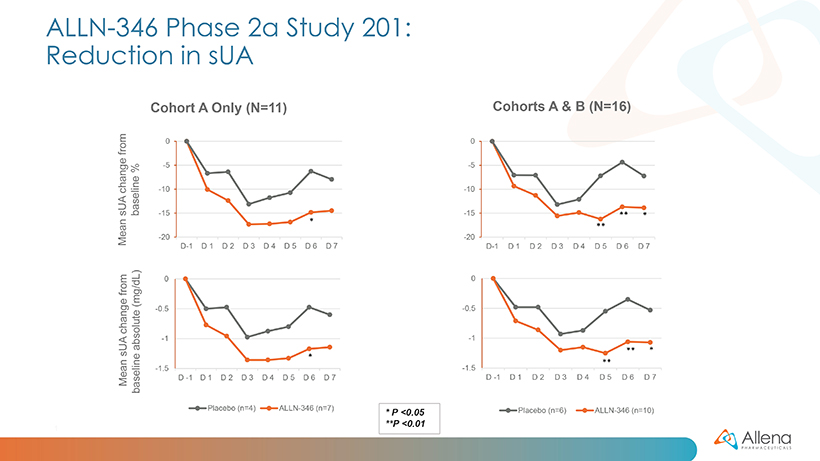
The sUA data analyzed from the full group of 16 patients during the one-week treatment period are shown graphically below:
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported):
(Exact name of registrant as specified in its charter)
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
| (Address of principal executive offices) | (Zip Code) |
Registrant’s telephone number, including area code (
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
Trading |
Name of each exchange on which registered | ||
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
| Item 8.01. | Other Events. |
On August 11, 2022, Allena Pharmaceuticals, Inc. (the “Company” or “Allena”), a biopharmaceutical company deploying its novel oral biologic platform to discover, develop and commercialize first-in-class, oral enzyme therapeutics for difficult-to-treat metabolic diseases, provided the following clinical and corporate update.
ALLN-346 Program Update
ALLN-346 is a potential first-in-class, non-absorbed, orally administered enzyme in development for the treatment of hyperuricemia and gout in the setting of advanced chronic kidney disease (CKD). In November 2021, the Company announced Fast Track Designation for ALLN-346 from the U.S. Food and Drug Administration (FDA).
The ALLN-346 Phase 2a clinical program consists of two trials: Study 201, a one-week inpatient study conducted at a clinical pharmacology unit, and Study 202, a two-week outpatient study being conducted at 23 sites across the U.S. These studies follow previously reported Phase 1 studies, including both a single-ascending dose and multiple-ascending dose study in healthy volunteers. In both Phase 1 studies, ALLN-346 was well tolerated with no clinically significant safety signals and no dose-limiting toxicities observed in any cohort up to the highest administered dose.
For Study 201, patients with hyperuricemia were randomized (2:1) to receive either five capsules of ALLN-346 or matching placebo three times daily for one week. The trial enrolled 16 patients (11 in cohort A and 5 in cohort B), the majority of whom had stage 2 CKD. Preliminary, topline data from the cohort A patients were reported in January 2022.
As announced in January, data from the first group of patients (cohort A) demonstrated a statistically significant reduction in serum uric acid (sUA) from baseline (p<0.05) in patients treated with ALLN-346 compared to placebo. The results from the additional five patients (cohort B) were consistent with those seen in the first 11 patients. In the full group of 16 patients, statistically significant reductions in sUA were seen from days five to seven (p<0.01 on days five and six, and p<0.05 on day seven). There was approximately a 15% reduction from baseline seen in sUA in patients treated with ALLN-346 vs. an approximately 8% reduction from baseline seen in the placebo group. The results also provide support for the potential GI-based mechanism of action of ALLN-346. Specifically, there was a positive correlation between the effect of ALLN-346 on sUA reduction and the degree of renal impairment as measured by estimated glomerular filtration rate (eGFR) (correlation coefficient r=0.84; p=0.002). In gout patients with advanced CKD, the intestinal tract becomes the primary route of elimination for urate, and ALLN-346 is specifically designed to capitalize on this physiologic adaptation by enhancing the breakdown and secretion of urate in the intestinal tract.
The sUA data analyzed from the full group of 16 patients during the one-week treatment period are shown graphically below:


The second Phase 2a trial of ALLN-346, Study 202, is a two-week, outpatient study designed to assess safety and tolerability in hyperuricemic patients with gout and CKD. Patients are randomized (2:1) to receive either five capsules of ALLN-346 or a matching placebo three times daily. Cohort A enrolled seven patients with Stage 2, or mild CKD (eGFR of 60-89 mL/minute) and Cohort B enrolled 12 patients with Stage 3, or moderate CKD (eGFR of 30-59 mL/minute). The planned Cohort C (patients with Stage 4 CKD, eGFR of 15-29 ml/min) and Cohort D (patients with Stage 3 CKD, eGFR of 30-59 ml/min, in combination with allopurinol treatment) are designed to evaluate up to 24 subjects per cohort to assess safety and serum urate changes in the patient populations which the Company believes are most representative of the expected target populations for ALLN-346.
Data from Cohorts A and B are summarized graphically below. In Study 202 mean percent reductions from baseline in sUA on days seven and fourteen were between zero and five percent for patients treated with ALLN-346, vs. an increase of two to five percent from baseline seen in the placebo group. These changes were not statistically significant. Numerically larger responses were observed in Cohort B, which included patients with more severe CKD, compared with Cohort A, which the Company believes provides further evidence for the GI-based mechanism of action of ALLN-346. However, the positive correlation between the effect of ALLN-346 on sUA reduction and the degree of renal impairment as measured by estimated glomerular filtration rate seen in Study 201 was not seen in Study 202. Two out of eleven ALLN-346 treated patients, and no placebo treated patients, experienced a gout flare. There are several possible triggers for gout flares, including the initiation of urate lowering therapy.
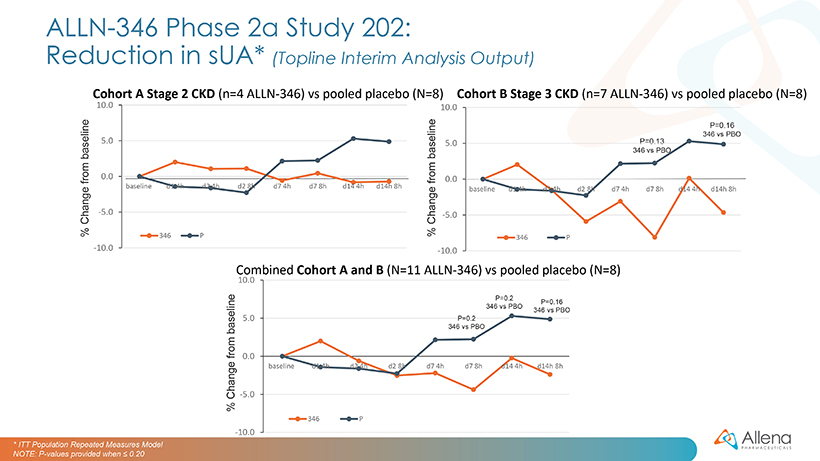
ALLN-346 was well tolerated, and analysis of clinical and laboratory parameters revealed no significant safety signals. The Company believes that the aggregate safety data from Study 201 and cohorts A and B of Study 202 allow for the possibility of opening cohort C in patients with more advanced CKD and cohort D in patients being treated with allopurinol.
Corporate Update
The Company is continuing to evaluate the data from cohorts A and B of Study 202 and the long-term viability of the ALLN-346 clinical program. In light of the clinical data observed to date, in particular the data from Study 201, which demonstrated a potential GI-based mechanism of action, it is possible that the clinical effect could be more pronounced in a more acute patient population (cohort C) or when used in combination with allopurinol (cohort D). However, the Company currently lacks the financial resources to conduct these studies.
As previously disclosed, the Company has initiated a strategic process to explore a range of strategic and financing alternatives to maximize shareholder value, including but not limited to securing financing to enable further development of the ALLN-346 clinical program. However, there can be no assurance that this strategic process will result in the Company pursuing any transaction or that any transaction, if pursued, will be completed.
The Company needs to raise capital imminently to continue as a going concern. Adequate financing may not be available on acceptable terms, or at all. The failure to obtain sufficient funds on commercially acceptable terms to fund the Company’s operations and satisfy the Company’s obligations to creditors will likely have a material adverse effect on the Company’s business, results of operations and financial condition and jeopardize its ability to continue operations in the near-term. Unless the Company can raise capital to fund operations, it will need to implement additional cost reduction strategies, including, among others, amending, delaying, limiting, reducing, or terminating the development program for ALLN-346, and it will likely need to seek an in-court or out-of-court restructuring of its liabilities. In the event of such future restructuring activities, holders of the Company’s common stock and other securities will likely suffer a total loss of their investment.
Forward-Looking Statements
This Current Report on Form 8-K release contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including, without limitation, statements concerning the future clinical, regulatory and commercial potential of ALLN-346; and statements regarding Allena’s financial position and need for capital. Any forward-looking statements in this Current Report on Form 8-K are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. Additional risks and uncertainties include, but are not limited to: market and other conditions, the timing for completion of Allena’s clinical trials of its product candidates, risks associated with obtaining, maintaining and protecting intellectual property; risks associated with Allena’s ability to enforce its patents against infringers and defend its patent portfolio against challenges from third parties; the risk of competition from other companies developing products for similar uses; risks associated with Allena’s financial condition and its need to obtain additional funding to support its business activities, including the future clinical development of ALLN-346, and its ability to continue as a going concern; risks associated with Allena’s dependence on third parties; risks related to the COVID-19 coronavirus; risks associated with Allena’s ability to identify and consummate financing and strategic alternatives that yield additional value for shareholders; the timing, benefits and outcome of the Allena’s strategic alternatives review process, including the determination of whether or not to pursue or consummate any strategic alternative, the structure, terms and specific risks and uncertainties associated with any potential strategic transaction, potential disruptions in Allena’s business and stock price as a result of its exploration, review and pursuit of strategic alternatives or the public announcement thereof and any decision or transaction resulting from such review. For a discussion of other risks and uncertainties, and other important factors, any of which could cause Allena’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in Allena’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2022, as well as discussions of potential risks, uncertainties and other important factors in Allena’s subsequent filings with the Securities and Exchange Commission. All information in this Current Report on Form 8-K is as of the date of the release, and Allena undertakes no duty to update this information unless required by law.
| Item 9.01. | Financial Statements and Exhibits. |
| (d) | Exhibits. |
| Exhibit Number |
Description | |
| 104 | Cover Page Interactive Data File (Embedded within the Inline XBRL Document). | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: August 11, 2022 | Allena Pharmaceuticals, Inc. | |||||
| By: | /s/ Richard Katz | |||||
| Richard Katz, M.D. | ||||||
| Chief Financial Officer | ||||||