UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2017
Commission file number 001-37437
XBIOTECH INC.
(Exact name of Registrant as specified in its charter)
| British Columbia, Canada | N/A |
| (State or other jurisdiction of incorporation or organization) | (IRS Employer Identification No.) |
8201 E. Riverside Drive, Bldg. 4, Suite 100
Austin TX 78744
(Address of principal executive offices, including zip code)
Telephone Number (512) 386-2900
(Registrant's telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered |
| Common Stock, par value $0.0001 per share | NASDAQ Global Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No ☐
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ý
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the
Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ý | Non-accelerated filer ☐ | Smaller Reporting Company ☐ | |||
| Emerging growth company ý |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No ý
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant as of December 31, 2017, was approximately $139,630,732, based upon the closing sales price for the registrant’s common stock, as reported on the NASDAQ Global Market. The calculation of the aggregate market value of voting and non-voting common equity excludes 10,387,160 shares of common stock the registrant held by executive officers, directors and shareholders that the registrant concluded were affiliates of the registrant on that date. Exclusion of such shares should not be construed to indicate that any such person possesses the power, direct or indirect, to direct or cause the direction of management or policies of the registrant or that such person is controlled by or under common control with the registrant.
As of March 15, 2018, 35,439,272 shares of the registrant’s Common Stock were outstanding.
Documents incorporated by reference:
Certain portions, as expressly described in this Annual Report on Form 10-K, of the registrant’s Proxy Statement for the 2018 Annual Meeting of the Stockholders, to be filed not later than 120 days after the end of the year covered by this Annual Report, are incorporated by reference into Part III of this Annual Report where indicated.
TABLE OF CONTENTS
PART I
| 2 |
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This annual report contain forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, included in this annual report, including, without limitation, statements regarding the assumptions we make about our business and economic model, our dividend policy, business strategy and other plans and objectives for our future operations, are forward-looking statements.
These forward-looking statements include declarations regarding our management’s beliefs and current expectations. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “would,” “could,” “expects,” “plans,” “contemplate,” “anticipates,” “believes,” “estimates,” “predicts,” “projects,” “intend” or “continue” or the negative of such terms or other comparable terminology, although not all forward-looking statements contain these identifying words. Forward-looking statements are subject to inherent risks and uncertainties in predicting future results and conditions that could cause the actual results to differ materially from those projected in these forward-looking statements. Some, but not all, of the forward-looking statements contained in this annual report include, among other things, statements about the following:
| · | our ability to obtain regulatory approval to market and sell Xilonix™ in the United States, Europe and elsewhere; |
| · | the initiation, timing, cost, progress and success of our research and development programs, preclinical studies and clinical trials for Xilonix™ and other product candidates; |
| · | our ability to advance product candidates into, and successfully complete, clinical trials; |
| · | our ability to successfully commercialize the sale of Xilonix™ in the United States, Europe and elsewhere; |
| · | our ability to recruit sufficient numbers of patients for our future clinical trials for our pharmaceutical products; |
| · | our ability to achieve profitability; |
| · | our ability to obtain funding for our operations, including research funding; |
| · | our ability to identify additional new products using our True Human™ antibody discovery platform; |
| · | the implementation of our business model and strategic plans; |
| · | our ability to develop and commercialize product candidates for orphan and niche indications independently; |
| · | our commercialization, marketing and manufacturing capabilities and strategy; |
| · | our ability to protect our intellectual property and operate our business without infringing upon the intellectual property rights of others; |
| · | our expectations regarding federal, state and foreign regulatory requirements; |
| 3 |
| · | the therapeutic benefits, effectiveness and safety of our product candidates; |
| · | the accuracy of our estimates of the size and characteristics of the markets that may be addressed by our products and product candidates; |
| · | the rate and degree of market acceptance and clinical utility of Xilonix™ and future products, if any; |
| · | the timing of and our collaborators’ ability to obtain and maintain regulatory approvals for our product candidates; |
| · | our expectations regarding market risk, including interest rate changes and foreign currency fluctuations; |
| · | our belief in the sufficiency of our cash flows to meet our needs for at least the next 12 to 24 months; |
| · | our expectations regarding the timing during which we will be an emerging growth company under the JOBS Act; |
| · | our ability to engage and retain the employees required to grow our business; |
| · | our future financial performance and projected expenditures; |
| · | developments relating to our competitors and our industry, including the success of competing therapies that are or become available; and |
| · | estimates of our expenses, future revenue, capital requirements and our needs for additional financing. |
You should also read the matters described in the “Risk Factors” and the other cautionary statements made in this annual report as being applicable to all related forward-looking statements wherever they appear in this annual report. We cannot assure you that the forward-looking statements in this annual report will prove to be accurate and therefore you are encouraged not to place undue reliance on forward-looking statements. You should read this annual report completely.
| 4 |
PART I
| ITEM 1 | BUSINESS |
Overview
XBiotech Inc. (“XBiotech” or the “Company) is a pre-market biopharmaceutical company engaged in discovering and developing True Human™ monoclonal antibodies for treating a variety of diseases. True Human™ monoclonal antibodies are those which occur naturally in human beings—as opposed to being derived from animal immunization or otherwise engineered. We believe that naturally occurring monoclonal antibodies have the potential to be safer and more effective than their non-naturally occurring counterparts. XBiotech is focused on developing its True Human™ pipeline and manufacturing system.
The majority of our efforts to date have been concentrated on developing our lead product candidate, MABp1 (also known as Xilonix™, CA-18C3, CV-18C3, RA-18C3, and T2-18C3), a therapeutic antibody which specifically neutralizes interleukin-1 alpha (IL-1 a). IL-1a is a pro-inflammatory protein produced by leukocytes and other cells, where it plays a key role in inflammation. When unchecked, inflammation can contribute to the development and progression of a variety of different diseases, such as cancer, vascular disease, inflammatory skin disease, and diabetes. Our clinical studies have shown that blocking IL-1a with our lead product candidate may have a beneficial effect on several diseases.
In June of 2017, the Company announced discontinuation of its global phase III study for treatment of advanced colorectal cancer (CRC) patients under U.S. FDA regulatory guidance after an Independent Data Monitoring Committee (IDMC) performed the second prospectively planned, unblinded analysis and recommended the early termination of the study since the findings were not sufficient to meet efficacy or the threshold for continuation, which involved a prospectively defined acceptance boundary for the interim analysis. XBiotech completed a Phase III study in Europe under European Medicine Agency (EMA) regulatory guidance for the treatment of symptomatic CRC in which the study’s primary endpoint was met. XBiotech submitted a Marketing Authorization Application (MAA) to the EMA in March 2016. In May 2017, the Company announced that it received a negative opinion from the EMA’s Committee for Medicinal Products for Human Use (“CHMP”) for the MAA in Europe. XBiotech subsequently pursued the EMA’s re-examination procedure in which new Rapporteurs were assigned to reevaluate the initial opinion after receiving the Company’s grounds for re-examination. In September 2017, the CHMP issued its opinion on the re-examination of the Company’s MAA and maintained its initial negative opinion issued in May 2017. No further EMA procedures are available to us for the present application. Although all statutory procedures available to the Company as provided by the EMA are exhausted in the application process, the Company continues to pursue clinical development of its oncology program.
An investigator sponsored study was launched in September 2017 at Cedars Sinai Medical Center located in Los Angeles, California for MABp1 to be used in combination with OnivydeⓇ (Irinotecan liposome injection) and 5-fluorouracil/folinic acid for treatment of advanced pancreatic adenocarcinoma. Andrew Hendifar, M.D., Principal Investigator of the study, Medical Oncology lead for the Gastrointestinal Disease Research Group at Cedars-Sinai and Co-Director of Pancreas Oncology, will be leading the study which is planned to enroll a total of 16 patients at the Cedars-Sinai Medical Center. The Phase I study will determine a maximum tolerated dose as well as assess efficacy. Other studies with MABp1 in oncology are also being considered and these will be announced if and when further progress is made in these directions.
We have also investigated our lead product candidate in clinical trials for other inflammatory conditions, including vascular disease (which led to fast track designation from the FDA to develop MABp1 as a therapy to reduce the need for re-intervention after treatment of peripheral vascular disease with angioplasty or other endovascular methods of treatment), type II diabetes, acne, psoriasis, pyoderma gangrenosum (PG) and hidradenitis suppurativa (HS). Data from each of these trials have been published in journals, with the exception of PG. A listing of these publications is included in the Summary of Clinical Findings to Date section of this document.
The most recent publication comes from a completed phase II, double-blinded, placebo-controlled investigator-sponsored study evaluating MABp1 for the treatment of hidradenitis suppurativa (HS), an inflammatory skin disease of such severity that it is often treated through surgical removal of lesions. In October 2017, data from this study was published in the Journal of Investigative Dermatology, which highlights results of twenty patients enrolled in the study with moderate to severe hidradenitis that had progressed on standard therapies. Patients received MABp1 for 12 weeks and were then followed an additional 12 weeks to observe durability of treatment. Efficacy measures include assessment of Hidradenitis Suppurativa Clinical Response (HiSCR) scores, a validated method for evaluating efficacy in HS patients, as well as quality of life assessment (as measured by the Dermatology Life Quality Index, (DLQI). Study results demonstrated a response rate in patients treated with MABp1 versus placebo of 60% vs 10%, respectively (p=0.035).
| 5 |
The Company has completed its Phase I/II study evaluating dosing, safety and efficacy of its novel antibody, 514G3. 514G3 was developed from a healthy human donor with natural antibodies effective at neutralizing Methicillin-resistant Staphylococcus aureus (MRSA) and non-MRSA forms of Staphylococcus aureus (S. aureus). 514G3 works to eliminate the principle immune evasion mechanism of the bacteria, allowing white blood cells to detect and destroy the bacteria. 514G3 has potential to treat all strains of MRSA and can be used without consideration for strain-specific resistance to various antibiotics. As a True Human monoclonal antibody, 514G3 is expected to be well tolerated without the side effects or risks of antibiotics, including the lack of risk of antibiotic resistance. This proprietary antibody received Fast Track Designation by the FDA for the treatment of all forms of S. aureus infections, including Methicillin-resistant S. aureus (MRSA). Top line results from the Phase I/II study were announced in April 2017 and reported a reduction in adverse events and shorter hospitalization associated with the 514G3 therapy, even with 514G3-treated subjects tending to be sicker than those receiving placebo. Research involving 514G3 was published in January 2018 in the journal PLOS ONE in a manuscript titled, “A Natural Human Monoclonal Antibody Targeting Staphylococcus Protein A Protects Against Staphylococcus aureus (S. aureus) Bacteremia.”
The Company is also developing other infectious disease therapies in its pipeline. XBiotech is using its True Human™ antibody technology to develop a first-in-class oral monoclonal antibody against clostridium difficile (C. diff). C. diff is a bacterium that can cause severe infections in the gastrointestinal tract. The infection is greatest for individuals who are being treated with antibiotics, those that are hospitalized or the elderly, particularly those in care facilities. Additionally, about 1 in 5 patients that become infected with C. diff experience a relapse and need to be re-treated. Recent outbreaks and increased virulence of C. diff suggest the urgent need to identify novel approaches to treat the disease. The Company has now shortlisted fourteen anti-C. diff antibody candidates against three different bacterial surface proteins, which are now being tested in vitro and in-vivo efficacy studies in C. diff infection models. The Company has shortlisted eight therapeutic antibody candidates against herpes zoster that are being tested in vitro for efficacy. In parallel, the Company is also working on generating cell lines for large scale manufacturing of these therapeutics, and working on formulating the same. The isolation of candidate True HumanTM therapeutic antibodies against influenza are ongoing, and the Company already has shortlisted a set of antibodies to be tested in vitro.
In November 2017, data was presented at the American Heart Association’s Scientific Sessions which provided the first evidence that IL-1a is associated with Neutrophil Extracellular Traps (NETs) and plays a key role in endothelial activation and thrombogenesis. The data stems from a Material Transfer Agreement (MTA) signed in 2016 with Brigham and Women’s Hospital and Massachusetts General Hospital in which Dr. Peter Libby, a renowned Cardiovascular medicine specialist at Brigham and Women’s Hospital (BWH) and the Mallinckrodt Professor of Medicine at Harvard Medical School, was named principal investigator of the research. The study probed the influence of NETs on the endothelial cell (EC) functions related to erosion-associated thrombosis. The data shows that exposure of human saphenous veins ECs (HSVECs) to NETs cause an increase in expression of cell surface adhesions such as VCAM-1 and ICAM-1, which may participate in atherogenesis. In addition, pre-treatment of NETs with MABp1or IL-1R antagonist, but not with an anti-IL-1β-neutralizing antibody, blocked the initiation of VCAM-1, ICAM-1, and TF expression, each of which have been linked to coronary thrombosis. In conclusion, it was found that NETs act to increase thrombogenicity in vitro through a response mediated by IL-1⍺. These data suggest a potentially important role for MABp1 therapy in heart disease. It also expands treatment opportunities for other inflammatory diseases in which NETs play a deleterious role, such as cancer as well as pulmonary, autoimmune and gastrointestinal diseases. The Company is currently conducting internal research to identify the presence of IL-1alpha in proteins extracted from NET preparations by mass sepectrometry.
Our True Human™ antibody therapeutics are developed in-house using our proprietary discovery platform. Identifying True Human™ antibodies useful for therapeutics may involve screening thousands of blood donors. To distinguish the clinically relevant antibodies from irrelevant background antibody molecules in donor bloods, we use our Super High Stringency Antibody Mining (SHSAM™) technology. After we identify donors, we undertake the complex process of identifying the unique genes for producing the native antibody. Once the nucleic acid sequence is isolated, we are able to introduce these sequences into engineered production cells to manufacture large quantities of product candidate for use in humans. All patents and other intellectual property relating to both the composition of matter and methods of use of our True Human™ antibodies were developed internally by us. We manufacture these antibodies using a proprietary expression system licensed from Lonza Sales AG. The manufacturing process we have developed incorporates both proprietary and non-proprietary technology.
A key aspect of our manufacturing system involves the use of simple disposable bioreactor technology. Our manufacturing operations are currently located within our 86,000 ft2 operations in Austin, Texas. Part of this includes a nearly 40,000 ft2 commercial manufacturing facility that the Company opened in August 2016. This new facility is located on XBiotech’s own 48-acre location just 15 minutes from the Texas capital in Austin. The building will provide a significant increase in the Company’s manufacturing and quality operations in anticipation of commercialization of the Company’s product pipeline. The new facility will increase about ten-fold the Company’s current production capacity. Completion of this building is the first phase in the Company’s plan to develop the 48-acre property to house additional production facilities, laboratories and administrative operations, creating a headquarter campus as the center of its global operations.
| 6 |
A Background on Therapeutic Antibodies
A century ago scientists and physicians envisioned being able to custom design therapeutic agents that were highly specific for a single biological target. By selectively attacking disease while sparing healthy tissue, these “magic bullets” were thought to be ideal therapeutic agents. It was not until the early 1970’s, however, that this vision was realized when Kohler and Milstein developed a ground-breaking method for making target-specific monoclonal antibodies—a Nobel prize-winning endeavor. Using this new approach, numerous monoclonal antibody-based research, diagnostic, and therapeutic products have been developed.
Kohler and Milstein’s discovery was based on their knowledge that the immune system of higher animals produces antibodies as a method of protecting them from various, potentially damaging, agents, such as viruses, bacteria, and diseased cells. White blood cells, known as B cells, produce billions of different types of antibodies, each with a unique potential to selectively attach to and neutralize different disease targets. The vast array of possible treatments based on antibodies led to the development of what is now a major industry around the use of therapeutic antibodies.
True Human™ Antibodies
White blood cells in the human body secrete billions of different antibodies that circulate through the blood to react and protect us from toxins, infectious agents or even other unwanted substances produced by our body. True Human™ antibodies, as the name implies, are simply those that are derived from a natural antibody identified from the blood of an individual. To develop a True Human™ antibody therapy, donors are screened to find an individual that has a specific antibody that matches the desired characteristics needed to obtain the intended medical benefit. White blood cells from that individual are obtained, the unique gene that produced the antibody is cloned, and the genetic information is used to produce an exact replica of the antibody sequence. A True Human™ antibody is, therefore, not to be confused with other marketed antibodies, such as so-called fully human antibodies—where antibody reactivity is developed through gene sequence engineering in the laboratory.
| 7 |
Fundamental Science of True Human™ Antibodies
To appreciate the background safety and tolerability of True Human™ antibodies, it is important to consider the fundamental biology of natural antibody production.
Billions of different white blood cells secrete billions of unique antibodies every day into circulation. The vast number of different antibodies (and cells that produce them), are essential to enable adequate molecular diversity to ward off all potential infectious or toxic threats. In other words, since antibodies act to bind and thereby neutralize unwanted agents, any given circulating antibody must be able to react with a potentially limitless number of existing or evolving disease entities.
The staggering number of different antibodies needed to achieve this level of preparedness, however, is a daunting concept from a genetics point of view. If an individual antibody gene was needed to encode each of a billion different antibodies, there would be 20,000 times as many genes needed just for antibodies as there would be needed to encode the rest of the entire human genome. Individual cells would need to be gigantic, and monumental resources would be required to make, copy and maintain all of the DNA. Clearly, the system of antibodies could not have evolved to protect us, had not an elegant solution emerged to deal with this genetic conundrum.
Thus, a hallmark of the immune physiology of all vertebrates (all have antibodies) is the ability to recombine and selectively mutate a relatively small number of gene segments to create a phenomenal and effectively unlimited number of antibody genes. By rearranging, recombining and mutating the genetic code, specialized white blood cells, or B lymphocytes, are able to create an unlimited array of antibody genes. The consequence of this genetic engineering, however, is that each antibody gene is unique to the individual B lymphocyte that created it—and no copy of the gene exists in the human germline. The only place to find a unique antibody gene is in the individual cells that created it.
The extraordinary process of gene rearrangement and mutation results in a multitude of unique B lymphocytes and consequently an incredibly diverse repertoire of antibodies in any given individual.
Elucidating the mechanisms behind the production of unique antibody genes must be considered one of the major achievements of medical research in the 20th century. Yet unfolding this mystery created another problem to solve: If antibodies were not produced from genes encoded in the human genome and the products of these genes were new to the body, why were these antibody molecules not recognized by the immune system as foreign substances—like any other foreign substance that they were intended to eradicate? How could the body distinguish the apparently “foreign” antibody molecules from the bona fide infectious intruders?
Unraveling the genetics of antibody production led to another major advance in medicine: the discovery of how an endless array of antibody proteins could be made in a way that individual molecules were always tolerated by the body.
In the early 1990s, research began to demonstrate that the production of antibodies was not an unregulated process. Rather, it was learned that the antibodies produced by each and every B lymphocyte were subject to intense scrutiny. Studies showed that B lymphocytes which produced acceptable antibodies were stimulated to grow while those that produced “autoreactive” antibodies were not. B lymphocytes that produced “good” antibodies were stimulated to proliferate, and enabled to produce copious amounts of antibody in the event it was needed to ward off a harmful agent. B lymphocytes that rearranged genes to produce antibodies that were ineffective or were autoreactive were given signals that instructed them to engage in a process of programmed cell death. Thus B lymphocytes producing harmful or useless antibodies are simply killed off. This mechanism for creating antibody diversity on the one hand, while protecting the individual from a mass of unwanted or intolerable antibody molecules on the other, was as elegant as it was fundamental to the success of vertebrate immune physiology.
This process of “selection” has been elucidated in great detail. There can be no more important feature of immune physiology than the process of selection. Selection is a fundamental step to enable the body to produce an extremely diverse set of antibody molecules without, in the process, producing an array of novel molecules that cause harm.
| 8 |
Industry Context
Until now each and every therapeutic antibody on the market has been derived from animals and/or through gene sequence modification in the laboratory to produce a desired antibody reactivity. Marketed antibodies to date, described as “fully human”, are not derived from human gene sequences that have undergone the crucial process of selection in a human.
Without exception, all marketed products to date that are described as “fully human”, are in fact engineered and are not selected based on natural tolerance in the human body. The use of the term “fully human” to describe these products has thus created considerable confusion. To our knowledge, there are at present no True Human™ antibodies manufactured, using recombinant protein technology, currently marketed. If successful in clinical development, our lead product candidate is expected to be the first True Human™ therapeutic antibody to be commercialized.
Platform Technology
There are significant technical challenges in identifying and cloning genes for True Human™ antibodies. A key problem to overcome can be to first identify individuals with the desired antibody reactivity. This can involve screening hundreds of donors to enable the identification of a single, clinically relevant antibody—discovered from literally trillions of irrelevant background antibody molecules in the blood of donors. We screen human donors to find an individual who has in his or her blood a specific antibody that we believe will be protective against a certain disease. White blood cells from that individual can then be isolated, and the unique gene that produced the antibody obtained. We currently obtain blood donor samples through a Research and Collaboration Agreement with the South Texas Blood & Tissue Center, a Texas 501(c)(3) non-profit corporation. See "Intellectual Property- Other Commercial Licenses."
Novel cloning technologies developed at XBiotech have enabled us to clone the crucial antibody gene sequences from these donors in order to reproduce a True Human™ antibody for use in clinical therapy. A True Human™ monoclonal antibody should therefore not be confused with other marketed therapeutic monoclonal antibodies, such as those currently referred to as “fully human” antibodies.
Market Opportunity
We have a number of indications in various stages of clinical or pre-clinical development with significant market opportunities. These include oncology, dermatology, diabetes, and other inflammatory conditions, as well as infectious disease indications. The potential market opportunities in these various indications are vast and we believe our research and manufacturing technologies, designed to more rapidly, cost-effectively and flexibly produce new therapies, will be advantageous in each market space.
Our Strategy
Our objective is to fundamentally change the way drugs are developed and commercialized, and become a leading biopharmaceutical company focused on the discovery, development and commercialization of therapeutic True Human™ antibodies. The key goals of our business strategy are to:
| • | Obtain regulatory approval to market and sell our lead product candidate and/or our other product candidates in the United States, Europe and other markets, and begin commercial sale; |
| • | Continue our research and clinical work on infectious diseases, including S. aureus; |
| • | Advance our pipeline of therapeutic antibodies and other possible clinical programs in strategic therapeutic areas; |
| • | Discover other True Human™ antibody therapies using our proprietary platform; and |
| • | Leverage our manufacturing technology. |
| 9 |
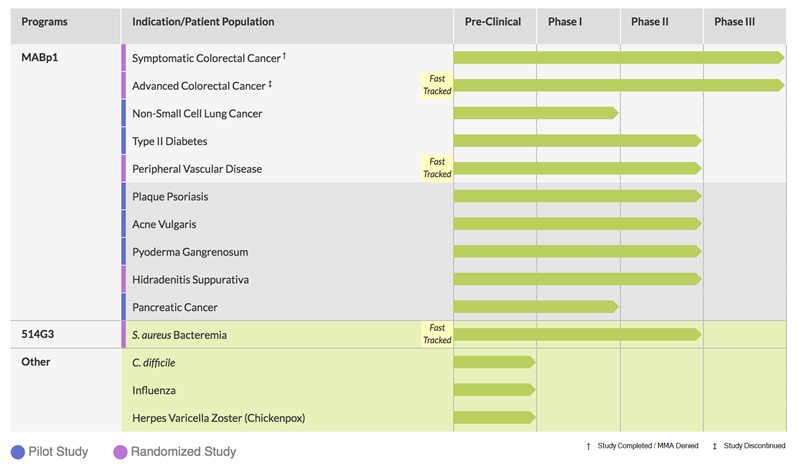
Product Pipeline
Our product development status for the fourth quarter of 2017 was as follows:
Competition
The therapeutic antibody space is dynamic as there continues to be a highly active commercial pipeline of therapeutic antibodies globally, involving a complex array of development cycles as products reach the end of their patent life and as new candidate products proceed into pivotal studies and approach registration. There are numerous independent reviews on the subject in both trade journals and academic press (one such example being Reichert JM, Antibodies to watch in 2018 MAbs. 2018 Jan 4:1-21).
We believe True Human™ therapeutic antibodies have important differentiating factors from other monoclonal antibodies currently marketed. The unique activity of our lead anti-cancer therapeutic has the potential ability to both improve well-being and extend life. We feel our product candidates will be highly differentiated in the market place for therapeutics in various indications including but not limited to cancer and dermatology. However, regardless of the potential advantages or uniqueness of our lead product candidate in the market, we do expect these products to compete head-to-head with the numerous existing candidate antibody products in development, including emerging biosimilar therapeutic antibodies.
Current Clinical and/or Regulatory Activity
European Registration Study Oncology
| 10 |
XBiotech has completed a Phase III study in Europe for treatment of symptomatic CRC. XBiotech proceeded with the submission of a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) in March 2016. In May 2017, the Company announced that it received a negative opinion from the EMA’s Committee for Medicinal Products for Human Use (“CHMP”) for the MAA in Europe. XBiotech subsequently pursued the EMA’s re-examination procedure in which new Rapporteurs were assigned to reevaluate the initial opinion after receiving the Company’s grounds for re-examination. In September 2017, the CHMP issued its opinion on the re-examination of the Company’s MAA and maintained its initial negative opinion issued in May 2017. No further EMA procedures are available to us for the present application.
In the double-blind placebo-controlled Phase III study, 309 patients were randomized (2:1) to receive Xilonix™ plus best supportive care (BSC) versus placebo plus BSC. Study participants had failed all available chemotherapy and had metastatic disease with one or more symptoms of metabolic dysfunction and functional impairment (i.e., elevated systemic inflammation, unintentional weight loss, pain, fatigue, anorexia). Patients were required to have an Eastern Cooperative Oncology Group (ECOG) function status of only 1 or 2. Elderly patients (>70 years of age) were eligible as well, in contrast to many studies of anti-cancer therapies in advanced disease. The primary endpoint in the study was a composite measure of stable or increased lean body mass (as measured by dual energy X-ray absorptiometry (DEXA)) and stability or improvement of two of three symptom measures of the EORTC QLQ-C30 (pain, fatigue, or anorexia) at week 8 compared with baseline measurements. Secondary endpoints evaluated paraneoplastic thrombocytosis and systemic inflammation. The results of this study were published in The Lancet Oncology in January 2017 in an article titled, “MABp1 as a Novel Antibody Treatment for Advanced Colorectal Cancer: A Randomised, Double-Blind, Placebo-Controlled, Phase 3 Study.”
US Registration Study Oncology
Enrollment was recently completed in the global randomized, double-blind, placebo-controlled Phase III study evaluating the Company’s lead product candidate as a treatment for advanced CRC. The Phase III study is being conducted under Fast Track designation from the FDA and involves over 600 advanced cancer patients from various countries worldwide.
In February 2017, the Company announced an affirmative outcome in the first interim analysis of this study, prospectively planned to occur at 50% of events. The Independent Data Monitoring Committee (IDMC) performed the unblinded analysis and reported the study had no safety concerns and that indications of efficacy thus far were sufficient to recommend proceeding with the study without modification. Per the DMC charter, this was the first of two interim efficacy analyses planned prior to the final analysis for overall survival. In June 2017, the Company announced discontinuation of the study after the IDMC performed the second prospectively planned, unblinded analysis at 75% of events and recommended the early termination of the study since the findings were not sufficient to meet efficacy or the threshold for continuation, which involved a prospectively defined acceptance boundary for the interim analysis (≤ p=0.08).
The double-blind, placebo-controlled Phase III study was randomized 2:1 with patients receiving Xilonix™ or placebo plus best supportive care. Patients were required to have metastatic CRC, and were required to have failed regimens that include flouropyrimidines, oxaliplatin, irinotecan, and Cetuximab or Panitumumab for patients with V-Ki-ras2Kirsten rat sarcoma (KRAS) mutation. Patients continued on the study until there was evidence of radiographic progression. The primary endpoint of this study was overall survival, with secondary endpoints including objective response rate, progression free survival, change in lean body mass as measured by DEXA, and patient reported quality of life using the validated European Organization for Research and Treatment of Cancer Quality of Life in cancer Questionnaire (EORTC QLQ C30 questionnaire).
| 11 |
Phase I Pancreatic Cancer Combination Study
In September 2017, an investigator sponsored study was launched at Cedars Sinai Medical Center located in Los Angeles, California for MABp1 to be used in combination with OnivydeⓇ (Irinotecan liposome injection) and 5-fluorouracil/folinic acid for treatment of advanced pancreatic adenocarcinoma. Andrew Hendifar, M.D., Principal Investigator of the study, Medical Oncology lead for the Gastrointestinal Disease Research Group at Cedars-Sinai and Co-Director of Pancreas Oncology, will be leading the study which is planned to enroll a total of 16 patients at the Cedars-Sinai Medical Center. The Phase I study will determine a maximum tolerated dose as well as assess efficacy.
Phase I/II Study for Staphylococcus aureus
In December 2016, the Company completed enrollment in its randomized, placebo-controlled Phase I/II study evaluating dosing, safety and efficacy of the Company’s novel antibody therapy, 514G3. This proprietary antibody therapy has received Fast Track Designation by the FDA for the treatment of all forms of Staphylococcus aureus infections, including Methicillin-resistant S. aureus (MRSA). Top line results from this study were announced in April 2017 and reported a reduction in adverse events and shorter hospitalization associated with the 514G3 therapy, even with 514G3-treated subjects tending to be sicker than those receiving placebo.
In the study, hospitalized adult patients with confirmed blood infections were randomized 3:1 (514G3 vs placebo) during a dose escalation phase to establish a phase II dose. The phase II portion was randomized 2:1 at the established phase II dose of 40mg/kg. A total of 52 patients were enrolled: 36 received 514G3 and 16 received placebo. Thirty of the 36 patients that were given 514G3 received the established phase II dose (40mg/kg).
| 12 |
Phase II Study for Hidradenitis Suppurativa (HS)
The Company completed enrollment in a double-blinded, placebo-controlled investigator-sponsored study for HS, an inflammatory skin disease of such severity that is often treated through surgical removal of lesions. Twenty patients with moderate to severe HS that had progressed on standard therapies were enrolled in this study. Patients received MABp1 for 12 weeks and were then followed for an additional 12 weeks to observe durability of treatment. Efficacy measures include assessment of Hidradenitis Suppurativa Clinical Response (HiSCR) scores, a validated method for evaluating efficacy in HS patients, as well as quality of life assessment (as measured by DLQI. The Company announced topline results from this study in February 2017, reporting a response rate in patients treated with MABp1 versus placebo of 60% vs 10%, respectively (p=0.035). Results from this study were published in the peer-reviewed, Journal of Investigative Dermatology in November 2017 in an article titled, “MABp1 Targeting IL-1α for Moderate to Severe Hidradenitis Suppurativa Not Eligible for Adalimumab: a Randomized Study.”
Summary of Clinical Findings to Date
Safety
Our lead product under development is derived from a natural human immune response. We expected that this would facilitate better tolerability when used as a therapeutic compared to humanized or “fully human” monoclonal antibodies. Antibody therapies are known to be associated with significant risk for infusion reactions, including serious anaphylactic reactions. We believe that these reactions are the result of using antibodies that were not derived from natural human immunity but rather had engineered specificities. Based on scientific principles of antibody physiology, a fundamentally important premise was that our True HumanTM antibody therapy should be safer and result in less infusion-related complications than engineered human antibodies when used in clinical studies.
Therapeutic monoclonal antibodies, even those so-called “fully human,” have been associated with infusion reactions. Comparably administration of our lead product candidate is associated with a reduced number of infusion related reactions.
To date the Company has published data from 7 of its clinical studies. The table below outlines each of these publications.
| Indication | Journal | Title |
| Oncology | The Lancet Oncology | MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study |
| Oncology | The Lancet Oncology | MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose-escalation and expansion study |
| Oncology | Investigational New Drugs | Xilonix, a novel true human antibody targeting the inflammatory cytokine interleukin-1 alpha, in non-small cell lung cancer |
| Psoriasis | JAMA Dermatology | Open-label trial of MABp1, a true human monoclonal antibody targeting interleukin 1α, for the treatment of psoriasis |
| Acne | Journal of Drugs in Dermatology | An open label, phase 2 study of MABp1 monotherapy for the treatment of acne vulgaris and psychiatric comorbidity |
| Cardiovascular | Journal of Vascular Surgery | A randomized phase II study of Xilonix, a targeted therapy against interleukin 1α, for the prevention of superficial femoral artery restenosis after percutaneous revascularization |
| Diabetes | Journal of Diabetes and Its Complications | Safety, pharmacokinetics, and preliminary efficacy of a specific anti-IL-1alpha therapeutic antibody (MABp1) in patients with type 2 diabetes mellitus |
| Hidradenitis Suppurativa | Journal of Investigative Dermatology | MABp1 Targeting Interleukin-1Alpha for Moderate to Severe Hidradenitis Suppurativa not Eligible for Adalimumab: A Randomized Study |
| 13 |
Intellectual Property
XBiotech has developed a large international intellectual property (IP) portfolio to protect important aspects of its technology, services and products, including patents, trademarks and trade secrets. As of December 31, 2017, XBiotech’s patent portfolio consisted of 18 patent families, and included 97 issued patents or allowed patent applications, and 111 (not including allowed) pending patent applications in various countries around the world. XBiotech’s IP portfolio is designed to protect XBiotech’s drug products, therapies and to some extent, its discovery technology. It includes patents and applications that protect MABp1 as a composition of matter and methods of using anti-IL-1a antibodies for the treatment of various diseases including cancer, vascular disorders, inflammatory skin diseases, diabetes, and arthritis. XBiotech’s IP portfolio also includes patents and applications directed to some aspects of our proprietary antibody discovery platform, as well as treating and preventing S. aureus infections.
XBiotech owns or licenses the rights to the patent families described in more detail below.
A. Interleukin-1 Alpha Antibodies and Methods of Use. This patent family relates to the development of IL-1a-specific True HumanTM monoclonal antibodies, including MABp1. As of December 31, 2017, XBiotech has been granted 31 patents in this family; including nine in the U.S., and others in Australia, Chile, China, Europe, Hong Kong, Indonesia, Israel, Japan, South Korea, Malaysia, Mexico, New Zealand, the Philippines, Russia, Singapore, and South Africa. Unless extended, patents in this family expire in 2029.
B. Treatment of Cancer with Anti- IL-1α Antibodies. This patent family relates to the use of anti- IL-1α antibodies to inhibit the metastatic potential of tumors by interrupting the role that tumor-derived IL-1α plays in tumor metastasis. As of December 31, 2017, XBiotech has been granted four patents for this family; including one in Australia, one in Canada, one in Japan, and one in Europe. Unless extended, patents in this family expire in 2027.
C. Treatment of Neoplastic Diseases. This patent family relates to the administration of anti- IL-1α antibodies to treat various tumor-associated diseases and the administration of a monoclonal antibody that specifically binds IL-1α to reduce the size of tumors in human patients suffering from cancer. As of December 31, 2017, XBiotech has been granted nine patents for this family including two in Australia, one in Europe, one in Japan, one in Mexico, one in New Zealand, one in the Philippines, one in Singapore, and one in South Africa.
D. Diagnosis, Treatment, and Prevention of Vascular Disorders. This patent family relates to methods of diagnosing, treating and preventing a variety of vascular disorder using IL-1a autoantibody. As of December 31, 2017, Xbiotech has been granted seven patents in this family, including two in the U.S., one in Australia, one in Europe and two in Japan. Unless extended, patents in this family expire in 2026.
E. IL-1 Alpha Immunization Induces Autoantibodies Protective Against Atherosclerosis. This patent family relates to the use of IL-1α in a vaccine to generate anti-IL-1a antibodies to protect against atherosclerosis. As of December 31, 2017, XBiotech has been granted patents for this family in Australia and Europe. Unless extended, patents in this family expire in 2027.
F. Targeting Pathogenic Monocytes. This patent family relates to the discovery that IL-1α is expressed on the proinflammatory, disease-associated CD14+CD16+ monocyte subset in humans, and describes targeting IL-1α to deplete these pathogenic cells or to modulate their function. As of December 31, 2017, XBiotech has been granted four patents in this family; including two in the U.S., one in Australia, and one in Japan. Unless extended, patents in this family expire in 2029.
G. Arthritis Treatment. This patent family relates to the administration of anti- IL-1α antibodies to treat conditions associated with arthritis. As of December 31, 2017, XBiotech has been granted three patents in this family, including two in Australia and one in Israel,. Unless extended, patents in this family expire in 2031.
H. Cachexia Treatment. This patent family relates to the administration of anti- IL-1α antibodies to treat cachexia. As of December 31, 2017, XBiotech has been granted four patents in this family, including one in the U.S., one in Japan, one in Russia, and one in South Africa. A further patent application has been allowed in Australia. Unless extended, patents in this family expire in 2032.
| 14 |
I. Treatment of Diabetes. This patent family relates to the administration of anti- IL-1α antibodies to treat diabetes. As of December 31, 2017, XBiotech has been granted one U.S. patent. Unless extended, patents in this family expire in 2033.
J. Treating Vascular Disease and Complications Thereof. This patent family relates to the administration of IL-1α targeting agents to reduce the chance or severity of a major adverse clinical event occurring in a patient who has received or is expected to receive surgical treatment for a stenosed blood vessel. As of December 31, 2017, XBiotech has been granted one patent in South Africa. Applications in this family were pending in the U.S., Australia, Canada, China, Europe, Israel, Japan, Mexico, New Zealand, Russia, South Korea, and Hong Kong. Unless extended, patents in this family expire in 2033.
K. Treatment of Inflammatory Skin Disease and Psychiatric Conditions. This patent family relates to the administration of anti- IL-1α antibodies to treat inflammatory skin diseases such as acne and psoriasis, as well as to treat psychiatric conditions such as anxiety. As of December 31, 2017, XBiotech has been granted three patents in this family, including one in the U.S., one in Australia, and one in Japan. A further application was allowed in the U.S. Other applications were pending in the U.S., Australia, Canada, China, Europe, Japan, Hong Kong, and South Korea. Unless extended, patents in this family expire in 2033.
L. Methods, compositions, and kits for reducing anti-antibody responses. This patent family relates to methods and compositions for reducing immune system-mediated reactions to allotypic determinants on administered antibody products. As of December 31, 2017, XBiotech has been granted one Australian patent in this family. Unless extended, patents in this family expire in 2030.
M. Identifying Affinity-Matured Human Antibodies. This patent family relates to methods and compositions for identifying affinity-matured True HumanTM monoclonal antibodies from donors. As of December 31, 2017, XBiotech has been granted eight patents in this family (four in the U.S., one in Australia, one in China, one in Russia, and one in Hong Kong)), and applications were pending in Australia, Canada, China, Europe, India, Israel, Japan, Mexico, Russia, and South Korea. Unless extended, patents in this family expire in 2032.
N. Compositions and Methods for Treating S. Aureus Infections. This patent family relates to antibodies for preventing and treating S. aureus infections. As of December 31, 2017, XBiotech has been granted eight patents, in this family, including three in the U.S, one in Colombia, one in Japan, one in Russia, one in Singapore, and one in South Korea. Two further applications were allowed in China and Mexico. Applications were pending in the U.S., Australia, Brazil, Canada, Chile, China, Europe, India, Indonesia, Israel, Japan, Malaysia, Mexico, New Zealand, the Philippines, Russia, Singapore, South Korea, and South Africa. Unless extended, patents in this family expire in 2035.
O. Antibacterial antibodies and Methods of Use. This patent family relates to antibodies for preventing and treating S. aureus infections. XBiotech acquired the use of patents within this family pursuant to its exclusive license agreement with STROX Biopharmaceuticals, LLC. As of December 31, 2017, this patent family includes three patents in the U.S., each expiring in 2019, unless extended.
P. Staphylococcus Aureus-Specific Antibody Preparations. This patent family relates to antibodies for preventing and treating S. aureus infections. XBiotech acquired use of patents within this family pursuant to its exclusive license agreement with STROX Biopharmaceuticals, LLC. As of December 31, 2017, this patent family includes one Australian and one Israeli patent. Unless extended, patents in this family expire in through 2029.
Q. Treatment of Hidradenitis Suppurativa. This invention relates to the use of antibodies (Abs) which specifically bind interleukin-1α (IL-1α) to treat Hidradenitis suppurativa. As of December 31, 2017, XBiotech has one pending U.S. application.
R. Treatment of S. Aureus Infections. This invention relates to the use of antibodies (Abs) which specifically bind interleukin-1α (IL-1α) for treating S. aureus infections. As of December 31, 2017, XBiotech has one pending U.S. application.
Because the patent positions of pharmaceutical, biotechnology, and diagnostics companies are highly uncertain and involve complex legal and factual questions, the patents owned and licensed by us, or any future patents, may not prevent other companies from developing similar or therapeutically equivalent products or ensure that others will not be issued patents that may prevent the sale of our products or require licensing and the payment of significant fees or royalties. Furthermore, to the extent that any of our future products or methods are not patentable, that such products or methods infringe upon the patents of third parties, or that our patents or future patents fail to give us an exclusive position in the subject matter claimed by those patents, we will be adversely affected. We may be unable to avoid infringement of third party patents and may have to obtain a license, defend an infringement action, or challenge the validity of the patents in court. A license may be unavailable on terms and conditions acceptable to us, if at all. Patent litigation is costly and time consuming, and we may be unable to prevail in any such patent litigation or devote sufficient resources to even pursue such litigation.
| 15 |
Employees
At December 31, 2017, we had 51 employees, 8 of whom hold a Ph.D. or M.D. (or equivalent) degree. None of our employees are represented by a labor union or covered by a collective bargaining agreement, nor have we experienced work stoppages. We believe that relations with our employees are good.
Corporate Information
XBiotech Inc. was incorporated in Canada on March 22, 2005. XBiotech USA Inc., a wholly-owned subsidiary of the Company, was incorporated in Delaware, United States in November 2007. XBiotech Switzerland AG, a wholly-owned subsidiary of the Company, was incorporated in Zug, Switzerland in August 2010. XBiotech Japan KK, a wholly-owned subsidiary of the Company, was incorporated in Tokyo, Japan in March 2013. XBiotech Germany GmbH, a wholly-owned subsidiary of the Company, was incorporated in Germany in January 2014. The Company’s headquarters are located in Austin, Texas.
Investor Information
We maintain an Internet website at http://www.xbiotech.com. The information on our website is not incorporated by reference into this annual report on Form 10-K and should not be considered to be a part of this annual report on Form 10-K. Our reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, our quarterly reports on Form 10-Q and our current reports on Form 8-K, and amendments to those reports, are accessible through our website, free of charge, as soon as reasonably practicable after these reports are filed electronically with, or otherwise furnished to, the SEC. We also make available on our website the charters of our audit committee, compensation committee, nominating and corporate governance committee, as well as our corporate governance guidelines and our code of business conduct and ethics. In addition, we intend to disclose on our web site any amendments to, or waivers from, our code of business conduct and ethics that are required to be disclosed pursuant to the SEC rules.
| 16 |
| ITEM 1A | RISK FACTORS |
Risks Related to our Financial Condition and Capital Requirements
We have incurred significant losses every quarter since our inception and anticipate that we will continue to incur significant losses in the future.
We are a pre-market pharmaceutical company with no revenue and a limited operating history. Investment in pharmaceutical product development is highly speculative because it entails substantial upfront capital expenditures and significant risk that any potential product candidate will fail to demonstrate adequate efficacy or an acceptable safety profile, gain regulatory approval or become commercially viable. We do not have any products approved by regulatory authorities for marketing or commercial sale and have not generated any revenue from product sales, or otherwise, to date, and we continue to incur significant research, development and other expenses related to our ongoing operations. As a result, we are not profitable and have incurred losses in every reporting period since our inception in 2005. For the years ended December 31, 2015, 2016, and 2017, we reported a net loss of $37.5 million, $52.8 million and $33.2 million respectively. As of December 31, 2017, we had an accumulated deficit since inception of approximately $216.6 million.
We expect to continue to incur significant expenses and operating losses for the foreseeable future. We anticipate these losses will increase as we continue the research and development of, and seek regulatory approvals for our lead product candidate in various indications and any of our other product candidates, and potentially begin to commercialize any products that may achieve regulatory approval. We may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our financial condition. The amount of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenues. Our prior losses and expected future losses have had, and will continue to have, an adverse effect on our financial condition. If our lead product candidate or any other product candidate fails in clinical trials or does not gain regulatory approval, or if approved and fails to achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods. We will need to raise significant additional funding, which may not be available on acceptable terms, if at all. Failure to obtain this necessary capital when needed may force us to delay, limit or terminate our product development efforts or other operations.
Since inception, we have dedicated a majority of our resources to the discovery and development of our proprietary preclinical and clinical product candidates, and we expect to continue to expend substantial resources doing so for the foreseeable future. These expenditures will include costs associated with conducting research and development, manufacturing product candidates and products approved for sale, conducting preclinical experiments and clinical trials and obtaining and maintaining regulatory approvals, as well as commercializing any products later approved for sale. During the year ending December 31, 2017, we recognized approximately $26.4 million in expenses associated with research and development and clinical trials.
We completed our initial public offering on April 15, 2015 and a registered direct offering in March 2017. However, the net proceeds from these offerings and cash on hand may not be sufficient to complete clinical development of any of our product candidates nor may it be sufficient to commercialize any product candidate. Accordingly, we may require substantial additional capital beyond the offering to continue our clinical development and potential commercialization activities. Our future capital requirements depend on many factors, including but not limited to:
| • | the number and characteristics of the future product candidates we pursue; |
| • | the scope, progress, results and costs of researching and developing any of our future product candidates, and conducting preclinical research and clinical trials; |
| • | the timing of, and the costs involved in, obtaining regulatory approvals for any future product candidates we develop; |
| • | the cost of future commercialization activities for our lead product candidate and the cost of commercializing any future products approved for sale; |
| 17 |
| • | the cost of manufacturing our future products; and |
| • | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patents, including litigation costs and the outcome of any such litigation. |
We are unable to estimate the funds we will actually require to complete research and development of our product candidates or the funds required to commercialize any resulting product in the future or the funds that will be required to meet other expenses. Our operating plan may change as a result of many factors currently unknown to us, and our expenses may be higher than expected. We may need to seek additional funds sooner than planned, through public or private equity or debt financings, government or other third-party funding, marketing and distribution arrangements and other collaborations, strategic alliances and licensing arrangements or a combination of these approaches. Raising funds in the future may present additional challenges and future financing may not be available in sufficient amounts or on terms acceptable to us, if at all.
Raising additional capital may cause dilution to our existing shareholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
The terms of any financing arrangements we enter into may adversely affect the holdings or the rights of our shareholders and the issuance of additional securities, by us, or the possibility of such issuance, may cause the market price of our shares to decline. The sale of additional equity or convertible securities would dilute all of our shareholders. The incurrence of indebtedness would result in increased fixed payment obligations and, potentially, the imposition of restrictive covenants. Those covenants may include limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. We could also be required to seek funds through arrangements with collaborators or otherwise at an earlier stage than otherwise would be desirable resulting in the loss of rights to some of our product candidates or other unfavorable terms, any of which may have a material adverse effect on our business, operating results and prospects. Additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our products.
We are subject to an ongoing shareholder class action lawsuit in California, which may adversely affect our business, financial condition, results of operations and cash flows.
We and certain of our executive officers and directors are defendants in a federal securities class action lawsuit filed in state court in California. The federal securities class action lawsuit in Texas was dismissed in October 2016. The California lawsuit and the previous lawsuit in Texas, including the current status, are described in Part I, Item 3 “Legal Proceedings” in this Form 10-K. We continue to believe the ongoing lawsuit to be without merit and intend to vigorously defend ourselves, however we cannot guarantee any particular outcome. These and any similar future matters may divert our attention from our ordinary business operations, and we may incur significant expenses associated with them (including, without limitation, substantial attorneys’ fees and other fees of professional advisors and potential obligations to indemnify the underwriter for our initial public offering and current and former officers and directors who are or may become parties to or involved in such matters). Depending on the outcome of such matters, we could be required to pay material damages and/or suffer other penalties, remedies or sanctions. Accordingly, the ultimate resolution of this pending matter or any similar future matters could have a material adverse effect on our business, financial condition, results of operations, cash flows, liquidity and could negatively impact the trading price of our common stock. Any existing or future shareholder lawsuits could also adversely impact our reputation, our relationships with our customers and our ability to generate revenue.
Risks Related to Our Business
We currently have no source of product revenue and may never become profitable.
To date, we have not generated any revenues from commercial product sales, or otherwise. Our ability to generate revenue in the future from product sales and achieve profitability will depend upon our ability, alone or with any future collaborators, to commercialize products successfully, including our lead product candidate or any future product candidates that we may develop, in-license or acquire in the future. Even if we are able to achieve regulatory approval successfully for our lead product candidate or any future product candidates, we do not know when any of these products will generate revenue from product sales, if at all. Our ability to generate revenue from product sales from our lead product candidate or any of our other product candidates also depends on a number of additional factors, including our ability to:
| 18 |
| • | complete development activities, including the necessary clinical trials; |
| • | complete and submit new drug applications, or NDAs, to the US Food and Drug Administration, or FDA, and obtain regulatory approval for indications for which there is a commercial market; |
| • | complete and submit applications to, and obtain regulatory approval from, foreign regulatory authorities such as the European Medicines Agency, or EMA; |
| • | establish our manufacturing operations; |
| • | develop a commercial organization capable of sales, marketing and distribution for our lead product candidate and any products for which we obtain marketing approval and intend to sell ourselves in the markets in which we choose to commercialize on our own; |
| • | find suitable distribution partners to help us market, sell and distribute our approved products in other markets; |
| • | obtain coverage and adequate reimbursement from third-party payers, including government and private payers; |
| • | achieve market acceptance for our products, if any; |
| • | establish, maintain and protect our intellectual property rights; and |
| • | attract, hire and retain qualified personnel. |
In addition, because of the numerous risks and uncertainties associated with pharmaceutical product development, including that our lead product candidate or any other product candidates may not advance through development or achieve the endpoints of applicable clinical trials, we are unable to predict the timing or amount of increased expenses, or when or if we will be able to achieve or maintain profitability. In addition, our expenses could increase beyond expectations if we decide to or are required by the FDA, or foreign regulatory authorities, to perform studies or trials in addition to those that we currently anticipate. Even if we are able to complete the development and regulatory process for our lead product candidate or any other product candidates, we anticipate incurring significant costs associated with commercializing these products.
Even if we are able to generate revenues from the sale of our lead product candidate or any other product candidates that may be approved, we may not become profitable and may need to obtain additional funding to continue operations. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and be forced to reduce our operations.
Our future success is dependent on the regulatory approval and commercialization of our lead product candidate and any of our other product candidates.
We do not have any products that have gained regulatory approval. The Company’s Phase III symptomatic colorectal cancer study has been completed and XBiotech proceeded with the submission of a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) in March 2016. In May 2017, the Company announced that it received a negative opinion from the EMA’s Committee for Medicinal Products for Human Use (“CHMP”) for the MAA in Europe. XBiotech subsequently pursued the EMA’s re-examination procedure in which new Rapporteurs were assigned to reevaluate the initial opinion after receiving the Company’s grounds for re-examination. In September 2017, the CHMP issued its opinion on the re-examination of the Company’s MAA and maintained its initial negative opinion issued in May 2017. In June 2017, XBiotech reported discontinuation of its second Phase III study, a double-blind placebo controlled study for improving survival in metastatic colorectal cancer, following the Independent Data Monitoring Committee’s (IDMC) second prospectively planned, unblinded interim analysis at 75% of events in the study.
| 19 |
As a result, our ability to finance our operations and generate revenue, are substantially dependent on our ability to obtain regulatory approval for, and, if approved, to successfully commercialize our lead product candidate in a timely manner. We cannot commercialize our lead product candidate or our other product candidates in the U.S. without first obtaining regulatory approval for each product from the FDA; similarly, we cannot commercialize our lead product candidate or our other product candidates outside of the U.S. without obtaining regulatory approval from comparable foreign regulatory authorities, including the EMA. The FDA review process typically takes years to complete and approval is never guaranteed. Before obtaining regulatory approvals for the commercial sale of our lead product candidate or any of our other potential product candidates for a target indication, we must demonstrate with substantial evidence gathered in preclinical and well-controlled clinical studies, including two well-controlled Phase III studies, and, with respect to approval in the U.S. to the satisfaction of the FDA, and in Europe, to the satisfaction of the EMA, that the product candidate is safe and effective for use for that target indication; and that the manufacturing facilities, processes and controls are adequate. Obtaining regulatory approval for marketing of our lead product candidate or our future product candidates in one country does not ensure we will be able to obtain regulatory approval in other countries. A failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in other countries.
Even if our lead product candidate or any of our other product candidates were to successfully obtain approval from the FDA or comparable foreign regulatory authorities, any approval might contain significant limitations related to use restrictions for specified age groups, warnings, precautions or contraindications, or may be subject to burdensome post-approval studies or risk management requirements. If we are unable to obtain regulatory approval for our lead product candidate in one or more jurisdictions, or any approval contains significant limitations, we may not be able to obtain sufficient funding or generate sufficient revenue to continue the development of any of our other product candidates that we are developing or may discover, in-license, develop or acquire in the future. Also, any regulatory approval of our lead product candidate or our other product candidates, once obtained, may be withdrawn. Furthermore, even if we obtain regulatory approval for our lead product candidate or our other product candidates, its commercial success will depend on a number of factors, including the following:
| • | development of a commercial organization within XBiotech or establishment of a commercial collaboration with a commercial infrastructure; |
| • | establishment of commercially viable pricing and obtaining approval for adequate reimbursement from third-party and government payers; |
| • | our ability to manufacture quantities of our lead product candidate using commercially satisfactory processes and at a scale sufficient to meet anticipated demand and enable us to reduce our cost of manufacturing; |
| • | our success in educating physicians and patients about the benefits, administration and use of our lead product candidate; |
| • | the availability, perceived advantages, relative cost, relative safety and relative efficacy of alternative and competing treatments; |
| • | the effectiveness of our own or our potential strategic collaborators’ marketing, sales and distribution strategy and operations; |
| • | acceptance as a safe and effective therapy by patients and the medical community; and |
| • | a continued acceptable safety profile following approval. |
Many of these factors are beyond our control. If we are unable to successfully commercialize our lead product candidate, we may not be able to earn sufficient revenues to continue our business.
New laws or regulations may be promulgated or modified in the United States, in Europe, or other jurisdictions that could impact our ability to receive the necessary approvals to successfully market and commercialize our lead product candidate or any of our other product candidates.
| 20 |
The pharmaceutical and biotechnology industry is one of the most regulated on a state, federal and international level. There are a number of laws, regulations, and court decisions which impact the daily activities of our business. As a result, we must ensure that strategies and planning in relation to our product candidates are in line with the current regulations governing our industry. When there are changes in leadership, whether within the U.S., or elsewhere, we must anticipate the possibility of shifts in regulatory policies as they pertain to our business. New or modified regulations may impact our ability to quickly respond with updates to our programs. While we may be able to anticipate certain changes, policy statements often are not always translated into actionable legislation. We continue to track updates and changes internally to ensure we are in compliance with regulatory authority guidelines and expectations. Court decisions at both the state and federal level can also impact the way in which we operate and make specific product related program decisions. New laws, regulations, or court orders could materially alter or impact our ability to receive necessary approvals from regulatory authorities to market and commercialize our lead product candidate or any of our other product candidates.
We submitted a Marketing Authorization Application to the EMA for our lead product candidate after successfully completing a Phase III study in Europe which was ultimately denied by the Agency. Even if the EMA or FDA approves our lead product candidate in the future, there are a number of obstacles to consider in the post-marketing approval and commercialization processes in Europe and/or the U.S.
In March 2016, we submitted a Marketing Authorization Application to the EMA Committee for Human Medicinal Products, or CHMP, for the Phase III study of our lead product candidate completed in Europe during Q4 2015. In May 2017, the Company announced that it received a negative opinion from the EMA’s Committee for Medicinal Products for Human Use (“CHMP”) for the MAA in Europe. XBiotech subsequently pursued the EMA’s re-examination procedure in which new Rapporteurs were assigned to reevaluate the initial opinion after receiving the Company’s grounds for re-examination. In September 2017, the CHMP issued its opinion on the re-examination of the Company’s MAA and maintained its initial negative opinion issued in May 2017. In June 2017, XBiotech reported discontinuation of its second Phase III study, a double-blind placebo controlled study for improving survival in metastatic colorectal cancer, following the Independent Data Monitoring Committee’s (IDMC) second prospectively planned, unblinded interim analysis at 75% of events in the study. Therefore, the Company does not currently have any marketing applications under review with any regulatory agencies.
During the EMA’s assessment period, our manufacturing facilities were audited. They were determined, by the EMA, to have met the standards of Good Manufacturing Practices, (GMP) in October 2016. Additionally, our new manufacturing facility, which opened in September 2016, must go through validation with the appropriate regulatory agency prior to commercial production. The new facility might fail validation or not meet regulatory standards for a commercial manufacturing facility. Also, during the assessment period, our clinical research sites engaged to recruit patients into the clinical trial were audited to ensure standards of Good Clinical Practice, (GCP). Even though there were no major findings resulting from the audit of the selected clinical research site, this was merely a sampling by the EMA and may not be representative of other research sites that participated in the clinical trial.
If the Company does seek approval in the EU in the future, we must also gain reimbursement approval in specific EU countries, as well as, buy-in from patients and health care professionals alike for the use of lead product candidate or our other product candidates to treat any relevant indication(s). If we do not receive reimbursement from countries or private payers in the EU, our lead product candidate may not reach or be accessible to patients or health care professionals. Even if our lead product candidate or our other product candidates is approved for reimbursement in EU countries, it may not always maintain its reimbursement status. There are a number of scenarios where we may encounter tight price controls, continuous negotiations, and other variety of outcomes that could challenge our ability to effectively sell the product in certain EU countries. Some countries may decide to no longer reimburse our lead product candidate or our other product candidates for a number of reasons. Further, patients and health care professionals may reject one lead product candidate or our other product candidates as a standard of care treatment for any relevant indication(s). If patients and healthcare professionals reject one of our product candidates, then it will be difficult to generate revenue for the company. There will be a similar scenario if the Company seeks approval in the U.S.
If one of our product candidates is approved by the EMA and/or FDA, we do not have a sufficient number of personnel engaged as employees to conduct an effective marketing and commercialization strategy. We would need to build a larger team to execute wide-ranging commercialization efforts in the EU and or U.S. As a result, we must build an in-house team of seasoned commercialization professionals, or pursue a strategic partnership through a contractual arrangement with an organization with the appropriate expertise, or a combination of both. The cost-benefit of such an arrangement may not actualize profit and generate revenues on a short-term basis. If we entrusted commercialization to an outside organization, there may be any number of issues that arise that we cannot foresee. We may not find a suitable strategic partner or fail to identify appropriate candidates to hire onto a commercialization team, thus potentially limiting our ability to effectively commercialize our lead product candidate.
| 21 |
Because the results of earlier clinical trials are not necessarily predictive of future results, product candidates we advance into clinical trials, may not have favorable results in later clinical trials or receive regulatory approval.
Success in preclinical testing and early clinical trials does not ensure that later clinical trials will generate adequate data to demonstrate the efficacy and safety of an investigational drug. A number of companies in the pharmaceutical and biotechnology industries, including those with greater resources and experience, have suffered significant setbacks in clinical trials, even after seeing promising results in earlier clinical trials. Despite the results reported in earlier clinical trials for our lead product candidate, we do not know whether the clinical trials we are conducting, or may conduct, will demonstrate adequate efficacy and safety to result in regulatory approval to market our lead product candidate or any of our other product candidates in any particular jurisdiction. Even if we believe that we have adequate data to support an application for regulatory approval to market our product candidates, the FDA or other comparable foreign regulatory authorities may not agree and could require us to conduct additional research studies, including late-stage clinical trials. If late-stage clinical trials do not produce favorable results, our ability to achieve regulatory approval for any of our product candidates may be adversely impacted.
If we are unable to enroll subjects in clinical trials, we will be unable to complete these trials on a timely basis.
Patient enrollment, a significant factor in the timing of clinical trials, is affected by many factors including the size and nature of the patient population, the proximity of subjects to clinical sites, the eligibility criteria for the trial, the design of the clinical trial, ability to obtain and maintain patient consents, risk that enrolled subjects will drop out before completion, competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating. Furthermore, we rely on Clinical Research Organizations (CRO’s) and clinical trial sites to ensure the proper and timely conduct of our clinical trials, and while we have agreements governing their committed activities, we have limited influence over their actual, day-to-day performance. We may experience delays in starting-up clinical trial sites in a timely manner, enrolling subjects in our trials, and may not be able to enroll a sufficient number of subjects to complete the trials.
If we experience delays in the completion or if there is termination of, any clinical trial of our lead product candidate or any future product candidates, the commercial prospects of our product candidates will be harmed, and our ability to generate product revenues from any of these product candidates will be delayed. In addition, any delays in completing our clinical trials will increase our costs, slow down our product candidate development and approval process and could shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, and jeopardize our ability to commence product sales, which would impair our ability to generate revenues and may harm our business, results of operations, financial condition and cash flows and future prospects. In addition, many of the factors that could cause a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our lead product candidate or our other product candidates.
The regulatory approval processes of the FDA and comparable foreign regulatory authorities are lengthy, time consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our lead product candidate or our other product candidates, our business may fail.
The time required to obtain approval by the FDA and comparable foreign regulatory authorities is unpredictable, but typically takes several years following the commencement of preclinical studies and clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities and any shifts in regulatory policy. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. We have not obtained regulatory approval for any product candidate, and it is possible that neither our lead product candidates nor any other product candidates we are developing or may discover, in-license or acquire and seek to develop in the future will ever obtain regulatory approval.
| 22 |
Our product candidates could fail to receive marketing approval from the FDA or a comparable foreign regulatory authority for many reasons, including but not limited to:
| • | disagreement over the design or implementation of our clinical trials; |
| • | failure to demonstrate that a product candidate is safe and effective; |
| • | failure of clinical trials to meet the level of statistical significance required for approval; |
| • | failure to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| • | disagreement over our interpretation of data from preclinical studies or clinical trials; |
| • | disagreement over whether to accept efficacy results from clinical trial sites outside the United States where the standard of care is potentially different from that in the United States; |
| • | the insufficiency of data collected from clinical trials of our lead product candidate or our other product candidates to support the submission and filing of an NDA or other submission or to obtain regulatory approval; |
| • | irreparable or critical compliance issues relating to our manufacturing and/clinical trial processes; or |
| • | changes in the approval policies or regulations that render our preclinical and clinical data insufficient for approval. |
The FDA or a comparable foreign regulatory authority may require more information, including additional preclinical or clinical data to support approval, which may delay or prevent approval and our commercialization plans, or we may decide to abandon the development program altogether. Even if we do obtain regulatory approval, our lead product candidate or our other product candidates may be approved for fewer or more limited indications than we request, approved contingent on the performance of costly post-marketing clinical trials, or approved with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. In addition, if our lead product candidate or our other product candidate produces undesirable side effects or safety issues, the FDA may require the establishment of Risk Evaluation Mitigation Strategies, or REMS, or a comparable foreign regulatory authority may require the establishment of a similar strategy, that may, restrict distribution of our products and impose burdensome implementation requirements. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates.
Even if we believe any completed, current or planned clinical trials are successful, the FDA or a comparable foreign regulatory authority may not agree that our completed clinical trials provide adequate data on the safety or efficacy of our lead product candidate or our other product candidates, permitting us to proceed to additional clinical trials. Approval by comparable foreign regulatory authorities does not ensure approval by the FDA and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative impact on the regulatory process in others. We may not be able to file for regulatory approvals, and even if we file we may not receive the necessary approvals to commercialize our products in any market.
Our lead product candidate or our other product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following any marketing approval.
Undesirable side effects caused by our lead product candidate or our other product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authority. If toxicities occur in our current or future clinical trials they could cause delay or even the discontinuation of further development of our lead product candidate or other product candidates, which would impair our ability to generate revenues and would have a material adverse effect our business, results of operations, financial condition and cash flows and future prospects. There can be no assurance that side effects from our lead product candidate or our other product candidates in future clinical trials or that side effects in general will not prompt the discontinued development or possible market approval of our lead product candidate or other product candidates. If serious side effects or other safety or toxicity issues are experienced in our clinical trials in the future, we may not receive approval to market our lead product candidate or any other product candidates, which could prevent us from ever generating revenues or achieving profitability. Results of our trials could reveal an unacceptably high severity and prevalence of side effects. In such an event, our trials could be suspended or terminated and the FDA or comparable foreign regulatory authorities could order us to cease further development of or deny approval of our product candidates for any or all targeted indications. The drug-related side effects could affect patient recruitment or the ability of enrolled subjects to complete the trial or result in potential product liability claims. Any of these occurrences may have a material adverse effect on our business, results of operations, financial condition and cash flows and future prospects.
| 23 |
Additionally, if our lead product candidate or any of our other product candidates receives marketing approval, and we or others later identify undesirable side effects caused by such product, a number of potentially significant negative consequences could result, including:
| • | we may be forced to suspend marketing of such product; |
| • | regulatory authorities may withdraw their approvals of such product; |
| • | regulatory authorities may require additional warnings on the label that could diminish the usage or otherwise limit the commercial success of such product; |
| • | the FDA or other regulatory bodies may issue safety alerts, Dear Healthcare Provider letters, press releases or other communications containing warnings about such product; |
| • | the FDA may require the establishment or modification of REMS or a comparable foreign regulatory authority may require the establishment or modification of a similar strategy that may, for instance, restrict distribution of our product and impose burdensome implementation requirements on us; |
| • | we may be required to change the way the product is administered or conduct additional clinical trials; |
| • | we could be sued and held liable for harm caused to subjects or patients; |
| • | we may be subject to litigation or product liability claims; and |
| • | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved.
Even if our lead product candidate or our other product candidates receive regulatory approval, they may still face future development and regulatory difficulties.
Even if we obtain regulatory approval for our lead product candidate or another product candidate, it would be subject to ongoing requirements by the FDA and comparable foreign regulatory authorities governing the manufacture, quality control, further development, labeling, packaging, storage, distribution, safety surveillance, import, export, advertising, promotion, recordkeeping and reporting of safety and other post-market information. The safety profile of any product will continue to be closely monitored by the FDA and comparable foreign regulatory authorities after approval. If the FDA or comparable foreign regulatory authorities become aware of new safety information after approval of our lead product candidate or any other product candidate, they may require labeling changes or establishment of a REMS or similar strategy, impose significant restrictions on a product’s indicated uses or marketing, or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance. For example, the label ultimately approved for our lead product candidate, if it achieves marketing approval, may include restrictions on use.
In addition, manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with current good manufacturing practices, or cGMP, and other regulations. If we or a regulatory agency discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions on that product, our manufacturing facility, including requiring recall or withdrawal of the product from the market or suspension of manufacturing. If we, our product candidates or our manufacturing facilities for our product candidates fail to comply with applicable regulatory requirements, a regulatory agency may:
| 24 |
| • | issue warning letters or untitled letters; |
| • | impose restrictions on the marketing or manufacturing of the product candidates; |
| • | mandate modifications to promotional materials or require us to provide corrective information to healthcare practitioners; |
| • | require us or any future collaborator to enter into a consent decree, which can include imposition of various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance; |
| • | seek an injunction or impose civil or criminal penalties or monetary fines; |
| • | suspend or withdraw regulatory approval; |
| • | suspend any ongoing clinical trials; |
| • | refuse to approve pending applications or supplements to applications filed by us; |
| • | suspend or impose restrictions on operations, including costly new manufacturing requirements; or |
| • | seize or detain products, refuse to permit the import or export of products, or require us to initiate a product recall. |
The occurrence of any event or penalty described above may inhibit our ability to commercialize our lead product candidate or any other product candidates and generate revenue.
The FDA strictly regulates the advertising and promotion of drug products, and drug products may only be marketed or promoted for their FDA approved uses, consistent with the product’s approved labeling. Advertising and promotion of any product candidate that obtains approval in the U.S., and is covered by federal insurance programs such as Medicare or Medicaid, will be heavily scrutinized by the FDA, the Department of Justice, (DOJ), the Office of Inspector General of the Department of Health and Human Services, (HHS), state attorneys general, members of Congress and the public. Violations, including promotion of our products for unapproved or off-label uses, are subject to enforcement letters, inquiries and investigations, and civil, criminal and/or administrative sanctions by the FDA and/or the DOJ. Additionally, advertising and promotion of, any product candidate that obtains approval outside of the U.S. will be heavily scrutinized by comparable foreign regulatory authorities.
In the U.S., engaging in impermissible promotion of our future products for off-label uses can also subject us to false claims litigation under federal and state statutes, which can lead to civil, criminal and/or administrative penalties and fines and corporate integrity agreements that materially restrict the manner in which we promote or distribute our drug products. The federal False Claims Act, allows any individual to bring a lawsuit against a pharmaceutical company on behalf of the federal government alleging submission of false or fraudulent claims, or causing to present such false or fraudulent claims, for payment by a federal program, such as Medicare or Medicaid. If the government prevails in the lawsuit, the individual may share in any fines or settlement funds. Since 2004, False Claims Act lawsuits against pharmaceutical companies have increased significantly in volume and breadth, leading to several substantial civil and criminal settlements based on certain sales practices promoting off-label drug uses. This growth in litigation has increased the risk that a pharmaceutical company will have to defend a false claims action, pay settlement fines or restitution, agree to comply with burdensome reporting and compliance obligations, and be excluded from Medicare, Medicaid and other federal and state healthcare programs. If we do not lawfully promote our approved products, we may become subject to such litigation and, if we are not successful in defending against such actions, those actions could have a material adverse effect on our business, results of operations, financial condition and cash flows and future prospects.
| 25 |
Existing government regulations may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our lead product candidate or any other product candidates. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and/or be subject to fines or enhanced government oversight and reporting obligations, which would adversely affect our business, prospects and ability to achieve or sustain profitability.
Failure to obtain regulatory approval in foreign jurisdictions would prevent our lead product candidate or any other product candidates from being marketed in those jurisdictions.
In order to market and sell our products in the European Union and many other jurisdictions, we must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the U.S. generally includes all of the risks associated with obtaining FDA approval. Additionally, in many countries outside the U.S., it is required that the product be approved for reimbursement before the product can be effectively commercialized in that country. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. We may not obtain approvals from regulatory authorities outside the U.S. on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the U.S. does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. A failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory approval process in others. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market. If we are unable to obtain approval of our lead product candidate for any of our other product candidates by regulatory authorities in the European Union or another jurisdiction, the commercial prospects of that product candidate may be significantly diminished and our business prospects could decline.
Even if we are able to commercialize our lead product candidate or our other product candidates, the products may not receive coverage and adequate reimbursement from third-party payers, which could harm our business.
Our ability to commercialize any products successfully will depend, in part, on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from government authorities, private health insurers, health maintenance organizations and third-party payers. Patients who are prescribed medications for the treatment of their conditions generally rely on third-party payers to reimburse all or part of the costs associated with their prescription drugs. Coverage and adequate reimbursement from government healthcare programs, such as Medicare and Medicaid, and private health insurers are critical to new product acceptance. Patients are unlikely to use our lead product candidate or our other product candidates unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our product candidates. A primary trend in the US healthcare industry and elsewhere is cost containment. As a result, government authorities and other third-party payers have attempted to control costs by limiting coverage and the amount of reimbursement for particular medications. Increasingly, third-party payers are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. Third-party payers may also seek additional clinical evidence, beyond the data required to obtain marketing approval, demonstrating clinical benefits and value in specific patient populations before covering our products for those patients. We cannot be sure that coverage and adequate reimbursement will be available for any product that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Coverage and reimbursement may impact the demand for, or the price of, any product candidate for which we obtain marketing approval. If coverage and reimbursement are not available or are available only at limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval.
There may be significant delays in obtaining coverage and reimbursement for newly approved drugs, and coverage may be more limited than the purposes for which the drug is approved by the FDA or comparable foreign regulatory authorities. Moreover, obtaining coverage does not imply that any drug will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sales and distribution. Interim reimbursement levels for new drugs, if applicable, may also be insufficient to cover our costs, and may only be temporary. Reimbursement rates may vary according to the use of the drug and the clinical setting in which it is used. Reimbursement rates may also be based in part on existing reimbursement amounts for lower cost drugs or may be bundled into the payments for other services. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payers and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S.. Coverage and reimbursement for drug products can differ significantly from payer to payer. As a result, the coverage and reimbursement determination process is often a time-consuming and costly process with no assurance that coverage and adequate reimbursement will be obtained or applied consistently. Third-party payers often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies. Our inability to promptly obtain coverage and profitable reimbursement rates from both government-funded and private payers for any approved products that we develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products, and our overall financial condition.
| 26 |
We have never marketed a drug before, and if we are unable to establish an effective sales force and marketing infrastructure, or enter into acceptable third-party sales and marketing or licensing arrangements, we may be unable to generate any revenue.
We do not currently have a comprehensive infrastructure for the sales, marketing and distribution of pharmaceutical drug products. The cost of establishing and maintaining such an infrastructure may exceed the cost-effectiveness of doing so. In order to market any products that may be approved by the FDA and comparable foreign regulatory authorities, we must build our sales, marketing, managerial and other non-technical capabilities or make arrangements with third parties to perform these services for which we would incur substantial costs. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, we may not be able to generate product revenue and may not become profitable. We will be competing with many companies that have extensive and well-funded sales and marketing operations. Without an internal commercial organization or the support of a third party to perform sales and marketing functions, or a combination of both, we may be unable to compete successfully against more established companies.
Our lead product candidate and our other product candidates, if approved, may not achieve adequate market acceptance among physicians, patients, and healthcare payers and others in the medical community necessary for commercial success.
Even if we obtain regulatory approval for our lead product candidate or any of our other product candidates, such product(s) may not gain market acceptance among physicians, healthcare payers, patients or the medical community within the U.S. or globally. Our commercial success also depends on coverage and adequate reimbursement of our product candidates by third-party payers, including government payers, generally, which may be difficult or time-consuming to obtain, may be limited in scope and may not be obtained in all jurisdictions in which we may seek to market our products. Market acceptance of any of our product candidates for which we receive approval depends on a number of factors, including:
| • | the efficacy and safety of such product candidates as demonstrated in clinical trials; |
| • | the clinical indications for which the product candidate is approved; |
| • | acceptance by physicians and patients of the product candidate as a safe and effective treatment; |
| • | the potential and perceived advantages of product candidates over alternative treatments; |
| • | the safety of product candidates seen in a broader patient group, including a product candidate’s use outside the approved indications; |
| • | the prevalence and severity of any side effects; |
| • | product labeling or product insert requirements of the FDA or other regulatory authorities; |
| • | the timing of market introduction of our products as well as competitive products; |
| • | the cost of treatment in relation to alternative treatments; |
| • | the availability of coverage and adequate reimbursement and pricing by third-party payers and government authorities; |
| 27 |
| • | relative convenience and ease of administration; |
| • | the effectiveness of our sales and marketing efforts and those of our collaborators; and |
| • | unfavorable publicity relating to the product candidate or the Company. |
If any of our product candidates are approved but fail to achieve market acceptance among physicians, patients, or healthcare payers, we will not be able to generate significant revenues, which would compromise our ability to become profitable.
Our research programs may not succeed.
In the last couple of years, XBiotech has positioned itself with a pipeline of potential drug candidates at all stages of development, from pre-clinical through Phase III clinical trial stage. Even though we have many drugs in development at this time, none of these research programs may succeed. There are several reasons why a drug program may fail:
| · | In the development stage, we may be unable to develop a therapy, which would mean us succeeding in isolating appropriate antibodies to reach the clinical trial stage |
| · | Any partnerships for the development of antibodies could fail to produce results that would necessitate clinical trials |
| · | We may not receive approval from regulatory bodies to move from early stage clinical trials to later stage clinical trials |
| · | Even if we are able to move to later stage clinical trials, it may prove to be difficult to enroll patients into the studies according to schedule, or at all |
| · | During the clinical trial, there could be unexpected serious adverse events causing severe injury or death in patients, requiring us to cease further enrollment or causing regulatory authorities to place the trial on clinical hold for an indefinite period of time |
| · | If a clinical trial is completed, we may not have the appropriate personnel to submit a marketing application to regulatory authorities for approval, and to further respond to the variety of follow up questions that regulatory authorities may have during the review process |
| · | Regulatory authorities may reject drug candidates for a variety of reasons, preventing us from proceeding with marketing and commercialization of approved products |
| · | We may run out of the funds necessary to complete development for any of our potential drug candidates |
Even an effective drug candidate might not be commercially successful.
Even if we ultimately succeed in creating a safe and effective drug, as determined by regulatory authorities, based on our current product pipeline, there is no assurance it would be commercially successful. Competitive products might become available faster or with lower costs or adverse risks to patients, resulting in few sales of any product developed by XBiotech. Occurrences of certain disease indications, such as those in our pipeline, might become sufficiently rare, or victims might be sufficiently impoverished, that commercial production is uneconomic Furthermore, we must have sufficient buy-in from patients and healthcare professionals to guarantee market exposure for our drug candidates. If the end-users are not reached with our products, then it will be difficult to generate revenue from our development efforts. And even though we could obtain regulatory approval for any of our drug candidates, it is not necessarily the case that government or third-party payers will decide to add our products to their respective prescription drug formularies for reimbursement, thus inhibiting the ability for our drug candidates to reach the target patient populations, and health care professionals serving those patients.
| 28 |
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to our current lead product candidate or our other product candidates to treat any relevant indication(s). There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the disease indications for which we are developing our future product candidates. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach, and others are based on entirely different approaches. Potential competitors include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
More established companies may have a competitive advantage over us due to their greater size, cash flows and institutional experience. Compared to us, many of our competitors may have significantly greater financial, technical and human resources. As a result of these factors, our competitors may obtain regulatory approval of their products before we do, which will limit our ability to develop or commercialize our lead product candidate or any of our other product candidates. In addition, many companies are developing new therapeutics to supplant or expand upon the standard of care for a number of diseases, as a result, we cannot predict what the standard of care will be as our product candidates progress through clinical development.
Our failure to successfully identify, acquire, develop and commercialize additional product candidates or approved products other than our lead product candidate could impair our ability to grow.
Although a substantial amount of our efforts will focus on the continued clinical testing and potential approval of our most advanced lead product candidate, a key element of our growth strategy is to acquire, develop and/or market additional products and product candidates. All of these potential product candidates remain in the discovery and clinical study stages. Research programs to identify product candidates require substantial technical, financial and human resources, whether or not any product candidates are ultimately identified. Because our internal research capabilities are limited, we may be dependent upon pharmaceutical and biotechnology companies, academic scientists and other researchers to sell or license products or technology to us. The success of this strategy depends partly upon our ability to identify, select and acquire promising pharmaceutical product candidates and products. The process of proposing, negotiating and implementing a license or acquisition of a product candidate or approved product is lengthy and complex. Other companies, including some with substantially greater financial, marketing and sales resources, may compete with us for the license or acquisition of product candidates and approved products. We have limited resources to identify and execute the acquisition or in-licensing of third-party products, businesses and technologies and integrate them into our current infrastructure. Moreover, we may devote resources to potential acquisitions or in-licensing opportunities that are never completed, or we may fail to realize the anticipated benefits of such efforts. Any product candidate that we acquire may require additional development efforts prior to commercial sale, including extensive clinical testing and approval by the FDA and applicable foreign regulatory authorities. All product candidates are prone to risks of failure typical of pharmaceutical product development, including the possibility that a product candidate will not be shown to be sufficiently safe and effective for approval by regulatory authorities. In addition, we cannot provide assurance that any products that we develop or approved products that we acquire will be manufactured profitably or achieve market acceptance.
Product liability lawsuits against us could cause us to incur substantial liabilities and to limit commercialization of any products that we may develop.
We face an inherent risk of product liability exposure related to the testing of our lead product candidate and any other product candidates in clinical trials and will face an even greater risk if we commercially sell any products that we may develop. Product liability claims may be brought against us by subjects enrolled in our clinical trials, patients, healthcare providers or others using, administering or selling our products. If we cannot successfully defend ourselves against claims that our product candidates or products caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for any product candidates or products that we may develop; |
| • | termination of clinical trial sites or entire clinical trial programs; |
| 29 |
| • | injury to our reputation and significant negative media attention; |
| • | withdrawal of clinical trial participants; |
| • | significant costs to defend the related litigation; |
| • | substantial monetary awards to clinical trial subjects or patients; |
| • | loss of revenue; |
| • | diversion of management and scientific resources from our business operations; and |
| • | the inability to commercialize our product candidates. |
We will obtain insurance coverage for products to include the sale of commercial products if we obtain marketing approval for our lead product candidate or our other product candidates, but we may be unable to obtain commercially reasonable product liability insurance for any products approved for marketing. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
We will need to expand our operations and grow the size of our organization in the future, and we may experience difficulties in managing this growth.
As of March 16, 2018, we had 42 employees. As our development and commercialization plans and strategies develop, or as a result of any future acquisitions, we will need additional managerial, operational, sales, marketing, scientific, and financial headcount and other resources. Our management, personnel and systems currently in place may not be adequate to support this future growth. Future growth would impose significant added responsibilities on members of management, including:
| • | managing our clinical trials effectively, which we anticipate potentially being conducted at numerous clinical sites on a global scale; |
| • | identifying, recruiting, maintaining, motivating and integrating additional employees with the expertise and experience we will require; |
| • | managing our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors and other third parties; |
| • | managing additional relationships with various strategic partners, suppliers and other third parties; |
| • | improving our managerial, development, operational and finance reporting systems and procedures; and |
| • | expanding our facilities. |
Our failure to accomplish any of these tasks could prevent us from successfully growing our Company.
We are highly dependent on our Chief Executive Officer.
Our future success depends in significant part on the continued service of our Chief Executive Officer, John Simard. Mr. Simard is critical to the strategic direction and overall management of our company as well as our research and development process. Although we have an employment agreement with Mr. Simard, it has no specific duration. The loss of Mr. Simard could adversely affect our business, financial condition and operating results.
| 30 |
We depend on key personnel to operate our business, and many members of our current management team are new. If we are unable to retain, attract and integrate qualified personnel, our ability to develop and successfully grow our business could be harmed.
In addition to the continued services of Mr. Simard, we believe that our future success is highly dependent on the contributions of our significant employees, as well as our ability to attract and retain highly skilled and experienced sales, research and development and other personnel in the United States and abroad. Some of our significant employees include our Medical Director, our Chief Scientific Officer, our Vice President of Quality Assurance, our Vice President of Quality Control, and our Vice President of Finance and Human Resources. Changes in our management team may be disruptive to our business.
All of our employees, including our Chief Executive Officer, are free to terminate their employment relationship with us at any time, subject to any applicable notice requirements, and their knowledge of our business and industry may be difficult to replace. If one or more of our executive officers or significant employees leaves, we may not be able to fully integrate new personnel or replicate the prior working relationships, and our operations could suffer. Qualified individuals with the breadth of skills and experience in the pharmaceutical industry that we require are in high demand, and we may incur significant costs to attract them. Many of the other pharmaceutical companies that we compete against for qualified personnel have greater financial and other resources, different risk profiles and a longer history in the industry than we do. They also may provide more diverse opportunities and better chances for career advancement. Our failure to attract and retain key personnel could impede the achievement of our research, development and commercialization objectives.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We are subject to numerous environmental, health and safety laws and regulations in the U.S. and elsewhere, including, as a result of our leased laboratory space, those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. Our operations involve the use of hazardous and flammable materials, including chemicals and biological materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes.
We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
Although we maintain insurance for employee injury to cover us for costs and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not maintain insurance for environmental liability or toxic tort claims that may be asserted against us in connection with our storage or disposal of biological or hazardous materials. In addition, we may incur substantial costs in order to comply with current or future environmental, health and safety laws and regulations. These current or future laws and regulations may impair our research, development or production efforts. Failure to comply with these laws and regulations may also result in substantial fines, penalties or other sanctions.
Business disruptions could seriously harm our future revenues and financial condition and increase our costs and expenses.
Our operations could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or manmade disasters or business interruptions, for which we are predominantly self-insured. We do not carry insurance for all categories of risk that our business may encounter. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-parties to supply various items which are critical for producing our product candidates. Our ability to produce clinical supplies of product candidates could be disrupted, if the operations of these suppliers are affected by a man-made or natural disaster or other business interruption. The ultimate impact on us, our significant suppliers and our general infrastructure of being consolidated in certain geographical areas is unknown, but our operations and financial condition could suffer in the event of a major earthquake, fire or other natural disaster. Further, any significant uninsured liability may require us to pay substantial amounts, which would adversely affect our business, results of operations, financial condition and cash flows from future prospects.
| 31 |
Risks Related to Intellectual Property
If we are unable to obtain or protect intellectual property rights, our competitive position could be harmed.
We depend on our ability to protect our proprietary technology. We rely on trade secret, patent, copyright and trademark laws, and confidentiality, licensing and other agreements with employees and third parties, all of which offer only limited protection. Our commercial success will depend in large part on our ability to obtain and maintain patent protection in the U.S. and other countries with respect to our proprietary technology and products. Where we deem appropriate, we seek to protect our proprietary position by filing patent applications in the U.S. and abroad related to our novel technologies and products that are important to our business. The patent positions of biotechnology and pharmaceutical companies generally are highly uncertain, involve complex legal and factual questions and have in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patents, including those patent rights licensed to us by third parties, are highly uncertain.
The steps we have taken to protect our proprietary rights may not be adequate to preclude misappropriation of our proprietary information or infringement of our intellectual property rights, both inside and outside the U.S. The rights already granted under any of our currently issued patents and those that may be granted under future issued patents may not provide us with the proprietary protection or competitive advantages we are seeking. If we are unable to obtain and maintain patent protection for our technology and products, or if the scope of the patent protection obtained is not sufficient, our competitors could develop and commercialize technology and products similar or superior to ours, and our ability to successfully commercialize our technology and products may be adversely affected.
With respect to patent rights, we do not know whether our pending patent applications for any of our technologies or product candidates will result in the issuance of patents that protect such technologies or products candidates, or if any of our issued patents will effectively prevent others from commercializing competitive technologies and products. Our pending patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. Further, the examination process may require us to narrow the claims for our pending patent applications, which may limit the scope of patent protection that may be obtained if these applications issue. Because the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, issued patents that we own or have licensed from third parties may be challenged in the courts or patent offices in the U.S. and abroad. Such challenges may result in the loss of patent protection, the narrowing of claims in such patents or the invalidity or unenforceability of such patents, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection for our technology and products. Protecting against the unauthorized use of our patented technology, trademarks and other intellectual property rights is expensive, difficult and, in some cases, not be possible. In some cases, it may be difficult or impossible to detect third-party infringement or misappropriation of our intellectual property rights, even in relation to issued patent claims, and proving any such infringement may be even more difficult.
Intellectual property rights do not necessarily address all potential threats to any competitive advantage we may have.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
| • | Others may be able to make compounds that are the same as or similar to our lead product candidate or our future product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed. |
| • | We might not have been the first to make the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed. |
| • | We or any of our licensors or strategic partners might not have been the first to file patent applications covering certain of our inventions. |
| 32 |
| • | Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights. |
It is possible that our pending patent applications will not lead to issued patents.
| • | Issued patents that we own or have exclusively licensed may not provide us with any competitive advantages, or may be held invalid or unenforceable, as a result of legal challenges by our competitors. |
| • | Our competitors might conduct research and development activities in the U.S. and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets. |
| • | We may not develop additional proprietary technologies that are patentable. |
| • | The patents of others may have an adverse effect on our business. |
Our technology may be found to infringe upon third-party intellectual property rights.
Third parties, may in the future, assert claims or initiate litigation related to their patent, copyright, trademark and other intellectual property rights in technology that is important to us. The asserted claims and/or litigation could include claims against us, our licensors or our suppliers alleging infringement of intellectual property rights with respect to our products or components of those products. Regardless of the merit of the claims, they could be time consuming, result in costly litigation and diversion of technical and management personnel, or require us to develop a non-infringing technology or enter into license agreements. We cannot assure you that licenses will be available on acceptable terms, if at all. Furthermore, because of the potential for significant damage awards, which are not necessarily predictable, it is not unusual to find even arguably unmeritorious claims resulting in large settlements. If any infringement or other intellectual property claim made against us by any third party is successful, or if we fail to develop non-infringing technology or license the proprietary rights on commercially reasonable terms and conditions, our business, operating results and financial condition could be materially and adversely affected.
If our products, methods, processes and other technologies infringe upon the proprietary rights of other parties, we could incur substantial costs and we may have to:
| • | obtain licenses, which may not be available on commercially reasonable terms, if at all; |
| • | abandon an infringing drug or therapy candidate; |
| • | redesign our products or processes to avoid infringement; |
| • | stop using the subject matter claimed in the patents held by others; |
| • | pay damages; or |
| • | defend litigation or administrative proceedings which may be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources. |
We may need to license intellectual property from third parties, and such licenses may not be available or may not be available on commercially reasonable terms.
A third party may hold intellectual property, including patent rights that are important or necessary to the development of our products. It may be necessary for us to use the patented or proprietary technology of a third party to manufacture, or otherwise commercialize, our own technology or products, in which case we would be required to obtain a license from such third party. Licensing such intellectual property may not be available or may not be available on commercially reasonable terms, which could have a material adverse effect on our business and financial condition. Several companies in our industry have licensed U.S. patent nos. 6,331,415 and 7,923,221. These patents appear to relate to antibody production techniques, and have been reported to remain in force until December 18, 2018, unless determined to be invalid or unenforceable before that date. Should a license to these patents be necessary, we cannot be certain that such a license would be available on commercially reasonable terms.
| 33 |
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and product candidates, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering into non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We also enter into confidentiality and invention or patent assignment agreements with our employees and consultants. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside of the U.S.are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate it, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
Risks Related to Owning Shares of Our Common Stock
Our share price may be volatile, which could subject us to additional securities class action lawsuits and prevent you from being able to sell your shares at or above the offering price.
Our stock could be subject to wide fluctuations in response to many risk factors listed in this section, and others beyond our control, including:
| • | results of our clinical trials; |
| • | results of clinical trials of our competitors’ products; |
| • | regulatory actions with respect to our products or our competitors’ products; |
| • | actual or anticipated fluctuations in our financial condition and operating results; |
| • | actual or anticipated changes in our growth rate relative to our competitors; |
| • | actual or anticipated fluctuations in our competitors’ operating results or changes in their growth rate; |
| • | competition from existing products or new products that may emerge; |
| • | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures, collaborations or capital commitments; |
| • | issuance of new or updated research or reports by securities analysts; |
| • | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| • | delisting of the Company’s security from the exchange on which it trades due to the Company not being in compliance with the listing requirements of the exchange; |
| • | share price and volume fluctuations attributable to inconsistent trading volume levels of our shares; |
| • | additions or departures of key management or scientific personnel; |
| 34 |
| • | disputes or other developments related to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| • | announcement or expectation of additional financing efforts; |
| • | sales of our common stock by us, our insiders or our other shareholders; |
| • | market conditions for biopharmaceutical stocks in general; and |
| • | general economic and market conditions. |
Furthermore, the stock markets have experienced extreme price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many companies. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These broad market and industry fluctuations, as well as general economic, political and market conditions such as recessions, interest rate changes or international currency fluctuations, may negatively impact the market price of shares of our common stock. In addition, such fluctuations could subject us to securities class action litigation, which could result in substantial costs and divert our management’s attention from other business concerns, which could seriously harm our business. If the market price of shares of our common stock does not exceed your buying price, you may not realize any return on your investment in us and may lose some or all of your investment.
Insiders continue to have substantial control over our company since our initial public offering in April 2015 and could delay or prevent a change in corporate control.
As of December 31, 2017, our directors, executive officers and principal shareholders, together with their affiliates, beneficially own, in the aggregate, at least 10 million shares or approximately 29% of our outstanding common stock, and could own approximately 13.2 million shares or approximately 37% of our outstanding common stock if they fully exercise their outstanding stock options. As a result, these shareholders, if acting together, have the ability to determine the outcome of matters submitted to our shareholders for approval, including the election of directors and any merger, consolidation or sale of all or substantially all of our assets. In addition, these persons, acting together, have the ability to control the management and affairs of the Company. Accordingly, this concentration of ownership may harm the market price of our common stock by:
| • | delaying, deferring or preventing a change in control of the Company; |
| • | impeding a merger, consolidation, takeover or other business combination involving the Company; or |
| • | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of the Company. |
We have broad discretion in the use of the net proceeds from our initial public offering in April 2015 and subsequent offerings and may not use them effectively.
We intend to continue to allocate the net proceeds that we received from the April 2015 offering and subsequent offerings as described below “Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities—Use of Proceeds from IPO.” However, our management will have broad discretion in the actual application of the net proceeds, and we may elect to allocate proceeds differently from that described in “Use of Proceeds” if we believe it would be in our best interests to do so. Our shareholders may not agree with the manner in which our management chooses to allocate and spend the net proceeds. The failure by our management to apply these funds effectively could have a material adverse effect on our business. We may invest the net proceeds from this offering in a manner that does not produce income or that loses value.
Provisions in our charter documents under Canadian law could make an acquisition of us, which may be beneficial to our shareholders, more difficult.
Our authorized preferred capital stock is available for issuance from time to time at the discretion of our Board of Directors, without shareholder approval. Our Articles of Incorporation (“Articles”) grant our Board of Directors the authority, subject to the corporate law of British Columbia, to determine or alter the special rights and restrictions granted to or imposed on any wholly unissued series of preferred shares, and such rights may be superior to those of our common stock.
| 35 |
Limitations on the ability to acquire and hold our common stock may be imposed by the Competition Act (Canada). This legislation permits the Commissioner of Competition of Canada to review any acquisition of a significant interest in us. This legislation grants the Commissioner jurisdiction to challenge such an acquisition before the Canadian Competition Tribunal if the Commissioner believes that it would, or would be likely to, result in a substantial lessening or prevention of competition in any market in Canada. The Investment Canada Act (Canada) subjects an acquisition of control of a company by a non-Canadian to government review if the value of our assets as calculated pursuant to the legislation exceeds a threshold amount. A reviewable acquisition may not proceed unless the relevant minister is satisfied that the investment is likely to be a net benefit to Canada.
Any of the foregoing could prevent or delay a change of control and may deprive or limit strategic opportunities for our shareholders to sell their shares and/or affect the market price of our shares.
We may be a passive foreign investment company for US tax purposes which may negatively affect US investors.
For US federal income taxation purposes, we will be a passive foreign investment company (PFIC) if in any taxable year either: (a) 75% or more of our gross income consists of passive income; or (b) 50% or more of the value of our assets is attributable to assets that produce, or are held for the production of, passive income. If we meet either test, our shares held by a US person in that year will be PFIC shares for that year and all subsequent years in which they are held by that person. In previous taxable years, our gross income consisted mostly of interest, and we have been considered a PFIC. We may also be a PFIC in future taxable years. Gain realized by a US investor from the sale of PFIC shares is taxed as ordinary income, as opposed to a capital gain, and subject to an interest charge unless the US person timely made certain tax elections.
We are governed by the corporate laws in British Columbia, Canada which in some cases have a different effect on shareholders than the corporate laws in Delaware, United States.
The material differences between the BCBCA as compared to the Delaware General Corporation Law (DGCL) which may be of most interest to shareholders include the following: (i) for material corporate transactions (ie mergers and amalgamations, other extraordinary corporate transactions, amendments to our Articles) the BCBCA generally requires two-thirds majority vote by shareholders, whereas DGCL generally only requires a majority vote of shareholders; (ii) the quorum for shareholders meetings is not prescribed under the BCBCA and is only two persons representing 20% of the issued shares under our Articles, whereas under DGCL, quorum requires a minimum of one-third of the shares entitled to vote to be present and companies’ certificates of incorporation frequently require a higher percentage to be present; (iii) under the BCBCA, a holder of 5% or more of our common stock can requisition a special meeting at which any matters that can be voted on at our annual meeting can be considered, whereas the DGCL does not give this right; (iv) our Articles require two-thirds majority vote by shareholders to pass a resolution for one or more directors to be removed, whereas DGCL only requires the affirmative vote of a majority of the shareholders; however, many public company charters limit removal of directors to a removal for cause; and (v) our Articles may be amended by resolution of our directors to alter our authorized share structure, including to consolidate or subdivide any of our shares, whereas under DGCL, a majority vote by shareholders is generally required to amend a corporation’s certificate of incorporation and a separate class vote may be required to authorize alterations to a corporation’s authorized share structure. We cannot predict if investors will find our common stock less attractive because of these material differences. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our share price may be more volatile.
Future sales, or the possibility of future sales, of a substantial number of our common stock could adversely affect the price of the shares and dilute shareholders.
Future sales of a substantial number of our common stock, or the perception that such sales will occur, could cause a decline in the market price of our common stock. As of December 31, 2017, we had 35,439,272 common stock outstanding.
In the future, we may issue additional common stock or other equity or debt securities convertible into common stock in connection with a financing, acquisition, litigation settlement, employee arrangements or otherwise. Any such issuance could result in substantial dilution to our existing shareholders and could cause our common share price to decline.
| 36 |
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups Act of 2012 ( JOBS Act) and we intend to take advantage of reduced disclosure and governance requirements applicable to emerging growth companies, which could result in our common stock being less attractive to investors and adversely affect the market price of our common stock or make it more difficult to raise capital as and when we need it.
We are an “emerging growth company” as that term is used in the JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved and exemptions from any rules that the Public Company Accounting Oversight Board may adopt requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements. We currently intend to take advantage of some, but not all, of the reduced regulatory and reporting requirements that will be available to us under the JOBS Act, so long as we qualify as an “emerging growth company.” For example, so long as we qualify as an “emerging growth company,” we may elect not to provide you with certain information, including certain financial information and certain information regarding compensation of our executive officers, that we would otherwise have been required to provide in filings we make with the Securities and Exchange Commission (SEC) which may make it more difficult for investors and securities analysts to evaluate the Company.
We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. We may take advantage of these reporting exemptions until we are no longer an emerging growth company, which in certain circumstances could be for up to five years.
Due to the exemptions from various reporting requirements provided to us as an “emerging growth company” we may be less attractive to investors and it may be difficult for us to raise additional capital as and when we need it. Investors may be unable to compare our business with other companies in our industry if they believe that our financial statements are not as transparent as other companies in our industry. If we are unable to raise additional capital as and when we need it, our business, results of operations, financial condition and cash flows and future prospects may be materially and adversely affected.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, shareholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 or any subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock.
We will be required to disclose changes made in our internal controls and procedures on a quarterly basis and our management will be required to assess the effectiveness of these controls annually. However, for as long as we are an “emerging growth company” under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404. We could be an “emerging growth company” for up to five years. An independent assessment of the effectiveness of our internal controls could detect problems that our management’s assessment might not.
| 37 |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
| ITEM 2. | PROPERTIES |
Our operations are based primarily in Austin, Texas. On January 12, 2008, the Company entered a lease agreement to lease its facility in Austin, Texas, USA. On September 15, 2010, the Company entered into a second lease agreement to lease additional space in Austin, Texas, USA. On March 20, 2013, the Company extended the lease for another 21 months with the same terms and rental rates as the current lease. To accommodate future potential larger-scale commercial manufacturing needs, the Company purchased 48 acres of industrial-zoned property located five miles from Austin’s central business district. In 2016 construction of a new manufacturing facility on this property was completed. The Company continues to prepare this manufacturing facility to produce registration batches of product in the event of potential commercialization in the future.
| ITEM 3. | LEGAL PROCEEDINGS |
On December 1, 2015, a purported securities class action complaint captioned Yogina Rezko v. XBiotech Inc., John Simard, Queena Han and WR Hambrecht & Co., LLC was filed against us, certain of our officers and directors and the underwriter for our initial public offering in the Superior Court for the State of California, Los Angeles County. On December 2, 2015, a purported securities class action complaint captioned Linh Tran v. XBiotech Inc., John Simard and Queena Han was filed against us and certain of our officers and directors in U.S. District Court for the Western District of Texas. The lawsuits are based on substantially similar factual allegations and purport to be class actions brought on behalf of purchasers of the Company's securities during the period from April 15, 2015 through November 23, 2015. The complaint filed in California state court alleges that the defendants violated the Securities Act of 1933, as amended (the "Securities Act"), and the complaint filed in federal court alleges that the defendants violated the Securities Exchange Act of 1934, as amended (the "Exchange Act"), in each case by making materially false and misleading statements concerning the Company's Phase III clinical trial conducted in Europe to assess Xilonix™ as a treatment for colorectal cancer. The California complaint purports to assert claims for violations of Sections 11, 12(a)(2) and 15 of the Securities Act, and the federal complaint purports to assert claims for violation of Sections 10(b) and 20(a) of the Exchange Act and Rule 10b-5 promulgated thereunder. Both complaints seek, on behalf of the purported class, an unspecified amount of monetary damages, interest, fees and expenses of attorneys and experts, and other relief.
In September 2016, a stay was granted at the Superior Court for the State of California, Los Angeles County, in Yogina Rezko v. XBiotech Inc., John Simard, Queena Han and WR Hambrecht & Co., LLC, in light of the ongoing case, Linh Tran v. XBiotech Inc., John Simard and Queena Han, in the U.S. District Court for the Western District of Texas, leaving plaintiffs with an opportunity re-file a complaint in Texas. In October 2016, the Texas securities class action lawsuit was dismissed with prejudice. At the June 7, 2017 hearing at the Superior Court for the State of California, Los Angeles County, we were granted a motion on the grounds of forum non conveniens. The judge ruled that the case belonged in Texas, not in California. The case is nevertheless stayed, due to California procedural rules, and not dismissed.
On July 6, 2017, plaintiffs proceeded to re-file in Travis County district court (located in Austin, Texas). A hearing date has yet to be scheduled. Subsequently, XBiotech won on its motion to remove the case from state court to federal court at the U.S. District Court for the Western District of Texas, Austin Division. Counsel further argued for a motion to stay the case, which was granted on October 6, 2017, taking into account the upcoming hearings at the U.S. Supreme Court for Cyan, Inc. v. Beaver County Employees Retirement Fund. At issue in this is case is whether state courts lack subject matter jurisdiction over covered class actions that allege only Securities Act of 1933 claims. Arguments before the Supreme Court are scheduled for November 28, 2017. A decision from the Court is expected in the first half of 2018, at which time we will learn more about its impact on whether plaintiffs may return to litigate Rezko v. XBiotech in state court. We are unable to estimate the outcome of the Texas state court matter or the resulting financial impact to us, if any.
| 38 |
On October 26, 2017, XBiotech Corporate Officers, Queena Han (VP of Finance) and John Simard (President and CEO), and XBiotech, Inc. were named defendants in a securities class action civil suit filed in federal court at the U.S. District Court for the Western District of Texas in Austin, Texas, claiming that officers of the Company made false and misleading statements, violating securities laws, in documents filed with the Securities Exchange Commission (SEC), in regulatory filings, press releases, and other public statements. The deadline for lead plaintiff applications expired for this case in December 2017, with no one filing a motion to be appointed lead plaintiff for the putative class. The Company announced on January 5, 2018 that the United States District Court for the Western District of Texas Austin Division filed an order of dismissal granting in all respects a Notice of Voluntary Dismissal filed by the sole plaintiff in the putative class action complaint (Case 1:17-cv-01023-SS). The abovementioned lawsuit has therefore officially been dismissed without prejudice by the Court.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
| 39 |
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES. |
Market Information
Our common stock began trading on the NASDAQ Global Select Market on April 15, 2015 under the symbol “XBIT.” Prior to that time, there was no established public trading market for our common stock. The following table sets forth the high and low close prices per share for our common stock on the NASDAQ Global Select Market for the periods indicated:
| Year Ended December 31, 2015: | High | Low | ||||||
| Second Quarter (commencing April 15, 2015) | $ | 31.50 | $ | 17.63 | ||||
| Third Quarter | $ | 20.71 | $ | 13.87 | ||||
| Fourth Quarter | $ | 15.77 | $ | 7.47 | ||||
| Year Ended December 31, 2016: | High | Low | ||||||
| First Quarter | $ | 10.44 | $ | 6.99 | ||||
| Second Quarter | $ | 20.92 | $ | 9.46 | ||||
| Third Quarter | $ | 24.90 | $ | 12.81 | ||||
| Fourth Quarter | $ | 16.90 | $ | 8.90 | ||||
| Year Ended December 31, 2017: | High | Low | ||||||
| First Quarter | $ | 19.20 | $ | 9.44 | ||||
| Second Quarter | $ | 17.17 | $ | 3.20 | ||||
| Third Quarter | $ | 5.58 | $ | 4.14 | ||||
| Fourth Quarter | $ | 4.66 | $ | 3.90 | ||||
Holders of record
As of February 8, 2018, there were 2,382 holders of record of our common stock. Because many of our shares are held by brokers and other institutions on behalf of stockholders, we are unable to estimate the total number of beneficial holders represented by these record holders.
Dividends
We have never paid or declared any cash dividends on our common stock. We currently intend to retain any earnings for future growth and, therefore, do not expect to pay cash dividends in the foreseeable future.
Use of Proceeds from IPO
On April 14, 2015, our registration statement on Form S-1 (File No. 333-201813) was declared effective by the Securities and Exchange Commission for our initial public offering pursuant to which we sold an aggregate of 4,000,000 shares of our common stock to investors at a price of $19.00 per share. W.R. Hambrecht + Co., Inc. acted as the sole underwriter. The offering commenced as of April 14, 2015 and did not terminate before all of the securities registered in the registration statement were sold. On April 17, 2015, we closed the sale of such shares, resulting in net proceeds to us of approximately $70.6 million after deducting underwriting discounts and commissions of $3.8 million and other offering expenses of approximately $1.6 million. No payments were made by us to directors, officers or persons owning ten percent or more of our common stock or to their associates, or to our affiliates.
| 40 |
| ITEM 6. | SELECTED FINANCIAL DATA |
The following selected consolidated financial data for each of the five years ended December 31, 2017 are derived from our audited consolidated financial statements. The selected consolidated financial data set forth below should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements, and the related Notes, included elsewhere in this annual report on Form 10-K. Historical results are not necessarily indicative of future results. Set forth below are our selected consolidated financial data (in thousands, except share and per share amounts)
| Year Ended December 31, | ||||||||||||||||||||
| 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||||
| Statement of Operations Data | ||||||||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Research and development | $ | 26,424 | $ | 42,486 | $ | 31,310 | $ | 14,329 | $ | 7,935 | ||||||||||
| General and administrative | 7,635 | 10,277 | 6,200 | 7,449 | 1,990 | |||||||||||||||
| Total operating expenses | 34,059 | 52,763 | 37,510 | 21,778 | 9,925 | |||||||||||||||
| Loss from operations | (34,059 | ) | (52,763 | ) | (37,510 | ) | (21,778 | ) | (9,925 | ) | ||||||||||
| Other income (loss): | ||||||||||||||||||||
| Interest income | 354 | 49 | - | 1 | 1 | |||||||||||||||
| Foreign exchange gain (loss) | 555 | (47 | ) | 6 | 53 | (3 | ) | |||||||||||||
| Other income | - | - | 21 | - | - | |||||||||||||||
| Total other income (loss): | 909 | 2 | 27 | 54 | (2 | ) | ||||||||||||||
| Net loss | (33,150 | ) | (52,761 | ) | (37,483 | ) | (21,724 | ) | (9,927 | ) | ||||||||||
| Net loss per common share—basic and diluted | (0.95 | ) | (1.63 | ) | (1.22 | ) | (0.90 | ) | (0.45 | ) | ||||||||||
| Weighted average number of common shares—basic and diluted | 34,875,814 | 32,403,391 | 30,801,994 | 24,162,700 | 22,220,416 | |||||||||||||||
| As of December 31, | ||||||||||||||||||||
| 2017 | 2016 | 2015 | 2014 | 2013 | ||||||||||||||||
| Balance sheet data | ||||||||||||||||||||
| Cash and cash equivalents | $ | 31,768 | $ | 34,324 | $ | 91,051 | $ | 57,329 | $ | 7,244 | ||||||||||
| Working capital | 30,540 | 28,967 | 86,750 | 54,917 | 6,848 | |||||||||||||||
| Total assets | 62,972 | 67,050 | 109,358 | 62,177 | 11,073 | |||||||||||||||
| Total shareholders’ equity | 60,162 | 59,064 | 103,050 | 59,030 | 10,228 | |||||||||||||||
| 41 |
| ITEM 7 | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION |
AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes thereto included elsewhere in this annual report on Form 10-K. Some of the information contained in this discussion and analysis or set forth elsewhere in this annual report on Form 10-K, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties. As a result of many factors, including those factors set forth in the “Risk Factors” section of this annual report on Form 10-K, our actual results could differ materially from the results described in or implied by the forward-looking statements contained in the following discussion and analysis.
Overview
XBiotech Inc. (“XBiotech” or the “Company) is a pre-market biopharmaceutical company engaged in discovering and developing True Human™ monoclonal antibodies for treating a variety of diseases. True Human™ monoclonal antibodies are those which occur naturally in human beings—as opposed to being derived from animal immunization or otherwise engineered. We believe that naturally occurring monoclonal antibodies have the potential to be safer and more effective than their non-naturally occurring counterparts. XBiotech is focused on developing its True Human™ pipeline and manufacturing system.
We have never been profitable and, as of December 31, 2017, we had an accumulated deficit of $216.6 million. We had a net loss of $33.2 million for the year ended December 31, 2017, compared to $52.8 million for the year ended December 31, 2016, and $37.5 million for the year ended December 31, 2015. We expect to incur significant and increasing operating losses for the foreseeable future as we advance our drug candidates from discovery through preclinical testing and clinical trials and seek regulatory approval and eventual commercialization. In addition to these increasing research and development expenses, we expect general and administrative costs to increase as we continue to operate as a public company. We will need to generate significant revenues to achieve profitability, and we may never do so. As of December 31, 2017, we had 51 employees.
Revenues
To date, we have not generated any revenue. Our ability to generate revenue and become profitable depends on our ability to successfully commercialize our lead product candidate or any other product candidate we may advance in the future.
Research and Development Expenses
Research and development expense consists of expenses incurred in connection with identifying and developing our drug candidates. These expenses consist primarily of salaries and related expenses, stock-based compensation, the purchase of equipment, laboratory and manufacturing supplies, facility costs, costs for preclinical and clinical research, development of quality control systems, quality assurance programs and manufacturing processes. We charge all research and development expenses to operating expenses as incurred.
Clinical development timelines, likelihood of success and total costs vary widely. We do not currently track our internal research and development costs or our personnel and related costs on an individual drug candidate basis. We use our research and development resources, including employees and our drug discovery technology, across multiple drug development programs. As a result, we cannot state precisely the costs incurred for each of our research and development programs or our clinical and preclinical drug candidates. From inception through December 31, 2017, we have recorded total research and development expenses, including share-based compensation, of $170.0 million. Our total research and development expenses for the year ended December 31, 2017 was $26.4 million, compared to $42.5 million the year ended December 31, 2016, and $31.3 million for the year ended December 31, 2015. Share-based compensation accounted for $0.4 million for the year ended December 31, 2017, $2.1 million for the year ended December 31, 2016 and $2.2 million for the year ended December 31, 2015.
| 42 |
Research and development expenses as a percentage of total operating expenses was 78% for the year ended December 31, 2017, 81% for the year ended December 31, 2016, and 83% for the year ended December 31, 2015. The percentages, excluding stock-based compensation, were 82% for the year ended December 31, 2017, 86% for the year ended December 31, 2016 and 88% for the year ended December 31, 2015.
Our clinical development costs decreased with the completion and discontinuation of our phase III colorectal cancer clinical trials under EMA and FDA jurisdictions, respectively.
The clinical research and development costs may increase going forward as we evaluate our pipeline and plan potential new studies.
Based on the results of our preclinical studies, we anticipate that we will select drug candidates and research projects for further development on an ongoing basis in response to their preclinical and clinical success and commercial potential. For research and development candidates in early stages of development, it is premature to estimate when material net cash inflows from these projects might occur.
General and Administrative Expenses
General and administrative expense consists primarily of salaries and related expenses for personnel in administrative, finance, business development and human resource functions, as well as the legal costs of pursuing patent protection of our intellectual property and patent filing and maintenance expenses, share–based compensation, and professional fees for legal services. Our total general and administration expenses was $7.6 million for the year ended December 31, 2017, $10.3 million for the year ended December 31, 2016 and $6.2 million for the year ended December 31, 2015. Share-based compensation accounted for $2.1 million for the year ended December 31, 2017, $3.5 million for the year ended December 31, 2016 and $2.2 million for the year ended December 31, 2015.
Critical Accounting Policies
Our Management’s Discussion and Analysis of Financial Condition and Results of Operations is based on our financial statements, which have been prepared in conformity with generally accepted accounting principles in the United States (US GAAP). The preparation of our financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and expenses incurred during the reported periods.
We base estimates on our historical experience, known trends and various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in the notes to our financial statements appearing in this Annual Report on Form 10-K, we believe that the following accounting policies are the most critical to understanding and evaluating our reported financial results.
Stock-Based Compensation
Stock-based awards are measured at fair value at each grant date. We recognize stock-based compensation expenses ratably over the requisite service period of the option award.
Determination of the Fair Value of Stock-Based Compensation Grants
The determination of the fair value of stock-based compensation arrangements is affected by a number of variables, including estimates of the expected stock price volatility, risk-free interest rate and the expected life of the award. We value stock options using the Black-Scholes option-pricing model, which was developed for use in estimating the fair value of traded options that are fully transferable and have no vesting restrictions. Black-Scholes option-pricing model and other option valuation models require the input of highly subjective assumptions, including the expected stock price volatility. If we made different assumptions, our stock-based compensation expenses, net loss, and net loss per common share could be significantly different. Prior to our initial public offering in April 2015, we issued common stock for cash consideration to investors. We believe that such transactions represent the best evidence of fair value of our common stock. Therefore, we used the sales price of our common stock prior to our initial public offering (IPO) in April 2015 as the fair value of our common stock. After our IPO, we determine that the fair value of common stock is equal to the closing price of the Company’s common stock as reported by NASDAQ on the option grant date.
| 43 |
The following summarizes the assumptions used for estimating the fair value of stock options granted during the periods indicated:
| Year Ended December 31, | ||||||
| 2017 | 2016 | 2015 | ||||
| Weighted-average grant date fair value per share | $4.85 | $7.29 | $17.95 | |||
| Expected volatility | 65%-67% | 65%-70% | 66%-71% | |||
| Risk-free interest rate | 1.83%-2.41% | 1.09%-2.44% | 1.07%-2.42% | |||
| Expected life (in years) | 5.38–10 | 5–10 | 3–10 | |||
| Dividend yield | — | — | — | |||
We have assumed no dividend yield because we do not expect to pay dividends in the foreseeable future, which is consistent with our past practice. The risk-free interest rate assumption is based on observed interest rates for U.S. Treasury securities with maturities consistent with the expected life of our stock options. The expected life represents the period of time the stock options are expected to be outstanding and is based on the simplified method when the stock option includes “plain vanilla” terms. Under the simplified method, the expected life of an option is presumed to be the midpoint between the vesting date and the end of the agreement term. We used the simplified method due to the lack of sufficient historical exercise data to provide a reasonable basis upon which to otherwise estimate the expected life of the stock options. For stock options that did not include “plain vanilla” terms, we used the contractual life of the stock option as the expected life. Such stock options consisted primarily of options issued to our board of directors that were immediately vested at issuance. Expected volatility is based on historical volatilities for publicly traded stock of comparable companies over the estimated expected life of the stock options. Due to the adoption of ASU No. 2016-09, “Stock Compensation,” effective January 1, 2017, the Company accounts for forfeitures as they occur rather than on an estimated basis.
Results of Operations
Revenue
We did not record any revenue during the years ended December 31, 2017, 2016 and 2015.
Expenses
Research and Development
Research and Development costs are summarized as follows (in thousands):
| Year Ended December 31, | Increase | % Increase | Year Ended December 31, | Increase | % Increase | |||||||||||||||||||||||||||
| 2017 | 2016 | (Decrease) | (Decrease) | 2016 | 2015 | (Decrease) | (Decrease) | |||||||||||||||||||||||||
| Salaries and related expenses | $ | 6,327 | $ | 8,402 | $ | (2,075 | ) | (25 | %) | $ | 8,402 | $ | 6,200 | $ | 2,202 | 36 | % | |||||||||||||||
| Laboratory and manufacturing supplies | 2,915 | 7,458 | (4,543 | ) | (61 | %) | 7,458 | 4,325 | 3,133 | 72 | % | |||||||||||||||||||||
| Clinical trials and sponsored research | 11,129 | 19,792 | (8,663 | ) | (44 | %) | 19,792 | 14,542 | 5,250 | 36 | % | |||||||||||||||||||||
| Stock-based compensation | 413 | 2,095 | (1,682 | ) | (80 | %) | 2,095 | 2,204 | (109 | ) | (5 | %) | ||||||||||||||||||||
| Other | 5,640 | 4,739 | 901 | 19 | % | 4,739 | 4,039 | 700 | 17 | % | ||||||||||||||||||||||
| Total | $ | 26,424 | $ | 42,486 | $ | (16,062 | ) | (38 | %) | $ | 42,486 | $ | 31,310 | $ | 11,176 | 36 | % | |||||||||||||||
| 44 |
We do not currently track our internal research and development costs or our personnel and related costs on an individual drug candidate basis. We use our research and development resources, including employees and our drug discovery technology, across multiple drug development programs. As a result, we cannot state precisely the costs incurred for each of our research and development programs or our clinical and preclinical drug candidates.
Research and development expenses decreased by 38% to $26.4 million for year ended December 31, 2017 compared to $42.5 million for the year ended December 31, 2016. The decrease in research and development expenses for the year ended December 31, 2017 compared to the year ended December 31, 2016 was due to a $8.7 million decrease of clinical trial activities and sponsored research expense, related to the completion of all active trials. In addition, there was a decrease in laboratory and manufacturing supplies expense due to a reduction in clinical trial drug manufacturing. Salary and related expenses also decreased due to the reduction of our research and development workforce from 96 to 44. The decrease of stock-based compensation expenses was mainly due to the forfeiture of terminated employees’ stock options.
Research and development expenses increased by 36% to $42.5 million for year ended December 31, 2016 compared to $31.3 million for the year ended December 31, 2015. The increase in research and development expenses for the year ended December 31, 2016 compared to the year ended December 31, 2015 was due to a $5.2 million increase of clinical trial activities and sponsored research expense, related to an expansion of clinical sites globally. In addition, there was a continued increase in laboratory and manufacturing supplies expense due to the increase of manufacturing processing development activities, research activities, quality control activities and the validation of equipment in the new manufacturing facility. The increase is also due to higher salaries and related expenses in 2016 due to the growing size of our workforce from 70 to 96. We also incurred increased allocated facility expense due to the facility expense incurred in the new manufacturing facility. Stock–based compensation increased due to the issuance of stock options to new employees.
General and Administrative
General and administrative costs are summarized as follows (in thousands):
| Year Ended December 31, | Increase | % Increase | Year Ended December 31, | Increase | % Increase | |||||||||||||||||||||||||||
| 2017 | 2016 | (Decrease) | (Decrease) | 2016 | 2015 | (Decrease) | (Decrease) | |||||||||||||||||||||||||
| Salaries and related expenses | $ | 1,497 | $ | 1,759 | $ | (262 | ) | (15 | %) | $ | 1,759 | $ | 1,167 | $ | 592 | 51 | % | |||||||||||||||
| Patent filing expense | 659 | 690 | (31 | ) | (4 | %) | 690 | 793 | (103 | ) | (13 | %) | ||||||||||||||||||||
| Stock-based compensation | 2,063 | 3,478 | (1,415 | ) | (41 | %) | 3,478 | 2,203 | 1,275 | 58 | % | |||||||||||||||||||||
| Professional fees | 1,676 | 2,479 | (803 | ) | (32 | %) | 2,478 | 802 | 1,676 | 209 | % | |||||||||||||||||||||
| Other | 1,740 | 1,871 | (131 | ) | (7 | %) | 1,872 | 1,235 | 637 | 52 | % | |||||||||||||||||||||
| Total | $ | 7,635 | $ | 10,277 | $ | (2,642 | ) | (26 | %) | $ | 10,277 | $ | 6,200 | $ | 4,077 | 66 | % | |||||||||||||||
General and administrative expenses decreased 26% to $7.6 million for the year ended December 31, 2017 compared to $10.3 million for the year ended December 31, 2016. The $1.4 million decrease in stock-based compensation is due to the grant of stock options to board members in the first quarter of 2016 that were immediately vested. Professional fees also decreased $0.8 million due to the reduction of public relations activities. The decrease in labor costs was mainly due to the reduction of our general and administrative workforce from 10 to 7.
| 45 |
General and administrative expenses increased 66% to $10.3 million for the year ended December 31, 2016 compared to $6.2 million for the year ended December 31, 2015. The increase was principally due to a $0.6 million salary increase from the growth of our workforce and a $135 thousand bonus payment to an executive officer in March 2016. Share-based compensation also increased by $1.3 million due to the grant of stock options to board members in March, September and December 2016. The increase in professional fees was attributable to the commercialization activities in Europe and public relationship activity. Other reasons for increases included insurance, travel expenses, recruiting activities and allocated facility expense.
Other Income
The following table summarizes other income (in thousands):
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Interest income | $ | 354 | $ | 49 | $ | - | ||||||
| Other income | - | - | 21 | |||||||||
| Foreign exchange gain (loss) | 555 | (47 | ) | 6 | ||||||||
| Total | $ | 909 | $ | 2 | $ | 27 | ||||||
The $354 thousand of interest income for the year ended December 31, 2017 is mainly from the interest generated from the Company’s Canadian bank account. Foreign exchange gain was mainly due to the fluctuation between US dollar and Euro in the year ended December 31, 2017 compared to the year ended December 31, 2016.
The $49 thousand of interest income for the year ended December 31, 2016 is mainly from the interest generated from the Company’s Canadian bank account. Other income consists primarily of a $21 thousand gain from the sale of fully-depreciated scientific equipment for the year ended December 31, 2015. Foreign exchange loss was mainly due to the fluctuation between US dollar and Euro in the year ended December 31, 2016 compared to the year ended December 31, 2015
Liquidity and Capital Resources
Our cash requirements could change materially as a result of the progress of our research and development and clinical programs, licensing activities, acquisitions, divestitures or other corporate developments.
Since our inception on March 22, 2005 through December 31, 2017, we have funded our operations principally through the private placement of equity securities and our initial public offering, which have provided aggregate cash proceeds of approximately $257.2 million. At December 31, 2017, we had cash and cash equivalents of $31.8 million as compared to cash and cash equivalents of $34.3 million at December 31, 2016. The following table summarizes our sources and uses of cash (in thousands):
| Year Ended December 31, | ||||||||||||
| Net cash (used in) provided by: | 2017 | 2016 | 2015 | |||||||||
| Operating activities | $ | (33,649 | ) | $ | (46,015 | ) | $ | (33,308 | ) | |||
| Investing activities | (1,405 | ) | (13,914 | ) | (10,392 | ) | ||||||
| Financing activities | 33,323 | 2,944 | 77,470 | |||||||||
| Effect of foreign exchange rate on cash and cash equivalents | (825 | ) | 258 | (48 | ) | |||||||
| Net change in cash and cash equivalents | $ | (2,556 | ) | $ | (56,727 | ) | $ | 33,722 | ||||
| 46 |
During the years ended December 31, 2017, 2016 and 2015, our operating activities used net cash of $33.6 million, $46.0 million and $33.3 million, respectively. The use of net cash in each of these periods primarily resulted from our net losses. The decrease in net loss from operations for the year ended December 31, 2017 as compared to the year ended December 31, 2016 was mainly due to the decrease in clinical trial and manufacturing activities, as well as, the reducing size of our workforce. The increase in net loss from operations for the year ended December 31, 2016 as compared to the year ended December 31, 2015 was mainly due to the increase in clinical trial and manufacturing activities, as well as, the growing size of our workforce.
During the years ended December 31, 2017, 2016 and 2015, our investing activities used net cash of $1.4 million, $13.9 million, and $10.4 million, respectively. The decrease for the year ended December 31, 2017 as compared to the year ended December 31, 2016 was principally due to the new manufacturing facility being completed in 2016. The increase for the year ended December 31, 2016 as compared to the year ended December 31, 2015 was primarily due to construction on the new manufacturing facility and equipment.
During the years ended December 31, 2017, 2016 and 2015, our financing activities provided net cash proceeds of $33.3 million, $2.9 million and $77.5 million, respectively. During the year ended December 31, 2017, we entered into subscription agreements with accredited investors, and sold 2.4 million common shares at $13 per share for approximately $31.6 million in net proceeds. Also, we sold 87 thousand shares under a Common Stock Sales Agreement with H.C. Wainwright & Co. LLC for net proceeds of approximately $1.0 million. Employees exercised stock options to purchase a total of 290 thousand shares of common stock for a total of approximately $0.7 million in net proceeds. During the year ended December 31, 2016, employees exercised stock options to purchase a total of 204,159 shares of our common stock for approximately $1.1 million in net proceeds. Also, we sold 144,426 shares under a Common Stock Sales Agreement with H.C. Wainwright & Co. LLC for net proceeds of approximately $1.8 million. During the year ended December 31, 2015, we received IPO proceeds of $76.0 million and incurred offering costs of $5.4 million, which consisted of underwriters’ commission direct incremental legal, accounting and other professional service fees related to our IPO.
We expect to continue to incur substantial operating losses in the future. We will not receive any product revenue until a drug candidate has been approved by the EMA or similar regulatory agencies in other countries and successfully commercialized. As of December 31, 2017, our principal sources of liquidity were our cash and cash equivalents, which totaled approximately $31.8 million.
Contractual Obligations and Commitments
On January 12, 2008, we entered a lease agreement to lease our office space in Austin, Texas. On September 15, 2010, we entered into a second lease agreement to lease additional space in Austin, Texas. On March 20, 2014, we extended the lease for an additional 21 months on the same terms and rental rates as the current lease. On February 28, 2015, we extended the lease for another 4 years. The future minimum lease payments are as follows as of December 31, 2017 (in thousands):
Contractual Obligations | Total | Less than | 1 - 3 Years | 3 - 5 Years | More than | |||||||||||||||
| Operating facility leases | $ | 549 | $ | 470 | $ | 79 | $ | — | $ | — | ||||||||||
| Total contractual obligations | $ | 549 | $ | 470 | $ | 79 | $ | — | $ | — | ||||||||||
Rent expense was approximately $741,000, $761,000 and $688,000 for the years ended December 31, 2017, 2016 and 2015, respectively.
Off-Balance Sheet Arrangements
Since inception, we have not engaged in any off-balance sheet activities, including the use of structured finance, special purpose entities or variable interest entities.
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURE OF MARKET RISKS |
The Company is not currently exposed to material market risk arising from financial instruments, changes in interest rates or commodity prices, or fluctuations in foreign currencies. The Company has no need to hedge against any of the foregoing risks and therefore currently engages in no hedging activities.
| 47 |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
Index to Financial Statements
| 48 |
Report of Independent Registered Public Accounting Firm
To the Shareholders and the Board of Directors of XBiotech Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of XBiotech Inc. (the Company) as of December 31, 2017 and 2016, the related consolidated statements of operations, comprehensive loss, shareholders' equity and cash flows for each of the three years in the period ended December 31, 2017, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company at December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Ernst & Young LLP
We have served as the Company’s auditor since 2005.
Houston, Texas
March 16, 2018
| 49 |
Consolidated Balance Sheets
(in thousands, except share data)
| December 31, 2017 | December 31, 2016 | |||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 31,768 | $ | 34,324 | ||||
| Prepaid expenses and other current assets | 1,564 | 2,606 | ||||||
| Total current assets | 33,332 | 36,930 | ||||||
| Property and equipment, net | 29,640 | 10,142 | ||||||
| Building construction in progress | - | 19,978 | ||||||
| Total assets | $ | 62,972 | $ | 67,050 | ||||
| Liabilities and shareholders’ equity | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 1,730 | $ | 4,431 | ||||
| Accrued expenses | 1,062 | 3,532 | ||||||
| Total current liabilities | 2,792 | 7,963 | ||||||
| Long-term liabilities: | ||||||||
| Deferred rent | 18 | 23 | ||||||
| Total liabilities | 2,810 | 7,986 | ||||||
| Shareholders’ equity: | ||||||||
| Preferred Stock, no par value, unlimited shares authorized, no shares outstanding | - | - | ||||||
| Common stock, no par value, unlimited shares authorized, 35,439,272 and 32,627,691 shares outstanding at December 31, 2017 and December 31, 2016, respectively | 277,492 | 242,419 | ||||||
| Accumulated other comprehensive loss | (768 | ) | 57 | |||||
| Accumulated deficit | (216,562 | ) | (183,412 | ) | ||||
| Total shareholders’ equity | 60,162 | 59,064 | ||||||
| Total liabilities and shareholders’ equity | $ | 62,972 | $ | 67,050 | ||||
See accompanying notes.
| 50 |
Consolidated Statements of Operations
(in thousands, except share and per share data)
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Operating expenses: | ||||||||||||
| Research and development | $ | 26,424 | $ | 42,486 | $ | 31,310 | ||||||
| General and administrative | 7,635 | 10,277 | 6,200 | |||||||||
| Total operating expenses | 34,059 | 52,763 | 37,510 | |||||||||
| Loss from operations | (34,059 | ) | (52,763 | ) | (37,510 | ) | ||||||
| Other income (loss): | ||||||||||||
| Interest income | 354 | 49 | - | |||||||||
| Other income | - | - | 21 | |||||||||
| Foreign exchange gain (loss) | 555 | (47 | ) | 6 | ||||||||
| Total other income (loss) | 909 | 2 | 27 | |||||||||
| Net loss | $ | (33,150 | ) | $ | (52,761 | ) | $ | (37,483 | ) | |||
| Net loss per share—basic and diluted | $ | (0.95 | ) | $ | (1.63 | ) | $ | (1.22 | ) | |||
| Shares used to compute basic and diluted net loss per share | 34,875,814 | 32,403,391 | 30,801,994 | |||||||||
See accompanying notes.
| 51 |
Consolidated Statements of Comprehensive Loss
(in thousands)
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Net loss | $ | (33,150 | ) | $ | (52,761 | ) | $ | (37,483 | ) | |||
| Foreign currency translation adjustment | (825 | ) | 258 | (48 | ) | |||||||
| Comprehensive loss | $ | (33,975 | ) | $ | (52,503 | ) | $ | (37,531 | ) | |||
See accompanying notes.
| 52 |
Consolidated Statements of Shareholders' Equity
(in thousands)
| Number of Shares | Common Stock Amount | Accumulated Other Comprehensive Income (Loss) | Accumulated Deficit | Total | ||||||||||||||||
| Balance at January 1, 2015 | 27,547 | 152,351 | (153 | ) | (93,168 | ) | 59,030 | |||||||||||||
| Net loss | - | - | - | (37,483 | ) | (37,483 | ) | |||||||||||||
| Foreign currency translation adjustment | - | - | (48 | ) | - | (48 | ) | |||||||||||||
| Issuance of common stock, net of issuance cost | 4,373 | 75,386 | - | - | 75,386 | |||||||||||||||
| Issuance of common stock under stock option plan | 359 | 1,348 | - | - | 1,348 | |||||||||||||||
| Stock subscription receivable | - | 410 | - | - | 410 | |||||||||||||||
| Share-based compensation expense | - | 4,407 | - | - | 4,407 | |||||||||||||||
| Balance at December 31, 2015 | 32,279 | 233,902 | (201 | ) | (130,651 | ) | 103,050 | |||||||||||||
| Net loss | - | - | - | (52,761 | ) | (52,761 | ) | |||||||||||||
| Foreign currency translation adjustment | - | - | 258 | - | 258 | |||||||||||||||
| Issuance of common stock, net of issuance cost | 145 | 1,808 | - | - | 1,808 | |||||||||||||||
| Issuance of common stock under stock option plan | 204 | 1,136 | - | - | 1,136 | |||||||||||||||
| Share-based compensation expense | - | 5,573 | - | - | 5,573 | |||||||||||||||
| Balance at December 31, 2016 | 32,628 | $ | 242,419 | $ | 57 | $ | (183,412 | ) | $ | 59,064 | ||||||||||
| Net loss | - | - | - | (33,150 | ) | (33,150 | ) | |||||||||||||
| Foreign currency translation adjustment | - | - | (825 | ) | - | (825 | ) | |||||||||||||
| Issuance of common stock, net of issuance cost | 2,521 | 32,620 | - | - | 32,620 | |||||||||||||||
| Issuance of common stock under stock option plan | 290 | 818 | - | - | 818 | |||||||||||||||
| Share-based compensation expense | - | 1,635 | - | - | 1,635 | |||||||||||||||
| Balance at December 31, 2017 | 35,439 | $ | 277,492 | $ | (768 | ) | $ | (216,562 | ) | $ | 60,162 | |||||||||
See accompanying notes.
| 53 |
Consolidated Statements of Cash Flows
(in thousands)
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Operating activities | ||||||||||||
| Net loss | $ | (33,150 | ) | $ | (52,761 | ) | $ | (37,483 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation | 1,484 | 698 | 699 | |||||||||
| Share-based compensation expense | 1,750 | 5,573 | 4,407 | |||||||||
| Gain on disposal of property and equipment | - | - | (157 | ) | ||||||||
| Other non-cash adjustments | 401 | - | - | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Prepaid expenses and other current | 1,041 | (617 | ) | (1,579 | ) | |||||||
| Accounts payable | (2,700 | ) | (981 | ) | 840 | |||||||
| Accrued expenses | (2,470 | ) | 2,066 | (52 | ) | |||||||
| Deferred rent | (5 | ) | 7 | 17 | ||||||||
| Net cash used in operating activities | (33,649 | ) | (46,015 | ) | (33,308 | ) | ||||||
| Investing activities | ||||||||||||
| Purchase of property and equipment | (1,405 | ) | (4,746 | ) | (2,322 | ) | ||||||
| Expenditures on building construction | - | (9,168 | ) | (8,070 | ) | |||||||
| Net cash used in investing activities | (1,405 | ) | (13,914 | ) | (10,392 | ) | ||||||
| Financing activities | ||||||||||||
| Issuance of common stock and warrants, net | 32,620 | 1,808 | 75,712 | |||||||||
| Issuance of common stock under stock option plan | 703 | 1,136 | 1,348 | |||||||||
| Collection of subscription receivable | - | - | 410 | |||||||||
| Net cash provided by financing activities | 33,323 | 2,944 | 77,470 | |||||||||
| Effect of foreign exchange rate on cash and cash equivalents | (825 | ) | 258 | (48 | ) | |||||||
| Net change in cash and cash equivalents | (2,556 | ) | (56,727 | ) | 33,722 | |||||||
| Cash and cash equivalents, beginning of period | 34,324 | 91,051 | 57,329 | |||||||||
| Cash and cash equivalents, end of period | $ | 31,768 | $ | 34,324 | $ | 91,051 | ||||||
| Supplemental Information: | ||||||||||||
| Accrued purchases of property and equipment | $ | - | $ | 148 | $ | 940 | ||||||
| Accrued expenditures on building construction | - | 439 | 1,416 | |||||||||
See accompanying notes.
| 54 |
Notes to Consolidated Financial Statements
1. Organization
XBiotech Inc. (XBiotech or the Company) was incorporated in Canada on March 22, 2005. XBiotech USA, Inc., a wholly-owned subsidiary of the Company, was incorporated in Delaware, United States in November 2007. XBiotech Switzerland AG, a wholly-owned subsidiary of the Company, was incorporated in Zug, Switzerland in August 2010. XBiotech Japan K.K., a wholly-owned subsidiary of the Company, was incorporated in Tokyo, Japan in March 2013. XBiotech Germany GmbH, a wholly-owned subsidiary of the Company, was incorporated in Germany in January 2014. The Company’s headquarters are located in Austin, Texas.
Since its inception, XBiotech has focused on advancing technology to rapidly identify and clone antibodies from individuals that have resistance to disease. At the heart of the Company is a proprietary technical knowhow to translate natural human immunity into therapeutic product candidates.
In 2005, the Company began to develop a new framework for commercial manufacturing, using technology that required less capital, fewer operators and provided greater flexibility than standard industry practices.
With the manufacturing capability to produce its True Human™ antibody therapy, in 2010, the Company began a clinical trial program. The first clinical trial program at MD Anderson Cancer Center began treating the sickest cancer patients irrespective of tumor type. Soon thereafter, the Company used the same antibody therapy in various clinical studies at treatment centers around the United States (U.S.) and abroad to investigate the antibody effect in patients that had vascular disease, leukemia, type 2 diabetes, psoriasis or acne.
The Company continues to be subject to a number of risks common to companies in similar stages of development. Principal among these risks are the uncertainties of technological innovations, dependence on key individuals, development of the same or similar technological innovations by the Company’s competitors and protection of proprietary technology. The Company’s ability to fund its planned clinical operations, including completion of its planned trials, is expected to depend on the amount and timing of cash receipts from future collaboration or product sales and/or financing transactions. The Company believes that its cash and cash equivalents of $31.8 million at December 31, 2017, will enable the Company to achieve several major inflection points, including potential new clinical studies with our lead product candidate. We expect to have sufficient cash through one year from the report issuance date.
2. Significant Accounting Policies
Basis of Presentation
These consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States (US GAAP).
Basis of Consolidation
The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries. All significant intercompany transactions have been eliminated upon consolidation.
Use of Estimates
The preparation of financial statements in accordance with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the reported values of amounts in the financial statements and accompanying notes. Actual results could differ from those estimates.
Prior to its initial public offering on April 15, 2015, the Company utilized significant estimates and assumptions in determining the fair value of its common stock. The board of directors determined the estimated fair value of the Company’s common stock based on a number of objective and subjective factors, including the prices at which the Company sold shares of its common stock to third parties and external market conditions affecting the biotechnology industry sector. After the initial public offering, the fair market value is calculated by using the closing price of the Company’s common stock as reported by NASDAQ.
| 55 |
Research and Development Costs
All research and development costs are charged to expense as incurred. Research and development costs include salaries and personnel-related costs, consulting fees, fees paid for contract clinical trial research services, the costs of laboratory consumables, equipment and facilities, license fees and other external costs. Costs incurred to acquire licenses for intellectual property to be used in research and development activities with no alternative future use are expensed as incurred as research and development costs.
Nonrefundable advance payments for goods or services to be received in the future for use in research and development activities are deferred and capitalized. The capitalized amounts are expensed as the related goods are delivered or the services are performed.
Income Taxes
The Company makes estimates and judgments in determining the need for a provision for income taxes, including the estimation of its taxable income or loss for the full fiscal year. The Company has accumulated significant deferred tax assets that reflect the tax effects of net operating losses and tax credit carryovers and temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Realization of certain deferred tax assets is dependent upon future earnings. The Company is uncertain about the timing and amount of any future earnings. Accordingly, the Company offsets these deferred tax assets with a valuation allowance. The Company may in the future determine that certain deferred tax assets will likely be realized, in which case the Company will reduce its valuation allowance in the period in which such determination is made. If the valuation allowance is reduced, the Company may recognize a benefit from income taxes in its statement of operations in that period.
The GAAP guidance requires recognition of the impact of a tax position in our financial statements only if that position is more likely than not to be sustained upon examination by taxing authorities, based on the technical merits of the position. Any interest and penalties related to uncertain tax positions will be reflected in income tax expense. Determining the consolidated provision for income taxes involves judgments, estimates and the application of complex tax regulations. We are required to provide for income taxes in each of the jurisdictions where we operate, including estimated liabilities for uncertain tax positions. Although we believe that we have provided adequate liabilities for uncertain tax positions, the actual liability resulting from examinations by taxing authorities could differ from the recorded income tax liabilities and could result in additional income tax expense having a material impact on our consolidated results of operations. Changes of estimates in our income tax liabilities are reflected in our income tax provision in the period in which the factors resulting in the change to our estimate become known to us. We benefit from the tax credit incentives under the U.S. research and experimentation tax credit extended to taxpayers engaged in qualified research and experimental activities while carrying on a trade or business.
Share-Based Compensation
The Company accounts for its share-based compensation awards in accordance with ASC Topic 718, Compensation-Stock Compensation (“ASC 718”). ASC 718, which requires all share-based payments to employees, including grants of employee stock options, to be recognized in the statements of operations based on their grant date fair values. For stock options granted to employees and to members of the board of directors for their services on the board of directors, the Company estimates the grant date fair value of each option award using the Black-Scholes option-pricing model. The use of the Black-Scholes option-pricing model requires management to make assumptions with respect to the expected term of the option, the expected volatility of the common stock consistent with the expected life of the option, risk-free interest rates and expected dividend yields of the common stock. For awards subject to service-based vesting conditions, the Company recognizes share-based compensation expense, equal to the grant date fair value of stock options on a straight-line basis over the requisite service period. The Company accounts for forfeitures as they occur rather than on an estimated basis.
Share-based compensation expense recognized for the years ended December 31, 2017, 2016 and 2015 was included in the following line items on the Consolidated Statements of Operations (in thousands).
| 56 |
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Research and development | $ | 413 | $ | 2,095 | $ | 2,204 | ||||||
| General and administrative | 2,063 | 3,478 | 2,203 | |||||||||
| Total share-based compensation expense | $ | 2,476 | $ | 5,573 | $ | 4,407 | ||||||
No related tax benefits were recognized for the years ended December 31, 2017, 2016 and 2015.
The fair value of each option is estimated on the date of grant using the Black-Scholes method with the following assumptions:
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Weighted-average grant date fair value per share | $4.85 | $7.29 | $17.95 | |||||||||
| Expected volatility | 65% | - | 67% | 65% | - | 70% | 66% | - | 71% | |||
| Risk-free interest rate | 1.83% | - | 2.41% | 1.09% | - | 2.44% | 1.07% | - | 2.42% | |||
| Expected life (in years) | 5.38 | - | 10 | 5 | - | 10 | 3 | - | 10 | |||
| Dividend yield | - | - | - | |||||||||
Due to the adoption of ASU No. 2016-09, “Stock Compensation,” the Company accounts for forfeitures as they occur rather than on an estimated basis.
Cash and Cash Equivalents
The Company considers highly liquid investments with a maturity of 90 days or less when purchased to be cash equivalents. Cash and cash equivalents consisted primarily of cash on deposit in U.S., German, Swiss and Canadian banks. Cash and cash equivalents are stated at cost which approximates fair value.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to credit risk consist primarily of cash and cash equivalents. The Company holds these investments in highly-rated financial institutions, and limits the amounts of credit exposure to any one financial institution. These amounts at times may exceed federally insured limits. The Company has not experienced any credit losses in such accounts and does not believe it is exposed to any significant credit risk on these funds. The Company has no off-balance sheet concentrations of credit risk, such as foreign currency exchange contracts, option contracts or other hedging arrangements.
Fair Value Measurements
The Company follows ASC Topic 820, Fair Value Measurements and Disclosures, which establishes a fair value hierarchy for those instruments measured at fair value that distinguishes between assumptions based on market date (observable inputs) and the Company’s own assumptions (unobservable inputs). The hierarchy consists of three levels:
| • | Level 1—Unadjusted quoted prices in active markets for identical assets or liabilities. |
| • | Level 2—Quoted prices for similar assets and liabilities in active markets, quoted prices in markets that are not active, or inputs which are observable, either directly or indirectly, for substantially the full term of the asset or liability. |
| • | Level 3—Unobservable inputs that reflect the Company’s own assumptions about the assumptions market participants would use in pricing the asset or liability in which there is little, if any, market activity for the asset or liability at the measurement date. |
At December 31, 2017 and 2016, the Company did not have any assets or liabilities that are measured at fair value on a recurring basis. The carrying amounts reflected in the balance sheets for cash and cash equivalents, prepaid expenses and other current assets, accounts payable, and accrued expenses approximate their fair values at December 31, 2017 and 2016, due to their short-term nature.
| 57 |
Property and Equipment
Property and equipment, which consists of land, construction in process, furniture and fixtures, computers and office equipment, scientific equipment, leasehold improvements, vehicles and building are stated at cost and depreciated over the estimated useful lives of the assets, with the exception of land and construction in process which are not depreciated, using the straight line method. The useful lives are as follows:
| • Furniture and fixtures | 7 years |
| • Office equipment | 5 years |
| • Leasehold improvements | Shorter of asset’s useful life or remaining lease term |
| • Scientific equipment | 5 years |
| • Vehicles | 5 years |
| • Building | 39 years |
Costs of major additions and betterments are capitalized; maintenance and repairs, which do not improve or extend the life of the respective assets, are charged to expense as incurred. Upon retirement or sale, the cost of the disposed asset and the related accumulated depreciation are removed from the accounts and the resulting gain or loss is recognized.
Impairment of Long-Lived Assets
The Company periodically evaluates its long-lived assets for potential impairment in accordance with ASC Topic 360, Property, Plant and Equipment. Potential impairment is assessed when there is evidence that events or changes in circumstances indicate that the carrying amount of an asset may not be recovered. Recoverability of these assets is assessed based on undiscounted expected future cash flows from the assets, considering a number of factors, including past operating results, budgets and economic projections, market trends and product development cycles. If impairments are identified, assets are written down to their estimated fair value. The Company has not recognized any impairment through December 31, 2017.
Foreign Currency Transactions
Certain transactions are denominated in a currency other than the Company’s functional currency of the U.S. dollar, and the Company generates assets and liabilities that are fixed in terms of the amount of foreign currency that will be received or paid. At each balance sheet date, the Company adjusts the assets and liabilities to reflect the current exchange rate, resulting in a translation gain or loss. Transaction gains and losses are also realized upon a settlement of a foreign currency transaction in determining net loss for the period in which the transaction is settled.
Comprehensive Income (Loss)
ASC Topic 220, Comprehensive Income, requires that all components of comprehensive income (loss), including net income (loss), be reported in the financial statements in the period in which they are recognized. Comprehensive income (loss) is defined as the change in equity during a period from transactions and other events and circumstances from non-owner sources, including unrealized gains and losses on investments and foreign currency translation adjustments.
Segment and Geographic Information
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision making group, in making decisions on how to allocate resources and assess performance. The Company’s chief operating decision maker is the Chief Executive Officer. The Company and the chief operating decision maker view the Company’s operations and manage its business as one operating segment. Substantially all of the Company’s operations are in the U.S. geographic segment.
| 58 |
Net Loss per Share
Net loss per share (“EPS”) is computed by dividing net loss by the weighted average number of common shares outstanding during each period. Diluted EPS is computed by dividing net loss by the weighted average number of common shares and common share equivalents outstanding (if dilutive) during each period. The number of common share equivalents, which include stock options, is computed using the treasury stock method.
Subsequent Events
The Company considered events or transactions occurring after the balance sheet date but prior to the date the consolidated financial statements are available to be issued for potential recognition or disclosure in its consolidated financial statements. We have evaluated subsequent events through the date of filing this Form 10-K.
Recent Accounting Pronouncements
In February 2016, the FASB issued final guidance that will change the accounting for leases ASU No. 2016-02, “Leases.” ASU 2016-02. The FASB issued final guidance that requires lessees to put most leases on their balance sheets but recognize expenses on their income statements in a manner similar to today’s accounting. The guidance also eliminates today’s real estate-specific provisions for all entities. For lessors, the guidance modifies the classification criteria and the accounting for sales-type and direct financing leases. All entities classify leases to determine how to recognize lease-related revenue and expense. Classification continues to affect what lessors record on the balance sheet. For calendar-year public business entities and certain calendar-year not-for-profit entities and employee benefit plans, the guidance is effective in 2019, and interim periods within that year. For other calendar-year entities, it is effective in 2020, and interim periods in 2021. Early adoption is permitted for all entities. The adoption of this standard will require the Company to record its operating leases on the balance sheet. The Company is currently evaluating the impact of this pronouncement on the Company's consolidated financial statements
In March 2016, the FASB issued ASU No. 2016-09, “Stock Compensation,” which is intended to simplify several aspects of the accounting for share-based payment award transactions. The guidance requires the recognition of the income tax effects of awards in the income statement when the awards vest or are settled, thus eliminating additional paid in capital pools. The Company adopted this pronouncement effective January 1, 2017. Upon adoption, the Company recognized approximately $491 thousand of accumulated excess tax benefits as deferred tax assets that under the previous guidance could not be recognized until the benefits were realized through a reduction in cash taxes paid. This part of the guidance is applied using a modified retrospective method with a cumulative-effect adjustment to the accumulated deficit for the excess tax benefits not previously recognized. However, given the full valuation allowance placed on the additional $491 thousand of deferred tax assets, the recognition of this provision of ASU 2016-09 had no impact to the Company’s accumulated deficit as of January 1, 2017. In addition, the guidance allows for a policy election to account for forfeitures as they occur rather than on an estimated basis. The Company elected to account for forfeitures as they occur using a modified retrospective transition method. The adoption of this one-time accounting policy election did not have a material impact on the Company’s financial statements.
3. Property and Equipment and Building Construction in Progress
Property and equipment consisted of the following as of December 31, 2017 and 2016 (in thousands):
| 2017 | 2016 | |||||||
| Computer and office equipment | $ | 461 | $ | 456 | ||||
| Furniture and fixtures | 183 | 132 | ||||||
| Land | 1,418 | 1,418 | ||||||
| Leasehold improvements | 802 | 794 | ||||||
| Scientific equipment | 12,194 | 6,116 | ||||||
| Vehicle | 30 | 30 | ||||||
| Building | 21,013 | 19,978 | ||||||
| Construction in process | 472 | 6,645 | ||||||
| Accumulated depreciation | (6,933 | ) | (5,449 | ) | ||||
| Total | $ | 29,640 | $ | 30,120 | ||||
| 59 |
Depreciation expenses related to property and equipment amounted to approximately $1,484,000, $698,000, and $699,000 and for the years ended December 31, 2017, 2016 and 2015, respectively. Construction in process is related to research and development and manufactory equipment.
4. Accrued Expenses
Accrued expenses consist of the following as of December 31, 2017, and 2016 (in thousands):
| 2017 | 2016 | |||||||
| Accrued compensation and related expenses | $ | 234 | $ | 413 | ||||
| Accrued professional fees | 32 | 95 | ||||||
| Accrued building construction fees | - | 521 | ||||||
| Accrued clinical trial expenses | 766 | 2,402 | ||||||
| Other | 30 | 101 | ||||||
| Total | $ | 1,062 | $ | 3,532 | ||||
5. Common Stock
Pursuant to its Articles, the Company has an unlimited number of shares available for issuance with no par value.
From January through December 2016, 204 thousand shares of common stock were issued upon the exercise of stock options at a price of $0.74 to $19.09 per share for total proceeds of $1.1 million.
From November through December 2016, under the Common Stock Sales Agreement with H.C. Wainwright & Co. LLC, the Company sold 145 thousand shares of common stock at a price between $13.60 to $14.17 per share for total proceeds of $1.8 million.
In February 2017, under the Common Stock Sales Agreement with H.C. Wainwright & Co. LLC, the Company sold 87 thousand shares of common stock at a price between $12.09 to $12.37 per share for total proceeds of $1.0 million.
In March 2017, the Company sold 2.4 million shares of common stock at a net price of $13.00 for total proceeds of approximately $31.6 million from investors.
From January through December 2017, 290 thousand shares of common stock were issued upon the exercise of stock options at a price of $2.50 to $14.71 per share for a total of $703 thousand.
6. Common Stock Options
On November 11, 2005, the board of directors of the Company adopted a stock option plan (“the Plan”) pursuant to which the Company may grant incentive stock and non-qualified stock options to directors, officers, employees or consultants of the Company or an affiliate or other persons as the Compensation Committee may approve.
All options will be non-transferable and may be exercised only by the participant, or in the event of the death of the participant, a legal representative until the earlier of the options’ expiry date or the first anniversary of the participant’s death, or such other date as may be specified by the Compensation Committee.
The term of the options is at the discretion of the Compensation Committee, but may not exceed 10 years from the grant date. The options expire on the earlier of the expiration date or the date three months following the day on which the participant ceases to be an officer or employee of or consultant to the Company, or in the event of the termination of the participant with cause, the date of such termination. Options held by non-employee Directors have an exercise period coterminous with the term of the options.
| 60 |
The number of common shares reserved for issuance to any one person pursuant to this Plan shall not, in aggregate, exceed 5% of the total number of outstanding common shares. The exercise price per common share under each option will be the fair market value of such shares at the time of the grant. Upon stock option exercise, the Company issues new shares of common stock.
A summary of changes in common stock options issued under the Plan is as follows:
| Options | Exercise Price | Weighted-Average Exercise Price | ||||||||||||
| Options outstanding at December 31, 2014 | 4,884,165 | $0.55 | - | $15.00 | $ | 7.03 | ||||||||
| Granted | 375,928 | 8.47 | - | 21.99 | 17.95 | |||||||||
| Exercised | (359,141 | ) | 0.53 | - | 10.00 | 3.75 | ||||||||
| Forfeitures | (114,375 | ) | 0.55 | - | 20.93 | 11.05 | ||||||||
| Options outstanding at December 31, 2015 | 4,786,577 | $0.53 | - | $21.99 | $ | 8.56 | ||||||||
| Granted | 1,059,990 | 7.71 | - | 19.10 | 11.72 | |||||||||
| Exercised | (204,159 | ) | 0.74 | - | 19.09 | 5.56 | ||||||||
| Forfeitures | (453,750 | ) | 0.52 | - | 16.91 | 12.79 | ||||||||
| Options outstanding at December 31, 2016 | 5,188,658 | $0.52 | - | $21.99 | $ | 8.49 | ||||||||
| Granted | 1,251,000 | 4.15 | - | 12.62 | 4.85 | |||||||||
| Exercised | (406,667 | ) | 0.93 | - | 14.71 | 2.28 | ||||||||
| Forfeitures | (729,367 | ) | 0.94 | - | 21.99 | 11.90 | ||||||||
| Options outstanding at December 31, 2017 | 5,303,624 | $2.5 | - | $21.99 | $ | 7.69 | ||||||||
The weighted average fair value of the options issued to directors, employees and consultants during the fiscal years ended December 31, 2017, 2016 and 2015, was $2.97, $7.29 and $17.95, respectively. Options with an intrinsic value of $(3.75), $1.63 and $2.86, became vested during 2017, 2016 and 2015, respectively. The total intrinsic value of options exercisable and total options outstanding at December 31, 2017 was $746,000. The total fair value of options vested during the years ended December 31, 2017, 2016 and 2015 was $1,601,000, $5,728,000and $5,463,000, respectively.
As of December 31, 2017, there was approximately $3.1 million of unrecognized compensation cost, related to stock options granted under the Plan which will be amortized to stock compensation expense over the next 2.23 years.
7. Net Loss Per Share
The following summarizes the computation of basic and diluted net loss per share for the years ended December 31, 2017, 2016 and 2015 (in thousands, except share and per share data):
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Net loss | $ | (33,150 | ) | $ | (52,761 | ) | $ | (37,483 | ) | |||
| Weighted-average number of common shares—basic and diluted | 34,875,814 | 32,403,391 | 30,801,994 | |||||||||
| Net loss per share—basic and diluted | $ | (0.95 | ) | $ | (1.63 | ) | $ | (1.22 | ) | |||
The following potentially dilutive securities outstanding, prior to the use of the treasury stock method or if-converted method, have been excluded from the computation of diluted weighted-average common shares outstanding, because including them would have had an anti-dilutive effect due to the losses reported.
| 61 |
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Stock options | 5,303,624 | 5,188,658 | 4,786,577 | |||||||||
| Warrants to purchase common stock | - | - | - | |||||||||
| Total | 5,303,624 | 5,188,658 | 4,786,577 | |||||||||
8. Income Taxes
The Company recorded no provision for income taxes for the years ended December 31, 2017, 2016 and 2015 due to the reported net losses in each year and reported valuation allowance.
A reconciliation of the Company’s Canadian federal statutory income tax rate to the Company’s effective income tax rate is as follows for the years ended December 31, 2017, 2016 and 2015:
| 2017 | 2016 | 2015 | ||||||||||
| Income tax benefit computed at federal tax rate | 26.0 | % | 26.0 | % | 26.0 | % | ||||||
| Change in valuation allowance | (15.3 | %) | (22.1 | %) | (27.2 | %) | ||||||
| Impacts of US tax reform | (13.4 | %) | - | - | ||||||||
| Stock compensation and other | 2.7 | % | (3.9 | %) | 1.2 | % | ||||||
| Total | — | % | — | % | — | % | ||||||
During the years ended December 31, 2017, 2016 and 2015, the Company had no interest and penalties related to income taxes.
As of December 31, 2017, and 2016, the Company has unused net operating losses of approximately $167.6 million (approximately $132.3 million in Canada, $27.7 million in the U.S., $6.7 million in Germany and $0.9 million in Switzerland and Japan) and $133.0 million (approximately $108.9 million in Canada, $18.6 million in the U.S., $4.8 million in Germany and $0.6 million in Switzerland and Japan), respectively, available to reduce taxable income of future years. The tax benefit of net operating losses begin to expire in 2025 in Canada, 2028 in U.S., 2034 in Germany 2018 in Switzerland, and 2022 in Japan.
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amount of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The Company has established a valuation allowance due to uncertainties regarding the realization of deferred tax assets based upon the Company’s lack of earnings history. Significant components of the Company’s deferred tax assets and liabilities as of December 31, 2017, 2016 and 2015 as follows (in thousands):
| 2017 | 2016 | 2015 | ||||||||||
| Deferred tax assets: | ||||||||||||
| Noncapital losses | $ | 41,551 | $ | 35,064 | $ | 23,038 | ||||||
| Qualifying research and development credits | 2,833 | 2,408 | 1,591 | |||||||||
| Stock based compensation | 1,618 | 2,737 | 3,982 | |||||||||
| Share issue costs | 42 | 27 | 30 | |||||||||
| Accrued liabilities | 49 | 286 | 356 | |||||||||
| Deferred rent | 4 | 8 | 6 | |||||||||
| Total deferred tax assets | 46,097 | 40,530 | 29,003 | |||||||||
| Deferred tax liabilities: | ||||||||||||
| Depreciation | 282 | 31 | 13 | |||||||||
| Share issuance costs | 35 | 16 | 23 | |||||||||
| Total deferred tax liabilities | 317 | 47 | 36 | |||||||||
| Net deferred tax asset | 45,780 | 40,483 | 28,967 | |||||||||
| Valuation allowance for deferred tax assets | (45,780 | ) | (40,483 | ) | (28,967 | ) | ||||||
| Net deferred tax asset including valuation allowance | $ | — | $ | — | $ | — | ||||||
On December 22, 2017, the President of the United States signed into law the Tax Cuts and Jobs Act, or TCJA, tax reform legislation. The TCJA makes significant changes in U.S. tax law including a reduction in the corporate tax rates, changes to net operating loss carryforwards and carrybacks, and a repeal of the corporate alternative minimum tax. The TCJA reduced the U.S. corporate tax rate from the current rate of 34 percent down to 21 percent starting on January 1, 2018. As a result of the TCJA, the Company was required to revalue deferred tax assets and liabilities at 21 percent. This revaluation resulted in a provision of $4.5 million to income tax expense in continuing operations and a corresponding reduction in the valuation allowance. As a result, there was no impact to the Company’s consolidated statements of comprehensive loss as a result of the reduction in tax rates.
| 62 |
As the Company does not have all of the necessary information to analyze all income tax effects of the TCJA, the Company will continue to make and refine calculations and estimates as additional information is obtained, which could potentially affect the provisional amounts relating to the deferred income taxes, including but not limited to deferred tax assets related to share-based compensation expenses. Where the Company has not yet been able to make reasonable estimates of the impact of certain elements, the Company has not recorded any amounts related to those elements and has continued accounting for them in accordance with ASC 740 on the basis of the tax laws in effect immediately prior to the enactment of the TCJA. The Company expects to complete a detailed analysis no later than the fourth quarter of 2018.
Due to additional current year losses, offset by the decrease of the U.S. tax rate from 34% to 21% in 2017, the valuation allowance increased by approximately $5.3 million and $11.5 million during the year ended December 31, 2017 and 2016 respectively.
The Company applies the accounting guidance in ASC 740 related to accounting for uncertainty in income taxes. The Company’s reserves related to taxes are based on a determination of whether, and how much of, a tax benefit taken by the Company in its tax filings or positions is more likely than not to be realized following resolution of any potential contingencies present related to the tax benefit. As of December 31, 2017 and 2016, the Company had no unrecognized tax benefits.
The Company files federal income tax returns in Canada, U.S, Switzerland, Germany, and Japan. The Company also files income tax returns in the state of Texas in the U.S. The statute of limitations for assessment by local taxing authorities is open for tax years ended after December 2011. There are currently no federal or state income tax audits in progress.
The components of income before income taxes are as follows:
| (In thousands) | Years Ended December 31, | |||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Domestic | $ | (5,765 | ) | $ | (8,749 | ) | $ | (6,544 | ) | |||
| Canada | (26,034 | ) | (41,625 | ) | (28,129 | ) | ||||||
| Other Foreign | (1,351 | ) | (2,387 | ) | (2,810 | ) | ||||||
| Total | $ | (33,150 | ) | $ | (52,761 | ) | $ | (37,483 | ) | |||
In November 2015, the FASB issued ASU 2015-17, “Balance Sheet Classification of Deferred Taxes”. The Company adopted these accounting changes on a prospective basis during the three months ended December 31, 2016. To simplify the presentation of deferred income taxes, the amendments in this update require that deferred tax liabilities and assets be classified as noncurrent in a classified statement of financial position. The adoption of this standard did not have a material effect on the Company’s financial statements or disclosures.
9. Commitments and Contingencies
On January 12, 2008, the Company entered a lease agreement to lease its facility in Austin, Texas, U.S. On September 15, 2010, the Company entered into a second lease agreement to lease additional space in Austin, Texas, U.S. On March 20, 2013, the company extended the lease for another 21 months with the same terms and rental rates as the current leases. On February 28, 2015, the Company extended the leases for another four years with two years early termination right. The future minimum lease payments are as follows as of December 31, 2017 (in thousands):
| 63 |
| 2018 | $ | 470 | ||
| 2019 | $ | 79 |
Rent expense was approximately $741,000, $761,000 and $688,000 for the years ended December 31, 2017, 2016 and 2015, respectively.
XBiotech Corporate officers, Queena Han (VP of Finance) and John Simard (President and CEO), XBiotech Inc., and certain directors were named defendants in securities class action civil suits filed in federal court at the U.S. District Court for the Western District of Texas, in Austin, Texas and state court at the Los Angeles County Superior Court, in California. In the California action, the underwriter WR Hambrecht & Co., LLC was also named as a defendant. These civil suits were filed on December 1, 2015. The foundation for both suits are similar in that the plaintiffs allege the officers of the Company made false and misleading statements, violating the securities laws, in the IPO documents in April 2015. Specifically, these alleged false statements in the IPO documents are in relation to the European Phase III clinical trial for Xilonix™. The allegations focus on a press release posted by XBiotech on November 23, 2015, explaining certain issues with patient data. Plaintiffs allege the company knew of these issues during the IPO and neglected to disclose them in supporting documentation filed with the Security and Exchange Commission (SEC). As a result of the news release, XBiotech (traded on the NASDAQ) stock declined. The resulting securities class action lawsuits are seeking relief for plaintiffs who report financial losses due to the alleged false and misleading statements. In September 2016, a stay was granted in the California case. Plaintiffs were, at that time, left with the opportunity to re-file in Texas prior to the decision on the motion to dismiss. Plaintiffs did not re-file in Texas before the case was dismissed with prejudice in October 2016. Plaintiffs sought to re-open the case in California. A hearing to address whether the case should be dismissed, was scheduled June 7, 2017. At the hearing, counsel for XBiotech argued that California was a forum non conveniens given the nexus of the allegations in the suit took place in Texas. The judge granted the forum non conveniens motion, finding that the case does not belong in California. As a result, the case is stayed rather than dismissed per California procedural rules. Plaintiffs were compelled to re-file in Texas.
The plaintiffs re-filed their suit in Travis County district court, located in Austin, Texas, on July 6, 2017. A hearing date has yet to be scheduled in this matter. Subsequently, XBiotech won on its motion to remove the case from state court to federal court at the U.S. District Court for the Western District of Texas, Austin Division. Counsel further argued for a motion to stay the case, which was granted on October 6, 2017, taking into account the upcoming hearings at the U.S. Supreme Court for Cyan, Inc. v. Beaver County Employees Retirement Fund. At issue in this is case is whether state courts lack subject matter jurisdiction over covered class actions that allege only Securities Act of 1933 claims. Arguments before the Supreme Court were held in November 2017. A decision from the Court is expected in the first half of 2018, at which time we will learn more about its impact on whether plaintiffs may return to litigate Rezko v. XBiotech in state court.
10. Subsequent Events
On January 16, 2018, Daniel Vasella resigned from the Board of Directors of XBiotech Inc. effective immediately. On January 16th, 2018, the Company promptly notified the NASDAQ Listing Qualifications Department (“NLQD”) of this event. Subsequently, the Company received a letter from the NLQD which noted the Company’s non-compliance with NASDAQ’s independent director and audit committee composition requirements set forth in Listing Rules 6505(b)(1) and 5605(c)(2), respectively. The Company was given 45 days to submit a plan to regain compliance related to NASDAQ’s independent director requirement and a cure period to regain compliance with the compensation committee requirements.
| 64 |
As described in a Form 8-K filing on March 1, 2018, on February 27, 2018, Mr. Jan-Paul Waldin was elected as a member of the Board of Directors the Company, as well as appointed as a member of the Company’s Audit Committee and Compensation Committee, to serve until his successor is duly elected and qualified or until his earlier resignation, removal or death. With the addition of Mr. Waldin to the Company’s Board of Directors, Audit Committee and Compensation Committee, the Company has regained compliance and is no longer subject to the requirements set forth in the Letter from NASDAQ dated January 18, 2018 and referenced in a Form 8-K filing on January 19, 2018. Therefore, the Company is currently only subject to the requirements set forth in the Letter from NASDAQ dated November 9, 2017 and referenced in the Company’s Form 10-Q filing on that same day in which it has been given a cure period until the earlier of the Company’s next annual shareholders’ meeting or November 8, 2018 in order to regain compliance with NASDAQ’s audit committee requirements as set forth in Listing Rule 5605. The Company plans to fill the vacancy and regain compliance on this matter within the cure period provided by NASDAQ.
11. Selected Quarterly Financial Data (Unaudited)
Selected Quarterly Financial Data (Unaudited) for the year ended December 31, 2017 and 2016 is presented below (in thousands except per share data):
| 2017 | First Quarter | Second Quarter | Third Quarter | Fourth Quarter | ||||||||||||
| Loss from operations | (10,271 | ) | (9,688 | ) | (6,593 | ) | (7,507 | ) | ||||||||
| Net Loss | (10,563 | ) | (9,133 | ) | (6,205 | ) | (7,249 | ) | ||||||||
| Net loss per share—basic and diluted | (0.32 | ) | (0.26 | ) | (0.18 | ) | (0.20 | ) | ||||||||
| 2016 | First Quarter | Second Quarter | Third Quarter | Fourth Quarter | ||||||||||||
| Loss from operations | (10,248 | ) | (13,615 | ) | (12,459 | ) | (16,441 | ) | ||||||||
| Net Loss | (10,257 | ) | (13,622 | ) | (12,484 | ) | (16,398 | ) | ||||||||
| Net loss per share—basic and diluted | (0.32 | ) | (0.42 | ) | (0.38 | ) | (0.50 | ) | ||||||||
| 65 |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
| None. |
| ITEM 9A. | CONTROLS AND PROCEDURES. |
Management's Evaluation of our Disclosure Controls and Procedures
As of the end of the year covered by this Annual Report on Form 10-K, an evaluation was carried out by the Company’s management, with the participation of the Chief Executive Officer and Principal Financial Officer, of the effectiveness of the Company’s disclosure controls and procedures, as defined in Rule 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934. Based on such evaluation, the Chief Executive Officer and Principal Financial Officer concluded that the Company’s disclosure controls and procedures are effective to ensure that information required to be disclosed in the reports the Company files or furnishes under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and regulations, and are operating in an effective manner.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). We conducted an assessment of the effectiveness of our internal control over financial reporting based on the criteria set forth in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on our assessment, we have concluded that our internal control over financial reporting was effective as of December 31, 2017, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with GAAP.
Changes in Internal Control Over Financial Reporting
There was no change in our internal control over financial reporting that occurred during the fourth quarter of the year ended December 31, 2017 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs.
| ITEM 9B. | OTHER INFORMATION |
None.
| 66 |
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
We incorporate by reference the information required by this Item with respect to directors and the Audit Committee from the information under the caption “ELECTION OF DIRECTORS,” including in particular the information under “Nominating and Corporate, Governance and Review Committee” , “Audit Committee”, “Report of the Audit Committee & the Board of Directors”, “Code of Ethics” and “Section 16(0) Beneficial Ownership Reporting Compliance” and “EXECUTIVE OFFICERS” contained in our definitive Proxy Statement (the “Proxy Statement”), which we will file on or about May 2, 2018 with the Securities and Exchange Commission in connection with the solicitation of proxies for our 2018 Annual Meeting of Stockholders to be held on June 19, 2018.
| ITEM 11. | EXECUTIVE COMPENSATION |
The information required by this item is incorporated herein by reference to the information contained under the sections captioned “EXECUTIVE COMPENSATION”, “DIRECTOR COMPENSATION”, “Compensation Committee Interlocks and Insider Participation,” “Employment Arrangements” and “Compensation Committee Report” of the Proxy Statement.
| ITEM 12. | SECURITY OWNERSHIP OF CERTATIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The information required by this item will be set forth under the heading “Security Ownership of Certain Beneficial Owners and Management" in our Proxy Statement and is incorporated herein by reference.
The information required by Item 201(d) of Regulation S-K will be set forth in the section headed “Equity Compensation Plan Information” in our Proxy Statement and is incorporated herein by reference.
| ITEM 13. | CERTAIN RELATIONSHPS AND RELATED TRANSACTIONS AND DIRECTOR INDENPENDENCE |
The information required by this item will be set forth in the section headed “Transactions with Related Persons” in our Proxy Statement and is incorporated herein by reference.
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES |
The information required by this item will be set forth in the section headed “Ratification of Selection of Independent Registered Public Accounting Firm” in our Proxy Statement and is incorporated herein by reference.
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
Financial Statements
See Index to Consolidated Financial Statements under Item 8 of Part II.
Financial Statement Schedules
None
| 67 |
| ITEM 16. | FORM 10–K SUMMARY |
Not applicable.
| 68 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized on March 16, 2018.
| XBIOTECH INC., | ||
|
/S/ JOHN SIMARD | ||
| Name: | John Simard | |
| Title: | President and Chief Executive Officer | |
| (Principal Executive Officer) | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signature and Title |
Date | |
|
/S/ JOHN SIMARD John Simard, Chief Executive Officer (Principal Executive Officer) and Director |
March 16, 2018 | |
|
/S/ QUEENA HAN Queena Han, Vice President of Finance & Human Resources (Principal Financial Officer and Principal Accounting Officer) |
March 16, 2018 | |
|
/S/ W. THORPE MCKENZIE W. Thorpe McKenzie, Director |
March 16, 2018 | |
|
/S/ JAN-PAUL WALDIN Jan-Paul Waldin, Director
|
March 16, 2018 | |
| 69 |
EXHIBIT INDEX
| 70 |
71