UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
________________________________________
FORM
Commission file number
________________________________________
XBIOTECH INC.
(Exact name of Registrant as specified in its charter)
| | N/A | |
| (State or other jurisdiction of incorporation or organization) | (IRS Employer Identification No.) |
(Address of principal executive offices, including zip code)
Telephone Number (
(Registrant's telephone number, including area code)
________________________________________
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| | | |
Securities registered pursuant to Section 12(g) of the Act:
None
________________________________________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically, every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | | Smaller Reporting Company | |||
| Emerging Growth Company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant as of June 30, 2021, was approximately $
As of March 15, 2022,
________________________________________
Documents incorporated by reference:
Certain portions, as expressly described in this Annual Report on Form 10-K, of the registrant’s Proxy Statement for the 2021 Annual Meeting of the Stockholders, to be filed not later than 120 days after the end of the year covered by this Annual Report, are incorporated by reference into Part III of this Annual Report where indicated.
TABLE OF CONTENTS
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This annual report contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this annual report, including, without limitation, statements regarding the assumptions we make about our business and economic model, our dividend policy, business strategy and other plans and objectives for our future operations, are forward-looking statements.
These forward-looking statements include declarations regarding our management’s beliefs and current expectations. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “would,” “could,” “expects,” “plans,” “contemplate,” “anticipates,” “believes,” “estimates,” “predicts,” “projects,” “intend” or “continue” or the negative of such terms or other comparable terminology, although not all forward-looking statements contain these identifying words. Forward-looking statements are subject to inherent risks and uncertainties in predicting future results and conditions that could cause the actual results to differ materially from those projected in these forward-looking statements. Some, but not all, of the forward-looking statements contained in this annual report include, among other things, statements about the following:
| ● |
our ability to obtain regulatory approval to market and sell our product candidates in the United States, Europe and elsewhere; |
| ● |
the initiation, timing, cost, progress and success of our research and development programs, preclinical studies and clinical trials for our product candidates; |
| ● |
our ability to advance product candidates into, and successfully complete, clinical trials; |
| ● |
our ability to successfully commercialize the sale of our product candidates in the United States, Europe and elsewhere; |
| ● |
our ability to recruit sufficient numbers of patients for our future clinical trials for our pharmaceutical products; |
| ● |
our ability to achieve profitability; |
| ● |
the implementation of our business model and strategic plans; |
| ● |
our ability to develop and commercialize product candidates for orphan and niche indications independently; |
| ● |
our commercialization, marketing and manufacturing capabilities and strategy; |
| ● |
our ability to protect our intellectual property and operate our business without infringing upon the intellectual property rights of others; |
| ● |
our expectations regarding federal, state and foreign regulatory requirements; |
| ● |
the therapeutic benefits, effectiveness and safety of our product candidates; |
| ● |
the accuracy of our estimates of the size and characteristics of the markets that may be addressed by our products and product candidates; |
| ● |
the rate and degree of market acceptance and clinical utility of our future products, if any; |
| ● |
our expectations regarding market risk, including interest rate changes, foreign currency fluctuations and regional or global economic impacts caused by public health threats, such as the outbreak of coronavirus or other infectious diseases; |
| ● |
our ability to engage and retain the employees required to grow our business; |
| ● |
our future financial performance and projected expenditures; |
| ● |
developments relating to our competitors and our industry, including the success of competing therapies that are or become available; and |
| ● |
estimates of our expenses, future revenue, capital requirements and our needs for additional financing. |
You should also read the matters described in the “Risk Factors” and the other cautionary statements made in this annual report as being applicable to all related forward-looking statements wherever they appear in this annual report. We cannot assure you that the forward-looking statements in this annual report will prove to be accurate and therefore you are encouraged not to place undue reliance on forward-looking statements. You should read this annual report completely.
Overview
XBiotech Inc. (“XBiotech” or the “Company) is a biopharmaceutical company that discovers and develops True Human™ monoclonal antibodies for treating a variety of diseases. XBiotech was incorporated in Canada on March 22, 2005.
True Human™ monoclonal antibodies are derived from natural human immune responses—as opposed to being derived from animal immunization or otherwise engineered. XBiotech’s belief is that naturally occurring monoclonal antibodies have the potential to be safer, more effective and faster to develop than engineered counterparts. XBiotech has developed a pipeline of product candidates targeting both inflammatory and infectious diseases. The Company has also developed manufacturing technology that reduces the cost and time to launch new product candidates. Moreover, a state-of-the-art physical plant and infrastructure to manufacture product has been constructed and is operational at the Company’s 48 acre campus in Austin, Texas. XBiotech also has an in-house clinical operations group, with the capability to launch and manage clinical development of its candidate products. The Company thus represents a fully integrated developer of biopharmaceuticals.
An area of medical focus for XBiotech are therapies that block a potent substance naturally produced by body, known as interleukin-1 alpha (IL-1a), that mediates tissue breakdown, angiogenesis, the formation of blood clots, malaise, muscle wasting and inflammation. IL-1a is a protein that is on or in cells of the body and is involved in the body’s response to injury or trauma. In almost all chronic and in some acute injury scenarios (such as stroke or heart attack), IL-1a may mediate harmful disease-related activity.
XBiotech sold bermekimab, a True Human™ antibody that blocks IL-1a activity, for $1.35 billion in cash and potential milestone payments (the “Janssen Transaction”). As part of the Janssen Transaction, XBiotech agreed not to further develop any anti-IL-1a antibodies in dermatology. The Company is pursuing the development of other True Human™ antibodies targeting IL-1a for areas of medicine outside of dermatology. The Company has already identified new IL-1a targeting product candidates that it is has brought into clinical studies. While the Company previously was focused on a single True Human™ antibody targeting IL-1a, it is currently developing two product candidates, and may develop one or more others, that target IL-1a and are used in specific areas of medicine.
Financial
XBiotech received $675 million on December 30, 2019 and $75 million on June 30, 2021 from the sale of bermekimab. In February 2020, XBiotech used approximately $420 million to repurchase stock from its shareholders. In July 2021, XBiotech distributed $75 million cash dividend to its shareholders. The remaining cash was reserved for ongoing operations as part of its multi-year business plan to identify and develop True Human™ antibodies, including anti-Il-1a therapies, aiming for new potential transactions for these drug candidates.
Since January 1, 2020 XBiotech also used its proprietary manufacturing technology, its manufacturing plant and infrastructure to produce drug product for under a supply agreement with Janssen. In addition, during 2020 and 2021 XBiotech provided clinical trial operations services for two Phase II clinical studies. In 2022 XBiotech executed a new manufacturing supply agreement with a Janssen related company to extend its production of bermekimab. Revenue from contract manufacturing and clinical services has and continues to offset a significant portion of operating expenses. We believe that XBiotech is in a very favorable position for an R&D stage biopharmaceutical, with a strong cash position in its balance sheet related to sale of its drug candidate, the absence of debt, a robust pipeline and ongoing contract income.
Further Development of IL-1a Therapies
IL-1a plays a key role in disease. While it is produced naturally by the body, when not properly controlled, in situations of acute or chronic injury, IL-1a can contribute to the development and progression of a variety of medical conditions, such as cancer, stroke, heart attack or arthritis, to name a few. Completed clinical studies and a myriad of scientific research have shown that blocking IL-1a may have a beneficial effect in some or most of these medical conditions. The potential unmet medical need for blocking IL-1a is therefore very significant. In 2021, the Company entered the clinic with a molecule targeting IL-1⍺ in Oncology (Pancreatic Cancer). The company has also filed an INDs and started a clinical program in Rheumatology; and has filed IND for a different anti-IL-1⍺ antibody in cardiovascular medicine.
Because the potential medical uses for anti-IL-1a therapy is so large, the Company is developing more than one True Human™ antibody, each neutralizing IL-1a, but designating different antibodies for use in specific areas of medicine. This will potentially allow XBiotech to individually partner different antibodies according to unique medical areas. The Company expects this will diversify risk for anti-IL-1a therapies, allow multiple partnerships, maximize value and facilitate greater resource dedication to these True Human™ therapeutics.
Infectious Disease Pipeline
XBiotech continued to achieve significant milestones with its infectious disease pipeline in 2021. The Company has identified several major areas of urgent unmet medical need for True Human™ anti-infective antibody therapies. True Human™ antibodies may be used therapeutically or prophylactically to supplement immunity in aging individuals where a natural decline in the robustness of the immune system leaves gaps and vulnerability to infectious diseases. True Human™ antibodies derived from healthy individuals with strong natural immunity to specific diseases are the source of our product candidates, which we believe can be used to supplement the weakening immune system that occurs with age. The Company believes this can be a highly effective means for providing protection against viral diseases such as shingles or intestinal bacterial infections such as C. difficile.
True Human™ antibodies may also be used to provide highly potent and targeted immunity against infectious diseases in young otherwise healthy individuals where infectious agents have overwhelmed natural immunity. For example, this can occur during intravenous drug use, from a deep puncture wound, or from the result of surgery, where bacteria has gained unnatural entry into a body compartment where it can establish and evade the immune system. Staphylococcus aureus, including methicillin resistant (MRSA) variant, is an example of our candidate anti-infective therapeutics.
Another patient population where True Human™ antibodies may be particularly useful is in infants. Primary to developing strong immunity, infants can be vulnerable to infections. Particularly premature infants may need supplemental immunity against specific infectious agents, such as respiratory syncytial virus (RSV). In 2021, the Company continued making progress in its search for a True Human antibody therapeutic candidate for RSV.
In 2021, the Company also started antibody discovery projects to isolate neutralizing antibodies against Norovirus and Measles virus. Norovirus continues to cause outbreaks in the US over the years. According to the CDC report, during August 1, 2021 – January 8, 2022, there were 156 norovirus outbreaks reported by NoroSTAT-participating states. During the same period in 2020, there were 16 norovirus outbreaks reported by these states. A Norovirus therapeutic antibody is expected to decrease morbidity and mortality in high-risk populations. Similarly, measles outbreaks have occurred sporadically in the US due to the following reasons: an increase in the number of travelers who get measles abroad and bring it into the U.S., and further spread of measles in U.S. communities with pockets of unvaccinated people. Therefore a therapeutic candidate against measles continues to be an unmet need and is a part of the XBiotech R&D pipeline.
We believe True Human™ antibody therapies have a very important application in the case of potent viruses, like for example, influenza, where the aggressive nature of the virus takes even relatively strong immune systems to the limits. Here again, in elderly, the young or those with weakened immune systems, an aggressive virus like influenza can be life threatening. In 2020, XBiotech announced that it had developed an antibody cocktail that is capable of neutralizing all known forms of influenza— from the deadly 1918 strain to the most recent versions of flu.
In 2019, XBiotech completed the construction of a new infectious disease and animal facility laboratory. Located in a separate building on our campus, just a short walk from the Company’s main manufacturing headquarters, the new facility incorporates an animal biological safety level 2 (ABSL2) laboratory and other laboratories for in vitro and in vivo testing of the Company’s True Human™ antibodies against infectious disease targets.
The ABSL2 laboratory is currently being used to test XBiotech’s True Human™ antibodies derived from healthy persons with natural immunity to the varicella virus. Natural human immunity is very effective at neutralizing varicella for most of our lives, when we might have billions of different antibodies. Later in life, as we age, the quantity and diversity of our antibody repertoires diminishes. We produce less antibodies against fewer targets. Even though we may have had decades of antibodies protecting us against varicella, as we age a “hole” can develop in the antibody repertoire that was responsible for controlling varicella. When this happens, varicella virus that has been “hiding” in cells of our body can resurge, spread about the body and cause debilitating disease. XBiotech has isolated potent True Human™ antibodies from healthy donors with potent neutralizing antibodies against varicella. We believe that patients suffering from resurgent varicella due to aging immune systems may be effectively treated by supplementing with True Human™ antibody therapy against varicella. There is a substantial unmet medical need for shingles therapy; and other indications where uncontrolled varicella infections, such in infants whose immune systems are not yet adequately developed.
Infrastructure
During 2021 XBiotech undertook to expand its main integrated manufacturing and R&D center. The expansion of the main R&D center was ongoing throughout 2021 and is now complete. The expansion has resulted the creation of two new wings, one that provides laboratory research infrastructure for scientists and another general work areas for manufacturing, clinical personnel and others. The building additions have enhanced the Company’s ability to house a larger workforce, expand R&D activities and orchestrate the production of multiple drug products from its existing manufacturing and R&D center. XBiotech owns its 48-acre campus—and all structures on the property—debt-free.
Sale of Drug Candidate and Equity Transaction
The sale of the anti-IL-1a antibody provided sufficient capital to allow the Company to complete a modified Dutch auction tender offer in February 2020, in which the Company repurchased $420 million of its stock from shareholders. The tender offer provided liquidity to the Company’s shareholders while allowing the Company to consolidate its equity ownership and retain sufficient capital to continue pursuing its business objectives. Final results of the tender offer, announced on February 19, 2020, reported that the Company purchased 14,000,000 common shares at a price of $30.00 per share for an aggregate price of approximately $420 million (excluding fees and expenses related to the offer). The shares repurchased represented approximately 32.67% of the common shares outstanding. The final proration factor for shares that XBiotech purchased pursuant to the tender offer was ultimately deemed to be approximately 33.25%. No equity offerings were made during 2020. In July 2021, XBiotech distributed a cash dividend, in the aggregate amount of $75 million or approximately $2.50 per share, to its shareholders.
A Background on Therapeutic Antibodies
A century ago, scientists and physicians envisioned being able to custom design therapeutic "antibodies” that were highly specific for a single target. By selectively attacking disease while sparing healthy tissue, these “magic bullets” were anticipated to be ideal therapeutic agents. It was not until the early 1970’s, however, that this vision was realized when Kohler and Milstein developed a ground-breaking method for making target-specific monoclonal antibodies—a Nobel prize-winning endeavor. Using this new approach, numerous monoclonal antibody-based research, diagnostic, and therapeutic products developed.
Kohler and Milstein’s discovery was based on their knowledge that the immune system of higher animals produces antibodies as a method of protecting them from various harmful agents, such as viruses, bacteria, and diseased cells. White blood cells, known as B cells, produce billions of different types of antibodies, each with a unique potential to bind and neutralize different disease targets. The vast array of possible treatments based on antibodies led to the development of what is now a major industry around the use of therapeutic antibodies.
True Human™ Antibodies
White blood cells in the human body secrete billions of different antibodies that circulate through the blood to react and protect us from toxins, infectious agents or even other unwanted substances produced by our body. True Human™ antibodies, as the name implies, are simply those that are derived from a natural antibody identified from the blood of an individual. To develop a True Human™ antibody therapy, donors are screened to find an individual that has a specific antibody that matches the desired characteristics needed to obtain the intended medical benefit. White blood cells from that individual are obtained, the unique gene that produced the antibody is cloned, and the genetic information is used to produce an exact replica of the antibody sequence. A True Human™ antibody is, therefore, not to be confused with other marketed antibodies, such as so-called “fully human” antibodies—where antibody reactivity is developed through gene sequence engineering in the laboratory.
Fundamental Science of True Human™ Antibodies
To appreciate the background safety and tolerability of True Human™ antibodies, it is important to consider the fundamental biology of natural antibody production.
Billions of different white blood cells secrete billions of unique antibodies every day into circulation. The vast number of different antibodies (and cells that produce them), are essential to enable adequate molecular diversity to ward off a vast range of potential infectious or toxic threats. In other words, since antibodies act to bind and thereby neutralize unwanted agents, any given circulating antibody must be able to react with a potentially limitless number of existing or evolving disease entities.
The staggering number of different antibodies needed to achieve this level of preparedness, however, is a daunting concept from a genetics point of view. If an individual antibody gene was needed to encode each of a billion different antibodies, there would be approximately 20,000 times as many genes needed just for antibodies as there would be needed to encode the rest of the entire human genome. Individual cells would need to be gigantic, and monumental resources of the body would be required to make, copy and maintain all of the DNA. Clearly, the system of antibodies could not have evolved to protect us, had not an elegant solution emerged to deal with this genetic conundrum.
Thus, a hallmark of the immune physiology of all vertebrates (all have antibodies) is the ability to recombine and selectively mutate a relatively small number of gene segments to create a phenomenal and effectively unlimited number of antibody genes. By rearranging, recombining and mutating the genetic code, specialized white blood cells, or B lymphocytes, are able to create an unlimited array of antibody genes. The consequence of this genetic engineering, however, is that each antibody gene is unique to the individual B lymphocyte that created it—and no copy of the gene exists in the human germline. The only place to find a unique antibody gene is in the individual cells that created it.
The extraordinary process of gene rearrangement and mutation results in a multitude of unique B lymphocytes and consequently an incredibly diverse repertoire of antibodies in any given individual.
Elucidating the mechanisms behind the production of unique antibody genes must be considered one of the major achievements of medical research in the 20th century. Yet unfolding this mystery created another problem to solve: If antibodies were not produced from genes encoded in the human genome and the products of these genes were new to the body, why were these antibody molecules not recognized by the immune system as foreign substances—like any other foreign substance that they were intended to eradicate? How could the body distinguish the apparently “foreign” antibody molecules from the bona fide infectious intruders?
Unraveling the genetics of antibody production led to another major advance in medicine: the discovery of how an endless array of antibody proteins could be made in a way that individual molecules were always tolerated by the body.
In the early 1990s, research began to demonstrate that the production of antibodies was not an unregulated process. Rather, it was learned that the antibodies produced by each and every B lymphocyte were subject to intense scrutiny. Studies showed that B lymphocytes which produced acceptable antibodies were stimulated to grow while those that produced “autoreactive” antibodies were not. B lymphocytes that produced “good” antibodies were stimulated to proliferate and enabled to produce copious amounts of antibody in the event it was needed to ward off a harmful agent. B lymphocytes that rearranged genes to produce antibodies that were ineffective or were autoreactive were given signals that instructed them to engage in a process of programmed cell death. Thus, B lymphocytes producing harmful or useless antibodies are simply killed off. This mechanism for creating antibody diversity on the one hand, while protecting the individual from a mass of unwanted or intolerable antibody molecules on the other, was as elegant as it was fundamental to the success of vertebrate immune physiology.
This process of “selection” has been elucidated in great detail. There can be no more important feature of immune physiology than the process of selection. Selection is a fundamental step to enable the body to produce an extremely diverse set of antibody molecules without, in the process, producing an array of novel molecules that cause harm.
Industry Context
Until now each and every therapeutic antibody on the market has been derived from animals and/or through gene sequence modification in the laboratory to produce a desired antibody reactivity. Marketed antibodies to date, described as “fully human”, are not derived from human gene sequences that have undergone the crucial process of selection in a human.
Without exception, all marketed products to date that are described as “fully human”, are in fact engineered and are not selected based on natural tolerance in the human body. The use of the term “fully human” to describe these products has thus created considerable confusion. To our knowledge, there are at present no True Human™ antibodies manufactured, using recombinant protein technology, currently marketed.
Platform Technology
Our True Human™ antibody therapeutics are developed in-house using our proprietary discovery platform. There are significant technical challenges in identifying and cloning genes for True Human™ antibodies. A key problem to overcome can be to first identify individuals with the desired antibody reactivity. This can involve screening thousands of blood donors to enable the identification of a single, clinically relevant antibody—discovered from literally trillions of irrelevant background antibody molecules in the blood of donors. To distinguish the clinically relevant antibodies from irrelevant background antibody molecules in donor bloods, we use our Super High Stringency Antibody Mining (SHSAM™) technology. White blood cells from that individual can then be isolated, and the unique gene that produced the native antibody obtained. We currently obtain blood donor samples through a Research and Collaboration Agreement with the South Texas Blood & Tissue Center, a Texas 501(c)(3) non-profit corporation.
Novel cloning technologies developed at XBiotech have enabled us to clone the crucial antibody gene sequences from these donors in order to reproduce a True Human™ antibody for use in clinical therapy. A True Human™ monoclonal antibody should therefore not be confused with other marketed therapeutic monoclonal antibodies, such as those currently referred to as “fully human” antibodies.
Market Opportunity
We have a number of indications in various stages of clinical or pre-clinical development with significant market opportunities. These include an array of inflammatory conditions as well as infectious disease indications. The potential market opportunities in these various indications are vast and we believe our research and manufacturing technologies, designed to more rapidly, cost-effectively and flexibly produce new therapies, will be advantageous in each market space.
Our Strategy
Our objective is to fundamentally change the way drugs are developed and commercialized and become a leading biopharmaceutical company focused on the discovery, development and commercialization of therapeutic True Human™ antibodies. The key goals of our business strategy are to:
| • |
Advance our pipeline of therapeutic antibodies and initiate clinical programs in strategic therapeutic areas; |
| • |
Discover other True Human™ antibody therapies using our proprietary platform; and |
| • |
Leverage our manufacturing technology. |
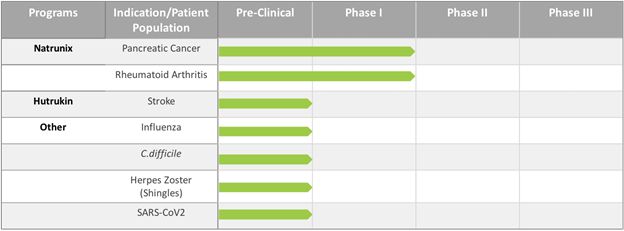
Product Pipeline
Our product development status for the end of the year 2021 was as follows:
Employees
As of December 31, 2021, we had 97 employees, all full-time employees.
Competition
The therapeutic antibody space is dynamic as there continues to be a highly active commercial pipeline of therapeutic antibodies globally, involving a complex array of development cycles as products reach the end of their patent life and as new candidate products proceed into pivotal studies and approach registration. There are numerous independent reviews on the subject in both trade journals and academic press (one such example being Reichert JM, Antibodies to watch in 2018 MAbs. 2018 Jan 4:1-21).
We believe True Human™ therapeutic antibodies have important differentiating factors from other monoclonal antibodies currently marketed. However, regardless of the potential advantages or uniqueness of our current or future product candidates in the market, we do expect these products to compete head-to-head with the numerous existing candidate antibody products in development, including emerging biosimilar therapeutic antibodies.
Safety
The Company’s True Human™ antibodies are derived from a natural human immune response. It is expected that this will facilitate better tolerability when used as a therapeutic compared to humanized or “fully human” monoclonal antibodies. Antibody therapies are known to be associated with significant risk for infusion reactions, including serious anaphylactic reactions. It is the Company’s belief that these reactions are, in large part, the result of using antibodies that were not derived from natural human immunity but rather had engineered specificities.
Intellectual Property
XBiotech has developed a large international intellectual property (IP) portfolio to protect important aspects of its technology, services, and products, including patents, trademarks and trade secrets.
Sale of a Portion of XBiotech’s IL-1a Patent Portfolio and Retention of Certain Rights Thereto.
On December 7, 2019, XBiotech entered into an Asset Purchase Agreement (the “Purchase Agreement”) under which it received $750 million upfront payment and the potential to receive another $600 million in milestone payments. Under the Purchase Agreement, XBiotech assigned a substantial portion of its patent portfolio covering the drug product candidate, bermekimab, that blocks the action of interleukin-1 alpha (IL-1a). Pursuant to the Purchase Agreement, on December 30, 2019, XBiotech assigned to the purchaser 12 patent families related to bermekimab and the use of IL-1α-specific antibodies for treating various conditions, including arthritis, neoplastic diseases, dermatological pathologies, vascular diseases, inflammatory skin disease and psychiatric conditions, cachexia, diabetes, atopic dermatitis and hidradenitis suppurativa.
An IP non-assertion and license agreement (the “IP Agreement”) incorporated into the Purchase Agreement allows XBiotech to develop and commercialize new antibody products which block IL-1a for all areas of medicine outside of dermatology without infringing the patent rights assigned under the Purchase Agreement. The IP Agreement further provides XBiotech the opportunity to enforce certain patent rights within the assigned portfolio that relate to the use of anti-IL-1a antibodies to treat non-dermatological disease against third parties in the event the purchaser elects not to do so.
Patent Rights Controlled by XBiotech.
As of December 31, 2021, XBiotech directly owns or controls through licenses the rights to several patent families. As set forth below, these include IL-1a related patent rights not assigned in the Purchase Agreement as well as others directed to our proprietary antibody discovery platform and to antibodies for treating and preventing S. aureus infections and cancer.
Patent Rights owned by XBiotech.
A. Treatment of Cancer with Anti-IL-1α Antibodies. This patent family relates to the use of anti-IL-1α antibodies to inhibit the metastatic potential of tumors by interrupting the role that tumor-derived IL-1α plays in tumor metastasis. As of December 31, 2021, XBiotech has been granted five patents for this family; including one in Australia, one in Canada, two in Japan, and one in Europe. As of December 31, 2021, this family has three pending patent applications (US, China, and Hong Kong). Unless extended, patents in this family expire in 2027.
B. Diagnosis, Treatment, and Prevention of Vascular Disorders. This patent family relates to methods of diagnosing, treating and preventing a variety of vascular disorder using IL-1a autoantibody. As of December 31, 2021, XBiotech has been granted seven patents in this family, including two in the U.S., one in Australia, one in Canada, one in Europe and two in Japan. Unless extended, patents in this family expire in 2026.
C. IL-1 Alpha Immunization Induces Autoantibodies Protective Against Atherosclerosis. This patent family relates to the use of IL-1α in a vaccine to generate anti-IL-1a antibodies to protect against atherosclerosis. As of December 31, 2021, XBiotech has been granted patents for this family in Australia and Europe. Unless extended, patents in this family expire in 2027.
D. Methods, Compositions, and Kits For Reducing Anti-Antibody Responses. This patent family relates to methods and compositions for reducing immune system-mediated reactions to allotypic determinants on administered antibody products. As of December 31, 2021, XBiotech has been granted one Australian patent in this family. Unless extended, patents in this family expire in 2030.
E. Identifying Affinity-Matured Human Antibodies. This patent family relates to methods and compositions for identifying affinity-matured True HumanTM monoclonal antibodies from donors. As of December 31, 2021, XBiotech has been granted 17 patents in this family (four in the U.S., two in Australia, one in China, one in Europe, one in India, , one in Japan, two in Israel, , one in South Korea, one in Mexico, two in Russia, and one in Hong Kong). As of December 31, 2021, this family has 3 pending patent applications (US, Canada, China, , Mexico, and Russia). Unless extended, patents in this family expire in 2032.
F. Compositions and Methods for Treating S. Aureus Infections. This patent family relates to antibodies for preventing and treating S. aureus infections. As of December 31, 2021, XBiotech has been granted 22 patents in this family, including five in the U.S, two in Australia, one in China, one in Colombia, one in Europe, one in Hong Kong, one in India, one in Indonesia, two in Japan, one in Mexico, one in the Philippines, one in Russia, one in Singapore, one in South Africa, and two in South Korea. As of December 31, 2021, this family has 17 pending patent applications (US, Brazil, Canada, China, Chile, Europe, India, Israel, Japan, Malaysia, Mexico, two in New Zealand, Philippines, Singapore, South Korea, and Russia) of which three (IL, RU, and PH) have been allowed. Unless extended, patents in this family expire in 2035.
G. Treatment of S. Aureus Infections. This patent family relates to the use of antibodies (Abs) which specifically bind interleukin-1α (IL-1α) for treating S. aureus bloodstream infections in human patients. As of December 31, 2021, XBiotech has one pending US application. Unless extended, patents in this family expire in 2038.
H. True Human Antibody Specific for Interleukin 1alpha (IL-1α). This patent family relates to fully human monoclonal Abs including an antigen-binding variable region that exhibits very high binding affinity for IL-1α. As of December 31 2021, XBiotech has one pending international patent application. Unless extended, patents in this family expire in 2041.
I. True Human Antibody Specific for Interleukin 1alpha (IL-1α). This patent family relates to fully human monoclonal Abs including an antigen-binding variable region that exhibits very high binding affinity for IL-1α. As of December 31 2021, XBiotech has one pending international patent application. Unless extended, patents in this family expire in 2041.
J. True Human Antibody Specific for Interleukin 1alpha (IL-1α). This patent family relates to fully human monoclonal Abs including an antigen-binding variable region that exhibits very high binding affinity for IL-1α. As of December 31 2021, XBiotech has one pending international patent application. Unless extended, patents in this family expire in 2041.
K. True Human Antibody Specific for Interleukin 1alpha (IL-1α). This patent family relates to fully human monoclonal Abs including an antigen-binding variable region that exhibits very high binding affinity for IL-1α. As of December 31 2021, XBiotech has one pending international patent application. Unless extended, patents in this family expire in 2041.
XBiotech has licensed exclusive rights to the intellectual property described below.
L. Monoclonal Human Tumor-Specific Antibody. This patent family relates generally to human tumor-specific antibodies as well as fragments, derivatives and variants thereof that recognize tumor-associated antigen NY-ESO-1. XBiotech acquired the use of patents within this family pursuant to its exclusive license agreement with CT Atlantic AG. As of December 31, 2021, this patent family includes 15 issued patents, including one in the U.S., one in Australia, one in Brazil, one in Canada, two in Europe, one in China, one in Israel, one in India, one in Japan, one in South Korea, one in New Zealand, one in Mexico, one in Russia, and one in South Africa. Unless extended, patents in this family expire in 2028.
M. Combination Therapy Including Tumor Associated Antigen-Binding Antibodies. This patent family relates generally to a combination therapy including tumor associated antigen binding antibodies. XBiotech acquired the use of patents within this family pursuant to its exclusive license agreement with CT Atlantic AG. As of December 31, 2021, this patent family includes pending applications in India and the U.S., and six issued patents, including one in Canada, one in China, one in Europe, one in India, one in Japan, and one in South Korea. Unless extended, patents in this family expire in 2032.
N. Treatment of Brain Ischemia-Reperfusion Injury. This patent family relates generally to reducing the sequelae of cerebral ischemia-reperfusion injury by administering to a subject an agent that selectively binds IL-1α. XBiotech and University of Zurich co-own this patent family. XBiotech acquired exclusive rights to this patent family pursuant to a license agreement with University of Zurich. As of December 31, 2021, this patent family includes seven pending patent applications (U.S., Australia, Canada, China, Europe, Japan, and South Korea). Unless extended, patents in this family expire in 2040.
Summary
The following summarizes some of the key risks and uncertainties that could materially adversely affect us. You should read this summary together with the more detailed description of each risk factor contained below.
Risks Related to our Business, Financial Condition and Capital Requirements
| ● |
We may incur significant losses during development of our current pipeline over the forseeable future. |
| ● |
Our business may be adversely affected by the ongoing COVID-19 pandemic. |
| ● |
We currently have limited source of revenue and may never sustain profitability. |
| ● |
Our future success is dependent on the regulatory approval and commercialization of our product candidates. |
| ● |
New laws or regulations could impact our ability to receive the necessary approvals to successfully market and commercialize our product candidates. |
| ● |
Product candidates we advance into clinical trials may not have favorable results in clinical trials or receive regulatory approval. |
| ● |
If we are unable to enroll subjects in clinical trials, we will be unable to complete these trials on a timely basis. |
| ● |
The regulatory approval processes of the FDA and comparable foreign regulatory authorities are lengthy, time consuming and inherently unpredictable. |
| ● |
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following any marketing approval. |
| ● |
Any product candidates that we commercialize may not receive coverage and adequate reimbursement from third-party payers. |
| ● |
If we are unable to establish an effective sales force and marketing infrastructure, or enter into acceptable third-party sales and marketing or licensing arrangements, we may be unable to generate any revenue. |
| ● |
Any approved product candidates may not achieve adequate market acceptance for commercial success. |
| ● |
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do. |
| ● |
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop. |
| ● |
Any failure to meet our ongoing obligations to manufacture bermekimab for Janssen would adversely affect our financial condition and business reputation. |
| ● |
We may never achieve any of the potential milestone payments that were negotiated as a part of the Janssen Transaction. |
| ● |
We are highly dependent on our Chief Executive Officer. |
| ● |
We depend on key personnel to operate our business, and we may be unable to retain, attract and integrate qualified personnel. |
| ● |
Failure to comply with environmental, health and safety laws and regulations could subject us to fines, penalties or other costs. |
| ● |
Our business may be disrupted by natural disasters, infrastructure interruptions, COVID-19 or other public health threats. |
Risks Related to Intellectual Property
| ● |
We may be unable to obtain or protect intellectual property rights. |
| ● |
Intellectual property rights do not necessarily address all potential threats to any competitive advantage we may have. |
| ● |
Our technology may be found to infringe upon third-party intellectual property rights. |
| ● |
We may be unable to license needed intellectual property from third parties on commercially reasonable terms or at all. |
| ● |
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position may be harmed. |
Risks Related to Owning Shares of our Common Stock
| ● |
Our share price may be volatile, which could subject us to securities class action lawsuits and prevent you from being able to sell your shares at or above the price at which you purchased them. |
| ● |
Our directors, executive officers and principal shareholders continue to have substantial control over our company and could hinder appropriate corporate control. |
| ● |
We have broad discretion in the use of the net proceeds from the Janssen Transaction and may not use them effectively. |
| ● |
Provisions in our charter documents under Canadian law could make an acquisition of us, which may be beneficial to our shareholders, more difficult. |
| ● |
Against the judgment of the Company, we may be considered a passive foreign investment company for US tax purposes which may negatively affect US investors. |
| ● |
We are governed by the corporate laws in British Columbia, Canada which in some cases have a different effect on shareholders than the corporate laws in Delaware. |
General Risk Factors
| ● |
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights to our technologies or product candidates. |
| ● |
Future sales, or the possibility of future sales, of a substantial number of our common stock could adversely affect the price of the shares and dilute shareholders. |
| ● |
Any inability to accurately report our financial results or prevent fraud due to a failure to maintain effective internal control over financial reporting could cause shareholders to lose confidence in our financial and other public reporting. |
Risks Related to our Business, Financial Condition and Capital Requirements
We have incurred significant losses since our inception and may incur significant losses in the future.
We are a pre-market pharmaceutical company with a limited operating history. We had no net income prior to the fourth quarter of 2019, when we sold certain assets to Janssen Biotech, Inc. and entered into certain related commercial agreements (the “Janssen Transaction”). Investment in pharmaceutical product development is highly speculative because it entails substantial upfront capital expenditures and significant risk that any potential product candidate will fail to demonstrate adequate efficacy or an acceptable safety profile, gain regulatory approval or become commercially viable. We do not have any products approved by regulatory authorities for marketing or commercial sale and have not generated any revenue from product sales, or otherwise, to date, and we continue to incur significant research, development and other expenses related to our ongoing operations. As a result, we incurred losses in every reporting period from our inception in 2005 through the third quarter of 2019. Although we were profitable during the fourth quarter and fiscal year ended December 31, 2019 due to the cash received in the Janssen Transaction, that was an extraordinary transaction outside of normal business operations that had never previously occurred and may not be repeated. We incurred a net loss for the fiscal year ended December 31, 2021.
We expect to continue to incur significant expenses and may incur operating losses for the foreseeable future. We anticipate these expenses will increase as we continue the research and development of, and seek regulatory approvals for our current and future product candidates in various indications, and potentially begin to commercialize any products that may achieve regulatory approval. We may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our financial condition. The amount of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues. Our prior losses have had, and any future losses may continue to have, an adverse effect on our financial condition. If any of our product candidates fail in clinical trials or do not gain regulatory approval, or if approved fail to achieve market acceptance, we may never sustain profitability.
Since inception, we have dedicated a majority of our resources to the discovery and development of our proprietary preclinical and clinical product candidates, and we expect to continue to expend substantial resources doing so for the foreseeable future. These expenditures will include costs associated with conducting research and development, manufacturing product candidates and products approved for sale, conducting preclinical experiments and clinical trials and obtaining and maintaining regulatory approvals, as well as commercializing any products later approved for sale. During the year ending December 31, 2021, we recognized approximately $28.3 million in expenses associated with research and development.
We completed our initial public offering on April 15, 2015 and additional registered offerings in March 2017 and May 2019. We also received a significant amount of cash proceeds from the Janssen Transaction. However, the net proceeds from these transactions and cash on hand may not be sufficient to complete clinical development of any of our product candidates nor may it be sufficient to commercialize any product candidate. In addition, we completed a modified Dutch auction tender offer for our common shares in February 2020, which consumed $420 million of our cash resources. We also distributed $75 million cash dividend to our investors in July 2021. Accordingly, we may require substantial additional capital to continue our clinical development and potential commercialization activities. Our future capital requirements depend on many factors, including but not limited to:
| • |
the number and characteristics of the future product candidates we pursue; |
| • |
the scope, progress, results and costs of researching and developing any of our future product candidates, and conducting preclinical research and clinical trials; |
| • |
the timing of, and the costs involved in, obtaining regulatory approvals for any future product candidates we develop; |
| • |
the cost of future commercialization activities for our product candidates and the cost of commercializing any future products approved for sale; |
| • |
the cost of manufacturing our future products; and |
| • |
the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patents, including litigation costs and the outcome of any such litigation. |
We are unable to accurately estimate the funds we will actually require to complete research and development of our product candidates or the funds required to commercialize any resulting product in the future or the funds that will be required to meet other expenses. Our operating plan may change as a result of many factors currently unknown to us, and our expenses may be higher than expected. Raising funds in the future may present additional challenges and future financing may not be available in sufficient amounts or on terms acceptable to us, if at all.
Our business may be adversely affected by the ongoing COVID-19 pandemic.
Beginning in late 2019, the outbreak of COVID-19 has evolved into a global pandemic that is disrupting our business operations, which we expect to continue throughout the remainder of 2022 and possibly beyond. Depending upon the length and severity of the pandemic, which cannot be predicted, we may continue to experience disruptions that could materially and adversely impact our business including:
| • |
If any third parties in our supply chain are or continue to be adversely impacted by restrictions resulting from the COVID-19 pandemic, including staffing shortages, production slowdowns, or disruptions in freight and other transportation services and delivery distribution systems, our supply chain may be disrupted, which would limit our ability to manufacture our product candidates for our clinical trials, to meet our obligations to manufacture drugs for Janssen under our clinical manufacturing agreement, or to conduct our research, development and clinical operations. |
| • |
The pandemic has already affected and may continue to affect our obligations and performance under our agreements with Janssen. We cannot predict the likely potential adverse impact of COVID-19 on Janssen’s future purchase orders or our ability to complete the manufacturing required by those purchase orders. |
| • |
Various aspects of our clinical trials could be limited or take longer than expected, including delays or difficulties in enrolling patients in our clinical trials, in clinical trial site initiation, and in recruiting clinical site investigators and clinical site staff; increased rates of patients withdrawing from clinical trials; diversion of healthcare resources away from the conduct of clinical trials; interruption of key clinical trial activities such as clinical trials site data monitoring due to limitations on travel imposed or recommended by governmental authorities; impact on employees and others or interruption of clinical trial visits or study procedures which may impact the integrity of subject data and clinical study endpoints; and interruption or delays in the operations of the FDA and comparable foreign regulatory agencies, which may impact regulatory review and approval timelines. |
| • |
The FDA and comparable foreign regulatory agencies may experience disruptions, have slower response times or be under-resourced to continue to monitor our clinical trials or to conduct required activities and review of our product candidates seeking regulatory review, or may prioritize review and approval of COVID-19 treatments and vaccines over other product candidates, and such disruptions could materially affect the development, timing and approval of our product candidates. |
| • |
As a result of market volatility caused by continued effects of COVID-19 affecting the global economy, we may face difficulties raising capital through sales of our common stock or other securities at acceptable prices, on acceptable terms or at all. |
The COVID-19 pandemic continues to evolve. The ultimate impact of the pandemic on us is highly uncertain and subject to change and will depend on future developments, which cannot be accurately predicted. We do not yet know the full extent of potential delays or impacts on our business, our clinical trials, our research programs, our clinical manufacturing or clinical trial management agreements, the healthcare system or the global economy. Given the uncertainties, we may be unable to maintain operations as planned prior to the COVID-19 pandemic.
We currently have no source of product revenue and may never sustain profitability.
To date, we have not generated any revenues from commercial product sales. Our ability to generate revenue in the future from product sales and achieve profitability will depend upon our ability, alone or with any future collaborators, to commercialize products successfully, including any current product candidates or any product candidates that we may develop, in-license or acquire in the future. Even if we are able to achieve regulatory approval for any current or future product candidates, we do not know when any of these products will generate revenue from product sales, if at all. Our ability to generate revenue from product sales from any of our product candidates also depends on a number of additional factors, including our ability to:
| • |
complete development activities, including the necessary clinical trials; |
| • |
complete and submit new drug applications, or NDAs, to the US Food and Drug Administration, or FDA, and obtain regulatory approval for indications for which there is a commercial market; |
| • |
complete and submit applications to, and obtain regulatory approval from, foreign regulatory authorities such as the European Medicines Agency, or EMA; |
| • |
establish our manufacturing operations; |
| • |
develop a commercial organization capable of sales, marketing and distribution for our product candidates and any products for which we obtain marketing approval and intend to sell ourselves in the markets in which we choose to commercialize on our own; |
| • |
find suitable distribution partners to help us market, sell and distribute our approved products in other markets; |
| • |
obtain coverage and adequate reimbursement from third-party payers, including government and private payers; |
| • |
achieve market acceptance for our products, if any; |
| • |
establish, maintain and protect our intellectual property rights; and |
| • |
attract, hire and retain qualified personnel. |
In addition, because of the numerous risks and uncertainties associated with pharmaceutical product development, including that our product candidates may not advance through development or achieve the endpoints of applicable clinical trials, we are unable to predict the timing or amount of increased expenses, or if we will be able to sustain profitability. In addition, our expenses could increase beyond expectations if we decide to or are required by the FDA, or foreign regulatory authorities, to perform studies or trials in addition to those that we currently anticipate. Even if we are able to complete the development and regulatory process for our product candidates, we anticipate incurring significant costs associated with commercializing these products.
Even if we are able to generate revenues from the sale of any of our product candidates that may be approved, we may not become profitable and may need to obtain additional funding to continue operations. If we are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce our operations.
Our future success is dependent on the regulatory approval and commercialization of our product candidates.
We do not have any products that have gained regulatory approval. As a result, our ability to finance our operations and generate revenue, are substantially dependent on our ability to obtain regulatory approval for, and, if approved, to successfully commercialize our product candidates in a timely manner. We cannot commercialize our other product candidates in the U.S. without first obtaining regulatory approval for each product from the FDA; similarly, we cannot commercialize any product candidates outside of the U.S. without obtaining regulatory approval from comparable foreign regulatory authorities, including the EMA. The FDA review process typically takes years to complete and approval is never guaranteed. Before obtaining regulatory approvals for the commercial sale of any of our potential product candidates for a target indication, we must demonstrate with substantial evidence gathered in preclinical and well-controlled clinical studies, including two well-controlled Phase III studies, and, with respect to approval in the U.S. to the satisfaction of the FDA, and in Europe, to the satisfaction of the EMA, that the product candidate is safe and effective for use for that target indication; and that the manufacturing facilities, processes and controls are adequate. Obtaining regulatory approval for marketing of our current or future product candidates in one country does not ensure we will be able to obtain regulatory approval in other countries. A failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in other countries.
Even if any of our product candidates were to successfully obtain approval from the FDA or comparable foreign regulatory authorities, any approval might contain significant limitations related to use restrictions for specified age groups, warnings, precautions or contraindications, or may be subject to burdensome post-approval studies or risk management requirements. If we are unable to obtain regulatory approval for our product candidates in one or more jurisdictions, or any approval contains significant limitations, we may not be able to obtain sufficient funding or generate sufficient revenue to continue the development of any of our other product candidates that we are developing or may discover, in-license, develop or acquire in the future. Also, any regulatory approval of our product candidates, once obtained, may be withdrawn. Furthermore, even if we obtain regulatory approval for any of our product candidates, their commercial success will depend on a number of factors, including the following:
| • |
development of a commercial organization within XBiotech or establishment of a commercial collaboration with a commercial infrastructure; |
| • |
establishment of commercially viable pricing and obtaining approval for adequate reimbursement from third-party and government payers; |
| • |
our ability to manufacture quantities of our product candidates using commercially satisfactory processes and at a scale sufficient to meet anticipated demand and enable us to reduce our cost of manufacturing; |
| • |
our success in educating physicians and patients about the benefits, administration and use of our product candidates; |
| • |
the availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing treatments; |
| • |
the effectiveness of our own or our potential strategic collaborators’ marketing, sales and distribution strategy and operations; |
| • |
acceptance as a safe and effective therapy by patients and the medical community; and |
| • |
a continued acceptable safety profile following approval. |
Many of these factors are beyond our control. If we are unable to successfully commercialize our product candidates, we may not be able to earn sufficient revenues to continue our business.
New laws or regulations may be promulgated or modified in the United States, in Europe, or other jurisdictions that could impact our ability to receive the necessary approvals to successfully market and commercialize our product candidates.
The pharmaceutical and biotechnology industry is one of the most regulated on a state, federal and international level. There are a number of laws, regulations, and court decisions which impact the daily activities of our business. As a result, we must ensure that strategies and planning in relation to our product candidates are in line with the current regulations governing our industry. When there are changes in leadership, whether within the U.S., or elsewhere, we must anticipate the possibility of shifts in regulatory policies as they pertain to our business. New or modified regulations may impact our ability to quickly respond with updates to our programs. While we may be able to anticipate certain changes, policy statements often are not always translated into actionable legislation. We continue to track updates and changes internally to ensure we are in compliance with regulatory authority guidelines and expectations. Court decisions at both the state and federal level can also impact the way in which we operate and make specific product related program decisions. New laws, regulations, or court orders could materially alter or impact our ability to receive necessary approvals from regulatory authorities to market and commercialize our product candidates.
Because the results of earlier clinical trials are not necessarily predictive of future results, product candidates we advance into clinical trials, may not have favorable results in later clinical trials or receive regulatory approval.
Success in preclinical testing and early clinical trials does not ensure that later clinical trials will generate adequate data to demonstrate the efficacy and safety of an investigational drug. A number of companies in the pharmaceutical and biotechnology industries, including those with greater resources and experience, have suffered significant setbacks in clinical trials, even after seeing promising results in earlier clinical trials. We do not know whether the clinical trials we are conducting, or may conduct, will demonstrate adequate efficacy and safety to result in regulatory approval to market any of our product candidates in any particular jurisdiction. Even if we believe that we have adequate data to support an application for regulatory approval to market our product candidates, the FDA or other comparable foreign regulatory authorities may not agree and could require us to conduct additional research studies, including late-stage clinical trials. If late-stage clinical trials do not produce favorable results, our ability to achieve regulatory approval for any of our product candidates may be adversely impacted.
If we are unable to enroll subjects in clinical trials, we will be unable to complete these trials on a timely basis.
Patient enrollment, a significant factor in the timing of clinical trials, is affected by many factors including the size and nature of the patient population, the proximity of subjects to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, ability to obtain and maintain patient consents, risk that enrolled subjects will drop out before completion, competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating. Furthermore, we rely on clinical trial sites to ensure the proper and timely conduct of our clinical trials, and while we have agreements governing their committed activities, we have limited influence over their actual, day-to-day performance. We may experience delays in starting-up clinical trial sites in a timely manner, enrolling subjects in our trials, and may not be able to enroll a sufficient number of subjects to complete the trials. In addition, travel restrictions, shutdowns of or occupancy limitations on certain businesses, bans or restrictions on large public gatherings and declarations of states of emergency remain in effect in some cities, states and countries around the world in response to the ongoing COVID-19 pandemic. Spikes in the numbers of infected patients, the rise and spread of new COVID-19 variants, delays in vaccine distribution or administration, adverse reactions to existing or future COVID-19 vaccines or future similar regional or global health concerns could negatively affect our ability to recruit and retain subjects in clinical trials if they disproportionately impact the sites in which we conduct any of our trials, which would have a material adverse effect on our business and our results of operation and financial condition.
If we experience delays in the completion or if there is termination of, any clinical trial of any current or future product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of these product candidates will be delayed. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, and jeopardize our ability to commence product sales, which would impair our ability to generate revenues and may harm our business, results of operations, financial condition and cash flows and future prospects. In addition, many of the factors that could cause a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
The regulatory approval processes of the FDA and comparable foreign regulatory authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business may fail.
The time required to obtain approval by the FDA and comparable foreign regulatory authorities is unpredictable, but typically takes several years following the commencement of preclinical studies and clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities and any shifts in regulatory policy. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate, and it is possible that none of the product candidates we are developing or may discover, in-license or acquire and seek to develop in the future will ever obtain regulatory approval.
Our product candidates could fail to receive marketing approval from the FDA or a comparable foreign regulatory authority for many reasons, including but not limited to:
| • |
disagreement over the design or implementation of our clinical trials; |
| • |
failure to demonstrate that a product candidate is safe and effective; |
| • |
failure of clinical trials to meet the level of statistical significance required for approval; |
| • |
failure to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| • |
disagreement over our interpretation of data from preclinical studies or clinical trials; |
| • |
disagreement over whether to accept efficacy results from clinical trial sites outside the United States where the standard of care is potentially different from that in the United States; |
| • |
the insufficiency of data collected from clinical trials of our product candidates to support the submission and filing of an NDA or other submission or to obtain regulatory approval; |
| • |
irreparable or critical compliance issues relating to our manufacturing and/clinical trial processes; or |
| • |
changes in the approval policies or regulations that render our preclinical and clinical data insufficient for approval. |
The FDA or a comparable foreign regulatory authority may require more information, including additional preclinical or clinical data to support approval, which may delay or prevent approval and our commercialization plans, or we may decide to abandon the development program altogether. Even if we do obtain regulatory approval, our product candidates may be approved for fewer or more limited indications than we request, approved contingent on the performance of costly post-marketing clinical trials, or approved with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. In addition, if any of our product candidates produce undesirable side effects or safety issues, the FDA may require the establishment of Risk Evaluation Mitigation Strategies, or REMS, or a comparable foreign regulatory authority may require the establishment of a similar strategy, that may, restrict distribution of our products and impose burdensome implementation requirements. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates.
Even if we believe any completed, current or planned clinical trials are successful, the FDA or a comparable foreign regulatory authority may not agree that our completed clinical trials provide adequate data on the safety or efficacy of our product candidates, permitting us to proceed to additional clinical trials. Approval by comparable foreign regulatory authorities does not ensure approval by the FDA and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative impact on the regulatory process in others. We may not be able to file for regulatory approvals, and even if we file, we may not receive the necessary approvals to commercialize our products in any market.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following any marketing approval.
Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authority. If toxicities occur in our current or future clinical trials they could cause delay or even the discontinuation of further development of our product candidates, which would impair our ability to generate revenues and would have a material adverse effect our business, results of operations, financial condition and cash flows and future prospects. There can be no assurance that side effects from our product candidates in future clinical trials or that side effects in general will not prompt the discontinued development or possible market approval of our product candidates. If serious side effects or other safety or toxicity issues are experienced in our clinical trials in the future, we may not receive approval to market any of our product candidates, which could prevent us from ever generating revenues from commercial product sales or sustaining profitability. Results of our trials could reveal an unacceptably high severity and prevalence of side effects. In such an event, our trials could be suspended or terminated and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our product candidates for any or all targeted indications. The drug-related side effects could affect patient recruitment or the ability of enrolled subjects to complete the trial or result in potential product liability claims. Any of these occurrences may have a material adverse effect on our business, results of operations, financial condition and cash flows and future prospects.
Additionally, if any of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused by such product, a number of potentially significant negative consequences could result, including:
| • |
we may be forced to suspend marketing of such product; |
| • |
regulatory authorities may withdraw their approvals of such product; |
| • |
regulatory authorities may require additional warnings on the label that could diminish the usage or otherwise limit the commercial success of such product; |
| • |
the FDA or other regulatory bodies may issue safety alerts, Dear Healthcare Provider letters, press releases or other communications containing warnings about such product; |
| • |
the FDA may require the establishment or modification of REMS or a comparable foreign regulatory authority may require the establishment or modification of a similar strategy that may, for instance, restrict distribution of our product and impose burdensome implementation requirements on us; |
| • |
we may be required to change the way the product is administered or conduct additional clinical trials; |
| • |
we could be sued and held liable for harm caused to subjects or patients; |
| • |
we may be subject to litigation or product liability claims; and |
| • |
our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved.
Even if our product candidates receive regulatory approval, they may still face future challenges, including ongoing regulatory oversight and marketing challenges.
Even if we obtain regulatory approval for any of our product candidates, it would be subject to ongoing requirements by the FDA and comparable foreign regulatory authorities governing the manufacture, quality control, further development, labeling, packaging, storage, distribution, safety surveillance, import, export, advertising, promotion, recordkeeping and reporting of safety and other post-market information. The safety profile of any product will continue to be closely monitored by the FDA and comparable foreign regulatory authorities after approval. If the FDA or comparable foreign regulatory authorities become aware of new safety information after approval of any product candidate, they may require labeling changes or establishment of a REMS or similar strategy, impose significant restrictions on a product’s indicated uses or marketing, or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance. For example, the label ultimately approved for any product candidate, if it achieves marketing approval, may include restrictions on use.
In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with current good manufacturing practices, or cGMP, and other regulations. If we or a regulatory agency discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions on that product, our manufacturing facility, including requiring recall or withdrawal of the product from the market or suspension of manufacturing. If we, our product candidates or our manufacturing facilities for our product candidates fail to comply with applicable regulatory requirements, a regulatory agency may:
| • |
issue warning letters or untitled letters; |
| • |
impose restrictions on the marketing or manufacturing of the product candidates; |
| • |
mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners; |
| • |
require us or any future collaborator to enter into a consent decree, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance; |
| • |
seek an injunction or impose civil or criminal penalties or monetary fines; |
| • |
suspend or withdraw regulatory approval; |
| • |
suspend any ongoing clinical trials; |
| • |
refuse to approve pending applications or supplements to applications filed by us; |
| • |
suspend or impose restrictions on operations, including costly new manufacturing requirements; or |
| • |
seize or detain products, refuse to permit the import or export of products, or require us to initiate a product recall. |
The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and generate revenue.
The FDA strictly regulates the advertising and promotion of drug products, and drug products may only be marketed or promoted for their FDA approved uses, consistent with the product’s approved labeling. Advertising and promotion of any product candidate that obtains approval in the U.S., and is covered by federal insurance programs such as Medicare or Medicaid, will be heavily scrutinized by the FDA, the Department of Justice, (DOJ), the Office of Inspector General of the Department of Health and Human Services, (HHS), state attorneys general, members of Congress and the public. Violations, including promotion of our products for unapproved or off-label uses, are subject to enforcement letters, inquiries and investigations, and civil, criminal and/or administrative sanctions by the FDA and/or the DOJ. Additionally, advertising and promotion of, any product candidate that obtains approval outside of the U.S. will be heavily scrutinized by comparable foreign regulatory authorities.
In the U.S., engaging in impermissible promotion of our future products for off-label uses can also subject us to false claims litigation under federal and state statutes, which can lead to civil, criminal and/or administrative penalties and fines and corporate integrity agreements that materially restrict the manner in which we promote or distribute our drug products. The federal False Claims Act, allows any individual to bring a lawsuit against a pharmaceutical company on behalf of the federal government alleging submission of false or fraudulent claims, or causing to present such false or fraudulent claims, for payment by a federal program, such as Medicare or Medicaid. If the government prevails in the lawsuit, the individual may share in any fines or settlement funds. Since 2004, False Claims Act lawsuits against pharmaceutical companies have increased significantly in volume and breadth, leading to several substantial civil and criminal settlements based on certain sales practices promoting off-label drug uses. This growth in litigation has increased the risk that a pharmaceutical company will have to defend a false claims action, pay settlement fines or restitution, agree to comply with burdensome reporting and compliance obligations, and be excluded from Medicare, Medicaid and other federal and state healthcare programs. If we do not lawfully promote our approved products, we may become subject to such litigation and, if we are not successful in defending against such actions, those actions could have a material adverse effect on our business, results of operations, financial condition and cash flows and future prospects.
Existing government regulations may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and/or be subject to fines or enhanced government oversight and reporting obligations, which would adversely affect our business, prospects and ability to sustain profitability.
Failure to obtain regulatory approval in foreign jurisdictions would prevent our product candidates from being marketed in those jurisdictions.
In order to market and sell our products in the European Union and many other jurisdictions, we must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the U.S. generally includes all of the risks associated with obtaining FDA approval. Additionally, in many countries outside the U.S., it is required that the product be approved for reimbursement before the product can be effectively commercialized in that country. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. We may not obtain approvals from regulatory authorities outside the U.S. on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the U.S. does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. A failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory approval process in others. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market. If we are unable to obtain approval of any of our product candidates by regulatory authorities in the European Union or another jurisdiction, the commercial prospects of that product candidate may be significantly diminished and our business prospects could decline.
Even if we are able to commercialize our product candidates, the products may not receive coverage and adequate reimbursement from third-party payers, which could harm our business.
Our ability to commercialize any products successfully will depend, in part, on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from government authorities, private health insurers, health maintenance organizations and third-party payers. Patients who are prescribed medications for the treatment of their conditions generally rely on third-party payers to reimburse all or part of the costs associated with their prescription drugs. Coverage and adequate reimbursement from government healthcare programs, such as Medicare and Medicaid, and private health insurers are critical to new product acceptance. Patients are unlikely to use our product candidates unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our product candidates. A primary trend in the US healthcare industry and elsewhere is cost containment. As a result, government authorities and other third-party payers have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payers are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. Third-party payers may also seek additional clinical evidence, beyond the data required to obtain marketing approval, demonstrating clinical benefits and value in specific patient populations before covering our products for those patients. We cannot be sure that coverage and adequate reimbursement will be available for any product that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Coverage and reimbursement may impact the demand for, or the price of, any product candidate for which we obtain marketing approval. If coverage and reimbursement are not available or are available only at limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval.
There may be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or comparable foreign regulatory authorities. Moreover, obtaining coverage does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sales and distribution. Interim reimbursement levels for new drugs, if applicable, may also be insufficient to cover our costs, and may only be temporary. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used. Reimbursement rates may also be based in part on existing reimbursement amounts for lower cost drugs or may be bundled into the payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payers and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. Coverage and reimbursement for drug products can differ significantly from payer to payer. As a result, the coverage and reimbursement determination process is often a time-consuming and costly process with no assurance that coverage and adequate reimbursement will be obtained or applied consistently. Third-party payers often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies. Our inability to promptly obtain coverage and profitable reimbursement rates from both government-funded and private payers for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products, and our overall financial condition.
We have never marketed a drug before, and if we are unable to establish an effective sales force and marketing infrastructure, or enter into acceptable third-party sales and marketing or licensing arrangements, we may be unable to generate any revenue.
We do not currently have a comprehensive infrastructure for the sales, marketing and distribution of pharmaceutical drug products. The cost of establishing and maintaining such an infrastructure may exceed the cost-effectiveness of doing so. In order to market any products that may be approved by the FDA and comparable foreign regulatory authorities, we must build our sales, marketing, managerial and other non-technical capabilities or make arrangements with third parties to perform these services for which we would incur substantial costs. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, we may not be able to generate product revenue and may not sustain profitability. We will be competing with many companies that have extensive and well-funded sales and marketing operations. Without an internal commercial organization or the support of a third party to perform sales and marketing functions, or a combination of both, we may be unable to compete successfully against more established companies.
Our product candidates, if approved, may not achieve adequate market acceptance among physicians, patients, and healthcare payers and others in the medical community necessary for commercial success.
Even if we obtain regulatory approval for any of our product candidates, such product(s) may not gain market acceptance among physicians, healthcare payers, patients or the medical community within the U.S. or globally. Our commercial success also depends on coverage and adequate reimbursement of our product candidates by third-party payers, including government payers, generally, which may be difficult or time-consuming to obtain, may be limited in scope and may not be obtained in all jurisdictions in which we may seek to market our products. Market acceptance of any of our product candidates for which we receive approval depends on a number of factors, including:
| • |
the efficacy and safety of such product candidates as demonstrated in clinical trials; |
| • |
the clinical indications for which the product candidate is approved; |
| • |
acceptance by physicians and patients of the product candidate as a safe and effective treatment; |
| • |
the potential and perceived advantages of product candidates over alternative treatments; |
| • |
the safety of product candidates seen in a broader patient group, including a product candidate’s use outside the approved indications; |
| • |
the prevalence and severity of any side effects; |
| • |
product labeling or product insert requirements of the FDA or other regulatory authorities; |
| • |
the timing of market introduction of our products as well as competitive products; |
| • |
the cost of treatment in relation to alternative treatments; |
| • |
the availability of coverage and adequate reimbursement and pricing by third-party payers and government authorities; |
| • |
relative convenience and ease of administration; |
| • |
the effectiveness of our sales and marketing efforts and those of our collaborators; and |
| • |
unfavorable publicity relating to the product candidate or the Company. |
If any of our product candidates are approved but fail to achieve market acceptance among physicians, patients, or healthcare payers, we will not be able to generate significant revenues, which would compromise our ability to sustain profitability.
Our research programs may not succeed.
In the last couple of years, XBiotech has positioned itself with a pipeline of potential drug candidates at all stages of development, from pre-clinical through Phase III clinical trial stage. Even though we have many drugs in development at this time, none of these research programs may succeed. There are several reasons why a drug program may fail, including the following:
| ● |
In the development stage, we may be unable to develop a therapy, which would mean us succeeding in isolating appropriate antibodies to reach the clinical trial stage; |
| ● |
Any partnerships for the development of antibodies could fail to produce results that would necessitate clinical trials; |
| ● |
We may not receive approval from regulatory bodies to move from early stage clinical trials to later stage clinical trials; |
| ● |
Even if we are able to move to later stage clinical trials, it may prove to be difficult to enroll patients into the studies according to schedule, or at all; |
| ● |
During the clinical trial, there could be unexpected serious adverse events causing severe injury or death in patients, requiring us to cease further enrollment or causing regulatory authorities to place the trial on clinical hold for an indefinite period of time; |
| ● |
If a clinical trial is completed, we may not have the appropriate personnel to submit a marketing application to regulatory authorities for approval, and to further respond to the variety of follow up questions that regulatory authorities may have during the review process; |
| ● |
Regulatory authorities may reject drug candidates for a variety of reasons, preventing us from proceeding with marketing and commercialization of approved products; and |
| ● |
We may run out of the funds necessary to complete development for any of our potential drug candidates. |
Even an effective drug candidate might not be commercially successful.
Even if we ultimately succeed in creating a safe and effective drug, as determined by regulatory authorities, based on our current product pipeline, there is no assurance it would be commercially successful. Competitive products might become available faster or with lower costs or adverse risks to patients, resulting in few sales of any product developed by XBiotech. Occurrences of certain disease indications, such as those in our pipeline, might become sufficiently rare, or victims might be sufficiently impoverished, that commercial production is uneconomic. Furthermore, we must have sufficient buy-in from patients and healthcare professionals to guarantee market exposure for our drug candidates. If the end-users are not reached with our products, then it will be difficult to generate revenue from our development efforts. And even though we could obtain regulatory approval for any of our drug candidates, it is not necessarily the case that government or third-party payers will decide to add our products to their respective prescription drug formularies for reimbursement, thus inhibiting the ability for our drug candidates to reach the target patient populations, and health care professionals serving those patients.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to our current or future product candidates to treat any relevant indication(s). There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the disease indications for which we are developing our future product candidates. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach, and others are based on entirely different approaches. Potential competitors include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
More established companies may have a competitive advantage over us due to their greater size, cash flows and institutional experience. Compared to us, many of our competitors may have significantly greater financial, technical and human resources. As a result of these factors, our competitors may obtain regulatory approval of their products before we do, which will limit our ability to develop or commercialize any of our product candidates. In addition, many companies are developing new therapeutics to supplant or expand upon the standard of care for a number of diseases, as a result, we cannot predict what the standard of care will be as our product candidates progress through clinical development.
Our failure to successfully identify, acquire, develop and commercialize additional product candidates or approved products could impair our ability to grow.
Although a substantial amount of our efforts will focus on the continued clinical testing and potential approval of our current product candidates, a key element of our growth strategy is to acquire, develop and/or market additional products and product candidates. All of these potential product candidates remain in the discovery and clinical study stages. Research programs to identify product candidates require substantial technical, financial and human resources, whether or not any product candidates are ultimately identified. Because our internal research capabilities are limited, we may be dependent upon pharmaceutical and biotechnology companies, academic scientists and other researchers to sell or license products or technology to us. The success of this strategy depends partly upon our ability to identify, select and acquire promising pharmaceutical product candidates and products. The process of proposing, negotiating and implementing a license or acquisition of a product candidate or approved product is lengthy and complex. Other companies, including some with substantially greater financial, marketing and sales resources, may compete with us for the license or acquisition of product candidates and approved products. We have limited resources to identify and execute the acquisition or in-licensing of third-party products, businesses and technologies and integrate them into our current infrastructure. Moreover, we may devote resources to potential acquisitions or in-licensing opportunities that are never completed, or we may fail to realize the anticipated benefits of such efforts. Any product candidate that we acquire may require additional development efforts prior to commercial sale, including extensive clinical testing and approval by the FDA and applicable foreign regulatory authorities. All product candidates are prone to risks of failure typical of pharmaceutical product development, including the possibility that a product candidate will not be shown to be sufficiently safe and effective for approval by regulatory authorities. In addition, we cannot provide assurance that any products that we develop or approved products that we acquire will be manufactured profitably or achieve market acceptance.
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the testing of our product candidates in clinical trials and will face an even greater risk if we commercially sell any products that we may develop. Product liability claims may be brought against us by subjects enrolled in our clinical trials, patients, healthcare providers or others using, administering or selling our products. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • |
decreased demand for any product candidates or products that we may develop; |
| • |
termination of clinical trial sites or entire clinical trial programs; |
| • |
injury to our reputation and significant negative media attention; |
| • |
withdrawal of clinical trial participants; |
| • |
significant costs to defend the related litigation; |
| • |
substantial monetary awards to clinical trial subjects or patients; |
| • |
loss of revenue; |
| • |
diversion of management and scientific resources from our business operations; and |
| • |
the inability to commercialize our product candidates. |
We will obtain insurance coverage for products to include the sale of commercial products if we obtain marketing approval for our product candidates, but we may be unable to obtain commercially reasonable product liability insurance for any products approved for marketing. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
We will need to expand our operations and grow the size of our organization in the future, and we may experience difficulties in managing this growth.
As of December 31, 2021, we had 97 employees. As our development and commercialization plans and strategies develop, or as a result of any future acquisitions, we will need additional managerial, operational, sales, marketing, scientific, and financial headcount and other resources. Our management, personnel and systems currently in place may not be adequate to support this future growth. Future growth would impose significant added responsibilities on members of management, including:
| • |
managing our clinical trials effectively, which we anticipate potentially being conducted at numerous clinical sites on a global scale; |
| • |
identifying, recruiting, maintaining, motivating and integrating additional employees with the expertise and experience we will require; |
| • |
managing our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors and other third parties; |
| • |
managing additional relationships with various strategic partners, suppliers and other third parties; |
| • |
improving our managerial, development, operational and finance reporting systems and procedures; and |
| • |
expanding our facilities. |
Our failure to accomplish any of these tasks could prevent us from successfully growing our Company.
We have ongoing obligations to manufacture bermekimab for use by Janssen in clinical trials, and any failure to satisfy our obligations under that agreement would have a material adverse effect on our financial condition and business reputation.
In connection with the Janssen Transaction, one of our subsidiaries entered into a clinical manufacturing agreement with an affiliate of Janssen, under which our subsidiary agreed to manufacture bermekimab for use by Janssen in clinical trials in return for quarterly cash payments. If we fail to manufacture bermekimab in sufficient quantities to satisfy our obligations under the clinical manufacturing agreement, we would be in breach of our contractual obligations, in which case Janssen could cease making payments to us and could also sue us for default. Any such litigation would be expensive and time-consuming and could damage our business reputation, causing potential business partners to be less willing to do business with us in the future. Any of these events could adversely affect our business, financial condition and operating results.
We may never achieve any of the potential milestone payments that were negotiated as a part of the Janssen Transaction.
As part of the Janssen Transaction, we are eligible to receive milestone payments of $150 million for each instance that Janssen, in its sole and absolute discretion, develops pharmaceutical products that contain bermekimab and that are for non-dermatological indications, provided that Janssen receives certain required commercial authorizations for such products within a specified timeframe. We are entitled to earn up to four milestone payments, for a maximum of $600 million. However, because the payment of these funds is subject to Janssen’s business decisions and discretion, as well as regulatory approvals and other factors outside our control, we may never receive any of these amounts. If we do not receive all or any of the milestone payments, we may be required to seek additional funding from other sources, which may not be available on terms acceptable to us or at all.
We are highly dependent on our Chief Executive Officer.
Our future success depends in significant part on the continued service of our Chief Executive Officer, John Simard. Mr. Simard is critical to the strategic direction and overall management of our company as well as our research and development process. Although we have an employment agreement with Mr. Simard, it has no specific duration. The loss of Mr. Simard could adversely affect our business, financial condition and operating results.
We depend on key personnel to operate our business. If we are unable to retain, attract and integrate qualified personnel, our ability to develop and successfully grow our business could be harmed.
In addition to the continued services of Mr. Simard, we believe that our future success is highly dependent on the contributions of our significant employees, as well as our ability to attract and retain highly skilled and experienced sales, research and development and other personnel in the United States and abroad. Some of our significant employees include our Chief Scientific Officer, our Vice President of Quality Assurance, our Vice President of Quality Control, and our Vice President of Finance and Human Resources. Changes in our management team may be disruptive to our business.
All of our employees, including our Chief Executive Officer, are free to terminate their employment relationship with us at any time, subject to any applicable notice requirements, and their knowledge of our business and industry may be difficult to replace. If one or more of our executive officers or significant employees leaves, we may not be able to fully integrate new personnel or replicate the prior working relationships, and our operations could suffer. Qualified individuals with the breadth of skills and experience in the pharmaceutical industry that we require are in high demand, and we may incur significant costs to attract them. Many of the other pharmaceutical companies that we compete against for qualified personnel have greater financial and other resources, different risk profiles and a longer history in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Our failure to attract and retain key personnel could impede the achievement of our research, development and commercialization objectives.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations in the U.S. and elsewhere, including, as a result of our leased laboratory space, those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes.
We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain insurance for employee injury to cover us for costs and expenses, we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological or hazardous materials. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations may also result in substantial fines, penalties or other sanctions.
Business disruptions caused by natural disasters, infrastructure interruptions, COVID-19 or other public health threats could seriously harm our future revenues and financial condition and increase our costs and expenses.
Our operations could be subject to earthquakes, power shortages or outages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics such as contagious disease outbreaks, and other natural or manmade disasters or business interruptions, for which we are predominantly self-insured. We do not carry insurance for all categories of risk that our business may encounter. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-parties to supply various items which are critical for producing our product candidates. Our ability to produce clinical supplies of product candidates could be disrupted, if the operations of these suppliers are affected by a man-made or natural disaster, a public health crisis or other business interruption. For example, the ongoing coronavirus threat has spread to a number of countries, including the United States and various countries in Europe, resulting in the declaration by the World Health Organization of a global pandemic and the announcement of extended travel restrictions, business shutdowns, cancellations and prohibitions of large public gatherings and declarations of states of emergency in cities, states and countries around the world. The imposition of any of these restrictions in one of the regions where our facilities or those of our third-party suppliers are located would have a disproportionately negative impact on us. The extent of the ultimate impact to us, our significant suppliers and our general infrastructure resulting from concentration in certain geographical areas is unknown and cannot be estimated, but our operations and financial condition would likely suffer in the event of a major earthquake, fire or other natural disaster or public health threat such as the coronavirus pandemic in one or more of those areas. Further, any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our business, results of operations, financial condition and cash flows from future prospects.
Risks Related to Intellectual Property
If we are unable to obtain or protect intellectual property rights, our competitive position could be harmed.
We depend on our ability to protect our proprietary technology. We rely on trade secret, patent, copyright and trademark laws, and confidentiality, licensing and other agreements with employees and third parties, all of which offer only limited protection. Our commercial success will depend in large part on our ability to obtain and maintain patent protection in the U.S. and other countries with respect to our proprietary technology and products. Where we deem appropriate, we seek to protect our proprietary position by filing patent applications in the U.S. and abroad related to our novel technologies and products that are important to our business. The patent positions of biotechnology and pharmaceutical companies generally are highly uncertain, involve complex legal and factual questions and have in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patents, including those patent rights licensed to us by third parties, are highly uncertain.
The steps we have taken to protect our proprietary rights may not be adequate to preclude misappropriation of our proprietary information or infringement of our intellectual property rights, both inside and outside the U.S. The rights already granted under any of our currently issued patents and those that may be granted under future issued patents may not provide us with the proprietary protection or competitive advantages we are seeking. If we are unable to obtain and maintain patent protection for our technology and products, or if the scope of the patent protection obtained is not sufficient, our competitors could develop and commercialize technology and products similar or superior to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
With respect to patent rights, we do not know whether our pending patent applications for any of our technologies or product candidates will result in the issuance of patents that protect such technologies or product candidates, or if any of our issued patents will effectively prevent others from commercializing competitive technologies and products. Our pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. Further, the examination process may require us to narrow the claims for our pending patent applications, which may limit the scope of patent protection that may be obtained if these applications issue. Because the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, issued patents that we own or have licensed from third parties may be challenged in the courts or patent offices in the U.S. and abroad. Such challenges may result in the loss of patent protection, the narrowing of claims in such patents or the invalidity or unenforceability of such patents, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection for our technology and products. Protecting against the unauthorized use of our patented technology, trademarks and other intellectual property rights is expensive, difficult and, in some cases, not be possible. In some cases, it may be difficult or impossible to detect third-party infringement or misappropriation of our intellectual property rights, even in relation to issued patent claims, and proving any such infringement may be even more difficult.
Intellectual property rights do not necessarily address all potential threats to any competitive advantage we may have.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
| • |
Others may be able to make compounds that are the same as or similar to our current or future product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed. |
| • |
We might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed. |
| • |
We or any of our licensors or strategic partners might not have been the first to file patent applications covering certain of our inventions. |
| • |
Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights. |
| • |
It is possible that our pending patent applications will not lead to issued patents. |
| • |
Issued patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors. |
| • |
Our competitors might conduct research and development activities in the U.S. and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets. |
| • |
We may not develop additional proprietary technologies that are patentable. |
| • |
The patents of others may have an adverse effect on our business. |
Our technology may be found to infringe upon third-party intellectual property rights.
Third parties, may in the future, assert claims or initiate litigation related to their patent, copyright, trademark and other intellectual property rights in technology that is important to us. The asserted claims and/or litigation could include claims against us, our licensors or our suppliers alleging infringement of intellectual property rights with respect to our products or components of those products. Regardless of the merit of the claims, they could be time consuming, result in costly litigation and diversion of technical and management personnel, or require us to develop a non-infringing technology or enter into license agreements. We cannot assure you that licenses will be available on acceptable terms, if at all. Furthermore, because of the potential for significant damage awards, which are not necessarily predictable, it is not unusual to find even arguably unmeritorious claims resulting in large settlements. If any infringement or other intellectual property claim made against us by any third party is successful, or if we fail to develop non-infringing technology or license the proprietary rights on commercially reasonable terms and conditions, our business, operating results and financial condition could be materially and adversely affected.
If our products, methods, processes and other technologies infringe upon the proprietary rights of other parties, we could incur substantial costs and we may have to:
| • |
obtain licenses, which may not be available on commercially reasonable terms, if at all; |
| • |
abandon an infringing drug or therapy candidate; |
| • |
redesign our products or processes to avoid infringement; |
| • |
stop using the subject matter claimed in the patents held by others; |
| • |
pay damages; or |
| • |
defend litigation or administrative proceedings which may be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources. |
We may need to license intellectual property from third parties, and such licenses may not be available or may not be available on commercially reasonable terms.
A third party may hold intellectual property, including patent rights that are important or necessary to the development of our products. It may be necessary for us to use the patented or proprietary technology of a third party to manufacture, or otherwise commercialize, our own technology or products, in which case we would be required to obtain a license from such third party. Licensing such intellectual property may not be available or may not be available on commercially reasonable terms, which could have a material adverse effect on our business and financial condition.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and product candidates, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside of the U.S. are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
Risks Related to Owning Shares of Our Common Stock
Our share price may be volatile, which could subject us to securities class action lawsuits and prevent you from being able to sell your shares at or above the price at which you purchased them.
Our stock could be subject to wide fluctuations in response to many risk factors listed in this section, and others beyond our control, including:
| • |
results of our clinical trials; |
| • |
results of clinical trials of our competitors’ products; |
| • |
regulatory actions with respect to our products or our competitors’ products; |
| • |
actual or anticipated fluctuations in our financial condition and operating results; |
| • |
actual or anticipated changes in our growth rate relative to our competitors; |
| • |
actual or anticipated fluctuations in our competitors’ operating results or changes in their growth rate; |
| • |
competition from existing products or new products that may emerge; |
| • |
announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments; |
| • |
issuance of new or updated research or reports by securities analysts; |
| • |
fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| • |
delisting of the Company’s common shares from the exchange on which they trade due to the Company not being in compliance with the listing requirements of the exchange; |
| • |
share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| • |
additions or departures of key management or scientific personnel; |
| • |
disputes or other developments related to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| • |
announcement or expectation of additional financing efforts; |
| • |
sales of our common stock by us, our insiders or our other shareholders; |
| • |
market conditions for biopharmaceutical stocks in general; and |
| • |
general economic and market conditions. |
Furthermore, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. In particular, stock markets have experienced extreme volatility in the first quarter of 2020 due to the ongoing coronavirus pandemic and investor concerns and uncertainty related to the impact of the outbreak on the economies of countries worldwide. These broad market and industry fluctuations, as well as general economic, political and market conditions such as recessions, interest rate changes or international currency fluctuations, may negatively impact the market price of shares of our common stock. In addition, such fluctuations could subject us to securities class action litigation, which could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business. If the market price of shares of our common stock does not exceed your buying price, you may not realize any return on your investment in us and may lose some or all of your investment.
Our directors, executive officers and principal shareholders continue to have substantial control over our company and could delay or prevent a change in corporate control.
As of March 15, 2022 our directors, executive officers and principal shareholders, together with their affiliates, beneficially own, in the aggregate, at least 6.8 million shares or approximately 22.53% of our outstanding common stock, and could own approximately 9.05 million shares or approximately 28% of our outstanding common stock if they fully exercise their outstanding stock options. As a result, these shareholders, if acting together, have the ability to determine the outcome of matters submitted to our shareholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. In addition, these persons, acting together, have the ability to control the management and affairs of the Company. Accordingly, this concentration of ownership may harm the market price of our common stock by:
| • |
delaying, deferring or preventing a change in control of the Company; |
| • |
impeding a merger, consolidation, takeover or other business combination involving the Company; or |
| • |
discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of the Company. |
We have broad discretion in the use of the net proceeds from the Janssen Transaction and may not use them effectively.
We intend to continue to allocate the net proceeds that we received from our public offerings and the Janssen Transaction to fund discovery and development of our next generation True Human™ anti-IL-1⍺ antibody program and to advance other antibody therapeutics in our pipeline. However, our management will have broad discretion in the actual application of the net proceeds, and we may elect to allocate proceeds differently if we believe it would be in our best interests to do so. For example, in February 2020, we completed a cash tender offer in which we repurchased $420 million of our common shares. In July 2021, we distributed $75 million cash dividend to our shareholders. Our shareholders may not agree with the manner in which our management chooses to allocate and spend the net proceeds. Our management may also fail to apply these funds effectively, which could have a material adverse effect on our business. We may invest our cash on hand in a manner that does not produce income or that loses value.
Provisions in our charter documents under Canadian law could make an acquisition of us, which may be beneficial to our shareholders, more difficult.
Our authorized preferred capital stock is available for issuance from time to time at the discretion of our Board of Directors, without shareholder approval. Our Articles of Incorporation (“Articles”) grant our Board of Directors the authority, subject to the corporate law of British Columbia, to determine or alter the special rights and restrictions granted to or imposed on any wholly unissued series of preferred shares, and such rights may be superior to those of our common stock.
Limitations on the ability to acquire and hold our common stock may be imposed by the Competition Act (Canada). This legislation permits the Commissioner of Competition of Canada to review any acquisition of a significant interest in us. This legislation grants the Commissioner jurisdiction to challenge such an acquisition before the Canadian Competition Tribunal if the Commissioner believes that it would, or would be likely to, result in a substantial lessening or prevention of competition in any market in Canada. The Investment Canada Act (Canada) subjects an acquisition of control of a company by a non-Canadian to government review if the value of our assets as calculated pursuant to the legislation exceeds a threshold amount. A reviewable acquisition may not proceed unless the relevant minister is satisfied that the investment is likely to be a net benefit to Canada.
Any of the foregoing could prevent or delay a change of control and may deprive or limit strategic opportunities for our shareholders to sell their shares and/or affect the market price of our shares.
We may be a passive foreign investment company for US tax purposes which may negatively affect US investors.
Although XBiotech does not meet the definition of “Investment Company”, for US federal income taxation purposes, we will be a passive foreign investment company (PFIC) if in any taxable year either: (a) 75% or more of our gross income consists of passive income; or (b) 50% or more of the value of our assets is attributable to assets that produce, or are held for the production of, passive income. If we meet either test, our shares held by a US person in that year will be PFIC shares for that year and all for subsequent years in which they are held by that person. In previous taxable years, we likely were a PFIC because our gross income consisted principally of interest. In 2019, however, the character of our gross income changed significantly as a result of the Janssen Agreements, and the determination of whether we were a PFIC in 2021 is uncertain. Moreover, the PFIC rules can apply differently to different US shareholders depending on whether a specific shareholder has made certain elections with respect to the ownership of PFIC shares. Because these rules are extremely complex and apply differently based upon whether and when a US shareholder has made certain elections, new and existing US shareholders should consult with their tax advisors as to the tax implications of acquiring, owning and disposing of our stock.
We are governed by the corporate laws in British Columbia, Canada which in some cases have a different effect on shareholders than the corporate laws in Delaware, United States.
The material differences between the BCBCA as compared to the Delaware General Corporation Law (DGCL) which may be of most interest to shareholders include the following:
| (i) |
for material corporate transactions (i.e. mergers and amalgamations, other extraordinary corporate transactions, amendments to our Articles) the BCBCA generally requires two-thirds majority vote by shareholders, whereas DGCL generally only requires a majority vote of shareholders; |
| (ii) |
the quorum for shareholders meetings is not prescribed under the BCBCA and is only two persons representing 20% of the issued shares under our Articles, whereas under DGCL, quorum requires a minimum of one-third of the shares entitled to vote to be present and companies’ certificates of incorporation frequently require a higher percentage to be present; |
| (iii) |
under the BCBCA, a holder of 5% or more of our common stock can requisition a special meeting at which any matters that can be voted on at our annual meeting can be considered, whereas the DGCL does not give this right; |
| (iv) |
our Articles require two-thirds majority vote by shareholders to pass a resolution for one or more directors to be removed, whereas DGCL only requires the affirmative vote of a majority of the shareholders; however, many public company charters limit removal of directors to a removal for cause; and |
| (v) |
our Articles may be amended by resolution of our directors to alter our authorized share structure, including to consolidate or subdivide any of our shares, whereas under DGCL, a majority vote by shareholders is generally required to amend a corporation’s certificate of incorporation and a separate class vote may be required to authorize alterations to a corporation’s authorized share structure. |
We cannot predict if investors will find our common stock less attractive because of these material differences. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our share price may be more volatile.
General Risk Factors
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
The terms of any financing arrangements we enter into may adversely affect the holdings or the rights of our shareholders and the issuance of additional securities, by us, or the possibility of such issuance, may cause the market price of our shares to decline. The sale of additional equity or convertible securities would dilute all of our shareholders. The incurrence of indebtedness would result in increased fixed payment obligations and, potentially, the imposition of restrictive covenants. Those covenants may include limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborators or otherwise at an earlier stage than otherwise would be desirable resulting in the loss of rights to some of our product candidates or other unfavorable terms, any of which may have a material adverse effect on our business, operating results and prospects. Additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our products.
Future sales, or the possibility of future sales, of a substantial number of our common stock could adversely affect the price of the shares and dilute shareholders.
We may have a limited ability to use some or all of our net operating loss and research tax credit carryforwards in the future.
As a result of prior operating losses and research and development activities, we have net operating loss, or “NOL,” and research tax credit carryforwards (collectively, the “carryforwards”) for U.S. federal income tax purposes. Under Section 382 of the Internal Revenue Code of 1986, as amended, substantial changes in the Company’s ownership may limit the amount of carryforwards that could be utilized annually in the future to offset U.S. taxable income and/or income tax. Specifically, this limitation may arise in the event of a cumulative change in ownership of the Company of more than 50% within a three-year period. Any such annual limitation may significantly reduce the utilization of the carryforwards before they expire.
Future sales of a substantial number of our common stock, or the perception that such sales will occur, could cause a decline in the market price of our common stock. As of March 15, 2022, we had 30,439,275 common shares outstanding.
In the future, we may issue additional common stock or other equity or debt securities convertible into common stock in connection with a financing, acquisition, litigation settlement, employee arrangements or otherwise. Any such issuance could result in substantial dilution to our existing shareholders and could cause our common share price to decline.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, shareholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 or any subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock.
We are required to disclose changes made in our internal controls and procedures on a quarterly basis and our management is required to assess the effectiveness of these controls annually. However, for as long as we are a “smaller reporting company” with under $100 million in annual revenue, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404. An independent assessment of the effectiveness of our internal controls could detect problems that our management’s assessment might not.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
The Company owns 48 acres of industrial-zoned property located five miles from Austin’s central business district. In 2016, construction of a new manufacturing facility on this property was completed. The Company uses this facility to produce product under the clinical manufacturing agreement entered into in connection with the Janssen Transaction and to support its drug development and other activities. In 2019, XBiotech completed the construction of a new infectious disease and animal facility laboratory. Located in a separate building on our campus, just a short walk from the Company’s main manufacturing headquarters, the new facility incorporates an animal biological safety level 2 (ABSL2) laboratory and other laboratories for in vitro and in vivo testing of the Company’s True Human™ antibodies against infectious disease targets. XBiotech owns the 48-acre campus—and all structures on the property—debt-free and envisions further expansion of facilities on the property.
The Company is not currently subject to any material legal proceedings.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market Information
Our common stock began trading on the NASDAQ Global Select Market on April 15, 2015 under the symbol “XBIT.” Prior to that time, there was no established public trading market for our common stock.
Holders of record
Because many of our shares are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of beneficial holders represented by these record holders.
Dividends
In July 2021, we paid $2.50 per share in dividends to shareholders. We currently intend to retain any earnings for future growth and, therefore, do not expect the comparable cash dividends will continue to be paid in the foreseeable future.
ITEM 6. SELECTED FINANCIAL DATA
Not applicable
ITEM 7 MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes thereto included elsewhere in this annual report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this annual report on Form 10-K, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this annual report on Form 10-K, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
XBiotech Inc. (“XBiotech” or the “Company) is a pre-market biopharmaceutical company engaged in discovering and developing True Human™ monoclonal antibodies for treating a variety of diseases. True Human™ monoclonal antibodies are those which occur naturally in human beings—as opposed to being derived from animal immunization or otherwise engineered. We believe that naturally occurring monoclonal antibodies have the potential to be safer and more effective than their non-naturally occurring counterparts. XBiotech is focused on developing its True Human™ pipeline and manufacturing system.
Following the Janssen Transaction in December 2019, the tender offer in February 2020, and the dividends paid in July 2021, retained earnings as of December 31, 2021 were $5.2 million. We had a net loss of $17.4 million for the year ended December 31, 2021, compared to a net loss of $11.2 million for the year ended December 31, 2020, and a net income of $668.6 million for the year ended December 31, 2019. During the next year, we expect the revenues from a new manufacturing contract with a Janssen-related company will generate offset operating expenses for our research and development activities. In addition, we expect to incur significant and increasing operating losses for the foreseeable future as we advance our drug candidates from discovery through preclinical testing and clinical. In addition to these increasing research and development expenses, we expect general and administrative costs to increase, particularly in consideration of current inflationary trends. We will need to generate significant revenues to achieve or sustain profitability, and we may never do so. As of December 31, 2021, we had 97 employees.
Revenues
Prior to receiving payments under the clinical manufacturing agreement entered into in connection with the Janssen Transaction, we had not generated any revenue. Under the clinical manufacturing agreement, we manufacture bermekimab for use by Janssen in clinical trials, in exchange for fixed payments, paid in quarterly installments through 2021. In Febuary 2022, we entered a new manufacturing contract with a Janssen-related company whereby we will continue to manufacture bermekimab through December 2023. The contract terminates in December 2023.
Research and Development Expenses
Research and development expense consists of expenses incurred in connection with identifying and developing our drug candidates. These expenses consist primarily of salaries and related expenses, share-based compensation, the purchase of equipment, laboratory and manufacturing supplies, facility costs, costs for preclinical and clinical research, development of quality control systems, quality assurance programs and manufacturing processes. We charge all research and development expenses to operating expenses as incurred.
Clinical development timelines, likelihood of success and total costs vary widely. We do not currently track our internal research and development costs or our personnel and related costs on an individual drug candidate basis. We use our research and development resources, including employees and our drug discovery technology, across multiple drug development programs. As a result, we cannot state precisely the costs incurred for each of our research and development programs or our clinical and preclinical drug candidates. From inception through December 31, 2021, we have recorded total research and development expenses, including share-based compensation, of $247.6 million. Our total research and development expenses for the year ended December 31, 2021 was $28.3 million, compared to $9.5 million the year ended December 31, 2020, and $24.1 million for the year ended December 31, 2019. Share-based compensation accounted for $2.0 million for the year ended December 31, 2021, $3.6 million for the year ended December 31, 2020 and $1.6 million for the year ended December 31, 2019.
Research and development expenses as a percentage of total operating expenses was 75% for the year ended December 31, 2021, 34% for the year ended December 31, 2020, and 77% for the year ended December 31, 2019. The percentages, excluding share-based compensation, were 79% for the year ended December 31, 2021, 36% for the year ended December 31, 2020 and 81% for the year ended December 31, 2019.
We will select drug candidates and research projects for further development on an ongoing basis in response to their preclinical and clinical success and commercial potential. For research and development candidates in early stages of development, it is premature to estimate when material net cash inflows from these projects might occur.
General and Administrative Expenses
General and administrative expense consists primarily of salaries and related expenses for personnel in administrative, finance, business development and human resource functions, as well as the legal costs of pursuing patent protection of our intellectual property and patent filing and maintenance expenses, share–based compensation, and professional fees for legal services. Our total general and administration expenses was $9.4 million for the year ended December 31, 2021, $18.2 million for the year ended December 31, 2020 and $7.1 million for the year ended December 31, 2019. Share-based compensation accounted for $2.2 million for the year ended December 31, 2021, $7.5 million for the year ended December 31, 2020 and $1.7 million for the year ended December 31, 2019.
Critical Accounting Policies
Our Management’s Discussion and Analysis of Financial Condition and Results of Operations is based on our financial statements, which have been prepared in conformity with generally accepted accounting principles in the United States (US GAAP). The preparation of our financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and expenses incurred during the reported periods.
We base estimates on our historical experience, known trends and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in the notes to our financial statements appearing in this Annual Report on Form 10-K, we believe that the following accounting policies are the most critical to understanding and evaluating our reported financial results.
Share-Based Compensation
Stock-based awards are measured at fair value at each grant date. We recognize share-based compensation expenses ratably over the requisite service period of the option award.
Determination of the Fair Value of Share-Based Compensation Grants
The determination of the fair value of share-based compensation arrangements is affected by a number of variables, including estimates of the expected stock price volatility, risk-free interest rate and the expected life of the award. We value stock options using the Black-Scholes option-pricing model, which was developed for use in estimating the fair value of traded options that are fully transferable and have no vesting restrictions. Black-Scholes option-pricing model and other option valuation models require the input of highly subjective assumptions, including the expected stock price volatility. If we made different assumptions, our share-based compensation expenses, net loss, and net loss per common share could be significantly different. We determine that the fair value of common stock as the closing price of the Company’s common stock as reported by NASDAQ on the option grant date.
The following summarizes the assumptions used for estimating the fair value of stock options granted during the periods indicated:
| Year Ended December 31, |
||||||||||||
| 2021 |
2020 |
2019 |
||||||||||
| Weighted-average grant date fair value per share |
$ | 9.29 | $ | 12.23 | $ | 9.36 | ||||||
| Expected volatility |
83%-91 | % | 87%-97 | % | 80%-97 | % | ||||||
| Risk-free interest rate |
0.5%-1.4 | % | 0.3%-1.9 | % | 1.4%-2.7 | % | ||||||
| Expected life (in years) |
5.38–6.25 | 5.38–10 | 5.38–10 | |||||||||
| Dividend yield |
— | — | — | |||||||||
With the exception of the dividend paid in 2021, we have assumed no dividend yield because we do not expect to pay dividends in the foreseeable future. The risk-free interest rate assumption is based on observed interest rates for U.S. Treasury securities with maturities consistent with the expected life of our stock options. The expected life represents the period of time the stock options are expected to be outstanding and is based on the simplified method when the stock option includes “plain vanilla” terms. Under the simplified method, the expected life of an option is presumed to be the midpoint between the vesting date and the end of the agreement term. We used the simplified method due to the lack of sufficient historical exercise data to provide a reasonable basis upon which to otherwise estimate the expected life of the stock options. For stock options that did not include “plain vanilla” terms, we used the contractual life of the stock option as the expected life. Such stock options consisted primarily of options issued to our board of directors that were immediately vested at issuance. Expected volatility is based on historical volatilities for publicly traded stock of comparable companies over the estimated expected life of the stock options. The Company accounts for forfeitures as they occur rather than on an estimated basis.
Income Taxes
We account for income taxes under the asset and liability method. We record deferred tax assets and liabilities for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, as well as for operating loss and tax credit carryforwards. We measure deferred tax assets and liabilities using enacted tax rates expected to apply to taxable income in the years in which we expect to recover or settle those temporary differences. We recognize the effect of a change in tax rates on deferred tax assets and liabilities in the results of operations in the period that includes the enactment date. We assess the likelihood that deferred tax assets will be realized, and we recognize a valuation allowance if it is more likely than not that some portion of the deferred tax assets will not be realized. This assessment requires judgment as to the likelihood and amounts of future taxable income by tax jurisdiction. To date, with the exception of certain Canada deferred tax assets that will reverse in a period in which they may be carried back, we have provided a valuation allowance against our deferred tax assets as we believe the objective and verifiable evidence of our historical pretax net losses outweighs any positive evidence of our forecasted future results. Although we believe that our tax estimates are reasonable, the ultimate tax determination involves significant judgment. We will continue to monitor the positive and negative evidence and will adjust the valuation allowance as sufficient objective positive evidence becomes available.
We account for uncertain tax positions by recognizing the financial statement effects of a tax position only when, based upon technical merits, it is more likely than not that the position will be sustained upon examination. We recognize potential accrued interest and penalties associated with unrecognized tax positions within our global operations in income tax expense.
Clinical Trial Accruals
Expense accruals related to clinical trials are based on the Company’s estimates of services received and efforts expended pursuant to contracts with third party service providers conduct and manage clinical trials on the Company’s behalf. The financial terms of these agreements vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing costs, the Company estimates the period over which services will be performed and the level of effort to be expended in each period based upon patient enrollment, clinical site activations, or information provided to the Company by its vendors on their actual costs incurred. Any estimates of the level of services performed or the costs of these services could differ from actual results.
Results of Operations
Revenue
Revenue during the year ended December 31, 2021, 2020 and 2019 are summarized as follows (in thousands):
| Year Ended December 31, |
||||||||||||
| 2021 |
2020 |
2019 |
||||||||||
| Revenue |
||||||||||||
| Manufacturing revenue |
$ | 18,000 | $ | 18,000 | $ | - | ||||||
| Clinical Trial revenue |
394 | 25,975 | - | |||||||||
| Other revenue |
- | 22 | - | |||||||||
| Total revenue |
$ | 18,394 | $ | 43,997 | $ | - | ||||||
We had not generated any revenue before the year 2020. Under the clinical manufacturing agreement with Janssen, for the year ended December 31, 2021, we have recorded $18.0 million as manufacturing revenue. Clinical trial revenue for the year ended December 31, 2021 is based on the Transition Services Agreement under which we agreed to continue operational management, on a fee-for-service basis, of certain clinical trials completed on December 2020 related to bermekimab, which includes $303 thousand pass-through revenue and $91 thousand mark-up revenue.
Under the same agreement with Janssen, for the year ended December 31, 2020, we have recorded $18.0 million as manufacturing revenue. Clinical trial revenue for the year ended December 31, 2020 includes $20.0 million pass-through revenue for two ongoing trials and $6.0 million mark-up revenue. The other revenue $22 thousand is generated from the grant of a non-exclusive license.
Cost of Goods Sold
Cost of goods sold during the year ended December 31, 2021, 2020 and 2019 are summarized as follows (in thousands):
| Year Ended December 31, |
||||||||||||
| 2021 |
2020 |
2019 |
||||||||||
| Cost of goods sold |
||||||||||||
| Manufacturing cost |
$ | 5,517 | $ | 12,859 | $ | - | ||||||
| Clinical trial cost |
303 | 21,689 | - | |||||||||
| Total cost of goods sold |
$ | 5,820 | $ | 34,548 | $ | - | ||||||
We had not incurred any cost of goods sold before the year 2020.The manufacturing cost for the year ended December 31, 2021 and 2020 represents period expense for manufacturing, quality assurance and quality control departments. Drug for the Janssen Transaction was mainly manufactured in the year 2020. Part of the cost was deferred to the year 2021. Clinical trial cost for the year ended December 31, 2021 and 2020, is $303 thousand and 21.7 million respectively, which is the pass-through expenses for two trials. These two trials were completed in December 2020.
Expenses
Research and Development
Research and Development costs are summarized as follows (in thousands):
| Year Ended December 31, |
Increase | % Increase |
Year Ended December 31, |
Increase | % Increase |
|||||||||||||||||||||||||||
| 2021 |
2020 |
(Decrease) |
(Decrease) |
2020 |
2019 |
(Decrease) |
(Decrease) |
|||||||||||||||||||||||||
| Salaries and related expenses |
$ | 12,235 | $ | 2,621 | $ | 9,614 | 367 | % | $ | 2,621 | $ | 6,291 | $ | (3,670 | ) | -58 | % | |||||||||||||||
| Laboratory and manufacturing supplies |
4,749 | 879 | 3,870 | 440 | % | 879 | 4,699 | (3,820 | ) | -81 | % | |||||||||||||||||||||
| Clinical trials and sponsored research |
1,390 | 84 | 1,306 | 1555 | % | 84 | 4,787 | (4,703 | ) | -98 | % | |||||||||||||||||||||
| Share-based compensation |
2,020 | 3,558 | (1,538 | ) | -43 | % | 3,558 | 1,635 | 1,923 | 118 | % | |||||||||||||||||||||
| Other |
7,874 | 2,385 | 5,489 | 230 | % | 2,385 | 6,678 | (4,293 | ) | -64 | % | |||||||||||||||||||||
| Total |
$ | 28,268 | $ | 9,527 | $ | 18,741 | 197 | % | $ | 9,527 | $ | 24,090 | $ | (14,563 | ) | -60 | % | |||||||||||||||
We do not currently track our internal research and development costs or our personnel and related costs on an individual drug candidate basis. We use our research and development resources, including employees and our drug discovery technology, across multiple drug development programs. As a result, we cannot state precisely the costs incurred for each of our research and development programs or our clinical and preclinical drug candidates.
Research and development expenses increased 197% to $28.3 million for year ended December 31, 2021 compared to $9.5 million for the year ended December 31, 2020. The increase was mainly due to classification of certain expenses incurred in connection with the Janssen. A portion of manufacturing department expenses were reclassed to cost of goods sold in 2021 compared to all manufacturing department expensed being reclassed to cost of goods sold in 2020, as all such expense incurred in 2020 were related to the clinical trial manufacturing agreement in the Janssen Transaction. The decrease of share-based compensation is due to the new grants to employees and Chief Executive Officer in the fourth quarter of 2019, being fully amortized in 2021. The other expense in 2021 includes the depreciation, facility allocation and other miscellaneous expense.
Research and development expenses decreased 60% to $9.5 million for year ended December 31, 2020 compared to $24.1 million for the year ended December 31, 2019. The decrease was mainly due to classification of certain expenses incurred in connection with the Janssen Transaction to cost of goods sold rather than research and development expense. Manufacturing department expenses related to the clinical manufacture agreement we entered into as part of the Janssen Transaction, as well as expenses pursuant to the Transition Services Agreement entered into as part of the Janssen Transaction were classified as cost of goods sold. Stock–based compensation expenses increased due to the new grants to employees and Chief Executive Officer in the fourth quarter of 2019.
General and Administrative
General and administrative costs are summarized as follows (in thousands):
| Year Ended December 31, |
Increase |
% Increase |
Year Ended December 31, |
Increase |
% Increase |
|||||||||||||||||||||||||||
| 2021 |
2020 |
(Decrease) |
(Decrease) |
2020 |
2019 |
(Decrease) |
(Decrease) |
|||||||||||||||||||||||||
| Salaries and related expenses |
$ | 3,971 | $ | 3,969 | $ | 2 | 0 | % | $ | 3,969 | $ | 1,345 | $ | 2,624 | 195 | % | ||||||||||||||||
| Patent filing expense |
794 | 525 | 269 | 51 | % | 525 | 801 | (276 | ) | -34 | % | |||||||||||||||||||||
| Share-based compensation |
2,162 | 7,537 | (5,375 | ) | -71 | % | 7,537 | 1,723 | 5,814 | 337 | % | |||||||||||||||||||||
| Professional fees |
1,469 | 2,678 | (1,209 | ) | -45 | % | 2,678 | 1,701 | 977 | 57 | % | |||||||||||||||||||||
| Other |
992 | 3,489 | (2,497 | ) | -72 | % | 3,489 | 1,573 | 1,916 | 122 | % | |||||||||||||||||||||
| Total |
$ | 9,388 | $ | 18,198 | $ | (8,810 | ) | -48 | % | $ | 18,198 | $ | 7,143 | $ | 11,055 | 155 | % | |||||||||||||||
General and administrative expenses decreased 48% to $9.4 million for the year ended December 31, 2021 compared to $18.2 million for the year ended December 31, 2020. The share-based compensation decrease was mainly due to the new grants to employees and Chief Executive Officer in the fourth quarter of 2019 fully amortized in 2021. Professional fees decreased $1.2 million mainly due to the Company no longer requiring professional and legal fees related to the tender offer completed in February 2020. Also, there was a $2.5 million decrease in other expense, mainly due to the reduced D&O insurance cost.
General and administrative expenses increased 155% to $18.2 million for the year ended December 31, 2020 compared to $7.1 million for the year ended December 31, 2019. The share-based compensation increase was mainly due to the new grants to employees and Chief Executive Officer in the fourth quarter of 2019. Salaries and related expense increased due to the bonus to the Chief Executive Officer in June 2020. Also, professional fees increased $1.0 million mainly due to professional and legal fees related to the tender offer completed in February 2020.
Other Income
The following table summarizes other income (in thousands):
| Year Ended December 31, |
||||||||||||
| 2021 |
2020 |
2019 |
||||||||||
| Interest income |
$ | 467 | $ | 2,456 | $ | 564 | ||||||
| Gain from Janssen Transaction |
- | - | 750,000 | |||||||||
| Other income (loss) |
(132 | ) | (503 | ) | 10 | |||||||
| Foreign exchange gain (loss) |
(711 | ) | 3,496 | (888 | ) | |||||||
| Total |
$ | (376 | ) | $ | 5,449 | $ | 749,686 | |||||
The interest income for the year ended December 31, 2021 and 2020 was mainly from the interest generated from the Company’s Canadian bank account and escrow account. The other expense for the year ended December 31, 2021 was a Canada Revenue Agency charge on tender offer tax withholding and for the year ended December 31, 2020 was a charitable donation. Foreign exchange gain (loss) was mainly due to the fluctuation between the US dollar and the Euro, and the US dollar and the Canadian dollar in the year ended December 31, 2021 compared to 2020.
Other income for the year ended December 31, 2019 includes a $750 million gain from the business purchase agreement with Janssen in December. The interest income for the year ended December 31, 2020 and 2019 was mainly from the interest generated from the Company’s Canadian bank account and escrow account. The other expense for the year ended December 31, 2020 was a charitable donation. Foreign exchange gain (loss) was mainly due to the fluctuation between the US dollar and the Euro, and the US dollar and the Canadian dollar in the year ended December 31, 2020 compared to 2019.
Income Taxes
Overall income tax benefit for the year ended December 31, 2021 is $8.0 million primarily due to an estimated $9.5 million refund of prior year Canadian income taxes through a carryback of tax losses to 2019. Income tax benefit for the year ended December 31, 2020 was $1.6 million primarily due to an estimated $1.8 million refund of prior year Canadian taxes through a carryback of tax losses to 2019. Income tax expense for the year ended December 31, 2019 was $49.8 million due to the income generated from the Janssen Transaction.
Liquidity and Capital Resources
Our cash requirements could change materially as a result of the progress of our research and development and clinical programs, licensing activities, acquisitions, divestitures or other corporate developments.
Since our inception on March 22, 2005 through December 31, 2021, we have funded our operations principally through private placements and public offerings of equity securities, which have provided aggregate cash proceeds of approximately $118.2 million. We received $675 million in cash proceeds from the Janssen Transaction in the year ended December 31, 2019 (Another $75 million was placed into escrow and released in 2021, as noted below), and $18.4 million revenue from the manufacturing agreement and clinical trial agreement for the year ended December 31, 2021. In June 2021, we received the remaining $75 million cash as the escrow receivable from the same transaction. In July 2021, we paid $75 million in dividends to shareholders. During the following 12 months, we expect that the revenues from Janssen Transaction Addendum will generate partial of the cash for our research and development activities. At December 31, 2021, we had cash and cash equivalents of $237.0 million as compared to cash and cash equivalents of $237.4 million at December 31, 2020 and $714.6 million at December 31, 2019.The following table summarizes our sources and uses of cash (in thousands):
| Year Ended December 31, |
||||||||||||
| Net cash (used in) provided by: |
2021 |
2020 |
2019 |
|||||||||
| Operating activities |
$ | 69,445 | $ | (65,149 | ) | $ | (18,270 | ) | ||||
| Investing activities |
(3,525 | ) | (3,727 | ) | 674,796 | |||||||
| Financing activities |
(67,008 | ) | (409,724 | ) | 42,096 | |||||||
| Effect of foreign exchange rate on cash and cash equivalents |
705 | 1,372 | 149 | |||||||||
| Net change in cash and cash equivalents |
$ | (383 | ) | $ | (477,228 | ) | $ | 698,771 | ||||
During the years ended December 31, 2021, 2020 and 2019, our operating activities generated net cash of $69.4 million and used net cash of $65.1 million, and $18.3 million respectively. The change in cash from operations for the year ended December 31, 2021 primarily resulted from the $75 million escrow received. The use of net cash in the year ended December 31, 2020 and 2019 primarily resulted from our net incomes and losses and the payment for income tax. The increase in the year ended December 31, 2020 compared with the year ended December 31, 2019 is mainly caused by the 2019 income tax payment $54.1 million paid in the year 2020. Also, we generated revenue from Janssen Transaction in the year 2021 and 2020.
During the years ended December 31, 2021, 2020 and 2019, our investing activities changed net cash of $3.5 million, $3.7 million, and $674.8 million, respectively. The use of cash in the year 2021 and 2020 was for building expansion and the warehouse in construction. The decrease for the year ended December 31, 2020 compared to the year ended December 31, 2019 was primarily due to no $675 million gain from the Janssen Transaction in the year 2019.
During the year ended December 31, 2021 and 2020, our financing activities used net cash proceeds of $67 million and $409.7 million, respectively and during the years ended December 31, 2019, our financing activities provided $42.1 million. In July 2021, we paid $75 million in dividends to shareholders. During the year ended December 31, 2021, employee exercised stock options to purchase a total of 1.1 million shares of our common stock for approximately $8.0 million in net proceeds. During the year ended December 31, 2020, employees exercised stock options to purchase a total of 1.8 million shares of our common stock for approximately $10.3 million in net proceeds. On February 19, 2020, we used approximately $420 million to purchase 14,000,000 common shares at a price of $30.00 per share, relating to the tender offer completed in February 2020. During the year ended December 31, 2019, we sold 4.8 million shares under the Common Shares Purchase Agreement with Piper Jaffray & Co for net proceeds of approximately $38 million. Employees exercised stock options to purchase a total of 771 thousand shares of our common stock for approximately $3.8 million in net proceeds.
We expect to continue to incur operating losses in the future. Further, the clinical manufacturing agreement with Janssen expires on December 2023, after which we do not expect to receive any additional revenue under that agreement. As of December 31, 2021, our principal sources of liquidity were our cash and cash equivalents, which totaled approximately $237.0 million.
Off-Balance Sheet Arrangements
Since inception, we have not engaged in any off-balance sheet activities, including the use of structured finance, special purpose entities or variable interest entities.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURE OF MARKET RISKS
The Company is not currently exposed to material market risk arising from financial instruments, changes in interest rates or commodity prices, or fluctuations in foreign currencies. The Company has no need to hedge against any of the foregoing risks and therefore currently engages in no hedging activities.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
Index to Financial Statements
| Report of Independent Registered Public Accounting Firm (PCAOB Firm ID: |
51 |
| 53 | |
| 54 | |
| 55 | |
| 56 | |
| 57 | |
| 58 | |
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of XBiotech Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of XBiotech Inc. (the Company) as of December 31, 2021 and 2020, the related consolidated statements of operations, comprehensive income (loss), shareholders' equity and cash flows for each of the three years in the period ended December 31, 2021, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2021, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that: (1) relates to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective or complex judgements. The communication of the critical audit matter does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
| Accrued Clinical Trial Cost |
||
| Description of the Matter |
As discussed in Note 2 to the consolidated financial statements, the Company records accruals for clinical trial activities based upon estimates of costs incurred through the balance sheet date that have yet to be invoiced by the clinical trial sites and third-party service providers. The Company’s accrual for clinical trial costs totaled $618 thousand at December 31, 2021.
Auditing the Company’s accruals for clinical trials is challenging due to the fact that information necessary to estimate the accrual includes significant assumptions derived from patient visits and project duration, and such assumptions are accumulated from multiple sources. Specifically, in certain circumstances, the determination of the nature and level of services received during the reporting period requires judgement because the timing and pattern of the vendor invoicing may not correspond to the timing and level of services provided and there may be a delay in invoicing from clinical trial sites and third party service providers. |
|
| How we Addressed the Matter in Our Audit |
To evaluate the accrual for clinical trial cost, our audit procedures included, among others, testing the completeness and accuracy of the underlying data used in the estimates and evaluating the significant assumptions including, but not limited to, patient visits and project duration, that are used by management to estimate the recorded accruals. To assess the reasonableness of the significant assumptions, we corroborated the progress of clinical trials with the Company’s clinical trial management team by performing inquiries of management, obtaining evidence directly from active patient sites, and verifying the active patient sites and currently enrolled patients to contracts or other third party documentation. We performed a look back analysis to identify and evaluate material inconsistencies within the historically recorded accruals and actual amounts realized through the current period. We also obtained subsequent invoices received from such third parties and any pending change orders to assess the impact to the recorded accruals through our report date and compared that to the accrued clinical trial cost as of the balance sheet date. |
/s/
We have served as the Company‘s auditor since 2005.
March 15, 2022
Consolidated Balance Sheets
(in thousands, except share data)
| December 31, 2021 | December 31, 2020 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Accounts receivable | ||||||||
| Escrow receivable | ||||||||
| Deferred cost of goods sold | ||||||||
| Income tax receivable | ||||||||
| Prepaid expenses and other current assets | ||||||||
| Total current assets | ||||||||
| Deferred tax asset | ||||||||
| Property and equipment, net | ||||||||
| Total assets | $ | $ | ||||||
| Liabilities and shareholders’ equity | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued expenses | ||||||||
| Income tax payable | ||||||||
| Total current liabilities | ||||||||
| Long-term liabilities: | ||||||||
| Income tax payable | ||||||||
| Deferred tax liability | ||||||||
| Total liabilities | ||||||||
| Shareholders’ equity: | ||||||||
| Preferred Stock, par value, shares authorized, shares outstanding | ||||||||
| Common stock, par value, shares authorized, and shares outstanding at December 31, 2021 and December 31, 2020, respectively | ||||||||
| Accumulated other comprehensive loss | ||||||||
| Retained earnings | ||||||||
| Total shareholders’ equity | ||||||||
| Total liabilities and shareholders’ equity | $ | $ | ||||||
See accompanying notes.
Consolidated Statements of Operations
(in thousands, except share and per share data)
| Year Ended December 31, | ||||||||||||
| 2021 | 2020 | 2019 | ||||||||||
| Revenue: | ||||||||||||
| Manufacturing revenue | $ | $ | $ | |||||||||
| Clinical trial service revenue | ||||||||||||
| Other revenue | ||||||||||||
| Total revenue | ||||||||||||
| Cost of goods sold: | ||||||||||||
| Manufacturing cost | ||||||||||||
| Clinical trial cost | ||||||||||||
| Total cost of goods sold | ||||||||||||
| Gross margin | ||||||||||||
| Operating expenses: | ||||||||||||
| Research and development | ||||||||||||
| General and administrative | ||||||||||||
| Total operating expenses | ||||||||||||
| Loss from operations | ( | ) | ( | ) | ( | ) | ||||||
| Other income (loss): | ||||||||||||
| Interest income | ||||||||||||
| Gain from Janssen Transaction | ||||||||||||
| Other income (loss) | ( | ) | ( | ) | ||||||||
| Foreign exchange gain (loss) | ( | ) | ( | ) | ||||||||
| Total other income (loss) | ( | ) | ||||||||||
| Income before income taxes | ( | ) | ( | ) | ||||||||
| Benefit (Provision) for income taxes | ( | ) | ||||||||||
| Net income (loss) | $ | ( | ) | $ | ( | ) | $ | |||||
| Net income (loss) per share—basic | $ | ( | ) | $ | ( | ) | $ | |||||
| Shares used to compute basic net income (loss) per share | ||||||||||||
| Net income (loss) per share—diluted | $ | ( | ) | $ | ( | ) | $ | |||||
| Shares used to compute diluted net income (loss) per share | ||||||||||||
See accompanying notes.
Consolidated Statements of Comprehensive Income (Loss)
(in thousands)
| Year Ended December 31, |
||||||||||||
| 2021 |
2020 |
2019 |
||||||||||
| Net income (loss) |
$ | ( |
) | $ | ( |
) | $ | |||||
| Foreign currency translation adjustment |
||||||||||||
| Comprehensive income (loss) |
$ | ( |
) | $ | ( |
) | $ | |||||
See accompanying notes.
Consolidated Statements of Shareholders' Equity
(in thousands)
| Number of Shares | Common Stock Amount | Accumulated Other Comprehensive Income (Loss) |
Retained Earnings | Total |
||||||||||||||||
| Balance at December 31, 2018 |
$ | $ | ( |
) | $ | ( |
) | $ | ||||||||||||
| Net income |
- | |||||||||||||||||||
| Foreign currency translation adjustment |
- | |||||||||||||||||||
| Issuance of common stock, net of issuance cost |
||||||||||||||||||||
| Issuance of common stock under stock option plan |
||||||||||||||||||||
| Subscription receivable |
- | ( |
) | ( |
) | |||||||||||||||
| Collection of stock subscription receivable |
- | |||||||||||||||||||
| Share-based compensation expense |
- | |||||||||||||||||||
| Balance at December 31, 2019 |
$ | ( |
) | |||||||||||||||||
| Net loss |
- | ( |
) | ( |
) | |||||||||||||||
| Tender offer |
( |
) | ( |
) | ( |
) | ( |
) | ||||||||||||
| Foreign currency translation adjustment |
- | |||||||||||||||||||
| Issuance of common stock under stock option plan |
||||||||||||||||||||
| Share-based compensation expense |
- | |||||||||||||||||||
| Balance at December 31, 2020 |
$ | $ | $ | $ | ||||||||||||||||
| Net loss |
- | ( |
) | ( |
) | |||||||||||||||
| Foreign currency translation adjustment |
- | |||||||||||||||||||
| Issuance of common stock under stock option plan |
||||||||||||||||||||
| Dividends |
- | ( |
) | ( |
) | |||||||||||||||
| Share-based compensation expense |
- | |||||||||||||||||||
| Balance at December 31, 2021 |
||||||||||||||||||||
See accompanying notes.
Consolidated Statements of Cash Flows
(in thousands)
| Year Ended December 31, |
||||||||||||
| 2021 |
2020 |
2019 |
||||||||||
| Operating activities |
||||||||||||
| Net income (loss) |
$ | ( |
) | $ | ( |
) | $ | |||||
| Adjustments to reconcile net loss to net cash provided by (used in) operating activities: |
||||||||||||
| Depreciation |
||||||||||||
| Share-based compensation expense |
||||||||||||
| Gain from Janssen Transaction |
( |
) | ||||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Account receivable |
( |
) | ||||||||||
| Income tax receivable |
( |
) | ( |
) | ||||||||
| Deferred cost of goods sold |
( |
) | ||||||||||
| Escrow receivable |
( |
) | ( |
) | ||||||||
| Prepaid expenses and other current assets |
( |
) | ( |
) | ||||||||
| Deferred tax asset |
( |
) | ( |
) | ||||||||
| Accounts payable |
( |
) | ( |
) | ||||||||
| Accrued expenses |
( |
) | ||||||||||
| Contract liabilities |
( |
) | ||||||||||
| Deferred rent |
( |
) | ||||||||||
| Income tax payable |
||||||||||||
| Deferred tax liability |
( |
) | ||||||||||
| Net cash used in operating activities |
( |
) | ( |
) | ||||||||
| Investing activities |
||||||||||||
| Purchase of property and equipment |
( |
) | ( |
) | ( |
) | ||||||
| Cash received from Janssen Transaction |
||||||||||||
| Net cash provided by investing activities |
( |
) | ( |
) | ||||||||
| Financing activities |
||||||||||||
| Issuance of common stock and warrants, net |
( |
) | ||||||||||
| Dividends |
( |
) | ||||||||||
| Issuance of common stock under stock option plan |
||||||||||||
| Collection of subscription receivable |
||||||||||||
| Net cash provided by financing activities |
( |
) | ( |
) | ||||||||
| Effect of foreign exchange rate on cash and cash equivalents |
||||||||||||
| Net change in cash and cash equivalents |
( |
) | ( |
) | ||||||||
| Cash and cash equivalents, beginning of period |
||||||||||||
| Cash and cash equivalents, end of period |
$ | $ | $ | |||||||||
| Supplemental Information: |
||||||||||||
| Accrued purchases of property and equipment |
||||||||||||
| Subscription receivable |
||||||||||||
| Income tax paid |
||||||||||||
See accompanying notes.
Notes to Consolidated Financial Statements
1. Organization
XBiotech Inc. (XBiotech or the Company) was incorporated in Canada on March 22, 2005. XBiotech USA, Inc., a wholly-owned subsidiary of the Company, was incorporated in Delaware, United States in November 2007. XBiotech Germany GmbH, a wholly-owned subsidiary of the Company, was incorporated in Germany in January 2014. The Company’s headquarters are located in Austin, Texas.
Since its inception, XBiotech has focused on advancing technology to rapidly identify and clone antibodies from individuals that have resistance to disease. At the heart of the Company is a proprietary technical knowhow to translate natural human immunity into therapeutic product candidates. The Company has in its pipeline both anti-infective and anti-inflammatory candidate therapeutics derived from this technology.
An area of medical focus for XBiotech are therapies that block a potent substance naturally produced by body, known as interleukin-1 alpha (IL-1a), that mediates tissue breakdown, angiogenesis, the formation of blood clots and inflammation. IL-1a is a protein that is on or in cells of the body and is involved in the body’s response to injury or trauma. In almost all chronic and in some acute injury scenarios (such as stroke or heart attack), IL-1a may mediate harmful disease-related activity.
At the end of 2019, XBiotech sold a True Human™ antibody that blocked IL-1a activity for $
The Company continues to be subject to a number of risks common to companies in similar stages of development. Principal among these risks are the uncertainties of technological innovations, dependence on key individuals, development of the same or similar technological innovations by the Company’s competitors and protection of proprietary technology. The Company’s ability to fund its planned clinical operations, including completion of its planned trials, is expected to depend on the amount and timing of cash receipts from future collaboration or product sales and/or financing transactions. The Company believes that its cash and cash equivalents of $
2. Significant Accounting Policies
Basis of Presentation
These consolidated financial statements have been prepared in conformity with U.S. Generally Accepted Accounting Principles (“US GAAP”). In the opinion of management, the accompanying consolidated financial statements reflect all adjustments (consisting only of normal recurring items) considered necessary to present fairly the Company’s financial position at December 31, 2021 and 2020, the results of its operations and comprehensive loss for the year ended December 31, 2021, 2020 and 2019, and the cash flows for the year ended December 31, 2021, 2020 and 2019.
Basis of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All significant intercompany transactions have been eliminated upon consolidation.
Use of Estimates
The preparation of financial statements in accordance with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the reported values of amounts in the financial statements and accompanying notes. Actual results could differ from those estimates.
Revenue
Revenue from Janssen Agreements
The Company recognizes revenues from its Janssen Agreements as follows.
The Company entered into its clinical manufacturing and clinical trial services arrangements in connection with its sale of certain intellectual property on December 30, 2019. These contracts commenced January 1, 2020. The Company executed a new related manufacturing agreement, which is expected to generate revenue through December 2023. While these agreements are not considered contracts with a customer based on the terms thereof, the Company is applying the revenue recognition guidance by analogy.
XBiotech is still in the research and development phase; however, the eventual output of the Company’s intended ordinary activities will be the licensing of intellectual property and/or sale of commercialized compounds for use in pharmaceutical treatment of disease, not the performance of manufacturing of development stage compounds or clinical trials for others. Although Janssen is not a customer, as these services are not the output of XBiotech’s ordinary activities, the Company evaluated the terms of the agreements and has analogized to Accounting Standards Codification, Topic 606, Revenue from Contracts with Customers (“ASC 606”) for clinical manufacturing and clinical trial services revenue recognition.
Under ASC 606, an entity recognizes revenue when (or as) its customer obtains control of promised goods or services, in an amount that reflects the consideration that the entity expects to receive in exchange for those goods or services. To determine revenue recognition for arrangements that an entity determines are within the scope of ASC 606 (or for those analogized to it), the Company performs the following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenue when (or as) the entity satisfies a performance obligation. The Company only applies the five-step model to contracts (including by analogy) when it is probable that the Company will collect the consideration it is entitled to in exchange for the goods or services it transfers to the counterparty. At contract inception, once the contract is determined to be within the scope of or analogized to ASC 606, the Company assesses the goods or services promised within each contract and determine those that are performance obligations, and assesses whether each promised good or service is distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective performance obligation when (or as) the performance obligation is satisfied.
Manufacturing Revenue
The Company has a Clinical Manufacturing Agreement that it accounts for by analogy to ASC 606, under which it agreed to manufacture bermekimab for use by Janssen in clinical trials, in exchange for payments of $
Clinical Trial Service Revenue
On December 30, 2019, the Company entered into a Transition Services Agreement with Janssen. Pursuant to the Transition Services Agreement, the Company has agreed to continue operational management, on a fee-for-service basis, of two ongoing clinical trials related to bermekimab. The arrangement may continue as long as the clinical trials are ongoing.
Research and Development Costs
All research and development costs are charged to expense as incurred. Research and development costs include salaries and personnel-related costs, consulting fees, fees paid for contract clinical trial research services, the costs of laboratory consumables, equipment and facilities, license fees and other external costs. Costs incurred to acquire licenses for intellectual property to be used in research and development activities with no alternative future use are expensed as incurred as research and development costs.
Nonrefundable advance payments for goods or services to be received in the future for use in research and development activities are deferred and capitalized. The capitalized amounts are expensed as the related goods are delivered or the services are performed.
Clinical Trial Accruals
Expense accruals related to clinical trials are based on the Company’s estimates of services received and efforts expended pursuant to contracts with third party service providers conduct and manage clinical trials on the Company’s behalf. The financial terms of these agreements vary from contract to contract and may result in uneven payment flows. Payments under some of these contracts depend on factors such as the successful enrollment of patients and the completion of clinical trial milestones. In accruing costs, the Company estimates the period over which services will be performed and the level of effort to be expended in each period based upon patient enrollment, clinical site activations, or information provided to the Company by its vendors on their actual costs incurred. Any estimates of the level of services performed or the costs of these services could differ from actual results.
Income Taxes
Income taxes are recorded in accordance with ASC 740, Accounting for Income Taxes (“ASC 740”), which provides for deferred taxes using an asset and liability approach. The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. The Company determines its deferred tax assets and liabilities based on differences between financial reporting and tax bases of assets and liabilities, which are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Valuation allowances are provided if based upon the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized.
The Company makes estimates and judgments in determining the need for a provision for income taxes, including the estimation of its taxable income or loss for the full fiscal year. The Company has accumulated significant deferred tax assets that reflect the tax effects of net operating losses and tax credit carryovers and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Realization of deferred tax assets is dependent upon future earnings. The Company is uncertain about the timing and amount of any future earnings. Accordingly, the Company offsets certain deferred tax assets with a valuation allowance. The Company may in the future determine that certain deferred tax assets are more-likely-than-not to be realized, in which case the Company will reduce its valuation allowance in the period in which such determination is made. If the valuation allowance is reduced, the Company may recognize a benefit from income taxes in its statement of operations in that period.
ASC 740 clarifies the accounting for uncertainty in income taxes recognized in the financial statements and provides that a tax benefit from an uncertain tax position may be recognized when it is more likely than not that the position will be sustained upon examination, including resolutions of any related appeals or litigation processes, based on the technical merits. This interpretation also provides guidance on measurement, derecognition, classification, interest and penalties, accounting in interim periods and disclosure. The Company’s policy for recording interest and penalties associated with uncertain tax positions is to record such items as a component of tax expense.
Share-Based Compensation
The Company accounts for its share-based compensation awards in accordance with ASC Topic 718, Compensation-Stock Compensation (“ASC 718”). ASC 718, which requires all share-based payments to employees, including grants of employee stock options, to be recognized in the statements of operations based on their grant date fair values. For stock options granted to employees and to members of the board of directors for their services on the board of directors, the Company estimates the grant date fair value of each option award using the Black-Scholes option-pricing model. The use of the Black-Scholes option-pricing model requires management to make assumptions with respect to the expected term of the option, the expected volatility of the common stock consistent with the expected life of the option, risk-free interest rates and expected dividend yields of the common stock. To determine the fair value of its common stock, the Company uses the closing price of the Company’s common stock as reported by NASDAQ. For awards subject to service-based vesting conditions, the Company recognizes share-based compensation expense, equal to the grant date fair value of stock options on a straight-line basis over the requisite service period. The Company accounts for forfeitures as they occur rather than on an estimated basis.
Share-based compensation expense recognized for the years ended December 31, 2021, 2020 and 2019 was included in the following line items on the Consolidated Statements of Operations (in thousands).
| Year Ended December 31, | ||||||||||||
| 2021 | 2020 | 2019 | ||||||||||
| Research and development | $ | $ | $ | |||||||||
| General and administrative | ||||||||||||
| Cost of goods sold | ||||||||||||
| Total share-based compensation expense | $ | $ | $ | |||||||||
The fair value of each option is estimated on the date of grant using the Black-Scholes method with the following assumptions:
| Year Ended December 31, | ||||||||||||||||||
| 2021 | 2020 | 2019 | ||||||||||||||||
| Weighted-average grant date fair value per share | $ | $ | $ | |||||||||||||||
| Expected volatility | - | - | ||||||||||||||||
| Risk-free interest rate | - | - | - | |||||||||||||||
| Expected life (in years) | - | - | - | |||||||||||||||
| Dividend yield | ||||||||||||||||||
Due to the adoption of ASU No. 2016-09, “Stock Compensation” on January 1, 2017, the Company accounts for forfeitures as they occur rather than on an estimated basis.
Cash and Cash Equivalents
The Company considers highly liquid investments with a maturity of 90 days or less when purchased to be cash equivalents. Cash and cash equivalents consisted primarily of cash on deposit in U.S., German, and Canadian banks. Cash and cash equivalents are stated at cost which approximates fair value.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to credit risk consist primarily of cash and cash equivalents. The Company holds these investments in highly-rated financial institutions, and limits the amounts of credit exposure to any one financial institution. These amounts at times may exceed federally insured limits. The Company has not experienced any credit losses in such accounts and does not believe it is exposed to any significant credit risk on these funds. The Company has no off-balance sheet concentrations of credit risk, such as foreign currency exchange contracts, option contracts or other hedging arrangements.
Fair Value Measurements
The consolidated financial statements include financial instruments for which the fair market value of such instruments may differ from amounts reflected on a historical cost basis. Financial instruments of the Company consist of cash deposits, accounts and other receivables, accounts payable, and certain accrued liabilities. These financial instruments are held at cost, which generally approximates fair value due to their short-term nature.
The Company follows ASC Topic 820, Fair Value Measurements and Disclosures, which establishes a fair value hierarchy for those instruments measured at fair value that distinguishes between assumptions based on market date (observable inputs) and the Company’s own assumptions (unobservable inputs). The hierarchy consists of three levels:
| • | Level 1—Unadjusted quoted prices in active markets for identical assets or liabilities. |
| • | Level 2—Quoted prices for similar assets and liabilities in active markets, quoted prices in markets that are not active, or inputs which are observable, either directly or indirectly, for substantially the full term of the asset or liability. |
| • | Level 3—Unobservable inputs that reflect the Company’s own assumptions about the assumptions market participants would use in pricing the asset or liability in which there is little, if any, market activity for the asset or liability at the measurement date. |
At December 31, 2021 and 2020, the Company did have any assets or liabilities that are measured at fair value on a recurring basis. The carrying amounts reflected in the balance sheets for cash and cash equivalents, prepaid expenses and other current assets, accounts payable, and accrued expenses approximate their fair values at December 31, 2021 and 2020, due to their short-term nature.
Property and Equipment
Property and equipment, which consists of land, construction in process, furniture and fixtures, computers and office equipment, scientific equipment, leasehold improvements, vehicles and building are stated at cost and depreciated over the estimated useful lives of the assets, with the exception of land and construction in process which are not depreciated, using the straight line method. The useful lives are as follows:
| • Furniture and fixtures | |
| • Office equipment | |
| • Scientific equipment | |
| • Vehicles | |
| • Mobile facility | |
| • Building | |
Costs of major additions and betterments are capitalized; maintenance and repairs, which do not improve or extend the life of the respective assets, are charged to expense as incurred. Upon retirement or sale, the cost of the disposed asset and the related accumulated depreciation are removed from the accounts and the resulting gain or loss is recognized.
Impairment of Long-Lived Assets
The Company periodically evaluates its long-lived assets for potential impairment in accordance with ASC Topic 360, Property, Plant and Equipment. Potential impairment is assessed when there is evidence that events or changes in circumstances indicate that the carrying amount of an asset may not be recovered. Recoverability of these assets is assessed based on undiscounted expected future cash flows from the assets, considering a number of factors, including past operating results, budgets and economic projections, market trends and product development cycles. If impairments are identified, assets are written down to their estimated fair value. The Company has recognized any impairment through December 31, 2021.
Foreign Currency Transactions
Certain transactions are denominated in a currency other than the Company’s functional currency of the U.S. dollar, and the Company generates assets and liabilities that are fixed in terms of the amount of foreign currency that will be received or paid. At each balance sheet date, the Company adjusts the assets and liabilities to reflect the current exchange rate, resulting in a translation gain or loss. Transaction gains and losses are also realized upon a settlement of a foreign currency transaction in determining net loss for the period in which the transaction is settled.
Comprehensive Income (Loss)
ASC Topic 220, Comprehensive Income, requires that all components of comprehensive income (loss), including net income (loss), be reported in the financial statements in the period in which they are recognized. Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources, including unrealized gains and losses on investments and foreign currency translation adjustments.
Segment and Geographic Information
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions on how to allocate resources and assess performance. The Company’s chief operating decision maker is the Chief Executive Officer. The Company and the chief operating decision maker view the Company’s operations and manage its business as operating segment. Substantially all of the Company’s operations are in the U.S. geographic segment.
Net Income/Loss per Share
Net income/loss per share (“EPS”) is computed by dividing net loss by the weighted average number of common shares outstanding during each period. Diluted EPS is computed by dividing net income/loss by the weighted average number of common shares and common share equivalents outstanding (if dilutive) during each period. The number of common share equivalents, which include stock options, is computed using the treasury stock method.
Recent Accounting Pronouncements
Recently Issued Accounting Pronouncements
In June 2016, the FASB issued ASU No. 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. This ASU requires instruments measured at amortized cost to be presented at the net amount expected to be collected. Entities are also required to record allowances for available-for-sale debt securities rather than reduce the carrying amount. On November 15, 2019, the FASB delayed the effective date of the standard for certain small public companies and other private companies. As amended, the effective date of ASC Topic 326 was delayed until fiscal years beginning after December 15, 2022 for SEC filers that are eligible to be smaller reporting companies under the SEC’s definition, as well as private companies and not-for-profit entities. The Company expects that the adoption will not have a material impact on its consolidated financial statements.
3. Revenue
On December 30, 2019, the Company entered into a Transition Services Agreement with Janssen. Pursuant to the Transition Services Agreement, the Company has agreed to continue operational management, on a fee-for-service basis, of ongoing clinical trials related to bermekimab. In consideration for all of the services to be provided, for each calendar quarter during the term of such agreement, Janssen shall pay the Company a fee for such quarter equal to all pass-through costs incurred by the Company during such calendar quarter, exclusive of the allocation of certain internal costs that are not considered pass-through pursuant to the agreement, plus a markup of
In the year 2020, we received $
4. Property and Equipment and Building Construction in Progress
Property and equipment consisted of the following as of December 31, 2021 and 2020 (in thousands):
| 2021 | 2020 | |||||||
| Computer and office equipment | $ | $ | ||||||
| Furniture and fixtures | ||||||||
| Land | ||||||||
| Scientific equipment | ||||||||
| Vehicle | ||||||||
| Building | ||||||||
| Mobile facility | ||||||||
| Construction in process | ||||||||
| Accumulated depreciation | ( | ) | ( | ) | ||||
| $ | $ | |||||||
Depreciation expenses related to property and equipment amounted to approximately $
5. Accrued Expenses
Accrued expenses consist of the following as of December 31, 2021, and 2020 (in thousands):
| 2021 | 2020 | |||||||
| Accrued compensation and related expenses | $ | $ | ||||||
| Accrued professional fees | ||||||||
| Accrued clinical trial expenses | ||||||||
| Other | ||||||||
| $ | $ | |||||||
6. Common Stock
Pursuant to its Articles, the Company has an unlimited number of shares available for issuance with par value.
On January 4, 2020, XBiotech announced that it had commenced a “modified Dutch auction” tender offer to purchase up to $
On February 19, 2020, the Company announced the final results of its “modified Dutch Auction” tender offer. The Company accepted for purchase
From January through December 31, 2020,
From January through December 31, 2021,
7. Common Stock Options
On November 11, 2005, the Board of Directors of the Company adopted the XBiotech Inc. 2005 Incentive Stock Option Plan (the “2005 Plan”), and on March 24, 2015, the board of directors of the Company adopted the XBiotech Inc. 2015 Equity Incentive Plan (the 2015 Plan”) pursuant to which the Company may grant incentive stock and non-qualified stock options to directors, officers, employees or consultants of the Company or an affiliate or other persons as the Compensation Committee may approve.
All options under both Plans will be non-transferable and may be exercised only by the participant, or in the event of the death of the participant, a legal representative until the earlier of the options’ expiry date or the first anniversary of the participant’s death, or such other date as may be specified by the Compensation Committee.
The term of the options is at the discretion of the Compensation Committee, but may not exceed
The number of common shares reserved for issuance to any one person pursuant to the 2005 Plan shall not, in aggregate, exceed
A summary of changes in common stock options issued under the 2005 Plan and under the 2015 Plan is as follows:
| Options | Exercise Price | Weighted-Average | |||||||||||
| Options outstanding at December 31, 2018 | $ | - | $ | $ | |||||||||
| Granted | - | ||||||||||||
| Exercised | ( | ) | - | ||||||||||
| Forfeitures | ( | ) | - | ||||||||||
| Options outstanding at December 31, 2019 | $ | - | $ | ||||||||||
| Granted | - | ||||||||||||
| Exercised | ( | ) | - | ||||||||||
| Forfeitures | ( | ) | - | ||||||||||
| Options outstanding at December 31, 2020 | $ | - | $ | ||||||||||
| Granted | $ | - | $ | ||||||||||
| Exercised | ( | ) | $ | - | $ | ||||||||
| Forfeitures | ( | ) | $ | - | $ | ||||||||
| Options outstanding at December 31, 2021 | $ | - | $ | ||||||||||
The weighted average fair value of the options issued to directors, employees and consultants during the fiscal years ended December 31, 2021, 2020 and 2019, was $
As of December 31, 2021, there was approximately $
8. Net Income/Loss Per Share
The following summarizes the computation of basic and diluted net income(loss) per share for the years ended December 31, 2021, 2020 and 2019 (in thousands, except share and per share data):
| Year Ended December 31, | ||||||||||||
| 2021 | 2020 | 2019 | ||||||||||
| Net income (loss) | $ | ( | ) | $ | ( | ) | $ | |||||
| Weighted-average number of common shares—basic | ||||||||||||
| Net income (loss) per share—basic | $ | ( | ) | $ | ( | ) | $ | |||||
| Weighted-average number of common shares—diluted | ||||||||||||
| Net income (loss) per share—diluted | $ | ( | ) | $ | ( | ) | $ | |||||
The following potentially dilutive securities outstanding, prior to the use of the treasury stock method or if-converted method, have been excluded from the computation of diluted weighted-average common shares outstanding, because including them would have had an anti-dilutive effect due to the losses reported.
| Year Ended December 31, | ||||||||||||
| 2021 | 2020 | 2019 | ||||||||||
| Stock options | ||||||||||||
| Warrants to purchase common stock | ||||||||||||
| Total | ||||||||||||
9. Income Taxes
The components of income before income taxes are as follows (in thousands):
|
| Years Ended December 31, | |||||||||||
| 2021 | 2020 | 2019 | ||||||||||
| United States | $ | $ | ( | ) | $ | |||||||
| Canada | ( | ) | ( | ) | ||||||||
| Other Foreign | ( | ) | ( | ) | ||||||||
| Total | $ | ( | ) | $ | ( | ) | $ | |||||
The components of the provision for income taxes are as follows for the years ended December 31, 2021, 2020, and 2019 (in thousands):
| Current | 2021 | 2020 | 2019 | |||||||||
| United States | $ | $ | $ | |||||||||
| Canada | ( | ) | ( | ) | ||||||||
| Other Foreign | ||||||||||||
| Total | $ | ( | ) | $ | ( | ) | ||||||
| Deferred | ||||||||||||
| United States | ||||||||||||
| Canada | ( | ) | ( | ) | ||||||||
| Other Foreign | ( | ) | ||||||||||
| Total | ( | ) | ( | ) | ||||||||
| Total income tax expense | $ | ) | $ | ( | ) | $ | ||||||
The provision for income taxes differs from the amount computed by applying the Canada statutory rate to pre-tax income as follows for the years ended December 31, 2021, 2020, and 2019:
| 2021 | 2020 | 2019 | ||||||||||
| Income tax benefit computed at federal tax rate | % | % | % | |||||||||
| Capital gains and foreign exchange | % | % | ( | )% | ||||||||
| Change in valuation allowance | % | ( | )% | ( | )% | |||||||
| Tax Credits generated | % | % | ||||||||||
| Prior year adjustments | % | % | ( | )% | ||||||||
| Changes in Uncertain Tax Positions | ( | )% | ( | )% | % | |||||||
| Stock compensation and other | ( | )% | ( | )% | ( | )% | ||||||
| Total | % | % | % | |||||||||
Significant components of the Company’s deferred tax assets and liabilities as of December 31, 2021, 2020, and 2019 are as follows (in thousands):
| 2021 | 2020 | |||||||
| Deferred tax assets: | ||||||||
| Noncapital losses | $ | $ | ||||||
| Research and other credits | ||||||||
| Stock based compensation | ||||||||
| Share issue costs | ||||||||
| Accrued liabilities | ||||||||
| Foreign Exchange | ||||||||
| Total deferred tax assets | ||||||||
| Less: Valuation allowance | ( | ) | ( | ) | ||||
| Total deferred tax asset including valuation allowance | ||||||||
| Deferred tax liabilities: | ||||||||
| Depreciation | ||||||||
| Prepaid assets | ||||||||
| Uncollectible debts | ||||||||
| Foreign exchange | ||||||||
| Total deferred tax liabilities | ||||||||
| Net deferred tax asset (liabilities) | $ | ( | ) | $ | ||||
As of December 31, 2021 and 2020, the Company had unused net operating losses of approximately $
As of December 31, 2021 and 2020, the Company had unused gross research and other tax credits of approximately $
The valuation allowance decreased by $
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. With the exception of certain items that will generate cash refunds through loss carryback claims in the next year, the Company has established a full valuation allowance in the U.S and Canada due to uncertainties regarding the realization of these deferred tax assets based upon the Company’s lack of earnings history and the Company’s expectation to continue incurring significant expenses for the foreseeable future. The valuation allowances in Japan and Switzerland were released in 2021 as both companies were dissolved during 2021.
The Company has not provided Canadian income taxes or withholding taxes on the undistributed earnings of the U.S. subsidiary or the excess of the financial reporting over the tax basis of our investment in the U.S. subsidiary as of December 31, 2021 because these amounts are considered to be indefinitely reinvested in the U.S. The Company intends to use available cash to expand our research and development and manufacturing facilities, and also fund the development of our manufacturing processes. The amount of the outside basis difference that is indefinitely reinvested is $
The Company applies the accounting guidance in ASC 740 related to accounting for uncertainty in income taxes. The Company’s reserves related to taxes are based on a determination of whether, and how much of, a tax benefit taken by the Company in its tax filings or positions is more likely than not to be realized following resolution of any potential contingencies present related to the tax benefit. A reconciliation of the beginning and ending amount of unrecognized tax benefits as of December 31, 2021, 2020 and 2019 is as follows (in thousands):
| 2021 | 2020 | 2019 | ||||||||||
| Balance as of January 1 | ||||||||||||
| Additions based on tax positions related to the current year | ||||||||||||
| Additions for tax positions of prior years | ||||||||||||
| Reductions for tax positions of prior years | ||||||||||||
| Settlements | ||||||||||||
| Balance at December 31 | ||||||||||||
The Company recognized interest and penalties related to unrecognized tax benefits of $
The Company files federal income tax returns in Canada, U.S, Switzerland, Germany, and Japan. The Company filed final tax returns in Switzerland and Japan during 2021. The Company also files income tax returns in the state of Texas in the U.S. The statute of limitations for assessment by local taxing authorities is open for tax years ended after December 2012 and all years since 2008 for the U.S. remain open until such time the 2019 tax year statute of limitation closes. There are currently no federal or state income tax audits in progress.
10. Subsequent Events
On February 2, 2022, XBiotech Inc. announced the Company “Inks Clinical Manufacturing Deal with Janssen”, which is the 2019 Janssen Transaction Addendum. XBiotech will continue to manufacture bermekimab for use by Janssen in its clinical trials through December 2023 in exchange for total payments of about $
11. Selected Quarterly Financial Data (Unaudited)
Selected Quarterly Financial Data (Unaudited) for the year ended December 31, 2021 and 2020 is presented below (in thousands except per share data):
| 2021 | First Quarter | Second Quarter | Third Quarter | Fourth Quarter | ||||||||||||
| Loss from operations | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| Net loss | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Net loss per share—basic and diluted | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| 2020 | First Quarter | Second Quarter | Third Quarter | Fourth Quarter | ||||||||||||
| Loss from operations | $ | ( | ) | $ | ( | ) | $ | ( | ) | $ | ( | ) | ||||
| Net loss | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Net loss per share—basic and diluted | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES.
Management's Evaluation of our Disclosure Controls and Procedures
As of the end of the year covered by this Annual Report on Form 10-K, an evaluation was carried out by the Company’s management, with the participation of the Chief Executive Officer and Principal Financial Officer, of the effectiveness of the Company’s disclosure controls and procedures, as defined in Rule 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934. Based on such evaluation, the Chief Executive Officer and Principal Financial Officer concluded that the Company’s disclosure controls and procedures are effective to ensure that information required to be disclosed in the reports the Company files or furnishes under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and regulations, and are operating in an effective manner.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). We conducted an assessment of the effectiveness of our internal control over financial reporting based on the criteria set forth in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on our assessment, we have concluded that our internal control over financial reporting was effective as of December 31, 2021, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with GAAP.
Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting that occurred during the fourth quarter of the year ended December 31, 2021 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints, and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs.
None.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
We incorporate by reference the information required by this Item with respect to directors and the Audit Committee from the information under the caption “ELECTION OF DIRECTORS,” including in particular the information under “Nominating and Corporate, Governance and Review Committee” , “Audit Committee”, “Report of the Audit Committee & the Board of Directors”, “Code of Ethics” and “Delinquent Section 16(a) Reports” and “EXECUTIVE OFFICERS” contained in our definitive Proxy Statement (the “Proxy Statement”), which we will file on or about April 29, 2022 with the Securities and Exchange Commission in connection with the solicitation of proxies for our 2022 Annual Meeting of Stockholders to be held on June 18, 2022.
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item is incorporated herein by reference to the information contained under the sections captioned “EXECUTIVE COMPENSATION”, “DIRECTOR COMPENSATION”, “Compensation Committee Interlocks and Insider Participation,” “Employment Arrangements” and “Compensation Committee Report” of the Proxy Statement.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The information required by this item will be set forth under the heading “Security Ownership of Certain Beneficial Owners and Management" in our Proxy Statement and is incorporated herein by reference.
The information required by Item 201(d) of Regulation S-K will be set forth in the section headed “Equity Compensation Plan Information” in our Proxy Statement and is incorporated herein by reference.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
The information required by this item will be set forth in the section headed “Transactions with Related Persons” in our Proxy Statement and is incorporated herein by reference.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The information required by this item will be set forth in the section headed “Ratification of Selection of Independent Registered Public Accounting Firm” in our Proxy Statement and is incorporated herein by reference.
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
Financial Statements
See Index to Consolidated Financial Statements under Item 8 of Part II.
Financial Statement Schedules
None
| Exhibit |
Description |
|
| 101 |
The following financial statements from the XBiotech Inc. Quarterly Report on Form 10-Q for the year ended December 31, 2021, formatted in Inline Xtensible Business Reporting Language (iXBRL): (i) condensed consolidated balance sheets, (ii) condensed consolidated statements of operations, (iii) condensed consolidated statements of comprehensive loss, (iv) ) condensed consolidated statements of shareholders’ equity; (v) condensed consolidated statements of cash flows and (vi) notes to condensed consolidated financial statements (detail tagged). |
|
| 104 |
Cover Page Interactive Data File (embedded within the iXBRL document). |
|
| + |
Indicates management contract or compensatory plan |
|
| * |
Filed herewith |
Not applicable.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on March 15, 2022.
| XBIOTECH INC., |
||
| /S/ JOHN SIMARD | ||
| Name: |
John Simard |
|
| Title: |
President and Chief Executive Officer |
|
| (Principal Executive Officer) |
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature and Title |
Date |
| /S/ JOHN SIMARD John Simard, Chief Executive Officer (Principal Executive Officer) and Director |
March 15, 2022 |
| /S/ QUEENA HAN Queena Han, Vice President of Finance & Human Resources (Principal Financial Officer and Principal Accounting Officer) |
March 15, 2022 |
| /S/ W. THORPE MCKENZIE W. Thorpe McKenzie, Director |
March 15, 2022 |
| /S/ Jan-Paul Waldin Jan-Paul Waldin, Director |
March 15, 2022 |
| /S/ Donald MacAdam Donald MacAdam, Director |
March 15, 2022 |
| /S/ Peter Libby Peter Libby, Director |
March 15, 2022 |