Use these links to rapidly review the document
TABLE OF CONTENTS
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS COLLPLANT HOLDINGS LTD. AS OF DECEMBER 31, 2014
As confidentially submitted to the U.S. Securities and Exchange Commission on May 26, 2015.
This draft registration statement has not been filed publicly with the Securities and Exchange Commission
and all information contained herein remains confidential.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
CollPlant Holdings Ltd.
(Exact name of registrant as specified in its charter)
| State of Israel (State or other jurisdiction of incorporation or organization) |
3842 (Primary Standard Industrial Classification Code Number) |
Not applicable (I.R.S. Employer Identification No.) |
3 Sapir Street, Weizmann Science Park
Ness-Ziona 74140, Israel
Tel: +972 (0)73 2325600
(Address, including zip code, and telephone number, including area code, of registrant's principal executive offices)
Puglisi & Associates
850 Library Avenue, Suite 204
Newark, Delaware
(302) 738-6680
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Mark S. Selinger, Esq. Joel L. Rubinstein, Esq. McDermott Will & Emery LLP 340 Madison Avenue New York, NY 10173 +1 212 547 5400 |
Yuval Horn, Adv. Roy Ribon, Adv. Horn & Co. Law Offices Amot Investments Tower 2 Weizmann Street, 24th Floor Tel Aviv 6423902, Israel +972 3 637 8200 |
Ivan K. Blumenthal, Esq. Merav Gershtenman, Esq. Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. 666 Third Avenue New York, NY 10017 +1 212 935 3000 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this registration statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
CALCULATION OF REGISTRATION FEE
|
||||
| Title of each class of securities to be registered |
Proposed maximum aggregate offering price(2)(3) |
Amount of registration fee(4) |
||
|---|---|---|---|---|
Ordinary Shares, par value NIS 0.01 per share(1) |
$ | $ | ||
|
||||
- (1)
- The
ordinary shares will be represented by American Depositary Shares ("ADSs"), which have been registered under a separate registration statement on
Form F-6 and are issuable upon deposit of the ordinary shares registered hereby. Each ADS represents ordinary shares.
- (2)
- Estimated
solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended.
- (3)
- Includes
ordinary shares that the underwriters have the option to purchase to cover over-allotments, if any.
- (4)
- Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the Securities and Exchange Commission has declared this registration statement effective. This preliminary prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state or jurisdiction where such offer or sale is not permitted.
PRELIMINARY PROSPECTUS SUBJECT TO COMPLETION DATED MAY 26, 2015
CollPlant Holdings Ltd.

AMERICAN DEPOSITARY SHARES
EACH REPRESENTING ORDINARY SHARES
This is our initial public offering in the United States. We are offering American Depositary Shares, or ADSs. Each ADS represents of our ordinary shares, par value NIS 0.01 per share.
We intend to apply to list our ADSs on the NASDAQ Capital Market, under the symbol " ". Our ordinary shares currently trade on the Tel Aviv Stock Exchange, or TASE, under the symbol "CLPT," and our ADSs are currently quoted on the OTCQX marketplace, or OTCQX, under the symbol "CQPTY." On , 2015, the closing price of our ordinary shares on TASE was NIS , or $ per share (based on the exchange rate reported by the Bank of Israel on such date), and the last reported bid price of our ADSs on OTCQX was $ per ADS. Assuming that our ADSs are listed for trading on the NASDAQ Capital Market, the quoting of our ADSs on OTCQX will be discontinued prior to the completion of this offering.
We are an emerging growth company, as defined in the U.S. Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and, as such, have elected to comply with certain reduced public company reporting requirements.
Investing in our ADSs involves a high degree of risk. See "Risk Factors" beginning on page 11 of this prospectus.
|
||||
| |
Per ADS |
Total |
||
|---|---|---|---|---|
Initial public offering price |
$ | $ | ||
Underwriting discounts and commissions |
$ | $ | ||
Proceeds to us (before expenses) |
$ | $ | ||
|
||||
- (1)
- See "Underwriting" beginning on page 164 for additional information regarding underwriting compensation.
The underwriters have an option to purchase up to additional ADSs from us at the initial public offering price, less the underwriting discounts and commissions payable by us, for 30 days after the date of this prospectus to cover over-allotments, if any.
None of the United States Securities and Exchange Commission, the Israel Securities Authority, or any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the ADSs to the purchasers in this offering on or about , 2015.
Book-Running Manager
Ladenburg Thalmann
Co-Manager
Brean Capital
The date of this prospectus is , 2015.
Until and including , 2015 (25 days after the date of this prospectus), all dealers that buy, sell, or trade the ADSs, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to the dealer's obligation to deliver a prospectus when acting as an underwriter and with respect to unsold allotments or subscriptions.
You should rely only on the information contained in this prospectus and any related free-writing prospectus that we authorize to be distributed to you. We have not, and the underwriters have not, authorized any person to provide you with information different from that contained in this prospectus or any related free-writing prospectus that we authorize to be distributed to you. This prospectus is not
i
an offer to sell, nor is it seeking an offer to buy, these securities in any state where the offer or sale is not permitted. The information in this prospectus speaks only as of the date of this prospectus unless the information specifically indicates that another date applies, regardless of the time of delivery of this prospectus or of any sale of the securities offered hereby.
We own various trademark registrations, trademark applications, unregistered trademarks, and trade names, including, among others: "collage" and "Vergenix." All other trademarks or trade names referred to in this prospectus are the property of their respective owners. Solely for convenience, trademarks, and trade names in this prospectus may be referred to without the symbols ® and TM, but such references should not be construed as any indication that their respective owners will not assert, to the fullest extent under applicable law, their rights to those trademarks or trade names.
Notice to Prospective Investors in Israel
This document does not constitute a public offering or selling or a solicitation of an offer to sell any kind of securities under the Israeli Securities Law, 5728-1968, and has not been filed with or approved by the Israeli Securities Authority. Any public offering in Israel requires a pre-approved permit by the Israeli Securities Authority or an exemption thereof. In Israel, this prospectus is being distributed only to, and is directed only at the types of, investors listed in the first addendum, or the Addendum, to the Israeli Securities Law, consisting primarily of a fund for joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriters purchasing for their own account, venture capital funds, entities with equity in excess of NIS 50.0 million, and "qualified individuals," each as defined in the Addendum (as it may be amended from time to time), collectively referred to as qualified investors. Qualified investors may be required to submit written confirmation that they fall within the scope of the Addendum.
ii
This summary highlights selected information about us and the ADSs that we are offering. This summary does not contain all of the information you should consider before investing in our ADSs. Before making an investment in our ADSs, you should read the entire prospectus, carefully for a more complete understanding of our business and this offering, including our consolidated financial statements, and the sections entitled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" included in this prospectus. Unless the context requires otherwise, the terms "CollPlant," "we," "us," "our," "the Company," and similar designations refer to CollPlant Holdings Ltd. and its subsidiary CollPlant Ltd. Unless derived from our financial statements or otherwise indicated, U.S. dollar translations of NIS amounts presented in this prospectus are translated using the rate of NIS 3.889 to one U.S. dollar, the exchange rate reported by the Bank of Israel for December 31, 2014.
Overview
We are a clinical-stage regenerative medicine company focused on developing and commercializing tissue repair products, initially for the orthobiologics and advanced wound care markets. Our product candidates, two of which are in registration trials, are based on our proprietary plant-based collagen technology, which we believe is the only viable technology available for the production of recombinant type I human collagen, or rhCollagen. We believe that our rhCollagen, which is identical to the type I collagen produced by the human body, has significant advantages compared to currently marketed tissue-derived collagen, including improved biofunctionality, superior homogeneity, and reduced risk of immune response. We believe the attributes of our rhCollagen make it suitable for numerous tissue repair applications throughout the human body. We believe that the market opportunity for our current product candidates utilizing our rhCollagen within the orthobiologics and advanced wound care markets exceeds $5 billion.
Our first rhCollagen-based product candidate is VergenixSTR, a soft tissue repair matrix composed of our rhCollagen and platelet-rich plasma, or PRP, extracted from a patient's blood, and intended for the treatment of tendinopathy. VergenixSTR is currently in a multi-center registration trial in Israel. Our second clinical product candidate, VergenixFG, is a wound-filling flowable gel made from our rhCollagen intended for treatment of deep surgical incisions and deep wounds, including diabetic ulcers, burns, bedsores, and other chronic wounds. VergenixFG is currently in a multi-center registration trial in Israel. To bring our initial two product candidates to market, we intend to first seek CE marking certification, which is required for a product to be sold within the European Union. After receiving CE marking for our initial products, we plan to request a pre-Investigational Device Exemption, or IDE, meeting with the U.S. Food and Drug Administration, or FDA, and pursue FDA approval for our rhCollagen-based products. Our third product candidate is a preclinical product based on VergenixBVF, a product platform we are developing for bone repair indications such as spinal fusion and trauma. VergenixBVF is a novel absorbable scaffold composed of our rhCollagen and minerals, which can be charged with growth factors to help accelerate bone formation. We are collaborating with a U.S.-based corporate collaborator in the development of VergenixBVF.
Our rhCollagen outperforms any tissue-derived collagen, whether from animal or human tissues, in biofunctionality and has certain useful physical characteristics, including thermal stability and a pristine triple helix, according to data published in peer-reviewed scientific publications. Our rhCollagen can be fabricated in different forms and shapes including gels, pastes, sponges, sheets, membranes, fibers, and thin coats, all of which have been tested in vitro and in animal models and proven superior to tissue-derived products. These different forms of our rhCollagen broaden the potential applications of our products. For example, collagen gels made of our rhCollagen are more homogenous and less viscous compared to tissue-derived collagen, making the rhCollagen gels ideal for any injectable product. We have demonstrated that, due to its homogeneity, rhCollagen can produce fibers and membranes with high molecular order, which enables the formation of tissue repair products with distinctive physical
1
properties, including improved tensile strength due to regional fibril alignment, higher levels of transparency, and the ability to achieve high concentrations of collagen at low viscosities. The unique properties of our rhCollagen make it an ideal building block for many products that we believe cannot currently be produced using tissue-derived collagen, such as BioInks for 3-D printing, artificial tendons, and transparent ophthalmic products.
The production of our rhCollagen begins when five human genes essential for the production of collagen are introduced into the tobacco plant. The genetically engineered tobacco plantlets are distributed to qualified greenhouses across Israel, where they are grown to maturity, which takes about eight weeks. The tobacco leaves are then harvested and processed to an extract, which undergoes purification until the final rhCollagen product is produced. Cost-effective production and the abundant supply of raw materials are two of the most important features of plant-based production. We are advancing a new production model that will result in labor cost reductions and higher yields, assuring adequate supply as demand for rhCollagen increases.
Advantages of our rhCollagen and rhCollagen-based Products
Collagen is the main component of connective tissue, comprising approximately 30% of the protein found in the human body. Its biofunctionality, biocompatibility, and biodegradability make it a logical choice for regenerative medicine products. We estimate the size of the market for human collagen-based tissue repair products for use in orthobiologics and advanced wound care applications is approximately $20 billion. Currently, collagen for medical use is primarily derived from bovine (cow) and porcine (pig) sources, as well as from human cadavers. It is extracted from the tissues using mechanical processes and chemical treatments. However, all of our product candidates are based on our proprietary plant-based recombinant type I human collagen, rhCollagen, which is identical to the
2
type I collagen produced by the human body. Our rhCollagen has many advantages over tissue-derived collagen, as outlined below:
| Tissue-Derived Collagen | rhCollagen | |
|---|---|---|
• Random defects in the protein structure, resulting in significant damage to binding sites for progenitor cells, which are required for cell proliferation and differentiation into tissue. |
• A pristine triple helix structure, resulting in optimal binding sites supporting endothelial, fibroblast, and keratinocyte cell attachment and proliferation. |
|
Advantage: In all cell types tested in vitro, cell proliferation was significantly better in scaffolds made of rhCollagen than in commercially available scaffolds made of bovine collagen. The accelerated cell proliferation achieved with rhCollagen results in faster wound healing, less scarring and overall high-quality tissue regeneration. |
||
• High proportions of cross-linked collagen species with high molecular weight, which can impair the collagen's ability to self-assemble homogenous scaffolds and impede its rate of degradation. |
• Allows for the precise control over the degree of cross-linked collagen due to the homogeneity of rhCollagen, enabling consistent and reproducible products with a controlled degradation rate. |
|
Advantage: Precise control over the proportion of cross-linked collagen allows us to optimize the degradation rate of rhCollagen to the indication targeted. Achieving the same level of engineered performance would be difficult, if not impossible, with tissue-derived collagen that varies from batch to batch. |
||
• Tissue-derived collagen, in many cases, contains residual contaminant proteins, growth factors and cytokines and carries a risk of disease transmission. As a result, scaffolds made of tissue-derived collagen may provoke inflammation, as well as undesirable immune and foreign body responses that may cause adverse effects and unpredictable biological outcomes. |
• Our plant-derived collagen is composed of pure molecules that are identical to type I human collagen. It has no residues of growth factors which can lead to potential side effects, does not induce an immunogenic response, and carries no risk of transmitting diseases and pathogens. |
|
Advantage: Preclinical studies have demonstrated that rhCollagen incubated with activated THP1-macrophages produce significantly lower levels of inflammatory cytokines when compared with bovine collagen, demonstrating that animal-derived collagen can provoke a foreign body response not seen with rhCollagen. This foreign body response delays healing and increases scarring. |
||
Our Strategy
We plan to exploit the unique characteristics of our rhCollagen technology platform to develop and commercialize an extensive portfolio of regenerative medicine products. The key elements of our strategy include the following:
- •
- Position our rhCollagen as the "gold standard" platform technology for collagen-based products in a broad range of markets. We believe that our rhCollagen represents a significant advance in collagen technology, demonstrated by its improved biofunctionality, superior homogeneity, and reduced risk of immune response. We also believe that our platform technology, and the knowledge and expertise we have gained in its development, will enable the development, both independently and with collaborators, of differentiated products in emerging industries such as bio-printing which we believe cannot be adequately addressed with currently available collagen technologies.
3
- •
- Establish a regulatory process for rhCollagen-based end products using VergenixSTR and VergenixFG as
precedent. We intend to rapidly seek marketing clearance of our initial product candidates, VergenixSTR and VergenixFG, through CE marking in Europe, and then pursue FDA
approval for marketing our rhCollagen-based products in the United States. We believe that this strategy will allow us to gain earlier market access and thereby more rapid industry acceptance for our
rhCollagen-based end products, since the timeline to achieve CE marking is generally shorter than the FDA approval route. Utilizing this strategy is expected to result in more physicians gaining
exposure to rhCollagen-based products like VergenixSTR and VergenixFG sooner.
- •
- Utilize collaborative partners and distributors to develop and commercialize our technology and
products. We believe the market-leading characteristics of our rhCollagen will create attractive collaboration opportunities for our product candidates. We intend to
selectively establish collaborations and strategic partnerships with well-established companies whose distribution networks are deeply entrenched, as well as with local and regional distributors in
certain markets.
- •
- Expand our manufacturing capacity to support commercialization of rhCollagen-based end
products. We intend to utilize a portion of the proceeds from this offering to construct a manufacturing facility in Israel that will enable us to manufacture commercial
quantities of our rhCollagen and rhCollagen-based end products in a cost-effective manner for application in both premium and commodity markets.
- •
- Expand our pipeline through ongoing development of new
products. We intend to continue to develop additional products, initially in the orthobiologics and advanced wound care markets and
subsequently in other high value markets, based on our rhCollagen, both independently and with strategic collaborators.
- •
- Advance our leadership position in recombinant protein production through our plant-based technology. As tissue engineering and regenerative medicine continue to evolve and expand, we expect that the demand for high-quality biomaterials will grow. In response to this demand, we may expand the use of our proprietary plant-based protein production know-how to other recombinant proteins.
Our Product Candidates
VergenixSTR—Tendinopathy Treatment
VergenixSTR is a soft tissue repair matrix composed of our rhCollagen and platelet-rich plasma, or PRP, extracted from a patient's blood. VergenixSTR is intended for the treatment of tendinopathy, such as in the elbow tendon (for treatment of "tennis elbow"), rotator cuff, patellar tendon, Achilles tendon, and hand tendons. VergenixSTR is injected into the affected area, and forms a viscous gel matrix which serves as a scaffold in the vicinity of a tendon injury site, allowing the platelet concentrate to remain in place at the injured area, enabling optimal healing. In a preclinical study in rats, VergenixSTR was shown to accelerate the healing of tendons in comparison to the control group which was treated with an injection of PRP only.
VergenixSTR is currently in a clinical trial in Israel intended to demonstrate safety and to evaluate the performance of VergenixSTR in patients suffering from tennis elbow, an inflammation of the tendons that join the forearm muscles on the outside of the elbow. Patients enrolled in the trial receive a one-time injection of VergenixSTR and are assessed for the level of pain, tendon healing measured by ultrasound, and recovery of hand movement over a period of six months. We expect to complete the trial in the second half of 2015, after which we will seek CE marking with the expectation of product
4
sales commencing in the first quarter of 2016. After receiving CE marking in the European Union, we intend to pursue regulatory approval for VergenixSTR in the United States.
VergenixFG—Wound Filler
VergenixFG is an advanced wound care product candidate intended for the treatment of deep surgical incisions and deep wounds, including diabetic ulcers, burns, bedsores, and other chronic wounds that are difficult to heal. The VergenixFG formulation provides a scaffold of pure human collagen that fills the wound bed and is engineered to create maximal contact with the surrounding tissue. In a cutaneous full-thickness wound pig model, 95% wound closure was observed with VergenixFG at day 21 compared to 68% closure in wounds treated with the benchmark product. The researchers concluded that VergenixFG proved effective in animal wound models, and it is expected to be capable of reducing the healing time of human wounds.
VergenixFG is currently in a clinical trial in Israel intended to demonstrate safety and evaluate performance of VergenixFG in patients with hard-to-heal chronic wounds of the lower limbs. Patients enrolled in the trial receive a single treatment of VergenixFG and are assessed for percentage of wound closure and degree of pain at a follow-up examination four weeks post-treatment. Interim results of the first ten patients in the trial showed the product was safe and produced excellent wound closure rates, with 80% to 100% healing within four weeks of the beginning of treatment in 7 of 10 patients. We expect to complete the trial in the fourth quarter of 2015, after which we will seek CE marking with the expectation of product sales commencing in the first quarter of 2016. After receiving CE marking in the European Union, we intend to pursue regulatory approval for VergenixFG in the United States.
VergenixBVF—Bone Healing Implant
VergenixBVF is a novel absorbable scaffold composed of our rhCollagen and synthetic minerals that mimics bone structure intended for use in posterolateral spinal fusion and trauma and other orthopedic applications. We intend to develop VergenixBVF as a product platform for the development of bone void filler products which can be used as a one-time treatment that can be charged with a growth factor to stimulate bone and tissue growth in a controlled manner.
In the initial VergenixBVF product candidate, which is in preclinical development with a U.S.-based corporate collaborator, the scaffold is intended to act as a carrier to enable sustained release of a recombinant bone growth protein to induce cell infiltration and proliferation. VergenixBVF combined with a growth factor has been tested in different animal models to verify its performance in bone healing. In these studies, the product candidate showed more rapid bone growth when compared with currently marketed products, an advantage we believe will be translated to human bone repair.
Future Product Candidates
We have several additional projects which are in different stages of development. We currently have in-house research projects related to the use of VergenixSTR for tendon rupture and the use of VergenixFG for surgical wounds, and are actively seeking collaborators in these indications. We are also developing BioInks consisting of our rhCollagen suitable for 3-D printing. Our researchers have chemically modified the gelling behavior of the collagen to adapt the biological molecules for printing. In addition, we are researching the production of other extracellular proteins through our plant-based production system.
Our Market Opportunity
We are initially focused on the orthobiologics and advanced wound care markets, and we believe the market opportunity for our current product candidates utilizing our rhCollagen platform technology exceeds $5 billion. Our VergenixSTR and VergenixBVF products address indications within the
5
orthobiologics market. GlobalData recently estimated that the major segments of the orthobiologics market currently comprise an annual $6.7 billion worldwide market. The overall increase in prevalence of musculoskeletal disorders combined with technological advancements in the orthobiologics field are fueling the growth of this market, resulting in a compound annual growth rate or CAGR of 7.7% in the North American market from 2014 to 2019 as predicted by MicroMarket Monitor.
VergenixSTR addresses indications within the soft tissue repair market, which was valued at $5.6 billion globally in 2013 by Transparency Market Research, and forecasted to grow to $8.5 billion in 2019, a CAGR of 7.2%. We estimate the size of the target market for VergenixSTR for treating tendonitis is three million procedures per year, or approximately $2.0 billion.
VergenixBVF addresses indications within the bone repair market. According to GlobalData, a total of 1.8 million bone-grafting procedures were performed in 2013 worldwide. We estimate the size of the target market for the initial VergenixBVF product candidate at one million procedures per year, representing a market size of approximately $3.5 billion.
VergenixFG addresses indications within the advanced wound care market, which Espicom estimates at $6.2 billion globally in 2013, representing a growth rate of approximately 5% since 2012. We estimate the size of the target market for VergenixFG in diabetic foot ulcers to be 300,000 patients and $500 million annually. Diabetic foot ulcers represent about one quarter of the total chronic wound market, indicating that our initial target market is several magnitudes greater than this market alone.
Risk Factors
Our business is subject to numerous risks, as more fully described in the section titled "Risk Factors" immediately following this prospectus summary. You should read and carefully consider these risks and all of the other information in this prospectus, including the financial statements and the related notes included elsewhere in this prospectus, before deciding whether to invest in the ADSs. In particular, such risks include, but are not limited to, the following:
- •
- We are a clinical-stage regenerative medicine company, and we have not yet generated any revenue from product sales.
- •
- Our product candidates are all at the clinical trial or preclinical study phase. Clinical trials are expensive and complex to
structure and operate, and failure can occur at any stage of clinical development, including a failure to receive approval for the conduct of clinical trials from governmental regulatory authorities
such as the FDA.
- •
- We have not yet commercialized any products or product platforms, and we may never do so.
- •
- We have limited experience in manufacturing products, and we must expand our capacity to do so.
- •
- Our product candidates are subject to extensive regulation and will remain subject to ongoing regulatory requirements even if they
receive marketing approval.
- •
- We have no experience in marketing or distributing our products, and we need to establish our distribution channels.
- •
- If we, or the parties from whom we license intellectual property, fail to adequately protect, enforce, or secure rights to the patents
which we own or may own in the future or that were licensed to us, the value of our intellectual property rights would diminish and our business and competitive position would suffer.
- •
- We face significant competition, and if we cannot successfully compete with new or existing products from our competitors, our product candidates may be rendered noncompetitive or obsolete.
6
Implications of Our Emerging Growth Company and Foreign Private Issuer Status
As a company with less than $1.0 billion in revenue for our year ending December 31, 2014, we qualify as an "emerging growth company" under Section 2(a) of the Securities Act of 1933, as amended, or the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. As an emerging growth company, we may take advantage of certain exemptions from reporting requirements that generally apply to public companies, including the auditor attestation requirements with respect to internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, compliance with new standards adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or auditor discussion and analysis, exemption from say-on-pay, say-on-frequency and say-on-golden parachute voting requirements, and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We have elected not to avail ourselves of an exemption that allows emerging growth companies to extend the transition period for complying with new or revised financial accounting standards. This election is irrevocable.
We will remain an emerging growth company until the earliest of (a) the last day of our fiscal year during which we have total annual gross revenue of at least $1.0 billion; (b) the last day of our fiscal year following the fifth anniversary of the completion of this offering; (c) the date on which we have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt; or (d) the date on which we are deemed to be a "large accelerated filer" under the Securities Exchange Act of 1934, as amended. Once we cease to be an emerging growth company, we will not be entitled to the exemptions provided in the JOBS Act.
We are also a foreign private issuer and are exempt from certain rules and regulations under the Securities Exchange Act of 1934, as amended, or the Exchange Act, that are applicable to other public companies that are not foreign private issuers. Most of these requirements for domestic public companies relate to disclosures that we would only be required to make if we cease to be a foreign private issuer in the future. As a foreign private issuer that is an emerging growth company, we will not be required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act for up to five fiscal years after the date of this offering, until we are no longer deemed to be an emerging growth company.
With respect to home country corporate governance practices under the listing rules of the NASDAQ Capital Market, or NASDAQ Listing Rules, we intend to follow home country practice in Israel with regard to, among other things, director nomination procedures and approval of compensation for officers. In addition, we may follow our home country law instead of the NASDAQ Listing Rules that require shareholder approval for certain dilutive events, such as the establishment or amendment of certain equity based compensation plans, an issuance that will result in a change of control of the company, certain transactions other than a public offering involving issuances of a 20% or greater interest in the company, and certain acquisitions of the stock or assets of another company, amending our compensation policy from time to time, and the approval of certain interested-parties transactions.
In relation to the Exchange Act, as a foreign private issuer, we are exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly, and current reports and financial statements with the Securities and Exchange Commission, or the SEC, as frequently or as promptly as domestic U.S. issuers whose securities are registered under the Exchange Act.
7
We may choose to take advantage of any, some, or all of the exemptions available to us as an emerging growth company or as a foreign private issuer. We have taken advantage of reduced reporting requirements in this prospectus.
Accordingly, the information contained in this prospectus may be different from the information you receive from other public companies in which you hold stock. Please see the section of this prospectus titled "Risk Factors—Risks Related to the Offering and Ownership of our ADSs" for a description of exemptions that apply to emerging growth companies and foreign private issuers.
Corporate Information
We were incorporated under the laws of the State of Israel in 1981. CollPlant Ltd., our subsidiary, was incorporated under the laws of the State of Israel in 2004 and merged with us in 2010. Our principal executive office is located at 3 Sapir Street, Weizmann Science Park, Ness-Ziona 74140, Israel, and our telephone number is +972 (0) 73 2325600. Our website address is www.collplant.com. We have included our website address in this prospectus solely as an inactive textual reference. We do not incorporate the information on or accessible through our website into this prospectus, and you should not consider any information on or accessible through our website a part of this prospectus.
8
ADSs offered by us |
ADSs | |
ADSs to be outstanding immediately after this offering |
ADSs (or ADSs if the underwriters exercise in full their option to purchase additional ADSs). |
|
Ordinary shares to be outstanding immediately after this offering |
ordinary shares |
|
Over-allotment option |
We have granted the underwriters an option for a period of 30 days from the date of this prospectus to purchase up to additional ADSs from us to cover over-allotments, if any. |
|
The ADSs |
Each ADS represents ordinary shares, par value NIS 0.01 per share. You will have the rights of an ADS holder as provided in the deposit agreement among us, the depositary and all holders and beneficial owners of ADSs issued thereunder. To better understand the terms of the ADSs, you should carefully read the section in this prospectus titled "Description of American Depositary Shares." We also encourage you to read the deposit agreement, which is filed as an exhibit to the registration statement that includes this prospectus. |
|
Depositary |
The Bank of New York Mellon |
|
Use of proceeds |
We intend to use the proceeds from this offering to continue the development of our product candidates, to conduct research and development activities, to expand and enhance our manufacturing capabilities, to establish our marketing capabilities, and for working capital and general corporate purposes. See the section of this prospectus titled "Use of Proceeds". |
|
Risk factors |
You should read the "Risk Factors" section starting on page 11 of this prospectus for a discussion of factors to consider carefully before deciding to invest in the ADSs. |
|
Proposed NASDAQ Capital Market symbol |
" " |
|
Tel Aviv Stock Exchange symbol |
CLPT |
|
OTCQX symbol |
CQPTY |
Assuming that our ADSs are listed for trading on the NASDAQ Capital Market, the quoting of our ADSs on OTCQX will be discontinued prior to the completion of this offering.
Unless otherwise stated, the number of ordinary shares to be outstanding after this offering is based on 241,392,352 ordinary shares outstanding as of December 31, 2014, excluding, as of such date, 17,963,346 ordinary shares issuable upon the exercise of options at a weighted average exercise price of NIS 0.56 ($0.14) per share, and 88,337,260 ordinary shares issuable upon the exercise of outstanding warrants at an exercise price of NIS 0.70 ($0.18) per share.
Unless otherwise indicated, all information in this prospectus:
- •
- assumes an initial public offering price of $ per ADS; and
- •
- assumes no exercise by the underwriters of their option to purchase up to an additional ADSs from us.
9
The following summary financial information should be read together with our audited financial statements and accompanying notes, as well as the information under the section of this prospectus titled "Management's Discussion and Analysis of Financial Condition and Results of Operations". Our historical results are not necessarily indicative of results that may be expected in the future.
We have derived the following summary statements of operations data for the years ended December 31, 2014 and December 31, 2013 from our audited financial statements, which have been prepared in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB, included elsewhere in this prospectus.
We prepare our financial statements in NIS. This prospectus contains conversions of NIS amounts into U.S. dollars at specific rates solely for the convenience of the reader. Unless otherwise noted, for the purposes of the presentation of financial data as of December 31, 2014, and for the year then ended, all conversions from NIS to U.S. dollars and from U.S. dollars to NIS were made at a rate of 3.889 NIS to 1.00 U.S. dollar, the daily representative rate in effect as of December 31, 2014. The dollar amounts presented in this prospectus should not be construed as representing amounts that are receivable or payable in dollars or convertible into dollars, unless otherwise indicated.
| |
Year ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | 2014 | |||||||
| |
(NIS in thousands except per share data) |
(Convenience translation into USD in thousands(1)) |
||||||||
Statement of comprehensive loss data: |
||||||||||
Research and development expenses |
16,151 | 14,879 | 3,826 | |||||||
Participation in research and development expenses |
(3,717 | ) | (5,145 | ) | (1,323 | ) | ||||
| | | | | | | | | | | |
Research and development expenses, net |
12,434 | 9,734 | 2,503 | |||||||
General and administrative and marketing expenses |
3,747 | 3,906 | 1,004 | |||||||
Operating loss |
16,181 |
13,640 |
3,507 |
|||||||
Financial income |
(25 |
) |
(642 |
) |
(165 |
) |
||||
Financial expenses: |
314 | 25 | 6 | |||||||
| | | | | | | | | | | |
Financial expenses (income), net |
289 | (617 | ) | (159 | ) | |||||
Loss |
16,470 |
13,023 |
3,348 |
|||||||
Loss per ordinary share, basic and diluted |
0.11 |
0.05 |
0.01 |
|||||||
Weighted average ordinary shares outstanding, basic and diluted |
155,590,908 |
241,280,958 |
||||||||
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | 2014 | |||||||
| |
(NIS in thousands) |
(Convenience translation into USD in thousands(1)) |
||||||||
Statement of financial position data: |
||||||||||
Cash and cash equivalents and short-term bank deposits |
23,777 | 11,062 | 2,845 | |||||||
Total assets |
30,273 | 16,958 | 4,361 | |||||||
Total liabilities |
3,189 | 2,647 | 681 | |||||||
Total equity |
27,084 | 14,311 | 3,680 | |||||||
- (1)
- Calculated using the exchange rate reported by the Bank of Israel for December 31, 2014 at the rate of one U.S. dollar per NIS 3.889.
10
Investing in our ADSs involves a high degree of risk. You should carefully consider the risks we describe below, along with all of the other information set forth in this prospectus, including the section entitled "Cautionary Note Regarding Forward-Looking Statements" and our financial statements and the related notes beginning on page F-1, before deciding to purchase our securities. The risks and uncertainties described below are those significant risk factors, currently known and specific to us, that we believe are relevant to an investment in our securities. If any of these risks materialize, our business, results of operations or financial condition could suffer, the price of the ADSs could decline substantially and you could lose part or all of your investment. Additional risks and uncertainties not currently known to us or that we now deem immaterial may also harm us and adversely affect your investment in the ADSs.
Risks Related to Our Financial Condition and Capital Requirements
We have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future.
We are a clinical-stage regenerative medicine company, and we have not yet generated any revenue from product sales. We have incurred losses in each year since our inception in 2004, including a net loss of $3,348,000 for the year ended December 31, 2014. As of December 31, 2014, we had an accumulated deficit of $30,605,000.
We have devoted most of our financial resources to research and development, including our clinical and preclinical development activities. To date, we have financed our operations primarily through the sale of equity securities, grants from government authorities and proceeds from strategic collaborators. The amount of our future net losses will depend, in part, on the rate of our future expenditures. If and when we obtain regulatory approval to market any of our product candidates, our future revenues will depend upon the size of any markets in which our product candidates have received approval, and our ability to achieve sufficient market acceptance, reimbursement from third-party payors and adequate market share for our product candidates in those markets.
We expect to continue to incur significant expenses and operating losses for the foreseeable future. We anticipate that our expenses will increase substantially if and as we:
- •
- continue our research and preclinical and clinical development of our product candidates;
- •
- initiate additional preclinical, clinical, or other studies for our product candidates;
- •
- seek marketing approvals for any of our product candidates that successfully complete clinical trials;
- •
- further develop and expand the manufacturing process for our product candidates;
- •
- establish a sales, marketing, and distribution infrastructure to commercialize our product candidates for which we may obtain
marketing approval;
- •
- seek to identify and validate additional product candidates;
- •
- maintain, protect, and expand our intellectual property portfolio;
- •
- attract and retain skilled personnel;
- •
- create additional infrastructure to support our operations as a public company; and
- •
- experience any delays or encounter issues with any of the above.
The net losses we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. In any particular quarter or quarters, our operating results could be below the expectations of securities analysts or investors, which could cause our share price to decline.
11
Even if this offering is successful, we may need to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain additional capital when needed may force us to delay, limit, or terminate our product development efforts or other operations.
Our product candidates are currently in clinical and preclinical development and we intend to continue advancing their development. Developing medical products is expensive, and we expect our research and development expenses to continue to be a material part of our expenses, and may increase substantially in connection with our ongoing activities, particularly as we advance our product candidates in clinical trials.
As of December 31, 2014, our cash and cash equivalents were $2,845,000. We estimate that the net proceeds from this offering will be approximately $ , assuming an initial public offering price of $ per ADS after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. We estimate that these net proceeds, together with our existing cash and cash equivalents, will be sufficient to fund our operations for at least the next three years. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements, and other collaborations, strategic alliances, and licensing arrangements, or a combination of these approaches. In any event, we will require additional capital to obtain regulatory approval for our product candidates and to commercialize any product that receives regulatory approval. Even if we believe we have sufficient funds for our current or future operating plans, we may seek additional capital if market conditions are favorable or if we have specific strategic considerations.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may compromise our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our shareholders, and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our ordinary shares or ADSs to decline. The sale of additional equity or convertible securities would dilute all of our shareholders. The incurrence of indebtedness would result in increased fixed payment obligations, and we may be required to agree to certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights, and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborative partners or otherwise at an earlier stage than otherwise would be desirable, and we may be required to relinquish rights to some of our technologies or products or otherwise agree to terms unfavorable to us.
If we are unable to obtain funding on a timely basis, we may be required to significantly curtail, delay, or discontinue one or more of our research or development programs or the commercialization of any product candidates, and we may be unable to expand our operations or otherwise capitalize on our business opportunities, as desired.
The report of our independent registered public accounting firm on our 2014 audited consolidated financial statements contains an explanatory paragraph regarding our ability to continue as a going concern.
Our recurring losses from operations and negative cash flows from operations raise substantial doubt about our ability to continue as a going concern without additional debt or equity financing. As a result, our independent registered public accounting firm included an explanatory paragraph in its report on our audited consolidated financial statements for 2014 with respect to this uncertainty. Substantial doubt about our ability to continue as a going concern may materially and adversely affect the price per share of our ordinary shares or ADSs and make it more difficult for us to obtain financing. If we are unable to obtain sufficient capital in this offering, our business, financial condition, and results of operations will be materially and adversely affected, and we will need to obtain
12
alternative financing or significantly modify our operational plans to continue as a going concern. Further, if we successfully complete and receive the net proceeds from this offering, given our planned expenditures for the next several years, including without limitation, expenditures in connection with our planned clinical trials of our lead product candidates, our independent registered public accounting firm may conclude, in connection with the preparation of our financial statements for 2015 or any subsequent period that there continues to be substantial doubt regarding our ability to continue as a going concern.
We have prepared our financial statements on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or amounts of liabilities that might be necessary should we be unable to continue in existence.
We have received and may continue to receive Israeli governmental grants to assist in the funding of our research and development activities. If we lose our funding from these research and development grants, we may encounter difficulties in the funding of future research and development projects and implementing technological improvements, which would harm our operating results.
Through December 31, 2014 we had received an aggregate of $6.4 million in the form of grants from the Israeli Office of the Chief Scientist, or OCS. The requirements and restrictions for such grants are found in the Israeli Encouragement of Industrial Research and Development Law, or the Research Law. Under the Research Law, royalties of 3% to 5% on the revenues derived from sales of products or services developed in whole or in part using these OCS grants are payable to the Israeli government. We developed our platform technologies, at least in part, with funds from these grants, and accordingly we would be obligated to pay these royalties on sales of any of our products that achieve regulatory approval. The maximum aggregate royalties paid generally cannot exceed 100% of the grants made to us, plus annual interest equal to the 12-month LIBOR applicable to dollar deposits, as published on the first business day of each calendar year. As of December 31, 2014, the maximum royalty amount that would be payable by us, excluding interest, is $6.3 million (assuming 100% of the royalties are payable). As of December 31, 2014, we paid non-material amounts in royalties to the OCS, relating mainly to the participation of our strategic collaborators in the product development. For the year ended December 31, 2014, we recorded grants totaling $923,000 from the OCS. The grants represented 24% of our gross research and development expenditures for the year ended December 31, 2014.
As part of funding our current and planned product development activities, we have submitted a follow-up grant application of approximately $1.5 million for fiscal year 2015. There is no assurance that this grant application will be approved.
These grants have funded some of our personnel, development activities with subcontractors, and other research and development costs and expenses. However, if these grants are not funded in their entirety or if new grants are not awarded in the future, due to, for example, OCS budget constraints or governmental policy decisions, our ability to fund future research and development and implement technological improvements would be impaired, which would negatively impact our ability to develop our product candidates.
The Israeli government grants we have received for research and development expenditures restrict our ability to manufacture products and transfer technologies outside of Israel and require us to satisfy specified conditions. If we fail to satisfy these conditions, we may be required to refund grants previously received together with interest and penalties.
Our research and development efforts have been financed, in part, through the grants that we have received from the OCS. We, therefore, must comply with the requirements of the Research Law.
13
Under the Research Law, we are prohibited from manufacturing products developed using these grants outside of the State of Israel without special approvals. We may not receive the required approvals for any proposed transfer of manufacturing activities. Even if we do receive approval to manufacture products developed with government grants outside of Israel, the royalty rate may be increased, and we may be required to pay up to 300% of the grant amounts plus interest, depending on the manufacturing volume that is performed outside of Israel. This restriction may impair our ability to outsource manufacturing or engage in our own manufacturing operations for our products or technologies. See "Management's Discussion and Analysis of Financial Condition and Results of Operations—Financial Overview—Research and Development Expenses" for additional information.
Additionally, under the Research Law, we are prohibited from transferring, including by way of license, the OCS-financed technologies and related intellectual property rights and know-how outside of the State of Israel, except under limited circumstances and only with the approval of the OCS Research Committee. We may not receive the required approvals for any proposed transfer, and even if received, we may be required to pay the OCS a portion of the consideration that we receive upon any sale of such technology to a non-Israeli entity of up to 600% of the grant amounts plus interest. The scope of the support received, the royalties that we have already paid to the OCS, the amount of time that has elapsed between the date on which the know-how or the related intellectual property rights were transferred and the date on which the OCS grants were received, the sale price, and the form of transaction will be taken into account in order to calculate the amount of the payment to the OCS. Approval of the transfer of technology to residents of the State of Israel is required, and may be granted in specific circumstances only if the recipient abides by the provisions of applicable laws, including the restrictions on the transfer of know-how and the obligation to pay royalties. No assurance can be given that approval to any such transfer, if requested, will be granted.
These restrictions may impair our ability to sell our technology assets or to perform or outsource manufacturing outside of Israel, engage in change of control transactions, or otherwise transfer our know-how outside of Israel. It may also require us to obtain the approval of the OCS for certain actions and transactions and pay additional royalties and other amounts to the OCS. In addition, any change of control and any change of ownership of our ordinary shares that would make a non-Israeli citizen or resident an "interested party" holding 5% or more of our ordinary shares, as defined in the Research Law, requires prior written approval from the OCS, and our failure to comply with this requirement could result in criminal liability.
These restrictions will continue to apply even after we have repaid the full amount of royalties on the grants. If we fail to satisfy the conditions of the Research Law, we may be required to refund certain grants previously received along with interest and penalties, and we may become subject to criminal charges.
We may not be able to correctly estimate or control our future operating expenses, which could lead to cash shortfalls.
Our operating expenses may fluctuate significantly in the future for various reasons, many of which are outside of our control. These reasons may include:
- •
- the time, resources, and expenses required to conduct clinical trials of, seek regulatory approvals for, manufacture, market, and sell
our current product candidates and any additional product candidates we may develop;
- •
- the time, resources, and expenses required to research and develop, conduct clinical trials of, and seek regulatory approvals for
additional indications of our current product candidates;
- •
- the costs of preparing, filing, prosecuting, defending, and enforcing patent claims and other patent-related costs, including
litigation costs or the results of such litigation;
- •
- any product liability or other lawsuits related to our product candidates and the costs associated with defending them or the results of such lawsuits;
14
- •
- the costs to attract and retain personnel with the skills required for effective operations; and
- •
- the costs associated with being a public company in the United States.
It is difficult to forecast our future performance, which may cause our financial results to fluctuate unpredictably.
Because we do not yet have a commercial operating history, and because the market for our product candidates may rapidly evolve, it is hard for us to predict our future performance. A number of factors, many of which are outside of our control, may contribute to fluctuations in our financial results assuming that we receive marketing authorizations and begin selling our product candidates. These factors may include variations in:
- •
- market demand for, and acceptance of, our product candidates;
- •
- our ability to obtain or maintain regulatory approvals;
- •
- our sales and marketing operations, or the effectiveness of these operations;
- •
- performance of our third-party contractors;
- •
- the availability of procedures or products that compete with our product candidates;
- •
- media coverage of our technologies, the procedures or products of our competitors or our industry; and
- •
- general economic and political conditions, including changes in general consumer confidence.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, our shareholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our ADSs.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports. Together with adequate disclosure controls and procedures, effective internal controls are designed to prevent fraud. Any failure to implement required new or improved controls or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses, may require prospective or retroactive changes to our financial statements, or may identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our ADSs.
We are required to disclose changes made in our internal controls and procedures on a quarterly basis and our management is required to assess the effectiveness of these controls annually. However, for as long as we are an "emerging growth company" under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act. We could be an emerging growth company for up to five years. An independent assessment of the effectiveness of our internal controls could detect problems that our management's assessment might not. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur the expense of remediation.
15
Risks Related to the Clinical Development and Regulatory Approval of Our Product Candidates
We currently depend heavily on the future success of our lead product candidates, VergenixSTR, VergenixFG, and VergenixBVF. Any failure to successfully develop, obtain regulatory approval for, and commercialize, independently or in cooperation with a third party collaborator, or the experience of significant delays in doing so, would compromise our ability to generate revenue and become profitable.
We have invested a significant portion of our efforts and financial resources in the development of VergenixSTR, VergenixFG, and VergenixBVF. Our ability to generate product revenue from our product candidates depends heavily on the successful development, approval, and commercialization of our product candidates, which, in turn, depend on several factors, including the following:
- •
- our ability to continue and support our rhCollagen platform technology and programs;
- •
- successfully completing our ongoing and future clinical trials and other studies required for our product candidates;
- •
- demonstrating and maintaining the safety and efficacy of our product candidates at a sufficient level of statistical or clinical
significance and otherwise obtaining marketing approvals from regulatory authorities;
- •
- establishing successful sales and marketing arrangements for our product candidates VergenixSTR and VergenixFG if approved;
- •
- the availability of coverage and reimbursement by healthcare payors for our product candidates, if approved;
- •
- establishing successful manufacturing arrangements with third-party manufacturers that are compliant with current good manufacturing
practices, or cGMP, and which will ensure the development of a large scale manufacturing process and adequate facilities or being able to conduct such manufacturing ourselves;
- •
- establishing a large scale facility as a second source for the manufacture of commercial quantities of our product candidates, if
approved; and
- •
- other risks described in this "Risk Factors" section.
Our product candidates are based on novel technology, which makes it difficult to predict the time and cost of product development and potential regulatory approval.
We have concentrated our product research and development efforts on our novel rhCollagen technology. We may experience development challenges in the future related to our technology, which could cause significant delays or unanticipated costs, and we may not be able to solve such development challenges. We may also experience delays in developing a sustainable, reproducible, and scalable manufacturing process or transferring that process to commercial partners, if we decide to do so, which may prevent us from completing our clinical trials or commercializing our product candidates on a timely or profitable basis, if at all.
In addition, the clinical trial requirements of European regulatory authorities, the FDA, and other regulatory authorities and the criteria these regulators use to determine the safety and efficacy of a product vary substantially according to the type, complexity, novelty, and intended use and market of the potential product candidates. The regulatory approval process for novel products such as ours can be more expensive and take longer than for other, better known or extensively studied medical devices or other products. Our product candidates may also be designated by the FDA or other regulatory authorities as Combination Products, which are products composed of two or more regulated components, such as a drug and a medical device, and then may be regulated as drug or biologic product, resulting in a longer regulatory approval process than the regulatory approval process for a medical device. Approvals by any regulatory authorities may not be indicative of what the FDA or other regulatory agencies may require for approval, and vice versa.
16
Regulatory requirements governing medical devices and other products for medical use have changed frequently and may continue to change in the future. Also, before a clinical trial can begin, an institutional review board, or IRB, at each institution at which a clinical trial will be performed must review the proposed clinical trial to assess the safety of the trial. In addition, adverse developments in clinical trials of medical devices and products conducted by others may cause European regulatory authorities, the FDA, or other regulatory authorities to change the requirements for approval of any of our product candidates.
These regulatory agencies and additional or new requirements may lengthen the regulatory review process, require us to perform additional studies, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of our product candidates, or lead to significant approval and post-approval limitations or restrictions. As we advance our product candidates, we will be required to consult with these regulatory authorities, and comply with applicable requirements. If we fail to do so, we may be required to delay or discontinue development of our product candidates. Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approval necessary to bring a potential product to market could impair our ability to generate product revenue and to become profitable.
We may find it difficult to enroll patients in our clinical trials, and patients could discontinue their participation in our clinical trials, which could delay or prevent clinical trials of our product candidates.
Identifying and qualifying patients to participate in clinical trials of our product candidates is critical to our success. The timing of our clinical trials depends on our ability to recruit patients to participate in our clinical trials. We may experience delays in patient enrollment in the future. If patients are unwilling to participate in our clinical trials because of negative publicity from adverse events in the biotechnology, pharmaceutical or medical technology industries, or for other reasons, including competitive clinical trials for similar patient populations, the timeline for recruiting patients, conducting trials, and obtaining regulatory approval of potential products may be delayed. These delays could result in increased costs, delays in advancing our product development, delays in testing the effectiveness of our technology, or termination of the clinical trials altogether.
We may not be able to identify, recruit, and enroll a sufficient number of patients, or those with required or desired characteristics to achieve diversity in a trial, to complete our clinical trials in a timely manner. Patient enrollment is affected by factors including:
- •
- design of the trial protocol;
- •
- size of the patient population;
- •
- eligibility criteria for the trial in question;
- •
- severity of the disease/wounds under investigation;
- •
- perceived risks and benefits of the product under study;
17
- •
- proximity and availability of clinical trial sites for prospective patients;
- •
- availability of competing therapies, product candidates, and clinical trials;
- •
- efforts to facilitate timely enrollment in clinical trials;
- •
- patient referral practices of physicians; and
- •
- ability to monitor patients adequately during and after treatment.
We are currently conducting clinical trials in Israel and intend to seek marketing approval in Europe, China and the United States. We may not be able to initiate or continue clinical trials if we cannot enroll a sufficient number of eligible patients to participate in the clinical trials required by European regulatory authorities, FDA, or other regulatory authorities.
In addition, patients enrolled in our clinical trials may discontinue their participation at any time during the trial as a result of a number of factors, including withdrawing their consent or experiencing adverse clinical events, which may or may not be related to our product candidates under evaluation. The discontinuation of patients in any one of our trials may cause us to delay or abandon such clinical trial, or cause the results from that trial not to be positive or sufficient to support a filing for regulatory approval of the applicable product.
Our clinical trials may not be successful or may be delayed.
As stated above, we are currently conducting registration trials in Israel for our product candidates VergenixSTR and VergenixFG. Before obtaining marketing approval from regulatory authorities for the sale of our product candidates or any future product, we must conduct extensive clinical trials to demonstrate the safety of these two product candidates in humans for European CE marking certification, and the safety and efficacy of our product candidates or any future products in humans for other regulatory authorities such as China and the United States. Clinical trials are expensive, time consuming, and uncertain as to outcome. We cannot guarantee that any clinical trials will be conducted as planned or completed on schedule, if at all. We may not receive FDA regulatory approval for the conduct of any particular clinical trial in the United States or regulatory approval for conduct of such clinical trial in other countries. A failure of one or more clinical trials can occur at any stage of testing. Events that may prevent successful or timely completion of clinical development include:
- •
- delays in reaching a consensus with regulatory agencies on trial design;
- •
- delays in reaching agreement on acceptable terms with prospective CROs and clinical trial sites;
- •
- delays in obtaining required IRB approval at each clinical trial site;
- •
- delays in recruiting suitable patients to participate in our clinical trials;
- •
- imposition of a clinical hold by regulatory agencies, including after an inspection of our clinical trial operations or trial sites;
- •
- failure by our CROs, other third parties or us to perform in accordance with clinical trial requirements or the FDA's good clinical
practices, or GCP, or applicable regulatory requirements in other countries;
- •
- delays in the testing, validation, manufacturing, and delivery of our product candidates to the clinical sites;
- •
- delays in having patients complete participation in a trial or return for post-treatment follow-up;
- •
- clinical trial sites or patients dropping out of a trial;
- •
- occurrence of serious adverse events associated with the product candidates that are viewed to outweigh their potential benefits; or
18
- •
- changes in regulatory requirements and guidance that require amending or submitting new clinical trial protocols.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to generate revenue from product sales. In addition, if we make manufacturing or design changes to our product candidates, we may need to conduct additional studies to bridge our modified product candidates to earlier versions. Clinical trial delays could also shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates.
If the results of our clinical trials are inconclusive or if there are safety concerns or adverse events associated with our product candidates, we may:
- •
- fail to obtain, or be delayed in obtaining, marketing approval for our product candidates;
- •
- obtain approval for indications or patient populations that are not as broad as intended or desired;
- •
- obtain approval with labeling that includes significant use or distribution restrictions or safety warnings;
- •
- be required to perform additional clinical trials to support approval or be subject to additional post-marketing testing requirements;
- •
- have regulatory authorities withdraw their approval of the product or impose restrictions on its distribution;
- •
- be subject to the addition of labeling statements, such as warnings or contraindications;
- •
- be sued; or
- •
- experience damage to our reputation.
Any of these events could prevent us from achieving or maintaining market acceptance of our product candidates and impair our ability to commercialize our product candidates.
Success in early clinical trials may not be indicative of results obtained in later trials.
There is a high failure rate for medical devices, medical products, and biologics proceeding through clinical trials. A number of companies in the pharmaceutical, biotechnology, and medical technology industries have suffered significant setbacks in later stage clinical trials even after achieving promising results in earlier stage clinical trials. Data obtained from preclinical and clinical activities are subject to varying interpretations, which may delay, limit, or prevent regulatory approval. In addition, regulatory delays or rejections may be encountered as a result of many factors, including the novelty of the product and changes in regulatory policy during the period of product development.
Even if we complete the necessary preclinical studies and clinical trials, we cannot predict when or if we will obtain regulatory approval to commercialize a product, or the approval may be for a more narrow indication than we expect.
We cannot commercialize a product until the appropriate regulatory authorities have reviewed and approved the product. Even if our product candidates demonstrate safety and efficacy in clinical trials, the regulatory agencies may not complete their review processes in a timely manner, or we may not be able to obtain regulatory approval. Additional delays may result if an FDA Advisory Committee or other regulatory authority recommends non-approval or restrictions on approval. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory agency policy during the period of product development, clinical trials, and the review process. Regulatory agencies also may approve a treatment for fewer or
19
more limited indications than requested or may grant approval subject to the performance of post-marketing studies. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our treatment.
Side effects may occur following treatment with our product candidates, which could make it more difficult for our product candidates to receive regulatory approval.
Treatment with our product candidates may cause side effects or other adverse events. In addition, since our product candidates are in some cases administered in combination with other therapies, patients, or clinical trial participants may experience side effects or other adverse events that are unrelated to our product, but may still impact the success of our clinical trials. Additionally, our product candidates could potentially cause other adverse events that have not yet been predicted. The experience of side effects and adverse events in our clinical trials could make it more difficult to achieve regulatory approval of our product candidates or, if approved, could negatively impact the market acceptance of such product candidates.
Even if we obtain regulatory approval for a product, our product candidates will remain subject to regulatory scrutiny.
Even if we obtain regulatory approval in a jurisdiction, the regulatory authority may still impose significant restrictions on the indicated uses or marketing of our product candidates, or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance. Advertising and promotional materials must comply with FDA, Federal Trade Commission, or FTC, and European and Chinese regulatory requirements and are subject to review by FDA, FTC or other governmental authorities, in addition to other potentially applicable federal and state laws.
In addition, product manufacturers and their facilities are subject to continual review and periodic inspections by the European regulatory authorities, the FDA, or other regulatory authorities for compliance with cGMP or any applicable European or other governmental regulations. If we or a regulatory agency discover previously unknown problems with a product such as adverse events of unanticipated severity or frequency or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions relative to that product or the manufacturing facility, including requiring recall or withdrawal of the product from the market or suspension of manufacturing.
If we fail to comply with applicable regulatory requirements following approval of any of our product candidates, one or more of regulatory authorities, such as the FDA, European regulatory authorities, or any other regulatory authorities, could:
- •
- issue a warning letter asserting that we are in violation of the law;
- •
- seek an injunction or impose civil or criminal penalties or monetary fines;
- •
- suspend or withdraw regulatory approval;
- •
- suspend any ongoing clinical trials;
- •
- seize our product; or
- •
- refuse to allow us to enter into supply contracts, including government contracts.
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity and potentially lead to private litigation. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and generate revenues.
20
We have only limited experience in regulatory affairs and intend to rely on consultants and other third parties for regulatory matters, which may affect our ability or the time we require to obtain necessary regulatory approvals.
Between 2010 and 2012, we had limited interactions with the FDA for a predecessor wound healing product candidate and have not had any discussions with the FDA regarding our current product candidates. We have limited experience in preparing and filing the applications necessary to gain regulatory approvals for our product candidates. Moreover, the products that are likely to result from our development programs are based on new technologies that have not been extensively used in humans. The regulatory requirements governing these types of product may be less well defined or more rigorous than for conventional products. As a result, we may experience a longer regulatory review process in connection with obtaining regulatory approvals, if any, of products that we develop. We intend to rely on independent consultants for regulatory services and compliance and product development and filings in Europe, the United States and elsewhere. Any failure by our consultants to properly advise us regarding, or properly perform tasks related to, regulatory submission and other requirements could compromise our ability to develop and obtain regulatory approval of our product candidates.
We are subject to stringent regulation and any adverse regulatory action may materially adversely affect our financial condition and business operations.
Our product candidates, development activities, and manufacturing processes are subject to extensive and rigorous regulation by numerous government agencies, including European regulatory authorities, the FDA, and other regulatory authorities. To varying degrees, each of these agencies monitors and enforces our compliance with laws and regulations governing the development, testing, manufacturing, labeling, marketing, and distribution of our products. The process of obtaining marketing approval or clearance in Europe, the United States, and other countries for new products or enhancements or modifications to existing products, could
- •
- take a significant amount of time;
- •
- require the expenditure of substantial resources;
- •
- involve rigorous and expensive preclinical and clinical testing, as well as increased post-market surveillance;
- •
- involve modifications, repairs, or replacements of our product candidates; and
- •
- result in limitations on the indicated uses of our product candidates.
We cannot be certain that we will receive required approval or clearance from European regulatory authorities, the FDA, or other regulatory authorities for new products or modifications to existing products on a timely basis. The failure to receive approval or clearance for significant new products or modifications to existing products on a timely basis could have a material adverse effect on our financial condition and results of operations.
Both before and after a product is commercially released, we have ongoing responsibilities under FDA regulations. For example, we are required to comply with the FDA's Quality System Regulation, or QSR, which are the good manufacturing requirements that the FDA applies to medical devices, and which mandates that manufacturers of medical devices adhere to certain requirements pertaining to, among other things, validation of manufacturing processes, controls for purchasing product components, and documentation practices. As another example, FDA regulations require us to provide information to the FDA whenever there is evidence that reasonably suggests that a product may have caused or contributed to a death or serious injury, or that a malfunction occurred which would be likely to cause or contribute to a death or serious injury upon recurrence. Compliance with applicable regulatory requirements is subject to continual review and is monitored rigorously through, among
21
other things, periodic inspections by the FDA, which may result in observations on Form 483, and in some cases warning letters, that require corrective action. If the FDA were to conclude that we are not in compliance with applicable laws or regulations, or that any of our medical devices are ineffective or pose an unreasonable health risk, the FDA could ban such medical devices, detain or seize such medical devices, order a recall, repair, replacement, or refund of such devices, or require us to notify health professionals and others that the devices present unreasonable risks of substantial harm to the public health.
The FDA has been increasing its scrutiny of the medical device, medical products, and biologics industries, and regulatory agencies are expected to continue to scrutinize the industry closely with inspections, with possible enforcement actions by the FDA or other agencies. Additionally, the FDA may restrict manufacturing and impose other operating restrictions, enjoin, and restrain certain violations of applicable law pertaining to medical devices, and assess civil or criminal penalties against our officers, employees, or us. The FDA may also recommend prosecution to the Department of Justice. Any adverse regulatory action, depending on its magnitude, may restrict us from effectively manufacturing, marketing, and selling our product candidates. In addition, negative publicity and product liability claims resulting from any adverse regulatory action could have a material adverse effect on our financial condition and results of operations.
Finally, the FDA issued regulations regarding "Current Good Manufacturing Practice Requirements for Combination Products" on January 22, 2013. These regulations may apply to some of our product candidates if they are designated by the FDA as Combination Products, which are products composed of two or more regulated components, such as a drug and a medical device. There have been and will be additional costs associated with compliance with the FDA Good Manufacturing Practice Requirements regulations for Combination Products.
Governmental regulations have become increasingly stringent and more common, and we may become subject to even more rigorous regulation by governmental authorities in various countries in the future. Penalties for a company's noncompliance with governmental regulation could be severe, including revocation or suspension of a company's business license and criminal sanctions.
If our product candidates obtain marketing approval, we will be subject to more extensive healthcare laws, regulation, and enforcement, and our failure to comply with those laws could have a material adverse effect on our results of operations and financial condition.
If we obtain approval for any of our product candidates, the regulatory requirements applicable to our operations, in particular our sales and marketing efforts, will increase significantly with respect to our operations, and the potential for civil and criminal enforcement by the federal government, state governments, and foreign governments will increase with respect to the conduct of our business. The laws that may affect our operations in the United States include:
- •
- the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving,
offering, or paying remuneration, directly or indirectly, to induce, or in return for, the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the
Medicare and Medicaid programs;
- •
- federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or
entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third party payors that are false or fraudulent;
- •
- the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters;
22
- •
- HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and its implementing regulations, which
imposes certain requirements relating to the privacy, security, and transmission of individually identifiable health information;
- •
- the federal physician sunshine requirements under The Patient Protection and Affordable Care Act, or ACA, which requires manufacturers
of drugs, devices, biologics, and medical supplies to report annually to the Centers for Medicare and Medicaid Services, or CMS, information related to payments and other transfers of value to
physicians, other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members; and
- •
- foreign and state law equivalents of each of the above federal laws, such as the U.S. Foreign Corrupt Practices Act, or FCPA, anti-kickback and false claims laws that may apply to items or services reimbursed by any third party payor, including commercial insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways, thus complicating compliance efforts.
The scope of these laws and our lack of experience in establishing the compliance programs necessary to comply with this complex and evolving regulatory environment increase the risks that we may violate the applicable laws and regulations. If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, the exclusion from participation in federal and state healthcare programs, and imprisonment, any of which could materially adversely affect our ability to operate our business and our financial results.
The impact of healthcare reform and other changes in the healthcare industry and in healthcare spending is currently unknown, and may adversely affect our business model.
The commercial potential for our approved product candidates, if any, could be affected by changes in healthcare spending and policy in Europe, in the United States, and in other countries. We operate in a highly regulated industry and new laws, regulations or judicial decisions, or new interpretations of existing laws, regulations, or decisions, related to healthcare availability, the method of delivery, or payment for healthcare products and services could negatively impact our business, operations, and financial condition.
In addition to the level of commercial success of our product candidates, if approved, our future prospects are also dependent on our ability to successfully develop a pipeline of additional products, and we may not be successful in our efforts in using our platform technologies to identify or discover additional products.
The success of our business depends primarily upon our ability to identify, develop, and commercialize products based on our platform technology. Although we have two product candidates currently in clinical development, and a third product in preclinical studies with a U.S.-based corporate collaborator, our research programs may fail to identify other potential products for clinical development for a number of reasons. Our research methodology may be unsuccessful in identifying potential products or our potential products may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval.
23
If any of these events occur, we may be forced to abandon our development efforts for a program or programs. Research programs to identify new products require substantial technical, financial, and human resources. We may focus our efforts and resources on potential programs or products that ultimately prove to be unsuccessful.
Risks Related to Our Reliance on Third Parties
We expect to rely on third parties to conduct some or all aspects of our product manufacturing, protocol development, research, and preclinical and clinical testing, and these third parties may not perform satisfactorily.
We do not expect to independently conduct all aspects of our product manufacturing, protocol development, research, and preclinical and clinical testing. We currently rely, and expect to continue to rely, on third parties with respect to parts of these items.
Any of these third parties may terminate their engagements with us at any time. If we need to enter into alternative arrangements, it could delay our product development activities. Our reliance on these third parties for research and development activities will reduce our control over these activities but will not relieve us of our responsibility to ensure compliance with all required regulations and study protocols.
If these third parties do not successfully carry out their contractual duties, meet expected deadlines, or conduct our studies in accordance with regulatory requirements or our stated study plans and protocols, we will not be able to complete, or may be delayed in completing, the preclinical studies and clinical trials required to support future FDA, European, or other approvals of our product candidates.
Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured the products ourselves, including:
- •
- the inability to negotiate manufacturing agreements with third parties under commercially reasonable terms;
- •
- reduced control as a result of using third-party manufacturers for all aspects of manufacturing activities;
- •
- termination or non-renewal of manufacturing agreements with third parties in a manner or at a time that is costly or damaging to us;
and
- •
- disruptions to the operations of our third-party manufacturers or suppliers caused by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer or supplier.
Any of these events could lead to clinical trial delays or failure to obtain regulatory approval, or impact our ability to successfully commercialize future products. Some of these events could be the basis of action from European regulatory authorities, the FDA, or other regulatory authorities, including injunction, recall, seizure, or total or partial suspension of production.
If we or our third-party manufacturers on which we rely cannot manufacture our product candidates at sufficient yields, we may experience delays in development, regulatory approval and commercialization.
Completion of our clinical trials and commercialization of our product candidates require access to, or development of, facilities to manufacture our product candidates at sufficient yields and at commercial scale. We have limited experience in large scale manufacturing, or managing third parties in manufacturing any of our product candidates in the volumes that are expected to be necessary to support large-scale clinical trials and sales. Our efforts to establish these capabilities may not meet our requirements as to scale-up, yield, cost, potency, or quality in compliance with cGMP. Our clinical trials should be conducted with product produced under applicable cGMP regulations. Failure to comply with
24
these regulations would delay the regulatory approval process. Even an experienced third-party manufacturer may encounter difficulties in production, including:
- •
- costs and challenges associated with scale-up and attaining sufficient manufacturing yields;
- •
- supply chain issues, including the timely availability and shelf life requirements of raw materials and supplies;
- •
- quality control and assurance;
- •
- shortages of qualified personnel and capital required to manufacture large quantities of product;
- •
- compliance with regulatory requirements that vary in each country where a product might be sold;
- •
- capacity limitations and scheduling availability in contracted facilities; and
- •
- natural disasters that affect facilities and possibly limit production.
Any delay or interruption in the supply of our product candidates could have a material adverse effect on our business and operations.
The regulatory authorities also may, at any time following approval of a product for sale, audit our manufacturing facilities or those of our third-party contractors. If any such inspection or audit identifies a failure to comply with applicable regulations or our product specifications or if a violation of applicable regulations, including a failure to comply with the product specifications, occurs independent of such an inspection or audit, we or the relevant regulatory authority may require remedial measures that may be costly or time consuming for us or a third party to implement and that may include the temporary or permanent suspension of a clinical trial or commercial sales or the temporary or permanent closure of a facility.
If we or any of our third-party manufacturers fail to maintain regulatory compliance, the FDA or the European authorities can impose regulatory sanctions including, among other things, refusal to approve a pending application for a new product or revocation of a pre-existing approval.
Additionally, if supply from one approved manufacturer is interrupted, there could be a significant disruption in commercial supply. Switching manufacturers may involve substantial costs and is likely to result in a delay in our desired clinical and commercial timelines.
These factors could cause the delay of clinical trials, regulatory submissions, required approvals, or commercialization of our product candidates, cause us to incur higher costs and prevent us from commercializing our product candidates successfully. Furthermore, if our suppliers fail to meet contractual requirements, and we are unable to secure one or more replacement suppliers capable of production at a substantially equivalent cost, our clinical trials may be delayed or we could lose potential revenue.
We expect to rely on third parties to conduct, supervise, and monitor our clinical trials, and if these third parties perform in an unsatisfactory manner, it may harm our business.
We rely heavily on hospitals, clinic centers, and other institutions and third parties, including the principal investigators and their staff, to carry out our clinical trials in accordance with our clinical protocols and designs. We also rely on a number of CROs to assist in undertaking, managing, monitoring, and executing our ongoing clinical trials. We expect to continue to rely on CROs, clinical data management organizations, medical institutions, and clinical investigators to conduct our development efforts in the future. We compete with many other companies for the resources of these third parties, and large pharmaceutical and medical device companies often have significantly more extensive agreements and relationships with such third-party providers, and such third-party providers may prioritize the requirements of such large pharmaceutical and medical device companies over ours. The third parties on whom we rely may terminate their engagements with us at any time, which may
25
cause delay in the development and commercialization of our product candidates. If any such third party terminates its engagement with us or fails to perform as agreed, we may be required to enter into alternative arrangements, which would result in significant cost and delay to our product development program. Moreover, our agreements with such third parties generally do not provide assurances regarding employee turnover and availability, which may cause interruptions in the research on our product candidates by such third parties.
Moreover, while our reliance on these third parties for certain development and management activities will reduce our control over these activities, it will not relieve us of our responsibilities. For example, European regulatory authorities, the FDA, and other regulatory authorities require compliance with regulations and standards, including GCP requirements, for designing, conducting, monitoring, recording, analyzing, and reporting the results of clinical trials to ensure that the data and results from trials are credible and accurate and that the rights, integrity, and confidentiality of trial participants are protected. Although we rely on third parties to conduct our clinical trials, we are responsible for ensuring that each of these clinical trials is conducted in accordance with its general investigational plan and protocol under legal and regulatory requirements. Regulatory authorities enforce these GCP requirements through periodic inspections of trial sponsors, principal investigators, and trial sites. If we or any of our CROs fail to comply with applicable GCP requirements, the clinical data generated in our clinical trials may be deemed unreliable, and European regulatory authorities, the FDA, or other regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a regulatory authority, such regulatory authority will determine that any of our clinical trials comply with GCP requirements.
If CROs and other third parties do not successfully carry out their duties under their agreements with us, if the quality or accuracy of the data they obtain is compromised due to their failure to adhere to trial protocols or to regulatory requirements, or if they otherwise fail to comply with regulations and trial protocols or meet expected standards or deadlines, the trials of our product candidates may not meet regulatory requirements. If trials do not meet regulatory requirements or if these third parties need to be replaced, the development of our product candidates may be delayed, suspended, or terminated, or the results may not be acceptable. If any of these events occur, we may not be able to obtain regulatory approval of our product candidates on a timely basis, at a reasonable cost, or at all.
Our reliance on third parties may require us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we rely on third parties to manufacture our product candidates, and because we collaborate with various organizations and academic institutions on the advancement of our technology, we must, at times, share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements, or other similar agreements with our collaborators, advisors, employees, and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, such as trade secrets. Despite these contractual provisions, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by potential competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, discovery by a third party of our trade secrets or other unauthorized use or disclosure would impair our intellectual property rights and protections in our product candidates.
In addition, these agreements typically restrict the ability of our collaborators, advisors, employees, and consultants to publish data potentially relating to our trade secrets. Our academic collaborators
26
typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. In other cases, publication rights are controlled exclusively by us, although in some cases we may share these rights with other parties. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication.
It could be difficult to replace some of our suppliers and equipment vendors.
Outside vendors provide key components, raw materials, and equipment used in the manufacture of our product candidates. An uncorrected defect or supplier's variation in a component or raw material, either unknown to us or incompatible with our manufacturing process, could harm our ability to manufacture product candidates. We may not be able to find a sufficient alternative supplier in a reasonable time period, or on commercially reasonable terms, if at all, and our ability to produce and supply our product candidates could be impaired.
If we were suddenly unable to purchase from one or more of these companies, we would need a significant period of time to qualify a replacement, and the production of any affected product candidates could be disrupted. While it is our policy to maintain sufficient inventory of components so that our production will not be significantly disrupted even if a particular component or material is not available for a period of time, we remain at risk that we will not be able to qualify new components or materials quickly enough to prevent a disruption if one or more of our suppliers ceases production of important components or materials, or if we are unable to quickly procure replacement equipment.
If we fail to enter into any needed collaboration agreements for our product candidates, we may be unable to commercialize them effectively or at all. However, there are risks associated with entering into any collaboration agreement.
To successfully develop and commercialize our product candidates, we will need substantial financial resources as well as expertise and physical resources and systems. We may elect to develop some or all of these physical resources and systems and expertise ourselves, or we may seek to collaborate with another company that can provide some or all of such physical resources and systems as well as financial resources and expertise. If we are not able to enter into a collaboration for one or more of our product candidates on acceptable terms, we might elect to delay or scale back the development and commercialization of the product candidate in order to preserve our financial resources or to allow us adequate time to develop the required physical resources and systems and expertise ourselves.
The risks in a collaboration agreement include the following:
- •
- the collaborator may not apply the expected financial resources, efforts, or required expertise in developing the physical resources and systems necessary to successfully develop and commercialize a product candidate;
27
- •
- the collaborator may not invest in the development of a sales and marketing force and the related infrastructure at levels that ensure
that sales of the product candidate reach their full potential;
- •
- we may be required to undertake the expenditure of substantial operational, financial, and management resources;
- •
- we may be required to issue equity securities that would dilute our existing shareholders' percentage ownership;
- •
- we may be required to assume substantial actual or contingent liabilities;
- •
- strategic partners could decide to move forward with a competing product developed either independently or in collaboration with
others, including our competitors;
- •
- disputes may arise between us and a collaborator that delay the development or commercialization or adversely affect the sales or
profitability of the product candidate; or
- •
- the collaborator may independently develop, or develop with third parties, products that could compete with our product candidates.
In addition, a collaborator for one or more of our product candidates may have the right to terminate the collaboration at its discretion. Any termination may require us to seek a new collaborator, which we may not be able to do on a timely basis, if at all, or require us to delay or scale back our development and commercialization efforts. The occurrence of any of these events could adversely affect the development and commercialization of our product candidates and materially harm our business and stock price by delaying the development of our product candidates, and the sale of any products that may be approved by the FDA or other regulatory agencies, by slowing the growth of such sales, by reducing the profitability of the product and/or by adversely affecting the reputation of the product.
We expect to depend upon third-party distributors and resellers for a significant portion of our sales.
We expect to rely primarily upon sales through independent distributors and resellers. While we are highly dependent upon acceptance of our product candidates and solutions by such third parties and their active marketing and sales efforts relating to our product candidates and solutions, most of our distributors and resellers will not be obligated to deal with us exclusively and are not contractually subject to minimum purchase requirements. In addition, some of our distributors and resellers may sell competing products or solutions. As a result, our distributors and resellers may give higher priority to products or services of our competitors, thereby reducing their efforts in selling our product candidates and services.
There can be no assurance that such distributors and resellers will act as effective sales agents for us, that they will remain our partners, or that, if we terminate or lose any of them, we will be successful in replacing them. Any such disruption in our distribution channels could adversely affect our business, operating results, and financial condition.
Risks Related to Commercialization of Our Product Candidates
We have limited experience in producing our core components and product candidates, and if we are unable to manufacture our core components and products in high-quality commercial quantities successfully and consistently to meet demand, our growth will be limited.
We have experience manufacturing only limited quantities of rhCollagen, the plant-based recombinant human type I collagen used in our product candidates. Our manufacturing capabilities will need to be further improved to meet the standard requirements for future clinical studies and for commercialization of our product candidates. To manufacture our rhCollagen in quantities that we believe will be sufficient to produce our end products and meet anticipated market demand, we will
28
need to increase manufacturing capacity, which will involve significant challenges. In addition, the development of commercial-scale, regulation-compliant manufacturing capabilities will require us to invest substantial additional funds and hire and retain the technical personnel who have the necessary manufacturing experience. We may not successfully complete any required increase to existing manufacturing processes in a timely manner, or at all. Our costs will be higher, and our challenges greater, if we decide to develop internal manufacturing capabilities to produce our end products.
If there is a disruption to our internal manufacturing operations, we will have no other means of production for the components and products from such operations until we restore the affected facilities or develop alternative manufacturing facilities, which would delay our clinical trials or cause us to be unable to meet commercial demand for our product candidates. In such case, we may need to arrange for third-party manufacturing of our components and product candidates, which would be expensive and time-consuming, assuming we can identify an appropriate third party manufacturer. Additionally, any damage to or destruction of our facilities or equipment may significantly impair our ability to manufacture our components and products on a timely basis.
If we are unable to produce our product candidates in sufficient quantities to meet anticipated customer demand, our revenues, business, and financial prospects would be harmed. The lack of experience we have in producing commercial quantities of our components and product candidates may also result in quality issues and product recalls. Any product recall could be expensive and generate negative publicity, which could impair our ability to market our product candidates and further affect our results of operations. Manufacturing delays related to quality control could negatively impact our ability to bring our technologies to market, harm our reputation, and decrease our revenues.
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell any of our product candidates that obtain regulatory approval, we may be unable to generate any revenue.
We have no experience selling and marketing our product candidates or any other products. To successfully commercialize our product candidates we will need to develop these capabilities, either on our own or with others. We are seeking to enter into commercial alliances with third-party collaborators and distributors to utilize their marketing and distribution capabilities, but we may be unable to do so on favorable terms, if at all. If any future collaboration or distribution partners do not commit sufficient resources to commercialize our future product candidates, and if we are unable to develop the necessary marketing capabilities on our own, we will be unable to generate sufficient product revenue to sustain our business. We will be competing with many companies that currently have extensive and well-funded marketing and sales operations. Without an internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies or successfully commercialize any of our product candidates.
We face competition and rapid technological change and the possibility that our competitors may develop therapies/product candidates that are more advanced or effective than ours, which could impair our ability to successfully commercialize our product candidates.
We operate in the regenerative medicine field, which is rapidly changing. We have competitors both in the United States and internationally, including major multinational pharmaceutical companies, biotechnology companies, medical technology companies, and universities and other research institutions.
Many of our potential competitors have substantially greater financial, technical and other resources, such as larger research and development staff and experienced marketing and manufacturing organizations. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our potential
29
competitors may succeed in developing, acquiring, or licensing on an exclusive basis, products that are more effective or less costly than any products that we may develop, or achieve earlier patent protection, regulatory approval, product commercialization, and market penetration than us. Additionally, technologies developed by others may render our potential products uneconomical or obsolete, and we may not be successful in marketing our product candidates against competitors.
We are not aware of any competitors that produce collagen from plants or that produce recombinant type I human collagen. However, our collagen-based product candidates will compete with alternative solutions; for example, our VergenixSTR product candidate will compete with companies that sell PRP kits. Our VergenixFG product candidate will compete with companies that produce and market animal collagen-based products and collagen products produced from skin donations. Our VergenixBVF product platform will compete with products that combine a recombinant bone growth protein and bovine-based collagen and with allograft products.
The commercial success of any current or future product, if approved, will depend upon the degree of market acceptance by physicians, patients, third-party payors and others in the medical community.
Even if we obtain the requisite regulatory approvals, the commercial success of our product candidates will depend in part on the medical community, patients, and third-party payors accepting our product candidates as medically useful, cost-effective, and safe. Any product that we bring to the market may not gain market acceptance by physicians, patients, third-party payors, and others in the medical community. If these product candidates do not achieve an adequate level of acceptance, we may not generate significant product revenue and may not become profitable. The degree of market acceptance of these product candidates, if approved for commercial sale, will depend on a number of factors, including:
- •
- the cost, safety, efficacy, and convenience of our product candidates in relation to alternative treatments and products;
- •
- the ability of third parties to enter into relationships with us without violating their existing agreements;
- •
- the effectiveness of our sales and marketing efforts;
- •
- the prevalence and severity of any side effects, including any limitations or warnings contained in a product's approved labeling;
- •
- the prevalence and severity of any side effects resulting from the procedure by which our product candidates are administered;
- •
- the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies;
- •
- the strength of marketing and distribution support for, and timing of market introduction of, competing products;
- •
- publicity concerning our product candidates or competing products and treatments; and
- •
- sufficient third-party insurance coverage or reimbursement.
Even if a potential product displays a favorable safety and efficacy profile in clinical trials, market acceptance of the product will not be known until after it is launched. Our efforts to educate the medical community and third-party payors on the benefits of the products may require significant resources and may never be successful. Such efforts to educate the marketplace may require more resources than are required by conventional technologies.
30
A variety of risks associated with international operations could harm our business.
If any of our product candidates are approved for commercialization, it is our current intention to market them on a worldwide basis, either alone or in collaboration with third parties. In addition, we may conduct development activities in various jurisdictions throughout the world. We expect that we will be subject to additional risks related to engaging in international operations, including:
- •
- different regulatory requirements for product approval in foreign countries;
- •
- reduced protection for intellectual property rights;
- •
- unexpected changes in tariffs, trade barriers, and regulatory requirements;
- •
- economic weakness, including inflation, or political instability in particular foreign economies and markets;
- •
- compliance with tax, employment, immigration, and labor laws for employees living or traveling abroad;
- •
- foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident
to doing business in another country;
- •
- workforce uncertainty in countries where labor unrest is more common than in the United States and Israel;
- •
- production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and
- •
- business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters including earthquakes, typhoons, floods, and fires.
The insurance coverage and reimbursement status of newly approved products is uncertain. Failure to obtain or maintain adequate coverage and reimbursement for any of our product candidates that are approved could limit our ability to market those product candidates and compromise our ability to generate revenue.
The availability of reimbursement by governmental and private payors is essential for most patients to be able to afford expensive treatments. Sales of our product candidates will depend substantially, both in Europe and in the United States, on the extent to which the costs of our product candidates will be paid by health maintenance, managed care, pharmacy benefit, and similar healthcare management organizations, or reimbursed by government health administration authorities, private health coverage insurers, and other third-party payors. If reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our product candidates. Even if we obtain coverage for our product candidates, third-party payors may not establish adequate reimbursement amounts, which may reduce the demand for, or the price of, our product candidates. If reimbursement is not available or is available only to limited levels, we may not be able to commercialize certain of our product candidates.
Furthermore, publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If reimbursement of our product candidates is unavailable or limited in scope or amount, or if pricing is set at unacceptable levels, we or our partner may elect not to commercialize our product candidates in such countries, and our business and financial condition could be adversely affected.
Promotion of off-label uses of our product candidates by physicians could adversely affect our business.
Any regulatory approval of our product candidates is limited to those specific indications for which our product candidates have been deemed safe and effective by the regulatory authorities. In addition, any new indication for an approved product also requires regulatory approval. If we produce an approved therapeutic product, we will rely on physicians to use and administer it as we have directed
31
and for the indications described on the labeling. It is not, however, uncommon for physicians to use in unapproved, or "off-label," uses or in a manner that is inconsistent with the manufacturer's directions. To the extent such off-label uses and departures from our administration directions become pervasive and produce results such as reduced efficacy or other adverse effects, the reputation of our product candidates in the marketplace may suffer. In addition, off-label uses may cause a decline in our revenue or potential revenue, to the extent that there is a difference between the prices of our product for different indications.
Furthermore, while physicians may choose to use our product candidates for off-label uses, our ability to promote the products is limited to those indications that are specifically approved by the regulators. Although regulatory authorities generally do not regulate the behavior of physicians, they do restrict communications by companies with respect to off-label use. If our promotional activities fail to comply with these regulations or guidelines, we may be subject to warnings from, or enforcement action by, these authorities. In addition, failure to follow regulation authorities rules and guidelines relating to promotion and advertising can result in the regulation authorities refusal to approve a product, the suspension or withdrawal of an approved product from the market, product recalls, fines, disgorgement of money, operating restrictions, injunctions, or criminal prosecution.
Risks Related to Our Business Operations
Our future success depends on our ability to retain key employees, consultants, and advisors and to attract, retain and motivate qualified personnel.
We are dependent on principal members of our executive team listed under "Management" in this prospectus, the loss of whose services may adversely impact the achievement of our objectives. While we have entered into employment agreements with each of our executive officers, any of them could leave our employment at any time, as all of our employees are "at will" employees. Recruiting and retaining other qualified employees, consultants, and advisors for our business, including scientific and technical personnel, will also be critical to our success. There is currently a shortage of skilled executives in our industry, which is likely to continue. As a result, competition for skilled personnel is intense and the turnover rate can be high. We may not be able to attract and retain personnel on acceptable terms given the competition among numerous medical device companies for individuals with similar skill sets. In addition, failure to succeed in clinical trials may make it more challenging to recruit and retain qualified personnel. The inability to recruit or loss of the services of any executive, key employee, consultant, or advisor may impede the progress of our research, development and commercialization objectives.
Our collaborations with outside scientists and consultants may be subject to restriction and change.
We work with medical experts, chemists, biologists, and other scientists at academic and other institutions, and consultants who assist us in our research, development, and regulatory efforts, including the members of our scientific advisory board. In addition, these scientists and consultants have provided, and we expect that they will continue to provide, valuable advice regarding our programs and regulatory approval processes. These scientists and consultants are not our employees and may have other commitments that would limit their future availability to us. If a conflict of interest arises between their work for us and their work for another entity, we may lose their services. In addition, we are limited in our ability to prevent them from establishing competing businesses or developing competing products. For example, if a key scientist acting as a principal investigator in any of our clinical trials identifies a potential product that is more scientifically interesting to his or her professional interests, his or her availability to remain involved in our clinical trials could be restricted or eliminated.
32
We will need to expand our organization and we may experience difficulties in managing this growth, which could disrupt our operations.
As of March 31, 2015, we had 34 employees. As we mature and undertake the activities required to advance our product candidates into later stage clinical development and to operate as a public company in the United States, we expect to expand our full-time employee base and to hire more consultants and contractors. Our management may need to divert a disproportionate amount of its attention away from our day-to-day activities and devote a substantial amount of time to managing these growth activities. We may not be able to effectively manage the expansion of our operations, which may result in weaknesses in our infrastructure, operational setbacks, loss of business opportunities, loss of employees, and reduced productivity among remaining employees. Our expected growth could require significant capital expenditures and may divert financial resources from other projects, such as the development of additional product candidates. If our management is unable to effectively manage our growth, our expenses may increase more than expected, our ability to generate or grow revenue could be compromised, and we may not be able to implement our business strategy. Our future financial performance and our ability to commercialize product candidates and compete effectively will depend, in part, on our ability to effectively manage any future growth.
Our employees, principal investigators, consultants, and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, principal investigators, consultants, and commercial partners. Misconduct by these parties could include intentional failures to comply with regulations, provide accurate information to European regulatory authorities, FDA and other regulatory authorities, comply with healthcare fraud and abuse laws and regulations, report financial information or data accurately, or disclose unauthorized activities to us. In particular, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing, and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and cause serious harm to our reputation. We have adopted a code of conduct applicable to all of our employees, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of significant fines or other sanctions.
We face potential product liability, and, if successful claims are brought against us, we may incur substantial liability and costs. If the use of our product candidates harms patients, or is perceived to harm patients even when such harm is unrelated to our product candidates, our regulatory approvals could be revoked or otherwise negatively impacted and we could be subject to costly and damaging product liability claims.
The use of our product candidates in clinical trials and the sale of any products for which we obtain marketing approval exposes us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, medical device companies, or others that sell or otherwise come into contact with our product candidates. There is a risk that our product candidates may induce adverse events. If we cannot successfully defend against product liability claims,
33
we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
- •
- impairment of our business reputation;
- •
- withdrawal of clinical trial participants;
- •
- costs due to related litigation;
- •
- distraction of management's attention from our primary business;
- •
- substantial monetary awards to patients or other claimants;
- •
- the inability to commercialize our product candidates;
- •
- decreased demand for our product candidates, if approved for commercial sale; and
- •
- impairment of our ability to obtain product liability insurance coverage.
We currently carry human clinical trials liability insurance of $3,000,000 for each clinical trial. We will acquire product liability insurance before commercializing our product candidates. We believe our clinical trials liability insurance coverage is sufficient in light of our current clinical programs; however, we may not be able to obtain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to product liability. If we obtain marketing approval for any products, we intend to obtain insurance coverage to include the sale of commercial products, but we may not be able to obtain product liability insurance on commercially reasonable terms or in adequate amounts. On occasion, large judgments have been awarded in class action lawsuits based on medical treatments that had unanticipated adverse effects. A successful product liability claim or series of claims brought against us could cause our ADS price to decline and, if judgments exceed our insurance coverage, could materially and adversely affect our financial position.
Our development of rhCollagen relies upon the continued availability of tobacco plants, and any interruption in availability or supply of tobacco plants may delay production and adversely affect commercial utilization of our rhCollagen-based product candidates, if any such product candidates are approved and marketed in the future.
Our product candidates are all based on our recombinant human collagen extracted from tobacco plants. Any disruption to the supply of tobacco plants or any change in its availability for use would delay our production of collagen and adversely affect commercial utilization of our product candidates, if any such product candidates are approved and marketed in the future.
The occurrence of severe adverse weather conditions or crop diseases may have a potentially devastating impact upon our tobacco production. The effect of severe adverse weather conditions or the occurrence and effect of crop disease may reduce yields in our plants or require higher levels of investment to maintain yields, even when only a portion of the crop is damaged. We cannot assure you that severe future adverse weather conditions will not adversely impact our operating results and financial condition. Although some crop diseases are treatable, the cost of treatment is high, and we cannot assure that such events in the future will not adversely affect our operating results and financial condition.
If our existing rhCollagen production site is damaged or destroyed, or production at this facility is otherwise interrupted, our business and prospects would be negatively affected.
We currently have a single, small-scale production site in Israel where we manufacture rhCollagen. If our existing production facility, or the equipment in it, is damaged or destroyed, we likely would not be able to quickly or inexpensively replace our production capacity. Any new facility needed to replace our existing production facility would need to comply with the necessary regulatory requirements and be tailored to our production requirements and processes. We would need regulatory approval before using any products manufactured at a new facility in clinical trials or selling any products that are ultimately approved. Such an event could delay our clinical trials or, if any of our product candidates are approved by the regulator, reduce or eliminate our product sales.
34
If we fail to comply with environmental, health, and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse impact on the success of our business.
We are subject to numerous environmental, health, and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment, and disposal of hazardous materials and wastes. Our operations involve the use of hazardous materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain workers' compensation insurance to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials or other work-related injuries, this insurance may not provide adequate coverage against potential liabilities. In addition, we may incur substantial costs in order to comply with current or future environmental, health, and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations also may result in substantial fines, penalties, or other sanctions.
We may use our financial and human resources to pursue a particular research program or product and fail to capitalize on programs or products that may be more profitable or for which there is a greater likelihood of success.
Because we have limited resources, we may forego or delay pursuit of opportunities with certain programs or products or for indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs for products may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product, we may relinquish valuable rights to that product through strategic collaboration, licensing, or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product, or we may allocate internal resources to a product in a therapeutic area in which it would have been more advantageous to enter into a collaboration arrangement.
We are subject to foreign currency exchange risk, and fluctuations between the U.S. dollar and the NIS, the Euro, and other non-U.S. currencies may adversely affect our earnings and results of operations.
We currently operate in two different currencies. While the NIS is our functional and reporting currency and investments in our share capital have been denominated in NIS, our financial results may be adversely affected by fluctuations in currency exchange rates as a significant portion of our operating expenses, including development and manufacturing expenses, are denominated in U.S. dollars.
We are exposed to the risks that the U.S. dollar may appreciate relative to the NIS, In such event, the dollar-denominated results of operations would be adversely affected. We cannot predict any future trends in the rate of inflation in Israel or the rate of devaluation (if any) of the NIS against the dollar. For example, the average exchange rate of the dollar against the NIS increased in 2014 and decreased in 2013. Market volatility and currency fluctuations may limit our ability to cost-effectively hedge against our foreign currency exposure. Hedging strategies may not eliminate our exposure to foreign exchange rate fluctuations and may involve costs and risks of their own, such as devotion of management time, external costs to implement the strategies, and potential accounting implications. Foreign currency fluctuations, independent of the performance of our underlying business, could lead to materially adverse results or could lead to positive results that are not repeated in future periods.
35
Risks Related to Our Intellectual Property
We have an extensive worldwide patent portfolio. The cost of maintaining our patent protection is high and maintaining our patent protection requires continuous review and compliance in order to maintain worldwide patent protection. We may not be able to effectively maintain our intellectual property position throughout the major markets of the world.
The U.S. Patent and Trademark Office, or U.S. PTO, and foreign patent authorities require maintenance fees and payments as well as continued compliance with a number of procedural and documentary requirements. Non-compliance may result in abandonment or lapse of the subject patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance may result in reduced royalty payments for lack of patent coverage in a particular jurisdiction from our collaboration partners or may result in competition, either of which could have a material adverse effect on our business.
We have made, and will continue to make, certain strategic decisions in balancing costs and the potential protection afforded by the patent laws of certain countries. As a result, we may not be able to prevent third parties from practicing our inventions in all countries throughout the world, or from selling or importing products made using our inventions in and into the United States or other countries. Third parties may use our technologies in territories in which we have not obtained patent protection to develop their own products and, further, may infringe our patents in territories which provide inadequate enforcement mechanisms, even if we have patent protection. Such third party products may compete with our product candidates, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
If we are unable to obtain or protect intellectual property rights related to our product candidates, we may not be able to obtain exclusivity for our product candidates or prevent others from developing similar competitive products.
We rely upon a combination of granted patents, pending patent applications, trade secret protection, and confidentiality agreements to protect the intellectual property related to our product candidates. The strength of patents in the field of regenerative medicine involves complex legal and scientific questions and can be uncertain. The patent applications that we own may fail to result in issued patents with claims that cover our product candidates in the United States or in other countries. There is no assurance that all of the potentially relevant prior art relating to our patents and patent applications has been found, which can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue and even if such patents cover our product candidates, third parties may challenge their validity, enforceability, or scope, which may result in the patent claims being narrowed or invalidated. Furthermore, even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property, provide exclusivity for our product candidates, or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties.
Our ability to attract third parties to collaborate with us to develop products and our ability to commercialize future products may be adversely affected if the patent applications we hold with respect to our techniques or product candidates fail to issue, if the breadth or strength of our patent protection is threatened, or if our patent portfolio fails to provide meaningful exclusivity for our product candidates. Third parties may challenge their validity or enforceability of our patents or patents that issue in the future from our patent applications, which may result in such patents being narrowed, invalidated, or held unenforceable. Even if our patents and patent applications are not challenged by third parties, they may not prevent others from designing around our claims and may not otherwise adequately protect our product candidates. If the breadth or strength of protection provided by the patents and patent applications we hold with respect to our product candidates is threatened, our ability to commercialize our product candidates may be adversely effected.
36
Discoveries are generally published in the scientific literature well after their actual development, and patent applications in the United States and other countries are typically not published until 18 months after filing and in some cases are never published. Therefore, we cannot be certain that we were the first to make the inventions claimed in our owned granted patents or patent applications, or that we were the first to file for patent protection covering such inventions. Subject to meeting other requirements for patentability, for United States patent applications filed prior to March 16, 2013, the first to invent the claimed invention is entitled to receive patent protection for that invention while, outside the United States, the first to file a patent application encompassing the invention is entitled to patent protection for the invention. In addition, patents have a limited lifespan. In the United States, the expiration of a patent is generally 20 years from the earliest non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Once the patent life has expired for a product, we may be open to competition from third party products, including products that are copies of our products. This risk is material in light of the length of the development process of our product candidates and lifespan of our current patent portfolio.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect our proprietary know-how and other proprietary information that is not patentable or that we elect not to patent. For example, many of our discovery, development, and manufacturing processes involve proprietary know-how, information, or technology that is not covered by patents. We seek to protect our trade secrets and proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors, and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. Security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. Although we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors, and any third parties who have access to our proprietary know-how, information, or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed, that our trade secrets and other confidential proprietary information will not be disclosed, or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret. In addition, others may independently discover our trade secrets and proprietary information. For example, the FDA, as part of its Transparency Initiative, is currently considering whether to make additional information publicly available on a routine basis, including information that we may consider to be trade secrets or other proprietary information, and it is not clear at the present time how the FDA's disclosure policies may change in the future, if at all.
Further, the laws of some countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and in other countries. If we are unable to prevent material disclosure of the non-patented intellectual property related to our technologies to third parties, and there is no guarantee that we will have any such enforceable trade secret protection, we may not be able to establish or maintain a competitive advantage in our market.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There is a substantial amount of litigation, both within and outside
37
the United States, involving patents and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions, and inter partes review proceedings before the U.S. PTO, and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are pursuing development technologies. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture, or methods for treatment related to the use or manufacture of our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our product candidates may be accused of infringing. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of any of our product candidates or any final product itself, the holders of any such patents may be able to block our ability to commercialize such product unless we obtained a license under the applicable patents, or until such patents expire. Similarly, if any third-party patents were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture, or methods of use, the holders of any such patents may be able to block our ability to develop and commercialize the applicable product unless we obtained a license or until such patent expires. In either case, such a license may not be available on commercially reasonable terms or at all.
The patent landscape in competitive product areas is highly complex and there may be patents of third parties of which we are unaware that may result in claims of infringement. Accordingly, there can be no assurance that our product candidates do not infringe proprietary rights of third parties. Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates. Defense of such claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of financial and employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys' fees for willful infringement, pay royalties, redesign our infringing products, or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure.
We intend, if necessary, to vigorously enforce our intellectual property in order to protect the proprietary position of our product candidates. Active efforts to enforce our patents may include litigation, post-grant patent challenges, administrative proceedings, or all of the foregoing, depending on the potential benefits that might be available from those actions and the costs associated with undertaking those efforts against third parties. We review and monitor publicly available information regarding products that may be competitive with our product candidates and intend to assert our intellectual property rights where appropriate.
We may enter into license agreements with third parties, and if we fail to comply with our obligations in such agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose license rights that are important to our business.
We may need to obtain licenses from third parties to advance our research or allow commercialization of our product candidates. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we may be required to expend
38
significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected products.
We may be involved in lawsuits or administrative proceedings to obtain, protect or enforce our patents, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents. To counter infringement or unauthorized use, we may be required to file an infringement suit, which can be expensive and time-consuming. In addition, in an infringement proceeding, the defendant may file a countersuit, challenging the validity or enforceability of our patent. In that case, a court may decide that a patent of ours is not valid, is unenforceable, or is not infringed, or it may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
We may not be able to prevent misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights.
We may be involved in interference proceedings in the U.S. PTO that are provoked by third parties or provoked by us when there appears to be the same subject matter claimed in our patents or patent applications and the third parties' patents or patent applications, in order to determine the priority of inventions. An unfavorable outcome could require us to cease using the related technology, to lose our patent claims partially or in entirety, or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Our defense of interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the trading price of our ordinary shares or ADSs.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications are prosecuted and also affect patent litigation. The United States Patent and Trademark Office, or U.S. PTO, has developed regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions which were enacted March 16, 2013. However, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. The Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. We may become involved in post-grant proceedings challenging our patents or the patents of others, and the outcome of any such proceedings are highly uncertain. An unfavorable outcome in any such proceedings could reduce the scope of, or invalidate, our patent rights, allow third parties to commercialize our technology and compete directly with us, or result in our inability to manufacture, develop, or commercialize our product candidates without infringing the patent rights of others.
39
We may be subject to claims that our employees, consultants, or independent contractors have wrongfully used or disclosed confidential information of third parties or, that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Certain of our employees and personnel are or were previously employed at universities, medical institutions, or other biotechnology or pharmaceutical companies. Although we try to ensure that our employees, consultants, and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants, or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee's former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees. Furthermore, universities or medical institutions who employ some of our key employees and personnel in parallel to their engagement by us may claim that intellectual property developed by such person is owned by the respective academic or medical institution under the respective institution intellectual property policy or applicable law.
We may become subject to claims for remuneration or royalties for assigned service invention rights by our employees, which could result in litigation and adversely affect our business.
A significant portion of our intellectual property has been developed by our employees in the course of their employment for us. Under the Israeli Patent Law, 5727-1967, or the Patent Law, inventions conceived by an employee during the term and as part of the scope of his or her employment with a company are regarded as "service inventions," which belong to the employer, absent a specific agreement between the employee and employer giving the employee service invention rights. The Patent Law also provides that if there is no such agreement between an employer and an employee, the Israeli Compensation and Royalties Committee, or the Committee, a body constituted under the Patent Law, shall determine whether the employee is entitled to remuneration for his inventions. Recent decisions by the Committee (which have been upheld by the Israeli Supreme Court on appeal) have created uncertainty in this area, as it held that employees may be entitled to remuneration for their service inventions despite having specifically waived any such rights. Further, the Committee has not yet determined the method for calculating this remuneration nor the criteria or circumstances under which an employee's waiver of his right to remuneration will be disregarded. We generally enter into assignment-of-invention agreements with our employees pursuant to which such individuals assign to us all rights to any inventions created in the scope of their employment or engagement with us. Although our employees have agreed to assign to us service invention rights, we may face claims demanding remuneration in consideration for assigned inventions. As a consequence of such claims, we could be required to pay additional remuneration or royalties to our current or former employees, or be forced to litigate such claims, which could negatively affect our business.
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators, or other third parties have an ownership interest in our patents or other intellectual property. Ownership disputes may arise in the future, for example, from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on
40
our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Obtaining and maintaining our patent protection require compliance with various procedural, document submissions, fee payment, and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees, and various other governmental fees on patents and applications are and will be due to be paid to the U.S. PTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and applications. The U.S. PTO and various non-U.S. governmental patent agencies require compliance with a number of procedural, documentary, fee payment, and other similar provisions during the patent application process. There are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction.
Issued patents covering our product candidates could be found invalid or unenforceable if challenged in court or in administrative proceedings.
If we initiate legal proceedings against a third party to enforce a patent covering one of our product candidates, the defendant may contend that the patent covering our product is invalid, unenforceable, or fails to cover the product or the infringing product. In patent litigation in the United States, defendants commonly allege that asserted patent claims are invalid and unenforceable. Grounds for a validity challenge could be an alleged failure to meet one or more of several statutory requirements, including lack of novelty, obviousness, lack of written description, indefiniteness, and non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the U.S. PTO, or made a misleading statement, during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, and equivalent proceedings in foreign jurisdictions, such as opposition proceedings. Such proceedings could result in revocation, amendments to our patent claims, or statements being made on the record such that our claims may no longer be construed to cover our product candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity, unenforceability, or non-infringement, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Even if resolved in our favor, litigation, or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. As further described below, we previously prevailed in an administrative challenge initiated by Fibrogen, Inc., a major biopharmaceutical company regarding our intellectual property rights, maintaining our intellectual property in all relevant scope, and will continue to protect and enforce our intellectual property rights. Moreover, third parties may continue to initiate new proceedings in the United States and foreign jurisdictions to challenge our patents from time to time.
41
In addition, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the market price of our ordinary shares or ADSs. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities.
An issued patent covering our product was administratively challenged by Fibrogen Inc., and the outcome of the challenge may result in our patent being revoked or amended.
Our European Patent No. 1 809 751 entitled "Collagen Producing Plants and Methods of Generating and Using Same," was granted by the European Patent Office, or EPO, on September 1, 2010. On June 1, 2011, Fibrogen, Inc. initiated an opposition proceeding with the EPO, seeking revocation of the patent in its entirety on the grounds that the claims were not supported by the contents of the patent, were not novel, and were not inventive. On January 22, 2013, the EPO issued is decision to maintain the patent in amended form with claims that cover genetically modified plants that produce collagen.
On June 3, 2013, Fibrogen, Inc. appealed the decision. On August 1, 2013, we filed an appeal, seeking to expand the scope of the patent. While we believe that we will prevail in our appeal and that Fibrogen's appeal will be rejected, the ultimate outcome of these proceedings remain uncertain, and final resolution of the proceeding may take a number of years and result in substantial costs to us.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our product candidates.
As is the case with other companies in our industry, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biotechnology industry involve both technological and legal complexity, and is therefore is costly, time-consuming, and inherently uncertain. In addition, in recent years the United States enacted and implemented wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in some situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents that had already been granted. The patent laws and regulations may changes in unpredictable ways through actions of the U.S. Congress, the federal courts, and the U.S. PTO, in the future, and any changes may adversely affect our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting, and defending patents on products in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Potential competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as in the United States. These products may compete with our product candidates, if approved, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
42
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Intellectual property rights do not address all potential threats to any competitive advantage we may have.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and intellectual property rights may not adequately protect our business or permit us to maintain our competitive advantage. The following examples are illustrative:
- •
- Others may be able to make products that are the same as or similar to our current or future products but that are not covered by the
claims of the patents that we own or have exclusively licensed.
- •
- We or any of our licensors or strategic partners might not have been the first to make the inventions covered by the issued patent or
pending patent application that we own or have exclusively licensed.
- •
- We or any of our licensors or strategic partners might not have been the first to file patent applications covering certain of our
inventions.
- •
- Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our
intellectual property rights.
- •
- The prosecution of our pending patent applications may not result in granted patents.
- •
- Granted patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or
unenforceable, as a result of legal challenges by our competitors.
- •
- Patent protection on our product candidates may expire before we are able to develop and commercialize the product, or before we are
able to recover our investment in the product.
- •
- Our competitors might conduct research and development activities in the United States and other countries that provide a safe harbor from patent infringement claims for such activities, as well as in countries in which we do not have patent rights, and may then use the information learned from such activities to develop competitive products for sale in markets where we intend to market our product candidates.
43
Risks Related to the Offering and Ownership of Our ADSs
The market price of our ADSs may be highly volatile, and you may not be able to resell your ADSs at or above the initial public offering price.
Prior to this offering, there has not been a public market in the United States for our ordinary shares, and an active market has not developed for our ADSs, which have been quoted on the OTCQX since March 2015. The initial public offering price of our ADSs in this offering will be based, in part, on the price of our ordinary shares on the Tel Aviv Stock Exchange, or the TASE, and on the price of our ADSs on the OTCQX, as well as on negotiations between us and the representative of the underwriters, which may not be indicative of prices that will prevail in the trading market. An active trading market for our ADSs may not develop following this offering. You may not be able to sell your ADSs quickly or at the market price if trading in our ADSs is not active.
The market price of our ADSs is likely to be volatile. Our ADS price could be subject to wide fluctuations in response to a variety of factors, including the following:
- •
- adverse results or delays in preclinical studies or clinical trials;
- •
- reports of adverse events in other similar products or clinical trials of such products;
- •
- inability to obtain additional funding;
- •
- any delay in filing a regulatory submission for any of our product candidates and any adverse development or perceived adverse
development with respect to the FDA's review or European authorities review of that regulatory submission;
- •
- failure to develop successfully and commercialize our product and future product;
- •
- failure to enter into strategic collaborations;
- •
- failure by us or strategic collaboration partners to prosecute, maintain, or enforce our intellectual property rights;
- •
- changes in laws or regulations applicable to future products;
- •
- inability to scale up our manufacturing capabilities (including in Israel), inability to obtain adequate product supply for our
product candidates, or the inability to do so at acceptable prices;
- •
- adverse regulatory decisions, including by the OCS under the Research Law;
- •
- introduction of new products, services, or technologies by our competitors;
- •
- failure to meet or exceed financial projections we may provide to the public;
- •
- failure to meet or exceed the financial expectations of the investment community;
- •
- the perception of the biotechnology industry by the public, legislatures, regulators, and the investment community;
- •
- announcements of significant acquisitions, strategic partnerships, joint ventures, or capital commitments by us or our competitors;
- •
- disputes or other developments relating to proprietary rights, including patents, litigation matters, and our ability to obtain patent
protection for our technologies;
- •
- additions or departures of key scientific or management personnel;
- •
- significant lawsuits, including patent or shareholder litigation;
- •
- changes in the market valuations of similar companies;
44
- •
- sales of our ordinary shares or ADSs by us or our shareholders in the future; and
- •
- trading volumes of our ordinary shares and ADSs.
In addition, companies trading in the stock market in general, and medical device companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our ordinary shares, regardless of our actual operating performance.
As a new investor, you will experience immediate dilution in the book value of any ADSs you purchase.
Because the initial public offering price per ADS being offered is substantially higher than our net tangible book value per ADS, you will suffer immediate and substantial dilution in the net tangible book value of any ADSs you purchase in this offering. Consequently, if you purchase ADSs in this offering at an initial public offering price of $ per ADS, you will incur immediate dilution of $ per ADS. In addition, you may experience further dilution to the extent that additional ordinary shares are issued upon exercise of outstanding options and warrants. If the underwriters exercise their over-allotment option, you may experience additional dilution. For further information regarding the dilution resulting from this offering, please see the section entitled "Dilution" in this prospectus.
We will incur additional significant costs as a result of the listing of the ADSs for trading on the NASDAQ Capital Market and thereby becoming a public company subject to SEC reporting requirements in the United States, and our management will be required to devote substantial additional time to new compliance initiatives as well as to compliance with ongoing United States and Israeli reporting requirements.
In addition to the costs associated with being an Israeli public company, upon the successful completion of this offering and the listing of our ADSs on the NASDAQ Capital Market, we will become a publicly reporting company in the United States. As a U.S. public reporting company, we will incur additional significant accounting, legal and other expenses that we did not incur before the offering. We also anticipate that we will incur costs associated with corporate governance requirements of the SEC and the NASDAQ Capital Market. We expect these rules and regulations to increase our legal and financial compliance costs, introduce new costs such as investor relations, stock exchange listing fees and shareholder reporting, and to make some activities more time consuming and costly. Our management and other personnel will need to devote substantial time to these compliance requirements; in addition, the implementation of such compliance processes and systems may require us to hire outside consultants and incur other significant costs. Any future changes in the laws and regulations affecting public companies in the United States and the rules and regulations adopted by the SEC and the NASDAQ Capital Market, for so long as they apply to us, will result in increased costs to us as we respond to such changes. These laws, rules, and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, on our board committees, if any, or as executive officers.
Our securities will be traded on more than one market or exchange and this may result in price variations.
Our ordinary shares have been trading on the TASE since May 2010, and our ADSs have been quoted on the OTCQX since March 2015. In conjunction with this offering, we will apply to list our ADSs on NASDAQ. Trading in ordinary shares and ADSs, as applicable, on these markets will take place in different currencies (U.S. dollars on NASDAQ and NIS on the TASE), and at different times
45
(resulting from different time zones, trading days, and public holidays in the United States and Israel). The trading prices of our shares on these two markets may differ due to these and other factors. Any decrease in the price of our ordinary shares on the TASE could cause a decrease in the trading price of our ADSs on NASDAQ.
Our principal shareholders and management own a significant percentage of our ordinary shares and will be able to exert significant control over matters subject to shareholder approval.
As of March 31, 2015, our executive officers, directors, five percent or more shareholders and their affiliates beneficially owned approximately 37.55% of our voting shares and, upon closing of this offering, that same group will beneficially own approximately % of our outstanding voting shares. These shareholders will have the ability to control us through their ownership positions. These shareholders may be able to determine all matters requiring shareholder approval. For example, these shareholders, if they were to act together, may be able to control elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our ordinary shares that you may believe are in your best interest as one of our shareholders.
We are an "emerging growth company" and a "foreign private issuer," and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies and foreign private issuers will make our ADSs less attractive to investors.
We are an "emerging growth company," as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements, extended transition periods for adopting new or revised accounting standards, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our ordinary shares held by non-affiliates exceeds $700.0 million as of any June 30 before that time or if we have total annual gross revenue of $1.0 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31 or, if we issue more than $1.0 billion in non-convertible debt during any three-year period before that time, we would cease to be an emerging growth company immediately.
Furthermore, as a foreign private issuer, we are not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. Under the Securities Exchange Act of 1934, or the Exchange Act, we will be subject to reporting obligations that, in certain respects, are less detailed and less frequent than those of U.S. domestic reporting companies. For example, we will not be required to issue proxy statements that comply with the requirements applicable to U.S. domestic reporting companies. We will also have four months after the end of each fiscal year to file our annual reports with the SEC and will not be required to file current reports as frequently or promptly as U.S. domestic reporting companies. Furthermore, our officers, directors, and principal shareholders will be exempt from the requirements to report transactions in our equity securities and from the short-swing profit liability provisions contained in Section 16 of the Exchange Act. These exemptions and leniencies, along with other corporate governance exemptions resulting from our ability to rely on home country rules, will reduce the frequency and scope of information and protections to which you may otherwise have been eligible in relation to a U.S. domestic reporting companies. See "Management—Corporate Governance Practices" for more information.
46
We cannot predict if investors will find our ADSs less attractive because we may rely on these reduced requirements. If some investors find our ADSs less attractive as a result, there may be a less active trading market for our ADSs and our share price may be more volatile.
Sales of a substantial number of our ordinary shares or ADSs in the public market could cause our share price to fall.
If our existing shareholders sell, indicate an intention to sell or the market perceives that they intend to sell, substantial amounts of our securities, either on the TASE or on the NASDAQ after this offering, the market price of our securities could decline significantly. On an as-converted basis as of March 31, 2015, upon the closing of this offering, we will have outstanding a total of ordinary shares, all of which are registered and available for sale in Israel, assuming no exercise of the underwriters' option to purchase additional shares. As of the date of this prospectus, the ADSs being sold in this offering, plus any ADSs sold upon exercise of the underwriters' option to purchase additional shares, will be freely tradable, without restriction, on NASDAQ immediately following this offering, assuming that current shareholders do not purchase ADSs in this offering. publicly held ordinary shares are freely tradable as of the date of this prospectus, and substantially all of the ordinary shares will be available for sale on TASE beginning 180 days from the date of this prospectus following the expiration of lock-up agreements between our executive officers, directors, shareholders, and option holders and the underwriters. Ladenburg Thalmann may, however, in their discretion, permit our officers, directors, and other shareholders who are subject to these lock-up agreements to sell shares prior to the expiration of the lock-up agreements.
After the lock-up agreements expire, based upon the number of ordinary shares, on an as-converted basis, outstanding as of March 31, 2015 up to an additional ordinary shares will be eligible for sale in the public market, of which shares are held by directors, executive officers, and other affiliates and will be subject to Rule 144 under the Securities Act of 1933, as amended, or the Securities Act.
In addition, as of March 31, 2015, an aggregate of 106,300,606 ordinary shares that are either subject to outstanding options or subject to outstanding warrants will become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules, the lock-up agreements and Rule 144 and Rule 701 under the Securities Act. If these additional ordinary shares are sold, or if it is perceived that they will be sold, in the public market, the market price of our ordinary shares could decline.
Future sales and issuances of our securities or rights to purchase securities, including pursuant to our equity incentive plans, could result in additional dilution of the percentage ownership of our shareholders and could cause the prices of our securities to fall.
Additional capital will be needed in the future to continue our planned operations. To the extent we raise additional capital by issuing equity securities, our shareholders may experience substantial dilution. We may sell ordinary shares, ADSs, convertible securities, or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell ordinary shares, ADSs, convertible securities, or other equity securities in one or more transactions, existing investors may be materially diluted by subsequent sales, and new investors could gain rights superior to our existing shareholders.
Pursuant to our Share Ownership and Option Plan (2010), our management is authorized to grant share options and other equity-based awards to our employees, directors, and consultants. As of March 31, 2015, our employees, officers, and consultants hold 17,963,346 options. If our board of directors elects to increase the number of shares available for future grant by the maximum amount
47
each year, our shareholders may experience additional dilution, which could cause our share price to fall.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering, including for any of the purposes described in the section entitled "Use of Proceeds," and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. The failure by our management to apply these funds effectively could harm our business. Pending their use, we may invest the net proceeds from this offering in investment-grade, interest-bearing securities. These investments may not yield a favorable return to our shareholders.
We do not intend to pay dividends on our securities, so any returns will be limited to the value of our shares.
We have never declared or paid any cash dividends on our share capital. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any return to shareholders will therefore be limited to the appreciation of their shares. In addition, Israeli law limits our ability to declare and pay dividends, and may subject our dividends to Israeli withholding taxes. As a result, investors in the ADSs or ordinary shares will not be able to benefit from owning these securities unless their market price becomes greater than the price paid by such investors and they are able to sell such securities. We cannot assure you that you will ever be able to resell our securities at a price in excess of the price paid.
In the event we make distributions or dividends, you may not receive the same distributions or dividends as those we make to the holders of our ordinary shares, and, in some limited circumstances, you may not receive dividends or other distributions, or receive any value for them, if it is illegal or impractical to make them available to you.
The depositary for the ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on ordinary shares or other deposited securities underlying the ADSs, after deducting its fees and expenses. You will receive these distributions in proportion to the number of ordinary shares your ADSs represent. However, the depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any holders of ADSs. For example, it would be unlawful to make a distribution to a holder of ADSs if it consists of securities that require registration under the Securities Act, but that are not properly registered or distributed under an applicable exemption from registration. In addition, conversion into U.S. dollars from foreign currency that was part of a dividend made in respect of deposited ordinary shares may require the approval or license of, or a filing with, any government or agency thereof, which may be unobtainable. In these cases, the depositary may determine not to distribute such property and hold it as "deposited securities" or may seek to effect a substitute dividend or distribution, including net cash proceeds from the sale of the dividends that the depositary deems an equitable and practicable substitute. We have no obligation to register under U.S. securities laws any ordinary shares, rights, or other securities made available through such distributions. We also have no obligation to take any other action to permit the distribution of ADSs, ordinary shares, rights, or anything else to holders of ADSs. In addition, the depositary may withhold from such dividends or distributions its fees and an amount on account of taxes or other governmental charges to the extent the depositary believes it is required to make such withholding. This means that you may not receive the same distributions or dividends as those we make to the holders of our ordinary shares, and, in some limited circumstances, you may not receive any
48
value for such distributions or dividends if it is illegal or impractical for us to make them available to you. These restrictions may cause a material decline in the value of the ADSs.
Holders of ADSs must act through the depositary to exercise their rights.
Holders of the ADSs do not have the same rights of our shareholders and may only exercise the voting rights with respect to the underlying ordinary shares in accordance with the provisions of the deposit agreement for the ADSs. Under Israeli law, the minimum notice period required to convene a shareholders meeting is no less than 35 or 21 calendar days, depending on the proposals on the agenda for the shareholders meeting. When a shareholder meeting is convened, holders of the ADSs may not receive sufficient notice of a shareholders' meeting to permit them to withdraw their ordinary shares to allow them to cast their vote with respect to any specific matter. In addition, the depositary and its agents may not be able to send voting materials to holders of the ADSs or carry out their voting instructions in a timely manner. We will make all reasonable efforts to cause the depositary to extend voting rights to holders of the ADSs in a timely manner, but we cannot assure holders that they will receive the voting materials in time to ensure that they can instruct the depositary to vote their ADSs. Furthermore, the depositary and its agents will not be responsible for any failure to carry out any instructions to vote, for the manner in which any vote is cast or for the effect of any such vote. As a result, holders of the ADSs may not be able to exercise their right to vote, and they may lack recourse if their ADSs are not voted as they requested. In addition, in the capacity as a holder of ADSs, they will not be able to call a shareholders' meeting.
You may be subject to limitations on transfer of your ADSs.
Your ADSs are transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer, or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deems it advisable to do so because of any requirement of law or of any government or governmental body or for any other reason in accordance with the terms of the deposit agreement. See the section of this prospectus titled "Description of American Depositary Shares."
Your percentage ownership in us may be diluted by future issuances of share capital, which could reduce your influence over matters on which shareholders vote.
Following the completion of this offering, our board of directors will have the authority, in most cases without action or vote of our shareholders, to issue all or any part of our authorized but unissued shares, including ordinary shares issuable upon the exercise of outstanding warrants and options. Issuances of additional shares would reduce your influence over matters on which our shareholders vote.
If equity research analysts do not publish research reports about our business or if they issue unfavorable commentary or downgrade our ADSs, the price of our ADSs could decline.
The trading market for our ADSs will rely in part on the research and reports that equity research analysts publish about us and our business. The price of our ADSs could decline if we do not obtain research analyst coverage or if one or more securities analysts downgrade our ADSs, issue other unfavorable commentary, or cease publishing reports about us or our business.
49
Risks Related to Our Incorporation and Operations in Israel
We are a "foreign private issuer" and intend to follow certain home country corporate governance practices, and our shareholders may not have the same protections afforded to shareholders of companies that are subject to all NASDAQ corporate governance requirements.
As a foreign private issuer, we will be permitted, and intend, to follow certain home country corporate governance practices instead of those otherwise required under the NASDAQ Stock Market for domestic U.S. issuers. For instance, we intend to follow home country practice in Israel with regard to the quorum requirement for shareholder meetings. As permitted under the Israeli Companies Law, 5759-1999, or the Companies Law, our articles of association provide that the quorum for any meeting of shareholders shall be the presence of at least two shareholders present in person, by proxy, or by a voting instrument, who hold at least 25% of the voting power of our shares. We may in the future (or may be required to) elect to follow home country practices in Israel (and consequently avoid the requirements that would otherwise apply to a U.S. company listed on The NASDAQ Capital Market) with regard to other matters, as well, such as the formation of compensation, nominating, and governance committees, separate executive sessions of independent directors and non-management directors, and the requirement to obtain shareholder approval for certain dilutive events (such as for the establishment or amendment of certain equity-based compensation plans, issuances that will result in a change of control of the company, certain transactions other than a public offering involving issuances of a 20% or more interest in the company, and certain acquisitions of the stock or assets of another company), amending our compensation policy from time to time, and the approval of certain interested-parties transactions. Following our home country governance practices as opposed to the requirements that would otherwise apply to a U.S. company listed on The NASDAQ Capital Market may provide less protection to you than what is accorded to investors under the NASDAQ Stock Market rules applicable to domestic U.S. issuers. See "Management—Corporate Governance Practices" for more information.
In addition, as a foreign private issuer, we will be exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements, including the requirement for an emerging growth company to disclose the compensation of the chief executive officer and other two highest compensated executive officers on an individual, rather than aggregate, basis. As long as our securities are traded on TASE and to the extent that we are not required to report under Israeli reporting regulations pursuant to the Israeli Securities Law, we will be required to disclose under the Companies Law, in the notice for our next annual meeting of shareholders at the latest, the annual compensation of our five most highly compensated officers on an individual, rather than aggregate, basis. However, this disclosure will not be as extensive as that required of a U.S. domestic issuer.
We would lose our foreign private issuer status if a majority of our directors or executive officers are U.S. citizens or residents, and we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. Although we have elected to comply with certain U.S. regulatory provisions, our loss of foreign private issuer status would make such provisions mandatory. The regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic reporting company may be significantly higher. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic reporting company forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. We may also be required to modify certain of our policies to comply with accepted governance practices associated with U.S. domestic reporting companies. Such conversion and modifications will involve additional costs. In addition, we may lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers.
50
Potential political, economic, and military instability in the State of Israel, where the majority of our senior management and our research and development facilities are located, may adversely impact our results of operations.
We are incorporated under Israeli law and our offices and operations are located in the State of Israel. In addition, our employees, officers, and all but two of our directors are residents of Israel. Accordingly, political, economic, and military conditions in Israel directly affect our business. Since the State of Israel was established in 1948, a number of armed conflicts have occurred between Israel and its neighboring countries. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its present trading partners, or a significant downturn in the economic or financial condition of Israel, could adversely impact our operations. Since October 2000, there have been increasing occurrences of terrorist violence. Ongoing and revived hostilities or other Israeli political or economic factors could harm our operations, product development and results of operations.
Although Israel has entered into various agreements with Egypt, Jordan, and the Palestinian Authority, there has been an increase in unrest and terrorist activity, which began in October 2000 and has continued with varying levels of severity. The establishment in 2006 of a government in the Palestinian Authority by representatives of the Hamas militant group has created additional unrest and uncertainty in the region. In 2006, a conflict between Israel and the Hezbollah in Lebanon resulted in thousands of rockets being fired from Lebanon up to 50 miles into Israel. Starting in December 2008, for approximately three weeks, Israel engaged in an armed conflict with Hamas in the Gaza Strip, which involved missile strikes against civilian targets in various parts of Israel and negatively affected business conditions in Israel. In November 2012, for approximately one week, Israel experienced a similar armed conflict, resulting in hundreds of rockets being fired from the Gaza Strip and disrupting most day-to-day civilian activity in southern Israel. Most recently, in July 2014, Israel yet again experienced rocket strikes against civilian targets in various parts of Israel, as part of an armed conflict commenced between Israel and Hamas. If continued or resumed, these hostilities may negatively affect business conditions in Israel in general and our business in particular. Our insurance policies do not cover us for the damages incurred in connection with these conflicts or for any resulting disruption in our operations. The Israeli government, as a matter of law, provides coverage for the reinstatement value of direct damages that are caused by terrorist attacks or acts of war; however, the government may cease providing such coverage or the coverage might not be enough to cover potential damages. In the event that hostilities disrupt the ongoing operation of our facilities or the airports and seaports on which we depend to import and export our supplies and product candidates, our operations may be materially adversely affected.
In addition, since the end of 2010, numerous acts of protest and civil unrest have taken place in several countries in the Middle East and North Africa, many of which involved significant violence. The civil unrest in Egypt, which borders Israel, resulted in the resignation of its president Hosni Mubarak, and to significant changes to the country's government. In Syria, also bordering Israel, a civil war is continuing to take place. The ultimate effect of these developments on the political and security situation in the Middle East and on Israel's position within the region is not clear at this time. Such instability may lead to deterioration in the political and trade relationships that exist between the State of Israel and certain other countries.
Popular uprisings in various countries in the Middle East and North Africa are affecting the political stability of those countries. Such instability may lead to deterioration in the political and trade relationships that exist between the State of Israel and these countries. Several countries, principally in the Middle East, still restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies if hostilities in Israel or political instability in the region continues or increases. Any hostilities involving Israel, interruption or curtailment of trade between Israel and its present trading partners, or significant downturns in the economic or financial condition of Israel could adversely affect our operations and product
51
development and adversely affect our share price. Similarly, Israeli companies are limited in conducting business with entities from several countries. For instance, in 2008, the Israeli legislature passed a law forbidding any investments in entities that transact business with Iran.
In addition, Iran has threatened to attack Israel and is widely believed to be developing nuclear weapons. Iran is also believed to have a strong influence among extremist groups in the region, such as Hamas in Gaza, Hezbollah in Lebanon, and various rebel militia groups in Syria. Additionally, a violent jihadist group named Islamic State of Iraq and Levant (ISIL) is involved in hostilities in Iraq and Syria and has been growing in influence. Although ISIL's activities have not directly affected the political and economic conditions in Israel, ISIL's stated purpose is to take control of the Middle East, including Israel. These situations may potentially escalate in the future to more violent events which may affect Israel and us. Any armed conflicts, terrorist activities, or political instability in the region could adversely affect business conditions and could harm our results of operations and could make it more difficult for us to raise capital. Parties with whom we do business may decline to travel to Israel during periods of heightened unrest or tension, forcing us to make alternative arrangements when necessary in order to meet our business partners face to face. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements. Further, in the past, the State of Israel and Israeli companies have been subjected to economic boycotts. Several countries still restrict business with the State of Israel and with Israeli companies. These restrictive laws and policies may have an adverse impact on our operating results, financial condition, or the expansion of our business.
Our operations may be disrupted by the obligations of personnel to perform military service.
As of March 31, 2015, we had 34 employees, all of whom were based in Israel. Some of our employees may be called upon to perform up to 36 days (and in some cases more) of annual military reserve duty until they reach the age of 40 (and in some cases, up to 45 or older) and, in emergency circumstances, could be called to immediate and unlimited active duty. In the event of severe unrest or other conflict, individuals could be required to serve in the military for extended periods of time. Since September 2000, in response to increased tension and hostilities, there have been occasional call-ups of military reservists, including in connection with the 2006 conflict in Lebanon, and the December 2008, November 2012 and July 2014 conflicts with Hamas, and it is possible that there will be additional call-ups in the future. Our operations could be disrupted by the absence of a significant number of our employees related to military service or the absence for extended periods of one or more of our key employees for military service. Such disruption could materially adversely affect our business and results of operations. Additionally, the absence of a significant number of the employees of our Israeli suppliers and contractors related to military service or the absence for extended periods of one or more of their key employees for military service may disrupt their operations.
The tax benefits that are available to us if and when we generate taxable income require us to meet various conditions and may be prevented or reduced in the future, which could increase our costs and taxes.
If and when we generate taxable income, we may be eligible for certain tax benefits provided to "Preferred Enterprises" under the Israeli Law for the Encouragement of Capital Investments, 5719-1959, as amended, or the Investment Law. The benefits that may be available to us under the Investment Law are subject to the fulfillment of conditions stipulated in the Investment Law. Further, in the future these tax benefits may be reduced or discontinued. If these tax benefits are reduced, cancelled, or discontinued, our Israeli taxable income would be subject to regular Israeli corporate tax rates. The standard corporate tax rate for Israeli companies is 26.5% for 2014 and thereafter. Additionally, if we increase our activities outside of Israel through acquisitions, for example, our expanded activities might not be eligible for inclusion in future Israeli tax benefit programs. See
52
"Taxation and Government Programs—Israeli Tax Considerations and Government Programs—Law for the Encouragement of Capital Investments, 5719-1959."
It may be difficult to enforce a U.S. judgment against us, our officers and directors, and the Israeli experts named in this prospectus in Israel or the United States, or to assert U.S. securities laws claims in Israel or serve process on our officers and directors and these experts.
We were incorporated in Israel, and our corporate headquarters and substantially all of our operations are located in Israel. All of our executive officers and all but two of our directors, and the Israeli experts named in this prospectus, are located in Israel. All of our assets are located outside the United States. Therefore, it may be difficult for an investor, or any other person or entity, to enforce a U.S. court judgment based upon the civil liability provisions of the U.S. federal securities laws against us or any of these persons in a U.S. or Israeli court, or to effect service of process upon these persons in the United States. Additionally, it may be difficult for an investor, or any other person or entity, to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws against us or our officers and directors on the grounds that Israel is not the most appropriate forum in which to bring such a claim. Even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel addressing the matters described above. See "Enforceability of Civil Liabilities."
Your rights and responsibilities as our shareholder will be governed by Israeli law, which may differ in some respects from the rights and responsibilities of shareholders of U.S. corporations.
Since we are incorporated under Israeli law, the rights and responsibilities of our shareholders are governed by our articles of association and Israeli law. These rights and responsibilities differ in some material respects from the rights and responsibilities of shareholders of U.S. corporations. In particular, a shareholder of an Israeli company has a duty to act in good faith and in a customary manner in exercising its rights and performing its obligations towards the company and other shareholders and to refrain from abusing its power in the company, including, among other things, in voting at the general meeting of shareholders on certain matters, such as an amendment to the company's articles of association, an increase of the company's authorized share capital, a merger of the company, and approval of related party transactions that require shareholder approval. A shareholder also has a general duty to refrain from discriminating against other shareholders. In addition, a controlling shareholder or a shareholder who knows that it possesses the power to determine the outcome of a shareholder vote or to appoint or prevent the appointment of an officer of the company has a duty to act in fairness towards the company with regard to such vote or appointment. However, Israeli law does not define the substance of this duty of fairness. There is limited case law available to assist us in understanding the nature of this duty or the implications of these provisions. These provisions may be interpreted to impose additional obligations and liabilities on our shareholders that are not typically imposed on shareholders of U.S. corporations. See "Management—Approval of Related Party Transactions under Israeli Law—Shareholders' Duties."
Provisions of Israeli law and our amended and restated articles of association could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our shareholders.
Israeli law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for transactions involving directors, officers, or significant shareholders and regulates other matters that may be relevant to such types of transactions. For
53
example, a tender offer for all of a company's issued and outstanding shares can only be completed if the acquirer receives positive responses from the holders of at least 95% of the issued share capital. Completion of the tender offer also requires approval of a majority of the offerees that do not have a personal interest in the tender offer, unless at least 98% of the company's outstanding shares are tendered. Furthermore, the shareholders, including those who indicated their acceptance of the tender offer (unless the acquirer stipulated in its tender offer that a shareholder that accepts the offer may not seek appraisal rights), may, at any time within six months following the completion of the tender offer, petition an Israeli court to alter the consideration for the acquisition. See "Description of Our Ordinary Shares—Acquisitions under Israeli Law" for additional information.
Further, Israeli tax considerations may make potential transactions undesirable to us or to some of our shareholders whose country of residence does not have a tax treaty with Israel granting tax relief to such shareholders from Israeli tax. For example, Israeli tax law does not recognize tax-free share exchanges to the same extent as U.S. tax law. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of a number of conditions, including, in some cases, a holding period of two years from the date of the transaction during which sales and dispositions of shares of the participating companies are subject to certain restrictions. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no disposition of the shares has occurred.
We have received Israeli government grants for certain research and development expenditures. The terms of these grants and loans may require us to satisfy specified conditions in order to manufacture products and transfer technologies outside of Israel. In addition, under the Encouragement of Industrial, Research and Development Law, 5744-1984, or the Research Law, to which we are subject due to our receipt of grants from the Office of the Chief Scientist of the Israeli Ministry of Economy, or OCS, a recipient of OCS grants such as us must receive the approval of the applicable authority of the OCS regarding any change of control or any change in the holding of the means of control of our Company which transforms any non-Israeli citizen or resident into an "interested party", as defined in the Research Law, in our Company, and in the latter event, the non-Israeli citizen or resident shall execute an undertaking in favor of the OCS, in a form provided under the OCS guidelines.
We expect to be classified as a passive foreign investment company for U.S. federal income tax purposes, and our U.S. shareholders may suffer adverse tax consequences as a result.
Generally, if, for any taxable year, either, at least 75% of our gross income is passive income (including our pro-rata share of the gross income of our 25% or more-owned corporate subsidiaries), or at least 50% of the average value of our assets (including our pro-rata share of the assets of our 25% or more-owned corporate subsidiaries) is attributable to assets that produce passive income or are held for the production of passive income, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. Passive income generally includes dividends, interest, and gains from disposition of passive assets and rents and royalties.
If we are characterized as a PFIC for any taxable year (or portion thereof) that is included in the holding period of a U.S. holder (as defined below) of our securities, our U.S. shareholders may suffer adverse tax consequences, including increased U.S. federal income tax liability upon a sale or other disposition of our securities or the receipt of certain excess distributions from the loss of the preferential rate applicable to dividends received on our ordinary shares, interest charges that apply to distributions by us, and additional reporting requirements. See "Taxation—Material U.S. Federal Income Tax Consequences—Passive Foreign Investment Company Consequences."
54
Our status as a PFIC may also depend, in part, on how quickly we utilize the cash proceeds from this offering in our business. Since PFIC status depends on the composition of our income and the composition and value of our assets (which, assuming we are not a CFC for the year being tested, may be determined in large part by reference to the market value of our ordinary shares, which may be volatile) from time to time, there can be no assurance that we will not be considered a PFIC for any taxable year. However, because we had no revenue-producing operations to date, we believe that we were a PFIC for our 2014 taxable year. Unless and until we generate sufficient revenue from sales and other non-passive sources and otherwise satisfy the asset test above, we expect to be treated as a PFIC.
U.S. investors are urged to consult their own tax advisors regarding the possible application of the PFIC rules. For more information, see "Taxation—Material U.S. Federal Income Tax Consequences—Passive Foreign Investment Company Consequences."
Our facilities in Israel are subject to local Business Licensing and Planning and Building regulations and we may be subject to fines if not complied with.
Under the Israeli Licensing of Businesses Law, to which our production site and offices and laboratories are subject, operating a business without a license or temporary permit is a criminal offense. We have a business license for our laboratories and offices, in effect until December 31, 2019. We are also examining the possibility of obtaining a business license for our plant growth and production site at Yessod Hama'ala. To date we have no valid business license for this site.
Our production sites and laboratories are subject to the Israeli Planning and Building Law, which sets provisions and obligations, inter alia, regarding the licensing process for a new building, including building permits, non-conforming use and easements, the supervision over its construction, and the required occupancy permits. According to the Planning and Building Law, work or use of land without a permit, where such permit is required, a deviation from the permit granted, or use of agricultural land in violation of the law constitute criminal offenses. As stated above, to date we have no valid business license for our site in Yessod Ha'maala, which may subject us to criminal procedures.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements made under "Prospectus Summary," "Risk Factors," "Use of Proceeds," "Management's Discussion and Analysis of Financial Condition and Results of Operations," "Business" and elsewhere in this prospectus constitute forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as "may," "will," "should," "expects," "plans," "project," "anticipates," "believes," "estimates," "predicts," "potential" "intends" or "continue," or the negative of these terms or other comparable terminology.
These forward-looking statements may include, but are not limited to, statements relating to our objectives, plans and strategies, statements that contain projections of results of operations or of financial condition, statements relating to the research, development and use of our products, and all statements (other than statements of historical facts) that address activities, events, or developments that we intend, expect, project, believe, or anticipate will or may occur in the future.
Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties. We have based these forward-looking statements on assumptions and assessments made by our management in light of their experience and their perception of historical trends, current conditions, expected future developments, and other factors they believe to be appropriate.
Important factors that could cause actual results, developments and business decisions to differ materially from those anticipated in these forward-looking statements include, among other things:
- •
- the overall global economic environment;
55
- •
- the impact of competition and new technologies;
- •
- general market, political, and economic conditions in the countries in which we operate;
- •
- projected capital expenditures and liquidity;
- •
- changes in our strategy;
- •
- government regulations and approvals;
- •
- litigation and regulatory proceedings; and
- •
- those factors referred to in "Risk Factors," "Business," and "Management's Discussion and Analysis of Financial Condition and Results of Operations," as well as in this prospectus generally.
These statements are only current predictions and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry's actual results, levels of activity, performance, or achievements to be materially different from those anticipated by the forward-looking statements. We discuss many of these risks in this prospectus in greater detail under the heading "Risk Factors" and elsewhere in this prospectus. You should not rely upon forward-looking statements as predictions of future events.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, or achievements. Except as required by law, we are under no duty to update or revise any of the forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this prospectus.
56
The following table sets forth information regarding the exchange rates of U.S. dollars per NIS for the periods indicated. Average rates are calculated by using the daily representative rates as reported by the Bank of Israel on the last day of each month during the periods presented.
| |
NIS per U.S. dollar | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Year Ended December 31,
|
High | Low | Average | Period End | |||||||||
2014 |
3.994 | 3.402 | 3.592 | 3.889 | |||||||||
2013 |
3.791 | 3.471 | 3.600 | 3.471 | |||||||||
2012 |
4.084 | 3.700 | 3.843 | 3.733 | |||||||||
2011 |
3.821 | 3.363 | 3.581 | 3.821 | |||||||||
2010 |
3.894 | 3.549 | 3.732 | 3.549 | |||||||||
The following table sets forth the high and low daily representative rates for the NIS as reported by the Bank of Israel for each of the prior six months.
| |
NIS per U.S. dollar | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Month Ended
|
High | Low | Average | Period End | |||||||||
May 2015 (through May 19, 2015) |
3.890 | 3.819 | 3.856 | 3.852 | |||||||||
April 2015 |
4.014 | 3.861 | 3.938 | 3.861 | |||||||||
March 2015 |
4.053 | 3.926 | 3.998 | 3.980 | |||||||||
February 2015 |
3.966 | 3.844 | 3.893 | 3.966 | |||||||||
January 2015 |
3.998 | 3.899 | 3.946 | 3.924 | |||||||||
December 2014 |
3.994 | 3.889 | 3.935 | 3.889 | |||||||||
PRICE RANGE OF OUR ORDINARY SHARES
Our ordinary shares have traded on the TASE under the symbol "CLPT" since May 2010. As of December 31, 2014, we had 241,392,352 ordinary shares outstanding, excluding, as of such date, 17,963,346 ordinary shares issuable upon the exercise of options and 88,337,260 ordinary shares issuable upon the exercise of outstanding warrants.
The following table shows the annual, quarterly and monthly ranges of the high and low per share sale price for our ordinary shares as reported by the TASE in NIS and U.S. dollars. U.S. dollar
57
amounts per ordinary share are provided using the U.S. dollar representative rate of exchange on the date to which the high or low market price is applicable, as reported by the Bank of Israel.
| |
NIS Price Per Ordinary Share |
U.S. Dollar Price Per Ordinary Share |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
High | Low | High | Low | |||||||||
Annual: |
|||||||||||||
2014 |
0.30 | 0.15 | 0.08 | 0.04 | |||||||||
2013 |
0.41 | 0.20 | 0.12 | 0.05 | |||||||||
2012 |
0.61 | 0.36 | 0.16 | 0.09 | |||||||||
2011 |
1.31 | 0.43 | 0.37 | 0.12 | |||||||||
2010 |
1.66 | 1.00 | 0.46 | 0.26 | |||||||||
Quarterly: |
|||||||||||||
Second Quarter 2015 (through May 19, 2015) |
0.48 | 0.36 | 0.12 | 0.09 | |||||||||
First Quarter 2015 |
0.52 | 0.22 | 0.13 | 0.06 | |||||||||
Fourth Quarter 2014 |
0.23 | 0.15 | 0.06 | 0.04 | |||||||||
Third Quarter 2014 |
0.26 | 0.22 | 0.08 | 0.06 | |||||||||
Second Quarter 2014 |
0.28 | 0.24 | 0.08 | 0.07 | |||||||||
First Quarter 2014 |
0.30 | 0.25 | 0.08 | 0.07 | |||||||||
Fourth Quarter 2013 |
0.41 | 0.29 | 0.12 | 0.08 | |||||||||
Third Quarter 2013 |
0.35 | 0.20 | 0.10 | 0.06 | |||||||||
Second Quarter 2013 |
0.31 | 0.22 | 0.09 | 0.06 | |||||||||
First Quarter 2013 |
0.39 | 0.31 | 0.10 | 0.08 | |||||||||
Fourth Quarter 2012 |
0.52 | 0.36 | 0.13 | 0.09 | |||||||||
Third Quarter 2012 |
0.54 | 0.44 | 0.13 | 0.11 | |||||||||
Second Quarter 2012 |
0.57 | 0.45 | 0.15 | 0.12 | |||||||||
First Quarter 2012 |
0.61 | 0.38 | 0.16 | 0.10 | |||||||||
Most Recent Six Months: |
|||||||||||||
May 2015 (through May 19, 2015) |
0.48 | 0.43 | 0.12 | 0.11 | |||||||||
April 2015 |
0.45 | 0.36 | 0.11 | 0.09 | |||||||||
March 2015 |
0.51 | 0.35 | 0.13 | 0.09 | |||||||||
February 2015 |
0.51 | 0.34 | 0.13 | 0.09 | |||||||||
January 2015 |
0.33 | 0.22 | 0.08 | 0.05 | |||||||||
December 2014 |
0.21 | 0.16 | 0.05 | 0.04 | |||||||||
Since March 2015, our ADSs have been quoted on OTCQX under the symbol "CQPTY." On , 2015, the last reported bid price of our ADSs on OTCQX was $ per ADS. Assuming that our ADSs are listed for trading on the NASDAQ Capital Market, the quoting of our ADSs on OTCQX will be discontinued prior to the completion of this offering.
58
We estimate that the net proceeds to us from our issuance and sale of of our ADSs in this offering will be approximately $ , based on an assumed initial public offering price of $ per ADS, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters' option to purchase additional ADSs in this offering is exercised in full, we estimate that the net proceeds from this offering will be approximately $ , based on an assumed offering price of $ per ADS, after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
We currently expect to use the net proceeds from this offering for:
- •
- continuing the development of our product candidates, and to conduct research and development activities, estimated at approximately
$ ;
- •
- the expansion and enhancement of our manufacturing capabilities, estimated at approximately $ ;
- •
- the establishment of marketing capabilities for the European market, estimated at approximately $ ; and
- •
- the remainder for working capital and general corporate purposes, including funding the costs of operating as a public company in the United States.
These expected uses of net proceeds from this offering represent our intentions based upon our current plans and business conditions which could change in the future as our plans and business conditions evolve. The amounts and timing of our actual expenditures may vary significantly and will depend upon numerous factors, including the progress of our development and commercialization efforts, the status of and results from our clinical trials and preclinical studies, whether or not we enter into strategic collaborations or partnerships, the amount of cash available from other sources, and our operating costs and expenditures. Accordingly, our management will have significant flexibility and broad discretion in applying the net proceeds of this offering.
We expect proceeds from this offering to meet our capital requirements for approximately three years.
Pending these uses, we intend to invest the net proceeds in low-risk, high-quality, investment-grade instruments, certificates of deposit, or direct or guaranteed obligations of the U.S. government or other governments, or hold as cash.
We have no current commitments or binding agreements with respect to any material acquisition of or investment in any technologies, products, or companies other than our own plans and business.
59
Since our merger in 2010 with CollPlant Ltd., we have not declared or paid any cash dividends on our ordinary shares and do not anticipate paying any cash dividends in the foreseeable future. We currently intend to retain future earnings, if any, for use in the operation of our business and to fund future growth. Payment of cash dividends, if any, in the future will depend on our financial condition, operating results, contractual restrictions, capital requirements, business prospects, and other factors our board of directors may deem relevant.
If we do decide to declare or pay any cash dividend, the depositary has agreed to pay the ADS holders the dividends it receives, after deducting its fees and expenses. See "Description of American Depositary Shares—Dividends and Other Distributions."
The Israeli Companies Law imposes further restrictions on our ability to declare and pay dividends. See "Description of Our Ordinary Shares—Dividend and Liquidation Rights" for additional information.
Payment of dividends may be subject to Israeli withholding taxes. See "Taxation—Israeli Tax Considerations" for additional information.
60
The following table sets forth our cash and cash equivalents and capitalization as of December 31, 2014:
- •
- on an actual basis; and
- •
- on an as adjusted basis, to reflect the issuance and sale of ADSs in this offering at an assumed initial public offering price of $ per ADS, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us as if the sale of ADSs had occurred on December 31, 2014.
The as adjusted column below is illustrative only. Our cash and cash equivalents and capitalization following the closing of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at the pricing of this offering. You should read the following table in conjunction with the sections titled "Selected Financial Data", "Management's Discussion and Analysis of Financial Condition and Results of Operations", "Description of Our Ordinary Shares", "Description Of American Depositary Shares" and our financial statements and related notes included elsewhere in this prospectus.
| |
Actual, as of December 31, 2014 (NIS in thousands) |
As adjusted, as of December 31, 2014 (NIS in thousands) |
Actual, as of December 31, 2014 (Convenience translation into USD in thousands(1)) |
As adjusted, as of December 31, 2014 (Convenience translation into USD in thousands(1)) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
Cash and cash equivalents |
11,062 | 2,845 | ||||||||
Total liabilities |
2,647 | 681 | ||||||||
Shareholders' Equity: |
||||||||||
Ordinary shares, par value NIS 0.01 per |
2,414 | 621 | ||||||||
Additional paid-in capital |
130,918 | 33,664 | ||||||||
Accumulated deficit |
(119,021 | ) | (30,605 | ) | ||||||
Total shareholders' equity |
14,311 | 3,680 | ||||||||
Total liabilities and equity |
16,958 | 4,361 | ||||||||
- (1)
- Calculated using the exchange rate reported by the Bank of Israel for December 31, 2014 at the rate of one U.S. dollar per NIS 3.889.
The outstanding share information in the table above is based on 241,392,352 ordinary shares outstanding as of December 31, 2014, excluding the following as of such date:
- •
- 17,963,346 ordinary shares issuable upon the exercise of outstanding options at a weighted average exercise price of NIS 0.56 ($0.14)
per share; and
- •
- 88,337,260 ordinary shares issuable upon the exercise of outstanding warrants at an exercise price of NIS 0.70 ($0.18) per share.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per ADS, would increase (decrease) the as adjusted amount of each of cash and cash equivalents, additional paid-in capital, total shareholders' equity (deficit), and total capitalization by approximately $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus,
61
remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
An increase (decrease) of 100,000 in the number of ADSs we are offering would increase (decrease) the as adjusted amount of cash and cash equivalents, additional paid-in capital, working capital, total shareholders' equity (deficit), and total capitalization by approximately $ million, assuming the assumed initial public offering price per ADS, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts and commissions and estimated offering expenses payable by us.
62
If you invest in our ADSs, you will experience immediate and substantial dilution to the extent of the difference between the initial public offering price of our ADSs and the pro forma as adjusted net tangible book value (deficit) per share of our ADSs immediately after the offering.
Our historical net tangible book value per share or per ADS is determined by dividing our total tangible assets, less total liabilities, by the actual number of outstanding ordinary shares or the total number of ADSs that would represent such actual number of shares based on a share-to-ADS ratio of -to-1. Our historical net tangible book value as of December 31, 2014 was $3,236,000 representing $0.013 per ordinary share or $ per ADS (using the ratio of ordinary shares to one ADS).
Our pro forma as adjusted net tangible book value as of December 31, 2014 was $ , representing $ per ordinary share or $ per ADS (using the ratio of ordinary shares to one ADS). The pro forma as adjusted net tangible book value gives effect to the sale of ADSs in this offering at an assumed initial public offering price of $ per ADS, after deducting underwriting discounts and commissions and estimated offering expenses payable by us. The difference between the initial public offering price per ADS and the pro forma as adjusted net tangible book value (deficit) per ADS represents an immediate dilution of $ per ADS to new investors purchasing ADSs in this offering.
The following table illustrates this dilution on a per ADS basis to new investors:
Assumed initial public offering price per ADS |
$ | ||||||
Historical net tangible book value per ADS before this offering, as of December 31, 2014 |
$ | ||||||
Pro forma increase in net tangible book value per ADS attributable to new investors participating in this offering |
$ | ||||||
Pro forma net tangible book value per ADS after this offering |
$ | ||||||
Dilution per ADS to new investors participating in this offering |
$ | ||||||
Percentage of dilution in net tangible book value per ADS for new investors participating in this offering |
% |
If the underwriters' over-allotment option to purchase additional ADSs from us is exercised in full, and based on an assumed initial public offering price of $ per ADS, the pro forma as adjusted net tangible book value per ADS after this offering would be approximately $ per ADS, the increase in the pro forma net tangible book value per ADS attributable to new investors would be approximately $ per ADS, and the dilution to new investors purchasing ADSs in this offering would be approximately $ per ADS.
The table below summarizes as of December 31, 2014, on the pro forma as adjusted basis described above, the number of ordinary shares we issued and sold (treating each ADS as ordinary shares), the total consideration we received and the average price per ordinary share (1) paid by our existing shareholders and (2) to be paid by new investors purchasing our ADSs in this offering at the initial public offering price of $ per ADS (treating each ADS as ordinary shares),
63
before deducting underwriting discounts and commissions and estimated offering expenses payable by us.
| |
Ordinary Shares | Total Consideration |
Average Price |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Number | Percent | Amount | Percent | Per Share | ||||||||||
Existing shareholders |
% | $ | % | $ | |||||||||||
New investors (treating each ADS as ordinary shares) |
% | $ | % | $ | |||||||||||
Total |
100.0 | % | $ | 100.0 | % | $ | |||||||||
If the underwriters' over-allotment option is exercised in full, the percentage of ordinary shares held by existing shareholders will be reduced to % of the total number of shares of our ordinary shares outstanding after this offering, and the number of shares held by new investors (treating each ADS as ordinary shares) will increase to shares, or % of the total number of ordinary shares outstanding after this offering.
The outstanding share information in the table above is based on 241,392,352 shares of ordinary shares outstanding as of December 31, 2014, excluding the following, as of such date:
- •
- 17,963,346 ordinary shares issuable upon the exercise of share options outstanding as of December 31, 2014 at a weighted
average exercise price of NIS 0.56 ($0.14) per share; and
- •
- 88,337,260 ordinary shares issuable upon the exercise of outstanding warrants at an exercise price of NIS 0.70 ($0.18) per share.
To the extent that options or warrants are exercised, new options or other share-based awards are issued, or we issue additional ordinary shares in the future, there will be further dilution to investors participating in this offering. In addition, we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our shareholders and ADS holders.
64
SELECTED CONSOLIDATED FINANCIAL DATA
You should read the following selected financial data in conjunction with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and the financial statements, related notes and other financial information included elsewhere in this prospectus.
The statement of comprehensive loss data for the years ended December 31, 2013 and 2014 and the statement of financial position data as of December 31, 2013 and 2014 are derived from our audited financial statements included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results that should be expected in the future. Our financial statements have been prepared in accordance with IFRS, as issued by the International Accounting Standards Board.
| |
Year ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | 2014 | |||||||
| |
(NIS in thousands except per share data) |
(Convenience translation into USD in thousands(1)) |
||||||||
Statement of comprehensive loss data: |
||||||||||
Research and development expenses |
16,151 | 14,879 | 3,826 | |||||||
Participation in research and development expenses |
(3,717 | ) | (5,145 | ) | (1,323 | ) | ||||
Research and development expenses, net |
12,434 | 9,734 | 2,503 | |||||||
General and administrative and marketing expenses |
3,747 | 3,906 | 1,004 | |||||||
Operating loss |
16,181 |
13,640 |
3,507 |
|||||||
Financial income |
(25 |
) |
(642 |
) |
(165 |
) |
||||
Financial expenses: |
314 | 25 | 6 | |||||||
Financial expenses (income), net |
289 |
(617 |
) |
(159 |
) |
|||||
Loss |
16,470 |
13,023 |
3,348 |
|||||||
Loss per ordinary share, basic and diluted |
0.11 |
0.05 |
0.01 |
|||||||
Weighted average ordinary shares outstanding, basic and diluted |
155,590,908 |
241,280,958 |
||||||||
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | 2014 | |||||||
| |
(NIS in thousands) |
(Convenience translation into USD in thousands(1)) |
||||||||
Statement of financial position data: |
||||||||||
Cash and cash equivalents and short-term bank deposits |
23,777 | 11,062 | 2,845 | |||||||
Total assets |
30,273 | 16,958 | 4,361 | |||||||
Total liabilities |
3,189 | 2,647 | 681 | |||||||
Total equity |
27,084 | 14,311 | 3,680 | |||||||
- (1)
- Calculated using the exchange rate reported by the Bank of Israel for December 31, 2014 at the rate of one U.S. dollar per NIS 3.889.
65
MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with the section titled "Selected Consolidated Financial Data" and our consolidated financial statements and related notes included elsewhere in this prospectus. This discussion and other parts of this prospectus contain forward-looking statements that involve risk and uncertainties, such as statements of our plans, objectives, expectations, and intentions. Our actual results could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the section titled "Risk Factors."
Overview
We are a clinical-stage regenerative medicine company focused on developing and commercializing tissue repair products, initially for the orthobiologics and advanced would care markets. Our product candidates, two of which are in registration trials, are based on our proprietary plant-based collagen technology, which we believe is the only viable technology available for the production of recombinant type I human collagen, or rhCollagen. We believe that our rhCollagen, which is identical to the type I collagen produced by the human body, has significant advantages compared to currently available tissue-derived collagen, including improved biofunctionality, superior homogeneity, and reduced risk of transmission of disease. We believe the attributes of our rhCollagen technology make it suitable for numerous tissue repair applications throughout the human body. We believe that the market opportunity for our current product candidates utilizing our rhCollagen within the orthobiologics and advanced wound care markets exceeds $5 billion.
Our first rhCollagen-based product candidate is VergenixSTR, a soft tissue repair matrix composed of our rhCollagen and platelet-rich plasma, or PRP, extracted from a patient's blood and intended for the treatment of tendinopathy. VergenixSTR is currently in a multi-center registration trial in Israel. Our second clinical product candidate, VergenixFG, is a wound-filling flowable gel made from our rhCollagen intended for treatment of deep surgical incisions and deep wounds, including diabetic ulcers, burns, bedsores, and other chronic wounds. VergenixFG is currently in a multi-center registration trial in Israel. To bring our initial two product candidates to market, we intend to first seek CE marking certification, which is required for a product to be sold within the European Union. After receiving CE marking for our initial products, we plan to request a pre-Investigational Device Exemption, or IDE, meeting with the U.S. FDA and pursue a U.S. FDA approval for our rhCollagen-based products. Our third product candidate is a preclinical product based on VergenixBVF, a product platform we are developing for bone repair indications such as spinal fusion and trauma. VergenixBVF is a novel absorbable scaffold composed of our rhCollagen and minerals, which can be charged with growth factors to help accelerate bone formation. We are collaborating with a U.S.-based corporate collaborator in the development of VergenixBVF.
Since incorporation of our wholly owned subsidiary CollPlant Ltd. in 2004, which merged with and into us in 2010, we have achieved a number of significant milestones:
- •
- From 2005 to 2011, we developed our based-plant technology, which we believe is the only viable technology available for the
production of recombinant type I human collagen, or rhCollagen.
- •
- In December 2011, we entered into a joint development agreement with Pfizer for the development of a product for the orthopedic market, comprised of a recombinant bone growth protein and our rhCollagen, along with other components. This co-development is now continuing with another U.S.-based company, which acquired the rights for commercialization of the protein from Pfizer, and our agreement with Pfizer expired. The work with Pfizer and now with the U.S.-based company promotes our strategy to position rhCollagen as the "gold
66
- •
- In December 2012, following a successful clinical trial, we received a CE mark for a predecessor wound healing product. This is the
first medical device in the world to receive a CE marking that is based on plant-derived rhCollagen.
- •
- In 2014, we completed preclinical studies with two of our product candidates, VergenixSTR and VergenixFG, and launched clinical trials
in Israel.
- •
- In March 2015, we announced successful interim results with VergenixFG. This clinical trial is necessary to support the applications required for commercialization of VergenixFG.
standard" platform technology for collagen-based products in a broad range of markets, and to commercialize our product candidates through a network of collaborative partners.
To date, we have financed our operations primarily with the net proceeds from private placements and from our public offerings of our ordinary shares on TASE, participation in product development collaborations, and government grants from the OCS.
Since our inception, we have incurred significant operating losses. Our net operating losses were NIS 16,181,000 and NIS 13,640,000 for the years ended December 31, 2013 and 2014, respectively. As of December 31, 2014, we had an accumulated deficit of NIS 119,021,000. We have not generated any revenue to date from sales of our products.
We expect to continue to incur expenses and operating losses for the foreseeable future. We expect to commence sales of VergenixSTR and VergenixFG in Europe in 2016. The net losses we incur may fluctuate significantly from quarter to quarter. We anticipate that our expenses will increase substantially if and as we:
- •
- continue our research and preclinical and clinical development of our pipeline products;
- •
- seek marketing approvals for VergenixSTR and VergenixFG and any other products in the United States and other new territories;
- •
- maintain, expand and protect our intellectual property portfolio;
- •
- hire additional operational, clinical, quality control, and scientific personnel;
- •
- establish plant infrastructure to accommodate product capacity increase;
- •
- add operational, financial and management information systems and personnel, including personnel to support our product development,
any future commercialization efforts and our transition to a public reporting company in the United States; and
- •
- identify additional product candidates.
Financial Operations Overview
Revenue
To date, we have not generated any revenue from sales of our products. We expect to start generating revenue from sales of VergenixSTR and VergenixFG in Europe in 2016. Our ability to generate revenue will depend on achieving CE marking and successful commercialization of those two products.
67
Operating Expenses
Research and development expenses
Research and development expenses consist of costs incurred for the development of both of our rhCollagen and our product candidates. Those expenses include:
- •
- employee-related expenses, including salaries and share-based compensation expenses for employees in research and development
functions;
- •
- expenses incurred in operating our laboratories and small-scale manufacturing facility;
- •
- expenses incurred under agreements with CROs and investigative sites that conduct our clinical trials;
- •
- expenses relating to outsourced and contracted services, such as external laboratories, consulting, and advisory services;
- •
- supply, development, and manufacturing costs relating to clinical trial materials;
- •
- maintenance of facilities, depreciation, and other expenses, which include direct and allocated expenses for rent and insurance; and
- •
- costs associated with preclinical and clinical activities.
Research and development activities are the primary focus of our business. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect that our research and development expenses will increase in absolute dollars in future periods as we continue to invest in research and development activities related to the development of our product candidates.
In the years 2013 and 2014, we spent approximately NIS 16,151,000 and NIS 14,879,000, respectively, on research and development of our rhCollagen technology and our product candidates, of which NIS 3,717,000 and NIS 5,145,000, respectively, were funded by participation by our strategic collaborators Pfizer and its U.S.-based successor to the commercialization rights to a recombinant bone growth protein, and government grants. We charge all research and development expenses to operations as they are incurred.
There are numerous factors associated with the successful commercialization of any of our product candidates, including future trial design and various regulatory requirements, many of which cannot be determined with accuracy at this time. Additionally, future commercial and regulatory factors beyond our control will affect our clinical development programs and plans.
Participation in Research and Development Expenses
Our research and development expenses are net of the following participations by third parties.
Participation by Pfizer and by our U.S.-based corporate collaborator. Starting in 2010, we entered into two agreements with Pfizer related to collaboration in the development of VergenixBVF, novel absorbable scaffold composed of our rhCollagen and synthetic minerals that mimics bone structure intended for use in posterolateral spinal fusion and trauma and other orthopedic applications. The product under development in our collaboration with Pfizer was VergenixBVF charged with a recombinant bone growth protein developed by Pfizer. The collaboration agreement with Pfizer expired in 2013, and since then we have continued the development of VergenixBVF under a strategic collaboration arrangement with another U.S.-based corporate collaborator that now owns the commercialization rights to this protein. This collaborator, as Pfizer before, is bearing all the research and development cost related to VergenixBVF.
68
Participation by the OCS. We have received grants from the OCS as part of the research and development programs for our rhCollagen technology and our product candidates. The requirements and restrictions for such grants are found in the Research Law. These grants are subject to repayment through future royalty payments on any products resulting from these research and development programs, including VergenixSTR and VergenixFG. Under the Research Law, and related regulations, royalties of 3% - 5% on the revenues derived from sales of products or services developed in whole or in part using these OCS grants are payable to the Israeli government. The maximum aggregate royalties paid generally cannot exceed 100% of the grants made to us, plus annual interest generally equal to the 12-month LIBOR applicable to dollar deposits, as published on the first business day of each calendar year. The total gross amount of grants actually received by us from the OCS as of December 31, 2014, totaled approximately NIS 24,700,000. As of December 31, 2014, we paid non-material royalty amounts to the OCS.
In addition to paying any royalty due, we must abide by other restrictions associated with receiving such grants under the Research Law, or the Research Law, that continue to apply following repayment to the OCS. These restrictions may impair our ability to outsource manufacturing, engage in change of control transactions, or otherwise transfer our know-how outside of Israel and may require us to obtain the approval of the OCS for certain actions and transactions and pay additional royalties and other amounts to the OCS. In addition, any change of control and any change of ownership of our ordinary shares that would make a non-Israeli citizen or resident an "interested party," as defined in the Research Law, require prior approval by the OCS. If we fail to comply with the Research Law, we may be subject to criminal charges.
Research and development grants received from the OCS are recognized upon receipt as a liability if future economic benefits are expected from the project that will result in royalty-bearing sales. The amount of the liability for the loan is first measured at fair value using a discount rate that reflects a market rate of interest that reflects the appropriate degree of risks inherent in our business. The change in the fair value of the liability associated with grants from the OCS is reflected as an increase or decrease in our research and development expenses for the relevant quarter.
Under applicable accounting rules, the grants from the OCS have been accounted for as an off-set against the related research and development expenses in our financial statements. Our balance sheet liabilities do not include obligations regarding royalties that we are obligated to pay to the OCS based on future sales of our products. As a result, our research and development expenses are shown on our financial statements net of the OCS grants. See Note 2G in our consolidated financial statements for more information.
General, administrative, and marketing expenses
Our general and administrative expenses consist principally of:
- •
- employee-related expenses, including salaries, benefits, and related expenses, including equity-based compensation expenses;
- •
- legal and professional fees for auditors and other consulting expenses not related to research and development activities;
- •
- cost of offices, communication, and office expenses;
- •
- information technology expenses; and
- •
- business development and marketing activities.
We expect that our general and administrative and marketing expenses will increase in the future as our business expands and we incur additional general and administrative costs associated with being a public company in the United States, including compliance under the Sarbanes-Oxley Act and rules
69
promulgated by the U.S. Securities and Exchange Commission. These public company-related increases will likely include costs of additional personnel, additional legal fees, audit fees, directors' liability insurance premiums, and costs related to investor relations. We as well expect that our marketing expenses will increase, as we will incur additional marketing costs associated with the commencement of sales, when and if our product candidates are approved.
Financial Income/Financial Expense
Financial income includes interest income regarding short term deposits and exchange rate differences. Financial expense consists primarily of exchange rate differences and bank commissions.
Taxes on income
We do not generate taxable income in Israel, as we have historically incurred operating losses resulting in carry forward tax losses of approximately NIS 106,500,000 as of December 31, 2014. We anticipate that we will be able to carry forward these tax losses indefinitely to future tax years assuming that we utilize them at the first opportunity. Accordingly, we do not expect to pay taxes in Israel until we have taxable income after the full utilization of our carry forward tax losses.
The standard corporate tax rate in Israel is 26.5%. Under the Investment Law, and other Israeli legislation, we may be entitled to certain additional tax benefits, including reduced tax rates, accelerated depreciation, and amortization rates for tax purposes on certain assets and amortization of other intangible property rights for tax purposes.
Results of Operations
Comparison of the years ended December 31, 2013 and 2014
The following table summarizes our results of operations for the years ended December 31, 2013 and 2014:
| |
Year ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | 2014 | |||||||
| |
(NIS in thousands) |
(Convenience translation into USD in thousands(1)) |
||||||||
Statement of comprehensive loss data: |
||||||||||
Research and development expenses |
16,151 | 14,879 | 3,826 | |||||||
Participation in research and development expenses |
(3,717 | ) | (5,145 | ) | (1,323 | ) | ||||
| | | | | | | | | | | |
Research and development expenses, net |
12,434 | 9,734 | 2,503 | |||||||
General and administrative and marketing expenses |
3,747 | 3,906 | 1,004 | |||||||
Operating loss |
16,181 | 13,640 | 3,507 | |||||||
Financial income: |
(25 | ) | (642 | ) | (165 | ) | ||||
Financial expenses: |
314 | 25 | 6 | |||||||
| | | | | | | | | | | |
Financial expenses (income), net |
289 | (617 | ) | (159 | ) | |||||
Loss |
16,470 | 13,023 | 3,348 | |||||||
- (1)
- Calculated using the exchange rate reported by the Bank of Israel for December 31, 2014 at the rate of one U.S. dollar per NIS 3.889.
70
Research and development expenses
Research and development expenses decreased 8% from NIS 16,151,000 in the year ended December 31, 2013 to NIS 14,879,000 in the year ended December 31, 2014. The expenses primarily related to the development of VergenixBVF, VergenixSTR and VergenixFG. The decrease in expenses relates to better efficiency in our process development procedures in 2014 versus 2013. Such efficiency included reductions in employee-related expenses and cost-effective use of subcontractors for the year ended December 31, 2014 compared with the same period in 2013.
The participation in the research and development expenses amounted to NIS 5,145,000 in 2014 compared to NIS 3,717,000 in 2013. The majority of the increase is due to higher OCS participation in our development plans and the participation of our U.S.-based corporate collaborator in the development of VergenixBVF.
General, administrative and marketing expenses
General, administrative, and marketing expenses increased from NIS 3,747,000 in the year ended December 31, 2013 to NIS 3,906,000 in the year ended December 31, 2014. The increase is primarily attributable to management-related compensation payments.
Financial expenses (income),net
Financial expenses (income), net, totalled NIS 289,000 in the year ended December 31, 2013 compared to financial income of NIS 617,000 in the year ended December 31, 2014. The vast majority of the change is due to exchange rate differences following an approximately 12% rise in the dollar exchange rate against the shekel, in the course of the year. Such increase resulted in financial income on our dollar currency deposits during 2014.
Liquidity and Capital Resources
To date, we have financed our operations primarily with the net proceeds from private placements and from our public offerings of our ordinary shares on TASE, participation from product development collaborations, and government grants from the OCS.
We believe that based on our current business plan, our existing cash, cash equivalents, and the net proceeds from this offering will be sufficient to meet our currently anticipated cash requirements for the next three years.
71
Cash flows
The following table summarizes our consolidated statement of cash flows for the years ended December 31, 2013 and 2014.
| |
Year ended December 31, |
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | 2014 | |||||||
| |
(NIS in thousands) |
(Convenience translation into USD in thousands(1)) |
||||||||
Net cash provided by (used in): |
||||||||||
Operating activities |
(13,244 | ) | (12,958 | ) | (3,331 | ) | ||||
Investing activities |
(397 | ) | (397 | ) | (102 | ) | ||||
Financing activities |
27,397 | 45 | 12 | |||||||
- (1)
- Calculated using the exchange rate reported by the Bank of Israel for December 31, 2014 at the rate of one U.S. dollar per NIS 3.889.
Net cash used in operating activities
The use of cash in all periods resulted primarily from our net losses adjusted for non-cash charges and measurements and changes in components of working capital. Adjustments to net income for non-cash items include depreciation and amortization and share-based compensation.
Net cash used in operating activities resulted primarily from our net losses adjusted for non-cash charges and measurements and changes in components of working capital. Adjustments to net loss for non-cash items include depreciation and amortization, equity-based compensation and exchange differences on cash and cash equivalents. This cash flow mainly reflects the cash needed for funding the product candidates and pipeline products development and management costs of the Company during the applicable periods.
Net cash used in operating activities in 2014 totaled NIS 12,958,000 and consisted primarily of net loss of NIS 13,023,000, adjusted for non-cash items including depreciation and amortization of NIS 802,000 and share based compensation of NIS 205,000, and a net increase in operating assets and liabilities of NIS 347,000, mainly attributed to decrease in trade payables of NIS 214,000 and other payables of NIS 328,000.
Net cash used in operating activities in 2013 totaled NIS 13,244,000 and consisted primarily of net loss of NIS 16,470,000, adjusted for non-cash items including depreciation and amortization of NIS 951,000, share based compensation of NIS 462,000, and a net decrease in operating assets and liabilities of NIS 1,526,000. The decrease in operating assets and liabilities is primarily attributed to the decrease in other receivables of NIS 1,301,000. A portion of this decrease represents the payments to us in 2013 by the OCS and by Pfizer for participation in research and development activities.
Net cash used in investing activities
The use of cash in investing activities primarily related to the purchases of property and equipment. Net cash used in investing activities was NIS 397,000 during the year ended December 31, 2013 and NIS 397,000 during the year ended December 31, 2014.
Net cash provided by financing activities
Net cash provided by financing activities amounted to approximately NIS 45,000 for 2014 and NIS 27,397,000 in 2013. In 2013 we consummated an equity raise in the Israeli capital market, and raised a net NIS 19,357,000 in return for the issuance of our shares and options. In addition, we raised from a strategic investor in a private placement NIS 8,040,000. Cash flow from financing activities in 2014 amounted to NIS 45,000, resulting from the exercise of options to share capital.
72
Cash and funding sources
The table below summarizes our sources of financing for the years ended December 31, 2013 and 2014.
| |
Issuance of Ordinary Shares and Warrants |
Government Grants and Strategic Collaboration |
Total | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(NIS in thousands) |
(Convenience translation into USD in thousands(1)) |
|||||||||||
Year ended December 31, 2014 |
45 | 5,145 | 5,190 | 1,335 | |||||||||
Year ended December 31, 2013 |
27,397 | 3,717 | 31,114 | ||||||||||
| | | | | | | | | | | | | | |
- (1)
- Calculated using the exchange rate reported by the Bank of Israel for December 31, 2014 at the rate of one U.S. dollar per NIS 3.889.
Funding requirements
We believe that our existing cash and cash equivalents, together with the net proceeds of this offering will enable us to fund our operating expenses and capital expenditure requirements for at least the next three years. We have based this estimate on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect.
Our present and future funding requirements will depend on many factors, including, among other things:
- •
- the progress, timing and completion of preclinical testing and clinical trials for VergenixSTR and VergenixFG or any future pipeline
product;
- •
- selling and marketing activities undertaken in connection with the anticipated commercialization of VergenixSTR and VergenixFG and any
other product candidates
- •
- costs involved in the development of distribution channels, and for an effective sales and marketing organization, for the
commercialization of our product candidates in Europe;
- •
- the time and costs involved in obtaining regulatory approval for VergenixSTR and VergenixFG and our pipeline products and any delays
we may encounter as a result of evolving regulatory requirements or adverse results with respect to any of these products;
- •
- the number of potential new products we identify and decide to develop; and
- •
- the costs involved in filing patent applications and maintaining and enforcing patents or defending against claims or infringements raised by third parties.
For more information as to the risks associated with our future funding needs, see "Risk Factors—Even if this offering is successful, we may need to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain additional capital when needed may force us to delay, limit, or terminate our product development efforts or other operations."
73
Contractual obligations and commitments
Our significant contractual obligations as of December 31, 2014 are summarized in the following table.
| |
Payments due by period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Less than 1 year |
1 to 2 years | 2 to 5 years | More than 5 years |
Total | |||||||||||
| |
(NIS) |
|||||||||||||||
Operating lease obligations(1) |
787,000 | 770,000 | 472,000 | 2,029,000 | ||||||||||||
- (1)
- Operating lease obligations consist of payments pursuant to lease agreements for office and laboratory facilities, as well as lease agreements for 6 vehicles, which generally run for a period of three years.
Our balance sheet liabilities do not include obligations regarding royalties that we are obligated to pay to the OCS based on future sales of our products. As of December 31, 2014, the maximum royalty amount that would be payable by us, before interest, is approximately NIS 24,500,000 (assuming 100% of the royalties are payable), contingent upon sales of our rhCollagen-based products.
Off-Balance Sheet Arrangements
As of December 31, 2014, we do not have any, and during the periods presented we did not have any, off-balance sheet arrangements.
Significant Accounting Estimates and Judgments
Estimates and judgments are reviewed on an ongoing basis and are based on past experience and other factors, including expectations of future events, which are considered reasonable in view of current circumstances.
Significant accounting estimate
We make estimates and assumptions with respect to the future. By nature, the accounting estimates are rarely identical to actual results. The estimate that has a significant risk of resulting in a material adjustment to carrying amounts of assets and liabilities in the next financial year is listed below.
Impairment of In Process Research and Development
We annually review the need to record impairment of IPR&D. To test for impairment, we as a whole have been identified as the smallest cash-generating unit to which the intangible asset can be attributed. Accordingly, we measure our recoverable amount as a whole. The recoverable amount is the higher of value in use and fair value less costs of disposal. In accordance with IFRS 13, the quoted market price in an active market provides the most reliable evidence of fair value. Since fair value less costs of disposal, which is based on our market price, is significantly higher than the carrying amount of the cash-generating unit, we determined that no impairment exists.
Significant judgments made when applying our accounting policy
Grants from the OCS
In accordance with the accounting treatment prescribed in Note 2G to our financial statements appearing elsewhere in this prospectus, our management is required to examine whether there is reasonable assurance that the OCS grant that was received will be repaid. In addition, if, at the date of
74
initial recognition, the grant is recognized in the statement of comprehensive income (loss), then in subsequent periods our management is required to evaluate whether the payment of royalties to the OCS is considered to be more likely than not. In such a case, a liability would be recognized based on our best estimate of the amount required to settle our royalty obligation to the OCS.
Since our products have yet to receive marketing clearance or regulatory approval, grants received are recorded against the related research and development expenses in the statement of comprehensive loss and a liability is not included in our financial statements.
Development costs
Development costs are capitalized in accordance with the accounting policy described in Note 2E(3) to our financial statements appearing elsewhere in this prospectus. Capitalization of costs is based on management's judgment about technological and economic feasibility.
Our management believes that as of December 31, 2014, the above conditions were not met; therefore development costs were not capitalized.
Quantitative and Qualitative Disclosures About Market Risks
We are exposed to market risk in the ordinary course of our business. Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our market risk exposure is primarily a result of fluctuations in foreign currency exchange rates and interest rates.
Foreign Currency Exchange Risk
Our functional and reporting currency is the New Israeli Shekel (NIS) which is the local currency in Israel. Our foreign currency exposures give rise to market risk associated with exchange rate movements of the NIS, mainly against the U.S. dollar and the Euro. Although the NIS is our functional currency, a small portion of our expenses consist principally of payments made to subcontractors and consultants for clinical trials, other research and development activities, and purchase of new equipment. A material portion of our research and development is conducted through collaboration agreements denominated in U.S. dollars, and therefore our net research and development expenses are subject to significant foreign currency risk. If the NIS fluctuates significantly against either the U.S. dollar or the Euro, it may have a negative impact on our results of operations. To date, such fluctuations in exchange rates have not materially affected our results of operations or financial condition for the periods under review.
To date, we have not entered into any hedging arrangements with respect to foreign currency risk or other derivative financial instruments. In the future, we may enter into currency hedging transactions to decrease the risk of financial exposure from fluctuations in the exchange rates of our principal operating currencies. These measures, however, may not adequately protect us from the material adverse effects of such fluctuations.
Interest Rate Risk
At present, our investments consist primarily of cash and cash equivalents in short-term deposits. The primary objective of our investment activities is to preserve our capital to fund our operations. Our investments are exposed to market risk due to fluctuation in interest rates, which may affect our interest income and the fair market value of our investments, if any. We manage this exposure by performing ongoing evaluations of our investments. Due to the short-term maturities, if any, of our investments to date, their carrying value has always approximated their fair value. We believe that our
75
exposure to interest rate risk is not significant and a 1% change in market interest rates would not have a material impact on our assets.
Recent Accounting Pronouncements
IFRS 9 Financial Instruments
The complete version of IFRS 9 replaces most of the guidance in IAS 39. IFRS 9 retains but simplifies the mixed measurement model and establishes three primary measurement categories or financial assets: amortized cost, fair value through other comprehensive income, or OCI, and fair value through profit and loss. The basis of classification depends on the entity's business model and the contractual cash flow characteristics of the financial asset. Investments in equity instruments are required to be measured at fair value through profit or loss with the irrevocable option at inception to present changes in fair value in OCI. There is now a new expected credit losses model that replaces the incurred loss impairment model used in IAS 39.
For financial liabilities there were no changes to classification and measurement except for the recognition in other comprehensive income of changes resulting from own credit risk, in liabilities designated at fair value, through profit or loss.
IFRS 9 relaxes the requirements for hedge effectiveness by replacing the bright line hedge effectiveness tests. It requires an economic relationship between the hedged item and hedging instrument and for the 'hedged ratio' to be the same as the one management actually use for risk management purposes. Contemporaneous documentation is still required but is different to that currently prepared under IAS 39.
The standard is effective for accounting periods beginning on or after 1 January 2018. Early adoption is permitted. We have not yet assessed IFRS 9's full impact.
JOBS Act
With less than $1.0 billion in revenues during our last fiscal year, we qualify as an emerging growth company under the JOBS Act. An emerging growth company may take advantage of specified provisions in the JOBS ACT that provide exemptions or reductions of its regulatory burdens related to reporting and other requirements that are otherwise applicable generally to public companies. These provisions include an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act. We may take advantage of some, but not necessarily all, of these provisions to reduce our burdens or exempt ourselves from regulatory requirements for up to five years or such earlier time that we are no longer deemed an emerging growth company. We have elected not to avail ourselves of an exemption that allows emerging growth companies to extend the transition period for complying with new or revised financial accounting standards. This election is irrevocable. We would cease to be an emerging growth company if we have more than $1.0 billion in annual revenue, our ordinary shares held by non-affiliates have a market value in excess of $700 million, or we issue more than $1.0 billion of non-convertible debt over a three-year period.
76
Overview
We are a clinical-stage regenerative medicine company focused on developing and commercializing tissue repair products, initially for the orthobiologics and advanced wound care markets. Our product candidates, two of which are in registration trials, are based on our proprietary plant-based collagen technology, which we believe is the only viable technology available for the production of recombinant type I human collagen, or rhCollagen. We believe that our rhCollagen, which is identical to the type I collagen produced by the human body, has significant advantages compared to currently marketed tissue-derived collagen, including improved biofunctionality, superior homogeneity, and reduced risk of immune response. We believe the attributes of our rhCollagen make it suitable for numerous tissue repair applications throughout the human body. We believe that the market opportunity for our current product candidates utilizing our rhCollagen within the orthobiologics and advanced wound care markets exceeds $5 billion.
Our rhCollagen outperforms any tissue-derived collagen, whether from animal or human tissues, in biofunctionality and certain useful physical characteristics, according to data published in peer-reviewed scientific publications. Our rhCollagen can be fabricated in different forms and shapes including gels, pastes, sponges, sheets, membranes, fibers, and thin coats, all of which have been tested in vitro and in animal models and proven superior to tissue-derived products. We have demonstrated that, due to its homogeneity, rhCollagen can produce fibers and membranes with high molecular order, which enables the formation of tissue repair products with distinctive physical properties. We produce our rhCollagen in genetically engineered tobacco plants, assuring an abundant supply of high quality raw materials.
Our three leading rhCollagen-based product candidates are:
- •
- VergenixSTR, a soft tissue repair matrix composed of our rhCollagen
and platelet-rich plasma, or PRP, extracted from a patient's blood. VergenixSTR is intended for the treatment of tendinopathy, such as
in the elbow tendon (for treatment of "tennis elbow"), rotator cuff, patellar tendon, Achilles tendon, and hand tendons. VergenixSTR forms a viscous gel matrix to serve as a scaffold in the
vicinity of a tendon injury site. Following the scaffold formation, our rhCollagen activates the platelets in PRP to provide sustained release of growth factors, which promote healing and repair of
tendon injuries. VergenixSTR is currently in a multi-center registration trial in Israel. These trial results will support an application for Conformité Européenne, or
CE, marking in the European Union. After receiving CE marking in Europe, we intend to pursue U.S. regulatory approval.
- •
- VergenixFG, a wound-filling flowable gel made from our
rhCollagen. VergenixFG is intended for treatment of deep surgical incisions and deep wounds, including diabetic ulcers, burns,
bedsores, and other chronic wounds. The VergenixFG formulation provides a scaffold that fills the wound site and establishes intimate contact with the surrounding tissue. VergenixFG provides complete
coverage of the wound site, facilitates wound closure through an engineered synchronization between scaffold degradation and growth of new tissue, and offers a non-allergenic and pathogen-free
scaffold for safe and efficacious wound care therapy. VergenixFG is currently in a multi-center registration trial in Israel. These trial results will support an application for CE marking in the
European Union. After receiving CE marking in Europe, we intend to pursue U.S. regulatory approval.
- •
- VergenixBVF, a bone void filler designed to help accelerate bone healing and formation. VergenixBVF is a product platform intended for bone repair indications such as spinal fusion and trauma. VergenixBVF is a novel absorbable scaffold composed of our rhCollagen and minerals, and when charged with growth factors (autologous or recombinant), it will stimulate the recruitment and differentiation of bone-forming cells, which can heal existing bone and produce new natural
77
bone. VergenixBVF is engineered to enable a sustained, optimal release of the charged growth factors to accelerate bone healing and new bone formation. We are collaborating with a U.S.-based company in the development of VergenixBVF.
Collagen and Collagen-Based Products
Collagen is the main component of connective tissue and is the most abundant protein in mammals. In humans, it comprises approximately 30% of the protein found in the body. Its biofunctionality, biocompatibility, and biodegradability make it a logical choice for regenerative medicine products. Due to its unique characteristics and diverse profile in human body functions, collagen is frequently selected from a variety of biocompatible materials for use in tissue repair to support structural integrity, induce cellular infiltration and promote healing. We estimate the size of the market for human collagen-based tissue repair products for use in orthobiologics and advanced wound care applications is approximately $20 billion.
Type I collagen is the most abundant form of collagen in the human body. It is the dominant constituent of connective tissue and serves as the primary scaffold in tissue or organ repair processes. It is found in tendons, skin, artery walls, corneas, the endomysium surrounding muscle fibers, fibrocartilage, and the organic part of bones and teeth. Type II collagen is primarily found in articular cartilage. Type III collagen, which is produced quickly by young fibroblasts before the tougher type I collagen is synthesized, is found in granulation tissue such as artery walls, skin, intestines, and the uterus. While there may be some niche applications in the future where type III or possibly type II collagen is appropriate, type I collagen is best suited for applications associated with regenerative medicine. Type III recombinant human collagen is currently available for the research market, and is not used in any products currently approved for medical use.
Disadvantages of Current Collagen-Based Products
Currently, type I collagen for medical use is primarily derived from bovine (cow) and porcine (pig) sources, as well as from human cadavers. It is extracted from the tissues using mechanical processes and chemical treatments. Tissue-derived collagen suffers from a number of disadvantages:
- •
- The harsh chemical conditions required to recycle collagen from mature tissue results in a collagen product with random defects in its
protein structure, leading to a compromised triple helix. Consequently, tissue-derived collagen results in significant damage to binding sites for progenitor cells, which are required for cell
proliferation and differentiation into tissue.
- •
- Tissue-derived collagen is non-homogenous and contains high proportions of cross-linked collagen species with high molecular weight.
The rate of degradation of collagen is based on the proportion of cross-linked collagen species within the product. Excessive proportions of cross-linked collagen can impair the collagen's ability to
self-assemble homogenous scaffolds with a high surface area and fully functional integrin-binding capacity, and can also impede its rate of degradation. The inability to effectively control the level
of cross-linked collagen species in tissue-derived collagen results in variability of performance for a given product, and affects the rate of infiltration of cells into the scaffold, which can delay
healing.
- •
- The extraction of collagen from mature mammalian tissues leaves, in many cases, contaminant proteins, growth factors, and cytokines.
As a result, scaffolds made of tissue-derived collagen may provoke inflammation, as well as undesirable immune and foreign body responses that may cause adverse effects and unpredictable biological
outcomes.
- •
- Extraction from animals or humans is also associated with risk of disease transmission. Since 2007 the FDA has highlighted the risks of transmissible diseases to humans in medical devices that contain materials derived from animal sources In January 2014, the FDA released draft
78
- •
- Although collagen molecules are similar among various animal species, slight differences in the protein sequence between species may result in different biological behavior when applied to humans, and in some cases, invoke specific immune responses; for example, bovine collagen is associated with hypersensitivity and allergic reactions in approximately 3% of people.
guidance suggesting precautionary procedures to be used in the production of medical devices containing materials derived from animal sources.
Bone Graft Products
According to GlobalData, the size of the global bone graft and substitute products market was approximately $2.1 billion in 2013, and is expected to grow to $2.7 billion in 2020. Bone grafts serve four mechanistic functions: structural, osteoconduction, osteoinduction, and osteogenesis. Structural grafts, such as a tricortical iliac crest bone graft (ICBG) or a femoral ring allograft, provide mechanical support. Osteoconductive grafts provide a scaffold for bone formation. Osteoinductive grafts induce differentiation of progenitor cells into bone-forming cells, or osteoblasts. Osteogenic grafts directly contribute cells for bone formation.
Autograft, which is a graft that uses bone taken directly from another site in the patient's body, has long been considered the gold standard for bone grafting. Autograft can serve all of the above functions; however, it requires an additional surgical procedure, and its efficacy is based on a number of factors, including age and health of the patient. In addition, the availability of bone in a patient's body for autograft procedures is limited; the most common source of harvesting is the ICBG. Autograft has been demonstrated to be successful, particularly in spine fusion, but the paucity of supply as well as the morbidity and pain associated with its harvest has led to demand for development of alternatives and supplements.
Allograft, which is a graft obtained from a human cadaver, provides an osteoconductive scaffold and is weakly osteoinductive. It does not, however, have any osteogenic potential, as the cells are killed during the part of allograft processing aimed at decreasing the risk of infection transmission and antigenicity. One type of allograft is Demineralized Bone Matrix (DBM), a bone graft substitute with osteoconductive and some osteoinductive qualities. It is extracted from allograft bone, resulting in the loss of mineralized bone components and consisting mostly of type I collagen with numerous retained growth factors.
Synthetic bone graft substitutes mimic the structure of bone. They can be made of calcium-based substances, hydroxyapatite, or collagen-based matrices, a combination of tissue-derived collagen and minerals. Synthetic bone graft substitutes are by nature only osteoconductive, as they lack any cells or growth factors. They can be charged with cells or growth factors in order to add osteoinductivity to the substance. The bone graft substitute market is increasingly becoming a highly lucrative market for manufacturers due to the significant growth opportunities; however, producing a quality substitute that effectively mimics natural bone remains a challenge. For example, more than 50 DBM products are currently on the market, but less than 10 have shown clinical evidence of their benefits in orthopedic and spine surgeries.
Advantages of our rhCollagen and rhCollagen-based Products
All of our product candidates are based on our proprietary plant-based recombinant type I human collagen, rhCollagen, which is identical to the type I collagen produced by the human body. The
79
graphic below illustrates the structural differences between our plant-derived collagen and currently marketed tissue-derived collagens.

The key advantages of products using our rhCollagen, as compared to those using collagen derived from animals or human cadaveric tissue, include:
- •
- Better biofunctionality in tissue
regeneration. Our rhCollagen outperforms animal or human tissue-derived collagen in biofunctionality and has a number of useful physical
characteristics, including thermal stability and a pristine triple helix, according to data published in peer-reviewed scientific publications. Because rhCollagen presents a pristine triple helix
structure, it enables superior binding, which accelerates primary human cell proliferation. Collagen scaffolds of our rhCollagen support endothelial, fibroblast, and keratinocyte cell attachment and
proliferation. In all cell types tested, cell proliferation was significantly better in scaffolds made of rhCollagen than in commercially available scaffolds made of bovine collagen. The accelerated
cell proliferation achieved with our rhCollagen results in faster wound healing, less scarring, and higher quality tissue regeneration.
- •
- Superior
homogeneity. Because our rhCollagen is synthesized by five human genes in tobacco plants producing pure molecules that are repeatable
and identical to type I human collagen, it is more homogenous than collagen derived from animal or human tissue sources. The high level of homogeneity of our rhCollagen allows the formulation
of extremely high concentrations of monomeric collagen, up to 150-200mg/ml, which is at least 10 to 100 times higher than the concentration achieved with tissue-derived collagen. The high
concentration of monomeric collagen is of particular importance where strong collagen fibers are needed for 3-D scaffolds. The homogeneity of our rhCollagen enables us to engineer consistent and
reproducible products with a controlled degradation rate which can be optimized to the targeted indication. Achieving the same level of engineered performance would be difficult, if not impossible,
with tissue-derived collagen that varies from batch to batch.
- •
- Improved safety and greater purity. Our pure plant-derived collagen does not induce an immunogenic response, whereas impurities carried over from the source of tissue-derived
80
- •
- Novel applications. Due to our ability to control the protein at the molecular level, it is possible to use our rhCollagen to produce products with unique physical features, as well as high repeatability, which is not possible with tissue-derived collagen. As compared to tissue-derived collagen, rhCollagen membranes have shown better thermal stability, improved tensile strength due to regional fibril alignment, and higher levels of transparency. In addition, rhCollagen can be used to produce high concentration solutions of collagen at low viscosities. The unique properties of our rhCollagen make it an ideal building block for many products that we believe cannot currently be produced using tissue-derived collagen, such as BioInks for 3-D printing, artificial tendons, and transparent ophthalmic products.
collagen can lead to immune system rejection. Preclinical studies have demonstrated that rhCollagen incubated with activated THP1-macrophages produces significantly lower levels of inflammatory cytokines when compared with bovine collagen that is similarly incubated. This demonstrates that animal-derived collagen can provoke a foreign body response not seen with rhCollagen, which delays healing and increases scarring. Further, with our rhCollagen, there are no potential side effects in the growth of tissue because there are no residues of growth factors. In addition, with tissue-derived collagen, there is a possibility that the animal or human from which the collagen was produced was infected with a virus, prion, or other pathogen. With our plant-derived rhCollagen there is no risk of transmitting diseases and pathogens.
We believe the clinical attributes of our rhCollagen will translate into benefits for patients, payors, and physicians, and will be adopted rapidly by the market once our product candidates receive regulatory approval. The improved biofunctionality of our products is intended to lead to faster recovery, better clinical outcomes, and reduced hospitalization time. Our in vivo studies have shown faster tissue remodeling, faster wound closure, and reduced scarring compared to competing products made from tissue-derived collagen.
We can produce our rhCollagen cost-effectively and have access to an abundant supply of raw materials. Tobacco is a relatively easy plant to grow, and can be cultivated in a wide range of climates and soils. The tobacco plant is an extremely hardy plant, may be grown in very large volumes and its growth time to reach desired maturity is relatively short (about eight weeks). Under our current production technology, we are able to achieve a cost of goods that allows us to offer products at prices that are competitive with tissue-derived collagen. We are advancing a new production model that will result in labor cost reductions and higher yields, assuring an abundant raw material supply as demand for rhCollagen increases.
Collagen-based products are already used extensively in the marketplace; therefore, we expect our product candidates will be eligible for reimbursement by third-party payors, including government agencies and insurance companies. We believe that the demand for tissue-derived collagen will decrease as the market recognizes the significant advantages of our plant-based rhCollagen.
Our Market Opportunity
Our rhCollagen represents a platform for the development of products addressing significant opportunities in multiple therapeutic, aesthetic, and other medical markets. We are initially focused on the orthobiologics and advanced wound care markets, and we believe the market opportunity for our current product candidates utilizing our rhCollagen platform technology exceeds $5 billion. However, we also see a significant opportunity to use our rhCollagen platform to develop products to address additional indications in these markets as well as in new markets, including cardiovascular and ophthalmic, as well as to advance new enabling technologies such as 3-D bio-printing. We believe that the potential addressable market opportunity for products using our technology is even greater than the market size served by currently available collagen-based products, mainly due to continued unmet medical needs and the shortcomings of tissue-derived collagen.
81
Orthobiologics Market
The established orthopedic market—estimated by QiG Group at more than $40 billion annual revenue worldwide in 2012—continues to offer exceptional growth opportunities. An aging population, active demographics, innovative technology, and emerging geographic areas will continue to drive growth in the global orthopedic market. Top market segments within orthopedics include reconstructive devices, such as joint replacements; spinal implants and instruments, used to treat joint pain; fracture repair, including the use of plates and screws; and arthroscopy and soft tissue repair, primarily for sports and movement related injuries.
Chronic complex musculoskeletal injuries that are slow to heal pose challenges to physicians and patients alike. Orthobiologics are substances that orthopedic surgeons use to help injuries heal more rapidly with a superior outcome. These products are made from substances that are naturally found in the body, which dynamically interact with the musculoskeletal system to facilitate the healing of bone, cartilage, meniscus, tendons, and ligaments affected by disease or injury. Orthobiologics products are spread across all segments of the larger orthopedic market, generating much of the growth within orthopedics. GlobalData recently estimated that the major segments of the orthobiologics market currently comprise an annual $6.7 billion worldwide market.
The orthobiologics market is segmented as follows:
- •
- Bone allografts;
- •
- Bone graft substitutes;
- •
- Viscosupplementation;
- •
- Growth factors, such as BMP; and
- •
- Cell-based therapies, such as PRP.
It is estimated that bone and joint disorders account for approximately half of all chronic conditions in individuals above 50 years of age in developed countries, and they are the most common cause of severe, long-term pain and disability. Moreover, the U.S. population aged 60 years and above is projected to increase by 33% this decade, which represents a key driver of this market as elderly patients are slower to heal and more in need of products that enhance and speed recovery. A rise in the geriatric population along with lifestyle changes such as increased obesity and growing participation in sports and outdoor activities among the older as well as younger generation all contribute to the increase in musculoskeletal disorders. The overall increase in prevalence of musculoskeletal disorders combined with technological advancements in the orthobiologics field are fueling the growth of this market, resulting in a CAGR of 7.7% in the North American market from 2014 to 2019 as predicted by MicroMarket Monitor.
Advanced Wound Care Market
The global market for wound care encompasses traditional dressings and bandages, as well as advanced wound care products such as bioengineered skin and skin substitutes and wound care growth factors. Over the past 30 years, there has been a shift from traditional wound dressings towards advanced therapies that aim to optimize the wound healing environment. Attempts to reduce the duration of hospital stays in order to limit healthcare costs and the goal of enhancing therapeutic outcomes are driving the demand for advanced wound care and closure products. One of the primary market drivers for advanced wound care products is the increasing incidence of chronic wounds, which are on the rise due to an aging population and a sharp rise in the incidence of diabetes and obesity worldwide. Both advanced age and chronic medical conditions are associated with a slower healing process, and all phases of wound healing are affected. The inflammatory response is decreased or delayed, as is the proliferative response.
82
Espicom estimates that the global market for advanced wound care in 2013 had reached $6.2 billion, representing a growth rate of approximately 5% since 2012. The three major market segments are device-based wound care, comprised of negative-pressure wound therapy and hydrosurgery systems; moist wound care, comprised of dressings that create and maintain a moist environment; and biologics, comprised of bioactive technologies that provide new approaches to debridement and dermal repair and regeneration.
With a wide range of dressings to choose from, dressing selection is a significant challenge for wound care clinicians. The ideal dressing should induce rapid healing at reasonable cost with minimal inconvenience to the patient. In a healing wound, a cascade of events occurs that includes platelet accumulation, inflammation, fibroblast proliferation, cell contraction, angiogenesis, and re-epithelization, ultimately leading to scar formation and wound remodeling. Collagen plays an important role in each of these phases of wound healing. Native intact collagen provides a natural scaffold or substrate for new tissue growth. Dressings containing collagen are thought to provide the wound with an alternative collagen source that is degraded over time, leaving the endogenous native collagen to continue normal wound healing.
Biological wound dressings have the benefit of forming part of the natural tissue matrix and some of them play an important role in natural wound healing and new tissue formation. These characteristics make them the most attractive and fastest growing segment of the overall advanced wound care market, experiencing double digit growth in 2013. In certain instances, these bioactive matrices are incorporated with compounds such as growth factors and antimicrobials for delivery to the wound site. There are a number of biological wound care dressings available that incorporate tissue-derived collagen to enhance wound bed preparation.
Our Strategy
We plan to exploit the unique characteristics of our rhCollagen technology platform to develop and commercialize an extensive portfolio of regenerative medicine products. The key elements of our strategy include the following:
- •
- Position our rhCollagen as the "gold standard" platform technology for
collagen-based products in a broad range of markets. We believe that our rhCollagen represents a significant advance in collagen
technology, demonstrated by its improved biofunctionality, superior homogeneity, and reduced risk of immune response. Our rhCollagen is a platform technology which can be utilized in a broad range of
therapeutic, aesthetic, and other medical applications, as well as in emerging industries such as bio-printing which we believe cannot be adequately addressed with currently available collagen
technologies. We intend to expand the awareness of rhCollagen through partnerships and collaborations with leading commercial and academic partners around the world and further clinical trials which
we will seek to have published in peer-reviewed journals, as well as through our participation in academic and industry conferences, to position rhCollagen as the "gold standard" platform technology
for collagen-based products. We believe our platform technology, and the knowledge and expertise we have gained in its development, will enable the development, both independently and with
collaborators, of differentiated products in multiple industries with a short time to market.
- •
- Establish a regulatory process for rhCollagen-based end products using VergenixSTR and VergenixFG as precedent. We intend to rapidly seek marketing clearance of our initial product candidates, VergenixSTR and VergenixFG, through CE marking in Europe upon completion of our clinical trials currently being conducted in Israel. The CE mark is a symbol that indicates that a product conforms with all applicable EU requirements and, once affixed, enables a product to be sold within the European Union and other countries that recognize the CE mark, subject to compliance with applicable submission and approval requirements in such other
83
- •
- Utilize collaborative partners and distributors to develop and
commercialize our technology and products. We believe the market-leading characteristics of our rhCollagen will create attractive
collaboration opportunities for our product candidates, and we intend to selectively establish collaborations and strategic partnerships with respect to our current and future product candidates in
order to accelerate their development and commercialization. We intend to create a commercial organization, initially in Europe, with well-established companies whose distribution networks are deeply
entrenched. Our commercial organization will be comprised of the distribution networks of our collaboration partners, particularly in the United States and China, as well as local and regional
distributors in certain markets.
- •
- Expand our manufacturing capacity to support commercialization of
rhCollagen-based end products. We cultivate the tobacco plants used in the production of our rhCollagen in a network of farms in
Israel, and we extract the raw materials used to manufacture our rhCollagen from these tobacco plants. We intend to utilize a portion of the proceeds from this offering to construct a manufacturing
facility in Israel that will enable us to manufacture commercial quantities of our rhCollagen and rhCollagen-based end products in a cost-effective manner for application in both premium and commodity
markets.
- •
- Expand our pipeline through ongoing development of new
products. We intend to continue to develop additional products, initially in the orthobiologics and advanced wound care markets and
subsequently in other high value markets, based on our rhCollagen, both independently and with strategic collaborators. Our product candidate pipeline and our research and development program are
expected to yield new product candidates in the coming years. Some of these new product candidates are derivatives of current product candidates, and therefore may benefit from an easier regulatory
pathway and shorter time to market, should our current product candidates receive regulatory approval.
- •
- Advance our leadership position in recombinant protein production through our plant-based technology. We continually seek to expand our knowledge of plant-based protein production systems and introduce improvements into our process. We are shifting production to an enhanced line of tobacco plants with higher collagen yield, along with improvements in the growing and cultivation process as well as collagen extraction and purification. As tissue engineering and regenerative medicine continue to evolve and expand, we expect that the demand for high-quality biomaterials will grow. We intend to collaborate with commercial and academic partners in order to identify and develop other recombinant proteins beyond collagen which may be produced with our proprietary plant-based protein production know-how.
countries. After receiving CE marking for our initial products, we plan to hold a pre-Investigational Device Exemption, or IDE, meeting with the FDA and pursue FDA approval for our rhCollagen-based products. We believe that this strategy will allow us to gain earlier market access and thereby more rapid industry acceptance for our rhCollagen-based end products, since the timeline to achieve CE marking is generally shorter than the FDA approval route. Utilizing this strategy is expected to result in more physicians gaining exposure to rhCollagen-based products sooner. Following receipt of a CE mark, we will be able to conduct post-marketing surveillance studies of our products with key opinion leaders, resulting in physicians gaining more hands-on experience with rhCollagen. Should these post-marketing surveillance studies successfully demonstrate the efficacy of our initial product candidates, we will endeavor to have these results published in peer-reviewed medical journals as a means of expanding the clinical credibility of rhCollagen and rhCollagen-based end products.
84
Our Product Candidates
VergenixSTR—Tendinopathy Treatment
VergenixSTR is a soft tissue repair matrix which combines cross-linked rhCollagen with PRP, a concentrated blood plasma that contains high levels of platelets, a critical component of the wound healing process. Platelets contain growth factors that are responsible for stimulating tissue generation and repair, including soft tissue repair, bone regeneration, development of new blood vessels, and stimulation of the wound healing process. VergenixSTR is injected into the affected area, and forms a viscous gel matrix which serves as a scaffold in the vicinity of a tendon injury site, allowing the platelet concentrate to remain in place at the injured area. The matrix formed has the capabilities to activate the platelets in PRP, thereby releasing growth factors in a controlled manner and controlled biodegradation time, enabling optimal healing.
The following graphic illustrates the VergenixSTR kit and application:

Market for Tendinopathy Treatment
VergenixSTR is intended for the treatment of tendinopathy by promoting healing and repair of tendon injuries in a variety of tendons including the elbow tendon (for treatment of "tennis elbow"), rotator cuffs, patellar tendons, Achilles tendon, and hand tendon.
85

Tendonitis: Annual procedures per indication in the United States
Today, the main treatments offered for tendinopathy are local steroid injection, shock wave therapy, and PRP alone. PRP is an orthobiologic that has recently gained popularity as an adjuvant treatment for musculoskeletal injuries. PRP has found application in diverse surgical fields to enhance bone and soft-tissue healing by placing high concentrations of autologous platelets at the site of tissue damage. The platelets contain alpha granules that are rich in several growth factors and play key roles in tissue repair mechanisms. The relative ease of preparation, applicability in the clinical setting, favorable safety profile, and possible beneficial outcome make PRP a promising therapeutic approach for regenerative treatments. One of the challenges in utilizing PRP for tissue repair is the localization of the platelets in the vicinity of the injured tissue. When administrated into large cavities with synovial fluids such as the knee and shoulder joints, PRP cannot be effective without addressing the issue of platelets localization.
We estimate the size of the target market for VergenixSTR for treating tendonitis is three million procedures per year, or approximately $2.0 billion. While our initial focus for VergenixSTR is in tendinopathy, the product platform of VergenixSTR may be applicable to other soft tissue indications such as tendon rupture, meniscus tear, and cartilage repair, as well as in the aesthetic market as a dermal filler. Transparency Market Research valued the global orthopedic soft tissue market at $5.6 billion in 2013. Globally, the aging population is playing a major role in increasing the incidence of sports injuries as the reduced flexibility and mobility associated with aging can make the body more prone to injury. Consequently, Transparency Market Research forecasts that the orthopedic soft tissue market will grow to $8.5 billion in 2019, a CAGR of 7.2%. The difficulties associated with healing in an aging population highlight the need for advanced orthobiologics products to serve this market.
VergenixSTR Product Development
As part of the VergenixSTR development, we conducted a preclinical study in rats, which was completed in August 2013. The purpose of the preclinical study was to demonstrate the healing ability of VergenixSTR in the treatment of injured and inflamed tendons such as the Achilles tendon, elbow tendon, shoulder tendon and hamstring. The control group participating in the VergenixSTR testing was treated with an injection of PRP only. The study findings demonstrated that VergenixSTR resulted in lower initial inflammatory mononuclear cell levels, which correlates with a reduction in pain. This effect, along with observations on the appearance of mature fibrosis, suggests that VergenixSTR may accelerate the healing of tendons in comparison with the control product.
In the fourth quarter of 2014, we commenced a single-arm registration trial of VergenixSTR in Israel to demonstrate the safety of treatments with VergenixSTR and to evaluate the performance of
86
VergenixSTR in people suffering from tennis elbow, or lateral epicondylitis. Tennis elbow is an inflammation of the tendons that join the forearm muscles on the outside of the elbow. The forearm muscles and tendons become damaged from overuse, leading to pain and tenderness on the outside of the elbow. Tennis, racquet sports and other sports and activities are a common cause of this condition. Tennis elbow affects 1% to 3% of population in the United States and Europe.
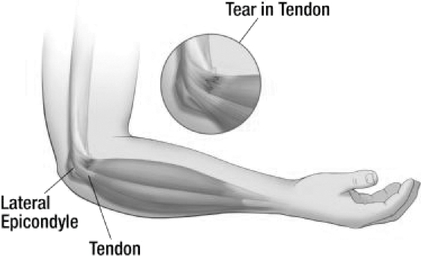
Patients enrolled in the trial receive a one-time injection of VergenixSTR and are monitored for a period of six months. The efficacy of the treatment is assessed according to several indicators, with endpoints being the level of pain, tendon healing assessed by ultrasound and recovery of patient's hand movement.
To date, we have enrolled 14 out of 20 patients participating in the trial. The trial is being conducted at three hospitals in Israel, and we expect to complete the trial in the second half of 2015. We intend to apply for a CE marking in the second half of 2015, and anticipate approval in the fourth quarter of 2015. We anticipate that, if CE marking is received when expected, sales of our VergenixSTR product candidate will commence in the first quarter of 2016. We have commenced discussions with an international distributor for VergenixSTR's distribution in Europe. After receiving CE marking, we intend to pursue regulatory approval for VergenixSTR in the United States and will request a pre-IDE meeting with the FDA to determine clinical study requirements and the appropriate regulatory pathway.
VergenixFG—Wound Filler
VergenixFG is an advanced wound care product candidate based on our rhCollagen technology. VergenixFG is intended for the treatment of deep surgical incisions and deep wounds, including diabetic ulcers, burns, bedsores, and other chronic wounds that are difficult to heal. VergenixFG is designed to be easy to use and to be administrated through a cannula by a doctor or nurse. The VergenixFG formulation provides a scaffold of pure human collagen that fills the wound bed and is engineered to create maximal contact with the surrounding tissue. VergenixFG provides complete coverage of the wound site, facilitates wound closure through an engineered synchronization between scaffold degradation and growth of new tissue, and offers a non-allergenic and pathogen-free scaffold for safe and efficacious wound care therapy. Other flowable gel products are available on the market, but they are based on tissue-derived collagen.
Market for Chronic Wounds
VergenixFG is designed to meet the needs of the advanced wound care market, initially in the treatment of chronic wounds. Chronic wounds are rarely seen in individuals who are otherwise healthy.
87
Major chronic diseases such as peripheral vascular diseases, cardiovascular diseases, diabetes, and other debilitating diseases have led to an increase in the incidence of chronic wounds. In wound healing, a cascade of events occurs that includes platelet accumulation, inflammation, fibroblast proliferation, cell contraction, angiogenesis, and re-epithelization, ultimately leading to scar formation. A chronic wound is stalled at one of these healing stages. This usually occurs during the inflammatory phase and is linked to elevated levels of the enzyme matrix metalloproteinase (MMPs) in the wound. During normal wound healing, proteases such as MMPs are attracted to the wound during the inflammatory phase and have an important role in breaking down unhealthy extracellular matrices (ECMs) so that new tissue forms. However, when MMPs are present in a wound at elevated levels for a prolonged period of time, this results in the destruction of healthy ECMs, which is associated with delayed wound healing and an increase in wound size. When the excess of MMPs is not balanced by normal physiological processes, alternative methods are required to reduce protease levels in the wound. This suggests a role for dressings containing collagen in the management of wounds where healing is stalled, as dressings containing collagen are thought to provide the wound with an alternative collagen source that can be degraded by the high levels of MMPs as a sacrificial substrate, leaving the body's native collagen to continue normal wound healing
We plan on selling VergenixFG at a competitive price to the other advanced healing products in the market. One of the most common areas where chronic wounds are seen is with diabetic foot ulcers. We estimate the diabetic foot ulcer market for VergenixFG to be approximately 300,000 patients and $500 million annually. Diabetic foot ulcers represent about one quarter of the total chronic wound market, indicating that our initial target market is several magnitudes greater than this market alone. We also see the opportunity for expansion of VergenixFG beyond chronic wounds into the treatment of deep surgical incisions. The National Center for Health Statistics reported a total of 51.4 million inpatient surgical procedures took place in the United States in 2010, and we believe at least half of those resulted in a major surgical wound that could benefit from an advanced wound closure product such as VergenixFG to facilitate healing.
VergenixFG Product Development
As part of our product development of VergenixFG during the years 2011 to 2013, preclinical studies were conducted by an external laboratory under Good Laboratory Practices, or GLPs. The purpose of the studies was to investigate the performance of VergenixFG in the treatment of wounds in large animals in comparison to a competing product produced from bovine collagen. In a cutaneous full-thickness wound pig model, 95% wound closure was observed with VergenixFG at day 21 compared to 68% closure in wounds treated with the benchmark product. Moreover, VergenixFG treatment induced an early angiogenic response and induced a significantly lower inflammatory response than in the control group. The researchers concluded that VergenixFG proved effective in animal wound models and is expected to be capable of reducing the healing time of human wounds.
In the fourth quarter of 2014, we commenced an open-label registration trial of VergenixFG to demonstrate the safety of treatments with VergenixFG and to evaluate its performance in patients with hard-to-heal chronic wounds of the lower limbs. Patients enrolled in the trial receive a one-time treatment of VergenixFG and have a follow-up examination four weeks post-treatment. The efficacy of the treatment is assessed according to several indicators, with the primary endpoints being percentage of wound closure and degree of pain.
To date, we have enrolled 15 out of 20 patients participating in the trial. On March 18, 2015, we reported interim results from the first ten patients in the VergenixFG trial. An analysis of the interim results demonstrates excellent wound closure rates, with 80% to 100% healing within four weeks of the beginning of treatment in 7 of 10 patients. In addition, the interim results indicate that VergenixFG appears safe for use on human subjects. The clinical trial is being conducted at three wound clinics of a leading HMO in Israel, and we expect to complete the trial in the fourth quarter of 2015. We intend to
88
apply for CE marking in the second quarter of 2015, and anticipate approval in the fourth quarter of 2015. We anticipate that, if CE marking is received when expected, sales of our VergenixFG product candidate will commence in the first quarter of 2016. We have commenced discussions with international distributors for VergenixFG's distribution in Europe. After receiving CE marking, we intend to pursue regulatory approval for VergenixFG in the United States, and will request a pre-IDE meeting with the FDA to determine clinical trial requirements and the appropriate regulatory pathway.
VergenixBVF—Bone Healing Implant
VergenixBVF is a novel absorbable scaffold composed of our rhCollagen and synthetic minerals that mimics bone structure intended for use in posterolateral spinal fusion and trauma and other orthopedic applications. We intend to develop VergenixBVF as a product platform for the development of bone void filler products which can be used as a one-time treatment that is easy to implant during open surgery and will cover a broad spectrum of bone repair indications. (see figure below).

The VergenixBVF platform covers a broad range of bone fractures
The scaffold is designed to be charged with a growth factor which will stimulate bone growth and tissue growth in a controlled manner. The platform also has the potential to support additional product applications where it is charged with cells or therapeutics, further expanding the potential market for VergenixBVF-related products.
Market for Bone Repair Products
The initial VergenixBVF product candidate is intended to be used as a bone void filler, initially targeting trauma. According to GlobalData, there were an estimated 414 million trauma-related bone grafting procedures across 10 major markets in 2013, accounting for 22% of all bone grafting procedures.
This product candidate will have wide applicability in skeletal procedures, and we expect that it will be used in multiple markets, including spinal fusion. GlobalData estimates that a total of 1.8 million bone-grafting procedures were performed in 2013 worldwide, with approximately one million related to spinal fusion. Spinal fusion procedures have the highest utilization rates of bone graft substitutes across all bone grafting procedures. We estimate the size of the worldwide target market for the initial VergenixBVF product candidate at one million procedures per year, representing a market size of approximately $3.5 billion.
89
VergenixBVF Product Development
VergenixBVF was initially developed by us in collaboration with Pfizer, Inc. The product under development in our collaboration with Pfizer was VergenixBVF charged with a recombinant bone growth protein developed by Pfizer. The collaboration agreement with Pfizer expired in 2013, and since then we have continued the development of VergenixBVF under a strategic collaboration arrangement with another U.S.-based corporate collaborator that now owns the commercialization rights to this protein.
In the initial VergenixBVF product candidate, the scaffold will act as a carrier to enable sustained release of the recombinant bone growth protein to induce cell infiltration and proliferation. VergenixBVF combined with a growth factor was tested in different animal models to verify its performance in bone healing. The tests, most of which have been conducted by our U.S.-based corporate collaborator, included rats, rabbits, and non-human primates. Rapid bone growth was observed in these studies, indicating that VergenixBVF combined with the growth factor has superior efficacy as compared to currently marketed products in the above-mentioned models. We believe this advantage will be translated to human bone repair.
The initial VergenixBVF product candidate is in the preclinical development stages with a U.S.-based corporate collaborator.
Technology
Our rhCollagen technology is based upon research conducted by our founder and Chief Scientific Officer, Prof. Oded Shoseyov. We believe our technology is the only viable technology available for the production of recombinant type I human collagen, the most abundant collagen in the human body.
The production of our rhCollagen begins with the creation of genetically engineered cultures, which are transferred to selected greenhouses across Israel, and continues with the harvesting of tobacco leaves, and the processing of such leaves to an extract, which then undergoes purification until the completion of the rhCollagen.
Five human genes encoding heterotrimeric type I collagen are introduced into tobacco plants. The three protein chains that make up type I collagen—two a1 protein chains and one a2 protein chain—are encoded by two genes. The other three genes encode the human prolyl-4-hydroxylase (P4Ha and P4Hb) as well as lysyl hydroxylase 3 (LH3) enzymes. These enzymes are responsible for key post-translational modifications of collagen, and plants co-expressing all five of these vacuole-targeted genes generate intact procollagen. The plants are grown in a greenhouse under strict growing protocols and mature leaves are transported to a protein extraction facility. Upon extraction, pro-collagen is enzymatically converted to atelocollagen using a plant-derived protease. The protein is purified to homogeneity through a cost effective industrial process taking advantage of collagen's unique properties which make it soluble at a very low pH.
Plant-derived rhCollagen forms thermally stable triple helix structures which readily fibrillate at natural pH and low sodium chloride concentrations, making it ideal for use in the manufacture of products for tissue repair in the human body. Binding of integrins (transmembrane receptors) presented by the cells to a specific 3-D structure on type I collagen fibrils requires a perfect triple helix. This binding is essential for binding and proliferation of cells on tissue repair scaffolds. In a recent study published in the Journal of Biomedical Materials Research Part B: Applied Biomaterials, rhCollagen was compared with acid-solubilized collagen from bovine dermis and pepsin-solubilized collagen from human fibroblast cell culture. Tested samples of the tissue-derived collagens had random fibrillar organization, whereas rhCollagen membranes showed far greater regional fibril alignment and transparency. RhCollagen membranes also showed better thermal stability compared with the tissue-derived collagens. The authors concluded that cross-linked rhCollagen membranes had a superior
90
combination of desirable properties, namely higher transparency, higher thermal and tensile strengths, and adequate hydration.
We have selected tobacco as the medium for production of rhCollagen due to certain attributes of the tobacco plant that provide us with a number of advantages:
- •
- The genetic structure of tobacco is well understood and therefore can be effectively manipulated.
- •
- We can monitor the effect of weather conditions on the accumulation of proteins in the plants, which allows us to make optimal use of
the growing area. We control the growing process in order to maximize yields.
- •
- Because tobacco is not part of the food chain, there are no concerns about cross-contamination of the food supply that could result
from genetically modified plants, which eases the regulatory burden.
- •
- Tobacco plants may be grown in very large volumes and its growth time until reaching the desired maturity is relatively short (about eight weeks).
We have developed a large portfolio of configurations and composites based on our rhCollagen that are used to create high-quality products, including our three product candidates, as follows:

Our Development Activities
Development History
Our rhCollagen was first developed as a collaboration among several commercial partners and the Hebrew University of Jerusalem, a major academic institution in Israel, under the direction of our founder, Professor Oded Shoseyov. Prof. Shoseyov is a faculty member at the Robert Smith Institute of Plant Science and Genetics at the Hebrew University of Jerusalem. The intellectual property was transferred to our subsidiary, CollPlant Ltd.
As part of our regulatory strategy, we first developed and achieved a CE marking for a collagen-based non- invasive dressing, VergenixWD. We believe that VergenixWD is the first medical device in
91
the world based on plant-derived rhCollagen to be authorized for marketing. VergenixWD is a sterile, biodegradable advanced wound care sheet supplied in various sizes, composed of rhCollagen that provides a moist wound healing environment. Currently, we are not promoting a marketing strategy for VergenixWD, which is considered a commodity product, and it is not part of the advanced wound care market that is our target market. We pursued a CE mark for this product as a predicate product for our intended CE marking for our VergenixSTR and VergenixFG product candidates in the European Union.
Future Development
To facilitate efficient development, our management holds annual research and development meetings where they prioritize development projects and determine future product candidates. The prioritization process is based on several factors, including our business plan, commercial potential of the product candidate, time to market, cost of development, feasibility of the project, and our established strategic objectives. We have several development projects which are in different stages of development.
Future Product Candidates
We periodically examine the continued development of other collagen-based product candidates that we have conceived. Each one of our current product candidates offers a platform to product derivatives that can address other indications and contribute to our pipeline and revenues. These derivative product candidates include, for example, the use of VergenixSTR for ACL repair and ophthalmology applications, and the use of VergenixFG for the treatment of deep surgical incisions. We currently have in-house research projects related to tendon rupture and surgical wounds and are actively seeking collaborators in these indications. We are also pursuing other platforms for our rhCollagen, such as biomaterial coatings in order to reduce foreign body response and tissue adhesion, through ongoing research and business development discussions.
BioInk for 3-D Bioprinting
3-D bio-printing is being applied to regenerative medicine to address the need for complex scaffolds and tissues and organs suitable for transplantation. We are developing BioInks suitable for 3-D printing using our rhCollagen. Our researchers have chemically modified the gelling behavior of the collagen to adapt the biological molecules for printing. Instead of gelling like unmodified collagen, the BioInks remain fluid during printing. Once the BioInks are irradiated with UV light, they crosslink and cure to form hydrogels. We have the ability to control the chemical modification of the biological molecules so that the resulting gels have differing strengths and swelling characteristics. The properties of natural tissue can therefore be imitated—from solid cartilage to soft adipose tissue.
Other Recombinant Proteins
As tissue engineering and regenerative medicine continue to evolve and expand, we expect that the demand for high-quality biomaterials will grow. There are a number of other extracellular proteins such as elastin, fibronectin, and different types of collagen which may be produced through our plant production system. Another protein, Resilin, has been produced using another proprietary technology for the production of recombinant proteins. Resilin is a polymeric rubber-like protein secreted by insects to specialized cuticle regions, in areas where high resilience and low stiffness are required. Combining collagen at the nano-scale with Resilin to produce fibers resulted in super-performing fibers with greater tensile strength and elasticity exceeding that of natural collagen fibers. This composite biomaterial can be used in indications where elasticity, strength, and memory shape properties are required, such as tendons, meniscus, and nucleus polyposis.
92
Manufacturing, Supply and Production
The majority of our product research and development work is carried out at our offices and research laboratories in the Weizmann Science Park in Ness-Ziona, Israel. The agricultural research and development and extraction activities for our rhCollagen are carried out at our site in the north of Israel.
We work with subcontractors with greenhouses for growing the tobacco plant containing human collagen in several locations in Israel. This tobacco growth occurs year-round and is optimized to the climate conditions in order to achieve the maximum amount of the protein in the leaves. The growers use our protocols and are monitored by our agronomists to ensure their compliance with these protocols. Each grower has the infrastructure that can be scaled-up to accommodate future demand without additional capital expenditures.
We perform the extraction process by which rhCollagen is extracted from the tobacco plants at our manufacturing facility in the north of Israel. The collagen purification process which produces rhCollagen is carried out by dedicated subcontractors spread across Israel. Our rhCollagen-based product candidates are currently manufactured in the United States by a subcontractor using rhCollagen we supply to them under our production protocols.
We currently have the ability to produce sufficient quantities of quality recombinant type I human collagen to support our product development activities and the expected commercial launch of the VergenixSTR and VergenixFG product candidates in Europe in 2016. We are undertaking development and optimization of the production process, which will enable us to increase production capacity and reduce production costs. Our activities are focused on yield improvement, scale-up and cost reduction.
While our upstream and downstream processes are quite robust and efficient, we continuously invest in further yield improvement and scalability, in order to reduce costs. In order to increase yield, we plan to increase biomass per growing area by using new genetic derivatives, improvement and optimization of growing techniques and introduction of online controls. Our next-generation tobacco plants have been created through improved genetics and cross-breeding, and produce three times the amount of collagen as our first-generation plants. Shifting our growing process from tissue culture techniques to cultivation of plants from seed, which we anticipate implementing in 2016, will also streamline the production process and reduce costs. In addition, increased growing areas will reduce overall cost per harvest. We also plan further process optimization of our extraction process to increase yields.
We are currently developing a full in-house purification capability. Following the purification process development, and in order to accommodate upcoming commercialization requirements, we will increase our overall production capacity through the establishment of a new facility which will be equipped with the production equipment and infrastructure to support the larger scale (i.e., clean rooms, water and air systems). We intend to utilize a portion of the proceeds of this offering to construct this manufacturing facility in Israel, which will enable us to produce large commercial quantities of our rhCollagen and rhCollagen-based end products.
Under our current production techniques, we achieve a cost of goods that allow us to offer competitive pricing in the orthobiologics, advanced wound care, and other premium collagen-based products markets. We anticipate that the above-mentioned production enhancements will reduce the production cost of our rhCollagen to a level that will enable us to be competitive in both premium and commodity markets for collagen-based products.
Sales, Marketing and Distribution
Our plan for marketing and distributing VergenixSTR and VergenixFG in the initial European market is to partner with large, established distributers to sell our products. We are currently in
93
discussions with distributors in Europe for the commencement of sales after receiving CE marking for these products. These potential distributors are active in the orthobiologics market and have the appropriate sales infrastructure in place to successfully market our products in targeted territories.
Following CE marking for our proprietary end products, we intend to initiate with our European Key Opinion Leaders a Post Marketing Surveillance study in order to generate additional clinical data that demonstrates the efficacy and superiority of our products. The study will facilitate market penetration of our products in Europe, as well as provide additional support for the submission package to other regulatory agencies, such as the FDA. We anticipate that any products we develop in collaboration with a strategic partner or collaborator, such as the VergenixBVF product candidate, will be marketed by such partner's sales force.
During 2013, we entered into a non-binding memorandum of understanding with Trauwin, a strategic shareholder based in China. We agreed in principle, subject to the negotiation of a definitive agreement, that Trauwin or its affiliate would distribute our products (excluding orthopedic products) in China.
Our proprietary end products will be marketed to physicians, hospitals, and clinics. We plan to expand the awareness of rhCollagen and our rhCollagen-based products to the end users through the publication of clinical trial data as well as marketing studies we may conduct, along with participation in academic and industry conferences. We will also market our rhCollagen to companies developing products using collagen which do not compete with our primary end products. We anticipate entering into collaborations or partnerships with these companies where we would supply them with rhCollagen for use in their products in return for royalties.
We are a clinical stage company and to date, we do not have customers making commercial purchases, and accordingly, we do not have an order backlog. To date, our rhCollagen has not been sold commercially, but rather individually to different consumers in the research market. We sell our rhCollagen in the research market under the brand name Collage. Sigma-Aldrich Company distributes Collage in the global research market, which includes, among others, academic institutions and hospitals worldwide. The Collage that we sell to Sigma-Aldrich under this framework is intended only for research laboratories (in vitro) and not for preclinical or clinical (in vivo) uses. To date, sales to Sigma-Aldrich were immaterial in scope and amount.
Competition
We are not aware of any competitors that produce human collagen from plants or that produce recombinant type I human collagen. However, our industry is characterized by rapidly evolving technology and intense competition, and our rhCollagen-based product candidates will compete with several alternative tissue-derived or synthetic products. Adequate protection of intellectual property, successful product development, adequate funding, and retention of skilled, experienced, and professional personal are among the many factors critical to success in the pharmaceutical industry.
Generally, our competitors currently include large fully integrated companies, as well as academic research institutes and companies in various developmental stages that develop alternative sources and forms of collagen and tissue-derived products.
Our VergenixSTR product candidate will compete with companies that sell PRP kits, including Biomet Inc., Arthrex Inc., Harvest Technologies Corporation, MTF Sports Medicine, and Arteriocyte Medical Systems Inc.
94
The primary competitors to our VergenixFG product candidate are products based on tissue-derived collagen. The lead competitors in this area are Integra Lifesciences Corporation, which manufactures products used in tissue regeneration, spine and nervous system regeneration and the treatment of wounds, and Wright Medical Technology Inc., which manufactures and markets tissue-derived collagen and sells collagen products (sponges, sheets, gel) produced from skin donations for a wide range of medical treatments such as curing ulcers, wounds and orthopedics.
Our VergenixBVF product platform will compete primarily with Medtronic Inc.'s INFUSE bone graft product, which combines a recombinant bone growth protein and bovine-based collagen; Nuvasive Inc.'s Osteocel, a cellular bone matrix product; and Othrofix's TRINITY ELITE allograft product.
Intellectual Property
Our success depends, in part, on our ability to protect our proprietary technology and intellectual property. We rely on a combination of patent, trade secret, and trademark laws in the United States and other jurisdictions to protect our intellectual property rights. In addition, we rely on proprietary processes and know-how, intellectual property licenses, and other contractual rights, including confidentiality and invention assignment agreements, to protect our intellectual property rights and develop and maintain our competitive position.
Patents
We have a global patent portfolio that is comprised of eight patent families. Almost two dozen of our patent applications have issued as patents or will issue soon, having been allowed by the relevant patent office. We have exclusive ownership of 14 issued patents in our patent family that cover methods of creating collagen-producing plants and two issued patents in our patent family that cover methods of processing plant-based collagen. These issued patents and others that may issue in the future in these patent families, assuming timely payment of annual fees, are expected to expire beginning in 2025. Our patent portfolio also includes patent families that cover production and use of collagen.
In addition, our patent portfolio includes pending applications, some of which are jointly owned with Yissum, as well as issued patents that are jointly owned with Yissum, which cover production of other biomaterials. Our more recently filed patent applications, if granted, could provide patent protection for our rhCollagen through 2034.
We are not aware of any impediments to the patent applications being granted in the United States or other jurisdictions. However, our patent applications may never issue as patents, and our issued patents and any that may issue in the future may be challenged, invalidated or circumvented.
Trade Secrets and Confidential Information
In addition to patented technology, we rely on our trade secrets and continuing technological innovations to develop and maintain our competitive position. In an effort to protect our trade secrets, we rely on, among other safeguards, confidentiality and invention assignment agreements to protect our proprietary technology, know-how and other intellectual property that may not be patentable or that we believe is best protected by means that do not require public disclosure. For example, we require our employees, consultants and advisors to execute confidentiality agreements in connection with their employment or consulting relationships with us, and to disclose and assign to us inventions conceived in connection with their services to us. These agreements also provide that all confidential information developed or made known to the individual during the course of their relationship with us must be kept confidential, except in specified circumstances.
95
Trademarks
We rely on trade names, trademarks and service marks to protect our name brands. Our registered trademarks in several countries include the following: "collage" and "Vergenix."
Materials Transfer Agreements
We periodically enter into materials transfer agreements with commercial organizations, medical institutions and research and development institutions to transfer materials and product candidates developed by us. These agreements include provisions that are customary for such agreements concerning the permitted use of the transferred material and any results obtained using the material, confidentiality, the rights in the transferred materials and in the results of the research and/or development in which the materials are used, and instructions concerning care and usage of the materials. These agreements may be used as a basis for further cooperation between us and the counterparties.
We may be unable to obtain, maintain and protect the intellectual property rights necessary to conduct our business, and may be subject to claims that we infringe or otherwise violate the intellectual property rights of others, which could materially harm our business. For a more comprehensive summary of the risks related to our intellectual property, see "Risk Factors."
Agreement with Yissum Research Development Company of the Hebrew University of Jerusalem Ltd. with Respect to Our rhCollagen
Under an agreement dated July 13, 2004 among Meytav—Technological Innovation Center Ltd., Yehuda Zafrir Fagin, Yissum Research Development Company of the Hebrew University of Jerusalem Ltd., or Yissum, and Prof. Oded Shoseyov (our chief scientific officer and a director), we carried out a research and development project to develop a process for the production of quality human collagen in plants and further developed the resulting product candidates created by us, Professor Shoseyov and Zafrir, for commercial applications. Yissum and Professor Shoseyov have assigned all intellectual property rights owned by them to us, including the intellectual property rights in connection with the development of the method for production of quality human collagen in plants. Pursuant to this agreement, in the event of (1) our ceasing development of a product for more than 18 months without Yissum consent, (2) the appointment of a receiver or liquidator of all or substantially all of our assets, which appointment is not removed within sixty days, (3) our passing a resolution for a voluntary winding up, or a winding up application being made against us and not set aside within sixty days, or (4) our making an assignment of rights or other assets for the benefit of our creditors, all rights in such patents and trade secrets revert to Yissum. In connection with this reversion right, we agreed that all intellectual property rights that can be registered in connection with such project would be registered with a 1% ownership interest in Yissum's name, solely in order to protect Yissum's reversion right. However, to date, all of our patents regarding our rhCollagen have been registered (or are proposed to be registered) with our wholly owned subsidiary, CollPlant Ltd., as 100% owner, without reflecting Yissum's 1% interest. Accordingly, Yissum may claim that such intellectual property rights should be registered in accordance with the terms of the agreement.
Government Regulation
We are a developer of tissue products which are subject to extensive regulation as medical devices and medical products in the United States, the European Union and other jurisdictions. These regulations govern, among other things, the introduction of new medical devices, the observance of certain standards with respect to the design, manufacture, testing, promotion and sales of the devices, the maintenance of certain records, the ability to track devices, the reporting of potential product defects, the import and export of devices, and other matters.
96
As a medical device company that wishes to obtain marketing authorization in the United States, we are subject to extensive regulation by the FDA and other federal, state, and local regulatory agencies. The Federal Food, Drug, and Cosmetic Act, or FD&C Act, the Public Health Service Act, or the PHS Act, and their implementing regulations set forth, among others, requirements for the research, testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record keeping, reporting, distribution, import, export, advertising, and promotion of our product candidates. A failure to comply with relevant requirements may lead to administrative, civil, or criminal sanctions. These sanctions could include the imposition by the FDA, of a clinical hold or other suspension on clinical trials, refusal to approve pending marketing applications or supplements, withdrawal of approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties, or criminal prosecution.
Although the discussion below focuses on regulation in the United States, we anticipate seeking approval for the marketing of our product candidates in other countries which have their own regulatory requirements. Generally, our activities in other countries will be subject to regulations that are similar in nature and scope as that imposed in the United States such as medical device approval, quality system requirements, product data and certifications, although there can be important differences and the number and scope of these regulatory requirements are generally increasing.
We must obtain approval by comparable regulatory authorities of foreign countries outside of the European Union and the United States before we can commence clinical trials or marketing of our product candidates in those countries. The approval process varies from country to country and the process may be longer or shorter than that required for FDA approval. In addition, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country. In all cases, clinical trials must be conducted in accordance with the FDA's regulations, commonly referred to as good clinical practices, or GCPs, and the applicable regulatory requirements and ethical principles that have their origin in the Declaration of Helsinki.
Government regulation may delay or prevent testing or marketing of our product candidates and impose costly procedures upon our activities. The testing and approval process, and the subsequent compliance with appropriate statutes and regulations, require substantial time, effort, and financial resources, and we cannot be certain that the FDA or any other regulatory agency will grant approvals for our product candidates or any future product candidates on a timely basis, or at all. The policies of the FDA or any other regulatory agency may change and additional governmental regulations may be enacted that could prevent or delay regulatory approval of our product candidates or any future product candidates or approval of new indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative, judicial, or administrative action, either in the United States or abroad.
Approval by Health Authorities
The following is a summary review of the laws and regulations governing our operations. Our product candidates are medical product candidates, and their marketing, once development is complete, is contingent upon approval of the health authorities in every country in which the product candidates will be marketed:
Israel
Conducting clinical trials on human subjects in Israel requires approval from the IRB operating at the medical institution in which the trials are to be carried out. In certain cases, it is also necessary to obtain the approval of the Ministry of Health. A similar procedure exists in most countries. We have received all of the IRB and Ministry of Health approvals required for conducting the clinical trials on our product candidates in Israel from 2012 to 2015.
97
The Medical Devices and Instruments Unit at the Ministry of Health is the body responsible for granting permits to import various kinds of medical devices and instruments according to its registry, monitoring the marketing of medical devices and instruments in Israel, and approving clinical trials in medical devices and instruments.
United States
The regulatory process of obtaining product approvals and clearances can be onerous and costly. Foreign companies manufacturing medical devices intended for sale in the United States are required to meet the FDA's regulatory requirements. The FDA does not recognize the regulatory certification provided by institutions of other countries.
Pre-Marketing Regulation
In the United States, medical devices are regulated by the FDA. Unless an exemption applies, a new medical device will require either prior 510(k) clearance or approval of a premarket approval application, or PMA, before it can be marketed in the United States. The information that must be submitted to the FDA in order to obtain clearance or approval to market a new medical device varies depending on how the medical device is classified by the FDA. Medical devices are classified into one of three classes on the basis of the controls deemed by the FDA to be necessary to reasonably ensure their safety and effectiveness. Class I devices, which are those that have the lowest level or risk associated with them, are subject to general controls, including labeling, premarket notification, and adherence to the QSR. Class II devices are subject to general controls and special controls, including performance standards. Class III devices, which have the highest level of risk associated with them, are subject to most of the previously identified requirements as well as to premarket approval. Most Class I devices and some Class II devices are exempt from the 510(k) requirement, although manufacturers of these devices are still subject to registration, listing, labeling and QSR requirements.
A 510(k) premarket notification must demonstrate that the device in question is substantially equivalent to another legally marketed device, or predicate device, that did not require premarket approval. In evaluating the 510(k), the FDA will determine whether the device has the same intended use as the predicate device, and (a) has the same technological characteristics as the predicate device, or (b) has different technological characteristics, and (i) the data supporting the substantial equivalence contains information, including appropriate clinical or scientific data, if deemed necessary by the FDA, that demonstrates that the device is as safe and as effective as a legally marketed device, and (ii) does not raise different questions of safety and effectiveness than the predicate device. Most 510(k)s do not require clinical data for clearance, but the FDA may request such data. If the FDA does not agree that the new device is substantially equivalent to the predicate device, the new device will be classified in Class III, and the manufacturer must submit a PMA.
The PMA process is more complex, costly, and time consuming than the 510(k) clearance procedure. A PMA must be supported by extensive data including, but not limited to, technical, preclinical, clinical, manufacturing, control, and labeling information to demonstrate to the FDA's satisfaction the safety and effectiveness of the device for its intended use. After a PMA is submitted, the FDA has 45 days to determine whether it is sufficiently complete to permit a substantive review. If the PMA is complete, the FDA will file the PMA. The FDA is subject to performance goal review times for PMAs and may issue a decision letter as a first action on a PMA within 180 days of filing, but if it has questions, it will likely issue a first major deficiency letter within 150 days of filing. It may also refer the PMA to an FDA advisory panel for additional review, and will conduct a preapproval inspection of the manufacturing facility to ensure compliance with the QSR, either of which could extend the 180-day response target. A PMA can take several years to complete, and there is no assurance that any submitted PMA will ever be approved. Even when approved, the FDA may limit the
98
indication for which the medical device may be marketed. Changes to the device, including changes to its manufacturing process, may require the approval of a supplemental PMA.
If a medical device is determined to present a "significant risk," the manufacturer may not begin a clinical trial until it submits an investigational device exemption, or IDE, to the FDA and obtains approval of the IDE from the FDA. The IDE must be supported by appropriate data, such as animal and laboratory testing results and include a proposed clinical protocol. The clinical trials must be conducted in accordance with applicable regulations, including but not limited to the FDA's IDE regulations and current good clinical practices. A clinical trial may be suspended by the FDA or the sponsor at any time for various reasons, including a belief that the risks to the study participants outweigh the benefits of participation in the trial. Even if a clinical trial is completed, the results may not demonstrate the safety and efficacy of a device, or may be equivocal or otherwise not be sufficient to obtain approval.
In August 2010, we submitted a 510(k) notification to the FDA for VergenixWD, a collagen-based non-invasive dressing. In October 2010, we received notice that the Center for Devices and Radiological Health, or CDRH, which is the FDA center with jurisdiction over medical devices, determined that the product candidate required a submission of a PMA for regulatory approval and not a 510(k). We filed an appeal of this decision which was denied, and in April 2012, the FDA confirmed its previous determination that our product candidate would require PMA approval prior to its marketing in the United States.
We expect, based on our prior limited interaction with the FDA in connection with our predecessor wound healing product candidate, that our current product candidates will be regulated as medical devices through a PMA process; however, no assurance can be given that the FDA will not impose additional, more stringent, regulatory requirements with respect to one or more of our current or future product candidates. Conducting clinical trials for our pipeline product candidates that are required to undergo the PMA process may take one to three years, depending on the composition of the product candidate under development and its designation.
To date, we are not conducting any discussions with the FDA in respect of any of our product candidates.
European Union
Under the European Union Medical Device Directive, or EU MDD, medical devices must meet the EU MDD requirements and receive a CE marking certification prior to marketing in the European Union, or EU. CE marking is the uniform labeling system of products designed to facilitate the supervision and control of the EU concerning manufacturers' compliance with the various regulations and directives of the EU and to clarify the obligations imposed in the various legislative provisions in the EU. Use of a uniform product labeling indicates compliance with all of the directives and regulations required for the application of such labeling, and it is effective as a manufacturer's declaration that the product meets the required criteria and technical specifications of the relevant authorities such as health, safety, and environmental protection. CE marking ensures free trade between the EU and European Free Trade Association countries (Switzerland, Iceland, Liechtenstein, and Norway) and permits the enforcement and customs authorities in European countries not to allow the marketing of similar products that do not bear the CE marking sign. Such certification allows, among other things, marking the products (according to various categories) with the CE marking and their sale and marketing in the EU.
CE marking certification requires a comprehensive quality system program, comprehensive technical documentation and data on the product, which are then reviewed by a Notified Body, or NB. An NB is an organization designated by the national governments of the EU member states to make independent judgments about whether a product complies with the EU MDD requirements and to
99
grant the CE marking if we, and our product, comply with specified terms. After receiving the CE marking, we must pass a review carried out by the competent NB annually, under which it audits our facilities to verify our compliance with the ISO 13485 quality system standard.
Compliance with the ISO 13485 standard, for medical device quality management systems, is required for regulatory purposes. ISO standards are recognized international quality standards that are designed to ensure that we develop and manufacture quality medical devices. Other countries are also instituting regulations regarding medical devices. Compliance with these regulations requires extensive documentation and clinical reports for all of our product candidates, revisions to labeling, and other requirements such as facility inspections to comply with the registration requirements.
In December 2012, we received the CE mark permitting the sale and marketing of VergenixWD in Europe. VergenixWD is our first medical product candidate based on collagen protein derived from plants that is authorized for sale and marketing in Europe, but we are not currently promoting a marketing strategy for VergenixWD, which is considered a commodity product and is not targeted towards the advanced wound care market, which is our target market. To date, we are also in contact with an NB in Europe in respect of VergenixSTR and VergenixFG.
China
China's medical device market, currently in a rapid state of expansion, is overseen by the China Food and Drug Administration, or CFDA (formerly the State Food and Drug Administration). The CFDA issues registration certificates required for all medical devices sold in China. The CFDA uses a risk-based system, and its approval process requires mandatory testing for Class II and III devices. Class II devices are moderate-risk devices and class III devices are high-risk medical devices. Third-party reviews of devices are currently not allowed in China; only the CFDA is authorized to approve devices. The registration process requires the submission of a registration standard along with device samples for testing. Manufacturers of Class II and Class III medical devices are also required to demonstrate that the device has been approved by the country of origin with documents like a CE certificate, 510(k) letter and PMA approval and compliance with ISO 13485, and they may also be required to submit clinical data in support of their application. In addition to these requirements, all medical device manufacturers must also include product information in Chinese on all packaging and labeling. Manufacturers exporting medical devices to China must appoint several China-based agents to act on their behalf. These include a registration agent to coordinate the CFDA registration process, a legal agent to handle any adverse events reported with a registered device, including a product recall, and an after-sales agent to provide technical service and maintenance support.
Other U.S. Federal Healthcare Laws and Regulations
Healthcare providers, physicians, and third-party payors play a primary role in the recommendation and medical devices that are granted marketing approval. In the United States, we are subject to laws and regulations pertaining to healthcare fraud and abuse, including anti-kickback laws and physician self-referral laws that regulate the means by which companies in the health care industry may market their products to hospitals and health care providers and may compete by discounting the prices of their products. The delivery of our product candidates is subject to regulation regarding reimbursement, and federal healthcare laws apply when a customer submits a claim for a product that is reimbursed under a federally funded healthcare program. These rules require that we exercise care in structuring our sales and marketing practices and customer discount arrangements.
Arrangements with healthcare providers, third-party payors, and other customers are subject to broadly applicable fraud and abuse and other healthcare laws and regulations, including the following:
- •
- the federal healthcare Anti-Kickback Law prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in
100
- •
- the U.S. False Claims Act imposes civil penalties, and provides for civil whistleblower or qui tam actions, against individuals or
entities for knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease, or conceal an
obligation to pay money to the federal government;
- •
- the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for executing
a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
- •
- HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act and its implementing regulations, also
imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
- •
- the federal false statements statute prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or
making any materially false statement in connection with the delivery of or payment for healthcare benefits, items, or services;
- •
- the federal transparency requirements under the Health Care Reform Law require manufacturers of drugs, devices, and medical supplies
to report to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians and teaching hospitals and physician ownership and investment
interests; and
- •
- analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payers, including private insurers.
cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order, or recommendation of, any good or service for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid;
Healthcare providers that purchase medical devices generally rely on third-party payors, including, in the United States, the Medicare and Medicaid programs and private payors, such as indemnity insurers, employer group health insurance programs, and managed care plans, to reimburse all or part of the cost of the products. As a result, demand for our product candidates is and will continue to be dependent in part on the coverage and reimbursement policies of these payors. The manner in which reimbursement is sought and obtained varies based upon the type of payor involved and the setting in which the product is furnished and utilized. Reimbursement from Medicare, Medicaid, and other third-party payors may be subject to periodic adjustments as a result of legislative, regulatory, and policy changes as well as budgetary pressures. Possible reductions in, or eliminations of, coverage or reimbursement by third-party payors, or denial of, or provision of uneconomical reimbursement for new products, may affect our customers' revenue and ability to purchase our product candidates. Any changes in the healthcare regulatory, payment, or enforcement landscape relative to our customers' healthcare services has the potential to significantly affect our operations and revenue.
Other Approvals
Our international operations as well as being an Israeli company subject us to laws regarding sanctioned countries, entities, and persons; customs, import-export, and laws regarding transactions in foreign countries; and the U.S. Foreign Corrupt Practices Act and local anti-bribery and other laws regarding interactions with healthcare providers. Among other things, these laws restrict, and in some cases can prevent, United States companies from directly or indirectly selling goods, technology, or services to people or entities in certain countries. In addition, these laws require that we exercise care in structuring our sales and marketing practices in foreign countries.
101
In addition to the above regulations, we are and may be subject to regulation under country-specific federal and state laws, including, but not limited to, requirements regarding record keeping, and the maintenance of personal information, including personal health information. As a public company whose securities will be registered pursuant to the Securities Act of 1933, as amended, we will be subject to U.S. securities laws and regulations, including the Sarbanes-Oxley Act of 2002. We also are subject to other present, and could be subject to possible future, local, state, federal, and non-U.S. regulations in countries in which we will distribute our products.
Israeli Ministry of Agriculture
The process of growth of transgenic plants and the treatment thereof is subject to the regulations published by the Israeli Ministry of Agriculture and the approval of the Ministry of Agriculture to engage in the cultivation of recombinant plants. Although the Ministry of Agriculture requirements do not necessarily apply to our operations, we hold a valid permit from the Plant Protection and Inspection Services Administration, or PPIS, for growing tobacco plants in greenhouses in the north of Israel, as well as in all of our subcontractors' facilities.
Business Licensing
Under the Israeli Licensing of Businesses Law, to which our production site and laboratories are subject, operating a business without a license or temporary permit is a criminal offense. We have a business license for our laboratories and offices, in effect until December 31, 2019. We are also examining the possibility of obtaining a business license for our plant growth and production site at Yessod Hama'ala. To date we have no valid business license for this site.
Planning and Building
Our production sites and laboratories are subject to the Israeli Planning and Building Law, which sets provisions and obligations, inter alia, regarding the licensing process for a new building, including building permits, non-conforming use and easements, the supervision over its construction, and the required occupancy permits. According to the Planning and Building Law, work or use of land without a permit where such permit is required, a deviation from the permit granted, or use of agricultural land in violation of the law, constitutes a criminal offense. As stated above, to date we have no valid business license for our site in Yessod Hama'ala.
Employees
As of March 31, 2015, we had 34 full-time employees, including 19 in research and development, six in manufacturing and five in general and administrative positions. 12 of our employees have either MDs or PhDs. All of our employees are located in Israel. We believe our employee relations are good.
In addition, we employ a limited number of part-time employees on a temporary basis, as well as consultants and service providers.
Israeli labor laws govern the length of the workday, minimum wages for employees, procedures for hiring and dismissing employees, determination of severance pay, annual leave, sick days, advance notice of termination of employment, equal opportunity and anti-discrimination laws, and other conditions of employment. Subject to specified exceptions, Israeli law generally requires severance pay upon the retirement, death, or dismissal of an employee, and requires us and our employees to make payments to the National Insurance Institute, which is similar to the U.S. Social Security Administration. Our employees have defined benefit pension plans that comply with the applicable Israeli legal requirements.
None of our employees currently work under any collective bargaining agreements.
102
Facilities
Our corporate headquarters and research facilities are located in Weizmann—the Science Park in Ness-Ziona, Israel, where we lease an aggregate of approximately 7,653 square feet of office and laboratory space, pursuant to lease agreements that expire on August 30, 2017. We rent additional areas in Yessod Hama'ala of approximately 64,583 square feet of greenhouse and manufacturing facility pursuant to a lease agreement that expire on April 30, 2017.
The majority of our research and development work is carried out at our offices and research laboratories in the Science Park—Kiryat Weizmann in Ness-Ziona, Israel. The plant research process and production of our rhCollagen are carried out at our site in the north of Israel, while the tobacco plant cultivation and collagen purification are carried out in various areas in Israel. Our greenhouses for tobacco growing are located in several areas in Israel, where we are using subcontractors under several agreements. The greenhouses are used by us for growing tobacco plants and other development services.
We believe that our existing facilities are adequate for our near-term needs. When our leases expire, we may look for additional or alternate space for our operations. We believe that suitable additional or alternative space and area would be available if required in the future on commercially reasonable terms.
Environmental, Health and Safety Matters
Our research, development, and manufacturing processes involve the controlled use of certain hazardous materials. Therefore, we are subject to extensive environmental, health, and safety laws and regulations in a number of jurisdictions, in Israel, governing, among other things: the use, storage, registration, handling, emission, and disposal of chemicals, waste materials, and sewage; chemicals, air, water and ground contamination; air emissions, and the cleanup of contaminated sites, including any contamination that results from spills due to our failure to properly dispose of chemicals, waste materials, and sewage. Our operations at our Ness-Ziona manufacturing facility use chemicals and produce waste materials and sewage. Our activities require permits from various governmental authorities including local municipal authorities, the Ministry of Environmental Protection, and the Ministry of Health. The Ministry of Environmental Protection and the Ministry of Health, local authorities and the municipal water and sewage company conduct periodic inspections in order to review and ensure our compliance with various regulations.
These laws, regulations and permits could potentially require the expenditure by us of significant amounts for compliance or remediation. We believe that our environmental, health, and safety procedures for handling and disposing of these materials comply with the standards prescribed by the controlling laws and regulations. If we fail to comply with such laws, regulations, or permits, we may be subject to fines and other civil, administrative, or criminal sanctions, including the revocation of permits and licenses necessary to continue our business activities. In addition, we may be required to pay damages or civil judgments in respect of third-party claims, including those relating to personal injury (including exposure to hazardous substances we use, store, handle, transport, manufacture, or dispose of), property damage, or contribution claims. These risks are managed to minimize or eliminate associated business impacts. Some environmental, health, and safety laws allow for strict joint and several liability for remediation costs, regardless of comparative fault. We may be identified as a responsible party under such laws. Such developments could have a material adverse effect on our business, financial condition, and results of operations as these kinds of liabilities could exceed our resources. We could be subject to a regulatory shutdown of a facility that could prevent the distribution and sale of product candidates manufactured in such facility for a significant period of time and we could suffer a casualty loss that could require a shutdown of the facility in order to repair it, any of which could have a material, adverse effect on our business. Although we continuously strive to
103
maintain full compliance with respect to all applicable global environmental, health, and safety laws and regulations, we could incur substantial costs to fully comply with future laws and regulations, and our operations, business, or assets may be negatively affected.
In addition, compliance with laws and regulations relating to environmental, health, and safety matters is an ongoing process and are often subject to change. In the event of any changes or new laws or regulations, we could be subject to new compliance measures or to penalties for activities which were previously permitted. For instance, Israeli regulations were promulgated in 2012 relating to the discharge of industrial sewage into the sewer system. These regulations establish new and potentially significant fines for discharging forbidden or irregular sewage into the sewage system. We have compliance procedures in place for employee health and safety programs, driven by a centrally led organizational structure that ensures proper implementation, which is essential to our overall business objectives.
We invest resources in creating a green production environment, and in the treatment and disposal of waste using environmentally friendly processes. We have received all the necessary permits from the Ministry of Environmental Protection regarding our operations in Yessod Hama'ala and Ness-Ziona. We consult with environmental consultants for direction on environmental issues.
Legal and Corporate Structure
Our legal and commercial name is CollPlant Holdings Ltd. We were incorporated in Israel on November 9, 1981 as a private company limited by shares. As of 1993, we are a public company and all of our ordinary shares are listed on the Tel Aviv Stock Exchange. Our name has changed several times, but has been CollPlant Holdings Ltd. since May 30, 2010, immediately after the consummation of the merger transaction with CollPlant Ltd.
We hold all of the issued and outstanding shares of CollPlant Ltd. and have no holdings in other companies.
CollPlant Ltd. was incorporated in Israel on August 12, 2004 as a private company limited by shares and began its operations as a technology incubator company under the Israeli Chief Scientist's technology incubators program. CollPlant Ltd. owns all of our intellectual property.
Legal Proceedings
To date, we are a party to the following legal proceedings:
Opposition proceedings to European patent No. 0 951 537 B1
On August 2, 2006, we initiated at the European Patent Office, or EPO, opposition proceedings to European patent EPO published in the name of Meristem Therapeutics SA, or Meristem, relating to the production of recombinant collagen in plants. To the best of our knowledge, patent opposition proceedings were also initiated by Fibrogen Inc. In addition, to the best of our knowledge, Meristem's patent rights in Europe and Canada expired as a result of failure to make payment of the annual renewal fees. The patent application filed by Meristem in the United States matured into a patent (U.S. 6,617,431) which, to the best of our knowledge, does not limit our business. To the best of our knowledge, the opposition proceedings in Europe continued at the request of the second entity opposing these proceedings (Fibrogen Inc.), and in the absence of a defense on the part of Meristem, on October 4, 2010, notice was received from the EPO that the patent was revoked. To the best of our knowledge, on January 30, 2011, Meristem's window for appealing the cancellation of the patent expired.
104
Opposition proceedings to European Patent No. 1 809 751 B1
Our European Patent No. 1 809 751 entitled "Collagen Producing Plants and Methods of Generating and Using Same," was granted by the EPO on September 1, 2010. On June 1, 2011, Fibrogen, Inc. initiated an opposition proceeding with the EPO, seeking revocation of the patent in its entirety on the grounds that the claims were not supported by the contents of the patent, were not novel, and were not inventive. On January 22, 2013, the EPO issued is decision to maintain the patent in amended form with claims that cover genetically modified plants that produce collagen.
On June 3, 2013, Fibrogen, Inc. appealed the decision. On August 1, 2013, we filed an appeal, seeking to expand the scope of the patent. While we believe that we will prevail in our appeal and that Fibrogen's appeal will be rejected, the ultimate outcome of these proceedings remain uncertain, and final resolution of the proceeding may take a number of years and result in substantial costs to us.
105
Executive Officers and Directors
The following table sets forth certain information relating to our executive officers and directors, including their ages as of the date of this prospectus. Unless otherwise stated, the address for our directors and executive officers is at the Company's registered address c/o 3 Sapir Street, Weizmann Science Park, P.O. Box 4132, Ness-Ziona 74140, Israel.
Name
|
Age | Position | |||
|---|---|---|---|---|---|
Yaron Yaniv(6)(7)(8) |
59 | Chairman and Director Chairman of the Financing Committee |
|||
Yehiel Tal |
63 | Chief Executive Officer | |||
Eran Rotem, CPA |
47 | Chief Financial Officer | |||
Prof. Oded Shoseyov |
59 | Founder, Chief Scientific Officer and Director | |||
Xiaojin Qian |
33 | Director | |||
Ira Leiderman(5)(6)(7)(8) |
58 | Director | |||
Nira Dror(1)(2)(3)(5)(6)(7)(8) |
61 | Director Chairman of the Nominating and Corporate Governance Committee |
|||
Adi Goldin(6) |
41 | Director | |||
Orli Tori(1)(2)(3)(4)(7)(8) |
50 | Director | |||
Rami Armon(1)(2)(3)(4)(8) |
44 | Director Chairman of the Audit Committee Chairman of the Financial Statements Committee Chairman of the Compensation Committee |
|||
Dr. Nadav Orr |
58 | Vice President, Research and Development | |||
Dr. Philippe Bensimon |
50 | Vice President, Regulatory Affairs and Quality Assurance | |||
- (1)
- Member
of the Compensation Committee
- (2)
- Member
of the Audit Committee
- (3)
- Member
of Financial Statements Committee
- (4)
- External
Director under Israeli Law
- (5)
- Independent
Director under Israeli Law
- (6)
- Member
of Financing Committee
- (7)
- Member
of the Nominating and Corporate Governance Committee
- (8)
- Independent Director under NASDAQ Listing Rules
Executive Officers
Yaron Yaniv has served on our board of directors since January 2014, and as Chairman since May 2014. Mr. Yaniv has 30 years of experience in managing commercial and industrial companies. Between 2000-2013, as chief executive officer of three companies in parallel, Eldan Electronic Instruments Ltd., Israel Pharma Services, and Cure Medical & Technical Supply Ltd., he specialized in the distribution of cutting edge technology medical devices and equipment as well as biotechnology products. Mr. Yaniv has a track record of proven success in company recovery—from chronic losses to significant profits through leadership, proactivity and solid relations at all levels with employees, customers, suppliers, and business partners.
106
Yehiel Tal has served as our chief executive officer since January 2010. Mr. Tal possesses over 22 years of management experience in the Israeli and American high-tech and biotechnology industries. Prior to joining us, Mr. Tal was the chief executive officer and co-founder of Regentis Biomaterials Ltd. Prior to that Mr. Tal served as vice-president of business development at ProChon BioTech Ltd. He has also served as vice president of marketing and business development at OrthoScan Technologies Ltd. and director of business development and business unit manager at Kulicke and Soffa Industries, Inc. Mr. Tal holds a Bachelor's and a Master's degree in mechanical engineering from the Technion, Israel Institute of Technology.
Eran Rotem has served as our chief financial officer since January 2012. Mr. Rotem possesses 20 years of broad financial and operational experience, primarily with biotechnology and industrial companies. Prior to joining us, Mr. Rotem served as the chief financial officer of Tefron Ltd., an industrial global company traded on both the Tel Aviv Stock Exchange (TASE:TFRN) and on the OTCBB (OTC:TFRFF) in the United States. Before Tefron, Mr. Rotem served as chief financial officer of Healthcare Technologies, Ltd. (NASDAQ:HCTL) and Gamida Ltd., a group of companies that specialize in the development, manufacturing, and marketing of clinical diagnostic test kits, as well as medical equipment and services to the biotechnology and high-tech industries. Prior to joining Healthcare Technologies, Ltd., Mr. Rotem served as a senior manager at Ernst & Young. Mr. Rotem holds a Bachelor's degree in Accounting and Business Administration from the Tel Aviv College of Management and is a Certified Public Accountant.
Prof. Oded Shoseyov founded our company in 2004 and has served as our chief scientific officer since August 2008. Prof. Shoseyov is a faculty member of the Hebrew University of Jerusalem. He has extensive experience with plant transformation systems and protein engineering. Prof. Shoseyov has authored or co-authored over 160 scientific publications and is the inventor or co-inventor of 45 patents. Prof. Shoseyov holds a Ph.D. from The Hebrew University of Jerusalem, Israel. Prof. Shoseyov received the Outstanding Scientist Polak Award for 2002, the 1999 and 2010 Kay Awards for Innovative and Applied Research, and The 2012 Israel Prime Minister Citation for Entrepreneurship and Innovation. He is the scientific founder of nine companies, including: Fulcrum Materials Ltd., a nano-biotech company which manufactures SP1-Carbon Nano Tube coated fabrics for the composite industry; CBD-Technologies/FuturaGene, a forestry agro-biotech company that develops and commercializes transgenic trees for the pulp and paper and the bio-fuel industry; Melodea Ltd., a nano-biotech company that develops and manufactures Nano Crystaline Cellulose from sludge for structural foam and fuel for combustion and jet engines as well as explosives; and Valentis Nanotech. Ltd., a nanotechnology company that develops and manufactures nano-bio-based transparent films for food packaging and agriculture.
Xiaojin Qian has served on our board of directors since November 2013. Mr. Qian is the managing director of Flon (China) Medical Material Co., Ltd. and a board member of Jiangsu Traumark Holdings Group Co., Ltd. and Trauwin Pte Ltd. Until March 2013, Mr. Qian served as vice president of Trauson Holding Co., Limited, a leading manufacturer of orthopedic products in China. Previously, Mr. Qian was director of public relations, secretary of the board, and secretary to the president of Trauson. Mr. Qian is experienced in maintaining relationships with public and other companies and in carrying out operational management duties. Mr. Qian graduated from Simon Fraser University, Canada, with a Master of Business Administration in 2009.
Ira Leiderman has served on our board of directors as an independent director since February 2015. Mr. Leiderman is a founder and managing partner of Long Trail Advisors LLC, a life sciences advisory firm and M&A boutique. Mr. Leiderman formed Long Trail after his departure from Ladenburg Thalmann in New York City where he was co-head of the healthcare group. Mr. Leiderman joined Ladenburg Thalmann when that firm acquired Punk Ziegel & Company in May 2008. At Punk Ziegel, Mr. Leiderman served as head of healthcare and sat on the firm's management committee. Prior to Punk Ziegel, Mr. Leiderman was at the Palladin Group, an investment management firm
107
where he oversaw investment transactions in public and private life sciences companies. Mr. Leiderman joined Palladin after leading the healthcare practice at Gerard Klauer Mattison (now part of BMO). Mr. Leiderman currently serves on the board of directors of MarginSurgical, Inc. and was chairman of the board of directors of Apthera Inc. Mr. Leiderman also serves on the boards of several not-for-profit organizations.
Nira Dror has served on our board of directors as an independent director since February 2015. Ms. Dror is the owner and the general manager of Nira Dror Ltd., a company which she founded in 2006 and which provides consulting and representation services for companies. From 2003 to 2005, Ms. Dror served as deputy general manager and as general manager of North America at El Al Israel Airlines Ltd. From 1999 to 2003, Ms. Dror served as general manager of Eastern Europe and East Mediterranean, and from 1989 to 1999, as general manager for Israel, at British Airways Plc. From 1986 to 1989, Ms. Dror served as the general manager of Histour Ltd., a tourism company, and from 1979 to 1985, Ms. Dror served as the chief economist for Teus Azorei Pituach Ltd. Ms. Dror currently serves as chairperson of the board of BHI Global Investments Advisory (Israel) . Ms. Dror also currently serves on the board of directors and as a member of the audit committee of the following companies: ClickSoftware Technologies Ltd., S. Shlomo Insurance Company Ltd., S. Shlomo Holding Ltd., and Sharonim Ltd. From 2006 to 2015, Ms. Dror served on the board of the directors and as a member of the audit committee of Dikla Insurance Company Ltd. and from 2012 to 2014, on the board of directors and as chairperson of the audit committee of Shemen Oil and Gas Resources Ltd. Ms. Dror served on the board of directors and as a member of the audit committee of Tzur Shamir Holdings Ltd. from 2006 to 2013, and as a member of the board and a chairperson of the audit committee of Bank Hapoalim Ltd. from 2006 to 2012. Ms. Dror was a member of the board of directors of H&O Ltd. from 2005 to 2008, and of Yoav Mineral Bath Tourism (1992) Ltd. from 2005 to 2008. Ms. Dror is a partner in AfterDox Ltd., a group of smart angels that invests in early stage Israeli High-Tech companies. Ms. Dror was also a member of the executive committee of the panel of airlines in Israel from 1990 to 1999. Ms. Dror holds a B.A. in Economics and Business Administration and an M.B.A. from Tel Aviv University, Israel.
Adi Goldin has served on our board of directors since May 2010. Mr. Goldin has over 15 years of experience in the life science, industrial, and technology industries in the areas of investments, business strategy, deal structure, and company management. For the last 10 years Mr. Goldin has served as a vice president at Docor International BV, and has played a key role in investing, managing, and nurturing technology-driven companies and startups in the information technology, industrial, and life science industries. Until 2010, Mr. Goldin was the chief executive officer of Softlib Ltd., an information technology company. Previously, Mr. Goldin was VP of investments and analysis at Inventech Central Hotels Ltd. (TASE: IVTC), where he took an active role in building startup companies and was involved in public offerings, M&A, and all aspects of the capital markets. Mr. Goldin practiced law in the law office of Israeli, Blustein, Kogan & Co. In addition, Mr. Goldin was part of the teaching staff of the Master's in Business Administration program run jointly by Tel Aviv University and Northwestern University's Kellogg School of Management (Chicago, USA). Mr. Goldin participated in the International Marketing and Global Consulting Program, a joint project of the University of Pennsylvania's Wharton Business School and Tel Aviv University's Business School. Mr. Goldin is a member of the Israel Bar Association. Mr. Goldin holds Bachelor's and Master's degrees in economics, summa cum laude, and an LL.B. in law from Tel Aviv University, Israel.
Orli Tori has served on our board of directors as an external director since March 2014 and is a member of our audit, compensation, and financial statements committees. Ms. Tori has a strong background in both life sciences and business. Ms.Tori is currently the CEO of Bar Ilan Research and Development Company Ltd. Prior to that she served as General Manager of Neopharm Israel, a diversified company combining activity in pharmaceuticals, medical and scientific devices, diagnostics, and consumer health care products and services. Ms. Tori obtained her Master's degree in microbiology, cum laude, and Bachelor's degree in Life Sciences at Tel Aviv University in Tel Aviv,
108
Israel, and has studied economics and management of health systems at Ben Gurion University in Beer Sheva, Israel. Ms. Tori graduated from the Executive Program for senior business managers at the Tel Aviv University School of Business.
Rami Armon has served on our board of directors as an external director since October 2011, and is the chairman of our audit, compensation, and financial statements committees. Mr. Armon is the chief executive officer and founder of Armon Capital Management Ltd., a company that offers services in the area of private equity, investment services, and corporate finance. Previously, Mr. Armon was vice president and chief investment officer of Menora Mivtachim Pension Ltd. Mr. Armon holds a Bachelor's degree from Bar Ilan University, Israel, where he studied investments and securities.
Dr. Nadav Orr has served as our vice president of research and development since September 2014. Dr. Orr has over 15 years of experience in research and development, including nine years in the development of biosurgery products. Prior joining us, Dr. Orr served as the associate director of research and development at Omrix Biopharmaceuticals Ltd., a subsidiary of Ethicon US LLC, part of the Johnson & Johnson family of companies. As part of his role at Omrix, Dr. Orr led an international team in the development of hemostatic combination products and led base business support for production processes and products. Dr. Orr holds a PhD from the Weizmann Institute of Science, Israel.
Dr. Philippe Bensimon has served as our vice president of regulatory affairs and quality assurance since February 2011. Dr. Bensimon has 19 years of experience in regulatory affairs, quality assurance and clinical affairs in international medical device companies. Prior to joining us Dr. Bensimon served for 14 years at InterVascular Datascope (now Maquet-Getinge Group), a manufacturer of long-term cardiovascular implants, including as director of regulatory affairs, quality assurance, and clinical affairs. Dr. Bensimon also served for five years at 3M Medical as manager of regulatory affairs. Dr. Bensimon holds a PharmD degree from the University of Pharmacy, Marseille, France.
Advisory Boards
We have established two advisory boards with specific expertise which include a scientific advisory board and a clinical advisory board.
Scientific Advisory Board
Prof. Avraham Heshko
Prof. Vicki Rosen
Prof. Abhay Pandit
Arthur Gertzman
Prof. Ofer Levy, MD, MCh (Orth)
Joseph M. Lane, MD
Clinical Advisory Board
Prof. Ofer Levy, MD, MCh (Orth)
Joseph M. Lane, MD
Scott Rodeo, MD
Thomas Serena, MD
Gabi Agar, MD
Corporate Governance Practices
Under the Companies Law, companies incorporated under the laws of the State of Israel, whose shares are publicly traded, including companies whose shares are listed on the NASDAQ Stock Market, or NASDAQ, are considered public companies under Israeli law and are required to comply with various corporate governance requirements under Israeli law relating to such matters as external directors, the audit committee, compensation, policy, company's auditors, and an internal auditor. This
109
is the case even if our shares are not listed on the Tel Aviv Stock Exchange. These requirements are in addition to the corporate governance requirements imposed by NASDAQ rules also referred to as the NASDAQ listing requirements, and other applicable provisions of U.S. securities laws to which we will become subject (as a foreign private issuer) upon the closing of this offering and the listing of our ADSs on NASDAQ. Under the NASDAQ listing requirements, a foreign private issuer, such as us, may generally follow its home country rules of corporate governance in lieu of the comparable requirements of NASDAQ, except for certain matters including (among others) the composition and responsibilities of the audit committee and the independence of its members within the meaning of the rules and regulations of the SEC.
We intend to rely on this "home country practice exemption" with respect to the following NASDAQ rules:
- •
- Quorum requirements. As permitted under the Companies Law
pursuant to our articles of association, the quorum required for an ordinary meeting of shareholders will consist of at least two shareholders present in person, by proxy or by other voting instrument
in accordance with the Companies Law, who hold at least 25% of the voting power of our shares (and in an adjourned meeting, with some exceptions, any number of shareholders), instead of
331/3% of the issued share capital required under the NASDAQ Listing Rules.
- •
- Nomination of our directors. With the exception of our
external directors and directors elected by our board of directors due to vacancy, according to our articles of association, our directors are elected by an annual meeting of our shareholders to hold
office until the next annual meeting is adjourned, which annual meeting is to be convened before 15 months following the last annual meeting. See "Management—Board of Directors."
The nominations for directors, which are presented to our shareholders by our board of directors, are generally made by the board of directors itself, in accordance with the provisions of our articles
of association and the Companies Law. Nominations need not be made by a nominating committee of our board of directors consisting solely of independent directors or a majority of the independent
directors in a vote in which only independent directors can participate, as required under the NASDAQ Listing Rules. Nominations may also be made by one or more of our shareholders, as permitted in
our articles of association and under the Companies Law.
- •
- Distribution of certain reports to shareholders. As
opposed to the NASDAQ Listing Rules, which require listed issuers to make certain reports, such as annual reports, interim reports, and quarterly reports, available to shareholders in one of a number
of specific manners, Israeli law does not require us to distribute periodic reports directly to shareholders. The generally accepted business practice in Israel is not to distribute such reports to
shareholders, but to make such reports available through a public website. In addition to making such reports available on a public website, we plan to make our audited financial statements available
to our shareholders at our offices and will only mail such reports to shareholders upon request. As a foreign private issuer, we are generally exempt from the SEC's proxy solicitation rules. See
"Where You Can Find More Information" for a description of our Exchange Act reporting obligations.
- •
- Compensation of officers. We follow the provisions of the Companies Law with respect to matters in connection with the composition and responsibilities of our compensation committee, office holder compensation and any required approval by the shareholders of such compensation. Israeli law and our articles of association do not require that the independent members of our board of directors, or a compensation committee composed solely of independent members of our board of directors, determine an executive officer's compensation, as is generally required under the NASDAQ Listing Rules with respect to the Chief Executive Officer and all other executive officers of a company. However, Israeli law and our articles of association do require that our audit and compensation committee each contain two external directors. In addition, Israeli law requires that additional members of the compensation
110
- •
- Independent directors. Israeli law does not require that
a majority of the directors serving on our board of directors be "independent," as defined under NASDAQ Listing Rule 5605(a)(2), but rather requires we have at least two external directors who
meet the requirements of the Companies Law. We are required, however, to ensure that all members of our audit committee are "independent" under the Companies Law and the applicable NASDAQ and SEC
criteria for independence, and under Israeli law, the audit committee and compensation committee must each include all external directors then serving on our board of directors. We must also ensure
that a majority of the members of our audit committee are "unaffiliated directors" as defined in the Companies Law. However, while not required by Israeli law, as of the date of this prospectus, a
majority of our directors are independent.
- •
- Shareholder approval. We will seek shareholder approval for all corporate actions requiring such approval under the requirements of the Companies Law, rather than seeking approval for corporate actions in accordance with NASDAQ Listing Rule 5635. In particular, under this NASDAQ Listing Rule, shareholder approval is generally required for: (i) an acquisition of shares or assets of another company that involves the issuance of 20% or more of the acquirer's shares or voting rights or if a director, officer or 5% shareholder has greater than a 5% interest in the target company or the consideration to be received; (ii) the issuance of shares leading to a change of control; (iii) adoption or amendment of equity compensation arrangements; and (iv) issuances of 20% or more of the shares or voting rights (including securities convertible into, or exercisable for, equity) of a listed company via a private placement (or via sales by directors, officers or 5% shareholders) if such equity is issued (or sold) at below the greater of the book or market value of shares. By contrast, under the Companies Law, shareholder approval is required for, among other things: (a) transactions with directors concerning the terms of their service or indemnification, exemption and insurance for their service (or for any other position that they may hold at a company), for which approvals of the compensation committee, board of directors and shareholders are all required; (b) extraordinary transactions with controlling shareholders of publicly held companies, which require the special approval described below under "Disclosure of personal interests of controlling shareholders and approval of certain transactions"; (c) terms of office and employment or other engagement of our controlling shareholder, if any, or such controlling shareholder's relative, which require the special approval described below under "Disclosure of personal interests of controlling shareholders and approval of certain transactions; (d) approval of transactions with Company's Chief Executive Officer with respect to his or hers compensation, whether in accordance with the approved compensation policy of the Company or not in accordance with the approved compensation policy of the Company, or transactions with officers of the Company not in accordance with the approved compensation policy; and (e) approval of the compensation policy of the Company for office holders. In addition, under the Companies Law, a merger requires approval of the shareholders of each of the merging companies.
committee and the external directors be compensated equally. Our compensation committee has been established and conducts itself in accordance with the provisions governing the composition of and the responsibilities of a compensation committee as set forth in the Companies Law. Furthermore, compensation of office holders is determined and approved by our compensation committee, and in general, by our board of directors as well, and in certain circumstances by our shareholders. Thus, we will seek shareholder approval for all corporate actions with respect to office holder compensation (including the compensation required to be approved for our Chief Executive Officer) requiring such approval under the requirements of the Companies Law, including seeking prior approval of the shareholders for the compensation policy and for certain office holder compensation, rather than seeking approval for such corporate actions in accordance with NASDAQ Listing Rules.
111
Except as stated above, we intend to comply with the rules generally applicable to U.S. domestic companies listed on NASDAQ, subject to certain exemptions the JOBS Act provides to emerging growth companies. We may in the future decide to use other foreign private issuer exemptions with respect to some or all of the other NASDAQ listing requirements. Following our home country governance practices, as opposed to the requirements that would otherwise apply to a company listed on NASDAQ, may provide less protection than is accorded to investors under NASDAQ listing requirements applicable to domestic issuers.
Board of Directors
Under the Companies Law, the overseeing of the management of our business is vested in our board of directors. Our board of directors may exercise all powers and may take all actions that are not specifically granted to our shareholders or to management. Our executive officers are responsible for our day-to-day management and have individual responsibilities established by our board of directors and specified in their specific employment agreements. Our chief executive officer is appointed by, and serves at the discretion of, our board of directors, subject to the employment agreement that we have entered into with him. All other executive officers are appointed by our chief executive officer with the prior review of our board of directors and compensation committee, and are subject to the terms of any applicable employment agreements that we may enter into with them.
Under our articles of association, our board of directors must consist of at least three and not more than twelve directors, including at least two external directors. Currently our board of directors consists of eight directors, including two external directors. The external directors were nominated by our board of directors and were subject to election at a meeting of our shareholders. Other than external directors, for whom special election requirements apply under the Companies Law, as detailed below, our articles of association provide that directors (other than external directors) are elected annually at the general meeting of our shareholders by a vote of the holders of a majority of the voting power present and voting, in person or by proxy, at that meeting.
According to Israeli Law, we have three classes of directors: independent directors, external directors (who are also independent in nature), and "regular" directors. For purposes of complying with NASDAQ Listing Rules, upon the closing of this offering and the listing on the NASDAQ Capital Market our board of directors will be comprised of five independent directors (of which two are external directors). Mr. Yaron Yaniv, the chairman of our board of directors, is considered an independent director according to the NASDAQ Listing Rules.
Our board of directors has determined that with the exception of Prof. Oded Shoseyov, Mr. Adi Goldin, and Mr. Xiojan Qian, all of our directors are independent under such rules. The definition of "independent director" under NASDAQ rules and "external director" under the Companies Law overlap to a significant degree such that we would generally expect the two directors serving as external directors to satisfy the requirements to be independent under NASDAQ rules. However, it is possible for a director to qualify as an "external director" under the Companies Law without qualifying as an "independent director" under NASDAQ rules, or vice-versa. The definition of external director under the Companies Law includes a set of statutory criteria that must be satisfied, including criteria whose aim is to ensure that there is no factor that would impair the ability of the external director to exercise independent judgment. The definition of independent director under NASDAQ rules specifies similar, if slightly less stringent, requirements in addition to the requirement that the board of directors consider any factor which would impair the ability of the independent director to exercise independent judgment. In addition, external directors serve for a period of three years pursuant to the requirements of the Companies Law. However, external directors must be elected by a special majority of shareholders while independent directors may be elected by an ordinary majority. See "—External Directors" for a description of the requirements under the Companies Law for a director to serve as an external director. In accordance with the exemption available to foreign private issuers under NASDAQ
112
rules, we do not intend to follow the requirements of NASDAQ rules with regard to the process of nominating directors, and instead, will follow Israeli law and practice, in accordance with which our board of directors (or a committee thereof) is authorized to recommend to our shareholders director nominees for election. See "—Corporate Governance Practices" for more information.
Under the Companies Law and our articles of association, nominees for directors may also be proposed by any shareholder holding at least 1% of our outstanding voting power. However, any such shareholder may propose a nominee only if a written notice of such shareholder's intent to propose a nominee has been duly submitted to us. Any such notice must include certain information, including, among other things, a description of all arrangements between the nominating shareholder and the proposed director nominee(s) and any other person pursuant to which the nomination(s) are to be made by the nominating shareholder, the consent of the proposed director nominee(s) to serve as our director(s) if elected and a declaration signed by the nominee(s) declaring that there is no limitation under the Companies Law preventing their election, and that all of the information that is required under the Companies Law and the Israeli Securities Law (if applicable) to be provided to us in connection with such election has been provided.
In addition, our articles of association allow our board of directors to appoint directors to fill vacancies on our board of directors, for a term of office equal to the remaining period of the term of office of the director(s) whose office(s) have been vacated. According to the Companies Law, external directors are elected for an initial term of three years and may be elected for additional three-year terms under the circumstances described below. External directors may be removed from office only under the limited circumstances set forth in the Companies Law. See "—External Directors."
Under the Companies Law, our board of directors must determine the minimum number of directors who are required to have accounting and financial expertise. See "—External Directors" below. In determining the number of directors required to have such expertise, our board of directors must consider, among other things, the type and size of the company and the scope and complexity of its operations. Our board of directors has determined that the minimum number of directors who are required to have accounting and financial expertise is one.
External Directors
Under the Companies Law, we are required to have at least two directors who qualify as external directors. The appointment of external directors was made by a resolution of the general meeting of our shareholders, and our external directors are Mr. Rami Armon and Ms. Orli Tori.
The Companies Law provides that external directors must be elected by a majority vote of the shares present and voting at a shareholders meeting, provided that either:
- •
- such majority includes at least a majority of the shares held by all shareholders who are not controlling shareholders and do not have
a personal interest in the election of the external director (other than a personal interest not deriving from a relationship with a controlling shareholder) that are voted at the meeting, excluding
abstentions, to which we refer as a disinterested majority; or
- •
- the total number of shares voted against the election of the external director by non-controlling shareholders and by shareholders who do not have a personal interest in the election of the external director (other than a personal interest not deriving from a relationship with a controlling shareholder) does not exceed 2% of the aggregate voting rights in the company.
Under the Companies Law, the term "controlling shareholder" is defined in the Companies Law as a shareholder with the ability to direct the activities of the company, other than by virtue of being an office holder. A shareholder is presumed to be a controlling shareholder if the shareholder holds 50% or more of the voting rights in a company or has the right to appoint the majority of the directors of the company or its general manager. With respect to certain matters, a controlling shareholder is
113
deemed to include any shareholder that holds 25% or more of the voting rights in a public company if no other shareholder holds more than 50% of the voting rights in the company, but excludes a shareholder whose power derives solely from his or her position as a director of the company or from any other position with the company.
Under the Companies Law, the initial term of an external director is three years. Thereafter, an external director may be reelected to serve in that capacity for no more than two additional three-year terms, provided that either (i) his or her service for each such additional term is recommended by one or more shareholders holding at least 1% of the company's voting rights and is approved at a shareholders meeting by a disinterested majority, where the total number of shares held by non-controlling, disinterested shareholders voting for such reelection exceeds 2% of the aggregate voting rights in the company, provided that the nominating shareholder, the external director and certain of their related parties meet additional independence requirements; (ii) his or her service for each such additional term is recommended by the board of directors and is approved at a shareholders meeting by the same majority required for the initial election of an external director (as described above); or (iii) the external director has recommended that he or she be nominated for each such additional term and such nomination is approved at a shareholders meeting by the same majority and under the same criteria required as if he had been recommended by a shareholder.
The term of office for external directors for companies traded on certain foreign stock exchanges, including The NASDAQ Capital Market, may be further extended, in increments of additional three-years' term (but no more than additional total of two consecutive such three-years' terms, unless restricted for only one additional consecutive three-years' term under the articles of association), in each case provided that, in addition to reelection in such manner described above, (i) the audit committee and subsequently the board of directors of the company confirm that, in light of the external director's expertise and special contribution to the work of the board of directors and its committees, the reelection for such additional period is beneficial to the company, and provided that (ii) the external director is reelected subject to the same shareholder vote requirements as if elected for the first time (as described above). Prior to the approval of the reelection of the external director at a general shareholders meeting, the company's shareholders must be informed of the term previously served by him or her and of the reasons why the board of directors and audit committee recommended the extension of his or her term.
External directors may be removed from office by a special general meeting of shareholders called by the board of directors, which approves such dismissal by the same shareholder vote percentage required for their election or by a court, in each case, only under limited circumstances, including ceasing to meet the statutory qualifications for appointment, or violating their duty of loyalty to the company. If an external directorship becomes vacant and there are fewer than two external directors on the board of directors at the time, then the board of directors is required under the Companies Law to call a shareholders' meeting as soon as possible (and no longer than in three-months' time following said vacancy) to appoint a replacement external director.
Each committee of the board of directors that exercises the powers of the board of directors must include at least one external director. The audit committee and the compensation committee must include all external directors then serving on the board of directors and should be comprised of a majority of independent directors, the external directors must be the majority of the members of the compensation committee, and the financial statements committee's chairman (as well as of the audit committee's and of the compensation committee's) must be an external director. See "—Committees of the Board of Directors". Under the Companies Law, external directors of a company and all members of the compensation committee are prohibited from receiving, directly or indirectly, any compensation for their services as external directors, other than for their services as external directors pursuant to the Companies Law and the regulations promulgated thereunder. Compensation of an external director is determined prior to his or her appointment and may not be changed during his or her term subject to
114
certain exceptions. Under the regulations pursuant to the Companies Law, certain exemptions and reliefs are granted to companies which securities are traded outside of Israel. We may use those exemptions and reliefs after the registration of our ADSs with the NASDAQ under this offering.
The Companies Law provides that a person is not qualified to serve as an external director if (i) the person is a relative of a controlling shareholder of the company, or (ii) if that person or his or her relative, partner, employer, another person to whom he or she was directly or indirectly subject, or any entity under the person's control, has or had, during the two years preceding the date of appointment as an external director: (a) any affiliation or other disqualifying relationship with the company, with any person or entity controlling the company or a relative of such person, or with any entity controlled by or under common control with the company; or (b) in the case of a company with no shareholder holding 25% or more of its voting rights, had at the date of appointment as external director, any affiliation or other disqualifying relationship with a person then serving as chairman of the board or chief executive officer, a holder of 5% or more of the issued share capital or voting power in the company, or the most senior financial officer.
The term "relative" is defined under the Companies Law as a spouse, sibling, parent, grandparent, or descendant; spouse's sibling, parent, or descendant; and the spouse of each of the foregoing persons. Under the Companies Law, the term "affiliation" and the similar types of prohibited relationships include (subject to certain exceptions):
- •
- an employment relationship;
- •
- a business or professional relationship even if not maintained on a regular basis (excluding insignificant relationships);
- •
- control; and
- •
- service as an office holder, excluding service as a director in a private company prior to the initial public offering of its shares if such director were appointed as a director of the private company in order to serve as an external director following the initial public offering.
The term office holder is defined under the Companies Law as the general manager, chief executive officer, chief business manager, deputy general manager, vice general manager, any other person assuming the responsibilities of any of these positions regardless of that person's title, and a director, or a manager directly subordinate to the general manager.
The external directors must be of Israeli residency (unless the company on which he or she serves, had offered shares (or bonds) to public outside of Israel or are registered on a Stock Exchange outside of Israel) and must possess the minimal criteria required for the directorship of a "regular" director. In addition, no person may serve as an external director if that person's position or professional or other activities create, or may create, a conflict of interest with that person's responsibilities as a director or otherwise interfere with that person's ability to serve as an external director or if the person is an employee of the Israel Securities Authority or of an Israeli stock exchange. A person may furthermore not continue to serve as an external director if he or she received direct or indirect compensation from the company including amounts paid pursuant to indemnification or exculpation contracts or commitments and insurance coverage for his or her service as an external director, other than as permitted by the Companies Law and the regulations promulgated thereunder.
Following the termination of an external director's service on a board of directors, such former external director and his or her spouse and children may not be provided with a direct or indirect benefit by the company, its controlling shareholder, or any entity under its controlling shareholder's control. This includes engagement as an office holder or director of the company or a company controlled by its controlling shareholder or employment by, or provision of services to, any such company for consideration, either directly or indirectly, including through a corporation controlled by the former external director. This restriction extends for a period of two years with regard to the
115
former external director and his or her spouse or child, and for one year with respect to other relatives of the former external director.
If, at the time at which an external director is appointed, all members of the board of directors, who are not controlling shareholders or relatives of controlling shareholders of the company are of the same gender, the external director to be appointed must be of the other gender. A director of one company may not be appointed as an external director of another company if a director of the other company is acting as an external director of the first company at such time.
According to regulations promulgated under the Companies Law, a person may be appointed as an external director only if he or she has professional qualifications or if he or she has accounting and financial expertise (each, as defined below). In addition, at least one of the external directors must be determined by our board of directors to have accounting and financial expertise.
A director with accounting and financial expertise is a director who, due to his or her education, experience, and skills, possesses an expertise in, and an understanding of, financial and accounting matters and financial statements, such that he or she is able to understand the financial statements of the company and initiate a discussion about the presentation of financial data. A director is deemed to have professional qualifications if he or she has: (i) an academic degree in economics, business management, accounting, law, or public administration, (ii) an academic degree or has completed other higher education, in the primary field of business of the company or a field which is relevant to his or her position in the company, or (iii) at least five years of experience serving in one of the following capacities, or at least five years cumulative experience serving in two or more of the following capacities: (a) a senior business management position in a company with a significant volume of business, (b) a senior position in a company's primary field of business, or (c) a senior position in public administration or service. The board of directors is charged with determining whether a director possesses financial and accounting expertise or professional qualifications.
Our board of directors has determined that Mr. Rami Armon, who serves as an external director, and Ms. Nira Dror, who serves as an independent director, have accounting and financial expertise and possesses professional qualifications as required under the Companies Law.
Role of Board of Directors in Risk Oversight Process
Risk assessment and oversight are an integral part of our governance and management processes. Our board of directors encourages management to promote a culture that incorporates risk management into our corporate strategy and day-to-day business operations. Management discusses strategic and operational risks at regular management meetings, and conducts specific strategic planning and review sessions during the year that include a focused discussion and analysis of the risks facing us. Throughout the year, senior management reviews these risks with the board of directors at regular board meetings as part of management presentations that focus on particular business functions, operations or strategies, and presents the steps taken by management to mitigate or eliminate such risks.
Leadership Structure of the Board of Directors
In accordance with the Companies Law and our articles of association, our board of directors is required to appoint one of its members to serve as chairman of the board of directors. Our board of directors has appointed Mr. Yaron Yaniv to serve as chairman of the board of directors and has approved his terms as an active chairman.
116
Committees of the Board of Directors
Currently, our board of directors has four active committees: audit committee, compensation committee, financial statements committee, and financing committee. The first three committees are mandatory and regulated under the Companies Law provisions.
Audit Committee
Under the Companies Law, we are required to appoint an audit committee. The audit committee must be comprised of at least three directors, including all of the external directors, one of whom must serve as chairman of the committee. The audit committee may not include the chairman of the board, any director employed by or otherwise providing services on a regular basis to the company, to a controlling shareholder or to any entity controlled by a controlling shareholder, any director who derives most of his or her income from a controlling shareholder, nor a controlling shareholder or a relative thereof.
In addition, under the Companies Law, the audit committee of a publicly traded company must consist of a majority of unaffiliated directors. In general, an "unaffiliated director" under the Companies Law is defined as either an external director or as a director who meets the following criteria:
- •
- he or she meets the qualifications for being appointed as an external director, except for the requirement (i) that the
director be an Israeli resident (which does not apply to companies such as ours whose securities have been offered (under this offering) outside of Israel to date or are listed outside of Israel) and
(ii) for accounting and financial expertise or professional qualifications; and
- •
- he or she has not served as a director of the company for a period exceeding nine consecutive years. For this purpose, a break of less than two years in the service shall not be deemed to interrupt the continuation of the service.
Under the NASDAQ listing requirements, we are required to maintain an audit committee consisting of at least three independent directors, all of whom are financially literate and at least one of whom has accounting or related financial management expertise.
Our audit committee consists of Mr. Rami Armon, Ms. Nira Dror and Ms. Orli Tori and is chaired by Mr. Armon. Mr. Armon and Ms. Dror possess accounting and financial expertise and are audit committee financial experts as defined by the Securities and Exchange Commission rules, and all of the members of our audit committee have the requisite financial literacy as defined by the NASDAQ Stock Market rules. Mr. Armon and Ms. Dror are "independent" as such term is defined in Rule 10A-3(b)(1) under the Exchange Act and under the listing standards of NASDAQ.
Our board of directors has adopted an audit committee charter to be effective upon the listing of our ADSs on The NASDAQ Capital Market setting forth the responsibilities of the audit committee consistent with the rules of the Securities and Exchange Commission and NASDAQ rules as well as the requirements for such committee under the Companies Law, including the following:
- •
- oversight of our independent registered public accounting firm and recommending the engagement, compensation or termination of
engagement of our independent registered public accounting firm to the board of directors in accordance with Israeli law;
- •
- recommending the engagement or termination of the person filling the office of our internal auditor; and
- •
- recommending the terms of audit and non-audit services provided by the independent registered public accounting firm for pre-approval by our board of directors.
117
Our audit committee provides assistance to our board of directors in fulfilling its legal and fiduciary obligations in matters involving our accounting, auditing, financial reporting, internal control, and legal compliance functions by pre-approving the services performed by our independent accountants and reviewing their reports regarding our accounting practices and systems of internal control over financial reporting. Our audit committee also oversees the audit efforts of our independent accountants and takes those actions that it deems necessary to satisfy itself that the accountants are independent of management.
Under the Companies Law, our audit committee is responsible for:
- •
- determining whether there are deficiencies in our business management practices, including in consultation with our internal auditor
or the independent auditor, and making recommendations to the board of directors to improve such practices;
- •
- determining whether certain acts of an office holder not in accordance with his or her fiduciary duty owed to the Company are
extraordinary or material and to approve such acts and certain related party transactions (including transactions in which an office holder has a personal interest) and whether such transaction is
extraordinary or material under the Companies Law (see "—Approval of Related Party Transactions Under Israeli Law");
- •
- where the board of directors approves the work plan of the internal auditor, to examine such work plan before its submission to the
board and propose amendments thereto;
- •
- establishing the approval process for certain transactions with a controlling shareholder or in which a controlling shareholder has a
personal interest;
- •
- examining our internal controls and internal auditor's performance, including whether the internal auditor has sufficient resources
and tools to dispose of its responsibilities;
- •
- examining the scope of our auditor's work and compensation and submitting a recommendation with respect thereto to our board of
directors or shareholders, depending on which of them is considering the appointment of our auditor; and
- •
- establishing procedures for the handling of employees' complaints as to deficiencies in the management of our business and the protection to be provided to such employees.
Our audit committee may not approve any actions requiring its approval (see "—Approval of Related Party Transactions Under Israeli Law"), unless at the time of approval a majority of the committee's members are present, which majority consists of unaffiliated directors including at least one external director.
Compensation Committee
Our compensation committee consists of Mr. Rami Armon, Ms. Nira Dror and Ms. Orli Tori. Mr. Armon is the chairman of the compensation committee. As prescribed by the Companies Law, our compensation committee includes our two external directors, one of whom is the chairperson of the committee. Ms. Dror is an independent director, as defined in "—Board of Directors."
Under the Companies Law, the board of directors of a public company must appoint a compensation committee. Subject to certain exceptions compensation committee must be comprised of at least three directors, including all of the external directors, which shall be a majority of the members of the compensation committee and one of whom must serve as chairman of the committee. Our compensation committee includes all of the external directors, who also constitute a majority of the compensation committee.
Each compensation committee member who is not an external director must be a director whose compensation does not exceed an amount that may be paid to an external director. The compensation
118
committee is subject to the same Companies Law restrictions as the audit committee as to who may not be a member of the committee.
The duties of the compensation committee include the recommendation to the company's board of directors of a policy regarding the terms of engagement of office holders, to which we refer as a compensation policy. That policy must be adopted by the company's board of directors, after considering the recommendations of the compensation committee, and must be approved by the company's shareholders, which approval requires what we refer to as a Special Majority. A "Special Majority" approval requires shareholder approval by a majority vote of the shares present and voting at a meeting of shareholders called for such purpose, provided that either: (a) such majority includes at least a majority of the shares held by all shareholders who are not controlling shareholders and do not have a personal interest in such compensation arrangement; or (b) the total number of shares of non-controlling shareholders and shareholders who do not have a personal interest in the compensation arrangement and who vote against the arrangement does not exceed 2% of the company's aggregate voting rights. Our compensation policy was approved by our shareholders on January 23, 2014.
Our compensation policy serves as the basis for decisions concerning the financial terms of employment or engagement of office holders, including exculpation, insurance, indemnification or any monetary payment or obligation of payment in respect of employment or engagement. According to the Companies Law, the compensation policy must be approved (or reapproved) not longer than every three years, relate to certain factors, including advancement of the company's objectives, the company's business plan and its long term strategy, and creation of appropriate incentives for office holders. It must also consider, among other things, the company's risk management, size and nature of its operations. The compensation policy must furthermore consider the following additional factors:
- •
- the knowledge, skills, expertise, and accomplishments of the relevant office holder;
- •
- the office holder's roles and responsibilities and prior compensation agreements with him or her;
- •
- the relationship between the terms offered and the average compensation of the other employees of the company, including those
employed through manpower companies;
- •
- the impact of disparities in salary upon work relationships in the company;
- •
- the possibility of reducing variable compensation at the discretion of the board of directors;
- •
- the possibility of setting a limit on the exercise value of non-cash variable equity-based compensation; and
- •
- as to severance compensation, the period of service of the office holder, the terms of his or her compensation during such service period, the company's performance during that period of service, the person's contributions towards the company's achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company.
The compensation policy must also include the following principles:
- •
- the link between variable compensation and long term performance and measurable criteria;
- •
- the relationship between variable and fixed compensation, and the ceiling for the value of variable compensation;
- •
- the conditions under which an office holder would be required to repay compensation paid to him or her if it was later shown that the
data upon which such compensation was based was inaccurate and was required to be restated in the company's financial statements;
- •
- the minimum holding or vesting period for variable, equity-based compensation; and
119
- •
- maximum limits for severance compensation.
The compensation committee is responsible for (a) recommending the compensation policy to a company's board of directors for its approval (and subsequent approval by its shareholders) and (b) duties related to the compensation policy and to the compensation of a company's office holders as well as functions previously fulfilled by a company's audit committee with respect to matters related to approval of the terms of engagement of office holders, including:
- •
- recommending whether a compensation policy should continue in effect, if the then-current policy has a term of greater than three
years (approval of either a new compensation policy or the continuation of an existing compensation policy must in any case occur every three years);
- •
- recommending to the board of directors periodic updates to the compensation policy;
- •
- assessing implementation of the compensation policy; and
- •
- determining whether the compensation terms of the chief executive officer of the company need not be brought to approval of the shareholders.
Our board of directors has adopted a compensation committee charter setting forth the responsibilities of the committee, which include:
- •
- the responsibilities set forth in the compensation policy;
- •
- reviewing and approving the granting of options and other incentive awards to the extent such authority is delegated by our board of
directors; and
- •
- reviewing, evaluating, and making recommendations regarding the compensation and benefits for our non-employee directors.
Financial Statements Committee
Our financial statements committee, which complies with the Israeli Companies Regulations (Provisions and Conditions Regarding the Financial Statements' Authorization Process), 2010, is responsible for considering and making recommendations to the board of directors on our financial statements. Prior to the approval of our financial statements by our board of directors, the financial statements committee reviews and discusses the financial statements and presents its recommendations with respect to the financial statements to the board of directors. Our financial statements committee currently consists of Mr. Armon, Ms. Dror, and Ms. Tori. Our audit committee serves as our financial statements committee as well.
Financing Committee
Our board of directors has formed a finance committee, on which Mr. Yaron Yaniv, Mr. Ira Leiderman, Ms. Nira Dror and Mr. Adi Goldin serve as members. The financing committee assists our board of directors in fulfilling its responsibilities across the principal areas of corporate finance for our company and its subsidiary.
Nominating and Corporate Governance Committee
Following the listing of our ADSs on The NASDAQ Capital Market, our nominating and corporate governance committee will consist of Mr. Yaron Yaniv, Mr. Ira Leiderman, Ms. Nira Dror, and Ms. Orly Tori and will be chaired by Ms. Dror. Each of the members of our nominating and corporate governance committee is independent under the listing requirements of the NASDAQ Capital Market.
120
Our board of directors has adopted a nominating and governance committee charter to be effective upon the listing of our shares on the NASDAQ Capital Market that will set forth the responsibilities of the nominating and governance committee which include:
- •
- overseeing and assisting our board in reviewing and recommending nominees for election as directors;
- •
- assessing the performance of the members of our board; and
- •
- establishing and maintaining effective corporate governance policies and practices, including, but not limited to, developing and recommending to our board a set of corporate governance guidelines applicable to our company.
Internal Auditor
Under the Companies Law, the board of directors of a public company must appoint an internal auditor based on the recommendation of the audit committee. The role of the internal auditor is to examine, among other things, our compliance with applicable law and orderly business procedures. The audit committee is required to oversee the activities and to assess the performance of the internal auditor as well as to review the internal auditor's work plan.
An internal auditor may not be:
- •
- a person (or a relative of a person) who holds more than 5% of the company's outstanding shares or voting rights;
- •
- a person (or a relative of a person) who has the power to appoint a director or the general manager of the company;
- •
- an office holder or director of the company; or
- •
- a member of the company's independent accounting firm, or anyone on its behalf.
Ms. Dana Gottesman, has been serving as our Internal Auditor since November 2013. Ms. Gottesman is a CPA, CIA, MA, Partner in the Risk Advisory Services (RAS) Group at the BDO Ziv Haft accounting firm. Ms. Gottesman has more than 10 years of experience in the provision of internal audit and risk management consulting services to public and private companies, government agencies, municipalities, non-profit organizations, and more. Ms. Gottesman specializes in the analysis and specification of work procedures and their assimilation in the organization, the internal audit of work procedures in different organizations, including the performance of risk surveys and fraud and embezzlement surveys. Ms. Gottesman holds a BA in Accounting and Business Administration and an MA in Internal Audit and Public Administration. Ms. Gottesman's nomination satisfies the requirements of the Companies Law.
Approval of Related Party Transactions Under Israeli Law
Fiduciary Duties of Directors and Executive Officers
The Companies Law codifies the fiduciary duties that office holders owe to a company. Each person listed in the table under "Management—Executive Officers and Directors" is an office holder under the Companies Law.
An office holder's fiduciary duties consist of a duty of care and a duty of loyalty. The duty of care requires an office holder to act with the level of care with which a reasonable office holder in the same position would have acted under the same circumstances. The duty of loyalty requires that an office holder act in good faith and in the best interests of the company.
121
The duty of care includes a duty to use reasonable means to obtain:
- •
- information on the advisability of a given action brought for his or her approval or performed by virtue of his or her position; and
- •
- all other important information pertaining to these actions.
The duty of loyalty includes a duty to:
- •
- refrain from any conflict of interest between the performance of his or her duties to the company and his or her other duties or
personal affairs;
- •
- refrain from any activity that is competitive with the company;
- •
- refrain from exploiting any business opportunity of the company to receive a personal gain for himself or herself or others; and
- •
- disclose to the company any information or documents relating to the company's affairs which the office holder received as a result of his or her position as an office holder.
Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions
The Companies Law requires that an office holder promptly disclose to the company any personal interest that he or she may be aware of and all related material information or documents concerning any existing or proposed transaction by the company. An interested office holder's disclosure must be made promptly and in any event no later than the first meeting of the board of directors at which the transaction is considered. An office holder is not obliged to disclose a personal interest if it derives solely from the personal interest of his or her relative in a transaction that is not considered as an extraordinary transaction.
A "personal interest" is defined under the Companies Law to include a personal interest of any person in an act or transaction of a company, including the personal interest of such person's relative or of a corporate body in which such person or a relative of such person is a 5% or greater shareholder, director, or general manager or in which he or she has the right to appoint at least one director or the general manager, but excluding a personal interest stemming from one's ownership of shares in the company.
A personal interest furthermore includes the personal interest of a person for whom the office holder holds a voting proxy or the personal interest of the office holder with respect to his or her vote on behalf of a person for whom he or she holds a proxy even if such shareholder has no personal interest in the matter. An office holder is not, however, obliged to disclose a personal interest if it derives solely from the personal interest of his or her relative in a transaction that is not considered an extraordinary transaction.
Under the Companies Law, an extraordinary transaction is defined as any of the following:
- •
- a transaction other than in the ordinary course of business;
- •
- a transaction that is not on market terms; or
- •
- a transaction that may have a material impact on the company's profitability, assets, or liabilities.
If it is determined that an office holder has a personal interest in a transaction, approval by the board of directors is required for the transaction, unless the company's articles of association provide for a different method of approval. Further, so long as an office holder has disclosed his or her personal interest in a transaction, the board of directors may approve an action by the office holder that would otherwise be deemed a breach of duty of loyalty. However, a company may not approve a transaction or action that is adverse to the company's interest or that is not performed by the office
122
holder in good faith. An extraordinary transaction in which an office holder has a personal interest requires approval first by the company's audit committee and subsequently by the board of directors. The compensation of, or an undertaking to indemnify or insure, an office holder who is not a director requires approval first by the company's compensation committee, then by the company's board of directors, and, if such compensation arrangement or an undertaking to indemnify or insure is inconsistent with the company's stated compensation policy or if the office holder is the chief executive officer (apart from a number of specific exceptions), then such arrangement is subject to a Special Majority approval. Arrangements regarding the compensation, indemnification, or insurance of a director require the approval of the compensation committee, board of directors, and shareholders by ordinary majority, in that order, and under certain circumstances, a Special Majority approval. If shareholders of a company do not approve the compensation terms of office holders, other than directors, but including the chief executive officer, the compensation committee, and board of directors may override the shareholders' decision, subject to certain conditions.
Generally, a person who has a personal interest in a matter which is considered at a meeting of the board of directors or the audit committee may not be present at such a meeting or vote on that matter unless the chairman of the relevant committee or board of directors (as applicable) determines that he or she should be present in order to present the transaction that is subject to approval. If a majority of the members of the audit committee or the board of directors (as applicable) has a personal interest in the approval of a transaction, then all directors may participate in discussions of the audit committee or the board of directors (as applicable) on such transaction and the voting on approval thereof, but shareholder approval is also required for such transaction.
Disclosure of Personal Interests of Controlling Shareholders, Approval of Certain Transactions
Under Israeli Law, the term "controlling shareholder" means a shareholder with the ability to direct the activities of our company, other than by virtue of being an executive officer or director. A shareholder is presumed to be a controlling shareholder if the shareholder holds 50% or more of the voting rights in a company or has the right to appoint the majority of the directors of the company or its general manager. For the purpose of approving transactions with controlling shareholders, the term also includes any shareholder that holds 25% or more of the voting rights of a company if the company has no shareholder that owns more than 50% of its voting rights. The transactions described below were entered into prior to the adoption of this policy.
Pursuant to Israeli law, the disclosure requirements regarding personal interests that apply to directors and executive officers also apply to a controlling shareholder of a public company. See "—External Directors" for a definition of controlling shareholder. In the context of a transaction involving a shareholder of the company, a controlling shareholder also includes a shareholder who holds 25% or more of the voting rights in the company if no other shareholder holds more than 50% of the voting rights in the company. For this purpose, the holdings of all shareholders who have a personal interest in the same transaction will be aggregated. The approval of the audit committee, the board of directors, and a Special Majority, in that order, is required for (a) extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, (b) the engagement with a controlling shareholder or his or her relative, directly or indirectly, for the provision of services to the company, (c) the terms of engagement and compensation of a controlling shareholder or his or her relative who is not an office holder, or (d) the employment of a controlling shareholder or his or her relative by the company, other than as an office holder.
To the extent that any such transaction with a controlling shareholder is for a period extending beyond three years, approval is required once every three years, unless, with respect to certain transactions, the audit committee determines that the duration of the transaction is reasonable given the circumstances related thereto.
123
Arrangements regarding the compensation, indemnification, or insurance of a controlling shareholder in his or her capacity as an office holder require the approval of the compensation committee, board of directors, and shareholders by a Special Majority and the terms thereof may not be inconsistent with the company's stated compensation policy.
Pursuant to regulations promulgated under the Companies Law, certain transactions with a controlling shareholder or his or her relative, or with directors, that would otherwise require approval of a company's shareholders may be exempt from shareholder approval upon certain determinations of the audit committee and board of directors. Under these regulations, a shareholder holding at least 1% of the issued share capital of the company may require, within 14 days of the publication of such determinations, that despite such determinations by the audit committee and the board of directors, such transaction will require shareholder approval under the same majority requirements that would otherwise apply to such transactions.
Shareholders' Duties
Under the Companies Law, a shareholder has a duty to act in good faith and in a customary manner toward the company and other shareholders and to refrain from abusing his or her power in the company, including, among other things, in voting at general meetings of shareholders and class meetings of shareholders with respect the following matters:
- •
- an amendment of the articles of association or memorandum of association of the company;
- •
- an increase in the company's authorized share capital;
- •
- a merger; or
- •
- the approval of related party transactions and acts of office holders that require shareholder approval.
A shareholder also has a general duty to refrain from discriminating against other shareholders. In addition, certain shareholders have a duty of fairness toward the company. These shareholders include any controlling shareholder, any shareholder who knows that he or she has the power to determine the outcome of a shareholder vote and any shareholder who has the power to appoint or to prevent the appointment of an office holder of the company or other power. The Companies Law does not define the substance of the duty of fairness, except to state that the remedies generally available upon a breach of contract will also apply in the event of a breach of the duty to act with fairness.
Exculpation, Insurance and Indemnification of Directors and Officers
Under the Companies Law, a company may not exculpate an office holder from liability for a breach of the duty of loyalty. An Israeli company may exculpate an office holder in advance from liability to the company, in whole or in part, for damages caused to the company as a result of a breach of duty of care but only if a provision authorizing such exculpation is included in its articles of association. Our articles of association include such a provision. A company may not exculpate a director from liability arising out of a prohibited dividend or distribution to shareholders.
Under the Companies Law, an Israeli company may indemnify an office holder in respect of the following liabilities and expenses incurred for acts performed as an office holder, either in advance of an event or following an event, provided a provision authorizing such indemnification is contained in its articles of association:
- •
- financial liability imposed on him or her in favor of another person pursuant to a judgment, including a settlement or arbitrator's award approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company's activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking must detail the abovementioned foreseen events and amount or criteria;
124
- •
- reasonable litigation expenses, including attorneys' fees, incurred by the office holder (1) as a result of an investigation or
proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no indictment was filed against such office holder as a result
of such investigation or proceeding and (ii) no financial liability was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or,
if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; and (2) in connection with a monetary sanction; and
- •
- reasonable litigation expenses, including attorneys' fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf or by a third party or in connection with criminal proceedings in which the office holder was acquitted or as a result of a conviction for an offense that does not require proof of criminal intent.
Under the Companies Law, a company may insure an office holder against the following liabilities incurred for acts performed as an office holder if and to the extent provided in the company's articles of association:
- •
- a breach of duty of care to the company or to a third party, including a breach arising out of the negligent conduct of the office
holder;
- •
- a breach of the duty of loyalty to the company, to the extent that the office holder acted in good faith and had a reasonable basis to
believe that the act would not prejudice the company; and
- •
- a financial liability imposed on the office holder in favor of a third party.
Under the Companies Law, a company may not indemnify or insure an office holder against any of the following:
- •
- a breach of duty of loyalty, except for indemnification and insurance for a breach of the duty of loyalty to the company and to the
extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company;
- •
- a breach of duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office
holder;
- •
- an act or omission committed with intent to derive illegal personal benefit; or
- •
- a fine or forfeit levied against the office holder.
Under the Companies Law, exculpation, indemnification, and insurance of office holders in a public company must be approved by the compensation committee and the board of directors and, with respect to certain office holders or under certain circumstances, by the shareholders.
Our articles of association and compensation policy allow us to exculpate, indemnify, and insure our office holders to the fullest extent permitted or to be permitted by the Companies Law.
As of the date of this offering, no claims for directors' and officers' liability insurance have been filed under this policy and we are not aware of any pending or threatened litigation or proceeding involving any of our directors or officers in which indemnification is sought.
We have obtained directors and officers liability insurance for the benefit of our office holders and intend to continue to maintain such coverage and pay all premiums thereunder to the fullest extent permitted by the Companies Law. In addition we have entered into agreements with each of our current office holders undertaking to indemnify them to the fullest extent permitted by the Companies Law and our articles of association, including with respect to liabilities resulting from this offering to the extent that these liabilities are not covered by insurance.
125
In the opinion of the Securities and Exchange Commission, indemnification of directors and office holders for liabilities arising under the Securities Act of 1933, as amended, or the Securities Act, however, is against public policy and therefore unenforceable.
There is no pending litigation or proceeding against any of our directors or officers as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics applicable to all of our directors and employees, including our chief executive officer, chief financial officer, controller or principal accounting officer, and other persons performing similar functions, which is a "code of ethics" as defined in Item 16B of Form 20-F promulgated by the Securities and Exchange Commission. Upon the effectiveness of the registration statement of which this prospectus forms a part, the full text of the Code of Business Conduct and Ethics will be posted on our website at www.collplant.com. Information contained on, or that can be accessed through, our website does not constitute a part of this prospectus and is not incorporated by reference herein. If we make any amendment to the Code of Business Conduct and Ethics or grant any waivers, including any implicit waiver, from a provision of the code of ethics, we will disclose the nature of such amendment or waiver on our website to the extent required by the rules and regulations of the Securities and Exchange Commission. Under Item 16B of the SEC's Form 20-F, if a waiver or amendment of the Code of Business Conduct and Ethics applies to our principal executive officer, principal financial officer, principal accounting officer, or controller and relates to standards promoting any of the values described in Item 16B(b) of such Form 20-F, we will disclose such waiver or amendment on our website in accordance with the requirements of Instruction 4 to such Item 16B.
Compensation of Executive Officers and Directors
The aggregate compensation paid by us to our current directors and executive officers, including share based compensation, for the year ended December 31, 2014, was $1,012,000. This amount includes any amounts set aside or accrued to provide pension, severance, retirement, annual leave, and recuperation or similar benefits or expenses. It does not include any business travel, relocation, professional, and business association dues and expenses reimbursed to office holders, and other benefits commonly reimbursed or paid by companies in Israel. The above also includes the provision for bonuses for the years ended December 31, 2014 in the amount $54,000, and the estimated fair value of share based compensation (options to buy ordinary shares) in the amount of $26,000. In addition, as of December 31, 2014, options to purchase an aggregate of 14,951,911 ordinary shares granted to our directors and executive officers were outstanding under the Employee Share Ownership and Option Plan (2010), or the 2010 Plan, at a weighted average exercise price of NIS 0.43 ($0.11) per share.
According to our obligations under certain employment agreements and our 2015 objectives compensation plan, certain members of our management may be granted bonuses in an aggregate amount of up to $ . Furthermore, according our 2015 objectives compensation plan upon the completion of this offering, certain members of our management will be eligible for bonuses in an aggregate amount of up to $ .
Employment and Services Agreements with Executive Officers and Directors
We have entered into written employment agreements with each of Yehiel Tal, Eran Rotem, Dr. Nadav Orr, and Dr. Philippe Bensimon. All such agreements contain provisions regarding non-competition, confidentiality of information, and assignment of inventions. The non-competition
126
provisions apply for a period of 12 months following termination of the respective officer's employment. In addition, we are required to provide 90 days' notice prior to terminating the employment of such executive officers other than in the case of a termination for Cause. Pursuant to his employment agreement, "Cause" means a breach by Mr. Tal of any of the material terms or conditions of this, or any other agreement, between him and us, Mr. Tal's willful misconduct, or action of personal dishonesty, bad faith, or breach of trust towards us or any of our subsidiaries and/or affiliates, the commission by Mr. Tal of a criminal offense, or fraud against us and/or any of our subsidiaries and/or affiliates or circumstances that would otherwise deny Mr. Tal the severance payments due to him under applicable law. These agreements do not provide for benefits upon the termination of these executives' respective employment with us, other than payment of salary and benefits during the required notice period for termination of these agreements, which varies under these individual agreements. Mr. Tal's agreement also provides for annual bonus payments based upon criteria determined by the board of directors, as well as special bonuses which may be payable upon the achievement of specified milestones, such as the execution of an income-generating commercial agreement or consummation of an initial public offering (subject to the satisfaction of certain conditions).
We have entered into written consulting and option agreements with Prof. Oded Shoseyov. Prof. Shoseyov's services agreement creates an independent contractor relationship between the parties and therefore does not provide for severance or other employment related benefits. We are required to provide 90 days' notice prior to terminating the services of Prof. Shoseyov other than in the case of a termination for Cause. Pursuant to his employment agreement, "Cause" means a breach by Prof. Shoseyov of any of the material terms or conditions of this, or any other agreement between him and us, Prof. Shoseyov' s willful misconduct, or action of personal dishonesty, bad faith, or breach of trust towards us or any of our subsidiaries and/or affiliates, or the commission by Prof. Shoseyov of a criminal offense, or fraud against us and/or any of our subsidiaries and/or affiliates. As consideration for the agreement, in addition to a monthly services fee, Prof. Shoseyov was granted the option to purchase 2,258,813 ordinary shares of the Company par value NIS 0.01 each, at an exercise price of NIS 0.69 ($0.18). Prof. Shoseyov is also entitled to payment of a special bonus under certain conditions, such as the execution of a substantial commercial agreement with a pharmaceutical company (which entitlement was waived by Prof. Shoseyov upon the grant of additional options to him in March 2015) and our initial public offering on the TASE (which occurred, and for which Prof. Shoseyov agreed to postpone said bonus payment in the amount of $50,000 to a later period). The services agreement further sets provisions regarding confidentiality, non-competition and the ownership of the parties' intellectual property.
Under the provisions of the services agreement we have complete ownership in any invention which is derived from our operations and businesses as well as first rights (for the development and commercialization) in any invention that is not our invention and that may be a result of Prof. Shoseyov's activity in the course of providing the services. In March 2011, Prof. Shoseyov was granted an option to purchase 2,258,813 of our ordinary shares at an exercise price of NIS 0.69 ($0.18) per share. On March 22, 2015, our compensation committee and board of directors recommended to our shareholders to approve the grant to Prof. Shoseyov of an option to purchase 10,000,000 of our ordinary shares, at an exercise price of NIS 0.60 ($0.15), with a vesting period and other terms which are in compliance with our compensation policy, and subject to continued provision of services to us.
In addition, we have entered into compensation agreements with certain of our directors. The amounts payable pursuant to these arrangements have been approved by our board of directors and shareholders.
We have entered into a written services agreement with Yaron Yaniv for the provision of management services as chairman of our board of directors in consideration for monthly management fees. Mr. Yaniv was granted an option to purchase 7,241,770 of our ordinary shares, at an exercise price
127
of NIS 0.26 ($0.07) per share. The options shall vest over a period of three years, when a third will vest upon the end of a year from the time of granting, and the rest in equal parts at the end of every quarter thereafter. The agreement is for an indefinite period and may be canceled by the parties with three months prior written notice.
Under the Companies Law, directors do not receive compensation for their service as our directors or otherwise, unless such compensation is approved by our compensation committee, then by the board of directors followed by the shareholders. The compensation of our directors may be fixed, as an all-inclusive payment or as payment for participation in meetings, or as a combination thereof. In addition, such compensation may include: (i) in the case of a director who is also an officer, a salary or other compensation in respect of his or her work as an officer, as may be agreed upon by the director and us; and (ii) reimbursement of expenses, including travel expenses, expended in connection with his or her duties as a member of the board of directors. To date, except for the external directors, the independent directors, our Chairman and CSO, none of the remaining directors receives any compensation.
Share Incentive Plan
In May 2010, we adopted the 2010 Plan, an option plan for employees and senior officers, and as part of the acquisition of CollPlant Ltd., all of the options under the Employee Share Ownership and Option Plan (2004) of CollPlant Ltd. were substituted with and assumed by options under our 2010 Plan, while any restriction periods under Sections 102(b)(2) and 102(b)(3) of the Israeli Income Tax Ordinance, or the Ordinance were calculated as of their original grant date. The 2010 Plan allows us to grant options to purchase our ordinary shares to our officers, employees, and consultants. The 2010 Plan is intended to enhance our ability to attract and retain desirable individuals by increasing their ownership interests in us. As of March 31, 2015, our employees, officers, and consultants hold an aggregate of 17,963,346 options to purchase ordinary shares under the 2010 Plan. On March 22, 2015, our board of directors approved the issuance of options to purchase an aggregate of 10,000,000 ordinary shares under the 2010 Plan, which grant remains subject to shareholder approval. As of March 31, 2015, options to purchase an aggregate of 6,665,898 ordinary shares had been exercised and transferred to the beneficial holders. The 2010 Plan is designed to reflect the provisions of the Israeli Income Tax Ordinance, or the Ordinance, mainly Sections 102 and 3(i), which affords certain tax advantages to Israeli employees, officers, and directors that are granted options in accordance with its terms. Section 102 of the Ordinance allows employees, directors, and officers, who are not controlling shareholders and who are Israeli residents, to receive favorable tax treatment for compensation in the form of shares or options. Section 102 of the Ordinance includes two alternatives for tax treatment involving the issuance of options or shares to a trustee for the benefit of the grantees and also includes an additional alternative for the issuance of options or shares directly to the grantee. Sections 102(b)(2) and 102(b)(3) of the Ordinance, which provide the most favorable tax treatment for grantees, permit the issuance to a trustee under the "capital gains track." In order to comply with the terms of the capital gains track, all options granted under a specific plan and subject to the provisions of Section 102 of the Ordinance, as well as the shares issued upon exercise of such options and other shares received following any realization of rights with respect to such options, such as share dividends and share splits, must be registered in the name of a trustee selected by the board of directors and held in trust for the benefit of the relevant employee, director, or officer. The trustee may not release these options or shares to the relevant grantee before the second anniversary of the registration of the options in the name of the trustee. However, under this track, our ability to deduct an expense with respect to the issuance of the options or shares might be limited. Section 3(i) of the Ordinance does not provide for similar tax benefits.
The plans may be administered by our board of directors either directly or upon the recommendation of a committee appointed by our board of directors.
128
The compensation committee recommends to the board of directors, and the board of directors determines or approves the eligible individuals who receive options under the plan, the number of ordinary shares covered by those options, the terms under which such options may be exercised, and other terms and conditions of the options, all in accordance with the provisions of the plans. Option holders may not transfer their options except in the event of death or transfer to an Administrator in accordance with law in the event of the absence of legal competency. Our compensation committee or board of directors may at any time amend or terminate each of the plans; however, any amendment or termination may not adversely affect any options or shares granted under such plan prior to such action.
The option exercise price is determined by the compensation committee, following the approval of the board of directors, and specified in each option award agreement. In general, and according to our compensation policy, the option exercise price is the market value of the shares on the date of grant as traded on TASE.
Awards under the 2010 Plan may be granted until 2020, 10 years from the date on which the 2010 Plan was approved by our board of directors.
Options granted under the 2010 Plan generally vest over four years commencing on the date of grant such that 25% vest on the first anniversary of the date of grant and an additional 6.25% vest at the end of each subsequent three-month period thereafter for 36 months and some every calendar year, unless otherwise provided in a specific allocation agreement.
Options, other than certain incentive share options, that are not exercised within 10 years from the grant date expire, unless otherwise determined by our board of directors. Except as otherwise determined by the board of directors or as set forth in an individual's award agreement, in the event of termination of employment or services for reasons of disability or death, or retirement, the grantee, or in the case of death, his or her legal successor, may exercise options that have vested prior to termination within a period of one year from the date of disability, death, or retirement. If we terminate a grantee's employment or service for cause, all of the grantee's unvested options will expire on the date of termination, yet options which by that date the offeree's eligibility to exercise has already been formed shall remain exercisable. If a grantee's employment or service is terminated for any other reason, the grantee may exercise his or her vested options within 90 days of the date of termination. Any expired or unvested options return to the pool for reissuance.
In the event of: (i) a sale of all or substantially all of our assets; or (ii) our consolidation or merger in which we are not the ongoing or surviving corporation, then, and unless otherwise determined in the agreement or by the board, we shall be entitled to determine that all of the outstanding unexercised options held by or for the benefit of any grantee shall be assumed or substituted for an appropriate number of options of the successor company, provided that the aggregate amount of the exercise price for such options shall be equal to the aggregate amount of the exercise price of our unexercised options held by each grantee at such time.
In the event of termination of the employment or the director or service-provider relationship by us or by a related company within 12 months after a significant event in which the options were assumed, then the unvested portion of the options shall become fully vested, and shall remain exercisable for a period of three months following the termination or notice of termination. For such purposes, a "Significant Event" would include our consolidation or merger with or into another corporation in which we are the ongoing or surviving corporation or in which, the ongoing or surviving corporation (or, if such transaction is effected through a subsidiary, the parent of such ongoing or surviving corporation) assumes the option or substitutes it with an appropriate option in the surviving corporation (or in the parent as aforesaid) in the manner set forth above.
129
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following is a description of the material terms of those transactions with related parties to which we are party to date.
Issuances of Securities over the Past Three Years
In each of the following issuances of securities, one or more of our officers, directors, and owners of 5% or more of our ordinary shares participated in the acquisition of our securities.
- •
- On March 15, 2012, we issued and sold 7,033,639 ordinary shares to our shareholders pursuant to a rights offering. The
following owners of our ordinary shares participated in this offering: Docor Levi Lassen BV, Prof. Oded Shoseyov, Pontifax (Israel) L.P., and Efi Cohen Arazi (former chairman).
- •
- On November 29, 2012, we issued and sold 23,987,200 ordinary shares and options to purchase 13,112,960 ordinary shares at an
exercise price of NIS 0.70 ($0.18) per share to the Israeli public pursuant to a public offering. The following owners of our ordinary shares participated in this offering: Docor Levi
Lassen BV, Prof. Oded Shoseyov, Pontifax (Israel) L.P., and Efi Cohen Arazi (former chairman).
- •
- On November 26, 2013, we issued and sold 16,856,173 ordinary shares to Trauwin Pte Ltd., pursuant to a private offering.
- •
- On December 18 and 24, 2013, we issued and sold 68,313,000 ordinary shares and options to purchase 75,144,300 ordinary shares
at an exercise price of NIS 0.70 ($0.18) per share to Israeli institutional investors and the Israeli public pursuant to two consecutive public offerings. The following owners of our ordinary shares
participated in these offerings: Docor Levi Lassen BV, Prof. Oded Shoseyov, and Pontifax (Israel) L.P.
- •
- On January 9, 2014, we issued and sold 4,517,626 ordinary shares to Yehiel Tal, our chief executive officer, pursuant to his exercise of options.
Agreement with Flon China Medical; Trauwin Pte Ltd. Investment Agreement
On August 25, 2013, we signed a non-binding memorandum of understanding, or MOU with Flon China Medical, or Flon, regarding an investment and a license agreement in connection with our product candidates in China. According to the MOU, Flon agreed to invest a total of $2.5 million in exchange for 10% of our issued share capital at the time of the execution of a binding agreement. The MOU also contemplates that we will supply rhCollagen to Flon for the production of our products in China. In addition, according to the MOU and upon execution of a binding agreement, Flon agreed to pay us an additional $1.5 million in exchange for a license for the exclusive distribution rights of our product candidates in China, both existing and future (excluding the field of orthopedics). The exclusivity period for our product candidates pursuant to this contemplated license agreement was set for 10 years from the execution of a final and binding agreement. License fees are scheduled in three equal installments ($0.5 million in each payment) upon the occurrence of each one of the following milestones: (1) the execution of a final and binding agreement; (2) obtaining registration approval in China for the first of our product candidates; and (3) receipt of registration approval in China for our second product candidate. In addition, the MOU established single-digit rate royalties for the sale of our product candidate in China, to be payable to us by Flon.
On October 2, 2013, we signed an investment agreement with Trauwin Pte Ltd., or Trauwin, a related party to Flon, pursuant to which on November 10, 2013, Trauwin invested a total of $2.5 million in exchange for approximately 10% of our issued and outstanding share capital as of such date. Execution of the license agreement and the supply agreement contemplated by the MOU was a
130
condition of the consummation of the investment transaction contemplated by the MOU. However, Trauwin orally waived this condition contemporaneously to its execution of the investment agreement. The license agreement and the supply agreement contemplated by the MOU are expected to be entered into at a future date, but to date, the parties are unable to estimate when such agreements will be signed, if at all.
Agreements with Yissum
We have entered into certain agreements with Yissum, in which Prof. Oded Shoseyov, our chief scientific officer, has or might have a personal interest, including an agreement dated July 13, 2004 with respect to the intellectual property rights relating to our rhCollagen. See "Business—Agreement with Yissum Research Development Company of the Hebrew University of Jerusalem Ltd. with Respect to Our RhCollagen" See "Management—Approval of Related Party Transactions Under Israeli Law."
On July 29, 2010, we signed a joint development agreement with Yissum. The agreement governs the relationship between the parties in connection with the invention protected by a patent application for the Resilin protein and future results from development work related to Resilin conducted jointly by us and Yissum or solely by us or Yissum. The Resilin protein and its patent are not related to our collagen protein and its related patents. The agreement stipulates that the parties will be co-owners of the Resilin patent and its associated know-how developed prior to the date of execution of the agreement. Future developments made by one party (without the contribution of the other) within that party's field of use will be owned by the developing party. Each party has granted the other an exclusive worldwide license, which can be sub-licensed, to make use of the Resilin patent and its associated know-how, for the purposes of research, development, production, marketing, distribution, license or sale of products limited to the licensee's field of use. Accordingly, we have exclusive rights to the technology for all human and veterinary uses, including therapeutic and diagnostic. Yissum has exclusivity in any other field. We were also granted first rights to develop and commercialize products in Yissum's field of exclusivity where a sub-license has not yet been given by Yissum to a third party.
On April 20, 2015 we entered into a consortium agreement with several international companies and academic institutions, outlining the framework of a tissue research and development project using nanotechnology, our rhCollagen, and stem cell technology. The project is expected to last approximately three years. The Hebrew University of Jerusalem together with Yissum and Prof. Oded Shoseyov, our chief scientist and the project manager on behalf of Yissum, will also take part in the project.
As part of the project, we will supply an insignificant amount of our rhCollagen, and become a member of the steering committee of the project. The agreement contains provisions protecting each consortium member's rights including with respect to the intellectual property to be developed as part of the project, and protecting us, our rhCollagen, and any intellectual property developed as part of the project with respect to our rhCollagen whether by the Hebrew University or by other parties participating in the consortium, as applicable.
Rights of Appointment
Our current board of directors consists of eight directors. See "Management—Board of Directors." Currently-serving directors that were appointed prior to this offering (other than the external directors) will continue to serve pursuant to their appointment until the first annual meeting of shareholders held after this offering. We are not a party to, and are not aware of, any voting agreements among our shareholders.
Registration Rights
There are no registration rights applicable to our ordinary shares.
131
Agreements with Directors and Officers
Insurance, Exculpation, and Indemnification Agreements
We have entered into indemnification agreements with each of our current directors and executive officers exculpating them from a breach of their duty of care to us to the fullest extent permitted by law, subject to limited exceptions, and undertaking to indemnify them to the fullest extent permitted by Israeli law, subject to limited exceptions, and including with respect to liabilities resulting from this offering to the extent such liabilities are not covered by insurance. See "Management—Approval of Related Party Transactions Under Israeli Law—Exculpation, Insurance and Indemnification of Directors and Officers."
Employment and Services Agreements
We have entered into employment or services agreements with our executive officers and certain of our directors. See "Management—Employment and Services Agreements with Executive Officers and Directors."
Options
Since our inception, we have granted options to purchase our ordinary shares to certain of our officers. We describe our option plans under "Management—Share Option Plans."
132
The following table sets forth information with respect to the beneficial ownership of our shares as of March 31, 2015 by:
- •
- each of our directors and executive officers;
- •
- each person or entity known by us to beneficially own more than 5% of our outstanding shares; and
- •
- all of our directors, director nominees, and executive officers as a group.
Our major shareholders do not have voting rights that are different from our shareholders in general.
Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting or investment power with respect to those securities, and include shares subject to options and warrants that are exercisable within 60 days after March 31, 2015. Such shares are also deemed outstanding for purposes of computing the percentage ownership of the person holding the option, but not the percentage ownership of any other person. As of March 31, 2015, there were no holders of record of our ordinary shares in the United States.
Unless otherwise indicated below, to our knowledge, all persons named in the table have sole voting and investment power with respect to their shares, except to the extent that authority is shared by spouses under community property laws. All percentages in this table assume no exercise by the underwriters of their option to purchase up to an additional shares from us. None of our shareholders has informed us that he, she, or it is affiliated with a registered broker-dealer or is in the business of underwriting securities. None of our shareholders has different voting rights from other shareholders.
133
Unless otherwise indicated, the address of each beneficial owner is c/o 3 Sapir Street, Weizmann Science Park, P.O. Box 4132, Ness-Ziona 74140, Israel.
| |
|
Percentage of Ordinary Shares Beneficially Owned |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Ordinary Shares Beneficially Owned |
Prior to the Offering |
After the Offering |
|||||||
5% Shareholders |
||||||||||
Meitav Dash Investment Ltd.(1) |
49,861,926 | 18.57 | % | % | ||||||
Trauwin Pte Ltd. |
16,856,173 | 6.90 | % | |||||||
Docor Levi Lassen BV(2) |
21,426,916 | 8.64 | % | |||||||
Ami Sagi |
16,619,472 | 6.81 | % | |||||||
Executive Officers and Directors |
||||||||||
Yaron Yaniv(3) |
1,408,914 | * | ||||||||
Oded Shoseyov(4) |
10,682,868 | 4.33 | % | |||||||
Adi Goldin |
— | — | ||||||||
Xiaojin Qian |
— | — | ||||||||
Yehiel Tal(5) |
4,670,667 | 1.91 | % | |||||||
Eran Rotem(6) |
492,232 | * | ||||||||
Philippe Bensimon(7) |
340,625 | * | ||||||||
Nadav Orr |
— | — | ||||||||
All executive officers and directors as a group (eight persons) |
17,595,306 | 7.16 | % | |||||||
- *
- Less
than 1%
- (1)
- Consists
of warrants to purchase 24,384,700 ordinary shares exercisable within 60 days of March 31, 2015 and 25,477,266 ordinary
shares.
- (2)
- Consists
of warrants to purchase 3,857,000 ordinary shares exercisable within 60 days of March 31, 2015 and 17,569,916 ordinary shares.
- (3)
- Consists
of options to purchase 1,810,442 ordinary shares exercisable within 60 days of March 31, 2015 and 1,408,914 ordinary shares.
- (4)
- Consists
of: (i) options to purchase 2,367,904 ordinary shares exercisable within 60 days of March 31, 2015, (ii) warrants to
purchase 110,000 ordinary shares exercisable within 60 days of March 31, 2015; and (iii) 8,204,964 ordinary shares.
- (5)
- Consists
of options to purchase 153,041 ordinary shares exercisable within 60 days of March 31, 2015 and 4,517,626 ordinary shares.
- (6)
- Consists
of options to purchase 492,232 ordinary shares exercisable within 60 days of March 31, 2015.
- (7)
- Consists of options to purchase 340,625 ordinary shares exercisable within 60 days of March 31, 2015.
134
DESCRIPTION OF OUR ORDINARY SHARES
The following description of our ordinary shares and provisions of our articles of association are summaries and do not purport to be complete.
General
As of March 31, 2015, our authorized share capital consisted of 1,500,000,000 ordinary shares, of which 241,392,352 ordinary shares were issued and outstanding. All of our outstanding ordinary shares have been validly issued, fully paid and non-assessable.
Upon the closing of this offering, our authorized share capital will consist solely of ordinary shares, par value NIS 0.01 per share, of which shares will be issued and outstanding (assuming that the underwriters do not exercise their option to purchase additional ordinary shares). Our ordinary shares are not redeemable and do not have any preemptive rights.
Options
As of March 31, 2015, an aggregate of 34,629,244 ordinary shares were reserved for issuance under our equity plans, of which options to purchase 17,963,341 ordinary shares have been granted and are outstanding, options to purchase 6,665,848 ordinary shares have been exercised and transferred to the beneficiary holders, and additional options to purchase 10,000,000 ordinary shares have been approved by our board of directors, pending the approval of our shareholders. No options for ordinary shares are currently reserved under our equity plans for future option grants.
Warrants
As of March 31, 2015, warrants to purchase 88,337,260 ordinary shares were issued and outstanding at a weighted average exercise price of NIS 0.70 ($0.18) per ordinary share. The expiration date of these warrants is December 31, 2016.
Share History
The following is a summary of the issuances of our ordinary shares for the last three years.
- •
- On March 15, 2012, we issued and sold 7,033,639 ordinary shares to our shareholders pursuant to a rights offering.
- •
- On November 29, 2012, we issued and sold 23,987,200 ordinary shares and options to purchase 13,112,960 ordinary shares
at an exercise price of NIS 0.70 ($0.18) per share to the Israeli public pursuant to a public offering. In connection with this offering, options to purchase 1,199,360 ordinary shares (series
F), at an exercise price of NIS 0.70 ($0.18) per share, were issued to the distributors of the offering.
- •
- On November 26, 2013, we issued and sold 16,856,173 ordinary shares to Trauwin Pte, pursuant to a private offering.
- •
- On December 18 and 24, 2013, we issued and sold 68,313,000 ordinary shares and options to purchase 75,144,300 ordinary shares,
at an exercise price of NIS 0.70 ($0.18) per share, to Israeli institutional investors and the Israeli public pursuant to two consecutive public offerings. In connection with these offerings,
options to purchase 6,831,300 ordinary shares, at an exercise price of NIS 0.70 ($0.18) per share, were issued to the underwriters of the offerings.
- •
- On January 9, 2014 we issued and sold 4,517,626 ordinary shares to Yehiel Tal, our chief executive officer, pursuant to his exercise of options.
135
Registration Number and Purposes of the Company
Our registration number with the Israeli Registrar of Companies is 52-0039785. Our purpose as set forth in our articles of association is to engage in any lawful activity.
Voting Rights and Conversion
All ordinary shares have identical voting and other rights in all respects.
Transfer of Shares
Our fully paid ordinary shares are issued in registered form and may be freely transferred under our articles of association, unless the transfer is restricted or prohibited by another instrument, applicable law, or the rules of a stock exchange on which the shares are listed for trade. The ownership or voting of our ordinary shares by non-residents of Israel is not restricted in any way by our articles of association or the laws of the State of Israel, except for ownership by nationals of some countries that are, or have been, in a state of war with Israel.
Election of Directors
Our ordinary shares do not have cumulative voting rights for the election of directors. As a result, the holders of a majority of the voting power represented at a shareholders meeting have the power to elect all of our directors, subject to the special approval requirements for external directors described under "Management—External Directors."
Under our articles of association, our board of directors must consist of not less than five, including two external directors, but no more than twelve directors, including two external directors, as required by the Companies Law. Pursuant to our articles of association, other than the external directors, for whom special election requirements apply under the Companies Law, the vote required to appoint a director is a simple majority vote of holders of our voting shares, participating and voting at the relevant meeting. Each director will serve until his or her successor is duly elected and qualified or until his or her earlier death, resignation, or removal by a vote of the majority voting power of our shareholders at a general meeting of our shareholders or until his or her office expires by operation of law, in accordance with the Companies Law. In addition, our articles of association allow our board of directors to appoint directors to fill vacancies on the board of directors to serve for a term of office equal to the remaining period of the term of office of the directors(s) whose office(s) have been vacated. External directors are elected for an initial term of three years, may be elected for additional terms of three years each under certain circumstances, and may be removed from office pursuant to the terms of the Companies Law. See "Management—External Directors." for more information.
Dividend and Liquidation Rights
We may declare a dividend to be paid to the holders of our ordinary shares in proportion to their respective shareholdings. Under the Companies Law, dividend distributions are determined by the board of directors and do not require the approval of the shareholders of a company unless the company's articles of association provide otherwise. Our articles of association do not require shareholder approval of a dividend distribution and provide that dividend distributions may be determined by our board of directors.
Pursuant to the Companies Law, the distribution amount is limited to the greater of retained earnings or earnings generated over the previous two years, according to our then last reviewed or audited financial statements, provided that the date of the financial statements is not more than six months prior to the date of the distribution, or we may otherwise only distribute dividends that do not meet such criteria only with court approval. In each case, we are only permitted to distribute a dividend
136
if our board of directors and the court, if applicable, determines that there is no reasonable concern that payment of the dividend will prevent us from satisfying our existing and foreseeable obligations as they become due.
In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of our ordinary shares in proportion to their shareholdings. This right, as well as the right to receive dividends, may be affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future.
With respect to non-exculpation of a director from liability arising out of a prohibited dividend or distribution to shareholders see "Management—Approval of Related Party Transactions Under Israeli Law—Exculpation, Insurance and Indemnification of Directors and Officers."
Exchange Controls
There are currently no Israeli currency control restrictions on remittances of dividends on our ordinary shares, proceeds from the sale of the shares or interest or other payments to non-residents of Israel, except for shareholders who are subjects of countries that are, or have been, in a state of war with Israel.
Shareholder Meetings
Under Israeli law, we are required to hold an annual general meeting of our shareholders once every calendar year that must be held no later than 15 months after the date of the previous annual general meeting. All meetings other than the annual general meeting of shareholders are referred to in our articles of association as extraordinary general meetings. Our board of directors may call extraordinary general meetings whenever it sees fit, at such time and place, within or outside of Israel, as it may determine. In addition, the Companies Law provides that our board of directors is required to convene an extraordinary general meeting upon the written request of (i) any two of our directors or one-quarter of the members of our board of directors or (ii) one or more shareholders holding, in the aggregate, either (a) 5% or more of our outstanding issued shares and 1% of our outstanding voting power or (b) 5% or more of our outstanding voting power. One or more shareholders, holding 1% or more of the outstanding voting power, may ask the board to add an item to the agenda of a prospective meeting, if the proposal merits discussion at the general meeting.
Subject to the provisions of the Companies Law and the regulations promulgated thereunder, shareholders entitled to participate and vote at general meetings are the shareholders of record on a date to be decided by the board of directors, which may be between four and 40 days prior to the date of the meeting. Furthermore, the Companies Law requires that resolutions regarding the following matters must be passed at a general meeting of our shareholders:
- •
- amendments to our articles of association;
- •
- appointment or termination of our auditors;
- •
- appointment of external directors;
- •
- approval of certain related party transactions;
- •
- increases or reductions of our authorized share capital;
- •
- a merger; and
- •
- the exercise of our board of director's powers by a general meeting, if our board of directors is unable to exercise its powers and the exercise of any of its powers is required for our proper management.
137
The Companies Law and our articles of association require that a notice of any annual general meeting or extraordinary general meeting be provided to shareholders at least 21 days prior to the meeting and if the agenda of the meeting includes the appointment or removal of directors, the approval of transactions with office holders or interested or related parties, or an approval of a merger, notice must be provided at least 35 days prior to the meeting.
All shareholder decisions are to be taken by votes in a shareholders' meeting. Under the Companies Law and our articles of association, shareholders are not permitted to take action via written consent in lieu of a meeting.
Voting Rights
Quorum Requirements
Pursuant to our articles of association, holders of our ordinary shares have one vote for each ordinary share held on all matters submitted to a vote before the shareholders at a general meeting. As a foreign private issuer, the quorum required for our general meetings of shareholders consists of at least two shareholders present in person, by proxy or written ballot who hold or represent between them at least 25% of the total outstanding voting rights. A meeting adjourned for lack of a quorum is generally adjourned to the same day in the following week at the same time and place or to a later time or date if so specified in the notice of the meeting. At the reconvened meeting, any two or more shareholders present in person or by proxy shall constitute a lawful quorum. See "Management—Corporate Governance Practices" for more information.
Vote Requirements
Our articles of association provide that all resolutions of our shareholders require a simple majority vote, unless otherwise required by the Companies Law or by our articles of association. Under the Companies Law, each of (i) the approval of an extraordinary transaction with a controlling shareholder and (ii) the terms of employment or other engagement of the controlling shareholder of the company or such controlling shareholder's relative (even if not extraordinary) requires, the approval described above under "Management—Approval of Related Party Transactions Under Israeli Law—Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions." Under our articles of association, the alteration of the rights, privileges, preferences, or obligations of any class of our shares requires a simple majority vote of the class so affected (or such other percentage of the relevant class that may be set forth in the governing documents relevant to such class), in addition to the ordinary majority vote of all classes of shares voting together as a single class at a shareholder meeting. An exception to the simple majority vote requirement is a resolution for the voluntary winding up, or an approval of a scheme of arrangement or reorganization, of the company pursuant to Section 350 of the Companies Law, which requires the approval of holders of 75% of the voting rights represented at the meeting, in person, by proxy, or by voting deed and voting on the resolution.
Access to Corporate Records
Under the Companies Law, shareholders are provided access to: minutes of our general meetings; our shareholders register and principal shareholders register, articles of association and financial statements; and any document that we are required by law to file publicly with the Israeli Companies Registrar or the Israel Securities Authority. In addition, shareholders may request to be provided with any document related to an action or transaction requiring shareholder approval under the related party transaction provisions of the Companies Law. We may deny this request if we believe it has not been made in good faith or if such denial is necessary to protect our interest or protect a trade secret or patent.
138
Modification of Class Rights
Under the Companies Law and our articles of association, the rights attached to any class of share, such as voting, liquidation, and dividend rights, may be amended by adoption of a resolution by the holders of a majority of the shares of that class present at a separate class meeting, or otherwise in accordance with the rights attached to such class of shares, as set forth in our articles of association.
Registration Rights
None of our shareholders is entitled to registration rights.
Acquisitions under Israeli Law
Full Tender Offer
A person wishing to acquire shares of an Israeli public company and who would as a result hold over 90% of the target company's issued and outstanding share capital is required by the Companies Law to make a tender offer to all of the company's shareholders for the purchase of all of the issued and outstanding shares of the company. A person wishing to acquire shares of a public Israeli company and who would as a result hold over 90% of the issued and outstanding share capital of a certain class of shares is required to make a tender offer to all of the shareholders who hold shares of the relevant class for the purchase of all of the issued and outstanding shares of that class. If the shareholders who do not accept the offer hold less than 5% of the issued and outstanding share capital of the company or of the applicable class, and more than half of the shareholders who do not have a personal interest in the offer accept the offer, all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law. However, a tender offer will also be accepted if the shareholders who do not accept the offer hold less than 2% of the issued and outstanding share capital of the company or of the applicable class of shares.
Upon a successful completion of such a full tender offer, any shareholder that was an offeree in such tender offer, whether such shareholder accepted the tender offer or not, may, within six months from the date of acceptance of the tender offer, petition an Israeli court to determine whether the tender offer was for less than fair value and that the fair value should be paid as determined by the court. However, under certain conditions, the offeror may include in the terms of the tender offer that an offeree who accepted the offer will not be entitled to petition the Israeli court as described above.
If (a) the shareholders who did not respond or accept the tender offer hold at least 5% of the issued and outstanding share capital of the company or of the applicable class or the shareholders who accept the offer constitute less than a majority of the offerees that do not have a personal interest in the acceptance of the tender offer, or (b) the shareholders who did not accept the tender offer hold 2% or more of the issued and outstanding share capital of the company (or of the applicable class), the acquirer may not acquire shares of the company that will increase its holdings to more than 90% of the company's issued and outstanding share capital or of the applicable class from shareholders who accepted the tender offer.
Special Tender Offer
The Companies Law provides that an acquisition of shares of an Israeli public company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of 25% or more of the voting rights in the company. This requirement does not apply if there is already another holder of at least 25% of the voting rights in the company. Similarly, the Companies Law provides that an acquisition of shares in a public company must be made by means of a special tender offer if, as a result of the acquisition, the purchaser would become a holder of more than 45%
139
of the voting rights in the company, provided that there is no other shareholder of the company who holds more than 45% of the voting rights in the company, subject to certain exceptions.
A special tender offer must be extended to all shareholders of a company but the offeror is not required to purchase shares representing more than 5% of the voting power attached to the company's outstanding shares, regardless of how many shares are tendered by shareholders. A special tender offer may be consummated only if (i) outstanding shares representing at least 5% of the voting power of the company will be acquired by the offeror and (ii) the number of shares tendered in the offer exceeds the number of shares whose holders objected to the offer (excluding the purchaser, controlling shareholders, holders of 25% or more of the voting rights in the company or any person having a personal interest in the acceptance of the tender offer). If a special tender offer is accepted, then the purchaser or any person or entity controlling it or under common control with the purchaser or such controlling person or entity may not make a subsequent tender offer for the purchase of shares of the target company and may not enter into a merger with the target company for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect such an offer or merger in the initial special tender offer.
Merger
The Companies Law permits merger transactions if approved by each party's board of directors and, unless certain requirements described under the Companies Law are met, by a majority vote of each party's shareholders, and, in the case of the target company, a majority vote of each class of its shares, voted on the proposed merger at a shareholders meeting.
For purposes of the shareholder vote, unless a court rules otherwise, the merger will not be deemed approved if a majority of the votes of shares represented at the shareholders meeting that are held by parties other than the other party to the merger, or by any person (or group of persons acting in concert) who holds (or hold, as the case may be) 25% or more of the voting rights or the right to appoint 25% or more of the directors of the other party, vote against the merger. If, however, the merger involves a merger with a company's own controlling shareholder or if the controlling shareholder has a personal interest in the merger, then the merger is instead subject to the same Special Majority approval that governs all extraordinary transactions with controlling shareholders (as described under "Management—Approval of Related Party Transactions Under Israeli Law—Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions").
If the transaction would have been approved by the shareholders of a merging company but for the separate approval of each class or the exclusion of the votes of certain shareholders as provided above, a court may still approve the merger upon the request of holders of at least 25% of the voting rights of a company, if the court holds that the merger is fair and reasonable, taking into account the value of the parties to the merger and the consideration offered to the shareholders of the target company.
Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of the merging entities, and may further give instructions to secure the rights of creditors.
In addition, a merger may not be consummated unless at least 50 days have passed from the date on which a proposal for approval of the merger was filed by each party with the Israeli Registrar of Companies and at least 30 days have passed from the date on which the merger was approved by the shareholders of each party.
140
Anti-Takeover Measures under Israeli Law
For as long as our securities are traded on TASE, the Israeli Securities Law does not allow us, being a public company traded on TASE, to create and issue shares having rights different from those attached to our ordinary shares, including shares providing certain preferred rights with respect to voting, distributions, or other matters and shares having preemptive rights. As of the closing of this offering and for as long as our shares are traded on TASE, no preferred shares will be authorized under the Israeli Securities Law and our articles of association. The authorization and designation of a class of preferred shares will require an amendment to our articles of association, which requires the prior approval of the holders of a majority of the voting power attaching to our issued and outstanding shares at a general meeting. The convening of the meeting, the shareholders entitled to participate and the majority vote required to be obtained at such a meeting will be subject to the requirements set forth in the Companies Law as described above in "—Voting Rights."
Borrowing Powers
Pursuant to the Companies Law and our articles of association, our board of directors may exercise all powers and take all actions that are not required under law or under our articles of association to be exercised or taken by our shareholders, including the power to borrow money for company purposes.
Changes in Capital
Our articles of association enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the Companies Law and must be approved by a resolution duly passed by our shareholders at a general meeting by voting on such change in the capital. In addition, transactions that have the effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings or profits, require the approval of both our board of directors and an Israeli court.
141
DESCRIPTION OF AMERICAN DEPOSITARY SHARES
American Depositary Shares
The Bank of New York Mellon, as depositary, will register and deliver American Depositary Shares, also referred to as ADSs. Each ADS will represent shares (or a right to receive shares) deposited with Bank Hapoalim, as custodian for the depositary in Israel. Each ADS will also represent any other securities, cash or other property which may be held by the depositary. The depositary's office at which the ADSs will be administered is located at 101 Barclay Street, New York, New York 10286. The Bank of New York Mellon's principal executive office is located at One Wall Street, New York, New York 10286.
You may hold ADSs either (A) directly (i) by having an American Depositary Receipt, also referred to as an ADR, which is a certificate evidencing a specific number of ADSs, registered in your name, or (ii) by having uncertificated ADSs registered in your name, or (B) indirectly by holding a security entitlement in ADSs through your broker or other financial institution that is a direct or indirect participant in The Depository Trust Company, also called DTC. If you hold ADSs directly, you are a registered ADS holder, also referred to as an ADS holder. This description assumes you are an ADS holder. If you hold the ADSs indirectly, you must rely on the procedures of your broker or other financial institution to assert the rights of ADS holders described in this section. You should consult with your broker or financial institution to find out what those procedures are.
Registered holders of uncertificated ADSs will receive statements from the depositary confirming their holdings.
As an ADS holder, we will not treat you as one of our shareholders and you will not have shareholder rights. Israeli law governs shareholder rights. The depositary will be the holder of the shares underlying your ADSs. As a registered holder of ADSs, you will have ADS holder rights. A deposit agreement among us, the depositary, ADS holders and all other persons indirectly or beneficially holding ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs.
The following is a summary of the material provisions of the deposit agreement. For more complete information, you should read the entire deposit agreement and the form of ADR. Directions on how to obtain copies of those documents are provided under "Where You Can Find More Information" on page 174.
Dividends and Other Distributions
How will you receive dividends and other distributions on the shares?
The depositary has agreed to pay to ADS holders the cash dividends or other distributions it or the custodian receives on shares or other deposited securities, after deducting its fees and expenses. You will receive these distributions in proportion to the number of shares your ADSs represent.
Cash. The depositary will convert any cash dividend or other cash distribution we pay on the shares into U.S. dollars, if it can do so on a reasonable basis and can transfer the U.S. dollars to the United States. If that is not possible or if any government approval is needed and cannot be obtained, the deposit agreement allows the depositary to distribute the foreign currency only to those ADS holders to whom it is possible to do so. It will hold the foreign currency it cannot convert for the account of the ADS holders who have not been paid. It will not invest the foreign currency and it will not be liable for any interest.
Before making a distribution, any withholding taxes, or other governmental charges that must be paid will be deducted. See "Taxation—Israeli Tax Considerations" and "Taxation—U.S. Federal Income Tax Consequences." The depositary will distribute only whole U.S. dollars and cents and will round
142
fractional cents to the nearest whole cent. If the exchange rates fluctuate during a time when the depositary cannot convert the foreign currency, you may lose some or all of the value of the distribution.
Shares. The depositary may distribute additional ADSs representing any shares we distribute as a dividend or free distribution. The depositary will only distribute whole ADSs. It will sell shares which would require it to deliver a fractional ADS (or ADSs representing those shares) and distribute the net proceeds in the same way as it does with cash. If the depositary does not distribute additional ADSs, the outstanding ADSs will also represent the new shares. The depositary may sell a portion of the distributed shares (or ADSs representing those shares) sufficient to pay its fees and expenses, and to pay taxes or charges that the depositary is obligated to withhold, in connection with that distribution.
Rights to Purchase Additional Shares. If we offer holders of our securities any rights to subscribe for additional shares or any other rights, the depositary may (i) exercise those rights on behalf of ADS holders, (ii) distribute those rights to ADS holders, or (iii) sell those rights and distribute the net proceeds to ADS holders, in each case after deduction or upon payment of its fees and expenses. To the extent the depositary does not do any of those things, it will allow the rights to lapse. In that case, you will receive no value for them. The depositary will exercise or distribute rights only if we ask it to and provide satisfactory assurances to the depositary that it is legal to do so. If the depositary will exercise rights, it will purchase the securities to which the rights relate and distribute those securities or, in the case of shares, new ADSs representing the new shares, to subscribing ADS holders, but only if ADS holders have paid the exercise price to the depositary. U.S. securities laws may restrict the ability of the depositary to distribute rights or ADSs or other securities issued on exercise of rights to all or certain ADS holders, and the securities distributed may be subject to restrictions on transfer.
Other Distributions. The depositary will send to ADS holders anything else we distribute on deposited securities by any means it thinks is legal, fair, and practical. If it cannot make the distribution in that way, the depositary has a choice. It may decide to sell what we distributed and distribute the net proceeds, in the same way as it does with cash. Or, it may decide to hold what we distributed, in which case ADSs will also represent the newly distributed property. However, the depositary is not required to distribute any securities (other than ADSs) to ADS holders unless it receives satisfactory evidence from us that it is legal to make that distribution. The depositary may sell a portion of the distributed securities or property sufficient to pay its fees and expenses in connection with that distribution. U.S. securities laws may restrict the ability of the depositary to distribute securities to all or certain ADS holders, and the securities distributed may be subject to restrictions on transfer.
The depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any ADS holders. We have no obligation to register ADSs, shares, rights, or other securities under the Securities Act. We also have no obligation to take any other action to permit the distribution of ADSs, shares, rights, or anything else to ADS holders. This means that you may not receive the distributions we make on our shares or any value for them if it is illegal or impractical for us to make them available to you.
Deposit, Withdrawal and Cancellation
How are ADSs issued?
The depositary will deliver ADSs if you or your broker deposits shares or evidence of rights to receive shares with the custodian. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will register the appropriate number of ADSs in the names you request and will deliver the ADSs to or upon the order of the person or persons that made the deposit.
143
How can ADS holders withdraw the deposited securities?
You may surrender your ADSs at the depositary's corporate trust office. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will deliver the shares and any other deposited securities underlying the ADSs to the ADS holder or a person the ADS holder designates at the office of the custodian. Or, at your request, risk and expense, the depositary will deliver the deposited securities at its corporate trust office, if feasible. The depositary may charge you a fee and its expenses for instructing the custodian regarding delivery of deposited securities.
How do ADS holders interchange between certificated ADSs and uncertificated ADSs?
You may surrender your ADR to the depositary for the purpose of exchanging your ADR for uncertificated ADSs. The depositary will cancel that ADR and will send to the ADS holder a statement confirming that the ADS holder is the registered holder of uncertificated ADSs. Alternatively, upon receipt by the depositary of a proper instruction from a registered holder of uncertificated ADSs requesting the exchange of uncertificated ADSs for certificated ADSs, the depositary will execute and deliver to the ADS holder an ADR evidencing those ADSs.
Voting Rights
How do you vote?
ADS holders may instruct the depositary how to vote the number of deposited shares their ADSs represent. If we request the depositary to solicit your voting instructions (and we are not required to do so), the depositary will notify you of a shareholders' meeting and send or make voting materials available to you. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary how to vote. For instructions to be valid, they much reach the depositary by a date set by the depositary. The depositary will try, as far as practicable, subject to the laws of Israel and the provisions of our articles of association or similar documents, to vote or to have its agents vote the shares or other deposited securities as instructed by ADS holders. If we do not request the depositary to solicit your voting instructions, you can still send voting instructions, and, in that case, the depositary may try to vote as you instruct, but it is not required to do so.
Except by instructing the depositary as described above, you won't be able to exercise voting rights unless you surrender your ADSs and withdraw the shares. However, you may not know about the meeting enough in advance to withdraw the shares. In any event, the depositary will not exercise any discretion in voting deposited securities and it will only vote or attempt to vote as instructed.
We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your shares. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that you may not be able to exercise voting rights and there may be nothing you can do if your shares are not voted as you requested.
In order to give you a reasonable opportunity to instruct the depositary as to the exercise of voting rights relating to Deposited Securities, if we request the Depositary to act, we agree to give the depositary notice of any such meeting and details concerning the matters to be voted upon at least 30 days in advance of the meeting date.
144
Fees and Expenses
| Persons depositing or withdrawing ordinary shares or ADS holders must pay: | For: | |
$5.00 (or less) per ADSs (or portion of ADSs) |
Issuance of ADSs, including issuances resulting from a distribution of ordinary shares or rights or other property; or cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
|
$.05 (or less) per ADS |
Any cash distribution to ADS holders |
|
A fee equivalent to the fee that would be payable if securities distributed to you had been ordinary shares and the ordinary shares had been deposited for issuance of ADSs |
Distribution of securities distributed to holders of deposited securities which are distributed by the depositary to ADS holders |
|
$.05 (or less) per ADS per calendar year |
Depositary services |
|
Registration or transfer fees |
Transfer and registration of ordinary shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw ordinary shares |
|
Expenses of the depositary |
Cable (including SWIFT) and facsimile transmissions (when expressly provided in the deposit agreement); conversion of foreign currency to U.S. dollars |
|
Taxes and other governmental charges the depositary or the custodian has to pay on any ADSs or ordinary shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes |
As necessary |
|
Any charges incurred by the depositary or its agents for servicing the deposited securities |
As necessary |
The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may collect any of its fees by deduction from any cash distribution payable (or by selling a portion of securities or other property distributable) to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid.
From time to time, the depositary may make payments to us to reimburse us for costs and expenses generally arising out of establishment and maintenance of the ADS program, waive fees and expenses for services provided to us by the depositary, or share revenue from the fees collected from ADS holders. In performing its duties under the deposit agreement, the depositary may use brokers, dealers, or other service providers that are affiliates of the depositary and that may earn or share fees or commissions.
145
The depositary may convert currency itself or through any of its affiliates and, in those cases, acts as principal for its own account and not as an agent, fiduciary, or broker on behalf of any other person and earns revenue, including, without limitation, fees and spreads that it will retain for its own account. The depositary makes no representation that the exchange rate used or obtained in any currency conversion will be the most favorable rate that could be obtained at the time or as to the method by which that rate will be determined, subject to its obligations under the deposit agreement.
Payment of Taxes
You will be responsible for any taxes or other governmental charges payable on your ADSs or on the deposited securities represented by any of your ADSs. The depositary may refuse to register any transfer of your ADSs or allow you to withdraw the deposited securities represented by your ADSs until such taxes or other charges are paid. It may apply payments owed to you or sell deposited securities represented by your ADSs to pay any taxes owed and you will remain liable for any deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number of ADSs to reflect the sale and pay to ADS holders any proceeds, or send to ADS holders any property, remaining after it has paid the taxes.
Tender and Exchange Offers; Redemption, Replacement or Cancellation of Deposited Securities
The depositary will not tender deposited securities in any voluntary tender or exchange offer unless instructed to do by an ADS holder surrendering ADSs and subject to any conditions or procedures the depositary may establish.
If deposited securities are redeemed for cash in a transaction that is mandatory for the depositary as a holder of deposited securities, the depositary will call for surrender of a corresponding number of ADSs and distribute the net redemption money to the holders of called ADSs upon surrender of those ADSs.
If there is any change in the deposited securities such as a sub-division, combination, or other reclassification, or any merger, consolidation, recapitalization, or reorganization affecting the issuer of deposited securities in which the depositary receives new securities in exchange for or in lieu of the old deposited securities, the depositary will hold those replacement securities as deposited securities under the deposit agreement. However, if the depositary decides it would not be lawful and to hold the replacement securities because those securities could not be distributed to ADS holders or for any other reason, the depositary may instead sell the replacement securities and distribute the net proceeds upon surrender of the ADSs.
If there is a replacement of the deposited securities and the depositary will continue to hold the replacement securities, the depositary may distribute new ADSs representing the new deposited securities or ask you to surrender your outstanding ADSs in exchange for new ADSs identifying the new deposited securities.
If there are no deposited securities underlying ADSs, including if the deposited securities are cancelled, or if the deposited securities underlying ADSs have become apparently worthless, the depositary may call for surrender or of those ADSs or cancel those ADSs upon notice to the ADS holders.
Amendment and Termination
How may the deposit agreement be amended?
We may agree with the depositary to amend the deposit agreement and the ADSs without your consent for any reason. If an amendment adds or increases fees or charges, except for taxes and other governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery
146
charges, or similar items, or prejudices a substantial right of ADS holders, it will not become effective for outstanding ADSs until 30 days after the depositary notifies ADS holders of the amendment. At the time an amendment becomes effective, you are considered, by continuing to hold your ADSs, to agree to the amendment and to be bound by the ADSs and the deposit agreement as amended.
How may the deposit agreement be terminated?
The depositary will initiate termination of the deposit agreement if we instruct it to do so. The depositary may initiate termination of the deposit agreement if
- •
- 60 days have passed since the depositary told us it wants to resign but a successor depositary has not been appointed and
accepted its appointment;
- •
- we delist our shares from an exchange on which they were listed and do not list the shares on another exchange;
- •
- we appear to be insolvent or enter insolvency proceedings;
- •
- all or substantially all the value of the deposited securities has been distributed either in cash or in the form of securities;
- •
- there are no deposited securities underlying the ADSs or the underlying deposited securities have become apparently worthless; or
- •
- there has been a replacement of deposited securities.
If the deposit agreement will terminate, the depositary will notify ADS holders at least 90 days before the termination date. At any time after the termination date, the depositary may sell the deposited securities. After that, the depositary will hold the money it received on the sale, as well as any other cash it is holding under the deposit agreement, unsegregated and without liability for interest, for the pro rata benefit of the ADS holders that have not surrendered their ADSs. Normally, the depositary will sell as soon as practicable after the termination date.
After the termination date and before the depositary sells, ADS holders can still surrender their ADSs and receive delivery of deposited securities, except that the depositary may refuse to accept a surrender for the purpose of withdrawing deposited securities if it would interfere with the selling process. The depositary may refuse to accept a surrender for the purpose of withdrawing sale proceeds until all the deposited securities have been sold. The depositary will continue to collect distributions on deposited securities, but, after the termination date, the depositary is not required to register any transfer of ADSs or distribute any dividends or other distributions on deposited securities to the ADSs holder (until they surrender their ADSs) or give any notices or perform any other duties under the deposit agreement except as described in this paragraph.
Limitations on Obligations and Liability
Limits on our Obligations and the Obligations of the Depositary; Limits on Liability to Holders of ADSs
The deposit agreement expressly limits our obligations and the obligations of the depositary. It also limits our liability and the liability of the depositary. We and the depositary:
- •
- are only obligated to perform obligations specifically set forth in the deposit agreement without negligence or bad faith;
- •
- are not liable if we are, or it is, prevented or delayed by law or circumstances beyond our or its control from performing our or its
obligations under the deposit agreement;
- •
- are not liable if we exercise or it exercises discretion permitted under the deposit agreement;
147
- •
- are not liable for the inability of any holder of ADSs to benefit from any distribution on deposited securities that is not made
available to holders of ADSs under the terms of the deposit agreement, or for any special, consequential, or punitive damages for any breach of the terms of the deposit agreement;
- •
- have no obligation to become involved in a lawsuit or other proceeding related to the ADSs or the deposit agreement on your behalf or
on behalf of any other person;
- •
- are not liable for the acts or omissions of any securities depository, clearing agency or settlement system; and
- •
- may rely upon any documents we believe or it believes in good faith to be genuine and to have been signed or presented by the proper person.
In the deposit agreement, we and the depositary agree to indemnify each other under certain circumstances.
Requirements for Depositary Actions
Before the depositary will deliver or register a transfer of ADSs, make a distribution on ADSs, or permit withdrawal of shares, the depositary may require:
- •
- payment of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for
the transfer of any shares or other deposited securities;
- •
- satisfactory proof of the identity and genuineness of any signature or other information it deems necessary; and
- •
- compliance with regulations it may establish, from time to time, consistent with the deposit agreement, including presentation of transfer documents.
The depositary may refuse to deliver ADSs or register transfers of ADSs when the transfer books of the depositary or our transfer books are closed or at any time if the depositary or we think it advisable to do so.
Your Right to Receive the Shares Underlying Your ADSs
ADS holders have the right to cancel their ADSs and withdraw the underlying shares at any time except:
- •
- when temporary delays arise because: (i) the depositary has closed its transfer books or we have closed our transfer books;
(ii) the transfer of ordinary shares is blocked to permit voting at a shareholders' meeting; or (iii) we are paying a dividend on our shares;
- •
- when you owe money to pay fees, taxes, and similar charges; or
- •
- when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of ordinary shares or other deposited securities.
This right of withdrawal may not be limited by any other provision of the deposit agreement.
Pre-release of ADSs
The deposit agreement permits the depositary to deliver ADSs before deposit of the underlying shares. This is called a pre-release of the ADSs. The depositary may also deliver shares upon cancellation of pre-released ADSs (even if the ADSs are canceled before the pre-release transaction
148
has been closed out). A pre-release is closed out as soon as the underlying shares are delivered to the depositary. The depositary may receive ADSs instead of shares to close out a pre-release. The depositary may pre-release ADSs only under the following conditions: (1) before or at the time of the pre-release, the person to whom the pre-release is being made represents to the depositary in writing that it or its customer owns the shares or ADSs to be deposited; (2) the pre-release is fully collateralized with cash or other collateral that the depositary considers appropriate; and (3) the depositary must be able to close out the pre-release on not more than five business days' notice. In addition, the depositary will limit the number of ADSs that may be outstanding at any time as a result of pre-release, although the depositary may disregard the limit from time to time if it thinks it is appropriate to do so.
Direct Registration System
In the deposit agreement, all parties to the deposit agreement acknowledge that the Direct Registration System, also referred to as DRS, and Profile Modification System, also referred to as Profile, will apply to the ADSs. DRS is a system administered by DTC that facilitates interchange between registered holding of uncertificated ADSs and holding of security entitlements in ADSs through DTC and a DTC participant. Profile is feature of DRSs that allows a DTC participant, claiming to act on behalf of a registered holder of uncertificated ADSs, to direct the depositary to register a transfer of those ADSs to DTC or its nominee and to deliver those ADSs to the DTC account of that DTC participant without receipt by the depositary of prior authorization from the ADS holder to register that transfer.
In connection with and in accordance with the arrangements and procedures relating to DRS/Profile, the parties to the deposit agreement understand that the depositary will not determine whether the DTC participant that is claiming to be acting on behalf of an ADS holder in requesting registration of transfer and delivery as described in the paragraph above has the actual authority to act on behalf of the ADS holder (notwithstanding any requirements under the Uniform Commercial Code). In the deposit agreement, the parties agree that the depositary's reliance on and compliance with instructions received by the depositary through the DRS/Profile system and in accordance with the deposit agreement will not constitute negligence or bad faith on the part of the depositary.
Shareholder Communications; Inspection of Register of Holders of ADSs; Disclosure of Beneficial Ownership
The depositary will make available for your inspection at its office all communications that it receives from us as a holder of deposited securities that we make generally available to holders of deposited securities. The depositary will send you copies of those communications or otherwise make those communications available to you if we ask it to. You have a right to inspect the register of holders of ADSs, but not for the purpose of contacting those holders about a matter unrelated to our business or the ADSs.
Each ADS holder and each indirect or beneficial owner agrees to comply with any applicable law, including in both the United States and Israel, with regard to the notification to us of the holding or proposed holding of certain interests in shares and the obtaining of certain consents, to the same extent as if such holder or owner were a registered holder or beneficial owner of shares. Each ADS holder and each indirect or beneficial owner agrees to provide all information known to it in response to a request made to provide beneficial ownership information. Each indirect and beneficial owner consents to the disclosure by the ADS holder or any other person through which it holds ADSs, of all information responsive to a request of that kind that is known to that ADS holder or other person.
149
SHARES ELIGIBLE FOR FUTURE SALE
Upon closing of this offering, we will have outstanding ordinary shares and ADSs ( ADSs if the underwriters exercise their over-allotted options). All of the ADSs issued in this offering will be freely transferable by persons other than our "affiliates" without restriction or further registration under the Securities Act. Sales of substantial numbers of our ADSs in the public market could adversely affect prevailing market prices of our ADSs. While our ADSs have been approved to be quoted on the NASDAQ Capital Market, we cannot assure you that a regular trading market will develop in the ADSs. All of the ordinary shares and ADSs held by our existing shareholders upon closing of this offering will be available for sale in the public market after the expiration or waiver of the lock-up agreements described below.
Rule 144
In general, under Rule 144 of the Securities Act (as in effect on the date of this prospectus), beginning 90 days after the date of this prospectus, an "affiliate" who has beneficially owned our shares for a period of at least six months is entitled to sell upon expiration or waiver of the lock-up agreements described below within any three-month period a number of shares that does not exceed the greater of either 1% of the then outstanding ADSs, or approximately immediately after this offering, or the average weekly trading volume of our ADSs on the NASDAQ Capital Market during the four calendar weeks preceding the filing with the SEC of a notice on Form 144 with respect to such sale. Such sales under Rule 144 of the Securities Act are also subject to prescribed requirements relating to the manner of sale, notice and availability of current public information about us.
Under Rule 144, a person who is not deemed to have been an affiliate of ours at any time during the 90 days preceding a sale, and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior holder other than an affiliate, is entitled to sell such shares without restriction, provided we have been in compliance with our reporting requirements under the Exchange Act for the six months following satisfaction of the six-month holding period. To the extent that our affiliates sell their shares, other than pursuant to Rule 144 or a registration statement, the purchaser's holding period for the purpose of effecting a sale under Rule 144 commences on the date of transfer from the affiliate.
Rule 701
In general, under Rule 701 of the Securities Act as in effect on the date of this prospectus, each of our employees, consultants or advisors who acquires our ordinary shares from us in connection with a compensatory share plan or other written agreement executed prior to the closing of this offering is eligible to resell such ordinary shares in reliance on Rule 144, but without compliance with some of the restrictions, including the holding period, contained in Rule 144.
Regulation S
Regulation S provides generally that sales made in offshore transactions are not subject to the registration or prospectus-delivery requirements of the Securities Act.
Lock-up Agreements
For a description of the lock-up agreements that we and our shareholders have entered into in connection with this offering, see "Underwriting."
150
Form S-8 Registration Statements
Following the completion of this offering, we intend to file one or more registration statements on Form S-8 under the Securities Act to register up to ordinary shares, in the aggregate, issued or reserved for issuance under our equity plans. The registration statement on Form S-8 will become effective automatically upon filing. Ordinary shares issued upon exercise of a share option and registered pursuant to the Form S-8 registration statement will, subject to vesting provisions and Rule 144 volume limitations applicable to our affiliates, be available for sale in the open market immediately unless they are subject to the 180-day lock-up or, if subject to the lock-up, immediately after the 180-day lock-up period expires.
As of March 31, 2015, an aggregate of 34,629,244 ordinary shares were reserved for issuance under our equity plans, of which options to purchase 17,963,341 ordinary shares have been granted and are outstanding, options to purchase 6,665,848 ordinary shares have been exercised, and transferred to the beneficiary holders, and additional options to purchase 10,000,000 ordinary shares have been approved by our board of directors, pending the approval of our shareholders. No options for ordinary shares are currently reserved under our plans for future option grants. Upon the closing of this offering, options held by our directors and executive officers, and holders of 5% and more of our outstanding securities, will be subject to lock-up agreements with the underwriters.
151
The following description is not intended to constitute a complete analysis of all tax consequences relating to the acquisition, ownership and disposition of our ordinary shares and ADSs. You should consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
Israeli Tax Considerations and Government Programs
The following is a brief summary of the material Israeli tax laws applicable to us, and certain Israeli Government programs that benefit us. This section also contains a discussion of material Israeli tax consequences concerning the ownership and disposition of our ordinary shares. This summary does not discuss all the aspects of Israeli tax law that may be relevant to a particular investor in light of his or her personal investment circumstances or to some types of investors subject to special treatment under Israeli law. Examples of such investors include residents of Israel or traders in securities who are subject to special tax regimes not covered in this discussion. To the extent that the discussion is based on new tax legislation that has not yet been subject to judicial or administrative interpretation, we cannot assure you that the appropriate tax authorities or the courts will accept the views expressed in this discussion. The discussion below is subject to change, including due to amendments under Israeli law or changes to the applicable judicial or administrative interpretations of Israeli law, which change could affect the tax consequences described below.
General Corporate Tax Structure in Israel
Israeli resident (as defined below) companies, such as us, are generally subject to corporate tax at the rate of 26.5% as of 2014. However, the effective tax rate payable by a company that derives income from a Preferred Enterprise (as discussed below) may be considerably less. Capital gains derived by an Israeli company are subject to tax at the prevailing corporate tax rate.
Law for the Encouragement of Industry (Taxes), 5729-1969
The Law for the Encouragement of Industry (Taxes), 5729-1969, or the Industry Encouragement Law, provides several tax benefits for "Industrial Companies." As we have not yet generated income, it is uncertain whether we qualify as an Industrial Company within the meaning of the Industry Encouragement Law.
The Industry Encouragement Law defines an "Industrial Company" as a company resident in Israel, of which 90% or more of its income in any tax year, other than income from defense loans, is derived from an "Industrial Enterprise" owned by it. An "Industrial Enterprise" is defined as an enterprise whose principal activity in a given tax year is industrial production.
The following corporate tax benefits, among others, are available to Industrial Companies:
- •
- amortization over an eight-year period of the cost of patents and rights to use a patent and know-how which were purchased in good
faith and are used for the development or advancement of the Industrial Enterprise; and
- •
- under certain conditions, an election to file consolidated tax returns with related Israeli Industrial Companies.
There can be no assurance that we currently qualify, or will continue to qualify, as an Industrial Company or that the benefits described above will be available in the future.
152
Law for the Encouragement of Capital Investments, 5719-1959
The Law for the Encouragement of Capital Investments, 5719-1959, or the Investment Law, currently provides certain tax benefits for income generated by "Preferred Companies" from their "Preferred Enterprises." The definition of a Preferred Company includes, inter alia, a company incorporated in Israel that is not wholly-owned by a governmental entity, which:
- •
- owns a Preferred Enterprise, which is defined as an "Industrial Enterprise" (as defined under the Investment Law) that is classified
as either a "Competitive Enterprise" (as defined under the Investment Law) or a "Competitive Enterprise in the Field of Renewable Energy" (as defined under the Investment Law);
- •
- is controlled and managed from Israel;
- •
- is not a "Family Company," a "Home Company," or a "Kibbutz" (collective community) as defined under the Income Tax Ordinance;
- •
- keeps acceptable books of account and files reports in accordance with the provisions of the Investment Law and the Income Tax
Ordinance; and
- •
- was not, and certain officers of which were not, convicted of certain crimes in the 10 years prior to the tax year with respect to which benefits are being claimed.
As of January 1, 2014, a Preferred Company is entitled to a reduced corporate tax rate of 16% with respect to its income derived by its Preferred Enterprise, unless the Preferred Enterprise is located in development area A, in which case the rate will be 9% (our operations are currently not located in development area A).
Dividends paid out of income attributed to a Preferred Enterprise are generally subject to tax at the rate of 20% or such lower rate as may be provided in an applicable tax treaty. However, if such dividends are paid to an Israeli company, such dividends should be exempt from tax (although, if such dividends are subsequently distributed to individuals or a non-Israeli company, tax at a rate of 20% or such lower rate as may be provided in an applicable tax treaty will apply).
If in the future we generate taxable income, to the extent that we qualify as a "Preferred Company," the benefits provided under the Investment Law could potentially reduce our corporate tax liabilities. Therefore, the termination or substantial reduction of the benefits available under the Investment Law could materially increase our tax liabilities.
The Encouragement of Industrial Research and Development Law, 5744-1984
Under the Encouragement of Industrial Research and Development Law, 5744-1984, or Research Law, research and development programs which meet specified criteria and are approved by a committee of the Office of the Chief Scientist, or OCS of the Israeli Ministry of Economy (formerly named the Ministry of Industry, Trade, and Labor) are eligible for grants. The grants awarded are typically up to 50% of the project's expenditures, as determined by the research committee. The grantee is required to pay royalties to the State of Israel from the sale of products developed under the program. Regulations under the Research Law generally provide for the payment of royalties of 3% to 5% on sales of products and services based on technology developed using grants, until 100% of the grant, linked to the dollar and bearing interest at the LIBOR rate, is repaid. The terms of the Israeli government participation also require that products developed with government grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel, unless approval is received from the OCS and additional payments are made to the State of Israel. However, this does not restrict the export of products that incorporate the funded technology. The royalty repayment ceiling can reach up to three times the amount of the grant received if manufacturing is
153
moved outside of Israel, and repayment of up to six times the amount of the grant may be required if the technology itself is transferred outside of Israel.
Taxation of our Shareholders
Capital Gains Tax
Israeli law generally imposes a capital gains tax (i) on the sale of any capital assets by residents of Israel, as defined for Israeli tax purposes, and (ii) on the sale of capital assets located in Israel, including shares of Israeli companies, by non-residents of Israel, unless a specific exemption is available or unless a tax treaty between Israel and the shareholder's country of residence provides otherwise. The law distinguishes between real gain and inflationary surplus. The inflationary surplus is a portion of the total capital gain that is equivalent to the increase of the relevant asset's purchase price which is attributable to the increase in the Israeli consumer price index or a foreign currency exchange rate between the date of purchase and the date of sale. The real gain is the excess of the total capital gain over the inflationary surplus.
Israeli Residents
Generally, as of January 1, 2012, the tax rate applicable to real capital gains derived from the sale of shares, whether listed on a stock market or not, is 25% for Israeli individuals, unless such shareholder claims a deduction for financing expenses in connection with such shares, in which case the gain will generally be taxed at a rate of 30%. Additionally, if such shareholder is considered a "substantial shareholder" at the time of the sale or at any time during the 12-month period preceding such sale, the tax rate will be 30%. A "substantial shareholder" is defined as one who holds, directly or indirectly, alone or "together with another" (i.e., together with a relative, or together with someone who is not a relative but with whom, according to an agreement, there is regular cooperation in material matters of the company, directly or indirectly), holds, directly or indirectly, at least 10% of any of the "means of control" in the company. "Means of control" generally include the right to vote, receive profits, nominate a director or an executive officer, receive assets upon liquidation, or instruct someone who holds any of the aforementioned rights regarding the manner in which such rights are to be exercised. However, different tax rates will apply to dealers in securities and shareholders who acquired their shares prior to the company's initial public offering. Israeli companies are subject to capital gains tax at the regular corporate tax rate (i.e., currently 26.5%) on real capital gains derived from the sale of listed shares.
As of January 1, 2013, Israeli resident shareholders who are individuals with taxable income that exceeds NIS 800,000 in a tax year (linked to the Israeli consumer price index each year) will be subject to an additional tax at the rate of 2% on the portion of their taxable income for such tax year that is in excess of NIS 800,000 (linked to the Israeli consumer price index each year). For this purpose, taxable income includes taxable capital gains from the sale of our shares and taxable income from dividend distributions.
In some instances where our shareholders are liable for Israeli tax on the sale of their ordinary shares, the payment of the consideration may be subject to the withholding of Israeli tax at source.
Non-Israeli Residents
A non-Israeli resident who derives capital gains from the sale of shares in an Israeli resident company that were purchased after the company was listed for trading on a stock exchange outside of Israel will be exempt from Israeli tax so long as the shares were not held through a permanent establishment that the non-resident maintains in Israel. However, non-Israeli resident corporations will not be entitled to the foregoing exemption if (i) an Israeli resident has a controlling interest, directly or indirectly, alone, "together with another" (as defined above), or together with another Israeli resident,
154
of more than 25% in one or more of the "means of control" (as defined above) in such non-Israeli resident corporation, or (ii) Israeli residents are the beneficiaries of, or are entitled to, 25% or more of the revenues or profits of such non-Israeli resident corporation, whether directly or indirectly.
In addition, a sale of securities by a non-Israeli resident may be exempt from Israeli capital gains tax under the provisions of an applicable tax treaty. For example, pursuant to the provisions of the Convention between the Government of the United States of America and the Government of the State of Israel with respect to Taxes on Income, as amended, or the U.S.-Israel Tax Treaty, capital gains arising from the sale, exchange or disposition of our ordinary shares by (i) a person who qualifies as a resident of the United States within the meaning of the U.S.-Israel Tax Treaty, (ii) who holds the shares as a capital asset, and (iii) who is entitled to claim the benefits afforded to such person by the U.S.-Israel Tax Treaty generally is generally exempt from Israeli capital gains tax. Such exemption will not apply if: (i) such person holds, directly or indirectly, shares representing 10% or more of our voting power during any part of the 12-month period preceding such sale, exchange, or disposition, subject to particular conditions; (ii) the capital gains from such sale, exchange, or disposition are attributable to a permanent establishment in Israel; or (iii) such person is an individual and was present in Israel for 183 days or more during the relevant tax year. In such case, the capital gain arising from the sale, exchange, or disposition of our ordinary shares would be subject to Israeli tax, to the extent applicable; however, under the U.S.-Israel Tax Treaty, the taxpayer may be permitted to claim a credit for such taxes against the U.S. federal income tax imposed with respect to such sale, exchange, or disposition, subject to the limitations under U.S. law applicable to foreign tax credits. The U.S.-Israel Tax Treaty does not relate to U.S. state or local taxes.
It should be noted that in the event that the real capital gain realized by an individual shareholder is not exempt from tax in Israel, an additional 2% tax might be applicable if certain conditions are met.
In some instances where our shareholders may be liable for Israeli tax on the sale of their ordinary shares, the payment of the consideration may be subject to the withholding of Israeli tax at source. Shareholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding at source at the time of sale.
Taxation of Dividend Distributions
Israeli Residents
Israeli resident individuals are generally subject to Israeli income tax on the receipt of dividends paid on our ordinary shares, other than bonus shares (share dividends). As of January 1, 2012, the tax rate applicable to such dividends is generally 25%. With respect to a person who is a "substantial shareholder" (as defined above) at the time the dividend is received or at any time during the preceding 12-month period, the applicable tax rate is 30%. Dividends paid from income derived from Preferred Enterprises accrued following December 31, 2013 will be subject to income tax at a rate of 20%.
As of January 1, 2013, Israeli resident shareholders who are individuals with taxable income that exceeds NIS 800,000 in a tax year (linked to the Israeli consumer price index each year) will be subject to an additional tax at the rate of 2% on the portion of their taxable income for such tax year that is in excess of NIS 800,000 (linked to the Israeli consumer price index each year). For this purpose, taxable income includes taxable capital gains from the sale of our shares and taxable income from dividend distributions.
Dividends paid to an Israeli resident individual shareholder on our ordinary shares will generally be subject to withholding tax at the rates corresponding with the income tax rates detailed above unless we are provided in advance with a withholding tax certificate issued by the Israel Tax Authority stipulating a different rate.
155
Notwithstanding the above, dividends paid to an Israeli resident "substantial shareholder" (as defined above) on publicly traded shares, like our ordinary shares, which are held via a "nominee company" (as defined under the Israeli Securities Law) are generally subject to Israeli withholding tax at a rate of 25%, unless a different rate is provided under an applicable tax treaty, provided that a certificate from the Israel Tax Authority allowing for a reduced withholding tax rate is obtained in advance. That said, in the event that such dividends paid to a "substantial shareholder" are sourced from regular earnings (i.e., not from Preferred Enterprise earnings) and a 25% withholding tax rate is applied, the "substantial shareholder" in question is generally required to file an Israeli tax return for the tax year in which the distribution was executed. Within the framework of such return, the dividend income shall be reported and pursuant thereto an additional tax liability in the amount of 5% of the gross dividend (for an overall income tax rate of 30%) should be paid.
If the dividend is attributable partly to income derived from a Preferred Enterprise and partly to other sources of income, the tax rate will be a blended rate reflecting the relative portions of the two types of income. We cannot assure you that we will designate the profits that are being distributed in a way that will reduce shareholders' tax liability.
Israeli resident companies are generally exempt from tax on the receipt of dividends paid on our ordinary shares.
Non-Israeli Residents
Unless relief is provided in a treaty between Israel and the shareholder's country of residence, non-Israeli residents are generally subject to Israeli income tax on the receipt of dividends paid on our ordinary shares at the rate of 25%. With respect to a person (including a corporation) who is a "substantial shareholder" (as defined above) at the time of receiving the dividend or at any time during the preceding 12-month period, absent treaty relief as mentioned above, the applicable Israeli income tax rate is 30%. Notwithstanding the above, dividends paid from income derived from Preferred Enterprises accrued following December 31, 2013 will be subject to Israeli income tax at a rate of 20%.
In this regard, dividends paid to a non-Israeli resident shareholder on our ordinary shares will generally be subject to withholding tax at the rates corresponding with the income tax rates detailed above unless we are provided in advance with a withholding tax certificate issued by the Israel Tax Authority stipulating a different rate (e.g., in accordance with the provisions of an applicable tax treaty).
Notwithstanding the above, dividends paid to a non-Israeli resident "substantial shareholder" (as defined above) on publicly traded shares, like our ordinary shares, which are held via a "nominee company" (as defined under the Israeli Securities Law) are generally subject to Israeli withholding tax at a rate of 25%, unless a different rate is provided under an applicable tax treaty, provided that a certificate from the Israel Tax Authority allowing for a reduced withholding tax rate is obtained in advance. That said, in the event that such dividends paid to a "substantial shareholder" are sourced from regular earnings (i.e., not from Preferred Enterprise earnings) and a 25% withholding tax rate is applied, the "substantial shareholder" in question is generally required to file an Israeli tax return for the tax year in which the distribution was executed. Within the framework of such return, the dividend income shall be reported and pursuant thereto an additional tax liability in the amount of 5% of the gross dividend (for an overall income tax rate of 30%) should be paid.
In addition, it should be noted that an additional 2% tax might be applicable to individual shareholders if certain conditions are met.
Under the U.S.-Israel Tax Treaty, the maximum Israeli tax on dividends paid to a holder of ordinary shares who qualifies as a resident of the United States within the meaning of the U.S.-Israel Tax Treaty is 25%. Such tax rate is generally reduced to 12.5% if (i) the shareholder is a U.S.
156
corporation and holds at least 10% of the outstanding shares of our voting stock during the part of our tax year that precedes the date of payment of the dividends and during the whole of our prior tax year; (ii) not more than 25% of our gross income in the tax year preceding the payment of the dividends consists of interest or dividends, other than dividends or interest received from subsidiary corporations 50% or more of the outstanding shares of voting stock of which is owned by us at the time such dividends or interest are received by us; and (iii) the dividends are not sourced from income derived during a period for which we were entitled to the reduced tax rate applicable to a Preferred Enterprise under the Investment Law. If the dividends are sourced from income derived during a period for which we are entitled to the reduced tax rate applicable to a Preferred Enterprise under the Investment Law, to the extent that the first two conditions detailed above are met, the Israeli tax rate applicable to such dividends should be 15%.
If the dividend is attributable partly to income derived from a Preferred Enterprise and partly to other sources of income, the tax rate will be a blended rate reflecting the relative portions of the two types of income. We cannot assure you that we will designate the profits that are being distributed in a way that will reduce shareholders' tax liability.
Estate and gift tax
Israeli law presently does not impose estate or gift taxes.
Material U.S. Federal Income Tax Consequences
The following summary describes certain material U.S. federal income tax consequences relating to an investment in our ADSs and ordinary shares. This summary deals only with ADSs and ordinary shares that are purchased pursuant to the offering and that are held as capital assets within the meaning of section 1221 of the U.S. Internal Revenue Code of 1986, as amended, or the Code, and does not address tax considerations of holders that may be subject to special tax rules, such as dealers or traders in securities or currencies, financial institutions, tax-exempt organizations, insurance companies, regulated investment companies, real estate investment trusts, individual retirement and tax-deferred accounts, persons holding ADSs or ordinary shares as part of a hedging, integrated, conversion or constructive sale transaction or a straddle, persons subject to the alternative minimum tax, or persons who have a functional currency other than the U.S. dollar. In addition, this discussion does not address the tax treatment of U.S. holders (as defined below) who own, directly, indirectly or constructively, 10% or more of our outstanding voting stock. The summary sets forth below relating to U.S. holders (as defined below) is applicable only to such U.S. holders (i) who are residents of the United States for purposes of the United States-Israel Tax Treaty, (ii) whose ordinary shares or ADSs are not, for purposes of the United States-Israel Tax Treaty, effectively connected with or attributable to a permanent establishment in Israel, and (iii) who otherwise qualify for the full benefits of the United States-Israel Tax Treaty. The discussion below is based upon the Code, existing and proposed Treasury regulations promulgated thereunder, and applicable administrative rulings and judicial decisions now in effect, all of which are subject to change, possibly on a retroactive basis, so as to result in U.S. federal income tax consequences different from those discussed below. In addition, this summary does not consider the possible application of U.S. federal gift or estate taxes or any aspect of state, local or non-U.S. tax laws. Furthermore, we can provide no assurance that the tax consequences contained in this summary will not be challenged by the Internal Revenue Service or will be sustained in a court if challenged.
As used in this summary the term "U.S. holder" means a beneficial owner of ADSs or ordinary shares that is, for U.S. federal income tax purposes: (1) an individual citizen or resident of the United States, (2) a corporation (or other entity taxable as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States or any political subdivision thereof, (3) an estate the income of which is subject to U.S. federal income taxation regardless of its source, or
157
(4) a trust if either (a) a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have the authority to control all substantial decisions of the trust, or (b) the trust has a valid election in effect under applicable Treasury regulations to be treated as a United States person. Except to the limited extent discussed below, this summary does not consider the U.S. federal tax considerations to a person that is not a U.S. holder (a "non-U.S. holder"). In addition, the tax treatment of persons who hold ADSs or ordinary shares through a partnership or other pass-through entity treated as a partnership for U.S. federal income tax purposes generally depends upon the status of the partner and the activities of the partnership. The tax consequences to such a partner or partnership are not considered in this summary and partners and partnerships should consult their tax advisors with respect to the U.S. federal tax consequences of investing in our ADSs or ordinary shares.
This summary does not discuss all aspects of U.S. federal income taxation that may be relevant to a particular investor in light of its circumstances. Prospective purchasers of our ADSs or ordinary shares should consult their own tax advisors with respect to the specific U.S. federal income tax consequences to such person of purchasing, holding or disposing of the ADSs or ordinary shares, as well as the effect of any state, local or other tax laws.
ADSs
If you hold ADSs, for United States federal income tax purposes, you generally will be treated as the owner of the underlying ordinary shares that are represented by such ADSs. Accordingly, deposits or withdrawals of ordinary shares for ADSs will not be subject to United States federal income tax.
Distributions on ADSs
Subject to the discussion under the heading "Passive Foreign Investment Company Consequences", U.S. holders are required to include in gross income the amount of any distribution paid on ordinary shares to the extent the distribution is paid out of our current and accumulated earnings and profits, as determined for U.S. federal income tax purposes. To the extent a distribution paid with respect to our ordinary shares exceeds our current and accumulated earnings and profits, such amount will be treated first as a non-taxable return of capital, reducing a U.S. holder's tax basis for the ordinary shares to the extent thereof, and thereafter as either long-term or short-term capital gain depending upon whether the U.S. holder has held our ordinary shares for more than one year as of the time such distribution is received. Preferential tax rates for long-term capital gains are applicable for U.S. holders that are individuals, estates or trusts. However, we do not expect to maintain calculations of our earnings and profits under United States federal income tax principles. Therefore, U.S. holders should expect that the entire amount of any distribution generally will be reported as dividend income. The amount of the dividend will generally be treated as foreign-source dividend income to U.S. holders. A non-corporate U.S. holder that meets certain eligibility requirements may qualify for a lower rate of U.S. federal income taxation on dividends paid if we are a "qualified foreign corporation" for U.S. federal income tax purposes. We generally will be treated as a qualified foreign corporation if we are not a passive foreign investment company (see discussion below), and (i) we are eligible for benefits under the United States-Israel income tax treaty or (ii) our ordinary shares are listed on an established securities market in the United States (which includes the NASDAQ Capital Market). We believe that we currently are treated as a qualified foreign corporation. However, no assurance can be given that a change in circumstances will not affect our treatment as a qualified foreign corporation for U.S. federal income tax purposes in any taxable year. In addition, a non-corporate U.S. holder will not be eligible for a reduced U.S. federal income tax rate with respect to dividend distributions on ordinary shares if (a) such U.S. holder has not held the ordinary shares for at least 61 days during the 121-day period starting on the date which is 60 days before, and ending 60 days after the ex-dividend date, (b) to the extent the U.S. holder is under an obligation to make related payments on substantially similar or
158
related property, or (c) with respect to any portion of a dividend that is taken into account by the U.S. holder as investment income under Section 163(d)(4)(B) of the Code. Any days during which the U.S. holder has diminished its risk of loss with respect to ordinary shares (for example, by holding an option to sell the ordinary shares) are not counted towards meeting the 61-day holding period. Non-corporate U.S. holders should consult their own tax advisors concerning whether dividends received by them qualify for the reduced rate of tax.
Non-corporate U.S. holders will not be eligible for reduced rates of taxation on any dividends received from us if we are a PFIC in the taxable year in which such dividends are paid or in the preceding taxable year. Corporate U.S. holders will not be allowed a deduction for dividends received from us.
The amount of a distribution with respect to our ordinary shares equals the amount of cash and the fair market value of any property distributed plus the amount of any Israeli taxes withheld therefrom. The amount of any cash distributions paid in NIS equals the U.S. dollar value of the NIS on the date of distribution based upon the exchange rate in effect on such date, regardless of whether the NIS are converted into U.S. dollars at that time, and U.S. holders who include such distribution in income on such date will have a tax basis in such NIS for U.S. federal income tax purposes equal to such U.S. dollar value. If the dividend is converted to U.S. dollars on the date of receipt, a U.S. holder generally will not recognize a foreign currency gain or loss. However, if the U.S. holder converts the NIS into U.S. dollars on a later date, the U.S. holder must include, in computing its income, any gain or loss resulting from any exchange rate fluctuations. The gain or loss will be equal to the difference between (i) the U.S. dollar value of the amount included in income when the dividend was received and (ii) the amount received on the conversion of the NIS into U.S. dollars. Such gain or loss will generally be ordinary income or loss and United States source income for U.S. foreign tax credit purposes. U.S. holders should consult their own tax advisors regarding the tax consequences to them if we pay dividends in NIS or any other non-U.S. currency.
Subject to certain significant conditions and limitations, including potential limitations under the U.S.-Israel Tax Treaty, U.S. holders may be entitled to a credit against their U.S. federal income tax liability or a deduction against U.S. federal taxable income in an amount equal to the Israeli tax withheld on distributions on our ordinary shares. U.S. holders should consult their own tax advisors to determine whether and to what extent they would be entitled to such credit. Distributions paid on our ordinary shares will generally be treated as passive income that is foreign source for U.S. foreign tax credit purposes, which may be relevant in calculating a U.S. holder's foreign tax credit limitation.
Disposition of ADSs
Subject to the discussion under the heading "Passive Foreign Investment Company Consequences," upon the sale, exchange or other disposition of ADSs, a U.S. holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized on the disposition and such U.S. holder's adjusted tax basis in the ADSs. The adjusted tax basis in an ADS generally will be equal to the cost of such ADS. The capital gain or loss realized on the sale, exchange, or other disposition of ADSs will be long-term capital gain or loss if the U.S. holder held the ADSs for more than one year as of the time of disposition. Preferential tax rates for long-term capital gain will generally apply to non-corporate U.S. holders. Any gain or loss realized by a U.S. holder on the sale, exchange, or other disposition of ADSs generally will be treated as from sources within the United States for U.S. foreign tax credit purposes, except for certain losses which will be treated as foreign source to the extent certain dividends were received (or certain inclusion amounts were taken into account) by the U.S. holder within the 24-month period preceding the date on which the U.S. holder recognized the loss. The deductibility of capital losses for U.S. federal income tax purposes is subject to limitations.
159
Disclosure of Reportable Transactions
If a U.S. holder sells or disposes of the ADSs at a loss or otherwise incurs certain losses that meet certain thresholds, such U.S. holder may be required to file a disclosure statement with the Internal Revenue Service, or the IRS. Failure to comply with these and other reporting requirements could result in the imposition of significant penalties.
Passive Foreign Investment Company Consequences
Generally, a non-U.S. corporation will be a PFIC for U.S. federal income tax purposes in any taxable year in which either (1) 75% or more of its gross income for such year consists of certain types of "passive" income or (2) 50% or more of the average fair market value of its assets during such year (based on quarterly valuations) produce or are held for the production of passive income. Passive income for this purpose generally includes dividends, interest, rents, royalties, annuities, income from certain commodities transactions and from notional principal contracts, and the excess of gains over losses from the disposition of assets that produce passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in a public offering. Assets that produce or are held for the production of passive income include cash, even if held as working capital or raised in a public offering, marketable securities and other assets that may produce passive income. In determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
A foreign corporation's PFIC status is an annual determination that is based on tests that are factual in nature, and our PFIC status for any year will depend on the composition of our income, fair market value of our assets, and our activities for such year. Because we had no revenue-producing operations to date, we believe that we were a PFIC for our 2014 taxable year. Unless and until we generate sufficient revenue from sales and other non-passive sources and otherwise satisfy the asset test above, we expect to be treated as a PFIC. Because the PFIC determination is highly fact intensive, there can be no assurance that we will not be a PFIC in 2015 or any other year. Even if we determine that we are not a PFIC after the close of a taxable year, there can be no assurance that the IRS or a court will agree with our conclusion.
If we were a PFIC for any taxable year during which a U.S. holder held ADSs, then unless an election has been made by a U.S. holder to be taxed under one of the alternative regimes discussed below, gain recognized by a U.S. holder on a sale or other disposition (including certain pledges) of our ADSs would be allocated ratably over the U.S. holder's holding period for the ADSs. The amounts allocated to the taxable year of the sale or other disposition and to any year before we became a PFIC would be taxed as ordinary income. The amount allocated to each other taxable year would be subject to tax at the highest rate in effect for individuals or corporations, as appropriate, for that taxable year, and an interest charge would be imposed on the amount allocated to that taxable year. Similar rules would apply to any distribution in respect of our ADSs in excess of 125% of the average of the annual distributions received by a U.S. holder during the preceding three years or such U.S. holder's holding period, whichever is shorter. In addition, non-corporate U.S. holders will not be eligible for reduced rates of taxation on any dividends received from us if we are a PFIC in the taxable year in which such dividends are paid or in the preceding taxable year.
If we are a PFIC for any taxable year during which you hold our ADSs and our non-United States subsidiary is also a PFIC, a U.S. holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC for purposes of the application of these rules. U.S. holders are urged to consult their tax advisors about the application of the PFIC rules to our subsidiary.
If we are treated as a PFIC for any taxable year during the holding period of a non-electing U.S. holder (i.e., a U.S. holder that does not elect to be taxed under one of the alternative regimes
160
discussed below), we will continue to be treated as a PFIC for all succeeding years during which such non-electing U.S. holder is treated as a direct or indirect holder even if we are not a PFIC for such years. A U.S. holder is encouraged to consult its tax advisor with respect to any available elections that may be applicable in such a situation, including the "deemed sale" election of Section 1298(b)(1) of the Code.
Notwithstanding the default PFIC rules described in the preceding paragraphs, certain elections may be available that would result in alternative tax consequences; i.e., the "qualified electing fund" or "QEF" election and the "mark to market" election. If a U.S. holder makes a timely and valid mark-to-market election, the U.S. holder generally will recognize as ordinary income any excess of the fair market value of the ADSs at the end of each taxable year over their adjusted tax basis, and will recognize an ordinary loss in respect of any excess of the adjusted tax basis of the ADSs over their fair market value at the end of the taxable year (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). The U.S. holder's tax basis in the ADSs will be adjusted to reflect the income or loss resulting from the mark-to-market election. Any gain recognized on the sale or other disposition of ADSs in a year when we are a PFIC will be treated as ordinary income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included as a result of the mark-to-market election and any loss in excess of such amount will be treated as capital loss). The mark-to-market election is available only if we are a PFIC and our ADSs are "regularly traded" on a "qualified exchange" within the meaning of applicable U.S. Treasury regulations. Our ADSs will be treated as "regularly traded" in any calendar year in which more than a de minimis quantity of the ADSs are traded on a qualified exchange on at least 15 days during each calendar quarter. Although the IRS has not published any authority identifying specific exchanges that may constitute "qualified exchanges," Treasury Regulations provide that a qualified exchange is (a) a U.S. securities exchange that is registered with the Securities and Exchange Commission, (b) the U.S. market system established pursuant to section 11A of the Securities and Exchange Act of 1934, or (c) a non-U.S. securities exchange that is regulated or supervised by a governmental authority of the country in which the market is located, provided that (i) such non-U.S. exchange has trading volume, listing, financial disclosure, surveillance, and other requirements designed to prevent fraudulent and manipulative acts and practices, to remove impediments to and perfect the mechanism of a free and open, fair and orderly, market, and to protect investors, and the laws of the country in which such non-U.S. exchange is located and the rules of such non-U.S. exchange ensure that such requirements are actually enforced; and (ii) the rules of such non-U.S. exchange effectively promote active trading of listed shares. No assurance can be given that the ADSs will meet the requirements to be treated as "regularly traded" for purposes of the mark-to-market election. The NASDAQ Capital Market is a qualified exchange for this purpose and, consequently, if the ADSs are regularly traded, the mark-to-market election will be available to a U.S. holder. Our ordinary shares currently trade on the Tel Aviv Stock Exchange, which must meet the requirements described above in order to allow for a mark-to-market election with respect to our ordinary shares. A mark-to-market election will not apply to ADSs held by a U.S. holder for any taxable year during which we are not a PFIC, but will remain in effect with respect to any subsequent taxable year in which we become a PFIC unless the ADSs are no longer regularly traded on a qualified exchange or the IRS consents to the revocation of the election. Such election will not apply to any PFIC subsidiary that we own. Each U.S. holder is encouraged to consult its own tax advisor with respect to the availability and tax consequences of a mark-to-market election with respect to our ADSs.
Another way in which certain of the adverse consequences of PFIC status can be mitigated is for a U.S. holder to make a QEF election. Generally, a shareholder making the QEF election is required for each taxable year to include in income a pro rata share of the ordinary earnings and net capital gain of the QEF, subject to a separate election to defer payment of taxes, which deferral is subject to an interest charge. An election to treat us as a QEF will not be available if we do not provide the information necessary to make such an election. We are not obligated and do not currently intend to
161
provide the information necessary to make a QEF election and thus it is not expected that a QEF election will be available for U.S. holders of our ADSs if we were a PFIC in any prior year, the current year or any future year.
U.S. holders should consult their tax advisors to determine under what circumstances these elections would be available and, if available, what the consequences of the alternative treatments would be in their particular circumstances.
If a U.S. holder holds ADSs in any year in which we are treated as a PFIC, the U.S. holder will be required to file Internal Revenue Service Form 8621 and may be subject to certain other information reporting requirements.
The U.S. federal income tax rules relating to PFICs are complex. Prospective U.S. holders are urged to consult their own tax advisors with respect to the consequences to them of an investment in a PFIC, any elections available with respect to our ADSs or ordinary shares and the IRS information reporting obligations with respect to the purchase, ownership and disposition of our ADSs or ordinary shares in the event we are determined to be a PFIC.
Medicare Tax on Investment Income
In addition to the income taxes described above, U.S. holders that are individuals, estates or trusts and whose income exceeds certain thresholds will be subject to a 3.8% tax on all or a portion of their "net investment income," which generally results from dividends and dispositions of ADSs. U.S. holders should consult their tax advisors with respect to the applicability of the 3.8% Medicare tax to their income and gains, if any, resulting from their investment in our ADSs.
Information Reporting and Backup Withholding
A U.S. holder may be subject to backup withholding and information reporting requirements with respect to cash distributions and proceeds from a disposition of ADSs or ordinary shares. In general, backup withholding will apply only if a U.S. holder fails to comply with certain identification procedures. Information reporting and backup withholding will not apply with respect to payments made to certain exempt recipients, such as corporations and tax-exempt organizations. Backup withholding is not an additional tax and may be claimed as a credit against the U.S. federal income tax liability of a U.S. holder, provided that the required information is furnished to the Internal Revenue Service.
Tax Reporting
Certain U.S. holders will be required to file an IRS Form 926 (Return by a U.S. Transferor of Property to a Foreign Corporation) to report a transfer of cash or other property to us. Substantial penalties may be imposed on a U.S. holder that fails to comply with this reporting requirement. Each U.S. holder is urged to consult with its own tax advisor regarding this reporting obligation.
Foreign Asset Reporting
Certain U.S. holders who are individuals may be required to report information relating to an interest in our ADSs or ordinary shares, subject to certain exceptions. For example, individuals that own "specified foreign financial assets" with an aggregate value in excess of $50,000 are generally required to file Form 8938 with respect to such assets with their tax returns. "Specified foreign financial assets" include any financial accounts maintained by foreign financial institutions, as well as any of the following, but only if they are not held in accounts maintained by financial institutions: (i) stocks and securities issued by non-United States persons; (ii) financial instruments and contracts held for investment that have non-United States issuers or counterparties; and (iii) interests in foreign entities.
162
Certain domestic entities that are U.S. holders may also be required to file Form 8938 in the near future. In addition, a U.S. holder should consider the possible obligation to file FinCEN Form 114, Report of Foreign Bank and Financial Accounts, as a result of holding ADSs or ordinary shares. U.S. holders are urged to consult their tax advisors regarding the application of these and other reporting requirements that may apply to their ownership of ADSs or ordinary shares.
Non-U.S. Holders of Ordinary Shares
Except as provided below, a non-U.S. holder of ordinary shares or ADSs generally will not be subject to U.S. income or withholding tax on the payment of dividends on and the proceeds from the disposition of ADSs or ordinary shares.
A non-U.S. holder may be subject to U.S. federal income tax on dividends received on ADSs or ordinary shares or upon the receipt of income from the disposition of ADSs or ordinary shares if (1) such income is effectively connected with the conduct by the non-U.S. holder of a trade or business in the United States or, in the case of a resident of a country which has an applicable income tax treaty with the United States, such item is attributable to a permanent establishment or a fixed place of business of the non-U.S. holder in the United States; (2) with respect to a U.S. holder that is an individual, the non-U.S. holder is an individual who is present in the United States for 183 days or more in the taxable year of the sale and certain other conditions are met; or (3) the non-U.S. holder is subject to tax pursuant to the provisions of the U.S. tax laws applicable to U.S. expatriates.
Payments to non-U.S. holders of distributions on, or proceeds from the disposition of, ADSs or ordinary shares are generally exempt from information reporting and backup withholding. However, a non-U.S. holder may be required, under certain circumstances, to establish that exemption by providing certification of non-U.S. status on an appropriate IRS Form W-8.
Backup withholding is not an additional tax.
The amount of any backup withholding from a payment to a non-U.S. holder may be claimed as a credit against such holder's U.S. federal income tax liability, provided that the required information is furnished to the Internal Revenue Service.
THE DISCUSSION ABOVE IS A GENERAL SUMMARY AND IS NOT INTENDED TO CONSTITUTE A COMPLETE ANALYSIS OF ALL TAX CONSEQUENCES RELATING TO THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR ADSs OR ORDINARY SHARES. IT DOES NOT COVER ALL TAX MATTERS THAT MAY BE OF IMPORTANCE TO A PROSPECTIVE INVESTOR. EACH PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT RELATING TO THE PURCHASE, OWNERSHIP, AND DISPOSITION OF ADSs OR ORDINARY SHARES IN LIGHT OF THE INVESTOR'S OWN CIRCUMSTANCES.
163
Ladenburg Thalmann & Co. Inc. is acting as book-running manager of the offering and the representative of the underwriters. Under the terms and subject to the terms and conditions set forth in an underwriting agreement, the underwriters named below have agreed, severally and not jointly, to purchase, and we have agreed to sell to them, the number of ADSs set forth opposite their name below.
Underwriter
|
Number of ADSs | |
|---|---|---|
Ladenburg Thalmann & Co. Inc. |
||
Brean Capital, LLC |
||
| | | |
Total |
||
| | | |
| | | |
| | | |
Subject to the terms and conditions set forth in the underwriting agreement, the underwriters have agreed, severally and not jointly, to purchase all of the ADSs sold under the underwriting agreement if any of these ADSs are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the non-defaulting underwriters may be increased or the underwriting agreement may be terminated.
The underwriters are offering the ADSs, subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel, including the validity of the ADSs, and other conditions contained in the underwriting agreement, such as the receipt by the underwriters of officers' certificates and legal opinions. The underwriters reserve the right to withdraw, cancel, or modify offers to the public and to reject orders in whole or in part.
Discounts and Commissions
The underwriters propose initially to offer the ADSs to the public at the public offering price set forth on the cover page of this prospectus and to dealers at that price less a concession not in excess of $ per ADS. After the initial offering of the ADSs, the public offering price and other selling terms may be changed by the representative.
The following table shows the underwriting discounts and commissions payable to the underwriters by us in connection with this offering (assuming both the exercise and non-exercise of the over-allotment option to purchase additional ADSs we have granted to the underwriters):
| |
Per ADS | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Without Over-allotment |
With Over-allotment |
Without Over-allotment |
With Over-allotment |
|||||||||
Public offering price |
$ | $ | |||||||||||
Underwriting discounts and commissions paid by us |
$ | $ | |||||||||||
We have also agreed to reimburse Ladenburg Thalmann & Co. Inc. for legal expenses relating to this offering in an amount of up to $ .
The expenses of the offering, not including the underwriting discounts and commissions, are estimated at and are payable by us.
Option to Purchase Additional ADSs
We have granted the underwriters an option to purchase up to additional ADSs at the public offering price, less underwriting discounts and commissions. The underwriters may exercise this option for 30 days from the date of this prospectus solely to cover sales of ADSs by the underwriters in
164
excess of the total number of ADSs set forth in the table above. If any ADSs are purchased pursuant to this over-allotment option, the underwriters will purchase the additional ADSs in approximately the same proportions as shown in the table above. If any of these additional ADSs are purchased, the underwriters will offer the additional ADSs on the same terms as those on which the ADSs are being offered. We will pay the expenses associated with the exercise of the over-allotment option.
Lock-Up Agreements
We, all of our directors and executive officers, and holders of 5% and more of our outstanding securities have agreed that, for a period of 180 days after the date of this prospectus, or the lock-up period, subject to certain limited exceptions, we and they will not directly or indirectly, without the prior written consent of the representatives of the underwriters, (1) offer for sale, sell, pledge, or otherwise dispose of (or enter into any transaction or device that is designed to, or could be expected to, result in the disposition by any person at any time in the future of) any ADSs or ordinary shares (including, without limitation, ADSs or ordinary shares that may be deemed to be beneficially owned by us or them in accordance with the rules and regulations of the SEC and ADSs or ordinary shares that may be issued upon exercise of any options or warrants) or securities convertible into or exercisable or exchangeable for ADSs or ordinary shares, (2) enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of ADSs or ordinary shares, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of ADSs, ordinary shares, or other securities, in cash or otherwise, (3) make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any ADSs, ordinary shares or securities convertible into or exercisable or exchangeable for ADSs, ordinary shares or any of our other securities, or (4) publicly disclose the intention to do any of the foregoing.
The representative of the underwriters may release the ADSs, ordinary shares and other securities subject to the lock-up agreements described above in whole or in part at any time. When determining whether or not to release ADSs, ordinary shares and other securities from lock-up agreements, the representative of the underwriters will consider, among other factors, the holder's reasons for requesting the release, the number of ADSs, ordinary shares and other securities for which the release is being requested and market conditions at the time.
At least three business days before the effectiveness of any release or waiver of any of the restrictions described above with respect to an officer or director of the Company, the representative of the underwriters will notify us of the impending release or waiver and we have agreed to announce the impending release or waiver by press release through a major news service at least two business days before the effective date of the release or waiver.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make for these liabilities.
Stabilization, Short Positions and Penalty Bids
In order to facilitate the offering of our ADSs, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of our ADSs. In connection with the offering, the underwriters may purchase and sell our ADSs in the open market. These transactions may include short sales, purchases on the open market to cover positions created by short sales and stabilizing transactions. Short sales involve the sale by the underwriters of a greater number of ADSs than they are required to purchase in the offering. "Covered" short sales are sales made in an amount not
165
greater than the underwriters' option to purchase additional ADSs in the offering. The underwriters may close out any covered short position by either exercising their over-allotment option or purchasing ADSs in the open market. In determining the source of ADSs to close out the covered short position, the underwriters will consider, among other things, the price of ADSs available for purchase in the open market as compared to the price at which they may purchase ADSs through the over-allotment option. "Naked" short sales are sales in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing ADSs in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of our ADSs in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of ADSs made by the underwriters in the open market prior to the completion of the offering.
Similar to other purchase transactions, the underwriters' purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our ADSs or preventing or retarding a decline in the market price of our ADSs. As result, the price of our ADSs may be higher than the price that might otherwise exist in the open market.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act, they may also engage in other activities that stabilize, maintain or otherwise affect the price of our ADSs, including the imposition of penalty bids. This means that if the representative of the underwriters purchases ADSs in the open market in stabilizing transactions or to cover short sales, the representative can require the underwriters that sold those ADSs as part of this offering to repay the underwriting discount received by them.
The underwriters make no representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our ADSs. In addition, neither we nor the underwriters make any representation that the underwriters will engage in these transactions or that these transactions, once commenced, will not be discontinued without notice.
Electronic Offer, Sale and Distribution
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of ADSs to underwriters for sale to their online brokerage account holders. Internet distributions will be allocated by the representative to the underwriters and selling group members that may make Internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters' websites and any information contained in any other website maintained by the underwriters is not part of this prospectus or the registration statement of which this prospectus forms a part.
Listing on The NASDAQ Capital Market
We intend to apply to list our ADSs on the NASDAQ Capital Market, under the symbol " ". Our ordinary shares currently trade on the Tel Aviv Stock Exchange, or TASE, under the symbol "CLPT," and our ADSs currently quoted on the OTCQX marketplace, or OTCQX, under the symbol "CQPTY." Assuming that our ADSs are listed for trading on the NASDAQ Capital Market, the quoting of our ADSs on OTCQX will be discontinued prior to the completion of this offering.
Electronic Offer, Sale and Distribution
A prospectus in electronic format may be made available on the websites maintained by one or more underwriters or selling group members, if any, participating in the offering. The underwriters may agree to allocate a number of ADSs to underwriters for sale to their online brokerage account holders. Internet distributions will be allocated by the representative to the underwriters and selling group
166
members that may make Internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters' websites and any information contained in any other website maintained by the underwriters is not part of this prospectus or the registration statement of which this prospectus forms a part.
Other Relationships
From time to time, certain of the underwriters and their affiliates have provided, and may provide in the future, certain financial advisory, investment banking and other services in the ordinary course of their business, for which they have received and may continue to receive customary fees and commissions. In addition, from time to time, the underwriters and their affiliates may effect transactions for their own account or the accounts of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future.
Notice to Non-U.S. Investors
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer for the offeree under this prospectus.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People's Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region, and Taiwan). The securities may not be offered or sold directly or indirectly in the People's Republic of China to legal or natural persons other than directly to "qualified domestic institutional investors."
167
European Economic Area—Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC ("Prospectus Directive"), as implemented in Member States of the European Economic Area (each, a "Relevant Member State"), from the requirement to produce a prospectus for offers of securities.
An offer to the public of securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
(a) to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
(b) to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements); and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statements);
(c) to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to obtaining the prior consent of the Company or any underwriter for any such offer; or
(d) in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Directive.
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers ("AMF"). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D.744-1, D.754-1, and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d'investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1, and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1, and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
168
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the "Prospectus Regulations"). The securities have not been offered or sold, and will not be offered, sold, or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
This document does not constitute a public offering or selling or a solicitation of an offer to sell any kind of securities under the Israeli Securities Law. This document does not constitute a prospectus under the Israeli Securities Law and has not been filed with or approved by the Israel Securities Authority. Any public offering in Israel requires a pre-approved permit by the Israel Securities Authority or an exemption thereof. In Israel, this document is being distributed only to, and is directed only at the types of, investors listed in the first addendum, or the Addendum, to the Israeli Securities Law, consisting primarily of a fund for joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriters purchasing for their own account, venture capital funds, entities with equity in excess of NIS 50.0 million, and "qualified individuals", each as defined in the Addendum (as it may be amended from time to time), collectively referred to as qualified investors. Qualified investors may be required to submit written confirmation that they fall within the scope of the Addendum.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Società e la Borsa, "CONSOB" pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 ("Decree No. 58"), other than:
- •
- to Italian qualified investors, as defined in Article 100 of Decree no. 58 by reference to Article 34-ter of
CONSOB Regulation no. 11971 of 14 May 1999 ("Regulation no. 1197l") as amended ("Qualified Investors"); and
- •
- in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended.
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
- •
- made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with
Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and
- •
- in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws.
169
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the "FIEL") pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissã do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are "qualified investors" (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are "qualified investors" (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange ("SIX") or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material
170
relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority.
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the UAE or any other governmental authority in the UAE, nor has the Company received authorization or licensing from the Central Bank of the UAE or any other governmental authority in the UAE to market or sell the securities within the UAE. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the UAE by the Company.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended ("FSMA")) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to "qualified investors" (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA.
This document should not be distributed, published, or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 ("FPO"), (ii) who fall within the categories of persons referred to in Article 49 (2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO, or (iii) to whom it may otherwise be lawfully communicated (together "relevant persons"). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
171
EXPENSES RELATED TO THIS OFFERING
We estimate that the total expenses of this offering payable by us, excluding the underwriting discounts and commissions, will be approximately $[ ], as follows:
| SEC registration fee | * | |||
| FINRA filing fee | * | |||
| NASDAQ listing fee | * | |||
| Printing and engraving expenses | * | |||
| Transfer agent fees and expenses | * | |||
| Legal fees and expenses | * | |||
| Data room and diligence expenses | * | |||
| Accounting fees and expenses | * | |||
| Miscellaneous | * | |||
| Total | * |
- *
- To be completed by amendment
The validity of our ordinary shares and certain matters governed by Israeli law will be passed on for us by Horn & Co. Law Offices, Tel Aviv, Israel, our Israeli counsel. The validity of the ADSs and certain other matters governed by U.S. federal and New York state law will be passed on for us by McDermott Will & Emery LLP, New York, New York, our U.S. counsel. Certain legal matters in connection with this offering will be passed on for the underwriters by Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., New York, New York.
ENFORCEABILITY OF CIVIL LIABILITIES
We are incorporated under the laws of the State of Israel. Service of process upon us and upon our directors and officers and the Israeli experts named in this registration statement, a substantial majority of whom reside outside of the United States, may be difficult to obtain within the United States. Furthermore, because substantially all of our assets and a substantial majority of our directors and officers are located outside of the United States, any judgment obtained in the United States against us or any of our directors and officers may not be collectible within the United States.
We have been informed by our Israeli legal counsel, Horn & Co. Law Offices, that it may be difficult to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on a violation of U.S. securities laws because Israel is not the most appropriate forum to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Israeli law.
Subject to specified time limitations and legal procedures, Israeli courts may enforce a United States judgment in a civil matter which, subject to certain exceptions, is non-appealable, including judgments based upon the civil liability provisions of the Securities Act and the Exchange Act and including a monetary or compensatory judgment in a non-civil matter, provided that among other things:
- •
- the judgment is obtained after due process before a court of competent jurisdiction, according to the laws of the state in which the judgment is given and the rules of private international law currently prevailing in Israel;
172
- •
- the judgment is final and is not subject to any right of appeal;
- •
- the prevailing law of the foreign state in which the judgment was rendered allows for the enforcement of judgments of Israeli courts;
- •
- adequate service of process has been effected and the defendant has had a reasonable opportunity to be heard and to present his or her
evidence;
- •
- the liabilities under the judgment are enforceable according to the laws of the State of Israel and the judgment and the enforcement
of the civil liabilities set forth in the judgment is not contrary to the law or public policy in Israel nor likely to impair the security or sovereignty of Israel;
- •
- the judgment was not obtained by fraud and does not conflict with any other valid judgments in the same matter between the same
parties;
- •
- an action between the same parties in the same matter is not pending in any Israeli court at the time the lawsuit is instituted in the
foreign court; and
- •
- the judgment is enforceable according to the law of the foreign state in which the relief was granted.
If a foreign judgment is enforced by an Israeli court, it generally will be payable in Israeli currency, which can then be converted into non-Israeli currency and transferred out of Israel. The usual practice in an action before an Israeli court to recover an amount in a non-Israeli currency is for the Israeli court to issue a judgment for the equivalent amount in Israeli currency at the rate of exchange in force on the date of the judgment, but the judgment debtor may make payment in foreign currency. Pending collection, the amount of the judgment of an Israeli court stated in Israeli currency ordinarily will be linked to the Israeli consumer price index plus interest at the annual statutory rate set by Israeli regulations prevailing at the time. Judgment creditors must bear the risk of unfavorable exchange rates.
The financial statements as of December 31, 2014 and 2013, and for each of the two years in the period ended December 31, 2014, included in this prospectus have been so included in reliance on the report (which contains an explanatory paragraph relating to our ability to continue as a going concern, as described in Note 1a to the financial statements) of Kesselman & Kesselman, Certified Public Accountants (Isr.), a member firm of PricewaterhouseCoopers International Limited, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting. The offices of Kesselman & Kesselman are located at Trade Tower, 25 Hamered Street, Tel-Aviv 68125, Israel.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form F-1 under the Securities Act relating to this offering of our ADSs. This prospectus does not contain all of the information contained in the registration statement. The rules and regulations of the SEC allow us to omit certain information from this prospectus that is included in the registration statement. Statements made in this prospectus concerning the contents of any contract, agreement, or other document are summaries of all material information about the documents summarized, but are not complete descriptions of all terms of these documents. If we filed any of these documents as an exhibit to the registration statement, you may read the document itself for a complete description of its terms.
You may read and copy the registration statement, including the related exhibits and schedules, and any document we file with the SEC without charge at the SEC's public reference room at 100 F Street, N.E., Room 1580, Washington, DC 20549. You may also obtain copies of the documents
173
at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Room 1580, Washington, DC 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference room. The SEC also maintains an Internet website that contains reports and other information regarding issuers that file electronically with the SEC. Our filings with the SEC are also available to the public through the SEC's website at http://www.sec.gov.
We are subject to the information reporting requirements of the Exchange Act that are applicable to foreign private issuers, and under those requirements are filing reports with the SEC. Those other reports or other information may be inspected without charge at the locations described above. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors, and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly, and current reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act. However, we will file with the SEC, within 120 days after the end of each fiscal year, or such applicable time as required by the SEC, an annual report on Form 20-F containing financial statements audited by an independent registered public accounting firm, and will submit to the SEC, on Form 6-K, unaudited quarterly financial information. As long as we are traded on the TASE, and are a public company pursuant to the Companies Law, we are considered a "Reporting Corporation", under the Israeli Securities Law and until decided otherwise by our shareholders or until we are exempt from such duties by the Israeli Securities Authority, we are required to file annual, quarterly, and immediate reports and financial statements with the Israeli Securities Authority and TASE as frequently or as promptly as Israeli public companies whose securities are registered under the Israeli Securities Law are required to.
We maintain a corporate website at www.collplant.com. Information contained on, or that can be accessed through, our website does not constitute a part of this prospectus. We have included our website address in this prospectus solely as an inactive textual reference. We will post on our website any materials required to be posted on such website under corporate or securities regulations, including posting any XBRL interactive financial data required to be filed with the SEC or any other regulatory authority, and any notices of general meetings of our shareholders.
174
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
COLLPLANT HOLDINGS LTD.
AS OF DECEMBER 31, 2014
F-1

Report of Independent Registered Public Accounting Firm
To the Shareholders of CollPlant Holdings Ltd.
We have audited the accompanying consolidated statements of financial position of CollPlant Holdings Ltd. and its subsidiary ("the Company") as of December 31, 2014 and 2013 and the consolidated statements of comprehensive loss, changes in equity and cash flows for the years then ended. These financial statements are the responsibility of the Company's board of directors and management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by the Company's board of directors and management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, these financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2014 and 2013 and the results of the Company's operations, changes in equity and cash flows for the years then ended, in accordance with International Financial Reporting Standards (IFRS), as issued by the International Accounting Standards Board ("IASB").
As discussed in Note 1a to the consolidated financial statements, the Company has an accumulated deficit as of December 31, 2014 and presently does not have sufficient cash and other resources to meet its plans in the following twelve months. These factors raise substantial doubt as to the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 1a. The accompanying financial statements have been prepared assuming that the Company will continue as a going concern and do not include any adjustments that might result from the outcome of this uncertainty.
| Tel-Aviv, Israel | Kesselman & Kesselman | |
| May 21, 2015 | Certified Public Accountants (lsr.) A member firm of PricewaterhouseCoopers International Limited |
Kesselman & Kesselman, Trade Tower, 25 Hamered Street, Tel-Aviv 6812508, Israel,
P.O Box 50005 Tel-Aviv 6150001 Telephone: +972 -3- 7954555, Fax:+972 -3- 7954556, www.pwc.com/il
F-2
CollPlant Holdings Ltd.
Consolidated Statements of Financial Position
| |
|
|
|
Convenience translation into USD (note 1b) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
December 31 | ||||||||||
| |
|
December 31, 2014 |
||||||||||
| |
Note | 2013 | 2014 | |||||||||
| |
|
NIS in thousands |
In thousands |
|||||||||
Assets |
||||||||||||
Current assets: |
||||||||||||
Cash and cash equivalents |
5 | 23,777 | 11,062 | 2,845 | ||||||||
Receivables |
6 | 1,728 | 1,548 | 398 | ||||||||
| | | | | | | | | | | | | |
|
25,505 | 12,610 | 3,243 | |||||||||
| | | | | | | | | | | | | |
Non-current assets: |
||||||||||||
Restricted deposit |
12A(1)(a) | 503 | 564 | 145 | ||||||||
Long-term receivables |
67 | 52 | 13 | |||||||||
Property and equipment |
7 | 2,462 | 2,007 | 516 | ||||||||
Intangible assets |
8 | 1,736 | 1,725 | 444 | ||||||||
| | | | | | | | | | | | | |
|
4,768 | 4,348 | 1,118 | |||||||||
| | | | | | | | | | | | | |
Total assets |
30,273 | 16,958 | 4,361 | |||||||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Liabilities and equity |
||||||||||||
Current liabilities: |
||||||||||||
Accounts payable |
10 | |||||||||||
Trade payables |
1,856 | 1,642 | 422 | |||||||||
Other |
1,333 | 1,005 | 259 | |||||||||
| | | | | | | | | | | | | |
|
3,189 | 2,647 | 681 | |||||||||
| | | | | | | | | | | | | |
Commitments and contingent liabilities |
12 | |||||||||||
| | | | | | | | | | | | | |
Total liabilities |
3,189 | 2,647 | 681 | |||||||||
| | | | | | | | | | | | | |
Equity: |
13 | |||||||||||
Ordinary shares |
2,369 | 2,414 | 621 | |||||||||
Additional paid in capital |
130,918 | 130,918 | 33,664 | |||||||||
Accumulated deficit |
(106,203 | ) | (119,021 | ) | (30,605 | ) | ||||||
| | | | | | | | | | | | | |
Total equity |
27,084 | 14,311 | 3,680 | |||||||||
| | | | | | | | | | | | | |
Total liabilities and equity |
30,273 | 16,958 | 4,361 | |||||||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
The accompanying notes are an integral part of the financial statements.
F-3
CollPlant Holdings Ltd.
Consolidated Statements of Comprehensive Loss
| |
|
Year ended December 31 | Convenience translation into USD (note 1b) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Note | 2013 | 2014 | 2014 | ||||||||
| |
|
NIS in thousands |
In thousands |
|||||||||
Research and development expenses, net: |
14 | |||||||||||
Research and development expenses |
16,151 | 14,879 | 3,826 | |||||||||
Participation in research and development expenses |
(3,717 | ) | (5,145 | ) | (1,323 | ) | ||||||
| | | | | | | | | | | | | |
Research and development expenses, net |
12,434 | 9,734 | 2,503 | |||||||||
General, administrative and marketing expenses |
15 | 3,747 | 3,906 | 1,004 | ||||||||
| | | | | | | | | | | | | |
Operating loss |
16,181 | 13,640 | 3,507 | |||||||||
| | | | | | | | | | | | | |
Financial income |
16 | 25 | 642 | 165 | ||||||||
Financial expenses |
16 | 314 | 25 | 6 | ||||||||
| | | | | | | | | | | | | |
Financial expenses (income), net |
289 | (617 | ) | (159 | ) | |||||||
| | | | | | | | | | | | | |
Loss and comprehensive loss for the year |
16,470 | 13,023 | 3,348 | |||||||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Basic and diluted loss per ordinary share (NIS/USD) |
17 | 0.11 | 0.05 | 0.01 | ||||||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
Weighted average ordinary shares outstanding |
155,590,908 | 241,280,958 | ||||||||||
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
| | | | | | | | | | | | | |
The accompanying notes are an integral part of the financial statements.
F-4
CollPlant Holdings Ltd.
Consolidated Statements of Changes in Equity
| |
Note | Ordinary shares |
Additional paid in capital |
Accumulated deficit |
Total equity |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
NIS in thousands |
|||||||||||||
Balance as at January 1, 2013 |
1,517 | 104,373 | (90,195 | ) | 15,695 | ||||||||||
Movement in 2013: |
|
||||||||||||||
Comprehensive loss for the year |
(16,470 | ) | (16,470 | ) | |||||||||||
Share-based compensation to employees and consultants |
462 | 462 | |||||||||||||
Proceeds from issuing shares, less issuance expenses of NIS 668 thousand |
13(A)(4) | 169 | 7,871 | 8,040 | |||||||||||
Proceeds from issuance of shares and warrants, less issuance expenses of NIS 1,963 thousand |
13(A)(5) | 683 | 18,674 | 19,357 | |||||||||||
| | | | | | | | | | | | | | | | |
Balance as at December 31, 2013 |
2,369 | 130,918 | (106,203 | ) | 27,084 | ||||||||||
Movement in 2014: |
|
||||||||||||||
Comprehensive loss for the year |
(13,023 | ) | (13,023 | ) | |||||||||||
Share-based compensation to employees and consultants |
205 | 205 | |||||||||||||
Exercise of options into shares |
13B(5) | 45 | 45 | ||||||||||||
| | | | | | | | | | | | | | | | |
Balance as at December 31, 2014 |
2,414 | 130,918 | (119,021 | ) | 14,311 | ||||||||||
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| |
|
Convenience translation into USD (note 1b) in thousands |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balance as at December 31, 2013 |
609 | 33,664 | (27,310 | ) | 6,963 | ||||||||||
Movement in 2014: |
|
||||||||||||||
Comprehensive loss for the year |
(3,348 | ) | (3,348 | ) | |||||||||||
Share-based compensation to employees and consultants |
53 | 53 | |||||||||||||
Exercise of options into shares |
13B(5) | 12 | 12 | ||||||||||||
| | | | | | | | | | | | | | | | |
Balance as at December 31, 2014 |
621 | 33,664 | (30,605 | ) | 3,680 | ||||||||||
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
The accompanying notes are an integral part of the financial statements.
F-5
CollPlant Holdings Ltd.
Consolidated Statements of Cash Flows
| |
Year ended December 31 |
Convenience translation into USD (note 1b) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | 2014 | |||||||
| |
NIS in thousands |
In thousands |
||||||||
Cash flows from operating activities: |
||||||||||
Net cash used in operations (see appendix) |
(13,269 | ) | (12,993 | ) | (3,340 | ) | ||||
Interest received |
25 | 35 | 9 | |||||||
| | | | | | | | | | | |
Net cash used in operating activities |
(13,244 | ) | (12,958 | ) | (3,331 | ) | ||||
| | | | | | | | | | | |
Cash flows from investing activities: |
||||||||||
Purchase of property, plant and equipment |
(474 | ) | (336 | ) | (86 | ) | ||||
Change in restricted deposit |
77 | (61 | ) | (16 | ) | |||||
| | | | | | | | | | | |
Net cash used in investing activities |
(397 | ) | (397 | ) | (102 | ) | ||||
| | | | | | | | | | | |
Cash flows from financing activities: |
||||||||||
Proceeds from issue of shares and options, less issue expenses |
27,397 | |||||||||
Exercise of options into shares |
45 | 12 | ||||||||
| | | | | | | | | | | |
Net cash provided by financing activities |
27,397 | 45 | 12 | |||||||
| | | | | | | | | | | |
Increase (decrease) in cash and cash equivalents |
13,756 | (13,310 | ) | (3,421 | ) | |||||
Cash and cash equivalents at the beginning of the period |
10,308 | 23,777 | 6,113 | |||||||
Exchange differences on cash and cash equivalents |
(287 | ) | 595 | 153 | ||||||
| | | | | | | | | | | |
Cash and cash equivalents at the end of the period |
23,777 | 11,062 | 2,845 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
F-6
CollPlant Holdings Ltd.
Appendix to the Consolidated Statements of Cash Flows
| |
Year ended December 31 |
Convenience translation into USD (note 1b) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | 2014 | |||||||
| |
NIS in thousands |
In thousands |
||||||||
Appendix to the statement of cash flow: |
||||||||||
Cash flows from operating activities: |
||||||||||
Loss for the year |
(16,470 | ) | (13,023 | ) | (3,348 | ) | ||||
Adjustments for: |
||||||||||
Depreciation and amortization |
951 | 802 | 206 | |||||||
Share-based compensation to employees and service providers |
462 | 205 | 53 | |||||||
Exchange differences on cash and cash equivalents |
287 | (595 | ) | (153 | ) | |||||
Interest received |
(25 | ) | (35 | ) | (9 | ) | ||||
| | | | | | | | | | | |
|
(14,795 | ) | (12,646 | ) | (3,251 | ) | ||||
| | | | | | | | | | | |
Changes in operating asset and liability items: |
||||||||||
Decrease in other receivables |
1,301 | 180 | 46 | |||||||
Decrease in other long-term receivables |
15 |
15 |
4 |
|||||||
Increase (decrease) in trade payables |
173 | (214 | ) | (55 | ) | |||||
Increase (decrease) in other payables |
37 | (328 | ) | (84 | ) | |||||
| | | | | | | | | | | |
|
1,526 | (347 | ) | (89 | ) | |||||
| | | | | | | | | | | |
Net cash used in activities |
(13,269 | ) | (12,993 | ) | (3,340 | ) | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The accompanying notes are an integral part of the financial statements
F-7
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements
NOTE 1—GENERAL
A. Operations
CollPlant Holdings Ltd. is clinical-stage regenerative medicine company focused on developing and commercializing tissue repair products, initially for the orthobiologics and advanced wound care markets. CollPlant's products, are based on its proprietary plant-based technology, for the production of recombinant type I human collagen, or rhCollagen. The Company operates through CollPlant Ltd., a wholly-owned subsidiary (CollPlant Holdings Ltd. and CollPlant Ltd. will be referred to hereinafter as "the Company" or "CollPlant").
The address of the Company's registered office is 3 Sapir St., Science Park, Ness–Ziona, Israel.
The Company has not yet generated income from its operations and as of December 31, 2014, has accrued losses of approximately NIS 119 million. The Company plans to continue research and development, production and marketing in the coming year (focusing on orthopedics, soft and hard tissue repair and wound healing), supported by funding sources as the Company's cash balances, grants from government authorities and proceeds from strategic partners. Presently, the Company does not have sufficient cash resources to meet its plans in the twelve months following December 31, 2014.
The Company is taking steps to raise additional financing sources to allowing the continuation of operations. These steps include efforts towards (1) signing and implementing product-development and licensing agreements with strategic collaborators that include full financing of development costs and payments to the Company for a license to sell the Company's products in the future; and (2) raising funds from private, public and/or institutional investors in Israel and overseas. It is uncertain whether the Company will be able to raise additional funds as aforesaid.
These factors raise substantial doubt regarding the Company's ability to continue as a going concern. The financial statements do not include adjustments for assets and liabilities and their classification which may be required if the Company is unable to continue as a going concern. If the Company is unable to raise the necessary funds, the Company may need to to curtail or cease operations.
B. Convenience translation into U.S. dollars ("dollars", "USD" or "$")
For the convenience of the reader, the reported New Israeli Shekel (NIS) amounts as of December 31, 2014 and for the year then ended have been translated into dollars at the Bank of Israel's representative rate of exchange for December 31, 2014 ($1 = NIS 3.889). The dollar amounts presented in these financial statements should not be construed as representing amounts that are receivable or payable in dollars or convertible into dollars, unless otherwise indicated.
C. Approval of financial statements
These financial statements were approved by the board of directors on May 21, 2015.
NOTE 2—SIGNIFICANT ACCOUNTING POLICIES
A. Basis of presentation of the financial statements
The Company's financial statements as at December 31, 2014 and 2013 and for the years then ended were prepared in conformity with International Financial Reporting Standards ("IFRS"), as issued by the International Accounting Standard Board (IASB).
F-8
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 2—SIGNIFICANT ACCOUNTING POLICIES (Continued)
The significant accounting policies described below have been applied consistently to all the years presented, unless otherwise stated.
The consolidated financial statements have been prepared on the basis of historical cost.
The preparation of financial statements in conformity with IFRS requires the use of certain critical accounting estimates. It also requires management to exercise judgment when applying the Company's accounting policies. Note 3 provides disclosure of areas involving a considerable degree of judgment or complexity, or areas where assumptions and estimates have a material effect on the financial statements. Actual results may differ materially from the estimates and assumptions used by the Company's management.
B. Consolidated financial statements
A subsidiary is an entity over which the Company has control. The Company controls an entity when the Company is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity. The subsidiary is fully consolidated from the date on which control is transferred to the Company. They are deconsolidated from the date that control ceases.
C. Translation of foreign currency balances and transactions
- 1)
- Functional currency and presentation currency
- 2)
- Transactions and balances
Items included in the financial statements are measured using the currency of the primary economic environment in which the Company operates ("the Functional Currency"). The financial statements are stated in New Israeli Shekels (NIS), which is the functional and presentation currency of the Company and its subsidiary.
Transactions in currencies other than the Functional Currency (foreign currencies) are translated into the Functional Currency at exchange rates at the dates of transaction.
Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation at year-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognised in the profit or loss for the year.
Gains and losses arising from changes in exchange rates are recognized in the statement of comprehensive loss under financing expenses (income).
D. Property and equipment
- 1)
- All property and equipment (including leasehold improvements) are stated at historical cost less accumulated depreciation and impairment. Historical cost includes expenditures that are directly attributable to the acquisition of the items.
Repairs and maintenance are charged to the income statement during the period in which they are incurred.
F-9
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 2—SIGNIFICANT ACCOUNTING POLICIES (Continued)
- 2)
- The assets are depreciated using the straight-line method to allocate their cost over their estimated useful lives, as follows:
| |
Years | |
|---|---|---|
Computer equipment |
3 | |
Greenhouse equipment* |
4 - 10 | |
Office furniture |
7 - 17 | |
Laboratory equipment |
4 - 5 |
- *
- Greenhouse equipment—agricultural equipment used in the tobacco production greenhouse
- 3)
- Gains and losses on disposals are determined by comparing proceeds with the associated carrying amount. These are included in the statement of comprehensive loss.
Leasehold improvements are depreciated over the lease period or the expected useful life of the improvements, whichever is shorter.
Impairment of the asset to its recoverable amount is recognized as incurred, if the carrying amount of the asset is greater than its estimated recoverable amount (see also section F below).
E. Intangible assets
- 1)
- In process research and development ("IPR&D")
- 2)
- Software
- 3)
- Research and development ("R&D")
Acquired IPR&D is presented based on the fair value at the date of the acquisition and is not depreciated during the research and development period. Such assets are tested annually for impairment, see F. below. The assessment is carried out more frequently if there are indications of impairment. The intangible asset balance remained unchanged as at December 31, 2014 and 2013.
Acquired software licenses are capitalized on the basis of the cost incurred to acquire and bring to use the specific software. These costs are amortized on a straight-line basis over the estimated useful life of licenses (three years).
- •
- It is technically feasible to complete the intangible asset so that it will be available for use.
- •
- Management intends to complete the development of the intangible asset and to use or sell the asset.
- •
- The intangible asset can be used or sold.
Research expenses are recognized as an expense as incurred. Costs incurred for development projects (referring to design and testing of new or improved products) are recognized as intangible assets when the following conditions exist:
F-10
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 2—SIGNIFICANT ACCOUNTING POLICIES (Continued)
- •
- It is possible to demonstrate how the intangible asset will generate probable future economic benefits.
- •
- There are adequate technical, financial and other resources to complete development and to use or sell the intangible
asset.
- •
- The expenditure attributable to the intangible asset can be reliably measured during its development.
Other development costs that do not meet these criteria are recognized as an expense when incurred. Development costs previously recognized as an expense are not recognized as an asset in subsequent periods.
As of December 31, 2014, the Company has not met the rules for capitalizing development costs as an intangible asset and accordingly, no asset whatsoever has been recognized in the financial statements for such costs.
F. Impairment of non-monetary assets
Assets that have indefinite useful life, such as goodwill or intangible assets not ready for use, are not subject to amortization and are tested annually for impairment.
Assets that are subject to amortization are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. An impairment loss is recognized for the amount by which the asset's carrying amount exceeds its recoverable amount. The recoverable amount of an asset is the higher of its fair value less cost to sell and value in use. For the purpose of assessing impairment, assets are grouped together at the lowest levels for which there are separately identifiable cash flows (cash-generating units). Non-financial assets other than goodwill that suffered impairment are reviewed for possible reversal of the impairment at each reporting date.
For the years ended December 31, 2014 and 2013, no impairment has been recognized.
G. Government grants
Government grants, which are received from the Israeli Office of Chief Scientist (the "OCS") by way of participation in research and development that is conducted by the Company, fall within the scope of "forgivable loans," as set forth in International Accounting Standard 20 "Accounting for Government Grants and Disclosure of Government Assistance" ("IAS 20").
As approved by the OCS, the grants are received in installments as the program progresses. The Company recognizes each forgivable loan on a systematic basis at the same time the Company records as an expense the related research and development costs for which the grant is received, provided that there is reasonable assurance that (a) the Company complies with the conditions attached to the grant, and (b) it is probable that the grant will be received (usually upon receipt of approval notice). The amount of the forgivable loan is recognized based on the participation rate approved by the OCS; thus, a forgivable loan is recognized as a receivable when approved research and development costs have been incurred before grant funds are received.
Since at the time of grant approval there is reasonable assurance that the Company will comply with the forgivable loan conditions attached to the grant, and it is not probable that the related
F-11
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 2—SIGNIFICANT ACCOUNTING POLICIES (Continued)
research and development will generate revenue, grant income is recorded against the related research and development expenses in the statement of comprehensive loss.
If forgivable loans are initially carried to income, as described above, and, in subsequent periods, it appears more likely than not that royalties will be paid to the OCS, the Company recognizes a liability which is measured based on the Company's best estimate of the amount required to settle the Company's obligation at the end of each reporting period.
H. Cash and cash equivalents
Cash and cash equivalents include cash on hand, short-term bank deposits, and other short-term highly liquid investments with maturities of three months or less.
I. Share capital
The Company's ordinary shares are classified as share capital. Incremental costs directly attributable to the issue of new shares or options are recognized in equity net of issue proceeds.
J. Trade payables
Trade payables include the Company's liabilities to pay for goods or services purchased from suppliers in the ordinary course of business. Trade payables are classified as current liabilities if payment is due within one year; otherwise they are recognized as non-current liabilities.
Trade payables are recognized initially at fair value and subsequently measured at amortized cost based on the effective interest method.
K. Deferred taxes
The Company recognizes deferred taxes based on the liability method, for temporary differences between the carrying amounts of assets and liabilities included in the consolidated financial statements and the amounts used for tax purposes. However, deferred tax liabilities are not recognized if they arise from the initial recognition of goodwill. In addition, deferred taxes are not recognized if the temporary differences arise on initial recognition of an asset or a liability, other than in a business combination, which, at the time of the transaction, have no effect on profit or loss—whether for accounting or tax purposes. The amount of deferred taxes is determined in accordance with the tax rates (and tax laws) that have been enacted or substantively enacted as at the date of the financial statements and are expected to apply when the deferred tax assets will be realized or when the deferred tax liabilities will be settled.
Deferred tax assets are recognized for deductible temporary differences, to the extent that it is probable that future taxable profits will be available against which they can be utilized.
In the absence of a forecast of future taxable income, a deferred tax asset was not recognized in the Company's financial statements.
L. Employee benefits
- 1)
- Liability for severance pay
F-12
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 2—SIGNIFICANT ACCOUNTING POLICIES (Continued)
- 2)
- Vacation and recreation pay
In accordance with labor laws and labor agreements in effect, the Company and its subsidiary are required to pay severance and pension benefits to employees who are dismissed or retire under certain circumstances.
The liability to pay pension and severance pay to employees in Israel who are covered by Section 14 of the Severance Pay Law, are covered by regular contributions to defined contribution plans. The amounts contributed are not included in the statement of financial position.
By law, all employees are entitled to vacation and recreation pay, calculated on a monthly basis. The right is based on the employment period.
M. Share-based payment
The Company has a share-based payment plan for employees and service providers, settled by the Company's equity instruments, whereby the Company receives services from employees and service providers in exchange for the Company's equity instruments (options). The fair value of services received from employees and service providers in exchange for the options is recognized as an expense in the statement of comprehensive loss. The total amount recognized as an expense in profit or loss is based on the fair value of the options granted, without taking into account the effect of service conditions and non-market vesting conditions.
Non-market vesting conditions are included in the assumptions used to estimate the number of options expected to vest. The total expense is recognized in the vesting period, which is the period for fulfillment of all the defined vesting terms of the share-based payment arrangement.
At each reporting date, the Company adjusts its estimates of the number of options that are expected to vest, based on the non-market vesting conditions, and recognizes the effect of the change compared to original estimates, if any, in the statement of comprehensive loss, and a corresponding adjustment in equity.
When exercising the options, the Company issues new shares, the proceeds, net of directly attributable transaction costs, are recognized in share capital (par value) and premium.
N. Segment reporting
Operating segments are reported in a manner consistent with the internal reporting provided to the chief operating decision-maker, who is responsible for allocating resources and assessing performance of the operating segments. The Company operates in one operating segment.
O. Loss per share
Basic loss per share is the net loss for the year divided by the weighted average number of ordinary shares outstanding in the period, net of shares held by the Company.
When calculating diluted loss per share, the Company adjusts the loss attributable to ordinary shareholders of the Company and the weighted average number of ordinary shares outstanding, for the effects of all dilutive potential ordinary shares.
Potential shares are only taken into account if their effect is dilutive (reduces earnings per share or increases loss per share).
F-13
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 2—SIGNIFICANT ACCOUNTING POLICIES (Continued)
P. New standards and interpretations not yet adopted
IFRS 9 Financial Instruments
The complete version of IFRS 9 replaces most of the guidance in IAS 39. IFRS 9 retains but simplifies the mixed measurement model and establishes three primary measurement categories or financial assets: amortized cost, fair value through OCI and fair value through P&L. The basis of classification depends on the entity's business model and the contractual cash flow characteristics of the financial asset. Investments in equity instruments are required to be measured at fair value through profit or loss with the irrevocable option at inception to present changes in fair value in OCI. There is now a new expected credit losses model that replaces the incurred loss impairment model used in IAS 39.
For financial liabilities there were no changes to classification and measurement except for the recognition in other comprehensive income of changes resulting from own credit risk, in liabilities designated at fair value, through profit or loss.
IFRS 9 relaxes the requirements for hedge effectiveness by replacing the bright line hedge effectiveness tests. It requires an economic relationship between the hedged item and hedging instrument and for the 'hedged ratio' to be the same as the one management actually use for risk management purposes. Contemporaneous documentation is still required but is different to that currently prepared under IAS 39.
The standard is effective for accounting periods beginning on or after 1 January 2018. Early adoption is permitted. The Company has not yet assessed IFRS 9's full impact.
NOTE 3—SIGNIFICANT ACCOUNTING ESTIMATES AND JUDGMENTS
Estimates and judgments are reviewed on an ongoing basis and are based on past experience and other factors, including expectations of future events, which are considered reasonable in view of current circumstances.
A. Significant accounting estimate
The Company makes estimates and assumptions with respect to the future. By nature, the accounting estimates are rarely identical to actual results. The estimate that has a significant risk of resulting in a material adjustment to carrying amounts of assets and liabilities in the next financial year is listed below.
Impairment of IPR&D
The Company reviews annually the need to record impairment of IPR&D.
To test for impairment, the Company as a whole has been identified as the smallest cash-generating unit to which the intangible asset can be attributed. Accordingly, the Company measured the recoverable amount of the Company as a whole. The recoverable amount is the higher of value in use and fair value less costs of disposal. In accordance with IFRS 13, the quoted market price in an active market provides the most reliable evidence of fair value. Since fair value less costs of disposal, which is based on the market price of the Company, is significantly higher than the carrying
F-14
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 3—SIGNIFICANT ACCOUNTING ESTIMATES AND JUDGMENTS (Continued)
amount of the cash-generating unit, the Company determined that no impairment exists. See also Note 2E(1).
B. Significant judgments made when applying the Company's accounting policy
- 1)
- Grants
from the OCS
In accordance with the accounting treatment prescribed in Note 2G, the Company's management is required to examine whether there is reasonable assurance that the grant that was received will be repaid. In addition, if, at the date of initial recognition, the grant is recognized in the statement of income, then in subsequent periods the Company's management is required to evaluate whether the payment of royalties to the OCS is considered to be more likely than not.
- 2)
- Development
costs
Development costs are capitalized in accordance with the accounting policy described in Note 2E(3). Capitalization of costs is based on management's judgment about technological and economic feasibility.
The Company's management believes that as at December 31, 2014, the above conditions were not met; therefore development costs were not capitalized.
NOTE 4—FINANCIAL INSTRUMENTS AND FINANCIAL RISK MANAGEMENT
- 1)
- Financial
risk factors
The Company's activities expose it to diverse financial risks: currency risk, credit risk, and liquidity risk. The Company's comprehensive risk management plan focuses on the unpredictability of financial markets and the attempt to minimize potential adverse effects on the Company's financial performance.
The Company's CFO is responsible for risk management in accordance with the policy approved by the board of directors.
- A)
- Market
risks
Exchange rate risk
The Company is exposed to exchange rate risks arising from exposure to various currencies, primarily the U.S. dollar. The exchange rate risk is due to future commercial transactions and assets or liabilities denominated in foreign currency.
On December 31, 2014, if the Company's Functional Currency had depreciated by 5% against the U.S. dollar, and if all the other variables had remained the constant, the post-tax loss for the year would have been lower by NIS 220 thousand (December 31, 2013, NIS 212 thousand), mainly due to losses from exchange rate differences for translation of cash balances, receivables and trade payables.
Financial risk management
F-15
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 4—FINANCIAL INSTRUMENTS AND FINANCIAL RISK MANAGEMENT (Continued)
- B)
- Liquidity
risk
The Company has not yet generated profits or positive cash flows from its operating activities, and the continuation of its operations in the current format is subject to raising financing sources until a positive cash flow is generated from its operations. See also note 1A.
- 2)
- Capital
risk management
The objectives of the Company's capital risk management are to maintain the Company's ability to continue as a going concern in order to provide shareholders with a return on their investment and to maintain an optimal capital structure to minimize the cost of capital.
NOTE 5—CASH AND CASH EQUIVALENTS
| |
December 31 | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
| |
NIS in thousands |
||||||
Breakdown by currency: |
|||||||
NIS |
18,983 | 6,563 | |||||
In foreign currency (mainly USD) |
4,794 | 4,499 | |||||
| | | | | | | | |
|
23,777 | 11,062 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
NOTE 6—RECEIVABLES
| |
December 31 | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
| |
NIS in thousands |
||||||
Value added tax |
781 | 227 | |||||
Receivables for participation in R&D expenses |
726 | 1,122 | |||||
Prepaid expenses |
170 | 164 | |||||
Other |
51 | 35 | |||||
| | | | | | | | |
|
1,728 | 1,548 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Most financial balances are in NIS and are unlinked.
The carrying amount of other receivables is a reasonable approximation of their fair value since the effect of discounting is insignificant.
The maximum exposure to credit risk as at December 31, 2014 for receivables that are financial assets is their fair value. The Company does not hold any collateral for these receivables.
F-16
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 7—PROPERTY AND EQUIPMENT
Composition of property and equipment and accumulated depreciation, by principal groups, and the movements therein in 2013:
| |
Cost | Accumulated depreciation | |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Carrying amount at beginning of year |
Additions | Carrying amount at end of year |
Carrying amount at beginning of year |
Additions | Carrying amount at end of year |
Depreciated balance as at December 31, 2013 |
|||||||||||||||
| |
NIS in thousands |
NIS in thousands |
NIS in thousands |
|||||||||||||||||||
Computer equipment |
517 | 86 | 603 | 440 | 57 | 497 | 106 | |||||||||||||||
Office furniture |
426 | 12 | 438 | 108 | 27 | 135 | 303 | |||||||||||||||
Laboratory equipment |
3,574 | 144 | 3,718 | 2,610 | 429 | 3,039 | 679 | |||||||||||||||
Greenhouse equipment |
2,973 | 9 | 2,982 | 1,628 | 305 | 1,933 | 1,049 | |||||||||||||||
Leasehold improvements |
715 | 223 | 938 | 496 | 117 | 613 | 325 | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
|
8,205 | 474 | 8,679 | 5,282 | 935 | 6,217 | 2,462 | |||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
Composition of property and equipment and accumulated depreciation, by principal groups, and the movements therein in 2014:
| |
Cost | Accumulated depreciation | |
|||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Carrying amount at beginning of year |
Additions | Disposals | Carrying amount at end of year |
Carrying amount at beginning of year |
Additions | Disposals | Carrying amount at end of year |
Depreciated balance as at December 31, 2014 |
|||||||||||||||||||
| |
NIS in thousands |
NIS in thousands |
NIS in thousands |
|||||||||||||||||||||||||
Computer equipment |
603 | 30 | 35 | 598 | 497 | 58 | 35 | 520 | 78 | |||||||||||||||||||
Office furniture |
438 | 438 | 135 | 26 | 161 | 277 | ||||||||||||||||||||||
Laboratory equipment |
3,718 | 268 | 3 | 3,983 | 3,039 | 335 | 3 | 3,371 | 612 | |||||||||||||||||||
Greenhouse equipment |
2,982 | 2,982 | 1,933 | 266 | 2,199 | 783 | ||||||||||||||||||||||
Leasehold improvements |
938 | 38 | 976 | 613 | 106 | 719 | 257 | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
|
8,679 | 336 | 38 | 8,977 | 6,217 | 791 | 38 | 6,970 | 2,007 | |||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
F-17
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 8—INTANGIBLE ASSETS
Composition of intangible assets and accumulated amortization, by principal groups, and the movements therein in 2013:
| |
Cost | Accumulated depreciation | |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Carrying amount at beginning of year |
Carrying amount at end of year |
Carrying amount at beginning of year |
Additions | Carrying amount at end of year |
Depreciated balance as at December 31, 2013 |
|||||||||||||
| |
NIS in thousands |
NIS in thousands |
NIS in thousands |
||||||||||||||||
Software |
104 | 104 | 72 | 16 | 88 | 16 | |||||||||||||
IPR&D |
1,720 | 1,720 | 1,720 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
|
1,824 | 1,824 | 72 | 16 | 88 | 1,736 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
Composition of intangible assets and accumulated amortization, by principal groups, and the movements therein in 2014:
| |
Cost | Accumulated depreciation | |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Carrying amount at beginning of year |
Carrying amount at end of year |
Carrying amount at beginning of year |
Additions | Carrying amount at end of year |
Depreciated balance as at December 31, 2014 |
|||||||||||||
| |
NIS in thousands |
NIS in thousands |
NIS in thousands |
||||||||||||||||
Software |
104 | 104 | 88 | 11 | 99 | 5 | |||||||||||||
IPR&D |
1,720 | 1,720 | 1,720 | ||||||||||||||||
| | | | | | | | | | | | | | | | | | | | |
|
1,824 | 1,824 | 88 | 11 | 99 | 1,725 | |||||||||||||
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | |
NOTE 9—INCOME TAX
A. Taxation of the Company and its subsidiary
1) Tax rates
The income of the Company and its subsidiary is taxable at the regular rate of corporate tax.
The rate of corporate tax in 2013 is 25% and in 2014 and thereafter is 26.5%.
B. Carry-forward tax losses
Deferred tax assets for carry-forward tax losses are recognized if it is expected that the tax benefit will be realized through the existence of future taxable profits.
The carry-forward losses of CollPlant Holdings Ltd. (without capital losses) as at December 31, 2014 and 2013 amounted to approximately NIS 5.7 million and NIS 5.1 million, respectively.
The carry-forward losses of CollPlant Ltd. (without capital losses) as at December 31, 2014 and 2013 amounted to approximately NIS 100.8 million and NIS 89.0 million, respectively.
The Company did not recognize deferred taxes on the losses as it is not probable that the differences will be realized in the foreseeable future.
F-18
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 9—INCOME TAX (Continued)
C. Tax assessments
In accordance with the Income Tax Ordinance, tax assessments filed by the Company and its subsidiary up to 2010 are considered final.
D. Value added tax
The Company and its subsidiary are registered as authorized dealers for VAT purposes.
NOTE 10—ACCOUNTS PAYABLE
| |
December 31 | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
| |
NIS in thousands |
||||||
A. Trade payables |
|||||||
Breakdown by currency: |
|||||||
NIS |
1,300 | 1,546 | |||||
In foreign currency (mainly USD) |
556 | 96 | |||||
| | | | | | | | |
|
1,856 | 1,642 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
B. Other payables: |
|||||||
Employees and institutions for employees |
965 |
667 |
|||||
Provisions for vacation and others |
368 | 338 | |||||
| | | | | | | | |
|
1,333 | 1,005 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The carrying amount of accounts payable is a reasonable approximation of their fair value since the effect of discounting is insignificant.
NOTE 11—RETIREMENT BENEFIT OBLIGATION
The amount recognized as an expense for defined contribution plans in 2014 and 2013 is NIS 958 thousand and NIS 855 thousand, respectively.
NOTE 12—COMMITMENTS AND CONTINGENT LIABILITIES
A. Agreements:
1) Operating lease agreements:
- a)
- On
August 19, 2013, an agreement was signed to extend the lease, which commenced in June 2008, of the Company's offices. The lease ends on
August 18, 2015, and the monthly rent amounts to NIS 54 thousand.
As collateral for the lease agreement, a restricted deposit was pledged in favor of the property owner. The balance of the restricted deposit as at December 31, 2014 amounts to NIS 564 thousand. The deposit is classified as a non-current asset.
F-19
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 12—COMMITMENTS AND CONTINGENT LIABILITIES (Continued)
- b)
- In April 2007, CollPlant signed an agreement with a third party for lease of land in Yessod Hama'ala. The lease was for a three-year period, with an option for renewal every year for up to another seven years. The Company extends the agreement annually in accordance with the terms of the option for renewal.
2) Commitment to pay royalties to the Government of Israel
The Company is committed to pay royalties to the Government of Israel on proceeds from sales of products in the research and development of which the Government participates by way of grants through the OCS. At time the grants were received, successful development of the related project was not assumed. In the case of failure of the project that was partly financed by the Government of Israel, the Company is not obligated to pay any such royalties. Under the terms of Company's funding from the Israeli Government, royalties of 3%-5% are payable on sales of products developed from projects so funded up to 100% of the amount of the grant received by the Company (dollar linked) with the addition of an annual interest based on LIBOR. Because the Company's products have yet to receive marketing clearance or regulatory approval, the Company's management believes that, as of December 31, 2014, the payment of royalties to the OCS is not considered to be more likely than not. Therefore, a liability is not included in the Company's financial statements. As of December 31, 2014, the maximum royalty amount that would be payable by the Company, before the additional LIBOR interest, is approximately NIS 24.5 million (assuming 100% of the grants are payable).
B. Development agreements with pharmaceutical and medical device companies
On November 17, 2010, CollPlant and Pfizer Inc. ("Pfizer") signed an agreement for joint development of prototype products for the treatment of orthopedic problems. The agreement refers, among other things, to the allocation of the rights of the project outcomes. In accordance with the agreement, Pfizer paid CollPlant immaterial amounts for the development of prototypes.
On December 22, 2011, CollPlant and Pfizer signed another joint development agreement for development of a product for the orthopedic market ("the Development Agreement"). In accordance with the Development Agreement, the parties will collaborate in the development of a product that contains Pfizer's therapeutic proteins and compounds based on CollPlant's recombinant human collagen (rhCollagen) ("the Product").
In accordance with the Development Agreement, the development plan was divided into two periods (each period is comprised of two stages) over a total period of three years. CollPlant was to receive a total consideration of $1.9 million for its activity in accordance with the Development Agreement, and subject to compliance with milestones and fulfillment of the conditions under the Development Agreement for each of the two agreement periods. In accordance with the Development Agreement, Pfizer was granted an exclusive right, limited in time, to negotiate the continuation of development and commercialization of the Product with CollPlant. In February 2012, an amount of $0.4 million was received, and the same amount was received at the beginning of 2013. The amounts received are offset against the Company's R&D expenses.
F-20
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 12—COMMITMENTS AND CONTINGENT LIABILITIES (Continued)
To the best of the Company's knowledge, based partially on public sources, in July 2013, Pfizer signed an agreement with a U.S.-based company ("the U.S. Company"), which specializes in orthobiologics, whereby Pfizer granted the U.S. Company an exclusive, global license for the portfolio of projects related to Pfizer's recombinant bone growth protein ("the Protein"). Pfizer will also continue to manufacture the Protein and supply it to the U.S. Company. The Company is currently involved in joint development with the U.S. Company for the Product, including work of development teams from both companies on samples for a bone treatment product, instead of the cooperation with Pfizer, which expired during 2014.
The Company believes that the work and negotiations between the parties will continue over the coming months, and if negotiations are successful, the Company believes that a joint development agreement will be signed with the U.S. Company (or with the U.S. Company and Pfizer, together), which would include milestones until commercialization of the Product and royalties on future sales. However, there is no assurance that the negotiations between these parties will culminate in a binding agreement on the said date or at all, as well as what the final terms of the agreement will be.
C. Research grants from external sources
- 1)
- On
January 20, 2010, a consortium, including CollPlant, received funding from the European Union Seventh Framework Program ("the Program"). The
research subject is tendon regeneration. The program continued for four years, including joint research and exchange of personnel between CollPlant and the other partners in Europe. The amounts
received are offset against the Company's R&D expenses. As at December 31, 2014, the program has ended and proceeds of EUR 36 thousand were received.
- 2)
- On August 17, 2010, a consortium, including CollPlant, received additional funding from the Program. The objective of this research is to developing hernia meshes using human recombinant collagen. The total funding for CollPlant in this research program amounts to EUR 274 thousand. As at December 31, 2014, proceeds amounting to EUR 235 thousand were received. The amounts received are offset against the Company's R&D expenses. The program has ended in April 2015.
NOTE 13—EQUITY
A. Ordinary shares and warrants:
- 1)
- Composition
| |
Number of shares | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Issued and paid up** | ||||||||
| |
Registered | |||||||||
| |
December 31 | |||||||||
| |
December 31 2014 and 2013 |
|||||||||
| |
2013 | 2014 | ||||||||
Ordinary shares of par value NIS 0.01* |
1,500,000,000 | 236,874,726 | 241,392,352 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
F-21
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 13—EQUITY (Continued)
| |
Amount in NIS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
|
Issued and paid up | ||||||||
| |
Registered | |||||||||
| |
December 31 | |||||||||
| |
December 31 2014 and 2013 |
|||||||||
| |
2013 | 2014 | ||||||||
Ordinary shares of par value NIS 0.01* |
15,000,000 | 2,368,747 | 2,413,923 | |||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
- *
- Traded
on the Tel Aviv Stock Exchange.
- **
- Not including 2,761,384 shares held by the Company. These shares are considered to be dormant.
- 2)
- The
ordinary shares confer on their holders the right to vote and participate in shareholder meetings (with one vote for each NIS 0.01 share), the right to
receive profits and the right to participate in surplus assets on liquidation of the Company.
- 3)
- In
2014, Series B, C, D, and E warrants expired without exercise.
- 4)
- In
October 2013, the Company signed an investment agreement, according to which $2.5 million was invested in the Company in exchange for 16,856,173
ordinary shares (the issue expenses amounted to $190 thousand).
- 5)
- In December 2013, the Company raised a gross amount of NIS 21.3 million for the issuance of 68,313,000 ordinary shares and 68,313,000 Series F warrants. The issuance expenses amounted to NIS 1.96 million. The Company also granted 6,831,300 Series F warrants to underwriters. Series F warrants are exercisable up to December 31, 2016, with an exercise price of NIS 0.7 per share.
B. Share-based payments
- 1)
- In accordance with an option plan for employees and consultants ("the Option Plan"), as amended from time to time, employees and consultants of the Company, will be granted options, each exercisable into one ordinary share of the Company of NIS 0.01. The ordinary shares that will be issued in accordance with the Option Plan will have the same rights as the other ordinary shares of the Company, immediately subsequent to their issue. An option that is not exercised within 10 years from the allotment date will expire, unless the board of directors extends its validity.
- 2)
- On May 29, 2013, options to purchase 1,268,487 ordinary shares were granted to employees and officers of the Company (who are not the CEO and/or a director), and 354,177 options were granted to officers of the Company. On July 4, 2013, options to purchase 270,000
Grants to employees are made in accordance with the Option Plan, and are carried out within the provisions set out for this matter in Section 102 of the Income Tax Ordinance. In accordance with the track selected by the Company and these provisions, the Company is not entitled to claim a tax deduction carried to employee benefits.
For those who are not employees of the Company, and for the Company's controlling shareholders (as defined in the Income Tax Ordinance) options are granted in accordance with section 3(I) of the Income Tax Ordinance.
F-22
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 13—EQUITY (Continued)
- 3)
- On September 8, 2014, the board of directors approved the grant of options to purchase 400,000 ordinary shares to the VP R&D. The options will vest over four years. One quarter will vest one year after the grant date, and the balance will vest in equal parts at the end of each subsequent quarter. The exercise price of each option is NIS 0.25 (unlinked).
ordinary shares were granted to two employees, including an officer who is not the CEO and/or a director. Total number of options granted in 2013 accumulated to 1,892,664.
480,783 options included in the above 2013 grants vested over one year. The exercise price of each option is NIS 0.3 (unlinked). The fair value of the options at the grant date was NIS 34 thousand.
1,411,881 options included in the above 2013 grants will vest over a four-year period. One quarter vests one year after the grant date, and the balance vests in equal parts at the end of each subsequent quarter. The exercise price of each option is NIS 0.44 (unlinked). The fair value of the options at the grant date was NIS 180 thousand.
The fair value of each option, calculated according to the Black-Scholes formula, is between NIS 0.07 and NIS 0.13. This value is based on the following assumptions: expected dividend of 0%, expected volatility of 85%, risk-free interest rate of 1.85%, and period up to exercise of 4 years.
- 4)
- On October 29, 2014, the Company's general meeting approved a grant for the chairman of the board of directors. The grant is for options to purchase 7,241,770 ordinary shares for an exercise price of NIS 0.26 per share. The options will vest over three years. One third will vest one year after grant date, and the balance will vest in equal installments at the end of each subsequent quarter. The fair value of the options at the grant date was NIS 340 thousand.
- 5)
- On January 9, 2014, the Company's CEO exercised a total of options to purchase 4,517,626 ordinary shares at an exercise price of NIS 0.01 per share, for a total consideration of NIS 45 thousand.
The fair value of the options at the grant date was NIS 42 thousand.
The fair value of each option, calculated according to the Black-Scholes formula, amounts to NIS 0.11. This value is based on the following assumptions: expected dividend of 0%, expected volatility of 51.24%, risk-free interest rate of 2%, and period to exercise of 4 years.
The fair value of each option, calculated according to the Black-Scholes formula, amounts to NIS 0.05. This value is based on the following assumptions: expected dividend of 0%, expected volatility of 51.24%, risk-free interest rate of 2%, and period to exercise of 4 years.
Exercise of options
F-23
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 13—EQUITY (Continued)
Changes in number of options and weighted average exercise prices are as follows:
| |
Year ended December 31, 2013 |
Year ended December 31, 2014 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
No. of options |
Average weighted exercise price |
No. of options |
Average weighted exercise price |
|||||||||
Outstanding at the beginning of the year |
15,722,201 | 0.7 | 15,535,762 | 0.57 | |||||||||
Granted |
1,892,664 | 0.41 | 7,641,770 | 0.26 | |||||||||
Expired |
(1,434,214 | ) | 1.39 | (376,091 | ) | 1.35 | |||||||
Forfeited |
(644,889 | ) | 1.39 | (320,469 | ) | 0.83 | |||||||
Exercised |
(4,517,626 | ) | 0.01 | ||||||||||
| | | | | | | | | | | | | | |
Outstanding at the end of the period |
15,535,762 | 0.57 | 17,963,346 | 0.56 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Exercisable at the end of the period |
13,170,611 | 0.53 | 9,042,670 | 0.75 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
The following is information about the exercise price and remaining contractual life of outstanding options:
| December 31, 2013 | December 31, 2014 | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of outstanding options |
Exercise price range |
Weighted average of remaining contractual life |
No. of outstanding options |
Exercise price range |
Weighted average of remaining contractual life |
||||||||||||
| 15,535,762 | 0.01 - 1.39 | 6.35 | 17,963,346 | 0.26 - 1.39 | 7.32 | ||||||||||||
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | |
The expenses recognized in the Company's statements of income in 2014 and 2013 for options granted to employees amounted to NIS 205 thousand and 462 thousand, respectively.
F-24
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 14—RESEARCH AND DEVELOPMENT EXPENSES, NET
| |
Year ended December 31 |
||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
| |
NIS in thousands |
||||||
Payroll and related expenses |
7,462 | 6,246 | |||||
Share-based payments |
341 | 137 | |||||
Subcontractors and consultants |
4,395 | 4,429 | |||||
Consumables and materials |
765 | 659 | |||||
Depreciation |
901 | 750 | |||||
Rent and maintenance |
1,842 | 2,056 | |||||
Other |
445 | 602 | |||||
| | | | | | | | |
|
16,151 | 14,879 | |||||
Less: |
|||||||
Participation in R&D expenses, see Notes 12B(1) and 12C |
(511 | ) | (1,554 | ) | |||
OCS participation in R&D expenses, see Note 12A(2) |
(3,206 | ) | (3,591 | ) | |||
| | | | | | | | |
|
12,434 | 9,734 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
NOTE 15—GENERAL, ADMINISTRATIVE AND MARKETING EXPENSES
| |
Year ended December 31 |
||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
| |
NIS in thousands |
||||||
Payroll and related expenses |
1,405 | 1,803 | |||||
Share-based payments |
121 | 68 | |||||
Directors' salary and insurance |
514 | 590 | |||||
Rent and office maintenance |
368 | 314 | |||||
Professional services |
833 | 859 | |||||
Depreciation |
50 | 52 | |||||
Other |
456 | 220 | |||||
| | | | | | | | |
|
3,747 | 3,906 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
F-25
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 16—FINANCING EXPENSES (INCOME), NET
| |
Year ended December 31 |
||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
| |
NIS in thousands |
||||||
Financing expenses: |
|||||||
Bank fees |
27 | 25 | |||||
Foreign exchange losses, net |
287 | ||||||
| | | | | | | | |
Total financing expenses |
314 | 25 | |||||
| | | | | | | | |
Financing income: |
|||||||
Interest income on cash equivalents and deposits |
25 | 35 | |||||
Foreign exchange gains, net |
607 | ||||||
| | | | | | | | |
Total financing income |
25 | 642 | |||||
| | | | | | | | |
|
289 | (617 | ) | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
NOTE 17—LOSS PER SHARE
Basic loss per share is calculated by dividing the loss attributable to the Company's shareholders by the weighted average number of ordinary shares issued, after taking into account, retrospectively, the benefit component in a rights offering. The calculation of the diluted loss per share did not take into account 17,963,346 options for employees and service providers, and 88,337,260 Series F warrants, since their effect is anti-dilutive.
NOTE 18—TRANSACTIONS AND BALANCES WITH RELATED PARTIES
"Related Party"—as defined in IAS 24R.
The Company's key management personnel include members of the executive management and board of directors, in accordance with the definition of Related Parties in IAS 24.
A. Transactions with and benefits to related parties
| |
Year ended December 31 |
||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
| |
NIS in thousands |
||||||
CEO's salary* |
1,249 | 1,209 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Of which share-based payments |
237 | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Remuneration of directors** |
879 | 893 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Of which share-based payments |
8 | 44 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
- *
- In accordance with the CEO's employment agreement, the CEO will be eligible for a bonus based on qualitative criteria and parameters determined by the Company, which
F-26
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 18—TRANSACTIONS AND BALANCES WITH RELATED PARTIES (Continued)
will amount to a maximum of four salaries, plus a special bonus based on the fulfillment of additional conditions.
- **
- The Company entered into an agreement with one of its shareholders (who also serves as a director of the Company as from May 20, 2010) for research consulting services in consideration of a monthly amount of NIS 32,000.
B. Balances with related parties:
| |
Year ended December 31 |
||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
| |
NIS in thousands |
||||||
For payroll and related expenses, the balance is included among other accounts payable |
(448 | ) | (339 | ) | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
C. Benefits for key officers
Compensation for the CFO, VP Research and Development, COO (up to May 2013), and VP Quality Assurance, defined as key management personnel for their services provided to the Company, is as follows:
| |
Year ended December 31 |
||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
| |
NIS in thousands |
||||||
Salary and other short-term benefits |
2,234 | 1,776 | |||||
Share-based compensation |
78 | 56 | |||||
| | | | | | | | |
|
2,312 | 1,832 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Number of key managers |
4 | 3 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
NOTE 19—SUBSEQUENT EVENTS
- A.
- On March 4, 2015, the Company announced that its ADR level 1 program became effective in the
United States. Each ADR comprises of 100 ordinary shares, traded over the counter (OTC) in the United States, under the symbol CQPTY.
- B.
- On March 22, 2015, the board of directors approved the grant of options to purchase 10,000,000
ordinary shares to its Director and Chief Scientific Officer. The grant is subject to the Company's general meeting approval. The options will vest over 5 years. One-fifth will vest one year
after the grant date, and the balance will vest in equal parts at the end of each subsequent quarter. The exercise price of each option is NIS 0.60. The fair value of the options at the date of board
of directors approval was NIS 1,748,000.
- C.
- On May 18, 2015, options to purchase 7,450,000 ordinary shares were granted to employees and officers of the Company (who are not the CEO and/or a director). The options will vest over 4 years. One quarter will vest one year after the grant date, and the balance will vest in equal parts
F-27
CollPlant Holdings Ltd.
Notes to the Consolidated Financial Statements (Continued)
NOTE 19—SUBSEQUENT EVENTS (Continued)
at the end of each subsequent quarter. The exercise price of each option is NIS 0.60. The fair value of the options at the grant date was NIS 1,597,000.
- D.
- On May 18, 2015, the board of directors approved the grant of options to purchase 5,670,000 ordinary
shares to the CEO of the company. The grant is subject to the Company's general meeting approval. The options will vest over 4 years. One quarter will vest one year after the grant date, and
the balance will vest in equal parts at the end of each subsequent quarter. The exercise price of each option is NIS 0.60. The fair value of the options at the date of board of directors approval was
NIS 1,216,000.
- E.
- On May 18, 2015, options to purchase 1,000,000 ordinary shares were granted to a consultant of the
Company. The options will vest according to certain milestones. The exercise price of each option is NIS 0.60. The fair value of the options at the grant date was NIS 240,000.
- F.
- On May 21, 2015, the board of directors approved the grant of options to purchase a total of 2,680,000 ordinary shares to four members of the board of directors, at 670,000 options each. The grant is subject to the Company's general meeting approval. The options will vest over 4 years. Half of the amount will vest two years after the date of the Board decision, and the balance will vest in equal parts at the end of each subsequent month. The exercise price of each option is NIS 0.60. The fair value of the options at the date of the approval of the board of directors was NIS 643,000.
F-28

American Depositary Shares
|
Each Representing |
Ordinary Shares |
Book-Running Manager
Ladenburg Thalmann
Co-Manager
Brean Capital
The date of this prospectus is , 2015.
Until and including , 2015 (25 days after the date of this prospectus), all dealers that buy, sell or trade our ordinary shares or ADSs, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors and Officers
An Israeli company may indemnify an office holder in respect of certain liabilities either in advance of an event or following an event provided that a provision authorizing such indemnification is inserted in its articles of association. Our articles of association contain such a provision.
An undertaking provided in advance by an Israeli company to indemnify an office holder with respect to a financial liability imposed on him or her in favor of another person pursuant to a judgment, settlement or arbitrator's award approved by a court must be limited to events which in the opinion of the board of directors can be foreseen based on the Company's activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking must detail the abovementioned events and amount or criteria.
In addition, a company may indemnify an office holder against the following liabilities incurred for acts performed as an office holder:
- •
- monetary liability incurred by the office holder in favor of another person according to judgment, including judgment following
settlement or arbitral proceeding.
- •
- reasonable litigation expenses, including attorneys' fees, incurred by the office holder as a result of an investigation or proceeding
instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that:
- •
- no indictment was filed against such office holder as a result of such investigation or proceeding; and
- •
- no financial liability, such as a criminal penalty, was imposed upon him or her as a substitute for the criminal
proceeding as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent or as a
monetary sanction; and
- •
- reasonable litigation expenses, including attorneys' fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the Company, on its behalf or by a third party or in connection with criminal proceedings in which the office holder was acquitted or as a result of a conviction for a crime that does not require proof of criminal intent.
An Israeli company may insure a director or officer against the following liabilities incurred for acts performed as a director or officer:
- •
- a breach of duty of care to the Company or to a third party, including a breach arising out of the negligent conduct of an office
holder;
- •
- a breach of duty of loyalty to the Company, provided the director or officer acted in good faith and had a reasonable basis to believe
that the act would not prejudice the interests of the Company; and
- •
- financial liabilities imposed on the office holder for the benefit of a third party.
An Israeli company may not, however, indemnify or insure an office holder against any of the following:
- •
- a breach of duty of loyalty, except to the extent that the office holder acted in good faith and had a reasonable basis to believe
that the act would not prejudice the interests of the Company;
- •
- a breach of duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office
holder;
- •
- an act or omission committed with intent to derive unlawful personal benefit; or
II-1
- •
- a fine, monetary sanction, penalty or forfeit levied against the office holder.
The Israeli Securities Law, provides that a company cannot obtain insurance against or indemnify a third party (including its officers and/or employees) for any administrative procedure conducted by the Israeli Securities Authority and/or monetary fine (other than for certain legal expenses and payments of damages to an injured party). The Israeli Securities Law permits insurance coverage and/or indemnification for certain liabilities incurred in connection with an administrative procedure, such as reasonable legal fees and certain compensation payable to injured parties for damages suffered by them, provided that such insurance and/or indemnification is permitted under the company's articles of association. Our articles of association contain such a provision.
Under the Israeli Companies Law, indemnification and insurance of office holders must be approved by our compensation committee, our board of directors and, in certain circumstances, also by our shareholders.
We have obtained directors' and officers' liability insurance for the benefit of our office holders and intend to continue to maintain such coverage and pay all premiums thereunder to the fullest extent permitted by the Israeli Companies Law, Securities Law and our articles of association. In addition, we have entered into indemnification and exculpation agreements with each of our directors and office holders providing them with indemnification for liabilities or expenses incurred as a result of acts performed by them in their capacity as our, or our subsidiaries', directors and officers. This indemnification is limited both in terms of amount and coverage. In the opinion of the SEC, however, indemnification of directors and office holders for liabilities arising under the Securities Act is against public policy and therefore unenforceable. In addition, we have entered into exculpation agreements with each of our directors and office holders providing them with exculpation from any liability for any negligent wrongdoing against us, as a result of acts performed by them in their capacity as our, or our subsidiaries', directors and officers.
The Israeli Securities Law provides that a company cannot obtain insurance against or indemnify a third party (including its officers and/or employees) for any administrative procedure conducted by the Israeli Securities Authority and/or monetary fine (other than for certain legal expenses and payments of damages to an injured party). The Israeli Securities Law permits insurance coverage and/or indemnification for certain liabilities incurred in connection with an administrative procedure, such as reasonable legal fees and certain compensation payable to injured parties for damages suffered by them, provided that such insurance and/or indemnification is permitted under the company's articles of association.
We have included in our amended and restated articles of association and in our compensation policy, applicable provisions with respect to directors' and officers' liability insurance for the benefit of our office holders, as well as with respect to indemnification and exculpation of office holders.
Item 7. Recent Sales of Unregistered Securities
Set forth below are the sales of all securities by the Company since January 1, 2012.
- •
- On March 15, 2012 we issued and sold 7,033,639 ordinary shares to our shareholders pursuant to a rights offering for aggregate
proceeds of NIS 3,225,000 ($829,000).
- •
- On November 29, 2012 we issued and sold 23,987,200 ordinary shares and options to purchase 11,993,600 ordinary shares
(Series E, which expired on December 31, 2014) and options to purchase 11,993,600 ordinary shares (Series F) at an exercise price of NIS 0.70 ($ 0.18) per share to the Israeli
public pursuant to a public offering, for aggregate proceeds of NIS 9,761,000 ($2,510,000). The distributors of such offering received a fee equal to 7% of aggregate proceeds, and were issued options
to purchase 1,199,360 ordinary shares (Series F), at an exercise price of NIS 0.70 ($0.18) per share.
- •
- On November 26, 2013 we issued and sold 16,856,173 ordinary shares to Trauwin Pte Ltd., pursuant to a private offering, for aggregate proceeds at NIS 8,040,000 ($2,067,000).
II-2
- •
- On December 18 and 24, 2013 we issued and sold 68,313,000 ordinary shares and options to purchase 75,144,300 ordinary shares
(Series F) at an exercise price of NIS 0.70 ($0.18) per share to Israeli institutional investors and the Israeli public pursuant to two consecutive public offerings, for aggregate proceeds of
NIS 19,357,000 ($4,977,000). The underwriters of such offerings received fees equal to 8% of such proceeds, and were issued options to purchase 6,831,300 ordinary shares (Series F), at an
exercise price of NIS 0.70 ($0.18) per share.
- •
- On January 9, 2014 we issued and sold 4,517,626 ordinary shares to Mr. Yehiel Tal, our chief executive officer, pursuant to his exercise of options, for aggregate proceeds of NIS 45,000 ($11,571).
The above-mentioned sale of securities were offered and sold for cash for the aggregate sum of approximately NIS 43.98 million ($11,308,819) (excluding consideration received for Mr. Tal's exercise of options of NIS 45,000 ($11,571)).
Item 8. Exhibits and Financial Statement Schedules.
The exhibit index attached hereto is incorporated herein by reference.
The undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
- (1)
- For
purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this
registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the
Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
- (2)
- For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-3
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Ness-Ziona, State of Israel, on , 2015.
|
COLLPLANT HOLDINGS LTD. |
|||||||||
By: |
|
By: |
|
|||||||
|
Name: | Yehiel Tal | Name: | Eran Rotem | ||||||
|
Title: | Chief Executive Officer | Title: | Chief Financial Officer | ||||||
The undersigned officers and directors of CollPlant Holdings Ltd. hereby constitute and appoint Yehiel Tal and Eran Rotem, and each of them singly, with full power of substitution, our true and lawful attorneys-in-fact and agents to take any actions to enable the Company to comply with the Securities Act, and any rules, regulations and requirements of the SEC, in connection with this registration statement on Form F-1, including the power and authority to sign for us in our names in the capacities indicated below any and all further amendments to this registration statement and any other registration statement filed pursuant to the provisions of Rule 462 under the Securities Act.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
Signatures
|
Title
|
Date
|
||
|---|---|---|---|---|
| Name: Yehiel Tal |
Chief Executive Officer (Principal Executive Officer) | |||
Name: Eran Rotem |
Chief Financial Officer (Principal Financial and Accounting Officer) |
|||
Name: Yaron Yaniv |
Chairman of the Board |
|||
Name: Orly Tori Trubowicz |
Director |
|||
Name: Xiaojin (Tony) Qian |
Director |
|||
Name: Rami Armon |
Director |
II-4
Signatures
|
Title
|
Date
|
||
|---|---|---|---|---|
| Name: Oded Shoseyov |
Director | |||
Name: Nira Dror |
Director |
|||
Name: Adi Goldin |
Director |
|||
Name: Ira Leiderman |
Director |
II-5
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the Securities Act of 1933, as amended, the undersigned, the duly authorized representative in the United States of CollPlant Holdings Ltd., has signed this registration statement on , 2015.
|
PUGLISI & ASSOCIATES | |||||
|
By: |
|||||
|
Name: | Donald J. Puglisi | ||||
|
Title: | Managing Director | ||||
II-6
| Exhibit Number |
Exhibit Description | ||
|---|---|---|---|
| 1.1* | Form of Underwriting Agreement by and among the Company and the underwriters named therein | ||
3.1* |
Memorandum of Association of the Company |
||
3.2* |
Amended and Restated Articles of Association of the Company, as currently in effect |
||
3.3* |
Articles of Association of the Company, to be in effect upon completion of this offering |
||
5.1* |
Opinion of Horn & Co. Law Offices, Israeli counsel to the Company, as to the validity of the ordinary shares being offered (including consent) |
||
10.1* |
Form of Indemnification Agreement and Form of Exculpation Agreement |
||
21.1* |
List of subsidiaries of the Registrant |
||
23.1* |
Consent of Kesselman & Kesselmen, Independent Registered Public Accounting Firm |
||
23.2* |
Consent of Horn & Co. Law Offices (included in Exhibit 5.1) |
||
24.1* |
Power of Attorney (included on the signature page of the Registration Statement) |
||
- *
- To be filed by amendment
II-7