that the collective data from prior trials and our preclinical studies provide support for concluding that lefamulin is well suited for treatment of CABP, we may fail to obtain favorable results in our Phase 3 clinical trials of lefamulin for CABP. If the results of our Phase 3 clinical trials are not favorable, including failure to achieve the primary efficacy endpoints of the trials, we may need to conduct additional clinical trials at significant cost or altogether abandon development of lefamulin for CABP and potentially other indications.
If we are required to conduct additional clinical trials or other testing of lefamulin or any other product candidate that we develop beyond those that we contemplate, if we are unable to successfully complete our clinical trials or other testing, if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may:
- •
- be delayed in obtaining marketing approval for our product candidates;
- •
- not obtain marketing approval at all;
S-17
- •
- obtain approval for indications or patient populations that are not as broad as intended or desired;
- •
- obtain approval with labeling that includes significant use or distribution restrictions or safety warnings, including boxed warnings;
- •
- be subject to additional post-marketing testing requirements or restrictions; or
- •
- have the product removed from the market after obtaining marketing approval.
The occurrence of any of the developments listed above could materially harm our business, financial condition, results of operations and prospects.
If we experience any of a number of possible unforeseen events in connection with our Phase 3 clinical trials of lefamulin for CABP or other clinical trials, the potential marketing approval or commercialization of lefamulin or other product candidates could be delayed or prevented.
We may experience numerous unforeseen events during, or as a result of, our Phase 3 clinical trials of lefamulin for CABP or other clinical trials we conduct that could delay or prevent our ability to receive marketing approval or commercialize lefamulin or our other product candidates, including:
- •
- clinical trials of lefamulin or our other product candidates may produce negative or inconclusive results, and we may decide, or regulators may
require us, to conduct additional clinical trials or abandon product development programs;
- •
- the number of patients required for clinical trials of lefamulin for CABP, lefamulin for other indications or our other product candidates may
be larger than we anticipate, enrollment in these clinical trials may be slower than we anticipate or participants may drop out of these clinical trials at a higher rate than we anticipate;
- •
- we may be unable to enroll a sufficient number of patients in our Phase 3 clinical trials of lefamulin for CABP or other clinical trials
we conduct to ensure adequate statistical power to detect any statistically significant treatment effects;
- •
- our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or
at all;
- •
- regulators, institutional review boards or independent ethics committees may not authorize us or our investigators to commence a clinical trial
or conduct a clinical trial at a prospective trial site or may require that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory
requirements or a finding that the participants are being exposed to unacceptable health risks;
- •
- we may experience delays in reaching or fail to reach agreement on acceptable clinical trial contracts or clinical trial protocols with
prospective trial sites;
- •
- we may have to suspend or terminate our Phase 3 clinical trials of lefamulin for CABP or other clinical trials of our product candidates
for various reasons, including a finding that the participants are being exposed to unacceptable health risks;
- •
- the cost of clinical trials of our product candidates may be greater than we anticipate;
- •
- the supply or quality of our product candidates or other materials necessary to conduct clinical trials of our product candidates may be
insufficient or inadequate; and
- •
- our product candidates may have undesirable side effects or other unexpected characteristics, causing us or our investigators, regulators, institutional review boards or independent ethics committees to suspend or terminate the trials.
S-18
For example, we may need to enroll more patients in our Phase 3 clinical trials of lefamulin for CABP than we currently anticipate if, among other reasons, an interim analysis indicates that a larger number of patients is required to generate additional data. We plan to conduct an interim analysis of 60% of the blinded enrolled patients in our first Phase 3 clinical trial to determine if an increase in the number of patients is required based on whether the observed clinical response is consistent with the point estimate used in determining the trial sample size. We may also increase the number of patients in either of our Phase 3 clinical trials in order to supplement the safety database. An increase in the number of patients in either of our Phase 3 clinical trials could delay our expected clinical development and regulatory approval timelines of lefamulin for CABP. We expect to reach 60% enrollment for our first Phase 3 clinical trial of lefamulin for CABP by the end of 2016 and complete the interim analysis in early 2017.
Our product development costs will increase if we experience delays in enrollment in our clinical development program or our non-clinical development program or in obtaining marketing approvals. We do not know whether any additional non-clinical tests or clinical trials will be required, or if they will begin as planned, or if they will need to be restructured or will be completed on schedule, or at all. Significant non-clinical study or clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do and impair our ability to successfully commercialize our product candidates and may harm our business and results of operations.
If we experience delays or difficulties in the enrollment of patients in our clinical trials, our receipt of necessary marketing approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials of lefamulin or any other product candidate that we develop if we are unable to locate and enroll a sufficient number of eligible patients to participate in these clinical trials. In particular, we may experience enrollment challenges at trial sites in the United States, where it is a common practice to place patients with potential moderate to severe CABP on antibiotics very shortly after examination. This practice could prevent potential U.S. trial patients from being enrolled in our clinical trials based on our eligibility criteria. In addition, some of our competitors have ongoing clinical trials for product candidates that could be competitive with lefamulin, and patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors' product candidates.
Patient enrollment is affected by other factors including:
- •
- severity of the disease under investigation;
- •
- eligibility criteria for the clinical trial in question;
- •
- perceived risks and benefits of the product candidate under study;
- •
- approval of other therapies to treat the disease under investigation;
- •
- efforts to facilitate timely enrollment in clinical trials;
- •
- patient referral practices of physicians;
- •
- the time of year in which the trial is initiated or conducted;
- •
- the geographic distribution of global trial sites, given the timing of pneumonia season globally, and the seasonal variation in the number of
patients suffering from pneumonia, including a decline in the number of patients with CABP during the summer months;
- •
- the ability to monitor patients adequately during and after treatment;
- •
- proximity and availability of clinical trial sites for prospective patients;
S-19
- •
- delays in the receipt of required regulatory approvals, or the failure to receive required regulatory approvals, in the jurisdictions in which
clinical trials are expected to be conducted; and
- •
- delays in the receipt of approvals, or the failure to receive approvals, from the relevant institutional review board or ethics committee at clinical trial sites.
For example, in each of our Phase 3 clinical trials of lefamulin, patients who have previously taken no more than one dose of a short acting, potentially effective antibiotic for the treatment of the current CABP episode within 24 hours of receiving the first dose of study medication will be allowed to participate in the trial but will comprise only up to 25% of the total intent to treat populations. Depending upon a region's or a clinical trial site's standard of care for the administration of antibiotics, this could affect our ability to enroll patients in these clinical trials in a timely fashion. Also, enrollment for our Phase 3 clinical trials may be negatively impacted by delays in opening clinical trial sites or the duration and/or severity of the influenza season. Enrollment delays in our clinical trials may result in increased development costs for our product candidates, which would cause the value of the company to decline and limit our ability to obtain additional financing. Our inability to enroll a sufficient number of patients in our Phase 3 clinical trials of lefamulin for CABP or any of our other clinical trials would result in significant delays or may require us to abandon one or more clinical trials altogether.
If serious adverse or undesirable side effects are identified during the development of lefamulin or any other product candidate that we develop, we may need to abandon or limit our development of that product candidate.
All of our product candidates are in clinical or preclinical development and their risk of failure is high. It is impossible to predict when or if any of our product candidates will prove effective or safe in humans or will receive marketing approval. If our product candidates are associated with undesirable side effects or have characteristics that are unexpected, we may need to abandon their development or limit development to certain uses or subpopulations in which the undesirable side effects or other characteristics are less prevalent, less severe or more acceptable from a risk-benefit perspective. Many compounds that initially showed promise in clinical or earlier stage testing have later been found to cause side effects or other safety issues that prevented further development of the compound.
Lefamulin was well tolerated in our Phase 2 clinical trial for ABSSSI. No patient in the trial suffered any serious adverse events that were found to be related to lefamulin, and no patient in the trial died. Some patients experienced adverse events that were assessed by the investigator as possibly or probably related to study medication. The majority of their symptoms were mild in severity. Four patients discontinued study medication following a drug-related event, three of whom were in a lefamulin treatment group: one patient experienced events of hyperhidrosis, vomiting and headache; one patient experienced infusion site pain; and one patient experienced dyspnea.
Because the potential for mild effect on electrocardiogram, or ECG, measurements was observed in preclinical studies, we have continued to assess this potential in all human clinical trials we have conducted. In the Phase 2 clinical trial, no change in ECG measurements was considered to be of clinical significance, and no drug-related cardiovascular adverse event was reported. Both lefamulin and vancomycin treatment were associated with a small increase in the QT interval. The QT interval is a measure of the heart's electrical cycle, with a lengthened QT interval representing a marker for potential ventricular arrhythmia. We are continuing to evaluate the effect of lefamulin on the QT interval in our Phase 3 clinical trials of lefamulin for CABP.
There were no systemic adverse events of clinical concern and no drug-related serious adverse events observed in any of our Phase 1 clinical trials of lefamulin. In these trials, the most commonly observed adverse effects with oral administration of lefamulin were related to the gastrointestinal tract,
S-20
including nausea and abdominal discomfort, while the most commonly observed adverse effects related to IV administration were related to irritation at the infusion site. In addition, lefamulin produced a transient, predictable and reproducible prolongation of the QT interval based on the maximum concentration of the drug in the blood plasma. At therapeutic doses, we expect that the drug will not produce large effects on the QT interval that would be of clinical relevance. We did not observe any drug-related cardiac adverse events, such as increase in ectopic ventricular activity or other cardiac arrhythmia, or clinically relevant ECG findings during the conduct of any of our Phase 1 clinical trials. None of the ECG stopping criteria defined in the trial protocols was reached in any clinical trial. However, if we observe clinically relevant effects on the QT interval in our Phase 3 clinical trials of lefamulin for CABP or in any other clinical trial of lefamulin, our ability to successfully develop lefamulin for CABP or any other indication may be significantly delayed or prevented.
If we elect or are forced to suspend or terminate any clinical trial of lefamulin or any other product candidates that we are developing, the commercial prospects of lefamulin or such other product candidates will be harmed and our ability to generate product revenues, if at all, from lefamulin or any of these other product candidates will be delayed or eliminated. Any of these occurrences could materially harm our business, financial condition, results of operations and prospects.
Even if lefamulin or any other product candidate receives marketing approval, it may fail to achieve the degree of market acceptance by physicians, patients, third-party payors and others in the medical community necessary for commercial success and the market opportunity for lefamulin may be smaller than we estimate.
If lefamulin or any of our other product candidates receive marketing approval, they may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors and others in the medical community. For example, current treatments for pneumonia, including generic options, are well established in the medical community, and doctors may continue to rely on these treatments without lefamulin. In addition, our efforts to effectively communicate lefamulin's differentiating characteristics and key attributes to clinicians and hospital pharmacies with the goal of establishing favorable formulary status for lefamulin may fail or may be less successful than we expect. If lefamulin does not achieve an adequate level of acceptance, we may not generate significant product revenues or any profits from operations. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
- •
- the efficacy and potential advantages compared to alternative treatments;
- •
- lefamulin's ability to limit the development of bacterial resistance in the pathogens it targets;
- •
- the prevalence and severity of any side effects;
- •
- the ability to offer our product candidates for sale at competitive prices, including in comparison to generic competition;
- •
- convenience and ease of administration compared to alternative treatments;
- •
- the willingness of the target patient population to try new therapies, physicians to prescribe these therapies and hospitals to approve the
cost and use by their physicians of these therapies;
- •
- the strength of marketing and distribution support;
- •
- the availability of third-party coverage and adequate reimbursement; and
- •
- the timing of any marketing approval in relation to other product approvals.
Although we believe that the mechanism of action for pleuromutilin antibiotics may result in slow development of bacterial resistance to lefamulin or our other pleuromutilin product candidates over time, bacteria might nevertheless develop resistance to lefamulin or our other pleuromutilin product candidates more rapidly or to a greater degree than we anticipate. If bacteria develop such resistance,
S-21
or if lefamulin is not effective against drug-resistant bacteria, the efficacy of these product candidates would decline, which would negatively affect our potential to generate revenues from these product candidates.
Our ability to negotiate, secure and maintain third-party coverage and reimbursement may be affected by political, economic and regulatory developments in the United States, the European Union and other jurisdictions. Governments continue to impose cost containment measures, and third-party payors are increasingly challenging prices charged for medicines and examining their cost effectiveness, in addition to their safety and efficacy. If the level of reimbursement is below our expectations, our revenue and gross margins would be adversely affected. Obtaining formulary approval from third-party payors can be an expensive and time-consuming process. We cannot be certain if and when we will obtain formulary approval to allow us to sell lefamulin or any future product candidates into our target markets. Even if we do obtain formulary approval, third-party payors, such as government or private health care insurers, carefully review and increasingly question the coverage of, and challenge the prices charged for, drugs. These and other similar developments could significantly limit the degree of market acceptance of lefamulin or any of our other product candidates that receive marketing approval.
If we are unable to establish sales, marketing and distribution capabilities or enter into sales, marketing and distribution agreements with third parties, we may not be successful in commercializing lefamulin or any other product candidate if and when they are approved.
We do not have a sales, marketing or distribution infrastructure, and as a company we have no experience in the sale, marketing or distribution of pharmaceutical products. To achieve commercial success for any approved product, we must either develop a sales, marketing and distribution organization or outsource these functions to third parties. If lefamulin receives marketing approval, we plan to commercialize it in the United States with our own targeted hospital sales and marketing organization that we plan to establish. In addition, we expect to utilize a variety of types of collaboration, distribution and other marketing arrangements with one or more third parties to commercialize lefamulin in markets outside the United States.
There are risks involved with establishing our own sales, marketing and distribution capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing and distribution capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our sales and marketing personnel.
Factors that may inhibit our efforts to commercialize our products on our own include:
- •
- our inability to recruit, train and retain adequate numbers of effective sales and marketing personnel;
- •
- the inability of sales personnel to obtain access to or persuade adequate numbers of physicians to prescribe any future products;
- •
- the lack of complementary products to be offered by sales personnel, which may put our sales representatives at a competitive disadvantage
relative to sales representatives from companies with more extensive product lines; and
- •
- unforeseen costs and expenses associated with creating an independent sales, marketing and distribution organization.
If we enter into arrangements with third parties to perform sales, marketing and distribution services, our product revenues or the profitability of these product revenues to us are likely to be lower
S-22
than if we were to market, sell and distribute ourselves any products that we develop. In addition, we may not be successful in entering into arrangements with third parties to sell, market and distribute our product candidates or may be unable to do so on terms that are favorable to us. We likely will have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish sales, marketing and distribution capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to lefamulin and any other products we may seek to develop or commercialize in the future from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
There are a variety of available therapies marketed for the treatment of CABP. Currently the treatment of CABP is dominated by generic products. For hospitalized patients, combination therapy is frequently used. Many currently approved drugs are well-established therapies and are widely accepted by physicians, patients and third-party payors. We also are aware of various drugs under development for the treatment of CABP, including solithromycin, (Phase 3 clinical trials completed and a New Drug Application, or NDA, filed by Cempra Inc.), omadacycline (under Phase 3 clinical development by Paratek Pharmaceuticals Inc.), delafloxacin (under Phase 3 clinical development by Melinta Therapeutics Inc.) and oral nafithromycin (under Phase 2 clinical development by Wockhardt Ltd.).
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are approved for broader indications or patient populations, are more convenient or are less expensive than any products that we may develop. Our competitors may also obtain marketing approvals for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected because in some cases insurers or other third-party payors seek to encourage the use of generic products. This may have the effect of making branded products less attractive, from a cost perspective, to buyers. We expect that if lefamulin is approved for CABP, it will be priced at a significant premium over competitive generic products. This may make it difficult for us to replace existing therapies with lefamulin. The key competitive factors affecting the success of our product candidates are likely to be their efficacy, safety, convenience, price and the availability of coverage and reimbursement from government and other third-party payors.
Many of our competitors may have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining approvals from regulatory authorities and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to or necessary for our programs.
S-23
Even if we are able to commercialize lefamulin or any other product candidate that we develop, the product may become subject to unfavorable pricing regulations, third-party reimbursement practices or healthcare reform initiatives, which would harm our business.
The regulations that govern marketing approvals, pricing, coverage and reimbursement for new drug products vary widely from country to country. Current and future legislation may significantly change the approval requirements in ways that could involve additional costs and cause delays in obtaining approvals. Some countries require approval of the sale price of a drug before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay our commercial launch of the product, possibly for lengthy time periods, and negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more product candidates, even if our product candidates obtain marketing approval.
Our ability to commercialize lefamulin or any other product candidate successfully also will depend in part on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Government authorities and other third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. A major trend in the E.U. and U.S. healthcare industries and elsewhere is cost containment. Government authorities and other third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We cannot be sure that coverage and reimbursement will be available for lefamulin or any other product that we commercialize and, if coverage and reimbursement are available, the level of reimbursement. Reimbursement may impact the demand for, or the price of, any product candidate for which we obtain marketing approval. Obtaining and maintaining adequate reimbursement for lefamulin may be particularly difficult because of the number of generic drugs, which are typically available at lower prices, that are available to treat CABP. In addition, third-party payors are likely to impose strict requirements for reimbursement of a higher priced drug, such as lefamulin. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize lefamulin or other product candidates for which we obtain marketing approval.
There may be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the applicable regulatory authority. Moreover, eligibility for coverage and reimbursement does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale and distribution. Interim reimbursement levels for new drugs, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost drugs, and may be incorporated into existing payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. In the United States, third-party payors often rely upon Medicare coverage policy and payment limitations in setting their own reimbursement policies. In the European Union, reference pricing systems and other measures may lead to cost containment and reduced prices. Our inability to promptly obtain coverage and adequate reimbursement rates from both government-funded
S-24
and private payors for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products and our overall financial condition.
Product liability lawsuits against us could divert our resources, cause us to incur substantial liabilities and to limit commercialization of any products that we may develop or in-license.
We face an inherent risk of product liability exposure related to the testing of lefamulin and any other product candidate that we develop in human clinical trials and will face an even greater risk if we commercially sell any products that we may develop or in-license. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we will incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
- •
- reduced resources of our management to pursue our business strategy;
- •
- decreased demand for any product candidates or products that we may develop;
- •
- injury to our reputation and significant negative media attention;
- •
- withdrawal of clinical trial participants;
- •
- significant costs to defend the related litigation;
- •
- substantial monetary awards to trial participants or patients;
- •
- loss of revenue; and
- •
- the inability to commercialize any products that we may develop.
We maintain product liability insurance that covers bodily injury to patients participating in our clinical trials up to a $10.0 million annual aggregate limit and subject to a per event deductible. This amount of insurance may not be adequate to cover all liabilities that we may incur. We will need to increase our insurance coverage when and if we begin commercializing lefamulin or any other product candidate that receives marketing approval. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
Risks Related to Our Dependence on Third Parties
Use of third parties to manufacture our product candidates may increase the risk that we will not have sufficient quantities of our product candidates or products or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We do not own or operate manufacturing facilities for the production of lefamulin that could be used in product candidate development, including clinical trial supply, or for commercial supply, or for the supply of any other compound that we are developing or evaluating in our research program. We have limited personnel with experience in drug manufacturing and lack the resources and the capabilities to manufacture any of our product candidates on a clinical or commercial scale. We currently rely on third parties for supply of lefamulin, and our strategy is to outsource all manufacturing of our product candidates and products to third parties.
We do not currently have any agreements with third-party manufacturers for the long-term commercial supply of any of our product candidates. We obtained the pleuromutilin starting material for the clinical trial supply of lefamulin from a single third-party manufacturer, Sandoz GmbH, or Sandoz, a division of Novartis AG, or Novartis. We have identified an alternative supplier that we believe will be able to provide pleuromutilin starting material for the commercial supply of lefamulin. Another third-party manufacturer synthesizes lefamulin from the pleuromutilin starting material and
S-25
provides our supply of the active pharmaceutical ingredient. We engage separate manufacturers to provide fill and finish services for the finished product that we are using in our clinical trials of lefamulin. We may be unable to conclude agreements for commercial supply with third-party manufacturers, or may be unable to do so on acceptable terms.
Even if we are able to establish and maintain arrangements with third-party manufacturers, reliance on third-party manufacturers entails risks, including:
- •
- reliance on the third party for regulatory compliance and quality assurance;
- •
- the possible breach of the manufacturing agreement by the third party;
- •
- the possible misappropriation of our proprietary information, including our trade secrets and know-how; and
- •
- the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us.
Third-party manufacturers may not be able to comply with current good manufacturing practice, or cGMP, regulations or similar regulatory requirements outside the United States. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of product candidates or products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our product candidates.
Our product candidates and any products that we may develop may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us.
If the third parties that we engage to supply any materials or manufacture product for our non-clinical testing and clinical trials should cease to continue to do so for any reason, we likely would experience delays in advancing these trials while we identify and qualify replacement suppliers and we may be unable to obtain replacement supplies on terms that are favorable to us. For example, there are only a limited number of known manufacturers that produce the pleuromutilin starting material used in the synthesis of lefamulin. In early 2015, Novartis announced the sale of its animal health division, including its veterinary products, to a third party. As a result, we have identified an alternative supplier that currently manufactures pleuromutilin starting material for veterinary products, that we believe will be able to provide pleuromutilin starting material for the commercial scale manufacture of lefamulin. In addition, if we are not able to obtain adequate supplies of our product candidates or the drug substances used to manufacture them, it will be more difficult for us to develop our product candidates and compete effectively.
Our current and anticipated future dependence upon others for the manufacture of our product candidates may adversely affect our future profit margins and our ability to develop product candidates and commercialize any products that receive marketing approval on a timely and competitive basis.
We rely on third parties to conduct our clinical trials and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials.
We do not independently conduct clinical trials for our product candidates. We rely on third parties, such as contract research organizations, clinical data management organizations, medical institutions and clinical investigators, to perform this function. We expect to continue to rely on such third parties in conducting our clinical trials of lefamulin, and expect to rely on these third parties to conduct clinical trials of any other product candidate that we develop. Any of these third parties may
S-26
terminate their engagements with us at any time. If we need to enter into alternative arrangements, it would delay our product development activities.
Our reliance on these third parties for clinical development activities reduces our control over these activities but does not relieve us of our responsibilities. For example, we remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA requires us to comply with standards, commonly referred to as Good Clinical Practices, or GCP, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a U.S. government-sponsored database, ClinicalTrials.gov, within certain timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions. Similar GCP and transparency requirements apply in the European Union. Failure to comply with such requirements, including with respect to clinical trials conducted outside the European Union, can also lead regulatory authorities to refuse to take into account clinical trial data submitted as part of a marketing authorization application, or MAA.
Furthermore, third parties that we rely on for our clinical development activities may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with regulatory requirements or our stated protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates. Our product development costs will increase if we experience delays in testing or obtaining marketing approvals.
We also rely on other third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our product candidates or commercialization of our products, producing additional losses and depriving us of potential product revenue.
We may enter into collaborations with third parties for the development or commercialization of lefamulin and our other product candidates. If those collaborations are not successful, we may not be able to capitalize on the market potential of these product candidates.
If lefamulin receives marketing approval, we plan to commercialize it in the United States with our own targeted hospital sales and marketing organization. Outside the United States, we expect to utilize a variety of types of collaboration, distribution and other marketing arrangements with one or more third parties to commercialize lefamulin. We also may seek third-party collaborators for development and commercialization of other product candidates or for lefamulin for indications other than CABP. Our likely collaborators for any sales, marketing, distribution, development, licensing or broader collaboration arrangements include large and mid-size pharmaceutical companies, regional and national pharmaceutical companies and biotechnology companies. We are not currently party to any such arrangement. However, if we do enter into any such arrangements with any third parties in the future, we will likely have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of our product candidates. Our ability to generate revenues from these arrangements will depend on our collaborators' abilities and efforts to successfully perform the functions assigned to them in these arrangements.
Collaborations involving our product candidates would pose numerous risks to us, including the following:
- •
- collaborators have significant discretion in determining the efforts and resources that they will apply to these collaborations and may not perform their obligations as expected;
S-27
- •
- collaborators may deemphasize or not pursue development and commercialization of our product candidates or may elect not to continue or renew
development or commercialization programs based on clinical trial results, changes in the collaborators' strategic focus, product and product candidate priorities, available funding, or external
factors such as an acquisition that diverts resources or creates competing priorities;
- •
- collaborators may delay clinical trials, provide insufficient funding for a clinical trial program, stop a clinical trial or abandon a product
candidate, repeat or conduct new clinical trials or require a new formulation of a product candidate for clinical testing;
- •
- collaborators could independently develop, or develop with third parties, products that compete directly or indirectly with our products or
product candidates if the collaborators believe that competitive products are more likely to be successfully developed or can be commercialized under terms that are more economically attractive than
ours;
- •
- a collaborator with marketing and distribution rights to one or more products may not commit sufficient resources to the marketing and
distribution of such product or products;
- •
- collaborators may not properly maintain or defend our intellectual property rights or may use our proprietary information in such a way as to
invite litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential litigation;
- •
- collaborators may infringe the intellectual property rights of third parties, which may expose us to litigation and potential liability;
- •
- disputes may arise between the collaborator and us as to the ownership of intellectual property arising during the collaboration;
- •
- we may grant exclusive rights to our collaborators, which would prevent us from collaborating with others;
- •
- disputes may arise between the collaborators and us that result in the delay or termination of the research, development or commercialization
of our products or product candidates or that result in costly litigation or arbitration that diverts management attention and resources; and
- •
- collaborations may be terminated and, if terminated, may result in a need for additional capital to pursue further development or commercialization of the applicable product candidates.
For example, in 2012, we entered into a stock purchase agreement with Forest pursuant to which Forest reimbursed us for certain external research and development costs and provided us with a $25.0 million loan in exchange for an exclusive right to acquire 100% of our outstanding shares for a one-year period. However, in 2013, Forest decided not to exercise its right to acquire us and terminated the stock purchase agreement. In connection with this termination, we repurchased the $25.0 million loan for €1.00. We no longer have a commercial relationship with Forest, and no rights or obligations remain outstanding under the stock purchase agreement.
Collaboration agreements may not lead to development or commercialization of product candidates in the most efficient manner or at all. If a collaborator of ours were to be involved in a business combination, the continued pursuit and emphasis on our product development or commercialization program could be delayed, diminished or terminated.
If we are not able to establish collaborations, we may have to alter our development and commercialization plans.
The potential commercialization of lefamulin and the development and potential commercialization of other product candidates will require substantial additional cash to fund expenses.
S-28
For some of our product candidates, we may decide to collaborate with pharmaceutical and biotechnology companies for the development and potential commercialization of those product candidates. For example, we intend to seek to commercialize lefamulin through a variety of types of collaboration arrangements outside the United States.
We face significant competition in seeking appropriate collaborators. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator's resources and expertise, the terms and conditions of the proposed collaboration and the proposed collaborator's evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge, and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us for our product candidate. We may also be restricted under future license agreements from entering into agreements on certain terms with potential collaborators. Collaborations are complex and time-consuming to negotiate and document. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators.
If we are unable to reach agreements with suitable collaborators on a timely basis, on acceptable terms, or at all, we may have to curtail the development of a product candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to fund and undertake development or commercialization activities on our own, we may need to obtain additional expertise and additional capital, which may not be available to us on acceptable terms or at all. If we fail to enter into collaborations and do not have sufficient funds or expertise to undertake the necessary development and commercialization activities, we may not be able to further develop our product candidates or bring them to market and generate product revenue.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain patent protection for our technology and products, or if the scope of the patent protection is not sufficiently broad, our competitors could develop and commercialize technology and products similar or identical to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
Our success depends in large part on our ability to obtain and maintain patent protection in the United States and other countries with respect to our proprietary technology and products. We seek to protect our proprietary position by filing patent applications in the United States, Europe and in certain additional foreign jurisdictions related to our novel technologies and product candidates that are important to our business. This process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we will fail to identify patentable aspects of our research and development output before it is too late to obtain patent protection. Moreover, if we license technology or product candidates from third parties in the future, these license agreements may not permit us to control the preparation, filing and prosecution of patent applications, or to maintain or enforce the patents, covering this intellectual property. These agreements could also give our licensors the right to enforce the licensed patents without our involvement, or to decide not to enforce the patents at all.
S-29
Therefore, in these circumstances, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of our business.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patent rights are highly uncertain. Our pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection.
The laws of foreign countries may not protect our rights to the same extent as the laws of the United States. For example, European patent law restricts the patentability of methods of treatment of the human body more than U.S. law does. In addition, we may not pursue or obtain patent protection in all major markets or may not obtain protection that enables us to prevent the entry of third parties onto the market. Assuming the other requirements for patentability are met, currently, the first to file a patent application is generally entitled to the patent. However, prior to March 16, 2013, in the United States, the first to invent was entitled to the patent. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot know with certainty whether we were the first to make the inventions claimed in our U.S. patents or pending U.S. patent applications, or that we were the first to file for patent protection of such inventions.
Moreover, we may be subject to a third party preissuance submission of prior art to the U.S. Patent and Trademark Office, or USPTO, or become involved in opposition, derivation, reexamination, inter partes review, post grant review, interference proceedings or other patent office proceedings or litigation, in the United States or elsewhere, challenging our patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
Even if our patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner. In addition, other companies may attempt to circumvent any regulatory data protection or market exclusivity that we obtain under applicable legislation, which may require us to allocate significant resources to preventing such circumvention. Legal and regulatory developments in the European Union and elsewhere may also result in clinical trial data submitted as part of an MAA becoming publicly available. Such developments could enable other companies to circumvent our intellectual property rights and use our clinical trial data to obtain marketing authorizations in the European Union and in other jurisdictions. Such developments may also require us to allocate significant resources to prevent other companies from circumventing or violating our intellectual property rights. Our attempts to prevent third parties from circumventing our intellectual property and other rights may ultimately be unsuccessful. We may also fail to take the required actions or pay the necessary fees to maintain our patents.
S-30
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our owned and licensed patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and products. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may become involved in lawsuits to protect or enforce our patents or other intellectual property, which could be expensive, time consuming and unsuccessful.
Competitors may infringe our patents, trademarks, copyrights or other intellectual property. To counter infringement or unauthorized use, we may be required to file claims, which can be expensive and time consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their intellectual property. In addition, in a patent infringement proceeding, a court may decide that a patent of ours is invalid or unenforceable, in whole or in part, construe the patent's claims narrowly or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain and could have a material adverse effect on the success of our business.
Our commercial success depends upon our ability and the ability of our collaborators to develop, manufacture, market and sell our product candidates and use our proprietary technologies without infringing the intellectual property and other proprietary rights of third parties. There is considerable intellectual property litigation in the biotechnology and pharmaceutical industries, and we may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, including interference, derivation, inter partes review or post-grant review proceedings before the USPTO. The risks of being involved in such litigation and proceedings may also increase as our product candidates approach commercialization, and as we gain greater visibility as a public company. Third parties may assert infringement claims against us based on existing or future intellectual property rights. We may not be aware of all such intellectual property rights potentially relating to our product candidates. Any freedom-to-operate search or analysis previously conducted may not have uncovered all relevant patents and patent applications, and there may be pending or future patent applications that, if issued, would block us from commercializing lefamulin. Thus, we do not know with certainty whether lefamulin, any other product candidate, or our commercialization thereof, does not and will not infringe any third party's intellectual property.
If we are found to infringe a third party's intellectual property rights, or in order to avoid or settle litigation, we could be required to obtain a license to continue developing and marketing our products and technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us, and could require us to make substantial payments. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages, including treble damages and attorneys' fees if we are found to have willfully infringed a patent or other intellectual property right. A finding of infringement could prevent us from commercializing our
S-31
product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
We may be subject to claims by third parties asserting that we or our employees have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Many of our employees were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee's former employer. Litigation may be necessary to defend against these claims.
In addition, while we typically require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our business was founded as a spin-off from Sandoz. Although all patent applications are fully owned by us and were either filed by Sandoz with all rights fully transferred to us, or filed in our sole name, because we acquired certain of our patents from Sandoz, we must rely on their prior practices, with regard to the assignment of such intellectual property. Our and their assignment agreements may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and be a distraction to management.
Intellectual property litigation could cause us to spend substantial resources and could distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of the ADSs. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development, sales, marketing or distribution activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and products, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate
S-32
collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. However, we cannot guarantee that we have executed these agreements with each party that may have or have had access to our trade secrets or that the agreements we have executed will provide adequate protection. Any party with whom we have executed such an agreement may breach that agreement and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be obtained or independently developed by a competitor, our competitive position would be harmed.
We have not yet registered our trademarks in all of our potential markets, and failure to secure those registrations could adversely affect our business.
Our trademark applications may not be allowed for registration, and our registered trademarks may not be maintained or enforced. During trademark registration proceedings, we may receive rejections. Although we are given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in the USPTO and in comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. If we do not secure registrations for our trademarks, we may encounter more difficulty in enforcing them against third parties than we otherwise would.
Risks Related to Regulatory Approval and Marketing of Our Product Candidates and Other Legal Compliance Matters
Even if we complete the necessary non-clinical studies and clinical trials, the marketing approval process is expensive, time-consuming and uncertain and may prevent us from obtaining approvals for the commercialization of some or all of our product candidates. If we are not able to obtain, or if there are delays in obtaining, required regulatory approvals, in particular in the United States or the European Union, we will not be able to commercialize our product candidates, and our ability to generate revenue will be materially impaired.
Our product candidates, including lefamulin, and the activities associated with their development and commercialization, including their design, testing, manufacture, safety, efficacy, recordkeeping, labeling, storage, approval, advertising, promotion, sale and distribution, are subject to comprehensive regulation by the FDA and by comparable authorities in other countries. Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate. We have not received approval to market lefamulin or any of our other product candidates from regulatory authorities in any jurisdiction.
We have no experience in filing and supporting the applications necessary to obtain marketing approvals for product candidates and expect to rely on third-party contract research organizations to assist us in this process. Securing marketing approval requires the submission of extensive non-clinical and clinical data and supporting information to various regulatory authorities for each therapeutic indication to establish the product candidate's safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory authorities. Regulatory authorities may determine that
S-33
lefamulin or any of our other product candidates are not effective or only moderately effective, or have undesirable or unintended side effects, toxicities, safety profiles or other characteristics that preclude us from obtaining marketing approval or that prevent or limit commercial use.
The process of obtaining marketing approvals is expensive, may take many years, if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. For example, on June 23, 2016, eligible members of the electorate in the United Kingdom decided by referendum to leave the European Union, commonly referred to as "Brexit". Since a significant proportion of the regulatory framework in the United Kingdom is derived from European Union directives and regulations, the referendum could materially change the regulatory regime applicable to the approval of any of our product candidates in the United Kingdom. In addition, since the European Medicines Agency, or EMA, is located in the U.K., the implications for the regulatory review process in the European Union has not been clarified and could result in relocation of the EMA or a disruption in the EMA review process. The FDA and comparable regulatory authorities in other countries have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from non-clinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
Accordingly, if we or our collaborators experience delays in obtaining approval or if we or they fail to obtain approval of our product candidates, the commercial prospects for our product candidates may be harmed and our ability to generate revenues will be materially impaired.
Our failure to obtain marketing approval in jurisdictions other than the United States and Europe would prevent our product candidates from being marketed in these other jurisdictions, and any approval we are granted for our product candidates in the United States and Europe would not assure approval of product candidates in other jurisdictions.
In order to market and sell lefamulin and our other product candidates in jurisdictions other than the United States and Europe, we must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval process varies among countries and can involve additional testing. The time required to obtain approval may differ from that required to obtain FDA approval or approvals from regulatory authorities in the European Union. The regulatory approval process outside the United States and Europe generally includes all of the risks associated with obtaining FDA approval or approvals from regulatory authorities in the European Union. In addition, some countries outside the United States and Europe require approval of the sales price of a drug before it can be marketed. In many countries, separate procedures must be followed to obtain reimbursement and a product may not be approved for sale in the country until it is also approved for reimbursement. We may not obtain marketing, pricing or reimbursement approvals outside the United States and Europe on a timely basis, if at all. Approval by the FDA or regulatory authorities in the European Union does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States and Europe does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA or regulatory authorities in the European Union. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market. Marketing approvals in countries outside the United States and Europe do not ensure pricing approvals in those countries or
S-34
in any other countries, and marketing approvals and pricing approvals do not ensure that reimbursement will be obtained.
Even if we obtain marketing approvals for our product candidates, the terms of approvals and ongoing regulation of our products may limit how we manufacture and market our products and compliance with such requirements may involve substantial resources, which could materially impair our ability to generate revenue.
Even if marketing approval of a product candidate is granted, an approved product and its manufacturer and marketer are subject to ongoing review and extensive regulation, including the potential requirements to implement a risk evaluation and mitigation strategy or to conduct costly post-marketing studies or clinical trials and surveillance to monitor the safety or efficacy of the product. We must also comply with requirements concerning advertising and promotion for any of our product candidates for which we obtain marketing approval. Promotional communications with respect to prescription drugs are subject to a variety of legal and regulatory restrictions and must be consistent with the information in the product's approved labeling. Thus, we will not be able to promote any products we develop for indications or uses for which they are not approved. In addition, manufacturers of approved products and those manufacturers' facilities are required to comply with extensive FDA requirements including ensuring that quality control and manufacturing procedures conform to cGMP, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation and reporting requirements. We and our contract manufacturers could be subject to periodic unannounced inspections by the FDA to monitor and ensure compliance with cGMP.
Accordingly, assuming we receive marketing approval for one or more of our product candidates, we and our contract manufacturers will continue to expend time, money and effort in all areas of regulatory compliance, including manufacturing, production, product surveillance and quality control. If we are not able to comply with post-approval regulatory requirements, we could have the marketing approvals for our products withdrawn by regulatory authorities and our ability to market any future products could be limited, which could adversely affect our ability to achieve or sustain profitability. Thus, the cost of compliance with post-approval regulations may have a negative effect on our operating results and financial condition.
Any product candidate for which we obtain marketing approval will be subject to strict enforcement of post-marketing requirements and we could be subject to substantial penalties, including withdrawal of our product from the market, if we fail to comply with all regulatory requirements or if we experience unanticipated problems with our product candidates, when and if any of them are approved.
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, advertising and promotional activities for such product, will be subject to continual requirements of and review by the FDA and other regulatory authorities. These requirements include, but are not limited to, restrictions governing promotion of an approved product, submissions of safety and other post-marketing information and reports, registration and listing requirements, cGMP requirements relating to manufacturing, quality control, quality assurance and corresponding maintenance of records and documents, and requirements regarding the distribution of samples to physicians and recordkeeping. In addition, even if marketing approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval.
The FDA and other federal and state agencies, including the U.S. Department of Justice, or DOJ, closely regulate compliance with all requirements governing prescription drug products, including requirements pertaining to marketing and promotion of drugs in accordance with the provisions of the approved labeling and manufacturing of products in accordance with cGMP requirements. The FDA and DOJ impose stringent restrictions on manufacturers' communications regarding off-label use and if
S-35
we do not market our products for their approved indications, we may be subject to enforcement action for off-label marketing. Violations of such requirements may lead to investigations alleging violations of the Food, Drug and Cosmetic Act and other statutes, including the False Claims Act and other federal and state health care fraud and abuse laws as well as state consumer protection laws. Our failure to comply with all regulatory requirements, and later discovery of previously unknown adverse events or other problems with our products, manufacturers or manufacturing processes, may yield various results, including:
- •
- litigation involving patients taking our products;
- •
- restrictions on such products, manufacturers or manufacturing processes;
- •
- restrictions on the labeling or marketing of a product;
- •
- restrictions on product distribution or use;
- •
- requirements to conduct post-marketing studies or clinical trials;
- •
- warning or untitled letters;
- •
- withdrawal of the products from the market;
- •
- refusal to approve pending applications or supplements to approved applications that we submit;
- •
- recall of products;
- •
- fines, restitution or disgorgement of profits or revenues;
- •
- suspension or withdrawal of marketing approvals;
- •
- damage to relationships with any potential collaborators;
- •
- unfavorable press coverage and damage to our reputation;
- •
- refusal to permit the import or export of our products;
- •
- product seizure; or
- •
- injunctions or the imposition of civil or criminal penalties.
Non-compliance by us or any future collaborator with regulatory requirements regarding safety monitoring or pharmacovigilance, and with requirements related to the development of products for the pediatric population, can also result in significant financial penalties. Similarly, failure to comply with regulatory requirements regarding the protection of personal information can also lead to significant penalties and sanctions.
Non-compliance with E.U. requirements regarding safety monitoring or pharmacovigilance, and with requirements related to the development of products for the pediatric population, can also result in significant financial penalties. Similarly, failure to comply with the European Union's requirements regarding the protection of personal information can also lead to significant penalties and sanctions.
Governments outside the United States tend to impose strict price controls, which may adversely affect our revenues, if any.
In some countries, particularly the member states of the European Union, the pricing of prescription pharmaceuticals is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after the receipt of marketing approval for a product. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost containment measures. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may
S-36
continue after reimbursement has been obtained. Reference pricing used by various E.U. member states and parallel distribution, or arbitrage between low-priced and high-priced member states, can further reduce prices. In some countries, we may be required to conduct a clinical trial or other studies that compare the cost-effectiveness of our product candidate to other available therapies in order to obtain or maintain reimbursement or pricing approval. Publication of discounts by third-party payors or authorities may lead to further pressure on prices or reimbursement levels within the country of publication and other countries. If reimbursement of our products is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business could be adversely affected.
The FDA's agreement to a Special Protocol Assessment, or SPA, with respect to the study design of our first Phase 3 clinical trial of lefamulin for CABP does not guarantee any particular outcome from regulatory review, including ultimate approval, and may not lead to a faster development or regulatory review or approval process.
We reached agreement with the FDA in September 2015 on a SPA regarding the study design of our first Phase 3 clinical trial of lefamulin for the treatment of CABP. The SPA process is designed to facilitate the FDA's review and approval of drugs by allowing the FDA to evaluate the proposed design and size of Phase 3 clinical trials that are intended to form the primary basis for determining a product candidate's efficacy and safety. Upon specific request by a clinical trial sponsor, the FDA will evaluate the protocol and respond to a sponsor's questions regarding, among other things, primary efficacy endpoints, trial conduct and data analysis, within 45 days of receipt of the request. The FDA ultimately assesses whether the protocol design and planned analysis of the trial are acceptable to support regulatory approval of the product candidate with respect to the effectiveness in the indication studied.
Our agreement with the FDA regarding the SPA may not lead to faster development, regulatory review or approval for lefamulin. Once the FDA and an applicant reach an agreement under the special protocol assessment process regarding the design and size of a clinical trial, the agreement generally cannot be changed after the clinical trial begins. Nevertheless, the FDA may revoke or alter a SPA under defined circumstances, such as changes in the relevant data or assumptions provided by the sponsor or the emergence of new public health concerns. A revocation or alteration in our SPA could significantly delay or prevent approval of any marketing applications we submit for lefamulin. In addition, any significant change to the protocols for our clinical trial subject to the SPA would require prior FDA approval, which could delay implementation of such a change and the conduct of the trial.
Fast track designation by the FDA may not actually lead to a faster development or regulatory review or approval process and does not assure FDA approval of our product candidate.
If a drug is intended for the treatment of a serious or life threatening condition and the drug demonstrates the potential to address unmet medical need for this condition, the drug sponsor may apply for FDA fast track designation. The FDA has designated each of the IV and oral formulations of lefamulin as a qualified infectious disease product, or QIDP, and granted fast track designations to each of these formulations of lefamulin. However, neither the QIDP nor the fast track designation ensures that lefamulin will receive marketing approval or that approval will be granted within any particular timeframe. We may also seek fast track designation for our other product candidates. We may not experience a faster development process, review or approval compared to conventional FDA procedures. In addition, the FDA may withdraw fast track designation if it believes that the designation is no longer supported by data from our clinical development program. Fast track designation alone does not guarantee qualification for the FDA's priority review procedures.
S-37
Priority review designation by the FDA may not lead to a faster regulatory review or approval process and, in any event, does not assure FDA approval of our product candidate.
If the FDA determines that a product candidate offers major advances in treatment or provides a treatment where no adequate therapy exists, the FDA may designate the product candidate for priority review. A priority review designation means that the goal for the FDA to review an application is six months, rather than the standard review period of ten months. Because the FDA designated each of the IV and oral formulations of lefamulin as a QIDP, lefamulin also will receive priority review. We may also request priority review for other product candidates. The FDA has broad discretion with respect to whether or not to grant priority review status to a product candidate, so even if we believe a particular product candidate is eligible for such designation or status, the FDA may decide not to grant it. Moreover, a priority review designation does not necessarily mean a faster regulatory review process or necessarily confer any advantage with respect to approval compared to conventional FDA procedures. Receiving priority review from the FDA does not guarantee approval within the six-month review cycle or thereafter.
Designation of our product candidate, lefamulin, as a Qualified Infectious Disease Product does not assure FDA approval of this product candidate.
A QIDP is "an antibacterial or antifungal drug intended to treat serious or life-threatening infections, including those caused by an antibacterial or antifungal resistant pathogen, including novel or emerging infectious pathogens or certain "qualifying pathogens." Upon the approval of an NDA for a drug product designated by the FDA as a QIDP, the product is granted an additional period of five years of regulatory exclusivity. Even though we have received QIDP designation for the IV and oral formulations of lefamulin, there is no assurance that this product candidate will be approved by the FDA.
Our relationships with healthcare providers, physicians and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which in the event of a violation could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors will play a primary role in the recommendation and prescription of any product candidates, including lefamulin, for which we obtain marketing approval. Our future arrangements with healthcare providers physicians and third-party payors may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute any products for which we obtain marketing approval. Restrictions under applicable federal and state healthcare laws and regulations, include the following:
- •
- the federal Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or
providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation or
arranging of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid.
- •
- the federal False Claims Act imposes criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented, false or fraudulent claims for payment by a federal healthcare program or making a false statement or record material to payment of a false claim or avoiding, decreasing or concealing an obligation to pay money to the federal government,with potential liability including mandatory treble damages and significant per-claim penalties, currently set at $5,500 to $11,000 per false claim;
S-38
- •
- the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for executing a scheme
to defraud any healthcare benefit program or making false statements relating to healthcare matters;
- •
- HIPPA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also imposes
obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
- •
- the federal Physician Payments Sunshine Act requires applicable manufacturers of covered products to report payments and other transfers of
value to physicians and teaching hospitals, with data collection beginning in August 2013; and
- •
- analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws and transparency statutes, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third party payors, including private insurers.
Some state laws require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require product manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. State and foreign laws also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
If our operations are found to be in violation of any of the laws described above or any governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our financial results. We are developing and implementing a corporate compliance program designed to ensure that we will market and sell any future products that we successfully develop from our product candidates in compliance with all applicable laws and regulations, but we cannot guarantee that this program will protect us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion of products from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
S-39
Current and future legislation may increase the difficulty and cost for us and any collaborators to obtain marketing approval of our product candidates and affect the prices we, or they, may obtain.
In the United States and a number of foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of lefamulin or any of our other product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any product candidates, including lefamulin, for which we obtain marketing approval. We expect that current laws, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we, or any collaborators, may receive for any approved products.
For example, in March 2010, President Obama signed into law the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or collectively the PPACA. Among the provisions of the PPACA of potential importance to our business and our product candidates are the following:
- •
- an annual, non-deductible fee on any entity that manufactures or imports specified branded prescription products and biologic agents;
- •
- an increase in the statutory minimum rebates a manufacturer must pay under the Medicaid Drug Rebate Program;
- •
- a new methodology by which rebates owed by manufacturers under the Medicaid Drug Rebate Program are calculated for products that are inhaled,
infused, instilled, implanted or injected;
- •
- expansion of healthcare fraud and abuse laws, including the civil False Claims Act and the federal Anti-Kickback Statute, new government
investigative powers and enhanced penalties for noncompliance;
- •
- a new Medicare Part D coverage gap discount program, in which manufacturers must agree to offer 50% point-of-sale discounts off
negotiated prices of applicable brand products to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer's outpatient products to be covered under Medicare
Part D;
- •
- extension of manufacturers' Medicaid rebate liability to individuals enrolled in Medicaid managed care organizations;
- •
- expansion of eligibility criteria for Medicaid programs;
- •
- expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program;
- •
- new requirements to report certain financial arrangements with physicians and teaching hospitals;
- •
- a new requirement to annually report product samples that manufacturers and distributors provide to physicians;
- •
- a new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research,
along with funding for such research;
- •
- a new Independent Payment Advisory Board, or IPAB, which has authority to recommend certain changes to the Medicare program to reduce
expenditures by the program that could result in reduced payments for prescription products; and
- •
- established the Center for Medicare and Medicaid Innovation within CMS to test innovative payment and service delivery models.
S-40
Other legislative changes have been proposed and adopted since the PPACA was enacted. These changes include the Budget Control Act of 2011, which, among other things, led to aggregate reductions to Medicare payments to providers of up to 2% per fiscal year that started in 2013 and, due to subsequent legislation, will continue until 2025. In addition, the American Taxpayer Relief Act of 2012, among other things, reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding and otherwise affect the prices we may obtain for any of our product candidates for which regulatory approval is obtained. In addition, there have also been recent public announcements by members of the U.S. Congress and the incoming presidential administration regarding their plans to repeal and replace the PPACA and Medicare. However, it remains unclear how a repeal or replacements of these programs might affect the prices we may obtain for any of our product candidates for which regulatory approval is obtained.
We expect that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in additional reductions in Medicare and other healthcare funding, more rigorous coverage criteria, new payment methodologies and in additional downward pressure on the price that we receive for any approved product. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability, or commercialize our products. Moreover, legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We cannot be sure whether additional legislative changes will be enacted, or whether the FDA regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be. In addition, increased scrutiny by the U.S. Congress of the FDA's approval process may significantly delay or prevent marketing approval, as well as subject us and any collaborators to more stringent product labeling and post-marketing testing and other requirements.
We are subject to anti-corruption laws, as well as export control laws, customs laws, sanctions laws and other laws governing our operations. If we fail to comply with these laws, we could be subject to civil or criminal penalties, other remedial measures and legal expenses, which could adversely affect our business, results of operations and financial condition.
Our operations are subject to anti-corruption laws, including the U.S. Foreign Corrupt Practices Act, or FCPA, and other anti-corruption laws that apply in countries where we do business and may do business in the future. The FCPA and these other laws generally prohibit us, our officers, and our employees and intermediaries from bribing, being bribed or making other prohibited payments to government officials or other persons to obtain or retain business or gain some other business advantage. We may in the future operate in jurisdictions that pose a high risk of potential FCPA violations, and we may participate in collaborations and relationships with third parties whose actions could potentially subject us to liability under the FCPA or local anti-corruption laws. In addition, we cannot predict the nature, scope or effect of future regulatory requirements to which our international operations might be subject or the manner in which existing laws might be administered or interpreted.
Compliance with the FCPA is expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition, the FCPA presents particular challenges in the pharmaceutical industry, because, in many countries, hospitals are operated by the government, and doctors and other hospital employees are considered foreign officials. Certain payments to hospitals in connection with clinical trials and other work have been deemed to be improper payments to government officials and have led to FCPA enforcement actions.
S-41
We are also subject to other laws and regulations governing our international operations, including regulations administered by the governments of the United States, and authorities in the European Union, including applicable export control regulations, economic sanctions on countries and persons, customs requirements and currency exchange regulations, collectively referred to as the trade control laws.
There is no assurance that we will be effective in ensuring our compliance with all applicable anti-corruption laws, including the FCPA or other legal requirements, including trade control laws. If we are not in compliance with the FCPA and other anti-corruption laws or trade control laws, we may be subject to criminal and civil penalties, disgorgement and other sanctions and remedial measures, and legal expenses, which could have an adverse impact on our business, financial condition, results of operations and liquidity. Likewise, any investigation of any potential violations of the FCPA, other anti-corruption laws or trade control laws by U.S. or other authorities could also have an adverse impact on our reputation, our business, results of operations and financial condition.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations currently, and may in the future, involve the use of hazardous and flammable materials, including chemicals and medical and biological materials, and produce hazardous waste products. Even if we contract with third parties for the disposal of these materials and wastes, we cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials or disposal of hazardous wastes, we could be held liable for any resulting damages, and any liability could exceed our resources.
Although we maintain workers' compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We also maintain a general liability program for some of the risks, but our program includes limited environmental damage coverage and has an annual aggregate coverage limit of $2.0 million.
In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties or other sanctions.
Our employees may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, which could cause significant liability for us and harm our reputation.
We are exposed to the risk of employee fraud or other misconduct, including intentional failures to comply with FDA regulations or similar regulations of comparable non-U.S. regulatory authorities, provide accurate information to the FDA or comparable non-U.S. regulatory authorities, comply with manufacturing standards we have established, comply with federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable non-U.S. regulatory authorities, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements.
S-42
Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws, standards or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
We rely significantly on information technology and any failure, inadequacy, interruption or security lapse of that technology, including any cyber security incidents, could harm our ability to operate our business effectively.
Despite the implementation of security measures, our internal computer systems and those of third parties with which we contract are vulnerable to damage from cyber-attacks, computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. System failures, accidents or security breaches could cause interruptions in our operations, and could result in a material disruption of our clinical and commercialization activities and business operations, in addition to possibly requiring substantial expenditures of resources to remedy. The loss of clinical trial data could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and our product research, development and commercialization efforts could be delayed.
Risks Related to Employee Matters and Managing Growth
Our future success depends on our ability to retain our chief executive officer and other key executives and to attract, retain and motivate qualified personnel.
We are highly dependent on Dr. Colin Broom, our Chief Executive Officer, and the other principal members of our management and scientific teams. Although we have formal employment agreements with each of our executive officers, these agreements do not prevent our executives from terminating their employment with us at any time. We do not maintain "key person" insurance on any of our executive officers. The unplanned loss of the services of any of these persons might impede the achievement of our research, development and commercialization objectives.
Recruiting and retaining qualified scientific, clinical, manufacturing and sales and marketing personnel, including in the United States where we plan to expand our physical presence, will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
S-43
We expect to expand our development, regulatory and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of drug development, regulatory affairs and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The physical expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
Risks Related to the Offering and Ownership of the ADSs or Common Shares
An active trading market for the ADSs may not be sustained, and our common shares are not listed on any national securities exchange.
The ADSs began trading on the NASDAQ Global Market on September 18, 2015. Given the limited trading history of the ADSs, there is a risk that an active trading market for the ADSs will not be sustained, which could put downward pressure on the market price of the ADSs and thereby affect the ability of our security holders to sell their ADSs. In addition, our common shares are not directly listed on any national securities exchange. As a result, common shareholders who exercise their rights to subscribe for new common shares in this offering may not be able to sell their common shares, unless they are deposited for delivery of ADSs to be sold on the NASDAQ Global Market.
The trading price of the ADSs may be volatile and fluctuate substantially and may decline below the subscription price, which could result in substantial losses for purchasers of new common shares and new ADSs in this offering.
The trading price of the ADSs has been and is likely to continue to be volatile. The stock market in general and the market for smaller biopharmaceutical companies in particular have experienced significant volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, you may not be able to sell the ADSs, including the common shares underlying the ADSs, at or above the offering price. The market price for the ADSs may be influenced by many factors, including:
- •
- the success of competitive products or technologies;
- •
- results of clinical trials of our product candidates or those of our competitors;
- •
- regulatory delays and greater government regulation of potential products due to adverse events;
- •
- regulatory or legal developments in the United States, the European Union and other countries;
- •
- developments or disputes concerning patent applications, issued patents or other proprietary rights;
- •
- the recruitment or departure of key scientific or management personnel;
- •
- the level of expenses related to any of our product candidates or clinical development programs;
- •
- the results of our efforts to discover, develop, acquire or in-license additional product candidates or products;
S-44
- •
- actual or anticipated changes in estimates as to financial results, development timelines or recommendations by securities analysts;
- •
- variations in our financial results or those of companies that are perceived to be similar to us;
- •
- changes in the structure of healthcare payment systems;
- •
- market conditions in the pharmaceutical and biotechnology sectors;
- •
- general economic, industry and market conditions; and
- •
- the other factors described in this "Risk Factors" section.
In the past, following periods of volatility in the market price of a company's securities, securities class-action litigation has often been instituted against that company. We also may face securities class-action litigation if we cannot obtain regulatory approvals for or if we otherwise fail to commercialize lefamulin or any of our other product candidates. Such litigation, if instituted against us, could cause us to incur substantial costs to defend such claims and divert management's attention and resources.
After this offering, our senior managers, supervisory board members and principal shareholders, if they choose to act together, will continue to have the ability to control most matters submitted to shareholders for approval.
Upon the closing of this offering, assuming the full exercise of the common share rights and ADSs rights in this offering, our senior managers and supervisory board members, combined with our shareholders, and their respective affiliates who owned more than 5% of our outstanding common shares before this offering, will, in the aggregate, beneficially own approximately 79.2% of our share capital. If our senior managers and supervisory board members, combined with our existing principal shareholders and their affiliated entities, purchase all of the ADSs and common shares they have indicated an interest in purchasing in this offering, including the purchase from Cantor Fitzgerald & Co., if Cantor Fitzgerald & Co. agrees to enter into an underwriting agreement following the expiration of the offerings of ADS rights and common share rights, of common shares that are not subscribed for in this offering, the number of common shares beneficially owned by our supervisory board members, senior managers, existing principal shareholders, and their respective affiliates will be, in the aggregate, approximately 77.4% of our share capital. As a result, if these shareholders were to choose to act together, they would be able to control most matters submitted to our shareholders for approval, as well as our management and affairs. For example, these persons, if they choose to act together, would control the election of supervisory board members and approval of any merger, consolidation or sale of all or substantially all of our assets.
We do not expect to pay dividends in the foreseeable future.
We have not paid any dividends on our common shares since our incorporation. Even if future operations lead to significant levels of distributable profits, we currently intend that earnings, if any, will be reinvested in our business and that dividends will not be paid until we have an established revenue stream to support continuing dividends. Payment of future dividends to securityholders will be at the discretion of the management board, subject to the approval of the supervisory board after taking into account various factors including our business prospects, cash requirements, financial performance, debt covenant limitations and new product development. In addition, Austrian law imposes limitations on our ability to pay dividends. Under Austrian law, a company may only pay dividends if the distribution of dividends is proposed by the management board and the supervisory board and resolved by the company's shareholders at a general meeting. Our ability to pay dividends is assessed by our management board based primarily on our unconsolidated financial statements prepared in accordance with the Austrian Commercial Code (Unternehmensgesetzbuch). Dividends may be paid only after the relevant balance sheet date from the net profit (Bilanzgewinn) recorded in our unconsolidated annual financial statements as approved by our supervisory board or by our
S-45
shareholders at a general meeting. In determining the amount available for distribution, the annual net income must be adjusted to account for any accumulated undistributed net profit or loss from previous years as well as for withdrawals from or allocations to reserves. Certain reserves must be established by law, and allocation to such reserves must therefore be deducted from the annual net income in order to calculate the annual net profit.
We expect that ADSs representing only a relatively small percentage of our common shares will be publicly traded following this offering, which may limit the liquidity of your investment and may have a material adverse effect on the price of the ADSs.
After this offering, assuming the full exercise of the common share rights and ADSs rights in this offering, only 20.8% of our common shares will be beneficially owned by parties other than our supervisory board members, senior management, shareholders holding 5% or more of our common shares, and their respective affiliates. If our existing principal shareholders and their affiliated entities purchase all of the ADSs and common shares they have indicated an interest in purchasing in this offering, including the purchase from Cantor Fitzgerald & Co., if Cantor Fitzgerald & Co. agrees to enter into an underwriting agreement following the expiration of the offerings of ADS rights and common share rights, of common shares that are not subscribed for in this offering, only 22.6% of our shares will be beneficially owned by parties other than our supervisory board members, senior management, existing principal shareholders, and their respective affiliates. As a result, ADSs representing only a relatively small number of our common shares will be actively traded in the public market. Limited liquidity may increase the volatility of the prices of the ADSs and the underlying common shares.
The ADSs and our common shares do not trade on any exchange outside of the United States.
The ADSs are listed only in the United States on The NASDAQ Global Market, and we have no plans to list the ADSs or our common shares in any other jurisdiction. As a result, a holder of ADSs or common shares outside of the United States may not be able to effect transactions in the ADSs as readily as the holder may if the ADSs were listed on an exchange in that holder's home jurisdiction. Additionally, a holder of common shares may not be able to effect transactions in our common shares without depositing such common shares with our depositary in exchange for the issuance of ADSs representing such common shares.
The sale of a substantial number of ADSs may cause the market price of the ADSs to decline.
Sales of a substantial number of our common shares or ADS, or the perception in the market that these sales could occur, could reduce the market price of the ADSs. After this offering, assuming all of the common shares offered in this offering are sold, including common shares represented by ADSs, we will have 2,719,025 common shares outstanding, including the common shares underlying ADSs. All of the 588,127 common shares offered hereby may be resold in the public market, after depositing them for delivery of ADSs, immediately without restriction, unless purchased by our affiliates. Of the remaining 2,130,898 common shares, 951,926 common shares are currently restricted as a result of securities laws or lock-up agreements, but will become eligible to be sold at various times after this offering. Moreover, holders of an aggregate of 951,306 common shares have rights, subject to specified conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other shareholders. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described in the "Plan of Distribution" section of this prospectus supplement. To the extent any of these shares are sold into the market, particularly in substantial quantities, the market price of the ADSs could decline.
S-46
Future issuances of common shares pursuant to our equity incentive plans could also result in additional dilution of the percentage ownership of our shareholders. We filed a registration statement on Form S-8 on November 18, 2015 that covers an aggregate of 201,632 common shares reserved for issuance pursuant to our equity incentive plans. Additionally, the majority of common shares that may be issued under our equity compensation plans also remain subject to vesting in tranches over a four year period. As of September 30, 2016, an aggregate of 49,161 options to purchase our common shares had vested and become exercisable.
If a large number of the ADSs are sold in the public market after they become eligible for sale, the sales could reduce the trading price of the ADSs and impede our ability to raise future capital.
We are an "emerging growth company," and the reduced disclosure requirements applicable to emerging growth companies may make the ADSs and our common shares less attractive to investors.
We are an "emerging growth company," as that term is used in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and may remain an emerging growth company until December 31, 2020 or such earlier time that we are no longer an emerging growth company. For so long as we remain an emerging growth company, we are permitted and may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. These provisions include:
- •
- an exemption from compliance with the auditor attestation requirement of Section 404 of the Sarbanes-Oxley Act of 2002, or the
Sarbanes-Oxley Act, on the design and effectiveness of our internal controls over financial reporting;
- •
- an exemption from compliance with any requirement that the Public Company Accounting Oversight Board may adopt regarding mandatory audit firm
rotation or a supplement to the auditor's report providing additional information about the audit and the financial statements;
- •
- reduced disclosure about the company's executive compensation arrangements; and
- •
- exemptions from the requirements to obtain a non-binding advisory vote on executive compensation or a shareholder approval of any golden parachute arrangements.
We may choose to take advantage of some, but not all, of the available exemptions. We may take advantage of these provisions until December 31, 2020 or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company upon the earlier to occur of: the last day of the fiscal year in which we have more than $1 billion in annual revenues; the date we qualify as a "large accelerated filer," with more than $700 million in market value of our share capital held by non-affiliates; or the issuance by us of more than $1 billion of non-convertible debt over a three-year period.
We may choose to take advantage of some, but not all, of the available benefits under the JOBS Act. We cannot predict whether investors will find the ADSs or common shares less attractive if we rely on these exemptions. If some investors find the ADSs less attractive as a result, there may be a less active trading market for the ADSs and the market price of the ADSs may be more volatile.
In addition, the JOBS Act also provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this exemption and, therefore, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required by the International Accounting Standards Board. Furthermore, beginning on January 1, 2017, when we expect to prepare our financial statements in accordance with generally accepted accounting principles in the United States, or U.S. GAAP, rather than the International Financial Reporting Standards, or IFRS, we will also adopt new or revised
S-47
U.S. GAAP accounting standards on the relevant dates on which adoption of such standards is required for other public companies that are not emerging growth companies.
We expect to lose our foreign private issuer status on January 1, 2017, which will require us to comply with the Exchange Act's domestic reporting regime, as well as NASDAQ's domestic company corporate governance requirements, which we expect will cause us to incur significant legal, accounting and other expenses.
We determined that, as of June 30, 2016, we no longer qualified as a "foreign private issuer" under the rules and regulations of the SEC. As a result, beginning January 1, 2017, we anticipate that our future annual filings with the SEC will be made on Form 10-K (including our annual report for the year ending December 31, 2016) rather than on Form 20-F. In addition, commencing on January 1, 2017, we plan to expand reporting consistent with that of a domestic U.S. filer, including filing quarterly reports on Form 10-Q and current reports on Form 8-K. In addition, we expect to prepare our financial statements in accordance with U.S. GAAP, rather than IFRS, and we will also adopt new or revised U.S. GAAP accounting standards on the relevant dates on which adoption of such standards is required for other public companies that are not emerging growth companies. We will also be subject to SEC rules governing the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; the provisions of Regulation Fair Disclosure, which regulate the selective disclosure of material information; and the sections of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities and establishing insider liability for profits realized from any "short-swing" transactions in our equity securities. In addition, beginning January 1, 2017, we will also be subject to the NASDAQ Stock Market listing requirements applicable to domestic U.S. issuers.
We expect the regulatory and compliance costs to comply with the reporting and corporate governance requirements applicable to a U.S. domestic issuer will be significantly higher than the costs we have historically incurred as a foreign private issuer. As a result, we expect that the loss of our foreign private issuer status will increase our legal and financial compliance costs and may make some activities highly time consuming and costly. We also expect that our loss of foreign private issuer status may make it more difficult and expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified members of our supervisory board.
Until January 1, 2017, when we lose our foreign private issuer status, we will be exempt from a number of rules under the U.S. securities laws and are permitted to file less information with the SEC than U.S. companies. This may limit the information available to holders of the ADSs.
We are currently a "foreign private issuer," as defined in the rules and regulations of the SEC, and, consequently, we are not subject to all of the disclosure requirements applicable to companies organized within the United States. We will cease to be able to rely on the foreign private issuer exemptions on January 1, 2017. While we remain a foreign private issuer, we are exempt, for example, from certain rules under the U.S. Securities Exchange Act of 1934, as amended, or the Exchange Act, that regulate disclosure obligations and procedural requirements related to the solicitation of proxies, consents or authorizations applicable to a security registered under the Exchange Act. In addition, our senior management and supervisory board members are exempt from the reporting and "short-swing" profit recovery provisions of Section 16 of the Exchange Act and related rules with respect to their purchases and sales of our securities. Foreign private issuers are also exempt from Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information. Moreover, foreign private issuers are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. public companies. Accordingly, while we are a foreign private issuer, there may be less publicly available information concerning our company than there is
S-48
for U.S. public companies, and you may not have the same protections afforded to shareholders of companies that are not foreign private issuers.
Until January 1, 2017, when we lose our foreign private issuer exemption, we intend to rely on NASDAQ Stock Market rules that permit us to comply with applicable Austrian corporate governance practices, rather than the corresponding domestic U.S. corporate governance practices, and therefore your rights as a shareholder will differ from the rights you would have as a shareholder of a domestic U.S. issuer.
As a foreign private issuer whose ADSs are listed on The NASDAQ Global Market, we are permitted in certain cases to follow Austrian corporate governance practices instead of the corresponding requirements of the NASDAQ Stock Market rules. A foreign private issuer that elects to follow a home country practice instead of NASDAQ requirements must submit to NASDAQ in advance a written statement from an independent counsel in such issuer's home country certifying that the issuer's practices are not prohibited by the home country's laws. In addition, a foreign private issuer must disclose in its annual report filed with the SEC each such requirement that it does not follow and describe the home country practice followed instead of any such requirement. We do not intend to follow NASDAQ's requirements to seek shareholder approval for the implementation of certain equity compensation plans and issuances of our common shares under such plans. In accordance with Austrian law, we are not required to seek shareholder approval in connection with the implementation of employee equity compensation plans unless such plans provide for the issuance of common shares to supervisory board members or the management board does not hold a valid authorization to issue common shares for such purpose. In addition, we do not intend to follow NASDAQ's requirements to seek shareholder approval for certain issuances of equity securities, including this offering. Accordingly, our shareholders may not be afforded the same protection as provided under NASDAQ's corporate governance rules while we are a foreign private issuer.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common shares and ADSs. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the trading price of the ADSs to decline and delay the development of our product candidates. Pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
We incur increased costs as a result of operating as a public company, and our management is required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company we incur, and particularly after we are no longer an emerging growth company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The NASDAQ Global Market and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. Our management and other personnel need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations have increased our legal and financial compliance costs and make some activities more time-consuming and costly. For example, these rules and regulations have made it more expensive for us to obtain director and officer liability insurance, which in turn could make it more difficult for us to attract and retain qualified members of our supervisory board.
For as long as we remain an emerging growth company, we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are
S-49
not emerging growth companies as described elsewhere in this "Risk Factors" section. We may remain an emerging growth company until December 31, 2020, although if the market value of our share capital that is held by non-affiliates exceeds $700 million as of any June 30 before that time or if we have annual gross revenues of $1 billion or more in any fiscal year, we would cease to be an emerging growth company as of December 31 of the applicable year. We also would cease to be an emerging growth company if we issue more than $1 billion of non-convertible debt over a three-year period.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, security holders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of the ADSs.
Effective internal control over financial reporting is necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, is designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us, as and when required, conducted in connection with Section 404 of the Sarbanes-Oxley Act, or Section 404, or any subsequent testing by our independent registered public accounting firm, as and when required, may reveal deficiencies in our internal control over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of the ADSs.
Pursuant to Section 404, we will be required to furnish a report by our management on our internal control over financial reporting. However, as an emerging growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm until we are no longer an emerging growth company. To achieve compliance with Section 404 within the prescribed period, we are engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants and adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude within the prescribed timeframe that our internal control over financial reporting is effective as required by Section 404. This could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
U.S. investors may have difficulty enforcing civil liabilities against us, our supervisory board members or senior management and the experts named in this prospectus supplement.
We are incorporated under the laws of Austria, and our registered offices and a substantial portion of our assets are located outside of the United States. In addition, many of the members of our management board and our supervisory board and our senior management are residents of Austria and jurisdictions other than the United States. As a result, it may not be possible to effect service of process on such persons or us in the United States or to enforce judgments obtained in U.S. courts against them or us based on civil liability provisions of the securities laws of the United States. In addition, it is questionable whether a court in Austria would accept jurisdiction and impose civil liability if proceedings were commenced in such court predicated solely upon U.S. federal securities laws. As the United States and Austria do not currently have a treaty providing for reciprocal recognition and enforcement of judgments in civil and commercial matters (other than arbitration awards in such matters), a final judgment for payment of money rendered by a federal or state court in the United States based on civil liability, whether or not predicated solely upon U.S. federal securities laws, will
S-50
not be enforceable, either in whole or in part, in Austria. However, if the party in whose favor such final judgment is rendered brings a new suit in a competent court in Austria, such party may submit to the Austrian court the final judgment rendered in the United States. Under such circumstances, a judgment by a federal or state court of the United States against the company will be regarded by an Austrian court only as evidence of the outcome of the dispute to which such judgment relates, and an Austrian court may choose to re-hear the dispute. In addition, awards of punitive damages in actions brought in the United States or elsewhere may be unenforceable in Austria. An award for monetary damages under the U.S. securities laws would be considered punitive if it does not seek to compensate the claimant for loss or damage suffered and is intended to punish the defendant. For more information, see the "Service of Process and Enforcement of Judgments" section in the accompanying prospectus.
Holders of ADSs may not have the same voting rights as the holders of our common shares and may not receive voting materials in time to be able to exercise their right to vote.
Except as described in this prospectus supplement and the accompanying prospectus, holders of the ADSs will not be able to exercise voting rights attaching to the common shares evidenced by the ADSs. Holders of the ADSs will have the right to instruct the depositary with respect to the voting of the common shares represented by the ADSs. If we tell the depositary to solicit your voting instructions, the depositary is required to endeavor to carry out your instructions. If we do not tell the depositary to solicit your voting instructions (and we are not required to do so), you can still send instructions, and, in that case, the depositary may, but is not required to, carry out those instructions. You may not receive voting materials in time to instruct the depositary to vote, and it is possible that you, or persons who hold their ADSs through brokers, dealers or other third parties, will not have the opportunity to exercise a right to vote.
Holders of ADSs may not have the same rights to participate in subscription rights offering as holders of our common shares.
Under Austrian law, whenever we issue new common shares, we are required by law, subject to certain limited exceptions, to grant subscription rights to all holders of our common shares, giving them the right to purchase a sufficient number of new common shares to maintain their existing ownership percentage. Although we have taken steps to offer common shares (in the form of ADSs) to holders of ADSs in connection with this offering, we are not required to do so, and we are not required to ensure that holders of ADSs have an opportunity to participate in this subscription rights offering or any future subscription rights offering on the same terms as holders of our common shares. For example, the period during which ADS holders may subscribe for and purchase new common shares (in the form of ADSs) in this subscription rights offering is shorter than the time period allowed for holders of our common shares. In addition, if we tell the depositary for the ADSs to provide holders of ADSs an opportunity to participate in any subscription rights offering, the depositary is required to endeavor to do so. However, if we do not tell the depositary to provide holders of ADSs an opportunity to participate in any subscription rights offering (and we are not required to do so), the depositary may, but is not required to, assist such ADS holder in subscribing for and purchasing the number of common shares representing such holder's pro rata ownership percentage in us.
Holders of ADSs may not receive subscription materials related to this subscription rights offering or any future subscription rights offering in time to instruct the depositary to subscribe for and purchase new common shares to which such ADS holders might otherwise be entitled, if they directly held our common shares. It is possible that registered holders of ADSs and persons who hold their ADSs through brokers, dealers or other third parties, will not have the opportunity to participate in this subscription rights offering or any future subscription rights offering. To the extent holders of ADSs are unable to exercise subscription rights, they will expire and holders of ADSs will not realize
S-51
any value from the grant of such subscription rights. In any such case, such holder's equity interest in our company would be diluted proportionately.
Holders of ADSs may not receive distributions on our common shares represented by the ADSs or any value for them if it is illegal or impractical to make them available to such holders.
The depositary for the ADSs has agreed to pay to holders of ADSs or distribute the cash dividends or other distributions it or the custodian receives on our common shares or other deposited securities after deducting its fees and expenses. Holders of ADSs will receive these distributions in proportion to the number of our common shares their ADSs represent. However, in accordance with the limitations set forth in the deposit agreement, it may be unlawful or impractical to make a distribution available to holders of ADSs. We have no obligation to take any other action to permit the distribution of the ADSs, common shares, rights or anything else to holders of the ADSs. This means that holders of ADSs may not receive the distributions we make on our common shares or any value from them if it is unlawful or impractical to make them available to such holders. These restrictions may have a material adverse effect on the value of the ADSs.
We are exposed to risks related to currency exchange rates.
A significant portion of our expenses are denominated in currencies other than euros. Because our financial statements are presented in euros, changes in currency exchange rates have had and could have a significant effect on our operating results when our operating results are translated into U.S. dollars. Exchange rate fluctuations between local currencies and the euro create risk in several ways, including the following:
- •
- weakening of the euro may increase the euro cost of overseas research and development expenses and the cost of sourced product components
outside of the euro zone;
- •
- strengthening of the euro may decrease the value of our revenues denominated in other currencies;
- •
- the exchange rates on non-euro transactions and cash deposits can distort our financial results; and
- •
- commercial pricing and profit margins are affected by currency fluctuations.
In addition, we expect to receive the net proceeds from this offering in euro. The strengthening of the U.S. dollar against the euro following this offering may impact our ability to use the net proceeds from this offering for general corporate purposes in the United States, as a strengthening of the U.S. dollar may increase the cost of using such proceeds in the United States. See "Use of Proceeds."
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation.
We are organized as a stock corporation (Aktiengesellschaft) and incorporated under Austrian law. The rights of holders of our common shares and, therefore, certain of the rights of holders of the ADSs, are governed by Austrian law, including the provisions of the Austrian Stock Corporation Act, and by our articles of association. These rights differ in important respects from the rights of shareholders in typical U.S. corporations. These differences include, in particular:
- •
- Under Austrian law, certain important resolutions, including, for example, capital decreases, mergers, conversions and spin-offs, the issuance of convertible bonds or bonds with warrants attached and the dissolution of the stock corporation (apart from insolvency and certain other proceedings), require the vote of a 75% majority (and, in some cases, as high as a 90% majority) of the capital present or represented at the relevant general meeting of shareholders. Therefore, the holder or holders of a blocking minority of 25% or, depending on the attendance level at
S-52
- •
- As a general rule under Austrian law, a shareholder has no direct recourse against the members of the management board or supervisory board of an Austrian stock corporation in the event that it is alleged that any of them have breached their duty of loyalty or duty of care to the Austrian stock corporation. Apart from insolvency or other special circumstances, only the Austrian stock corporation itself has the right to claim damages from members of the management or supervisory board. An Austrian stock corporation may waive or settle these damages claims only after five years, if the shareholders approve the waiver or settlement at the general meeting with a simple majority of the votes cast and no group of shareholders holding, in the aggregate, at least 20% (and in some cases, 5%) of the Austrian stock corporation's share capital objects to such waiver or settlement and has its opposition formally noted in the minutes of the general meeting. However, Austrian courts acknowledge a waiver or settlement of claims for damages earlier if all shareholders consent to such waiver. See "Description of Share Capital—Differences in Corporate Law" in the accompanying prospectus for a description of the principal differences between the provisions of the Austrian Stock Corporation Act and, for example, the Delaware General Corporation Law relating to shareholders' rights and protections.
the general meeting, the holder or holders of a smaller percentage of the shares in an Austrian stock corporation may be able to block any such votes, possibly to our detriment or the detriment of our other shareholders.
We may be classified as a passive foreign investment company for our tax year ending December 31, 2016, which may result in adverse U.S. federal income tax consequence to U.S. holders.
Based on our estimated gross income and average value of our gross assets and the nature of our business, we do not believe that we were a "passive foreign investment company," or PFIC, for U.S. federal income tax purposes for our tax years ended December 31, 2014 or December 31, 2015. Although a final determination regarding our PFIC status for our tax year ended December 31, 2016 cannot be made until our year-end results are known, it currently appears that there is a higher likelihood than in prior years that we may be a PFIC for the 2016 tax year. A corporation organized outside the United States generally will be classified as a PFIC for U.S. federal income tax purposes in any taxable year in which at least 75% of its gross income is passive income or on average at least 50% of the gross value of its assets is attributable to assets that produce passive income or are held for the production of passive income. Passive income for this purpose generally includes dividends, interest, royalties, rents and gains from commodities and securities transactions. Our status in any taxable year will depend on our assets and activities in each year, and because this is a factual determination made annually after the end of each taxable year, there can be no assurance that we will not be considered a PFIC for the current taxable year or any future taxable year. The market value of our assets may be determined in large part by reference to the market price of the ADSs, which may fluctuate considerably given that market prices of biotechnology companies have been especially volatile. If we were to be treated as a PFIC for the tax year ending December 31, 2016, or any other future taxable year during which a U.S. holder held the ADSs, however, certain adverse U.S. federal income tax consequences could apply to the U.S. holder. We currently intend to make available the information necessary to permit a U.S. holder to make a valid QEF election, which may mitigate some of the adverse U.S. federal income tax consequences that could apply to a U.S. holder of ADSs. See "Taxation—Taxation in the United States—Taxation of Rights Shares and ADSs—Passive Foreign Investment Company Considerations."
There is no certainty that the issuance of the new common shares or new ADSs will occur when or as we expect.
Until the issuance of the new common shares, including those represented by new ADSs, that are subscribed for in this offering are registered with the commercial register in Austria, you will not
S-53
receive any common shares or ADSs for which you have subscribed. We cannot assure you that the registration of such common shares, which must be approved by a judge of the commercial court in Vienna prior to being issued, will take place when or as anticipated or at all. If the judge does not approve the creation of the new common shares, this offering will need to be cancelled, and you will not receive any common shares or ADSs for which you have subscribed, or purchased from Cantor Fitzgerald & Co., as the case may be, in this financing. Further, if there is a delay in the judge's approval from the time we currently expect, you may receive the common shares or ADSs you purchase later than December 19, 2016.
Common shareholder and ADS holders who do not subscribe for all of the new common shares or new ADSs to which they are entitled to subscribe will experience a reduction in their proportionate ownership interest in us.
If you do not subscribe for all of the new common shares or new ADSs you are entitled to subscribe for, your proportionate ownership interest in us will be reduced and the percentage that your existing common shares, in the form of common shares or ADSs, represents of our total share capital will be reduced accordingly. If all of our common shareholder and ADS holders fully exercised their rights to subscribe for new common shares and new ADSs in this offering, we expect this offering will result in an immediate dilution of our as adjusted net tangible book value of €9.48 ($8.28) per common share and $0.83 per ADS to existing common shareholders and ADS holders who exercise subscription rights in this offering. See "Dilution" in this prospectus supplement for more information.
Rights to subscribe for new common shares and new ADSs are not transferable, except to the extent otherwise provided under Austrian law.
The rights to subscribe for new common shares and new ADSs in this offering are not transferable, except to the extent otherwise provided under Austrian law. As a result, you will not be able to realize the inherent value of your subscription rights without exercising such subscription rights and paying the applicable subscription price for any new common shares or new ADSs for which you subscribe. Existing common shareholders and ADSs holders who do not exercise their subscription rights will receive no economic value for their subscription rights, as such unexercised subscription rights will become void and worthless at the end of the subscription period.
Furthermore, the value of subscription rights depends largely on the trading price of the ADSs. Therefore, a significant decline in the trading price of the ADSs could also adversely affect the value of such subscription rights.
Holders of ADSs are subject to exchange rate risk in connection with the ADS rights offering.
In the event that the U.S. dollar weakens against the euro during the ADS rights exercise period, holders of ADSs subscribing for new ADSs may pay more than the ADS subscription payment of $4.68 per new ADS and will receive fewer new ADSs than they have subscribed for.
The estimated ADS subscription price is $4.25 per new ADS. As each ADS represents one tenth (1/10) of a common share, the estimated subscription price per ADS is one tenth (1/10) the U.S. dollar equivalent of the per common share subscription price of €40.14, translated into U.S. dollars at the rate of €1.00 to $1.0588, the exchange rate at the European Central Bank on November 28, 2016. A subscriber of new ADSs must pay an ADS subscription payment of $4.68 per ADS, which is the estimated ADS subscription price of $4.25 per new ADS, plus an additional 10% which represents an allowance for potential fluctuations in the exchange rate between the euro and the U.S. dollar, currency conversion expenses and an ADS issuance fee of the depositary of $0.05 per new ADS. The 10% allowance is meant to increase the likelihood that the depositary will have sufficient funds to pay the actual ADS subscription price in light of a possible appreciation of the euro against the U.S. dollar between the date of this prospectus supplement and the end of the ADS rights exercise period and to
S-54
pay currency conversion expenses as well as the ADS issuance fee of the depositary of $0.05 per ADS. On or about December 13, 2016, the ADS rights agent will determine the actual U.S. dollar ADS subscription price by converting the euro common share subscription price into U.S. dollars at the exchange rate assigned by it on that date. If the actual U.S. dollar ADS subscription price (plus the ADS issuance fee and the currency conversion expenses) is less than the ADS subscription payment, the ADS rights agent will refund such excess amount to the subscribing ADS holder without interest. However, if the amount of the ADS subscription payment you paid to the ADS rights agent is, for any reason, including due to currency exchange rate fluctuations, insufficient to pay the subscription price in euro plus conversion expenses and ADS issuance fees for all of the ADSs you are subscribing for, the ADS rights agent will require you to pay the deficiency as a condition of receiving your new ADSs, or will instruct the depositary to subscribe on your behalf for only the number of whole ADSs that can be subscribed for with the amount you have paid and will refund to you as soon as practicable the excess amount without interest.
If there is insufficient interest in the rights offering, we may determine not to proceed with the offering.
There is no minimum condition at which we are required to consummate the offering described in this prospectus supplement, and we reserve the right to cancel or terminate the offering at any time. If there is insufficient interest from existing holders of our common shares or holders of ADSs to subscribe for new common shares or new ADSs, as applicable, in the rights offering, we may determine not to proceed with the offering. If we do not proceed with the rights offering, the common share rights and ADS rights offered hereby will be cancelled and will have no further value.
Cantor Fitzgerald & Co. is not obligated to purchase any ADSs representing new common shares that are unsubscribed in the rights offering.
If any new common shares are not subscribed for pursuant to the common share rights and ADS rights described in this prospectus supplement, following the expiration of the offering of such common share rights and ADS rights, we may, at the discretion of Cantor Fitzgerald & Co., based on market conditions and demand, enter into an underwriting agreement pursuant to which Cantor Fitzgerald & Co. would agree to subscribe for and purchase up to all of the unsubscribed common shares at a purchase price of €40.14 per common share for purposes of resale of ADSs representing the unsubscribed common shares. However, entry into an underwriting agreement for all or a portion of the unsubscribed shares remains at the discretion of Cantor Fitzgerald & Co. and Cantor Fitzgerald & Co. is not obligated to purchase all or any of the unsubscribed common shares. See "Plan of Distribution." If Cantor Fitzgerald & Co. chooses not to enter into an underwriting agreement for the purchase and resale of ADSs representing unsubscribed common shares or if Cantor Fitzgerald & Co. chooses to purchase for resale less than all of any unsubscribed common shares, the net proceeds to us from this financing may be lower than we currently expect. If Cantor Fitzgerald & Co. does not enter into an underwriting agreement to purchase a sufficient number of ADSs representing new common shares that are unsubscribed in the rights offering, we may determine not to proceed with this offering.
The market price of the ADSs may decline below the ADS subscription price prior to the expiration of the applicable subscription period.
Once you exercise your rights pursuant to this offering, you may not revoke the exercise. The market price of the ADSs may decline below the ADS subscription price prior to the expiration of the ADS subscription period as a result of, among other things, market factors and the number of new common shares, including those represented by ADSs, to be issued in this offering. If you exercise your rights and the market price of the ADSs trades below the ADS subscription price on the date the new ADSs are issued to you in respect of such rights, then you will have purchased those ADSs at a price that is higher than the market price. Any decrease in market prices may continue after the completion of this offering. On November 28, 2016, the closing price of the ADSs on the NASDAQ Global Market was $4.25 per ADS.
S-55
We estimate that we will receive net proceeds from this offering of approximately $20.9 million (€19.8 million), assuming the (i) full exercise of subscription rights for the common shares and ADSs offered in this offering and (ii) issuance and sale of an aggregate of 588,127 common shares, including the sale of 114,658 common shares at the subscription price of €40.14 per new common share and 4,734,690 ADSs, representing 473,469 common shares, at the estimated subscription price of $4.25 per new ADS, and assuming a euro-to-U.S. dollar exchange rate of €1.00 to $1.0588, after deducting estimated fees and offering expenses payable by us.
Each decrease of 50,000 in the number of common shares sold in this offering, including common shares represented by ADSs, would decrease the net proceeds to us from this offering by approximately $2.0 million (€1.8 million), assuming a euro-to-U.S. dollar exchange rate of €1.00 to $1.0588, after deducting estimated fees and offering expenses payable by us.
We intend to use the net proceeds from this offering for general corporate purposes, including working capital and pre-commercial activities.
The expected use of net proceeds from this offering represents our intentions based upon our current plans and business conditions, which could change in the future as our plans and business conditions evolve. The amounts and timing of our actual expenditures may vary significantly depending on numerous factors, including the progress of our development, the status of and results from clinical trials, as well as any collaborations that we may enter into with third parties for our product candidates, and any unforeseen cash needs. As a result, our management retains broad discretion over the allocation of the net proceeds from this offering. We have no current agreements, commitments or understandings for any material acquisitions or licenses of any products, businesses or technologies.
As of September 30, 2016, we had cash, cash equivalents, marketable securities and term deposits of €66.2 million ($73.9 million). If the gross proceeds from this offering are $20.0 million or more, we estimate that such funds, after deducting estimated fees and offering expenses payable by us, together with our existing cash, cash equivalents, marketable securities and term deposits, will be sufficient to enable us to fund our operating expenses and capital expenditure requirements at least into the second quarter of 2018. If the gross proceeds from this offering are $5.0 million or more, we estimate that such funds, after deducting estimated fees and offering expenses payable by us, together with our existing cash, cash equivalents, marketable securities and term deposits, will be sufficient to enable us to fund our operating expenses and capital expenditure requirements at least into the first quarter of 2018. If the gross proceeds from this offering are less than such amounts, our capital resources may not be sufficient to enable us to fund our operating expenses and capital expenditure requirements for the time periods indicated. We have based these estimates on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect. While we anticipate that the net proceeds from this offering, together with our existing cash and cash equivalents, will be sufficient to allow us to obtain top-line data for our two international Phase 3 clinical trials of lefamulin for the treatment of moderate to severe CABP, the net proceeds may not be sufficient to allow us to submit applications for marketing approval for lefamulin for the treatment of CABP in the United States and Europe. We also do not expect the net proceeds from this offering to be sufficient to complete the clinical development of lefamulin for any other indications.
Pending our use of the net proceeds from this offering, we intend to invest the net proceeds in a variety of capital preservation investments, including term deposits, short-term, investment-grade, interest-bearing instruments and U.S. government securities.
S-56
We have never declared or paid any cash dividends on our common shares. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. We do not plan to declare or pay cash dividends on our common shares in the foreseeable future.
In addition, Austrian law imposes limitations on our ability to pay dividends. Under Austrian law, a company may only pay dividends if the distribution of dividends is proposed by the management board and the supervisory board and resolved by the company's shareholders at a general meeting. Our ability to pay dividends is assessed by our management board based primarily on our unconsolidated financial statements prepared in accordance with the Austrian Commercial Code (Unternehmensgesetzbuch). Dividends may be paid only after the relevant balance sheet date from the net profit (Bilanzgewinn) recorded in our unconsolidated annual financial statements as approved by our supervisory board or by our shareholders at a general meeting. In determining the amount available for distribution, the annual net income must be adjusted to account for any accumulated undistributed net profit or loss from previous years as well as for withdrawals from or allocations to reserves. Certain reserves must be established by law, and allocation to such reserves must therefore be deducted from the annual net income in order to calculate the annual net profit. Dividends paid by us may be subject to Austrian withholding tax.
S-57
The following table sets forth our cash and cash equivalents and capitalization as of September 30, 2016:
- •
- on an actual basis; and
- •
- on an as adjusted basis to give effect to the assumed (i) full exercise of subscription rights for the common shares and ADSs offered in this offering and (ii) issuance and sale of an aggregate of 588,127 common shares, including the sale of 114,658 common shares at the subscription price of €40.14 per new common share and 4,734,690 ADSs, representing 473,469 common shares, at the estimated subscription price of $4.25 per new ADS, and assuming a euro-to-U.S. dollar exchange rate of €1.00 to $1.0588, after deducting estimated fees and offering expenses payable by us.
This table should be read with our consolidated financial statements and the related notes and "Operating and Financial Review and Prospects" in our most recent Annual Report on Form 20-F and our unaudited consolidated interim financial statements and the related notes for the nine months ended September 30, 2016, each of which is incorporated by reference in this prospectus supplement.
| |
As of September 30, 2016 | ||||||
|---|---|---|---|---|---|---|---|
(in thousands, except share and per share data)
|
Actual | As Adjusted(1) | |||||
Cash, cash equivalents, marketable securities and term deposits |
€ | 66,233 | € | 85,991 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Total debt |
— | — | |||||
Equity |
|||||||
Share capital |
2,128 | 2,716 | |||||
Capital reserves |
225,042 | 248,061 | |||||
Other reserves(2) |
(2,452 | ) | (6,301 | ) | |||
Accumulated losses |
(161,035 | ) | (161,035 | ) | |||
| | | | | | | | |
Total equity |
63,683 | 83,441 | |||||
| | | | | | | | |
Total capitalization |
€ | 63,683 | € | 83,441 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
- (1)
- Each
decrease of 50,000 in the number of common shares sold in this offering, including common shares represented by ADSs, would decrease the as adjusted amount of
cash, cash equivalents, marketable securities and term deposits, capital and other reserves, total equity and total capitalization by approximately $2.0 million
(€1.8 million), assuming a euro-to-U.S. dollar exchange rate of €1.00 to $1.0588, after deducting estimated fees and offering expenses payable by
us.
- (2)
- Other reserves includes treasury shares and accumulated other comprehensive income. On an as adjusted basis, other reserves also includes capitalized estimated offering expenses payable by us.
The table above excludes:
- •
- 197,604 common shares issuable upon the exercise of options outstanding as of September 30, 2016 at a weighted average exercise price of
€66.00 per share;
- •
- 159,340 additional common shares reserved for future issuance as of September 30, 2016 under our Stock Option Plan 2007 and Stock Option
Plan 2015; and
- •
- 319 common shares held in treasury as of September 30, 2016.
S-58
If you do not exercise your rights to subscribe for common shares or ADSs in this offering, your ownership interest will be diluted. In addition, even if you exercise your rights to subscribe for common shares or ADSs in this offering, your ownership interest will be diluted to the extent of the difference between the subscription price per common share (or ADS) in this offering and our as adjusted net tangible book value per common share (or ADS) after this offering.
Our historical net tangible book value as of September 30, 2016 was €63.5 million ($70.9 million), or €29.85 ($33.31) per common share and $3.33 per ADS. Each ADS represents one tenth (1/10) of a common share. Historical net tangible book value represents the amount of our total tangible assets less our total liabilities. Historical net tangible book value per share represents historical net tangible book value divided by 2,128,006 common shares of our outstanding share capital as of September 30, 2016.
Assuming (i) the full exercise of subscription rights for the common shares and ADSs offered in this offering and (ii) the issuance and sale of 588,127 common shares, including the sale of 114,658 common shares at the subscription price of €40.14 per new common share and 4,734,690 ADSs, representing 473,469 common shares, at the estimated subscription price of $4.25 per new ADS, and assuming a euro-to-U.S. dollar exchange rate of €1.00 to $1.0588, after deducting estimated fees and offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2016 would have been €83.3 million ($92.9 million), or €30.66 ($34.22) per common share and $3.42 per ADS. This would represent an immediate dilution of our as adjusted net tangible book value of €9.48 ($8.28) per common share and $0.83 per ADS to existing common shareholders and ADS holders who exercise subscription rights in this offering, or 19.5%, assuming full exercise of subscription rights for the common shares and ADSs offered hereby.
Each decrease of 50,000 in the number of common shares sold in this offering, including common shares represented by ADSs, would further decrease our as adjusted net tangible book value by €0.12 ($0.13) per common share and $0.01 per ADS to existing common shareholders and ADS holders who exercise subscription rights in this offering.
In addition, assuming the issuance and sale of 588,127 common shares in this offering (including common shares represented by ADSs), existing common shareholder and ADS holders who do not exercise their subscription rights in this offering will be diluted such that a shareholder holding 10% of our issued and outstanding common share capital prior to this offering will have such shareholding reduced to approximately 7.8% of our issued and outstanding common share capital immediately following this offering.
S-59
American Depositary Shares, or ADSs, representing our common shares, have been trading on the NASDAQ Global Market under the symbol "NBRV" since September 18, 2015. The following table sets forth, for the periods indicated, the reported high and low sale prices of ADSs on the NASDAQ Global Market in U.S. dollars. Each ADS represents one tenth (1/10) of a common share.
| |
Price Per ADS | ||||||
|---|---|---|---|---|---|---|---|
| |
$ | ||||||
| |
High | Low | |||||
Annual (Fiscal Year Ended December 31): |
|||||||
2015 (from September 18 through December 31) |
17.48 | 8.51 | |||||
Quarterly: |
|||||||
Third Quarter 2015 (from September 18 through September 30) |
17.48 | 8.75 | |||||
Fourth Quarter 2015 |
10.69 | 8.51 | |||||
First Quarter 2016 |
9.80 | 6.61 | |||||
Second Quarter 2016 |
9.70 | 6.85 | |||||
Third Quarter 2016 |
8.85 | 7.01 | |||||
Monthly: |
|||||||
May 2016 |
9.02 | 7.01 | |||||
June 2016 |
7.99 | 6.85 | |||||
July 2016 |
8.47 | 7.31 | |||||
August 2016 |
8.40 | 7.12 | |||||
September 2016 |
8.85 | 7.01 | |||||
October 2016 |
7.50 | 4.99 | |||||
November 2016 (through November 28) |
6.06 | 3.52 | |||||
On November 28, 2016, the last reported sales price of ADSs on the NASDAQ Global Market was $4.25 per ADS.
S-60
General Information
We are offering to our common shareholders rights to subscribe for new common shares and, through The Bank of New York Mellon, our depositary and the ADS rights agent, are offering to American Depositary Share, or ADS, holders non-transferable rights to subscribe for new ADSs pursuant to a rights offering. Except to the extent otherwise provided under Austrian law, the common share rights will be non-transferable. We expect to issue up to 588,127 common shares in this offering, including common shares represented by ADSs. Each ADS represents one tenth (1/10) of a common share, nominal value €1.00 per share.
As of November 29, 2016, we had 2,130,898 common shares issued and outstanding (including common shares represented by ADSs). Assuming all of the common shares offered in this offering are sold, including common shares represented by ADSs, immediately following the closing of this offering, we will have 2,719,025 common shares (including common shares represented by ADSs) issued and outstanding.
If you are a holder of ADSs at 5:00 p.m. (New York City time) on November 29, 2016, which is the ADS record date, you will receive 0.276 ADS rights for each ADS owned on that date. One ADS right will entitle you to subscribe for and purchase one new ADS at the U.S. dollar equivalent of €4.014 per ADS. Based on a euro-to-U.S. dollar exchange rate of €1.00 to $1.0588, we estimate that subscription price at $4.25 per ADS. To subscribe for new ADSs, a holder of ADS rights must pay to The Bank of New York Mellon $4.68 per ADS so subscribed, which represents 110% of the estimated subscription price to account for currency conversion expense, ADS issuance fees payable to the depositary and potential fluctuations in the exchange rate between the euro and the U.S. dollar, payable as described below. On or about December 13, 2016, the ADS rights agent will determine the actual U.S. dollar ADS subscription price by converting the euro subscription price into U.S. dollars at an exchange rate assigned by it on that date. Fractional ADSs rights will not be issued, and ADS right entitlements will be reduced to the next smaller whole number of ADS rights. ADS rights will not be assignable or transferable. The depositary will send to each registered holder of ADSs on or about the record date an ADS subscription form, together with a letter of instructions, for exercising ADS rights.
If you are a holder of common shares on November 29, 2016, which is the common share record date, you will have the common share right to subscribe for and purchase 0.276 new common shares, at a subscription price of €40.14 per new common share, for each common share held on the common share record date, payable as described below. No fractional common shares will be issued and common share right entitlements will be reduced to the next smaller whole number of common shares.
Common shareholders and ADS holders generally will be treated alike in the rights offering, except that:
- •
- The timing of certain actions and periods will differ for holders of ADS rights and holders of common share rights. In particular, the last
date for exercise and payment is earlier for holders of ADS rights.
- •
- Holders of common share rights must pay the subscription price in euro, while holders of ADS rights must pay an ADS subscription payment in U.S. dollars under an arrangement with the ADS rights agent. The ADS subscription payment includes an allowance for potential fluctuations between the euro and the U.S. dollar, currency conversion expenses and the payment of ADS issuance fees of the depositary.
If any new common shares are not subscribed for pursuant to the common share rights and ADS rights described above, following the expiration of the offering of such common share rights and ADS rights, we may, at the discretion of Cantor Fitzgerald & Co., based on market conditions and demand, enter into an underwriting agreement pursuant to which Cantor Fitzgerald & Co. would agree to subscribe for and purchase up to all of the unsubscribed common shares at a purchase price of €
S-61
40.14 per common share for purposes of resale of ADSs representing the unsubscribed common shares. However, entry into an underwriting agreement for all or a portion of the unsubscribed shares remains at the discretion of Cantor Fitzgerald & Co., and Cantor Fitzgerald & Co. is not obligated to purchase all or any of the unsubscribed common shares. In the event that Cantor Fitzgerald & Co. and we do enter into an underwriting agreement for unsubscribed shares, Cantor Fitzgerald & Co. may sell ADSs at variable prices, which may be more or less than the purchase price. See "Plan of Distribution."
In addition, certain of our existing principal shareholders and their affiliated entities, holding approximately 39.7% of our outstanding share capital, have indicated an interest in purchasing up to an aggregate of approximately $17.5 million of the securities we are offering. However, indications of interest are not binding agreements or commitments to subscribe and purchase. These shareholders may determine to subscribe for and purchase fewer common shares and/or ADSs than they indicate an interest in subscribing for and purchasing or not to subscribe for any common shares and/or ADSs in the rights offering or in the potential offering of ADSs representing any unsubscribed common shares. In addition, because these investors are only entitled to subscribe for and purchase their pro rata portion of any new common shares or ADSs offered as part of the offerings of ADS rights and common share rights, any purchases above this pro rata amount would occur through the potential offering of ADSs representing any unsubscribed shares. Following the expiration of the offering of ADS rights and common share rights, Cantor Fitzgerald & Co. may determine not to proceed with the potential offering of ADSs representing unsubscribed common shares or may determine to sell fewer ADSs representing unsubscribed common shares to any of these shareholders or not to sell any such ADSs to these shareholders.
The Information Agent
Georgeson LLC is acting as information agent for this offering. If you have any questions regarding the ADS rights or the common share rights, please contact Georgeson LLC toll free at (866) 278-8941 or by e-mail at Nabriva@georgeson.com.
Offering to Holders of ADSs
Summary Timetable
The summary timetable below lists some important dates relating to the ADS rights offering. All times referred to in this timetable are New York City time, unless stated otherwise.
ADS record date—date for determining holders of ADSs receiving ADS rights |
5:00 p.m. on November 29, 2016 | |
ADS subscription form and letters of instructions sent to registered ADS holders and made available to beneficial owners of ADSs |
On or about November 30, 2016 | |
ADS rights offering commencement date—beginning of period during which ADS rights holders can subscribe for and purchase new ADSs |
November 30, 2016 | |
ADS rights expiration date—end of period during which ADS rights holders can subscribe for and purchase new ADSs. Please note that your broker, bank or other securities intermediary may impose an earlier date by which instructions must be received from you |
5:00 p.m. on December 12, 2016 | |
New common shares expected to be deposited with the custodian |
On or about December 19, 2016 | |
New ADSs expected to be delivered |
On or about December 19, 2016 |
S-62
The following is a summary of the important provisions of the ADS rights offering.
Offering to Holders of ADSs
If you hold ADSs on the ADS record date, you will receive 0.276 ADS rights for each ADS you hold on that date. One ADS right will entitle you to subscribe for and purchase one new ADS. Fractional ADS rights will not be issued, and ADS right entitlements will be reduced to the next smaller whole number of ADS rights.
ADS Record Date
The record date for determining the holders of ADSs entitled to receive ADS rights is November 29, 2016. Only holders of record of ADSs at 5:00 p.m. (New York City time) on the ADS record date will be entitled to receive ADS rights.
ADS Rights Exercise Period
ADS rights may be exercised during the period from November 30, 2016 through 5:00 p.m. (New York City time) on December 12, 2016, which is the ADS rights expiration time. If you do not exercise your ADS rights within the ADS rights exercise period, your ADS rights will expire and will have no further value.
ADS Subscription Price
The estimated ADS subscription price is $4.25 per ADS, which is the subscription price of €40.14 per common share for one tenth (1/10) of a common share translated into U.S. dollars at the rate of €1.00 to $1.0588, the exchange rate at the European Central Bank on November 28, 2016. On or about December 13, 2016, the ADS rights agent will determine the actual U.S. dollar ADS subscription price by converting the euro common share subscription price into U.S. dollars at the exchange rate assigned by it on that date. The ADS rights agent may convert currency itself or through any of its affiliates and, in those cases, acts as principal for its own account and not as an agent, fiduciary or broker on behalf of any other person and earns revenue, including, without limitation, fees and spreads that it will retain for its own account. The spread is the difference between the exchange rate assigned to the currency conversion made by the ADS rights agent and the rate that agent or its affiliate receives in an offsetting foreign currency trade. The ADS rights agent makes no representation that the exchange rate used or obtained in any currency conversion will be the most favorable rate that could be obtained at the time or that the method by which that rate will be determined will be the most favorable to ADS holders, except that the ADS rights agent will agree to act without gross negligence or willful misconduct.
ADS Subscription Payment
In order to exercise your ADS rights and to subscribe for and purchase any additional ADSs, you must pay the ADS subscription payment of $4.68 per ADS, which represents 110% of the estimated subscription price to account for potential fluctuations in the exchange rate between the euro and the U.S. dollar, currency conversion expenses and an ADS issuance fee of the depositary of $0.05 per new ADS. The ADS rights agent will deduct the ADS issuance fee from each subscribing holder's ADS subscription payment. You must make the ADS subscription payment in U.S. dollars.
If the actual U.S. dollar ADS subscription price (plus the ADS issuance fee and the currency conversion expense) is less than the ADS subscription payment, the ADS rights agent will refund such excess amount to the subscribing ADS holder without interest. However, if the amount of the ADS subscription payment you paid to the ADS rights agent is, for any reason, including due to currency exchange rate fluctuations, insufficient to pay the subscription price in euro plus conversion expenses and ADS issuance fees for all of the ADSs you are subscribing for, the ADS rights agent will require you to pay the deficiency as a condition of receiving your new ADSs, or will instruct the depositary to
S-63
subscribe on your behalf for only the number of whole ADSs that can be subscribed for with the amount you have paid and will refund to you as soon as practicable the excess amount without interest.
No Revocation of Exercise
The exercise of ADS rights is irrevocable and may not be cancelled, modified or revoked.
Procedure for Exercising ADS Rights
Subscription by DTC Participants. If you hold ADSs through a broker or other securities intermediary and wish to exercise your ADS rights, you should contact your securities intermediary and instruct it to subscribe on your behalf through the automated system of The Depository Trust Company, or DTC, prior to 5:00 p.m. (New York City time) on December 12, 2016, also referred to as the ADS rights expiration time. Your securities intermediary will charge the applicable ADS subscription payment to your account. Your broker or other securities intermediary may set a cutoff date and time to receive instructions that is earlier than the ADS rights expiration time stated above. You should contact your securities intermediary to determine the cutoff date and time that applies to you.
Subscription by Registered ADS Holders. If you are a registered holder of ADSs, you can exercise your ADS rights by delivering to the depositary a properly completed and original signed ADS subscription form and paying in full the estimated ADS subscription payment for the new ADSs. You may make such payment by certified or official bank check payable to "The Bank of New York Mellon".
The properly completed ADS subscription form and payment should be delivered to:
| By registered, certified or express mail: |
By overnight courier: |
|
|---|---|---|
| The Bank of New York Mellon | The Bank of New York Mellon | |
| Voluntary Corporate Actions—Suite V | Voluntary Corporate Actions—Suite V | |
| P.O. Box 43031 | 250 Royall Street | |
| Providence, Rhode Island 02940-3031 | Canton, Massachusetts 02021 | |
| United States of America | United States of America |
The ADS rights agent must receive the original ADS subscription form and ADS subscription payment on or before the ADS rights expiration time. Deposit in the mail will not constitute delivery to the ADS rights agent.
The ADS rights agent has discretion to refuse to accept any improperly completed or unsigned ADS subscription form. Please see the ADS subscription form and instruction booklet for additional information.
Because the depositary will not be able to subscribe for any fraction of a common share and each ADS represents one tenth (1/10) of one common share, we will instruct the ADS rights agent to reduce the number of new ADSs for which an ADS holder is permitted to subscribe pursuant to ADS rights to the extent necessary so that the depositary will not subscribe, in the aggregate, for any fraction of a common share.
We and the ADS rights agent will determine all questions about the timeliness, validity, form and eligibility of any exercise of ADS rights. We, in our sole discretion, may waive any defect or irregularity, or permit you to correct a defect or irregularity within the time we determine. ADS subscription forms will not be considered received or accepted until we have waived all irregularities or you have cured them in time. Neither we nor the ADS rights agent have to notify you of any defect or irregularity in submitting ADS subscription forms. We and the ADS rights agent will not incur any liability for failing to do so.
S-64
You will elect the method of delivering ADS subscription forms and making the ADS subscription payment to the ADS rights agent, and you will bear any risk associated with it. If you send ADS subscription forms or payments by mail, you should use registered mail, properly insured, with return receipt requested, and allow sufficient time to ensure delivery to the ADS rights agent and clearance of payment before the appropriate time.
Non-transferability of ADS Rights
ADS rights are not transferable, and any purported transfer of ADS rights will be null and void.
ADS Issuance Fee
Subscribing holders will be charged an ADS issuance fee of $0.05 per new ADS issued, payable to the depositary. The ADS rights agent will deduct the ADS issuance fee from the ADS subscription payment in respect of each subscription.
Delivery of ADSs
The depositary will deliver new ADSs purchased pursuant to the ADS rights offering as soon as practicable after the receipt of the common shares by the depositary's custodian, which is expected to be on or about December 19, 2016.
ADS Rights Agent
The Bank of New York Mellon, in addition to acting as depositary in respect of our ADS program, will act as the ADS rights agent. The ADS rights are to be issued under the terms of a rights agent agreement relating to this offering between us and The Bank of New York Mellon. We have filed a copy of the deposit agreement as an exhibit to the registration statement of which this prospectus supplement forms a part and a copy of the rights agent agreement on a Form 6-K.
Offering to Holders of Common Shares
Summary Timetable
The timetable below lists some important dates relating to the common share rights offering. All times referred to in this timetable are Vienna time, unless stated otherwise.
Common share record date—date for determining holders of common shares receiving common share rights |
November 29, 2016 | |
Common share rights commencement date—beginning of period during which common share rights holders can subscribe for and purchase new common shares |
November 30, 2016 | |
Common share rights expiration date—end of period during which common share rights holders can subscribe for new common shares |
5:00 p.m. December 14, 2016 | |
Common share subscription price payment date—end of period during which common share rights holders can pay for new common shares (on a same day funds basis) |
5:00 p.m. December 14, 2016 | |
Delivery of new common shares to common shareholders |
On or about December 19, 2016 |
S-65
Rights Offering to Holders of Common Shares
If you are a holder of common shares on November 29, 2016, which is the common share record date, you will have the common share right to subscribe for and purchase 0.276 new common shares, at a subscription price of €40.14 per new common share, for each common share held on the common share record date, payable as described below. No fractional common shares will be issued and common share right entitlements will be reduced to the next smaller whole number of common shares.
Common Share Rights
Common share rights will not be evidenced by rights certificates and will not be listed on any national securities market or exchange. Common share rights will not be transferable or assignable, except to the extent otherwise provided under Austrian law.
Common Share Record Date
The record date for the determination of common shareholders entitled to common share rights is November 29, 2016. Only common shareholders of record on the common share record date will be entitled to common share rights.
Share Rights Exercise Period
Common share rights may be exercised during the period from November 30, 2016 through 5:00 p.m. (Vienna time) on December 14, 2016. Following the common share rights expiration date, the common share rights will expire and common shareholders will have no rights to participate in the rights offering.
Common Share Subscription Price
The common share subscription price for new common shares purchased upon the exercise of common share rights is €40.14 per common share.
Procedure for Exercising Common Share Rights
The exercise of common share rights is irrevocable and may not be cancelled or modified. You may exercise your common share rights by delivering to us, at our executive offices at Leberstrasse 20, 1110 Vienna, Austria, a properly completed subscription form and full payment of the common share subscription price for the new common shares being purchased.
If you fail to exercise your common share rights by 5:00 p.m. (Vienna time) on December 14, 2016 and to arrange for payment for your new common shares so that we receive full payment by 5:00 p.m. (Vienna time) on December 14, 2016, your rights will lapse and will have no further value.
We will determine all questions about the timeliness, validity, form and eligibility of exercising the common share rights. Our determinations will be final and binding. We may decide to waive a defect or irregularity in subscriptions for new common shares, or permit you to correct a defect or irregularity within the time we determine. Instructions will not be considered, received or accepted until we have waived all irregularities or you have cured them in time. We have no obligation to notify you of any defect or irregularity in submitting instructions, and we will not incur any liability for failing to do so.
Non-transferability of Common Share Rights
Common share rights will not be transferable, and any purported transfer of common share rights will be null and void, except to the extent otherwise provided under Austrian law.
Delivery of New Common Shares
We intend to issue the new common shares on or about December 19, 2016, at which time you should receive physical delivery of the share certificate representing the new common shares you subscribed for and purchased in the common share rights offering.
S-66
Taxation in Austria
This taxation summary solely addresses the principal Austrian tax consequences of the acquisition, ownership and disposal of ADSs or our common shares. It does not purport to describe all potential tax aspects that may be relevant to a prospective holder of ADSs or our common shares and does not deal with specific situations which may be of relevance for certain potential shareholders. Where in this summary English terms and expressions are used to refer to Austrian concepts, the meaning to be attributed to such terms and expressions shall be the meaning to be attributed to the equivalent Austrian concepts under Austrian tax law.
General Remarks
Individuals having a permanent domicile (Wohnsitz) and/or their habitual abode (gewöhnlicher Aufenthalt) in Austria are subject to income tax (Einkommensteuer) in Austria on their worldwide income (unlimited income tax liability; unbeschränkte Einkommensteuerpflicht). Individuals having neither a permanent domicile nor their habitual abode in Austria are subject to income tax only on income from certain Austrian sources (limited income tax liability; beschränkte Einkommensteuerpflicht).
Corporations having their place of effective management (Ort der Geschäftsleitung) and/or their legal seat (Sitz) in Austria are subject to corporate income tax (Körperschaftsteuer) in Austria on their worldwide income (unlimited corporate income tax liability; unbeschränkte Körperschaftsteuerpflicht). Corporations having neither their place of effective management nor their legal seat in Austria are subject to corporate income tax only on income from certain Austrian sources (limited corporate income tax liability; beschränkte Körperschaftsteuerpflicht).
Both in case of unlimited and limited (corporate) income tax liability, Austria's right to tax may be restricted by double taxation treaties.
Except for Austrian withholding taxes which have to be withheld at source, the responsibility for adherence to obligations under applicable tax legislation is always the responsibility of the relevant holder of our common shares.
American Depositary Shares
For Austrian tax purposes, a holder of ADSs who is entitled to claim the full number of shares represented by the ADSs held at any time and who may freely dispose of and exercise the shareholder rights, in particular voting rights, inherent to our common shares (or instruct the depositary acting as an agent for the holder in light of the ADSs to do so) is in general considered to be the economic owner of common shares represented by such ADSs. As a result, dividend income resulting from our common shares should be attributable to the holder of the ADSs for Austrian tax purposes. Consequently, any distribution received by an Austrian resident or non-resident ADS holder with respect to our common shares will therefore be taxable in Austria as a dividend under the principles set out for common shares below. Capital gains realized upon the disposal of the ADSs will equally be subject to tax in Austria as outlined below with respect to capital gains realized through the sale of common shares.
Income Taxation of Shareholders Tax Resident in Austria (Residents)
Taxation of Dividends
Dividends distributed by an Austrian corporation (whether open or disguised) are generally subject to a withholding tax (Kapitalertragsteuer), levied at a rate of currently 27.5% (which may be reduced to 25% in case the recipient is a corporation). This tax is withheld by the company paying the dividend or
S-67
the bank paying out the dividend on the company's behalf. The shareholder is entitled to receive a certificate showing the gross dividend, the amount of tax withheld, the date of payment and the period in respect of which the dividend is payable, and also the tax office to which the tax withheld was remitted.
For Austrian resident individual shareholders (unbeschränkt steuerpflichtige natürliche Personen) of the common shares who are subject to unlimited income tax liability, a 27.5% withholding tax is levied which fully discharges any further income tax liability on such dividend income (final taxation—Endbesteuerung), which means that no further income tax is due and the dividends do not have to be included in the shareholder's income tax return. This applies irrespective of whether the common shares are held as non-business assets (Privatvermögen) or as business assets (Betriebsvermögen) of a resident individual shareholder. If the applicable income tax rate of an individual shareholder is less than 27.5%, the individual shareholder may opt to include the dividends (together with any other income from capital investments subject to the special 27.5% or 25% tax rate) in his regular annual tax assessment (Regelbesteuerungsoption). In this case, the dividends are taxed at the regular progressive income tax rate applicable to the shareholder's total income and any Austrian withholding tax on such dividend income will be credited against the shareholder's personal income tax liability and, if exceeding, refunded. Expenses incurred by the holder in connection with the common shares (including interest expenses for third party financing for the acquisition of the shares) may not be deducted for income tax purposes.
For Austrian resident corporations (unbeschränkt steuerpflichtige Körperschaften), dividend income is exempt from corporate income tax and the Austrian dividend withholding tax is credited against the corporate income tax liability of the shareholder or refunded. No withholding tax has to be deducted by the distributing company if the Austrian resident corporate shareholder directly or indirectly (e.g. via a partnership) holds at least 10% of the share capital of the distributing company. Expenses in connection with tax exempt dividend income are generally not deductible. As an exception to this general rule, interest accrued for financing raised for the acquisition of the shares may—under certain restrictions—be deductible provided that the shares qualify as fixed assets of the recipient and were acquired from an independent third party (i.e. no acquisition from group companies).
Private foundations (Privatstiftungen) pursuant to the Austrian Private Foundations Act (Privatstiftungsgesetz Federal Law Gazette No. 694/1993 "Austrian Private Foundations Act") fulfilling the prerequisites contained in sec. 13(3) and (6) of the Austrian Corporate Income Tax Act (Körperschaftsteuergesetz, KStG) are subject to special provisions that exempt dividends distributed by Austrian entities from corporate income tax. Austrian withholding tax is credited against the Austrian corporate income tax assessed; excess amounts may be reclaimed. Under the conditions set forth in sec 94(12) of the Austrian Income Tax Act (Einkommensteuergesetz, EStG) no withholding tax is levied.
Dividend distributions have to be distinguished from a repayment of equity pursuant to sec. 4(12) EStG. A repayment of equity leads to a reduction of the tax basis of the shares. Insofar as the repayment exceeds the acquisition costs/tax basis of the shares, a capital gain will be deemed realized (see as to the taxation of capital gains below). The option to (freely) choose whether the distribution of retained earnings is to be regarded as distribution of dividends or repayment of equity for Austrian tax purposes requires the distributions to be covered by a positive level of internal financing of the distributing company (positiver Stand der Innenfinanzierung) or—in case of an intended repayment of equity—a positive level of external financing (positiver Einlagenstand), both to be evidenced by respective documentation (Innenfinanzierungsevidenzkonto, Einlagenevidenzkonto). In contrast, payments qualified as so-called constructive dividends (verdeckte Gewinnausschüttungen) may be taxed as dividends for tax purposes regardless of the level of internal or external financing.
S-68
Taxation of Capital Gains
Capital gains, i.e. the difference between the sale proceeds or the redemption amount of the common shares and their acquisition costs, are generally subject to Austrian (corporate) income tax. For shares held as non-business assets, the acquisition costs do not include ancillary acquisition cost (Anschaffungsnebenkosten).
For holders of common shares who are subject to unlimited income tax liability, holding the common shares as non-business assets, capital gains realized upon a sale are subject to Austrian income tax. In the case of capital gains with nexus relevant for Austrian withholding tax purposes, basically income that is paid by an Austrian custodian agent (depotführende Stelle) or, without an Austrian custodian agent, by an Austrian paying agent (auszahlende Stelle), provided the non-Austrian custodian agent is a non-Austrian branch or group company of such paying agent and the Austrian paying agent executes the transaction in cooperation with the non-Austrian custodian agent and processes the payment, a final withholding tax of 27.5% is levied, i.e. no further income tax is due. An Austrian custodian or paying agent within the present context may be a credit institution within the meaning of sec. 1 of the Austrian Banking Act (Bankwesengesetz), an Austrian branch of a non-Austrian credit institution from another Member State within the meaning of sec. 9 of the Austrian Banking Act or an Austrian branch of certain investment services providers. In the case of income from capital gains without a nexus relevant for Austrian withholding tax purposes (i.e. in the absence of an Austrian custodian or paying agent), the income must be included in the shareholder's income tax return and is subject to a flat income tax rate of 27.5%. In both cases, the individual shareholder has the option to include the capital gains in his regular annual tax return and apply for taxation at the progressive income tax rate on the shareholder's total income in which case the Austrian withholding tax will be credited against the shareholder's personal income tax liability and, if exceeding, refunded. A deduction of expenses that are directly economically connected to income that is subject to the (special) 27.5% tax rate is generally not allowed. Subject to certain restrictions, losses realized by individual shareholders upon the disposal of common shares may be offset with investment income subject to the 27.5% Austrian income tax rate (including dividends from our common shares but neither with interest income from savings accounts or other debt claims against credit institutions nor with distributions effected by private foundations). For such set-off of losses the shareholder generally has to opt for assessment to income tax (in particular as regards securities deposited with different banks): In case of an Austrian depository, the set-off of losses has to be effected by the Austrian custodian agent taking into account all of a taxpayer's accounts with this custodian agent. Losses from the sale of shares held as non-business assets may not be carried forward to subsequent years.
For holders of common shares who are subject to unlimited income tax liability, holding the common shares as business assets, capital gains realized upon a sale (including, inter alia, a redemption or withdrawal of the common shares from the business) are subject to Austrian income tax. In the case of capital gains with an Austrian nexus relevant for Austrian withholding tax purposes (as described above), such income is subject to a withholding tax of 27.5%. In this case, the Austrian withholding tax does not discharge of Austrian income tax liability but may be credited against the income tax liability assessed. The capital gains must always be included in the income tax return (even if Austrian withholding tax is triggered), but are nevertheless taxed at a flat income tax rate of 27.5%, with any withholding tax being credited. In addition, the option exists to include income subject to the tax rate of 27.5% in the annual tax return at the progressive income tax rate, i.e. if a lower than 27.5% progressive income tax rate applies. Losses realized upon the disposal of common shares may preferentially be offset with other capital gains of the same business (or appreciations in value of such assets), only 55% of any residual loss may be offset with other types of income (and carried forward).
For holders of our common shares who are subject to unlimited corporate income tax liability, capital gains realized upon the sale of the common shares are taxed at the normal corporate income tax rate of 25%. According to sec. 93(1a) EStG, the withholding tax may be levied at a rate of 25%
S-69
(instead of 27.5%). A corporation may file an exemption declaration in order to avoid that Austrian withholding tax is levied. On the level of an Austrian resident corporate shareholder the restrictions on the offset of losses outlined above do in general not apply. However, sec. 12(3) KStG provides for certain restrictions with respect to the tax deductibility of impairment losses as well as of losses from the sale or other disposal of participations in the meaning of sec. 10 KStG.
Private foundations pursuant to the Austrian Private Foundations Act fulfilling the prerequisites contained in sec. 13(3) and (6) KStG and holding the common shares as a non-business asset are subject to interim taxation (Zwischenbesteuerung) at a rate of 25% on income from realized increases in value of the common shares. Interim tax does not become due insofar as distributions subject to withholding tax are made to beneficiaries in the same tax period. Reference is made to the possibility to achieve a tax deferral upon transferring hidden reserves realized upon the sale of shares by way of a qualified replacement acquisition pursuant to sec. 13(4) KStG. In the case of capital gains with nexus relevant for Austrian withholding tax purposes (as described above), the income is, in general, subject to a withholding tax of 25%, which can be credited against the tax due. Under the conditions set forth in sec. 94(12) EStG, no withholding tax is levied.
Withdrawals (Entnahmen oder sonstiges Ausscheiden aus dem Depot), the transfer of the investor's tax residence (Wegzug) outside of Austria, the transfer of the common shares to a non-resident without consideration (unentgeltliche Übertragung) or any other circumstances which lead to a restriction of Austria's existing taxation right with respect to the common shares are in general deemed as a disposal resulting in exit taxation. As an exception to this general rule withdrawals and other transfers of common shares from an investor's account are not deemed to be a disposal if Austria's taxation right is not being restricted and certain other requirements pursuant to § 27(6)(2) EStG are met. Upon application of the taxpayer, the exit taxation of the common shares held as private assets can be deferred until the actual disposal of the common shares in case the investor being an individual transfers his or her tax residence outside of Austria to an EU member state or certain member states of the European Economic Area or transfers the common shares for no consideration to another individual resident in an EU member state or certain member states of the European Economic Area. In all other cases leading to a restriction of Austria's taxation right with respect to an EU member state or certain member states of the European Economic Area the taxpayer may apply for a payment of the triggered income tax in instalments over a period of seven years. In case the common shares are held as business assets and represent current assets (Umlaufvermögen), a payment period of two years applies instead.
Income Taxation of Shareholders Tax Resident Outside of Austria (Non-Residents)
Taxation of Dividends
For non-resident individual holders of our common shares, dividends distributed by an Austrian corporation are, in principle, subject to 27.5% withholding tax, and dividends distributed to a corporate holder are subject to a 25% withholding tax.
Double taxation treaties may, however, provide for a reduction of the Austrian taxation right for dividends distributed by an Austrian entity to a non-Austrian resident (individual or corporate) shareholder. Austria has entered into tax treaties with more than 90 countries. Most of the Austrian tax treaties in principle follow the OECD Model Tax Convention and provide for a reduction of Austrian dividend withholding tax to 15% in case of portfolio dividends and for a further reduction in case of qualified participations. For example, the tax treaty with the United States as currently in place provides for a reduction of Austrian withholding tax to 15% or, in case of a company (other than a partnership) owning directly at least 10% of the voting stock of the company the dividends, to 5%.
A non-resident shareholder who is entitled to a reduced rate under an applicable tax treaty may apply for refund of the difference between the 27.5% (25%) withholding tax and the lower rate
S-70
provided for by the tax treaty in the course of a refund procedure. In order to obtain such refund, an eligible non-resident shareholder will generally have to provide a certificate of residence issued by the tax authorities of its country of residence. Claims for refund of the Austrian withholding tax may be filed using the forms ZS-RD 1 and ZS RD 1A (German) or ZS-RE 1 and ZS RE 1A (English). Tax treaty relief from Austrian withholding tax may also be granted by the distributing company already at source provided that the requirements of the Austrian relief at source rules under an ordinance of the Austrian Ministry of Finance (DBA-Entlastungsverordnung) are met. However, an Austrian corporation is under no obligation to grant tax treaty relief at source, and it is common practice that listed companies do not grant such relief at source. Further, a relief at source is not possible under the mentioned ordinance of the Austrian Ministry of Finance if the dividends are paid by a bank acting as custodian of the shares for the shareholder.
Dividends paid to a company qualifying under the Council Directive 2011/96/EU dated 30 November 2011 (EC Parent-Subsidiary Directive) are exempt from withholding tax if the EU company has held directly at least 10% of the share capital for an uninterrupted period of at least one year and meets certain additional criteria. Dividends which are attributable to an Austrian permanent establishment (Betriebsstätte) of a company resident in an EU or a EEA member state are exempt from corporate income tax, and the 25% withholding tax is credited against a given Austrian corporate income tax liability of the EU or EEA company and, if exceeding or in case of a lack of an Austrian corporate income tax liability, refunded.
In addition, corporate shareholders resident in (i) an EU member state or (ii) a EEA member state with which Austria has entered into an agreement on comprehensive mutual assistance in tax administration and tax enforcement (currently Norway and Liechtenstein) are entitled to a refund of the Austrian dividend withholding tax that would otherwise be due under the applicable tax treaty if and to the extent the shareholder provides evidence that in its country of residence no (full) tax credit for such withholding tax was possible pursuant to the applicable tax treaty. A refund of the remaining Austrian withholding tax that exceeds the tax rate provided for by the applicable tax treaty can be obtained from the competent Austrian tax office Bruck Eisenstadt Oberwart upon application.
Taxation of Capital Gains
For non-resident individuals and corporations, capital gains on the sale of shares are taxable in Austria if (i) the shares are attributable to an Austrian permanent establishment or (ii) the selling shareholder has held a qualified shareholding (i.e. he held at one point in time during the last five years preceding the sale at least 1% of the Austrian corporation's capital). Capital gains are generally subject to 27.5% withholding tax (or 25% in case of corporations) in Austria provided that there is a sufficient nexus relevant for Austrian withholding tax purposes (as outlined above). However, pursuant to sec. 94(13) EStG, such capital gains realized by non-residents shareholders are exempt from Austrian withholding tax. As a consequence, capital gains realized by non-residents shareholders must be included in the annual tax return.
In this respect, it has to be noted that most of Austria's tax treaties follow the OECD Model Tax Convention thus attributing the right of taxation on capital gains to the state of residence of the seller (unless the shares are attributable to an Austrian permanent establishment) and providing for (full) exemption from taxation in Austria. Consequently, under such circumstances, the capital gains are not taxable in Austria.
Taxation of Subscription Rights
The granting of subscription rights (Bezugsrechte) to existing shareholders against contributions in cash in the course of an increase of the share capital does not constitute income within the EStG. The sale of such subscription right could result in capital gains within the meaning of sec. 27(3) EStG (as
S-71
outlined above for Austrian and non-Austrian resident individual and corporate shareholders). According to sec. 5 of the Capital Measures Directive (KapitalmaBnahmen-Verordnung), the acquisition cost of the subscription right is assumed to be zero in that case, which constitutes the tax basis for any capital gains. At the moment, the subscription right is exercised, the acquisition cost of the existing shares is assumed to have the value of the shares as of the day the subscription right is exercised (the subscription right itself is assumed to have no value). In contrast, the acquisition cost for the newly issued shares is deemed to be the cash payment made for them.
Tax Cooperation Agreements Austria/Switzerland and Austria/Liechtenstein
On 1 January 2013, the Treaty between the Republic of Austria and the Swiss Confederation on Cooperation in the Areas of Taxation and Capital Markets entered into force. A similar treaty between the Republic of Austria and the Principality of Liechtenstein is in force since 1 January 2014. These treaties provide that a Swiss, or as the case may be Liechtenstein, paying agent has to withhold a tax amounting to 25%, on, inter alia, dividends and capital gains from assets booked with an account or deposit of such Swiss, or as the case may be Liechtenstein, paying agent if the relevant holder of such assets (i.e., generally individuals on their own behalf and as beneficial owners of assets held by a domiciliary company (Sitzgesellschaft)) is tax resident in Austria. The same applies to such income from assets managed by a Liechtenstein paying agent if the relevant holder of these assets (i.e. in general individuals as beneficial owners of a transparent structure) is tax resident in Austria. For Austrian income tax purposes, the withholding tax has the effect of final taxation regarding the underlying income if the EStG provides for the effect of final taxation for such income. The taxpayer can opt for voluntary disclosure instead of the withholding tax by expressly authorizing the Swiss, or as the case may be Liechtenstein, paying agent to disclose to the competent Austrian authority such income which subsequently has to be included in the income tax return.
Given the Agreement between the European Community and the Swiss Confederation providing for measures equivalent to those laid down in Council Directive 2003/48/EC on taxation of savings income in the form of interest payments as amended by the Amending Protocol dated 19 December 2015, the Treaty between the Republic of Austria and the Swiss Confederation on Cooperation in the Areas of Taxation and Capital Markets (in force since 1 January 2013) will be repealed with effect from 1 January 2017. Therefore, as of 1 January 2017, no withholding tax will be levied by Swiss paying agents. As a consequence, dividends and capital gains derived from assets booked with an account or deposit of a Swiss paying agent after 31 December 2016 must be included in the income tax return. Furthermore, given the Amending Protocol to the Agreement between the European Community and the Principality of Liechtenstein providing for measures equivalent to those laid down in Council Directive 2003/48/EC on taxation of savings income in the form of interest payments dated 24 December 2015, also the Treaty between the Republic of Austria and the Principality of Liechtenstein on Cooperation in the Area of Taxation (in force since 1 January 2014) will be, in general, repealed as of 1 January 2017. As a consequence, dividends and capital gains derived from assets booked with an account or deposit of a Liechtenstein paying agent after 31 December 2016 must be included in the income tax return. However, this does not apply with regard to income derived from assets booked with an account or deposit of a Liechtenstein paying agent held by certain investment vehicles (Vermögensstrukturen).
Inheritance and Gift Tax
Austria does not levy inheritance or gift tax.
However, it should be noted that certain gratuitous transfers of assets to (Austrian or foreign) private law foundations and comparable legal estates (privatrechtliche Stiftungen und damit vergleichbare Vermögensmassen) are subject to a foundation entry tax (Stiftungseingangssteuer) pursuant to the Austrian Foundation Entry Tax Act (Stiftungseingangssteuergesetz, StiftEG). Such tax is triggered if, at the
S-72
time of transfer, the transferor and/or the transferee have a domicile, their habitual abode, their legal seat or their place of effective management in Austria. Certain exemptions apply in the event of a transfer mortis causa of financial assets within the meaning of sec. 27(3) and (4) EStG (except for participations in corporations) if income from such financial assets is subject to the special tax rate of 27.5 or 25%. The tax base is the fair market value of the assets transferred minus any debts, calculated at the time of transfer. In general, a tax rate of 2.5% applies, in certain cases, however, a tax rate of 25% applies. Since 1 January 2014, special provisions apply to transfers of assets to non-transparent foundations and similar vehicles (Vermögensstrukturen) falling within the scope of the tax treaty between Austria and Liechtenstein (see above).
In addition, a special notification obligation exists for gifts of money, receivables, shares in corporations, participations in partnerships, businesses, movable tangible assets and intangibles if the donor and/or the donee have a domicile, their habitual abode, their legal seat or their place of effective management in Austria. Not all gifts are covered by the notification obligation. In case of gifts to certain related parties, a threshold of EUR 50,000 per year applies; in all other cases, a notification is obligatory if the value of gifts made exceeds an amount of EUR 15,000 during a period of five years. Furthermore, gratuitous transfers to foundations within the scope of the StiftEG described above are also exempt from the notification obligation. Intentional violation of the notification obligation may lead to the levying of fines of up to 10% of the fair market value of the assets transferred.
Further, it should be noted that gratuitous transfers of common shares may trigger income tax at the level of the transferor pursuant to sec. 27(6)(1) EStG (see above).
Capital Contribution Tax
The capital contribution tax (Gesellschaftsteuer) has been abolished in Austria with effect from 1 January 2016.
Value Added Tax
Sale and purchase of the shares is exempt from value added tax, or VAT, in Austria with no right to deduct input VAT for related expenses.
Other taxes
Currently, the introduction of a financial transaction tax on the transfer of shares is discussed and envisaged on a European level. It is recommended for investors to get in touch with their tax advisors with respect to potential tax consequences which may be triggered by an introduction of a financial transaction tax.
Taxation in the United States
The following is a general summary of the material U.S. federal income tax considerations applicable to a U.S. holder (as defined below) arising from and relating to the receipt, ownership, exercise, termination or disposition of ADS rights and/or common share rights (which, for purposes of this discussion, are sometimes collectively referred to as "rights") pursuant to the Offering, and the acquisition, ownership and disposition of ADSs and/or common shares received upon the exercise of the rights (which, for purposes of this discussion, are sometimes collectively referred to as "rights shares"). This summary does not purport to be a comprehensive discussion of all the tax considerations that may be relevant to a particular U.S. holder, as defined below, of the rights or the rights shares. This summary is based on current provisions of the Internal Revenue Code of 1986, as amended, or the Code, existing, final, temporary and proposed U.S. Treasury Regulations, administrative rulings and judicial decisions, in each case as in effect and available on the date of this prospectus supplement; all of the foregoing are subject to change, which change could apply retroactively and could affect the tax consequences described below.
S-73
This summary is addressed only to U.S. holders, as defined below, that acquire rights at their original issuance or rights shares in connection with the exercise of rights, and hold the rights and the rights shares so acquired as capital assets. This summary does not address all U.S. federal income tax matters that may be relevant to a particular U.S. holder, and each prospective investor should consult a professional tax advisor with respect to the tax consequences arising from and relating to the receipt, ownership, exercise, termination or disposition of rights and the acquisition, ownership and disposition of rights shares. This summary does not address tax considerations applicable to a holder of rights or rights shares that may be subject to special tax rules including, without limitation, the following:
- •
- banks or other financial institutions;
- •
- insurance companies;
- •
- dealers or traders in securities, currencies, or notional principal contracts;
- •
- tax-exempt entities, including an "individual retirement account" or "Roth IRA" retirement plan;
- •
- regulated investment companies or real estate investment trusts;
- •
- persons that hold the rights and/or rights shares as part of a hedge, straddle, conversion, constructive sale or similar transaction involving
more than one position;
- •
- an entity classified as a partnership and persons that hold the rights and/or rights shares through partnerships or certain other pass-through
entities;
- •
- holders (whether individuals, corporations or partnerships) that are treated as expatriates for some or all U.S. federal income tax purposes;
- •
- persons who acquired rights or rights shares as compensation for the performance of services;
- •
- persons holding rights or rights shares in connection with a trade or business conducted outside of the United States;
- •
- a U.S. holder who holds rights or rights shares through a financial account at a foreign financial institution that does not meet the
requirements for avoiding future withholding with respect to certain payments under Sections 1471 through 1474 of the Internal Revenue Code of 1986, as amended, or the Code;
- •
- holders that own (or are deemed to own) 10% or more of our voting shares; and
- •
- holders that have a "functional currency" other than the U.S. dollar.
Further, this summary does not address alternative minimum tax, gift or estate consequences or the indirect effects on the holders of equity interests in entities that own rights or rights shares. In addition, this discussion does not consider the U.S. tax consequences to holders of rights or rights shares other than U.S. holders, as defined below.
For the purposes of this summary, a "U.S. holder" is a beneficial owner of rights or rights shares that is (or is treated as), for U.S. federal income tax purposes:
- •
- an individual who is either a citizen or resident of the United States;
- •
- a corporation, or other entity that is treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws
of the United States or any state of the United States or the District of Columbia;
- •
- an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or
S-74
- •
- a trust, if a court within the United States is able to exercise primary supervision over its administration and one or more U.S. persons have the authority to control all of the substantial decisions of such trust or has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a "United States person" within the meaning of the Code.
If a partnership holds rights or rights shares, the tax treatment of a partner will generally depend upon the status of the partner and upon the activities of the partnership.
We will not seek a ruling from the U.S. Internal Revenue Service, or IRS, with regard to the U.S. federal income tax treatment of an investment in the rights or rights shares, and we cannot assure you that that the IRS will agree with the conclusions set forth below.
Taxation of Rights
The following discussion is subject in its entirety to the rules described below under the heading "—Passive Foreign Investment Corporation Considerations".
Receipt of Rights
Generally, a U.S. holder that receives a right to acquire shares will not be treated as receiving a taxable dividend. However, under certain circumstances, a U.S. holder that receives a right to acquire shares may be treated as receiving a taxable dividend in an amount equal to the value of such right. For example, a U.S. holder that receives a right to acquire shares of common stock of the issuing corporation generally will be treated as having received a taxable dividend if such U.S. holder's proportionate interest in the earnings and profits or assets of the corporation is increased and any other shareholder receives a distribution of cash or other property. For purposes of the preceding sentence, the term "shareholder" includes holders of warrants, options and convertible securities.
During the last 36 months, the Company has not made any distributions of cash or non-stock property with respect to: (i) any shares or ADSs of the Company or (ii) options or warrants to acquire any shares or ADSs of the Company. The distribution of the rights hereunder is an isolated transaction and is not part of a plan to increase any shareholder's proportionate interest in the Company's earnings and profits. The Company does not have any convertible debt outstanding. Nor does the Company currently intend to issue any convertible debt or pay any dividends on its common shares (other than the issuance of rights in connection with the Offering). Accordingly, while the issue is not free from doubt, the Company believes that the distribution of the rights should be treated as a non-taxable stock distribution under the Code and the Company and its agents (including the depositary) intend to treat the distribution of the rights consistently with this belief. The following discussion is based upon the treatment of the right issuance as a non-recognition event for federal income tax purposes under the Code. The following discussion assumes that the IRS and U.S. courts will respect the Company's position and that the U.S. holder is not subject to U.S. federal income tax on the receipt (or deemed receipt) of a right. However, the Company's position is not binding on the IRS and no assurance can be made that the IRS will not disagree with such position. If the Company's position were finally determined by the IRS or a U.S. court to be incorrect, the fair market value of the rights a U.S. holder receives would likely be treated as a distribution to that U.S. holder, with the consequences described below under "Taxation of Rights Shares and ADSs—Distributions". Although no assurance can be given, it is anticipated that the Company will not have current and accumulated earnings and profits through the end of the current taxable year.
If the fair market value of the rights on the date of their distribution equals or exceeds 15% of the fair market value on such date of the common shares or ADSs with respect to which the rights are distributed, a U.S. holder's tax basis in such common shares or ADSs shares must be allocated between such common shares or ADSs and the rights. Such an allocation must be made in proportion to the
S-75
fair market value of the rights and the fair market value of the common shares or ADSs on the date the rights are distributed.
If the fair market value of the rights on the date the Company distributes them is less than 15% of the fair market value of the common shares or ADSs with respect to which the rights are distributed on the same date, a U.S. holder's tax basis in such rights will be zero and the U.S. holder's basis in the common shares or ADSs with respect to which the rights are distributed will remain unchanged. Notwithstanding the foregoing, a U.S. holder may elect (in a statement attached to the U.S. holder's U.S. federal income tax return for the year in which the rights were received) to allocate to the rights a portion of the U.S. holder's basis in such common shares or ADSs shares in the manner described in the immediately preceding paragraph. Any such election is irrevocable and must be applied to all of the rights an investor receives pursuant to the Offering.
Sale or Other Disposition of Rights
A U.S. holder will generally recognize gain or loss for U.S. federal income tax purposes upon the sale or exchange of a right in an amount equal to the difference between the U.S. dollar value of the amount realized from such sale or exchange and the U.S. holder's tax basis for that right. Subject to the discussion under "—Passive Foreign Investment Company Considerations" below, this gain or loss will generally be a capital gain or loss and will generally be treated as from sources within the United States. Such capital gain or loss will be treated as long-term capital gain or loss if the U.S. holder has held the right for more than one year at the time of the sale or exchange; a U.S. holder's holding period in a right will be deemed to have begun on the same date as that of the common share or ADS with respect to which the U.S. holder received that right. Long-term capital gains of non-corporate U.S. holders may be eligible for a preferential tax rate; the deductibility of capital losses is subject to limitations. The so-called "Medicare tax" described below under under "Taxation of Rights Shares and ADSs—Medicare Tax" may also apply to gain from the sale or disposition of rights.
For a cash basis taxpayer, units of foreign currency paid or received are translated into U.S. dollars at the spot rate on the settlement date of the purchase or sale; in that case, no foreign currency exchange gain or loss will result from currency fluctuations between the trade date and the settlement date of such a purchase or sale. An accrual basis taxpayer may elect the same treatment required of cash basis taxpayers with respect to purchases and sales of rights that are traded on an established securities market, provided the election is applied consistently from year to year. Such election may not be changed without the consent of the IRS. For an accrual basis taxpayer who does not make such election, units of foreign currency paid or received are translated into U.S. dollars at the spot rate on the trade date of the purchase or sale. Such an accrual basis taxpayer may recognize exchange gain or loss based on currency fluctuations between the trade date and settlement date. Any foreign currency gain or loss a U.S. holder realizes will be U.S. source ordinary income or loss.
Termination of Rights
If a U.S. holder allows a right to expire without such right being exercised, sold or exchanged by the U.S. holder or on the U.S. holder's behalf, no portion of the tax basis in the rights shares owned by such holder with respect to which such rights were distributed will be allocated to the unexercised rights. If a U.S. holder allocates a portion of its tax basis in the rights shares held by such holder to the rights and the rights expire unexercised after the holder disposes of the rights shares with respect to which the rights were received, then the tax consequences are unclear: the U.S. holder should either recompute its gain or loss from the sale of the rights share as if no basis were allocated to the rights or recognize a capital loss from the expiration of the rights equal to the tax basis allocated to such rights.
S-76
Exercise of Rights
The exercise of a right by a U.S. holder will not be a taxable transaction for U.S. federal income tax purposes. A U.S. holder's initial basis in rights shares acquired upon exercise of a right generally will be equal to the ADS subscription price or the common share subscription price, as applicable, plus the U.S. holder's basis (if any) in the right (determined in U.S. dollars). Subject to the PFIC rules discussed below, the holding period for rights shares acquired on the exercise of a right will begin on the date of exercise.
Passive Foreign Investment Company Considerations
A corporation organized outside the United States generally will be classified as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes in any taxable year in which, after applying the applicable look-through rules, either: (i) at least 75% of its gross income is passive income, or (ii) on average at least 50% of the gross value of its assets is attributable to assets that produce passive income or are held for the production of passive income. In arriving at this calculation, a pro rata portion of the income and assets of each corporation in which we own, directly or indirectly, at least a 25% interest, as determined by the value of such corporation, must be taken into account. Passive income for this purpose generally includes dividends, interest, royalties, rents and gains from commodities and securities transactions.
We believe that we were not a PFIC for any previous taxable year. Based on our estimated gross income, the composition and average value of our gross assets, and the nature of the active businesses conducted by our "25% or greater" owned subsidiaries, and although a final determination regarding our PFIC status for our tax year ended December 31, 2016 cannot be made until our year-end results are known, it currently appears that there is a higher likelihood than in prior years that we may be a PFIC for the 2016 tax year. Our status for any taxable year will depend on our assets and activities in each year, and because this is a factual determination made annually after the end of each taxable year, there can be no assurance that we will not be considered a PFIC for the current taxable year or any future taxable year. The market value of our assets may be determined in large part by reference to the market price of the ADSs and our common shares, which is likely to fluctuate (and may fluctuate considerably given that market prices of life sciences companies can be especially volatile).
If we were (or are) a PFIC for any taxable year during which a U.S. holder held (or holds) rights or rights shares, under the "default PFIC regime" (i.e., in the absence of one of the elections described below) gain recognized by the U.S. holder on a sale or other disposition (including a pledge) of rights will be allocated ratably over the U.S. holder's combined holding period for the rights and the rights shares. The amounts allocated to the taxable year of the sale or other disposition and to any year before we became a PFIC would be taxed as ordinary income. The amount allocated to each other taxable year would be subject to tax at the highest rate in effect for individuals or corporations, as appropriate, for that taxable year, and an interest charge would be imposed on the resulting tax liability for that taxable year.
If we were considered a PFIC for the current taxable year or any future taxable year, a U.S. holder would be required to file annual information returns for such year, whether or not the U.S. holder disposed of any rights during such year.
For the treatment of holders of rights shares under the PFIC rules, see in the discussion under "Taxation of Rights Shares and ADSs—Passive Foreign Investment Company Considerations" below.
S-77
Taxation of Rights Shares and ADSs
Ownership of ADSs
For U.S. federal income tax purposes, a holder of ADSs generally will be treated as the owner of the common shares represented by such ADSs. Gain or loss will generally not be recognized on account of exchanges of common shares for ADSs, or of ADSs for common shares. References to common shares (or to rights shares) in the discussion below are deemed to include ADSs (or ADSs received upon exercise of an ADS right), unless the context otherwise requires.
Distributions
Subject to the discussion under "—Passive Foreign Investment Company Considerations" below, the gross amount of any distribution actually or constructively received by a U.S. holder with respect to rights shares will be taxable to the U.S. holder as a dividend to the extent of such U.S. holder's pro rata share of our current and accumulated earnings and profits as determined under U.S. federal income tax principles. Distributions in excess of such pro rata share of our earnings and profits will be non-taxable to the U.S. holder to the extent of, and will be applied against and reduce, the U.S. holder's adjusted tax basis in the rights shares. Distributions in excess of the sum of such pro rata share of our earnings and profits and such adjusted tax basis will generally be taxable to the U.S. holder as capital gain from the sale or exchange of property. However, since we do not calculate our earnings and profits under U.S. federal income tax principles, it is expected that any distribution made with respect to rights shares will be reported as a dividend, even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above. The amount of any distribution of property other than cash will be the fair market value of that property on the date of distribution. A corporate U.S. holder will not be eligible for any dividends-received deduction in respect of a dividend received with respect to rights shares.
Subject to the discussion below regarding the "Medicare tax," qualified dividends received by non-corporate U.S. holders (i.e., individuals and certain trusts and estates) are currently subject to a maximum U.S. federal income tax rate of 20%. This reduced income tax rate is applicable to dividends paid by "qualified foreign corporations" to non-corporate U.S. holders that meet the applicable requirements, including a minimum holding period (generally, at least 61 days without protection from the risk of loss during the 121-day period beginning 60 days before the ex-dividend date). A non-U.S. corporation (other than a corporation that is classified as a PFIC for the taxable year in which the dividend is paid or the preceding taxable year) generally will be considered to be a qualified foreign corporation (a) if it is eligible for the benefits of a comprehensive tax treaty with the United States which the Secretary of Treasury of the United States determines is satisfactory for purposes of this provision and which includes an exchange of information provision, or (b) with respect to any dividend it pays on shares of stock which are readily tradable on an established securities market in the United States. Our ADSs are listed on The NASDAQ Global Market, which has been determined to be an established securities market in the United States. The Company, which is incorporated under the laws of Austria, believes that it qualifies as a resident of Austria for the purposes of, and is eligible for the benefits of, the Convention between the United States of America and the Republic of Austria for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income, signed on May 31, 1996, or the U.S.-Austria Tax Treaty, although there can be no assurance in this regard. Further, the IRS has determined that the U.S.-Austria Tax Treaty is satisfactory for purposes of the qualified dividend rules and that it includes an exchange-of-information program. Based on the foregoing, we expect to be considered a qualified foreign corporation under the Code. Accordingly, dividends paid by us to non-corporate U.S. holders with respect to rights shares that meet the minimum holding period and other requirements are expected to be treated as "qualified dividend income." However, dividends paid by us will not qualify for the 20% maximum U.S. federal income tax
S-78
rate if we are treated, for the tax year in which the dividends are paid or the preceding tax year, as a PFIC for U.S. federal income tax purposes, as discussed below.
Dividends received by a U.S. holder with respect to rights shares generally will be treated as foreign source income for the purposes of calculating that holder's foreign tax credit limitation. For these purposes, dividends distributed by us generally will constitute "passive category income" (but, in the case of some U.S. holders, may constitute "general category income").
Sale or Other Disposition of Rights Shares
A U.S. holder will generally recognize gain or loss for U.S. federal income tax purposes upon the sale or exchange of rights shares in an amount equal to the difference between the U.S. dollar value of the amount realized from such sale or exchange and the U.S. holder's tax basis for those rights shares. Subject to the discussion under "—Passive Foreign Investment Company Considerations" below, this gain or loss will generally be a capital gain or loss and will generally be treated as from sources within the United States. Such capital gain or loss will be treated as long-term capital gain or loss if the U.S. holder has held (or is deemed to have held) the rights shares for more than one year at the time of the sale or exchange; for this purpose, the U.S. holder's holding period for rights shares will begin on the date on which the U.S. holder received the rights upon the exercise of which the rights shares were received. Long-term capital gains of non-corporate U.S. holders may be eligible for a preferential tax rate; the deductibility of capital losses is subject to limitations.
For a cash basis taxpayer, units of foreign currency paid or received are translated into U.S. dollars at the spot rate on the settlement date of the purchase or sale; in that case, no foreign currency exchange gain or loss will result from currency fluctuations between the trade date and the settlement date of such a purchase or sale. An accrual basis taxpayer may elect the same treatment required of cash basis taxpayers with respect to purchases and sales of rights shares that are traded on an established securities market, provided the election is applied consistently from year to year. Such election may not be changed without the consent of the IRS. For an accrual basis taxpayer who does not make such election, units of foreign currency paid or received are translated into U.S. dollars at the spot rate on the trade date of the purchase or sale. Such an accrual basis taxpayer may recognize exchange gain or loss based on currency fluctuations between the trade date and settlement date. Any foreign currency gain or loss a U.S. holder realizes will be U.S. source ordinary income or loss.
Medicare Tax
An additional 3.8% tax, or "Medicare Tax," is imposed on all or a portion of the "net investment income" (which includes taxable dividends and net capital gains, adjusted for deductions properly allocable to such dividends or net capital gains) received by (i) U.S. holders that are individuals with modified adjusted gross income of over $200,000 ($250,000 in the case of joint filers, $125,000 in the case of married individuals filing separately) and (ii) certain trusts or estates.
Passive Foreign Investment Company Considerations
The definition of a PFIC and our belief as to our PFIC status for the current taxable year and any other year are as set forth in the discussion under "Taxation of Rights—Passive Foreign Investment Company Considerations" above.
If we were (or are) a PFIC for any taxable year during which a U.S. holder held (or holds) rights shares, under the default PFIC regime described in the discussion "Taxation of Rights—Passive Foreign Investment Company Considerations" above (i.e., in the absence of one of the elections described below), gain recognized by the U.S. holder on a sale or other disposition (including a pledge) of the rights shares would be determined and taxed as described in that discussion (substituting references to rights shares for each reference to rights in that discussion, as applicable). The U.S. holder's holding
S-79
period for the rights shares for this purpose will include its holding period for the rights whose exercise resulted in the U.S. holder's receipt of the rights shares.
In the event we were treated as a PFIC, the tax consequences under the default PFIC regime described above could be mitigated by either a "mark-to-market" or "qualified electing fund" election.
A U.S. holder making a mark-to-market election (if the eligibility requirements for such an election were satisfied) generally would not be subject to the PFIC rules discussed above, except with respect to any portion of the holder's holding period that preceded the effective date of the election (which portion would include the U.S. holder's holding period for the rights whose exercise resulted in the U.S. holder's receipt of the rights shares). Instead, the electing holder would include in ordinary income, for each taxable year in which we are a PFIC and to which the election applies, an amount equal to any excess of (a) the fair market value of the rights shares as of the close of such taxable year over (b) the electing holder's adjusted tax basis in such rights shares. In addition, an electing holder would be allowed a deduction in an amount equal to the lesser of (a) the excess, if any, of (i) the electing holder's adjusted tax basis in the rights shares over (ii) the fair market value of such rights shares as of the close of such taxable year or (b) the excess, if any, of (i) the amount included in ordinary income because of the election for prior taxable years over (ii) the amount allowed as a deduction because of the election for prior taxable years. The election would cause adjustments in the electing holder's tax basis in the rights shares (and possibly, in some cases, any unexercised rights) to reflect the amount included in gross income or allowed as a deduction because of the election. In addition, upon a sale or other taxable disposition of rights shares, an electing holder would recognize ordinary income or loss (not to exceed the excess, if any, of (a) the amount included in ordinary income because of the election for prior taxable years over (b) the amount allowed as a deduction because of the election for prior taxable years).
Alternatively, a U.S. holder making a valid and timely "QEF election" generally would not be subject to the default PFIC regime discussed above. Instead, for each PFIC year to which such an election applied, the electing holder would be subject to U.S. federal income tax on the electing holder's pro rata share of our net capital gain and ordinary earnings, regardless of whether such amounts were actually distributed to the electing holder. We currently intend to make available the information necessary to permit a U.S. holder to make a valid QEF election, however, there can be no assurance that we will continue to do so in future years. Since, for this purpose, the U.S. holder's holding period for the rights shares will include its holding period for the rights whose exercise resulted in the U.S. holder's receipt of the rights shares, any QEF election will have to be accompanied by a timely "purging" election that will effectively treat the rights shares as sold, and then reacquired, in the year the rights shares are acquired.
If we were considered a PFIC for the current taxable year or any future taxable year, a U.S. holder would be required to file annual information returns for such year, whether or not the U.S. holder disposed of any rights shares or received any distributions in respect of rights shares during such year.
Backup Withholding and Information Reporting
U.S. holders generally will be subject to information reporting requirements with respect to dividends on rights shares and on the proceeds from the sale, exchange or disposition of rights shares that are paid within the United States or through U.S.-related financial intermediaries, unless the U.S. holder is an "exempt recipient." In addition, U.S. holders may be subject to backup withholding (at a 28% rate) on such payments, unless the U.S. holder provides a taxpayer identification number and a duly executed IRS Form W-9 or otherwise establishes an exemption. Backup withholding is not an additional tax, and the amount of any backup withholding will be allowed as a credit against a U.S.
S-80
holder's U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Foreign Account Tax Compliance Act, or FATCA, and Related Provisions
Under certain circumstances, the Company or its paying agent may be required, pursuant to the FATCA provisions of the Code (or analogous provisions of non-U.S. law) and regulations or pronouncements thereunder, any "intergovernmental agreement" entered into pursuant to those provisions or any U.S. or non-U.S. fiscal or regulatory legislation, rules, guidance notes or practices adopted pursuant to any such agreement, to withhold U.S. tax at a rate of 30% on all or a portion of payments of dividends or other corporate distributions which are treated as "foreign pass-thru payments" made on or after January 1, 2019, if such payments are not exempt from such withholding. The Company believes, and this discussion assumes, that the Company is not a "foreign financial institution" for purposes of FATCA. The rules regarding FATCA and "foreign pass-thru payments," including the treatment of proceeds from the disposition of rights shares, are not completely clear, and further guidance may be issued by the IRS that would clarify how FATCA might apply to dividends or other amounts paid on or with respect to rights shares.
Foreign Asset Reporting
In addition, certain individuals who are U.S. holders may be required to file IRS Form 8938 to report the ownership of "specified foreign financial assets" if the total value of those assets exceeds an applicable threshold amount (subject to certain exceptions). For these purposes, a specified foreign financial asset may include not only a financial account (as defined for these purposes) maintained by a non-U.S. financial institution, but also stock or securities issued by a non-U.S. corporation (such as the Company). Certain U.S. entities may also be required to file IRS Form 8938 in the future.
S-81
General Overview
We are offering to our common shareholders rights to subscribe for new common shares and, through The Bank of New York Mellon, our depositary and the ADS rights agent, are offering to holders of American Depositary Shares, or ADS, non-transferable rights to subscribe for new ADSs pursuant to a rights offering. Except to the extent otherwise provided under Austrian law, the common share rights will be non-transferable. We expect to issue up to 588,127 common shares in this offering, including common shares represented by ADSs. Each ADS represents one tenth (1/10) of a common share, nominal value €1.00 per share.
If you are a holder of ADSs at 5:00 p.m. (New York City time) on November 29, 2016, which is the ADS record date, you will receive 0.276 ADS rights for each ADS owned on that date. One ADS right will entitle you to subscribe for and purchase one new ADS at the U.S. dollar equivalent of €4.014 per ADS. Based on a euro-to-U.S. dollar exchange rate of €1.00 to $1.0588, we estimate that subscription price at $4.25 per ADS. To subscribe for new ADSs, a holder of ADS rights must pay to The Bank of New York Mellon $4.68 per ADS so subscribed, which represents 110% of the estimated subscription price to account for currency conversion expense, ADS issuance fees payable to the depositary and potential fluctuations in the exchange rate between the euro and the U.S. dollar. On or about December 13, 2016, the ADS rights agent will determine the actual U.S. dollar ADS subscription price by converting the euro subscription price into U.S. dollars at an exchange rate assigned by it on that date. Fractional ADSs rights will not be issued, and ADS right entitlements will be reduced to the next smaller whole number of ADS rights. ADS rights will not be assignable or transferable. The depositary will send to each registered holder of ADSs on or about the record date an ADS subscription form, together with a letter of instructions, for exercising ADS rights.
If you are a holder of common shares on November 29, 2016, which is the common share record date, you will have the common share right to subscribe for and purchase 0.276 new common shares, at a subscription price of €40.14 per new common share, for each common share held on the common share record date, payable as described below. No fractional common shares will be issued and common share right entitlements will be reduced to the next smaller whole number of common shares. Common share rights will not be assignable or transferable, except to the extent otherwise provided under Austrian law.
If any new common shares are not subscribed for pursuant to the common share rights and ADS rights described above, following the expiration of the offering of such common share rights and ADS rights, we may, at the discretion of Cantor Fitzgerald & Co., based on market conditions and demand, enter into an underwriting agreement pursuant to which Cantor Fitzgerald & Co. would agree to subscribe for and purchase up to all of the unsubscribed common shares at a purchase price of €40.14 per common share for purposes of resale of ADSs representing the unsubscribed common shares. However, entry into an underwriting agreement for all or a portion of the unsubscribed shares remains at the discretion of Cantor Fitzgerald & Co. and Cantor Fitzgerald & Co. is not obligated to purchase all or any of the unsubscribed common shares. In the event that Cantor Fitzgerald & Co. and we do enter into an underwriting agreement for unsubscribed shares, Cantor Fitzgerald & Co. may sell ADSs at variable prices, which may be more or less than the purchase price and will be determined based on market conditions and demand.
In consideration for services provided to us in connection with these offerings, we have agreed to pay Cantor Fitzgerald & Co. a financial advisory fee equal to 8.0% of the U.S. dollar gross proceeds raised from these offerings.
In addition, certain of our existing principal shareholders and their affiliated entities, holding approximately 39.7% of our outstanding share capital, have indicated an interest in purchasing up to an
S-82
aggregate of approximately $17.5 million of the securities we are offering. However, indications of interest are not binding agreements or commitments to subscribe and purchase. These shareholders may determine to subscribe for and purchase fewer common shares and/or ADSs than they indicate an interest in subscribing for and purchasing or not to subscribe for any common shares and/or ADSs in the rights offering or in the potential offering of ADSs representing any unsubscribed common shares. In addition, because these investors are only entitled to subscribe for and purchase their pro rata portion of any new common shares or ADSs offered as part of the offerings of ADS rights and common share rights, any purchases above this pro rata amount would occur through the potential offering of ADSs representing any unsubscribed shares. Following the expiration of the offering of ADS rights and common share rights, Cantor Fitzgerald & Co. may determine not to proceed with the potential offering of ADSs representing unsubscribed common shares or may determine to sell fewer ADSs representing unsubscribed common shares to any of these shareholders or not to sell any such ADSs to these shareholders.
Until the issuance of the new common shares, including those representing new ADSs that are subscribed for in this offering, are registered with the commercial register in Austria, you will not receive any common shares or ADSs for which you have subscribed. We cannot assure you that the registration of such common shares, which must be approved by a judge of the commercial court in Vienna prior to being issued, will take place when or as anticipated or at all. If the judge does not approve the creation of the new common shares, this offering will need to be cancelled, and you will not receive any common shares or ADSs for which you have subscribed, or purchased from Cantor Fitzgerald & Co., as the case may be, in this financing. Further, if there is a delay in the judge's approval from the time we currently expect, you may receive the common shares or ADSs you purchase later than December 19, 2016.
Below, we set forth the plan of distribution for each of (1) the offering of ADS rights, (2) the offering of common share rights and (3) the potential offering of ADSs representing unsubscribed common shares.
Offering to Holders of ADSs
See "Description of the Offering—Offering to Holders of ADSs" for the plan of distribution in connection with this offering. In consideration for services provided to us in connection with the offering of ADS rights, we have agreed to pay Cantor Fitzgerald & Co. a financial advisory fee equal to 8.0% of the U.S. dollar gross proceeds raised from this offering.
Offering to Holders of Common Shares
See "Description of the Offering—Offering to Holders of Common Shares" for the plan of distribution in connection with this offering. In consideration for services provided to us in connection with the offering of common share rights, we have agreed to pay Cantor Fitzgerald & Co. a financial advisory fee equal to 8.0% of the U.S. dollar gross proceeds raised from this offering.
Potential Offering of ADSs Representing Unsubscribed Common Shares
Underwriting
If any new common shares are not subscribed for pursuant to the offering of the common share rights and ADS rights described above, following the expiration of the offering of such common share rights and ADS rights, we may, at the discretion of Cantor Fitzgerald & Co., based on market conditions and demand, enter into an underwriting agreement pursuant to which Cantor Fitzgerald & Co. would agree to subscribe for and purchase up to all of the unsubscribed common shares at a purchase price of €40.14 per common share for purposes of resale of ADSs representing the unsubscribed common shares. However, entry into an underwriting agreement for all or a portion of
S-83
the unsubscribed shares remains at the discretion of Cantor Fitzgerald & Co. and Cantor Fitzgerald & Co. is not obligated to purchase all or any of the unsubscribed common shares. In the event that Cantor Fitzgerald & Co. and we do enter into an underwriting agreement for unsubscribed shares, Cantor Fitzgerald & Co. may sell ADSs at variable prices, which may be more or less than the purchase price and will be determined based on market conditions and demand. Cantor Fitzgerald & Co. will act as sole bookrunner in connection with any such offering. Subject to the terms and conditions set forth in the underwriting agreement that may be entered into in connection with this offering, Cantor Fitzgerald & Co. will agree to purchase all of the ADSs sold under the underwriting agreement if any of the ADSs are purchased.
We will agree to indemnify Cantor Fitzgerald & Co. against certain liabilities, including liabilities under the Securities Act, or to contribute to payments Cantor Fitzgerald & Co. may be required to make in respect of those liabilities.
In compliance with the Austrian Stock Corporation Act applicable when issuing new shares, Cantor Fitzgerald & Co. or an affiliate of Cantor Fitzgerald & Co., will subscribe for all of the common shares underlying the ADSs to be sold by Cantor Fitzgerald & Co. at a purchase price of €40.14 per common share, and transfer such shares to the depositary for purposes of the resale by Cantor Fitzgerald & Co. of ADSs representing such common shares.
Cantor Fitzgerald & Co. will offer the ADSs representing common shares that they may subscribe for pursuant to the underwriting agreement, subject to prior issue, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the ADSs and the common shares underlying the ADSs, and other conditions to be contained in the underwriting agreement, such as the receipt by Cantor Fitzgerald & Co. of officers' certificates and legal opinions. Cantor Fitzgerald & Co. reserves the right to withdraw, cancel or modify offers to the public with respect to the ADSs representing common shares not subscribed for in the rights offering, and to reject such orders in whole or in part.
Fees and Expenses
Under Austrian law, if Cantor Fitzgerald & Co. purchases any common shares that are not subscribed for pursuant to the common share rights and ADS rights, it will be required to purchase and pay for such common shares in advance at the euro-denominated subscription price of €40.14 per share. We have agreed to pay Cantor Fitzgerald & Co. a cash fee equal to equal to 8.0% of the U.S. dollar gross proceeds to us from resale by Cantor Fitzgerald & Co. of any ADSs representing such common shares.
We estimate expenses payable by us in connection with this offering, other than the fees paid to Cantor Fitzgerald & Co. referred to above, will be approximately $2.1 million.
No Sales of Similar Securities
We, our supervisory board members, management board members and one other existing security holder have agreed not to sell or transfer any of our common shares or securities convertible into or exchangeable or exercisable for our common shares, which includes ADSs, for 90 days after the date of the final prospectus supplement for this offering without first obtaining the written consent of Cantor Fitzgerald & Co. Specifically, we and these other persons have agreed, with certain limited exceptions, not to directly or indirectly:
- •
- offer, pledge, sell or contract to sell any of our equity securities;
- •
- sell any option or contract to purchase any of our equity securities;
- •
- purchase any option or contract to sell any of our equity securities;
S-84
- •
- grant any option, right or warrant for the sale of any of our equity securities;
- •
- otherwise dispose of or transfer any of our equity securities;
- •
- request or demand that we file a registration statement related to any of our equity securities;
or
- •
- enter into any swap or other agreement or any transaction that transfers, in whole or in part, the economic consequence of ownership of any of our equity securities, whether any such swap, agreement or transaction is to be settled by equity securities, in cash or otherwise.
This lock-up provision applies to our equity securities, which includes our common shares and securities convertible into or exchangeable or exercisable for our common shares, which also includes ADSs. It also applies to our equity securities owned now or acquired later by the person executing the agreement or for which the person executing the agreement later acquires the power of disposition.
NASDAQ Global Market Listing
Our ADSs are listed on The NASDAQ Global Market under the symbol "NBRV." On November 28, 2016, the last reported sale price of our ADSs was $4.25 per ADS.
Stamp Taxes
If you purchase securities offered in this prospectus supplement, you may be required to pay stamp taxes and other charges under the laws and practices of the country of purchase, in addition to the offering price listed on the cover page of this prospectus supplement.
Price Stabilization
Until the distribution of the ADSs representing common shares not subscribed for in the rights offering is completed, Securities and Exchange Commission rules may limit Cantor Fitzgerald & Co. from bidding for and purchasing ADSs. However, Cantor Fitzgerald & Co. may engage in transactions that stabilize the price of the ADSs, such as bids or purchases to peg, fix or maintain that price. Stabilizing transactions consist of various bids for or purchases of ADSs made by Cantor Fitzgerald & Co. in the open market prior to the closing of the offering.
Stabilizing bids may have the effect of preventing or retarding a decline in the market price of the ADSs. As a result, the price of the ADSs may be higher than the price that might otherwise exist in the open market. Cantor Fitzgerald & Co. may conduct these transactions on The NASDAQ Global Market, in the over-the-counter market or otherwise.
Neither we nor Cantor Fitzgerald & Co. make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of the ADSs. In addition, neither we nor Cantor Fitzgerald & Co. make any representation that Cantor Fitzgerald & Co. will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Distribution
In connection with the offering, Cantor Fitzgerald & Co. or securities dealers may distribute prospectuses by electronic means, such as e-mail.
Other Relationships
Cantor Fitzgerald & Co. and certain of its affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and
S-85
brokerage activities. Cantor Fitzgerald & Co. and certain of its affiliates may in the future engage in investment banking and other commercial dealings in the ordinary course of business with us and our affiliates, for which they may in the future receive customary fees, commissions and expenses.
In addition, in the ordinary course of their business activities, Cantor Fitzgerald & Co. and its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. Cantor Fitzgerald & Co. and its affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Selling Restrictions
European Economic Area
In relation to each member state of the European Economic Area which has implemented the Prospectus Directive, each referred to herein as a Relevant Member State, with effect from and including the date on which the Prospectus Directive is implemented in that Relevant Member State, referred to herein as the Relevant Implementation Date, no offer of any securities which are the subject of the offering contemplated by this prospectus has been or will be made to the public in that Relevant Member State other than any offer where a prospectus has been or will be published in relation to such securities that has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and notified to the relevant competent authority in that Relevant Member State in accordance with the Prospectus Directive, except that with effect from and including the Relevant Implementation Date, an offer of such securities may be made to the public in that Relevant Member State:
- •
- to any legal entity which is a "qualified investor" as defined in the Prospectus Directive;
- •
- to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or
legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representatives of the
underwriters for any such offer; or
- •
- in any other circumstances falling within Article 3(2) of the Prospectus Directive,
provided that no such offer of securities shall require the Company or any of the underwriters to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
This prospectus has been prepared on the basis that any offer of ADSs or common shares in any Relevant Member State will be made pursuant to an exemption under the Prospectus Directive from the requirement to publish a prospectus for offers of ADSs or common shares. Accordingly, any person making or intending to make an offer in that Relevant Member State of the ADSs or common shares which are the subject of the offering contemplated in this prospectus may only do so in circumstances in which no obligation arises for us or Cantor Fitzgerald & Co. to publish a prospectus pursuant to Article 3 of the Prospectus Directive in relation to such offer. Neither we nor Cantor Fitzgerald & Co. have authorized, nor do we or they authorize, the making of any offer of the ADSs or common shares in circumstances in which an obligation arises for us or Cantor Fitzgerald & Co. to publish a prospectus for such offer.
For the purposes of this provision, the expression an "offer to the public" in relation to any securities in any Relevant Member State means the communication in any form and by any means of
S-86
sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe the securities, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State and the expression "Prospectus Directive" means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the Relevant Member State and the expression "2010 PD Amending Directive" means Directive 2010/73/EU.
Hong Kong
No securities have been offered or sold, and no securities may be offered or sold, in Hong Kong, by means of any document, other than to persons whose ordinary business is to buy or sell shares or debentures, whether as principal or agent; or to "professional investors" as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or in other circumstances which do not result in the document being a "prospectus" as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32) of Hong Kong. No document, invitation or advertisement relating to the securities has been issued or may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted under the securities laws of Hong Kong) other than with respect to securities which are or are intended to be disposed of only to persons outside Hong Kong or only to "professional investors" as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance.
This prospectus has not been registered with the Registrar of Companies in Hong Kong. Accordingly, this prospectus may not be issued, circulated or distributed in Hong Kong, and the securities may not be offered for subscription to members of the public in Hong Kong. Each person acquiring the securities will be required, and is deemed by the acquisition of the securities, to confirm that he is aware of the restriction on offers of the securities described in this prospectus and the relevant offering documents and that he is not acquiring, and has not been offered any securities in circumstances that contravene any such restrictions.
Japan
The offering has not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948 of Japan, as amended), or FIEL, and the Initial Purchaser will not offer or sell any securities, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means, unless otherwise provided herein, any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the FIEL and any other applicable laws, regulations and ministerial guidelines of Japan.
Singapore
This prospectus has not been and will not be lodged or registered with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or the invitation for subscription or purchase of the securities may not be issued, circulated or distributed, nor may the securities be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to the public or any member of the public in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (ii) to a relevant person as defined under
S-87
Section 275(2), or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions, specified in Section 275 of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of any other applicable provision of the SFA.
Where the securities are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
- •
- a corporation (which is not an accredited investor as defined under Section 4A of the SFA) the sole business of which is to hold
investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
- •
- a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor,
shares, debentures and units of shares and debentures of that corporation or the beneficiaries' rights and interest in that trust shall not be transferable for six months after that corporation or that trust has acquired the Offer Shares under Section 275 of the SFA except:
- •
- to an institutional investor under Section 274 of the SFA or to a relevant person defined in Section 275(2) of the SFA, or to any
person pursuant to an offer that is made on terms that such shares, debentures and units of shares and debentures of that corporation or such rights and interest in that trust are acquired at a
consideration of not less than $200,000 (or its equivalent in a foreign currency) for each transaction, whether such amount is to be paid for in cash or by exchange of securities or other assets, and
further for corporations, in accordance with the conditions, specified in Section 275 of the SFA;
- •
- where no consideration is given for the transfer; or
- •
- where the transfer is by operation of law.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or SIX, or on any other stock exchange or regulated trading facility in Switzerland. This prospectus has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this prospectus nor any other offering or marketing material relating to the securities or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this prospectus nor any other offering or marketing material relating to the offering, the Company or the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this prospectus will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, or FINMA, and the offer of securities has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or CISA. The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of securities.
United Kingdom
This prospectus is only being distributed to, and is only directed at, persons in the United Kingdom that are
qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive that are also (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000
S-88
(Financial Promotion) Order 2005, as amended, referred to herein as the Order, and/or (ii) high net worth entities falling within Article 49(2)(a) to (d) of the Order and other persons to whom it may lawfully be communicated. Each such person is referred to herein as a Relevant Person.
This prospectus and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom. Any person in the United Kingdom that is not a Relevant Person should not act or rely on this document or any of its contents.
Israel
This document does not constitute a prospectus under the Israeli Securities Law, 5728-1968, or the Securities Law, and has not been filed with or approved by the Israel Securities Authority. In the State of Israel, this document is being distributed only to, and is directed only at, and any offer of the ordinary shares is directed only at, investors listed in the first addendum, or the Addendum, to the Israeli Securities Law, consisting primarily of joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriters, venture capital funds, entities with equity in excess of NIS 50 million and "qualified individuals", each as defined in the Addendum (as it may be amended from time to time), collectively referred to as qualified investors (in each case purchasing for their own account or, where permitted under the Addendum, for the accounts of their clients who are investors listed in the Addendum). Qualified investors will be required to submit written confirmation that they fall within the scope of the Addendum, are aware of the meaning of same and agree to it.
S-89
EXPENSES RELATED TO THE OFFERING
We estimate that our expenses in connection with this offering, other than fees paid to Cantor Fitzgerald & Co., will be as follows:
Expenses
|
Amount | |||
|---|---|---|---|---|
ADS rights agent fees and expenses |
$ | 50,000 | ||
Information agent fees and expenses |
10,000 | |||
Printing and engraving expenses |
10,000 | |||
Legal fees and expenses |
970,000 | |||
Accounting fees and expenses |
106,000 | |||
Auditor insurance coverage expenses |
847,000 | |||
Miscellaneous costs |
82,000 | |||
| | | | | |
Total |
$ | 2,075,000 | ||
| | | | | |
| | | | | |
| | | | | |
All amounts in the table are estimates. We will pay all of the expenses of this offering.
Legal matters with respect to U.S. federal and New York State law in connection with this offering will be passed upon for us by Wilmer Cutler Pickering Hale and Dorr LLP, New York, New York. Wilmer Cutler Pickering Hale and Dorr LLP, New York, New York has also provided an opinion as to matters of U.S. federal income tax. Certain legal matters with respect to Austrian law in connection with the validity of the shares being offered by this prospectus supplement and other legal matters will be passed upon for us by Freshfields Bruckhaus Deringer LLP, Vienna, Austria. Freshfields Bruckhaus Deringer LLP, Vienna, Austria has also provided an opinion as to matters of Austrian income tax. Covington & Burling LLP, New York, New York is U.S. federal and New York State law counsel for Cantor Fitzgerald & Co. in connection with this offering. Schönherr Rechtsanwälte GmbH, Vienna, Austria is counsel to Cantor Fitzgerald & Co. with respect to Austrian law.
The financial statements incorporated in this prospectus supplement by reference to the Annual Report on Form 20-F for the year ended December 31, 2015 have been so incorporated in reliance on the report of PwC Wirtschaftsprüfung GmbH, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
The registered offices of PwC Wirtschaftsprüfung GmbH are located at Erdbergstrasse 200, 1030 Vienna, Austria.
S-90
WHERE YOU CAN FIND ADDITIONAL INFORMATION
As of the date of this prospectus supplement, we are subject to the periodic reporting and other informational requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, applicable to foreign private issuers. Accordingly, we are required to file reports, including annual reports on Form 20-F, and other information with the SEC. As a foreign private issuer, we are exempt from the rules of the Exchange Act prescribing the furnishing and content of proxy statements to shareholders under the federal proxy rules contained in Sections 14(a), (b) and (c) of the Exchange Act, and our "insiders" are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. However, we have determined that, as of June 30, 2016, we no longer qualified as a "foreign private issuer" under the rules and regulations of the SEC. As a result, beginning January 1, 2017, we anticipate that our future annual filings with the SEC will be made on Form 10-K rather than on Form 20-F. In addition, commencing on January 1, 2017, we plan to expand our reporting consistent with that of a domestic U.S. filer, including filing quarterly reports on Form 10-Q, current reports on Form 8-K and proxy statements under Section 14 of the Exchange Act. We will also prepare our financial statements in accordance with generally accepted accounting principles in the United States rather than the International Financial Reporting Standards. In addition, after January 1, 2017, our "insiders" will also be subject to the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
This prospectus supplement and the accompanying prospectus are part of the registration statement on Form F-3 we filed with the SEC under the Securities Act of 1933, and do not contain all the information set forth in the registration statement. Whenever a reference is made in this prospectus supplement or the accompanying prospectus to any of our contracts, agreements or other documents, the reference may not be complete and you should refer to the exhibits that are a part of the registration statement or the exhibits to the reports or other documents incorporated by reference in this prospectus supplement and the accompanying prospectus for a copy of such contract, agreement or other document. You may read and copy these documents at the SEC's Public Reference Room at 100 F Street N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an internet website at www.sec.gov, from which you can electronically access the registration statement and its exhibits.
Copies of certain information filed by us with the SEC are also available on our website at http://www.nabriva.com. The information contained on, or that can be accessed from, our website does not form a part of this prospectus supplement.
The SEC allows us to incorporate by reference in this prospectus supplement much of the information we file with the SEC, which means that we can disclose important information to you by referring you to those publicly available documents. The information that we incorporate by reference in this prospectus supplement is considered to be part of this prospectus supplement and the accompanying prospectus. Because we are incorporating by reference future filings with the SEC, this prospectus supplement is continually updated and those future filings may modify or supersede some of the information included or incorporated by reference in this prospectus supplement. This means that you must look at all of the SEC filings that we incorporate by reference to determine if any of the statements in this prospectus supplement, the accompanying prospectus or in any document incorporated by reference herein or therein have been modified or superseded. This prospectus supplement incorporates by reference the documents listed below (File No 001-37558) and any future filings we make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act (other than those documents or the portions of those documents not deemed to be filed) and, to the extent designated therein, reports on Form 6-K that we
S-91
furnish to the SEC, in each case, after the date of this prospectus supplement and until the offering of securities offered hereby is terminated or completed:
- •
- Annual Report on Form 20-F for the fiscal year ended December 31, 2015, filed with the SEC on April 28, 2016;
- •
- Report on Form 6-K, furnished to the SEC on July 25, 2016;
- •
- The information contained in Exhibits 10.1, 10.2, 10.3, 10.4 and 10.5 to our Report on Form 6-K, furnished to the SEC on
August 9, 2016;
- •
- Report on Form 6-K, furnished to the SEC on October 17, 2016;
- •
- Report on Form 6-K, furnished to the SEC on October 21, 2016;
- •
- Report on Form 6-K, furnished to the SEC on November 29, 2016; and
- •
- The description of our common shares and ADSs contained in our registration statement on Form 8-A, filed with the SEC under the Exchange Act on September 15, 2015, including any amendment or report filed for the purpose of updating such description.
You may request a copy of these filings, at no cost, by writing or telephoning us at the following address or phone number:
Nabriva
Therapeutics AG
Leberstrasse 20, 1110 Vienna, Austria
+43 (0)1 740 930
S-92
$75,000,000
PROSPECTUS

NABRIVA THERAPEUTICS AG
Common Shares
American Depositary Shares representing Common Shares
Rights to Subscribe for Common Shares
We may issue securities from time to time in one or more offerings of up to $75,000,000 in aggregate offering price. This prospectus describes the general terms of these securities and the general manner in which these securities will be offered. We will provide the specific terms of these securities in supplements to this prospectus. The prospectus supplements will also describe the specific manner in which these securities will be offered and may also supplement, update or amend information contained in this document. You should read this prospectus and any applicable prospectus supplement carefully before you invest.
We may offer these securities in amounts, at prices and on terms determined at the time of offering. The securities may be sold directly to you, through agents, or through underwriters and dealers. If agents, underwriters or dealers are used to sell the securities, we will name them and describe their compensation in a prospectus supplement.
Our ADSs are listed on The NASDAQ Global Market under the symbol "NBRV."
Investing in these securities involves significant risks. See "Risk Factors" included in any accompanying prospectus supplement and in the documents incorporated by reference in this prospectus for a discussion of the factors you should carefully consider before deciding to purchase these securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is November 9, 2016.
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission, which we refer to as the SEC, utilizing a "shelf" registration process. Under this shelf registration process, we may from time to time sell any combination of the securities described in this prospectus in one or more offerings for an aggregate initial offering price of up to $75,000,000.
This prospectus provides you with a general description of the securities we may offer. Each time we sell securities, we will provide one or more prospectus supplements that will contain specific information about the terms of the offering. The prospectus supplement may also add, update or change information contained in this prospectus. You should read both this prospectus and the accompanying prospectus supplement together with the additional information described under the heading "Where You Can Find More Information" beginning on page 3 of this prospectus.
We have not authorized anyone to provide you with information different from that contained in or incorporated by reference in this prospectus, any accompanying prospectus supplement or in any related free writing prospectus filed by us with the SEC. We do not take any responsibility for, and cannot provide any assurance as to the reliability of, any information other than the information in this prospectus, any accompanying prospectus supplement or in any related free writing prospectus filed by us with the SEC. This prospectus and the accompanying prospectus supplement do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the securities described in the accompanying prospectus supplement or an offer to sell or the solicitation of an offer to buy such securities in any circumstances in which such offer or solicitation is unlawful. You should assume that the information appearing in this prospectus, any prospectus supplement, the documents incorporated by reference and any related free writing prospectus is accurate only as of their respective dates. Our business, financial condition, results of operations and prospects may have changed materially since those dates.
Unless the context specifically indicates otherwise, references in this prospectus to "Nabriva Therapeutics AG," "Nabriva," "we," "our," "ours," "us," "our company," "our group" or similar terms refer to Nabriva Therapeutics AG and its consolidated subsidiary, Nabriva Therapeutics US, Inc., a Delaware corporation.
1
Investing in our securities involves significant risks. You should carefully consider the risks and uncertainties described in this prospectus and any accompanying prospectus supplement, including the risk factors set forth in our filings with the SEC that are incorporated by reference herein, including the risk factors in our Annual Report on Form 20-F for the fiscal year ended December 31, 2015, before making an investment decision pursuant to this prospectus and any accompanying prospectus supplement relating to a specific offering.
Our business, financial condition and results of operations could be materially and adversely affected by any or all of these risks or by additional risks and uncertainties not presently known to us or that we currently deem immaterial that may adversely affect us in the future.
2
WHERE YOU CAN FIND MORE INFORMATION
As of the date of this prospectus, we are subject to the periodic reporting and other informational requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, applicable to foreign private issuers. Accordingly, we are required to file reports, including annual reports on Form 20-F, and other information with the SEC. As a foreign private issuer, we are exempt from the rules of the Exchange Act prescribing the furnishing and content of proxy statements to shareholders under the federal proxy rules contained in Sections 14(a), (b) and (c) of the Exchange Act, and our "insiders" are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. However, we have determined that, as of June 30, 2016, we no longer qualified as a "foreign private issuer" under the rules and regulations of the SEC. As a result, beginning January 1, 2017, we anticipate that our future annual filings with the SEC will be made on Form 10-K rather than on Form 20-F. In addition, commencing on January 1, 2017, we plan to expand our reporting consistent with that of a domestic U.S. filer, including filing quarterly reports on Form 10-Q, current reports on Form 8-K and proxy statements under Section 14 of the Exchange Act. We will also prepare our financial statements in accordance with generally accepted accounting principles in the United States rather than the International Financial Reporting Standards. In addition, after January 1, 2017, our "insiders" will also be subject to the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
Our SEC filings are available to the public over the Internet at the SEC's website at http://www.sec.gov. Copies of certain information filed by us with the SEC are also available on our website at http://www.nabriva.com. Our website is not a part of this prospectus and is not incorporated by reference in this prospectus. You may also read and copy any document we file at the SEC's Public Reference Room, 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the Public Reference Room.
This prospectus is part of a registration statement we filed with the SEC. This prospectus omits some information contained in the registration statement in accordance with SEC rules and regulations. You should review the information and exhibits in the registration statement for further information on us and our consolidated subsidiary and the securities we are offering. Statements in this prospectus concerning any document we filed as an exhibit to the registration statement or that we otherwise filed with the SEC are not intended to be comprehensive and are qualified by reference to these filings. You should review the complete document to evaluate these statements. You can obtain a copy of the registration statement from the SEC at the address listed above or from the SEC's website.
The SEC allows us to incorporate by reference in this prospectus much of the information we file with the SEC, which means that we can disclose important information to you by referring you to those publicly available documents. The information that we incorporate by reference in this prospectus is considered to be part of this prospectus. Because we are incorporating by reference future filings with the SEC, this prospectus is continually updated and those future filings may modify or supersede some of the information included or incorporated in this prospectus. This means that you must look at all of the SEC filings that we incorporate by reference to determine if any of the statements in this prospectus or in any document previously incorporated by reference have been modified or superseded. This prospectus incorporates by reference the documents listed below (File No 001-37558) and any future filings we make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Securities Exchange Act of 1934, as amended, or the Exchange Act (other than those documents or the portions of those documents not deemed to be filed) and, to the extent designated therein, reports on Form 6-K that we furnish to the SEC, in each case, between the date of the initial registration statement and the
3
effectiveness of the registration statement and following the effectiveness of the registration statement until the offering of the securities under the registration statement is terminated or completed:
- •
- Annual Report on Form 20-F for the fiscal year ended December 31, 2015, filed with the SEC on April 28, 2016;
- •
- Report on Form 6-K, furnished to the SEC on October 17, 2016;
- •
- Report on Form 6-K, furnished to the SEC on October 21, 2016; and
- •
- The description of our common shares and ADSs contained in our registration statement on Form 8-A, filed with the SEC under the Exchange Act on September 15, 2015, including any amendment or report filed for the purpose of updating such description.
You may request a copy of these filings, at no cost, by writing or telephoning us at the following address or phone number:
Nabriva
Therapeutics AG
1000 Continental Drive, Suite 600
King of Prussia, PA 19406
(610) 816-6640
4
This prospectus and the information incorporated by reference in this prospectus include "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Exchange Act. All statements contained or incorporated by reference herein, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth, other than statements of historical facts, are forward-looking statements. The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "predict," "project," "potential," "will," "would," "could," "should," "continue," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. You are cautioned that these forward-looking statements are only predictions and are subject to risks, uncertainties and assumptions that are referenced in the section of any accompanying prospectus supplement entitled "Risk Factors." You should also carefully review the risk factors and cautionary statements described in the other documents we file from time to time with the SEC, specifically our most recent Annual Report on Form 20-F and our Reports on Form 6-K. We undertake no obligation to revise or update any forward-looking statements, except to the extent required by law.
5
We are a clinical stage biopharmaceutical company engaged in the research and development of novel anti-infective agents to treat serious infections, with a focus on the pleuromutilin class of antibiotics. We are developing our lead product candidate, lefamulin, to be the first pleuromutilin antibiotic available for systemic administration in humans.
Our principal executive offices are located at Leberstrasse 20, 1110 Vienna, Austria, and our telephone number is +43 (0)1 740 930.
6
We intend to use the net proceeds from the sale of any securities offered under this prospectus for general corporate purposes unless otherwise indicated in the applicable prospectus supplement. General corporate purposes may include research and development costs, the acquisition or licensing of other products, businesses or technologies, working capital and capital expenditures. We may temporarily invest the net proceeds from the sale of any securities offered under this prospectus in a variety of capital preservation instruments, including term deposits and short-term, investment-grade, interest-bearing instruments and U.S. and certain European government securities, until they are used for their stated purpose. We have not determined the amount of net proceeds to be used specifically for such purposes. As a result, management will retain broad discretion over the allocation of net proceeds.
7
The following describes our issued share capital, summarizes the material provisions of our articles of association and highlights certain differences in corporate law in Austria and the United States. The description of our articles of association is based upon, and is qualified by reference to, our articles of association. This summary is not complete. You should read our articles of association, which are filed as an exhibit to the registration statement of which this prospectus forms a part, for the provisions that are important to you.
General
We were incorporated in October 2005 in Austria under the name Nabriva Therapeutics Forschungs GmbH, a limited liability company organized under Austrian law, as a spin-off from Sandoz GmbH and commenced operations in February 2006. In 2007, we subsequently transformed into a stock corporation under the name Nabriva Therapeutics AG. We are incorporated under the laws of the Republic of Austria and registered at the Commercial Register of the Commercial Court of Vienna. Our executive offices are located at Leberstrasse 20, 1110 Vienna, Austria.
Issued Share Capital
Under our articles of association, our share capital consists solely of common shares. As of October 1, 2016, there were 2,128,006 common shares outstanding, nominal value €1.00 per share.
Authorized and Conditional Capital
On August 25, 2016, our shareholders authorized (1) our management board, subject to the approval of the supervisory board, to increase our share capital by issuing up to 918,033 new common shares against contribution in cash or in-kind with or without subscription rights, within five years following the registration of such authorized increase (authorized capital 2016), (2) our management board to increase our registered share capital within a period of five years, with the approval of the supervisory board, by issuing up to 146,129 common shares against contribution in cash or in-kind in full or in part by exclusion of subscription rights for the purposes of fulfilling the subscription rights under our Stock Option Plan 2015 (authorized capital 2016—SOP); (3) to effect a conditional capital increase of up to a nominal value of €197,770 for stock options granted under our Stock Option Plan 2015 (conditional capital 2016—SOP); and (4) to effect a conditional capital increase of up to a nominal value of €704,162 to grant conversion or subscription rights to holders of convertible bonds, participation rights or profit participating bonds (conditional capital 2016—convertible bonds). The authorizations described above became effective upon their registration with the commercial register.
The issuance of common shares based on the authorizations described above will only become effective upon payment of the issue price of such common shares and, in the case of common shares issued out of authorized capital, registration with the commercial register. The authorized common shares issued out of conditional capital may become effective after payment of the issue price but without registration with the commercial register.
As of October 1, 2016, there were 359,280 common shares reserved for future issuance under our Stock Option Plan 2007 and our Stock Option Plan 2015, including the common shares described above that were authorized for issuance by our shareholders in August 2016.
Common Shares
Our shareholders are entitled to one vote per share at each general meeting of shareholders. Our shareholders are entitled to receive dividends, if any, in proportion to their respective ownership out of our distributable profits if such a resolution is passed by the general meeting of shareholders. See
8
"—General Regulations on Earnings Appropriation and Dividend Payments." Our articles of association provide that any dividends that remain unclaimed after three years from the date of the general meeting of shareholders at which they were approved will revert to our unrestricted capital reserves.
In the event the company is liquidated, dissolved or wound up, our shareholders will be entitled to share pro rata in all assets remaining after payment of liabilities. Our shares have no preemptive, subscription or conversion rights, and there are no redemption provisions applicable to our shares, except for the mandatory subscription rights provided for under the Austrian Stock Corporation Act. The rights, preferences and privileges of holders of our shares will be subject to, and may be adversely affected by, the rights of holders of shares of any series of preferred shares that we may designate and issue in the future.
Legal title to the shares represented by one or more global share certificates is transferred by agreement (Titel), endorsement of the share certificates (Indossament) and physical handover of the share certificates (tatsächliche Übergabe) in accordance with Austrian law. Neither Austrian law nor our articles of association restrict the transferability of our shares. Each shareholder is entitled to receive physical share certificates evidencing its individual ownership of its shares in the company.
Options
As of October 1, 2016, options to purchase an aggregate of 197,604 common shares, at a weighted average exercise price of €66.00 per share, were outstanding.
Articles of Association
Our articles of association, which were most recently amended on August 25, 2016, contain provisions relating to shareholders' meetings, management and financial accounting that are customary for Austrian stock corporations and do not deviate from the rules provided for by statutory law.
Pursuant to the articles of association our business objective is:
- (i)
- research
or development in the area of medicine and pharmaceuticals, in particular in the field of development of, anti-infective agents, the registration and
commercial exploitation of intellectual property rights in these areas as well as the trade in goods of any kind; and
- (ii)
- the participation in other enterprises of the same or similar kind including the takeover of management in such enterprises as well as asset management (except for banking transactions); its activities extend to Austria and abroad.
We are further entitled to engage in all business activities and to carry out all measures that are deemed necessary or useful for the company's business objective.
Pursuant to the articles of association (and as provided for by statutory Austrian law), the following matters must be approved by the general meeting of shareholders: (i) the distribution of profits, (ii) the release from liability of the members of the management and supervisory boards, (iii) the election of members of the supervisory board, (iv) the appointment of auditors, (v) the amendment of the company's articles of association, and (vi) the increase of the company's share capital (and the corresponding exclusion of subscription rights, if applicable). The shareholders may adopt the respective resolutions at a general meeting by a simple majority of the votes cast, unless a higher majority is mandatorily required by law.
General meetings take place at either (i) our registered offices in Vienna, (ii) at the seat of any domestic branch establishment or domestic subsidiary, or (iii) at an Austrian provincial capital. We must give at least 28 days' (with respect to the convocation of the annual general meeting) and at least 21 days' (with respect to any extraordinary general meeting) prior notice to our shareholders for a
9
general meeting. Our annual general meeting must take place within the first eight months of our financial year and typically includes the following resolutions:
- (i)
- approving
the annual financial statements, if required by law;
- (ii)
- distribution
of profits from the preceding financial year;
- (iii)
- discharge
of members of the management board and the supervisory board for their activities in the preceding financial year; and
- (iv)
- appointment of the auditor for the next financial year.
Pursuant to the articles of association, the supervisory board consists of a minimum of three and a maximum of ten members to be elected by the general meeting for a maximum period of five years. The members of the supervisory board must elect a chairman and one deputy chairman from among its members. The supervisory board must appoint at least one and a maximum of five members of the management board for a maximum term of five years. If the management board consists of more than a single member, the supervisory board has competence to decide whether each member of the management board is permitted to act singly on behalf of the company or jointly with other members of the management board.
Other Austrian Law Considerations
Austrian Stock Corporation Act
The following summary provides information on certain relevant provisions of the Austrian Stock Corporation Act. The summary of relevant provisions under Austrian law set forth hereunder is for general information only. It does not purport to be a comprehensive and complete description of all the topics discussed below.
General Regulations on Earnings Appropriation and Dividend Payments
During the first five months of each financial year, the management board must prepare annual financial statements for the previous financial year, including the notes thereto and the management report. After the financial statements have been audited, the management board must present them, along with a proposal for the distribution of any net profit, to the supervisory board. The supervisory board must provide the management board with a statement on the annual financial statements within two months of their presentation. The supervisory board must also file a report to the general meeting. Pursuant to the Austrian Commercial Code and the Austrian Stock Corporation Act, we may only pay dividends out of distributable profits. Distributable profits are based on accumulated profits, as shown in our unconsolidated financial statements in accordance with the Austrian Commercial Code, after allocations have been made to reserves, including retained earnings. On the basis of the management board's proposal and the supervisory board's report, the general meeting resolves whether dividends will be paid for any financial year and the amount and timing of any such dividend payment. The general meeting, in its resolution, is bound to the annual financial statements as prepared by the management board and approved by the supervisory board. In case the supervisory board does not approve the annual financial statements as prepared by the management board or if the management board and the supervisory board so decide, the general meeting is competent for approving the annual financial statements. It is, however, not bound to the management board's proposal for the distribution of the net profit. Pursuant to the articles of association, the general meeting may also resolve not to distribute all or parts of the net profit among the shareholders. Dividends that have not been collected by the shareholder within three years after becoming due are deemed forfeited and accrue to our free reserve.
10
Liquidation Rights
In the case of our liquidation, any assets remaining after discharge of liabilities and repayment of supplementary capital will be distributed to the shareholders on a pro rata basis. Pursuant to statutory law, a vote of at least 75% of the share capital present or represented at the general meeting is required to pass a resolution regarding the liquidation of the company.
General Provisions Concerning Changes in Share Capital
Pursuant to the provisions of the Austrian Stock Corporation Act, an increase of our share capital is permitted in particular by way of a resolution of the general meeting:
- •
- to issue new shares against contributions in cash or in kind adopted by the general meeting (ordinary capital increase
(ordentliche Kapitalerhöhung) pursuant to Section 149 et seq. of the Austrian Stock Corporation Act);
- •
- authorizing the management board, on the basis of the articles of association and subject to approval of the supervisory board, to issue new
shares up to a specified amount not exceeding 50% of the issued share capital at the time of authorization within a specified period, which may not exceed five years (authorized capital
(genehmigtes Kapital) pursuant to Section 169 et seq. of the Austrian Stock Corporation Act);
- •
- authorizing the issuance of new shares up to a specified amount for specific purposes, such as granting stock options to employees, executives
and members of the management board and the supervisory board or of an affiliated company not exceeding 10% of the issued share capital at the time of authorization, to prepare a merger, or in order
to grant conversion rights or subscription rights to holders of convertible bonds not exceeding 50% of the issued share capital at the time of authorization (conditional capital
(bedingtes Kapital) pursuant to Section 159 et seq. of the Austrian Stock Corporation Act);
- •
- authorizing the management board, subject to the approval of the supervisory board, to effect a conditional capital increase in order to grant
stock options to employees, executives and members of the management board up to a certain amount not exceeding 10% of the issued share capital at the time of authorization (authorized conditional
capital (genehmigtes bedingtes Kapital) pursuant to Section 159 paragraph 3 of the Austrian Stock Corporation Act); or
- •
- authorizing the conversion of free reserves or profit carried forward into share capital (capital adjustment (Kapitalberichtigung) pursuant to the Austrian Capital Adjustment Act (Kapitalberichtigungsgesetz) (the "Capital Adjustment Act")).
Resolutions of the general meeting regarding an ordinary increase of our share capital or regarding authorized or conditional capital or authorized conditional capital as well as the exclusion of subscription rights of existing shareholders require a vote of at least 75% of the share capital represented in the respective general meeting.
Except in the case of certain capital reductions effected by a repurchase of shares by the company, a resolution to decrease the share capital requires a majority of at least 75% of the share capital represented in the respective general meeting (Section 175 paragraph 1 of the Austrian Stock Corporation Act).
Following any of the resolutions described above, a judge in Austria must approve the creation of the new common shares, including the new common shares that will underlie any ADSs offered by us. A delay in approval by the judge could result in a delay in the settlement of such an offering.
11
General Provisions Concerning Subscription Rights
Pursuant to Section 153 paragraph 1 of the Austrian Stock Corporation Act, our existing shareholders are entitled to subscribe for and to be allocated such number of new shares to allow them to maintain their existing participation in our share capital. The subscription rights (Bezugsrechte) of existing shareholders are therefore proportionate to the number of shares held by them prior to the offering of new shares. Similarly, Section 174 paragraph 4 of the Austrian Stock Corporation Act provides for subscription rights of our existing shareholders in relation to securities convertible into shares, securities with warrants to purchase shares, securities with profit participation or participation certificates to allow them to maintain their existing participation in our share capital. Shareholders may waive or choose not to exercise their subscription rights. Furthermore, subscription rights may fully or partially be excluded by resolution of the general meeting (Section 153 paragraph 3 of the Austrian Stock Corporation Act). If subscription rights are to be excluded, a majority of at least 75% of the share capital present or represented at the general meeting must approve the respective resolution. In addition, the proposal to exclude subscription rights must be announced prior to the respective general meeting and must be based on a written report by the management board justifying such exclusion. A shareholders' resolution in respect of authorized capital may either directly exclude subscription rights or authorize the management board to exclude subscription rights with a majority of 75% of the share capital present or represented at the respective general meeting. If the management board is authorized to exclude subscription rights, the management board's resolution to exclude subscription rights requires approval by the supervisory board, as well as an additional reasoned statement justifying the exclusion. If shares are issued out of conditional capital, existing shareholders are not entitled to subscription rights.
Existing shareholders are entitled to exercise their rights within a specified subscription period (Bezugsfrist), which must last for at least two weeks. When issuing new shares, the management board must publish a notice in the official gazette (Amtsblatt zur Wiener Zeitung) specifying the beginning and the duration of the subscription period, as well as the subscription price. Shareholders may transfer their subscription rights during the subscription period. If subscription rights are not exercised during the subscription period, they will be deemed forfeited. Subscription rights are not considered excluded if new shares are initially subscribed for by a credit institution which undertakes to offer the new shares to existing shareholders in proportion to their subscription rights (intermediate subscription right, mittelbares Bezugsrecht).
Authorization to Purchase and Sell Treasury Shares
Pursuant to the Austrian Stock Corporation Act, a stock corporation may generally only purchase and sell its treasury shares in the following limited circumstances:
- •
- upon prior authorization by the general meeting, for a period not exceeding 30 months and limited to a total of 10% of the overall share
capital, if the shares are listed on a regulated market, or if the shares are intended to be offered to the company's employees, members of its management board or supervisory board or employees of
certain affiliated companies; the resolution must determine a minimum and a maximum consideration, provided that the company keeps sufficient reserves;
- •
- if the shares are acquired without payment of consideration or if the stock corporation is acting as agent on a commission basis;
- •
- to prevent substantial, immediately threatening damage to the stock corporation (subject to the limitation of 10% of the overall share
capital), provided the stock corporation keeps sufficient reserves;
- •
- by way of a universal legal succession (e.g., succession by merger);
12
- •
- for the purpose of indemnifying minority shareholders, if required by law, provided that the stock corporation keeps sufficient reserves;
- •
- as part of a redemption of shares in accordance with the rules for capital decreases approved by the shareholders' meeting; or
- •
- if the stock corporation is a credit institution authorized by the shareholders' meeting to purchase treasury shares for the purpose of trading in securities.
Shareholder Rights
Voting Rights and Majority Requirements
Each share entitles its holder to one vote at the shareholders' meeting. There is no minimum attendance quorum at the shareholders' meeting under Austrian corporation law. Resolutions of the shareholders' meeting are passed by a simple majority of the votes cast or, in matters which require a majority of the share capital, by a simple majority of the share capital present, unless the stock corporation law requires a higher majority.
Under the Austrian stock corporation law, the following measures require the affirmative vote of at least 75% of the share capital present or represented at a shareholders' meeting:
- •
- change of the business objectives;
- •
- increase of share capital with a simultaneous exclusion of subscription rights;
- •
- creation of authorized capital or conditional capital;
- •
- decrease of share capital;
- •
- exclusion of subscription rights for convertible bonds, participating bonds and participation rights;
- •
- dissolution of the company or continuation of the dissolved company;
- •
- transformation of the company into a limited liability company (Gesellschaft mit beschränkter
Haftung—GmbH);
- •
- approval of a merger or a spin-off (proportionate to shareholdings);
- •
- transfer of all or a majority of the assets of the company;
- •
- approval of profit pools or agreements on the operation of the business;
- •
- post-formation acquisition (Nachgründung);
- •
- revocation of the appointment of supervisory board members; and
- •
- sale of treasury shares other than by a stock exchange or a public offer.
Under Austrian law, approval by shareholders holding at least 90% of the share capital is required for an upstream merger, with certain exceptions, for a spin-off disproportionate to shareholdings or for a squeeze-out of minority shareholders.
A shareholder or a group of shareholders holding at least one-third of the share capital present or represented at the shareholders' meeting can elect a person to the supervisory board provided that the same shareholders' meeting has to elect at least three members of the supervisory board.
A shareholder or a group of shareholders holding at least 20% of the share capital may object to settlements or waivers of liability claims of the company against its founders or members of the management board or the supervisory board.
13
A shareholder or a group of shareholders holding at least 10% of the share capital may in particular:
- •
- request special audits of activities with respect to the management of the company, if these activities took place within the previous two
years;
- •
- veto the appointment of a special auditor and request a court to appoint another special auditor;
- •
- request an adjournment of the shareholders' meeting if the annual financial statements are found to be incorrect by the shareholders who
require such adjournment;
- •
- request a court to recall a member of the supervisory board for cause; and
- •
- request the assertion of damage claims by the company against members of the management board or the supervisory board or certain other parties, if the claim is not obviously unfounded.
A shareholder or a group of shareholders holding at least 5% of the share capital may in particular:
- •
- request that a shareholders' meeting be convened or, if the management board and the supervisory board do not comply with such request to
convene a shareholders' meeting or, upon court approval, convene a shareholders' meeting themselves;
- •
- request that a topic be put on the agenda of the shareholders' meeting and be made public;
- •
- request the assertion of damage claims of the company against members of the management board or the supervisory board or certain other
parties, if a special report reveals facts which may entitle the company to such damage claims;
- •
- request court appointment of another auditor of the financial statements for cause;
- •
- appeal a shareholders' resolution, if such resolution provides for amortization, accumulated depreciation, reserves and accruals exceeding the
limit set by law or the articles of association;
- •
- apply for the appointment or removal of liquidators for cause; and
- •
- apply for an audit of the annual financial statements during liquidation.
A shareholder or a group of shareholders holding at least 1% of the share capital in a listed company may communicate to the company propositions for resolutions with respect to each topic on the agenda of the shareholders' meeting and request for these propositions to be made public on the internet site of the company. Under our articles of association, such proposals must be made in the German language.
Change or Impairment of Shareholders' Rights
The Austrian Stock Corporation Act contains provisions that protect the rights of individual shareholders. As a general rule, shareholders must be treated equally under equal circumstances, unless the concerned shareholders agree otherwise. Furthermore, measures affecting shareholders' rights, such as capital increases and the exclusion of subscription rights, generally require a shareholders' resolution.
The articles of association do not provide for more stringent conditions for the exercise of shareholders' rights than those provided by the Austrian Stock Corporation Act. In addition, the articles of association do not allow changes to, or restriction on shareholders' rights under less stringent conditions than those provided by the Austrian Stock Corporation Act.
Neither Austrian law nor the articles of association restrict the right of non-resident or foreign holders of the shares to hold or vote their shares.
14
Shareholders' Meeting
The shareholders' meeting is convened by the management board or the supervisory board. The shareholders' meeting may take place at the corporate seat of the company in Vienna, Austria, at the seat of any branch office or subsidiary in Austria or in a capital of an Austrian province.
A company must publish an invitation notice of the shareholders' meeting; the minimum period between the publication of the invitation notice and the day of the general shareholders' meeting must be 28 days, or 21 days in case of an extraordinary shareholders' meeting. Shareholders may appoint proxies to represent them at shareholders' meetings.
The general shareholders' meeting must take place within the first eight months of each financial year.
Redemption or Conversion of Shares
A redemption of shares is possible in the course of a decrease of the stated share capital resolved by the shareholders' meeting, or by the company's purchase of its own shares. A capital decrease requires a shareholders' resolution with a majority of at least 75% of the share capital present or represented at the shareholders' meeting.
Pursuant to the Austrian Stock Corporation Act, a company may acquire its own shares only under specific circumstances. See "—Authorization to Purchase and Sell Treasury Shares." The shares may be converted into a different class of shares, such as non-voting preferred shares, but only with the consent of the respective holder or, in case of a conversion negatively affecting other shareholders whose shares are not converted, the consent of such shareholders.
Neither our articles of association nor the Austrian Stock Corporation Act contain an obligation of the shareholders to make further commitments, such as equity contributions, to the company.
Austrian Insolvency Act
The following summary provides information on certain relevant provisions of the Austrian Insolvency Act (Insolvenzordnung), or the AIA. The summary of relevant provisions under Austrian law set forth hereunder is for general information only. It does not purport to be a comprehensive and complete description of all the topics discussed below.
As we are incorporated under the laws of Austria, a rebuttable presumption exists that we also have our "center of main interests" in Austria. In the event of an insolvency of a company having its "center of main interests" in Austria, insolvency proceedings may be initiated in Austria. Such proceedings will be governed by Austrian law. Under certain circumstances, insolvency proceedings may also be opened in Austria in accordance with Austrian law with respect to the assets of companies that are not organized under Austrian law.
The following is a brief description of certain aspects of Austrian insolvency law. The law relating to insolvency is regulated by the AIA which entered into force on July 1, 2010.
Insolvency proceedings (Insolvenzverfahren) are opened by a court in the event that the debtor is insolvent (zahlungsunfähig) (i.e., unable to pay its debts as and when they fall due) or over-indebted within the meaning of the AIA (überschuldet) (i.e., its liabilities exceed the liquidation value of its assets in combination with a negative prognosis on its ability to continue as a going concern (negative Fortbestehensprognose)). Under Austrian law, insolvency proceedings may be initiated either by the debtor or a creditor by filing an application to that effect with a court of competent jurisdiction. If insolvency proceedings are initiated upon a creditor's request, such creditor will have to show that the debtor is insolvent or over-indebted. In the event that the debtor is at imminent risk of being unable to
15
pay its debts as and when they fall due (drohende Zahlungsunfähigkeit), insolvency proceedings may be initiated only upon the debtor's request.
If the debtor has submitted, together with its application requesting the opening of insolvency proceedings, an application for the commencement of restructuring proceedings (Sanierungsverfahren), the court may order the opening of either insolvency proceedings or restructuring proceedings. The legal provisions regulating restructuring proceedings do not apply to insolvency proceedings.
Depending on whether the debtor submits a restructuring plan (Sanierungsplan) together with the application for the opening of insolvency proceedings, the initiated proceedings may be in the form of restructuring proceedings (Sanierungsverfahren) or insolvency proceedings. If it is the debtor that has applied for the initiation of insolvency proceedings and has submitted to the court a restructuring plan (Sanierungsplan) that offers a recovery rate of at least 20% payable to the unsecured creditors over a maximum period of two years, any proceedings so initiated by the court will be in the form of restructuring proceedings. A debtor may also submit a restructuring plan in the course of insolvency proceedings that are already in progress whereupon such proceedings will continue as restructuring proceedings. For the debtor's restructuring plan to be approved by the court it should meet certain criteria specified by law.
The purpose of a restructuring plan is to enable a debtor to be released from a portion of its debts (not to exceed 80% of the aggregate amount thereof) and to continue its business operations. A restructuring plan has to be approved by a "qualified majority" of the debtor's unsecured creditors. A "qualified majority" refers to a majority of the debtor's unsecured creditors present at the respective court hearing, provided that such majority represents more than 50% of the aggregate amount of all claims of the unsecured creditors being present at such hearing. Once the debtor has complied with the terms of a restructuring plan that was duly approved by the creditors and confirmed by the court, it will be released from its remaining outstanding unsecured debts. Unsecured creditors whose claims under the restructuring plan have not been satisfied in accordance with the plan's terms may enforce their individual claims against the debtor, in which case the restructuring proceedings will be continued as insolvency proceedings.
If the restructuring proceedings have been initiated and the debtor has submitted a restructuring plan that offers a recovery rate of at least 30% to the unsecured creditors over a maximum two-year period after the approval of such restructuring plan, the debtor qualifies for self-administration (Sanierungsverfahren mit Eigenverwaltung).
Unless the debtor qualifies for self-administration, it is not allowed as of the date of the opening of the insolvency or the restructuring proceedings, as the case may be, to dispose of the assets belonging to the insolvency estate (Insolvenzmasse). The opening of insolvency proceedings takes effect on the day following the publication of the court's order opening such proceedings in the official online database of Austrian insolvencies. After the opening of insolvency proceedings, transactions of the debtor with respect to assets belonging to the insolvency estate have no effect against the creditors of the insolvency estate.
With its decision to open the insolvency proceedings, the court will appoint an insolvency administrator (Insolvenzverwalter) and may, depending on the nature and the size of the debtor's business (either ex officio or upon the request of the creditors' meeting (Gläubigerversammlung)), appoint a creditors' committee (Gläubigerausschuss) charged with monitoring and assisting the insolvency administrator in the discharge of its duties. After the opening of insolvency proceedings (and unless the debtor qualifies for self-administration) only the insolvency administrator is entitled to act on behalf of the insolvency estate.
16
Under Austrian law, an insolvency administrator's role is to continue the debtor's business with a view to enabling a potential reorganization of the debtor's business either by implementing the debtor's restructuring plan or by a sale of the debtor's business. If neither a restructuring plan nor a sale of the debtor's business is possible, the insolvency administrator will discontinue the debtor's business operations. As a result of the ensuing insolvency proceedings, the debtor's assets will be liquidated and the proceeds realized thereby will be distributed to the debtor's creditors, with the debtor remaining liable for any portion of its debts not satisfied by such proceeds.
If the debtor qualifies for self-administration, the court will proceed with the appointment of a restructuring administrator (Sanierungsverwalter) to monitor the activities of the debtor. In such case, certain transactions are either subject to the restructuring administrator's approval or may be performed only by the restructuring administrator.
Unsecured creditors (Insolvenzgläubiger) wishing to assert their claims against the debtor need to participate in the insolvency proceedings and must file their claim with the competent court within the time period set out in the court order opening the insolvency proceedings. At the respective hearing (examination hearing (Prüfungstagsatzung)), the insolvency administrator has to declare whether it acknowledges or contests each of the claims filed with the court. If the insolvency administrator acknowledges a creditor's claim, such creditor will be entitled to participate in the insolvency proceedings and the pro rata distribution to unsecured creditors that will follow. If a creditor's claim is contested by the insolvency administrator, the creditor will have to seek enforcement of its claim in civil proceedings and only then participate in the insolvency proceedings.
Claims of unsecured creditors which were created before the opening of the insolvency proceedings rank pari passu with each other. Certain claims which lawfully arose against the insolvency estate after the opening of the insolvency proceedings (privileged claims (Masseforderungen)) enjoy priority in insolvency proceedings. Claims which are secured by collateral, such as a mortgage, a pledge over bank accounts or shares, an assignment of receivables for security purposes or a security transfer of moveable assets (preferential claims (Absonderungsrechte)), are entitled to preferential payment in the distribution of the proceeds resulting from the realization of the charged asset. Creditors who have a right to preferential treatment may participate in the pro rata distribution to the unsecured creditors only to the extent that the proceeds from the realization of the assets charged to them did not cover their claims or if they have waived their right to preferential treatment. Secured creditors do not have a voting right with respect to the approval of the restructuring plan to the extent their claim is covered by security. Claims relating to the payment of taxes, social security contributions and employee compensation are not, as such, privileged or preferential claims under Austrian law.
The costs of the insolvency proceedings and certain liabilities accrued during such proceedings constitute privileged claims (Masseforderungen) and rank senior to all other unsecured claims (Insolvenzforderungen). Claims of creditors with a right of segregation of assets (Aussonderungsberechtigte), such as creditors with a retention of title or trustors, remain unaffected by the opening of insolvency proceedings.
Once insolvency proceedings have been opened, it is no longer possible to obtain an execution lien with respect to assets belonging to the insolvency estate. All execution proceedings against the debtor are subject to an automatic stay (Vollstreckungssperre). Execution liens obtained within the last 60 days prior to the opening of insolvency proceedings expire upon the opening of such insolvency proceedings unless the insolvency proceedings are terminated due to lack of funds to cover the cost of such proceedings.
Under the voidance rules of the AIA, an insolvency administrator may, by action of voidance or by defense of voidance, under certain circumstances as set forth in the AIA, challenge any transaction, which includes, without limitation, the granting of security and the guaranteeing, assuming and/or paying of debt.
17
Exchange Controls
There are currently no legal restrictions in Austria on international capital movements and foreign-exchange transactions, except in limited embargo circumstances (Teilembargo) relating to certain areas, entities or persons as a result of applicable resolutions adopted by the United Nations and the European Union. Restrictions currently exist with respect to, among others, Afghanistan, Belarus, Burma/Myanmar, Central African Republic, Congo, Egypt, Eritrea, Guinea, Guinea-Bissau, Iran, Iraq, Ivory Coast, Lebanon, Liberia, Libya, North Korea, Somalia, South Sudan, Sudan, Syria, Tunisia, Ukraine and Zimbabwe.
For statistical purposes, there are, however, limited notification requirements regarding transactions involving cross-border monetary transfers. With some exceptions, every corporation or individual residing in Austria must report to the Austrian National Bank (Österreichische Nationalbank) any active or passive cross-border direct investment transaction exceeding €500,000 (or the equivalent in a foreign currency), provided that special rules apply for securities held in custody by or through Austrian custodians.
Differences in Corporate Law
The applicable provisions of the Austrian Stock Corporation Act differ from laws applicable to U.S. corporations and their shareholders. Set forth below is a summary of certain differences between the provisions of the Austrian Stock Corporation Act applicable to us and the Delaware General Corporation Law relating to shareholders' rights and protections. This summary is not intended to be a complete discussion of the respective rights and it is qualified in its entirety by reference to Delaware law and Austrian law.
| |
Austria | Delaware | ||
|---|---|---|---|---|
Board System |
Under Austrian law, a stock corporation has a two-tiered board structure composed of the management board (Vorstand) and the supervisory board (Aufsichtsrat). The management board is responsible for running the company's affairs and representing the company in dealings with third parties. The members of the management board are appointed by the company's supervisory board for a definite term, and their appointment can only be revoked under certain circumstances. The supervisory board is elected by the company's shareholders and has a strategic and monitoring role. The supervisory board does not actively manage the company but grants prior approval before the management board takes certain actions |
Under Delaware law, a corporation has a unitary board structure and it is the responsibility of the board of directors to appoint and oversee the management of the corporation on behalf of and in the best interests of the shareholders of the corporation. |
18
| |
Austria | Delaware | ||
|---|---|---|---|---|
Number of Directors |
Under Austrian law, a stock corporation must have at least one member on its management board and the number of member shall be fixed by or in the manner provided in the company's articles of association. A stock corporation must have at least three but no more than 20 supervisory board members, who are elected or delegated by the company's shareholders. The articles of association of the company must specify if the supervisory board will have more than three members. If the company has a works council, a body which represents the company's employees, then the company's works council is entitled to nominate one member to the supervisory board for every two supervisory board members elected or delegated by the company's shareholders. Consequently, supervisory boards are often split such that two-thirds of supervisory board members are representatives of the shareholders, while one-third are representatives of the works council. |
Under Delaware law, a corporation must have at least one director and the number of directors shall be fixed by or in the manner provided in the bylaws. |
||
Removal of Directors |
The management board of an Austrian stock corporation is appointed by the supervisory board for a maximum period of five years with an opportunity to be re-elected. The supervisory board may remove a member of the management board prior to the expiration of his or her term only for a significant cause, such as gross negligence (grobe Fahrlässigkeit) in carrying out their duties, the inability to manage the business properly or a vote of no-confidence during the shareholders' meeting (Vertrauensentzug). The shareholders themselves are not entitled to appoint or dismiss the members of the management board. Supervisory board members may be removed by a resolution of three-quarters of the shareholders unless otherwise provided by the company's articles of association. The company's first supervisory board, however, may be removed by a simple majority of votes cast. Supervisory board members who are delegated by a shareholder or the works council by a special right of such shareholder or works council may be revoked and the resulting vacancy filled only at the sole discretion of such shareholder or works council. |
Under Delaware law, any director or the entire board of directors may be removed, with or without cause, by the holders of a majority of the shares then entitled to vote at an election of directors, except (a) unless the certificate of incorporation provides otherwise, in the case of a corporation whose board of directors is classified, shareholders may effect such removal only for cause, or (b) in the case of a corporation having cumulative voting, if less than the entire board of directors is to be removed, no director may be removed without cause if the votes cast against his removal would be sufficient to elect him if then cumulatively voted at an election of the entire board of directors, or, if there are classes of directors, at an election of the class of directors of which he is a part. |
19
| |
Austria | Delaware | ||
|---|---|---|---|---|
Vacancies on the Board of Directors |
Under Austrian law, vacant positions on the management board are filled by the supervisory board in accordance with the general rules of appointment, which provide that vacancies are filled by (i) the simple majority of supervisory board votes cast and (ii) in any case also the majority of votes of members who represent the shareholders (in order to protect shareholders against excessive influence of employee representatives on the supervisory board). In case of emergencies, a vacant position on the management board may be temporarily filled by a supervisory board member or by an individual appointed by the court. If the number of supervisory board members representing the shareholders falls below three, the statutory minimum required, the company's management board is required to convene an extraordinary general meeting, during which the vacant positions of the supervisory board will be filled. If the vacancy persists for longer than three months, the court shall appoint the necessary number of supervisory board members. Supervisory board members, who are delegated by a shareholder (special right of such stockholder) may be revoked and the resulting vacancy filled again at the sole discretion of such shareholder. The same applies—mutatis mutandis—to supervisory board members, who are delegated by the works council. |
Under Delaware law, vacancies and newly created directorships may be filled by a majority of the directors then in office (even though less than a quorum) or by a sole remaining director unless (a) otherwise provided in the certificate of incorporation or by-laws of the corporation or (b) the certificate of incorporation directs that a particular class of stock is to elect such director, in which case a majority of the other directors elected by such class, or a sole remaining director elected by such class, will fill such vacancy. |
||
Annual General Meeting |
Under Austrian law, a stock corporation must hold an annual general meeting within eight months of the end of its fiscal year. The annual general meeting must be held in Austria at a location determined by the articles of association. If the articles of association do not provide for a specific location, the general meeting shall be held at the company's seat or, if applicable, at the venue where its shares are listed. |
Under Delaware law, the annual meeting of stockholders shall be held at such place, on such date and at such time as may be designated from time to time by the board of directors or as provided in the certificate of incorporation or by the bylaws. |
||
General Meeting |
Under Austrian law, extraordinary general meetings, in addition to the annual general meeting, may be convened by either the management board or the supervisory board if it is in the best interest of the company. Shareholders holding at least 5% of the company's share capital are entitled to request that the management board convene an extraordinary general meeting, and may also address their request to the court, which then may authorize the requesting minority shareholders to convene a special meeting by themselves. |
Under Delaware law, special meetings of the stockholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the bylaws. |
20
| |
Austria | Delaware | ||
|---|---|---|---|---|
Notice of General Meetings |
Under Austrian law, unless a longer period is otherwise provided for in the articles of association, the shareholders must be given advance notice of at least 28 days, with respect to the annual general meeting, and 21 days with respect to extraordinary general meetings. Such notices must specify the location, date, hour, and purpose or purposes of the meeting, including the respective items on the agenda. The management board and the supervisory board are furthermore required to prepare proposals for each item on the agenda. Such proposals must be provided to the shareholders at least 21 days before the general meeting. The shareholders of a non-listed company may in all cases consent to a shorter notice period or waive all formality requirements with respect to the convention of a general meeting, if all shareholders entitled to attend so consent. |
Under Delaware law, unless otherwise provided in the certificate of incorporation or bylaws, written notice of any meeting of the stockholders must be given to each stockholder entitled to vote at the meeting not less than ten nor more than 60 days before the date of the meeting and shall specify the place, date, hour, and purpose or purposes of the meeting. |
||
Proxy |
Under Austrian law, a shareholder may designate another person to attend, speak and vote at a general meeting of the company on their behalf by proxy. A management board member may issue a proxy to another management board member representing the member's voting rights as a management board member. Except for the chairman of the supervisory board, who may not issue a proxy, a supervisory board member may only issue a proxy to another supervisory board member representing the member's voting rights as a supervisory board member only if so allowed by the company's articles of association. |
Under Delaware law, at any meeting of stockholders, a stockholder may designate another person to act for such stockholder by proxy, but no such proxy shall be voted or acted upon after three years from its date, unless the proxy provides for a longer period. A director of a Delaware corporation may not issue a proxy representing the director's voting rights as a director. |
||
Pre-emptive Rights |
Under Austrian law, existing shareholders have a statutory pre-emptive subscription right for any additional issue of shares or any security convertible into shares pro rata to the nominal value of their respective holdings in the company, unless (i) shareholders representing three-quarters of the registered share capital present at the general meeting have resolved upon the whole or partial exclusion of the pre-emptive subscription right and (ii) there exists good and objective cause for such exclusion. No separate resolution on the exclusion of subscription rights is required if all shareholders waive their statutory pre-emptive subscription right. |
Under Delaware law, stockholders have no pre-emptive rights to subscribe to additional issues of stock or to any security convertible into such stock unless, and except to the extent that, such rights are expressly provided for in the certificate of incorporation. |
21
| |
Austria | Delaware | ||
|---|---|---|---|---|
Authority to Allot |
Under Austrian law, the management board may not allot shares, grant rights to subscribe for or to convert any security into shares unless a shareholder resolution to that effect has been passed at the company's general meeting, in each case in accordance with the provisions of the Austrian Stock Corporation Act. |
Under Delaware law, if the corporation's charter or certificate of incorporation so provides, the board of directors has the power to authorize the issuance of stock. It may authorize capital stock to be issued for consideration consisting of cash, any tangible or intangible property or any benefit to the corporation or any combination thereof. It may determine the amount of such consideration by approving a formula. In the absence of actual fraud in the transaction, the judgment of the directors as to the value of such consideration is conclusive. |
||
Liability of Directors and Officers |
Under Austrian law, any provision, whether contained in the company's articles of association or any contract or otherwise, that purports to exempt a management or supervisory board member from any liability that would otherwise attach to such board member in connection with any negligence, default, breach of duty or breach of trust in relation to the company is void. |
Under Delaware law, a corporation's certificate of incorporation may include a provision eliminating or limiting the personal liability of a director to the corporation and its stockholders for damages arising from a breach of fiduciary duty as a
director. However, no provision can limit the liability of a director for: • any breach of the director's duty of loyalty to the corporation or its stockholders;
• acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law;
• intentional or negligent payment of unlawful dividends or stock purchases or redemptions; or
• any transaction from which the director derives an improper personal benefit. |
||
Voting Rights |
Under Austrian law, each company share, except statutory preferred shares, entitles its holder to one vote at the general meeting. While Austrian law does not provide for a minimum attendance quorum for general meetings, the company's articles of association may so provide. In general, resolutions adopted at a general meeting of shareholders may be passed by a simple majority of votes cast, unless a higher majority is required by law. |
Delaware law provides that, unless otherwise provided in the certificate of incorporation, each stockholder is entitled to one vote for each share of capital stock held by such stockholder. |
22
| |
Austria | Delaware | ||
|---|---|---|---|---|
Shareholder Vote on Certain Transactions |
Majority approval by holders of at least 90% of the share capital is required for certain types of reorganizations, in particular: • dissolution without liquidation and transfer of assets and liabilities by way of universal legal succession to the 90%—shareholder (verschmelzende Umwandlung);
• demerger whereby the distribution of new shares to shareholders of the demerging company is disproportional (nicht-verhältniswahrende Spaltung); and
• squeeze-out of minority shareholders by a shareholders' resolution (Gesellschafterausschluss). |
Generally, under Delaware law, unless the certificate of incorporation provides for the vote of a larger portion of the stock, completion of a merger, consolidation, sale, lease or exchange of all or substantially all of a corporation's assets or dissolution requires: • the approval of the board of directors; and
• approval by the vote of the holders of a majority of the outstanding stock or, if the certificate of incorporation provides for more or less than one vote per share, a majority of the votes of the outstanding stock of a corporation entitled to vote on the matter. |
||
|
Unless otherwise provided for in the company's articles of association, an affirmative vote of holders of at least three-quarters of the share capital is required for: |
|||
|
an amendment to the company's articles of association; |
|||
|
the issuance of convertible bonds and similar hybrid instruments; |
|||
|
an ordinary increase in the stated share capital; and |
|||
|
the removal of members of the supervisory board. |
|||
|
Unless higher voting requirements are provided for in the company's articles of association, an affirmative vote of holders of at least three-quarters of the votes cast is required for: |
|||
|
amendment of the object of the business of the company; |
|||
|
post-formation acquisition (Nachgründung); |
|||
|
transfer of substantially all assets of the company or similar situations; |
|||
|
certain capital reorganizations, such as conditional capital increases, authorization of additional capital, and reduction in the capital stock; |
|||
|
dissolution of the company and continuation of the dissolved company; |
|||
|
transformation into a limited liability company; |
|||
|
certain types of reorganizations such as mergers and demergers; and |
23
| |
Austria | Delaware | ||
|---|---|---|---|---|
|
• exclusion of shareholders' subscription rights in case of the issuance of new shares. |
|||
Standard of Conduct for Directors |
Under Austrian law, both management and supervisory board members must conduct their affairs with "the care and diligence of a prudent manager" and act in the best interests of the company, which includes the interests of the employees and shareholders. The scope of the fiduciary duties of management and supervisory board members is generally determined by the Austrian courts.
• to act in accordance with the law and the company's articles of association and to exercise their powers only for the purposes for which they are conferred;
• to report to the supervisory board on a regular basis as well as on certain important occasions;
• to exercise reasonable care, skill and diligence;
• to maintain a proper accounting system;
• to not compete, directly or indirectly, with the company without permission by the supervisory board; and
• to not effect transactions for the purpose of deriving an improper personal benefit. Members of the supervisory board owe substantially the same statutory and fiduciary duties to the company as members of the management board, including:
• to effectively supervise the company's affairs and the management board;
• to evaluate and issue a resolution on certain transactions which can only be conducted by the management board after approval of the supervisory board;
• to approve the company's financial statements; and
• to represent the company in transactions between the company and members of the management board. |
Delaware law does not contain specific provisions setting forth the standard of conduct of a director. The scope of the fiduciary duties of directors is generally determined by the courts of the State of Delaware. In general, directors have a duty to act without self-interest, on a well-informed basis and in a manner they reasonably believe to be in the best interest of the stockholders. In addition, under Delaware law, when the board of directors of a Delaware corporation approves the sale or break-up of a corporation, the board of directors may, in certain circumstances, have a duty to obtain the highest value reasonably available to the shareholders. |
24
| |
Austria | Delaware | ||
|---|---|---|---|---|
Stockholder Suits |
Under Austrian law, generally, the company, rather than its shareholders, is the proper claimant in an action with respect to a wrong committed against the company or in cases where there is an irregularity in the company's internal management or supervision. Therefore, such claims may only be raised by the company represented by its management board, or, in the case of a wrong committed by a member of the management board, by the supervisory board. The management board, or, if a claim is against a member of the management board, the supervisory board, is obliged to pursue the company's claims against the designated individuals if so resolved by a simple majority of votes cast during a shareholders meeting. A vote by holders of 10% of the share capital of the company may request the court to appoint a representative to pursue the claim on behalf of the company. If the company is unable to fulfil its third party obligations, the company's creditors may pursue the company's damage claims against members of the management board for certain wrongdoings. For certain wrongdoings of members of the management board and subject to the company not being able to fulfil its obligations vis-a-vis third parties, the company's creditors may pursue the company's damage claims against members of the management board themselves. In general, shareholders cannot bring forward damage claims against the company or its management or supervisory board since under Austrian law, they only suffer an indirect damage, as the decrease of their share value is merely a reflection of the decrease of the value of the company (which is the primarily damaged party) as a result of wrongdoings to the company. Only if the damage of a shareholder is the result of a breach of certain laws, which specifically aim at the protection of shareholders (Schutzgesetz), such claims may be pursued by the shareholder himself (e.g. wilful misrepresentation of financial numbers in reports or to the auditor by a member of the management board). |
Under Delaware law, a stockholder may initiate a derivative action to enforce a right of a corporation if the corporation fails to enforce the right itself. The complaint must: • state that the plaintiff was a stockholder at the time of the transaction of which the plaintiff complains or that the plaintiffs shares thereafter devolved on the plaintiff by operation of law; and
• allege with particularity the efforts made by the plaintiff to obtain the action the plaintiff desires from the directors and the reasons for the plaintiff's failure to obtain the action; or
• state the reasons for not making the effort. Additionally, the plaintiff must remain a stockholder through the duration of the derivative suit. The action will not be dismissed or compromised without the approval of the Delaware Court of Chancery. |
25
DESCRIPTION OF AMERICAN DEPOSITARY SHARES
General
The Bank of New York Mellon, as depositary, will register and deliver American Depositary Shares, also referred to as ADSs. Each ADS will represent one tenth (1/10) of a common share (or a right to receive one tenth (1/10) of a common share) deposited with UniCredit Bank Austria AG, as custodian for the depositary in Vienna, Austria. Each ADS will also represent any other securities, cash or other property that may be held by the depositary. The depositary's office at which the ADSs will be administered is located at 101 Barclay Street, New York, New York 10286. The Bank of New York Mellon's principal executive office is located at 225 Liberty Street, New York, New York 10286.
You may hold ADSs either (A) directly (i) by having an American Depositary Receipt, also referred to as an ADR, which is a certificate evidencing a specific number of ADSs, registered in your name, or (ii) by having uncertificated ADSs registered in your name, or (B) indirectly by holding a security entitlement in ADSs through your broker or other financial institution that is a direct or indirect participant in The Depository Trust Company, also called DTC. If you hold ADSs directly, you are a registered ADS holder, also referred to as an ADS holder. This description assumes you are an ADS holder. If you hold the ADSs indirectly, you must rely on the procedures of your broker or other financial institution to assert the rights of ADS holders described in this section. You should consult with your broker or financial institution to find out what those procedures are.
Registered holders of uncertificated ADSs will receive statements from the depositary confirming their holdings.
As an ADS holder, you will not be treated as one of our shareholders and you will not have shareholder rights. Austrian law governs shareholder rights. The depositary will be the holder of the shares underlying your ADSs. As a registered holder of ADSs, you will have ADS holder rights. A deposit agreement among us, the depositary, ADS holders and all other persons indirectly or beneficially holding ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs.
The following is a summary of the material provisions of the deposit agreement. For more complete information, you should read the entire deposit agreement and the form of ADR. Directions on how to obtain copies of those documents are provided in "Where You Can Find More Information" in this prospectus.
Dividends and Other Distributions
How will you receive dividends and other distributions on the shares?
The depositary has agreed to pay or distribute to ADS holders the cash dividends or other distributions it or the custodian receives on shares or other deposited securities, upon payment or deduction of its fees and expenses. You will receive these distributions in proportion to the number of shares your ADSs represent.
Cash. The depositary will convert any cash dividend or other cash distribution we pay on the shares into U.S. dollars, if it can do so on a reasonable basis and can transfer the U.S. dollars to the United States. If that is not possible or if any government approval is needed and cannot be obtained, the deposit agreement allows the depositary to distribute the foreign currency only to those ADS holders to whom it is possible to do so. It will hold the foreign currency it cannot convert for the account of the ADS holders who have not been paid. It will not invest the foreign currency and it will not be liable for any interest.
Before making a distribution, any withholding taxes, or other governmental charges that must be paid will be deducted. See "Taxation" in the applicable accompanying prospectus supplement. It will
26
distribute only whole U.S. dollars and cents and will round fractional cents to the nearest whole cent. If the exchange rates fluctuate during a time when the depositary cannot convert the foreign currency, you may lose some of the value of the distribution.
Shares. The depositary may distribute additional ADSs representing any shares we distribute as a dividend or free distribution. The depositary will only distribute whole ADSs. It will sell shares which would require it to deliver a fraction of an ADS (or ADSs representing those shares) and distribute the net proceeds in the same way as it does with cash. If the depositary does not distribute additional ADSs, the outstanding ADSs will also represent the new shares. The depositary may sell a portion of the distributed shares (or ADSs representing those shares) sufficient to pay its fees and expenses in connection with that distribution.
Rights to purchase additional shares. If we offer holders of our securities any rights to subscribe for additional shares or any other rights, the depositary may (i) exercise those rights on behalf of ADS holders, (ii) distribute those rights to ADS holders or (iii) sell those rights and distribute the net proceeds to ADS holders, in each case after deduction or upon payment of its fees and expenses. To the extent the depositary does not do any of those things, it will allow the rights to lapse. In that case, you will receive no value for them. The depositary will exercise or distribute rights only if we ask it to and provide satisfactory assurances to the depositary that it is legal to do so. If the depositary will exercise rights, it will purchase the securities to which the rights relate and distribute those securities or, in the case of shares, new ADSs representing the new shares, to subscribing ADS holders, but only if ADS holders have paid the exercise price to the depositary. U.S. securities laws may restrict the ability of the depositary to distribute rights or ADSs or other securities issued on exercise of rights to all or certain ADS holders, and the securities distributed may be subject to restrictions on transfer.
Other Distributions. The depositary will send to ADS holders anything else we distribute on deposited securities by any means it thinks is legal, fair and practical. If it cannot make the distribution in that way, the depositary has a choice. It may decide to sell what we distributed and distribute the net proceeds, in the same way as it does with cash. Or, it may decide to hold what we distributed, in which case ADSs will also represent the newly distributed property. However, the depositary is not required to distribute any securities (other than ADSs) to ADS holders unless it receives satisfactory evidence from us that it is legal to make that distribution. The depositary may sell a portion of the distributed securities or property sufficient to pay its fees and expenses in connection with that distribution. U.S. securities laws may restrict the ability of the depositary to distribute securities to all or certain ADS holders, and the securities distributed may be subject to restrictions on transfer.
The depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any ADS holders. We have no obligation to register ADSs, shares, rights or other securities under the Securities Act. We also have no obligation to take any other action to permit the distribution of ADSs, shares, rights or anything else to ADS holders. This means that you may not receive the distributions we make on our shares or any value for them if it is illegal or impractical for us to make them available to you.
Deposit, Withdrawal and Cancellation
How are ADSs issued?
The depositary will deliver ADSs if you or your broker deposits shares or evidence of rights to receive shares with the custodian. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will register the appropriate number of ADSs in the names you request and will deliver the ADSs to or upon the order of the person or persons that made the deposit.
27
How can ADS holders withdraw the deposited securities?
You may surrender your ADSs for the purpose of withdrawal at the depositary's office. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will deliver the shares and any other deposited securities underlying the ADSs to the ADS holder or a person the ADS holder designates at the office of the custodian. Or, at your request, risk and expense, the depositary will deliver the deposited securities at its office, if feasible. The depositary may charge you a fee and its expenses for instructing the custodian regarding delivery of deposited securities.
How do ADS holders interchange between certificated ADSs and uncertificated ADSs?
You may surrender your ADR to the depositary for the purpose of exchanging your ADR for uncertificated ADSs. The depositary will cancel that ADR and will send to the ADS holder a statement confirming that the ADS holder is the registered holder of uncertificated ADSs. Alternatively, upon receipt by the depositary of a proper instruction from a registered holder of uncertificated ADSs requesting the exchange of uncertificated ADSs for certificated ADSs, the depositary will execute and deliver to the ADS holder an ADR evidencing those ADSs.
Voting Rights
How do you vote?
ADS holders may instruct the depositary how to vote the number of deposited shares their ADSs represent. If we request the depositary to solicit your voting instructions (and we are not required to do so), the depositary will notify you of a shareholders' meeting and send or make voting materials available to you. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary how to vote. For instructions to be valid, they much reach the depositary by a date set by the depositary. The depositary will try, as far as practical, subject to the laws of Austria and the provisions of our articles of association or similar documents, to vote or to have its agents vote the shares or other deposited securities as instructed by ADS holders. If we do not tell the depositary to solicit your voting instructions, you can still send voting instructions, and, in that case, the depositary may try to vote as you instruct, but it is not required to do so.
Except by instructing the depositary as described above, you will not be able to exercise voting rights unless you surrender your ADSs and withdraw the shares. However, you may not know about the meeting far enough in advance to withdraw the shares. In any event, the depositary will not exercise any discretion in voting deposited securities and it will only vote or attempt to vote as instructed.
We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your shares. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that you may not be able to exercise voting rights and there may be nothing you can do if your shares are not voted as you requested.
28
Fees and Expenses
Persons depositing or withdrawing shares or ADS holders must pay: |
For: | |
|---|---|---|
$5.00 (or less) per 100 ADSs (or portion of 100 ADSs) |
Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property | |
|
Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
|
$.05 (or less) per ADS |
Any cash distribution to ADS holders |
|
A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs |
Distribution of securities distributed to holders of deposited securities (including rights) that are distributed by the depositary to ADS holders |
|
$.05 (or less) per ADS per calendar year |
Depositary services |
|
Registration or transfer fees |
Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares |
|
Expenses of the depositary |
Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement) |
|
|
Converting foreign currency to U.S. dollars |
|
Taxes and other governmental charges the depositary or the custodian has to pay on any ADSs or shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes |
As necessary |
|
Any charges incurred by the depositary or its agents for servicing the deposited securities |
As necessary |
The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may collect any of its fees by deduction from any cash distribution payable (or by selling a portion of securities or other property distributable) to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid.
From time to time, the depositary may make payments to us to reimburse us for costs and expenses generally arising out of establishment and maintenance of the ADS program, waive fees and expenses for services provided to us by the depositary or share revenue from the fees collected from ADS holders. In performing its duties under the deposit agreement, the depositary may use brokers,
29
dealers or other service providers that are affiliates of the depositary and that may earn or share fees or commissions.
Payment of Taxes
You will be responsible for any taxes or other governmental charges payable on your ADSs or on the deposited securities represented by any of your ADSs. The depositary may refuse to register any transfer of your ADSs or allow you to withdraw the deposited securities represented by your ADSs until those taxes or other charges are paid. It may apply payments owed to you or sell deposited securities represented by your ADSs to pay any taxes owed and you will remain liable for any deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number of ADSs to reflect the sale and pay to ADS holders any proceeds, or send to ADS holders any property, remaining after it has paid the taxes.
Tender and Exchange Offers; Redemption, Replacement or Cancellation of Deposited Securities
The depositary will not tender deposited securities in any voluntary tender or exchange offer unless instructed to do so by an ADS holder surrendering ADSs and subject to any conditions or procedures the depositary may establish.
If deposited securities are redeemed for cash in a transaction that is mandatory for the depositary as a holder of deposited securities, the depositary will call for surrender of a corresponding number of ADSs and distribute the net redemption money to the holders of called ADSs upon surrender of those ADSs.
If there is any change in the deposited securities such as a sub-division, combination or other reclassification, or any merger, consolidation, recapitalization or reorganization affecting the issuer of deposited securities in which the depositary receives new securities in exchange for or in lieu of the old deposited securities, the depositary will hold those replacement securities as deposited securities under the deposit agreement. However, if the depositary decides it would not be lawful to hold the replacement securities because those securities could not be distributed to ADS holders or for any other reason, the depositary may instead sell the replacement securities and distribute the net proceeds upon surrender of the ADSs.
If there is a replacement of the deposited securities and the depositary will continue to hold the replacement securities, the depositary may distribute new ADSs representing the new deposited securities or ask you to surrender your outstanding ADRs in exchange for new ADRs identifying the new deposited securities.
If there are no deposited securities underlying ADSs, including if the deposited securities are cancelled, or if the deposited securities underlying ADSs have become apparently worthless, the depositary may call for surrender or of those ADSs or cancel those ADSs upon notice to the ADS holders.
Amendment and Termination
How may the deposit agreement be amended?
We may agree with the depositary to amend the deposit agreement and the ADRs without your consent for any reason. If an amendment adds or increases fees or charges, except for taxes and other governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery charges or similar items, or prejudices a substantial right of ADS holders, it will not become effective for outstanding ADSs until 30 days after the depositary notifies ADS holders of the amendment. At the time an amendment becomes effective, you are considered, by continuing to hold your ADSs, to agree to the amendment and to be bound by the ADRs and the deposit agreement as amended.
30
How may the deposit agreement be terminated?
The depositary will terminate the deposit agreement if we instruct it to do so. The depositary may terminate the deposit agreement if:
- •
- 60 days have passed since the depositary told us it wants to resign but a successor depositary has not been appointed and accepted its
appointment;
- •
- we delist our shares from an exchange on which they were listed and do not list the shares on another exchange;
- •
- we appear to be insolvent or enter insolvency proceedings;
- •
- all or substantially all the value of the deposited securities has been distributed either in cash or in the form of securities;
- •
- there are no deposited securities underlying the ADSs or the underlying deposited securities have become apparently worthless; or
- •
- there has been a replacement of deposited securities.
If the deposit agreement will terminate, the depositary will notify ADS holders at least 90 days before the termination date. At any time after the termination date, the depositary may sell the deposited securities. After that, the depositary will hold the money it received on the sale, as well as any other cash it is holding under the deposit agreement, unsegregated and without liability for interest, for the pro rata benefit of the ADS holders that have not surrendered their ADSs. Normally, the depositary will sell as soon as practicable after the termination date.
After the termination date and before the depositary sells, ADS holders can still surrender their ADSs and receive delivery of deposited securities, except that the depositary may refuse to accept a surrender for the purpose of withdrawing deposited securities if it would interfere with the selling process. The depositary may refuse to accept a surrender for the purpose of withdrawing sale proceeds until all the deposited securities have been sold. The depositary will continue to collect distributions on deposited securities, but, after the termination date, the depositary is not required to register any transfer of ADSs or distribute any dividends or other distributions on deposited securities to the ADSs holder (until they surrender their ADSs) or give any notices or perform any other duties under the deposit agreement except as described in this paragraph.
Limitations on Obligations and Liability
Limits on our Obligations and the Obligations of the Depositary; Limits on Liability to Holders of ADSs
The deposit agreement expressly limits our obligations and the obligations of the depositary. It also limits our liability and the liability of the depositary. We and the depositary:
- •
- are only obligated to take the actions specifically set forth in the deposit agreement without negligence or bad faith;
- •
- are not liable if we are or it is prevented or delayed by law or circumstances beyond our or its control from performing our or its obligations
under the deposit agreement;
- •
- are not liable if we or it exercises discretion permitted under the deposit agreement;
- •
- are not liable for the inability of any holder of ADSs to benefit from any distribution on deposited securities that is not made available to holders of ADSs under the terms of the deposit agreement, or for any special, consequential or punitive damages for any breach of the terms of the deposit agreement;
31
- •
- have no obligation to become involved in a lawsuit or other proceeding related to the ADSs or the deposit agreement on your behalf or on behalf
of any other person;
- •
- are not liable for the acts or omissions of any securities depository, clearing agency or settlement system; and
- •
- may rely upon any documents we believe or it believes in good faith to be genuine and to have been signed or presented by the proper person.
In the deposit agreement, we and the depositary agree to indemnify each other under certain circumstances.
Requirements for Depositary Actions
Before the depositary will deliver or register a transfer of ADSs, make a distribution on ADSs, or permit withdrawal of shares, the depositary may require:
- •
- payment of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for the
transfer of any shares or other deposited securities;
- •
- satisfactory proof of the identity and genuineness of any signature or other information it deems necessary; and
- •
- compliance with regulations it may establish, from time to time, consistent with the deposit agreement, including presentation of transfer documents.
The depositary may refuse to deliver ADSs or register transfers of ADSs when the transfer books of the depositary or our transfer books are closed or at any time if the depositary or we think it advisable to do so.
Your Right to Receive the Shares Underlying your ADSs
ADS holders have the right to cancel their ADSs and withdraw the underlying shares at any time except:
- •
- when temporary delays arise because: (i) the depositary has closed its transfer books or we have closed our transfer books;
(ii) the transfer of shares is blocked to permit voting at a shareholders' meeting; or (iii) we are paying a dividend on our shares;
- •
- when you owe money to pay fees, taxes and similar charges; or
- •
- when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of shares or other deposited securities.
This right of withdrawal may not be limited by any other provision of the deposit agreement.
Pre-release of ADSs
The deposit agreement permits the depositary to deliver ADSs before deposit of the underlying shares. This is called a pre-release of the ADSs. The depositary may also deliver shares upon cancellation of pre-released ADSs (even if the ADSs are canceled before the pre-release transaction has been closed out). A pre-release is closed out as soon as the underlying shares are delivered to the depositary. The depositary may receive ADSs instead of shares to close out a pre-release. The depositary may pre-release ADSs only under the following conditions: (1) before or at the time of the pre-release, the person to whom the pre-release is being made represents to the depositary in writing that it or its customer owns the shares or ADSs to be deposited; (2) the pre-release is fully collateralized with cash or other collateral that the depositary considers appropriate; and (3) the
32
depositary must be able to close out the pre-release on not more than five business days' notice. The depositary will normally limit the number of ADSs that may be outstanding at any time as a result of pre-release to no more than 30% of the total ADSs outstanding. However, the depositary may disregard that limit from time to time as it deems appropriate. For example, a large cancellation of ADSs at a time when pre-releases are outstanding could cause the percentage of pre-released ADSs to temporarily exceed 30%. Although it is possible that this 30% limit may be exceeded from time to time, it is neither typical nor the intention of the ADS program for such limit to be significantly exceeded for an extended period of time.
Direct Registration System
In the deposit agreement, all parties to the deposit agreement acknowledge that the Direct Registration System, also referred to as DRS, and Profile Modification System, also referred to as Profile, will apply to the ADSs. DRS is a system administered by DTC that facilitates interchange between registered holding of uncertificated ADSs and holding of security entitlements in ADSs through DTC and a DTC participant. Profile is a feature of DRSs that allows a DTC participant, claiming to act on behalf of a registered holder of uncertificated ADSs, to direct the depositary to register a transfer of those ADSs to DTC or its nominee and to deliver those ADSs to the DTC account of that DTC participant without receipt by the depositary of prior authorization from the ADS holder to register that transfer.
In connection with and in accordance with the arrangements and procedures relating to DRS/Profile, the parties to the deposit agreement understand that the depositary will not determine whether the DTC participant that is claiming to be acting on behalf of an ADS holder in requesting registration of transfer and delivery as described in the paragraph above has the actual authority to act on behalf of the ADS holder (notwithstanding any requirements under the Uniform Commercial Code). In the deposit agreement, the parties agree that the depositary's reliance on and compliance with instructions received by the depositary through the DRS/Profile system and in accordance with the deposit agreement will not constitute negligence or bad faith on the part of the depositary.
Shareholder Communications; Inspection of Register of Holders of ADSs
The depositary will make available for your inspection at its office all communications that it receives from us as a holder of deposited securities that we make generally available to holders of deposited securities. The depositary will send you copies of those communications or otherwise make those communications available to you if we ask it to. You have a right to inspect the register of holders of ADSs, but not for the purpose of contacting those holders about a matter unrelated to our business or the ADSs.
33
DESCRIPTION OF RIGHTS TO SUBSCRIBE FOR COMMON SHARES OR ADSs
We may issue rights to subscribe for our common shares or our ADSs. These rights may or may not be transferable. In connection with any offering of rights, we may enter into a standby arrangement with one or more underwriters or other purchasers pursuant to which the underwriters or other purchasers may be required to purchase any securities remaining unsubscribed after such offering.
The terms of the rights to subscribe for shares of our common shares or ADSs will be set forth in a prospectus supplement which, will describe, among other things:
- •
- the exercise price;
- •
- the aggregate number of rights to be issued;
- •
- the number of common shares or ADSs purchasable upon exercise of each right;
- •
- the procedures and limitations relating to the exercise of the rights;
- •
- the date upon which the exercise of rights will commence;
- •
- the record date, if any, to determine who is entitled to the rights;
- •
- the expiration date;
- •
- the extent to which the rights are transferable;
- •
- information regarding the trading of rights, including the stock exchanges, if any, on which the rights will be listed;
- •
- the extent to which the rights may include an over-subscription privilege with respect to unsubscribed common shares or ADSs;
- •
- if applicable, the material terms of any standby underwriting or purchase arrangement entered into by us in connection with the offering of the
rights; and
- •
- any other material terms of the rights.
34
Taxation in Austria
A general summary of certain Austrian tax considerations relating to the purchase, ownership and disposition of any of the securities offered by this prospectus will be set forth in a prospectus supplement relating to the offering of those securities.
Taxation in the United States
A general summary of the material U.S. federal income tax consequences relating to the purchase, ownership and disposition of any of the securities offered by this prospectus will be set forth in a prospectus supplement relating to the offering of those securities.
35
We may sell securities:
- •
- to or through underwriters, brokers or dealers;
- •
- through agents;
- •
- directly to one or more other purchasers in negotiated sales or competitively bid transactions;
- •
- through a block trade in which the broker or dealer engaged to handle the block trade will attempt to sell the securities as agent, but may
position and resell a portion of the block as principal to facilitate the transaction; or
- •
- through a combination of any of the above methods of sale.
In addition, we may issue the securities as a dividend or distribution or in a subscription rights offering to our existing security holders, which may or may not be transferable. In any distribution of subscription rights to our shareholders, if all of the underlying securities are not subscribed for, we may then sell the unsubscribed securities directly to third parties or may engage the services of one or more underwriters, dealers or agents, including standby underwriters, to sell the unsubscribed securities to third parties.
We may directly solicit offers to purchase securities, or agents may be designated to solicit such offers. We will, in the prospectus supplement relating to such offering, name any agent that could be viewed as an underwriter under the Securities Act, and describe any commissions that we must pay. Any such agent will be acting on a best efforts basis for the period of its appointment or, if indicated in the applicable prospectus supplement, on a firm commitment basis. This prospectus may be used in connection with any offering of our securities through any of these methods or other methods described in the applicable prospectus supplement.
The distribution of the securities may be effected from time to time in one or more transactions:
- •
- at a fixed price, or prices, which may be changed from time to time;
- •
- at market prices prevailing at the time of sale;
- •
- at prices related to such prevailing market prices; or
- •
- at negotiated prices.
Each prospectus supplement will describe the method of distribution of the securities and any applicable restrictions.
The prospectus supplement with respect to the securities of a particular series will describe the terms of the offering of the securities, including the following:
- •
- the name of the agent or any underwriters;
- •
- the public offering or purchase price;
- •
- the proceeds we will receive from the sales of securities;
- •
- any discounts and commissions to be allowed or paid to the agent or underwriters;
- •
- all other items constituting underwriting compensation;
- •
- any discounts and commissions to be allowed or re-allowed or paid to dealers; and
- •
- any exchanges on which the securities will be listed.
36
If any underwriters or agents are utilized in the sale of the securities in respect of which this prospectus is delivered, we will enter into an underwriting agreement or other agreement with them at the time of sale to them, and we will set forth in the prospectus supplement relating to such offering the names of the underwriters or agents and the terms of the related agreement with them.
If a dealer is utilized in the sale of the securities in respect of which the prospectus is delivered, we will sell such securities to the dealer, as principal. The dealer may then resell such securities to the public at varying prices to be determined by such dealer at the time of resale.
If we offer securities in a subscription rights offering to our existing security holders, we may enter into a standby underwriting agreement with dealers, acting as standby underwriters. We may pay the standby underwriters a commitment fee for the securities they commit to purchase on a standby basis. If we do not enter into a standby underwriting arrangement, we may retain a dealer-manager to manage a subscription rights offering for us.
Remarketing firms, agents, underwriters, dealers and other persons may be entitled under agreements which they may enter into with us to indemnification by us against certain civil liabilities, including liabilities under the Securities Act, and may be customers of, engage in transactions with or perform services for us in the ordinary course of business.
If so indicated in the applicable prospectus supplement, we will authorize underwriters or other persons acting as our agents to solicit offers by certain institutions to purchase securities from us pursuant to delayed delivery contracts providing for payment and delivery on the date stated in the prospectus supplement. Each contract will be for an amount not less than, and the aggregate amount of securities sold pursuant to such contracts shall not be less nor more than, the respective amounts stated in the prospectus supplement. Institutions with whom the contracts, when authorized, may be made include commercial and savings banks, insurance companies, pension funds, investment companies, educational and charitable institutions and other institutions, but shall in all cases be subject to our approval. Delayed delivery contracts will not be subject to any conditions except that:
- •
- the purchase by an institution of the securities covered under that contract shall not at the time of delivery be prohibited under the laws of
the jurisdiction to which that institution is subject; and
- •
- if the securities are also being sold to underwriters acting as principals for their own account, the underwriters shall have purchased such securities not sold for delayed delivery. The underwriters and other persons acting as our agents will not have any responsibility in respect of the validity or performance of delayed delivery contracts.
Certain agents, underwriters and dealers, and their associates and affiliates may be customers of, have borrowing relationships with, engage in other transactions with, or perform services, including investment banking services, for us or one or more of our respective affiliates in the ordinary course of business.
In order to facilitate the offering of the securities, any underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of the securities or any other securities the prices of which may be used to determine payments on such securities. Specifically, any underwriters may overallot in connection with the offering, creating a short position for their own accounts. In addition, to cover overallotments or to stabilize the price of the securities or of any such other securities, the underwriters may bid for, and purchase, the securities or any such other securities in the open market. Finally, in any offering of the securities through a syndicate of underwriters, the underwriting syndicate may reclaim selling concessions allowed to an underwriter or a dealer for distributing the securities in the offering if the syndicate repurchases previously distributed securities in transactions to cover syndicate short positions, in stabilization transactions or otherwise. Any of these activities may stabilize
37
or maintain the market price of the securities above independent market levels. Any such underwriters are not required to engage in these activities and may end any of these activities at any time.
Under Rule 15c6-1 of the Exchange Act, trades in the secondary market generally are required to settle in three business days, unless the parties to any such trade expressly agree otherwise. The applicable prospectus supplement may provide that the original issue date for your securities may be more than three scheduled business days after the trade date for your securities. Accordingly, in such a case, if you wish to trade securities on any date prior to the third business day before the original issue date for your securities, you will be required, by virtue of the fact that your securities initially are expected to settle in more than three scheduled business days after the trade date for your securities, to make alternative settlement arrangements to prevent a failed settlement.
To comply with the securities laws of some states, if applicable, the securities may be sold in such jurisdictions only through registered or licensed brokers or dealers. In addition, in some states the securities may not be sold unless they have been registered or qualified for sale or an exemption from registration or qualification requirements is available and is complied with.
The securities may be new issues of securities and may have no established trading market. The securities may or may not be listed on a national securities exchange. We can make no assurance as to the liquidity of or the existence of trading markets for any of the securities.
In compliance with the guidelines of the Financial Industry Regulatory Authority, or FINRA, the aggregate maximum discount, commission or agency fees or other items constituting underwriting compensation to be received by any FINRA member or independent broker-dealer will not exceed 8% of the proceeds from any offering pursuant to this prospectus and any applicable prospectus supplement.
38
Unless the applicable prospectus supplement indicates otherwise, certain legal matters of U.S. federal law and New York State law will be passed upon for us by Wilmer Cutler Pickering Hale and Dorr LLP. Unless the applicable prospectus supplement indicates otherwise, the validity of the securities in respect of which this prospectus is being delivered and certain legal matters with respect to Austrian law will be passed upon by Freshfields Bruckhaus Deringer LLP.
The financial statements incorporated in this prospectus by reference to the Annual Report on Form 20-F for the year ended December 31, 2015 have been so incorporated in reliance on the report of PwC Wirtschaftsprüfung GmbH, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
The following table sets forth the expenses (other than underwriting discounts and commissions) expected to be incurred by us in connection with a possible offering of $75,000,000 of the securities registered under this registration statement. All amounts other than the SEC registration fee are estimates.
SEC registration fee |
$ | 8,693 | ||
FINRA filing fee |
* | |||
Printing and engraving |
* | |||
Accounting services |
* | |||
NASDAQ fees |
* | |||
Legal fees of registrant's counsel |
* | |||
Transfer agent's, trustee's and depository's fees and expenses |
* | |||
Miscellaneous |
* | |||
| | | | | |
Total |
$ | * | ||
| | | | | |
| | | | | |
| | | | | |
- *
- To be provided by a prospectus supplement or as an exhibit to a Report on Form 6-K that is incorporated by reference into this prospectus.
39
SERVICE OF PROCESS AND ENFORCEMENT OF JUDGMENTS
We are incorporated under the laws of Austria, and our registered offices and a significant portion of our assets are located outside of the United States. In addition, a member of our supervisory board is a residents of a jurisdiction other than the United States. As a result, it may be difficult for you to effect service of process upon such supervisory board member or upon us or to enforce judgments obtained in U.S. courts based on the civil liability provisions of the U.S. securities laws against us in the United States.
We have appointed C T Corporation System as our authorized agent upon whom process may be served in any action instituted in any U.S. federal or state court having subject matter jurisdiction arising out of or based upon the securities offered by this prospectus.
Litigation in Austria is also subject to rules of procedure that differ from the U.S. rules, including with respect to the taking and admissibility of evidence, the conduct of the proceedings and the allocation of costs. According to the Austrian Enforcement Act (Exekutionsordnung—EO), foreign judgments are only enforceable if reciprocity is warranted by a bilateral or multilateral treaty between the countries involved or by an ordinance (Verordnung) of the Austrian government (in which ordinance the Austrian government confirms reciprocity). As the United States and Austria do not currently have a treaty providing for reciprocal recognition and enforcement of judgments in civil and commercial matters (except for arbitration awards in such matters), a final judgment for payment of money rendered by a federal or state court in the United States based on civil liability, whether or not predicated solely upon U.S. federal securities laws, will not be enforceable, either in whole or in part, in Austria. However, if the party in whose favor such final judgment is rendered brings a new suit in a competent court in Austria, such party may submit to the Austrian court the final judgment rendered in the United States. Under such circumstances, a judgment by a federal or state court of the United States against us or our managing directors will be regarded by an Austrian court only as evidence of the outcome of the dispute to which such judgment relates, and an Austrian court may choose to re-hear the dispute. In addition, awards of punitive damages in actions brought in the United States or elsewhere are generally not enforceable in Austria. Furthermore, actions brought in an Austrian court against us or any non-U.S. members of our management board, supervisory board or senior management to enforce liabilities based on U.S. federal securities laws may be subject to certain restrictions. For example, proceedings in Austria would have to be conducted in the German language, and all documents submitted to the court would, in principle, have to be translated into the German language. For these reasons, it may be difficult for a U.S. investor to bring an original action in an Austrian court predicated upon the civil liability provisions of the U.S. federal securities laws against us, the members of our management board and supervisory board and senior management. In addition, even if a judgment against our company or any non-U.S. members of our management board, supervisory board or senior management based on the civil liability provisions of the U.S. federal securities laws is obtained in the United States, a U.S. investor may not be able to enforce it in Austrian courts.
40

NABRIVA THERAPEUTICS AG
Rights Offering for Common Shares
Including Common Shares Represented by American Depositary Shares
Offering of American Depositary Shares Representing Common Shares
Not Subscribed for in the Rights Offering
PROSPECTUS SUPPLEMENT
Cantor Fitzgerald & Co.