
1 MASTER DEVELOPMENT AND SUPPLY AGREEMENT This MASTER DEVELOPMENT AND SUPPLY AGREEMENT ( Agreement ) is made effective the first (1st) day of August 2023 , by and between Curia New Mexico, LLC Curia 4401 Alexander Blvd. NE, Albuquerque, New Mexico 87107 and Indivior UK Limited ( Indivior ), with its registered address at Chapleo Building, Henry Boot Way, Priory Park, Hull HU4 7BY, UK. WHEREAS, Indivior is engaged in the business of developing pharmaceutical products; WHEREAS, Curia is engaged in the business of providing contract pharmaceutical development, manufacturing, packaging and analytical services to the pharmaceutical industry; WHEREAS, Indivior desires to engage Curia to manufacture and supply certain Product(s) (as defined below), and to provide related development services, and Curia desires to provide such manufacture, supply, and development services, pursuant to the terms and conditions set forth in this Agreement; WHEREAS, Indivior and Curia desire for this Agreement to supersede the Supply Agreement entered into by and between Curia Global, Inc., an Affiliate of Curia (formerly known as Albany Molecular Research, Inc.) and Indivior dated August 1, 2017, as amended ; WHEREAS, Indivior and Curia executed this Agreement as of the date of last signature below but each intend for this Agreement to be effective and binding on the parties as of the Effective Date above; and WHEREAS, Indivior and Curia each conducted themselves in accordance with the terms of this Agreement as of the Effective Date, notwithstanding the Execution Date. NOW, THEREFORE, in consideration of the mutual covenants, terms and conditions set forth below, the parties agree as follows: ARTICLE 1 DEFINITIONS The following terms have the following meanings in this Agreement: 1.1 Affiliate(s) means any corporation, firm, partnership or other entity which controls, is controlled by or is under common control with a party for as long as such control exists. For purposes of this definition, "control" shall mean the ownership of at least fifty percent (50%) of the voting share capital of such entity or any other comparable equity or ownership interest, provided that Affiliates of Curia shall be limited to its direct and indirect subsidiaries. Exhibit 4.17.2 PORTIONS OF THIS EXHIBIT HAVE BEEN REDACTED. CERTAIN IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH (i) NOT MATERIAL AND (ii) WOULD LIKELY TO CAUSE COMPETITIVE HARM IF PUBLICLY DISCLOSED. REDACTED MATERIAL IS MARKED WITH [***].

2 1.2 twelve (12) month forecast of projected total volume of Product to be ordered in the applicable year (beginning 2024) for April 1 March 31; such forecast to be provided by Indivior to Curia by October 1 of the prior year (beginning in 2023). 1.3 has been released by Indivior and provided to Curia, along with a Certificate of Analysis. 1.4 Section 2.4. 1.5 Applicable Laws means all laws, ordinances, rules and regulations within the Territory applicable to the Processing of the Product or any aspect thereof and the obligations of Curia or Indivior, as the context requires under this Agreement, including, without limitation, (i) all applicable federal, state and local laws and regulations of each Territory; (ii) the U.S. Federal Food, Drug and Cosmetic Act, and (iii) cGMPs. 1.6 means the quantity of Product comprising a specified number of units (e.g., syringes) as mutually agreed upon between the parties, that (a) is intended to have uniform character and quality within specified limits, and (b) is Processed according to a single manufacturing order during the same cycle of Processing. 1.7 o shall have the meaning set forth in Section 7.1(i). 1.8 5.2. 1.9 Cale means a period of three (3) consecutive months commencing on January 1, April 1, July 1 or October 1 of any Calendar Year. 1.10 1.11 1.12 (i) with reference to a Batch of Product, the certificate that accompanies each shipment of a Batch of Product and which lists the test methods, acceptance limits and release test results for that specific Batch of Product; and (ii) with reference to Indivior Materials, the certificate that accompanies each shipment of Indivior Materials and which lists the test methods, acceptance limits and release test results for that specific batch of Indivior Materials. 1.13 means (i) with reference to a Batch of Product, a certificate attesting that the particular Batch of Product was Processed in accordance with the applicable Manufacturing Standards, and (ii) with reference to Indivior Materials, a certificate attesting that the Indivior Materials were manufactured in accordance with cGMPs as applicable to such Indivior Materials and conform to applicable regulatory requirements.

3 1.14 cGMP means the current good manufacturing practices in the United States for the manufacture, control and storage of human pharmaceutical products, and equivalent regulations in such other Territories as are expressly agreed upon by the parties in writing, in each case as applicable to Product or Indivior Materials, as the context may require, provided that in the event of any conflict among the applicable laws of the United States and other Territories, current good manufacturing practices in the applicable Territory shall be applied unless the parties otherwise agree in writing. In the event Indivior does not provide Curia with advance notice of the intended destination Territory for any Batch of Product, Curia shall default to current good manufacturing practices in the United States. For clarity, the current good manufacturing practices in the United States applicable to Product shall mean those set forth in 21 C.F.R. 210 and 21 C.F.R. 211, as may be amended or supplemented, and the related regulations and FDA guidance documents in effect from time to time. 1.15 quired clinical trials, if any, and has been approved by an applicable Regulatory Authority for commercial distribution. 1.16 shall mean, with respect to the efforts expended by a party under this Agreement, that degree of effort consistent with the practices and standards of the pharmaceutical industry in the United States normally used to achieve the fulfillment of obligations similar to those assumed by a party under this Agreement as expeditiously as possible. 1.17 means a product used for generic versions of the Product or Brixadi . 1.18 Confidential Information shall have the meaning set forth in Section 11.1. 1.19 Term of this Agreement, with the first Contract Year commencing on the Effective Date, and with each subsequent Contract Year commencing on the anniversary of the Effective Date. 1.20 Background Technology by Curia or any of its Affiliates as of the Effective Date; or (ii) developed or obtained by or on behalf of Curia or any of its Affiliates after the Effective Date independent of this Agreement, and all intellectual property rights in any of the foregoing. 1.21 Curia Section 13.2. 1.22 improvements to Curia Background Technology, (ii) is developed using Confidential Information of Curia and does not incorporate Indivior Confidential Information, or (iii) consists of improvements to the manufacturing process that are generally applicable to multiple products (but which do not incorporate Indivior Confidential Information), and in each case of (i), (ii) and (iii), is not Product-specific Program Technology, and all intellectual property rights in any of the foregoing clauses (i), (ii) and (iii).

4 1.23 Curia Curia Background Technology and Curia Program Technology. 1.24 all have the meaning set forth in Section 5.3. 1.25 Effective Date shall have the meaning set forth in the Preamble. 1.26 Curia Albuquerque, New Mexico, located at 4401 Alexander Blvd. NE, Albuquerque, New Mexico 87107. 1.27 thereto. 1.28 Section 4.2. 1.29 Section 4.1. 1.30 1.31 Section 3.3. 1.32 means (i) API; (ii) any intermediates or derivatives of API; (iii) any other Technology owned or controlled by Indivior or any of its Affiliates as of the Effective Date; or (iv) any Technology, other than Program Technology, developed or obtained by or on behalf of Indivior or any of its Affiliates after the Effective Date, and all intellectual property rights in any of the foregoing. 1.33 Section 13.1. 1.34 s API, Raw Materials, including polymer, and syringe, components (e.g., plunger and syringe) provided by Indivior and, in the future, any other reference standards used in the Processing of Product that are to be provided by Indivior approved qualified suppliers or materials that may become necessary for the Processing of the Product and that which is agreed in writing by the parties. 1.35 under Section 12.3 of this Agreement in Product-specific Program Technology. 1.36 -conforming Product which could not have been ascertained by Indivior by the exercise of reasonable diligence regarding the cause of such defect in accordance with Section 6.1, such defect is determined . 1.37 Section 13.1.

5 1.38 of Product in accordance with procedures set forth in the Master Batch Record and cGMP (if applicable as per intended use of Product), and in conformance with Specifications, provided that a failure to conform to Specifications due to the use of any (i) defective, adulterated or misbranded API or other Indivior Materials as supplied by Indivior (including, but not limited to failure of API or other Indivior Materials to meet applicable Specifications or to have been manufactured in accordance with cGMP), or (ii) defective Other Raw Materials (other than Indivior Materials), where the defects were not reasonably discoverable by Curia as a result of testing in accordance with its standard operating procedures shall not prevent Product from being considered to have been Processed in accordance with Manufacturing Standards. 1.39 as may be amended from time to time in accordance with this Agreement and the Quality Agreement, specifying or referencing the complete set of formal instructions agreed upon by the parties for the Processing of Product, including, but not limited to material descriptions, the formula, processing procedures, in-process testing specifications, Product Specifications and packaging and shipping specifications. For the avoidance of doubt, upon finalization, Indivior shall have full and sole rights, titles, and ownership to, in and of the Master Batch Record, provided, however, Indivior shall in no event have any . As of the Effective Date, the parties agree that no Curia Confidential Information is included in the Master Batch Record. In the event Curia does add any of its Confidential Information to the Master Batch Record, Curia shall give Indivior written notice of which such information Information. 1.40 - shall have the meaning set forth in Section 18.15. 1.41 1.42 Section 3.1. 1.43 - Product which is in developmental/clinical trial phases and has not yet been approved by an applicable Regulatory Authority for commercial distribution. Non-commercial Product includes Stability/Validation Batches. 1.44 the meaning set forth in Section 4.1. 1.45 -conforming Product, which, at the time of Delivery, was not Processed in accordance with Manufacturing Standards. 1.46 Section 6.1. 1.47 shall have the meaning set forth in Section 18.15. 1.48 shall have the meaning set forth in Section 18.15.

6 1.49 have the meaning set forth in Section 3.2. 1.50 syringes, and bulk packaging of naked syringes into containers for Delivery. For the avoidance of doubt, this shall exclude syringe labelling and Secondary Packaging. 1.51 S performance i performance will be continually measured as set out in Exhibit D. 1.52 Section 7.2(i). 1.53 Section 7.2(i). 1.54 Process, , Processing means the compounding, filling, producing and/or Packaging of the API and Raw Materials into Product in accordance with the Specifications, Master Batch Record, Manufacturing Standards (if applicable) and the terms and conditions set forth in this Agreement. 1.55 Processing Date means the day on which Curia will commence Processing a given Batch of Product. 1.56 means the fully compounded bulk drug product in its final dosage form Processed under this Agreement as further described in Exhibit B, attached hereto, and includes both Commercial Product and Non-commercial Product. 1.57 - an improvement, modification, derivative, or new use of Indivior API, Product, or , but which is not an improvement of general applicability to the manufacturing process, and, in each case, all intellectual property rights in any of the foregoing. 1.58 oped by or on behalf of either party or any of its Affiliates in the course of the activities contemplated by this Agreement. 1.59 Section 2.1. 1.60 the written order placed by Indivior for quantities of Product required to be Processed and released by Curia under this Agreement as well as for any services to be provided by Curia. 1.61 the parties with respect to quality assurance/quality control activities, in a form mutually agreeable to both parties. 1.62 to manufacture and ship the Product in accordance with the Specifications, but not including the API.

7 1.63 meaning set forth in Section 7.6. 1.64 Regulatory Authority means any governmental regulatory authority within a Territory involved in regulating any aspect of the development, manufacture, market approval, sale, distribution, packaging or use of the Product. 1.65 Section 4.2. 1.66 shall have the meaning set forth in Section 18.15. 1.67 1.68 Secondary Packaging specifically included under the definition for Packaging, which activities are not and will not be in direct contact with the dosage form (for the avoidance of doubt, Secondary Packaging shall include the labelling of syringes containing the Product). 1.69 shall mean, (a) with respect to Product, the written specifications and quality standards, including tests, analytical procedures and acceptance criteria established to confirm the characteristics and quality of Product, as set forth in the Master Batch Record applicable to the Product, and as amended from time to time in accordance with the terms of Article 8 of this Agreement; and (b) with respect to Indivior Materials, including API, the written specifications and quality standards, including tests, analytical procedures and acceptance criteria, to which such Indivior Materials must conform in order to be considered acceptable for use in Processing of Product. 1.70 all discoveries, inventions, know-how, developments, methods, techniques, trade secrets, innovations, updates, modifications, enhancements, improvements, copyrights, data, documentation, processes, procedures, specifications and other intellectual property of any kind, whether or not protectable under patent, trademark, copyright or similar laws. 1.71 means the transfer from Curia to Indivior or any third party designated by Indivior of the full and complete procedures and tangible and intangible information that is reasonably necessary to Process Product, inclusive of, without limitation, documents, Process instructions, Master Batch Records, analytical methods, stability samples, retention samples and materials (including Specifications for Raw Materials). 1.72 Term shall have the meaning set forth in Section 15.1. 1.73 , as of the Effective Date and as applicable, the United States, Canada, countries within the European Union, and Australia where such country or countries may be amended or added to from time to time by Indivior, as mutually agreed by the parties, where such consent shall not be unreasonably withheld by Curia.

8 1.74 Pricing shall have the meaning set forth in Section 7.1(i). 1.75 the validation protocol, that have been processed in accordance with the Manufacturing Standards and such Validation Batch will be intended to be commercializable unless specified otherwise in the relevant validation protocol or SOW (defined below) for such Validation Batch. 1.76 the manufacturing process for Product that is established after the completion of Processing, at scale, of the Validation Batches in accordance with the Manufacturing Standards. ARTICLE 2 DEVELOPMENT, VALIDATION, PROCESSING & RELATED SERVICES 2.1 Development and Stability/Validation Services. Curia shall perform any process development, qualification, validation and stability services described in the applicable SOW for the prices specified therein. With respect to the development and validation services described in the SOW, the parties shall agree on a project Gantt chart at the commencement of services (the dates for receipt of information and materials, review and approval of documentation, scheduled engineering and manufacturing run dates and dates for other tasks for which Indivior is responsible consistent with the terms of this Agreement and the SOW. Upon mutual written approval of the Project Plan, any delay or departure from the specified dates caused solely by Indivior for Validation Batches shall result in the application of rescheduling or cancellation fees under Section 7.3. 2.2 Failures Prior to Validation and Additional Validation Batches. Notwithstanding anything in this Agreement to the contrary, Indivior shall pay for all Validation Batches that fail to meet the Manufacturing Standards in accordance with this Agreement and assume responsibility for all costs, including but not limited to the cost of API or other Indivior Materials, associated with such Validation Batch failures until such time as there exists a Validated Manufacturing Process for the Product, provided, however, that Curia shall be responsible for Validation Batch failures due to gross negligence or willful misconduct. In the event of a Validation Batch failure due to gross negligence or willful misconduct, Indivior shall have, in its sole discretion, the right to either (i) have Curia re-make the relevant Validation Batch at no further cost to Indivior (provided Indivior pays for the conforming replacement services solely in the event Indivior has not already paid for the non-conforming services) or (ii) to have Curia credit to Indivior the amount paid by Indivior for such failed Validation Batch. Indivior shall be responsible for supplying, at Indivior Indivior Materials, as necessary for Curia to complete such replacement. The foregoing remedy shall be Indivior for any failure of a Validation Batch to conform to the Manufacturing Standards. Indivior acknowledges that a change in Specifications, manufacturing Process or Master Batch Records may require a new Validated Manufacturing Process using the new Specifications, manufacturing Process or Master Batch Records, provided, however, the parties agree that Validation Batches Processed without changes to the Specifications or Process which are required solely for Indivior to make use of a previously validated Process for a Product in a new Territory shall not constitute

9 a need for a new Validated Manufacturing Process and in such circumstances, each such Batch Processed by Curia shall remain subject to the terms of Article 6. In the event that Purchase Orders do not provide a continuity of processing so as to keep Curia nel trained on Product, the processing of additional Validation Batches before the next commercial manufacturing run may be necessary at Curia Indivior . 2.3 Supply and Purchase of Product. During the Term, Curia shall Process the Product in accordance with the terms and conditions of this Agreement. Indivior shall purchase the Product from Curia in accordance with Article 4 and other terms and conditions of this Agreement. 2.4 Annual Product Maintenance Fee. Indivior shall pay to Curia an Annual Product Maintenance Fee (“APMF”) for each Product manufactured per Facility. For clarity, the APMF shall apply per Product family per Facility. The APMF will cover an array of Product support activities, which are irrespective of manufactured Product volumes, and include the following: dedicated primary point of contact for all commercial manufacturing activities; scheduling, planning, and communicating all commercial manufacturing activities; Drug Master File (DMF) updates with the FDA and EU; annual audit conducted by Indivior; annual Product Review in accordance with 21 CFR § 211.180; host all person-in-plant activities; Product license or permits from local, state and all federal authorities; access to document library (additional copies of Batch paperwork or other Batch documentation when requested); storage of Indivior dedicated equipment; access to Curia common change parts for filling equipment; Product documentation and sample storage (retains) relating to cGMP requirements; re-qualification of Raw Material vendors; maintenance and storage of Raw Material vendor audit reports; and storage of project dedicated components and excipients in Curia W.I.P. cages. The APMF will be payable within sixty (60) days after the Effective Date, and then within sixty (60) days after the start of every subsequent Contract Year for the remainder of the Term. The APMF for the first Product manufactured at a Facility is fixed at [***] per twelve (12) months, for the Term of the Agreement. Each additional Product at the same Facility will incur an APMF of [***] per twelve (12) months, for the Term of the Agreement. As of the Effective Date, the APMF under this Agreement shall be [***], subject to adjustment in the event additional Product families are added or removed at any time during the Term. In the event that this Agreement is terminated prior to the expiration of the Term, Curia would provide a credit to Indivior for the pro-rated portion of the APMF for such partial Contract Year, unless Curia terminates based on Indivior's material breach of this Agreement, in which case, no such pro-ration shall occur. 2.5 Technology Transfer. Indivior may, during the course of this Agreement, name an alternate manufacturer for supply of the Product. Curia will cooperate in any Technology Transfer,

10 and will cooperate in all reasonable requests made by Indivior to coordinate supply of Product, Indivior Materials, applicable Technology (including but not limited to Indivior Technology), and all other information or materials necessary to complete the Technology Transfer between such alternate manufacturer and Curia. All costs associated with a Technology Transfer shall be borne by Indivior in accordance with then-current market rates and reasonable man-hours as agreed by both parties in writing; provided, however, Curia shall use Commercially Reasonable Efforts to help complete the Technology Transfer. Each party shall continue to fulfill its obligations under the terms of this Agreement during any Technology Transfer. 2.6 Other Related Services. Curia shall provide services other than the Processing of Product upon terms and conditions agreed to by the parties in writing from time to time, such other services to be set forth in a Scope of Work (each, an SOW ) signed by both parties. A sample form of an SOW is attached hereto as Exhibit C. ARTICLE 3 MATERIALS 3.1 API and Indivior Materials. Indivior shall supply to Curia for Processing, at Indivior s sole cost, the API and other applicable Indivior Materials in quantities sufficient to meet Indivior s requirements for each Product as further set forth in Article 4. Prior to delivery of any of the API or other Indivior Material to Curia for Processing, Indivior shall provide to Curia a copy of the Material Safety Data Sheet (“MSDS”) for such material, and follow up with any subsequent revisions thereto. Indivior shall supply the API, other Indivior Materials, and Certificates of Analysis and Certificates of Compliance DDP the Facility (Incoterm 2020). Indivior shall use Commercially Reasonable Efforts to at all times maintain at least a sixty (60) day supply of Indivior Materials with Curia. Upon receipt of the API/other Indivior Materials, Curia shall conduct the testing set forth in Exhibit A, attached hereto. Curia shall not be responsible for any further testing or for confirming that the API and other Indivior Materials meet applicable Specifications, unless otherwise agreed by the parties in writing. Curia shall use the Indivior Materials solely and exclusively for Processing under this Agreement. Title to and risk of loss of API and other Indivior Materials shall at all times remain with Indivior, and Curia shall have no liability with respect to cost, or loss of, API or other Indivior Materials, except to the extent of any losses attributable to Curia’s gross negligible or willful misconduct. In the event of any API or Indivior Materials loss caused by Curia’s gross negligible or willful misconduct, Curia shall provide Indivior with a credit for such API or Indivior Materials in the amount of the lesser of the replacement value of the API or Indivior Materials; or (ii) [***] per event. The remedy set forth in the immediately foregoing sentence shall be Indivior’s sole remedy in the event of any loss or damage to API or other Indivior Materials. 3.2 Other Raw Materials. The parties may agree in writing that Curia shall be responsible for procuring, inspecting and releasing certain Raw Materials necessary to meet Purchase Orders (“Other Raw Materials”). Curia shall procure such Other Raw Materials in sufficient quantities to meet the Firm Period Forecast. If Indivior requires a specific supplier for any Other Raw Material, Indivior will be responsible for all costs associated with qualification of that supplier, if not previously qualified by Curia, and such Other Raw Material will be deemed Indivior Material. If

11 Indivior does not so request a specific supplier, then Curia shall be responsible for the costs of qualifying any supplier it so selects to the extent Curia has not already qualified such supplier. Unless a particular Other Raw Material can be replaced with the same raw material from another supplier, Curia shall not be liable for any delay in Processing of Product if (i) Curia is unable to obtain that Other Raw Material in a timely manner, and (ii) Curia placed orders for such Other Raw Material promptly following receipt of Indivior’s Firm Period Forecast/Purchase Order. Curia shall test and release the Other Raw Materials for use in Processing activities in accordance with its standard operating procedures. Provided Curia has complied with such standard operating procedures with regard to testing of any batch of Other Raw Materials, Curia shall not be responsible for the consequences of any defects in that batch of Other Raw Materials that are not reasonably discoverable as a result of such testing. 3.3 Equipment. Indivior shall provide, or has provided, to Curia, at Indivior’s expense, the equipment set forth in the applicable SOW if, any (“Indivior Equipment”). Indivior may alternatively request that such equipment be purchased by Curia on behalf of Indivior in which case, Indivior shall pay for such equipment [***]. Indivior shall be responsible for all freight, insurance and other costs of transporting Indivior Equipment to the Facility. Title to, and risk of loss of, all such Indivior Equipment shall be retained by Indivior at all times and Indivior shall obtain adequate insurance for such equipment if desired. The parties agree that they shall put into place appropriate documentation, such as a Purchase Order, specifically authorizing and identifying Indivior Equipment. Curia shall keep Indivior Equipment free and clear of any liens and/or encumbrances and provide reasonable documentation supporting the purchase (or allocation) of such equipment on behalf of Indivior. Curia shall be responsible for keeping all Indivior Equipment in good repair and in working order, scheduling and performing maintenance on Indivior Equipment as recommended by the applicable equipment manufacturer, and repairing Indivior Equipment, in each case, at Indivior’s cost and expense. Indivior agrees that any delay in providing Indivior Equipment or reimbursing Curia for acquiring Indivior Equipment may cause a delay in Processing activities and that Curia will not be responsible for such a delay. In the event Curia’s failure to properly maintain Indivior Equipment causes a delay in any scheduled Processing Date, Curia shall not charge Indivior any applicable rescheduling or cancellation fee for such Batch. Upon completion of the use of the Indivior Equipment, unless otherwise mutually agreed by the parties, the Indivior Equipment shall be shipped to Indivior, at Indivior’s cost and expenses. Notwithstanding the above, Curia shall be responsible for any repair or replacement costs for Indivior Equipment in the event such repair or replacement is necessary as a result of Curia’s gross negligence, willful misconduct, or failure to perform its responsibilities as described in this Section 3.3. 3.4 Packaging. Indivior shall provide or approve, prior to the procurement of applicable components, all Packaging information necessary to Process the Product. For purposes of this Agreement, Curia’s obligations relating to packaging shall be limited to Packaging. Such Packaging information is and shall remain the exclusive property of Indivior, and Indivior shall be solely responsible for the content thereof. Any changes or supplements to Packaging information should be submitted to Curia, in accordance with the applicable Curia SOP, in writing at least ninety (90) days prior to the desired implementation date (or as otherwise agreed by the parties in writing), together with the required documentation. Notwithstanding the forgoing, in the event of a required emergency change or supplement, changes may be requested by Indivior with less than

12 ninety (90) days’ prior written notice. Indivior shall reimburse Curia for any costs and/or expenses related to any such change, amendment or supplement and its implementation. Indivior shall also reimburse Curia for [***] for any prior versions of Packaging that become obsolete due to the implementation of changes. Curia shall not use any Packaging information provided by Indivior or any reproduction thereof following the termination of this Agreement, or during the Term of this Agreement in any manner other than solely for the purpose of performing its obligations hereunder. Curia shall make changes to the syringes, for the purpose of Packaging, as may be requested by Indivior from time to time. Curia will make no changes to the syringes for Packaging without the prior written approval of Indivior. Any changes or supplements pursuant hereto should be submitted to Curia in writing at least one hundred twenty (120) days prior to the desired implementation date, together with the required documentation, provided that any changes or supplements that require new equipment, change parts, requalification of the manufacturing line may require a longer lead time, and with such longer lead time being established through good faith discussions by the parties. Indivior shall reimburse Curia for any costs and/or expenses related to any such change, amendment or supplement and its implementation. Indivior shall also reimburse Curia for any prior syringes that become obsolete due to the implementation of changes to the extent such stock of syringes does not exceed Curia requirements for the Firm Period Forecast or any minimum supplier pack/delivery quantity required by the suppliers. Moreover, Indivior acknowledges that the manufacturing line may not be able to accommodate certain changes to the syringes. 3.5 Reimbursement for Other Raw Materials. In the event of (i) a Specification change for any reason, (ii) termination or expiration of this Agreement; or (iii) obsolescence of any Other Raw Material, Indivior shall bear the cost of any unused Other Raw Materials, provided that Curia purchased such Other Raw Materials in quantities consistent with the Firm Period Forecast/Purchase Order and any minimum purchase obligations required by the Other Raw Material supplier. In the event Indivior wishes to instead purchase such unused Other Raw Materials from Curia, Indivior shall pay to Curia the cost of such Other Raw Materials, plus 20%. Indivior shall also pay for all shipping costs associated with shipping such unused Other Raw Materials to Indivior and title to and risk of loss of such Other Raw Materials shall transfer to Indivior upon Curia’s submission of an invoice to Indivior for such Other Raw Materials. 3.6 Storage of Indivior Materials. During the Term of the Agreement, Curia will store all quantities of Indivior Materials required for the Firm Period Forecast, not to exceed the quantity of Indivior Materials required to produce [***] units of Product, at no cost to Indivior. For any request by Indivior for Curia to store Indivior Materials at a quantity greater than that required to produce [***] units of Product, Curia shall use Commercially Reasonably efforts to store at the Facility at a monthly storage charge at Curia’s current rates plus ten percent. To the extent Curia’s Facility does not have the capacity to store such Indivior Materials required to produce [***] units Curia shall store such excess Indivior Materials at a third party warehouse (qualified by Curia to store such Indivior Materials) at a monthly storage charge to Indivior for the duration of storage, billed at the third party’s then current standard monthly storage fees and minimums, plus 10%, pro- rated for any partial month. For the avoidance of doubt, in no event shall Curia charge Indivior any amounts in excess of the cost of such third party storage services plus 10% and Curia shall absorb any excess costs associated with storing Indivior Materials at a third party facility (e.g., transportation costs). In the event that a monthly storage

13 fee will be incurred as described above, Curia will provide reasonable advance notice to Indivior of the amount of such fee(s) and projected date of implementation of the same. 3.1 Physical Inventory Count. -to- during the Term at the Facility. To schedule the Inventory Count, Indivior shall give Curia reasonable Indivior, and its external auditor(s) , to conduct a full physical count of its Inventory (defined below) as close to the year-end as possible, preferably during the year-end shut down at Curia, to minimize inventory movements in the Facility. The number of visitors during the Inventory Count shall be limited to two (2) Indivior personnel, and one (1) Inventory Auditor, for two (2) days. The Inventory Count shall be conducted in a manner that is consistent with the method historically used by Indivior to conduct a full physical count of its inventory, as conducted du -end book closing process. Curia agrees to provide reasonable support to Indivior during the Inventory Count. Indivior agrees to provide Curia with reasonable compensation for their efforts in preparation and execution of the Inventory Count, such compensation to be set forth in an SOW or other written documentation signed by both parties. The cost of such Inventory Count per Facility shall be $15,000 per day for Calendar Year 2023 and thereafter $15,000 shall be the base cost of an Inventory Count, subject to a price increase based on PPI for each subsequent Calendar Year during the Term. 3.2 Inventory and Consumption Reports. (i) Inventory Reports. Month-end closing inventory reports shall be provided by Curia to Indivior no less than three (3) business days before each month-end; and no less than four (4) business days before each quarter-end. The Inventory Reports shall be inclusive of all Indivior owned inventory including but not limited to API and Indivior Materials and . (ii) Consumption Reports. Curia shall use Commercially Reasonable Efforts to provide Consumption Reports (defined below) to Indivior within forty-five (45) days of the Processing Date of the applicable Batch. Consumption Reports shall detail the Inventory consumption quantities allocated to the Purchase Orders still being Processed, or pending Quality Assurance approval, net of any items returned to stock ption . Curia shall include the following information in each Consumption Report to ensure records are maintained with integrity: Material Description Curia Lot Number Indivior Batch Number Quantity issued (net of returns) For those Products already Processed, released, and in the process of being shipped, or have already shipped, Curia will provide to Indivior the relevant Consumption Reports.

14 ARTICLE 4 FORECASTS & PURCHASE ORDERS 4.1 Capacity Guarantee. In connection with this Agreement and in exchange for Indivior funding, Curia arranged for the design and construction of a new specialized, prefilled syringe manufacturing line at the Facility, (the “New Manufacturing Line”). Title to, and risk of loss of, the New Manufacturing Line shall be retained by Curia. Furthermore, during the Term and in exchange for the Indivior funding, Curia agrees that the New Manufacturing Line will have a minimum capacity of 1.6 million units per Annual Forecast period available for Indivior (“Minimum Annual Capacity”); provided, however, Curia may fill any unused capacity above the Capacity Guarantee (as hereinafter defined) with product for other customers, subject to its limitations in Section 4.8. On October 1st of each year, beginning in the year 2023, Indivior shall furnish to Curia the Annual Forecast for the following Annual Forecast period and Curia agrees to commit to make such quantity of Product for the following Annual Forecast period, provided that such Annual Forecast does not exceed the Minimum Annual Capacity. Indivior agrees to purchase up to [***] of the Annual Forecast, (“Indivior Commitment”) and Curia agrees to make available to Indivior up [***] additional capacity above the Annual Forecast during the Annual Forecast period up to the Minimum Annual Capacity (“Capacity Guarantee”). In the event that the quantities of Product requested through Purchase Orders by Indivior in any given Annual Forecast period (“Indivior Actual Demand”) are less than the Indivior Commitment for such year, Curia shall invoice Indivior for and Indivior shall pay for the difference in the quantity of Product actually requested through Purchase Orders by Indivior and the Product in the Indivior Commitment on a take-or-pay basis at the then applicable pricing. Any Indivior Annual Forecast to Deliver greater than [***] will be discussed and mutually agreed upon by the parties. A table is provided below to show various examples of possible scenarios under this Section 4.1.
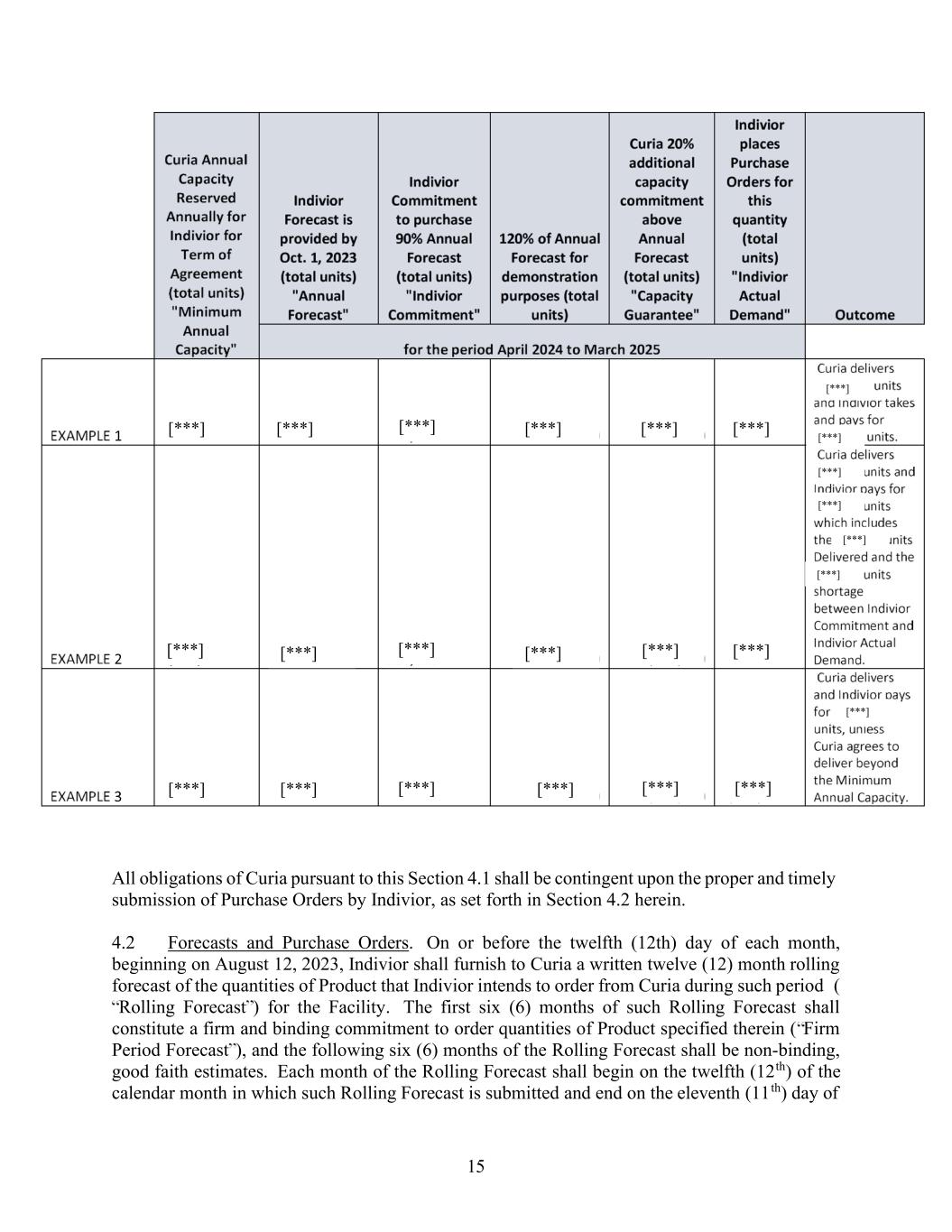
15 All obligations of Curia pursuant to this Section 4.1 shall be contingent upon the proper and timely submission of Purchase Orders by Indivior, as set forth in Section 4.2 herein. 4.2 Forecasts and Purchase Orders. On or before the twelfth (12th) day of each month, beginning on August 12, 2023, Indivior shall furnish to Curia a written twelve (12) month rolling forecast of the quantities of Product that Indivior intends to order from Curia during such period ( Rolling Forecast ) for the Facility. The first six (6) months of such Rolling Forecast shall constitute a firm and binding commitment to order quantities of Product specified therein ( Firm Period Forecast ), and the following six (6) months of the Rolling Forecast shall be non-binding, good faith estimates. Each month of the Rolling Forecast shall begin on the twelfth (12th) of the calendar month in which such Rolling Forecast is submitted and end on the eleventh (11th) day of [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***]

16 the following calendar month. Curia reserves the right to adjust the Rolling Forecast based on available resources, Facility capacity and other relevant factors. Indivior shall have the right to request an increase of the Firm Period Forecast to include additional units of Product. Curia may, in its sole discretion, supply such additional Curia agrees to supply such additional quantities, Indivior shall submit a Purchase Order for such inability to fulfill Purchase Orders for quantities in excess of the Firm Period Forecast be deemed a breach of this Agreement, nor relieve Indivior of its obligations under this Agreement. Indivior shall submit with each Rolling Forecast, a non-cancelable Purchase Order for the Firm Period Forecast (or such portion of the Firm Period Forecast not covered by previously submitted Purchase Orders). Indivior may alternatively submit Purchase Orders for certain portions of the Firm Period Forecast subsequent to the submission of the Rolling Forecast, provided the Purchase Orders provide the required lead time for Processing as set forth below. Curia shall notify Indivior of acceptance of the Rolling Forecast and any Purchase Order within seven (7) business days of receipt. Curia shall be deemed to have accepted Purchase Orders which it does not acknowledge within seven (7) business days of receipt. Curia shall have the right to reject Rolling Forecasts and Purchase Orders that are inconsistent with this Agreement. Each Purchase Order shall specify the quantity of Product being ordered, and the desired delivery date. Upon mutual agreement in writing for additional quantities of Product beyond the Firm Period Forecast, including projected delivery date(s), Indivior shall issue the applicable Purchase Order to be accepted by Curia as described above. Once placed, all Purchase Orders for Product shall be non-cancelable. No different or additional terms or conditions set forth in any Purchase Order shall modify in any way the terms and conditions of this Agreement, and in the event of a conflict between terms in any Purchase Order and this Agreement, the terms of this Agreement shall control. All Purchase Orders submitted in accordance with the terms of this Agreement shall be effective and binding on the parties upon acceptance by Curia. Except as otherwise provided herein, neither party shall have the right or power to refuse, reduce, or otherwise modify their obligations under any Purchase Order; however, Purchase Orders may be amended (i) upon written mutual agreement regarding such modification that is signed by both parties; or (ii) as otherwise provided in this Section 4.2 or Section 4.3. 4.3 Right to Modify. For the avoidance of doubt, the parties expressly acknowledge and agree that (a) the Firm Period Forecast constitutes a reservation by Indivior of the corresponding manufacturing slots and that Indivior shall have the right to schedule the Processing of any Product (whether Commercial Product or a Validation Batch) in each such manufacturing 5); and (b) Indivior shall be permitted to change the Product, if applicable, to be Processed in each such manufacturing slot, provided, (i) Curia otherwise possesses or can timely obtain the necessary Raw Materials and Indivior Materials necessary to Process the same; (ii) the alternative Product is on the same manufacturing line; and (iii) Indivior provides at least thirty (3 of the requested change.

17 4.4 Failure to Supply Purchase Order for the Firm Period Forecast. Any failure by Indivior to comply with its obligations under this Agreement to issue Purchase Orders for quantities of Product in any Firm Period Forecast shall not relieve Indivior of its obligations to pay for those quantities on a take-or-pay basis. Curia shall not be deemed to have failed to supply quantities or otherwise fulfill its obligations pursuant to this Agreement. In the event that Indivior does provide Purchase Orders in accordance the Firm Period Forecast obligation, but Curia is unable to Deliver the full quantity of Product in accordance with the Firm Period Forecast timely, Indivior shall not be responsible to pay for any deficit resulting fr the fault of Indivior, until such Product is delivered. 4.5 Curia or Purchase Orders Not Placed. In the event Indivior refuses or fails to make scheduled deliveries of the API or other Indivior Materials with sufficient time before the scheduled Processing Date (which failure shall be deemed to include the delivery of API or other Indivior Materials which do satisfy the applicable Specifications), Curia reserves the right to cancel all, or any part of, a Purchase Order upon as much advance notice as possible, in writing, to Indivior, and Curia shall have no further obligations or liability with respect to such Purchase Order. Curia shall invoice Indivior for such Purchase Order amount not supplied for reasons outlined herein and payments shall be made by Indivior within sixty (60) days of the receipt of the invoice. Notwithstanding the forgoing, the parties shall work together in good faith to substitute a Product in any affected manufacturing slots pursuant to Section 4.3 cancellation of any such affected Purchase Order, taking into consideration whether Curia has sufficient API, Indivior Materials, and Raw Materials in its possession to Process the requested Product. For the avoidance of doubt, Purchase Orders for Commercial Product are non-cancellable and, except as set forth in Section 4.3, any rescheduling or delays by Indivior will be deemed a cancellation by Indivior of the applicable Purchase Order, unless otherwise agreed by both parties in writing. In the event of any such cancellation or rescheduling of Purchase Orders by Indivior for Commercial Product, Indivior shall be charged 100% of the Unit or Batch Price based on the specific Purchase Order value unless such rescheduling or cancellation of a Purchase Order for . 4.6 Notice for Unplanned Delay. Curia shall provide Indivior with as much advance notice as possible (and will use its best efforts to provide at least fifteen (15) days advance notice where possible) if Curia determines that any Processing will be delayed, cancelled, or will deviate from the terms of an accepted Purchase Order for any reason. 4.7 Penalties for Delay. Beginning on January 1, 2024 and for the remainder of the Term, in the event Curia does not Deliver Product on or before the date set forth in the applicable Purchase Order, the penalties set forth in the table below shall apply to the Unit or Batch Price of the Product required documentation or information in accordance with this Agreement which is required for Curia to Process the Product on the scheduled Processing Date; (ii) the applicable Product fails to meet the target molecular weight as defined in the Specifications; (iii) there is a delay caused by third party testing of the applicable Product; or (iv) Indivior exercises its right to modify a Purchase Order in accordance with Section 4.3 above within four (4) months of the scheduled Processing Date of the applicable Batch of Product.

18 Number of Days Delivery is Late % of Price Deducted* [***] 4.8 Exclusivity. Nothing in this Agreement shall prevent Curia from processing product for parties other than Indivior, provided, however, Curia shall not process any Competing Product for another customer at the Facility during the Term, or shall prevent Indivior from engaging other contractors to provide services with respect to Product, subject in each case to the obligations of Confidential Information and Technology. ARTICLE 5 RELEASE; BATCH RECORD REVIEW; DELIVERY AND STORAGE 5.1 Release. Curia shall be responsible for release testing of the Product, and, subject to the terms of the Quality Agreement, shall release each Batch to Indivior on the basis of its manufacturing and controls documentation review. In connection with the release of the Product, Curia shall deliver Product documentation (such as executed Batch records, Certificate of Analysis, Certificate of Compliance or other quality document as determined by Curia) to its customer portal. Notwithstanding anything in this Agreement to the contrary, Indivior shall be responsible for final release of Product prior to distribution for its intended use. 5.2 Review of Batch Records. Curia shall provide written notice to Indivior that the Batch review. Period the parties agree to respond to any reasonable inquiry by the other party with respect to such Batch records in accordance with the timelines as set forth in the Quality Agreement. 5.3 Delivery. Title to and risk of loss of Product shall transfer from Curia to Indivior upon delivery of the Product EXW the Facility (Incoterms 202 will be through the issuance of the completed stamped Batch record, Certificate of Conformance and Certificate of Analysis to Indivior. Indivior is responsible for transportation of the Product to Indivior Indivior. For avoidance of doubt, Indivior is responsible for arranging pick up by the carrier and all shipping costs and risks. Should Indivior request Curia to assist with any arrangements with the carrier, such arrangements will be made by Curia on behalf of Indivior in accordance applicable instructions and at the sole risk and expense of Indivior. 5.4 Failure to Take Delivery and Storage. If Indivior does not pick up Product upon Delivery by Curia, Curia shall store such Product at the Facility or a third-party storage location for thirty (30) days at no cost and, thereafter, at a monthly storage charge to Indivior for the duration of storage, billed at Curia - [***][***] [***]

19 and minimums, pro-rated for any partial month. For all Product stored by Curia upon Delivery, Indivior agrees that: (i) Indivior has title and risk of ownership; (ii) Indivior has made a fixed commitment to purchase such Product; (iii) Indivior is responsible for any decrease in market value of such Product that relates to factors and circumstances outside of Curia Indivior is responsible for obtaining insurance for such Product during the storage period, if desired; and (v) Indivior is responsible for transportation of the Product to Indivior sole risk and expense of the Indivior. 5.5 . Curia shall perform all release activities for which it is responsible (i) within five (5) months of the Processing Date for all MOW Batches of Product, and (ii) within six (6) months of the Processing Date for all other Batches of Product that are not MOW Batches (each, as applicable, the agrees to use Commercially Reasonable Efforts to respond promptly to the other party during all release activities. In addition, Curia shall not be deemed to have failed to meet the Release documentation or information in accordance with this Agreement which is required for Curia to Process the Product on the scheduled Processing Date; (ii) there is a molecular weight issue associated with the applicable Batch of Product; or (iii) there is a delay caused by third party testing (e.g. . In the event Curia does not meet the Release Deadline for an applicable Batch of Product, Indivior shall have the right, in its sole discretion, to (a) have Curia Process a replacement Batch at no additional cost to Indivior (provided Indivior provides sufficient quantities of Indivior Materials to perform such replacement), or (b) have Curia credit to Indivior the amount paid for such Batch of Product. remedy for any Batch of Product for which Curia does not meet the Release Deadline. ARTICLE 6 REMEDIES FOR NON-CONFORMING PRODUCT 6.1 Notice of Rejection; Remedies. All Batches and/or Products are deemed accepted upon Delivery unless rejected by Indivior in accordance with this Article. Indivior may reject a Batch or Product solely if such Batch is determined to be Non-conforming Product. Indivior shall notify Curia in writing of its rejection of any Batch or Product by (a) the earlier to occur of (i) the end of the Batch Record Review Period or (ii) ten (10) business days from notification of the availability for testing of quality control samples, if applicable, and (b) with respect to Latent Defects, promptly upon discovery of such Latent Defect but no later than fourteen (14) days following such discovery; provided that Indivior must so inform Curia of any Latent Defects within three (3) months of Delivery of such Product (as applicable, by Indivior shall specify Indivior r rejection and be accompanied by any supporting analyses or documentation. Within (30) days of receiving a notice of rejection from Indivior, Curia shall respond stating whether (i) it accepts the rejection or (ii) it disputes the rejection, in which case the parties shall, after good faith negotiation as to whether the rejection is justified, refer such dispute to a mutually acceptable independent third party with the appropriate expertise to assess the conformity or non-conformity of rejected Product to the Manufacturing Standards at the time of Delivery. Such independent third party shall test the applicable Product and shall review the relevant Batch Records and other relevant documentation to determine whether such Product was

20 Processed in accordance with the Manufacturing Standards or not. The parties agree that such third party’s determination shall be final and binding upon the parties. The party against whom the independent third party rules shall bear the costs of testing and review by such independent third party. If such third party determines that Indivior’s rejection of Product was incorrect, Indivior shall purchase and pay for both the initially rejected Product and any replacement Product produced at Indivior’s request. In the event that Indivior rightfully rejects a Batch after a Validated Manufacturing Process has been established, Curia shall have, in its sole discretion, the right to either (a) replace, as soon as possible, the rejected Batch or portion thereof (provided that Indivior pays for the conforming replacement Product solely to the extent it has not already paid for the Non-conforming Product), or (b) credit Indivior a pro-rata portion of the amount paid by Indivior to Curia with respect to such Batch based on the percentage of such Batch that is unusable. In the event Curia replaces or credits Indivior for Non-conforming Product per the immediately foregoing sentence, Curia shall provide Indivior with a credit for API or Indivior Materials in the amount of the lesser of (i) the replacement value of the API or Indivior Materials; or (ii) [***] per Batch. The remedy set forth in the two immediately foregoing sentences shall be Indivior’s sole remedy for failure of Non-conforming Product to have been Processed in accordance with the Manufacturing Standards and the other terms of this Agreement. 6.2 Supply of Material for Replacement Product. In the event Curia replaces Non-conforming Product pursuant to Section 6.1 above, Indivior shall supply Curia with sufficient quantities of API and other Indivior Materials at its sole cost as necessary for Curia to complete such replacement. 6.3 Other Latent Defects. In the event of any discovery of any latent defect other than a Latent Defect, each party shall use Commercially Reasonable Efforts to determine the cause of such latent defect and, in the event Curia is determined to have contributed to any such latent defect, the parties will negotiate in good faith how to address the issue, taking into account the extent to which such latent defect is reasonably associated with Curia’s Processing failures under this Agreement. ARTICLE 7 PRICING AND PAYMENT 7.1 Pricing. (i) Batch and/or Unit Pricing. Indivior shall pay for Product upon Delivery in the manner set forth in Exhibit B, attached hereto and incorporated by reference. Indivior and Curia agree that Indivior shall pay for Product on a batch basis (“Batch Price” or “Batch Pricing”) a and/or on a per-unit basis (“Unit Price” or “Unit Pricing”) both which include all costs to complete Delivery of the Product, including but not limited to, planning, Processing, release testing, Other Raw Materials, and documentation. Indivior shall also pay Curia for any development, validation and regulatory support services as set forth in an SOW or change order. The parties acknowledge and agree that the pricing set forth in this Agreement benefits from the investments Indivior has made in the Facility and that this statement is applicable to this Agreement only and shall not set a precedent or impact any other agreement between the parties.

21 7.2 (ii) Other Services. In the event Indivior requests any other services in connection with the Processing of Product, Curia shall provide a written quote of the proposed fee, for such additional services and Indivior shall advise Curia whether it wishes to have such additional services performed by Curia. If the parties mutually agree that such additional services shall be performed by Curia, the parties shall enter into an SOW defining the services and fee in sufficient detail, and Curia shall perform, and Indivior shall pay Curia for, such additional services as set forth in the applicable, fully executed SOW. (iii) Third Party Costs. For any third party expense, excluding expenses related to the Delivery of the Product as set forth in 7.1(i) assuming EXW Delivery, incurred by Curia on behalf of Indivior, in accordance with this Agreement, an applicable SOW or change order, Indivior agrees to reimburse Curia for such expense and pay Curia an additional fee of not more than fifteen percent (15%) of such third party cost. Price Increase [***]

22 7.3 Rescheduling and Cancellation Fees for other Services and Validation Batches. (i) If Indivior cancels or reschedules any services that make use of Processing equipment but are not manufacture of Product services, then Indivior shall pay to Curia 100% of the fees of such services having a scheduled start date within thirty rescheduling, as applicable. (ii) If Indivior cancels or reschedules any Validation Batch for which the parties have signed a slot booking form or other form of manufacturing date confirmation, or if Curia needs to cancel or reschedule any Validation Batch due to delay by Indivior s obligation to provide documentation, approvals, API and other materials) in Processing such Validation Batch, then Indivior shall pay to Curia the fee set forth in the table below. For clarity, upon full execution by the parties of any slot booking form or other form of manufacturing date confirmation, the date(s) set forth in the applicable slot booking form or other form of manufacturing date confirmation are firm and binding on both parties. Curia may not reschedule or cancel the date set forth in a fully executed slot booking form or other form of manufacturing date confirmation without the prior written consent of Indivior, provided that in no event shall Indivior incur a rescheduling/cancellation fee for a Processing date modification agreed to, in For the avoidance of doubt, in the event the manufacturing slot for a Validation Batch is control, or pursuant to Section 4.3 above, Indivior shall not pay a rescheduling/cancellation fee for such rescheduled/cancelled Validation Batch. Number of days before scheduled Processing Date or when delay, rescheduling or cancellation occurs* Rescheduling/Cancellation Fee (expressed as a percentage of the Validation Batch fee) Greater than 60 days 30 to 60 days 14 to 29 days Less than 14 days *In the event any cancellation by Indivior occurs after the commencement of Processing Further, tasks/services rescheduled, delayed or put on hold more than once or rescheduled, [***] [***] [***] [***]

23 delayed or put on hold beyond ninety (90) days of the initial scheduled date shall be deemed to have been cancelled, unless otherwise mutually agreed upon in writing. 7.4 Invoicing Schedule. Indivior shall be invoiced for payments due under this Agreement as follows: (i) Processing and Supply of Commercial Product: Purchase Orders for Commercial Product will be invoiced upon Delivery and due within sixty (60) days of the date of receipt of the invoice (which date shall be deemed to be the same date the invoice is issued to Indivior, provided the invoice is issued electronically). For clarity, administrative changes to invoices do not reset the clock with respect to the sixty (60) day payment window. (ii) Other Services: Any additional services (other than Processing of Commercial Product) shall be invoiced as set forth in the applicable SOW. Payment schedules shall be as set forth in the applicable SOW. Unless otherwise set forth in the applicable SOW, any payments in advance of performance shall not exceed twenty percent (20%), with any remaining balance to be invoiced upon performance and/or delivery by Curia. 7.5 Taxes; Duty. All taxes, duties and other amounts assessed on the Raw Materials, API or otherwise in connection with delivery of Product and the other services prior to or upon sale to Indivior are the responsibility of Indivior, and Indivior shall reimburse Curia for any such taxes, duties or other expenses paid by Curia. 7.6 Product Approval. Notwithstanding the terms set forth above, Indivior shall use its best efforts to expedite and obtain all regulatory approvals necessary for Curia to commence production at a Facility, as applicable . 7.7 Payment Terms. Unless otherwise set forth in this Agreement, Indivior shall pay all undisputed invoices under this Agreement in full, or the portion of any invoice not in dispute, within sixty (60) days after the date of receipt of such invoice (which date shall be deemed to be the same date the invoice is issued to Indivior provided the invoice is issued electronically). All invoices and payments required to be paid hereunder shall be paid in U.S. dollars, and all such payments shall be made electronically in immediately available funds to an account designated by Curia, unless the parties agree to settle such payments through other means. Indivior shall notify Curia of any errors in of such invoice and if such notice of any errors is not provided to Curia within such fifteen (15) day period, the invoice shall be deemed accepted as-is by Indivior and shall be paid within sixty (60) days of receipt by Indivior. Curia shall resolve any errors in an invoice of which it receives notice within fifteen (15) days of receipt of notice from Indivior. In the event such error is resolved by Curia within such fifteen (15) day period, payment of such invoice shall remain due within sixty (60) days of the date on which the defective invoice was received by Indivior. In the event such error is not resolved by Curia within its fifteen (15) day cure period, the payment due date shall be automatically invoice. In the event payment is not received by Curia on or before the sixtieth (60th) day after the date of receipt of any undisputed invoice, then such unpaid amount shall accrue interest at the rate

24 of one percent (1%) per month compounded monthly until paid in full. In addition, if Indivior fails to pay undisputed invoices when due, in addition to its other rights under this Agreement, in law or under equity, Curia will have the right, upon written notice to Indivior, to cease all activities hereunder until all outstanding invoices have been paid in full; provided, however, the foregoing shall not apply to any portions of an invoice that are subject to a good faith dispute. For purposes invoice, Indivior has substantiated with sufficient documentation the reason for any disputed portion of an invoice in writing to Curia. ARTICLE 8 SPECIFICATIONS 8.1 Curia will maintain, as part of its quality documentation, all Specifications as listed within global drug product applications and, additionally, will maintain internal Specifications for Raw Materials as agreed in writing between both parties. The parties agree that internal Specifications for molecular weight of the Indivior polymer may define stricter criteria for certain identified parameters than comparable parameters within global drug product Specifications and/or may be used to group together certain parameters or criteria to meet multiple-country specification commitments. 8.2 Any changes to the Specifications as agreed to by the parties from time to time shall be in writing, dated and signed by the parties through the appropriate change control processes. No change in the Specifications, Facility, or Processes shall be implemented by Curia, whether requested by Indivior or requested or required by any Regulatory Authority, until the parties have agreed in writing to such change, the implementation date of such change, and any increase or decrease in fees associated with such change. Curia shall respond promptly to any request made by Indivior for a change in the Specifications, and both parties shall use commercially reasonable, good faith efforts to agree to the terms of such change in a timely manner. As soon as possible after a request is made for any change in Specifications or Processes, Curia shall notify Indivior of the fees associated with such change. Indivior shall pay all fees associated with such agreed upon changes. Curia shall notify Indivior as soon as reasonably practical after Curia becomes aware of any changes in Applicable Laws which are likely to affect the supply of the Product under this Agreement (including rationale, expected timings, risk assessment, price impact and any impact on business continuity). ARTICLE 9 QUALITY & REGULATORY MATTERS; RECORDS; BUSINESS CONTINUITY 9.1 Quality Agreement. Prior to Processing of the first Batch of Product, or at such other time as the parties may mutually agree upon, the parties shall execute a Quality Agreement. The Quality Agreement shall in no way determine liability or financial responsibility of the parties for the responsibilities set forth therein. In the event of a conflict between the terms of this Agreement and the Quality Agreement, this Agreement shall control except to the extent that such provision

25 relates to compliance with cGMP requirements and/or applicable regulatory laws and regulations, 9.2 Regulatory Compliance. Indivior shall be solely responsible for all permits and licenses required by any regulatory agency with respect to Product and the Processing under this Agreement, including any product licenses, applications and amendments in connection therewith. Curia will be responsible for maintaining all applicable licensures and permits required by any Regulatory Authority with respect to a Facility so required for Curia to meet its obligations under set forth in the first sentence of this Section 9.2. During the Term, Curia will assist Indivior with all regulatory matters relating to Processing under this Agreement, at Indivior s request and at Indivior s expense. Each party intends and commits to cooperate to satisfy all Applicable Laws relating to Processing under this Agreement. 9.3 Regulatory Correspondence. Indivior shall make available to Curia the Curia-specific portions of all regulatory applications and amendments thereto and all correspondence with a Regulatory Authority, in each case relating to the Product, including without limitation, an IND, NDA, ANDA, 505(b)(2) and DMF or their equivalent applications in foreign jurisdictions. For Curia- regulatory applications, amendments or correspondence that reference Curia documentation, facilities or capabilities. Indivior agrees to incorporate all changes provided by Curia which correct for factual inaccuracies and to reasonably consider all other comments. Indivior shall provide this information, and Curia shall review, in accordance with the following: (a) Original Applications/ Amendments Not Requested by Regulatory Authority: Indivior shall provide sixty (60) days prior notice of its intent to file. Upon receipt of the draft application or amendment, Curia shall provide any comments to Indivior within ten (10) business days. (b) Amendments/ Responses Requested by a Regulatory Authority: Indivior shall notify Curia of the request by the Regulatory Authority within twenty-four hours of receipt of request. Upon receipt of the draft amendment or response, Curia shall provide any comments to Indivior within four (4) business days. 9.4 GMP Audits. Indivior shall have the right to conduct one GMP audit at the Facility, at no cost to Indivior, during each twelve (12) month period during the Term (i) to observe, inspect, and audit the manner in which Curia conducts Processing of Product; and (ii) inspect Curia and records relating to Processing of Product, including Curia to its Processing of Product. which shall be conducted at no cost to Indivior, Indivior shall be charged for all additional audits, including any pre-approval inspections, at rates agreed upon by the parties based on timing of the audit, resource demand and any production disruption that may be caused by such an audit. The cost of any such additional GMP audit during the applicable twelve (12) month period shall be $10,000 per day for Calendar Year 2023 and thereafter such amount shall be the base cost and subject to a price increase based on PPI for each subsequent Calendar Year during the Term. To schedule any audit under this Article,

26 Indivior shall give Curia reasonable advance notice, but not less than four (4) performed in such a manner as not to unduly delay the performance of the services or interfere with Curia of visitors during any audit is limited to two (2) persons for two (2) days. No financial information shall be made available for audit except with respect to supporting documentation for specific out-of-pocket expenses charged to Indivior. All findings from any audit conducted under this Article shall be communicated to Curia, in writing, within thirty (30) business days of such audit. Curia shall notify Indivior of any audits by a Regulatory Authority which are Product- specific. Indivior shall cooperate with Curia with respect to any Product-specific audits and, at Curia site during any such audit to answer questions in connection with any such audit. 9.5 Facility Audit. Curia shall give Indivior or each of its authorized representatives, which are not competitors of Curia, access to the Facility for a site and/or Facility risk evaluation, any such audit to be at and expense, as set forth in an SOW or other written documentation signed by the parties. The aforementioned access shall be granted if requested by Indivior, once per Calendar Year. The cost of such Facility audit shall be $6,700 per day for Calendar Year 2023 and thereafter such amount shall be the base cost and subject to a price increase based on PPI for each subsequent Calendar Year during the Term. Curia shall use reasonable endeavors to ensure that its employees, its Affiliates and their employees cooperate with and provide reasonable assistance to Indivior during such audit. To schedule any audit under this Section 9.5, Indivior shall give Curia reasonable advance notice, but not less than two (2) prior written notice. All audits shall be carried out during normal business hours and performed in such a manner as not to unduly delay the performance of the services hereunder or interfere with Curia business. The number of visitors during any audit is limited to two (2) persons for two (2) days. Indivior shall ensure that any of its representatives who are sent to the Facility to perform an audit pursuant to this Section 9.5 shall comply with all confidentiality, security, safety, quality or similar guidelines that apply to persons present in the Facility and that are communicated by Curia, and Indivior shall be responsible for any breach of these guidelines by such representatives. 9.6 Maintenance of Records. Curia shall maintain complete and accurate records of all data obtained or generated by Curia relating to its performance of services under this Agreement. Curia shall retain such records in a secure area, reasonably protected from fire, theft, and destruction, for at least three (3) years following the completion of the relevant services, or as otherwise provided by Applicable Law, whichever time period is longer. 9.7 Recalls. In the event Curia believes a recall, field alert, Product withdrawal or field correction may be necessary with respect to any Product Processed and supplied under this Agreement, Curia shall immediately notify Indivior in writing. Curia will not act to initiate a recall, field alert, Product withdrawal or field correction without the express prior written approval of Indivior, unless otherwise required by Applicable Laws. In the event Indivior believes a recall, field alert, Product withdrawal or field correction may be necessary with respect to any Product Processed and supplied under this Agreement, Indivior shall immediately notify Curia in writing and Curia shall provide all reasonable cooperation and assistance to Indivior, at Indivior .

27 Indivior shall be financially responsible for the cost of, or expenses incurred in connection with, any recall, field alert, Product withdrawal or field correction, unless a recall, field alert, Product llful misconduct. In the negligence or willful misconduct, Curia shall bear the reasonable, actual and documented administrative costs incurred by Indivior for such recall, field alert, Product withdrawal or field correction (e.g., expenses related to communications with regulatory authorities, cost of notifying customers, reasonable professional fees). Further, in the event of any recall, each party shall use Commercially Reasonable Efforts to determine the cause of such recall and, in the event Curia is determined to have contributed to any necessary recall, the parties will negotiate in good faith how to address such recall, taking into account the extent to which such recall is reasonably associated failures under this Agreement. For the avoidance of doubt, in the event -conforming Product results in a recall, field alert, Product withdrawal or field correction, Indivior shall be entitled to both the remedy set forth in this Section 9.7 with respect to the recall and the remedy set forth in Section 6.1 with respect to the Non-conforming Product. 9.8 Business Continuity Plan. the Facility at which Product is Processed under this Agreement. The parties agree that in Calendar Year 2023, they will align on the expectations and timeline for implementation of the BCP. Curia shall share broadly what it may cover in any current, applicable BCP so the parties may work together to address any gaps in an applicable BCP. ARTICLE 10 REPRESENTATIONS AND WARRANTIES; FLOWDOWN TERMS 10.1 Indivior. Indivior represents and warrants to Curia that: (i) Indivior Materials will comply with all applicable Specifications and will have been produced in compliance with the Applicable Laws; (ii) it has all necessary authority and all right, title and interest in and to any Technology related to each Product as well as any Technology that is provided by Indivior to Curia under this Agreement, and Curia , in accordance with this Agreement, of any Technology provided by Indivior will not, to the best of infringe any third party intellectual property rights; (iii) it has provided all safe handling instructions, health and environmental information and MSDS(s) applicable to the Product or to any Indivior Materials, in sufficient time for review and training by Curia prior to delivery; (iv) all Product delivered to Indivior by Curia will be held, used and/or disposed of by Indivior in accordance with all Applicable Laws; (v) it will comply with all Applicable Laws relating to Indivior this Agreement, and in particular, Indivior has obtained, and shall maintain, all

28 necessary approvals from Regulatory Authorities for its use of any materials or Products provided by Curia under this Agreement; (vi) it will not release Product for further distribution or use if the Batch record for a particular Batch of Product indicates that the Product does not comply with the Specifications or Applicable Laws; and (vii) prior to distributing any Batch of Commercial Product, Indivior will review all Product-specific validation records and confirm that the Product has been validated in compliance with Applicable Laws. 10.2 Curia. Curia represents and warrants to Indivior that: (i) at the time of Delivery, Product will have been Processed in accordance with the Manufacturing Standards, provided, however, this Section 10.2(i) shall in no event apply to Product that is Processed by Curia in accordance with Section 2.2 of this Agreement; (ii) it shall perform the services hereunder in a professional manner and in accordance with all Applicable Laws at a Facility that complies with all Applicable Laws; (iii) Any services supplied by Curia under this Agreement will be supplied by appropriately qualified and trained personnel, whom, to the best of Curia knowledge: (1) are not currently listed on the U.S. Department of Health Office of Inspector (2) have not been or are currently the subject of a proceeding that could lead to their becoming a debarred individual or debarred entity under Section 306 of the Food Drug and Cosmetic Act (21 U.S.C § 335a). Curia further agrees that if, at any time after execution of this Agreement, it becomes aware of itself or any employees providing services under this Agreement that (a) become excluded from participation in any federal healthcare program (as that term is defined by 42 U.S.C § 1320a-7b(f)); (b) are convicted of any crime that could result in exclusion from federal health care programs under 42 U.S.C. §§ 1320a-7, 1320-7a, or (c) become excluded, suspended, debarred or otherwise ineligible to participate in federal procurement or non-procurement programs, or (d) becomes listed on the General Services Administration System or Award Management, then Curia shall promptly provide notice of such to Indivior in accordance with the following sentence. In the event that Curia or any of its officers, directors, or employees performing services under this Agreement has become or, to the best of its knowledge, is in the process of being charged, convicted, debarred, excluded, proposed to be excluded, suspended or otherwise rendered ineligible, or is on an enforcement list, Curia will immediately, but in any event within two (2) business days of becoming aware of such event, notify Indivior in writing via email to ciacompliance@indivior.com and a hard-copy sent to Indivior Inc., Attn: Chief Compliance Officer, 10710 Midlothian Turnpike, Suite

29 125, North Chesterfield, VA 23235. Curia understands that debarment or exclusion of itself or an employee performing services under this Agreement may result in termination by Indivior in accordance with Section 15.2(i) below; and (iv) Curia will not use the intellectual property of any third party in the Processing of the Product under this Agreement, without the prior written consent of Indivior. 10.3 Mutual Representations. Each party represents and warrants that: (i) It is duly organized and validly existing under the laws of the jurisdiction of its incorporation and has full corporate power and authority to enter into this Agreement and to carry out the provisions hereof. (ii) This Agreement is a legal and valid obligation of it, binding upon it and enforceable against it in accordance with the terms of this Agreement. (iii) The execution, delivery and performance of this Agreement by it does not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which such party may be bound, and does not violate any law or regulation of any court, governmental body or other administrative authority having authority over it. (iv) Compliance with Anti-Corruption Laws. (a) Indivior and Curia are committed to conduct business with the highest degree of ethics and integrity and will comply with all applicable local and international laws and regulations relating to anti-corruption including the U.S. Foreign Corrupt Practices Act (FCPA) and the U.K. Bribery Act 2010, as well as any applicable laws implementing the UN Convention Against Corruption and the OECD Anti-Bribery Convention. (b) Each party undertakes that in connection with its obligations under this Agreement, it, its directors, employees and officers have not and shall not directly or indirectly (a) offer, provide, authorize for or promise to another person, or (b) request, accept or agree to accept from another person, any such Benefit is for the purpose of influencing the receiving person improperly in his/her official capacity for the purpose of obtaining a business advantage, or where such Benefit would constitute a violation of any Applicable Law. (c) In order to demonstrate compliance with Section 10.3(iv)(b) of this Agreement, each party shall keep books and records complete and accurate in order to reflect in reasonable detail the character and value of transactions and expenditures made under this Agreement. Each party shall give prompt written notice to the other party if it has failed to comply with or has breached Section 10.4(iv)(b) of this Agreement.

30 (d) If in the reasonable opinion of a party, the other party has failed to comply with Section 10.4(iv)(b) of this Agreement in any material respect, then the non-breaching party may be entitled to terminate this Agreement in accordance with Section 15.2(i) below. 10.4 Limitations; Disclaimer. THE REPRESENTATIONS AND WARRANTIES SET FORTH IN THIS ARTICLE 10 ARE THE SOLE AND EXCLUSIVE REPRESENTATIONS AND WARRANTIES MADE BY EACH PARTY TO THE OTHER AND NEITHER PARTY MAKES ANY OTHER REPRESENTATIONS, WARRANTIES OR GUARANTEES OF ANY KIND WHATSOEVER, EXPRESS OR IMPLIED, INCLUDING WITHOUT LIMITATION ANY IMPLIED WARRANTIES OF MERCHANTABILITY, NON-INFRINGEMENT OR FITNESS FOR A PARTICULAR PURPOSE. 10.5 Flowdown Terms. The parties acknowledge and agree that this Agreement will be Federal Supply Schedule (Contract#: 36F79720D0156) with the US Government and that this Agreement is subject to FAR 52.244-6 (Subcontracts for Commercial Items). Curia understands and agrees that its performance of services under this Agreement will be subject to certain applicable government requirements Exhibit E, attached hereto. The parties further agree and acknowledge that (i) Curia is at all times responsible for determining which government requirements may apply as Flowdown Terms and ensuring its own compliance with the same; and (ii) the Flowdown Terms may be updated from time to time upon written notice from Indivior to Curia, which such written notice shall not require an amendment to this Agreement but shall be set forth in a separate writing. ARTICLE 11 CONFIDENTIAL INFORMATION 11.1 Confidential Information includes all confidential or proprietary scientific, technical, trade, business and/or financial information furnished by Curia or Indivior, or any of their respective representatives or Affiliates , to the other or its representatives or Affiliates (collectively , whether furnished before, on or after the Effective Date and furnished in any form, including but not limited to written, verbal, visual, electronic or in any other media or manner, and whether or not identified as confidential. Confidential Information of Indivior includes, but is not limited to Indivior Existing Technology and Product-specific Program Technology. Confidential Information of Curia includes, but is not limited to Curia Background Technology, Curia Program Technology, the pricing information contained in any SOW, and the portions of the Master Batch Record and information provided in connection with Indivior regulatory filings that are not Product-specific. Notwithstanding the other provisions of this Agreement, a p ion which the receiving party can establish by competent proof (A) is or becomes generally available to the public other than as a result of a breach of this Agreement, or (B) is already known by the receiving party at available to the receiving party on a non-confidential basis from a source that is entitled to disclose it on a non-confidential basis, or (D) was or is independently developed by or for the receiving

31 party without reference to the disclosing party’s Confidential Information, as evidenced by the receiving party’s written records.. 11.2 Confidentiality Obligation. The receiving party agrees to hold the Confidential Information of the disclosing party in trust and confidence and not to disclose such Confidential Information except (i) to those of its employees (including employees of its Affiliates) who have a need to know such information for purposes of performing such party’s obligations under this Agreement and who are under an obligation of confidentiality no less restrictive than the obligations set forth in this Agreement that would apply to such information, (ii) by Curia to third parties who are involved in performing services under this Agreement and who are bound by written confidentiality restrictions no less stringent than those contained in this Agreement, (iii) by either party to Regulatory Authorities in connection with regulatory filings related to the Facilities or to Product Processed under this Agreement or (iv) as otherwise approved by the disclosing party in writing. Notwithstanding the foregoing limitations on disclosure, the receiving party may disclose such Confidential Information of the disclosing party as the receiving party reasonably determines is required by any law, rule, regulation, order, decision, decree, subpoena or other legal process to be disclosed, including but not limited to the U.S. Securities and Exchange Commission (“SEC or other public disclosure requirements. If such disclosure is required by legal process, rule, regulation (including but not limited to SEC or other public disclosure requirements), order, decision, or decree, the receiving party shall, if legally permitted, notify the disclosing party promptly with a copy of such proposed redactions sent to [***] if sending to Curia and shall provide such opportunity as is reasonable in the circumstances for the disclosing party to object to, or limit, such disclosure and will cooperate with the disclosing party in seeking to prevent or limit such disclosure (i.e., no less than 72 hours unless the SEC rules require a shorter timeline), except that no such act by the disclosing party to object to or limit, or assistance of the receiving party requested by the disclosing party, shall interfere with, delay or hinder the receiving party’s obligations to the SEC or other public disclosure requirements.. 11.3 Restrictions on Use; No Implied License. The receiving party agrees that it shall not use the disclosing party’s Confidential Information except for purposes of fulfilling its obligations under this Agreement or as otherwise expressly contemplated by this Agreement. The receiving party will obtain no right of any kind or license under any patent application or patent by reason of this Agreement, except as explicitly granted under this Agreement. All Confidential Information will remain the sole property of the party disclosing such information or data. 11.4 Protective Measures. In protecting the secrecy of and avoiding disclosure and unauthorized use of disclosing party’s Confidential Information, the receiving party shall take at least those measures that it uses to protect its own confidential information; however, in no event, shall less than a reasonable standard of care be used. The receiving party shall immediately notify the disclosing party in the event of any unauthorized use or disclosure of the disclosing p Confidential Information of which the receiving party is or becomes aware, provided that in no event shall such notification be deemed an admission for evidentiary purposes. 11.5 Return of Confidential Information. Upon the written request of the disclosing party, except as set forth in Article 9, the receiving party shall promptly return to disclosing party or destroy all of the tangible Confidential Information of the disclosing party in its possession or

32 control, except that one (1) copy may be retained by receiving party solely for the purpose of determining the scope of obligations incurred under this Agreement and except that neither party shall have any obligation to return or destroy computer files that are created during automatic system back-up and that cannot be reasonably returned or destroyed. Any Confidential Information of the disclosing party retained by the receiving party under this section shall continue to be subject to the confidentiality and non-use obligations according to the terms and conditions set forth herein. 11.6 Terms of Agreement. The parties hereby acknowledge and agree that the terms of this Agreement and any SOW executed hereunder, but not the existence thereof, shall be considered Confidential Information of both parties. 11.7 Term of Obligation. The parties obligations under this Article 11 shall continue in effect during the Term and for a period of seven (7) years following termination or expiration of the applicable SOW. Notwithstanding the foregoing, the confidentiality and non-disclosure of Trade Secrets shall survive the termination or expiration of this Agreement until such time as the Trade Secret is no longer a Trade Secret under Applicable Law. shall be defined as defined by Applicable Law. ARTICLE 12 INTELLECTUAL PROPERTY 12.1 Indivior Pre-Existing Technology. Curia agrees that Indivior has and shall retain sole and exclusive rights of ownership in and to any Indivior Confidential Information and other Indivior Existing Technology. Curia does not acquire any license or other right to Indivior Confidential Information or Indivior Existing Technology except for the limited purpose of carrying out its obligations under this Agreement. 12.2 Curia Pre-Existing Technology. Indivior agrees that Curia has and shall retain sole and exclusive rights of ownership in and to any Curia Confidential Information and other Curia Background Technology whether or not incorporated into the services under this Agreement. Indivior does not acquire any license or other right to Curia Confidential Information or Curia Background Technology except for the limited purpose of using Product Processed under this Agreement as expressly set forth in this Agreement. 12.3 Program Technology. All Product-specific Program Technology shall be the exclusive property of Indivior, and Curia hereby assigns any rights it may have in Product-specific Program Technology to Indivior, and further agrees to take such actions as are reasonably requested by Indivior, at Indivior with Indivior -specific Program Technology. All Curia Program Technology shall be the exclusive property of Curia, and Indivior agrees to assign its rights in Curia Program Technology to Curia, and to take such actions as are reasonably requested by Curia, at Curia expense, to effect the foregoing assignment and in connection with Curia efforts to secure patent protection for such Curia Program Technology.

33 12.4 No Other Licenses. Except as expressly set forth in this Agreement, nothing in this Agreement shall be deemed to grant to either party any right or license under any patents, patent applications, know-how, technology, inventions or other Technology of the other party. ARTICLE 13 INDEMNIFICATION 13.1 Indemnification by Curia. Curia shall indemnify, defend and hold harmless Indivior, its Affiliates, and their respective directors, officers, employees, agents, successors and assigns Indivior Indemnitees all suits, claims, losses, demands, liabilities, resulting from or arising out of, directly or indirectly, Losses incurred in connection with, or arising out, of (A) any material breach by Curia of any of its representations, warranties, or covenants set forth in this Agreement, (B) any gross negligence or willful misconduct of any Curia Indemnitee (defined below), or (C) any violation of Applicable Laws, including cGMP (if applicable), by Curia or its Affiliates in performing obligations under this Agreement; except, in each case, to the extent that any of the foregoing Losses arises out of claims for which Indivior is obligated to indemnify Curia pursuant to Section 13.2. 13.2 Indemnification by Indivior. Indivior shall indemnify, defend and hold harmless Curia, its Affiliates, and their Curia Indemnitees from and against all Losses incurred in connection with, or arising out of (A) any material breach by Indivior of any of its representations, warranties or covenants set forth in this Agreement; (B) the marketing, sale, distribution or use of Product, including, but not limited to, use in clinical trials (if applicable), or any side effects, contraindications, illness, and/or death resulting from use of Product (whether based on strict liability, inherent design defect, negligence, failure to warn, breach of contracts or any other theory of liability); (C) the gross negligence or willful misconduct of any Indivior Indemnitees, as defined in Section 13.1, or (D) a claim by a third party that Curia use of API or other Indivior Materials or of Technology provided to Curia by Indivior infringes ; or (E) any violation of Applicable Laws by Indivior or its Affiliates in performing obligations under this Agreement; except, in each case, to the extent that any of the foregoing Losses arises out of claims for which Curia is obligated to indemnify Indivior pursuant to Section 13.1. 13.3 Indemnification Procedures. In the event that either party seeks indemnification under the terms of Sections promptly, but in any event within fifteen (15) days, or liability of which the Indemnified Party becomes aware (including a copy of any related complaint, summons, notice or other instrument ), provided that failure to provide such notice shall not eliminate the Article 13 except to the extent the Indemnifying Party has been prejudiced by such failure. The Indemnifying Party shall have the right to assume direction and control of the defense of any indemnified claim, provided that if the Indemnifying Party does not assume direction and control of the defense, the Indemnified Party shall do so, provided that such defense shall be, in both cases, solely a . The Indemnified Party shall cooperate as requested by, and at the expense of, the Indemnifying Party, in the defense of the claim. The Indemnifying Party shall not settle or otherwise compromise any

34 claim or suit in any manner which requires the Indemnified Party to provide any consideration, admit fault or take any other action that would be binding on such Indemnified Party without the prior written consent of the Indemnified Party. The Indemnifying Party shall not have any obligation to the Indemnified Party under this Article 13 for any claim settled by the Indemnified Party without the Indemnifying Party’s prior written consent. ARTICLE 14 INSURANCE 14.1 Curia. Curia shall, at its own cost and expense, obtain and maintain in full force and effect the following insurance during the term of this Agreement: (i) Commercial General Liability insurance with per-occurrence and general aggregate limits of not less than [***]; (ii) Products and Completed Operations Liability Insurance with per-occurrence and general aggregate limits of not less than [***]; (iii) Workers’ Compensation and Employer’s Liability Insurance with statutory limits of not less than [***]; (iv) Professional Services Errors & Omissions Liability Insurance with per claim and aggregate limits of not less than [***] covering sums that Curia becomes legally obligated to pay as damages resulting from claims made by Indivior for errors or omissions committed in the conduct of the services outlined in the Agreement. In lieu of insurance, Curia may self-insure any or a portion of the above required insurance. In the event that any of the required policies of insurance are written on a claims-made basis, then such policies shall be maintained during the entire term of this Agreement and for a period of not less than three (3) years following the termination or expiration of this Agreement. Each insurance policy that is required under this Article shall be obtained from an insurance carrier with an A.M. Best rating of at least A- VII. 14.2 Indivior Insurance. Indivior shall, at its own cost and expense, obtain and maintain in full force and effect the following insurance during the term of this Agreement: (i) Products and Completed Operations Liability Insurance with per-occurrence and general aggregate limits of not less than [***]; (ii) Workers’ Compensation and Employer’s Liability Insurance with statutory limits for Workers’ Compensation and Employer’s Liability Insurance limits of not less than [***]; (iii) All Risk Property Insurance, including transit coverage, in an amount equal to full replacement value covering Indivior’s property, including Indivior Materials, while it is at a Facility or in transit to or from a Facility. In the event that any of the required policies of insurance are written on claims-made basis, then such policies shall be maintained during the entire term of this Agreement and for a period of not less than three (3) years following the termination or expiration of this Agreement. Indivior shall not seek reimbursement for any property claim, or portion thereof that is not fully recovered from Indivior’s Property Insurance policy. Each insurance policy that is required under this Article shall be obtained from an insurance carrier with an A.M. Best rating of at least A- VII. ARTICLE 15 TERM AND TERMINATION 15.1 Term. This Agreement shall commence on the Effective Date and shall continue for five (5) years and eight (8) months unless earlier terminated under Section 15.2 below (the “Term”). The term of this Agreement may be extended upon mutual agreement of the parties.

35 15.2 Termination by Either Party. (i) Material Breach. Either party may terminate this Agreement effective upon sixty (60) days prior written notice to the other party, if the other party commits a material breach of this Agreement and fails to cure such breach by the end of such sixty (60) day period. (ii) Bankruptcy. Either party may terminate this Agreement effective upon written notice to the other party, if the other party becomes insolvent or admits in writing its inability to pay its debts as they become due, files a petition for bankruptcy, makes an assignment for the benefit of its creditors or has a receiver, trustee or other court officer appointed for its properties or assets; and such filings or assignments are not voided within sixty (60) days. 15.3 Effect of Termination. In the event of any termination or expiration of this Agreement, Indivior shall be responsible for payment of (i) any outstanding invoices, (ii) any in-process Product not yet delivered or Product ordered pursuant to a Purchase Order, (iii) any Product specified in the most recent Firm Period Forecast for which a Purchase Order has not yet been submitted, (iv) any applicable cancellation fees pursuant to Section 7.3, (v) other actual costs and expenses incurred by Curia prior to the date of notice of termination, and (vi) costs relating to decommissioning activities, including Curia documented costs, including the costs of packaging equipment and inventory. Without limiting the foregoing, upon termination of this Agreement, Curia shall ship to Indivior all Indivior Materials, Indivior Equipment and Product in Curia possession and Indivior shall pay all costs associated therewith. Payment shall be due within sixty (60) days of the date of the final invoice. In the event any credit towards future payments is or will by owed by Curia to Indivior as of the date of termination or expiration of this Agreement, Curia shall pay Indivior directly the full balance of any such unused credit(s). Such payment shall be made no less than sixty (60) days after the date of termination or expiration of this Agreement. 15.4 Termination by Indivior. This Agreement may be terminated by Indivior upon twelve (12) written notice to Curia in the event a court of competent jurisdiction or a Regulatory Authority (1) withholds marketing approval of a Product; and/or (2) infringement litigation makes it impracticable for Indivior to continue production of the Product. ARTICLE 16 LIMITATIONS OF LIABILITY 16.1 EXCEPT AS SET FORTH IN SECTION 3.1 AND 6.1, OR AS MAY OTHERWISE BE AGREED BY THE PARTIES IN A SEPARATE WRITING SIGNED BY THE PARTIES PURSUANT TO SECTIONS 6.3 OR 9.7, CURIA SHALL HAVE NO LIABILITY FOR THE COST OF, OR LOSS OR DAMAGE TO, API AND OTHER INDIVIOR MATERIALS, AT ANY TIME, WHETHER OR NOT SUCH API OR OTHER INDIVIOR MATERIALS ARE INCORPORATED INTO PRODUCT. 16.2 MISCONDUCT, CURIA AGGREGATE LIABILITY UNDER THIS AGREEMENT SHALL IN NO EVENT EXCEED THE TOTAL FEES PAID BY INDIVIOR TO CURIA FOR THE SERVICES AT ISSUE/BATCH OF PRODUCT GIVING RISE TO SUCH LIABILITIES,

36 CLAIMS OR OBLIGATIONS, MINUS ANY THIRD-PARTY PASS THROUGH EXPENSES. THE FOREGOING CAP SHALL NOT INCLUDE ANY AMOUNTS PAID BY CURIA TO INDIVIOR IN ACCORDANCE WITH SECTION 16.1. 16.3 (I) GROSS NELIGENCE OR WILLFUL MISCONDUCT, (II) BREACH OF ITS CONFIDENTIALITY OBLIGATIONS PURSUANT TO ARTICLE 11, AND/OR (III) INDEMNITY OBLIGATIONS PURSUANT TO ARTICLE 13, NEITHER PARTY SHALL BE LIABLE TO THE OTHER PARTY FOR INDIRECT, INCIDENTAL, SPECIAL OR CONSEQUENTIAL DAMAGES OF THE OTHER PARTY OR ITS RELATED INDEMNIFIED PARTIES ARISING OUT OF PERFORMANCE UNDER THIS AGREEMENT, INCLUDING WITHOUT LIMITATION, LOSS OF REVENUES, PROFITS OR DATA, OR PENALTIES ARISING UNDER THIRD PARTY CONTRACTS, WHETHER IN CONTRACT OR TORT, EVEN IF SUCH PARTY HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. THE FOREGOING WAIVER SHALL IN NO EVENT ARTICLE 17 NOTICE All notices and other communications hereunder shall be in writing and shall be deemed given: (A) when delivered personally; (B) when delivered by facsimile transmission (receipt verified); (C) when received or refused, if mailed by registered or certified mail (return receipt requested), postage prepaid; or (D) when delivered if sent by express courier service, to the parties at the following addresses (or at such other address for a party as shall be specified by like notice; provided, that notices of a change of address shall be effective only upon receipt thereof): To Indivior: Indivior UK Limited Chapleo Building, Henry Boot Way, Priory Park, Hull HU4 7BY, United Kingdom Attn: Assistant General Counsel, EMEA With a copy to: Indivior Inc. 10710 Midlothian Turnpike, Suite 125 North Chesterfield, VA 23235 Attn: Chief Legal Officer To Curia: Curia Global, Inc. 26 Corporate Circle Albany, New York 12203 Attn: Legal Department

37 ARTICLE 18 MISCELLANEOUS 18.1 Entire Agreement; Amendments. This Agreement, the attachments/Exhibits, and any amendments thereto constitute the entire understanding between the parties and supersede any contracts, agreements or understanding (oral or written) of the parties with respect to the subject matter hereof. The 2017 ABQ Agreement is hereby terminated, replaced and superseded by this Agreement, notwithstanding any provisions therein to the contrary, and the rights and obligations of the parties with respect to the services set forth in the 2017 ABQ Agreement shall be subject to the terms and conditions of this Agreement. Any outstanding Purchase Orders issued pursuant to the 2017 ABQ Agreement shall be governed by this Agreement as of the Effective Date without the need to amend the same. For the avoidance of doubt, the Master Development and Supply Agreement entered into between Curia Massachusetts, Inc., an Affiliate of Curia, and Indivior is not superseded by this Agreement and shall remain in full force and effect in accordance with its terms. No term of this Agreement may be amended except upon written agreement of both parties, unless otherwise provided in this Agreement. Except as expressly set forth herein, the terms and conditions set forth in this Agreement shall govern over any conflicting terms and conditions. Any additional terms and conditions proposed by or contained in any form provided by Indivior (including a purchase order, order acknowledgement/acceptance, or otherwise) are hereby rejected. The recitals at the start of this Agreement are hereby incorporated into and made a part of this Agreement. 18.2 Captions. The captions and Section headers in this Agreement are for convenience only and are not to be interpreted or construed as a substantive part of this Agreement. 18.3 Further Assurances. The parties agree to execute, acknowledge and deliver such further instruments and to take all such other incidental acts as may be reasonably necessary or appropriate to carry out the purpose and intent of this Agreement. 18.4 No Waiver. Failure by either party to insist upon strict compliance with any term of this Agreement in any one or more instances will not be deemed to be a waiver of its rights to insist upon such strict compliance with respect to any subsequent failure. 18.5 Severability. If any term of this Agreement is declared invalid or unenforceable by a court or other body of competent jurisdiction, the remaining terms of this Agreement will continue in full force and effect. 18.6 Independent Contractors. The relationship of the parties is that of independent contractors, and neither party will incur any debts or make any commitments for or on behalf of the other party except to the extent expressly provided in this Agreement. Nothing in this Agreement is intended to create or will be construed as creating between the parties the relationship of joint venturers, co- partners, employer/employee or principal and agent. 18.7 Successors and Assigns. This Agreement will be binding upon and inure to the benefit of the parties, their successors and permitted assigns, and any entity that acquires rights to the services and/or Products that are subject hereof. Neither party may assign this Agreement, in whole or in

38 part, without the prior written consent of the other party, except that either party may, without the other party's consent, assign this Agreement to an Affiliate or to a successor to all of the business or assets of the assigning party (which for the avoidance of doubt, shall include a sale of a division, site or specific business unit), provided that the assigning party provides written notice to the non- assigning party within thirty (30) days of such assignment not requiring consent. 18.8 Governing Law. This Agreement shall be governed by and construed under the laws of the State of Delaware, excluding its conflicts of law provisions. 18.9 Dispute Resolution. The parties shall first attempt in good faith to resolve any claim or controversy between the parties that arises out of this Agreement or breach thereof promptly by negotiations between senior executives who have authority to settle the controversy and who are not the persons with direct responsibility for the day-to-day administration of services provided under and received pursuant to this Agreement. Any such dispute that has not been resolved by negotiation as provided above shall be determined by binding arbitration. All disputes arising from or related to this Agreement, which the parties are unable to resolve pursuant to the paragraph above, shall be submitted to arbitration before a single arbitrator, mutually agreeable to the parties, and administered by the American Arbitration Association (AAA) in Wilmington, DE (or at a location mutually agreed to by the parties) under the Commercial Arbitration Rules then existing of the AAA, and judgment on the award may be entered in a court of competent jurisdiction. The parties acknowledge and agree that the United Nations Convention on Contracts for the International Sale of Goods is specifically excluded from application to this Agreement. The arbitrator shall have exclusive authority to resolve any dispute relating to the interpretation, applicability, enforceability, or formation of the agreement to arbitrate, including but not limited to, any claim that all or part of the agreement to arbitrate is void or voidable for any reason. Unless the parties agree otherwise, the parties and the arbitrator shall treat the proceedings, any related discovery, and the decisions, as confidential, except in connection with judicial proceedings ancillary to the arbitration, such as a judicial challenge to, or enforcement of, an award, and unless otherwise required by law or to protect a legal right of a party. The arbitrator is empowered to impose reasonable limits on discovery, if any, and the time and manner for presenting evidence, with the goal of an efficient and economical arbitration process. The arbitrator must follow the rule of law in entering any award or relief in the arbitration. Except as may be required by law, neither party nor an arbitrator may disclose the existence, content, or results of any arbitration hereunder without the prior written consent of both parties. 18.10 Counterparts. This Agreement may be executed in one or more counterparts, each of which will be deemed an original but all of which together will constitute one and the same instrument.

39 Any photocopy, facsimile or electronic reproduction of the executed Agreement shall constitute an original. 18.11 Publicity. Neither party will make any press release or other public disclosure regarding this Agreement or the transactions contemplated hereby without the other party's express prior written consent, except as required under applicable law or by any governmental agency, in which case the party required to make the press release or public disclosure shall use commercially reasonable efforts to obtain the approval of the other party as to the form, nature and extent of the press release or public disclosure prior to issuing the press release or making the public disclosure. Neither party shall use the names, logos, or trademarks of any other party for publicity or advertising purposes without the prior written consent of such other party. 18.12 Survival. Termination or expiration of this Agreement will not affect any rights and obligation of the parties that arose prior to such termination or expiration. In addition, Articles 6 (Remedies for Non-conforming Product), 11 (Confidentiality), 12 (Intellectual Property), 13 (Indemnification), 14 (Insurance), 16 (Limitations of Liability), 17 (Notice), 18 (Miscellaneous), and Sections 9.7 (Recalls) and 15.3 (Effect of Termination), and any other provisions of this Agreement which by their nature are intended to survive shall survive expiration or termination of this Agreement, provided that the obligations under Articles 11 and 6 shall survive only for the period stated therein. 18.13 Force Majeure. Except as to payments required under this Agreement, neither party shall be liable in damages for, nor shall this Agreement be terminable or cancelable by reason of, any caused by events or other action or failure to act of any government or agency thereof, war or insurrection, civil commotion, destruction of production facilities or materials by earthquake, fire, flood or storm, labor disturbances, epidemic, epidemic, pandemic, public utilities or common carriers; provided however, that the party seeking relief hereunder shall immediately notify the other party of such caus The party that may invoke this Section 18.13 shall use all reasonable endeavors to reinstate its ongoing obligations to the other . If the cause(s) shall continue unabated for one hundred eighty (180) days, then both parties shall discuss and negotiate in good faith any required modifications to this Agreement. 18.14 Data Protection. Indivior and Curia acknowledge and agree as follows: (i) in the performance of this Agreement, and the delivery of any documentation hereunder, certain Personal Data may be generated, disclosed to a party to this Agreement, and may be incorporated into files processed by either party or by the Affiliates of either party; (ii) Personal Data will be processed and stored solely for purposes of performing this Agreement and will be maintained for no longer than is necessary; (iii) each party represents and warrants that it has all legal right and authority to disclose any Personal Data of any third party it discloses to the other party to this Agreement, and that it has obtained the necessary consents from the relevant third party data subjects to so disclose such Personal Data; (iv) each party is aware that an individual whose Personal Data is processed hereunder has certain rights to request access to, removal of or restriction on the processing of its Personal Data, and each party will provide reasonable cooperation to the other as may be requested

40 in the processing of any such request from such an individual. shall be as defined in Article 4 of Regulation (EU) 2016/679 (General Data Protection Regulation). 18.15 Sanctions, Trade Controls and Anti-Money Laundering Compliance. Each party represents and warrants that (i) neither it nor any of its affiliates, officers, directors or employees involved in the execution of this contract (including those participating in negotiations, meetings or the Sectoral Sanctions List or the Foreign Sanctions Evaders List maintained by the U.S. Treasury other blocked, restricted, sanctioned or denied parties list maintained by the U.S. government (including any department thereof) or any other government having jurisdiction over the transaction between the parties or a person or entity with whom a U.S. person (as defined by the laws and regulations administered by OFAC, 31 C.F.R. Parts 500- of the United States (as defined by the OFAC Regulations) is otherwise prohibited from dealing with or more owned in the aggregate by one or more Sanctions Target(s) or controlled by, or under common control with, or acting for the benefit of or on behalf of, any Sanctions Target; and (iii) it is in compliance with all applicable U.S. and global anti-money laundering laws and regulations, and the financial transactions and/or financial institutions involved in the transaction with the other party, were, and is and will be, at all times, in full compliance with any special designations, rules or proposed rules promulgated pursuant to Section 311 of the USA Patriot Act. Further, each party represents and warrants that it will not export or re-export any goods, technology or software in any manner in connection with this Agreement that violates any applicable national or international export control law, executive order, regulation, rule or sanction, including, but not limited to, the OFAC Regulations, the United States Export Administration Regulations, 15 C.F.R. Parts 730- 774, the International Traffic in Arms Regulations, 22 C.F.R. Part 120 et seq., the Export Administration Act, the International Emergency Economic Powers Act, the Trading with the Enemy Act, the Iran Sanctions Act, the Comprehensive Iran Sanctions, Accountability, and Divestment Act, the Trade Sanctions Reform and Export Enhancement Act of 2000 or any OFAC sanctions program; specifically, each party certifies that it Military End- Users Supplier are not intended for, and will not be diverted to, any military use, and will not be further transferred or re-exported to a military end-user. Either party shall be entitled to terminate the Agreement or stop or withhold performance (including Delivery) hereunder, without any liability whatsoever, in the event of any breach of the statements, representations and warranties contained hereinabove by applicable sanctions or trade control or anti-money laundering laws.

41 IN WITNESS WHEREOF, the parties have caused their duly authorized representative to execute this Agreement as of the Execution Date, the same to binding and effective as of the Effective Date. Indivior UK Limited By: ___________________________ Name:__________________________ Title: __________________________ Curia New Mexico, LLC By: ________________________________ Name:______________________________ Title: _________________________________ Date:_________________________________ Date:________________________ Contract No. /s/ Scott Wagner /s/ Hillel West

42 E X H IB IT A IN D IV IO R M A T E R IA L S T E ST IN G C ur ia s ha ll p er fo rm t he t es ti ng l is te d in t he t ab le b el ow , as a pp li ca bl e, f or I nd iv io r M at er ia ls . T he p ar ti es a gr ee a ll s uc h te st in g is in cl ud ed i n th e U ni t an d/ or B at ch P ri ce a nd s ha ll n ot b e a se pa ra te c ha rg e to I nd iv io r, u nl es s th e pa rt ie s ag re e in w ri ti ng t o an y m od if ic at io ns to th e te st in g an d th e co st s as so ci at ed w it h su ch m od if ic at io ns . In di vi or M at er ia ls N am e T es t T es ti ng S it e Id en tif ic at io n by ¹H N M R A lb an y C op ol ym er M ol e R at io b y ¹H N M R T in C on te nt A pp ea ra nc e A B Q C ol or R es id ua l M on om er C on te nt C -N M R C op lo ym er B lo ck L en gt h A B Q M ol ec ul ar W ei gh t Po ly di sp er si ty W at er B io bu rd en N el so n B ac te ri al E nd ot ox in s Id en tit y A B Q Id en tif ic at io n by F T IR R el at ed S ub st an ce s A ss ay ID b y H PL C R et en ti on T im e W at er C on te nt R es id ua l S ol ve nt : E th yl A ce ta te R es id ua l o n Ig ni tio n A pp ea ra nc e Sp ec if ic O pt ic al R ot at io n B io bu rd en N el so n B ac te ri al E nd ot ox in s Fo re ig n M at te r A B Q V is ua l I ns pe ct io n Ph ys ic al D im en si on s Id en tif ic at io n by F T IR [* ** ] [* ** ] [* ** ]

43 E X H IB IT B C O M M E R C IA L B A T C H A N D U N IT P R IC IN G P ro d u ct : S u b lo ca d e® IB A 1 00 m g: P ri ci n g p er T ie r B at ch S ch ed u le b el ow In cl u d es : al l c os ts to c om pl et e D el iv er y of th e Pr od uc t a s pr ov id ed in S ec ti on 7 .1 D oe s n ot in cl u d e: In di vi or M at er ia ls In di vi or E qu ip m en t P ro ce ss c ha ng es IB C 30 0m g: P ri ci n g p er T ie r B at ch S ch ed u le b el ow In cl u d es : al l c os ts to c om pl et e D el iv er y of th e Pr od uc t a s pr ov id ed in S ec ti on 7 .1 D oe s n ot in cl u d e: In di vi or M at er ia ls In di vi or E qu ip m en t P ro ce ss c ha ng es T w o- P er io d P ri ci ng M od el In di vi or w ill p ay f or , an d C ur ia w il l su pp ly , Pr od uc t un de r a tw o- pe ri od p ri ci ng m od el . T he m od el w ill b e di vi de d in to t w o pe ri od s: P er io d 1 an d P er io d 2. P er io d 1 w ill s ta rt w ith b at ch p ri ce ti er s un ti l c on ve rs io n to p er U ni t P ri ci ng in P er io d 2.

44 U nd er th e P er io d 1 pr ic in g, B at ch P ri ci ng w il l a pp ly . I nd iv io r w il l i ss ue P O s at T ie r B p ri ci ng , w hi ch is th e st an da rd P O p ri ce a t t he P O qu an ti ty . C ur ia w il l t he n is su e an d in vo ic e at T ie r A , B o r C p ri ci ng b as ed o n ac tu al q ua nt it y of P ro du ct o f D el iv er ed . I nd iv io r w il l v er if y th e in vo ic e qu an ti ty a nd u pd at e th e P O to th e ap pr op ri at e ti er p ri ce , i f ne ce ss ar y. T he P er io d 1 pr ic in g m od el is p ro vi de d be lo w . P er io d 1 P ri ci ng - T ie r B at ch S ch ed ul e: ** F or t ra ns ac ti on al p ro ce ss in g, t he P O a nd I nv oi ce q ua nt it ie s m ay n ee d to r ef le ct b at ch c ou nt o f 1 w ith te xt d es cr ip ti on o f th e un it qu an ti ty o n th e P O a nd I nv oi ce . E X A M PL E S Pr e- Pr oc es si ng A ct ua l B at ch Y ie ld Po st -P ro ce ss in g PO P ri ce PO Q ua nt ity In vo ic e Q ua nt ity ** In vo ic e Pr ic e E X A M PL E 1 IB A 1 00 m g [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] E X A M PL E 2 IB A 1 00 m g [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] E X A M PL E 3 IB A 1 00 m g [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] E X A M PL E 4 IB C 3 00 m g [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] E X A M PL E 5 IB C 3 00 m g [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] E X A M PL E 6 IB C 3 00 m g [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ]
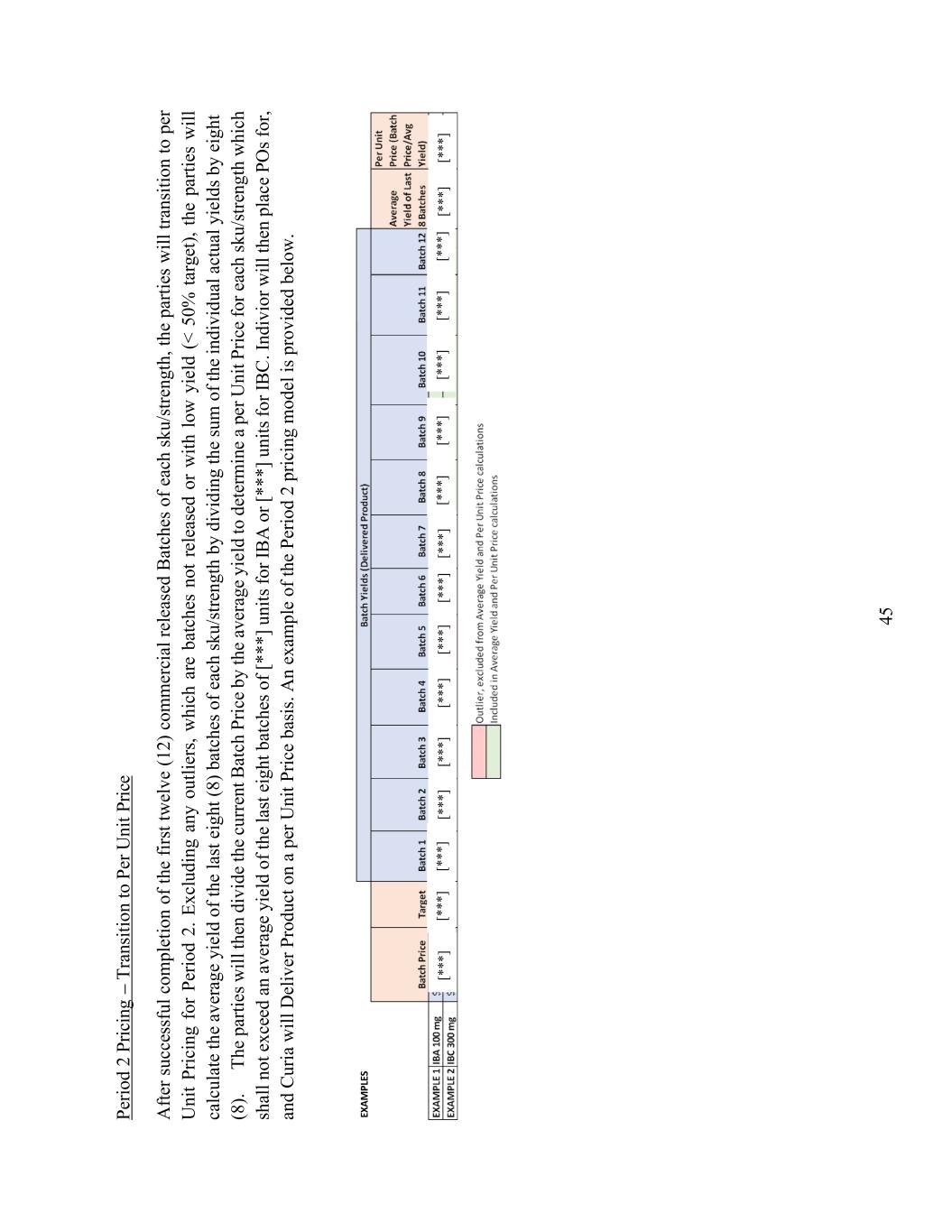
45 P er io d 2 Pr ic in g T ra ns it io n to P er U ni t P ri ce A ft er s uc ce ss fu l c om pl et io n of th e fi rs t t w el ve ( 12 ) co m m er ci al r el ea se d B at ch es o f ea ch s ku /s tr en gt h, th e pa rt ie s w il l t ra ns it io n to p er U ni t P ri ci ng f or P er io d 2. E xc lu di ng a ny o ut li er s, w hi ch a re b at ch es n ot r el ea se d or w it h lo w y ie ld ( < 50 % t ar ge t) , th e pa rt ie s w il l ca lc ul at e th e av er ag e yi el d of th e la st e ig ht ( 8) b at ch es o f ea ch s ku /s tr en gt h by d iv id in g th e su m o f t he in di vi du al a ct ua l y ie ld s by e ig ht (8 ). T he p ar tie s w il l t he n di vi de th e cu rr en t B at ch P ri ce b y th e av er ag e yi el d to d et er m in e a pe r U ni t P ri ce fo r ea ch s ku /s tr en gt h w hi ch sh al l n ot e xc ee d an a ve ra ge y ie ld o f th e la st e ig ht b at ch es o f [* ** ] un it s fo r IB A o r [* ** ] un it s fo r IB C . I nd iv io r w ill th en p la ce P O s fo r, an d C ur ia w il l D el iv er P ro du ct o n a pe r U ni t P ri ce b as is . A n ex am pl e of th e P er io d 2 pr ic in g m od el is p ro vi de d be lo w . [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ] [* ** ]

46 EXHIBIT C FORM OF SCOPE OF WORK Scope of Work This Statement of Work SOW , effective as of the date of last signature hereto (the SOW Effective Date , is entered into by and between [Curia New Mexico, LLC or other Curia entity performing the services], having an address of [4401 Alexander Blvd. NE, Albuquerque, New Mexico 87107 or other Curia entity address] Curia Indivior UK Limited, having an address of Chapleo Building, Henry Boot Way, Priory Park, Hull HU4 7BY, UK Indivior and is issued pursuant to the Master Development and Supply Agreement by and between Curia and Indivior, effective January 1, 2023 (the Agreement . This SOW is subject to the terms and conditions of the Agreement and is made a part thereof by reference. Any term not otherwise defined herein shall have the meaning specified in the Agreement. To the extent any terms or conditions of this SOW conflict with the terms and conditions of the Agreement, the terms and conditions of the Agreement shall control, unless this SOW expressly states the intent of the parties that a particular provision of this SOW supersede the Agreement with respect to a particular matter. 1. . [describe services with specificity] 2. a. [describe fees with specificity] b. Fees shall not exceed: [AMOUNT] ($XXX); and Expenses shall not exceed: [AMOUNT] ($XXX), for a total not to exceed amount of [AMOUNT] ($XXX) (the Expenses may include travel, shipping, conference calls, materials duplication, and other expenses related to performing the services. The NTE Amount may be increased upon written approval by Indivior, which must be documented in an amendment to this SOW and signed by both parties. 3. The term of this SOW shall commence on the SOW Effective Date and shall continue until completion of the Services, unless earlier terminated by a party in accordance with the Agreement. 4. This SOW and the Agreement form the basis for the . Any change to this SOW shall be documented in a written change order mutually agreed upon and executed by the parties. 5. This SOW may be executed in one or more counterparts, each of which will be deemed an original but all of which together will constitute one and the same instrument. Any photocopy, facsimile or electronic reproduction of the executed SOW shall constitute an original.

47 6. Any alterations or amendments to this SOW (including any handwritten changes) will be null and void except by an instrument in writing, signed by authorized representatives of both parties. IN WITNESS WHEREOF, this SOW has been executed by the parties hereto through their duly authorized representatives effective as of the SOW Effective Date. [Curia New Mexico, LLC Indivior UK Limited or other Curia entity performing the services] By: By: Name: Name: Title: Title: Date: Date:
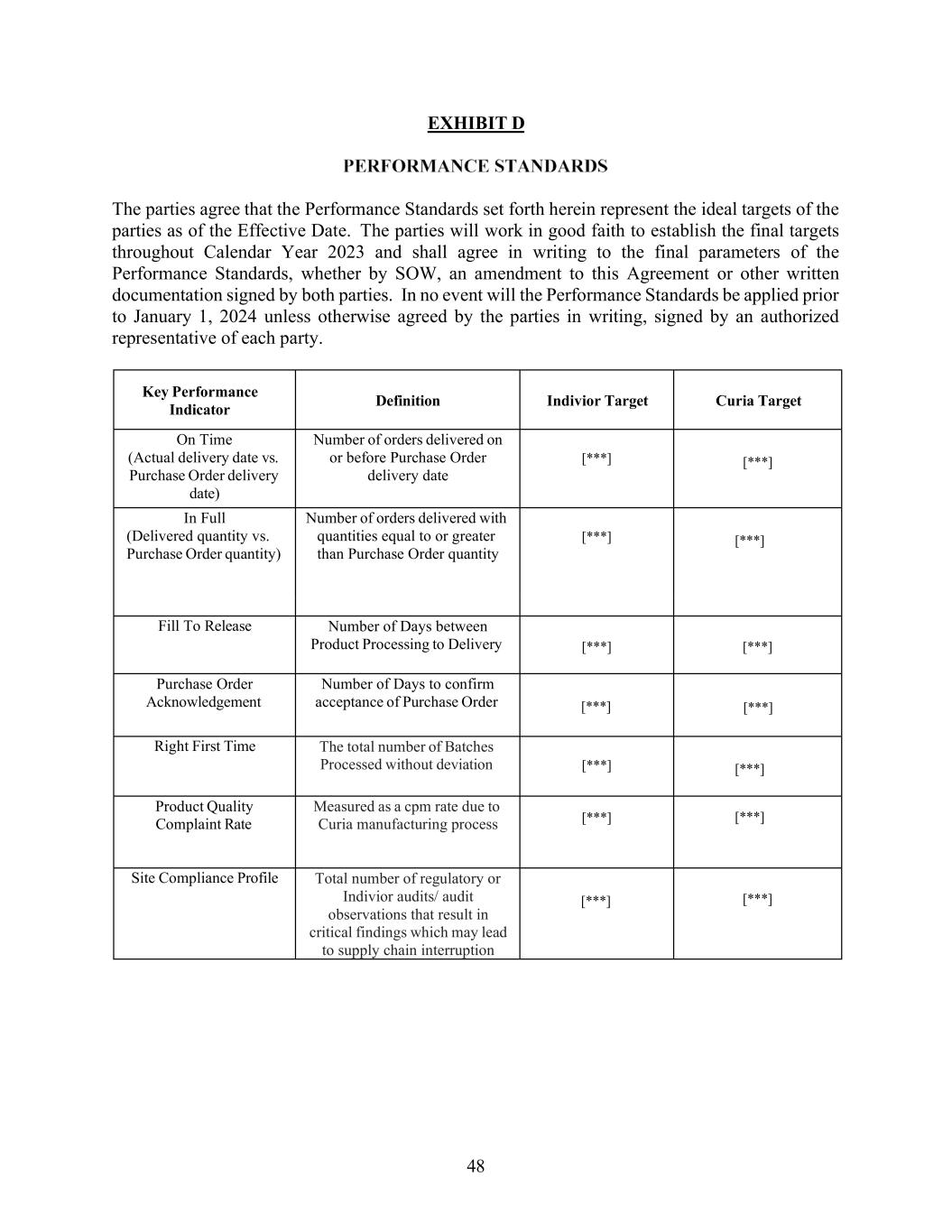
48 EXHIBIT D The parties agree that the Performance Standards set forth herein represent the ideal targets of the parties as of the Effective Date. The parties will work in good faith to establish the final targets throughout Calendar Year 2023 and shall agree in writing to the final parameters of the Performance Standards, whether by SOW, an amendment to this Agreement or other written documentation signed by both parties. In no event will the Performance Standards be applied prior to January 1, 2024 unless otherwise agreed by the parties in writing, signed by an authorized representative of each party. Key Performance Indicator Definition Indivior Target Curia Target On Time (Actual delivery date vs. Purchase Order delivery date) Number of orders delivered on or before Purchase Order delivery date [***] [***] In Full (Delivered quantity vs. Purchase Order quantity) Number of orders delivered with quantities equal to or greater than Purchase Order quantity [***] [***] Fill To Release Number of Days between Product Processing to Delivery [***] [***] Purchase Order Acknowledgement Number of Days to confirm acceptance of Purchase Order [***] [***] Right First Time The total number of Batches Processed without deviation [***] [***] Product Quality Complaint Rate Measured as a cpm rate due to Curia manufacturing process [***] [***] Site Compliance Profile Total number of regulatory or Indivior audits/ audit observations that result in critical findings which may lead to supply chain interruption [***] [***]
49 EXHIBIT E Citation Title FAR 52.203-6 (2020) Restrictions on Subcontractor Sales to the Government FAR 52.203-13 (2020) Contractor Code of Business Ethics and Conduct FAR 52.203-17 (2020) Contractor Employee Whistleblower Rights and Requirement to Inform Employees of Whistleblower Rights FAR 52.203-19 (2017) Prohibition on Requiring Certain Internal Confidentiality Agreements or Statements FAR 52.204-10 (2016) Reporting Executive Compensation and First- Tier Subcontract Awards FAR 52.204-21 (2016) Basic Safeguarding of Covered Contract Information Systems FAR 52.204-23 (2018) Prohibition on Contracting for Hardware, Software, and Services Developed or Provided by Kaspersky Lab and Other Covered Entities FAR 52.204-25 (2020) Prohibition on Contracting for Certain Telecommunications and Video Surveillance Services or Equipment FAR 52.209-6 (2020) Subcontracting with Contractors Debarred, Suspended, or Proposed for Debarment FAR 52.222-17 (2014) Nondisplacement of Qualified Workers FAR 52.222-21 (2015) Prohibition of Segregated Facilities FAR 52.222-26 (2015) Equal Opportunity FAR 52.222-35 (2020) Equal Opportunity for Veterans FAR 52.222-36 (2020) Equal Opportunity for Workers with Disabilities FAR 52.222-37 (2020) Employment Reports on Veterans FAR 52.222-40 (2010) Notification of Employee Rights Under the National Labor Relations Act FAR 52.222-41 (2018) Service Contract Labor Standards FAR 52.222-50 (2020) Combating Trafficking in Persons FAR 52.222-51 (2014) Exemption from Application of the Service Contract Labor Standards to Contracts for Maintenance, Calibration, or Repair of Certain Equipment-Requirements FAR 52.222-53 (2014) Exemption from Application of the Service Contract Labor Standards to Contracts for Certain Services-Requirements

50 FAR 52.222-54 (2015) Employment Eligibility Verification FAR 52.222-55 (2020) Minimum Wages Under Executive Order 13658 FAR 52.222-62 (2017) Paid Sick Leave Under Executive Order 13706 FAR 52.223-18 (2011) Encouraging Contractor Policies to Ban Text Messaging While Driving FAR 52.225-13 (2008) Restriction of Certain Foreign Purchases FAR 52.232-40 (2013) Providing Accelerated Payment to Small Business Subcontractors